User login
Correcting pelvic organ prolapse with robotic sacrocolpopexy
- Promontory dissection and creation of the retroperitoneal tunnel
- Dissection of the rectovaginal and vesicovaginal spaces
- Attachment of y-mesh to vagina: Start anteriorly
- Posterior y-mesh attachment
- Attachment of mesh to sacral promontory
These videos were provided by Catherine A. Matthews, MD.
Recent years have seen growing recognition that adequate support of the vaginal apex is an essential component of durable surgical repair of pelvic organ prolapse.1,2 Sacrocolpopexy is now considered the gold standard for repair of Level-1 defects of pelvic support, providing excellent long-term results.3-5
A recent randomized, controlled trial demonstrated the superior efficacy of laparoscopic sacrocolpopexy to a total vaginal mesh procedure in women who have vaginal vault prolapse—further evidence that sacrocolpopexy is the procedure of choice for these patients.6
The advantages of sacrocolpopexy include:
- reduced risk of mesh exposure, compared to insertion of vaginal mesh
- preservation of vaginal length
- reduced risk of re-operation for symptomatic recurrent prolapse
- reduced risk of de novo dyspareunia secondary to contraction of mesh.
Obstacles. Although a small number of surgeons are able to accomplish sacrocolpopexy using standard laparoscopic techniques, most of these procedures are still performed by laparotomy because the extensive suturing and knot-tying present a surgical challenge. Open sacrocolpopexy has disadvantages, too, including more pain, longer recovery, and longer length of stay.7-9
With the introduction of the da Vinci robot (Intuitive Surgical), the feasibility of having more surgeons perform this operation using a reproducible, minimally-invasive technique is much greater. The steep learning curve associated with standard laparoscopy in regard to mastering intracorporeal knot-tying and suturing is greatly diminished by articulating instruments. This makes robotic sacrocolpopexy an accessible option for all gynecologic surgeons who treat women with pelvic organ prolapse.
In this article, I detail the steps—with tips and tricks from my experience—to completing an efficient robotic-assisted sacrocolpopexy—modeled exactly after the open technique—that utilizes a y-shaped polypropylene mesh graft. Included is capsule advice from OBG Management’s coding consultant on obtaining reimbursement for robotic procedures (see “ Coding tips for robotic sacrocolpopexy”).
- Two proficient tableside assistants are needed
- Use steep Trendelenburg to remove the bowel from the operative field
- A fan retractor is necessary in some cases to gain access to the promontory
- Correct identification of the sacral promontory is key
- In the absence of haptic feedback, novice surgeons must be aware of the potential danger in dissecting too far laterally and entering the common iliac vessels
- Y-shaped grafts should be fashioned individually
- Know the exit point of the needle at the promontory
- Adequate spacing between the robotic arms is essential to avoiding interference among instruments during the procedure.
Details of the procedure
1. Surgical preparation, set-up
The patient completes a bowel prep using two bottles of magnesium citrate and taking only clear liquids 1 day before surgery. Although mechanical bowel cleansing has not been shown to decrease operative morbidity, manipulation and retraction of the sigmoid colon may be easier with the bowel empty.
Perioperative antibiotics are administered 30 minutes prior to the procedure. Heparin, 5,000 U, is injected subcutaneously for thromboprophylaxis as the patient is en route to the operating suite.
The patient is placed in the dorsal lithotomy position, buttocks extending one inch over the end of the operating table. The table should be covered with egg-crate foam to avoid having her slip down while in steep Trendelenburg position.
After the patient is prepped and draped, a Foley catheter is placed into the bladder. EEA sizers (Covidien) are inserted into the vagina and rectum.
Two experienced surgical assistants are necessary:
- One on the patient’s right side to assist with tissue retraction and introduction of suture material
- Another seated between the patient’s legs to provide adequate vaginal and rectal manipulation during surgery.
2. Port placement, docking, and instrumentation
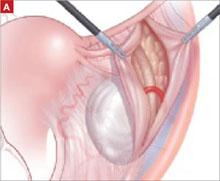
Pneumoperitoneum is obtained with a Veress needle. Five trocars are then placed (FIGURE 1).
Careful port placement is integral to the success of this procedure because:
- Inadequate distance between robotic arms and the camera results in arm collisions and interference
- Visualization and access to the sacral promontory may be compromised if the camera is inserted too low on the anterior abdominal wall
- Bowel retraction may be compromised if the fourth arm of the robot isn’t at least 3 cm above the anterior superior iliac crest.
My experience evaluating the abdomen before trocar insertion is that at least 15 cm is required between the pubic bone and the umbilicus to rely on this landmark for locating the 12-mm camera port.10 If this distance is shorter (as it is in many obese women), insertion above the umbilicus is necessary.
An accessory 12-mm port, used to introduce sutures and the mesh graft, is placed approximately 10 cm lateral and 4 cm cephalad to the camera in the right-upper quadrant.
An 8-mm robotic port is placed in the right lower quadrant, 10 cm lateral to the accessory port and approximately 3 cm above the anterior superior iliac crest.
The third and fourth robotic arms are placed 10 cm apart in the left lower quadrant, with the fourth arm typically as far lateral as possible.
Docking. After the patient has been placed in steep Trendelenburg position and the table is locked, the robot is docked from the patient’s left side at a 45° angle to the table. Side-docking permits easy access to the vagina to 1) evaluate graft tension and 2) complete cystoscopy to ensure ureteral and bladder integrity.
TIP Take care to ensure that the spine of the robot is positioned right next to the bed at the level of the patient’s hip; driving it up too high in relation to the abdomen can compromise the mobility of the fourth arm. In addition, if the robot is not close enough to the bed, the reach of the first (right) arm may be limited.
Next, introduce monopolar scissors through the right arm; a bipolar PK Dissector (Intuitive Surgical) through the left arm; and an atraumatic bowel grasper, such as Cadiere Forceps (Intuitive Surgical), through the fourth arm.
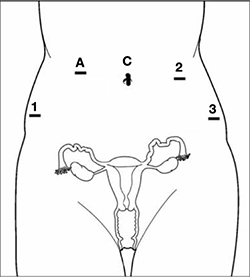
FIGURE 1 Placement of 5 ports for robotic sacrocolpopexy
Key: C, camera; A, accessory port; 1, right arm (monopolar shears); 2, left arm (PK Dissector); 3, fourth arm (Cadiere Forceps).
3. Dissect the sacral promontory and create a retroperitoneal tunnel
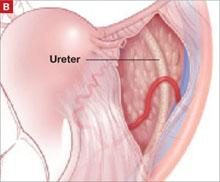
With the use of a 0° scope or 30° down-scope, retract the sigmoid colon laterally using Cadiere forceps and identify the right ureter.
TIP When you attempt robotic sacrocolpopexy for the first time, it may help to identify the sacral promontory, using a standard laparoscopic instrument with haptic feedback, before you dock the robot.
Elevate the peritoneum overlying the sacral promontory and open it using monopolar cautery. Expose the fat pad that overlays the anterior longitudinal ligament and gently dissect it away (FIGURE 2; VIDEO 1). Often, the middle sacral artery is visualized; it can be coagulated using the PK Dissector if necessary.
TIP In a case in which the promontory is difficult to find, dissecting the retrorectal space is a simple way to mobilize the bowel away from the sacrum, thus exposing the promontory.
TRICK Instead of opening the peritoneum from the sacrum to the cul-de-sac, I create a retroperitoneal tunnel along that right paracolic gutter, from the promontory to just medial to the right uterosacral ligament (VIDEO 1). Doing so has three benefits:
- It is quicker and less bloody
- It allows the mesh to lay flat in the hollow of the sacrum when you bring the sacral arm up to the promontory
- There is much less peritoneum to close over the mesh at the end of the procedure.
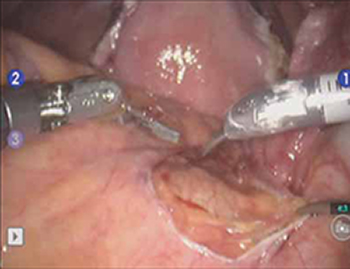
FIGURE 2 Entering the peritoneum
Open the peritoneum at the sacral promontory and dissect the fat pad. This reveals the anterior longitudinal ligament.
4. Dissect the vesicovaginal and rectovaginal spaces
Effective vaginal and rectal manipulation is critical to complete this part of the procedure safely. To gain access to the rectovaginal space, the vaginal assistant needs to push the vagina all the way in and up toward the anterior abdominal wall (the handle of the EEA sizer will be pushing hard up against the perineum) while simultaneously pushing the rectal probe downward (effectively scissoring the two apart).
From the exit point of the retroperitoneal tunnel that was created at the beginning of the case, then extend the peritoneal incision transversely in the shape of a “T” to expose the posterior vaginal wall (FIGURE 3, VIDEO 2). If indicated, dissect the rectovaginal space all the way down to the perineal body.
Deviate the vagina posteriorly to facilitate dissection of the bladder from the anterior vaginal wall. Use sharp dissection with scissors and short bursts of energy with monopolar cautery.
TIP If you encounter significant scarring between the bladder and vagina, retrograde-fill the bladder with 300 mL of saline mixed with methylene blue dye to identify the surgical plane.
Expose approximately 4 to 6 cm of anterior vaginal wall, depending on the degree of anterior vaginal wall prolapse. Try to leave the peritoneum intact at the apex of the vagina to reduce the chance that mesh will erode.
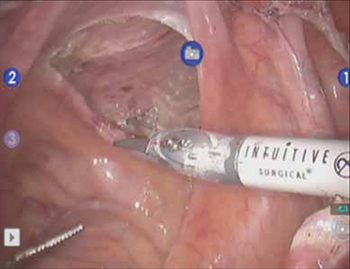
FIGURE 3 The peritoneal incision
Extend the peritoneal incision along the cul-de-sac to the posterior vaginal wall in a T-shaped configuration to gain access the rectovaginal space. When perorming cervicosacropexy, it is easiest to develop this surgical plane before amputating the cervix.
5. Attach the y-mesh to the vagina
Several mesh options exist: IntePro (American Medical Systems), Alyte (Bard Medical), and Restorelle Y (Mpathy Medical) are preformed Type-1 polypropylene mesh products. A correctly sized mesh can easily be fashioned by suturing together two strips of Gynemesh (Ethicon) that are approximately 3 cm wide.
Because there can be significant variability in the relative dimensions of the anterior and posterior segments of mesh, I recommend fashioning the graft after dissection is complete: When posterior wall prolapse is extensive, for example, preformed y-mesh strips may not be long enough to reach all the way down to the perineal body. After having assessed the differences in graft placement and manipulation when the two arms are sutured together 1) before the grafts are placed intracorporeally and 2) after they are placed, I’ve concluded that the first method—suturing before placement—is far easier.
Introduce the mesh graft through the accessory port after exchanging the scissors and PK dissector for a suture cutter and a large needle driver. Retract the bladder using the fourth arm, and place the anterior mesh arm over the anterior vaginal wall; suture it in place using 2-0 Gore-Tex sutures on CT-2 needles that are each cut to 6 inches long.
For greatest efficiency, anchor the two distal corners first (FIGURE 4; VIDEO 3), then place a series of interrupted stitches towards the vaginal apex. Tie the knots using 2 surgeon’s knots, followed by 2 half-hitches. Attempt to achieve healthy bites through the vaginal muscularis without perforating the epithelium.
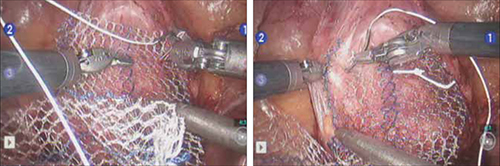
FIGURE 4 Suturing the mesh graft to the vaginal wall
Left and right: Suture the y-shaped polypropylene mesh graft to the anterior vaginal wall first, starting at the distal corners. The bladder is retracted cephalad using the fourth arm.After you’ve adequately secured the anterior mesh arm, deviate the vagina anteriorly and drape the posterior mesh arm over the posterior vaginal wall with the assistance of the fourth robotic arm that can hold upward traction on the sacral end of the mesh graft. Starting at the vaginal apex, place 6 to 8 interrupted sutures to secure the mesh to the posterior vaginal wall (FIGURE 5; VIDEO 4). If necessary, exchange the 0° scope for a 30° up-scope so that you can fully visualize the rectovaginal space.
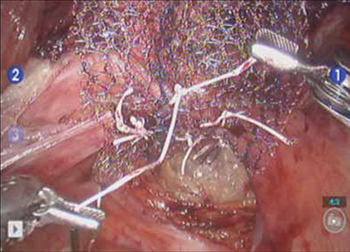
FIGURE 5 Attachment of the posterior arm of the mesh
The fourth arm of the robot provides upward traction on the sacral portion of the mesh graft.
6. Attach the graft to the sacrum
Again retract the sigmoid laterally to expose the promontory dissection. Retrieve the sacral arm of the mesh through the retroperitoneal tunnel and pull it up toward the promontory. Deviate the vagina toward the sacrum and, ensuring that there is no excessive tension, suture the sacral portion of the mesh graft to the anterior longitudinal ligament at the promontory, using 2 or 3 interrupted sutures (FIGURE 6, VIDEO 5).
When placing the needle during this critical juncture, it is important to rotate through the ligament along the curvature of the needle—as opposed to driving the needle forward and risking exiting farther laterally than expected.
TIP Because of the slight traction that exists on the mesh, a slip-knot is preferred instead of a surgeon’s knot when suturing the sacral portion of the mesh graft to the anterior longitudinal ligament. Take care to visualize the middle sacral artery and either suture around it or cauterize it.
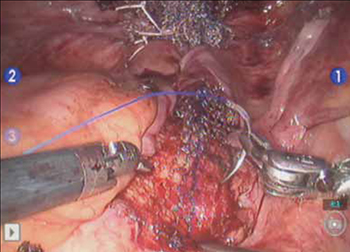
FIGURE 6 Suture the mesh directly to the anterior longitudinal ligament
Use two or three stitches, secured with slip-knots. Take care not to create excessive tension on the mesh graft.
7. Extraperitonealize the mesh
I no longer routinely close the peritoneum over the mesh at the vaginal apex because I have not had a single case of small-bowel obstruction since I began performing this procedure laparoscopically. You should close the peritoneal window at the promontory, however, if the mesh is tented up at all, because tenting creates the potential for bowel to get caught beneath the mesh. Perform this closure using 2-0 monofilament suture or 2-0 Vicryl suture (Ethicon) cut to 8 inches (FIGURE 7). It is always easiest to start distally and suture towards the camera and operative instruments.
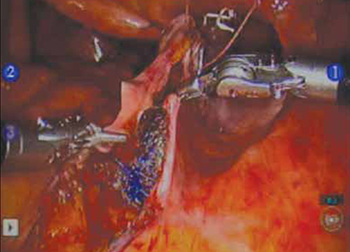
FIGURE 7 Extraperitonealize the mesh
Close the peritoneum from the apex of the vagina with a purse-string—like stitch, continuing it to the promontory in running fashion.
8. Ensure that mesh is not in the bladder or rectum
Perform cystoscopy and a rectal examination at the end of each case to confirm that no sutures or mesh material are within either viscus. It is much easier to remove these before leaving the operating room.
Modifying the procedure for uterovaginal prolapse
If the patient has an intact uterus and benign cervical cytology, perform supracervical hysterectomy before proceeding with Steps 1–8 above.
TIP Leaving the cervix in situ may reduce the chance of mesh erosion and provides an excellent platform for mesh attachment.
TRICK I find it most helpful to fully dissect the anterior and posterior vaginal walls before cervical amputation because upward traction on the corpus improves visualization of the surgical planes.
Once the cervix is amputated, however, effective vaginal manipulation can present a surgical challenge. Some surgeons use a tenaculum attached to the fourth arm of the robot to apply traction on the cervix, but this eliminates this arm from performing other necessary tasks. Malleable or Breisky-Navratil retractors can be used to delineate the anterior and posterior vaginal fornices, but are not always satisfactory—especially if an assistant isn’t seated between the legs.
TIP A useful and inexpensive instrument is the Colpo-Probe vaginal fornix delineator (Cooper Surgical) (FIGURE 8), which not only assists in dissecting the vagina from the bladder and rectum but also provides a stable surface during attachment of mesh.

FIGURE 8 Colpo-Probe
This device delineates the anterior and posterior vaginal fornices. It provides a stable platform against which to suture the mesh graft.
Tips and tricks for managing hemorrhage during sacrocolpopexy
Four potential areas of bleeding danger exist:
- In trying to find the sacral promontory, you risk entering the right common iliac vein if dissection is too far cephalad and lateral.
TIP I strongly advise novice robotic surgeons to try to identify the site of the promontory with a standard laparoscopic instrument with haptic feedback before moving to the surgical console.
TRICK Another trick that can help with safe identification of the promontory is mobilization of the sigmoid colon away from the sacrum by developing the retrorectal space. - During dissection of the fat pad from the promontory, you can encounter the middle sacral artery.
TRICK Spreading carefully in a caudal–cephalad direction until the level of the ligament is reached, instead of spreading in a lateral dimension, can decrease the chances of lacerating of this vessel. - A dangerous plexus of veins traverses the hollow of the sacrum. If you are trying to affix mesh at the level of S2-3, therefore, you may encounter significant bleeding.
TIP Work above the level of S1 to avoid these veins completely. - In securing the mesh to the sacral promontory, you can puncture the left common iliac vein if you are not aware of the exit point of the needle and it traverses too far medially.
TIP If you encounter bleeding, introduce a RAY-TEC sponge (Johnson & Johnson) through the accessory port. Apply manual compression for at least 5 minutes. If bleeding persists, I recommend Floseal Hemostatic Matrix (Baxter) to control hemorrhage that arises from arterial and venous sources.
TRICK As last resort, perform emergency undocking in rapid fashion while the bedside assistant maintains pressure through the accessory port.
Conclusion
The da Vinci robotic surgical system facilitates completion of sacrocolpopexy and cervicosacropexy, in a manner identical to the open technique, by surgeons who may not possess advanced laparoscopic skills. Full knowledge of the relevant anatomy is critical; there is the potential for significant morbidity during the procedure if surgical planes are created incorrectly.
Robotic surgery in on the rise, but coding for robotic procedures is still limited to the basic, conventional procedure. Why? Because insurers reimburse the hospital for use of the equipment but still refuse to reimburse the surgeon any additional amount for incorporating the robot into the surgical technique.
Coding for your work when performing robotic sacrocolpopexy is straightforward: Report laparoscopic code 57425 (laparoscopy, surgical, colpopexy [suspension of vaginal apex]) for the procedure. Mesh that might be used as part of the repair is not reported separately.
Blue Cross/Blue Shield (BC/BS) added the Healthcare Common Procedure Coding System Level-II code S2900 (surgical techniques requiring use of robotic surgical system) to the national code set a few years ago. Although BC/BS and some other payers accept this code on the claim, there is no reimbursement attached: It was developed for informational purposes only.
Remember, however, that coding is complete only when you have an appropriate linking diagnosis to establish the medical necessity of laparoscopic sacrocolpopexy. Diagnostic coding options for this surgical procedure include documentation of uterovaginal prolapse (incomplete prolapse is 618.2; complete prolapse is 618.3); vaginal vault prolapse after hysterectomy (618.5); and uterine prolapse without vaginal wall prolapse (618.1).
—MELANIE WITT, RN, CPC, COBGC, MA
We want to hear from you! Tell us what you think.
1. Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438-1443.
2. Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2007;(3):CD004014.-
3. Sullivan ES, Longaker CJ, Lee PY. Total pelvic mesh repair: a ten-year experience. Dis Colon Rectum. 2001;44(6):857-863.
4. Culligan PJ, Murphy M, Blackwell L, Hammons G, Graham C, Heit MH. Long-term success of abdominal sacral colpopexy using synthetic mesh. Am J Obstet Gynecol. 2002;187(6):1473-1482.
5. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104(4):805-823.
6. Maher CF, Feiner B, DeCuyper EM, Nichlos CJ, Hickey KV, O’Rourke P. Laparoscopic sacral colpopexy versus total vaginal mesh for vaginal vault prolapse: a randomized trial. Am J Obstet Gynecol. 2011;204(4):360.e1-7.
7. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic sacral colpopexy for vaginal vault prolapse. Obstet Gynecol. 1994;84(5):885-888.
8. Ross JW. Techniques of laparoscopic repair of total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4(2):173-183.
9. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy hysterectomy, and burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6(2):115-119.
10. Matthews CA, Schubert CM, Woodward AP, Gill EJ. Variance in abdominal wall anatomy and port placement in women undergoing robotic gynecologic surgery. J Minim Invasive Gynecol. 2010;17(5):583-586.
- Promontory dissection and creation of the retroperitoneal tunnel
- Dissection of the rectovaginal and vesicovaginal spaces
- Attachment of y-mesh to vagina: Start anteriorly
- Posterior y-mesh attachment
- Attachment of mesh to sacral promontory
These videos were provided by Catherine A. Matthews, MD.
Recent years have seen growing recognition that adequate support of the vaginal apex is an essential component of durable surgical repair of pelvic organ prolapse.1,2 Sacrocolpopexy is now considered the gold standard for repair of Level-1 defects of pelvic support, providing excellent long-term results.3-5
A recent randomized, controlled trial demonstrated the superior efficacy of laparoscopic sacrocolpopexy to a total vaginal mesh procedure in women who have vaginal vault prolapse—further evidence that sacrocolpopexy is the procedure of choice for these patients.6
The advantages of sacrocolpopexy include:
- reduced risk of mesh exposure, compared to insertion of vaginal mesh
- preservation of vaginal length
- reduced risk of re-operation for symptomatic recurrent prolapse
- reduced risk of de novo dyspareunia secondary to contraction of mesh.
Obstacles. Although a small number of surgeons are able to accomplish sacrocolpopexy using standard laparoscopic techniques, most of these procedures are still performed by laparotomy because the extensive suturing and knot-tying present a surgical challenge. Open sacrocolpopexy has disadvantages, too, including more pain, longer recovery, and longer length of stay.7-9
With the introduction of the da Vinci robot (Intuitive Surgical), the feasibility of having more surgeons perform this operation using a reproducible, minimally-invasive technique is much greater. The steep learning curve associated with standard laparoscopy in regard to mastering intracorporeal knot-tying and suturing is greatly diminished by articulating instruments. This makes robotic sacrocolpopexy an accessible option for all gynecologic surgeons who treat women with pelvic organ prolapse.
In this article, I detail the steps—with tips and tricks from my experience—to completing an efficient robotic-assisted sacrocolpopexy—modeled exactly after the open technique—that utilizes a y-shaped polypropylene mesh graft. Included is capsule advice from OBG Management’s coding consultant on obtaining reimbursement for robotic procedures (see “ Coding tips for robotic sacrocolpopexy”).
- Two proficient tableside assistants are needed
- Use steep Trendelenburg to remove the bowel from the operative field
- A fan retractor is necessary in some cases to gain access to the promontory
- Correct identification of the sacral promontory is key
- In the absence of haptic feedback, novice surgeons must be aware of the potential danger in dissecting too far laterally and entering the common iliac vessels
- Y-shaped grafts should be fashioned individually
- Know the exit point of the needle at the promontory
- Adequate spacing between the robotic arms is essential to avoiding interference among instruments during the procedure.
Details of the procedure
1. Surgical preparation, set-up
The patient completes a bowel prep using two bottles of magnesium citrate and taking only clear liquids 1 day before surgery. Although mechanical bowel cleansing has not been shown to decrease operative morbidity, manipulation and retraction of the sigmoid colon may be easier with the bowel empty.
Perioperative antibiotics are administered 30 minutes prior to the procedure. Heparin, 5,000 U, is injected subcutaneously for thromboprophylaxis as the patient is en route to the operating suite.
The patient is placed in the dorsal lithotomy position, buttocks extending one inch over the end of the operating table. The table should be covered with egg-crate foam to avoid having her slip down while in steep Trendelenburg position.
After the patient is prepped and draped, a Foley catheter is placed into the bladder. EEA sizers (Covidien) are inserted into the vagina and rectum.
Two experienced surgical assistants are necessary:
- One on the patient’s right side to assist with tissue retraction and introduction of suture material
- Another seated between the patient’s legs to provide adequate vaginal and rectal manipulation during surgery.
2. Port placement, docking, and instrumentation
Pneumoperitoneum is obtained with a Veress needle. Five trocars are then placed (FIGURE 1).
Careful port placement is integral to the success of this procedure because:
- Inadequate distance between robotic arms and the camera results in arm collisions and interference
- Visualization and access to the sacral promontory may be compromised if the camera is inserted too low on the anterior abdominal wall
- Bowel retraction may be compromised if the fourth arm of the robot isn’t at least 3 cm above the anterior superior iliac crest.
My experience evaluating the abdomen before trocar insertion is that at least 15 cm is required between the pubic bone and the umbilicus to rely on this landmark for locating the 12-mm camera port.10 If this distance is shorter (as it is in many obese women), insertion above the umbilicus is necessary.
An accessory 12-mm port, used to introduce sutures and the mesh graft, is placed approximately 10 cm lateral and 4 cm cephalad to the camera in the right-upper quadrant.
An 8-mm robotic port is placed in the right lower quadrant, 10 cm lateral to the accessory port and approximately 3 cm above the anterior superior iliac crest.
The third and fourth robotic arms are placed 10 cm apart in the left lower quadrant, with the fourth arm typically as far lateral as possible.
Docking. After the patient has been placed in steep Trendelenburg position and the table is locked, the robot is docked from the patient’s left side at a 45° angle to the table. Side-docking permits easy access to the vagina to 1) evaluate graft tension and 2) complete cystoscopy to ensure ureteral and bladder integrity.
TIP Take care to ensure that the spine of the robot is positioned right next to the bed at the level of the patient’s hip; driving it up too high in relation to the abdomen can compromise the mobility of the fourth arm. In addition, if the robot is not close enough to the bed, the reach of the first (right) arm may be limited.
Next, introduce monopolar scissors through the right arm; a bipolar PK Dissector (Intuitive Surgical) through the left arm; and an atraumatic bowel grasper, such as Cadiere Forceps (Intuitive Surgical), through the fourth arm.

FIGURE 1 Placement of 5 ports for robotic sacrocolpopexy
Key: C, camera; A, accessory port; 1, right arm (monopolar shears); 2, left arm (PK Dissector); 3, fourth arm (Cadiere Forceps).
3. Dissect the sacral promontory and create a retroperitoneal tunnel
With the use of a 0° scope or 30° down-scope, retract the sigmoid colon laterally using Cadiere forceps and identify the right ureter.
TIP When you attempt robotic sacrocolpopexy for the first time, it may help to identify the sacral promontory, using a standard laparoscopic instrument with haptic feedback, before you dock the robot.
Elevate the peritoneum overlying the sacral promontory and open it using monopolar cautery. Expose the fat pad that overlays the anterior longitudinal ligament and gently dissect it away (FIGURE 2; VIDEO 1). Often, the middle sacral artery is visualized; it can be coagulated using the PK Dissector if necessary.
TIP In a case in which the promontory is difficult to find, dissecting the retrorectal space is a simple way to mobilize the bowel away from the sacrum, thus exposing the promontory.
TRICK Instead of opening the peritoneum from the sacrum to the cul-de-sac, I create a retroperitoneal tunnel along that right paracolic gutter, from the promontory to just medial to the right uterosacral ligament (VIDEO 1). Doing so has three benefits:
- It is quicker and less bloody
- It allows the mesh to lay flat in the hollow of the sacrum when you bring the sacral arm up to the promontory
- There is much less peritoneum to close over the mesh at the end of the procedure.

FIGURE 2 Entering the peritoneum
Open the peritoneum at the sacral promontory and dissect the fat pad. This reveals the anterior longitudinal ligament.
4. Dissect the vesicovaginal and rectovaginal spaces
Effective vaginal and rectal manipulation is critical to complete this part of the procedure safely. To gain access to the rectovaginal space, the vaginal assistant needs to push the vagina all the way in and up toward the anterior abdominal wall (the handle of the EEA sizer will be pushing hard up against the perineum) while simultaneously pushing the rectal probe downward (effectively scissoring the two apart).
From the exit point of the retroperitoneal tunnel that was created at the beginning of the case, then extend the peritoneal incision transversely in the shape of a “T” to expose the posterior vaginal wall (FIGURE 3, VIDEO 2). If indicated, dissect the rectovaginal space all the way down to the perineal body.
Deviate the vagina posteriorly to facilitate dissection of the bladder from the anterior vaginal wall. Use sharp dissection with scissors and short bursts of energy with monopolar cautery.
TIP If you encounter significant scarring between the bladder and vagina, retrograde-fill the bladder with 300 mL of saline mixed with methylene blue dye to identify the surgical plane.
Expose approximately 4 to 6 cm of anterior vaginal wall, depending on the degree of anterior vaginal wall prolapse. Try to leave the peritoneum intact at the apex of the vagina to reduce the chance that mesh will erode.

FIGURE 3 The peritoneal incision
Extend the peritoneal incision along the cul-de-sac to the posterior vaginal wall in a T-shaped configuration to gain access the rectovaginal space. When perorming cervicosacropexy, it is easiest to develop this surgical plane before amputating the cervix.
5. Attach the y-mesh to the vagina
Several mesh options exist: IntePro (American Medical Systems), Alyte (Bard Medical), and Restorelle Y (Mpathy Medical) are preformed Type-1 polypropylene mesh products. A correctly sized mesh can easily be fashioned by suturing together two strips of Gynemesh (Ethicon) that are approximately 3 cm wide.
Because there can be significant variability in the relative dimensions of the anterior and posterior segments of mesh, I recommend fashioning the graft after dissection is complete: When posterior wall prolapse is extensive, for example, preformed y-mesh strips may not be long enough to reach all the way down to the perineal body. After having assessed the differences in graft placement and manipulation when the two arms are sutured together 1) before the grafts are placed intracorporeally and 2) after they are placed, I’ve concluded that the first method—suturing before placement—is far easier.
Introduce the mesh graft through the accessory port after exchanging the scissors and PK dissector for a suture cutter and a large needle driver. Retract the bladder using the fourth arm, and place the anterior mesh arm over the anterior vaginal wall; suture it in place using 2-0 Gore-Tex sutures on CT-2 needles that are each cut to 6 inches long.
For greatest efficiency, anchor the two distal corners first (FIGURE 4; VIDEO 3), then place a series of interrupted stitches towards the vaginal apex. Tie the knots using 2 surgeon’s knots, followed by 2 half-hitches. Attempt to achieve healthy bites through the vaginal muscularis without perforating the epithelium.

FIGURE 4 Suturing the mesh graft to the vaginal wall
Left and right: Suture the y-shaped polypropylene mesh graft to the anterior vaginal wall first, starting at the distal corners. The bladder is retracted cephalad using the fourth arm.After you’ve adequately secured the anterior mesh arm, deviate the vagina anteriorly and drape the posterior mesh arm over the posterior vaginal wall with the assistance of the fourth robotic arm that can hold upward traction on the sacral end of the mesh graft. Starting at the vaginal apex, place 6 to 8 interrupted sutures to secure the mesh to the posterior vaginal wall (FIGURE 5; VIDEO 4). If necessary, exchange the 0° scope for a 30° up-scope so that you can fully visualize the rectovaginal space.

FIGURE 5 Attachment of the posterior arm of the mesh
The fourth arm of the robot provides upward traction on the sacral portion of the mesh graft.
6. Attach the graft to the sacrum
Again retract the sigmoid laterally to expose the promontory dissection. Retrieve the sacral arm of the mesh through the retroperitoneal tunnel and pull it up toward the promontory. Deviate the vagina toward the sacrum and, ensuring that there is no excessive tension, suture the sacral portion of the mesh graft to the anterior longitudinal ligament at the promontory, using 2 or 3 interrupted sutures (FIGURE 6, VIDEO 5).
When placing the needle during this critical juncture, it is important to rotate through the ligament along the curvature of the needle—as opposed to driving the needle forward and risking exiting farther laterally than expected.
TIP Because of the slight traction that exists on the mesh, a slip-knot is preferred instead of a surgeon’s knot when suturing the sacral portion of the mesh graft to the anterior longitudinal ligament. Take care to visualize the middle sacral artery and either suture around it or cauterize it.

FIGURE 6 Suture the mesh directly to the anterior longitudinal ligament
Use two or three stitches, secured with slip-knots. Take care not to create excessive tension on the mesh graft.
7. Extraperitonealize the mesh
I no longer routinely close the peritoneum over the mesh at the vaginal apex because I have not had a single case of small-bowel obstruction since I began performing this procedure laparoscopically. You should close the peritoneal window at the promontory, however, if the mesh is tented up at all, because tenting creates the potential for bowel to get caught beneath the mesh. Perform this closure using 2-0 monofilament suture or 2-0 Vicryl suture (Ethicon) cut to 8 inches (FIGURE 7). It is always easiest to start distally and suture towards the camera and operative instruments.

FIGURE 7 Extraperitonealize the mesh
Close the peritoneum from the apex of the vagina with a purse-string—like stitch, continuing it to the promontory in running fashion.
8. Ensure that mesh is not in the bladder or rectum
Perform cystoscopy and a rectal examination at the end of each case to confirm that no sutures or mesh material are within either viscus. It is much easier to remove these before leaving the operating room.
Modifying the procedure for uterovaginal prolapse
If the patient has an intact uterus and benign cervical cytology, perform supracervical hysterectomy before proceeding with Steps 1–8 above.
TIP Leaving the cervix in situ may reduce the chance of mesh erosion and provides an excellent platform for mesh attachment.
TRICK I find it most helpful to fully dissect the anterior and posterior vaginal walls before cervical amputation because upward traction on the corpus improves visualization of the surgical planes.
Once the cervix is amputated, however, effective vaginal manipulation can present a surgical challenge. Some surgeons use a tenaculum attached to the fourth arm of the robot to apply traction on the cervix, but this eliminates this arm from performing other necessary tasks. Malleable or Breisky-Navratil retractors can be used to delineate the anterior and posterior vaginal fornices, but are not always satisfactory—especially if an assistant isn’t seated between the legs.
TIP A useful and inexpensive instrument is the Colpo-Probe vaginal fornix delineator (Cooper Surgical) (FIGURE 8), which not only assists in dissecting the vagina from the bladder and rectum but also provides a stable surface during attachment of mesh.

FIGURE 8 Colpo-Probe
This device delineates the anterior and posterior vaginal fornices. It provides a stable platform against which to suture the mesh graft.
Tips and tricks for managing hemorrhage during sacrocolpopexy
Four potential areas of bleeding danger exist:
- In trying to find the sacral promontory, you risk entering the right common iliac vein if dissection is too far cephalad and lateral.
TIP I strongly advise novice robotic surgeons to try to identify the site of the promontory with a standard laparoscopic instrument with haptic feedback before moving to the surgical console.
TRICK Another trick that can help with safe identification of the promontory is mobilization of the sigmoid colon away from the sacrum by developing the retrorectal space. - During dissection of the fat pad from the promontory, you can encounter the middle sacral artery.
TRICK Spreading carefully in a caudal–cephalad direction until the level of the ligament is reached, instead of spreading in a lateral dimension, can decrease the chances of lacerating of this vessel. - A dangerous plexus of veins traverses the hollow of the sacrum. If you are trying to affix mesh at the level of S2-3, therefore, you may encounter significant bleeding.
TIP Work above the level of S1 to avoid these veins completely. - In securing the mesh to the sacral promontory, you can puncture the left common iliac vein if you are not aware of the exit point of the needle and it traverses too far medially.
TIP If you encounter bleeding, introduce a RAY-TEC sponge (Johnson & Johnson) through the accessory port. Apply manual compression for at least 5 minutes. If bleeding persists, I recommend Floseal Hemostatic Matrix (Baxter) to control hemorrhage that arises from arterial and venous sources.
TRICK As last resort, perform emergency undocking in rapid fashion while the bedside assistant maintains pressure through the accessory port.
Conclusion
The da Vinci robotic surgical system facilitates completion of sacrocolpopexy and cervicosacropexy, in a manner identical to the open technique, by surgeons who may not possess advanced laparoscopic skills. Full knowledge of the relevant anatomy is critical; there is the potential for significant morbidity during the procedure if surgical planes are created incorrectly.
Robotic surgery in on the rise, but coding for robotic procedures is still limited to the basic, conventional procedure. Why? Because insurers reimburse the hospital for use of the equipment but still refuse to reimburse the surgeon any additional amount for incorporating the robot into the surgical technique.
Coding for your work when performing robotic sacrocolpopexy is straightforward: Report laparoscopic code 57425 (laparoscopy, surgical, colpopexy [suspension of vaginal apex]) for the procedure. Mesh that might be used as part of the repair is not reported separately.
Blue Cross/Blue Shield (BC/BS) added the Healthcare Common Procedure Coding System Level-II code S2900 (surgical techniques requiring use of robotic surgical system) to the national code set a few years ago. Although BC/BS and some other payers accept this code on the claim, there is no reimbursement attached: It was developed for informational purposes only.
Remember, however, that coding is complete only when you have an appropriate linking diagnosis to establish the medical necessity of laparoscopic sacrocolpopexy. Diagnostic coding options for this surgical procedure include documentation of uterovaginal prolapse (incomplete prolapse is 618.2; complete prolapse is 618.3); vaginal vault prolapse after hysterectomy (618.5); and uterine prolapse without vaginal wall prolapse (618.1).
—MELANIE WITT, RN, CPC, COBGC, MA
We want to hear from you! Tell us what you think.
- Promontory dissection and creation of the retroperitoneal tunnel
- Dissection of the rectovaginal and vesicovaginal spaces
- Attachment of y-mesh to vagina: Start anteriorly
- Posterior y-mesh attachment
- Attachment of mesh to sacral promontory
These videos were provided by Catherine A. Matthews, MD.
Recent years have seen growing recognition that adequate support of the vaginal apex is an essential component of durable surgical repair of pelvic organ prolapse.1,2 Sacrocolpopexy is now considered the gold standard for repair of Level-1 defects of pelvic support, providing excellent long-term results.3-5
A recent randomized, controlled trial demonstrated the superior efficacy of laparoscopic sacrocolpopexy to a total vaginal mesh procedure in women who have vaginal vault prolapse—further evidence that sacrocolpopexy is the procedure of choice for these patients.6
The advantages of sacrocolpopexy include:
- reduced risk of mesh exposure, compared to insertion of vaginal mesh
- preservation of vaginal length
- reduced risk of re-operation for symptomatic recurrent prolapse
- reduced risk of de novo dyspareunia secondary to contraction of mesh.
Obstacles. Although a small number of surgeons are able to accomplish sacrocolpopexy using standard laparoscopic techniques, most of these procedures are still performed by laparotomy because the extensive suturing and knot-tying present a surgical challenge. Open sacrocolpopexy has disadvantages, too, including more pain, longer recovery, and longer length of stay.7-9
With the introduction of the da Vinci robot (Intuitive Surgical), the feasibility of having more surgeons perform this operation using a reproducible, minimally-invasive technique is much greater. The steep learning curve associated with standard laparoscopy in regard to mastering intracorporeal knot-tying and suturing is greatly diminished by articulating instruments. This makes robotic sacrocolpopexy an accessible option for all gynecologic surgeons who treat women with pelvic organ prolapse.
In this article, I detail the steps—with tips and tricks from my experience—to completing an efficient robotic-assisted sacrocolpopexy—modeled exactly after the open technique—that utilizes a y-shaped polypropylene mesh graft. Included is capsule advice from OBG Management’s coding consultant on obtaining reimbursement for robotic procedures (see “ Coding tips for robotic sacrocolpopexy”).
- Two proficient tableside assistants are needed
- Use steep Trendelenburg to remove the bowel from the operative field
- A fan retractor is necessary in some cases to gain access to the promontory
- Correct identification of the sacral promontory is key
- In the absence of haptic feedback, novice surgeons must be aware of the potential danger in dissecting too far laterally and entering the common iliac vessels
- Y-shaped grafts should be fashioned individually
- Know the exit point of the needle at the promontory
- Adequate spacing between the robotic arms is essential to avoiding interference among instruments during the procedure.
Details of the procedure
1. Surgical preparation, set-up
The patient completes a bowel prep using two bottles of magnesium citrate and taking only clear liquids 1 day before surgery. Although mechanical bowel cleansing has not been shown to decrease operative morbidity, manipulation and retraction of the sigmoid colon may be easier with the bowel empty.
Perioperative antibiotics are administered 30 minutes prior to the procedure. Heparin, 5,000 U, is injected subcutaneously for thromboprophylaxis as the patient is en route to the operating suite.
The patient is placed in the dorsal lithotomy position, buttocks extending one inch over the end of the operating table. The table should be covered with egg-crate foam to avoid having her slip down while in steep Trendelenburg position.
After the patient is prepped and draped, a Foley catheter is placed into the bladder. EEA sizers (Covidien) are inserted into the vagina and rectum.
Two experienced surgical assistants are necessary:
- One on the patient’s right side to assist with tissue retraction and introduction of suture material
- Another seated between the patient’s legs to provide adequate vaginal and rectal manipulation during surgery.
2. Port placement, docking, and instrumentation
Pneumoperitoneum is obtained with a Veress needle. Five trocars are then placed (FIGURE 1).
Careful port placement is integral to the success of this procedure because:
- Inadequate distance between robotic arms and the camera results in arm collisions and interference
- Visualization and access to the sacral promontory may be compromised if the camera is inserted too low on the anterior abdominal wall
- Bowel retraction may be compromised if the fourth arm of the robot isn’t at least 3 cm above the anterior superior iliac crest.
My experience evaluating the abdomen before trocar insertion is that at least 15 cm is required between the pubic bone and the umbilicus to rely on this landmark for locating the 12-mm camera port.10 If this distance is shorter (as it is in many obese women), insertion above the umbilicus is necessary.
An accessory 12-mm port, used to introduce sutures and the mesh graft, is placed approximately 10 cm lateral and 4 cm cephalad to the camera in the right-upper quadrant.
An 8-mm robotic port is placed in the right lower quadrant, 10 cm lateral to the accessory port and approximately 3 cm above the anterior superior iliac crest.
The third and fourth robotic arms are placed 10 cm apart in the left lower quadrant, with the fourth arm typically as far lateral as possible.
Docking. After the patient has been placed in steep Trendelenburg position and the table is locked, the robot is docked from the patient’s left side at a 45° angle to the table. Side-docking permits easy access to the vagina to 1) evaluate graft tension and 2) complete cystoscopy to ensure ureteral and bladder integrity.
TIP Take care to ensure that the spine of the robot is positioned right next to the bed at the level of the patient’s hip; driving it up too high in relation to the abdomen can compromise the mobility of the fourth arm. In addition, if the robot is not close enough to the bed, the reach of the first (right) arm may be limited.
Next, introduce monopolar scissors through the right arm; a bipolar PK Dissector (Intuitive Surgical) through the left arm; and an atraumatic bowel grasper, such as Cadiere Forceps (Intuitive Surgical), through the fourth arm.

FIGURE 1 Placement of 5 ports for robotic sacrocolpopexy
Key: C, camera; A, accessory port; 1, right arm (monopolar shears); 2, left arm (PK Dissector); 3, fourth arm (Cadiere Forceps).
3. Dissect the sacral promontory and create a retroperitoneal tunnel
With the use of a 0° scope or 30° down-scope, retract the sigmoid colon laterally using Cadiere forceps and identify the right ureter.
TIP When you attempt robotic sacrocolpopexy for the first time, it may help to identify the sacral promontory, using a standard laparoscopic instrument with haptic feedback, before you dock the robot.
Elevate the peritoneum overlying the sacral promontory and open it using monopolar cautery. Expose the fat pad that overlays the anterior longitudinal ligament and gently dissect it away (FIGURE 2; VIDEO 1). Often, the middle sacral artery is visualized; it can be coagulated using the PK Dissector if necessary.
TIP In a case in which the promontory is difficult to find, dissecting the retrorectal space is a simple way to mobilize the bowel away from the sacrum, thus exposing the promontory.
TRICK Instead of opening the peritoneum from the sacrum to the cul-de-sac, I create a retroperitoneal tunnel along that right paracolic gutter, from the promontory to just medial to the right uterosacral ligament (VIDEO 1). Doing so has three benefits:
- It is quicker and less bloody
- It allows the mesh to lay flat in the hollow of the sacrum when you bring the sacral arm up to the promontory
- There is much less peritoneum to close over the mesh at the end of the procedure.

FIGURE 2 Entering the peritoneum
Open the peritoneum at the sacral promontory and dissect the fat pad. This reveals the anterior longitudinal ligament.
4. Dissect the vesicovaginal and rectovaginal spaces
Effective vaginal and rectal manipulation is critical to complete this part of the procedure safely. To gain access to the rectovaginal space, the vaginal assistant needs to push the vagina all the way in and up toward the anterior abdominal wall (the handle of the EEA sizer will be pushing hard up against the perineum) while simultaneously pushing the rectal probe downward (effectively scissoring the two apart).
From the exit point of the retroperitoneal tunnel that was created at the beginning of the case, then extend the peritoneal incision transversely in the shape of a “T” to expose the posterior vaginal wall (FIGURE 3, VIDEO 2). If indicated, dissect the rectovaginal space all the way down to the perineal body.
Deviate the vagina posteriorly to facilitate dissection of the bladder from the anterior vaginal wall. Use sharp dissection with scissors and short bursts of energy with monopolar cautery.
TIP If you encounter significant scarring between the bladder and vagina, retrograde-fill the bladder with 300 mL of saline mixed with methylene blue dye to identify the surgical plane.
Expose approximately 4 to 6 cm of anterior vaginal wall, depending on the degree of anterior vaginal wall prolapse. Try to leave the peritoneum intact at the apex of the vagina to reduce the chance that mesh will erode.

FIGURE 3 The peritoneal incision
Extend the peritoneal incision along the cul-de-sac to the posterior vaginal wall in a T-shaped configuration to gain access the rectovaginal space. When perorming cervicosacropexy, it is easiest to develop this surgical plane before amputating the cervix.
5. Attach the y-mesh to the vagina
Several mesh options exist: IntePro (American Medical Systems), Alyte (Bard Medical), and Restorelle Y (Mpathy Medical) are preformed Type-1 polypropylene mesh products. A correctly sized mesh can easily be fashioned by suturing together two strips of Gynemesh (Ethicon) that are approximately 3 cm wide.
Because there can be significant variability in the relative dimensions of the anterior and posterior segments of mesh, I recommend fashioning the graft after dissection is complete: When posterior wall prolapse is extensive, for example, preformed y-mesh strips may not be long enough to reach all the way down to the perineal body. After having assessed the differences in graft placement and manipulation when the two arms are sutured together 1) before the grafts are placed intracorporeally and 2) after they are placed, I’ve concluded that the first method—suturing before placement—is far easier.
Introduce the mesh graft through the accessory port after exchanging the scissors and PK dissector for a suture cutter and a large needle driver. Retract the bladder using the fourth arm, and place the anterior mesh arm over the anterior vaginal wall; suture it in place using 2-0 Gore-Tex sutures on CT-2 needles that are each cut to 6 inches long.
For greatest efficiency, anchor the two distal corners first (FIGURE 4; VIDEO 3), then place a series of interrupted stitches towards the vaginal apex. Tie the knots using 2 surgeon’s knots, followed by 2 half-hitches. Attempt to achieve healthy bites through the vaginal muscularis without perforating the epithelium.

FIGURE 4 Suturing the mesh graft to the vaginal wall
Left and right: Suture the y-shaped polypropylene mesh graft to the anterior vaginal wall first, starting at the distal corners. The bladder is retracted cephalad using the fourth arm.After you’ve adequately secured the anterior mesh arm, deviate the vagina anteriorly and drape the posterior mesh arm over the posterior vaginal wall with the assistance of the fourth robotic arm that can hold upward traction on the sacral end of the mesh graft. Starting at the vaginal apex, place 6 to 8 interrupted sutures to secure the mesh to the posterior vaginal wall (FIGURE 5; VIDEO 4). If necessary, exchange the 0° scope for a 30° up-scope so that you can fully visualize the rectovaginal space.

FIGURE 5 Attachment of the posterior arm of the mesh
The fourth arm of the robot provides upward traction on the sacral portion of the mesh graft.
6. Attach the graft to the sacrum
Again retract the sigmoid laterally to expose the promontory dissection. Retrieve the sacral arm of the mesh through the retroperitoneal tunnel and pull it up toward the promontory. Deviate the vagina toward the sacrum and, ensuring that there is no excessive tension, suture the sacral portion of the mesh graft to the anterior longitudinal ligament at the promontory, using 2 or 3 interrupted sutures (FIGURE 6, VIDEO 5).
When placing the needle during this critical juncture, it is important to rotate through the ligament along the curvature of the needle—as opposed to driving the needle forward and risking exiting farther laterally than expected.
TIP Because of the slight traction that exists on the mesh, a slip-knot is preferred instead of a surgeon’s knot when suturing the sacral portion of the mesh graft to the anterior longitudinal ligament. Take care to visualize the middle sacral artery and either suture around it or cauterize it.

FIGURE 6 Suture the mesh directly to the anterior longitudinal ligament
Use two or three stitches, secured with slip-knots. Take care not to create excessive tension on the mesh graft.
7. Extraperitonealize the mesh
I no longer routinely close the peritoneum over the mesh at the vaginal apex because I have not had a single case of small-bowel obstruction since I began performing this procedure laparoscopically. You should close the peritoneal window at the promontory, however, if the mesh is tented up at all, because tenting creates the potential for bowel to get caught beneath the mesh. Perform this closure using 2-0 monofilament suture or 2-0 Vicryl suture (Ethicon) cut to 8 inches (FIGURE 7). It is always easiest to start distally and suture towards the camera and operative instruments.

FIGURE 7 Extraperitonealize the mesh
Close the peritoneum from the apex of the vagina with a purse-string—like stitch, continuing it to the promontory in running fashion.
8. Ensure that mesh is not in the bladder or rectum
Perform cystoscopy and a rectal examination at the end of each case to confirm that no sutures or mesh material are within either viscus. It is much easier to remove these before leaving the operating room.
Modifying the procedure for uterovaginal prolapse
If the patient has an intact uterus and benign cervical cytology, perform supracervical hysterectomy before proceeding with Steps 1–8 above.
TIP Leaving the cervix in situ may reduce the chance of mesh erosion and provides an excellent platform for mesh attachment.
TRICK I find it most helpful to fully dissect the anterior and posterior vaginal walls before cervical amputation because upward traction on the corpus improves visualization of the surgical planes.
Once the cervix is amputated, however, effective vaginal manipulation can present a surgical challenge. Some surgeons use a tenaculum attached to the fourth arm of the robot to apply traction on the cervix, but this eliminates this arm from performing other necessary tasks. Malleable or Breisky-Navratil retractors can be used to delineate the anterior and posterior vaginal fornices, but are not always satisfactory—especially if an assistant isn’t seated between the legs.
TIP A useful and inexpensive instrument is the Colpo-Probe vaginal fornix delineator (Cooper Surgical) (FIGURE 8), which not only assists in dissecting the vagina from the bladder and rectum but also provides a stable surface during attachment of mesh.

FIGURE 8 Colpo-Probe
This device delineates the anterior and posterior vaginal fornices. It provides a stable platform against which to suture the mesh graft.
Tips and tricks for managing hemorrhage during sacrocolpopexy
Four potential areas of bleeding danger exist:
- In trying to find the sacral promontory, you risk entering the right common iliac vein if dissection is too far cephalad and lateral.
TIP I strongly advise novice robotic surgeons to try to identify the site of the promontory with a standard laparoscopic instrument with haptic feedback before moving to the surgical console.
TRICK Another trick that can help with safe identification of the promontory is mobilization of the sigmoid colon away from the sacrum by developing the retrorectal space. - During dissection of the fat pad from the promontory, you can encounter the middle sacral artery.
TRICK Spreading carefully in a caudal–cephalad direction until the level of the ligament is reached, instead of spreading in a lateral dimension, can decrease the chances of lacerating of this vessel. - A dangerous plexus of veins traverses the hollow of the sacrum. If you are trying to affix mesh at the level of S2-3, therefore, you may encounter significant bleeding.
TIP Work above the level of S1 to avoid these veins completely. - In securing the mesh to the sacral promontory, you can puncture the left common iliac vein if you are not aware of the exit point of the needle and it traverses too far medially.
TIP If you encounter bleeding, introduce a RAY-TEC sponge (Johnson & Johnson) through the accessory port. Apply manual compression for at least 5 minutes. If bleeding persists, I recommend Floseal Hemostatic Matrix (Baxter) to control hemorrhage that arises from arterial and venous sources.
TRICK As last resort, perform emergency undocking in rapid fashion while the bedside assistant maintains pressure through the accessory port.
Conclusion
The da Vinci robotic surgical system facilitates completion of sacrocolpopexy and cervicosacropexy, in a manner identical to the open technique, by surgeons who may not possess advanced laparoscopic skills. Full knowledge of the relevant anatomy is critical; there is the potential for significant morbidity during the procedure if surgical planes are created incorrectly.
Robotic surgery in on the rise, but coding for robotic procedures is still limited to the basic, conventional procedure. Why? Because insurers reimburse the hospital for use of the equipment but still refuse to reimburse the surgeon any additional amount for incorporating the robot into the surgical technique.
Coding for your work when performing robotic sacrocolpopexy is straightforward: Report laparoscopic code 57425 (laparoscopy, surgical, colpopexy [suspension of vaginal apex]) for the procedure. Mesh that might be used as part of the repair is not reported separately.
Blue Cross/Blue Shield (BC/BS) added the Healthcare Common Procedure Coding System Level-II code S2900 (surgical techniques requiring use of robotic surgical system) to the national code set a few years ago. Although BC/BS and some other payers accept this code on the claim, there is no reimbursement attached: It was developed for informational purposes only.
Remember, however, that coding is complete only when you have an appropriate linking diagnosis to establish the medical necessity of laparoscopic sacrocolpopexy. Diagnostic coding options for this surgical procedure include documentation of uterovaginal prolapse (incomplete prolapse is 618.2; complete prolapse is 618.3); vaginal vault prolapse after hysterectomy (618.5); and uterine prolapse without vaginal wall prolapse (618.1).
—MELANIE WITT, RN, CPC, COBGC, MA
We want to hear from you! Tell us what you think.
1. Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438-1443.
2. Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2007;(3):CD004014.-
3. Sullivan ES, Longaker CJ, Lee PY. Total pelvic mesh repair: a ten-year experience. Dis Colon Rectum. 2001;44(6):857-863.
4. Culligan PJ, Murphy M, Blackwell L, Hammons G, Graham C, Heit MH. Long-term success of abdominal sacral colpopexy using synthetic mesh. Am J Obstet Gynecol. 2002;187(6):1473-1482.
5. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104(4):805-823.
6. Maher CF, Feiner B, DeCuyper EM, Nichlos CJ, Hickey KV, O’Rourke P. Laparoscopic sacral colpopexy versus total vaginal mesh for vaginal vault prolapse: a randomized trial. Am J Obstet Gynecol. 2011;204(4):360.e1-7.
7. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic sacral colpopexy for vaginal vault prolapse. Obstet Gynecol. 1994;84(5):885-888.
8. Ross JW. Techniques of laparoscopic repair of total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4(2):173-183.
9. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy hysterectomy, and burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6(2):115-119.
10. Matthews CA, Schubert CM, Woodward AP, Gill EJ. Variance in abdominal wall anatomy and port placement in women undergoing robotic gynecologic surgery. J Minim Invasive Gynecol. 2010;17(5):583-586.
1. Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438-1443.
2. Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2007;(3):CD004014.-
3. Sullivan ES, Longaker CJ, Lee PY. Total pelvic mesh repair: a ten-year experience. Dis Colon Rectum. 2001;44(6):857-863.
4. Culligan PJ, Murphy M, Blackwell L, Hammons G, Graham C, Heit MH. Long-term success of abdominal sacral colpopexy using synthetic mesh. Am J Obstet Gynecol. 2002;187(6):1473-1482.
5. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104(4):805-823.
6. Maher CF, Feiner B, DeCuyper EM, Nichlos CJ, Hickey KV, O’Rourke P. Laparoscopic sacral colpopexy versus total vaginal mesh for vaginal vault prolapse: a randomized trial. Am J Obstet Gynecol. 2011;204(4):360.e1-7.
7. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic sacral colpopexy for vaginal vault prolapse. Obstet Gynecol. 1994;84(5):885-888.
8. Ross JW. Techniques of laparoscopic repair of total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4(2):173-183.
9. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy hysterectomy, and burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6(2):115-119.
10. Matthews CA, Schubert CM, Woodward AP, Gill EJ. Variance in abdominal wall anatomy and port placement in women undergoing robotic gynecologic surgery. J Minim Invasive Gynecol. 2010;17(5):583-586.
Take this simplified approach to correcting exposure of vaginal mesh
CASE: Pain during intercourse, well after mesh implantation
Your patient, 61 years old, para 3, has come to your office by referral with a complaint of dyspareunia. The history includes placement of a synthetic vaginal mesh kit 14 months earlier for prolapse.
The medical record shows that the referring physician performed a “mesh excision” 1 year after the original procedure.
The woman reports that she is “very frustrated” that she is still dealing with this problem so long after the original procedure.
On examination, you note a 2.5-cm diameter area of exposed mesh in the anterior vagina, with healthy surrounding tissue and without inflammation or purulence (FIGURE 1). You are unable to reproduce her complaint of pain on vaginal examination.
What options can you offer to this woman? And will those options meet her therapeutic expectations?
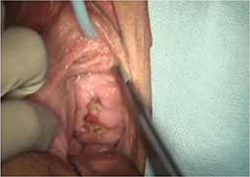
FIGURE 1 Examination of your referred patient: Mesh is noticeably exposedThe recent increase in the use of mesh grafts to reconstruct pelvic anatomy has been directed mainly at improving surgical outcomes. Yet, at the same time, gynecologic surgeons find themselves facing a rise in associated complications of such surgery that they did not see previously.
Among the most troublesome and concerning of those complications are 1) exposure of mesh through the vaginal epithelium and 2) contraction or hardening of mesh (or both) that can result in dyspareunia and chronic pelvic pain. Other, rare complications include infection and fistula.
Our goal in this article is to address the management of graft-healing abnormalities in which a segment of the mesh is palpable or visible, or both, within the vaginal canal. Our focus is on simple abnormalities that can be managed by most generalist gynecologists; to be clear, more complex abnormalities, and those that provoke more serious or lasting symptoms, belong under the care of a specialist.
A recent shift in terminology is significant
Early on, this complication was called “erosion” as understanding of the mechanism of its development grew, however, terminology applied to the problem has changed.
In fact, mesh itself very rarely erodes into the vagina or an underlying viscus. Instead, the complication occurs most commonly as a result of disruption of a suture line—most likely the result of a hematoma or localized inflammation that develops postoperatively.
“Exposure” (our preference here) and “extrusion” are now the recommended terms, based on a consensus terminology document published this year jointly by the International Urogynecological Association and the International Continence Society.1
Exposure of implanted mesh is considered a “simple” healing abnormality because it typically
- occurs along the suture line and early in the course of healing
- is not associated with infection of the graft.2
The typical physical appearance is one of visible mesh along an open suture line without granulation tissue or purulence—again, see FIGURE 1. The mesh is firmly adherent to the vaginal epithelial edges and underlying fascia.
The reported incidence of mesh exposures—in regard to currently used meshes, which are all Type-1, monofilament, macroporous polypropylene grafts—is approximately 10% but as high as 15% to 20% in some reported series.3,4 The higher rates of exposure are usually seen in series in which some patients have had a synthetic graft implanted as an overlay to fascial midline plication. When the graft is implanted in the subfascial layer of the vaginal wall (i.e., without midline plication), however, the reported rate of exposure falls—to 5% to 10%.5-7
Recommendations for management
Initially, recommendations for “erosion” management were based on concerns about underlying mesh infection or rejection, and included a need to remove the entire graft. That recommendation still applies to multifilament, microporous grafts that present with inflammatory infiltrates, granulation tissue, and purulence. Although these kinds of grafts (known as “Type-2/3 grafts”—e.g., GoreTex, IVS) have not been marketed for pelvic reconstruction over the past 3 to 5 years, their behavior post-implantation is less predictable—and patients who have delayed healing abnormalities are, therefore, still being seen. It’s fortunate that development of an overlying biofilm prevents tissue incorporation into these types of graft, allowing them to be removed easily.
Exposures related to Type-1 mesh—currently used in pelvic reconstruction—that occur without surrounding infection do not require extensive removal. Rather, they can be managed conservatively or, when necessary, with outpatient surgery. In patients who are not sexually active, exposures are usually asymptomatic; they might only be observed by the physician on vaginal examination and are amenable to simple monitoring. In sexually active patients, exposure of Type-1 mesh usually results in dyspareunia or a complaint that the partner “can feel the mesh.” Depending on the size and the nature of symptoms and the extent of the defect, these commonly seen exposures can be managed by following a simple algorithm.
Palpable or visible mesh fibrils can be trimmed in the office; they might even respond to local estrogen alone. Consider these options if the patient displays vaginal atrophy.
Typically, vaginal estrogen is prescribed as 1 g nightly for 2 weeks and then 1 g two or three nights a week. Re-examine the patient in 3 months; if symptoms of mesh exposure persist, it’s unlikely that continued conservative therapy will be successful, and outpatient surgery is recommended.
When exposure is asymptomatic, you can simply monitor the condition for 3 to 6 months; if complaints or findings arise, consider intervention.
Small (<0.5 cm in diameter) exposures can also be managed in the office, including excision of exposed mesh and local estrogen. If the exposure is easily reachable, we recommend grasping the exposed area with pick-ups or a hemostat and with gentle traction, using Metzenbaum scissors to trim exposed mesh as close to the vaginal epithelium as possible. Local topical or injected anesthesia may be needed. Bleeding should be minimal because no dissection is necessary. Silver nitrate can be applied for any minor bleeding. Larger (0.5–4.0 cm) exposures are unlikely to heal on their own. They require outpatient excision in the operating room.
Preoperative tissue preparation with local estrogen is key to successful repair of these exposures. Vaginal estrogen increases blood flow to the epithelium; as tissue becomes well-estrogenized, risk of recurrence diminishes.
The technique we employ includes:
- circumferential infiltration of vaginal epithelium surrounding the exposed mesh with 1% lidocaine with epinephrine
- sharp circumscription of the area of exposure, using a scalpel, with a 0.5-cm margin of vaginal epithelium (FIGURE 2)
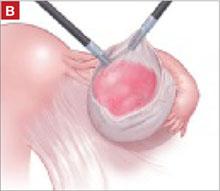
- wide dissection, with undermining and mobilization of surrounding healthy vaginal epithelium around the exposure (FIGURE 3)
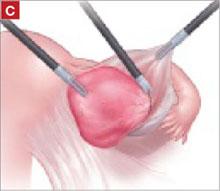
- excision of the exposed mesh and attached vaginal mucosa, with careful dissection of the mesh off underlying tissues with Metzenbaum scissors—being careful to avoid injury to underlying bladder or rectum (FIGURE 4)
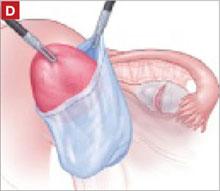
- reapproximation of mesh edges, using 2-0 polypropylene suture to close the resulting defect so that prolapse does not recur (FIGURE 5)
- closing of the previously mobilized vaginal epithelium with 2-0 Vicryl suture, without tension, to cover the reapproximated mesh edges—after irrigation and assurance of adequate hemostasis (FIGURE 6).
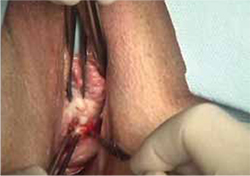
FIGURE 2 Incision of vaginal epithelium
Allow for a 0.5-cm margin.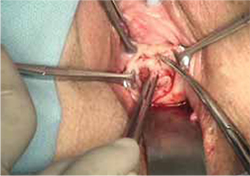
FIGURE 3 Undermining and mobilization of epithelium
Perform wide dissection.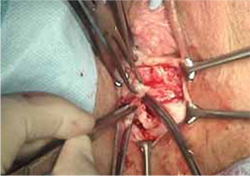
FIGURE 4 Dissection of mesh from underlying tissue
Keep clear of underlying bladder and rectum!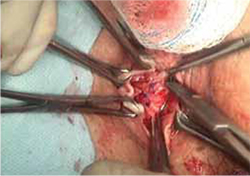
FIGURE 5 Reapproximation of edges to re-establish support
Our choice of suture is 2-0 polypropylene.
FIGURE 6 Irrigation of vaginal epithelium, followed by closure
Before you close, ensure that hemostasis is adequate.The choice of closure—vertical or horizontal—depends on the nature of the original defect.
You can watch a video of this technique that we’ve provided.
Several cautions should be taken with this technique, including:
- avoiding narrowing the vaginal canal
- minimizing trauma to healthy vaginal epithelium that will be used for closure
- maintaining hemostasis to avoid formation of hematomas.
Largest (>4 cm) exposures are likely the result of devascularized sloughing of vaginal epithelium. They are, fortunately, uncommon.
It’s unlikely that, after excision of exposed mesh, the vaginal epithelial edges can be approximated without significantly narrowing or shortening the vaginal canal. Proposed techniques for managing these large exposures include covering the defect with a biologic graft, such as small intestinal submucosa, to allow epithelium to re-grow. Regrettably, prolapse is likely to recur in the unprotected area that results.
Contraction and localized pain
Hardening and contraction typically occur along the fixation arms of the mesh. These complications might result from mesh shrinkage or from mesh being placed too tight, so to speak, at implantation. Rarely does the entire implanted mesh contract.
Severe mesh contraction can result in localized pain and de novo dyspareunia. Symptoms usually resolve after identification of the painful area and removal of the involved mesh segment.8
Diagnostic maneuver. In-office trigger-point injection of bupivacaine with triamcinolone is useful to accurately identify the location of pain that is causing dyspareunia. After injection, the patient is asked to return home and resume sexual intercourse; if dyspareunia diminishes significantly, surgical removal of the involved mesh segment is likely to ameliorate symptoms.
If dyspareunia persists after injection, however, the problem either 1) originates in a different location along the graft or 2) may not be related to the mesh—that is, it may be introital pain or preexisting vaginal pain.
The findings of trigger-point injection and a subsequent trial of sexual intercourse are useful for counseling the patient and developing realistic expectations that surgery will be successful.
Management note: Mesh contraction should be managed by a surgeon who is experienced in extensive deep pelvic dissection, which is necessary to remove the mesh arms.
Chronic pain
Diffuse vaginal pain after mesh implantation is unusual; typically, the patient’s report of pain has been preceded by recognition of another, underlying pelvic pain syndrome. Management of such pain is controversial, and many patients will not be satisfied until the entire graft is removed. Whether such drastic intervention actually resolves the pain is unclear; again, work with the patient to create realistic expectations before surgery—including the risk that prolapse will recur and that reoperation will be necessary.
Management note: An existing pelvic pain syndrome should be considered a relative contraindication to implantation of mesh.
Infection of the graft
Rarely, infection has been reported after implantation of Type-1 mesh—the result of either multi-microbial colonization or isolated infection by Bacteriodes melaninogenicus, Actinomyces spp, or Staphylococcus aureus. Untreated preoperative bacterial vaginitis is likely the underlying cause, and should be considered a contraindication to mesh implantation.
Typically, these patients complain of vaginal discharge and bleeding early postoperatively. Vaginal exposure of the mesh results from local inflammation and necrosis of tissue.
Management note: In these cases, it is necessary to 1) prescribe antimicrobial therapy that covers gram-negative and anaerobic bacteria and 2) undertake surgical removal of the exposed mesh, as we outlined above.9
Visceral erosion or fistula
Many experts believe that what is recorded as “erosion” of synthetic mesh into bladder or rectum is, in fact, a result of unrecognized visceral perforation at original implantation. This is a rare complication of mesh implantation.
Patients who experience mesh erosion into the bladder may have lower urinary-tract symptoms (LUTS) of urgency, frequency, dysuria, and hematuria. Any patient who reports de novo LUTS in the early postoperative period after a vaginal mesh procedure should receive office cystourethroscopy to ensure that no foreign body is present in the bladder or urethra.
Management note: Operative cystourethroscopy, with removal of exposed mesh, is the management of choice when mesh is found in the bladder or urethra.
Patients who have constant urinary or fecal incontinence immediately after surgery should be evaluated for vesicovaginal or rectovaginal fistula.
The presence of any of these complications necessitates removal of the involved mesh in its entirety, with concomitant repair of fistula. Typically, the procedures are performed by a specialist.
Our experience with correcting simple mesh exposures
During the past year at our tertiary referral center, 26 patients have undergone mesh revision because of exposure, using the technique we described above (FIGURE 2-6). The problem resolved in all; none had persistent dyspareunia. Many of these patients had already undergone attempts at correction of the exposure elsewhere—mostly, in the office, using techniques appropriate for that setting. Prolapse has not recurred in the 10 patients who required reapproximation of mesh edges because of a defect >2.5 cm.
CASE RESOLVED: Treatment, improvement
Under your care, the patient undergoes simplified outpatient excision of the exposed area of mesh. Mesh edges are reapproximated to support the resulting 3-cm defect.
At a 12-week postop visit, you note complete resolution of the exposure and normal vaginal caliber. The patient continues to apply estrogen cream and reports sustained improvement in sexual function.
- Preoperatively, prepare the vaginal epithelium with local estrogen cream (recommended dosage: 1 g, two nights every week for a trial of at least 6 weeks)
- Use hydrodissection to facilitate placement of the graft deep to the vaginal epithelial fibromuscular fascial layer
- Do not place a synthetic mesh as an overlay to a midline fascial plication
- Be fastidious about hemostasis
- Close the vaginal epithelium without tension
- Leave vaginal packing in place for 24 hours
- Consider using biologic grafts when appropriate (as an overlay to midline plication when used on the anterior vaginal wall).
For simple presentations, success is within reach
Simple mesh exposure can (as in the case we described) be managed by most gynecologists, utilizing the simple stepwise approach that we outlined above (for additional tips based on our experience, see “Pearls for avoiding mesh exposures”). In the case of more significant symptoms, de novo dyspareunia, visceral erosion, or fistula, however, referral to a specialist is warranted.
Transvaginal mesh surgery reduces pelvic organ prolapse
But dyspareunia may develop in premenopausal women
Transvaginal mesh (TVM) surgery is effective in treating pelvic organ prolapse (POP) in both pre- and postmenopausal women but dyspareunia may worsen in premenopausal women, according to a study published online May 23 in the Journal of Sexual Medicine.
Cheng-Yu Long, MD, PhD, from Kaohsiung Medical University in Taiwan, and colleagues compared the changes in sexual function of premenopausal and postmenopausal women after TVM surgery. A total of 68 sexually active women, categorized as premenopausal (36) and postmenopausal (32), with symptomatic POP stages II to IV were referred for TVM surgery. Preoperative and postoperative assessments included pelvic examination using the POP quantification (POP-Q) system, and completing the Female Sexual Function Index (FSFI), Urogenital Distress Inventory (UDI-6), and Incontinence Impact Questionnaire (IIQ-7).
The investigators found significant improvement in the POP-Q analysis at points Aa, Ba, C, Ap, and Bp in both groups but not in total vaginal length. The UDI-6 and IIQ-7 scores decreased significantly after TVM surgery. The dyspareunia domain score decreased significantly after surgery only in the premenopausal group. Reports of diminished scores of the dyspareunia domain and total scores were more common among women in the premenopausal group, but there were no significant differences in FSFI domains or total scores between the groups.
Copyright © 2011 HealthDay. All rights reserved.
We want to hear from you! Tell us what you think.
1. Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prosthesis (meshes, implants, tapes) and grafts in female pelvic floor surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2011;22(1):3-15.
2. Davila GW, Drutz H, Deprest J. Clinical implications of the biology of grafts: conclusions of the 2005 IUGA Grafts Roundtable. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(suppl 1):S51-55.
3. Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol. 2010;116(2 pt 1):293-303.
4. Hiltunen R, Nieminen K, Takala T, et al. Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 2):455-462.
5. Fatton B, Amblard J, Debodiance P, Cosson M, Jacquetin B. Transvaginal repair of genital prolapse: preliminary results of a new tension-free vaginal mesh (Prolift technique)—a case series multicentric study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):743-752.
6. Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair. Obstet Gynecol. 2009;113(2):367-373.
7. Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111(4):891-898.
8. Feiner B, Maher C. Vaginal mesh contraction: definition clinical presentation, and management. Obstet Gynecol. 2010;115(2 pt 2):325-330.
9. Athanasiou S, Matthaiou DK, Falagas ME. Vaginal mesh infection due to Bacteroides melaninogenicus: a case report of another emerging foreign body related infection. Scand J Infect Dis. 2006;38(11-12):1108-1110.
CASE: Pain during intercourse, well after mesh implantation
Your patient, 61 years old, para 3, has come to your office by referral with a complaint of dyspareunia. The history includes placement of a synthetic vaginal mesh kit 14 months earlier for prolapse.
The medical record shows that the referring physician performed a “mesh excision” 1 year after the original procedure.
The woman reports that she is “very frustrated” that she is still dealing with this problem so long after the original procedure.
On examination, you note a 2.5-cm diameter area of exposed mesh in the anterior vagina, with healthy surrounding tissue and without inflammation or purulence (FIGURE 1). You are unable to reproduce her complaint of pain on vaginal examination.
What options can you offer to this woman? And will those options meet her therapeutic expectations?

FIGURE 1 Examination of your referred patient: Mesh is noticeably exposedThe recent increase in the use of mesh grafts to reconstruct pelvic anatomy has been directed mainly at improving surgical outcomes. Yet, at the same time, gynecologic surgeons find themselves facing a rise in associated complications of such surgery that they did not see previously.
Among the most troublesome and concerning of those complications are 1) exposure of mesh through the vaginal epithelium and 2) contraction or hardening of mesh (or both) that can result in dyspareunia and chronic pelvic pain. Other, rare complications include infection and fistula.
Our goal in this article is to address the management of graft-healing abnormalities in which a segment of the mesh is palpable or visible, or both, within the vaginal canal. Our focus is on simple abnormalities that can be managed by most generalist gynecologists; to be clear, more complex abnormalities, and those that provoke more serious or lasting symptoms, belong under the care of a specialist.
A recent shift in terminology is significant
Early on, this complication was called “erosion” as understanding of the mechanism of its development grew, however, terminology applied to the problem has changed.
In fact, mesh itself very rarely erodes into the vagina or an underlying viscus. Instead, the complication occurs most commonly as a result of disruption of a suture line—most likely the result of a hematoma or localized inflammation that develops postoperatively.
“Exposure” (our preference here) and “extrusion” are now the recommended terms, based on a consensus terminology document published this year jointly by the International Urogynecological Association and the International Continence Society.1
Exposure of implanted mesh is considered a “simple” healing abnormality because it typically
- occurs along the suture line and early in the course of healing
- is not associated with infection of the graft.2
The typical physical appearance is one of visible mesh along an open suture line without granulation tissue or purulence—again, see FIGURE 1. The mesh is firmly adherent to the vaginal epithelial edges and underlying fascia.
The reported incidence of mesh exposures—in regard to currently used meshes, which are all Type-1, monofilament, macroporous polypropylene grafts—is approximately 10% but as high as 15% to 20% in some reported series.3,4 The higher rates of exposure are usually seen in series in which some patients have had a synthetic graft implanted as an overlay to fascial midline plication. When the graft is implanted in the subfascial layer of the vaginal wall (i.e., without midline plication), however, the reported rate of exposure falls—to 5% to 10%.5-7
Recommendations for management
Initially, recommendations for “erosion” management were based on concerns about underlying mesh infection or rejection, and included a need to remove the entire graft. That recommendation still applies to multifilament, microporous grafts that present with inflammatory infiltrates, granulation tissue, and purulence. Although these kinds of grafts (known as “Type-2/3 grafts”—e.g., GoreTex, IVS) have not been marketed for pelvic reconstruction over the past 3 to 5 years, their behavior post-implantation is less predictable—and patients who have delayed healing abnormalities are, therefore, still being seen. It’s fortunate that development of an overlying biofilm prevents tissue incorporation into these types of graft, allowing them to be removed easily.
Exposures related to Type-1 mesh—currently used in pelvic reconstruction—that occur without surrounding infection do not require extensive removal. Rather, they can be managed conservatively or, when necessary, with outpatient surgery. In patients who are not sexually active, exposures are usually asymptomatic; they might only be observed by the physician on vaginal examination and are amenable to simple monitoring. In sexually active patients, exposure of Type-1 mesh usually results in dyspareunia or a complaint that the partner “can feel the mesh.” Depending on the size and the nature of symptoms and the extent of the defect, these commonly seen exposures can be managed by following a simple algorithm.
Palpable or visible mesh fibrils can be trimmed in the office; they might even respond to local estrogen alone. Consider these options if the patient displays vaginal atrophy.
Typically, vaginal estrogen is prescribed as 1 g nightly for 2 weeks and then 1 g two or three nights a week. Re-examine the patient in 3 months; if symptoms of mesh exposure persist, it’s unlikely that continued conservative therapy will be successful, and outpatient surgery is recommended.
When exposure is asymptomatic, you can simply monitor the condition for 3 to 6 months; if complaints or findings arise, consider intervention.
Small (<0.5 cm in diameter) exposures can also be managed in the office, including excision of exposed mesh and local estrogen. If the exposure is easily reachable, we recommend grasping the exposed area with pick-ups or a hemostat and with gentle traction, using Metzenbaum scissors to trim exposed mesh as close to the vaginal epithelium as possible. Local topical or injected anesthesia may be needed. Bleeding should be minimal because no dissection is necessary. Silver nitrate can be applied for any minor bleeding. Larger (0.5–4.0 cm) exposures are unlikely to heal on their own. They require outpatient excision in the operating room.
Preoperative tissue preparation with local estrogen is key to successful repair of these exposures. Vaginal estrogen increases blood flow to the epithelium; as tissue becomes well-estrogenized, risk of recurrence diminishes.
The technique we employ includes:
- circumferential infiltration of vaginal epithelium surrounding the exposed mesh with 1% lidocaine with epinephrine
- sharp circumscription of the area of exposure, using a scalpel, with a 0.5-cm margin of vaginal epithelium (FIGURE 2)
- wide dissection, with undermining and mobilization of surrounding healthy vaginal epithelium around the exposure (FIGURE 3)
- excision of the exposed mesh and attached vaginal mucosa, with careful dissection of the mesh off underlying tissues with Metzenbaum scissors—being careful to avoid injury to underlying bladder or rectum (FIGURE 4)
- reapproximation of mesh edges, using 2-0 polypropylene suture to close the resulting defect so that prolapse does not recur (FIGURE 5)
- closing of the previously mobilized vaginal epithelium with 2-0 Vicryl suture, without tension, to cover the reapproximated mesh edges—after irrigation and assurance of adequate hemostasis (FIGURE 6).

FIGURE 2 Incision of vaginal epithelium
Allow for a 0.5-cm margin.
FIGURE 3 Undermining and mobilization of epithelium
Perform wide dissection.
FIGURE 4 Dissection of mesh from underlying tissue
Keep clear of underlying bladder and rectum!
FIGURE 5 Reapproximation of edges to re-establish support
Our choice of suture is 2-0 polypropylene.
FIGURE 6 Irrigation of vaginal epithelium, followed by closure
Before you close, ensure that hemostasis is adequate.The choice of closure—vertical or horizontal—depends on the nature of the original defect.
You can watch a video of this technique that we’ve provided.
Several cautions should be taken with this technique, including:
- avoiding narrowing the vaginal canal
- minimizing trauma to healthy vaginal epithelium that will be used for closure
- maintaining hemostasis to avoid formation of hematomas.
Largest (>4 cm) exposures are likely the result of devascularized sloughing of vaginal epithelium. They are, fortunately, uncommon.
It’s unlikely that, after excision of exposed mesh, the vaginal epithelial edges can be approximated without significantly narrowing or shortening the vaginal canal. Proposed techniques for managing these large exposures include covering the defect with a biologic graft, such as small intestinal submucosa, to allow epithelium to re-grow. Regrettably, prolapse is likely to recur in the unprotected area that results.
Contraction and localized pain
Hardening and contraction typically occur along the fixation arms of the mesh. These complications might result from mesh shrinkage or from mesh being placed too tight, so to speak, at implantation. Rarely does the entire implanted mesh contract.
Severe mesh contraction can result in localized pain and de novo dyspareunia. Symptoms usually resolve after identification of the painful area and removal of the involved mesh segment.8
Diagnostic maneuver. In-office trigger-point injection of bupivacaine with triamcinolone is useful to accurately identify the location of pain that is causing dyspareunia. After injection, the patient is asked to return home and resume sexual intercourse; if dyspareunia diminishes significantly, surgical removal of the involved mesh segment is likely to ameliorate symptoms.
If dyspareunia persists after injection, however, the problem either 1) originates in a different location along the graft or 2) may not be related to the mesh—that is, it may be introital pain or preexisting vaginal pain.
The findings of trigger-point injection and a subsequent trial of sexual intercourse are useful for counseling the patient and developing realistic expectations that surgery will be successful.
Management note: Mesh contraction should be managed by a surgeon who is experienced in extensive deep pelvic dissection, which is necessary to remove the mesh arms.
Chronic pain
Diffuse vaginal pain after mesh implantation is unusual; typically, the patient’s report of pain has been preceded by recognition of another, underlying pelvic pain syndrome. Management of such pain is controversial, and many patients will not be satisfied until the entire graft is removed. Whether such drastic intervention actually resolves the pain is unclear; again, work with the patient to create realistic expectations before surgery—including the risk that prolapse will recur and that reoperation will be necessary.
Management note: An existing pelvic pain syndrome should be considered a relative contraindication to implantation of mesh.
Infection of the graft
Rarely, infection has been reported after implantation of Type-1 mesh—the result of either multi-microbial colonization or isolated infection by Bacteriodes melaninogenicus, Actinomyces spp, or Staphylococcus aureus. Untreated preoperative bacterial vaginitis is likely the underlying cause, and should be considered a contraindication to mesh implantation.
Typically, these patients complain of vaginal discharge and bleeding early postoperatively. Vaginal exposure of the mesh results from local inflammation and necrosis of tissue.
Management note: In these cases, it is necessary to 1) prescribe antimicrobial therapy that covers gram-negative and anaerobic bacteria and 2) undertake surgical removal of the exposed mesh, as we outlined above.9
Visceral erosion or fistula
Many experts believe that what is recorded as “erosion” of synthetic mesh into bladder or rectum is, in fact, a result of unrecognized visceral perforation at original implantation. This is a rare complication of mesh implantation.
Patients who experience mesh erosion into the bladder may have lower urinary-tract symptoms (LUTS) of urgency, frequency, dysuria, and hematuria. Any patient who reports de novo LUTS in the early postoperative period after a vaginal mesh procedure should receive office cystourethroscopy to ensure that no foreign body is present in the bladder or urethra.
Management note: Operative cystourethroscopy, with removal of exposed mesh, is the management of choice when mesh is found in the bladder or urethra.
Patients who have constant urinary or fecal incontinence immediately after surgery should be evaluated for vesicovaginal or rectovaginal fistula.
The presence of any of these complications necessitates removal of the involved mesh in its entirety, with concomitant repair of fistula. Typically, the procedures are performed by a specialist.
Our experience with correcting simple mesh exposures
During the past year at our tertiary referral center, 26 patients have undergone mesh revision because of exposure, using the technique we described above (FIGURE 2-6). The problem resolved in all; none had persistent dyspareunia. Many of these patients had already undergone attempts at correction of the exposure elsewhere—mostly, in the office, using techniques appropriate for that setting. Prolapse has not recurred in the 10 patients who required reapproximation of mesh edges because of a defect >2.5 cm.
CASE RESOLVED: Treatment, improvement
Under your care, the patient undergoes simplified outpatient excision of the exposed area of mesh. Mesh edges are reapproximated to support the resulting 3-cm defect.
At a 12-week postop visit, you note complete resolution of the exposure and normal vaginal caliber. The patient continues to apply estrogen cream and reports sustained improvement in sexual function.
- Preoperatively, prepare the vaginal epithelium with local estrogen cream (recommended dosage: 1 g, two nights every week for a trial of at least 6 weeks)
- Use hydrodissection to facilitate placement of the graft deep to the vaginal epithelial fibromuscular fascial layer
- Do not place a synthetic mesh as an overlay to a midline fascial plication
- Be fastidious about hemostasis
- Close the vaginal epithelium without tension
- Leave vaginal packing in place for 24 hours
- Consider using biologic grafts when appropriate (as an overlay to midline plication when used on the anterior vaginal wall).
For simple presentations, success is within reach
Simple mesh exposure can (as in the case we described) be managed by most gynecologists, utilizing the simple stepwise approach that we outlined above (for additional tips based on our experience, see “Pearls for avoiding mesh exposures”). In the case of more significant symptoms, de novo dyspareunia, visceral erosion, or fistula, however, referral to a specialist is warranted.
Transvaginal mesh surgery reduces pelvic organ prolapse
But dyspareunia may develop in premenopausal women
Transvaginal mesh (TVM) surgery is effective in treating pelvic organ prolapse (POP) in both pre- and postmenopausal women but dyspareunia may worsen in premenopausal women, according to a study published online May 23 in the Journal of Sexual Medicine.
Cheng-Yu Long, MD, PhD, from Kaohsiung Medical University in Taiwan, and colleagues compared the changes in sexual function of premenopausal and postmenopausal women after TVM surgery. A total of 68 sexually active women, categorized as premenopausal (36) and postmenopausal (32), with symptomatic POP stages II to IV were referred for TVM surgery. Preoperative and postoperative assessments included pelvic examination using the POP quantification (POP-Q) system, and completing the Female Sexual Function Index (FSFI), Urogenital Distress Inventory (UDI-6), and Incontinence Impact Questionnaire (IIQ-7).
The investigators found significant improvement in the POP-Q analysis at points Aa, Ba, C, Ap, and Bp in both groups but not in total vaginal length. The UDI-6 and IIQ-7 scores decreased significantly after TVM surgery. The dyspareunia domain score decreased significantly after surgery only in the premenopausal group. Reports of diminished scores of the dyspareunia domain and total scores were more common among women in the premenopausal group, but there were no significant differences in FSFI domains or total scores between the groups.
Copyright © 2011 HealthDay. All rights reserved.
We want to hear from you! Tell us what you think.
CASE: Pain during intercourse, well after mesh implantation
Your patient, 61 years old, para 3, has come to your office by referral with a complaint of dyspareunia. The history includes placement of a synthetic vaginal mesh kit 14 months earlier for prolapse.
The medical record shows that the referring physician performed a “mesh excision” 1 year after the original procedure.
The woman reports that she is “very frustrated” that she is still dealing with this problem so long after the original procedure.
On examination, you note a 2.5-cm diameter area of exposed mesh in the anterior vagina, with healthy surrounding tissue and without inflammation or purulence (FIGURE 1). You are unable to reproduce her complaint of pain on vaginal examination.
What options can you offer to this woman? And will those options meet her therapeutic expectations?

FIGURE 1 Examination of your referred patient: Mesh is noticeably exposedThe recent increase in the use of mesh grafts to reconstruct pelvic anatomy has been directed mainly at improving surgical outcomes. Yet, at the same time, gynecologic surgeons find themselves facing a rise in associated complications of such surgery that they did not see previously.
Among the most troublesome and concerning of those complications are 1) exposure of mesh through the vaginal epithelium and 2) contraction or hardening of mesh (or both) that can result in dyspareunia and chronic pelvic pain. Other, rare complications include infection and fistula.
Our goal in this article is to address the management of graft-healing abnormalities in which a segment of the mesh is palpable or visible, or both, within the vaginal canal. Our focus is on simple abnormalities that can be managed by most generalist gynecologists; to be clear, more complex abnormalities, and those that provoke more serious or lasting symptoms, belong under the care of a specialist.
A recent shift in terminology is significant
Early on, this complication was called “erosion” as understanding of the mechanism of its development grew, however, terminology applied to the problem has changed.
In fact, mesh itself very rarely erodes into the vagina or an underlying viscus. Instead, the complication occurs most commonly as a result of disruption of a suture line—most likely the result of a hematoma or localized inflammation that develops postoperatively.
“Exposure” (our preference here) and “extrusion” are now the recommended terms, based on a consensus terminology document published this year jointly by the International Urogynecological Association and the International Continence Society.1
Exposure of implanted mesh is considered a “simple” healing abnormality because it typically
- occurs along the suture line and early in the course of healing
- is not associated with infection of the graft.2
The typical physical appearance is one of visible mesh along an open suture line without granulation tissue or purulence—again, see FIGURE 1. The mesh is firmly adherent to the vaginal epithelial edges and underlying fascia.
The reported incidence of mesh exposures—in regard to currently used meshes, which are all Type-1, monofilament, macroporous polypropylene grafts—is approximately 10% but as high as 15% to 20% in some reported series.3,4 The higher rates of exposure are usually seen in series in which some patients have had a synthetic graft implanted as an overlay to fascial midline plication. When the graft is implanted in the subfascial layer of the vaginal wall (i.e., without midline plication), however, the reported rate of exposure falls—to 5% to 10%.5-7
Recommendations for management
Initially, recommendations for “erosion” management were based on concerns about underlying mesh infection or rejection, and included a need to remove the entire graft. That recommendation still applies to multifilament, microporous grafts that present with inflammatory infiltrates, granulation tissue, and purulence. Although these kinds of grafts (known as “Type-2/3 grafts”—e.g., GoreTex, IVS) have not been marketed for pelvic reconstruction over the past 3 to 5 years, their behavior post-implantation is less predictable—and patients who have delayed healing abnormalities are, therefore, still being seen. It’s fortunate that development of an overlying biofilm prevents tissue incorporation into these types of graft, allowing them to be removed easily.
Exposures related to Type-1 mesh—currently used in pelvic reconstruction—that occur without surrounding infection do not require extensive removal. Rather, they can be managed conservatively or, when necessary, with outpatient surgery. In patients who are not sexually active, exposures are usually asymptomatic; they might only be observed by the physician on vaginal examination and are amenable to simple monitoring. In sexually active patients, exposure of Type-1 mesh usually results in dyspareunia or a complaint that the partner “can feel the mesh.” Depending on the size and the nature of symptoms and the extent of the defect, these commonly seen exposures can be managed by following a simple algorithm.
Palpable or visible mesh fibrils can be trimmed in the office; they might even respond to local estrogen alone. Consider these options if the patient displays vaginal atrophy.
Typically, vaginal estrogen is prescribed as 1 g nightly for 2 weeks and then 1 g two or three nights a week. Re-examine the patient in 3 months; if symptoms of mesh exposure persist, it’s unlikely that continued conservative therapy will be successful, and outpatient surgery is recommended.
When exposure is asymptomatic, you can simply monitor the condition for 3 to 6 months; if complaints or findings arise, consider intervention.
Small (<0.5 cm in diameter) exposures can also be managed in the office, including excision of exposed mesh and local estrogen. If the exposure is easily reachable, we recommend grasping the exposed area with pick-ups or a hemostat and with gentle traction, using Metzenbaum scissors to trim exposed mesh as close to the vaginal epithelium as possible. Local topical or injected anesthesia may be needed. Bleeding should be minimal because no dissection is necessary. Silver nitrate can be applied for any minor bleeding. Larger (0.5–4.0 cm) exposures are unlikely to heal on their own. They require outpatient excision in the operating room.
Preoperative tissue preparation with local estrogen is key to successful repair of these exposures. Vaginal estrogen increases blood flow to the epithelium; as tissue becomes well-estrogenized, risk of recurrence diminishes.
The technique we employ includes:
- circumferential infiltration of vaginal epithelium surrounding the exposed mesh with 1% lidocaine with epinephrine
- sharp circumscription of the area of exposure, using a scalpel, with a 0.5-cm margin of vaginal epithelium (FIGURE 2)
- wide dissection, with undermining and mobilization of surrounding healthy vaginal epithelium around the exposure (FIGURE 3)
- excision of the exposed mesh and attached vaginal mucosa, with careful dissection of the mesh off underlying tissues with Metzenbaum scissors—being careful to avoid injury to underlying bladder or rectum (FIGURE 4)
- reapproximation of mesh edges, using 2-0 polypropylene suture to close the resulting defect so that prolapse does not recur (FIGURE 5)
- closing of the previously mobilized vaginal epithelium with 2-0 Vicryl suture, without tension, to cover the reapproximated mesh edges—after irrigation and assurance of adequate hemostasis (FIGURE 6).

FIGURE 2 Incision of vaginal epithelium
Allow for a 0.5-cm margin.
FIGURE 3 Undermining and mobilization of epithelium
Perform wide dissection.
FIGURE 4 Dissection of mesh from underlying tissue
Keep clear of underlying bladder and rectum!
FIGURE 5 Reapproximation of edges to re-establish support
Our choice of suture is 2-0 polypropylene.
FIGURE 6 Irrigation of vaginal epithelium, followed by closure
Before you close, ensure that hemostasis is adequate.The choice of closure—vertical or horizontal—depends on the nature of the original defect.
You can watch a video of this technique that we’ve provided.
Several cautions should be taken with this technique, including:
- avoiding narrowing the vaginal canal
- minimizing trauma to healthy vaginal epithelium that will be used for closure
- maintaining hemostasis to avoid formation of hematomas.
Largest (>4 cm) exposures are likely the result of devascularized sloughing of vaginal epithelium. They are, fortunately, uncommon.
It’s unlikely that, after excision of exposed mesh, the vaginal epithelial edges can be approximated without significantly narrowing or shortening the vaginal canal. Proposed techniques for managing these large exposures include covering the defect with a biologic graft, such as small intestinal submucosa, to allow epithelium to re-grow. Regrettably, prolapse is likely to recur in the unprotected area that results.
Contraction and localized pain
Hardening and contraction typically occur along the fixation arms of the mesh. These complications might result from mesh shrinkage or from mesh being placed too tight, so to speak, at implantation. Rarely does the entire implanted mesh contract.
Severe mesh contraction can result in localized pain and de novo dyspareunia. Symptoms usually resolve after identification of the painful area and removal of the involved mesh segment.8
Diagnostic maneuver. In-office trigger-point injection of bupivacaine with triamcinolone is useful to accurately identify the location of pain that is causing dyspareunia. After injection, the patient is asked to return home and resume sexual intercourse; if dyspareunia diminishes significantly, surgical removal of the involved mesh segment is likely to ameliorate symptoms.
If dyspareunia persists after injection, however, the problem either 1) originates in a different location along the graft or 2) may not be related to the mesh—that is, it may be introital pain or preexisting vaginal pain.
The findings of trigger-point injection and a subsequent trial of sexual intercourse are useful for counseling the patient and developing realistic expectations that surgery will be successful.
Management note: Mesh contraction should be managed by a surgeon who is experienced in extensive deep pelvic dissection, which is necessary to remove the mesh arms.
Chronic pain
Diffuse vaginal pain after mesh implantation is unusual; typically, the patient’s report of pain has been preceded by recognition of another, underlying pelvic pain syndrome. Management of such pain is controversial, and many patients will not be satisfied until the entire graft is removed. Whether such drastic intervention actually resolves the pain is unclear; again, work with the patient to create realistic expectations before surgery—including the risk that prolapse will recur and that reoperation will be necessary.
Management note: An existing pelvic pain syndrome should be considered a relative contraindication to implantation of mesh.
Infection of the graft
Rarely, infection has been reported after implantation of Type-1 mesh—the result of either multi-microbial colonization or isolated infection by Bacteriodes melaninogenicus, Actinomyces spp, or Staphylococcus aureus. Untreated preoperative bacterial vaginitis is likely the underlying cause, and should be considered a contraindication to mesh implantation.
Typically, these patients complain of vaginal discharge and bleeding early postoperatively. Vaginal exposure of the mesh results from local inflammation and necrosis of tissue.
Management note: In these cases, it is necessary to 1) prescribe antimicrobial therapy that covers gram-negative and anaerobic bacteria and 2) undertake surgical removal of the exposed mesh, as we outlined above.9
Visceral erosion or fistula
Many experts believe that what is recorded as “erosion” of synthetic mesh into bladder or rectum is, in fact, a result of unrecognized visceral perforation at original implantation. This is a rare complication of mesh implantation.
Patients who experience mesh erosion into the bladder may have lower urinary-tract symptoms (LUTS) of urgency, frequency, dysuria, and hematuria. Any patient who reports de novo LUTS in the early postoperative period after a vaginal mesh procedure should receive office cystourethroscopy to ensure that no foreign body is present in the bladder or urethra.
Management note: Operative cystourethroscopy, with removal of exposed mesh, is the management of choice when mesh is found in the bladder or urethra.
Patients who have constant urinary or fecal incontinence immediately after surgery should be evaluated for vesicovaginal or rectovaginal fistula.
The presence of any of these complications necessitates removal of the involved mesh in its entirety, with concomitant repair of fistula. Typically, the procedures are performed by a specialist.
Our experience with correcting simple mesh exposures
During the past year at our tertiary referral center, 26 patients have undergone mesh revision because of exposure, using the technique we described above (FIGURE 2-6). The problem resolved in all; none had persistent dyspareunia. Many of these patients had already undergone attempts at correction of the exposure elsewhere—mostly, in the office, using techniques appropriate for that setting. Prolapse has not recurred in the 10 patients who required reapproximation of mesh edges because of a defect >2.5 cm.
CASE RESOLVED: Treatment, improvement
Under your care, the patient undergoes simplified outpatient excision of the exposed area of mesh. Mesh edges are reapproximated to support the resulting 3-cm defect.
At a 12-week postop visit, you note complete resolution of the exposure and normal vaginal caliber. The patient continues to apply estrogen cream and reports sustained improvement in sexual function.
- Preoperatively, prepare the vaginal epithelium with local estrogen cream (recommended dosage: 1 g, two nights every week for a trial of at least 6 weeks)
- Use hydrodissection to facilitate placement of the graft deep to the vaginal epithelial fibromuscular fascial layer
- Do not place a synthetic mesh as an overlay to a midline fascial plication
- Be fastidious about hemostasis
- Close the vaginal epithelium without tension
- Leave vaginal packing in place for 24 hours
- Consider using biologic grafts when appropriate (as an overlay to midline plication when used on the anterior vaginal wall).
For simple presentations, success is within reach
Simple mesh exposure can (as in the case we described) be managed by most gynecologists, utilizing the simple stepwise approach that we outlined above (for additional tips based on our experience, see “Pearls for avoiding mesh exposures”). In the case of more significant symptoms, de novo dyspareunia, visceral erosion, or fistula, however, referral to a specialist is warranted.
Transvaginal mesh surgery reduces pelvic organ prolapse
But dyspareunia may develop in premenopausal women
Transvaginal mesh (TVM) surgery is effective in treating pelvic organ prolapse (POP) in both pre- and postmenopausal women but dyspareunia may worsen in premenopausal women, according to a study published online May 23 in the Journal of Sexual Medicine.
Cheng-Yu Long, MD, PhD, from Kaohsiung Medical University in Taiwan, and colleagues compared the changes in sexual function of premenopausal and postmenopausal women after TVM surgery. A total of 68 sexually active women, categorized as premenopausal (36) and postmenopausal (32), with symptomatic POP stages II to IV were referred for TVM surgery. Preoperative and postoperative assessments included pelvic examination using the POP quantification (POP-Q) system, and completing the Female Sexual Function Index (FSFI), Urogenital Distress Inventory (UDI-6), and Incontinence Impact Questionnaire (IIQ-7).
The investigators found significant improvement in the POP-Q analysis at points Aa, Ba, C, Ap, and Bp in both groups but not in total vaginal length. The UDI-6 and IIQ-7 scores decreased significantly after TVM surgery. The dyspareunia domain score decreased significantly after surgery only in the premenopausal group. Reports of diminished scores of the dyspareunia domain and total scores were more common among women in the premenopausal group, but there were no significant differences in FSFI domains or total scores between the groups.
Copyright © 2011 HealthDay. All rights reserved.
We want to hear from you! Tell us what you think.
1. Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prosthesis (meshes, implants, tapes) and grafts in female pelvic floor surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2011;22(1):3-15.
2. Davila GW, Drutz H, Deprest J. Clinical implications of the biology of grafts: conclusions of the 2005 IUGA Grafts Roundtable. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(suppl 1):S51-55.
3. Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol. 2010;116(2 pt 1):293-303.
4. Hiltunen R, Nieminen K, Takala T, et al. Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 2):455-462.
5. Fatton B, Amblard J, Debodiance P, Cosson M, Jacquetin B. Transvaginal repair of genital prolapse: preliminary results of a new tension-free vaginal mesh (Prolift technique)—a case series multicentric study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):743-752.
6. Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair. Obstet Gynecol. 2009;113(2):367-373.
7. Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111(4):891-898.
8. Feiner B, Maher C. Vaginal mesh contraction: definition clinical presentation, and management. Obstet Gynecol. 2010;115(2 pt 2):325-330.
9. Athanasiou S, Matthaiou DK, Falagas ME. Vaginal mesh infection due to Bacteroides melaninogenicus: a case report of another emerging foreign body related infection. Scand J Infect Dis. 2006;38(11-12):1108-1110.
1. Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prosthesis (meshes, implants, tapes) and grafts in female pelvic floor surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2011;22(1):3-15.
2. Davila GW, Drutz H, Deprest J. Clinical implications of the biology of grafts: conclusions of the 2005 IUGA Grafts Roundtable. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(suppl 1):S51-55.
3. Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol. 2010;116(2 pt 1):293-303.
4. Hiltunen R, Nieminen K, Takala T, et al. Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 2):455-462.
5. Fatton B, Amblard J, Debodiance P, Cosson M, Jacquetin B. Transvaginal repair of genital prolapse: preliminary results of a new tension-free vaginal mesh (Prolift technique)—a case series multicentric study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):743-752.
6. Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair. Obstet Gynecol. 2009;113(2):367-373.
7. Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111(4):891-898.
8. Feiner B, Maher C. Vaginal mesh contraction: definition clinical presentation, and management. Obstet Gynecol. 2010;115(2 pt 2):325-330.
9. Athanasiou S, Matthaiou DK, Falagas ME. Vaginal mesh infection due to Bacteroides melaninogenicus: a case report of another emerging foreign body related infection. Scand J Infect Dis. 2006;38(11-12):1108-1110.
High uterosacral vaginal vault suspension to repair enterocele and apical prolapse
- Pelvic anatomy of high intraperitoneal vaginal vault suspension
- Anatomy of the uterosacral ligament
- High uterosacral suspension (complete uterine procidentia)
- High uterosacral suspension (post-hysterectomy vaginal vault prolapse)
These videos were selected by Mickey Karram, MD, and Christine Vaccaro, DO, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS).
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
The concept of utilizing the uterosacral ligaments to support the vaginal cuff and correct an enterocele is nothing new: As early as 1957, Milton McCall described what became known as the McCall culdoplasty, in which sutures incorporated the uterosacral ligaments into the posterior vaginal vault to obliterate the cul-de-sac and suspend or support the vaginal apex at the time of vaginal hysterectomy.1
Later, in the 1990s, Richardson promoted the concept that, in patients who have pelvic organ prolapse, the uterosacral ligaments do not become attenuated, instead, they break at specific points.
Shull and colleagues took this idea and described how utilizing uterosacral ligaments to support the vaginal cuff can be performed vaginally—by passing sutures bilaterally through the uterosacral ligaments near the level of the ischial spine.2
Since Shull described this procedure, numerous published studies have demonstrated outcomes similar to other vaginal suspension procedures, such as sacrospinous ligament suspension.3-5
Potential advantages of a high uterosacral vaginal vault suspension are that:
- it provides good apical support without significantly distorting the vaginal axis, making it applicable to all types of vaginal prolapse
- intraperitoneal passage of sutures can be a lot cleaner and simpler than passing sutures, or anchors, through retroperitoneal structures, such as the sacrospinous ligament (FIGURE 1).
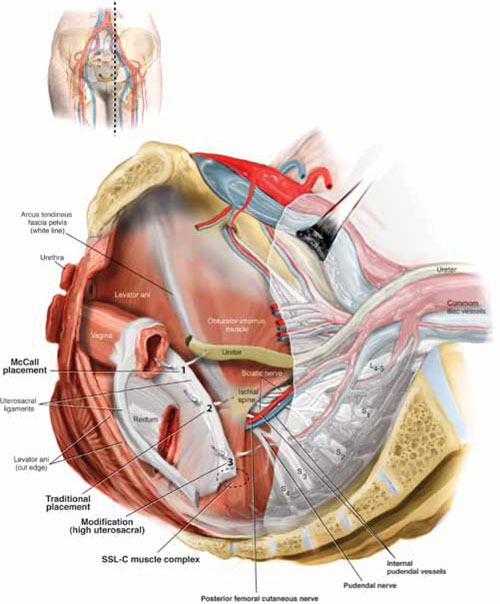
FIGURE 1 Locating intraperitoneal sutures during uterosacral suspension
Cross-section of the pelvic floor shows where sutures are placed as part of McCall culdoplasty (1), traditional uterosacral suspension (2), and modified high uterosacral suspension (3). Note: High uterosacral suspension may involve passing the suture through the sacrospinous ligament–coccygeus (SSL-C) muscle complex (dashed oval) because a segment of the uterosacral ligament inserts into that structure.
A disadvantage of the procedure is that the uterosacral ligament may, at times, lie in close proximity to the ureter. Studies have shown that the ureter can become kinked when sutures in this procedure are passed too far laterally.2-5
High uterosacral suspension has been our operation of choice for 11 years for patients who have pelvic organ prolapse in which the peritoneum is accessible (see “How this procedure evolved in our hands”). In this article, we provide a step-by-step description of the procedure. Four accompanying videos that further illuminate those steps are noted in the text here at appropriate places.(For example, Video #1, immediately below, sets the stage for the step-by-step discussion by reviewing pertinent pelvic anatomy.)
- When we first performed high uterosacral vaginal vault suspension as described by Shull and colleagues,1 we mobilized vaginal muscularis off the epithelium and suspended the epithelium and muscularis separately, making sure that sutures were passed through the anterior and Posterior vaginal walls.
- Initially, we thought that a large cul-de-sac needed to be obliterated in the midline with internal McCall-type stitches that were separate and distinct from the uterosacral suspension sutures. We no longer do this routinely because we believe that the numerous sutures that are passed through the full thickness of the posterior vaginal wall, including the peritoneum, effectively obliterate the enterocele and keep down the incidence of recurrent enterocele and high rectocele.
- We have come to realize that sutures placed medial and cephalad to the ischial spine are often passed through a portion of the coccygeus muscle-sacrospinous ligament complex. At times, a small window can be made in the peritoneum that provides direct access to this complex (FIGURE 1; FIGURE 3).
References
1. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
1. Enter the peritoneum
It’s our opinion that, even though extraperitoneal uterosacral suspension procedures have been described, the pertinent anatomic structures (again, see Video #1) are not easily identifiable unless suspension is undertaken intraperitoneally. Entering the peritoneum is, obviously, not a concern if the patient is undergoing vaginal hysterectomy. If the patient has post-hysterectomy prolapse, however, you must be able to isolate an enterocele and enter the peritoneum (follow FIGURE 2, beginning here and through subsequent steps of the procedure).
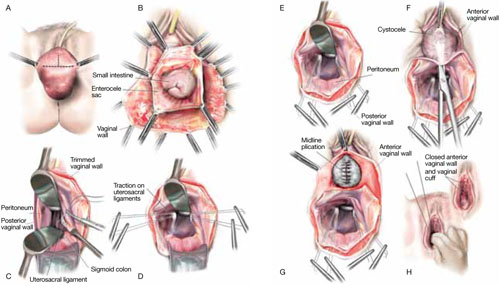
FIGURE 2 Step by step: High uterosacral vaginal vault suspension
A The most prominent portion of the prolapsed vaginal vault is grasped with two Allis clamps. B The vaginal wall is opened up and the enterocele sac is identified and entered. C The bowel is packed high into the pelvis using large laparotomy sponges. The retractor lifts the sponges out of the lower pelvis, thus completely exposing the cul-de-sac. When appropriate traction is placed downward on the uterosacral ligaments with an Allis clamp, the uterosacral ligaments are easily palpated bilaterally. D Delayed absorbable sutures have been passed through the uppermost portion of the uterosacral ligaments on each side, and have been individually tagged.
E Each end of the previously passed sutures is brought out through the posterior peritoneum and the posterior vaginal wall. (A free needle is used to pass both ends of these delayed absorbable sutures through the full thickness of the vaginal wall.) F Anterior colporrhaphy is begun by initiating dissection between the prolapsed bladder and the anterior vaginal wall. G Anterior colporrhaphy is complete. H The vagina has been appropriately trimmed and closed with interrupted or continuous delayed absorbable sutures. Delayed absorbable sutures that were previously brought out through the full thickness of the posterior vaginal wall are then tied; doing so elevates the prolapsed vaginal vault high up into the hollow of the sacrum.Once you have entered the peritoneum, the cul-de-sac must be relatively free of adhesive disease if you are to be able to continue with this procedure. (See “5 surgical pearls for high ureterosacral vaginal vault suspension”)
- Be prepared to convert to a sacrospinous fixation if you cannot enter the enterocele sac or if the posterior cul-de-sac is obliterated with adhesions
- Pass the sutures through durable tissue so that, when traction is placed on the sutures, there is minimal movement of peritoneum. Doing so might avoid kinking of the ureter.
- Pass the sutures through the full thickness of the posterior vaginal wall, including the peritoneum. Doing so not only suspends the apex but tremendously facilitates support for the posterior vaginal wall (FIGURE 4).
- When prolapse is very large, excise redundant portions of the upper part of the posterior vaginal wall and peritoneum—making sure, however, that you keep all layers together for performing the suspension. (See VIDEO #4, showing high uterosacral suspension in a patient who has complete uterine procidentia.)
- Do not try to pass a ureteral stent if you do not see indigo carmine dye spill from the ureteral orifices; to do so can be difficult after repair of prolapse, even in the hands of a skilled urologist. It is best instead to:
- identify the offending suture
- cut it
- visualize the spill of dye-colored urine
- proceed with either replacing the cut suture or maintaining the suspension with other, remaining sutures.
In our experience, when we have also performed an anterior repair, the ureter is kinked in at least 50% of cases because of one of the sutures that was used to correct the cystocele.
2. Pack the bowel; expose the uterosacral ligaments
Next, pack the small bowel out of the cul-de-sac to allow easy access and visualization of the uppermost portions of the uterosacral ligament. This is best accomplished by passing large, moistened laparotomy sponges intraperitoneally and elevating them with a large retractor (e.g., Deaver, Breisky-Navrital, Sweetheart).
When the bowel is appropriately packed, the retractor lifts the intestinal contents out of the pelvis, usually allowing easy access to the proximal or uppermost portion of the uterosacral ligaments (see Video #3, which focuses on the anatomy of the uterosacral ligament).
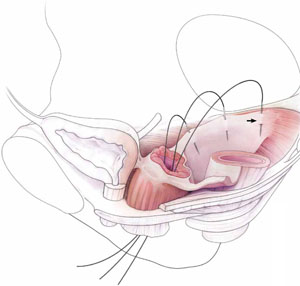
When performing high uterosacral suspension, it is possible to pass sutures through the coccygeus muscle-sacrospinous ligament complex (arrow) because a segment of the uterosacral ligament inserts into that structure.
3. Palpate the ischial spines bilaterally
It’s important that you palpate the ischial spines. Often, the ureter can be palpated against the pelvic sidewall. If you palpate the ischial spines and continue to palpate medially and cephalad, you can usually palpate the coccygeus muscle-sacrospinous ligament complex transperitoneally because a portion of the uterosacral ligament inserts into the sacrospinous ligament.6
If sutures can be passed at this level, the result will (usually) be a vagina that is, at minimum, approximately 9 cm long.
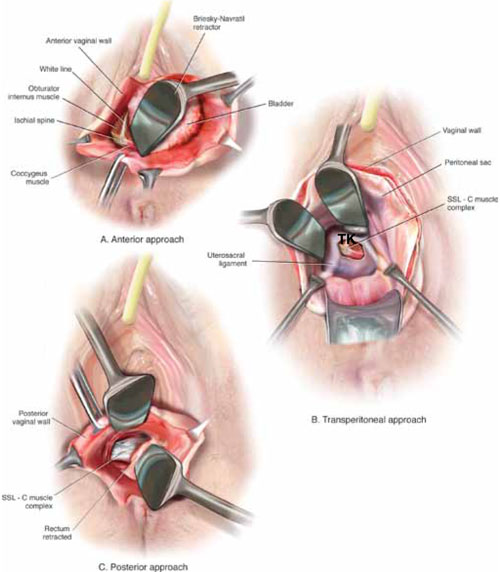
FIGURE 3 Access to the sacrospinous ligament
The sacrospinous ligament can be palpated and exposed along any one of three approaches: anterior paravaginally (A), transperitoneally (B), and posterior pararectally (C).
4. Pass the sutures
We prefer to pass two or three sutures on each side, utilizing a long, straight needle holder. Because we eventually pass the sutures through the full thickness of the posterior vaginal wall, we’ve opted for a delayed absorbable suture—preferably, 0 Vicryl on a CT-2 needle.
A Breisky-Navrital retractor is utilized to retract the sigmoid colon in the opposite direction of the ligament in which the sutures are being passed. At times, attaching a light to a suction device or a retractor is also helpful to visualize this area.
Use an Allis clamp to elevate and apply traction on the distal uterosacral ligament; this facilitates palpation and visualization of the appropriate site for placement of the sutures. The exact area of suture passage is best identified by palpation.
(Note: In early descriptions of this procedure, permanent sutures were utilized; again, we use delayed absorbable sutures because all sutures are brought out through the full thickness of the posterior vaginal wall. Permanent suture in our approach would be unacceptable because the sutures are tied in the lumen of the vagina. In some other modifications of this procedure, sutures are passed through the muscular layer of the vagina to exclude epithelium; under those circumstances, permanent sutures can be utilized.)
Once the sutures are brought through the full thickness of the posterior vaginal wall—including the peritoneum, if possible—tag them individually. If the anterior segment is well-supported, close the vaginal incision with a continuous delayed absorbable suture.
Tie the suspension sutures, elevating the apex into the hollow of the sacrum.
If anterior colporrhaphy is needed, perform that repair. Close the anterior vaginal wall as well as the vaginal cuff before tying off the suspension sutures.
5. Ensure that the ureters are patent
After the sutures are tied, instruct the anesthesiologist to administer 5 cc of indigo carmine dye intravenously. Assuming no renal compromise, you should see dye in the bladder 5 to 10 minutes later. If the patient is elderly or if you want to expedite this step, furosemide, 5 to 10 mg, can be given by IV push.
Next, perform cystoscopy to ensure ureteral patency. You should observe a spill of dye-colored urine out of both ureteral orifices. If dye does not spill from either orifice after a reasonable wait (usually, 20 minutes), assume that the ureter on that side is obstructed.
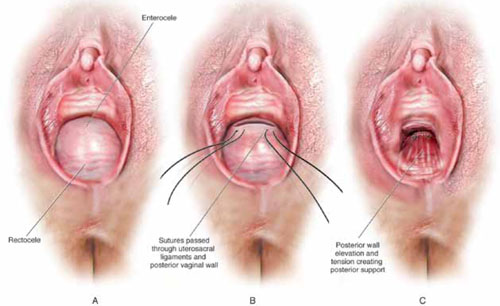
FIGURE 4 Providing support for the posterior vaginal wall
A View of a posterior vaginal wall defect secondary to an enterocele and rectocele. B After entry into the enterocele sac, intraperitoneal suspension sutures are brought out through the full thickness of the vaginal wall at the level of the apex. C Tying these sutures after the vaginal incision is closed at the apex not only results in greater vaginal length but also contributes to overall support of the entire posterior vaginal wall.
6. Completely reconstruct the vagina
The remainder of steps required to complete the procedure usually involve posterior colporrhaphy and perineoplasty. We also reserve placement of a synthetic midurethral sling (if one is needed) until after the vault procedure is complete.
Refer to FIGURE 2 for a step-by step guide to how best to perform high uterosacral vaginal vault suspension.
Questions often asked about this procedure
What do I do if I can’t isolate an enterocele sac and enter it?
Perform a unilateral or bilateral sacrospinous ligament colpopexy.
Is it always possible to identify a usable uterosacral ligament in patients who have advanced prolapse?
We’ve found it extremely rare not to be able identify a usable and durable structure.
The trick to identifying the ligament is to pass an Allis clamp so that one end is positioned intraperitoneally, as high up as possible, and the other end is on the vaginal mucosa side. Elevating the clamp puts the ligament on tension. These clamps are usually placed between 4 and 5 o’clock on the left side and between 7 and 8 o’clock on the right side.
With appropriate traction, the ligament can usually be easily palpated.
If I don’t see indigo carmine dye spilling from one side during cystoscopy, what sequence of events should I undertake?
If the only sutures placed on that side were the uterosacral ligament sutures, cut them individually. If the ureter spills dye after a suture is cut, decide whether you think it is appropriate to replace that suture. Sometimes, unilateral suspension or a suspension with one remaining suture on the side where you cut a suture or two is sufficient.
If you do want to replace a cut suture, ureteral patency must be confirmed again after it is replaced.
No further management of the ureter is required—that is, it isn’t necessary to catheterize the ureter or perform postoperative imaging studies. If anterior colporrhaphy has also been performed, however, apply your highest index of suspicion to determine the source of the offending suture: the uterosacral suspension or the anterior repair.*
If the patient has severe hip or leg pain postoperatively, what should I suspect is wrong? How should I manage this complication?
The nerve to the levator ani runs within the coccygeus muscle. In a thin patient, in whom deep bites are taken, the nerve is often injured or trapped. Such trauma can cause hip pain that is fairly severe but that is almost always self-limiting and requires only nonsteroidal anti-inflammatory medication. Usually, this complication resolves within 2 weeks after surgery.
Significant postoperative pain that radiates down the back of the thigh or down the leg all the way to the foot is of greater concern because one of the sacral nerve segments has most likely been injured or stretched. Obtain a neurology consult; rarely, it becomes necessary to take the patient back to surgery to cut the offending suture.
*For detailed discussion of this subject, see the International Academy of Pelvic Surgery’s August 2010 “Case of the month” at www.academyofpelvicsurgery.com.
We want to hear from you! Tell us what you think.
1. McCall ML. Posterior culdeplasty; surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10(6):595-602.
2. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
3. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183(6):1402-1411.
4. Karram M, Goldwasser S, Kleeman S, Steele A, Vassallo B, Walsh P. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185(6):1339-1343.
5. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006;108(2):255-263.
6. Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JOL. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;13(3):447-451.
- Pelvic anatomy of high intraperitoneal vaginal vault suspension
- Anatomy of the uterosacral ligament
- High uterosacral suspension (complete uterine procidentia)
- High uterosacral suspension (post-hysterectomy vaginal vault prolapse)
These videos were selected by Mickey Karram, MD, and Christine Vaccaro, DO, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS).
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
The concept of utilizing the uterosacral ligaments to support the vaginal cuff and correct an enterocele is nothing new: As early as 1957, Milton McCall described what became known as the McCall culdoplasty, in which sutures incorporated the uterosacral ligaments into the posterior vaginal vault to obliterate the cul-de-sac and suspend or support the vaginal apex at the time of vaginal hysterectomy.1
Later, in the 1990s, Richardson promoted the concept that, in patients who have pelvic organ prolapse, the uterosacral ligaments do not become attenuated, instead, they break at specific points.
Shull and colleagues took this idea and described how utilizing uterosacral ligaments to support the vaginal cuff can be performed vaginally—by passing sutures bilaterally through the uterosacral ligaments near the level of the ischial spine.2
Since Shull described this procedure, numerous published studies have demonstrated outcomes similar to other vaginal suspension procedures, such as sacrospinous ligament suspension.3-5
Potential advantages of a high uterosacral vaginal vault suspension are that:
- it provides good apical support without significantly distorting the vaginal axis, making it applicable to all types of vaginal prolapse
- intraperitoneal passage of sutures can be a lot cleaner and simpler than passing sutures, or anchors, through retroperitoneal structures, such as the sacrospinous ligament (FIGURE 1).

FIGURE 1 Locating intraperitoneal sutures during uterosacral suspension
Cross-section of the pelvic floor shows where sutures are placed as part of McCall culdoplasty (1), traditional uterosacral suspension (2), and modified high uterosacral suspension (3). Note: High uterosacral suspension may involve passing the suture through the sacrospinous ligament–coccygeus (SSL-C) muscle complex (dashed oval) because a segment of the uterosacral ligament inserts into that structure.
A disadvantage of the procedure is that the uterosacral ligament may, at times, lie in close proximity to the ureter. Studies have shown that the ureter can become kinked when sutures in this procedure are passed too far laterally.2-5
High uterosacral suspension has been our operation of choice for 11 years for patients who have pelvic organ prolapse in which the peritoneum is accessible (see “How this procedure evolved in our hands”). In this article, we provide a step-by-step description of the procedure. Four accompanying videos that further illuminate those steps are noted in the text here at appropriate places.(For example, Video #1, immediately below, sets the stage for the step-by-step discussion by reviewing pertinent pelvic anatomy.)
- When we first performed high uterosacral vaginal vault suspension as described by Shull and colleagues,1 we mobilized vaginal muscularis off the epithelium and suspended the epithelium and muscularis separately, making sure that sutures were passed through the anterior and Posterior vaginal walls.
- Initially, we thought that a large cul-de-sac needed to be obliterated in the midline with internal McCall-type stitches that were separate and distinct from the uterosacral suspension sutures. We no longer do this routinely because we believe that the numerous sutures that are passed through the full thickness of the posterior vaginal wall, including the peritoneum, effectively obliterate the enterocele and keep down the incidence of recurrent enterocele and high rectocele.
- We have come to realize that sutures placed medial and cephalad to the ischial spine are often passed through a portion of the coccygeus muscle-sacrospinous ligament complex. At times, a small window can be made in the peritoneum that provides direct access to this complex (FIGURE 1; FIGURE 3).
References
1. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
1. Enter the peritoneum
It’s our opinion that, even though extraperitoneal uterosacral suspension procedures have been described, the pertinent anatomic structures (again, see Video #1) are not easily identifiable unless suspension is undertaken intraperitoneally. Entering the peritoneum is, obviously, not a concern if the patient is undergoing vaginal hysterectomy. If the patient has post-hysterectomy prolapse, however, you must be able to isolate an enterocele and enter the peritoneum (follow FIGURE 2, beginning here and through subsequent steps of the procedure).

FIGURE 2 Step by step: High uterosacral vaginal vault suspension
A The most prominent portion of the prolapsed vaginal vault is grasped with two Allis clamps. B The vaginal wall is opened up and the enterocele sac is identified and entered. C The bowel is packed high into the pelvis using large laparotomy sponges. The retractor lifts the sponges out of the lower pelvis, thus completely exposing the cul-de-sac. When appropriate traction is placed downward on the uterosacral ligaments with an Allis clamp, the uterosacral ligaments are easily palpated bilaterally. D Delayed absorbable sutures have been passed through the uppermost portion of the uterosacral ligaments on each side, and have been individually tagged.
E Each end of the previously passed sutures is brought out through the posterior peritoneum and the posterior vaginal wall. (A free needle is used to pass both ends of these delayed absorbable sutures through the full thickness of the vaginal wall.) F Anterior colporrhaphy is begun by initiating dissection between the prolapsed bladder and the anterior vaginal wall. G Anterior colporrhaphy is complete. H The vagina has been appropriately trimmed and closed with interrupted or continuous delayed absorbable sutures. Delayed absorbable sutures that were previously brought out through the full thickness of the posterior vaginal wall are then tied; doing so elevates the prolapsed vaginal vault high up into the hollow of the sacrum.Once you have entered the peritoneum, the cul-de-sac must be relatively free of adhesive disease if you are to be able to continue with this procedure. (See “5 surgical pearls for high ureterosacral vaginal vault suspension”)
- Be prepared to convert to a sacrospinous fixation if you cannot enter the enterocele sac or if the posterior cul-de-sac is obliterated with adhesions
- Pass the sutures through durable tissue so that, when traction is placed on the sutures, there is minimal movement of peritoneum. Doing so might avoid kinking of the ureter.
- Pass the sutures through the full thickness of the posterior vaginal wall, including the peritoneum. Doing so not only suspends the apex but tremendously facilitates support for the posterior vaginal wall (FIGURE 4).
- When prolapse is very large, excise redundant portions of the upper part of the posterior vaginal wall and peritoneum—making sure, however, that you keep all layers together for performing the suspension. (See VIDEO #4, showing high uterosacral suspension in a patient who has complete uterine procidentia.)
- Do not try to pass a ureteral stent if you do not see indigo carmine dye spill from the ureteral orifices; to do so can be difficult after repair of prolapse, even in the hands of a skilled urologist. It is best instead to:
- identify the offending suture
- cut it
- visualize the spill of dye-colored urine
- proceed with either replacing the cut suture or maintaining the suspension with other, remaining sutures.
In our experience, when we have also performed an anterior repair, the ureter is kinked in at least 50% of cases because of one of the sutures that was used to correct the cystocele.
2. Pack the bowel; expose the uterosacral ligaments
Next, pack the small bowel out of the cul-de-sac to allow easy access and visualization of the uppermost portions of the uterosacral ligament. This is best accomplished by passing large, moistened laparotomy sponges intraperitoneally and elevating them with a large retractor (e.g., Deaver, Breisky-Navrital, Sweetheart).
When the bowel is appropriately packed, the retractor lifts the intestinal contents out of the pelvis, usually allowing easy access to the proximal or uppermost portion of the uterosacral ligaments (see Video #3, which focuses on the anatomy of the uterosacral ligament).

When performing high uterosacral suspension, it is possible to pass sutures through the coccygeus muscle-sacrospinous ligament complex (arrow) because a segment of the uterosacral ligament inserts into that structure.
3. Palpate the ischial spines bilaterally
It’s important that you palpate the ischial spines. Often, the ureter can be palpated against the pelvic sidewall. If you palpate the ischial spines and continue to palpate medially and cephalad, you can usually palpate the coccygeus muscle-sacrospinous ligament complex transperitoneally because a portion of the uterosacral ligament inserts into the sacrospinous ligament.6
If sutures can be passed at this level, the result will (usually) be a vagina that is, at minimum, approximately 9 cm long.

FIGURE 3 Access to the sacrospinous ligament
The sacrospinous ligament can be palpated and exposed along any one of three approaches: anterior paravaginally (A), transperitoneally (B), and posterior pararectally (C).
4. Pass the sutures
We prefer to pass two or three sutures on each side, utilizing a long, straight needle holder. Because we eventually pass the sutures through the full thickness of the posterior vaginal wall, we’ve opted for a delayed absorbable suture—preferably, 0 Vicryl on a CT-2 needle.
A Breisky-Navrital retractor is utilized to retract the sigmoid colon in the opposite direction of the ligament in which the sutures are being passed. At times, attaching a light to a suction device or a retractor is also helpful to visualize this area.
Use an Allis clamp to elevate and apply traction on the distal uterosacral ligament; this facilitates palpation and visualization of the appropriate site for placement of the sutures. The exact area of suture passage is best identified by palpation.
(Note: In early descriptions of this procedure, permanent sutures were utilized; again, we use delayed absorbable sutures because all sutures are brought out through the full thickness of the posterior vaginal wall. Permanent suture in our approach would be unacceptable because the sutures are tied in the lumen of the vagina. In some other modifications of this procedure, sutures are passed through the muscular layer of the vagina to exclude epithelium; under those circumstances, permanent sutures can be utilized.)
Once the sutures are brought through the full thickness of the posterior vaginal wall—including the peritoneum, if possible—tag them individually. If the anterior segment is well-supported, close the vaginal incision with a continuous delayed absorbable suture.
Tie the suspension sutures, elevating the apex into the hollow of the sacrum.
If anterior colporrhaphy is needed, perform that repair. Close the anterior vaginal wall as well as the vaginal cuff before tying off the suspension sutures.
5. Ensure that the ureters are patent
After the sutures are tied, instruct the anesthesiologist to administer 5 cc of indigo carmine dye intravenously. Assuming no renal compromise, you should see dye in the bladder 5 to 10 minutes later. If the patient is elderly or if you want to expedite this step, furosemide, 5 to 10 mg, can be given by IV push.
Next, perform cystoscopy to ensure ureteral patency. You should observe a spill of dye-colored urine out of both ureteral orifices. If dye does not spill from either orifice after a reasonable wait (usually, 20 minutes), assume that the ureter on that side is obstructed.

FIGURE 4 Providing support for the posterior vaginal wall
A View of a posterior vaginal wall defect secondary to an enterocele and rectocele. B After entry into the enterocele sac, intraperitoneal suspension sutures are brought out through the full thickness of the vaginal wall at the level of the apex. C Tying these sutures after the vaginal incision is closed at the apex not only results in greater vaginal length but also contributes to overall support of the entire posterior vaginal wall.
6. Completely reconstruct the vagina
The remainder of steps required to complete the procedure usually involve posterior colporrhaphy and perineoplasty. We also reserve placement of a synthetic midurethral sling (if one is needed) until after the vault procedure is complete.
Refer to FIGURE 2 for a step-by step guide to how best to perform high uterosacral vaginal vault suspension.
Questions often asked about this procedure
What do I do if I can’t isolate an enterocele sac and enter it?
Perform a unilateral or bilateral sacrospinous ligament colpopexy.
Is it always possible to identify a usable uterosacral ligament in patients who have advanced prolapse?
We’ve found it extremely rare not to be able identify a usable and durable structure.
The trick to identifying the ligament is to pass an Allis clamp so that one end is positioned intraperitoneally, as high up as possible, and the other end is on the vaginal mucosa side. Elevating the clamp puts the ligament on tension. These clamps are usually placed between 4 and 5 o’clock on the left side and between 7 and 8 o’clock on the right side.
With appropriate traction, the ligament can usually be easily palpated.
If I don’t see indigo carmine dye spilling from one side during cystoscopy, what sequence of events should I undertake?
If the only sutures placed on that side were the uterosacral ligament sutures, cut them individually. If the ureter spills dye after a suture is cut, decide whether you think it is appropriate to replace that suture. Sometimes, unilateral suspension or a suspension with one remaining suture on the side where you cut a suture or two is sufficient.
If you do want to replace a cut suture, ureteral patency must be confirmed again after it is replaced.
No further management of the ureter is required—that is, it isn’t necessary to catheterize the ureter or perform postoperative imaging studies. If anterior colporrhaphy has also been performed, however, apply your highest index of suspicion to determine the source of the offending suture: the uterosacral suspension or the anterior repair.*
If the patient has severe hip or leg pain postoperatively, what should I suspect is wrong? How should I manage this complication?
The nerve to the levator ani runs within the coccygeus muscle. In a thin patient, in whom deep bites are taken, the nerve is often injured or trapped. Such trauma can cause hip pain that is fairly severe but that is almost always self-limiting and requires only nonsteroidal anti-inflammatory medication. Usually, this complication resolves within 2 weeks after surgery.
Significant postoperative pain that radiates down the back of the thigh or down the leg all the way to the foot is of greater concern because one of the sacral nerve segments has most likely been injured or stretched. Obtain a neurology consult; rarely, it becomes necessary to take the patient back to surgery to cut the offending suture.
*For detailed discussion of this subject, see the International Academy of Pelvic Surgery’s August 2010 “Case of the month” at www.academyofpelvicsurgery.com.
We want to hear from you! Tell us what you think.
- Pelvic anatomy of high intraperitoneal vaginal vault suspension
- Anatomy of the uterosacral ligament
- High uterosacral suspension (complete uterine procidentia)
- High uterosacral suspension (post-hysterectomy vaginal vault prolapse)
These videos were selected by Mickey Karram, MD, and Christine Vaccaro, DO, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS).
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
The concept of utilizing the uterosacral ligaments to support the vaginal cuff and correct an enterocele is nothing new: As early as 1957, Milton McCall described what became known as the McCall culdoplasty, in which sutures incorporated the uterosacral ligaments into the posterior vaginal vault to obliterate the cul-de-sac and suspend or support the vaginal apex at the time of vaginal hysterectomy.1
Later, in the 1990s, Richardson promoted the concept that, in patients who have pelvic organ prolapse, the uterosacral ligaments do not become attenuated, instead, they break at specific points.
Shull and colleagues took this idea and described how utilizing uterosacral ligaments to support the vaginal cuff can be performed vaginally—by passing sutures bilaterally through the uterosacral ligaments near the level of the ischial spine.2
Since Shull described this procedure, numerous published studies have demonstrated outcomes similar to other vaginal suspension procedures, such as sacrospinous ligament suspension.3-5
Potential advantages of a high uterosacral vaginal vault suspension are that:
- it provides good apical support without significantly distorting the vaginal axis, making it applicable to all types of vaginal prolapse
- intraperitoneal passage of sutures can be a lot cleaner and simpler than passing sutures, or anchors, through retroperitoneal structures, such as the sacrospinous ligament (FIGURE 1).

FIGURE 1 Locating intraperitoneal sutures during uterosacral suspension
Cross-section of the pelvic floor shows where sutures are placed as part of McCall culdoplasty (1), traditional uterosacral suspension (2), and modified high uterosacral suspension (3). Note: High uterosacral suspension may involve passing the suture through the sacrospinous ligament–coccygeus (SSL-C) muscle complex (dashed oval) because a segment of the uterosacral ligament inserts into that structure.
A disadvantage of the procedure is that the uterosacral ligament may, at times, lie in close proximity to the ureter. Studies have shown that the ureter can become kinked when sutures in this procedure are passed too far laterally.2-5
High uterosacral suspension has been our operation of choice for 11 years for patients who have pelvic organ prolapse in which the peritoneum is accessible (see “How this procedure evolved in our hands”). In this article, we provide a step-by-step description of the procedure. Four accompanying videos that further illuminate those steps are noted in the text here at appropriate places.(For example, Video #1, immediately below, sets the stage for the step-by-step discussion by reviewing pertinent pelvic anatomy.)
- When we first performed high uterosacral vaginal vault suspension as described by Shull and colleagues,1 we mobilized vaginal muscularis off the epithelium and suspended the epithelium and muscularis separately, making sure that sutures were passed through the anterior and Posterior vaginal walls.
- Initially, we thought that a large cul-de-sac needed to be obliterated in the midline with internal McCall-type stitches that were separate and distinct from the uterosacral suspension sutures. We no longer do this routinely because we believe that the numerous sutures that are passed through the full thickness of the posterior vaginal wall, including the peritoneum, effectively obliterate the enterocele and keep down the incidence of recurrent enterocele and high rectocele.
- We have come to realize that sutures placed medial and cephalad to the ischial spine are often passed through a portion of the coccygeus muscle-sacrospinous ligament complex. At times, a small window can be made in the peritoneum that provides direct access to this complex (FIGURE 1; FIGURE 3).
References
1. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
1. Enter the peritoneum
It’s our opinion that, even though extraperitoneal uterosacral suspension procedures have been described, the pertinent anatomic structures (again, see Video #1) are not easily identifiable unless suspension is undertaken intraperitoneally. Entering the peritoneum is, obviously, not a concern if the patient is undergoing vaginal hysterectomy. If the patient has post-hysterectomy prolapse, however, you must be able to isolate an enterocele and enter the peritoneum (follow FIGURE 2, beginning here and through subsequent steps of the procedure).

FIGURE 2 Step by step: High uterosacral vaginal vault suspension
A The most prominent portion of the prolapsed vaginal vault is grasped with two Allis clamps. B The vaginal wall is opened up and the enterocele sac is identified and entered. C The bowel is packed high into the pelvis using large laparotomy sponges. The retractor lifts the sponges out of the lower pelvis, thus completely exposing the cul-de-sac. When appropriate traction is placed downward on the uterosacral ligaments with an Allis clamp, the uterosacral ligaments are easily palpated bilaterally. D Delayed absorbable sutures have been passed through the uppermost portion of the uterosacral ligaments on each side, and have been individually tagged.
E Each end of the previously passed sutures is brought out through the posterior peritoneum and the posterior vaginal wall. (A free needle is used to pass both ends of these delayed absorbable sutures through the full thickness of the vaginal wall.) F Anterior colporrhaphy is begun by initiating dissection between the prolapsed bladder and the anterior vaginal wall. G Anterior colporrhaphy is complete. H The vagina has been appropriately trimmed and closed with interrupted or continuous delayed absorbable sutures. Delayed absorbable sutures that were previously brought out through the full thickness of the posterior vaginal wall are then tied; doing so elevates the prolapsed vaginal vault high up into the hollow of the sacrum.Once you have entered the peritoneum, the cul-de-sac must be relatively free of adhesive disease if you are to be able to continue with this procedure. (See “5 surgical pearls for high ureterosacral vaginal vault suspension”)
- Be prepared to convert to a sacrospinous fixation if you cannot enter the enterocele sac or if the posterior cul-de-sac is obliterated with adhesions
- Pass the sutures through durable tissue so that, when traction is placed on the sutures, there is minimal movement of peritoneum. Doing so might avoid kinking of the ureter.
- Pass the sutures through the full thickness of the posterior vaginal wall, including the peritoneum. Doing so not only suspends the apex but tremendously facilitates support for the posterior vaginal wall (FIGURE 4).
- When prolapse is very large, excise redundant portions of the upper part of the posterior vaginal wall and peritoneum—making sure, however, that you keep all layers together for performing the suspension. (See VIDEO #4, showing high uterosacral suspension in a patient who has complete uterine procidentia.)
- Do not try to pass a ureteral stent if you do not see indigo carmine dye spill from the ureteral orifices; to do so can be difficult after repair of prolapse, even in the hands of a skilled urologist. It is best instead to:
- identify the offending suture
- cut it
- visualize the spill of dye-colored urine
- proceed with either replacing the cut suture or maintaining the suspension with other, remaining sutures.
In our experience, when we have also performed an anterior repair, the ureter is kinked in at least 50% of cases because of one of the sutures that was used to correct the cystocele.
2. Pack the bowel; expose the uterosacral ligaments
Next, pack the small bowel out of the cul-de-sac to allow easy access and visualization of the uppermost portions of the uterosacral ligament. This is best accomplished by passing large, moistened laparotomy sponges intraperitoneally and elevating them with a large retractor (e.g., Deaver, Breisky-Navrital, Sweetheart).
When the bowel is appropriately packed, the retractor lifts the intestinal contents out of the pelvis, usually allowing easy access to the proximal or uppermost portion of the uterosacral ligaments (see Video #3, which focuses on the anatomy of the uterosacral ligament).

When performing high uterosacral suspension, it is possible to pass sutures through the coccygeus muscle-sacrospinous ligament complex (arrow) because a segment of the uterosacral ligament inserts into that structure.
3. Palpate the ischial spines bilaterally
It’s important that you palpate the ischial spines. Often, the ureter can be palpated against the pelvic sidewall. If you palpate the ischial spines and continue to palpate medially and cephalad, you can usually palpate the coccygeus muscle-sacrospinous ligament complex transperitoneally because a portion of the uterosacral ligament inserts into the sacrospinous ligament.6
If sutures can be passed at this level, the result will (usually) be a vagina that is, at minimum, approximately 9 cm long.

FIGURE 3 Access to the sacrospinous ligament
The sacrospinous ligament can be palpated and exposed along any one of three approaches: anterior paravaginally (A), transperitoneally (B), and posterior pararectally (C).
4. Pass the sutures
We prefer to pass two or three sutures on each side, utilizing a long, straight needle holder. Because we eventually pass the sutures through the full thickness of the posterior vaginal wall, we’ve opted for a delayed absorbable suture—preferably, 0 Vicryl on a CT-2 needle.
A Breisky-Navrital retractor is utilized to retract the sigmoid colon in the opposite direction of the ligament in which the sutures are being passed. At times, attaching a light to a suction device or a retractor is also helpful to visualize this area.
Use an Allis clamp to elevate and apply traction on the distal uterosacral ligament; this facilitates palpation and visualization of the appropriate site for placement of the sutures. The exact area of suture passage is best identified by palpation.
(Note: In early descriptions of this procedure, permanent sutures were utilized; again, we use delayed absorbable sutures because all sutures are brought out through the full thickness of the posterior vaginal wall. Permanent suture in our approach would be unacceptable because the sutures are tied in the lumen of the vagina. In some other modifications of this procedure, sutures are passed through the muscular layer of the vagina to exclude epithelium; under those circumstances, permanent sutures can be utilized.)
Once the sutures are brought through the full thickness of the posterior vaginal wall—including the peritoneum, if possible—tag them individually. If the anterior segment is well-supported, close the vaginal incision with a continuous delayed absorbable suture.
Tie the suspension sutures, elevating the apex into the hollow of the sacrum.
If anterior colporrhaphy is needed, perform that repair. Close the anterior vaginal wall as well as the vaginal cuff before tying off the suspension sutures.
5. Ensure that the ureters are patent
After the sutures are tied, instruct the anesthesiologist to administer 5 cc of indigo carmine dye intravenously. Assuming no renal compromise, you should see dye in the bladder 5 to 10 minutes later. If the patient is elderly or if you want to expedite this step, furosemide, 5 to 10 mg, can be given by IV push.
Next, perform cystoscopy to ensure ureteral patency. You should observe a spill of dye-colored urine out of both ureteral orifices. If dye does not spill from either orifice after a reasonable wait (usually, 20 minutes), assume that the ureter on that side is obstructed.

FIGURE 4 Providing support for the posterior vaginal wall
A View of a posterior vaginal wall defect secondary to an enterocele and rectocele. B After entry into the enterocele sac, intraperitoneal suspension sutures are brought out through the full thickness of the vaginal wall at the level of the apex. C Tying these sutures after the vaginal incision is closed at the apex not only results in greater vaginal length but also contributes to overall support of the entire posterior vaginal wall.
6. Completely reconstruct the vagina
The remainder of steps required to complete the procedure usually involve posterior colporrhaphy and perineoplasty. We also reserve placement of a synthetic midurethral sling (if one is needed) until after the vault procedure is complete.
Refer to FIGURE 2 for a step-by step guide to how best to perform high uterosacral vaginal vault suspension.
Questions often asked about this procedure
What do I do if I can’t isolate an enterocele sac and enter it?
Perform a unilateral or bilateral sacrospinous ligament colpopexy.
Is it always possible to identify a usable uterosacral ligament in patients who have advanced prolapse?
We’ve found it extremely rare not to be able identify a usable and durable structure.
The trick to identifying the ligament is to pass an Allis clamp so that one end is positioned intraperitoneally, as high up as possible, and the other end is on the vaginal mucosa side. Elevating the clamp puts the ligament on tension. These clamps are usually placed between 4 and 5 o’clock on the left side and between 7 and 8 o’clock on the right side.
With appropriate traction, the ligament can usually be easily palpated.
If I don’t see indigo carmine dye spilling from one side during cystoscopy, what sequence of events should I undertake?
If the only sutures placed on that side were the uterosacral ligament sutures, cut them individually. If the ureter spills dye after a suture is cut, decide whether you think it is appropriate to replace that suture. Sometimes, unilateral suspension or a suspension with one remaining suture on the side where you cut a suture or two is sufficient.
If you do want to replace a cut suture, ureteral patency must be confirmed again after it is replaced.
No further management of the ureter is required—that is, it isn’t necessary to catheterize the ureter or perform postoperative imaging studies. If anterior colporrhaphy has also been performed, however, apply your highest index of suspicion to determine the source of the offending suture: the uterosacral suspension or the anterior repair.*
If the patient has severe hip or leg pain postoperatively, what should I suspect is wrong? How should I manage this complication?
The nerve to the levator ani runs within the coccygeus muscle. In a thin patient, in whom deep bites are taken, the nerve is often injured or trapped. Such trauma can cause hip pain that is fairly severe but that is almost always self-limiting and requires only nonsteroidal anti-inflammatory medication. Usually, this complication resolves within 2 weeks after surgery.
Significant postoperative pain that radiates down the back of the thigh or down the leg all the way to the foot is of greater concern because one of the sacral nerve segments has most likely been injured or stretched. Obtain a neurology consult; rarely, it becomes necessary to take the patient back to surgery to cut the offending suture.
*For detailed discussion of this subject, see the International Academy of Pelvic Surgery’s August 2010 “Case of the month” at www.academyofpelvicsurgery.com.
We want to hear from you! Tell us what you think.
1. McCall ML. Posterior culdeplasty; surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10(6):595-602.
2. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
3. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183(6):1402-1411.
4. Karram M, Goldwasser S, Kleeman S, Steele A, Vassallo B, Walsh P. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185(6):1339-1343.
5. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006;108(2):255-263.
6. Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JOL. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;13(3):447-451.
1. McCall ML. Posterior culdeplasty; surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10(6):595-602.
2. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
3. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183(6):1402-1411.
4. Karram M, Goldwasser S, Kleeman S, Steele A, Vassallo B, Walsh P. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185(6):1339-1343.
5. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006;108(2):255-263.
6. Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JOL. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;13(3):447-451.
Applying single-incision laparoscopic surgery to gyn practice: What’s involved
- Update on minimally invasive surgery
Amy Garcia, MD (April 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
The benefits of minimally invasive surgery—including less pain, faster recovery, and improved cosmesis—are well known.1,2 Standard laparotomy has been replaced by multiple-port operative laparoscopy for a great array of procedures, and advances in medical technology allow for a minimally invasive surgical approach even when a surgeon is faced with complex pathology.
Single-port laparoscopic surgery (SPLS) represents the latest advance in minimally invasive surgery. Using flexible endoscopes and articulating instruments, the surgeon can complete complex procedures through a single 2-cm incision in the abdomen. The incision is usually placed in the umbilicus, where it is easily hidden.3-8
Since the first laparoscopic hysterectomy through a single incision was performed 20 years ago, SPLS has been used successfully to perform nephrectomy, prostatectomy, hemicolectomy, cholecystectomy, splenectomy, intussusception reduction, gastrostomy tube placement, thoracoscopic lung biopsy, thoracoscopic decortication, and appendectomy.4-7
In gynecology, SPLS has been used to perform oophorectomy, salpingectomy, bilateral tubal ligation, ovarian cystectomy, surgical treatment of ectopic pregnancy, and both total and partial hysterectomy.7-11 At least two recent studies have concluded that SPLS is an acceptable way to treat many benign and malignant gynecologic conditions that are currently treated using multiport laparoscopy.3,11
This article outlines our approach to SPLS in the gynecologic patient and provides an overview of instrumentation, with the aim of allowing you to consider whether this approach might be feasible in your surgical practice, at your institution.
Unique setup required
When SPLS is performed through the umbilicus, the instruments must be held closer to the midline and more cephalad than during conventional laparoscopy to permit adequate visualization and manipulation. For this reason, the surgeon needs to assume a position higher over the torso and thorax of the patient, and both of the patient’s arms need to be tucked. Place the patient in a dorsal lithotomy position with a uterine manipulator in place to facilitate surgery—even when the uterus will be preserved and surgery involves only the adnexae.
With appropriate equipment and positioning, visualization and manipulation of anatomy are comparable to those of standard multiport laparoscopy.
New instruments simplify SPLS
Innovative surgical instruments allow for appropriate hand positioning outside the abdomen and minimize the internal collision of instruments brought through a single midline incision (FIGURE 1). A variety of single-port options are available, each with a unique patented design and method of insertion. In fact, the development of ports with multiple instrument channels has revolutionized SPLS.
FIGURE 1 Setup
Desired triangulation of instruments in SPLS setup.Before true single ports became available, it was necessary to place three 5-mm low-profile trocars in the fascia at three separate sites through a single skin incision. Pneumoperitoneum was established with a Veress needle, but the fascial incisions gradually merged with repeated cannula manipulation, producing air leaks.
Today, multiple-channel ports are placed using an open technique into a single skin and fascial incision. Trocars and instruments of varying size can be exchanged with ease without jeopardizing pneumoperitoneum.
Among the options:
- the SILS Port (Covidien) – a soft, flexible, three-channel port that allows for placement of blunt trocars ranging in size from 5 mm to 12 mm (FIGURE 2)
- the TriPort (Olympus America) – two flexible rings joined by a sleeve and multiple-channel port (FIGURE 3)
- GelPOINT Advanced Access Platform (Applied Medical) – a system constructed of synthetic gel material and consisting of a “GelSeal” cap, cannulas, and seals to accommodate 5-mm to 10-mm instrumentation (FIGURE 4).
We have found that all three devices allow for good range of motion while maintaining pneumoperitoneum.
(A recent article from Korea reports an inventive technique to perform single-incision laparoscopy using standard instrumentation: The authors fitted a self-retaining ring retractor with a surgical glove that had three of the fingers cut off and replaced by trocars.12)
FIGURE 2 SILS Port
The SILS Port is a soft, flexible, three-channel port that allows for placement of blunt trocars ranging in size from 5 mm to 12 mm.
FIGURE 3 TriPort
The TriPort system comprises two flexible rings joined by a sleeve and multiple-channel port.
FIGURE 4 GelPOINT
The GelPOINT Advanced Access Platform is constructed of synthetic gel material and accommodates 5-mm to 10-mm instrumentation.
A flexible laparoscope improves visualization
The ability to visualize the operative field is vital to any surgery, including SPLS. Use of a flexible laparoscope facilitates uncompromised visualization of the entire pelvis (FIGURE 5). Outside the abdomen, the flexible camera can be held laterally and away from the midline to help reduce the clashing of instruments and hands.
FIGURE 5 Flexible-tip laparoscope
A flexible-tip laparoscope ensures good visualization of the surgical field.If a flexible laparoscope is not available, a rigid 30-degree or 45-degree angled scope can be used, although visualization may be limited and adequate triangulation of instruments may be difficult to achieve.
When using a rigid laparoscope, a light cord that inserts into the back of the camera is necessary; otherwise, a 90-degree light cord adapter can be purchased.
Design enhancements facilitate coordination of instruments
One of the disadvantages of SPLS has been the restriction of movement that arises because of the close proximity of instruments and instrument handles. The latest designs have made articulation possible for tissue graspers, scissors, vessel sealers, and scopes.9,10 The value of articulation is apparent inside the abdomen, where it allows perfect positioning of the area of dissection. Outside the abdomen, the handles can be arranged in an angled pattern to allow the surgeon and assistant to operate comfortably (FIGURE 1).
In a four-handed procedure, one hand is on the camera, one on the uterine manipulator, and the remaining two hands operate the articulating grasper, vessel sealer, or needle driver, depending on the task.
Standard straight instruments can also be used for portions of the procedure.
Dissection and hemostasis are achieved in a manner similar to that of conventional laparoscopy. Our instrument of choice is an enhanced bipolar instrument, although harmonic and traditional biopolar energy can be used as well, depending on the preference of the surgeon.
The latest instruments are designed to dissect, cauterize, and cut, thereby decreasing the number of instrument exchanges necessary.
With the right aids, suturing can be simplified
Suturing through a single port can be a challenge. When possible, closure of the vaginal cuff following a total laparoscopic or laparoscopic-assisted vaginal hysterectomy should be performed from below. When endoscopic suturing is required, standard suturing using both intracorporeal and extracorporeal methods is possible.
Suturing aids such as the Endo Stitch (Covidien) or Lapra-Ty (Ethicon) are helpful. One author recommends Quill bidirectional, self-retaining suture with barbs (Angiotech) to avoid the need for knot-tying.6 (For more on self-retaining suture, see “Barbed suture, now in the toolbox of minimally invasive gyn surgery,” by Jon I. Einarsson, MD, MPH, and James A. Greenberg, MD, in the September 2009 issue at obgmanagement.com.)
The MiniLap (Stryker) is a 2.3-mm grasper that is inserted percutaneously directly through the abdominal wall without an incision. It can be used to set the needle on the needle driver or manipulate tissue while suturing. The resulting skin incision is barely visible and does not require closure.
Options are varied for specimen removal
Small specimens can be removed directly through a single-port system that has been opened, or they can be extracted after the system is removed, with rapid desufflation (FIGURE 6).
FIGURE 6 Single-incision oophorectomy
An ovary and tube removed through a single incision using the A) TriPort and B) SILS Port systems.Compared with conventional laparoscopy, the larger incision associated with single-port surgery facilitates specimen removal. Larger or potentially malignant specimens can be placed into an EndoCatch bag (Covidien) inserted through the single-incision 10-mm cannula (FIGURE 7).
FIGURE 7 Specimen removal
In this case, the specimen was placed in a 10-mm EndoCatch bag and removed through the 10-mm cannula of the SILS Port.In total laparoscopic hysterectomy or laparoscopic-assisted vaginal hysterectomy, the uterus is removed through the vagina. In supracervical hysterectomy, a small uterus can be removed through the cul-de-sac or directly through the single incision after placement in a bag.
When morcellation is required, the instrument can be placed through the cul-desac, cervix, or a single port. The morcellator can be placed directly through the SPLS port while utilizing the flexible scope in an angled direction (looking back toward the morcellator) for complete visualization (note: Covidien does not recommend this usage).
Transcervical tissue morcellation has also been described. In this approach, the cervix is dilated once the uterine body has been amputated, and the tissue morcellator is inserted through the cervix while the surgeon maintains visualization from above.6,13
How to master the technique
Many patients desire SPLS for its superior cosmetic outcome, but the approach may not always be appropriate. Depending on the procedure and characteristics of the patient (TABLE), multiple-port laparoscopy may be a better option. When a surgeon first attempts SPLS, we recommend that it be limited to the treatment of adnexal pathology only.
In your early SPlS cases, look for these patient characteristics
|
By using the techniques described in this article and selecting patients carefully, the surgeon can develop expertise in SPLS.11,14 During the learning process, the use of additional ports or Mini-Lap instruments (Stryker) can reduce the challenges of more difficult procedures and should be considered without reservation, as should the use of articulating accessory instruments and flexible or angled laparoscopes.
Although the clinical benefits of SPLS have yet to be determined, the cosmetic advantage of a single, hidden, umbilical incision likely increases patient satisfaction.
Clearly, the goal of SPLS is to use technology in a way that offers all of the benefits of traditional multiport laparoscopy without any of the limitations. Further study is required to determine whether SPLS meets this standard and, more important, whether it has any advantages over conventional techniques.
We want to hear from you! Tell us what you think.
1. Medeiros LR, Rosa DD, Bozzerri MC, et al. Laparoscopy versus laparotomy for benign ovarian tumour. Cochrane Database Syst Rev. 2009;(2):CD004751.-
2. Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynecological pathology. Results of a metaanalysis. Hum Reprod. 2002;17(5):1334-1342.
3. Fader AN, Escobar PF. Laparoendoscopic single-site surgery (LESS) in gynecologic oncology: technique and initial report. Gynecol Oncol. 2009;114(2):157-161.
4. Pelosi MA, Pelosi MA 3rd. Laparoscopic hysterectomy with bilateral salpingo-oophorectomy using a single umbilical puncture. N J Med. 1991;88(1):721-726.
5. Curcillo PG, King SA, Podolsky ER, Rottman SJ. Single port access (SPA) minimal access surgery through a single incision. Surg Technol Int. 2009;18:19-25.
6. Romanelli JR, Earle DB. Single-port laparoscopic surgery: an overview. Surg Endosc. 2009;23(7):1419-1427.
7. Ponsky TA. Single port laparoscopic cholecystectomy in adults and children: tools and techniques. J Am Coll Surg. 2009;209(5):e1-6.
8. Kim YW. Single port transumbilical myomectomy and ovarian cystectomy. J Minim Invasive Gynecol. 2009;16(6 suppl):S74.-
9. Ghezzi F, Cromi A, Fasola M, Bolis P. One-trocar salpingectomy for the treatment of tubal pregnancy: a “marionette-like” technique. BJOG. 2005;112(10):1417-1419.
10. Lim MC, Kim TJ, Kang S, Bae DS, Park SY, Seo SS. Embryonic natural orifice transumbilical endoscopic surgery (E-NOTES) for adnexal tumors. Surg Endosc. 2009;23(11):2445-2449.
11. Escobar PF, Starks DC, Fader AN, et al. Single-port risk-reducing salpingo-oophorectomy with and without hysterectomy: surgical outcomes and learning curve analysis. Gynecol Oncol. 2010;119(1):43-47.
12. Jeon HG, Jeong W, Oh CK. Initial experience with 50 laparoendoscopic single site surgeries using a homemade, single port device at a single center. J Urol. 2010;183(5):1866-1871.
13. Yoon G, Kim TJ, Lee YY, et al. Single port access subtotal hysterectomy with transcervical morcellation: a pilot study. J Minim Invasive Gynecol. 2010;17(1):78-81.
14. Ghomi A, Littman P, Prasad A, et al. Assessing the learning curve for laparoscopic supracervical hysterectomy. JSLS. 2007;11(2):190-194.
- Update on minimally invasive surgery
Amy Garcia, MD (April 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
The benefits of minimally invasive surgery—including less pain, faster recovery, and improved cosmesis—are well known.1,2 Standard laparotomy has been replaced by multiple-port operative laparoscopy for a great array of procedures, and advances in medical technology allow for a minimally invasive surgical approach even when a surgeon is faced with complex pathology.
Single-port laparoscopic surgery (SPLS) represents the latest advance in minimally invasive surgery. Using flexible endoscopes and articulating instruments, the surgeon can complete complex procedures through a single 2-cm incision in the abdomen. The incision is usually placed in the umbilicus, where it is easily hidden.3-8
Since the first laparoscopic hysterectomy through a single incision was performed 20 years ago, SPLS has been used successfully to perform nephrectomy, prostatectomy, hemicolectomy, cholecystectomy, splenectomy, intussusception reduction, gastrostomy tube placement, thoracoscopic lung biopsy, thoracoscopic decortication, and appendectomy.4-7
In gynecology, SPLS has been used to perform oophorectomy, salpingectomy, bilateral tubal ligation, ovarian cystectomy, surgical treatment of ectopic pregnancy, and both total and partial hysterectomy.7-11 At least two recent studies have concluded that SPLS is an acceptable way to treat many benign and malignant gynecologic conditions that are currently treated using multiport laparoscopy.3,11
This article outlines our approach to SPLS in the gynecologic patient and provides an overview of instrumentation, with the aim of allowing you to consider whether this approach might be feasible in your surgical practice, at your institution.
Unique setup required
When SPLS is performed through the umbilicus, the instruments must be held closer to the midline and more cephalad than during conventional laparoscopy to permit adequate visualization and manipulation. For this reason, the surgeon needs to assume a position higher over the torso and thorax of the patient, and both of the patient’s arms need to be tucked. Place the patient in a dorsal lithotomy position with a uterine manipulator in place to facilitate surgery—even when the uterus will be preserved and surgery involves only the adnexae.
With appropriate equipment and positioning, visualization and manipulation of anatomy are comparable to those of standard multiport laparoscopy.
New instruments simplify SPLS
Innovative surgical instruments allow for appropriate hand positioning outside the abdomen and minimize the internal collision of instruments brought through a single midline incision (FIGURE 1). A variety of single-port options are available, each with a unique patented design and method of insertion. In fact, the development of ports with multiple instrument channels has revolutionized SPLS.
FIGURE 1 Setup
Desired triangulation of instruments in SPLS setup.Before true single ports became available, it was necessary to place three 5-mm low-profile trocars in the fascia at three separate sites through a single skin incision. Pneumoperitoneum was established with a Veress needle, but the fascial incisions gradually merged with repeated cannula manipulation, producing air leaks.
Today, multiple-channel ports are placed using an open technique into a single skin and fascial incision. Trocars and instruments of varying size can be exchanged with ease without jeopardizing pneumoperitoneum.
Among the options:
- the SILS Port (Covidien) – a soft, flexible, three-channel port that allows for placement of blunt trocars ranging in size from 5 mm to 12 mm (FIGURE 2)
- the TriPort (Olympus America) – two flexible rings joined by a sleeve and multiple-channel port (FIGURE 3)
- GelPOINT Advanced Access Platform (Applied Medical) – a system constructed of synthetic gel material and consisting of a “GelSeal” cap, cannulas, and seals to accommodate 5-mm to 10-mm instrumentation (FIGURE 4).
We have found that all three devices allow for good range of motion while maintaining pneumoperitoneum.
(A recent article from Korea reports an inventive technique to perform single-incision laparoscopy using standard instrumentation: The authors fitted a self-retaining ring retractor with a surgical glove that had three of the fingers cut off and replaced by trocars.12)
FIGURE 2 SILS Port
The SILS Port is a soft, flexible, three-channel port that allows for placement of blunt trocars ranging in size from 5 mm to 12 mm.
FIGURE 3 TriPort
The TriPort system comprises two flexible rings joined by a sleeve and multiple-channel port.
FIGURE 4 GelPOINT
The GelPOINT Advanced Access Platform is constructed of synthetic gel material and accommodates 5-mm to 10-mm instrumentation.
A flexible laparoscope improves visualization
The ability to visualize the operative field is vital to any surgery, including SPLS. Use of a flexible laparoscope facilitates uncompromised visualization of the entire pelvis (FIGURE 5). Outside the abdomen, the flexible camera can be held laterally and away from the midline to help reduce the clashing of instruments and hands.
FIGURE 5 Flexible-tip laparoscope
A flexible-tip laparoscope ensures good visualization of the surgical field.If a flexible laparoscope is not available, a rigid 30-degree or 45-degree angled scope can be used, although visualization may be limited and adequate triangulation of instruments may be difficult to achieve.
When using a rigid laparoscope, a light cord that inserts into the back of the camera is necessary; otherwise, a 90-degree light cord adapter can be purchased.
Design enhancements facilitate coordination of instruments
One of the disadvantages of SPLS has been the restriction of movement that arises because of the close proximity of instruments and instrument handles. The latest designs have made articulation possible for tissue graspers, scissors, vessel sealers, and scopes.9,10 The value of articulation is apparent inside the abdomen, where it allows perfect positioning of the area of dissection. Outside the abdomen, the handles can be arranged in an angled pattern to allow the surgeon and assistant to operate comfortably (FIGURE 1).
In a four-handed procedure, one hand is on the camera, one on the uterine manipulator, and the remaining two hands operate the articulating grasper, vessel sealer, or needle driver, depending on the task.
Standard straight instruments can also be used for portions of the procedure.
Dissection and hemostasis are achieved in a manner similar to that of conventional laparoscopy. Our instrument of choice is an enhanced bipolar instrument, although harmonic and traditional biopolar energy can be used as well, depending on the preference of the surgeon.
The latest instruments are designed to dissect, cauterize, and cut, thereby decreasing the number of instrument exchanges necessary.
With the right aids, suturing can be simplified
Suturing through a single port can be a challenge. When possible, closure of the vaginal cuff following a total laparoscopic or laparoscopic-assisted vaginal hysterectomy should be performed from below. When endoscopic suturing is required, standard suturing using both intracorporeal and extracorporeal methods is possible.
Suturing aids such as the Endo Stitch (Covidien) or Lapra-Ty (Ethicon) are helpful. One author recommends Quill bidirectional, self-retaining suture with barbs (Angiotech) to avoid the need for knot-tying.6 (For more on self-retaining suture, see “Barbed suture, now in the toolbox of minimally invasive gyn surgery,” by Jon I. Einarsson, MD, MPH, and James A. Greenberg, MD, in the September 2009 issue at obgmanagement.com.)
The MiniLap (Stryker) is a 2.3-mm grasper that is inserted percutaneously directly through the abdominal wall without an incision. It can be used to set the needle on the needle driver or manipulate tissue while suturing. The resulting skin incision is barely visible and does not require closure.
Options are varied for specimen removal
Small specimens can be removed directly through a single-port system that has been opened, or they can be extracted after the system is removed, with rapid desufflation (FIGURE 6).
FIGURE 6 Single-incision oophorectomy
An ovary and tube removed through a single incision using the A) TriPort and B) SILS Port systems.Compared with conventional laparoscopy, the larger incision associated with single-port surgery facilitates specimen removal. Larger or potentially malignant specimens can be placed into an EndoCatch bag (Covidien) inserted through the single-incision 10-mm cannula (FIGURE 7).
FIGURE 7 Specimen removal
In this case, the specimen was placed in a 10-mm EndoCatch bag and removed through the 10-mm cannula of the SILS Port.In total laparoscopic hysterectomy or laparoscopic-assisted vaginal hysterectomy, the uterus is removed through the vagina. In supracervical hysterectomy, a small uterus can be removed through the cul-de-sac or directly through the single incision after placement in a bag.
When morcellation is required, the instrument can be placed through the cul-desac, cervix, or a single port. The morcellator can be placed directly through the SPLS port while utilizing the flexible scope in an angled direction (looking back toward the morcellator) for complete visualization (note: Covidien does not recommend this usage).
Transcervical tissue morcellation has also been described. In this approach, the cervix is dilated once the uterine body has been amputated, and the tissue morcellator is inserted through the cervix while the surgeon maintains visualization from above.6,13
How to master the technique
Many patients desire SPLS for its superior cosmetic outcome, but the approach may not always be appropriate. Depending on the procedure and characteristics of the patient (TABLE), multiple-port laparoscopy may be a better option. When a surgeon first attempts SPLS, we recommend that it be limited to the treatment of adnexal pathology only.
In your early SPlS cases, look for these patient characteristics
|
By using the techniques described in this article and selecting patients carefully, the surgeon can develop expertise in SPLS.11,14 During the learning process, the use of additional ports or Mini-Lap instruments (Stryker) can reduce the challenges of more difficult procedures and should be considered without reservation, as should the use of articulating accessory instruments and flexible or angled laparoscopes.
Although the clinical benefits of SPLS have yet to be determined, the cosmetic advantage of a single, hidden, umbilical incision likely increases patient satisfaction.
Clearly, the goal of SPLS is to use technology in a way that offers all of the benefits of traditional multiport laparoscopy without any of the limitations. Further study is required to determine whether SPLS meets this standard and, more important, whether it has any advantages over conventional techniques.
We want to hear from you! Tell us what you think.
- Update on minimally invasive surgery
Amy Garcia, MD (April 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
The benefits of minimally invasive surgery—including less pain, faster recovery, and improved cosmesis—are well known.1,2 Standard laparotomy has been replaced by multiple-port operative laparoscopy for a great array of procedures, and advances in medical technology allow for a minimally invasive surgical approach even when a surgeon is faced with complex pathology.
Single-port laparoscopic surgery (SPLS) represents the latest advance in minimally invasive surgery. Using flexible endoscopes and articulating instruments, the surgeon can complete complex procedures through a single 2-cm incision in the abdomen. The incision is usually placed in the umbilicus, where it is easily hidden.3-8
Since the first laparoscopic hysterectomy through a single incision was performed 20 years ago, SPLS has been used successfully to perform nephrectomy, prostatectomy, hemicolectomy, cholecystectomy, splenectomy, intussusception reduction, gastrostomy tube placement, thoracoscopic lung biopsy, thoracoscopic decortication, and appendectomy.4-7
In gynecology, SPLS has been used to perform oophorectomy, salpingectomy, bilateral tubal ligation, ovarian cystectomy, surgical treatment of ectopic pregnancy, and both total and partial hysterectomy.7-11 At least two recent studies have concluded that SPLS is an acceptable way to treat many benign and malignant gynecologic conditions that are currently treated using multiport laparoscopy.3,11
This article outlines our approach to SPLS in the gynecologic patient and provides an overview of instrumentation, with the aim of allowing you to consider whether this approach might be feasible in your surgical practice, at your institution.
Unique setup required
When SPLS is performed through the umbilicus, the instruments must be held closer to the midline and more cephalad than during conventional laparoscopy to permit adequate visualization and manipulation. For this reason, the surgeon needs to assume a position higher over the torso and thorax of the patient, and both of the patient’s arms need to be tucked. Place the patient in a dorsal lithotomy position with a uterine manipulator in place to facilitate surgery—even when the uterus will be preserved and surgery involves only the adnexae.
With appropriate equipment and positioning, visualization and manipulation of anatomy are comparable to those of standard multiport laparoscopy.
New instruments simplify SPLS
Innovative surgical instruments allow for appropriate hand positioning outside the abdomen and minimize the internal collision of instruments brought through a single midline incision (FIGURE 1). A variety of single-port options are available, each with a unique patented design and method of insertion. In fact, the development of ports with multiple instrument channels has revolutionized SPLS.
FIGURE 1 Setup
Desired triangulation of instruments in SPLS setup.Before true single ports became available, it was necessary to place three 5-mm low-profile trocars in the fascia at three separate sites through a single skin incision. Pneumoperitoneum was established with a Veress needle, but the fascial incisions gradually merged with repeated cannula manipulation, producing air leaks.
Today, multiple-channel ports are placed using an open technique into a single skin and fascial incision. Trocars and instruments of varying size can be exchanged with ease without jeopardizing pneumoperitoneum.
Among the options:
- the SILS Port (Covidien) – a soft, flexible, three-channel port that allows for placement of blunt trocars ranging in size from 5 mm to 12 mm (FIGURE 2)
- the TriPort (Olympus America) – two flexible rings joined by a sleeve and multiple-channel port (FIGURE 3)
- GelPOINT Advanced Access Platform (Applied Medical) – a system constructed of synthetic gel material and consisting of a “GelSeal” cap, cannulas, and seals to accommodate 5-mm to 10-mm instrumentation (FIGURE 4).
We have found that all three devices allow for good range of motion while maintaining pneumoperitoneum.
(A recent article from Korea reports an inventive technique to perform single-incision laparoscopy using standard instrumentation: The authors fitted a self-retaining ring retractor with a surgical glove that had three of the fingers cut off and replaced by trocars.12)
FIGURE 2 SILS Port
The SILS Port is a soft, flexible, three-channel port that allows for placement of blunt trocars ranging in size from 5 mm to 12 mm.
FIGURE 3 TriPort
The TriPort system comprises two flexible rings joined by a sleeve and multiple-channel port.
FIGURE 4 GelPOINT
The GelPOINT Advanced Access Platform is constructed of synthetic gel material and accommodates 5-mm to 10-mm instrumentation.
A flexible laparoscope improves visualization
The ability to visualize the operative field is vital to any surgery, including SPLS. Use of a flexible laparoscope facilitates uncompromised visualization of the entire pelvis (FIGURE 5). Outside the abdomen, the flexible camera can be held laterally and away from the midline to help reduce the clashing of instruments and hands.
FIGURE 5 Flexible-tip laparoscope
A flexible-tip laparoscope ensures good visualization of the surgical field.If a flexible laparoscope is not available, a rigid 30-degree or 45-degree angled scope can be used, although visualization may be limited and adequate triangulation of instruments may be difficult to achieve.
When using a rigid laparoscope, a light cord that inserts into the back of the camera is necessary; otherwise, a 90-degree light cord adapter can be purchased.
Design enhancements facilitate coordination of instruments
One of the disadvantages of SPLS has been the restriction of movement that arises because of the close proximity of instruments and instrument handles. The latest designs have made articulation possible for tissue graspers, scissors, vessel sealers, and scopes.9,10 The value of articulation is apparent inside the abdomen, where it allows perfect positioning of the area of dissection. Outside the abdomen, the handles can be arranged in an angled pattern to allow the surgeon and assistant to operate comfortably (FIGURE 1).
In a four-handed procedure, one hand is on the camera, one on the uterine manipulator, and the remaining two hands operate the articulating grasper, vessel sealer, or needle driver, depending on the task.
Standard straight instruments can also be used for portions of the procedure.
Dissection and hemostasis are achieved in a manner similar to that of conventional laparoscopy. Our instrument of choice is an enhanced bipolar instrument, although harmonic and traditional biopolar energy can be used as well, depending on the preference of the surgeon.
The latest instruments are designed to dissect, cauterize, and cut, thereby decreasing the number of instrument exchanges necessary.
With the right aids, suturing can be simplified
Suturing through a single port can be a challenge. When possible, closure of the vaginal cuff following a total laparoscopic or laparoscopic-assisted vaginal hysterectomy should be performed from below. When endoscopic suturing is required, standard suturing using both intracorporeal and extracorporeal methods is possible.
Suturing aids such as the Endo Stitch (Covidien) or Lapra-Ty (Ethicon) are helpful. One author recommends Quill bidirectional, self-retaining suture with barbs (Angiotech) to avoid the need for knot-tying.6 (For more on self-retaining suture, see “Barbed suture, now in the toolbox of minimally invasive gyn surgery,” by Jon I. Einarsson, MD, MPH, and James A. Greenberg, MD, in the September 2009 issue at obgmanagement.com.)
The MiniLap (Stryker) is a 2.3-mm grasper that is inserted percutaneously directly through the abdominal wall without an incision. It can be used to set the needle on the needle driver or manipulate tissue while suturing. The resulting skin incision is barely visible and does not require closure.
Options are varied for specimen removal
Small specimens can be removed directly through a single-port system that has been opened, or they can be extracted after the system is removed, with rapid desufflation (FIGURE 6).
FIGURE 6 Single-incision oophorectomy
An ovary and tube removed through a single incision using the A) TriPort and B) SILS Port systems.Compared with conventional laparoscopy, the larger incision associated with single-port surgery facilitates specimen removal. Larger or potentially malignant specimens can be placed into an EndoCatch bag (Covidien) inserted through the single-incision 10-mm cannula (FIGURE 7).
FIGURE 7 Specimen removal
In this case, the specimen was placed in a 10-mm EndoCatch bag and removed through the 10-mm cannula of the SILS Port.In total laparoscopic hysterectomy or laparoscopic-assisted vaginal hysterectomy, the uterus is removed through the vagina. In supracervical hysterectomy, a small uterus can be removed through the cul-de-sac or directly through the single incision after placement in a bag.
When morcellation is required, the instrument can be placed through the cul-desac, cervix, or a single port. The morcellator can be placed directly through the SPLS port while utilizing the flexible scope in an angled direction (looking back toward the morcellator) for complete visualization (note: Covidien does not recommend this usage).
Transcervical tissue morcellation has also been described. In this approach, the cervix is dilated once the uterine body has been amputated, and the tissue morcellator is inserted through the cervix while the surgeon maintains visualization from above.6,13
How to master the technique
Many patients desire SPLS for its superior cosmetic outcome, but the approach may not always be appropriate. Depending on the procedure and characteristics of the patient (TABLE), multiple-port laparoscopy may be a better option. When a surgeon first attempts SPLS, we recommend that it be limited to the treatment of adnexal pathology only.
In your early SPlS cases, look for these patient characteristics
|
By using the techniques described in this article and selecting patients carefully, the surgeon can develop expertise in SPLS.11,14 During the learning process, the use of additional ports or Mini-Lap instruments (Stryker) can reduce the challenges of more difficult procedures and should be considered without reservation, as should the use of articulating accessory instruments and flexible or angled laparoscopes.
Although the clinical benefits of SPLS have yet to be determined, the cosmetic advantage of a single, hidden, umbilical incision likely increases patient satisfaction.
Clearly, the goal of SPLS is to use technology in a way that offers all of the benefits of traditional multiport laparoscopy without any of the limitations. Further study is required to determine whether SPLS meets this standard and, more important, whether it has any advantages over conventional techniques.
We want to hear from you! Tell us what you think.
1. Medeiros LR, Rosa DD, Bozzerri MC, et al. Laparoscopy versus laparotomy for benign ovarian tumour. Cochrane Database Syst Rev. 2009;(2):CD004751.-
2. Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynecological pathology. Results of a metaanalysis. Hum Reprod. 2002;17(5):1334-1342.
3. Fader AN, Escobar PF. Laparoendoscopic single-site surgery (LESS) in gynecologic oncology: technique and initial report. Gynecol Oncol. 2009;114(2):157-161.
4. Pelosi MA, Pelosi MA 3rd. Laparoscopic hysterectomy with bilateral salpingo-oophorectomy using a single umbilical puncture. N J Med. 1991;88(1):721-726.
5. Curcillo PG, King SA, Podolsky ER, Rottman SJ. Single port access (SPA) minimal access surgery through a single incision. Surg Technol Int. 2009;18:19-25.
6. Romanelli JR, Earle DB. Single-port laparoscopic surgery: an overview. Surg Endosc. 2009;23(7):1419-1427.
7. Ponsky TA. Single port laparoscopic cholecystectomy in adults and children: tools and techniques. J Am Coll Surg. 2009;209(5):e1-6.
8. Kim YW. Single port transumbilical myomectomy and ovarian cystectomy. J Minim Invasive Gynecol. 2009;16(6 suppl):S74.-
9. Ghezzi F, Cromi A, Fasola M, Bolis P. One-trocar salpingectomy for the treatment of tubal pregnancy: a “marionette-like” technique. BJOG. 2005;112(10):1417-1419.
10. Lim MC, Kim TJ, Kang S, Bae DS, Park SY, Seo SS. Embryonic natural orifice transumbilical endoscopic surgery (E-NOTES) for adnexal tumors. Surg Endosc. 2009;23(11):2445-2449.
11. Escobar PF, Starks DC, Fader AN, et al. Single-port risk-reducing salpingo-oophorectomy with and without hysterectomy: surgical outcomes and learning curve analysis. Gynecol Oncol. 2010;119(1):43-47.
12. Jeon HG, Jeong W, Oh CK. Initial experience with 50 laparoendoscopic single site surgeries using a homemade, single port device at a single center. J Urol. 2010;183(5):1866-1871.
13. Yoon G, Kim TJ, Lee YY, et al. Single port access subtotal hysterectomy with transcervical morcellation: a pilot study. J Minim Invasive Gynecol. 2010;17(1):78-81.
14. Ghomi A, Littman P, Prasad A, et al. Assessing the learning curve for laparoscopic supracervical hysterectomy. JSLS. 2007;11(2):190-194.
1. Medeiros LR, Rosa DD, Bozzerri MC, et al. Laparoscopy versus laparotomy for benign ovarian tumour. Cochrane Database Syst Rev. 2009;(2):CD004751.-
2. Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynecological pathology. Results of a metaanalysis. Hum Reprod. 2002;17(5):1334-1342.
3. Fader AN, Escobar PF. Laparoendoscopic single-site surgery (LESS) in gynecologic oncology: technique and initial report. Gynecol Oncol. 2009;114(2):157-161.
4. Pelosi MA, Pelosi MA 3rd. Laparoscopic hysterectomy with bilateral salpingo-oophorectomy using a single umbilical puncture. N J Med. 1991;88(1):721-726.
5. Curcillo PG, King SA, Podolsky ER, Rottman SJ. Single port access (SPA) minimal access surgery through a single incision. Surg Technol Int. 2009;18:19-25.
6. Romanelli JR, Earle DB. Single-port laparoscopic surgery: an overview. Surg Endosc. 2009;23(7):1419-1427.
7. Ponsky TA. Single port laparoscopic cholecystectomy in adults and children: tools and techniques. J Am Coll Surg. 2009;209(5):e1-6.
8. Kim YW. Single port transumbilical myomectomy and ovarian cystectomy. J Minim Invasive Gynecol. 2009;16(6 suppl):S74.-
9. Ghezzi F, Cromi A, Fasola M, Bolis P. One-trocar salpingectomy for the treatment of tubal pregnancy: a “marionette-like” technique. BJOG. 2005;112(10):1417-1419.
10. Lim MC, Kim TJ, Kang S, Bae DS, Park SY, Seo SS. Embryonic natural orifice transumbilical endoscopic surgery (E-NOTES) for adnexal tumors. Surg Endosc. 2009;23(11):2445-2449.
11. Escobar PF, Starks DC, Fader AN, et al. Single-port risk-reducing salpingo-oophorectomy with and without hysterectomy: surgical outcomes and learning curve analysis. Gynecol Oncol. 2010;119(1):43-47.
12. Jeon HG, Jeong W, Oh CK. Initial experience with 50 laparoendoscopic single site surgeries using a homemade, single port device at a single center. J Urol. 2010;183(5):1866-1871.
13. Yoon G, Kim TJ, Lee YY, et al. Single port access subtotal hysterectomy with transcervical morcellation: a pilot study. J Minim Invasive Gynecol. 2010;17(1):78-81.
14. Ghomi A, Littman P, Prasad A, et al. Assessing the learning curve for laparoscopic supracervical hysterectomy. JSLS. 2007;11(2):190-194.
Colpocleisis: A simple, effective, and underutilized procedure
CASE 1: Problematic prolapse, but no incontinence
An 81-year-old multiparous woman, who has a history of recurrent stage-III pelvic organ prolapse (POP), reports worsening discomfort that makes it difficult for her to care for her ailing husband. She also has “trouble” with bladder emptying and constipation, but denies any loss of urine. She has not had vaginal intercourse in more than a decade because of her husband’s medical condition.
Aside from health issues—she suffers from obesity, coronary artery disease, hypertension, and diabetes—the patient is content with her marriage of 58 years.
Urodynamic testing fails to demonstrate detrusor overactivity, stress urinary incontinence, or intrinsic sphincteric deficiency. A cough stress test is repeated after reduction of her prolapse using a large cotton swab, and confirms the findings of the urodynamic tests.
Is reconstructive surgery appropriate for this patient?
Traditional reconstructive surgical procedures for treating POP fail in as many as 30% of patients, and new approaches—some involving grafts—are proposed every day, often without much data behind them.1
Regardless of the approach, reconstructive surgery is a lengthy procedure that subjects patients who are already medically compromised to significant risk, including bleeding, infection, and fluid shifts. Delayed return to normal activity may be especially costly among elderly women because of the risk of venous thromboembolism.
Because of the high failure rate, slow recovery, and risk of complications, reconstructive surgery may not be as appropriate as colpocleisis for the woman described above. Colpocleisis—suturing the inside walls of the vagina together—has an efficacy rate exceeding 90%.2 This relatively simple operation has been around for almost two centuries and has a good track record, but is often overlooked when counseling a patient about her options.
Any frail, elderly woman who has stage-III or -IV POP who does not desire to preserve coital ability is a candidate for colpocleisis (TABLE). Advantages include:
- a short operating time
- few complications
- amenability of local anesthesia
- short hospitalization
- speedy recovery
- high success rate
- low rate of regret.2-5
Because it precludes coital activity, however, colpocleisis may cause problems with self-image. It also may lead to de novo or worsening urinary incontinence and complicate or delay the diagnosis of cervical and endometrial pathology.
This article explores these issues through a case-based discussion of colpocleisis, including a detailed description of surgical technique.
TABLE
Requirements for colpocleisis
Both of the following must be present
|
Plus at least one of the following
|
Colpocleisis, as noted, entails suturing the inside walls of the vagina together. It is controversial because of its impact on coital activity. With careful patient selection, however, colpocleisis is considered a valid option for frail and elderly women who have POP and do not desire or foresee the possibility of future vaginal intercourse. Such women may represent a surprising percentage of the elderly population. A community-based survey found that 78% of married women 70 to 79 years old are not sexually active,6 and a study from The Netherlands found a prevalence of symptomatic POP of 11.4% among white women 45 to 85 years old.7
The fundamental reason for choosing an obliterative procedure such as colpocleisis over total pelvic reconstruction is to treat the prolapse with the least invasive technique in the shortest time. Hysterectomy, which often adds 30 to 80 minutes to the procedure, should therefore be performed only in patients who have a suspicious finding upon initial evaluation. For the same reason, partial colpocleisis—performed using the LeFort technique with limited dissection—has become the most popular obliterative approach. We try to avoid a total colpocleisis procedure—also known as colpectomy—in which the entire vaginal epithelium is stripped, because it is feasible only when the uterus is already absent or scheduled to be removed concomitantly.
(Note: The term vaginectomy should be reserved for gynecologic oncology procedures performed to remove vaginal cancer. Vaginectomy entails full-thickness excision of the vaginal walls, including the fibromuscular layer, as opposed to excision of the epithelial layer only, as in colpocleisis. In this article, we present the LeFort method, a partial colpocleisis technique, because we believe it is more easily adapted by the general gynecologist.8)
CASE 1 RESOLVED
After detailed counseling, which includes family members, the patient opts to undergo colpocleisis. The procedure takes 45 minutes. She is discharged on postoperative Day 1, and reports substantially improved quality of life.
CASE 2: Recurrent prolapse and problems with a pessary
A 72-year-old multiparous, widowed woman experiences recurrent stage-III isolated apical prolapse. She has already undergone two reconstructive procedures, and was discouraged from undergoing a third because of her chronic obstructive lung disease. She tried to use a Gellhorn-type pessary, which required a doctor’s intervention to insert and remove. Frustrated by the many office visits involved in having the pessary checked, she now demands surgical therapy. Another gynecologist has offered to repair the prolapse using mesh, but the patient has concerns about the safety and efficacy of the procedure because it is a relatively new approach.
In addition to the recurrent prolapse, she loses urine with stress and urge. She often has a postvoid residual volume >100 cc; urodynamic assessment confirms mixed urinary incontinence. The patient does not foresee any change in her social status (unmarried, sexually inactive).
Is colpocleisis a reasonable option?
Although the pessary is a helpful conservative alternative for women who are either unable or unwilling to undergo complex surgical pelvic repair and is considered first-line treatment by a majority of urogynecologists, it sometimes becomes more difficult to maintain than the patient is willing to tolerate.9 When a woman cannot remove and reinsert the device herself, the pessary requires a lifelong commitment to doctor’s visits every 2 or 3 months. This commitment is especially problematic for patients who become unable to drive or who lack social support.
Maintenance of the pessary becomes more frustrating as the patient becomes more dependent. Many gynecologists have seen a patient who developed a serious complication such as vesicovaginal or rectovaginal fistula because of a neglected pessary.10
In Case 2, the patient appears to be a potential candidate for colpocleisis, given her age and single status. Although pelvic floor repair appears to be safe in older women, any perioperative complication in a patient 70 years of age or older doubles the risk of discharge to a care facility.11,12 Women who have already undergone several surgeries or who have advanced medical problems such as coronary artery disease or cancer should be counseled thoroughly about the safety and efficacy of colpocleisis.
As for self-image, colpocleisis eliminates prolapse and reduces the genital hiatus. If the patient understands that colpocleisis is obliterative for the vagina but may improve the external appearance of the genital area, she may be more accepting of the procedure. One recent prospective, multicenter study found that only 2% of women thought their body looked worse 1 year after colpocleisis; 60% thought their body looked better.5
When reviewing treatment options, inform the patient that the pessary is a palliative option, whereas surgical therapy aims to be definitive.
CASE 2 RESOLVED
After comprehensive counseling, the patient elects to undergo colpocleisis, along with placement of a midurethral sling. She is discharged 1 day after surgery, and reports substantially improved urinary function, including bladder emptying, and quality of life. She says she would recommend the procedure to any woman who has a similar condition.
CASE 3: Pessary-related complications, incontinence, and underlying medical conditions
A 92-year-old multiparous widow, whose stage-IV uterovaginal prolapse has been managed by a pessary, develops vaginal ulcers in both anterior and posterior walls. After removal of the pessary and 4 weeks of treatment with vaginal estrogen, a smaller pessary is inserted, but she again develops ulcers and bleeding.
The patient’s medical condition is complicated by hypertension and generalized arthritis. She has urodynamically confirmed mixed urinary incontinence. She lives with her daughter and does not want to be placed in a nursing home.
What treatment options should you offer to her?
Because of this patient’s advanced age, poor health, and pessary-related problems, she is an ideal candidate for colpocleisis, provided she consents to the procedure after thorough counseling about its benefits and limitations.
Preoperative concerns
A thorough history, physical examination, and normal Pap test are necessary. If a suspicious pelvic mass or uterine bleeding is present, transvaginal ultrasonography (US) is crucial. In-office endometrial sampling also is necessary in any woman who has unexplained vaginal bleeding. More invasive procedures such as dilatation and curettage and hysteroscopy are needed only when the biopsy is inadequate or endometrial thickness exceeds 4 mm on transvaginal US.13
All elderly women who have high-risk medical problems must be cleared for surgery, with the necessary cardiac and pulmonary workup completed before the procedure.
Because colpocleisis is an extraperitoneal procedure, we have adapted use of over-the-counter enema products on the day before surgery in lieu of mechanical bowel preparation, which may lead to dehydration in very elderly women.
Coordinated consultation between the surgeon and anesthesiologist is necessary to determine the type of anesthesia to be used. Sedation and local anesthesia can be adequate for extremely high-risk women.14,15 Antibiotic prophylaxis is conventional for all patients.
Surgical technique
The LeFort method involves denudation and approximation of the midportions of the anterior and posterior vaginal walls.8 This operation creates a longitudinal vaginal septum with bilateral channels on each side, which serve as conduits for any secretion or bleeding from the apical vagina (FIGURE 1A AND B). Aggressive perineorraphy is also needed to shorten the genital hiatus. The following description incorporates perineorraphy into the LeFort technique.
FIGURE 1 Principles of LeFort colpocleisis
The depiction here is not anatomically precise: The vagina is illustrated as a rectangular prism to clarify the relationship between tissues.
Patient positioning
Place the patient in the dorsal lithotomy position, using stirrups to support the entire leg up to the knee. Let the patient’s buttocks overhang the edge of the table by 1 to 2 inches. A slight Trendelenburg position is imperative, especially when operating on the anterior compartment of the vagina. The bladder should be only partially emptied because the leakage of urine from the bladder makes it easier to identify inadvertent cystotomy. Infiltration of local anesthetic solution to develop the surgical planes is acceptable.
Initiating the procedure
Remove a rectangular piece of vaginal epithelium from the anterior vaginal wall, beginning 2 to 3 cm distal to the vaginal apex (or cervix, if the uterus is present) and ending immediately proximal to the urethrovesical junction to leave space for midurethral sling placement. Remove a similarly sized piece of epithelium from the posterior vaginal wall. This posterior rectangle is an almost geometric projection of the anterior rectangle, but is somewhat longer (2 to 3 cm) (FIGURE 1).
When removing the vaginal epithelium, it may be helpful to use the skills developed for anterior and posterior colporraphy. Our operation begins with a 5- to 6-cm transverse incision at the anterior vaginal apex, which creates the proximal side of the anterior rectangle described above (FIGURE 2A).
As you develop the plane between the epithelium and fibromuscular layer, make a midline sagittal incision and extend it to the urethrovesical junction (FIGURE 2B). Dissect the epithelium off the fibromuscular layer approximately 3 cm bilaterally, then make a transverse incision at the urethrovesical junction. Finally, remove the anterior rectangle in two pieces by cutting along the lateral sides (FIGURE 2C AND D). Remove the posterior rectangle using the same technique, but also excise a triangular piece of skin from the posterior fourchette for the perineorraphy portion of the procedure (FIGURE 2E).
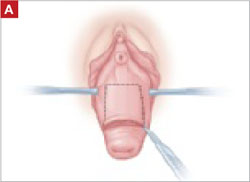
FIGURE 2 LeFort technique, step by step
Begin with a 5–6 cm transverse incision at the anterior vaginal apex.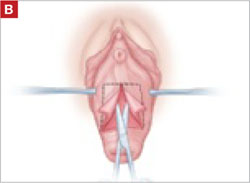
Dissect the epithelium off the fibromuscular layer, with a midline sagittal incision extending to the urethrovesical junction.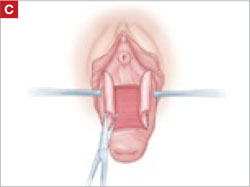
After dissection is completed, make a transverse incision at the urethrovesical junction, and remove the anterior rectangle in two pieces by cutting along the lateral sides.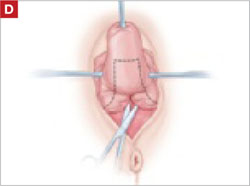
Denude the posterior rectangle using the same technique. In addition, excise a triangular piece of skin from the perineum.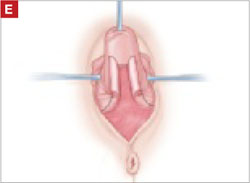
The posterior rectangle is ready for removal.
Suturing
Suture the apical sides of the anterior and posterior rectangles together using a continuous running technique (FIGURE 2F). Then approximate the lateral sides bilaterally using continuous sutures.
To ensure adherence of the anterior and posterior rectangles, stitch the raw surfaces together in three rows (FIGURE 2G). Do not include the distal 2 cm of the posterior vagina because you will need to leave room for perineorraphy.
Using several sutures, reapproximate the torn perineal fibromuscular structures in the midline to perform perineorraphy (FIGURE 2H). Close the distal vagina, beginning at the midpoint of the anterior transverse side, which lies at the urethrovesical junction (FIGURE 2I). Continue this suture on the posterior vagina and then the perineal body, sagittally, creating a small invagination in the distal vagina (FIGURE 2J).
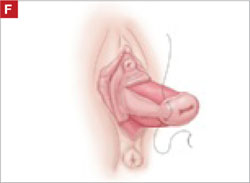
FIGURE 2 LeFort technique, step by step
Suture all but the distal sides of the rectangles between the anterior and posterior vaginal walls.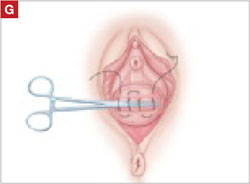
Also stitch together the raw surfaces in three rows in an imbricating fashion.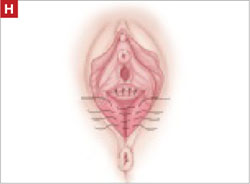
Perform perineorraphy.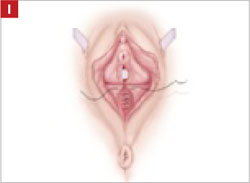
Close the distal vagina, starting at the midpoint of the anterior transverse side. If indicated, place a midurethral sling.
Final appearance.
Sling procedure
We place a midurethral sling as part of most colpocleisis operations. It is best to do this after the colpocleisis but before the perineorraphy.
In our cases, cystoscopy with simultaneous intravenous indigo carmine injection is standard before perineorraphy, even when a sling procedure is not planned. This safeguard ensures ureteral patency, which can be compromised (although rarely) in these procedures. Cutting and replacement of one of the sutures that approximate the raw tissues typically resolve the problem.16
Special considerations
Here are additional key points about colpocleisis, based on our experience:
- If an ulcer lies within the area designated to be denuded, some debridement to freshen up the surface will suffice. An ulcer is not an indication to deviate from the standard procedure.
- A modification developed by Goodall and Power may allow coitus by removing only a triangular piece of epithelium from each wall, leaving more room for the channels.17
- We have been unable to find any report of uterine or cervical cancer after colpocleisis, despite a MEDLINE search of the literature in English. Even so, the lateral channels created by the LeFort procedure allow any bleeding to escape the vagina, and may therefore enable recognition of malignancy. When noninvasive imaging techniques such as US or magnetic resonance are inadequate, vaginoscopy and hysteroscopy may be accomplished via these channels.
- When colpocleisis is performed in a hysterectomized woman, no lateral channel is necessary. Therefore, it is appropriate to do total colpocleisis.18,19
- When a patient with POP has a rectovaginal or vesicovaginal fistula caused by a neglected pessary, the addition of LeFort colpocleisis to the fistula repair may provide an effective treatment for both problems.10
Surgical outcomes
Success rate
Evidence concerning colpocleisis comes from case series, some of which are more than 30 years old. Although the definition of success is not clear in some series, the reported success rate has always exceeded 90% over the past three decades.2,18-22 Moreover, some of these reports involve as many as 30 years of follow-up.
Perioperative complication
In a recent review of the literature, the procedure-related mortality rate was 0.025%.2 When the authors focused only on studies published since 1980, major complications due to the patient’s underlying cardiovascular and pulmonary condition were seen in 2% of cases. Major surgical complications such as pyelonephritis and bleeding requiring transfusion occurred in 4% of cases, and less severe complications occurred in 15%.
In a study that included women who underwent concomitant vaginal hysterectomy, hysterectomy prolonged the surgery by 52 minutes, with a 5% rate of laparotomy as a result of intraoperative bleeding.22
In our series of 40 colpocleisis cases, we noted no instance in which a patient regretted the procedure.18 Others have also reported a low rate of regret—the highest being 9%.3-5,19-21
Using validated questionnaires, FitzGerald and colleagues found significant improvement in mental and physical quality of life, as well as urinary, colorectal, and bulge-related pelvic floor symptoms, 1 year after colpocleisis.5
De novo or worsening urinary incontinence is one of the drawbacks of colpocleisis. However, the same risk is present in approximately 40% of women who undergo surgical reconstructive procedures for POP without a continence operation.23 Because preoperative urinary retention is common in women who have POP, the decision to add a potentially harmful continence procedure is complicated in colpocleisis candidates. A small case series reported that the success rate ranged from 90% to 94% in women who underwent a midurethral tension-free sling procedure for the treatment of urinary incontinence at the time of colpocleisis.5
Preoperative urodynamic studies to detect urethral intrinsic deficiency and detrusor dysfunction are prudent, and detailed counseling of the patient about urinary control is vital. We perform a midurethral sling procedure in most of our colpocleisis cases, and have had pleasing results.
CASE 3 RESOLVED
The patient decides to undergo partial colpocleisis using the LeFort procedure, along with placement of a midurethral sling, for a total operative time of 75 minutes. She is discharged 1 day later and reports substantial improvement in urinary function and quality of life.
1. Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol. 2001;184:1496-1503.
2. FitzGerald MP, Richter HE, Siddique S, Thompson P, Zyczynski H, Weber A. For the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271.
3. Wheeler TL, Jr, Richter HE, Burgio KL, et al. Regret, satisfaction, and symptom improvement: analysis of the impact of partial colpocleisis for the management of severe pelvic organ prolapse. Am J Obstet Gynecol. 2005;193:2067-2070.
4. Hullfish KL, Bovbjerg VE, Steers WD. Colpocleisis for pelvic organ prolapse: patient goals, quality of life, and satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
5. FitzGerald MP, Richter HE, Bradley CS, et al. For the Pelvic Floor Disorders Network. Pelvic support, pelvic symptoms, and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1603-1609.
6. Patel D, Gillespie B, Foxman B. Sexual behavior of older women: results of a random-digit-dialing survey of 2,000 women in the United States. Sex Transm Dis. 2003;30:216-220.
7. Slieker-ten Hove MC, Pool-Goudzwaard AL, Eijkemans MJ, Steegers-Theunissen RP, Burger CW, Vierhout ME. Symptomatic pelvic organ prolapse and possible risk factors in a general population. Am J Obstet Gynecol. 2009;200:184.e1-184.e7.
8. Berlin F. Three cases of complete prolapsus uteri operated upon according to the method of Leon LeFort. Am J Obstet Gynecol. 1881;14:866-868.
9. Cundiff GW, Weidner AC, Visco AG, Bump RC, Addison WA. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 Pt 1):931-935.
10. Esin S, Harmanli OH. Large vesicovaginal fistula in women with pelvic organ prolapse: the role of colpocleisis revisited. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1711-1713.
11. Gerten KA, Markland AD, Lloyd LK, Richter HE. Prolapse and incontinence surgery in older women. J Urol. 2008;179:2111-2118.
12. Manku K, Bacchetti P, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. I. Long-term survival. Anesth Analg. 2003;96:583-589.
13. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 426: The role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol. 2009;113(2 Pt 1):462-464.
14. Moore RD, Miklos JR. Colpocleisis and tension-free vaginal tape sling for severe uterine and vaginal prolapse and stress urinary incontinence under local anesthesia. J Am Assoc Gynecol Laparosc. 2003;10:276-280.
15. Buchsbaum GM, Albushies DT, Schoenecker E, Duecy EE, Glantz JC. Local anesthesia with sedation for vaginal reconstructive surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:211-214.
16. Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194:1478-1485.
17. Goodall JR, Power RMH. A modification of the Le Fort operation for increasing its scope. Am J Obstet Gynecol. 1937;34:968-976.
18. Harmanli OH, Dandolu V, Chatwani AJ, Grody MT. Total colpocleisis for severe pelvic organ prolapse. J Reprod Med. 2003;48:703-706.
19. DeLancey JOL, Morley GW. Total colpocleisis for vaginal eversion. Am J Obstet Gynecol. 1997;176:1228-1232.
20. Goldman J, Ovadia J, Feldberg D. The Neugebauer-Le Fort operation: a review of 118 partial colpocleises. Eur J Obstet Gynecol Reprod Biol. 1981;12:31-35.
21. Ubachs JM, van Sante TJ, Schellekens LA. Partial colpocleisis by a modification of Le Fort’s operation. Obstet Gynecol. 1973;42:415-420.
22. Von Pechmann WS, Mutone MD, Fyffe J, Hale DS. Total colpocleisis with high levator plication for the treatment of advanced pelvic organ prolapse. Am J Obstet Gynecol. 2003;189:121-126.
23. Albo ME, Richter HE, Brubaker L, et al. For Urinary Incontinence Treatment Network. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.
CASE 1: Problematic prolapse, but no incontinence
An 81-year-old multiparous woman, who has a history of recurrent stage-III pelvic organ prolapse (POP), reports worsening discomfort that makes it difficult for her to care for her ailing husband. She also has “trouble” with bladder emptying and constipation, but denies any loss of urine. She has not had vaginal intercourse in more than a decade because of her husband’s medical condition.
Aside from health issues—she suffers from obesity, coronary artery disease, hypertension, and diabetes—the patient is content with her marriage of 58 years.
Urodynamic testing fails to demonstrate detrusor overactivity, stress urinary incontinence, or intrinsic sphincteric deficiency. A cough stress test is repeated after reduction of her prolapse using a large cotton swab, and confirms the findings of the urodynamic tests.
Is reconstructive surgery appropriate for this patient?
Traditional reconstructive surgical procedures for treating POP fail in as many as 30% of patients, and new approaches—some involving grafts—are proposed every day, often without much data behind them.1
Regardless of the approach, reconstructive surgery is a lengthy procedure that subjects patients who are already medically compromised to significant risk, including bleeding, infection, and fluid shifts. Delayed return to normal activity may be especially costly among elderly women because of the risk of venous thromboembolism.
Because of the high failure rate, slow recovery, and risk of complications, reconstructive surgery may not be as appropriate as colpocleisis for the woman described above. Colpocleisis—suturing the inside walls of the vagina together—has an efficacy rate exceeding 90%.2 This relatively simple operation has been around for almost two centuries and has a good track record, but is often overlooked when counseling a patient about her options.
Any frail, elderly woman who has stage-III or -IV POP who does not desire to preserve coital ability is a candidate for colpocleisis (TABLE). Advantages include:
- a short operating time
- few complications
- amenability of local anesthesia
- short hospitalization
- speedy recovery
- high success rate
- low rate of regret.2-5
Because it precludes coital activity, however, colpocleisis may cause problems with self-image. It also may lead to de novo or worsening urinary incontinence and complicate or delay the diagnosis of cervical and endometrial pathology.
This article explores these issues through a case-based discussion of colpocleisis, including a detailed description of surgical technique.
TABLE
Requirements for colpocleisis
Both of the following must be present
|
Plus at least one of the following
|
Colpocleisis, as noted, entails suturing the inside walls of the vagina together. It is controversial because of its impact on coital activity. With careful patient selection, however, colpocleisis is considered a valid option for frail and elderly women who have POP and do not desire or foresee the possibility of future vaginal intercourse. Such women may represent a surprising percentage of the elderly population. A community-based survey found that 78% of married women 70 to 79 years old are not sexually active,6 and a study from The Netherlands found a prevalence of symptomatic POP of 11.4% among white women 45 to 85 years old.7
The fundamental reason for choosing an obliterative procedure such as colpocleisis over total pelvic reconstruction is to treat the prolapse with the least invasive technique in the shortest time. Hysterectomy, which often adds 30 to 80 minutes to the procedure, should therefore be performed only in patients who have a suspicious finding upon initial evaluation. For the same reason, partial colpocleisis—performed using the LeFort technique with limited dissection—has become the most popular obliterative approach. We try to avoid a total colpocleisis procedure—also known as colpectomy—in which the entire vaginal epithelium is stripped, because it is feasible only when the uterus is already absent or scheduled to be removed concomitantly.
(Note: The term vaginectomy should be reserved for gynecologic oncology procedures performed to remove vaginal cancer. Vaginectomy entails full-thickness excision of the vaginal walls, including the fibromuscular layer, as opposed to excision of the epithelial layer only, as in colpocleisis. In this article, we present the LeFort method, a partial colpocleisis technique, because we believe it is more easily adapted by the general gynecologist.8)
CASE 1 RESOLVED
After detailed counseling, which includes family members, the patient opts to undergo colpocleisis. The procedure takes 45 minutes. She is discharged on postoperative Day 1, and reports substantially improved quality of life.
CASE 2: Recurrent prolapse and problems with a pessary
A 72-year-old multiparous, widowed woman experiences recurrent stage-III isolated apical prolapse. She has already undergone two reconstructive procedures, and was discouraged from undergoing a third because of her chronic obstructive lung disease. She tried to use a Gellhorn-type pessary, which required a doctor’s intervention to insert and remove. Frustrated by the many office visits involved in having the pessary checked, she now demands surgical therapy. Another gynecologist has offered to repair the prolapse using mesh, but the patient has concerns about the safety and efficacy of the procedure because it is a relatively new approach.
In addition to the recurrent prolapse, she loses urine with stress and urge. She often has a postvoid residual volume >100 cc; urodynamic assessment confirms mixed urinary incontinence. The patient does not foresee any change in her social status (unmarried, sexually inactive).
Is colpocleisis a reasonable option?
Although the pessary is a helpful conservative alternative for women who are either unable or unwilling to undergo complex surgical pelvic repair and is considered first-line treatment by a majority of urogynecologists, it sometimes becomes more difficult to maintain than the patient is willing to tolerate.9 When a woman cannot remove and reinsert the device herself, the pessary requires a lifelong commitment to doctor’s visits every 2 or 3 months. This commitment is especially problematic for patients who become unable to drive or who lack social support.
Maintenance of the pessary becomes more frustrating as the patient becomes more dependent. Many gynecologists have seen a patient who developed a serious complication such as vesicovaginal or rectovaginal fistula because of a neglected pessary.10
In Case 2, the patient appears to be a potential candidate for colpocleisis, given her age and single status. Although pelvic floor repair appears to be safe in older women, any perioperative complication in a patient 70 years of age or older doubles the risk of discharge to a care facility.11,12 Women who have already undergone several surgeries or who have advanced medical problems such as coronary artery disease or cancer should be counseled thoroughly about the safety and efficacy of colpocleisis.
As for self-image, colpocleisis eliminates prolapse and reduces the genital hiatus. If the patient understands that colpocleisis is obliterative for the vagina but may improve the external appearance of the genital area, she may be more accepting of the procedure. One recent prospective, multicenter study found that only 2% of women thought their body looked worse 1 year after colpocleisis; 60% thought their body looked better.5
When reviewing treatment options, inform the patient that the pessary is a palliative option, whereas surgical therapy aims to be definitive.
CASE 2 RESOLVED
After comprehensive counseling, the patient elects to undergo colpocleisis, along with placement of a midurethral sling. She is discharged 1 day after surgery, and reports substantially improved urinary function, including bladder emptying, and quality of life. She says she would recommend the procedure to any woman who has a similar condition.
CASE 3: Pessary-related complications, incontinence, and underlying medical conditions
A 92-year-old multiparous widow, whose stage-IV uterovaginal prolapse has been managed by a pessary, develops vaginal ulcers in both anterior and posterior walls. After removal of the pessary and 4 weeks of treatment with vaginal estrogen, a smaller pessary is inserted, but she again develops ulcers and bleeding.
The patient’s medical condition is complicated by hypertension and generalized arthritis. She has urodynamically confirmed mixed urinary incontinence. She lives with her daughter and does not want to be placed in a nursing home.
What treatment options should you offer to her?
Because of this patient’s advanced age, poor health, and pessary-related problems, she is an ideal candidate for colpocleisis, provided she consents to the procedure after thorough counseling about its benefits and limitations.
Preoperative concerns
A thorough history, physical examination, and normal Pap test are necessary. If a suspicious pelvic mass or uterine bleeding is present, transvaginal ultrasonography (US) is crucial. In-office endometrial sampling also is necessary in any woman who has unexplained vaginal bleeding. More invasive procedures such as dilatation and curettage and hysteroscopy are needed only when the biopsy is inadequate or endometrial thickness exceeds 4 mm on transvaginal US.13
All elderly women who have high-risk medical problems must be cleared for surgery, with the necessary cardiac and pulmonary workup completed before the procedure.
Because colpocleisis is an extraperitoneal procedure, we have adapted use of over-the-counter enema products on the day before surgery in lieu of mechanical bowel preparation, which may lead to dehydration in very elderly women.
Coordinated consultation between the surgeon and anesthesiologist is necessary to determine the type of anesthesia to be used. Sedation and local anesthesia can be adequate for extremely high-risk women.14,15 Antibiotic prophylaxis is conventional for all patients.
Surgical technique
The LeFort method involves denudation and approximation of the midportions of the anterior and posterior vaginal walls.8 This operation creates a longitudinal vaginal septum with bilateral channels on each side, which serve as conduits for any secretion or bleeding from the apical vagina (FIGURE 1A AND B). Aggressive perineorraphy is also needed to shorten the genital hiatus. The following description incorporates perineorraphy into the LeFort technique.

FIGURE 1 Principles of LeFort colpocleisis
The depiction here is not anatomically precise: The vagina is illustrated as a rectangular prism to clarify the relationship between tissues.
Patient positioning
Place the patient in the dorsal lithotomy position, using stirrups to support the entire leg up to the knee. Let the patient’s buttocks overhang the edge of the table by 1 to 2 inches. A slight Trendelenburg position is imperative, especially when operating on the anterior compartment of the vagina. The bladder should be only partially emptied because the leakage of urine from the bladder makes it easier to identify inadvertent cystotomy. Infiltration of local anesthetic solution to develop the surgical planes is acceptable.
Initiating the procedure
Remove a rectangular piece of vaginal epithelium from the anterior vaginal wall, beginning 2 to 3 cm distal to the vaginal apex (or cervix, if the uterus is present) and ending immediately proximal to the urethrovesical junction to leave space for midurethral sling placement. Remove a similarly sized piece of epithelium from the posterior vaginal wall. This posterior rectangle is an almost geometric projection of the anterior rectangle, but is somewhat longer (2 to 3 cm) (FIGURE 1).
When removing the vaginal epithelium, it may be helpful to use the skills developed for anterior and posterior colporraphy. Our operation begins with a 5- to 6-cm transverse incision at the anterior vaginal apex, which creates the proximal side of the anterior rectangle described above (FIGURE 2A).
As you develop the plane between the epithelium and fibromuscular layer, make a midline sagittal incision and extend it to the urethrovesical junction (FIGURE 2B). Dissect the epithelium off the fibromuscular layer approximately 3 cm bilaterally, then make a transverse incision at the urethrovesical junction. Finally, remove the anterior rectangle in two pieces by cutting along the lateral sides (FIGURE 2C AND D). Remove the posterior rectangle using the same technique, but also excise a triangular piece of skin from the posterior fourchette for the perineorraphy portion of the procedure (FIGURE 2E).

FIGURE 2 LeFort technique, step by step
Begin with a 5–6 cm transverse incision at the anterior vaginal apex.
Dissect the epithelium off the fibromuscular layer, with a midline sagittal incision extending to the urethrovesical junction.
After dissection is completed, make a transverse incision at the urethrovesical junction, and remove the anterior rectangle in two pieces by cutting along the lateral sides.
Denude the posterior rectangle using the same technique. In addition, excise a triangular piece of skin from the perineum.
The posterior rectangle is ready for removal.
Suturing
Suture the apical sides of the anterior and posterior rectangles together using a continuous running technique (FIGURE 2F). Then approximate the lateral sides bilaterally using continuous sutures.
To ensure adherence of the anterior and posterior rectangles, stitch the raw surfaces together in three rows (FIGURE 2G). Do not include the distal 2 cm of the posterior vagina because you will need to leave room for perineorraphy.
Using several sutures, reapproximate the torn perineal fibromuscular structures in the midline to perform perineorraphy (FIGURE 2H). Close the distal vagina, beginning at the midpoint of the anterior transverse side, which lies at the urethrovesical junction (FIGURE 2I). Continue this suture on the posterior vagina and then the perineal body, sagittally, creating a small invagination in the distal vagina (FIGURE 2J).

FIGURE 2 LeFort technique, step by step
Suture all but the distal sides of the rectangles between the anterior and posterior vaginal walls.
Also stitch together the raw surfaces in three rows in an imbricating fashion.
Perform perineorraphy.
Close the distal vagina, starting at the midpoint of the anterior transverse side. If indicated, place a midurethral sling.
Final appearance.
Sling procedure
We place a midurethral sling as part of most colpocleisis operations. It is best to do this after the colpocleisis but before the perineorraphy.
In our cases, cystoscopy with simultaneous intravenous indigo carmine injection is standard before perineorraphy, even when a sling procedure is not planned. This safeguard ensures ureteral patency, which can be compromised (although rarely) in these procedures. Cutting and replacement of one of the sutures that approximate the raw tissues typically resolve the problem.16
Special considerations
Here are additional key points about colpocleisis, based on our experience:
- If an ulcer lies within the area designated to be denuded, some debridement to freshen up the surface will suffice. An ulcer is not an indication to deviate from the standard procedure.
- A modification developed by Goodall and Power may allow coitus by removing only a triangular piece of epithelium from each wall, leaving more room for the channels.17
- We have been unable to find any report of uterine or cervical cancer after colpocleisis, despite a MEDLINE search of the literature in English. Even so, the lateral channels created by the LeFort procedure allow any bleeding to escape the vagina, and may therefore enable recognition of malignancy. When noninvasive imaging techniques such as US or magnetic resonance are inadequate, vaginoscopy and hysteroscopy may be accomplished via these channels.
- When colpocleisis is performed in a hysterectomized woman, no lateral channel is necessary. Therefore, it is appropriate to do total colpocleisis.18,19
- When a patient with POP has a rectovaginal or vesicovaginal fistula caused by a neglected pessary, the addition of LeFort colpocleisis to the fistula repair may provide an effective treatment for both problems.10
Surgical outcomes
Success rate
Evidence concerning colpocleisis comes from case series, some of which are more than 30 years old. Although the definition of success is not clear in some series, the reported success rate has always exceeded 90% over the past three decades.2,18-22 Moreover, some of these reports involve as many as 30 years of follow-up.
Perioperative complication
In a recent review of the literature, the procedure-related mortality rate was 0.025%.2 When the authors focused only on studies published since 1980, major complications due to the patient’s underlying cardiovascular and pulmonary condition were seen in 2% of cases. Major surgical complications such as pyelonephritis and bleeding requiring transfusion occurred in 4% of cases, and less severe complications occurred in 15%.
In a study that included women who underwent concomitant vaginal hysterectomy, hysterectomy prolonged the surgery by 52 minutes, with a 5% rate of laparotomy as a result of intraoperative bleeding.22
In our series of 40 colpocleisis cases, we noted no instance in which a patient regretted the procedure.18 Others have also reported a low rate of regret—the highest being 9%.3-5,19-21
Using validated questionnaires, FitzGerald and colleagues found significant improvement in mental and physical quality of life, as well as urinary, colorectal, and bulge-related pelvic floor symptoms, 1 year after colpocleisis.5
De novo or worsening urinary incontinence is one of the drawbacks of colpocleisis. However, the same risk is present in approximately 40% of women who undergo surgical reconstructive procedures for POP without a continence operation.23 Because preoperative urinary retention is common in women who have POP, the decision to add a potentially harmful continence procedure is complicated in colpocleisis candidates. A small case series reported that the success rate ranged from 90% to 94% in women who underwent a midurethral tension-free sling procedure for the treatment of urinary incontinence at the time of colpocleisis.5
Preoperative urodynamic studies to detect urethral intrinsic deficiency and detrusor dysfunction are prudent, and detailed counseling of the patient about urinary control is vital. We perform a midurethral sling procedure in most of our colpocleisis cases, and have had pleasing results.
CASE 3 RESOLVED
The patient decides to undergo partial colpocleisis using the LeFort procedure, along with placement of a midurethral sling, for a total operative time of 75 minutes. She is discharged 1 day later and reports substantial improvement in urinary function and quality of life.
CASE 1: Problematic prolapse, but no incontinence
An 81-year-old multiparous woman, who has a history of recurrent stage-III pelvic organ prolapse (POP), reports worsening discomfort that makes it difficult for her to care for her ailing husband. She also has “trouble” with bladder emptying and constipation, but denies any loss of urine. She has not had vaginal intercourse in more than a decade because of her husband’s medical condition.
Aside from health issues—she suffers from obesity, coronary artery disease, hypertension, and diabetes—the patient is content with her marriage of 58 years.
Urodynamic testing fails to demonstrate detrusor overactivity, stress urinary incontinence, or intrinsic sphincteric deficiency. A cough stress test is repeated after reduction of her prolapse using a large cotton swab, and confirms the findings of the urodynamic tests.
Is reconstructive surgery appropriate for this patient?
Traditional reconstructive surgical procedures for treating POP fail in as many as 30% of patients, and new approaches—some involving grafts—are proposed every day, often without much data behind them.1
Regardless of the approach, reconstructive surgery is a lengthy procedure that subjects patients who are already medically compromised to significant risk, including bleeding, infection, and fluid shifts. Delayed return to normal activity may be especially costly among elderly women because of the risk of venous thromboembolism.
Because of the high failure rate, slow recovery, and risk of complications, reconstructive surgery may not be as appropriate as colpocleisis for the woman described above. Colpocleisis—suturing the inside walls of the vagina together—has an efficacy rate exceeding 90%.2 This relatively simple operation has been around for almost two centuries and has a good track record, but is often overlooked when counseling a patient about her options.
Any frail, elderly woman who has stage-III or -IV POP who does not desire to preserve coital ability is a candidate for colpocleisis (TABLE). Advantages include:
- a short operating time
- few complications
- amenability of local anesthesia
- short hospitalization
- speedy recovery
- high success rate
- low rate of regret.2-5
Because it precludes coital activity, however, colpocleisis may cause problems with self-image. It also may lead to de novo or worsening urinary incontinence and complicate or delay the diagnosis of cervical and endometrial pathology.
This article explores these issues through a case-based discussion of colpocleisis, including a detailed description of surgical technique.
TABLE
Requirements for colpocleisis
Both of the following must be present
|
Plus at least one of the following
|
Colpocleisis, as noted, entails suturing the inside walls of the vagina together. It is controversial because of its impact on coital activity. With careful patient selection, however, colpocleisis is considered a valid option for frail and elderly women who have POP and do not desire or foresee the possibility of future vaginal intercourse. Such women may represent a surprising percentage of the elderly population. A community-based survey found that 78% of married women 70 to 79 years old are not sexually active,6 and a study from The Netherlands found a prevalence of symptomatic POP of 11.4% among white women 45 to 85 years old.7
The fundamental reason for choosing an obliterative procedure such as colpocleisis over total pelvic reconstruction is to treat the prolapse with the least invasive technique in the shortest time. Hysterectomy, which often adds 30 to 80 minutes to the procedure, should therefore be performed only in patients who have a suspicious finding upon initial evaluation. For the same reason, partial colpocleisis—performed using the LeFort technique with limited dissection—has become the most popular obliterative approach. We try to avoid a total colpocleisis procedure—also known as colpectomy—in which the entire vaginal epithelium is stripped, because it is feasible only when the uterus is already absent or scheduled to be removed concomitantly.
(Note: The term vaginectomy should be reserved for gynecologic oncology procedures performed to remove vaginal cancer. Vaginectomy entails full-thickness excision of the vaginal walls, including the fibromuscular layer, as opposed to excision of the epithelial layer only, as in colpocleisis. In this article, we present the LeFort method, a partial colpocleisis technique, because we believe it is more easily adapted by the general gynecologist.8)
CASE 1 RESOLVED
After detailed counseling, which includes family members, the patient opts to undergo colpocleisis. The procedure takes 45 minutes. She is discharged on postoperative Day 1, and reports substantially improved quality of life.
CASE 2: Recurrent prolapse and problems with a pessary
A 72-year-old multiparous, widowed woman experiences recurrent stage-III isolated apical prolapse. She has already undergone two reconstructive procedures, and was discouraged from undergoing a third because of her chronic obstructive lung disease. She tried to use a Gellhorn-type pessary, which required a doctor’s intervention to insert and remove. Frustrated by the many office visits involved in having the pessary checked, she now demands surgical therapy. Another gynecologist has offered to repair the prolapse using mesh, but the patient has concerns about the safety and efficacy of the procedure because it is a relatively new approach.
In addition to the recurrent prolapse, she loses urine with stress and urge. She often has a postvoid residual volume >100 cc; urodynamic assessment confirms mixed urinary incontinence. The patient does not foresee any change in her social status (unmarried, sexually inactive).
Is colpocleisis a reasonable option?
Although the pessary is a helpful conservative alternative for women who are either unable or unwilling to undergo complex surgical pelvic repair and is considered first-line treatment by a majority of urogynecologists, it sometimes becomes more difficult to maintain than the patient is willing to tolerate.9 When a woman cannot remove and reinsert the device herself, the pessary requires a lifelong commitment to doctor’s visits every 2 or 3 months. This commitment is especially problematic for patients who become unable to drive or who lack social support.
Maintenance of the pessary becomes more frustrating as the patient becomes more dependent. Many gynecologists have seen a patient who developed a serious complication such as vesicovaginal or rectovaginal fistula because of a neglected pessary.10
In Case 2, the patient appears to be a potential candidate for colpocleisis, given her age and single status. Although pelvic floor repair appears to be safe in older women, any perioperative complication in a patient 70 years of age or older doubles the risk of discharge to a care facility.11,12 Women who have already undergone several surgeries or who have advanced medical problems such as coronary artery disease or cancer should be counseled thoroughly about the safety and efficacy of colpocleisis.
As for self-image, colpocleisis eliminates prolapse and reduces the genital hiatus. If the patient understands that colpocleisis is obliterative for the vagina but may improve the external appearance of the genital area, she may be more accepting of the procedure. One recent prospective, multicenter study found that only 2% of women thought their body looked worse 1 year after colpocleisis; 60% thought their body looked better.5
When reviewing treatment options, inform the patient that the pessary is a palliative option, whereas surgical therapy aims to be definitive.
CASE 2 RESOLVED
After comprehensive counseling, the patient elects to undergo colpocleisis, along with placement of a midurethral sling. She is discharged 1 day after surgery, and reports substantially improved urinary function, including bladder emptying, and quality of life. She says she would recommend the procedure to any woman who has a similar condition.
CASE 3: Pessary-related complications, incontinence, and underlying medical conditions
A 92-year-old multiparous widow, whose stage-IV uterovaginal prolapse has been managed by a pessary, develops vaginal ulcers in both anterior and posterior walls. After removal of the pessary and 4 weeks of treatment with vaginal estrogen, a smaller pessary is inserted, but she again develops ulcers and bleeding.
The patient’s medical condition is complicated by hypertension and generalized arthritis. She has urodynamically confirmed mixed urinary incontinence. She lives with her daughter and does not want to be placed in a nursing home.
What treatment options should you offer to her?
Because of this patient’s advanced age, poor health, and pessary-related problems, she is an ideal candidate for colpocleisis, provided she consents to the procedure after thorough counseling about its benefits and limitations.
Preoperative concerns
A thorough history, physical examination, and normal Pap test are necessary. If a suspicious pelvic mass or uterine bleeding is present, transvaginal ultrasonography (US) is crucial. In-office endometrial sampling also is necessary in any woman who has unexplained vaginal bleeding. More invasive procedures such as dilatation and curettage and hysteroscopy are needed only when the biopsy is inadequate or endometrial thickness exceeds 4 mm on transvaginal US.13
All elderly women who have high-risk medical problems must be cleared for surgery, with the necessary cardiac and pulmonary workup completed before the procedure.
Because colpocleisis is an extraperitoneal procedure, we have adapted use of over-the-counter enema products on the day before surgery in lieu of mechanical bowel preparation, which may lead to dehydration in very elderly women.
Coordinated consultation between the surgeon and anesthesiologist is necessary to determine the type of anesthesia to be used. Sedation and local anesthesia can be adequate for extremely high-risk women.14,15 Antibiotic prophylaxis is conventional for all patients.
Surgical technique
The LeFort method involves denudation and approximation of the midportions of the anterior and posterior vaginal walls.8 This operation creates a longitudinal vaginal septum with bilateral channels on each side, which serve as conduits for any secretion or bleeding from the apical vagina (FIGURE 1A AND B). Aggressive perineorraphy is also needed to shorten the genital hiatus. The following description incorporates perineorraphy into the LeFort technique.

FIGURE 1 Principles of LeFort colpocleisis
The depiction here is not anatomically precise: The vagina is illustrated as a rectangular prism to clarify the relationship between tissues.
Patient positioning
Place the patient in the dorsal lithotomy position, using stirrups to support the entire leg up to the knee. Let the patient’s buttocks overhang the edge of the table by 1 to 2 inches. A slight Trendelenburg position is imperative, especially when operating on the anterior compartment of the vagina. The bladder should be only partially emptied because the leakage of urine from the bladder makes it easier to identify inadvertent cystotomy. Infiltration of local anesthetic solution to develop the surgical planes is acceptable.
Initiating the procedure
Remove a rectangular piece of vaginal epithelium from the anterior vaginal wall, beginning 2 to 3 cm distal to the vaginal apex (or cervix, if the uterus is present) and ending immediately proximal to the urethrovesical junction to leave space for midurethral sling placement. Remove a similarly sized piece of epithelium from the posterior vaginal wall. This posterior rectangle is an almost geometric projection of the anterior rectangle, but is somewhat longer (2 to 3 cm) (FIGURE 1).
When removing the vaginal epithelium, it may be helpful to use the skills developed for anterior and posterior colporraphy. Our operation begins with a 5- to 6-cm transverse incision at the anterior vaginal apex, which creates the proximal side of the anterior rectangle described above (FIGURE 2A).
As you develop the plane between the epithelium and fibromuscular layer, make a midline sagittal incision and extend it to the urethrovesical junction (FIGURE 2B). Dissect the epithelium off the fibromuscular layer approximately 3 cm bilaterally, then make a transverse incision at the urethrovesical junction. Finally, remove the anterior rectangle in two pieces by cutting along the lateral sides (FIGURE 2C AND D). Remove the posterior rectangle using the same technique, but also excise a triangular piece of skin from the posterior fourchette for the perineorraphy portion of the procedure (FIGURE 2E).

FIGURE 2 LeFort technique, step by step
Begin with a 5–6 cm transverse incision at the anterior vaginal apex.
Dissect the epithelium off the fibromuscular layer, with a midline sagittal incision extending to the urethrovesical junction.
After dissection is completed, make a transverse incision at the urethrovesical junction, and remove the anterior rectangle in two pieces by cutting along the lateral sides.
Denude the posterior rectangle using the same technique. In addition, excise a triangular piece of skin from the perineum.
The posterior rectangle is ready for removal.
Suturing
Suture the apical sides of the anterior and posterior rectangles together using a continuous running technique (FIGURE 2F). Then approximate the lateral sides bilaterally using continuous sutures.
To ensure adherence of the anterior and posterior rectangles, stitch the raw surfaces together in three rows (FIGURE 2G). Do not include the distal 2 cm of the posterior vagina because you will need to leave room for perineorraphy.
Using several sutures, reapproximate the torn perineal fibromuscular structures in the midline to perform perineorraphy (FIGURE 2H). Close the distal vagina, beginning at the midpoint of the anterior transverse side, which lies at the urethrovesical junction (FIGURE 2I). Continue this suture on the posterior vagina and then the perineal body, sagittally, creating a small invagination in the distal vagina (FIGURE 2J).

FIGURE 2 LeFort technique, step by step
Suture all but the distal sides of the rectangles between the anterior and posterior vaginal walls.
Also stitch together the raw surfaces in three rows in an imbricating fashion.
Perform perineorraphy.
Close the distal vagina, starting at the midpoint of the anterior transverse side. If indicated, place a midurethral sling.
Final appearance.
Sling procedure
We place a midurethral sling as part of most colpocleisis operations. It is best to do this after the colpocleisis but before the perineorraphy.
In our cases, cystoscopy with simultaneous intravenous indigo carmine injection is standard before perineorraphy, even when a sling procedure is not planned. This safeguard ensures ureteral patency, which can be compromised (although rarely) in these procedures. Cutting and replacement of one of the sutures that approximate the raw tissues typically resolve the problem.16
Special considerations
Here are additional key points about colpocleisis, based on our experience:
- If an ulcer lies within the area designated to be denuded, some debridement to freshen up the surface will suffice. An ulcer is not an indication to deviate from the standard procedure.
- A modification developed by Goodall and Power may allow coitus by removing only a triangular piece of epithelium from each wall, leaving more room for the channels.17
- We have been unable to find any report of uterine or cervical cancer after colpocleisis, despite a MEDLINE search of the literature in English. Even so, the lateral channels created by the LeFort procedure allow any bleeding to escape the vagina, and may therefore enable recognition of malignancy. When noninvasive imaging techniques such as US or magnetic resonance are inadequate, vaginoscopy and hysteroscopy may be accomplished via these channels.
- When colpocleisis is performed in a hysterectomized woman, no lateral channel is necessary. Therefore, it is appropriate to do total colpocleisis.18,19
- When a patient with POP has a rectovaginal or vesicovaginal fistula caused by a neglected pessary, the addition of LeFort colpocleisis to the fistula repair may provide an effective treatment for both problems.10
Surgical outcomes
Success rate
Evidence concerning colpocleisis comes from case series, some of which are more than 30 years old. Although the definition of success is not clear in some series, the reported success rate has always exceeded 90% over the past three decades.2,18-22 Moreover, some of these reports involve as many as 30 years of follow-up.
Perioperative complication
In a recent review of the literature, the procedure-related mortality rate was 0.025%.2 When the authors focused only on studies published since 1980, major complications due to the patient’s underlying cardiovascular and pulmonary condition were seen in 2% of cases. Major surgical complications such as pyelonephritis and bleeding requiring transfusion occurred in 4% of cases, and less severe complications occurred in 15%.
In a study that included women who underwent concomitant vaginal hysterectomy, hysterectomy prolonged the surgery by 52 minutes, with a 5% rate of laparotomy as a result of intraoperative bleeding.22
In our series of 40 colpocleisis cases, we noted no instance in which a patient regretted the procedure.18 Others have also reported a low rate of regret—the highest being 9%.3-5,19-21
Using validated questionnaires, FitzGerald and colleagues found significant improvement in mental and physical quality of life, as well as urinary, colorectal, and bulge-related pelvic floor symptoms, 1 year after colpocleisis.5
De novo or worsening urinary incontinence is one of the drawbacks of colpocleisis. However, the same risk is present in approximately 40% of women who undergo surgical reconstructive procedures for POP without a continence operation.23 Because preoperative urinary retention is common in women who have POP, the decision to add a potentially harmful continence procedure is complicated in colpocleisis candidates. A small case series reported that the success rate ranged from 90% to 94% in women who underwent a midurethral tension-free sling procedure for the treatment of urinary incontinence at the time of colpocleisis.5
Preoperative urodynamic studies to detect urethral intrinsic deficiency and detrusor dysfunction are prudent, and detailed counseling of the patient about urinary control is vital. We perform a midurethral sling procedure in most of our colpocleisis cases, and have had pleasing results.
CASE 3 RESOLVED
The patient decides to undergo partial colpocleisis using the LeFort procedure, along with placement of a midurethral sling, for a total operative time of 75 minutes. She is discharged 1 day later and reports substantial improvement in urinary function and quality of life.
1. Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol. 2001;184:1496-1503.
2. FitzGerald MP, Richter HE, Siddique S, Thompson P, Zyczynski H, Weber A. For the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271.
3. Wheeler TL, Jr, Richter HE, Burgio KL, et al. Regret, satisfaction, and symptom improvement: analysis of the impact of partial colpocleisis for the management of severe pelvic organ prolapse. Am J Obstet Gynecol. 2005;193:2067-2070.
4. Hullfish KL, Bovbjerg VE, Steers WD. Colpocleisis for pelvic organ prolapse: patient goals, quality of life, and satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
5. FitzGerald MP, Richter HE, Bradley CS, et al. For the Pelvic Floor Disorders Network. Pelvic support, pelvic symptoms, and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1603-1609.
6. Patel D, Gillespie B, Foxman B. Sexual behavior of older women: results of a random-digit-dialing survey of 2,000 women in the United States. Sex Transm Dis. 2003;30:216-220.
7. Slieker-ten Hove MC, Pool-Goudzwaard AL, Eijkemans MJ, Steegers-Theunissen RP, Burger CW, Vierhout ME. Symptomatic pelvic organ prolapse and possible risk factors in a general population. Am J Obstet Gynecol. 2009;200:184.e1-184.e7.
8. Berlin F. Three cases of complete prolapsus uteri operated upon according to the method of Leon LeFort. Am J Obstet Gynecol. 1881;14:866-868.
9. Cundiff GW, Weidner AC, Visco AG, Bump RC, Addison WA. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 Pt 1):931-935.
10. Esin S, Harmanli OH. Large vesicovaginal fistula in women with pelvic organ prolapse: the role of colpocleisis revisited. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1711-1713.
11. Gerten KA, Markland AD, Lloyd LK, Richter HE. Prolapse and incontinence surgery in older women. J Urol. 2008;179:2111-2118.
12. Manku K, Bacchetti P, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. I. Long-term survival. Anesth Analg. 2003;96:583-589.
13. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 426: The role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol. 2009;113(2 Pt 1):462-464.
14. Moore RD, Miklos JR. Colpocleisis and tension-free vaginal tape sling for severe uterine and vaginal prolapse and stress urinary incontinence under local anesthesia. J Am Assoc Gynecol Laparosc. 2003;10:276-280.
15. Buchsbaum GM, Albushies DT, Schoenecker E, Duecy EE, Glantz JC. Local anesthesia with sedation for vaginal reconstructive surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:211-214.
16. Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194:1478-1485.
17. Goodall JR, Power RMH. A modification of the Le Fort operation for increasing its scope. Am J Obstet Gynecol. 1937;34:968-976.
18. Harmanli OH, Dandolu V, Chatwani AJ, Grody MT. Total colpocleisis for severe pelvic organ prolapse. J Reprod Med. 2003;48:703-706.
19. DeLancey JOL, Morley GW. Total colpocleisis for vaginal eversion. Am J Obstet Gynecol. 1997;176:1228-1232.
20. Goldman J, Ovadia J, Feldberg D. The Neugebauer-Le Fort operation: a review of 118 partial colpocleises. Eur J Obstet Gynecol Reprod Biol. 1981;12:31-35.
21. Ubachs JM, van Sante TJ, Schellekens LA. Partial colpocleisis by a modification of Le Fort’s operation. Obstet Gynecol. 1973;42:415-420.
22. Von Pechmann WS, Mutone MD, Fyffe J, Hale DS. Total colpocleisis with high levator plication for the treatment of advanced pelvic organ prolapse. Am J Obstet Gynecol. 2003;189:121-126.
23. Albo ME, Richter HE, Brubaker L, et al. For Urinary Incontinence Treatment Network. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.
1. Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol. 2001;184:1496-1503.
2. FitzGerald MP, Richter HE, Siddique S, Thompson P, Zyczynski H, Weber A. For the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271.
3. Wheeler TL, Jr, Richter HE, Burgio KL, et al. Regret, satisfaction, and symptom improvement: analysis of the impact of partial colpocleisis for the management of severe pelvic organ prolapse. Am J Obstet Gynecol. 2005;193:2067-2070.
4. Hullfish KL, Bovbjerg VE, Steers WD. Colpocleisis for pelvic organ prolapse: patient goals, quality of life, and satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
5. FitzGerald MP, Richter HE, Bradley CS, et al. For the Pelvic Floor Disorders Network. Pelvic support, pelvic symptoms, and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1603-1609.
6. Patel D, Gillespie B, Foxman B. Sexual behavior of older women: results of a random-digit-dialing survey of 2,000 women in the United States. Sex Transm Dis. 2003;30:216-220.
7. Slieker-ten Hove MC, Pool-Goudzwaard AL, Eijkemans MJ, Steegers-Theunissen RP, Burger CW, Vierhout ME. Symptomatic pelvic organ prolapse and possible risk factors in a general population. Am J Obstet Gynecol. 2009;200:184.e1-184.e7.
8. Berlin F. Three cases of complete prolapsus uteri operated upon according to the method of Leon LeFort. Am J Obstet Gynecol. 1881;14:866-868.
9. Cundiff GW, Weidner AC, Visco AG, Bump RC, Addison WA. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 Pt 1):931-935.
10. Esin S, Harmanli OH. Large vesicovaginal fistula in women with pelvic organ prolapse: the role of colpocleisis revisited. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1711-1713.
11. Gerten KA, Markland AD, Lloyd LK, Richter HE. Prolapse and incontinence surgery in older women. J Urol. 2008;179:2111-2118.
12. Manku K, Bacchetti P, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. I. Long-term survival. Anesth Analg. 2003;96:583-589.
13. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 426: The role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol. 2009;113(2 Pt 1):462-464.
14. Moore RD, Miklos JR. Colpocleisis and tension-free vaginal tape sling for severe uterine and vaginal prolapse and stress urinary incontinence under local anesthesia. J Am Assoc Gynecol Laparosc. 2003;10:276-280.
15. Buchsbaum GM, Albushies DT, Schoenecker E, Duecy EE, Glantz JC. Local anesthesia with sedation for vaginal reconstructive surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:211-214.
16. Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194:1478-1485.
17. Goodall JR, Power RMH. A modification of the Le Fort operation for increasing its scope. Am J Obstet Gynecol. 1937;34:968-976.
18. Harmanli OH, Dandolu V, Chatwani AJ, Grody MT. Total colpocleisis for severe pelvic organ prolapse. J Reprod Med. 2003;48:703-706.
19. DeLancey JOL, Morley GW. Total colpocleisis for vaginal eversion. Am J Obstet Gynecol. 1997;176:1228-1232.
20. Goldman J, Ovadia J, Feldberg D. The Neugebauer-Le Fort operation: a review of 118 partial colpocleises. Eur J Obstet Gynecol Reprod Biol. 1981;12:31-35.
21. Ubachs JM, van Sante TJ, Schellekens LA. Partial colpocleisis by a modification of Le Fort’s operation. Obstet Gynecol. 1973;42:415-420.
22. Von Pechmann WS, Mutone MD, Fyffe J, Hale DS. Total colpocleisis with high levator plication for the treatment of advanced pelvic organ prolapse. Am J Obstet Gynecol. 2003;189:121-126.
23. Albo ME, Richter HE, Brubaker L, et al. For Urinary Incontinence Treatment Network. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.
Postmenopausal dyspareunia— a problem for the 21st century
The author reports that he serves on the speaker’s bureau for Novogyne, TherRx, Warner-Chilcott, and Solvay, and on the advisory board for Upsher-Smith, Novogyne, QuatRx, and Wyeth.
CASE: History of dyspareunia
At her latest visit, a 56-year-old woman who is 7 years postmenopausal relates that she has been experiencing worsening pain with intercourse to the point that she now has very little sex drive at all. This problem began approximately 1 year after she discontinued hormone therapy in the wake of reports that it causes cancer and heart attack. She has been offered both local vaginal and systemic hormone therapy, but is too frightened to use any hormones at all. Sexual lubricants no longer seem to work.
How do you counsel her about these symptoms? And what therapy do you offer?
Physicians and other health-care practitioners are seeing a large and growing number of genitourinary and sexual-related complaints among menopausal women—so much so that it has reached epidemic proportions. Yet dyspareunia is underreported and undertreated, and quality of life suffers for these women.
In this article, I focus on two interrelated causes of this epidemic:
- vaginal dryness and vulvovaginal atrophy (VVA) and the impact of these conditions on women’s sexual function and psychosocial well-being
- barriers to optimal treatment.
I also explore how ObGyns’ role in this area of care is evolving—as a way to understand how you can better serve this expanding segment of our patient population.
Dyspareunia can have many causes, including endometriosis, interstitial cystitis, surgical scarring, injury that occurs during childbirth, and psychosocial origin (such as a history of sexual abuse). Our focus here is on dyspareunia due to VVA.
during sex. What should you do?
- Sexual pain as a category of female sexual dysfunction is relevant at any age; for postmenopausal women dealing with vaginal dryness as a result of estrogen deficiency, it may well be the dominant issue. When determining the cause of a sexual problem in a postmenopausal woman, put dyspareunia caused by vaginal dryness (as well as its psychosocial consequences) at the top of the list of possibilities.
- Bring up the topic of vaginal dryness and sexual pain with postmenopausal patients as part of the routine yearly exam, and explain the therapeutic capabilities of all available options.
- Estrogen therapy, either local or systemic, remains the standard when lubricants are inadequate. Make every effort to counsel the patient about the real risk:benefit ratio of estrogen use.
- If the patient is reluctant to use estrogen therapy, discuss with her the option of short-term local estrogen use, with the understanding that more acceptable options may become available in the near future. This may facilitate acceptance of short-term hormonal treatment and allow the patient to maintain her vaginal health and much of her vaginal sexual function.
- Keep abreast of both present and future options for therapy.
Just how sizable is the postmenopausal population?
About 32% of the female population is older than 50 years.1 That means that around 48 million women are currently menopausal, or will become so over the next few years.
Because average life expectancy approaches 80 years in the United States and other countries of the industrialized world,2 many women will live approximately 40 years beyond menopause or their final menstrual period. Their quality of life during the second half of their life is dependent on both physical and psychosocial health.
Postmenopausal dyspareunia isn’t new
Sexual issues arising from physical causes—dyspareunia among them—have long accounted for a large share of medical concerns reported by postmenopausal women. In a 1985 survey, for example, dyspareunia accounted for 42.5% of their complaints.3
But epidemiologic studies to determine the prevalence of female sexual dysfunction in postmenopausal women are difficult to carry out. Why? Because researchers would need to 1) address changes over time and 2) distinguish problems of sexual function from those brought on by aging.4
The techniques and methodology for researching female sexual dysfunction continue to evolve, creating new definitions of the stages of menopause and new diagnostic approaches to female sexual dysfunction.
However, based on available studies, Dennerstein and Hayes concluded that:
- postmenopausal women report a high rate of sexual dysfunction (higher than men)
- psychosocial factors can ameliorate a decline in sexual function
- “vaginal dryness and dyspareunia seem to be driven primarily by declining estradiol.”4
The WHI and its domino effect
Millions of postmenopausal women stopped taking estrogen-based therapy in the wake of widespread media coverage after 2002 publication of data from the estrogen–progestin arm of the Women’s Health Initiative (WHI), which purported to show, among other things, an increased risk of breast cancer.5
For decades, many postmenopausal women achieved medical management of VVA through long-term use of systemic hormone replacement therapy (HRT), which they used primarily to control other chronic symptoms of menopause, such as hot flashes.
After the WHI data were published (and misrepresented), reduced usage of estrogen-based HRT “unmasked” vaginal symptoms, including sexual pain, due to the effects of estrogen deficiency on the vaginal epithelium and vaginal blood flow. Since then, we have been forced to examine anew the natural history of menopause.
Within days or weeks of discontinuing HRT, women may reexperience the acute vasomotor symptoms that accompany estrogen withdrawal—most commonly hot flashes, night sweats, sleeplessness, palpitations, and headaches. Over time—anywhere from 6 months to several years—the body adjusts to the loss or withdrawal of estrogen, and these vasomotor symptoms eventually diminish or resolve. Not so for the longer-term physical effects of chronic low serum levels of estrogen, which worsen over time.
Approximately 6 months after discontinuing estrogen therapy, postmenopausal women may begin to experience vaginal dryness and VVA. As the years pass, other side effects of estrogen deficiency arise: bone loss, joint pain, mood alteration (including depression), change in skin tone, hair loss, and cardiac and central nervous system changes. These side effects do not resolve spontaneously; in fact, they grow worse as a woman ages. They may have deleterious psychosocial as well as physical impacts on her life—especially on the quality of her intimate relationship.
Clarify the report (adjust appropriately for same-sex partner)
- Where does it hurt? Describe the pain.
- When does it hurt? Does the pain occur 1) with penile contact at the opening of the vagina, 2) once the penis is partially in, 3) with full entry, 4) after some thrusting, 5) after deep thrusting, 6) with the partner’s ejaculation, 7) after withdrawal, or 8) with subsequent micturition?
- Does your body tense when your partner is attempting, or you are attempting, to insert his penis? What are your thoughts and feelings at this time?
- How long does the pain last?
- Does touching cause pain? Does it hurt when you ride a bicycle or wear tight clothes? Does penetration by tampons or fingers hurt?
Assess the pelvic floor
- Do you recognize the feeling of pelvic floor muscle tension during sexual contact?
- Do you recognize the feeling of pelvic floor muscle tension in other (nonsexual) situations?
Evaluate arousal
- Do you feel subjectively excited when you attempt intercourse?
- Does your vagina become sufficiently moist? Do you recognize the feeling of drying up?
Determine the consequences of the complaint
- What do you do when you experience pain during sexual contact? Do you continue? Or do you stop whatever is causing the pain?
- Do you continue to include intercourse or attempts at intercourse in your lovemaking, or do you use other methods of achieving sexual fulfillment? If you use other ways to make love, do you and your partner clearly understand that intercourse will not be attempted?
- What other effect does the pain have on your sexual relationship?
Explore biomedical antecedents
- When and how did the pain start?
- What tests have you undergone?
- What treatment have you received?
Source: Adapted from Basson R, et al.12
Is 60 the new 40?
Many women and men in the large cohort known as the Baby Boomer generation continue to be sexually active into their 60s, 70s, and 80s, as demonstrated by a 2007 study of sexuality and health in older adults.6 In the 57- to 64-year-old age group, 61.6% of women and 83.7% of men were sexually active (defined as sexual activity with a partner within the past 12 months). In the 65- to 74-year-old group, 39.5% of women and 67% of men were sexually active; and in the 75- to 85-year-old group, 16.7% of women and 38.5% of men were sexually active (TABLE).
These findings indicate that fewer women than men remain sexually active during their later years. One reason may be the epidemic of sexual-related symptoms among postmenopausal women. In the same survey, 34.3% of women 57 to 64 years old reported avoiding sex because of:
- pain during intercourse (17.8%)
- difficulty with lubrication (35.9%).
Across all groups, the most prevalent sexual problem was low desire (43%).6 Around 40% of postmenopausal women reported no sexual activity in the past 12 months, as well as lack of interest in sex. This number may include women who have ceased to have sex because of vaginal dryness and dyspareunia, thereby reducing the percentage reporting these symptoms (TABLE).
TABLE
Older adults are having sex—and experiencing sexual problems
| Activity or problem by gender | Number of respondents | Report, by age group (95% confidence interval*) | ||
|---|---|---|---|---|
| 57–64 yr (%) | 65–74 yr (%) | 75–85 yr (%) | ||
| Sexually active in previous 12 months† | ||||
| Men | 1,385 | 83.7 (77.6–89.8) | 67.0 (62.1–72.0) | 38.5 (33.6–43.5) |
| Women | 1,501 | 61.6 (56.7–66.4) | 39.5 (34.6–44.4) | 16.7 (12.5–21.0) |
| Difficulty with lubrication | ||||
| Women | 495 | 35.9 (29.6–42.2) | 43.2 (34.8–51.5) | 43.6 (27.0–60.2) |
| Pain during intercourse | ||||
| Men | 878 | 3.0 (1.1–4.8) | 3.2 (1.2–5.3) | 1.0 (0–2.5) |
| Women | 506 | 17.8 (13.3–22.2) | 18.6 (10.8–26.3) | 11.8 (4.3–19.4) |
| Avoidance of sex due to sexual problems** | ||||
| Men | 533 | 22.1 (17.3–26.9) | 30.1 (23.2–37.0) | 25.7 (14.9–36.4) |
| Women | 357 | 34.3 (25.0–43.7) | 30.5 (21.5–39.4) | 22.7 (9.4–35.9) |
| Source: Adapted from Lindau ST, et al.6 | ||||
| Adjusted odds ratios are based on a logistic regression including the age group and self-rated health status as covariates, estimated separately for men and women. The confidence interval is based on the inversion of the Wald tests constructed with the use of design-based standard errors. | ||||
| † These data exclude 107 respondents who reported at least one sexual problem. | ||||
| ** This question was asked only of respondents who reported at least one sexual problem. | ||||
Assessing menopause-related sexual function is a challenge
Although the transition phases of menopause have been well studied and reported for decades, few of these studies have included questions about the impact of menopause on sexual function.7 When longitudinal studies that included the classification of female sexual dysfunction began to appear, they provided evidence of the important role that VVA and psychosocial factors play in female sexual dysfunction.8
In the fourth year of the Melbourne Women’s Midlife Health Project longitudinal study, six variables related to sexual function were identified. Three were determinate of sexual function:
- feelings for the partner
- problems related to the partner
- vaginal dryness/dyspareunia.
The other three variables—sexual responsiveness, frequency of sexual activity, and libido—were dependent or outcome variables.
By the sixth year of this study, two variables had increased in significance: vaginal dryness/dyspareunia and partner problems.7
Sexual pain and relationship problems can create a vicious cycle
The interrelationship of vaginal dryness, sexual pain, flagging desire, and psychosocial parameters can produce a vicious cycle. A woman experiencing or anticipating pain may have diminished sexual desire or avoid sex altogether. During intercourse, the brain’s awareness of vaginal pain may trigger a physiologic response that can cause the muscles of the vagina to tighten and lubrication to decrease. The result? Greater vaginal pain.
This vicious cycle can contribute to relationship issues with the sexual partner and harm a woman’s psychosocial well-being. Resentment, anger, and misunderstanding may arise when a couple is dealing with problems of sexual function, and these stressors can damage many aspects of the relationship, further exacerbating sexual difficulties.
An additional and very important dimension of these issues is their potential impact on the family unit.
VVA can diminish overall well-being
In a 2007 survey reported at the North American Menopause Society (NAMS), one third to one half of 506 respondents said that VVA had a bad effect on their sexual interest, mood, self-esteem, and the intimate relationship (FIGURE 1).9 Reports from in-depth interviews were consistent with survey results and offered further insight into a woman’s emotional response to the condition of vaginal dryness and its impact on her life. Women found the condition “embarrassing,” something they had to endure but didn’t talk about, and felt that it had a major impact on their self-esteem and intimate relationship.
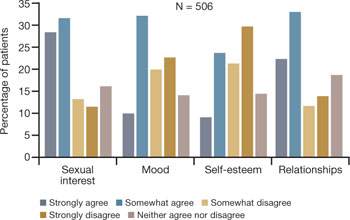
FIGURE 1 Dyspareunia affects more than interest in sex—relationships, mood, and self-esteem suffer
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Clinicians often don’t ask about VVA, and patients are reluctant to talk
Among women of all ages, dyspareunia is underreported and undertreated. In the survey reported at NAMS, 40% of respondents said that their physician had never asked them about the problem of VVA (FIGURE 2).9
Women themselves may be reluctant to discuss the problem with physicians, nurse practitioners, or other health-care providers out of embarrassment or the assumption that there is nothing to be done about the problem. Nevertheless, more than 40% of respondents said they would be highly likely to seek treatment for VVA if they had a concern about urogenital complications of the condition (FIGURE 3).9
Another barrier may be the sense that asking the health-care provider about sex may embarrass him or her. As a result, sufferers do not anticipate help from their physician and other members of the health-care profession and fail to seek treatment or counseling for this chronic medical condition.10,11
In a 1999 telephone survey of 500 adults 25 years of age or older, 71% said they thought that their doctor would dismiss concerns about sexual problems, but 85% said they would talk to their physician anyway if they had a problem, even though they might not get treatment.11 In that survey, 91% of married men and 84% of married women rated a satisfying sex life as important to quality of life.11
Another important and often overlooked limitation on this type of discussion is the time constraints that busy clinicians face, especially with the low reimbursement offered by managed care. Sexual problems can hardly be adequately discussed in 7 to 10 minutes.
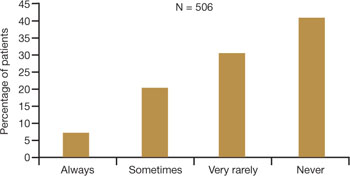
FIGURE 2 Do physicians ask about dyspareunia? Most women surveyed said “rarely” or “never”
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
FIGURE 3 Are these women likely to seek treatment?
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Women have performance anxiety, too
It is well known that men with even a mild degree of erectile dysfunction can suffer from performance anxiety, but the fact that women can also suffer from this phenomenon is not given as much attention. Such anxiety can be a factor in relationship difficulties. With both partners perhaps feeling anxious about sexual performance, a couple may avoid even simple acts of affection, such as holding hands, to avoid raising the other’s expectations.
Exacerbating the situation is the fact that many men use widely prescribed phosphodiesterase type 5 (PDE5) inhibitors, whereas women are contending with barriers to continued sexual activity as they age. It does not take a psychologist to understand that this imbalance often adds to emotional strain and tension between partners.
Popular media address the issue
Look beyond what our postmenopausal patients tell us directly—to the popular media and online forums—to appreciate the scope of sexual pain as a major issue among postmenopausal women. Evidence of psychosocial effects is found on numerous Web sites—some from organizations, others designed by women seeking help from each other.
Red Hot Mamas
This organization aims to empower women through menopause education. Highlighted in the Winter 2007/2008 Red Hot Mamas Report is a survey done in conjunction with Harris Interactive exploring the impact of menopausal symptoms on a woman’s sex life, which found that 47% of women who have VVA have avoided or stopped sex completely because it was uncomfortable, compared with 23% of normal women.
Power Surge
This Web site offers a list of strategies for dealing with sexual pain, including an overview of hormone-based prescription and nonprescription products, along with a variety of over-the-counter, natural, holistic, and herbal therapies for treating dyspareunia.
What is the physician’s role?
Given the epidemic of sexual pain, it is crucial that physicians and others who care for postmenopausal women increase their awareness of this issue and pay special attention to its psychosocial parameters.
Ask patients about sexual function in general and dyspareunia in particular as part of the routine annual visit. A simple opening “Yes/No” question, such as “Are you sexually active?” can lead to further questions appropriate to the patient. For example, if the answer is “No,” the follow-up question might be, “Does that bother you or your partner?” Further discussion may uncover whether the lack of sexual activity is a cause of distress and identify which variables are involved.
If, instead, the answer is “Yes,” follow-up questions can identify the presence of common postmenopausal physical issues, such as vaginal dryness and difficulty with lubrication. The visit then can turn to strategies to ameliorate those conditions.
When a patient reports dyspareunia, further diagnostic information such as precise location, degree of arousal, and reaction to pain can help determine the appropriate course of treatment. For an approach to this aspect of ascertaining patient history, see the list of sample questions above.12
During the physical, pay particular attention to any physical abnormalities or organic causes of sexual pain. Questions designed to characterize the location and nature of the pain can pinpoint the cause. Sexual pain arising from VVA is likely to 1) be localized at the introitus and 2) occur with penile entry.
Since the mid-1990s, the availability of validated scales to measure female sexual function has increased rapidly and enabled researchers to better identify, quantify, and evaluate treatments for female sexual dysfunction.7 Over time, we have moved away from the somewhat mechanical sequence inherent in the linear progression of desire leading to genital stimulation followed by arousal and orgasm toward an appreciation of the multiple physical, emotional, and subjective factors that are at play in women’s sexual function.
By 1998, a classification scheme was developed to further the means to study and discuss disorders of desire, arousal, orgasm, and sexual pain.8 Further contextual definitions of sexual dysfunction are under consideration.13
Basson proposed one new model of female sexual function (see the diagram), and observed that
"…women identify many reasons they are sexual over and beyond inherent sexual drive or “hunger.” Women tell of wanting to increase emotional closeness, commitment, sharing, tenderness, and tolerance, and to show the partner that he or she has been missed (emotionally or physically). Such intimacy-based reasons motivate the woman to find a way to become sexually aroused. This arousal is not spontaneous but triggered by deliberately sought sexual stimuli."13
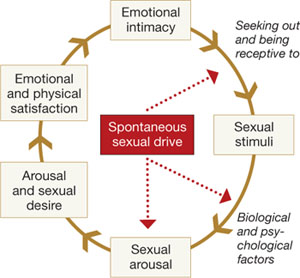
Intimacy-based model of female sexual response cycle
In this flow of physical and emotional variables involved in female sexual function, categories interact. For example, low desire can be and is frequently secondary to the anticipation of pain during sexual intercourse. Arousal can be hampered by lack of vaginal lubrication—perhaps inhibited by the anticipation of pain. Secondary orgasmic disorders can result from low desire, difficulty of arousal, and sexual pain.14 Sexual pain can affect sexual function at any point on this continuum.
Treatments in the pipeline
For decades, hormone-based treatments have been the predominant therapeutic option for vaginal dryness. Often they are a secondary benefit of hormone therapy for vasomotor symptoms and osteoporosis. Estrogen can be delivered in the form of oral tablet, transdermal patch, gel, spray, or vaginal ring for systemic use, or as vaginal cream, ring, or tablet for local use.
However, despite data to the contrary and our reassurances to the patient about overall safety, a large number of women, and many primary care providers, are no longer inclined to use short- or long-term HRT in any presentation.
Other women may have risk factors that contraindicate exogenous hormones.
Nonhormonal options for vaginal dryness and dyspareunia are limited, and there are no approved systemic or oral nonestrogen options. Over-the-counter topical lubricants can ease some of the symptoms of VVA temporarily and allow successful vaginal penetration in many cases. Some may cause vaginal warming and pleasant sensations, but overall they treat the symptom rather than the source of pain. Moreover, many patients consider local lubricants messy and inconvenient and claim they “ruin the mood.”
The use of vaginal dilators along with estrogen or lubricant therapy is an often-forgotten adjunct to therapy for dyspareunia caused by VVA (FIGURE 4).
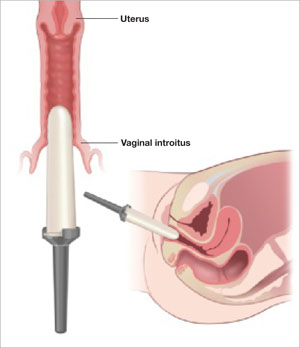
FIGURE 4 Mechanical dilation of the vagina is a useful adjunct
Mechanical dilation is often needed to restore penetration capability in the vagina, even after hormonal treatment. The focus should be on the vaginal introitus, with the top 25% to 35% of the dilator inserted into the opening once a day for 15 minutes, increasing the dilator diameter over time.
New SERMs are in development
Preclinical and clinical research into the diverse class of selective estrogen receptor modulators (SERMs) to treat estrogen-mediated disease produced tamoxifen for breast cancer prevention and raloxifene for both vertebral osteoporosis and breast cancer prevention. Each SERM seems to have unique tissue selectivity. The antiestrogenic activity of tamoxifen and raloxifene extends to the vagina and can exacerbate vaginal dryness.
A new generation of orally active SERMs is under investigation specifically for the treatment of chronic vaginal symptoms. These new agents target the nonvaginal treatment of VVA and associated symptoms. The first oral SERM for long-term treatment of these symptoms, ospemifene (Ophena), may become available in the near future. It is a novel SERM that has both anti-estrogenic and estrogenic actions, depending on the tissue. It was shown to significantly improve both vaginal dryness and dyspareunia in a large placebo-controlled trial.15
1. US Census Bureau. 2006 American community survey. S0101. Age and sex. Available at: http://fact-finder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenuId=&_lang=en&_ts.
2. National Center for Health Statistics. Health, United States, 2007, with Chartbook on Trends in the Health of Americans. Hyattsville, Md: NCHS; 2007. Available at: http://www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed February 2, 2009.
3. Sarrel PM, Whitehead MI. Sex and menopause: defining the issues. Maturitas. 1985;7:217-224.
4. Dennerstein L, Hayes RD. Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med. 2005;2(suppl 3):118-132.
5. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
6. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
7. Dennerstein L, Alexander JL, Kotz K. The menopause and sexual functioning: a review of the population-based studies. Annu Rev Sex Res. 2003;14:64-82.
8. Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888-993.
9. Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
10. Heim LJ. Evaluation and differential diagnosis of dyspareunia. Am Fam Physician. 2001;63:1535-1552.
11. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173-2174.
12. Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med. 2004;1:24-34.
13. Basson R. Female sexual response: the role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-353.
14. Walsh KE, Berman JR. Sexual dysfunction in the older woman: an overview of the current understanding and management. Drugs Aging. 2004;21:655-675.
15. Bachmann GA, Komi J, Hanley R. A new SERM, Ophena (ospemifene), effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Presented at the Endocrine Society annual meeting, San Francisco, Calif, June 2008.
The author reports that he serves on the speaker’s bureau for Novogyne, TherRx, Warner-Chilcott, and Solvay, and on the advisory board for Upsher-Smith, Novogyne, QuatRx, and Wyeth.
CASE: History of dyspareunia
At her latest visit, a 56-year-old woman who is 7 years postmenopausal relates that she has been experiencing worsening pain with intercourse to the point that she now has very little sex drive at all. This problem began approximately 1 year after she discontinued hormone therapy in the wake of reports that it causes cancer and heart attack. She has been offered both local vaginal and systemic hormone therapy, but is too frightened to use any hormones at all. Sexual lubricants no longer seem to work.
How do you counsel her about these symptoms? And what therapy do you offer?
Physicians and other health-care practitioners are seeing a large and growing number of genitourinary and sexual-related complaints among menopausal women—so much so that it has reached epidemic proportions. Yet dyspareunia is underreported and undertreated, and quality of life suffers for these women.
In this article, I focus on two interrelated causes of this epidemic:
- vaginal dryness and vulvovaginal atrophy (VVA) and the impact of these conditions on women’s sexual function and psychosocial well-being
- barriers to optimal treatment.
I also explore how ObGyns’ role in this area of care is evolving—as a way to understand how you can better serve this expanding segment of our patient population.
Dyspareunia can have many causes, including endometriosis, interstitial cystitis, surgical scarring, injury that occurs during childbirth, and psychosocial origin (such as a history of sexual abuse). Our focus here is on dyspareunia due to VVA.
during sex. What should you do?
- Sexual pain as a category of female sexual dysfunction is relevant at any age; for postmenopausal women dealing with vaginal dryness as a result of estrogen deficiency, it may well be the dominant issue. When determining the cause of a sexual problem in a postmenopausal woman, put dyspareunia caused by vaginal dryness (as well as its psychosocial consequences) at the top of the list of possibilities.
- Bring up the topic of vaginal dryness and sexual pain with postmenopausal patients as part of the routine yearly exam, and explain the therapeutic capabilities of all available options.
- Estrogen therapy, either local or systemic, remains the standard when lubricants are inadequate. Make every effort to counsel the patient about the real risk:benefit ratio of estrogen use.
- If the patient is reluctant to use estrogen therapy, discuss with her the option of short-term local estrogen use, with the understanding that more acceptable options may become available in the near future. This may facilitate acceptance of short-term hormonal treatment and allow the patient to maintain her vaginal health and much of her vaginal sexual function.
- Keep abreast of both present and future options for therapy.
Just how sizable is the postmenopausal population?
About 32% of the female population is older than 50 years.1 That means that around 48 million women are currently menopausal, or will become so over the next few years.
Because average life expectancy approaches 80 years in the United States and other countries of the industrialized world,2 many women will live approximately 40 years beyond menopause or their final menstrual period. Their quality of life during the second half of their life is dependent on both physical and psychosocial health.
Postmenopausal dyspareunia isn’t new
Sexual issues arising from physical causes—dyspareunia among them—have long accounted for a large share of medical concerns reported by postmenopausal women. In a 1985 survey, for example, dyspareunia accounted for 42.5% of their complaints.3
But epidemiologic studies to determine the prevalence of female sexual dysfunction in postmenopausal women are difficult to carry out. Why? Because researchers would need to 1) address changes over time and 2) distinguish problems of sexual function from those brought on by aging.4
The techniques and methodology for researching female sexual dysfunction continue to evolve, creating new definitions of the stages of menopause and new diagnostic approaches to female sexual dysfunction.
However, based on available studies, Dennerstein and Hayes concluded that:
- postmenopausal women report a high rate of sexual dysfunction (higher than men)
- psychosocial factors can ameliorate a decline in sexual function
- “vaginal dryness and dyspareunia seem to be driven primarily by declining estradiol.”4
The WHI and its domino effect
Millions of postmenopausal women stopped taking estrogen-based therapy in the wake of widespread media coverage after 2002 publication of data from the estrogen–progestin arm of the Women’s Health Initiative (WHI), which purported to show, among other things, an increased risk of breast cancer.5
For decades, many postmenopausal women achieved medical management of VVA through long-term use of systemic hormone replacement therapy (HRT), which they used primarily to control other chronic symptoms of menopause, such as hot flashes.
After the WHI data were published (and misrepresented), reduced usage of estrogen-based HRT “unmasked” vaginal symptoms, including sexual pain, due to the effects of estrogen deficiency on the vaginal epithelium and vaginal blood flow. Since then, we have been forced to examine anew the natural history of menopause.
Within days or weeks of discontinuing HRT, women may reexperience the acute vasomotor symptoms that accompany estrogen withdrawal—most commonly hot flashes, night sweats, sleeplessness, palpitations, and headaches. Over time—anywhere from 6 months to several years—the body adjusts to the loss or withdrawal of estrogen, and these vasomotor symptoms eventually diminish or resolve. Not so for the longer-term physical effects of chronic low serum levels of estrogen, which worsen over time.
Approximately 6 months after discontinuing estrogen therapy, postmenopausal women may begin to experience vaginal dryness and VVA. As the years pass, other side effects of estrogen deficiency arise: bone loss, joint pain, mood alteration (including depression), change in skin tone, hair loss, and cardiac and central nervous system changes. These side effects do not resolve spontaneously; in fact, they grow worse as a woman ages. They may have deleterious psychosocial as well as physical impacts on her life—especially on the quality of her intimate relationship.
Clarify the report (adjust appropriately for same-sex partner)
- Where does it hurt? Describe the pain.
- When does it hurt? Does the pain occur 1) with penile contact at the opening of the vagina, 2) once the penis is partially in, 3) with full entry, 4) after some thrusting, 5) after deep thrusting, 6) with the partner’s ejaculation, 7) after withdrawal, or 8) with subsequent micturition?
- Does your body tense when your partner is attempting, or you are attempting, to insert his penis? What are your thoughts and feelings at this time?
- How long does the pain last?
- Does touching cause pain? Does it hurt when you ride a bicycle or wear tight clothes? Does penetration by tampons or fingers hurt?
Assess the pelvic floor
- Do you recognize the feeling of pelvic floor muscle tension during sexual contact?
- Do you recognize the feeling of pelvic floor muscle tension in other (nonsexual) situations?
Evaluate arousal
- Do you feel subjectively excited when you attempt intercourse?
- Does your vagina become sufficiently moist? Do you recognize the feeling of drying up?
Determine the consequences of the complaint
- What do you do when you experience pain during sexual contact? Do you continue? Or do you stop whatever is causing the pain?
- Do you continue to include intercourse or attempts at intercourse in your lovemaking, or do you use other methods of achieving sexual fulfillment? If you use other ways to make love, do you and your partner clearly understand that intercourse will not be attempted?
- What other effect does the pain have on your sexual relationship?
Explore biomedical antecedents
- When and how did the pain start?
- What tests have you undergone?
- What treatment have you received?
Source: Adapted from Basson R, et al.12
Is 60 the new 40?
Many women and men in the large cohort known as the Baby Boomer generation continue to be sexually active into their 60s, 70s, and 80s, as demonstrated by a 2007 study of sexuality and health in older adults.6 In the 57- to 64-year-old age group, 61.6% of women and 83.7% of men were sexually active (defined as sexual activity with a partner within the past 12 months). In the 65- to 74-year-old group, 39.5% of women and 67% of men were sexually active; and in the 75- to 85-year-old group, 16.7% of women and 38.5% of men were sexually active (TABLE).
These findings indicate that fewer women than men remain sexually active during their later years. One reason may be the epidemic of sexual-related symptoms among postmenopausal women. In the same survey, 34.3% of women 57 to 64 years old reported avoiding sex because of:
- pain during intercourse (17.8%)
- difficulty with lubrication (35.9%).
Across all groups, the most prevalent sexual problem was low desire (43%).6 Around 40% of postmenopausal women reported no sexual activity in the past 12 months, as well as lack of interest in sex. This number may include women who have ceased to have sex because of vaginal dryness and dyspareunia, thereby reducing the percentage reporting these symptoms (TABLE).
TABLE
Older adults are having sex—and experiencing sexual problems
| Activity or problem by gender | Number of respondents | Report, by age group (95% confidence interval*) | ||
|---|---|---|---|---|
| 57–64 yr (%) | 65–74 yr (%) | 75–85 yr (%) | ||
| Sexually active in previous 12 months† | ||||
| Men | 1,385 | 83.7 (77.6–89.8) | 67.0 (62.1–72.0) | 38.5 (33.6–43.5) |
| Women | 1,501 | 61.6 (56.7–66.4) | 39.5 (34.6–44.4) | 16.7 (12.5–21.0) |
| Difficulty with lubrication | ||||
| Women | 495 | 35.9 (29.6–42.2) | 43.2 (34.8–51.5) | 43.6 (27.0–60.2) |
| Pain during intercourse | ||||
| Men | 878 | 3.0 (1.1–4.8) | 3.2 (1.2–5.3) | 1.0 (0–2.5) |
| Women | 506 | 17.8 (13.3–22.2) | 18.6 (10.8–26.3) | 11.8 (4.3–19.4) |
| Avoidance of sex due to sexual problems** | ||||
| Men | 533 | 22.1 (17.3–26.9) | 30.1 (23.2–37.0) | 25.7 (14.9–36.4) |
| Women | 357 | 34.3 (25.0–43.7) | 30.5 (21.5–39.4) | 22.7 (9.4–35.9) |
| Source: Adapted from Lindau ST, et al.6 | ||||
| Adjusted odds ratios are based on a logistic regression including the age group and self-rated health status as covariates, estimated separately for men and women. The confidence interval is based on the inversion of the Wald tests constructed with the use of design-based standard errors. | ||||
| † These data exclude 107 respondents who reported at least one sexual problem. | ||||
| ** This question was asked only of respondents who reported at least one sexual problem. | ||||
Assessing menopause-related sexual function is a challenge
Although the transition phases of menopause have been well studied and reported for decades, few of these studies have included questions about the impact of menopause on sexual function.7 When longitudinal studies that included the classification of female sexual dysfunction began to appear, they provided evidence of the important role that VVA and psychosocial factors play in female sexual dysfunction.8
In the fourth year of the Melbourne Women’s Midlife Health Project longitudinal study, six variables related to sexual function were identified. Three were determinate of sexual function:
- feelings for the partner
- problems related to the partner
- vaginal dryness/dyspareunia.
The other three variables—sexual responsiveness, frequency of sexual activity, and libido—were dependent or outcome variables.
By the sixth year of this study, two variables had increased in significance: vaginal dryness/dyspareunia and partner problems.7
Sexual pain and relationship problems can create a vicious cycle
The interrelationship of vaginal dryness, sexual pain, flagging desire, and psychosocial parameters can produce a vicious cycle. A woman experiencing or anticipating pain may have diminished sexual desire or avoid sex altogether. During intercourse, the brain’s awareness of vaginal pain may trigger a physiologic response that can cause the muscles of the vagina to tighten and lubrication to decrease. The result? Greater vaginal pain.
This vicious cycle can contribute to relationship issues with the sexual partner and harm a woman’s psychosocial well-being. Resentment, anger, and misunderstanding may arise when a couple is dealing with problems of sexual function, and these stressors can damage many aspects of the relationship, further exacerbating sexual difficulties.
An additional and very important dimension of these issues is their potential impact on the family unit.
VVA can diminish overall well-being
In a 2007 survey reported at the North American Menopause Society (NAMS), one third to one half of 506 respondents said that VVA had a bad effect on their sexual interest, mood, self-esteem, and the intimate relationship (FIGURE 1).9 Reports from in-depth interviews were consistent with survey results and offered further insight into a woman’s emotional response to the condition of vaginal dryness and its impact on her life. Women found the condition “embarrassing,” something they had to endure but didn’t talk about, and felt that it had a major impact on their self-esteem and intimate relationship.

FIGURE 1 Dyspareunia affects more than interest in sex—relationships, mood, and self-esteem suffer
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Clinicians often don’t ask about VVA, and patients are reluctant to talk
Among women of all ages, dyspareunia is underreported and undertreated. In the survey reported at NAMS, 40% of respondents said that their physician had never asked them about the problem of VVA (FIGURE 2).9
Women themselves may be reluctant to discuss the problem with physicians, nurse practitioners, or other health-care providers out of embarrassment or the assumption that there is nothing to be done about the problem. Nevertheless, more than 40% of respondents said they would be highly likely to seek treatment for VVA if they had a concern about urogenital complications of the condition (FIGURE 3).9
Another barrier may be the sense that asking the health-care provider about sex may embarrass him or her. As a result, sufferers do not anticipate help from their physician and other members of the health-care profession and fail to seek treatment or counseling for this chronic medical condition.10,11
In a 1999 telephone survey of 500 adults 25 years of age or older, 71% said they thought that their doctor would dismiss concerns about sexual problems, but 85% said they would talk to their physician anyway if they had a problem, even though they might not get treatment.11 In that survey, 91% of married men and 84% of married women rated a satisfying sex life as important to quality of life.11
Another important and often overlooked limitation on this type of discussion is the time constraints that busy clinicians face, especially with the low reimbursement offered by managed care. Sexual problems can hardly be adequately discussed in 7 to 10 minutes.

FIGURE 2 Do physicians ask about dyspareunia? Most women surveyed said “rarely” or “never”
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
FIGURE 3 Are these women likely to seek treatment?
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Women have performance anxiety, too
It is well known that men with even a mild degree of erectile dysfunction can suffer from performance anxiety, but the fact that women can also suffer from this phenomenon is not given as much attention. Such anxiety can be a factor in relationship difficulties. With both partners perhaps feeling anxious about sexual performance, a couple may avoid even simple acts of affection, such as holding hands, to avoid raising the other’s expectations.
Exacerbating the situation is the fact that many men use widely prescribed phosphodiesterase type 5 (PDE5) inhibitors, whereas women are contending with barriers to continued sexual activity as they age. It does not take a psychologist to understand that this imbalance often adds to emotional strain and tension between partners.
Popular media address the issue
Look beyond what our postmenopausal patients tell us directly—to the popular media and online forums—to appreciate the scope of sexual pain as a major issue among postmenopausal women. Evidence of psychosocial effects is found on numerous Web sites—some from organizations, others designed by women seeking help from each other.
Red Hot Mamas
This organization aims to empower women through menopause education. Highlighted in the Winter 2007/2008 Red Hot Mamas Report is a survey done in conjunction with Harris Interactive exploring the impact of menopausal symptoms on a woman’s sex life, which found that 47% of women who have VVA have avoided or stopped sex completely because it was uncomfortable, compared with 23% of normal women.
Power Surge
This Web site offers a list of strategies for dealing with sexual pain, including an overview of hormone-based prescription and nonprescription products, along with a variety of over-the-counter, natural, holistic, and herbal therapies for treating dyspareunia.
What is the physician’s role?
Given the epidemic of sexual pain, it is crucial that physicians and others who care for postmenopausal women increase their awareness of this issue and pay special attention to its psychosocial parameters.
Ask patients about sexual function in general and dyspareunia in particular as part of the routine annual visit. A simple opening “Yes/No” question, such as “Are you sexually active?” can lead to further questions appropriate to the patient. For example, if the answer is “No,” the follow-up question might be, “Does that bother you or your partner?” Further discussion may uncover whether the lack of sexual activity is a cause of distress and identify which variables are involved.
If, instead, the answer is “Yes,” follow-up questions can identify the presence of common postmenopausal physical issues, such as vaginal dryness and difficulty with lubrication. The visit then can turn to strategies to ameliorate those conditions.
When a patient reports dyspareunia, further diagnostic information such as precise location, degree of arousal, and reaction to pain can help determine the appropriate course of treatment. For an approach to this aspect of ascertaining patient history, see the list of sample questions above.12
During the physical, pay particular attention to any physical abnormalities or organic causes of sexual pain. Questions designed to characterize the location and nature of the pain can pinpoint the cause. Sexual pain arising from VVA is likely to 1) be localized at the introitus and 2) occur with penile entry.
Since the mid-1990s, the availability of validated scales to measure female sexual function has increased rapidly and enabled researchers to better identify, quantify, and evaluate treatments for female sexual dysfunction.7 Over time, we have moved away from the somewhat mechanical sequence inherent in the linear progression of desire leading to genital stimulation followed by arousal and orgasm toward an appreciation of the multiple physical, emotional, and subjective factors that are at play in women’s sexual function.
By 1998, a classification scheme was developed to further the means to study and discuss disorders of desire, arousal, orgasm, and sexual pain.8 Further contextual definitions of sexual dysfunction are under consideration.13
Basson proposed one new model of female sexual function (see the diagram), and observed that
"…women identify many reasons they are sexual over and beyond inherent sexual drive or “hunger.” Women tell of wanting to increase emotional closeness, commitment, sharing, tenderness, and tolerance, and to show the partner that he or she has been missed (emotionally or physically). Such intimacy-based reasons motivate the woman to find a way to become sexually aroused. This arousal is not spontaneous but triggered by deliberately sought sexual stimuli."13

Intimacy-based model of female sexual response cycle
In this flow of physical and emotional variables involved in female sexual function, categories interact. For example, low desire can be and is frequently secondary to the anticipation of pain during sexual intercourse. Arousal can be hampered by lack of vaginal lubrication—perhaps inhibited by the anticipation of pain. Secondary orgasmic disorders can result from low desire, difficulty of arousal, and sexual pain.14 Sexual pain can affect sexual function at any point on this continuum.
Treatments in the pipeline
For decades, hormone-based treatments have been the predominant therapeutic option for vaginal dryness. Often they are a secondary benefit of hormone therapy for vasomotor symptoms and osteoporosis. Estrogen can be delivered in the form of oral tablet, transdermal patch, gel, spray, or vaginal ring for systemic use, or as vaginal cream, ring, or tablet for local use.
However, despite data to the contrary and our reassurances to the patient about overall safety, a large number of women, and many primary care providers, are no longer inclined to use short- or long-term HRT in any presentation.
Other women may have risk factors that contraindicate exogenous hormones.
Nonhormonal options for vaginal dryness and dyspareunia are limited, and there are no approved systemic or oral nonestrogen options. Over-the-counter topical lubricants can ease some of the symptoms of VVA temporarily and allow successful vaginal penetration in many cases. Some may cause vaginal warming and pleasant sensations, but overall they treat the symptom rather than the source of pain. Moreover, many patients consider local lubricants messy and inconvenient and claim they “ruin the mood.”
The use of vaginal dilators along with estrogen or lubricant therapy is an often-forgotten adjunct to therapy for dyspareunia caused by VVA (FIGURE 4).

FIGURE 4 Mechanical dilation of the vagina is a useful adjunct
Mechanical dilation is often needed to restore penetration capability in the vagina, even after hormonal treatment. The focus should be on the vaginal introitus, with the top 25% to 35% of the dilator inserted into the opening once a day for 15 minutes, increasing the dilator diameter over time.
New SERMs are in development
Preclinical and clinical research into the diverse class of selective estrogen receptor modulators (SERMs) to treat estrogen-mediated disease produced tamoxifen for breast cancer prevention and raloxifene for both vertebral osteoporosis and breast cancer prevention. Each SERM seems to have unique tissue selectivity. The antiestrogenic activity of tamoxifen and raloxifene extends to the vagina and can exacerbate vaginal dryness.
A new generation of orally active SERMs is under investigation specifically for the treatment of chronic vaginal symptoms. These new agents target the nonvaginal treatment of VVA and associated symptoms. The first oral SERM for long-term treatment of these symptoms, ospemifene (Ophena), may become available in the near future. It is a novel SERM that has both anti-estrogenic and estrogenic actions, depending on the tissue. It was shown to significantly improve both vaginal dryness and dyspareunia in a large placebo-controlled trial.15
The author reports that he serves on the speaker’s bureau for Novogyne, TherRx, Warner-Chilcott, and Solvay, and on the advisory board for Upsher-Smith, Novogyne, QuatRx, and Wyeth.
CASE: History of dyspareunia
At her latest visit, a 56-year-old woman who is 7 years postmenopausal relates that she has been experiencing worsening pain with intercourse to the point that she now has very little sex drive at all. This problem began approximately 1 year after she discontinued hormone therapy in the wake of reports that it causes cancer and heart attack. She has been offered both local vaginal and systemic hormone therapy, but is too frightened to use any hormones at all. Sexual lubricants no longer seem to work.
How do you counsel her about these symptoms? And what therapy do you offer?
Physicians and other health-care practitioners are seeing a large and growing number of genitourinary and sexual-related complaints among menopausal women—so much so that it has reached epidemic proportions. Yet dyspareunia is underreported and undertreated, and quality of life suffers for these women.
In this article, I focus on two interrelated causes of this epidemic:
- vaginal dryness and vulvovaginal atrophy (VVA) and the impact of these conditions on women’s sexual function and psychosocial well-being
- barriers to optimal treatment.
I also explore how ObGyns’ role in this area of care is evolving—as a way to understand how you can better serve this expanding segment of our patient population.
Dyspareunia can have many causes, including endometriosis, interstitial cystitis, surgical scarring, injury that occurs during childbirth, and psychosocial origin (such as a history of sexual abuse). Our focus here is on dyspareunia due to VVA.
during sex. What should you do?
- Sexual pain as a category of female sexual dysfunction is relevant at any age; for postmenopausal women dealing with vaginal dryness as a result of estrogen deficiency, it may well be the dominant issue. When determining the cause of a sexual problem in a postmenopausal woman, put dyspareunia caused by vaginal dryness (as well as its psychosocial consequences) at the top of the list of possibilities.
- Bring up the topic of vaginal dryness and sexual pain with postmenopausal patients as part of the routine yearly exam, and explain the therapeutic capabilities of all available options.
- Estrogen therapy, either local or systemic, remains the standard when lubricants are inadequate. Make every effort to counsel the patient about the real risk:benefit ratio of estrogen use.
- If the patient is reluctant to use estrogen therapy, discuss with her the option of short-term local estrogen use, with the understanding that more acceptable options may become available in the near future. This may facilitate acceptance of short-term hormonal treatment and allow the patient to maintain her vaginal health and much of her vaginal sexual function.
- Keep abreast of both present and future options for therapy.
Just how sizable is the postmenopausal population?
About 32% of the female population is older than 50 years.1 That means that around 48 million women are currently menopausal, or will become so over the next few years.
Because average life expectancy approaches 80 years in the United States and other countries of the industrialized world,2 many women will live approximately 40 years beyond menopause or their final menstrual period. Their quality of life during the second half of their life is dependent on both physical and psychosocial health.
Postmenopausal dyspareunia isn’t new
Sexual issues arising from physical causes—dyspareunia among them—have long accounted for a large share of medical concerns reported by postmenopausal women. In a 1985 survey, for example, dyspareunia accounted for 42.5% of their complaints.3
But epidemiologic studies to determine the prevalence of female sexual dysfunction in postmenopausal women are difficult to carry out. Why? Because researchers would need to 1) address changes over time and 2) distinguish problems of sexual function from those brought on by aging.4
The techniques and methodology for researching female sexual dysfunction continue to evolve, creating new definitions of the stages of menopause and new diagnostic approaches to female sexual dysfunction.
However, based on available studies, Dennerstein and Hayes concluded that:
- postmenopausal women report a high rate of sexual dysfunction (higher than men)
- psychosocial factors can ameliorate a decline in sexual function
- “vaginal dryness and dyspareunia seem to be driven primarily by declining estradiol.”4
The WHI and its domino effect
Millions of postmenopausal women stopped taking estrogen-based therapy in the wake of widespread media coverage after 2002 publication of data from the estrogen–progestin arm of the Women’s Health Initiative (WHI), which purported to show, among other things, an increased risk of breast cancer.5
For decades, many postmenopausal women achieved medical management of VVA through long-term use of systemic hormone replacement therapy (HRT), which they used primarily to control other chronic symptoms of menopause, such as hot flashes.
After the WHI data were published (and misrepresented), reduced usage of estrogen-based HRT “unmasked” vaginal symptoms, including sexual pain, due to the effects of estrogen deficiency on the vaginal epithelium and vaginal blood flow. Since then, we have been forced to examine anew the natural history of menopause.
Within days or weeks of discontinuing HRT, women may reexperience the acute vasomotor symptoms that accompany estrogen withdrawal—most commonly hot flashes, night sweats, sleeplessness, palpitations, and headaches. Over time—anywhere from 6 months to several years—the body adjusts to the loss or withdrawal of estrogen, and these vasomotor symptoms eventually diminish or resolve. Not so for the longer-term physical effects of chronic low serum levels of estrogen, which worsen over time.
Approximately 6 months after discontinuing estrogen therapy, postmenopausal women may begin to experience vaginal dryness and VVA. As the years pass, other side effects of estrogen deficiency arise: bone loss, joint pain, mood alteration (including depression), change in skin tone, hair loss, and cardiac and central nervous system changes. These side effects do not resolve spontaneously; in fact, they grow worse as a woman ages. They may have deleterious psychosocial as well as physical impacts on her life—especially on the quality of her intimate relationship.
Clarify the report (adjust appropriately for same-sex partner)
- Where does it hurt? Describe the pain.
- When does it hurt? Does the pain occur 1) with penile contact at the opening of the vagina, 2) once the penis is partially in, 3) with full entry, 4) after some thrusting, 5) after deep thrusting, 6) with the partner’s ejaculation, 7) after withdrawal, or 8) with subsequent micturition?
- Does your body tense when your partner is attempting, or you are attempting, to insert his penis? What are your thoughts and feelings at this time?
- How long does the pain last?
- Does touching cause pain? Does it hurt when you ride a bicycle or wear tight clothes? Does penetration by tampons or fingers hurt?
Assess the pelvic floor
- Do you recognize the feeling of pelvic floor muscle tension during sexual contact?
- Do you recognize the feeling of pelvic floor muscle tension in other (nonsexual) situations?
Evaluate arousal
- Do you feel subjectively excited when you attempt intercourse?
- Does your vagina become sufficiently moist? Do you recognize the feeling of drying up?
Determine the consequences of the complaint
- What do you do when you experience pain during sexual contact? Do you continue? Or do you stop whatever is causing the pain?
- Do you continue to include intercourse or attempts at intercourse in your lovemaking, or do you use other methods of achieving sexual fulfillment? If you use other ways to make love, do you and your partner clearly understand that intercourse will not be attempted?
- What other effect does the pain have on your sexual relationship?
Explore biomedical antecedents
- When and how did the pain start?
- What tests have you undergone?
- What treatment have you received?
Source: Adapted from Basson R, et al.12
Is 60 the new 40?
Many women and men in the large cohort known as the Baby Boomer generation continue to be sexually active into their 60s, 70s, and 80s, as demonstrated by a 2007 study of sexuality and health in older adults.6 In the 57- to 64-year-old age group, 61.6% of women and 83.7% of men were sexually active (defined as sexual activity with a partner within the past 12 months). In the 65- to 74-year-old group, 39.5% of women and 67% of men were sexually active; and in the 75- to 85-year-old group, 16.7% of women and 38.5% of men were sexually active (TABLE).
These findings indicate that fewer women than men remain sexually active during their later years. One reason may be the epidemic of sexual-related symptoms among postmenopausal women. In the same survey, 34.3% of women 57 to 64 years old reported avoiding sex because of:
- pain during intercourse (17.8%)
- difficulty with lubrication (35.9%).
Across all groups, the most prevalent sexual problem was low desire (43%).6 Around 40% of postmenopausal women reported no sexual activity in the past 12 months, as well as lack of interest in sex. This number may include women who have ceased to have sex because of vaginal dryness and dyspareunia, thereby reducing the percentage reporting these symptoms (TABLE).
TABLE
Older adults are having sex—and experiencing sexual problems
| Activity or problem by gender | Number of respondents | Report, by age group (95% confidence interval*) | ||
|---|---|---|---|---|
| 57–64 yr (%) | 65–74 yr (%) | 75–85 yr (%) | ||
| Sexually active in previous 12 months† | ||||
| Men | 1,385 | 83.7 (77.6–89.8) | 67.0 (62.1–72.0) | 38.5 (33.6–43.5) |
| Women | 1,501 | 61.6 (56.7–66.4) | 39.5 (34.6–44.4) | 16.7 (12.5–21.0) |
| Difficulty with lubrication | ||||
| Women | 495 | 35.9 (29.6–42.2) | 43.2 (34.8–51.5) | 43.6 (27.0–60.2) |
| Pain during intercourse | ||||
| Men | 878 | 3.0 (1.1–4.8) | 3.2 (1.2–5.3) | 1.0 (0–2.5) |
| Women | 506 | 17.8 (13.3–22.2) | 18.6 (10.8–26.3) | 11.8 (4.3–19.4) |
| Avoidance of sex due to sexual problems** | ||||
| Men | 533 | 22.1 (17.3–26.9) | 30.1 (23.2–37.0) | 25.7 (14.9–36.4) |
| Women | 357 | 34.3 (25.0–43.7) | 30.5 (21.5–39.4) | 22.7 (9.4–35.9) |
| Source: Adapted from Lindau ST, et al.6 | ||||
| Adjusted odds ratios are based on a logistic regression including the age group and self-rated health status as covariates, estimated separately for men and women. The confidence interval is based on the inversion of the Wald tests constructed with the use of design-based standard errors. | ||||
| † These data exclude 107 respondents who reported at least one sexual problem. | ||||
| ** This question was asked only of respondents who reported at least one sexual problem. | ||||
Assessing menopause-related sexual function is a challenge
Although the transition phases of menopause have been well studied and reported for decades, few of these studies have included questions about the impact of menopause on sexual function.7 When longitudinal studies that included the classification of female sexual dysfunction began to appear, they provided evidence of the important role that VVA and psychosocial factors play in female sexual dysfunction.8
In the fourth year of the Melbourne Women’s Midlife Health Project longitudinal study, six variables related to sexual function were identified. Three were determinate of sexual function:
- feelings for the partner
- problems related to the partner
- vaginal dryness/dyspareunia.
The other three variables—sexual responsiveness, frequency of sexual activity, and libido—were dependent or outcome variables.
By the sixth year of this study, two variables had increased in significance: vaginal dryness/dyspareunia and partner problems.7
Sexual pain and relationship problems can create a vicious cycle
The interrelationship of vaginal dryness, sexual pain, flagging desire, and psychosocial parameters can produce a vicious cycle. A woman experiencing or anticipating pain may have diminished sexual desire or avoid sex altogether. During intercourse, the brain’s awareness of vaginal pain may trigger a physiologic response that can cause the muscles of the vagina to tighten and lubrication to decrease. The result? Greater vaginal pain.
This vicious cycle can contribute to relationship issues with the sexual partner and harm a woman’s psychosocial well-being. Resentment, anger, and misunderstanding may arise when a couple is dealing with problems of sexual function, and these stressors can damage many aspects of the relationship, further exacerbating sexual difficulties.
An additional and very important dimension of these issues is their potential impact on the family unit.
VVA can diminish overall well-being
In a 2007 survey reported at the North American Menopause Society (NAMS), one third to one half of 506 respondents said that VVA had a bad effect on their sexual interest, mood, self-esteem, and the intimate relationship (FIGURE 1).9 Reports from in-depth interviews were consistent with survey results and offered further insight into a woman’s emotional response to the condition of vaginal dryness and its impact on her life. Women found the condition “embarrassing,” something they had to endure but didn’t talk about, and felt that it had a major impact on their self-esteem and intimate relationship.

FIGURE 1 Dyspareunia affects more than interest in sex—relationships, mood, and self-esteem suffer
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Clinicians often don’t ask about VVA, and patients are reluctant to talk
Among women of all ages, dyspareunia is underreported and undertreated. In the survey reported at NAMS, 40% of respondents said that their physician had never asked them about the problem of VVA (FIGURE 2).9
Women themselves may be reluctant to discuss the problem with physicians, nurse practitioners, or other health-care providers out of embarrassment or the assumption that there is nothing to be done about the problem. Nevertheless, more than 40% of respondents said they would be highly likely to seek treatment for VVA if they had a concern about urogenital complications of the condition (FIGURE 3).9
Another barrier may be the sense that asking the health-care provider about sex may embarrass him or her. As a result, sufferers do not anticipate help from their physician and other members of the health-care profession and fail to seek treatment or counseling for this chronic medical condition.10,11
In a 1999 telephone survey of 500 adults 25 years of age or older, 71% said they thought that their doctor would dismiss concerns about sexual problems, but 85% said they would talk to their physician anyway if they had a problem, even though they might not get treatment.11 In that survey, 91% of married men and 84% of married women rated a satisfying sex life as important to quality of life.11
Another important and often overlooked limitation on this type of discussion is the time constraints that busy clinicians face, especially with the low reimbursement offered by managed care. Sexual problems can hardly be adequately discussed in 7 to 10 minutes.

FIGURE 2 Do physicians ask about dyspareunia? Most women surveyed said “rarely” or “never”
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
FIGURE 3 Are these women likely to seek treatment?
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Women have performance anxiety, too
It is well known that men with even a mild degree of erectile dysfunction can suffer from performance anxiety, but the fact that women can also suffer from this phenomenon is not given as much attention. Such anxiety can be a factor in relationship difficulties. With both partners perhaps feeling anxious about sexual performance, a couple may avoid even simple acts of affection, such as holding hands, to avoid raising the other’s expectations.
Exacerbating the situation is the fact that many men use widely prescribed phosphodiesterase type 5 (PDE5) inhibitors, whereas women are contending with barriers to continued sexual activity as they age. It does not take a psychologist to understand that this imbalance often adds to emotional strain and tension between partners.
Popular media address the issue
Look beyond what our postmenopausal patients tell us directly—to the popular media and online forums—to appreciate the scope of sexual pain as a major issue among postmenopausal women. Evidence of psychosocial effects is found on numerous Web sites—some from organizations, others designed by women seeking help from each other.
Red Hot Mamas
This organization aims to empower women through menopause education. Highlighted in the Winter 2007/2008 Red Hot Mamas Report is a survey done in conjunction with Harris Interactive exploring the impact of menopausal symptoms on a woman’s sex life, which found that 47% of women who have VVA have avoided or stopped sex completely because it was uncomfortable, compared with 23% of normal women.
Power Surge
This Web site offers a list of strategies for dealing with sexual pain, including an overview of hormone-based prescription and nonprescription products, along with a variety of over-the-counter, natural, holistic, and herbal therapies for treating dyspareunia.
What is the physician’s role?
Given the epidemic of sexual pain, it is crucial that physicians and others who care for postmenopausal women increase their awareness of this issue and pay special attention to its psychosocial parameters.
Ask patients about sexual function in general and dyspareunia in particular as part of the routine annual visit. A simple opening “Yes/No” question, such as “Are you sexually active?” can lead to further questions appropriate to the patient. For example, if the answer is “No,” the follow-up question might be, “Does that bother you or your partner?” Further discussion may uncover whether the lack of sexual activity is a cause of distress and identify which variables are involved.
If, instead, the answer is “Yes,” follow-up questions can identify the presence of common postmenopausal physical issues, such as vaginal dryness and difficulty with lubrication. The visit then can turn to strategies to ameliorate those conditions.
When a patient reports dyspareunia, further diagnostic information such as precise location, degree of arousal, and reaction to pain can help determine the appropriate course of treatment. For an approach to this aspect of ascertaining patient history, see the list of sample questions above.12
During the physical, pay particular attention to any physical abnormalities or organic causes of sexual pain. Questions designed to characterize the location and nature of the pain can pinpoint the cause. Sexual pain arising from VVA is likely to 1) be localized at the introitus and 2) occur with penile entry.
Since the mid-1990s, the availability of validated scales to measure female sexual function has increased rapidly and enabled researchers to better identify, quantify, and evaluate treatments for female sexual dysfunction.7 Over time, we have moved away from the somewhat mechanical sequence inherent in the linear progression of desire leading to genital stimulation followed by arousal and orgasm toward an appreciation of the multiple physical, emotional, and subjective factors that are at play in women’s sexual function.
By 1998, a classification scheme was developed to further the means to study and discuss disorders of desire, arousal, orgasm, and sexual pain.8 Further contextual definitions of sexual dysfunction are under consideration.13
Basson proposed one new model of female sexual function (see the diagram), and observed that
"…women identify many reasons they are sexual over and beyond inherent sexual drive or “hunger.” Women tell of wanting to increase emotional closeness, commitment, sharing, tenderness, and tolerance, and to show the partner that he or she has been missed (emotionally or physically). Such intimacy-based reasons motivate the woman to find a way to become sexually aroused. This arousal is not spontaneous but triggered by deliberately sought sexual stimuli."13

Intimacy-based model of female sexual response cycle
In this flow of physical and emotional variables involved in female sexual function, categories interact. For example, low desire can be and is frequently secondary to the anticipation of pain during sexual intercourse. Arousal can be hampered by lack of vaginal lubrication—perhaps inhibited by the anticipation of pain. Secondary orgasmic disorders can result from low desire, difficulty of arousal, and sexual pain.14 Sexual pain can affect sexual function at any point on this continuum.
Treatments in the pipeline
For decades, hormone-based treatments have been the predominant therapeutic option for vaginal dryness. Often they are a secondary benefit of hormone therapy for vasomotor symptoms and osteoporosis. Estrogen can be delivered in the form of oral tablet, transdermal patch, gel, spray, or vaginal ring for systemic use, or as vaginal cream, ring, or tablet for local use.
However, despite data to the contrary and our reassurances to the patient about overall safety, a large number of women, and many primary care providers, are no longer inclined to use short- or long-term HRT in any presentation.
Other women may have risk factors that contraindicate exogenous hormones.
Nonhormonal options for vaginal dryness and dyspareunia are limited, and there are no approved systemic or oral nonestrogen options. Over-the-counter topical lubricants can ease some of the symptoms of VVA temporarily and allow successful vaginal penetration in many cases. Some may cause vaginal warming and pleasant sensations, but overall they treat the symptom rather than the source of pain. Moreover, many patients consider local lubricants messy and inconvenient and claim they “ruin the mood.”
The use of vaginal dilators along with estrogen or lubricant therapy is an often-forgotten adjunct to therapy for dyspareunia caused by VVA (FIGURE 4).

FIGURE 4 Mechanical dilation of the vagina is a useful adjunct
Mechanical dilation is often needed to restore penetration capability in the vagina, even after hormonal treatment. The focus should be on the vaginal introitus, with the top 25% to 35% of the dilator inserted into the opening once a day for 15 minutes, increasing the dilator diameter over time.
New SERMs are in development
Preclinical and clinical research into the diverse class of selective estrogen receptor modulators (SERMs) to treat estrogen-mediated disease produced tamoxifen for breast cancer prevention and raloxifene for both vertebral osteoporosis and breast cancer prevention. Each SERM seems to have unique tissue selectivity. The antiestrogenic activity of tamoxifen and raloxifene extends to the vagina and can exacerbate vaginal dryness.
A new generation of orally active SERMs is under investigation specifically for the treatment of chronic vaginal symptoms. These new agents target the nonvaginal treatment of VVA and associated symptoms. The first oral SERM for long-term treatment of these symptoms, ospemifene (Ophena), may become available in the near future. It is a novel SERM that has both anti-estrogenic and estrogenic actions, depending on the tissue. It was shown to significantly improve both vaginal dryness and dyspareunia in a large placebo-controlled trial.15
1. US Census Bureau. 2006 American community survey. S0101. Age and sex. Available at: http://fact-finder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenuId=&_lang=en&_ts.
2. National Center for Health Statistics. Health, United States, 2007, with Chartbook on Trends in the Health of Americans. Hyattsville, Md: NCHS; 2007. Available at: http://www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed February 2, 2009.
3. Sarrel PM, Whitehead MI. Sex and menopause: defining the issues. Maturitas. 1985;7:217-224.
4. Dennerstein L, Hayes RD. Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med. 2005;2(suppl 3):118-132.
5. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
6. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
7. Dennerstein L, Alexander JL, Kotz K. The menopause and sexual functioning: a review of the population-based studies. Annu Rev Sex Res. 2003;14:64-82.
8. Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888-993.
9. Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
10. Heim LJ. Evaluation and differential diagnosis of dyspareunia. Am Fam Physician. 2001;63:1535-1552.
11. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173-2174.
12. Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med. 2004;1:24-34.
13. Basson R. Female sexual response: the role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-353.
14. Walsh KE, Berman JR. Sexual dysfunction in the older woman: an overview of the current understanding and management. Drugs Aging. 2004;21:655-675.
15. Bachmann GA, Komi J, Hanley R. A new SERM, Ophena (ospemifene), effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Presented at the Endocrine Society annual meeting, San Francisco, Calif, June 2008.
1. US Census Bureau. 2006 American community survey. S0101. Age and sex. Available at: http://fact-finder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenuId=&_lang=en&_ts.
2. National Center for Health Statistics. Health, United States, 2007, with Chartbook on Trends in the Health of Americans. Hyattsville, Md: NCHS; 2007. Available at: http://www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed February 2, 2009.
3. Sarrel PM, Whitehead MI. Sex and menopause: defining the issues. Maturitas. 1985;7:217-224.
4. Dennerstein L, Hayes RD. Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med. 2005;2(suppl 3):118-132.
5. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
6. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
7. Dennerstein L, Alexander JL, Kotz K. The menopause and sexual functioning: a review of the population-based studies. Annu Rev Sex Res. 2003;14:64-82.
8. Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888-993.
9. Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
10. Heim LJ. Evaluation and differential diagnosis of dyspareunia. Am Fam Physician. 2001;63:1535-1552.
11. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173-2174.
12. Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med. 2004;1:24-34.
13. Basson R. Female sexual response: the role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-353.
14. Walsh KE, Berman JR. Sexual dysfunction in the older woman: an overview of the current understanding and management. Drugs Aging. 2004;21:655-675.
15. Bachmann GA, Komi J, Hanley R. A new SERM, Ophena (ospemifene), effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Presented at the Endocrine Society annual meeting, San Francisco, Calif, June 2008.
Expert tips for adnexal surgery through the laparoscope
The authors report no financial relationships relevant to this article.
CASE 1: Cystic mass in patient’s only remaining ovary
Mrs. R is a 29-year-old G1P1 who underwent a right oophorectomy, with a midline incision, for a dermoid cyst at the time of cesarean delivery. She now has a left ovarian cyst. Preoperative ultrasonography (US) reveals that it measures 3.5×4.2×3.7 cm and has both solid components and a multiloculated appearance, consistent with a dermoid cyst.
How common is this scenario?
Studies predict that one of every three women will undergo surgical management of an adnexal mass at some point in her life.1 This troubling statistic prompts several critical questions:
- How do we handle the workup for these women so that only appropriate patients undergo surgery?
- How often will a mass be malignant?
- How can we safely remove an adnexal mass to maximize patient safety, reduce overall recovery time, and prevent less favorable outcomes in women who are eventually found to have a malignancy?
A thorough workup and, sometimes, conservative management can prevent unnecessary surgery that may lead to early menopause or surgical complications. And maximizing the use of minimally invasive techniques in women who do require surgery can shorten hospital stay and recovery time. At the time of surgery, careful abdominal entry and meticulous surgical dissection and mass removal can limit the potential risks of laparoscopic excision in women who have an ultimate diagnosis of cancer.
In this article, we review the workup for women who have an adnexal mass, describe patient-selection criteria for laparoscopic surgery, including the risks and benefits of this approach ( TABLE 1 ), and present several techniques to safely manage a mass with potentially malignant histology via laparoscopy.
TABLE 1
There are benefits and risks to managing an adnexal mass laparoscopically
| Benefit | Risk |
|---|---|
| Shorter recovery Fewer adhesions Decreased overall cost Magnification Decreased pain and narcotic use Fewer wound complications | Expensive equipment* Loss of tactile sensation Concern for malignancy Risk of tumor dissemination/spillage/chemical peritonitis Trocar-site metastasis |
| *Though greater expense is not a risk per se, it does enter into decision making. | |
Begin with the physical
When a woman is known to have a pelvic mass, the aim of the office exam is to 1) identify characteristics that suggest malignancy and 2) rule out nongynecologic causes of the mass. Physical findings that are worrisome for a malignant process include:
- fixed or nodular pelvic mass
- bilateral masses
- nodular abdominal mass
- ascites
- pleural effusion on auscultation or percussion of the lung.
Although these findings can be present under benign conditions, they increase the risk that a malignancy will be detected at surgery.
Other causes of a pelvic mass should also be considered, including infection (pelvic abscess) and tumors of the colon, particularly when the pelvic mass occurs on the left side.
Some symptoms, though vague, are worth noting
Although ovarian cancer was once thought to be a silent disease, recent research has shown that bloating, pelvic or abdominal pain, early satiety, and urinary frequency and urgency are more common among women with ovarian cancer than among healthy controls and patients in high-risk screening clinics.2-4 Although these symptoms are generally nonspecific, they merit attention if they occur more than 12 times a month and have been present for less than 1 year. When they meet these criteria, the symptoms have a sensitivity for diagnosing early- and late-stage ovarian cancer of 56.7% and 79.5%, respectively.4
Sensitivity for the diagnosis of early-stage ovarian cancer may be as high as 80% when the symptom index score is combined with an elevated level of the tumor marker CA 125.3
Transvaginal US is crucial
Transvaginal US is now standard practice to obtain high-resolution images of an adnexal mass. Grayscale US has traditionally been used alone for evaluation.
Specificity is typically lower in women who are premenopausal because many benign lesions, such as endometrioma, have a similar sonographic appearance to cancer.
A number of US scoring algorithms have now been proposed to aid in the triage of women who have an adnexal mass. Sensitivity of these algorithms ranges from 65% to 100%; specificity, from 77% to 95%.5
CA 125 is the standard tumor marker
For the past two decades, CA 125 has been the standard serum marker in the screening of high-risk women for ovarian cancer and the triage of women who have an adnexal mass.
This blood test has been studied widely since its introduction in 1983. It typically has sensitivity of 75% to 85% and specificity of 85% to 95% in identifying women who have ovarian cancer. However, it is elevated in only 50% to 60% of women who have stage I ovarian cancer. Its lack of specificity and poor positive predictive value have kept researchers busy trying to identify other serum markers, for both ovarian cancer and identification of high-risk pelvic masses.
Our recommended workup and management of adnexal masses In postmenopausal women who had a pelvic mass, one study found that a CA 125 level above 65 IU/mL had sensitivity of 71% and specificity of 92.5% in the identification of ovarian cancer.6 Another group found that CA 125 levels above 65 IU/mL were more than 95% sensitive in the diagnosis of ovarian cancer in postmenopausal women.7
Several studies have combined CA 125 with other markers or with US to screen high-risk women or triage those who have an adnexal mass. These studies have shown modest improvements in sensitivity but usually lower specificity than with CA 125 testing alone.
Markers that may be used for suspected sex cord stromas and germ-cell tumors are:
- lactate dehydrogenase (LDH) for dysgerminomas
- alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) for yolk sac tumors
- testosterone for Sertoli-Leydig cell tumors
- inhibin A and B for granulosa cell tumors.
An algorithm for working up and managing adnexal masses appears above.
How to gain abdominal access
In the opening case, the patient clearly has a benign mass. The treatment? Safe entry into the peritoneal cavity to remove the cyst and as little normal tissue as possible. This is critical in this patient because she has only one ovary.
Peritoneal access for abdominal and pelvic laparoscopy has been studied widely. Options include:
- direct insertion using a Veress needle
- open laparoscopy
- direct trocar insertion.
The technique usually depends on the preference of the surgeon. The primary goal of abdominal entry is to minimize the risk of injury, particularly unrecognized injury.
Data on complication rates show no definite benefit for open versus closed techniques in the prevention of injury to underlying viscera. However, evidence does suggest that the open technique may lower the risk of major vascular injury.8
We employ direct trocar insertion using radially expanding or optical trocars.
The Veress needle option
When the Veress needle is used to gain intraperitoneal access, data indicate that initial intraperitoneal pressure below 10 mm Hg is a reliable marker for peritoneal entry, even in obese patients.9 Insufflation pressure as high as 25 to 30 mm Hg prior to placement of the initial trocar is safe from a cardiopulmonary standpoint and may allow easier entry with a nonbladed trocar.10
Tests to confirm intraperitoneal placement of the Veress needle, such as the hanging-drop test or saline flush, do not appear to offer any additional useful information.11
Open laparoscopy is suitable when adhesions are unlikely
Open laparoscopy is typically performed by making a minilaparotomy incision at the umbilicus and then dissecting and entering the peritoneal cavity. A blunt-tip trocar is inserted.
The disadvantage of this approach is that there may be extensive adhesions under the umbilicus, and it is difficult to dissect such adhesions sufficiently to introduce a cannula and laparoscope. Adhesions left behind often obscure the field of view after introduction of the trocar.
Our preference? Left upper-quadrant insertion
In Case 1, the previous midline incision mandates an alternative approach. When abdominal entry at the umbilicus is unsuccessful or potentially difficult because of an earlier midline incision, umbilical hernia repair, or history of multiple lower abdominal or pelvic surgeries, a left upper-quadrant insertion is useful. It is, in fact, our preferred technique, and involves a small incision at the midclavicular line 5 cm below the left costal margin, at a site called Palmer’s point.
The direction of insertion usually ranges from 45° to 90°, depending on the patient’s body weight. If the trocar is placed properly, the closest organs are the stomach and the left lobe of the liver (4 to 6 cm).12 Given the stomach’s close proximity, it should be decompressed with an orogastric tube prior to trocar insertion.
Several studies have demonstrated the safety and efficacy of this entry technique.12-14 It can be helpful in cases that involve difficult access. We usually use a 5-mm primary trocar site for a 5-mm laparoscope. Modern optics allow for a pristine view with these small scopes, eliminating the need to close fascia and perhaps causing less pain at the incision.
Accessory trocar sites facilitate complex technique
We usually use three accessory sites. Two of them are lower-quadrant ports that are placed 2 cm medial and 2 cm cephalad to the anterior superior iliac spine. This area generally lies well away from the inferior epigastric vessels and remains above the area of the ilioinguinal and iliohypogastric nerves, making it a safer point of insertion.15 One trocar is 5 mm in size and the other is 10 mm. The larger one is used to extract the specimen.
We place an additional 5-mm port lateral to the rectus muscle at the level of the umbilicus. This allows the principal surgeon to use two instruments (a toothed forceps and scissors) comfortably while the assistant holds the laparoscope and assists with a grasper.
Does the type of trocar matter?
No randomized studies have directly compared all types of trocars. Options include:
- a pyramidal tip (as in reusable trocars) or shielded tip
- radial expansion
- visible entry
- blunt (Hasson-type) trocar.
Safety data on direct comparison of trocars are limited, but it appears that a radially expanding trocar may offer less port-site pain and potentially less bleeding than a traditional cutting trocar.16 Moreover, the rate of hernia at the port site appears to be relatively low with a radially expanding trocar, even when fascia is left unclosed at a 10-mm site.17
None of these trocars appears to be clearly superior at avoiding visceral or vascular injury.
Technique of laparoscopic cyst removal
A video clip of the surgery is linked to this article in the Video Library at www.obgmanagement.com. In this case, a trocar was inserted in the left upper quadrant, and a laparoscopic cystectomy was initiated using the trocars already specified.
The peritoneal cavity and adnexa were inspected, followed by pelvic washings, as detailed in TABLE 2 . Next, the ovarian cortex was incised ( FIGURE 1A ) with scissors using bipolar or unipolar energy, typically at a low power setting, such as 12 to 15 watts.
It was relatively easy to develop a tissue plane between the cortex and underlying dermoid cyst using simple or aqua-dissection ( FIGURE 1B and C ). The cyst was enucleated without rupture and inserted into a specimen bag through a 10-mm port ( FIGURE 1D ). (The specimen bags generally are available in 10-mm and 15-mm sizes.) Once the trocar was removed, the entire specimen was brought out through the incision ( FIGURE 1E ).
Cystic masses can generally be carefully aspirated using a laparoscopic needle or angiocath, or they can be incised and drained using a standard suction device. Manufacturers of most specimen bags do not recommend the morcellation of tissue within the bag because of the potential for rupture of the bag with tumor spillage or injury to underlying structures.
Occasionally, the mass remains too large to remove after drainage of the cyst fluid. Remedies include enlarging the port site with a scalpel or using a gallbladder speculum to increase the diameter of the port site ( FIGURE 1E ). If possible, the incision should be large enough to deliver the entire bag intact. Use of excessive force will rupture the bag and may cause the specimen to be lost or malignant cells to be inadvertently spilled (in the case of a cancer diagnosis).
TABLE 2
Take these 10 steps to safe laparoscopic surgery
| Examine the anesthetized patient |
| Enter the abdomen |
| Inspect the mass and peritoneal surfaces, including the diaphragm. Biopsy sites suspicious for metastasis and obtain frozen section |
| Perform pelvic and abdominal washings |
Closely inspect adnexa. If findings are not suggestive of malignancy, proceed with laparoscopy. If findings indicate obvious malignancy
|
Perform cystectomy or oophorectomy
|
| Inspect for hemostasis |
| Place cyst/ovary in endopouch |
| Open bag at abdominal wall and remove for frozen section |
| Reinspect and close |
Avoid:
|
FIGURE 1 Laparoscopic cyst removal
Begin by incising the cortex using scissors with or without an energy source.
Dissect the cyst free from the cortex using sharp dissection.
Remove the cyst from the ovary.
Place the cyst in a specimen bag, and …
… bring it to the surface for extraction. The abdominal incision may have to be enlarged to accommodate the specimen.
CASE 1: OUTCOME
The patient’s cyst is removed intact and she is discharged home. Rupture of a dermoid cyst is not associated with any problems as long as copious irrigation is used to aspirate the cyst content.18 Other cysts, such as endometriomas, may not be as easily dissected, and rupture is inevitable.
CASE 2: Symptoms suggestive of cancer
Mrs. B is a 47-year-old woman who reports abdominal bloating for the past 3 weeks. She also complains of early satiety and occasional constipation. She has no history of cancer, but her sister was given a diagnosis of breast cancer at 41 years of age, and her maternal aunt had breast cancer at 55 years.
Mrs. B is moderately obese, with a nontender abdomen and no palpable mass. Her pelvic exam also is negative for a mass or nodularity, but the extent of the exam is limited by body habitus. Her physician orders a transvaginal US, which reveals a 6-cm complex mass with thin septation and a 1-cm solid nodule, with no definite blood flow. The patient’s CA 125 level is 80 IU/mL, which we consider to be within the low-risk range for a premenopausal woman.
The patient is counseled about the need to have the mass removed and is scheduled for laparoscopic right salpingooophorectomy. Given the family history of breast cancer, the physician also requests consultation with a gynecologic oncologist, who agrees to assist with surgery and perform a laparotomy and staging in the event that a malignancy is diagnosed.
Is the mass likely to be malignant?
Given the patient’s family history of breast cancer, the recent onset of symptoms associated with ovarian cancer,2 and the characteristics of the mass (complex, with a nodule), malignancy is possible. This patient has an intermediate risk of cancer and requires additional counseling and planning.
However, most women who undergo laparoscopy for removal of an adnexal mass have benign pathologic findings.
What is the real risk of ovarian cancer?
The lifetime risk of developing ovarian cancer in the general population remains stable at approximately 1 in 70 women, with a mean age at diagnosis of 63 years.19 Ninety percent of ovarian cancer cases are sporadic, and less than 10% can be linked to genetic syndromes.
Women who have mutations in the BRCA1 gene carry a lifetime risk of ovarian cancer of up to 50%, and women who have mutations in BRCA2 have a lifetime risk of up to 25%.20,21 Women who have mutations associated with Lynch II syndrome or Hereditary Nonpolyposis Colorectal Cancer syndrome may have a lifetime risk of ovarian cancer of 12%.22,23
Some women who have a strong family history of breast and ovarian cancer do not carry a known mutation, but are likely to be at increased risk.
Additional risk factors known to be associated with ovarian cancer are nulliparity and infertility. However, the single most important risk factor for epithelial ovarian cancer is age.
Risk-reducing strategies include:
In the case of PBSO, it is imperative to ensure that all ovarian surface epithelium is removed. This means excising the infundibulopelvic ligament at least 1.5 cm above the proximal end of the ovary and excising any adjacent tissue to which the ovary is adherent (including pelvic sidewall peritoneum). Both requirements are easily achieved using the techniques outlined here.
Who should perform surgery?
The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) have published guidelines for referral to a gynecologic oncologist ( TABLE 3 ). When Im and colleagues reviewed the records of more than 1,000 women who underwent surgery for a pelvic mass at six institutions over 12 months, they found that 70% of premenopausal women and 94% of postmenopausal women who were diagnosed with ovarian cancer were properly referred to a qualified subspecialist.27 “Over”-referral occurred in 30% to 40% of women who had a benign mass, but may be preferable given the importance of proper staging and debulking to survival.
ACOG and the SGO recommend referral for women who have:
- elevated tumor markers
- ascites
- a fixed or nodular mass
- a strong family history of breast or ovarian cancer.
Consider preoperative referral of all high-risk and, probably, intermediate-risk women, depending on the availability of qualified specialists for complete surgical staging.
In addition, women need to be counseled thoroughly about the possibility that a malignancy will be diagnosed by frozen section, necessitating additional surgical procedures.
TABLE 3
Your patient has a newly diagnosed pelvic mass. Should you refer her?
| Is she premenopausal? Then refer her when… | Is she postmenopausal? Then refer her when… |
|---|---|
| CA125 >200 IU/mL Ascites is present Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative | CA125 >35 IU/mL Ascites is present Nodular or fixed pelvic mass Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative |
Technique of laparoscopic adnexectomy
In Case 2, an umbilical site was chosen for the primary cannula. In general, the direction of insertion depends on the patient’s body habitus. Heavier patients have a thicker abdominal wall and an umbilicus below the level of the aortic bifurcation. In these women, the angle of insertion should be adjusted from the usual 45° (for patients of normal weight) to an angle nearer to 90°. Lateral ports are typical, as in Case 1.
Treat every mass like cancer
Laparoscopic excision of an adnexal mass can be safe and effective, with better outcomes and recovery than with laparotomy, provided the surgeon adheres to basic principles ( TABLE 2 ). This means treating every mass as though it is potentially malignant, and thoroughly inspecting the abdominal cavity before and after excision of the mass.
Know the retroperitoneal space
As with Case 1, successful laparoscopic excision of an adnexal mass begins with inspection of the peritoneal cavity, abdominopelvic washings, and identification of both the infundibulopelvic ligament and ureter. Knowledge of the retroperitoneal space can be of great value in difficult cases that involve significant pelvic adhesions or sidewall fibrosis. We generally use a retroperitoneal approach for laparoscopic adnexectomy ( FIGURE 2A ).
In our typical approach, we incise the peritoneum lateral to the uteroovarian ligament and continue the incision up the pelvic sidewall lateral to the infundibulopelvic ligament and up along the paracolic gutter, if needed ( FIGURE 2A ). We then mobilize the medial leaf of the broad ligament from the sidewall using blunt dissection between the external iliac vessels laterally and the ureter medially ( FIGURE 2B ).
Once we have identified the ureter, we use scissors to create a window in the medial leaf of the broad ligament just beneath the gonadal vessels. We then use an energy source to occlude and transect the pedicle. Using this technique, we secure the infundibulopelvic ligaments and safely mobilize the ureter before initiating more aggressive sidewall dissection distally for adhesions or fibrosis.
Once the ureter is mobilized, we excise the involved peritoneum along with the mass. We then seal and transect the uteroovarian ligament.
FIGURE 2 The retroperitoneal approach
Grasp and incise the peritoneum just lateral to the adnexal mass and enter the retroperitoneal space, where loose areolar tissue is visible, with the ureter seen on the medial leaf of the broad ligament.
After dissecting this tissue, identify the ureter and internal iliac vessels.
Remove the mass in a bag
Once the mass is excised, we place it in a laparoscopic bag, as described for Case 1. Solid adnexal masses are problematic because they are not amenable to drainage, and morcellation is usually discouraged. Laparoscopic excision can still be carried out, and a minilaparotomy or posterior colpotomy can be used to extract the mass in a bag. Patients managed in this way still have a complication rate similar to or lower than that of patients undergoing laparotomy for oophorectomy; they also recover faster.
Risks in cases of malignancy
The risk of tumor spillage in laparoscopic surgery can be lowered using laparoscopically guided minilaparotomy techniques. Preoperative rupture appears to be more predictive of outcome.28
The concern about metastatic implants in a laparoscopic port site in patients who have gynecologic cancer is real, with an incidence ranging from 0.97% to 1.1%. The phenomenon usually affects women who had ovarian cancer and is most common after laparoscopy with findings of ascites, carcinomatosis, or persistent disease (in the case of second-look laparoscopy performed after completion of primary therapy for ovarian cancer).
These data suggest that port-site implantation is not a concern that should deter clinicians from laparoscopic evaluation of a suspected ovarian neoplasm unless a patient presents with ascites or carcinomatosis suggestive of advanced disease.29
Data are limited regarding delays in definitive therapy. One study suggests that definitive staging on the day of rupture does not influence overall outcome, but delay by more than 2 weeks may lead to poorer prognosis.30
CASE 2: OUTCOME
Laparoscopy is performed. On initial inspection, the peritoneal cavity is unremarkable. The right ovary is multicystic with normal surface anatomy. The mass is somewhat adherent to the pelvic sidewall. Adnexectomy is performed, and frozen section reveals the mass to be a serous cystadenoma.
1. Curtin JP. Management of the adnexal mass. Gynecol Oncol. 1994;55:S42-S46.
2. Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712.
3. Andersen MR, Goff BA, Lowe KA, et al. Combining a symptoms index with CA 125 to improve detection of ovarian cancer. Cancer. 2008;113:484-489.
4. Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221-227.
5. Alcázar JL, Mercé LT, Laparte C, et al. A new scoring system to differentiate benign from malignant adnexal masses. Am J Obstet Gynecol. 2003;188:685-692.
6. Maggino T, Gadducci A, D’Addario V, et al. Prospective multicenter study on CA 125 in postmenopausal pelvic masses. Gynecol Oncol. 1994;54:117-123.
7. Malkasian GD, Jr, Knapp RC, Lavin PT, et al. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988;159:341-346.
8. Larobina M, Nottle P. Complete evidence regarding major vascular injuries during laparoscopic access. Surg Laparosc Endosc Percutan Tech. 2005;15:119-123.
9. Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10:415-420.
10. Vilos GA, Vilos AG, Abu-Rafea B, Hollett-Caines J, Nikkhah-Abyaneh Z, Edris F. Three simple steps during closed laparoscopic entry may minimize major injuries. Surg Endosc. 2008 July 15. [Epub ahead of print]
11. Vilos GA, Ternamian A, Dempster J, Laberge PY. The Society of Obstetricians and Gynaecologists of Canada. Laparoscopic entry: a review of techniques, technologies, and complications. J Obstet Gynaecol Can. 2007;29:433-465.
12. Tulikangas PK, Nicklas A, Falcone T, Price LL. Anatomy of the left upper quadrant for cannula insertion. J Am Assoc Gynecol Laparosc. 2000;7:211-214.
13. Stepp KJ, Tulikangas PK, Goldberg JM, Attaran M, Falcone T. Laparoscopy for adnexal masses in the second trimester of pregnancy. J Am Assoc Gynecol Laparosc. 2003;10:55-59.
14. Tulikangas PK, Robinson DS, Falcone T. Left upper quadrant cannula insertion. Fertil Steril. 2003;79:411-412.
15. Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189:1574-1578.
16. Yim SF, Yuen PM. Randomized double-masked comparison of radially expanding access device and conventional cutting tip trocar in laparoscopy. Obstet Gynecol. 2001;97:435-438.
17. Johnson WH, Fecher AM, McMahon RL, et al. VersaStep trocar hernia rate in unclosed fascial defects in bariatric patients. Surg Endosc. 2006;20:1584-1586.
18. Lin P, Falcone T, Tulandi T. Excision of ovarian dermoid cyst by laparoscopy and by laparotomy. Am J Obstet Gynecol. 1995;173:769-771.
19. Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5-26.
20. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingooophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
21. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643-646.
22. Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214-218.
23. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
24. Dowdy SC, Stefanek M, Hartmann LC. Surgical risk reduction: prophylactic salpingooophorectomy and prophylactic mastectomy. Am J Obstet Gynecol. 2004;191:1113-1123.
25. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424-428.
26. Whittemore AS, Balise RR, Pharoah PD, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer. 2004;91:1911-1915.
27. Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol. 2005;105:35-41.
28. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
29. Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003;91:179-189.
30. Lehner R, Wenzl R, Heinzl H, Husslein P, Sevelda P. Influence of delayed staging laparotomy after laparoscopic removal of ovarian masses later found malignant. Obstet Gynecol. 1998;92:967-971.
The authors report no financial relationships relevant to this article.
CASE 1: Cystic mass in patient’s only remaining ovary
Mrs. R is a 29-year-old G1P1 who underwent a right oophorectomy, with a midline incision, for a dermoid cyst at the time of cesarean delivery. She now has a left ovarian cyst. Preoperative ultrasonography (US) reveals that it measures 3.5×4.2×3.7 cm and has both solid components and a multiloculated appearance, consistent with a dermoid cyst.
How common is this scenario?
Studies predict that one of every three women will undergo surgical management of an adnexal mass at some point in her life.1 This troubling statistic prompts several critical questions:
- How do we handle the workup for these women so that only appropriate patients undergo surgery?
- How often will a mass be malignant?
- How can we safely remove an adnexal mass to maximize patient safety, reduce overall recovery time, and prevent less favorable outcomes in women who are eventually found to have a malignancy?
A thorough workup and, sometimes, conservative management can prevent unnecessary surgery that may lead to early menopause or surgical complications. And maximizing the use of minimally invasive techniques in women who do require surgery can shorten hospital stay and recovery time. At the time of surgery, careful abdominal entry and meticulous surgical dissection and mass removal can limit the potential risks of laparoscopic excision in women who have an ultimate diagnosis of cancer.
In this article, we review the workup for women who have an adnexal mass, describe patient-selection criteria for laparoscopic surgery, including the risks and benefits of this approach ( TABLE 1 ), and present several techniques to safely manage a mass with potentially malignant histology via laparoscopy.
TABLE 1
There are benefits and risks to managing an adnexal mass laparoscopically
| Benefit | Risk |
|---|---|
| Shorter recovery Fewer adhesions Decreased overall cost Magnification Decreased pain and narcotic use Fewer wound complications | Expensive equipment* Loss of tactile sensation Concern for malignancy Risk of tumor dissemination/spillage/chemical peritonitis Trocar-site metastasis |
| *Though greater expense is not a risk per se, it does enter into decision making. | |
Begin with the physical
When a woman is known to have a pelvic mass, the aim of the office exam is to 1) identify characteristics that suggest malignancy and 2) rule out nongynecologic causes of the mass. Physical findings that are worrisome for a malignant process include:
- fixed or nodular pelvic mass
- bilateral masses
- nodular abdominal mass
- ascites
- pleural effusion on auscultation or percussion of the lung.
Although these findings can be present under benign conditions, they increase the risk that a malignancy will be detected at surgery.
Other causes of a pelvic mass should also be considered, including infection (pelvic abscess) and tumors of the colon, particularly when the pelvic mass occurs on the left side.
Some symptoms, though vague, are worth noting
Although ovarian cancer was once thought to be a silent disease, recent research has shown that bloating, pelvic or abdominal pain, early satiety, and urinary frequency and urgency are more common among women with ovarian cancer than among healthy controls and patients in high-risk screening clinics.2-4 Although these symptoms are generally nonspecific, they merit attention if they occur more than 12 times a month and have been present for less than 1 year. When they meet these criteria, the symptoms have a sensitivity for diagnosing early- and late-stage ovarian cancer of 56.7% and 79.5%, respectively.4
Sensitivity for the diagnosis of early-stage ovarian cancer may be as high as 80% when the symptom index score is combined with an elevated level of the tumor marker CA 125.3
Transvaginal US is crucial
Transvaginal US is now standard practice to obtain high-resolution images of an adnexal mass. Grayscale US has traditionally been used alone for evaluation.
Specificity is typically lower in women who are premenopausal because many benign lesions, such as endometrioma, have a similar sonographic appearance to cancer.
A number of US scoring algorithms have now been proposed to aid in the triage of women who have an adnexal mass. Sensitivity of these algorithms ranges from 65% to 100%; specificity, from 77% to 95%.5
CA 125 is the standard tumor marker
For the past two decades, CA 125 has been the standard serum marker in the screening of high-risk women for ovarian cancer and the triage of women who have an adnexal mass.
This blood test has been studied widely since its introduction in 1983. It typically has sensitivity of 75% to 85% and specificity of 85% to 95% in identifying women who have ovarian cancer. However, it is elevated in only 50% to 60% of women who have stage I ovarian cancer. Its lack of specificity and poor positive predictive value have kept researchers busy trying to identify other serum markers, for both ovarian cancer and identification of high-risk pelvic masses.
Our recommended workup and management of adnexal masses In postmenopausal women who had a pelvic mass, one study found that a CA 125 level above 65 IU/mL had sensitivity of 71% and specificity of 92.5% in the identification of ovarian cancer.6 Another group found that CA 125 levels above 65 IU/mL were more than 95% sensitive in the diagnosis of ovarian cancer in postmenopausal women.7
Several studies have combined CA 125 with other markers or with US to screen high-risk women or triage those who have an adnexal mass. These studies have shown modest improvements in sensitivity but usually lower specificity than with CA 125 testing alone.
Markers that may be used for suspected sex cord stromas and germ-cell tumors are:
- lactate dehydrogenase (LDH) for dysgerminomas
- alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) for yolk sac tumors
- testosterone for Sertoli-Leydig cell tumors
- inhibin A and B for granulosa cell tumors.
An algorithm for working up and managing adnexal masses appears above.
How to gain abdominal access
In the opening case, the patient clearly has a benign mass. The treatment? Safe entry into the peritoneal cavity to remove the cyst and as little normal tissue as possible. This is critical in this patient because she has only one ovary.
Peritoneal access for abdominal and pelvic laparoscopy has been studied widely. Options include:
- direct insertion using a Veress needle
- open laparoscopy
- direct trocar insertion.
The technique usually depends on the preference of the surgeon. The primary goal of abdominal entry is to minimize the risk of injury, particularly unrecognized injury.
Data on complication rates show no definite benefit for open versus closed techniques in the prevention of injury to underlying viscera. However, evidence does suggest that the open technique may lower the risk of major vascular injury.8
We employ direct trocar insertion using radially expanding or optical trocars.
The Veress needle option
When the Veress needle is used to gain intraperitoneal access, data indicate that initial intraperitoneal pressure below 10 mm Hg is a reliable marker for peritoneal entry, even in obese patients.9 Insufflation pressure as high as 25 to 30 mm Hg prior to placement of the initial trocar is safe from a cardiopulmonary standpoint and may allow easier entry with a nonbladed trocar.10
Tests to confirm intraperitoneal placement of the Veress needle, such as the hanging-drop test or saline flush, do not appear to offer any additional useful information.11
Open laparoscopy is suitable when adhesions are unlikely
Open laparoscopy is typically performed by making a minilaparotomy incision at the umbilicus and then dissecting and entering the peritoneal cavity. A blunt-tip trocar is inserted.
The disadvantage of this approach is that there may be extensive adhesions under the umbilicus, and it is difficult to dissect such adhesions sufficiently to introduce a cannula and laparoscope. Adhesions left behind often obscure the field of view after introduction of the trocar.
Our preference? Left upper-quadrant insertion
In Case 1, the previous midline incision mandates an alternative approach. When abdominal entry at the umbilicus is unsuccessful or potentially difficult because of an earlier midline incision, umbilical hernia repair, or history of multiple lower abdominal or pelvic surgeries, a left upper-quadrant insertion is useful. It is, in fact, our preferred technique, and involves a small incision at the midclavicular line 5 cm below the left costal margin, at a site called Palmer’s point.
The direction of insertion usually ranges from 45° to 90°, depending on the patient’s body weight. If the trocar is placed properly, the closest organs are the stomach and the left lobe of the liver (4 to 6 cm).12 Given the stomach’s close proximity, it should be decompressed with an orogastric tube prior to trocar insertion.
Several studies have demonstrated the safety and efficacy of this entry technique.12-14 It can be helpful in cases that involve difficult access. We usually use a 5-mm primary trocar site for a 5-mm laparoscope. Modern optics allow for a pristine view with these small scopes, eliminating the need to close fascia and perhaps causing less pain at the incision.
Accessory trocar sites facilitate complex technique
We usually use three accessory sites. Two of them are lower-quadrant ports that are placed 2 cm medial and 2 cm cephalad to the anterior superior iliac spine. This area generally lies well away from the inferior epigastric vessels and remains above the area of the ilioinguinal and iliohypogastric nerves, making it a safer point of insertion.15 One trocar is 5 mm in size and the other is 10 mm. The larger one is used to extract the specimen.
We place an additional 5-mm port lateral to the rectus muscle at the level of the umbilicus. This allows the principal surgeon to use two instruments (a toothed forceps and scissors) comfortably while the assistant holds the laparoscope and assists with a grasper.
Does the type of trocar matter?
No randomized studies have directly compared all types of trocars. Options include:
- a pyramidal tip (as in reusable trocars) or shielded tip
- radial expansion
- visible entry
- blunt (Hasson-type) trocar.
Safety data on direct comparison of trocars are limited, but it appears that a radially expanding trocar may offer less port-site pain and potentially less bleeding than a traditional cutting trocar.16 Moreover, the rate of hernia at the port site appears to be relatively low with a radially expanding trocar, even when fascia is left unclosed at a 10-mm site.17
None of these trocars appears to be clearly superior at avoiding visceral or vascular injury.
Technique of laparoscopic cyst removal
A video clip of the surgery is linked to this article in the Video Library at www.obgmanagement.com. In this case, a trocar was inserted in the left upper quadrant, and a laparoscopic cystectomy was initiated using the trocars already specified.
The peritoneal cavity and adnexa were inspected, followed by pelvic washings, as detailed in TABLE 2 . Next, the ovarian cortex was incised ( FIGURE 1A ) with scissors using bipolar or unipolar energy, typically at a low power setting, such as 12 to 15 watts.
It was relatively easy to develop a tissue plane between the cortex and underlying dermoid cyst using simple or aqua-dissection ( FIGURE 1B and C ). The cyst was enucleated without rupture and inserted into a specimen bag through a 10-mm port ( FIGURE 1D ). (The specimen bags generally are available in 10-mm and 15-mm sizes.) Once the trocar was removed, the entire specimen was brought out through the incision ( FIGURE 1E ).
Cystic masses can generally be carefully aspirated using a laparoscopic needle or angiocath, or they can be incised and drained using a standard suction device. Manufacturers of most specimen bags do not recommend the morcellation of tissue within the bag because of the potential for rupture of the bag with tumor spillage or injury to underlying structures.
Occasionally, the mass remains too large to remove after drainage of the cyst fluid. Remedies include enlarging the port site with a scalpel or using a gallbladder speculum to increase the diameter of the port site ( FIGURE 1E ). If possible, the incision should be large enough to deliver the entire bag intact. Use of excessive force will rupture the bag and may cause the specimen to be lost or malignant cells to be inadvertently spilled (in the case of a cancer diagnosis).
TABLE 2
Take these 10 steps to safe laparoscopic surgery
| Examine the anesthetized patient |
| Enter the abdomen |
| Inspect the mass and peritoneal surfaces, including the diaphragm. Biopsy sites suspicious for metastasis and obtain frozen section |
| Perform pelvic and abdominal washings |
Closely inspect adnexa. If findings are not suggestive of malignancy, proceed with laparoscopy. If findings indicate obvious malignancy
|
Perform cystectomy or oophorectomy
|
| Inspect for hemostasis |
| Place cyst/ovary in endopouch |
| Open bag at abdominal wall and remove for frozen section |
| Reinspect and close |
Avoid:
|
FIGURE 1 Laparoscopic cyst removal
Begin by incising the cortex using scissors with or without an energy source.
Dissect the cyst free from the cortex using sharp dissection.
Remove the cyst from the ovary.
Place the cyst in a specimen bag, and …
… bring it to the surface for extraction. The abdominal incision may have to be enlarged to accommodate the specimen.
CASE 1: OUTCOME
The patient’s cyst is removed intact and she is discharged home. Rupture of a dermoid cyst is not associated with any problems as long as copious irrigation is used to aspirate the cyst content.18 Other cysts, such as endometriomas, may not be as easily dissected, and rupture is inevitable.
CASE 2: Symptoms suggestive of cancer
Mrs. B is a 47-year-old woman who reports abdominal bloating for the past 3 weeks. She also complains of early satiety and occasional constipation. She has no history of cancer, but her sister was given a diagnosis of breast cancer at 41 years of age, and her maternal aunt had breast cancer at 55 years.
Mrs. B is moderately obese, with a nontender abdomen and no palpable mass. Her pelvic exam also is negative for a mass or nodularity, but the extent of the exam is limited by body habitus. Her physician orders a transvaginal US, which reveals a 6-cm complex mass with thin septation and a 1-cm solid nodule, with no definite blood flow. The patient’s CA 125 level is 80 IU/mL, which we consider to be within the low-risk range for a premenopausal woman.
The patient is counseled about the need to have the mass removed and is scheduled for laparoscopic right salpingooophorectomy. Given the family history of breast cancer, the physician also requests consultation with a gynecologic oncologist, who agrees to assist with surgery and perform a laparotomy and staging in the event that a malignancy is diagnosed.
Is the mass likely to be malignant?
Given the patient’s family history of breast cancer, the recent onset of symptoms associated with ovarian cancer,2 and the characteristics of the mass (complex, with a nodule), malignancy is possible. This patient has an intermediate risk of cancer and requires additional counseling and planning.
However, most women who undergo laparoscopy for removal of an adnexal mass have benign pathologic findings.
What is the real risk of ovarian cancer?
The lifetime risk of developing ovarian cancer in the general population remains stable at approximately 1 in 70 women, with a mean age at diagnosis of 63 years.19 Ninety percent of ovarian cancer cases are sporadic, and less than 10% can be linked to genetic syndromes.
Women who have mutations in the BRCA1 gene carry a lifetime risk of ovarian cancer of up to 50%, and women who have mutations in BRCA2 have a lifetime risk of up to 25%.20,21 Women who have mutations associated with Lynch II syndrome or Hereditary Nonpolyposis Colorectal Cancer syndrome may have a lifetime risk of ovarian cancer of 12%.22,23
Some women who have a strong family history of breast and ovarian cancer do not carry a known mutation, but are likely to be at increased risk.
Additional risk factors known to be associated with ovarian cancer are nulliparity and infertility. However, the single most important risk factor for epithelial ovarian cancer is age.
Risk-reducing strategies include:
In the case of PBSO, it is imperative to ensure that all ovarian surface epithelium is removed. This means excising the infundibulopelvic ligament at least 1.5 cm above the proximal end of the ovary and excising any adjacent tissue to which the ovary is adherent (including pelvic sidewall peritoneum). Both requirements are easily achieved using the techniques outlined here.
Who should perform surgery?
The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) have published guidelines for referral to a gynecologic oncologist ( TABLE 3 ). When Im and colleagues reviewed the records of more than 1,000 women who underwent surgery for a pelvic mass at six institutions over 12 months, they found that 70% of premenopausal women and 94% of postmenopausal women who were diagnosed with ovarian cancer were properly referred to a qualified subspecialist.27 “Over”-referral occurred in 30% to 40% of women who had a benign mass, but may be preferable given the importance of proper staging and debulking to survival.
ACOG and the SGO recommend referral for women who have:
- elevated tumor markers
- ascites
- a fixed or nodular mass
- a strong family history of breast or ovarian cancer.
Consider preoperative referral of all high-risk and, probably, intermediate-risk women, depending on the availability of qualified specialists for complete surgical staging.
In addition, women need to be counseled thoroughly about the possibility that a malignancy will be diagnosed by frozen section, necessitating additional surgical procedures.
TABLE 3
Your patient has a newly diagnosed pelvic mass. Should you refer her?
| Is she premenopausal? Then refer her when… | Is she postmenopausal? Then refer her when… |
|---|---|
| CA125 >200 IU/mL Ascites is present Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative | CA125 >35 IU/mL Ascites is present Nodular or fixed pelvic mass Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative |
Technique of laparoscopic adnexectomy
In Case 2, an umbilical site was chosen for the primary cannula. In general, the direction of insertion depends on the patient’s body habitus. Heavier patients have a thicker abdominal wall and an umbilicus below the level of the aortic bifurcation. In these women, the angle of insertion should be adjusted from the usual 45° (for patients of normal weight) to an angle nearer to 90°. Lateral ports are typical, as in Case 1.
Treat every mass like cancer
Laparoscopic excision of an adnexal mass can be safe and effective, with better outcomes and recovery than with laparotomy, provided the surgeon adheres to basic principles ( TABLE 2 ). This means treating every mass as though it is potentially malignant, and thoroughly inspecting the abdominal cavity before and after excision of the mass.
Know the retroperitoneal space
As with Case 1, successful laparoscopic excision of an adnexal mass begins with inspection of the peritoneal cavity, abdominopelvic washings, and identification of both the infundibulopelvic ligament and ureter. Knowledge of the retroperitoneal space can be of great value in difficult cases that involve significant pelvic adhesions or sidewall fibrosis. We generally use a retroperitoneal approach for laparoscopic adnexectomy ( FIGURE 2A ).
In our typical approach, we incise the peritoneum lateral to the uteroovarian ligament and continue the incision up the pelvic sidewall lateral to the infundibulopelvic ligament and up along the paracolic gutter, if needed ( FIGURE 2A ). We then mobilize the medial leaf of the broad ligament from the sidewall using blunt dissection between the external iliac vessels laterally and the ureter medially ( FIGURE 2B ).
Once we have identified the ureter, we use scissors to create a window in the medial leaf of the broad ligament just beneath the gonadal vessels. We then use an energy source to occlude and transect the pedicle. Using this technique, we secure the infundibulopelvic ligaments and safely mobilize the ureter before initiating more aggressive sidewall dissection distally for adhesions or fibrosis.
Once the ureter is mobilized, we excise the involved peritoneum along with the mass. We then seal and transect the uteroovarian ligament.
FIGURE 2 The retroperitoneal approach
Grasp and incise the peritoneum just lateral to the adnexal mass and enter the retroperitoneal space, where loose areolar tissue is visible, with the ureter seen on the medial leaf of the broad ligament.
After dissecting this tissue, identify the ureter and internal iliac vessels.
Remove the mass in a bag
Once the mass is excised, we place it in a laparoscopic bag, as described for Case 1. Solid adnexal masses are problematic because they are not amenable to drainage, and morcellation is usually discouraged. Laparoscopic excision can still be carried out, and a minilaparotomy or posterior colpotomy can be used to extract the mass in a bag. Patients managed in this way still have a complication rate similar to or lower than that of patients undergoing laparotomy for oophorectomy; they also recover faster.
Risks in cases of malignancy
The risk of tumor spillage in laparoscopic surgery can be lowered using laparoscopically guided minilaparotomy techniques. Preoperative rupture appears to be more predictive of outcome.28
The concern about metastatic implants in a laparoscopic port site in patients who have gynecologic cancer is real, with an incidence ranging from 0.97% to 1.1%. The phenomenon usually affects women who had ovarian cancer and is most common after laparoscopy with findings of ascites, carcinomatosis, or persistent disease (in the case of second-look laparoscopy performed after completion of primary therapy for ovarian cancer).
These data suggest that port-site implantation is not a concern that should deter clinicians from laparoscopic evaluation of a suspected ovarian neoplasm unless a patient presents with ascites or carcinomatosis suggestive of advanced disease.29
Data are limited regarding delays in definitive therapy. One study suggests that definitive staging on the day of rupture does not influence overall outcome, but delay by more than 2 weeks may lead to poorer prognosis.30
CASE 2: OUTCOME
Laparoscopy is performed. On initial inspection, the peritoneal cavity is unremarkable. The right ovary is multicystic with normal surface anatomy. The mass is somewhat adherent to the pelvic sidewall. Adnexectomy is performed, and frozen section reveals the mass to be a serous cystadenoma.
The authors report no financial relationships relevant to this article.
CASE 1: Cystic mass in patient’s only remaining ovary
Mrs. R is a 29-year-old G1P1 who underwent a right oophorectomy, with a midline incision, for a dermoid cyst at the time of cesarean delivery. She now has a left ovarian cyst. Preoperative ultrasonography (US) reveals that it measures 3.5×4.2×3.7 cm and has both solid components and a multiloculated appearance, consistent with a dermoid cyst.
How common is this scenario?
Studies predict that one of every three women will undergo surgical management of an adnexal mass at some point in her life.1 This troubling statistic prompts several critical questions:
- How do we handle the workup for these women so that only appropriate patients undergo surgery?
- How often will a mass be malignant?
- How can we safely remove an adnexal mass to maximize patient safety, reduce overall recovery time, and prevent less favorable outcomes in women who are eventually found to have a malignancy?
A thorough workup and, sometimes, conservative management can prevent unnecessary surgery that may lead to early menopause or surgical complications. And maximizing the use of minimally invasive techniques in women who do require surgery can shorten hospital stay and recovery time. At the time of surgery, careful abdominal entry and meticulous surgical dissection and mass removal can limit the potential risks of laparoscopic excision in women who have an ultimate diagnosis of cancer.
In this article, we review the workup for women who have an adnexal mass, describe patient-selection criteria for laparoscopic surgery, including the risks and benefits of this approach ( TABLE 1 ), and present several techniques to safely manage a mass with potentially malignant histology via laparoscopy.
TABLE 1
There are benefits and risks to managing an adnexal mass laparoscopically
| Benefit | Risk |
|---|---|
| Shorter recovery Fewer adhesions Decreased overall cost Magnification Decreased pain and narcotic use Fewer wound complications | Expensive equipment* Loss of tactile sensation Concern for malignancy Risk of tumor dissemination/spillage/chemical peritonitis Trocar-site metastasis |
| *Though greater expense is not a risk per se, it does enter into decision making. | |
Begin with the physical
When a woman is known to have a pelvic mass, the aim of the office exam is to 1) identify characteristics that suggest malignancy and 2) rule out nongynecologic causes of the mass. Physical findings that are worrisome for a malignant process include:
- fixed or nodular pelvic mass
- bilateral masses
- nodular abdominal mass
- ascites
- pleural effusion on auscultation or percussion of the lung.
Although these findings can be present under benign conditions, they increase the risk that a malignancy will be detected at surgery.
Other causes of a pelvic mass should also be considered, including infection (pelvic abscess) and tumors of the colon, particularly when the pelvic mass occurs on the left side.
Some symptoms, though vague, are worth noting
Although ovarian cancer was once thought to be a silent disease, recent research has shown that bloating, pelvic or abdominal pain, early satiety, and urinary frequency and urgency are more common among women with ovarian cancer than among healthy controls and patients in high-risk screening clinics.2-4 Although these symptoms are generally nonspecific, they merit attention if they occur more than 12 times a month and have been present for less than 1 year. When they meet these criteria, the symptoms have a sensitivity for diagnosing early- and late-stage ovarian cancer of 56.7% and 79.5%, respectively.4
Sensitivity for the diagnosis of early-stage ovarian cancer may be as high as 80% when the symptom index score is combined with an elevated level of the tumor marker CA 125.3
Transvaginal US is crucial
Transvaginal US is now standard practice to obtain high-resolution images of an adnexal mass. Grayscale US has traditionally been used alone for evaluation.
Specificity is typically lower in women who are premenopausal because many benign lesions, such as endometrioma, have a similar sonographic appearance to cancer.
A number of US scoring algorithms have now been proposed to aid in the triage of women who have an adnexal mass. Sensitivity of these algorithms ranges from 65% to 100%; specificity, from 77% to 95%.5
CA 125 is the standard tumor marker
For the past two decades, CA 125 has been the standard serum marker in the screening of high-risk women for ovarian cancer and the triage of women who have an adnexal mass.
This blood test has been studied widely since its introduction in 1983. It typically has sensitivity of 75% to 85% and specificity of 85% to 95% in identifying women who have ovarian cancer. However, it is elevated in only 50% to 60% of women who have stage I ovarian cancer. Its lack of specificity and poor positive predictive value have kept researchers busy trying to identify other serum markers, for both ovarian cancer and identification of high-risk pelvic masses.
Our recommended workup and management of adnexal masses In postmenopausal women who had a pelvic mass, one study found that a CA 125 level above 65 IU/mL had sensitivity of 71% and specificity of 92.5% in the identification of ovarian cancer.6 Another group found that CA 125 levels above 65 IU/mL were more than 95% sensitive in the diagnosis of ovarian cancer in postmenopausal women.7
Several studies have combined CA 125 with other markers or with US to screen high-risk women or triage those who have an adnexal mass. These studies have shown modest improvements in sensitivity but usually lower specificity than with CA 125 testing alone.
Markers that may be used for suspected sex cord stromas and germ-cell tumors are:
- lactate dehydrogenase (LDH) for dysgerminomas
- alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) for yolk sac tumors
- testosterone for Sertoli-Leydig cell tumors
- inhibin A and B for granulosa cell tumors.
An algorithm for working up and managing adnexal masses appears above.
How to gain abdominal access
In the opening case, the patient clearly has a benign mass. The treatment? Safe entry into the peritoneal cavity to remove the cyst and as little normal tissue as possible. This is critical in this patient because she has only one ovary.
Peritoneal access for abdominal and pelvic laparoscopy has been studied widely. Options include:
- direct insertion using a Veress needle
- open laparoscopy
- direct trocar insertion.
The technique usually depends on the preference of the surgeon. The primary goal of abdominal entry is to minimize the risk of injury, particularly unrecognized injury.
Data on complication rates show no definite benefit for open versus closed techniques in the prevention of injury to underlying viscera. However, evidence does suggest that the open technique may lower the risk of major vascular injury.8
We employ direct trocar insertion using radially expanding or optical trocars.
The Veress needle option
When the Veress needle is used to gain intraperitoneal access, data indicate that initial intraperitoneal pressure below 10 mm Hg is a reliable marker for peritoneal entry, even in obese patients.9 Insufflation pressure as high as 25 to 30 mm Hg prior to placement of the initial trocar is safe from a cardiopulmonary standpoint and may allow easier entry with a nonbladed trocar.10
Tests to confirm intraperitoneal placement of the Veress needle, such as the hanging-drop test or saline flush, do not appear to offer any additional useful information.11
Open laparoscopy is suitable when adhesions are unlikely
Open laparoscopy is typically performed by making a minilaparotomy incision at the umbilicus and then dissecting and entering the peritoneal cavity. A blunt-tip trocar is inserted.
The disadvantage of this approach is that there may be extensive adhesions under the umbilicus, and it is difficult to dissect such adhesions sufficiently to introduce a cannula and laparoscope. Adhesions left behind often obscure the field of view after introduction of the trocar.
Our preference? Left upper-quadrant insertion
In Case 1, the previous midline incision mandates an alternative approach. When abdominal entry at the umbilicus is unsuccessful or potentially difficult because of an earlier midline incision, umbilical hernia repair, or history of multiple lower abdominal or pelvic surgeries, a left upper-quadrant insertion is useful. It is, in fact, our preferred technique, and involves a small incision at the midclavicular line 5 cm below the left costal margin, at a site called Palmer’s point.
The direction of insertion usually ranges from 45° to 90°, depending on the patient’s body weight. If the trocar is placed properly, the closest organs are the stomach and the left lobe of the liver (4 to 6 cm).12 Given the stomach’s close proximity, it should be decompressed with an orogastric tube prior to trocar insertion.
Several studies have demonstrated the safety and efficacy of this entry technique.12-14 It can be helpful in cases that involve difficult access. We usually use a 5-mm primary trocar site for a 5-mm laparoscope. Modern optics allow for a pristine view with these small scopes, eliminating the need to close fascia and perhaps causing less pain at the incision.
Accessory trocar sites facilitate complex technique
We usually use three accessory sites. Two of them are lower-quadrant ports that are placed 2 cm medial and 2 cm cephalad to the anterior superior iliac spine. This area generally lies well away from the inferior epigastric vessels and remains above the area of the ilioinguinal and iliohypogastric nerves, making it a safer point of insertion.15 One trocar is 5 mm in size and the other is 10 mm. The larger one is used to extract the specimen.
We place an additional 5-mm port lateral to the rectus muscle at the level of the umbilicus. This allows the principal surgeon to use two instruments (a toothed forceps and scissors) comfortably while the assistant holds the laparoscope and assists with a grasper.
Does the type of trocar matter?
No randomized studies have directly compared all types of trocars. Options include:
- a pyramidal tip (as in reusable trocars) or shielded tip
- radial expansion
- visible entry
- blunt (Hasson-type) trocar.
Safety data on direct comparison of trocars are limited, but it appears that a radially expanding trocar may offer less port-site pain and potentially less bleeding than a traditional cutting trocar.16 Moreover, the rate of hernia at the port site appears to be relatively low with a radially expanding trocar, even when fascia is left unclosed at a 10-mm site.17
None of these trocars appears to be clearly superior at avoiding visceral or vascular injury.
Technique of laparoscopic cyst removal
A video clip of the surgery is linked to this article in the Video Library at www.obgmanagement.com. In this case, a trocar was inserted in the left upper quadrant, and a laparoscopic cystectomy was initiated using the trocars already specified.
The peritoneal cavity and adnexa were inspected, followed by pelvic washings, as detailed in TABLE 2 . Next, the ovarian cortex was incised ( FIGURE 1A ) with scissors using bipolar or unipolar energy, typically at a low power setting, such as 12 to 15 watts.
It was relatively easy to develop a tissue plane between the cortex and underlying dermoid cyst using simple or aqua-dissection ( FIGURE 1B and C ). The cyst was enucleated without rupture and inserted into a specimen bag through a 10-mm port ( FIGURE 1D ). (The specimen bags generally are available in 10-mm and 15-mm sizes.) Once the trocar was removed, the entire specimen was brought out through the incision ( FIGURE 1E ).
Cystic masses can generally be carefully aspirated using a laparoscopic needle or angiocath, or they can be incised and drained using a standard suction device. Manufacturers of most specimen bags do not recommend the morcellation of tissue within the bag because of the potential for rupture of the bag with tumor spillage or injury to underlying structures.
Occasionally, the mass remains too large to remove after drainage of the cyst fluid. Remedies include enlarging the port site with a scalpel or using a gallbladder speculum to increase the diameter of the port site ( FIGURE 1E ). If possible, the incision should be large enough to deliver the entire bag intact. Use of excessive force will rupture the bag and may cause the specimen to be lost or malignant cells to be inadvertently spilled (in the case of a cancer diagnosis).
TABLE 2
Take these 10 steps to safe laparoscopic surgery
| Examine the anesthetized patient |
| Enter the abdomen |
| Inspect the mass and peritoneal surfaces, including the diaphragm. Biopsy sites suspicious for metastasis and obtain frozen section |
| Perform pelvic and abdominal washings |
Closely inspect adnexa. If findings are not suggestive of malignancy, proceed with laparoscopy. If findings indicate obvious malignancy
|
Perform cystectomy or oophorectomy
|
| Inspect for hemostasis |
| Place cyst/ovary in endopouch |
| Open bag at abdominal wall and remove for frozen section |
| Reinspect and close |
Avoid:
|
FIGURE 1 Laparoscopic cyst removal
Begin by incising the cortex using scissors with or without an energy source.
Dissect the cyst free from the cortex using sharp dissection.
Remove the cyst from the ovary.
Place the cyst in a specimen bag, and …
… bring it to the surface for extraction. The abdominal incision may have to be enlarged to accommodate the specimen.
CASE 1: OUTCOME
The patient’s cyst is removed intact and she is discharged home. Rupture of a dermoid cyst is not associated with any problems as long as copious irrigation is used to aspirate the cyst content.18 Other cysts, such as endometriomas, may not be as easily dissected, and rupture is inevitable.
CASE 2: Symptoms suggestive of cancer
Mrs. B is a 47-year-old woman who reports abdominal bloating for the past 3 weeks. She also complains of early satiety and occasional constipation. She has no history of cancer, but her sister was given a diagnosis of breast cancer at 41 years of age, and her maternal aunt had breast cancer at 55 years.
Mrs. B is moderately obese, with a nontender abdomen and no palpable mass. Her pelvic exam also is negative for a mass or nodularity, but the extent of the exam is limited by body habitus. Her physician orders a transvaginal US, which reveals a 6-cm complex mass with thin septation and a 1-cm solid nodule, with no definite blood flow. The patient’s CA 125 level is 80 IU/mL, which we consider to be within the low-risk range for a premenopausal woman.
The patient is counseled about the need to have the mass removed and is scheduled for laparoscopic right salpingooophorectomy. Given the family history of breast cancer, the physician also requests consultation with a gynecologic oncologist, who agrees to assist with surgery and perform a laparotomy and staging in the event that a malignancy is diagnosed.
Is the mass likely to be malignant?
Given the patient’s family history of breast cancer, the recent onset of symptoms associated with ovarian cancer,2 and the characteristics of the mass (complex, with a nodule), malignancy is possible. This patient has an intermediate risk of cancer and requires additional counseling and planning.
However, most women who undergo laparoscopy for removal of an adnexal mass have benign pathologic findings.
What is the real risk of ovarian cancer?
The lifetime risk of developing ovarian cancer in the general population remains stable at approximately 1 in 70 women, with a mean age at diagnosis of 63 years.19 Ninety percent of ovarian cancer cases are sporadic, and less than 10% can be linked to genetic syndromes.
Women who have mutations in the BRCA1 gene carry a lifetime risk of ovarian cancer of up to 50%, and women who have mutations in BRCA2 have a lifetime risk of up to 25%.20,21 Women who have mutations associated with Lynch II syndrome or Hereditary Nonpolyposis Colorectal Cancer syndrome may have a lifetime risk of ovarian cancer of 12%.22,23
Some women who have a strong family history of breast and ovarian cancer do not carry a known mutation, but are likely to be at increased risk.
Additional risk factors known to be associated with ovarian cancer are nulliparity and infertility. However, the single most important risk factor for epithelial ovarian cancer is age.
Risk-reducing strategies include:
In the case of PBSO, it is imperative to ensure that all ovarian surface epithelium is removed. This means excising the infundibulopelvic ligament at least 1.5 cm above the proximal end of the ovary and excising any adjacent tissue to which the ovary is adherent (including pelvic sidewall peritoneum). Both requirements are easily achieved using the techniques outlined here.
Who should perform surgery?
The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) have published guidelines for referral to a gynecologic oncologist ( TABLE 3 ). When Im and colleagues reviewed the records of more than 1,000 women who underwent surgery for a pelvic mass at six institutions over 12 months, they found that 70% of premenopausal women and 94% of postmenopausal women who were diagnosed with ovarian cancer were properly referred to a qualified subspecialist.27 “Over”-referral occurred in 30% to 40% of women who had a benign mass, but may be preferable given the importance of proper staging and debulking to survival.
ACOG and the SGO recommend referral for women who have:
- elevated tumor markers
- ascites
- a fixed or nodular mass
- a strong family history of breast or ovarian cancer.
Consider preoperative referral of all high-risk and, probably, intermediate-risk women, depending on the availability of qualified specialists for complete surgical staging.
In addition, women need to be counseled thoroughly about the possibility that a malignancy will be diagnosed by frozen section, necessitating additional surgical procedures.
TABLE 3
Your patient has a newly diagnosed pelvic mass. Should you refer her?
| Is she premenopausal? Then refer her when… | Is she postmenopausal? Then refer her when… |
|---|---|
| CA125 >200 IU/mL Ascites is present Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative | CA125 >35 IU/mL Ascites is present Nodular or fixed pelvic mass Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative |
Technique of laparoscopic adnexectomy
In Case 2, an umbilical site was chosen for the primary cannula. In general, the direction of insertion depends on the patient’s body habitus. Heavier patients have a thicker abdominal wall and an umbilicus below the level of the aortic bifurcation. In these women, the angle of insertion should be adjusted from the usual 45° (for patients of normal weight) to an angle nearer to 90°. Lateral ports are typical, as in Case 1.
Treat every mass like cancer
Laparoscopic excision of an adnexal mass can be safe and effective, with better outcomes and recovery than with laparotomy, provided the surgeon adheres to basic principles ( TABLE 2 ). This means treating every mass as though it is potentially malignant, and thoroughly inspecting the abdominal cavity before and after excision of the mass.
Know the retroperitoneal space
As with Case 1, successful laparoscopic excision of an adnexal mass begins with inspection of the peritoneal cavity, abdominopelvic washings, and identification of both the infundibulopelvic ligament and ureter. Knowledge of the retroperitoneal space can be of great value in difficult cases that involve significant pelvic adhesions or sidewall fibrosis. We generally use a retroperitoneal approach for laparoscopic adnexectomy ( FIGURE 2A ).
In our typical approach, we incise the peritoneum lateral to the uteroovarian ligament and continue the incision up the pelvic sidewall lateral to the infundibulopelvic ligament and up along the paracolic gutter, if needed ( FIGURE 2A ). We then mobilize the medial leaf of the broad ligament from the sidewall using blunt dissection between the external iliac vessels laterally and the ureter medially ( FIGURE 2B ).
Once we have identified the ureter, we use scissors to create a window in the medial leaf of the broad ligament just beneath the gonadal vessels. We then use an energy source to occlude and transect the pedicle. Using this technique, we secure the infundibulopelvic ligaments and safely mobilize the ureter before initiating more aggressive sidewall dissection distally for adhesions or fibrosis.
Once the ureter is mobilized, we excise the involved peritoneum along with the mass. We then seal and transect the uteroovarian ligament.
FIGURE 2 The retroperitoneal approach
Grasp and incise the peritoneum just lateral to the adnexal mass and enter the retroperitoneal space, where loose areolar tissue is visible, with the ureter seen on the medial leaf of the broad ligament.
After dissecting this tissue, identify the ureter and internal iliac vessels.
Remove the mass in a bag
Once the mass is excised, we place it in a laparoscopic bag, as described for Case 1. Solid adnexal masses are problematic because they are not amenable to drainage, and morcellation is usually discouraged. Laparoscopic excision can still be carried out, and a minilaparotomy or posterior colpotomy can be used to extract the mass in a bag. Patients managed in this way still have a complication rate similar to or lower than that of patients undergoing laparotomy for oophorectomy; they also recover faster.
Risks in cases of malignancy
The risk of tumor spillage in laparoscopic surgery can be lowered using laparoscopically guided minilaparotomy techniques. Preoperative rupture appears to be more predictive of outcome.28
The concern about metastatic implants in a laparoscopic port site in patients who have gynecologic cancer is real, with an incidence ranging from 0.97% to 1.1%. The phenomenon usually affects women who had ovarian cancer and is most common after laparoscopy with findings of ascites, carcinomatosis, or persistent disease (in the case of second-look laparoscopy performed after completion of primary therapy for ovarian cancer).
These data suggest that port-site implantation is not a concern that should deter clinicians from laparoscopic evaluation of a suspected ovarian neoplasm unless a patient presents with ascites or carcinomatosis suggestive of advanced disease.29
Data are limited regarding delays in definitive therapy. One study suggests that definitive staging on the day of rupture does not influence overall outcome, but delay by more than 2 weeks may lead to poorer prognosis.30
CASE 2: OUTCOME
Laparoscopy is performed. On initial inspection, the peritoneal cavity is unremarkable. The right ovary is multicystic with normal surface anatomy. The mass is somewhat adherent to the pelvic sidewall. Adnexectomy is performed, and frozen section reveals the mass to be a serous cystadenoma.
1. Curtin JP. Management of the adnexal mass. Gynecol Oncol. 1994;55:S42-S46.
2. Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712.
3. Andersen MR, Goff BA, Lowe KA, et al. Combining a symptoms index with CA 125 to improve detection of ovarian cancer. Cancer. 2008;113:484-489.
4. Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221-227.
5. Alcázar JL, Mercé LT, Laparte C, et al. A new scoring system to differentiate benign from malignant adnexal masses. Am J Obstet Gynecol. 2003;188:685-692.
6. Maggino T, Gadducci A, D’Addario V, et al. Prospective multicenter study on CA 125 in postmenopausal pelvic masses. Gynecol Oncol. 1994;54:117-123.
7. Malkasian GD, Jr, Knapp RC, Lavin PT, et al. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988;159:341-346.
8. Larobina M, Nottle P. Complete evidence regarding major vascular injuries during laparoscopic access. Surg Laparosc Endosc Percutan Tech. 2005;15:119-123.
9. Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10:415-420.
10. Vilos GA, Vilos AG, Abu-Rafea B, Hollett-Caines J, Nikkhah-Abyaneh Z, Edris F. Three simple steps during closed laparoscopic entry may minimize major injuries. Surg Endosc. 2008 July 15. [Epub ahead of print]
11. Vilos GA, Ternamian A, Dempster J, Laberge PY. The Society of Obstetricians and Gynaecologists of Canada. Laparoscopic entry: a review of techniques, technologies, and complications. J Obstet Gynaecol Can. 2007;29:433-465.
12. Tulikangas PK, Nicklas A, Falcone T, Price LL. Anatomy of the left upper quadrant for cannula insertion. J Am Assoc Gynecol Laparosc. 2000;7:211-214.
13. Stepp KJ, Tulikangas PK, Goldberg JM, Attaran M, Falcone T. Laparoscopy for adnexal masses in the second trimester of pregnancy. J Am Assoc Gynecol Laparosc. 2003;10:55-59.
14. Tulikangas PK, Robinson DS, Falcone T. Left upper quadrant cannula insertion. Fertil Steril. 2003;79:411-412.
15. Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189:1574-1578.
16. Yim SF, Yuen PM. Randomized double-masked comparison of radially expanding access device and conventional cutting tip trocar in laparoscopy. Obstet Gynecol. 2001;97:435-438.
17. Johnson WH, Fecher AM, McMahon RL, et al. VersaStep trocar hernia rate in unclosed fascial defects in bariatric patients. Surg Endosc. 2006;20:1584-1586.
18. Lin P, Falcone T, Tulandi T. Excision of ovarian dermoid cyst by laparoscopy and by laparotomy. Am J Obstet Gynecol. 1995;173:769-771.
19. Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5-26.
20. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingooophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
21. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643-646.
22. Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214-218.
23. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
24. Dowdy SC, Stefanek M, Hartmann LC. Surgical risk reduction: prophylactic salpingooophorectomy and prophylactic mastectomy. Am J Obstet Gynecol. 2004;191:1113-1123.
25. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424-428.
26. Whittemore AS, Balise RR, Pharoah PD, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer. 2004;91:1911-1915.
27. Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol. 2005;105:35-41.
28. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
29. Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003;91:179-189.
30. Lehner R, Wenzl R, Heinzl H, Husslein P, Sevelda P. Influence of delayed staging laparotomy after laparoscopic removal of ovarian masses later found malignant. Obstet Gynecol. 1998;92:967-971.
1. Curtin JP. Management of the adnexal mass. Gynecol Oncol. 1994;55:S42-S46.
2. Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712.
3. Andersen MR, Goff BA, Lowe KA, et al. Combining a symptoms index with CA 125 to improve detection of ovarian cancer. Cancer. 2008;113:484-489.
4. Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221-227.
5. Alcázar JL, Mercé LT, Laparte C, et al. A new scoring system to differentiate benign from malignant adnexal masses. Am J Obstet Gynecol. 2003;188:685-692.
6. Maggino T, Gadducci A, D’Addario V, et al. Prospective multicenter study on CA 125 in postmenopausal pelvic masses. Gynecol Oncol. 1994;54:117-123.
7. Malkasian GD, Jr, Knapp RC, Lavin PT, et al. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988;159:341-346.
8. Larobina M, Nottle P. Complete evidence regarding major vascular injuries during laparoscopic access. Surg Laparosc Endosc Percutan Tech. 2005;15:119-123.
9. Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10:415-420.
10. Vilos GA, Vilos AG, Abu-Rafea B, Hollett-Caines J, Nikkhah-Abyaneh Z, Edris F. Three simple steps during closed laparoscopic entry may minimize major injuries. Surg Endosc. 2008 July 15. [Epub ahead of print]
11. Vilos GA, Ternamian A, Dempster J, Laberge PY. The Society of Obstetricians and Gynaecologists of Canada. Laparoscopic entry: a review of techniques, technologies, and complications. J Obstet Gynaecol Can. 2007;29:433-465.
12. Tulikangas PK, Nicklas A, Falcone T, Price LL. Anatomy of the left upper quadrant for cannula insertion. J Am Assoc Gynecol Laparosc. 2000;7:211-214.
13. Stepp KJ, Tulikangas PK, Goldberg JM, Attaran M, Falcone T. Laparoscopy for adnexal masses in the second trimester of pregnancy. J Am Assoc Gynecol Laparosc. 2003;10:55-59.
14. Tulikangas PK, Robinson DS, Falcone T. Left upper quadrant cannula insertion. Fertil Steril. 2003;79:411-412.
15. Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189:1574-1578.
16. Yim SF, Yuen PM. Randomized double-masked comparison of radially expanding access device and conventional cutting tip trocar in laparoscopy. Obstet Gynecol. 2001;97:435-438.
17. Johnson WH, Fecher AM, McMahon RL, et al. VersaStep trocar hernia rate in unclosed fascial defects in bariatric patients. Surg Endosc. 2006;20:1584-1586.
18. Lin P, Falcone T, Tulandi T. Excision of ovarian dermoid cyst by laparoscopy and by laparotomy. Am J Obstet Gynecol. 1995;173:769-771.
19. Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5-26.
20. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingooophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
21. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643-646.
22. Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214-218.
23. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
24. Dowdy SC, Stefanek M, Hartmann LC. Surgical risk reduction: prophylactic salpingooophorectomy and prophylactic mastectomy. Am J Obstet Gynecol. 2004;191:1113-1123.
25. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424-428.
26. Whittemore AS, Balise RR, Pharoah PD, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer. 2004;91:1911-1915.
27. Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol. 2005;105:35-41.
28. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
29. Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003;91:179-189.
30. Lehner R, Wenzl R, Heinzl H, Husslein P, Sevelda P. Influence of delayed staging laparotomy after laparoscopic removal of ovarian masses later found malignant. Obstet Gynecol. 1998;92:967-971.
ROUNDTABLE PART 2 OF 2: Using mesh to repair prolapse: Averting, managing complications
Hear Dr Phillips discuss the key points of this series
Vaginal placement of mesh for the correction of pelvic organ prolapse is not an entirely benign procedure. As Mickey M. Karram, MD, and an expert panel discuss in this article—the second of a two-part series—complications secondary to mesh placement can be a challenge to correct and often make life miserable for patients who experience them. Here, these experts address mesh erosion, extrusion, and other serious complications; discuss ways to prevent them; and offer strategies for managing them when they arise.
In Part 1, which appeared in the January 2009 issue of OBG Management, the panel discussed the increasing use of mesh in prolapse repair—in particular, the proliferation of mesh kits.
How common is erosion?
DR. KARRAM: The literature seems to indicate that, even in the best of hands, there is an extrusion, or erosion, rate of between 5% and 17% when mesh is used. Would you agree with this statistic?
DR. LUCENTE: Not completely. The vaginal exposure rate can be as low as 2%, as reported by our center and others, when the mesh is properly placed below all histologic layers of the vaginal wall, as it is when it is “delivered” to the pelvis via the transabdominal route.1,2
At the other end of the scale, an exposure rate above 17% has been reported when mesh is improperly placed within the vaginal wall—that is, just below the mucosa, as some surgeons have described in the methodology section of their abstract or article.3,4

MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.

SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.

VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.

MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
We have found that complete, full-thickness dissection of the vaginal wall into the true pelvic space (vesicovaginal and rectovaginal), utilizing small vaginal incisions and limiting hysterectomy and the trimming of vaginal mucosa, can promote a very low vaginal-exposure rate.
DR. WALTERS: Some surgeons tell me that their own extrusion or erosion rate is lower than the published rate of 5% to 17%, but it is impossible to be certain of the long-term outcome in any patient unless she is followed carefully. The patient may consult another physician about her complications. The primary surgeon—even an expert—often does not know the actual mesh complication rate.
That said, I am sure that some surgeons are particularly adept at using mesh kits for prolapse repair, thereby keeping their mesh complication rate low. The 5% to 17% number is what most gynecologic surgeons should expect for their patients.
DR. RAZ: The complication rates are clearly underreported since very few centers of excellence report on complications and the majority of users don’t report them. Also, the reported complication rate concerns short-term erosion. I imagine that, as time passes and vaginal tissue becomes more atrophic, the incidence of erosion will increase.
Are simple measures enough to resolve erosion?
DR. KARRAM: There seems to be a general perception that most extrusions or erosions can be easily managed in the office by placing estrogen or trimming. In our experience, that approach has been successful in a minority of cases only.
What have you seen?
DR. WALTERS: At the Cleveland Clinic, as at most tertiary care referral centers, we often see the worst cases of extrusion or erosion related to mesh. Estrogen helps in some cases of simple mesh exposure, especially after sacrocolpopexy. If estrogen is going to be effective, however, the problem should clear up relatively quickly; if it isn’t effective after a month or two of therapy, estrogen is unlikely to ever be successful.
When it comes to related problems, such as ridges or strictures in the vagina, dyspareunia, penile pain with insertion, and vaginal burning pain, I have not found simple trimming and estrogen to be effective.
DR. KARRAM: It’s also unlikely that simple excision or placement of estrogen will be successful over the long term. When an extrusion or erosion occurs, we are generally seeing only the tip of the iceberg. That’s because mesh is placed in a certain plane. Although only part of the mesh may be exposed, the entire mesh is likely to be affected because it lies in the same plane.
Also, because of the special nature of vaginal flora, it is unlikely that a foreign body is going to be successfully managed by simple excision or placement of estrogen.
DR. LUCENTE: Management of vaginal exposure really depends on the size of the exposure, its location, and whether there is underlying infection or ischemia of host tissue. When the exposure is small (<1 cm in diameter) and in the midline, with the mesh lying flat below the plane of the vaginal wall, we have been very successful using a conservative approach.
However, even the tiniest of exposures needs to be surgically excised if it traverses the vaginal sulcus. Obviously, any mesh erosion into viscera such as the bladder and bowel also requires surgical intervention. Host-tissue factors always play a contributing role.
I also want to point out that the manner in which exposure is managed depends to some extent on whether the mesh was properly placed. Exposures that arise when mesh is implanted improperly are difficult to correct and usually require complete removal.
Although we, too, started off with an exposure rate around 8%, it is now very low, thanks to technical advancements.
DR. RAZ: A very small vaginal erosion of a mesh sling can sometimes be managed in the office by excision. The cases referred to our service generally involve more extensive areas of exposure that will not be resolved by local treatment.
Is risk of injury operator-dependent?
DR. KARRAM: We’re all seeing very severe complications secondary to mesh placement. Would each of you give your opinion as to whether the severe complications such as significant pain, dyspareunia, and injury of important structures are mostly technical or inherent to mesh placement. Would they happen in the best of hands?
DR. LUCENTE: The more severe complications, for the most part, are very much related to technique. Not that they cannot happen in the very best of hands, but they are extremely rare when technique is meticulous.
Over a 4-year period, after well over 1,000 transvaginal mesh surgeries at our center, we had no death, ICU admission, or transfusion, and our intraoperative complication rate was only 3%, most commonly involving simple cystotomy without long-term consequence. This compares very favorably to the nearly 12% complication rate reported recently in the CARE trial for abdominal sacral colpopexy.5
Our primary challenge today is preventing postoperative dyspareunia. Our rate of new-onset dyspareunia is approximately 3.5%. This complication is, I think, more likely to be related to the inherent material properties of mesh, such as elasticity and flexural rigidity, and to host-tissue response to the material itself.
DR. RAZ: I think that the majority of complications are operator-dependent. Thin dissection of the vaginal wall and unrecognized bladder, urethral, and vaginal perforation are the most common reasons for the complications. Mesh does not move after surgery; if there is a problem, it means that the mesh was misplaced.
Another problem is that industry, in an effort to sell more kits, is pushing physicians who are unfamiliar with the principles of pelvic reconstruction to perform this complex procedure. Repair of major vaginal prolapse is not a simple sling procedure.
In addition, there is a greater likelihood of complications in patients who have severe atrophic tissues. These patients should not be candidates for mesh reconstruction.
DR. WALTERS: Many of the complications that we see with mesh are certainly operator-dependent. For example, mesh that is placed under too much tension leaves the vagina tight and stiff, and mesh that is placed with ripples and ridges causes irregularities in the vagina that are often painful, especially during intercourse.
I do not believe that mesh “erodes” into the bladder, urethra, or rectum, but that it is placed there inadvertently and overlooked intraoperatively (FIGURES 1 and 2), Visceral erosion can occur if the primary surgeon made a cystotomy or proctotomy before proceeding with the mesh kit, and the mesh eventually wore through the repaired area.
There are also some problems that are inherent to mesh, and that occur even in the best hands and after surgeries that are performed very competently. Some mesh exposures are inevitable, as are some cases of dyspareunia and rare cases of vaginal burning and pain. In addition, I am seeing more de novo SUI [stress urinary incontinence] with anterior mesh kits. Although this is not really a complication, it does lead to dissatisfaction in patients and merits efforts to prevent it.
DR. KARRAM: Yes. With the current state of mesh, I believe pain and dyspareunia are almost inevitable in some cases.
DR. LUCENTE: Another problem that is currently underaddressed is scar plating along the surface of the mesh. Such plating forms more readily in the absence of mechanical movement or distention during the early stages of wound healing. To make a comparison, even the best reconstructive orthopedic surgeons cannot achieve optimal functional outcomes with an implant surgery without intense postoperative physical therapy, which may simply involve range of motion or movement.
Most everyone is familiar with the capsular fibrosis and contraction that develop around a breast implant if there isn’t immediate postoperative massaging of the breast tissue and implant during wound repair. I am confident that the rate of dyspareunia will decline over time if specialists in reconstructive pelvic surgery pay closer attention to optimizing vaginal length, preserving the cervix (in women with relatively shorter vaginal length), and ensuring optimal apical attachment (that is, above the ischial spine) in younger, sexually active patients.
DR. RAZ: I think it is the surgeon rather than the surgery who causes most complications. In its effort to sell kits, industry sometimes puts them in the hands of surgeons who are not well prepared for the task. This operation can be quite complex, and you cannot create a pelvic surgeon from a physician who is unfamiliar with the anatomy. If you cannot manage the potential complications, you should not perform this type of surgery.
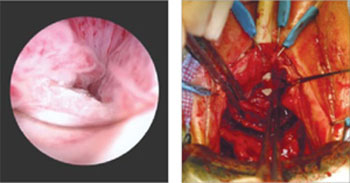
FIGURE 1 When mesh “erodes” into the urethra
Two images of mesh in the urethra. There is some uncertainty here whether mesh that has penetrated the urethra eroded through vaginal tissue or was placed there inadvertently and overlooked intraoperatively.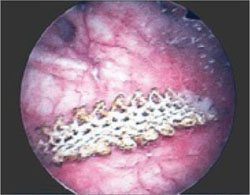
FIGURE 2 Mesh in the bladder
A segment of tension-free vaginal tape has penetrated into the bladder.
Should mesh be removed at the time of injury?
DR. KARRAM: As we discuss specific complications, let’s start with the most severe, which I would say relate to the inadvertent placement of mesh through important structures such as bowel, bladder, or ureters. If this were to happen and be diagnosed intraoperatively, what would you recommend that the surgeon do—abort the procedure or simply remove the mesh or trocar and attempt to pass it again safely?
DR. LUCENTE: That is a difficult question to answer because so much depends on various intraoperative factors.
I am much more comfortable proceeding with surgery after intraoperative bladder injury than after bowel or rectal injury. We have successfully corrected cystotomies that were small, did not encroach on the ureter, and were easily repaired without tension—and we have seen no fistula formation as a result.
The key is to maintain a high index of suspicion throughout the procedure. We have always diagnosed injuries before mesh is delivered—either during dissection or during passage of the needle or trocar. We have not experienced any ureteral injuries aside from “kinking” of one ureter, which was easily corrected with simple readjustment of the mesh.
If, at any time, we were concerned about potential infection, fistula, or a more severe complication that would be aggravated by proceeding with the operation, we would abort the procedure. However, we would be likely to proceed with an alternative operation to address the pelvic-support defect so that the patient would not awaken with intraoperative injury and no surgical treatment for her primary complaint.
We conduct informed consent in such a way as to preserve our flexibility to adapt the surgical plan to execute the reparative work that is necessary despite the development of a non–life-threatening complication during surgery. In the event of any injury to the bowel that would involve gross spillage of fecal material, of course, I would abort placement of synthetic mesh.
DR. WALTERS: If I placed one of the trocars through the bladder or bowel, I would probably remove it, reposition it, and continue with the surgery. With bladder perforation, this approach is generally no problem, but I would usually leave a Foley catheter in place for 1 week of continuous bladder drainage.
If I placed the trocar through the rectum, I would probably oversew the proctotomy, irrigate the space, and continue with the mesh repair. If I had an outright laceration in the bladder or rectum as part of the dissection, I would repair it and consider converting the surgery to prolapse repair without mesh.
The most dreaded complication: the foreshortened vagina
DR. KARRAM: It would seem that the most difficult complication to deal with is the foreshortened, firm, painful vagina. A patient who has these problems may be perceived, at times, as a pelvic “cripple.” Is this an accepted, albeit rare, complication? Or can it be avoided?
DR. LUCENTE: This is the most feared complication arising from the use of synthetic mesh. I do believe it can almost always be avoided—but I never say never. The key is to pay full attention to considerations of vaginal length before surgery, including, first, preservation of the cervix, and, second, placing the mesh loosely, properly sized, and attached with optimization of apical support to preserve vaginal length.
I also believe that use of second-generation meshes that are lighter, more elastic, and more flexible helps reduce this complication when the mesh is properly placed by a surgeon well trained in the technique.
When the vagina is foreshortened, the sooner it is revised, the better the chance that pain will resolve, whether the mesh is removed or released.
DR. RAZ: Mesh infection, capsular formation, dissection of a thin vaginal wall, and excess vaginal-wall excision lead to the short, firm, and painful vagina. The use and abuse of mesh has created a new subspecialty to manage mesh complications. The PFS syndrome (painful, firm, and short vagina) is one of the most difficult complications to treat because, in many cases, it cannot be reversed without major surgery.
DR. WALTERS: Women who have a foreshortened, firm, or painful vagina after mesh augmentation almost always need to have the mesh removed with reconstruction of the vaginal canal. I have never seen a successful outcome in this type of patient without complete or near-complete removal of the mesh.
1. van Raalte H, Lucente V, Haff R, Murphy M. Prolift: an innovative delivery system for transvaginal placement of synthetic grafts for the repair of pelvic organ prolapse. J Pelvic Med Surg .2007;13:351-360.
2. Murphy M, Raders JL, Haff R, Yeager M, Lucente V. Early U.S. experience with vaginal extraperitoneal colpopexy using propylene graft (Prolift) for the treatment of pelvic organ prolapse. J Pelvic Med Surg .2006;12:104-105.
3. Nguyen JM, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111:891-898.
4. Nieminen K, Hiltunen R, Heiskanen E, et al. Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1611-1616.
5. Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112:49-55.
Hear Dr Phillips discuss the key points of this series
Vaginal placement of mesh for the correction of pelvic organ prolapse is not an entirely benign procedure. As Mickey M. Karram, MD, and an expert panel discuss in this article—the second of a two-part series—complications secondary to mesh placement can be a challenge to correct and often make life miserable for patients who experience them. Here, these experts address mesh erosion, extrusion, and other serious complications; discuss ways to prevent them; and offer strategies for managing them when they arise.
In Part 1, which appeared in the January 2009 issue of OBG Management, the panel discussed the increasing use of mesh in prolapse repair—in particular, the proliferation of mesh kits.
How common is erosion?
DR. KARRAM: The literature seems to indicate that, even in the best of hands, there is an extrusion, or erosion, rate of between 5% and 17% when mesh is used. Would you agree with this statistic?
DR. LUCENTE: Not completely. The vaginal exposure rate can be as low as 2%, as reported by our center and others, when the mesh is properly placed below all histologic layers of the vaginal wall, as it is when it is “delivered” to the pelvis via the transabdominal route.1,2
At the other end of the scale, an exposure rate above 17% has been reported when mesh is improperly placed within the vaginal wall—that is, just below the mucosa, as some surgeons have described in the methodology section of their abstract or article.3,4

MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.

SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.

VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.

MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
We have found that complete, full-thickness dissection of the vaginal wall into the true pelvic space (vesicovaginal and rectovaginal), utilizing small vaginal incisions and limiting hysterectomy and the trimming of vaginal mucosa, can promote a very low vaginal-exposure rate.
DR. WALTERS: Some surgeons tell me that their own extrusion or erosion rate is lower than the published rate of 5% to 17%, but it is impossible to be certain of the long-term outcome in any patient unless she is followed carefully. The patient may consult another physician about her complications. The primary surgeon—even an expert—often does not know the actual mesh complication rate.
That said, I am sure that some surgeons are particularly adept at using mesh kits for prolapse repair, thereby keeping their mesh complication rate low. The 5% to 17% number is what most gynecologic surgeons should expect for their patients.
DR. RAZ: The complication rates are clearly underreported since very few centers of excellence report on complications and the majority of users don’t report them. Also, the reported complication rate concerns short-term erosion. I imagine that, as time passes and vaginal tissue becomes more atrophic, the incidence of erosion will increase.
Are simple measures enough to resolve erosion?
DR. KARRAM: There seems to be a general perception that most extrusions or erosions can be easily managed in the office by placing estrogen or trimming. In our experience, that approach has been successful in a minority of cases only.
What have you seen?
DR. WALTERS: At the Cleveland Clinic, as at most tertiary care referral centers, we often see the worst cases of extrusion or erosion related to mesh. Estrogen helps in some cases of simple mesh exposure, especially after sacrocolpopexy. If estrogen is going to be effective, however, the problem should clear up relatively quickly; if it isn’t effective after a month or two of therapy, estrogen is unlikely to ever be successful.
When it comes to related problems, such as ridges or strictures in the vagina, dyspareunia, penile pain with insertion, and vaginal burning pain, I have not found simple trimming and estrogen to be effective.
DR. KARRAM: It’s also unlikely that simple excision or placement of estrogen will be successful over the long term. When an extrusion or erosion occurs, we are generally seeing only the tip of the iceberg. That’s because mesh is placed in a certain plane. Although only part of the mesh may be exposed, the entire mesh is likely to be affected because it lies in the same plane.
Also, because of the special nature of vaginal flora, it is unlikely that a foreign body is going to be successfully managed by simple excision or placement of estrogen.
DR. LUCENTE: Management of vaginal exposure really depends on the size of the exposure, its location, and whether there is underlying infection or ischemia of host tissue. When the exposure is small (<1 cm in diameter) and in the midline, with the mesh lying flat below the plane of the vaginal wall, we have been very successful using a conservative approach.
However, even the tiniest of exposures needs to be surgically excised if it traverses the vaginal sulcus. Obviously, any mesh erosion into viscera such as the bladder and bowel also requires surgical intervention. Host-tissue factors always play a contributing role.
I also want to point out that the manner in which exposure is managed depends to some extent on whether the mesh was properly placed. Exposures that arise when mesh is implanted improperly are difficult to correct and usually require complete removal.
Although we, too, started off with an exposure rate around 8%, it is now very low, thanks to technical advancements.
DR. RAZ: A very small vaginal erosion of a mesh sling can sometimes be managed in the office by excision. The cases referred to our service generally involve more extensive areas of exposure that will not be resolved by local treatment.
Is risk of injury operator-dependent?
DR. KARRAM: We’re all seeing very severe complications secondary to mesh placement. Would each of you give your opinion as to whether the severe complications such as significant pain, dyspareunia, and injury of important structures are mostly technical or inherent to mesh placement. Would they happen in the best of hands?
DR. LUCENTE: The more severe complications, for the most part, are very much related to technique. Not that they cannot happen in the very best of hands, but they are extremely rare when technique is meticulous.
Over a 4-year period, after well over 1,000 transvaginal mesh surgeries at our center, we had no death, ICU admission, or transfusion, and our intraoperative complication rate was only 3%, most commonly involving simple cystotomy without long-term consequence. This compares very favorably to the nearly 12% complication rate reported recently in the CARE trial for abdominal sacral colpopexy.5
Our primary challenge today is preventing postoperative dyspareunia. Our rate of new-onset dyspareunia is approximately 3.5%. This complication is, I think, more likely to be related to the inherent material properties of mesh, such as elasticity and flexural rigidity, and to host-tissue response to the material itself.
DR. RAZ: I think that the majority of complications are operator-dependent. Thin dissection of the vaginal wall and unrecognized bladder, urethral, and vaginal perforation are the most common reasons for the complications. Mesh does not move after surgery; if there is a problem, it means that the mesh was misplaced.
Another problem is that industry, in an effort to sell more kits, is pushing physicians who are unfamiliar with the principles of pelvic reconstruction to perform this complex procedure. Repair of major vaginal prolapse is not a simple sling procedure.
In addition, there is a greater likelihood of complications in patients who have severe atrophic tissues. These patients should not be candidates for mesh reconstruction.
DR. WALTERS: Many of the complications that we see with mesh are certainly operator-dependent. For example, mesh that is placed under too much tension leaves the vagina tight and stiff, and mesh that is placed with ripples and ridges causes irregularities in the vagina that are often painful, especially during intercourse.
I do not believe that mesh “erodes” into the bladder, urethra, or rectum, but that it is placed there inadvertently and overlooked intraoperatively (FIGURES 1 and 2), Visceral erosion can occur if the primary surgeon made a cystotomy or proctotomy before proceeding with the mesh kit, and the mesh eventually wore through the repaired area.
There are also some problems that are inherent to mesh, and that occur even in the best hands and after surgeries that are performed very competently. Some mesh exposures are inevitable, as are some cases of dyspareunia and rare cases of vaginal burning and pain. In addition, I am seeing more de novo SUI [stress urinary incontinence] with anterior mesh kits. Although this is not really a complication, it does lead to dissatisfaction in patients and merits efforts to prevent it.
DR. KARRAM: Yes. With the current state of mesh, I believe pain and dyspareunia are almost inevitable in some cases.
DR. LUCENTE: Another problem that is currently underaddressed is scar plating along the surface of the mesh. Such plating forms more readily in the absence of mechanical movement or distention during the early stages of wound healing. To make a comparison, even the best reconstructive orthopedic surgeons cannot achieve optimal functional outcomes with an implant surgery without intense postoperative physical therapy, which may simply involve range of motion or movement.
Most everyone is familiar with the capsular fibrosis and contraction that develop around a breast implant if there isn’t immediate postoperative massaging of the breast tissue and implant during wound repair. I am confident that the rate of dyspareunia will decline over time if specialists in reconstructive pelvic surgery pay closer attention to optimizing vaginal length, preserving the cervix (in women with relatively shorter vaginal length), and ensuring optimal apical attachment (that is, above the ischial spine) in younger, sexually active patients.
DR. RAZ: I think it is the surgeon rather than the surgery who causes most complications. In its effort to sell kits, industry sometimes puts them in the hands of surgeons who are not well prepared for the task. This operation can be quite complex, and you cannot create a pelvic surgeon from a physician who is unfamiliar with the anatomy. If you cannot manage the potential complications, you should not perform this type of surgery.

FIGURE 1 When mesh “erodes” into the urethra
Two images of mesh in the urethra. There is some uncertainty here whether mesh that has penetrated the urethra eroded through vaginal tissue or was placed there inadvertently and overlooked intraoperatively.
FIGURE 2 Mesh in the bladder
A segment of tension-free vaginal tape has penetrated into the bladder.
Should mesh be removed at the time of injury?
DR. KARRAM: As we discuss specific complications, let’s start with the most severe, which I would say relate to the inadvertent placement of mesh through important structures such as bowel, bladder, or ureters. If this were to happen and be diagnosed intraoperatively, what would you recommend that the surgeon do—abort the procedure or simply remove the mesh or trocar and attempt to pass it again safely?
DR. LUCENTE: That is a difficult question to answer because so much depends on various intraoperative factors.
I am much more comfortable proceeding with surgery after intraoperative bladder injury than after bowel or rectal injury. We have successfully corrected cystotomies that were small, did not encroach on the ureter, and were easily repaired without tension—and we have seen no fistula formation as a result.
The key is to maintain a high index of suspicion throughout the procedure. We have always diagnosed injuries before mesh is delivered—either during dissection or during passage of the needle or trocar. We have not experienced any ureteral injuries aside from “kinking” of one ureter, which was easily corrected with simple readjustment of the mesh.
If, at any time, we were concerned about potential infection, fistula, or a more severe complication that would be aggravated by proceeding with the operation, we would abort the procedure. However, we would be likely to proceed with an alternative operation to address the pelvic-support defect so that the patient would not awaken with intraoperative injury and no surgical treatment for her primary complaint.
We conduct informed consent in such a way as to preserve our flexibility to adapt the surgical plan to execute the reparative work that is necessary despite the development of a non–life-threatening complication during surgery. In the event of any injury to the bowel that would involve gross spillage of fecal material, of course, I would abort placement of synthetic mesh.
DR. WALTERS: If I placed one of the trocars through the bladder or bowel, I would probably remove it, reposition it, and continue with the surgery. With bladder perforation, this approach is generally no problem, but I would usually leave a Foley catheter in place for 1 week of continuous bladder drainage.
If I placed the trocar through the rectum, I would probably oversew the proctotomy, irrigate the space, and continue with the mesh repair. If I had an outright laceration in the bladder or rectum as part of the dissection, I would repair it and consider converting the surgery to prolapse repair without mesh.
The most dreaded complication: the foreshortened vagina
DR. KARRAM: It would seem that the most difficult complication to deal with is the foreshortened, firm, painful vagina. A patient who has these problems may be perceived, at times, as a pelvic “cripple.” Is this an accepted, albeit rare, complication? Or can it be avoided?
DR. LUCENTE: This is the most feared complication arising from the use of synthetic mesh. I do believe it can almost always be avoided—but I never say never. The key is to pay full attention to considerations of vaginal length before surgery, including, first, preservation of the cervix, and, second, placing the mesh loosely, properly sized, and attached with optimization of apical support to preserve vaginal length.
I also believe that use of second-generation meshes that are lighter, more elastic, and more flexible helps reduce this complication when the mesh is properly placed by a surgeon well trained in the technique.
When the vagina is foreshortened, the sooner it is revised, the better the chance that pain will resolve, whether the mesh is removed or released.
DR. RAZ: Mesh infection, capsular formation, dissection of a thin vaginal wall, and excess vaginal-wall excision lead to the short, firm, and painful vagina. The use and abuse of mesh has created a new subspecialty to manage mesh complications. The PFS syndrome (painful, firm, and short vagina) is one of the most difficult complications to treat because, in many cases, it cannot be reversed without major surgery.
DR. WALTERS: Women who have a foreshortened, firm, or painful vagina after mesh augmentation almost always need to have the mesh removed with reconstruction of the vaginal canal. I have never seen a successful outcome in this type of patient without complete or near-complete removal of the mesh.
Hear Dr Phillips discuss the key points of this series
Vaginal placement of mesh for the correction of pelvic organ prolapse is not an entirely benign procedure. As Mickey M. Karram, MD, and an expert panel discuss in this article—the second of a two-part series—complications secondary to mesh placement can be a challenge to correct and often make life miserable for patients who experience them. Here, these experts address mesh erosion, extrusion, and other serious complications; discuss ways to prevent them; and offer strategies for managing them when they arise.
In Part 1, which appeared in the January 2009 issue of OBG Management, the panel discussed the increasing use of mesh in prolapse repair—in particular, the proliferation of mesh kits.
How common is erosion?
DR. KARRAM: The literature seems to indicate that, even in the best of hands, there is an extrusion, or erosion, rate of between 5% and 17% when mesh is used. Would you agree with this statistic?
DR. LUCENTE: Not completely. The vaginal exposure rate can be as low as 2%, as reported by our center and others, when the mesh is properly placed below all histologic layers of the vaginal wall, as it is when it is “delivered” to the pelvis via the transabdominal route.1,2
At the other end of the scale, an exposure rate above 17% has been reported when mesh is improperly placed within the vaginal wall—that is, just below the mucosa, as some surgeons have described in the methodology section of their abstract or article.3,4

MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.

SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.

VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.

MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
We have found that complete, full-thickness dissection of the vaginal wall into the true pelvic space (vesicovaginal and rectovaginal), utilizing small vaginal incisions and limiting hysterectomy and the trimming of vaginal mucosa, can promote a very low vaginal-exposure rate.
DR. WALTERS: Some surgeons tell me that their own extrusion or erosion rate is lower than the published rate of 5% to 17%, but it is impossible to be certain of the long-term outcome in any patient unless she is followed carefully. The patient may consult another physician about her complications. The primary surgeon—even an expert—often does not know the actual mesh complication rate.
That said, I am sure that some surgeons are particularly adept at using mesh kits for prolapse repair, thereby keeping their mesh complication rate low. The 5% to 17% number is what most gynecologic surgeons should expect for their patients.
DR. RAZ: The complication rates are clearly underreported since very few centers of excellence report on complications and the majority of users don’t report them. Also, the reported complication rate concerns short-term erosion. I imagine that, as time passes and vaginal tissue becomes more atrophic, the incidence of erosion will increase.
Are simple measures enough to resolve erosion?
DR. KARRAM: There seems to be a general perception that most extrusions or erosions can be easily managed in the office by placing estrogen or trimming. In our experience, that approach has been successful in a minority of cases only.
What have you seen?
DR. WALTERS: At the Cleveland Clinic, as at most tertiary care referral centers, we often see the worst cases of extrusion or erosion related to mesh. Estrogen helps in some cases of simple mesh exposure, especially after sacrocolpopexy. If estrogen is going to be effective, however, the problem should clear up relatively quickly; if it isn’t effective after a month or two of therapy, estrogen is unlikely to ever be successful.
When it comes to related problems, such as ridges or strictures in the vagina, dyspareunia, penile pain with insertion, and vaginal burning pain, I have not found simple trimming and estrogen to be effective.
DR. KARRAM: It’s also unlikely that simple excision or placement of estrogen will be successful over the long term. When an extrusion or erosion occurs, we are generally seeing only the tip of the iceberg. That’s because mesh is placed in a certain plane. Although only part of the mesh may be exposed, the entire mesh is likely to be affected because it lies in the same plane.
Also, because of the special nature of vaginal flora, it is unlikely that a foreign body is going to be successfully managed by simple excision or placement of estrogen.
DR. LUCENTE: Management of vaginal exposure really depends on the size of the exposure, its location, and whether there is underlying infection or ischemia of host tissue. When the exposure is small (<1 cm in diameter) and in the midline, with the mesh lying flat below the plane of the vaginal wall, we have been very successful using a conservative approach.
However, even the tiniest of exposures needs to be surgically excised if it traverses the vaginal sulcus. Obviously, any mesh erosion into viscera such as the bladder and bowel also requires surgical intervention. Host-tissue factors always play a contributing role.
I also want to point out that the manner in which exposure is managed depends to some extent on whether the mesh was properly placed. Exposures that arise when mesh is implanted improperly are difficult to correct and usually require complete removal.
Although we, too, started off with an exposure rate around 8%, it is now very low, thanks to technical advancements.
DR. RAZ: A very small vaginal erosion of a mesh sling can sometimes be managed in the office by excision. The cases referred to our service generally involve more extensive areas of exposure that will not be resolved by local treatment.
Is risk of injury operator-dependent?
DR. KARRAM: We’re all seeing very severe complications secondary to mesh placement. Would each of you give your opinion as to whether the severe complications such as significant pain, dyspareunia, and injury of important structures are mostly technical or inherent to mesh placement. Would they happen in the best of hands?
DR. LUCENTE: The more severe complications, for the most part, are very much related to technique. Not that they cannot happen in the very best of hands, but they are extremely rare when technique is meticulous.
Over a 4-year period, after well over 1,000 transvaginal mesh surgeries at our center, we had no death, ICU admission, or transfusion, and our intraoperative complication rate was only 3%, most commonly involving simple cystotomy without long-term consequence. This compares very favorably to the nearly 12% complication rate reported recently in the CARE trial for abdominal sacral colpopexy.5
Our primary challenge today is preventing postoperative dyspareunia. Our rate of new-onset dyspareunia is approximately 3.5%. This complication is, I think, more likely to be related to the inherent material properties of mesh, such as elasticity and flexural rigidity, and to host-tissue response to the material itself.
DR. RAZ: I think that the majority of complications are operator-dependent. Thin dissection of the vaginal wall and unrecognized bladder, urethral, and vaginal perforation are the most common reasons for the complications. Mesh does not move after surgery; if there is a problem, it means that the mesh was misplaced.
Another problem is that industry, in an effort to sell more kits, is pushing physicians who are unfamiliar with the principles of pelvic reconstruction to perform this complex procedure. Repair of major vaginal prolapse is not a simple sling procedure.
In addition, there is a greater likelihood of complications in patients who have severe atrophic tissues. These patients should not be candidates for mesh reconstruction.
DR. WALTERS: Many of the complications that we see with mesh are certainly operator-dependent. For example, mesh that is placed under too much tension leaves the vagina tight and stiff, and mesh that is placed with ripples and ridges causes irregularities in the vagina that are often painful, especially during intercourse.
I do not believe that mesh “erodes” into the bladder, urethra, or rectum, but that it is placed there inadvertently and overlooked intraoperatively (FIGURES 1 and 2), Visceral erosion can occur if the primary surgeon made a cystotomy or proctotomy before proceeding with the mesh kit, and the mesh eventually wore through the repaired area.
There are also some problems that are inherent to mesh, and that occur even in the best hands and after surgeries that are performed very competently. Some mesh exposures are inevitable, as are some cases of dyspareunia and rare cases of vaginal burning and pain. In addition, I am seeing more de novo SUI [stress urinary incontinence] with anterior mesh kits. Although this is not really a complication, it does lead to dissatisfaction in patients and merits efforts to prevent it.
DR. KARRAM: Yes. With the current state of mesh, I believe pain and dyspareunia are almost inevitable in some cases.
DR. LUCENTE: Another problem that is currently underaddressed is scar plating along the surface of the mesh. Such plating forms more readily in the absence of mechanical movement or distention during the early stages of wound healing. To make a comparison, even the best reconstructive orthopedic surgeons cannot achieve optimal functional outcomes with an implant surgery without intense postoperative physical therapy, which may simply involve range of motion or movement.
Most everyone is familiar with the capsular fibrosis and contraction that develop around a breast implant if there isn’t immediate postoperative massaging of the breast tissue and implant during wound repair. I am confident that the rate of dyspareunia will decline over time if specialists in reconstructive pelvic surgery pay closer attention to optimizing vaginal length, preserving the cervix (in women with relatively shorter vaginal length), and ensuring optimal apical attachment (that is, above the ischial spine) in younger, sexually active patients.
DR. RAZ: I think it is the surgeon rather than the surgery who causes most complications. In its effort to sell kits, industry sometimes puts them in the hands of surgeons who are not well prepared for the task. This operation can be quite complex, and you cannot create a pelvic surgeon from a physician who is unfamiliar with the anatomy. If you cannot manage the potential complications, you should not perform this type of surgery.

FIGURE 1 When mesh “erodes” into the urethra
Two images of mesh in the urethra. There is some uncertainty here whether mesh that has penetrated the urethra eroded through vaginal tissue or was placed there inadvertently and overlooked intraoperatively.
FIGURE 2 Mesh in the bladder
A segment of tension-free vaginal tape has penetrated into the bladder.
Should mesh be removed at the time of injury?
DR. KARRAM: As we discuss specific complications, let’s start with the most severe, which I would say relate to the inadvertent placement of mesh through important structures such as bowel, bladder, or ureters. If this were to happen and be diagnosed intraoperatively, what would you recommend that the surgeon do—abort the procedure or simply remove the mesh or trocar and attempt to pass it again safely?
DR. LUCENTE: That is a difficult question to answer because so much depends on various intraoperative factors.
I am much more comfortable proceeding with surgery after intraoperative bladder injury than after bowel or rectal injury. We have successfully corrected cystotomies that were small, did not encroach on the ureter, and were easily repaired without tension—and we have seen no fistula formation as a result.
The key is to maintain a high index of suspicion throughout the procedure. We have always diagnosed injuries before mesh is delivered—either during dissection or during passage of the needle or trocar. We have not experienced any ureteral injuries aside from “kinking” of one ureter, which was easily corrected with simple readjustment of the mesh.
If, at any time, we were concerned about potential infection, fistula, or a more severe complication that would be aggravated by proceeding with the operation, we would abort the procedure. However, we would be likely to proceed with an alternative operation to address the pelvic-support defect so that the patient would not awaken with intraoperative injury and no surgical treatment for her primary complaint.
We conduct informed consent in such a way as to preserve our flexibility to adapt the surgical plan to execute the reparative work that is necessary despite the development of a non–life-threatening complication during surgery. In the event of any injury to the bowel that would involve gross spillage of fecal material, of course, I would abort placement of synthetic mesh.
DR. WALTERS: If I placed one of the trocars through the bladder or bowel, I would probably remove it, reposition it, and continue with the surgery. With bladder perforation, this approach is generally no problem, but I would usually leave a Foley catheter in place for 1 week of continuous bladder drainage.
If I placed the trocar through the rectum, I would probably oversew the proctotomy, irrigate the space, and continue with the mesh repair. If I had an outright laceration in the bladder or rectum as part of the dissection, I would repair it and consider converting the surgery to prolapse repair without mesh.
The most dreaded complication: the foreshortened vagina
DR. KARRAM: It would seem that the most difficult complication to deal with is the foreshortened, firm, painful vagina. A patient who has these problems may be perceived, at times, as a pelvic “cripple.” Is this an accepted, albeit rare, complication? Or can it be avoided?
DR. LUCENTE: This is the most feared complication arising from the use of synthetic mesh. I do believe it can almost always be avoided—but I never say never. The key is to pay full attention to considerations of vaginal length before surgery, including, first, preservation of the cervix, and, second, placing the mesh loosely, properly sized, and attached with optimization of apical support to preserve vaginal length.
I also believe that use of second-generation meshes that are lighter, more elastic, and more flexible helps reduce this complication when the mesh is properly placed by a surgeon well trained in the technique.
When the vagina is foreshortened, the sooner it is revised, the better the chance that pain will resolve, whether the mesh is removed or released.
DR. RAZ: Mesh infection, capsular formation, dissection of a thin vaginal wall, and excess vaginal-wall excision lead to the short, firm, and painful vagina. The use and abuse of mesh has created a new subspecialty to manage mesh complications. The PFS syndrome (painful, firm, and short vagina) is one of the most difficult complications to treat because, in many cases, it cannot be reversed without major surgery.
DR. WALTERS: Women who have a foreshortened, firm, or painful vagina after mesh augmentation almost always need to have the mesh removed with reconstruction of the vaginal canal. I have never seen a successful outcome in this type of patient without complete or near-complete removal of the mesh.
1. van Raalte H, Lucente V, Haff R, Murphy M. Prolift: an innovative delivery system for transvaginal placement of synthetic grafts for the repair of pelvic organ prolapse. J Pelvic Med Surg .2007;13:351-360.
2. Murphy M, Raders JL, Haff R, Yeager M, Lucente V. Early U.S. experience with vaginal extraperitoneal colpopexy using propylene graft (Prolift) for the treatment of pelvic organ prolapse. J Pelvic Med Surg .2006;12:104-105.
3. Nguyen JM, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111:891-898.
4. Nieminen K, Hiltunen R, Heiskanen E, et al. Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1611-1616.
5. Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112:49-55.
1. van Raalte H, Lucente V, Haff R, Murphy M. Prolift: an innovative delivery system for transvaginal placement of synthetic grafts for the repair of pelvic organ prolapse. J Pelvic Med Surg .2007;13:351-360.
2. Murphy M, Raders JL, Haff R, Yeager M, Lucente V. Early U.S. experience with vaginal extraperitoneal colpopexy using propylene graft (Prolift) for the treatment of pelvic organ prolapse. J Pelvic Med Surg .2006;12:104-105.
3. Nguyen JM, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111:891-898.
4. Nieminen K, Hiltunen R, Heiskanen E, et al. Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1611-1616.
5. Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112:49-55.
ROUNDTABLE: PART 1 OF 2: Using mesh to repair prolapse calls for more than a kit—it takes skill
MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.
SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.
VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.
MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
Mesh kits for repairing prolapse are proliferating like crazy, just as they did for midurethral sling procedures. But mesh augmentation of prolapse surgeries requires more than a prepackaged assortment of tools and materials. In this article, moderator Mickey M. Karram, MD, and a panel of nationally recognized urogynecologists and urologists describe the literature on mesh augmentation and discuss indications, contraindications, techniques, applicable cases, and the considerable training required.
In Part 2, which will appear in the February issue of OBG Management, the panel tackles the thorny topic of complications, including erosion, extrusion, foreshortening of the vagina, dyspareunia, and pain. Their discussion focuses on ways to avoid these problems, and methods for correcting them.
Do we have enough data?
DR. KARRAM: To start, let’s quickly review the peer-reviewed literature on the use of mesh augmentation during surgery for pelvic organ prolapse.
DR. WALTERS: Until recently, most data concerned open abdominal sacrocolpopexy (ASC) using polypropylene or Merseline mesh. There is significant clinical experience with this operation, and multiple cohort studies show long-term cure rates of 78% to 100% for apical prolapse.1
At least two randomized controlled trials have compared open ASC with sutured vaginal colpopexy procedures, and ASC is certainly equal to—perhaps better than—all transvaginal sutured repairs.2,3
With ASC, most recurrences affect the distal half of the vagina and involve one or more of the following:
- anterior or posterior vaginal wall prolapse (or both)
- stress urinary incontinence (SUI)
- distal rectocele.1,3
Mesh erosion occurs in 3.4% of cases and is usually easily managed.1 Other complications, including bowel injury, tend to be related to access, regardless of whether the operation is performed via laparotomy or laparoscopy.
Robotic sacrocolpopexy has become popular in recent years, and we will probably see data on this approach as we gain experience.
When it comes to vaginal mesh kits, the peer-reviewed literature is just beginning to expand, with many studies being presented at international meetings. For anterior and, possibly, apical vaginal prolapse, the cure rate after use of a mesh kit appears to be as high as, or higher than, the rate for sutured repairs.4 This high rate of anatomic cure is balanced somewhat by additional cost and complications involving mesh and the kits.
For posterior vaginal wall prolapse and rectocele, I firmly believe, based on our research and that of others, that sutured repairs are superior to graft-augmented surgery.5
DR. KARRAM: What are the indications and contraindications for mesh augmentation of prolapse repair ( FIGURES 1 and 2 )?
DR. LUCENTE: I believe mesh is indicated in any patient in need of surgical repair of pelvic organ prolapse who is seeking optimal durability and is willing to accept the known risks of the surgery.
The issue becomes more complex when it comes to contraindications. Absolute contraindications are fairly obvious; they include medically unstable patients and those who may have an inactive infectious process within the pelvis or even undiagnosed abnormal uterine bleeding.
At our center, because the potential for dyspareunia and pelvic discomfort is our biggest concern, we have developed a profile of the patient who is more likely to develop these complaints. The profile includes any patient who has a chronic pain disorder of any type, but especially chronic pelvic pain disorders such as endometriosis and vulvodynia. Other risk factors appear to be a history of pelvic surgery involving any permanent material, suture or mesh, and young age.
So if we have a patient in her late 30s who has undergone reconstructive surgery using permanent sutures and who has an element of chronic pelvic pain, we would counsel her strongly to consider surgical options other than the use of synthetic mesh.
DR. WALTERS: The main indications for mesh-augmented prolapse repair are recurrent posthysterectomy vaginal vault prolapse, for which I usually perform ASC, and recurrent cystocele or anteriorapical prolapse, for which I use one of the anterior mesh kits.
I still think sutured repairs—by that, I mean uterosacral ligament or sacrospinous colpopexy with sutured rectocele repair—work best for recurrent posterior wall and posteriorapical prolapse. I don’t use mesh augmentation for rectocele.
The main contraindication to mesh augmentation, as I see it, is a history of mesh complications. If I am repairing a mesh complication such as erosion or pain, I do not place another mesh.
Medical issues that might increase mesh complications, such as diabetes, steroid use, or severe vaginal atrophy, would, at the very least, make me consider carefully whether mesh augmentation is appropriate. The literature is not clear on this, so mesh could still be used if the surgeon thinks it is necessary.
DR. KARRAM: I haven’t found a definitive indication for mesh augmentation. We have used biologic meshes empirically, but I am not convinced that they really add long-term durability, regardless of whether they are used in the anterior or posterior vaginal segment.
Our published durability rate for traditional suture-type repairs is in the range of 85% at 5 years out.6 Even if I assumed that mesh would give me 100% 5-year durability, this rate would have to be at the expense of some erosion, pain, and other complications unique to mesh. I do not think that the potential improvement in durability is worth these potential complications.
FIGURE 1 When the pelvic support system is intact, prolapse is rare
In the normal pelvis, organs are supported by a complex web of muscles, fascia, and connective tissue. 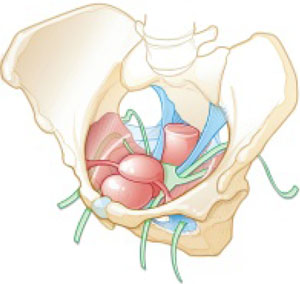
FIGURE 2 Mesh augmentation seeks to enhance the durability of repair
One type of mesh in final position. Mesh-augmented repair restores the vaginal apex and lends support to the walls of the vagina.
DR. KARRAM: If you are doing a lot of mesh repairs, you are obviously content with the results and feel that the few complications you are seeing are outweighed by the advantages mesh confers. How do you avoid extrusion and avert creation of a painful vagina?
DR. RAZ: Most of our cases are recurrent prolapse after failed vaginal or abdominal repair. I am indeed using a significant amount of soft polypropylene mesh for reconstructive procedures. As with the use of any other synthetic material, low-grade infection can develop after a few weeks or months. I use copious irrigation with antibiotic solution during reconstruction.
To avoid extrusion, I perform deep, rather than superficial, dissection of the vaginal wall to allow for better coverage of the mesh. For posterior mesh reconstruction, I cover the mesh with pararectal fascia to prevent erosion.
For mesh-augmented procedures, I cut the mesh myself in the operating room ( FIGURE 3 ). For a sling, I use a 10 cm × 1 cm soft polypropylene mesh. For a grade 3 or 4 cystocele, I use a trapezoid of soft polypropylene mesh with several points of fixation:
- at the sacrouterine ligament
- lateral to the obturator fascia
- distal to the bladder neck.
I always repair the vault at the same time.
For vault prolapse, I use a segment of soft polypropylene mesh in the shape of an apron with two arms (1 cm × 4 cm) and a central segment (4 cm × 7 cm). I support the vault using number 1-0 delayed absorbable suture and mesh. From outside the vaginal wall, in the posterolateral deep vaginal wall (inside the peritoneum), I incorporate the origin of the sacrouterine ligament and one arm of the mesh in the groove between the colon and levator ani, 15 cm from the introitus. I bring the suture 1 cm from the original entrance. A separate set of sutures brings the perirectal fascia together with the sacrouterine ligaments and perivesical fascia to close the peritoneal cavity. I tie the vault-suspension sutures, providing support to the cuff in a high posterior position (12 to 15 cm from the introitus).
In selected cases of significant recurrent rectocele, I use a rectangle of soft polypropylene mesh anchored to the origin of the sacrouterine ligament and distal to the perineal membrane. The mesh is covered by the pararectal fascia.
We have not seen vaginal, urethral, or bladder erosion in 1,800 cases of our distal urethral Prolene sling procedure using 10 cm × 1 cm soft mesh. In patients who have significant cystocele, vault prolapse, and recurrent rectocele, our vaginal erosion rate is 3%. We have never encountered rectal, bladder, or bowel perforation using our technique.
DR. LUCENTE: We often use mesh and are more than simply content with our results—we are extremely pleased, and so are our patients. Having said that, our techniques have definitely evolved over the past few years, as we’ve focused on how to decrease exposure and, more recently, optimize sexual function and vaginal comfort.
First, to avoid exposure, the most critical step is precise hydrodissection and distention of the true vesicovaginal space. This step can only be achieved through careful tactile guidance of the needle tip into the space, where it should remain while hydrodissection is performed. Always remember, sharp dissection “follows” hydrodissection. If you place the needle bevel within the vaginal wall, you will “split” the vaginal wall—as during standard colporrhaphy—which will lead to a high exposure rate.
Second, to avoid dyspareunia, it’s essential to pay close attention to POP-Q measurements, especially vaginal length, to ensure that the reconstruction restores the same length without foreshortening. This approach entails leaving the cervix in most patients who have a shorter vagina, and making sure that the mesh is secured above the ischial spine in younger, sexually active patients who have demonstrated a higher risk of postoperative deep, penetrating dyspareunia, compared with older, less sexually active patients.
Also paramount is to ensure that you have manually displaced the vagina inwardly as much as possible before deploying or setting the mesh. If you simply try to suture secure the mesh with the vagina incised open, without the ability to deploy the mesh with a closed, displaced vagina (to mimic deep penetration), it is difficult, if not impossible, to properly set the mesh for optimal comfort.
In the early days of midurethral pubovaginal slings using polypropylene, the adage was “looser is better than tighter.” This is even truer for transvaginal mesh.
DR. KARRAM: Dr. Walters, please describe your current surgical procedure of choice without mesh and explain why you haven’t adopted mesh for routine repairs.
DR. WALTERS: About 20% of my prolapse surgeries—usually for posthysterectomy or recurrent vaginal vault prolapse—involve ASC with placement of polypropylene mesh. I perform most of these cases through a Pfannenstiel incision, but I’ve also done them laparoscopically. Several of my partners perform ASC laparoscopically and robotically.
For the other 80% of my patients who have prolapse, I perform repairs transvaginally, usually using high bilateral uterosacralligament vaginal-vault suspension. We have learned to suture higher and slightly more medial on the uterosacral ligaments to attain greater vaginal depth and minimize ureteral obstruction. We use two or three sutures on each uterosacral ligament, usually a combination of permanent and delayed absorbable sutures.
I am also performing more sacrospinous ligament suspensions because this operation is being studied by the Pelvic Floor Disorders Network. Properly performed, it is an excellent surgery for apical prolapse. But, as with most of our surgeries for prolapse, recurrent anterior wall prolapse remains a problem.
Like you, Dr. Karram, we’ve studied our group’s anatomic and functional outcomes very carefully for more than 10 years and are mostly satisfied with our cure and complication rates. Although our anatomic outcomes with these surgeries are not always perfect, our reoperation rate for prolapse is only about 5%, with a high level of satisfaction in 88% to 92% of patients.
DR. RAZ: Unaugmented reconstruction fails in more than 30% of cases. Some patients who have significant prolapse and attenuated tissue think that this tissue will become healthier or stronger after reconstructive surgery, but that isn’t the case. In these situations, excision and plication make no clinical sense.
The problem is that we have yet to identify the ideal surrogate for poor-quality tissue. Most of us use polypropylene mesh in different variations. We need a better material that will be nonimmunogenic, well tolerated, and easily incorporated without erosion. Xenograft-like derivatives of dermis, or allografts such as cadaveric fascia, have failed over the long term because the body reabsorbs the graft without forming any new connective tissue.
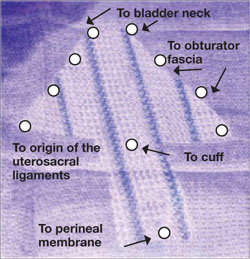
FIGURE 3 Mesh can be cut in the OR to custom-fit a patient
Hand-cut mesh and points of placement.
PHOTO: SHLOMO RAZ, MD
Is a kit a valuable aid?
DR. KARRAM: If a surgeon wants to augment a repair, what are the advantages of a packaged mesh kit, compared with simply cutting the mesh and performing surgery without a kit?
DR. WALTERS: The advantages of a packaged mesh kit are the convenience involved and the ability to consistently perform the same operation with the same product. That facilitates learning, teaching, and research. It also helps us understand the published literature a little better because “custom” prolapse repairs are operator-dependent and difficult to apply generally to a population of surgeons.
These advantages are most clearly apparent with midurethral sling mesh kits, which have almost revolutionized surgery for stress incontinence. I don’t believe mesh kits for prolapse are there yet, but they certainly have potential.
DR. RAZ: I’m opposed to the use of kits. They are industry-driven. One company has made $1 billion selling them. Imagine a patient who undergoes placement of a sling kit ($1,000), cystocele kit ($1,500), and posterior mesh kit ($1,500). How can our healthcare system sustain this burden, especially when there is no real evidence that a kit improves the operation, and given the incredible complication rate that we see?
Moreover, the kits contain a single-use needle and passer and a precut segment of polypropylene mesh. But every patient is different and requires a unique size or shape of mesh. I don’t believe that a surgeon who knows pelvic anatomy needs a kit to perform mesh-augmented reconstruction. We can buy the same segment of mesh for $200 to $400, cut it as needed, and perform the same operation advertised by industry.
For surgeons who prefer a kit, the tools that are included should be made reusable.
DR. LUCENTE: In my opinion, the primary advantage of a commercially available transvaginal mesh delivery system—notice, I avoided the word “kit,” because I think there are plenty of negative connotations associated with it—is the ability to deliver the mesh in a “tension”-free manner.
One alternative that many people pursue is cutting the mesh to size and using sutures to hold it in place while tissue ingrowth occurs. However, the hernia literature suggests that suturing mesh in place increases the risk of postoperative discomfort at the site of implantation. The true cause of the discomfort remains unclear, but it is thought to arise from nerve tethering or traction at the pre-committed points of attachment before the host tissue and mesh interface have adjusted or settled with tissue ingrowth.
All neuropathic complications of mesh implantation have been shown in the current hernia literature to be increased with the use of sutures.7 Also, as previously mentioned, it is extremely difficult to set or adjust the mesh with the vaginal incision remaining “open,” which is a downside to suture techniques.
What training is necessary to use a kit?
DR. KARRAM: Mesh kits are aggressively promoted by industry, with close to half a dozen different kits to be available soon. What is the minimum amount of training one should have before utilizing these kits?
DR. WALTERS: The surgeon should at least know how to perform traditional sutured prolapse repairs and SUI surgery and be able to perform cystoscopy. Ideally, the surgeon should undergo training on a cadaver with a skilled and experienced user of the mesh kit. The surgeon also should carefully review the risks and benefits of mesh kits with the patient and inform the patient that he or she is in the early learning curve of a particular surgery. The informed patient should have a right to refuse mesh-augmented prolapse surgery after the consent process.
DR. LUCENTE: I’m glad you asked this question. I strongly believe that surgical expertise and proficiency within gynecology need to be more effectively addressed by us all. We have a situation in our field in which techniques and technology are widening the gap between what is possible and what the surgeon is comfortable doing safely.
It’s incumbent on all of us, especially those who are in a leadership position as a chairperson or chief of a division, to work with our physician staff and faculty to optimize surgical skill and patient outcomes, including safety, with new technologies.
As for the minimal amount of training needed, that’s extremely variable. It depends on the current skill set of the physician and his or her ability to pick up the mechanics of the surgery as it is taught through a cadaver lab or preceptorship. It’s regrettable that some physicians lack the objectivity and insight to judge their own skill set. This, again, is the time for a chairperson or chief of a division to step up to the plate and ensure proper credentialing and demonstration of proficiency.
It is unrealistic to expect industry to decide who should or should not utilize this truly breakthrough technology. That is our responsibility as physicians.
DR. KARRAM: At a minimum, I think any surgeon utilizing a kit should have a firm understanding of pelvic floor anatomy and experience performing traditional repairs:
- intraperitoneal procedures such as Mc-Call culdoplasty and uterosacral suspension
- sacrospinous suspension
- retropubic procedures and anti-incontinence operations such as pubovaginal slings.
This three-dimensional understanding of the pelvic floor is mandatory if one is to assume that blind passage of trocars through potentially dangerous spaces is the wave of the future.
DR. RAZ: You need to be a pelvic surgeon, know your anatomy, and know how to manage complications if you are going to use one of these kits. You should stick to the surgery that works best in your hands. Industry cannot teach you to be a good pelvic surgeon; it takes lifelong experience.
DR. KARRAM: If you have a patient who is sexually inactive with pelvic organ prolapse, would you prefer a mesh repair or an obliterative procedure? And why?
DR. WALTERS: If the patient is sexually inactive—especially if she is older and definitely will not be in the future—it makes absolutely no sense to perform a mesh-augmented repair. A traditional, somewhat tight, sutured repair works fine in this setting and carries very low risk.
In fact, our group and others have found that, in carefully selected patients, partial colpectomy and colpocleisis procedures (without grafts) have among the highest cure and satisfaction rates of all surgeries we perform for prolapse; they also have relatively low risk.8 Recurrent prolapse after an obliterative procedure is rare; most of the dissatisfaction relates to postoperative voiding difficulties or persistent or de novo urinary incontinence.
DR. KARRAM: I also prefer an obliterative procedure. I see no reason to bring in the cost and potential for complications that mesh repair entails. An obliterative procedure should produce an anatomic success rate close to 100%, with minimal complications. It also can be performed quickly with minimal anesthesia and convalescence.
DR. LUCENTE: My response is based on a clinical study that my associate, Dr. Miles Murphy, has performed, comparing a transvaginal mesh procedure with a LaForte operation for severe pelvic organ prolapse.9 Both patient groups were well satisfied with the result, and success rates were comparable. However, the group that underwent the transvaginal mesh procedure had a shorter operative time.
As a result of these studies, we tend to prefer transvaginal mesh repair. Even though the woman may be sexually inactive, the procedure preserves vaginal function, and we all know that life has a way of being unpredictable. Her situation may change so that she once again desires sexual function.
However, for a very elderly woman—one in her late 80s or 90s—who has severe or extreme prolapse with a very large procidentia and vaginal length measuring, say, 13 cm beyond the introitus, I do prefer an obliterative procedure.
DR. RAZ: I agree. I would not offer a sexually inactive patient an obliterative procedure. You never know what the future will hold.
Mesh repair can be performed safely, provided the surgeon has good knowledge of anatomic landmarks and knows how to manage any potential complications that may arise.
1. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
2. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421;discussion 1421-1422.
3. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
4. Murphy M. Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines on vaginal graft use from the Society of Gynecologic Surgeons. Obstet Gynecol. 2008;112:1123-1130.
5. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical procedures including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
6. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 2006;108:255-263.
7. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322-332.
8. Barber MD, Amundsen C, Paraiso MFR, Weidner A, Romero A, Walters MD. Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J. 2007;18:799-806.
9. Murphy M, van Raalte H, Mercurio E, Haff R, Wiseman B, Lucente VR. Incontinence-related quality of life and sexual function following the tension-free vaginal tape vs the “inside-out” tension-free vaginal tape obturator. Int Urogynecol J. 2008;19:481-487.
MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.
SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.
VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.
MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
Mesh kits for repairing prolapse are proliferating like crazy, just as they did for midurethral sling procedures. But mesh augmentation of prolapse surgeries requires more than a prepackaged assortment of tools and materials. In this article, moderator Mickey M. Karram, MD, and a panel of nationally recognized urogynecologists and urologists describe the literature on mesh augmentation and discuss indications, contraindications, techniques, applicable cases, and the considerable training required.
In Part 2, which will appear in the February issue of OBG Management, the panel tackles the thorny topic of complications, including erosion, extrusion, foreshortening of the vagina, dyspareunia, and pain. Their discussion focuses on ways to avoid these problems, and methods for correcting them.
Do we have enough data?
DR. KARRAM: To start, let’s quickly review the peer-reviewed literature on the use of mesh augmentation during surgery for pelvic organ prolapse.
DR. WALTERS: Until recently, most data concerned open abdominal sacrocolpopexy (ASC) using polypropylene or Merseline mesh. There is significant clinical experience with this operation, and multiple cohort studies show long-term cure rates of 78% to 100% for apical prolapse.1
At least two randomized controlled trials have compared open ASC with sutured vaginal colpopexy procedures, and ASC is certainly equal to—perhaps better than—all transvaginal sutured repairs.2,3
With ASC, most recurrences affect the distal half of the vagina and involve one or more of the following:
- anterior or posterior vaginal wall prolapse (or both)
- stress urinary incontinence (SUI)
- distal rectocele.1,3
Mesh erosion occurs in 3.4% of cases and is usually easily managed.1 Other complications, including bowel injury, tend to be related to access, regardless of whether the operation is performed via laparotomy or laparoscopy.
Robotic sacrocolpopexy has become popular in recent years, and we will probably see data on this approach as we gain experience.
When it comes to vaginal mesh kits, the peer-reviewed literature is just beginning to expand, with many studies being presented at international meetings. For anterior and, possibly, apical vaginal prolapse, the cure rate after use of a mesh kit appears to be as high as, or higher than, the rate for sutured repairs.4 This high rate of anatomic cure is balanced somewhat by additional cost and complications involving mesh and the kits.
For posterior vaginal wall prolapse and rectocele, I firmly believe, based on our research and that of others, that sutured repairs are superior to graft-augmented surgery.5
DR. KARRAM: What are the indications and contraindications for mesh augmentation of prolapse repair ( FIGURES 1 and 2 )?
DR. LUCENTE: I believe mesh is indicated in any patient in need of surgical repair of pelvic organ prolapse who is seeking optimal durability and is willing to accept the known risks of the surgery.
The issue becomes more complex when it comes to contraindications. Absolute contraindications are fairly obvious; they include medically unstable patients and those who may have an inactive infectious process within the pelvis or even undiagnosed abnormal uterine bleeding.
At our center, because the potential for dyspareunia and pelvic discomfort is our biggest concern, we have developed a profile of the patient who is more likely to develop these complaints. The profile includes any patient who has a chronic pain disorder of any type, but especially chronic pelvic pain disorders such as endometriosis and vulvodynia. Other risk factors appear to be a history of pelvic surgery involving any permanent material, suture or mesh, and young age.
So if we have a patient in her late 30s who has undergone reconstructive surgery using permanent sutures and who has an element of chronic pelvic pain, we would counsel her strongly to consider surgical options other than the use of synthetic mesh.
DR. WALTERS: The main indications for mesh-augmented prolapse repair are recurrent posthysterectomy vaginal vault prolapse, for which I usually perform ASC, and recurrent cystocele or anteriorapical prolapse, for which I use one of the anterior mesh kits.
I still think sutured repairs—by that, I mean uterosacral ligament or sacrospinous colpopexy with sutured rectocele repair—work best for recurrent posterior wall and posteriorapical prolapse. I don’t use mesh augmentation for rectocele.
The main contraindication to mesh augmentation, as I see it, is a history of mesh complications. If I am repairing a mesh complication such as erosion or pain, I do not place another mesh.
Medical issues that might increase mesh complications, such as diabetes, steroid use, or severe vaginal atrophy, would, at the very least, make me consider carefully whether mesh augmentation is appropriate. The literature is not clear on this, so mesh could still be used if the surgeon thinks it is necessary.
DR. KARRAM: I haven’t found a definitive indication for mesh augmentation. We have used biologic meshes empirically, but I am not convinced that they really add long-term durability, regardless of whether they are used in the anterior or posterior vaginal segment.
Our published durability rate for traditional suture-type repairs is in the range of 85% at 5 years out.6 Even if I assumed that mesh would give me 100% 5-year durability, this rate would have to be at the expense of some erosion, pain, and other complications unique to mesh. I do not think that the potential improvement in durability is worth these potential complications.

FIGURE 1 When the pelvic support system is intact, prolapse is rare
In the normal pelvis, organs are supported by a complex web of muscles, fascia, and connective tissue. 
FIGURE 2 Mesh augmentation seeks to enhance the durability of repair
One type of mesh in final position. Mesh-augmented repair restores the vaginal apex and lends support to the walls of the vagina.
DR. KARRAM: If you are doing a lot of mesh repairs, you are obviously content with the results and feel that the few complications you are seeing are outweighed by the advantages mesh confers. How do you avoid extrusion and avert creation of a painful vagina?
DR. RAZ: Most of our cases are recurrent prolapse after failed vaginal or abdominal repair. I am indeed using a significant amount of soft polypropylene mesh for reconstructive procedures. As with the use of any other synthetic material, low-grade infection can develop after a few weeks or months. I use copious irrigation with antibiotic solution during reconstruction.
To avoid extrusion, I perform deep, rather than superficial, dissection of the vaginal wall to allow for better coverage of the mesh. For posterior mesh reconstruction, I cover the mesh with pararectal fascia to prevent erosion.
For mesh-augmented procedures, I cut the mesh myself in the operating room ( FIGURE 3 ). For a sling, I use a 10 cm × 1 cm soft polypropylene mesh. For a grade 3 or 4 cystocele, I use a trapezoid of soft polypropylene mesh with several points of fixation:
- at the sacrouterine ligament
- lateral to the obturator fascia
- distal to the bladder neck.
I always repair the vault at the same time.
For vault prolapse, I use a segment of soft polypropylene mesh in the shape of an apron with two arms (1 cm × 4 cm) and a central segment (4 cm × 7 cm). I support the vault using number 1-0 delayed absorbable suture and mesh. From outside the vaginal wall, in the posterolateral deep vaginal wall (inside the peritoneum), I incorporate the origin of the sacrouterine ligament and one arm of the mesh in the groove between the colon and levator ani, 15 cm from the introitus. I bring the suture 1 cm from the original entrance. A separate set of sutures brings the perirectal fascia together with the sacrouterine ligaments and perivesical fascia to close the peritoneal cavity. I tie the vault-suspension sutures, providing support to the cuff in a high posterior position (12 to 15 cm from the introitus).
In selected cases of significant recurrent rectocele, I use a rectangle of soft polypropylene mesh anchored to the origin of the sacrouterine ligament and distal to the perineal membrane. The mesh is covered by the pararectal fascia.
We have not seen vaginal, urethral, or bladder erosion in 1,800 cases of our distal urethral Prolene sling procedure using 10 cm × 1 cm soft mesh. In patients who have significant cystocele, vault prolapse, and recurrent rectocele, our vaginal erosion rate is 3%. We have never encountered rectal, bladder, or bowel perforation using our technique.
DR. LUCENTE: We often use mesh and are more than simply content with our results—we are extremely pleased, and so are our patients. Having said that, our techniques have definitely evolved over the past few years, as we’ve focused on how to decrease exposure and, more recently, optimize sexual function and vaginal comfort.
First, to avoid exposure, the most critical step is precise hydrodissection and distention of the true vesicovaginal space. This step can only be achieved through careful tactile guidance of the needle tip into the space, where it should remain while hydrodissection is performed. Always remember, sharp dissection “follows” hydrodissection. If you place the needle bevel within the vaginal wall, you will “split” the vaginal wall—as during standard colporrhaphy—which will lead to a high exposure rate.
Second, to avoid dyspareunia, it’s essential to pay close attention to POP-Q measurements, especially vaginal length, to ensure that the reconstruction restores the same length without foreshortening. This approach entails leaving the cervix in most patients who have a shorter vagina, and making sure that the mesh is secured above the ischial spine in younger, sexually active patients who have demonstrated a higher risk of postoperative deep, penetrating dyspareunia, compared with older, less sexually active patients.
Also paramount is to ensure that you have manually displaced the vagina inwardly as much as possible before deploying or setting the mesh. If you simply try to suture secure the mesh with the vagina incised open, without the ability to deploy the mesh with a closed, displaced vagina (to mimic deep penetration), it is difficult, if not impossible, to properly set the mesh for optimal comfort.
In the early days of midurethral pubovaginal slings using polypropylene, the adage was “looser is better than tighter.” This is even truer for transvaginal mesh.
DR. KARRAM: Dr. Walters, please describe your current surgical procedure of choice without mesh and explain why you haven’t adopted mesh for routine repairs.
DR. WALTERS: About 20% of my prolapse surgeries—usually for posthysterectomy or recurrent vaginal vault prolapse—involve ASC with placement of polypropylene mesh. I perform most of these cases through a Pfannenstiel incision, but I’ve also done them laparoscopically. Several of my partners perform ASC laparoscopically and robotically.
For the other 80% of my patients who have prolapse, I perform repairs transvaginally, usually using high bilateral uterosacralligament vaginal-vault suspension. We have learned to suture higher and slightly more medial on the uterosacral ligaments to attain greater vaginal depth and minimize ureteral obstruction. We use two or three sutures on each uterosacral ligament, usually a combination of permanent and delayed absorbable sutures.
I am also performing more sacrospinous ligament suspensions because this operation is being studied by the Pelvic Floor Disorders Network. Properly performed, it is an excellent surgery for apical prolapse. But, as with most of our surgeries for prolapse, recurrent anterior wall prolapse remains a problem.
Like you, Dr. Karram, we’ve studied our group’s anatomic and functional outcomes very carefully for more than 10 years and are mostly satisfied with our cure and complication rates. Although our anatomic outcomes with these surgeries are not always perfect, our reoperation rate for prolapse is only about 5%, with a high level of satisfaction in 88% to 92% of patients.
DR. RAZ: Unaugmented reconstruction fails in more than 30% of cases. Some patients who have significant prolapse and attenuated tissue think that this tissue will become healthier or stronger after reconstructive surgery, but that isn’t the case. In these situations, excision and plication make no clinical sense.
The problem is that we have yet to identify the ideal surrogate for poor-quality tissue. Most of us use polypropylene mesh in different variations. We need a better material that will be nonimmunogenic, well tolerated, and easily incorporated without erosion. Xenograft-like derivatives of dermis, or allografts such as cadaveric fascia, have failed over the long term because the body reabsorbs the graft without forming any new connective tissue.

FIGURE 3 Mesh can be cut in the OR to custom-fit a patient
Hand-cut mesh and points of placement.
PHOTO: SHLOMO RAZ, MD
Is a kit a valuable aid?
DR. KARRAM: If a surgeon wants to augment a repair, what are the advantages of a packaged mesh kit, compared with simply cutting the mesh and performing surgery without a kit?
DR. WALTERS: The advantages of a packaged mesh kit are the convenience involved and the ability to consistently perform the same operation with the same product. That facilitates learning, teaching, and research. It also helps us understand the published literature a little better because “custom” prolapse repairs are operator-dependent and difficult to apply generally to a population of surgeons.
These advantages are most clearly apparent with midurethral sling mesh kits, which have almost revolutionized surgery for stress incontinence. I don’t believe mesh kits for prolapse are there yet, but they certainly have potential.
DR. RAZ: I’m opposed to the use of kits. They are industry-driven. One company has made $1 billion selling them. Imagine a patient who undergoes placement of a sling kit ($1,000), cystocele kit ($1,500), and posterior mesh kit ($1,500). How can our healthcare system sustain this burden, especially when there is no real evidence that a kit improves the operation, and given the incredible complication rate that we see?
Moreover, the kits contain a single-use needle and passer and a precut segment of polypropylene mesh. But every patient is different and requires a unique size or shape of mesh. I don’t believe that a surgeon who knows pelvic anatomy needs a kit to perform mesh-augmented reconstruction. We can buy the same segment of mesh for $200 to $400, cut it as needed, and perform the same operation advertised by industry.
For surgeons who prefer a kit, the tools that are included should be made reusable.
DR. LUCENTE: In my opinion, the primary advantage of a commercially available transvaginal mesh delivery system—notice, I avoided the word “kit,” because I think there are plenty of negative connotations associated with it—is the ability to deliver the mesh in a “tension”-free manner.
One alternative that many people pursue is cutting the mesh to size and using sutures to hold it in place while tissue ingrowth occurs. However, the hernia literature suggests that suturing mesh in place increases the risk of postoperative discomfort at the site of implantation. The true cause of the discomfort remains unclear, but it is thought to arise from nerve tethering or traction at the pre-committed points of attachment before the host tissue and mesh interface have adjusted or settled with tissue ingrowth.
All neuropathic complications of mesh implantation have been shown in the current hernia literature to be increased with the use of sutures.7 Also, as previously mentioned, it is extremely difficult to set or adjust the mesh with the vaginal incision remaining “open,” which is a downside to suture techniques.
What training is necessary to use a kit?
DR. KARRAM: Mesh kits are aggressively promoted by industry, with close to half a dozen different kits to be available soon. What is the minimum amount of training one should have before utilizing these kits?
DR. WALTERS: The surgeon should at least know how to perform traditional sutured prolapse repairs and SUI surgery and be able to perform cystoscopy. Ideally, the surgeon should undergo training on a cadaver with a skilled and experienced user of the mesh kit. The surgeon also should carefully review the risks and benefits of mesh kits with the patient and inform the patient that he or she is in the early learning curve of a particular surgery. The informed patient should have a right to refuse mesh-augmented prolapse surgery after the consent process.
DR. LUCENTE: I’m glad you asked this question. I strongly believe that surgical expertise and proficiency within gynecology need to be more effectively addressed by us all. We have a situation in our field in which techniques and technology are widening the gap between what is possible and what the surgeon is comfortable doing safely.
It’s incumbent on all of us, especially those who are in a leadership position as a chairperson or chief of a division, to work with our physician staff and faculty to optimize surgical skill and patient outcomes, including safety, with new technologies.
As for the minimal amount of training needed, that’s extremely variable. It depends on the current skill set of the physician and his or her ability to pick up the mechanics of the surgery as it is taught through a cadaver lab or preceptorship. It’s regrettable that some physicians lack the objectivity and insight to judge their own skill set. This, again, is the time for a chairperson or chief of a division to step up to the plate and ensure proper credentialing and demonstration of proficiency.
It is unrealistic to expect industry to decide who should or should not utilize this truly breakthrough technology. That is our responsibility as physicians.
DR. KARRAM: At a minimum, I think any surgeon utilizing a kit should have a firm understanding of pelvic floor anatomy and experience performing traditional repairs:
- intraperitoneal procedures such as Mc-Call culdoplasty and uterosacral suspension
- sacrospinous suspension
- retropubic procedures and anti-incontinence operations such as pubovaginal slings.
This three-dimensional understanding of the pelvic floor is mandatory if one is to assume that blind passage of trocars through potentially dangerous spaces is the wave of the future.
DR. RAZ: You need to be a pelvic surgeon, know your anatomy, and know how to manage complications if you are going to use one of these kits. You should stick to the surgery that works best in your hands. Industry cannot teach you to be a good pelvic surgeon; it takes lifelong experience.
DR. KARRAM: If you have a patient who is sexually inactive with pelvic organ prolapse, would you prefer a mesh repair or an obliterative procedure? And why?
DR. WALTERS: If the patient is sexually inactive—especially if she is older and definitely will not be in the future—it makes absolutely no sense to perform a mesh-augmented repair. A traditional, somewhat tight, sutured repair works fine in this setting and carries very low risk.
In fact, our group and others have found that, in carefully selected patients, partial colpectomy and colpocleisis procedures (without grafts) have among the highest cure and satisfaction rates of all surgeries we perform for prolapse; they also have relatively low risk.8 Recurrent prolapse after an obliterative procedure is rare; most of the dissatisfaction relates to postoperative voiding difficulties or persistent or de novo urinary incontinence.
DR. KARRAM: I also prefer an obliterative procedure. I see no reason to bring in the cost and potential for complications that mesh repair entails. An obliterative procedure should produce an anatomic success rate close to 100%, with minimal complications. It also can be performed quickly with minimal anesthesia and convalescence.
DR. LUCENTE: My response is based on a clinical study that my associate, Dr. Miles Murphy, has performed, comparing a transvaginal mesh procedure with a LaForte operation for severe pelvic organ prolapse.9 Both patient groups were well satisfied with the result, and success rates were comparable. However, the group that underwent the transvaginal mesh procedure had a shorter operative time.
As a result of these studies, we tend to prefer transvaginal mesh repair. Even though the woman may be sexually inactive, the procedure preserves vaginal function, and we all know that life has a way of being unpredictable. Her situation may change so that she once again desires sexual function.
However, for a very elderly woman—one in her late 80s or 90s—who has severe or extreme prolapse with a very large procidentia and vaginal length measuring, say, 13 cm beyond the introitus, I do prefer an obliterative procedure.
DR. RAZ: I agree. I would not offer a sexually inactive patient an obliterative procedure. You never know what the future will hold.
Mesh repair can be performed safely, provided the surgeon has good knowledge of anatomic landmarks and knows how to manage any potential complications that may arise.
MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.
SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.
VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.
MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
Mesh kits for repairing prolapse are proliferating like crazy, just as they did for midurethral sling procedures. But mesh augmentation of prolapse surgeries requires more than a prepackaged assortment of tools and materials. In this article, moderator Mickey M. Karram, MD, and a panel of nationally recognized urogynecologists and urologists describe the literature on mesh augmentation and discuss indications, contraindications, techniques, applicable cases, and the considerable training required.
In Part 2, which will appear in the February issue of OBG Management, the panel tackles the thorny topic of complications, including erosion, extrusion, foreshortening of the vagina, dyspareunia, and pain. Their discussion focuses on ways to avoid these problems, and methods for correcting them.
Do we have enough data?
DR. KARRAM: To start, let’s quickly review the peer-reviewed literature on the use of mesh augmentation during surgery for pelvic organ prolapse.
DR. WALTERS: Until recently, most data concerned open abdominal sacrocolpopexy (ASC) using polypropylene or Merseline mesh. There is significant clinical experience with this operation, and multiple cohort studies show long-term cure rates of 78% to 100% for apical prolapse.1
At least two randomized controlled trials have compared open ASC with sutured vaginal colpopexy procedures, and ASC is certainly equal to—perhaps better than—all transvaginal sutured repairs.2,3
With ASC, most recurrences affect the distal half of the vagina and involve one or more of the following:
- anterior or posterior vaginal wall prolapse (or both)
- stress urinary incontinence (SUI)
- distal rectocele.1,3
Mesh erosion occurs in 3.4% of cases and is usually easily managed.1 Other complications, including bowel injury, tend to be related to access, regardless of whether the operation is performed via laparotomy or laparoscopy.
Robotic sacrocolpopexy has become popular in recent years, and we will probably see data on this approach as we gain experience.
When it comes to vaginal mesh kits, the peer-reviewed literature is just beginning to expand, with many studies being presented at international meetings. For anterior and, possibly, apical vaginal prolapse, the cure rate after use of a mesh kit appears to be as high as, or higher than, the rate for sutured repairs.4 This high rate of anatomic cure is balanced somewhat by additional cost and complications involving mesh and the kits.
For posterior vaginal wall prolapse and rectocele, I firmly believe, based on our research and that of others, that sutured repairs are superior to graft-augmented surgery.5
DR. KARRAM: What are the indications and contraindications for mesh augmentation of prolapse repair ( FIGURES 1 and 2 )?
DR. LUCENTE: I believe mesh is indicated in any patient in need of surgical repair of pelvic organ prolapse who is seeking optimal durability and is willing to accept the known risks of the surgery.
The issue becomes more complex when it comes to contraindications. Absolute contraindications are fairly obvious; they include medically unstable patients and those who may have an inactive infectious process within the pelvis or even undiagnosed abnormal uterine bleeding.
At our center, because the potential for dyspareunia and pelvic discomfort is our biggest concern, we have developed a profile of the patient who is more likely to develop these complaints. The profile includes any patient who has a chronic pain disorder of any type, but especially chronic pelvic pain disorders such as endometriosis and vulvodynia. Other risk factors appear to be a history of pelvic surgery involving any permanent material, suture or mesh, and young age.
So if we have a patient in her late 30s who has undergone reconstructive surgery using permanent sutures and who has an element of chronic pelvic pain, we would counsel her strongly to consider surgical options other than the use of synthetic mesh.
DR. WALTERS: The main indications for mesh-augmented prolapse repair are recurrent posthysterectomy vaginal vault prolapse, for which I usually perform ASC, and recurrent cystocele or anteriorapical prolapse, for which I use one of the anterior mesh kits.
I still think sutured repairs—by that, I mean uterosacral ligament or sacrospinous colpopexy with sutured rectocele repair—work best for recurrent posterior wall and posteriorapical prolapse. I don’t use mesh augmentation for rectocele.
The main contraindication to mesh augmentation, as I see it, is a history of mesh complications. If I am repairing a mesh complication such as erosion or pain, I do not place another mesh.
Medical issues that might increase mesh complications, such as diabetes, steroid use, or severe vaginal atrophy, would, at the very least, make me consider carefully whether mesh augmentation is appropriate. The literature is not clear on this, so mesh could still be used if the surgeon thinks it is necessary.
DR. KARRAM: I haven’t found a definitive indication for mesh augmentation. We have used biologic meshes empirically, but I am not convinced that they really add long-term durability, regardless of whether they are used in the anterior or posterior vaginal segment.
Our published durability rate for traditional suture-type repairs is in the range of 85% at 5 years out.6 Even if I assumed that mesh would give me 100% 5-year durability, this rate would have to be at the expense of some erosion, pain, and other complications unique to mesh. I do not think that the potential improvement in durability is worth these potential complications.

FIGURE 1 When the pelvic support system is intact, prolapse is rare
In the normal pelvis, organs are supported by a complex web of muscles, fascia, and connective tissue. 
FIGURE 2 Mesh augmentation seeks to enhance the durability of repair
One type of mesh in final position. Mesh-augmented repair restores the vaginal apex and lends support to the walls of the vagina.
DR. KARRAM: If you are doing a lot of mesh repairs, you are obviously content with the results and feel that the few complications you are seeing are outweighed by the advantages mesh confers. How do you avoid extrusion and avert creation of a painful vagina?
DR. RAZ: Most of our cases are recurrent prolapse after failed vaginal or abdominal repair. I am indeed using a significant amount of soft polypropylene mesh for reconstructive procedures. As with the use of any other synthetic material, low-grade infection can develop after a few weeks or months. I use copious irrigation with antibiotic solution during reconstruction.
To avoid extrusion, I perform deep, rather than superficial, dissection of the vaginal wall to allow for better coverage of the mesh. For posterior mesh reconstruction, I cover the mesh with pararectal fascia to prevent erosion.
For mesh-augmented procedures, I cut the mesh myself in the operating room ( FIGURE 3 ). For a sling, I use a 10 cm × 1 cm soft polypropylene mesh. For a grade 3 or 4 cystocele, I use a trapezoid of soft polypropylene mesh with several points of fixation:
- at the sacrouterine ligament
- lateral to the obturator fascia
- distal to the bladder neck.
I always repair the vault at the same time.
For vault prolapse, I use a segment of soft polypropylene mesh in the shape of an apron with two arms (1 cm × 4 cm) and a central segment (4 cm × 7 cm). I support the vault using number 1-0 delayed absorbable suture and mesh. From outside the vaginal wall, in the posterolateral deep vaginal wall (inside the peritoneum), I incorporate the origin of the sacrouterine ligament and one arm of the mesh in the groove between the colon and levator ani, 15 cm from the introitus. I bring the suture 1 cm from the original entrance. A separate set of sutures brings the perirectal fascia together with the sacrouterine ligaments and perivesical fascia to close the peritoneal cavity. I tie the vault-suspension sutures, providing support to the cuff in a high posterior position (12 to 15 cm from the introitus).
In selected cases of significant recurrent rectocele, I use a rectangle of soft polypropylene mesh anchored to the origin of the sacrouterine ligament and distal to the perineal membrane. The mesh is covered by the pararectal fascia.
We have not seen vaginal, urethral, or bladder erosion in 1,800 cases of our distal urethral Prolene sling procedure using 10 cm × 1 cm soft mesh. In patients who have significant cystocele, vault prolapse, and recurrent rectocele, our vaginal erosion rate is 3%. We have never encountered rectal, bladder, or bowel perforation using our technique.
DR. LUCENTE: We often use mesh and are more than simply content with our results—we are extremely pleased, and so are our patients. Having said that, our techniques have definitely evolved over the past few years, as we’ve focused on how to decrease exposure and, more recently, optimize sexual function and vaginal comfort.
First, to avoid exposure, the most critical step is precise hydrodissection and distention of the true vesicovaginal space. This step can only be achieved through careful tactile guidance of the needle tip into the space, where it should remain while hydrodissection is performed. Always remember, sharp dissection “follows” hydrodissection. If you place the needle bevel within the vaginal wall, you will “split” the vaginal wall—as during standard colporrhaphy—which will lead to a high exposure rate.
Second, to avoid dyspareunia, it’s essential to pay close attention to POP-Q measurements, especially vaginal length, to ensure that the reconstruction restores the same length without foreshortening. This approach entails leaving the cervix in most patients who have a shorter vagina, and making sure that the mesh is secured above the ischial spine in younger, sexually active patients who have demonstrated a higher risk of postoperative deep, penetrating dyspareunia, compared with older, less sexually active patients.
Also paramount is to ensure that you have manually displaced the vagina inwardly as much as possible before deploying or setting the mesh. If you simply try to suture secure the mesh with the vagina incised open, without the ability to deploy the mesh with a closed, displaced vagina (to mimic deep penetration), it is difficult, if not impossible, to properly set the mesh for optimal comfort.
In the early days of midurethral pubovaginal slings using polypropylene, the adage was “looser is better than tighter.” This is even truer for transvaginal mesh.
DR. KARRAM: Dr. Walters, please describe your current surgical procedure of choice without mesh and explain why you haven’t adopted mesh for routine repairs.
DR. WALTERS: About 20% of my prolapse surgeries—usually for posthysterectomy or recurrent vaginal vault prolapse—involve ASC with placement of polypropylene mesh. I perform most of these cases through a Pfannenstiel incision, but I’ve also done them laparoscopically. Several of my partners perform ASC laparoscopically and robotically.
For the other 80% of my patients who have prolapse, I perform repairs transvaginally, usually using high bilateral uterosacralligament vaginal-vault suspension. We have learned to suture higher and slightly more medial on the uterosacral ligaments to attain greater vaginal depth and minimize ureteral obstruction. We use two or three sutures on each uterosacral ligament, usually a combination of permanent and delayed absorbable sutures.
I am also performing more sacrospinous ligament suspensions because this operation is being studied by the Pelvic Floor Disorders Network. Properly performed, it is an excellent surgery for apical prolapse. But, as with most of our surgeries for prolapse, recurrent anterior wall prolapse remains a problem.
Like you, Dr. Karram, we’ve studied our group’s anatomic and functional outcomes very carefully for more than 10 years and are mostly satisfied with our cure and complication rates. Although our anatomic outcomes with these surgeries are not always perfect, our reoperation rate for prolapse is only about 5%, with a high level of satisfaction in 88% to 92% of patients.
DR. RAZ: Unaugmented reconstruction fails in more than 30% of cases. Some patients who have significant prolapse and attenuated tissue think that this tissue will become healthier or stronger after reconstructive surgery, but that isn’t the case. In these situations, excision and plication make no clinical sense.
The problem is that we have yet to identify the ideal surrogate for poor-quality tissue. Most of us use polypropylene mesh in different variations. We need a better material that will be nonimmunogenic, well tolerated, and easily incorporated without erosion. Xenograft-like derivatives of dermis, or allografts such as cadaveric fascia, have failed over the long term because the body reabsorbs the graft without forming any new connective tissue.

FIGURE 3 Mesh can be cut in the OR to custom-fit a patient
Hand-cut mesh and points of placement.
PHOTO: SHLOMO RAZ, MD
Is a kit a valuable aid?
DR. KARRAM: If a surgeon wants to augment a repair, what are the advantages of a packaged mesh kit, compared with simply cutting the mesh and performing surgery without a kit?
DR. WALTERS: The advantages of a packaged mesh kit are the convenience involved and the ability to consistently perform the same operation with the same product. That facilitates learning, teaching, and research. It also helps us understand the published literature a little better because “custom” prolapse repairs are operator-dependent and difficult to apply generally to a population of surgeons.
These advantages are most clearly apparent with midurethral sling mesh kits, which have almost revolutionized surgery for stress incontinence. I don’t believe mesh kits for prolapse are there yet, but they certainly have potential.
DR. RAZ: I’m opposed to the use of kits. They are industry-driven. One company has made $1 billion selling them. Imagine a patient who undergoes placement of a sling kit ($1,000), cystocele kit ($1,500), and posterior mesh kit ($1,500). How can our healthcare system sustain this burden, especially when there is no real evidence that a kit improves the operation, and given the incredible complication rate that we see?
Moreover, the kits contain a single-use needle and passer and a precut segment of polypropylene mesh. But every patient is different and requires a unique size or shape of mesh. I don’t believe that a surgeon who knows pelvic anatomy needs a kit to perform mesh-augmented reconstruction. We can buy the same segment of mesh for $200 to $400, cut it as needed, and perform the same operation advertised by industry.
For surgeons who prefer a kit, the tools that are included should be made reusable.
DR. LUCENTE: In my opinion, the primary advantage of a commercially available transvaginal mesh delivery system—notice, I avoided the word “kit,” because I think there are plenty of negative connotations associated with it—is the ability to deliver the mesh in a “tension”-free manner.
One alternative that many people pursue is cutting the mesh to size and using sutures to hold it in place while tissue ingrowth occurs. However, the hernia literature suggests that suturing mesh in place increases the risk of postoperative discomfort at the site of implantation. The true cause of the discomfort remains unclear, but it is thought to arise from nerve tethering or traction at the pre-committed points of attachment before the host tissue and mesh interface have adjusted or settled with tissue ingrowth.
All neuropathic complications of mesh implantation have been shown in the current hernia literature to be increased with the use of sutures.7 Also, as previously mentioned, it is extremely difficult to set or adjust the mesh with the vaginal incision remaining “open,” which is a downside to suture techniques.
What training is necessary to use a kit?
DR. KARRAM: Mesh kits are aggressively promoted by industry, with close to half a dozen different kits to be available soon. What is the minimum amount of training one should have before utilizing these kits?
DR. WALTERS: The surgeon should at least know how to perform traditional sutured prolapse repairs and SUI surgery and be able to perform cystoscopy. Ideally, the surgeon should undergo training on a cadaver with a skilled and experienced user of the mesh kit. The surgeon also should carefully review the risks and benefits of mesh kits with the patient and inform the patient that he or she is in the early learning curve of a particular surgery. The informed patient should have a right to refuse mesh-augmented prolapse surgery after the consent process.
DR. LUCENTE: I’m glad you asked this question. I strongly believe that surgical expertise and proficiency within gynecology need to be more effectively addressed by us all. We have a situation in our field in which techniques and technology are widening the gap between what is possible and what the surgeon is comfortable doing safely.
It’s incumbent on all of us, especially those who are in a leadership position as a chairperson or chief of a division, to work with our physician staff and faculty to optimize surgical skill and patient outcomes, including safety, with new technologies.
As for the minimal amount of training needed, that’s extremely variable. It depends on the current skill set of the physician and his or her ability to pick up the mechanics of the surgery as it is taught through a cadaver lab or preceptorship. It’s regrettable that some physicians lack the objectivity and insight to judge their own skill set. This, again, is the time for a chairperson or chief of a division to step up to the plate and ensure proper credentialing and demonstration of proficiency.
It is unrealistic to expect industry to decide who should or should not utilize this truly breakthrough technology. That is our responsibility as physicians.
DR. KARRAM: At a minimum, I think any surgeon utilizing a kit should have a firm understanding of pelvic floor anatomy and experience performing traditional repairs:
- intraperitoneal procedures such as Mc-Call culdoplasty and uterosacral suspension
- sacrospinous suspension
- retropubic procedures and anti-incontinence operations such as pubovaginal slings.
This three-dimensional understanding of the pelvic floor is mandatory if one is to assume that blind passage of trocars through potentially dangerous spaces is the wave of the future.
DR. RAZ: You need to be a pelvic surgeon, know your anatomy, and know how to manage complications if you are going to use one of these kits. You should stick to the surgery that works best in your hands. Industry cannot teach you to be a good pelvic surgeon; it takes lifelong experience.
DR. KARRAM: If you have a patient who is sexually inactive with pelvic organ prolapse, would you prefer a mesh repair or an obliterative procedure? And why?
DR. WALTERS: If the patient is sexually inactive—especially if she is older and definitely will not be in the future—it makes absolutely no sense to perform a mesh-augmented repair. A traditional, somewhat tight, sutured repair works fine in this setting and carries very low risk.
In fact, our group and others have found that, in carefully selected patients, partial colpectomy and colpocleisis procedures (without grafts) have among the highest cure and satisfaction rates of all surgeries we perform for prolapse; they also have relatively low risk.8 Recurrent prolapse after an obliterative procedure is rare; most of the dissatisfaction relates to postoperative voiding difficulties or persistent or de novo urinary incontinence.
DR. KARRAM: I also prefer an obliterative procedure. I see no reason to bring in the cost and potential for complications that mesh repair entails. An obliterative procedure should produce an anatomic success rate close to 100%, with minimal complications. It also can be performed quickly with minimal anesthesia and convalescence.
DR. LUCENTE: My response is based on a clinical study that my associate, Dr. Miles Murphy, has performed, comparing a transvaginal mesh procedure with a LaForte operation for severe pelvic organ prolapse.9 Both patient groups were well satisfied with the result, and success rates were comparable. However, the group that underwent the transvaginal mesh procedure had a shorter operative time.
As a result of these studies, we tend to prefer transvaginal mesh repair. Even though the woman may be sexually inactive, the procedure preserves vaginal function, and we all know that life has a way of being unpredictable. Her situation may change so that she once again desires sexual function.
However, for a very elderly woman—one in her late 80s or 90s—who has severe or extreme prolapse with a very large procidentia and vaginal length measuring, say, 13 cm beyond the introitus, I do prefer an obliterative procedure.
DR. RAZ: I agree. I would not offer a sexually inactive patient an obliterative procedure. You never know what the future will hold.
Mesh repair can be performed safely, provided the surgeon has good knowledge of anatomic landmarks and knows how to manage any potential complications that may arise.
1. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
2. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421;discussion 1421-1422.
3. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
4. Murphy M. Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines on vaginal graft use from the Society of Gynecologic Surgeons. Obstet Gynecol. 2008;112:1123-1130.
5. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical procedures including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
6. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 2006;108:255-263.
7. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322-332.
8. Barber MD, Amundsen C, Paraiso MFR, Weidner A, Romero A, Walters MD. Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J. 2007;18:799-806.
9. Murphy M, van Raalte H, Mercurio E, Haff R, Wiseman B, Lucente VR. Incontinence-related quality of life and sexual function following the tension-free vaginal tape vs the “inside-out” tension-free vaginal tape obturator. Int Urogynecol J. 2008;19:481-487.
1. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
2. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421;discussion 1421-1422.
3. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
4. Murphy M. Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines on vaginal graft use from the Society of Gynecologic Surgeons. Obstet Gynecol. 2008;112:1123-1130.
5. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical procedures including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
6. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 2006;108:255-263.
7. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322-332.
8. Barber MD, Amundsen C, Paraiso MFR, Weidner A, Romero A, Walters MD. Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J. 2007;18:799-806.
9. Murphy M, van Raalte H, Mercurio E, Haff R, Wiseman B, Lucente VR. Incontinence-related quality of life and sexual function following the tension-free vaginal tape vs the “inside-out” tension-free vaginal tape obturator. Int Urogynecol J. 2008;19:481-487.
6 office tests to assess ovarian reserve, and what they tell you
The University of Medicine and Dentistry of New Jersey (UMDNJ) owns a patent relating to the use of anti-Müllerian hormone/Müllerian inhibiting substance for predicting ovarian response in women with infertility. The patent is based in part on work that Dr. Seifer carried out while employed at UMDNJ. In accordance with UMDNJ policy, Dr. Seifer, a named inventor on this patent, assigned his interest in the invention to UMDNJ. UMDNJ has a licensing agreement with Diagnostic Systems Laboratory for the use of the claimed invention. Dr. Seifer receives a portion of the royalties, as determined by UMDNJ policy, that UMDNJ gains from this licensing agreement.
CASE: Borderline test result prompts referral
A 36-year-old nulliparous woman is seen in your office for evaluation after 6 months of infertility. She is ovulatory, and has been using an ovulation-prediction kit to time intercourse. You learn that she had Chlamydia trachomatis infection in the distant past, but elicit no other significant medical or surgical history. She reports that she smoked approximately one pack of cigarettes a day for 15 years but gave up smoking 5 years ago.
You order a hysterosalpingogram, followed by day 3 testing of follicle-stimulating hormone (FSH). The hysterosalpingogram is normal; the FSH level is 7.5 mIU/mL and the estradiol level is 30 pg/mL—both in the normal range.
The patient asks for testing of anti-Müllerian hormone (AMH; also known as Müllerian-inhibiting substance) because she has read that it is a new marker of fertility. The result is 0.5 ng/mL, a borderline value. After reviewing these results, you refer her to a reproductive endocrinologist for further management.
Was the test for AMH indicated? And is this referral appropriate?
The referral is entirely appropriate, even though the patient has not been trying to conceive for a full year. Why? The AMH value suggests that her ovarian reserve is in early decline. She would benefit from evaluation by a subspecialist who can review the entire spectrum of treatments, including aggressive options such as ovulation induction and in vitro fertilization (IVF), to optimize her reproductive success.
This article reviews the various biomarkers available to assess ovarian reserve in women who experience infertility:
- day 3 (basal) FSH
- clomiphene citrate challenge
- gonadotropin-releasing hormone (GnRH) agonist stimulation
- inhibin-B
- antral follicle count (AFC)
- AMH.
The AFC and AMH tend to detect the earliest changes in ovarian reserve, followed, sequentially, by inhibin-B, the clomiphene citrate challenge test (CCCT), and basal FSH.
The tests we describe are used primarily to assess treatment prognosis in infertile women. In time, however, appropriate population screening of ovarian reserve may be feasible to provide many more women with information about their reproductive potential and help them shape their life plan.
What makes a test valuable?
Ovarian reserve describes a woman’s reproductive potential—specifically, the number and quality of oocytes she possesses.1 Biochemical tests of ovarian reserve emerged during the rise of assisted reproductive technologies (ART) in the late 1980s to predict both responsiveness to superovulation drugs and the odds of pregnancy with treatment.
Ideally, a test that assesses ovarian reserve should be affordable, straightforward, rapidly interpretable, and minimally invasive. It also should be able to detect changes that begin early in reproductive life. To be applicable to large populations of reproductive-age women, it should be of use anytime in the menstrual cycle, and should provide reproducible and highly accurate assessment of the reproductive aging process.
Our ability to offer tests that accurately measure ovarian reserve has a significant impact on women at risk of infertility and early menopause and on those who choose to delay childbearing for personal (nonmedical) reasons. These tests have become increasingly relevant because women are choosing to have their first child at a later age than their counterparts did 20 years ago:
- In 1980, 40% of women having their first baby were younger than 25 years, and only 5% were older than 35
- In 2000, 25% of women were younger than 25 when their first child was born, and 15% were older than 35.
Who should be tested?
Ovarian reserve is a complex clinical phenomenon that is influenced by age, genetics, and environmental variables. The decline in a woman’s ovarian reserve over time is irreversible; the trajectory of this decline is fundamental to the odds of fertility with age and the timing of the menopausal transition. At present, the markers used most often in clinical practice have some utility but also suffer from several drawbacks ( TABLE ).
For the general practitioner performing an infertility evaluation, we recommend focusing on the following groups of women for ovarian reserve testing:
- women over 30 years of age
- women with a history of exposure to a confirmed gonadotoxin, i.e., tobacco smoke, chemotherapy, radiation therapy
- women with a strong family history of early menopause or premature ovarian failure
- women who have had extensive ovarian surgery, i.e., cystectomy and unilateral oophorectomy.
Testing tends to have the highest yield in these groups. Women who have abnormal results should be referred to a reproductive endocrinologist for further evaluation and treatment.
The six tests are described below.
TABLE
How six markers of ovarian reserve stack up
| Test (year described) | Timing | Intracycle and intercycle variability | Sensitivity (specificity) | Reflects changes in ovarian reserve | Normal levels | Confounders | Out-of-pocket cost |
|---|---|---|---|---|---|---|---|
| Basal follicle-stimulating hormone (FSH) (1988) | Day 3 of menstrual cycle | Clinically significant | 7%–8% (98%–99%) | Late | • Early follicular phase FSH level <10 mIU/mL • Estradiol level <80 pg/mL | • High estradiol level (decreases) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $125–$150 |
| Clomiphene challenge test (1989) | Days 3 and 10 of menstrual cycle | Clinically significant | 25%–40% (98%–99%) | Late | • Day 3 FSH level <10 mIU/mL; day 3 estradiol level <80 pg/mL • Day 10 FSH level <10 mIU/mL | • High day 3 estradiol level (decreases day 3 FSH) • Low day 10 estradiol (increases day 10 FSH) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $550–$600 |
| GnRH agonist (1988) | Early follicular phase of menstrual cycle | Clinically significant | 32%–89% (79%–97%) | Late | Variable | • Oral contraceptives (decrease estradiol levels) • Pregnancy (increases estrogens) | $300–$350 |
| Inhibin-B (1997) | Early follicular phase of menstrual cycle | Clinically significant | 33%–81% (29%–95%) | Early | Variable in the literature; normal cutoffs range from ≥45–80 pg/mL | • Obesity (decreases) • PCOS (increases) • Exogenous FSH administration (increases) • Oral contraceptive use (decreases) | $150–$200 |
| Antral follicle count (1997) | Early follicular phase of menstrual cycle | Clinically significant (includes interobserver variability) | 8%–60% (33%–96%) | Earliest | ≥5–10 total antral follicles | • Oral contraceptive use (decreases) • Polycystic ovary syndrome (PCOS) (increases) | $300–$500 |
| Anti–Müllerian hormone/Müllerian-inhibiting substance (2002) | At any time; not cycle-dependent | Minimal | 49%–76% (89%–94%) | Earliest | >0.7 ng/mL | • PCOS (increases) • Obesity (decreases) • Exogenous FSH administration (decreases) | $150–$400 |
1 | Basal FSH—widely used but only moderately informative
Day 3 FSH and the CCCT are the most widely used measures of ovarian reserve in ART practice. The use of early follicular-phase FSH as a marker of ovarian reserve and fertility was proposed 20 years ago with the emergence of IVF.2-4 The test is an indirect assessment of ovarian reserve in that it measures pituitary production of FSH in response to feedback from ovarian hormones. Estradiol and inhibin-B reach a nadir early in the menstrual cycle; measuring FSH on day 3 offers a glimpse of the functioning of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise later in the cycle ( FIGURE 1 ).5,6
FIGURE 1 The HPO axis
The FSH level opens a window onto the function of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise in the cycle. Women who have normal ovarian reserve have sufficient ovarian hormone production early in the menstrual cycle to maintain FSH levels within the normal range. Conversely, a “monotropic” elevation in FSH—one that is unaccompanied by a rise in luteinizing hormone (LH)—reflects poor hormone production from an aging pool of ovarian follicles and disinhibition of FSH production.5,6
FSH measurements are typically combined with estradiol to enhance the sensitivity of testing ( FIGURE 2, ). Premature elevations of estradiol early in the follicular phase are driven by rising FSH levels in women with declining ovarian reserve. Abnormally elevated estrogen levels then feed back negatively on pituitary production of FSH and mask an elevation that might otherwise reveal diminished ovarian reserve. Measurement of both FSH and estradiol on cycle day 3 may therefore help decrease the incidence of false-negative testing.
Commonly cited criteria for normal ovarian reserve are:
- early follicular phase FSH, <10 mIU/mL
- estradiol, <80 pg/mL1
It is extremely important to note, however, that these are general guidelines and that cutoffs are both laboratory- and practice-specific.
FIGURE 2 Monthly and lifetime variations in estradiol and FSH
How 17ß-estradiol and follicle-stimulating hormone levels vary over the menstrual cycle (top) and a woman’s lifetime (bottom).
2 | Clomiphene citrate—more sensitive than FSH testing
Like basal FSH testing, the CCCT is an indirect assessment of ovarian reserve. Unlike FSH testing, the CCCT is provocative. It involves administration of 100 mg of clomiphene citrate (Clomid) on days 5 through 9 of the menstrual cycle, with FSH and estradiol measured on days 3 and 10. Once clomiphene citrate is administered, FSH and LH levels rise, followed by an increase in estradiol and inhibin. Evidence suggests that the smaller follicular cohorts in women with diminished ovarian reserve produce less inhibin-B and estradiol and, therefore, less negative feedback on clomiphene-induced pituitary FSH release.6,7 The result: persistent elevation of the day 10 FSH value and a positive screen for diminished ovarian reserve.
In some women, day 10 FSH is elevated even after a normal day 3 value. This makes the CCCT more sensitive than basal FSH testing; it can identify women who might go unrecognized if evaluated by day 3 FSH and estradiol levels alone.
More expensive and labor-intensive than the alternatives
Interpretation of the CCCT requires that FSH and estradiol both be assessed on days 3 and 10. An elevated FSH (≥10 mIU/mL) on either day indicates diminished ovarian reserve. As with basal FSH testing, elevated estradiol (≥80 pg/mL) on day 3 is considered abnormal. The day 10 estradiol value of the CCCT reflects whether or not clomiphene citrate was administered appropriately, and should be elevated. However, the significance of the day 10 estradiol level has been debated with respect to its predictive value for pregnancy in infertile populations.8
The addition of day 10 FSH assessment improves the sensitivity of the CCCT over basal FSH measurement, but makes it a more expensive and labor-intensive test ( TABLE ).5,6 The CCCT involves administration of clomiphene citrate, a safe drug (though it can have side effects), and two blood draws instead of one. Nevertheless, both tests are relatively noninvasive, rapid measures of ovarian reserve.
Drawbacks of the tests
Both basal FSH testing and the CCCT are widely used, although support for their ability to predict ovarian reserve in the infertile population has been challenged recently. Newer data demonstrate that these tests are limited in their ability to predict outcome (pregnancy and response to superovulation drugs) in all but a narrow group of patients undergoing IVF. Performance is particularly limited in:
Additional drawbacks of basal FSH testing and the CCCT include:
- significant variability of test results from cycle to cycle (intercycle variability)
- limited time frame within which the tests can be performed (intracycle variability).
The basal FSH test and CCCT have high specificity (98% to 99% for each) as an assessment of reproductive performance in infertile women and generate few false-positive results.5,6 However, the high screen cutoffs that allow for such specificity come at a price: Few women will screen positive, and sensitivity of the tests is low (between 7% and 8% for basal FSH and between 25% and 40% for the CCCT). Such low sensitivity means that many women will not conceive after infertility treatment despite a normal test result.5,11 Overall, the tests are not highly informative for many women who get tested.
Once abnormal, normal results are meaningless
Once an FSH level or the CCCT has ever been abnormal, the patient has diminished ovarian reserve; normal values in subsequent menstrual cycles do not improve the odds of pregnancy with treatment.14 This fact can be a significant source of confusion and frustration for patients.
3 | GnRH agonist stimulation —no better than FSH testing
This test was developed in the search for a very sensitive assessment of ovarian reserve. It was designed to uncover subtle abnormalities in pituitary and ovarian dynamics. It involves administering a gonadotropin-releasing hormone (GnRH) agonist such as leuprolide acetate (Lupron) on day 2 or 3 of the menstrual cycle and measuring pituitary and ovarian hormone responses.5,15
One group of investigators demonstrated a correlation between stimulated estradiol levels and responsiveness during IVF,16 but other studies have shown that the test does not perform significantly better than day 3 FSH in predicting ovarian reserve.17,18
The sensitivity of GnRH agonist testing for pregnancy is moderate (32% to 89%); specificity ranges from 79% to 97%.19
4 | Inhibin-B—not helpful when used alone
This glycoprotein hormone produced by granulosa cells of developing follicles is a direct measure of ovarian reserve when assessed in the early follicular phase of the menstrual cycle.20 Women treated with IVF who have a low inhibin-B level—particularly when using cutoffs below the range of 45–80 pg/mL—have been shown to respond poorly to superovulation and have a lower pregnancy rate than women with high inhibin-B.21,22 One group of investigators demonstrated that women with clinical evidence of diminished ovarian reserve but a normal FSH level also had low inhibin-B production, suggesting that it may be a more sensitive marker than FSH.22
Inhibin-B testing involves a simple blood draw. However, the test has been incorporated into clinical assessment of ovarian reserve only to a limited degree, due to the lack of reliable assays and controversy concerning its prognostic value.23
Because of these limitations, routine testing of serum inhibin-B in isolation of other markers of ovarian reserve is not recommended.
5 | Antral follicle count—good predictor of IVF outcome
Transvaginal ultrasonographic determination of the number of ovarian follicles that measure between 2 mm and 10 mm in diameter in the early follicular phase of the cycle yields the AFC. As a direct marker of the cohort of growing follicles in the early menstrual cycle, the AFC is believed to correlate strongly with the number of primordial follicles present in the ovary and, therefore, ovarian reserve. Total AFCs of less than 5 to 10 are suggestive of diminished ovarian reserve.24,25
In IVF cycles, AFC has proven to be an accurate predictor of number of oocytes retrieved, risk of cycle cancellation, and odds of conception.24,25 Some investigators have even suggested that, compared with other markers of ovarian reserve, AFC is the best independent predictor of outcome in IVF cycles.7,26-27
In a group of normally cycling women with proven fertility, AFC also showed a strong correlation with age, declining slowly until age 37 and more rapidly thereafter.28,29
AFC sensitivity for pregnancy is moderate and varies widely in published reports (8% to 60%), whereas specificity tends to be higher (33% to 96%).19
Drawbacks of AFC
- Because of the need to perform transvaginal ultrasonography, AFC is a more invasive and often more expensive test than hormonal biomarkers
- Accurate assessment of AFC requires an experienced sonographer and can be limited in patients who have had pelvic surgery or uterine fibroids and in those who are obese
- Moderate interobserver and intercycle variability of AFC determinations limits its reproducibility29,30
- As with basal FSH measurement, the intercycle variability of AFC does not correlate well with IVF outcome in individual patients.30
6 | Anti-Müllerian hormone— many advantages
The drawbacks of the tests just described— e.g., intercycle variability, lack of uniform cutoffs, and limited ability to predict IVF outcomes—make the development of more reliable measures of ovarian reserve a priority in reproductive medicine. AMH is a highly promising marker that appears to have many advantages over other tests and may have the greatest power to predict ovarian aging in women of reproductive age.
How it works
AMH is a glycoprotein growth factor and a member of the transforming growth factor-ß superfamily.31 It is primarily produced by the pool of early-growing follicles, which are believed to serve as a proxy for the number of primordial follicles in the ovary. The number of primordial follicles at a given point in time represents the ovarian reserve. AMH levels above 0.7 ng/mL are considered normal; values between 0.3 ng/mL and 0.7 ng/mL are consistent with borderline ovarian reserve, according to 2007 data from Reprosource Corp.
AMH has been studied as a marker of ovarian reserve for 6 years, with multiple reports describing declines in levels with age and with diminishing oocyte numbers. It is undetectable at menopause.32
The age-related decline in AMH is gradual but measurable even in young women, consistently preceding changes in other markers of ovarian reserve such as FSH and inhibin-B.32-35 The longitudinal changes in AMH have been demonstrated in ovulatory premenopausal women and healthy volunteers with proven fertility.33,34 In one series of women followed over a mean of 4 years (ages 25 to 46), AMH testing was superior to day 3 FSH, inhibin-B, and AFC in its ability to predict the onset of cycle irregularity and the menopausal transition.33
Does it predict oocyte quality?
AMH has performed well as a biomarker, comparable in most series to AFC and superior to FSH. AMH levels are strongly correlated with the number of oocytes retrieved during IVF and the odds of cycle cancellation due to poor response35-41 —but does it accurately characterize oocyte quality, the other element of ovarian reserve?
Some reports have shown a strong association between AMH levels and surrogates of oocyte quality, including fertilization, oocyte morphology, embryo quality, and pregnancy and miscarriage rates,36-41 but others have not.42 Some reports demonstrate a relationship between AMH and some but not all surrogate markers of oocyte quality.40
Advantages of AMH
- It demonstrates minimal intracycle variability.32,43-45 Compared with other markers of ovarian reserve, which must be measured early in the follicular phase of the menstrual cycle, AMH can be assessed at random times, making it a more convenient method for patients and physicians
- It demonstrates minimal intercycle variability32,34
- AMH levels are not significantly affected by the hormonal changes of pregnancy, oral contraceptive use, or GnRH treatment, and can be measured in these settings.46,47
Utility of AMH is limited in PCOS and obesity
The ability to use AMH as a marker of ovarian aging in women who have polycystic ovary syndrome (PCOS) and in women who are obese may be limited by the ovulatory dysfunction in these populations. Circulating levels of AMH are higher in women with PCOS than in unaffected women, a finding thought to be indicative of oligo-ovulation and poor follicular development in polycystic ovaries.48-53
In a recent series investigating AMH levels in women with PCOS, AMH and the degree of insulin resistance were positively correlated, and the AMH level was negatively correlated with the number of menses in a year.49 The consistently positive correlation between AMH and PCOS may suggest a future role for this marker as a diagnostic tool.
In obese women who do not have PCOS, AMH production may be lower than in women of normal weight. In a recent series, normally cycling obese women in the later reproductive years were shown to have an AMH level 70% lower than those in women who were not obese.54 These differences have not been well studied in younger obese women.
Which test is best?
AMH may be preferable to the other tests to assess ovarian reserve because it can be measured any time during the menstrual cycle or between cycles. AMH measurement is also useful if a woman is taking oral contraceptives or leuprolide acetate because these medications may confound the results of the other test methods. In addition, AMH may be the earliest indicator of decline in ovarian reproductive function. As such, it may highlight cases that merit a search for other causes of infertility and make it possible to treat them in a timely manner.
Elevated AMH may reveal occult PCOS and warn of significant risk of ovarian hyperstimulation prior to ovulation induction with gonadotrophins, so that the clinician can plan smaller doses.
A normal female is born with 1 million to 2 million oocytes, a number that declines continuously, primarily through the process of follicular atresia. By the onset of puberty, the number of oocytes has declined to approximately 300,000. As a woman enters her late 30s, when the total number of oocytes is approximately 25,000, the pace of oocyte depletion begins to increase, as does the rate of spontaneous miscarriage.1,55,56
The effect of age on fertility is believed to arise from changes in both oocyte number and quality. Multiple investigators have found a greater frequency of cellular abnormalities in oocytes from older women.1,2,5,15,57
Although ovarian reserve declines with age in all women, women of similar ages can have very different degrees of ovarian reserve, and some women who have very poor ovarian reserve may never conceive, despite aggressive fertility treatment.
The biologic basis for differences in ovarian reserve among similar groups of women is not completely understood, but is probably rooted in genetic, lifestyle, and environmental factors that affect granulosa cell and oocyte function. Identifying sensitive biomarkers that can determine ovarian reserve independent of age is critical to predict fertility and age at menopause.5
1. Practice Committee of the American Society for Reproductive Medicine. Age and infertility in women. Fertil Steril. 2006;86:S248-S252.
2. Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu H-C. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50:298-307.
3. Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651-654.
4. Toner JP, Philiput CB, Jones GS, Muasher SJ. Basal follicle stimulating hormone level is a better predictor of in vitro fertilization outcome than age. Fertil Steril. 1991;55:784-791.
5. Barnhart K, Osheroff J. Follicle stimulating hormone as a predictor of fertility. Curr Opin Obstet Gynecol. 1998;10:227-232.
6. Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: the physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;69:474-477.
7. Yong PY, Baird DT, Thong KJ, McNeilly AS, Anderson RA. Prospective analysis of the relationships between the ovarian follicle cohort and basal FSH concentration, the inhibin response to exogenous FSH and ovarian follicle number at different stages of the normal menstrual cycle and after pituitary down-regulation. Hum Reprod. 2003;18:35-44.
8. Scott RT, Jr, Illions EH, Kost ER, Dellinger C, Hofmann GE, Navot D. Evaluation of the significance of the estradiol response during the clomiphene citrate challenge test. Fertil Steril. 1993;60:242-246.
9. Esposito MA, Coutifaris C, Barnhart KT. A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod. 2002;17:118-123.
10. Bancsi L, Broekmans FJM, Wol BWJ, Habbema DK, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003;79:1091-1100.
11. Jain T, Soules MR, Collins JA. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 2004;82:180-185.
12. Toner JP. Modest follicle-stimulating hormone elevations in younger women: warn but don’t disqualify. Fertil Steril. 2004;81:1493-1495.
13. Van Rooij IAJ, de Jong E, Broekmans FJM, Looman CWN, Habbeman DK, te Velde ER. High follicle-stimulating hormone levels should not necessarily lead to the exclusion of subfertile patients from treatment. Fertil Steril. 2004;81:1478-1485.
14. Scott RT, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54:297-302.
15. Bulkulmez O, Arici A. Assessment of ovarian reserve. Curr Opin Obstet Gynecol. 2004;16:231-237.
16. Ranieri DM, Quinn F, Makhlouf A, et al. Simultaneous evaluation of basal follicle-stimulating hormone and 17-beta-estradiol response to gonadotropin-releasing hormone analogue stimulation: an improved predictor of ovarian reserve. Fertil Steril. 1998;70:227-233.
17. Fujimoto VY, Klein NA, Battaglia DE, Bremmer WJ, Soules MR. The anterior pituitary response to a gonadotropin-releasing hormone challenge test in normal older reproductive age women. Fertil Steril. 1996;65:539-544.
18. Galtier-Dereure F, De Bouard V, Picto MC, et al. Ovarian reserve test with the gonadotrophin-releasing hormone agonist buserelin: correlation with in-vitro fertilization outcome. Hum Reprod. 1996;11:1393-1398.
19. Broekmans FJ, Fwee J, Hendricks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685-718.
20. Klein NA, Illingworth PJ, Groome NP, NcNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81:2742-2745.
21. Seifer DB, Lambert-Messerlian G, Hogan JW, et al. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67:110-114.
22. Seifer DB, Scott RT, Jr, Bergh PA, et al. Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin B before a rise in day 3 follicle-stimulating hormone. Fertil Steril. 1999;72:63-65.
23. Corson SL, Gutmann J, Batzer FR, Wallace H, Klein N, Soules MR. Inhibin-B as a test of ovarian reserve for infertile women. Hum Reprod. 1999;14:2818-2821.
24. Tomas C, Nuojua-Huttunen S, Martikainen H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum Reprod. 1997;12:220-223.
25. Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505-510.
26. Hung E, Tang OS, Ho PC. The significance of the number of antral follicles prior to stimulation in predicting ovarian responses in an IVF programme. Hum Reprod. 2000;15:1937-1942.
27. Bancsi LFJMM, Broekmans FJM, Eijkemans MJC, de Jong FH, Habbema JDF, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328-336.
28. Ng EH, Yeung WS, Fong DY, Ho PC. Effects of age on hormonal and ultrasound markers of ovarian reserve in Chinese women with proven fertility. Hum Reprod. 2003;18:2169-2174.
29. Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845-851.
30. Hansen KR, Morris JL, Thyer AC, Soules MR. Reproductive aging and the variability in the ovarian antral follicle count: application in the clinical setting. Fertil Steril. 2003;80:577-583.
31. Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685-698.
32. de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM. Anti-Müllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357-362.
33. van Rooij IAJ, Broekmans FJM, Scheffer GJ, et al. Serum anti-Müllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979-987.
34. van Rooij IAJ, Tonkelaar I, Broekmans FJ, et al. Anti-Müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601-606.
35. Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Müllerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20-24.
36. Silberstein T, MacLaughlin DT, Shai I, et al. Müllerian-inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159-163.
37. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum Müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468-471.
38. Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022-2026.
39. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum anti-Müllerian hormone/Müllerian-inhibiting substance appears to be a more discriminatory marker of ART outcome than follicular stimulating hormone, inhibin B or estradiol. Fertil Steril. 2004;82:1323-1329.
40. Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22:2414-2421.
41. Fanchin R, Mendez DH, Frydman N, et al. Anti-Müllerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796-1802.
42. Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Th omas CM, Braat DD. Anti-Müllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87:223-226.
43. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FM, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057-4063.
44. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Müllerian hormone throughout the menstrual cycle. Hum Reprod. 2006;21:3103-3107.
45. Tsepelidis S, Devreker F, Demeestere F, Flahaut I, Gervy A, Englert C. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837-1840.
46. La Marca A, Giulini Orvieto R, De Leo V, Volpe A. Anti-Müllerian hormone concentrations in maternal serum during pregnancy. Hum Reprod. 2005;20:1569-1572.
47. Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Müllerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196-201.
48. Al-Qahtani A, Groome NP. Anti-Müllerian hormone: Cinderella finds new admirers. J Clin Endocrinol Metab. 2006;91:3760-3762.
49. La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Müllerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970-971.
50. Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820-1836.
51. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-Müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957-5962.
52. Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum anti-Müllerian substance and other reproductive hormones in untreated women with polycystic ovary syndrome and endometriosis. Fertil Steril. 1997;67:962-965.
53. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240-245.
54. Freeman EW, Gracia CG, Sammel MD, Lin H, Lim LC, Strauss JF, 3rd. Association of anti-Müllerian hormone levels with obesity in later reproductive-age women. Fertil Steril. 2007;87:101-106.
55. Scott RT, Opsahl MS, Leonardi MR, Neall GS, Illions EH, Navot D. Life table analysis of pregnancy rates in a general infertility population relative to ovarian reserve and patient age. Hum Reprod. 1995;10:1706-1710.
56. Speroff L. Fritz M. eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
57. Lim AS, Tsakok MFH. Age-related decline in fertility: a link to degenerative oocytes? Fertil Steril. 1997;68:265-271.
The University of Medicine and Dentistry of New Jersey (UMDNJ) owns a patent relating to the use of anti-Müllerian hormone/Müllerian inhibiting substance for predicting ovarian response in women with infertility. The patent is based in part on work that Dr. Seifer carried out while employed at UMDNJ. In accordance with UMDNJ policy, Dr. Seifer, a named inventor on this patent, assigned his interest in the invention to UMDNJ. UMDNJ has a licensing agreement with Diagnostic Systems Laboratory for the use of the claimed invention. Dr. Seifer receives a portion of the royalties, as determined by UMDNJ policy, that UMDNJ gains from this licensing agreement.
CASE: Borderline test result prompts referral
A 36-year-old nulliparous woman is seen in your office for evaluation after 6 months of infertility. She is ovulatory, and has been using an ovulation-prediction kit to time intercourse. You learn that she had Chlamydia trachomatis infection in the distant past, but elicit no other significant medical or surgical history. She reports that she smoked approximately one pack of cigarettes a day for 15 years but gave up smoking 5 years ago.
You order a hysterosalpingogram, followed by day 3 testing of follicle-stimulating hormone (FSH). The hysterosalpingogram is normal; the FSH level is 7.5 mIU/mL and the estradiol level is 30 pg/mL—both in the normal range.
The patient asks for testing of anti-Müllerian hormone (AMH; also known as Müllerian-inhibiting substance) because she has read that it is a new marker of fertility. The result is 0.5 ng/mL, a borderline value. After reviewing these results, you refer her to a reproductive endocrinologist for further management.
Was the test for AMH indicated? And is this referral appropriate?
The referral is entirely appropriate, even though the patient has not been trying to conceive for a full year. Why? The AMH value suggests that her ovarian reserve is in early decline. She would benefit from evaluation by a subspecialist who can review the entire spectrum of treatments, including aggressive options such as ovulation induction and in vitro fertilization (IVF), to optimize her reproductive success.
This article reviews the various biomarkers available to assess ovarian reserve in women who experience infertility:
- day 3 (basal) FSH
- clomiphene citrate challenge
- gonadotropin-releasing hormone (GnRH) agonist stimulation
- inhibin-B
- antral follicle count (AFC)
- AMH.
The AFC and AMH tend to detect the earliest changes in ovarian reserve, followed, sequentially, by inhibin-B, the clomiphene citrate challenge test (CCCT), and basal FSH.
The tests we describe are used primarily to assess treatment prognosis in infertile women. In time, however, appropriate population screening of ovarian reserve may be feasible to provide many more women with information about their reproductive potential and help them shape their life plan.
What makes a test valuable?
Ovarian reserve describes a woman’s reproductive potential—specifically, the number and quality of oocytes she possesses.1 Biochemical tests of ovarian reserve emerged during the rise of assisted reproductive technologies (ART) in the late 1980s to predict both responsiveness to superovulation drugs and the odds of pregnancy with treatment.
Ideally, a test that assesses ovarian reserve should be affordable, straightforward, rapidly interpretable, and minimally invasive. It also should be able to detect changes that begin early in reproductive life. To be applicable to large populations of reproductive-age women, it should be of use anytime in the menstrual cycle, and should provide reproducible and highly accurate assessment of the reproductive aging process.
Our ability to offer tests that accurately measure ovarian reserve has a significant impact on women at risk of infertility and early menopause and on those who choose to delay childbearing for personal (nonmedical) reasons. These tests have become increasingly relevant because women are choosing to have their first child at a later age than their counterparts did 20 years ago:
- In 1980, 40% of women having their first baby were younger than 25 years, and only 5% were older than 35
- In 2000, 25% of women were younger than 25 when their first child was born, and 15% were older than 35.
Who should be tested?
Ovarian reserve is a complex clinical phenomenon that is influenced by age, genetics, and environmental variables. The decline in a woman’s ovarian reserve over time is irreversible; the trajectory of this decline is fundamental to the odds of fertility with age and the timing of the menopausal transition. At present, the markers used most often in clinical practice have some utility but also suffer from several drawbacks ( TABLE ).
For the general practitioner performing an infertility evaluation, we recommend focusing on the following groups of women for ovarian reserve testing:
- women over 30 years of age
- women with a history of exposure to a confirmed gonadotoxin, i.e., tobacco smoke, chemotherapy, radiation therapy
- women with a strong family history of early menopause or premature ovarian failure
- women who have had extensive ovarian surgery, i.e., cystectomy and unilateral oophorectomy.
Testing tends to have the highest yield in these groups. Women who have abnormal results should be referred to a reproductive endocrinologist for further evaluation and treatment.
The six tests are described below.
TABLE
How six markers of ovarian reserve stack up
| Test (year described) | Timing | Intracycle and intercycle variability | Sensitivity (specificity) | Reflects changes in ovarian reserve | Normal levels | Confounders | Out-of-pocket cost |
|---|---|---|---|---|---|---|---|
| Basal follicle-stimulating hormone (FSH) (1988) | Day 3 of menstrual cycle | Clinically significant | 7%–8% (98%–99%) | Late | • Early follicular phase FSH level <10 mIU/mL • Estradiol level <80 pg/mL | • High estradiol level (decreases) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $125–$150 |
| Clomiphene challenge test (1989) | Days 3 and 10 of menstrual cycle | Clinically significant | 25%–40% (98%–99%) | Late | • Day 3 FSH level <10 mIU/mL; day 3 estradiol level <80 pg/mL • Day 10 FSH level <10 mIU/mL | • High day 3 estradiol level (decreases day 3 FSH) • Low day 10 estradiol (increases day 10 FSH) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $550–$600 |
| GnRH agonist (1988) | Early follicular phase of menstrual cycle | Clinically significant | 32%–89% (79%–97%) | Late | Variable | • Oral contraceptives (decrease estradiol levels) • Pregnancy (increases estrogens) | $300–$350 |
| Inhibin-B (1997) | Early follicular phase of menstrual cycle | Clinically significant | 33%–81% (29%–95%) | Early | Variable in the literature; normal cutoffs range from ≥45–80 pg/mL | • Obesity (decreases) • PCOS (increases) • Exogenous FSH administration (increases) • Oral contraceptive use (decreases) | $150–$200 |
| Antral follicle count (1997) | Early follicular phase of menstrual cycle | Clinically significant (includes interobserver variability) | 8%–60% (33%–96%) | Earliest | ≥5–10 total antral follicles | • Oral contraceptive use (decreases) • Polycystic ovary syndrome (PCOS) (increases) | $300–$500 |
| Anti–Müllerian hormone/Müllerian-inhibiting substance (2002) | At any time; not cycle-dependent | Minimal | 49%–76% (89%–94%) | Earliest | >0.7 ng/mL | • PCOS (increases) • Obesity (decreases) • Exogenous FSH administration (decreases) | $150–$400 |
1 | Basal FSH—widely used but only moderately informative
Day 3 FSH and the CCCT are the most widely used measures of ovarian reserve in ART practice. The use of early follicular-phase FSH as a marker of ovarian reserve and fertility was proposed 20 years ago with the emergence of IVF.2-4 The test is an indirect assessment of ovarian reserve in that it measures pituitary production of FSH in response to feedback from ovarian hormones. Estradiol and inhibin-B reach a nadir early in the menstrual cycle; measuring FSH on day 3 offers a glimpse of the functioning of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise later in the cycle ( FIGURE 1 ).5,6
FIGURE 1 The HPO axis
The FSH level opens a window onto the function of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise in the cycle. Women who have normal ovarian reserve have sufficient ovarian hormone production early in the menstrual cycle to maintain FSH levels within the normal range. Conversely, a “monotropic” elevation in FSH—one that is unaccompanied by a rise in luteinizing hormone (LH)—reflects poor hormone production from an aging pool of ovarian follicles and disinhibition of FSH production.5,6
FSH measurements are typically combined with estradiol to enhance the sensitivity of testing ( FIGURE 2, ). Premature elevations of estradiol early in the follicular phase are driven by rising FSH levels in women with declining ovarian reserve. Abnormally elevated estrogen levels then feed back negatively on pituitary production of FSH and mask an elevation that might otherwise reveal diminished ovarian reserve. Measurement of both FSH and estradiol on cycle day 3 may therefore help decrease the incidence of false-negative testing.
Commonly cited criteria for normal ovarian reserve are:
- early follicular phase FSH, <10 mIU/mL
- estradiol, <80 pg/mL1
It is extremely important to note, however, that these are general guidelines and that cutoffs are both laboratory- and practice-specific.
FIGURE 2 Monthly and lifetime variations in estradiol and FSH
How 17ß-estradiol and follicle-stimulating hormone levels vary over the menstrual cycle (top) and a woman’s lifetime (bottom).
2 | Clomiphene citrate—more sensitive than FSH testing
Like basal FSH testing, the CCCT is an indirect assessment of ovarian reserve. Unlike FSH testing, the CCCT is provocative. It involves administration of 100 mg of clomiphene citrate (Clomid) on days 5 through 9 of the menstrual cycle, with FSH and estradiol measured on days 3 and 10. Once clomiphene citrate is administered, FSH and LH levels rise, followed by an increase in estradiol and inhibin. Evidence suggests that the smaller follicular cohorts in women with diminished ovarian reserve produce less inhibin-B and estradiol and, therefore, less negative feedback on clomiphene-induced pituitary FSH release.6,7 The result: persistent elevation of the day 10 FSH value and a positive screen for diminished ovarian reserve.
In some women, day 10 FSH is elevated even after a normal day 3 value. This makes the CCCT more sensitive than basal FSH testing; it can identify women who might go unrecognized if evaluated by day 3 FSH and estradiol levels alone.
More expensive and labor-intensive than the alternatives
Interpretation of the CCCT requires that FSH and estradiol both be assessed on days 3 and 10. An elevated FSH (≥10 mIU/mL) on either day indicates diminished ovarian reserve. As with basal FSH testing, elevated estradiol (≥80 pg/mL) on day 3 is considered abnormal. The day 10 estradiol value of the CCCT reflects whether or not clomiphene citrate was administered appropriately, and should be elevated. However, the significance of the day 10 estradiol level has been debated with respect to its predictive value for pregnancy in infertile populations.8
The addition of day 10 FSH assessment improves the sensitivity of the CCCT over basal FSH measurement, but makes it a more expensive and labor-intensive test ( TABLE ).5,6 The CCCT involves administration of clomiphene citrate, a safe drug (though it can have side effects), and two blood draws instead of one. Nevertheless, both tests are relatively noninvasive, rapid measures of ovarian reserve.
Drawbacks of the tests
Both basal FSH testing and the CCCT are widely used, although support for their ability to predict ovarian reserve in the infertile population has been challenged recently. Newer data demonstrate that these tests are limited in their ability to predict outcome (pregnancy and response to superovulation drugs) in all but a narrow group of patients undergoing IVF. Performance is particularly limited in:
Additional drawbacks of basal FSH testing and the CCCT include:
- significant variability of test results from cycle to cycle (intercycle variability)
- limited time frame within which the tests can be performed (intracycle variability).
The basal FSH test and CCCT have high specificity (98% to 99% for each) as an assessment of reproductive performance in infertile women and generate few false-positive results.5,6 However, the high screen cutoffs that allow for such specificity come at a price: Few women will screen positive, and sensitivity of the tests is low (between 7% and 8% for basal FSH and between 25% and 40% for the CCCT). Such low sensitivity means that many women will not conceive after infertility treatment despite a normal test result.5,11 Overall, the tests are not highly informative for many women who get tested.
Once abnormal, normal results are meaningless
Once an FSH level or the CCCT has ever been abnormal, the patient has diminished ovarian reserve; normal values in subsequent menstrual cycles do not improve the odds of pregnancy with treatment.14 This fact can be a significant source of confusion and frustration for patients.
3 | GnRH agonist stimulation —no better than FSH testing
This test was developed in the search for a very sensitive assessment of ovarian reserve. It was designed to uncover subtle abnormalities in pituitary and ovarian dynamics. It involves administering a gonadotropin-releasing hormone (GnRH) agonist such as leuprolide acetate (Lupron) on day 2 or 3 of the menstrual cycle and measuring pituitary and ovarian hormone responses.5,15
One group of investigators demonstrated a correlation between stimulated estradiol levels and responsiveness during IVF,16 but other studies have shown that the test does not perform significantly better than day 3 FSH in predicting ovarian reserve.17,18
The sensitivity of GnRH agonist testing for pregnancy is moderate (32% to 89%); specificity ranges from 79% to 97%.19
4 | Inhibin-B—not helpful when used alone
This glycoprotein hormone produced by granulosa cells of developing follicles is a direct measure of ovarian reserve when assessed in the early follicular phase of the menstrual cycle.20 Women treated with IVF who have a low inhibin-B level—particularly when using cutoffs below the range of 45–80 pg/mL—have been shown to respond poorly to superovulation and have a lower pregnancy rate than women with high inhibin-B.21,22 One group of investigators demonstrated that women with clinical evidence of diminished ovarian reserve but a normal FSH level also had low inhibin-B production, suggesting that it may be a more sensitive marker than FSH.22
Inhibin-B testing involves a simple blood draw. However, the test has been incorporated into clinical assessment of ovarian reserve only to a limited degree, due to the lack of reliable assays and controversy concerning its prognostic value.23
Because of these limitations, routine testing of serum inhibin-B in isolation of other markers of ovarian reserve is not recommended.
5 | Antral follicle count—good predictor of IVF outcome
Transvaginal ultrasonographic determination of the number of ovarian follicles that measure between 2 mm and 10 mm in diameter in the early follicular phase of the cycle yields the AFC. As a direct marker of the cohort of growing follicles in the early menstrual cycle, the AFC is believed to correlate strongly with the number of primordial follicles present in the ovary and, therefore, ovarian reserve. Total AFCs of less than 5 to 10 are suggestive of diminished ovarian reserve.24,25
In IVF cycles, AFC has proven to be an accurate predictor of number of oocytes retrieved, risk of cycle cancellation, and odds of conception.24,25 Some investigators have even suggested that, compared with other markers of ovarian reserve, AFC is the best independent predictor of outcome in IVF cycles.7,26-27
In a group of normally cycling women with proven fertility, AFC also showed a strong correlation with age, declining slowly until age 37 and more rapidly thereafter.28,29
AFC sensitivity for pregnancy is moderate and varies widely in published reports (8% to 60%), whereas specificity tends to be higher (33% to 96%).19
Drawbacks of AFC
- Because of the need to perform transvaginal ultrasonography, AFC is a more invasive and often more expensive test than hormonal biomarkers
- Accurate assessment of AFC requires an experienced sonographer and can be limited in patients who have had pelvic surgery or uterine fibroids and in those who are obese
- Moderate interobserver and intercycle variability of AFC determinations limits its reproducibility29,30
- As with basal FSH measurement, the intercycle variability of AFC does not correlate well with IVF outcome in individual patients.30
6 | Anti-Müllerian hormone— many advantages
The drawbacks of the tests just described— e.g., intercycle variability, lack of uniform cutoffs, and limited ability to predict IVF outcomes—make the development of more reliable measures of ovarian reserve a priority in reproductive medicine. AMH is a highly promising marker that appears to have many advantages over other tests and may have the greatest power to predict ovarian aging in women of reproductive age.
How it works
AMH is a glycoprotein growth factor and a member of the transforming growth factor-ß superfamily.31 It is primarily produced by the pool of early-growing follicles, which are believed to serve as a proxy for the number of primordial follicles in the ovary. The number of primordial follicles at a given point in time represents the ovarian reserve. AMH levels above 0.7 ng/mL are considered normal; values between 0.3 ng/mL and 0.7 ng/mL are consistent with borderline ovarian reserve, according to 2007 data from Reprosource Corp.
AMH has been studied as a marker of ovarian reserve for 6 years, with multiple reports describing declines in levels with age and with diminishing oocyte numbers. It is undetectable at menopause.32
The age-related decline in AMH is gradual but measurable even in young women, consistently preceding changes in other markers of ovarian reserve such as FSH and inhibin-B.32-35 The longitudinal changes in AMH have been demonstrated in ovulatory premenopausal women and healthy volunteers with proven fertility.33,34 In one series of women followed over a mean of 4 years (ages 25 to 46), AMH testing was superior to day 3 FSH, inhibin-B, and AFC in its ability to predict the onset of cycle irregularity and the menopausal transition.33
Does it predict oocyte quality?
AMH has performed well as a biomarker, comparable in most series to AFC and superior to FSH. AMH levels are strongly correlated with the number of oocytes retrieved during IVF and the odds of cycle cancellation due to poor response35-41 —but does it accurately characterize oocyte quality, the other element of ovarian reserve?
Some reports have shown a strong association between AMH levels and surrogates of oocyte quality, including fertilization, oocyte morphology, embryo quality, and pregnancy and miscarriage rates,36-41 but others have not.42 Some reports demonstrate a relationship between AMH and some but not all surrogate markers of oocyte quality.40
Advantages of AMH
- It demonstrates minimal intracycle variability.32,43-45 Compared with other markers of ovarian reserve, which must be measured early in the follicular phase of the menstrual cycle, AMH can be assessed at random times, making it a more convenient method for patients and physicians
- It demonstrates minimal intercycle variability32,34
- AMH levels are not significantly affected by the hormonal changes of pregnancy, oral contraceptive use, or GnRH treatment, and can be measured in these settings.46,47
Utility of AMH is limited in PCOS and obesity
The ability to use AMH as a marker of ovarian aging in women who have polycystic ovary syndrome (PCOS) and in women who are obese may be limited by the ovulatory dysfunction in these populations. Circulating levels of AMH are higher in women with PCOS than in unaffected women, a finding thought to be indicative of oligo-ovulation and poor follicular development in polycystic ovaries.48-53
In a recent series investigating AMH levels in women with PCOS, AMH and the degree of insulin resistance were positively correlated, and the AMH level was negatively correlated with the number of menses in a year.49 The consistently positive correlation between AMH and PCOS may suggest a future role for this marker as a diagnostic tool.
In obese women who do not have PCOS, AMH production may be lower than in women of normal weight. In a recent series, normally cycling obese women in the later reproductive years were shown to have an AMH level 70% lower than those in women who were not obese.54 These differences have not been well studied in younger obese women.
Which test is best?
AMH may be preferable to the other tests to assess ovarian reserve because it can be measured any time during the menstrual cycle or between cycles. AMH measurement is also useful if a woman is taking oral contraceptives or leuprolide acetate because these medications may confound the results of the other test methods. In addition, AMH may be the earliest indicator of decline in ovarian reproductive function. As such, it may highlight cases that merit a search for other causes of infertility and make it possible to treat them in a timely manner.
Elevated AMH may reveal occult PCOS and warn of significant risk of ovarian hyperstimulation prior to ovulation induction with gonadotrophins, so that the clinician can plan smaller doses.
A normal female is born with 1 million to 2 million oocytes, a number that declines continuously, primarily through the process of follicular atresia. By the onset of puberty, the number of oocytes has declined to approximately 300,000. As a woman enters her late 30s, when the total number of oocytes is approximately 25,000, the pace of oocyte depletion begins to increase, as does the rate of spontaneous miscarriage.1,55,56
The effect of age on fertility is believed to arise from changes in both oocyte number and quality. Multiple investigators have found a greater frequency of cellular abnormalities in oocytes from older women.1,2,5,15,57
Although ovarian reserve declines with age in all women, women of similar ages can have very different degrees of ovarian reserve, and some women who have very poor ovarian reserve may never conceive, despite aggressive fertility treatment.
The biologic basis for differences in ovarian reserve among similar groups of women is not completely understood, but is probably rooted in genetic, lifestyle, and environmental factors that affect granulosa cell and oocyte function. Identifying sensitive biomarkers that can determine ovarian reserve independent of age is critical to predict fertility and age at menopause.5
The University of Medicine and Dentistry of New Jersey (UMDNJ) owns a patent relating to the use of anti-Müllerian hormone/Müllerian inhibiting substance for predicting ovarian response in women with infertility. The patent is based in part on work that Dr. Seifer carried out while employed at UMDNJ. In accordance with UMDNJ policy, Dr. Seifer, a named inventor on this patent, assigned his interest in the invention to UMDNJ. UMDNJ has a licensing agreement with Diagnostic Systems Laboratory for the use of the claimed invention. Dr. Seifer receives a portion of the royalties, as determined by UMDNJ policy, that UMDNJ gains from this licensing agreement.
CASE: Borderline test result prompts referral
A 36-year-old nulliparous woman is seen in your office for evaluation after 6 months of infertility. She is ovulatory, and has been using an ovulation-prediction kit to time intercourse. You learn that she had Chlamydia trachomatis infection in the distant past, but elicit no other significant medical or surgical history. She reports that she smoked approximately one pack of cigarettes a day for 15 years but gave up smoking 5 years ago.
You order a hysterosalpingogram, followed by day 3 testing of follicle-stimulating hormone (FSH). The hysterosalpingogram is normal; the FSH level is 7.5 mIU/mL and the estradiol level is 30 pg/mL—both in the normal range.
The patient asks for testing of anti-Müllerian hormone (AMH; also known as Müllerian-inhibiting substance) because she has read that it is a new marker of fertility. The result is 0.5 ng/mL, a borderline value. After reviewing these results, you refer her to a reproductive endocrinologist for further management.
Was the test for AMH indicated? And is this referral appropriate?
The referral is entirely appropriate, even though the patient has not been trying to conceive for a full year. Why? The AMH value suggests that her ovarian reserve is in early decline. She would benefit from evaluation by a subspecialist who can review the entire spectrum of treatments, including aggressive options such as ovulation induction and in vitro fertilization (IVF), to optimize her reproductive success.
This article reviews the various biomarkers available to assess ovarian reserve in women who experience infertility:
- day 3 (basal) FSH
- clomiphene citrate challenge
- gonadotropin-releasing hormone (GnRH) agonist stimulation
- inhibin-B
- antral follicle count (AFC)
- AMH.
The AFC and AMH tend to detect the earliest changes in ovarian reserve, followed, sequentially, by inhibin-B, the clomiphene citrate challenge test (CCCT), and basal FSH.
The tests we describe are used primarily to assess treatment prognosis in infertile women. In time, however, appropriate population screening of ovarian reserve may be feasible to provide many more women with information about their reproductive potential and help them shape their life plan.
What makes a test valuable?
Ovarian reserve describes a woman’s reproductive potential—specifically, the number and quality of oocytes she possesses.1 Biochemical tests of ovarian reserve emerged during the rise of assisted reproductive technologies (ART) in the late 1980s to predict both responsiveness to superovulation drugs and the odds of pregnancy with treatment.
Ideally, a test that assesses ovarian reserve should be affordable, straightforward, rapidly interpretable, and minimally invasive. It also should be able to detect changes that begin early in reproductive life. To be applicable to large populations of reproductive-age women, it should be of use anytime in the menstrual cycle, and should provide reproducible and highly accurate assessment of the reproductive aging process.
Our ability to offer tests that accurately measure ovarian reserve has a significant impact on women at risk of infertility and early menopause and on those who choose to delay childbearing for personal (nonmedical) reasons. These tests have become increasingly relevant because women are choosing to have their first child at a later age than their counterparts did 20 years ago:
- In 1980, 40% of women having their first baby were younger than 25 years, and only 5% were older than 35
- In 2000, 25% of women were younger than 25 when their first child was born, and 15% were older than 35.
Who should be tested?
Ovarian reserve is a complex clinical phenomenon that is influenced by age, genetics, and environmental variables. The decline in a woman’s ovarian reserve over time is irreversible; the trajectory of this decline is fundamental to the odds of fertility with age and the timing of the menopausal transition. At present, the markers used most often in clinical practice have some utility but also suffer from several drawbacks ( TABLE ).
For the general practitioner performing an infertility evaluation, we recommend focusing on the following groups of women for ovarian reserve testing:
- women over 30 years of age
- women with a history of exposure to a confirmed gonadotoxin, i.e., tobacco smoke, chemotherapy, radiation therapy
- women with a strong family history of early menopause or premature ovarian failure
- women who have had extensive ovarian surgery, i.e., cystectomy and unilateral oophorectomy.
Testing tends to have the highest yield in these groups. Women who have abnormal results should be referred to a reproductive endocrinologist for further evaluation and treatment.
The six tests are described below.
TABLE
How six markers of ovarian reserve stack up
| Test (year described) | Timing | Intracycle and intercycle variability | Sensitivity (specificity) | Reflects changes in ovarian reserve | Normal levels | Confounders | Out-of-pocket cost |
|---|---|---|---|---|---|---|---|
| Basal follicle-stimulating hormone (FSH) (1988) | Day 3 of menstrual cycle | Clinically significant | 7%–8% (98%–99%) | Late | • Early follicular phase FSH level <10 mIU/mL • Estradiol level <80 pg/mL | • High estradiol level (decreases) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $125–$150 |
| Clomiphene challenge test (1989) | Days 3 and 10 of menstrual cycle | Clinically significant | 25%–40% (98%–99%) | Late | • Day 3 FSH level <10 mIU/mL; day 3 estradiol level <80 pg/mL • Day 10 FSH level <10 mIU/mL | • High day 3 estradiol level (decreases day 3 FSH) • Low day 10 estradiol (increases day 10 FSH) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $550–$600 |
| GnRH agonist (1988) | Early follicular phase of menstrual cycle | Clinically significant | 32%–89% (79%–97%) | Late | Variable | • Oral contraceptives (decrease estradiol levels) • Pregnancy (increases estrogens) | $300–$350 |
| Inhibin-B (1997) | Early follicular phase of menstrual cycle | Clinically significant | 33%–81% (29%–95%) | Early | Variable in the literature; normal cutoffs range from ≥45–80 pg/mL | • Obesity (decreases) • PCOS (increases) • Exogenous FSH administration (increases) • Oral contraceptive use (decreases) | $150–$200 |
| Antral follicle count (1997) | Early follicular phase of menstrual cycle | Clinically significant (includes interobserver variability) | 8%–60% (33%–96%) | Earliest | ≥5–10 total antral follicles | • Oral contraceptive use (decreases) • Polycystic ovary syndrome (PCOS) (increases) | $300–$500 |
| Anti–Müllerian hormone/Müllerian-inhibiting substance (2002) | At any time; not cycle-dependent | Minimal | 49%–76% (89%–94%) | Earliest | >0.7 ng/mL | • PCOS (increases) • Obesity (decreases) • Exogenous FSH administration (decreases) | $150–$400 |
1 | Basal FSH—widely used but only moderately informative
Day 3 FSH and the CCCT are the most widely used measures of ovarian reserve in ART practice. The use of early follicular-phase FSH as a marker of ovarian reserve and fertility was proposed 20 years ago with the emergence of IVF.2-4 The test is an indirect assessment of ovarian reserve in that it measures pituitary production of FSH in response to feedback from ovarian hormones. Estradiol and inhibin-B reach a nadir early in the menstrual cycle; measuring FSH on day 3 offers a glimpse of the functioning of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise later in the cycle ( FIGURE 1 ).5,6
FIGURE 1 The HPO axis
The FSH level opens a window onto the function of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise in the cycle. Women who have normal ovarian reserve have sufficient ovarian hormone production early in the menstrual cycle to maintain FSH levels within the normal range. Conversely, a “monotropic” elevation in FSH—one that is unaccompanied by a rise in luteinizing hormone (LH)—reflects poor hormone production from an aging pool of ovarian follicles and disinhibition of FSH production.5,6
FSH measurements are typically combined with estradiol to enhance the sensitivity of testing ( FIGURE 2, ). Premature elevations of estradiol early in the follicular phase are driven by rising FSH levels in women with declining ovarian reserve. Abnormally elevated estrogen levels then feed back negatively on pituitary production of FSH and mask an elevation that might otherwise reveal diminished ovarian reserve. Measurement of both FSH and estradiol on cycle day 3 may therefore help decrease the incidence of false-negative testing.
Commonly cited criteria for normal ovarian reserve are:
- early follicular phase FSH, <10 mIU/mL
- estradiol, <80 pg/mL1
It is extremely important to note, however, that these are general guidelines and that cutoffs are both laboratory- and practice-specific.
FIGURE 2 Monthly and lifetime variations in estradiol and FSH
How 17ß-estradiol and follicle-stimulating hormone levels vary over the menstrual cycle (top) and a woman’s lifetime (bottom).
2 | Clomiphene citrate—more sensitive than FSH testing
Like basal FSH testing, the CCCT is an indirect assessment of ovarian reserve. Unlike FSH testing, the CCCT is provocative. It involves administration of 100 mg of clomiphene citrate (Clomid) on days 5 through 9 of the menstrual cycle, with FSH and estradiol measured on days 3 and 10. Once clomiphene citrate is administered, FSH and LH levels rise, followed by an increase in estradiol and inhibin. Evidence suggests that the smaller follicular cohorts in women with diminished ovarian reserve produce less inhibin-B and estradiol and, therefore, less negative feedback on clomiphene-induced pituitary FSH release.6,7 The result: persistent elevation of the day 10 FSH value and a positive screen for diminished ovarian reserve.
In some women, day 10 FSH is elevated even after a normal day 3 value. This makes the CCCT more sensitive than basal FSH testing; it can identify women who might go unrecognized if evaluated by day 3 FSH and estradiol levels alone.
More expensive and labor-intensive than the alternatives
Interpretation of the CCCT requires that FSH and estradiol both be assessed on days 3 and 10. An elevated FSH (≥10 mIU/mL) on either day indicates diminished ovarian reserve. As with basal FSH testing, elevated estradiol (≥80 pg/mL) on day 3 is considered abnormal. The day 10 estradiol value of the CCCT reflects whether or not clomiphene citrate was administered appropriately, and should be elevated. However, the significance of the day 10 estradiol level has been debated with respect to its predictive value for pregnancy in infertile populations.8
The addition of day 10 FSH assessment improves the sensitivity of the CCCT over basal FSH measurement, but makes it a more expensive and labor-intensive test ( TABLE ).5,6 The CCCT involves administration of clomiphene citrate, a safe drug (though it can have side effects), and two blood draws instead of one. Nevertheless, both tests are relatively noninvasive, rapid measures of ovarian reserve.
Drawbacks of the tests
Both basal FSH testing and the CCCT are widely used, although support for their ability to predict ovarian reserve in the infertile population has been challenged recently. Newer data demonstrate that these tests are limited in their ability to predict outcome (pregnancy and response to superovulation drugs) in all but a narrow group of patients undergoing IVF. Performance is particularly limited in:
Additional drawbacks of basal FSH testing and the CCCT include:
- significant variability of test results from cycle to cycle (intercycle variability)
- limited time frame within which the tests can be performed (intracycle variability).
The basal FSH test and CCCT have high specificity (98% to 99% for each) as an assessment of reproductive performance in infertile women and generate few false-positive results.5,6 However, the high screen cutoffs that allow for such specificity come at a price: Few women will screen positive, and sensitivity of the tests is low (between 7% and 8% for basal FSH and between 25% and 40% for the CCCT). Such low sensitivity means that many women will not conceive after infertility treatment despite a normal test result.5,11 Overall, the tests are not highly informative for many women who get tested.
Once abnormal, normal results are meaningless
Once an FSH level or the CCCT has ever been abnormal, the patient has diminished ovarian reserve; normal values in subsequent menstrual cycles do not improve the odds of pregnancy with treatment.14 This fact can be a significant source of confusion and frustration for patients.
3 | GnRH agonist stimulation —no better than FSH testing
This test was developed in the search for a very sensitive assessment of ovarian reserve. It was designed to uncover subtle abnormalities in pituitary and ovarian dynamics. It involves administering a gonadotropin-releasing hormone (GnRH) agonist such as leuprolide acetate (Lupron) on day 2 or 3 of the menstrual cycle and measuring pituitary and ovarian hormone responses.5,15
One group of investigators demonstrated a correlation between stimulated estradiol levels and responsiveness during IVF,16 but other studies have shown that the test does not perform significantly better than day 3 FSH in predicting ovarian reserve.17,18
The sensitivity of GnRH agonist testing for pregnancy is moderate (32% to 89%); specificity ranges from 79% to 97%.19
4 | Inhibin-B—not helpful when used alone
This glycoprotein hormone produced by granulosa cells of developing follicles is a direct measure of ovarian reserve when assessed in the early follicular phase of the menstrual cycle.20 Women treated with IVF who have a low inhibin-B level—particularly when using cutoffs below the range of 45–80 pg/mL—have been shown to respond poorly to superovulation and have a lower pregnancy rate than women with high inhibin-B.21,22 One group of investigators demonstrated that women with clinical evidence of diminished ovarian reserve but a normal FSH level also had low inhibin-B production, suggesting that it may be a more sensitive marker than FSH.22
Inhibin-B testing involves a simple blood draw. However, the test has been incorporated into clinical assessment of ovarian reserve only to a limited degree, due to the lack of reliable assays and controversy concerning its prognostic value.23
Because of these limitations, routine testing of serum inhibin-B in isolation of other markers of ovarian reserve is not recommended.
5 | Antral follicle count—good predictor of IVF outcome
Transvaginal ultrasonographic determination of the number of ovarian follicles that measure between 2 mm and 10 mm in diameter in the early follicular phase of the cycle yields the AFC. As a direct marker of the cohort of growing follicles in the early menstrual cycle, the AFC is believed to correlate strongly with the number of primordial follicles present in the ovary and, therefore, ovarian reserve. Total AFCs of less than 5 to 10 are suggestive of diminished ovarian reserve.24,25
In IVF cycles, AFC has proven to be an accurate predictor of number of oocytes retrieved, risk of cycle cancellation, and odds of conception.24,25 Some investigators have even suggested that, compared with other markers of ovarian reserve, AFC is the best independent predictor of outcome in IVF cycles.7,26-27
In a group of normally cycling women with proven fertility, AFC also showed a strong correlation with age, declining slowly until age 37 and more rapidly thereafter.28,29
AFC sensitivity for pregnancy is moderate and varies widely in published reports (8% to 60%), whereas specificity tends to be higher (33% to 96%).19
Drawbacks of AFC
- Because of the need to perform transvaginal ultrasonography, AFC is a more invasive and often more expensive test than hormonal biomarkers
- Accurate assessment of AFC requires an experienced sonographer and can be limited in patients who have had pelvic surgery or uterine fibroids and in those who are obese
- Moderate interobserver and intercycle variability of AFC determinations limits its reproducibility29,30
- As with basal FSH measurement, the intercycle variability of AFC does not correlate well with IVF outcome in individual patients.30
6 | Anti-Müllerian hormone— many advantages
The drawbacks of the tests just described— e.g., intercycle variability, lack of uniform cutoffs, and limited ability to predict IVF outcomes—make the development of more reliable measures of ovarian reserve a priority in reproductive medicine. AMH is a highly promising marker that appears to have many advantages over other tests and may have the greatest power to predict ovarian aging in women of reproductive age.
How it works
AMH is a glycoprotein growth factor and a member of the transforming growth factor-ß superfamily.31 It is primarily produced by the pool of early-growing follicles, which are believed to serve as a proxy for the number of primordial follicles in the ovary. The number of primordial follicles at a given point in time represents the ovarian reserve. AMH levels above 0.7 ng/mL are considered normal; values between 0.3 ng/mL and 0.7 ng/mL are consistent with borderline ovarian reserve, according to 2007 data from Reprosource Corp.
AMH has been studied as a marker of ovarian reserve for 6 years, with multiple reports describing declines in levels with age and with diminishing oocyte numbers. It is undetectable at menopause.32
The age-related decline in AMH is gradual but measurable even in young women, consistently preceding changes in other markers of ovarian reserve such as FSH and inhibin-B.32-35 The longitudinal changes in AMH have been demonstrated in ovulatory premenopausal women and healthy volunteers with proven fertility.33,34 In one series of women followed over a mean of 4 years (ages 25 to 46), AMH testing was superior to day 3 FSH, inhibin-B, and AFC in its ability to predict the onset of cycle irregularity and the menopausal transition.33
Does it predict oocyte quality?
AMH has performed well as a biomarker, comparable in most series to AFC and superior to FSH. AMH levels are strongly correlated with the number of oocytes retrieved during IVF and the odds of cycle cancellation due to poor response35-41 —but does it accurately characterize oocyte quality, the other element of ovarian reserve?
Some reports have shown a strong association between AMH levels and surrogates of oocyte quality, including fertilization, oocyte morphology, embryo quality, and pregnancy and miscarriage rates,36-41 but others have not.42 Some reports demonstrate a relationship between AMH and some but not all surrogate markers of oocyte quality.40
Advantages of AMH
- It demonstrates minimal intracycle variability.32,43-45 Compared with other markers of ovarian reserve, which must be measured early in the follicular phase of the menstrual cycle, AMH can be assessed at random times, making it a more convenient method for patients and physicians
- It demonstrates minimal intercycle variability32,34
- AMH levels are not significantly affected by the hormonal changes of pregnancy, oral contraceptive use, or GnRH treatment, and can be measured in these settings.46,47
Utility of AMH is limited in PCOS and obesity
The ability to use AMH as a marker of ovarian aging in women who have polycystic ovary syndrome (PCOS) and in women who are obese may be limited by the ovulatory dysfunction in these populations. Circulating levels of AMH are higher in women with PCOS than in unaffected women, a finding thought to be indicative of oligo-ovulation and poor follicular development in polycystic ovaries.48-53
In a recent series investigating AMH levels in women with PCOS, AMH and the degree of insulin resistance were positively correlated, and the AMH level was negatively correlated with the number of menses in a year.49 The consistently positive correlation between AMH and PCOS may suggest a future role for this marker as a diagnostic tool.
In obese women who do not have PCOS, AMH production may be lower than in women of normal weight. In a recent series, normally cycling obese women in the later reproductive years were shown to have an AMH level 70% lower than those in women who were not obese.54 These differences have not been well studied in younger obese women.
Which test is best?
AMH may be preferable to the other tests to assess ovarian reserve because it can be measured any time during the menstrual cycle or between cycles. AMH measurement is also useful if a woman is taking oral contraceptives or leuprolide acetate because these medications may confound the results of the other test methods. In addition, AMH may be the earliest indicator of decline in ovarian reproductive function. As such, it may highlight cases that merit a search for other causes of infertility and make it possible to treat them in a timely manner.
Elevated AMH may reveal occult PCOS and warn of significant risk of ovarian hyperstimulation prior to ovulation induction with gonadotrophins, so that the clinician can plan smaller doses.
A normal female is born with 1 million to 2 million oocytes, a number that declines continuously, primarily through the process of follicular atresia. By the onset of puberty, the number of oocytes has declined to approximately 300,000. As a woman enters her late 30s, when the total number of oocytes is approximately 25,000, the pace of oocyte depletion begins to increase, as does the rate of spontaneous miscarriage.1,55,56
The effect of age on fertility is believed to arise from changes in both oocyte number and quality. Multiple investigators have found a greater frequency of cellular abnormalities in oocytes from older women.1,2,5,15,57
Although ovarian reserve declines with age in all women, women of similar ages can have very different degrees of ovarian reserve, and some women who have very poor ovarian reserve may never conceive, despite aggressive fertility treatment.
The biologic basis for differences in ovarian reserve among similar groups of women is not completely understood, but is probably rooted in genetic, lifestyle, and environmental factors that affect granulosa cell and oocyte function. Identifying sensitive biomarkers that can determine ovarian reserve independent of age is critical to predict fertility and age at menopause.5
1. Practice Committee of the American Society for Reproductive Medicine. Age and infertility in women. Fertil Steril. 2006;86:S248-S252.
2. Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu H-C. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50:298-307.
3. Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651-654.
4. Toner JP, Philiput CB, Jones GS, Muasher SJ. Basal follicle stimulating hormone level is a better predictor of in vitro fertilization outcome than age. Fertil Steril. 1991;55:784-791.
5. Barnhart K, Osheroff J. Follicle stimulating hormone as a predictor of fertility. Curr Opin Obstet Gynecol. 1998;10:227-232.
6. Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: the physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;69:474-477.
7. Yong PY, Baird DT, Thong KJ, McNeilly AS, Anderson RA. Prospective analysis of the relationships between the ovarian follicle cohort and basal FSH concentration, the inhibin response to exogenous FSH and ovarian follicle number at different stages of the normal menstrual cycle and after pituitary down-regulation. Hum Reprod. 2003;18:35-44.
8. Scott RT, Jr, Illions EH, Kost ER, Dellinger C, Hofmann GE, Navot D. Evaluation of the significance of the estradiol response during the clomiphene citrate challenge test. Fertil Steril. 1993;60:242-246.
9. Esposito MA, Coutifaris C, Barnhart KT. A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod. 2002;17:118-123.
10. Bancsi L, Broekmans FJM, Wol BWJ, Habbema DK, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003;79:1091-1100.
11. Jain T, Soules MR, Collins JA. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 2004;82:180-185.
12. Toner JP. Modest follicle-stimulating hormone elevations in younger women: warn but don’t disqualify. Fertil Steril. 2004;81:1493-1495.
13. Van Rooij IAJ, de Jong E, Broekmans FJM, Looman CWN, Habbeman DK, te Velde ER. High follicle-stimulating hormone levels should not necessarily lead to the exclusion of subfertile patients from treatment. Fertil Steril. 2004;81:1478-1485.
14. Scott RT, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54:297-302.
15. Bulkulmez O, Arici A. Assessment of ovarian reserve. Curr Opin Obstet Gynecol. 2004;16:231-237.
16. Ranieri DM, Quinn F, Makhlouf A, et al. Simultaneous evaluation of basal follicle-stimulating hormone and 17-beta-estradiol response to gonadotropin-releasing hormone analogue stimulation: an improved predictor of ovarian reserve. Fertil Steril. 1998;70:227-233.
17. Fujimoto VY, Klein NA, Battaglia DE, Bremmer WJ, Soules MR. The anterior pituitary response to a gonadotropin-releasing hormone challenge test in normal older reproductive age women. Fertil Steril. 1996;65:539-544.
18. Galtier-Dereure F, De Bouard V, Picto MC, et al. Ovarian reserve test with the gonadotrophin-releasing hormone agonist buserelin: correlation with in-vitro fertilization outcome. Hum Reprod. 1996;11:1393-1398.
19. Broekmans FJ, Fwee J, Hendricks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685-718.
20. Klein NA, Illingworth PJ, Groome NP, NcNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81:2742-2745.
21. Seifer DB, Lambert-Messerlian G, Hogan JW, et al. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67:110-114.
22. Seifer DB, Scott RT, Jr, Bergh PA, et al. Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin B before a rise in day 3 follicle-stimulating hormone. Fertil Steril. 1999;72:63-65.
23. Corson SL, Gutmann J, Batzer FR, Wallace H, Klein N, Soules MR. Inhibin-B as a test of ovarian reserve for infertile women. Hum Reprod. 1999;14:2818-2821.
24. Tomas C, Nuojua-Huttunen S, Martikainen H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum Reprod. 1997;12:220-223.
25. Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505-510.
26. Hung E, Tang OS, Ho PC. The significance of the number of antral follicles prior to stimulation in predicting ovarian responses in an IVF programme. Hum Reprod. 2000;15:1937-1942.
27. Bancsi LFJMM, Broekmans FJM, Eijkemans MJC, de Jong FH, Habbema JDF, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328-336.
28. Ng EH, Yeung WS, Fong DY, Ho PC. Effects of age on hormonal and ultrasound markers of ovarian reserve in Chinese women with proven fertility. Hum Reprod. 2003;18:2169-2174.
29. Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845-851.
30. Hansen KR, Morris JL, Thyer AC, Soules MR. Reproductive aging and the variability in the ovarian antral follicle count: application in the clinical setting. Fertil Steril. 2003;80:577-583.
31. Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685-698.
32. de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM. Anti-Müllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357-362.
33. van Rooij IAJ, Broekmans FJM, Scheffer GJ, et al. Serum anti-Müllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979-987.
34. van Rooij IAJ, Tonkelaar I, Broekmans FJ, et al. Anti-Müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601-606.
35. Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Müllerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20-24.
36. Silberstein T, MacLaughlin DT, Shai I, et al. Müllerian-inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159-163.
37. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum Müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468-471.
38. Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022-2026.
39. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum anti-Müllerian hormone/Müllerian-inhibiting substance appears to be a more discriminatory marker of ART outcome than follicular stimulating hormone, inhibin B or estradiol. Fertil Steril. 2004;82:1323-1329.
40. Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22:2414-2421.
41. Fanchin R, Mendez DH, Frydman N, et al. Anti-Müllerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796-1802.
42. Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Th omas CM, Braat DD. Anti-Müllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87:223-226.
43. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FM, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057-4063.
44. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Müllerian hormone throughout the menstrual cycle. Hum Reprod. 2006;21:3103-3107.
45. Tsepelidis S, Devreker F, Demeestere F, Flahaut I, Gervy A, Englert C. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837-1840.
46. La Marca A, Giulini Orvieto R, De Leo V, Volpe A. Anti-Müllerian hormone concentrations in maternal serum during pregnancy. Hum Reprod. 2005;20:1569-1572.
47. Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Müllerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196-201.
48. Al-Qahtani A, Groome NP. Anti-Müllerian hormone: Cinderella finds new admirers. J Clin Endocrinol Metab. 2006;91:3760-3762.
49. La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Müllerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970-971.
50. Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820-1836.
51. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-Müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957-5962.
52. Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum anti-Müllerian substance and other reproductive hormones in untreated women with polycystic ovary syndrome and endometriosis. Fertil Steril. 1997;67:962-965.
53. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240-245.
54. Freeman EW, Gracia CG, Sammel MD, Lin H, Lim LC, Strauss JF, 3rd. Association of anti-Müllerian hormone levels with obesity in later reproductive-age women. Fertil Steril. 2007;87:101-106.
55. Scott RT, Opsahl MS, Leonardi MR, Neall GS, Illions EH, Navot D. Life table analysis of pregnancy rates in a general infertility population relative to ovarian reserve and patient age. Hum Reprod. 1995;10:1706-1710.
56. Speroff L. Fritz M. eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
57. Lim AS, Tsakok MFH. Age-related decline in fertility: a link to degenerative oocytes? Fertil Steril. 1997;68:265-271.
1. Practice Committee of the American Society for Reproductive Medicine. Age and infertility in women. Fertil Steril. 2006;86:S248-S252.
2. Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu H-C. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50:298-307.
3. Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651-654.
4. Toner JP, Philiput CB, Jones GS, Muasher SJ. Basal follicle stimulating hormone level is a better predictor of in vitro fertilization outcome than age. Fertil Steril. 1991;55:784-791.
5. Barnhart K, Osheroff J. Follicle stimulating hormone as a predictor of fertility. Curr Opin Obstet Gynecol. 1998;10:227-232.
6. Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: the physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;69:474-477.
7. Yong PY, Baird DT, Thong KJ, McNeilly AS, Anderson RA. Prospective analysis of the relationships between the ovarian follicle cohort and basal FSH concentration, the inhibin response to exogenous FSH and ovarian follicle number at different stages of the normal menstrual cycle and after pituitary down-regulation. Hum Reprod. 2003;18:35-44.
8. Scott RT, Jr, Illions EH, Kost ER, Dellinger C, Hofmann GE, Navot D. Evaluation of the significance of the estradiol response during the clomiphene citrate challenge test. Fertil Steril. 1993;60:242-246.
9. Esposito MA, Coutifaris C, Barnhart KT. A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod. 2002;17:118-123.
10. Bancsi L, Broekmans FJM, Wol BWJ, Habbema DK, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003;79:1091-1100.
11. Jain T, Soules MR, Collins JA. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 2004;82:180-185.
12. Toner JP. Modest follicle-stimulating hormone elevations in younger women: warn but don’t disqualify. Fertil Steril. 2004;81:1493-1495.
13. Van Rooij IAJ, de Jong E, Broekmans FJM, Looman CWN, Habbeman DK, te Velde ER. High follicle-stimulating hormone levels should not necessarily lead to the exclusion of subfertile patients from treatment. Fertil Steril. 2004;81:1478-1485.
14. Scott RT, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54:297-302.
15. Bulkulmez O, Arici A. Assessment of ovarian reserve. Curr Opin Obstet Gynecol. 2004;16:231-237.
16. Ranieri DM, Quinn F, Makhlouf A, et al. Simultaneous evaluation of basal follicle-stimulating hormone and 17-beta-estradiol response to gonadotropin-releasing hormone analogue stimulation: an improved predictor of ovarian reserve. Fertil Steril. 1998;70:227-233.
17. Fujimoto VY, Klein NA, Battaglia DE, Bremmer WJ, Soules MR. The anterior pituitary response to a gonadotropin-releasing hormone challenge test in normal older reproductive age women. Fertil Steril. 1996;65:539-544.
18. Galtier-Dereure F, De Bouard V, Picto MC, et al. Ovarian reserve test with the gonadotrophin-releasing hormone agonist buserelin: correlation with in-vitro fertilization outcome. Hum Reprod. 1996;11:1393-1398.
19. Broekmans FJ, Fwee J, Hendricks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685-718.
20. Klein NA, Illingworth PJ, Groome NP, NcNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81:2742-2745.
21. Seifer DB, Lambert-Messerlian G, Hogan JW, et al. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67:110-114.
22. Seifer DB, Scott RT, Jr, Bergh PA, et al. Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin B before a rise in day 3 follicle-stimulating hormone. Fertil Steril. 1999;72:63-65.
23. Corson SL, Gutmann J, Batzer FR, Wallace H, Klein N, Soules MR. Inhibin-B as a test of ovarian reserve for infertile women. Hum Reprod. 1999;14:2818-2821.
24. Tomas C, Nuojua-Huttunen S, Martikainen H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum Reprod. 1997;12:220-223.
25. Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505-510.
26. Hung E, Tang OS, Ho PC. The significance of the number of antral follicles prior to stimulation in predicting ovarian responses in an IVF programme. Hum Reprod. 2000;15:1937-1942.
27. Bancsi LFJMM, Broekmans FJM, Eijkemans MJC, de Jong FH, Habbema JDF, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328-336.
28. Ng EH, Yeung WS, Fong DY, Ho PC. Effects of age on hormonal and ultrasound markers of ovarian reserve in Chinese women with proven fertility. Hum Reprod. 2003;18:2169-2174.
29. Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845-851.
30. Hansen KR, Morris JL, Thyer AC, Soules MR. Reproductive aging and the variability in the ovarian antral follicle count: application in the clinical setting. Fertil Steril. 2003;80:577-583.
31. Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685-698.
32. de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM. Anti-Müllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357-362.
33. van Rooij IAJ, Broekmans FJM, Scheffer GJ, et al. Serum anti-Müllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979-987.
34. van Rooij IAJ, Tonkelaar I, Broekmans FJ, et al. Anti-Müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601-606.
35. Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Müllerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20-24.
36. Silberstein T, MacLaughlin DT, Shai I, et al. Müllerian-inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159-163.
37. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum Müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468-471.
38. Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022-2026.
39. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum anti-Müllerian hormone/Müllerian-inhibiting substance appears to be a more discriminatory marker of ART outcome than follicular stimulating hormone, inhibin B or estradiol. Fertil Steril. 2004;82:1323-1329.
40. Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22:2414-2421.
41. Fanchin R, Mendez DH, Frydman N, et al. Anti-Müllerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796-1802.
42. Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Th omas CM, Braat DD. Anti-Müllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87:223-226.
43. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FM, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057-4063.
44. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Müllerian hormone throughout the menstrual cycle. Hum Reprod. 2006;21:3103-3107.
45. Tsepelidis S, Devreker F, Demeestere F, Flahaut I, Gervy A, Englert C. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837-1840.
46. La Marca A, Giulini Orvieto R, De Leo V, Volpe A. Anti-Müllerian hormone concentrations in maternal serum during pregnancy. Hum Reprod. 2005;20:1569-1572.
47. Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Müllerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196-201.
48. Al-Qahtani A, Groome NP. Anti-Müllerian hormone: Cinderella finds new admirers. J Clin Endocrinol Metab. 2006;91:3760-3762.
49. La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Müllerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970-971.
50. Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820-1836.
51. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-Müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957-5962.
52. Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum anti-Müllerian substance and other reproductive hormones in untreated women with polycystic ovary syndrome and endometriosis. Fertil Steril. 1997;67:962-965.
53. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240-245.
54. Freeman EW, Gracia CG, Sammel MD, Lin H, Lim LC, Strauss JF, 3rd. Association of anti-Müllerian hormone levels with obesity in later reproductive-age women. Fertil Steril. 2007;87:101-106.
55. Scott RT, Opsahl MS, Leonardi MR, Neall GS, Illions EH, Navot D. Life table analysis of pregnancy rates in a general infertility population relative to ovarian reserve and patient age. Hum Reprod. 1995;10:1706-1710.
56. Speroff L. Fritz M. eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
57. Lim AS, Tsakok MFH. Age-related decline in fertility: a link to degenerative oocytes? Fertil Steril. 1997;68:265-271.