User login
FDA database reveals many rheumatic and musculoskeletal adverse events on immunotherapies
AMSTERDAM – Mining of the Food and Drug Administration adverse events database revealed a more substantial risk of rheumatic and musculoskeletal events on checkpoint inhibitor therapy than has been previously reported, according to Xerxes N. Pundole, PhD, an instructor in the research faculty at the University of Texas MD Anderson Cancer Center, Houston.
In a video interview, Dr. Pundole summarized data he presented at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
So far, according to Dr. Pundole, there have been a relatively limited number of reports in the medical literature of inflammatory rheumatic or musculoskeletal events from checkpoint inhibitors. However, other inflammatory conditions, such as colitis and pneumonitis, are known to occur commonly with these agents. The FDA adverse event database provided an opportunity to evaluate how often rheumatic and musculoskeletal events are reported in the real world.
In this interview, Dr. Pundole explained that rheumatic and musculoskeletal events do occur at higher rates than would be expected in patients not treated with a checkpoint inhibitor. With data from more than 30,000 unique patients, the relative risks of some of these adverse events, such as polymyositis, were more than doubled, although the event rates were not evenly distributed.
Specifically, rheumatic and musculoskeletal adverse events were far less common with the cytotoxic T-lymphocyte antigen 4 checkpoint inhibitor ipilimumab (Yervoy) relative to programmed cell death protein 1 inhibitors, particularly nivolumab (Opdivo).
In another notable finding, a demographic stratification of the FDA database found elderly men to be overrepresented among patients developing adverse events related to musculoskeletal inflammation.
Overall, his data do support a relationship between checkpoint inhibitors and a greater risk of rheumatic and musculoskeletal adverse events than has been previously reported, but he noted that these data provide no specific guidance for those who already have RA or another inflammatory condition.
“Can you identify these adverse events early on to keep the patients on immune checkpoint inhibitor therapy and not have to stop their cancer treatment? That’s a question,” Dr. Pundole said. However, he suggested that the FDA data support clinician awareness of the problem and the studies that will establish strategies for preserving the benefit-to-risk ratio of checkpoint inhibitors in patients who are at greater risk of adverse events relative to immune function because of a preexisting inflammatory condition.
SOURCE: Pundole XN et al. Ann Rheum Dis. 2018;77(Suppl 2):147-148. Abstract OP0197.
AMSTERDAM – Mining of the Food and Drug Administration adverse events database revealed a more substantial risk of rheumatic and musculoskeletal events on checkpoint inhibitor therapy than has been previously reported, according to Xerxes N. Pundole, PhD, an instructor in the research faculty at the University of Texas MD Anderson Cancer Center, Houston.
In a video interview, Dr. Pundole summarized data he presented at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
So far, according to Dr. Pundole, there have been a relatively limited number of reports in the medical literature of inflammatory rheumatic or musculoskeletal events from checkpoint inhibitors. However, other inflammatory conditions, such as colitis and pneumonitis, are known to occur commonly with these agents. The FDA adverse event database provided an opportunity to evaluate how often rheumatic and musculoskeletal events are reported in the real world.
In this interview, Dr. Pundole explained that rheumatic and musculoskeletal events do occur at higher rates than would be expected in patients not treated with a checkpoint inhibitor. With data from more than 30,000 unique patients, the relative risks of some of these adverse events, such as polymyositis, were more than doubled, although the event rates were not evenly distributed.
Specifically, rheumatic and musculoskeletal adverse events were far less common with the cytotoxic T-lymphocyte antigen 4 checkpoint inhibitor ipilimumab (Yervoy) relative to programmed cell death protein 1 inhibitors, particularly nivolumab (Opdivo).
In another notable finding, a demographic stratification of the FDA database found elderly men to be overrepresented among patients developing adverse events related to musculoskeletal inflammation.
Overall, his data do support a relationship between checkpoint inhibitors and a greater risk of rheumatic and musculoskeletal adverse events than has been previously reported, but he noted that these data provide no specific guidance for those who already have RA or another inflammatory condition.
“Can you identify these adverse events early on to keep the patients on immune checkpoint inhibitor therapy and not have to stop their cancer treatment? That’s a question,” Dr. Pundole said. However, he suggested that the FDA data support clinician awareness of the problem and the studies that will establish strategies for preserving the benefit-to-risk ratio of checkpoint inhibitors in patients who are at greater risk of adverse events relative to immune function because of a preexisting inflammatory condition.
SOURCE: Pundole XN et al. Ann Rheum Dis. 2018;77(Suppl 2):147-148. Abstract OP0197.
AMSTERDAM – Mining of the Food and Drug Administration adverse events database revealed a more substantial risk of rheumatic and musculoskeletal events on checkpoint inhibitor therapy than has been previously reported, according to Xerxes N. Pundole, PhD, an instructor in the research faculty at the University of Texas MD Anderson Cancer Center, Houston.
In a video interview, Dr. Pundole summarized data he presented at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
So far, according to Dr. Pundole, there have been a relatively limited number of reports in the medical literature of inflammatory rheumatic or musculoskeletal events from checkpoint inhibitors. However, other inflammatory conditions, such as colitis and pneumonitis, are known to occur commonly with these agents. The FDA adverse event database provided an opportunity to evaluate how often rheumatic and musculoskeletal events are reported in the real world.
In this interview, Dr. Pundole explained that rheumatic and musculoskeletal events do occur at higher rates than would be expected in patients not treated with a checkpoint inhibitor. With data from more than 30,000 unique patients, the relative risks of some of these adverse events, such as polymyositis, were more than doubled, although the event rates were not evenly distributed.
Specifically, rheumatic and musculoskeletal adverse events were far less common with the cytotoxic T-lymphocyte antigen 4 checkpoint inhibitor ipilimumab (Yervoy) relative to programmed cell death protein 1 inhibitors, particularly nivolumab (Opdivo).
In another notable finding, a demographic stratification of the FDA database found elderly men to be overrepresented among patients developing adverse events related to musculoskeletal inflammation.
Overall, his data do support a relationship between checkpoint inhibitors and a greater risk of rheumatic and musculoskeletal adverse events than has been previously reported, but he noted that these data provide no specific guidance for those who already have RA or another inflammatory condition.
“Can you identify these adverse events early on to keep the patients on immune checkpoint inhibitor therapy and not have to stop their cancer treatment? That’s a question,” Dr. Pundole said. However, he suggested that the FDA data support clinician awareness of the problem and the studies that will establish strategies for preserving the benefit-to-risk ratio of checkpoint inhibitors in patients who are at greater risk of adverse events relative to immune function because of a preexisting inflammatory condition.
SOURCE: Pundole XN et al. Ann Rheum Dis. 2018;77(Suppl 2):147-148. Abstract OP0197.
REPORTING FROM THE EULAR 2018 CONGRESS
Baricitinib shows potential as lupus treatment
AMSTERDAM – A significantly higher proportion of patients with lupus experienced improvements in joint and skin symptoms if they were treated with baricitinib (Olumiant) than if they received placebo in a phase 2 trial.
The primary endpoint of arthritis or rash resolution as measured by the Systemic Lupus Erythematosus (SLE) Disease Activity Index 2000 (SLEDAI-2K) was met by approximately 67% of patients who were treated with 4 mg baricitinib once daily and by around 53% of patients given a matching placebo (P less than .05).
With no new safety concerns, these findings suggest that baricitinib could be of benefit in patients with SLE and further study is warranted in a phase 3 trial, said the presenting study investigator Daniel J. Wallace, MD, at the European Congress of Rheumatology. Dr. Wallace is the associate director of the Rheumatology Fellowship Program at Cedars-Sinai Medical Center, Los Angeles.
Baricitinib is already approved for use as a treatment for RA in more than 40 countries. On June 1, Eli Lilly announced that the Food and Drug Administration had given the green light for its use in RA in the United States, but only at a dose of 2 mg once daily, whereas a 2-mg and 4-mg once-daily dose is approved in most other countries.
Data from the phase 2 trial presented by Dr. Wallace did include a 2-mg dose arm, but the difference in treatment response rates versus placebo was not statistically significant.
“I think the placebo response is mainly inflated by the use of corticosteroids,” said Dr. Dörner, professor of medicine at Charité–Universitätsmedizin Berlin. “If one would have applied a steroid tapering regimen, I would have expected a larger effect size, and possibly also the 2-mg [dose] be more effective as compared to placebo.” This is something to consider when moving into a phase 3 trial, he suggested.
For inclusion in the phase 2 trial, patients had to meet the following criteria: Be positive for antinuclear antibodies and/or a positive anti-dsDNA test, have a SLEDAI-2K clinical score of 4 or more, and have active SLEDAI arthritis and/or rash. Patients with severe active lupus nephritis or CNS involvement were excluded.
The mean age of patients was around 44 years, and as might be expected, the study population was predominantly female (99%). Around two-thirds of patients were white, 19% were of Asian descent, and the rest were designated as “other”. The average time to SLE onset was 9.7 years in the placebo group and just over 11 years in the baricitinib arms, with similar SLEDAI-2K scores of about 8-9, about 7-8 tender joints, and about five swollen joints at baseline.
A number of other secondary endpoints were also met by the 4 mg baricitinib group, Dr. Wallace reported. This included the relatively new Lupus Low Disease Activity State, he said, which was met by 38% (n = 27) of patients treated with 4 mg baricitinib, 33% (n = 35) treated with 2 mg baricitinib, and 26% (n = 27) of those given placebo (P less than .05 for the 4-mg dose vs. placebo). There were also numerically fewer SLE flares, including fewer severe flares.
“Some of the other outcomes demonstrated statistical significance: Physician Global Assessment, tender joint count, worst joint pain, and worst pain on a numeric rating scale,” Dr. Wallace said. A trend towards improvement was seen in the swollen joint count, with modest improvement in fatigue.
Treatment-emergent adverse events were seen in around 71%-73% of patients given baricitinib and 65% of patients given placebo. Most were mild or moderate in nature, but serious adverse events did occur in approximately 10% of patients who received baricitinib and in 4% of those who received placebo.
What’s noteworthy, Dr. Dörner said during a press briefing, is the very low rate of venous thromboembolism seen in the trial. “We’d have expected to see more deep vein thrombosis,” he said. Only one case occurred, in a patent taking the 4-mg dose, but this patient had preexisting antiphospholipid antibodies.
Additionally, although the percentage of patients with serious infections was slightly higher in the 2 and 4 mg baricitinib arms than for placebo (1.9% and 5.8% vs. 1%, respectively) “this is what we expect for lupus patients,” Dr. Dörner said. Furthermore, herpes zoster infection, which is very often reactivated in lupus because of the disease or its treatment, was only reported in one patient in the placebo group and in one patient in the 4 mg group.
“I think there is a very promising outlook, at least for the 4-mg dose of baricitinib,” Dr. Dörner said. “There have been no new safety or tolerability issues when compared to the RA population, and we’re looking forward to seeing subsequent studies in this [SLE] patient population where we have a need for more efficacious therapies.”
The study was funded by Eli Lilly. Dr. Dörner was part of the trial’s steering committee and has acted as a consultant for Eli Lilly. He has also received grant or research support from Roche/Chugai, Janssen, and Sanofi-Aventis; consulted for AbbVie, Celgene, Roche, UCB, Merck Sharp & Dohme, Pfizer/Hospira, and Novartis; and he is part of the speakers bureaus for Amgen, Celgene, and Biogen. Dr. Wallace has acted as a consultant for Eli Lilly, as well as EMD Serono, Pfizer, and GlaxoSmithKline.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Wallace DJ et al. Ann Rheum Dis. 2018;77(Suppl 2):59. Abstract OP0019.
AMSTERDAM – A significantly higher proportion of patients with lupus experienced improvements in joint and skin symptoms if they were treated with baricitinib (Olumiant) than if they received placebo in a phase 2 trial.
The primary endpoint of arthritis or rash resolution as measured by the Systemic Lupus Erythematosus (SLE) Disease Activity Index 2000 (SLEDAI-2K) was met by approximately 67% of patients who were treated with 4 mg baricitinib once daily and by around 53% of patients given a matching placebo (P less than .05).
With no new safety concerns, these findings suggest that baricitinib could be of benefit in patients with SLE and further study is warranted in a phase 3 trial, said the presenting study investigator Daniel J. Wallace, MD, at the European Congress of Rheumatology. Dr. Wallace is the associate director of the Rheumatology Fellowship Program at Cedars-Sinai Medical Center, Los Angeles.
Baricitinib is already approved for use as a treatment for RA in more than 40 countries. On June 1, Eli Lilly announced that the Food and Drug Administration had given the green light for its use in RA in the United States, but only at a dose of 2 mg once daily, whereas a 2-mg and 4-mg once-daily dose is approved in most other countries.
Data from the phase 2 trial presented by Dr. Wallace did include a 2-mg dose arm, but the difference in treatment response rates versus placebo was not statistically significant.
“I think the placebo response is mainly inflated by the use of corticosteroids,” said Dr. Dörner, professor of medicine at Charité–Universitätsmedizin Berlin. “If one would have applied a steroid tapering regimen, I would have expected a larger effect size, and possibly also the 2-mg [dose] be more effective as compared to placebo.” This is something to consider when moving into a phase 3 trial, he suggested.
For inclusion in the phase 2 trial, patients had to meet the following criteria: Be positive for antinuclear antibodies and/or a positive anti-dsDNA test, have a SLEDAI-2K clinical score of 4 or more, and have active SLEDAI arthritis and/or rash. Patients with severe active lupus nephritis or CNS involvement were excluded.
The mean age of patients was around 44 years, and as might be expected, the study population was predominantly female (99%). Around two-thirds of patients were white, 19% were of Asian descent, and the rest were designated as “other”. The average time to SLE onset was 9.7 years in the placebo group and just over 11 years in the baricitinib arms, with similar SLEDAI-2K scores of about 8-9, about 7-8 tender joints, and about five swollen joints at baseline.
A number of other secondary endpoints were also met by the 4 mg baricitinib group, Dr. Wallace reported. This included the relatively new Lupus Low Disease Activity State, he said, which was met by 38% (n = 27) of patients treated with 4 mg baricitinib, 33% (n = 35) treated with 2 mg baricitinib, and 26% (n = 27) of those given placebo (P less than .05 for the 4-mg dose vs. placebo). There were also numerically fewer SLE flares, including fewer severe flares.
“Some of the other outcomes demonstrated statistical significance: Physician Global Assessment, tender joint count, worst joint pain, and worst pain on a numeric rating scale,” Dr. Wallace said. A trend towards improvement was seen in the swollen joint count, with modest improvement in fatigue.
Treatment-emergent adverse events were seen in around 71%-73% of patients given baricitinib and 65% of patients given placebo. Most were mild or moderate in nature, but serious adverse events did occur in approximately 10% of patients who received baricitinib and in 4% of those who received placebo.
What’s noteworthy, Dr. Dörner said during a press briefing, is the very low rate of venous thromboembolism seen in the trial. “We’d have expected to see more deep vein thrombosis,” he said. Only one case occurred, in a patent taking the 4-mg dose, but this patient had preexisting antiphospholipid antibodies.
Additionally, although the percentage of patients with serious infections was slightly higher in the 2 and 4 mg baricitinib arms than for placebo (1.9% and 5.8% vs. 1%, respectively) “this is what we expect for lupus patients,” Dr. Dörner said. Furthermore, herpes zoster infection, which is very often reactivated in lupus because of the disease or its treatment, was only reported in one patient in the placebo group and in one patient in the 4 mg group.
“I think there is a very promising outlook, at least for the 4-mg dose of baricitinib,” Dr. Dörner said. “There have been no new safety or tolerability issues when compared to the RA population, and we’re looking forward to seeing subsequent studies in this [SLE] patient population where we have a need for more efficacious therapies.”
The study was funded by Eli Lilly. Dr. Dörner was part of the trial’s steering committee and has acted as a consultant for Eli Lilly. He has also received grant or research support from Roche/Chugai, Janssen, and Sanofi-Aventis; consulted for AbbVie, Celgene, Roche, UCB, Merck Sharp & Dohme, Pfizer/Hospira, and Novartis; and he is part of the speakers bureaus for Amgen, Celgene, and Biogen. Dr. Wallace has acted as a consultant for Eli Lilly, as well as EMD Serono, Pfizer, and GlaxoSmithKline.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Wallace DJ et al. Ann Rheum Dis. 2018;77(Suppl 2):59. Abstract OP0019.
AMSTERDAM – A significantly higher proportion of patients with lupus experienced improvements in joint and skin symptoms if they were treated with baricitinib (Olumiant) than if they received placebo in a phase 2 trial.
The primary endpoint of arthritis or rash resolution as measured by the Systemic Lupus Erythematosus (SLE) Disease Activity Index 2000 (SLEDAI-2K) was met by approximately 67% of patients who were treated with 4 mg baricitinib once daily and by around 53% of patients given a matching placebo (P less than .05).
With no new safety concerns, these findings suggest that baricitinib could be of benefit in patients with SLE and further study is warranted in a phase 3 trial, said the presenting study investigator Daniel J. Wallace, MD, at the European Congress of Rheumatology. Dr. Wallace is the associate director of the Rheumatology Fellowship Program at Cedars-Sinai Medical Center, Los Angeles.
Baricitinib is already approved for use as a treatment for RA in more than 40 countries. On June 1, Eli Lilly announced that the Food and Drug Administration had given the green light for its use in RA in the United States, but only at a dose of 2 mg once daily, whereas a 2-mg and 4-mg once-daily dose is approved in most other countries.
Data from the phase 2 trial presented by Dr. Wallace did include a 2-mg dose arm, but the difference in treatment response rates versus placebo was not statistically significant.
“I think the placebo response is mainly inflated by the use of corticosteroids,” said Dr. Dörner, professor of medicine at Charité–Universitätsmedizin Berlin. “If one would have applied a steroid tapering regimen, I would have expected a larger effect size, and possibly also the 2-mg [dose] be more effective as compared to placebo.” This is something to consider when moving into a phase 3 trial, he suggested.
For inclusion in the phase 2 trial, patients had to meet the following criteria: Be positive for antinuclear antibodies and/or a positive anti-dsDNA test, have a SLEDAI-2K clinical score of 4 or more, and have active SLEDAI arthritis and/or rash. Patients with severe active lupus nephritis or CNS involvement were excluded.
The mean age of patients was around 44 years, and as might be expected, the study population was predominantly female (99%). Around two-thirds of patients were white, 19% were of Asian descent, and the rest were designated as “other”. The average time to SLE onset was 9.7 years in the placebo group and just over 11 years in the baricitinib arms, with similar SLEDAI-2K scores of about 8-9, about 7-8 tender joints, and about five swollen joints at baseline.
A number of other secondary endpoints were also met by the 4 mg baricitinib group, Dr. Wallace reported. This included the relatively new Lupus Low Disease Activity State, he said, which was met by 38% (n = 27) of patients treated with 4 mg baricitinib, 33% (n = 35) treated with 2 mg baricitinib, and 26% (n = 27) of those given placebo (P less than .05 for the 4-mg dose vs. placebo). There were also numerically fewer SLE flares, including fewer severe flares.
“Some of the other outcomes demonstrated statistical significance: Physician Global Assessment, tender joint count, worst joint pain, and worst pain on a numeric rating scale,” Dr. Wallace said. A trend towards improvement was seen in the swollen joint count, with modest improvement in fatigue.
Treatment-emergent adverse events were seen in around 71%-73% of patients given baricitinib and 65% of patients given placebo. Most were mild or moderate in nature, but serious adverse events did occur in approximately 10% of patients who received baricitinib and in 4% of those who received placebo.
What’s noteworthy, Dr. Dörner said during a press briefing, is the very low rate of venous thromboembolism seen in the trial. “We’d have expected to see more deep vein thrombosis,” he said. Only one case occurred, in a patent taking the 4-mg dose, but this patient had preexisting antiphospholipid antibodies.
Additionally, although the percentage of patients with serious infections was slightly higher in the 2 and 4 mg baricitinib arms than for placebo (1.9% and 5.8% vs. 1%, respectively) “this is what we expect for lupus patients,” Dr. Dörner said. Furthermore, herpes zoster infection, which is very often reactivated in lupus because of the disease or its treatment, was only reported in one patient in the placebo group and in one patient in the 4 mg group.
“I think there is a very promising outlook, at least for the 4-mg dose of baricitinib,” Dr. Dörner said. “There have been no new safety or tolerability issues when compared to the RA population, and we’re looking forward to seeing subsequent studies in this [SLE] patient population where we have a need for more efficacious therapies.”
The study was funded by Eli Lilly. Dr. Dörner was part of the trial’s steering committee and has acted as a consultant for Eli Lilly. He has also received grant or research support from Roche/Chugai, Janssen, and Sanofi-Aventis; consulted for AbbVie, Celgene, Roche, UCB, Merck Sharp & Dohme, Pfizer/Hospira, and Novartis; and he is part of the speakers bureaus for Amgen, Celgene, and Biogen. Dr. Wallace has acted as a consultant for Eli Lilly, as well as EMD Serono, Pfizer, and GlaxoSmithKline.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Wallace DJ et al. Ann Rheum Dis. 2018;77(Suppl 2):59. Abstract OP0019.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: Baricitinib at 4 mg was associated with significant clinical improvements versus placebo and had an acceptable safety and tolerability profile.
Major finding: A higher percentage of patients receiving 4 mg of baricitinib than those receiving placebo achieved the primary endpoint of arthritis and/or rash remission as defined by the Systemic Lupus Erythematosus Disease Activity Index 2000 at week 24 (P less than .05).
Study details: A phase 2, multinational, double-blind, placebo-controlled, parallel group study of once-daily, oral baricitinib (2 mg and 4 mg) in 314 patients with SLE receiving standard therapy.
Disclosures: The study was funded by Eli Lilly. Dr. Dörner was part of the trial’s steering committee and has acted as a consultant for Eli Lilly. He has also received grant or research support from Roche/Chugai, Janssen, and Sanofi-Aventis; consulted for AbbVie, Celgene, Roche, UCB, Merck Sharp & Dohme, Pfizer/Hospira, and Novartis; and he is part of the speakers bureaus for Amgen, Celgene, and Biogen. Dr. Wallace has acted as a consultant for Eli Lilly, as well as EMD Serono, Pfizer, and GlaxoSmithKline.
Source: Wallace DJ et al. Ann Rheum Dis. 2018;77(Suppl 2):59. Abstract OP0019.
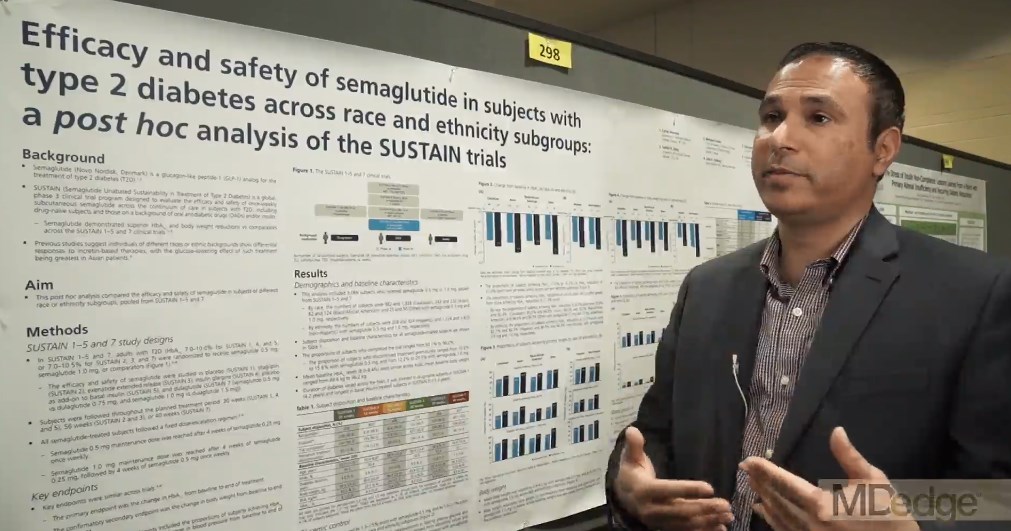
Semaglutide drops HbA1c, weight, across ethnicities
BOSTON – studied in a series of clinical trials; the efficacy did not come at the cost of frequent hypoglycemia or other serious adverse events, according to a pooled subgroup analysis of the SUSTAIN trials.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The trials investigated the safety and efficacy of semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, in the treatment of T2DM. Cyrus V. Desouza, MBBS, presented results of a post hoc analysis of racial and ethnic subgroups, drawing on SUSTAIN trials 1-5 and 7 (SUSTAIN 6 had a different design, focusing on cardiovascular outcomes).
“The trials incorporated patients on the whole spectrum of diabetes, starting from people who are newly diagnosed ... all the way to patients who were on a combination of oral antidiabetic drugs plus insulin,” Dr. Desouza explained in an interview at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists.
The mean time since diagnosis in the SUSTAIN trials varied from 4.2 years in SUSTAIN 1 to 13.3 years in SUSTAIN 5. Dr. Desouza and his colleagues pooled data from the six trials to conduct the subgroup analyses.
Patients in the intervention arms of all trials received once weekly subcutaneous semaglutide, at a dose of either 0.5 mg or 1.0 mg, according to Dr. Desouza, professor of diabetes, endocrinology, and metabolism and Schultz Professor of Diabetes Research, Diabetes, Endocrinology, and Metabolism at the University of Nebraska, Lincoln.
In all, data from 3,066 patients were available. In the racial analysis, 982 low- and 1,328 high-dose semaglutide recipients were white, 243 and 232 were Asian, 82 and 124 were African American, and 25 and 50 identified as “other,” respectively.
An analysis by ethnicity found that 208 low- and 324 high-dose recipients were Hispanic.
At baseline in all trials, mean hemoglobin A1c levels were similar, ranging from 8% to 8.4%; weights at baseline were a mean 89.6 kg to 96.2 kg across the trials.
The range of reductions in HbA1c was similar across racial and ethnic groups. “If you look at the proportion of patients who actually achieved an A1c below 7[%], it’s pretty impressive – it’s between 70% to 80%.” Between 50% and 60% of patients reached an HbA1c less than 6.5%, said Dr. Desouza.
Looking at the data another way, 62.2%-72.4% of patients saw an HbA1c reduction of at least 1% on low-dose semaglutide; the range across ethnicities was 74.2%-87.1% on high-dose semaglutide. Dr. Desouza said that the sample sizes weren’t large enough to calculate statistical significance for these subgroup differences.
“But I think what is impressive is that over 50% of patients in all the races and ethnicities were able to achieve a 5% body weight loss, which is metabolically significant in terms of improving outcomes,” he said. “I think that’s a really important fact.” A smaller proportion – around 20% – lost at least 10% of body weight, mostly on high-dose semaglutide.
Severe hypoglycemia, as defined by American Diabetes Association classification, was very rare across trials, except that 4.7% of African Americans saw this adverse event on high-dose semaglutide. Incidence in other subgroups, at either dose, ranged from 0% to 2.4%.
Otherwise, the medication was generally well tolerated, though gastrointestinal side effects were seen. “Asian people have a little higher GI side effects – up to 50% of Asians did develop GI side effects, and between 10% and 13% of Asians had to stop medication due to side effects,” said Dr. Desouza. “So I think that would be the one caveat in terms of tolerance that we did learn.”
The SUSTAIN trials were sponsored by Novo Nordisk. Dr. Desouza has received consulting fees for Novo Nordisk and has received grant support from several other pharmaceutical companies. Two coauthors are Novo Nordisk employees.
SOURCE: Desouza C et al. AACE 2018, Abstract 298
BOSTON – studied in a series of clinical trials; the efficacy did not come at the cost of frequent hypoglycemia or other serious adverse events, according to a pooled subgroup analysis of the SUSTAIN trials.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The trials investigated the safety and efficacy of semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, in the treatment of T2DM. Cyrus V. Desouza, MBBS, presented results of a post hoc analysis of racial and ethnic subgroups, drawing on SUSTAIN trials 1-5 and 7 (SUSTAIN 6 had a different design, focusing on cardiovascular outcomes).
“The trials incorporated patients on the whole spectrum of diabetes, starting from people who are newly diagnosed ... all the way to patients who were on a combination of oral antidiabetic drugs plus insulin,” Dr. Desouza explained in an interview at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists.
The mean time since diagnosis in the SUSTAIN trials varied from 4.2 years in SUSTAIN 1 to 13.3 years in SUSTAIN 5. Dr. Desouza and his colleagues pooled data from the six trials to conduct the subgroup analyses.
Patients in the intervention arms of all trials received once weekly subcutaneous semaglutide, at a dose of either 0.5 mg or 1.0 mg, according to Dr. Desouza, professor of diabetes, endocrinology, and metabolism and Schultz Professor of Diabetes Research, Diabetes, Endocrinology, and Metabolism at the University of Nebraska, Lincoln.
In all, data from 3,066 patients were available. In the racial analysis, 982 low- and 1,328 high-dose semaglutide recipients were white, 243 and 232 were Asian, 82 and 124 were African American, and 25 and 50 identified as “other,” respectively.
An analysis by ethnicity found that 208 low- and 324 high-dose recipients were Hispanic.
At baseline in all trials, mean hemoglobin A1c levels were similar, ranging from 8% to 8.4%; weights at baseline were a mean 89.6 kg to 96.2 kg across the trials.
The range of reductions in HbA1c was similar across racial and ethnic groups. “If you look at the proportion of patients who actually achieved an A1c below 7[%], it’s pretty impressive – it’s between 70% to 80%.” Between 50% and 60% of patients reached an HbA1c less than 6.5%, said Dr. Desouza.
Looking at the data another way, 62.2%-72.4% of patients saw an HbA1c reduction of at least 1% on low-dose semaglutide; the range across ethnicities was 74.2%-87.1% on high-dose semaglutide. Dr. Desouza said that the sample sizes weren’t large enough to calculate statistical significance for these subgroup differences.
“But I think what is impressive is that over 50% of patients in all the races and ethnicities were able to achieve a 5% body weight loss, which is metabolically significant in terms of improving outcomes,” he said. “I think that’s a really important fact.” A smaller proportion – around 20% – lost at least 10% of body weight, mostly on high-dose semaglutide.
Severe hypoglycemia, as defined by American Diabetes Association classification, was very rare across trials, except that 4.7% of African Americans saw this adverse event on high-dose semaglutide. Incidence in other subgroups, at either dose, ranged from 0% to 2.4%.
Otherwise, the medication was generally well tolerated, though gastrointestinal side effects were seen. “Asian people have a little higher GI side effects – up to 50% of Asians did develop GI side effects, and between 10% and 13% of Asians had to stop medication due to side effects,” said Dr. Desouza. “So I think that would be the one caveat in terms of tolerance that we did learn.”
The SUSTAIN trials were sponsored by Novo Nordisk. Dr. Desouza has received consulting fees for Novo Nordisk and has received grant support from several other pharmaceutical companies. Two coauthors are Novo Nordisk employees.
SOURCE: Desouza C et al. AACE 2018, Abstract 298
BOSTON – studied in a series of clinical trials; the efficacy did not come at the cost of frequent hypoglycemia or other serious adverse events, according to a pooled subgroup analysis of the SUSTAIN trials.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The trials investigated the safety and efficacy of semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, in the treatment of T2DM. Cyrus V. Desouza, MBBS, presented results of a post hoc analysis of racial and ethnic subgroups, drawing on SUSTAIN trials 1-5 and 7 (SUSTAIN 6 had a different design, focusing on cardiovascular outcomes).
“The trials incorporated patients on the whole spectrum of diabetes, starting from people who are newly diagnosed ... all the way to patients who were on a combination of oral antidiabetic drugs plus insulin,” Dr. Desouza explained in an interview at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists.
The mean time since diagnosis in the SUSTAIN trials varied from 4.2 years in SUSTAIN 1 to 13.3 years in SUSTAIN 5. Dr. Desouza and his colleagues pooled data from the six trials to conduct the subgroup analyses.
Patients in the intervention arms of all trials received once weekly subcutaneous semaglutide, at a dose of either 0.5 mg or 1.0 mg, according to Dr. Desouza, professor of diabetes, endocrinology, and metabolism and Schultz Professor of Diabetes Research, Diabetes, Endocrinology, and Metabolism at the University of Nebraska, Lincoln.
In all, data from 3,066 patients were available. In the racial analysis, 982 low- and 1,328 high-dose semaglutide recipients were white, 243 and 232 were Asian, 82 and 124 were African American, and 25 and 50 identified as “other,” respectively.
An analysis by ethnicity found that 208 low- and 324 high-dose recipients were Hispanic.
At baseline in all trials, mean hemoglobin A1c levels were similar, ranging from 8% to 8.4%; weights at baseline were a mean 89.6 kg to 96.2 kg across the trials.
The range of reductions in HbA1c was similar across racial and ethnic groups. “If you look at the proportion of patients who actually achieved an A1c below 7[%], it’s pretty impressive – it’s between 70% to 80%.” Between 50% and 60% of patients reached an HbA1c less than 6.5%, said Dr. Desouza.
Looking at the data another way, 62.2%-72.4% of patients saw an HbA1c reduction of at least 1% on low-dose semaglutide; the range across ethnicities was 74.2%-87.1% on high-dose semaglutide. Dr. Desouza said that the sample sizes weren’t large enough to calculate statistical significance for these subgroup differences.
“But I think what is impressive is that over 50% of patients in all the races and ethnicities were able to achieve a 5% body weight loss, which is metabolically significant in terms of improving outcomes,” he said. “I think that’s a really important fact.” A smaller proportion – around 20% – lost at least 10% of body weight, mostly on high-dose semaglutide.
Severe hypoglycemia, as defined by American Diabetes Association classification, was very rare across trials, except that 4.7% of African Americans saw this adverse event on high-dose semaglutide. Incidence in other subgroups, at either dose, ranged from 0% to 2.4%.
Otherwise, the medication was generally well tolerated, though gastrointestinal side effects were seen. “Asian people have a little higher GI side effects – up to 50% of Asians did develop GI side effects, and between 10% and 13% of Asians had to stop medication due to side effects,” said Dr. Desouza. “So I think that would be the one caveat in terms of tolerance that we did learn.”
The SUSTAIN trials were sponsored by Novo Nordisk. Dr. Desouza has received consulting fees for Novo Nordisk and has received grant support from several other pharmaceutical companies. Two coauthors are Novo Nordisk employees.
SOURCE: Desouza C et al. AACE 2018, Abstract 298
REPORTING FROM AACE 2018
Web portal does not reduce phone encounters or office visits for IBD patients
WASHINGTON – Inflammatory bowel disease patients may love web-based portals that allow them to interact with their doctors and records, but it does not seem to reduce their trips to the doctor.
“There was actually no decrease in office visits or phone encounters with patients that are utilizing MyChart [a web-based patient portal],” said Alexander Hristov, MD, a resident at the University of Wisconsin–Madison, in a video interview at the annual Digestive Disease Week®. “So in fact, the patients that had MyChart use were also the patients that were calling in more frequently and visiting the clinic more frequently, which is interesting because we did not see that there was an offset for emergency room visits or hospitalizations.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Out of the 616 total patients with either Crohn’s disease (355 patients) or ulcerative colitis (261 patients) analyzed in the study, 28% used MyChart. MyChart users also had higher number of prednisone prescriptions, compared with nonusers (51.9% vs. 40.8%, P = .01). There was no difference between MyChart users and nonusers for emergency room visits (P = .11) or hospitalizations (P = .16).
Interestingly, most messages sent via MyChart were for administrative reasons (54%), with both symptoms (28%) and education (18%) lagging behind.
Even though patients seem to like the portal, there is no billable time set aside for physicians to add the data for patients to access or respond to patient comments and requests through the portal. Unless MyChart can be shown to improve outcomes in some way, it is only an added burden for physicians.
Dr. Hristov mentioned that further work should be done to understand how web-based portals like MyChart can help both doctors and patients utilize this technology.
“We want to see the actual, measurable clinical outcomes of MyChart use,” he said. “So we want to set up a protocol where we can actually have measurable statistics looking at disease activity, inflammatory markers, and is there an impact that we are having on the patients disease course.”
Dr. Hristov had no financial disclosures to report.
SOURCE: Hristov A et al. Gastroenterology. 2018 May. doi: 0.1016/S0016-5085(18)32737-9.
WASHINGTON – Inflammatory bowel disease patients may love web-based portals that allow them to interact with their doctors and records, but it does not seem to reduce their trips to the doctor.
“There was actually no decrease in office visits or phone encounters with patients that are utilizing MyChart [a web-based patient portal],” said Alexander Hristov, MD, a resident at the University of Wisconsin–Madison, in a video interview at the annual Digestive Disease Week®. “So in fact, the patients that had MyChart use were also the patients that were calling in more frequently and visiting the clinic more frequently, which is interesting because we did not see that there was an offset for emergency room visits or hospitalizations.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Out of the 616 total patients with either Crohn’s disease (355 patients) or ulcerative colitis (261 patients) analyzed in the study, 28% used MyChart. MyChart users also had higher number of prednisone prescriptions, compared with nonusers (51.9% vs. 40.8%, P = .01). There was no difference between MyChart users and nonusers for emergency room visits (P = .11) or hospitalizations (P = .16).
Interestingly, most messages sent via MyChart were for administrative reasons (54%), with both symptoms (28%) and education (18%) lagging behind.
Even though patients seem to like the portal, there is no billable time set aside for physicians to add the data for patients to access or respond to patient comments and requests through the portal. Unless MyChart can be shown to improve outcomes in some way, it is only an added burden for physicians.
Dr. Hristov mentioned that further work should be done to understand how web-based portals like MyChart can help both doctors and patients utilize this technology.
“We want to see the actual, measurable clinical outcomes of MyChart use,” he said. “So we want to set up a protocol where we can actually have measurable statistics looking at disease activity, inflammatory markers, and is there an impact that we are having on the patients disease course.”
Dr. Hristov had no financial disclosures to report.
SOURCE: Hristov A et al. Gastroenterology. 2018 May. doi: 0.1016/S0016-5085(18)32737-9.
WASHINGTON – Inflammatory bowel disease patients may love web-based portals that allow them to interact with their doctors and records, but it does not seem to reduce their trips to the doctor.
“There was actually no decrease in office visits or phone encounters with patients that are utilizing MyChart [a web-based patient portal],” said Alexander Hristov, MD, a resident at the University of Wisconsin–Madison, in a video interview at the annual Digestive Disease Week®. “So in fact, the patients that had MyChart use were also the patients that were calling in more frequently and visiting the clinic more frequently, which is interesting because we did not see that there was an offset for emergency room visits or hospitalizations.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Out of the 616 total patients with either Crohn’s disease (355 patients) or ulcerative colitis (261 patients) analyzed in the study, 28% used MyChart. MyChart users also had higher number of prednisone prescriptions, compared with nonusers (51.9% vs. 40.8%, P = .01). There was no difference between MyChart users and nonusers for emergency room visits (P = .11) or hospitalizations (P = .16).
Interestingly, most messages sent via MyChart were for administrative reasons (54%), with both symptoms (28%) and education (18%) lagging behind.
Even though patients seem to like the portal, there is no billable time set aside for physicians to add the data for patients to access or respond to patient comments and requests through the portal. Unless MyChart can be shown to improve outcomes in some way, it is only an added burden for physicians.
Dr. Hristov mentioned that further work should be done to understand how web-based portals like MyChart can help both doctors and patients utilize this technology.
“We want to see the actual, measurable clinical outcomes of MyChart use,” he said. “So we want to set up a protocol where we can actually have measurable statistics looking at disease activity, inflammatory markers, and is there an impact that we are having on the patients disease course.”
Dr. Hristov had no financial disclosures to report.
SOURCE: Hristov A et al. Gastroenterology. 2018 May. doi: 0.1016/S0016-5085(18)32737-9.
REPORTING FROM DDW 2018
Key clinical point: Inflammatory bowel disease patients had more office visits and phone calls with physicians, and had worse outcomes.
Major finding: MyChart patients averaged 7.2 office visits and 19.2 phone encounters, compared with 5.6 office visits and 13.7 phone encounters in nonusers.
Study details: A review of patient electronic health records from Jan. 1, 2012, to December 31, 2015.
Disclosures: Dr. Hristov had no relevant financial disclosures to report.
Source: Hristov A et al Gastroenterology. 2018 May. doi: 10.1016/S0016-5085(18)32737-9.
VIDEO: Hepatitis C eradication cuts nonliver cancer rate
WASHINGTON – Treatment of hepatitis C infection with a direct-acting antiviral drug strongly linked with a rapid, 14% drop in the incidence of all nonhepatic cancers, based on analysis of data from more than 30,000 U.S. patients.
The data also showed compared with infected patients who had been treated with an interferon-based regimen during the period immediately preceding the availability of DAAs in late 2013. This included a 45% cut in lung cancers, a 49% cut in bladder cancer, a 62% relative risk reduction in leukemia, and a 29% drop in prostate cancer, Michael B. Charlton, MD, said at the annual Digestive Disease Week.®
The relative reductions in nonhepatic cancer incidence appeared soon after DAA treatment. The data Dr. Charlton reported reflected a median follow-up of 1 year for DAA-treated patients and 2.6 years for the hepatitis C–infected patients who had received interferon and did not get a DAA. A major difference between these two regimens is their efficacy, with DAA regimens producing sustained virologic response rates of 90% or better, while the interferon regimens produced substantially lower eradication rates.
“The most obvious hypothesis” to explain the observed effects is that “hepatitis C is a potent carcinogen,” possibly acting by inhibiting immune surveillance for new cancers in infected people, Dr. Charlton said in a video interview.
The study he reported used insurance-claims data from more than 146 million U.S. residents during 2007-2017 in the IQVIA PharMetrics Plus database, which included more than 367,000 adults infected with hepatitis C. Dr. Charlton and his associates pulled from this claims data on 10,989 of the infected patients who received interferon during January 2007-May 2011 (and followed through November 2013), and 22,894 infected patients treated with any type of DAA during December 2013 through March 2017. They used these two discrete time windows to completely separate the patients who received a DAA from those who did not.
The primary analysis calculated a hazard ratio for the development of any nonhepatic cancer after adjustment for a number of demographic and clinical covariates including age, smoking history, and weight, and also applied propensity-score weighting to the data. The Kaplan-Meier analysis of the data showed clear separation of the cancer-free survival curves of the two subgroups by 6 months of follow-up, and then showed steady further separation over time suggesting an ongoing carcinogenic effect from continued hepatitis C infection in patients who had received the less effective antiviral regimen. The analysis was able to reveal this effect because it had data from many thousands of treated hepatitis C patients, far more than had been enrolled in the pivotal trials for the DAAs, noted Dr. Charlton, professor and director of the Center for Liver Diseases at the University of Chicago.
The Centers for Disease Control and Prevention estimates that 3.5 million Americans have a chronic hepatitis C infection. Dr. Charlton believed the number today might be more like 1-2 million remaining chronic U.S. cases because of the strong impact of DAA treatment. These chronic infections largely remain because hepatitis C is mostly silent and many clinicians fail to act on screening recommendations. The new findings provide even greater incentive for more rigorous screening and treatment, Dr. Charlton suggested.
“As if you needed another reason to get rid of hepatitis C, lowering your cancer risk is now added to the list,” he said.
WASHINGTON – Treatment of hepatitis C infection with a direct-acting antiviral drug strongly linked with a rapid, 14% drop in the incidence of all nonhepatic cancers, based on analysis of data from more than 30,000 U.S. patients.
The data also showed compared with infected patients who had been treated with an interferon-based regimen during the period immediately preceding the availability of DAAs in late 2013. This included a 45% cut in lung cancers, a 49% cut in bladder cancer, a 62% relative risk reduction in leukemia, and a 29% drop in prostate cancer, Michael B. Charlton, MD, said at the annual Digestive Disease Week.®
The relative reductions in nonhepatic cancer incidence appeared soon after DAA treatment. The data Dr. Charlton reported reflected a median follow-up of 1 year for DAA-treated patients and 2.6 years for the hepatitis C–infected patients who had received interferon and did not get a DAA. A major difference between these two regimens is their efficacy, with DAA regimens producing sustained virologic response rates of 90% or better, while the interferon regimens produced substantially lower eradication rates.
“The most obvious hypothesis” to explain the observed effects is that “hepatitis C is a potent carcinogen,” possibly acting by inhibiting immune surveillance for new cancers in infected people, Dr. Charlton said in a video interview.
The study he reported used insurance-claims data from more than 146 million U.S. residents during 2007-2017 in the IQVIA PharMetrics Plus database, which included more than 367,000 adults infected with hepatitis C. Dr. Charlton and his associates pulled from this claims data on 10,989 of the infected patients who received interferon during January 2007-May 2011 (and followed through November 2013), and 22,894 infected patients treated with any type of DAA during December 2013 through March 2017. They used these two discrete time windows to completely separate the patients who received a DAA from those who did not.
The primary analysis calculated a hazard ratio for the development of any nonhepatic cancer after adjustment for a number of demographic and clinical covariates including age, smoking history, and weight, and also applied propensity-score weighting to the data. The Kaplan-Meier analysis of the data showed clear separation of the cancer-free survival curves of the two subgroups by 6 months of follow-up, and then showed steady further separation over time suggesting an ongoing carcinogenic effect from continued hepatitis C infection in patients who had received the less effective antiviral regimen. The analysis was able to reveal this effect because it had data from many thousands of treated hepatitis C patients, far more than had been enrolled in the pivotal trials for the DAAs, noted Dr. Charlton, professor and director of the Center for Liver Diseases at the University of Chicago.
The Centers for Disease Control and Prevention estimates that 3.5 million Americans have a chronic hepatitis C infection. Dr. Charlton believed the number today might be more like 1-2 million remaining chronic U.S. cases because of the strong impact of DAA treatment. These chronic infections largely remain because hepatitis C is mostly silent and many clinicians fail to act on screening recommendations. The new findings provide even greater incentive for more rigorous screening and treatment, Dr. Charlton suggested.
“As if you needed another reason to get rid of hepatitis C, lowering your cancer risk is now added to the list,” he said.
WASHINGTON – Treatment of hepatitis C infection with a direct-acting antiviral drug strongly linked with a rapid, 14% drop in the incidence of all nonhepatic cancers, based on analysis of data from more than 30,000 U.S. patients.
The data also showed compared with infected patients who had been treated with an interferon-based regimen during the period immediately preceding the availability of DAAs in late 2013. This included a 45% cut in lung cancers, a 49% cut in bladder cancer, a 62% relative risk reduction in leukemia, and a 29% drop in prostate cancer, Michael B. Charlton, MD, said at the annual Digestive Disease Week.®
The relative reductions in nonhepatic cancer incidence appeared soon after DAA treatment. The data Dr. Charlton reported reflected a median follow-up of 1 year for DAA-treated patients and 2.6 years for the hepatitis C–infected patients who had received interferon and did not get a DAA. A major difference between these two regimens is their efficacy, with DAA regimens producing sustained virologic response rates of 90% or better, while the interferon regimens produced substantially lower eradication rates.
“The most obvious hypothesis” to explain the observed effects is that “hepatitis C is a potent carcinogen,” possibly acting by inhibiting immune surveillance for new cancers in infected people, Dr. Charlton said in a video interview.
The study he reported used insurance-claims data from more than 146 million U.S. residents during 2007-2017 in the IQVIA PharMetrics Plus database, which included more than 367,000 adults infected with hepatitis C. Dr. Charlton and his associates pulled from this claims data on 10,989 of the infected patients who received interferon during January 2007-May 2011 (and followed through November 2013), and 22,894 infected patients treated with any type of DAA during December 2013 through March 2017. They used these two discrete time windows to completely separate the patients who received a DAA from those who did not.
The primary analysis calculated a hazard ratio for the development of any nonhepatic cancer after adjustment for a number of demographic and clinical covariates including age, smoking history, and weight, and also applied propensity-score weighting to the data. The Kaplan-Meier analysis of the data showed clear separation of the cancer-free survival curves of the two subgroups by 6 months of follow-up, and then showed steady further separation over time suggesting an ongoing carcinogenic effect from continued hepatitis C infection in patients who had received the less effective antiviral regimen. The analysis was able to reveal this effect because it had data from many thousands of treated hepatitis C patients, far more than had been enrolled in the pivotal trials for the DAAs, noted Dr. Charlton, professor and director of the Center for Liver Diseases at the University of Chicago.
The Centers for Disease Control and Prevention estimates that 3.5 million Americans have a chronic hepatitis C infection. Dr. Charlton believed the number today might be more like 1-2 million remaining chronic U.S. cases because of the strong impact of DAA treatment. These chronic infections largely remain because hepatitis C is mostly silent and many clinicians fail to act on screening recommendations. The new findings provide even greater incentive for more rigorous screening and treatment, Dr. Charlton suggested.
“As if you needed another reason to get rid of hepatitis C, lowering your cancer risk is now added to the list,” he said.
REPORTING FROM DDW 2018
Key clinical point: Eradicating hepatitis C with direct-acting antivirals significantly cut the incidence of many nonliver cancers.
Major finding: Direct-acting antiviral treatment linked with a 14% drop in nonhepatic cancers, compared with patients not getting this treatment.
Study details: Analysis of 33,883 Americans treated for hepatitis C during 2007-2017 in an insurance claims database.
Disclosures: The study was funded by Gilead, a company that markets direct-acting antiviral drugs for hepatitis C virus. Dr. Charlton has been a consultant to and has received research funding from Gilead and several other companies that market drugs from this class.
NAFLD patients with abnormal liver tests may not get statins when indicated
WASHINGTON – Though the liver safety of statins in patients with low-level liver enzyme elevations has long been established, some providers still hesitate to prescribe them to the patients with the conditions for which they are indicated.
Nonalcoholic fatty liver disease (NAFLD), hyperlipidemia, metabolic syndrome, and diabetes, which often co-occur, are also involved in cardiovascular disease. Cardiovascular disease is the most common cause of mortality in NAFLD, before liver disease.
Sonal Kumar, MD, MPH, of New York–Presbyterian Hospital described in a video interview at the annual Digestive Disease Week® a study she and her colleagues conducted to evaluate statin use in patients with hyperlipidemia by using data from the National Health and Nutrition Examination Survey during 2005-2014 (NHANES). Adult patients aged over 18 years were included if they did not have viral hepatitis, did not excessively consume alcohol, were not pregnant, and did not have transaminase levels over 500 IU/L.
Statin use was assessed in 136,833,627 participants by NHANES interviewers. Of these participants, 74.6% had hyperlipidemia (defined as LDL cholesterol greater than 130 mg/dL) and 93.5% were taking a statin. Patients with hyperlipidemia with abnormal alanine aminotransferase values were significantly less likely to be taking a statin (86.3% vs. 89.1%, P = .001). With multivariate analysis, abnormal ALT significantly decreased the odds of patients receiving a statin if they had diabetes (odds ratio, 0.75; 95% confidence interval, 0.57-0.99) when sex and age were controlled for.
Statins are underutilized in patients with NAFLD and diabetes, patient groups in whom they could help control cardiovascular disease risk factors, said Dr. Kumar. Providers need to be educated on the safety of statins in these patients to improve cardiovascular outcomes.
Dr. Kumar reported receiving support from Gilead and AbbVie.
WASHINGTON – Though the liver safety of statins in patients with low-level liver enzyme elevations has long been established, some providers still hesitate to prescribe them to the patients with the conditions for which they are indicated.
Nonalcoholic fatty liver disease (NAFLD), hyperlipidemia, metabolic syndrome, and diabetes, which often co-occur, are also involved in cardiovascular disease. Cardiovascular disease is the most common cause of mortality in NAFLD, before liver disease.
Sonal Kumar, MD, MPH, of New York–Presbyterian Hospital described in a video interview at the annual Digestive Disease Week® a study she and her colleagues conducted to evaluate statin use in patients with hyperlipidemia by using data from the National Health and Nutrition Examination Survey during 2005-2014 (NHANES). Adult patients aged over 18 years were included if they did not have viral hepatitis, did not excessively consume alcohol, were not pregnant, and did not have transaminase levels over 500 IU/L.
Statin use was assessed in 136,833,627 participants by NHANES interviewers. Of these participants, 74.6% had hyperlipidemia (defined as LDL cholesterol greater than 130 mg/dL) and 93.5% were taking a statin. Patients with hyperlipidemia with abnormal alanine aminotransferase values were significantly less likely to be taking a statin (86.3% vs. 89.1%, P = .001). With multivariate analysis, abnormal ALT significantly decreased the odds of patients receiving a statin if they had diabetes (odds ratio, 0.75; 95% confidence interval, 0.57-0.99) when sex and age were controlled for.
Statins are underutilized in patients with NAFLD and diabetes, patient groups in whom they could help control cardiovascular disease risk factors, said Dr. Kumar. Providers need to be educated on the safety of statins in these patients to improve cardiovascular outcomes.
Dr. Kumar reported receiving support from Gilead and AbbVie.
WASHINGTON – Though the liver safety of statins in patients with low-level liver enzyme elevations has long been established, some providers still hesitate to prescribe them to the patients with the conditions for which they are indicated.
Nonalcoholic fatty liver disease (NAFLD), hyperlipidemia, metabolic syndrome, and diabetes, which often co-occur, are also involved in cardiovascular disease. Cardiovascular disease is the most common cause of mortality in NAFLD, before liver disease.
Sonal Kumar, MD, MPH, of New York–Presbyterian Hospital described in a video interview at the annual Digestive Disease Week® a study she and her colleagues conducted to evaluate statin use in patients with hyperlipidemia by using data from the National Health and Nutrition Examination Survey during 2005-2014 (NHANES). Adult patients aged over 18 years were included if they did not have viral hepatitis, did not excessively consume alcohol, were not pregnant, and did not have transaminase levels over 500 IU/L.
Statin use was assessed in 136,833,627 participants by NHANES interviewers. Of these participants, 74.6% had hyperlipidemia (defined as LDL cholesterol greater than 130 mg/dL) and 93.5% were taking a statin. Patients with hyperlipidemia with abnormal alanine aminotransferase values were significantly less likely to be taking a statin (86.3% vs. 89.1%, P = .001). With multivariate analysis, abnormal ALT significantly decreased the odds of patients receiving a statin if they had diabetes (odds ratio, 0.75; 95% confidence interval, 0.57-0.99) when sex and age were controlled for.
Statins are underutilized in patients with NAFLD and diabetes, patient groups in whom they could help control cardiovascular disease risk factors, said Dr. Kumar. Providers need to be educated on the safety of statins in these patients to improve cardiovascular outcomes.
Dr. Kumar reported receiving support from Gilead and AbbVie.
REPORTING FROM DDW 2018
Key clinical point: Patients diagnosed with hyperlipidemia who had abnormal ALT levels were less likely to take a statin (86.3% vs. 89.1%, P = .001).
Major finding: Abnormal ALT significantly decreased the odds of patients receiving a statin if they had diabetes (odds ratio, 0.75; 95% confidence interval, 0.57-0.99) when sex and age were controlled for.
Data source: Data from 136,833,627 adult patients from the National Health and Nutrition Examination Survey collected during 2005-2014.
Disclosures: Dr. Kumar reported receiving support from Gilead and AbbVie.
Maintenance chemo boosts survival for youth with high-risk rhabdomyosarcoma
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Maintenance chemotherapy is life-prolonging for youth with high-risk rhabdomyosarcoma, finds a trial of 371 patients aged 0 to 21 years who had completed standard intensive therapy.
The 5-year rate of overall survival was 86.5% for those who received maintenance therapy with the combination of low-dose intravenous vinorelbine and oral cyclophosphamide, compared with 73.7% for those who did not, translating to a near halving of the risk of death (hazard ratio, 0.52; P = .0111). The regimen was well tolerated.
In an interview at the annual meeting of the American Society of Clinical Oncology, lead study author Gianni Bisogno, MD, PhD, discussed the risk-benefit profile of maintenance chemotherapy and the practice-changing nature of the new data. Dr. Bisogno, a professor at the University Hospital of Padova in Italy and chair of the European Paediatric Soft tissue Sarcoma Study Group, also described plans for a new trial that will explore alternate maintenance schedules and collaboration with colleagues in North America to further improve pediatric rhabdomyosarcoma outcomes.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Maintenance chemotherapy is life-prolonging for youth with high-risk rhabdomyosarcoma, finds a trial of 371 patients aged 0 to 21 years who had completed standard intensive therapy.
The 5-year rate of overall survival was 86.5% for those who received maintenance therapy with the combination of low-dose intravenous vinorelbine and oral cyclophosphamide, compared with 73.7% for those who did not, translating to a near halving of the risk of death (hazard ratio, 0.52; P = .0111). The regimen was well tolerated.
In an interview at the annual meeting of the American Society of Clinical Oncology, lead study author Gianni Bisogno, MD, PhD, discussed the risk-benefit profile of maintenance chemotherapy and the practice-changing nature of the new data. Dr. Bisogno, a professor at the University Hospital of Padova in Italy and chair of the European Paediatric Soft tissue Sarcoma Study Group, also described plans for a new trial that will explore alternate maintenance schedules and collaboration with colleagues in North America to further improve pediatric rhabdomyosarcoma outcomes.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Maintenance chemotherapy is life-prolonging for youth with high-risk rhabdomyosarcoma, finds a trial of 371 patients aged 0 to 21 years who had completed standard intensive therapy.
The 5-year rate of overall survival was 86.5% for those who received maintenance therapy with the combination of low-dose intravenous vinorelbine and oral cyclophosphamide, compared with 73.7% for those who did not, translating to a near halving of the risk of death (hazard ratio, 0.52; P = .0111). The regimen was well tolerated.
In an interview at the annual meeting of the American Society of Clinical Oncology, lead study author Gianni Bisogno, MD, PhD, discussed the risk-benefit profile of maintenance chemotherapy and the practice-changing nature of the new data. Dr. Bisogno, a professor at the University Hospital of Padova in Italy and chair of the European Paediatric Soft tissue Sarcoma Study Group, also described plans for a new trial that will explore alternate maintenance schedules and collaboration with colleagues in North America to further improve pediatric rhabdomyosarcoma outcomes.
REPORTING FROM ASCO 2018
TAILORx: Most women with intermediate risk ER+ breast cancer can safely skip chemo
CHICAGO – New data from the TAILORx trial are welcome news for women with HR-positive, HER2-negative, axillary node–negative early-stage breast cancer and their oncologists caught in the gray area surrounding the need for adjuvant chemotherapy.
Results of the noninferiority phase 3 trial—the largest adjuvant breast cancer treatment trial ever conducted—show that among the 6,711 women with an intermediate Oncotype DX Breast Recurrence Score (11-25), those who received only endocrine therapy and skipped adjuvant chemotherapy did not have worse invasive disease-free survival than counterparts who received both (hazard ratio, 1.08; P=.26).
The 9-year rate of invasive disease–free survival was 83.3% with endocrine therapy alone and 84.3% with both chemotherapy and endocrine therapy, and the pattern was essentially the same for freedom from any recurrence and distant recurrence, and overall survival.
The findings are practice changing, according to lead study author Joseph A. Sparano, MD, associate director for clinical research at the Albert Einstein Cancer Center and Montefiore Health System in New York, and vice-chair of the ECOG-ACRIN Cancer Research Group.
In a video interview at the annual meeting of the American Society of Clinical Oncology, he discussed implications of the new data for decision making, results of interaction analyses showing that one size does not fit all and certain women with intermediate recurrence scores do derive benefit from adjuvant chemotherapy, as well as plans to use the tumor samples for future analyses on those that do recur.
CHICAGO – New data from the TAILORx trial are welcome news for women with HR-positive, HER2-negative, axillary node–negative early-stage breast cancer and their oncologists caught in the gray area surrounding the need for adjuvant chemotherapy.
Results of the noninferiority phase 3 trial—the largest adjuvant breast cancer treatment trial ever conducted—show that among the 6,711 women with an intermediate Oncotype DX Breast Recurrence Score (11-25), those who received only endocrine therapy and skipped adjuvant chemotherapy did not have worse invasive disease-free survival than counterparts who received both (hazard ratio, 1.08; P=.26).
The 9-year rate of invasive disease–free survival was 83.3% with endocrine therapy alone and 84.3% with both chemotherapy and endocrine therapy, and the pattern was essentially the same for freedom from any recurrence and distant recurrence, and overall survival.
The findings are practice changing, according to lead study author Joseph A. Sparano, MD, associate director for clinical research at the Albert Einstein Cancer Center and Montefiore Health System in New York, and vice-chair of the ECOG-ACRIN Cancer Research Group.
In a video interview at the annual meeting of the American Society of Clinical Oncology, he discussed implications of the new data for decision making, results of interaction analyses showing that one size does not fit all and certain women with intermediate recurrence scores do derive benefit from adjuvant chemotherapy, as well as plans to use the tumor samples for future analyses on those that do recur.
CHICAGO – New data from the TAILORx trial are welcome news for women with HR-positive, HER2-negative, axillary node–negative early-stage breast cancer and their oncologists caught in the gray area surrounding the need for adjuvant chemotherapy.
Results of the noninferiority phase 3 trial—the largest adjuvant breast cancer treatment trial ever conducted—show that among the 6,711 women with an intermediate Oncotype DX Breast Recurrence Score (11-25), those who received only endocrine therapy and skipped adjuvant chemotherapy did not have worse invasive disease-free survival than counterparts who received both (hazard ratio, 1.08; P=.26).
The 9-year rate of invasive disease–free survival was 83.3% with endocrine therapy alone and 84.3% with both chemotherapy and endocrine therapy, and the pattern was essentially the same for freedom from any recurrence and distant recurrence, and overall survival.
The findings are practice changing, according to lead study author Joseph A. Sparano, MD, associate director for clinical research at the Albert Einstein Cancer Center and Montefiore Health System in New York, and vice-chair of the ECOG-ACRIN Cancer Research Group.
In a video interview at the annual meeting of the American Society of Clinical Oncology, he discussed implications of the new data for decision making, results of interaction analyses showing that one size does not fit all and certain women with intermediate recurrence scores do derive benefit from adjuvant chemotherapy, as well as plans to use the tumor samples for future analyses on those that do recur.
REPORTING FROM ASCO 2018
App monitoring improves quality of IBD care
WASHINGTON – in a single-center randomized study with 320 patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Based on this success, the app will soon be made available to all of the roughly 5,000 inflammatory bowel disease (IBD) patients managed at Mount Sinai Medical Center in New York as well as IBD patients at several other North American centers that plan to adopt the app, Ashish Atreja, MD, said at the annual Digestive Disease Week.®
Home monitoring of IBD patients “is feasible with high adoption,” said Dr. Atreja, a gastroenterologist at Mount Sinai who directs the Sinai AppLab. The 162 IBD patients randomized to regularly use the HealthPROMISE app had their quality-of-care metric rise from 50% at baseline to 84% after an average follow-up of 575 days (19 months), a statistically significant improvement over the 158 control patients whose metric rose from 50% to 65% for the study’s primary endpoint, he reported. The results also showed a trend toward improved quality of life among the patients using the HealthPROMISE app, compared with the controls, who used an IBD educational app that produced less patient engagement than did the HealthPROMISE app, Dr Atreja said.
Dr. Atreja and his associates modeled the app on remote monitoring methods developed for patients with other types of chronic disease, such as diabetes and heart failure.
“You can’t provide proactive IBD care without remote monitoring,” Dr. Atreja explained in a video interview. “Reactive care is not best practice anymore. The only way to do treat-to-target is with remote monitoring.”
Care coordinators monitor the entries that IBD patients send in via the app. Dr. Atreja estimated that about five care coordinators will be able to track the inputs from the roughly 5,000 IBD patients at Mount Sinai who will soon begin using the app. The financial feasibility of this approach depends in part on the $45/patient per month reimbursement that U.S. health insurers now provide to centers that run remote monitoring programs, he said.
“The direction for managing chronic diseases is increasingly looking at home monitoring as a way to streamline costs and improve patient care,” commented Gil Y. Melmed, MD, director of Clinical Inflammatory Bowel Disease at Cedars-Sinai Medical Center in Los Angeles. The results that Dr. Atreja reported came from “a highly selected population that was well educated and largely white.” The study needs replication in different patient groups to establish its reproducibility and generalizability, Dr. Melmed said in an interview.
Dr. Melmed had no relevant disclosures.
[email protected]
On Twitter @mitchelzoler
SOURCE: Atreja A et al. Digestive Disease Week 2018 abstract 17.
*This story was updated on June 7, 2018.
WASHINGTON – in a single-center randomized study with 320 patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Based on this success, the app will soon be made available to all of the roughly 5,000 inflammatory bowel disease (IBD) patients managed at Mount Sinai Medical Center in New York as well as IBD patients at several other North American centers that plan to adopt the app, Ashish Atreja, MD, said at the annual Digestive Disease Week.®
Home monitoring of IBD patients “is feasible with high adoption,” said Dr. Atreja, a gastroenterologist at Mount Sinai who directs the Sinai AppLab. The 162 IBD patients randomized to regularly use the HealthPROMISE app had their quality-of-care metric rise from 50% at baseline to 84% after an average follow-up of 575 days (19 months), a statistically significant improvement over the 158 control patients whose metric rose from 50% to 65% for the study’s primary endpoint, he reported. The results also showed a trend toward improved quality of life among the patients using the HealthPROMISE app, compared with the controls, who used an IBD educational app that produced less patient engagement than did the HealthPROMISE app, Dr Atreja said.
Dr. Atreja and his associates modeled the app on remote monitoring methods developed for patients with other types of chronic disease, such as diabetes and heart failure.
“You can’t provide proactive IBD care without remote monitoring,” Dr. Atreja explained in a video interview. “Reactive care is not best practice anymore. The only way to do treat-to-target is with remote monitoring.”
Care coordinators monitor the entries that IBD patients send in via the app. Dr. Atreja estimated that about five care coordinators will be able to track the inputs from the roughly 5,000 IBD patients at Mount Sinai who will soon begin using the app. The financial feasibility of this approach depends in part on the $45/patient per month reimbursement that U.S. health insurers now provide to centers that run remote monitoring programs, he said.
“The direction for managing chronic diseases is increasingly looking at home monitoring as a way to streamline costs and improve patient care,” commented Gil Y. Melmed, MD, director of Clinical Inflammatory Bowel Disease at Cedars-Sinai Medical Center in Los Angeles. The results that Dr. Atreja reported came from “a highly selected population that was well educated and largely white.” The study needs replication in different patient groups to establish its reproducibility and generalizability, Dr. Melmed said in an interview.
Dr. Melmed had no relevant disclosures.
[email protected]
On Twitter @mitchelzoler
SOURCE: Atreja A et al. Digestive Disease Week 2018 abstract 17.
*This story was updated on June 7, 2018.
WASHINGTON – in a single-center randomized study with 320 patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Based on this success, the app will soon be made available to all of the roughly 5,000 inflammatory bowel disease (IBD) patients managed at Mount Sinai Medical Center in New York as well as IBD patients at several other North American centers that plan to adopt the app, Ashish Atreja, MD, said at the annual Digestive Disease Week.®
Home monitoring of IBD patients “is feasible with high adoption,” said Dr. Atreja, a gastroenterologist at Mount Sinai who directs the Sinai AppLab. The 162 IBD patients randomized to regularly use the HealthPROMISE app had their quality-of-care metric rise from 50% at baseline to 84% after an average follow-up of 575 days (19 months), a statistically significant improvement over the 158 control patients whose metric rose from 50% to 65% for the study’s primary endpoint, he reported. The results also showed a trend toward improved quality of life among the patients using the HealthPROMISE app, compared with the controls, who used an IBD educational app that produced less patient engagement than did the HealthPROMISE app, Dr Atreja said.
Dr. Atreja and his associates modeled the app on remote monitoring methods developed for patients with other types of chronic disease, such as diabetes and heart failure.
“You can’t provide proactive IBD care without remote monitoring,” Dr. Atreja explained in a video interview. “Reactive care is not best practice anymore. The only way to do treat-to-target is with remote monitoring.”
Care coordinators monitor the entries that IBD patients send in via the app. Dr. Atreja estimated that about five care coordinators will be able to track the inputs from the roughly 5,000 IBD patients at Mount Sinai who will soon begin using the app. The financial feasibility of this approach depends in part on the $45/patient per month reimbursement that U.S. health insurers now provide to centers that run remote monitoring programs, he said.
“The direction for managing chronic diseases is increasingly looking at home monitoring as a way to streamline costs and improve patient care,” commented Gil Y. Melmed, MD, director of Clinical Inflammatory Bowel Disease at Cedars-Sinai Medical Center in Los Angeles. The results that Dr. Atreja reported came from “a highly selected population that was well educated and largely white.” The study needs replication in different patient groups to establish its reproducibility and generalizability, Dr. Melmed said in an interview.
Dr. Melmed had no relevant disclosures.
[email protected]
On Twitter @mitchelzoler
SOURCE: Atreja A et al. Digestive Disease Week 2018 abstract 17.
*This story was updated on June 7, 2018.
REPORTING FROM DDW 2018
Key clinical point: Regular remote monitoring of IBD patients improved the medical care they received.
Major finding: Quality of care rose from 50% at baseline to 84% in app-monitored patients and to 65% in controls.
Study details: A single-center randomized study with 320 IBD patients.
Disclosures: The study had no commercial funding. Dr. Atreja had no disclosures.
Source: Atreja A et al. Digestive Disease Week 2018 abstract 17.
A blood test to detect lung cancer inches toward the clinic
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO - Uptake of recommended low-dose CT for lung cancer screening has been dismal. Blood-based assays are an attractive alternative being explored by the Circulating Cell–Free Genome Atlas (CCGA) project. Interim results of a CCGA study of 561 individuals without cancer and 118 patients with lung cancers of all stages have found that a trio of assays searching for molecular signatures in plasma cell-free DNA achieved roughly 50% sensitivity for detection of early-stage (stage I-IIIA) lung cancers and 91% sensitivity for detection of late-stage (stage IIIB-IV) lung cancers.
In this video interview from the annual meeting of the American Society of Clinical Oncology, lead study author Geoffrey R. Oxnard, MD, of the Dana-Farber Cancer Institute, Boston, discusses the science behind these assays, how they may fill an unmet medical need, and ongoing work to bring them into the clinic.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO - Uptake of recommended low-dose CT for lung cancer screening has been dismal. Blood-based assays are an attractive alternative being explored by the Circulating Cell–Free Genome Atlas (CCGA) project. Interim results of a CCGA study of 561 individuals without cancer and 118 patients with lung cancers of all stages have found that a trio of assays searching for molecular signatures in plasma cell-free DNA achieved roughly 50% sensitivity for detection of early-stage (stage I-IIIA) lung cancers and 91% sensitivity for detection of late-stage (stage IIIB-IV) lung cancers.
In this video interview from the annual meeting of the American Society of Clinical Oncology, lead study author Geoffrey R. Oxnard, MD, of the Dana-Farber Cancer Institute, Boston, discusses the science behind these assays, how they may fill an unmet medical need, and ongoing work to bring them into the clinic.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO - Uptake of recommended low-dose CT for lung cancer screening has been dismal. Blood-based assays are an attractive alternative being explored by the Circulating Cell–Free Genome Atlas (CCGA) project. Interim results of a CCGA study of 561 individuals without cancer and 118 patients with lung cancers of all stages have found that a trio of assays searching for molecular signatures in plasma cell-free DNA achieved roughly 50% sensitivity for detection of early-stage (stage I-IIIA) lung cancers and 91% sensitivity for detection of late-stage (stage IIIB-IV) lung cancers.
In this video interview from the annual meeting of the American Society of Clinical Oncology, lead study author Geoffrey R. Oxnard, MD, of the Dana-Farber Cancer Institute, Boston, discusses the science behind these assays, how they may fill an unmet medical need, and ongoing work to bring them into the clinic.
REPORTING FROM ASCO 2018