User login
Why is buprenorphine use flatlining?
Opioid overdose deaths are at a record high in the United States, and many of these deaths can be prevented with medications such as buprenorphine, said lead author Kao-Ping Chua, MD, of the University of Michigan, Ann Arbor, in an interview. “However, buprenorphine cannot prevent opioid overdose deaths if patients are never started on the medication or only stay on the medication for a short time. For that reason, rates of buprenorphine initiation and retention are critical metrics for measuring how well the U.S. health care system is responding to the opioid epidemic,” he said.
“At the time we started our study, several other research groups had evaluated U.S. rates of buprenorphine initiation and retention using data through 2020. However, more recent national data were lacking,” Dr. Chua told this news organization. “We felt that this was an important knowledge gap given the many changes in society that have occurred since 2020,” he noted. “For example, it was possible that the relaxation of social distancing measures during 2021 and 2022 might have reduced barriers to health care visits, thereby increasing opportunities to initiate treatment for opioid addiction with buprenorphine,” he said.
Dr. Chua and colleagues used data from the IQVIA Longitudinal Prescription Database, which reports 92% of prescriptions dispensed from retail pharmacies in the United States. “Buprenorphine products included immediate-release and extended-release formulations approved for opioid use disorder but not formulations primarily used to treat pain,” they write.
Monthly buprenorphine initiation was defined as the number of patients initiating therapy per 100,000 individuals. For retention, the researchers used a National Quality Forum-endorsed quality measure that defined retention as continuous use of buprenorphine for at least 180 days.
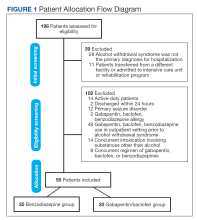
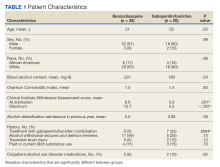
A total of 3,006,629 patients began buprenorphine therapy during the study period; approximately 43% were female.
During the first years of the study period, from January 2016 through September 2018, the monthly buprenorphine initiation rate increased from 12.5 per 100,000 to 15.9 per 100,000, with a statistically significant monthly percentage change of 0.62% (P < .001).
However, from October 2018 through October 2022, the monthly percentage remained essentially the same (P = .62) with a monthly percentage change of −0.03%.
From March 2020 through December 2020, the median monthly buprenorphine initiation rate was 14.4 per 100,000, only slightly lower than the rates from January 2019 through February 2020 and from January 2021 through October 2022 (15.5 per 100,000 and 15.0 per 100,000, respectively).
Over the entire study period from January 2016 through October 2022, the median monthly retention rate for buprenorphine use was 22.2%. This rate increased minimally, with no significant changes in slope and a monthly percentage change of 0.08% (P = .04).
The study findings were limited by several factors, including a lack of data on race and ethnicity, in-clinic administration of buprenorphine, and buprenorphine dispensing through methadone outpatient programs, the researchers note. Also, data did not indicate whether some patients began buprenorphine to treat pain, they say. The timing of the flattening of buprenorphine use also suggests the influence of factors beyond the COVID-19 pandemic, they write.
However, the results were strengthened by the large sample size and suggest that efforts to date to increase buprenorphine use have been unsuccessful, the researchers write. “A comprehensive approach is needed to eliminate barriers to buprenorphine initiation and retention, such as stigma and uneven access to prescribers,” they conclude.
Study highlights underuse of buprenorphine option
“Our study shows that buprenorphine initiation rates have been flat since the end of 2018 and that rates of 180-day retention in buprenorphine therapy have remained low throughout 2016-2022,” Dr. Chua told this news organization. “Neither of these findings are particularly surprising, but they are disappointing,” he said. “There were a lot of policy and clinical efforts to maintain and expand access to buprenorphine during the COVID-19 pandemic, such as allowing buprenorphine to be prescribed via telehealth without an in-person visit and eliminating training requirements for the waiver that previously was required to prescribe buprenorphine.
“The fact that buprenorphine initiation and retention did not rise after these efforts were implemented suggests that they were insufficient to meet the rising need for this medication,” he said.
The current study “adds to a growing body of research suggesting that clinicians are not maximizing opportunities to initiate buprenorphine treatment among patients with opioid addiction,” Dr. Chua said. He cited another of his recent studies in which 1 in 12 patients were prescribed buprenorphine within 30 days of an emergency department visit for opioid overdose from August 2019 to April 2021, but half of patients with emergency department visits with anaphylaxis were prescribed anepinephrine auto-injector.
“My hope is that our new study will further underscore to clinicians how much the health care system is underusing a critical tool to prevent opioid overdose deaths,” he said.
The federal government’s recent elimination of the waiver needed to prescribe buprenorphine may move the needle, but to what degree remains to be seen, Dr. Chua added. “It is possible this intervention will be insufficient to overcome the many other barriers to buprenorphine initiation and retention, such as stigma about the drug among clinicians, patients, and pharmacists,” he said.
Lack of education remains a barrier to buprenorphine use
The current study is important to determine whether attempts to increase buprenorphine initiation and treatment retention are working, said Reuben J. Strayer, MD, director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York, in an interview.
Dr. Strayer was not involved in the current study, but said he was surprised that initiation of buprenorphine didn’t decrease more dramatically during the pandemic, given the significant barriers to accessing care during that time.
However, “efforts to increase buprenorphine initiation and retention have not been sufficiently effective,” Dr. Strayer said. “The rise of fentanyl as a primary street opioid, replacing heroin, has dissuaded both patients and providers from initiating buprenorphine for fear of precipitated withdrawal.”
The elimination of the DATA 2000 (X) waiver was the removal of a potential barrier to increased buprenorphine use, said Dr. Strayer. “Now that the DATA 2000 (X) waiver has been eliminated, the focus of buprenorphine access is educating primary care and inpatient providers on its use, so that patients with OUD [opioid use disorder] can be treated, regardless of the venue at which they seek care,” he said.
Looking ahead, “The priority in buprenorphine research is determining the most effective way to initiate buprenorphine without the risk of precipitated withdrawal,” Dr. Strayer added.
The study was supported in part by the Benter Foundation, the Michigan Department of Health and Human Services, and the Susan B. Meister Child Health Evaluation and Research Center in the department of pediatrics at the University of Michigan. Dr. Chua was supported by the National Institute on Drug Abuse. Dr. Strayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths are at a record high in the United States, and many of these deaths can be prevented with medications such as buprenorphine, said lead author Kao-Ping Chua, MD, of the University of Michigan, Ann Arbor, in an interview. “However, buprenorphine cannot prevent opioid overdose deaths if patients are never started on the medication or only stay on the medication for a short time. For that reason, rates of buprenorphine initiation and retention are critical metrics for measuring how well the U.S. health care system is responding to the opioid epidemic,” he said.
“At the time we started our study, several other research groups had evaluated U.S. rates of buprenorphine initiation and retention using data through 2020. However, more recent national data were lacking,” Dr. Chua told this news organization. “We felt that this was an important knowledge gap given the many changes in society that have occurred since 2020,” he noted. “For example, it was possible that the relaxation of social distancing measures during 2021 and 2022 might have reduced barriers to health care visits, thereby increasing opportunities to initiate treatment for opioid addiction with buprenorphine,” he said.
Dr. Chua and colleagues used data from the IQVIA Longitudinal Prescription Database, which reports 92% of prescriptions dispensed from retail pharmacies in the United States. “Buprenorphine products included immediate-release and extended-release formulations approved for opioid use disorder but not formulations primarily used to treat pain,” they write.
Monthly buprenorphine initiation was defined as the number of patients initiating therapy per 100,000 individuals. For retention, the researchers used a National Quality Forum-endorsed quality measure that defined retention as continuous use of buprenorphine for at least 180 days.
A total of 3,006,629 patients began buprenorphine therapy during the study period; approximately 43% were female.
During the first years of the study period, from January 2016 through September 2018, the monthly buprenorphine initiation rate increased from 12.5 per 100,000 to 15.9 per 100,000, with a statistically significant monthly percentage change of 0.62% (P < .001).
However, from October 2018 through October 2022, the monthly percentage remained essentially the same (P = .62) with a monthly percentage change of −0.03%.
From March 2020 through December 2020, the median monthly buprenorphine initiation rate was 14.4 per 100,000, only slightly lower than the rates from January 2019 through February 2020 and from January 2021 through October 2022 (15.5 per 100,000 and 15.0 per 100,000, respectively).
Over the entire study period from January 2016 through October 2022, the median monthly retention rate for buprenorphine use was 22.2%. This rate increased minimally, with no significant changes in slope and a monthly percentage change of 0.08% (P = .04).
The study findings were limited by several factors, including a lack of data on race and ethnicity, in-clinic administration of buprenorphine, and buprenorphine dispensing through methadone outpatient programs, the researchers note. Also, data did not indicate whether some patients began buprenorphine to treat pain, they say. The timing of the flattening of buprenorphine use also suggests the influence of factors beyond the COVID-19 pandemic, they write.
However, the results were strengthened by the large sample size and suggest that efforts to date to increase buprenorphine use have been unsuccessful, the researchers write. “A comprehensive approach is needed to eliminate barriers to buprenorphine initiation and retention, such as stigma and uneven access to prescribers,” they conclude.
Study highlights underuse of buprenorphine option
“Our study shows that buprenorphine initiation rates have been flat since the end of 2018 and that rates of 180-day retention in buprenorphine therapy have remained low throughout 2016-2022,” Dr. Chua told this news organization. “Neither of these findings are particularly surprising, but they are disappointing,” he said. “There were a lot of policy and clinical efforts to maintain and expand access to buprenorphine during the COVID-19 pandemic, such as allowing buprenorphine to be prescribed via telehealth without an in-person visit and eliminating training requirements for the waiver that previously was required to prescribe buprenorphine.
“The fact that buprenorphine initiation and retention did not rise after these efforts were implemented suggests that they were insufficient to meet the rising need for this medication,” he said.
The current study “adds to a growing body of research suggesting that clinicians are not maximizing opportunities to initiate buprenorphine treatment among patients with opioid addiction,” Dr. Chua said. He cited another of his recent studies in which 1 in 12 patients were prescribed buprenorphine within 30 days of an emergency department visit for opioid overdose from August 2019 to April 2021, but half of patients with emergency department visits with anaphylaxis were prescribed anepinephrine auto-injector.
“My hope is that our new study will further underscore to clinicians how much the health care system is underusing a critical tool to prevent opioid overdose deaths,” he said.
The federal government’s recent elimination of the waiver needed to prescribe buprenorphine may move the needle, but to what degree remains to be seen, Dr. Chua added. “It is possible this intervention will be insufficient to overcome the many other barriers to buprenorphine initiation and retention, such as stigma about the drug among clinicians, patients, and pharmacists,” he said.
Lack of education remains a barrier to buprenorphine use
The current study is important to determine whether attempts to increase buprenorphine initiation and treatment retention are working, said Reuben J. Strayer, MD, director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York, in an interview.
Dr. Strayer was not involved in the current study, but said he was surprised that initiation of buprenorphine didn’t decrease more dramatically during the pandemic, given the significant barriers to accessing care during that time.
However, “efforts to increase buprenorphine initiation and retention have not been sufficiently effective,” Dr. Strayer said. “The rise of fentanyl as a primary street opioid, replacing heroin, has dissuaded both patients and providers from initiating buprenorphine for fear of precipitated withdrawal.”
The elimination of the DATA 2000 (X) waiver was the removal of a potential barrier to increased buprenorphine use, said Dr. Strayer. “Now that the DATA 2000 (X) waiver has been eliminated, the focus of buprenorphine access is educating primary care and inpatient providers on its use, so that patients with OUD [opioid use disorder] can be treated, regardless of the venue at which they seek care,” he said.
Looking ahead, “The priority in buprenorphine research is determining the most effective way to initiate buprenorphine without the risk of precipitated withdrawal,” Dr. Strayer added.
The study was supported in part by the Benter Foundation, the Michigan Department of Health and Human Services, and the Susan B. Meister Child Health Evaluation and Research Center in the department of pediatrics at the University of Michigan. Dr. Chua was supported by the National Institute on Drug Abuse. Dr. Strayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths are at a record high in the United States, and many of these deaths can be prevented with medications such as buprenorphine, said lead author Kao-Ping Chua, MD, of the University of Michigan, Ann Arbor, in an interview. “However, buprenorphine cannot prevent opioid overdose deaths if patients are never started on the medication or only stay on the medication for a short time. For that reason, rates of buprenorphine initiation and retention are critical metrics for measuring how well the U.S. health care system is responding to the opioid epidemic,” he said.
“At the time we started our study, several other research groups had evaluated U.S. rates of buprenorphine initiation and retention using data through 2020. However, more recent national data were lacking,” Dr. Chua told this news organization. “We felt that this was an important knowledge gap given the many changes in society that have occurred since 2020,” he noted. “For example, it was possible that the relaxation of social distancing measures during 2021 and 2022 might have reduced barriers to health care visits, thereby increasing opportunities to initiate treatment for opioid addiction with buprenorphine,” he said.
Dr. Chua and colleagues used data from the IQVIA Longitudinal Prescription Database, which reports 92% of prescriptions dispensed from retail pharmacies in the United States. “Buprenorphine products included immediate-release and extended-release formulations approved for opioid use disorder but not formulations primarily used to treat pain,” they write.
Monthly buprenorphine initiation was defined as the number of patients initiating therapy per 100,000 individuals. For retention, the researchers used a National Quality Forum-endorsed quality measure that defined retention as continuous use of buprenorphine for at least 180 days.
A total of 3,006,629 patients began buprenorphine therapy during the study period; approximately 43% were female.
During the first years of the study period, from January 2016 through September 2018, the monthly buprenorphine initiation rate increased from 12.5 per 100,000 to 15.9 per 100,000, with a statistically significant monthly percentage change of 0.62% (P < .001).
However, from October 2018 through October 2022, the monthly percentage remained essentially the same (P = .62) with a monthly percentage change of −0.03%.
From March 2020 through December 2020, the median monthly buprenorphine initiation rate was 14.4 per 100,000, only slightly lower than the rates from January 2019 through February 2020 and from January 2021 through October 2022 (15.5 per 100,000 and 15.0 per 100,000, respectively).
Over the entire study period from January 2016 through October 2022, the median monthly retention rate for buprenorphine use was 22.2%. This rate increased minimally, with no significant changes in slope and a monthly percentage change of 0.08% (P = .04).
The study findings were limited by several factors, including a lack of data on race and ethnicity, in-clinic administration of buprenorphine, and buprenorphine dispensing through methadone outpatient programs, the researchers note. Also, data did not indicate whether some patients began buprenorphine to treat pain, they say. The timing of the flattening of buprenorphine use also suggests the influence of factors beyond the COVID-19 pandemic, they write.
However, the results were strengthened by the large sample size and suggest that efforts to date to increase buprenorphine use have been unsuccessful, the researchers write. “A comprehensive approach is needed to eliminate barriers to buprenorphine initiation and retention, such as stigma and uneven access to prescribers,” they conclude.
Study highlights underuse of buprenorphine option
“Our study shows that buprenorphine initiation rates have been flat since the end of 2018 and that rates of 180-day retention in buprenorphine therapy have remained low throughout 2016-2022,” Dr. Chua told this news organization. “Neither of these findings are particularly surprising, but they are disappointing,” he said. “There were a lot of policy and clinical efforts to maintain and expand access to buprenorphine during the COVID-19 pandemic, such as allowing buprenorphine to be prescribed via telehealth without an in-person visit and eliminating training requirements for the waiver that previously was required to prescribe buprenorphine.
“The fact that buprenorphine initiation and retention did not rise after these efforts were implemented suggests that they were insufficient to meet the rising need for this medication,” he said.
The current study “adds to a growing body of research suggesting that clinicians are not maximizing opportunities to initiate buprenorphine treatment among patients with opioid addiction,” Dr. Chua said. He cited another of his recent studies in which 1 in 12 patients were prescribed buprenorphine within 30 days of an emergency department visit for opioid overdose from August 2019 to April 2021, but half of patients with emergency department visits with anaphylaxis were prescribed anepinephrine auto-injector.
“My hope is that our new study will further underscore to clinicians how much the health care system is underusing a critical tool to prevent opioid overdose deaths,” he said.
The federal government’s recent elimination of the waiver needed to prescribe buprenorphine may move the needle, but to what degree remains to be seen, Dr. Chua added. “It is possible this intervention will be insufficient to overcome the many other barriers to buprenorphine initiation and retention, such as stigma about the drug among clinicians, patients, and pharmacists,” he said.
Lack of education remains a barrier to buprenorphine use
The current study is important to determine whether attempts to increase buprenorphine initiation and treatment retention are working, said Reuben J. Strayer, MD, director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York, in an interview.
Dr. Strayer was not involved in the current study, but said he was surprised that initiation of buprenorphine didn’t decrease more dramatically during the pandemic, given the significant barriers to accessing care during that time.
However, “efforts to increase buprenorphine initiation and retention have not been sufficiently effective,” Dr. Strayer said. “The rise of fentanyl as a primary street opioid, replacing heroin, has dissuaded both patients and providers from initiating buprenorphine for fear of precipitated withdrawal.”
The elimination of the DATA 2000 (X) waiver was the removal of a potential barrier to increased buprenorphine use, said Dr. Strayer. “Now that the DATA 2000 (X) waiver has been eliminated, the focus of buprenorphine access is educating primary care and inpatient providers on its use, so that patients with OUD [opioid use disorder] can be treated, regardless of the venue at which they seek care,” he said.
Looking ahead, “The priority in buprenorphine research is determining the most effective way to initiate buprenorphine without the risk of precipitated withdrawal,” Dr. Strayer added.
The study was supported in part by the Benter Foundation, the Michigan Department of Health and Human Services, and the Susan B. Meister Child Health Evaluation and Research Center in the department of pediatrics at the University of Michigan. Dr. Chua was supported by the National Institute on Drug Abuse. Dr. Strayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Cocaine damage can be misdiagnosed as nasal vasculitis
Nasal damage from cocaine use can be misdiagnosed as a rare, nonthreatening nasal disease, according to researchers from the United Kingdom.
Granulomatosis with polyangiitis (GPA), a disorder which causes inflammation in the nose, sinuses, throat, lungs, and kidneys, can have similar symptoms to cocaine-induced vasculitis, the researchers wrote. Drug testing can help identify patients who have cocaine-induced disease, they argued.
“Patients with destructive nasal lesions, especially young patients, should have urine toxicology performed for cocaine before diagnosing GPA and considering immunosuppressive therapy,” the authors wrote.
The paper was published in Rheumatology Advances in Practice.
Cocaine is the second-most popular drug in the United Kingdom, with 2.0% of people aged 16-59 years reporting using the drug in the past year. In the United States, about 1.7% of people aged 12 years and older (about 4.8 million people) used cocaine in the last 12 months, according to the 2021 National Survey on Drug Use and Health. The drug can cause midline destructive lesions, skin rash, and other vascular problems, and it can also trigger the production of antineutrophil cytoplasmic antibodies (ANCA) that lead to a clinical presentation that mimics GPA, which can make diagnosis more difficult. Treating cocaine-induced disease with immunosuppressant medication can be ineffective if the patient does not stop using the drug, and can have dangerous side effects, previous case studies suggest.
To better understand cocaine-induced disease, researchers conducted a review of patients who visited vasculitis clinics at Queen Elizabeth Hospital in Birmingham, England, and at the Royal Free Hospital in London between 2016 and 2021. They identified 42 patients with GPA-like symptoms who disclosed cocaine use or tested positive for the drug in urine toxicology test. The study included 23 men, 18 women, and 1 individual who did not identify with either gender. The median age was 41 years, and most patients were white.
Of those who underwent drug testing, more than 85% were positive. Nine patients who denied ever using cocaine were positive for the drug and 11 patients who said they were ex-users also tested positive via urine analysis. During clinical examinations, 30 patients had evidence of septal perforation, of which 6 had oronasal fistulas. Most patients’ symptoms were limited to the upper respiratory tract, though 12 did have other systemic symptoms, including skin lesions, joint pain, breathlessness, fatigue, and diplopia. Of the patients who received blood tests for ANCA, 87.5% tested positive for the antibodies.
The researchers noted that patients who continued cocaine use did not see improvement of symptoms, even if they were treated with immunosuppressant drugs.
“The experience in our two different centers suggests that discontinuation of cocaine is required to manage patients and that symptoms will persist despite immunosuppression if there is ongoing cocaine use,” the authors wrote.
“It can feel like chasing your tail at times if you’re trying to treat the inflammation but the real culprit – what’s driving the inflammation – is persistent,” Lindsay S. Lally, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. She was not involved with the work.
Dr. Lally said the paper had a decent-sized cohort, and “helps us recognize that cocaine use is probably an under-recognized mimic of GPA, even though it’s something we all learn about and talk about.” She added that routine toxicology screening for patients deserves some consideration, though asking patients to complete a drug test could also undermine trust in the doctor-patient relationship. Patients who deny cocaine use may leave the office without providing a urine sample.
If Dr. Lally does suspect cocaine may be the cause of a patient’s systems, having a candid conversation with the patient may have a better chance at getting a patient to open up about their potential drug use. In practice, this means explaining “why it’s so important for me as their partner in this treatment to understand what factors are at play, and how dangerous it could potentially be if I was giving strong immunosuppressive medications [for a condition] that is being induced by a drug,” she said. “I do think that partnership and talking to the patients, at least in many patients, is more helpful than sort of the ‘gotcha’ moment” that can happen with drug testing.
The study authors disclosed no relevant financial relationships. Dr. Lally reported receiving consulting fees from Amgen.
A version of this article first appeared on Medscape.com.
Nasal damage from cocaine use can be misdiagnosed as a rare, nonthreatening nasal disease, according to researchers from the United Kingdom.
Granulomatosis with polyangiitis (GPA), a disorder which causes inflammation in the nose, sinuses, throat, lungs, and kidneys, can have similar symptoms to cocaine-induced vasculitis, the researchers wrote. Drug testing can help identify patients who have cocaine-induced disease, they argued.
“Patients with destructive nasal lesions, especially young patients, should have urine toxicology performed for cocaine before diagnosing GPA and considering immunosuppressive therapy,” the authors wrote.
The paper was published in Rheumatology Advances in Practice.
Cocaine is the second-most popular drug in the United Kingdom, with 2.0% of people aged 16-59 years reporting using the drug in the past year. In the United States, about 1.7% of people aged 12 years and older (about 4.8 million people) used cocaine in the last 12 months, according to the 2021 National Survey on Drug Use and Health. The drug can cause midline destructive lesions, skin rash, and other vascular problems, and it can also trigger the production of antineutrophil cytoplasmic antibodies (ANCA) that lead to a clinical presentation that mimics GPA, which can make diagnosis more difficult. Treating cocaine-induced disease with immunosuppressant medication can be ineffective if the patient does not stop using the drug, and can have dangerous side effects, previous case studies suggest.
To better understand cocaine-induced disease, researchers conducted a review of patients who visited vasculitis clinics at Queen Elizabeth Hospital in Birmingham, England, and at the Royal Free Hospital in London between 2016 and 2021. They identified 42 patients with GPA-like symptoms who disclosed cocaine use or tested positive for the drug in urine toxicology test. The study included 23 men, 18 women, and 1 individual who did not identify with either gender. The median age was 41 years, and most patients were white.
Of those who underwent drug testing, more than 85% were positive. Nine patients who denied ever using cocaine were positive for the drug and 11 patients who said they were ex-users also tested positive via urine analysis. During clinical examinations, 30 patients had evidence of septal perforation, of which 6 had oronasal fistulas. Most patients’ symptoms were limited to the upper respiratory tract, though 12 did have other systemic symptoms, including skin lesions, joint pain, breathlessness, fatigue, and diplopia. Of the patients who received blood tests for ANCA, 87.5% tested positive for the antibodies.
The researchers noted that patients who continued cocaine use did not see improvement of symptoms, even if they were treated with immunosuppressant drugs.
“The experience in our two different centers suggests that discontinuation of cocaine is required to manage patients and that symptoms will persist despite immunosuppression if there is ongoing cocaine use,” the authors wrote.
“It can feel like chasing your tail at times if you’re trying to treat the inflammation but the real culprit – what’s driving the inflammation – is persistent,” Lindsay S. Lally, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. She was not involved with the work.
Dr. Lally said the paper had a decent-sized cohort, and “helps us recognize that cocaine use is probably an under-recognized mimic of GPA, even though it’s something we all learn about and talk about.” She added that routine toxicology screening for patients deserves some consideration, though asking patients to complete a drug test could also undermine trust in the doctor-patient relationship. Patients who deny cocaine use may leave the office without providing a urine sample.
If Dr. Lally does suspect cocaine may be the cause of a patient’s systems, having a candid conversation with the patient may have a better chance at getting a patient to open up about their potential drug use. In practice, this means explaining “why it’s so important for me as their partner in this treatment to understand what factors are at play, and how dangerous it could potentially be if I was giving strong immunosuppressive medications [for a condition] that is being induced by a drug,” she said. “I do think that partnership and talking to the patients, at least in many patients, is more helpful than sort of the ‘gotcha’ moment” that can happen with drug testing.
The study authors disclosed no relevant financial relationships. Dr. Lally reported receiving consulting fees from Amgen.
A version of this article first appeared on Medscape.com.
Nasal damage from cocaine use can be misdiagnosed as a rare, nonthreatening nasal disease, according to researchers from the United Kingdom.
Granulomatosis with polyangiitis (GPA), a disorder which causes inflammation in the nose, sinuses, throat, lungs, and kidneys, can have similar symptoms to cocaine-induced vasculitis, the researchers wrote. Drug testing can help identify patients who have cocaine-induced disease, they argued.
“Patients with destructive nasal lesions, especially young patients, should have urine toxicology performed for cocaine before diagnosing GPA and considering immunosuppressive therapy,” the authors wrote.
The paper was published in Rheumatology Advances in Practice.
Cocaine is the second-most popular drug in the United Kingdom, with 2.0% of people aged 16-59 years reporting using the drug in the past year. In the United States, about 1.7% of people aged 12 years and older (about 4.8 million people) used cocaine in the last 12 months, according to the 2021 National Survey on Drug Use and Health. The drug can cause midline destructive lesions, skin rash, and other vascular problems, and it can also trigger the production of antineutrophil cytoplasmic antibodies (ANCA) that lead to a clinical presentation that mimics GPA, which can make diagnosis more difficult. Treating cocaine-induced disease with immunosuppressant medication can be ineffective if the patient does not stop using the drug, and can have dangerous side effects, previous case studies suggest.
To better understand cocaine-induced disease, researchers conducted a review of patients who visited vasculitis clinics at Queen Elizabeth Hospital in Birmingham, England, and at the Royal Free Hospital in London between 2016 and 2021. They identified 42 patients with GPA-like symptoms who disclosed cocaine use or tested positive for the drug in urine toxicology test. The study included 23 men, 18 women, and 1 individual who did not identify with either gender. The median age was 41 years, and most patients were white.
Of those who underwent drug testing, more than 85% were positive. Nine patients who denied ever using cocaine were positive for the drug and 11 patients who said they were ex-users also tested positive via urine analysis. During clinical examinations, 30 patients had evidence of septal perforation, of which 6 had oronasal fistulas. Most patients’ symptoms were limited to the upper respiratory tract, though 12 did have other systemic symptoms, including skin lesions, joint pain, breathlessness, fatigue, and diplopia. Of the patients who received blood tests for ANCA, 87.5% tested positive for the antibodies.
The researchers noted that patients who continued cocaine use did not see improvement of symptoms, even if they were treated with immunosuppressant drugs.
“The experience in our two different centers suggests that discontinuation of cocaine is required to manage patients and that symptoms will persist despite immunosuppression if there is ongoing cocaine use,” the authors wrote.
“It can feel like chasing your tail at times if you’re trying to treat the inflammation but the real culprit – what’s driving the inflammation – is persistent,” Lindsay S. Lally, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. She was not involved with the work.
Dr. Lally said the paper had a decent-sized cohort, and “helps us recognize that cocaine use is probably an under-recognized mimic of GPA, even though it’s something we all learn about and talk about.” She added that routine toxicology screening for patients deserves some consideration, though asking patients to complete a drug test could also undermine trust in the doctor-patient relationship. Patients who deny cocaine use may leave the office without providing a urine sample.
If Dr. Lally does suspect cocaine may be the cause of a patient’s systems, having a candid conversation with the patient may have a better chance at getting a patient to open up about their potential drug use. In practice, this means explaining “why it’s so important for me as their partner in this treatment to understand what factors are at play, and how dangerous it could potentially be if I was giving strong immunosuppressive medications [for a condition] that is being induced by a drug,” she said. “I do think that partnership and talking to the patients, at least in many patients, is more helpful than sort of the ‘gotcha’ moment” that can happen with drug testing.
The study authors disclosed no relevant financial relationships. Dr. Lally reported receiving consulting fees from Amgen.
A version of this article first appeared on Medscape.com.
FROM RHEUMATOLOGY ADVANCES IN PRACTICE
You’ve quit smoking with vaping. Now what?
This article is part of a series from Medscape on vaping.
Every day, Sonia Sharma, PA, meets people like Natalie H., who is trying to quit vaping.
Natalie, a member of the nicotine addiction support group at the University of California San Francisco’s Fontana Tobacco Treatment Center, switched from traditional cigarettes to vaping but found the electronic version just as addictive and eventually decided to quit using nicotine completely.
“I went from being an occasional cigarette smoker, a few a month, to a daily vaper,” said Natalie, who preferred not to give her last name to protect her privacy. “Vaping made my nicotine addiction worse, not better.”
“We have people tell us they vape before their feet hit the ground in the morning,” said Ms. Sharma, who coleads Natalie’s support group at UCSF. Ms. Sharma has met individuals who had smoked four to five cigarettes a day, switched to e-cigarettes to quit smoking, then vaped the equivalent of a pack a day. Others had switched to vapes to quit but ended up both vaping and smoking again. And others picked up vaping without ever smoking. They want to quit, she said, but are not sure how.
Researchers from the National Institutes of Health in 2020 reported that 5.6 million adults in the United States vaped. A little over 57% of people said they started using e-cigarettes to quit smoking traditional cigarettes. Another study in 2021 based on survey data found that about 60% of e-cigarette users wanted to quit their vaping habit.
Vaping has been marketed as a way to help people kick their smoking habit. Research is inconclusive on this claim. But unlike cessation tools like nicotine gums or lozenges, using vapes for cessation is uncharted territory. Vapers lack guidance for how to use the devices to quit, and they have even less direction on what to do if they develop an addiction to the vapes themselves.
A new addiction?
Monica Hanna, MPH, assistant director of the Nicotine and Tobacco Recovery Program at RWJBarnabas Health’s Institute for Prevention and Recovery in New Jersey, said she has witnessed a higher level of nicotine addiction in the vapers with whom she has worked.
“When someone takes a hit from a vaping device, it doesn’t generate the burn it would from traditional tobacco,” Ms. Hanna said. “This causes people to take a deeper pull, and when they take a deeper pull, they establish a higher level of nicotine dependence over time.”
A 2019 study of nearly 900 people published in the New England Journal of Medicine found that smokers who used vapes for cessation were twice as likely to have quit smoking cigarettes as those who used other nicotine replacement therapy. However, 80% of people who switched to vaping were using e-cigarettes a year after they tried to quit smoking.
Given that potential for addiction, Nancy Rigotti, MD, director of Massachusetts General Hospital’s Tobacco Research and Treatment Center in Boston, said patients must use vapes “properly” for cessation. That means giving up smoking completely and quitting vapes as soon as patients are sure they will not go back to smoking tobacco.
“We are going to need to help these people to stop vaping,” said Dr. Rigotti, who is working with Achieve Life Sciences, a pharmaceutical company developing a prescription drug to treat nicotine addiction from vapes and cigarettes.
And many nicotine users who have tried vaping to quit smoking end up becoming dual users.
“It’s important to stress that health benefits [of switching to vaping] only occur if the switch to vapes is complete and permanent. So far, that appears difficult to do for most people who smoke, and in my anecdotal experience it has not worked,” said J. Taylor Hays, MD, the former medical director of Mayo Clinic’s Nicotine Dependence Center in Rochester, Minn.
Besides challenges in communicating the current evidence, no established method exists to help vapers quit, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There are some experimental methods like using social interventions, counseling, and some educational campaigns,” Dr. Nargis said. “[Little] progress has been done in terms of clinical interventions.”
Unlike cessation products such as gum or a nicotine patch, which have clear recommendations for duration of use, similar guidelines don’t exist for vapes, in part because the U.S. Food and Drug Administration hasn’t yet granted approval of vapes as cessation products.
Alex Clark, the CEO of Consumer Advocates for Smoke-free Alternatives Association, a nonprofit group that supports vaping, said people could vape for longer and still benefit from making the switch from traditional cigarettes.
“The most important thing is that people start replacing cigarettes with a smoke-free product and continue until they’ve completely switched,” said Mr. Clark, whose group accepts donations from the e-cigarette industry. “Following switching, people are encouraged to continue with the product for as long as they feel necessary.”
But 2013 guidelines from the FDA advised makers of nicotine-replacement therapies – including gums, patches, and lozenges – to include labeling that advises users to complete treatment. According to the agency, if a person feels like they “need to use [the NRT product] for a longer period to keep from smoking, talk to your health care provider.”
Dr. Hays, who is now an emeritus professor at the Mayo Clinic, said he would not recommend patients try vaping as a cessation device given the availability of more proven techniques such as patches and gums. If a patient insists, vaping could be considered under the medical guidance of a cessation professional. He also advised people purchase products only from large tobacco companies that are likely to have “reasonable quality control.” Hundreds of vaping devices are on the market, and they are not all equivalent, he said.
But when an e-cigarette user wants to quit vaping, guidance might boil down to using traditional tobacco cessation methods like gums and lozenges because few tools exist to help people with a vaping-specific addiction.
The long-term health outcomes of vaping are also unclear, and decades will pass before scientists are able to make conclusions, according to Thomas Eissenberg, PhD, codirector of Virginia Commonwealth University’s Center for the Study of Tobacco Products in Richmond.
“I don’t think anyone knows what the long-term effects of heated propylene glycol and vegetable glycerin and flavors intended as food ingredients are, especially when these compounds are inhaled hundreds of times a day, week after week, year after year,” Dr. Eissenberg said.
Dr. Rigotti reported that she receives no funding from the tobacco or e-cigarette industry. She is working with Achieve Life Sciences to develop a tool for vaping cessation. Dr. Eissenberg, Ms. Hanna, Dr. Hays, Dr. Nargis, and Ms. Sharma reported no funding from the tobacco or e-cigarette industry.
A version of this article first appeared on Medscape.com.
This article is part of a series from Medscape on vaping.
Every day, Sonia Sharma, PA, meets people like Natalie H., who is trying to quit vaping.
Natalie, a member of the nicotine addiction support group at the University of California San Francisco’s Fontana Tobacco Treatment Center, switched from traditional cigarettes to vaping but found the electronic version just as addictive and eventually decided to quit using nicotine completely.
“I went from being an occasional cigarette smoker, a few a month, to a daily vaper,” said Natalie, who preferred not to give her last name to protect her privacy. “Vaping made my nicotine addiction worse, not better.”
“We have people tell us they vape before their feet hit the ground in the morning,” said Ms. Sharma, who coleads Natalie’s support group at UCSF. Ms. Sharma has met individuals who had smoked four to five cigarettes a day, switched to e-cigarettes to quit smoking, then vaped the equivalent of a pack a day. Others had switched to vapes to quit but ended up both vaping and smoking again. And others picked up vaping without ever smoking. They want to quit, she said, but are not sure how.
Researchers from the National Institutes of Health in 2020 reported that 5.6 million adults in the United States vaped. A little over 57% of people said they started using e-cigarettes to quit smoking traditional cigarettes. Another study in 2021 based on survey data found that about 60% of e-cigarette users wanted to quit their vaping habit.
Vaping has been marketed as a way to help people kick their smoking habit. Research is inconclusive on this claim. But unlike cessation tools like nicotine gums or lozenges, using vapes for cessation is uncharted territory. Vapers lack guidance for how to use the devices to quit, and they have even less direction on what to do if they develop an addiction to the vapes themselves.
A new addiction?
Monica Hanna, MPH, assistant director of the Nicotine and Tobacco Recovery Program at RWJBarnabas Health’s Institute for Prevention and Recovery in New Jersey, said she has witnessed a higher level of nicotine addiction in the vapers with whom she has worked.
“When someone takes a hit from a vaping device, it doesn’t generate the burn it would from traditional tobacco,” Ms. Hanna said. “This causes people to take a deeper pull, and when they take a deeper pull, they establish a higher level of nicotine dependence over time.”
A 2019 study of nearly 900 people published in the New England Journal of Medicine found that smokers who used vapes for cessation were twice as likely to have quit smoking cigarettes as those who used other nicotine replacement therapy. However, 80% of people who switched to vaping were using e-cigarettes a year after they tried to quit smoking.
Given that potential for addiction, Nancy Rigotti, MD, director of Massachusetts General Hospital’s Tobacco Research and Treatment Center in Boston, said patients must use vapes “properly” for cessation. That means giving up smoking completely and quitting vapes as soon as patients are sure they will not go back to smoking tobacco.
“We are going to need to help these people to stop vaping,” said Dr. Rigotti, who is working with Achieve Life Sciences, a pharmaceutical company developing a prescription drug to treat nicotine addiction from vapes and cigarettes.
And many nicotine users who have tried vaping to quit smoking end up becoming dual users.
“It’s important to stress that health benefits [of switching to vaping] only occur if the switch to vapes is complete and permanent. So far, that appears difficult to do for most people who smoke, and in my anecdotal experience it has not worked,” said J. Taylor Hays, MD, the former medical director of Mayo Clinic’s Nicotine Dependence Center in Rochester, Minn.
Besides challenges in communicating the current evidence, no established method exists to help vapers quit, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There are some experimental methods like using social interventions, counseling, and some educational campaigns,” Dr. Nargis said. “[Little] progress has been done in terms of clinical interventions.”
Unlike cessation products such as gum or a nicotine patch, which have clear recommendations for duration of use, similar guidelines don’t exist for vapes, in part because the U.S. Food and Drug Administration hasn’t yet granted approval of vapes as cessation products.
Alex Clark, the CEO of Consumer Advocates for Smoke-free Alternatives Association, a nonprofit group that supports vaping, said people could vape for longer and still benefit from making the switch from traditional cigarettes.
“The most important thing is that people start replacing cigarettes with a smoke-free product and continue until they’ve completely switched,” said Mr. Clark, whose group accepts donations from the e-cigarette industry. “Following switching, people are encouraged to continue with the product for as long as they feel necessary.”
But 2013 guidelines from the FDA advised makers of nicotine-replacement therapies – including gums, patches, and lozenges – to include labeling that advises users to complete treatment. According to the agency, if a person feels like they “need to use [the NRT product] for a longer period to keep from smoking, talk to your health care provider.”
Dr. Hays, who is now an emeritus professor at the Mayo Clinic, said he would not recommend patients try vaping as a cessation device given the availability of more proven techniques such as patches and gums. If a patient insists, vaping could be considered under the medical guidance of a cessation professional. He also advised people purchase products only from large tobacco companies that are likely to have “reasonable quality control.” Hundreds of vaping devices are on the market, and they are not all equivalent, he said.
But when an e-cigarette user wants to quit vaping, guidance might boil down to using traditional tobacco cessation methods like gums and lozenges because few tools exist to help people with a vaping-specific addiction.
The long-term health outcomes of vaping are also unclear, and decades will pass before scientists are able to make conclusions, according to Thomas Eissenberg, PhD, codirector of Virginia Commonwealth University’s Center for the Study of Tobacco Products in Richmond.
“I don’t think anyone knows what the long-term effects of heated propylene glycol and vegetable glycerin and flavors intended as food ingredients are, especially when these compounds are inhaled hundreds of times a day, week after week, year after year,” Dr. Eissenberg said.
Dr. Rigotti reported that she receives no funding from the tobacco or e-cigarette industry. She is working with Achieve Life Sciences to develop a tool for vaping cessation. Dr. Eissenberg, Ms. Hanna, Dr. Hays, Dr. Nargis, and Ms. Sharma reported no funding from the tobacco or e-cigarette industry.
A version of this article first appeared on Medscape.com.
This article is part of a series from Medscape on vaping.
Every day, Sonia Sharma, PA, meets people like Natalie H., who is trying to quit vaping.
Natalie, a member of the nicotine addiction support group at the University of California San Francisco’s Fontana Tobacco Treatment Center, switched from traditional cigarettes to vaping but found the electronic version just as addictive and eventually decided to quit using nicotine completely.
“I went from being an occasional cigarette smoker, a few a month, to a daily vaper,” said Natalie, who preferred not to give her last name to protect her privacy. “Vaping made my nicotine addiction worse, not better.”
“We have people tell us they vape before their feet hit the ground in the morning,” said Ms. Sharma, who coleads Natalie’s support group at UCSF. Ms. Sharma has met individuals who had smoked four to five cigarettes a day, switched to e-cigarettes to quit smoking, then vaped the equivalent of a pack a day. Others had switched to vapes to quit but ended up both vaping and smoking again. And others picked up vaping without ever smoking. They want to quit, she said, but are not sure how.
Researchers from the National Institutes of Health in 2020 reported that 5.6 million adults in the United States vaped. A little over 57% of people said they started using e-cigarettes to quit smoking traditional cigarettes. Another study in 2021 based on survey data found that about 60% of e-cigarette users wanted to quit their vaping habit.
Vaping has been marketed as a way to help people kick their smoking habit. Research is inconclusive on this claim. But unlike cessation tools like nicotine gums or lozenges, using vapes for cessation is uncharted territory. Vapers lack guidance for how to use the devices to quit, and they have even less direction on what to do if they develop an addiction to the vapes themselves.
A new addiction?
Monica Hanna, MPH, assistant director of the Nicotine and Tobacco Recovery Program at RWJBarnabas Health’s Institute for Prevention and Recovery in New Jersey, said she has witnessed a higher level of nicotine addiction in the vapers with whom she has worked.
“When someone takes a hit from a vaping device, it doesn’t generate the burn it would from traditional tobacco,” Ms. Hanna said. “This causes people to take a deeper pull, and when they take a deeper pull, they establish a higher level of nicotine dependence over time.”
A 2019 study of nearly 900 people published in the New England Journal of Medicine found that smokers who used vapes for cessation were twice as likely to have quit smoking cigarettes as those who used other nicotine replacement therapy. However, 80% of people who switched to vaping were using e-cigarettes a year after they tried to quit smoking.
Given that potential for addiction, Nancy Rigotti, MD, director of Massachusetts General Hospital’s Tobacco Research and Treatment Center in Boston, said patients must use vapes “properly” for cessation. That means giving up smoking completely and quitting vapes as soon as patients are sure they will not go back to smoking tobacco.
“We are going to need to help these people to stop vaping,” said Dr. Rigotti, who is working with Achieve Life Sciences, a pharmaceutical company developing a prescription drug to treat nicotine addiction from vapes and cigarettes.
And many nicotine users who have tried vaping to quit smoking end up becoming dual users.
“It’s important to stress that health benefits [of switching to vaping] only occur if the switch to vapes is complete and permanent. So far, that appears difficult to do for most people who smoke, and in my anecdotal experience it has not worked,” said J. Taylor Hays, MD, the former medical director of Mayo Clinic’s Nicotine Dependence Center in Rochester, Minn.
Besides challenges in communicating the current evidence, no established method exists to help vapers quit, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There are some experimental methods like using social interventions, counseling, and some educational campaigns,” Dr. Nargis said. “[Little] progress has been done in terms of clinical interventions.”
Unlike cessation products such as gum or a nicotine patch, which have clear recommendations for duration of use, similar guidelines don’t exist for vapes, in part because the U.S. Food and Drug Administration hasn’t yet granted approval of vapes as cessation products.
Alex Clark, the CEO of Consumer Advocates for Smoke-free Alternatives Association, a nonprofit group that supports vaping, said people could vape for longer and still benefit from making the switch from traditional cigarettes.
“The most important thing is that people start replacing cigarettes with a smoke-free product and continue until they’ve completely switched,” said Mr. Clark, whose group accepts donations from the e-cigarette industry. “Following switching, people are encouraged to continue with the product for as long as they feel necessary.”
But 2013 guidelines from the FDA advised makers of nicotine-replacement therapies – including gums, patches, and lozenges – to include labeling that advises users to complete treatment. According to the agency, if a person feels like they “need to use [the NRT product] for a longer period to keep from smoking, talk to your health care provider.”
Dr. Hays, who is now an emeritus professor at the Mayo Clinic, said he would not recommend patients try vaping as a cessation device given the availability of more proven techniques such as patches and gums. If a patient insists, vaping could be considered under the medical guidance of a cessation professional. He also advised people purchase products only from large tobacco companies that are likely to have “reasonable quality control.” Hundreds of vaping devices are on the market, and they are not all equivalent, he said.
But when an e-cigarette user wants to quit vaping, guidance might boil down to using traditional tobacco cessation methods like gums and lozenges because few tools exist to help people with a vaping-specific addiction.
The long-term health outcomes of vaping are also unclear, and decades will pass before scientists are able to make conclusions, according to Thomas Eissenberg, PhD, codirector of Virginia Commonwealth University’s Center for the Study of Tobacco Products in Richmond.
“I don’t think anyone knows what the long-term effects of heated propylene glycol and vegetable glycerin and flavors intended as food ingredients are, especially when these compounds are inhaled hundreds of times a day, week after week, year after year,” Dr. Eissenberg said.
Dr. Rigotti reported that she receives no funding from the tobacco or e-cigarette industry. She is working with Achieve Life Sciences to develop a tool for vaping cessation. Dr. Eissenberg, Ms. Hanna, Dr. Hays, Dr. Nargis, and Ms. Sharma reported no funding from the tobacco or e-cigarette industry.
A version of this article first appeared on Medscape.com.
What will vaping lead to? Emerging research shows damage, and addiction
Jake Warn calls vaping “a toxic artificial love.”
Jake, of Winslow, Maine, was 16 years old when he began vaping. Unlike cigarettes, vaping can be odorless, and its smoke leaves no trace, which allowed him and his friends to use the devices in school bathrooms without fear of being caught.
He would use an entire cartridge containing the vape liquid, the equivalent of smoking one pack of tobacco cigarettes, within 1 school day. By the fall semester of his first year in college, Jake said his use had increased even more.
“It got pricey, so that’s when I really started to notice” the extent of his dependency, he said recently.
Vaping rates among teenagers in Maine doubled from 15.3% to 28.7% between 2017 and 2019, while Jake was in high school. In 2021, 11% of high schoolers across the nation said they regularly smoked e-cigarettes, and an estimated 28% have ever tried the devices, according to the Centers for Disease Control and Prevention.
The Food and Drug Administration classifies e-cigarettes as a tobacco product because many contain nicotine, which comes from tobacco. Like Jake, the habit is likely to carry into adulthood for many who start in their teenage years, experts say.
Electronic nicotine delivery systems (ENDS) such as vapes have been touted by their manufacturers and by some in the medical field as a healthier alternative to cigarettes and as a method to help smokers give up the habit.
But, that’s not how Jake – who had never used combustible cigarettes – picked up vaping, or how he sold the idea to his mother.
“It’s all organic and natural flavoring, it’s just flavored water,” Mary Lou Warn recalled her son saying to her. She researched the health effects of vaping but didn’t find much online. “I knew they were dangerous because you don’t put anything in your lungs that isn’t fresh air.”
A determined athlete in high school, Jake found that his asthma worsened as he transitioned to college, especially when he ran a track meet or during a soccer game.
Mrs. Warn noticed changes off the field, too.
“He was coughing constantly, he wasn’t sleeping well, he wasn’t eating well,” she said. “I knew the addiction was taking over.”
Vaping irritated Jake’s throat, and he would get nosebleeds that he couldn’t stop, she added.
Since Mrs. Warn first looked into the effects of e-cigarettes on respiratory health back in 2017, many studies have been conducted of the short-term health outcomes for first-time smokers who never used combustible tobacco products. Studies suggest that vaping may worsen bronchitis and asthma, raise blood pressure, interfere with brain development in young users, suppress the immune system, and increase the risk of developing a chronic lung disease (Am J Prev Med. 2020 Feb;58[2]:182-90). Studies of mice and cell cultures have found that the vapor or extracts from vapes damage the chemical structure of DNA.
Still, the limited number of long-term human studies has made it hard to know what the health outcomes of e-cigarette users will be in the future. Conclusive studies linking commercial cigarette use to deaths from heart disease and cancer didn’t emerge until the mid-1950s, decades after manufacturers began mass production and marketing in the early 20th century.
Years could pass before researchers gain a clearer understanding of the health implications of long-term e-cigarette use, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There hasn’t been any such study to establish the direct link from ENDS to cancer, but it is understood that it [vaping] may promote the development of cancer and lung damage and inflammation,” Dr. Nargis said.
For decades, advocates built awareness of the harms of tobacco use, which led to a sharp decline in tobacco-related illnesses such as lung cancer. But Hilary Schneider, Maine’s director of government relations for the ACS Cancer Action Network, said she fears the uptick in the use of vapes – especially among those who never smoked or those who use both combustible cigarettes and e-cigarettes – may reverse declines in the rates of smoking-relating diseases.
Multiple studies suggest that inhaling chemicals found in e-cigarettes – including nicotine-carrying aerosols – can damage arteries and inflame and injure the lungs.
Vapes “basically have created a pediatric tobacco-use epidemic,” Ms. Schneider said. “What we’re seeing is unprecedented tobacco use rates, higher rates than we’ve seen in decades.”
One reason many young people start vaping is the attraction to flavors, which range from classic menthol to fruits and sweets. A handful of states have enacted bans or restrictions on the sale of flavored vapes.
“It’s new, and it’s just been marketed in a way that we’re really fighting the false narrative put out there by makers of these products that are trying to make them appealing to kids,” said Rachel Boykan, MD, clinical professor of pediatrics and attending physician at Stony Brook (N.Y.) Children’s Hospital.
The flavor Red Bull, in particular, hooked Jake. And though he wasn’t aware of it at the time, nicotine packed into the pods may have kept him from quitting: The average nicotine concentration in e-cigarettes more than doubled from 2013 to 2018, according to a study by the Truth Initiative and the CDC.
The immediate risks of nicotine on the developing brain are well documented. Studies suggest that nicotine – which is found in ENDS products – may affect adolescents’ ability to learn, remember, and maintain attention.
But many adolescents and young adults who use e-cigarettes say that vaping helps alleviate anxiety and keep them attentive, which adds to the complexity of their dependency, according to Dr. Boykan.
Nicotine “actually interrupts neural circuits, that it can be associated with more anxiety, depression, attention to learning, and susceptibility to other addictive substances,” she said. “That is enough to make it very scary.”
Jake also said a social environment in which so many of his friends vaped also made it difficult for him to quit.
“You’re hanging out with your friends at night, and all of them are using it, and you’re trying not to,” he said.
Jake eventually took a semester off from college for an unrelated surgery. He moved home, away from his vaping classmates. He eventually transferred to a different college and lived at home, where no one vaped and where he wasn’t allowed to smoke in the house, he said.
“He came home and we took him to a doctor, and they didn’t know quite how to handle kids and addiction to e-cigarettes,” Mrs. Warn said.
Not fully understanding the long-term health implications of e-cigarette use has precluded many clinicians from offering clear messaging on the risk of vaping to current and potential users.
“It’s taken pediatricians time to ask the right questions and recognize nicotine addiction” from vaping, said Dr. Boykan, who serves as chair of the Section on Nicotine and Tobacco Prevention and Treatment of the American Academy of Pediatrics. “It’s just hit us so fast.”
But once pediatricians do identify a nicotine dependency, it can be difficult to treat, Dr. Boykan said. Many pediatricians now recognize that e-cigarette addiction may occur in children as early as middle school.
“We don’t have a lot of evidence-based treatments for kids to recommend,” Dr. Boykan said.
Will vaping be a ‘phase?’
Aware of his vaping dependency and the possible risks to his long-term health, Jake, now 23, said he’s lessened his use, compared with his college days, but still struggles to kick the habit for good.
“I’d like to not be able to use all the time, not to feel the urge,” Jake said. “But I think over time it’ll just kind of phase out.”
But his mother said quitting may not be that simple.
“This will be a lifelong journey,” she said. “When I think of who he is, addiction is something he will always have. It’s a part of him now.”
Dr. Boykan, Ms. Schneider, and Dr. Nardis reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Jake Warn calls vaping “a toxic artificial love.”
Jake, of Winslow, Maine, was 16 years old when he began vaping. Unlike cigarettes, vaping can be odorless, and its smoke leaves no trace, which allowed him and his friends to use the devices in school bathrooms without fear of being caught.
He would use an entire cartridge containing the vape liquid, the equivalent of smoking one pack of tobacco cigarettes, within 1 school day. By the fall semester of his first year in college, Jake said his use had increased even more.
“It got pricey, so that’s when I really started to notice” the extent of his dependency, he said recently.
Vaping rates among teenagers in Maine doubled from 15.3% to 28.7% between 2017 and 2019, while Jake was in high school. In 2021, 11% of high schoolers across the nation said they regularly smoked e-cigarettes, and an estimated 28% have ever tried the devices, according to the Centers for Disease Control and Prevention.
The Food and Drug Administration classifies e-cigarettes as a tobacco product because many contain nicotine, which comes from tobacco. Like Jake, the habit is likely to carry into adulthood for many who start in their teenage years, experts say.
Electronic nicotine delivery systems (ENDS) such as vapes have been touted by their manufacturers and by some in the medical field as a healthier alternative to cigarettes and as a method to help smokers give up the habit.
But, that’s not how Jake – who had never used combustible cigarettes – picked up vaping, or how he sold the idea to his mother.
“It’s all organic and natural flavoring, it’s just flavored water,” Mary Lou Warn recalled her son saying to her. She researched the health effects of vaping but didn’t find much online. “I knew they were dangerous because you don’t put anything in your lungs that isn’t fresh air.”
A determined athlete in high school, Jake found that his asthma worsened as he transitioned to college, especially when he ran a track meet or during a soccer game.
Mrs. Warn noticed changes off the field, too.
“He was coughing constantly, he wasn’t sleeping well, he wasn’t eating well,” she said. “I knew the addiction was taking over.”
Vaping irritated Jake’s throat, and he would get nosebleeds that he couldn’t stop, she added.
Since Mrs. Warn first looked into the effects of e-cigarettes on respiratory health back in 2017, many studies have been conducted of the short-term health outcomes for first-time smokers who never used combustible tobacco products. Studies suggest that vaping may worsen bronchitis and asthma, raise blood pressure, interfere with brain development in young users, suppress the immune system, and increase the risk of developing a chronic lung disease (Am J Prev Med. 2020 Feb;58[2]:182-90). Studies of mice and cell cultures have found that the vapor or extracts from vapes damage the chemical structure of DNA.
Still, the limited number of long-term human studies has made it hard to know what the health outcomes of e-cigarette users will be in the future. Conclusive studies linking commercial cigarette use to deaths from heart disease and cancer didn’t emerge until the mid-1950s, decades after manufacturers began mass production and marketing in the early 20th century.
Years could pass before researchers gain a clearer understanding of the health implications of long-term e-cigarette use, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There hasn’t been any such study to establish the direct link from ENDS to cancer, but it is understood that it [vaping] may promote the development of cancer and lung damage and inflammation,” Dr. Nargis said.
For decades, advocates built awareness of the harms of tobacco use, which led to a sharp decline in tobacco-related illnesses such as lung cancer. But Hilary Schneider, Maine’s director of government relations for the ACS Cancer Action Network, said she fears the uptick in the use of vapes – especially among those who never smoked or those who use both combustible cigarettes and e-cigarettes – may reverse declines in the rates of smoking-relating diseases.
Multiple studies suggest that inhaling chemicals found in e-cigarettes – including nicotine-carrying aerosols – can damage arteries and inflame and injure the lungs.
Vapes “basically have created a pediatric tobacco-use epidemic,” Ms. Schneider said. “What we’re seeing is unprecedented tobacco use rates, higher rates than we’ve seen in decades.”
One reason many young people start vaping is the attraction to flavors, which range from classic menthol to fruits and sweets. A handful of states have enacted bans or restrictions on the sale of flavored vapes.
“It’s new, and it’s just been marketed in a way that we’re really fighting the false narrative put out there by makers of these products that are trying to make them appealing to kids,” said Rachel Boykan, MD, clinical professor of pediatrics and attending physician at Stony Brook (N.Y.) Children’s Hospital.
The flavor Red Bull, in particular, hooked Jake. And though he wasn’t aware of it at the time, nicotine packed into the pods may have kept him from quitting: The average nicotine concentration in e-cigarettes more than doubled from 2013 to 2018, according to a study by the Truth Initiative and the CDC.
The immediate risks of nicotine on the developing brain are well documented. Studies suggest that nicotine – which is found in ENDS products – may affect adolescents’ ability to learn, remember, and maintain attention.
But many adolescents and young adults who use e-cigarettes say that vaping helps alleviate anxiety and keep them attentive, which adds to the complexity of their dependency, according to Dr. Boykan.
Nicotine “actually interrupts neural circuits, that it can be associated with more anxiety, depression, attention to learning, and susceptibility to other addictive substances,” she said. “That is enough to make it very scary.”
Jake also said a social environment in which so many of his friends vaped also made it difficult for him to quit.
“You’re hanging out with your friends at night, and all of them are using it, and you’re trying not to,” he said.
Jake eventually took a semester off from college for an unrelated surgery. He moved home, away from his vaping classmates. He eventually transferred to a different college and lived at home, where no one vaped and where he wasn’t allowed to smoke in the house, he said.
“He came home and we took him to a doctor, and they didn’t know quite how to handle kids and addiction to e-cigarettes,” Mrs. Warn said.
Not fully understanding the long-term health implications of e-cigarette use has precluded many clinicians from offering clear messaging on the risk of vaping to current and potential users.
“It’s taken pediatricians time to ask the right questions and recognize nicotine addiction” from vaping, said Dr. Boykan, who serves as chair of the Section on Nicotine and Tobacco Prevention and Treatment of the American Academy of Pediatrics. “It’s just hit us so fast.”
But once pediatricians do identify a nicotine dependency, it can be difficult to treat, Dr. Boykan said. Many pediatricians now recognize that e-cigarette addiction may occur in children as early as middle school.
“We don’t have a lot of evidence-based treatments for kids to recommend,” Dr. Boykan said.
Will vaping be a ‘phase?’
Aware of his vaping dependency and the possible risks to his long-term health, Jake, now 23, said he’s lessened his use, compared with his college days, but still struggles to kick the habit for good.
“I’d like to not be able to use all the time, not to feel the urge,” Jake said. “But I think over time it’ll just kind of phase out.”
But his mother said quitting may not be that simple.
“This will be a lifelong journey,” she said. “When I think of who he is, addiction is something he will always have. It’s a part of him now.”
Dr. Boykan, Ms. Schneider, and Dr. Nardis reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Jake Warn calls vaping “a toxic artificial love.”
Jake, of Winslow, Maine, was 16 years old when he began vaping. Unlike cigarettes, vaping can be odorless, and its smoke leaves no trace, which allowed him and his friends to use the devices in school bathrooms without fear of being caught.
He would use an entire cartridge containing the vape liquid, the equivalent of smoking one pack of tobacco cigarettes, within 1 school day. By the fall semester of his first year in college, Jake said his use had increased even more.
“It got pricey, so that’s when I really started to notice” the extent of his dependency, he said recently.
Vaping rates among teenagers in Maine doubled from 15.3% to 28.7% between 2017 and 2019, while Jake was in high school. In 2021, 11% of high schoolers across the nation said they regularly smoked e-cigarettes, and an estimated 28% have ever tried the devices, according to the Centers for Disease Control and Prevention.
The Food and Drug Administration classifies e-cigarettes as a tobacco product because many contain nicotine, which comes from tobacco. Like Jake, the habit is likely to carry into adulthood for many who start in their teenage years, experts say.
Electronic nicotine delivery systems (ENDS) such as vapes have been touted by their manufacturers and by some in the medical field as a healthier alternative to cigarettes and as a method to help smokers give up the habit.
But, that’s not how Jake – who had never used combustible cigarettes – picked up vaping, or how he sold the idea to his mother.
“It’s all organic and natural flavoring, it’s just flavored water,” Mary Lou Warn recalled her son saying to her. She researched the health effects of vaping but didn’t find much online. “I knew they were dangerous because you don’t put anything in your lungs that isn’t fresh air.”
A determined athlete in high school, Jake found that his asthma worsened as he transitioned to college, especially when he ran a track meet or during a soccer game.
Mrs. Warn noticed changes off the field, too.
“He was coughing constantly, he wasn’t sleeping well, he wasn’t eating well,” she said. “I knew the addiction was taking over.”
Vaping irritated Jake’s throat, and he would get nosebleeds that he couldn’t stop, she added.
Since Mrs. Warn first looked into the effects of e-cigarettes on respiratory health back in 2017, many studies have been conducted of the short-term health outcomes for first-time smokers who never used combustible tobacco products. Studies suggest that vaping may worsen bronchitis and asthma, raise blood pressure, interfere with brain development in young users, suppress the immune system, and increase the risk of developing a chronic lung disease (Am J Prev Med. 2020 Feb;58[2]:182-90). Studies of mice and cell cultures have found that the vapor or extracts from vapes damage the chemical structure of DNA.
Still, the limited number of long-term human studies has made it hard to know what the health outcomes of e-cigarette users will be in the future. Conclusive studies linking commercial cigarette use to deaths from heart disease and cancer didn’t emerge until the mid-1950s, decades after manufacturers began mass production and marketing in the early 20th century.
Years could pass before researchers gain a clearer understanding of the health implications of long-term e-cigarette use, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There hasn’t been any such study to establish the direct link from ENDS to cancer, but it is understood that it [vaping] may promote the development of cancer and lung damage and inflammation,” Dr. Nargis said.
For decades, advocates built awareness of the harms of tobacco use, which led to a sharp decline in tobacco-related illnesses such as lung cancer. But Hilary Schneider, Maine’s director of government relations for the ACS Cancer Action Network, said she fears the uptick in the use of vapes – especially among those who never smoked or those who use both combustible cigarettes and e-cigarettes – may reverse declines in the rates of smoking-relating diseases.
Multiple studies suggest that inhaling chemicals found in e-cigarettes – including nicotine-carrying aerosols – can damage arteries and inflame and injure the lungs.
Vapes “basically have created a pediatric tobacco-use epidemic,” Ms. Schneider said. “What we’re seeing is unprecedented tobacco use rates, higher rates than we’ve seen in decades.”
One reason many young people start vaping is the attraction to flavors, which range from classic menthol to fruits and sweets. A handful of states have enacted bans or restrictions on the sale of flavored vapes.
“It’s new, and it’s just been marketed in a way that we’re really fighting the false narrative put out there by makers of these products that are trying to make them appealing to kids,” said Rachel Boykan, MD, clinical professor of pediatrics and attending physician at Stony Brook (N.Y.) Children’s Hospital.
The flavor Red Bull, in particular, hooked Jake. And though he wasn’t aware of it at the time, nicotine packed into the pods may have kept him from quitting: The average nicotine concentration in e-cigarettes more than doubled from 2013 to 2018, according to a study by the Truth Initiative and the CDC.
The immediate risks of nicotine on the developing brain are well documented. Studies suggest that nicotine – which is found in ENDS products – may affect adolescents’ ability to learn, remember, and maintain attention.
But many adolescents and young adults who use e-cigarettes say that vaping helps alleviate anxiety and keep them attentive, which adds to the complexity of their dependency, according to Dr. Boykan.
Nicotine “actually interrupts neural circuits, that it can be associated with more anxiety, depression, attention to learning, and susceptibility to other addictive substances,” she said. “That is enough to make it very scary.”
Jake also said a social environment in which so many of his friends vaped also made it difficult for him to quit.
“You’re hanging out with your friends at night, and all of them are using it, and you’re trying not to,” he said.
Jake eventually took a semester off from college for an unrelated surgery. He moved home, away from his vaping classmates. He eventually transferred to a different college and lived at home, where no one vaped and where he wasn’t allowed to smoke in the house, he said.
“He came home and we took him to a doctor, and they didn’t know quite how to handle kids and addiction to e-cigarettes,” Mrs. Warn said.
Not fully understanding the long-term health implications of e-cigarette use has precluded many clinicians from offering clear messaging on the risk of vaping to current and potential users.
“It’s taken pediatricians time to ask the right questions and recognize nicotine addiction” from vaping, said Dr. Boykan, who serves as chair of the Section on Nicotine and Tobacco Prevention and Treatment of the American Academy of Pediatrics. “It’s just hit us so fast.”
But once pediatricians do identify a nicotine dependency, it can be difficult to treat, Dr. Boykan said. Many pediatricians now recognize that e-cigarette addiction may occur in children as early as middle school.
“We don’t have a lot of evidence-based treatments for kids to recommend,” Dr. Boykan said.
Will vaping be a ‘phase?’
Aware of his vaping dependency and the possible risks to his long-term health, Jake, now 23, said he’s lessened his use, compared with his college days, but still struggles to kick the habit for good.
“I’d like to not be able to use all the time, not to feel the urge,” Jake said. “But I think over time it’ll just kind of phase out.”
But his mother said quitting may not be that simple.
“This will be a lifelong journey,” she said. “When I think of who he is, addiction is something he will always have. It’s a part of him now.”
Dr. Boykan, Ms. Schneider, and Dr. Nardis reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Should you recommend e-cigs to help patients quit smoking?
In 2014, after smoking cigarettes for 40 years, Kati Markowitz decided to switch to vaping. She had heard the newer electronic cigarettes might be less harmful. And, at the time, she said, she wasn’t aware of other options to try to quit smoking.
For 7 years, she vaped every day.
Then Ms. Markowitz received news she’d hoped never to hear: She had lung cancer. A nodule detected in a CT scan had grown. She was scheduled for treatment – the removal of an entire lobe from her right lung. But first, she said, her surgeon told her she had to quit vaping, which reduces the risk for post-operative complications and enables a healthy recovery.
Ms. Markowitz had thought switching to vaping would be less harmful than smoking cigarettes. Now, she no longer believes that’s true.
“Did I fool myself by hoping to get lucky and not have any bad repercussions? Yes, I did,” Ms. Markowitz said, adding that she wonders if vaping contributed to her lung cancer or if she’ll experience other negative health effects in the future.
Researchers are divided on if e-cigarettes are as effective in smoking cessation as other nicotine replacement therapies like gums and lozenges. They also say more research is needed on the long-term health impacts of vaping to ultimately determine if vapes are a safe replacement for cigarettes.
“There is scientific research to support vaping as a cessation tool, but we wouldn’t use it as a first line of defense because we still need longitudinal studies to understand the long-term risk of e-cigarettes,” said Monica Hanna, MPH, assistant director of the Nicotine and Tobacco Recovery Program at RWJBarnabas Health’s Institute for Prevention and Recovery, Eatontown, N.J. “We also need research to understand exactly how we could use e-cigarettes as a cessation device.”
Vaping to quit
The first prototypes of e-cigarettes were developed in the 1930s, although what are now known as vapes weren’t sold by manufacturers until the 2000s in the United States, following an invention by a former health official in China. The vape was touted by both researchers and manufacturers over the years of development as a way to quit smoking cigarettes.
The Consumer Advocates for Smoke-free Alternatives Association, a nonprofit group that supports vaping and accepts donations from the e-cigarette industry, has compiled more than 13,000 testimonials from people who say vaping helped them give up smoking.
Studies show mixed results that using vapes can help traditional smokers quit.
A November 2022 Cochrane review showed a “high certainty of evidence that people are more likely to stop smoking traditional cigarettes for at least 6 months using e-cigarettes, or ‘vapes,’ than using nicotine replacement therapies, such as patches and gums.” The meta-analysis examined 78 studies with more than 22,000 participants. And a 2019 study with 886 participants, published in the New England Journal Medicine, found smokers who tried vaping to quit were twice as likely after a year to have stopped smoking cigarettes than those who used nicotine replacement therapy.
“In terms of the global research, it’s pretty clear that vaping can help smokers quit,” said Peter Shields, MD, a professor in the department of internal medicine at The Ohio State University College of Medicine, Columbus, who specializes in the treatment of lung cancer.
But a 2013 study published in the Lancet, and another from the Lancet in 2019, found only a modest improvement in cessation outcomes when participants used e-cigarettes paired with patches, compared with patches alone.
“For a disruptive technology that was supposed to end combustible tobacco use, there seems very little large-scale disruption,” said Thomas Eissenberg, PhD, co-director of Virginia Commonwealth University’s Center for the Study of Tobacco Products, Richmond.
Michael Joseph Blaha, MD, MPH, director of clinical research at the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, pointed to research that shows a portion of people who start vaping to quit smoking end up using both products – or become so-called “dual” users.
“I do think there is fairly high-quality evidence that vaping can lead to more cessation, but at the tradeoff of more long-term dual users and more overall nicotine addiction,” Dr. Blaha said. “Vaping remains a third-line clinical tool after nicotine replacement therapy and FDA-approved cessation medications.”
The U.S. Food and Drug Administration has not approved any e-cigarette or vaping device for smoking cessation, like it has for patches and gums, which means manufacturers cannot market their products as helping tobacco smokers quit.
“ Ms. Hanna, from RWJBarnabas Health’s Institute for Prevention and Recovery, said.
Reducing harm and improving health?
Vapes have also been touted as a boon to individual and public health since cigarette smoking is the leading cause of preventable disease and disability in the United States, responsible for more than 480,000 deaths per year in the U.S., according to the U.S. Centers for Disease Control and Prevention.
Quitting smoking lowers the risk of developing various cancers, heart disease, stroke, and other serious diseases. The aim of nicotine replacement therapy is to help smokers quit by gradually providing the body with smaller doses of nicotine over time, without exposing the body to toxic chemicals found in cigarettes.
“No one should say that e-cigarettes are safe, but compared to cigarettes, the data is consistent: They are not as harmful, and when a smoker switches, it’s better for them,” Dr. Shields said. “Like with other nicotine replacement therapies, if there is a risk that someone stops vaping and returns to smoking, I would rather have them as long-term vapers since it is generally considered to be less harmful than combustible tobacco.”
The FDA has allowed a handful of companies to market their electronic nicotine delivery systems as safer than traditional cigarettes by gaining approval through the Premarket Tobacco Product Applications process. In 2021, the agency announced its first PMTA authorization of an electronic cigarette to R.J. Reynolds for three of its tobacco-flavored vaping products. Regulators approved more products from three additional companies in 2022.
But the FDA has also denied others, including two products in 2023 from R.J. Reynolds, stating that, “the applications lacked sufficient evidence to demonstrate that permitting the marketing of the products would be appropriate for the protection of the public health.”
Questions remain among some researchers on the effects of vaping if used long term. Data on the health effects of vapes are just beginning to emerge and are mainly from studies of animals or cells. Measuring health effects among vape users will entail decades more of study, since Americans only gained access to the products in the 2000s.
Dr. Eissenberg said vaping likely does not cause the same diseases as cigarette smoking, but that does not mean they are not harmful. Ingredients found in e-cigarettes, such as heated propylene glycol, vegetable glycerin, and flavors, have only been used as food ingredients. The potential diseases caused by vapes are still unknown, because inhaling these heated ingredients is new. He also said he had “no issue” with an adult smoker vaping to help them quit smoking – as long they do so for a short period.
“I am very concerned that long-term use in adults could lead to considerable disease and death,” Dr. Eissenberg said. “Simply put, the human lung evolved for one purpose: gas exchange of oxygen in, carbon dioxide out. Anything else that enters the lung is a challenge to the organ.”
But Kenneth Warner, PhD, dean emeritus at the University of Michigan School of Public Health, Ann Arbor, said breaking the addiction to traditional cigarettes could reduce high rates of lung cancer in lower income communities where rates of smoking are comparatively high.
About three times as many Americans smoked (12.6%) than vaped (4.7%) in 2021, but those who live in households with lower incomes are more likely to smoke. According to the CDC, use of tobacco is higher among adults who were uninsured (27.3%) or who had Medicaid coverage (28.6%) than among those with private insurance (16.4%). People with annual family incomes of less than $12,500 also are more likely to be diagnosed with lung cancer than those with family incomes of $50,000 or more. Public health researchers have attributed those disparities in part to higher rates of smoking in lower-income households.
Dr. Warner said many lower-income and other Americans may never quit smoking cigarettes because they believe making the switch to e-cigarettes will not benefit their health. A 2022 study, published in the American Journal of Preventive Medicine, found that the percent of Americans who thought vaping was more harmful than smoking quadrupled between 2018 and 2020, from 6.8% to 28.3%. A third of respondents thought vaping was as harmful as smoking.
“We’ve convinced a large percentage of the American public that vaping is as harmful as smoking when it could be helping people quit smoking,” Dr. Warner said. “People are dying right now.”
Ms. Markowitz did quit smoking by taking up vaping. But now she questions if her lung cancer prognosis would have been delayed, or even avoided, if she’d tried a traditional method like a lozenge or gum instead. She vaped once an hour for most of her 7 years of using the devices.
“For people who are trying to stop smoking, I would recommend something like the patch instead,” Ms. Markowitz said.
The Consumer Advocates for Smoke-free Alternatives receives funding from the vaping industry. Dr. Blaha, Dr. Eissenberg, Ms. Hanna, Dr. Shields, and Dr. Warner reported no funding from the tobacco or e-cigarette industry. Dr. Blaha and Dr. Warner receive tobacco-related research funding from the FDA. Dr. Warner is a member of the FDA’s Tobacco Products Scientific Advisory Committee.
A version of this article first appeared on Medscape.com.
In 2014, after smoking cigarettes for 40 years, Kati Markowitz decided to switch to vaping. She had heard the newer electronic cigarettes might be less harmful. And, at the time, she said, she wasn’t aware of other options to try to quit smoking.
For 7 years, she vaped every day.
Then Ms. Markowitz received news she’d hoped never to hear: She had lung cancer. A nodule detected in a CT scan had grown. She was scheduled for treatment – the removal of an entire lobe from her right lung. But first, she said, her surgeon told her she had to quit vaping, which reduces the risk for post-operative complications and enables a healthy recovery.
Ms. Markowitz had thought switching to vaping would be less harmful than smoking cigarettes. Now, she no longer believes that’s true.
“Did I fool myself by hoping to get lucky and not have any bad repercussions? Yes, I did,” Ms. Markowitz said, adding that she wonders if vaping contributed to her lung cancer or if she’ll experience other negative health effects in the future.
Researchers are divided on if e-cigarettes are as effective in smoking cessation as other nicotine replacement therapies like gums and lozenges. They also say more research is needed on the long-term health impacts of vaping to ultimately determine if vapes are a safe replacement for cigarettes.
“There is scientific research to support vaping as a cessation tool, but we wouldn’t use it as a first line of defense because we still need longitudinal studies to understand the long-term risk of e-cigarettes,” said Monica Hanna, MPH, assistant director of the Nicotine and Tobacco Recovery Program at RWJBarnabas Health’s Institute for Prevention and Recovery, Eatontown, N.J. “We also need research to understand exactly how we could use e-cigarettes as a cessation device.”
Vaping to quit
The first prototypes of e-cigarettes were developed in the 1930s, although what are now known as vapes weren’t sold by manufacturers until the 2000s in the United States, following an invention by a former health official in China. The vape was touted by both researchers and manufacturers over the years of development as a way to quit smoking cigarettes.
The Consumer Advocates for Smoke-free Alternatives Association, a nonprofit group that supports vaping and accepts donations from the e-cigarette industry, has compiled more than 13,000 testimonials from people who say vaping helped them give up smoking.
Studies show mixed results that using vapes can help traditional smokers quit.
A November 2022 Cochrane review showed a “high certainty of evidence that people are more likely to stop smoking traditional cigarettes for at least 6 months using e-cigarettes, or ‘vapes,’ than using nicotine replacement therapies, such as patches and gums.” The meta-analysis examined 78 studies with more than 22,000 participants. And a 2019 study with 886 participants, published in the New England Journal Medicine, found smokers who tried vaping to quit were twice as likely after a year to have stopped smoking cigarettes than those who used nicotine replacement therapy.
“In terms of the global research, it’s pretty clear that vaping can help smokers quit,” said Peter Shields, MD, a professor in the department of internal medicine at The Ohio State University College of Medicine, Columbus, who specializes in the treatment of lung cancer.
But a 2013 study published in the Lancet, and another from the Lancet in 2019, found only a modest improvement in cessation outcomes when participants used e-cigarettes paired with patches, compared with patches alone.
“For a disruptive technology that was supposed to end combustible tobacco use, there seems very little large-scale disruption,” said Thomas Eissenberg, PhD, co-director of Virginia Commonwealth University’s Center for the Study of Tobacco Products, Richmond.
Michael Joseph Blaha, MD, MPH, director of clinical research at the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, pointed to research that shows a portion of people who start vaping to quit smoking end up using both products – or become so-called “dual” users.
“I do think there is fairly high-quality evidence that vaping can lead to more cessation, but at the tradeoff of more long-term dual users and more overall nicotine addiction,” Dr. Blaha said. “Vaping remains a third-line clinical tool after nicotine replacement therapy and FDA-approved cessation medications.”
The U.S. Food and Drug Administration has not approved any e-cigarette or vaping device for smoking cessation, like it has for patches and gums, which means manufacturers cannot market their products as helping tobacco smokers quit.
“ Ms. Hanna, from RWJBarnabas Health’s Institute for Prevention and Recovery, said.
Reducing harm and improving health?
Vapes have also been touted as a boon to individual and public health since cigarette smoking is the leading cause of preventable disease and disability in the United States, responsible for more than 480,000 deaths per year in the U.S., according to the U.S. Centers for Disease Control and Prevention.
Quitting smoking lowers the risk of developing various cancers, heart disease, stroke, and other serious diseases. The aim of nicotine replacement therapy is to help smokers quit by gradually providing the body with smaller doses of nicotine over time, without exposing the body to toxic chemicals found in cigarettes.
“No one should say that e-cigarettes are safe, but compared to cigarettes, the data is consistent: They are not as harmful, and when a smoker switches, it’s better for them,” Dr. Shields said. “Like with other nicotine replacement therapies, if there is a risk that someone stops vaping and returns to smoking, I would rather have them as long-term vapers since it is generally considered to be less harmful than combustible tobacco.”
The FDA has allowed a handful of companies to market their electronic nicotine delivery systems as safer than traditional cigarettes by gaining approval through the Premarket Tobacco Product Applications process. In 2021, the agency announced its first PMTA authorization of an electronic cigarette to R.J. Reynolds for three of its tobacco-flavored vaping products. Regulators approved more products from three additional companies in 2022.
But the FDA has also denied others, including two products in 2023 from R.J. Reynolds, stating that, “the applications lacked sufficient evidence to demonstrate that permitting the marketing of the products would be appropriate for the protection of the public health.”
Questions remain among some researchers on the effects of vaping if used long term. Data on the health effects of vapes are just beginning to emerge and are mainly from studies of animals or cells. Measuring health effects among vape users will entail decades more of study, since Americans only gained access to the products in the 2000s.
Dr. Eissenberg said vaping likely does not cause the same diseases as cigarette smoking, but that does not mean they are not harmful. Ingredients found in e-cigarettes, such as heated propylene glycol, vegetable glycerin, and flavors, have only been used as food ingredients. The potential diseases caused by vapes are still unknown, because inhaling these heated ingredients is new. He also said he had “no issue” with an adult smoker vaping to help them quit smoking – as long they do so for a short period.
“I am very concerned that long-term use in adults could lead to considerable disease and death,” Dr. Eissenberg said. “Simply put, the human lung evolved for one purpose: gas exchange of oxygen in, carbon dioxide out. Anything else that enters the lung is a challenge to the organ.”
But Kenneth Warner, PhD, dean emeritus at the University of Michigan School of Public Health, Ann Arbor, said breaking the addiction to traditional cigarettes could reduce high rates of lung cancer in lower income communities where rates of smoking are comparatively high.
About three times as many Americans smoked (12.6%) than vaped (4.7%) in 2021, but those who live in households with lower incomes are more likely to smoke. According to the CDC, use of tobacco is higher among adults who were uninsured (27.3%) or who had Medicaid coverage (28.6%) than among those with private insurance (16.4%). People with annual family incomes of less than $12,500 also are more likely to be diagnosed with lung cancer than those with family incomes of $50,000 or more. Public health researchers have attributed those disparities in part to higher rates of smoking in lower-income households.
Dr. Warner said many lower-income and other Americans may never quit smoking cigarettes because they believe making the switch to e-cigarettes will not benefit their health. A 2022 study, published in the American Journal of Preventive Medicine, found that the percent of Americans who thought vaping was more harmful than smoking quadrupled between 2018 and 2020, from 6.8% to 28.3%. A third of respondents thought vaping was as harmful as smoking.
“We’ve convinced a large percentage of the American public that vaping is as harmful as smoking when it could be helping people quit smoking,” Dr. Warner said. “People are dying right now.”
Ms. Markowitz did quit smoking by taking up vaping. But now she questions if her lung cancer prognosis would have been delayed, or even avoided, if she’d tried a traditional method like a lozenge or gum instead. She vaped once an hour for most of her 7 years of using the devices.
“For people who are trying to stop smoking, I would recommend something like the patch instead,” Ms. Markowitz said.
The Consumer Advocates for Smoke-free Alternatives receives funding from the vaping industry. Dr. Blaha, Dr. Eissenberg, Ms. Hanna, Dr. Shields, and Dr. Warner reported no funding from the tobacco or e-cigarette industry. Dr. Blaha and Dr. Warner receive tobacco-related research funding from the FDA. Dr. Warner is a member of the FDA’s Tobacco Products Scientific Advisory Committee.
A version of this article first appeared on Medscape.com.
In 2014, after smoking cigarettes for 40 years, Kati Markowitz decided to switch to vaping. She had heard the newer electronic cigarettes might be less harmful. And, at the time, she said, she wasn’t aware of other options to try to quit smoking.
For 7 years, she vaped every day.
Then Ms. Markowitz received news she’d hoped never to hear: She had lung cancer. A nodule detected in a CT scan had grown. She was scheduled for treatment – the removal of an entire lobe from her right lung. But first, she said, her surgeon told her she had to quit vaping, which reduces the risk for post-operative complications and enables a healthy recovery.
Ms. Markowitz had thought switching to vaping would be less harmful than smoking cigarettes. Now, she no longer believes that’s true.
“Did I fool myself by hoping to get lucky and not have any bad repercussions? Yes, I did,” Ms. Markowitz said, adding that she wonders if vaping contributed to her lung cancer or if she’ll experience other negative health effects in the future.
Researchers are divided on if e-cigarettes are as effective in smoking cessation as other nicotine replacement therapies like gums and lozenges. They also say more research is needed on the long-term health impacts of vaping to ultimately determine if vapes are a safe replacement for cigarettes.
“There is scientific research to support vaping as a cessation tool, but we wouldn’t use it as a first line of defense because we still need longitudinal studies to understand the long-term risk of e-cigarettes,” said Monica Hanna, MPH, assistant director of the Nicotine and Tobacco Recovery Program at RWJBarnabas Health’s Institute for Prevention and Recovery, Eatontown, N.J. “We also need research to understand exactly how we could use e-cigarettes as a cessation device.”
Vaping to quit
The first prototypes of e-cigarettes were developed in the 1930s, although what are now known as vapes weren’t sold by manufacturers until the 2000s in the United States, following an invention by a former health official in China. The vape was touted by both researchers and manufacturers over the years of development as a way to quit smoking cigarettes.
The Consumer Advocates for Smoke-free Alternatives Association, a nonprofit group that supports vaping and accepts donations from the e-cigarette industry, has compiled more than 13,000 testimonials from people who say vaping helped them give up smoking.
Studies show mixed results that using vapes can help traditional smokers quit.
A November 2022 Cochrane review showed a “high certainty of evidence that people are more likely to stop smoking traditional cigarettes for at least 6 months using e-cigarettes, or ‘vapes,’ than using nicotine replacement therapies, such as patches and gums.” The meta-analysis examined 78 studies with more than 22,000 participants. And a 2019 study with 886 participants, published in the New England Journal Medicine, found smokers who tried vaping to quit were twice as likely after a year to have stopped smoking cigarettes than those who used nicotine replacement therapy.
“In terms of the global research, it’s pretty clear that vaping can help smokers quit,” said Peter Shields, MD, a professor in the department of internal medicine at The Ohio State University College of Medicine, Columbus, who specializes in the treatment of lung cancer.
But a 2013 study published in the Lancet, and another from the Lancet in 2019, found only a modest improvement in cessation outcomes when participants used e-cigarettes paired with patches, compared with patches alone.
“For a disruptive technology that was supposed to end combustible tobacco use, there seems very little large-scale disruption,” said Thomas Eissenberg, PhD, co-director of Virginia Commonwealth University’s Center for the Study of Tobacco Products, Richmond.
Michael Joseph Blaha, MD, MPH, director of clinical research at the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, pointed to research that shows a portion of people who start vaping to quit smoking end up using both products – or become so-called “dual” users.
“I do think there is fairly high-quality evidence that vaping can lead to more cessation, but at the tradeoff of more long-term dual users and more overall nicotine addiction,” Dr. Blaha said. “Vaping remains a third-line clinical tool after nicotine replacement therapy and FDA-approved cessation medications.”
The U.S. Food and Drug Administration has not approved any e-cigarette or vaping device for smoking cessation, like it has for patches and gums, which means manufacturers cannot market their products as helping tobacco smokers quit.
“ Ms. Hanna, from RWJBarnabas Health’s Institute for Prevention and Recovery, said.
Reducing harm and improving health?
Vapes have also been touted as a boon to individual and public health since cigarette smoking is the leading cause of preventable disease and disability in the United States, responsible for more than 480,000 deaths per year in the U.S., according to the U.S. Centers for Disease Control and Prevention.
Quitting smoking lowers the risk of developing various cancers, heart disease, stroke, and other serious diseases. The aim of nicotine replacement therapy is to help smokers quit by gradually providing the body with smaller doses of nicotine over time, without exposing the body to toxic chemicals found in cigarettes.
“No one should say that e-cigarettes are safe, but compared to cigarettes, the data is consistent: They are not as harmful, and when a smoker switches, it’s better for them,” Dr. Shields said. “Like with other nicotine replacement therapies, if there is a risk that someone stops vaping and returns to smoking, I would rather have them as long-term vapers since it is generally considered to be less harmful than combustible tobacco.”
The FDA has allowed a handful of companies to market their electronic nicotine delivery systems as safer than traditional cigarettes by gaining approval through the Premarket Tobacco Product Applications process. In 2021, the agency announced its first PMTA authorization of an electronic cigarette to R.J. Reynolds for three of its tobacco-flavored vaping products. Regulators approved more products from three additional companies in 2022.
But the FDA has also denied others, including two products in 2023 from R.J. Reynolds, stating that, “the applications lacked sufficient evidence to demonstrate that permitting the marketing of the products would be appropriate for the protection of the public health.”
Questions remain among some researchers on the effects of vaping if used long term. Data on the health effects of vapes are just beginning to emerge and are mainly from studies of animals or cells. Measuring health effects among vape users will entail decades more of study, since Americans only gained access to the products in the 2000s.
Dr. Eissenberg said vaping likely does not cause the same diseases as cigarette smoking, but that does not mean they are not harmful. Ingredients found in e-cigarettes, such as heated propylene glycol, vegetable glycerin, and flavors, have only been used as food ingredients. The potential diseases caused by vapes are still unknown, because inhaling these heated ingredients is new. He also said he had “no issue” with an adult smoker vaping to help them quit smoking – as long they do so for a short period.
“I am very concerned that long-term use in adults could lead to considerable disease and death,” Dr. Eissenberg said. “Simply put, the human lung evolved for one purpose: gas exchange of oxygen in, carbon dioxide out. Anything else that enters the lung is a challenge to the organ.”
But Kenneth Warner, PhD, dean emeritus at the University of Michigan School of Public Health, Ann Arbor, said breaking the addiction to traditional cigarettes could reduce high rates of lung cancer in lower income communities where rates of smoking are comparatively high.
About three times as many Americans smoked (12.6%) than vaped (4.7%) in 2021, but those who live in households with lower incomes are more likely to smoke. According to the CDC, use of tobacco is higher among adults who were uninsured (27.3%) or who had Medicaid coverage (28.6%) than among those with private insurance (16.4%). People with annual family incomes of less than $12,500 also are more likely to be diagnosed with lung cancer than those with family incomes of $50,000 or more. Public health researchers have attributed those disparities in part to higher rates of smoking in lower-income households.
Dr. Warner said many lower-income and other Americans may never quit smoking cigarettes because they believe making the switch to e-cigarettes will not benefit their health. A 2022 study, published in the American Journal of Preventive Medicine, found that the percent of Americans who thought vaping was more harmful than smoking quadrupled between 2018 and 2020, from 6.8% to 28.3%. A third of respondents thought vaping was as harmful as smoking.
“We’ve convinced a large percentage of the American public that vaping is as harmful as smoking when it could be helping people quit smoking,” Dr. Warner said. “People are dying right now.”
Ms. Markowitz did quit smoking by taking up vaping. But now she questions if her lung cancer prognosis would have been delayed, or even avoided, if she’d tried a traditional method like a lozenge or gum instead. She vaped once an hour for most of her 7 years of using the devices.
“For people who are trying to stop smoking, I would recommend something like the patch instead,” Ms. Markowitz said.
The Consumer Advocates for Smoke-free Alternatives receives funding from the vaping industry. Dr. Blaha, Dr. Eissenberg, Ms. Hanna, Dr. Shields, and Dr. Warner reported no funding from the tobacco or e-cigarette industry. Dr. Blaha and Dr. Warner receive tobacco-related research funding from the FDA. Dr. Warner is a member of the FDA’s Tobacco Products Scientific Advisory Committee.
A version of this article first appeared on Medscape.com.
Integrating addiction medicine with primary care cost effective: Study
Integrating buprenorphine and harm eduction tools into primary care may improve clinical outcomes, increase costs only modestly, and be cost effective in health systems, authors conclude in an original investigation in JAMA Network Open.
A team led by Raagini Jawa, MD, MPH, with the Center for Research on Healthcare, University of Pittsburgh, set out to analyze costs of the interventions versus increased benefit in extending life expectancy.
Their analysis found that, compared with the status quo, integrating buprenorphine and harm reduction kits (syringes, wound care supplies, etc.) reduced drug use–related deaths by 33% and was cost effective.
“Our results suggest that integrated addiction care in primary care has the potential to save lives and increase nonemergency health care use, which is consistent with prior literature,” the authors write. “Colocated addiction services within primary care is pragmatic and effective and has comparable quality to specialty care. We found that onsite BUP [buprenorphine prescribing] plus HR [harm reduction] provides better outcomes than BUP alone at a lower cost.”
Three strategies compared
Using a microsimulation model of 2.25 million people in the United States who inject opioids, with an average age of 44 (69% of them male), the researchers tested three strategies:
- Status quo. PCP refers to addiction care.
- BUP. PCP services plus onsite buprenorphine prescribing with referral to off-site harm reduction kits.
- BUP plus HR. PCP services plus on-site buprenorphine prescribing and harm reduction kits.
The model is the Reducing Infections Related to Drug Use Cost-Effectiveness (REDUCE) microsimulation model, which tracks serious injection-related infections, overdose, hospitalization, and death.
The status quo (referral for treatment) resulted in 1,162 overdose deaths per 10,000 people (95% credible interval, 1,144-2,303), whereas both BUP and BUP plus HR resulted in about 160 fewer deaths per 10,000 people (95% Crl for BUP, 802-1718; 95% CrI for BUP plus HR, 692-1,810).
Compared with the status quo strategy, life expectancy was lengthened with the BUP strategy by 2.65 years and BUP plus HR by 2.71 years.
Researchers found the average discounted lifetime cost per person of both the BUP strategy and the BUP plus HR strategy were higher than the average status quo.
“The dominating strategy was BUP plus HR,” the authors write. “Compared with status quo, BUP plus HR was cost effective (incremental cost-effectiveness ratio [ICER], $34,400 per life year).”
Cost for primary care practices
Comparatively, over a 5-year period, BUP plus HR was found to cost an individual PCP practice approximately $13,000.
That cost includes direct costs for resources and opportunity costs, the authors write. These costs could be offset by health care system savings.
“These costs included those for X-waiver training, which has been eliminated; thus, we expect this to cost less. Put another way, our findings inform ways to reinvest health care dollars as financial incentives for PCPs to adopt this new paradigm. Public health departments could provide grants or harm reduction kit supplies directly to PCPs to offset these costs as they do in some places with syringe service programs and/or increase Medicaid reimbursements for providing addiction care in primary care,” they write.
Data help make the case
Dinah Applewhite, MD, a primary care physician and addiction medicine specialist at Massachusetts General Hospital in Boston, who was not part of the study, said clinicians there have seen the benefits of integrating various aspects of addiction medicine into primary care but these data on outcomes and cost-effectiveness can help make the case to hospital leaders, legislators, and grant providers.
The primary care setting also provides a chance to engage patients around their injection practice and explore ways to minimize risk, she said.
“By offering them these kits, it lets them know your priority is their safety and well-being,” Dr. Applewhite said.
She noted that the linkage to primary care was low for patients who inject drugs, which speaks to the need for models in addition to this one, such as bringing primary care clinicians into syringe service programs.
“The medical establishment has a lot to learn from these programs,” she said.
Practices need support
She said it’s important to note that primary care practices need support from administrative leaders, philanthropists, and grant providers to help cover the costs.
“It’s one of the barriers to doing this,” she said. “There isn’t a mechanism to pay for this.”
Sarah Bagley, MD, a primary care physician at Boston Medical Center and medical director of BMC’s Center for Addiction Treatment for Adolescents/Young Adults Who Use Substances told this publication she was excited to see that the addition of harm reduction kits to buprenorphine seemed to have the optimal effect in improving outcomes. People with substance abuse disorders should feel they are welcome in primary care even if they are not yet ready to stop drug use, she said.
She said she was also glad to see increased life expectancy with these interventions. The news of overdose deaths contributing to a decrease in life expectancy can be overwhelming, she said.
But this study, she says, offers a road map for addressing the overdose crisis “by including harm reduction in the substance abuse care we provide.”
She pointed out that the study showed that costs increase per patient with both interventions, compared with the status quo. The study found that health care costs per person during a lifetime increased, compared with the status quo, by 69.1% for BUP and 74.3% for BUP plus HR.
But it’s important to understand the reason for that, she said: “The cost was higher because people were staying alive.”
She said it may help to compare giving optimal care to people who have substance abuse disorders with giving optimal care to people with other chronic conditions, such as diabetes, who may not always adhere to recommended diets or treatment regimens.
“We still invite those patients in and work with them based on where they are,” she said.
Growing epidemic
The researchers point to the urgent need for solutions given the U.S. opioid epidemic, which has led to increasing numbers of overdoses and injection drug use–related infections, such as infective endocarditis, and severe skin and soft tissue infections.
They point out that primary care providers are the largest clinical workforce in the United States, but few of their practices offer comprehensive addiction care onsite.
“Primary care practices are a practical place to integrate addiction services, where PCPs can prescribe buprenorphine and deliver harm reduction kits,” they write.
Coauthor Dr. Kimmel reports personal fees from Massachusetts Department of Public Health, Bureau of Substance Addiction Services Overdose Education and Prevention Program, and American Academy of Addiction Psychiatry, Opioid Response Network for harm reduction education outside the submitted work and previous consulting with Abt Associates on a Massachusetts Department of Public Health–funded project to improve access to medications for opioid use disorder treatment. Dr. Applewhite and Dr. Bagley report no relevant financial relationships.
Integrating buprenorphine and harm eduction tools into primary care may improve clinical outcomes, increase costs only modestly, and be cost effective in health systems, authors conclude in an original investigation in JAMA Network Open.
A team led by Raagini Jawa, MD, MPH, with the Center for Research on Healthcare, University of Pittsburgh, set out to analyze costs of the interventions versus increased benefit in extending life expectancy.
Their analysis found that, compared with the status quo, integrating buprenorphine and harm reduction kits (syringes, wound care supplies, etc.) reduced drug use–related deaths by 33% and was cost effective.
“Our results suggest that integrated addiction care in primary care has the potential to save lives and increase nonemergency health care use, which is consistent with prior literature,” the authors write. “Colocated addiction services within primary care is pragmatic and effective and has comparable quality to specialty care. We found that onsite BUP [buprenorphine prescribing] plus HR [harm reduction] provides better outcomes than BUP alone at a lower cost.”
Three strategies compared
Using a microsimulation model of 2.25 million people in the United States who inject opioids, with an average age of 44 (69% of them male), the researchers tested three strategies:
- Status quo. PCP refers to addiction care.
- BUP. PCP services plus onsite buprenorphine prescribing with referral to off-site harm reduction kits.
- BUP plus HR. PCP services plus on-site buprenorphine prescribing and harm reduction kits.
The model is the Reducing Infections Related to Drug Use Cost-Effectiveness (REDUCE) microsimulation model, which tracks serious injection-related infections, overdose, hospitalization, and death.
The status quo (referral for treatment) resulted in 1,162 overdose deaths per 10,000 people (95% credible interval, 1,144-2,303), whereas both BUP and BUP plus HR resulted in about 160 fewer deaths per 10,000 people (95% Crl for BUP, 802-1718; 95% CrI for BUP plus HR, 692-1,810).
Compared with the status quo strategy, life expectancy was lengthened with the BUP strategy by 2.65 years and BUP plus HR by 2.71 years.
Researchers found the average discounted lifetime cost per person of both the BUP strategy and the BUP plus HR strategy were higher than the average status quo.
“The dominating strategy was BUP plus HR,” the authors write. “Compared with status quo, BUP plus HR was cost effective (incremental cost-effectiveness ratio [ICER], $34,400 per life year).”
Cost for primary care practices
Comparatively, over a 5-year period, BUP plus HR was found to cost an individual PCP practice approximately $13,000.
That cost includes direct costs for resources and opportunity costs, the authors write. These costs could be offset by health care system savings.
“These costs included those for X-waiver training, which has been eliminated; thus, we expect this to cost less. Put another way, our findings inform ways to reinvest health care dollars as financial incentives for PCPs to adopt this new paradigm. Public health departments could provide grants or harm reduction kit supplies directly to PCPs to offset these costs as they do in some places with syringe service programs and/or increase Medicaid reimbursements for providing addiction care in primary care,” they write.
Data help make the case
Dinah Applewhite, MD, a primary care physician and addiction medicine specialist at Massachusetts General Hospital in Boston, who was not part of the study, said clinicians there have seen the benefits of integrating various aspects of addiction medicine into primary care but these data on outcomes and cost-effectiveness can help make the case to hospital leaders, legislators, and grant providers.
The primary care setting also provides a chance to engage patients around their injection practice and explore ways to minimize risk, she said.
“By offering them these kits, it lets them know your priority is their safety and well-being,” Dr. Applewhite said.
She noted that the linkage to primary care was low for patients who inject drugs, which speaks to the need for models in addition to this one, such as bringing primary care clinicians into syringe service programs.
“The medical establishment has a lot to learn from these programs,” she said.
Practices need support
She said it’s important to note that primary care practices need support from administrative leaders, philanthropists, and grant providers to help cover the costs.
“It’s one of the barriers to doing this,” she said. “There isn’t a mechanism to pay for this.”
Sarah Bagley, MD, a primary care physician at Boston Medical Center and medical director of BMC’s Center for Addiction Treatment for Adolescents/Young Adults Who Use Substances told this publication she was excited to see that the addition of harm reduction kits to buprenorphine seemed to have the optimal effect in improving outcomes. People with substance abuse disorders should feel they are welcome in primary care even if they are not yet ready to stop drug use, she said.
She said she was also glad to see increased life expectancy with these interventions. The news of overdose deaths contributing to a decrease in life expectancy can be overwhelming, she said.
But this study, she says, offers a road map for addressing the overdose crisis “by including harm reduction in the substance abuse care we provide.”
She pointed out that the study showed that costs increase per patient with both interventions, compared with the status quo. The study found that health care costs per person during a lifetime increased, compared with the status quo, by 69.1% for BUP and 74.3% for BUP plus HR.
But it’s important to understand the reason for that, she said: “The cost was higher because people were staying alive.”
She said it may help to compare giving optimal care to people who have substance abuse disorders with giving optimal care to people with other chronic conditions, such as diabetes, who may not always adhere to recommended diets or treatment regimens.
“We still invite those patients in and work with them based on where they are,” she said.
Growing epidemic
The researchers point to the urgent need for solutions given the U.S. opioid epidemic, which has led to increasing numbers of overdoses and injection drug use–related infections, such as infective endocarditis, and severe skin and soft tissue infections.
They point out that primary care providers are the largest clinical workforce in the United States, but few of their practices offer comprehensive addiction care onsite.
“Primary care practices are a practical place to integrate addiction services, where PCPs can prescribe buprenorphine and deliver harm reduction kits,” they write.
Coauthor Dr. Kimmel reports personal fees from Massachusetts Department of Public Health, Bureau of Substance Addiction Services Overdose Education and Prevention Program, and American Academy of Addiction Psychiatry, Opioid Response Network for harm reduction education outside the submitted work and previous consulting with Abt Associates on a Massachusetts Department of Public Health–funded project to improve access to medications for opioid use disorder treatment. Dr. Applewhite and Dr. Bagley report no relevant financial relationships.
Integrating buprenorphine and harm eduction tools into primary care may improve clinical outcomes, increase costs only modestly, and be cost effective in health systems, authors conclude in an original investigation in JAMA Network Open.
A team led by Raagini Jawa, MD, MPH, with the Center for Research on Healthcare, University of Pittsburgh, set out to analyze costs of the interventions versus increased benefit in extending life expectancy.
Their analysis found that, compared with the status quo, integrating buprenorphine and harm reduction kits (syringes, wound care supplies, etc.) reduced drug use–related deaths by 33% and was cost effective.
“Our results suggest that integrated addiction care in primary care has the potential to save lives and increase nonemergency health care use, which is consistent with prior literature,” the authors write. “Colocated addiction services within primary care is pragmatic and effective and has comparable quality to specialty care. We found that onsite BUP [buprenorphine prescribing] plus HR [harm reduction] provides better outcomes than BUP alone at a lower cost.”
Three strategies compared
Using a microsimulation model of 2.25 million people in the United States who inject opioids, with an average age of 44 (69% of them male), the researchers tested three strategies:
- Status quo. PCP refers to addiction care.
- BUP. PCP services plus onsite buprenorphine prescribing with referral to off-site harm reduction kits.
- BUP plus HR. PCP services plus on-site buprenorphine prescribing and harm reduction kits.
The model is the Reducing Infections Related to Drug Use Cost-Effectiveness (REDUCE) microsimulation model, which tracks serious injection-related infections, overdose, hospitalization, and death.
The status quo (referral for treatment) resulted in 1,162 overdose deaths per 10,000 people (95% credible interval, 1,144-2,303), whereas both BUP and BUP plus HR resulted in about 160 fewer deaths per 10,000 people (95% Crl for BUP, 802-1718; 95% CrI for BUP plus HR, 692-1,810).
Compared with the status quo strategy, life expectancy was lengthened with the BUP strategy by 2.65 years and BUP plus HR by 2.71 years.
Researchers found the average discounted lifetime cost per person of both the BUP strategy and the BUP plus HR strategy were higher than the average status quo.
“The dominating strategy was BUP plus HR,” the authors write. “Compared with status quo, BUP plus HR was cost effective (incremental cost-effectiveness ratio [ICER], $34,400 per life year).”
Cost for primary care practices
Comparatively, over a 5-year period, BUP plus HR was found to cost an individual PCP practice approximately $13,000.
That cost includes direct costs for resources and opportunity costs, the authors write. These costs could be offset by health care system savings.
“These costs included those for X-waiver training, which has been eliminated; thus, we expect this to cost less. Put another way, our findings inform ways to reinvest health care dollars as financial incentives for PCPs to adopt this new paradigm. Public health departments could provide grants or harm reduction kit supplies directly to PCPs to offset these costs as they do in some places with syringe service programs and/or increase Medicaid reimbursements for providing addiction care in primary care,” they write.
Data help make the case
Dinah Applewhite, MD, a primary care physician and addiction medicine specialist at Massachusetts General Hospital in Boston, who was not part of the study, said clinicians there have seen the benefits of integrating various aspects of addiction medicine into primary care but these data on outcomes and cost-effectiveness can help make the case to hospital leaders, legislators, and grant providers.
The primary care setting also provides a chance to engage patients around their injection practice and explore ways to minimize risk, she said.
“By offering them these kits, it lets them know your priority is their safety and well-being,” Dr. Applewhite said.
She noted that the linkage to primary care was low for patients who inject drugs, which speaks to the need for models in addition to this one, such as bringing primary care clinicians into syringe service programs.
“The medical establishment has a lot to learn from these programs,” she said.
Practices need support
She said it’s important to note that primary care practices need support from administrative leaders, philanthropists, and grant providers to help cover the costs.
“It’s one of the barriers to doing this,” she said. “There isn’t a mechanism to pay for this.”
Sarah Bagley, MD, a primary care physician at Boston Medical Center and medical director of BMC’s Center for Addiction Treatment for Adolescents/Young Adults Who Use Substances told this publication she was excited to see that the addition of harm reduction kits to buprenorphine seemed to have the optimal effect in improving outcomes. People with substance abuse disorders should feel they are welcome in primary care even if they are not yet ready to stop drug use, she said.
She said she was also glad to see increased life expectancy with these interventions. The news of overdose deaths contributing to a decrease in life expectancy can be overwhelming, she said.
But this study, she says, offers a road map for addressing the overdose crisis “by including harm reduction in the substance abuse care we provide.”
She pointed out that the study showed that costs increase per patient with both interventions, compared with the status quo. The study found that health care costs per person during a lifetime increased, compared with the status quo, by 69.1% for BUP and 74.3% for BUP plus HR.
But it’s important to understand the reason for that, she said: “The cost was higher because people were staying alive.”
She said it may help to compare giving optimal care to people who have substance abuse disorders with giving optimal care to people with other chronic conditions, such as diabetes, who may not always adhere to recommended diets or treatment regimens.
“We still invite those patients in and work with them based on where they are,” she said.
Growing epidemic
The researchers point to the urgent need for solutions given the U.S. opioid epidemic, which has led to increasing numbers of overdoses and injection drug use–related infections, such as infective endocarditis, and severe skin and soft tissue infections.
They point out that primary care providers are the largest clinical workforce in the United States, but few of their practices offer comprehensive addiction care onsite.
“Primary care practices are a practical place to integrate addiction services, where PCPs can prescribe buprenorphine and deliver harm reduction kits,” they write.
Coauthor Dr. Kimmel reports personal fees from Massachusetts Department of Public Health, Bureau of Substance Addiction Services Overdose Education and Prevention Program, and American Academy of Addiction Psychiatry, Opioid Response Network for harm reduction education outside the submitted work and previous consulting with Abt Associates on a Massachusetts Department of Public Health–funded project to improve access to medications for opioid use disorder treatment. Dr. Applewhite and Dr. Bagley report no relevant financial relationships.
JAMA NETWORK OPEN
Evaluation of Gabapentin and Baclofen Combination for Inpatient Management of Alcohol Withdrawal Syndrome
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7-9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
Methods
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
Results
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28).
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome.
Additionally, this study examined multiple secondary outcomes (Table 2).
Discussion
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate recordkeeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
Conclusions
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
1. National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. Updated April 2021. Accessed February 2, 2023. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder
2. Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3-4):175-177. doi:10.1080/19371918.2013.758987
3. Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. Accessed January 26, 2023. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis
4. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
5. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144-151. doi:10.1001/jama.278.2.144
6. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649-655.
7. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi:10.1111/j.1530-0277.2009.00986.x
8. Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics. 2018;59(5):496-505. doi:10.1016/j.psym.2018.03.002
9. Bates RE, Leung JG, Morgan RJ 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542-549. Published 2020 Aug 19. doi:10.1016/j.mayocpiqo.2020.06.002
10. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
11. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. doi:10.1177/1060028015585849
12. Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi:10.3389/fpsyt.2018.00773
13. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. Published 2019 Nov 6. doi:10.1002/14651858.CD008502.pub6
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7-9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
Methods
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
Results
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28).
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome.
Additionally, this study examined multiple secondary outcomes (Table 2).
Discussion
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate recordkeeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
Conclusions
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7-9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
Methods
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
Results
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28).
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome.
Additionally, this study examined multiple secondary outcomes (Table 2).
Discussion
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate recordkeeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
Conclusions
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
1. National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. Updated April 2021. Accessed February 2, 2023. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder
2. Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3-4):175-177. doi:10.1080/19371918.2013.758987
3. Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. Accessed January 26, 2023. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis
4. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
5. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144-151. doi:10.1001/jama.278.2.144
6. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649-655.
7. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi:10.1111/j.1530-0277.2009.00986.x
8. Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics. 2018;59(5):496-505. doi:10.1016/j.psym.2018.03.002
9. Bates RE, Leung JG, Morgan RJ 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542-549. Published 2020 Aug 19. doi:10.1016/j.mayocpiqo.2020.06.002
10. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
11. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. doi:10.1177/1060028015585849
12. Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi:10.3389/fpsyt.2018.00773
13. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. Published 2019 Nov 6. doi:10.1002/14651858.CD008502.pub6
1. National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. Updated April 2021. Accessed February 2, 2023. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder
2. Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3-4):175-177. doi:10.1080/19371918.2013.758987
3. Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. Accessed January 26, 2023. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis
4. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
5. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144-151. doi:10.1001/jama.278.2.144
6. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649-655.
7. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi:10.1111/j.1530-0277.2009.00986.x
8. Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics. 2018;59(5):496-505. doi:10.1016/j.psym.2018.03.002
9. Bates RE, Leung JG, Morgan RJ 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542-549. Published 2020 Aug 19. doi:10.1016/j.mayocpiqo.2020.06.002
10. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
11. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. doi:10.1177/1060028015585849
12. Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi:10.3389/fpsyt.2018.00773
13. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. Published 2019 Nov 6. doi:10.1002/14651858.CD008502.pub6
Battlefield Acupuncture vs Ketorolac for Treating Pain in the Emergency Department
Acute pain is a primary symptom for many patients who present to the emergency department (ED). The ED team is challenged with relieving pain while limiting harm from medications.1 A 2017 National Health Interview Survey showed that compared with nonveterans, more veterans reported pain in the previous 3 months, and the rate of severe pain was 40% higher in the veteran group especially among those who served during the era of wars in Afghanistan and Iraq.2
The American College of Emergency Physicians guidelines pain management guidelines recommend patient-centered shared decision making that includes patient education about treatment goals and expectations, and short- and long-term risks, as well as a preference toward pharmacologic treatment with nonopioid analgesics except for patients with severe pain or pain refractory to other drug and treatment modalities.3 There is a lack of evidence regarding superior efficacy of either opioid or nonopioid analgesics; therefore, the use of nonopioid analgesics, such as oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) or central analgesics, such as acetaminophen, is preferred for treating acute pain to mitigate adverse effects (AEs) and risks associated with opioid use.1,3,4 The US Department of Veterans Affairs (VA) and Department of Defense (DoD) guideline on managing opioid therapy for chronic pain, updated in 2017 and 2022, similarly recommends alternatives to opioids for mild-to-moderate acute pain and encourages multimodal pain care.5 However, use of other pharmacologic treatments, such as NSAIDs, is limited by AE profiles, patient contraindications, and severity of acute pain etiologies. There is a need for the expanded use of nonpharmacologic treatments for addressing pain in the veteran population.
The American College of Emergency Physicians guidelines recommend nonpharmacologic modalities, such as applying heat or cold, physical therapy, cognitive behavioral therapy, and acupuncture.3 A 2014 study reported that 37% to 46% of active duty and reserve military personnel use complementary and alternative medicine (CAM) for a variety of ailments, and there is increasing interest in the use of CAM as adjuncts to traditional therapies.6 According to one study, some CAM therapies are used significantly more by military personnel than used by civilians.7 However, the percentage of the veteran population using acupuncture in this study was small, and more information is needed to assess its use.
Auricular acupuncture originated in traditional Chinese medicine.8 Contemporary auricular acupuncture experts view this modality as a self-contained microsystem mapping portions of the ear to specific parts of the body and internal organs. The analgesic effects may be mediated through the central nervous system by local release of endorphins through nerve fiber activation and neurotransmitters—including serotonin, dopamine, and norepinephrine—leading to pre- and postsynaptic suppression of pain transmission.
Battlefield acupuncture (BFA) uses 5 set points anatomically located on each ear.9 Practitioners use small semipermanent, dartlike acupuncture needles. Patients could experience pain relief in a few minutes, which can last minutes, hours, days, weeks, or months depending on the pathology of the pain. This procedure developed in 2001 has been studied for different pain types and has shown benefit when used for postsurgical pain, chronic spinal cord injury−related neuropathic pain, and general chronic pain, as well as for other indications, such as insomnia, depression, and weight loss.8,10-13 In 2018, a randomized controlled trial compared postintervention numeric rating scale (NRS) pain scores in patients presenting to the ED with acute or acute-on-chronic lower back pain who received BFA as an adjunct to standard care vs standard care alone.14 Patients receiving BFA as an adjunct to standard care were found to have mean postintervention pain scores 1.7 points lower than those receiving standard care alone. This study demonstrated that BFA was feasible and well tolerated for lower back pain in the ED as an adjunct to standard care. The study was limited by the adjunct use of BFA rather than as monotherapy and by the practitioners’ discretion regarding standard care, which was not defined by the study’s authors.
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, offers several CAM modalities, such as exercise/movement therapy, chiropractic, art/music therapy, and relaxation workshops, which are widely used by veterans. Recent evidence suggests BFA could reduce pain scores as an adjunct or an alternative to pharmacologic therapy. We are interested in how CAM therapies, such as BFA, can help avoid AEs associated with opioid or NSAID therapy.
At the JBVAMC ED, ketorolac 15 mg is the preferred first-line treatment of acute, noncancer pain, based on the results of previous studies. In 2018 BFA was offered first to veterans presenting with acute or acute-on-chronic pain to the ED; however, its effectiveness for pain reduction vs ketorolac has not been evaluated in this patient population. Limited literature is available on BFA and its use in the ED. To our knowledge, this was the first observational study assessing the difference between a single session of BFA vs a single dose of ketorolac in treating noncancer acute or acute-on-chronic pain in the ED.
Methods
This study was a retrospective chart review of patients who presented to the JBVAMC ED with acute pain or acute-on-chronic pain, who received ketorolac or BFA. The study population was generated from a list of all IV and intramuscular (IM) ketorolac unit dose orders verified from June 1, 2018, through August 30, 2019, and a list of all BFA procedure notes signed from June 1, 2018, through August 30, 2019. Patients were included in the study if they had documented administration of IV or IM ketorolac or BFA between June 1, 2018, and August 30, 2019. Patients who received ketorolac doses other than 15 mg, the intervention was administered outside of the ED, received adjunct treatment in addition to the treatment intervention in the ED, had no baseline NRS pain score documented before the intervention, had an NRS pain score of < 4, had no postintervention NRS pain score documented within 6 hours, had a treatment indication other than pain, or had active cancer were excluded. As in previous JBVAMC studies, we used NRS pain score cutoffs (mild, moderate, severe, and very severe) based on Woo and colleagues’ meta-analysis and excluded scores < 4.15
Endpoints
The primary endpoint was the mean difference in NRS pain score before and after the intervention, determined by comparing the NRS pain score documented at triage to the ED with the first documented NRS pain score at least 30 minutes to 6 hours after treatment administration. The secondary endpoints included the number of patients prescribed pain medication at discharge, the number of patients who were discharged with no medications, and the number of patients admitted to the hospital. The safety endpoint included any AEs of the intervention. Subgroup analyses were performed comparing the mean difference in NRS pain score among subgroups classified by severity of baseline NRS pain score and pain location.
Statistical Analysis
Baseline characteristics and endpoints were analyzed using descriptive statistics. Categorical data were analyzed using Fisher exact test and z test for proportions, and continuous data were compared using t test and paired t test. An 80% power calculation determined that 84 patients per group were needed to detect a statistically significant difference in pain score reduction of 1.3 at a type-1 error rate of 0.05. The sample size was based on a calculation performed in a previously published study that compared IV ketorolac at 3 single-dose regimens for treating acute pain in the ED.16 The 1.3 pain score reduction is considered the minimum clinically significant difference in pain that could be detected with the NRS.17
Results
Sixty-one patients received BFA during the study period: 31 were excluded (26 received adjunct treatment in the ED, 2 had active cancer documented, 2 had an indication other than pain, and 1 received BFA outside of the ED), leaving 30 patients in the BFA cohort. During the study period, 1299 patients received ketorolac.
Baseline characteristics were similar between the 2 groups except for the average baseline NRS pain score, which was statistically significantly higher in the BFA vs ketorolac group (8.7 vs 7.7, respectively; P = .02). The mean age was 51 years in the BFA group and 48 years in the ketorolac group. Most patients in each cohort were male: 80% in the BFA group and 71% in the ketorolac group. The most common types of pain documented as the chief ED presentation included back, lower extremity, and head.
Endpoints
The mean difference in NRS pain score was 3.9 for the BFA group and 5.1 for the ketorolac group. Both were clinically and statistically significant reductions (P = .03 and P < .01), but the difference between the intervention groups in NRS score reduction was not statistically significant (P = .07).
For the secondary endpoint of outpatient prescriptions written at discharge, there was no significant difference between the groups except for oral NSAIDs, which were more likely to be prescribed to patients who received ketorolac (P = .01).
Subgroup Analysis
An analysis was performed for subgroups classified by baseline NRS pain score (mild: 4; moderate, 5 - 6; severe, 7 - 9; and very severe, 10). Data for mild pain was limited because a small number of patients received interventions. For moderate pain, the mean difference in NRS pain score for BFA and ketorolac was 3.5 and 3.8, respectively; for severe pain, 3.4 and 5.3; and for very severe pain, 4.6 and 6.4. There was a larger difference in the preintervention and postintervention NRS pain scores within severe pain and very severe pain groups.
Discussion
Both interventions resulted in a significant reduction in the mean NRS pain score of about 4 to 5 points within their group, and BFA resulted in a similar NRS pain score reduction compared with ketorolac 15 mg. Because the baseline NRS pain scores were significantly different between the BFA and ketorolac groups,
In this study, more patients in the BFA group presented to the ED with lower extremity pain, such as gout or neuropathy, compared with the ketorolac group; however, BFA did not result in a significantly different pain score reduction in this subgroup compared with ketorolac. Patients receiving BFA were more likely to receive topical analgesics or muscle relaxants at discharge; whereas those receiving ketorolac were significantly more likely to receive oral NSAIDs. Patients in this study also were more likely to be admitted to the hospital if they received ketorolac; however, for these patients, pain was secondary to their chief presentation, and the admitting physician’s familiarity with ketorolac might have been the reason for choosing this intervention. Reasons for the admissions were surgical observation, psychiatric stabilization, kidney/gallstones, rule out of acute coronary syndrome, pneumonia, and proctitis in the ketorolac group, and suicidal ideations in the BFA group.
Limitations
As a limited number of patients received BFA at JBVAMC, the study was not sufficiently powered to detect a difference in the primary outcome. Because BFA required a consultation to be entered in the electronic health record, in addition to time needed to perform the procedure, practitioners might have preferred IV/IM ketorolac during busy times in the ED, potentially leading to underrepresentation in the BFA group. Prescribing preferences might have differed among the rotating physicians, timing of the documentation of the NRS pain score could have differed based on the treatment intervention, and the investigators were unable to control or accurately assess whether patients had taken an analgesic medication before presenting to the ED.
Conclusions
NRS pain score reduction with BFA did not differ compared with ketorolac 15 mg for treating acute and acute-on-chronic pain in the ED. Although this study was underpowered, these results add to the limited existing literature, suggesting that both interventions could result in clinically significant pain score reductions for patients presenting to the ED with severe and very severe pain, making BFA a viable nonpharmacologic option. Future studies could include investigating the benefit of BFA in the veteran population by studying larger samples in the ED, surveying patients after their interventions to identify rates AEs, and exploring the use of BFA for chronic pain in the outpatient setting.
1. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. doi:10.1016/j.annemergmed.2012.06.013
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Motov S, Strayer R, Hayes BD, et al. The treatment of acute pain in the emergency department: a white paper position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2018;54(5):731-736. doi:10.1016/j.jemermed.2018.01.020
4. Samcam I, Papa L. Acute pain management in the emergency department. In: Prostran M, ed. Pain Management. IntechOpen; 2016. doi:10.5772/62861
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the use of opioids in the management of chronic pain. Accessed February 15, 2023. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
6. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population surveys. Med Care. 2014;52(12 suppl 5):S83-590. doi:10.1097/MLR.0000000000000227
7. Goertz C, Marriott BP, Finch FD, et al. Military report more complementary and alternative medicine use than civilians. J Altern Complement Med. 2013;19(6):509-517. doi:10.1089/acm.2012.0108
8. King HC, Hickey AH, Connelly C. Auricular acupuncture: a brief introduction for military providers. Mil Med. 2013;178(8):867-874. doi:10.7205/MILMED-D-13-00075
9. Niemtzow RC. Battlefield acupuncture. Medical Acupunct. 2007;19(4):225-228. doi:10.1089/acu.2007.0603
10. Collinsworth KM, Goss DL. Battlefield acupuncture and physical therapy versus physical therapy alone after shoulder surgery. Med Acupunct. 2019;31(4):228-238. doi:10.1089/acu.2019.1372
11. Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. 2017;40(4):432-438. doi:10.1080/10790268.2016.1141489
12. Federman DG, Radhakrishnan K, Gabriel L, Poulin LM, Kravetz JD. Group battlefield acupuncture in primary care for veterans with pain. South Med J. 2018;111(10):619-624. doi:10.14423/SMJ.0000000000000877
13. Garner BK, Hopkinson SG, Ketz AK, Landis CA, Trego LL. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Med Acupunct. 2018;30(5):262-272. doi:10.1089/acu.2018.1294
14. Fox LM, Murakami M, Danesh H, Manini AF. Battlefield acupuncture to treat low back pain in the emergency department. Am J Emerg Med. 2018; 36:1045-1048. doi:10.1016/j.ajem.2018.02.038
15. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
16. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177-184. doi:10.1016/j.annemergmed.2016.10.014
17. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390-392. doi:10.1111/j.1553-2712.2003.tb01355.
Acute pain is a primary symptom for many patients who present to the emergency department (ED). The ED team is challenged with relieving pain while limiting harm from medications.1 A 2017 National Health Interview Survey showed that compared with nonveterans, more veterans reported pain in the previous 3 months, and the rate of severe pain was 40% higher in the veteran group especially among those who served during the era of wars in Afghanistan and Iraq.2
The American College of Emergency Physicians guidelines pain management guidelines recommend patient-centered shared decision making that includes patient education about treatment goals and expectations, and short- and long-term risks, as well as a preference toward pharmacologic treatment with nonopioid analgesics except for patients with severe pain or pain refractory to other drug and treatment modalities.3 There is a lack of evidence regarding superior efficacy of either opioid or nonopioid analgesics; therefore, the use of nonopioid analgesics, such as oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) or central analgesics, such as acetaminophen, is preferred for treating acute pain to mitigate adverse effects (AEs) and risks associated with opioid use.1,3,4 The US Department of Veterans Affairs (VA) and Department of Defense (DoD) guideline on managing opioid therapy for chronic pain, updated in 2017 and 2022, similarly recommends alternatives to opioids for mild-to-moderate acute pain and encourages multimodal pain care.5 However, use of other pharmacologic treatments, such as NSAIDs, is limited by AE profiles, patient contraindications, and severity of acute pain etiologies. There is a need for the expanded use of nonpharmacologic treatments for addressing pain in the veteran population.
The American College of Emergency Physicians guidelines recommend nonpharmacologic modalities, such as applying heat or cold, physical therapy, cognitive behavioral therapy, and acupuncture.3 A 2014 study reported that 37% to 46% of active duty and reserve military personnel use complementary and alternative medicine (CAM) for a variety of ailments, and there is increasing interest in the use of CAM as adjuncts to traditional therapies.6 According to one study, some CAM therapies are used significantly more by military personnel than used by civilians.7 However, the percentage of the veteran population using acupuncture in this study was small, and more information is needed to assess its use.
Auricular acupuncture originated in traditional Chinese medicine.8 Contemporary auricular acupuncture experts view this modality as a self-contained microsystem mapping portions of the ear to specific parts of the body and internal organs. The analgesic effects may be mediated through the central nervous system by local release of endorphins through nerve fiber activation and neurotransmitters—including serotonin, dopamine, and norepinephrine—leading to pre- and postsynaptic suppression of pain transmission.
Battlefield acupuncture (BFA) uses 5 set points anatomically located on each ear.9 Practitioners use small semipermanent, dartlike acupuncture needles. Patients could experience pain relief in a few minutes, which can last minutes, hours, days, weeks, or months depending on the pathology of the pain. This procedure developed in 2001 has been studied for different pain types and has shown benefit when used for postsurgical pain, chronic spinal cord injury−related neuropathic pain, and general chronic pain, as well as for other indications, such as insomnia, depression, and weight loss.8,10-13 In 2018, a randomized controlled trial compared postintervention numeric rating scale (NRS) pain scores in patients presenting to the ED with acute or acute-on-chronic lower back pain who received BFA as an adjunct to standard care vs standard care alone.14 Patients receiving BFA as an adjunct to standard care were found to have mean postintervention pain scores 1.7 points lower than those receiving standard care alone. This study demonstrated that BFA was feasible and well tolerated for lower back pain in the ED as an adjunct to standard care. The study was limited by the adjunct use of BFA rather than as monotherapy and by the practitioners’ discretion regarding standard care, which was not defined by the study’s authors.
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, offers several CAM modalities, such as exercise/movement therapy, chiropractic, art/music therapy, and relaxation workshops, which are widely used by veterans. Recent evidence suggests BFA could reduce pain scores as an adjunct or an alternative to pharmacologic therapy. We are interested in how CAM therapies, such as BFA, can help avoid AEs associated with opioid or NSAID therapy.
At the JBVAMC ED, ketorolac 15 mg is the preferred first-line treatment of acute, noncancer pain, based on the results of previous studies. In 2018 BFA was offered first to veterans presenting with acute or acute-on-chronic pain to the ED; however, its effectiveness for pain reduction vs ketorolac has not been evaluated in this patient population. Limited literature is available on BFA and its use in the ED. To our knowledge, this was the first observational study assessing the difference between a single session of BFA vs a single dose of ketorolac in treating noncancer acute or acute-on-chronic pain in the ED.
Methods
This study was a retrospective chart review of patients who presented to the JBVAMC ED with acute pain or acute-on-chronic pain, who received ketorolac or BFA. The study population was generated from a list of all IV and intramuscular (IM) ketorolac unit dose orders verified from June 1, 2018, through August 30, 2019, and a list of all BFA procedure notes signed from June 1, 2018, through August 30, 2019. Patients were included in the study if they had documented administration of IV or IM ketorolac or BFA between June 1, 2018, and August 30, 2019. Patients who received ketorolac doses other than 15 mg, the intervention was administered outside of the ED, received adjunct treatment in addition to the treatment intervention in the ED, had no baseline NRS pain score documented before the intervention, had an NRS pain score of < 4, had no postintervention NRS pain score documented within 6 hours, had a treatment indication other than pain, or had active cancer were excluded. As in previous JBVAMC studies, we used NRS pain score cutoffs (mild, moderate, severe, and very severe) based on Woo and colleagues’ meta-analysis and excluded scores < 4.15
Endpoints
The primary endpoint was the mean difference in NRS pain score before and after the intervention, determined by comparing the NRS pain score documented at triage to the ED with the first documented NRS pain score at least 30 minutes to 6 hours after treatment administration. The secondary endpoints included the number of patients prescribed pain medication at discharge, the number of patients who were discharged with no medications, and the number of patients admitted to the hospital. The safety endpoint included any AEs of the intervention. Subgroup analyses were performed comparing the mean difference in NRS pain score among subgroups classified by severity of baseline NRS pain score and pain location.
Statistical Analysis
Baseline characteristics and endpoints were analyzed using descriptive statistics. Categorical data were analyzed using Fisher exact test and z test for proportions, and continuous data were compared using t test and paired t test. An 80% power calculation determined that 84 patients per group were needed to detect a statistically significant difference in pain score reduction of 1.3 at a type-1 error rate of 0.05. The sample size was based on a calculation performed in a previously published study that compared IV ketorolac at 3 single-dose regimens for treating acute pain in the ED.16 The 1.3 pain score reduction is considered the minimum clinically significant difference in pain that could be detected with the NRS.17
Results
Sixty-one patients received BFA during the study period: 31 were excluded (26 received adjunct treatment in the ED, 2 had active cancer documented, 2 had an indication other than pain, and 1 received BFA outside of the ED), leaving 30 patients in the BFA cohort. During the study period, 1299 patients received ketorolac.
Baseline characteristics were similar between the 2 groups except for the average baseline NRS pain score, which was statistically significantly higher in the BFA vs ketorolac group (8.7 vs 7.7, respectively; P = .02). The mean age was 51 years in the BFA group and 48 years in the ketorolac group. Most patients in each cohort were male: 80% in the BFA group and 71% in the ketorolac group. The most common types of pain documented as the chief ED presentation included back, lower extremity, and head.
Endpoints
The mean difference in NRS pain score was 3.9 for the BFA group and 5.1 for the ketorolac group. Both were clinically and statistically significant reductions (P = .03 and P < .01), but the difference between the intervention groups in NRS score reduction was not statistically significant (P = .07).
For the secondary endpoint of outpatient prescriptions written at discharge, there was no significant difference between the groups except for oral NSAIDs, which were more likely to be prescribed to patients who received ketorolac (P = .01).
Subgroup Analysis
An analysis was performed for subgroups classified by baseline NRS pain score (mild: 4; moderate, 5 - 6; severe, 7 - 9; and very severe, 10). Data for mild pain was limited because a small number of patients received interventions. For moderate pain, the mean difference in NRS pain score for BFA and ketorolac was 3.5 and 3.8, respectively; for severe pain, 3.4 and 5.3; and for very severe pain, 4.6 and 6.4. There was a larger difference in the preintervention and postintervention NRS pain scores within severe pain and very severe pain groups.
Discussion
Both interventions resulted in a significant reduction in the mean NRS pain score of about 4 to 5 points within their group, and BFA resulted in a similar NRS pain score reduction compared with ketorolac 15 mg. Because the baseline NRS pain scores were significantly different between the BFA and ketorolac groups,
In this study, more patients in the BFA group presented to the ED with lower extremity pain, such as gout or neuropathy, compared with the ketorolac group; however, BFA did not result in a significantly different pain score reduction in this subgroup compared with ketorolac. Patients receiving BFA were more likely to receive topical analgesics or muscle relaxants at discharge; whereas those receiving ketorolac were significantly more likely to receive oral NSAIDs. Patients in this study also were more likely to be admitted to the hospital if they received ketorolac; however, for these patients, pain was secondary to their chief presentation, and the admitting physician’s familiarity with ketorolac might have been the reason for choosing this intervention. Reasons for the admissions were surgical observation, psychiatric stabilization, kidney/gallstones, rule out of acute coronary syndrome, pneumonia, and proctitis in the ketorolac group, and suicidal ideations in the BFA group.
Limitations
As a limited number of patients received BFA at JBVAMC, the study was not sufficiently powered to detect a difference in the primary outcome. Because BFA required a consultation to be entered in the electronic health record, in addition to time needed to perform the procedure, practitioners might have preferred IV/IM ketorolac during busy times in the ED, potentially leading to underrepresentation in the BFA group. Prescribing preferences might have differed among the rotating physicians, timing of the documentation of the NRS pain score could have differed based on the treatment intervention, and the investigators were unable to control or accurately assess whether patients had taken an analgesic medication before presenting to the ED.
Conclusions
NRS pain score reduction with BFA did not differ compared with ketorolac 15 mg for treating acute and acute-on-chronic pain in the ED. Although this study was underpowered, these results add to the limited existing literature, suggesting that both interventions could result in clinically significant pain score reductions for patients presenting to the ED with severe and very severe pain, making BFA a viable nonpharmacologic option. Future studies could include investigating the benefit of BFA in the veteran population by studying larger samples in the ED, surveying patients after their interventions to identify rates AEs, and exploring the use of BFA for chronic pain in the outpatient setting.
Acute pain is a primary symptom for many patients who present to the emergency department (ED). The ED team is challenged with relieving pain while limiting harm from medications.1 A 2017 National Health Interview Survey showed that compared with nonveterans, more veterans reported pain in the previous 3 months, and the rate of severe pain was 40% higher in the veteran group especially among those who served during the era of wars in Afghanistan and Iraq.2
The American College of Emergency Physicians guidelines pain management guidelines recommend patient-centered shared decision making that includes patient education about treatment goals and expectations, and short- and long-term risks, as well as a preference toward pharmacologic treatment with nonopioid analgesics except for patients with severe pain or pain refractory to other drug and treatment modalities.3 There is a lack of evidence regarding superior efficacy of either opioid or nonopioid analgesics; therefore, the use of nonopioid analgesics, such as oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) or central analgesics, such as acetaminophen, is preferred for treating acute pain to mitigate adverse effects (AEs) and risks associated with opioid use.1,3,4 The US Department of Veterans Affairs (VA) and Department of Defense (DoD) guideline on managing opioid therapy for chronic pain, updated in 2017 and 2022, similarly recommends alternatives to opioids for mild-to-moderate acute pain and encourages multimodal pain care.5 However, use of other pharmacologic treatments, such as NSAIDs, is limited by AE profiles, patient contraindications, and severity of acute pain etiologies. There is a need for the expanded use of nonpharmacologic treatments for addressing pain in the veteran population.
The American College of Emergency Physicians guidelines recommend nonpharmacologic modalities, such as applying heat or cold, physical therapy, cognitive behavioral therapy, and acupuncture.3 A 2014 study reported that 37% to 46% of active duty and reserve military personnel use complementary and alternative medicine (CAM) for a variety of ailments, and there is increasing interest in the use of CAM as adjuncts to traditional therapies.6 According to one study, some CAM therapies are used significantly more by military personnel than used by civilians.7 However, the percentage of the veteran population using acupuncture in this study was small, and more information is needed to assess its use.
Auricular acupuncture originated in traditional Chinese medicine.8 Contemporary auricular acupuncture experts view this modality as a self-contained microsystem mapping portions of the ear to specific parts of the body and internal organs. The analgesic effects may be mediated through the central nervous system by local release of endorphins through nerve fiber activation and neurotransmitters—including serotonin, dopamine, and norepinephrine—leading to pre- and postsynaptic suppression of pain transmission.
Battlefield acupuncture (BFA) uses 5 set points anatomically located on each ear.9 Practitioners use small semipermanent, dartlike acupuncture needles. Patients could experience pain relief in a few minutes, which can last minutes, hours, days, weeks, or months depending on the pathology of the pain. This procedure developed in 2001 has been studied for different pain types and has shown benefit when used for postsurgical pain, chronic spinal cord injury−related neuropathic pain, and general chronic pain, as well as for other indications, such as insomnia, depression, and weight loss.8,10-13 In 2018, a randomized controlled trial compared postintervention numeric rating scale (NRS) pain scores in patients presenting to the ED with acute or acute-on-chronic lower back pain who received BFA as an adjunct to standard care vs standard care alone.14 Patients receiving BFA as an adjunct to standard care were found to have mean postintervention pain scores 1.7 points lower than those receiving standard care alone. This study demonstrated that BFA was feasible and well tolerated for lower back pain in the ED as an adjunct to standard care. The study was limited by the adjunct use of BFA rather than as monotherapy and by the practitioners’ discretion regarding standard care, which was not defined by the study’s authors.
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, offers several CAM modalities, such as exercise/movement therapy, chiropractic, art/music therapy, and relaxation workshops, which are widely used by veterans. Recent evidence suggests BFA could reduce pain scores as an adjunct or an alternative to pharmacologic therapy. We are interested in how CAM therapies, such as BFA, can help avoid AEs associated with opioid or NSAID therapy.
At the JBVAMC ED, ketorolac 15 mg is the preferred first-line treatment of acute, noncancer pain, based on the results of previous studies. In 2018 BFA was offered first to veterans presenting with acute or acute-on-chronic pain to the ED; however, its effectiveness for pain reduction vs ketorolac has not been evaluated in this patient population. Limited literature is available on BFA and its use in the ED. To our knowledge, this was the first observational study assessing the difference between a single session of BFA vs a single dose of ketorolac in treating noncancer acute or acute-on-chronic pain in the ED.
Methods
This study was a retrospective chart review of patients who presented to the JBVAMC ED with acute pain or acute-on-chronic pain, who received ketorolac or BFA. The study population was generated from a list of all IV and intramuscular (IM) ketorolac unit dose orders verified from June 1, 2018, through August 30, 2019, and a list of all BFA procedure notes signed from June 1, 2018, through August 30, 2019. Patients were included in the study if they had documented administration of IV or IM ketorolac or BFA between June 1, 2018, and August 30, 2019. Patients who received ketorolac doses other than 15 mg, the intervention was administered outside of the ED, received adjunct treatment in addition to the treatment intervention in the ED, had no baseline NRS pain score documented before the intervention, had an NRS pain score of < 4, had no postintervention NRS pain score documented within 6 hours, had a treatment indication other than pain, or had active cancer were excluded. As in previous JBVAMC studies, we used NRS pain score cutoffs (mild, moderate, severe, and very severe) based on Woo and colleagues’ meta-analysis and excluded scores < 4.15
Endpoints
The primary endpoint was the mean difference in NRS pain score before and after the intervention, determined by comparing the NRS pain score documented at triage to the ED with the first documented NRS pain score at least 30 minutes to 6 hours after treatment administration. The secondary endpoints included the number of patients prescribed pain medication at discharge, the number of patients who were discharged with no medications, and the number of patients admitted to the hospital. The safety endpoint included any AEs of the intervention. Subgroup analyses were performed comparing the mean difference in NRS pain score among subgroups classified by severity of baseline NRS pain score and pain location.
Statistical Analysis
Baseline characteristics and endpoints were analyzed using descriptive statistics. Categorical data were analyzed using Fisher exact test and z test for proportions, and continuous data were compared using t test and paired t test. An 80% power calculation determined that 84 patients per group were needed to detect a statistically significant difference in pain score reduction of 1.3 at a type-1 error rate of 0.05. The sample size was based on a calculation performed in a previously published study that compared IV ketorolac at 3 single-dose regimens for treating acute pain in the ED.16 The 1.3 pain score reduction is considered the minimum clinically significant difference in pain that could be detected with the NRS.17
Results
Sixty-one patients received BFA during the study period: 31 were excluded (26 received adjunct treatment in the ED, 2 had active cancer documented, 2 had an indication other than pain, and 1 received BFA outside of the ED), leaving 30 patients in the BFA cohort. During the study period, 1299 patients received ketorolac.
Baseline characteristics were similar between the 2 groups except for the average baseline NRS pain score, which was statistically significantly higher in the BFA vs ketorolac group (8.7 vs 7.7, respectively; P = .02). The mean age was 51 years in the BFA group and 48 years in the ketorolac group. Most patients in each cohort were male: 80% in the BFA group and 71% in the ketorolac group. The most common types of pain documented as the chief ED presentation included back, lower extremity, and head.
Endpoints
The mean difference in NRS pain score was 3.9 for the BFA group and 5.1 for the ketorolac group. Both were clinically and statistically significant reductions (P = .03 and P < .01), but the difference between the intervention groups in NRS score reduction was not statistically significant (P = .07).
For the secondary endpoint of outpatient prescriptions written at discharge, there was no significant difference between the groups except for oral NSAIDs, which were more likely to be prescribed to patients who received ketorolac (P = .01).
Subgroup Analysis
An analysis was performed for subgroups classified by baseline NRS pain score (mild: 4; moderate, 5 - 6; severe, 7 - 9; and very severe, 10). Data for mild pain was limited because a small number of patients received interventions. For moderate pain, the mean difference in NRS pain score for BFA and ketorolac was 3.5 and 3.8, respectively; for severe pain, 3.4 and 5.3; and for very severe pain, 4.6 and 6.4. There was a larger difference in the preintervention and postintervention NRS pain scores within severe pain and very severe pain groups.
Discussion
Both interventions resulted in a significant reduction in the mean NRS pain score of about 4 to 5 points within their group, and BFA resulted in a similar NRS pain score reduction compared with ketorolac 15 mg. Because the baseline NRS pain scores were significantly different between the BFA and ketorolac groups,
In this study, more patients in the BFA group presented to the ED with lower extremity pain, such as gout or neuropathy, compared with the ketorolac group; however, BFA did not result in a significantly different pain score reduction in this subgroup compared with ketorolac. Patients receiving BFA were more likely to receive topical analgesics or muscle relaxants at discharge; whereas those receiving ketorolac were significantly more likely to receive oral NSAIDs. Patients in this study also were more likely to be admitted to the hospital if they received ketorolac; however, for these patients, pain was secondary to their chief presentation, and the admitting physician’s familiarity with ketorolac might have been the reason for choosing this intervention. Reasons for the admissions were surgical observation, psychiatric stabilization, kidney/gallstones, rule out of acute coronary syndrome, pneumonia, and proctitis in the ketorolac group, and suicidal ideations in the BFA group.
Limitations
As a limited number of patients received BFA at JBVAMC, the study was not sufficiently powered to detect a difference in the primary outcome. Because BFA required a consultation to be entered in the electronic health record, in addition to time needed to perform the procedure, practitioners might have preferred IV/IM ketorolac during busy times in the ED, potentially leading to underrepresentation in the BFA group. Prescribing preferences might have differed among the rotating physicians, timing of the documentation of the NRS pain score could have differed based on the treatment intervention, and the investigators were unable to control or accurately assess whether patients had taken an analgesic medication before presenting to the ED.
Conclusions
NRS pain score reduction with BFA did not differ compared with ketorolac 15 mg for treating acute and acute-on-chronic pain in the ED. Although this study was underpowered, these results add to the limited existing literature, suggesting that both interventions could result in clinically significant pain score reductions for patients presenting to the ED with severe and very severe pain, making BFA a viable nonpharmacologic option. Future studies could include investigating the benefit of BFA in the veteran population by studying larger samples in the ED, surveying patients after their interventions to identify rates AEs, and exploring the use of BFA for chronic pain in the outpatient setting.
1. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. doi:10.1016/j.annemergmed.2012.06.013
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Motov S, Strayer R, Hayes BD, et al. The treatment of acute pain in the emergency department: a white paper position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2018;54(5):731-736. doi:10.1016/j.jemermed.2018.01.020
4. Samcam I, Papa L. Acute pain management in the emergency department. In: Prostran M, ed. Pain Management. IntechOpen; 2016. doi:10.5772/62861
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the use of opioids in the management of chronic pain. Accessed February 15, 2023. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
6. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population surveys. Med Care. 2014;52(12 suppl 5):S83-590. doi:10.1097/MLR.0000000000000227
7. Goertz C, Marriott BP, Finch FD, et al. Military report more complementary and alternative medicine use than civilians. J Altern Complement Med. 2013;19(6):509-517. doi:10.1089/acm.2012.0108
8. King HC, Hickey AH, Connelly C. Auricular acupuncture: a brief introduction for military providers. Mil Med. 2013;178(8):867-874. doi:10.7205/MILMED-D-13-00075
9. Niemtzow RC. Battlefield acupuncture. Medical Acupunct. 2007;19(4):225-228. doi:10.1089/acu.2007.0603
10. Collinsworth KM, Goss DL. Battlefield acupuncture and physical therapy versus physical therapy alone after shoulder surgery. Med Acupunct. 2019;31(4):228-238. doi:10.1089/acu.2019.1372
11. Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. 2017;40(4):432-438. doi:10.1080/10790268.2016.1141489
12. Federman DG, Radhakrishnan K, Gabriel L, Poulin LM, Kravetz JD. Group battlefield acupuncture in primary care for veterans with pain. South Med J. 2018;111(10):619-624. doi:10.14423/SMJ.0000000000000877
13. Garner BK, Hopkinson SG, Ketz AK, Landis CA, Trego LL. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Med Acupunct. 2018;30(5):262-272. doi:10.1089/acu.2018.1294
14. Fox LM, Murakami M, Danesh H, Manini AF. Battlefield acupuncture to treat low back pain in the emergency department. Am J Emerg Med. 2018; 36:1045-1048. doi:10.1016/j.ajem.2018.02.038
15. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
16. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177-184. doi:10.1016/j.annemergmed.2016.10.014
17. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390-392. doi:10.1111/j.1553-2712.2003.tb01355.
1. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. doi:10.1016/j.annemergmed.2012.06.013
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Motov S, Strayer R, Hayes BD, et al. The treatment of acute pain in the emergency department: a white paper position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2018;54(5):731-736. doi:10.1016/j.jemermed.2018.01.020
4. Samcam I, Papa L. Acute pain management in the emergency department. In: Prostran M, ed. Pain Management. IntechOpen; 2016. doi:10.5772/62861
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the use of opioids in the management of chronic pain. Accessed February 15, 2023. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
6. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population surveys. Med Care. 2014;52(12 suppl 5):S83-590. doi:10.1097/MLR.0000000000000227
7. Goertz C, Marriott BP, Finch FD, et al. Military report more complementary and alternative medicine use than civilians. J Altern Complement Med. 2013;19(6):509-517. doi:10.1089/acm.2012.0108
8. King HC, Hickey AH, Connelly C. Auricular acupuncture: a brief introduction for military providers. Mil Med. 2013;178(8):867-874. doi:10.7205/MILMED-D-13-00075
9. Niemtzow RC. Battlefield acupuncture. Medical Acupunct. 2007;19(4):225-228. doi:10.1089/acu.2007.0603
10. Collinsworth KM, Goss DL. Battlefield acupuncture and physical therapy versus physical therapy alone after shoulder surgery. Med Acupunct. 2019;31(4):228-238. doi:10.1089/acu.2019.1372
11. Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. 2017;40(4):432-438. doi:10.1080/10790268.2016.1141489
12. Federman DG, Radhakrishnan K, Gabriel L, Poulin LM, Kravetz JD. Group battlefield acupuncture in primary care for veterans with pain. South Med J. 2018;111(10):619-624. doi:10.14423/SMJ.0000000000000877
13. Garner BK, Hopkinson SG, Ketz AK, Landis CA, Trego LL. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Med Acupunct. 2018;30(5):262-272. doi:10.1089/acu.2018.1294
14. Fox LM, Murakami M, Danesh H, Manini AF. Battlefield acupuncture to treat low back pain in the emergency department. Am J Emerg Med. 2018; 36:1045-1048. doi:10.1016/j.ajem.2018.02.038
15. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
16. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177-184. doi:10.1016/j.annemergmed.2016.10.014
17. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390-392. doi:10.1111/j.1553-2712.2003.tb01355.
New insight into the growing problem of gaming disorder
A team of international researchers led by Orsolya Király, PhD, of the Institute of Psychology, Eötvös Loránd University, Budapest, reviewed the characteristics and etiology of GD. They concluded that its genesis arises from the interaction of environmental factors, game-specific factors and individual factors, including personality traits, comorbid psychopathology, and genetic predisposition.
“The development of GD is a complex process and we identified three major factors involved,” study coauthor Mark Griffiths, PhD, distinguished professor of behavioral addiction and director of the international gaming research unit, psychology department, Nottingham (England) Trent University, said in an interview. Because of this complexity, “prevention and intervention in GD require multiprofessional action.”
The review was published in Comprehensive Psychiatry.
In a second paper, published online in Frontiers in Psychiatry, Chinese investigators reviewing randomized controlled trials (RCTs) presented “compelling evidence” to support four effective interventions for GD: group counseling, acceptance and cognitive restructuring intervention program (ACRIP), short-term cognitive-behavioral therapy (CBT), and craving behavioral intervention (CBI).
A third paper, published online in the Journal of Behavioral Addictions, in which researchers analyzed close to 50 studies of GD, found that the concept of “recovery” is rarely mentioned in GD research. Lead author Belle Gavriel-Fried, PhD, senior professor, Bob Shapell School of Social Work, Tel Aviv University, said in an interview that recovery is a “holistic concept that taps into many aspects of life.”
Understanding the “differences in the impact and availability” of negative and positive human resources and their effect on recovery “can help clinicians to customize treatment,” she said.
Complex interplay
GD is garnering increasing attention in the clinical community, especially since 2019, when the World Health Organization included it in the ICD-11.
“Although for most individuals, gaming is a recreational activity or even a passion, a small group of gamers experiences negative symptoms which impact their mental and physical health and cause functional impairment,” wrote Dr. Király and colleagues.
Dr. Griffiths explained that his team wanted to provide an “up-to-date primer – a ‘one-stop shop’ – on all things etiologic concerning gaming disorder for academics and practitioners” as well as others, such as health policy makers, teachers, and individuals in the gaming industry.
The researchers identified three factors that increase the risk of developing GD, the first being gaming-related factors, which make video games “addictive in a way that vulnerable individuals may develop GD.”
For example, GD is more prevalent among online versus offline game players, possibly because online multiplayer games “provide safe environments in which players can fulfill their social needs while remaining invisible and anonymous.”
Game genre also matters, with massively multiplayer online role-playing games, first-person/third-person shooter games, real-time strategy games, and multiplayer online battle arena games most implicated in problematic gaming. Moreover, the “monetization techniques” of certain games also increase their addictive potential.
The researchers point to individual factors that increase the risk of developing GD, including male sex and younger age, personality traits like impulsivity and sensation-seeking, and comorbidities including ADHD, anxiety, and depression.
Poor self-esteem and lack of social competencies make gaming “an easy and efficient way to compensate for these deficiencies, which in turn, heightens the risk for developing GD,” they add. Neurobiological processes and genetic predisposition also play a role.
Lastly, the authors mentioned environmental factors, including family and peer-group issues, problems at work or school, and cultural factors.
“The take-home messages are that problematic gaming has had a long history of empirical research; that the psychiatric community now views GD as a legitimate mental health issue; and that the reasons for GD are complex, with many different factors involved in the acquisition, development, and maintenance of GD,” said Dr. Griffiths.
Beneficial behavioral therapies
Yuzhou Chen and colleagues, Southwest University, Chongqing, China, conducted a systematic review of RCTs investigating interventions for treating GD. Despite the “large number of intervention approaches developed over the past decade, as yet, there are no authoritative guidelines for what makes an effective GD intervention,” they wrote.
Few studies have focused specifically on GD but instead have focused on a combination of internet addiction and GD. But the interventions used to treat internet addiction may not apply to GD. And few studies have utilized an RCT design. The researchers therefore set out to review studies that specifically used an RCT design to investigate interventions for GD.
They searched six databases to identify RCTs that tested GD interventions from the inception of each database until the end of 2021. To be included, participants had to be diagnosed with GD and receive either a “complete and systematic intervention” or be in a comparator control group receiving no intervention or placebo.
Seven studies met the inclusion criteria (n = 332 participants). The studies tested five interventions:
- Group counseling with three different themes (interpersonal interaction, acceptance and commitment, cognition and behavior)
- CBI, which addresses cravings
- Transcranial direct current stimulation (tDCS)
- ACRIP with the main objectives of reducing GD symptoms and improving psychological well-being
- Short-term CBT, which addresses maladaptive cognitions
The mean duration of the interventions ranged from 3 to 15 weeks.
The primary outcome was GD severity, with secondary outcomes including depression, anxiety, cognition, game time, self-esteem, self-compassion, shyness, impulsivity, and psychological well-being.
Group counseling, CBI, ACRIP, and short-term CBT interventions had “a significant effect on decreasing the severity of GD,” while tDCS had “no significant effect.”
Behavioral therapy “exerts its effect on the behavioral mechanism of GD; for example, by reducing the association between game-related stimuli and the game player’s response to them,” the authors suggested.
Behavioral therapy “exerts its effect on the behavioral mechanism of GD; for example, by reducing the association between game-related stimuli and the game-player’s response to them,” the authors suggested.
Recovery vs. pathology
Recovery “traditionally represents the transition from trauma and illness to health,” Dr. Gavriel-Fried and colleagues noted.
Two paradigms of recovery are “deficit based” and “strength based.” The first assesses recovery in terms of abstinence, sobriety, and symptom reduction; and the second focuses on “growth, rather than a reduction in pathology.”
But although recovery is “embedded within mental health addiction policies and practice,” the concept has received “scant attention” in GD research.
The researchers therefore aimed to “map and summarize the state of the art on recovery from GD,” defining “recovery” as the “ability to handle conflicting feelings and emotions without external mediation.”
They conducted a scoping review of all literature regarding GD or internet GD published before February 2022 (47 studies, 2,924 participants with GD; mean age range, 13-26 years).
Most studies (n = 32) consisted of exclusively male subjects. Only 10 included both sexes, and female participants were in the minority.
Most studies (n = 42) did not address the concept of recovery, although all studies did report significant improvements in gaming-related pathology. Typical terminology used to describe changes in participants’ GD were “reduction” and/or “decrease” in symptom severity.
Although 18 studies mentioned the word “recovery,” only 5 actually discussed issues related to the notion of recovery, and only 5 used the term “abstinence.”
In addition, only 13 studies examined positive components of life in patients with GD, such as increased psychological well-being, life satisfaction, quality of life, improved emotional state, relational skills, and executive control, as well as improved self-care, hygiene, sleep, and interest in school studies.
“As a person and researcher who believes that words shape the way we perceive things, I think we should use the word ‘recovery’ rather than ‘pathology’ much more in research, therapy, and policy,” said Dr. Gavriel-Fried.
She noted that, because GD is a “relatively new behavioral addictive disorder, theories are still being developed and definitions of the symptoms are still being fine-tuned.”
“The field as a whole will benefit from future theoretical work that will lead to practical solutions for treating GD and ways to identify the risk factors,” Dr. Gavriel-Fried said.
Filling a research gap
In a comment, David Greenfield, MD, founder and medical director of the Connecticut-based Center for Internet and Technology Addiction, noted that 3 decades ago, there was almost no research into this area.
“The fact that we have these reviews and studies is good because all of the research adds to the science providing more data about an area we still don’t know that much about, where research is still in its infancy,” said Dr. Greenfield, who was not involved with the present study.
“Although we have definitions, there’s no complete agreement about the definitions of GD, and we do not yet have a unified approach,” continued Dr. Greenfield, who wrote the books Overcoming Internet Addiction for Dummies and Virtual Addiction.
He suggested that “recovery” is rarely used as a concept in GD research perhaps because there’s a “bifurcation in the field of addiction medicine in which behavioral addictions are not seen as equivalent to substance addictions,” and, particularly with GD, the principles of “recovery” have not yet matured.
“Recovery means meaningful life away from the screen, not just abstinence from the screen,” said Dr. Greenfield.
The study by Mr. Chen and colleagues was supported by grants from the National Social Science Foundation of China, the Chongqing Research Program of Basic Research and Frontier Technology, and the Fundamental Research Funds for the Central Universities. Dr. Griffiths has reported receiving research funding from Norsk Tipping (the gambling operator owned by the Norwegian government). The study by Dr. Király and colleagues received support from the Hungarian National Research Development and Innovation Office and the Janos Bolyai Research Scholarship Academy of Sciences to individual investigators. The study by Dr. Gavriel-Fried and colleagues received support from the Hungarian National Research Development and Innovation Office and the Janos Bolyai Research Scholarship Academy of Sciences to individual investigators. Dr. Gavriel-Fried has reported receiving grants from the Israel National Insurance Institute and the Committee for Independent Studies of the Israel Lottery. Dr. Greenfield reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A team of international researchers led by Orsolya Király, PhD, of the Institute of Psychology, Eötvös Loránd University, Budapest, reviewed the characteristics and etiology of GD. They concluded that its genesis arises from the interaction of environmental factors, game-specific factors and individual factors, including personality traits, comorbid psychopathology, and genetic predisposition.
“The development of GD is a complex process and we identified three major factors involved,” study coauthor Mark Griffiths, PhD, distinguished professor of behavioral addiction and director of the international gaming research unit, psychology department, Nottingham (England) Trent University, said in an interview. Because of this complexity, “prevention and intervention in GD require multiprofessional action.”
The review was published in Comprehensive Psychiatry.
In a second paper, published online in Frontiers in Psychiatry, Chinese investigators reviewing randomized controlled trials (RCTs) presented “compelling evidence” to support four effective interventions for GD: group counseling, acceptance and cognitive restructuring intervention program (ACRIP), short-term cognitive-behavioral therapy (CBT), and craving behavioral intervention (CBI).
A third paper, published online in the Journal of Behavioral Addictions, in which researchers analyzed close to 50 studies of GD, found that the concept of “recovery” is rarely mentioned in GD research. Lead author Belle Gavriel-Fried, PhD, senior professor, Bob Shapell School of Social Work, Tel Aviv University, said in an interview that recovery is a “holistic concept that taps into many aspects of life.”
Understanding the “differences in the impact and availability” of negative and positive human resources and their effect on recovery “can help clinicians to customize treatment,” she said.
Complex interplay
GD is garnering increasing attention in the clinical community, especially since 2019, when the World Health Organization included it in the ICD-11.
“Although for most individuals, gaming is a recreational activity or even a passion, a small group of gamers experiences negative symptoms which impact their mental and physical health and cause functional impairment,” wrote Dr. Király and colleagues.
Dr. Griffiths explained that his team wanted to provide an “up-to-date primer – a ‘one-stop shop’ – on all things etiologic concerning gaming disorder for academics and practitioners” as well as others, such as health policy makers, teachers, and individuals in the gaming industry.
The researchers identified three factors that increase the risk of developing GD, the first being gaming-related factors, which make video games “addictive in a way that vulnerable individuals may develop GD.”
For example, GD is more prevalent among online versus offline game players, possibly because online multiplayer games “provide safe environments in which players can fulfill their social needs while remaining invisible and anonymous.”
Game genre also matters, with massively multiplayer online role-playing games, first-person/third-person shooter games, real-time strategy games, and multiplayer online battle arena games most implicated in problematic gaming. Moreover, the “monetization techniques” of certain games also increase their addictive potential.
The researchers point to individual factors that increase the risk of developing GD, including male sex and younger age, personality traits like impulsivity and sensation-seeking, and comorbidities including ADHD, anxiety, and depression.
Poor self-esteem and lack of social competencies make gaming “an easy and efficient way to compensate for these deficiencies, which in turn, heightens the risk for developing GD,” they add. Neurobiological processes and genetic predisposition also play a role.
Lastly, the authors mentioned environmental factors, including family and peer-group issues, problems at work or school, and cultural factors.
“The take-home messages are that problematic gaming has had a long history of empirical research; that the psychiatric community now views GD as a legitimate mental health issue; and that the reasons for GD are complex, with many different factors involved in the acquisition, development, and maintenance of GD,” said Dr. Griffiths.
Beneficial behavioral therapies
Yuzhou Chen and colleagues, Southwest University, Chongqing, China, conducted a systematic review of RCTs investigating interventions for treating GD. Despite the “large number of intervention approaches developed over the past decade, as yet, there are no authoritative guidelines for what makes an effective GD intervention,” they wrote.
Few studies have focused specifically on GD but instead have focused on a combination of internet addiction and GD. But the interventions used to treat internet addiction may not apply to GD. And few studies have utilized an RCT design. The researchers therefore set out to review studies that specifically used an RCT design to investigate interventions for GD.
They searched six databases to identify RCTs that tested GD interventions from the inception of each database until the end of 2021. To be included, participants had to be diagnosed with GD and receive either a “complete and systematic intervention” or be in a comparator control group receiving no intervention or placebo.
Seven studies met the inclusion criteria (n = 332 participants). The studies tested five interventions:
- Group counseling with three different themes (interpersonal interaction, acceptance and commitment, cognition and behavior)
- CBI, which addresses cravings
- Transcranial direct current stimulation (tDCS)
- ACRIP with the main objectives of reducing GD symptoms and improving psychological well-being
- Short-term CBT, which addresses maladaptive cognitions
The mean duration of the interventions ranged from 3 to 15 weeks.
The primary outcome was GD severity, with secondary outcomes including depression, anxiety, cognition, game time, self-esteem, self-compassion, shyness, impulsivity, and psychological well-being.
Group counseling, CBI, ACRIP, and short-term CBT interventions had “a significant effect on decreasing the severity of GD,” while tDCS had “no significant effect.”
Behavioral therapy “exerts its effect on the behavioral mechanism of GD; for example, by reducing the association between game-related stimuli and the game player’s response to them,” the authors suggested.
Behavioral therapy “exerts its effect on the behavioral mechanism of GD; for example, by reducing the association between game-related stimuli and the game-player’s response to them,” the authors suggested.
Recovery vs. pathology
Recovery “traditionally represents the transition from trauma and illness to health,” Dr. Gavriel-Fried and colleagues noted.
Two paradigms of recovery are “deficit based” and “strength based.” The first assesses recovery in terms of abstinence, sobriety, and symptom reduction; and the second focuses on “growth, rather than a reduction in pathology.”
But although recovery is “embedded within mental health addiction policies and practice,” the concept has received “scant attention” in GD research.
The researchers therefore aimed to “map and summarize the state of the art on recovery from GD,” defining “recovery” as the “ability to handle conflicting feelings and emotions without external mediation.”
They conducted a scoping review of all literature regarding GD or internet GD published before February 2022 (47 studies, 2,924 participants with GD; mean age range, 13-26 years).
Most studies (n = 32) consisted of exclusively male subjects. Only 10 included both sexes, and female participants were in the minority.
Most studies (n = 42) did not address the concept of recovery, although all studies did report significant improvements in gaming-related pathology. Typical terminology used to describe changes in participants’ GD were “reduction” and/or “decrease” in symptom severity.
Although 18 studies mentioned the word “recovery,” only 5 actually discussed issues related to the notion of recovery, and only 5 used the term “abstinence.”
In addition, only 13 studies examined positive components of life in patients with GD, such as increased psychological well-being, life satisfaction, quality of life, improved emotional state, relational skills, and executive control, as well as improved self-care, hygiene, sleep, and interest in school studies.
“As a person and researcher who believes that words shape the way we perceive things, I think we should use the word ‘recovery’ rather than ‘pathology’ much more in research, therapy, and policy,” said Dr. Gavriel-Fried.
She noted that, because GD is a “relatively new behavioral addictive disorder, theories are still being developed and definitions of the symptoms are still being fine-tuned.”
“The field as a whole will benefit from future theoretical work that will lead to practical solutions for treating GD and ways to identify the risk factors,” Dr. Gavriel-Fried said.
Filling a research gap
In a comment, David Greenfield, MD, founder and medical director of the Connecticut-based Center for Internet and Technology Addiction, noted that 3 decades ago, there was almost no research into this area.
“The fact that we have these reviews and studies is good because all of the research adds to the science providing more data about an area we still don’t know that much about, where research is still in its infancy,” said Dr. Greenfield, who was not involved with the present study.
“Although we have definitions, there’s no complete agreement about the definitions of GD, and we do not yet have a unified approach,” continued Dr. Greenfield, who wrote the books Overcoming Internet Addiction for Dummies and Virtual Addiction.
He suggested that “recovery” is rarely used as a concept in GD research perhaps because there’s a “bifurcation in the field of addiction medicine in which behavioral addictions are not seen as equivalent to substance addictions,” and, particularly with GD, the principles of “recovery” have not yet matured.
“Recovery means meaningful life away from the screen, not just abstinence from the screen,” said Dr. Greenfield.
The study by Mr. Chen and colleagues was supported by grants from the National Social Science Foundation of China, the Chongqing Research Program of Basic Research and Frontier Technology, and the Fundamental Research Funds for the Central Universities. Dr. Griffiths has reported receiving research funding from Norsk Tipping (the gambling operator owned by the Norwegian government). The study by Dr. Király and colleagues received support from the Hungarian National Research Development and Innovation Office and the Janos Bolyai Research Scholarship Academy of Sciences to individual investigators. The study by Dr. Gavriel-Fried and colleagues received support from the Hungarian National Research Development and Innovation Office and the Janos Bolyai Research Scholarship Academy of Sciences to individual investigators. Dr. Gavriel-Fried has reported receiving grants from the Israel National Insurance Institute and the Committee for Independent Studies of the Israel Lottery. Dr. Greenfield reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A team of international researchers led by Orsolya Király, PhD, of the Institute of Psychology, Eötvös Loránd University, Budapest, reviewed the characteristics and etiology of GD. They concluded that its genesis arises from the interaction of environmental factors, game-specific factors and individual factors, including personality traits, comorbid psychopathology, and genetic predisposition.
“The development of GD is a complex process and we identified three major factors involved,” study coauthor Mark Griffiths, PhD, distinguished professor of behavioral addiction and director of the international gaming research unit, psychology department, Nottingham (England) Trent University, said in an interview. Because of this complexity, “prevention and intervention in GD require multiprofessional action.”
The review was published in Comprehensive Psychiatry.
In a second paper, published online in Frontiers in Psychiatry, Chinese investigators reviewing randomized controlled trials (RCTs) presented “compelling evidence” to support four effective interventions for GD: group counseling, acceptance and cognitive restructuring intervention program (ACRIP), short-term cognitive-behavioral therapy (CBT), and craving behavioral intervention (CBI).
A third paper, published online in the Journal of Behavioral Addictions, in which researchers analyzed close to 50 studies of GD, found that the concept of “recovery” is rarely mentioned in GD research. Lead author Belle Gavriel-Fried, PhD, senior professor, Bob Shapell School of Social Work, Tel Aviv University, said in an interview that recovery is a “holistic concept that taps into many aspects of life.”
Understanding the “differences in the impact and availability” of negative and positive human resources and their effect on recovery “can help clinicians to customize treatment,” she said.
Complex interplay
GD is garnering increasing attention in the clinical community, especially since 2019, when the World Health Organization included it in the ICD-11.
“Although for most individuals, gaming is a recreational activity or even a passion, a small group of gamers experiences negative symptoms which impact their mental and physical health and cause functional impairment,” wrote Dr. Király and colleagues.
Dr. Griffiths explained that his team wanted to provide an “up-to-date primer – a ‘one-stop shop’ – on all things etiologic concerning gaming disorder for academics and practitioners” as well as others, such as health policy makers, teachers, and individuals in the gaming industry.
The researchers identified three factors that increase the risk of developing GD, the first being gaming-related factors, which make video games “addictive in a way that vulnerable individuals may develop GD.”
For example, GD is more prevalent among online versus offline game players, possibly because online multiplayer games “provide safe environments in which players can fulfill their social needs while remaining invisible and anonymous.”
Game genre also matters, with massively multiplayer online role-playing games, first-person/third-person shooter games, real-time strategy games, and multiplayer online battle arena games most implicated in problematic gaming. Moreover, the “monetization techniques” of certain games also increase their addictive potential.
The researchers point to individual factors that increase the risk of developing GD, including male sex and younger age, personality traits like impulsivity and sensation-seeking, and comorbidities including ADHD, anxiety, and depression.
Poor self-esteem and lack of social competencies make gaming “an easy and efficient way to compensate for these deficiencies, which in turn, heightens the risk for developing GD,” they add. Neurobiological processes and genetic predisposition also play a role.
Lastly, the authors mentioned environmental factors, including family and peer-group issues, problems at work or school, and cultural factors.
“The take-home messages are that problematic gaming has had a long history of empirical research; that the psychiatric community now views GD as a legitimate mental health issue; and that the reasons for GD are complex, with many different factors involved in the acquisition, development, and maintenance of GD,” said Dr. Griffiths.
Beneficial behavioral therapies
Yuzhou Chen and colleagues, Southwest University, Chongqing, China, conducted a systematic review of RCTs investigating interventions for treating GD. Despite the “large number of intervention approaches developed over the past decade, as yet, there are no authoritative guidelines for what makes an effective GD intervention,” they wrote.
Few studies have focused specifically on GD but instead have focused on a combination of internet addiction and GD. But the interventions used to treat internet addiction may not apply to GD. And few studies have utilized an RCT design. The researchers therefore set out to review studies that specifically used an RCT design to investigate interventions for GD.
They searched six databases to identify RCTs that tested GD interventions from the inception of each database until the end of 2021. To be included, participants had to be diagnosed with GD and receive either a “complete and systematic intervention” or be in a comparator control group receiving no intervention or placebo.
Seven studies met the inclusion criteria (n = 332 participants). The studies tested five interventions:
- Group counseling with three different themes (interpersonal interaction, acceptance and commitment, cognition and behavior)
- CBI, which addresses cravings
- Transcranial direct current stimulation (tDCS)
- ACRIP with the main objectives of reducing GD symptoms and improving psychological well-being
- Short-term CBT, which addresses maladaptive cognitions
The mean duration of the interventions ranged from 3 to 15 weeks.
The primary outcome was GD severity, with secondary outcomes including depression, anxiety, cognition, game time, self-esteem, self-compassion, shyness, impulsivity, and psychological well-being.
Group counseling, CBI, ACRIP, and short-term CBT interventions had “a significant effect on decreasing the severity of GD,” while tDCS had “no significant effect.”
Behavioral therapy “exerts its effect on the behavioral mechanism of GD; for example, by reducing the association between game-related stimuli and the game player’s response to them,” the authors suggested.
Behavioral therapy “exerts its effect on the behavioral mechanism of GD; for example, by reducing the association between game-related stimuli and the game-player’s response to them,” the authors suggested.
Recovery vs. pathology
Recovery “traditionally represents the transition from trauma and illness to health,” Dr. Gavriel-Fried and colleagues noted.
Two paradigms of recovery are “deficit based” and “strength based.” The first assesses recovery in terms of abstinence, sobriety, and symptom reduction; and the second focuses on “growth, rather than a reduction in pathology.”
But although recovery is “embedded within mental health addiction policies and practice,” the concept has received “scant attention” in GD research.
The researchers therefore aimed to “map and summarize the state of the art on recovery from GD,” defining “recovery” as the “ability to handle conflicting feelings and emotions without external mediation.”
They conducted a scoping review of all literature regarding GD or internet GD published before February 2022 (47 studies, 2,924 participants with GD; mean age range, 13-26 years).
Most studies (n = 32) consisted of exclusively male subjects. Only 10 included both sexes, and female participants were in the minority.
Most studies (n = 42) did not address the concept of recovery, although all studies did report significant improvements in gaming-related pathology. Typical terminology used to describe changes in participants’ GD were “reduction” and/or “decrease” in symptom severity.
Although 18 studies mentioned the word “recovery,” only 5 actually discussed issues related to the notion of recovery, and only 5 used the term “abstinence.”
In addition, only 13 studies examined positive components of life in patients with GD, such as increased psychological well-being, life satisfaction, quality of life, improved emotional state, relational skills, and executive control, as well as improved self-care, hygiene, sleep, and interest in school studies.
“As a person and researcher who believes that words shape the way we perceive things, I think we should use the word ‘recovery’ rather than ‘pathology’ much more in research, therapy, and policy,” said Dr. Gavriel-Fried.
She noted that, because GD is a “relatively new behavioral addictive disorder, theories are still being developed and definitions of the symptoms are still being fine-tuned.”
“The field as a whole will benefit from future theoretical work that will lead to practical solutions for treating GD and ways to identify the risk factors,” Dr. Gavriel-Fried said.
Filling a research gap
In a comment, David Greenfield, MD, founder and medical director of the Connecticut-based Center for Internet and Technology Addiction, noted that 3 decades ago, there was almost no research into this area.
“The fact that we have these reviews and studies is good because all of the research adds to the science providing more data about an area we still don’t know that much about, where research is still in its infancy,” said Dr. Greenfield, who was not involved with the present study.
“Although we have definitions, there’s no complete agreement about the definitions of GD, and we do not yet have a unified approach,” continued Dr. Greenfield, who wrote the books Overcoming Internet Addiction for Dummies and Virtual Addiction.
He suggested that “recovery” is rarely used as a concept in GD research perhaps because there’s a “bifurcation in the field of addiction medicine in which behavioral addictions are not seen as equivalent to substance addictions,” and, particularly with GD, the principles of “recovery” have not yet matured.
“Recovery means meaningful life away from the screen, not just abstinence from the screen,” said Dr. Greenfield.
The study by Mr. Chen and colleagues was supported by grants from the National Social Science Foundation of China, the Chongqing Research Program of Basic Research and Frontier Technology, and the Fundamental Research Funds for the Central Universities. Dr. Griffiths has reported receiving research funding from Norsk Tipping (the gambling operator owned by the Norwegian government). The study by Dr. Király and colleagues received support from the Hungarian National Research Development and Innovation Office and the Janos Bolyai Research Scholarship Academy of Sciences to individual investigators. The study by Dr. Gavriel-Fried and colleagues received support from the Hungarian National Research Development and Innovation Office and the Janos Bolyai Research Scholarship Academy of Sciences to individual investigators. Dr. Gavriel-Fried has reported receiving grants from the Israel National Insurance Institute and the Committee for Independent Studies of the Israel Lottery. Dr. Greenfield reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New guidelines for cannabis in chronic pain management released
New clinical practice guidelines for cannabis in chronic pain management have been released.
Developed by a group of Canadian researchers, clinicians, and patients, the guidelines note that cannabinoid-based medicines (CBM) may help clinicians offer an effective, less addictive, alternative to opioids in patients with chronic noncancer pain and comorbid conditions.
“We don’t recommend using CBM first line for anything pretty much because there are other alternatives that may be more effective and also offer fewer side effects,” lead guideline author Alan Bell, MD, assistant professor of family and community medicine at the University of Toronto, told this news organization.
“But I would strongly argue that I would use cannabis-based medicine over opioids every time. Why would you use a high potency-high toxicity agent when there’s a low potency-low toxicity alternative?” he said.
The guidelines were published online in the journal Cannabis and Cannabinoid Research.
Examining the evidence
A consistent criticism of CBM has been the lack of quality research supporting its therapeutic utility. To develop the current recommendations, the task force reviewed 47 pain management studies enrolling more than 11,000 patients. Almost half of the studies (n = 22) were randomized controlled trials (RCTs) and 12 of the 19 included systematic reviews focused solely on RCTs.
Overall, 38 of the 47 included studies demonstrated that CBM provided at least moderate benefits for chronic pain, resulting in a “strong” recommendation – mostly as an adjunct or replacement treatment in individuals living with chronic pain.
Overall, the guidelines place a high value on improving chronic pain and functionality, and addressing co-occurring conditions such as insomnia, anxiety and depression, mobility, and inflammation. They also provide practical dosing and formulation tips to support the use of CBM in the clinical setting.
When it comes to chronic pain, CBM is not a panacea. However, prior research suggests cannabinoids and opioids share several pharmacologic properties, including independent but possibly related mechanisms for antinociception, making them an intriguing combination.
In the current guidelines, all of the four studies specifically addressing combined opioids and vaporized cannabis flower demonstrated further pain reduction, reinforcing the conclusion that the benefits of CBM for improving pain control in patients taking opioids outweigh the risk of nonserious adverse events (AEs), such as dry mouth, dizziness, increased appetite, sedation, and concentration difficulties.
The recommendations also highlighted evidence demonstrating that a majority of participants were able to reduce use of routine pain medications with concomitant CBM/opioid administration, while simultaneously offering secondary benefits such as improved sleep, anxiety, and mood, as well as prevention of opioid tolerance and dose escalation.
Importantly, the guidelines offer an evidence-based algorithm with a clear framework for tapering patients off opioids, especially those who are on > 50 mg MED, which places them with a twofold greater risk for fatal overdose.
An effective alternative
Commenting on the new guidelines, Mark Wallace, MD, who has extensive experience researching and treating pain patients with medical cannabis, said the genesis of his interest in medical cannabis mirrors the guidelines’ focus.
“What got me interested in medical cannabis was trying to get patients off of opioids,” said Dr. Wallace, professor of anesthesiology and chief of the division of pain medicine in the department of anesthesiology at the University of California, San Diego. Dr. Wallace, who was not involved in the guidelines’ development study, said that he’s “titrated hundreds of patients off of opioids using cannabis.”
Dr. Wallace said he found the guidelines’ dosing recommendations helpful.
“If you stay within the 1- to 5-mg dosing range, the risks are so incredibly low, you’re not going to harm the patient.”
While there are patients who abuse cannabis and CBMs, Dr. Wallace noted that he has seen only one patient in the past 20 years who was overusing the medical cannabis. He added that his patient population does not use medical cannabis to get high and, in fact, wants to avoid doses that produce that effect at all costs.
Also commenting on the guidelines, Christopher Gilligan, MD, MBA, associate chief medical officer and a pain medicine physician at Brigham and Women’s Hospital in Boston, who was not involved in the guidelines’ development, points to the risks.
“When we have an opportunity to use cannabinoids in place of opioids for our patients, I think that that’s a positive thing ... and a wise choice in terms of risk benefit,” Dr. Gilligan said.
On the other hand, he cautioned that “freely prescribing” cannabinoids for chronic pain in patients who aren’t on opioids is not good practice.
“We have to take seriously the potential adverse effects of [cannabis], including marijuana use disorder, interference with learning, memory impairment, and psychotic breakthroughs,” said Dr. Gilligan.
Given the current climate, it would appear that CBM is a long way from being endorsed by the Food and Drug Administration, but for clinicians interested in trying CBM for chronic pain patients, the guidelines may offer a roadmap for initiation and an alternative to prescribing opioids.
Dr. Bell, Dr. Gilligan, and Dr. Wallace report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New clinical practice guidelines for cannabis in chronic pain management have been released.
Developed by a group of Canadian researchers, clinicians, and patients, the guidelines note that cannabinoid-based medicines (CBM) may help clinicians offer an effective, less addictive, alternative to opioids in patients with chronic noncancer pain and comorbid conditions.
“We don’t recommend using CBM first line for anything pretty much because there are other alternatives that may be more effective and also offer fewer side effects,” lead guideline author Alan Bell, MD, assistant professor of family and community medicine at the University of Toronto, told this news organization.
“But I would strongly argue that I would use cannabis-based medicine over opioids every time. Why would you use a high potency-high toxicity agent when there’s a low potency-low toxicity alternative?” he said.
The guidelines were published online in the journal Cannabis and Cannabinoid Research.
Examining the evidence
A consistent criticism of CBM has been the lack of quality research supporting its therapeutic utility. To develop the current recommendations, the task force reviewed 47 pain management studies enrolling more than 11,000 patients. Almost half of the studies (n = 22) were randomized controlled trials (RCTs) and 12 of the 19 included systematic reviews focused solely on RCTs.
Overall, 38 of the 47 included studies demonstrated that CBM provided at least moderate benefits for chronic pain, resulting in a “strong” recommendation – mostly as an adjunct or replacement treatment in individuals living with chronic pain.
Overall, the guidelines place a high value on improving chronic pain and functionality, and addressing co-occurring conditions such as insomnia, anxiety and depression, mobility, and inflammation. They also provide practical dosing and formulation tips to support the use of CBM in the clinical setting.
When it comes to chronic pain, CBM is not a panacea. However, prior research suggests cannabinoids and opioids share several pharmacologic properties, including independent but possibly related mechanisms for antinociception, making them an intriguing combination.
In the current guidelines, all of the four studies specifically addressing combined opioids and vaporized cannabis flower demonstrated further pain reduction, reinforcing the conclusion that the benefits of CBM for improving pain control in patients taking opioids outweigh the risk of nonserious adverse events (AEs), such as dry mouth, dizziness, increased appetite, sedation, and concentration difficulties.
The recommendations also highlighted evidence demonstrating that a majority of participants were able to reduce use of routine pain medications with concomitant CBM/opioid administration, while simultaneously offering secondary benefits such as improved sleep, anxiety, and mood, as well as prevention of opioid tolerance and dose escalation.
Importantly, the guidelines offer an evidence-based algorithm with a clear framework for tapering patients off opioids, especially those who are on > 50 mg MED, which places them with a twofold greater risk for fatal overdose.
An effective alternative
Commenting on the new guidelines, Mark Wallace, MD, who has extensive experience researching and treating pain patients with medical cannabis, said the genesis of his interest in medical cannabis mirrors the guidelines’ focus.
“What got me interested in medical cannabis was trying to get patients off of opioids,” said Dr. Wallace, professor of anesthesiology and chief of the division of pain medicine in the department of anesthesiology at the University of California, San Diego. Dr. Wallace, who was not involved in the guidelines’ development study, said that he’s “titrated hundreds of patients off of opioids using cannabis.”
Dr. Wallace said he found the guidelines’ dosing recommendations helpful.
“If you stay within the 1- to 5-mg dosing range, the risks are so incredibly low, you’re not going to harm the patient.”
While there are patients who abuse cannabis and CBMs, Dr. Wallace noted that he has seen only one patient in the past 20 years who was overusing the medical cannabis. He added that his patient population does not use medical cannabis to get high and, in fact, wants to avoid doses that produce that effect at all costs.
Also commenting on the guidelines, Christopher Gilligan, MD, MBA, associate chief medical officer and a pain medicine physician at Brigham and Women’s Hospital in Boston, who was not involved in the guidelines’ development, points to the risks.
“When we have an opportunity to use cannabinoids in place of opioids for our patients, I think that that’s a positive thing ... and a wise choice in terms of risk benefit,” Dr. Gilligan said.
On the other hand, he cautioned that “freely prescribing” cannabinoids for chronic pain in patients who aren’t on opioids is not good practice.
“We have to take seriously the potential adverse effects of [cannabis], including marijuana use disorder, interference with learning, memory impairment, and psychotic breakthroughs,” said Dr. Gilligan.
Given the current climate, it would appear that CBM is a long way from being endorsed by the Food and Drug Administration, but for clinicians interested in trying CBM for chronic pain patients, the guidelines may offer a roadmap for initiation and an alternative to prescribing opioids.
Dr. Bell, Dr. Gilligan, and Dr. Wallace report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New clinical practice guidelines for cannabis in chronic pain management have been released.
Developed by a group of Canadian researchers, clinicians, and patients, the guidelines note that cannabinoid-based medicines (CBM) may help clinicians offer an effective, less addictive, alternative to opioids in patients with chronic noncancer pain and comorbid conditions.
“We don’t recommend using CBM first line for anything pretty much because there are other alternatives that may be more effective and also offer fewer side effects,” lead guideline author Alan Bell, MD, assistant professor of family and community medicine at the University of Toronto, told this news organization.
“But I would strongly argue that I would use cannabis-based medicine over opioids every time. Why would you use a high potency-high toxicity agent when there’s a low potency-low toxicity alternative?” he said.
The guidelines were published online in the journal Cannabis and Cannabinoid Research.
Examining the evidence
A consistent criticism of CBM has been the lack of quality research supporting its therapeutic utility. To develop the current recommendations, the task force reviewed 47 pain management studies enrolling more than 11,000 patients. Almost half of the studies (n = 22) were randomized controlled trials (RCTs) and 12 of the 19 included systematic reviews focused solely on RCTs.
Overall, 38 of the 47 included studies demonstrated that CBM provided at least moderate benefits for chronic pain, resulting in a “strong” recommendation – mostly as an adjunct or replacement treatment in individuals living with chronic pain.
Overall, the guidelines place a high value on improving chronic pain and functionality, and addressing co-occurring conditions such as insomnia, anxiety and depression, mobility, and inflammation. They also provide practical dosing and formulation tips to support the use of CBM in the clinical setting.
When it comes to chronic pain, CBM is not a panacea. However, prior research suggests cannabinoids and opioids share several pharmacologic properties, including independent but possibly related mechanisms for antinociception, making them an intriguing combination.
In the current guidelines, all of the four studies specifically addressing combined opioids and vaporized cannabis flower demonstrated further pain reduction, reinforcing the conclusion that the benefits of CBM for improving pain control in patients taking opioids outweigh the risk of nonserious adverse events (AEs), such as dry mouth, dizziness, increased appetite, sedation, and concentration difficulties.
The recommendations also highlighted evidence demonstrating that a majority of participants were able to reduce use of routine pain medications with concomitant CBM/opioid administration, while simultaneously offering secondary benefits such as improved sleep, anxiety, and mood, as well as prevention of opioid tolerance and dose escalation.
Importantly, the guidelines offer an evidence-based algorithm with a clear framework for tapering patients off opioids, especially those who are on > 50 mg MED, which places them with a twofold greater risk for fatal overdose.
An effective alternative
Commenting on the new guidelines, Mark Wallace, MD, who has extensive experience researching and treating pain patients with medical cannabis, said the genesis of his interest in medical cannabis mirrors the guidelines’ focus.
“What got me interested in medical cannabis was trying to get patients off of opioids,” said Dr. Wallace, professor of anesthesiology and chief of the division of pain medicine in the department of anesthesiology at the University of California, San Diego. Dr. Wallace, who was not involved in the guidelines’ development study, said that he’s “titrated hundreds of patients off of opioids using cannabis.”
Dr. Wallace said he found the guidelines’ dosing recommendations helpful.
“If you stay within the 1- to 5-mg dosing range, the risks are so incredibly low, you’re not going to harm the patient.”
While there are patients who abuse cannabis and CBMs, Dr. Wallace noted that he has seen only one patient in the past 20 years who was overusing the medical cannabis. He added that his patient population does not use medical cannabis to get high and, in fact, wants to avoid doses that produce that effect at all costs.
Also commenting on the guidelines, Christopher Gilligan, MD, MBA, associate chief medical officer and a pain medicine physician at Brigham and Women’s Hospital in Boston, who was not involved in the guidelines’ development, points to the risks.
“When we have an opportunity to use cannabinoids in place of opioids for our patients, I think that that’s a positive thing ... and a wise choice in terms of risk benefit,” Dr. Gilligan said.
On the other hand, he cautioned that “freely prescribing” cannabinoids for chronic pain in patients who aren’t on opioids is not good practice.
“We have to take seriously the potential adverse effects of [cannabis], including marijuana use disorder, interference with learning, memory impairment, and psychotic breakthroughs,” said Dr. Gilligan.
Given the current climate, it would appear that CBM is a long way from being endorsed by the Food and Drug Administration, but for clinicians interested in trying CBM for chronic pain patients, the guidelines may offer a roadmap for initiation and an alternative to prescribing opioids.
Dr. Bell, Dr. Gilligan, and Dr. Wallace report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANNABIS AND CANNABINOID RESEARCH