User login
Control of COPD Symptoms: Addressing an Unmet Need
This series for primary care physicians covers key topics in the management of chronic obstructive pulmonary disease (COPD) and asthma within the context of current national guidelines and clinical practice.
Click here to read the supplement
Randall Brown, MD, MPH, AE-C
Center for Managing Chronic Disease
University of Michigan, Ann Arbor
This series for primary care physicians covers key topics in the management of chronic obstructive pulmonary disease (COPD) and asthma within the context of current national guidelines and clinical practice.
Click here to read the supplement
Randall Brown, MD, MPH, AE-C
Center for Managing Chronic Disease
University of Michigan, Ann Arbor
This series for primary care physicians covers key topics in the management of chronic obstructive pulmonary disease (COPD) and asthma within the context of current national guidelines and clinical practice.
Click here to read the supplement
Randall Brown, MD, MPH, AE-C
Center for Managing Chronic Disease
University of Michigan, Ann Arbor
Cardiovascular disease: Innovations in devices and techniques
Supplement Editor:
Maan A. Fares, MD
Contents
Cardiovascular disease: Innovations in devices and techniques
Maan A. Fares
Transcatheter mitral valve replacement: A frontier in cardiac intervention
Amar Krishnaswamy, Stephanie Mick, Jose Navia, A. Marc Gillinov, E. Murrat Tuzcu, and Samir R. Kapadia
Bioresorbable stents: The future of Interventional cardiology?
Stephen G. Ellis and Haris Riaz
Leadless cardiac pacing: What primary care providers and non-EP cardiologists should know
Erich L. Kiehl and Daniel J. Cantillon
PCSK9 inhibition: A promise fulfilled?
Khendi White, Chaitra Mohan, and Michael Rocco
Fibromuscular dysplasia: Advances in understanding and management
Ellen K. Brinza and Heather L. Gornik
Supplement Editor:
Maan A. Fares, MD
Contents
Cardiovascular disease: Innovations in devices and techniques
Maan A. Fares
Transcatheter mitral valve replacement: A frontier in cardiac intervention
Amar Krishnaswamy, Stephanie Mick, Jose Navia, A. Marc Gillinov, E. Murrat Tuzcu, and Samir R. Kapadia
Bioresorbable stents: The future of Interventional cardiology?
Stephen G. Ellis and Haris Riaz
Leadless cardiac pacing: What primary care providers and non-EP cardiologists should know
Erich L. Kiehl and Daniel J. Cantillon
PCSK9 inhibition: A promise fulfilled?
Khendi White, Chaitra Mohan, and Michael Rocco
Fibromuscular dysplasia: Advances in understanding and management
Ellen K. Brinza and Heather L. Gornik
Supplement Editor:
Maan A. Fares, MD
Contents
Cardiovascular disease: Innovations in devices and techniques
Maan A. Fares
Transcatheter mitral valve replacement: A frontier in cardiac intervention
Amar Krishnaswamy, Stephanie Mick, Jose Navia, A. Marc Gillinov, E. Murrat Tuzcu, and Samir R. Kapadia
Bioresorbable stents: The future of Interventional cardiology?
Stephen G. Ellis and Haris Riaz
Leadless cardiac pacing: What primary care providers and non-EP cardiologists should know
Erich L. Kiehl and Daniel J. Cantillon
PCSK9 inhibition: A promise fulfilled?
Khendi White, Chaitra Mohan, and Michael Rocco
Fibromuscular dysplasia: Advances in understanding and management
Ellen K. Brinza and Heather L. Gornik
Role of the Kidney in Type 2 Diabetes and Mechanism of Action of Sodium Glucose Cotransporter-2 Inhibitors
While type 2 diabetes (T2D) is commonly seen in primary care, it is difficult to manage successfully over time. This series offers brief eNewsletters written by clinical experts that are designed to assist in the clinical management of patients with T2D.
While type 2 diabetes (T2D) is commonly seen in primary care, it is difficult to manage successfully over time. This series offers brief eNewsletters written by clinical experts that are designed to assist in the clinical management of patients with T2D.
While type 2 diabetes (T2D) is commonly seen in primary care, it is difficult to manage successfully over time. This series offers brief eNewsletters written by clinical experts that are designed to assist in the clinical management of patients with T2D.
The Role of Hysteroscopy in Minimally Invasive Management of Intrauterine Health
Faculty/Faculty Disclosure
Linda D. Bradley, MD
Obstetrics, Gynecology and Women’s
Health Institute,
Cleveland Clinic,
Cleveland, Ohio, USA
Competing Interest and Financial Disclosures: Dr. Bradley reports that she has received grant/research/clinical trial support from Bayer Healthcare Pharmaceuticals Inc. She is a consultant and on the advisory board for Bayer Healthcare Pharmaceuticals Inc., Boston Scientific Corporation, and Smith & Nephew; she is on the advisory board for Patient-Centered Outcomes Research Institute; she is on the speakers' bureau for Smith & Nephew (Medtronic); she is on the scientific advisory panel for Karl Storz; and she is on the data safety and monitoring board for Gynesonics.
Faculty/Faculty Disclosure
Linda D. Bradley, MD
Obstetrics, Gynecology and Women’s
Health Institute,
Cleveland Clinic,
Cleveland, Ohio, USA
Competing Interest and Financial Disclosures: Dr. Bradley reports that she has received grant/research/clinical trial support from Bayer Healthcare Pharmaceuticals Inc. She is a consultant and on the advisory board for Bayer Healthcare Pharmaceuticals Inc., Boston Scientific Corporation, and Smith & Nephew; she is on the advisory board for Patient-Centered Outcomes Research Institute; she is on the speakers' bureau for Smith & Nephew (Medtronic); she is on the scientific advisory panel for Karl Storz; and she is on the data safety and monitoring board for Gynesonics.
Faculty/Faculty Disclosure
Linda D. Bradley, MD
Obstetrics, Gynecology and Women’s
Health Institute,
Cleveland Clinic,
Cleveland, Ohio, USA
Competing Interest and Financial Disclosures: Dr. Bradley reports that she has received grant/research/clinical trial support from Bayer Healthcare Pharmaceuticals Inc. She is a consultant and on the advisory board for Bayer Healthcare Pharmaceuticals Inc., Boston Scientific Corporation, and Smith & Nephew; she is on the advisory board for Patient-Centered Outcomes Research Institute; she is on the speakers' bureau for Smith & Nephew (Medtronic); she is on the scientific advisory panel for Karl Storz; and she is on the data safety and monitoring board for Gynesonics.
Large Healthcare System VTE Prevention
Venous thromboembolism (VTE), including both deep vein thrombosis (DVT) and pulmonary embolism, is a major cause of preventable hospital death and long‐term morbidity. VTE accounts for approximately 100,000 to 200,000 hospital deaths annually,[1] and preventable DVT costs an estimated $2.5 billion annually, with each case resulting in direct hospital costs of an estimated $25,977.[2] Although VTE is less common in children, its incidence is increasing in the medically ill hospitalized pediatric patient. The most recent analysis of a large national children's hospital database showed VTE rates increasing from 34 to 58 per 10,000 admissions from 2001 to 2007.[3] Rates in pediatric trauma patients are higher, at 60 to 100 per 10,000 admissions.[4, 5, 6]
The Joint Commission, the Surgeon General, and the Centers for Disease Control and Prevention have supported initiatives to increase awareness and promote strategies designed to prevent hospital acquired VTE.[7, 8] There are several high‐quality, evidence‐based VTE prophylaxis (VTE‐P) guidelines for adult hospitalized populations.[9, 10, 11] Pediatric VTE‐P guidelines are not well established, but the literature regarding VTE risk stratification and prophylaxis guidelines for medically complex children is growing.[12, 13, 14, 15, 16, 17]
A significant challenge has been developing systems that ensure that evidence and consensus‐based care recommendations are reliably implemented. This summary will describe the methods applied across an integrated health system that includes 22 acute care facilities and 1 pediatric hospital across 5 states that have resulted in a significant reduction in preventable VTE.
SETTING
Mayo Clinic is an integrated health system that owns 22 acute care facilities across 5 states, housing 3971 beds with approximately 122,000 admissions per year. Mayo Clinic Rochester, Arizona, and Florida are all tertiary academic medical centers with trauma and transplant programs. During this project, Arizona and Florida utilized a common build of the Cerner (Kansas City, MO) electronic health record (EHR). The other facilities, collectively referred to as the Mayo Clinic Health System (MCHS) hospitals, include 1 level II trauma center and 11 critical access hospitals and serve communities of varying sizes in Minnesota, Wisconsin, and Iowa. A different build of the Cerner EHR served the MCHS during this project.
Mayo Clinic Rochester is responsible for nearly 50% of all admissions and procedures. Mayo Eugenio Litta Children's Hospital, Rochester, Minnesota is a tertiary children's hospital facility housing 44 general pediatric beds, 26 neonatal intensive care unit beds, 24 intermediate nursery special care beds, and 16 pediatric intensive care unit beds. Mayo Clinic Rochester used the GE Centricity (GE Healthcare, Wauwatosa, WI) EHR and custom‐designed computerized decision support.
METHODS
Mayo Clinic has developed a system to deliberately speed the diffusion of best practices across our system to drive reliable, evidence‐based care, reduce unwanted variation in processes and outcomes, and improve value.[18] The 3 main components of this system are (1) discovery: wherein we learn a practice that demonstrably solves the clinical problem well in at least 1 of our facilities, (2) assessment of readiness for diffusion, and (3) diffusion of the best practice across all of Mayo Clinic. The diffusion process is active and equipped with a project team and execution timeline. Both adult and pediatric projects began with discovery phases. The adult program has fully diffused; the pediatric program is diffusing at this writing.
ADULT ACUTE CARE PATIENTS
Discovery Through Pilot Projects
Beginning in 2006, 2 spontaneously convened interdisciplinary teams worked independently in selected medical and surgical practices in our Rochester hospital to improve VTE‐P. Each team's work resulted in the reduction of defect rates on pilot hospital services to <10%. Key findings were: (1) the vast majority of patients in the pilot had at least 1 risk factor for VTE and (2) when physicians explicitly determined a VTE‐P plan, they made the correct decision 98% of the time without any specific risk rule or point system.[18] Both teams found that efforts to ensure declaration of VTE‐P plans in the workflow of admission resulted in the most improvement in appropriate VTE‐P rates.
Creation of VTE‐Prevention Plans and the VTE Prophylaxis Tollgate
Based on lessons learned from the pilot projects, multidisciplinary improvement teams focused on adaptation of optimal VTE‐P plans for individual practices (eg, preferred VTE‐P for a neurosurgery patient is not the same as for a medical patient), and a VTE‐P tollgatea requirement for providers to complete a VTE‐P plan for each patientwas integrated into the clinical workflow of all order sets used for admissions, transfers, and for selected postoperative order sets. As we moved from the paper systems to computerized order entry, tollgates were subsequently converted to the GE Centricity electronic environment. To minimize burden on clinicians, designs were tested in a usability laboratory prior to operational deployment to ensure that they were as clear and easy as our software would allow.
Alerts
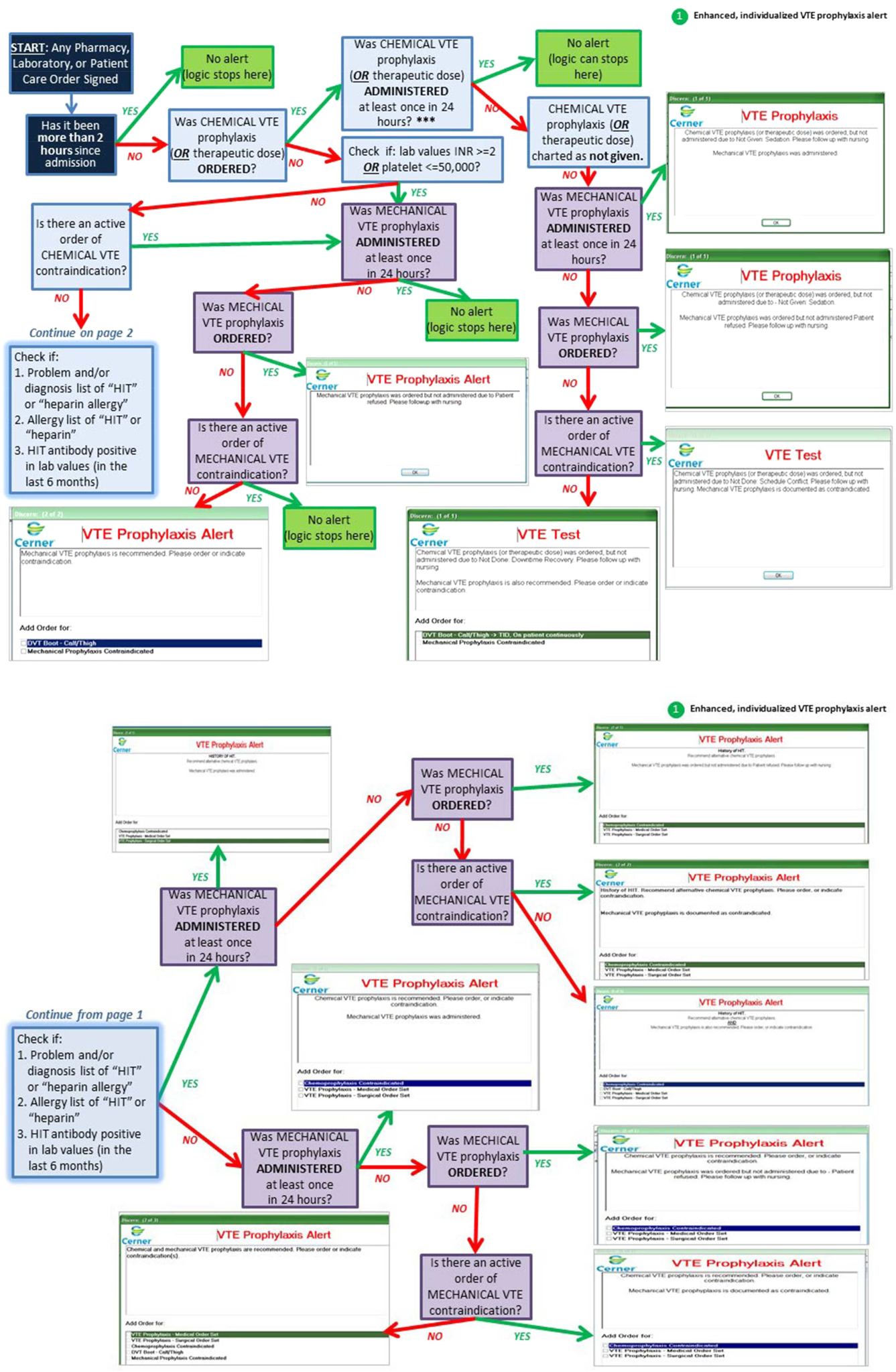
Based on initial reports and feedback, our clinical decision support (CDS) team designed alerts that notify the clinician when (1) any patient previously declared as at least moderate risk for VTE did not have a valid VTE‐P plan in place for any 24‐hour period, or (2) when any patient carried a low‐risk categorization for >3 days (because this should prompt reconsideration of risk status). Alerts were designed to be clear and to facilitate steps to correct the situation.
VTE‐P alerts would present to any member of the patient's provider team who accessed the patient's EHR, and would continue to alert with each access until conditions were rendered to satisfy the requirements of the alert. Each alert provided easy access to an abbreviated VTE‐P tollgate order that would allow the provider to select a clinically appropriate response: either restate the low‐risk status, change the low‐risk status and add an active VTE‐P order, specify why neither mechanical nor pharmacologic VTE‐P may be given, or restart a VTE‐P order.
Monitoring
Both process and outcome measures were used to monitor the effectiveness of VTE‐P activities. During initial roll‐out, the teams measured and reported the proportion of patients where either (1) VTE risk factors were present (patient is determined to be at least at moderate risk for VTE) and either pharmacologic or mechanical VTE‐P was ordered within 24 hours of admission, or (2) VTE risk factors were not present, and VTE‐P not indicated was documented within 24 hours of admission. The CDS system also provided ongoing monitoring of CDS‐alert firing frequency, which closely correlated with the prevalence of patients without a valid VTE‐P plan.
Diffusion Across All Units of Mayo Rochester
Diffusion teams included physician champions, project managers, a pharmacist, and a nurse. To emphasize the engagement of institutional leadership, the project was commissioned by the institution's Clinical Practice Quality Oversight Committee and co‐chaired by the Department of Medicine Associate Chair for Quality and the Chair of the Surgical Quality and Safety Subcommittee.
Implementation of this integrated system resulted in substantial improvement to 97% hospital‐wide VTE‐P rates that were sustained over 3 quarters. At that time, a decision was made to diffuse this new best practice across all Mayo Clinic acute care facilities.
Diffusion to All 22 Mayo Clinic Acute Care Facilities
After readiness for diffusion assessment,[18] an enterprise diffusion team, this time led by the Mayo Clinic Patient Safety Officer (an MD), 3 other physician champions (1 from each region of the Mayo Clinic), a project manager, a pharmacist, a computerized physician order entry system content specialist, and the institutional quality office personnel who assisted with the measurement, analysis, and display of data at the work sites. The best practices diffused were: (1) All admission or transfer order sets will have a VTE‐P tollgate. (2) All VTE‐P tollgates will be a force function (ie, they cannot be bypassed). (3) Over 95% of all eligible patients in the facility at any given moment will have a valid VTE‐P plan in place. (4) Ongoing compliance monitoring must be available as an automatic feed, not by chart review. The end goal of our diffusion process was to ensure that all best practices were ensured at all of our facilities.
Key issues in the diffusion process included implementation of the VTE‐P tollgates into all admission or transfer order sets and the computer decision support logic that had been developed in the GE Centricity system into the Cerner EHR. Each system had slightly different constraints to 4 best practices to be diffused. We had difficulty designing the GE Centricity order sets or flags in such a way that absolutely forced an action (best practice items 1 and 2). Instead, our design had to alert the ordering provider until the appropriate conditions were met. This is suboptimal in that it creates the potential for alarm fatigue and subsequent error. It is for that reason that a tight monitoring system was necessary to provide feedback on a per‐provider level if the alerts were too numerous (suggesting that alarm fatigue or misunderstanding might be leading to failure to correct the unsafe situation producing the alarm).
In contrast, the Cerner system did not have as much capability for our IT support to provide as much customization of decision support but was fully capable of forcing functions. Therefore, we needed to provide a more rigid logic into the order sets. This led to a less than optimal user interface each time a patient was admitted or transferred, but fulfilled mission goals.
Pediatric Patients
Pediatric Discovery Project
Development of Pediatric VTE Risk‐Assessment Tool
To develop a VTE‐P system for our pediatric hospital, our first task was to design a VTE risk‐stratification tool. The improvement team included a physician, pharmacist, and clinical nurse specialists from pediatric intensive care unit (PICU), cardiac intensive care units, and general pediatric services. A literature review identified the most common published risk factors for VTE in children. We next performed a retrospective review of pediatric hospital‐acquired VTE in 2011 to 2012. Eight VTEs were identified (infants to age 18 years). All were related to central venous catheters, sepsis, congenital heart disease, leukemia, myocarditis, and extreme prematurity (Table 1). In contrast to other series, our patients were younger (80% less than 14 years of age). Based on these reviews and iterative consensus with our pediatric staff, an initial pediatric VTE risk‐screening tool was designed and piloted first in the PICU for usability and to assess face validity.
| Age/Gender | Main Diagnosis | Comorbidities | Central Lines Prior to VTE Event | VTE Event |
|---|---|---|---|---|
| ||||
| 18 y/M | Congenital heart disease | Heart transplant | Right and left IJV, right arterial, left femoral vein and artery | DVT left IJV, innominate, subclavian, axillary veins |
| 3 y/M | Idiopathic myocarditis | ECMO | Left radial arterial, right brachial PICC, RIJ venous, left arterial femoral, right venous femoral, | Cerebral embolism with multiple infarcts |
| 0.2 y/F | Premature | NEC | Right IJ PICC | Right axillary, subclavian DVT, left greater saphenous vein |
| 0.5 y/M | Premature | Hypoxic‐ischemic encephalopathy | Umbilical artery and vein catheters, right IJ PICC, right femoral venous | IVC thrombosis and bilateral renal veins |
| 0.1 y/F | Sepsis | RSV pneumonia | Right femoral venous | Right common femoral and right external iliac DVT |
| 12 y/M | Acute lymphoblastic leukemia | Renal dysfunction | Left femoral arterial, right femoral venous | Right common femoral DVT |
| 0.1 y/M | Congenital heart disease | Umbilical artery and vein catheters, left femoral artery, right IJV | Left femoral artery thrombosis | |
| 1 y/M | Seizures | Partially treated meningitis/hyponatremia | Left femoral vein | Left common femoral and external iliac DVT |
Developing Consensus About Appropriate VTE‐P
The risk of even low‐dose anticoagulation may be higher in children than in adults. Therefore, in addition to first estimating the risk for VTE, we also incorporated into the risk‐assessment tool an estimate of risk for bleeding (Table 2). Physicians were responsible for using the VTE‐P screening. Bleeding risk‐assessment categories included: intracranial bleed, premature infant, internal injury (eg, organ injury, splenic laceration), planned surgery within 24 hours, renal failure, liver dysfunction, coagulopathy, thrombocytopenia (eg, platelets <50,000), disseminated intravascular coagulation, congenital bleeding disorder, and neurosurgical and spine fusion patients. If any of these were present, pharmacologic prophylaxis was contraindicated. If a patient was considered at risk for VTE, a pediatric hematology consult was recommended or advised. If there was no increased bleeding risk and the child had 2 or more risk factors or a central venous catheter with additional thrombosis risk factors, the consensus was to use appropriately dosed low‐molecular‐weight heparin or unfractionated heparin in addition to mechanical prophylaxis. A patient considered at increased risk for bleeding but with risk factors for thrombosis would receive early ambulation and/or mechanical prophylaxis. In all cases, removal of central catheters was recommended within 72 hours if possible.
|
| Risk factors |
| Central venous catheter 7 days |
| High‐risk orthopedic surgery |
| Complex fracture of pelvis or lower extremity |
| Projected immobility for 7 days |
| History of prior VTE |
| History of prior thrombophilia |
| ECMO |
| Malignancy |
| Multiple body trauma |
| Use of hormonal therapy |
| BMI > 95th percentile |
| Continuous BPAP/CPAP or mechanical ventilation |
| Inflammatory bowel disease |
| Guidance if no increased bleeding risk |
| 2 risk factorsmechanical combined with pharmacologic prophylaxis |
| Central venous catheter 7 days and additional thrombosis risk factorsmechanical combined with pharmacologic prophylaxis |
| Pharmacologic prophylaxis generally not utilized in spine or neurosurgery patients |
| Guidance if increased bleeding risk |
| 2 risk factors or central venous catheter and additional thrombosis risk factors 7 days hematology consult |
| 2 risk factors or central venous catheter 7 days and additional thrombosis risk factorsearly ambulation + mechanical VTE‐P |
Pilot Implementation
We initiated use of the risk‐assessment tool and VTE‐P algorithm in the PICU using a paper system at first, and measured via chart review (1) the proportion of patients for whom a VTE‐P risk assessment was completed according to the recommended plan and (2) the proportion with the appropriate VTE‐P plan selected based upon risk factors present. The risk‐assessment tool was iteratively improved and built into the electronic order system (Table 2). This would ensure diffusion across the children's hospital, and would be subsequently diffused across the rest of Mayo Clinic.
Metrics
During the system diffusion for the adult system, we relied on 2 metrics to measure improvement: the CDS alert frequency and Centers for Medicare and Medicaid Services (CMS) VTE Core Measures. The CDS alert frequency is cross‐sectional and can be used to estimate what percentage of patients at any given moment in time in our hospital have a valid VTE‐P. From chart audits, we anticipate that at target, approximately 4% of patients would generate CDS alerts because needs and plans change in the dynamic care environment. For example, VTE‐P may be held for a procedure, or during transition from 1 to another unit. Or, observation patients may have been classified as low risk, but when converted to admission status there may be a lag while the VTE risk status is changed. These data can be provided by service and provider, and are reported back to the providers to help reduce practice variation.
In addition, the CMS Core Measures provided a manual chart review metric to supplement the automated data. VTE‐1 and VTE‐2 measures the proportion of sampled charts demonstrating either delivery of VTE‐P or declaration of low risk in non‐ICU and ICU patients, respectively. VTE‐6, the proportion of patients acquiring a VTE who did not receive prophylaxis, served as our outcome measure. For the pediatric efforts, manual chart review served during the improvement pilots, but will be supplanted by a similar automated system.
RESULTS
Adult Acute Care Patients
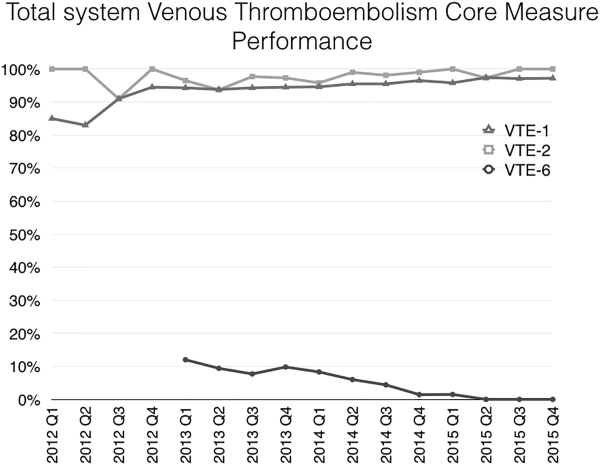
Mayo Clinic used CMS Core Measures in all 22 hospitals in the system from 2013 onward. The results are shown in Figure 1. Of note, VTE‐1 has improved from its project start values in the mid‐80% range to consistently above 95% for the last 6 quarters (most recently above 97%), VTE‐2 has averaged 97.3%, and most recently is at 100%, and VTE‐6 has declined from about 12% to 0% in the recent quarters.
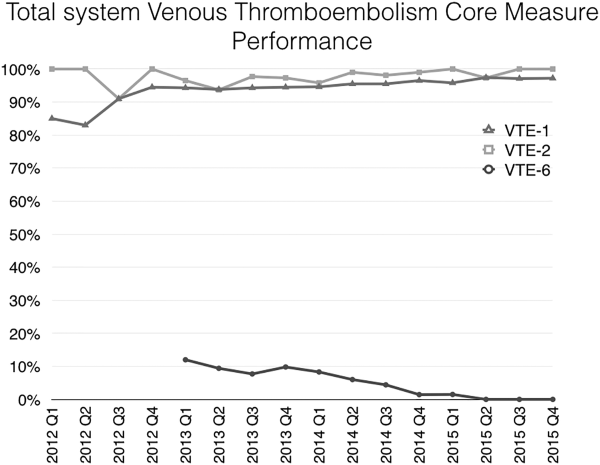
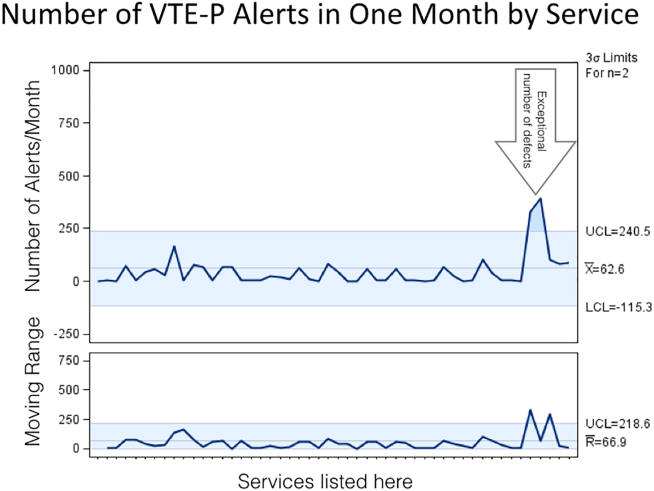
Figure 2 shows the number of VTE‐P alerts generated during 1 month by service in Mayo Clinic Rochester. We display these data as control charts so that practices on services with a statistically excessive number of alerts can be targeted for improvement. Similar data are available at all institutions.
Pediatric Patients
The PICU had an average of 101 admissions per month during study period (range, 72120) with a mean of 11 patients per day (range, 912 patients). Prior to the VTE‐P pilot, none had VTE risks documented. A total of 773 patients were screened for VTE in the intensive care unit during the study period, of which 194 were identified with 2 or greater VTE risk factors (25%). Sixty‐six of 194 patients (34%) had pharmacologic and/or mechanical prophylaxis (n = 83, 44%) selected for VTE‐P. No bleeding events were reported among these patients. During the discovery pilot, the VTE screening tool resulted in >92% compliance with risk documentation, >64% appropriate VTE‐P use, and 0 VTE events. The subsequently improved screening tool resulted in approximately 88% compliance over the subsequent 6 months of use, and in 9 months 2 VTE were diagnosed (both occurring in hospital units not using the screening tool).
An electronic VTE‐P tollgate for pediatric patients went live on March 17, 2016 (Figure 3). We have also developed a CDS alert for pediatric patients not having an appropriate VTE‐P plan documented, and alert frequency reports will allow focused improvement efforts if needed.

DISCUSSION
Our VTE‐P system has resulted in significant reductions in preventable VTE. The key components of our system are: (1) Ensure that a VTE‐P is declared at admission by providing a mandatory VTE‐P tollgate that requires the provider to assess the risk for VTE and provide an appropriate order for VTE‐P. (2) Use clinical decision support to provide ongoing surveillance and alerting providers when there is a lapse in the VTE‐P plan. With these, we have driven CMS Core Measures VTE‐6 to 0 over 3 quarters.
Different VTE‐P strategies have been implemented among hospitalized medically ill patients. Despite the morbidity and mortality risks inherent to VTE, some studies have shown that more than half and nearly 79% of high‐risk hospitalized medical patients received no VTE prevention.[19] Among those who received prophylactic therapy, inadequate duration or type was prescribed in nearly 44%.[20] Electronic orders have resulted in improved prophylaxis in some literature reports.[21, 22] One study showed that a physician alert reduced VTE incidence from 4.13 to 2.23 events per 10,000 patients.[21] Our system, combining prompted electronic orders with clinical decision support for ongoing real‐time monitoring for VTE‐P plans appears to have been effective in producing reliable ordering of VTE‐P in both adults and children.
However, our system has limitations, some inherent in its design and others not addressed yet. Intrinsically, we depend upon clinicians to rightly gauge the patient risk for VTE‐P. Because a significant majority of our patients have at least moderate risk for VTE, the construction of the order sets tend to guide the clinician to select some form of prophylaxis. However, our system does not specifically provide guidance as to what VTE‐P to choose. If the clinician deems the patient at low risk, the CDS criteria will accept this judgment for up to 3 days without questioning the provider. Similarly, by national criteria, some patients at very high risk would ideally receive both mechanical and pharmacologic VTE‐P.[23] Our monitoring system does not distinguish between very high risk and moderately high risk when determining if a valid VTE‐P is in place. Audits of clinician decision making have shown that at present the appropriate decisions are being made 98% of the time, but this could change over time and with new guideline recommendations. Another challenge concerns the difference between ordering and delivering prophylaxis. When ordered, pharmacologic VTE‐P is reliably delivered. In contrast, providing ongoing delivery of ordered mechanical VTE‐P is more challenging. In addition, our current system does not extend to VTE‐P plans for discharge. Future clinical decision support might suggest which patients should receive combined prophylaxis while in the hospital or which home‐going prophylaxis plans should be considered.
We acknowledged the limitations of diffusing a VTE and bleeding risk‐assessment tool that has not been validated in our hospitalized pediatric population. Validation of pediatric VTE risk assessment tools have been recently developed but not widely validated in large prospective studies to be considered the standard of care.[6, 12] Based on our own institutional experience, the vast majority of VTE events occurred in pediatric patients with a central venous catheter (CVC) and other risk factors for thrombosis, and this category was arbitrarily chosen as one to consider pharmacologic prophylaxis if no bleeding risk factors and a central line to be in placed greater than 7 days duration. Although, pediatric evidence guidelines do not support the use of pharmacologic prophylaxis in patients with CVC,[15] risk factors for thrombosis in children, although less frequent than adults, are still present, and VTE‐P should be assessed and individualized in each patient considered at risk for thrombosis. Other groups have attempted a similar approach as the one taken by our group, with variations in the criteria used for thromboprophylaxis in the pediatric population.[5, 14] Our data illustrate that not all pediatric patients require pharmacologic prophylaxis (34%), and VTE‐P should be individualized based on patient risk factors for thrombosis and bleeding risk.
A strength of our system is derived from the substantial clinician and expert input, the codification of consensus, and the hard wiring of that consensus into the electronic ordering, clinical decision rules, and reporting environment. As new advances to VTE‐P are developed, we will strive to codify those new processes into our workflow, building on our past success.
Acknowledgements
The authors acknowledge all of the members involved in the VTE prevention effort at Mayo Clinic including nurses, pharmacists, and information technology support staff.
Disclosure: Nothing to report.
- U.S. Department of Health and Human Services. Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Available at: https://www.ncbi.nlm.nih.gov/books/NBK44178. Published 2008. Accessed May 23, 2016.
- , , , , . Deep‐vein thrombosis: a United States cost model for a preventable and costly adverse event. Thromb Haemost. 2011;106:405–415.
- , , , . Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–1008.
- , , , , . Risk factors for venous thromboembolism in pediatric trauma. J Trauma. 2002;52:922–927.
- , , , , , . Incidence and risk factors for venous thromboembolism in critically ill children after trauma. J Trauma. 2010;68:52–56.
- , , , , , . Risk factors for in‐hospital venous thromboembolism in children: a case‐control study employing diagnostic validation. Haematologica. 2012;97:509–515.
- . Prevention of deep vein thrombosis and pulmonary embolism. Public Health Rep. 2008;123(4):420–421.
- . Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process. J Gen Intern Med. 2007;22:1762–1770.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;14(10):901–907.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88:545–549.
- , , , et al. Risk‐prediction tool for identifying hospitalized children with a predisposition for development of venous thromboembolism: Peds‐Clot clinical Decision Rule. J Thromb Haemost. 2012;10:1326–1334.
- , , , et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72:1292–1297.
- , , , . Thromboprophylaxis in a pediatric hospital: a patient‐safety and quality‐improvement initiative. Pediatrics. 2011;127:e1326–e1332.
- , , , et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e737S–e801S.
- , , , et al. Safety and efficacy of low molecular weight heparins in children: a systematic review of the literature and meta‐analysis of single‐arm studies. Semin Thromb Hemost. 2011;37:814–825.
- , , , . Safety of prophylactic anticoagulation at a pediatric hospital. J Pediatr Hematol Oncol. 2013;35:e287–e291.
- , , , et al. Accelerating the use of best practices: the Mayo Clinic Model of Diffusion. Jt Comm J Qual Patient Saf. 2013;39:167–176.
- , , , , . Disease burden and unmet needs for prevention of venous thromboembolism in medically ill patients in Europe show underutilisation of preventive therapies. Thromb Haemost. 2011;106:600–608.
- , , , . Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement. J Gen Intern Med. 2002;17:788–791.
- , , , et al. Maintained effectiveness of an electronic alert system to prevent venous thromboembolism among hospitalized patients. Thromb Haemost. 2008;100:699–704.
- , , , et al. Validation of a clinical guideline on prevention of venous thromboembolism in medical inpatients: a before‐and‐after study with systematic ultrasound examination. J Intern Med. 2004;256:338–348.
- , , , et al. Low‐molecular‐weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365:26: 2463–2472.
Venous thromboembolism (VTE), including both deep vein thrombosis (DVT) and pulmonary embolism, is a major cause of preventable hospital death and long‐term morbidity. VTE accounts for approximately 100,000 to 200,000 hospital deaths annually,[1] and preventable DVT costs an estimated $2.5 billion annually, with each case resulting in direct hospital costs of an estimated $25,977.[2] Although VTE is less common in children, its incidence is increasing in the medically ill hospitalized pediatric patient. The most recent analysis of a large national children's hospital database showed VTE rates increasing from 34 to 58 per 10,000 admissions from 2001 to 2007.[3] Rates in pediatric trauma patients are higher, at 60 to 100 per 10,000 admissions.[4, 5, 6]
The Joint Commission, the Surgeon General, and the Centers for Disease Control and Prevention have supported initiatives to increase awareness and promote strategies designed to prevent hospital acquired VTE.[7, 8] There are several high‐quality, evidence‐based VTE prophylaxis (VTE‐P) guidelines for adult hospitalized populations.[9, 10, 11] Pediatric VTE‐P guidelines are not well established, but the literature regarding VTE risk stratification and prophylaxis guidelines for medically complex children is growing.[12, 13, 14, 15, 16, 17]
A significant challenge has been developing systems that ensure that evidence and consensus‐based care recommendations are reliably implemented. This summary will describe the methods applied across an integrated health system that includes 22 acute care facilities and 1 pediatric hospital across 5 states that have resulted in a significant reduction in preventable VTE.
SETTING
Mayo Clinic is an integrated health system that owns 22 acute care facilities across 5 states, housing 3971 beds with approximately 122,000 admissions per year. Mayo Clinic Rochester, Arizona, and Florida are all tertiary academic medical centers with trauma and transplant programs. During this project, Arizona and Florida utilized a common build of the Cerner (Kansas City, MO) electronic health record (EHR). The other facilities, collectively referred to as the Mayo Clinic Health System (MCHS) hospitals, include 1 level II trauma center and 11 critical access hospitals and serve communities of varying sizes in Minnesota, Wisconsin, and Iowa. A different build of the Cerner EHR served the MCHS during this project.
Mayo Clinic Rochester is responsible for nearly 50% of all admissions and procedures. Mayo Eugenio Litta Children's Hospital, Rochester, Minnesota is a tertiary children's hospital facility housing 44 general pediatric beds, 26 neonatal intensive care unit beds, 24 intermediate nursery special care beds, and 16 pediatric intensive care unit beds. Mayo Clinic Rochester used the GE Centricity (GE Healthcare, Wauwatosa, WI) EHR and custom‐designed computerized decision support.
METHODS
Mayo Clinic has developed a system to deliberately speed the diffusion of best practices across our system to drive reliable, evidence‐based care, reduce unwanted variation in processes and outcomes, and improve value.[18] The 3 main components of this system are (1) discovery: wherein we learn a practice that demonstrably solves the clinical problem well in at least 1 of our facilities, (2) assessment of readiness for diffusion, and (3) diffusion of the best practice across all of Mayo Clinic. The diffusion process is active and equipped with a project team and execution timeline. Both adult and pediatric projects began with discovery phases. The adult program has fully diffused; the pediatric program is diffusing at this writing.
ADULT ACUTE CARE PATIENTS
Discovery Through Pilot Projects
Beginning in 2006, 2 spontaneously convened interdisciplinary teams worked independently in selected medical and surgical practices in our Rochester hospital to improve VTE‐P. Each team's work resulted in the reduction of defect rates on pilot hospital services to <10%. Key findings were: (1) the vast majority of patients in the pilot had at least 1 risk factor for VTE and (2) when physicians explicitly determined a VTE‐P plan, they made the correct decision 98% of the time without any specific risk rule or point system.[18] Both teams found that efforts to ensure declaration of VTE‐P plans in the workflow of admission resulted in the most improvement in appropriate VTE‐P rates.
Creation of VTE‐Prevention Plans and the VTE Prophylaxis Tollgate
Based on lessons learned from the pilot projects, multidisciplinary improvement teams focused on adaptation of optimal VTE‐P plans for individual practices (eg, preferred VTE‐P for a neurosurgery patient is not the same as for a medical patient), and a VTE‐P tollgatea requirement for providers to complete a VTE‐P plan for each patientwas integrated into the clinical workflow of all order sets used for admissions, transfers, and for selected postoperative order sets. As we moved from the paper systems to computerized order entry, tollgates were subsequently converted to the GE Centricity electronic environment. To minimize burden on clinicians, designs were tested in a usability laboratory prior to operational deployment to ensure that they were as clear and easy as our software would allow.
Alerts
Based on initial reports and feedback, our clinical decision support (CDS) team designed alerts that notify the clinician when (1) any patient previously declared as at least moderate risk for VTE did not have a valid VTE‐P plan in place for any 24‐hour period, or (2) when any patient carried a low‐risk categorization for >3 days (because this should prompt reconsideration of risk status). Alerts were designed to be clear and to facilitate steps to correct the situation.
VTE‐P alerts would present to any member of the patient's provider team who accessed the patient's EHR, and would continue to alert with each access until conditions were rendered to satisfy the requirements of the alert. Each alert provided easy access to an abbreviated VTE‐P tollgate order that would allow the provider to select a clinically appropriate response: either restate the low‐risk status, change the low‐risk status and add an active VTE‐P order, specify why neither mechanical nor pharmacologic VTE‐P may be given, or restart a VTE‐P order.
Monitoring
Both process and outcome measures were used to monitor the effectiveness of VTE‐P activities. During initial roll‐out, the teams measured and reported the proportion of patients where either (1) VTE risk factors were present (patient is determined to be at least at moderate risk for VTE) and either pharmacologic or mechanical VTE‐P was ordered within 24 hours of admission, or (2) VTE risk factors were not present, and VTE‐P not indicated was documented within 24 hours of admission. The CDS system also provided ongoing monitoring of CDS‐alert firing frequency, which closely correlated with the prevalence of patients without a valid VTE‐P plan.
Diffusion Across All Units of Mayo Rochester
Diffusion teams included physician champions, project managers, a pharmacist, and a nurse. To emphasize the engagement of institutional leadership, the project was commissioned by the institution's Clinical Practice Quality Oversight Committee and co‐chaired by the Department of Medicine Associate Chair for Quality and the Chair of the Surgical Quality and Safety Subcommittee.
Implementation of this integrated system resulted in substantial improvement to 97% hospital‐wide VTE‐P rates that were sustained over 3 quarters. At that time, a decision was made to diffuse this new best practice across all Mayo Clinic acute care facilities.
Diffusion to All 22 Mayo Clinic Acute Care Facilities
After readiness for diffusion assessment,[18] an enterprise diffusion team, this time led by the Mayo Clinic Patient Safety Officer (an MD), 3 other physician champions (1 from each region of the Mayo Clinic), a project manager, a pharmacist, a computerized physician order entry system content specialist, and the institutional quality office personnel who assisted with the measurement, analysis, and display of data at the work sites. The best practices diffused were: (1) All admission or transfer order sets will have a VTE‐P tollgate. (2) All VTE‐P tollgates will be a force function (ie, they cannot be bypassed). (3) Over 95% of all eligible patients in the facility at any given moment will have a valid VTE‐P plan in place. (4) Ongoing compliance monitoring must be available as an automatic feed, not by chart review. The end goal of our diffusion process was to ensure that all best practices were ensured at all of our facilities.
Key issues in the diffusion process included implementation of the VTE‐P tollgates into all admission or transfer order sets and the computer decision support logic that had been developed in the GE Centricity system into the Cerner EHR. Each system had slightly different constraints to 4 best practices to be diffused. We had difficulty designing the GE Centricity order sets or flags in such a way that absolutely forced an action (best practice items 1 and 2). Instead, our design had to alert the ordering provider until the appropriate conditions were met. This is suboptimal in that it creates the potential for alarm fatigue and subsequent error. It is for that reason that a tight monitoring system was necessary to provide feedback on a per‐provider level if the alerts were too numerous (suggesting that alarm fatigue or misunderstanding might be leading to failure to correct the unsafe situation producing the alarm).
In contrast, the Cerner system did not have as much capability for our IT support to provide as much customization of decision support but was fully capable of forcing functions. Therefore, we needed to provide a more rigid logic into the order sets. This led to a less than optimal user interface each time a patient was admitted or transferred, but fulfilled mission goals.
Pediatric Patients
Pediatric Discovery Project
Development of Pediatric VTE Risk‐Assessment Tool
To develop a VTE‐P system for our pediatric hospital, our first task was to design a VTE risk‐stratification tool. The improvement team included a physician, pharmacist, and clinical nurse specialists from pediatric intensive care unit (PICU), cardiac intensive care units, and general pediatric services. A literature review identified the most common published risk factors for VTE in children. We next performed a retrospective review of pediatric hospital‐acquired VTE in 2011 to 2012. Eight VTEs were identified (infants to age 18 years). All were related to central venous catheters, sepsis, congenital heart disease, leukemia, myocarditis, and extreme prematurity (Table 1). In contrast to other series, our patients were younger (80% less than 14 years of age). Based on these reviews and iterative consensus with our pediatric staff, an initial pediatric VTE risk‐screening tool was designed and piloted first in the PICU for usability and to assess face validity.
| Age/Gender | Main Diagnosis | Comorbidities | Central Lines Prior to VTE Event | VTE Event |
|---|---|---|---|---|
| ||||
| 18 y/M | Congenital heart disease | Heart transplant | Right and left IJV, right arterial, left femoral vein and artery | DVT left IJV, innominate, subclavian, axillary veins |
| 3 y/M | Idiopathic myocarditis | ECMO | Left radial arterial, right brachial PICC, RIJ venous, left arterial femoral, right venous femoral, | Cerebral embolism with multiple infarcts |
| 0.2 y/F | Premature | NEC | Right IJ PICC | Right axillary, subclavian DVT, left greater saphenous vein |
| 0.5 y/M | Premature | Hypoxic‐ischemic encephalopathy | Umbilical artery and vein catheters, right IJ PICC, right femoral venous | IVC thrombosis and bilateral renal veins |
| 0.1 y/F | Sepsis | RSV pneumonia | Right femoral venous | Right common femoral and right external iliac DVT |
| 12 y/M | Acute lymphoblastic leukemia | Renal dysfunction | Left femoral arterial, right femoral venous | Right common femoral DVT |
| 0.1 y/M | Congenital heart disease | Umbilical artery and vein catheters, left femoral artery, right IJV | Left femoral artery thrombosis | |
| 1 y/M | Seizures | Partially treated meningitis/hyponatremia | Left femoral vein | Left common femoral and external iliac DVT |
Developing Consensus About Appropriate VTE‐P
The risk of even low‐dose anticoagulation may be higher in children than in adults. Therefore, in addition to first estimating the risk for VTE, we also incorporated into the risk‐assessment tool an estimate of risk for bleeding (Table 2). Physicians were responsible for using the VTE‐P screening. Bleeding risk‐assessment categories included: intracranial bleed, premature infant, internal injury (eg, organ injury, splenic laceration), planned surgery within 24 hours, renal failure, liver dysfunction, coagulopathy, thrombocytopenia (eg, platelets <50,000), disseminated intravascular coagulation, congenital bleeding disorder, and neurosurgical and spine fusion patients. If any of these were present, pharmacologic prophylaxis was contraindicated. If a patient was considered at risk for VTE, a pediatric hematology consult was recommended or advised. If there was no increased bleeding risk and the child had 2 or more risk factors or a central venous catheter with additional thrombosis risk factors, the consensus was to use appropriately dosed low‐molecular‐weight heparin or unfractionated heparin in addition to mechanical prophylaxis. A patient considered at increased risk for bleeding but with risk factors for thrombosis would receive early ambulation and/or mechanical prophylaxis. In all cases, removal of central catheters was recommended within 72 hours if possible.
|
| Risk factors |
| Central venous catheter 7 days |
| High‐risk orthopedic surgery |
| Complex fracture of pelvis or lower extremity |
| Projected immobility for 7 days |
| History of prior VTE |
| History of prior thrombophilia |
| ECMO |
| Malignancy |
| Multiple body trauma |
| Use of hormonal therapy |
| BMI > 95th percentile |
| Continuous BPAP/CPAP or mechanical ventilation |
| Inflammatory bowel disease |
| Guidance if no increased bleeding risk |
| 2 risk factorsmechanical combined with pharmacologic prophylaxis |
| Central venous catheter 7 days and additional thrombosis risk factorsmechanical combined with pharmacologic prophylaxis |
| Pharmacologic prophylaxis generally not utilized in spine or neurosurgery patients |
| Guidance if increased bleeding risk |
| 2 risk factors or central venous catheter and additional thrombosis risk factors 7 days hematology consult |
| 2 risk factors or central venous catheter 7 days and additional thrombosis risk factorsearly ambulation + mechanical VTE‐P |
Pilot Implementation
We initiated use of the risk‐assessment tool and VTE‐P algorithm in the PICU using a paper system at first, and measured via chart review (1) the proportion of patients for whom a VTE‐P risk assessment was completed according to the recommended plan and (2) the proportion with the appropriate VTE‐P plan selected based upon risk factors present. The risk‐assessment tool was iteratively improved and built into the electronic order system (Table 2). This would ensure diffusion across the children's hospital, and would be subsequently diffused across the rest of Mayo Clinic.
Metrics
During the system diffusion for the adult system, we relied on 2 metrics to measure improvement: the CDS alert frequency and Centers for Medicare and Medicaid Services (CMS) VTE Core Measures. The CDS alert frequency is cross‐sectional and can be used to estimate what percentage of patients at any given moment in time in our hospital have a valid VTE‐P. From chart audits, we anticipate that at target, approximately 4% of patients would generate CDS alerts because needs and plans change in the dynamic care environment. For example, VTE‐P may be held for a procedure, or during transition from 1 to another unit. Or, observation patients may have been classified as low risk, but when converted to admission status there may be a lag while the VTE risk status is changed. These data can be provided by service and provider, and are reported back to the providers to help reduce practice variation.
In addition, the CMS Core Measures provided a manual chart review metric to supplement the automated data. VTE‐1 and VTE‐2 measures the proportion of sampled charts demonstrating either delivery of VTE‐P or declaration of low risk in non‐ICU and ICU patients, respectively. VTE‐6, the proportion of patients acquiring a VTE who did not receive prophylaxis, served as our outcome measure. For the pediatric efforts, manual chart review served during the improvement pilots, but will be supplanted by a similar automated system.
RESULTS
Adult Acute Care Patients
Mayo Clinic used CMS Core Measures in all 22 hospitals in the system from 2013 onward. The results are shown in Figure 1. Of note, VTE‐1 has improved from its project start values in the mid‐80% range to consistently above 95% for the last 6 quarters (most recently above 97%), VTE‐2 has averaged 97.3%, and most recently is at 100%, and VTE‐6 has declined from about 12% to 0% in the recent quarters.

Figure 2 shows the number of VTE‐P alerts generated during 1 month by service in Mayo Clinic Rochester. We display these data as control charts so that practices on services with a statistically excessive number of alerts can be targeted for improvement. Similar data are available at all institutions.

Pediatric Patients
The PICU had an average of 101 admissions per month during study period (range, 72120) with a mean of 11 patients per day (range, 912 patients). Prior to the VTE‐P pilot, none had VTE risks documented. A total of 773 patients were screened for VTE in the intensive care unit during the study period, of which 194 were identified with 2 or greater VTE risk factors (25%). Sixty‐six of 194 patients (34%) had pharmacologic and/or mechanical prophylaxis (n = 83, 44%) selected for VTE‐P. No bleeding events were reported among these patients. During the discovery pilot, the VTE screening tool resulted in >92% compliance with risk documentation, >64% appropriate VTE‐P use, and 0 VTE events. The subsequently improved screening tool resulted in approximately 88% compliance over the subsequent 6 months of use, and in 9 months 2 VTE were diagnosed (both occurring in hospital units not using the screening tool).
An electronic VTE‐P tollgate for pediatric patients went live on March 17, 2016 (Figure 3). We have also developed a CDS alert for pediatric patients not having an appropriate VTE‐P plan documented, and alert frequency reports will allow focused improvement efforts if needed.

DISCUSSION
Our VTE‐P system has resulted in significant reductions in preventable VTE. The key components of our system are: (1) Ensure that a VTE‐P is declared at admission by providing a mandatory VTE‐P tollgate that requires the provider to assess the risk for VTE and provide an appropriate order for VTE‐P. (2) Use clinical decision support to provide ongoing surveillance and alerting providers when there is a lapse in the VTE‐P plan. With these, we have driven CMS Core Measures VTE‐6 to 0 over 3 quarters.
Different VTE‐P strategies have been implemented among hospitalized medically ill patients. Despite the morbidity and mortality risks inherent to VTE, some studies have shown that more than half and nearly 79% of high‐risk hospitalized medical patients received no VTE prevention.[19] Among those who received prophylactic therapy, inadequate duration or type was prescribed in nearly 44%.[20] Electronic orders have resulted in improved prophylaxis in some literature reports.[21, 22] One study showed that a physician alert reduced VTE incidence from 4.13 to 2.23 events per 10,000 patients.[21] Our system, combining prompted electronic orders with clinical decision support for ongoing real‐time monitoring for VTE‐P plans appears to have been effective in producing reliable ordering of VTE‐P in both adults and children.
However, our system has limitations, some inherent in its design and others not addressed yet. Intrinsically, we depend upon clinicians to rightly gauge the patient risk for VTE‐P. Because a significant majority of our patients have at least moderate risk for VTE, the construction of the order sets tend to guide the clinician to select some form of prophylaxis. However, our system does not specifically provide guidance as to what VTE‐P to choose. If the clinician deems the patient at low risk, the CDS criteria will accept this judgment for up to 3 days without questioning the provider. Similarly, by national criteria, some patients at very high risk would ideally receive both mechanical and pharmacologic VTE‐P.[23] Our monitoring system does not distinguish between very high risk and moderately high risk when determining if a valid VTE‐P is in place. Audits of clinician decision making have shown that at present the appropriate decisions are being made 98% of the time, but this could change over time and with new guideline recommendations. Another challenge concerns the difference between ordering and delivering prophylaxis. When ordered, pharmacologic VTE‐P is reliably delivered. In contrast, providing ongoing delivery of ordered mechanical VTE‐P is more challenging. In addition, our current system does not extend to VTE‐P plans for discharge. Future clinical decision support might suggest which patients should receive combined prophylaxis while in the hospital or which home‐going prophylaxis plans should be considered.
We acknowledged the limitations of diffusing a VTE and bleeding risk‐assessment tool that has not been validated in our hospitalized pediatric population. Validation of pediatric VTE risk assessment tools have been recently developed but not widely validated in large prospective studies to be considered the standard of care.[6, 12] Based on our own institutional experience, the vast majority of VTE events occurred in pediatric patients with a central venous catheter (CVC) and other risk factors for thrombosis, and this category was arbitrarily chosen as one to consider pharmacologic prophylaxis if no bleeding risk factors and a central line to be in placed greater than 7 days duration. Although, pediatric evidence guidelines do not support the use of pharmacologic prophylaxis in patients with CVC,[15] risk factors for thrombosis in children, although less frequent than adults, are still present, and VTE‐P should be assessed and individualized in each patient considered at risk for thrombosis. Other groups have attempted a similar approach as the one taken by our group, with variations in the criteria used for thromboprophylaxis in the pediatric population.[5, 14] Our data illustrate that not all pediatric patients require pharmacologic prophylaxis (34%), and VTE‐P should be individualized based on patient risk factors for thrombosis and bleeding risk.
A strength of our system is derived from the substantial clinician and expert input, the codification of consensus, and the hard wiring of that consensus into the electronic ordering, clinical decision rules, and reporting environment. As new advances to VTE‐P are developed, we will strive to codify those new processes into our workflow, building on our past success.
Acknowledgements
The authors acknowledge all of the members involved in the VTE prevention effort at Mayo Clinic including nurses, pharmacists, and information technology support staff.
Disclosure: Nothing to report.
Venous thromboembolism (VTE), including both deep vein thrombosis (DVT) and pulmonary embolism, is a major cause of preventable hospital death and long‐term morbidity. VTE accounts for approximately 100,000 to 200,000 hospital deaths annually,[1] and preventable DVT costs an estimated $2.5 billion annually, with each case resulting in direct hospital costs of an estimated $25,977.[2] Although VTE is less common in children, its incidence is increasing in the medically ill hospitalized pediatric patient. The most recent analysis of a large national children's hospital database showed VTE rates increasing from 34 to 58 per 10,000 admissions from 2001 to 2007.[3] Rates in pediatric trauma patients are higher, at 60 to 100 per 10,000 admissions.[4, 5, 6]
The Joint Commission, the Surgeon General, and the Centers for Disease Control and Prevention have supported initiatives to increase awareness and promote strategies designed to prevent hospital acquired VTE.[7, 8] There are several high‐quality, evidence‐based VTE prophylaxis (VTE‐P) guidelines for adult hospitalized populations.[9, 10, 11] Pediatric VTE‐P guidelines are not well established, but the literature regarding VTE risk stratification and prophylaxis guidelines for medically complex children is growing.[12, 13, 14, 15, 16, 17]
A significant challenge has been developing systems that ensure that evidence and consensus‐based care recommendations are reliably implemented. This summary will describe the methods applied across an integrated health system that includes 22 acute care facilities and 1 pediatric hospital across 5 states that have resulted in a significant reduction in preventable VTE.
SETTING
Mayo Clinic is an integrated health system that owns 22 acute care facilities across 5 states, housing 3971 beds with approximately 122,000 admissions per year. Mayo Clinic Rochester, Arizona, and Florida are all tertiary academic medical centers with trauma and transplant programs. During this project, Arizona and Florida utilized a common build of the Cerner (Kansas City, MO) electronic health record (EHR). The other facilities, collectively referred to as the Mayo Clinic Health System (MCHS) hospitals, include 1 level II trauma center and 11 critical access hospitals and serve communities of varying sizes in Minnesota, Wisconsin, and Iowa. A different build of the Cerner EHR served the MCHS during this project.
Mayo Clinic Rochester is responsible for nearly 50% of all admissions and procedures. Mayo Eugenio Litta Children's Hospital, Rochester, Minnesota is a tertiary children's hospital facility housing 44 general pediatric beds, 26 neonatal intensive care unit beds, 24 intermediate nursery special care beds, and 16 pediatric intensive care unit beds. Mayo Clinic Rochester used the GE Centricity (GE Healthcare, Wauwatosa, WI) EHR and custom‐designed computerized decision support.
METHODS
Mayo Clinic has developed a system to deliberately speed the diffusion of best practices across our system to drive reliable, evidence‐based care, reduce unwanted variation in processes and outcomes, and improve value.[18] The 3 main components of this system are (1) discovery: wherein we learn a practice that demonstrably solves the clinical problem well in at least 1 of our facilities, (2) assessment of readiness for diffusion, and (3) diffusion of the best practice across all of Mayo Clinic. The diffusion process is active and equipped with a project team and execution timeline. Both adult and pediatric projects began with discovery phases. The adult program has fully diffused; the pediatric program is diffusing at this writing.
ADULT ACUTE CARE PATIENTS
Discovery Through Pilot Projects
Beginning in 2006, 2 spontaneously convened interdisciplinary teams worked independently in selected medical and surgical practices in our Rochester hospital to improve VTE‐P. Each team's work resulted in the reduction of defect rates on pilot hospital services to <10%. Key findings were: (1) the vast majority of patients in the pilot had at least 1 risk factor for VTE and (2) when physicians explicitly determined a VTE‐P plan, they made the correct decision 98% of the time without any specific risk rule or point system.[18] Both teams found that efforts to ensure declaration of VTE‐P plans in the workflow of admission resulted in the most improvement in appropriate VTE‐P rates.
Creation of VTE‐Prevention Plans and the VTE Prophylaxis Tollgate
Based on lessons learned from the pilot projects, multidisciplinary improvement teams focused on adaptation of optimal VTE‐P plans for individual practices (eg, preferred VTE‐P for a neurosurgery patient is not the same as for a medical patient), and a VTE‐P tollgatea requirement for providers to complete a VTE‐P plan for each patientwas integrated into the clinical workflow of all order sets used for admissions, transfers, and for selected postoperative order sets. As we moved from the paper systems to computerized order entry, tollgates were subsequently converted to the GE Centricity electronic environment. To minimize burden on clinicians, designs were tested in a usability laboratory prior to operational deployment to ensure that they were as clear and easy as our software would allow.
Alerts
Based on initial reports and feedback, our clinical decision support (CDS) team designed alerts that notify the clinician when (1) any patient previously declared as at least moderate risk for VTE did not have a valid VTE‐P plan in place for any 24‐hour period, or (2) when any patient carried a low‐risk categorization for >3 days (because this should prompt reconsideration of risk status). Alerts were designed to be clear and to facilitate steps to correct the situation.
VTE‐P alerts would present to any member of the patient's provider team who accessed the patient's EHR, and would continue to alert with each access until conditions were rendered to satisfy the requirements of the alert. Each alert provided easy access to an abbreviated VTE‐P tollgate order that would allow the provider to select a clinically appropriate response: either restate the low‐risk status, change the low‐risk status and add an active VTE‐P order, specify why neither mechanical nor pharmacologic VTE‐P may be given, or restart a VTE‐P order.
Monitoring
Both process and outcome measures were used to monitor the effectiveness of VTE‐P activities. During initial roll‐out, the teams measured and reported the proportion of patients where either (1) VTE risk factors were present (patient is determined to be at least at moderate risk for VTE) and either pharmacologic or mechanical VTE‐P was ordered within 24 hours of admission, or (2) VTE risk factors were not present, and VTE‐P not indicated was documented within 24 hours of admission. The CDS system also provided ongoing monitoring of CDS‐alert firing frequency, which closely correlated with the prevalence of patients without a valid VTE‐P plan.
Diffusion Across All Units of Mayo Rochester
Diffusion teams included physician champions, project managers, a pharmacist, and a nurse. To emphasize the engagement of institutional leadership, the project was commissioned by the institution's Clinical Practice Quality Oversight Committee and co‐chaired by the Department of Medicine Associate Chair for Quality and the Chair of the Surgical Quality and Safety Subcommittee.
Implementation of this integrated system resulted in substantial improvement to 97% hospital‐wide VTE‐P rates that were sustained over 3 quarters. At that time, a decision was made to diffuse this new best practice across all Mayo Clinic acute care facilities.
Diffusion to All 22 Mayo Clinic Acute Care Facilities
After readiness for diffusion assessment,[18] an enterprise diffusion team, this time led by the Mayo Clinic Patient Safety Officer (an MD), 3 other physician champions (1 from each region of the Mayo Clinic), a project manager, a pharmacist, a computerized physician order entry system content specialist, and the institutional quality office personnel who assisted with the measurement, analysis, and display of data at the work sites. The best practices diffused were: (1) All admission or transfer order sets will have a VTE‐P tollgate. (2) All VTE‐P tollgates will be a force function (ie, they cannot be bypassed). (3) Over 95% of all eligible patients in the facility at any given moment will have a valid VTE‐P plan in place. (4) Ongoing compliance monitoring must be available as an automatic feed, not by chart review. The end goal of our diffusion process was to ensure that all best practices were ensured at all of our facilities.
Key issues in the diffusion process included implementation of the VTE‐P tollgates into all admission or transfer order sets and the computer decision support logic that had been developed in the GE Centricity system into the Cerner EHR. Each system had slightly different constraints to 4 best practices to be diffused. We had difficulty designing the GE Centricity order sets or flags in such a way that absolutely forced an action (best practice items 1 and 2). Instead, our design had to alert the ordering provider until the appropriate conditions were met. This is suboptimal in that it creates the potential for alarm fatigue and subsequent error. It is for that reason that a tight monitoring system was necessary to provide feedback on a per‐provider level if the alerts were too numerous (suggesting that alarm fatigue or misunderstanding might be leading to failure to correct the unsafe situation producing the alarm).
In contrast, the Cerner system did not have as much capability for our IT support to provide as much customization of decision support but was fully capable of forcing functions. Therefore, we needed to provide a more rigid logic into the order sets. This led to a less than optimal user interface each time a patient was admitted or transferred, but fulfilled mission goals.
Pediatric Patients
Pediatric Discovery Project
Development of Pediatric VTE Risk‐Assessment Tool
To develop a VTE‐P system for our pediatric hospital, our first task was to design a VTE risk‐stratification tool. The improvement team included a physician, pharmacist, and clinical nurse specialists from pediatric intensive care unit (PICU), cardiac intensive care units, and general pediatric services. A literature review identified the most common published risk factors for VTE in children. We next performed a retrospective review of pediatric hospital‐acquired VTE in 2011 to 2012. Eight VTEs were identified (infants to age 18 years). All were related to central venous catheters, sepsis, congenital heart disease, leukemia, myocarditis, and extreme prematurity (Table 1). In contrast to other series, our patients were younger (80% less than 14 years of age). Based on these reviews and iterative consensus with our pediatric staff, an initial pediatric VTE risk‐screening tool was designed and piloted first in the PICU for usability and to assess face validity.
| Age/Gender | Main Diagnosis | Comorbidities | Central Lines Prior to VTE Event | VTE Event |
|---|---|---|---|---|
| ||||
| 18 y/M | Congenital heart disease | Heart transplant | Right and left IJV, right arterial, left femoral vein and artery | DVT left IJV, innominate, subclavian, axillary veins |
| 3 y/M | Idiopathic myocarditis | ECMO | Left radial arterial, right brachial PICC, RIJ venous, left arterial femoral, right venous femoral, | Cerebral embolism with multiple infarcts |
| 0.2 y/F | Premature | NEC | Right IJ PICC | Right axillary, subclavian DVT, left greater saphenous vein |
| 0.5 y/M | Premature | Hypoxic‐ischemic encephalopathy | Umbilical artery and vein catheters, right IJ PICC, right femoral venous | IVC thrombosis and bilateral renal veins |
| 0.1 y/F | Sepsis | RSV pneumonia | Right femoral venous | Right common femoral and right external iliac DVT |
| 12 y/M | Acute lymphoblastic leukemia | Renal dysfunction | Left femoral arterial, right femoral venous | Right common femoral DVT |
| 0.1 y/M | Congenital heart disease | Umbilical artery and vein catheters, left femoral artery, right IJV | Left femoral artery thrombosis | |
| 1 y/M | Seizures | Partially treated meningitis/hyponatremia | Left femoral vein | Left common femoral and external iliac DVT |
Developing Consensus About Appropriate VTE‐P
The risk of even low‐dose anticoagulation may be higher in children than in adults. Therefore, in addition to first estimating the risk for VTE, we also incorporated into the risk‐assessment tool an estimate of risk for bleeding (Table 2). Physicians were responsible for using the VTE‐P screening. Bleeding risk‐assessment categories included: intracranial bleed, premature infant, internal injury (eg, organ injury, splenic laceration), planned surgery within 24 hours, renal failure, liver dysfunction, coagulopathy, thrombocytopenia (eg, platelets <50,000), disseminated intravascular coagulation, congenital bleeding disorder, and neurosurgical and spine fusion patients. If any of these were present, pharmacologic prophylaxis was contraindicated. If a patient was considered at risk for VTE, a pediatric hematology consult was recommended or advised. If there was no increased bleeding risk and the child had 2 or more risk factors or a central venous catheter with additional thrombosis risk factors, the consensus was to use appropriately dosed low‐molecular‐weight heparin or unfractionated heparin in addition to mechanical prophylaxis. A patient considered at increased risk for bleeding but with risk factors for thrombosis would receive early ambulation and/or mechanical prophylaxis. In all cases, removal of central catheters was recommended within 72 hours if possible.
|
| Risk factors |
| Central venous catheter 7 days |
| High‐risk orthopedic surgery |
| Complex fracture of pelvis or lower extremity |
| Projected immobility for 7 days |
| History of prior VTE |
| History of prior thrombophilia |
| ECMO |
| Malignancy |
| Multiple body trauma |
| Use of hormonal therapy |
| BMI > 95th percentile |
| Continuous BPAP/CPAP or mechanical ventilation |
| Inflammatory bowel disease |
| Guidance if no increased bleeding risk |
| 2 risk factorsmechanical combined with pharmacologic prophylaxis |
| Central venous catheter 7 days and additional thrombosis risk factorsmechanical combined with pharmacologic prophylaxis |
| Pharmacologic prophylaxis generally not utilized in spine or neurosurgery patients |
| Guidance if increased bleeding risk |
| 2 risk factors or central venous catheter and additional thrombosis risk factors 7 days hematology consult |
| 2 risk factors or central venous catheter 7 days and additional thrombosis risk factorsearly ambulation + mechanical VTE‐P |
Pilot Implementation
We initiated use of the risk‐assessment tool and VTE‐P algorithm in the PICU using a paper system at first, and measured via chart review (1) the proportion of patients for whom a VTE‐P risk assessment was completed according to the recommended plan and (2) the proportion with the appropriate VTE‐P plan selected based upon risk factors present. The risk‐assessment tool was iteratively improved and built into the electronic order system (Table 2). This would ensure diffusion across the children's hospital, and would be subsequently diffused across the rest of Mayo Clinic.
Metrics
During the system diffusion for the adult system, we relied on 2 metrics to measure improvement: the CDS alert frequency and Centers for Medicare and Medicaid Services (CMS) VTE Core Measures. The CDS alert frequency is cross‐sectional and can be used to estimate what percentage of patients at any given moment in time in our hospital have a valid VTE‐P. From chart audits, we anticipate that at target, approximately 4% of patients would generate CDS alerts because needs and plans change in the dynamic care environment. For example, VTE‐P may be held for a procedure, or during transition from 1 to another unit. Or, observation patients may have been classified as low risk, but when converted to admission status there may be a lag while the VTE risk status is changed. These data can be provided by service and provider, and are reported back to the providers to help reduce practice variation.
In addition, the CMS Core Measures provided a manual chart review metric to supplement the automated data. VTE‐1 and VTE‐2 measures the proportion of sampled charts demonstrating either delivery of VTE‐P or declaration of low risk in non‐ICU and ICU patients, respectively. VTE‐6, the proportion of patients acquiring a VTE who did not receive prophylaxis, served as our outcome measure. For the pediatric efforts, manual chart review served during the improvement pilots, but will be supplanted by a similar automated system.
RESULTS
Adult Acute Care Patients
Mayo Clinic used CMS Core Measures in all 22 hospitals in the system from 2013 onward. The results are shown in Figure 1. Of note, VTE‐1 has improved from its project start values in the mid‐80% range to consistently above 95% for the last 6 quarters (most recently above 97%), VTE‐2 has averaged 97.3%, and most recently is at 100%, and VTE‐6 has declined from about 12% to 0% in the recent quarters.

Figure 2 shows the number of VTE‐P alerts generated during 1 month by service in Mayo Clinic Rochester. We display these data as control charts so that practices on services with a statistically excessive number of alerts can be targeted for improvement. Similar data are available at all institutions.

Pediatric Patients
The PICU had an average of 101 admissions per month during study period (range, 72120) with a mean of 11 patients per day (range, 912 patients). Prior to the VTE‐P pilot, none had VTE risks documented. A total of 773 patients were screened for VTE in the intensive care unit during the study period, of which 194 were identified with 2 or greater VTE risk factors (25%). Sixty‐six of 194 patients (34%) had pharmacologic and/or mechanical prophylaxis (n = 83, 44%) selected for VTE‐P. No bleeding events were reported among these patients. During the discovery pilot, the VTE screening tool resulted in >92% compliance with risk documentation, >64% appropriate VTE‐P use, and 0 VTE events. The subsequently improved screening tool resulted in approximately 88% compliance over the subsequent 6 months of use, and in 9 months 2 VTE were diagnosed (both occurring in hospital units not using the screening tool).
An electronic VTE‐P tollgate for pediatric patients went live on March 17, 2016 (Figure 3). We have also developed a CDS alert for pediatric patients not having an appropriate VTE‐P plan documented, and alert frequency reports will allow focused improvement efforts if needed.

DISCUSSION
Our VTE‐P system has resulted in significant reductions in preventable VTE. The key components of our system are: (1) Ensure that a VTE‐P is declared at admission by providing a mandatory VTE‐P tollgate that requires the provider to assess the risk for VTE and provide an appropriate order for VTE‐P. (2) Use clinical decision support to provide ongoing surveillance and alerting providers when there is a lapse in the VTE‐P plan. With these, we have driven CMS Core Measures VTE‐6 to 0 over 3 quarters.
Different VTE‐P strategies have been implemented among hospitalized medically ill patients. Despite the morbidity and mortality risks inherent to VTE, some studies have shown that more than half and nearly 79% of high‐risk hospitalized medical patients received no VTE prevention.[19] Among those who received prophylactic therapy, inadequate duration or type was prescribed in nearly 44%.[20] Electronic orders have resulted in improved prophylaxis in some literature reports.[21, 22] One study showed that a physician alert reduced VTE incidence from 4.13 to 2.23 events per 10,000 patients.[21] Our system, combining prompted electronic orders with clinical decision support for ongoing real‐time monitoring for VTE‐P plans appears to have been effective in producing reliable ordering of VTE‐P in both adults and children.
However, our system has limitations, some inherent in its design and others not addressed yet. Intrinsically, we depend upon clinicians to rightly gauge the patient risk for VTE‐P. Because a significant majority of our patients have at least moderate risk for VTE, the construction of the order sets tend to guide the clinician to select some form of prophylaxis. However, our system does not specifically provide guidance as to what VTE‐P to choose. If the clinician deems the patient at low risk, the CDS criteria will accept this judgment for up to 3 days without questioning the provider. Similarly, by national criteria, some patients at very high risk would ideally receive both mechanical and pharmacologic VTE‐P.[23] Our monitoring system does not distinguish between very high risk and moderately high risk when determining if a valid VTE‐P is in place. Audits of clinician decision making have shown that at present the appropriate decisions are being made 98% of the time, but this could change over time and with new guideline recommendations. Another challenge concerns the difference between ordering and delivering prophylaxis. When ordered, pharmacologic VTE‐P is reliably delivered. In contrast, providing ongoing delivery of ordered mechanical VTE‐P is more challenging. In addition, our current system does not extend to VTE‐P plans for discharge. Future clinical decision support might suggest which patients should receive combined prophylaxis while in the hospital or which home‐going prophylaxis plans should be considered.
We acknowledged the limitations of diffusing a VTE and bleeding risk‐assessment tool that has not been validated in our hospitalized pediatric population. Validation of pediatric VTE risk assessment tools have been recently developed but not widely validated in large prospective studies to be considered the standard of care.[6, 12] Based on our own institutional experience, the vast majority of VTE events occurred in pediatric patients with a central venous catheter (CVC) and other risk factors for thrombosis, and this category was arbitrarily chosen as one to consider pharmacologic prophylaxis if no bleeding risk factors and a central line to be in placed greater than 7 days duration. Although, pediatric evidence guidelines do not support the use of pharmacologic prophylaxis in patients with CVC,[15] risk factors for thrombosis in children, although less frequent than adults, are still present, and VTE‐P should be assessed and individualized in each patient considered at risk for thrombosis. Other groups have attempted a similar approach as the one taken by our group, with variations in the criteria used for thromboprophylaxis in the pediatric population.[5, 14] Our data illustrate that not all pediatric patients require pharmacologic prophylaxis (34%), and VTE‐P should be individualized based on patient risk factors for thrombosis and bleeding risk.
A strength of our system is derived from the substantial clinician and expert input, the codification of consensus, and the hard wiring of that consensus into the electronic ordering, clinical decision rules, and reporting environment. As new advances to VTE‐P are developed, we will strive to codify those new processes into our workflow, building on our past success.
Acknowledgements
The authors acknowledge all of the members involved in the VTE prevention effort at Mayo Clinic including nurses, pharmacists, and information technology support staff.
Disclosure: Nothing to report.
- U.S. Department of Health and Human Services. Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Available at: https://www.ncbi.nlm.nih.gov/books/NBK44178. Published 2008. Accessed May 23, 2016.
- , , , , . Deep‐vein thrombosis: a United States cost model for a preventable and costly adverse event. Thromb Haemost. 2011;106:405–415.
- , , , . Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–1008.
- , , , , . Risk factors for venous thromboembolism in pediatric trauma. J Trauma. 2002;52:922–927.
- , , , , , . Incidence and risk factors for venous thromboembolism in critically ill children after trauma. J Trauma. 2010;68:52–56.
- , , , , , . Risk factors for in‐hospital venous thromboembolism in children: a case‐control study employing diagnostic validation. Haematologica. 2012;97:509–515.
- . Prevention of deep vein thrombosis and pulmonary embolism. Public Health Rep. 2008;123(4):420–421.
- . Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process. J Gen Intern Med. 2007;22:1762–1770.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;14(10):901–907.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88:545–549.
- , , , et al. Risk‐prediction tool for identifying hospitalized children with a predisposition for development of venous thromboembolism: Peds‐Clot clinical Decision Rule. J Thromb Haemost. 2012;10:1326–1334.
- , , , et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72:1292–1297.
- , , , . Thromboprophylaxis in a pediatric hospital: a patient‐safety and quality‐improvement initiative. Pediatrics. 2011;127:e1326–e1332.
- , , , et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e737S–e801S.
- , , , et al. Safety and efficacy of low molecular weight heparins in children: a systematic review of the literature and meta‐analysis of single‐arm studies. Semin Thromb Hemost. 2011;37:814–825.
- , , , . Safety of prophylactic anticoagulation at a pediatric hospital. J Pediatr Hematol Oncol. 2013;35:e287–e291.
- , , , et al. Accelerating the use of best practices: the Mayo Clinic Model of Diffusion. Jt Comm J Qual Patient Saf. 2013;39:167–176.
- , , , , . Disease burden and unmet needs for prevention of venous thromboembolism in medically ill patients in Europe show underutilisation of preventive therapies. Thromb Haemost. 2011;106:600–608.
- , , , . Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement. J Gen Intern Med. 2002;17:788–791.
- , , , et al. Maintained effectiveness of an electronic alert system to prevent venous thromboembolism among hospitalized patients. Thromb Haemost. 2008;100:699–704.
- , , , et al. Validation of a clinical guideline on prevention of venous thromboembolism in medical inpatients: a before‐and‐after study with systematic ultrasound examination. J Intern Med. 2004;256:338–348.
- , , , et al. Low‐molecular‐weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365:26: 2463–2472.
- U.S. Department of Health and Human Services. Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Available at: https://www.ncbi.nlm.nih.gov/books/NBK44178. Published 2008. Accessed May 23, 2016.
- , , , , . Deep‐vein thrombosis: a United States cost model for a preventable and costly adverse event. Thromb Haemost. 2011;106:405–415.
- , , , . Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–1008.
- , , , , . Risk factors for venous thromboembolism in pediatric trauma. J Trauma. 2002;52:922–927.
- , , , , , . Incidence and risk factors for venous thromboembolism in critically ill children after trauma. J Trauma. 2010;68:52–56.
- , , , , , . Risk factors for in‐hospital venous thromboembolism in children: a case‐control study employing diagnostic validation. Haematologica. 2012;97:509–515.
- . Prevention of deep vein thrombosis and pulmonary embolism. Public Health Rep. 2008;123(4):420–421.
- . Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process. J Gen Intern Med. 2007;22:1762–1770.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;14(10):901–907.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88:545–549.
- , , , et al. Risk‐prediction tool for identifying hospitalized children with a predisposition for development of venous thromboembolism: Peds‐Clot clinical Decision Rule. J Thromb Haemost. 2012;10:1326–1334.
- , , , et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72:1292–1297.
- , , , . Thromboprophylaxis in a pediatric hospital: a patient‐safety and quality‐improvement initiative. Pediatrics. 2011;127:e1326–e1332.
- , , , et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e737S–e801S.
- , , , et al. Safety and efficacy of low molecular weight heparins in children: a systematic review of the literature and meta‐analysis of single‐arm studies. Semin Thromb Hemost. 2011;37:814–825.
- , , , . Safety of prophylactic anticoagulation at a pediatric hospital. J Pediatr Hematol Oncol. 2013;35:e287–e291.
- , , , et al. Accelerating the use of best practices: the Mayo Clinic Model of Diffusion. Jt Comm J Qual Patient Saf. 2013;39:167–176.
- , , , , . Disease burden and unmet needs for prevention of venous thromboembolism in medically ill patients in Europe show underutilisation of preventive therapies. Thromb Haemost. 2011;106:600–608.
- , , , . Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement. J Gen Intern Med. 2002;17:788–791.
- , , , et al. Maintained effectiveness of an electronic alert system to prevent venous thromboembolism among hospitalized patients. Thromb Haemost. 2008;100:699–704.
- , , , et al. Validation of a clinical guideline on prevention of venous thromboembolism in medical inpatients: a before‐and‐after study with systematic ultrasound examination. J Intern Med. 2004;256:338–348.
- , , , et al. Low‐molecular‐weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365:26: 2463–2472.
© 2016 Society of Hospital Medicine
Prevention of Healthcare‐Associated VTE
Venous thromboembolism (VTE), blood clots occurring as deep vein thrombosis, pulmonary embolism, or both, is an important and growing public health issue. The precise number of people affected by VTE is unknown; however, estimates suggest that up to 900,000 events resulting in as many as 100,000 premature deaths occur in the United States yearly with healthcare costs as high as $10 billion.[1, 2, 3] Although anyone can develop VTE, research has shown that half of VTE events occurring in the outpatient setting are directly linked to a recent hospitalization or surgery.[4] In patients with cancer, VTE is a leading cause of death after the cancer itself.[5, 6] Fortunately, many of these healthcare‐associated VTE (HA‐VTE) cases can be prevented. Recent analyses have shown that as many as 70% of HA‐VTE cases are preventable through appropriate prophylaxis,[7, 8, 9] yet reports suggest that fewer than half of hospital patients receive VTE prophylaxis in accordance with accepted evidence‐based guidelines.[10] Appropriate prevention of HA‐VTE can result in a significant reduction in overall VTE occurrence, thereby decreasing healthcare burden and unnecessary deaths.
In November 2015, the Centers for Disease Control and Prevention (CDC) released the Healthcare‐Associated VTE Prevention Challenge (
This issue of the Journal of Hospital Medicine showcases the initiatives of several of the CDC's HA‐VTE prevention champions. These champions range from a small community hospital to some of the country's largest health systems, and they represent both rural and urban areas. Together they cared for more than 450,000 patients admitted to hospitals across the United States in 2014. They were able to improve VTE prevention within their institutions and organizations by implementing successful and sustainable VTE prevention strategies such as engaging teams of different healthcare experts to support and promote prevention activities, informing patients and providers about the need for and benefits of VTE prevention, and using technology (such as electronic risk assessment and clinical decision support tools and alerts) to ensure that all patients are assessed for their risk for VTE and bleeding. These tools also help ensure that patients, when appropriate, are provided with and use appropriate prevention measures for their level of risk. Moreover, they provided real‐time feedback, scorecards, and dashboards for providers and organizations to monitor performance and identify areas for improvement.
The CDC and the Agency for Healthcare Research and Quality (AHRQ) are partnering to disseminate and promote these best practices. In addition to this challenge, the CDC, AHRQ and the Joint Commission Center for Transforming Healthcare are working on activities and programs dedicated to improving prevention of HA‐VTE. These are summarized below.
AGENCY FOR HEALTHCARE RESEARCH AND QUALITY
The AHRQ works to reduce the incidence of VTE among patients at risk of developing the condition by conducting research and providing support for quality‐improvement efforts. In 2008, AHRQ published an evidence‐based resource for prevention of VTE based on quality‐improvement principles that were successfully applied by the University of California, San Diego Medical Center, and Emory University Hospitals in an AHRQ‐funded research project. This resource, Preventing Hospital‐Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement, was updated in 2014 and is available at
Since its original release, hospital quality‐improvement teams have used the guide to close the gap between available evidence about how to prevent VTE and successful implementation of that knowledge so that hospitals can improve care as effectively and efficiently as possible. The guide includes examples of risk assessment methodologies and evaluation metrics along with other key elements that help front‐line providers establish or enhance VTE prevention programs.
AHRQ maintains an active patient safety program that spans a wide range of patient safety problems including VTE that is aimed at making healthcare safer. The AHRQ works with many different healthcare stakeholders to (1) better understand threats to patient safety and (2) develop and refine strategies that put this knowledge to work to prevent patient harm. For example, the AHRQ provides extramural research grants to investigators studying new methods of identifying patients at risk for VTE and ways to improve VTE prophylaxis in hospital patients undergoing medical treatment and surgical procedures.
In addition to a collaborative partnership with the CDC on the 2015 Healthcare‐Associated VTE Prevention Challenge, the AHRQ also partners with other federal agencies and is an active contributor to the Department of Health and Human Services National Action Plan for Adverse Drug Event Prevention. The plan outlines opportunities to advance patient safety through the prevention of adverse drug events and the promotion of medication safety among 3 drug classes including anticoagulants, a key component for VTE prevention. Patients and their families are critically important partners for improving patient safety, and the significant potential for their impact is represented by the AHRQ tools and resources such as an information video and guide for patients about the safe use of blood thinners (
THE JOINT COMMISSION CENTER FOR TRANSFORMING HEALTHCARE
The Joint Commission Center for Transforming Healthcare commenced the Preventing Venous Thromboembolism project in October 2014, with 5 participating hospitals and health centers in collaboration with the CDC. The hospitals and health centers participating on this project include: Cleveland Clinic, The Johns Hopkins Hospital, Kaiser Permanente South Bay Medical Center, Massachusetts General Hospital, and Texas Health Resources.
Often, VTE risk factors are not consistently assessed across all hospital patients, and there is much variation to the selection of appropriate mechanical and/or pharmacological prophylaxis. In addition, the current accepted guidelines are not implemented consistently across hospitals. VTE rates can be reduced with accurate risk assessment and appropriate utilization of pharmacological and/or mechanical prophylaxis. However, there are multiple barriers to consistent, successful implementation of preventative measures. During the first year of this project, the participants utilized Robust Process Improvement, a fact‐based, systematic, and data‐driven problem‐solving methodology that incorporates tools and concepts from Lean, Six Sigma, and change management, to assist them in identifying the root causes and barriers to preventing VTE in at‐risk patients.
Aggregate preliminary findings show that some of the contributing factors to VTE prevention included issues with staff attitudes and beliefs, staff and patient education, risk assessments, order sets, ineffective mechanical and pharmacological prophylaxis, and patient refusal. The participating organizations are currently developing and implementing solutions targeted to their specific root causes. The solutions will be tested, validated, and then spread to other healthcare organizations. Project findings are tentatively scheduled for release in 2017.
The Joint Commission has been committed to preventing VTE for over a decade, beginning with the development of the VTE measures, which were the result of the National Consensus Standards for the Prevention and Care of Deep Vein Thrombosis project with the National Quality Forum that formally began in January 2005. In addition, The Joint Commission and Joint Commission Resources have also aimed to reduce harm and improve the quality of care for VTE patients through the Hospital Engagement Network collaborative, publications related to improving VTE patient safety, and research on improving transitions of care and discharge instructions for patients with VTE.
CENTERS FOR DISEASE CONTROL AND PREVENTION
The CDC's Division of Blood Disorders works to prevent VTE and to reduce sickness and death among those who develop VTE. The CDC's primary VTE activities focus on developing improved methods and innovative tools for monitoring and understanding VTE occurrence and the effectiveness of VTE prevention activities, conducting epidemiologic studies on the causes and outcomes of VTE and its complications, and working internally and with partners to develop and promote education and awareness materials to inform the public and healthcare providers about the importance of knowing VTE risk factors to improve prevention, and the importance of knowing the signs and symptoms of VTE to ensure early and accurate diagnosis and treatment of VTE. Recently, the CDC collaborated with the National Blood Clot Alliance, through their Stop the Clot, Spread the Word Campaign, to develop and promote prevention of VTE among hospitalized patients (
The CDC, AHRQ, and The Joint Commission hope that readers will find the successes of the HA‐VTE Prevention Champions' initiatives informative and encouraging. They are examples of how any healthcare setting, from a small hospital to a large healthcare system, can implement approaches and tools to improve prevention of HA‐VTE.
Disclosures
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality, or the Joint Commission Center for Transforming Healthcare. The authors declare no conflicts of interest.
- U.S. Department of Health and Human Services, Office of the Surgeon General. The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: National Heart, Lung, and Blood Institute; 2008.
- , , , . Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501.
- , , , , . Surveillance for deep vein thrombosis and pulmonary embolism: recommendations from a national workshop. Am J Prev Med. 2010;38(4 suppl):S502–S509.
- , , , , . Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007;167(14):1471–1475.
- , . Cancer‐associated thrombosis. Hematology Am Soc Hematol Educ Program. 2013;2013:684–691.
- . Cancer‐associated thrombosis: updates and controversies. Hematology Am Soc Hematol Educ Program. 2012;2012:626–630.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88:545–549.
- , , , . A simple reminder system improves venous thromboembolism prophylaxis rates and reduces thrombotic events for hospitalized patients. J Thromb Haemost. 2012;10:236–243.
- , . Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23:187–195.
- , , , et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201.
Venous thromboembolism (VTE), blood clots occurring as deep vein thrombosis, pulmonary embolism, or both, is an important and growing public health issue. The precise number of people affected by VTE is unknown; however, estimates suggest that up to 900,000 events resulting in as many as 100,000 premature deaths occur in the United States yearly with healthcare costs as high as $10 billion.[1, 2, 3] Although anyone can develop VTE, research has shown that half of VTE events occurring in the outpatient setting are directly linked to a recent hospitalization or surgery.[4] In patients with cancer, VTE is a leading cause of death after the cancer itself.[5, 6] Fortunately, many of these healthcare‐associated VTE (HA‐VTE) cases can be prevented. Recent analyses have shown that as many as 70% of HA‐VTE cases are preventable through appropriate prophylaxis,[7, 8, 9] yet reports suggest that fewer than half of hospital patients receive VTE prophylaxis in accordance with accepted evidence‐based guidelines.[10] Appropriate prevention of HA‐VTE can result in a significant reduction in overall VTE occurrence, thereby decreasing healthcare burden and unnecessary deaths.
In November 2015, the Centers for Disease Control and Prevention (CDC) released the Healthcare‐Associated VTE Prevention Challenge (
This issue of the Journal of Hospital Medicine showcases the initiatives of several of the CDC's HA‐VTE prevention champions. These champions range from a small community hospital to some of the country's largest health systems, and they represent both rural and urban areas. Together they cared for more than 450,000 patients admitted to hospitals across the United States in 2014. They were able to improve VTE prevention within their institutions and organizations by implementing successful and sustainable VTE prevention strategies such as engaging teams of different healthcare experts to support and promote prevention activities, informing patients and providers about the need for and benefits of VTE prevention, and using technology (such as electronic risk assessment and clinical decision support tools and alerts) to ensure that all patients are assessed for their risk for VTE and bleeding. These tools also help ensure that patients, when appropriate, are provided with and use appropriate prevention measures for their level of risk. Moreover, they provided real‐time feedback, scorecards, and dashboards for providers and organizations to monitor performance and identify areas for improvement.
The CDC and the Agency for Healthcare Research and Quality (AHRQ) are partnering to disseminate and promote these best practices. In addition to this challenge, the CDC, AHRQ and the Joint Commission Center for Transforming Healthcare are working on activities and programs dedicated to improving prevention of HA‐VTE. These are summarized below.
AGENCY FOR HEALTHCARE RESEARCH AND QUALITY
The AHRQ works to reduce the incidence of VTE among patients at risk of developing the condition by conducting research and providing support for quality‐improvement efforts. In 2008, AHRQ published an evidence‐based resource for prevention of VTE based on quality‐improvement principles that were successfully applied by the University of California, San Diego Medical Center, and Emory University Hospitals in an AHRQ‐funded research project. This resource, Preventing Hospital‐Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement, was updated in 2014 and is available at
Since its original release, hospital quality‐improvement teams have used the guide to close the gap between available evidence about how to prevent VTE and successful implementation of that knowledge so that hospitals can improve care as effectively and efficiently as possible. The guide includes examples of risk assessment methodologies and evaluation metrics along with other key elements that help front‐line providers establish or enhance VTE prevention programs.
AHRQ maintains an active patient safety program that spans a wide range of patient safety problems including VTE that is aimed at making healthcare safer. The AHRQ works with many different healthcare stakeholders to (1) better understand threats to patient safety and (2) develop and refine strategies that put this knowledge to work to prevent patient harm. For example, the AHRQ provides extramural research grants to investigators studying new methods of identifying patients at risk for VTE and ways to improve VTE prophylaxis in hospital patients undergoing medical treatment and surgical procedures.
In addition to a collaborative partnership with the CDC on the 2015 Healthcare‐Associated VTE Prevention Challenge, the AHRQ also partners with other federal agencies and is an active contributor to the Department of Health and Human Services National Action Plan for Adverse Drug Event Prevention. The plan outlines opportunities to advance patient safety through the prevention of adverse drug events and the promotion of medication safety among 3 drug classes including anticoagulants, a key component for VTE prevention. Patients and their families are critically important partners for improving patient safety, and the significant potential for their impact is represented by the AHRQ tools and resources such as an information video and guide for patients about the safe use of blood thinners (
THE JOINT COMMISSION CENTER FOR TRANSFORMING HEALTHCARE
The Joint Commission Center for Transforming Healthcare commenced the Preventing Venous Thromboembolism project in October 2014, with 5 participating hospitals and health centers in collaboration with the CDC. The hospitals and health centers participating on this project include: Cleveland Clinic, The Johns Hopkins Hospital, Kaiser Permanente South Bay Medical Center, Massachusetts General Hospital, and Texas Health Resources.
Often, VTE risk factors are not consistently assessed across all hospital patients, and there is much variation to the selection of appropriate mechanical and/or pharmacological prophylaxis. In addition, the current accepted guidelines are not implemented consistently across hospitals. VTE rates can be reduced with accurate risk assessment and appropriate utilization of pharmacological and/or mechanical prophylaxis. However, there are multiple barriers to consistent, successful implementation of preventative measures. During the first year of this project, the participants utilized Robust Process Improvement, a fact‐based, systematic, and data‐driven problem‐solving methodology that incorporates tools and concepts from Lean, Six Sigma, and change management, to assist them in identifying the root causes and barriers to preventing VTE in at‐risk patients.
Aggregate preliminary findings show that some of the contributing factors to VTE prevention included issues with staff attitudes and beliefs, staff and patient education, risk assessments, order sets, ineffective mechanical and pharmacological prophylaxis, and patient refusal. The participating organizations are currently developing and implementing solutions targeted to their specific root causes. The solutions will be tested, validated, and then spread to other healthcare organizations. Project findings are tentatively scheduled for release in 2017.
The Joint Commission has been committed to preventing VTE for over a decade, beginning with the development of the VTE measures, which were the result of the National Consensus Standards for the Prevention and Care of Deep Vein Thrombosis project with the National Quality Forum that formally began in January 2005. In addition, The Joint Commission and Joint Commission Resources have also aimed to reduce harm and improve the quality of care for VTE patients through the Hospital Engagement Network collaborative, publications related to improving VTE patient safety, and research on improving transitions of care and discharge instructions for patients with VTE.
CENTERS FOR DISEASE CONTROL AND PREVENTION
The CDC's Division of Blood Disorders works to prevent VTE and to reduce sickness and death among those who develop VTE. The CDC's primary VTE activities focus on developing improved methods and innovative tools for monitoring and understanding VTE occurrence and the effectiveness of VTE prevention activities, conducting epidemiologic studies on the causes and outcomes of VTE and its complications, and working internally and with partners to develop and promote education and awareness materials to inform the public and healthcare providers about the importance of knowing VTE risk factors to improve prevention, and the importance of knowing the signs and symptoms of VTE to ensure early and accurate diagnosis and treatment of VTE. Recently, the CDC collaborated with the National Blood Clot Alliance, through their Stop the Clot, Spread the Word Campaign, to develop and promote prevention of VTE among hospitalized patients (
The CDC, AHRQ, and The Joint Commission hope that readers will find the successes of the HA‐VTE Prevention Champions' initiatives informative and encouraging. They are examples of how any healthcare setting, from a small hospital to a large healthcare system, can implement approaches and tools to improve prevention of HA‐VTE.
Disclosures
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality, or the Joint Commission Center for Transforming Healthcare. The authors declare no conflicts of interest.
Venous thromboembolism (VTE), blood clots occurring as deep vein thrombosis, pulmonary embolism, or both, is an important and growing public health issue. The precise number of people affected by VTE is unknown; however, estimates suggest that up to 900,000 events resulting in as many as 100,000 premature deaths occur in the United States yearly with healthcare costs as high as $10 billion.[1, 2, 3] Although anyone can develop VTE, research has shown that half of VTE events occurring in the outpatient setting are directly linked to a recent hospitalization or surgery.[4] In patients with cancer, VTE is a leading cause of death after the cancer itself.[5, 6] Fortunately, many of these healthcare‐associated VTE (HA‐VTE) cases can be prevented. Recent analyses have shown that as many as 70% of HA‐VTE cases are preventable through appropriate prophylaxis,[7, 8, 9] yet reports suggest that fewer than half of hospital patients receive VTE prophylaxis in accordance with accepted evidence‐based guidelines.[10] Appropriate prevention of HA‐VTE can result in a significant reduction in overall VTE occurrence, thereby decreasing healthcare burden and unnecessary deaths.
In November 2015, the Centers for Disease Control and Prevention (CDC) released the Healthcare‐Associated VTE Prevention Challenge (
This issue of the Journal of Hospital Medicine showcases the initiatives of several of the CDC's HA‐VTE prevention champions. These champions range from a small community hospital to some of the country's largest health systems, and they represent both rural and urban areas. Together they cared for more than 450,000 patients admitted to hospitals across the United States in 2014. They were able to improve VTE prevention within their institutions and organizations by implementing successful and sustainable VTE prevention strategies such as engaging teams of different healthcare experts to support and promote prevention activities, informing patients and providers about the need for and benefits of VTE prevention, and using technology (such as electronic risk assessment and clinical decision support tools and alerts) to ensure that all patients are assessed for their risk for VTE and bleeding. These tools also help ensure that patients, when appropriate, are provided with and use appropriate prevention measures for their level of risk. Moreover, they provided real‐time feedback, scorecards, and dashboards for providers and organizations to monitor performance and identify areas for improvement.
The CDC and the Agency for Healthcare Research and Quality (AHRQ) are partnering to disseminate and promote these best practices. In addition to this challenge, the CDC, AHRQ and the Joint Commission Center for Transforming Healthcare are working on activities and programs dedicated to improving prevention of HA‐VTE. These are summarized below.
AGENCY FOR HEALTHCARE RESEARCH AND QUALITY
The AHRQ works to reduce the incidence of VTE among patients at risk of developing the condition by conducting research and providing support for quality‐improvement efforts. In 2008, AHRQ published an evidence‐based resource for prevention of VTE based on quality‐improvement principles that were successfully applied by the University of California, San Diego Medical Center, and Emory University Hospitals in an AHRQ‐funded research project. This resource, Preventing Hospital‐Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement, was updated in 2014 and is available at
Since its original release, hospital quality‐improvement teams have used the guide to close the gap between available evidence about how to prevent VTE and successful implementation of that knowledge so that hospitals can improve care as effectively and efficiently as possible. The guide includes examples of risk assessment methodologies and evaluation metrics along with other key elements that help front‐line providers establish or enhance VTE prevention programs.
AHRQ maintains an active patient safety program that spans a wide range of patient safety problems including VTE that is aimed at making healthcare safer. The AHRQ works with many different healthcare stakeholders to (1) better understand threats to patient safety and (2) develop and refine strategies that put this knowledge to work to prevent patient harm. For example, the AHRQ provides extramural research grants to investigators studying new methods of identifying patients at risk for VTE and ways to improve VTE prophylaxis in hospital patients undergoing medical treatment and surgical procedures.
In addition to a collaborative partnership with the CDC on the 2015 Healthcare‐Associated VTE Prevention Challenge, the AHRQ also partners with other federal agencies and is an active contributor to the Department of Health and Human Services National Action Plan for Adverse Drug Event Prevention. The plan outlines opportunities to advance patient safety through the prevention of adverse drug events and the promotion of medication safety among 3 drug classes including anticoagulants, a key component for VTE prevention. Patients and their families are critically important partners for improving patient safety, and the significant potential for their impact is represented by the AHRQ tools and resources such as an information video and guide for patients about the safe use of blood thinners (
THE JOINT COMMISSION CENTER FOR TRANSFORMING HEALTHCARE
The Joint Commission Center for Transforming Healthcare commenced the Preventing Venous Thromboembolism project in October 2014, with 5 participating hospitals and health centers in collaboration with the CDC. The hospitals and health centers participating on this project include: Cleveland Clinic, The Johns Hopkins Hospital, Kaiser Permanente South Bay Medical Center, Massachusetts General Hospital, and Texas Health Resources.
Often, VTE risk factors are not consistently assessed across all hospital patients, and there is much variation to the selection of appropriate mechanical and/or pharmacological prophylaxis. In addition, the current accepted guidelines are not implemented consistently across hospitals. VTE rates can be reduced with accurate risk assessment and appropriate utilization of pharmacological and/or mechanical prophylaxis. However, there are multiple barriers to consistent, successful implementation of preventative measures. During the first year of this project, the participants utilized Robust Process Improvement, a fact‐based, systematic, and data‐driven problem‐solving methodology that incorporates tools and concepts from Lean, Six Sigma, and change management, to assist them in identifying the root causes and barriers to preventing VTE in at‐risk patients.
Aggregate preliminary findings show that some of the contributing factors to VTE prevention included issues with staff attitudes and beliefs, staff and patient education, risk assessments, order sets, ineffective mechanical and pharmacological prophylaxis, and patient refusal. The participating organizations are currently developing and implementing solutions targeted to their specific root causes. The solutions will be tested, validated, and then spread to other healthcare organizations. Project findings are tentatively scheduled for release in 2017.
The Joint Commission has been committed to preventing VTE for over a decade, beginning with the development of the VTE measures, which were the result of the National Consensus Standards for the Prevention and Care of Deep Vein Thrombosis project with the National Quality Forum that formally began in January 2005. In addition, The Joint Commission and Joint Commission Resources have also aimed to reduce harm and improve the quality of care for VTE patients through the Hospital Engagement Network collaborative, publications related to improving VTE patient safety, and research on improving transitions of care and discharge instructions for patients with VTE.
CENTERS FOR DISEASE CONTROL AND PREVENTION
The CDC's Division of Blood Disorders works to prevent VTE and to reduce sickness and death among those who develop VTE. The CDC's primary VTE activities focus on developing improved methods and innovative tools for monitoring and understanding VTE occurrence and the effectiveness of VTE prevention activities, conducting epidemiologic studies on the causes and outcomes of VTE and its complications, and working internally and with partners to develop and promote education and awareness materials to inform the public and healthcare providers about the importance of knowing VTE risk factors to improve prevention, and the importance of knowing the signs and symptoms of VTE to ensure early and accurate diagnosis and treatment of VTE. Recently, the CDC collaborated with the National Blood Clot Alliance, through their Stop the Clot, Spread the Word Campaign, to develop and promote prevention of VTE among hospitalized patients (
The CDC, AHRQ, and The Joint Commission hope that readers will find the successes of the HA‐VTE Prevention Champions' initiatives informative and encouraging. They are examples of how any healthcare setting, from a small hospital to a large healthcare system, can implement approaches and tools to improve prevention of HA‐VTE.
Disclosures
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality, or the Joint Commission Center for Transforming Healthcare. The authors declare no conflicts of interest.
- U.S. Department of Health and Human Services, Office of the Surgeon General. The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: National Heart, Lung, and Blood Institute; 2008.
- , , , . Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501.
- , , , , . Surveillance for deep vein thrombosis and pulmonary embolism: recommendations from a national workshop. Am J Prev Med. 2010;38(4 suppl):S502–S509.
- , , , , . Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007;167(14):1471–1475.
- , . Cancer‐associated thrombosis. Hematology Am Soc Hematol Educ Program. 2013;2013:684–691.
- . Cancer‐associated thrombosis: updates and controversies. Hematology Am Soc Hematol Educ Program. 2012;2012:626–630.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88:545–549.
- , , , . A simple reminder system improves venous thromboembolism prophylaxis rates and reduces thrombotic events for hospitalized patients. J Thromb Haemost. 2012;10:236–243.
- , . Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23:187–195.
- , , , et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201.
- U.S. Department of Health and Human Services, Office of the Surgeon General. The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: National Heart, Lung, and Blood Institute; 2008.
- , , , . Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501.
- , , , , . Surveillance for deep vein thrombosis and pulmonary embolism: recommendations from a national workshop. Am J Prev Med. 2010;38(4 suppl):S502–S509.
- , , , , . Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007;167(14):1471–1475.
- , . Cancer‐associated thrombosis. Hematology Am Soc Hematol Educ Program. 2013;2013:684–691.
- . Cancer‐associated thrombosis: updates and controversies. Hematology Am Soc Hematol Educ Program. 2012;2012:626–630.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88:545–549.
- , , , . A simple reminder system improves venous thromboembolism prophylaxis rates and reduces thrombotic events for hospitalized patients. J Thromb Haemost. 2012;10:236–243.
- , . Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23:187–195.
- , , , et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201.
© 2016 Society of Hospital Medicine
The Johns Hopkins VTE Collaborative
Venous thromboembolism (VTE), which encompasses deep venous thrombosis and pulmonary embolism, is an important cause of preventable morbidity and mortality.[1] Each year it is estimated as many as 600,000 American's suffer VTE and as many as 100,000 die.[2] Consequently, patient safety and healthcare quality, accrediting organizations such as The Joint Commission, and federal agencies such as the Centers for Disease Control and Prevention and Agency for Healthcare Research and Quality (AHRQ) have made VTE prevention a priority.[3, 4, 5]
Despite widespread recognition that VTE prophylaxis is an important patient safety measure, poor performance is common. The ENDORSE (Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting) study of over 68,000 hospitalized patients in 32 countries noted only 58.5% of surgical patients and 39.5% medical patients received American College of Chest Physicians (ACCP) guideline‐appropriate VTE prophylaxis.[6] In 2005, an audit of the surgical services at The Johns Hopkins Hospital found that only 33% of 322 randomly selected patients were prescribed prophylaxis consistent with the ACCP guidelines.
Achieving defect‐free VTE prevention requires attention to each step in the process: (1) assessment of both VTE and bleeding risk, (2) prescription of risk‐appropriate VTE prophylaxis, and (3) administration of risk‐appropriate VTE prophylaxis. In 2005, to improve our VTE prevention performance at Johns Hopkins Hospital, the Center for Innovations organized a VTE Collaborative of 2 physicians, 1 nurse, and 1 pharmacist dedicated to VTE quality improvement. Since then, the group has grown dramatically, adding a clinical informatics expert and numerous other members and coming under the auspices of The Armstrong Institute for Patient Safety. Recognizing that many, though not all, VTEs are potentially preventable,[7, 8] the mission of the Johns Hopkins VTE Collaborative is to ensure that all hospitalized patients receive risk‐appropriate, best‐practice VTE prophylaxis. This article chronicles the innovative strategies that the Johns Hopkins VTE Collaborative has employed over the past decade to improve our hospital's performance in VTE prevention (Table 1).
|
| Strategies to improve VTE prophylaxis ordering |
| Paper‐based patient risk assessment forms (before computer order entry) |
| Mandatory evidence‐based specialty‐specific computer clinical decision support smart order sets |
| Group data and competitions |
| 1‐on‐1 provider feedback |
| Pay for performance |
| Individualized feedback with resident scorecards |
| Strategies to improve VTE prophylaxis administration |
| Identification of missed doses as a major contributor to preventable VTE |
| Identification of physician, nurse and patient contributors to missed doses |
| Collaboration with patients to create patient‐centered educational materials |
| Novel web‐based module for nursing education |
| Real‐time missed doses alert |
| Targeted 1‐on‐1 patient education |
ENSURING EVERY PATIENT IS PRESCRIBED RISK‐APPROPRIATE PROPHYLAXIS
With the support of hospital leadership, the VTE Collaborative held a series of events in 2005 with medical and surgical providers to review the current evidence supporting VTE prophylaxis and achieve consensus on appropriate practice based upon the 2004 ACCP VTE Prophylaxis Guideline. The result was the development of 5 evidence‐based, paper VTE prophylaxis order sets that guided the ordering provider on the assessment of VTE and bleeding risk and facilitated the selection of risk‐appropriate VTE prophylaxis. Because there were no validated VTE or bleeding risk assessment tools at the time we developed our order sets, we used specialty‐specific VTE risk factors derived from the 2004 ACCP Guideline. To identify patients inappropriate for pharmacologic prophylaxis, we used exclusion criteria derived from contemporary randomized clinical trials of pharmacologic prophylaxis in the target populations (ie, active bleeding, abnormal activated partial thromboplastin time not due to a lupus inhibitor) or mutually agreed upon thresholds after discussion with individual provider groups (platelet count <50,000/L). On the Johns Hopkins Hospital inpatient acute rehabilitation unit, introduction of the paper order sets increased adherence with ACCP guidelines from 27% to 98% (P < 0.0001) and reduced symptomatic VTE from 49 per 1000 admissions to 8 per 1000 admissions (P = 0.0001).[9] This study demonstrated that paper order sets used consistently by a dedicated group of providers can result in sustained improvements in practice. Paper order sets remain a low‐tech, easy‐to‐implement strategy that can be applied in any healthcare setting. Other services also saw improvements in risk‐appropriate prophylaxis prescription. In a follow‐up cross‐sectional analysis of the surgical services at Johns Hopkins, we found that appropriate VTE prophylaxis prescription improved from 33% to 62% in a sample of 226 patients. Unfortunately, paper order sets had several disadvantages including (1) the inherent difficulty of making them a mandatory part of the admission or transfer process, (2) their existence outside the usual clinical workflow, and (3) the labor‐ and time‐intensive data collection that made it difficult to provide credible, timely performance reports to providers and leadership.
These disadvantages and our adoption of a computerized provider order entry system prompted us to pursue the development and implementation of mandatory, evidence‐based, specialty‐specific computerized clinical decision support (CCDS) VTE prophylaxis order sets. Using the Translating Research Into Practice approach to quality improvement,[10] we collaborated with providers to design 16 different evidence‐based specialty‐specific CCDS VTE order sets. These CCDS VTE order sets, which are imbedded in the specialty‐specific admission and transfer order sets, assist providers in assessing patients' VTE and bleeding risk factors and provide evidence‐based risk‐appropriate VTE prophylaxis (see Supporting Figure 1 in the online version of this article). Individual patient data are saved in an administrative database and can be easily aggregated for research analyses and quality improvement/performance reporting. A detailed discussion of our strategy for change is discussed in Streiff et al.[11] Because pharmacologic prophylaxis is not appropriate for every patient, and not all VTE are preventable, even with perfect prophylaxis, the goals of our collaborative are to ensure that every patient is ordered VTE prophylaxis consistent with their risk profile (risk‐appropriate prophylaxis) and to eliminate preventable episodes of VTE (VTE that occurs in the setting of suboptimal prophylaxis). In a prepost quasi‐experimental study of 1599 trauma patients, the CCDS VTE order set increased risk‐appropriate prophylaxis prescription from 66.2% to 84.4% (P < .001) and reduced the incidence of potentially preventable harm from VTE from 1% to 0.17% (P = 0.04) (Figure 1).[12] On the medical service, the CCDS VTE order set improved risk‐appropriate VTE prophylaxis prescription from 65.6% to 90.1% (P < 0.0001) and reduced the incidence of potentially preventable harm attributable to VTE from 1.1% to 0% (P = 0.001). There was no increase in major bleeding (International Society of Thrombosis and Hemostasis definition: hemoglobin decline of 2 grams/dL or transfusion of 2 or more units of blood or bleeding into a critical organ such as brain, gastrointestinal tract, or eye) postorder set implementation (0.3% vs 0.1%, P = 0.625) or all‐cause mortality (1.3% vs 2.0%, P = 0.285).[13]
These order sets demonstrated that CCDS tools can lead to significant improvements in prescribing practices and reductions in preventable harm from VTE without increasing the risk of major bleeding complications. In addition to improving the quality of care, the order sets also improved the consistency of care. In a retrospective analysis, we found that implementation of CCDS VTE order sets eliminated racial disparities in prescribing practices. In the preimplementation group, risk‐appropriate VTE prophylaxis was prescribed for 70.1% of black patients and 56.6% of white patients on the trauma service (P = 0.025) and 69.5% of black patients and 61.7% of white patients on the medical service (P = 0.015). After implementation of the CCDS VTE order sets, care improved for all patients such that the previously observed disparities were eliminated (trauma service 84.5% vs 85.5%, P = 0.99 and medical service 91.8% vs 88.0%, P = 0.082).[14] These data indicate that standardizing care can potentially eliminate disparities in clinical practice.
Although implementation of mandatory evidence‐based, specialty‐specific CDSS VTE order sets led to substantial improvements in VTE prophylaxis ordering, high performance was not uniform across our institution. On the medical service, substantial disparities in adherence to order set recommendations existed. On the housestaff services, over 90% of patients consistently received risk‐appropriate VTE prophylaxis compared with only 85% on the hospitalist service. Examination of individual provider performance found that some providers only ordered risk‐appropriate prophylaxis 50% of the time, whereas others were doing so 98% of the time. To address this disparity, we conducted a retrospective analysis of a prospective performance improvement project conducted on the Johns Hopkins Hospitalist service studying the impact of individualized hospitalist attending feedback on VTE prevention practices. During the preintervention period (January 2009December 2010), guideline‐adherent VTE prophylaxis was ordered for 86% (95% confidence interval [CI]: 85%‐88%) of patients. Six months after initiation of direct face‐to‐face provider feedback (January 2011June 2011), guideline‐adherent VTE prophylaxis rates rose to 90% (95% CI: 88‐93). Subsequently (July 2011December 2012), a pay‐for‐performance (P4P) initiative was added to direct face‐to‐face provider feedback. During the P4P initiative, provider incentive per relative value unit (RVU) was progressively increased with increasing performance on provision of risk‐appropriate VTE prophylaxis (adherence <80% = no bonus to $0.50 per RVU for adherence 95%). During this period, prescription of guideline‐adherent prophylaxis rose to 94% (95% CI: 93%‐96%).[15] These initiatives transformed the hospitalist unit from a consistently low‐performance unit to a high‐performance unit.
Similar findings were noted on the trauma service. Although the original plan was to provide feedback to attending trauma surgeons, that plan changed when we found that performance was driven entirely by resident practice; residents write the VTE prophylaxis orders, which is then attributed to attending performance. Resident performance varied widely; 42 of 75 (56%) residents on the trauma service ordered risk‐appropriate prophylaxis for 100% of their patients. In contrast, 7 (9.3%) residents never ordered optimal prophylaxis for any of their patients.[16] To motivate all residents to prescribe optimal prophylaxis, we developed an individualized resident VTE prophylaxis scorecard (Figure 2). This prospective cohort study of 2420 patients and 49 general surgery residents compared resident VTE prophylaxis performance on the general surgery service during 3 periods: period 1 (baseline, July 2013September 2013), period 2 (surgery resident scorecard, October 2013December 2013), period 3 (resident scorecard plus individualized 1‐on‐1 coaching, January 2014March 2014). At baseline, 89.4% of patients were prescribed appropriate VTE prophylaxis, and only 45% of residents prescribed risk‐appropriate prophylaxis for all their patients. During the scorecard period, 95.4% of patients were prescribed risk‐appropriate VTE prophylaxis (P < 0.001). During the scorecard plus coaching period, risk‐appropriate prophylaxis rose to 96.4%. These prescribing practice changes were durable. During the 15 months prior to issuing scorecards, 88.0% of patients (3718/4226) were prescribed risk‐appropriate prophylaxis. After implementation, 95.8% of patients (3799/3966) were prescribed risk‐appropriate prophylaxis (P < 0.001) (see Supporting Figure 2 in the online version of this article). During the baseline period, 7 of 865 patients (0.81%) had a VTE during their hospital stay, of which 3 (0.35%) were potentially preventable. In contrast, none of the 3 of 784 patients who suffered VTE during the postimplementation period had a potentially preventable event (0.35% vs 0%, P = 0.046).[17] These studies demonstrate that providing physicians with their own specific data can be a powerful tool for performance improvement that may be applicable to many other quality and safety measures. Our group recently received funding from the AHRQ to scale this work to other residents, nurse practitioners, physician assistants, and attending physicians (1R01HS024547, Individualized Performance Feedback on Venous Thromboembolism Prevention Practice).
IMPROVING VTE PROPHYLAXIS ADMINISTRATION
Ordering VTE prophylaxis does not ensure its administration. We conducted a retrospective review of electronic administration records of 10,526 consecutive patients admitted over a 7‐month period at The Johns Hopkins Hospital. Twelve percent of the over 100,000 ordered doses of VTE prophylaxis were not administered, and the proportion of nonadministered doses on individual floors varied 5‐fold from 5.4% to 26.9%. The proportion of nonadministered doses was significantly higher on medical floors compared with all other services (17.5% vs 8.1%, odds ratio [OR]: 2.1 [95% CI: 2.0‐2.2]). Patient or family member refusal was the most common cause for nonadministered doses of VTE prophylaxis accounting for 59% of all missed doses. Eight percent of patients missed more than half their prescribed doses, and 5% of patients missed over 75% of ordered doses of VTE prophylaxis. Consistent with the Pareto principle, over 80% of the missed doses of prophylaxis were accounted for by just 20% of the patients.[18] A retrospective analysis of hospital‐acquired VTE at Johns Hopkins found that 39% of events occurred in patients who missed 1 or more doses of appropriate VTE prophylaxis.[19] Louis et al. noted that nonadministration of 1 dose of VTE prophylaxis was associated with a significant increase in risk for hospital acquired VTE.[20] These data indicate the need for more aggressive interventions to reduce missed doses to improve VTE prevention.
To fully understand the root causes of VTE prophylaxis non‐administration, we conducted a series of studies examining each of the participants in the VTE prevention care pathway, physicians, nurses, and patients. In a survey of 122 resident physicians, we found significant differences in clinical practice between medicine and surgery residents. Medicine residents were more likely to believe that VTE prophylaxis was overprescribed, and that it was appropriate for nurses to make judgement calls about whether patients needed the prophylaxis that was prescribed.[21] In a mixed methods study that included a written survey and qualitative observations of nursing practice, we found that some nurses presented pharmacologic VTE prophylaxis injections as optional to patients. Furthermore, nurses on units where nonadministration was higher were more likely to believe that VTE prophylaxis was prescribed for patients unnecessarily, and that they could use their clinical judgement to determine when it was appropriate to omit doses of pharmacologic prophylaxis.[22] Our team also examined patient preferences in regard to VTE prophylaxis. In a survey of 227 consecutive medical and surgical inpatients, we found that 60% of patients would prefer an oral route of administration if available. Patients with a preference for a parenteral route of administration (27.5%) were less likely to refuse prophylaxis (37.5% vs 51.3%, P < 0.0001).[23] These findings underscore the fact that unit culture, nursing attitudes and beliefs, and patient preferences have an important influence on medication administration, and that nursepatient communication is an important target for modifying adherence.
PATIENT‐CENTERED APPROACHES TO IMPROVE VTE PROPHYLAXIS ADMINISTRATION
To address nurse‐ and patient‐related factors that influence VTE prophylaxis administration, we applied for and received a Patient Centered Outcomes Research Institute contract to develop patient‐centered interventions to engage and empower patients to take an active role in their preventive care. To achieve these aims, we partnered with 3 national patient advocacy organizations, the National Blood Clot Alliance, the North American Thrombosis Forum, and ClotCare, as well as our local Johns Hopkins Patient and Family Advisory Council. Using a modified Delphi method, we engaged patient stakeholders from the 4 collaborating organizations to build consensus on patient‐centered VTE education methods. Input from this Delphi assessment was used to build educational materials including paper brochures published in 8 different languages and a 10‐minute educational video filmed by an Oscar‐winning documentary director featuring both clinicians and patients relating their VTE experience and the importance of VTE prevention.[24] These educational materials are available for public use (
ENGAGING TRAINEES IN MULTIDISCIPLINARY PATIENT SAFETY/QUALITY IMPROVEMENT INITIATIVES
Trainees from many healthcare‐related disciplines have played a critical role in our quest to improve VTE prevention. Over the past 10 years, we have mentored countless medical students, public health graduate students, nursing students, residents, and postdoctoral fellows in research projects that have resulted in numerous high‐quality publications. Trainees have helped to dispel staff concerns about patient falls in connection of intermittent pneumatic compression devices,[25] identify the weaknesses of current publicly reported VTE measures,[26, 27, 28, 29] identify opportunities to improve VTE prevention practices within clinical specialties,[30, 31, 32] define the role of surveillance bias in VTE outcomes reporting,[33, 34, 35] discover and fully explore the important problem of missed doses of VTE prophylaxis,[18, 21, 22, 23, 36] and summarize knowledge about VTE prevention via systematic reviews and meta‐analyses.[37, 38, 39] These collaborations have been a classic win‐win. The mentees learn critical skills while growing their curriculum vitae with contributions to the literature, allowing them to progress in their careers (ie, obtain a residency match, faculty positions). The faculty have leveraged this work to obtain over $3 million in extramural funding to develop interventions to study and improve the quality of VTE preventive care for hospitalized patients.
In healthcare, we have not yet achieved defect‐free VTE prevention; however, we have a better understanding of the path to accomplishing this goal. In this article we describe our goal of zero harm from VTE and our learning journey to realize that goal. Although the journey never ends, a critical ingredient to the success of our program has been the multidisciplinary nature of our VTE collaborative team. The combination of expertise from medicine, surgery, nursing, pharmacy, clinical informatics, and public health has facilitated the development of innovative strategies to improve VTE prevention that integrate seamlessly into clinical workflow. The approach used for VTE can be applied to eliminate other types of harms.
Disclosures
Mr. Lau, Dr. Streiff, and Dr. Haut are supported by a grant from the Agency for Healthcare Research and Quality (1R01HS024547) titled Individualized Performance Feedback on Venous Thromboembolism Prevention Practice and a contract from the Patient‐Centered Outcomes Research Institute titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Mr. Lau is supported by the Institute for Excellence in Education Berkheimer Faculty Education Scholar Grant and a contract (AD‐1306‐03980) from the Patient‐Centered Outcomes Research Institute titled Patient Centered Approaches to Collect Sexual Orientation/Gender Identity Information in the Emergency Department. Ms. Hobson has given expert witness testimony in various medical malpractice cases. Dr. Streiff has received research funding from Portola and Janssen; consulted for Bio2Medical, CSL Behring, Merck, and Janssen HealthCare; and has given expert witness testimony in various medical malpractice cases. Dr. Haut receives royalties from Lippincott, Williams, and Wilkins for a book titled Avoiding Common ICU Errors. Dr. Haut is a paid consultant and speaker for the Preventing Avoidable Venous ThromboembolismEvery Patient, Every Time VHA/Vizient IMPERATIV Advantage Performance Improvement Collaborative. Dr. Haut is a paid consultant and speaker for the Illinois Surgical Quality Improvement Collaborative. All other authors report no disclosures.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- Office of the Surgeon General (US); National Heart, Lung, and Blood Institute (US). The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008.
- , . Prevention of venous thromboembolism: brief update review. In: Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , et al. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med. 2013;158:365–368.
- , . Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23:187–195.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371:387–394.
- , . The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009;301:1063–1065.
- , , , et al. ICD‐9 code‐based venous thromboembolism performance targets fail to measure up. Am J Med Qual. 2016;31(5):448–453.
- , , , , . Evidence‐based venous thromboembolism prophylaxis is associated with a six‐fold decrease in numbers of symptomatic venous thromboembolisms in rehabilitation inpatients. PM R. 2011;3:1111–1115.e1.
- , , . Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;147:901–907.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , , , et al. Eliminating health care disparities with mandatory clinical decision support: the venous thromboembolism (VTE) example. Med Care. 2015;53:18–24.
- , , , et al. Use of provider‐level dashboards and pay‐for‐performance in venous thromboembolism prophylaxis. J Hosp Med. 2015;10:172–178.
- , , , , , . Attending physician performance measure scores and resident physicians' ordering practices. JAMA Surg. 2015;150:813–814.
- , , , et al. Individualized performance feedback to surgical residents improves appropriate venous thromboembolism prophylaxis prescription and reduces potentially preventable VTE: a prospective cohort study [published online November 25, 2015]. Ann Surg. doi: 10.1097/SLA.0000000000001512.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8:e66311.
- , , , et al. Preventability of hospital‐acquired venous thromboembolism. JAMA Surg. 2015;150(9):912–915.
- , , , et al. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 2014;149:365–370.
- , , , et al. Prescriber knowledge and attitudes regarding non‐administration of prescribed pharmacologic venous thromboembolism prophylaxis [published online May 21, 2016]. J Thromb Thrombolysis. doi:10.1007/s11239-016-1378-8.
- , , , et al. Hidden barriers to delivery of pharmacological venous thromboembolism prophylaxis: the role of nursing beliefs and practices. J Patient Saf. 2016;12:63–68.
- , , , et al. Patient preferences regarding pharmacologic venous thromboembolism prophylaxis. J Hosp Med. 2015;10:108–111.
- , , , et al. Patient preferences for receiving education on venous thromboembolism prevention—a survey of stakeholder organizations. PLoS One. 2016;11:e0152084.
- , , , , , . Are sequential compression devices commonly associated with in‐hospital falls? A myth‐busters review using the patient safety net database. J Patient Saf. 2011;7:77–79.
- , , , , . No association between hospital‐reported perioperative venous thromboembolism prophylaxis and outcome rates in publicly reported data. JAMA Surg. 2014;149:400–401.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148:299–300.
- , , , et al. Hazards of benchmarking complications with the National Trauma Data Bank: numerators in search of denominators. J Trauma. 2008;64:273–277; discussion 277–279.
- , , , et al. Is the meaningful use venous thromboembolism VTE‐6 measure meaningful? A retrospective analysis of one hospital's VTE‐6 cases. Jt Comm J Qual Patient Saf. 2016;42(9):410–416.
- , , , et al. Is venous thromboembolism in colorectal surgery patients preventable or inevitable? One institution's experience. J Am Coll Surg. 2013;216:395–401.e1.
- , , , et al. Venous thromboembolic prophylaxis after a hepatic resection: patterns of care among liver surgeons. HPB (Oxford). 2014;16:892–898.
- , , , et al. Defining incidence and risk factors of venous thromboembolism after hepatectomy. J Gastrointest Surg. 2014;18:1116–1124.
- , , , et al. Can increased incidence of deep vein thrombosis (DVT) be used as a marker of quality of care in the absence of standardized screening? The potential effect of surveillance bias on reported DVT rates after trauma. J Trauma. 2007;63:1132–1135; discussion 1135–1137.
- , . Surveillance bias in outcomes reporting. JAMA. 2011;305:2462–2463.
- , , , et al. Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. J Trauma. 2008;64:932–936; discussion 936–937.
- , , , et al. Nonadministration of thromboprophylaxis in hospitalized patients with HIV: a missed opportunity for prevention? J Hosp Med. 2014;9:215–220.
- , , , et al. Pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Comparative effectiveness review No. 116. Prepared by the Johns Hopkins University Evidence‐based Practice Center under Contract No. 290‐2007‐10061‐I.) AHRQ Publication No. 13‐EHC082–1. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , et al. Pharmacologic and mechanical strategies for preventing venous thromboembolism after bariatric surgery: a systematic review and meta‐analysis. JAMA Surg. 2013;148:675–686.
- , , , et al. The effectiveness of prophylactic inferior vena cava filters in trauma patients: a systematic review and meta‐analysis. JAMA Surg. 2014;149:194–202.
Venous thromboembolism (VTE), which encompasses deep venous thrombosis and pulmonary embolism, is an important cause of preventable morbidity and mortality.[1] Each year it is estimated as many as 600,000 American's suffer VTE and as many as 100,000 die.[2] Consequently, patient safety and healthcare quality, accrediting organizations such as The Joint Commission, and federal agencies such as the Centers for Disease Control and Prevention and Agency for Healthcare Research and Quality (AHRQ) have made VTE prevention a priority.[3, 4, 5]
Despite widespread recognition that VTE prophylaxis is an important patient safety measure, poor performance is common. The ENDORSE (Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting) study of over 68,000 hospitalized patients in 32 countries noted only 58.5% of surgical patients and 39.5% medical patients received American College of Chest Physicians (ACCP) guideline‐appropriate VTE prophylaxis.[6] In 2005, an audit of the surgical services at The Johns Hopkins Hospital found that only 33% of 322 randomly selected patients were prescribed prophylaxis consistent with the ACCP guidelines.
Achieving defect‐free VTE prevention requires attention to each step in the process: (1) assessment of both VTE and bleeding risk, (2) prescription of risk‐appropriate VTE prophylaxis, and (3) administration of risk‐appropriate VTE prophylaxis. In 2005, to improve our VTE prevention performance at Johns Hopkins Hospital, the Center for Innovations organized a VTE Collaborative of 2 physicians, 1 nurse, and 1 pharmacist dedicated to VTE quality improvement. Since then, the group has grown dramatically, adding a clinical informatics expert and numerous other members and coming under the auspices of The Armstrong Institute for Patient Safety. Recognizing that many, though not all, VTEs are potentially preventable,[7, 8] the mission of the Johns Hopkins VTE Collaborative is to ensure that all hospitalized patients receive risk‐appropriate, best‐practice VTE prophylaxis. This article chronicles the innovative strategies that the Johns Hopkins VTE Collaborative has employed over the past decade to improve our hospital's performance in VTE prevention (Table 1).
|
| Strategies to improve VTE prophylaxis ordering |
| Paper‐based patient risk assessment forms (before computer order entry) |
| Mandatory evidence‐based specialty‐specific computer clinical decision support smart order sets |
| Group data and competitions |
| 1‐on‐1 provider feedback |
| Pay for performance |
| Individualized feedback with resident scorecards |
| Strategies to improve VTE prophylaxis administration |
| Identification of missed doses as a major contributor to preventable VTE |
| Identification of physician, nurse and patient contributors to missed doses |
| Collaboration with patients to create patient‐centered educational materials |
| Novel web‐based module for nursing education |
| Real‐time missed doses alert |
| Targeted 1‐on‐1 patient education |
ENSURING EVERY PATIENT IS PRESCRIBED RISK‐APPROPRIATE PROPHYLAXIS
With the support of hospital leadership, the VTE Collaborative held a series of events in 2005 with medical and surgical providers to review the current evidence supporting VTE prophylaxis and achieve consensus on appropriate practice based upon the 2004 ACCP VTE Prophylaxis Guideline. The result was the development of 5 evidence‐based, paper VTE prophylaxis order sets that guided the ordering provider on the assessment of VTE and bleeding risk and facilitated the selection of risk‐appropriate VTE prophylaxis. Because there were no validated VTE or bleeding risk assessment tools at the time we developed our order sets, we used specialty‐specific VTE risk factors derived from the 2004 ACCP Guideline. To identify patients inappropriate for pharmacologic prophylaxis, we used exclusion criteria derived from contemporary randomized clinical trials of pharmacologic prophylaxis in the target populations (ie, active bleeding, abnormal activated partial thromboplastin time not due to a lupus inhibitor) or mutually agreed upon thresholds after discussion with individual provider groups (platelet count <50,000/L). On the Johns Hopkins Hospital inpatient acute rehabilitation unit, introduction of the paper order sets increased adherence with ACCP guidelines from 27% to 98% (P < 0.0001) and reduced symptomatic VTE from 49 per 1000 admissions to 8 per 1000 admissions (P = 0.0001).[9] This study demonstrated that paper order sets used consistently by a dedicated group of providers can result in sustained improvements in practice. Paper order sets remain a low‐tech, easy‐to‐implement strategy that can be applied in any healthcare setting. Other services also saw improvements in risk‐appropriate prophylaxis prescription. In a follow‐up cross‐sectional analysis of the surgical services at Johns Hopkins, we found that appropriate VTE prophylaxis prescription improved from 33% to 62% in a sample of 226 patients. Unfortunately, paper order sets had several disadvantages including (1) the inherent difficulty of making them a mandatory part of the admission or transfer process, (2) their existence outside the usual clinical workflow, and (3) the labor‐ and time‐intensive data collection that made it difficult to provide credible, timely performance reports to providers and leadership.
These disadvantages and our adoption of a computerized provider order entry system prompted us to pursue the development and implementation of mandatory, evidence‐based, specialty‐specific computerized clinical decision support (CCDS) VTE prophylaxis order sets. Using the Translating Research Into Practice approach to quality improvement,[10] we collaborated with providers to design 16 different evidence‐based specialty‐specific CCDS VTE order sets. These CCDS VTE order sets, which are imbedded in the specialty‐specific admission and transfer order sets, assist providers in assessing patients' VTE and bleeding risk factors and provide evidence‐based risk‐appropriate VTE prophylaxis (see Supporting Figure 1 in the online version of this article). Individual patient data are saved in an administrative database and can be easily aggregated for research analyses and quality improvement/performance reporting. A detailed discussion of our strategy for change is discussed in Streiff et al.[11] Because pharmacologic prophylaxis is not appropriate for every patient, and not all VTE are preventable, even with perfect prophylaxis, the goals of our collaborative are to ensure that every patient is ordered VTE prophylaxis consistent with their risk profile (risk‐appropriate prophylaxis) and to eliminate preventable episodes of VTE (VTE that occurs in the setting of suboptimal prophylaxis). In a prepost quasi‐experimental study of 1599 trauma patients, the CCDS VTE order set increased risk‐appropriate prophylaxis prescription from 66.2% to 84.4% (P < .001) and reduced the incidence of potentially preventable harm from VTE from 1% to 0.17% (P = 0.04) (Figure 1).[12] On the medical service, the CCDS VTE order set improved risk‐appropriate VTE prophylaxis prescription from 65.6% to 90.1% (P < 0.0001) and reduced the incidence of potentially preventable harm attributable to VTE from 1.1% to 0% (P = 0.001). There was no increase in major bleeding (International Society of Thrombosis and Hemostasis definition: hemoglobin decline of 2 grams/dL or transfusion of 2 or more units of blood or bleeding into a critical organ such as brain, gastrointestinal tract, or eye) postorder set implementation (0.3% vs 0.1%, P = 0.625) or all‐cause mortality (1.3% vs 2.0%, P = 0.285).[13]

These order sets demonstrated that CCDS tools can lead to significant improvements in prescribing practices and reductions in preventable harm from VTE without increasing the risk of major bleeding complications. In addition to improving the quality of care, the order sets also improved the consistency of care. In a retrospective analysis, we found that implementation of CCDS VTE order sets eliminated racial disparities in prescribing practices. In the preimplementation group, risk‐appropriate VTE prophylaxis was prescribed for 70.1% of black patients and 56.6% of white patients on the trauma service (P = 0.025) and 69.5% of black patients and 61.7% of white patients on the medical service (P = 0.015). After implementation of the CCDS VTE order sets, care improved for all patients such that the previously observed disparities were eliminated (trauma service 84.5% vs 85.5%, P = 0.99 and medical service 91.8% vs 88.0%, P = 0.082).[14] These data indicate that standardizing care can potentially eliminate disparities in clinical practice.
Although implementation of mandatory evidence‐based, specialty‐specific CDSS VTE order sets led to substantial improvements in VTE prophylaxis ordering, high performance was not uniform across our institution. On the medical service, substantial disparities in adherence to order set recommendations existed. On the housestaff services, over 90% of patients consistently received risk‐appropriate VTE prophylaxis compared with only 85% on the hospitalist service. Examination of individual provider performance found that some providers only ordered risk‐appropriate prophylaxis 50% of the time, whereas others were doing so 98% of the time. To address this disparity, we conducted a retrospective analysis of a prospective performance improvement project conducted on the Johns Hopkins Hospitalist service studying the impact of individualized hospitalist attending feedback on VTE prevention practices. During the preintervention period (January 2009December 2010), guideline‐adherent VTE prophylaxis was ordered for 86% (95% confidence interval [CI]: 85%‐88%) of patients. Six months after initiation of direct face‐to‐face provider feedback (January 2011June 2011), guideline‐adherent VTE prophylaxis rates rose to 90% (95% CI: 88‐93). Subsequently (July 2011December 2012), a pay‐for‐performance (P4P) initiative was added to direct face‐to‐face provider feedback. During the P4P initiative, provider incentive per relative value unit (RVU) was progressively increased with increasing performance on provision of risk‐appropriate VTE prophylaxis (adherence <80% = no bonus to $0.50 per RVU for adherence 95%). During this period, prescription of guideline‐adherent prophylaxis rose to 94% (95% CI: 93%‐96%).[15] These initiatives transformed the hospitalist unit from a consistently low‐performance unit to a high‐performance unit.
Similar findings were noted on the trauma service. Although the original plan was to provide feedback to attending trauma surgeons, that plan changed when we found that performance was driven entirely by resident practice; residents write the VTE prophylaxis orders, which is then attributed to attending performance. Resident performance varied widely; 42 of 75 (56%) residents on the trauma service ordered risk‐appropriate prophylaxis for 100% of their patients. In contrast, 7 (9.3%) residents never ordered optimal prophylaxis for any of their patients.[16] To motivate all residents to prescribe optimal prophylaxis, we developed an individualized resident VTE prophylaxis scorecard (Figure 2). This prospective cohort study of 2420 patients and 49 general surgery residents compared resident VTE prophylaxis performance on the general surgery service during 3 periods: period 1 (baseline, July 2013September 2013), period 2 (surgery resident scorecard, October 2013December 2013), period 3 (resident scorecard plus individualized 1‐on‐1 coaching, January 2014March 2014). At baseline, 89.4% of patients were prescribed appropriate VTE prophylaxis, and only 45% of residents prescribed risk‐appropriate prophylaxis for all their patients. During the scorecard period, 95.4% of patients were prescribed risk‐appropriate VTE prophylaxis (P < 0.001). During the scorecard plus coaching period, risk‐appropriate prophylaxis rose to 96.4%. These prescribing practice changes were durable. During the 15 months prior to issuing scorecards, 88.0% of patients (3718/4226) were prescribed risk‐appropriate prophylaxis. After implementation, 95.8% of patients (3799/3966) were prescribed risk‐appropriate prophylaxis (P < 0.001) (see Supporting Figure 2 in the online version of this article). During the baseline period, 7 of 865 patients (0.81%) had a VTE during their hospital stay, of which 3 (0.35%) were potentially preventable. In contrast, none of the 3 of 784 patients who suffered VTE during the postimplementation period had a potentially preventable event (0.35% vs 0%, P = 0.046).[17] These studies demonstrate that providing physicians with their own specific data can be a powerful tool for performance improvement that may be applicable to many other quality and safety measures. Our group recently received funding from the AHRQ to scale this work to other residents, nurse practitioners, physician assistants, and attending physicians (1R01HS024547, Individualized Performance Feedback on Venous Thromboembolism Prevention Practice).

IMPROVING VTE PROPHYLAXIS ADMINISTRATION
Ordering VTE prophylaxis does not ensure its administration. We conducted a retrospective review of electronic administration records of 10,526 consecutive patients admitted over a 7‐month period at The Johns Hopkins Hospital. Twelve percent of the over 100,000 ordered doses of VTE prophylaxis were not administered, and the proportion of nonadministered doses on individual floors varied 5‐fold from 5.4% to 26.9%. The proportion of nonadministered doses was significantly higher on medical floors compared with all other services (17.5% vs 8.1%, odds ratio [OR]: 2.1 [95% CI: 2.0‐2.2]). Patient or family member refusal was the most common cause for nonadministered doses of VTE prophylaxis accounting for 59% of all missed doses. Eight percent of patients missed more than half their prescribed doses, and 5% of patients missed over 75% of ordered doses of VTE prophylaxis. Consistent with the Pareto principle, over 80% of the missed doses of prophylaxis were accounted for by just 20% of the patients.[18] A retrospective analysis of hospital‐acquired VTE at Johns Hopkins found that 39% of events occurred in patients who missed 1 or more doses of appropriate VTE prophylaxis.[19] Louis et al. noted that nonadministration of 1 dose of VTE prophylaxis was associated with a significant increase in risk for hospital acquired VTE.[20] These data indicate the need for more aggressive interventions to reduce missed doses to improve VTE prevention.
To fully understand the root causes of VTE prophylaxis non‐administration, we conducted a series of studies examining each of the participants in the VTE prevention care pathway, physicians, nurses, and patients. In a survey of 122 resident physicians, we found significant differences in clinical practice between medicine and surgery residents. Medicine residents were more likely to believe that VTE prophylaxis was overprescribed, and that it was appropriate for nurses to make judgement calls about whether patients needed the prophylaxis that was prescribed.[21] In a mixed methods study that included a written survey and qualitative observations of nursing practice, we found that some nurses presented pharmacologic VTE prophylaxis injections as optional to patients. Furthermore, nurses on units where nonadministration was higher were more likely to believe that VTE prophylaxis was prescribed for patients unnecessarily, and that they could use their clinical judgement to determine when it was appropriate to omit doses of pharmacologic prophylaxis.[22] Our team also examined patient preferences in regard to VTE prophylaxis. In a survey of 227 consecutive medical and surgical inpatients, we found that 60% of patients would prefer an oral route of administration if available. Patients with a preference for a parenteral route of administration (27.5%) were less likely to refuse prophylaxis (37.5% vs 51.3%, P < 0.0001).[23] These findings underscore the fact that unit culture, nursing attitudes and beliefs, and patient preferences have an important influence on medication administration, and that nursepatient communication is an important target for modifying adherence.
PATIENT‐CENTERED APPROACHES TO IMPROVE VTE PROPHYLAXIS ADMINISTRATION
To address nurse‐ and patient‐related factors that influence VTE prophylaxis administration, we applied for and received a Patient Centered Outcomes Research Institute contract to develop patient‐centered interventions to engage and empower patients to take an active role in their preventive care. To achieve these aims, we partnered with 3 national patient advocacy organizations, the National Blood Clot Alliance, the North American Thrombosis Forum, and ClotCare, as well as our local Johns Hopkins Patient and Family Advisory Council. Using a modified Delphi method, we engaged patient stakeholders from the 4 collaborating organizations to build consensus on patient‐centered VTE education methods. Input from this Delphi assessment was used to build educational materials including paper brochures published in 8 different languages and a 10‐minute educational video filmed by an Oscar‐winning documentary director featuring both clinicians and patients relating their VTE experience and the importance of VTE prevention.[24] These educational materials are available for public use (
ENGAGING TRAINEES IN MULTIDISCIPLINARY PATIENT SAFETY/QUALITY IMPROVEMENT INITIATIVES
Trainees from many healthcare‐related disciplines have played a critical role in our quest to improve VTE prevention. Over the past 10 years, we have mentored countless medical students, public health graduate students, nursing students, residents, and postdoctoral fellows in research projects that have resulted in numerous high‐quality publications. Trainees have helped to dispel staff concerns about patient falls in connection of intermittent pneumatic compression devices,[25] identify the weaknesses of current publicly reported VTE measures,[26, 27, 28, 29] identify opportunities to improve VTE prevention practices within clinical specialties,[30, 31, 32] define the role of surveillance bias in VTE outcomes reporting,[33, 34, 35] discover and fully explore the important problem of missed doses of VTE prophylaxis,[18, 21, 22, 23, 36] and summarize knowledge about VTE prevention via systematic reviews and meta‐analyses.[37, 38, 39] These collaborations have been a classic win‐win. The mentees learn critical skills while growing their curriculum vitae with contributions to the literature, allowing them to progress in their careers (ie, obtain a residency match, faculty positions). The faculty have leveraged this work to obtain over $3 million in extramural funding to develop interventions to study and improve the quality of VTE preventive care for hospitalized patients.
In healthcare, we have not yet achieved defect‐free VTE prevention; however, we have a better understanding of the path to accomplishing this goal. In this article we describe our goal of zero harm from VTE and our learning journey to realize that goal. Although the journey never ends, a critical ingredient to the success of our program has been the multidisciplinary nature of our VTE collaborative team. The combination of expertise from medicine, surgery, nursing, pharmacy, clinical informatics, and public health has facilitated the development of innovative strategies to improve VTE prevention that integrate seamlessly into clinical workflow. The approach used for VTE can be applied to eliminate other types of harms.
Disclosures
Mr. Lau, Dr. Streiff, and Dr. Haut are supported by a grant from the Agency for Healthcare Research and Quality (1R01HS024547) titled Individualized Performance Feedback on Venous Thromboembolism Prevention Practice and a contract from the Patient‐Centered Outcomes Research Institute titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Mr. Lau is supported by the Institute for Excellence in Education Berkheimer Faculty Education Scholar Grant and a contract (AD‐1306‐03980) from the Patient‐Centered Outcomes Research Institute titled Patient Centered Approaches to Collect Sexual Orientation/Gender Identity Information in the Emergency Department. Ms. Hobson has given expert witness testimony in various medical malpractice cases. Dr. Streiff has received research funding from Portola and Janssen; consulted for Bio2Medical, CSL Behring, Merck, and Janssen HealthCare; and has given expert witness testimony in various medical malpractice cases. Dr. Haut receives royalties from Lippincott, Williams, and Wilkins for a book titled Avoiding Common ICU Errors. Dr. Haut is a paid consultant and speaker for the Preventing Avoidable Venous ThromboembolismEvery Patient, Every Time VHA/Vizient IMPERATIV Advantage Performance Improvement Collaborative. Dr. Haut is a paid consultant and speaker for the Illinois Surgical Quality Improvement Collaborative. All other authors report no disclosures.
Venous thromboembolism (VTE), which encompasses deep venous thrombosis and pulmonary embolism, is an important cause of preventable morbidity and mortality.[1] Each year it is estimated as many as 600,000 American's suffer VTE and as many as 100,000 die.[2] Consequently, patient safety and healthcare quality, accrediting organizations such as The Joint Commission, and federal agencies such as the Centers for Disease Control and Prevention and Agency for Healthcare Research and Quality (AHRQ) have made VTE prevention a priority.[3, 4, 5]
Despite widespread recognition that VTE prophylaxis is an important patient safety measure, poor performance is common. The ENDORSE (Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting) study of over 68,000 hospitalized patients in 32 countries noted only 58.5% of surgical patients and 39.5% medical patients received American College of Chest Physicians (ACCP) guideline‐appropriate VTE prophylaxis.[6] In 2005, an audit of the surgical services at The Johns Hopkins Hospital found that only 33% of 322 randomly selected patients were prescribed prophylaxis consistent with the ACCP guidelines.
Achieving defect‐free VTE prevention requires attention to each step in the process: (1) assessment of both VTE and bleeding risk, (2) prescription of risk‐appropriate VTE prophylaxis, and (3) administration of risk‐appropriate VTE prophylaxis. In 2005, to improve our VTE prevention performance at Johns Hopkins Hospital, the Center for Innovations organized a VTE Collaborative of 2 physicians, 1 nurse, and 1 pharmacist dedicated to VTE quality improvement. Since then, the group has grown dramatically, adding a clinical informatics expert and numerous other members and coming under the auspices of The Armstrong Institute for Patient Safety. Recognizing that many, though not all, VTEs are potentially preventable,[7, 8] the mission of the Johns Hopkins VTE Collaborative is to ensure that all hospitalized patients receive risk‐appropriate, best‐practice VTE prophylaxis. This article chronicles the innovative strategies that the Johns Hopkins VTE Collaborative has employed over the past decade to improve our hospital's performance in VTE prevention (Table 1).
|
| Strategies to improve VTE prophylaxis ordering |
| Paper‐based patient risk assessment forms (before computer order entry) |
| Mandatory evidence‐based specialty‐specific computer clinical decision support smart order sets |
| Group data and competitions |
| 1‐on‐1 provider feedback |
| Pay for performance |
| Individualized feedback with resident scorecards |
| Strategies to improve VTE prophylaxis administration |
| Identification of missed doses as a major contributor to preventable VTE |
| Identification of physician, nurse and patient contributors to missed doses |
| Collaboration with patients to create patient‐centered educational materials |
| Novel web‐based module for nursing education |
| Real‐time missed doses alert |
| Targeted 1‐on‐1 patient education |
ENSURING EVERY PATIENT IS PRESCRIBED RISK‐APPROPRIATE PROPHYLAXIS
With the support of hospital leadership, the VTE Collaborative held a series of events in 2005 with medical and surgical providers to review the current evidence supporting VTE prophylaxis and achieve consensus on appropriate practice based upon the 2004 ACCP VTE Prophylaxis Guideline. The result was the development of 5 evidence‐based, paper VTE prophylaxis order sets that guided the ordering provider on the assessment of VTE and bleeding risk and facilitated the selection of risk‐appropriate VTE prophylaxis. Because there were no validated VTE or bleeding risk assessment tools at the time we developed our order sets, we used specialty‐specific VTE risk factors derived from the 2004 ACCP Guideline. To identify patients inappropriate for pharmacologic prophylaxis, we used exclusion criteria derived from contemporary randomized clinical trials of pharmacologic prophylaxis in the target populations (ie, active bleeding, abnormal activated partial thromboplastin time not due to a lupus inhibitor) or mutually agreed upon thresholds after discussion with individual provider groups (platelet count <50,000/L). On the Johns Hopkins Hospital inpatient acute rehabilitation unit, introduction of the paper order sets increased adherence with ACCP guidelines from 27% to 98% (P < 0.0001) and reduced symptomatic VTE from 49 per 1000 admissions to 8 per 1000 admissions (P = 0.0001).[9] This study demonstrated that paper order sets used consistently by a dedicated group of providers can result in sustained improvements in practice. Paper order sets remain a low‐tech, easy‐to‐implement strategy that can be applied in any healthcare setting. Other services also saw improvements in risk‐appropriate prophylaxis prescription. In a follow‐up cross‐sectional analysis of the surgical services at Johns Hopkins, we found that appropriate VTE prophylaxis prescription improved from 33% to 62% in a sample of 226 patients. Unfortunately, paper order sets had several disadvantages including (1) the inherent difficulty of making them a mandatory part of the admission or transfer process, (2) their existence outside the usual clinical workflow, and (3) the labor‐ and time‐intensive data collection that made it difficult to provide credible, timely performance reports to providers and leadership.
These disadvantages and our adoption of a computerized provider order entry system prompted us to pursue the development and implementation of mandatory, evidence‐based, specialty‐specific computerized clinical decision support (CCDS) VTE prophylaxis order sets. Using the Translating Research Into Practice approach to quality improvement,[10] we collaborated with providers to design 16 different evidence‐based specialty‐specific CCDS VTE order sets. These CCDS VTE order sets, which are imbedded in the specialty‐specific admission and transfer order sets, assist providers in assessing patients' VTE and bleeding risk factors and provide evidence‐based risk‐appropriate VTE prophylaxis (see Supporting Figure 1 in the online version of this article). Individual patient data are saved in an administrative database and can be easily aggregated for research analyses and quality improvement/performance reporting. A detailed discussion of our strategy for change is discussed in Streiff et al.[11] Because pharmacologic prophylaxis is not appropriate for every patient, and not all VTE are preventable, even with perfect prophylaxis, the goals of our collaborative are to ensure that every patient is ordered VTE prophylaxis consistent with their risk profile (risk‐appropriate prophylaxis) and to eliminate preventable episodes of VTE (VTE that occurs in the setting of suboptimal prophylaxis). In a prepost quasi‐experimental study of 1599 trauma patients, the CCDS VTE order set increased risk‐appropriate prophylaxis prescription from 66.2% to 84.4% (P < .001) and reduced the incidence of potentially preventable harm from VTE from 1% to 0.17% (P = 0.04) (Figure 1).[12] On the medical service, the CCDS VTE order set improved risk‐appropriate VTE prophylaxis prescription from 65.6% to 90.1% (P < 0.0001) and reduced the incidence of potentially preventable harm attributable to VTE from 1.1% to 0% (P = 0.001). There was no increase in major bleeding (International Society of Thrombosis and Hemostasis definition: hemoglobin decline of 2 grams/dL or transfusion of 2 or more units of blood or bleeding into a critical organ such as brain, gastrointestinal tract, or eye) postorder set implementation (0.3% vs 0.1%, P = 0.625) or all‐cause mortality (1.3% vs 2.0%, P = 0.285).[13]

These order sets demonstrated that CCDS tools can lead to significant improvements in prescribing practices and reductions in preventable harm from VTE without increasing the risk of major bleeding complications. In addition to improving the quality of care, the order sets also improved the consistency of care. In a retrospective analysis, we found that implementation of CCDS VTE order sets eliminated racial disparities in prescribing practices. In the preimplementation group, risk‐appropriate VTE prophylaxis was prescribed for 70.1% of black patients and 56.6% of white patients on the trauma service (P = 0.025) and 69.5% of black patients and 61.7% of white patients on the medical service (P = 0.015). After implementation of the CCDS VTE order sets, care improved for all patients such that the previously observed disparities were eliminated (trauma service 84.5% vs 85.5%, P = 0.99 and medical service 91.8% vs 88.0%, P = 0.082).[14] These data indicate that standardizing care can potentially eliminate disparities in clinical practice.
Although implementation of mandatory evidence‐based, specialty‐specific CDSS VTE order sets led to substantial improvements in VTE prophylaxis ordering, high performance was not uniform across our institution. On the medical service, substantial disparities in adherence to order set recommendations existed. On the housestaff services, over 90% of patients consistently received risk‐appropriate VTE prophylaxis compared with only 85% on the hospitalist service. Examination of individual provider performance found that some providers only ordered risk‐appropriate prophylaxis 50% of the time, whereas others were doing so 98% of the time. To address this disparity, we conducted a retrospective analysis of a prospective performance improvement project conducted on the Johns Hopkins Hospitalist service studying the impact of individualized hospitalist attending feedback on VTE prevention practices. During the preintervention period (January 2009December 2010), guideline‐adherent VTE prophylaxis was ordered for 86% (95% confidence interval [CI]: 85%‐88%) of patients. Six months after initiation of direct face‐to‐face provider feedback (January 2011June 2011), guideline‐adherent VTE prophylaxis rates rose to 90% (95% CI: 88‐93). Subsequently (July 2011December 2012), a pay‐for‐performance (P4P) initiative was added to direct face‐to‐face provider feedback. During the P4P initiative, provider incentive per relative value unit (RVU) was progressively increased with increasing performance on provision of risk‐appropriate VTE prophylaxis (adherence <80% = no bonus to $0.50 per RVU for adherence 95%). During this period, prescription of guideline‐adherent prophylaxis rose to 94% (95% CI: 93%‐96%).[15] These initiatives transformed the hospitalist unit from a consistently low‐performance unit to a high‐performance unit.
Similar findings were noted on the trauma service. Although the original plan was to provide feedback to attending trauma surgeons, that plan changed when we found that performance was driven entirely by resident practice; residents write the VTE prophylaxis orders, which is then attributed to attending performance. Resident performance varied widely; 42 of 75 (56%) residents on the trauma service ordered risk‐appropriate prophylaxis for 100% of their patients. In contrast, 7 (9.3%) residents never ordered optimal prophylaxis for any of their patients.[16] To motivate all residents to prescribe optimal prophylaxis, we developed an individualized resident VTE prophylaxis scorecard (Figure 2). This prospective cohort study of 2420 patients and 49 general surgery residents compared resident VTE prophylaxis performance on the general surgery service during 3 periods: period 1 (baseline, July 2013September 2013), period 2 (surgery resident scorecard, October 2013December 2013), period 3 (resident scorecard plus individualized 1‐on‐1 coaching, January 2014March 2014). At baseline, 89.4% of patients were prescribed appropriate VTE prophylaxis, and only 45% of residents prescribed risk‐appropriate prophylaxis for all their patients. During the scorecard period, 95.4% of patients were prescribed risk‐appropriate VTE prophylaxis (P < 0.001). During the scorecard plus coaching period, risk‐appropriate prophylaxis rose to 96.4%. These prescribing practice changes were durable. During the 15 months prior to issuing scorecards, 88.0% of patients (3718/4226) were prescribed risk‐appropriate prophylaxis. After implementation, 95.8% of patients (3799/3966) were prescribed risk‐appropriate prophylaxis (P < 0.001) (see Supporting Figure 2 in the online version of this article). During the baseline period, 7 of 865 patients (0.81%) had a VTE during their hospital stay, of which 3 (0.35%) were potentially preventable. In contrast, none of the 3 of 784 patients who suffered VTE during the postimplementation period had a potentially preventable event (0.35% vs 0%, P = 0.046).[17] These studies demonstrate that providing physicians with their own specific data can be a powerful tool for performance improvement that may be applicable to many other quality and safety measures. Our group recently received funding from the AHRQ to scale this work to other residents, nurse practitioners, physician assistants, and attending physicians (1R01HS024547, Individualized Performance Feedback on Venous Thromboembolism Prevention Practice).

IMPROVING VTE PROPHYLAXIS ADMINISTRATION
Ordering VTE prophylaxis does not ensure its administration. We conducted a retrospective review of electronic administration records of 10,526 consecutive patients admitted over a 7‐month period at The Johns Hopkins Hospital. Twelve percent of the over 100,000 ordered doses of VTE prophylaxis were not administered, and the proportion of nonadministered doses on individual floors varied 5‐fold from 5.4% to 26.9%. The proportion of nonadministered doses was significantly higher on medical floors compared with all other services (17.5% vs 8.1%, odds ratio [OR]: 2.1 [95% CI: 2.0‐2.2]). Patient or family member refusal was the most common cause for nonadministered doses of VTE prophylaxis accounting for 59% of all missed doses. Eight percent of patients missed more than half their prescribed doses, and 5% of patients missed over 75% of ordered doses of VTE prophylaxis. Consistent with the Pareto principle, over 80% of the missed doses of prophylaxis were accounted for by just 20% of the patients.[18] A retrospective analysis of hospital‐acquired VTE at Johns Hopkins found that 39% of events occurred in patients who missed 1 or more doses of appropriate VTE prophylaxis.[19] Louis et al. noted that nonadministration of 1 dose of VTE prophylaxis was associated with a significant increase in risk for hospital acquired VTE.[20] These data indicate the need for more aggressive interventions to reduce missed doses to improve VTE prevention.
To fully understand the root causes of VTE prophylaxis non‐administration, we conducted a series of studies examining each of the participants in the VTE prevention care pathway, physicians, nurses, and patients. In a survey of 122 resident physicians, we found significant differences in clinical practice between medicine and surgery residents. Medicine residents were more likely to believe that VTE prophylaxis was overprescribed, and that it was appropriate for nurses to make judgement calls about whether patients needed the prophylaxis that was prescribed.[21] In a mixed methods study that included a written survey and qualitative observations of nursing practice, we found that some nurses presented pharmacologic VTE prophylaxis injections as optional to patients. Furthermore, nurses on units where nonadministration was higher were more likely to believe that VTE prophylaxis was prescribed for patients unnecessarily, and that they could use their clinical judgement to determine when it was appropriate to omit doses of pharmacologic prophylaxis.[22] Our team also examined patient preferences in regard to VTE prophylaxis. In a survey of 227 consecutive medical and surgical inpatients, we found that 60% of patients would prefer an oral route of administration if available. Patients with a preference for a parenteral route of administration (27.5%) were less likely to refuse prophylaxis (37.5% vs 51.3%, P < 0.0001).[23] These findings underscore the fact that unit culture, nursing attitudes and beliefs, and patient preferences have an important influence on medication administration, and that nursepatient communication is an important target for modifying adherence.
PATIENT‐CENTERED APPROACHES TO IMPROVE VTE PROPHYLAXIS ADMINISTRATION
To address nurse‐ and patient‐related factors that influence VTE prophylaxis administration, we applied for and received a Patient Centered Outcomes Research Institute contract to develop patient‐centered interventions to engage and empower patients to take an active role in their preventive care. To achieve these aims, we partnered with 3 national patient advocacy organizations, the National Blood Clot Alliance, the North American Thrombosis Forum, and ClotCare, as well as our local Johns Hopkins Patient and Family Advisory Council. Using a modified Delphi method, we engaged patient stakeholders from the 4 collaborating organizations to build consensus on patient‐centered VTE education methods. Input from this Delphi assessment was used to build educational materials including paper brochures published in 8 different languages and a 10‐minute educational video filmed by an Oscar‐winning documentary director featuring both clinicians and patients relating their VTE experience and the importance of VTE prevention.[24] These educational materials are available for public use (
ENGAGING TRAINEES IN MULTIDISCIPLINARY PATIENT SAFETY/QUALITY IMPROVEMENT INITIATIVES
Trainees from many healthcare‐related disciplines have played a critical role in our quest to improve VTE prevention. Over the past 10 years, we have mentored countless medical students, public health graduate students, nursing students, residents, and postdoctoral fellows in research projects that have resulted in numerous high‐quality publications. Trainees have helped to dispel staff concerns about patient falls in connection of intermittent pneumatic compression devices,[25] identify the weaknesses of current publicly reported VTE measures,[26, 27, 28, 29] identify opportunities to improve VTE prevention practices within clinical specialties,[30, 31, 32] define the role of surveillance bias in VTE outcomes reporting,[33, 34, 35] discover and fully explore the important problem of missed doses of VTE prophylaxis,[18, 21, 22, 23, 36] and summarize knowledge about VTE prevention via systematic reviews and meta‐analyses.[37, 38, 39] These collaborations have been a classic win‐win. The mentees learn critical skills while growing their curriculum vitae with contributions to the literature, allowing them to progress in their careers (ie, obtain a residency match, faculty positions). The faculty have leveraged this work to obtain over $3 million in extramural funding to develop interventions to study and improve the quality of VTE preventive care for hospitalized patients.
In healthcare, we have not yet achieved defect‐free VTE prevention; however, we have a better understanding of the path to accomplishing this goal. In this article we describe our goal of zero harm from VTE and our learning journey to realize that goal. Although the journey never ends, a critical ingredient to the success of our program has been the multidisciplinary nature of our VTE collaborative team. The combination of expertise from medicine, surgery, nursing, pharmacy, clinical informatics, and public health has facilitated the development of innovative strategies to improve VTE prevention that integrate seamlessly into clinical workflow. The approach used for VTE can be applied to eliminate other types of harms.
Disclosures
Mr. Lau, Dr. Streiff, and Dr. Haut are supported by a grant from the Agency for Healthcare Research and Quality (1R01HS024547) titled Individualized Performance Feedback on Venous Thromboembolism Prevention Practice and a contract from the Patient‐Centered Outcomes Research Institute titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Mr. Lau is supported by the Institute for Excellence in Education Berkheimer Faculty Education Scholar Grant and a contract (AD‐1306‐03980) from the Patient‐Centered Outcomes Research Institute titled Patient Centered Approaches to Collect Sexual Orientation/Gender Identity Information in the Emergency Department. Ms. Hobson has given expert witness testimony in various medical malpractice cases. Dr. Streiff has received research funding from Portola and Janssen; consulted for Bio2Medical, CSL Behring, Merck, and Janssen HealthCare; and has given expert witness testimony in various medical malpractice cases. Dr. Haut receives royalties from Lippincott, Williams, and Wilkins for a book titled Avoiding Common ICU Errors. Dr. Haut is a paid consultant and speaker for the Preventing Avoidable Venous ThromboembolismEvery Patient, Every Time VHA/Vizient IMPERATIV Advantage Performance Improvement Collaborative. Dr. Haut is a paid consultant and speaker for the Illinois Surgical Quality Improvement Collaborative. All other authors report no disclosures.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- Office of the Surgeon General (US); National Heart, Lung, and Blood Institute (US). The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008.
- , . Prevention of venous thromboembolism: brief update review. In: Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , et al. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med. 2013;158:365–368.
- , . Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23:187–195.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371:387–394.
- , . The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009;301:1063–1065.
- , , , et al. ICD‐9 code‐based venous thromboembolism performance targets fail to measure up. Am J Med Qual. 2016;31(5):448–453.
- , , , , . Evidence‐based venous thromboembolism prophylaxis is associated with a six‐fold decrease in numbers of symptomatic venous thromboembolisms in rehabilitation inpatients. PM R. 2011;3:1111–1115.e1.
- , , . Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;147:901–907.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , , , et al. Eliminating health care disparities with mandatory clinical decision support: the venous thromboembolism (VTE) example. Med Care. 2015;53:18–24.
- , , , et al. Use of provider‐level dashboards and pay‐for‐performance in venous thromboembolism prophylaxis. J Hosp Med. 2015;10:172–178.
- , , , , , . Attending physician performance measure scores and resident physicians' ordering practices. JAMA Surg. 2015;150:813–814.
- , , , et al. Individualized performance feedback to surgical residents improves appropriate venous thromboembolism prophylaxis prescription and reduces potentially preventable VTE: a prospective cohort study [published online November 25, 2015]. Ann Surg. doi: 10.1097/SLA.0000000000001512.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8:e66311.
- , , , et al. Preventability of hospital‐acquired venous thromboembolism. JAMA Surg. 2015;150(9):912–915.
- , , , et al. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 2014;149:365–370.
- , , , et al. Prescriber knowledge and attitudes regarding non‐administration of prescribed pharmacologic venous thromboembolism prophylaxis [published online May 21, 2016]. J Thromb Thrombolysis. doi:10.1007/s11239-016-1378-8.
- , , , et al. Hidden barriers to delivery of pharmacological venous thromboembolism prophylaxis: the role of nursing beliefs and practices. J Patient Saf. 2016;12:63–68.
- , , , et al. Patient preferences regarding pharmacologic venous thromboembolism prophylaxis. J Hosp Med. 2015;10:108–111.
- , , , et al. Patient preferences for receiving education on venous thromboembolism prevention—a survey of stakeholder organizations. PLoS One. 2016;11:e0152084.
- , , , , , . Are sequential compression devices commonly associated with in‐hospital falls? A myth‐busters review using the patient safety net database. J Patient Saf. 2011;7:77–79.
- , , , , . No association between hospital‐reported perioperative venous thromboembolism prophylaxis and outcome rates in publicly reported data. JAMA Surg. 2014;149:400–401.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148:299–300.
- , , , et al. Hazards of benchmarking complications with the National Trauma Data Bank: numerators in search of denominators. J Trauma. 2008;64:273–277; discussion 277–279.
- , , , et al. Is the meaningful use venous thromboembolism VTE‐6 measure meaningful? A retrospective analysis of one hospital's VTE‐6 cases. Jt Comm J Qual Patient Saf. 2016;42(9):410–416.
- , , , et al. Is venous thromboembolism in colorectal surgery patients preventable or inevitable? One institution's experience. J Am Coll Surg. 2013;216:395–401.e1.
- , , , et al. Venous thromboembolic prophylaxis after a hepatic resection: patterns of care among liver surgeons. HPB (Oxford). 2014;16:892–898.
- , , , et al. Defining incidence and risk factors of venous thromboembolism after hepatectomy. J Gastrointest Surg. 2014;18:1116–1124.
- , , , et al. Can increased incidence of deep vein thrombosis (DVT) be used as a marker of quality of care in the absence of standardized screening? The potential effect of surveillance bias on reported DVT rates after trauma. J Trauma. 2007;63:1132–1135; discussion 1135–1137.
- , . Surveillance bias in outcomes reporting. JAMA. 2011;305:2462–2463.
- , , , et al. Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. J Trauma. 2008;64:932–936; discussion 936–937.
- , , , et al. Nonadministration of thromboprophylaxis in hospitalized patients with HIV: a missed opportunity for prevention? J Hosp Med. 2014;9:215–220.
- , , , et al. Pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Comparative effectiveness review No. 116. Prepared by the Johns Hopkins University Evidence‐based Practice Center under Contract No. 290‐2007‐10061‐I.) AHRQ Publication No. 13‐EHC082–1. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , et al. Pharmacologic and mechanical strategies for preventing venous thromboembolism after bariatric surgery: a systematic review and meta‐analysis. JAMA Surg. 2013;148:675–686.
- , , , et al. The effectiveness of prophylactic inferior vena cava filters in trauma patients: a systematic review and meta‐analysis. JAMA Surg. 2014;149:194–202.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- Office of the Surgeon General (US); National Heart, Lung, and Blood Institute (US). The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008.
- , . Prevention of venous thromboembolism: brief update review. In: Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , et al. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med. 2013;158:365–368.
- , . Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23:187–195.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371:387–394.
- , . The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009;301:1063–1065.
- , , , et al. ICD‐9 code‐based venous thromboembolism performance targets fail to measure up. Am J Med Qual. 2016;31(5):448–453.
- , , , , . Evidence‐based venous thromboembolism prophylaxis is associated with a six‐fold decrease in numbers of symptomatic venous thromboembolisms in rehabilitation inpatients. PM R. 2011;3:1111–1115.e1.
- , , . Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;147:901–907.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , , , et al. Eliminating health care disparities with mandatory clinical decision support: the venous thromboembolism (VTE) example. Med Care. 2015;53:18–24.
- , , , et al. Use of provider‐level dashboards and pay‐for‐performance in venous thromboembolism prophylaxis. J Hosp Med. 2015;10:172–178.
- , , , , , . Attending physician performance measure scores and resident physicians' ordering practices. JAMA Surg. 2015;150:813–814.
- , , , et al. Individualized performance feedback to surgical residents improves appropriate venous thromboembolism prophylaxis prescription and reduces potentially preventable VTE: a prospective cohort study [published online November 25, 2015]. Ann Surg. doi: 10.1097/SLA.0000000000001512.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8:e66311.
- , , , et al. Preventability of hospital‐acquired venous thromboembolism. JAMA Surg. 2015;150(9):912–915.
- , , , et al. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 2014;149:365–370.
- , , , et al. Prescriber knowledge and attitudes regarding non‐administration of prescribed pharmacologic venous thromboembolism prophylaxis [published online May 21, 2016]. J Thromb Thrombolysis. doi:10.1007/s11239-016-1378-8.
- , , , et al. Hidden barriers to delivery of pharmacological venous thromboembolism prophylaxis: the role of nursing beliefs and practices. J Patient Saf. 2016;12:63–68.
- , , , et al. Patient preferences regarding pharmacologic venous thromboembolism prophylaxis. J Hosp Med. 2015;10:108–111.
- , , , et al. Patient preferences for receiving education on venous thromboembolism prevention—a survey of stakeholder organizations. PLoS One. 2016;11:e0152084.
- , , , , , . Are sequential compression devices commonly associated with in‐hospital falls? A myth‐busters review using the patient safety net database. J Patient Saf. 2011;7:77–79.
- , , , , . No association between hospital‐reported perioperative venous thromboembolism prophylaxis and outcome rates in publicly reported data. JAMA Surg. 2014;149:400–401.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148:299–300.
- , , , et al. Hazards of benchmarking complications with the National Trauma Data Bank: numerators in search of denominators. J Trauma. 2008;64:273–277; discussion 277–279.
- , , , et al. Is the meaningful use venous thromboembolism VTE‐6 measure meaningful? A retrospective analysis of one hospital's VTE‐6 cases. Jt Comm J Qual Patient Saf. 2016;42(9):410–416.
- , , , et al. Is venous thromboembolism in colorectal surgery patients preventable or inevitable? One institution's experience. J Am Coll Surg. 2013;216:395–401.e1.
- , , , et al. Venous thromboembolic prophylaxis after a hepatic resection: patterns of care among liver surgeons. HPB (Oxford). 2014;16:892–898.
- , , , et al. Defining incidence and risk factors of venous thromboembolism after hepatectomy. J Gastrointest Surg. 2014;18:1116–1124.
- , , , et al. Can increased incidence of deep vein thrombosis (DVT) be used as a marker of quality of care in the absence of standardized screening? The potential effect of surveillance bias on reported DVT rates after trauma. J Trauma. 2007;63:1132–1135; discussion 1135–1137.
- , . Surveillance bias in outcomes reporting. JAMA. 2011;305:2462–2463.
- , , , et al. Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. J Trauma. 2008;64:932–936; discussion 936–937.
- , , , et al. Nonadministration of thromboprophylaxis in hospitalized patients with HIV: a missed opportunity for prevention? J Hosp Med. 2014;9:215–220.
- , , , et al. Pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Comparative effectiveness review No. 116. Prepared by the Johns Hopkins University Evidence‐based Practice Center under Contract No. 290‐2007‐10061‐I.) AHRQ Publication No. 13‐EHC082–1. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , et al. Pharmacologic and mechanical strategies for preventing venous thromboembolism after bariatric surgery: a systematic review and meta‐analysis. JAMA Surg. 2013;148:675–686.
- , , , et al. The effectiveness of prophylactic inferior vena cava filters in trauma patients: a systematic review and meta‐analysis. JAMA Surg. 2014;149:194–202.
© 2016 Society of Hospital Medicine
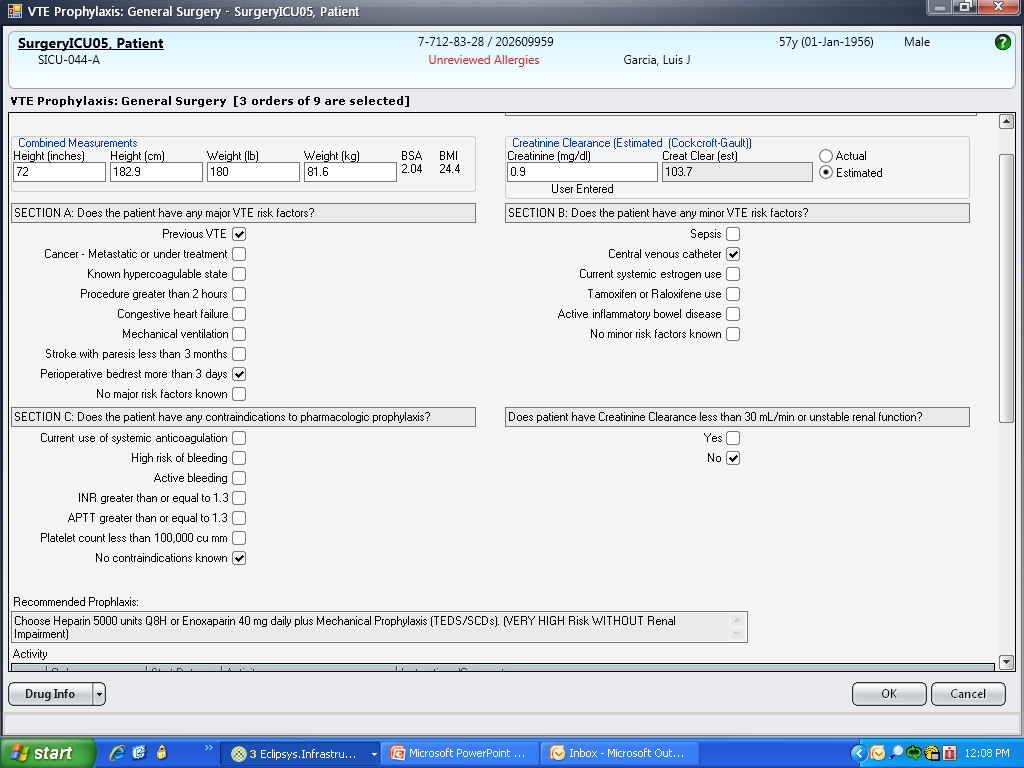
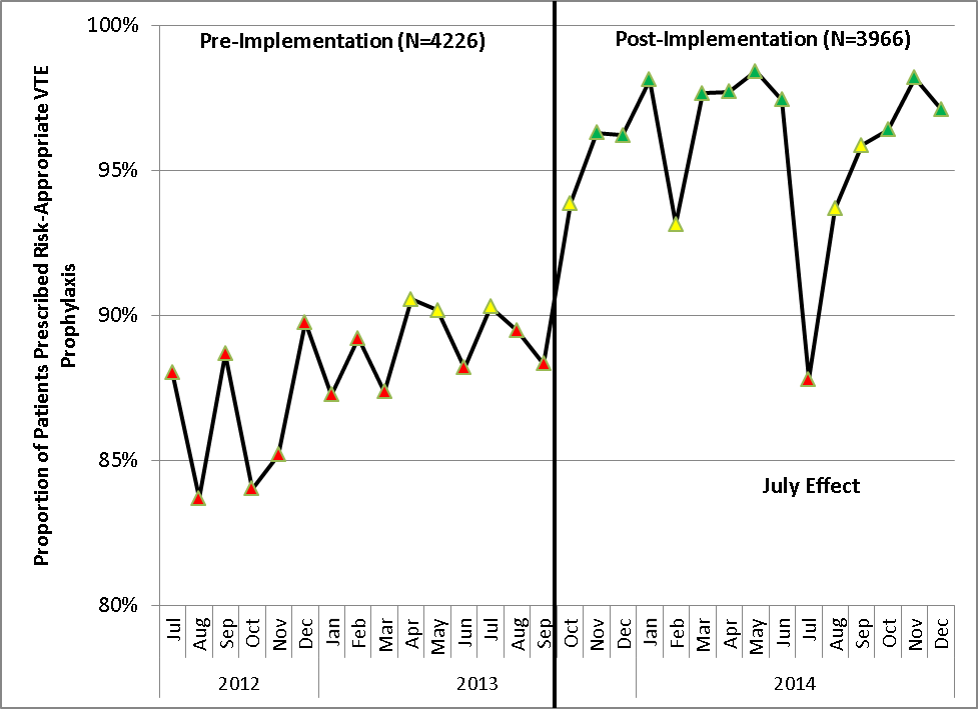
Prevention of Venous Thromboembolism
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a serious and growing public health problem. In the United States an estimated 900,000 people are affected and more than 100,000 die from VTE or related complications each year. More than half of VTE events occur in association with hospitalization or major surgery; many are thought to be preventable.[1, 2, 3, 4, 5] The Centers for Medicare and Medicaid Services (CMS), Centers for Disease Control and Prevention (CDC), and the Agency for Healthcare Research and Quality (AHRQ),[6, 7, 8, 9] among other organizations, have identified VTE as a potentially preventable never event. Evidence‐based guidelines and resources exist to help support hospital‐acquired venous thromboembolism (HA‐VTE) prevention.[1, 2, 3, 4, 5, 6, 7, 8, 9, 10] Harborview Medical Center, a tertiary referral center with more than 17,000 patients hospitalized annually, many requiring surgery, serves one of the highest‐risk populations for HA‐VTE development. Despite high rates of VTE prophylaxis in accordance with an established institutional guideline,[11, 12] VTE remains the most common hospital‐acquired condition in our institution.
OBJECTIVES
To improve the safety and care of all patients in our medical center and eliminate preventable HA‐VTE events, we set out to: (1) incorporate evidence‐based best practices in VTE prevention and treatment into current practice in alignment with institutional guidelines, (2) standardize the review process for all HA‐VTE events to identify opportunities for improvement, (3) utilize quality improvement (QI) analytics and information technology (IT) to actively improve our processes at the point of care, and (4) share process and outcome performance relating to VTE prevention transparently across our institution
METHODS
To prevent HA‐VTE, we employ a multifactorial strategy that includes designated clinical leadership, active engagement of all care team members, decision support tools embedded in the electronic health record (EHR), QI analytics, and retrospective and prospective reporting that provides ongoing measurement and analysis of the effectiveness of implemented interventions.
Setting/Patients
Harborview Medical Center, a 413‐bed academic tertiary referral center and the only level 1 adult and pediatric trauma and burn center for a 5‐state area, also serves as the primary safety‐net provider in the region. Harborview has centers of excellence in trauma, neurosciences, orthopedic and vascular surgery and rehabilitation, and is the only certified comprehensive stroke center in 5 states. With more than 17,000 admissions annually, including over 6000 trauma cases, HA‐VTE is a disease that spans critical and acute care settings and impacts patients on all clinical services. Harborview serves a population that is at extremely high risk for VTE as well as bleeding, particularly patients who have sustained central nervous system trauma or polytrauma.
Intervention
In 2010, at the request of the Harborview Medical Executive Board and Medical Director, we formed the Harborview VTE Task Force to assess VTE prevention practices across services and identify improvement opportunities for all hospitalized patients. This multidisciplinary team, co‐chaired by a hospitalist and trauma surgeon, includes representatives from trauma/general surgery, orthopedic surgery, hospital medicine, nursing, pharmacy, and QI. Task force members represent critical and acute care as well as the ambulatory setting. Additional stakeholders and local experts including IT directors and analysts, continuity of care nurses, and other clinical service representatives participate on an ad hoc basis.
Since its inception, the VTE Task Force has met monthly to review performance data and develop improvement initiatives. Initially we collaborated with experts across our health system to update an existing institutional VTE prophylaxis guideline to reflect current evidence‐based standards.[1, 3, 4, 5, 12] We met with all clinical services to ensure that the guidelines incorporated departmental best practices. These guidelines were integrated into our Cerner‐based (Cerner Corp., North Kansas City, MO) computerized provider order entry (CPOE) system to support accurate VTE risk assessment and appropriate ordering of prophylaxis.
The VTE Task Force collaborated with QI programmers to develop an electronic tool, the Harborview VTE Tool (Figure 1),[13] that allows for efficient, standardized review of all HA‐VTE at monthly meetings. The tool uses word and phrase search capabilities to identify PEs and DVTs from imaging and vascular studies and links those events with pertinent demographic and clinical data from the EHR in a timeline. Information about VTE risk assigned by physicians in the CPOE system is extracted as well as specific VTE prophylaxis and treatment (drug, dose, timing of administration of medications, reason for doses being held, and orders for and application of mechanical prophylaxis). Using the VTE tool, the task force reviews each VTE event to assess the accuracy of VTE risk assignment, the appropriateness of prophylaxis received relative to guidelines, and the adequacy of VTE treatment and follow‐up. This tool has facilitated our review process, decreasing time from >30 minutes of manual chart review per event to several minutes. In recent months, a quality analyst has prescreened all VTEs prior to task force discussion to further improve efficiency. The tool allows the team to assess the case together and reach consensus regarding VTE prevention.
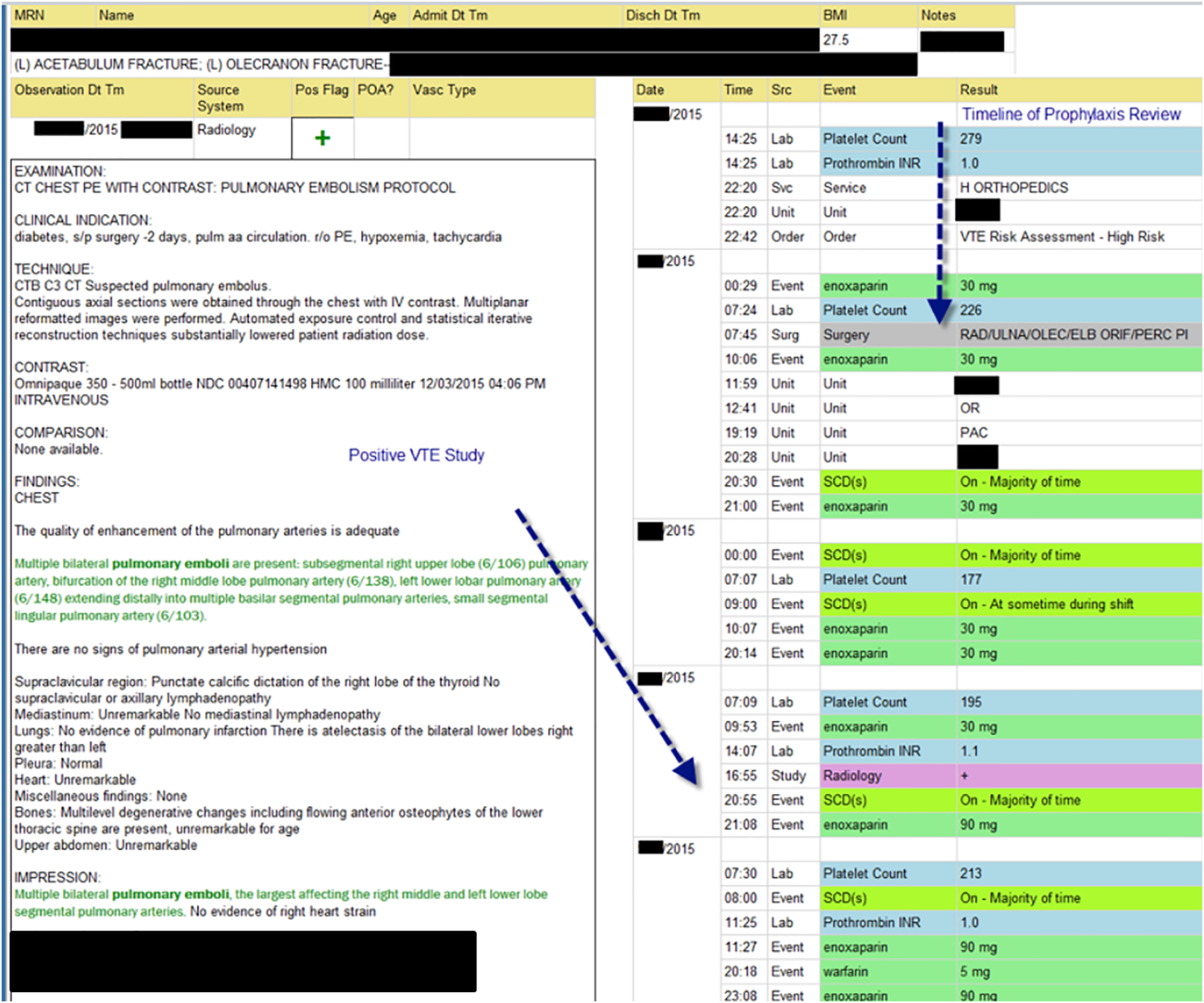
Prompt event reviews allow the task force to provide timely feedback about specific VTE events to physicians, nurses, and pharmacists. Cases with potential opportunities for improvement are referred to a medical center‐wide QI committee for secondary review. Areas of opportunity identified are tracked and trended to direct ongoing system improvement cycles. In 2014, as a result of reviewing patient cases with VTE diagnosed after discharge, we began a similar review process to assess current practice and standardize prophylaxis across care transitions.
In response to opportunities identified from reviews, the VTE Task Force developed multiple reporting tools that provide real‐time, actionable information to clinicians at the bedside. Daily electronic lists highlight patients who have not received chemical or mechanical prophylaxis in 24 hours and are utilized by nursing, pharmacy, and physician groups. Patients receiving new start vitamin K antagonists or direct oral anticoagulants are identified for pharmacists and discharge care coordinators to support early patient/family education and ensure appropriate follow‐up. Based on input from frontline providers, tools are continually refined to improve their clinical utility. A timeline of initiatives that the Harborview VTE Task Force has championed is outlined in Figure 2.
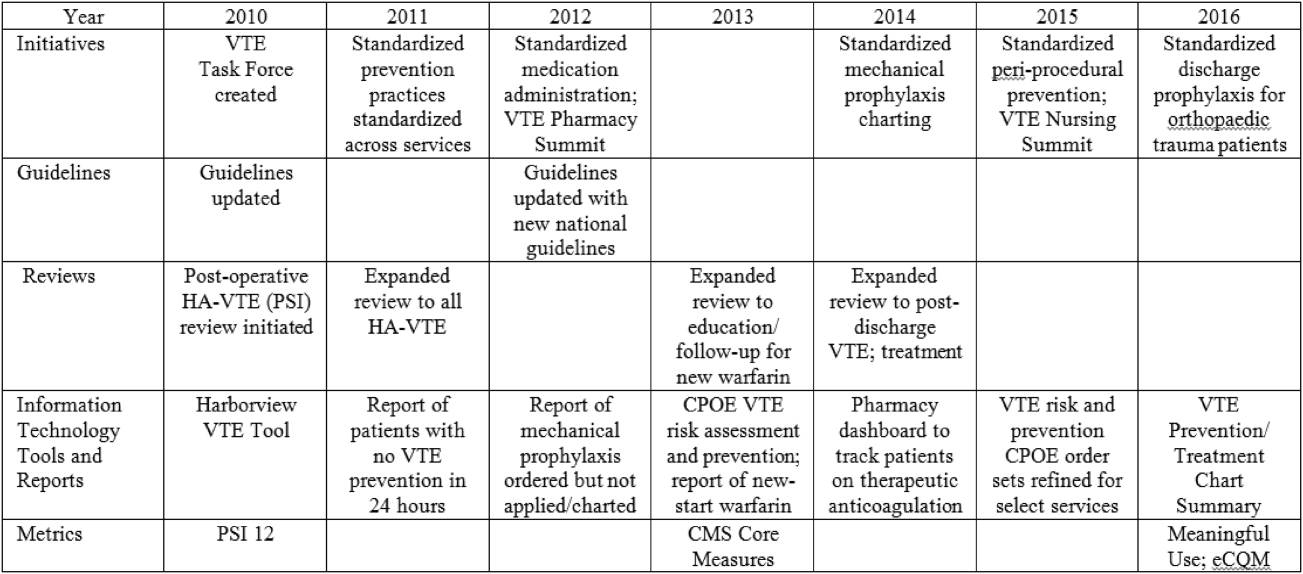
To bring HA‐VTE prevention information to the point of care, we developed a VTE Prevention/Treatment Summary within the EHR (Figure 3). Information about VTE risk assigned by the physician based on guidelines, current prophylaxis orders (pharmacologic/nonpharmacologic) and administration status, therapeutic anticoagulation and pertinent laboratory values are imported into a summary snapshot that can be accessed on demand by any member of the care team from within the patient's chart. The same data elements are being imbedded in resident physician and nursing handoff tools to highlight VTE prevention for all hospitalized patients and ensure optimal prophylaxis at transitions of care.
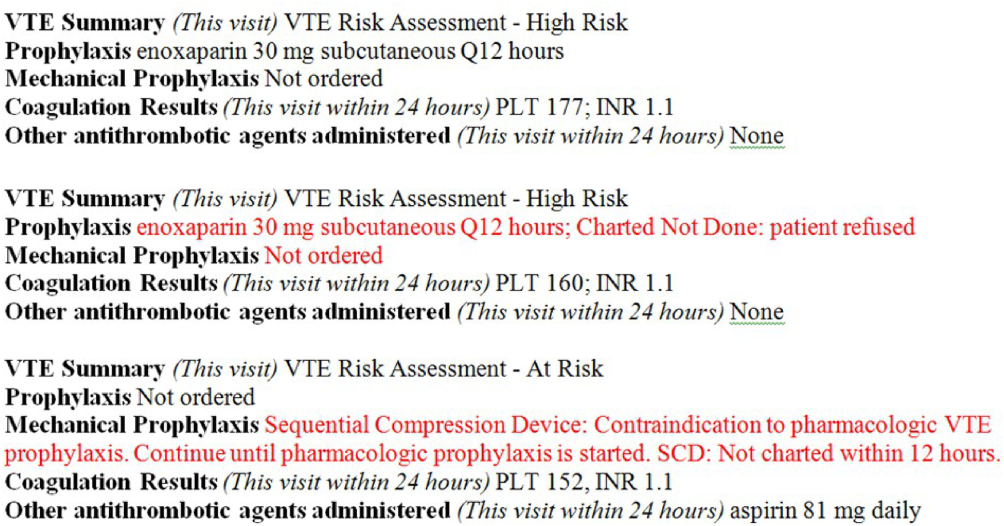
To emphasize Harborview's commitment to VTE prevention and ensure that care providers across the institution are aware of and engaged in this effort, we utilize our intranet to disseminate information in a fully transparent manner. Both process and outcome measures are available to all physicians and staff at service and unit levels on a Web‐based institutional dashboard. Data are updated monthly by QI analysts and improvement opportunities are highlighted in multiple fora. Descriptions of the quality metrics that are tracked are summarized in Table 1.
| Quality Metric | Description |
|---|---|
| |
| AHRQ PSI 12 | Cases of VTE not present on admission per 1000 surgical discharges with select operating room procedures |
| CMS Core Measure VTE‐1 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an acute care area, random sample |
| CMS Core Measure VTE‐2 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an intensive care unit or surgery date, random sample |
| CMS Core Measure VTE 5 | Percent of patients with hospital acquired VTE discharged to home on warfarin who received education and written discharge instructions |
| CMS Core Measure VTE‐6 | Percent of patients with hospital‐acquired VTE who received VTE prophylaxis prior to the event diagnosis |
MEASUREMENTS
Outcomes
Harborview benchmarks performance against hospitals nationally using the CMS Hospital Compare data and with peer academic institutions through Vizient data (Vizient, Irving, TX). To measure the impact of our initiatives, the task force began tracking postoperative VTE rates based on the AHRQ Patient Safety Indicator (PSI) 12 and expanded to include HA‐VTE rates for all hospitalized patients. We also report performance on Core Measure VTE‐6: incidence of potentially preventable VTE.
Process
We monitor VTE prophylaxis compliance based on the CMS Core Measures VTE‐1 and 2, random samples of acute and critical care patients without VTE. Internally, we measure compliance with guideline‐directed therapy for all HA‐VTE cases reviewed by the task force. With the upcoming retirement of the CMS chart‐abstracted measures, we are developing methods to track appropriate VTE prophylaxis provided to all eligible patients and will replace the sampled populations with this more expansive dataset. This approach will provide information for further improvements in VTE prophylaxis and act as an important step for success with the Electronic Clinical Quality Measures under the Meaningful Use program.
RESULTS
Our VTE prevention initiatives have resulted in improved compliance with our institutional guideline‐directed VTE prophylaxis and a decrease in HA‐VTE at our institution.
VTE Core Measures
Since the inception of VTE Core Measures in 2013, our annual performance on VTE‐1: prophylaxis for acute care patients has been above 95% and VTE‐2: prophylaxis for critical care patients has been above 98%. This performance has been consistently above the national mean for both measures (VTE‐1: 91% among Washington state hospitals and 93% nationally; VTE‐2: 95% among Washington state hospitals and 97% nationally). The CMS Hospital Compare current public reporting period is based on information collected from July 2014 through June 2015. Our internal performance for calendar year 2015 was 96% (289 of 302) for VTE‐1 and 98% (235 of 241) for VTE‐2.
Harborview has had zero potentially preventable VTE events (VTE‐6) compared with a reported national average of 4% since the inception of these measures in January 2013.
Guideline‐Directed VTE Prevention: Patients Diagnosed With HA‐VTE
The task force reviews each case to determine if the patient received guideline‐adherent prophylaxis on every day prior to the event. Patients with active bleeding or those with high bleeding risk should have mechanical prophylaxis ordered and applied until pharmacologic prophylaxis is appropriate. Any missed single dose of pharmacologic prophylaxis or missed day of applied mechanical prophylaxis is considered a possible opportunity for improvement, and the case is referred to the appropriate clinical service for additional review.
Since task force launch, the percent of all patients diagnosed with HA‐VTE who received guideline‐directed prophylaxis increased 7% from 86% (105 of 122) in 2012 to 92% (80 of 87) in the first 9 months of 2015. Of events with possible opportunities, most were deemed not to have been preventable. Some trauma patients were ineligible for pharmacologic and mechanical prophylaxis, some were prophylaxed according to the best available evidence, and some had risk factors (for example, active malignancy) only identified after the VTE event. The few remaining events highlighted opportunities regarding standardization of pharmacologic prophylaxis periprocedurally, documentation of application of mechanical prophylaxis, and communication of patient refusal of doses, all ongoing focus areas for improvement.
Reduction in HA‐VTE
Improved VTE prophylaxis has contributed to a 15% reduction in HA‐VTE in all hospitalized patients over 5 years from a rate of 7.5 events/1000 inpatients in 2011 to 6.4/1000 inpatients for the first 9 months of 2015. Among postoperative patients (AHRQ PSI 12), the rate of VTE decreased 21% from 11.7/1000 patients in 2011 to 9.3/1000 patients in the first 9 months of 2015.
Patient/Family Engagement
We further improved our processes to ensure that patients with HA‐VTE who discharge to home receive written discharge instructions for warfarin use (VTE‐5). In 2014, performance on this measure was 91% (51 of 56 eligible patients) and in 2015 performance improved to 96% (78 of 81 eligible patients) compared with a reported national average of 91%. Additionally, 97% (79 of 81) of patients who discharged home on warfarin after HA‐VTE now have outpatient anticoagulation follow‐up arranged prior to hospital discharge. We are developing new initiatives for patient and family education regarding direct oral anticoagulants.
Discussion/Conclusions
With interdisciplinary teamwork and use of QI analytics to drive transparency, we have improved VTE prevention and reduced rates of HA‐VTE. Harborview's HA‐VTE prevention initiative can be duplicated by other organizations given the structured nature of the intervention. The multidisciplinary approach, clinical presence of task force members, and support and engagement of senior clinical leadership have been key elements to our program's success. The existence of a standard institutional guideline based on evidence‐based national guidelines and incorporation of these standards into the EHR is vital. The VTE task force has consistently used QI analytics both for retrospective review and real‐time data feedback. Complete and easy accessibility and transparency of performance at the service and unit level supports accountability. Integration of the task force work into existing institutional QI structures has further led to improvements in patient safety.
Ongoing task force collaboration and communication with frontline providers and clinical departments has been critical to engagement and sustained improvements in VTE prevention and treatment. The work of the VTE task force represents the steadfast commitment of Harborview and our clinical staff to prevent preventable harm. This multidisciplinary effort has served as a model for other QI initiatives across our institution and health system.
Disclosure
Nothing to report.
- , , , et al. Executive Summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , . Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of VTE in nonsurgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al; American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
- Centers for Medicare and Medicaid Services. Core measures. Available at: https://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityMeasures/Core‐Measures.html. Accessed September 1, 2016.
- Centers for Disease Control and Prevention. Venous thromboembolism. Available at: http://www.cdc.gov/ncbddd/dvt/index.html. Accessed September 1, 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. AHRQ Publication No. 16‐0001‐EF. Rockville MD: Agency for Healthcare Research and Quality; 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/vtguide/index.html. Accessed September 1, 2016.
- , . Preventing hospital‐acquired venous‐thromboembolism, a guide for effective quality improvement. Version 3.3. Venous Thromboembolism Quality Improvement Implementation Toolkit. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed September 1, 2016.
- , , , , . Adherence to guideline‐directed venous thromboembolism prophylaxis among medical and surgical inpatients at 33 academic medical centers in the United States. Am J Med Qual. 2010;26(3):174–180.
- UW Medicine guidelines for prevention of venous thromboembolism (VTE) in hospitalized patients. Available at: https://depts.washington.edu/anticoag/home. Accessed June 13, 2016.
- , , , , , . Upper extremity deep vein thrombosis in hospitalized patients: a descriptive study. J Hosp Med. 2014;9(1):48–53.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a serious and growing public health problem. In the United States an estimated 900,000 people are affected and more than 100,000 die from VTE or related complications each year. More than half of VTE events occur in association with hospitalization or major surgery; many are thought to be preventable.[1, 2, 3, 4, 5] The Centers for Medicare and Medicaid Services (CMS), Centers for Disease Control and Prevention (CDC), and the Agency for Healthcare Research and Quality (AHRQ),[6, 7, 8, 9] among other organizations, have identified VTE as a potentially preventable never event. Evidence‐based guidelines and resources exist to help support hospital‐acquired venous thromboembolism (HA‐VTE) prevention.[1, 2, 3, 4, 5, 6, 7, 8, 9, 10] Harborview Medical Center, a tertiary referral center with more than 17,000 patients hospitalized annually, many requiring surgery, serves one of the highest‐risk populations for HA‐VTE development. Despite high rates of VTE prophylaxis in accordance with an established institutional guideline,[11, 12] VTE remains the most common hospital‐acquired condition in our institution.
OBJECTIVES
To improve the safety and care of all patients in our medical center and eliminate preventable HA‐VTE events, we set out to: (1) incorporate evidence‐based best practices in VTE prevention and treatment into current practice in alignment with institutional guidelines, (2) standardize the review process for all HA‐VTE events to identify opportunities for improvement, (3) utilize quality improvement (QI) analytics and information technology (IT) to actively improve our processes at the point of care, and (4) share process and outcome performance relating to VTE prevention transparently across our institution
METHODS
To prevent HA‐VTE, we employ a multifactorial strategy that includes designated clinical leadership, active engagement of all care team members, decision support tools embedded in the electronic health record (EHR), QI analytics, and retrospective and prospective reporting that provides ongoing measurement and analysis of the effectiveness of implemented interventions.
Setting/Patients
Harborview Medical Center, a 413‐bed academic tertiary referral center and the only level 1 adult and pediatric trauma and burn center for a 5‐state area, also serves as the primary safety‐net provider in the region. Harborview has centers of excellence in trauma, neurosciences, orthopedic and vascular surgery and rehabilitation, and is the only certified comprehensive stroke center in 5 states. With more than 17,000 admissions annually, including over 6000 trauma cases, HA‐VTE is a disease that spans critical and acute care settings and impacts patients on all clinical services. Harborview serves a population that is at extremely high risk for VTE as well as bleeding, particularly patients who have sustained central nervous system trauma or polytrauma.
Intervention
In 2010, at the request of the Harborview Medical Executive Board and Medical Director, we formed the Harborview VTE Task Force to assess VTE prevention practices across services and identify improvement opportunities for all hospitalized patients. This multidisciplinary team, co‐chaired by a hospitalist and trauma surgeon, includes representatives from trauma/general surgery, orthopedic surgery, hospital medicine, nursing, pharmacy, and QI. Task force members represent critical and acute care as well as the ambulatory setting. Additional stakeholders and local experts including IT directors and analysts, continuity of care nurses, and other clinical service representatives participate on an ad hoc basis.
Since its inception, the VTE Task Force has met monthly to review performance data and develop improvement initiatives. Initially we collaborated with experts across our health system to update an existing institutional VTE prophylaxis guideline to reflect current evidence‐based standards.[1, 3, 4, 5, 12] We met with all clinical services to ensure that the guidelines incorporated departmental best practices. These guidelines were integrated into our Cerner‐based (Cerner Corp., North Kansas City, MO) computerized provider order entry (CPOE) system to support accurate VTE risk assessment and appropriate ordering of prophylaxis.
The VTE Task Force collaborated with QI programmers to develop an electronic tool, the Harborview VTE Tool (Figure 1),[13] that allows for efficient, standardized review of all HA‐VTE at monthly meetings. The tool uses word and phrase search capabilities to identify PEs and DVTs from imaging and vascular studies and links those events with pertinent demographic and clinical data from the EHR in a timeline. Information about VTE risk assigned by physicians in the CPOE system is extracted as well as specific VTE prophylaxis and treatment (drug, dose, timing of administration of medications, reason for doses being held, and orders for and application of mechanical prophylaxis). Using the VTE tool, the task force reviews each VTE event to assess the accuracy of VTE risk assignment, the appropriateness of prophylaxis received relative to guidelines, and the adequacy of VTE treatment and follow‐up. This tool has facilitated our review process, decreasing time from >30 minutes of manual chart review per event to several minutes. In recent months, a quality analyst has prescreened all VTEs prior to task force discussion to further improve efficiency. The tool allows the team to assess the case together and reach consensus regarding VTE prevention.

Prompt event reviews allow the task force to provide timely feedback about specific VTE events to physicians, nurses, and pharmacists. Cases with potential opportunities for improvement are referred to a medical center‐wide QI committee for secondary review. Areas of opportunity identified are tracked and trended to direct ongoing system improvement cycles. In 2014, as a result of reviewing patient cases with VTE diagnosed after discharge, we began a similar review process to assess current practice and standardize prophylaxis across care transitions.
In response to opportunities identified from reviews, the VTE Task Force developed multiple reporting tools that provide real‐time, actionable information to clinicians at the bedside. Daily electronic lists highlight patients who have not received chemical or mechanical prophylaxis in 24 hours and are utilized by nursing, pharmacy, and physician groups. Patients receiving new start vitamin K antagonists or direct oral anticoagulants are identified for pharmacists and discharge care coordinators to support early patient/family education and ensure appropriate follow‐up. Based on input from frontline providers, tools are continually refined to improve their clinical utility. A timeline of initiatives that the Harborview VTE Task Force has championed is outlined in Figure 2.

To bring HA‐VTE prevention information to the point of care, we developed a VTE Prevention/Treatment Summary within the EHR (Figure 3). Information about VTE risk assigned by the physician based on guidelines, current prophylaxis orders (pharmacologic/nonpharmacologic) and administration status, therapeutic anticoagulation and pertinent laboratory values are imported into a summary snapshot that can be accessed on demand by any member of the care team from within the patient's chart. The same data elements are being imbedded in resident physician and nursing handoff tools to highlight VTE prevention for all hospitalized patients and ensure optimal prophylaxis at transitions of care.

To emphasize Harborview's commitment to VTE prevention and ensure that care providers across the institution are aware of and engaged in this effort, we utilize our intranet to disseminate information in a fully transparent manner. Both process and outcome measures are available to all physicians and staff at service and unit levels on a Web‐based institutional dashboard. Data are updated monthly by QI analysts and improvement opportunities are highlighted in multiple fora. Descriptions of the quality metrics that are tracked are summarized in Table 1.
| Quality Metric | Description |
|---|---|
| |
| AHRQ PSI 12 | Cases of VTE not present on admission per 1000 surgical discharges with select operating room procedures |
| CMS Core Measure VTE‐1 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an acute care area, random sample |
| CMS Core Measure VTE‐2 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an intensive care unit or surgery date, random sample |
| CMS Core Measure VTE 5 | Percent of patients with hospital acquired VTE discharged to home on warfarin who received education and written discharge instructions |
| CMS Core Measure VTE‐6 | Percent of patients with hospital‐acquired VTE who received VTE prophylaxis prior to the event diagnosis |
MEASUREMENTS
Outcomes
Harborview benchmarks performance against hospitals nationally using the CMS Hospital Compare data and with peer academic institutions through Vizient data (Vizient, Irving, TX). To measure the impact of our initiatives, the task force began tracking postoperative VTE rates based on the AHRQ Patient Safety Indicator (PSI) 12 and expanded to include HA‐VTE rates for all hospitalized patients. We also report performance on Core Measure VTE‐6: incidence of potentially preventable VTE.
Process
We monitor VTE prophylaxis compliance based on the CMS Core Measures VTE‐1 and 2, random samples of acute and critical care patients without VTE. Internally, we measure compliance with guideline‐directed therapy for all HA‐VTE cases reviewed by the task force. With the upcoming retirement of the CMS chart‐abstracted measures, we are developing methods to track appropriate VTE prophylaxis provided to all eligible patients and will replace the sampled populations with this more expansive dataset. This approach will provide information for further improvements in VTE prophylaxis and act as an important step for success with the Electronic Clinical Quality Measures under the Meaningful Use program.
RESULTS
Our VTE prevention initiatives have resulted in improved compliance with our institutional guideline‐directed VTE prophylaxis and a decrease in HA‐VTE at our institution.
VTE Core Measures
Since the inception of VTE Core Measures in 2013, our annual performance on VTE‐1: prophylaxis for acute care patients has been above 95% and VTE‐2: prophylaxis for critical care patients has been above 98%. This performance has been consistently above the national mean for both measures (VTE‐1: 91% among Washington state hospitals and 93% nationally; VTE‐2: 95% among Washington state hospitals and 97% nationally). The CMS Hospital Compare current public reporting period is based on information collected from July 2014 through June 2015. Our internal performance for calendar year 2015 was 96% (289 of 302) for VTE‐1 and 98% (235 of 241) for VTE‐2.
Harborview has had zero potentially preventable VTE events (VTE‐6) compared with a reported national average of 4% since the inception of these measures in January 2013.
Guideline‐Directed VTE Prevention: Patients Diagnosed With HA‐VTE
The task force reviews each case to determine if the patient received guideline‐adherent prophylaxis on every day prior to the event. Patients with active bleeding or those with high bleeding risk should have mechanical prophylaxis ordered and applied until pharmacologic prophylaxis is appropriate. Any missed single dose of pharmacologic prophylaxis or missed day of applied mechanical prophylaxis is considered a possible opportunity for improvement, and the case is referred to the appropriate clinical service for additional review.
Since task force launch, the percent of all patients diagnosed with HA‐VTE who received guideline‐directed prophylaxis increased 7% from 86% (105 of 122) in 2012 to 92% (80 of 87) in the first 9 months of 2015. Of events with possible opportunities, most were deemed not to have been preventable. Some trauma patients were ineligible for pharmacologic and mechanical prophylaxis, some were prophylaxed according to the best available evidence, and some had risk factors (for example, active malignancy) only identified after the VTE event. The few remaining events highlighted opportunities regarding standardization of pharmacologic prophylaxis periprocedurally, documentation of application of mechanical prophylaxis, and communication of patient refusal of doses, all ongoing focus areas for improvement.
Reduction in HA‐VTE
Improved VTE prophylaxis has contributed to a 15% reduction in HA‐VTE in all hospitalized patients over 5 years from a rate of 7.5 events/1000 inpatients in 2011 to 6.4/1000 inpatients for the first 9 months of 2015. Among postoperative patients (AHRQ PSI 12), the rate of VTE decreased 21% from 11.7/1000 patients in 2011 to 9.3/1000 patients in the first 9 months of 2015.
Patient/Family Engagement
We further improved our processes to ensure that patients with HA‐VTE who discharge to home receive written discharge instructions for warfarin use (VTE‐5). In 2014, performance on this measure was 91% (51 of 56 eligible patients) and in 2015 performance improved to 96% (78 of 81 eligible patients) compared with a reported national average of 91%. Additionally, 97% (79 of 81) of patients who discharged home on warfarin after HA‐VTE now have outpatient anticoagulation follow‐up arranged prior to hospital discharge. We are developing new initiatives for patient and family education regarding direct oral anticoagulants.
Discussion/Conclusions
With interdisciplinary teamwork and use of QI analytics to drive transparency, we have improved VTE prevention and reduced rates of HA‐VTE. Harborview's HA‐VTE prevention initiative can be duplicated by other organizations given the structured nature of the intervention. The multidisciplinary approach, clinical presence of task force members, and support and engagement of senior clinical leadership have been key elements to our program's success. The existence of a standard institutional guideline based on evidence‐based national guidelines and incorporation of these standards into the EHR is vital. The VTE task force has consistently used QI analytics both for retrospective review and real‐time data feedback. Complete and easy accessibility and transparency of performance at the service and unit level supports accountability. Integration of the task force work into existing institutional QI structures has further led to improvements in patient safety.
Ongoing task force collaboration and communication with frontline providers and clinical departments has been critical to engagement and sustained improvements in VTE prevention and treatment. The work of the VTE task force represents the steadfast commitment of Harborview and our clinical staff to prevent preventable harm. This multidisciplinary effort has served as a model for other QI initiatives across our institution and health system.
Disclosure
Nothing to report.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a serious and growing public health problem. In the United States an estimated 900,000 people are affected and more than 100,000 die from VTE or related complications each year. More than half of VTE events occur in association with hospitalization or major surgery; many are thought to be preventable.[1, 2, 3, 4, 5] The Centers for Medicare and Medicaid Services (CMS), Centers for Disease Control and Prevention (CDC), and the Agency for Healthcare Research and Quality (AHRQ),[6, 7, 8, 9] among other organizations, have identified VTE as a potentially preventable never event. Evidence‐based guidelines and resources exist to help support hospital‐acquired venous thromboembolism (HA‐VTE) prevention.[1, 2, 3, 4, 5, 6, 7, 8, 9, 10] Harborview Medical Center, a tertiary referral center with more than 17,000 patients hospitalized annually, many requiring surgery, serves one of the highest‐risk populations for HA‐VTE development. Despite high rates of VTE prophylaxis in accordance with an established institutional guideline,[11, 12] VTE remains the most common hospital‐acquired condition in our institution.
OBJECTIVES
To improve the safety and care of all patients in our medical center and eliminate preventable HA‐VTE events, we set out to: (1) incorporate evidence‐based best practices in VTE prevention and treatment into current practice in alignment with institutional guidelines, (2) standardize the review process for all HA‐VTE events to identify opportunities for improvement, (3) utilize quality improvement (QI) analytics and information technology (IT) to actively improve our processes at the point of care, and (4) share process and outcome performance relating to VTE prevention transparently across our institution
METHODS
To prevent HA‐VTE, we employ a multifactorial strategy that includes designated clinical leadership, active engagement of all care team members, decision support tools embedded in the electronic health record (EHR), QI analytics, and retrospective and prospective reporting that provides ongoing measurement and analysis of the effectiveness of implemented interventions.
Setting/Patients
Harborview Medical Center, a 413‐bed academic tertiary referral center and the only level 1 adult and pediatric trauma and burn center for a 5‐state area, also serves as the primary safety‐net provider in the region. Harborview has centers of excellence in trauma, neurosciences, orthopedic and vascular surgery and rehabilitation, and is the only certified comprehensive stroke center in 5 states. With more than 17,000 admissions annually, including over 6000 trauma cases, HA‐VTE is a disease that spans critical and acute care settings and impacts patients on all clinical services. Harborview serves a population that is at extremely high risk for VTE as well as bleeding, particularly patients who have sustained central nervous system trauma or polytrauma.
Intervention
In 2010, at the request of the Harborview Medical Executive Board and Medical Director, we formed the Harborview VTE Task Force to assess VTE prevention practices across services and identify improvement opportunities for all hospitalized patients. This multidisciplinary team, co‐chaired by a hospitalist and trauma surgeon, includes representatives from trauma/general surgery, orthopedic surgery, hospital medicine, nursing, pharmacy, and QI. Task force members represent critical and acute care as well as the ambulatory setting. Additional stakeholders and local experts including IT directors and analysts, continuity of care nurses, and other clinical service representatives participate on an ad hoc basis.
Since its inception, the VTE Task Force has met monthly to review performance data and develop improvement initiatives. Initially we collaborated with experts across our health system to update an existing institutional VTE prophylaxis guideline to reflect current evidence‐based standards.[1, 3, 4, 5, 12] We met with all clinical services to ensure that the guidelines incorporated departmental best practices. These guidelines were integrated into our Cerner‐based (Cerner Corp., North Kansas City, MO) computerized provider order entry (CPOE) system to support accurate VTE risk assessment and appropriate ordering of prophylaxis.
The VTE Task Force collaborated with QI programmers to develop an electronic tool, the Harborview VTE Tool (Figure 1),[13] that allows for efficient, standardized review of all HA‐VTE at monthly meetings. The tool uses word and phrase search capabilities to identify PEs and DVTs from imaging and vascular studies and links those events with pertinent demographic and clinical data from the EHR in a timeline. Information about VTE risk assigned by physicians in the CPOE system is extracted as well as specific VTE prophylaxis and treatment (drug, dose, timing of administration of medications, reason for doses being held, and orders for and application of mechanical prophylaxis). Using the VTE tool, the task force reviews each VTE event to assess the accuracy of VTE risk assignment, the appropriateness of prophylaxis received relative to guidelines, and the adequacy of VTE treatment and follow‐up. This tool has facilitated our review process, decreasing time from >30 minutes of manual chart review per event to several minutes. In recent months, a quality analyst has prescreened all VTEs prior to task force discussion to further improve efficiency. The tool allows the team to assess the case together and reach consensus regarding VTE prevention.

Prompt event reviews allow the task force to provide timely feedback about specific VTE events to physicians, nurses, and pharmacists. Cases with potential opportunities for improvement are referred to a medical center‐wide QI committee for secondary review. Areas of opportunity identified are tracked and trended to direct ongoing system improvement cycles. In 2014, as a result of reviewing patient cases with VTE diagnosed after discharge, we began a similar review process to assess current practice and standardize prophylaxis across care transitions.
In response to opportunities identified from reviews, the VTE Task Force developed multiple reporting tools that provide real‐time, actionable information to clinicians at the bedside. Daily electronic lists highlight patients who have not received chemical or mechanical prophylaxis in 24 hours and are utilized by nursing, pharmacy, and physician groups. Patients receiving new start vitamin K antagonists or direct oral anticoagulants are identified for pharmacists and discharge care coordinators to support early patient/family education and ensure appropriate follow‐up. Based on input from frontline providers, tools are continually refined to improve their clinical utility. A timeline of initiatives that the Harborview VTE Task Force has championed is outlined in Figure 2.

To bring HA‐VTE prevention information to the point of care, we developed a VTE Prevention/Treatment Summary within the EHR (Figure 3). Information about VTE risk assigned by the physician based on guidelines, current prophylaxis orders (pharmacologic/nonpharmacologic) and administration status, therapeutic anticoagulation and pertinent laboratory values are imported into a summary snapshot that can be accessed on demand by any member of the care team from within the patient's chart. The same data elements are being imbedded in resident physician and nursing handoff tools to highlight VTE prevention for all hospitalized patients and ensure optimal prophylaxis at transitions of care.

To emphasize Harborview's commitment to VTE prevention and ensure that care providers across the institution are aware of and engaged in this effort, we utilize our intranet to disseminate information in a fully transparent manner. Both process and outcome measures are available to all physicians and staff at service and unit levels on a Web‐based institutional dashboard. Data are updated monthly by QI analysts and improvement opportunities are highlighted in multiple fora. Descriptions of the quality metrics that are tracked are summarized in Table 1.
| Quality Metric | Description |
|---|---|
| |
| AHRQ PSI 12 | Cases of VTE not present on admission per 1000 surgical discharges with select operating room procedures |
| CMS Core Measure VTE‐1 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an acute care area, random sample |
| CMS Core Measure VTE‐2 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an intensive care unit or surgery date, random sample |
| CMS Core Measure VTE 5 | Percent of patients with hospital acquired VTE discharged to home on warfarin who received education and written discharge instructions |
| CMS Core Measure VTE‐6 | Percent of patients with hospital‐acquired VTE who received VTE prophylaxis prior to the event diagnosis |
MEASUREMENTS
Outcomes
Harborview benchmarks performance against hospitals nationally using the CMS Hospital Compare data and with peer academic institutions through Vizient data (Vizient, Irving, TX). To measure the impact of our initiatives, the task force began tracking postoperative VTE rates based on the AHRQ Patient Safety Indicator (PSI) 12 and expanded to include HA‐VTE rates for all hospitalized patients. We also report performance on Core Measure VTE‐6: incidence of potentially preventable VTE.
Process
We monitor VTE prophylaxis compliance based on the CMS Core Measures VTE‐1 and 2, random samples of acute and critical care patients without VTE. Internally, we measure compliance with guideline‐directed therapy for all HA‐VTE cases reviewed by the task force. With the upcoming retirement of the CMS chart‐abstracted measures, we are developing methods to track appropriate VTE prophylaxis provided to all eligible patients and will replace the sampled populations with this more expansive dataset. This approach will provide information for further improvements in VTE prophylaxis and act as an important step for success with the Electronic Clinical Quality Measures under the Meaningful Use program.
RESULTS
Our VTE prevention initiatives have resulted in improved compliance with our institutional guideline‐directed VTE prophylaxis and a decrease in HA‐VTE at our institution.
VTE Core Measures
Since the inception of VTE Core Measures in 2013, our annual performance on VTE‐1: prophylaxis for acute care patients has been above 95% and VTE‐2: prophylaxis for critical care patients has been above 98%. This performance has been consistently above the national mean for both measures (VTE‐1: 91% among Washington state hospitals and 93% nationally; VTE‐2: 95% among Washington state hospitals and 97% nationally). The CMS Hospital Compare current public reporting period is based on information collected from July 2014 through June 2015. Our internal performance for calendar year 2015 was 96% (289 of 302) for VTE‐1 and 98% (235 of 241) for VTE‐2.
Harborview has had zero potentially preventable VTE events (VTE‐6) compared with a reported national average of 4% since the inception of these measures in January 2013.
Guideline‐Directed VTE Prevention: Patients Diagnosed With HA‐VTE
The task force reviews each case to determine if the patient received guideline‐adherent prophylaxis on every day prior to the event. Patients with active bleeding or those with high bleeding risk should have mechanical prophylaxis ordered and applied until pharmacologic prophylaxis is appropriate. Any missed single dose of pharmacologic prophylaxis or missed day of applied mechanical prophylaxis is considered a possible opportunity for improvement, and the case is referred to the appropriate clinical service for additional review.
Since task force launch, the percent of all patients diagnosed with HA‐VTE who received guideline‐directed prophylaxis increased 7% from 86% (105 of 122) in 2012 to 92% (80 of 87) in the first 9 months of 2015. Of events with possible opportunities, most were deemed not to have been preventable. Some trauma patients were ineligible for pharmacologic and mechanical prophylaxis, some were prophylaxed according to the best available evidence, and some had risk factors (for example, active malignancy) only identified after the VTE event. The few remaining events highlighted opportunities regarding standardization of pharmacologic prophylaxis periprocedurally, documentation of application of mechanical prophylaxis, and communication of patient refusal of doses, all ongoing focus areas for improvement.
Reduction in HA‐VTE
Improved VTE prophylaxis has contributed to a 15% reduction in HA‐VTE in all hospitalized patients over 5 years from a rate of 7.5 events/1000 inpatients in 2011 to 6.4/1000 inpatients for the first 9 months of 2015. Among postoperative patients (AHRQ PSI 12), the rate of VTE decreased 21% from 11.7/1000 patients in 2011 to 9.3/1000 patients in the first 9 months of 2015.
Patient/Family Engagement
We further improved our processes to ensure that patients with HA‐VTE who discharge to home receive written discharge instructions for warfarin use (VTE‐5). In 2014, performance on this measure was 91% (51 of 56 eligible patients) and in 2015 performance improved to 96% (78 of 81 eligible patients) compared with a reported national average of 91%. Additionally, 97% (79 of 81) of patients who discharged home on warfarin after HA‐VTE now have outpatient anticoagulation follow‐up arranged prior to hospital discharge. We are developing new initiatives for patient and family education regarding direct oral anticoagulants.
Discussion/Conclusions
With interdisciplinary teamwork and use of QI analytics to drive transparency, we have improved VTE prevention and reduced rates of HA‐VTE. Harborview's HA‐VTE prevention initiative can be duplicated by other organizations given the structured nature of the intervention. The multidisciplinary approach, clinical presence of task force members, and support and engagement of senior clinical leadership have been key elements to our program's success. The existence of a standard institutional guideline based on evidence‐based national guidelines and incorporation of these standards into the EHR is vital. The VTE task force has consistently used QI analytics both for retrospective review and real‐time data feedback. Complete and easy accessibility and transparency of performance at the service and unit level supports accountability. Integration of the task force work into existing institutional QI structures has further led to improvements in patient safety.
Ongoing task force collaboration and communication with frontline providers and clinical departments has been critical to engagement and sustained improvements in VTE prevention and treatment. The work of the VTE task force represents the steadfast commitment of Harborview and our clinical staff to prevent preventable harm. This multidisciplinary effort has served as a model for other QI initiatives across our institution and health system.
Disclosure
Nothing to report.
- , , , et al. Executive Summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , . Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of VTE in nonsurgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al; American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
- Centers for Medicare and Medicaid Services. Core measures. Available at: https://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityMeasures/Core‐Measures.html. Accessed September 1, 2016.
- Centers for Disease Control and Prevention. Venous thromboembolism. Available at: http://www.cdc.gov/ncbddd/dvt/index.html. Accessed September 1, 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. AHRQ Publication No. 16‐0001‐EF. Rockville MD: Agency for Healthcare Research and Quality; 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/vtguide/index.html. Accessed September 1, 2016.
- , . Preventing hospital‐acquired venous‐thromboembolism, a guide for effective quality improvement. Version 3.3. Venous Thromboembolism Quality Improvement Implementation Toolkit. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed September 1, 2016.
- , , , , . Adherence to guideline‐directed venous thromboembolism prophylaxis among medical and surgical inpatients at 33 academic medical centers in the United States. Am J Med Qual. 2010;26(3):174–180.
- UW Medicine guidelines for prevention of venous thromboembolism (VTE) in hospitalized patients. Available at: https://depts.washington.edu/anticoag/home. Accessed June 13, 2016.
- , , , , , . Upper extremity deep vein thrombosis in hospitalized patients: a descriptive study. J Hosp Med. 2014;9(1):48–53.
- , , , et al. Executive Summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , . Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of VTE in nonsurgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al; American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
- Centers for Medicare and Medicaid Services. Core measures. Available at: https://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityMeasures/Core‐Measures.html. Accessed September 1, 2016.
- Centers for Disease Control and Prevention. Venous thromboembolism. Available at: http://www.cdc.gov/ncbddd/dvt/index.html. Accessed September 1, 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. AHRQ Publication No. 16‐0001‐EF. Rockville MD: Agency for Healthcare Research and Quality; 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/vtguide/index.html. Accessed September 1, 2016.
- , . Preventing hospital‐acquired venous‐thromboembolism, a guide for effective quality improvement. Version 3.3. Venous Thromboembolism Quality Improvement Implementation Toolkit. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed September 1, 2016.
- , , , , . Adherence to guideline‐directed venous thromboembolism prophylaxis among medical and surgical inpatients at 33 academic medical centers in the United States. Am J Med Qual. 2010;26(3):174–180.
- UW Medicine guidelines for prevention of venous thromboembolism (VTE) in hospitalized patients. Available at: https://depts.washington.edu/anticoag/home. Accessed June 13, 2016.
- , , , , , . Upper extremity deep vein thrombosis in hospitalized patients: a descriptive study. J Hosp Med. 2014;9(1):48–53.
© 2016 Society of Hospital Medicine
Improving VTE Prevention
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism, is a significant cause of morbidity and mortality in the United States among hospitalized patients.[1, 2, 3, 4, 5, 6] Although it may not be possible to completely eradicate VTE events,[7] chemical and/or mechanical prophylaxis can reduce VTE rates by up to 74% to 86%,[8, 9, 10] and meta‐analyses have demonstrated the benefit of VTE prophylaxis in the inpatient population.[11, 12] Despite evidence‐based guidelines regarding the appropriate type, duration, and dosing of prophylaxis, thromboprophylaxis has been found to be underutilized in the inpatient setting.[13, 14, 15]
Northwestern Memorial Hospital (NMH) historically performed poorly on VTE outcome measures. VTE in the surgical patient population was an especially glaring problem, as NMH was persistently found to be a risk‐adjusted poor performer in the American College of Surgeons National Surgical Quality Improvement Project (ACS‐NSQIP).
However, VTE outcome measures have been shown to be problematic due to their susceptibility to surveillance bias; that is, variation in the ordering of screening or diagnostic VTE imaging studies between hospitals leads to variable VTE rates (the more you look, the more you find).[16, 17, 18, 19] More vigilant hospitals that have a lower threshold to order an imaging study may find higher occurrences of VTE, and paradoxically be deemed a poor performer. Surveillance bias and the lack of validity of the VTE outcome measurement highlighted the importance of utilizing process‐of‐care measures in assessing hospital VTE prevention efforts.[20, 21] Thus, when the Joint Commission enacted 6 new VTE core process‐of‐care measures on January 1, 2013 to monitor hospital performance on VTE prophylaxis administration and VTE treatment (Table 1), NMH undertook a hospital‐wide quality‐improvement (QI) project utilizing the define‐measure‐analyze‐improve‐control (DMAIC) process improvement (PI) methodology to optimize their performance on these core measures as well as the Surgical Care Improvement Project (SCIP) SCIP‐VTE‐2 measure. In this article, we describe the QI effort undertaken at NMH to improve hospital‐level measure performance and the outcomes of this effort.
| VTE Measure | Measure Calculation | Description of Issues | Interventions | Preintervention Performance, % (N)* | Postintervention Performance, % (N) |
|---|---|---|---|---|---|
| |||||
| VTE‐1: VTE PPX | Patients who received VTE prophylaxis or have documentation why no VTE prophylaxis was given | Missing documentation (both chemical and mechanical); prophylaxis ordered, but not administered; patient refusals and opportunity to increase patient education regarding prophylaxis | 1. Enhanced, individualized VTE prophylaxis alert: alert incorporated order, administration, mechanical PPX, lab exclusion and contraindication details | NMH: 86.6% (174) | NMH: 93.6% (162) |
| All patients | Undocumented contraindication reasons | 2. Nursing education initiative: back‐to‐basics VTE education initiative to help increase the administration of VTE prophylaxis and improve patient education resulting in fewer patient refusals and missed doses | NMH general surgery: 94.4% (34) | NMH general surgery: 97.6% (41) | |
| Inconsistent monitoring and patient education | 3. Updated VTE prophylaxis surgical and medicine order set: updated order listing, heparin TID setting and contraindications | NMH general medicine: 82.5% (115) | NMH general medicine: 90.2% (85) | ||
| VTE‐2: ICU VTE PPX | Patients who received VTE prophylaxis or have documentation why no VTE prophylaxis was given | See interventions 1 through 3 | NMH: 100% (58) | NMH: 95.8% (69) | |
| Patients directly admitted or transferred to the ICU | NMH general surgery: 100% (11) | NMH general surgery: 100% (10) | |||
| NMH general medicine: 100% (40) | NMH general medicine: 100% (51) | ||||
| VTE‐3: VTE patients with anticoagulation overlap therapy | Patients who received overlap therapy of parenteral anticoagulation and warfarin therapy | Gaps in documentation and administration of overlap therapy for 5 days | 4. Overlap therapy alert at discharge: document VTE on diagnosis list with alert to either (1) document reason for discontinuation of parental therapy or (2) prescribe parental anticoagulation during hospitalization or at discharge | NMH: 95.8% (159) | NMH: 100% (105) |
| Patients with confirmed VTE who received warfarin | 5. Overlap therapy alert during hospitalization: documentation alert on the day therapy discontinued | NMH general surgery: 85.7% (12) | NMH general surgery: 100% (16) | ||
| NMH general medicine: 97.0% (129) | NMH general medicine: 100% (79) | ||||
| VTE‐4: VTE patients receiving unfractionated dosages/platelet count monitoring by protocol or nomogram | Patients who have IV UFH therapy dosages and platelet counts monitored according to defined parameters such as a nomogram or protocol | Missing required language on IV UFH orders and order may not include preselected CBC order | 6. Updated heparin order sets: reminder to monitor platelet counts per nomogram and preselect CBC order | NMH: 73.7% (98) | NMH: 100% (74) |
| Patients with confirmed VTE receiving IV UFH therapy | NMH general surgery: 56.3% (7) | NMH general surgery: 100% (9) | |||
| NMH general medicine: 83.8% (88) | NMH general medicine: 100% (52) | ||||
| VTE‐5: VTE warfarin discharge instructions | Patients with documentation that they or their caregivers were given written discharge instructions or other educational material about warfarin | Discharge process is not standardized | 7. Warfarin Patient Education Task: automate nursing task for warfarin order set, check individual warfarin education excluding consult orders | NMH: 9.6% (12) | NMH: 87.5% (63) |
| Patients with confirmed VTE discharged on warfarin therapy | Patient education during hospitalization varies | 8. Warfarin dotphrase: new warfarin/Coumadin dotphrase aligned with department and core measure requirements | NMH general surgery: 0% (0) | NMH general surgery: 100% (11) | |
| No standardized process for initiating and tracking warfarin education during hospitalization | 9. Department Warfarin Instructions Phase II: update department warfarin language, automate warfarin education task | NMH general medicine: 11.3% (12) | NMH general medicine: 85.5% (50) | ||
| Warfarin special instructions for discharge is not aligned with the EMR dotphrase | 10. Physician Referral Order Update: Add follow‐up reason to order | ||||
| Follow‐up appointments are inconsistent | |||||
| VTE‐6: Incidence of potentially preventable VTE | Patients who received no VTE PPX prior to the VTE diagnostic test order date | Failure reasons related to other measures | NMH: 8% (8) | NMH: 2.4% (2) | |
| Patients who developed confirmed VTE during hospitalization | NMH general surgery: 6.7% (1) | NMH general surgery: 0% (0) | |||
| NMH general medicine: 13.5% (7) | NMH general medicine: 0% (0) | ||||
| SCIP‐VTE‐2 | Surgery patients who receive appropriate VTE prophylaxis within 24 hours prior to anesthesia start time to 24 hours after anesthesia end time | Standard enoxaparin administration time is 1300 and there is a gap between surgery end time to enoxaparin administration (i.e. patient may wait up to 23 hours for prophylaxis) | 11. Updated VTE prophylaxis‐surgical and medicine order set: added 1‐time and 2‐time heparin doses to enoxaparin order section | NMH: 99.5% (202) | NMH: 100% (104) |
| All selected surgery patients | NMH General Surgery: 98.5% (67) | NMH General Surgery: 100% (100) | |||
| NMH General Medicine: N/A | NMH General Medicine: N/A | ||||
| Additional interventions | Incomplete VTE prophylaxis information | 12. Updated IPC view | |||
| Inconsistent documentation across forms | 13. Updated ADL forms and iView nursing responses updated | ||||
| 14. Updated unit snapshot to mirror IPC view | |||||
| 15. Updated MPET: updated nursing task: standardize Not Given and Not Done Nursing Responses | |||||
METHODS
Setting
NMH is a tertiary referral and teaching hospital affiliated with the Feinberg School of Medicine of Northwestern University. It is the flagship of Northwestern Medicine, which also includes 4 community hospitals, a dedicated women's hospital, and outpatient and urgent care centers.[22] NMH is an 885‐bed hospital with approximately 50,000 inpatients admitted annually. This project, to evaluate the outcomes of the NMH VTE QI initiative, was reviewed and approved by the Northwestern University Institutional Review Board as an exempt activity.
Measures
The Joint Commission VTE measures were a product of the National Consensus Standards for the Prevention and Care of Deep Vein Thrombosis project between the Joint Commission and National Quality Forum (NQF). These 6 measures are endorsed by the NQF and aligned with the Centers of Medicare and Medicaid Services.[23] SCIP also has measures focusing on VTE prophylaxis. SCIP‐VTE‐2 focuses on prophylaxis in the perioperative period (the 24 hours prior to anesthesia start time to 24 hours postanesthesia end time). Specific measure definitions are in Table 1. All patients hospitalized at NMH were eligible for case abstraction; specific inclusion and exclusion criteria were based on measure specifics set forth by The Joint Commission and SCIP, and random cases were selected for abstraction utilizing the standard sampling methodology required for these measures. Case abstraction was performed by a nurse and validated by physicians.
The Intervention
Review of baseline performance on the core measures began in January 2013. Common failure points were identified first by electronic medical record (EMR) evaluation. Subsequently, focus groups with front‐line staff, close examination of EMR ordering logic for chemical and mechanical prophylaxis with the IT department, hospital floor observations, and evaluation of the patient education process during discharge were performed to further define the reasons for common failure points.
Fifteen data‐driven, focused interventions were then designed, pilot tested, and implemented throughout the hospital in May 2013, with iterative improvement of each component over the next 18 months (Table 1). This project utilized DMAIC PI methodology, and was carried out by a multidisciplinary team with representatives from the departments of surgery, internal medicine, anesthesia, gynecology, PI, clinical quality, pharmacy, analytics, information technology (IT), and nursing. Broadly, the 15 interventions consisted of (1) EMR alerts, (2) education initiatives, (3) new EMR order sets, and (4) other EMR changes.
EMR Alerts
Novel provider alerts were built into NMH's inpatient EMR platform (Cerner PowerChart; Cerner Corp., North Kansas City, MO) to address common mistakes contributing to failures on VTE‐1 (chemoprophylaxis) and VTE‐3 (overlap therapy). Although VTE‐1 failures were often multifactorial, missing documentation regarding reasons for no chemoprophylaxis given and failures to order chemoprophylaxis were 2 common drivers of failures. To address these 2 problems, a logic‐driven alert to force patient‐specific ordering of appropriate VTE prophylaxis was developed (Figure 1). VTE‐3 (overlap therapy) failures occurred due to clinician failure to order a full 5 days of overlap therapy when switching from parenteral anticoagulation to warfarin therapy; hence, to target VTE‐3 performance, new alerts reminding clinicians to meticulously order and document the overlap of parenteral VTE therapy and warfarin were developed. As part of the logic‐driven alert to improve patient‐specific ordering of appropriate VTE prophylaxis, we allowed for the inclusion of documentation of a contraindication to explain why VTE prophylaxis was not ordered.
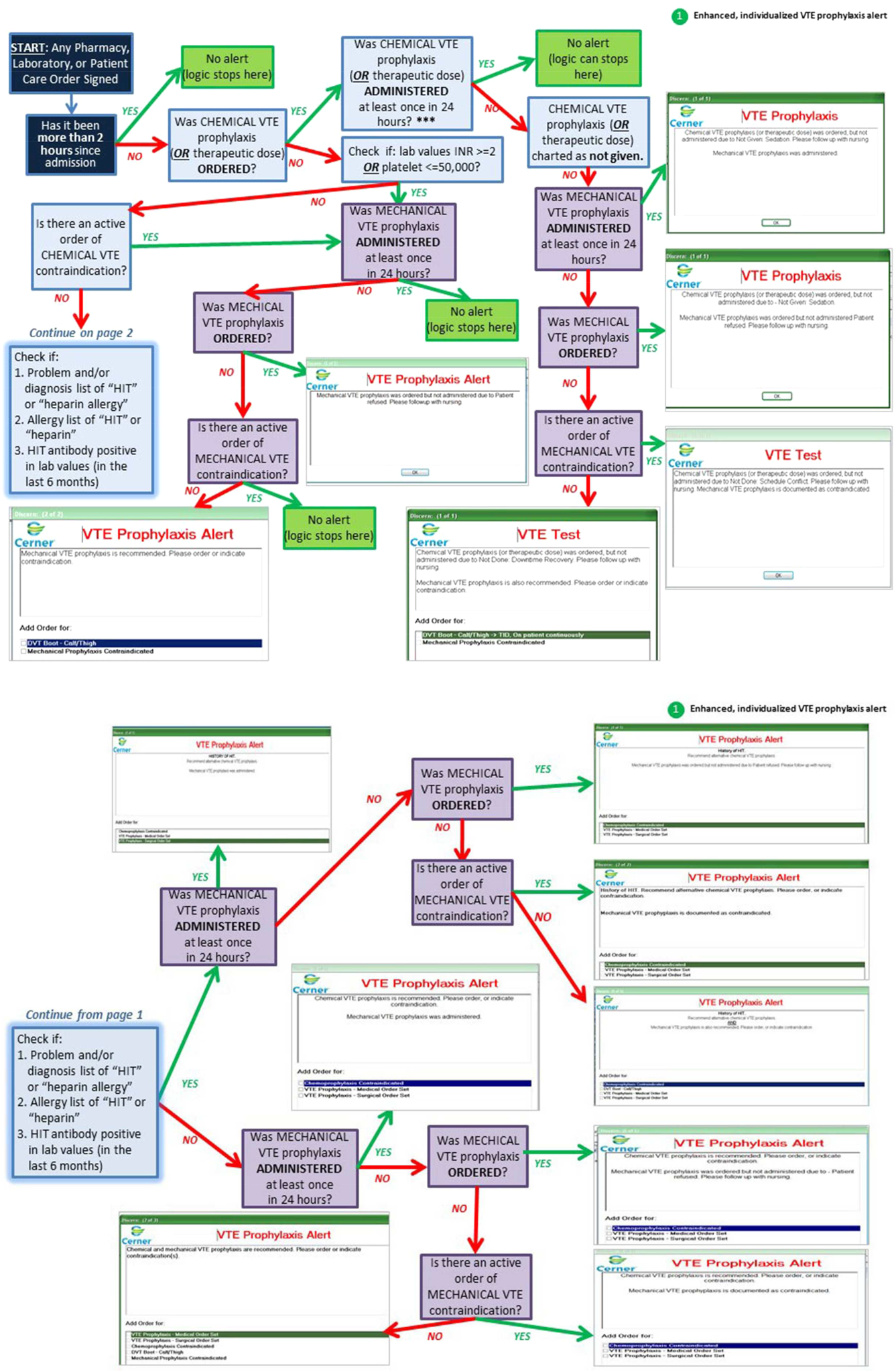
Educational Initiatives
After consulting with attending physicians, residents, nurses, and practice managers at NMH to understand the potential drivers of VTE‐1 (chemoprophylaxis) failures, a team of clinicians and PI experts held 2‐part interactive educational sessions with nurses to address knowledge deficits. The first part focused on general VTE education (eg, the significance of the problem nationwide as well as at NMH, general signs and symptoms of VTE, risk factors for VTE, and NMH‐specific failure rates for mechanical and chemoprophylaxis). The second portion used a myth‐busting approach, in which common misunderstandings that frequently impede VTE prophylaxis (eg, a patient capable of ambulating does not need sequential compression devices (SCDs), or SCDs cannot be applied to a patient with acute or chronic DVT) were discussed. Educational efforts also addressed VTE‐5 (warfarin discharge instructions) performance; although nurses provided patient education with regard to home warfarin use, the timing was inconsistent. The VTE‐5 education provided nurses with a standardized method and time for educating patients about postdischarge warfarin use. EMR changes ensured that when warfarin was ordered, warfarin education automatically populated the nurse's task list, reminding them to educate their patients prior to discharge.
New EMR Order Sets
Previously existing order sets often made it difficult for physicians to order the correct dosing and timing of VTE prophylaxis, document contraindications to prophylaxis, and lacked the appropriate laboratory orders with therapy orders. New order sets were designed to facilitate compliance with VTE‐1 (chemoprophylaxis), VTE‐4 (platelet monitoring), VTE‐5 (warfarin discharge instructions), and SCIP‐VTE‐2 (perioperative prophylaxis) by updating lab and medication order listings, dosing choices, prophylaxis contraindications, reminders to monitor platelet counts per nomogram, and physician follow‐up reasons. When we considered our hospital's specific local factors, we came to the conclusion that risk stratification would be a difficult strategy to apply effectively as a component of the new order sets, mainly due to barriers related to buy‐in from physicians and nurses.
Other EMR Changes
Other interventions targeted at specific issues were programmed into the EMR. For example, a shortcut (known as a dotphrase in Cerner PowerChart) for inserting warfarin instructions into patient care documentation was available to physicians, but was misaligned to the standard warfarin instructions. In addition, the physician responsible for following up on a patient's first outpatient international normalized ratio was often omitted from the discharge instructions, potentially leaving patients without a physician to adjust their dosing appropriately. Adding this physician information, as well as aligning and updating all discharge instructions, allowed for clear, consistent patient instructions for home warfarin use. Moreover, EMR forms used by physicians and forms used by nurses to check for VTE prophylaxis were inconsistent, thus leading to potential confusion between physicians and nurses. Accordingly, regularly used EMR forms (eg, the interdisciplinary plan of care, and the unit summary page or unit snapshot) were updated and standardized.
Control Mechanisms
Concurrent with the implementation of the 15 interventions was the development of several control mechanisms to ensure sustained improvement. These mechanisms consisted of (1) an electronic proxy measure for VTE‐1 (chemoprophylaxis) and (2) monitoring of clinician (including physicians, nurses, and midlevel providers) responses to the EMR alerts, and (3) a comprehensive EMR unit report (Figure 2).
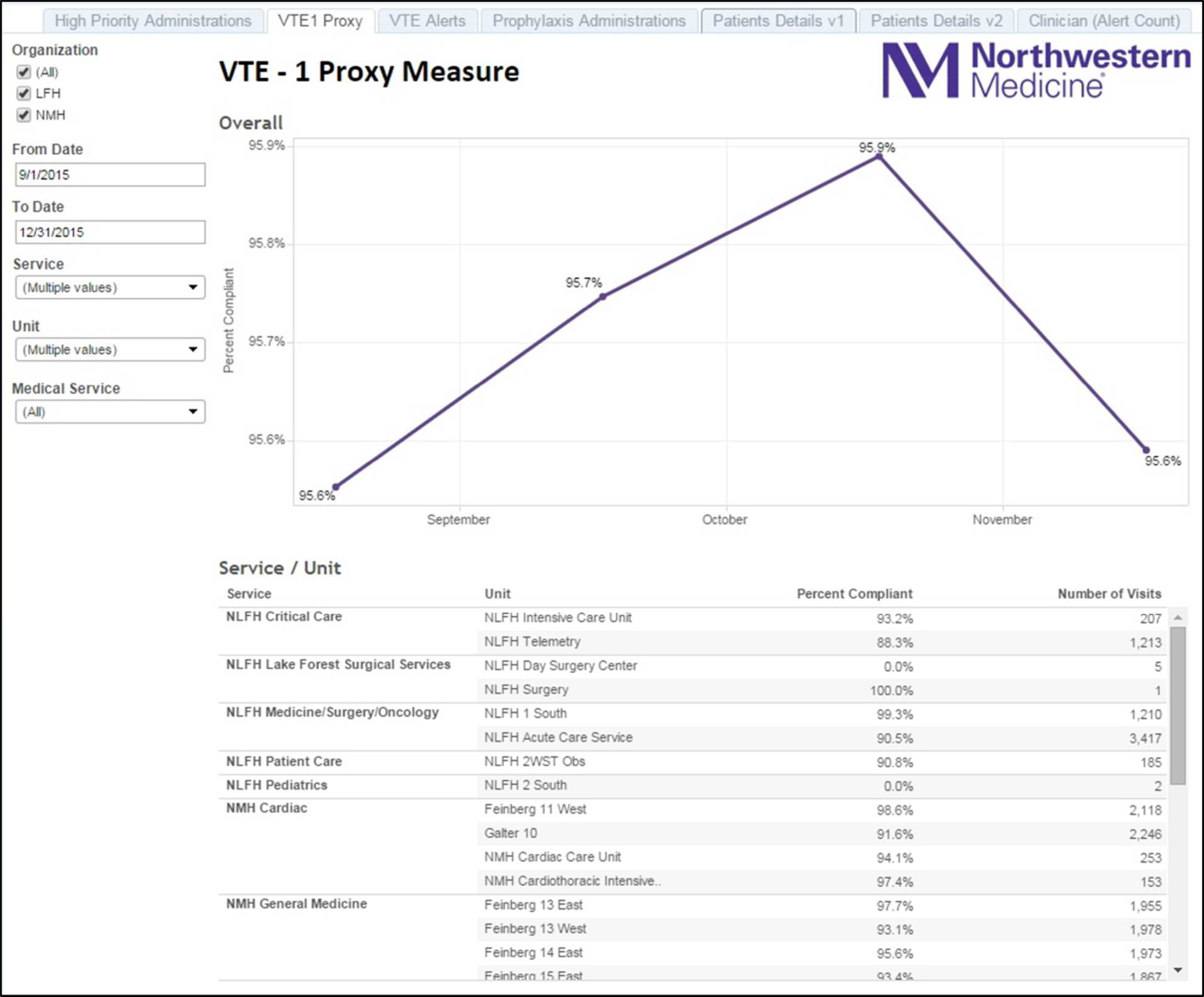
Proxy Measure
Because the Joint Commission core measures are abstracted from only a sample of cases, and a time lag existed between each failure on VTE‐1 (chemoprophylaxis) to the time the QI team learned of the failure, a proxy measure was created. This proxy measure is used as a stand‐in for actual VTE‐1 measure performance, but is generated in real time and reflects performance throughout the entire hospital instead of a random sample of cases. Using the Northwestern Electronic Data Warehouse (EDW), the NMH analytics team created a report reflecting thromboprophylaxis administration on each hospital unit currently and over time. Performance could also be examined for each individual hospital service line. Being able to track longitudinal performance by unit and by service line enabled the QI team to understand trends in performance. Having the ability to examine patients who missed doses over the preceding few hours allowed unit leadership to proactively act upon the failures in a timely fashion, instead of waiting to receive their performance on the Joint Commission core measures.
Physician Alert Response Monitoring
Monitoring of clinical responses to EMR alerts was embedded as standard practice. Because alert fatigue is a documented unintended consequence of heavy reliance on EMR alerts,[24, 25] physicians and nurses who failed to respond to alerts regarding VTE prophylaxis were identified. Interventions targeted toward this group of nonresponders are currently being developed and tested.
EDW Unit Report
This report allows unit managers to track potential failures real time and act prior to a failure occurring (eg, missed chemoprophylaxis dose) through the NMH EDW (Figure 2). These reports contained detailed order and administration data at the individual patient, nurse, and physician levels. Missed doses of VTE chemoprophylaxis were immediately fed back to unit nursing managers who utilized the report to perform a rapid drilldown to identify the root cause(s) of the failure, and then rectify the failure while the patient was still hospitalized.
Statistical Analyses
Hospital performance on the VTE core measures and SCIP‐VTE‐2 was determined by trained nurse abstractors, who abstract cases randomly sampled by the University of HealthCare Consortium, and adjudicate findings as per the Specifications Manual for National Hospital Inpatient Quality Measures. Performance in the period prior to the QI intervention and in the period following the QI intervention was documented as proportions of abstracted cases found to be compliant with measure specifications. Differences between the pre‐ and postintervention periods were compared using a binomial test, with a P value <0.05 considered significant. All analyses were performed using Stata version 13 (StataCorp, College Station, TX).
RESULTS
A total of 1679 cases were abstracted to obtain core measure performance in the time period before the DMAIC intervention phase (January 1, 2013May 1, 2013), and 1424 cases were abstracted to obtain core measure performance in the time period after the DMAIC intervention phase (October 1, 2014April 1, 2015).
Overall NMH performance on measures VTE‐1 (chemoprophylaxis) and VTE36 (overlap therapy, platelet monitoring, warfarin discharge instructions, hospital‐acquired [HA]‐VTE) improved significantly (P < 0.05) (Table 1). No improvement was seen on VTE‐2 (intensive care unit chemoprophylaxis) given that pre‐ and postintervention performance was 100%, which likely reflects previous hospital efforts to improve adherence to this measure. The percentage of patients who failed measure VTE‐6 (number of patients with HA‐VTE who did not have VTE prophylaxis ordered prior to diagnosis of their VTE) decreased from 8% to 2.4%, demonstrating improved VTE prevention prescribing habits in NMH providers rather than a change in VTE event rates (ie, if more patients receive prophylaxis, they cannot be included in the numerator). Performance on SCIP‐VTE‐2 (perioperative chemoprophylaxis) increased from 99.5% to 100% as well but did not reach significance given the baseline high performance.
Measure performance on the general surgery services was comparable to the general medical services, with 1 exception. VTE‐1 (chemoprophylaxis) performance was lower both prior to and following the QI intervention on general medicine services (medicine: 82.5% to 90.2% vs surgery: 94.4% to 97.6%). Recent performance on the VTE‐1 proxy measure has proven to be stable between 95% and 97% on surgery services. Physician response to alerts has increased slightly among the NMH general medicine practitioners (15.2%19.1%) but has been stable among NMH general surgery providers.
DISCUSSION
Our study demonstrates that a formal DMAIC QI project taken on by a multidisciplinary team (including clinicians from multiple specialties as well as personnel from IT, nursing, analytics, and PI) can be successfully implemented and can result in marked improvement in VTE core process measure performance. We used a multifaceted approach undertaken by the NMH VTE QI team, utilizing 15 data‐driven interventions including EMR alerts, education initiatives, and new EMR order sets. These were combined with strong control mechanisms to sustain gains.
Previously published studies on VTE prophylaxis practices found that projects combining both passive (ie, helping clinicians to remember to risk‐assess their patients' for VTE) and active (ie, assisting clinicians in appropriate prescribing practices) strategies are the most successful.[26] Our improvement on VTE‐1 can be compared to previous studies examining changes in ordering rates of VTE prophylaxis. Other QI projects that featured a combination of interventions observed similar significant increases in prophylaxis ordering.[27, 28] Our improvement on VTE‐1 (chemoprophylaxis) was significant, although the difference between pre‐ and postintervention performance varied by service type (general surgery vs general medicine vs other). The small increment of improvement on surgical services was likely attributable to a high baseline performance. Prior to 2013, surgically focused VTE prophylaxis QI efforts spurred by poor ACS‐NSQIP performance proved to be successful, thus resulting in high surgical prophylaxis rates at the outset of the hospital‐wide VTE DMAIC project.
One of the most significant unanticipated barriers to improving performance on VTE‐1 (chemoprophylaxis) included the different hospital subcultures on the medical floors as compared to the surgical floors. The surgical floors had higher rates of compliance with VTE‐1 than the general medicine floors both before and after the QI interventions. When the root causes were explored, the medical floors were found to have different ordering and administration patterns. These, in part, stemmed from differing guidelines[29] and standards in the literature regarding VTE prophylaxis for medical and surgical patients. Multiple discussions within the multidisciplinary QI team and with each involved department were held, focusing on the data regarding safe care in medical patients at low risk for a VTE. Subsequent EMR alerts alterations reflected the internal medicine VTE prophylaxis recommendations for medical patients, allowing that low‐risk patients could be assessed by the provider and given as a reason for foregoing VTE prophylaxis.
Barriers to VTE prophylaxis administration were encountered on the nursing front as well. Floor observations illustrated that chemoprophylaxis injections were often offered as an optional medication. Patients, when given the choice of receiving an injection or not, would understandably choose to forgo their heparin or enoxaparin shot. This missed dose was then documented as a patient refusal. This may not be a problem unique to NMH; 1 study demonstrated that almost 12% of chemoprophylaxis doses may not be administered, and a frequent reason may be due to patient refusal.[30] The lack of patient education regarding the importance of receiving chemical prophylaxis was an improvement opportunity at both the nursing and physician level. Not only did physicians and nurses take the responsibility to educate patients on the importance of receiving the proper prophylaxis, but nursing managers were made responsible for acting on missed doses that were listed on the real‐time performance reports for their units. Missed prophylaxis doses thus became an actionable item instead of an acceptable occurrence.
Culture change in an organization is difficult and necessitates sustained efforts. An important component of our project is our control mechanism, in which a real‐time, continuously updated unit report leverages data from our EDW to generate ongoing performance reports that are regularly reviewed by hospital leadership, clinical process owners, and, most importantly, frontline nurse managers. The unit‐specific reports allow nurse managers and clinical project owners to review prophylaxis failures on a case‐by‐case basis daily and to address and rectify the cause. In addition, the QI team tracks individual physician action taken in response to EMR alerts. As performance feedback to surgical trainees has been demonstrated to have a positive effect on ordering practices,[31] efforts to improve resident alert response rates by means of feedback and education are underway.
Limitations
Our results have to be interpreted within certain limitations. First, given that hospital performance on the VTE core measures is determined by abstracting only a sample of eligible cases, it is possible that our results were affected by sampling error. Second, because of problems with the VTE outcome measure due to surveillance bias, we are unable to draw any valid conclusions about changes in VTE event rates as a result of this QI project. Third, because many of our interventions were tailored to NMH's EMR platform and local hospital culture, it is possible that parts of our project are not readily generalizable to other hospitals; however, we believe that many components, such as the alert logics, can be easily tailored to other EMR platforms.
CONCLUSION
This institutional project was a large, multidisciplinary, and sustained undertaking that improved our performance on the VTE core measures. We believe that our bundle of EMR modifications, alerts (particularly the underlying alert logics), order sets, and standardization of summary EMR view can be adopted in other settings with appropriate adaptations to each hospital's specific local environment. Our focused educational interventions can also be easily adapted to other hospital settings. Perhaps the most important part of the project was the construction of novel control mechanisms that allow for tracking of physical alert response and for real‐time evaluation, audit, and feedback of prophylaxis ordering and administration practices at NMH. Taken as a whole, this bundle of resources to improve adherence to optimal VTE prophylaxis will facilitate future interventions targeted at reaching defect‐free care.
Disclosures: Nothing to report.
- , , , , . Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:7S–47S.
- , , , , . Estimated annual numbers of US acute‐care hospital patients at risk for venous thromboembolism. Am J Hematol. 2007;82:777–782.
- , , , et al. All‐cause and potentially disease‐related health care costs associated with venous thromboembolism in commercial, Medicare, and Medicaid beneficiaries. J Manag Care Pharm. 2012;18:363–374.
- , . Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835–1846.
- , , , . Long‐term outcomes after deep vein thrombosis: postphlebitic syndrome and quality of life. J Gen Intern Med. 2000;15:425–429.
- , , , et al. The long‐term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7.
- , . The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009;301:1063–1065.
- , , , et al. Hidden costs associated with venous thromboembolism: impact of lost productivity on employers and employees. J Occup Environ Med. 2014;56(9):979–985.
- , . Designing and implementing effective venous thromboembolism prevention protocols: lessons from collaborative efforts. J Thromb Thrombolysis. 2010;29:159–166.
- , , , . Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162–1173.
- , , , , . Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278–288.
- , , , , . Meta‐analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913–930.
- , , , et al. Preventability of hospital‐acquired venous thromboembolism. JAMA Surg. 2015;150(9):912–915.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE survey): findings in surgical patients. Ann Surg. 2010;251:330–338.
- , , , . Are surgical patients at risk of venous thromboembolism currently meeting the Surgical Care Improvement Project performance measure for appropriate and timely prophylaxis? J Thromb Thrombolysis. 2010;30:55–66.
- , , , et al. Evaluation of surveillance bias and the validity of the venous thromboembolism quality measure. JAMA. 2013;310:1482–1489.
- , , , , , . Evaluation of hospital factors associated with hospital postoperative venous thromboembolism imaging utilisation practices. BMJ Qual Saf. 2014;23(11):947–956.
- , , , et al. Association between hospital imaging use and venous thromboembolism events rates based on clinical data. Ann Surg. 2014;260:558–564; discussion 64–66.
- , , , , . Postoperative venous thromboembolism outcomes measure: analytic exploration of potential misclassification of hospital quality due to surveillance bias. Ann Surg. 2015;261(3):443–444.
- , , . The need to revisit VTE quality measures. JAMA. 2014;312:286–287.
- . Facilitating quality improvement: pushing the pendulum back toward process measures. JAMA. 2015;314:1333–1334.
- Northwestern Medicine website. Available at: https://www.nm.org/locations‐at‐northwestern‐medicine. Accessed February 23, 2016.
- Venous thromboembolism. The Joint Commission website. Available at: http://www.jointcommission.org/venous_thromboembolism. Accessed February 23, 2016.
- , , , , . Some unintended consequences of clinical decision support systems. AMIA Annu Symp Proc. 2007:26–30.
- , , , . Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13:138–147.
- , , , et al. A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals. Ann Surg. 2005;241:397–415.
- , , , et al. Optimizing prevention of hospital‐acquired venous thromboembolism (VTE): prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5:10–18.
- , , . Improving the use of venous thromboembolism prophylaxis in an Australian teaching hospital. Qual Saf Health Care. 2009;18:408–412.
- , , , , . Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155:625–632.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8:e66311.
- , , , et al. Individualized performance feedback to surgical residents improves appropriate venous thromboembolism prophylaxis prescription and reduces potentially preventable VTE: a prospective cohort study [published online November 25, 2015]. Ann Surg. doi: 10.1097/SLA.0000000000001512.
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism, is a significant cause of morbidity and mortality in the United States among hospitalized patients.[1, 2, 3, 4, 5, 6] Although it may not be possible to completely eradicate VTE events,[7] chemical and/or mechanical prophylaxis can reduce VTE rates by up to 74% to 86%,[8, 9, 10] and meta‐analyses have demonstrated the benefit of VTE prophylaxis in the inpatient population.[11, 12] Despite evidence‐based guidelines regarding the appropriate type, duration, and dosing of prophylaxis, thromboprophylaxis has been found to be underutilized in the inpatient setting.[13, 14, 15]
Northwestern Memorial Hospital (NMH) historically performed poorly on VTE outcome measures. VTE in the surgical patient population was an especially glaring problem, as NMH was persistently found to be a risk‐adjusted poor performer in the American College of Surgeons National Surgical Quality Improvement Project (ACS‐NSQIP).
However, VTE outcome measures have been shown to be problematic due to their susceptibility to surveillance bias; that is, variation in the ordering of screening or diagnostic VTE imaging studies between hospitals leads to variable VTE rates (the more you look, the more you find).[16, 17, 18, 19] More vigilant hospitals that have a lower threshold to order an imaging study may find higher occurrences of VTE, and paradoxically be deemed a poor performer. Surveillance bias and the lack of validity of the VTE outcome measurement highlighted the importance of utilizing process‐of‐care measures in assessing hospital VTE prevention efforts.[20, 21] Thus, when the Joint Commission enacted 6 new VTE core process‐of‐care measures on January 1, 2013 to monitor hospital performance on VTE prophylaxis administration and VTE treatment (Table 1), NMH undertook a hospital‐wide quality‐improvement (QI) project utilizing the define‐measure‐analyze‐improve‐control (DMAIC) process improvement (PI) methodology to optimize their performance on these core measures as well as the Surgical Care Improvement Project (SCIP) SCIP‐VTE‐2 measure. In this article, we describe the QI effort undertaken at NMH to improve hospital‐level measure performance and the outcomes of this effort.
| VTE Measure | Measure Calculation | Description of Issues | Interventions | Preintervention Performance, % (N)* | Postintervention Performance, % (N) |
|---|---|---|---|---|---|
| |||||
| VTE‐1: VTE PPX | Patients who received VTE prophylaxis or have documentation why no VTE prophylaxis was given | Missing documentation (both chemical and mechanical); prophylaxis ordered, but not administered; patient refusals and opportunity to increase patient education regarding prophylaxis | 1. Enhanced, individualized VTE prophylaxis alert: alert incorporated order, administration, mechanical PPX, lab exclusion and contraindication details | NMH: 86.6% (174) | NMH: 93.6% (162) |
| All patients | Undocumented contraindication reasons | 2. Nursing education initiative: back‐to‐basics VTE education initiative to help increase the administration of VTE prophylaxis and improve patient education resulting in fewer patient refusals and missed doses | NMH general surgery: 94.4% (34) | NMH general surgery: 97.6% (41) | |
| Inconsistent monitoring and patient education | 3. Updated VTE prophylaxis surgical and medicine order set: updated order listing, heparin TID setting and contraindications | NMH general medicine: 82.5% (115) | NMH general medicine: 90.2% (85) | ||
| VTE‐2: ICU VTE PPX | Patients who received VTE prophylaxis or have documentation why no VTE prophylaxis was given | See interventions 1 through 3 | NMH: 100% (58) | NMH: 95.8% (69) | |
| Patients directly admitted or transferred to the ICU | NMH general surgery: 100% (11) | NMH general surgery: 100% (10) | |||
| NMH general medicine: 100% (40) | NMH general medicine: 100% (51) | ||||
| VTE‐3: VTE patients with anticoagulation overlap therapy | Patients who received overlap therapy of parenteral anticoagulation and warfarin therapy | Gaps in documentation and administration of overlap therapy for 5 days | 4. Overlap therapy alert at discharge: document VTE on diagnosis list with alert to either (1) document reason for discontinuation of parental therapy or (2) prescribe parental anticoagulation during hospitalization or at discharge | NMH: 95.8% (159) | NMH: 100% (105) |
| Patients with confirmed VTE who received warfarin | 5. Overlap therapy alert during hospitalization: documentation alert on the day therapy discontinued | NMH general surgery: 85.7% (12) | NMH general surgery: 100% (16) | ||
| NMH general medicine: 97.0% (129) | NMH general medicine: 100% (79) | ||||
| VTE‐4: VTE patients receiving unfractionated dosages/platelet count monitoring by protocol or nomogram | Patients who have IV UFH therapy dosages and platelet counts monitored according to defined parameters such as a nomogram or protocol | Missing required language on IV UFH orders and order may not include preselected CBC order | 6. Updated heparin order sets: reminder to monitor platelet counts per nomogram and preselect CBC order | NMH: 73.7% (98) | NMH: 100% (74) |
| Patients with confirmed VTE receiving IV UFH therapy | NMH general surgery: 56.3% (7) | NMH general surgery: 100% (9) | |||
| NMH general medicine: 83.8% (88) | NMH general medicine: 100% (52) | ||||
| VTE‐5: VTE warfarin discharge instructions | Patients with documentation that they or their caregivers were given written discharge instructions or other educational material about warfarin | Discharge process is not standardized | 7. Warfarin Patient Education Task: automate nursing task for warfarin order set, check individual warfarin education excluding consult orders | NMH: 9.6% (12) | NMH: 87.5% (63) |
| Patients with confirmed VTE discharged on warfarin therapy | Patient education during hospitalization varies | 8. Warfarin dotphrase: new warfarin/Coumadin dotphrase aligned with department and core measure requirements | NMH general surgery: 0% (0) | NMH general surgery: 100% (11) | |
| No standardized process for initiating and tracking warfarin education during hospitalization | 9. Department Warfarin Instructions Phase II: update department warfarin language, automate warfarin education task | NMH general medicine: 11.3% (12) | NMH general medicine: 85.5% (50) | ||
| Warfarin special instructions for discharge is not aligned with the EMR dotphrase | 10. Physician Referral Order Update: Add follow‐up reason to order | ||||
| Follow‐up appointments are inconsistent | |||||
| VTE‐6: Incidence of potentially preventable VTE | Patients who received no VTE PPX prior to the VTE diagnostic test order date | Failure reasons related to other measures | NMH: 8% (8) | NMH: 2.4% (2) | |
| Patients who developed confirmed VTE during hospitalization | NMH general surgery: 6.7% (1) | NMH general surgery: 0% (0) | |||
| NMH general medicine: 13.5% (7) | NMH general medicine: 0% (0) | ||||
| SCIP‐VTE‐2 | Surgery patients who receive appropriate VTE prophylaxis within 24 hours prior to anesthesia start time to 24 hours after anesthesia end time | Standard enoxaparin administration time is 1300 and there is a gap between surgery end time to enoxaparin administration (i.e. patient may wait up to 23 hours for prophylaxis) | 11. Updated VTE prophylaxis‐surgical and medicine order set: added 1‐time and 2‐time heparin doses to enoxaparin order section | NMH: 99.5% (202) | NMH: 100% (104) |
| All selected surgery patients | NMH General Surgery: 98.5% (67) | NMH General Surgery: 100% (100) | |||
| NMH General Medicine: N/A | NMH General Medicine: N/A | ||||
| Additional interventions | Incomplete VTE prophylaxis information | 12. Updated IPC view | |||
| Inconsistent documentation across forms | 13. Updated ADL forms and iView nursing responses updated | ||||
| 14. Updated unit snapshot to mirror IPC view | |||||
| 15. Updated MPET: updated nursing task: standardize Not Given and Not Done Nursing Responses | |||||
METHODS
Setting
NMH is a tertiary referral and teaching hospital affiliated with the Feinberg School of Medicine of Northwestern University. It is the flagship of Northwestern Medicine, which also includes 4 community hospitals, a dedicated women's hospital, and outpatient and urgent care centers.[22] NMH is an 885‐bed hospital with approximately 50,000 inpatients admitted annually. This project, to evaluate the outcomes of the NMH VTE QI initiative, was reviewed and approved by the Northwestern University Institutional Review Board as an exempt activity.
Measures
The Joint Commission VTE measures were a product of the National Consensus Standards for the Prevention and Care of Deep Vein Thrombosis project between the Joint Commission and National Quality Forum (NQF). These 6 measures are endorsed by the NQF and aligned with the Centers of Medicare and Medicaid Services.[23] SCIP also has measures focusing on VTE prophylaxis. SCIP‐VTE‐2 focuses on prophylaxis in the perioperative period (the 24 hours prior to anesthesia start time to 24 hours postanesthesia end time). Specific measure definitions are in Table 1. All patients hospitalized at NMH were eligible for case abstraction; specific inclusion and exclusion criteria were based on measure specifics set forth by The Joint Commission and SCIP, and random cases were selected for abstraction utilizing the standard sampling methodology required for these measures. Case abstraction was performed by a nurse and validated by physicians.
The Intervention
Review of baseline performance on the core measures began in January 2013. Common failure points were identified first by electronic medical record (EMR) evaluation. Subsequently, focus groups with front‐line staff, close examination of EMR ordering logic for chemical and mechanical prophylaxis with the IT department, hospital floor observations, and evaluation of the patient education process during discharge were performed to further define the reasons for common failure points.
Fifteen data‐driven, focused interventions were then designed, pilot tested, and implemented throughout the hospital in May 2013, with iterative improvement of each component over the next 18 months (Table 1). This project utilized DMAIC PI methodology, and was carried out by a multidisciplinary team with representatives from the departments of surgery, internal medicine, anesthesia, gynecology, PI, clinical quality, pharmacy, analytics, information technology (IT), and nursing. Broadly, the 15 interventions consisted of (1) EMR alerts, (2) education initiatives, (3) new EMR order sets, and (4) other EMR changes.
EMR Alerts
Novel provider alerts were built into NMH's inpatient EMR platform (Cerner PowerChart; Cerner Corp., North Kansas City, MO) to address common mistakes contributing to failures on VTE‐1 (chemoprophylaxis) and VTE‐3 (overlap therapy). Although VTE‐1 failures were often multifactorial, missing documentation regarding reasons for no chemoprophylaxis given and failures to order chemoprophylaxis were 2 common drivers of failures. To address these 2 problems, a logic‐driven alert to force patient‐specific ordering of appropriate VTE prophylaxis was developed (Figure 1). VTE‐3 (overlap therapy) failures occurred due to clinician failure to order a full 5 days of overlap therapy when switching from parenteral anticoagulation to warfarin therapy; hence, to target VTE‐3 performance, new alerts reminding clinicians to meticulously order and document the overlap of parenteral VTE therapy and warfarin were developed. As part of the logic‐driven alert to improve patient‐specific ordering of appropriate VTE prophylaxis, we allowed for the inclusion of documentation of a contraindication to explain why VTE prophylaxis was not ordered.

Educational Initiatives
After consulting with attending physicians, residents, nurses, and practice managers at NMH to understand the potential drivers of VTE‐1 (chemoprophylaxis) failures, a team of clinicians and PI experts held 2‐part interactive educational sessions with nurses to address knowledge deficits. The first part focused on general VTE education (eg, the significance of the problem nationwide as well as at NMH, general signs and symptoms of VTE, risk factors for VTE, and NMH‐specific failure rates for mechanical and chemoprophylaxis). The second portion used a myth‐busting approach, in which common misunderstandings that frequently impede VTE prophylaxis (eg, a patient capable of ambulating does not need sequential compression devices (SCDs), or SCDs cannot be applied to a patient with acute or chronic DVT) were discussed. Educational efforts also addressed VTE‐5 (warfarin discharge instructions) performance; although nurses provided patient education with regard to home warfarin use, the timing was inconsistent. The VTE‐5 education provided nurses with a standardized method and time for educating patients about postdischarge warfarin use. EMR changes ensured that when warfarin was ordered, warfarin education automatically populated the nurse's task list, reminding them to educate their patients prior to discharge.
New EMR Order Sets
Previously existing order sets often made it difficult for physicians to order the correct dosing and timing of VTE prophylaxis, document contraindications to prophylaxis, and lacked the appropriate laboratory orders with therapy orders. New order sets were designed to facilitate compliance with VTE‐1 (chemoprophylaxis), VTE‐4 (platelet monitoring), VTE‐5 (warfarin discharge instructions), and SCIP‐VTE‐2 (perioperative prophylaxis) by updating lab and medication order listings, dosing choices, prophylaxis contraindications, reminders to monitor platelet counts per nomogram, and physician follow‐up reasons. When we considered our hospital's specific local factors, we came to the conclusion that risk stratification would be a difficult strategy to apply effectively as a component of the new order sets, mainly due to barriers related to buy‐in from physicians and nurses.
Other EMR Changes
Other interventions targeted at specific issues were programmed into the EMR. For example, a shortcut (known as a dotphrase in Cerner PowerChart) for inserting warfarin instructions into patient care documentation was available to physicians, but was misaligned to the standard warfarin instructions. In addition, the physician responsible for following up on a patient's first outpatient international normalized ratio was often omitted from the discharge instructions, potentially leaving patients without a physician to adjust their dosing appropriately. Adding this physician information, as well as aligning and updating all discharge instructions, allowed for clear, consistent patient instructions for home warfarin use. Moreover, EMR forms used by physicians and forms used by nurses to check for VTE prophylaxis were inconsistent, thus leading to potential confusion between physicians and nurses. Accordingly, regularly used EMR forms (eg, the interdisciplinary plan of care, and the unit summary page or unit snapshot) were updated and standardized.
Control Mechanisms
Concurrent with the implementation of the 15 interventions was the development of several control mechanisms to ensure sustained improvement. These mechanisms consisted of (1) an electronic proxy measure for VTE‐1 (chemoprophylaxis) and (2) monitoring of clinician (including physicians, nurses, and midlevel providers) responses to the EMR alerts, and (3) a comprehensive EMR unit report (Figure 2).

Proxy Measure
Because the Joint Commission core measures are abstracted from only a sample of cases, and a time lag existed between each failure on VTE‐1 (chemoprophylaxis) to the time the QI team learned of the failure, a proxy measure was created. This proxy measure is used as a stand‐in for actual VTE‐1 measure performance, but is generated in real time and reflects performance throughout the entire hospital instead of a random sample of cases. Using the Northwestern Electronic Data Warehouse (EDW), the NMH analytics team created a report reflecting thromboprophylaxis administration on each hospital unit currently and over time. Performance could also be examined for each individual hospital service line. Being able to track longitudinal performance by unit and by service line enabled the QI team to understand trends in performance. Having the ability to examine patients who missed doses over the preceding few hours allowed unit leadership to proactively act upon the failures in a timely fashion, instead of waiting to receive their performance on the Joint Commission core measures.
Physician Alert Response Monitoring
Monitoring of clinical responses to EMR alerts was embedded as standard practice. Because alert fatigue is a documented unintended consequence of heavy reliance on EMR alerts,[24, 25] physicians and nurses who failed to respond to alerts regarding VTE prophylaxis were identified. Interventions targeted toward this group of nonresponders are currently being developed and tested.
EDW Unit Report
This report allows unit managers to track potential failures real time and act prior to a failure occurring (eg, missed chemoprophylaxis dose) through the NMH EDW (Figure 2). These reports contained detailed order and administration data at the individual patient, nurse, and physician levels. Missed doses of VTE chemoprophylaxis were immediately fed back to unit nursing managers who utilized the report to perform a rapid drilldown to identify the root cause(s) of the failure, and then rectify the failure while the patient was still hospitalized.
Statistical Analyses
Hospital performance on the VTE core measures and SCIP‐VTE‐2 was determined by trained nurse abstractors, who abstract cases randomly sampled by the University of HealthCare Consortium, and adjudicate findings as per the Specifications Manual for National Hospital Inpatient Quality Measures. Performance in the period prior to the QI intervention and in the period following the QI intervention was documented as proportions of abstracted cases found to be compliant with measure specifications. Differences between the pre‐ and postintervention periods were compared using a binomial test, with a P value <0.05 considered significant. All analyses were performed using Stata version 13 (StataCorp, College Station, TX).
RESULTS
A total of 1679 cases were abstracted to obtain core measure performance in the time period before the DMAIC intervention phase (January 1, 2013May 1, 2013), and 1424 cases were abstracted to obtain core measure performance in the time period after the DMAIC intervention phase (October 1, 2014April 1, 2015).
Overall NMH performance on measures VTE‐1 (chemoprophylaxis) and VTE36 (overlap therapy, platelet monitoring, warfarin discharge instructions, hospital‐acquired [HA]‐VTE) improved significantly (P < 0.05) (Table 1). No improvement was seen on VTE‐2 (intensive care unit chemoprophylaxis) given that pre‐ and postintervention performance was 100%, which likely reflects previous hospital efforts to improve adherence to this measure. The percentage of patients who failed measure VTE‐6 (number of patients with HA‐VTE who did not have VTE prophylaxis ordered prior to diagnosis of their VTE) decreased from 8% to 2.4%, demonstrating improved VTE prevention prescribing habits in NMH providers rather than a change in VTE event rates (ie, if more patients receive prophylaxis, they cannot be included in the numerator). Performance on SCIP‐VTE‐2 (perioperative chemoprophylaxis) increased from 99.5% to 100% as well but did not reach significance given the baseline high performance.
Measure performance on the general surgery services was comparable to the general medical services, with 1 exception. VTE‐1 (chemoprophylaxis) performance was lower both prior to and following the QI intervention on general medicine services (medicine: 82.5% to 90.2% vs surgery: 94.4% to 97.6%). Recent performance on the VTE‐1 proxy measure has proven to be stable between 95% and 97% on surgery services. Physician response to alerts has increased slightly among the NMH general medicine practitioners (15.2%19.1%) but has been stable among NMH general surgery providers.
DISCUSSION
Our study demonstrates that a formal DMAIC QI project taken on by a multidisciplinary team (including clinicians from multiple specialties as well as personnel from IT, nursing, analytics, and PI) can be successfully implemented and can result in marked improvement in VTE core process measure performance. We used a multifaceted approach undertaken by the NMH VTE QI team, utilizing 15 data‐driven interventions including EMR alerts, education initiatives, and new EMR order sets. These were combined with strong control mechanisms to sustain gains.
Previously published studies on VTE prophylaxis practices found that projects combining both passive (ie, helping clinicians to remember to risk‐assess their patients' for VTE) and active (ie, assisting clinicians in appropriate prescribing practices) strategies are the most successful.[26] Our improvement on VTE‐1 can be compared to previous studies examining changes in ordering rates of VTE prophylaxis. Other QI projects that featured a combination of interventions observed similar significant increases in prophylaxis ordering.[27, 28] Our improvement on VTE‐1 (chemoprophylaxis) was significant, although the difference between pre‐ and postintervention performance varied by service type (general surgery vs general medicine vs other). The small increment of improvement on surgical services was likely attributable to a high baseline performance. Prior to 2013, surgically focused VTE prophylaxis QI efforts spurred by poor ACS‐NSQIP performance proved to be successful, thus resulting in high surgical prophylaxis rates at the outset of the hospital‐wide VTE DMAIC project.
One of the most significant unanticipated barriers to improving performance on VTE‐1 (chemoprophylaxis) included the different hospital subcultures on the medical floors as compared to the surgical floors. The surgical floors had higher rates of compliance with VTE‐1 than the general medicine floors both before and after the QI interventions. When the root causes were explored, the medical floors were found to have different ordering and administration patterns. These, in part, stemmed from differing guidelines[29] and standards in the literature regarding VTE prophylaxis for medical and surgical patients. Multiple discussions within the multidisciplinary QI team and with each involved department were held, focusing on the data regarding safe care in medical patients at low risk for a VTE. Subsequent EMR alerts alterations reflected the internal medicine VTE prophylaxis recommendations for medical patients, allowing that low‐risk patients could be assessed by the provider and given as a reason for foregoing VTE prophylaxis.
Barriers to VTE prophylaxis administration were encountered on the nursing front as well. Floor observations illustrated that chemoprophylaxis injections were often offered as an optional medication. Patients, when given the choice of receiving an injection or not, would understandably choose to forgo their heparin or enoxaparin shot. This missed dose was then documented as a patient refusal. This may not be a problem unique to NMH; 1 study demonstrated that almost 12% of chemoprophylaxis doses may not be administered, and a frequent reason may be due to patient refusal.[30] The lack of patient education regarding the importance of receiving chemical prophylaxis was an improvement opportunity at both the nursing and physician level. Not only did physicians and nurses take the responsibility to educate patients on the importance of receiving the proper prophylaxis, but nursing managers were made responsible for acting on missed doses that were listed on the real‐time performance reports for their units. Missed prophylaxis doses thus became an actionable item instead of an acceptable occurrence.
Culture change in an organization is difficult and necessitates sustained efforts. An important component of our project is our control mechanism, in which a real‐time, continuously updated unit report leverages data from our EDW to generate ongoing performance reports that are regularly reviewed by hospital leadership, clinical process owners, and, most importantly, frontline nurse managers. The unit‐specific reports allow nurse managers and clinical project owners to review prophylaxis failures on a case‐by‐case basis daily and to address and rectify the cause. In addition, the QI team tracks individual physician action taken in response to EMR alerts. As performance feedback to surgical trainees has been demonstrated to have a positive effect on ordering practices,[31] efforts to improve resident alert response rates by means of feedback and education are underway.
Limitations
Our results have to be interpreted within certain limitations. First, given that hospital performance on the VTE core measures is determined by abstracting only a sample of eligible cases, it is possible that our results were affected by sampling error. Second, because of problems with the VTE outcome measure due to surveillance bias, we are unable to draw any valid conclusions about changes in VTE event rates as a result of this QI project. Third, because many of our interventions were tailored to NMH's EMR platform and local hospital culture, it is possible that parts of our project are not readily generalizable to other hospitals; however, we believe that many components, such as the alert logics, can be easily tailored to other EMR platforms.
CONCLUSION
This institutional project was a large, multidisciplinary, and sustained undertaking that improved our performance on the VTE core measures. We believe that our bundle of EMR modifications, alerts (particularly the underlying alert logics), order sets, and standardization of summary EMR view can be adopted in other settings with appropriate adaptations to each hospital's specific local environment. Our focused educational interventions can also be easily adapted to other hospital settings. Perhaps the most important part of the project was the construction of novel control mechanisms that allow for tracking of physical alert response and for real‐time evaluation, audit, and feedback of prophylaxis ordering and administration practices at NMH. Taken as a whole, this bundle of resources to improve adherence to optimal VTE prophylaxis will facilitate future interventions targeted at reaching defect‐free care.
Disclosures: Nothing to report.
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism, is a significant cause of morbidity and mortality in the United States among hospitalized patients.[1, 2, 3, 4, 5, 6] Although it may not be possible to completely eradicate VTE events,[7] chemical and/or mechanical prophylaxis can reduce VTE rates by up to 74% to 86%,[8, 9, 10] and meta‐analyses have demonstrated the benefit of VTE prophylaxis in the inpatient population.[11, 12] Despite evidence‐based guidelines regarding the appropriate type, duration, and dosing of prophylaxis, thromboprophylaxis has been found to be underutilized in the inpatient setting.[13, 14, 15]
Northwestern Memorial Hospital (NMH) historically performed poorly on VTE outcome measures. VTE in the surgical patient population was an especially glaring problem, as NMH was persistently found to be a risk‐adjusted poor performer in the American College of Surgeons National Surgical Quality Improvement Project (ACS‐NSQIP).
However, VTE outcome measures have been shown to be problematic due to their susceptibility to surveillance bias; that is, variation in the ordering of screening or diagnostic VTE imaging studies between hospitals leads to variable VTE rates (the more you look, the more you find).[16, 17, 18, 19] More vigilant hospitals that have a lower threshold to order an imaging study may find higher occurrences of VTE, and paradoxically be deemed a poor performer. Surveillance bias and the lack of validity of the VTE outcome measurement highlighted the importance of utilizing process‐of‐care measures in assessing hospital VTE prevention efforts.[20, 21] Thus, when the Joint Commission enacted 6 new VTE core process‐of‐care measures on January 1, 2013 to monitor hospital performance on VTE prophylaxis administration and VTE treatment (Table 1), NMH undertook a hospital‐wide quality‐improvement (QI) project utilizing the define‐measure‐analyze‐improve‐control (DMAIC) process improvement (PI) methodology to optimize their performance on these core measures as well as the Surgical Care Improvement Project (SCIP) SCIP‐VTE‐2 measure. In this article, we describe the QI effort undertaken at NMH to improve hospital‐level measure performance and the outcomes of this effort.
| VTE Measure | Measure Calculation | Description of Issues | Interventions | Preintervention Performance, % (N)* | Postintervention Performance, % (N) |
|---|---|---|---|---|---|
| |||||
| VTE‐1: VTE PPX | Patients who received VTE prophylaxis or have documentation why no VTE prophylaxis was given | Missing documentation (both chemical and mechanical); prophylaxis ordered, but not administered; patient refusals and opportunity to increase patient education regarding prophylaxis | 1. Enhanced, individualized VTE prophylaxis alert: alert incorporated order, administration, mechanical PPX, lab exclusion and contraindication details | NMH: 86.6% (174) | NMH: 93.6% (162) |
| All patients | Undocumented contraindication reasons | 2. Nursing education initiative: back‐to‐basics VTE education initiative to help increase the administration of VTE prophylaxis and improve patient education resulting in fewer patient refusals and missed doses | NMH general surgery: 94.4% (34) | NMH general surgery: 97.6% (41) | |
| Inconsistent monitoring and patient education | 3. Updated VTE prophylaxis surgical and medicine order set: updated order listing, heparin TID setting and contraindications | NMH general medicine: 82.5% (115) | NMH general medicine: 90.2% (85) | ||
| VTE‐2: ICU VTE PPX | Patients who received VTE prophylaxis or have documentation why no VTE prophylaxis was given | See interventions 1 through 3 | NMH: 100% (58) | NMH: 95.8% (69) | |
| Patients directly admitted or transferred to the ICU | NMH general surgery: 100% (11) | NMH general surgery: 100% (10) | |||
| NMH general medicine: 100% (40) | NMH general medicine: 100% (51) | ||||
| VTE‐3: VTE patients with anticoagulation overlap therapy | Patients who received overlap therapy of parenteral anticoagulation and warfarin therapy | Gaps in documentation and administration of overlap therapy for 5 days | 4. Overlap therapy alert at discharge: document VTE on diagnosis list with alert to either (1) document reason for discontinuation of parental therapy or (2) prescribe parental anticoagulation during hospitalization or at discharge | NMH: 95.8% (159) | NMH: 100% (105) |
| Patients with confirmed VTE who received warfarin | 5. Overlap therapy alert during hospitalization: documentation alert on the day therapy discontinued | NMH general surgery: 85.7% (12) | NMH general surgery: 100% (16) | ||
| NMH general medicine: 97.0% (129) | NMH general medicine: 100% (79) | ||||
| VTE‐4: VTE patients receiving unfractionated dosages/platelet count monitoring by protocol or nomogram | Patients who have IV UFH therapy dosages and platelet counts monitored according to defined parameters such as a nomogram or protocol | Missing required language on IV UFH orders and order may not include preselected CBC order | 6. Updated heparin order sets: reminder to monitor platelet counts per nomogram and preselect CBC order | NMH: 73.7% (98) | NMH: 100% (74) |
| Patients with confirmed VTE receiving IV UFH therapy | NMH general surgery: 56.3% (7) | NMH general surgery: 100% (9) | |||
| NMH general medicine: 83.8% (88) | NMH general medicine: 100% (52) | ||||
| VTE‐5: VTE warfarin discharge instructions | Patients with documentation that they or their caregivers were given written discharge instructions or other educational material about warfarin | Discharge process is not standardized | 7. Warfarin Patient Education Task: automate nursing task for warfarin order set, check individual warfarin education excluding consult orders | NMH: 9.6% (12) | NMH: 87.5% (63) |
| Patients with confirmed VTE discharged on warfarin therapy | Patient education during hospitalization varies | 8. Warfarin dotphrase: new warfarin/Coumadin dotphrase aligned with department and core measure requirements | NMH general surgery: 0% (0) | NMH general surgery: 100% (11) | |
| No standardized process for initiating and tracking warfarin education during hospitalization | 9. Department Warfarin Instructions Phase II: update department warfarin language, automate warfarin education task | NMH general medicine: 11.3% (12) | NMH general medicine: 85.5% (50) | ||
| Warfarin special instructions for discharge is not aligned with the EMR dotphrase | 10. Physician Referral Order Update: Add follow‐up reason to order | ||||
| Follow‐up appointments are inconsistent | |||||
| VTE‐6: Incidence of potentially preventable VTE | Patients who received no VTE PPX prior to the VTE diagnostic test order date | Failure reasons related to other measures | NMH: 8% (8) | NMH: 2.4% (2) | |
| Patients who developed confirmed VTE during hospitalization | NMH general surgery: 6.7% (1) | NMH general surgery: 0% (0) | |||
| NMH general medicine: 13.5% (7) | NMH general medicine: 0% (0) | ||||
| SCIP‐VTE‐2 | Surgery patients who receive appropriate VTE prophylaxis within 24 hours prior to anesthesia start time to 24 hours after anesthesia end time | Standard enoxaparin administration time is 1300 and there is a gap between surgery end time to enoxaparin administration (i.e. patient may wait up to 23 hours for prophylaxis) | 11. Updated VTE prophylaxis‐surgical and medicine order set: added 1‐time and 2‐time heparin doses to enoxaparin order section | NMH: 99.5% (202) | NMH: 100% (104) |
| All selected surgery patients | NMH General Surgery: 98.5% (67) | NMH General Surgery: 100% (100) | |||
| NMH General Medicine: N/A | NMH General Medicine: N/A | ||||
| Additional interventions | Incomplete VTE prophylaxis information | 12. Updated IPC view | |||
| Inconsistent documentation across forms | 13. Updated ADL forms and iView nursing responses updated | ||||
| 14. Updated unit snapshot to mirror IPC view | |||||
| 15. Updated MPET: updated nursing task: standardize Not Given and Not Done Nursing Responses | |||||
METHODS
Setting
NMH is a tertiary referral and teaching hospital affiliated with the Feinberg School of Medicine of Northwestern University. It is the flagship of Northwestern Medicine, which also includes 4 community hospitals, a dedicated women's hospital, and outpatient and urgent care centers.[22] NMH is an 885‐bed hospital with approximately 50,000 inpatients admitted annually. This project, to evaluate the outcomes of the NMH VTE QI initiative, was reviewed and approved by the Northwestern University Institutional Review Board as an exempt activity.
Measures
The Joint Commission VTE measures were a product of the National Consensus Standards for the Prevention and Care of Deep Vein Thrombosis project between the Joint Commission and National Quality Forum (NQF). These 6 measures are endorsed by the NQF and aligned with the Centers of Medicare and Medicaid Services.[23] SCIP also has measures focusing on VTE prophylaxis. SCIP‐VTE‐2 focuses on prophylaxis in the perioperative period (the 24 hours prior to anesthesia start time to 24 hours postanesthesia end time). Specific measure definitions are in Table 1. All patients hospitalized at NMH were eligible for case abstraction; specific inclusion and exclusion criteria were based on measure specifics set forth by The Joint Commission and SCIP, and random cases were selected for abstraction utilizing the standard sampling methodology required for these measures. Case abstraction was performed by a nurse and validated by physicians.
The Intervention
Review of baseline performance on the core measures began in January 2013. Common failure points were identified first by electronic medical record (EMR) evaluation. Subsequently, focus groups with front‐line staff, close examination of EMR ordering logic for chemical and mechanical prophylaxis with the IT department, hospital floor observations, and evaluation of the patient education process during discharge were performed to further define the reasons for common failure points.
Fifteen data‐driven, focused interventions were then designed, pilot tested, and implemented throughout the hospital in May 2013, with iterative improvement of each component over the next 18 months (Table 1). This project utilized DMAIC PI methodology, and was carried out by a multidisciplinary team with representatives from the departments of surgery, internal medicine, anesthesia, gynecology, PI, clinical quality, pharmacy, analytics, information technology (IT), and nursing. Broadly, the 15 interventions consisted of (1) EMR alerts, (2) education initiatives, (3) new EMR order sets, and (4) other EMR changes.
EMR Alerts
Novel provider alerts were built into NMH's inpatient EMR platform (Cerner PowerChart; Cerner Corp., North Kansas City, MO) to address common mistakes contributing to failures on VTE‐1 (chemoprophylaxis) and VTE‐3 (overlap therapy). Although VTE‐1 failures were often multifactorial, missing documentation regarding reasons for no chemoprophylaxis given and failures to order chemoprophylaxis were 2 common drivers of failures. To address these 2 problems, a logic‐driven alert to force patient‐specific ordering of appropriate VTE prophylaxis was developed (Figure 1). VTE‐3 (overlap therapy) failures occurred due to clinician failure to order a full 5 days of overlap therapy when switching from parenteral anticoagulation to warfarin therapy; hence, to target VTE‐3 performance, new alerts reminding clinicians to meticulously order and document the overlap of parenteral VTE therapy and warfarin were developed. As part of the logic‐driven alert to improve patient‐specific ordering of appropriate VTE prophylaxis, we allowed for the inclusion of documentation of a contraindication to explain why VTE prophylaxis was not ordered.

Educational Initiatives
After consulting with attending physicians, residents, nurses, and practice managers at NMH to understand the potential drivers of VTE‐1 (chemoprophylaxis) failures, a team of clinicians and PI experts held 2‐part interactive educational sessions with nurses to address knowledge deficits. The first part focused on general VTE education (eg, the significance of the problem nationwide as well as at NMH, general signs and symptoms of VTE, risk factors for VTE, and NMH‐specific failure rates for mechanical and chemoprophylaxis). The second portion used a myth‐busting approach, in which common misunderstandings that frequently impede VTE prophylaxis (eg, a patient capable of ambulating does not need sequential compression devices (SCDs), or SCDs cannot be applied to a patient with acute or chronic DVT) were discussed. Educational efforts also addressed VTE‐5 (warfarin discharge instructions) performance; although nurses provided patient education with regard to home warfarin use, the timing was inconsistent. The VTE‐5 education provided nurses with a standardized method and time for educating patients about postdischarge warfarin use. EMR changes ensured that when warfarin was ordered, warfarin education automatically populated the nurse's task list, reminding them to educate their patients prior to discharge.
New EMR Order Sets
Previously existing order sets often made it difficult for physicians to order the correct dosing and timing of VTE prophylaxis, document contraindications to prophylaxis, and lacked the appropriate laboratory orders with therapy orders. New order sets were designed to facilitate compliance with VTE‐1 (chemoprophylaxis), VTE‐4 (platelet monitoring), VTE‐5 (warfarin discharge instructions), and SCIP‐VTE‐2 (perioperative prophylaxis) by updating lab and medication order listings, dosing choices, prophylaxis contraindications, reminders to monitor platelet counts per nomogram, and physician follow‐up reasons. When we considered our hospital's specific local factors, we came to the conclusion that risk stratification would be a difficult strategy to apply effectively as a component of the new order sets, mainly due to barriers related to buy‐in from physicians and nurses.
Other EMR Changes
Other interventions targeted at specific issues were programmed into the EMR. For example, a shortcut (known as a dotphrase in Cerner PowerChart) for inserting warfarin instructions into patient care documentation was available to physicians, but was misaligned to the standard warfarin instructions. In addition, the physician responsible for following up on a patient's first outpatient international normalized ratio was often omitted from the discharge instructions, potentially leaving patients without a physician to adjust their dosing appropriately. Adding this physician information, as well as aligning and updating all discharge instructions, allowed for clear, consistent patient instructions for home warfarin use. Moreover, EMR forms used by physicians and forms used by nurses to check for VTE prophylaxis were inconsistent, thus leading to potential confusion between physicians and nurses. Accordingly, regularly used EMR forms (eg, the interdisciplinary plan of care, and the unit summary page or unit snapshot) were updated and standardized.
Control Mechanisms
Concurrent with the implementation of the 15 interventions was the development of several control mechanisms to ensure sustained improvement. These mechanisms consisted of (1) an electronic proxy measure for VTE‐1 (chemoprophylaxis) and (2) monitoring of clinician (including physicians, nurses, and midlevel providers) responses to the EMR alerts, and (3) a comprehensive EMR unit report (Figure 2).

Proxy Measure
Because the Joint Commission core measures are abstracted from only a sample of cases, and a time lag existed between each failure on VTE‐1 (chemoprophylaxis) to the time the QI team learned of the failure, a proxy measure was created. This proxy measure is used as a stand‐in for actual VTE‐1 measure performance, but is generated in real time and reflects performance throughout the entire hospital instead of a random sample of cases. Using the Northwestern Electronic Data Warehouse (EDW), the NMH analytics team created a report reflecting thromboprophylaxis administration on each hospital unit currently and over time. Performance could also be examined for each individual hospital service line. Being able to track longitudinal performance by unit and by service line enabled the QI team to understand trends in performance. Having the ability to examine patients who missed doses over the preceding few hours allowed unit leadership to proactively act upon the failures in a timely fashion, instead of waiting to receive their performance on the Joint Commission core measures.
Physician Alert Response Monitoring
Monitoring of clinical responses to EMR alerts was embedded as standard practice. Because alert fatigue is a documented unintended consequence of heavy reliance on EMR alerts,[24, 25] physicians and nurses who failed to respond to alerts regarding VTE prophylaxis were identified. Interventions targeted toward this group of nonresponders are currently being developed and tested.
EDW Unit Report
This report allows unit managers to track potential failures real time and act prior to a failure occurring (eg, missed chemoprophylaxis dose) through the NMH EDW (Figure 2). These reports contained detailed order and administration data at the individual patient, nurse, and physician levels. Missed doses of VTE chemoprophylaxis were immediately fed back to unit nursing managers who utilized the report to perform a rapid drilldown to identify the root cause(s) of the failure, and then rectify the failure while the patient was still hospitalized.
Statistical Analyses
Hospital performance on the VTE core measures and SCIP‐VTE‐2 was determined by trained nurse abstractors, who abstract cases randomly sampled by the University of HealthCare Consortium, and adjudicate findings as per the Specifications Manual for National Hospital Inpatient Quality Measures. Performance in the period prior to the QI intervention and in the period following the QI intervention was documented as proportions of abstracted cases found to be compliant with measure specifications. Differences between the pre‐ and postintervention periods were compared using a binomial test, with a P value <0.05 considered significant. All analyses were performed using Stata version 13 (StataCorp, College Station, TX).
RESULTS
A total of 1679 cases were abstracted to obtain core measure performance in the time period before the DMAIC intervention phase (January 1, 2013May 1, 2013), and 1424 cases were abstracted to obtain core measure performance in the time period after the DMAIC intervention phase (October 1, 2014April 1, 2015).
Overall NMH performance on measures VTE‐1 (chemoprophylaxis) and VTE36 (overlap therapy, platelet monitoring, warfarin discharge instructions, hospital‐acquired [HA]‐VTE) improved significantly (P < 0.05) (Table 1). No improvement was seen on VTE‐2 (intensive care unit chemoprophylaxis) given that pre‐ and postintervention performance was 100%, which likely reflects previous hospital efforts to improve adherence to this measure. The percentage of patients who failed measure VTE‐6 (number of patients with HA‐VTE who did not have VTE prophylaxis ordered prior to diagnosis of their VTE) decreased from 8% to 2.4%, demonstrating improved VTE prevention prescribing habits in NMH providers rather than a change in VTE event rates (ie, if more patients receive prophylaxis, they cannot be included in the numerator). Performance on SCIP‐VTE‐2 (perioperative chemoprophylaxis) increased from 99.5% to 100% as well but did not reach significance given the baseline high performance.
Measure performance on the general surgery services was comparable to the general medical services, with 1 exception. VTE‐1 (chemoprophylaxis) performance was lower both prior to and following the QI intervention on general medicine services (medicine: 82.5% to 90.2% vs surgery: 94.4% to 97.6%). Recent performance on the VTE‐1 proxy measure has proven to be stable between 95% and 97% on surgery services. Physician response to alerts has increased slightly among the NMH general medicine practitioners (15.2%19.1%) but has been stable among NMH general surgery providers.
DISCUSSION
Our study demonstrates that a formal DMAIC QI project taken on by a multidisciplinary team (including clinicians from multiple specialties as well as personnel from IT, nursing, analytics, and PI) can be successfully implemented and can result in marked improvement in VTE core process measure performance. We used a multifaceted approach undertaken by the NMH VTE QI team, utilizing 15 data‐driven interventions including EMR alerts, education initiatives, and new EMR order sets. These were combined with strong control mechanisms to sustain gains.
Previously published studies on VTE prophylaxis practices found that projects combining both passive (ie, helping clinicians to remember to risk‐assess their patients' for VTE) and active (ie, assisting clinicians in appropriate prescribing practices) strategies are the most successful.[26] Our improvement on VTE‐1 can be compared to previous studies examining changes in ordering rates of VTE prophylaxis. Other QI projects that featured a combination of interventions observed similar significant increases in prophylaxis ordering.[27, 28] Our improvement on VTE‐1 (chemoprophylaxis) was significant, although the difference between pre‐ and postintervention performance varied by service type (general surgery vs general medicine vs other). The small increment of improvement on surgical services was likely attributable to a high baseline performance. Prior to 2013, surgically focused VTE prophylaxis QI efforts spurred by poor ACS‐NSQIP performance proved to be successful, thus resulting in high surgical prophylaxis rates at the outset of the hospital‐wide VTE DMAIC project.
One of the most significant unanticipated barriers to improving performance on VTE‐1 (chemoprophylaxis) included the different hospital subcultures on the medical floors as compared to the surgical floors. The surgical floors had higher rates of compliance with VTE‐1 than the general medicine floors both before and after the QI interventions. When the root causes were explored, the medical floors were found to have different ordering and administration patterns. These, in part, stemmed from differing guidelines[29] and standards in the literature regarding VTE prophylaxis for medical and surgical patients. Multiple discussions within the multidisciplinary QI team and with each involved department were held, focusing on the data regarding safe care in medical patients at low risk for a VTE. Subsequent EMR alerts alterations reflected the internal medicine VTE prophylaxis recommendations for medical patients, allowing that low‐risk patients could be assessed by the provider and given as a reason for foregoing VTE prophylaxis.
Barriers to VTE prophylaxis administration were encountered on the nursing front as well. Floor observations illustrated that chemoprophylaxis injections were often offered as an optional medication. Patients, when given the choice of receiving an injection or not, would understandably choose to forgo their heparin or enoxaparin shot. This missed dose was then documented as a patient refusal. This may not be a problem unique to NMH; 1 study demonstrated that almost 12% of chemoprophylaxis doses may not be administered, and a frequent reason may be due to patient refusal.[30] The lack of patient education regarding the importance of receiving chemical prophylaxis was an improvement opportunity at both the nursing and physician level. Not only did physicians and nurses take the responsibility to educate patients on the importance of receiving the proper prophylaxis, but nursing managers were made responsible for acting on missed doses that were listed on the real‐time performance reports for their units. Missed prophylaxis doses thus became an actionable item instead of an acceptable occurrence.
Culture change in an organization is difficult and necessitates sustained efforts. An important component of our project is our control mechanism, in which a real‐time, continuously updated unit report leverages data from our EDW to generate ongoing performance reports that are regularly reviewed by hospital leadership, clinical process owners, and, most importantly, frontline nurse managers. The unit‐specific reports allow nurse managers and clinical project owners to review prophylaxis failures on a case‐by‐case basis daily and to address and rectify the cause. In addition, the QI team tracks individual physician action taken in response to EMR alerts. As performance feedback to surgical trainees has been demonstrated to have a positive effect on ordering practices,[31] efforts to improve resident alert response rates by means of feedback and education are underway.
Limitations
Our results have to be interpreted within certain limitations. First, given that hospital performance on the VTE core measures is determined by abstracting only a sample of eligible cases, it is possible that our results were affected by sampling error. Second, because of problems with the VTE outcome measure due to surveillance bias, we are unable to draw any valid conclusions about changes in VTE event rates as a result of this QI project. Third, because many of our interventions were tailored to NMH's EMR platform and local hospital culture, it is possible that parts of our project are not readily generalizable to other hospitals; however, we believe that many components, such as the alert logics, can be easily tailored to other EMR platforms.
CONCLUSION
This institutional project was a large, multidisciplinary, and sustained undertaking that improved our performance on the VTE core measures. We believe that our bundle of EMR modifications, alerts (particularly the underlying alert logics), order sets, and standardization of summary EMR view can be adopted in other settings with appropriate adaptations to each hospital's specific local environment. Our focused educational interventions can also be easily adapted to other hospital settings. Perhaps the most important part of the project was the construction of novel control mechanisms that allow for tracking of physical alert response and for real‐time evaluation, audit, and feedback of prophylaxis ordering and administration practices at NMH. Taken as a whole, this bundle of resources to improve adherence to optimal VTE prophylaxis will facilitate future interventions targeted at reaching defect‐free care.
Disclosures: Nothing to report.
- , , , , . Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:7S–47S.
- , , , , . Estimated annual numbers of US acute‐care hospital patients at risk for venous thromboembolism. Am J Hematol. 2007;82:777–782.
- , , , et al. All‐cause and potentially disease‐related health care costs associated with venous thromboembolism in commercial, Medicare, and Medicaid beneficiaries. J Manag Care Pharm. 2012;18:363–374.
- , . Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835–1846.
- , , , . Long‐term outcomes after deep vein thrombosis: postphlebitic syndrome and quality of life. J Gen Intern Med. 2000;15:425–429.
- , , , et al. The long‐term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7.
- , . The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009;301:1063–1065.
- , , , et al. Hidden costs associated with venous thromboembolism: impact of lost productivity on employers and employees. J Occup Environ Med. 2014;56(9):979–985.
- , . Designing and implementing effective venous thromboembolism prevention protocols: lessons from collaborative efforts. J Thromb Thrombolysis. 2010;29:159–166.
- , , , . Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162–1173.
- , , , , . Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278–288.
- , , , , . Meta‐analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913–930.
- , , , et al. Preventability of hospital‐acquired venous thromboembolism. JAMA Surg. 2015;150(9):912–915.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE survey): findings in surgical patients. Ann Surg. 2010;251:330–338.
- , , , . Are surgical patients at risk of venous thromboembolism currently meeting the Surgical Care Improvement Project performance measure for appropriate and timely prophylaxis? J Thromb Thrombolysis. 2010;30:55–66.
- , , , et al. Evaluation of surveillance bias and the validity of the venous thromboembolism quality measure. JAMA. 2013;310:1482–1489.
- , , , , , . Evaluation of hospital factors associated with hospital postoperative venous thromboembolism imaging utilisation practices. BMJ Qual Saf. 2014;23(11):947–956.
- , , , et al. Association between hospital imaging use and venous thromboembolism events rates based on clinical data. Ann Surg. 2014;260:558–564; discussion 64–66.
- , , , , . Postoperative venous thromboembolism outcomes measure: analytic exploration of potential misclassification of hospital quality due to surveillance bias. Ann Surg. 2015;261(3):443–444.
- , , . The need to revisit VTE quality measures. JAMA. 2014;312:286–287.
- . Facilitating quality improvement: pushing the pendulum back toward process measures. JAMA. 2015;314:1333–1334.
- Northwestern Medicine website. Available at: https://www.nm.org/locations‐at‐northwestern‐medicine. Accessed February 23, 2016.
- Venous thromboembolism. The Joint Commission website. Available at: http://www.jointcommission.org/venous_thromboembolism. Accessed February 23, 2016.
- , , , , . Some unintended consequences of clinical decision support systems. AMIA Annu Symp Proc. 2007:26–30.
- , , , . Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13:138–147.
- , , , et al. A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals. Ann Surg. 2005;241:397–415.
- , , , et al. Optimizing prevention of hospital‐acquired venous thromboembolism (VTE): prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5:10–18.
- , , . Improving the use of venous thromboembolism prophylaxis in an Australian teaching hospital. Qual Saf Health Care. 2009;18:408–412.
- , , , , . Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155:625–632.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8:e66311.
- , , , et al. Individualized performance feedback to surgical residents improves appropriate venous thromboembolism prophylaxis prescription and reduces potentially preventable VTE: a prospective cohort study [published online November 25, 2015]. Ann Surg. doi: 10.1097/SLA.0000000000001512.
- , , , , . Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:7S–47S.
- , , , , . Estimated annual numbers of US acute‐care hospital patients at risk for venous thromboembolism. Am J Hematol. 2007;82:777–782.
- , , , et al. All‐cause and potentially disease‐related health care costs associated with venous thromboembolism in commercial, Medicare, and Medicaid beneficiaries. J Manag Care Pharm. 2012;18:363–374.
- , . Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835–1846.
- , , , . Long‐term outcomes after deep vein thrombosis: postphlebitic syndrome and quality of life. J Gen Intern Med. 2000;15:425–429.
- , , , et al. The long‐term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7.
- , . The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009;301:1063–1065.
- , , , et al. Hidden costs associated with venous thromboembolism: impact of lost productivity on employers and employees. J Occup Environ Med. 2014;56(9):979–985.
- , . Designing and implementing effective venous thromboembolism prevention protocols: lessons from collaborative efforts. J Thromb Thrombolysis. 2010;29:159–166.
- , , , . Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162–1173.
- , , , , . Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278–288.
- , , , , . Meta‐analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913–930.
- , , , et al. Preventability of hospital‐acquired venous thromboembolism. JAMA Surg. 2015;150(9):912–915.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE survey): findings in surgical patients. Ann Surg. 2010;251:330–338.
- , , , . Are surgical patients at risk of venous thromboembolism currently meeting the Surgical Care Improvement Project performance measure for appropriate and timely prophylaxis? J Thromb Thrombolysis. 2010;30:55–66.
- , , , et al. Evaluation of surveillance bias and the validity of the venous thromboembolism quality measure. JAMA. 2013;310:1482–1489.
- , , , , , . Evaluation of hospital factors associated with hospital postoperative venous thromboembolism imaging utilisation practices. BMJ Qual Saf. 2014;23(11):947–956.
- , , , et al. Association between hospital imaging use and venous thromboembolism events rates based on clinical data. Ann Surg. 2014;260:558–564; discussion 64–66.
- , , , , . Postoperative venous thromboembolism outcomes measure: analytic exploration of potential misclassification of hospital quality due to surveillance bias. Ann Surg. 2015;261(3):443–444.
- , , . The need to revisit VTE quality measures. JAMA. 2014;312:286–287.
- . Facilitating quality improvement: pushing the pendulum back toward process measures. JAMA. 2015;314:1333–1334.
- Northwestern Medicine website. Available at: https://www.nm.org/locations‐at‐northwestern‐medicine. Accessed February 23, 2016.
- Venous thromboembolism. The Joint Commission website. Available at: http://www.jointcommission.org/venous_thromboembolism. Accessed February 23, 2016.
- , , , , . Some unintended consequences of clinical decision support systems. AMIA Annu Symp Proc. 2007:26–30.
- , , , . Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13:138–147.
- , , , et al. A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals. Ann Surg. 2005;241:397–415.
- , , , et al. Optimizing prevention of hospital‐acquired venous thromboembolism (VTE): prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5:10–18.
- , , . Improving the use of venous thromboembolism prophylaxis in an Australian teaching hospital. Qual Saf Health Care. 2009;18:408–412.
- , , , , . Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155:625–632.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8:e66311.
- , , , et al. Individualized performance feedback to surgical residents improves appropriate venous thromboembolism prophylaxis prescription and reduces potentially preventable VTE: a prospective cohort study [published online November 25, 2015]. Ann Surg. doi: 10.1097/SLA.0000000000001512.
© 2016 Society of Hospital Medicine