User login
The authors report no financial relationships relevant to this article.
CASE 1: Cystic mass in patient’s only remaining ovary
Mrs. R is a 29-year-old G1P1 who underwent a right oophorectomy, with a midline incision, for a dermoid cyst at the time of cesarean delivery. She now has a left ovarian cyst. Preoperative ultrasonography (US) reveals that it measures 3.5×4.2×3.7 cm and has both solid components and a multiloculated appearance, consistent with a dermoid cyst.
How common is this scenario?
Studies predict that one of every three women will undergo surgical management of an adnexal mass at some point in her life.1 This troubling statistic prompts several critical questions:
- How do we handle the workup for these women so that only appropriate patients undergo surgery?
- How often will a mass be malignant?
- How can we safely remove an adnexal mass to maximize patient safety, reduce overall recovery time, and prevent less favorable outcomes in women who are eventually found to have a malignancy?
A thorough workup and, sometimes, conservative management can prevent unnecessary surgery that may lead to early menopause or surgical complications. And maximizing the use of minimally invasive techniques in women who do require surgery can shorten hospital stay and recovery time. At the time of surgery, careful abdominal entry and meticulous surgical dissection and mass removal can limit the potential risks of laparoscopic excision in women who have an ultimate diagnosis of cancer.
In this article, we review the workup for women who have an adnexal mass, describe patient-selection criteria for laparoscopic surgery, including the risks and benefits of this approach ( TABLE 1 ), and present several techniques to safely manage a mass with potentially malignant histology via laparoscopy.
TABLE 1
There are benefits and risks to managing an adnexal mass laparoscopically
| Benefit | Risk |
|---|---|
| Shorter recovery Fewer adhesions Decreased overall cost Magnification Decreased pain and narcotic use Fewer wound complications | Expensive equipment* Loss of tactile sensation Concern for malignancy Risk of tumor dissemination/spillage/chemical peritonitis Trocar-site metastasis |
| *Though greater expense is not a risk per se, it does enter into decision making. | |
Begin with the physical
When a woman is known to have a pelvic mass, the aim of the office exam is to 1) identify characteristics that suggest malignancy and 2) rule out nongynecologic causes of the mass. Physical findings that are worrisome for a malignant process include:
- fixed or nodular pelvic mass
- bilateral masses
- nodular abdominal mass
- ascites
- pleural effusion on auscultation or percussion of the lung.
Although these findings can be present under benign conditions, they increase the risk that a malignancy will be detected at surgery.
Other causes of a pelvic mass should also be considered, including infection (pelvic abscess) and tumors of the colon, particularly when the pelvic mass occurs on the left side.
Some symptoms, though vague, are worth noting
Although ovarian cancer was once thought to be a silent disease, recent research has shown that bloating, pelvic or abdominal pain, early satiety, and urinary frequency and urgency are more common among women with ovarian cancer than among healthy controls and patients in high-risk screening clinics.2-4 Although these symptoms are generally nonspecific, they merit attention if they occur more than 12 times a month and have been present for less than 1 year. When they meet these criteria, the symptoms have a sensitivity for diagnosing early- and late-stage ovarian cancer of 56.7% and 79.5%, respectively.4
Sensitivity for the diagnosis of early-stage ovarian cancer may be as high as 80% when the symptom index score is combined with an elevated level of the tumor marker CA 125.3
Transvaginal US is crucial
Transvaginal US is now standard practice to obtain high-resolution images of an adnexal mass. Grayscale US has traditionally been used alone for evaluation.
Specificity is typically lower in women who are premenopausal because many benign lesions, such as endometrioma, have a similar sonographic appearance to cancer.
A number of US scoring algorithms have now been proposed to aid in the triage of women who have an adnexal mass. Sensitivity of these algorithms ranges from 65% to 100%; specificity, from 77% to 95%.5
CA 125 is the standard tumor marker
For the past two decades, CA 125 has been the standard serum marker in the screening of high-risk women for ovarian cancer and the triage of women who have an adnexal mass.
This blood test has been studied widely since its introduction in 1983. It typically has sensitivity of 75% to 85% and specificity of 85% to 95% in identifying women who have ovarian cancer. However, it is elevated in only 50% to 60% of women who have stage I ovarian cancer. Its lack of specificity and poor positive predictive value have kept researchers busy trying to identify other serum markers, for both ovarian cancer and identification of high-risk pelvic masses.
Our recommended workup and management of adnexal masses In postmenopausal women who had a pelvic mass, one study found that a CA 125 level above 65 IU/mL had sensitivity of 71% and specificity of 92.5% in the identification of ovarian cancer.6 Another group found that CA 125 levels above 65 IU/mL were more than 95% sensitive in the diagnosis of ovarian cancer in postmenopausal women.7
Several studies have combined CA 125 with other markers or with US to screen high-risk women or triage those who have an adnexal mass. These studies have shown modest improvements in sensitivity but usually lower specificity than with CA 125 testing alone.
Markers that may be used for suspected sex cord stromas and germ-cell tumors are:
- lactate dehydrogenase (LDH) for dysgerminomas
- alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) for yolk sac tumors
- testosterone for Sertoli-Leydig cell tumors
- inhibin A and B for granulosa cell tumors.
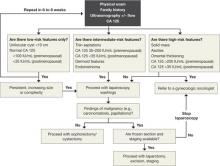
An algorithm for working up and managing adnexal masses appears above.
How to gain abdominal access
In the opening case, the patient clearly has a benign mass. The treatment? Safe entry into the peritoneal cavity to remove the cyst and as little normal tissue as possible. This is critical in this patient because she has only one ovary.
Peritoneal access for abdominal and pelvic laparoscopy has been studied widely. Options include:
- direct insertion using a Veress needle
- open laparoscopy
- direct trocar insertion.
The technique usually depends on the preference of the surgeon. The primary goal of abdominal entry is to minimize the risk of injury, particularly unrecognized injury.
Data on complication rates show no definite benefit for open versus closed techniques in the prevention of injury to underlying viscera. However, evidence does suggest that the open technique may lower the risk of major vascular injury.8
We employ direct trocar insertion using radially expanding or optical trocars.
The Veress needle option
When the Veress needle is used to gain intraperitoneal access, data indicate that initial intraperitoneal pressure below 10 mm Hg is a reliable marker for peritoneal entry, even in obese patients.9 Insufflation pressure as high as 25 to 30 mm Hg prior to placement of the initial trocar is safe from a cardiopulmonary standpoint and may allow easier entry with a nonbladed trocar.10
Tests to confirm intraperitoneal placement of the Veress needle, such as the hanging-drop test or saline flush, do not appear to offer any additional useful information.11
Open laparoscopy is suitable when adhesions are unlikely
Open laparoscopy is typically performed by making a minilaparotomy incision at the umbilicus and then dissecting and entering the peritoneal cavity. A blunt-tip trocar is inserted.
The disadvantage of this approach is that there may be extensive adhesions under the umbilicus, and it is difficult to dissect such adhesions sufficiently to introduce a cannula and laparoscope. Adhesions left behind often obscure the field of view after introduction of the trocar.
Our preference? Left upper-quadrant insertion
In Case 1, the previous midline incision mandates an alternative approach. When abdominal entry at the umbilicus is unsuccessful or potentially difficult because of an earlier midline incision, umbilical hernia repair, or history of multiple lower abdominal or pelvic surgeries, a left upper-quadrant insertion is useful. It is, in fact, our preferred technique, and involves a small incision at the midclavicular line 5 cm below the left costal margin, at a site called Palmer’s point.
The direction of insertion usually ranges from 45° to 90°, depending on the patient’s body weight. If the trocar is placed properly, the closest organs are the stomach and the left lobe of the liver (4 to 6 cm).12 Given the stomach’s close proximity, it should be decompressed with an orogastric tube prior to trocar insertion.
Several studies have demonstrated the safety and efficacy of this entry technique.12-14 It can be helpful in cases that involve difficult access. We usually use a 5-mm primary trocar site for a 5-mm laparoscope. Modern optics allow for a pristine view with these small scopes, eliminating the need to close fascia and perhaps causing less pain at the incision.
Accessory trocar sites facilitate complex technique
We usually use three accessory sites. Two of them are lower-quadrant ports that are placed 2 cm medial and 2 cm cephalad to the anterior superior iliac spine. This area generally lies well away from the inferior epigastric vessels and remains above the area of the ilioinguinal and iliohypogastric nerves, making it a safer point of insertion.15 One trocar is 5 mm in size and the other is 10 mm. The larger one is used to extract the specimen.
We place an additional 5-mm port lateral to the rectus muscle at the level of the umbilicus. This allows the principal surgeon to use two instruments (a toothed forceps and scissors) comfortably while the assistant holds the laparoscope and assists with a grasper.
Does the type of trocar matter?
No randomized studies have directly compared all types of trocars. Options include:
- a pyramidal tip (as in reusable trocars) or shielded tip
- radial expansion
- visible entry
- blunt (Hasson-type) trocar.
Safety data on direct comparison of trocars are limited, but it appears that a radially expanding trocar may offer less port-site pain and potentially less bleeding than a traditional cutting trocar.16 Moreover, the rate of hernia at the port site appears to be relatively low with a radially expanding trocar, even when fascia is left unclosed at a 10-mm site.17
None of these trocars appears to be clearly superior at avoiding visceral or vascular injury.
Technique of laparoscopic cyst removal
A video clip of the surgery is linked to this article in the Video Library at www.obgmanagement.com. In this case, a trocar was inserted in the left upper quadrant, and a laparoscopic cystectomy was initiated using the trocars already specified.
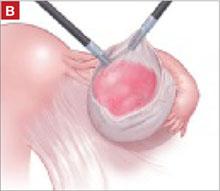
The peritoneal cavity and adnexa were inspected, followed by pelvic washings, as detailed in TABLE 2 . Next, the ovarian cortex was incised ( FIGURE 1A ) with scissors using bipolar or unipolar energy, typically at a low power setting, such as 12 to 15 watts.
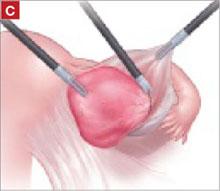
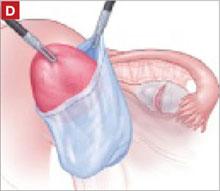
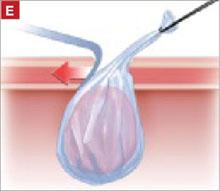
It was relatively easy to develop a tissue plane between the cortex and underlying dermoid cyst using simple or aqua-dissection ( FIGURE 1B and C ). The cyst was enucleated without rupture and inserted into a specimen bag through a 10-mm port ( FIGURE 1D ). (The specimen bags generally are available in 10-mm and 15-mm sizes.) Once the trocar was removed, the entire specimen was brought out through the incision ( FIGURE 1E ).
Cystic masses can generally be carefully aspirated using a laparoscopic needle or angiocath, or they can be incised and drained using a standard suction device. Manufacturers of most specimen bags do not recommend the morcellation of tissue within the bag because of the potential for rupture of the bag with tumor spillage or injury to underlying structures.
Occasionally, the mass remains too large to remove after drainage of the cyst fluid. Remedies include enlarging the port site with a scalpel or using a gallbladder speculum to increase the diameter of the port site ( FIGURE 1E ). If possible, the incision should be large enough to deliver the entire bag intact. Use of excessive force will rupture the bag and may cause the specimen to be lost or malignant cells to be inadvertently spilled (in the case of a cancer diagnosis).
TABLE 2
Take these 10 steps to safe laparoscopic surgery
| Examine the anesthetized patient |
| Enter the abdomen |
| Inspect the mass and peritoneal surfaces, including the diaphragm. Biopsy sites suspicious for metastasis and obtain frozen section |
| Perform pelvic and abdominal washings |
Closely inspect adnexa. If findings are not suggestive of malignancy, proceed with laparoscopy. If findings indicate obvious malignancy
|
Perform cystectomy or oophorectomy
|
| Inspect for hemostasis |
| Place cyst/ovary in endopouch |
| Open bag at abdominal wall and remove for frozen section |
| Reinspect and close |
Avoid:
|
FIGURE 1 Laparoscopic cyst removal
Begin by incising the cortex using scissors with or without an energy source.
Dissect the cyst free from the cortex using sharp dissection.
Remove the cyst from the ovary.
Place the cyst in a specimen bag, and …
… bring it to the surface for extraction. The abdominal incision may have to be enlarged to accommodate the specimen.
CASE 1: OUTCOME
The patient’s cyst is removed intact and she is discharged home. Rupture of a dermoid cyst is not associated with any problems as long as copious irrigation is used to aspirate the cyst content.18 Other cysts, such as endometriomas, may not be as easily dissected, and rupture is inevitable.
CASE 2: Symptoms suggestive of cancer
Mrs. B is a 47-year-old woman who reports abdominal bloating for the past 3 weeks. She also complains of early satiety and occasional constipation. She has no history of cancer, but her sister was given a diagnosis of breast cancer at 41 years of age, and her maternal aunt had breast cancer at 55 years.
Mrs. B is moderately obese, with a nontender abdomen and no palpable mass. Her pelvic exam also is negative for a mass or nodularity, but the extent of the exam is limited by body habitus. Her physician orders a transvaginal US, which reveals a 6-cm complex mass with thin septation and a 1-cm solid nodule, with no definite blood flow. The patient’s CA 125 level is 80 IU/mL, which we consider to be within the low-risk range for a premenopausal woman.
The patient is counseled about the need to have the mass removed and is scheduled for laparoscopic right salpingooophorectomy. Given the family history of breast cancer, the physician also requests consultation with a gynecologic oncologist, who agrees to assist with surgery and perform a laparotomy and staging in the event that a malignancy is diagnosed.
Is the mass likely to be malignant?
Given the patient’s family history of breast cancer, the recent onset of symptoms associated with ovarian cancer,2 and the characteristics of the mass (complex, with a nodule), malignancy is possible. This patient has an intermediate risk of cancer and requires additional counseling and planning.
However, most women who undergo laparoscopy for removal of an adnexal mass have benign pathologic findings.
What is the real risk of ovarian cancer?
The lifetime risk of developing ovarian cancer in the general population remains stable at approximately 1 in 70 women, with a mean age at diagnosis of 63 years.19 Ninety percent of ovarian cancer cases are sporadic, and less than 10% can be linked to genetic syndromes.
Women who have mutations in the BRCA1 gene carry a lifetime risk of ovarian cancer of up to 50%, and women who have mutations in BRCA2 have a lifetime risk of up to 25%.20,21 Women who have mutations associated with Lynch II syndrome or Hereditary Nonpolyposis Colorectal Cancer syndrome may have a lifetime risk of ovarian cancer of 12%.22,23
Some women who have a strong family history of breast and ovarian cancer do not carry a known mutation, but are likely to be at increased risk.
Additional risk factors known to be associated with ovarian cancer are nulliparity and infertility. However, the single most important risk factor for epithelial ovarian cancer is age.
Risk-reducing strategies include:
In the case of PBSO, it is imperative to ensure that all ovarian surface epithelium is removed. This means excising the infundibulopelvic ligament at least 1.5 cm above the proximal end of the ovary and excising any adjacent tissue to which the ovary is adherent (including pelvic sidewall peritoneum). Both requirements are easily achieved using the techniques outlined here.
Who should perform surgery?
The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) have published guidelines for referral to a gynecologic oncologist ( TABLE 3 ). When Im and colleagues reviewed the records of more than 1,000 women who underwent surgery for a pelvic mass at six institutions over 12 months, they found that 70% of premenopausal women and 94% of postmenopausal women who were diagnosed with ovarian cancer were properly referred to a qualified subspecialist.27 “Over”-referral occurred in 30% to 40% of women who had a benign mass, but may be preferable given the importance of proper staging and debulking to survival.
ACOG and the SGO recommend referral for women who have:
- elevated tumor markers
- ascites
- a fixed or nodular mass
- a strong family history of breast or ovarian cancer.
Consider preoperative referral of all high-risk and, probably, intermediate-risk women, depending on the availability of qualified specialists for complete surgical staging.
In addition, women need to be counseled thoroughly about the possibility that a malignancy will be diagnosed by frozen section, necessitating additional surgical procedures.
TABLE 3
Your patient has a newly diagnosed pelvic mass. Should you refer her?
| Is she premenopausal? Then refer her when… | Is she postmenopausal? Then refer her when… |
|---|---|
| CA125 >200 IU/mL Ascites is present Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative | CA125 >35 IU/mL Ascites is present Nodular or fixed pelvic mass Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative |
Technique of laparoscopic adnexectomy
In Case 2, an umbilical site was chosen for the primary cannula. In general, the direction of insertion depends on the patient’s body habitus. Heavier patients have a thicker abdominal wall and an umbilicus below the level of the aortic bifurcation. In these women, the angle of insertion should be adjusted from the usual 45° (for patients of normal weight) to an angle nearer to 90°. Lateral ports are typical, as in Case 1.
Treat every mass like cancer
Laparoscopic excision of an adnexal mass can be safe and effective, with better outcomes and recovery than with laparotomy, provided the surgeon adheres to basic principles ( TABLE 2 ). This means treating every mass as though it is potentially malignant, and thoroughly inspecting the abdominal cavity before and after excision of the mass.
Know the retroperitoneal space
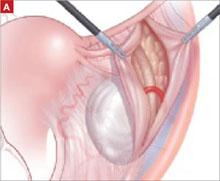
As with Case 1, successful laparoscopic excision of an adnexal mass begins with inspection of the peritoneal cavity, abdominopelvic washings, and identification of both the infundibulopelvic ligament and ureter. Knowledge of the retroperitoneal space can be of great value in difficult cases that involve significant pelvic adhesions or sidewall fibrosis. We generally use a retroperitoneal approach for laparoscopic adnexectomy ( FIGURE 2A ).
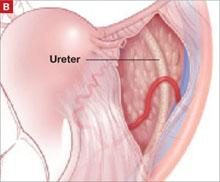
In our typical approach, we incise the peritoneum lateral to the uteroovarian ligament and continue the incision up the pelvic sidewall lateral to the infundibulopelvic ligament and up along the paracolic gutter, if needed ( FIGURE 2A ). We then mobilize the medial leaf of the broad ligament from the sidewall using blunt dissection between the external iliac vessels laterally and the ureter medially ( FIGURE 2B ).
Once we have identified the ureter, we use scissors to create a window in the medial leaf of the broad ligament just beneath the gonadal vessels. We then use an energy source to occlude and transect the pedicle. Using this technique, we secure the infundibulopelvic ligaments and safely mobilize the ureter before initiating more aggressive sidewall dissection distally for adhesions or fibrosis.
Once the ureter is mobilized, we excise the involved peritoneum along with the mass. We then seal and transect the uteroovarian ligament.
FIGURE 2 The retroperitoneal approach
Grasp and incise the peritoneum just lateral to the adnexal mass and enter the retroperitoneal space, where loose areolar tissue is visible, with the ureter seen on the medial leaf of the broad ligament.
After dissecting this tissue, identify the ureter and internal iliac vessels.
Remove the mass in a bag
Once the mass is excised, we place it in a laparoscopic bag, as described for Case 1. Solid adnexal masses are problematic because they are not amenable to drainage, and morcellation is usually discouraged. Laparoscopic excision can still be carried out, and a minilaparotomy or posterior colpotomy can be used to extract the mass in a bag. Patients managed in this way still have a complication rate similar to or lower than that of patients undergoing laparotomy for oophorectomy; they also recover faster.
Risks in cases of malignancy
The risk of tumor spillage in laparoscopic surgery can be lowered using laparoscopically guided minilaparotomy techniques. Preoperative rupture appears to be more predictive of outcome.28
The concern about metastatic implants in a laparoscopic port site in patients who have gynecologic cancer is real, with an incidence ranging from 0.97% to 1.1%. The phenomenon usually affects women who had ovarian cancer and is most common after laparoscopy with findings of ascites, carcinomatosis, or persistent disease (in the case of second-look laparoscopy performed after completion of primary therapy for ovarian cancer).
These data suggest that port-site implantation is not a concern that should deter clinicians from laparoscopic evaluation of a suspected ovarian neoplasm unless a patient presents with ascites or carcinomatosis suggestive of advanced disease.29
Data are limited regarding delays in definitive therapy. One study suggests that definitive staging on the day of rupture does not influence overall outcome, but delay by more than 2 weeks may lead to poorer prognosis.30
CASE 2: OUTCOME
Laparoscopy is performed. On initial inspection, the peritoneal cavity is unremarkable. The right ovary is multicystic with normal surface anatomy. The mass is somewhat adherent to the pelvic sidewall. Adnexectomy is performed, and frozen section reveals the mass to be a serous cystadenoma.
1. Curtin JP. Management of the adnexal mass. Gynecol Oncol. 1994;55:S42-S46.
2. Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712.
3. Andersen MR, Goff BA, Lowe KA, et al. Combining a symptoms index with CA 125 to improve detection of ovarian cancer. Cancer. 2008;113:484-489.
4. Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221-227.
5. Alcázar JL, Mercé LT, Laparte C, et al. A new scoring system to differentiate benign from malignant adnexal masses. Am J Obstet Gynecol. 2003;188:685-692.
6. Maggino T, Gadducci A, D’Addario V, et al. Prospective multicenter study on CA 125 in postmenopausal pelvic masses. Gynecol Oncol. 1994;54:117-123.
7. Malkasian GD, Jr, Knapp RC, Lavin PT, et al. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988;159:341-346.
8. Larobina M, Nottle P. Complete evidence regarding major vascular injuries during laparoscopic access. Surg Laparosc Endosc Percutan Tech. 2005;15:119-123.
9. Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10:415-420.
10. Vilos GA, Vilos AG, Abu-Rafea B, Hollett-Caines J, Nikkhah-Abyaneh Z, Edris F. Three simple steps during closed laparoscopic entry may minimize major injuries. Surg Endosc. 2008 July 15. [Epub ahead of print]
11. Vilos GA, Ternamian A, Dempster J, Laberge PY. The Society of Obstetricians and Gynaecologists of Canada. Laparoscopic entry: a review of techniques, technologies, and complications. J Obstet Gynaecol Can. 2007;29:433-465.
12. Tulikangas PK, Nicklas A, Falcone T, Price LL. Anatomy of the left upper quadrant for cannula insertion. J Am Assoc Gynecol Laparosc. 2000;7:211-214.
13. Stepp KJ, Tulikangas PK, Goldberg JM, Attaran M, Falcone T. Laparoscopy for adnexal masses in the second trimester of pregnancy. J Am Assoc Gynecol Laparosc. 2003;10:55-59.
14. Tulikangas PK, Robinson DS, Falcone T. Left upper quadrant cannula insertion. Fertil Steril. 2003;79:411-412.
15. Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189:1574-1578.
16. Yim SF, Yuen PM. Randomized double-masked comparison of radially expanding access device and conventional cutting tip trocar in laparoscopy. Obstet Gynecol. 2001;97:435-438.
17. Johnson WH, Fecher AM, McMahon RL, et al. VersaStep trocar hernia rate in unclosed fascial defects in bariatric patients. Surg Endosc. 2006;20:1584-1586.
18. Lin P, Falcone T, Tulandi T. Excision of ovarian dermoid cyst by laparoscopy and by laparotomy. Am J Obstet Gynecol. 1995;173:769-771.
19. Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5-26.
20. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingooophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
21. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643-646.
22. Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214-218.
23. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
24. Dowdy SC, Stefanek M, Hartmann LC. Surgical risk reduction: prophylactic salpingooophorectomy and prophylactic mastectomy. Am J Obstet Gynecol. 2004;191:1113-1123.
25. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424-428.
26. Whittemore AS, Balise RR, Pharoah PD, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer. 2004;91:1911-1915.
27. Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol. 2005;105:35-41.
28. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
29. Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003;91:179-189.
30. Lehner R, Wenzl R, Heinzl H, Husslein P, Sevelda P. Influence of delayed staging laparotomy after laparoscopic removal of ovarian masses later found malignant. Obstet Gynecol. 1998;92:967-971.
The authors report no financial relationships relevant to this article.
CASE 1: Cystic mass in patient’s only remaining ovary
Mrs. R is a 29-year-old G1P1 who underwent a right oophorectomy, with a midline incision, for a dermoid cyst at the time of cesarean delivery. She now has a left ovarian cyst. Preoperative ultrasonography (US) reveals that it measures 3.5×4.2×3.7 cm and has both solid components and a multiloculated appearance, consistent with a dermoid cyst.
How common is this scenario?
Studies predict that one of every three women will undergo surgical management of an adnexal mass at some point in her life.1 This troubling statistic prompts several critical questions:
- How do we handle the workup for these women so that only appropriate patients undergo surgery?
- How often will a mass be malignant?
- How can we safely remove an adnexal mass to maximize patient safety, reduce overall recovery time, and prevent less favorable outcomes in women who are eventually found to have a malignancy?
A thorough workup and, sometimes, conservative management can prevent unnecessary surgery that may lead to early menopause or surgical complications. And maximizing the use of minimally invasive techniques in women who do require surgery can shorten hospital stay and recovery time. At the time of surgery, careful abdominal entry and meticulous surgical dissection and mass removal can limit the potential risks of laparoscopic excision in women who have an ultimate diagnosis of cancer.
In this article, we review the workup for women who have an adnexal mass, describe patient-selection criteria for laparoscopic surgery, including the risks and benefits of this approach ( TABLE 1 ), and present several techniques to safely manage a mass with potentially malignant histology via laparoscopy.
TABLE 1
There are benefits and risks to managing an adnexal mass laparoscopically
| Benefit | Risk |
|---|---|
| Shorter recovery Fewer adhesions Decreased overall cost Magnification Decreased pain and narcotic use Fewer wound complications | Expensive equipment* Loss of tactile sensation Concern for malignancy Risk of tumor dissemination/spillage/chemical peritonitis Trocar-site metastasis |
| *Though greater expense is not a risk per se, it does enter into decision making. | |
Begin with the physical
When a woman is known to have a pelvic mass, the aim of the office exam is to 1) identify characteristics that suggest malignancy and 2) rule out nongynecologic causes of the mass. Physical findings that are worrisome for a malignant process include:
- fixed or nodular pelvic mass
- bilateral masses
- nodular abdominal mass
- ascites
- pleural effusion on auscultation or percussion of the lung.
Although these findings can be present under benign conditions, they increase the risk that a malignancy will be detected at surgery.
Other causes of a pelvic mass should also be considered, including infection (pelvic abscess) and tumors of the colon, particularly when the pelvic mass occurs on the left side.
Some symptoms, though vague, are worth noting
Although ovarian cancer was once thought to be a silent disease, recent research has shown that bloating, pelvic or abdominal pain, early satiety, and urinary frequency and urgency are more common among women with ovarian cancer than among healthy controls and patients in high-risk screening clinics.2-4 Although these symptoms are generally nonspecific, they merit attention if they occur more than 12 times a month and have been present for less than 1 year. When they meet these criteria, the symptoms have a sensitivity for diagnosing early- and late-stage ovarian cancer of 56.7% and 79.5%, respectively.4
Sensitivity for the diagnosis of early-stage ovarian cancer may be as high as 80% when the symptom index score is combined with an elevated level of the tumor marker CA 125.3
Transvaginal US is crucial
Transvaginal US is now standard practice to obtain high-resolution images of an adnexal mass. Grayscale US has traditionally been used alone for evaluation.
Specificity is typically lower in women who are premenopausal because many benign lesions, such as endometrioma, have a similar sonographic appearance to cancer.
A number of US scoring algorithms have now been proposed to aid in the triage of women who have an adnexal mass. Sensitivity of these algorithms ranges from 65% to 100%; specificity, from 77% to 95%.5
CA 125 is the standard tumor marker
For the past two decades, CA 125 has been the standard serum marker in the screening of high-risk women for ovarian cancer and the triage of women who have an adnexal mass.
This blood test has been studied widely since its introduction in 1983. It typically has sensitivity of 75% to 85% and specificity of 85% to 95% in identifying women who have ovarian cancer. However, it is elevated in only 50% to 60% of women who have stage I ovarian cancer. Its lack of specificity and poor positive predictive value have kept researchers busy trying to identify other serum markers, for both ovarian cancer and identification of high-risk pelvic masses.
Our recommended workup and management of adnexal masses In postmenopausal women who had a pelvic mass, one study found that a CA 125 level above 65 IU/mL had sensitivity of 71% and specificity of 92.5% in the identification of ovarian cancer.6 Another group found that CA 125 levels above 65 IU/mL were more than 95% sensitive in the diagnosis of ovarian cancer in postmenopausal women.7
Several studies have combined CA 125 with other markers or with US to screen high-risk women or triage those who have an adnexal mass. These studies have shown modest improvements in sensitivity but usually lower specificity than with CA 125 testing alone.
Markers that may be used for suspected sex cord stromas and germ-cell tumors are:
- lactate dehydrogenase (LDH) for dysgerminomas
- alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) for yolk sac tumors
- testosterone for Sertoli-Leydig cell tumors
- inhibin A and B for granulosa cell tumors.
An algorithm for working up and managing adnexal masses appears above.
How to gain abdominal access
In the opening case, the patient clearly has a benign mass. The treatment? Safe entry into the peritoneal cavity to remove the cyst and as little normal tissue as possible. This is critical in this patient because she has only one ovary.
Peritoneal access for abdominal and pelvic laparoscopy has been studied widely. Options include:
- direct insertion using a Veress needle
- open laparoscopy
- direct trocar insertion.
The technique usually depends on the preference of the surgeon. The primary goal of abdominal entry is to minimize the risk of injury, particularly unrecognized injury.
Data on complication rates show no definite benefit for open versus closed techniques in the prevention of injury to underlying viscera. However, evidence does suggest that the open technique may lower the risk of major vascular injury.8
We employ direct trocar insertion using radially expanding or optical trocars.
The Veress needle option
When the Veress needle is used to gain intraperitoneal access, data indicate that initial intraperitoneal pressure below 10 mm Hg is a reliable marker for peritoneal entry, even in obese patients.9 Insufflation pressure as high as 25 to 30 mm Hg prior to placement of the initial trocar is safe from a cardiopulmonary standpoint and may allow easier entry with a nonbladed trocar.10
Tests to confirm intraperitoneal placement of the Veress needle, such as the hanging-drop test or saline flush, do not appear to offer any additional useful information.11
Open laparoscopy is suitable when adhesions are unlikely
Open laparoscopy is typically performed by making a minilaparotomy incision at the umbilicus and then dissecting and entering the peritoneal cavity. A blunt-tip trocar is inserted.
The disadvantage of this approach is that there may be extensive adhesions under the umbilicus, and it is difficult to dissect such adhesions sufficiently to introduce a cannula and laparoscope. Adhesions left behind often obscure the field of view after introduction of the trocar.
Our preference? Left upper-quadrant insertion
In Case 1, the previous midline incision mandates an alternative approach. When abdominal entry at the umbilicus is unsuccessful or potentially difficult because of an earlier midline incision, umbilical hernia repair, or history of multiple lower abdominal or pelvic surgeries, a left upper-quadrant insertion is useful. It is, in fact, our preferred technique, and involves a small incision at the midclavicular line 5 cm below the left costal margin, at a site called Palmer’s point.
The direction of insertion usually ranges from 45° to 90°, depending on the patient’s body weight. If the trocar is placed properly, the closest organs are the stomach and the left lobe of the liver (4 to 6 cm).12 Given the stomach’s close proximity, it should be decompressed with an orogastric tube prior to trocar insertion.
Several studies have demonstrated the safety and efficacy of this entry technique.12-14 It can be helpful in cases that involve difficult access. We usually use a 5-mm primary trocar site for a 5-mm laparoscope. Modern optics allow for a pristine view with these small scopes, eliminating the need to close fascia and perhaps causing less pain at the incision.
Accessory trocar sites facilitate complex technique
We usually use three accessory sites. Two of them are lower-quadrant ports that are placed 2 cm medial and 2 cm cephalad to the anterior superior iliac spine. This area generally lies well away from the inferior epigastric vessels and remains above the area of the ilioinguinal and iliohypogastric nerves, making it a safer point of insertion.15 One trocar is 5 mm in size and the other is 10 mm. The larger one is used to extract the specimen.
We place an additional 5-mm port lateral to the rectus muscle at the level of the umbilicus. This allows the principal surgeon to use two instruments (a toothed forceps and scissors) comfortably while the assistant holds the laparoscope and assists with a grasper.
Does the type of trocar matter?
No randomized studies have directly compared all types of trocars. Options include:
- a pyramidal tip (as in reusable trocars) or shielded tip
- radial expansion
- visible entry
- blunt (Hasson-type) trocar.
Safety data on direct comparison of trocars are limited, but it appears that a radially expanding trocar may offer less port-site pain and potentially less bleeding than a traditional cutting trocar.16 Moreover, the rate of hernia at the port site appears to be relatively low with a radially expanding trocar, even when fascia is left unclosed at a 10-mm site.17
None of these trocars appears to be clearly superior at avoiding visceral or vascular injury.
Technique of laparoscopic cyst removal
A video clip of the surgery is linked to this article in the Video Library at www.obgmanagement.com. In this case, a trocar was inserted in the left upper quadrant, and a laparoscopic cystectomy was initiated using the trocars already specified.
The peritoneal cavity and adnexa were inspected, followed by pelvic washings, as detailed in TABLE 2 . Next, the ovarian cortex was incised ( FIGURE 1A ) with scissors using bipolar or unipolar energy, typically at a low power setting, such as 12 to 15 watts.
It was relatively easy to develop a tissue plane between the cortex and underlying dermoid cyst using simple or aqua-dissection ( FIGURE 1B and C ). The cyst was enucleated without rupture and inserted into a specimen bag through a 10-mm port ( FIGURE 1D ). (The specimen bags generally are available in 10-mm and 15-mm sizes.) Once the trocar was removed, the entire specimen was brought out through the incision ( FIGURE 1E ).
Cystic masses can generally be carefully aspirated using a laparoscopic needle or angiocath, or they can be incised and drained using a standard suction device. Manufacturers of most specimen bags do not recommend the morcellation of tissue within the bag because of the potential for rupture of the bag with tumor spillage or injury to underlying structures.
Occasionally, the mass remains too large to remove after drainage of the cyst fluid. Remedies include enlarging the port site with a scalpel or using a gallbladder speculum to increase the diameter of the port site ( FIGURE 1E ). If possible, the incision should be large enough to deliver the entire bag intact. Use of excessive force will rupture the bag and may cause the specimen to be lost or malignant cells to be inadvertently spilled (in the case of a cancer diagnosis).
TABLE 2
Take these 10 steps to safe laparoscopic surgery
| Examine the anesthetized patient |
| Enter the abdomen |
| Inspect the mass and peritoneal surfaces, including the diaphragm. Biopsy sites suspicious for metastasis and obtain frozen section |
| Perform pelvic and abdominal washings |
Closely inspect adnexa. If findings are not suggestive of malignancy, proceed with laparoscopy. If findings indicate obvious malignancy
|
Perform cystectomy or oophorectomy
|
| Inspect for hemostasis |
| Place cyst/ovary in endopouch |
| Open bag at abdominal wall and remove for frozen section |
| Reinspect and close |
Avoid:
|
FIGURE 1 Laparoscopic cyst removal
Begin by incising the cortex using scissors with or without an energy source.
Dissect the cyst free from the cortex using sharp dissection.
Remove the cyst from the ovary.
Place the cyst in a specimen bag, and …
… bring it to the surface for extraction. The abdominal incision may have to be enlarged to accommodate the specimen.
CASE 1: OUTCOME
The patient’s cyst is removed intact and she is discharged home. Rupture of a dermoid cyst is not associated with any problems as long as copious irrigation is used to aspirate the cyst content.18 Other cysts, such as endometriomas, may not be as easily dissected, and rupture is inevitable.
CASE 2: Symptoms suggestive of cancer
Mrs. B is a 47-year-old woman who reports abdominal bloating for the past 3 weeks. She also complains of early satiety and occasional constipation. She has no history of cancer, but her sister was given a diagnosis of breast cancer at 41 years of age, and her maternal aunt had breast cancer at 55 years.
Mrs. B is moderately obese, with a nontender abdomen and no palpable mass. Her pelvic exam also is negative for a mass or nodularity, but the extent of the exam is limited by body habitus. Her physician orders a transvaginal US, which reveals a 6-cm complex mass with thin septation and a 1-cm solid nodule, with no definite blood flow. The patient’s CA 125 level is 80 IU/mL, which we consider to be within the low-risk range for a premenopausal woman.
The patient is counseled about the need to have the mass removed and is scheduled for laparoscopic right salpingooophorectomy. Given the family history of breast cancer, the physician also requests consultation with a gynecologic oncologist, who agrees to assist with surgery and perform a laparotomy and staging in the event that a malignancy is diagnosed.
Is the mass likely to be malignant?
Given the patient’s family history of breast cancer, the recent onset of symptoms associated with ovarian cancer,2 and the characteristics of the mass (complex, with a nodule), malignancy is possible. This patient has an intermediate risk of cancer and requires additional counseling and planning.
However, most women who undergo laparoscopy for removal of an adnexal mass have benign pathologic findings.
What is the real risk of ovarian cancer?
The lifetime risk of developing ovarian cancer in the general population remains stable at approximately 1 in 70 women, with a mean age at diagnosis of 63 years.19 Ninety percent of ovarian cancer cases are sporadic, and less than 10% can be linked to genetic syndromes.
Women who have mutations in the BRCA1 gene carry a lifetime risk of ovarian cancer of up to 50%, and women who have mutations in BRCA2 have a lifetime risk of up to 25%.20,21 Women who have mutations associated with Lynch II syndrome or Hereditary Nonpolyposis Colorectal Cancer syndrome may have a lifetime risk of ovarian cancer of 12%.22,23
Some women who have a strong family history of breast and ovarian cancer do not carry a known mutation, but are likely to be at increased risk.
Additional risk factors known to be associated with ovarian cancer are nulliparity and infertility. However, the single most important risk factor for epithelial ovarian cancer is age.
Risk-reducing strategies include:
In the case of PBSO, it is imperative to ensure that all ovarian surface epithelium is removed. This means excising the infundibulopelvic ligament at least 1.5 cm above the proximal end of the ovary and excising any adjacent tissue to which the ovary is adherent (including pelvic sidewall peritoneum). Both requirements are easily achieved using the techniques outlined here.
Who should perform surgery?
The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) have published guidelines for referral to a gynecologic oncologist ( TABLE 3 ). When Im and colleagues reviewed the records of more than 1,000 women who underwent surgery for a pelvic mass at six institutions over 12 months, they found that 70% of premenopausal women and 94% of postmenopausal women who were diagnosed with ovarian cancer were properly referred to a qualified subspecialist.27 “Over”-referral occurred in 30% to 40% of women who had a benign mass, but may be preferable given the importance of proper staging and debulking to survival.
ACOG and the SGO recommend referral for women who have:
- elevated tumor markers
- ascites
- a fixed or nodular mass
- a strong family history of breast or ovarian cancer.
Consider preoperative referral of all high-risk and, probably, intermediate-risk women, depending on the availability of qualified specialists for complete surgical staging.
In addition, women need to be counseled thoroughly about the possibility that a malignancy will be diagnosed by frozen section, necessitating additional surgical procedures.
TABLE 3
Your patient has a newly diagnosed pelvic mass. Should you refer her?
| Is she premenopausal? Then refer her when… | Is she postmenopausal? Then refer her when… |
|---|---|
| CA125 >200 IU/mL Ascites is present Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative | CA125 >35 IU/mL Ascites is present Nodular or fixed pelvic mass Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative |
Technique of laparoscopic adnexectomy
In Case 2, an umbilical site was chosen for the primary cannula. In general, the direction of insertion depends on the patient’s body habitus. Heavier patients have a thicker abdominal wall and an umbilicus below the level of the aortic bifurcation. In these women, the angle of insertion should be adjusted from the usual 45° (for patients of normal weight) to an angle nearer to 90°. Lateral ports are typical, as in Case 1.
Treat every mass like cancer
Laparoscopic excision of an adnexal mass can be safe and effective, with better outcomes and recovery than with laparotomy, provided the surgeon adheres to basic principles ( TABLE 2 ). This means treating every mass as though it is potentially malignant, and thoroughly inspecting the abdominal cavity before and after excision of the mass.
Know the retroperitoneal space
As with Case 1, successful laparoscopic excision of an adnexal mass begins with inspection of the peritoneal cavity, abdominopelvic washings, and identification of both the infundibulopelvic ligament and ureter. Knowledge of the retroperitoneal space can be of great value in difficult cases that involve significant pelvic adhesions or sidewall fibrosis. We generally use a retroperitoneal approach for laparoscopic adnexectomy ( FIGURE 2A ).
In our typical approach, we incise the peritoneum lateral to the uteroovarian ligament and continue the incision up the pelvic sidewall lateral to the infundibulopelvic ligament and up along the paracolic gutter, if needed ( FIGURE 2A ). We then mobilize the medial leaf of the broad ligament from the sidewall using blunt dissection between the external iliac vessels laterally and the ureter medially ( FIGURE 2B ).
Once we have identified the ureter, we use scissors to create a window in the medial leaf of the broad ligament just beneath the gonadal vessels. We then use an energy source to occlude and transect the pedicle. Using this technique, we secure the infundibulopelvic ligaments and safely mobilize the ureter before initiating more aggressive sidewall dissection distally for adhesions or fibrosis.
Once the ureter is mobilized, we excise the involved peritoneum along with the mass. We then seal and transect the uteroovarian ligament.
FIGURE 2 The retroperitoneal approach
Grasp and incise the peritoneum just lateral to the adnexal mass and enter the retroperitoneal space, where loose areolar tissue is visible, with the ureter seen on the medial leaf of the broad ligament.
After dissecting this tissue, identify the ureter and internal iliac vessels.
Remove the mass in a bag
Once the mass is excised, we place it in a laparoscopic bag, as described for Case 1. Solid adnexal masses are problematic because they are not amenable to drainage, and morcellation is usually discouraged. Laparoscopic excision can still be carried out, and a minilaparotomy or posterior colpotomy can be used to extract the mass in a bag. Patients managed in this way still have a complication rate similar to or lower than that of patients undergoing laparotomy for oophorectomy; they also recover faster.
Risks in cases of malignancy
The risk of tumor spillage in laparoscopic surgery can be lowered using laparoscopically guided minilaparotomy techniques. Preoperative rupture appears to be more predictive of outcome.28
The concern about metastatic implants in a laparoscopic port site in patients who have gynecologic cancer is real, with an incidence ranging from 0.97% to 1.1%. The phenomenon usually affects women who had ovarian cancer and is most common after laparoscopy with findings of ascites, carcinomatosis, or persistent disease (in the case of second-look laparoscopy performed after completion of primary therapy for ovarian cancer).
These data suggest that port-site implantation is not a concern that should deter clinicians from laparoscopic evaluation of a suspected ovarian neoplasm unless a patient presents with ascites or carcinomatosis suggestive of advanced disease.29
Data are limited regarding delays in definitive therapy. One study suggests that definitive staging on the day of rupture does not influence overall outcome, but delay by more than 2 weeks may lead to poorer prognosis.30
CASE 2: OUTCOME
Laparoscopy is performed. On initial inspection, the peritoneal cavity is unremarkable. The right ovary is multicystic with normal surface anatomy. The mass is somewhat adherent to the pelvic sidewall. Adnexectomy is performed, and frozen section reveals the mass to be a serous cystadenoma.
The authors report no financial relationships relevant to this article.
CASE 1: Cystic mass in patient’s only remaining ovary
Mrs. R is a 29-year-old G1P1 who underwent a right oophorectomy, with a midline incision, for a dermoid cyst at the time of cesarean delivery. She now has a left ovarian cyst. Preoperative ultrasonography (US) reveals that it measures 3.5×4.2×3.7 cm and has both solid components and a multiloculated appearance, consistent with a dermoid cyst.
How common is this scenario?
Studies predict that one of every three women will undergo surgical management of an adnexal mass at some point in her life.1 This troubling statistic prompts several critical questions:
- How do we handle the workup for these women so that only appropriate patients undergo surgery?
- How often will a mass be malignant?
- How can we safely remove an adnexal mass to maximize patient safety, reduce overall recovery time, and prevent less favorable outcomes in women who are eventually found to have a malignancy?
A thorough workup and, sometimes, conservative management can prevent unnecessary surgery that may lead to early menopause or surgical complications. And maximizing the use of minimally invasive techniques in women who do require surgery can shorten hospital stay and recovery time. At the time of surgery, careful abdominal entry and meticulous surgical dissection and mass removal can limit the potential risks of laparoscopic excision in women who have an ultimate diagnosis of cancer.
In this article, we review the workup for women who have an adnexal mass, describe patient-selection criteria for laparoscopic surgery, including the risks and benefits of this approach ( TABLE 1 ), and present several techniques to safely manage a mass with potentially malignant histology via laparoscopy.
TABLE 1
There are benefits and risks to managing an adnexal mass laparoscopically
| Benefit | Risk |
|---|---|
| Shorter recovery Fewer adhesions Decreased overall cost Magnification Decreased pain and narcotic use Fewer wound complications | Expensive equipment* Loss of tactile sensation Concern for malignancy Risk of tumor dissemination/spillage/chemical peritonitis Trocar-site metastasis |
| *Though greater expense is not a risk per se, it does enter into decision making. | |
Begin with the physical
When a woman is known to have a pelvic mass, the aim of the office exam is to 1) identify characteristics that suggest malignancy and 2) rule out nongynecologic causes of the mass. Physical findings that are worrisome for a malignant process include:
- fixed or nodular pelvic mass
- bilateral masses
- nodular abdominal mass
- ascites
- pleural effusion on auscultation or percussion of the lung.
Although these findings can be present under benign conditions, they increase the risk that a malignancy will be detected at surgery.
Other causes of a pelvic mass should also be considered, including infection (pelvic abscess) and tumors of the colon, particularly when the pelvic mass occurs on the left side.
Some symptoms, though vague, are worth noting
Although ovarian cancer was once thought to be a silent disease, recent research has shown that bloating, pelvic or abdominal pain, early satiety, and urinary frequency and urgency are more common among women with ovarian cancer than among healthy controls and patients in high-risk screening clinics.2-4 Although these symptoms are generally nonspecific, they merit attention if they occur more than 12 times a month and have been present for less than 1 year. When they meet these criteria, the symptoms have a sensitivity for diagnosing early- and late-stage ovarian cancer of 56.7% and 79.5%, respectively.4
Sensitivity for the diagnosis of early-stage ovarian cancer may be as high as 80% when the symptom index score is combined with an elevated level of the tumor marker CA 125.3
Transvaginal US is crucial
Transvaginal US is now standard practice to obtain high-resolution images of an adnexal mass. Grayscale US has traditionally been used alone for evaluation.
Specificity is typically lower in women who are premenopausal because many benign lesions, such as endometrioma, have a similar sonographic appearance to cancer.
A number of US scoring algorithms have now been proposed to aid in the triage of women who have an adnexal mass. Sensitivity of these algorithms ranges from 65% to 100%; specificity, from 77% to 95%.5
CA 125 is the standard tumor marker
For the past two decades, CA 125 has been the standard serum marker in the screening of high-risk women for ovarian cancer and the triage of women who have an adnexal mass.
This blood test has been studied widely since its introduction in 1983. It typically has sensitivity of 75% to 85% and specificity of 85% to 95% in identifying women who have ovarian cancer. However, it is elevated in only 50% to 60% of women who have stage I ovarian cancer. Its lack of specificity and poor positive predictive value have kept researchers busy trying to identify other serum markers, for both ovarian cancer and identification of high-risk pelvic masses.
Our recommended workup and management of adnexal masses In postmenopausal women who had a pelvic mass, one study found that a CA 125 level above 65 IU/mL had sensitivity of 71% and specificity of 92.5% in the identification of ovarian cancer.6 Another group found that CA 125 levels above 65 IU/mL were more than 95% sensitive in the diagnosis of ovarian cancer in postmenopausal women.7
Several studies have combined CA 125 with other markers or with US to screen high-risk women or triage those who have an adnexal mass. These studies have shown modest improvements in sensitivity but usually lower specificity than with CA 125 testing alone.
Markers that may be used for suspected sex cord stromas and germ-cell tumors are:
- lactate dehydrogenase (LDH) for dysgerminomas
- alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) for yolk sac tumors
- testosterone for Sertoli-Leydig cell tumors
- inhibin A and B for granulosa cell tumors.
An algorithm for working up and managing adnexal masses appears above.
How to gain abdominal access
In the opening case, the patient clearly has a benign mass. The treatment? Safe entry into the peritoneal cavity to remove the cyst and as little normal tissue as possible. This is critical in this patient because she has only one ovary.
Peritoneal access for abdominal and pelvic laparoscopy has been studied widely. Options include:
- direct insertion using a Veress needle
- open laparoscopy
- direct trocar insertion.
The technique usually depends on the preference of the surgeon. The primary goal of abdominal entry is to minimize the risk of injury, particularly unrecognized injury.
Data on complication rates show no definite benefit for open versus closed techniques in the prevention of injury to underlying viscera. However, evidence does suggest that the open technique may lower the risk of major vascular injury.8
We employ direct trocar insertion using radially expanding or optical trocars.
The Veress needle option
When the Veress needle is used to gain intraperitoneal access, data indicate that initial intraperitoneal pressure below 10 mm Hg is a reliable marker for peritoneal entry, even in obese patients.9 Insufflation pressure as high as 25 to 30 mm Hg prior to placement of the initial trocar is safe from a cardiopulmonary standpoint and may allow easier entry with a nonbladed trocar.10
Tests to confirm intraperitoneal placement of the Veress needle, such as the hanging-drop test or saline flush, do not appear to offer any additional useful information.11
Open laparoscopy is suitable when adhesions are unlikely
Open laparoscopy is typically performed by making a minilaparotomy incision at the umbilicus and then dissecting and entering the peritoneal cavity. A blunt-tip trocar is inserted.
The disadvantage of this approach is that there may be extensive adhesions under the umbilicus, and it is difficult to dissect such adhesions sufficiently to introduce a cannula and laparoscope. Adhesions left behind often obscure the field of view after introduction of the trocar.
Our preference? Left upper-quadrant insertion
In Case 1, the previous midline incision mandates an alternative approach. When abdominal entry at the umbilicus is unsuccessful or potentially difficult because of an earlier midline incision, umbilical hernia repair, or history of multiple lower abdominal or pelvic surgeries, a left upper-quadrant insertion is useful. It is, in fact, our preferred technique, and involves a small incision at the midclavicular line 5 cm below the left costal margin, at a site called Palmer’s point.
The direction of insertion usually ranges from 45° to 90°, depending on the patient’s body weight. If the trocar is placed properly, the closest organs are the stomach and the left lobe of the liver (4 to 6 cm).12 Given the stomach’s close proximity, it should be decompressed with an orogastric tube prior to trocar insertion.
Several studies have demonstrated the safety and efficacy of this entry technique.12-14 It can be helpful in cases that involve difficult access. We usually use a 5-mm primary trocar site for a 5-mm laparoscope. Modern optics allow for a pristine view with these small scopes, eliminating the need to close fascia and perhaps causing less pain at the incision.
Accessory trocar sites facilitate complex technique
We usually use three accessory sites. Two of them are lower-quadrant ports that are placed 2 cm medial and 2 cm cephalad to the anterior superior iliac spine. This area generally lies well away from the inferior epigastric vessels and remains above the area of the ilioinguinal and iliohypogastric nerves, making it a safer point of insertion.15 One trocar is 5 mm in size and the other is 10 mm. The larger one is used to extract the specimen.
We place an additional 5-mm port lateral to the rectus muscle at the level of the umbilicus. This allows the principal surgeon to use two instruments (a toothed forceps and scissors) comfortably while the assistant holds the laparoscope and assists with a grasper.
Does the type of trocar matter?
No randomized studies have directly compared all types of trocars. Options include:
- a pyramidal tip (as in reusable trocars) or shielded tip
- radial expansion
- visible entry
- blunt (Hasson-type) trocar.
Safety data on direct comparison of trocars are limited, but it appears that a radially expanding trocar may offer less port-site pain and potentially less bleeding than a traditional cutting trocar.16 Moreover, the rate of hernia at the port site appears to be relatively low with a radially expanding trocar, even when fascia is left unclosed at a 10-mm site.17
None of these trocars appears to be clearly superior at avoiding visceral or vascular injury.
Technique of laparoscopic cyst removal
A video clip of the surgery is linked to this article in the Video Library at www.obgmanagement.com. In this case, a trocar was inserted in the left upper quadrant, and a laparoscopic cystectomy was initiated using the trocars already specified.
The peritoneal cavity and adnexa were inspected, followed by pelvic washings, as detailed in TABLE 2 . Next, the ovarian cortex was incised ( FIGURE 1A ) with scissors using bipolar or unipolar energy, typically at a low power setting, such as 12 to 15 watts.
It was relatively easy to develop a tissue plane between the cortex and underlying dermoid cyst using simple or aqua-dissection ( FIGURE 1B and C ). The cyst was enucleated without rupture and inserted into a specimen bag through a 10-mm port ( FIGURE 1D ). (The specimen bags generally are available in 10-mm and 15-mm sizes.) Once the trocar was removed, the entire specimen was brought out through the incision ( FIGURE 1E ).
Cystic masses can generally be carefully aspirated using a laparoscopic needle or angiocath, or they can be incised and drained using a standard suction device. Manufacturers of most specimen bags do not recommend the morcellation of tissue within the bag because of the potential for rupture of the bag with tumor spillage or injury to underlying structures.
Occasionally, the mass remains too large to remove after drainage of the cyst fluid. Remedies include enlarging the port site with a scalpel or using a gallbladder speculum to increase the diameter of the port site ( FIGURE 1E ). If possible, the incision should be large enough to deliver the entire bag intact. Use of excessive force will rupture the bag and may cause the specimen to be lost or malignant cells to be inadvertently spilled (in the case of a cancer diagnosis).
TABLE 2
Take these 10 steps to safe laparoscopic surgery
| Examine the anesthetized patient |
| Enter the abdomen |
| Inspect the mass and peritoneal surfaces, including the diaphragm. Biopsy sites suspicious for metastasis and obtain frozen section |
| Perform pelvic and abdominal washings |
Closely inspect adnexa. If findings are not suggestive of malignancy, proceed with laparoscopy. If findings indicate obvious malignancy
|
Perform cystectomy or oophorectomy
|
| Inspect for hemostasis |
| Place cyst/ovary in endopouch |
| Open bag at abdominal wall and remove for frozen section |
| Reinspect and close |
Avoid:
|
FIGURE 1 Laparoscopic cyst removal
Begin by incising the cortex using scissors with or without an energy source.
Dissect the cyst free from the cortex using sharp dissection.
Remove the cyst from the ovary.
Place the cyst in a specimen bag, and …
… bring it to the surface for extraction. The abdominal incision may have to be enlarged to accommodate the specimen.
CASE 1: OUTCOME
The patient’s cyst is removed intact and she is discharged home. Rupture of a dermoid cyst is not associated with any problems as long as copious irrigation is used to aspirate the cyst content.18 Other cysts, such as endometriomas, may not be as easily dissected, and rupture is inevitable.
CASE 2: Symptoms suggestive of cancer
Mrs. B is a 47-year-old woman who reports abdominal bloating for the past 3 weeks. She also complains of early satiety and occasional constipation. She has no history of cancer, but her sister was given a diagnosis of breast cancer at 41 years of age, and her maternal aunt had breast cancer at 55 years.
Mrs. B is moderately obese, with a nontender abdomen and no palpable mass. Her pelvic exam also is negative for a mass or nodularity, but the extent of the exam is limited by body habitus. Her physician orders a transvaginal US, which reveals a 6-cm complex mass with thin septation and a 1-cm solid nodule, with no definite blood flow. The patient’s CA 125 level is 80 IU/mL, which we consider to be within the low-risk range for a premenopausal woman.
The patient is counseled about the need to have the mass removed and is scheduled for laparoscopic right salpingooophorectomy. Given the family history of breast cancer, the physician also requests consultation with a gynecologic oncologist, who agrees to assist with surgery and perform a laparotomy and staging in the event that a malignancy is diagnosed.
Is the mass likely to be malignant?
Given the patient’s family history of breast cancer, the recent onset of symptoms associated with ovarian cancer,2 and the characteristics of the mass (complex, with a nodule), malignancy is possible. This patient has an intermediate risk of cancer and requires additional counseling and planning.
However, most women who undergo laparoscopy for removal of an adnexal mass have benign pathologic findings.
What is the real risk of ovarian cancer?
The lifetime risk of developing ovarian cancer in the general population remains stable at approximately 1 in 70 women, with a mean age at diagnosis of 63 years.19 Ninety percent of ovarian cancer cases are sporadic, and less than 10% can be linked to genetic syndromes.
Women who have mutations in the BRCA1 gene carry a lifetime risk of ovarian cancer of up to 50%, and women who have mutations in BRCA2 have a lifetime risk of up to 25%.20,21 Women who have mutations associated with Lynch II syndrome or Hereditary Nonpolyposis Colorectal Cancer syndrome may have a lifetime risk of ovarian cancer of 12%.22,23
Some women who have a strong family history of breast and ovarian cancer do not carry a known mutation, but are likely to be at increased risk.
Additional risk factors known to be associated with ovarian cancer are nulliparity and infertility. However, the single most important risk factor for epithelial ovarian cancer is age.
Risk-reducing strategies include:
In the case of PBSO, it is imperative to ensure that all ovarian surface epithelium is removed. This means excising the infundibulopelvic ligament at least 1.5 cm above the proximal end of the ovary and excising any adjacent tissue to which the ovary is adherent (including pelvic sidewall peritoneum). Both requirements are easily achieved using the techniques outlined here.
Who should perform surgery?
The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) have published guidelines for referral to a gynecologic oncologist ( TABLE 3 ). When Im and colleagues reviewed the records of more than 1,000 women who underwent surgery for a pelvic mass at six institutions over 12 months, they found that 70% of premenopausal women and 94% of postmenopausal women who were diagnosed with ovarian cancer were properly referred to a qualified subspecialist.27 “Over”-referral occurred in 30% to 40% of women who had a benign mass, but may be preferable given the importance of proper staging and debulking to survival.
ACOG and the SGO recommend referral for women who have:
- elevated tumor markers
- ascites
- a fixed or nodular mass
- a strong family history of breast or ovarian cancer.
Consider preoperative referral of all high-risk and, probably, intermediate-risk women, depending on the availability of qualified specialists for complete surgical staging.
In addition, women need to be counseled thoroughly about the possibility that a malignancy will be diagnosed by frozen section, necessitating additional surgical procedures.
TABLE 3
Your patient has a newly diagnosed pelvic mass. Should you refer her?
| Is she premenopausal? Then refer her when… | Is she postmenopausal? Then refer her when… |
|---|---|
| CA125 >200 IU/mL Ascites is present Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative | CA125 >35 IU/mL Ascites is present Nodular or fixed pelvic mass Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative |
Technique of laparoscopic adnexectomy
In Case 2, an umbilical site was chosen for the primary cannula. In general, the direction of insertion depends on the patient’s body habitus. Heavier patients have a thicker abdominal wall and an umbilicus below the level of the aortic bifurcation. In these women, the angle of insertion should be adjusted from the usual 45° (for patients of normal weight) to an angle nearer to 90°. Lateral ports are typical, as in Case 1.
Treat every mass like cancer
Laparoscopic excision of an adnexal mass can be safe and effective, with better outcomes and recovery than with laparotomy, provided the surgeon adheres to basic principles ( TABLE 2 ). This means treating every mass as though it is potentially malignant, and thoroughly inspecting the abdominal cavity before and after excision of the mass.
Know the retroperitoneal space
As with Case 1, successful laparoscopic excision of an adnexal mass begins with inspection of the peritoneal cavity, abdominopelvic washings, and identification of both the infundibulopelvic ligament and ureter. Knowledge of the retroperitoneal space can be of great value in difficult cases that involve significant pelvic adhesions or sidewall fibrosis. We generally use a retroperitoneal approach for laparoscopic adnexectomy ( FIGURE 2A ).
In our typical approach, we incise the peritoneum lateral to the uteroovarian ligament and continue the incision up the pelvic sidewall lateral to the infundibulopelvic ligament and up along the paracolic gutter, if needed ( FIGURE 2A ). We then mobilize the medial leaf of the broad ligament from the sidewall using blunt dissection between the external iliac vessels laterally and the ureter medially ( FIGURE 2B ).
Once we have identified the ureter, we use scissors to create a window in the medial leaf of the broad ligament just beneath the gonadal vessels. We then use an energy source to occlude and transect the pedicle. Using this technique, we secure the infundibulopelvic ligaments and safely mobilize the ureter before initiating more aggressive sidewall dissection distally for adhesions or fibrosis.
Once the ureter is mobilized, we excise the involved peritoneum along with the mass. We then seal and transect the uteroovarian ligament.
FIGURE 2 The retroperitoneal approach
Grasp and incise the peritoneum just lateral to the adnexal mass and enter the retroperitoneal space, where loose areolar tissue is visible, with the ureter seen on the medial leaf of the broad ligament.
After dissecting this tissue, identify the ureter and internal iliac vessels.
Remove the mass in a bag
Once the mass is excised, we place it in a laparoscopic bag, as described for Case 1. Solid adnexal masses are problematic because they are not amenable to drainage, and morcellation is usually discouraged. Laparoscopic excision can still be carried out, and a minilaparotomy or posterior colpotomy can be used to extract the mass in a bag. Patients managed in this way still have a complication rate similar to or lower than that of patients undergoing laparotomy for oophorectomy; they also recover faster.
Risks in cases of malignancy
The risk of tumor spillage in laparoscopic surgery can be lowered using laparoscopically guided minilaparotomy techniques. Preoperative rupture appears to be more predictive of outcome.28
The concern about metastatic implants in a laparoscopic port site in patients who have gynecologic cancer is real, with an incidence ranging from 0.97% to 1.1%. The phenomenon usually affects women who had ovarian cancer and is most common after laparoscopy with findings of ascites, carcinomatosis, or persistent disease (in the case of second-look laparoscopy performed after completion of primary therapy for ovarian cancer).
These data suggest that port-site implantation is not a concern that should deter clinicians from laparoscopic evaluation of a suspected ovarian neoplasm unless a patient presents with ascites or carcinomatosis suggestive of advanced disease.29
Data are limited regarding delays in definitive therapy. One study suggests that definitive staging on the day of rupture does not influence overall outcome, but delay by more than 2 weeks may lead to poorer prognosis.30
CASE 2: OUTCOME
Laparoscopy is performed. On initial inspection, the peritoneal cavity is unremarkable. The right ovary is multicystic with normal surface anatomy. The mass is somewhat adherent to the pelvic sidewall. Adnexectomy is performed, and frozen section reveals the mass to be a serous cystadenoma.
1. Curtin JP. Management of the adnexal mass. Gynecol Oncol. 1994;55:S42-S46.
2. Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712.
3. Andersen MR, Goff BA, Lowe KA, et al. Combining a symptoms index with CA 125 to improve detection of ovarian cancer. Cancer. 2008;113:484-489.
4. Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221-227.
5. Alcázar JL, Mercé LT, Laparte C, et al. A new scoring system to differentiate benign from malignant adnexal masses. Am J Obstet Gynecol. 2003;188:685-692.
6. Maggino T, Gadducci A, D’Addario V, et al. Prospective multicenter study on CA 125 in postmenopausal pelvic masses. Gynecol Oncol. 1994;54:117-123.
7. Malkasian GD, Jr, Knapp RC, Lavin PT, et al. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988;159:341-346.
8. Larobina M, Nottle P. Complete evidence regarding major vascular injuries during laparoscopic access. Surg Laparosc Endosc Percutan Tech. 2005;15:119-123.
9. Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10:415-420.
10. Vilos GA, Vilos AG, Abu-Rafea B, Hollett-Caines J, Nikkhah-Abyaneh Z, Edris F. Three simple steps during closed laparoscopic entry may minimize major injuries. Surg Endosc. 2008 July 15. [Epub ahead of print]
11. Vilos GA, Ternamian A, Dempster J, Laberge PY. The Society of Obstetricians and Gynaecologists of Canada. Laparoscopic entry: a review of techniques, technologies, and complications. J Obstet Gynaecol Can. 2007;29:433-465.
12. Tulikangas PK, Nicklas A, Falcone T, Price LL. Anatomy of the left upper quadrant for cannula insertion. J Am Assoc Gynecol Laparosc. 2000;7:211-214.
13. Stepp KJ, Tulikangas PK, Goldberg JM, Attaran M, Falcone T. Laparoscopy for adnexal masses in the second trimester of pregnancy. J Am Assoc Gynecol Laparosc. 2003;10:55-59.
14. Tulikangas PK, Robinson DS, Falcone T. Left upper quadrant cannula insertion. Fertil Steril. 2003;79:411-412.
15. Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189:1574-1578.
16. Yim SF, Yuen PM. Randomized double-masked comparison of radially expanding access device and conventional cutting tip trocar in laparoscopy. Obstet Gynecol. 2001;97:435-438.
17. Johnson WH, Fecher AM, McMahon RL, et al. VersaStep trocar hernia rate in unclosed fascial defects in bariatric patients. Surg Endosc. 2006;20:1584-1586.
18. Lin P, Falcone T, Tulandi T. Excision of ovarian dermoid cyst by laparoscopy and by laparotomy. Am J Obstet Gynecol. 1995;173:769-771.
19. Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5-26.
20. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingooophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
21. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643-646.
22. Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214-218.
23. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
24. Dowdy SC, Stefanek M, Hartmann LC. Surgical risk reduction: prophylactic salpingooophorectomy and prophylactic mastectomy. Am J Obstet Gynecol. 2004;191:1113-1123.
25. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424-428.
26. Whittemore AS, Balise RR, Pharoah PD, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer. 2004;91:1911-1915.
27. Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol. 2005;105:35-41.
28. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
29. Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003;91:179-189.
30. Lehner R, Wenzl R, Heinzl H, Husslein P, Sevelda P. Influence of delayed staging laparotomy after laparoscopic removal of ovarian masses later found malignant. Obstet Gynecol. 1998;92:967-971.
1. Curtin JP. Management of the adnexal mass. Gynecol Oncol. 1994;55:S42-S46.
2. Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712.
3. Andersen MR, Goff BA, Lowe KA, et al. Combining a symptoms index with CA 125 to improve detection of ovarian cancer. Cancer. 2008;113:484-489.
4. Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221-227.
5. Alcázar JL, Mercé LT, Laparte C, et al. A new scoring system to differentiate benign from malignant adnexal masses. Am J Obstet Gynecol. 2003;188:685-692.
6. Maggino T, Gadducci A, D’Addario V, et al. Prospective multicenter study on CA 125 in postmenopausal pelvic masses. Gynecol Oncol. 1994;54:117-123.
7. Malkasian GD, Jr, Knapp RC, Lavin PT, et al. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988;159:341-346.
8. Larobina M, Nottle P. Complete evidence regarding major vascular injuries during laparoscopic access. Surg Laparosc Endosc Percutan Tech. 2005;15:119-123.
9. Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10:415-420.
10. Vilos GA, Vilos AG, Abu-Rafea B, Hollett-Caines J, Nikkhah-Abyaneh Z, Edris F. Three simple steps during closed laparoscopic entry may minimize major injuries. Surg Endosc. 2008 July 15. [Epub ahead of print]
11. Vilos GA, Ternamian A, Dempster J, Laberge PY. The Society of Obstetricians and Gynaecologists of Canada. Laparoscopic entry: a review of techniques, technologies, and complications. J Obstet Gynaecol Can. 2007;29:433-465.
12. Tulikangas PK, Nicklas A, Falcone T, Price LL. Anatomy of the left upper quadrant for cannula insertion. J Am Assoc Gynecol Laparosc. 2000;7:211-214.
13. Stepp KJ, Tulikangas PK, Goldberg JM, Attaran M, Falcone T. Laparoscopy for adnexal masses in the second trimester of pregnancy. J Am Assoc Gynecol Laparosc. 2003;10:55-59.
14. Tulikangas PK, Robinson DS, Falcone T. Left upper quadrant cannula insertion. Fertil Steril. 2003;79:411-412.
15. Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189:1574-1578.
16. Yim SF, Yuen PM. Randomized double-masked comparison of radially expanding access device and conventional cutting tip trocar in laparoscopy. Obstet Gynecol. 2001;97:435-438.
17. Johnson WH, Fecher AM, McMahon RL, et al. VersaStep trocar hernia rate in unclosed fascial defects in bariatric patients. Surg Endosc. 2006;20:1584-1586.
18. Lin P, Falcone T, Tulandi T. Excision of ovarian dermoid cyst by laparoscopy and by laparotomy. Am J Obstet Gynecol. 1995;173:769-771.
19. Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5-26.
20. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingooophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
21. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643-646.
22. Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214-218.
23. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
24. Dowdy SC, Stefanek M, Hartmann LC. Surgical risk reduction: prophylactic salpingooophorectomy and prophylactic mastectomy. Am J Obstet Gynecol. 2004;191:1113-1123.
25. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424-428.
26. Whittemore AS, Balise RR, Pharoah PD, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer. 2004;91:1911-1915.
27. Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol. 2005;105:35-41.
28. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
29. Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003;91:179-189.
30. Lehner R, Wenzl R, Heinzl H, Husslein P, Sevelda P. Influence of delayed staging laparotomy after laparoscopic removal of ovarian masses later found malignant. Obstet Gynecol. 1998;92:967-971.