User login
CABG safe 3 days after stopping ticagrelor: RAPID CABG
Patients with acute coronary syndromes who have been taking the antiplatelet medication, ticagrelor, and who need coronary artery bypass surgery (CABG) may be able to safely have the procedure earlier than typically recommended, a new randomized trial suggests.
The RAPID CABG trial found that early surgery 2-3 days after ticagrelor cessation was noninferior in incurring severe or massive perioperative bleeding, compared with waiting 5-7 days. There was also no significant difference in TIMI CABG or Bleeding Academic Research Consortium (BARC) type 4 or 5 bleeding.
Patients in the delayed group had a numerically higher number of ischemic events requiring earlier surgery and had a longer hospital stay.
The study was presented at the American Heart Association scientific sessions.
“RAPID CABG is the first and only randomized controlled trial evaluating the safety of early surgery in patients taking ticagrelor,” said lead investigator Derek So, MD.
Dr. So, a cardiologist at the University of Ottawa Heart Institute and a professor at the University of Ottawa, explained that ticagrelor is a first-line antiplatelet agent for patients with acute coronary syndromes (ACS), but around 10% of patients presenting with ACS require CABG surgery.
A major concern among patients requiring bypass surgery is perioperative bleeding, and it has been shown that patients undergoing urgent bypass within 24 hours of the last dose of ticagrelor have increased mortality. Accordingly, guidelines suggest a waiting period for patients not requiring urgent bypass surgery, Dr. So noted.
Current North American guidelines suggest a waiting period of at least 5 days after stopping ticagrelor before bypass surgery. In contrast, the updated European and Japanese guidelines suggest a waiting period of 3 days.
Dr. So noted that all of the guidelines are based on cohort studies and pharmacodynamic studies, with no randomized evidence. Pharmacodynamic studies have shown that at 48 hours after the last dose of ticagrelor, the level of platelet inhibition drops to the same levels seen with long-term treatment with clopidogrel, a weaker antiplatelet drug, and after 120 hours (5 days) the effect has completely worn off.
Dr. So concluded that these new results from the RAPID CABG trial “may influence future iterations of North American guidelines with reduced waiting prior to bypass surgery” for patients receiving ticagrelor, and “they could also strengthen the level of evidence in European and Asian guidelines.”
Designated discussant of the RAPID CABG trial, Roxana Mehran, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, said this was a “very important study,” being the only randomized trial to look at this issue to date.
Dr. Mehran noted that the results showed a similar number of major life-threatening bleeding events in the early and delayed groups and met the noninferiority endpoint, but she pointed out that the trial had a small sample size and a small number of events. “Therefore, larger trials are needed to verify these important and encouraging results.”
However, she concluded that these results should be considered in decisions about the timing of bypass surgery in patients receiving ticagrelor. “I will be changing my practice and sending patients earlier based on this data,” she said.
RAPID CABG
RAPID CABG was a physician-initiated multicenter randomized study evaluating the safety of early surgery at 2-3 days after ticagrelor cessation, compared with a delay of 5-7 days among patients presenting with ACS who required nonemergency CABG surgery.
The study enrolled 143 patients with ACS who were receiving ticagrelor and needed CABG surgery. Patients with stenting for culprit lesions, those requiring urgent surgery (less than 24 hours after presentation), and those requiring valve surgery were excluded.
Three patients declined surgery, and several others underwent surgery outside the assigned time window, so the results were based on the per protocol analysis of patients who actually had CABG in the assigned time window: 65 patients in the early CABG group and 58 in the delayed group.
The mean time from last ticagrelor dose to surgery was 3 days in the early group and 6 days in the delayed group.
Platelet reactivity on the VerifyNow test showed more residual antiplatelet activity in the early group, with P2Y12 reaction unit (PRU) levels of 200 (vs. 251 in the delayed group). This test measures the extent of platelet aggregation in the presence of P2Y12-inhibitor drugs, with lower PRU levels showing stronger antiplatelet effects.
The primary outcome of the study was severe or massive bleeding by Universal Definition of Perioperative Bleeding (UDPB) class 3 or 4. This is defined as a blood transfusions of more than 5 units of red blood cells or plasma within 24 hours of surgical closure, chest tube drainage of over 1,000 mL in the first 12 hours, and reoperation for bleeding.
Results showed that 4.6% of the early-surgery group had a primary outcome bleeding event, compared with 5.2% of the delayed surgery group, meeting the criteria for noninferiority (P = .0253 for noninferiority).
Individual components of the primary endpoint showed three class 3 (severe) bleeding events in both groups and no class 4 (massive) bleeding events in either group.
In terms of other bleeding outcomes, TIMI CABG bleeding occurred in two patients (3.1%) in the early-surgery group vs. no patients in the delayed group; BARC 4 bleeding occurred in two patients (3.1%) in the early group versus none in the delayed group, and there were no BARC 5 bleeding events in either group.
In the intention-to-treat analysis, ischemic events before surgery occurred in six patients (8.7%) in the delayed group (one myocardial infarction, four cases of recurrent ischemia, and one ventricular tachycardia) versus none in the early group.
Cumulative 6-month ischemic events occurred in nine patients (13.0%) in the delayed group vs. four patients (5.6%) in the early group, the difference being driven by nonfatal MI and recurrent ischemia.
There were no cardiovascular deaths in either group and one all-cause death in both groups.
Patients undergoing early surgery also had a shorter hospitalization, with a median length of stay of 9 days versus 12 days in the delayed group.
Larger trial needed
Commenting on the RAPID CABG study at an AHA press conference, Joanna Chikwe, MD, chair of the cardiac surgery department at Cedars-Sinai Medical Center, Los Angeles, said the results were in line with her practice.
“These results confirm what I already think is safe,” she said. “I’m comfortable going within 48 hours. But we individualize our approach, so it was helpful that the study investigators included platelet reactivity data. The interesting thing for me in this study was the number of adverse events in patients who waited longer.”
Dr. Chikwe said her top-line message was that “Surgery looked incredibly safe; there was amazingly low mortality. And if a patient has an indication for surgery, waiting does not serve you well.”
However, she also cautioned that the trial was somewhat underpowered, with a small number of events that drove the primary outcome, leading to some uncertainty on the results.
“The RAPID trial was helpful, and although it confirms my practice, I think physicians may want to see a larger-powered trial to be convincingly compelled that they should change their practice,” Dr. Chikwe noted.
She added that clinical trials in cardiac surgery are driven by inherent challenges. “Cardiac surgery is not very common, and it is hard to recruit patients into these trials, so you are generally tied to a small number of patients, and you therefore have to be extremely thoughtful about the study design. It is almost a given that you will need to use surrogate endpoints, and the choice of the surrogate endpoint can determine which way the trial goes.”
The RAPID CABG study was funded by the Canadian Institutes of Health Research. Dr. So reports research support, consultancy, or speaker’s fees from AggreDyne, Roche Diagnostics, Fujimori Kogyo, and AstraZeneca Canada. Dr. Mehran reports that her institution has received significant trial funding from AstraZeneca (the manufacturer of ticagrelor).
A version of this article first appeared on Medscape.com.
Patients with acute coronary syndromes who have been taking the antiplatelet medication, ticagrelor, and who need coronary artery bypass surgery (CABG) may be able to safely have the procedure earlier than typically recommended, a new randomized trial suggests.
The RAPID CABG trial found that early surgery 2-3 days after ticagrelor cessation was noninferior in incurring severe or massive perioperative bleeding, compared with waiting 5-7 days. There was also no significant difference in TIMI CABG or Bleeding Academic Research Consortium (BARC) type 4 or 5 bleeding.
Patients in the delayed group had a numerically higher number of ischemic events requiring earlier surgery and had a longer hospital stay.
The study was presented at the American Heart Association scientific sessions.
“RAPID CABG is the first and only randomized controlled trial evaluating the safety of early surgery in patients taking ticagrelor,” said lead investigator Derek So, MD.
Dr. So, a cardiologist at the University of Ottawa Heart Institute and a professor at the University of Ottawa, explained that ticagrelor is a first-line antiplatelet agent for patients with acute coronary syndromes (ACS), but around 10% of patients presenting with ACS require CABG surgery.
A major concern among patients requiring bypass surgery is perioperative bleeding, and it has been shown that patients undergoing urgent bypass within 24 hours of the last dose of ticagrelor have increased mortality. Accordingly, guidelines suggest a waiting period for patients not requiring urgent bypass surgery, Dr. So noted.
Current North American guidelines suggest a waiting period of at least 5 days after stopping ticagrelor before bypass surgery. In contrast, the updated European and Japanese guidelines suggest a waiting period of 3 days.
Dr. So noted that all of the guidelines are based on cohort studies and pharmacodynamic studies, with no randomized evidence. Pharmacodynamic studies have shown that at 48 hours after the last dose of ticagrelor, the level of platelet inhibition drops to the same levels seen with long-term treatment with clopidogrel, a weaker antiplatelet drug, and after 120 hours (5 days) the effect has completely worn off.
Dr. So concluded that these new results from the RAPID CABG trial “may influence future iterations of North American guidelines with reduced waiting prior to bypass surgery” for patients receiving ticagrelor, and “they could also strengthen the level of evidence in European and Asian guidelines.”
Designated discussant of the RAPID CABG trial, Roxana Mehran, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, said this was a “very important study,” being the only randomized trial to look at this issue to date.
Dr. Mehran noted that the results showed a similar number of major life-threatening bleeding events in the early and delayed groups and met the noninferiority endpoint, but she pointed out that the trial had a small sample size and a small number of events. “Therefore, larger trials are needed to verify these important and encouraging results.”
However, she concluded that these results should be considered in decisions about the timing of bypass surgery in patients receiving ticagrelor. “I will be changing my practice and sending patients earlier based on this data,” she said.
RAPID CABG
RAPID CABG was a physician-initiated multicenter randomized study evaluating the safety of early surgery at 2-3 days after ticagrelor cessation, compared with a delay of 5-7 days among patients presenting with ACS who required nonemergency CABG surgery.
The study enrolled 143 patients with ACS who were receiving ticagrelor and needed CABG surgery. Patients with stenting for culprit lesions, those requiring urgent surgery (less than 24 hours after presentation), and those requiring valve surgery were excluded.
Three patients declined surgery, and several others underwent surgery outside the assigned time window, so the results were based on the per protocol analysis of patients who actually had CABG in the assigned time window: 65 patients in the early CABG group and 58 in the delayed group.
The mean time from last ticagrelor dose to surgery was 3 days in the early group and 6 days in the delayed group.
Platelet reactivity on the VerifyNow test showed more residual antiplatelet activity in the early group, with P2Y12 reaction unit (PRU) levels of 200 (vs. 251 in the delayed group). This test measures the extent of platelet aggregation in the presence of P2Y12-inhibitor drugs, with lower PRU levels showing stronger antiplatelet effects.
The primary outcome of the study was severe or massive bleeding by Universal Definition of Perioperative Bleeding (UDPB) class 3 or 4. This is defined as a blood transfusions of more than 5 units of red blood cells or plasma within 24 hours of surgical closure, chest tube drainage of over 1,000 mL in the first 12 hours, and reoperation for bleeding.
Results showed that 4.6% of the early-surgery group had a primary outcome bleeding event, compared with 5.2% of the delayed surgery group, meeting the criteria for noninferiority (P = .0253 for noninferiority).
Individual components of the primary endpoint showed three class 3 (severe) bleeding events in both groups and no class 4 (massive) bleeding events in either group.
In terms of other bleeding outcomes, TIMI CABG bleeding occurred in two patients (3.1%) in the early-surgery group vs. no patients in the delayed group; BARC 4 bleeding occurred in two patients (3.1%) in the early group versus none in the delayed group, and there were no BARC 5 bleeding events in either group.
In the intention-to-treat analysis, ischemic events before surgery occurred in six patients (8.7%) in the delayed group (one myocardial infarction, four cases of recurrent ischemia, and one ventricular tachycardia) versus none in the early group.
Cumulative 6-month ischemic events occurred in nine patients (13.0%) in the delayed group vs. four patients (5.6%) in the early group, the difference being driven by nonfatal MI and recurrent ischemia.
There were no cardiovascular deaths in either group and one all-cause death in both groups.
Patients undergoing early surgery also had a shorter hospitalization, with a median length of stay of 9 days versus 12 days in the delayed group.
Larger trial needed
Commenting on the RAPID CABG study at an AHA press conference, Joanna Chikwe, MD, chair of the cardiac surgery department at Cedars-Sinai Medical Center, Los Angeles, said the results were in line with her practice.
“These results confirm what I already think is safe,” she said. “I’m comfortable going within 48 hours. But we individualize our approach, so it was helpful that the study investigators included platelet reactivity data. The interesting thing for me in this study was the number of adverse events in patients who waited longer.”
Dr. Chikwe said her top-line message was that “Surgery looked incredibly safe; there was amazingly low mortality. And if a patient has an indication for surgery, waiting does not serve you well.”
However, she also cautioned that the trial was somewhat underpowered, with a small number of events that drove the primary outcome, leading to some uncertainty on the results.
“The RAPID trial was helpful, and although it confirms my practice, I think physicians may want to see a larger-powered trial to be convincingly compelled that they should change their practice,” Dr. Chikwe noted.
She added that clinical trials in cardiac surgery are driven by inherent challenges. “Cardiac surgery is not very common, and it is hard to recruit patients into these trials, so you are generally tied to a small number of patients, and you therefore have to be extremely thoughtful about the study design. It is almost a given that you will need to use surrogate endpoints, and the choice of the surrogate endpoint can determine which way the trial goes.”
The RAPID CABG study was funded by the Canadian Institutes of Health Research. Dr. So reports research support, consultancy, or speaker’s fees from AggreDyne, Roche Diagnostics, Fujimori Kogyo, and AstraZeneca Canada. Dr. Mehran reports that her institution has received significant trial funding from AstraZeneca (the manufacturer of ticagrelor).
A version of this article first appeared on Medscape.com.
Patients with acute coronary syndromes who have been taking the antiplatelet medication, ticagrelor, and who need coronary artery bypass surgery (CABG) may be able to safely have the procedure earlier than typically recommended, a new randomized trial suggests.
The RAPID CABG trial found that early surgery 2-3 days after ticagrelor cessation was noninferior in incurring severe or massive perioperative bleeding, compared with waiting 5-7 days. There was also no significant difference in TIMI CABG or Bleeding Academic Research Consortium (BARC) type 4 or 5 bleeding.
Patients in the delayed group had a numerically higher number of ischemic events requiring earlier surgery and had a longer hospital stay.
The study was presented at the American Heart Association scientific sessions.
“RAPID CABG is the first and only randomized controlled trial evaluating the safety of early surgery in patients taking ticagrelor,” said lead investigator Derek So, MD.
Dr. So, a cardiologist at the University of Ottawa Heart Institute and a professor at the University of Ottawa, explained that ticagrelor is a first-line antiplatelet agent for patients with acute coronary syndromes (ACS), but around 10% of patients presenting with ACS require CABG surgery.
A major concern among patients requiring bypass surgery is perioperative bleeding, and it has been shown that patients undergoing urgent bypass within 24 hours of the last dose of ticagrelor have increased mortality. Accordingly, guidelines suggest a waiting period for patients not requiring urgent bypass surgery, Dr. So noted.
Current North American guidelines suggest a waiting period of at least 5 days after stopping ticagrelor before bypass surgery. In contrast, the updated European and Japanese guidelines suggest a waiting period of 3 days.
Dr. So noted that all of the guidelines are based on cohort studies and pharmacodynamic studies, with no randomized evidence. Pharmacodynamic studies have shown that at 48 hours after the last dose of ticagrelor, the level of platelet inhibition drops to the same levels seen with long-term treatment with clopidogrel, a weaker antiplatelet drug, and after 120 hours (5 days) the effect has completely worn off.
Dr. So concluded that these new results from the RAPID CABG trial “may influence future iterations of North American guidelines with reduced waiting prior to bypass surgery” for patients receiving ticagrelor, and “they could also strengthen the level of evidence in European and Asian guidelines.”
Designated discussant of the RAPID CABG trial, Roxana Mehran, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, said this was a “very important study,” being the only randomized trial to look at this issue to date.
Dr. Mehran noted that the results showed a similar number of major life-threatening bleeding events in the early and delayed groups and met the noninferiority endpoint, but she pointed out that the trial had a small sample size and a small number of events. “Therefore, larger trials are needed to verify these important and encouraging results.”
However, she concluded that these results should be considered in decisions about the timing of bypass surgery in patients receiving ticagrelor. “I will be changing my practice and sending patients earlier based on this data,” she said.
RAPID CABG
RAPID CABG was a physician-initiated multicenter randomized study evaluating the safety of early surgery at 2-3 days after ticagrelor cessation, compared with a delay of 5-7 days among patients presenting with ACS who required nonemergency CABG surgery.
The study enrolled 143 patients with ACS who were receiving ticagrelor and needed CABG surgery. Patients with stenting for culprit lesions, those requiring urgent surgery (less than 24 hours after presentation), and those requiring valve surgery were excluded.
Three patients declined surgery, and several others underwent surgery outside the assigned time window, so the results were based on the per protocol analysis of patients who actually had CABG in the assigned time window: 65 patients in the early CABG group and 58 in the delayed group.
The mean time from last ticagrelor dose to surgery was 3 days in the early group and 6 days in the delayed group.
Platelet reactivity on the VerifyNow test showed more residual antiplatelet activity in the early group, with P2Y12 reaction unit (PRU) levels of 200 (vs. 251 in the delayed group). This test measures the extent of platelet aggregation in the presence of P2Y12-inhibitor drugs, with lower PRU levels showing stronger antiplatelet effects.
The primary outcome of the study was severe or massive bleeding by Universal Definition of Perioperative Bleeding (UDPB) class 3 or 4. This is defined as a blood transfusions of more than 5 units of red blood cells or plasma within 24 hours of surgical closure, chest tube drainage of over 1,000 mL in the first 12 hours, and reoperation for bleeding.
Results showed that 4.6% of the early-surgery group had a primary outcome bleeding event, compared with 5.2% of the delayed surgery group, meeting the criteria for noninferiority (P = .0253 for noninferiority).
Individual components of the primary endpoint showed three class 3 (severe) bleeding events in both groups and no class 4 (massive) bleeding events in either group.
In terms of other bleeding outcomes, TIMI CABG bleeding occurred in two patients (3.1%) in the early-surgery group vs. no patients in the delayed group; BARC 4 bleeding occurred in two patients (3.1%) in the early group versus none in the delayed group, and there were no BARC 5 bleeding events in either group.
In the intention-to-treat analysis, ischemic events before surgery occurred in six patients (8.7%) in the delayed group (one myocardial infarction, four cases of recurrent ischemia, and one ventricular tachycardia) versus none in the early group.
Cumulative 6-month ischemic events occurred in nine patients (13.0%) in the delayed group vs. four patients (5.6%) in the early group, the difference being driven by nonfatal MI and recurrent ischemia.
There were no cardiovascular deaths in either group and one all-cause death in both groups.
Patients undergoing early surgery also had a shorter hospitalization, with a median length of stay of 9 days versus 12 days in the delayed group.
Larger trial needed
Commenting on the RAPID CABG study at an AHA press conference, Joanna Chikwe, MD, chair of the cardiac surgery department at Cedars-Sinai Medical Center, Los Angeles, said the results were in line with her practice.
“These results confirm what I already think is safe,” she said. “I’m comfortable going within 48 hours. But we individualize our approach, so it was helpful that the study investigators included platelet reactivity data. The interesting thing for me in this study was the number of adverse events in patients who waited longer.”
Dr. Chikwe said her top-line message was that “Surgery looked incredibly safe; there was amazingly low mortality. And if a patient has an indication for surgery, waiting does not serve you well.”
However, she also cautioned that the trial was somewhat underpowered, with a small number of events that drove the primary outcome, leading to some uncertainty on the results.
“The RAPID trial was helpful, and although it confirms my practice, I think physicians may want to see a larger-powered trial to be convincingly compelled that they should change their practice,” Dr. Chikwe noted.
She added that clinical trials in cardiac surgery are driven by inherent challenges. “Cardiac surgery is not very common, and it is hard to recruit patients into these trials, so you are generally tied to a small number of patients, and you therefore have to be extremely thoughtful about the study design. It is almost a given that you will need to use surrogate endpoints, and the choice of the surrogate endpoint can determine which way the trial goes.”
The RAPID CABG study was funded by the Canadian Institutes of Health Research. Dr. So reports research support, consultancy, or speaker’s fees from AggreDyne, Roche Diagnostics, Fujimori Kogyo, and AstraZeneca Canada. Dr. Mehran reports that her institution has received significant trial funding from AstraZeneca (the manufacturer of ticagrelor).
A version of this article first appeared on Medscape.com.
FROM AHA 2021
VEST: External sheath for CABG vein grafts shows promise
A novel, stent-shaped device that provides external buttressing to saphenous vein grafts placed during coronary artery bypass surgery was safe, but failed to improve 12-month patency of vein grafts, in a prospective study with 224 patients.
Despite the neutral result, “we are cautiously optimistic” about the prospects for the device to reduce the risk for failure of coronary vein grafts caused by intimal hyperplasia of the internal lining of the vein graft that leads to graft occlusion, said John D. Puskas, MD, lead investigator of the study, who reported the results at the American Heart Association scientific sessions.
In the trial, called VEST, each buttressed vein graft was compared with a similar, unbuttressed graft in the same patient. Perhaps the biggest issue faced by the study was the unexpectedly high 42% rate of vein-graft occlusion or diffuse disease seen in the studied grafts 12 months after placement. This rate included both the vein grafts placed within the external buttressing device and control vein grafts that underwent the same postharvest preparation but weren’t placed within an external sheath, which is formed from woven cobalt chromium wire.
Dr. Puskas attributed this high failure rate to the need to remove all adventitia tissue and fat from the harvested saphenous vein segments before grafting, a step required to allow the vein conduit to fit inside the wire sheath. The potential exists to further optimize this step, he said in an interview.
“I was very surprised by the low 12-month patency rates” in both treatment arms of the study, commented Joanna Chikwe, MD, chair of cardiac surgery at Cedars-Sinai Medical Center in Los Angeles.
External scaffold to counter blood pressure
The concept behind the external buttressing sheath is that the walls of saphenous vein grafts are not structured to accommodate arterial blood pressure, and over time this pressure produces accelerated atherosclerotic changes and premature occlusion and graft failure. The external support is supposed to impede vein wall dilatation, reduce irregularities of the inner lumen surface, and improve hemodynamics and shear stress.
The VEST trial ran at 14 U.S. and 3 Canadian centers and enrolled 224 patients scheduled for coronary artery bypass grafting with planned use of at least two saphenous vein grafts, along with an internal mammary artery graft for the left anterior descending coronary artery. The patients averaged 66 years of age, 21% were women, and 51% had diabetes.
All patients successfully underwent their surgery, with 203 returning after 12 months for their primary follow-up examination by intravascular ultrasound. However, because of the high rate of vein occlusion or development of diffuse intragraft disease, successful intravascular ultrasound (IVUS) examination of both vein grafts occurred in only 113 patients.
The IVUS examinations showed that the study’s primary endpoint, the intimal hyperplasia area in all 224 patients who received vein grafts, averaged 5.11 mm2 in the grafts placed within the wire sleeve and 5.79 mm2 for control grafts not placed in the wire sheath, a difference that fell short of significance (P = .072). However, in a sensitivity analysis that focused on only the 113 patients who had both vein grafts successfully assayed by IVUS, the average area of intimal hyperplasia was 4.58 mm2 in the grafts within a wire sheath and 5.12 mm2 in the control grafts, a significant difference (P = .043).
The combined rate of major adverse cardiovascular events after 12 months was 7%, including a 2% mortality rate, a 3% stroke rate, and 3% rate of Mis, outcomes that suggested “no safety signals,” said Dr. Puskas, chair of cardiovascular surgery at Mount Sinai St. Luke’s in New York.
Although a large body of evidence has shown the superiority of arterial grafts for long-term graft patency, vein grafts have many advantages that have maintained them as the most widely used conduits worldwide for coronary artery bypass surgery, Dr. Puskas said.
Saphenous vein segments are readily available from patients and easy to harvest; they nicely conform to the coronary arteries that require bypass, rarely leak, are easy to work with, and can successfully hold stitches. Surgeons performing coronary artery bypass are unlikely to abandon vein grafts anytime soon, which makes improving the performance of vein grafts a priority, Dr. Puskas said.
The study was sponsored by Vascular Graft Solutions, the company developing the venous graft external support. Dr. Puskas and Dr. Chikwe had no disclosures related to the study.
A novel, stent-shaped device that provides external buttressing to saphenous vein grafts placed during coronary artery bypass surgery was safe, but failed to improve 12-month patency of vein grafts, in a prospective study with 224 patients.
Despite the neutral result, “we are cautiously optimistic” about the prospects for the device to reduce the risk for failure of coronary vein grafts caused by intimal hyperplasia of the internal lining of the vein graft that leads to graft occlusion, said John D. Puskas, MD, lead investigator of the study, who reported the results at the American Heart Association scientific sessions.
In the trial, called VEST, each buttressed vein graft was compared with a similar, unbuttressed graft in the same patient. Perhaps the biggest issue faced by the study was the unexpectedly high 42% rate of vein-graft occlusion or diffuse disease seen in the studied grafts 12 months after placement. This rate included both the vein grafts placed within the external buttressing device and control vein grafts that underwent the same postharvest preparation but weren’t placed within an external sheath, which is formed from woven cobalt chromium wire.
Dr. Puskas attributed this high failure rate to the need to remove all adventitia tissue and fat from the harvested saphenous vein segments before grafting, a step required to allow the vein conduit to fit inside the wire sheath. The potential exists to further optimize this step, he said in an interview.
“I was very surprised by the low 12-month patency rates” in both treatment arms of the study, commented Joanna Chikwe, MD, chair of cardiac surgery at Cedars-Sinai Medical Center in Los Angeles.
External scaffold to counter blood pressure
The concept behind the external buttressing sheath is that the walls of saphenous vein grafts are not structured to accommodate arterial blood pressure, and over time this pressure produces accelerated atherosclerotic changes and premature occlusion and graft failure. The external support is supposed to impede vein wall dilatation, reduce irregularities of the inner lumen surface, and improve hemodynamics and shear stress.
The VEST trial ran at 14 U.S. and 3 Canadian centers and enrolled 224 patients scheduled for coronary artery bypass grafting with planned use of at least two saphenous vein grafts, along with an internal mammary artery graft for the left anterior descending coronary artery. The patients averaged 66 years of age, 21% were women, and 51% had diabetes.
All patients successfully underwent their surgery, with 203 returning after 12 months for their primary follow-up examination by intravascular ultrasound. However, because of the high rate of vein occlusion or development of diffuse intragraft disease, successful intravascular ultrasound (IVUS) examination of both vein grafts occurred in only 113 patients.
The IVUS examinations showed that the study’s primary endpoint, the intimal hyperplasia area in all 224 patients who received vein grafts, averaged 5.11 mm2 in the grafts placed within the wire sleeve and 5.79 mm2 for control grafts not placed in the wire sheath, a difference that fell short of significance (P = .072). However, in a sensitivity analysis that focused on only the 113 patients who had both vein grafts successfully assayed by IVUS, the average area of intimal hyperplasia was 4.58 mm2 in the grafts within a wire sheath and 5.12 mm2 in the control grafts, a significant difference (P = .043).
The combined rate of major adverse cardiovascular events after 12 months was 7%, including a 2% mortality rate, a 3% stroke rate, and 3% rate of Mis, outcomes that suggested “no safety signals,” said Dr. Puskas, chair of cardiovascular surgery at Mount Sinai St. Luke’s in New York.
Although a large body of evidence has shown the superiority of arterial grafts for long-term graft patency, vein grafts have many advantages that have maintained them as the most widely used conduits worldwide for coronary artery bypass surgery, Dr. Puskas said.
Saphenous vein segments are readily available from patients and easy to harvest; they nicely conform to the coronary arteries that require bypass, rarely leak, are easy to work with, and can successfully hold stitches. Surgeons performing coronary artery bypass are unlikely to abandon vein grafts anytime soon, which makes improving the performance of vein grafts a priority, Dr. Puskas said.
The study was sponsored by Vascular Graft Solutions, the company developing the venous graft external support. Dr. Puskas and Dr. Chikwe had no disclosures related to the study.
A novel, stent-shaped device that provides external buttressing to saphenous vein grafts placed during coronary artery bypass surgery was safe, but failed to improve 12-month patency of vein grafts, in a prospective study with 224 patients.
Despite the neutral result, “we are cautiously optimistic” about the prospects for the device to reduce the risk for failure of coronary vein grafts caused by intimal hyperplasia of the internal lining of the vein graft that leads to graft occlusion, said John D. Puskas, MD, lead investigator of the study, who reported the results at the American Heart Association scientific sessions.
In the trial, called VEST, each buttressed vein graft was compared with a similar, unbuttressed graft in the same patient. Perhaps the biggest issue faced by the study was the unexpectedly high 42% rate of vein-graft occlusion or diffuse disease seen in the studied grafts 12 months after placement. This rate included both the vein grafts placed within the external buttressing device and control vein grafts that underwent the same postharvest preparation but weren’t placed within an external sheath, which is formed from woven cobalt chromium wire.
Dr. Puskas attributed this high failure rate to the need to remove all adventitia tissue and fat from the harvested saphenous vein segments before grafting, a step required to allow the vein conduit to fit inside the wire sheath. The potential exists to further optimize this step, he said in an interview.
“I was very surprised by the low 12-month patency rates” in both treatment arms of the study, commented Joanna Chikwe, MD, chair of cardiac surgery at Cedars-Sinai Medical Center in Los Angeles.
External scaffold to counter blood pressure
The concept behind the external buttressing sheath is that the walls of saphenous vein grafts are not structured to accommodate arterial blood pressure, and over time this pressure produces accelerated atherosclerotic changes and premature occlusion and graft failure. The external support is supposed to impede vein wall dilatation, reduce irregularities of the inner lumen surface, and improve hemodynamics and shear stress.
The VEST trial ran at 14 U.S. and 3 Canadian centers and enrolled 224 patients scheduled for coronary artery bypass grafting with planned use of at least two saphenous vein grafts, along with an internal mammary artery graft for the left anterior descending coronary artery. The patients averaged 66 years of age, 21% were women, and 51% had diabetes.
All patients successfully underwent their surgery, with 203 returning after 12 months for their primary follow-up examination by intravascular ultrasound. However, because of the high rate of vein occlusion or development of diffuse intragraft disease, successful intravascular ultrasound (IVUS) examination of both vein grafts occurred in only 113 patients.
The IVUS examinations showed that the study’s primary endpoint, the intimal hyperplasia area in all 224 patients who received vein grafts, averaged 5.11 mm2 in the grafts placed within the wire sleeve and 5.79 mm2 for control grafts not placed in the wire sheath, a difference that fell short of significance (P = .072). However, in a sensitivity analysis that focused on only the 113 patients who had both vein grafts successfully assayed by IVUS, the average area of intimal hyperplasia was 4.58 mm2 in the grafts within a wire sheath and 5.12 mm2 in the control grafts, a significant difference (P = .043).
The combined rate of major adverse cardiovascular events after 12 months was 7%, including a 2% mortality rate, a 3% stroke rate, and 3% rate of Mis, outcomes that suggested “no safety signals,” said Dr. Puskas, chair of cardiovascular surgery at Mount Sinai St. Luke’s in New York.
Although a large body of evidence has shown the superiority of arterial grafts for long-term graft patency, vein grafts have many advantages that have maintained them as the most widely used conduits worldwide for coronary artery bypass surgery, Dr. Puskas said.
Saphenous vein segments are readily available from patients and easy to harvest; they nicely conform to the coronary arteries that require bypass, rarely leak, are easy to work with, and can successfully hold stitches. Surgeons performing coronary artery bypass are unlikely to abandon vein grafts anytime soon, which makes improving the performance of vein grafts a priority, Dr. Puskas said.
The study was sponsored by Vascular Graft Solutions, the company developing the venous graft external support. Dr. Puskas and Dr. Chikwe had no disclosures related to the study.
FROM AHA 2021
FDA panel slams Endologix response to stent-graft safety issues
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
AHA 2021 puts scientific dialogue, health equity center stage
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
At 5 years, iFR found as effective and safe as FFR for guiding PCI intervention
The rate of major adverse cardiac events (MACE) over 5 years is similar whether revascularization is guided by instantaneous wave-free ratio (iFR) or fractional flow reserve (FFR), according to long-term results of the iFR-SWEDEHEART study.
“The results are about the same as reported at 12 months. There were no significant differences in any outcome we evaluated,” according to Matthias Götberg, MD, PhD.
When the initial results of the noninferiority iFR-SWEDEHEART trial were published after 1 year of follow-up, the primary MACE endpoint of death from any-cause nonfatal myocardial infarction, or unplanned revascularization, was met by 6.7% and 6.1% of those randomized to iFR or FFR, respectively.
These outcomes were not significantly different and placed iFR well within the predefined boundaries of noninferiority (P = .007).
In this new and final follow-up of iFR-SWEDEHEART, which evaluated the same 2,019 patients who were alive at 1 year (none were lost to follow-up), the MACE endpoint was met by 21.5% and 19.9% of those managed with iFR and FFR, respectively. The hazard ratio (1.09) had a wide 95% confidence interval (0.90-1.31) that did not approach statistical significance.
No differences seen across outcomes
When broken down into the MACE components, there were no differences between iFR and FFR, respectively, for all-cause death (9.4% vs. 7.9%), MI (5.8% vs. 5.7%) or unplanned revascularization (11.6% vs. 11.3%).
Across predefined subgroups, such as those defined by age, gender, stable versus unstable angina, and presence of risk factors such as diabetes, hypertension, hyperlipidemia, and smoking, there were also no significant differences in outcome.
At the time iFR-SWEDEHART was initiated, FFR had already been accepted as more effective than angiographic assessment to identify lesion ischemia and the need for percutaneous intervention (PCI). The iFR-SWEDEHEART trial tested iFR, a relatively new technology at the time, as a noninferior alternative. Unlike FFR, which requires adenosine to dilate the vessel, adding cost and patient discomfort, iFR measures the resting pressure gradient across the coronary lesion, and it is generally easier to perform.
“The advantage of iFR is that it provides an instantaneous lesion assessment without the need for adenosine,” Dr. Götberg explained in presenting the results at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
When the procedural results were compared in the published study at 1 year, it was noted that the mean number of lesions evaluated per patient was higher (1.55 vs. 1.43; P = .002), but the proportion of lesions found functionally significant was lower (29.2% vs. 36.8%; P < .0001) among those randomized to iFR than in the FFR group.
While most other procedural characteristics, such as PCI access route, fluoroscopy time, and contrast use did not differ significantly, fewer stents were placed in patients managed with iFR (1.58 vs. 1.73; P = .048), and a reduction in the average procedural time of a few minutes approached significance (P = .09).
Patient discomfort is greater with FFR
Patient discomfort measured during the procedure did differ, according to Dr. Götberg, an interventional cardiologist at Skåne University Hospital, Lund, Sweden.
Only about 30% in the FFR group reported no discomfort. Most of the others reported mild or moderate discomfort, but nearly 10% characterized the discomfort as severe. In the iFR group, more than 95% reported no discomfort. All of the remaining patients reported discomfort level as mild.
Because differences in MACE would be most likely to occur in the first year after revascularization, the similarity of the 1- and 5-year results were expected, according to Dr. Götberg. However, a 5-year follow-up was considered prudent given the relatively limited experience with iFR when the study was designed. This technique is now well established and widely used.
The study supports the premise that quicker and easier-to-obtain results with iFR are obtained without sacrificing greater relative risk of failing to identify a vulnerable lesion, according to Dr. Götberg.
Nevertheless, iFR and FFR “are not an exact match,” according to Jennifer A. Rymer, MD, an interventional cardiologist and assistant professor of medicine at Duke University, Durham, N.C. Although she called this trial an “excellent” demonstration of comparable utility in distinguishing lesions that do not require intervention from those that do, she implied that some clinicians might still prefer FFR for other reasons.
For example, FFR provides information about coronary flow reserve and microvascular resistance that are relevant to the underlying pathophysiology in a diseased vessel, according to Shmuel Banai, MD, head of interventional cardiology, Tel Aviv Medical Center. Recognizing that this information is not as readily generated by iFR, he is among those who plan to continue to use FFR despite these results.
However, for those who are now routinely performing iFR for the purposes of guiding revascularization, “these data are reassuring,” said David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta. The 5-year data essentially eliminate the likelihood that iFR relative to FFR increases the risk of missing functionally significant lesions for revascularization procedures.
Dr. Götberg reports financial relationships with Abbott, Boston Scientific, Medtronic, and Phillips Healthcare. Dr. Rymer reports no potential financial conflicts of interest. Dr. Banai has a financial relationship with Neovasc. Dr. Kandzari reports financial relationships with Ablative Solutions and Medtronic.
The rate of major adverse cardiac events (MACE) over 5 years is similar whether revascularization is guided by instantaneous wave-free ratio (iFR) or fractional flow reserve (FFR), according to long-term results of the iFR-SWEDEHEART study.
“The results are about the same as reported at 12 months. There were no significant differences in any outcome we evaluated,” according to Matthias Götberg, MD, PhD.
When the initial results of the noninferiority iFR-SWEDEHEART trial were published after 1 year of follow-up, the primary MACE endpoint of death from any-cause nonfatal myocardial infarction, or unplanned revascularization, was met by 6.7% and 6.1% of those randomized to iFR or FFR, respectively.
These outcomes were not significantly different and placed iFR well within the predefined boundaries of noninferiority (P = .007).
In this new and final follow-up of iFR-SWEDEHEART, which evaluated the same 2,019 patients who were alive at 1 year (none were lost to follow-up), the MACE endpoint was met by 21.5% and 19.9% of those managed with iFR and FFR, respectively. The hazard ratio (1.09) had a wide 95% confidence interval (0.90-1.31) that did not approach statistical significance.
No differences seen across outcomes
When broken down into the MACE components, there were no differences between iFR and FFR, respectively, for all-cause death (9.4% vs. 7.9%), MI (5.8% vs. 5.7%) or unplanned revascularization (11.6% vs. 11.3%).
Across predefined subgroups, such as those defined by age, gender, stable versus unstable angina, and presence of risk factors such as diabetes, hypertension, hyperlipidemia, and smoking, there were also no significant differences in outcome.
At the time iFR-SWEDEHART was initiated, FFR had already been accepted as more effective than angiographic assessment to identify lesion ischemia and the need for percutaneous intervention (PCI). The iFR-SWEDEHEART trial tested iFR, a relatively new technology at the time, as a noninferior alternative. Unlike FFR, which requires adenosine to dilate the vessel, adding cost and patient discomfort, iFR measures the resting pressure gradient across the coronary lesion, and it is generally easier to perform.
“The advantage of iFR is that it provides an instantaneous lesion assessment without the need for adenosine,” Dr. Götberg explained in presenting the results at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
When the procedural results were compared in the published study at 1 year, it was noted that the mean number of lesions evaluated per patient was higher (1.55 vs. 1.43; P = .002), but the proportion of lesions found functionally significant was lower (29.2% vs. 36.8%; P < .0001) among those randomized to iFR than in the FFR group.
While most other procedural characteristics, such as PCI access route, fluoroscopy time, and contrast use did not differ significantly, fewer stents were placed in patients managed with iFR (1.58 vs. 1.73; P = .048), and a reduction in the average procedural time of a few minutes approached significance (P = .09).
Patient discomfort is greater with FFR
Patient discomfort measured during the procedure did differ, according to Dr. Götberg, an interventional cardiologist at Skåne University Hospital, Lund, Sweden.
Only about 30% in the FFR group reported no discomfort. Most of the others reported mild or moderate discomfort, but nearly 10% characterized the discomfort as severe. In the iFR group, more than 95% reported no discomfort. All of the remaining patients reported discomfort level as mild.
Because differences in MACE would be most likely to occur in the first year after revascularization, the similarity of the 1- and 5-year results were expected, according to Dr. Götberg. However, a 5-year follow-up was considered prudent given the relatively limited experience with iFR when the study was designed. This technique is now well established and widely used.
The study supports the premise that quicker and easier-to-obtain results with iFR are obtained without sacrificing greater relative risk of failing to identify a vulnerable lesion, according to Dr. Götberg.
Nevertheless, iFR and FFR “are not an exact match,” according to Jennifer A. Rymer, MD, an interventional cardiologist and assistant professor of medicine at Duke University, Durham, N.C. Although she called this trial an “excellent” demonstration of comparable utility in distinguishing lesions that do not require intervention from those that do, she implied that some clinicians might still prefer FFR for other reasons.
For example, FFR provides information about coronary flow reserve and microvascular resistance that are relevant to the underlying pathophysiology in a diseased vessel, according to Shmuel Banai, MD, head of interventional cardiology, Tel Aviv Medical Center. Recognizing that this information is not as readily generated by iFR, he is among those who plan to continue to use FFR despite these results.
However, for those who are now routinely performing iFR for the purposes of guiding revascularization, “these data are reassuring,” said David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta. The 5-year data essentially eliminate the likelihood that iFR relative to FFR increases the risk of missing functionally significant lesions for revascularization procedures.
Dr. Götberg reports financial relationships with Abbott, Boston Scientific, Medtronic, and Phillips Healthcare. Dr. Rymer reports no potential financial conflicts of interest. Dr. Banai has a financial relationship with Neovasc. Dr. Kandzari reports financial relationships with Ablative Solutions and Medtronic.
The rate of major adverse cardiac events (MACE) over 5 years is similar whether revascularization is guided by instantaneous wave-free ratio (iFR) or fractional flow reserve (FFR), according to long-term results of the iFR-SWEDEHEART study.
“The results are about the same as reported at 12 months. There were no significant differences in any outcome we evaluated,” according to Matthias Götberg, MD, PhD.
When the initial results of the noninferiority iFR-SWEDEHEART trial were published after 1 year of follow-up, the primary MACE endpoint of death from any-cause nonfatal myocardial infarction, or unplanned revascularization, was met by 6.7% and 6.1% of those randomized to iFR or FFR, respectively.
These outcomes were not significantly different and placed iFR well within the predefined boundaries of noninferiority (P = .007).
In this new and final follow-up of iFR-SWEDEHEART, which evaluated the same 2,019 patients who were alive at 1 year (none were lost to follow-up), the MACE endpoint was met by 21.5% and 19.9% of those managed with iFR and FFR, respectively. The hazard ratio (1.09) had a wide 95% confidence interval (0.90-1.31) that did not approach statistical significance.
No differences seen across outcomes
When broken down into the MACE components, there were no differences between iFR and FFR, respectively, for all-cause death (9.4% vs. 7.9%), MI (5.8% vs. 5.7%) or unplanned revascularization (11.6% vs. 11.3%).
Across predefined subgroups, such as those defined by age, gender, stable versus unstable angina, and presence of risk factors such as diabetes, hypertension, hyperlipidemia, and smoking, there were also no significant differences in outcome.
At the time iFR-SWEDEHART was initiated, FFR had already been accepted as more effective than angiographic assessment to identify lesion ischemia and the need for percutaneous intervention (PCI). The iFR-SWEDEHEART trial tested iFR, a relatively new technology at the time, as a noninferior alternative. Unlike FFR, which requires adenosine to dilate the vessel, adding cost and patient discomfort, iFR measures the resting pressure gradient across the coronary lesion, and it is generally easier to perform.
“The advantage of iFR is that it provides an instantaneous lesion assessment without the need for adenosine,” Dr. Götberg explained in presenting the results at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
When the procedural results were compared in the published study at 1 year, it was noted that the mean number of lesions evaluated per patient was higher (1.55 vs. 1.43; P = .002), but the proportion of lesions found functionally significant was lower (29.2% vs. 36.8%; P < .0001) among those randomized to iFR than in the FFR group.
While most other procedural characteristics, such as PCI access route, fluoroscopy time, and contrast use did not differ significantly, fewer stents were placed in patients managed with iFR (1.58 vs. 1.73; P = .048), and a reduction in the average procedural time of a few minutes approached significance (P = .09).
Patient discomfort is greater with FFR
Patient discomfort measured during the procedure did differ, according to Dr. Götberg, an interventional cardiologist at Skåne University Hospital, Lund, Sweden.
Only about 30% in the FFR group reported no discomfort. Most of the others reported mild or moderate discomfort, but nearly 10% characterized the discomfort as severe. In the iFR group, more than 95% reported no discomfort. All of the remaining patients reported discomfort level as mild.
Because differences in MACE would be most likely to occur in the first year after revascularization, the similarity of the 1- and 5-year results were expected, according to Dr. Götberg. However, a 5-year follow-up was considered prudent given the relatively limited experience with iFR when the study was designed. This technique is now well established and widely used.
The study supports the premise that quicker and easier-to-obtain results with iFR are obtained without sacrificing greater relative risk of failing to identify a vulnerable lesion, according to Dr. Götberg.
Nevertheless, iFR and FFR “are not an exact match,” according to Jennifer A. Rymer, MD, an interventional cardiologist and assistant professor of medicine at Duke University, Durham, N.C. Although she called this trial an “excellent” demonstration of comparable utility in distinguishing lesions that do not require intervention from those that do, she implied that some clinicians might still prefer FFR for other reasons.
For example, FFR provides information about coronary flow reserve and microvascular resistance that are relevant to the underlying pathophysiology in a diseased vessel, according to Shmuel Banai, MD, head of interventional cardiology, Tel Aviv Medical Center. Recognizing that this information is not as readily generated by iFR, he is among those who plan to continue to use FFR despite these results.
However, for those who are now routinely performing iFR for the purposes of guiding revascularization, “these data are reassuring,” said David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta. The 5-year data essentially eliminate the likelihood that iFR relative to FFR increases the risk of missing functionally significant lesions for revascularization procedures.
Dr. Götberg reports financial relationships with Abbott, Boston Scientific, Medtronic, and Phillips Healthcare. Dr. Rymer reports no potential financial conflicts of interest. Dr. Banai has a financial relationship with Neovasc. Dr. Kandzari reports financial relationships with Ablative Solutions and Medtronic.
FROM TCT 2021
Short DAPT course beneficial after PCI in ‘bi-risk’ patients
Ischemic events not increased
Just months after the MASTER DAPT trial showed that abbreviated dual-antiplatelet therapy (DAPT) lowers the risk of bleeding after stent placement in patients at high bleeding risk, a new analysis showed the favorable benefit-to-risk ratio was about the same in the subgroup who also had an acute or recent myocardial infarction.
In the new prespecified MASTER DAPT analysis, the data show that the subgroup with both an increased bleeding risk and an increased risk of ischemic events benefited much like the entire study population from a shorter DAPT duration, reported Pieter C. Smits, MD, PhD, at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
“There was no signal towards increased ischemic risk in the abbreviated DAPT population presenting with recent acute MI,” said Dr. Smits, emphasizing the consistency of results in this “bi-risk” subgroup with objective criteria for increased risks of bleeding and ischemic events.
MASTER DAPT main results published
The main results of the MASTER DAPT trial were presented at the 2021 annual meeting of the European Society of Cardiology and published recently in the New England Journal of Medicine. The trial randomized 4,434 patients who met one or more criteria for high bleeding risk. These included age of at least 75 years, documented anemia, a clinical indication for oral anticoagulants, and previous bleeding episodes requiring hospitalization.
In the trial, all patients were maintained on DAPT for 1 month after implantation of a biodegradable-polymer, sirolimus-eluting coronary stent (Ultimaster, Terumo). At the end of the month, those randomized to abbreviated DAPT started immediately on single-agent antiplatelet therapy, while those in the standard DAPT group remained on DAPT for at least 2 additional months.
Over 1 year of follow-up, the bleeding event rate was lower in the abbreviated DAPT group (6.5% vs. 9.4%; P < .0001 for superiority). The slight increase in major ischemic events among those in the abbreviated DAPT group (6.1% vs. 5.9%) was not significantly different (P = .001 for noninferiority).
When compared on the basis of net adverse clinical events (NACE), which comprised all-case death, MI, stroke, or Bleeding Academic Research Consortium (BARC) level 3 or 5 bleeding, there was a slight advantage for abbreviated DAPT (7.5% vs. 7.7%). This did not reach significance, but it was similar (P < .001 for noninferiority), favoring the abbreviated course of DAPT because of the bleeding advantage.
Recent MI vs. no MI
In the new analysis, patients in both the abbreviated and standard DAPT group were stratified into those with no major cardiovascular event within the past 12 months and those with an acute MI or acute coronary syndrome within this time. There were somewhat more patients without a history of MI within the previous 12 months in both the abbreviated DAPT (1,381 vs. 914 patients) and standard DAPT (1,418 vs. 866) groups.
In those without a recent MI, NACE rates were nearly identical over 1-year follow-up for those who received abbreviated versus standard DAPT. In both, slightly more than 6% had a NACE event, producing a hazard ratio of 1.03 for abbreviated versus standard DAPT (P = 0.85).
For those with a recent MI, event rates began to separate within 30 days. By 1 year, NACE rates exceeded 10% in those on standard DAPT, but remained below 9% for those on abbreviated DAPT. The lower hazard ratio in the abbreviated DAPT group (HR, 0.83; P = .22) did not reach statistical significance, but it did echo the larger MASTER DAPT conclusion.
“An abbreviated DAPT strategy significantly reduced clinically relevant bleeding risk in these bi-risk patients without increasing risk of ischemic events,” reported Dr. Smits, director of interventional cardiology at Maasstad Hospital, Rotterdam, the Netherlands.
No difference in NACE components
In fact, when the components of NACE were evaluated individually in the subgroup of patients with prior MI, both stroke (HR, 0.47; P = .16) and all-cause death (HR, 0.78; P = .28), although not significant, numerically favored abbreviated DAPT.
There was no difference between abbreviated and standard DAPT for risk of MI at 1 year (HR, 1.03; P = .92).
As in the overall MASTER DAPT results, bleeding risk (BARC 2, 3, or 5 bleeding) was significantly reduced in the substudy among those with a recent prior MI (P = .013) or those with no MI in the prior 12 months (P = .01).
In MASTER DAPT, which was an open-label study that randomized participants in 30 countries, all patients received one type of drug-eluting stent. While Dr. Smits conceded that it is not clear whether the conclusions about abbreviated DAPT can be extrapolated to other stents, he noted that recent long-term outcomes for modern drug-eluting coronary stents have been similar, suggesting these results might be more broadly applicable.
According to Dr. Smit, the consistency of this subgroup analysis with the previously published MASTER DAPT study is mutually reinforcing for a role of abbreviated DAPT in patients at high bleeding risk. Other experts agreed.
“One of the concerns that people have had is exactly what has been addressed here in this subgroup analysis. These are the patients that are not only bleeding-risk high but ischemic-risk high. The question was whether the benefit of reducing bleeding risk is offset by increasing stent thrombosis or other ischemic event outcomes, and the answer from the analysis is really clearly no,” said Philippe Gabriel Steg, MD, chief, department of cardiology, Hôpital Bichat, Paris, at the meeting, sponsored by the Cardiovascular Research Foundation.
Dr. Smits reports financial relationships with Abiomed, Abbott Vascular, Daiichi-Sankyo, Microport, Opsense, and Terumo Medical. Dr. Steg reports financial relationships with Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Idorsia, Merck, Novartis, Regeneron, and Sanofi-Aventis.
Ischemic events not increased
Ischemic events not increased
Just months after the MASTER DAPT trial showed that abbreviated dual-antiplatelet therapy (DAPT) lowers the risk of bleeding after stent placement in patients at high bleeding risk, a new analysis showed the favorable benefit-to-risk ratio was about the same in the subgroup who also had an acute or recent myocardial infarction.
In the new prespecified MASTER DAPT analysis, the data show that the subgroup with both an increased bleeding risk and an increased risk of ischemic events benefited much like the entire study population from a shorter DAPT duration, reported Pieter C. Smits, MD, PhD, at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
“There was no signal towards increased ischemic risk in the abbreviated DAPT population presenting with recent acute MI,” said Dr. Smits, emphasizing the consistency of results in this “bi-risk” subgroup with objective criteria for increased risks of bleeding and ischemic events.
MASTER DAPT main results published
The main results of the MASTER DAPT trial were presented at the 2021 annual meeting of the European Society of Cardiology and published recently in the New England Journal of Medicine. The trial randomized 4,434 patients who met one or more criteria for high bleeding risk. These included age of at least 75 years, documented anemia, a clinical indication for oral anticoagulants, and previous bleeding episodes requiring hospitalization.
In the trial, all patients were maintained on DAPT for 1 month after implantation of a biodegradable-polymer, sirolimus-eluting coronary stent (Ultimaster, Terumo). At the end of the month, those randomized to abbreviated DAPT started immediately on single-agent antiplatelet therapy, while those in the standard DAPT group remained on DAPT for at least 2 additional months.
Over 1 year of follow-up, the bleeding event rate was lower in the abbreviated DAPT group (6.5% vs. 9.4%; P < .0001 for superiority). The slight increase in major ischemic events among those in the abbreviated DAPT group (6.1% vs. 5.9%) was not significantly different (P = .001 for noninferiority).
When compared on the basis of net adverse clinical events (NACE), which comprised all-case death, MI, stroke, or Bleeding Academic Research Consortium (BARC) level 3 or 5 bleeding, there was a slight advantage for abbreviated DAPT (7.5% vs. 7.7%). This did not reach significance, but it was similar (P < .001 for noninferiority), favoring the abbreviated course of DAPT because of the bleeding advantage.
Recent MI vs. no MI
In the new analysis, patients in both the abbreviated and standard DAPT group were stratified into those with no major cardiovascular event within the past 12 months and those with an acute MI or acute coronary syndrome within this time. There were somewhat more patients without a history of MI within the previous 12 months in both the abbreviated DAPT (1,381 vs. 914 patients) and standard DAPT (1,418 vs. 866) groups.
In those without a recent MI, NACE rates were nearly identical over 1-year follow-up for those who received abbreviated versus standard DAPT. In both, slightly more than 6% had a NACE event, producing a hazard ratio of 1.03 for abbreviated versus standard DAPT (P = 0.85).
For those with a recent MI, event rates began to separate within 30 days. By 1 year, NACE rates exceeded 10% in those on standard DAPT, but remained below 9% for those on abbreviated DAPT. The lower hazard ratio in the abbreviated DAPT group (HR, 0.83; P = .22) did not reach statistical significance, but it did echo the larger MASTER DAPT conclusion.
“An abbreviated DAPT strategy significantly reduced clinically relevant bleeding risk in these bi-risk patients without increasing risk of ischemic events,” reported Dr. Smits, director of interventional cardiology at Maasstad Hospital, Rotterdam, the Netherlands.
No difference in NACE components
In fact, when the components of NACE were evaluated individually in the subgroup of patients with prior MI, both stroke (HR, 0.47; P = .16) and all-cause death (HR, 0.78; P = .28), although not significant, numerically favored abbreviated DAPT.
There was no difference between abbreviated and standard DAPT for risk of MI at 1 year (HR, 1.03; P = .92).
As in the overall MASTER DAPT results, bleeding risk (BARC 2, 3, or 5 bleeding) was significantly reduced in the substudy among those with a recent prior MI (P = .013) or those with no MI in the prior 12 months (P = .01).
In MASTER DAPT, which was an open-label study that randomized participants in 30 countries, all patients received one type of drug-eluting stent. While Dr. Smits conceded that it is not clear whether the conclusions about abbreviated DAPT can be extrapolated to other stents, he noted that recent long-term outcomes for modern drug-eluting coronary stents have been similar, suggesting these results might be more broadly applicable.
According to Dr. Smit, the consistency of this subgroup analysis with the previously published MASTER DAPT study is mutually reinforcing for a role of abbreviated DAPT in patients at high bleeding risk. Other experts agreed.
“One of the concerns that people have had is exactly what has been addressed here in this subgroup analysis. These are the patients that are not only bleeding-risk high but ischemic-risk high. The question was whether the benefit of reducing bleeding risk is offset by increasing stent thrombosis or other ischemic event outcomes, and the answer from the analysis is really clearly no,” said Philippe Gabriel Steg, MD, chief, department of cardiology, Hôpital Bichat, Paris, at the meeting, sponsored by the Cardiovascular Research Foundation.
Dr. Smits reports financial relationships with Abiomed, Abbott Vascular, Daiichi-Sankyo, Microport, Opsense, and Terumo Medical. Dr. Steg reports financial relationships with Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Idorsia, Merck, Novartis, Regeneron, and Sanofi-Aventis.
Just months after the MASTER DAPT trial showed that abbreviated dual-antiplatelet therapy (DAPT) lowers the risk of bleeding after stent placement in patients at high bleeding risk, a new analysis showed the favorable benefit-to-risk ratio was about the same in the subgroup who also had an acute or recent myocardial infarction.
In the new prespecified MASTER DAPT analysis, the data show that the subgroup with both an increased bleeding risk and an increased risk of ischemic events benefited much like the entire study population from a shorter DAPT duration, reported Pieter C. Smits, MD, PhD, at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
“There was no signal towards increased ischemic risk in the abbreviated DAPT population presenting with recent acute MI,” said Dr. Smits, emphasizing the consistency of results in this “bi-risk” subgroup with objective criteria for increased risks of bleeding and ischemic events.
MASTER DAPT main results published
The main results of the MASTER DAPT trial were presented at the 2021 annual meeting of the European Society of Cardiology and published recently in the New England Journal of Medicine. The trial randomized 4,434 patients who met one or more criteria for high bleeding risk. These included age of at least 75 years, documented anemia, a clinical indication for oral anticoagulants, and previous bleeding episodes requiring hospitalization.
In the trial, all patients were maintained on DAPT for 1 month after implantation of a biodegradable-polymer, sirolimus-eluting coronary stent (Ultimaster, Terumo). At the end of the month, those randomized to abbreviated DAPT started immediately on single-agent antiplatelet therapy, while those in the standard DAPT group remained on DAPT for at least 2 additional months.
Over 1 year of follow-up, the bleeding event rate was lower in the abbreviated DAPT group (6.5% vs. 9.4%; P < .0001 for superiority). The slight increase in major ischemic events among those in the abbreviated DAPT group (6.1% vs. 5.9%) was not significantly different (P = .001 for noninferiority).
When compared on the basis of net adverse clinical events (NACE), which comprised all-case death, MI, stroke, or Bleeding Academic Research Consortium (BARC) level 3 or 5 bleeding, there was a slight advantage for abbreviated DAPT (7.5% vs. 7.7%). This did not reach significance, but it was similar (P < .001 for noninferiority), favoring the abbreviated course of DAPT because of the bleeding advantage.
Recent MI vs. no MI
In the new analysis, patients in both the abbreviated and standard DAPT group were stratified into those with no major cardiovascular event within the past 12 months and those with an acute MI or acute coronary syndrome within this time. There were somewhat more patients without a history of MI within the previous 12 months in both the abbreviated DAPT (1,381 vs. 914 patients) and standard DAPT (1,418 vs. 866) groups.
In those without a recent MI, NACE rates were nearly identical over 1-year follow-up for those who received abbreviated versus standard DAPT. In both, slightly more than 6% had a NACE event, producing a hazard ratio of 1.03 for abbreviated versus standard DAPT (P = 0.85).
For those with a recent MI, event rates began to separate within 30 days. By 1 year, NACE rates exceeded 10% in those on standard DAPT, but remained below 9% for those on abbreviated DAPT. The lower hazard ratio in the abbreviated DAPT group (HR, 0.83; P = .22) did not reach statistical significance, but it did echo the larger MASTER DAPT conclusion.
“An abbreviated DAPT strategy significantly reduced clinically relevant bleeding risk in these bi-risk patients without increasing risk of ischemic events,” reported Dr. Smits, director of interventional cardiology at Maasstad Hospital, Rotterdam, the Netherlands.
No difference in NACE components
In fact, when the components of NACE were evaluated individually in the subgroup of patients with prior MI, both stroke (HR, 0.47; P = .16) and all-cause death (HR, 0.78; P = .28), although not significant, numerically favored abbreviated DAPT.
There was no difference between abbreviated and standard DAPT for risk of MI at 1 year (HR, 1.03; P = .92).
As in the overall MASTER DAPT results, bleeding risk (BARC 2, 3, or 5 bleeding) was significantly reduced in the substudy among those with a recent prior MI (P = .013) or those with no MI in the prior 12 months (P = .01).
In MASTER DAPT, which was an open-label study that randomized participants in 30 countries, all patients received one type of drug-eluting stent. While Dr. Smits conceded that it is not clear whether the conclusions about abbreviated DAPT can be extrapolated to other stents, he noted that recent long-term outcomes for modern drug-eluting coronary stents have been similar, suggesting these results might be more broadly applicable.
According to Dr. Smit, the consistency of this subgroup analysis with the previously published MASTER DAPT study is mutually reinforcing for a role of abbreviated DAPT in patients at high bleeding risk. Other experts agreed.
“One of the concerns that people have had is exactly what has been addressed here in this subgroup analysis. These are the patients that are not only bleeding-risk high but ischemic-risk high. The question was whether the benefit of reducing bleeding risk is offset by increasing stent thrombosis or other ischemic event outcomes, and the answer from the analysis is really clearly no,” said Philippe Gabriel Steg, MD, chief, department of cardiology, Hôpital Bichat, Paris, at the meeting, sponsored by the Cardiovascular Research Foundation.
Dr. Smits reports financial relationships with Abiomed, Abbott Vascular, Daiichi-Sankyo, Microport, Opsense, and Terumo Medical. Dr. Steg reports financial relationships with Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Idorsia, Merck, Novartis, Regeneron, and Sanofi-Aventis.
FROM TCT 2021
FAVOR III China: QFR-guided PCI shows advantage over angiography
Percutaneous coronary intervention (PCI) guided by quantitative flow ratio (QFR) lesion assessment provided better clinical outcomes than visual assessment of the angiogram in the sham-controlled FAVOR III China study.
PCI success rates were about 95% with both strategies; however, QFR guidance was associated with fewer major adverse cardiac events (MACE) at 1 year, use of fewer stents, less contrast medium exposure, and fewer procedural complications.
“The simplicity and safety of QFR compared with wire-based physiologic measurements should facilitate the adoption of physiologic lesion assessment into routine clinical practice,” co–primary investigator Bo Xu, MBBS, Fuwai Hospital, Beijing, said.
The results were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held online and in Orlando, and published simultaneously in The Lancet.
Although pressure wire–based physiological assessment with fractional flow reserve (FFR) and instantaneous wave-free ratio (IFR) more accurately identify flow-limiting lesions than standard angiography and have been shown to improve outcomes after PCI, the authors note that it’s underused in practice because of prolonged procedural time, potential pressure wire complications, and side effects from hyperemic agents.
QFR, however, is derived from 3-dimensional coronary artery reconstruction and computational fluid dynamics from the angiogram, so FFR can be estimated without the need for a pressure wire or hyperemic drugs.
FAVOR III China was designed statistically for superiority and enrolled 3,847 patients with stable or unstable angina or a myocardial infarction (MI) at least 72 hours before screening if they had at least one coronary lesion with a diameter stenosis of 50% to 90% and a reference vessel diameter of at least 2.5 mm. The intention-to-treat population included 3,825 patients (mean age, 62.7 years; 29.4% female).
In the QFR group, QFR was measured in all coronary arteries with a lesion but PCI performed only in lesions with a QFR of at least 0.80 or diameter stenosis greater than 90%. Two angiographic imaging runs were taken and the data transmitted to the AngioPlus system (Pulse Medical Imaging Technology) by a local network of sites for QFR calculation.
PCI in the angiography-guided group was performed on the basis of visual angiographic assessment only. A 10-minute delay was used in both groups to preserve masking.
The primary endpoint of 1-year MACE, a composite of all-cause death, MI, or ischemia-driven revascularization, occurred in 5.8% of the QFR-guided group and 8.8% of the angiography-guided group (hazard ratio, 0.65; 95% CI, 0.51-0.83; P = .0004).
The curves separated within 48 hours, driven largely by fewer MIs (3.4% vs. 5.7%; P = .0008) and ischemia-driven revascularizations (2.0% vs. 3.1%; P = .0078) in the QFR-guided group, Mr. Xu said.
The major secondary endpoint of MACE excluding periprocedural MI occurred in 3.1% of QFR-guided patients and 4.8% of angiography-guided patients (HR, 0.64; 95% CI, 0.46-0.89; P = .0073).
The prerandomization revascularization plan was changed in 23.3% of patients with QFR and only 6.2% in the angiography group (P < .0001), mainly due to deferral of treatment of at least one vessel originally planned for PCI (19.6% vs. 5.2%; P < .0001).
“I think in the next guideline they will change the recommendation, not just to include FFR and IFR, but also to include QFR,” Giuseppe Tarantini, MD, PhD, University of Padua, Italy, said during a press briefing on the study.
“This is a milestone in our community, not only because it is easier to use compared to the other lesion-specific indexes like FFR, IFR, but also for the need to expand the use of physiology in the setting of interventional cardiology,” he added.
In an accompanying commentary, Robert A. Byrne, MBBCh, PhD, and Laurna McGovern, MBBCh, both from the Cardiovascular Research Institute Dublin, say the results are “relevant for cardiovascular disease researchers and clinicians and an important step forward for the field of angiography-derived flow measurements for guidance of PCI.”
They point out, however, that the control group did not receive pressure wire–guided PCI, which is the standard of care in contemporary practice and out of step with clinical practice guidelines, thus limiting external validity.
They also note that experiences to date suggest that up to 20% of patients may be unsuitable for the algorithm analysis because of coronary anatomy, presence of overlapping vessels, and insufficient image quality.
Commenting for this news organization, David E. Kandzari, MD, chief of the Piedmont Heart Institute, Atlanta, said “the technology isn’t readily available in catheterization labs today. Could it be assimilated into the cath labs at one point in the near term? I think absolutely, and that would be a welcome addition to expedite the procedure itself.”
Nevertheless, he said the results “need to be externally validated too, with what is the gold standard today of FFR in a larger experience.”
Session moderator Gregg W. Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said FAVOR III China has “advanced our knowledge” but pointed out that the ongoing randomized FAVOR III Europe Japan study is directly comparing QFR with invasive pressure-wire assessed FFR. The estimated primary completion date for that study is Dec. 31.
The study was supported by grants from the Beijing Municipal Science and Technology Commission, Chinese Academy of Medical Sciences, and the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital. Dr. Byrne reported institutional research or educational funding from Abbott Vascular, Biosensors, Biotronik, and Boston Scientific. Ms. McGovern has disclosed no relevant financial relationships. Dr. Kandzari reported minor consulting honoraria from the interventional device industry and institutional research grant support.
A version of this article first appeared on Medscape.com.
Percutaneous coronary intervention (PCI) guided by quantitative flow ratio (QFR) lesion assessment provided better clinical outcomes than visual assessment of the angiogram in the sham-controlled FAVOR III China study.
PCI success rates were about 95% with both strategies; however, QFR guidance was associated with fewer major adverse cardiac events (MACE) at 1 year, use of fewer stents, less contrast medium exposure, and fewer procedural complications.
“The simplicity and safety of QFR compared with wire-based physiologic measurements should facilitate the adoption of physiologic lesion assessment into routine clinical practice,” co–primary investigator Bo Xu, MBBS, Fuwai Hospital, Beijing, said.
The results were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held online and in Orlando, and published simultaneously in The Lancet.
Although pressure wire–based physiological assessment with fractional flow reserve (FFR) and instantaneous wave-free ratio (IFR) more accurately identify flow-limiting lesions than standard angiography and have been shown to improve outcomes after PCI, the authors note that it’s underused in practice because of prolonged procedural time, potential pressure wire complications, and side effects from hyperemic agents.
QFR, however, is derived from 3-dimensional coronary artery reconstruction and computational fluid dynamics from the angiogram, so FFR can be estimated without the need for a pressure wire or hyperemic drugs.
FAVOR III China was designed statistically for superiority and enrolled 3,847 patients with stable or unstable angina or a myocardial infarction (MI) at least 72 hours before screening if they had at least one coronary lesion with a diameter stenosis of 50% to 90% and a reference vessel diameter of at least 2.5 mm. The intention-to-treat population included 3,825 patients (mean age, 62.7 years; 29.4% female).
In the QFR group, QFR was measured in all coronary arteries with a lesion but PCI performed only in lesions with a QFR of at least 0.80 or diameter stenosis greater than 90%. Two angiographic imaging runs were taken and the data transmitted to the AngioPlus system (Pulse Medical Imaging Technology) by a local network of sites for QFR calculation.
PCI in the angiography-guided group was performed on the basis of visual angiographic assessment only. A 10-minute delay was used in both groups to preserve masking.
The primary endpoint of 1-year MACE, a composite of all-cause death, MI, or ischemia-driven revascularization, occurred in 5.8% of the QFR-guided group and 8.8% of the angiography-guided group (hazard ratio, 0.65; 95% CI, 0.51-0.83; P = .0004).
The curves separated within 48 hours, driven largely by fewer MIs (3.4% vs. 5.7%; P = .0008) and ischemia-driven revascularizations (2.0% vs. 3.1%; P = .0078) in the QFR-guided group, Mr. Xu said.
The major secondary endpoint of MACE excluding periprocedural MI occurred in 3.1% of QFR-guided patients and 4.8% of angiography-guided patients (HR, 0.64; 95% CI, 0.46-0.89; P = .0073).
The prerandomization revascularization plan was changed in 23.3% of patients with QFR and only 6.2% in the angiography group (P < .0001), mainly due to deferral of treatment of at least one vessel originally planned for PCI (19.6% vs. 5.2%; P < .0001).
“I think in the next guideline they will change the recommendation, not just to include FFR and IFR, but also to include QFR,” Giuseppe Tarantini, MD, PhD, University of Padua, Italy, said during a press briefing on the study.
“This is a milestone in our community, not only because it is easier to use compared to the other lesion-specific indexes like FFR, IFR, but also for the need to expand the use of physiology in the setting of interventional cardiology,” he added.
In an accompanying commentary, Robert A. Byrne, MBBCh, PhD, and Laurna McGovern, MBBCh, both from the Cardiovascular Research Institute Dublin, say the results are “relevant for cardiovascular disease researchers and clinicians and an important step forward for the field of angiography-derived flow measurements for guidance of PCI.”
They point out, however, that the control group did not receive pressure wire–guided PCI, which is the standard of care in contemporary practice and out of step with clinical practice guidelines, thus limiting external validity.
They also note that experiences to date suggest that up to 20% of patients may be unsuitable for the algorithm analysis because of coronary anatomy, presence of overlapping vessels, and insufficient image quality.
Commenting for this news organization, David E. Kandzari, MD, chief of the Piedmont Heart Institute, Atlanta, said “the technology isn’t readily available in catheterization labs today. Could it be assimilated into the cath labs at one point in the near term? I think absolutely, and that would be a welcome addition to expedite the procedure itself.”
Nevertheless, he said the results “need to be externally validated too, with what is the gold standard today of FFR in a larger experience.”
Session moderator Gregg W. Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said FAVOR III China has “advanced our knowledge” but pointed out that the ongoing randomized FAVOR III Europe Japan study is directly comparing QFR with invasive pressure-wire assessed FFR. The estimated primary completion date for that study is Dec. 31.
The study was supported by grants from the Beijing Municipal Science and Technology Commission, Chinese Academy of Medical Sciences, and the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital. Dr. Byrne reported institutional research or educational funding from Abbott Vascular, Biosensors, Biotronik, and Boston Scientific. Ms. McGovern has disclosed no relevant financial relationships. Dr. Kandzari reported minor consulting honoraria from the interventional device industry and institutional research grant support.
A version of this article first appeared on Medscape.com.
Percutaneous coronary intervention (PCI) guided by quantitative flow ratio (QFR) lesion assessment provided better clinical outcomes than visual assessment of the angiogram in the sham-controlled FAVOR III China study.
PCI success rates were about 95% with both strategies; however, QFR guidance was associated with fewer major adverse cardiac events (MACE) at 1 year, use of fewer stents, less contrast medium exposure, and fewer procedural complications.
“The simplicity and safety of QFR compared with wire-based physiologic measurements should facilitate the adoption of physiologic lesion assessment into routine clinical practice,” co–primary investigator Bo Xu, MBBS, Fuwai Hospital, Beijing, said.
The results were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held online and in Orlando, and published simultaneously in The Lancet.
Although pressure wire–based physiological assessment with fractional flow reserve (FFR) and instantaneous wave-free ratio (IFR) more accurately identify flow-limiting lesions than standard angiography and have been shown to improve outcomes after PCI, the authors note that it’s underused in practice because of prolonged procedural time, potential pressure wire complications, and side effects from hyperemic agents.
QFR, however, is derived from 3-dimensional coronary artery reconstruction and computational fluid dynamics from the angiogram, so FFR can be estimated without the need for a pressure wire or hyperemic drugs.
FAVOR III China was designed statistically for superiority and enrolled 3,847 patients with stable or unstable angina or a myocardial infarction (MI) at least 72 hours before screening if they had at least one coronary lesion with a diameter stenosis of 50% to 90% and a reference vessel diameter of at least 2.5 mm. The intention-to-treat population included 3,825 patients (mean age, 62.7 years; 29.4% female).
In the QFR group, QFR was measured in all coronary arteries with a lesion but PCI performed only in lesions with a QFR of at least 0.80 or diameter stenosis greater than 90%. Two angiographic imaging runs were taken and the data transmitted to the AngioPlus system (Pulse Medical Imaging Technology) by a local network of sites for QFR calculation.
PCI in the angiography-guided group was performed on the basis of visual angiographic assessment only. A 10-minute delay was used in both groups to preserve masking.
The primary endpoint of 1-year MACE, a composite of all-cause death, MI, or ischemia-driven revascularization, occurred in 5.8% of the QFR-guided group and 8.8% of the angiography-guided group (hazard ratio, 0.65; 95% CI, 0.51-0.83; P = .0004).
The curves separated within 48 hours, driven largely by fewer MIs (3.4% vs. 5.7%; P = .0008) and ischemia-driven revascularizations (2.0% vs. 3.1%; P = .0078) in the QFR-guided group, Mr. Xu said.
The major secondary endpoint of MACE excluding periprocedural MI occurred in 3.1% of QFR-guided patients and 4.8% of angiography-guided patients (HR, 0.64; 95% CI, 0.46-0.89; P = .0073).
The prerandomization revascularization plan was changed in 23.3% of patients with QFR and only 6.2% in the angiography group (P < .0001), mainly due to deferral of treatment of at least one vessel originally planned for PCI (19.6% vs. 5.2%; P < .0001).
“I think in the next guideline they will change the recommendation, not just to include FFR and IFR, but also to include QFR,” Giuseppe Tarantini, MD, PhD, University of Padua, Italy, said during a press briefing on the study.
“This is a milestone in our community, not only because it is easier to use compared to the other lesion-specific indexes like FFR, IFR, but also for the need to expand the use of physiology in the setting of interventional cardiology,” he added.
In an accompanying commentary, Robert A. Byrne, MBBCh, PhD, and Laurna McGovern, MBBCh, both from the Cardiovascular Research Institute Dublin, say the results are “relevant for cardiovascular disease researchers and clinicians and an important step forward for the field of angiography-derived flow measurements for guidance of PCI.”
They point out, however, that the control group did not receive pressure wire–guided PCI, which is the standard of care in contemporary practice and out of step with clinical practice guidelines, thus limiting external validity.
They also note that experiences to date suggest that up to 20% of patients may be unsuitable for the algorithm analysis because of coronary anatomy, presence of overlapping vessels, and insufficient image quality.
Commenting for this news organization, David E. Kandzari, MD, chief of the Piedmont Heart Institute, Atlanta, said “the technology isn’t readily available in catheterization labs today. Could it be assimilated into the cath labs at one point in the near term? I think absolutely, and that would be a welcome addition to expedite the procedure itself.”
Nevertheless, he said the results “need to be externally validated too, with what is the gold standard today of FFR in a larger experience.”
Session moderator Gregg W. Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said FAVOR III China has “advanced our knowledge” but pointed out that the ongoing randomized FAVOR III Europe Japan study is directly comparing QFR with invasive pressure-wire assessed FFR. The estimated primary completion date for that study is Dec. 31.
The study was supported by grants from the Beijing Municipal Science and Technology Commission, Chinese Academy of Medical Sciences, and the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital. Dr. Byrne reported institutional research or educational funding from Abbott Vascular, Biosensors, Biotronik, and Boston Scientific. Ms. McGovern has disclosed no relevant financial relationships. Dr. Kandzari reported minor consulting honoraria from the interventional device industry and institutional research grant support.
A version of this article first appeared on Medscape.com.
How applicable is ISCHEMIA trial to U.S. clinical practice?
The applicability of the results of the ISCHEMIA trial to real-world clinical practice in the United States has been called into question by a new study showing that less than a third of U.S. patients with stable ischemic heart disease (IHD) who currently undergo intervention would meet the trial’s inclusion criteria.
The ISCHEMIA trial, first reported in 2019, showed that, for stable patients with moderate to severe ischemia, an invasive approach using percutaneous coronary intervention (PCI) did not significantly reduce major cardiovascular events after a median 3.2 years of follow-up, compared with a conservative medical strategy.
For the current study, a group of interventionalists analyzed contemporary U.S. data on patients undergoing PCI and found that a large proportion of patients receiving PCI for stable ischemic heart disease in the United States would not have met criteria of the ISCHEMIA trial population.
The study was published online Nov. 1 in JACC: Cardiovascular Interventions).
“While ISCHEMIA was a very well-conducted trial, our results show that it only applies to about one-third of stable IHD patients undergoing intervention in U.S. clinical practice in the real world. In this group, while ISCHEMIA did not show a reduction in event rate in the intervention group, there was a reduction in symptoms,” lead author Saurav Chatterjee, MD, Long Island Jewish Medical Center, New York, told this news organization.
“But ISCHEMIA did not really answer the question for 67% of stable IHD in current U.S. practice. We may be abIe to defer PCI in these patients, but we don’t know that from the ISCHEMIA trial, as these patients were not included in the trial,” Chatterjee said.
“There is some concern that people will accept the ISCHEMIA results as being universal, but we cannot apply these results to all stable IHD patients who currently undergo intervention,” he added. “We believe that patients who do not fall into the ISCHEMIA population need a nuanced individual approach, taking into account symptom burden and patient preferences.”
In the new report, Dr. Chatterjee and associates note that the applicability of the ISCHEMIA findings to contemporary practice has been questioned by some, because of the exclusion of a significant proportion of patients that are routinely considered for revascularization, both within and outside of the United States.
They point out that the ISCHEMIA trial recruited 16.5% of its participants from the United States, and the proportion of patients in contemporary U.S. practice that would have qualified for the trial is not clear.
They therefore examined the proportion of stable IHD patients meeting inclusion criteria for the ISCHEMIA trial in a U.S. nationwide PCI registry.
The researchers used data from the National Cardiovascular Data Registry (NCDR) CathPCI Registry, which includes patients undergoing PCI at 1,662 institutions and accounts for more than 90% of PCI-capable hospitals in the United States.
All PCI procedures performed at institutions participating in the NCDR CathPCI Registry from October 2017 to June 2019 were identified. Patients presenting with acute coronary syndrome (ACS), cardiogenic shock, or cardiac arrest were excluded, as there is significant evidence in favor of revascularization in these groups, and they were not included in the ISCHEMIA trial.
Subsequently, all remaining stable IHD patients were classified into one of four groups.
- ISCHEMIA-like: These patients had intermediate- or high-risk findings on a stress test but no high-risk features that would have excluded enrollment into the ISCHEMIA trial
- High risk: This group comprised patients with stable IHD and left ventricular ejection fraction less than 35%, significant unprotected left main stenosis (>50%), preexisting dialysis, recent heart-failure exacerbation, or heart transplant. These patients would have met exclusion criteria for the ISCHEMIA trial
- Low risk: This group included patients with stable and negative or low-risk findings on stress test and would have met exclusion criteria for the ISCHEMIA trial
- Not classifiable: This group comprised patients with stable IHD not fitting any of the other cohorts, including no stress test or extent of ischemia not reported on stress testing. These patients would have not had enough information to clearly meet inclusion or exclusion criteria for the ISCHEMIA trial
Results showed that during the study period 927,011 patients underwent PCI as recorded in the NCDR CathPCI Registry. Of these, 58% had ACS, cardiogenic shock, or cardiac arrest and were excluded; the remaining 388,212 patients who underwent PCI for stable IHD comprised the study population.
Of these, 125,302 (32.28%) had a moderate- or high-risk stress test without high-risk anatomic or clinical features and met ISCHEMIA trial inclusion criteria.
Among stable IHD patients not meeting ISCHEMIA trial inclusion criteria, 71,852 (18.51%) had high-risk criteria that would have excluded them from the ISCHEMIA trial, a total of 67,159 (17.29%) patients had low-risk criteria that would have excluded them from the ISCHEMIA trial, and 123,899 (31.92%) were unclassifiable, either owing to lack of stress testing or the extent of ischemia not being reported on stress testing.
The authors suggest that the unclassifiable patients appear to represent a “higher-risk” population than those closely resembling the ISCHEMIA trial population, with more prior myocardial infarction and heart failure.
ISCHEMIA investigators respond
In an accompanying editorial, ISCHEMIA investigators David J. Maron, MD, Stanford (Calif.) University, and Sripal Bangalore, MD, and Judith S. Hochman, MD, New York University, argue that many of the patients highlighted by Dr. Chatterjee and associates were excluded from the ISCHEMIA trial for good reason.
They explain that ISCHEMIA was designed under the premise that prior stable IHD strategy trials such as COURAGE and BARI 2D included lower-risk patients, and the remaining gap was to evaluate the utility of invasive management in those at higher risk with moderate or severe stress-induced ischemia.
They point out that, among the NCDR patients with stable IHD in the current study by Dr. Chatterjee and associates who did not meet ISCHEMIA entry criteria, 18.5% had high-risk features, including 35.2% with left main coronary artery disease, 43.7% with left ventricular systolic dysfunction, and 16.8% with end-stage renal disease.
Although ISCHEMIA results do not apply to patients who were excluded from the trial, there is little controversy regarding the benefit of revascularization in patients with stable IHD with left main coronary artery disease or left ventricular ejection fraction <35%, which is why they were excluded from ISCHEMIA, the editorialists note.
They also report that patients with end-stage renal disease, who were also designated as not meeting ISCHEMIA inclusion criteria, were included in the companion ISCHEMIA CKD trial.
They further point out that, at the other end of the risk spectrum, 17.3% of stable IHD patients in the current analysis had negative or low-risk functional testing, and these patients were excluded from ISCHEMIA because they were shown in COURAGE and BARI 2D to not benefit from revascularization, and they do not meet guideline recommendations for elective PCI in the absence of symptoms.
Of the 31.9% of stable IHD patients who had missing data on ischemic burden, the ISCHEMIA investigators say that some of these would have qualified for the trial, although it is not possible to say how many. They suggest a conservative estimate of 50%.
Taking these arguments into account, the editorialists recalculated the proportion of NCDR PCI patients with stable IHD who would have been included in ISCHEMIA as between 62.1% and 68.6% of patients.
They say the current NCDR analysis by Dr. Chatterjee and associates should be interpreted as indicating, at worst, that the ISCHEMIA trial results apply to only 32% of patients undergoing elective PCI in the United States, and at best “that the results apply to a far higher proportion, excluding only those at high risk (18.5%) or with unacceptable symptoms despite maximal medical therapy (percentage unknown), for whom PCI is clearly indicated.”
The editorialists conclude: “The purpose of the analysis by Chatterjee et al. is to inform the cardiovascular community of the proportion of patients with stable IHD in clinical practice who would have been excluded from ISCHEMIA without regard for the logic of each exclusion criterion. The purpose of this editorial is to provide context for the analysis, admittedly from the perspective of ISCHEMIA investigators, with the hope that this helps readers clearly see the relevance of the trial to patients under their care.”
They add: “For practical and ethical reasons, ISCHEMIA excluded stable patients with high-risk features, angina inadequately controlled by medication, and low-risk features who do not meet evidence-based guidelines for revascularization. That leaves a large percentage of patients for whom the ISCHEMIA trial is highly relevant; exactly what percentage on the basis of NCDR data is hard to say.”
The ISCHEMIA trial was supported by the National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
The applicability of the results of the ISCHEMIA trial to real-world clinical practice in the United States has been called into question by a new study showing that less than a third of U.S. patients with stable ischemic heart disease (IHD) who currently undergo intervention would meet the trial’s inclusion criteria.
The ISCHEMIA trial, first reported in 2019, showed that, for stable patients with moderate to severe ischemia, an invasive approach using percutaneous coronary intervention (PCI) did not significantly reduce major cardiovascular events after a median 3.2 years of follow-up, compared with a conservative medical strategy.
For the current study, a group of interventionalists analyzed contemporary U.S. data on patients undergoing PCI and found that a large proportion of patients receiving PCI for stable ischemic heart disease in the United States would not have met criteria of the ISCHEMIA trial population.
The study was published online Nov. 1 in JACC: Cardiovascular Interventions).
“While ISCHEMIA was a very well-conducted trial, our results show that it only applies to about one-third of stable IHD patients undergoing intervention in U.S. clinical practice in the real world. In this group, while ISCHEMIA did not show a reduction in event rate in the intervention group, there was a reduction in symptoms,” lead author Saurav Chatterjee, MD, Long Island Jewish Medical Center, New York, told this news organization.
“But ISCHEMIA did not really answer the question for 67% of stable IHD in current U.S. practice. We may be abIe to defer PCI in these patients, but we don’t know that from the ISCHEMIA trial, as these patients were not included in the trial,” Chatterjee said.
“There is some concern that people will accept the ISCHEMIA results as being universal, but we cannot apply these results to all stable IHD patients who currently undergo intervention,” he added. “We believe that patients who do not fall into the ISCHEMIA population need a nuanced individual approach, taking into account symptom burden and patient preferences.”
In the new report, Dr. Chatterjee and associates note that the applicability of the ISCHEMIA findings to contemporary practice has been questioned by some, because of the exclusion of a significant proportion of patients that are routinely considered for revascularization, both within and outside of the United States.
They point out that the ISCHEMIA trial recruited 16.5% of its participants from the United States, and the proportion of patients in contemporary U.S. practice that would have qualified for the trial is not clear.
They therefore examined the proportion of stable IHD patients meeting inclusion criteria for the ISCHEMIA trial in a U.S. nationwide PCI registry.
The researchers used data from the National Cardiovascular Data Registry (NCDR) CathPCI Registry, which includes patients undergoing PCI at 1,662 institutions and accounts for more than 90% of PCI-capable hospitals in the United States.
All PCI procedures performed at institutions participating in the NCDR CathPCI Registry from October 2017 to June 2019 were identified. Patients presenting with acute coronary syndrome (ACS), cardiogenic shock, or cardiac arrest were excluded, as there is significant evidence in favor of revascularization in these groups, and they were not included in the ISCHEMIA trial.
Subsequently, all remaining stable IHD patients were classified into one of four groups.
- ISCHEMIA-like: These patients had intermediate- or high-risk findings on a stress test but no high-risk features that would have excluded enrollment into the ISCHEMIA trial
- High risk: This group comprised patients with stable IHD and left ventricular ejection fraction less than 35%, significant unprotected left main stenosis (>50%), preexisting dialysis, recent heart-failure exacerbation, or heart transplant. These patients would have met exclusion criteria for the ISCHEMIA trial
- Low risk: This group included patients with stable and negative or low-risk findings on stress test and would have met exclusion criteria for the ISCHEMIA trial
- Not classifiable: This group comprised patients with stable IHD not fitting any of the other cohorts, including no stress test or extent of ischemia not reported on stress testing. These patients would have not had enough information to clearly meet inclusion or exclusion criteria for the ISCHEMIA trial
Results showed that during the study period 927,011 patients underwent PCI as recorded in the NCDR CathPCI Registry. Of these, 58% had ACS, cardiogenic shock, or cardiac arrest and were excluded; the remaining 388,212 patients who underwent PCI for stable IHD comprised the study population.
Of these, 125,302 (32.28%) had a moderate- or high-risk stress test without high-risk anatomic or clinical features and met ISCHEMIA trial inclusion criteria.
Among stable IHD patients not meeting ISCHEMIA trial inclusion criteria, 71,852 (18.51%) had high-risk criteria that would have excluded them from the ISCHEMIA trial, a total of 67,159 (17.29%) patients had low-risk criteria that would have excluded them from the ISCHEMIA trial, and 123,899 (31.92%) were unclassifiable, either owing to lack of stress testing or the extent of ischemia not being reported on stress testing.
The authors suggest that the unclassifiable patients appear to represent a “higher-risk” population than those closely resembling the ISCHEMIA trial population, with more prior myocardial infarction and heart failure.
ISCHEMIA investigators respond
In an accompanying editorial, ISCHEMIA investigators David J. Maron, MD, Stanford (Calif.) University, and Sripal Bangalore, MD, and Judith S. Hochman, MD, New York University, argue that many of the patients highlighted by Dr. Chatterjee and associates were excluded from the ISCHEMIA trial for good reason.
They explain that ISCHEMIA was designed under the premise that prior stable IHD strategy trials such as COURAGE and BARI 2D included lower-risk patients, and the remaining gap was to evaluate the utility of invasive management in those at higher risk with moderate or severe stress-induced ischemia.
They point out that, among the NCDR patients with stable IHD in the current study by Dr. Chatterjee and associates who did not meet ISCHEMIA entry criteria, 18.5% had high-risk features, including 35.2% with left main coronary artery disease, 43.7% with left ventricular systolic dysfunction, and 16.8% with end-stage renal disease.
Although ISCHEMIA results do not apply to patients who were excluded from the trial, there is little controversy regarding the benefit of revascularization in patients with stable IHD with left main coronary artery disease or left ventricular ejection fraction <35%, which is why they were excluded from ISCHEMIA, the editorialists note.
They also report that patients with end-stage renal disease, who were also designated as not meeting ISCHEMIA inclusion criteria, were included in the companion ISCHEMIA CKD trial.
They further point out that, at the other end of the risk spectrum, 17.3% of stable IHD patients in the current analysis had negative or low-risk functional testing, and these patients were excluded from ISCHEMIA because they were shown in COURAGE and BARI 2D to not benefit from revascularization, and they do not meet guideline recommendations for elective PCI in the absence of symptoms.
Of the 31.9% of stable IHD patients who had missing data on ischemic burden, the ISCHEMIA investigators say that some of these would have qualified for the trial, although it is not possible to say how many. They suggest a conservative estimate of 50%.
Taking these arguments into account, the editorialists recalculated the proportion of NCDR PCI patients with stable IHD who would have been included in ISCHEMIA as between 62.1% and 68.6% of patients.
They say the current NCDR analysis by Dr. Chatterjee and associates should be interpreted as indicating, at worst, that the ISCHEMIA trial results apply to only 32% of patients undergoing elective PCI in the United States, and at best “that the results apply to a far higher proportion, excluding only those at high risk (18.5%) or with unacceptable symptoms despite maximal medical therapy (percentage unknown), for whom PCI is clearly indicated.”
The editorialists conclude: “The purpose of the analysis by Chatterjee et al. is to inform the cardiovascular community of the proportion of patients with stable IHD in clinical practice who would have been excluded from ISCHEMIA without regard for the logic of each exclusion criterion. The purpose of this editorial is to provide context for the analysis, admittedly from the perspective of ISCHEMIA investigators, with the hope that this helps readers clearly see the relevance of the trial to patients under their care.”
They add: “For practical and ethical reasons, ISCHEMIA excluded stable patients with high-risk features, angina inadequately controlled by medication, and low-risk features who do not meet evidence-based guidelines for revascularization. That leaves a large percentage of patients for whom the ISCHEMIA trial is highly relevant; exactly what percentage on the basis of NCDR data is hard to say.”
The ISCHEMIA trial was supported by the National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
The applicability of the results of the ISCHEMIA trial to real-world clinical practice in the United States has been called into question by a new study showing that less than a third of U.S. patients with stable ischemic heart disease (IHD) who currently undergo intervention would meet the trial’s inclusion criteria.
The ISCHEMIA trial, first reported in 2019, showed that, for stable patients with moderate to severe ischemia, an invasive approach using percutaneous coronary intervention (PCI) did not significantly reduce major cardiovascular events after a median 3.2 years of follow-up, compared with a conservative medical strategy.
For the current study, a group of interventionalists analyzed contemporary U.S. data on patients undergoing PCI and found that a large proportion of patients receiving PCI for stable ischemic heart disease in the United States would not have met criteria of the ISCHEMIA trial population.
The study was published online Nov. 1 in JACC: Cardiovascular Interventions).
“While ISCHEMIA was a very well-conducted trial, our results show that it only applies to about one-third of stable IHD patients undergoing intervention in U.S. clinical practice in the real world. In this group, while ISCHEMIA did not show a reduction in event rate in the intervention group, there was a reduction in symptoms,” lead author Saurav Chatterjee, MD, Long Island Jewish Medical Center, New York, told this news organization.
“But ISCHEMIA did not really answer the question for 67% of stable IHD in current U.S. practice. We may be abIe to defer PCI in these patients, but we don’t know that from the ISCHEMIA trial, as these patients were not included in the trial,” Chatterjee said.
“There is some concern that people will accept the ISCHEMIA results as being universal, but we cannot apply these results to all stable IHD patients who currently undergo intervention,” he added. “We believe that patients who do not fall into the ISCHEMIA population need a nuanced individual approach, taking into account symptom burden and patient preferences.”
In the new report, Dr. Chatterjee and associates note that the applicability of the ISCHEMIA findings to contemporary practice has been questioned by some, because of the exclusion of a significant proportion of patients that are routinely considered for revascularization, both within and outside of the United States.
They point out that the ISCHEMIA trial recruited 16.5% of its participants from the United States, and the proportion of patients in contemporary U.S. practice that would have qualified for the trial is not clear.
They therefore examined the proportion of stable IHD patients meeting inclusion criteria for the ISCHEMIA trial in a U.S. nationwide PCI registry.
The researchers used data from the National Cardiovascular Data Registry (NCDR) CathPCI Registry, which includes patients undergoing PCI at 1,662 institutions and accounts for more than 90% of PCI-capable hospitals in the United States.
All PCI procedures performed at institutions participating in the NCDR CathPCI Registry from October 2017 to June 2019 were identified. Patients presenting with acute coronary syndrome (ACS), cardiogenic shock, or cardiac arrest were excluded, as there is significant evidence in favor of revascularization in these groups, and they were not included in the ISCHEMIA trial.
Subsequently, all remaining stable IHD patients were classified into one of four groups.
- ISCHEMIA-like: These patients had intermediate- or high-risk findings on a stress test but no high-risk features that would have excluded enrollment into the ISCHEMIA trial
- High risk: This group comprised patients with stable IHD and left ventricular ejection fraction less than 35%, significant unprotected left main stenosis (>50%), preexisting dialysis, recent heart-failure exacerbation, or heart transplant. These patients would have met exclusion criteria for the ISCHEMIA trial
- Low risk: This group included patients with stable and negative or low-risk findings on stress test and would have met exclusion criteria for the ISCHEMIA trial
- Not classifiable: This group comprised patients with stable IHD not fitting any of the other cohorts, including no stress test or extent of ischemia not reported on stress testing. These patients would have not had enough information to clearly meet inclusion or exclusion criteria for the ISCHEMIA trial
Results showed that during the study period 927,011 patients underwent PCI as recorded in the NCDR CathPCI Registry. Of these, 58% had ACS, cardiogenic shock, or cardiac arrest and were excluded; the remaining 388,212 patients who underwent PCI for stable IHD comprised the study population.
Of these, 125,302 (32.28%) had a moderate- or high-risk stress test without high-risk anatomic or clinical features and met ISCHEMIA trial inclusion criteria.
Among stable IHD patients not meeting ISCHEMIA trial inclusion criteria, 71,852 (18.51%) had high-risk criteria that would have excluded them from the ISCHEMIA trial, a total of 67,159 (17.29%) patients had low-risk criteria that would have excluded them from the ISCHEMIA trial, and 123,899 (31.92%) were unclassifiable, either owing to lack of stress testing or the extent of ischemia not being reported on stress testing.
The authors suggest that the unclassifiable patients appear to represent a “higher-risk” population than those closely resembling the ISCHEMIA trial population, with more prior myocardial infarction and heart failure.
ISCHEMIA investigators respond
In an accompanying editorial, ISCHEMIA investigators David J. Maron, MD, Stanford (Calif.) University, and Sripal Bangalore, MD, and Judith S. Hochman, MD, New York University, argue that many of the patients highlighted by Dr. Chatterjee and associates were excluded from the ISCHEMIA trial for good reason.
They explain that ISCHEMIA was designed under the premise that prior stable IHD strategy trials such as COURAGE and BARI 2D included lower-risk patients, and the remaining gap was to evaluate the utility of invasive management in those at higher risk with moderate or severe stress-induced ischemia.
They point out that, among the NCDR patients with stable IHD in the current study by Dr. Chatterjee and associates who did not meet ISCHEMIA entry criteria, 18.5% had high-risk features, including 35.2% with left main coronary artery disease, 43.7% with left ventricular systolic dysfunction, and 16.8% with end-stage renal disease.
Although ISCHEMIA results do not apply to patients who were excluded from the trial, there is little controversy regarding the benefit of revascularization in patients with stable IHD with left main coronary artery disease or left ventricular ejection fraction <35%, which is why they were excluded from ISCHEMIA, the editorialists note.
They also report that patients with end-stage renal disease, who were also designated as not meeting ISCHEMIA inclusion criteria, were included in the companion ISCHEMIA CKD trial.
They further point out that, at the other end of the risk spectrum, 17.3% of stable IHD patients in the current analysis had negative or low-risk functional testing, and these patients were excluded from ISCHEMIA because they were shown in COURAGE and BARI 2D to not benefit from revascularization, and they do not meet guideline recommendations for elective PCI in the absence of symptoms.
Of the 31.9% of stable IHD patients who had missing data on ischemic burden, the ISCHEMIA investigators say that some of these would have qualified for the trial, although it is not possible to say how many. They suggest a conservative estimate of 50%.
Taking these arguments into account, the editorialists recalculated the proportion of NCDR PCI patients with stable IHD who would have been included in ISCHEMIA as between 62.1% and 68.6% of patients.
They say the current NCDR analysis by Dr. Chatterjee and associates should be interpreted as indicating, at worst, that the ISCHEMIA trial results apply to only 32% of patients undergoing elective PCI in the United States, and at best “that the results apply to a far higher proportion, excluding only those at high risk (18.5%) or with unacceptable symptoms despite maximal medical therapy (percentage unknown), for whom PCI is clearly indicated.”
The editorialists conclude: “The purpose of the analysis by Chatterjee et al. is to inform the cardiovascular community of the proportion of patients with stable IHD in clinical practice who would have been excluded from ISCHEMIA without regard for the logic of each exclusion criterion. The purpose of this editorial is to provide context for the analysis, admittedly from the perspective of ISCHEMIA investigators, with the hope that this helps readers clearly see the relevance of the trial to patients under their care.”
They add: “For practical and ethical reasons, ISCHEMIA excluded stable patients with high-risk features, angina inadequately controlled by medication, and low-risk features who do not meet evidence-based guidelines for revascularization. That leaves a large percentage of patients for whom the ISCHEMIA trial is highly relevant; exactly what percentage on the basis of NCDR data is hard to say.”
The ISCHEMIA trial was supported by the National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
FFR-guided PCI falls short vs. surgery in multivessel disease: FAME 3
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”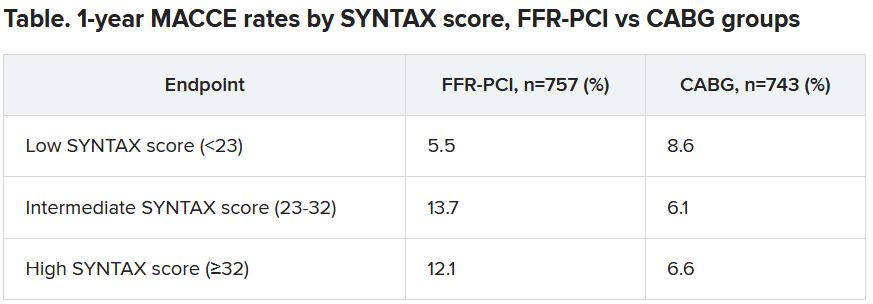
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
SUGAR trial finds superior stent for those with diabetes and CAD
Superiority shown on TLF endpoint
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Superiority shown on TLF endpoint
Superiority shown on TLF endpoint
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
FROM TCT 2021











