User login
6 office tests to assess ovarian reserve, and what they tell you
The University of Medicine and Dentistry of New Jersey (UMDNJ) owns a patent relating to the use of anti-Müllerian hormone/Müllerian inhibiting substance for predicting ovarian response in women with infertility. The patent is based in part on work that Dr. Seifer carried out while employed at UMDNJ. In accordance with UMDNJ policy, Dr. Seifer, a named inventor on this patent, assigned his interest in the invention to UMDNJ. UMDNJ has a licensing agreement with Diagnostic Systems Laboratory for the use of the claimed invention. Dr. Seifer receives a portion of the royalties, as determined by UMDNJ policy, that UMDNJ gains from this licensing agreement.
CASE: Borderline test result prompts referral
A 36-year-old nulliparous woman is seen in your office for evaluation after 6 months of infertility. She is ovulatory, and has been using an ovulation-prediction kit to time intercourse. You learn that she had Chlamydia trachomatis infection in the distant past, but elicit no other significant medical or surgical history. She reports that she smoked approximately one pack of cigarettes a day for 15 years but gave up smoking 5 years ago.
You order a hysterosalpingogram, followed by day 3 testing of follicle-stimulating hormone (FSH). The hysterosalpingogram is normal; the FSH level is 7.5 mIU/mL and the estradiol level is 30 pg/mL—both in the normal range.
The patient asks for testing of anti-Müllerian hormone (AMH; also known as Müllerian-inhibiting substance) because she has read that it is a new marker of fertility. The result is 0.5 ng/mL, a borderline value. After reviewing these results, you refer her to a reproductive endocrinologist for further management.
Was the test for AMH indicated? And is this referral appropriate?
The referral is entirely appropriate, even though the patient has not been trying to conceive for a full year. Why? The AMH value suggests that her ovarian reserve is in early decline. She would benefit from evaluation by a subspecialist who can review the entire spectrum of treatments, including aggressive options such as ovulation induction and in vitro fertilization (IVF), to optimize her reproductive success.
This article reviews the various biomarkers available to assess ovarian reserve in women who experience infertility:
- day 3 (basal) FSH
- clomiphene citrate challenge
- gonadotropin-releasing hormone (GnRH) agonist stimulation
- inhibin-B
- antral follicle count (AFC)
- AMH.
The AFC and AMH tend to detect the earliest changes in ovarian reserve, followed, sequentially, by inhibin-B, the clomiphene citrate challenge test (CCCT), and basal FSH.
The tests we describe are used primarily to assess treatment prognosis in infertile women. In time, however, appropriate population screening of ovarian reserve may be feasible to provide many more women with information about their reproductive potential and help them shape their life plan.
What makes a test valuable?
Ovarian reserve describes a woman’s reproductive potential—specifically, the number and quality of oocytes she possesses.1 Biochemical tests of ovarian reserve emerged during the rise of assisted reproductive technologies (ART) in the late 1980s to predict both responsiveness to superovulation drugs and the odds of pregnancy with treatment.
Ideally, a test that assesses ovarian reserve should be affordable, straightforward, rapidly interpretable, and minimally invasive. It also should be able to detect changes that begin early in reproductive life. To be applicable to large populations of reproductive-age women, it should be of use anytime in the menstrual cycle, and should provide reproducible and highly accurate assessment of the reproductive aging process.
Our ability to offer tests that accurately measure ovarian reserve has a significant impact on women at risk of infertility and early menopause and on those who choose to delay childbearing for personal (nonmedical) reasons. These tests have become increasingly relevant because women are choosing to have their first child at a later age than their counterparts did 20 years ago:
- In 1980, 40% of women having their first baby were younger than 25 years, and only 5% were older than 35
- In 2000, 25% of women were younger than 25 when their first child was born, and 15% were older than 35.
Who should be tested?
Ovarian reserve is a complex clinical phenomenon that is influenced by age, genetics, and environmental variables. The decline in a woman’s ovarian reserve over time is irreversible; the trajectory of this decline is fundamental to the odds of fertility with age and the timing of the menopausal transition. At present, the markers used most often in clinical practice have some utility but also suffer from several drawbacks ( TABLE ).
For the general practitioner performing an infertility evaluation, we recommend focusing on the following groups of women for ovarian reserve testing:
- women over 30 years of age
- women with a history of exposure to a confirmed gonadotoxin, i.e., tobacco smoke, chemotherapy, radiation therapy
- women with a strong family history of early menopause or premature ovarian failure
- women who have had extensive ovarian surgery, i.e., cystectomy and unilateral oophorectomy.
Testing tends to have the highest yield in these groups. Women who have abnormal results should be referred to a reproductive endocrinologist for further evaluation and treatment.
The six tests are described below.
TABLE
How six markers of ovarian reserve stack up
| Test (year described) | Timing | Intracycle and intercycle variability | Sensitivity (specificity) | Reflects changes in ovarian reserve | Normal levels | Confounders | Out-of-pocket cost |
|---|---|---|---|---|---|---|---|
| Basal follicle-stimulating hormone (FSH) (1988) | Day 3 of menstrual cycle | Clinically significant | 7%–8% (98%–99%) | Late | • Early follicular phase FSH level <10 mIU/mL • Estradiol level <80 pg/mL | • High estradiol level (decreases) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $125–$150 |
| Clomiphene challenge test (1989) | Days 3 and 10 of menstrual cycle | Clinically significant | 25%–40% (98%–99%) | Late | • Day 3 FSH level <10 mIU/mL; day 3 estradiol level <80 pg/mL • Day 10 FSH level <10 mIU/mL | • High day 3 estradiol level (decreases day 3 FSH) • Low day 10 estradiol (increases day 10 FSH) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $550–$600 |
| GnRH agonist (1988) | Early follicular phase of menstrual cycle | Clinically significant | 32%–89% (79%–97%) | Late | Variable | • Oral contraceptives (decrease estradiol levels) • Pregnancy (increases estrogens) | $300–$350 |
| Inhibin-B (1997) | Early follicular phase of menstrual cycle | Clinically significant | 33%–81% (29%–95%) | Early | Variable in the literature; normal cutoffs range from ≥45–80 pg/mL | • Obesity (decreases) • PCOS (increases) • Exogenous FSH administration (increases) • Oral contraceptive use (decreases) | $150–$200 |
| Antral follicle count (1997) | Early follicular phase of menstrual cycle | Clinically significant (includes interobserver variability) | 8%–60% (33%–96%) | Earliest | ≥5–10 total antral follicles | • Oral contraceptive use (decreases) • Polycystic ovary syndrome (PCOS) (increases) | $300–$500 |
| Anti–Müllerian hormone/Müllerian-inhibiting substance (2002) | At any time; not cycle-dependent | Minimal | 49%–76% (89%–94%) | Earliest | >0.7 ng/mL | • PCOS (increases) • Obesity (decreases) • Exogenous FSH administration (decreases) | $150–$400 |
1 | Basal FSH—widely used but only moderately informative
Day 3 FSH and the CCCT are the most widely used measures of ovarian reserve in ART practice. The use of early follicular-phase FSH as a marker of ovarian reserve and fertility was proposed 20 years ago with the emergence of IVF.2-4 The test is an indirect assessment of ovarian reserve in that it measures pituitary production of FSH in response to feedback from ovarian hormones. Estradiol and inhibin-B reach a nadir early in the menstrual cycle; measuring FSH on day 3 offers a glimpse of the functioning of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise later in the cycle ( FIGURE 1 ).5,6
FIGURE 1 The HPO axis
The FSH level opens a window onto the function of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise in the cycle. Women who have normal ovarian reserve have sufficient ovarian hormone production early in the menstrual cycle to maintain FSH levels within the normal range. Conversely, a “monotropic” elevation in FSH—one that is unaccompanied by a rise in luteinizing hormone (LH)—reflects poor hormone production from an aging pool of ovarian follicles and disinhibition of FSH production.5,6
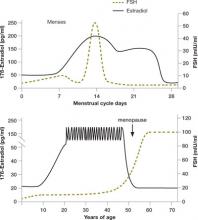
FSH measurements are typically combined with estradiol to enhance the sensitivity of testing ( FIGURE 2, ). Premature elevations of estradiol early in the follicular phase are driven by rising FSH levels in women with declining ovarian reserve. Abnormally elevated estrogen levels then feed back negatively on pituitary production of FSH and mask an elevation that might otherwise reveal diminished ovarian reserve. Measurement of both FSH and estradiol on cycle day 3 may therefore help decrease the incidence of false-negative testing.
Commonly cited criteria for normal ovarian reserve are:
- early follicular phase FSH, <10 mIU/mL
- estradiol, <80 pg/mL1
It is extremely important to note, however, that these are general guidelines and that cutoffs are both laboratory- and practice-specific.
FIGURE 2 Monthly and lifetime variations in estradiol and FSH
How 17ß-estradiol and follicle-stimulating hormone levels vary over the menstrual cycle (top) and a woman’s lifetime (bottom).
2 | Clomiphene citrate—more sensitive than FSH testing
Like basal FSH testing, the CCCT is an indirect assessment of ovarian reserve. Unlike FSH testing, the CCCT is provocative. It involves administration of 100 mg of clomiphene citrate (Clomid) on days 5 through 9 of the menstrual cycle, with FSH and estradiol measured on days 3 and 10. Once clomiphene citrate is administered, FSH and LH levels rise, followed by an increase in estradiol and inhibin. Evidence suggests that the smaller follicular cohorts in women with diminished ovarian reserve produce less inhibin-B and estradiol and, therefore, less negative feedback on clomiphene-induced pituitary FSH release.6,7 The result: persistent elevation of the day 10 FSH value and a positive screen for diminished ovarian reserve.
In some women, day 10 FSH is elevated even after a normal day 3 value. This makes the CCCT more sensitive than basal FSH testing; it can identify women who might go unrecognized if evaluated by day 3 FSH and estradiol levels alone.
More expensive and labor-intensive than the alternatives
Interpretation of the CCCT requires that FSH and estradiol both be assessed on days 3 and 10. An elevated FSH (≥10 mIU/mL) on either day indicates diminished ovarian reserve. As with basal FSH testing, elevated estradiol (≥80 pg/mL) on day 3 is considered abnormal. The day 10 estradiol value of the CCCT reflects whether or not clomiphene citrate was administered appropriately, and should be elevated. However, the significance of the day 10 estradiol level has been debated with respect to its predictive value for pregnancy in infertile populations.8
The addition of day 10 FSH assessment improves the sensitivity of the CCCT over basal FSH measurement, but makes it a more expensive and labor-intensive test ( TABLE ).5,6 The CCCT involves administration of clomiphene citrate, a safe drug (though it can have side effects), and two blood draws instead of one. Nevertheless, both tests are relatively noninvasive, rapid measures of ovarian reserve.
Drawbacks of the tests
Both basal FSH testing and the CCCT are widely used, although support for their ability to predict ovarian reserve in the infertile population has been challenged recently. Newer data demonstrate that these tests are limited in their ability to predict outcome (pregnancy and response to superovulation drugs) in all but a narrow group of patients undergoing IVF. Performance is particularly limited in:
Additional drawbacks of basal FSH testing and the CCCT include:
- significant variability of test results from cycle to cycle (intercycle variability)
- limited time frame within which the tests can be performed (intracycle variability).
The basal FSH test and CCCT have high specificity (98% to 99% for each) as an assessment of reproductive performance in infertile women and generate few false-positive results.5,6 However, the high screen cutoffs that allow for such specificity come at a price: Few women will screen positive, and sensitivity of the tests is low (between 7% and 8% for basal FSH and between 25% and 40% for the CCCT). Such low sensitivity means that many women will not conceive after infertility treatment despite a normal test result.5,11 Overall, the tests are not highly informative for many women who get tested.
Once abnormal, normal results are meaningless
Once an FSH level or the CCCT has ever been abnormal, the patient has diminished ovarian reserve; normal values in subsequent menstrual cycles do not improve the odds of pregnancy with treatment.14 This fact can be a significant source of confusion and frustration for patients.
3 | GnRH agonist stimulation —no better than FSH testing
This test was developed in the search for a very sensitive assessment of ovarian reserve. It was designed to uncover subtle abnormalities in pituitary and ovarian dynamics. It involves administering a gonadotropin-releasing hormone (GnRH) agonist such as leuprolide acetate (Lupron) on day 2 or 3 of the menstrual cycle and measuring pituitary and ovarian hormone responses.5,15
One group of investigators demonstrated a correlation between stimulated estradiol levels and responsiveness during IVF,16 but other studies have shown that the test does not perform significantly better than day 3 FSH in predicting ovarian reserve.17,18
The sensitivity of GnRH agonist testing for pregnancy is moderate (32% to 89%); specificity ranges from 79% to 97%.19
4 | Inhibin-B—not helpful when used alone
This glycoprotein hormone produced by granulosa cells of developing follicles is a direct measure of ovarian reserve when assessed in the early follicular phase of the menstrual cycle.20 Women treated with IVF who have a low inhibin-B level—particularly when using cutoffs below the range of 45–80 pg/mL—have been shown to respond poorly to superovulation and have a lower pregnancy rate than women with high inhibin-B.21,22 One group of investigators demonstrated that women with clinical evidence of diminished ovarian reserve but a normal FSH level also had low inhibin-B production, suggesting that it may be a more sensitive marker than FSH.22
Inhibin-B testing involves a simple blood draw. However, the test has been incorporated into clinical assessment of ovarian reserve only to a limited degree, due to the lack of reliable assays and controversy concerning its prognostic value.23
Because of these limitations, routine testing of serum inhibin-B in isolation of other markers of ovarian reserve is not recommended.
5 | Antral follicle count—good predictor of IVF outcome
Transvaginal ultrasonographic determination of the number of ovarian follicles that measure between 2 mm and 10 mm in diameter in the early follicular phase of the cycle yields the AFC. As a direct marker of the cohort of growing follicles in the early menstrual cycle, the AFC is believed to correlate strongly with the number of primordial follicles present in the ovary and, therefore, ovarian reserve. Total AFCs of less than 5 to 10 are suggestive of diminished ovarian reserve.24,25
In IVF cycles, AFC has proven to be an accurate predictor of number of oocytes retrieved, risk of cycle cancellation, and odds of conception.24,25 Some investigators have even suggested that, compared with other markers of ovarian reserve, AFC is the best independent predictor of outcome in IVF cycles.7,26-27
In a group of normally cycling women with proven fertility, AFC also showed a strong correlation with age, declining slowly until age 37 and more rapidly thereafter.28,29
AFC sensitivity for pregnancy is moderate and varies widely in published reports (8% to 60%), whereas specificity tends to be higher (33% to 96%).19
Drawbacks of AFC
- Because of the need to perform transvaginal ultrasonography, AFC is a more invasive and often more expensive test than hormonal biomarkers
- Accurate assessment of AFC requires an experienced sonographer and can be limited in patients who have had pelvic surgery or uterine fibroids and in those who are obese
- Moderate interobserver and intercycle variability of AFC determinations limits its reproducibility29,30
- As with basal FSH measurement, the intercycle variability of AFC does not correlate well with IVF outcome in individual patients.30
6 | Anti-Müllerian hormone— many advantages
The drawbacks of the tests just described— e.g., intercycle variability, lack of uniform cutoffs, and limited ability to predict IVF outcomes—make the development of more reliable measures of ovarian reserve a priority in reproductive medicine. AMH is a highly promising marker that appears to have many advantages over other tests and may have the greatest power to predict ovarian aging in women of reproductive age.
How it works
AMH is a glycoprotein growth factor and a member of the transforming growth factor-ß superfamily.31 It is primarily produced by the pool of early-growing follicles, which are believed to serve as a proxy for the number of primordial follicles in the ovary. The number of primordial follicles at a given point in time represents the ovarian reserve. AMH levels above 0.7 ng/mL are considered normal; values between 0.3 ng/mL and 0.7 ng/mL are consistent with borderline ovarian reserve, according to 2007 data from Reprosource Corp.
AMH has been studied as a marker of ovarian reserve for 6 years, with multiple reports describing declines in levels with age and with diminishing oocyte numbers. It is undetectable at menopause.32
The age-related decline in AMH is gradual but measurable even in young women, consistently preceding changes in other markers of ovarian reserve such as FSH and inhibin-B.32-35 The longitudinal changes in AMH have been demonstrated in ovulatory premenopausal women and healthy volunteers with proven fertility.33,34 In one series of women followed over a mean of 4 years (ages 25 to 46), AMH testing was superior to day 3 FSH, inhibin-B, and AFC in its ability to predict the onset of cycle irregularity and the menopausal transition.33
Does it predict oocyte quality?
AMH has performed well as a biomarker, comparable in most series to AFC and superior to FSH. AMH levels are strongly correlated with the number of oocytes retrieved during IVF and the odds of cycle cancellation due to poor response35-41 —but does it accurately characterize oocyte quality, the other element of ovarian reserve?
Some reports have shown a strong association between AMH levels and surrogates of oocyte quality, including fertilization, oocyte morphology, embryo quality, and pregnancy and miscarriage rates,36-41 but others have not.42 Some reports demonstrate a relationship between AMH and some but not all surrogate markers of oocyte quality.40
Advantages of AMH
- It demonstrates minimal intracycle variability.32,43-45 Compared with other markers of ovarian reserve, which must be measured early in the follicular phase of the menstrual cycle, AMH can be assessed at random times, making it a more convenient method for patients and physicians
- It demonstrates minimal intercycle variability32,34
- AMH levels are not significantly affected by the hormonal changes of pregnancy, oral contraceptive use, or GnRH treatment, and can be measured in these settings.46,47
Utility of AMH is limited in PCOS and obesity
The ability to use AMH as a marker of ovarian aging in women who have polycystic ovary syndrome (PCOS) and in women who are obese may be limited by the ovulatory dysfunction in these populations. Circulating levels of AMH are higher in women with PCOS than in unaffected women, a finding thought to be indicative of oligo-ovulation and poor follicular development in polycystic ovaries.48-53
In a recent series investigating AMH levels in women with PCOS, AMH and the degree of insulin resistance were positively correlated, and the AMH level was negatively correlated with the number of menses in a year.49 The consistently positive correlation between AMH and PCOS may suggest a future role for this marker as a diagnostic tool.
In obese women who do not have PCOS, AMH production may be lower than in women of normal weight. In a recent series, normally cycling obese women in the later reproductive years were shown to have an AMH level 70% lower than those in women who were not obese.54 These differences have not been well studied in younger obese women.
Which test is best?
AMH may be preferable to the other tests to assess ovarian reserve because it can be measured any time during the menstrual cycle or between cycles. AMH measurement is also useful if a woman is taking oral contraceptives or leuprolide acetate because these medications may confound the results of the other test methods. In addition, AMH may be the earliest indicator of decline in ovarian reproductive function. As such, it may highlight cases that merit a search for other causes of infertility and make it possible to treat them in a timely manner.
Elevated AMH may reveal occult PCOS and warn of significant risk of ovarian hyperstimulation prior to ovulation induction with gonadotrophins, so that the clinician can plan smaller doses.
A normal female is born with 1 million to 2 million oocytes, a number that declines continuously, primarily through the process of follicular atresia. By the onset of puberty, the number of oocytes has declined to approximately 300,000. As a woman enters her late 30s, when the total number of oocytes is approximately 25,000, the pace of oocyte depletion begins to increase, as does the rate of spontaneous miscarriage.1,55,56
The effect of age on fertility is believed to arise from changes in both oocyte number and quality. Multiple investigators have found a greater frequency of cellular abnormalities in oocytes from older women.1,2,5,15,57
Although ovarian reserve declines with age in all women, women of similar ages can have very different degrees of ovarian reserve, and some women who have very poor ovarian reserve may never conceive, despite aggressive fertility treatment.
The biologic basis for differences in ovarian reserve among similar groups of women is not completely understood, but is probably rooted in genetic, lifestyle, and environmental factors that affect granulosa cell and oocyte function. Identifying sensitive biomarkers that can determine ovarian reserve independent of age is critical to predict fertility and age at menopause.5
1. Practice Committee of the American Society for Reproductive Medicine. Age and infertility in women. Fertil Steril. 2006;86:S248-S252.
2. Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu H-C. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50:298-307.
3. Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651-654.
4. Toner JP, Philiput CB, Jones GS, Muasher SJ. Basal follicle stimulating hormone level is a better predictor of in vitro fertilization outcome than age. Fertil Steril. 1991;55:784-791.
5. Barnhart K, Osheroff J. Follicle stimulating hormone as a predictor of fertility. Curr Opin Obstet Gynecol. 1998;10:227-232.
6. Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: the physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;69:474-477.
7. Yong PY, Baird DT, Thong KJ, McNeilly AS, Anderson RA. Prospective analysis of the relationships between the ovarian follicle cohort and basal FSH concentration, the inhibin response to exogenous FSH and ovarian follicle number at different stages of the normal menstrual cycle and after pituitary down-regulation. Hum Reprod. 2003;18:35-44.
8. Scott RT, Jr, Illions EH, Kost ER, Dellinger C, Hofmann GE, Navot D. Evaluation of the significance of the estradiol response during the clomiphene citrate challenge test. Fertil Steril. 1993;60:242-246.
9. Esposito MA, Coutifaris C, Barnhart KT. A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod. 2002;17:118-123.
10. Bancsi L, Broekmans FJM, Wol BWJ, Habbema DK, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003;79:1091-1100.
11. Jain T, Soules MR, Collins JA. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 2004;82:180-185.
12. Toner JP. Modest follicle-stimulating hormone elevations in younger women: warn but don’t disqualify. Fertil Steril. 2004;81:1493-1495.
13. Van Rooij IAJ, de Jong E, Broekmans FJM, Looman CWN, Habbeman DK, te Velde ER. High follicle-stimulating hormone levels should not necessarily lead to the exclusion of subfertile patients from treatment. Fertil Steril. 2004;81:1478-1485.
14. Scott RT, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54:297-302.
15. Bulkulmez O, Arici A. Assessment of ovarian reserve. Curr Opin Obstet Gynecol. 2004;16:231-237.
16. Ranieri DM, Quinn F, Makhlouf A, et al. Simultaneous evaluation of basal follicle-stimulating hormone and 17-beta-estradiol response to gonadotropin-releasing hormone analogue stimulation: an improved predictor of ovarian reserve. Fertil Steril. 1998;70:227-233.
17. Fujimoto VY, Klein NA, Battaglia DE, Bremmer WJ, Soules MR. The anterior pituitary response to a gonadotropin-releasing hormone challenge test in normal older reproductive age women. Fertil Steril. 1996;65:539-544.
18. Galtier-Dereure F, De Bouard V, Picto MC, et al. Ovarian reserve test with the gonadotrophin-releasing hormone agonist buserelin: correlation with in-vitro fertilization outcome. Hum Reprod. 1996;11:1393-1398.
19. Broekmans FJ, Fwee J, Hendricks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685-718.
20. Klein NA, Illingworth PJ, Groome NP, NcNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81:2742-2745.
21. Seifer DB, Lambert-Messerlian G, Hogan JW, et al. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67:110-114.
22. Seifer DB, Scott RT, Jr, Bergh PA, et al. Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin B before a rise in day 3 follicle-stimulating hormone. Fertil Steril. 1999;72:63-65.
23. Corson SL, Gutmann J, Batzer FR, Wallace H, Klein N, Soules MR. Inhibin-B as a test of ovarian reserve for infertile women. Hum Reprod. 1999;14:2818-2821.
24. Tomas C, Nuojua-Huttunen S, Martikainen H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum Reprod. 1997;12:220-223.
25. Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505-510.
26. Hung E, Tang OS, Ho PC. The significance of the number of antral follicles prior to stimulation in predicting ovarian responses in an IVF programme. Hum Reprod. 2000;15:1937-1942.
27. Bancsi LFJMM, Broekmans FJM, Eijkemans MJC, de Jong FH, Habbema JDF, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328-336.
28. Ng EH, Yeung WS, Fong DY, Ho PC. Effects of age on hormonal and ultrasound markers of ovarian reserve in Chinese women with proven fertility. Hum Reprod. 2003;18:2169-2174.
29. Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845-851.
30. Hansen KR, Morris JL, Thyer AC, Soules MR. Reproductive aging and the variability in the ovarian antral follicle count: application in the clinical setting. Fertil Steril. 2003;80:577-583.
31. Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685-698.
32. de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM. Anti-Müllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357-362.
33. van Rooij IAJ, Broekmans FJM, Scheffer GJ, et al. Serum anti-Müllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979-987.
34. van Rooij IAJ, Tonkelaar I, Broekmans FJ, et al. Anti-Müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601-606.
35. Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Müllerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20-24.
36. Silberstein T, MacLaughlin DT, Shai I, et al. Müllerian-inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159-163.
37. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum Müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468-471.
38. Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022-2026.
39. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum anti-Müllerian hormone/Müllerian-inhibiting substance appears to be a more discriminatory marker of ART outcome than follicular stimulating hormone, inhibin B or estradiol. Fertil Steril. 2004;82:1323-1329.
40. Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22:2414-2421.
41. Fanchin R, Mendez DH, Frydman N, et al. Anti-Müllerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796-1802.
42. Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Th omas CM, Braat DD. Anti-Müllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87:223-226.
43. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FM, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057-4063.
44. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Müllerian hormone throughout the menstrual cycle. Hum Reprod. 2006;21:3103-3107.
45. Tsepelidis S, Devreker F, Demeestere F, Flahaut I, Gervy A, Englert C. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837-1840.
46. La Marca A, Giulini Orvieto R, De Leo V, Volpe A. Anti-Müllerian hormone concentrations in maternal serum during pregnancy. Hum Reprod. 2005;20:1569-1572.
47. Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Müllerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196-201.
48. Al-Qahtani A, Groome NP. Anti-Müllerian hormone: Cinderella finds new admirers. J Clin Endocrinol Metab. 2006;91:3760-3762.
49. La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Müllerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970-971.
50. Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820-1836.
51. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-Müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957-5962.
52. Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum anti-Müllerian substance and other reproductive hormones in untreated women with polycystic ovary syndrome and endometriosis. Fertil Steril. 1997;67:962-965.
53. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240-245.
54. Freeman EW, Gracia CG, Sammel MD, Lin H, Lim LC, Strauss JF, 3rd. Association of anti-Müllerian hormone levels with obesity in later reproductive-age women. Fertil Steril. 2007;87:101-106.
55. Scott RT, Opsahl MS, Leonardi MR, Neall GS, Illions EH, Navot D. Life table analysis of pregnancy rates in a general infertility population relative to ovarian reserve and patient age. Hum Reprod. 1995;10:1706-1710.
56. Speroff L. Fritz M. eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
57. Lim AS, Tsakok MFH. Age-related decline in fertility: a link to degenerative oocytes? Fertil Steril. 1997;68:265-271.
The University of Medicine and Dentistry of New Jersey (UMDNJ) owns a patent relating to the use of anti-Müllerian hormone/Müllerian inhibiting substance for predicting ovarian response in women with infertility. The patent is based in part on work that Dr. Seifer carried out while employed at UMDNJ. In accordance with UMDNJ policy, Dr. Seifer, a named inventor on this patent, assigned his interest in the invention to UMDNJ. UMDNJ has a licensing agreement with Diagnostic Systems Laboratory for the use of the claimed invention. Dr. Seifer receives a portion of the royalties, as determined by UMDNJ policy, that UMDNJ gains from this licensing agreement.
CASE: Borderline test result prompts referral
A 36-year-old nulliparous woman is seen in your office for evaluation after 6 months of infertility. She is ovulatory, and has been using an ovulation-prediction kit to time intercourse. You learn that she had Chlamydia trachomatis infection in the distant past, but elicit no other significant medical or surgical history. She reports that she smoked approximately one pack of cigarettes a day for 15 years but gave up smoking 5 years ago.
You order a hysterosalpingogram, followed by day 3 testing of follicle-stimulating hormone (FSH). The hysterosalpingogram is normal; the FSH level is 7.5 mIU/mL and the estradiol level is 30 pg/mL—both in the normal range.
The patient asks for testing of anti-Müllerian hormone (AMH; also known as Müllerian-inhibiting substance) because she has read that it is a new marker of fertility. The result is 0.5 ng/mL, a borderline value. After reviewing these results, you refer her to a reproductive endocrinologist for further management.
Was the test for AMH indicated? And is this referral appropriate?
The referral is entirely appropriate, even though the patient has not been trying to conceive for a full year. Why? The AMH value suggests that her ovarian reserve is in early decline. She would benefit from evaluation by a subspecialist who can review the entire spectrum of treatments, including aggressive options such as ovulation induction and in vitro fertilization (IVF), to optimize her reproductive success.
This article reviews the various biomarkers available to assess ovarian reserve in women who experience infertility:
- day 3 (basal) FSH
- clomiphene citrate challenge
- gonadotropin-releasing hormone (GnRH) agonist stimulation
- inhibin-B
- antral follicle count (AFC)
- AMH.
The AFC and AMH tend to detect the earliest changes in ovarian reserve, followed, sequentially, by inhibin-B, the clomiphene citrate challenge test (CCCT), and basal FSH.
The tests we describe are used primarily to assess treatment prognosis in infertile women. In time, however, appropriate population screening of ovarian reserve may be feasible to provide many more women with information about their reproductive potential and help them shape their life plan.
What makes a test valuable?
Ovarian reserve describes a woman’s reproductive potential—specifically, the number and quality of oocytes she possesses.1 Biochemical tests of ovarian reserve emerged during the rise of assisted reproductive technologies (ART) in the late 1980s to predict both responsiveness to superovulation drugs and the odds of pregnancy with treatment.
Ideally, a test that assesses ovarian reserve should be affordable, straightforward, rapidly interpretable, and minimally invasive. It also should be able to detect changes that begin early in reproductive life. To be applicable to large populations of reproductive-age women, it should be of use anytime in the menstrual cycle, and should provide reproducible and highly accurate assessment of the reproductive aging process.
Our ability to offer tests that accurately measure ovarian reserve has a significant impact on women at risk of infertility and early menopause and on those who choose to delay childbearing for personal (nonmedical) reasons. These tests have become increasingly relevant because women are choosing to have their first child at a later age than their counterparts did 20 years ago:
- In 1980, 40% of women having their first baby were younger than 25 years, and only 5% were older than 35
- In 2000, 25% of women were younger than 25 when their first child was born, and 15% were older than 35.
Who should be tested?
Ovarian reserve is a complex clinical phenomenon that is influenced by age, genetics, and environmental variables. The decline in a woman’s ovarian reserve over time is irreversible; the trajectory of this decline is fundamental to the odds of fertility with age and the timing of the menopausal transition. At present, the markers used most often in clinical practice have some utility but also suffer from several drawbacks ( TABLE ).
For the general practitioner performing an infertility evaluation, we recommend focusing on the following groups of women for ovarian reserve testing:
- women over 30 years of age
- women with a history of exposure to a confirmed gonadotoxin, i.e., tobacco smoke, chemotherapy, radiation therapy
- women with a strong family history of early menopause or premature ovarian failure
- women who have had extensive ovarian surgery, i.e., cystectomy and unilateral oophorectomy.
Testing tends to have the highest yield in these groups. Women who have abnormal results should be referred to a reproductive endocrinologist for further evaluation and treatment.
The six tests are described below.
TABLE
How six markers of ovarian reserve stack up
| Test (year described) | Timing | Intracycle and intercycle variability | Sensitivity (specificity) | Reflects changes in ovarian reserve | Normal levels | Confounders | Out-of-pocket cost |
|---|---|---|---|---|---|---|---|
| Basal follicle-stimulating hormone (FSH) (1988) | Day 3 of menstrual cycle | Clinically significant | 7%–8% (98%–99%) | Late | • Early follicular phase FSH level <10 mIU/mL • Estradiol level <80 pg/mL | • High estradiol level (decreases) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $125–$150 |
| Clomiphene challenge test (1989) | Days 3 and 10 of menstrual cycle | Clinically significant | 25%–40% (98%–99%) | Late | • Day 3 FSH level <10 mIU/mL; day 3 estradiol level <80 pg/mL • Day 10 FSH level <10 mIU/mL | • High day 3 estradiol level (decreases day 3 FSH) • Low day 10 estradiol (increases day 10 FSH) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $550–$600 |
| GnRH agonist (1988) | Early follicular phase of menstrual cycle | Clinically significant | 32%–89% (79%–97%) | Late | Variable | • Oral contraceptives (decrease estradiol levels) • Pregnancy (increases estrogens) | $300–$350 |
| Inhibin-B (1997) | Early follicular phase of menstrual cycle | Clinically significant | 33%–81% (29%–95%) | Early | Variable in the literature; normal cutoffs range from ≥45–80 pg/mL | • Obesity (decreases) • PCOS (increases) • Exogenous FSH administration (increases) • Oral contraceptive use (decreases) | $150–$200 |
| Antral follicle count (1997) | Early follicular phase of menstrual cycle | Clinically significant (includes interobserver variability) | 8%–60% (33%–96%) | Earliest | ≥5–10 total antral follicles | • Oral contraceptive use (decreases) • Polycystic ovary syndrome (PCOS) (increases) | $300–$500 |
| Anti–Müllerian hormone/Müllerian-inhibiting substance (2002) | At any time; not cycle-dependent | Minimal | 49%–76% (89%–94%) | Earliest | >0.7 ng/mL | • PCOS (increases) • Obesity (decreases) • Exogenous FSH administration (decreases) | $150–$400 |
1 | Basal FSH—widely used but only moderately informative
Day 3 FSH and the CCCT are the most widely used measures of ovarian reserve in ART practice. The use of early follicular-phase FSH as a marker of ovarian reserve and fertility was proposed 20 years ago with the emergence of IVF.2-4 The test is an indirect assessment of ovarian reserve in that it measures pituitary production of FSH in response to feedback from ovarian hormones. Estradiol and inhibin-B reach a nadir early in the menstrual cycle; measuring FSH on day 3 offers a glimpse of the functioning of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise later in the cycle ( FIGURE 1 ).5,6
FIGURE 1 The HPO axis
The FSH level opens a window onto the function of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise in the cycle. Women who have normal ovarian reserve have sufficient ovarian hormone production early in the menstrual cycle to maintain FSH levels within the normal range. Conversely, a “monotropic” elevation in FSH—one that is unaccompanied by a rise in luteinizing hormone (LH)—reflects poor hormone production from an aging pool of ovarian follicles and disinhibition of FSH production.5,6
FSH measurements are typically combined with estradiol to enhance the sensitivity of testing ( FIGURE 2, ). Premature elevations of estradiol early in the follicular phase are driven by rising FSH levels in women with declining ovarian reserve. Abnormally elevated estrogen levels then feed back negatively on pituitary production of FSH and mask an elevation that might otherwise reveal diminished ovarian reserve. Measurement of both FSH and estradiol on cycle day 3 may therefore help decrease the incidence of false-negative testing.
Commonly cited criteria for normal ovarian reserve are:
- early follicular phase FSH, <10 mIU/mL
- estradiol, <80 pg/mL1
It is extremely important to note, however, that these are general guidelines and that cutoffs are both laboratory- and practice-specific.
FIGURE 2 Monthly and lifetime variations in estradiol and FSH
How 17ß-estradiol and follicle-stimulating hormone levels vary over the menstrual cycle (top) and a woman’s lifetime (bottom).
2 | Clomiphene citrate—more sensitive than FSH testing
Like basal FSH testing, the CCCT is an indirect assessment of ovarian reserve. Unlike FSH testing, the CCCT is provocative. It involves administration of 100 mg of clomiphene citrate (Clomid) on days 5 through 9 of the menstrual cycle, with FSH and estradiol measured on days 3 and 10. Once clomiphene citrate is administered, FSH and LH levels rise, followed by an increase in estradiol and inhibin. Evidence suggests that the smaller follicular cohorts in women with diminished ovarian reserve produce less inhibin-B and estradiol and, therefore, less negative feedback on clomiphene-induced pituitary FSH release.6,7 The result: persistent elevation of the day 10 FSH value and a positive screen for diminished ovarian reserve.
In some women, day 10 FSH is elevated even after a normal day 3 value. This makes the CCCT more sensitive than basal FSH testing; it can identify women who might go unrecognized if evaluated by day 3 FSH and estradiol levels alone.
More expensive and labor-intensive than the alternatives
Interpretation of the CCCT requires that FSH and estradiol both be assessed on days 3 and 10. An elevated FSH (≥10 mIU/mL) on either day indicates diminished ovarian reserve. As with basal FSH testing, elevated estradiol (≥80 pg/mL) on day 3 is considered abnormal. The day 10 estradiol value of the CCCT reflects whether or not clomiphene citrate was administered appropriately, and should be elevated. However, the significance of the day 10 estradiol level has been debated with respect to its predictive value for pregnancy in infertile populations.8
The addition of day 10 FSH assessment improves the sensitivity of the CCCT over basal FSH measurement, but makes it a more expensive and labor-intensive test ( TABLE ).5,6 The CCCT involves administration of clomiphene citrate, a safe drug (though it can have side effects), and two blood draws instead of one. Nevertheless, both tests are relatively noninvasive, rapid measures of ovarian reserve.
Drawbacks of the tests
Both basal FSH testing and the CCCT are widely used, although support for their ability to predict ovarian reserve in the infertile population has been challenged recently. Newer data demonstrate that these tests are limited in their ability to predict outcome (pregnancy and response to superovulation drugs) in all but a narrow group of patients undergoing IVF. Performance is particularly limited in:
Additional drawbacks of basal FSH testing and the CCCT include:
- significant variability of test results from cycle to cycle (intercycle variability)
- limited time frame within which the tests can be performed (intracycle variability).
The basal FSH test and CCCT have high specificity (98% to 99% for each) as an assessment of reproductive performance in infertile women and generate few false-positive results.5,6 However, the high screen cutoffs that allow for such specificity come at a price: Few women will screen positive, and sensitivity of the tests is low (between 7% and 8% for basal FSH and between 25% and 40% for the CCCT). Such low sensitivity means that many women will not conceive after infertility treatment despite a normal test result.5,11 Overall, the tests are not highly informative for many women who get tested.
Once abnormal, normal results are meaningless
Once an FSH level or the CCCT has ever been abnormal, the patient has diminished ovarian reserve; normal values in subsequent menstrual cycles do not improve the odds of pregnancy with treatment.14 This fact can be a significant source of confusion and frustration for patients.
3 | GnRH agonist stimulation —no better than FSH testing
This test was developed in the search for a very sensitive assessment of ovarian reserve. It was designed to uncover subtle abnormalities in pituitary and ovarian dynamics. It involves administering a gonadotropin-releasing hormone (GnRH) agonist such as leuprolide acetate (Lupron) on day 2 or 3 of the menstrual cycle and measuring pituitary and ovarian hormone responses.5,15
One group of investigators demonstrated a correlation between stimulated estradiol levels and responsiveness during IVF,16 but other studies have shown that the test does not perform significantly better than day 3 FSH in predicting ovarian reserve.17,18
The sensitivity of GnRH agonist testing for pregnancy is moderate (32% to 89%); specificity ranges from 79% to 97%.19
4 | Inhibin-B—not helpful when used alone
This glycoprotein hormone produced by granulosa cells of developing follicles is a direct measure of ovarian reserve when assessed in the early follicular phase of the menstrual cycle.20 Women treated with IVF who have a low inhibin-B level—particularly when using cutoffs below the range of 45–80 pg/mL—have been shown to respond poorly to superovulation and have a lower pregnancy rate than women with high inhibin-B.21,22 One group of investigators demonstrated that women with clinical evidence of diminished ovarian reserve but a normal FSH level also had low inhibin-B production, suggesting that it may be a more sensitive marker than FSH.22
Inhibin-B testing involves a simple blood draw. However, the test has been incorporated into clinical assessment of ovarian reserve only to a limited degree, due to the lack of reliable assays and controversy concerning its prognostic value.23
Because of these limitations, routine testing of serum inhibin-B in isolation of other markers of ovarian reserve is not recommended.
5 | Antral follicle count—good predictor of IVF outcome
Transvaginal ultrasonographic determination of the number of ovarian follicles that measure between 2 mm and 10 mm in diameter in the early follicular phase of the cycle yields the AFC. As a direct marker of the cohort of growing follicles in the early menstrual cycle, the AFC is believed to correlate strongly with the number of primordial follicles present in the ovary and, therefore, ovarian reserve. Total AFCs of less than 5 to 10 are suggestive of diminished ovarian reserve.24,25
In IVF cycles, AFC has proven to be an accurate predictor of number of oocytes retrieved, risk of cycle cancellation, and odds of conception.24,25 Some investigators have even suggested that, compared with other markers of ovarian reserve, AFC is the best independent predictor of outcome in IVF cycles.7,26-27
In a group of normally cycling women with proven fertility, AFC also showed a strong correlation with age, declining slowly until age 37 and more rapidly thereafter.28,29
AFC sensitivity for pregnancy is moderate and varies widely in published reports (8% to 60%), whereas specificity tends to be higher (33% to 96%).19
Drawbacks of AFC
- Because of the need to perform transvaginal ultrasonography, AFC is a more invasive and often more expensive test than hormonal biomarkers
- Accurate assessment of AFC requires an experienced sonographer and can be limited in patients who have had pelvic surgery or uterine fibroids and in those who are obese
- Moderate interobserver and intercycle variability of AFC determinations limits its reproducibility29,30
- As with basal FSH measurement, the intercycle variability of AFC does not correlate well with IVF outcome in individual patients.30
6 | Anti-Müllerian hormone— many advantages
The drawbacks of the tests just described— e.g., intercycle variability, lack of uniform cutoffs, and limited ability to predict IVF outcomes—make the development of more reliable measures of ovarian reserve a priority in reproductive medicine. AMH is a highly promising marker that appears to have many advantages over other tests and may have the greatest power to predict ovarian aging in women of reproductive age.
How it works
AMH is a glycoprotein growth factor and a member of the transforming growth factor-ß superfamily.31 It is primarily produced by the pool of early-growing follicles, which are believed to serve as a proxy for the number of primordial follicles in the ovary. The number of primordial follicles at a given point in time represents the ovarian reserve. AMH levels above 0.7 ng/mL are considered normal; values between 0.3 ng/mL and 0.7 ng/mL are consistent with borderline ovarian reserve, according to 2007 data from Reprosource Corp.
AMH has been studied as a marker of ovarian reserve for 6 years, with multiple reports describing declines in levels with age and with diminishing oocyte numbers. It is undetectable at menopause.32
The age-related decline in AMH is gradual but measurable even in young women, consistently preceding changes in other markers of ovarian reserve such as FSH and inhibin-B.32-35 The longitudinal changes in AMH have been demonstrated in ovulatory premenopausal women and healthy volunteers with proven fertility.33,34 In one series of women followed over a mean of 4 years (ages 25 to 46), AMH testing was superior to day 3 FSH, inhibin-B, and AFC in its ability to predict the onset of cycle irregularity and the menopausal transition.33
Does it predict oocyte quality?
AMH has performed well as a biomarker, comparable in most series to AFC and superior to FSH. AMH levels are strongly correlated with the number of oocytes retrieved during IVF and the odds of cycle cancellation due to poor response35-41 —but does it accurately characterize oocyte quality, the other element of ovarian reserve?
Some reports have shown a strong association between AMH levels and surrogates of oocyte quality, including fertilization, oocyte morphology, embryo quality, and pregnancy and miscarriage rates,36-41 but others have not.42 Some reports demonstrate a relationship between AMH and some but not all surrogate markers of oocyte quality.40
Advantages of AMH
- It demonstrates minimal intracycle variability.32,43-45 Compared with other markers of ovarian reserve, which must be measured early in the follicular phase of the menstrual cycle, AMH can be assessed at random times, making it a more convenient method for patients and physicians
- It demonstrates minimal intercycle variability32,34
- AMH levels are not significantly affected by the hormonal changes of pregnancy, oral contraceptive use, or GnRH treatment, and can be measured in these settings.46,47
Utility of AMH is limited in PCOS and obesity
The ability to use AMH as a marker of ovarian aging in women who have polycystic ovary syndrome (PCOS) and in women who are obese may be limited by the ovulatory dysfunction in these populations. Circulating levels of AMH are higher in women with PCOS than in unaffected women, a finding thought to be indicative of oligo-ovulation and poor follicular development in polycystic ovaries.48-53
In a recent series investigating AMH levels in women with PCOS, AMH and the degree of insulin resistance were positively correlated, and the AMH level was negatively correlated with the number of menses in a year.49 The consistently positive correlation between AMH and PCOS may suggest a future role for this marker as a diagnostic tool.
In obese women who do not have PCOS, AMH production may be lower than in women of normal weight. In a recent series, normally cycling obese women in the later reproductive years were shown to have an AMH level 70% lower than those in women who were not obese.54 These differences have not been well studied in younger obese women.
Which test is best?
AMH may be preferable to the other tests to assess ovarian reserve because it can be measured any time during the menstrual cycle or between cycles. AMH measurement is also useful if a woman is taking oral contraceptives or leuprolide acetate because these medications may confound the results of the other test methods. In addition, AMH may be the earliest indicator of decline in ovarian reproductive function. As such, it may highlight cases that merit a search for other causes of infertility and make it possible to treat them in a timely manner.
Elevated AMH may reveal occult PCOS and warn of significant risk of ovarian hyperstimulation prior to ovulation induction with gonadotrophins, so that the clinician can plan smaller doses.
A normal female is born with 1 million to 2 million oocytes, a number that declines continuously, primarily through the process of follicular atresia. By the onset of puberty, the number of oocytes has declined to approximately 300,000. As a woman enters her late 30s, when the total number of oocytes is approximately 25,000, the pace of oocyte depletion begins to increase, as does the rate of spontaneous miscarriage.1,55,56
The effect of age on fertility is believed to arise from changes in both oocyte number and quality. Multiple investigators have found a greater frequency of cellular abnormalities in oocytes from older women.1,2,5,15,57
Although ovarian reserve declines with age in all women, women of similar ages can have very different degrees of ovarian reserve, and some women who have very poor ovarian reserve may never conceive, despite aggressive fertility treatment.
The biologic basis for differences in ovarian reserve among similar groups of women is not completely understood, but is probably rooted in genetic, lifestyle, and environmental factors that affect granulosa cell and oocyte function. Identifying sensitive biomarkers that can determine ovarian reserve independent of age is critical to predict fertility and age at menopause.5
The University of Medicine and Dentistry of New Jersey (UMDNJ) owns a patent relating to the use of anti-Müllerian hormone/Müllerian inhibiting substance for predicting ovarian response in women with infertility. The patent is based in part on work that Dr. Seifer carried out while employed at UMDNJ. In accordance with UMDNJ policy, Dr. Seifer, a named inventor on this patent, assigned his interest in the invention to UMDNJ. UMDNJ has a licensing agreement with Diagnostic Systems Laboratory for the use of the claimed invention. Dr. Seifer receives a portion of the royalties, as determined by UMDNJ policy, that UMDNJ gains from this licensing agreement.
CASE: Borderline test result prompts referral
A 36-year-old nulliparous woman is seen in your office for evaluation after 6 months of infertility. She is ovulatory, and has been using an ovulation-prediction kit to time intercourse. You learn that she had Chlamydia trachomatis infection in the distant past, but elicit no other significant medical or surgical history. She reports that she smoked approximately one pack of cigarettes a day for 15 years but gave up smoking 5 years ago.
You order a hysterosalpingogram, followed by day 3 testing of follicle-stimulating hormone (FSH). The hysterosalpingogram is normal; the FSH level is 7.5 mIU/mL and the estradiol level is 30 pg/mL—both in the normal range.
The patient asks for testing of anti-Müllerian hormone (AMH; also known as Müllerian-inhibiting substance) because she has read that it is a new marker of fertility. The result is 0.5 ng/mL, a borderline value. After reviewing these results, you refer her to a reproductive endocrinologist for further management.
Was the test for AMH indicated? And is this referral appropriate?
The referral is entirely appropriate, even though the patient has not been trying to conceive for a full year. Why? The AMH value suggests that her ovarian reserve is in early decline. She would benefit from evaluation by a subspecialist who can review the entire spectrum of treatments, including aggressive options such as ovulation induction and in vitro fertilization (IVF), to optimize her reproductive success.
This article reviews the various biomarkers available to assess ovarian reserve in women who experience infertility:
- day 3 (basal) FSH
- clomiphene citrate challenge
- gonadotropin-releasing hormone (GnRH) agonist stimulation
- inhibin-B
- antral follicle count (AFC)
- AMH.
The AFC and AMH tend to detect the earliest changes in ovarian reserve, followed, sequentially, by inhibin-B, the clomiphene citrate challenge test (CCCT), and basal FSH.
The tests we describe are used primarily to assess treatment prognosis in infertile women. In time, however, appropriate population screening of ovarian reserve may be feasible to provide many more women with information about their reproductive potential and help them shape their life plan.
What makes a test valuable?
Ovarian reserve describes a woman’s reproductive potential—specifically, the number and quality of oocytes she possesses.1 Biochemical tests of ovarian reserve emerged during the rise of assisted reproductive technologies (ART) in the late 1980s to predict both responsiveness to superovulation drugs and the odds of pregnancy with treatment.
Ideally, a test that assesses ovarian reserve should be affordable, straightforward, rapidly interpretable, and minimally invasive. It also should be able to detect changes that begin early in reproductive life. To be applicable to large populations of reproductive-age women, it should be of use anytime in the menstrual cycle, and should provide reproducible and highly accurate assessment of the reproductive aging process.
Our ability to offer tests that accurately measure ovarian reserve has a significant impact on women at risk of infertility and early menopause and on those who choose to delay childbearing for personal (nonmedical) reasons. These tests have become increasingly relevant because women are choosing to have their first child at a later age than their counterparts did 20 years ago:
- In 1980, 40% of women having their first baby were younger than 25 years, and only 5% were older than 35
- In 2000, 25% of women were younger than 25 when their first child was born, and 15% were older than 35.
Who should be tested?
Ovarian reserve is a complex clinical phenomenon that is influenced by age, genetics, and environmental variables. The decline in a woman’s ovarian reserve over time is irreversible; the trajectory of this decline is fundamental to the odds of fertility with age and the timing of the menopausal transition. At present, the markers used most often in clinical practice have some utility but also suffer from several drawbacks ( TABLE ).
For the general practitioner performing an infertility evaluation, we recommend focusing on the following groups of women for ovarian reserve testing:
- women over 30 years of age
- women with a history of exposure to a confirmed gonadotoxin, i.e., tobacco smoke, chemotherapy, radiation therapy
- women with a strong family history of early menopause or premature ovarian failure
- women who have had extensive ovarian surgery, i.e., cystectomy and unilateral oophorectomy.
Testing tends to have the highest yield in these groups. Women who have abnormal results should be referred to a reproductive endocrinologist for further evaluation and treatment.
The six tests are described below.
TABLE
How six markers of ovarian reserve stack up
| Test (year described) | Timing | Intracycle and intercycle variability | Sensitivity (specificity) | Reflects changes in ovarian reserve | Normal levels | Confounders | Out-of-pocket cost |
|---|---|---|---|---|---|---|---|
| Basal follicle-stimulating hormone (FSH) (1988) | Day 3 of menstrual cycle | Clinically significant | 7%–8% (98%–99%) | Late | • Early follicular phase FSH level <10 mIU/mL • Estradiol level <80 pg/mL | • High estradiol level (decreases) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $125–$150 |
| Clomiphene challenge test (1989) | Days 3 and 10 of menstrual cycle | Clinically significant | 25%–40% (98%–99%) | Late | • Day 3 FSH level <10 mIU/mL; day 3 estradiol level <80 pg/mL • Day 10 FSH level <10 mIU/mL | • High day 3 estradiol level (decreases day 3 FSH) • Low day 10 estradiol (increases day 10 FSH) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $550–$600 |
| GnRH agonist (1988) | Early follicular phase of menstrual cycle | Clinically significant | 32%–89% (79%–97%) | Late | Variable | • Oral contraceptives (decrease estradiol levels) • Pregnancy (increases estrogens) | $300–$350 |
| Inhibin-B (1997) | Early follicular phase of menstrual cycle | Clinically significant | 33%–81% (29%–95%) | Early | Variable in the literature; normal cutoffs range from ≥45–80 pg/mL | • Obesity (decreases) • PCOS (increases) • Exogenous FSH administration (increases) • Oral contraceptive use (decreases) | $150–$200 |
| Antral follicle count (1997) | Early follicular phase of menstrual cycle | Clinically significant (includes interobserver variability) | 8%–60% (33%–96%) | Earliest | ≥5–10 total antral follicles | • Oral contraceptive use (decreases) • Polycystic ovary syndrome (PCOS) (increases) | $300–$500 |
| Anti–Müllerian hormone/Müllerian-inhibiting substance (2002) | At any time; not cycle-dependent | Minimal | 49%–76% (89%–94%) | Earliest | >0.7 ng/mL | • PCOS (increases) • Obesity (decreases) • Exogenous FSH administration (decreases) | $150–$400 |
1 | Basal FSH—widely used but only moderately informative
Day 3 FSH and the CCCT are the most widely used measures of ovarian reserve in ART practice. The use of early follicular-phase FSH as a marker of ovarian reserve and fertility was proposed 20 years ago with the emergence of IVF.2-4 The test is an indirect assessment of ovarian reserve in that it measures pituitary production of FSH in response to feedback from ovarian hormones. Estradiol and inhibin-B reach a nadir early in the menstrual cycle; measuring FSH on day 3 offers a glimpse of the functioning of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise later in the cycle ( FIGURE 1 ).5,6
FIGURE 1 The HPO axis
The FSH level opens a window onto the function of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise in the cycle. Women who have normal ovarian reserve have sufficient ovarian hormone production early in the menstrual cycle to maintain FSH levels within the normal range. Conversely, a “monotropic” elevation in FSH—one that is unaccompanied by a rise in luteinizing hormone (LH)—reflects poor hormone production from an aging pool of ovarian follicles and disinhibition of FSH production.5,6
FSH measurements are typically combined with estradiol to enhance the sensitivity of testing ( FIGURE 2, ). Premature elevations of estradiol early in the follicular phase are driven by rising FSH levels in women with declining ovarian reserve. Abnormally elevated estrogen levels then feed back negatively on pituitary production of FSH and mask an elevation that might otherwise reveal diminished ovarian reserve. Measurement of both FSH and estradiol on cycle day 3 may therefore help decrease the incidence of false-negative testing.
Commonly cited criteria for normal ovarian reserve are:
- early follicular phase FSH, <10 mIU/mL
- estradiol, <80 pg/mL1
It is extremely important to note, however, that these are general guidelines and that cutoffs are both laboratory- and practice-specific.
FIGURE 2 Monthly and lifetime variations in estradiol and FSH
How 17ß-estradiol and follicle-stimulating hormone levels vary over the menstrual cycle (top) and a woman’s lifetime (bottom).
2 | Clomiphene citrate—more sensitive than FSH testing
Like basal FSH testing, the CCCT is an indirect assessment of ovarian reserve. Unlike FSH testing, the CCCT is provocative. It involves administration of 100 mg of clomiphene citrate (Clomid) on days 5 through 9 of the menstrual cycle, with FSH and estradiol measured on days 3 and 10. Once clomiphene citrate is administered, FSH and LH levels rise, followed by an increase in estradiol and inhibin. Evidence suggests that the smaller follicular cohorts in women with diminished ovarian reserve produce less inhibin-B and estradiol and, therefore, less negative feedback on clomiphene-induced pituitary FSH release.6,7 The result: persistent elevation of the day 10 FSH value and a positive screen for diminished ovarian reserve.
In some women, day 10 FSH is elevated even after a normal day 3 value. This makes the CCCT more sensitive than basal FSH testing; it can identify women who might go unrecognized if evaluated by day 3 FSH and estradiol levels alone.
More expensive and labor-intensive than the alternatives
Interpretation of the CCCT requires that FSH and estradiol both be assessed on days 3 and 10. An elevated FSH (≥10 mIU/mL) on either day indicates diminished ovarian reserve. As with basal FSH testing, elevated estradiol (≥80 pg/mL) on day 3 is considered abnormal. The day 10 estradiol value of the CCCT reflects whether or not clomiphene citrate was administered appropriately, and should be elevated. However, the significance of the day 10 estradiol level has been debated with respect to its predictive value for pregnancy in infertile populations.8
The addition of day 10 FSH assessment improves the sensitivity of the CCCT over basal FSH measurement, but makes it a more expensive and labor-intensive test ( TABLE ).5,6 The CCCT involves administration of clomiphene citrate, a safe drug (though it can have side effects), and two blood draws instead of one. Nevertheless, both tests are relatively noninvasive, rapid measures of ovarian reserve.
Drawbacks of the tests
Both basal FSH testing and the CCCT are widely used, although support for their ability to predict ovarian reserve in the infertile population has been challenged recently. Newer data demonstrate that these tests are limited in their ability to predict outcome (pregnancy and response to superovulation drugs) in all but a narrow group of patients undergoing IVF. Performance is particularly limited in:
Additional drawbacks of basal FSH testing and the CCCT include:
- significant variability of test results from cycle to cycle (intercycle variability)
- limited time frame within which the tests can be performed (intracycle variability).
The basal FSH test and CCCT have high specificity (98% to 99% for each) as an assessment of reproductive performance in infertile women and generate few false-positive results.5,6 However, the high screen cutoffs that allow for such specificity come at a price: Few women will screen positive, and sensitivity of the tests is low (between 7% and 8% for basal FSH and between 25% and 40% for the CCCT). Such low sensitivity means that many women will not conceive after infertility treatment despite a normal test result.5,11 Overall, the tests are not highly informative for many women who get tested.
Once abnormal, normal results are meaningless
Once an FSH level or the CCCT has ever been abnormal, the patient has diminished ovarian reserve; normal values in subsequent menstrual cycles do not improve the odds of pregnancy with treatment.14 This fact can be a significant source of confusion and frustration for patients.
3 | GnRH agonist stimulation —no better than FSH testing
This test was developed in the search for a very sensitive assessment of ovarian reserve. It was designed to uncover subtle abnormalities in pituitary and ovarian dynamics. It involves administering a gonadotropin-releasing hormone (GnRH) agonist such as leuprolide acetate (Lupron) on day 2 or 3 of the menstrual cycle and measuring pituitary and ovarian hormone responses.5,15
One group of investigators demonstrated a correlation between stimulated estradiol levels and responsiveness during IVF,16 but other studies have shown that the test does not perform significantly better than day 3 FSH in predicting ovarian reserve.17,18
The sensitivity of GnRH agonist testing for pregnancy is moderate (32% to 89%); specificity ranges from 79% to 97%.19
4 | Inhibin-B—not helpful when used alone
This glycoprotein hormone produced by granulosa cells of developing follicles is a direct measure of ovarian reserve when assessed in the early follicular phase of the menstrual cycle.20 Women treated with IVF who have a low inhibin-B level—particularly when using cutoffs below the range of 45–80 pg/mL—have been shown to respond poorly to superovulation and have a lower pregnancy rate than women with high inhibin-B.21,22 One group of investigators demonstrated that women with clinical evidence of diminished ovarian reserve but a normal FSH level also had low inhibin-B production, suggesting that it may be a more sensitive marker than FSH.22
Inhibin-B testing involves a simple blood draw. However, the test has been incorporated into clinical assessment of ovarian reserve only to a limited degree, due to the lack of reliable assays and controversy concerning its prognostic value.23
Because of these limitations, routine testing of serum inhibin-B in isolation of other markers of ovarian reserve is not recommended.
5 | Antral follicle count—good predictor of IVF outcome
Transvaginal ultrasonographic determination of the number of ovarian follicles that measure between 2 mm and 10 mm in diameter in the early follicular phase of the cycle yields the AFC. As a direct marker of the cohort of growing follicles in the early menstrual cycle, the AFC is believed to correlate strongly with the number of primordial follicles present in the ovary and, therefore, ovarian reserve. Total AFCs of less than 5 to 10 are suggestive of diminished ovarian reserve.24,25
In IVF cycles, AFC has proven to be an accurate predictor of number of oocytes retrieved, risk of cycle cancellation, and odds of conception.24,25 Some investigators have even suggested that, compared with other markers of ovarian reserve, AFC is the best independent predictor of outcome in IVF cycles.7,26-27
In a group of normally cycling women with proven fertility, AFC also showed a strong correlation with age, declining slowly until age 37 and more rapidly thereafter.28,29
AFC sensitivity for pregnancy is moderate and varies widely in published reports (8% to 60%), whereas specificity tends to be higher (33% to 96%).19
Drawbacks of AFC
- Because of the need to perform transvaginal ultrasonography, AFC is a more invasive and often more expensive test than hormonal biomarkers
- Accurate assessment of AFC requires an experienced sonographer and can be limited in patients who have had pelvic surgery or uterine fibroids and in those who are obese
- Moderate interobserver and intercycle variability of AFC determinations limits its reproducibility29,30
- As with basal FSH measurement, the intercycle variability of AFC does not correlate well with IVF outcome in individual patients.30
6 | Anti-Müllerian hormone— many advantages
The drawbacks of the tests just described— e.g., intercycle variability, lack of uniform cutoffs, and limited ability to predict IVF outcomes—make the development of more reliable measures of ovarian reserve a priority in reproductive medicine. AMH is a highly promising marker that appears to have many advantages over other tests and may have the greatest power to predict ovarian aging in women of reproductive age.
How it works
AMH is a glycoprotein growth factor and a member of the transforming growth factor-ß superfamily.31 It is primarily produced by the pool of early-growing follicles, which are believed to serve as a proxy for the number of primordial follicles in the ovary. The number of primordial follicles at a given point in time represents the ovarian reserve. AMH levels above 0.7 ng/mL are considered normal; values between 0.3 ng/mL and 0.7 ng/mL are consistent with borderline ovarian reserve, according to 2007 data from Reprosource Corp.
AMH has been studied as a marker of ovarian reserve for 6 years, with multiple reports describing declines in levels with age and with diminishing oocyte numbers. It is undetectable at menopause.32
The age-related decline in AMH is gradual but measurable even in young women, consistently preceding changes in other markers of ovarian reserve such as FSH and inhibin-B.32-35 The longitudinal changes in AMH have been demonstrated in ovulatory premenopausal women and healthy volunteers with proven fertility.33,34 In one series of women followed over a mean of 4 years (ages 25 to 46), AMH testing was superior to day 3 FSH, inhibin-B, and AFC in its ability to predict the onset of cycle irregularity and the menopausal transition.33
Does it predict oocyte quality?
AMH has performed well as a biomarker, comparable in most series to AFC and superior to FSH. AMH levels are strongly correlated with the number of oocytes retrieved during IVF and the odds of cycle cancellation due to poor response35-41 —but does it accurately characterize oocyte quality, the other element of ovarian reserve?
Some reports have shown a strong association between AMH levels and surrogates of oocyte quality, including fertilization, oocyte morphology, embryo quality, and pregnancy and miscarriage rates,36-41 but others have not.42 Some reports demonstrate a relationship between AMH and some but not all surrogate markers of oocyte quality.40
Advantages of AMH
- It demonstrates minimal intracycle variability.32,43-45 Compared with other markers of ovarian reserve, which must be measured early in the follicular phase of the menstrual cycle, AMH can be assessed at random times, making it a more convenient method for patients and physicians
- It demonstrates minimal intercycle variability32,34
- AMH levels are not significantly affected by the hormonal changes of pregnancy, oral contraceptive use, or GnRH treatment, and can be measured in these settings.46,47
Utility of AMH is limited in PCOS and obesity
The ability to use AMH as a marker of ovarian aging in women who have polycystic ovary syndrome (PCOS) and in women who are obese may be limited by the ovulatory dysfunction in these populations. Circulating levels of AMH are higher in women with PCOS than in unaffected women, a finding thought to be indicative of oligo-ovulation and poor follicular development in polycystic ovaries.48-53
In a recent series investigating AMH levels in women with PCOS, AMH and the degree of insulin resistance were positively correlated, and the AMH level was negatively correlated with the number of menses in a year.49 The consistently positive correlation between AMH and PCOS may suggest a future role for this marker as a diagnostic tool.
In obese women who do not have PCOS, AMH production may be lower than in women of normal weight. In a recent series, normally cycling obese women in the later reproductive years were shown to have an AMH level 70% lower than those in women who were not obese.54 These differences have not been well studied in younger obese women.
Which test is best?
AMH may be preferable to the other tests to assess ovarian reserve because it can be measured any time during the menstrual cycle or between cycles. AMH measurement is also useful if a woman is taking oral contraceptives or leuprolide acetate because these medications may confound the results of the other test methods. In addition, AMH may be the earliest indicator of decline in ovarian reproductive function. As such, it may highlight cases that merit a search for other causes of infertility and make it possible to treat them in a timely manner.
Elevated AMH may reveal occult PCOS and warn of significant risk of ovarian hyperstimulation prior to ovulation induction with gonadotrophins, so that the clinician can plan smaller doses.
A normal female is born with 1 million to 2 million oocytes, a number that declines continuously, primarily through the process of follicular atresia. By the onset of puberty, the number of oocytes has declined to approximately 300,000. As a woman enters her late 30s, when the total number of oocytes is approximately 25,000, the pace of oocyte depletion begins to increase, as does the rate of spontaneous miscarriage.1,55,56
The effect of age on fertility is believed to arise from changes in both oocyte number and quality. Multiple investigators have found a greater frequency of cellular abnormalities in oocytes from older women.1,2,5,15,57
Although ovarian reserve declines with age in all women, women of similar ages can have very different degrees of ovarian reserve, and some women who have very poor ovarian reserve may never conceive, despite aggressive fertility treatment.
The biologic basis for differences in ovarian reserve among similar groups of women is not completely understood, but is probably rooted in genetic, lifestyle, and environmental factors that affect granulosa cell and oocyte function. Identifying sensitive biomarkers that can determine ovarian reserve independent of age is critical to predict fertility and age at menopause.5
1. Practice Committee of the American Society for Reproductive Medicine. Age and infertility in women. Fertil Steril. 2006;86:S248-S252.
2. Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu H-C. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50:298-307.
3. Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651-654.
4. Toner JP, Philiput CB, Jones GS, Muasher SJ. Basal follicle stimulating hormone level is a better predictor of in vitro fertilization outcome than age. Fertil Steril. 1991;55:784-791.
5. Barnhart K, Osheroff J. Follicle stimulating hormone as a predictor of fertility. Curr Opin Obstet Gynecol. 1998;10:227-232.
6. Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: the physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;69:474-477.
7. Yong PY, Baird DT, Thong KJ, McNeilly AS, Anderson RA. Prospective analysis of the relationships between the ovarian follicle cohort and basal FSH concentration, the inhibin response to exogenous FSH and ovarian follicle number at different stages of the normal menstrual cycle and after pituitary down-regulation. Hum Reprod. 2003;18:35-44.
8. Scott RT, Jr, Illions EH, Kost ER, Dellinger C, Hofmann GE, Navot D. Evaluation of the significance of the estradiol response during the clomiphene citrate challenge test. Fertil Steril. 1993;60:242-246.
9. Esposito MA, Coutifaris C, Barnhart KT. A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod. 2002;17:118-123.
10. Bancsi L, Broekmans FJM, Wol BWJ, Habbema DK, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003;79:1091-1100.
11. Jain T, Soules MR, Collins JA. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 2004;82:180-185.
12. Toner JP. Modest follicle-stimulating hormone elevations in younger women: warn but don’t disqualify. Fertil Steril. 2004;81:1493-1495.
13. Van Rooij IAJ, de Jong E, Broekmans FJM, Looman CWN, Habbeman DK, te Velde ER. High follicle-stimulating hormone levels should not necessarily lead to the exclusion of subfertile patients from treatment. Fertil Steril. 2004;81:1478-1485.
14. Scott RT, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54:297-302.
15. Bulkulmez O, Arici A. Assessment of ovarian reserve. Curr Opin Obstet Gynecol. 2004;16:231-237.
16. Ranieri DM, Quinn F, Makhlouf A, et al. Simultaneous evaluation of basal follicle-stimulating hormone and 17-beta-estradiol response to gonadotropin-releasing hormone analogue stimulation: an improved predictor of ovarian reserve. Fertil Steril. 1998;70:227-233.
17. Fujimoto VY, Klein NA, Battaglia DE, Bremmer WJ, Soules MR. The anterior pituitary response to a gonadotropin-releasing hormone challenge test in normal older reproductive age women. Fertil Steril. 1996;65:539-544.
18. Galtier-Dereure F, De Bouard V, Picto MC, et al. Ovarian reserve test with the gonadotrophin-releasing hormone agonist buserelin: correlation with in-vitro fertilization outcome. Hum Reprod. 1996;11:1393-1398.
19. Broekmans FJ, Fwee J, Hendricks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685-718.
20. Klein NA, Illingworth PJ, Groome NP, NcNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81:2742-2745.
21. Seifer DB, Lambert-Messerlian G, Hogan JW, et al. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67:110-114.
22. Seifer DB, Scott RT, Jr, Bergh PA, et al. Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin B before a rise in day 3 follicle-stimulating hormone. Fertil Steril. 1999;72:63-65.
23. Corson SL, Gutmann J, Batzer FR, Wallace H, Klein N, Soules MR. Inhibin-B as a test of ovarian reserve for infertile women. Hum Reprod. 1999;14:2818-2821.
24. Tomas C, Nuojua-Huttunen S, Martikainen H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum Reprod. 1997;12:220-223.
25. Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505-510.
26. Hung E, Tang OS, Ho PC. The significance of the number of antral follicles prior to stimulation in predicting ovarian responses in an IVF programme. Hum Reprod. 2000;15:1937-1942.
27. Bancsi LFJMM, Broekmans FJM, Eijkemans MJC, de Jong FH, Habbema JDF, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328-336.
28. Ng EH, Yeung WS, Fong DY, Ho PC. Effects of age on hormonal and ultrasound markers of ovarian reserve in Chinese women with proven fertility. Hum Reprod. 2003;18:2169-2174.
29. Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845-851.
30. Hansen KR, Morris JL, Thyer AC, Soules MR. Reproductive aging and the variability in the ovarian antral follicle count: application in the clinical setting. Fertil Steril. 2003;80:577-583.
31. Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685-698.
32. de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM. Anti-Müllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357-362.
33. van Rooij IAJ, Broekmans FJM, Scheffer GJ, et al. Serum anti-Müllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979-987.
34. van Rooij IAJ, Tonkelaar I, Broekmans FJ, et al. Anti-Müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601-606.
35. Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Müllerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20-24.
36. Silberstein T, MacLaughlin DT, Shai I, et al. Müllerian-inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159-163.
37. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum Müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468-471.
38. Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022-2026.
39. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum anti-Müllerian hormone/Müllerian-inhibiting substance appears to be a more discriminatory marker of ART outcome than follicular stimulating hormone, inhibin B or estradiol. Fertil Steril. 2004;82:1323-1329.
40. Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22:2414-2421.
41. Fanchin R, Mendez DH, Frydman N, et al. Anti-Müllerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796-1802.
42. Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Th omas CM, Braat DD. Anti-Müllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87:223-226.
43. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FM, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057-4063.
44. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Müllerian hormone throughout the menstrual cycle. Hum Reprod. 2006;21:3103-3107.
45. Tsepelidis S, Devreker F, Demeestere F, Flahaut I, Gervy A, Englert C. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837-1840.
46. La Marca A, Giulini Orvieto R, De Leo V, Volpe A. Anti-Müllerian hormone concentrations in maternal serum during pregnancy. Hum Reprod. 2005;20:1569-1572.
47. Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Müllerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196-201.
48. Al-Qahtani A, Groome NP. Anti-Müllerian hormone: Cinderella finds new admirers. J Clin Endocrinol Metab. 2006;91:3760-3762.
49. La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Müllerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970-971.
50. Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820-1836.
51. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-Müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957-5962.
52. Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum anti-Müllerian substance and other reproductive hormones in untreated women with polycystic ovary syndrome and endometriosis. Fertil Steril. 1997;67:962-965.
53. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240-245.
54. Freeman EW, Gracia CG, Sammel MD, Lin H, Lim LC, Strauss JF, 3rd. Association of anti-Müllerian hormone levels with obesity in later reproductive-age women. Fertil Steril. 2007;87:101-106.
55. Scott RT, Opsahl MS, Leonardi MR, Neall GS, Illions EH, Navot D. Life table analysis of pregnancy rates in a general infertility population relative to ovarian reserve and patient age. Hum Reprod. 1995;10:1706-1710.
56. Speroff L. Fritz M. eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
57. Lim AS, Tsakok MFH. Age-related decline in fertility: a link to degenerative oocytes? Fertil Steril. 1997;68:265-271.
1. Practice Committee of the American Society for Reproductive Medicine. Age and infertility in women. Fertil Steril. 2006;86:S248-S252.
2. Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu H-C. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50:298-307.
3. Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651-654.
4. Toner JP, Philiput CB, Jones GS, Muasher SJ. Basal follicle stimulating hormone level is a better predictor of in vitro fertilization outcome than age. Fertil Steril. 1991;55:784-791.
5. Barnhart K, Osheroff J. Follicle stimulating hormone as a predictor of fertility. Curr Opin Obstet Gynecol. 1998;10:227-232.
6. Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: the physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;69:474-477.
7. Yong PY, Baird DT, Thong KJ, McNeilly AS, Anderson RA. Prospective analysis of the relationships between the ovarian follicle cohort and basal FSH concentration, the inhibin response to exogenous FSH and ovarian follicle number at different stages of the normal menstrual cycle and after pituitary down-regulation. Hum Reprod. 2003;18:35-44.
8. Scott RT, Jr, Illions EH, Kost ER, Dellinger C, Hofmann GE, Navot D. Evaluation of the significance of the estradiol response during the clomiphene citrate challenge test. Fertil Steril. 1993;60:242-246.
9. Esposito MA, Coutifaris C, Barnhart KT. A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod. 2002;17:118-123.
10. Bancsi L, Broekmans FJM, Wol BWJ, Habbema DK, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003;79:1091-1100.
11. Jain T, Soules MR, Collins JA. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 2004;82:180-185.
12. Toner JP. Modest follicle-stimulating hormone elevations in younger women: warn but don’t disqualify. Fertil Steril. 2004;81:1493-1495.
13. Van Rooij IAJ, de Jong E, Broekmans FJM, Looman CWN, Habbeman DK, te Velde ER. High follicle-stimulating hormone levels should not necessarily lead to the exclusion of subfertile patients from treatment. Fertil Steril. 2004;81:1478-1485.
14. Scott RT, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54:297-302.
15. Bulkulmez O, Arici A. Assessment of ovarian reserve. Curr Opin Obstet Gynecol. 2004;16:231-237.
16. Ranieri DM, Quinn F, Makhlouf A, et al. Simultaneous evaluation of basal follicle-stimulating hormone and 17-beta-estradiol response to gonadotropin-releasing hormone analogue stimulation: an improved predictor of ovarian reserve. Fertil Steril. 1998;70:227-233.
17. Fujimoto VY, Klein NA, Battaglia DE, Bremmer WJ, Soules MR. The anterior pituitary response to a gonadotropin-releasing hormone challenge test in normal older reproductive age women. Fertil Steril. 1996;65:539-544.
18. Galtier-Dereure F, De Bouard V, Picto MC, et al. Ovarian reserve test with the gonadotrophin-releasing hormone agonist buserelin: correlation with in-vitro fertilization outcome. Hum Reprod. 1996;11:1393-1398.
19. Broekmans FJ, Fwee J, Hendricks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685-718.
20. Klein NA, Illingworth PJ, Groome NP, NcNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81:2742-2745.
21. Seifer DB, Lambert-Messerlian G, Hogan JW, et al. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67:110-114.
22. Seifer DB, Scott RT, Jr, Bergh PA, et al. Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin B before a rise in day 3 follicle-stimulating hormone. Fertil Steril. 1999;72:63-65.
23. Corson SL, Gutmann J, Batzer FR, Wallace H, Klein N, Soules MR. Inhibin-B as a test of ovarian reserve for infertile women. Hum Reprod. 1999;14:2818-2821.
24. Tomas C, Nuojua-Huttunen S, Martikainen H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum Reprod. 1997;12:220-223.
25. Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505-510.
26. Hung E, Tang OS, Ho PC. The significance of the number of antral follicles prior to stimulation in predicting ovarian responses in an IVF programme. Hum Reprod. 2000;15:1937-1942.
27. Bancsi LFJMM, Broekmans FJM, Eijkemans MJC, de Jong FH, Habbema JDF, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328-336.
28. Ng EH, Yeung WS, Fong DY, Ho PC. Effects of age on hormonal and ultrasound markers of ovarian reserve in Chinese women with proven fertility. Hum Reprod. 2003;18:2169-2174.
29. Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845-851.
30. Hansen KR, Morris JL, Thyer AC, Soules MR. Reproductive aging and the variability in the ovarian antral follicle count: application in the clinical setting. Fertil Steril. 2003;80:577-583.
31. Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685-698.
32. de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM. Anti-Müllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357-362.
33. van Rooij IAJ, Broekmans FJM, Scheffer GJ, et al. Serum anti-Müllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979-987.
34. van Rooij IAJ, Tonkelaar I, Broekmans FJ, et al. Anti-Müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601-606.
35. Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Müllerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20-24.
36. Silberstein T, MacLaughlin DT, Shai I, et al. Müllerian-inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159-163.
37. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum Müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468-471.
38. Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022-2026.
39. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum anti-Müllerian hormone/Müllerian-inhibiting substance appears to be a more discriminatory marker of ART outcome than follicular stimulating hormone, inhibin B or estradiol. Fertil Steril. 2004;82:1323-1329.
40. Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22:2414-2421.
41. Fanchin R, Mendez DH, Frydman N, et al. Anti-Müllerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796-1802.
42. Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Th omas CM, Braat DD. Anti-Müllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87:223-226.
43. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FM, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057-4063.
44. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Müllerian hormone throughout the menstrual cycle. Hum Reprod. 2006;21:3103-3107.
45. Tsepelidis S, Devreker F, Demeestere F, Flahaut I, Gervy A, Englert C. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837-1840.
46. La Marca A, Giulini Orvieto R, De Leo V, Volpe A. Anti-Müllerian hormone concentrations in maternal serum during pregnancy. Hum Reprod. 2005;20:1569-1572.
47. Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Müllerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196-201.
48. Al-Qahtani A, Groome NP. Anti-Müllerian hormone: Cinderella finds new admirers. J Clin Endocrinol Metab. 2006;91:3760-3762.
49. La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Müllerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970-971.
50. Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820-1836.
51. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-Müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957-5962.
52. Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum anti-Müllerian substance and other reproductive hormones in untreated women with polycystic ovary syndrome and endometriosis. Fertil Steril. 1997;67:962-965.
53. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240-245.
54. Freeman EW, Gracia CG, Sammel MD, Lin H, Lim LC, Strauss JF, 3rd. Association of anti-Müllerian hormone levels with obesity in later reproductive-age women. Fertil Steril. 2007;87:101-106.
55. Scott RT, Opsahl MS, Leonardi MR, Neall GS, Illions EH, Navot D. Life table analysis of pregnancy rates in a general infertility population relative to ovarian reserve and patient age. Hum Reprod. 1995;10:1706-1710.
56. Speroff L. Fritz M. eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
57. Lim AS, Tsakok MFH. Age-related decline in fertility: a link to degenerative oocytes? Fertil Steril. 1997;68:265-271.