User login
No overall statin effect seen on dementia, cognition in ASPREE analysis
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”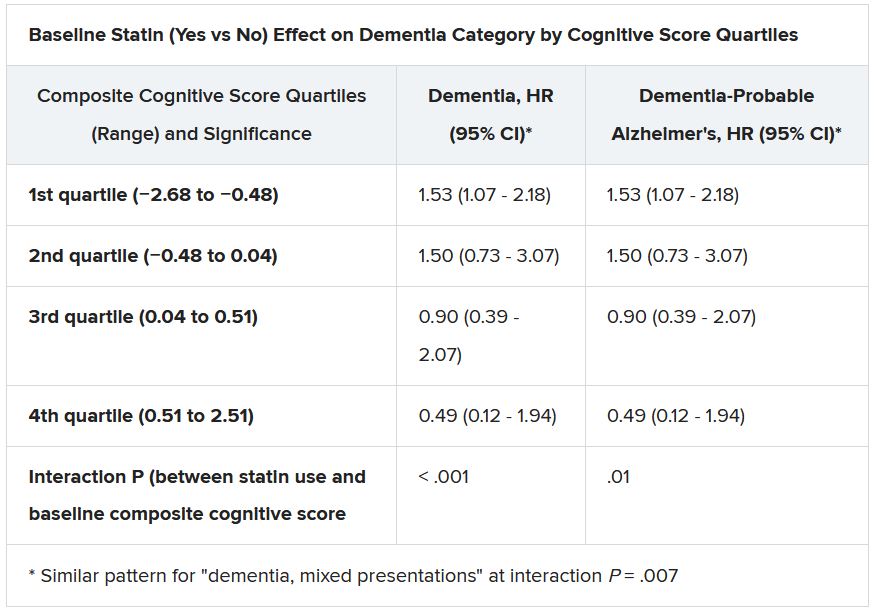
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Bariatric surgery’s cardiovascular benefit extends to 7 years
Patients with obesity who had bariatric surgery had a lower risk of having a major adverse cardiovascular event (MACE) or dying from all causes during a median 7-year follow-up, compared with similar patients who did not undergo surgery.
These findings, from a province-wide retrospective cohort study from Quebec, follow two recent, slightly shorter similar trials.
Now we need a large randomized clinical trial (RCT), experts say, to definitively establish cardiovascular and mortality benefits in people with obesity who have metabolic/bariatric surgery. And such a trial is just beginning.
Philippe Bouchard, MD, a general surgery resident from McGill University in Montreal presented the Quebec study in a top papers session at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings showed that, among obese patients with metabolic syndrome, bariatric/metabolic surgery is associated with a sustained decrease in the incidence of MACE and all-cause mortality of at least 5 years, Dr. Bouchard said.
“The results of this population-based observational study should be validated in randomized controlled trials,” he concluded.
In the meantime, “we believe our study adds to the body of evidence in mainly two ways,” Dr. Bouchard told this news organization in an email.
It has a longer follow-up than recent observational studies, “a median of 7 years, compared to 3.9 years in a study from the Cleveland Clinic, and 4.6 years in one from Ontario, he said.
“This allows us to [estimate] an absolute risk reduction of MACE of 5.11% at 10 years,” he added. This is a smaller risk reduction than the roughly 40% risk reduction seen in the other two studies, possibly because of selection bias, Dr. Bouchard speculated.
“Second, most of the larger cohorts are heavily weighted on Roux-en-Y gastric bypass,” he continued. In contrast, their study included diverse procedures, including sleeve gastrectomy, duodenal switch, and adjustable gastric banding.
“Given the rise in popularity of a derivative of the duodenal switch – the single-anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADi-S) – we believe this information is timely and relevant to clinicians,” Dr. Bouchard said.
RCT on the subject is coming
“I totally agree that we need a large randomized controlled trial of bariatric surgery versus optimal medical therapy to conclusively establish” the impact of bariatric surgery on cardiovascular outcomes, said the assigned discussant, Mehran Anvari, MD. And their research group is just about to begin one.
In the absence of RCT data, clinicians “may currently not refer [eligible] patients for bariatric surgery because of the high risk they pose,” said Dr. Anvari, professor and director of the Centre for Minimal Access Surgery of McMaster University, Hamilton, Ont., and senior author in the Ontario study.
Furthermore, an important point is that the current trial extended the follow-up to 7 years, he told this news organization in an email.
That study included patients with diabetes and hypertension, he added, whereas his group included patients with a history of cardiovascular disease and/or heart failure.
“We hope these studies encourage general practitioners and cardiologists to consider bariatric surgery as a viable treatment option to prevent and reduce the risk of MACE in the obese patients [body mass index >35 kg/m2] with significant cardiovascular disease,” he said.
“We have embarked on a pilot RCT among bariatric centers of excellence in Ontario,” Dr. Anvari added, which showed the feasibility and safety of such a study.
He estimates that the RCT will need to recruit 2,000 patients to demonstrate the safety and effectiveness of bariatric surgery in reducing MACE and cardiac and all-cause mortality among patients with existing cardiovascular disease.
This “will require international collaboration,” he added, “and our group is currently establishing collaboration with sites in North America, Europe, and Australia to conduct such a study.”
Patients matched for age, sex, number of comorbidities
Quebec has a single public health care system that covers the cost of bariatric surgery for eligible patients; that is, those with a BMI greater than 35 kg/m2 and comorbidities or a BMI greater than 40 kg/m2.
Using this provincial health care database, which covers over 97% of the population, the researchers identified 3,637 patients with diabetes and/or hypertension who had bariatric surgery during 2007-2012.
They matched the surgery patients with 5,420 control patients with obesity who lived in the same geographic region and had a similar age, sex, and number of Charlson Comorbidity Index comorbidities, but did not undergo bariatric surgery.
The patients had a mean age of 50 and 64% were women.
Half had zero to one comorbidities, a quarter had two comorbidities, and another quarter had at least three comorbidities.
Most patients in the surgery group had type 2 diabetes (70%) and 50% had hypertension, whereas in the control group, most patients had hypertension (82%) and 41% had diabetes.
The most common type of bariatric surgery was adjustable gastric banding (42% of patients), followed by duodenal switch (24%), sleeve gastrectomy (23%), and Roux-en-Y gastric bypass (11%).
The primary outcome was the incidence of MACE, defined as coronary artery events (including myocardial infarction, percutaneous coronary intervention, and coronary artery bypass graft), stroke, heart failure, and all-cause mortality,
After a median follow-up of 7-11 years, fewer patients in the surgical group than in the control group had MACE (20% vs. 25%) or died from all causes (4.1% vs. 6.3%, both statistically significant at P < .01)
Similarly, significantly fewer patients in the surgical group than in the control group had a coronary artery event or heart failure (each P < .01).
However, there were no significant between-group difference in the rate of stroke, possibly because of the small number of strokes.
The risk of MACE was 17% lower in the group that had bariatric surgery than in the control group (adjusted hazard ratio, 0.83; 95% confidence interval, 0.78-0.89), after adjusting for age, sex, and number of comorbidities.
In subgroup analysis, patients who had adjustable gastric banding, Roux-en-Y gastric bypass, or duodenal switch had a significantly lower risk of MACE than control patients.
The risk of MACE was similar in patients who had sleeve gastrectomy and in control patients.
However, these subgroup results need to be interpreted with caution since the surgery and control patients in each surgery type subgroup were not matched for age, sex, and comorbidities, said Dr. Bouchard.
He acknowledged that study limitations include a lack of information about the patients’ BMI, weight, medications, and glycemic control (hemoglobin A1c).
Dr. Bouchard and Dr. Anvari have no relevant financial disclosures.
Patients with obesity who had bariatric surgery had a lower risk of having a major adverse cardiovascular event (MACE) or dying from all causes during a median 7-year follow-up, compared with similar patients who did not undergo surgery.
These findings, from a province-wide retrospective cohort study from Quebec, follow two recent, slightly shorter similar trials.
Now we need a large randomized clinical trial (RCT), experts say, to definitively establish cardiovascular and mortality benefits in people with obesity who have metabolic/bariatric surgery. And such a trial is just beginning.
Philippe Bouchard, MD, a general surgery resident from McGill University in Montreal presented the Quebec study in a top papers session at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings showed that, among obese patients with metabolic syndrome, bariatric/metabolic surgery is associated with a sustained decrease in the incidence of MACE and all-cause mortality of at least 5 years, Dr. Bouchard said.
“The results of this population-based observational study should be validated in randomized controlled trials,” he concluded.
In the meantime, “we believe our study adds to the body of evidence in mainly two ways,” Dr. Bouchard told this news organization in an email.
It has a longer follow-up than recent observational studies, “a median of 7 years, compared to 3.9 years in a study from the Cleveland Clinic, and 4.6 years in one from Ontario, he said.
“This allows us to [estimate] an absolute risk reduction of MACE of 5.11% at 10 years,” he added. This is a smaller risk reduction than the roughly 40% risk reduction seen in the other two studies, possibly because of selection bias, Dr. Bouchard speculated.
“Second, most of the larger cohorts are heavily weighted on Roux-en-Y gastric bypass,” he continued. In contrast, their study included diverse procedures, including sleeve gastrectomy, duodenal switch, and adjustable gastric banding.
“Given the rise in popularity of a derivative of the duodenal switch – the single-anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADi-S) – we believe this information is timely and relevant to clinicians,” Dr. Bouchard said.
RCT on the subject is coming
“I totally agree that we need a large randomized controlled trial of bariatric surgery versus optimal medical therapy to conclusively establish” the impact of bariatric surgery on cardiovascular outcomes, said the assigned discussant, Mehran Anvari, MD. And their research group is just about to begin one.
In the absence of RCT data, clinicians “may currently not refer [eligible] patients for bariatric surgery because of the high risk they pose,” said Dr. Anvari, professor and director of the Centre for Minimal Access Surgery of McMaster University, Hamilton, Ont., and senior author in the Ontario study.
Furthermore, an important point is that the current trial extended the follow-up to 7 years, he told this news organization in an email.
That study included patients with diabetes and hypertension, he added, whereas his group included patients with a history of cardiovascular disease and/or heart failure.
“We hope these studies encourage general practitioners and cardiologists to consider bariatric surgery as a viable treatment option to prevent and reduce the risk of MACE in the obese patients [body mass index >35 kg/m2] with significant cardiovascular disease,” he said.
“We have embarked on a pilot RCT among bariatric centers of excellence in Ontario,” Dr. Anvari added, which showed the feasibility and safety of such a study.
He estimates that the RCT will need to recruit 2,000 patients to demonstrate the safety and effectiveness of bariatric surgery in reducing MACE and cardiac and all-cause mortality among patients with existing cardiovascular disease.
This “will require international collaboration,” he added, “and our group is currently establishing collaboration with sites in North America, Europe, and Australia to conduct such a study.”
Patients matched for age, sex, number of comorbidities
Quebec has a single public health care system that covers the cost of bariatric surgery for eligible patients; that is, those with a BMI greater than 35 kg/m2 and comorbidities or a BMI greater than 40 kg/m2.
Using this provincial health care database, which covers over 97% of the population, the researchers identified 3,637 patients with diabetes and/or hypertension who had bariatric surgery during 2007-2012.
They matched the surgery patients with 5,420 control patients with obesity who lived in the same geographic region and had a similar age, sex, and number of Charlson Comorbidity Index comorbidities, but did not undergo bariatric surgery.
The patients had a mean age of 50 and 64% were women.
Half had zero to one comorbidities, a quarter had two comorbidities, and another quarter had at least three comorbidities.
Most patients in the surgery group had type 2 diabetes (70%) and 50% had hypertension, whereas in the control group, most patients had hypertension (82%) and 41% had diabetes.
The most common type of bariatric surgery was adjustable gastric banding (42% of patients), followed by duodenal switch (24%), sleeve gastrectomy (23%), and Roux-en-Y gastric bypass (11%).
The primary outcome was the incidence of MACE, defined as coronary artery events (including myocardial infarction, percutaneous coronary intervention, and coronary artery bypass graft), stroke, heart failure, and all-cause mortality,
After a median follow-up of 7-11 years, fewer patients in the surgical group than in the control group had MACE (20% vs. 25%) or died from all causes (4.1% vs. 6.3%, both statistically significant at P < .01)
Similarly, significantly fewer patients in the surgical group than in the control group had a coronary artery event or heart failure (each P < .01).
However, there were no significant between-group difference in the rate of stroke, possibly because of the small number of strokes.
The risk of MACE was 17% lower in the group that had bariatric surgery than in the control group (adjusted hazard ratio, 0.83; 95% confidence interval, 0.78-0.89), after adjusting for age, sex, and number of comorbidities.
In subgroup analysis, patients who had adjustable gastric banding, Roux-en-Y gastric bypass, or duodenal switch had a significantly lower risk of MACE than control patients.
The risk of MACE was similar in patients who had sleeve gastrectomy and in control patients.
However, these subgroup results need to be interpreted with caution since the surgery and control patients in each surgery type subgroup were not matched for age, sex, and comorbidities, said Dr. Bouchard.
He acknowledged that study limitations include a lack of information about the patients’ BMI, weight, medications, and glycemic control (hemoglobin A1c).
Dr. Bouchard and Dr. Anvari have no relevant financial disclosures.
Patients with obesity who had bariatric surgery had a lower risk of having a major adverse cardiovascular event (MACE) or dying from all causes during a median 7-year follow-up, compared with similar patients who did not undergo surgery.
These findings, from a province-wide retrospective cohort study from Quebec, follow two recent, slightly shorter similar trials.
Now we need a large randomized clinical trial (RCT), experts say, to definitively establish cardiovascular and mortality benefits in people with obesity who have metabolic/bariatric surgery. And such a trial is just beginning.
Philippe Bouchard, MD, a general surgery resident from McGill University in Montreal presented the Quebec study in a top papers session at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings showed that, among obese patients with metabolic syndrome, bariatric/metabolic surgery is associated with a sustained decrease in the incidence of MACE and all-cause mortality of at least 5 years, Dr. Bouchard said.
“The results of this population-based observational study should be validated in randomized controlled trials,” he concluded.
In the meantime, “we believe our study adds to the body of evidence in mainly two ways,” Dr. Bouchard told this news organization in an email.
It has a longer follow-up than recent observational studies, “a median of 7 years, compared to 3.9 years in a study from the Cleveland Clinic, and 4.6 years in one from Ontario, he said.
“This allows us to [estimate] an absolute risk reduction of MACE of 5.11% at 10 years,” he added. This is a smaller risk reduction than the roughly 40% risk reduction seen in the other two studies, possibly because of selection bias, Dr. Bouchard speculated.
“Second, most of the larger cohorts are heavily weighted on Roux-en-Y gastric bypass,” he continued. In contrast, their study included diverse procedures, including sleeve gastrectomy, duodenal switch, and adjustable gastric banding.
“Given the rise in popularity of a derivative of the duodenal switch – the single-anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADi-S) – we believe this information is timely and relevant to clinicians,” Dr. Bouchard said.
RCT on the subject is coming
“I totally agree that we need a large randomized controlled trial of bariatric surgery versus optimal medical therapy to conclusively establish” the impact of bariatric surgery on cardiovascular outcomes, said the assigned discussant, Mehran Anvari, MD. And their research group is just about to begin one.
In the absence of RCT data, clinicians “may currently not refer [eligible] patients for bariatric surgery because of the high risk they pose,” said Dr. Anvari, professor and director of the Centre for Minimal Access Surgery of McMaster University, Hamilton, Ont., and senior author in the Ontario study.
Furthermore, an important point is that the current trial extended the follow-up to 7 years, he told this news organization in an email.
That study included patients with diabetes and hypertension, he added, whereas his group included patients with a history of cardiovascular disease and/or heart failure.
“We hope these studies encourage general practitioners and cardiologists to consider bariatric surgery as a viable treatment option to prevent and reduce the risk of MACE in the obese patients [body mass index >35 kg/m2] with significant cardiovascular disease,” he said.
“We have embarked on a pilot RCT among bariatric centers of excellence in Ontario,” Dr. Anvari added, which showed the feasibility and safety of such a study.
He estimates that the RCT will need to recruit 2,000 patients to demonstrate the safety and effectiveness of bariatric surgery in reducing MACE and cardiac and all-cause mortality among patients with existing cardiovascular disease.
This “will require international collaboration,” he added, “and our group is currently establishing collaboration with sites in North America, Europe, and Australia to conduct such a study.”
Patients matched for age, sex, number of comorbidities
Quebec has a single public health care system that covers the cost of bariatric surgery for eligible patients; that is, those with a BMI greater than 35 kg/m2 and comorbidities or a BMI greater than 40 kg/m2.
Using this provincial health care database, which covers over 97% of the population, the researchers identified 3,637 patients with diabetes and/or hypertension who had bariatric surgery during 2007-2012.
They matched the surgery patients with 5,420 control patients with obesity who lived in the same geographic region and had a similar age, sex, and number of Charlson Comorbidity Index comorbidities, but did not undergo bariatric surgery.
The patients had a mean age of 50 and 64% were women.
Half had zero to one comorbidities, a quarter had two comorbidities, and another quarter had at least three comorbidities.
Most patients in the surgery group had type 2 diabetes (70%) and 50% had hypertension, whereas in the control group, most patients had hypertension (82%) and 41% had diabetes.
The most common type of bariatric surgery was adjustable gastric banding (42% of patients), followed by duodenal switch (24%), sleeve gastrectomy (23%), and Roux-en-Y gastric bypass (11%).
The primary outcome was the incidence of MACE, defined as coronary artery events (including myocardial infarction, percutaneous coronary intervention, and coronary artery bypass graft), stroke, heart failure, and all-cause mortality,
After a median follow-up of 7-11 years, fewer patients in the surgical group than in the control group had MACE (20% vs. 25%) or died from all causes (4.1% vs. 6.3%, both statistically significant at P < .01)
Similarly, significantly fewer patients in the surgical group than in the control group had a coronary artery event or heart failure (each P < .01).
However, there were no significant between-group difference in the rate of stroke, possibly because of the small number of strokes.
The risk of MACE was 17% lower in the group that had bariatric surgery than in the control group (adjusted hazard ratio, 0.83; 95% confidence interval, 0.78-0.89), after adjusting for age, sex, and number of comorbidities.
In subgroup analysis, patients who had adjustable gastric banding, Roux-en-Y gastric bypass, or duodenal switch had a significantly lower risk of MACE than control patients.
The risk of MACE was similar in patients who had sleeve gastrectomy and in control patients.
However, these subgroup results need to be interpreted with caution since the surgery and control patients in each surgery type subgroup were not matched for age, sex, and comorbidities, said Dr. Bouchard.
He acknowledged that study limitations include a lack of information about the patients’ BMI, weight, medications, and glycemic control (hemoglobin A1c).
Dr. Bouchard and Dr. Anvari have no relevant financial disclosures.
FROM ASMBS 2021
Simple risk assessment predicts post-PCI ischemic events
A patient’s risk for ischemic events, but not bleeding, after percutaneous coronary intervention (PCI) can be predicted simply based on whether they have one or more guideline-based standardized risk criteria, a large-scale real-world analysis suggests.
Haoyu Wang, MD, and colleagues showed that having at least one high-risk feature, as outlined in the 2018 European Society of Cardiology and European Association for Cardiothoracic Surgery (ESC/EACTS) Guidelines on Myocardial Revascularization, was associated with an increased risk for target vessel failure by 48% and for a patient-oriented composite outcome by 44%.
Moreover, they showed that implantation of at least three stents and the presence of diabetes and diffuse multivessel disease were the only high-risk features from the guidelines that were independent predictors of the two outcomes.
The study of more than 10,000 PCI patients also showed that determining whether patients were at high bleeding risk (HBR) did not modify their ischemic risk.
This, said Dr. Wang, from the National Center for Cardiovascular Diseases, Fuwai Hospital, Beijing, underscores the importance of applying the high ischemic risk (HIR) criteria from the ESC/EACTS guidelines when tailoring dual antiplatelet therapy (DAPT).
The research was presented at the European Atherosclerosis Society 2021 Virtual Congress on June 2, and published online in the Journal of Atherosclerosis and Thrombosis.
Dr. Wang told theheart.org | Medscape Cardiology that they conducted the study to determine which – HIR or HBR – is “most important to balance when treating patients undergoing PCI and then having dual antiplatelet therapy.”
The results showed that when patients have both a HIR and HBR, it is the ESC/EACTS guideline HIR criteria that have “a higher impact” than the bleeding risk, and that this can be “used to guide our choice of the duration of dual anti-platelet therapy.”
“Maybe we can extend, or use more potent, P2Y12 inhibitors” in those situations, he said.
S. Lale Tokgözoglu, MD, PhD, professor of cardiology, Hacettepe University, Ankara, Turkey, who was not involved in the study, said the HIR assessment “performed well,” adding that the HBR score might have been expected to attenuate its “prognostic advantage.”
She told this news organization that the results “are interesting since previous observations have suggested that Asian patients may be more prone to medication side effects and bleeding.”
These findings emphasize the importance of assessing HIR in daily PCI practice and confirm that it “performs well in different populations in real life,” added Dr. Tokgözoglu, a former president of the EAS.
The ESC/EACTS guidelines aimed to standardize the definition of HIR, Dr. Wang said during the presentation.
They set out 10 high-risk features for ischemic events for patients undergoing revascularization, which included patient medical history, comorbid conditions, and the characteristics of the PCI procedure.
Although the goals of the criteria are to inform decision-making and stimulate research, Dr. Wang said that their “prevalence and prognostic association with clinical outcomes are yet to be established in real-world PCI practice.”
Alongside, the Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE-DAPT) score was developed to predict out-of-hospital bleeding in patients receiving DAPT after stent implantation.
Although a PRECISE-DAPT score of at least 25 constitutes a patient at high bleeding risk, Dr. Wang pointed out that such patients are typically also at risk for ischemic events after PCI, and it is “unclear” whether being at HBR modifies this risk.
To investigate further, they used the prospective, real-world Fuwai PCI registry to collate an all-comer patient population with unselected use of drug-eluting stents at the National Center for Cardiovascular Diseases at Fuwai Hospital.
They excluded individuals who were treated with balloon angioplasty alone, bioresorbable scaffolds, or bare metal stents, leaving a total population of 10,167 patients who were treated in 2013.
In that cohort, 5,149 patients (50.6%) met at least one risk criterion from the ESC/EACTS guidelines (HIR patients) and 5,018 (49.4%) met none of the risk criteria (non-HIR patients).
The most common criteria were implantation of at least three stents (23.5%); total stent length greater than 60 mm (20.2%); diffuse multivessel disease, especially in diabetic patients (18.5%); and a history of ST-segment elevation myocardial infarction (13.9%).
HIR patients were significantly older than non-HIR patients (average age, 58.86 vs. 57.77 years; P < .001), were more likely to have diabetes mellitus (42.6% vs. 16.9%; P < .001); and were more likely to have already had a myocardial infarction (32.2% vs. 5.2%; P < .001).
HIR patients also had higher average PRECISE-ADAPT scores than those without HIR (11.22 vs. 9.94; P < .001), and were conversely less likely to have the left anterior descending artery as the target vessel than non-HIR patients (86.0% vs. 94.6%; P < .001).
Cox regression analysis taking into account a range of patient and clinical factors revealed that HIR patients were significantly more likely than their non-HIR counterparts to experience target vessel failure (hazard ratio, 1.48; 95% confidence interval, 1.25-1.74; P < .001).
They were also significantly more likely to have a patient-oriented composite outcome, defined as all-cause death, any myocardial infarction, or any revascularization (HR, 1.44; 95% CI, 1.28-1.63; P < .001).
There was also a significantly higher risk for cardiac death in HIR than in non-HIR patients (HR, 1.95; 95% CI, 1.16-3.29; P = .012).
However, there was no significant association between HIR status and clinically relevant bleeding (HR, 0.84; 95% CI, 0.66-1.06; P = .143).
When the researchers looked at individual ischemic risk features, they found that, on fully adjusted analyses, only two were independent predictors of target vessel failure and the patient-oriented composite outcome.
Having at least three stents implanted was significantly associated with target vessel failure (HR, 1.36; 95% CI, 1.02-1.80; P = .038), and borderline significantly associated with the patient oriented composite outcome (HR, 1.23; 95% CI, 1.00-1.53; P = .056).
Diffuse multivessel disease, especially in diabetic patients, was significantly associated with both target vessel failure (HR, 1.24; 95% CI, 1.02-1.51; P = .035) and with the patient-oriented composite outcome (HR, 1.20; 95% CI, 1.04-1.39; P = .012).
Neither risk feature was significantly associated with clinically relevant bleeding, Dr. Wang noted.
Stratifying the patients by HBR status, the team found that rates of target vessel failure, the patient-oriented composite outcome, cardiac death, myocardial infarction, and definite/probable stent thrombosis were higher in patients with both HIR and HBR than those with neither HIR nor HBR (P < .001).
Further stratifying patients by PRECISE-ADAPT scores – 10 or less indicating very low risk, 11-17 indicating low risk, 18-24 indicating moderate risk, and at least 25 indicating high risk – showed that HIR features had a consistent effect on ischemic and bleeding outcomes, regardless of bleeding risk.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
A patient’s risk for ischemic events, but not bleeding, after percutaneous coronary intervention (PCI) can be predicted simply based on whether they have one or more guideline-based standardized risk criteria, a large-scale real-world analysis suggests.
Haoyu Wang, MD, and colleagues showed that having at least one high-risk feature, as outlined in the 2018 European Society of Cardiology and European Association for Cardiothoracic Surgery (ESC/EACTS) Guidelines on Myocardial Revascularization, was associated with an increased risk for target vessel failure by 48% and for a patient-oriented composite outcome by 44%.
Moreover, they showed that implantation of at least three stents and the presence of diabetes and diffuse multivessel disease were the only high-risk features from the guidelines that were independent predictors of the two outcomes.
The study of more than 10,000 PCI patients also showed that determining whether patients were at high bleeding risk (HBR) did not modify their ischemic risk.
This, said Dr. Wang, from the National Center for Cardiovascular Diseases, Fuwai Hospital, Beijing, underscores the importance of applying the high ischemic risk (HIR) criteria from the ESC/EACTS guidelines when tailoring dual antiplatelet therapy (DAPT).
The research was presented at the European Atherosclerosis Society 2021 Virtual Congress on June 2, and published online in the Journal of Atherosclerosis and Thrombosis.
Dr. Wang told theheart.org | Medscape Cardiology that they conducted the study to determine which – HIR or HBR – is “most important to balance when treating patients undergoing PCI and then having dual antiplatelet therapy.”
The results showed that when patients have both a HIR and HBR, it is the ESC/EACTS guideline HIR criteria that have “a higher impact” than the bleeding risk, and that this can be “used to guide our choice of the duration of dual anti-platelet therapy.”
“Maybe we can extend, or use more potent, P2Y12 inhibitors” in those situations, he said.
S. Lale Tokgözoglu, MD, PhD, professor of cardiology, Hacettepe University, Ankara, Turkey, who was not involved in the study, said the HIR assessment “performed well,” adding that the HBR score might have been expected to attenuate its “prognostic advantage.”
She told this news organization that the results “are interesting since previous observations have suggested that Asian patients may be more prone to medication side effects and bleeding.”
These findings emphasize the importance of assessing HIR in daily PCI practice and confirm that it “performs well in different populations in real life,” added Dr. Tokgözoglu, a former president of the EAS.
The ESC/EACTS guidelines aimed to standardize the definition of HIR, Dr. Wang said during the presentation.
They set out 10 high-risk features for ischemic events for patients undergoing revascularization, which included patient medical history, comorbid conditions, and the characteristics of the PCI procedure.
Although the goals of the criteria are to inform decision-making and stimulate research, Dr. Wang said that their “prevalence and prognostic association with clinical outcomes are yet to be established in real-world PCI practice.”
Alongside, the Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE-DAPT) score was developed to predict out-of-hospital bleeding in patients receiving DAPT after stent implantation.
Although a PRECISE-DAPT score of at least 25 constitutes a patient at high bleeding risk, Dr. Wang pointed out that such patients are typically also at risk for ischemic events after PCI, and it is “unclear” whether being at HBR modifies this risk.
To investigate further, they used the prospective, real-world Fuwai PCI registry to collate an all-comer patient population with unselected use of drug-eluting stents at the National Center for Cardiovascular Diseases at Fuwai Hospital.
They excluded individuals who were treated with balloon angioplasty alone, bioresorbable scaffolds, or bare metal stents, leaving a total population of 10,167 patients who were treated in 2013.
In that cohort, 5,149 patients (50.6%) met at least one risk criterion from the ESC/EACTS guidelines (HIR patients) and 5,018 (49.4%) met none of the risk criteria (non-HIR patients).
The most common criteria were implantation of at least three stents (23.5%); total stent length greater than 60 mm (20.2%); diffuse multivessel disease, especially in diabetic patients (18.5%); and a history of ST-segment elevation myocardial infarction (13.9%).
HIR patients were significantly older than non-HIR patients (average age, 58.86 vs. 57.77 years; P < .001), were more likely to have diabetes mellitus (42.6% vs. 16.9%; P < .001); and were more likely to have already had a myocardial infarction (32.2% vs. 5.2%; P < .001).
HIR patients also had higher average PRECISE-ADAPT scores than those without HIR (11.22 vs. 9.94; P < .001), and were conversely less likely to have the left anterior descending artery as the target vessel than non-HIR patients (86.0% vs. 94.6%; P < .001).
Cox regression analysis taking into account a range of patient and clinical factors revealed that HIR patients were significantly more likely than their non-HIR counterparts to experience target vessel failure (hazard ratio, 1.48; 95% confidence interval, 1.25-1.74; P < .001).
They were also significantly more likely to have a patient-oriented composite outcome, defined as all-cause death, any myocardial infarction, or any revascularization (HR, 1.44; 95% CI, 1.28-1.63; P < .001).
There was also a significantly higher risk for cardiac death in HIR than in non-HIR patients (HR, 1.95; 95% CI, 1.16-3.29; P = .012).
However, there was no significant association between HIR status and clinically relevant bleeding (HR, 0.84; 95% CI, 0.66-1.06; P = .143).
When the researchers looked at individual ischemic risk features, they found that, on fully adjusted analyses, only two were independent predictors of target vessel failure and the patient-oriented composite outcome.
Having at least three stents implanted was significantly associated with target vessel failure (HR, 1.36; 95% CI, 1.02-1.80; P = .038), and borderline significantly associated with the patient oriented composite outcome (HR, 1.23; 95% CI, 1.00-1.53; P = .056).
Diffuse multivessel disease, especially in diabetic patients, was significantly associated with both target vessel failure (HR, 1.24; 95% CI, 1.02-1.51; P = .035) and with the patient-oriented composite outcome (HR, 1.20; 95% CI, 1.04-1.39; P = .012).
Neither risk feature was significantly associated with clinically relevant bleeding, Dr. Wang noted.
Stratifying the patients by HBR status, the team found that rates of target vessel failure, the patient-oriented composite outcome, cardiac death, myocardial infarction, and definite/probable stent thrombosis were higher in patients with both HIR and HBR than those with neither HIR nor HBR (P < .001).
Further stratifying patients by PRECISE-ADAPT scores – 10 or less indicating very low risk, 11-17 indicating low risk, 18-24 indicating moderate risk, and at least 25 indicating high risk – showed that HIR features had a consistent effect on ischemic and bleeding outcomes, regardless of bleeding risk.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
A patient’s risk for ischemic events, but not bleeding, after percutaneous coronary intervention (PCI) can be predicted simply based on whether they have one or more guideline-based standardized risk criteria, a large-scale real-world analysis suggests.
Haoyu Wang, MD, and colleagues showed that having at least one high-risk feature, as outlined in the 2018 European Society of Cardiology and European Association for Cardiothoracic Surgery (ESC/EACTS) Guidelines on Myocardial Revascularization, was associated with an increased risk for target vessel failure by 48% and for a patient-oriented composite outcome by 44%.
Moreover, they showed that implantation of at least three stents and the presence of diabetes and diffuse multivessel disease were the only high-risk features from the guidelines that were independent predictors of the two outcomes.
The study of more than 10,000 PCI patients also showed that determining whether patients were at high bleeding risk (HBR) did not modify their ischemic risk.
This, said Dr. Wang, from the National Center for Cardiovascular Diseases, Fuwai Hospital, Beijing, underscores the importance of applying the high ischemic risk (HIR) criteria from the ESC/EACTS guidelines when tailoring dual antiplatelet therapy (DAPT).
The research was presented at the European Atherosclerosis Society 2021 Virtual Congress on June 2, and published online in the Journal of Atherosclerosis and Thrombosis.
Dr. Wang told theheart.org | Medscape Cardiology that they conducted the study to determine which – HIR or HBR – is “most important to balance when treating patients undergoing PCI and then having dual antiplatelet therapy.”
The results showed that when patients have both a HIR and HBR, it is the ESC/EACTS guideline HIR criteria that have “a higher impact” than the bleeding risk, and that this can be “used to guide our choice of the duration of dual anti-platelet therapy.”
“Maybe we can extend, or use more potent, P2Y12 inhibitors” in those situations, he said.
S. Lale Tokgözoglu, MD, PhD, professor of cardiology, Hacettepe University, Ankara, Turkey, who was not involved in the study, said the HIR assessment “performed well,” adding that the HBR score might have been expected to attenuate its “prognostic advantage.”
She told this news organization that the results “are interesting since previous observations have suggested that Asian patients may be more prone to medication side effects and bleeding.”
These findings emphasize the importance of assessing HIR in daily PCI practice and confirm that it “performs well in different populations in real life,” added Dr. Tokgözoglu, a former president of the EAS.
The ESC/EACTS guidelines aimed to standardize the definition of HIR, Dr. Wang said during the presentation.
They set out 10 high-risk features for ischemic events for patients undergoing revascularization, which included patient medical history, comorbid conditions, and the characteristics of the PCI procedure.
Although the goals of the criteria are to inform decision-making and stimulate research, Dr. Wang said that their “prevalence and prognostic association with clinical outcomes are yet to be established in real-world PCI practice.”
Alongside, the Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE-DAPT) score was developed to predict out-of-hospital bleeding in patients receiving DAPT after stent implantation.
Although a PRECISE-DAPT score of at least 25 constitutes a patient at high bleeding risk, Dr. Wang pointed out that such patients are typically also at risk for ischemic events after PCI, and it is “unclear” whether being at HBR modifies this risk.
To investigate further, they used the prospective, real-world Fuwai PCI registry to collate an all-comer patient population with unselected use of drug-eluting stents at the National Center for Cardiovascular Diseases at Fuwai Hospital.
They excluded individuals who were treated with balloon angioplasty alone, bioresorbable scaffolds, or bare metal stents, leaving a total population of 10,167 patients who were treated in 2013.
In that cohort, 5,149 patients (50.6%) met at least one risk criterion from the ESC/EACTS guidelines (HIR patients) and 5,018 (49.4%) met none of the risk criteria (non-HIR patients).
The most common criteria were implantation of at least three stents (23.5%); total stent length greater than 60 mm (20.2%); diffuse multivessel disease, especially in diabetic patients (18.5%); and a history of ST-segment elevation myocardial infarction (13.9%).
HIR patients were significantly older than non-HIR patients (average age, 58.86 vs. 57.77 years; P < .001), were more likely to have diabetes mellitus (42.6% vs. 16.9%; P < .001); and were more likely to have already had a myocardial infarction (32.2% vs. 5.2%; P < .001).
HIR patients also had higher average PRECISE-ADAPT scores than those without HIR (11.22 vs. 9.94; P < .001), and were conversely less likely to have the left anterior descending artery as the target vessel than non-HIR patients (86.0% vs. 94.6%; P < .001).
Cox regression analysis taking into account a range of patient and clinical factors revealed that HIR patients were significantly more likely than their non-HIR counterparts to experience target vessel failure (hazard ratio, 1.48; 95% confidence interval, 1.25-1.74; P < .001).
They were also significantly more likely to have a patient-oriented composite outcome, defined as all-cause death, any myocardial infarction, or any revascularization (HR, 1.44; 95% CI, 1.28-1.63; P < .001).
There was also a significantly higher risk for cardiac death in HIR than in non-HIR patients (HR, 1.95; 95% CI, 1.16-3.29; P = .012).
However, there was no significant association between HIR status and clinically relevant bleeding (HR, 0.84; 95% CI, 0.66-1.06; P = .143).
When the researchers looked at individual ischemic risk features, they found that, on fully adjusted analyses, only two were independent predictors of target vessel failure and the patient-oriented composite outcome.
Having at least three stents implanted was significantly associated with target vessel failure (HR, 1.36; 95% CI, 1.02-1.80; P = .038), and borderline significantly associated with the patient oriented composite outcome (HR, 1.23; 95% CI, 1.00-1.53; P = .056).
Diffuse multivessel disease, especially in diabetic patients, was significantly associated with both target vessel failure (HR, 1.24; 95% CI, 1.02-1.51; P = .035) and with the patient-oriented composite outcome (HR, 1.20; 95% CI, 1.04-1.39; P = .012).
Neither risk feature was significantly associated with clinically relevant bleeding, Dr. Wang noted.
Stratifying the patients by HBR status, the team found that rates of target vessel failure, the patient-oriented composite outcome, cardiac death, myocardial infarction, and definite/probable stent thrombosis were higher in patients with both HIR and HBR than those with neither HIR nor HBR (P < .001).
Further stratifying patients by PRECISE-ADAPT scores – 10 or less indicating very low risk, 11-17 indicating low risk, 18-24 indicating moderate risk, and at least 25 indicating high risk – showed that HIR features had a consistent effect on ischemic and bleeding outcomes, regardless of bleeding risk.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
Revised dispatch system boosts bystander CPR in those with limited English
The improved Los Angeles medical dispatch system prompted more callers with limited English proficiency to initiate telecommunicator-assisted cardiopulmonary resuscitation (T-CPR), compared with the previous system, a new study shows.
The Los Angeles Tiered Dispatch System (LA-TDS), adopted in late 2014, used simplified questions aimed at identifying cardiac arrest, compared with the city’s earlier Medical Priority Dispatch System (MPDS).
The result was substantially decreased call processing times, decreased “undertriage” of out-of-hospital cardiac arrest (OHCA), and improved overall T-CPR rates (Resuscitation. 2020 Oct;155:74-81).
But now, a secondary analysis of the data shows there was a much higher jump in T-CPR rates among a small subset of callers with limited English proficiency, compared with those proficient in English (JAMA Network Open. 2021;4[6]:e216827).
“This was an unanticipated, significant, and disproportionate change, but fortunately a very good change,” lead author Stephen Sanko, MD, said in an interview.
While the T-CPR rate among English-proficient callers increased from 55% with the MPDS to 67% with the LA-TDS (odds ratio, 1.66; P = .007), it rose from 28% to 69% (OR, 5.66; P = .003) among callers with limited English proficiency. In the adjusted analysis, the new LA-TDS was associated with a 69% higher prevalence of T-CPR among English-proficient callers, compared with a 350% greater prevalence among callers with limited English proficiency.
“The emergency communication process between a caller and 911 telecommunicator is more complex than we thought, and likely constitutes a unique subsubspecialty that interacts with fields as diverse as medicine, health equity, linguistics, sociology, consumer behavior and others,” said Dr. Sanko, who is from the division of emergency medical services at the University of Southern California in Los Angeles.
“Yet in spite of this complexity, we’re starting to be able to reproducibly classify elements of the emergency conversation that we believe are tied to outcomes we all care about. ... Modulators of health disparities are present as early as the dispatch conversation, and, importantly, they can be intervened upon to promote improved outcomes,” he continued.
The retrospective cohort study was a predefined secondary analysis of a previously published study comparing telecommunicator management of out-of-hospital cardiac arrest over 3 months with the MPDS versus 3 months with the LA-TDS. The primary outcome was the number of patients who received telecommunicator-assisted chest compressions from callers with limited English proficiency.
Of the 597 emergency calls that met the inclusion criteria, 289 (48%) were in the MPDS cohort and 308 (52%) were in the LA-TDS cohort. In the MPDS cohort, 263 callers had English proficiency and 26 had limited proficiency; in the latter cohort, those figures were 273 and 35, respectively.
There were no significant differences between cohorts in the use of real-time translation services, which were employed 27%-31% of the time.
The reason for the overall T-CPR improvement is likely that the LA-TDS was tailored to the community needs, said Dr. Sanko. “Most people, including doctors, think of 911 dispatch as something simple and straightforward, like ordering a pizza or calling a ride share. [But] LA-TDS is a ‘home grown’ dispatch system whose structure, questions, and emergency instructions were all developed by EMS medical directors and telecommunicators with extensive experience in our community.”
That being said, the researchers acknowledge that the reason behind the bigger T-CPR boost in LEP callers remains unclear. Although the link between language and system was statistically significant, they noted “it was not an a priori hypothesis and appeared to be largely attributable to the low T-CPR rates for callers with limited English proficiency using MPDS.” Additionally, such callers were “remarkably under-represented” in the sample, “which included approximately 600 calls over two quarters in a large city,” said Dr Sanko.
“We hypothesize that a more direct structure, earlier commitment to treating patients with abnormal life status indicators as being suspected cardiac arrest cases, and earlier reassurance may have improved caller confidence that telecommunicators knew what they were doing. This in turn may have translated into an increased likelihood of bystander caller willingness to perform immediate life-saving maneuvers.”
Despite a number of limitations, “the study is important and highlights instructive topics for discussion that suggest potential next-step opportunities,” noted Richard Chocron, MD, PhD, Miranda Lewis, MD, and Thomas Rea, MD, MPH, in an invited commentary that accompanied the publication. Dr. Chocron is from the Paris University, Paris Research Cardiovascular Center, INSERM; Dr. Lewis is from the Georges Pompidou European Hospital in Paris; and Dr. Rea is from the Division of Emergency Medical Services, Public Health–Seattle & King County. Both Dr. Lewis and Dr. Rea are also at the University of Washington, Seattle.
“Sanko et al. found that approximately 10% of all emergency calls were classified as limited English proficiency calls in a community in which 19% of the population was considered to have limited English proficiency,” they added. “This finding suggests the possibility that populations with limited English proficiency are less likely to activate 911 for incidence of cardiac arrest. If true, this finding would compound the health disparity observed among those with limited English proficiency. This topic is important in that it transcends the role of EMS personnel and engages a broad spectrum of societal stakeholders. We must listen, learn, and ultimately deliver public safety resources to groups who have not been well served by conventional approaches.”
None of the authors or editorialists reported any conflicts of interest.
The improved Los Angeles medical dispatch system prompted more callers with limited English proficiency to initiate telecommunicator-assisted cardiopulmonary resuscitation (T-CPR), compared with the previous system, a new study shows.
The Los Angeles Tiered Dispatch System (LA-TDS), adopted in late 2014, used simplified questions aimed at identifying cardiac arrest, compared with the city’s earlier Medical Priority Dispatch System (MPDS).
The result was substantially decreased call processing times, decreased “undertriage” of out-of-hospital cardiac arrest (OHCA), and improved overall T-CPR rates (Resuscitation. 2020 Oct;155:74-81).
But now, a secondary analysis of the data shows there was a much higher jump in T-CPR rates among a small subset of callers with limited English proficiency, compared with those proficient in English (JAMA Network Open. 2021;4[6]:e216827).
“This was an unanticipated, significant, and disproportionate change, but fortunately a very good change,” lead author Stephen Sanko, MD, said in an interview.
While the T-CPR rate among English-proficient callers increased from 55% with the MPDS to 67% with the LA-TDS (odds ratio, 1.66; P = .007), it rose from 28% to 69% (OR, 5.66; P = .003) among callers with limited English proficiency. In the adjusted analysis, the new LA-TDS was associated with a 69% higher prevalence of T-CPR among English-proficient callers, compared with a 350% greater prevalence among callers with limited English proficiency.
“The emergency communication process between a caller and 911 telecommunicator is more complex than we thought, and likely constitutes a unique subsubspecialty that interacts with fields as diverse as medicine, health equity, linguistics, sociology, consumer behavior and others,” said Dr. Sanko, who is from the division of emergency medical services at the University of Southern California in Los Angeles.
“Yet in spite of this complexity, we’re starting to be able to reproducibly classify elements of the emergency conversation that we believe are tied to outcomes we all care about. ... Modulators of health disparities are present as early as the dispatch conversation, and, importantly, they can be intervened upon to promote improved outcomes,” he continued.
The retrospective cohort study was a predefined secondary analysis of a previously published study comparing telecommunicator management of out-of-hospital cardiac arrest over 3 months with the MPDS versus 3 months with the LA-TDS. The primary outcome was the number of patients who received telecommunicator-assisted chest compressions from callers with limited English proficiency.
Of the 597 emergency calls that met the inclusion criteria, 289 (48%) were in the MPDS cohort and 308 (52%) were in the LA-TDS cohort. In the MPDS cohort, 263 callers had English proficiency and 26 had limited proficiency; in the latter cohort, those figures were 273 and 35, respectively.
There were no significant differences between cohorts in the use of real-time translation services, which were employed 27%-31% of the time.
The reason for the overall T-CPR improvement is likely that the LA-TDS was tailored to the community needs, said Dr. Sanko. “Most people, including doctors, think of 911 dispatch as something simple and straightforward, like ordering a pizza or calling a ride share. [But] LA-TDS is a ‘home grown’ dispatch system whose structure, questions, and emergency instructions were all developed by EMS medical directors and telecommunicators with extensive experience in our community.”
That being said, the researchers acknowledge that the reason behind the bigger T-CPR boost in LEP callers remains unclear. Although the link between language and system was statistically significant, they noted “it was not an a priori hypothesis and appeared to be largely attributable to the low T-CPR rates for callers with limited English proficiency using MPDS.” Additionally, such callers were “remarkably under-represented” in the sample, “which included approximately 600 calls over two quarters in a large city,” said Dr Sanko.
“We hypothesize that a more direct structure, earlier commitment to treating patients with abnormal life status indicators as being suspected cardiac arrest cases, and earlier reassurance may have improved caller confidence that telecommunicators knew what they were doing. This in turn may have translated into an increased likelihood of bystander caller willingness to perform immediate life-saving maneuvers.”
Despite a number of limitations, “the study is important and highlights instructive topics for discussion that suggest potential next-step opportunities,” noted Richard Chocron, MD, PhD, Miranda Lewis, MD, and Thomas Rea, MD, MPH, in an invited commentary that accompanied the publication. Dr. Chocron is from the Paris University, Paris Research Cardiovascular Center, INSERM; Dr. Lewis is from the Georges Pompidou European Hospital in Paris; and Dr. Rea is from the Division of Emergency Medical Services, Public Health–Seattle & King County. Both Dr. Lewis and Dr. Rea are also at the University of Washington, Seattle.
“Sanko et al. found that approximately 10% of all emergency calls were classified as limited English proficiency calls in a community in which 19% of the population was considered to have limited English proficiency,” they added. “This finding suggests the possibility that populations with limited English proficiency are less likely to activate 911 for incidence of cardiac arrest. If true, this finding would compound the health disparity observed among those with limited English proficiency. This topic is important in that it transcends the role of EMS personnel and engages a broad spectrum of societal stakeholders. We must listen, learn, and ultimately deliver public safety resources to groups who have not been well served by conventional approaches.”
None of the authors or editorialists reported any conflicts of interest.
The improved Los Angeles medical dispatch system prompted more callers with limited English proficiency to initiate telecommunicator-assisted cardiopulmonary resuscitation (T-CPR), compared with the previous system, a new study shows.
The Los Angeles Tiered Dispatch System (LA-TDS), adopted in late 2014, used simplified questions aimed at identifying cardiac arrest, compared with the city’s earlier Medical Priority Dispatch System (MPDS).
The result was substantially decreased call processing times, decreased “undertriage” of out-of-hospital cardiac arrest (OHCA), and improved overall T-CPR rates (Resuscitation. 2020 Oct;155:74-81).
But now, a secondary analysis of the data shows there was a much higher jump in T-CPR rates among a small subset of callers with limited English proficiency, compared with those proficient in English (JAMA Network Open. 2021;4[6]:e216827).
“This was an unanticipated, significant, and disproportionate change, but fortunately a very good change,” lead author Stephen Sanko, MD, said in an interview.
While the T-CPR rate among English-proficient callers increased from 55% with the MPDS to 67% with the LA-TDS (odds ratio, 1.66; P = .007), it rose from 28% to 69% (OR, 5.66; P = .003) among callers with limited English proficiency. In the adjusted analysis, the new LA-TDS was associated with a 69% higher prevalence of T-CPR among English-proficient callers, compared with a 350% greater prevalence among callers with limited English proficiency.
“The emergency communication process between a caller and 911 telecommunicator is more complex than we thought, and likely constitutes a unique subsubspecialty that interacts with fields as diverse as medicine, health equity, linguistics, sociology, consumer behavior and others,” said Dr. Sanko, who is from the division of emergency medical services at the University of Southern California in Los Angeles.
“Yet in spite of this complexity, we’re starting to be able to reproducibly classify elements of the emergency conversation that we believe are tied to outcomes we all care about. ... Modulators of health disparities are present as early as the dispatch conversation, and, importantly, they can be intervened upon to promote improved outcomes,” he continued.
The retrospective cohort study was a predefined secondary analysis of a previously published study comparing telecommunicator management of out-of-hospital cardiac arrest over 3 months with the MPDS versus 3 months with the LA-TDS. The primary outcome was the number of patients who received telecommunicator-assisted chest compressions from callers with limited English proficiency.
Of the 597 emergency calls that met the inclusion criteria, 289 (48%) were in the MPDS cohort and 308 (52%) were in the LA-TDS cohort. In the MPDS cohort, 263 callers had English proficiency and 26 had limited proficiency; in the latter cohort, those figures were 273 and 35, respectively.
There were no significant differences between cohorts in the use of real-time translation services, which were employed 27%-31% of the time.
The reason for the overall T-CPR improvement is likely that the LA-TDS was tailored to the community needs, said Dr. Sanko. “Most people, including doctors, think of 911 dispatch as something simple and straightforward, like ordering a pizza or calling a ride share. [But] LA-TDS is a ‘home grown’ dispatch system whose structure, questions, and emergency instructions were all developed by EMS medical directors and telecommunicators with extensive experience in our community.”
That being said, the researchers acknowledge that the reason behind the bigger T-CPR boost in LEP callers remains unclear. Although the link between language and system was statistically significant, they noted “it was not an a priori hypothesis and appeared to be largely attributable to the low T-CPR rates for callers with limited English proficiency using MPDS.” Additionally, such callers were “remarkably under-represented” in the sample, “which included approximately 600 calls over two quarters in a large city,” said Dr Sanko.
“We hypothesize that a more direct structure, earlier commitment to treating patients with abnormal life status indicators as being suspected cardiac arrest cases, and earlier reassurance may have improved caller confidence that telecommunicators knew what they were doing. This in turn may have translated into an increased likelihood of bystander caller willingness to perform immediate life-saving maneuvers.”
Despite a number of limitations, “the study is important and highlights instructive topics for discussion that suggest potential next-step opportunities,” noted Richard Chocron, MD, PhD, Miranda Lewis, MD, and Thomas Rea, MD, MPH, in an invited commentary that accompanied the publication. Dr. Chocron is from the Paris University, Paris Research Cardiovascular Center, INSERM; Dr. Lewis is from the Georges Pompidou European Hospital in Paris; and Dr. Rea is from the Division of Emergency Medical Services, Public Health–Seattle & King County. Both Dr. Lewis and Dr. Rea are also at the University of Washington, Seattle.
“Sanko et al. found that approximately 10% of all emergency calls were classified as limited English proficiency calls in a community in which 19% of the population was considered to have limited English proficiency,” they added. “This finding suggests the possibility that populations with limited English proficiency are less likely to activate 911 for incidence of cardiac arrest. If true, this finding would compound the health disparity observed among those with limited English proficiency. This topic is important in that it transcends the role of EMS personnel and engages a broad spectrum of societal stakeholders. We must listen, learn, and ultimately deliver public safety resources to groups who have not been well served by conventional approaches.”
None of the authors or editorialists reported any conflicts of interest.
FROM JAMA NETWORK OPEN
Single subcutaneous shot offers fast, potent platelet inhibition in STEMI
A subcutaneous dose of the second-generation glycoprotein IIb/IIIa inhibitor RUC-4 achieved rapid dose-dependent platelet inhibition in patients with ST-segment elevation MI (STEMI) undergoing stenting in the CEL-02 study.
Platelet inhibition occurred within 15 minutes among the 27 patients, and wore off rapidly, with almost 50% of platelet function recovered within 122 minutes.
The drug was well tolerated, with no thrombocytopenia in the first 72 hours after administration, one injection-site reaction, and two major bleeds likely caused by catheter-based trauma to the proximal radial artery, reported Jurrien ten Berg, MD, PhD, St. Antonius Hospital, Nieuwegein, the Netherlands.
The results were reported during the annual meeting of the European Association of Percutaneous Cardiovascular Interventions (EuroPCR 2021) and published simultaneously in EuroIntervention.
Dr. ten Berg noted that there is a need for drugs like RUC-4 in the early treatment of STEMI because oral P2Y12 inhibitors have a “seriously delayed” onset by about 2-4 hours. Prehospital use of the glycoprotein inhibitor (GPI) tirofiban was shown to improve reperfusion and late outcomes in the ON-TIME 2 trial, but GPIs require continuous intravenous administration and are associated with thrombocytopenia.
“Since RUC-4 is unique among small-molecule GPI in not inducing the receptor to undergo a major conformational change that has been implicated in the development of thrombocytopenia, it is possible that RUC-4 may be associated with fewer episodes of thrombocytopenia than current GPI,” the authors wrote.
RUC-4, also called zalunfiban, can be delivered with a single subcutaneous dose and, in a phase 1 study, demonstrated platelet inhibition within 15 minutes and was well tolerated up to a dose of 0.075 mg/kg among healthy volunteers and patients with stable coronary artery disease on aspirin.
In the CEL-02 study, 27 STEMI patients received a weight-adjusted subcutaneous injection of RUC-4 before primary percutaneous coronary intervention (PCI) in escalating doses of 0.075 mg/kg, 0.090 mg/kg, and 0.110 mg/kg. Patients were given standard treatment in the ambulance, which included aspirin (93%), ticagrelor (93%), and unfractionated heparin (96%). The activated clotting time was less than 200 seconds in 92% of patients who received additional heparin during cardiac catheterization.
The patients’ mean age was 62 years, 26% were women, and 96% were White. Pharmacodynamic data were available for 24 patients.
The average platelet inhibition 15 minutes after the injection was 77.5%, 87.5%, and 91.7%, respectively, for the three escalating doses (P = .002 for trend).
The primary endpoint of at least 77% inhibition of the iso-TRAP channel – which corresponds to 80% inhibition of light transmission aggregometry stimulated by 20 mcM adenosine diphosphate within 15 minutes – was achieved in three of eight patients at the lowest dose and in seven of eight patients at the middle and highest doses.
“Single-dose subcutaneous RUC-4 induces a fast, potent dose-dependent response of platelet inhibition in patients with STEMI presenting for primary PCI,” Dr. ten Berg concluded. “It is therefore promising for prehospital platelet inhibition in STEMI patients, and the results support further research on clinical benefit.”
The double-blind, randomized phase 2b CELEBRATE trial is underway, evaluating 1,668 STEMI patients treated with a 0.110 mg/kg or 0.130 mg/kg dose of RUC-4 or placebo in the ambulance. The coprimary outcomes are restoration of coronary artery blood flow and resolution of ST-segment deviation post-PCI/angiography. Primary completion is set for March 2023.
Marco Valgimigli, MD, who was not involved in the study, said in an interview that RUC-4 has “some theoretical advantages, compared with conventional IIb/IIIa inhibitors, namely the absence of thrombocytopenia which is, however, relatively rare, especially with tirofiban or eptifibatide.”
The subcutaneous approach may also offer an advantage. Yet, if the administration of RUC-4 is “to happen in the ambulance – a setting where an IV line is usually established – whether the subcutaneous versus IV administration of the treatment proves to be advantageous remains to be seen,” said Dr. Valgimigli, from Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano, Switzerland.
“We would need to see the results of large randomized trials embracing this treatment option before a clinical decision can be made, especially considering that IIb/IIa inhibitors in the ambulance have been tested in the past but ultimately abandoned,” he said.
Limitations of the study are its open-label design, the fact that iso-TRAP channel assay data were not reported by the VeryifyNow instrument and had to be calculated from the raw data, and the fact that the timing of the RUC-4 injection immediately before PCI does not fully resemble the expected use of RUC-4 in clinical practice, where RUC-4 would be administered at the same time as the aspirin, ticagrelor, and heparin, and about an hour before PCI, ten Berg and colleagues wrote.
CeleCor Therapeutics sponsored the study and provided study materials. Dr. ten Berg reported receiving lecture or consultancy fees from AstraZeneca, Eli Lilly, Daiichi Sankyo, The Medicines Company, AccuMetrics, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Bayer, Ferrer, and Idorsia, and institutional research grants from ZonMw and AstraZeneca. Coauthor Barry S. Coller is an inventor of RUC-4 and a founder, equity holder, and consultant to CeleCor. He also receives royalties from Centocor/Janssen and the VerifyNow assays. Dr. Valgimigli has received grants from Abbott, Terumo, Medicure, and AstraZeneca, and personal fees from Abbott, Chiesi, Bayer, Daiichi Sankyo, Amgen, Terumo, Alvimedica, AstraZeneca, Biosensors, and Idorsia.
A version of this article first appeared on Medscape.com.
A subcutaneous dose of the second-generation glycoprotein IIb/IIIa inhibitor RUC-4 achieved rapid dose-dependent platelet inhibition in patients with ST-segment elevation MI (STEMI) undergoing stenting in the CEL-02 study.
Platelet inhibition occurred within 15 minutes among the 27 patients, and wore off rapidly, with almost 50% of platelet function recovered within 122 minutes.
The drug was well tolerated, with no thrombocytopenia in the first 72 hours after administration, one injection-site reaction, and two major bleeds likely caused by catheter-based trauma to the proximal radial artery, reported Jurrien ten Berg, MD, PhD, St. Antonius Hospital, Nieuwegein, the Netherlands.
The results were reported during the annual meeting of the European Association of Percutaneous Cardiovascular Interventions (EuroPCR 2021) and published simultaneously in EuroIntervention.
Dr. ten Berg noted that there is a need for drugs like RUC-4 in the early treatment of STEMI because oral P2Y12 inhibitors have a “seriously delayed” onset by about 2-4 hours. Prehospital use of the glycoprotein inhibitor (GPI) tirofiban was shown to improve reperfusion and late outcomes in the ON-TIME 2 trial, but GPIs require continuous intravenous administration and are associated with thrombocytopenia.
“Since RUC-4 is unique among small-molecule GPI in not inducing the receptor to undergo a major conformational change that has been implicated in the development of thrombocytopenia, it is possible that RUC-4 may be associated with fewer episodes of thrombocytopenia than current GPI,” the authors wrote.
RUC-4, also called zalunfiban, can be delivered with a single subcutaneous dose and, in a phase 1 study, demonstrated platelet inhibition within 15 minutes and was well tolerated up to a dose of 0.075 mg/kg among healthy volunteers and patients with stable coronary artery disease on aspirin.
In the CEL-02 study, 27 STEMI patients received a weight-adjusted subcutaneous injection of RUC-4 before primary percutaneous coronary intervention (PCI) in escalating doses of 0.075 mg/kg, 0.090 mg/kg, and 0.110 mg/kg. Patients were given standard treatment in the ambulance, which included aspirin (93%), ticagrelor (93%), and unfractionated heparin (96%). The activated clotting time was less than 200 seconds in 92% of patients who received additional heparin during cardiac catheterization.
The patients’ mean age was 62 years, 26% were women, and 96% were White. Pharmacodynamic data were available for 24 patients.
The average platelet inhibition 15 minutes after the injection was 77.5%, 87.5%, and 91.7%, respectively, for the three escalating doses (P = .002 for trend).
The primary endpoint of at least 77% inhibition of the iso-TRAP channel – which corresponds to 80% inhibition of light transmission aggregometry stimulated by 20 mcM adenosine diphosphate within 15 minutes – was achieved in three of eight patients at the lowest dose and in seven of eight patients at the middle and highest doses.
“Single-dose subcutaneous RUC-4 induces a fast, potent dose-dependent response of platelet inhibition in patients with STEMI presenting for primary PCI,” Dr. ten Berg concluded. “It is therefore promising for prehospital platelet inhibition in STEMI patients, and the results support further research on clinical benefit.”
The double-blind, randomized phase 2b CELEBRATE trial is underway, evaluating 1,668 STEMI patients treated with a 0.110 mg/kg or 0.130 mg/kg dose of RUC-4 or placebo in the ambulance. The coprimary outcomes are restoration of coronary artery blood flow and resolution of ST-segment deviation post-PCI/angiography. Primary completion is set for March 2023.
Marco Valgimigli, MD, who was not involved in the study, said in an interview that RUC-4 has “some theoretical advantages, compared with conventional IIb/IIIa inhibitors, namely the absence of thrombocytopenia which is, however, relatively rare, especially with tirofiban or eptifibatide.”
The subcutaneous approach may also offer an advantage. Yet, if the administration of RUC-4 is “to happen in the ambulance – a setting where an IV line is usually established – whether the subcutaneous versus IV administration of the treatment proves to be advantageous remains to be seen,” said Dr. Valgimigli, from Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano, Switzerland.
“We would need to see the results of large randomized trials embracing this treatment option before a clinical decision can be made, especially considering that IIb/IIa inhibitors in the ambulance have been tested in the past but ultimately abandoned,” he said.
Limitations of the study are its open-label design, the fact that iso-TRAP channel assay data were not reported by the VeryifyNow instrument and had to be calculated from the raw data, and the fact that the timing of the RUC-4 injection immediately before PCI does not fully resemble the expected use of RUC-4 in clinical practice, where RUC-4 would be administered at the same time as the aspirin, ticagrelor, and heparin, and about an hour before PCI, ten Berg and colleagues wrote.
CeleCor Therapeutics sponsored the study and provided study materials. Dr. ten Berg reported receiving lecture or consultancy fees from AstraZeneca, Eli Lilly, Daiichi Sankyo, The Medicines Company, AccuMetrics, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Bayer, Ferrer, and Idorsia, and institutional research grants from ZonMw and AstraZeneca. Coauthor Barry S. Coller is an inventor of RUC-4 and a founder, equity holder, and consultant to CeleCor. He also receives royalties from Centocor/Janssen and the VerifyNow assays. Dr. Valgimigli has received grants from Abbott, Terumo, Medicure, and AstraZeneca, and personal fees from Abbott, Chiesi, Bayer, Daiichi Sankyo, Amgen, Terumo, Alvimedica, AstraZeneca, Biosensors, and Idorsia.
A version of this article first appeared on Medscape.com.
A subcutaneous dose of the second-generation glycoprotein IIb/IIIa inhibitor RUC-4 achieved rapid dose-dependent platelet inhibition in patients with ST-segment elevation MI (STEMI) undergoing stenting in the CEL-02 study.
Platelet inhibition occurred within 15 minutes among the 27 patients, and wore off rapidly, with almost 50% of platelet function recovered within 122 minutes.
The drug was well tolerated, with no thrombocytopenia in the first 72 hours after administration, one injection-site reaction, and two major bleeds likely caused by catheter-based trauma to the proximal radial artery, reported Jurrien ten Berg, MD, PhD, St. Antonius Hospital, Nieuwegein, the Netherlands.
The results were reported during the annual meeting of the European Association of Percutaneous Cardiovascular Interventions (EuroPCR 2021) and published simultaneously in EuroIntervention.
Dr. ten Berg noted that there is a need for drugs like RUC-4 in the early treatment of STEMI because oral P2Y12 inhibitors have a “seriously delayed” onset by about 2-4 hours. Prehospital use of the glycoprotein inhibitor (GPI) tirofiban was shown to improve reperfusion and late outcomes in the ON-TIME 2 trial, but GPIs require continuous intravenous administration and are associated with thrombocytopenia.
“Since RUC-4 is unique among small-molecule GPI in not inducing the receptor to undergo a major conformational change that has been implicated in the development of thrombocytopenia, it is possible that RUC-4 may be associated with fewer episodes of thrombocytopenia than current GPI,” the authors wrote.
RUC-4, also called zalunfiban, can be delivered with a single subcutaneous dose and, in a phase 1 study, demonstrated platelet inhibition within 15 minutes and was well tolerated up to a dose of 0.075 mg/kg among healthy volunteers and patients with stable coronary artery disease on aspirin.
In the CEL-02 study, 27 STEMI patients received a weight-adjusted subcutaneous injection of RUC-4 before primary percutaneous coronary intervention (PCI) in escalating doses of 0.075 mg/kg, 0.090 mg/kg, and 0.110 mg/kg. Patients were given standard treatment in the ambulance, which included aspirin (93%), ticagrelor (93%), and unfractionated heparin (96%). The activated clotting time was less than 200 seconds in 92% of patients who received additional heparin during cardiac catheterization.
The patients’ mean age was 62 years, 26% were women, and 96% were White. Pharmacodynamic data were available for 24 patients.
The average platelet inhibition 15 minutes after the injection was 77.5%, 87.5%, and 91.7%, respectively, for the three escalating doses (P = .002 for trend).
The primary endpoint of at least 77% inhibition of the iso-TRAP channel – which corresponds to 80% inhibition of light transmission aggregometry stimulated by 20 mcM adenosine diphosphate within 15 minutes – was achieved in three of eight patients at the lowest dose and in seven of eight patients at the middle and highest doses.
“Single-dose subcutaneous RUC-4 induces a fast, potent dose-dependent response of platelet inhibition in patients with STEMI presenting for primary PCI,” Dr. ten Berg concluded. “It is therefore promising for prehospital platelet inhibition in STEMI patients, and the results support further research on clinical benefit.”
The double-blind, randomized phase 2b CELEBRATE trial is underway, evaluating 1,668 STEMI patients treated with a 0.110 mg/kg or 0.130 mg/kg dose of RUC-4 or placebo in the ambulance. The coprimary outcomes are restoration of coronary artery blood flow and resolution of ST-segment deviation post-PCI/angiography. Primary completion is set for March 2023.
Marco Valgimigli, MD, who was not involved in the study, said in an interview that RUC-4 has “some theoretical advantages, compared with conventional IIb/IIIa inhibitors, namely the absence of thrombocytopenia which is, however, relatively rare, especially with tirofiban or eptifibatide.”
The subcutaneous approach may also offer an advantage. Yet, if the administration of RUC-4 is “to happen in the ambulance – a setting where an IV line is usually established – whether the subcutaneous versus IV administration of the treatment proves to be advantageous remains to be seen,” said Dr. Valgimigli, from Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano, Switzerland.
“We would need to see the results of large randomized trials embracing this treatment option before a clinical decision can be made, especially considering that IIb/IIa inhibitors in the ambulance have been tested in the past but ultimately abandoned,” he said.
Limitations of the study are its open-label design, the fact that iso-TRAP channel assay data were not reported by the VeryifyNow instrument and had to be calculated from the raw data, and the fact that the timing of the RUC-4 injection immediately before PCI does not fully resemble the expected use of RUC-4 in clinical practice, where RUC-4 would be administered at the same time as the aspirin, ticagrelor, and heparin, and about an hour before PCI, ten Berg and colleagues wrote.
CeleCor Therapeutics sponsored the study and provided study materials. Dr. ten Berg reported receiving lecture or consultancy fees from AstraZeneca, Eli Lilly, Daiichi Sankyo, The Medicines Company, AccuMetrics, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Bayer, Ferrer, and Idorsia, and institutional research grants from ZonMw and AstraZeneca. Coauthor Barry S. Coller is an inventor of RUC-4 and a founder, equity holder, and consultant to CeleCor. He also receives royalties from Centocor/Janssen and the VerifyNow assays. Dr. Valgimigli has received grants from Abbott, Terumo, Medicure, and AstraZeneca, and personal fees from Abbott, Chiesi, Bayer, Daiichi Sankyo, Amgen, Terumo, Alvimedica, AstraZeneca, Biosensors, and Idorsia.
A version of this article first appeared on Medscape.com.
Heart benefits of DASH low-sodium diet ‘swift and direct’
New data show for the first time that combining the DASH (Dietary Approaches to Stop Hypertension) diet with sodium restriction decreases myocardial injury and cardiac strain, which are associated with subclinical cardiac damage and long-term cardiovascular risk.
“The benefits of healthy eating are swift and direct. High sodium is not just about taste, it causes heart strain,” Stephen Juraschek, MD, PhD, from Beth Israel Deaconess Medical Center, Boston, said in an interview.
“We should consciously follow a diet enriched with fruit and vegetables and low in sodium. Collectively, we should think about how foods are promoted in society and what is an acceptable amount of sodium for food supplies,” said Dr. Juraschek.
The findings, from a secondary analysis of the DASH-Sodium trial, were published the Journal of the American College of Cardiology.
Renewed focus on diet
“These data should spur a renewed focus on the critical need for widespread adoption of the DASH–low-sodium diet in the United States,” wrote the coauthors of a linked editorial.
“The challenge remains moving the DASH–low-sodium diet from the research world into the real world, where its significant health benefits can be fully realized,” they added.
The researchers evaluated the impact of the DASH diet and sodium restriction, individually and combined, on biomarkers of cardiac injury (high-sensitivity cardiac troponin I [hs-cTnI]), cardiac strain (N-terminal of the prohormone brain natriuretic peptide [NT-proBNP]), and inflammation (high-sensitivity C-reactive protein [hs-CRP]).
The DASH-Sodium trial was a controlled feeding study that enrolled 412 adults (mean age, 48 years; 56% women, 56% Black) with untreated systolic blood pressure between 120 and 159 mm Hg and diastolic blood pressure between 80 and 95 mm Hg. Mean baseline BP was 135/86 mm Hg.
Participants were randomly allocated to a typical American diet (control) or the heart-healthy DASH diet. Further, participants in both groups were assigned to each of three sodium intake levels: low (0.5 mg/kcal), medium (1.1 mg/kcal) or high (1.6 mg/kcal) for 30 days using a crossover design with washout periods in between.
Compared with the control diet, the DASH diet reduced hs-cTnI by 18% and hs-CRP by 13% with no impact on NT-proBNP.
In contrast, lowering sodium from high to low levels reduced NT-proBNP independent of diet by 19%, but did not alter hs-cTnI and mildly increased hs-CRP (9%).
Combining the DASH diet with sodium reduction lowered hs-cTnI by 20% and NT-proBNP by 23%, with no significant change in hs-CRP, compared with the high-sodium-control diet.
“Together, these findings imply that two distinct dietary strategies might improve two key pathways of subclinical cardiac damage: injury and strain,” Dr. Juraschek and colleagues wrote.
“These findings should strengthen public resolve for public policies that promote the DASH dietary pattern and lower sodium intake in the United States and globally,” they concluded.
“We need to talk about DASH more. Most adults in the U.S. have never heard of it,” Dr. Juraschek said in an interview.
“We need to promote nutrition literacy with regard to nutrition facts. Labeling is not very transparent and hard to understand. Many people don’t know where salt is hiding in their diet,” he added.
It will also be important to address disparities in access to healthy foods and food insecurity, Dr. Juraschek said.
“If we don’t address food costs and access, disparities in healthy eating will persist. Greater equity is key. We should also be mindful about populations dependent on others for meal preparation [children in schools or older adults on meal plans]. This might be regulated in ways that promote healthier eating population wide, but for these patients, they may not have autonomy to choose what they eat,” Dr. Juraschek said.
In their editorial, Neha J. Pagidipati, MD, and Laura P. Svetkey, MD, from Duke University and Duke Clinical Research Institute, Durham, N.C., said an important caveat is that the beneficial effects of diet and sodium restriction on cardiac injury and strain occurred in people without any clinical evidence of coronary artery disease or heart failure at baseline, “suggesting that this dietary combination can improve subclinical metrics of cardiac health.”
“Further, the impact on these markers was seen within weeks, indicating a relatively rapid impact on cardiac damage,” they added.
The measurement of cardiac biomarkers was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute. The original DASH trial was supported by the NHLBI, the Office of Research on Minority Health, and the National Center for Research Resources. Dr. Juraschek and coauthors disclosed no relevant conflicts of interest. Dr. Pagidipati has received research support to the institution from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, Regeneron, Sanofi, and Verily Life Sciences; and has received consultation fees from Boehringer Ingelheim, Eli Lilly, AstraZeneca, and Novo Nordisk. Dr. Svetkey has no relevant disclosures.
A version of this article first appeared on Medscape.com.
New data show for the first time that combining the DASH (Dietary Approaches to Stop Hypertension) diet with sodium restriction decreases myocardial injury and cardiac strain, which are associated with subclinical cardiac damage and long-term cardiovascular risk.
“The benefits of healthy eating are swift and direct. High sodium is not just about taste, it causes heart strain,” Stephen Juraschek, MD, PhD, from Beth Israel Deaconess Medical Center, Boston, said in an interview.
“We should consciously follow a diet enriched with fruit and vegetables and low in sodium. Collectively, we should think about how foods are promoted in society and what is an acceptable amount of sodium for food supplies,” said Dr. Juraschek.
The findings, from a secondary analysis of the DASH-Sodium trial, were published the Journal of the American College of Cardiology.
Renewed focus on diet
“These data should spur a renewed focus on the critical need for widespread adoption of the DASH–low-sodium diet in the United States,” wrote the coauthors of a linked editorial.
“The challenge remains moving the DASH–low-sodium diet from the research world into the real world, where its significant health benefits can be fully realized,” they added.
The researchers evaluated the impact of the DASH diet and sodium restriction, individually and combined, on biomarkers of cardiac injury (high-sensitivity cardiac troponin I [hs-cTnI]), cardiac strain (N-terminal of the prohormone brain natriuretic peptide [NT-proBNP]), and inflammation (high-sensitivity C-reactive protein [hs-CRP]).
The DASH-Sodium trial was a controlled feeding study that enrolled 412 adults (mean age, 48 years; 56% women, 56% Black) with untreated systolic blood pressure between 120 and 159 mm Hg and diastolic blood pressure between 80 and 95 mm Hg. Mean baseline BP was 135/86 mm Hg.
Participants were randomly allocated to a typical American diet (control) or the heart-healthy DASH diet. Further, participants in both groups were assigned to each of three sodium intake levels: low (0.5 mg/kcal), medium (1.1 mg/kcal) or high (1.6 mg/kcal) for 30 days using a crossover design with washout periods in between.
Compared with the control diet, the DASH diet reduced hs-cTnI by 18% and hs-CRP by 13% with no impact on NT-proBNP.
In contrast, lowering sodium from high to low levels reduced NT-proBNP independent of diet by 19%, but did not alter hs-cTnI and mildly increased hs-CRP (9%).
Combining the DASH diet with sodium reduction lowered hs-cTnI by 20% and NT-proBNP by 23%, with no significant change in hs-CRP, compared with the high-sodium-control diet.
“Together, these findings imply that two distinct dietary strategies might improve two key pathways of subclinical cardiac damage: injury and strain,” Dr. Juraschek and colleagues wrote.
“These findings should strengthen public resolve for public policies that promote the DASH dietary pattern and lower sodium intake in the United States and globally,” they concluded.
“We need to talk about DASH more. Most adults in the U.S. have never heard of it,” Dr. Juraschek said in an interview.
“We need to promote nutrition literacy with regard to nutrition facts. Labeling is not very transparent and hard to understand. Many people don’t know where salt is hiding in their diet,” he added.
It will also be important to address disparities in access to healthy foods and food insecurity, Dr. Juraschek said.
“If we don’t address food costs and access, disparities in healthy eating will persist. Greater equity is key. We should also be mindful about populations dependent on others for meal preparation [children in schools or older adults on meal plans]. This might be regulated in ways that promote healthier eating population wide, but for these patients, they may not have autonomy to choose what they eat,” Dr. Juraschek said.
In their editorial, Neha J. Pagidipati, MD, and Laura P. Svetkey, MD, from Duke University and Duke Clinical Research Institute, Durham, N.C., said an important caveat is that the beneficial effects of diet and sodium restriction on cardiac injury and strain occurred in people without any clinical evidence of coronary artery disease or heart failure at baseline, “suggesting that this dietary combination can improve subclinical metrics of cardiac health.”
“Further, the impact on these markers was seen within weeks, indicating a relatively rapid impact on cardiac damage,” they added.
The measurement of cardiac biomarkers was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute. The original DASH trial was supported by the NHLBI, the Office of Research on Minority Health, and the National Center for Research Resources. Dr. Juraschek and coauthors disclosed no relevant conflicts of interest. Dr. Pagidipati has received research support to the institution from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, Regeneron, Sanofi, and Verily Life Sciences; and has received consultation fees from Boehringer Ingelheim, Eli Lilly, AstraZeneca, and Novo Nordisk. Dr. Svetkey has no relevant disclosures.
A version of this article first appeared on Medscape.com.
New data show for the first time that combining the DASH (Dietary Approaches to Stop Hypertension) diet with sodium restriction decreases myocardial injury and cardiac strain, which are associated with subclinical cardiac damage and long-term cardiovascular risk.
“The benefits of healthy eating are swift and direct. High sodium is not just about taste, it causes heart strain,” Stephen Juraschek, MD, PhD, from Beth Israel Deaconess Medical Center, Boston, said in an interview.
“We should consciously follow a diet enriched with fruit and vegetables and low in sodium. Collectively, we should think about how foods are promoted in society and what is an acceptable amount of sodium for food supplies,” said Dr. Juraschek.
The findings, from a secondary analysis of the DASH-Sodium trial, were published the Journal of the American College of Cardiology.
Renewed focus on diet
“These data should spur a renewed focus on the critical need for widespread adoption of the DASH–low-sodium diet in the United States,” wrote the coauthors of a linked editorial.
“The challenge remains moving the DASH–low-sodium diet from the research world into the real world, where its significant health benefits can be fully realized,” they added.
The researchers evaluated the impact of the DASH diet and sodium restriction, individually and combined, on biomarkers of cardiac injury (high-sensitivity cardiac troponin I [hs-cTnI]), cardiac strain (N-terminal of the prohormone brain natriuretic peptide [NT-proBNP]), and inflammation (high-sensitivity C-reactive protein [hs-CRP]).
The DASH-Sodium trial was a controlled feeding study that enrolled 412 adults (mean age, 48 years; 56% women, 56% Black) with untreated systolic blood pressure between 120 and 159 mm Hg and diastolic blood pressure between 80 and 95 mm Hg. Mean baseline BP was 135/86 mm Hg.
Participants were randomly allocated to a typical American diet (control) or the heart-healthy DASH diet. Further, participants in both groups were assigned to each of three sodium intake levels: low (0.5 mg/kcal), medium (1.1 mg/kcal) or high (1.6 mg/kcal) for 30 days using a crossover design with washout periods in between.
Compared with the control diet, the DASH diet reduced hs-cTnI by 18% and hs-CRP by 13% with no impact on NT-proBNP.
In contrast, lowering sodium from high to low levels reduced NT-proBNP independent of diet by 19%, but did not alter hs-cTnI and mildly increased hs-CRP (9%).
Combining the DASH diet with sodium reduction lowered hs-cTnI by 20% and NT-proBNP by 23%, with no significant change in hs-CRP, compared with the high-sodium-control diet.
“Together, these findings imply that two distinct dietary strategies might improve two key pathways of subclinical cardiac damage: injury and strain,” Dr. Juraschek and colleagues wrote.
“These findings should strengthen public resolve for public policies that promote the DASH dietary pattern and lower sodium intake in the United States and globally,” they concluded.
“We need to talk about DASH more. Most adults in the U.S. have never heard of it,” Dr. Juraschek said in an interview.
“We need to promote nutrition literacy with regard to nutrition facts. Labeling is not very transparent and hard to understand. Many people don’t know where salt is hiding in their diet,” he added.
It will also be important to address disparities in access to healthy foods and food insecurity, Dr. Juraschek said.
“If we don’t address food costs and access, disparities in healthy eating will persist. Greater equity is key. We should also be mindful about populations dependent on others for meal preparation [children in schools or older adults on meal plans]. This might be regulated in ways that promote healthier eating population wide, but for these patients, they may not have autonomy to choose what they eat,” Dr. Juraschek said.
In their editorial, Neha J. Pagidipati, MD, and Laura P. Svetkey, MD, from Duke University and Duke Clinical Research Institute, Durham, N.C., said an important caveat is that the beneficial effects of diet and sodium restriction on cardiac injury and strain occurred in people without any clinical evidence of coronary artery disease or heart failure at baseline, “suggesting that this dietary combination can improve subclinical metrics of cardiac health.”
“Further, the impact on these markers was seen within weeks, indicating a relatively rapid impact on cardiac damage,” they added.
The measurement of cardiac biomarkers was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute. The original DASH trial was supported by the NHLBI, the Office of Research on Minority Health, and the National Center for Research Resources. Dr. Juraschek and coauthors disclosed no relevant conflicts of interest. Dr. Pagidipati has received research support to the institution from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, Regeneron, Sanofi, and Verily Life Sciences; and has received consultation fees from Boehringer Ingelheim, Eli Lilly, AstraZeneca, and Novo Nordisk. Dr. Svetkey has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Benefit from cooling temps for cardiac arrest does not differ in randomized trial
The first randomized controlled trial to compare specific temperatures for therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest showed no differences in major outcomes, according to a single-center, double-blind study.
In the CAPITAL-CHILL trial, cooling temperatures of 31° C and 34° C were compared to explore the hypothesis that a lower temperature would improve major outcomes, explained Michel Le May, MD.
No differences for the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days were observed, he reported at the annual scientific sessions of the American College of Cardiology.
The study was completed over a period of almost 7 years in patients presumed to have had an out-of-hospital cardiac arrest and who were unconscious when they reached a center affiliated with the Ottawa Heart Institute, where Dr. Le May directs the regional STEMI (ST-elevation myocardial infarction) program. The initial rhythm at the time of the cardiac arrest was not an entry criterion.
Of 389 patients enrolled, the intention-to-treat analysis included 184 randomized to a cooling temperature of 31° C group and 183 to a temperature of 34° C. The assigned target temperature, reached with an endovascular device, was known only by the managing nurses.
31° C and 34° C are equivalent
There was a small numerical disadvantage for the lower temperature assignment, but none reached statistical significance. This was true of the primary outcome (48.4% vs. 45.4% for the higher temperature) and its components of mortality (43.5% vs. 41.0%) and poor neurologic outcome (4.9% vs. 4.4%). Poor neurologic outcome was defined as a Disability Rating Scale score of greater than 5.
Deaths were most common in the early part of the 180-day follow-up in both arms. On a Kaplan-Meier survival graph, Dr. Le May showed curves that he characterized as “almost superimposable.”
There were no significant differences for any subgroup stratifications, such as age 75 years or older versus younger, males versus females, presence versus absence or an initial shockable rhythm, percutaneous coronary intervention (PCI) within 24 hours versus later, and STEMI versus non-STEMI. In these analyses, the higher temperature was associated with a potential trend for benefit among females and those with a shockable rhythm.
There was no signal for a difference in neurologic outcomes on the Disability Rating Scale or the Modified Rankin Scale. On the latter, for example, 46% of those in the 31° C group and 44% of these in the 34° C group had a score of four or greater at the end of follow-up.
The baseline characteristics of the two groups were similar. About 80% were male; the average age was roughly 62 years. More than 80% of the cardiac arrests were witnessed with CPR being administered by bystanders in nearly 70%. Nearly 40% had a STEMI.
Interventions were similar. Almost all patients underwent coronary angiography, of which nearly 60% received a percutaneous coronary intervention. More than 50% received a stent. The time from arrest to randomization was slightly longer in the 31° C group (228 vs. 204 minutes). The time to balloon inflation from arrival at the cardiac center was also slightly longer (73 vs. 60 minutes).
There was a trend for an increased rate of seizures in the 31° C group (12.5% vs. 7.1%; P = .08), but other secondary outcomes, including pneumonia (67.8% vs. 63.4%), renal replacement therapy (9.2% vs. 9.3%), and stroke (4.4% vs. 1.6%), were similar in the 31° C and 34° C groups, respectively.
Bleeding, whether measured by transfusion (19.6% vs. 22.4%) or TIMI major bleed (23.4% vs. 19.7%) were similar in the 31° C and 34° C groups, respectively. Thrombosis, whether measured by stent thrombosis (1.2% vs. 2.2%) or deep venous thrombosis (11.4% vs. 10.9%) were similar in these two groups, respectively.
The length of stay in the cardiac intensive care unit was significantly greater in the 31° C group (10 vs. 7 days; P = .004). Some of this increased length of stay can be attributed to the longer rewarming process required for the greater cooling, according to Dr. Le May, but he acknowledged that it is not clear this provides a full explanation.
More trials like CAPITAL-CHILL needed
The validity of these findings is supported by several strengths of the methodology, according to Jeanne E. Poole, MD, director of the arrhythmia service and electrophysiology laboratory, University of Washington, Seattle. This includes the reliance of an endovascular device, which can accelerate the time to the target temperature and assure the precision with which it is reached and maintained.
Dr. Poole did note that many of the primary and secondary measures, including the rates of stroke, seizures, and major bleeds, even though not significantly different, favored the higher temperature. The slightly longer door-to-balloon times might have been a factor. For the higher rate of pneumonia in the 31° C group, she questioned whether the longer period of ventilation linked to a longer period of rewarming might have been a factor.
However, Dr. Poole praised the CAPITAL-CHILL trial for drawing attention to a group of patients for whom survival rates remain “dismally low.” She indicated that these types of high-level trials are needed to look for strategies to improve outcomes.
Dr. Le May and Dr. Poole report no potential conflicts of interest.
The first randomized controlled trial to compare specific temperatures for therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest showed no differences in major outcomes, according to a single-center, double-blind study.
In the CAPITAL-CHILL trial, cooling temperatures of 31° C and 34° C were compared to explore the hypothesis that a lower temperature would improve major outcomes, explained Michel Le May, MD.
No differences for the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days were observed, he reported at the annual scientific sessions of the American College of Cardiology.
The study was completed over a period of almost 7 years in patients presumed to have had an out-of-hospital cardiac arrest and who were unconscious when they reached a center affiliated with the Ottawa Heart Institute, where Dr. Le May directs the regional STEMI (ST-elevation myocardial infarction) program. The initial rhythm at the time of the cardiac arrest was not an entry criterion.
Of 389 patients enrolled, the intention-to-treat analysis included 184 randomized to a cooling temperature of 31° C group and 183 to a temperature of 34° C. The assigned target temperature, reached with an endovascular device, was known only by the managing nurses.
31° C and 34° C are equivalent
There was a small numerical disadvantage for the lower temperature assignment, but none reached statistical significance. This was true of the primary outcome (48.4% vs. 45.4% for the higher temperature) and its components of mortality (43.5% vs. 41.0%) and poor neurologic outcome (4.9% vs. 4.4%). Poor neurologic outcome was defined as a Disability Rating Scale score of greater than 5.
Deaths were most common in the early part of the 180-day follow-up in both arms. On a Kaplan-Meier survival graph, Dr. Le May showed curves that he characterized as “almost superimposable.”
There were no significant differences for any subgroup stratifications, such as age 75 years or older versus younger, males versus females, presence versus absence or an initial shockable rhythm, percutaneous coronary intervention (PCI) within 24 hours versus later, and STEMI versus non-STEMI. In these analyses, the higher temperature was associated with a potential trend for benefit among females and those with a shockable rhythm.
There was no signal for a difference in neurologic outcomes on the Disability Rating Scale or the Modified Rankin Scale. On the latter, for example, 46% of those in the 31° C group and 44% of these in the 34° C group had a score of four or greater at the end of follow-up.
The baseline characteristics of the two groups were similar. About 80% were male; the average age was roughly 62 years. More than 80% of the cardiac arrests were witnessed with CPR being administered by bystanders in nearly 70%. Nearly 40% had a STEMI.
Interventions were similar. Almost all patients underwent coronary angiography, of which nearly 60% received a percutaneous coronary intervention. More than 50% received a stent. The time from arrest to randomization was slightly longer in the 31° C group (228 vs. 204 minutes). The time to balloon inflation from arrival at the cardiac center was also slightly longer (73 vs. 60 minutes).
There was a trend for an increased rate of seizures in the 31° C group (12.5% vs. 7.1%; P = .08), but other secondary outcomes, including pneumonia (67.8% vs. 63.4%), renal replacement therapy (9.2% vs. 9.3%), and stroke (4.4% vs. 1.6%), were similar in the 31° C and 34° C groups, respectively.
Bleeding, whether measured by transfusion (19.6% vs. 22.4%) or TIMI major bleed (23.4% vs. 19.7%) were similar in the 31° C and 34° C groups, respectively. Thrombosis, whether measured by stent thrombosis (1.2% vs. 2.2%) or deep venous thrombosis (11.4% vs. 10.9%) were similar in these two groups, respectively.
The length of stay in the cardiac intensive care unit was significantly greater in the 31° C group (10 vs. 7 days; P = .004). Some of this increased length of stay can be attributed to the longer rewarming process required for the greater cooling, according to Dr. Le May, but he acknowledged that it is not clear this provides a full explanation.
More trials like CAPITAL-CHILL needed
The validity of these findings is supported by several strengths of the methodology, according to Jeanne E. Poole, MD, director of the arrhythmia service and electrophysiology laboratory, University of Washington, Seattle. This includes the reliance of an endovascular device, which can accelerate the time to the target temperature and assure the precision with which it is reached and maintained.
Dr. Poole did note that many of the primary and secondary measures, including the rates of stroke, seizures, and major bleeds, even though not significantly different, favored the higher temperature. The slightly longer door-to-balloon times might have been a factor. For the higher rate of pneumonia in the 31° C group, she questioned whether the longer period of ventilation linked to a longer period of rewarming might have been a factor.
However, Dr. Poole praised the CAPITAL-CHILL trial for drawing attention to a group of patients for whom survival rates remain “dismally low.” She indicated that these types of high-level trials are needed to look for strategies to improve outcomes.
Dr. Le May and Dr. Poole report no potential conflicts of interest.
The first randomized controlled trial to compare specific temperatures for therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest showed no differences in major outcomes, according to a single-center, double-blind study.
In the CAPITAL-CHILL trial, cooling temperatures of 31° C and 34° C were compared to explore the hypothesis that a lower temperature would improve major outcomes, explained Michel Le May, MD.
No differences for the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days were observed, he reported at the annual scientific sessions of the American College of Cardiology.
The study was completed over a period of almost 7 years in patients presumed to have had an out-of-hospital cardiac arrest and who were unconscious when they reached a center affiliated with the Ottawa Heart Institute, where Dr. Le May directs the regional STEMI (ST-elevation myocardial infarction) program. The initial rhythm at the time of the cardiac arrest was not an entry criterion.
Of 389 patients enrolled, the intention-to-treat analysis included 184 randomized to a cooling temperature of 31° C group and 183 to a temperature of 34° C. The assigned target temperature, reached with an endovascular device, was known only by the managing nurses.
31° C and 34° C are equivalent
There was a small numerical disadvantage for the lower temperature assignment, but none reached statistical significance. This was true of the primary outcome (48.4% vs. 45.4% for the higher temperature) and its components of mortality (43.5% vs. 41.0%) and poor neurologic outcome (4.9% vs. 4.4%). Poor neurologic outcome was defined as a Disability Rating Scale score of greater than 5.
Deaths were most common in the early part of the 180-day follow-up in both arms. On a Kaplan-Meier survival graph, Dr. Le May showed curves that he characterized as “almost superimposable.”
There were no significant differences for any subgroup stratifications, such as age 75 years or older versus younger, males versus females, presence versus absence or an initial shockable rhythm, percutaneous coronary intervention (PCI) within 24 hours versus later, and STEMI versus non-STEMI. In these analyses, the higher temperature was associated with a potential trend for benefit among females and those with a shockable rhythm.
There was no signal for a difference in neurologic outcomes on the Disability Rating Scale or the Modified Rankin Scale. On the latter, for example, 46% of those in the 31° C group and 44% of these in the 34° C group had a score of four or greater at the end of follow-up.
The baseline characteristics of the two groups were similar. About 80% were male; the average age was roughly 62 years. More than 80% of the cardiac arrests were witnessed with CPR being administered by bystanders in nearly 70%. Nearly 40% had a STEMI.
Interventions were similar. Almost all patients underwent coronary angiography, of which nearly 60% received a percutaneous coronary intervention. More than 50% received a stent. The time from arrest to randomization was slightly longer in the 31° C group (228 vs. 204 minutes). The time to balloon inflation from arrival at the cardiac center was also slightly longer (73 vs. 60 minutes).
There was a trend for an increased rate of seizures in the 31° C group (12.5% vs. 7.1%; P = .08), but other secondary outcomes, including pneumonia (67.8% vs. 63.4%), renal replacement therapy (9.2% vs. 9.3%), and stroke (4.4% vs. 1.6%), were similar in the 31° C and 34° C groups, respectively.
Bleeding, whether measured by transfusion (19.6% vs. 22.4%) or TIMI major bleed (23.4% vs. 19.7%) were similar in the 31° C and 34° C groups, respectively. Thrombosis, whether measured by stent thrombosis (1.2% vs. 2.2%) or deep venous thrombosis (11.4% vs. 10.9%) were similar in these two groups, respectively.
The length of stay in the cardiac intensive care unit was significantly greater in the 31° C group (10 vs. 7 days; P = .004). Some of this increased length of stay can be attributed to the longer rewarming process required for the greater cooling, according to Dr. Le May, but he acknowledged that it is not clear this provides a full explanation.
More trials like CAPITAL-CHILL needed
The validity of these findings is supported by several strengths of the methodology, according to Jeanne E. Poole, MD, director of the arrhythmia service and electrophysiology laboratory, University of Washington, Seattle. This includes the reliance of an endovascular device, which can accelerate the time to the target temperature and assure the precision with which it is reached and maintained.
Dr. Poole did note that many of the primary and secondary measures, including the rates of stroke, seizures, and major bleeds, even though not significantly different, favored the higher temperature. The slightly longer door-to-balloon times might have been a factor. For the higher rate of pneumonia in the 31° C group, she questioned whether the longer period of ventilation linked to a longer period of rewarming might have been a factor.
However, Dr. Poole praised the CAPITAL-CHILL trial for drawing attention to a group of patients for whom survival rates remain “dismally low.” She indicated that these types of high-level trials are needed to look for strategies to improve outcomes.
Dr. Le May and Dr. Poole report no potential conflicts of interest.
FROM ACC 2021
GALACTIC-HF: Novel drug most effective in sickest HFrEF patients
The greatest relative benefit from omecamtiv mecarbil, a member of the novel myotropic drug class that improves cardiac performance, is produced in heart failure patients with the lowest left ventricular ejection fraction (LVEF), a new analysis of the recently published phase 3 GALACTIC-HF trial has found.
The findings reinforce the potential for this drug to be helpful in the management of the most advanced stages of heart failure with reduced ejection fraction (HFrEF), reported John R. Teerlink, MD, director of heart failure at San Francisco Veterans Affairs Medical Center, at the annual scientific sessions of the American College of Cardiology.
The phase 3 multinational GALACTIC-HF trial, published earlier this year, linked omecamtiv mecarbil with an 8% reduction in the risk of a heart failure–related events or cardiovascular death, relative to placebo, which was the primary outcome. For entry, HFrEF patients were required to have a LVEF of 35% or less.
Drilling down on ejection fraction
The new analysis divided participants into quartiles of baseline LVEF and then compared relative outcomes and safety.
In the lowest quartile, defined by a LVEF of 22% or lower, the reduction in risk of events reached 17% (hazard ratio, 0.83; 95% confidence interval, 0.73-0.95) for omecamtiv mecarbil relative to placebo. In the highest, defined by a LVEF of 33% or greater, the benefit fell short of significance (HR 0.99; 95% CI, 0.84-1.16). Across quartiles, LVEF was the “strongest modifier of the treatment effect,” emerging in this analysis as a statistically significant (P = .004) continuous variable.
The comparison by LVEF quartiles also provided an opportunity to show that omecamtiv mecarbil was as safe and well tolerated in those with the most advanced disease as in those less sick. At the lowest levels of LVEF, like the higher levels, omecamtiv mecarbil did not produce any adverse effects on blood pressure, heart rate, potassium homeostasis, or renal function.
In GALACTIC-HF, 8,256 HFrEF patients with LVEF 35% or less were randomized to omecamtiv mecarbil or placebo. The primary composite outcome of hospitalization or urgent visit for heart failure or death from cardiovascular causes was evaluated after a median of 21.8 months on therapy.
When incidence rate per 100 patient years was graphed against the range of LVEF, the relative advantage of omecamtiv mecarbil became visible just below an LVEF of 30%, climbing steadily even to the lowest LVEF, which reached 10%.
Perhaps relevant to the reduction in events, there were also greater relative reductions in NT-proBNP (NT-proB-type natriuretic peptide) for omecamtiv mecarbil at lower relative to higher LVEF. Although omecamtiv mecarbil is not associated with any direct vascular, electrophysiologic, or neurohormonal effects, according to Dr. Teerlink, the indirect effects of selective binding to cardiac myosin has been associated with lower NT-proBNP and other biomarkers of cardiac remodeling in prior clinical studies.
Although Dr. Teerlink acknowledged that relatively few patients in GALACTIC-HF received an angiotensin-receptor neprilysin inhibitor (ARNI) or a sodium glucose cotransporter-2 (SGLT2) inhibitor, he said there is “every reason to believe that omecamtiv mecarbil would be complementary to these therapies.” He said the mechanism of action of omecamtiv mecarbil, which improves systolic function, has no overlap with these drugs.
Importantly, there is a particular need for new treatment options in patients with advanced LVEF, according to Dr. Teerlink, who cited evidence, for example, that “the beneficial effect of [the ARNI] sacubitril valsartan, while still significant, decreases in patients with LVEF less than 35%.”
Overall, based on these results, “we believe that omecamtiv mecarbil represents a novel therapy that holds the promise of improving clinical outcomes in patients with severely reduced ejection fraction, which are the very patients that are most challenging for us to treat,” Dr. Teerlink said.
Omecamtiv mecarbil may ‘buy you some time’
Ileana Piña, MD, clinical professor of medicine, Central Michigan University, Mount Pleasant, Mich., agreed. She said that omecamtiv mecarbil, if approved, will be an option for the type of HFrEF patients who are being considered for heart transplant or mechanical-assist devices.
“We are very loath to use inotropes in this population, because we know that ultimately the inotrope is not going to do well,” said Dr. Piña, calling these therapies a “Band-Aid.” Based on the evidence from GALACTIC-HF, she thinks that omecamtiv mecarbil will be more versatile.
“This drug does not increase myocardial oxygen demand as do the inotropes, and it can be given in the outpatient setting if need be, so I see this as a real advance,” Dr. Piña said. Although Dr. Piña acknowledged that omecamtiv mecarbil did not reduce mortality in the GALACTIC-HF trial, “at least it will buy you some time.”
Dr. Teerlink has financial relationships with multiple pharmaceutical companies, including Amgen, Cytogenetics, and Servier, which provided funding for the GALACTIC-HF trial. Dr. Piña reports no potential conflicts of interest.
The greatest relative benefit from omecamtiv mecarbil, a member of the novel myotropic drug class that improves cardiac performance, is produced in heart failure patients with the lowest left ventricular ejection fraction (LVEF), a new analysis of the recently published phase 3 GALACTIC-HF trial has found.
The findings reinforce the potential for this drug to be helpful in the management of the most advanced stages of heart failure with reduced ejection fraction (HFrEF), reported John R. Teerlink, MD, director of heart failure at San Francisco Veterans Affairs Medical Center, at the annual scientific sessions of the American College of Cardiology.
The phase 3 multinational GALACTIC-HF trial, published earlier this year, linked omecamtiv mecarbil with an 8% reduction in the risk of a heart failure–related events or cardiovascular death, relative to placebo, which was the primary outcome. For entry, HFrEF patients were required to have a LVEF of 35% or less.
Drilling down on ejection fraction
The new analysis divided participants into quartiles of baseline LVEF and then compared relative outcomes and safety.
In the lowest quartile, defined by a LVEF of 22% or lower, the reduction in risk of events reached 17% (hazard ratio, 0.83; 95% confidence interval, 0.73-0.95) for omecamtiv mecarbil relative to placebo. In the highest, defined by a LVEF of 33% or greater, the benefit fell short of significance (HR 0.99; 95% CI, 0.84-1.16). Across quartiles, LVEF was the “strongest modifier of the treatment effect,” emerging in this analysis as a statistically significant (P = .004) continuous variable.
The comparison by LVEF quartiles also provided an opportunity to show that omecamtiv mecarbil was as safe and well tolerated in those with the most advanced disease as in those less sick. At the lowest levels of LVEF, like the higher levels, omecamtiv mecarbil did not produce any adverse effects on blood pressure, heart rate, potassium homeostasis, or renal function.
In GALACTIC-HF, 8,256 HFrEF patients with LVEF 35% or less were randomized to omecamtiv mecarbil or placebo. The primary composite outcome of hospitalization or urgent visit for heart failure or death from cardiovascular causes was evaluated after a median of 21.8 months on therapy.
When incidence rate per 100 patient years was graphed against the range of LVEF, the relative advantage of omecamtiv mecarbil became visible just below an LVEF of 30%, climbing steadily even to the lowest LVEF, which reached 10%.
Perhaps relevant to the reduction in events, there were also greater relative reductions in NT-proBNP (NT-proB-type natriuretic peptide) for omecamtiv mecarbil at lower relative to higher LVEF. Although omecamtiv mecarbil is not associated with any direct vascular, electrophysiologic, or neurohormonal effects, according to Dr. Teerlink, the indirect effects of selective binding to cardiac myosin has been associated with lower NT-proBNP and other biomarkers of cardiac remodeling in prior clinical studies.
Although Dr. Teerlink acknowledged that relatively few patients in GALACTIC-HF received an angiotensin-receptor neprilysin inhibitor (ARNI) or a sodium glucose cotransporter-2 (SGLT2) inhibitor, he said there is “every reason to believe that omecamtiv mecarbil would be complementary to these therapies.” He said the mechanism of action of omecamtiv mecarbil, which improves systolic function, has no overlap with these drugs.
Importantly, there is a particular need for new treatment options in patients with advanced LVEF, according to Dr. Teerlink, who cited evidence, for example, that “the beneficial effect of [the ARNI] sacubitril valsartan, while still significant, decreases in patients with LVEF less than 35%.”
Overall, based on these results, “we believe that omecamtiv mecarbil represents a novel therapy that holds the promise of improving clinical outcomes in patients with severely reduced ejection fraction, which are the very patients that are most challenging for us to treat,” Dr. Teerlink said.
Omecamtiv mecarbil may ‘buy you some time’
Ileana Piña, MD, clinical professor of medicine, Central Michigan University, Mount Pleasant, Mich., agreed. She said that omecamtiv mecarbil, if approved, will be an option for the type of HFrEF patients who are being considered for heart transplant or mechanical-assist devices.
“We are very loath to use inotropes in this population, because we know that ultimately the inotrope is not going to do well,” said Dr. Piña, calling these therapies a “Band-Aid.” Based on the evidence from GALACTIC-HF, she thinks that omecamtiv mecarbil will be more versatile.
“This drug does not increase myocardial oxygen demand as do the inotropes, and it can be given in the outpatient setting if need be, so I see this as a real advance,” Dr. Piña said. Although Dr. Piña acknowledged that omecamtiv mecarbil did not reduce mortality in the GALACTIC-HF trial, “at least it will buy you some time.”
Dr. Teerlink has financial relationships with multiple pharmaceutical companies, including Amgen, Cytogenetics, and Servier, which provided funding for the GALACTIC-HF trial. Dr. Piña reports no potential conflicts of interest.
The greatest relative benefit from omecamtiv mecarbil, a member of the novel myotropic drug class that improves cardiac performance, is produced in heart failure patients with the lowest left ventricular ejection fraction (LVEF), a new analysis of the recently published phase 3 GALACTIC-HF trial has found.
The findings reinforce the potential for this drug to be helpful in the management of the most advanced stages of heart failure with reduced ejection fraction (HFrEF), reported John R. Teerlink, MD, director of heart failure at San Francisco Veterans Affairs Medical Center, at the annual scientific sessions of the American College of Cardiology.
The phase 3 multinational GALACTIC-HF trial, published earlier this year, linked omecamtiv mecarbil with an 8% reduction in the risk of a heart failure–related events or cardiovascular death, relative to placebo, which was the primary outcome. For entry, HFrEF patients were required to have a LVEF of 35% or less.
Drilling down on ejection fraction
The new analysis divided participants into quartiles of baseline LVEF and then compared relative outcomes and safety.
In the lowest quartile, defined by a LVEF of 22% or lower, the reduction in risk of events reached 17% (hazard ratio, 0.83; 95% confidence interval, 0.73-0.95) for omecamtiv mecarbil relative to placebo. In the highest, defined by a LVEF of 33% or greater, the benefit fell short of significance (HR 0.99; 95% CI, 0.84-1.16). Across quartiles, LVEF was the “strongest modifier of the treatment effect,” emerging in this analysis as a statistically significant (P = .004) continuous variable.
The comparison by LVEF quartiles also provided an opportunity to show that omecamtiv mecarbil was as safe and well tolerated in those with the most advanced disease as in those less sick. At the lowest levels of LVEF, like the higher levels, omecamtiv mecarbil did not produce any adverse effects on blood pressure, heart rate, potassium homeostasis, or renal function.
In GALACTIC-HF, 8,256 HFrEF patients with LVEF 35% or less were randomized to omecamtiv mecarbil or placebo. The primary composite outcome of hospitalization or urgent visit for heart failure or death from cardiovascular causes was evaluated after a median of 21.8 months on therapy.
When incidence rate per 100 patient years was graphed against the range of LVEF, the relative advantage of omecamtiv mecarbil became visible just below an LVEF of 30%, climbing steadily even to the lowest LVEF, which reached 10%.
Perhaps relevant to the reduction in events, there were also greater relative reductions in NT-proBNP (NT-proB-type natriuretic peptide) for omecamtiv mecarbil at lower relative to higher LVEF. Although omecamtiv mecarbil is not associated with any direct vascular, electrophysiologic, or neurohormonal effects, according to Dr. Teerlink, the indirect effects of selective binding to cardiac myosin has been associated with lower NT-proBNP and other biomarkers of cardiac remodeling in prior clinical studies.
Although Dr. Teerlink acknowledged that relatively few patients in GALACTIC-HF received an angiotensin-receptor neprilysin inhibitor (ARNI) or a sodium glucose cotransporter-2 (SGLT2) inhibitor, he said there is “every reason to believe that omecamtiv mecarbil would be complementary to these therapies.” He said the mechanism of action of omecamtiv mecarbil, which improves systolic function, has no overlap with these drugs.
Importantly, there is a particular need for new treatment options in patients with advanced LVEF, according to Dr. Teerlink, who cited evidence, for example, that “the beneficial effect of [the ARNI] sacubitril valsartan, while still significant, decreases in patients with LVEF less than 35%.”
Overall, based on these results, “we believe that omecamtiv mecarbil represents a novel therapy that holds the promise of improving clinical outcomes in patients with severely reduced ejection fraction, which are the very patients that are most challenging for us to treat,” Dr. Teerlink said.
Omecamtiv mecarbil may ‘buy you some time’
Ileana Piña, MD, clinical professor of medicine, Central Michigan University, Mount Pleasant, Mich., agreed. She said that omecamtiv mecarbil, if approved, will be an option for the type of HFrEF patients who are being considered for heart transplant or mechanical-assist devices.
“We are very loath to use inotropes in this population, because we know that ultimately the inotrope is not going to do well,” said Dr. Piña, calling these therapies a “Band-Aid.” Based on the evidence from GALACTIC-HF, she thinks that omecamtiv mecarbil will be more versatile.
“This drug does not increase myocardial oxygen demand as do the inotropes, and it can be given in the outpatient setting if need be, so I see this as a real advance,” Dr. Piña said. Although Dr. Piña acknowledged that omecamtiv mecarbil did not reduce mortality in the GALACTIC-HF trial, “at least it will buy you some time.”
Dr. Teerlink has financial relationships with multiple pharmaceutical companies, including Amgen, Cytogenetics, and Servier, which provided funding for the GALACTIC-HF trial. Dr. Piña reports no potential conflicts of interest.
FROM ACC 2021
SAFE-PAD: Endovascular paclitaxel-coated devices exonerated in real-world analysis
A cohort analysis using advanced strategies to minimize the impact of confounders has concluded that the current Food and Drug Administration warning about paclitaxel-coated devices used for femoropopliteal endovascular treatment should be lifted, according to investigators of a study called SAFE-PAD.
In early 2019, an FDA letter to clinicians warned that endovascular stents and balloons coated with paclitaxel might increase mortality, recounted the principal investigator of SAFE-PAD, Eric A. Secemsky, MD, director of vascular intervention, Beth Israel Deaconess Hospital, Boston.
An FDA advisory committee that was subsequently convened in 2019 did not elect to remove these devices from the market, but it did call for restrictions and for the collection of more safety data. In the absence of a clear mechanism of risk, and in the context of perceived problems with data suggesting harm, Dr. Secemsky said that there was interest in a conclusive answer.
The problem was that a randomized controlled trial, even if funding were available, was considered impractical, he noted in presenting SAFE-PAD at the annual scientific sessions of the American College of Cardiology.
In the initial meta-analysis that suggested an increased mortality risk, no risk was seen in the first year after exposure, and it climbed to only 3.5% after 2 years. As a result, the definitive 2-year study with sufficient power to produce conclusive results was an estimated 40,000 patients. Even if extended to 5 years, 20,000 patients would be needed, according to Dr. Secemsky.
SAFE-PAD born of collaboration
An alternative solution was required, which is why “we became engaged with the FDA to design a real-world study for use in making a regulatory decision,” Dr. Secemsky said.
SAFE-PAD, designed with feedback from the FDA, employed sophisticated methodologies to account for known and unknown confounding in the Medicare cohort data used for this study.
Of 168,553 Medicare fee-for-service patients undergoing femoropopliteal artery revascularization with a stent, a balloon, or both at 2,978 institutions, 70,584 (42%) were treated with a paclitaxel drug-coated device (DCD) and the remainder were managed with a non–drug-coated device (NDCD).
The groups were compared with a primary outcome of all-cause mortality in a design to evaluate DCD for noninferiority. Several secondary outcomes, such as repeated lower extremity revascularization, were also evaluated.
To create balanced groups, inverse probability of treatment weighting (IPTW) blinded to outcome was the primary analytic strategy. In addition, several sensitivity analyses were applied, including a technique that tests for the impact of a hypothetical variable that allows adjustment for an unknown confounder.
After a median follow-up of 2.7 years (longest more than 5 years), the cumulative mortality after weighting was 53.8% in the DCD group and 55.1% in the NDCD group. The 5% advantage for the DCD group (hazard ratio, 0.95; 95% confidence interval, 0.94-0.97) ensured noninferiority (P < .001).
On unweighted analysis, the mortality difference favoring DCD was even greater (HR, 0.85; 95% CI, 0.82–0.85).
None of the sensitivity analyses – including a multivariable Cox regression analysis, an instrumental variable analysis, and a falsification endpoints analysis that employed myocardial infarction, pneumonia, and heart failure – altered the conclusion. The hypothetical variable analysis produced the same result.
“A missing confounder would need to be more prevalent and more strongly associated to outcome than any measured variable in this analysis,” reported Dr. Secemsky, indicating that this ruled out essentially any probability of this occurring.
A subgroup analysis told the same story. By hazard ratio for the outcome of mortality, DCD was consistently favored over NDCD for groups characterized by low risk (HR, 0.98), stent implantation (HR, 0.97), receipt of balloon angioplasty alone (HR, 0.94), having critical limb ischemia (HR, 0.95) or no critical limb ischemia (HR, 0.97), and being managed inpatient (HR, 0.97) or outpatient (HR, 0.95).
The results of SAFE-PAD were simultaneously published with Dr. Secemsky’s ACC presentation.
Value of revascularization questioned
In an accompanying editorial, the coauthors Rita F. Redberg, MD, of the University of California, San Francisco, and Mary M. McDermott, MD, of Northwestern University, Chicago, reiterated the findings and the conclusions, but used the forum to draw attention to the low survival rates.
“Thus, while this well-done observational study provides new information,” they wrote, “a major conclusion should be that mortality is high among Medicare beneficiaries undergoing revascularization [for peripheral artery disease] with any devices.”
‘Very impressive’ methods
Marc P. Bonaca, MD, director of vascular research, University of Colorado at Denver, Aurora, called the methods to ensure the validity of the conclusions of this study “very impressive.” In situations where prospective randomized trials are impractical, he suggested that this type of approach might answer an unmet need.
“We have always desired the ability to look at these large datasets with a lot of power to answer important questions,” he said. While “the issue has always been residual confounding,” he expressed interest in further verifications that this type of methodology can serve as a template for data analysis to guide other regulatory decisions.
Dr. Secemsky reports financial relationships with Abbott, Bayer, Boston Scientific, Cook, CSI, Inari, Janssen, Medtronic, and Phillips. Dr. Redford reports no potential conflicts of interest. Dr. McDermott reports a financial relationship with Regeneron. Dr. Bonaca reports financial relationships with Amgen, AstraZeneca, Bayer, Janssen Merck, Novo Nordisk, Pfizer, and Sanofi.
A cohort analysis using advanced strategies to minimize the impact of confounders has concluded that the current Food and Drug Administration warning about paclitaxel-coated devices used for femoropopliteal endovascular treatment should be lifted, according to investigators of a study called SAFE-PAD.
In early 2019, an FDA letter to clinicians warned that endovascular stents and balloons coated with paclitaxel might increase mortality, recounted the principal investigator of SAFE-PAD, Eric A. Secemsky, MD, director of vascular intervention, Beth Israel Deaconess Hospital, Boston.
An FDA advisory committee that was subsequently convened in 2019 did not elect to remove these devices from the market, but it did call for restrictions and for the collection of more safety data. In the absence of a clear mechanism of risk, and in the context of perceived problems with data suggesting harm, Dr. Secemsky said that there was interest in a conclusive answer.
The problem was that a randomized controlled trial, even if funding were available, was considered impractical, he noted in presenting SAFE-PAD at the annual scientific sessions of the American College of Cardiology.
In the initial meta-analysis that suggested an increased mortality risk, no risk was seen in the first year after exposure, and it climbed to only 3.5% after 2 years. As a result, the definitive 2-year study with sufficient power to produce conclusive results was an estimated 40,000 patients. Even if extended to 5 years, 20,000 patients would be needed, according to Dr. Secemsky.
SAFE-PAD born of collaboration
An alternative solution was required, which is why “we became engaged with the FDA to design a real-world study for use in making a regulatory decision,” Dr. Secemsky said.
SAFE-PAD, designed with feedback from the FDA, employed sophisticated methodologies to account for known and unknown confounding in the Medicare cohort data used for this study.
Of 168,553 Medicare fee-for-service patients undergoing femoropopliteal artery revascularization with a stent, a balloon, or both at 2,978 institutions, 70,584 (42%) were treated with a paclitaxel drug-coated device (DCD) and the remainder were managed with a non–drug-coated device (NDCD).
The groups were compared with a primary outcome of all-cause mortality in a design to evaluate DCD for noninferiority. Several secondary outcomes, such as repeated lower extremity revascularization, were also evaluated.
To create balanced groups, inverse probability of treatment weighting (IPTW) blinded to outcome was the primary analytic strategy. In addition, several sensitivity analyses were applied, including a technique that tests for the impact of a hypothetical variable that allows adjustment for an unknown confounder.
After a median follow-up of 2.7 years (longest more than 5 years), the cumulative mortality after weighting was 53.8% in the DCD group and 55.1% in the NDCD group. The 5% advantage for the DCD group (hazard ratio, 0.95; 95% confidence interval, 0.94-0.97) ensured noninferiority (P < .001).
On unweighted analysis, the mortality difference favoring DCD was even greater (HR, 0.85; 95% CI, 0.82–0.85).
None of the sensitivity analyses – including a multivariable Cox regression analysis, an instrumental variable analysis, and a falsification endpoints analysis that employed myocardial infarction, pneumonia, and heart failure – altered the conclusion. The hypothetical variable analysis produced the same result.
“A missing confounder would need to be more prevalent and more strongly associated to outcome than any measured variable in this analysis,” reported Dr. Secemsky, indicating that this ruled out essentially any probability of this occurring.
A subgroup analysis told the same story. By hazard ratio for the outcome of mortality, DCD was consistently favored over NDCD for groups characterized by low risk (HR, 0.98), stent implantation (HR, 0.97), receipt of balloon angioplasty alone (HR, 0.94), having critical limb ischemia (HR, 0.95) or no critical limb ischemia (HR, 0.97), and being managed inpatient (HR, 0.97) or outpatient (HR, 0.95).
The results of SAFE-PAD were simultaneously published with Dr. Secemsky’s ACC presentation.
Value of revascularization questioned
In an accompanying editorial, the coauthors Rita F. Redberg, MD, of the University of California, San Francisco, and Mary M. McDermott, MD, of Northwestern University, Chicago, reiterated the findings and the conclusions, but used the forum to draw attention to the low survival rates.
“Thus, while this well-done observational study provides new information,” they wrote, “a major conclusion should be that mortality is high among Medicare beneficiaries undergoing revascularization [for peripheral artery disease] with any devices.”
‘Very impressive’ methods
Marc P. Bonaca, MD, director of vascular research, University of Colorado at Denver, Aurora, called the methods to ensure the validity of the conclusions of this study “very impressive.” In situations where prospective randomized trials are impractical, he suggested that this type of approach might answer an unmet need.
“We have always desired the ability to look at these large datasets with a lot of power to answer important questions,” he said. While “the issue has always been residual confounding,” he expressed interest in further verifications that this type of methodology can serve as a template for data analysis to guide other regulatory decisions.
Dr. Secemsky reports financial relationships with Abbott, Bayer, Boston Scientific, Cook, CSI, Inari, Janssen, Medtronic, and Phillips. Dr. Redford reports no potential conflicts of interest. Dr. McDermott reports a financial relationship with Regeneron. Dr. Bonaca reports financial relationships with Amgen, AstraZeneca, Bayer, Janssen Merck, Novo Nordisk, Pfizer, and Sanofi.
A cohort analysis using advanced strategies to minimize the impact of confounders has concluded that the current Food and Drug Administration warning about paclitaxel-coated devices used for femoropopliteal endovascular treatment should be lifted, according to investigators of a study called SAFE-PAD.
In early 2019, an FDA letter to clinicians warned that endovascular stents and balloons coated with paclitaxel might increase mortality, recounted the principal investigator of SAFE-PAD, Eric A. Secemsky, MD, director of vascular intervention, Beth Israel Deaconess Hospital, Boston.
An FDA advisory committee that was subsequently convened in 2019 did not elect to remove these devices from the market, but it did call for restrictions and for the collection of more safety data. In the absence of a clear mechanism of risk, and in the context of perceived problems with data suggesting harm, Dr. Secemsky said that there was interest in a conclusive answer.
The problem was that a randomized controlled trial, even if funding were available, was considered impractical, he noted in presenting SAFE-PAD at the annual scientific sessions of the American College of Cardiology.
In the initial meta-analysis that suggested an increased mortality risk, no risk was seen in the first year after exposure, and it climbed to only 3.5% after 2 years. As a result, the definitive 2-year study with sufficient power to produce conclusive results was an estimated 40,000 patients. Even if extended to 5 years, 20,000 patients would be needed, according to Dr. Secemsky.
SAFE-PAD born of collaboration
An alternative solution was required, which is why “we became engaged with the FDA to design a real-world study for use in making a regulatory decision,” Dr. Secemsky said.
SAFE-PAD, designed with feedback from the FDA, employed sophisticated methodologies to account for known and unknown confounding in the Medicare cohort data used for this study.
Of 168,553 Medicare fee-for-service patients undergoing femoropopliteal artery revascularization with a stent, a balloon, or both at 2,978 institutions, 70,584 (42%) were treated with a paclitaxel drug-coated device (DCD) and the remainder were managed with a non–drug-coated device (NDCD).
The groups were compared with a primary outcome of all-cause mortality in a design to evaluate DCD for noninferiority. Several secondary outcomes, such as repeated lower extremity revascularization, were also evaluated.
To create balanced groups, inverse probability of treatment weighting (IPTW) blinded to outcome was the primary analytic strategy. In addition, several sensitivity analyses were applied, including a technique that tests for the impact of a hypothetical variable that allows adjustment for an unknown confounder.
After a median follow-up of 2.7 years (longest more than 5 years), the cumulative mortality after weighting was 53.8% in the DCD group and 55.1% in the NDCD group. The 5% advantage for the DCD group (hazard ratio, 0.95; 95% confidence interval, 0.94-0.97) ensured noninferiority (P < .001).
On unweighted analysis, the mortality difference favoring DCD was even greater (HR, 0.85; 95% CI, 0.82–0.85).
None of the sensitivity analyses – including a multivariable Cox regression analysis, an instrumental variable analysis, and a falsification endpoints analysis that employed myocardial infarction, pneumonia, and heart failure – altered the conclusion. The hypothetical variable analysis produced the same result.
“A missing confounder would need to be more prevalent and more strongly associated to outcome than any measured variable in this analysis,” reported Dr. Secemsky, indicating that this ruled out essentially any probability of this occurring.
A subgroup analysis told the same story. By hazard ratio for the outcome of mortality, DCD was consistently favored over NDCD for groups characterized by low risk (HR, 0.98), stent implantation (HR, 0.97), receipt of balloon angioplasty alone (HR, 0.94), having critical limb ischemia (HR, 0.95) or no critical limb ischemia (HR, 0.97), and being managed inpatient (HR, 0.97) or outpatient (HR, 0.95).
The results of SAFE-PAD were simultaneously published with Dr. Secemsky’s ACC presentation.
Value of revascularization questioned
In an accompanying editorial, the coauthors Rita F. Redberg, MD, of the University of California, San Francisco, and Mary M. McDermott, MD, of Northwestern University, Chicago, reiterated the findings and the conclusions, but used the forum to draw attention to the low survival rates.
“Thus, while this well-done observational study provides new information,” they wrote, “a major conclusion should be that mortality is high among Medicare beneficiaries undergoing revascularization [for peripheral artery disease] with any devices.”
‘Very impressive’ methods
Marc P. Bonaca, MD, director of vascular research, University of Colorado at Denver, Aurora, called the methods to ensure the validity of the conclusions of this study “very impressive.” In situations where prospective randomized trials are impractical, he suggested that this type of approach might answer an unmet need.
“We have always desired the ability to look at these large datasets with a lot of power to answer important questions,” he said. While “the issue has always been residual confounding,” he expressed interest in further verifications that this type of methodology can serve as a template for data analysis to guide other regulatory decisions.
Dr. Secemsky reports financial relationships with Abbott, Bayer, Boston Scientific, Cook, CSI, Inari, Janssen, Medtronic, and Phillips. Dr. Redford reports no potential conflicts of interest. Dr. McDermott reports a financial relationship with Regeneron. Dr. Bonaca reports financial relationships with Amgen, AstraZeneca, Bayer, Janssen Merck, Novo Nordisk, Pfizer, and Sanofi.
FROM ACC 2021
FLOWER-MI: FFR-guided complete revascularization shows no advantage in STEMI
For patients with ST-elevated myocardial infarction (STEMI) undergoing complete revascularization, percutaneous coronary interventions (PCI) guided by fractional flow reserve (FFR) relative to angiography-guided PCI do not result in significantly lower risk of death or events, according to data from the randomized FLOWER-MI trial.
Rather, the events at 1 year were numerically lower among those randomized to the angiography-guided approach, according to the principal investigator of the trial, Etienne Puymirat, MD, PhD.
Prior studies showing an advantage for FFR-guided PCI in patients with coronary syndromes provided the hypothesis that FFR-guided PCI would also be superior for guiding PCI in STEMI patients. In the multicenter FAME trial, for example, FFR-guided PCI for patients with multivessel disease was associated with fewer stent placements (P < .001) and a nearly 30% lower rate of events at 1 year (P = .02).
While the advantage of complete revascularization, meaning PCI treatment of nonculprit as well as culprit lesions, has already been shown to be a better strategy than treatment of culprit lesions alone, FLOWER-MI is the first large study to compare FFR to angiography for guiding this approach to STEMI patients with multivessel disease, said Dr. Puymirat of Hôpital Européen George Pompidou, Paris, at the annual scientific sessions of the American College of Cardiology.
In this trial, involving multiple centers in France, STEMI patients were eligible for randomization if they had successful PCI of a culprit lesion and 50% or greater stenosis in at least one additional nonculprit lesion. The complete revascularization, whether patients were randomized to PCI guided by angiography or FFR, was performed during the index hospital admission. Patient management and follow-up was otherwise the same.
After a small number of exclusions, the intention-to-treat populations were 577 patients in the angiography-guided group and 586 in the FFR-guided group. The characteristics of the groups were well matched with an average age of about 62 years and similar rates of risk factors, such as hypertension and diabetes.
Angiography guidance just as good
The primary outcome was a composite of all-cause mortality, nonfatal MI, and unplanned revascularization. By hazard ratio, the risk of having one of these events within 1 year of PCI was numerically greater, at 32 in the FFR-guided group and 24 in the angiography-guided group, but the difference was not statistically significant (1.32; P = .31).
However, the total rate of events was low (5.5% vs. 4.2% for the angiography-guided and FFR-guided groups, respectively) and the confidence intervals were wide (95% CI, 0.78-2.23). This was also true of the components of the primary outcome.
No signal for a difference between strategies could be derived from these components, which included a higher rate of MI in the FFR-guided group (3.1% vs. 1.7%) but a lower rate of death (1.5% vs. 1.7%).
Unplanned hospitalizations leading to revascularization rates were also low (1.9% and 2.6% for angiography-guided and FFR-guided PCI, respectively), although it was reported that the rate of revascularization for nonculprit lesions was about twice as high in the FFR group (53.3% vs. 27.3%).
At 1 year, there were also low rates and no significant differences in a list of secondary outcomes that included hospitalization for recurrent ischemia or heart failure, stent thrombosis, and revascularization. As within the primary composite outcome, no pattern could be seen in the secondary events, some of which were numerically more common in the FFR-guided group and some numerically lower.
In a cost-efficacy analysis, the median per-patient cost of the FFR-guided strategy was about 500 Euros ($607) greater (8,832 vs. 8,322; P < .01), leading Dr. Puymirat to conclude that “the use of FFR for nonculprit lesions appears to be less effective but more expensive,” at least by costs derived in France.
Lack of statistical power limits interpretation
The conclusion of FLOWER-MI is that FFR-guided PCI in complete revascularization of nonculprit lesions in STEMI patients is not superior to an angiography-guided approach, but Dr. Puymirat cautioned that the low number of events precludes a definitive message.
William Fearon, MD, professor of cardiovascular medicine at Stanford (Calif.) University Medical Center, agreed. Based on his calculations, the trial was substantially underpowered. Evaluating the details of treatment in the FFR group, Dr. Fearon pointed out that a nonculprit lesion with a FFR of 0.80 or less was identified in about 55% of patients. Ultimately, 66% in the FFR group received PCI, eliminating the key distinction between strategies for the majority of patients enrolled.
“Only about one-third of the FFR-guided patients, or about 200 patients, did not receive nonculprit PCI, and therefore only in this small group could we expect a difference in outcomes from the angio-guided group,” Dr. Fearon said.
Fewer stents were placed in the FFR-guided than angiography-guided group (1.01 vs. 1.5), but Dr. Fearon suggested that it would be very difficult to show a difference in risk of events in a study of this size when event rates at 1 year reached only about 5%.
In response, Dr. Puymirat acknowledged that the rate of events for this trial, which was designed in 2015, were lower than expected. In recalculating the power needed based on the rate of events observed in FLOWER-MI, he estimated that about 8,000 patients would have been needed to show a meaningful difference in these PCI strategies.
Dr. Puymirat reports financial relationships with more than a dozen pharmaceutical companies, including Abbott, which provided some of the funding for this trial. Dr. Fearon reports financial relationships with Abbott, CathWorks, HeartFlow, and Medtronic.
For patients with ST-elevated myocardial infarction (STEMI) undergoing complete revascularization, percutaneous coronary interventions (PCI) guided by fractional flow reserve (FFR) relative to angiography-guided PCI do not result in significantly lower risk of death or events, according to data from the randomized FLOWER-MI trial.
Rather, the events at 1 year were numerically lower among those randomized to the angiography-guided approach, according to the principal investigator of the trial, Etienne Puymirat, MD, PhD.
Prior studies showing an advantage for FFR-guided PCI in patients with coronary syndromes provided the hypothesis that FFR-guided PCI would also be superior for guiding PCI in STEMI patients. In the multicenter FAME trial, for example, FFR-guided PCI for patients with multivessel disease was associated with fewer stent placements (P < .001) and a nearly 30% lower rate of events at 1 year (P = .02).
While the advantage of complete revascularization, meaning PCI treatment of nonculprit as well as culprit lesions, has already been shown to be a better strategy than treatment of culprit lesions alone, FLOWER-MI is the first large study to compare FFR to angiography for guiding this approach to STEMI patients with multivessel disease, said Dr. Puymirat of Hôpital Européen George Pompidou, Paris, at the annual scientific sessions of the American College of Cardiology.
In this trial, involving multiple centers in France, STEMI patients were eligible for randomization if they had successful PCI of a culprit lesion and 50% or greater stenosis in at least one additional nonculprit lesion. The complete revascularization, whether patients were randomized to PCI guided by angiography or FFR, was performed during the index hospital admission. Patient management and follow-up was otherwise the same.
After a small number of exclusions, the intention-to-treat populations were 577 patients in the angiography-guided group and 586 in the FFR-guided group. The characteristics of the groups were well matched with an average age of about 62 years and similar rates of risk factors, such as hypertension and diabetes.
Angiography guidance just as good
The primary outcome was a composite of all-cause mortality, nonfatal MI, and unplanned revascularization. By hazard ratio, the risk of having one of these events within 1 year of PCI was numerically greater, at 32 in the FFR-guided group and 24 in the angiography-guided group, but the difference was not statistically significant (1.32; P = .31).
However, the total rate of events was low (5.5% vs. 4.2% for the angiography-guided and FFR-guided groups, respectively) and the confidence intervals were wide (95% CI, 0.78-2.23). This was also true of the components of the primary outcome.
No signal for a difference between strategies could be derived from these components, which included a higher rate of MI in the FFR-guided group (3.1% vs. 1.7%) but a lower rate of death (1.5% vs. 1.7%).
Unplanned hospitalizations leading to revascularization rates were also low (1.9% and 2.6% for angiography-guided and FFR-guided PCI, respectively), although it was reported that the rate of revascularization for nonculprit lesions was about twice as high in the FFR group (53.3% vs. 27.3%).
At 1 year, there were also low rates and no significant differences in a list of secondary outcomes that included hospitalization for recurrent ischemia or heart failure, stent thrombosis, and revascularization. As within the primary composite outcome, no pattern could be seen in the secondary events, some of which were numerically more common in the FFR-guided group and some numerically lower.
In a cost-efficacy analysis, the median per-patient cost of the FFR-guided strategy was about 500 Euros ($607) greater (8,832 vs. 8,322; P < .01), leading Dr. Puymirat to conclude that “the use of FFR for nonculprit lesions appears to be less effective but more expensive,” at least by costs derived in France.
Lack of statistical power limits interpretation
The conclusion of FLOWER-MI is that FFR-guided PCI in complete revascularization of nonculprit lesions in STEMI patients is not superior to an angiography-guided approach, but Dr. Puymirat cautioned that the low number of events precludes a definitive message.
William Fearon, MD, professor of cardiovascular medicine at Stanford (Calif.) University Medical Center, agreed. Based on his calculations, the trial was substantially underpowered. Evaluating the details of treatment in the FFR group, Dr. Fearon pointed out that a nonculprit lesion with a FFR of 0.80 or less was identified in about 55% of patients. Ultimately, 66% in the FFR group received PCI, eliminating the key distinction between strategies for the majority of patients enrolled.
“Only about one-third of the FFR-guided patients, or about 200 patients, did not receive nonculprit PCI, and therefore only in this small group could we expect a difference in outcomes from the angio-guided group,” Dr. Fearon said.
Fewer stents were placed in the FFR-guided than angiography-guided group (1.01 vs. 1.5), but Dr. Fearon suggested that it would be very difficult to show a difference in risk of events in a study of this size when event rates at 1 year reached only about 5%.
In response, Dr. Puymirat acknowledged that the rate of events for this trial, which was designed in 2015, were lower than expected. In recalculating the power needed based on the rate of events observed in FLOWER-MI, he estimated that about 8,000 patients would have been needed to show a meaningful difference in these PCI strategies.
Dr. Puymirat reports financial relationships with more than a dozen pharmaceutical companies, including Abbott, which provided some of the funding for this trial. Dr. Fearon reports financial relationships with Abbott, CathWorks, HeartFlow, and Medtronic.
For patients with ST-elevated myocardial infarction (STEMI) undergoing complete revascularization, percutaneous coronary interventions (PCI) guided by fractional flow reserve (FFR) relative to angiography-guided PCI do not result in significantly lower risk of death or events, according to data from the randomized FLOWER-MI trial.
Rather, the events at 1 year were numerically lower among those randomized to the angiography-guided approach, according to the principal investigator of the trial, Etienne Puymirat, MD, PhD.
Prior studies showing an advantage for FFR-guided PCI in patients with coronary syndromes provided the hypothesis that FFR-guided PCI would also be superior for guiding PCI in STEMI patients. In the multicenter FAME trial, for example, FFR-guided PCI for patients with multivessel disease was associated with fewer stent placements (P < .001) and a nearly 30% lower rate of events at 1 year (P = .02).
While the advantage of complete revascularization, meaning PCI treatment of nonculprit as well as culprit lesions, has already been shown to be a better strategy than treatment of culprit lesions alone, FLOWER-MI is the first large study to compare FFR to angiography for guiding this approach to STEMI patients with multivessel disease, said Dr. Puymirat of Hôpital Européen George Pompidou, Paris, at the annual scientific sessions of the American College of Cardiology.
In this trial, involving multiple centers in France, STEMI patients were eligible for randomization if they had successful PCI of a culprit lesion and 50% or greater stenosis in at least one additional nonculprit lesion. The complete revascularization, whether patients were randomized to PCI guided by angiography or FFR, was performed during the index hospital admission. Patient management and follow-up was otherwise the same.
After a small number of exclusions, the intention-to-treat populations were 577 patients in the angiography-guided group and 586 in the FFR-guided group. The characteristics of the groups were well matched with an average age of about 62 years and similar rates of risk factors, such as hypertension and diabetes.
Angiography guidance just as good
The primary outcome was a composite of all-cause mortality, nonfatal MI, and unplanned revascularization. By hazard ratio, the risk of having one of these events within 1 year of PCI was numerically greater, at 32 in the FFR-guided group and 24 in the angiography-guided group, but the difference was not statistically significant (1.32; P = .31).
However, the total rate of events was low (5.5% vs. 4.2% for the angiography-guided and FFR-guided groups, respectively) and the confidence intervals were wide (95% CI, 0.78-2.23). This was also true of the components of the primary outcome.
No signal for a difference between strategies could be derived from these components, which included a higher rate of MI in the FFR-guided group (3.1% vs. 1.7%) but a lower rate of death (1.5% vs. 1.7%).
Unplanned hospitalizations leading to revascularization rates were also low (1.9% and 2.6% for angiography-guided and FFR-guided PCI, respectively), although it was reported that the rate of revascularization for nonculprit lesions was about twice as high in the FFR group (53.3% vs. 27.3%).
At 1 year, there were also low rates and no significant differences in a list of secondary outcomes that included hospitalization for recurrent ischemia or heart failure, stent thrombosis, and revascularization. As within the primary composite outcome, no pattern could be seen in the secondary events, some of which were numerically more common in the FFR-guided group and some numerically lower.
In a cost-efficacy analysis, the median per-patient cost of the FFR-guided strategy was about 500 Euros ($607) greater (8,832 vs. 8,322; P < .01), leading Dr. Puymirat to conclude that “the use of FFR for nonculprit lesions appears to be less effective but more expensive,” at least by costs derived in France.
Lack of statistical power limits interpretation
The conclusion of FLOWER-MI is that FFR-guided PCI in complete revascularization of nonculprit lesions in STEMI patients is not superior to an angiography-guided approach, but Dr. Puymirat cautioned that the low number of events precludes a definitive message.
William Fearon, MD, professor of cardiovascular medicine at Stanford (Calif.) University Medical Center, agreed. Based on his calculations, the trial was substantially underpowered. Evaluating the details of treatment in the FFR group, Dr. Fearon pointed out that a nonculprit lesion with a FFR of 0.80 or less was identified in about 55% of patients. Ultimately, 66% in the FFR group received PCI, eliminating the key distinction between strategies for the majority of patients enrolled.
“Only about one-third of the FFR-guided patients, or about 200 patients, did not receive nonculprit PCI, and therefore only in this small group could we expect a difference in outcomes from the angio-guided group,” Dr. Fearon said.
Fewer stents were placed in the FFR-guided than angiography-guided group (1.01 vs. 1.5), but Dr. Fearon suggested that it would be very difficult to show a difference in risk of events in a study of this size when event rates at 1 year reached only about 5%.
In response, Dr. Puymirat acknowledged that the rate of events for this trial, which was designed in 2015, were lower than expected. In recalculating the power needed based on the rate of events observed in FLOWER-MI, he estimated that about 8,000 patients would have been needed to show a meaningful difference in these PCI strategies.
Dr. Puymirat reports financial relationships with more than a dozen pharmaceutical companies, including Abbott, which provided some of the funding for this trial. Dr. Fearon reports financial relationships with Abbott, CathWorks, HeartFlow, and Medtronic.
FROM ACC 2021






















