User login
Optimizing diagnostic testing for venous thromboembolism
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
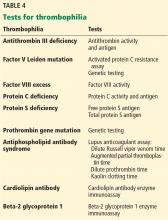
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
KEY POINTS
- A pretest clinical prediction tool such as the Wells score can help in deciding whether a patient with suspected venous thromboembolism warrants further workup.
- A clinical prediction tool should be used in concert with additional laboratory testing (eg, D-dimer) and imaging in patients at risk.
- In many cases, screening for thrombophilia to determine the cause of a venous thromboembolic event may be unwarranted.
- Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked.
Patients with challenging behaviors: Communication strategies
From time to time, all physicians encounter patients whose behavior evokes negative emotions. In 1978, in an article titled “Taking care of the hateful patient,”1 Groves detailed 4 types of patients—“dependent clingers, entitled demanders, manipulative help-rejecters, and self-destructive deniers”1—that even the most seasoned physicians dread, and provided suggestions for managing interactions with them. The topic was revisited and updated in 2006 by Strous et al.2
Now, more than 10 years later, the challenge of how to interact with difficult patients is more relevant than ever. A cultural environment in which every patient can become an “expert” via the Internet has added new challenges. Patients who are especially time-consuming and emotionally draining exacerbate the many other pressures physicians face today (eg, increased paperwork, cost-consciousness, shortened appointment times, and the move to electronic medical records), contributing to physician burnout.
This article further updates the topic of managing challenging patients to reflect the current practice climate. We provide a more modern view of challenging patients and provide guidance on handling them. Although it may be tempting to diagnose some of these patients as having a personality disorder, it can often be more helpful to recognize patterns of behavior and have a clear plan for management. We also discuss general coping strategies for avoiding physician burnout.
INTERNET-SEEKING, QUESTIONING
A 45-year-old man carries in an overstuffed briefcase for his first primary care visit. He is a medical editor for a national journal and recently worked on a case study involving a rare cancer. As he edited, he recognized that he had the same symptoms and diagnosed himself with the same disease. He has brought with him a sheaf of articles he found on the Internet detailing clinical trials for experimental treatments. When the doctor begins to ask questions, he says the answers are irrelevant: he explains that he would have gone straight to an oncologist, but his insurance policy requires that he also have a primary care physician. He now expects the doctor to order magnetic resonance imaging, refer him to an oncologist, and support his request for the treatment he has identified as best.
The Internet: A blessing and a curse
Patients now have access to enormous amounts of information of variable accuracy. As in this case, patients may come to an appointment carrying early research studies that the physician has not yet reviewed. Others get their information from patient blogs that frequently offer opinions without evidence. Often, based on an advertisement or Internet reading, a patient requests a particular medication or test that may not be cost-effective or medically justified.
In a survey more than 10 years ago, more than 75% of physicians reported that they had patients who brought in information from online sources.3 Hu et al4 reported that 70% of patients who had online information planned to discuss it with their physicians. This practice is only growing, including in older patients.5
Physicians may feel confused and frustrated by patients who come armed with information. They may infer that patients do not trust them to diagnose correctly or treat optimally. In addition, discussing such information takes time, causing others on the schedule to wait, adding to the stress of coping with over-booked appointments.
Why so overprepared?
Patients who have or fear that they have cancer may be particularly worried that an important treatment will be overlooked.6 Since they feel that their life is hanging in the balance, their search for a definitive cure is understandable.
Internet-seeking, intensely questioning patients clearly want more information about the treatments they are receiving, alternative medical or procedural options, and complementary therapies.7 The response to their desire for more information affects their impression of physician empathy.8
Adapting to a more informed patient
Approaching these patients as an opportunity to educate may result in a more trusting patient and one more likely to be open to physician guidance and more likely to adhere to an advised treatment plan. Triangulation of the 3 actors—the physician, the patient, and the Web—can help patients to make more-informed choices and foster an attitude of partnership with the physician to lead them to optimal healthcare.
In a review of the impact of Internet use on healthcare and the physician-patient relationship, Wald et al9 urged physicians to:
- Adopt a positive attitude toward discussing Internet contributions
- Encourage patients to take an active role in maintaining health
- Acknowledge patient concerns and fears
- Avoid becoming defensive
- Recommend credible Internet sources.
Laing et al10 urged physicians to recognize rather than deny the effects of patients’ online searching for information and support, and not to ignore the potential impact on treatment. Consumers are gaining autonomy and self-efficacy, and Laing et al encouraged healthcare providers to develop ways to incorporate this new reality into the services they provide.10
How Web-based interaction can assist in patient decision-making for colorectal cancer screening is being explored.11 Patients at home can use an online tool to learn about screening choices and would be more knowledgable and comfortable discussing the options with their care provider. The hope is to build in an automatic reminder for the clinician, who would better understand the patient’s preference before the office visit.
One approach to our patient is to say, “I can see how worried you are about having the same type of cancer you read about, and I want to help you. It is clear to me that you know a lot about healthcare, and I appreciate your engagement in your health. How about starting over? Let me ask a few questions so I can get a better perspective on your symptoms?” Many times, this strategy can help patients reframe their view and accept help.
DEMANDING, LITIGATION-THREATENING
A 60-year-old lawyer is admitted to the hospital for evaluation of abdominal pain. His physician recommends placing a nasogastric tube to provide nutrition while the evaluation is completed. His wife, a former nurse practitioner, insists that a nasogastric tube would be too dangerous and demands that he be allowed to eat instead. The couple declares the primary internal medicine physician incompetent, does not want any residents to be involved in his care, and antagonizes the nurses with constant demands. Soon, the entire team avoids the patient’s room.
Why so hostile?
People with demanding behavior often have a hostile and confrontational manner. They may use medical jargon and appear to believe that they know more than their healthcare team. Many demand to know why they have not been offered a particular test, diagnosis, or treatment, especially if they or a family member has a healthcare background. Such patients appear to feel that they are being treated incorrectly and leave us feeling vulnerable, wondering whether the patient might one day come back to haunt us with a lawsuit, especially if the medical outcome is unfavorable.
Understanding the motivation for the behavior can help a physician to empathize with the demanding patient.12 Although it may seem that the demanding patient is trying to intimidate the physician, the goal is usually the same: to find the best possible treatment. Anger and hostility are often motivated by fear and a sense of losing control.
Ironically, this maladaptive coping style may alienate the very people who can help the patient. Hostile behavior evokes defensiveness and resentment in others. A power struggle may ensue: as the patient makes more unreasonable demands and threats, the physician reacts by asserting his or her views in an attempt to maintain control. Or the physician and the rest of the healthcare team may simply avoid the patient as much as possible.
Collaboration can defuse anger
The best strategy is often to steer the encounter away from a power struggle by legitimizing the patient’s feeling of entitlement to the best possible treatment.13 Take a collaborative stance with the patient, with the common goal of finding and implementing the most effective and lowest-risk diagnostic and treatment plan. Empathy and exploration of the patient’s concerns are always in order.
Physicians can try several strategies to improve interactions with demanding patients and caregivers:
Be consistent. All members of the healthcare team, including nurses and specialists, should convey consistent messages regarding diagnostic testing and treatment plans.
Don’t play the game. Demanding patients often complain about being mistreated by other healthcare providers. When confronted with such complaints, acknowledge the patient’s feelings while refraining from blaming or criticizing other members of the healthcare team.
Clarify expectations. Clarifying expectations from the initial patient encounter can prevent conflicts later. Support a patient who must accept a diagnosis of a terminal illness, and then when appropriate, discuss goals moving forward. Collaboration within the framework of reasonable expectations is key.
For our case, the physician could say, “We want to work with you together as a team. We will work hard to address your concerns, but our nurses must have a safe environment in which to help you.” Such a statement highlights shared goals and expression of concern without judgment. The next step is to clarify expectations by describing the hospital routine and how decisions are made.
Offer choices. Offering choices whenever possible can help a demanding patient feel more in control. Rather than dismiss a patient’s ideas, explore the alternatives. While effective patient communication is preferable to repeated referrals to specialists,14 judicious referral can engender trust in the physician’s competence if a diagnosis is not forthcoming.15
A unique challenge in teaching hospitals is the patient who refuses to interact with residents and students. It is best to acknowledge the patient’s concerns and offer alternative options:
- If the patient is worried about lack of completed training, then clarify the residents’ roles and reassure the patient that you communicate with residents and supervisors regarding any clinical decisions
- If possible, offer to see the patient alone or have the resident interact only on an as-needed basis
- Consider transferring the patient to a nonteaching service or to another hospital.
Admit failings. Although not easy, admitting to and apologizing for things that have gone wrong can help to calm a demanding patient and even reduce the likelihood of a lawsuit.16 The physician should not convey defensiveness and instead should acknowledge the limitations of the healthcare system.
Legitimize concerns—to an extent. Legitimizing a demanding patient’s concerns is important, but never be bullied into taking actions that create unnecessary risk. Upsetting a demanding patient is better than ordering tests or providing treatments that are potentially harmful. Good physician-patient communication can go a long way toward preventing adverse outcomes.
CONSTANTLY SEEKING REASSURANCE
A 25-year-old professional presents to a new primary care provider concerned about a mole on her back. She discusses her sun exposure and family history of skin cancer and produces photographs documenting changes in the mole over time. Impressed with this level of detail, the physician takes time to explain his concerns before referring her to a dermatologist. Later that day, she calls to let the doctor know that her procedure has been scheduled and to thank him for his care. A few weeks after the mole is removed, she returns to discuss treatment options for the small remaining scar.
After this appointment, she calls the office repeatedly with a wide array of concerns, including an isolated symptom of fatigue that could indicate cancer and the relative merits of different sunscreens. She also sends the physician frequent e-mail messages through the personal health record system with pictures of inconsequential marks on her skin.
Needing reassurance is normal—to a point
Many patients seek reassurance from their physicians, and this can be done in a healthy and respectful manner. But requests for reassurance may escalate to becoming repeated, insistent, and even aggressive.1 This can elicit reactions from physicians ranging from feeling annoyed and burdened to feeling angry and overwhelmed.17 This can lead to significant stress, which, if not managed well, can lead to excessively control of physician behavior and substandard care.18
Reassurance-seeking behavior can manifest anywhere along the spectrum of health and disease.19 It may be a symptom of health anxiety (ie, an exaggerated fear of illness) or hypochondria (ie, the persistent conviction that one is currently or likely to become ill).20,21
Why so needy?
Attachment theory may help explain neediness. Parental bonding during childhood is associated with mental and physical health and health-related behaviors in adults.22,23 People with insecure-preoccupied attachment styles tend to be overly emotionally dependent on the acceptance of others and may exhibit dependent and care-seeking behaviors with a physician.24
Needy patients are often genuinely grateful for the care and attention from a physician.1 In the beginning, the doctor may appreciate the patient’s validation of care provided, but this positive feeling wanes as calls and requests become incessant. As the physician’s exhaustion increases with each request, the care and well-being of the patient may no longer be the primary focus.1
Set boundaries
Be alert to signs that a patient is crossing the line to an unhealthy need for reassurance. Address medical concerns appropriately, then institute clear guidelines for follow-up, which should be reinforced by the entire care team if necessary.22
The following strategies can be useful for defining boundaries:
- Instruct the patient to come to the office only for scheduled follow-up visits and to call only during office hours or in an emergency
- Be up-front about the time allowed for each appointment and ask the patient to help focus the discussion according to his or her main concerns25
- Consider telling the patient, “You seem really worried about a lot of physical symptoms. I want to reassure you that I find no evidence of a medical illness that would require intervention. I am concerned about your phone calls and e-mails, and I wonder what would be helpful at this point to address your concerns?”
- Consider treating the patient for anxiety.
It is important to remain responsive to all types of patient concerns. Setting boundaries will guide patients to express health concerns in an appropriate manner so that they can be heard and managed.18,19
SELF-INJURY
A 22-year-old woman presents to the emergency department complaining of abdominal pain. After a full workup, the physician clears her medically and orders a few laboratory tests. As the nurse draws blood samples, she notices multiple fresh cuts on the patient’s arm and informs the physician. The patient is questioned and examined again and acknowledges occasional thoughts of self-harm.
Her parents arrive and appear appropriately concerned. They report that she has been “cutting” for 4 years and is regularly seeing a therapist. However, they say that they are not worried for her safety and that she has an appointment with her therapist this week. Based on this, the emergency department physician discharges her.
Two weeks later, the patient returns to the emergency department with continued cutting and apparent cellulitis, prompting medical admission.
Self-injury presents in many ways
Self-injurious behaviors come in many forms other than the easily recognized one presented in this case: eg, a patient with cirrhosis who continues to drink, a patient with severe epilepsy who forgets to take medications and lands in the emergency department every week for status epilepticus, a patient with diabetes who eats a high-sugar diet, a patient with renal insufficiency who ignores water restrictions, or a patient with an organ transplant who misses medications and relapses.
There is an important psychological difference between patients who knowingly continue to challenge their luck and those who do not fully understand the severity of their condition and the consequences of their actions. The patient who simply does not “get it” can sometimes be managed effectively with education and by working with family members to create an environment to facilitate critical healthy behaviors.
Patients who willfully self-inflict injury are asking for help while doing everything to avoid being helped. They typically come to the office or the emergency department with assorted complaints, not divulging the real reason for their visit until the last minute as they are leaving. Then they drop a clue to the real concern, leaving the physician confused and frustrated.
Why deny an obvious problem?
Fear of the stigma of mental illness can be a major barrier to full disclosure of symptoms of psychological distress, and this especially tends to be the case for patients from some ethnic minorities.26
On the other hand, patients with borderline or antisocial personality disorder (and less often, schizotypal or narcissistic personality disorder) frequently use denial as their primary psychological defense. Self-destructive denial is sometimes associated with traumatic memories, feelings of worthlessness, or a desire to reduce self-awareness and rationalize harmful behaviors. Such patients usually need lengthy treatment, and although the likelihood of cure is low, therapy can be helpful.27–29
Lessons from psychiatry
It can be difficult to maintain empathy for patients who intentionally harm themselves. It is helpful to think of these patients as having a terminal illness and to recognize that they are suffering.
Different interventions have been studied for such patients. Dialectical behavior therapy, an approach that teaches patients better coping skills for regulating emotions, can help reduce maladaptive emotional distress and self-destructive behaviors.30–32 Lessons from this approach can be applied by general practitioners:
- Engage the patient and together establish an effective crisis management plan
- With patient permission, involve the family in the treatment plan
- Set clear limits about self-harm: once the patient values interaction with the doctor, he or she will be less likely to break the agreement.
Patients with severe or continuing issues can be referred to appropriate services that offer dialectical behavior therapy or other intensive outpatient programs.
To handle our patient, one might start by saying, “I am sorry to see you back in the ER. We need to treat the cellulitis and get your outpatient behavioral team on board, so we know the plan.” Then, it is critical that the entire team keep to that plan.
HOW TO STAY IN CONTROL AND IMPROVE INTERACTIONS
Patients with challenging behaviors will always be part of medical practice. Physicians should be aware of their reactions and feelings towards a patient (known in psychiatry as countertransference), as they can increase physician stress and interfere with providing optimal care. Finding effective ways to work with difficult patients will avoid these outcomes.
Physicians also feel loss of control
Most physicians are resilient, but they can feel overwhelmed under certain circumstances. According to Scudder and Shanafelt,33 a physician’s sense of well-being is influenced by several factors, including feelings of control in the workplace. It is easy to imagine how one or more difficult patients can create a sense of overwhelming demand and loss of control.
These tips can help maintain a sense of control and improve interactions with patients:
Have a plan for effective communication. Not having a plan for communicating in a difficult situation can contribute to loss of control in a hectic schedule that is already stretched to its limits. Practicing responses with a colleague for especially difficult patients or using a team approach can be helpful. Remaining compassionate while setting boundaries will result in the best outcome for the patient and physician.
Stop to analyze the situation. One of the tenets of cognitive-behavioral therapy is recognizing that negative thoughts can quickly take us down a dangerous path. Feeling angry and resentful without stopping to think and reflect on the causes can lead to the physician feeling victimized (just like many difficult patients feel).
It is important to step back and think, not just feel. While difficult patients present in different ways, all are reacting to losing control of their situation and want support. During a difficult interaction with a patient, pause to consider, “Why is he behaving this way? Is he afraid? Does he feel that no one cares?”
When a patient verbally attacks beyond what is appropriate, recognize that this is probably due less to anything the physician did than to the patient’s internal issues. Identifying the driver of a patient’s behavior makes it easier to control our own emotions.
Practice empathy. Difficult patients usually have something in their background that can help explain their inappropriate behavior, such as a lack of parental support or abuse. Being open to hearing their story facilitates an empathetic connection.
AVOIDING BURNOUT
Discuss problems
Sadly, physicians often neglect to talk with each other and with trainees about issues leading to burnout, thereby missing important opportunities for empathy, objectivity, reflection, and teaching moments.
Lessons can be gleaned from training in psychiatry, a field in which one must learn methods for working effectively in challenging situations. Dozens of scenarios are practiced using videotapes or observation through one-way mirrors. While not everyone has such opportunities, everyone can discuss issues with one another. Regularly scheduled facilitated groups devoted to discussing problems with colleagues can be enormously helpful.
Schedule quiet times
Mindfulness is an excellent way to spend a few minutes out of every 3- to 4-hour block. There are many ways to help facilitate such moments. Residents and students can be provided with a small book, Mindfulness on the Go,37 aimed for the busy person.
Deep, slow breathing can bring rapid relief to intense negative feelings. Not only does it reduce anxiety faster than medication, but it is also free, is easily taught to others, and can be done unobtrusively. A short description of the role of the blood pH in managing the locus ceruleus and vagus nerve’s balance of sympathetic and parasympathetic activity may capture the curiosity of someone who may otherwise be resistant to the exercise.
Increasingly, hospitals are developing mindfulness sessions and offering a variety of skills physicians can put into their toolbox. Lessons from cognitive-behavioral therapy, dialectical behavior therapy, imagery, and muscle relaxation can help physicians in responding to patients. Investing in communication skills training specific to challenging behaviors seen in different specialties better equips physicians with more effective strategies.
- Groves JE. Taking care of the hateful patient. N Engl J Med 1978; 298:883–887.
- Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med 2006; 17:387–393.
- Malone M, Mathes L, Dooley J, While AE. Health information seeking and its effect on the doctor-patient digital divide. J Telemed Telecare 2005; 11(suppl1):25–28.
- Hu X, Bell RA, Kravitz RL, Orrange S. The prepared patient: information seeking of online support group members before their medical appointments. J Health Commun 2012; 17:960–978.
- Tse MM, Choi KC, Leung RS. E-health for older people: the use of technology in health promotion. Cyberpsychol Behav 2008; 11:475–479.
- Pereira JL, Koski S, Hanson J, Bruera ED, Mackey JR. Internet usage among women with breast cancer: an exploratory study. Clin Breast Cancer 2000; 1:148–153.
- Brauer JA, El Sehamy A, Metz JN, Mao JJ. Complementary and alternative and supportive care at leading cancer centers: a systematic analysis of websites. J Altern Complement Med 2010; 16:183–186.
- Smith SG, Pandit A, Rush SR, Wolf MS, Simon C. The association between patient activation and accessing online health information: results from a national survey of US adults. Health Expect 2015; 18:3262–3273.
- Wald HS, Dube CE, Anthony DC. Untangling the Web—the impact of Internet use on health care and the physician-patient relationship. Patient Educ Couns 2007; 68:218–224.
- Laing A, Hogg G, Winkelman D. Healthcare and the information revolution: re-configuring the healthcare service encounter. Health Serv Manage Res 2004; 17:188–199.
- Jimbo M, Shultz CG, Nease DE, Fetters MD, Power D, Ruffin MT 4th. Perceived barriers and facilitators of using a Web-based interactive decision aid for colorectal cancer screening in community practice settings: findings from focus groups with primary care clinicians and medical office staff. J Med Internet Res 2013; 15:e286.
- Steinmetz D, Tabenkin H. The ‘difficult patient’ as perceived by family physicians. Fam Pract 2001; 18:495–500.
- Arciniegas DB, Beresford TP. Managing difficult interactions with patients in neurology practices: a practical approach. Neurology 2010; 75(suppl 1):S39–S44.
- Gallagher TH, Lo B, Chesney M, Christensen K. How do physicians respond to patient’s requests for costly, unindicated services? J Gen Intern Med 1997; 12:663–668.
- Breen KJ, Greenberg PB. Difficult physician-patient encounters. Intern Med J 2010; 40:682–688.
- Huntington B, Kuhn N. Communication gaffes: a root cause of malpractice claims. Proc (Bayl Univ Med Cent) 2003; 16:157–161.
- Maunder RG, Panzer A, Viljoen M, Owen J, Human S, Hunter JJ. Physicians’ difficulty with emergency department patients is related to patients’ attachment style. Soc Sci Med 2006; 63:552–562.
- Thompson D, Ciechanowski PS. Attaching a new understanding to the patient-physician relationship in family practice. J Am Board Fam Pract 2003; 16:219–226.
- Groves M, Muskin P. Psychological responses to illness. In: Levenson JL, ed. The American Psychiatric Publishing Textbook of Psychosomatic Medicine. Arlington, VA: American Psychiatric Publishing, Inc; 2004:68–88.
- Görgen SM, Hiller W, Witthöft M. Health anxiety, cognitive coping, and emotion regulation: a latent variable approach. Int J Behav Med 2014; 21:364–374.
- Strand J, Goulding A, Tidefors I. Attachment styles and symptoms in individuals with psychosis. Nord J Psychiatry 2015; 69:67–72.
- Hooper LM, Tomek S, Newman CR. Using attachment theory in medical settings: implications for primary care physicians. J Ment Health 2012; 21:23–37.
- Bowlby J. Attachment and loss. 1. Attachment. New York, NY: Basic Books; 1969.
- Fuertes JN, Anand P, Haggerty G, Kestenbaum M, Rosenblum GC. The physician-patient working alliance and patient psychological attachment, adherence, outcome expectations, and satisfaction in a sample of rheumatology patients. Behav Med 2015; 41:60–68.
- Frederiksen HB, Kragstrup J, Dehlholm-Lambertsen B. Attachment in the doctor-patient relationship in general practice: a qualitative study. Scand J Prim Health Care 2010; 28:185–190.
- Rastogi P, Khushalani S, Dhawan S, et al. Understanding clinician perception of common presentations in South Asians seeking mental health treatment and determining barriers and facilitators to treatment. Asian J Psychiatr 2014; 7:15–21.
- van der Kolk BA, Perry JC, Herman JL. Childhood origins of self-destructive behavior. Am J Psychiatry 1991; 148:1665–1671.
- Gacono CB, Meloy JR, Berg JL. Object relations, defensive operations, and affective states in narcissistic, borderline, and antisocial personality disorder. J Pers Assess 1992; 59:32–49.
- Perry JC, Presniak MD, Olson TR. Defense mechanisms in schizotypal, borderline, antisocial, and narcissistic personality disorders. Psychiatry 2013; 76:32–52.
- Gibson J, Booth R, Davenport J, Keogh K, Owens T. Dialectical behaviour therapy-informed skills training for deliberate self-harm: a controlled trial with 3-month follow-up data. Behav Res Ther 2014; 60:8–14.
- Fischer S, Peterson C. Dialectical behavior therapy for adolescent binge eating, purging, suicidal behavior, and non-suicidal self-injury: a pilot study. Psychotherapy (Chic). 2015; 52:78–92.
- Booth R, Keogh K, Doyle J, Owens T. Living through distress: a skills training group for reducing deliberate self-harm. Behav Cogn Psychother 2014; 42:156–165.
- Scudder L, Shanafelt TD. Two sides of the physician coin: burnout and well-being. Medscape. Feb 09, 2015. http://nbpsa.org/images/PRP/PhysicianBurnoutMedscape.pdf. Accessed June 2, 2017.
- McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg 2015; 123:161–173.
- Schneider S, Kingsolver K, Rosdahl J. Can physician self-care enhance patient-centered healthcare? Qualitative findings from a physician well-being coaching program. J Fam Med 2015; 2:6.
- Simonds G, Sotile W. Building Resilience in Neurosurgical Residents: A Primer. B Wright Publishing; 2015.
- Bays JC. Mindfulness on the Go: Simple Meditation Practices You Can Do Anywhere. Boulder, CO: Shambhala Publications; 2014.
From time to time, all physicians encounter patients whose behavior evokes negative emotions. In 1978, in an article titled “Taking care of the hateful patient,”1 Groves detailed 4 types of patients—“dependent clingers, entitled demanders, manipulative help-rejecters, and self-destructive deniers”1—that even the most seasoned physicians dread, and provided suggestions for managing interactions with them. The topic was revisited and updated in 2006 by Strous et al.2
Now, more than 10 years later, the challenge of how to interact with difficult patients is more relevant than ever. A cultural environment in which every patient can become an “expert” via the Internet has added new challenges. Patients who are especially time-consuming and emotionally draining exacerbate the many other pressures physicians face today (eg, increased paperwork, cost-consciousness, shortened appointment times, and the move to electronic medical records), contributing to physician burnout.
This article further updates the topic of managing challenging patients to reflect the current practice climate. We provide a more modern view of challenging patients and provide guidance on handling them. Although it may be tempting to diagnose some of these patients as having a personality disorder, it can often be more helpful to recognize patterns of behavior and have a clear plan for management. We also discuss general coping strategies for avoiding physician burnout.
INTERNET-SEEKING, QUESTIONING
A 45-year-old man carries in an overstuffed briefcase for his first primary care visit. He is a medical editor for a national journal and recently worked on a case study involving a rare cancer. As he edited, he recognized that he had the same symptoms and diagnosed himself with the same disease. He has brought with him a sheaf of articles he found on the Internet detailing clinical trials for experimental treatments. When the doctor begins to ask questions, he says the answers are irrelevant: he explains that he would have gone straight to an oncologist, but his insurance policy requires that he also have a primary care physician. He now expects the doctor to order magnetic resonance imaging, refer him to an oncologist, and support his request for the treatment he has identified as best.
The Internet: A blessing and a curse
Patients now have access to enormous amounts of information of variable accuracy. As in this case, patients may come to an appointment carrying early research studies that the physician has not yet reviewed. Others get their information from patient blogs that frequently offer opinions without evidence. Often, based on an advertisement or Internet reading, a patient requests a particular medication or test that may not be cost-effective or medically justified.
In a survey more than 10 years ago, more than 75% of physicians reported that they had patients who brought in information from online sources.3 Hu et al4 reported that 70% of patients who had online information planned to discuss it with their physicians. This practice is only growing, including in older patients.5
Physicians may feel confused and frustrated by patients who come armed with information. They may infer that patients do not trust them to diagnose correctly or treat optimally. In addition, discussing such information takes time, causing others on the schedule to wait, adding to the stress of coping with over-booked appointments.
Why so overprepared?
Patients who have or fear that they have cancer may be particularly worried that an important treatment will be overlooked.6 Since they feel that their life is hanging in the balance, their search for a definitive cure is understandable.
Internet-seeking, intensely questioning patients clearly want more information about the treatments they are receiving, alternative medical or procedural options, and complementary therapies.7 The response to their desire for more information affects their impression of physician empathy.8
Adapting to a more informed patient
Approaching these patients as an opportunity to educate may result in a more trusting patient and one more likely to be open to physician guidance and more likely to adhere to an advised treatment plan. Triangulation of the 3 actors—the physician, the patient, and the Web—can help patients to make more-informed choices and foster an attitude of partnership with the physician to lead them to optimal healthcare.
In a review of the impact of Internet use on healthcare and the physician-patient relationship, Wald et al9 urged physicians to:
- Adopt a positive attitude toward discussing Internet contributions
- Encourage patients to take an active role in maintaining health
- Acknowledge patient concerns and fears
- Avoid becoming defensive
- Recommend credible Internet sources.
Laing et al10 urged physicians to recognize rather than deny the effects of patients’ online searching for information and support, and not to ignore the potential impact on treatment. Consumers are gaining autonomy and self-efficacy, and Laing et al encouraged healthcare providers to develop ways to incorporate this new reality into the services they provide.10
How Web-based interaction can assist in patient decision-making for colorectal cancer screening is being explored.11 Patients at home can use an online tool to learn about screening choices and would be more knowledgable and comfortable discussing the options with their care provider. The hope is to build in an automatic reminder for the clinician, who would better understand the patient’s preference before the office visit.
One approach to our patient is to say, “I can see how worried you are about having the same type of cancer you read about, and I want to help you. It is clear to me that you know a lot about healthcare, and I appreciate your engagement in your health. How about starting over? Let me ask a few questions so I can get a better perspective on your symptoms?” Many times, this strategy can help patients reframe their view and accept help.
DEMANDING, LITIGATION-THREATENING
A 60-year-old lawyer is admitted to the hospital for evaluation of abdominal pain. His physician recommends placing a nasogastric tube to provide nutrition while the evaluation is completed. His wife, a former nurse practitioner, insists that a nasogastric tube would be too dangerous and demands that he be allowed to eat instead. The couple declares the primary internal medicine physician incompetent, does not want any residents to be involved in his care, and antagonizes the nurses with constant demands. Soon, the entire team avoids the patient’s room.
Why so hostile?
People with demanding behavior often have a hostile and confrontational manner. They may use medical jargon and appear to believe that they know more than their healthcare team. Many demand to know why they have not been offered a particular test, diagnosis, or treatment, especially if they or a family member has a healthcare background. Such patients appear to feel that they are being treated incorrectly and leave us feeling vulnerable, wondering whether the patient might one day come back to haunt us with a lawsuit, especially if the medical outcome is unfavorable.
Understanding the motivation for the behavior can help a physician to empathize with the demanding patient.12 Although it may seem that the demanding patient is trying to intimidate the physician, the goal is usually the same: to find the best possible treatment. Anger and hostility are often motivated by fear and a sense of losing control.
Ironically, this maladaptive coping style may alienate the very people who can help the patient. Hostile behavior evokes defensiveness and resentment in others. A power struggle may ensue: as the patient makes more unreasonable demands and threats, the physician reacts by asserting his or her views in an attempt to maintain control. Or the physician and the rest of the healthcare team may simply avoid the patient as much as possible.
Collaboration can defuse anger
The best strategy is often to steer the encounter away from a power struggle by legitimizing the patient’s feeling of entitlement to the best possible treatment.13 Take a collaborative stance with the patient, with the common goal of finding and implementing the most effective and lowest-risk diagnostic and treatment plan. Empathy and exploration of the patient’s concerns are always in order.
Physicians can try several strategies to improve interactions with demanding patients and caregivers:
Be consistent. All members of the healthcare team, including nurses and specialists, should convey consistent messages regarding diagnostic testing and treatment plans.
Don’t play the game. Demanding patients often complain about being mistreated by other healthcare providers. When confronted with such complaints, acknowledge the patient’s feelings while refraining from blaming or criticizing other members of the healthcare team.
Clarify expectations. Clarifying expectations from the initial patient encounter can prevent conflicts later. Support a patient who must accept a diagnosis of a terminal illness, and then when appropriate, discuss goals moving forward. Collaboration within the framework of reasonable expectations is key.
For our case, the physician could say, “We want to work with you together as a team. We will work hard to address your concerns, but our nurses must have a safe environment in which to help you.” Such a statement highlights shared goals and expression of concern without judgment. The next step is to clarify expectations by describing the hospital routine and how decisions are made.
Offer choices. Offering choices whenever possible can help a demanding patient feel more in control. Rather than dismiss a patient’s ideas, explore the alternatives. While effective patient communication is preferable to repeated referrals to specialists,14 judicious referral can engender trust in the physician’s competence if a diagnosis is not forthcoming.15
A unique challenge in teaching hospitals is the patient who refuses to interact with residents and students. It is best to acknowledge the patient’s concerns and offer alternative options:
- If the patient is worried about lack of completed training, then clarify the residents’ roles and reassure the patient that you communicate with residents and supervisors regarding any clinical decisions
- If possible, offer to see the patient alone or have the resident interact only on an as-needed basis
- Consider transferring the patient to a nonteaching service or to another hospital.
Admit failings. Although not easy, admitting to and apologizing for things that have gone wrong can help to calm a demanding patient and even reduce the likelihood of a lawsuit.16 The physician should not convey defensiveness and instead should acknowledge the limitations of the healthcare system.
Legitimize concerns—to an extent. Legitimizing a demanding patient’s concerns is important, but never be bullied into taking actions that create unnecessary risk. Upsetting a demanding patient is better than ordering tests or providing treatments that are potentially harmful. Good physician-patient communication can go a long way toward preventing adverse outcomes.
CONSTANTLY SEEKING REASSURANCE
A 25-year-old professional presents to a new primary care provider concerned about a mole on her back. She discusses her sun exposure and family history of skin cancer and produces photographs documenting changes in the mole over time. Impressed with this level of detail, the physician takes time to explain his concerns before referring her to a dermatologist. Later that day, she calls to let the doctor know that her procedure has been scheduled and to thank him for his care. A few weeks after the mole is removed, she returns to discuss treatment options for the small remaining scar.
After this appointment, she calls the office repeatedly with a wide array of concerns, including an isolated symptom of fatigue that could indicate cancer and the relative merits of different sunscreens. She also sends the physician frequent e-mail messages through the personal health record system with pictures of inconsequential marks on her skin.
Needing reassurance is normal—to a point
Many patients seek reassurance from their physicians, and this can be done in a healthy and respectful manner. But requests for reassurance may escalate to becoming repeated, insistent, and even aggressive.1 This can elicit reactions from physicians ranging from feeling annoyed and burdened to feeling angry and overwhelmed.17 This can lead to significant stress, which, if not managed well, can lead to excessively control of physician behavior and substandard care.18
Reassurance-seeking behavior can manifest anywhere along the spectrum of health and disease.19 It may be a symptom of health anxiety (ie, an exaggerated fear of illness) or hypochondria (ie, the persistent conviction that one is currently or likely to become ill).20,21
Why so needy?
Attachment theory may help explain neediness. Parental bonding during childhood is associated with mental and physical health and health-related behaviors in adults.22,23 People with insecure-preoccupied attachment styles tend to be overly emotionally dependent on the acceptance of others and may exhibit dependent and care-seeking behaviors with a physician.24
Needy patients are often genuinely grateful for the care and attention from a physician.1 In the beginning, the doctor may appreciate the patient’s validation of care provided, but this positive feeling wanes as calls and requests become incessant. As the physician’s exhaustion increases with each request, the care and well-being of the patient may no longer be the primary focus.1
Set boundaries
Be alert to signs that a patient is crossing the line to an unhealthy need for reassurance. Address medical concerns appropriately, then institute clear guidelines for follow-up, which should be reinforced by the entire care team if necessary.22
The following strategies can be useful for defining boundaries:
- Instruct the patient to come to the office only for scheduled follow-up visits and to call only during office hours or in an emergency
- Be up-front about the time allowed for each appointment and ask the patient to help focus the discussion according to his or her main concerns25
- Consider telling the patient, “You seem really worried about a lot of physical symptoms. I want to reassure you that I find no evidence of a medical illness that would require intervention. I am concerned about your phone calls and e-mails, and I wonder what would be helpful at this point to address your concerns?”
- Consider treating the patient for anxiety.
It is important to remain responsive to all types of patient concerns. Setting boundaries will guide patients to express health concerns in an appropriate manner so that they can be heard and managed.18,19
SELF-INJURY
A 22-year-old woman presents to the emergency department complaining of abdominal pain. After a full workup, the physician clears her medically and orders a few laboratory tests. As the nurse draws blood samples, she notices multiple fresh cuts on the patient’s arm and informs the physician. The patient is questioned and examined again and acknowledges occasional thoughts of self-harm.
Her parents arrive and appear appropriately concerned. They report that she has been “cutting” for 4 years and is regularly seeing a therapist. However, they say that they are not worried for her safety and that she has an appointment with her therapist this week. Based on this, the emergency department physician discharges her.
Two weeks later, the patient returns to the emergency department with continued cutting and apparent cellulitis, prompting medical admission.
Self-injury presents in many ways
Self-injurious behaviors come in many forms other than the easily recognized one presented in this case: eg, a patient with cirrhosis who continues to drink, a patient with severe epilepsy who forgets to take medications and lands in the emergency department every week for status epilepticus, a patient with diabetes who eats a high-sugar diet, a patient with renal insufficiency who ignores water restrictions, or a patient with an organ transplant who misses medications and relapses.
There is an important psychological difference between patients who knowingly continue to challenge their luck and those who do not fully understand the severity of their condition and the consequences of their actions. The patient who simply does not “get it” can sometimes be managed effectively with education and by working with family members to create an environment to facilitate critical healthy behaviors.
Patients who willfully self-inflict injury are asking for help while doing everything to avoid being helped. They typically come to the office or the emergency department with assorted complaints, not divulging the real reason for their visit until the last minute as they are leaving. Then they drop a clue to the real concern, leaving the physician confused and frustrated.
Why deny an obvious problem?
Fear of the stigma of mental illness can be a major barrier to full disclosure of symptoms of psychological distress, and this especially tends to be the case for patients from some ethnic minorities.26
On the other hand, patients with borderline or antisocial personality disorder (and less often, schizotypal or narcissistic personality disorder) frequently use denial as their primary psychological defense. Self-destructive denial is sometimes associated with traumatic memories, feelings of worthlessness, or a desire to reduce self-awareness and rationalize harmful behaviors. Such patients usually need lengthy treatment, and although the likelihood of cure is low, therapy can be helpful.27–29
Lessons from psychiatry
It can be difficult to maintain empathy for patients who intentionally harm themselves. It is helpful to think of these patients as having a terminal illness and to recognize that they are suffering.
Different interventions have been studied for such patients. Dialectical behavior therapy, an approach that teaches patients better coping skills for regulating emotions, can help reduce maladaptive emotional distress and self-destructive behaviors.30–32 Lessons from this approach can be applied by general practitioners:
- Engage the patient and together establish an effective crisis management plan
- With patient permission, involve the family in the treatment plan
- Set clear limits about self-harm: once the patient values interaction with the doctor, he or she will be less likely to break the agreement.
Patients with severe or continuing issues can be referred to appropriate services that offer dialectical behavior therapy or other intensive outpatient programs.
To handle our patient, one might start by saying, “I am sorry to see you back in the ER. We need to treat the cellulitis and get your outpatient behavioral team on board, so we know the plan.” Then, it is critical that the entire team keep to that plan.
HOW TO STAY IN CONTROL AND IMPROVE INTERACTIONS
Patients with challenging behaviors will always be part of medical practice. Physicians should be aware of their reactions and feelings towards a patient (known in psychiatry as countertransference), as they can increase physician stress and interfere with providing optimal care. Finding effective ways to work with difficult patients will avoid these outcomes.
Physicians also feel loss of control
Most physicians are resilient, but they can feel overwhelmed under certain circumstances. According to Scudder and Shanafelt,33 a physician’s sense of well-being is influenced by several factors, including feelings of control in the workplace. It is easy to imagine how one or more difficult patients can create a sense of overwhelming demand and loss of control.
These tips can help maintain a sense of control and improve interactions with patients:
Have a plan for effective communication. Not having a plan for communicating in a difficult situation can contribute to loss of control in a hectic schedule that is already stretched to its limits. Practicing responses with a colleague for especially difficult patients or using a team approach can be helpful. Remaining compassionate while setting boundaries will result in the best outcome for the patient and physician.
Stop to analyze the situation. One of the tenets of cognitive-behavioral therapy is recognizing that negative thoughts can quickly take us down a dangerous path. Feeling angry and resentful without stopping to think and reflect on the causes can lead to the physician feeling victimized (just like many difficult patients feel).
It is important to step back and think, not just feel. While difficult patients present in different ways, all are reacting to losing control of their situation and want support. During a difficult interaction with a patient, pause to consider, “Why is he behaving this way? Is he afraid? Does he feel that no one cares?”
When a patient verbally attacks beyond what is appropriate, recognize that this is probably due less to anything the physician did than to the patient’s internal issues. Identifying the driver of a patient’s behavior makes it easier to control our own emotions.
Practice empathy. Difficult patients usually have something in their background that can help explain their inappropriate behavior, such as a lack of parental support or abuse. Being open to hearing their story facilitates an empathetic connection.
AVOIDING BURNOUT
Discuss problems
Sadly, physicians often neglect to talk with each other and with trainees about issues leading to burnout, thereby missing important opportunities for empathy, objectivity, reflection, and teaching moments.
Lessons can be gleaned from training in psychiatry, a field in which one must learn methods for working effectively in challenging situations. Dozens of scenarios are practiced using videotapes or observation through one-way mirrors. While not everyone has such opportunities, everyone can discuss issues with one another. Regularly scheduled facilitated groups devoted to discussing problems with colleagues can be enormously helpful.
Schedule quiet times
Mindfulness is an excellent way to spend a few minutes out of every 3- to 4-hour block. There are many ways to help facilitate such moments. Residents and students can be provided with a small book, Mindfulness on the Go,37 aimed for the busy person.
Deep, slow breathing can bring rapid relief to intense negative feelings. Not only does it reduce anxiety faster than medication, but it is also free, is easily taught to others, and can be done unobtrusively. A short description of the role of the blood pH in managing the locus ceruleus and vagus nerve’s balance of sympathetic and parasympathetic activity may capture the curiosity of someone who may otherwise be resistant to the exercise.
Increasingly, hospitals are developing mindfulness sessions and offering a variety of skills physicians can put into their toolbox. Lessons from cognitive-behavioral therapy, dialectical behavior therapy, imagery, and muscle relaxation can help physicians in responding to patients. Investing in communication skills training specific to challenging behaviors seen in different specialties better equips physicians with more effective strategies.
From time to time, all physicians encounter patients whose behavior evokes negative emotions. In 1978, in an article titled “Taking care of the hateful patient,”1 Groves detailed 4 types of patients—“dependent clingers, entitled demanders, manipulative help-rejecters, and self-destructive deniers”1—that even the most seasoned physicians dread, and provided suggestions for managing interactions with them. The topic was revisited and updated in 2006 by Strous et al.2
Now, more than 10 years later, the challenge of how to interact with difficult patients is more relevant than ever. A cultural environment in which every patient can become an “expert” via the Internet has added new challenges. Patients who are especially time-consuming and emotionally draining exacerbate the many other pressures physicians face today (eg, increased paperwork, cost-consciousness, shortened appointment times, and the move to electronic medical records), contributing to physician burnout.
This article further updates the topic of managing challenging patients to reflect the current practice climate. We provide a more modern view of challenging patients and provide guidance on handling them. Although it may be tempting to diagnose some of these patients as having a personality disorder, it can often be more helpful to recognize patterns of behavior and have a clear plan for management. We also discuss general coping strategies for avoiding physician burnout.
INTERNET-SEEKING, QUESTIONING
A 45-year-old man carries in an overstuffed briefcase for his first primary care visit. He is a medical editor for a national journal and recently worked on a case study involving a rare cancer. As he edited, he recognized that he had the same symptoms and diagnosed himself with the same disease. He has brought with him a sheaf of articles he found on the Internet detailing clinical trials for experimental treatments. When the doctor begins to ask questions, he says the answers are irrelevant: he explains that he would have gone straight to an oncologist, but his insurance policy requires that he also have a primary care physician. He now expects the doctor to order magnetic resonance imaging, refer him to an oncologist, and support his request for the treatment he has identified as best.
The Internet: A blessing and a curse
Patients now have access to enormous amounts of information of variable accuracy. As in this case, patients may come to an appointment carrying early research studies that the physician has not yet reviewed. Others get their information from patient blogs that frequently offer opinions without evidence. Often, based on an advertisement or Internet reading, a patient requests a particular medication or test that may not be cost-effective or medically justified.
In a survey more than 10 years ago, more than 75% of physicians reported that they had patients who brought in information from online sources.3 Hu et al4 reported that 70% of patients who had online information planned to discuss it with their physicians. This practice is only growing, including in older patients.5
Physicians may feel confused and frustrated by patients who come armed with information. They may infer that patients do not trust them to diagnose correctly or treat optimally. In addition, discussing such information takes time, causing others on the schedule to wait, adding to the stress of coping with over-booked appointments.
Why so overprepared?
Patients who have or fear that they have cancer may be particularly worried that an important treatment will be overlooked.6 Since they feel that their life is hanging in the balance, their search for a definitive cure is understandable.
Internet-seeking, intensely questioning patients clearly want more information about the treatments they are receiving, alternative medical or procedural options, and complementary therapies.7 The response to their desire for more information affects their impression of physician empathy.8
Adapting to a more informed patient
Approaching these patients as an opportunity to educate may result in a more trusting patient and one more likely to be open to physician guidance and more likely to adhere to an advised treatment plan. Triangulation of the 3 actors—the physician, the patient, and the Web—can help patients to make more-informed choices and foster an attitude of partnership with the physician to lead them to optimal healthcare.
In a review of the impact of Internet use on healthcare and the physician-patient relationship, Wald et al9 urged physicians to:
- Adopt a positive attitude toward discussing Internet contributions
- Encourage patients to take an active role in maintaining health
- Acknowledge patient concerns and fears
- Avoid becoming defensive
- Recommend credible Internet sources.
Laing et al10 urged physicians to recognize rather than deny the effects of patients’ online searching for information and support, and not to ignore the potential impact on treatment. Consumers are gaining autonomy and self-efficacy, and Laing et al encouraged healthcare providers to develop ways to incorporate this new reality into the services they provide.10
How Web-based interaction can assist in patient decision-making for colorectal cancer screening is being explored.11 Patients at home can use an online tool to learn about screening choices and would be more knowledgable and comfortable discussing the options with their care provider. The hope is to build in an automatic reminder for the clinician, who would better understand the patient’s preference before the office visit.
One approach to our patient is to say, “I can see how worried you are about having the same type of cancer you read about, and I want to help you. It is clear to me that you know a lot about healthcare, and I appreciate your engagement in your health. How about starting over? Let me ask a few questions so I can get a better perspective on your symptoms?” Many times, this strategy can help patients reframe their view and accept help.
DEMANDING, LITIGATION-THREATENING
A 60-year-old lawyer is admitted to the hospital for evaluation of abdominal pain. His physician recommends placing a nasogastric tube to provide nutrition while the evaluation is completed. His wife, a former nurse practitioner, insists that a nasogastric tube would be too dangerous and demands that he be allowed to eat instead. The couple declares the primary internal medicine physician incompetent, does not want any residents to be involved in his care, and antagonizes the nurses with constant demands. Soon, the entire team avoids the patient’s room.
Why so hostile?
People with demanding behavior often have a hostile and confrontational manner. They may use medical jargon and appear to believe that they know more than their healthcare team. Many demand to know why they have not been offered a particular test, diagnosis, or treatment, especially if they or a family member has a healthcare background. Such patients appear to feel that they are being treated incorrectly and leave us feeling vulnerable, wondering whether the patient might one day come back to haunt us with a lawsuit, especially if the medical outcome is unfavorable.
Understanding the motivation for the behavior can help a physician to empathize with the demanding patient.12 Although it may seem that the demanding patient is trying to intimidate the physician, the goal is usually the same: to find the best possible treatment. Anger and hostility are often motivated by fear and a sense of losing control.
Ironically, this maladaptive coping style may alienate the very people who can help the patient. Hostile behavior evokes defensiveness and resentment in others. A power struggle may ensue: as the patient makes more unreasonable demands and threats, the physician reacts by asserting his or her views in an attempt to maintain control. Or the physician and the rest of the healthcare team may simply avoid the patient as much as possible.
Collaboration can defuse anger
The best strategy is often to steer the encounter away from a power struggle by legitimizing the patient’s feeling of entitlement to the best possible treatment.13 Take a collaborative stance with the patient, with the common goal of finding and implementing the most effective and lowest-risk diagnostic and treatment plan. Empathy and exploration of the patient’s concerns are always in order.
Physicians can try several strategies to improve interactions with demanding patients and caregivers:
Be consistent. All members of the healthcare team, including nurses and specialists, should convey consistent messages regarding diagnostic testing and treatment plans.
Don’t play the game. Demanding patients often complain about being mistreated by other healthcare providers. When confronted with such complaints, acknowledge the patient’s feelings while refraining from blaming or criticizing other members of the healthcare team.
Clarify expectations. Clarifying expectations from the initial patient encounter can prevent conflicts later. Support a patient who must accept a diagnosis of a terminal illness, and then when appropriate, discuss goals moving forward. Collaboration within the framework of reasonable expectations is key.
For our case, the physician could say, “We want to work with you together as a team. We will work hard to address your concerns, but our nurses must have a safe environment in which to help you.” Such a statement highlights shared goals and expression of concern without judgment. The next step is to clarify expectations by describing the hospital routine and how decisions are made.
Offer choices. Offering choices whenever possible can help a demanding patient feel more in control. Rather than dismiss a patient’s ideas, explore the alternatives. While effective patient communication is preferable to repeated referrals to specialists,14 judicious referral can engender trust in the physician’s competence if a diagnosis is not forthcoming.15
A unique challenge in teaching hospitals is the patient who refuses to interact with residents and students. It is best to acknowledge the patient’s concerns and offer alternative options:
- If the patient is worried about lack of completed training, then clarify the residents’ roles and reassure the patient that you communicate with residents and supervisors regarding any clinical decisions
- If possible, offer to see the patient alone or have the resident interact only on an as-needed basis
- Consider transferring the patient to a nonteaching service or to another hospital.
Admit failings. Although not easy, admitting to and apologizing for things that have gone wrong can help to calm a demanding patient and even reduce the likelihood of a lawsuit.16 The physician should not convey defensiveness and instead should acknowledge the limitations of the healthcare system.
Legitimize concerns—to an extent. Legitimizing a demanding patient’s concerns is important, but never be bullied into taking actions that create unnecessary risk. Upsetting a demanding patient is better than ordering tests or providing treatments that are potentially harmful. Good physician-patient communication can go a long way toward preventing adverse outcomes.
CONSTANTLY SEEKING REASSURANCE
A 25-year-old professional presents to a new primary care provider concerned about a mole on her back. She discusses her sun exposure and family history of skin cancer and produces photographs documenting changes in the mole over time. Impressed with this level of detail, the physician takes time to explain his concerns before referring her to a dermatologist. Later that day, she calls to let the doctor know that her procedure has been scheduled and to thank him for his care. A few weeks after the mole is removed, she returns to discuss treatment options for the small remaining scar.
After this appointment, she calls the office repeatedly with a wide array of concerns, including an isolated symptom of fatigue that could indicate cancer and the relative merits of different sunscreens. She also sends the physician frequent e-mail messages through the personal health record system with pictures of inconsequential marks on her skin.
Needing reassurance is normal—to a point
Many patients seek reassurance from their physicians, and this can be done in a healthy and respectful manner. But requests for reassurance may escalate to becoming repeated, insistent, and even aggressive.1 This can elicit reactions from physicians ranging from feeling annoyed and burdened to feeling angry and overwhelmed.17 This can lead to significant stress, which, if not managed well, can lead to excessively control of physician behavior and substandard care.18
Reassurance-seeking behavior can manifest anywhere along the spectrum of health and disease.19 It may be a symptom of health anxiety (ie, an exaggerated fear of illness) or hypochondria (ie, the persistent conviction that one is currently or likely to become ill).20,21
Why so needy?
Attachment theory may help explain neediness. Parental bonding during childhood is associated with mental and physical health and health-related behaviors in adults.22,23 People with insecure-preoccupied attachment styles tend to be overly emotionally dependent on the acceptance of others and may exhibit dependent and care-seeking behaviors with a physician.24
Needy patients are often genuinely grateful for the care and attention from a physician.1 In the beginning, the doctor may appreciate the patient’s validation of care provided, but this positive feeling wanes as calls and requests become incessant. As the physician’s exhaustion increases with each request, the care and well-being of the patient may no longer be the primary focus.1
Set boundaries
Be alert to signs that a patient is crossing the line to an unhealthy need for reassurance. Address medical concerns appropriately, then institute clear guidelines for follow-up, which should be reinforced by the entire care team if necessary.22
The following strategies can be useful for defining boundaries:
- Instruct the patient to come to the office only for scheduled follow-up visits and to call only during office hours or in an emergency
- Be up-front about the time allowed for each appointment and ask the patient to help focus the discussion according to his or her main concerns25
- Consider telling the patient, “You seem really worried about a lot of physical symptoms. I want to reassure you that I find no evidence of a medical illness that would require intervention. I am concerned about your phone calls and e-mails, and I wonder what would be helpful at this point to address your concerns?”
- Consider treating the patient for anxiety.
It is important to remain responsive to all types of patient concerns. Setting boundaries will guide patients to express health concerns in an appropriate manner so that they can be heard and managed.18,19
SELF-INJURY
A 22-year-old woman presents to the emergency department complaining of abdominal pain. After a full workup, the physician clears her medically and orders a few laboratory tests. As the nurse draws blood samples, she notices multiple fresh cuts on the patient’s arm and informs the physician. The patient is questioned and examined again and acknowledges occasional thoughts of self-harm.
Her parents arrive and appear appropriately concerned. They report that she has been “cutting” for 4 years and is regularly seeing a therapist. However, they say that they are not worried for her safety and that she has an appointment with her therapist this week. Based on this, the emergency department physician discharges her.
Two weeks later, the patient returns to the emergency department with continued cutting and apparent cellulitis, prompting medical admission.
Self-injury presents in many ways
Self-injurious behaviors come in many forms other than the easily recognized one presented in this case: eg, a patient with cirrhosis who continues to drink, a patient with severe epilepsy who forgets to take medications and lands in the emergency department every week for status epilepticus, a patient with diabetes who eats a high-sugar diet, a patient with renal insufficiency who ignores water restrictions, or a patient with an organ transplant who misses medications and relapses.
There is an important psychological difference between patients who knowingly continue to challenge their luck and those who do not fully understand the severity of their condition and the consequences of their actions. The patient who simply does not “get it” can sometimes be managed effectively with education and by working with family members to create an environment to facilitate critical healthy behaviors.
Patients who willfully self-inflict injury are asking for help while doing everything to avoid being helped. They typically come to the office or the emergency department with assorted complaints, not divulging the real reason for their visit until the last minute as they are leaving. Then they drop a clue to the real concern, leaving the physician confused and frustrated.
Why deny an obvious problem?
Fear of the stigma of mental illness can be a major barrier to full disclosure of symptoms of psychological distress, and this especially tends to be the case for patients from some ethnic minorities.26
On the other hand, patients with borderline or antisocial personality disorder (and less often, schizotypal or narcissistic personality disorder) frequently use denial as their primary psychological defense. Self-destructive denial is sometimes associated with traumatic memories, feelings of worthlessness, or a desire to reduce self-awareness and rationalize harmful behaviors. Such patients usually need lengthy treatment, and although the likelihood of cure is low, therapy can be helpful.27–29
Lessons from psychiatry
It can be difficult to maintain empathy for patients who intentionally harm themselves. It is helpful to think of these patients as having a terminal illness and to recognize that they are suffering.
Different interventions have been studied for such patients. Dialectical behavior therapy, an approach that teaches patients better coping skills for regulating emotions, can help reduce maladaptive emotional distress and self-destructive behaviors.30–32 Lessons from this approach can be applied by general practitioners:
- Engage the patient and together establish an effective crisis management plan
- With patient permission, involve the family in the treatment plan
- Set clear limits about self-harm: once the patient values interaction with the doctor, he or she will be less likely to break the agreement.
Patients with severe or continuing issues can be referred to appropriate services that offer dialectical behavior therapy or other intensive outpatient programs.
To handle our patient, one might start by saying, “I am sorry to see you back in the ER. We need to treat the cellulitis and get your outpatient behavioral team on board, so we know the plan.” Then, it is critical that the entire team keep to that plan.
HOW TO STAY IN CONTROL AND IMPROVE INTERACTIONS
Patients with challenging behaviors will always be part of medical practice. Physicians should be aware of their reactions and feelings towards a patient (known in psychiatry as countertransference), as they can increase physician stress and interfere with providing optimal care. Finding effective ways to work with difficult patients will avoid these outcomes.
Physicians also feel loss of control
Most physicians are resilient, but they can feel overwhelmed under certain circumstances. According to Scudder and Shanafelt,33 a physician’s sense of well-being is influenced by several factors, including feelings of control in the workplace. It is easy to imagine how one or more difficult patients can create a sense of overwhelming demand and loss of control.
These tips can help maintain a sense of control and improve interactions with patients:
Have a plan for effective communication. Not having a plan for communicating in a difficult situation can contribute to loss of control in a hectic schedule that is already stretched to its limits. Practicing responses with a colleague for especially difficult patients or using a team approach can be helpful. Remaining compassionate while setting boundaries will result in the best outcome for the patient and physician.
Stop to analyze the situation. One of the tenets of cognitive-behavioral therapy is recognizing that negative thoughts can quickly take us down a dangerous path. Feeling angry and resentful without stopping to think and reflect on the causes can lead to the physician feeling victimized (just like many difficult patients feel).
It is important to step back and think, not just feel. While difficult patients present in different ways, all are reacting to losing control of their situation and want support. During a difficult interaction with a patient, pause to consider, “Why is he behaving this way? Is he afraid? Does he feel that no one cares?”
When a patient verbally attacks beyond what is appropriate, recognize that this is probably due less to anything the physician did than to the patient’s internal issues. Identifying the driver of a patient’s behavior makes it easier to control our own emotions.
Practice empathy. Difficult patients usually have something in their background that can help explain their inappropriate behavior, such as a lack of parental support or abuse. Being open to hearing their story facilitates an empathetic connection.
AVOIDING BURNOUT
Discuss problems
Sadly, physicians often neglect to talk with each other and with trainees about issues leading to burnout, thereby missing important opportunities for empathy, objectivity, reflection, and teaching moments.
Lessons can be gleaned from training in psychiatry, a field in which one must learn methods for working effectively in challenging situations. Dozens of scenarios are practiced using videotapes or observation through one-way mirrors. While not everyone has such opportunities, everyone can discuss issues with one another. Regularly scheduled facilitated groups devoted to discussing problems with colleagues can be enormously helpful.
Schedule quiet times
Mindfulness is an excellent way to spend a few minutes out of every 3- to 4-hour block. There are many ways to help facilitate such moments. Residents and students can be provided with a small book, Mindfulness on the Go,37 aimed for the busy person.
Deep, slow breathing can bring rapid relief to intense negative feelings. Not only does it reduce anxiety faster than medication, but it is also free, is easily taught to others, and can be done unobtrusively. A short description of the role of the blood pH in managing the locus ceruleus and vagus nerve’s balance of sympathetic and parasympathetic activity may capture the curiosity of someone who may otherwise be resistant to the exercise.
Increasingly, hospitals are developing mindfulness sessions and offering a variety of skills physicians can put into their toolbox. Lessons from cognitive-behavioral therapy, dialectical behavior therapy, imagery, and muscle relaxation can help physicians in responding to patients. Investing in communication skills training specific to challenging behaviors seen in different specialties better equips physicians with more effective strategies.
- Groves JE. Taking care of the hateful patient. N Engl J Med 1978; 298:883–887.
- Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med 2006; 17:387–393.
- Malone M, Mathes L, Dooley J, While AE. Health information seeking and its effect on the doctor-patient digital divide. J Telemed Telecare 2005; 11(suppl1):25–28.
- Hu X, Bell RA, Kravitz RL, Orrange S. The prepared patient: information seeking of online support group members before their medical appointments. J Health Commun 2012; 17:960–978.
- Tse MM, Choi KC, Leung RS. E-health for older people: the use of technology in health promotion. Cyberpsychol Behav 2008; 11:475–479.
- Pereira JL, Koski S, Hanson J, Bruera ED, Mackey JR. Internet usage among women with breast cancer: an exploratory study. Clin Breast Cancer 2000; 1:148–153.
- Brauer JA, El Sehamy A, Metz JN, Mao JJ. Complementary and alternative and supportive care at leading cancer centers: a systematic analysis of websites. J Altern Complement Med 2010; 16:183–186.
- Smith SG, Pandit A, Rush SR, Wolf MS, Simon C. The association between patient activation and accessing online health information: results from a national survey of US adults. Health Expect 2015; 18:3262–3273.
- Wald HS, Dube CE, Anthony DC. Untangling the Web—the impact of Internet use on health care and the physician-patient relationship. Patient Educ Couns 2007; 68:218–224.
- Laing A, Hogg G, Winkelman D. Healthcare and the information revolution: re-configuring the healthcare service encounter. Health Serv Manage Res 2004; 17:188–199.
- Jimbo M, Shultz CG, Nease DE, Fetters MD, Power D, Ruffin MT 4th. Perceived barriers and facilitators of using a Web-based interactive decision aid for colorectal cancer screening in community practice settings: findings from focus groups with primary care clinicians and medical office staff. J Med Internet Res 2013; 15:e286.
- Steinmetz D, Tabenkin H. The ‘difficult patient’ as perceived by family physicians. Fam Pract 2001; 18:495–500.
- Arciniegas DB, Beresford TP. Managing difficult interactions with patients in neurology practices: a practical approach. Neurology 2010; 75(suppl 1):S39–S44.
- Gallagher TH, Lo B, Chesney M, Christensen K. How do physicians respond to patient’s requests for costly, unindicated services? J Gen Intern Med 1997; 12:663–668.
- Breen KJ, Greenberg PB. Difficult physician-patient encounters. Intern Med J 2010; 40:682–688.
- Huntington B, Kuhn N. Communication gaffes: a root cause of malpractice claims. Proc (Bayl Univ Med Cent) 2003; 16:157–161.
- Maunder RG, Panzer A, Viljoen M, Owen J, Human S, Hunter JJ. Physicians’ difficulty with emergency department patients is related to patients’ attachment style. Soc Sci Med 2006; 63:552–562.
- Thompson D, Ciechanowski PS. Attaching a new understanding to the patient-physician relationship in family practice. J Am Board Fam Pract 2003; 16:219–226.
- Groves M, Muskin P. Psychological responses to illness. In: Levenson JL, ed. The American Psychiatric Publishing Textbook of Psychosomatic Medicine. Arlington, VA: American Psychiatric Publishing, Inc; 2004:68–88.
- Görgen SM, Hiller W, Witthöft M. Health anxiety, cognitive coping, and emotion regulation: a latent variable approach. Int J Behav Med 2014; 21:364–374.
- Strand J, Goulding A, Tidefors I. Attachment styles and symptoms in individuals with psychosis. Nord J Psychiatry 2015; 69:67–72.
- Hooper LM, Tomek S, Newman CR. Using attachment theory in medical settings: implications for primary care physicians. J Ment Health 2012; 21:23–37.
- Bowlby J. Attachment and loss. 1. Attachment. New York, NY: Basic Books; 1969.
- Fuertes JN, Anand P, Haggerty G, Kestenbaum M, Rosenblum GC. The physician-patient working alliance and patient psychological attachment, adherence, outcome expectations, and satisfaction in a sample of rheumatology patients. Behav Med 2015; 41:60–68.
- Frederiksen HB, Kragstrup J, Dehlholm-Lambertsen B. Attachment in the doctor-patient relationship in general practice: a qualitative study. Scand J Prim Health Care 2010; 28:185–190.
- Rastogi P, Khushalani S, Dhawan S, et al. Understanding clinician perception of common presentations in South Asians seeking mental health treatment and determining barriers and facilitators to treatment. Asian J Psychiatr 2014; 7:15–21.
- van der Kolk BA, Perry JC, Herman JL. Childhood origins of self-destructive behavior. Am J Psychiatry 1991; 148:1665–1671.
- Gacono CB, Meloy JR, Berg JL. Object relations, defensive operations, and affective states in narcissistic, borderline, and antisocial personality disorder. J Pers Assess 1992; 59:32–49.
- Perry JC, Presniak MD, Olson TR. Defense mechanisms in schizotypal, borderline, antisocial, and narcissistic personality disorders. Psychiatry 2013; 76:32–52.
- Gibson J, Booth R, Davenport J, Keogh K, Owens T. Dialectical behaviour therapy-informed skills training for deliberate self-harm: a controlled trial with 3-month follow-up data. Behav Res Ther 2014; 60:8–14.
- Fischer S, Peterson C. Dialectical behavior therapy for adolescent binge eating, purging, suicidal behavior, and non-suicidal self-injury: a pilot study. Psychotherapy (Chic). 2015; 52:78–92.
- Booth R, Keogh K, Doyle J, Owens T. Living through distress: a skills training group for reducing deliberate self-harm. Behav Cogn Psychother 2014; 42:156–165.
- Scudder L, Shanafelt TD. Two sides of the physician coin: burnout and well-being. Medscape. Feb 09, 2015. http://nbpsa.org/images/PRP/PhysicianBurnoutMedscape.pdf. Accessed June 2, 2017.
- McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg 2015; 123:161–173.
- Schneider S, Kingsolver K, Rosdahl J. Can physician self-care enhance patient-centered healthcare? Qualitative findings from a physician well-being coaching program. J Fam Med 2015; 2:6.
- Simonds G, Sotile W. Building Resilience in Neurosurgical Residents: A Primer. B Wright Publishing; 2015.
- Bays JC. Mindfulness on the Go: Simple Meditation Practices You Can Do Anywhere. Boulder, CO: Shambhala Publications; 2014.
- Groves JE. Taking care of the hateful patient. N Engl J Med 1978; 298:883–887.
- Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med 2006; 17:387–393.
- Malone M, Mathes L, Dooley J, While AE. Health information seeking and its effect on the doctor-patient digital divide. J Telemed Telecare 2005; 11(suppl1):25–28.
- Hu X, Bell RA, Kravitz RL, Orrange S. The prepared patient: information seeking of online support group members before their medical appointments. J Health Commun 2012; 17:960–978.
- Tse MM, Choi KC, Leung RS. E-health for older people: the use of technology in health promotion. Cyberpsychol Behav 2008; 11:475–479.
- Pereira JL, Koski S, Hanson J, Bruera ED, Mackey JR. Internet usage among women with breast cancer: an exploratory study. Clin Breast Cancer 2000; 1:148–153.
- Brauer JA, El Sehamy A, Metz JN, Mao JJ. Complementary and alternative and supportive care at leading cancer centers: a systematic analysis of websites. J Altern Complement Med 2010; 16:183–186.
- Smith SG, Pandit A, Rush SR, Wolf MS, Simon C. The association between patient activation and accessing online health information: results from a national survey of US adults. Health Expect 2015; 18:3262–3273.
- Wald HS, Dube CE, Anthony DC. Untangling the Web—the impact of Internet use on health care and the physician-patient relationship. Patient Educ Couns 2007; 68:218–224.
- Laing A, Hogg G, Winkelman D. Healthcare and the information revolution: re-configuring the healthcare service encounter. Health Serv Manage Res 2004; 17:188–199.
- Jimbo M, Shultz CG, Nease DE, Fetters MD, Power D, Ruffin MT 4th. Perceived barriers and facilitators of using a Web-based interactive decision aid for colorectal cancer screening in community practice settings: findings from focus groups with primary care clinicians and medical office staff. J Med Internet Res 2013; 15:e286.
- Steinmetz D, Tabenkin H. The ‘difficult patient’ as perceived by family physicians. Fam Pract 2001; 18:495–500.
- Arciniegas DB, Beresford TP. Managing difficult interactions with patients in neurology practices: a practical approach. Neurology 2010; 75(suppl 1):S39–S44.
- Gallagher TH, Lo B, Chesney M, Christensen K. How do physicians respond to patient’s requests for costly, unindicated services? J Gen Intern Med 1997; 12:663–668.
- Breen KJ, Greenberg PB. Difficult physician-patient encounters. Intern Med J 2010; 40:682–688.
- Huntington B, Kuhn N. Communication gaffes: a root cause of malpractice claims. Proc (Bayl Univ Med Cent) 2003; 16:157–161.
- Maunder RG, Panzer A, Viljoen M, Owen J, Human S, Hunter JJ. Physicians’ difficulty with emergency department patients is related to patients’ attachment style. Soc Sci Med 2006; 63:552–562.
- Thompson D, Ciechanowski PS. Attaching a new understanding to the patient-physician relationship in family practice. J Am Board Fam Pract 2003; 16:219–226.
- Groves M, Muskin P. Psychological responses to illness. In: Levenson JL, ed. The American Psychiatric Publishing Textbook of Psychosomatic Medicine. Arlington, VA: American Psychiatric Publishing, Inc; 2004:68–88.
- Görgen SM, Hiller W, Witthöft M. Health anxiety, cognitive coping, and emotion regulation: a latent variable approach. Int J Behav Med 2014; 21:364–374.
- Strand J, Goulding A, Tidefors I. Attachment styles and symptoms in individuals with psychosis. Nord J Psychiatry 2015; 69:67–72.
- Hooper LM, Tomek S, Newman CR. Using attachment theory in medical settings: implications for primary care physicians. J Ment Health 2012; 21:23–37.
- Bowlby J. Attachment and loss. 1. Attachment. New York, NY: Basic Books; 1969.
- Fuertes JN, Anand P, Haggerty G, Kestenbaum M, Rosenblum GC. The physician-patient working alliance and patient psychological attachment, adherence, outcome expectations, and satisfaction in a sample of rheumatology patients. Behav Med 2015; 41:60–68.
- Frederiksen HB, Kragstrup J, Dehlholm-Lambertsen B. Attachment in the doctor-patient relationship in general practice: a qualitative study. Scand J Prim Health Care 2010; 28:185–190.
- Rastogi P, Khushalani S, Dhawan S, et al. Understanding clinician perception of common presentations in South Asians seeking mental health treatment and determining barriers and facilitators to treatment. Asian J Psychiatr 2014; 7:15–21.
- van der Kolk BA, Perry JC, Herman JL. Childhood origins of self-destructive behavior. Am J Psychiatry 1991; 148:1665–1671.
- Gacono CB, Meloy JR, Berg JL. Object relations, defensive operations, and affective states in narcissistic, borderline, and antisocial personality disorder. J Pers Assess 1992; 59:32–49.
- Perry JC, Presniak MD, Olson TR. Defense mechanisms in schizotypal, borderline, antisocial, and narcissistic personality disorders. Psychiatry 2013; 76:32–52.
- Gibson J, Booth R, Davenport J, Keogh K, Owens T. Dialectical behaviour therapy-informed skills training for deliberate self-harm: a controlled trial with 3-month follow-up data. Behav Res Ther 2014; 60:8–14.
- Fischer S, Peterson C. Dialectical behavior therapy for adolescent binge eating, purging, suicidal behavior, and non-suicidal self-injury: a pilot study. Psychotherapy (Chic). 2015; 52:78–92.
- Booth R, Keogh K, Doyle J, Owens T. Living through distress: a skills training group for reducing deliberate self-harm. Behav Cogn Psychother 2014; 42:156–165.
- Scudder L, Shanafelt TD. Two sides of the physician coin: burnout and well-being. Medscape. Feb 09, 2015. http://nbpsa.org/images/PRP/PhysicianBurnoutMedscape.pdf. Accessed June 2, 2017.
- McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg 2015; 123:161–173.
- Schneider S, Kingsolver K, Rosdahl J. Can physician self-care enhance patient-centered healthcare? Qualitative findings from a physician well-being coaching program. J Fam Med 2015; 2:6.
- Simonds G, Sotile W. Building Resilience in Neurosurgical Residents: A Primer. B Wright Publishing; 2015.
- Bays JC. Mindfulness on the Go: Simple Meditation Practices You Can Do Anywhere. Boulder, CO: Shambhala Publications; 2014.
KEY POINTS
- Patients who intensely question everything need validation of their need for information and a collaborative approach based on sound medical evidence.
- Patients whose behavior is hostile and demanding need limits placed on aggressive behavior and assurance that the healthcare team is working in their best interests.
- Patients who seek reassurance to the point of overuse of the doctor’s time need to have boundaries set.
- Many patients who injure themselves and deny the problem have a personality disorder. They need empathy and a clear plan for care, often involving behavioral therapy.
- Physicians should plan effective communication strategies for difficult patients, discuss issues with colleagues, and use relaxation methods to help avoid burnout.
Breast cancer screening: Does tomosynthesis augment mammography?
Each year, millions of women undergo mammography in the hope of decreasing their risk of dying of breast cancer. The effectiveness of screening mammography, however, continues to be debated.
While most randomized controlled trials have demonstrated significantly lower mortality rates in women who undergo screening, not all trials have. Most experts agree that screening mammography programs decrease breast cancer mortality rates by 12% to 33%.1,2 But some point out that although mammography programs clearly detect more cases of breast cancer, some proportion of this detection may include “overdiagnosis” of cancers that would not have caused morbidity or mortality, including ductal carcinoma in situ. Also, although deaths from breast cancer have decreased in the United States, at least some of the decrease may be due to more effective treatment rather than early detection.
Moreover, screening has well-documented harms. False-positive results cause alarm and expose women to needless follow-up imaging and biopsies, with their attendant inconvenience, discomfort, risks, and costs. Conversely, false-negative results (especially common in women with dense breasts) lead to missed diagnosis and a false sense of security.
How could programs and technology be improved to make screening more beneficial, both for patients and for society as a whole? A major improvement would be if mammography could be made more sensitive and specific for detecting invasive cancers, with fewer false-positive results. Lower cost and less frequent screening would also be major improvements.
Digital breast tomosynthesis (DBT), also known as 3-dimensional (3D) mammography, may be a way to improve the value of breast cancer screening programs. In 2011, the US Food and Drug Administration (FDA) approved DBT for all mammographic indications, including screening.
WHAT IS TOMOSYNTHESIS?
In DBT, the x-ray source is rotated in an arc around the patient’s breast (Figure 1), generating a 3D image.3 DBT is now routinely built into newer-generation mammography units. The 3D projections of DBT are obtained during the same breast compression required for standard 2D digital mammography. Thus, DBT requires minimal additional time on the part of the patient and the technologist.4
The 3D images are processed and sent to a viewing station, where a radiologist can interpret them next to 2D images. The radiologist has the ability to scroll through the DBT projections slice by slice, as in other cross-sectional imaging examinations. However, given the larger number of images compared with digital mammography, DBT requires more time for interpretation, interrupting the workflow. A population-based observational study suggested that combined digital mammography and DBT screening examinations take twice as long to interpret.5
The main advantage of DBT is that it can mitigate the problem of overlapping breast tissue on standard digital projections. These areas of focal asymmetry may represent suspicious masses—or merely overlapping breast parenchyma. When areas of focal asymmetry are found on 2D digital mammography without DBT, patients need to come back for further diagnostic imaging to resolve the finding.6 In addition, especially in women with dense breasts, areas of overlapping tissue can have a masking effect, obscuring small breast cancers.7
For breast cancer screening, DBT is read in conjunction with standard digital mammography. By allowing examination of the breast parenchyma in thin slices, DBT decreases the interpretive issue of overlapping breast parenchyma and the masking effect, potentially leading to fewer false-positive results and higher rates of cancer detection (Figure 2).
EFFECTIVENESS OF TOMOSYNTHESIS
There is limited evidence at this time to support the addition of DBT to digital mammography for primary breast cancer screening, with no published randomized trials that assessed outcomes. However, 2 population-based trials in Europe have prospectively assessed DBT plus digital mammography as a primary screening strategy: the Screening With Tomosynthesis or Standard Mammography (STORM) trial8 and the Oslo tomosynthesis screening trial.5 Only the STORM trial reported first-year interval cancer rates, from which the sensitivity and specificity of DBT plus 2D digital mammography could be calculated and compared with those of 2D digital mammography alone.8
The Oslo trial: Limited applicability in USA
In April 2013, the Oslo tomosynthesis screening trial published interim results of its prospective cohort study of 12,631 Norwegian women ages 50 to 69.5 Women were invited to participate based on the availability of technical staff and imaging systems at the time of screening, and all participants underwent digital mammography and DBT. Images were read independently by 4 radiologists using a double-reader protocol.
The interim results suggest that adding DBT to digital mammography increased cancer detection rates by 31% and decreased the false-positive rate by 13% compared with 2D digital mammography alone (Table 1). However, the double-reader protocol in this study differs from typical single-reader protocols in the United States, limiting the applicability of the findings.
The STORM trial: Low sensitivity
The STORM trial is a prospective cohort study that included 7,292 women without symptoms, at average risk, age 48 and older, who participated in national breast cancer screening services in northern Italy. Each participant underwent digital mammography and DBT. The examinations were read sequentially (digital mammography first, then DBT plus digital mammography) either by a single radiologist, as is most common in the United States, or by 2 radiologists, as is standard in Europe.
Using the single-reader strategy, adding DBT significantly increased cancer detection rates and reduced the total recall rate (Table 1). Sensitivity was 85% vs 54%, and specificity was 97% vs 96%.8,9
Of note, the sensitivity of 54% for digital mammography in the STORM trial is substantially lower than the sensitivity of digital mammography reported in the United States.10
Friedewald et al confirmed Oslo and STORM
To date, the largest US study of DBT plus digital mammography for breast cancer screening was a multicenter retrospective cohort study by Friedewald et al in 2014.11 This study compared cancer detection and recall rates before and after the implementation of DBT at 13 breast centers and evaluated a total of 454,850 examinations (173,663 with DBT plus digital mammography and 281,187 with digital mammography only).
Overall, the recall rate decreased significantly after DBT was adopted and the cancer detection rate increased, findings consistent with those of the STORM and Oslo trials (Table 1). Adding DBT detected invasive cancers at a higher rate than 2D digital mammography alone (4.1/1000 vs 2.9/1,000), while there was no significant difference in ductal carcinoma in situ detection rates. This suggests that the additional cancers detected by DBT may be more clinically important. Nevertheless, the number of biopsies with negative results also increased, suggesting that adding DBT may pose potential harms.
In 2016, Rafferty et al12 published an additional analysis of the data from Friedewald et al, concluding that adding DBT to 2D digital mammography increased the cancer detection rate more in women with heterogeneously dense breasts than in those with either nondense breasts or extremely dense breasts.12 The reduction in recall rate was also greatest in the heterogeneously dense subgroup.
Insufficient evidence to recommend
Most other cohort studies comparing DBT and digital mammography have had findings similar to those of the European prospective studies and the large US retrospective cohort study, with the addition of DBT to mammography reducing recall rates and increasing cancer detection rates.13 However, many of these studies were subject to potential selection bias and did not provide information on the cancer risk of the participants. In addition, no studies have assessed clinical outcomes such as breast cancer stage at diagnosis or interval cancers, let alone breast cancer mortality.
Rigorous studies need to be done in the United States, using the standard single-reader protocol most often used in this country, to ascertain the clinical outcomes of DBT plus digital mammography for breast cancer screening for women at average risk. A 2016 review cited a dearth of high-quality US studies assessing the role of DBT in primary breast cancer.13
The US Preventive Services Task Force, in its 2016 guidelines for breast cancer screening, concluded that there was insufficient evidence to assess the harms and benefits of DBT as a method of breast cancer screening, including adjunctive screening in women with dense breasts.1
Similarly, the American College of Physicians has advised against screening average-risk women for breast cancer using DBT.14
APPROVAL, DISSEMINATION, COSTS, AND CHOICE FOR PATIENTS
Even with early promising data suggesting that DBT can increase cancer detection rates and decrease false-positive results when added to routine screening mammography, the rapid diffusion of DBT into clinical practice is outpacing evidence of its effectiveness.4 This adoption was spurred in January 2015 when the Centers for Medicare and Medicaid Services added a Current Procedural Terminology code for DBT, allowing for additional reimbursement for it for all mammography indications.15 Still, the use of DBT in community settings is inconsistent, given the significant up-front costs associated with equipment purchases and variable reimbursement by private insurers who consider the technology experimental.
For the US healthcare system as a whole, it is uncertain whether the purported benefits of DBT will outweigh the additional costs associated with its use. The average reimbursement for a routine digital mammography examination is $135; adding DBT adds an average of $56 to the cost.15
Using an established, discrete-event breast cancer simulation model, a team of investigators evaluated the cost-effectiveness of combined biennial digital mammography and DBT screening compared with biennial digital mammography screening alone in US women with dense breasts.16 They found that biennial combined screening is likely to be cost-effective in US women with dense breasts. They also found that for every 2,000 women screened from age 50 to age 74, adding DBT would prevent 1 breast cancer death and 810 false-positive screening examinations.16
In addition, some have expressed concern that adding DBT to standard digital mammography increases radiation exposure. In fact, the radiation dose with DBT is similar to that with standard 2D digital mammography. Thus, when acquired together, combined digital mammography and DBT screening leads to twice the radiation dose compared with digital mammography alone.17 Nevertheless, this increased dose remains well below the FDA limits for a screening examination. In addition, the FDA has approved software that allows reconstruction of 2D synthetic views from the 3D data set, which will eventually bring radiation dose levels down to levels comparable to those of conventional digital mammography.17
Given that DBT is built into newer mammography units and is available as an add-on feature for existing units, its use is likely to increase even faster than digital mammography did when it replaced screen-film mammography in the previous decade.4 Its adoption by screening facilities, however, remains variable, and patients wishing to obtain combined DBT and digital mammography screening may have to travel to a different facility from their usual place of screening.18
Moreover, not all insurance companies cover DBT, resulting in additional out-of-pocket costs to the patient. It is currently unclear how individual facilities are offering DBT services, including how patients are selected for additional DBT and if they are offered the choice to add or forego DBT screening in combination with standard digital mammography.
SUMMARY: AN EMERGING TECHNOLOGY
DBT is an emerging imaging technology that allows the radiologist to view breast images in slices, as in computed tomography. DBT images can be obtained using the same breast compression that women already undergo for 2D digital mammography for breast cancer screening.
At this time, adding DBT to digital mammography screening nearly doubles the radiation exposure to the patient. However, new software is available that allows creation of synthetic 2D views from the 3D data set, resulting in radiation exposure that is similar to conventional digital mammography.
Although there are no published randomized controlled trials assessing the benefit of DBT over 2D digital mammography for breast cancer screening, prospective observational studies suggest that DBT may reduce false-positive recall rates and increase cancer detection rates when used in population-based screening programs. Assuming that additional breast cancer detection contributes to improved clinical outcomes, women with dense breasts may benefit more than women without dense breasts.
No national organizations currently recommend DBT for primary breast cancer screening. Ideally, future studies would determine whether DBT screening reduces breast cancer mortality. Since this research may not be feasible, surrogate clinical outcomes, such as a decrease in interval breast cancer rates and impact on stage at time of diagnosis, would allow us to more confidently recommend this new technology.
- Siu AL; US Preventive Services Task Force. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:279–296.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314:1599–1614.
- Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad Radiol 2011; 18:1298–1310.
- Lee CI, Lehman CD. Digital breast tomosynthesis and the challenges of implementing an emerging breast cancer screening technology into clinical practice. J Am Coll Radiol 2013; 10:913–917.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267:47–56.
- Helvie MA. Digital mammography imaging: breast tomosynthesis and advanced applications. Radiol Clin North Am 2010; 48:917–929.
- Gur D, Abrams GS, Chough DM, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol 2009; 193:586–591.
- Houssami N, Macaskill P, Bernardi D, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading—evidence to guide future screening strategies. Eur J Cancer 2014; 50:1799–1807.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14:583–589.
- Humphrey L, Chan BKS, Detlefsen S, Helfand M. Screening for Breast Cancer. Rockville, MD: Agency for Healthcare Research and Quality (US); 2002.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311:2499–2507.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315:1784–1786.
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164:268–278.
- Wilt TJ, Harris RP, Qaseem A; High Value Care Task Force of the American College of Physicians. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 2015; 162:718–725.
- American College of Radiology. CMS establishes breast tomosynthesis values in 2015 MPFS final rule. www.acr.org/News-Publications/News/News-Articles/2014/Economics/20141105-CMS-Establishes-Values-for-Breast-Tomosynthesis-in-2015-Final-Rule. Accessed June 14, 2017.
- Lee CI, Cevik M, Alagoz O, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology 2015; 274:772–780.
- Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015; 24:93–99.
- Lee CI, Bogart A, Hubbard RA, et al. Advanced breast imaging availability by screening facility characteristics. Acad Radiol 2015; 22:846–652.
Each year, millions of women undergo mammography in the hope of decreasing their risk of dying of breast cancer. The effectiveness of screening mammography, however, continues to be debated.
While most randomized controlled trials have demonstrated significantly lower mortality rates in women who undergo screening, not all trials have. Most experts agree that screening mammography programs decrease breast cancer mortality rates by 12% to 33%.1,2 But some point out that although mammography programs clearly detect more cases of breast cancer, some proportion of this detection may include “overdiagnosis” of cancers that would not have caused morbidity or mortality, including ductal carcinoma in situ. Also, although deaths from breast cancer have decreased in the United States, at least some of the decrease may be due to more effective treatment rather than early detection.
Moreover, screening has well-documented harms. False-positive results cause alarm and expose women to needless follow-up imaging and biopsies, with their attendant inconvenience, discomfort, risks, and costs. Conversely, false-negative results (especially common in women with dense breasts) lead to missed diagnosis and a false sense of security.
How could programs and technology be improved to make screening more beneficial, both for patients and for society as a whole? A major improvement would be if mammography could be made more sensitive and specific for detecting invasive cancers, with fewer false-positive results. Lower cost and less frequent screening would also be major improvements.
Digital breast tomosynthesis (DBT), also known as 3-dimensional (3D) mammography, may be a way to improve the value of breast cancer screening programs. In 2011, the US Food and Drug Administration (FDA) approved DBT for all mammographic indications, including screening.
WHAT IS TOMOSYNTHESIS?
In DBT, the x-ray source is rotated in an arc around the patient’s breast (Figure 1), generating a 3D image.3 DBT is now routinely built into newer-generation mammography units. The 3D projections of DBT are obtained during the same breast compression required for standard 2D digital mammography. Thus, DBT requires minimal additional time on the part of the patient and the technologist.4
The 3D images are processed and sent to a viewing station, where a radiologist can interpret them next to 2D images. The radiologist has the ability to scroll through the DBT projections slice by slice, as in other cross-sectional imaging examinations. However, given the larger number of images compared with digital mammography, DBT requires more time for interpretation, interrupting the workflow. A population-based observational study suggested that combined digital mammography and DBT screening examinations take twice as long to interpret.5
The main advantage of DBT is that it can mitigate the problem of overlapping breast tissue on standard digital projections. These areas of focal asymmetry may represent suspicious masses—or merely overlapping breast parenchyma. When areas of focal asymmetry are found on 2D digital mammography without DBT, patients need to come back for further diagnostic imaging to resolve the finding.6 In addition, especially in women with dense breasts, areas of overlapping tissue can have a masking effect, obscuring small breast cancers.7
For breast cancer screening, DBT is read in conjunction with standard digital mammography. By allowing examination of the breast parenchyma in thin slices, DBT decreases the interpretive issue of overlapping breast parenchyma and the masking effect, potentially leading to fewer false-positive results and higher rates of cancer detection (Figure 2).
EFFECTIVENESS OF TOMOSYNTHESIS
There is limited evidence at this time to support the addition of DBT to digital mammography for primary breast cancer screening, with no published randomized trials that assessed outcomes. However, 2 population-based trials in Europe have prospectively assessed DBT plus digital mammography as a primary screening strategy: the Screening With Tomosynthesis or Standard Mammography (STORM) trial8 and the Oslo tomosynthesis screening trial.5 Only the STORM trial reported first-year interval cancer rates, from which the sensitivity and specificity of DBT plus 2D digital mammography could be calculated and compared with those of 2D digital mammography alone.8
The Oslo trial: Limited applicability in USA
In April 2013, the Oslo tomosynthesis screening trial published interim results of its prospective cohort study of 12,631 Norwegian women ages 50 to 69.5 Women were invited to participate based on the availability of technical staff and imaging systems at the time of screening, and all participants underwent digital mammography and DBT. Images were read independently by 4 radiologists using a double-reader protocol.
The interim results suggest that adding DBT to digital mammography increased cancer detection rates by 31% and decreased the false-positive rate by 13% compared with 2D digital mammography alone (Table 1). However, the double-reader protocol in this study differs from typical single-reader protocols in the United States, limiting the applicability of the findings.
The STORM trial: Low sensitivity
The STORM trial is a prospective cohort study that included 7,292 women without symptoms, at average risk, age 48 and older, who participated in national breast cancer screening services in northern Italy. Each participant underwent digital mammography and DBT. The examinations were read sequentially (digital mammography first, then DBT plus digital mammography) either by a single radiologist, as is most common in the United States, or by 2 radiologists, as is standard in Europe.
Using the single-reader strategy, adding DBT significantly increased cancer detection rates and reduced the total recall rate (Table 1). Sensitivity was 85% vs 54%, and specificity was 97% vs 96%.8,9
Of note, the sensitivity of 54% for digital mammography in the STORM trial is substantially lower than the sensitivity of digital mammography reported in the United States.10
Friedewald et al confirmed Oslo and STORM
To date, the largest US study of DBT plus digital mammography for breast cancer screening was a multicenter retrospective cohort study by Friedewald et al in 2014.11 This study compared cancer detection and recall rates before and after the implementation of DBT at 13 breast centers and evaluated a total of 454,850 examinations (173,663 with DBT plus digital mammography and 281,187 with digital mammography only).
Overall, the recall rate decreased significantly after DBT was adopted and the cancer detection rate increased, findings consistent with those of the STORM and Oslo trials (Table 1). Adding DBT detected invasive cancers at a higher rate than 2D digital mammography alone (4.1/1000 vs 2.9/1,000), while there was no significant difference in ductal carcinoma in situ detection rates. This suggests that the additional cancers detected by DBT may be more clinically important. Nevertheless, the number of biopsies with negative results also increased, suggesting that adding DBT may pose potential harms.
In 2016, Rafferty et al12 published an additional analysis of the data from Friedewald et al, concluding that adding DBT to 2D digital mammography increased the cancer detection rate more in women with heterogeneously dense breasts than in those with either nondense breasts or extremely dense breasts.12 The reduction in recall rate was also greatest in the heterogeneously dense subgroup.
Insufficient evidence to recommend
Most other cohort studies comparing DBT and digital mammography have had findings similar to those of the European prospective studies and the large US retrospective cohort study, with the addition of DBT to mammography reducing recall rates and increasing cancer detection rates.13 However, many of these studies were subject to potential selection bias and did not provide information on the cancer risk of the participants. In addition, no studies have assessed clinical outcomes such as breast cancer stage at diagnosis or interval cancers, let alone breast cancer mortality.
Rigorous studies need to be done in the United States, using the standard single-reader protocol most often used in this country, to ascertain the clinical outcomes of DBT plus digital mammography for breast cancer screening for women at average risk. A 2016 review cited a dearth of high-quality US studies assessing the role of DBT in primary breast cancer.13
The US Preventive Services Task Force, in its 2016 guidelines for breast cancer screening, concluded that there was insufficient evidence to assess the harms and benefits of DBT as a method of breast cancer screening, including adjunctive screening in women with dense breasts.1
Similarly, the American College of Physicians has advised against screening average-risk women for breast cancer using DBT.14
APPROVAL, DISSEMINATION, COSTS, AND CHOICE FOR PATIENTS
Even with early promising data suggesting that DBT can increase cancer detection rates and decrease false-positive results when added to routine screening mammography, the rapid diffusion of DBT into clinical practice is outpacing evidence of its effectiveness.4 This adoption was spurred in January 2015 when the Centers for Medicare and Medicaid Services added a Current Procedural Terminology code for DBT, allowing for additional reimbursement for it for all mammography indications.15 Still, the use of DBT in community settings is inconsistent, given the significant up-front costs associated with equipment purchases and variable reimbursement by private insurers who consider the technology experimental.
For the US healthcare system as a whole, it is uncertain whether the purported benefits of DBT will outweigh the additional costs associated with its use. The average reimbursement for a routine digital mammography examination is $135; adding DBT adds an average of $56 to the cost.15
Using an established, discrete-event breast cancer simulation model, a team of investigators evaluated the cost-effectiveness of combined biennial digital mammography and DBT screening compared with biennial digital mammography screening alone in US women with dense breasts.16 They found that biennial combined screening is likely to be cost-effective in US women with dense breasts. They also found that for every 2,000 women screened from age 50 to age 74, adding DBT would prevent 1 breast cancer death and 810 false-positive screening examinations.16
In addition, some have expressed concern that adding DBT to standard digital mammography increases radiation exposure. In fact, the radiation dose with DBT is similar to that with standard 2D digital mammography. Thus, when acquired together, combined digital mammography and DBT screening leads to twice the radiation dose compared with digital mammography alone.17 Nevertheless, this increased dose remains well below the FDA limits for a screening examination. In addition, the FDA has approved software that allows reconstruction of 2D synthetic views from the 3D data set, which will eventually bring radiation dose levels down to levels comparable to those of conventional digital mammography.17
Given that DBT is built into newer mammography units and is available as an add-on feature for existing units, its use is likely to increase even faster than digital mammography did when it replaced screen-film mammography in the previous decade.4 Its adoption by screening facilities, however, remains variable, and patients wishing to obtain combined DBT and digital mammography screening may have to travel to a different facility from their usual place of screening.18
Moreover, not all insurance companies cover DBT, resulting in additional out-of-pocket costs to the patient. It is currently unclear how individual facilities are offering DBT services, including how patients are selected for additional DBT and if they are offered the choice to add or forego DBT screening in combination with standard digital mammography.
SUMMARY: AN EMERGING TECHNOLOGY
DBT is an emerging imaging technology that allows the radiologist to view breast images in slices, as in computed tomography. DBT images can be obtained using the same breast compression that women already undergo for 2D digital mammography for breast cancer screening.
At this time, adding DBT to digital mammography screening nearly doubles the radiation exposure to the patient. However, new software is available that allows creation of synthetic 2D views from the 3D data set, resulting in radiation exposure that is similar to conventional digital mammography.
Although there are no published randomized controlled trials assessing the benefit of DBT over 2D digital mammography for breast cancer screening, prospective observational studies suggest that DBT may reduce false-positive recall rates and increase cancer detection rates when used in population-based screening programs. Assuming that additional breast cancer detection contributes to improved clinical outcomes, women with dense breasts may benefit more than women without dense breasts.
No national organizations currently recommend DBT for primary breast cancer screening. Ideally, future studies would determine whether DBT screening reduces breast cancer mortality. Since this research may not be feasible, surrogate clinical outcomes, such as a decrease in interval breast cancer rates and impact on stage at time of diagnosis, would allow us to more confidently recommend this new technology.
Each year, millions of women undergo mammography in the hope of decreasing their risk of dying of breast cancer. The effectiveness of screening mammography, however, continues to be debated.
While most randomized controlled trials have demonstrated significantly lower mortality rates in women who undergo screening, not all trials have. Most experts agree that screening mammography programs decrease breast cancer mortality rates by 12% to 33%.1,2 But some point out that although mammography programs clearly detect more cases of breast cancer, some proportion of this detection may include “overdiagnosis” of cancers that would not have caused morbidity or mortality, including ductal carcinoma in situ. Also, although deaths from breast cancer have decreased in the United States, at least some of the decrease may be due to more effective treatment rather than early detection.
Moreover, screening has well-documented harms. False-positive results cause alarm and expose women to needless follow-up imaging and biopsies, with their attendant inconvenience, discomfort, risks, and costs. Conversely, false-negative results (especially common in women with dense breasts) lead to missed diagnosis and a false sense of security.
How could programs and technology be improved to make screening more beneficial, both for patients and for society as a whole? A major improvement would be if mammography could be made more sensitive and specific for detecting invasive cancers, with fewer false-positive results. Lower cost and less frequent screening would also be major improvements.
Digital breast tomosynthesis (DBT), also known as 3-dimensional (3D) mammography, may be a way to improve the value of breast cancer screening programs. In 2011, the US Food and Drug Administration (FDA) approved DBT for all mammographic indications, including screening.
WHAT IS TOMOSYNTHESIS?
In DBT, the x-ray source is rotated in an arc around the patient’s breast (Figure 1), generating a 3D image.3 DBT is now routinely built into newer-generation mammography units. The 3D projections of DBT are obtained during the same breast compression required for standard 2D digital mammography. Thus, DBT requires minimal additional time on the part of the patient and the technologist.4
The 3D images are processed and sent to a viewing station, where a radiologist can interpret them next to 2D images. The radiologist has the ability to scroll through the DBT projections slice by slice, as in other cross-sectional imaging examinations. However, given the larger number of images compared with digital mammography, DBT requires more time for interpretation, interrupting the workflow. A population-based observational study suggested that combined digital mammography and DBT screening examinations take twice as long to interpret.5
The main advantage of DBT is that it can mitigate the problem of overlapping breast tissue on standard digital projections. These areas of focal asymmetry may represent suspicious masses—or merely overlapping breast parenchyma. When areas of focal asymmetry are found on 2D digital mammography without DBT, patients need to come back for further diagnostic imaging to resolve the finding.6 In addition, especially in women with dense breasts, areas of overlapping tissue can have a masking effect, obscuring small breast cancers.7
For breast cancer screening, DBT is read in conjunction with standard digital mammography. By allowing examination of the breast parenchyma in thin slices, DBT decreases the interpretive issue of overlapping breast parenchyma and the masking effect, potentially leading to fewer false-positive results and higher rates of cancer detection (Figure 2).
EFFECTIVENESS OF TOMOSYNTHESIS
There is limited evidence at this time to support the addition of DBT to digital mammography for primary breast cancer screening, with no published randomized trials that assessed outcomes. However, 2 population-based trials in Europe have prospectively assessed DBT plus digital mammography as a primary screening strategy: the Screening With Tomosynthesis or Standard Mammography (STORM) trial8 and the Oslo tomosynthesis screening trial.5 Only the STORM trial reported first-year interval cancer rates, from which the sensitivity and specificity of DBT plus 2D digital mammography could be calculated and compared with those of 2D digital mammography alone.8
The Oslo trial: Limited applicability in USA
In April 2013, the Oslo tomosynthesis screening trial published interim results of its prospective cohort study of 12,631 Norwegian women ages 50 to 69.5 Women were invited to participate based on the availability of technical staff and imaging systems at the time of screening, and all participants underwent digital mammography and DBT. Images were read independently by 4 radiologists using a double-reader protocol.
The interim results suggest that adding DBT to digital mammography increased cancer detection rates by 31% and decreased the false-positive rate by 13% compared with 2D digital mammography alone (Table 1). However, the double-reader protocol in this study differs from typical single-reader protocols in the United States, limiting the applicability of the findings.
The STORM trial: Low sensitivity
The STORM trial is a prospective cohort study that included 7,292 women without symptoms, at average risk, age 48 and older, who participated in national breast cancer screening services in northern Italy. Each participant underwent digital mammography and DBT. The examinations were read sequentially (digital mammography first, then DBT plus digital mammography) either by a single radiologist, as is most common in the United States, or by 2 radiologists, as is standard in Europe.
Using the single-reader strategy, adding DBT significantly increased cancer detection rates and reduced the total recall rate (Table 1). Sensitivity was 85% vs 54%, and specificity was 97% vs 96%.8,9
Of note, the sensitivity of 54% for digital mammography in the STORM trial is substantially lower than the sensitivity of digital mammography reported in the United States.10
Friedewald et al confirmed Oslo and STORM
To date, the largest US study of DBT plus digital mammography for breast cancer screening was a multicenter retrospective cohort study by Friedewald et al in 2014.11 This study compared cancer detection and recall rates before and after the implementation of DBT at 13 breast centers and evaluated a total of 454,850 examinations (173,663 with DBT plus digital mammography and 281,187 with digital mammography only).
Overall, the recall rate decreased significantly after DBT was adopted and the cancer detection rate increased, findings consistent with those of the STORM and Oslo trials (Table 1). Adding DBT detected invasive cancers at a higher rate than 2D digital mammography alone (4.1/1000 vs 2.9/1,000), while there was no significant difference in ductal carcinoma in situ detection rates. This suggests that the additional cancers detected by DBT may be more clinically important. Nevertheless, the number of biopsies with negative results also increased, suggesting that adding DBT may pose potential harms.
In 2016, Rafferty et al12 published an additional analysis of the data from Friedewald et al, concluding that adding DBT to 2D digital mammography increased the cancer detection rate more in women with heterogeneously dense breasts than in those with either nondense breasts or extremely dense breasts.12 The reduction in recall rate was also greatest in the heterogeneously dense subgroup.
Insufficient evidence to recommend
Most other cohort studies comparing DBT and digital mammography have had findings similar to those of the European prospective studies and the large US retrospective cohort study, with the addition of DBT to mammography reducing recall rates and increasing cancer detection rates.13 However, many of these studies were subject to potential selection bias and did not provide information on the cancer risk of the participants. In addition, no studies have assessed clinical outcomes such as breast cancer stage at diagnosis or interval cancers, let alone breast cancer mortality.
Rigorous studies need to be done in the United States, using the standard single-reader protocol most often used in this country, to ascertain the clinical outcomes of DBT plus digital mammography for breast cancer screening for women at average risk. A 2016 review cited a dearth of high-quality US studies assessing the role of DBT in primary breast cancer.13
The US Preventive Services Task Force, in its 2016 guidelines for breast cancer screening, concluded that there was insufficient evidence to assess the harms and benefits of DBT as a method of breast cancer screening, including adjunctive screening in women with dense breasts.1
Similarly, the American College of Physicians has advised against screening average-risk women for breast cancer using DBT.14
APPROVAL, DISSEMINATION, COSTS, AND CHOICE FOR PATIENTS
Even with early promising data suggesting that DBT can increase cancer detection rates and decrease false-positive results when added to routine screening mammography, the rapid diffusion of DBT into clinical practice is outpacing evidence of its effectiveness.4 This adoption was spurred in January 2015 when the Centers for Medicare and Medicaid Services added a Current Procedural Terminology code for DBT, allowing for additional reimbursement for it for all mammography indications.15 Still, the use of DBT in community settings is inconsistent, given the significant up-front costs associated with equipment purchases and variable reimbursement by private insurers who consider the technology experimental.
For the US healthcare system as a whole, it is uncertain whether the purported benefits of DBT will outweigh the additional costs associated with its use. The average reimbursement for a routine digital mammography examination is $135; adding DBT adds an average of $56 to the cost.15
Using an established, discrete-event breast cancer simulation model, a team of investigators evaluated the cost-effectiveness of combined biennial digital mammography and DBT screening compared with biennial digital mammography screening alone in US women with dense breasts.16 They found that biennial combined screening is likely to be cost-effective in US women with dense breasts. They also found that for every 2,000 women screened from age 50 to age 74, adding DBT would prevent 1 breast cancer death and 810 false-positive screening examinations.16
In addition, some have expressed concern that adding DBT to standard digital mammography increases radiation exposure. In fact, the radiation dose with DBT is similar to that with standard 2D digital mammography. Thus, when acquired together, combined digital mammography and DBT screening leads to twice the radiation dose compared with digital mammography alone.17 Nevertheless, this increased dose remains well below the FDA limits for a screening examination. In addition, the FDA has approved software that allows reconstruction of 2D synthetic views from the 3D data set, which will eventually bring radiation dose levels down to levels comparable to those of conventional digital mammography.17
Given that DBT is built into newer mammography units and is available as an add-on feature for existing units, its use is likely to increase even faster than digital mammography did when it replaced screen-film mammography in the previous decade.4 Its adoption by screening facilities, however, remains variable, and patients wishing to obtain combined DBT and digital mammography screening may have to travel to a different facility from their usual place of screening.18
Moreover, not all insurance companies cover DBT, resulting in additional out-of-pocket costs to the patient. It is currently unclear how individual facilities are offering DBT services, including how patients are selected for additional DBT and if they are offered the choice to add or forego DBT screening in combination with standard digital mammography.
SUMMARY: AN EMERGING TECHNOLOGY
DBT is an emerging imaging technology that allows the radiologist to view breast images in slices, as in computed tomography. DBT images can be obtained using the same breast compression that women already undergo for 2D digital mammography for breast cancer screening.
At this time, adding DBT to digital mammography screening nearly doubles the radiation exposure to the patient. However, new software is available that allows creation of synthetic 2D views from the 3D data set, resulting in radiation exposure that is similar to conventional digital mammography.
Although there are no published randomized controlled trials assessing the benefit of DBT over 2D digital mammography for breast cancer screening, prospective observational studies suggest that DBT may reduce false-positive recall rates and increase cancer detection rates when used in population-based screening programs. Assuming that additional breast cancer detection contributes to improved clinical outcomes, women with dense breasts may benefit more than women without dense breasts.
No national organizations currently recommend DBT for primary breast cancer screening. Ideally, future studies would determine whether DBT screening reduces breast cancer mortality. Since this research may not be feasible, surrogate clinical outcomes, such as a decrease in interval breast cancer rates and impact on stage at time of diagnosis, would allow us to more confidently recommend this new technology.
- Siu AL; US Preventive Services Task Force. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:279–296.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314:1599–1614.
- Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad Radiol 2011; 18:1298–1310.
- Lee CI, Lehman CD. Digital breast tomosynthesis and the challenges of implementing an emerging breast cancer screening technology into clinical practice. J Am Coll Radiol 2013; 10:913–917.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267:47–56.
- Helvie MA. Digital mammography imaging: breast tomosynthesis and advanced applications. Radiol Clin North Am 2010; 48:917–929.
- Gur D, Abrams GS, Chough DM, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol 2009; 193:586–591.
- Houssami N, Macaskill P, Bernardi D, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading—evidence to guide future screening strategies. Eur J Cancer 2014; 50:1799–1807.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14:583–589.
- Humphrey L, Chan BKS, Detlefsen S, Helfand M. Screening for Breast Cancer. Rockville, MD: Agency for Healthcare Research and Quality (US); 2002.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311:2499–2507.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315:1784–1786.
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164:268–278.
- Wilt TJ, Harris RP, Qaseem A; High Value Care Task Force of the American College of Physicians. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 2015; 162:718–725.
- American College of Radiology. CMS establishes breast tomosynthesis values in 2015 MPFS final rule. www.acr.org/News-Publications/News/News-Articles/2014/Economics/20141105-CMS-Establishes-Values-for-Breast-Tomosynthesis-in-2015-Final-Rule. Accessed June 14, 2017.
- Lee CI, Cevik M, Alagoz O, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology 2015; 274:772–780.
- Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015; 24:93–99.
- Lee CI, Bogart A, Hubbard RA, et al. Advanced breast imaging availability by screening facility characteristics. Acad Radiol 2015; 22:846–652.
- Siu AL; US Preventive Services Task Force. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:279–296.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314:1599–1614.
- Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad Radiol 2011; 18:1298–1310.
- Lee CI, Lehman CD. Digital breast tomosynthesis and the challenges of implementing an emerging breast cancer screening technology into clinical practice. J Am Coll Radiol 2013; 10:913–917.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267:47–56.
- Helvie MA. Digital mammography imaging: breast tomosynthesis and advanced applications. Radiol Clin North Am 2010; 48:917–929.
- Gur D, Abrams GS, Chough DM, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol 2009; 193:586–591.
- Houssami N, Macaskill P, Bernardi D, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading—evidence to guide future screening strategies. Eur J Cancer 2014; 50:1799–1807.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14:583–589.
- Humphrey L, Chan BKS, Detlefsen S, Helfand M. Screening for Breast Cancer. Rockville, MD: Agency for Healthcare Research and Quality (US); 2002.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311:2499–2507.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315:1784–1786.
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164:268–278.
- Wilt TJ, Harris RP, Qaseem A; High Value Care Task Force of the American College of Physicians. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 2015; 162:718–725.
- American College of Radiology. CMS establishes breast tomosynthesis values in 2015 MPFS final rule. www.acr.org/News-Publications/News/News-Articles/2014/Economics/20141105-CMS-Establishes-Values-for-Breast-Tomosynthesis-in-2015-Final-Rule. Accessed June 14, 2017.
- Lee CI, Cevik M, Alagoz O, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology 2015; 274:772–780.
- Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015; 24:93–99.
- Lee CI, Bogart A, Hubbard RA, et al. Advanced breast imaging availability by screening facility characteristics. Acad Radiol 2015; 22:846–652.
KEY POINTS
- DBT creates 3-dimensional images of the breast that the radiologist can view slice by slice, as in other cross-sectional imaging examinations.
- Initial studies suggest that, when used in conjunction with standard 2-dimensional digital mammography as a screening test, DBT can reduce recall rates and increase cancer detection rates, but its impact on breast cancer mortality rates and cancer stage at diagnosis is not known.
- Drawbacks of DBT: it exposes the patient to more radiation, takes the radiologist longer to interpret, and costs more than standard digital mammography alone.
- Not all insurance companies cover DBT for breast cancer screening.
- Dr. Lee has received research grant funding from GE Healthcare. Dr. Lee’s time is supported in part by the American Cancer Society (126947-MRSG-14-160-01-CPHPS).
- The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the University of Washington, Seattle.
Living with hematologic cancer: Recommendations, solutions
Adults with leukemia, lymphoma, multiple myeloma, and other hematologic cancers are living longer, and more than 1.2 million patients with these cancers are alive in the United States.1 Most adults with nonpediatric cancers are diagnosed in the fifth to seventh decade, and many now survive more than 5 years. The survival rate of patients with most hematologic cancers has doubled since 1974, transforming once-terminal diagnoses into chronic conditions. According to one estimate, there will be 18 million cancer survivors (all types of cancer) by 2022, and nearly 2 million of these will be survivors of hematologic cancers.2
Although survivors of hematologic cancers are at risk of complications of their cancer treatment, they often do not receive routine health maintenance and see their primary care providers only for acute issues.
Primary care providers can play a major role in monitoring the health of hematologic cancer survivors. This requires staying up-to-date on diagnosis, management, and surveillance in this group and being able to address their survivorship issues.3
In this article, we focus on survivorship considerations in patients with previously treated hematologic cancers, including childhood, adolescent, and young-adult cancers. We discuss the role of primary care in the multidisciplinary approach to the continuing care of these patients, and we review innovative technologic solutions to the challenges of delivering care to this group.
SURVIVORSHIP BEGINS AT DIAGNOSIS
The definition of cancer survivorship has changed in the last decade, particularly with hematologic cancers.4
Survivorship was once considered the time after the patient successfully completed cancer treatment. But most patients with hematologic cancers will likely need to continue treatment until they die, with essentially unpredictable and intermittent periods of remission and relapse. Advances in cancer treatment and supportive care have led to longer life. Thus, a commonly recognized definition of survivorship begins at diagnosis rather than later in the disease course and continues through the balance of the patient’s life.5
The survivorship care plan
In 2005, the Institute of Medicine released a report6 calling attention to cancer survivors and their special needs. At that time, a growing number of patients were not returning to their primary care physicians to receive health maintenance after completing their cancer treatment. A proposed solution was for the oncologist to develop a personalized survivorship care plan, which would help the patient understand the treatments received, the importance of health maintenance, and the need for follow-up surveillance.5
The survivorship care plan was originally intended for patients who had completed their cancer treatment. But patients with hematologic cancers tend to need lifelong treatment. Nevertheless, major organizations such as the American Society of Hematology and the American Society of Clinical Oncology consider a survivorship care plan an essential part of cancer care for all patients and not just those with solid tumors.7 The plan should consist of a written treatment summary and recommendations for follow-up care.
EFFECTS OF HEMATOLOGIC CANCER AND ITS TREATMENT
Hematologic cancers and their treatment put patients at risk of many complications, including endocrinopathies, such as hypothyroidism or diabetes secondary to chronic steroid and immunosuppressant use, and cardiovascular events, such as congestive heart failure and stroke due to high-dose chemotherapy. Survivors are also at risk of secondary cancers and recurrence of the primary cancer.8–15
Despite the gravity of a cancer diagnosis, cancer patients do not always adhere to a healthy lifestyle. A survey of over 400,000 cancer survivors found that 15% were current cigarette smokers, 27.5% were obese, and 31.5% had not engaged in physical activity during the previous 30 days.16
THE PRIMARY CARE CLINICIAN AND SURVIVORSHIP CARE
Many hematologic oncology practices include not only medical oncologists but also ancillary team members such as nurse practitioners, nurse specialists, physician assistants, registered nurses, and in some cases a social worker or nutritionist. Patients with hematologic cancers often rely on this team for most of their care while undergoing cancer treatment.
Depending on the type of cancer, and especially after a period of stable disease or remission, some patients transition away from the oncology team, particularly if they live far away, and receive care from their local primary care clinician.
Although the Institute for Medicine intended the survivorship care plan6 to be a patient-focused tool, primary care providers can benefit from it too. In survey of oncologists and primary care providers in the United States,17 49% of the 1,130 oncologists said they almost always provided care plans to patients, and 85% perceived a greater benefit for primary care providers to have these plans than for cancer survivors. However, only 13% of the 1,120 primary care providers surveyed said they consistently received a care plan from the oncologist. The study suggests that oncologists should make a better effort to share these plans with primary care providers to enhance the coordination of care.
COMPONENTS OF A SURVIVORSHIP CARE PLAN AND SELF-MANAGEMENT
Although personalized survivorship care plans are not routinely used in patients with blood cancers,18 they are as important in hematologic cancer survivors as in patients with solid tumors.
The plan should consist of a treatment summary and information on essential components of a healthy lifestyle and should take into consideration coordination of care among primary and other providers, health maintenance recommendations, information on early detection and screening, and psychosocial welfare. Guidance on preventive screening for physical, financial, and psychosocial well-being should be generated by the oncology team or primary care provider and can be helpful to patients and caregivers as they navigate the healthcare system. (See https://cancercontrol.cancer.gov/pdf/ASCO-Survivorship-Care-Plan.pdf for a sample survivorship care plan.)
Although patients with hematologic cancer often have a highly variable course with multiple periods of remission and relapse, the survivorship care plan and treatment summary are essential components of their ongoing care.
Self-management of chronic illness refers to daily activities to keep the illness under control, minimize its impact on physical health and function, and help the patient cope with the psychosocial sequelae of the illness.19 Empowering patients and their caregivers to take control of their health is an essential component of survivorship care. Patients and caregivers can be valuable partners to primary care providers and the oncology team in ongoing care to ensure proper testing and monitoring for secondary illnesses.
INFORMATION TECHNOLOGY SOLUTIONS
Implementation of a survivorship care plan can be facilitated by integrating the plan and treatment summaries into the patient’s electronic medical record and encouraging the patient to be a part of the process.20 Many electronic medical record systems such as Epic can automatically fill in treatment summaries and provide patients access to a survivorship care plan tailored to their needs, but these features are not routinely used, and output can be lengthy and hard to follow.21,22
There has been a surge in research in information technology and care plan delivery since the Health Information Technology for Economic and Clinical Health (HITECH) Act was passed in 2009,23 specifically in innovative strategies to proactively screen for, assess, and manage disease- and treatment-related symptoms in cancer survivors. As a result, patients and families can be more engaged in their care, and providers can better guide survivorship concerns.
Providers can create their own survivorship care plans or use electronic resources to generate one. The American Society of Clinical Oncology and the National Comprehensive Cancer Network provide printed templates in which the patient, primary care provider, or oncology team can complete a care plan. Newer electronic platforms such as the Carevive system are also available. Brief electronic outcome questionnaires can be completed by the patient at home or in the waiting room to assess symptoms, evaluate health maintenance practices, and generate a plan of care to review with the patient.
EMERGING TECHNOLOGY: TELEMEDICINE, VIRTUAL VISITS
Technology can help patients and the healthcare team in survivorship monitoring. Telemedicine, the exchange of medical information via electronic communication, includes video conferencing for patient consultations, transmission of still images, patient portals, and remote monitoring of vital signs.24
This technology is critical to deliver high-quality acute and chronic care to patients in remote or rural areas, locally to patients unable to travel to the clinic, and internationally.25–28 As patients become more technologically savvy, providers can try novel strategies to provide patients access to care. As of September 2015, there were at least 165,000 health applications (apps) for smartphones to help patients better manage aspects of their care such as diet, exercise, blood pressure, and blood sugar levels.29
Video technology such as Express Care Online allows patients to connect with their healthcare providers for video and virtual visits without having to leave home or take time off from work. It also allows oncology providers to have virtual face-to-face contact with patients undergoing treatment phases, and primary care providers to have easier contact with patients during maintenance and remission phases. This technology allows for earlier detection of illness and provides broader access to care. Virtual visits may even prevent needless hospitalization in some cases or, conversely, alert the physician to tell the patient with alarming symptoms of an acute event, that it is time to go to the hospital.
SURVEILLANCE FOR LATE TREATMENT EFFECTS
Guidelines for surveillance for late treatment effects include the following:
- Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer30
- National Comprehensive Cancer Network Guidelines for Age-Related Recommendations: Adolescent and Young Adult Oncology31
- National Comprehensive Cancer Network Guidelines for Treatment of Cancer by Site and Survivorship31
- American Society for Blood and Marrow Transplantation, for survivors of hematopoietic cell transplantation.32
Survivors of childhood blood cancers are at increased risk of cardiac effects of high-dose or anthracycline chemotherapy (eg, doxorubicin for lymphoma, idarubicin for leukemia), skin cancer, sex-specific cancers (breast cancer, cervical cancer, prostate cancer), and osteoporosis.5,30,33,34
For adult survivors of childhood cancers, it is generally recommended to screen for secondary conditions according to the US Preventive Services Task Force. The clinician must also consider the age at cancer diagnosis (child, young adult, or adult), the length of time since chemotherapy (months vs years), and the type of chemotherapy received.
A myriad of recommendations exist according to cancer type, location, stage, and age at diagnosis, but no clear consensus for screening exists. The major survivorship surveillance guidelines of the Children’s Oncology Group, National Comprehensive Cancer Network, and American Society for Blood and Marrow Transplantation are very detailed and lengthy and therefore not user-friendly for the busy clinician. While these guidelines contain minor differences as to what to test for and when to test, they differ mainly in considerations of the length of exposure to chemotherapy and radiation (eg, children, young adults, and older adults), length of time from completion of treatment to assessment of late complications, and whether the patient underwent hematopoietic stem cell transplant.35,36
Table 1 reviews hematologic malignancies and conditions that blood cancer survivors are at risk for and general routine screening recommendations.5,22,30,33,34,36–39 In general, an assessment by a healthcare provider is recommended annually to screen for late effects of cancer and its treatment. Most important are screening for cardiac toxicity, giving immunizations, and preventing second cancers.
Table 1 reflects general recommendations for healthcare screening in childhood, adolescent, or young adult cancer survivors who see adult primary care physicians and for adult cancer survivors (acute leukemias, lymphomas, and multiple myeloma).
Table 2 focuses on screening and prevention specifically after hematopoietic cell transplantation.30,32 These tables are not meant to be all-inclusive but to provide evidence-based recommendations for health surveillance at a glance.
SURVIVORS NEED ONGOING CARE
Recent successes in the treatment of hematologic cancers have led to dramatic changes in the overall health of these patients. In many instances, cancer survivors in the United States are considered to have a chronic illness with survival rates surpassing those in the past. A longer life span is counterbalanced by cumulative physical, financial, and psychosocial issues that require a multidisciplinary team to monitor and manage.
Childhood cancer survivors face the same psychosocial and financial issues as survivors of adult-onset cancers and are at heightened risk of preventable conditions. Ultimately, it is up to the survivor to self-manage many long-term treatment-related symptoms.
A survivorship care plan and treatment summary to guide the patient, primary provider, and oncology team is an essential component of quality care. Screening guidelines vary according to the age at treatment and length of time from therapy, but general screening and the use of technology and information technology solutions to deliver care can help survivors. These solutions have the potential to transform healthcare delivery in the future and provide the opportunity for ongoing, comprehensive care.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29.
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012; 62:220–241.
- Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol 2014; 32:1578–1585.
- Bell K, Ristovski-Slijepcevic S. Cancer survivorship: why labels matter. J Clin Oncol 2013; 31:409–411.
- Denlinger CS, Carlson RW, Are M, et al. Survivorship: introduction and definition. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2014; 12:34–45.
- National Cancer Institute. About cancer survivorship research. http://cancercontrol.cancer.gov/ocs/. Accessed April 28, 2017.
- Cabe MS, Faithfull S, Makin W, Wengstrom Y. Survivorship programs and care planning. Cancer 2013; 119(suppl 11):2179–2186.
- Galindo RJ, Yoon J, Devoe C, Myers AK. PEG-asparaginase induced severe hypertriglyceridemia. Arch Endocrinol Metab 2016; 60:173–177.
- Pophali PA, Klotz JK, Ito S, et al. Male survivors of allogeneic hematopoietic stem cell transplantation have a long term persisting risk of cardiovascular events. Exp Hematol 2014; 42:83–89.
- Armenian SH, Sun CL, Shannon T, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood 2011; 118:6023–6029.
- Duncan CN, Majhail NS, Brazauskas R, et al. Long-term survival and late effects among one-year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant 2015; 21:151–158.
- Inamoto Y, Shah NN, Savani BN, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant 2015; 50:1013–1023.
- Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014; 14:61–70.
- Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol 2012; 30:3734–3745.
- Bhatia S. Genetic variation as a modifier of association between therapeutic exposure and subsequent malignant neoplasms in cancer survivors. Cancer 2015; 121:648–663.
- Underwood JM, Townsend JS, Stewart SL, et al; Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). Surveillance of demographic characteristics and health behaviors among adult cancer survivors—behavioral risk factor surveillance system, United States, 2009. MMWR Surveill Summ 2012; 61:1–23.
- Forsythe LP, Parry C, Alfano CM, et al. Use of survivorship care plans in the United States: associations with survivorship care. J Natl Cancer Inst 2013; 105:1579–1587.
- Taylor K, Monterosso L. Survivorship care plans and treatment summaries in adult patients with hematologic cancer: an integrative literature review. Oncol Nurs Forum 2015; 42:283–291.
- Faiman B. Medication self-management: important concepts for advanced practitioners in oncology. J Adv Pract Oncol 2011; 2:26–34.
- Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract 2014; 10:e150–e159.
- Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract 2015; 11:e329–e335.
- Mayer D. Integration of survivorship care plans into electronic health records. Chicago, IL: American Society of Clinical Oncology; 2015.
- US Department of Health and Human Services. HITECH Act Enforcement Interim Final Rule, 2015. www.hhs.gov/hipaa/for-professionals/special-topics/HITECH-act-enforcement-interim-final-rule/index.html. Accessed May 5, 2017.
- American Telemedicine Association. What is telemedicine? www.americantelemed.org/main/about/about-telemedicine/telemedicine-faqs. Accessed May 5, 2017.
- Sabesan S. Specialist cancer care through telehealth models. Aust J Rural Health 2015; 23:19–23.
- Jhaveri D, Larkins S, Sabesan S. Telestroke, tele-oncology and teledialysis: a systematic review to analyse the outcomes of active therapies delivered with telemedicine support. J Telemed Telecare 2015; 21:181–188.
- Adler E, Alexis C, Ali Z, et al. Bridging the distance in the Caribbean: telemedicine as a means to build capacity for care in paediatric cancer and blood disorders. Stud Health Technol Inform 2015; 209:1–8.
- Pesec M, Sherertz T. Global health from a cancer care perspective. Future Oncol 2015; 11:2235–2245.
- Murphy T. Report: health care apps available in US top 165,000. www.businessinsider.com/ap-report-health-care-apps-available-in-us-top-165000-2015-9. Accessed May 5, 2017.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf. Accessed May 5, 2017.
- Anderson KC, Alsina M, Bensinger W, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: multiple myeloma. J Natl Compr Canc Netw 2009; 7:908–942.
- Majhail NS, Rizzo JD, Lee SJ, et al; Center for International Blood and Marrow Transplant Research (CIBMTR); American Society for Blood and Marrow Transplantation (ASBMT); European Group for Blood and Marrow Transplantation (EBMT); Asia-Pacific Blood and Marrow Transplantation Group (APBMT); Bone Marrow Transplant Society of Australia and New Zealand (BMTSANZ); East Mediterranean Blood and Marrow Transplantation Group (EMBMT); Sociedade Brasileira de Transplante de Medula Ossea (SBTMO). Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18:348–371.
- Ligibel JA, Denlinger CS. New NCCN guidelines for survivorship care. J Natl Compr Canc Netw 2013; 11(suppl):640–644.
- Valdivieso M, Kujawa AM, Jones T, Baker LH. Cancer survivors in the United States: a review of the literature and a call to action. Int J Med Sci 2012; 9:163–173.
- Rizzo JD, Brouwers M, Hurley P, et al; American Society of Hematology and the American Society of Clinical Oncology Practice Guideline Update Committee. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 2010; 116:4045–4059.
- Barthel EM, Spencer K, Banco D, Kiernan E, Parsons S. Is the adolescent and young adult cancer survivor at risk for late effects? It depends on where you look. J Adolesc Young Adult Oncol 2016; 5:159–173.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726, W-236.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46: 1–24.
- Bilotti E, Faiman BM, Richards TA, et al; International Myeloma Foundation Nurse Leadership Board. Survivorship care guidelines for patients living with multiple myeloma: consensus statements of the International Myeloma Foundation Nurse Leadership Board. Clin J Oncol Nurs 2011; 15(suppl):5–8.
Adults with leukemia, lymphoma, multiple myeloma, and other hematologic cancers are living longer, and more than 1.2 million patients with these cancers are alive in the United States.1 Most adults with nonpediatric cancers are diagnosed in the fifth to seventh decade, and many now survive more than 5 years. The survival rate of patients with most hematologic cancers has doubled since 1974, transforming once-terminal diagnoses into chronic conditions. According to one estimate, there will be 18 million cancer survivors (all types of cancer) by 2022, and nearly 2 million of these will be survivors of hematologic cancers.2
Although survivors of hematologic cancers are at risk of complications of their cancer treatment, they often do not receive routine health maintenance and see their primary care providers only for acute issues.
Primary care providers can play a major role in monitoring the health of hematologic cancer survivors. This requires staying up-to-date on diagnosis, management, and surveillance in this group and being able to address their survivorship issues.3
In this article, we focus on survivorship considerations in patients with previously treated hematologic cancers, including childhood, adolescent, and young-adult cancers. We discuss the role of primary care in the multidisciplinary approach to the continuing care of these patients, and we review innovative technologic solutions to the challenges of delivering care to this group.
SURVIVORSHIP BEGINS AT DIAGNOSIS
The definition of cancer survivorship has changed in the last decade, particularly with hematologic cancers.4
Survivorship was once considered the time after the patient successfully completed cancer treatment. But most patients with hematologic cancers will likely need to continue treatment until they die, with essentially unpredictable and intermittent periods of remission and relapse. Advances in cancer treatment and supportive care have led to longer life. Thus, a commonly recognized definition of survivorship begins at diagnosis rather than later in the disease course and continues through the balance of the patient’s life.5
The survivorship care plan
In 2005, the Institute of Medicine released a report6 calling attention to cancer survivors and their special needs. At that time, a growing number of patients were not returning to their primary care physicians to receive health maintenance after completing their cancer treatment. A proposed solution was for the oncologist to develop a personalized survivorship care plan, which would help the patient understand the treatments received, the importance of health maintenance, and the need for follow-up surveillance.5
The survivorship care plan was originally intended for patients who had completed their cancer treatment. But patients with hematologic cancers tend to need lifelong treatment. Nevertheless, major organizations such as the American Society of Hematology and the American Society of Clinical Oncology consider a survivorship care plan an essential part of cancer care for all patients and not just those with solid tumors.7 The plan should consist of a written treatment summary and recommendations for follow-up care.
EFFECTS OF HEMATOLOGIC CANCER AND ITS TREATMENT
Hematologic cancers and their treatment put patients at risk of many complications, including endocrinopathies, such as hypothyroidism or diabetes secondary to chronic steroid and immunosuppressant use, and cardiovascular events, such as congestive heart failure and stroke due to high-dose chemotherapy. Survivors are also at risk of secondary cancers and recurrence of the primary cancer.8–15
Despite the gravity of a cancer diagnosis, cancer patients do not always adhere to a healthy lifestyle. A survey of over 400,000 cancer survivors found that 15% were current cigarette smokers, 27.5% were obese, and 31.5% had not engaged in physical activity during the previous 30 days.16
THE PRIMARY CARE CLINICIAN AND SURVIVORSHIP CARE
Many hematologic oncology practices include not only medical oncologists but also ancillary team members such as nurse practitioners, nurse specialists, physician assistants, registered nurses, and in some cases a social worker or nutritionist. Patients with hematologic cancers often rely on this team for most of their care while undergoing cancer treatment.
Depending on the type of cancer, and especially after a period of stable disease or remission, some patients transition away from the oncology team, particularly if they live far away, and receive care from their local primary care clinician.
Although the Institute for Medicine intended the survivorship care plan6 to be a patient-focused tool, primary care providers can benefit from it too. In survey of oncologists and primary care providers in the United States,17 49% of the 1,130 oncologists said they almost always provided care plans to patients, and 85% perceived a greater benefit for primary care providers to have these plans than for cancer survivors. However, only 13% of the 1,120 primary care providers surveyed said they consistently received a care plan from the oncologist. The study suggests that oncologists should make a better effort to share these plans with primary care providers to enhance the coordination of care.
COMPONENTS OF A SURVIVORSHIP CARE PLAN AND SELF-MANAGEMENT
Although personalized survivorship care plans are not routinely used in patients with blood cancers,18 they are as important in hematologic cancer survivors as in patients with solid tumors.
The plan should consist of a treatment summary and information on essential components of a healthy lifestyle and should take into consideration coordination of care among primary and other providers, health maintenance recommendations, information on early detection and screening, and psychosocial welfare. Guidance on preventive screening for physical, financial, and psychosocial well-being should be generated by the oncology team or primary care provider and can be helpful to patients and caregivers as they navigate the healthcare system. (See https://cancercontrol.cancer.gov/pdf/ASCO-Survivorship-Care-Plan.pdf for a sample survivorship care plan.)
Although patients with hematologic cancer often have a highly variable course with multiple periods of remission and relapse, the survivorship care plan and treatment summary are essential components of their ongoing care.
Self-management of chronic illness refers to daily activities to keep the illness under control, minimize its impact on physical health and function, and help the patient cope with the psychosocial sequelae of the illness.19 Empowering patients and their caregivers to take control of their health is an essential component of survivorship care. Patients and caregivers can be valuable partners to primary care providers and the oncology team in ongoing care to ensure proper testing and monitoring for secondary illnesses.
INFORMATION TECHNOLOGY SOLUTIONS
Implementation of a survivorship care plan can be facilitated by integrating the plan and treatment summaries into the patient’s electronic medical record and encouraging the patient to be a part of the process.20 Many electronic medical record systems such as Epic can automatically fill in treatment summaries and provide patients access to a survivorship care plan tailored to their needs, but these features are not routinely used, and output can be lengthy and hard to follow.21,22
There has been a surge in research in information technology and care plan delivery since the Health Information Technology for Economic and Clinical Health (HITECH) Act was passed in 2009,23 specifically in innovative strategies to proactively screen for, assess, and manage disease- and treatment-related symptoms in cancer survivors. As a result, patients and families can be more engaged in their care, and providers can better guide survivorship concerns.
Providers can create their own survivorship care plans or use electronic resources to generate one. The American Society of Clinical Oncology and the National Comprehensive Cancer Network provide printed templates in which the patient, primary care provider, or oncology team can complete a care plan. Newer electronic platforms such as the Carevive system are also available. Brief electronic outcome questionnaires can be completed by the patient at home or in the waiting room to assess symptoms, evaluate health maintenance practices, and generate a plan of care to review with the patient.
EMERGING TECHNOLOGY: TELEMEDICINE, VIRTUAL VISITS
Technology can help patients and the healthcare team in survivorship monitoring. Telemedicine, the exchange of medical information via electronic communication, includes video conferencing for patient consultations, transmission of still images, patient portals, and remote monitoring of vital signs.24
This technology is critical to deliver high-quality acute and chronic care to patients in remote or rural areas, locally to patients unable to travel to the clinic, and internationally.25–28 As patients become more technologically savvy, providers can try novel strategies to provide patients access to care. As of September 2015, there were at least 165,000 health applications (apps) for smartphones to help patients better manage aspects of their care such as diet, exercise, blood pressure, and blood sugar levels.29
Video technology such as Express Care Online allows patients to connect with their healthcare providers for video and virtual visits without having to leave home or take time off from work. It also allows oncology providers to have virtual face-to-face contact with patients undergoing treatment phases, and primary care providers to have easier contact with patients during maintenance and remission phases. This technology allows for earlier detection of illness and provides broader access to care. Virtual visits may even prevent needless hospitalization in some cases or, conversely, alert the physician to tell the patient with alarming symptoms of an acute event, that it is time to go to the hospital.
SURVEILLANCE FOR LATE TREATMENT EFFECTS
Guidelines for surveillance for late treatment effects include the following:
- Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer30
- National Comprehensive Cancer Network Guidelines for Age-Related Recommendations: Adolescent and Young Adult Oncology31
- National Comprehensive Cancer Network Guidelines for Treatment of Cancer by Site and Survivorship31
- American Society for Blood and Marrow Transplantation, for survivors of hematopoietic cell transplantation.32
Survivors of childhood blood cancers are at increased risk of cardiac effects of high-dose or anthracycline chemotherapy (eg, doxorubicin for lymphoma, idarubicin for leukemia), skin cancer, sex-specific cancers (breast cancer, cervical cancer, prostate cancer), and osteoporosis.5,30,33,34
For adult survivors of childhood cancers, it is generally recommended to screen for secondary conditions according to the US Preventive Services Task Force. The clinician must also consider the age at cancer diagnosis (child, young adult, or adult), the length of time since chemotherapy (months vs years), and the type of chemotherapy received.
A myriad of recommendations exist according to cancer type, location, stage, and age at diagnosis, but no clear consensus for screening exists. The major survivorship surveillance guidelines of the Children’s Oncology Group, National Comprehensive Cancer Network, and American Society for Blood and Marrow Transplantation are very detailed and lengthy and therefore not user-friendly for the busy clinician. While these guidelines contain minor differences as to what to test for and when to test, they differ mainly in considerations of the length of exposure to chemotherapy and radiation (eg, children, young adults, and older adults), length of time from completion of treatment to assessment of late complications, and whether the patient underwent hematopoietic stem cell transplant.35,36
Table 1 reviews hematologic malignancies and conditions that blood cancer survivors are at risk for and general routine screening recommendations.5,22,30,33,34,36–39 In general, an assessment by a healthcare provider is recommended annually to screen for late effects of cancer and its treatment. Most important are screening for cardiac toxicity, giving immunizations, and preventing second cancers.
Table 1 reflects general recommendations for healthcare screening in childhood, adolescent, or young adult cancer survivors who see adult primary care physicians and for adult cancer survivors (acute leukemias, lymphomas, and multiple myeloma).
Table 2 focuses on screening and prevention specifically after hematopoietic cell transplantation.30,32 These tables are not meant to be all-inclusive but to provide evidence-based recommendations for health surveillance at a glance.
SURVIVORS NEED ONGOING CARE
Recent successes in the treatment of hematologic cancers have led to dramatic changes in the overall health of these patients. In many instances, cancer survivors in the United States are considered to have a chronic illness with survival rates surpassing those in the past. A longer life span is counterbalanced by cumulative physical, financial, and psychosocial issues that require a multidisciplinary team to monitor and manage.
Childhood cancer survivors face the same psychosocial and financial issues as survivors of adult-onset cancers and are at heightened risk of preventable conditions. Ultimately, it is up to the survivor to self-manage many long-term treatment-related symptoms.
A survivorship care plan and treatment summary to guide the patient, primary provider, and oncology team is an essential component of quality care. Screening guidelines vary according to the age at treatment and length of time from therapy, but general screening and the use of technology and information technology solutions to deliver care can help survivors. These solutions have the potential to transform healthcare delivery in the future and provide the opportunity for ongoing, comprehensive care.
Adults with leukemia, lymphoma, multiple myeloma, and other hematologic cancers are living longer, and more than 1.2 million patients with these cancers are alive in the United States.1 Most adults with nonpediatric cancers are diagnosed in the fifth to seventh decade, and many now survive more than 5 years. The survival rate of patients with most hematologic cancers has doubled since 1974, transforming once-terminal diagnoses into chronic conditions. According to one estimate, there will be 18 million cancer survivors (all types of cancer) by 2022, and nearly 2 million of these will be survivors of hematologic cancers.2
Although survivors of hematologic cancers are at risk of complications of their cancer treatment, they often do not receive routine health maintenance and see their primary care providers only for acute issues.
Primary care providers can play a major role in monitoring the health of hematologic cancer survivors. This requires staying up-to-date on diagnosis, management, and surveillance in this group and being able to address their survivorship issues.3
In this article, we focus on survivorship considerations in patients with previously treated hematologic cancers, including childhood, adolescent, and young-adult cancers. We discuss the role of primary care in the multidisciplinary approach to the continuing care of these patients, and we review innovative technologic solutions to the challenges of delivering care to this group.
SURVIVORSHIP BEGINS AT DIAGNOSIS
The definition of cancer survivorship has changed in the last decade, particularly with hematologic cancers.4
Survivorship was once considered the time after the patient successfully completed cancer treatment. But most patients with hematologic cancers will likely need to continue treatment until they die, with essentially unpredictable and intermittent periods of remission and relapse. Advances in cancer treatment and supportive care have led to longer life. Thus, a commonly recognized definition of survivorship begins at diagnosis rather than later in the disease course and continues through the balance of the patient’s life.5
The survivorship care plan
In 2005, the Institute of Medicine released a report6 calling attention to cancer survivors and their special needs. At that time, a growing number of patients were not returning to their primary care physicians to receive health maintenance after completing their cancer treatment. A proposed solution was for the oncologist to develop a personalized survivorship care plan, which would help the patient understand the treatments received, the importance of health maintenance, and the need for follow-up surveillance.5
The survivorship care plan was originally intended for patients who had completed their cancer treatment. But patients with hematologic cancers tend to need lifelong treatment. Nevertheless, major organizations such as the American Society of Hematology and the American Society of Clinical Oncology consider a survivorship care plan an essential part of cancer care for all patients and not just those with solid tumors.7 The plan should consist of a written treatment summary and recommendations for follow-up care.
EFFECTS OF HEMATOLOGIC CANCER AND ITS TREATMENT
Hematologic cancers and their treatment put patients at risk of many complications, including endocrinopathies, such as hypothyroidism or diabetes secondary to chronic steroid and immunosuppressant use, and cardiovascular events, such as congestive heart failure and stroke due to high-dose chemotherapy. Survivors are also at risk of secondary cancers and recurrence of the primary cancer.8–15
Despite the gravity of a cancer diagnosis, cancer patients do not always adhere to a healthy lifestyle. A survey of over 400,000 cancer survivors found that 15% were current cigarette smokers, 27.5% were obese, and 31.5% had not engaged in physical activity during the previous 30 days.16
THE PRIMARY CARE CLINICIAN AND SURVIVORSHIP CARE
Many hematologic oncology practices include not only medical oncologists but also ancillary team members such as nurse practitioners, nurse specialists, physician assistants, registered nurses, and in some cases a social worker or nutritionist. Patients with hematologic cancers often rely on this team for most of their care while undergoing cancer treatment.
Depending on the type of cancer, and especially after a period of stable disease or remission, some patients transition away from the oncology team, particularly if they live far away, and receive care from their local primary care clinician.
Although the Institute for Medicine intended the survivorship care plan6 to be a patient-focused tool, primary care providers can benefit from it too. In survey of oncologists and primary care providers in the United States,17 49% of the 1,130 oncologists said they almost always provided care plans to patients, and 85% perceived a greater benefit for primary care providers to have these plans than for cancer survivors. However, only 13% of the 1,120 primary care providers surveyed said they consistently received a care plan from the oncologist. The study suggests that oncologists should make a better effort to share these plans with primary care providers to enhance the coordination of care.
COMPONENTS OF A SURVIVORSHIP CARE PLAN AND SELF-MANAGEMENT
Although personalized survivorship care plans are not routinely used in patients with blood cancers,18 they are as important in hematologic cancer survivors as in patients with solid tumors.
The plan should consist of a treatment summary and information on essential components of a healthy lifestyle and should take into consideration coordination of care among primary and other providers, health maintenance recommendations, information on early detection and screening, and psychosocial welfare. Guidance on preventive screening for physical, financial, and psychosocial well-being should be generated by the oncology team or primary care provider and can be helpful to patients and caregivers as they navigate the healthcare system. (See https://cancercontrol.cancer.gov/pdf/ASCO-Survivorship-Care-Plan.pdf for a sample survivorship care plan.)
Although patients with hematologic cancer often have a highly variable course with multiple periods of remission and relapse, the survivorship care plan and treatment summary are essential components of their ongoing care.
Self-management of chronic illness refers to daily activities to keep the illness under control, minimize its impact on physical health and function, and help the patient cope with the psychosocial sequelae of the illness.19 Empowering patients and their caregivers to take control of their health is an essential component of survivorship care. Patients and caregivers can be valuable partners to primary care providers and the oncology team in ongoing care to ensure proper testing and monitoring for secondary illnesses.
INFORMATION TECHNOLOGY SOLUTIONS
Implementation of a survivorship care plan can be facilitated by integrating the plan and treatment summaries into the patient’s electronic medical record and encouraging the patient to be a part of the process.20 Many electronic medical record systems such as Epic can automatically fill in treatment summaries and provide patients access to a survivorship care plan tailored to their needs, but these features are not routinely used, and output can be lengthy and hard to follow.21,22
There has been a surge in research in information technology and care plan delivery since the Health Information Technology for Economic and Clinical Health (HITECH) Act was passed in 2009,23 specifically in innovative strategies to proactively screen for, assess, and manage disease- and treatment-related symptoms in cancer survivors. As a result, patients and families can be more engaged in their care, and providers can better guide survivorship concerns.
Providers can create their own survivorship care plans or use electronic resources to generate one. The American Society of Clinical Oncology and the National Comprehensive Cancer Network provide printed templates in which the patient, primary care provider, or oncology team can complete a care plan. Newer electronic platforms such as the Carevive system are also available. Brief electronic outcome questionnaires can be completed by the patient at home or in the waiting room to assess symptoms, evaluate health maintenance practices, and generate a plan of care to review with the patient.
EMERGING TECHNOLOGY: TELEMEDICINE, VIRTUAL VISITS
Technology can help patients and the healthcare team in survivorship monitoring. Telemedicine, the exchange of medical information via electronic communication, includes video conferencing for patient consultations, transmission of still images, patient portals, and remote monitoring of vital signs.24
This technology is critical to deliver high-quality acute and chronic care to patients in remote or rural areas, locally to patients unable to travel to the clinic, and internationally.25–28 As patients become more technologically savvy, providers can try novel strategies to provide patients access to care. As of September 2015, there were at least 165,000 health applications (apps) for smartphones to help patients better manage aspects of their care such as diet, exercise, blood pressure, and blood sugar levels.29
Video technology such as Express Care Online allows patients to connect with their healthcare providers for video and virtual visits without having to leave home or take time off from work. It also allows oncology providers to have virtual face-to-face contact with patients undergoing treatment phases, and primary care providers to have easier contact with patients during maintenance and remission phases. This technology allows for earlier detection of illness and provides broader access to care. Virtual visits may even prevent needless hospitalization in some cases or, conversely, alert the physician to tell the patient with alarming symptoms of an acute event, that it is time to go to the hospital.
SURVEILLANCE FOR LATE TREATMENT EFFECTS
Guidelines for surveillance for late treatment effects include the following:
- Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer30
- National Comprehensive Cancer Network Guidelines for Age-Related Recommendations: Adolescent and Young Adult Oncology31
- National Comprehensive Cancer Network Guidelines for Treatment of Cancer by Site and Survivorship31
- American Society for Blood and Marrow Transplantation, for survivors of hematopoietic cell transplantation.32
Survivors of childhood blood cancers are at increased risk of cardiac effects of high-dose or anthracycline chemotherapy (eg, doxorubicin for lymphoma, idarubicin for leukemia), skin cancer, sex-specific cancers (breast cancer, cervical cancer, prostate cancer), and osteoporosis.5,30,33,34
For adult survivors of childhood cancers, it is generally recommended to screen for secondary conditions according to the US Preventive Services Task Force. The clinician must also consider the age at cancer diagnosis (child, young adult, or adult), the length of time since chemotherapy (months vs years), and the type of chemotherapy received.
A myriad of recommendations exist according to cancer type, location, stage, and age at diagnosis, but no clear consensus for screening exists. The major survivorship surveillance guidelines of the Children’s Oncology Group, National Comprehensive Cancer Network, and American Society for Blood and Marrow Transplantation are very detailed and lengthy and therefore not user-friendly for the busy clinician. While these guidelines contain minor differences as to what to test for and when to test, they differ mainly in considerations of the length of exposure to chemotherapy and radiation (eg, children, young adults, and older adults), length of time from completion of treatment to assessment of late complications, and whether the patient underwent hematopoietic stem cell transplant.35,36
Table 1 reviews hematologic malignancies and conditions that blood cancer survivors are at risk for and general routine screening recommendations.5,22,30,33,34,36–39 In general, an assessment by a healthcare provider is recommended annually to screen for late effects of cancer and its treatment. Most important are screening for cardiac toxicity, giving immunizations, and preventing second cancers.
Table 1 reflects general recommendations for healthcare screening in childhood, adolescent, or young adult cancer survivors who see adult primary care physicians and for adult cancer survivors (acute leukemias, lymphomas, and multiple myeloma).
Table 2 focuses on screening and prevention specifically after hematopoietic cell transplantation.30,32 These tables are not meant to be all-inclusive but to provide evidence-based recommendations for health surveillance at a glance.
SURVIVORS NEED ONGOING CARE
Recent successes in the treatment of hematologic cancers have led to dramatic changes in the overall health of these patients. In many instances, cancer survivors in the United States are considered to have a chronic illness with survival rates surpassing those in the past. A longer life span is counterbalanced by cumulative physical, financial, and psychosocial issues that require a multidisciplinary team to monitor and manage.
Childhood cancer survivors face the same psychosocial and financial issues as survivors of adult-onset cancers and are at heightened risk of preventable conditions. Ultimately, it is up to the survivor to self-manage many long-term treatment-related symptoms.
A survivorship care plan and treatment summary to guide the patient, primary provider, and oncology team is an essential component of quality care. Screening guidelines vary according to the age at treatment and length of time from therapy, but general screening and the use of technology and information technology solutions to deliver care can help survivors. These solutions have the potential to transform healthcare delivery in the future and provide the opportunity for ongoing, comprehensive care.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29.
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012; 62:220–241.
- Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol 2014; 32:1578–1585.
- Bell K, Ristovski-Slijepcevic S. Cancer survivorship: why labels matter. J Clin Oncol 2013; 31:409–411.
- Denlinger CS, Carlson RW, Are M, et al. Survivorship: introduction and definition. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2014; 12:34–45.
- National Cancer Institute. About cancer survivorship research. http://cancercontrol.cancer.gov/ocs/. Accessed April 28, 2017.
- Cabe MS, Faithfull S, Makin W, Wengstrom Y. Survivorship programs and care planning. Cancer 2013; 119(suppl 11):2179–2186.
- Galindo RJ, Yoon J, Devoe C, Myers AK. PEG-asparaginase induced severe hypertriglyceridemia. Arch Endocrinol Metab 2016; 60:173–177.
- Pophali PA, Klotz JK, Ito S, et al. Male survivors of allogeneic hematopoietic stem cell transplantation have a long term persisting risk of cardiovascular events. Exp Hematol 2014; 42:83–89.
- Armenian SH, Sun CL, Shannon T, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood 2011; 118:6023–6029.
- Duncan CN, Majhail NS, Brazauskas R, et al. Long-term survival and late effects among one-year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant 2015; 21:151–158.
- Inamoto Y, Shah NN, Savani BN, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant 2015; 50:1013–1023.
- Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014; 14:61–70.
- Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol 2012; 30:3734–3745.
- Bhatia S. Genetic variation as a modifier of association between therapeutic exposure and subsequent malignant neoplasms in cancer survivors. Cancer 2015; 121:648–663.
- Underwood JM, Townsend JS, Stewart SL, et al; Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). Surveillance of demographic characteristics and health behaviors among adult cancer survivors—behavioral risk factor surveillance system, United States, 2009. MMWR Surveill Summ 2012; 61:1–23.
- Forsythe LP, Parry C, Alfano CM, et al. Use of survivorship care plans in the United States: associations with survivorship care. J Natl Cancer Inst 2013; 105:1579–1587.
- Taylor K, Monterosso L. Survivorship care plans and treatment summaries in adult patients with hematologic cancer: an integrative literature review. Oncol Nurs Forum 2015; 42:283–291.
- Faiman B. Medication self-management: important concepts for advanced practitioners in oncology. J Adv Pract Oncol 2011; 2:26–34.
- Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract 2014; 10:e150–e159.
- Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract 2015; 11:e329–e335.
- Mayer D. Integration of survivorship care plans into electronic health records. Chicago, IL: American Society of Clinical Oncology; 2015.
- US Department of Health and Human Services. HITECH Act Enforcement Interim Final Rule, 2015. www.hhs.gov/hipaa/for-professionals/special-topics/HITECH-act-enforcement-interim-final-rule/index.html. Accessed May 5, 2017.
- American Telemedicine Association. What is telemedicine? www.americantelemed.org/main/about/about-telemedicine/telemedicine-faqs. Accessed May 5, 2017.
- Sabesan S. Specialist cancer care through telehealth models. Aust J Rural Health 2015; 23:19–23.
- Jhaveri D, Larkins S, Sabesan S. Telestroke, tele-oncology and teledialysis: a systematic review to analyse the outcomes of active therapies delivered with telemedicine support. J Telemed Telecare 2015; 21:181–188.
- Adler E, Alexis C, Ali Z, et al. Bridging the distance in the Caribbean: telemedicine as a means to build capacity for care in paediatric cancer and blood disorders. Stud Health Technol Inform 2015; 209:1–8.
- Pesec M, Sherertz T. Global health from a cancer care perspective. Future Oncol 2015; 11:2235–2245.
- Murphy T. Report: health care apps available in US top 165,000. www.businessinsider.com/ap-report-health-care-apps-available-in-us-top-165000-2015-9. Accessed May 5, 2017.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf. Accessed May 5, 2017.
- Anderson KC, Alsina M, Bensinger W, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: multiple myeloma. J Natl Compr Canc Netw 2009; 7:908–942.
- Majhail NS, Rizzo JD, Lee SJ, et al; Center for International Blood and Marrow Transplant Research (CIBMTR); American Society for Blood and Marrow Transplantation (ASBMT); European Group for Blood and Marrow Transplantation (EBMT); Asia-Pacific Blood and Marrow Transplantation Group (APBMT); Bone Marrow Transplant Society of Australia and New Zealand (BMTSANZ); East Mediterranean Blood and Marrow Transplantation Group (EMBMT); Sociedade Brasileira de Transplante de Medula Ossea (SBTMO). Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18:348–371.
- Ligibel JA, Denlinger CS. New NCCN guidelines for survivorship care. J Natl Compr Canc Netw 2013; 11(suppl):640–644.
- Valdivieso M, Kujawa AM, Jones T, Baker LH. Cancer survivors in the United States: a review of the literature and a call to action. Int J Med Sci 2012; 9:163–173.
- Rizzo JD, Brouwers M, Hurley P, et al; American Society of Hematology and the American Society of Clinical Oncology Practice Guideline Update Committee. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 2010; 116:4045–4059.
- Barthel EM, Spencer K, Banco D, Kiernan E, Parsons S. Is the adolescent and young adult cancer survivor at risk for late effects? It depends on where you look. J Adolesc Young Adult Oncol 2016; 5:159–173.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726, W-236.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46: 1–24.
- Bilotti E, Faiman BM, Richards TA, et al; International Myeloma Foundation Nurse Leadership Board. Survivorship care guidelines for patients living with multiple myeloma: consensus statements of the International Myeloma Foundation Nurse Leadership Board. Clin J Oncol Nurs 2011; 15(suppl):5–8.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29.
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012; 62:220–241.
- Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol 2014; 32:1578–1585.
- Bell K, Ristovski-Slijepcevic S. Cancer survivorship: why labels matter. J Clin Oncol 2013; 31:409–411.
- Denlinger CS, Carlson RW, Are M, et al. Survivorship: introduction and definition. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2014; 12:34–45.
- National Cancer Institute. About cancer survivorship research. http://cancercontrol.cancer.gov/ocs/. Accessed April 28, 2017.
- Cabe MS, Faithfull S, Makin W, Wengstrom Y. Survivorship programs and care planning. Cancer 2013; 119(suppl 11):2179–2186.
- Galindo RJ, Yoon J, Devoe C, Myers AK. PEG-asparaginase induced severe hypertriglyceridemia. Arch Endocrinol Metab 2016; 60:173–177.
- Pophali PA, Klotz JK, Ito S, et al. Male survivors of allogeneic hematopoietic stem cell transplantation have a long term persisting risk of cardiovascular events. Exp Hematol 2014; 42:83–89.
- Armenian SH, Sun CL, Shannon T, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood 2011; 118:6023–6029.
- Duncan CN, Majhail NS, Brazauskas R, et al. Long-term survival and late effects among one-year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant 2015; 21:151–158.
- Inamoto Y, Shah NN, Savani BN, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant 2015; 50:1013–1023.
- Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014; 14:61–70.
- Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol 2012; 30:3734–3745.
- Bhatia S. Genetic variation as a modifier of association between therapeutic exposure and subsequent malignant neoplasms in cancer survivors. Cancer 2015; 121:648–663.
- Underwood JM, Townsend JS, Stewart SL, et al; Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). Surveillance of demographic characteristics and health behaviors among adult cancer survivors—behavioral risk factor surveillance system, United States, 2009. MMWR Surveill Summ 2012; 61:1–23.
- Forsythe LP, Parry C, Alfano CM, et al. Use of survivorship care plans in the United States: associations with survivorship care. J Natl Cancer Inst 2013; 105:1579–1587.
- Taylor K, Monterosso L. Survivorship care plans and treatment summaries in adult patients with hematologic cancer: an integrative literature review. Oncol Nurs Forum 2015; 42:283–291.
- Faiman B. Medication self-management: important concepts for advanced practitioners in oncology. J Adv Pract Oncol 2011; 2:26–34.
- Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract 2014; 10:e150–e159.
- Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract 2015; 11:e329–e335.
- Mayer D. Integration of survivorship care plans into electronic health records. Chicago, IL: American Society of Clinical Oncology; 2015.
- US Department of Health and Human Services. HITECH Act Enforcement Interim Final Rule, 2015. www.hhs.gov/hipaa/for-professionals/special-topics/HITECH-act-enforcement-interim-final-rule/index.html. Accessed May 5, 2017.
- American Telemedicine Association. What is telemedicine? www.americantelemed.org/main/about/about-telemedicine/telemedicine-faqs. Accessed May 5, 2017.
- Sabesan S. Specialist cancer care through telehealth models. Aust J Rural Health 2015; 23:19–23.
- Jhaveri D, Larkins S, Sabesan S. Telestroke, tele-oncology and teledialysis: a systematic review to analyse the outcomes of active therapies delivered with telemedicine support. J Telemed Telecare 2015; 21:181–188.
- Adler E, Alexis C, Ali Z, et al. Bridging the distance in the Caribbean: telemedicine as a means to build capacity for care in paediatric cancer and blood disorders. Stud Health Technol Inform 2015; 209:1–8.
- Pesec M, Sherertz T. Global health from a cancer care perspective. Future Oncol 2015; 11:2235–2245.
- Murphy T. Report: health care apps available in US top 165,000. www.businessinsider.com/ap-report-health-care-apps-available-in-us-top-165000-2015-9. Accessed May 5, 2017.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf. Accessed May 5, 2017.
- Anderson KC, Alsina M, Bensinger W, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: multiple myeloma. J Natl Compr Canc Netw 2009; 7:908–942.
- Majhail NS, Rizzo JD, Lee SJ, et al; Center for International Blood and Marrow Transplant Research (CIBMTR); American Society for Blood and Marrow Transplantation (ASBMT); European Group for Blood and Marrow Transplantation (EBMT); Asia-Pacific Blood and Marrow Transplantation Group (APBMT); Bone Marrow Transplant Society of Australia and New Zealand (BMTSANZ); East Mediterranean Blood and Marrow Transplantation Group (EMBMT); Sociedade Brasileira de Transplante de Medula Ossea (SBTMO). Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18:348–371.
- Ligibel JA, Denlinger CS. New NCCN guidelines for survivorship care. J Natl Compr Canc Netw 2013; 11(suppl):640–644.
- Valdivieso M, Kujawa AM, Jones T, Baker LH. Cancer survivors in the United States: a review of the literature and a call to action. Int J Med Sci 2012; 9:163–173.
- Rizzo JD, Brouwers M, Hurley P, et al; American Society of Hematology and the American Society of Clinical Oncology Practice Guideline Update Committee. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 2010; 116:4045–4059.
- Barthel EM, Spencer K, Banco D, Kiernan E, Parsons S. Is the adolescent and young adult cancer survivor at risk for late effects? It depends on where you look. J Adolesc Young Adult Oncol 2016; 5:159–173.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726, W-236.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46: 1–24.
- Bilotti E, Faiman BM, Richards TA, et al; International Myeloma Foundation Nurse Leadership Board. Survivorship care guidelines for patients living with multiple myeloma: consensus statements of the International Myeloma Foundation Nurse Leadership Board. Clin J Oncol Nurs 2011; 15(suppl):5–8.
KEY POINTS
- The definition of survivorship is different in patients with hematologic cancer than in patients with solid tumors, as treatment is often ongoing and lacks a clear stopping point.
- Routine health maintenance is especially important for patients with hematologic cancers, who face a heightened risk of secondary cancers and other conditions.
- Survivorship plans can improve communication between the primary care provider, patient, and oncology team.
- Physicians should emphasize the importance of a healthy lifestyle and routine health maintenance for their patients who are cancer survivors.
Tickborne diseases other than Lyme in the United States
Ticks are responsible for most vector-borne infections in the United States. Most infections occur between April and October, when tick populations peak.1 However, infections can occur year-round.2,3
Tick bites are often unnoticed because the ticks are small when they are at the infective stage of their life cycle, and their attachment is characteristically painless and often in intertriginous body sites.1 Therefore, absence of a known tick bite never precludes the diagnosis of a tickborne infection.1,4,5
Although rural outdoor activities are recognized risk factors, tickborne infections also occur in urban areas.6 Thus, the lack of classic epidemiologic clues does not rule out a diagnosis of tickborne infection.
In most cases, tickborne illnesses present with nonspecific symptoms such as fever, malaise, headache, nausea, and myalgia. Accurate diagnosis of tickborne diseases can be challenging due to the similar clinical manifestations and overlapping geographic distributions of potential tick vectors.1
This review summarizes the epidemiology, clinical features, treatment, and prevention of the most prevalent non-Lyme tickborne diseases of the United States: Rocky Mountain spotted fever (RMSF), other spotted fever group rickettsial (SFGR) infections, ehrlichiosis, babesiosis, tickborne relapsing fever, Borrelia miyamotoi infection, southern tick-associated rash illness (STARI), tularemia, and tickborne viral infections.
ROCKY MOUNTAIN SPOTTED FEVER
Dermacentor variabilis, the American dog tick, is the major vector in the southern and eastern United States, and D andersoni, the Rocky Mountain wood tick, is the most common vector in the western United States.4,7,8 Rhipicephalus sanguineus, the brown dog tick, has also been found to transmit RMSF in Arizona.9,10
While most infections in humans are transmitted by tick bite, rare cases of RMSF are contracted through exposure to infective tick hemolymph during tick removal, parenteral inoculation or infectious aerosols in laboratory settings, and blood transfusion.7,8
RMSF is both the most common and the most likely cause of death among rickettsial infections in the United States.4,7,8 Most cases occur in children ages 5 to 9.10,11 The case-fatality rate is over 20% without antimicrobial therapy but less than 1% with timely and appropriate antibiotic treatment.7,8
Clinical manifestations of Rocky Mountain spotted fever
RMSF is transmitted after only 2 to 20 hours of tick attachment, and symptoms begin 3 to 12 days after inoculation.1,7,8 Unlike many other species that cause SFGR infection, R rickettsii does not cause an eschar at the site of inoculation.7,12
The classic triad of RMSF is fever, headache, and a rash. This triad is present in only 3% of early infections, but the prevalence increases to 60% to 70% by 2 weeks after the tick bite.1,7 Other common initial symptoms include generalized malaise, weakness, and myalgia.7,8,12 Gastrointestinal symptoms are common, and RMSF can be misdiagnosed as gastroenteritis, particularly in children.8
A rash usually occurs. It is due to systemic vasculitis and endothelial injury and often presents 2 to 5 days after the onset of fever, which can delay diagnosis.7,12,13 It usually progresses from macular to petechial and begins on the ankles, forearms, and wrists, spreading centripetally to the trunk and face and often including the palms and soles.7 Large areas of ecchymosis, ulceration, and (uncommonly) gangrene may occur as lesions coalesce.7,8 The 10% of patients who do not develop a rash (“spotless” fever) tend to have a poorer prognosis due to delayed diagnosis.8
Risk factors for severe disease include delay or lack of appropriate treatment, extremes of age, Native American descent, glucose-6-phosphate dehydrogenase deficiency, and immunocompromised states.1,10,11,13 Complications from the widespread Rickettsia-induced vasculitis may include a septic or toxic shock-like syndrome and neurovascular, cardiac, respiratory, and renal damage.7,11 Without appropriate therapy, death occurs 7 to 15 days after symptom onset.8
Laboratory evaluation may reveal thrombocytopenia and anemia.7 Leukocytosis or leukopenia may be present.8 Hyponatremia, elevated aminotransferase levels, elevated creatine kinase levels, prolonged coagulation times, and decreased fibrinogen may also be present.7,8
Diagnosis of Rocky Mountain spotted fever
No diagnostic studies are available for the acute phase of RMSF. Therefore, a high suspicion of RMSF is essential, and treatment should be started as soon as RMSF is suspected. Confirmatory testing can retrospectively validate a clinical diagnosis.4,7,11
Serologic testing with an immunofluorescence antibody assay remains the principal diagnostic test for RMSF, and paired testing (during the acute and convalescent phases) has a sensitivity of 94%.4 A 4-fold or greater increase in antibody titer (with a minimum titer of 1:64) between acute and convalescent samples is considered diagnostic of acute infection.4,7,8 Serology is often negative early in the disease course.4,7,8 The assay cross-reacts with other SFGR species, however.4,8
Amplification of R rickettsii DNA by polymerase chain reaction (PCR) from blood or biopsy sites can be done in some research settings, but its utility is limited because of low sensitivity early in the course of the infection.4,7
Immunohistochemical staining of a skin biopsy or autopsy specimen is a highly specific diagnostic test performed at a limited number of laboratories, though it has a sensitivity of only 60% to 92%.4,7,8
Cell culture can also be performed, but only in biosafety level 3 (scale of 1 to 4) laboratories.1
Treatment of Rocky Mountain spotted fever
Prompt initiation of antibiotic therapy greatly improves prognosis.1,13,14
Doxycycline for 7 days is the treatment of choice for RMSF, including in pregnant patients with life-threatening disease and in children.4,7,8,15,16
Tetracycline can also be used.
Chloramphenicol is an alternative treatment for pregnant patients with mild to moderate disease or those patients with a severe hypersensitivity reaction to doxycycline.1,4,7,9,15,16 In the United States, chloramphenicol is currently available only in an intravenous formulation.
Fever typically subsides within 24 to 48 hours of starting treatment.4,8 Failure to clinically improve within 48 hours suggests an alternative diagnosis.1,4 Long-term complications of severe infection may include hearing loss, blindness, and amputation of digits or extremities due to gangrene.1,8 Persistence of disease beyond acute infection has not been observed.1
OTHER SPOTTED FEVER GROUP RICKETTSIAl INFECTIONS
Both infections are characterized by an inoculation eschar. Symptoms include fever, headache, myalgia, and regional lymphadenopathy.1 Rash (most often maculopapular or vesicopustular) is characteristic of R parkeri, but it is not common in Rickettsia species 364D rickettsiosis.17,18 Mild thrombocytopenia, leukopenia, and elevated aminotransferase levels are common in R parkeri infection.1 Both infections appear to be milder than RMSF.
EHRLICHIOSES: EHRLICHIOSIS AND ANAPLASMOSIS
“Ehrlichiosis” is the generic name for infections caused by both the Ehrlichia and Anaplasma genera,19,20 which are small, gram-negative obligate intracellular bacterial pathogens.21 In the United States, infections are most commonly caused by A phagocytophilum, the causative organism of human granulocytic anaplasmosis (HGA) (Table 4), and E chaffeensis, the causative organism of human monocytic ehrlichiosis (HME) (Table 5). The incidence rates of these 2 infections have increased over the past decade, in part due to increased clinical awareness and improved diagnostic capabilities.3,22,23
E ewingii (Table 6) and E muris-like agent (Table 7) are lesser known causes of human ehrlichiosis in the United States.20,23–25 Initially, E ewingii was believed to primarily affect immunocompromised patients, but it was later recognized in immunocompetent hosts.23 E muris-like agent was first discovered as a cause of infection in 2009, and cases have been limited to Wisconsin and Minnesota.24,25
Human granulocytic anaplasmosis. A phagocytophilum is transmitted by Ixodes scapularis (the deer tick or blacklegged tick) in the northeastern and upper-midwestern regions of the United States, and I pacificus (the western blacklegged tick) along the northern Pacific coast.1,19,20,26 The 6 states accounting for most cases are New York, Connecticut, Massachusetts, Rhode Island, Minnesota, and Wisconsin.27 The white-footed mouse serves as the primary reservoir for A phagocytophilum, and humans are an accidental, “dead-end” host.21 Cases have also been reported to be transmitted via blood transfusion and transplacentally.20,26,28,29
Clinical manifestations of ehrlichiosis
After an incubation time of 5 to 21 days, ehrlichiosis typically presents as a febrile viral-like illness with nonspecific symptoms that include fever, chills, sweats, myalgia, headache, malaise, and cough.1,26,27,31
Gastrointestinal symptoms, arthralgia, photophobia, and nervous system involvement may also occur.1,20,29,32 Gastrointestinal symptoms tend to be more common in HME than HGA.20
Rash occurs in up to one-third of patients with HME, but it is rare in HGA.4,19,20,27 HME presents with more central nervous system involvement (such as meningitis or seizures) than HGA, in which central nervous system involvement is rare.
Severe complications of HME and HGA occur in a minority of cases and may include acute respiratory distress syndrome, renal failure, disseminated intravascular coagulopathy, and spontaneous hemorrhage.19 In general, HME is more severe than HGA and is more likely to progress to fulminant toxic or septic shocklike syndrome in rare instances.19
Laboratory tests may reveal leukopenia, lymphopenia, thrombocytopenia, and elevated liver-associated enzyme levels.1,19,20,26 Anemia and hyponatremia may also be present.4,30
Diagnosis of ehrlichiosis
The most rapid diagnostic method is examination of Wright- or Giemsa-stained peripheral blood smears for morulae, which are cytoplasmic intravacuolar inclusions of bacteria within leukocytes.20 However, its sensitivity is as low as 20% and declines even further after the first week of infection.4,20
PCR testing is the most sensitive and rapid tool available during acute infection.1,20,26,30,31 However, due to waning of the bacteremic phase, its sensitivity decreases after the first week of infection and after treatment is started.19,20
Serologic detection of antibodies with an indirect immunofluorescence assay is the most frequently used test for diagnosis of ehrlichiosis, and paired serology demonstrating seroconversion (at least a 4-fold increase in titer, with a minimal titer of 1:64) is most sensitive (82% to 100%).4,19,20,26 Cross-reactivity can occur, so testing for antibodies to both A phagocytophilum and E chaffeensis might assist in a more accurate diagnosis in areas where tick vectors overlap.4,19,20,26
HGA and HME can be isolated through cell culture in blood or cerebrospinal fluid. However, this is labor-intensive and performed in only a few specialized laboratories.4,19,20,27,31
Treatment of ehrlichiosis
If ehrlichiosis is suspected, treatment should not be delayed; the disease can be life-threatening and the ability to diagnose acute infection is often limited.20,26,32
Doxycycline is the treatment of choice, even in pregnant patients with severe infection and in children.1,19,26,27 Antibiotics are given for 5 to 10 days and continued for at least 3 days after the fever subsides.19,20,26,27,30 In HGA, a 10-day course of doxycycline is recommended to also provide the appropriate length of treatment for Borrelia burgdorferi.1,31
Rifampin is an alternative for those with severe tetracycline allergy, as well as those with mild to moderate infection during pregnancy.1,20,26,29–32
Fever typically resolves within 24 to 48 hours of starting treatment, and persistence of fever over 48 hours after starting antibiotics suggests an alternative diagnosis or possible coinfection.1,4,19,20,26,27,30,32
Persistence of chronic A phagocytophilum or E chaffeensis infection in humans beyond 2 months has not been demonstrated.20,26,30,33 Therefore, antibiotic treatment beyond the acute stage of infection is not indicated.30 Long-term prognosis is favorable, and patients are expected to make a full recovery.26,30
BABESIOSIS
Babesiosis occurs in the northeastern and upper midwestern states, with most cases reported in Massachusetts, Connecticut, Rhode Island, New York, New Jersey, Minnesota, and Wisconsin.31,32,34–36 Outbreaks have also been documented in Washington, California, and Missouri.31,32,35 The spread mimics that of Lyme disease, though it can be slower.34,36–39
Most cases in the Northeast and upper Midwest are caused by Babesia microti, while Babesia duncani has sporadically caused disease along the Pacific coast and Babesia divergens has been found in the Midwest and Northwest.34,36,39
Though babesiosis is usually a tickborne illness, it can also be transmitted through blood transfusion and, rarely, transplacental spread.31,32,34,36,39–41 The I scapularis tick is the host vector for Babesia microti, and transmission of disease requires 24 to 72 hours of attachment to a host.34,35 The primary reservoir for Babesia microti is the white-footed mouse, and humans are accidental hosts.32,34–36,39
Clinical manifestations of babesiosis
Babesia species cause illness by lysing erythrocytes, with resultant cytokine release.34
Symptoms typically appear 1 to 4 weeks after inoculation, after which most cases present as a viral-like illness with gradual onset of fever, chills, sweats, fatigue, malaise, headache, arthralgia, myalgia, nausea, anorexia, and nonproductive cough.32,34–36,39
Physical findings may include splenomegaly, hepatomegaly, jaundice, petechiae, and ecchymosis.32,34–36,39 Rash is seldom present and is not a characteristic feature of babesiosis.35,36
Laboratory features may include thrombocytopenia, hemolytic anemia, and elevated liver enzyme levels.32,34,36,39
Severe disease can occur in elderly, immunocompromised, or splenectomized individuals and can be life-threatening.34,39 Complications of severe infection can include acute respiratory distress syndrome, diffuse intravascular coagulation, and liver or renal failure.31,32,34–36,39 Splenic infarction or rupture may occur at lower levels of parasitemia in those without other manifestations of severe disease.31 The course can be prolonged and relapsing despite standard antibiotic therapy, typically in the setting of severe immunocompromise.32,34,42,43 Death occurs in up to 10% of severe cases.34
Diagnosis of babesiosis
Babesiosis should be considered if a patient presents with a febrile illness and nonspecific symptoms and comes from an endemic area or has received a blood transfusion within 6 months.34,35
The diagnosis of babesiosis is most commonly made by finding the intraerythrocytic ring form of the organism (trophozoite) on Giemsa- or Wright-stained thin blood smears.34,36,39 Babesia can be distinguished from Plasmodia (the agent of malaria) by the rare presence of tetrads of merozoites arranged in a cross-like pattern (the Maltese cross); the absence of hemozoin (brownish deposits) in the ring form; and the occasional presence of extracellular ring forms.34,36
The level of parasitemia (representing the number of parasites per microliter of blood) is generally between 1% and 10%, although it can be as high as 80%.36,39 Because parasitemia is often low early in disease (< 1%), multiple blood smears should be examined.34–36,39
Several real-time PCR assays are available to detect low-grade Babesia microti parasitemia in patients with negative blood smears during early infection.31 These assays have high diagnostic sensitivity and specificity and do not cross-react with other Babesia or Plasmodium species.34–36,39
Paired serology (immunoglobulin G) can confirm infection, although antibody may be absent early in the course of illness.31,34–36,39
Treatment of babesiosis
Current guidelines recommend antimicrobial therapy only for patients with symptoms and positive test results for Babesia.32 Treatment of asymptomatic patients should additionally be considered if parasitemia (not positive PCR or serology) persists for 3 months or longer.32,34–36,39
For mild to moderate babesiosis, the combination of oral atovaquone and azithromycin for 7 to 10 days has similar efficacy and a lower incidence of adverse effects than clindamycin plus quinine.31,32,34,44 For immunocompromised patients, higher doses of azithromycin can be used.31,32
For severe babesiosis or those with risk factors for severe disease, intravenous clindamycin and oral quinine are recommended for 7 to 10 days based on expert opinion.31,32,34–36,39,43 Adverse effects of this regimen include diarrhea, tinnitus, and hearing deficits.35,39 If necessary, intravenous quinidine can be used, but the patient should receive cardiac monitoring for possible prolongation of the QT interval.34,39 As quinine therapy is often interrupted due to the above side effects, alternative regimens such as intravenous azithromycin or clindamycin in combination with oral atovaquone should be considered for severe cases.31 However, these regimens are not well studied.31
Partial or complete exchange transfusion of whole blood or packed red blood cells should be considered in patients with a high level of parasitemia (≥ 10%), severe anemia (hemoglobin < 10 g/dL), or renal, hepatic, or pulmonary compromise.31,32,34–36,39 In critically ill patients, parasitemia should be monitored daily until it has decreased to less than 5%.32,34,39
Generally, symptoms improve within 48 hours of antimicrobial therapy initiation; however, parasitemia may take up to 3 months to resolve.32,34,39 In severely immunocompromised patients, babesiosis may persist or relapse despite appropriate therapy.34,39,42,43 In these cases, at least 6 weeks of antimicrobial therapy is recommended, including 2 weeks of therapy after Babesia organisms are no longer seen on blood smear.31,33,36,39,42
TICKBORNE RELAPSING FEVER
The illness is transmitted by either ticks or body lice. The tick-borne illness is caused by spirochetes of the genus Borrelia and transmitted to humans by the bite of an infected Ornithodoros soft tick.45 Approximately 70% of reported cases in the United States occur in California, Washington, and Colorado.46 Most cases are caused by Borrelia hermsii and are linked to sleeping in rodent-infested cabins in mountainous areas.46 Remarkably, tick-borne borreliae are transmitted within about 30 seconds of tick attachment.47,48
The hallmark of tickborne relapsing fever is febrile episodes lasting 3 to 5 days, with relapses after 5 to 7 days of apparent recovery.49 If untreated, several episodes of fever and nonspecific symptoms will occur before illness resolves spontaneously. Overall mortality rates are very low (< 5%).50
Laboratory confirmation of tickborne relapsing fever is made by detecting spirochetes in a blood smear during a febrile episode or serologic antibody confirmation. However, serologic testing is unhelpful in the acute setting and can yield false-positive results with prior exposure to other Borrelia species (eg, Lyme disease) or other spirochetes. Serologic antibody testing with a 4-fold increase between acute and convalescent samples or PCR can aid in diagnosis, though the latter is available only in research settings.47
The preferred treatment regimen for adults is an oral tetracycline for 10 days. Erythromycin is recommended when tetracyclines are contraindicated.51
When starting treatment, all patients should be monitored closely for the Jarisch-Herxheimer reaction (rigors, hypotension, and high fevers), which develops in over 50% of cases as a result of rapid spirochetal killing and massive cytokine release.52
BORRELIA MIYAMOTOI INFECTION
The most common clinical manifestations are similar to other tickborne relapsing fever infections, although a true “relapsing fever” itself is not usually present.53 The characteristic erythema migrans rash often found in Lyme disease is typically absent in B miyamotoi infection; however, when present, it should prompt investigation into coinfection.54 Cases of meningoencephalitis have been reported in immunosuppressed hosts.55
There is currently no validated test available for diagnosis of B miyamotoi; however, PCR and serology are available in a few specialized laboratories.31,53
The treatment of choice is doxycycline for 2 to 4 weeks. Amoxicillin and ceftriaxone also appear effective.53
SOUTHERN TICK-ASSOCIATED RASH ILLNESS
Infection can present similarly to Lyme disease with an erythema migrans-like rash and associated flulike symptoms, although systemic symptoms and multiple erythema migrans lesions are less likely with STARI. Also, the erythema migrans-like lesions tend to be smaller and more likely to have central clearing than those in Lyme disease.57 Nevertheless, it is difficult to distinguish the 2 illnesses, especially in mid-Atlantic states such as Maryland or Virginia, where both diseases coexist. The most reliable method of distinguishing STARI from Lyme disease is demonstrating that the patient was bitten by a Lone Star tick rather than an Ixodes tick. Numerous questions remain unanswered about the causative organism, pathophysiology, definitive diagnosis, geographic range of illness, and most effective treatment for STARI.
Most reported cases have responded promptly to doxycycline, though it is not known whether antibiotic treatment is necessary.58
TULAREMIA
Ticks are thought to be the most important vectors, and most cases occur in the south-central United States.59 The geographic distribution of disease is gradually shifting northward due to spread of the major tick vectors, A americanum, D variabilis, and D andersoni. Approximately 100 to 200 cases of tularemia are diagnosed each year in the United States, with most concentrated in Kansas, Oklahoma, Missouri, and Arkansas.60
Humans can acquire F tularensis by several routes, and the route of infection ultimately dictates the clinical syndrome. Ulceroglandular and glandular forms of the disease are the most common in the United States, and both frequently result from a tick bite. A few days after tick exposure, an erythematous, often painful papuloulcerative lesion with a central eschar manifests at the site of the tick bite. Additional symptoms may include fever, chills, headache, myalgia, malaise, and suppurative lymphadenitis.61
Diagnosis can be made by identifying F tularensis in blood, fluid, or tissue culture performed under biosafety level 3 conditions; however, serology is used in most cases.62
Streptomycin and gentamicin are considered drugs of choice and should be continued for at least 10 days. For relatively mild disease, oral doxycycline or ciprofloxacin can be considered for at least 14 days, although the latter is not approved for treatment.59,63
TICKBORNE VIRAL INFECTIONS
Powassan virus, an uncommon flavivirus, is found in the Great Lakes region and northeast United States. In the Great Lakes region, I cookei ticks transmit the traditional lineage of this virus. However, more recent cases have been identified in the Northeast and Midwest, where Powassan virus lineage II (or deer tick virus) is transmitted by I scapularis.31,64
The classic presentation is a viral encephalitis. Rash (most often maculopapular) and gastrointestinal symptoms have been reported as well. A high index of suspicion is needed for diagnosis because clinical features and laboratory findings resemble those of other arboviral infections.
Treatment for Powassan viral encephalitis is supportive, although corticosteroids have been used with some success.64 While asymptomatic infection has been documented, the reported mortality rate of Powassan virus encephalitis is 10% to 15%, and focal neurologic deficits can persist among survivors.65
Clinical and laboratory features appear to be very similar to those of the ehrlichioses.1 A clinical diagnosis should be considered in patients with A americanum exposure, fever, and cytopenias who lack PCR or serologic evidence for ehrlichiosis infection or who fail to respond to doxycycline therapy.24
COINFECTION
Some tick vectors transmit more than 1 type of infection, and therefore, coinfection with multiple pathogens may occur. For example, I scapularis transmits Borrelia burgdorferi (Lyme disease), HGA, Babesia microti, B miyamotoi, E muris-like agent, and Powassan virus lineage II, while A americanum transmits HME and Heartland virus.24,26,31,34,36,67 Coinfection may increase the severity of disease, often due to a delay in diagnosis, though more research is needed to understand the clinical manifestations of coinfection.31,35,67
PREVENTION
Unfortunately, there are no available human vaccines for tickborne illnesses in the United States, and the effectiveness of single-dose prophylaxis with doxycycline for non-Lyme infections has not been evaluated.4,7,26
Illness is best prevented by minimizing skin exposure to ticks, use of tick repellents containing DEET, use of long-legged and long-sleeved clothing impregnated with an acaricide such as permethrin, and conducting timely body checks for ticks after potential exposure.1,31,32 Light-colored clothing is suggested, since it allows for better visibility of crawling ticks.4,32 Bathing or showering within 2 hours of tick exposure helps prevent attachment of ticks.4,31,68 If camping outside, use of a bed net is recommended.68
Ticks are most easily removed by grasping the head of the tick as close to the skin surface as possible with fine-tipped tweezers.32,68 Removing or crushing ticks with bare hands should be avoided to prevent potential contamination, and hands should be washed thoroughly after tick removal.1,4
Blood donors are screened for a history of symptomatic tickborne disease; however, asymptomatic donors who are not identified at screening pose the greatest risk to the blood supply. Babesia microti is the most common reported transfusion-transmitted parasite in the United States, and transmission of R rickettsii, A phagocytophilum, and E ewingii have also been reported infrequently.28,40,69 Currently, no test is approved to screen blood for tickborne illnesses, though such a test would help prevent transmission of tickborne illnesses by blood transfusion in areas where these diseases are endemic.40,41
TAKE-HOME POINTS
Tickborne illnesses are increasing throughout the United States as a result of vector expansion and changes in human ecology.
It is essential that primary care clinicians consider tickborne illnesses in the differential diagnosis for any patient presenting with a fever and constitutional symptoms when the cause of symptoms is unclear and tick exposure is possible or known.
All the diseases discussed are nationally notifiable conditions, and confirmed cases should be reported.
Knowledge of the geographic locations of potential exposure is paramount to determining which tickborne infections to consider, and the absence of a tick bite history should not exclude the diagnosis in the correct clinical presentation.
In addition, it is important to recognize the limitations of diagnostic testing for many tickborne infections; empiric treatment is most often warranted before confirming the diagnosis.
Tick avoidance is the most effective way to prevent these often severe infections.
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep 2016; 65:1–44.
- Openshaw JJ, Swerdlow DL, Krebs JW, et al. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg 2010; 83:174–182.
- Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg 2011; 85:124–131.
- Chapman AS, Bakken JS, Folk SM, et al; Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep 2006; 55:1–27.
- Mukkada S, Buckingham SC. Recognition of and prompt treatment for tick-borne infections in children. Infect Dis Clin North Am 2015; 29:539–555.
- Schutze GE, Buckingham SC, Marshall GS, et al; Tick-borne Infections in Children Study (TICS) Group. Human monocytic ehrlichiosis in children. Pediatr Infect Dis J 2007; 26:475–479.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis 2007; 7:724–732.
- Lin L, Decker C. Rocky Mountain spotted fever. Dis Mon 2012; 58:361–369.
- Demma LJ, Traeger MS, Nicholson WL, et al. Rocky mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 2005; 353:587–594.
- Traeger MS, Regan JJ, Humpherys D, et al. Rocky mountain spotted fever characterization and comparison to similar illnesses in a highly endemic area—Arizona, 2002-2011. Clin Infect Dis 2015; 60:1650–1658.
- Dahlgren FS, Holman RC, Paddock CD, Callinan LS, McQuiston JH. Fatal Rocky Mountain spotted fever in the United States, 1999–2007. Am J Trop Med Hyg 2012; 86:713–719.
- Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 2013; 26:657–702.
- Regan JJ, Traeger MS, Humpherys D, et al. Risk factors for fatal outcome from rocky mountain spotted fever in a highly endemic area—Arizona, 2002-2011. Clin Infect Dis 2015; 60:1659–1666.
- Nelson R. Rocky Mountain spotted fever in Native Americans. Lancet Infect Dis 2015; 15:1013–1014.
- Botelho-Nevers E, Socolovschi C, Raoult D, Parola P. Treatment of Rickettsia spp. infections: a review. Expert Rev Anti Infect Ther 2012; 10:1425–1437.
- Dotters-Katz SK, Kuller J, Heine RP. Arthropod-borne bacterial diseases in pregnancy. Obstet Gynecol Surv 2013; 68:635–649.
- Paddock CD, Finley RW, Wright CS, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis 2008; 47:1188–1196.
- Shapiro MR, Fritz CL, Tait K, et al. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis 2010; 50:541–548.
- Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis 2007; 45(suppl 1):S45–S51.
- Thomas RJ, Dumler JS, Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther 2009; 7:709–722.
- Severo MS, Stephens KD, Kotsyfakis M, Pedra JH. Anaplasma phagocytophilum: deceptively simple or simply deceptive? Future Microbiol 2012; 7:719–731.
- Dahlgren FS, Heitman KN, Drexler NA, Massung RF, Behravesh CB. Human granulocytic anaplasmosis in the United States from 2008 to 2012: a summary of national surveillance data. Am J Trop Med Hyg 2015; 93:66–72.
- Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, Behravesh CB. Increasing Incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med 2016; 94:52–60.
- Wormser GP, Pritt B. Update and commentary on four emerging tick-borne infections: Ehrlichia muris-like agent, Borrelia miyamotoi, deer tick virus, heartland virus, and whether ticks play a role in transmission of Bartonella henselae. Infect Dis Clin North Am 2015; 29:371–381.
- Pritt BS, Sloan LM, Johnson DK, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med 2011; 365:422–429.
- Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am 2015; 29:341–355.
- St Clair K, Decker CF. Ehrlichioses: anaplasmosis and human ehrlichiosis. Dis Mon 2012; 58:346–354.
- Centers for Disease Control and Prevention (CDC). Anaplasma phagocytophilum transmitted through blood transfusion—Minnesota, 2007. MMWR Morb Mortal Wkly Rep 2008; 57:1145–1148.
- Dhand A, Nadelman RB, Aguero-Rosenfeld M, Haddad FA, Stokes DP, Horowitz HW. Human granulocytic anaplasmosis during pregnancy: case series and literature review. Clin Infect Dis 2007; 45:589–593.
- Stone JH, Dierberg K, Aram G, Dumler JS. Human monocytic ehrlichiosis. JAMA 2004; 292:2263–2270.
- Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis. JAMA 2016; 315:1767–1777.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–1134.
- Dumler JS, Choi KS, Garcia-Garcia JC, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis 2005; 11:1828–1834.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am 2015; 29:357–370.
- Kavanaugh MJ, Decker CF. Babesiosis. Dis Mon 2012; 58:355–360.
- Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am 2008; 22:469–488.
- Diuk-Wasser MA, Liu Y, Steeves TK, et al. Monitoring human babesiosis emergence through vector surveillance New England USA. Emerg Infect Dis 2014; 20:225–231.
- Dunn JM, Krause PJ, Davis S, et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS One 2014; 9:e115494.
- Vannier E, Krause PJ. Human babesiosis. N Engl J Med 2012; 366:2397–2407.
- Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011; 155:509–519.
- Wudhikarn K, Perry EH, Kemperman M, Jensen KA, Kline SE. Transfusion-transmitted babesiosis in an immunocompromised patient: a case report and review. Am J Med 2011; 124:800–805.
- Krause PJ, Gewurz BE, Hill D, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 2008; 46:370–376.
- Wormser GP, Prasad A, Neuhaus E, et al. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis 2010; 50:381–386.
- Krause PJ, Lepore T, Sikand VK, et al. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 2000; 343:1454–1458.
- Centers for Disease Control and Prevention (CDC). Tick-borne relapsing fever (TBRF): distribution. www.cdc.gov/relapsing-fever/distribution/index.html. Accessed June 7, 2017.
- Forrester JD, Kjemtrup AM, Fritz CL, et al; Centers for Disease Control and Prevention (CDC). Tickborne relapsing fever—United States, 1990-2011. MMWR Morb Mortal Wkly Rep 2015; 64:58–60.
- Dworkin MS, Schwan TG, Anderson DE Jr, Borchardt SM. Tick-borne relapsing fever. Infect Dis Clin North Am 2008; 22:449–468.
- Anderson JF. The natural history of ticks. Med Clin North Am 2002; 86:205–218.
- Barbour AG. Antigenic variation of a relapsing fever Borrelia species. Annu Rev Microbiol 1990; 44:155–171.
- Centers for Disease Control and Prevention (CDC). Acute respiratory distress syndrome in persons with tickborne relapsing fever—three states, 2004-2005. MMWR Morb Mortal Wkly Rep 2007; 56:1073–1076.
- Centers for Disease Control and Prevention (CDC). Tick-borne relapsing fever (TBRF): information for clinicians. www.cdc.gov/relapsing-fever/clinicians/index.html. Accessed June 7, 2017.
- Dworkin MS, Anderson DE Jr, Schwan TG, et al. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis 1998; 26:122–131.
- Wagemakers A, Staarink PJ, Sprong H, Hovius JW. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol 2015; 31:260–269.
- Krause PJ, Narasimhan S, Wormser GP, et al; Tick Borne Diseases Group. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis 2014; 20:1183–1190.
- Gugliotta JL, Goethert HK, Berardi VP, Telford SR 3rd. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med 2013; 368:240–245.
- Masters EJ, Grigery CN, Masters RW. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect Dis Clin North Am 2008; 22:361–376.
- Wormser GP, Masters E, Nowakowski J, et al. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin Infect Dis 2005; 41:958–965.
- Feder HM Jr, Hoss DM, Zemel L, Telford SR 3rd, Dias F, Wormser GP. Southern tick-associated rash illness (STARI) in the north: STARI following a tick bite in Long Island, New York. Clin Infect Dis 2011; 53:e142–e146.
- Carvalho CL, Lopes de Carvalho I, Ze-Ze L, Nuncio MS, Duarte EL. Tularaemia: a challenging zoonosis. Comp Immunol Microbiol Infect Dis 2014; 37):85–96.
- Centers for Disease Control and Prevention (CDC). Tularemia: statistics. www.cdc.gov/tularemia/statistics/index.html. Accessed June 7, 2017.
- Weber IB, Turabelidze G, Patrick S, Griffith KS, Kugeler KJ, Mead PS. Clinical recognition and management of tularemia in Missouri: a retrospective records review of 121 cases. Clin Infect Dis 2012; 55:1283–1290.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am 2008; 22:489–504.
- Johansson A, Berglund L, Sjostedt A, Tarnvik A. Ciprofloxacin for treatment of tularemia. Clin Infect Dis 2001; 33:267–268.
- Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 2016; 62:707–713.
- Ebel GD. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol 2010; 55:95–110.
- Pastula DM, Turabelidze G, Yates KF, et al; Centers for Disease Control and Prevention (CDC). Notes from the field: heartland virus disease—United States, 2012-–2013. MMWR Morb Mortal Wkly Rep 2014; 63:270–271.
- Knapp KL, Rice NA. Human coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res 2015; 2015:587131.
- Pujalte GG, Chua JV. Tick-borne infections in the United States. Prim Care 2013; 40:619–635.
- Regan J, Matthias J, Green-Murphy A, et al. A confirmed Ehrlichia ewingii infection likely acquired through platelet transfusion. Clin Infect Dis 2013; 56:e105–e107.
Ticks are responsible for most vector-borne infections in the United States. Most infections occur between April and October, when tick populations peak.1 However, infections can occur year-round.2,3
Tick bites are often unnoticed because the ticks are small when they are at the infective stage of their life cycle, and their attachment is characteristically painless and often in intertriginous body sites.1 Therefore, absence of a known tick bite never precludes the diagnosis of a tickborne infection.1,4,5
Although rural outdoor activities are recognized risk factors, tickborne infections also occur in urban areas.6 Thus, the lack of classic epidemiologic clues does not rule out a diagnosis of tickborne infection.
In most cases, tickborne illnesses present with nonspecific symptoms such as fever, malaise, headache, nausea, and myalgia. Accurate diagnosis of tickborne diseases can be challenging due to the similar clinical manifestations and overlapping geographic distributions of potential tick vectors.1
This review summarizes the epidemiology, clinical features, treatment, and prevention of the most prevalent non-Lyme tickborne diseases of the United States: Rocky Mountain spotted fever (RMSF), other spotted fever group rickettsial (SFGR) infections, ehrlichiosis, babesiosis, tickborne relapsing fever, Borrelia miyamotoi infection, southern tick-associated rash illness (STARI), tularemia, and tickborne viral infections.
ROCKY MOUNTAIN SPOTTED FEVER
Dermacentor variabilis, the American dog tick, is the major vector in the southern and eastern United States, and D andersoni, the Rocky Mountain wood tick, is the most common vector in the western United States.4,7,8 Rhipicephalus sanguineus, the brown dog tick, has also been found to transmit RMSF in Arizona.9,10
While most infections in humans are transmitted by tick bite, rare cases of RMSF are contracted through exposure to infective tick hemolymph during tick removal, parenteral inoculation or infectious aerosols in laboratory settings, and blood transfusion.7,8
RMSF is both the most common and the most likely cause of death among rickettsial infections in the United States.4,7,8 Most cases occur in children ages 5 to 9.10,11 The case-fatality rate is over 20% without antimicrobial therapy but less than 1% with timely and appropriate antibiotic treatment.7,8
Clinical manifestations of Rocky Mountain spotted fever
RMSF is transmitted after only 2 to 20 hours of tick attachment, and symptoms begin 3 to 12 days after inoculation.1,7,8 Unlike many other species that cause SFGR infection, R rickettsii does not cause an eschar at the site of inoculation.7,12
The classic triad of RMSF is fever, headache, and a rash. This triad is present in only 3% of early infections, but the prevalence increases to 60% to 70% by 2 weeks after the tick bite.1,7 Other common initial symptoms include generalized malaise, weakness, and myalgia.7,8,12 Gastrointestinal symptoms are common, and RMSF can be misdiagnosed as gastroenteritis, particularly in children.8
A rash usually occurs. It is due to systemic vasculitis and endothelial injury and often presents 2 to 5 days after the onset of fever, which can delay diagnosis.7,12,13 It usually progresses from macular to petechial and begins on the ankles, forearms, and wrists, spreading centripetally to the trunk and face and often including the palms and soles.7 Large areas of ecchymosis, ulceration, and (uncommonly) gangrene may occur as lesions coalesce.7,8 The 10% of patients who do not develop a rash (“spotless” fever) tend to have a poorer prognosis due to delayed diagnosis.8
Risk factors for severe disease include delay or lack of appropriate treatment, extremes of age, Native American descent, glucose-6-phosphate dehydrogenase deficiency, and immunocompromised states.1,10,11,13 Complications from the widespread Rickettsia-induced vasculitis may include a septic or toxic shock-like syndrome and neurovascular, cardiac, respiratory, and renal damage.7,11 Without appropriate therapy, death occurs 7 to 15 days after symptom onset.8
Laboratory evaluation may reveal thrombocytopenia and anemia.7 Leukocytosis or leukopenia may be present.8 Hyponatremia, elevated aminotransferase levels, elevated creatine kinase levels, prolonged coagulation times, and decreased fibrinogen may also be present.7,8
Diagnosis of Rocky Mountain spotted fever
No diagnostic studies are available for the acute phase of RMSF. Therefore, a high suspicion of RMSF is essential, and treatment should be started as soon as RMSF is suspected. Confirmatory testing can retrospectively validate a clinical diagnosis.4,7,11
Serologic testing with an immunofluorescence antibody assay remains the principal diagnostic test for RMSF, and paired testing (during the acute and convalescent phases) has a sensitivity of 94%.4 A 4-fold or greater increase in antibody titer (with a minimum titer of 1:64) between acute and convalescent samples is considered diagnostic of acute infection.4,7,8 Serology is often negative early in the disease course.4,7,8 The assay cross-reacts with other SFGR species, however.4,8
Amplification of R rickettsii DNA by polymerase chain reaction (PCR) from blood or biopsy sites can be done in some research settings, but its utility is limited because of low sensitivity early in the course of the infection.4,7
Immunohistochemical staining of a skin biopsy or autopsy specimen is a highly specific diagnostic test performed at a limited number of laboratories, though it has a sensitivity of only 60% to 92%.4,7,8
Cell culture can also be performed, but only in biosafety level 3 (scale of 1 to 4) laboratories.1
Treatment of Rocky Mountain spotted fever
Prompt initiation of antibiotic therapy greatly improves prognosis.1,13,14
Doxycycline for 7 days is the treatment of choice for RMSF, including in pregnant patients with life-threatening disease and in children.4,7,8,15,16
Tetracycline can also be used.
Chloramphenicol is an alternative treatment for pregnant patients with mild to moderate disease or those patients with a severe hypersensitivity reaction to doxycycline.1,4,7,9,15,16 In the United States, chloramphenicol is currently available only in an intravenous formulation.
Fever typically subsides within 24 to 48 hours of starting treatment.4,8 Failure to clinically improve within 48 hours suggests an alternative diagnosis.1,4 Long-term complications of severe infection may include hearing loss, blindness, and amputation of digits or extremities due to gangrene.1,8 Persistence of disease beyond acute infection has not been observed.1
OTHER SPOTTED FEVER GROUP RICKETTSIAl INFECTIONS
Both infections are characterized by an inoculation eschar. Symptoms include fever, headache, myalgia, and regional lymphadenopathy.1 Rash (most often maculopapular or vesicopustular) is characteristic of R parkeri, but it is not common in Rickettsia species 364D rickettsiosis.17,18 Mild thrombocytopenia, leukopenia, and elevated aminotransferase levels are common in R parkeri infection.1 Both infections appear to be milder than RMSF.
EHRLICHIOSES: EHRLICHIOSIS AND ANAPLASMOSIS
“Ehrlichiosis” is the generic name for infections caused by both the Ehrlichia and Anaplasma genera,19,20 which are small, gram-negative obligate intracellular bacterial pathogens.21 In the United States, infections are most commonly caused by A phagocytophilum, the causative organism of human granulocytic anaplasmosis (HGA) (Table 4), and E chaffeensis, the causative organism of human monocytic ehrlichiosis (HME) (Table 5). The incidence rates of these 2 infections have increased over the past decade, in part due to increased clinical awareness and improved diagnostic capabilities.3,22,23
E ewingii (Table 6) and E muris-like agent (Table 7) are lesser known causes of human ehrlichiosis in the United States.20,23–25 Initially, E ewingii was believed to primarily affect immunocompromised patients, but it was later recognized in immunocompetent hosts.23 E muris-like agent was first discovered as a cause of infection in 2009, and cases have been limited to Wisconsin and Minnesota.24,25
Human granulocytic anaplasmosis. A phagocytophilum is transmitted by Ixodes scapularis (the deer tick or blacklegged tick) in the northeastern and upper-midwestern regions of the United States, and I pacificus (the western blacklegged tick) along the northern Pacific coast.1,19,20,26 The 6 states accounting for most cases are New York, Connecticut, Massachusetts, Rhode Island, Minnesota, and Wisconsin.27 The white-footed mouse serves as the primary reservoir for A phagocytophilum, and humans are an accidental, “dead-end” host.21 Cases have also been reported to be transmitted via blood transfusion and transplacentally.20,26,28,29
Clinical manifestations of ehrlichiosis
After an incubation time of 5 to 21 days, ehrlichiosis typically presents as a febrile viral-like illness with nonspecific symptoms that include fever, chills, sweats, myalgia, headache, malaise, and cough.1,26,27,31
Gastrointestinal symptoms, arthralgia, photophobia, and nervous system involvement may also occur.1,20,29,32 Gastrointestinal symptoms tend to be more common in HME than HGA.20
Rash occurs in up to one-third of patients with HME, but it is rare in HGA.4,19,20,27 HME presents with more central nervous system involvement (such as meningitis or seizures) than HGA, in which central nervous system involvement is rare.
Severe complications of HME and HGA occur in a minority of cases and may include acute respiratory distress syndrome, renal failure, disseminated intravascular coagulopathy, and spontaneous hemorrhage.19 In general, HME is more severe than HGA and is more likely to progress to fulminant toxic or septic shocklike syndrome in rare instances.19
Laboratory tests may reveal leukopenia, lymphopenia, thrombocytopenia, and elevated liver-associated enzyme levels.1,19,20,26 Anemia and hyponatremia may also be present.4,30
Diagnosis of ehrlichiosis
The most rapid diagnostic method is examination of Wright- or Giemsa-stained peripheral blood smears for morulae, which are cytoplasmic intravacuolar inclusions of bacteria within leukocytes.20 However, its sensitivity is as low as 20% and declines even further after the first week of infection.4,20
PCR testing is the most sensitive and rapid tool available during acute infection.1,20,26,30,31 However, due to waning of the bacteremic phase, its sensitivity decreases after the first week of infection and after treatment is started.19,20
Serologic detection of antibodies with an indirect immunofluorescence assay is the most frequently used test for diagnosis of ehrlichiosis, and paired serology demonstrating seroconversion (at least a 4-fold increase in titer, with a minimal titer of 1:64) is most sensitive (82% to 100%).4,19,20,26 Cross-reactivity can occur, so testing for antibodies to both A phagocytophilum and E chaffeensis might assist in a more accurate diagnosis in areas where tick vectors overlap.4,19,20,26
HGA and HME can be isolated through cell culture in blood or cerebrospinal fluid. However, this is labor-intensive and performed in only a few specialized laboratories.4,19,20,27,31
Treatment of ehrlichiosis
If ehrlichiosis is suspected, treatment should not be delayed; the disease can be life-threatening and the ability to diagnose acute infection is often limited.20,26,32
Doxycycline is the treatment of choice, even in pregnant patients with severe infection and in children.1,19,26,27 Antibiotics are given for 5 to 10 days and continued for at least 3 days after the fever subsides.19,20,26,27,30 In HGA, a 10-day course of doxycycline is recommended to also provide the appropriate length of treatment for Borrelia burgdorferi.1,31
Rifampin is an alternative for those with severe tetracycline allergy, as well as those with mild to moderate infection during pregnancy.1,20,26,29–32
Fever typically resolves within 24 to 48 hours of starting treatment, and persistence of fever over 48 hours after starting antibiotics suggests an alternative diagnosis or possible coinfection.1,4,19,20,26,27,30,32
Persistence of chronic A phagocytophilum or E chaffeensis infection in humans beyond 2 months has not been demonstrated.20,26,30,33 Therefore, antibiotic treatment beyond the acute stage of infection is not indicated.30 Long-term prognosis is favorable, and patients are expected to make a full recovery.26,30
BABESIOSIS
Babesiosis occurs in the northeastern and upper midwestern states, with most cases reported in Massachusetts, Connecticut, Rhode Island, New York, New Jersey, Minnesota, and Wisconsin.31,32,34–36 Outbreaks have also been documented in Washington, California, and Missouri.31,32,35 The spread mimics that of Lyme disease, though it can be slower.34,36–39
Most cases in the Northeast and upper Midwest are caused by Babesia microti, while Babesia duncani has sporadically caused disease along the Pacific coast and Babesia divergens has been found in the Midwest and Northwest.34,36,39
Though babesiosis is usually a tickborne illness, it can also be transmitted through blood transfusion and, rarely, transplacental spread.31,32,34,36,39–41 The I scapularis tick is the host vector for Babesia microti, and transmission of disease requires 24 to 72 hours of attachment to a host.34,35 The primary reservoir for Babesia microti is the white-footed mouse, and humans are accidental hosts.32,34–36,39
Clinical manifestations of babesiosis
Babesia species cause illness by lysing erythrocytes, with resultant cytokine release.34
Symptoms typically appear 1 to 4 weeks after inoculation, after which most cases present as a viral-like illness with gradual onset of fever, chills, sweats, fatigue, malaise, headache, arthralgia, myalgia, nausea, anorexia, and nonproductive cough.32,34–36,39
Physical findings may include splenomegaly, hepatomegaly, jaundice, petechiae, and ecchymosis.32,34–36,39 Rash is seldom present and is not a characteristic feature of babesiosis.35,36
Laboratory features may include thrombocytopenia, hemolytic anemia, and elevated liver enzyme levels.32,34,36,39
Severe disease can occur in elderly, immunocompromised, or splenectomized individuals and can be life-threatening.34,39 Complications of severe infection can include acute respiratory distress syndrome, diffuse intravascular coagulation, and liver or renal failure.31,32,34–36,39 Splenic infarction or rupture may occur at lower levels of parasitemia in those without other manifestations of severe disease.31 The course can be prolonged and relapsing despite standard antibiotic therapy, typically in the setting of severe immunocompromise.32,34,42,43 Death occurs in up to 10% of severe cases.34
Diagnosis of babesiosis
Babesiosis should be considered if a patient presents with a febrile illness and nonspecific symptoms and comes from an endemic area or has received a blood transfusion within 6 months.34,35
The diagnosis of babesiosis is most commonly made by finding the intraerythrocytic ring form of the organism (trophozoite) on Giemsa- or Wright-stained thin blood smears.34,36,39 Babesia can be distinguished from Plasmodia (the agent of malaria) by the rare presence of tetrads of merozoites arranged in a cross-like pattern (the Maltese cross); the absence of hemozoin (brownish deposits) in the ring form; and the occasional presence of extracellular ring forms.34,36
The level of parasitemia (representing the number of parasites per microliter of blood) is generally between 1% and 10%, although it can be as high as 80%.36,39 Because parasitemia is often low early in disease (< 1%), multiple blood smears should be examined.34–36,39
Several real-time PCR assays are available to detect low-grade Babesia microti parasitemia in patients with negative blood smears during early infection.31 These assays have high diagnostic sensitivity and specificity and do not cross-react with other Babesia or Plasmodium species.34–36,39
Paired serology (immunoglobulin G) can confirm infection, although antibody may be absent early in the course of illness.31,34–36,39
Treatment of babesiosis
Current guidelines recommend antimicrobial therapy only for patients with symptoms and positive test results for Babesia.32 Treatment of asymptomatic patients should additionally be considered if parasitemia (not positive PCR or serology) persists for 3 months or longer.32,34–36,39
For mild to moderate babesiosis, the combination of oral atovaquone and azithromycin for 7 to 10 days has similar efficacy and a lower incidence of adverse effects than clindamycin plus quinine.31,32,34,44 For immunocompromised patients, higher doses of azithromycin can be used.31,32
For severe babesiosis or those with risk factors for severe disease, intravenous clindamycin and oral quinine are recommended for 7 to 10 days based on expert opinion.31,32,34–36,39,43 Adverse effects of this regimen include diarrhea, tinnitus, and hearing deficits.35,39 If necessary, intravenous quinidine can be used, but the patient should receive cardiac monitoring for possible prolongation of the QT interval.34,39 As quinine therapy is often interrupted due to the above side effects, alternative regimens such as intravenous azithromycin or clindamycin in combination with oral atovaquone should be considered for severe cases.31 However, these regimens are not well studied.31
Partial or complete exchange transfusion of whole blood or packed red blood cells should be considered in patients with a high level of parasitemia (≥ 10%), severe anemia (hemoglobin < 10 g/dL), or renal, hepatic, or pulmonary compromise.31,32,34–36,39 In critically ill patients, parasitemia should be monitored daily until it has decreased to less than 5%.32,34,39
Generally, symptoms improve within 48 hours of antimicrobial therapy initiation; however, parasitemia may take up to 3 months to resolve.32,34,39 In severely immunocompromised patients, babesiosis may persist or relapse despite appropriate therapy.34,39,42,43 In these cases, at least 6 weeks of antimicrobial therapy is recommended, including 2 weeks of therapy after Babesia organisms are no longer seen on blood smear.31,33,36,39,42
TICKBORNE RELAPSING FEVER
The illness is transmitted by either ticks or body lice. The tick-borne illness is caused by spirochetes of the genus Borrelia and transmitted to humans by the bite of an infected Ornithodoros soft tick.45 Approximately 70% of reported cases in the United States occur in California, Washington, and Colorado.46 Most cases are caused by Borrelia hermsii and are linked to sleeping in rodent-infested cabins in mountainous areas.46 Remarkably, tick-borne borreliae are transmitted within about 30 seconds of tick attachment.47,48
The hallmark of tickborne relapsing fever is febrile episodes lasting 3 to 5 days, with relapses after 5 to 7 days of apparent recovery.49 If untreated, several episodes of fever and nonspecific symptoms will occur before illness resolves spontaneously. Overall mortality rates are very low (< 5%).50
Laboratory confirmation of tickborne relapsing fever is made by detecting spirochetes in a blood smear during a febrile episode or serologic antibody confirmation. However, serologic testing is unhelpful in the acute setting and can yield false-positive results with prior exposure to other Borrelia species (eg, Lyme disease) or other spirochetes. Serologic antibody testing with a 4-fold increase between acute and convalescent samples or PCR can aid in diagnosis, though the latter is available only in research settings.47
The preferred treatment regimen for adults is an oral tetracycline for 10 days. Erythromycin is recommended when tetracyclines are contraindicated.51
When starting treatment, all patients should be monitored closely for the Jarisch-Herxheimer reaction (rigors, hypotension, and high fevers), which develops in over 50% of cases as a result of rapid spirochetal killing and massive cytokine release.52
BORRELIA MIYAMOTOI INFECTION
The most common clinical manifestations are similar to other tickborne relapsing fever infections, although a true “relapsing fever” itself is not usually present.53 The characteristic erythema migrans rash often found in Lyme disease is typically absent in B miyamotoi infection; however, when present, it should prompt investigation into coinfection.54 Cases of meningoencephalitis have been reported in immunosuppressed hosts.55
There is currently no validated test available for diagnosis of B miyamotoi; however, PCR and serology are available in a few specialized laboratories.31,53
The treatment of choice is doxycycline for 2 to 4 weeks. Amoxicillin and ceftriaxone also appear effective.53
SOUTHERN TICK-ASSOCIATED RASH ILLNESS
Infection can present similarly to Lyme disease with an erythema migrans-like rash and associated flulike symptoms, although systemic symptoms and multiple erythema migrans lesions are less likely with STARI. Also, the erythema migrans-like lesions tend to be smaller and more likely to have central clearing than those in Lyme disease.57 Nevertheless, it is difficult to distinguish the 2 illnesses, especially in mid-Atlantic states such as Maryland or Virginia, where both diseases coexist. The most reliable method of distinguishing STARI from Lyme disease is demonstrating that the patient was bitten by a Lone Star tick rather than an Ixodes tick. Numerous questions remain unanswered about the causative organism, pathophysiology, definitive diagnosis, geographic range of illness, and most effective treatment for STARI.
Most reported cases have responded promptly to doxycycline, though it is not known whether antibiotic treatment is necessary.58
TULAREMIA
Ticks are thought to be the most important vectors, and most cases occur in the south-central United States.59 The geographic distribution of disease is gradually shifting northward due to spread of the major tick vectors, A americanum, D variabilis, and D andersoni. Approximately 100 to 200 cases of tularemia are diagnosed each year in the United States, with most concentrated in Kansas, Oklahoma, Missouri, and Arkansas.60
Humans can acquire F tularensis by several routes, and the route of infection ultimately dictates the clinical syndrome. Ulceroglandular and glandular forms of the disease are the most common in the United States, and both frequently result from a tick bite. A few days after tick exposure, an erythematous, often painful papuloulcerative lesion with a central eschar manifests at the site of the tick bite. Additional symptoms may include fever, chills, headache, myalgia, malaise, and suppurative lymphadenitis.61
Diagnosis can be made by identifying F tularensis in blood, fluid, or tissue culture performed under biosafety level 3 conditions; however, serology is used in most cases.62
Streptomycin and gentamicin are considered drugs of choice and should be continued for at least 10 days. For relatively mild disease, oral doxycycline or ciprofloxacin can be considered for at least 14 days, although the latter is not approved for treatment.59,63
TICKBORNE VIRAL INFECTIONS
Powassan virus, an uncommon flavivirus, is found in the Great Lakes region and northeast United States. In the Great Lakes region, I cookei ticks transmit the traditional lineage of this virus. However, more recent cases have been identified in the Northeast and Midwest, where Powassan virus lineage II (or deer tick virus) is transmitted by I scapularis.31,64
The classic presentation is a viral encephalitis. Rash (most often maculopapular) and gastrointestinal symptoms have been reported as well. A high index of suspicion is needed for diagnosis because clinical features and laboratory findings resemble those of other arboviral infections.
Treatment for Powassan viral encephalitis is supportive, although corticosteroids have been used with some success.64 While asymptomatic infection has been documented, the reported mortality rate of Powassan virus encephalitis is 10% to 15%, and focal neurologic deficits can persist among survivors.65
Clinical and laboratory features appear to be very similar to those of the ehrlichioses.1 A clinical diagnosis should be considered in patients with A americanum exposure, fever, and cytopenias who lack PCR or serologic evidence for ehrlichiosis infection or who fail to respond to doxycycline therapy.24
COINFECTION
Some tick vectors transmit more than 1 type of infection, and therefore, coinfection with multiple pathogens may occur. For example, I scapularis transmits Borrelia burgdorferi (Lyme disease), HGA, Babesia microti, B miyamotoi, E muris-like agent, and Powassan virus lineage II, while A americanum transmits HME and Heartland virus.24,26,31,34,36,67 Coinfection may increase the severity of disease, often due to a delay in diagnosis, though more research is needed to understand the clinical manifestations of coinfection.31,35,67
PREVENTION
Unfortunately, there are no available human vaccines for tickborne illnesses in the United States, and the effectiveness of single-dose prophylaxis with doxycycline for non-Lyme infections has not been evaluated.4,7,26
Illness is best prevented by minimizing skin exposure to ticks, use of tick repellents containing DEET, use of long-legged and long-sleeved clothing impregnated with an acaricide such as permethrin, and conducting timely body checks for ticks after potential exposure.1,31,32 Light-colored clothing is suggested, since it allows for better visibility of crawling ticks.4,32 Bathing or showering within 2 hours of tick exposure helps prevent attachment of ticks.4,31,68 If camping outside, use of a bed net is recommended.68
Ticks are most easily removed by grasping the head of the tick as close to the skin surface as possible with fine-tipped tweezers.32,68 Removing or crushing ticks with bare hands should be avoided to prevent potential contamination, and hands should be washed thoroughly after tick removal.1,4
Blood donors are screened for a history of symptomatic tickborne disease; however, asymptomatic donors who are not identified at screening pose the greatest risk to the blood supply. Babesia microti is the most common reported transfusion-transmitted parasite in the United States, and transmission of R rickettsii, A phagocytophilum, and E ewingii have also been reported infrequently.28,40,69 Currently, no test is approved to screen blood for tickborne illnesses, though such a test would help prevent transmission of tickborne illnesses by blood transfusion in areas where these diseases are endemic.40,41
TAKE-HOME POINTS
Tickborne illnesses are increasing throughout the United States as a result of vector expansion and changes in human ecology.
It is essential that primary care clinicians consider tickborne illnesses in the differential diagnosis for any patient presenting with a fever and constitutional symptoms when the cause of symptoms is unclear and tick exposure is possible or known.
All the diseases discussed are nationally notifiable conditions, and confirmed cases should be reported.
Knowledge of the geographic locations of potential exposure is paramount to determining which tickborne infections to consider, and the absence of a tick bite history should not exclude the diagnosis in the correct clinical presentation.
In addition, it is important to recognize the limitations of diagnostic testing for many tickborne infections; empiric treatment is most often warranted before confirming the diagnosis.
Tick avoidance is the most effective way to prevent these often severe infections.
Ticks are responsible for most vector-borne infections in the United States. Most infections occur between April and October, when tick populations peak.1 However, infections can occur year-round.2,3
Tick bites are often unnoticed because the ticks are small when they are at the infective stage of their life cycle, and their attachment is characteristically painless and often in intertriginous body sites.1 Therefore, absence of a known tick bite never precludes the diagnosis of a tickborne infection.1,4,5
Although rural outdoor activities are recognized risk factors, tickborne infections also occur in urban areas.6 Thus, the lack of classic epidemiologic clues does not rule out a diagnosis of tickborne infection.
In most cases, tickborne illnesses present with nonspecific symptoms such as fever, malaise, headache, nausea, and myalgia. Accurate diagnosis of tickborne diseases can be challenging due to the similar clinical manifestations and overlapping geographic distributions of potential tick vectors.1
This review summarizes the epidemiology, clinical features, treatment, and prevention of the most prevalent non-Lyme tickborne diseases of the United States: Rocky Mountain spotted fever (RMSF), other spotted fever group rickettsial (SFGR) infections, ehrlichiosis, babesiosis, tickborne relapsing fever, Borrelia miyamotoi infection, southern tick-associated rash illness (STARI), tularemia, and tickborne viral infections.
ROCKY MOUNTAIN SPOTTED FEVER
Dermacentor variabilis, the American dog tick, is the major vector in the southern and eastern United States, and D andersoni, the Rocky Mountain wood tick, is the most common vector in the western United States.4,7,8 Rhipicephalus sanguineus, the brown dog tick, has also been found to transmit RMSF in Arizona.9,10
While most infections in humans are transmitted by tick bite, rare cases of RMSF are contracted through exposure to infective tick hemolymph during tick removal, parenteral inoculation or infectious aerosols in laboratory settings, and blood transfusion.7,8
RMSF is both the most common and the most likely cause of death among rickettsial infections in the United States.4,7,8 Most cases occur in children ages 5 to 9.10,11 The case-fatality rate is over 20% without antimicrobial therapy but less than 1% with timely and appropriate antibiotic treatment.7,8
Clinical manifestations of Rocky Mountain spotted fever
RMSF is transmitted after only 2 to 20 hours of tick attachment, and symptoms begin 3 to 12 days after inoculation.1,7,8 Unlike many other species that cause SFGR infection, R rickettsii does not cause an eschar at the site of inoculation.7,12
The classic triad of RMSF is fever, headache, and a rash. This triad is present in only 3% of early infections, but the prevalence increases to 60% to 70% by 2 weeks after the tick bite.1,7 Other common initial symptoms include generalized malaise, weakness, and myalgia.7,8,12 Gastrointestinal symptoms are common, and RMSF can be misdiagnosed as gastroenteritis, particularly in children.8
A rash usually occurs. It is due to systemic vasculitis and endothelial injury and often presents 2 to 5 days after the onset of fever, which can delay diagnosis.7,12,13 It usually progresses from macular to petechial and begins on the ankles, forearms, and wrists, spreading centripetally to the trunk and face and often including the palms and soles.7 Large areas of ecchymosis, ulceration, and (uncommonly) gangrene may occur as lesions coalesce.7,8 The 10% of patients who do not develop a rash (“spotless” fever) tend to have a poorer prognosis due to delayed diagnosis.8
Risk factors for severe disease include delay or lack of appropriate treatment, extremes of age, Native American descent, glucose-6-phosphate dehydrogenase deficiency, and immunocompromised states.1,10,11,13 Complications from the widespread Rickettsia-induced vasculitis may include a septic or toxic shock-like syndrome and neurovascular, cardiac, respiratory, and renal damage.7,11 Without appropriate therapy, death occurs 7 to 15 days after symptom onset.8
Laboratory evaluation may reveal thrombocytopenia and anemia.7 Leukocytosis or leukopenia may be present.8 Hyponatremia, elevated aminotransferase levels, elevated creatine kinase levels, prolonged coagulation times, and decreased fibrinogen may also be present.7,8
Diagnosis of Rocky Mountain spotted fever
No diagnostic studies are available for the acute phase of RMSF. Therefore, a high suspicion of RMSF is essential, and treatment should be started as soon as RMSF is suspected. Confirmatory testing can retrospectively validate a clinical diagnosis.4,7,11
Serologic testing with an immunofluorescence antibody assay remains the principal diagnostic test for RMSF, and paired testing (during the acute and convalescent phases) has a sensitivity of 94%.4 A 4-fold or greater increase in antibody titer (with a minimum titer of 1:64) between acute and convalescent samples is considered diagnostic of acute infection.4,7,8 Serology is often negative early in the disease course.4,7,8 The assay cross-reacts with other SFGR species, however.4,8
Amplification of R rickettsii DNA by polymerase chain reaction (PCR) from blood or biopsy sites can be done in some research settings, but its utility is limited because of low sensitivity early in the course of the infection.4,7
Immunohistochemical staining of a skin biopsy or autopsy specimen is a highly specific diagnostic test performed at a limited number of laboratories, though it has a sensitivity of only 60% to 92%.4,7,8
Cell culture can also be performed, but only in biosafety level 3 (scale of 1 to 4) laboratories.1
Treatment of Rocky Mountain spotted fever
Prompt initiation of antibiotic therapy greatly improves prognosis.1,13,14
Doxycycline for 7 days is the treatment of choice for RMSF, including in pregnant patients with life-threatening disease and in children.4,7,8,15,16
Tetracycline can also be used.
Chloramphenicol is an alternative treatment for pregnant patients with mild to moderate disease or those patients with a severe hypersensitivity reaction to doxycycline.1,4,7,9,15,16 In the United States, chloramphenicol is currently available only in an intravenous formulation.
Fever typically subsides within 24 to 48 hours of starting treatment.4,8 Failure to clinically improve within 48 hours suggests an alternative diagnosis.1,4 Long-term complications of severe infection may include hearing loss, blindness, and amputation of digits or extremities due to gangrene.1,8 Persistence of disease beyond acute infection has not been observed.1
OTHER SPOTTED FEVER GROUP RICKETTSIAl INFECTIONS
Both infections are characterized by an inoculation eschar. Symptoms include fever, headache, myalgia, and regional lymphadenopathy.1 Rash (most often maculopapular or vesicopustular) is characteristic of R parkeri, but it is not common in Rickettsia species 364D rickettsiosis.17,18 Mild thrombocytopenia, leukopenia, and elevated aminotransferase levels are common in R parkeri infection.1 Both infections appear to be milder than RMSF.
EHRLICHIOSES: EHRLICHIOSIS AND ANAPLASMOSIS
“Ehrlichiosis” is the generic name for infections caused by both the Ehrlichia and Anaplasma genera,19,20 which are small, gram-negative obligate intracellular bacterial pathogens.21 In the United States, infections are most commonly caused by A phagocytophilum, the causative organism of human granulocytic anaplasmosis (HGA) (Table 4), and E chaffeensis, the causative organism of human monocytic ehrlichiosis (HME) (Table 5). The incidence rates of these 2 infections have increased over the past decade, in part due to increased clinical awareness and improved diagnostic capabilities.3,22,23
E ewingii (Table 6) and E muris-like agent (Table 7) are lesser known causes of human ehrlichiosis in the United States.20,23–25 Initially, E ewingii was believed to primarily affect immunocompromised patients, but it was later recognized in immunocompetent hosts.23 E muris-like agent was first discovered as a cause of infection in 2009, and cases have been limited to Wisconsin and Minnesota.24,25
Human granulocytic anaplasmosis. A phagocytophilum is transmitted by Ixodes scapularis (the deer tick or blacklegged tick) in the northeastern and upper-midwestern regions of the United States, and I pacificus (the western blacklegged tick) along the northern Pacific coast.1,19,20,26 The 6 states accounting for most cases are New York, Connecticut, Massachusetts, Rhode Island, Minnesota, and Wisconsin.27 The white-footed mouse serves as the primary reservoir for A phagocytophilum, and humans are an accidental, “dead-end” host.21 Cases have also been reported to be transmitted via blood transfusion and transplacentally.20,26,28,29
Clinical manifestations of ehrlichiosis
After an incubation time of 5 to 21 days, ehrlichiosis typically presents as a febrile viral-like illness with nonspecific symptoms that include fever, chills, sweats, myalgia, headache, malaise, and cough.1,26,27,31
Gastrointestinal symptoms, arthralgia, photophobia, and nervous system involvement may also occur.1,20,29,32 Gastrointestinal symptoms tend to be more common in HME than HGA.20
Rash occurs in up to one-third of patients with HME, but it is rare in HGA.4,19,20,27 HME presents with more central nervous system involvement (such as meningitis or seizures) than HGA, in which central nervous system involvement is rare.
Severe complications of HME and HGA occur in a minority of cases and may include acute respiratory distress syndrome, renal failure, disseminated intravascular coagulopathy, and spontaneous hemorrhage.19 In general, HME is more severe than HGA and is more likely to progress to fulminant toxic or septic shocklike syndrome in rare instances.19
Laboratory tests may reveal leukopenia, lymphopenia, thrombocytopenia, and elevated liver-associated enzyme levels.1,19,20,26 Anemia and hyponatremia may also be present.4,30
Diagnosis of ehrlichiosis
The most rapid diagnostic method is examination of Wright- or Giemsa-stained peripheral blood smears for morulae, which are cytoplasmic intravacuolar inclusions of bacteria within leukocytes.20 However, its sensitivity is as low as 20% and declines even further after the first week of infection.4,20
PCR testing is the most sensitive and rapid tool available during acute infection.1,20,26,30,31 However, due to waning of the bacteremic phase, its sensitivity decreases after the first week of infection and after treatment is started.19,20
Serologic detection of antibodies with an indirect immunofluorescence assay is the most frequently used test for diagnosis of ehrlichiosis, and paired serology demonstrating seroconversion (at least a 4-fold increase in titer, with a minimal titer of 1:64) is most sensitive (82% to 100%).4,19,20,26 Cross-reactivity can occur, so testing for antibodies to both A phagocytophilum and E chaffeensis might assist in a more accurate diagnosis in areas where tick vectors overlap.4,19,20,26
HGA and HME can be isolated through cell culture in blood or cerebrospinal fluid. However, this is labor-intensive and performed in only a few specialized laboratories.4,19,20,27,31
Treatment of ehrlichiosis
If ehrlichiosis is suspected, treatment should not be delayed; the disease can be life-threatening and the ability to diagnose acute infection is often limited.20,26,32
Doxycycline is the treatment of choice, even in pregnant patients with severe infection and in children.1,19,26,27 Antibiotics are given for 5 to 10 days and continued for at least 3 days after the fever subsides.19,20,26,27,30 In HGA, a 10-day course of doxycycline is recommended to also provide the appropriate length of treatment for Borrelia burgdorferi.1,31
Rifampin is an alternative for those with severe tetracycline allergy, as well as those with mild to moderate infection during pregnancy.1,20,26,29–32
Fever typically resolves within 24 to 48 hours of starting treatment, and persistence of fever over 48 hours after starting antibiotics suggests an alternative diagnosis or possible coinfection.1,4,19,20,26,27,30,32
Persistence of chronic A phagocytophilum or E chaffeensis infection in humans beyond 2 months has not been demonstrated.20,26,30,33 Therefore, antibiotic treatment beyond the acute stage of infection is not indicated.30 Long-term prognosis is favorable, and patients are expected to make a full recovery.26,30
BABESIOSIS
Babesiosis occurs in the northeastern and upper midwestern states, with most cases reported in Massachusetts, Connecticut, Rhode Island, New York, New Jersey, Minnesota, and Wisconsin.31,32,34–36 Outbreaks have also been documented in Washington, California, and Missouri.31,32,35 The spread mimics that of Lyme disease, though it can be slower.34,36–39
Most cases in the Northeast and upper Midwest are caused by Babesia microti, while Babesia duncani has sporadically caused disease along the Pacific coast and Babesia divergens has been found in the Midwest and Northwest.34,36,39
Though babesiosis is usually a tickborne illness, it can also be transmitted through blood transfusion and, rarely, transplacental spread.31,32,34,36,39–41 The I scapularis tick is the host vector for Babesia microti, and transmission of disease requires 24 to 72 hours of attachment to a host.34,35 The primary reservoir for Babesia microti is the white-footed mouse, and humans are accidental hosts.32,34–36,39
Clinical manifestations of babesiosis
Babesia species cause illness by lysing erythrocytes, with resultant cytokine release.34
Symptoms typically appear 1 to 4 weeks after inoculation, after which most cases present as a viral-like illness with gradual onset of fever, chills, sweats, fatigue, malaise, headache, arthralgia, myalgia, nausea, anorexia, and nonproductive cough.32,34–36,39
Physical findings may include splenomegaly, hepatomegaly, jaundice, petechiae, and ecchymosis.32,34–36,39 Rash is seldom present and is not a characteristic feature of babesiosis.35,36
Laboratory features may include thrombocytopenia, hemolytic anemia, and elevated liver enzyme levels.32,34,36,39
Severe disease can occur in elderly, immunocompromised, or splenectomized individuals and can be life-threatening.34,39 Complications of severe infection can include acute respiratory distress syndrome, diffuse intravascular coagulation, and liver or renal failure.31,32,34–36,39 Splenic infarction or rupture may occur at lower levels of parasitemia in those without other manifestations of severe disease.31 The course can be prolonged and relapsing despite standard antibiotic therapy, typically in the setting of severe immunocompromise.32,34,42,43 Death occurs in up to 10% of severe cases.34
Diagnosis of babesiosis
Babesiosis should be considered if a patient presents with a febrile illness and nonspecific symptoms and comes from an endemic area or has received a blood transfusion within 6 months.34,35
The diagnosis of babesiosis is most commonly made by finding the intraerythrocytic ring form of the organism (trophozoite) on Giemsa- or Wright-stained thin blood smears.34,36,39 Babesia can be distinguished from Plasmodia (the agent of malaria) by the rare presence of tetrads of merozoites arranged in a cross-like pattern (the Maltese cross); the absence of hemozoin (brownish deposits) in the ring form; and the occasional presence of extracellular ring forms.34,36
The level of parasitemia (representing the number of parasites per microliter of blood) is generally between 1% and 10%, although it can be as high as 80%.36,39 Because parasitemia is often low early in disease (< 1%), multiple blood smears should be examined.34–36,39
Several real-time PCR assays are available to detect low-grade Babesia microti parasitemia in patients with negative blood smears during early infection.31 These assays have high diagnostic sensitivity and specificity and do not cross-react with other Babesia or Plasmodium species.34–36,39
Paired serology (immunoglobulin G) can confirm infection, although antibody may be absent early in the course of illness.31,34–36,39
Treatment of babesiosis
Current guidelines recommend antimicrobial therapy only for patients with symptoms and positive test results for Babesia.32 Treatment of asymptomatic patients should additionally be considered if parasitemia (not positive PCR or serology) persists for 3 months or longer.32,34–36,39
For mild to moderate babesiosis, the combination of oral atovaquone and azithromycin for 7 to 10 days has similar efficacy and a lower incidence of adverse effects than clindamycin plus quinine.31,32,34,44 For immunocompromised patients, higher doses of azithromycin can be used.31,32
For severe babesiosis or those with risk factors for severe disease, intravenous clindamycin and oral quinine are recommended for 7 to 10 days based on expert opinion.31,32,34–36,39,43 Adverse effects of this regimen include diarrhea, tinnitus, and hearing deficits.35,39 If necessary, intravenous quinidine can be used, but the patient should receive cardiac monitoring for possible prolongation of the QT interval.34,39 As quinine therapy is often interrupted due to the above side effects, alternative regimens such as intravenous azithromycin or clindamycin in combination with oral atovaquone should be considered for severe cases.31 However, these regimens are not well studied.31
Partial or complete exchange transfusion of whole blood or packed red blood cells should be considered in patients with a high level of parasitemia (≥ 10%), severe anemia (hemoglobin < 10 g/dL), or renal, hepatic, or pulmonary compromise.31,32,34–36,39 In critically ill patients, parasitemia should be monitored daily until it has decreased to less than 5%.32,34,39
Generally, symptoms improve within 48 hours of antimicrobial therapy initiation; however, parasitemia may take up to 3 months to resolve.32,34,39 In severely immunocompromised patients, babesiosis may persist or relapse despite appropriate therapy.34,39,42,43 In these cases, at least 6 weeks of antimicrobial therapy is recommended, including 2 weeks of therapy after Babesia organisms are no longer seen on blood smear.31,33,36,39,42
TICKBORNE RELAPSING FEVER
The illness is transmitted by either ticks or body lice. The tick-borne illness is caused by spirochetes of the genus Borrelia and transmitted to humans by the bite of an infected Ornithodoros soft tick.45 Approximately 70% of reported cases in the United States occur in California, Washington, and Colorado.46 Most cases are caused by Borrelia hermsii and are linked to sleeping in rodent-infested cabins in mountainous areas.46 Remarkably, tick-borne borreliae are transmitted within about 30 seconds of tick attachment.47,48
The hallmark of tickborne relapsing fever is febrile episodes lasting 3 to 5 days, with relapses after 5 to 7 days of apparent recovery.49 If untreated, several episodes of fever and nonspecific symptoms will occur before illness resolves spontaneously. Overall mortality rates are very low (< 5%).50
Laboratory confirmation of tickborne relapsing fever is made by detecting spirochetes in a blood smear during a febrile episode or serologic antibody confirmation. However, serologic testing is unhelpful in the acute setting and can yield false-positive results with prior exposure to other Borrelia species (eg, Lyme disease) or other spirochetes. Serologic antibody testing with a 4-fold increase between acute and convalescent samples or PCR can aid in diagnosis, though the latter is available only in research settings.47
The preferred treatment regimen for adults is an oral tetracycline for 10 days. Erythromycin is recommended when tetracyclines are contraindicated.51
When starting treatment, all patients should be monitored closely for the Jarisch-Herxheimer reaction (rigors, hypotension, and high fevers), which develops in over 50% of cases as a result of rapid spirochetal killing and massive cytokine release.52
BORRELIA MIYAMOTOI INFECTION
The most common clinical manifestations are similar to other tickborne relapsing fever infections, although a true “relapsing fever” itself is not usually present.53 The characteristic erythema migrans rash often found in Lyme disease is typically absent in B miyamotoi infection; however, when present, it should prompt investigation into coinfection.54 Cases of meningoencephalitis have been reported in immunosuppressed hosts.55
There is currently no validated test available for diagnosis of B miyamotoi; however, PCR and serology are available in a few specialized laboratories.31,53
The treatment of choice is doxycycline for 2 to 4 weeks. Amoxicillin and ceftriaxone also appear effective.53
SOUTHERN TICK-ASSOCIATED RASH ILLNESS
Infection can present similarly to Lyme disease with an erythema migrans-like rash and associated flulike symptoms, although systemic symptoms and multiple erythema migrans lesions are less likely with STARI. Also, the erythema migrans-like lesions tend to be smaller and more likely to have central clearing than those in Lyme disease.57 Nevertheless, it is difficult to distinguish the 2 illnesses, especially in mid-Atlantic states such as Maryland or Virginia, where both diseases coexist. The most reliable method of distinguishing STARI from Lyme disease is demonstrating that the patient was bitten by a Lone Star tick rather than an Ixodes tick. Numerous questions remain unanswered about the causative organism, pathophysiology, definitive diagnosis, geographic range of illness, and most effective treatment for STARI.
Most reported cases have responded promptly to doxycycline, though it is not known whether antibiotic treatment is necessary.58
TULAREMIA
Ticks are thought to be the most important vectors, and most cases occur in the south-central United States.59 The geographic distribution of disease is gradually shifting northward due to spread of the major tick vectors, A americanum, D variabilis, and D andersoni. Approximately 100 to 200 cases of tularemia are diagnosed each year in the United States, with most concentrated in Kansas, Oklahoma, Missouri, and Arkansas.60
Humans can acquire F tularensis by several routes, and the route of infection ultimately dictates the clinical syndrome. Ulceroglandular and glandular forms of the disease are the most common in the United States, and both frequently result from a tick bite. A few days after tick exposure, an erythematous, often painful papuloulcerative lesion with a central eschar manifests at the site of the tick bite. Additional symptoms may include fever, chills, headache, myalgia, malaise, and suppurative lymphadenitis.61
Diagnosis can be made by identifying F tularensis in blood, fluid, or tissue culture performed under biosafety level 3 conditions; however, serology is used in most cases.62
Streptomycin and gentamicin are considered drugs of choice and should be continued for at least 10 days. For relatively mild disease, oral doxycycline or ciprofloxacin can be considered for at least 14 days, although the latter is not approved for treatment.59,63
TICKBORNE VIRAL INFECTIONS
Powassan virus, an uncommon flavivirus, is found in the Great Lakes region and northeast United States. In the Great Lakes region, I cookei ticks transmit the traditional lineage of this virus. However, more recent cases have been identified in the Northeast and Midwest, where Powassan virus lineage II (or deer tick virus) is transmitted by I scapularis.31,64
The classic presentation is a viral encephalitis. Rash (most often maculopapular) and gastrointestinal symptoms have been reported as well. A high index of suspicion is needed for diagnosis because clinical features and laboratory findings resemble those of other arboviral infections.
Treatment for Powassan viral encephalitis is supportive, although corticosteroids have been used with some success.64 While asymptomatic infection has been documented, the reported mortality rate of Powassan virus encephalitis is 10% to 15%, and focal neurologic deficits can persist among survivors.65
Clinical and laboratory features appear to be very similar to those of the ehrlichioses.1 A clinical diagnosis should be considered in patients with A americanum exposure, fever, and cytopenias who lack PCR or serologic evidence for ehrlichiosis infection or who fail to respond to doxycycline therapy.24
COINFECTION
Some tick vectors transmit more than 1 type of infection, and therefore, coinfection with multiple pathogens may occur. For example, I scapularis transmits Borrelia burgdorferi (Lyme disease), HGA, Babesia microti, B miyamotoi, E muris-like agent, and Powassan virus lineage II, while A americanum transmits HME and Heartland virus.24,26,31,34,36,67 Coinfection may increase the severity of disease, often due to a delay in diagnosis, though more research is needed to understand the clinical manifestations of coinfection.31,35,67
PREVENTION
Unfortunately, there are no available human vaccines for tickborne illnesses in the United States, and the effectiveness of single-dose prophylaxis with doxycycline for non-Lyme infections has not been evaluated.4,7,26
Illness is best prevented by minimizing skin exposure to ticks, use of tick repellents containing DEET, use of long-legged and long-sleeved clothing impregnated with an acaricide such as permethrin, and conducting timely body checks for ticks after potential exposure.1,31,32 Light-colored clothing is suggested, since it allows for better visibility of crawling ticks.4,32 Bathing or showering within 2 hours of tick exposure helps prevent attachment of ticks.4,31,68 If camping outside, use of a bed net is recommended.68
Ticks are most easily removed by grasping the head of the tick as close to the skin surface as possible with fine-tipped tweezers.32,68 Removing or crushing ticks with bare hands should be avoided to prevent potential contamination, and hands should be washed thoroughly after tick removal.1,4
Blood donors are screened for a history of symptomatic tickborne disease; however, asymptomatic donors who are not identified at screening pose the greatest risk to the blood supply. Babesia microti is the most common reported transfusion-transmitted parasite in the United States, and transmission of R rickettsii, A phagocytophilum, and E ewingii have also been reported infrequently.28,40,69 Currently, no test is approved to screen blood for tickborne illnesses, though such a test would help prevent transmission of tickborne illnesses by blood transfusion in areas where these diseases are endemic.40,41
TAKE-HOME POINTS
Tickborne illnesses are increasing throughout the United States as a result of vector expansion and changes in human ecology.
It is essential that primary care clinicians consider tickborne illnesses in the differential diagnosis for any patient presenting with a fever and constitutional symptoms when the cause of symptoms is unclear and tick exposure is possible or known.
All the diseases discussed are nationally notifiable conditions, and confirmed cases should be reported.
Knowledge of the geographic locations of potential exposure is paramount to determining which tickborne infections to consider, and the absence of a tick bite history should not exclude the diagnosis in the correct clinical presentation.
In addition, it is important to recognize the limitations of diagnostic testing for many tickborne infections; empiric treatment is most often warranted before confirming the diagnosis.
Tick avoidance is the most effective way to prevent these often severe infections.
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep 2016; 65:1–44.
- Openshaw JJ, Swerdlow DL, Krebs JW, et al. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg 2010; 83:174–182.
- Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg 2011; 85:124–131.
- Chapman AS, Bakken JS, Folk SM, et al; Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep 2006; 55:1–27.
- Mukkada S, Buckingham SC. Recognition of and prompt treatment for tick-borne infections in children. Infect Dis Clin North Am 2015; 29:539–555.
- Schutze GE, Buckingham SC, Marshall GS, et al; Tick-borne Infections in Children Study (TICS) Group. Human monocytic ehrlichiosis in children. Pediatr Infect Dis J 2007; 26:475–479.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis 2007; 7:724–732.
- Lin L, Decker C. Rocky Mountain spotted fever. Dis Mon 2012; 58:361–369.
- Demma LJ, Traeger MS, Nicholson WL, et al. Rocky mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 2005; 353:587–594.
- Traeger MS, Regan JJ, Humpherys D, et al. Rocky mountain spotted fever characterization and comparison to similar illnesses in a highly endemic area—Arizona, 2002-2011. Clin Infect Dis 2015; 60:1650–1658.
- Dahlgren FS, Holman RC, Paddock CD, Callinan LS, McQuiston JH. Fatal Rocky Mountain spotted fever in the United States, 1999–2007. Am J Trop Med Hyg 2012; 86:713–719.
- Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 2013; 26:657–702.
- Regan JJ, Traeger MS, Humpherys D, et al. Risk factors for fatal outcome from rocky mountain spotted fever in a highly endemic area—Arizona, 2002-2011. Clin Infect Dis 2015; 60:1659–1666.
- Nelson R. Rocky Mountain spotted fever in Native Americans. Lancet Infect Dis 2015; 15:1013–1014.
- Botelho-Nevers E, Socolovschi C, Raoult D, Parola P. Treatment of Rickettsia spp. infections: a review. Expert Rev Anti Infect Ther 2012; 10:1425–1437.
- Dotters-Katz SK, Kuller J, Heine RP. Arthropod-borne bacterial diseases in pregnancy. Obstet Gynecol Surv 2013; 68:635–649.
- Paddock CD, Finley RW, Wright CS, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis 2008; 47:1188–1196.
- Shapiro MR, Fritz CL, Tait K, et al. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis 2010; 50:541–548.
- Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis 2007; 45(suppl 1):S45–S51.
- Thomas RJ, Dumler JS, Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther 2009; 7:709–722.
- Severo MS, Stephens KD, Kotsyfakis M, Pedra JH. Anaplasma phagocytophilum: deceptively simple or simply deceptive? Future Microbiol 2012; 7:719–731.
- Dahlgren FS, Heitman KN, Drexler NA, Massung RF, Behravesh CB. Human granulocytic anaplasmosis in the United States from 2008 to 2012: a summary of national surveillance data. Am J Trop Med Hyg 2015; 93:66–72.
- Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, Behravesh CB. Increasing Incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med 2016; 94:52–60.
- Wormser GP, Pritt B. Update and commentary on four emerging tick-borne infections: Ehrlichia muris-like agent, Borrelia miyamotoi, deer tick virus, heartland virus, and whether ticks play a role in transmission of Bartonella henselae. Infect Dis Clin North Am 2015; 29:371–381.
- Pritt BS, Sloan LM, Johnson DK, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med 2011; 365:422–429.
- Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am 2015; 29:341–355.
- St Clair K, Decker CF. Ehrlichioses: anaplasmosis and human ehrlichiosis. Dis Mon 2012; 58:346–354.
- Centers for Disease Control and Prevention (CDC). Anaplasma phagocytophilum transmitted through blood transfusion—Minnesota, 2007. MMWR Morb Mortal Wkly Rep 2008; 57:1145–1148.
- Dhand A, Nadelman RB, Aguero-Rosenfeld M, Haddad FA, Stokes DP, Horowitz HW. Human granulocytic anaplasmosis during pregnancy: case series and literature review. Clin Infect Dis 2007; 45:589–593.
- Stone JH, Dierberg K, Aram G, Dumler JS. Human monocytic ehrlichiosis. JAMA 2004; 292:2263–2270.
- Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis. JAMA 2016; 315:1767–1777.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–1134.
- Dumler JS, Choi KS, Garcia-Garcia JC, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis 2005; 11:1828–1834.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am 2015; 29:357–370.
- Kavanaugh MJ, Decker CF. Babesiosis. Dis Mon 2012; 58:355–360.
- Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am 2008; 22:469–488.
- Diuk-Wasser MA, Liu Y, Steeves TK, et al. Monitoring human babesiosis emergence through vector surveillance New England USA. Emerg Infect Dis 2014; 20:225–231.
- Dunn JM, Krause PJ, Davis S, et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS One 2014; 9:e115494.
- Vannier E, Krause PJ. Human babesiosis. N Engl J Med 2012; 366:2397–2407.
- Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011; 155:509–519.
- Wudhikarn K, Perry EH, Kemperman M, Jensen KA, Kline SE. Transfusion-transmitted babesiosis in an immunocompromised patient: a case report and review. Am J Med 2011; 124:800–805.
- Krause PJ, Gewurz BE, Hill D, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 2008; 46:370–376.
- Wormser GP, Prasad A, Neuhaus E, et al. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis 2010; 50:381–386.
- Krause PJ, Lepore T, Sikand VK, et al. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 2000; 343:1454–1458.
- Centers for Disease Control and Prevention (CDC). Tick-borne relapsing fever (TBRF): distribution. www.cdc.gov/relapsing-fever/distribution/index.html. Accessed June 7, 2017.
- Forrester JD, Kjemtrup AM, Fritz CL, et al; Centers for Disease Control and Prevention (CDC). Tickborne relapsing fever—United States, 1990-2011. MMWR Morb Mortal Wkly Rep 2015; 64:58–60.
- Dworkin MS, Schwan TG, Anderson DE Jr, Borchardt SM. Tick-borne relapsing fever. Infect Dis Clin North Am 2008; 22:449–468.
- Anderson JF. The natural history of ticks. Med Clin North Am 2002; 86:205–218.
- Barbour AG. Antigenic variation of a relapsing fever Borrelia species. Annu Rev Microbiol 1990; 44:155–171.
- Centers for Disease Control and Prevention (CDC). Acute respiratory distress syndrome in persons with tickborne relapsing fever—three states, 2004-2005. MMWR Morb Mortal Wkly Rep 2007; 56:1073–1076.
- Centers for Disease Control and Prevention (CDC). Tick-borne relapsing fever (TBRF): information for clinicians. www.cdc.gov/relapsing-fever/clinicians/index.html. Accessed June 7, 2017.
- Dworkin MS, Anderson DE Jr, Schwan TG, et al. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis 1998; 26:122–131.
- Wagemakers A, Staarink PJ, Sprong H, Hovius JW. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol 2015; 31:260–269.
- Krause PJ, Narasimhan S, Wormser GP, et al; Tick Borne Diseases Group. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis 2014; 20:1183–1190.
- Gugliotta JL, Goethert HK, Berardi VP, Telford SR 3rd. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med 2013; 368:240–245.
- Masters EJ, Grigery CN, Masters RW. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect Dis Clin North Am 2008; 22:361–376.
- Wormser GP, Masters E, Nowakowski J, et al. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin Infect Dis 2005; 41:958–965.
- Feder HM Jr, Hoss DM, Zemel L, Telford SR 3rd, Dias F, Wormser GP. Southern tick-associated rash illness (STARI) in the north: STARI following a tick bite in Long Island, New York. Clin Infect Dis 2011; 53:e142–e146.
- Carvalho CL, Lopes de Carvalho I, Ze-Ze L, Nuncio MS, Duarte EL. Tularaemia: a challenging zoonosis. Comp Immunol Microbiol Infect Dis 2014; 37):85–96.
- Centers for Disease Control and Prevention (CDC). Tularemia: statistics. www.cdc.gov/tularemia/statistics/index.html. Accessed June 7, 2017.
- Weber IB, Turabelidze G, Patrick S, Griffith KS, Kugeler KJ, Mead PS. Clinical recognition and management of tularemia in Missouri: a retrospective records review of 121 cases. Clin Infect Dis 2012; 55:1283–1290.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am 2008; 22:489–504.
- Johansson A, Berglund L, Sjostedt A, Tarnvik A. Ciprofloxacin for treatment of tularemia. Clin Infect Dis 2001; 33:267–268.
- Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 2016; 62:707–713.
- Ebel GD. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol 2010; 55:95–110.
- Pastula DM, Turabelidze G, Yates KF, et al; Centers for Disease Control and Prevention (CDC). Notes from the field: heartland virus disease—United States, 2012-–2013. MMWR Morb Mortal Wkly Rep 2014; 63:270–271.
- Knapp KL, Rice NA. Human coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res 2015; 2015:587131.
- Pujalte GG, Chua JV. Tick-borne infections in the United States. Prim Care 2013; 40:619–635.
- Regan J, Matthias J, Green-Murphy A, et al. A confirmed Ehrlichia ewingii infection likely acquired through platelet transfusion. Clin Infect Dis 2013; 56:e105–e107.
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep 2016; 65:1–44.
- Openshaw JJ, Swerdlow DL, Krebs JW, et al. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg 2010; 83:174–182.
- Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg 2011; 85:124–131.
- Chapman AS, Bakken JS, Folk SM, et al; Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep 2006; 55:1–27.
- Mukkada S, Buckingham SC. Recognition of and prompt treatment for tick-borne infections in children. Infect Dis Clin North Am 2015; 29:539–555.
- Schutze GE, Buckingham SC, Marshall GS, et al; Tick-borne Infections in Children Study (TICS) Group. Human monocytic ehrlichiosis in children. Pediatr Infect Dis J 2007; 26:475–479.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis 2007; 7:724–732.
- Lin L, Decker C. Rocky Mountain spotted fever. Dis Mon 2012; 58:361–369.
- Demma LJ, Traeger MS, Nicholson WL, et al. Rocky mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 2005; 353:587–594.
- Traeger MS, Regan JJ, Humpherys D, et al. Rocky mountain spotted fever characterization and comparison to similar illnesses in a highly endemic area—Arizona, 2002-2011. Clin Infect Dis 2015; 60:1650–1658.
- Dahlgren FS, Holman RC, Paddock CD, Callinan LS, McQuiston JH. Fatal Rocky Mountain spotted fever in the United States, 1999–2007. Am J Trop Med Hyg 2012; 86:713–719.
- Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 2013; 26:657–702.
- Regan JJ, Traeger MS, Humpherys D, et al. Risk factors for fatal outcome from rocky mountain spotted fever in a highly endemic area—Arizona, 2002-2011. Clin Infect Dis 2015; 60:1659–1666.
- Nelson R. Rocky Mountain spotted fever in Native Americans. Lancet Infect Dis 2015; 15:1013–1014.
- Botelho-Nevers E, Socolovschi C, Raoult D, Parola P. Treatment of Rickettsia spp. infections: a review. Expert Rev Anti Infect Ther 2012; 10:1425–1437.
- Dotters-Katz SK, Kuller J, Heine RP. Arthropod-borne bacterial diseases in pregnancy. Obstet Gynecol Surv 2013; 68:635–649.
- Paddock CD, Finley RW, Wright CS, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis 2008; 47:1188–1196.
- Shapiro MR, Fritz CL, Tait K, et al. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis 2010; 50:541–548.
- Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis 2007; 45(suppl 1):S45–S51.
- Thomas RJ, Dumler JS, Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther 2009; 7:709–722.
- Severo MS, Stephens KD, Kotsyfakis M, Pedra JH. Anaplasma phagocytophilum: deceptively simple or simply deceptive? Future Microbiol 2012; 7:719–731.
- Dahlgren FS, Heitman KN, Drexler NA, Massung RF, Behravesh CB. Human granulocytic anaplasmosis in the United States from 2008 to 2012: a summary of national surveillance data. Am J Trop Med Hyg 2015; 93:66–72.
- Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, Behravesh CB. Increasing Incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med 2016; 94:52–60.
- Wormser GP, Pritt B. Update and commentary on four emerging tick-borne infections: Ehrlichia muris-like agent, Borrelia miyamotoi, deer tick virus, heartland virus, and whether ticks play a role in transmission of Bartonella henselae. Infect Dis Clin North Am 2015; 29:371–381.
- Pritt BS, Sloan LM, Johnson DK, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med 2011; 365:422–429.
- Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am 2015; 29:341–355.
- St Clair K, Decker CF. Ehrlichioses: anaplasmosis and human ehrlichiosis. Dis Mon 2012; 58:346–354.
- Centers for Disease Control and Prevention (CDC). Anaplasma phagocytophilum transmitted through blood transfusion—Minnesota, 2007. MMWR Morb Mortal Wkly Rep 2008; 57:1145–1148.
- Dhand A, Nadelman RB, Aguero-Rosenfeld M, Haddad FA, Stokes DP, Horowitz HW. Human granulocytic anaplasmosis during pregnancy: case series and literature review. Clin Infect Dis 2007; 45:589–593.
- Stone JH, Dierberg K, Aram G, Dumler JS. Human monocytic ehrlichiosis. JAMA 2004; 292:2263–2270.
- Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis. JAMA 2016; 315:1767–1777.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–1134.
- Dumler JS, Choi KS, Garcia-Garcia JC, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis 2005; 11:1828–1834.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am 2015; 29:357–370.
- Kavanaugh MJ, Decker CF. Babesiosis. Dis Mon 2012; 58:355–360.
- Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am 2008; 22:469–488.
- Diuk-Wasser MA, Liu Y, Steeves TK, et al. Monitoring human babesiosis emergence through vector surveillance New England USA. Emerg Infect Dis 2014; 20:225–231.
- Dunn JM, Krause PJ, Davis S, et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS One 2014; 9:e115494.
- Vannier E, Krause PJ. Human babesiosis. N Engl J Med 2012; 366:2397–2407.
- Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011; 155:509–519.
- Wudhikarn K, Perry EH, Kemperman M, Jensen KA, Kline SE. Transfusion-transmitted babesiosis in an immunocompromised patient: a case report and review. Am J Med 2011; 124:800–805.
- Krause PJ, Gewurz BE, Hill D, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 2008; 46:370–376.
- Wormser GP, Prasad A, Neuhaus E, et al. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis 2010; 50:381–386.
- Krause PJ, Lepore T, Sikand VK, et al. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 2000; 343:1454–1458.
- Centers for Disease Control and Prevention (CDC). Tick-borne relapsing fever (TBRF): distribution. www.cdc.gov/relapsing-fever/distribution/index.html. Accessed June 7, 2017.
- Forrester JD, Kjemtrup AM, Fritz CL, et al; Centers for Disease Control and Prevention (CDC). Tickborne relapsing fever—United States, 1990-2011. MMWR Morb Mortal Wkly Rep 2015; 64:58–60.
- Dworkin MS, Schwan TG, Anderson DE Jr, Borchardt SM. Tick-borne relapsing fever. Infect Dis Clin North Am 2008; 22:449–468.
- Anderson JF. The natural history of ticks. Med Clin North Am 2002; 86:205–218.
- Barbour AG. Antigenic variation of a relapsing fever Borrelia species. Annu Rev Microbiol 1990; 44:155–171.
- Centers for Disease Control and Prevention (CDC). Acute respiratory distress syndrome in persons with tickborne relapsing fever—three states, 2004-2005. MMWR Morb Mortal Wkly Rep 2007; 56:1073–1076.
- Centers for Disease Control and Prevention (CDC). Tick-borne relapsing fever (TBRF): information for clinicians. www.cdc.gov/relapsing-fever/clinicians/index.html. Accessed June 7, 2017.
- Dworkin MS, Anderson DE Jr, Schwan TG, et al. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis 1998; 26:122–131.
- Wagemakers A, Staarink PJ, Sprong H, Hovius JW. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol 2015; 31:260–269.
- Krause PJ, Narasimhan S, Wormser GP, et al; Tick Borne Diseases Group. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis 2014; 20:1183–1190.
- Gugliotta JL, Goethert HK, Berardi VP, Telford SR 3rd. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med 2013; 368:240–245.
- Masters EJ, Grigery CN, Masters RW. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect Dis Clin North Am 2008; 22:361–376.
- Wormser GP, Masters E, Nowakowski J, et al. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin Infect Dis 2005; 41:958–965.
- Feder HM Jr, Hoss DM, Zemel L, Telford SR 3rd, Dias F, Wormser GP. Southern tick-associated rash illness (STARI) in the north: STARI following a tick bite in Long Island, New York. Clin Infect Dis 2011; 53:e142–e146.
- Carvalho CL, Lopes de Carvalho I, Ze-Ze L, Nuncio MS, Duarte EL. Tularaemia: a challenging zoonosis. Comp Immunol Microbiol Infect Dis 2014; 37):85–96.
- Centers for Disease Control and Prevention (CDC). Tularemia: statistics. www.cdc.gov/tularemia/statistics/index.html. Accessed June 7, 2017.
- Weber IB, Turabelidze G, Patrick S, Griffith KS, Kugeler KJ, Mead PS. Clinical recognition and management of tularemia in Missouri: a retrospective records review of 121 cases. Clin Infect Dis 2012; 55:1283–1290.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am 2008; 22:489–504.
- Johansson A, Berglund L, Sjostedt A, Tarnvik A. Ciprofloxacin for treatment of tularemia. Clin Infect Dis 2001; 33:267–268.
- Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 2016; 62:707–713.
- Ebel GD. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol 2010; 55:95–110.
- Pastula DM, Turabelidze G, Yates KF, et al; Centers for Disease Control and Prevention (CDC). Notes from the field: heartland virus disease—United States, 2012-–2013. MMWR Morb Mortal Wkly Rep 2014; 63:270–271.
- Knapp KL, Rice NA. Human coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res 2015; 2015:587131.
- Pujalte GG, Chua JV. Tick-borne infections in the United States. Prim Care 2013; 40:619–635.
- Regan J, Matthias J, Green-Murphy A, et al. A confirmed Ehrlichia ewingii infection likely acquired through platelet transfusion. Clin Infect Dis 2013; 56:e105–e107.
KEY POINTS
- Tickborne illnesses should be considered in patients with known or potential tick exposure presenting with fever or vague constitutional symptoms in tick-endemic regions.
- Given that tick-bite history is commonly unknown, absence of a known tick bite does not exclude the diagnosis of a tick-borne illness.
- Starting empiric treatment is usually warranted before the diagnosis of tickborne illness is confirmed.
- Tick avoidance is the most effective measure for preventing tickborne infections.
Dozing off: Examining excessive daytime sleepiness in psychiatric patients
Excessive daytime sleepiness (EDS) is “the inability to maintain wakefulness and alertness during the major waking periods of the day, with sleep occurring unintentionally or at inappropriate times, almost daily for at least 3 months,” according to the American Academy of Sleep Medicine.1 EDS is common, with a prevalence up to 25% to 30% in the general population.1-4 The prevalence rate varies in different studies, primarily because of inconsistent definitions of EDS, and therefore differences in diagnosis and assessment.1,2,4 In a study of 300 psychiatric outpatients, 34% had EDS.3 However, studies and evidence reviewing EDS in psychiatric patients are limited.
The causes of EDS are many and varied,1,8 including medical and psychiatric etiologies. A thorough history, screening at-risk patients, and timely sleep center referral are vital to detect and appropriately manage the cause of EDS.5
This article reviews the literature on EDS, with a focus on the risks of untreated EDS, common etiologies of the condition, as well as a brief description of screening and treatment strategies.
EDS vs fatigue
Many patients describe EDS as “fatigue”1; however, a patient’s report of fatigue could be mistaken for EDS.4 Although there is overlap, it is important for physicians to distinguish between these 2 entities for accurate identification and treatment.1,4
Risk of inadequate screening
A study of 117 patients with symptomatic coronary artery disease showed that EDS is associated with significantly greater incidence of cardiovascular adverse events at 16-month follow up.2 This study had limitations such as small sample size; therefore, more studies are needed. Because of these risks, timely and accurate diagnosis not only improves the patient’s quality of life and reduces polypharmacy but also can be life-saving.
Common causes of EDS in psychiatric patients
Because of the high prevalence and severity of impairments caused by EDS, it is essential for psychiatrists to be informed about causes of EDS and thoroughly assess for the potential underlying etiology before concluding that the sleep problem is a manifestation of the psychiatric disorder and prescribing psychotropic medication for it.
Some common causes of EDS in psychiatric patients include:
Sleep-disordered breathing.8 Obstructive sleep apnea (OSA) is often underdiagnosed,6,7 and considering how common it is,6 psychiatrists likely will see many patients with OSA in their practice.5 OSA has a higher prevalence among patients with psychiatric disorders such as depression6,9 and schizophrenia. Additionally, there is evidence suggesting that patients with OSA are more likely to suffer from depression and EDS than healthy controls6,9,10; some of the proposed mechanisms are sleep fragmentation and hypoxemia.6,9-11 OSA is the most common form of sleep-disordered breathing and is a common cause of EDS.1,2,12 Also, undiagnosed and untreated OSA in patients with depression could cause refractoriness to pharmacological treatment of depression.6,9,10
When unrecognized and untreated, OSA can be life-threatening. Despite this, OSA is not regularly screened for in clinical psychiatric practice.6,10 Therefore, it is imperative that psychiatrists be well-acquainted with measures to identify at-risk patients and refer to a sleep specialist when appropriate.
OSA is accompanied by irritability, cognitive difficulties, and poor sleep, creating an overlap with symptoms of depressive disorders.6,10 Use of sedative hypnotic medications, such as benzodiazepines, which further reduces muscle tone in the airway and suppresses respiratory effort, can worsen OSA symptoms5,6,10 and pose cerebrovascular, cardiovascular, and potentially life-threatening risks, and therefore is not indicated in this population.9,13
Obesity is a risk factor for OSA.6 Patients with mood disorders or schizophrenia or other psychotic disorders are at higher risk of obesity because of psychotropic-induced weight gain, stress-induced mechanisms, and/or lower levels of self-care. When these patients have unrecognized or untreated OSA and are prescribed sedative medications at night or stimulant medications during the day, they could be at increased cardiac or respiratory risks without resolving their underlying condition. A diligent psychiatrist can dramatically reduce the risks by referring a patient for nocturnal polysomnography,1 helping the patient implement lifestyle modifications (eg, exercise, weight loss, and healthy nutrition), prescribing judiciously, and monitoring closely for such risks. An accurate diagnosis of and treatment for OSA can improve sleep6 dramatically and help depressive symptoms through better sleep, more daytime energy and concentration, and adequate oxygenation of the brain while sleeping.
Psychiatrists can screen for OSA using the STOP-Bang (Snoring, Tired, Observed apnea, Pressure, Body mass index, Age, Neck circumference, Gender) Questionnaire, which is a quick, 8-item screening scale that helps to categorize OSA risk as mild, moderate, or severe.12 Hypertension, snoring, and/or gasping for breath (“observed apnea”)—a history which often is provided by spouses or significant others—daytime dozing and/or tiredness, having a large neck circumference or volume, body mass index, male sex, and age are items on the STOP-Bang Questionnaire and also are features that should raise high clinical suspicion of OSA.12 Referral for nocturnal polysomnography in at-risk patients should be the next step1,5 in any sleep-related breathing disorder.
Treatment for OSA involves continuous positive airway pressure (CPAP) therapy, which has been shown to relieve OSA and decrease related EDS.5,6 Other treatment modalities, such as oral appliances and surgery, may be used5 in some cases, but more studies are needed for conclusive results.
Several studies have shown improved depression, mood, and cognition after administering treatment such as CPAP6,9,14 in patients with OSA and depression. Considering the significant risks of cardiovascular,8 cerebrovascular,8 and overall morbidity and mortality associated with untreated OSA,12 it is important to routinely screen for sleep-disordered breathing in patients with depression9 or other psychiatric disorders and refer for specialized sleep evaluation and treatment, when indicated.
Medications. EDS can result from some prescription and over-the-counter medications.1,2,5,7 Sedating antidepressants, antihistamines, antipsychotics, anticonvulsants,1,8 and beta blockers2 could cause sedation, which can persist during daytime, although a few studies did not find an association between antipsychotic use and EDS.3 Benzodiazepines and other sedative-hypnotics,1,7 especially long-acting agents or higher dosages,5 can lead to EDS and decreased alertness. Non-psychotropics, such as opioid pain medications,1,7 antitussives, and skeletal muscle relaxants, also can contribute to or cause daytime sedation.7 When using these agents, psychiatrists should monitor and routinely assess patients while aiming for the lowest effective dosage when feasible.
This strategy creates a framework for psychiatrists to routinely educate patients about these commonly encountered side effects, reduce polypharmacy when possible, and help patients effectively manage or prevent these adverse effects.
Depression.1 Some studies found >45% patients with depression had EDS.3,13,15 Besides an association between depression and EDS,13,16 Chellappa and Araújo13 also found a significant association between EDS and suicidal ideation. The causes of EDS in patients with depression may be varied, ranging from restless legs syndrome, residual depressive symptoms,15 to OSA. Depression is often comorbid with OSA,6 with up to 20% of patients with depression suffering from OSA,10 creating higher risk for EDS. Depressive disorders are routinely assessed during an evaluation of OSA at sleep centers, but OSA often is not screened in psychiatric practice.10
There is a strong need for regular screening for OSA in patients with depression, particularly because most studies show a link between the 2 conditions.10 Both depression and OSA have some common risk factors, such as obesity, hypertension, and metabolic syndrome.10 Patients with these conditions are at greater risk for OSA, and therefore a psychiatrist should proactively screen and refer such patients for nocturnal polysomnography when they suspect OSA. Patients with OSA and depression often present to the psychiatrist with depressive symptoms that appear to be resistant to pharmacological treatment,10 therefore underscoring the importance of screening and ruling out OSA in patients with depression.
Circadian rhythm disorders, restless legs syndrome, alcohol and other substance use, and use of prescription sedative-hypnotics are more common in patients with depression; therefore, this population is at high risk for EDS.
Circadian rhythm disorders and insufficient sleep syndrome. Insufficient sleep syndrome1,2,8 frequently causes EDS and occurs more commonly in busy people who try to get by with less sleep.8 Over time, the effect of sleep loss is cumulative and can be accompanied by mood symptoms, such as irritability, fatigue, and problems with concentration.8 Shift workers1,8 commonly experience insufficient sleep as well as circadian rhythm disorders and EDS. Modafinil is FDA-approved for EDS in shift work sleep disorder.
Geriatric patients may experience advanced sleep phase syndrome involving early awakenings.8 Adolescents, on the other hand, often suffer from delayed sleep phase syndrome, which is a type of circadian rhythm disorder, related to increasing academic and social pressures, natural pubertal shift to later sleep onset, pervading technology use, and often nebulous bedtime routines. This can be a cause of sleep persisting into daytime.8 Taking a careful history and a sleep diary may be useful because this disorder might be confused for insomnia. Treatment involves gradual shifting of the time of sleep onset through bright light exposure and other modalities.8
Adolescents might not be forthcoming about the severity of their sleep problems; therefore, psychiatrists should screen proactively through clinical interviews of patients and parents and consider this possibility when encountering an adolescent with recent-onset attention or cognitive difficulties.
Treatment for circadian rhythm disorders usually includes planned or prescribed sleep scheduling, timed light exposure,8 and occasional use of melatonin or other sedative agents.17
Hypersomnia of central origin, which includes narcolepsy, idiopathic hypersomnia, and recurrent hypersomnia, can present with EDS.1,18,19 Narcolepsy is a rare, debilitating sleep disorder that manifests as EDS or sleep attacks, with or without cataplexy, and sleep paralysis.5,8,18,19 The Multiple Sleep Latency Test and polysomnography are used for diagnosis.1,5 Shortened REM latency is a classic finding often noted on polysomnography. Treatment involves pharmacologic and behavioral strategies and education.5,8 Modafinil is FDA-approved for EDS associated with narcolepsy. Stimulant medications have been used for narcolepsy in the past; further studies are needed to establish benefit–risk ratio of use in this population.18
Kleine-Levin syndrome is a form of recurrent hypersomnia, a less common sleep disorder, characterized by episodes of excessive sleepiness accompanied by hyperphagia and hypersexuality.5,18,19
Other medical conditions,1 such as the rare familial fatal insomnia, neurological conditions1 such as encephalitis,8 epilepsy,8 Alzheimer’s disease or other types of dementia,8 Parkinson’s disease,1 or multiple sclerosis,1,18 can cause excessive daytime fatigue by causing secondary insomnia or hypersomnia.
Treating the underlying disorder is an important first step in these cases. In addition, coordinating with neurologists or other specialists involved in caring for patients with these conditions is important. Regularly reviewing and simplifying the often complex medication regimen, when possible, can go a long way in mitigating EDS in this population.
Other disorders affecting sleep. Restless legs syndrome and periodic limb movement disorder are other causes of EDS.3 Treatment involves lifestyle modifications, iron supplementation in certain patients, and use of dopaminergic agents such as ropinirole, pramipexole, and other medications, depending on severity of the condition, comorbidities, and other factors.20
Alcohol or substance use. Substance use or withdrawal can be associated with sleep disorders, such as hypersomnia,19 insomnia,19 and related EDS.5 For example, alcohol use disorder affects REM sleep, and can cause EDS. Secondary central apnea can be the result of long-standing opioid use19 and can present like EDS.
Insomnia. Primary insomnia rarely causes EDS.5 Insomnia due to a medical or psychiatric condition may be an indirect cause of EDS by causing sleep deprivation.
Steps for timely and accurate diagnosis
Utilize the following steps for facilitating timely diagnosis and treatment of EDS:
Thorough history. Patients often describe “tiredness” instead of sleepiness.8 Therefore, the astute psychiatrist should explore further when patients are presenting with this concern, especially by asking more specific questions such as the tendency to doze off during daytime.8
Family members can be vital sources for obtaining a complete history,5 especially because patients might deny,8 minimize, or not be fully aware1 of the extent of their symptoms. Asking family members about patient’s snoring, irregular breathing, or gasping at night can be particularly valuable.5 Obtaining a family history of sleep disorders can be particularly important, especially in conditions such as OSA and narcolepsy.
Asking about any history of safety issues,8 including sleepiness during driving, cooking, or other activities, is also important.
Use of scales and other screening measures. Psychiatrists can use initial screening measures in the office setting. Epworth Sleepiness Scale15,21 is a validated,2 short, self-administered measure to assess the level of daytime sleepiness; however, it has some limitations such as not being able to measure changes in sleepiness from hour to hour or day to day. Because of its limitations, the Epworth Sleepiness Scale should not be used by itself as a diagnostic tool.3 It has been commonly used for detecting OSA2 and narcolepsy. The Stanford Sleepiness Scale is a self-rating scale that measures the subjective degree of sleepiness and alertness; it has limitations as well, such as having little correlation with chronic sleep loss.8 Other tools such as visual analogue scales also could be helpful.8 For more specialized testing, such as Multiple Sleep Latency Test or polysomnography, referral to a sleep specialist is ideal.8
Education. The assessment is an opportunity for the psychiatrist to educate patients about sleep hygiene, the importance of regular bedtimes, and getting adequate sleep to avoid accumulating a sleep deficit.
Urgent referral of at-risk populations. Prompt or urgent referral of at-risk populations, such as geriatric patients or those with a history of dozing off during driving, is invaluable in preventing morbidity and mortality from untreated sleep disorders.
Patients with severe daytime sleepiness should be advised to not drive or operate heavy machinery until this condition is adequately controlled.18
Bottom Line
1. Chervin RD. Approach to the patient with excessive daytime sleepiness. http://www.uptodate.com/contents/approach-to-the-patient-with-excessive-daytime-sleepiness. Updated January 2016. Accessed June 5, 2017.
2. Lee CH, Ng WY, Hau W, et al. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9(12):1267-1272.
3. Hawley CJ, Gale TM, Sivakumaran T, et al. Excessive daytime sleepiness in psychiatric disorders: prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1-2):138-141.
4. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54(1):61-69.
5. Krahn LE. Excessive daytime sleepiness: diagnosing the causes. Current Psychiatry. 2002;1(1):49-57.
6. Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17-25.
7. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
8. Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124(pt 8):1482-1491.
9. Cheng P, Casement M, Chen CF, et al. Sleep disordered breathing in major depressive disorder. J Sleep Res. 2013;22(4):459-462.
10. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.
11. Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47(6):583-596.
12. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638.
13. Chellappa SL, Araújo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28(2):126-129.
14. Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11(6):552-557.
15. Lundt L. Use of the Epworth Sleepiness Scale to evaluate the symptom of excessive sleepiness in major depressive disorder. Gen Hosp Psychiatry. 2005;27(2):146-148.
16. Hawley CJ. Excessive daytime sleepiness in psychiatry: a relevant focus for clinical attention and treatment? Int J Psychiatry Clin Pract. 2006;10(2):117-123.
17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701-715.
18. Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711.
19. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9(4):687-701.
20. National Institute of Neurological Disorders and Stroke. Restless legs syndrome information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Restless-Legs-Syndrome-Information-Page. Accessed June 2, 2017.
21. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381.
Excessive daytime sleepiness (EDS) is “the inability to maintain wakefulness and alertness during the major waking periods of the day, with sleep occurring unintentionally or at inappropriate times, almost daily for at least 3 months,” according to the American Academy of Sleep Medicine.1 EDS is common, with a prevalence up to 25% to 30% in the general population.1-4 The prevalence rate varies in different studies, primarily because of inconsistent definitions of EDS, and therefore differences in diagnosis and assessment.1,2,4 In a study of 300 psychiatric outpatients, 34% had EDS.3 However, studies and evidence reviewing EDS in psychiatric patients are limited.
The causes of EDS are many and varied,1,8 including medical and psychiatric etiologies. A thorough history, screening at-risk patients, and timely sleep center referral are vital to detect and appropriately manage the cause of EDS.5
This article reviews the literature on EDS, with a focus on the risks of untreated EDS, common etiologies of the condition, as well as a brief description of screening and treatment strategies.
EDS vs fatigue
Many patients describe EDS as “fatigue”1; however, a patient’s report of fatigue could be mistaken for EDS.4 Although there is overlap, it is important for physicians to distinguish between these 2 entities for accurate identification and treatment.1,4
Risk of inadequate screening
A study of 117 patients with symptomatic coronary artery disease showed that EDS is associated with significantly greater incidence of cardiovascular adverse events at 16-month follow up.2 This study had limitations such as small sample size; therefore, more studies are needed. Because of these risks, timely and accurate diagnosis not only improves the patient’s quality of life and reduces polypharmacy but also can be life-saving.
Common causes of EDS in psychiatric patients
Because of the high prevalence and severity of impairments caused by EDS, it is essential for psychiatrists to be informed about causes of EDS and thoroughly assess for the potential underlying etiology before concluding that the sleep problem is a manifestation of the psychiatric disorder and prescribing psychotropic medication for it.
Some common causes of EDS in psychiatric patients include:
Sleep-disordered breathing.8 Obstructive sleep apnea (OSA) is often underdiagnosed,6,7 and considering how common it is,6 psychiatrists likely will see many patients with OSA in their practice.5 OSA has a higher prevalence among patients with psychiatric disorders such as depression6,9 and schizophrenia. Additionally, there is evidence suggesting that patients with OSA are more likely to suffer from depression and EDS than healthy controls6,9,10; some of the proposed mechanisms are sleep fragmentation and hypoxemia.6,9-11 OSA is the most common form of sleep-disordered breathing and is a common cause of EDS.1,2,12 Also, undiagnosed and untreated OSA in patients with depression could cause refractoriness to pharmacological treatment of depression.6,9,10
When unrecognized and untreated, OSA can be life-threatening. Despite this, OSA is not regularly screened for in clinical psychiatric practice.6,10 Therefore, it is imperative that psychiatrists be well-acquainted with measures to identify at-risk patients and refer to a sleep specialist when appropriate.
OSA is accompanied by irritability, cognitive difficulties, and poor sleep, creating an overlap with symptoms of depressive disorders.6,10 Use of sedative hypnotic medications, such as benzodiazepines, which further reduces muscle tone in the airway and suppresses respiratory effort, can worsen OSA symptoms5,6,10 and pose cerebrovascular, cardiovascular, and potentially life-threatening risks, and therefore is not indicated in this population.9,13
Obesity is a risk factor for OSA.6 Patients with mood disorders or schizophrenia or other psychotic disorders are at higher risk of obesity because of psychotropic-induced weight gain, stress-induced mechanisms, and/or lower levels of self-care. When these patients have unrecognized or untreated OSA and are prescribed sedative medications at night or stimulant medications during the day, they could be at increased cardiac or respiratory risks without resolving their underlying condition. A diligent psychiatrist can dramatically reduce the risks by referring a patient for nocturnal polysomnography,1 helping the patient implement lifestyle modifications (eg, exercise, weight loss, and healthy nutrition), prescribing judiciously, and monitoring closely for such risks. An accurate diagnosis of and treatment for OSA can improve sleep6 dramatically and help depressive symptoms through better sleep, more daytime energy and concentration, and adequate oxygenation of the brain while sleeping.
Psychiatrists can screen for OSA using the STOP-Bang (Snoring, Tired, Observed apnea, Pressure, Body mass index, Age, Neck circumference, Gender) Questionnaire, which is a quick, 8-item screening scale that helps to categorize OSA risk as mild, moderate, or severe.12 Hypertension, snoring, and/or gasping for breath (“observed apnea”)—a history which often is provided by spouses or significant others—daytime dozing and/or tiredness, having a large neck circumference or volume, body mass index, male sex, and age are items on the STOP-Bang Questionnaire and also are features that should raise high clinical suspicion of OSA.12 Referral for nocturnal polysomnography in at-risk patients should be the next step1,5 in any sleep-related breathing disorder.
Treatment for OSA involves continuous positive airway pressure (CPAP) therapy, which has been shown to relieve OSA and decrease related EDS.5,6 Other treatment modalities, such as oral appliances and surgery, may be used5 in some cases, but more studies are needed for conclusive results.
Several studies have shown improved depression, mood, and cognition after administering treatment such as CPAP6,9,14 in patients with OSA and depression. Considering the significant risks of cardiovascular,8 cerebrovascular,8 and overall morbidity and mortality associated with untreated OSA,12 it is important to routinely screen for sleep-disordered breathing in patients with depression9 or other psychiatric disorders and refer for specialized sleep evaluation and treatment, when indicated.
Medications. EDS can result from some prescription and over-the-counter medications.1,2,5,7 Sedating antidepressants, antihistamines, antipsychotics, anticonvulsants,1,8 and beta blockers2 could cause sedation, which can persist during daytime, although a few studies did not find an association between antipsychotic use and EDS.3 Benzodiazepines and other sedative-hypnotics,1,7 especially long-acting agents or higher dosages,5 can lead to EDS and decreased alertness. Non-psychotropics, such as opioid pain medications,1,7 antitussives, and skeletal muscle relaxants, also can contribute to or cause daytime sedation.7 When using these agents, psychiatrists should monitor and routinely assess patients while aiming for the lowest effective dosage when feasible.
This strategy creates a framework for psychiatrists to routinely educate patients about these commonly encountered side effects, reduce polypharmacy when possible, and help patients effectively manage or prevent these adverse effects.
Depression.1 Some studies found >45% patients with depression had EDS.3,13,15 Besides an association between depression and EDS,13,16 Chellappa and Araújo13 also found a significant association between EDS and suicidal ideation. The causes of EDS in patients with depression may be varied, ranging from restless legs syndrome, residual depressive symptoms,15 to OSA. Depression is often comorbid with OSA,6 with up to 20% of patients with depression suffering from OSA,10 creating higher risk for EDS. Depressive disorders are routinely assessed during an evaluation of OSA at sleep centers, but OSA often is not screened in psychiatric practice.10
There is a strong need for regular screening for OSA in patients with depression, particularly because most studies show a link between the 2 conditions.10 Both depression and OSA have some common risk factors, such as obesity, hypertension, and metabolic syndrome.10 Patients with these conditions are at greater risk for OSA, and therefore a psychiatrist should proactively screen and refer such patients for nocturnal polysomnography when they suspect OSA. Patients with OSA and depression often present to the psychiatrist with depressive symptoms that appear to be resistant to pharmacological treatment,10 therefore underscoring the importance of screening and ruling out OSA in patients with depression.
Circadian rhythm disorders, restless legs syndrome, alcohol and other substance use, and use of prescription sedative-hypnotics are more common in patients with depression; therefore, this population is at high risk for EDS.
Circadian rhythm disorders and insufficient sleep syndrome. Insufficient sleep syndrome1,2,8 frequently causes EDS and occurs more commonly in busy people who try to get by with less sleep.8 Over time, the effect of sleep loss is cumulative and can be accompanied by mood symptoms, such as irritability, fatigue, and problems with concentration.8 Shift workers1,8 commonly experience insufficient sleep as well as circadian rhythm disorders and EDS. Modafinil is FDA-approved for EDS in shift work sleep disorder.
Geriatric patients may experience advanced sleep phase syndrome involving early awakenings.8 Adolescents, on the other hand, often suffer from delayed sleep phase syndrome, which is a type of circadian rhythm disorder, related to increasing academic and social pressures, natural pubertal shift to later sleep onset, pervading technology use, and often nebulous bedtime routines. This can be a cause of sleep persisting into daytime.8 Taking a careful history and a sleep diary may be useful because this disorder might be confused for insomnia. Treatment involves gradual shifting of the time of sleep onset through bright light exposure and other modalities.8
Adolescents might not be forthcoming about the severity of their sleep problems; therefore, psychiatrists should screen proactively through clinical interviews of patients and parents and consider this possibility when encountering an adolescent with recent-onset attention or cognitive difficulties.
Treatment for circadian rhythm disorders usually includes planned or prescribed sleep scheduling, timed light exposure,8 and occasional use of melatonin or other sedative agents.17
Hypersomnia of central origin, which includes narcolepsy, idiopathic hypersomnia, and recurrent hypersomnia, can present with EDS.1,18,19 Narcolepsy is a rare, debilitating sleep disorder that manifests as EDS or sleep attacks, with or without cataplexy, and sleep paralysis.5,8,18,19 The Multiple Sleep Latency Test and polysomnography are used for diagnosis.1,5 Shortened REM latency is a classic finding often noted on polysomnography. Treatment involves pharmacologic and behavioral strategies and education.5,8 Modafinil is FDA-approved for EDS associated with narcolepsy. Stimulant medications have been used for narcolepsy in the past; further studies are needed to establish benefit–risk ratio of use in this population.18
Kleine-Levin syndrome is a form of recurrent hypersomnia, a less common sleep disorder, characterized by episodes of excessive sleepiness accompanied by hyperphagia and hypersexuality.5,18,19
Other medical conditions,1 such as the rare familial fatal insomnia, neurological conditions1 such as encephalitis,8 epilepsy,8 Alzheimer’s disease or other types of dementia,8 Parkinson’s disease,1 or multiple sclerosis,1,18 can cause excessive daytime fatigue by causing secondary insomnia or hypersomnia.
Treating the underlying disorder is an important first step in these cases. In addition, coordinating with neurologists or other specialists involved in caring for patients with these conditions is important. Regularly reviewing and simplifying the often complex medication regimen, when possible, can go a long way in mitigating EDS in this population.
Other disorders affecting sleep. Restless legs syndrome and periodic limb movement disorder are other causes of EDS.3 Treatment involves lifestyle modifications, iron supplementation in certain patients, and use of dopaminergic agents such as ropinirole, pramipexole, and other medications, depending on severity of the condition, comorbidities, and other factors.20
Alcohol or substance use. Substance use or withdrawal can be associated with sleep disorders, such as hypersomnia,19 insomnia,19 and related EDS.5 For example, alcohol use disorder affects REM sleep, and can cause EDS. Secondary central apnea can be the result of long-standing opioid use19 and can present like EDS.
Insomnia. Primary insomnia rarely causes EDS.5 Insomnia due to a medical or psychiatric condition may be an indirect cause of EDS by causing sleep deprivation.
Steps for timely and accurate diagnosis
Utilize the following steps for facilitating timely diagnosis and treatment of EDS:
Thorough history. Patients often describe “tiredness” instead of sleepiness.8 Therefore, the astute psychiatrist should explore further when patients are presenting with this concern, especially by asking more specific questions such as the tendency to doze off during daytime.8
Family members can be vital sources for obtaining a complete history,5 especially because patients might deny,8 minimize, or not be fully aware1 of the extent of their symptoms. Asking family members about patient’s snoring, irregular breathing, or gasping at night can be particularly valuable.5 Obtaining a family history of sleep disorders can be particularly important, especially in conditions such as OSA and narcolepsy.
Asking about any history of safety issues,8 including sleepiness during driving, cooking, or other activities, is also important.
Use of scales and other screening measures. Psychiatrists can use initial screening measures in the office setting. Epworth Sleepiness Scale15,21 is a validated,2 short, self-administered measure to assess the level of daytime sleepiness; however, it has some limitations such as not being able to measure changes in sleepiness from hour to hour or day to day. Because of its limitations, the Epworth Sleepiness Scale should not be used by itself as a diagnostic tool.3 It has been commonly used for detecting OSA2 and narcolepsy. The Stanford Sleepiness Scale is a self-rating scale that measures the subjective degree of sleepiness and alertness; it has limitations as well, such as having little correlation with chronic sleep loss.8 Other tools such as visual analogue scales also could be helpful.8 For more specialized testing, such as Multiple Sleep Latency Test or polysomnography, referral to a sleep specialist is ideal.8
Education. The assessment is an opportunity for the psychiatrist to educate patients about sleep hygiene, the importance of regular bedtimes, and getting adequate sleep to avoid accumulating a sleep deficit.
Urgent referral of at-risk populations. Prompt or urgent referral of at-risk populations, such as geriatric patients or those with a history of dozing off during driving, is invaluable in preventing morbidity and mortality from untreated sleep disorders.
Patients with severe daytime sleepiness should be advised to not drive or operate heavy machinery until this condition is adequately controlled.18
Bottom Line
Excessive daytime sleepiness (EDS) is “the inability to maintain wakefulness and alertness during the major waking periods of the day, with sleep occurring unintentionally or at inappropriate times, almost daily for at least 3 months,” according to the American Academy of Sleep Medicine.1 EDS is common, with a prevalence up to 25% to 30% in the general population.1-4 The prevalence rate varies in different studies, primarily because of inconsistent definitions of EDS, and therefore differences in diagnosis and assessment.1,2,4 In a study of 300 psychiatric outpatients, 34% had EDS.3 However, studies and evidence reviewing EDS in psychiatric patients are limited.
The causes of EDS are many and varied,1,8 including medical and psychiatric etiologies. A thorough history, screening at-risk patients, and timely sleep center referral are vital to detect and appropriately manage the cause of EDS.5
This article reviews the literature on EDS, with a focus on the risks of untreated EDS, common etiologies of the condition, as well as a brief description of screening and treatment strategies.
EDS vs fatigue
Many patients describe EDS as “fatigue”1; however, a patient’s report of fatigue could be mistaken for EDS.4 Although there is overlap, it is important for physicians to distinguish between these 2 entities for accurate identification and treatment.1,4
Risk of inadequate screening
A study of 117 patients with symptomatic coronary artery disease showed that EDS is associated with significantly greater incidence of cardiovascular adverse events at 16-month follow up.2 This study had limitations such as small sample size; therefore, more studies are needed. Because of these risks, timely and accurate diagnosis not only improves the patient’s quality of life and reduces polypharmacy but also can be life-saving.
Common causes of EDS in psychiatric patients
Because of the high prevalence and severity of impairments caused by EDS, it is essential for psychiatrists to be informed about causes of EDS and thoroughly assess for the potential underlying etiology before concluding that the sleep problem is a manifestation of the psychiatric disorder and prescribing psychotropic medication for it.
Some common causes of EDS in psychiatric patients include:
Sleep-disordered breathing.8 Obstructive sleep apnea (OSA) is often underdiagnosed,6,7 and considering how common it is,6 psychiatrists likely will see many patients with OSA in their practice.5 OSA has a higher prevalence among patients with psychiatric disorders such as depression6,9 and schizophrenia. Additionally, there is evidence suggesting that patients with OSA are more likely to suffer from depression and EDS than healthy controls6,9,10; some of the proposed mechanisms are sleep fragmentation and hypoxemia.6,9-11 OSA is the most common form of sleep-disordered breathing and is a common cause of EDS.1,2,12 Also, undiagnosed and untreated OSA in patients with depression could cause refractoriness to pharmacological treatment of depression.6,9,10
When unrecognized and untreated, OSA can be life-threatening. Despite this, OSA is not regularly screened for in clinical psychiatric practice.6,10 Therefore, it is imperative that psychiatrists be well-acquainted with measures to identify at-risk patients and refer to a sleep specialist when appropriate.
OSA is accompanied by irritability, cognitive difficulties, and poor sleep, creating an overlap with symptoms of depressive disorders.6,10 Use of sedative hypnotic medications, such as benzodiazepines, which further reduces muscle tone in the airway and suppresses respiratory effort, can worsen OSA symptoms5,6,10 and pose cerebrovascular, cardiovascular, and potentially life-threatening risks, and therefore is not indicated in this population.9,13
Obesity is a risk factor for OSA.6 Patients with mood disorders or schizophrenia or other psychotic disorders are at higher risk of obesity because of psychotropic-induced weight gain, stress-induced mechanisms, and/or lower levels of self-care. When these patients have unrecognized or untreated OSA and are prescribed sedative medications at night or stimulant medications during the day, they could be at increased cardiac or respiratory risks without resolving their underlying condition. A diligent psychiatrist can dramatically reduce the risks by referring a patient for nocturnal polysomnography,1 helping the patient implement lifestyle modifications (eg, exercise, weight loss, and healthy nutrition), prescribing judiciously, and monitoring closely for such risks. An accurate diagnosis of and treatment for OSA can improve sleep6 dramatically and help depressive symptoms through better sleep, more daytime energy and concentration, and adequate oxygenation of the brain while sleeping.
Psychiatrists can screen for OSA using the STOP-Bang (Snoring, Tired, Observed apnea, Pressure, Body mass index, Age, Neck circumference, Gender) Questionnaire, which is a quick, 8-item screening scale that helps to categorize OSA risk as mild, moderate, or severe.12 Hypertension, snoring, and/or gasping for breath (“observed apnea”)—a history which often is provided by spouses or significant others—daytime dozing and/or tiredness, having a large neck circumference or volume, body mass index, male sex, and age are items on the STOP-Bang Questionnaire and also are features that should raise high clinical suspicion of OSA.12 Referral for nocturnal polysomnography in at-risk patients should be the next step1,5 in any sleep-related breathing disorder.
Treatment for OSA involves continuous positive airway pressure (CPAP) therapy, which has been shown to relieve OSA and decrease related EDS.5,6 Other treatment modalities, such as oral appliances and surgery, may be used5 in some cases, but more studies are needed for conclusive results.
Several studies have shown improved depression, mood, and cognition after administering treatment such as CPAP6,9,14 in patients with OSA and depression. Considering the significant risks of cardiovascular,8 cerebrovascular,8 and overall morbidity and mortality associated with untreated OSA,12 it is important to routinely screen for sleep-disordered breathing in patients with depression9 or other psychiatric disorders and refer for specialized sleep evaluation and treatment, when indicated.
Medications. EDS can result from some prescription and over-the-counter medications.1,2,5,7 Sedating antidepressants, antihistamines, antipsychotics, anticonvulsants,1,8 and beta blockers2 could cause sedation, which can persist during daytime, although a few studies did not find an association between antipsychotic use and EDS.3 Benzodiazepines and other sedative-hypnotics,1,7 especially long-acting agents or higher dosages,5 can lead to EDS and decreased alertness. Non-psychotropics, such as opioid pain medications,1,7 antitussives, and skeletal muscle relaxants, also can contribute to or cause daytime sedation.7 When using these agents, psychiatrists should monitor and routinely assess patients while aiming for the lowest effective dosage when feasible.
This strategy creates a framework for psychiatrists to routinely educate patients about these commonly encountered side effects, reduce polypharmacy when possible, and help patients effectively manage or prevent these adverse effects.
Depression.1 Some studies found >45% patients with depression had EDS.3,13,15 Besides an association between depression and EDS,13,16 Chellappa and Araújo13 also found a significant association between EDS and suicidal ideation. The causes of EDS in patients with depression may be varied, ranging from restless legs syndrome, residual depressive symptoms,15 to OSA. Depression is often comorbid with OSA,6 with up to 20% of patients with depression suffering from OSA,10 creating higher risk for EDS. Depressive disorders are routinely assessed during an evaluation of OSA at sleep centers, but OSA often is not screened in psychiatric practice.10
There is a strong need for regular screening for OSA in patients with depression, particularly because most studies show a link between the 2 conditions.10 Both depression and OSA have some common risk factors, such as obesity, hypertension, and metabolic syndrome.10 Patients with these conditions are at greater risk for OSA, and therefore a psychiatrist should proactively screen and refer such patients for nocturnal polysomnography when they suspect OSA. Patients with OSA and depression often present to the psychiatrist with depressive symptoms that appear to be resistant to pharmacological treatment,10 therefore underscoring the importance of screening and ruling out OSA in patients with depression.
Circadian rhythm disorders, restless legs syndrome, alcohol and other substance use, and use of prescription sedative-hypnotics are more common in patients with depression; therefore, this population is at high risk for EDS.
Circadian rhythm disorders and insufficient sleep syndrome. Insufficient sleep syndrome1,2,8 frequently causes EDS and occurs more commonly in busy people who try to get by with less sleep.8 Over time, the effect of sleep loss is cumulative and can be accompanied by mood symptoms, such as irritability, fatigue, and problems with concentration.8 Shift workers1,8 commonly experience insufficient sleep as well as circadian rhythm disorders and EDS. Modafinil is FDA-approved for EDS in shift work sleep disorder.
Geriatric patients may experience advanced sleep phase syndrome involving early awakenings.8 Adolescents, on the other hand, often suffer from delayed sleep phase syndrome, which is a type of circadian rhythm disorder, related to increasing academic and social pressures, natural pubertal shift to later sleep onset, pervading technology use, and often nebulous bedtime routines. This can be a cause of sleep persisting into daytime.8 Taking a careful history and a sleep diary may be useful because this disorder might be confused for insomnia. Treatment involves gradual shifting of the time of sleep onset through bright light exposure and other modalities.8
Adolescents might not be forthcoming about the severity of their sleep problems; therefore, psychiatrists should screen proactively through clinical interviews of patients and parents and consider this possibility when encountering an adolescent with recent-onset attention or cognitive difficulties.
Treatment for circadian rhythm disorders usually includes planned or prescribed sleep scheduling, timed light exposure,8 and occasional use of melatonin or other sedative agents.17
Hypersomnia of central origin, which includes narcolepsy, idiopathic hypersomnia, and recurrent hypersomnia, can present with EDS.1,18,19 Narcolepsy is a rare, debilitating sleep disorder that manifests as EDS or sleep attacks, with or without cataplexy, and sleep paralysis.5,8,18,19 The Multiple Sleep Latency Test and polysomnography are used for diagnosis.1,5 Shortened REM latency is a classic finding often noted on polysomnography. Treatment involves pharmacologic and behavioral strategies and education.5,8 Modafinil is FDA-approved for EDS associated with narcolepsy. Stimulant medications have been used for narcolepsy in the past; further studies are needed to establish benefit–risk ratio of use in this population.18
Kleine-Levin syndrome is a form of recurrent hypersomnia, a less common sleep disorder, characterized by episodes of excessive sleepiness accompanied by hyperphagia and hypersexuality.5,18,19
Other medical conditions,1 such as the rare familial fatal insomnia, neurological conditions1 such as encephalitis,8 epilepsy,8 Alzheimer’s disease or other types of dementia,8 Parkinson’s disease,1 or multiple sclerosis,1,18 can cause excessive daytime fatigue by causing secondary insomnia or hypersomnia.
Treating the underlying disorder is an important first step in these cases. In addition, coordinating with neurologists or other specialists involved in caring for patients with these conditions is important. Regularly reviewing and simplifying the often complex medication regimen, when possible, can go a long way in mitigating EDS in this population.
Other disorders affecting sleep. Restless legs syndrome and periodic limb movement disorder are other causes of EDS.3 Treatment involves lifestyle modifications, iron supplementation in certain patients, and use of dopaminergic agents such as ropinirole, pramipexole, and other medications, depending on severity of the condition, comorbidities, and other factors.20
Alcohol or substance use. Substance use or withdrawal can be associated with sleep disorders, such as hypersomnia,19 insomnia,19 and related EDS.5 For example, alcohol use disorder affects REM sleep, and can cause EDS. Secondary central apnea can be the result of long-standing opioid use19 and can present like EDS.
Insomnia. Primary insomnia rarely causes EDS.5 Insomnia due to a medical or psychiatric condition may be an indirect cause of EDS by causing sleep deprivation.
Steps for timely and accurate diagnosis
Utilize the following steps for facilitating timely diagnosis and treatment of EDS:
Thorough history. Patients often describe “tiredness” instead of sleepiness.8 Therefore, the astute psychiatrist should explore further when patients are presenting with this concern, especially by asking more specific questions such as the tendency to doze off during daytime.8
Family members can be vital sources for obtaining a complete history,5 especially because patients might deny,8 minimize, or not be fully aware1 of the extent of their symptoms. Asking family members about patient’s snoring, irregular breathing, or gasping at night can be particularly valuable.5 Obtaining a family history of sleep disorders can be particularly important, especially in conditions such as OSA and narcolepsy.
Asking about any history of safety issues,8 including sleepiness during driving, cooking, or other activities, is also important.
Use of scales and other screening measures. Psychiatrists can use initial screening measures in the office setting. Epworth Sleepiness Scale15,21 is a validated,2 short, self-administered measure to assess the level of daytime sleepiness; however, it has some limitations such as not being able to measure changes in sleepiness from hour to hour or day to day. Because of its limitations, the Epworth Sleepiness Scale should not be used by itself as a diagnostic tool.3 It has been commonly used for detecting OSA2 and narcolepsy. The Stanford Sleepiness Scale is a self-rating scale that measures the subjective degree of sleepiness and alertness; it has limitations as well, such as having little correlation with chronic sleep loss.8 Other tools such as visual analogue scales also could be helpful.8 For more specialized testing, such as Multiple Sleep Latency Test or polysomnography, referral to a sleep specialist is ideal.8
Education. The assessment is an opportunity for the psychiatrist to educate patients about sleep hygiene, the importance of regular bedtimes, and getting adequate sleep to avoid accumulating a sleep deficit.
Urgent referral of at-risk populations. Prompt or urgent referral of at-risk populations, such as geriatric patients or those with a history of dozing off during driving, is invaluable in preventing morbidity and mortality from untreated sleep disorders.
Patients with severe daytime sleepiness should be advised to not drive or operate heavy machinery until this condition is adequately controlled.18
Bottom Line
1. Chervin RD. Approach to the patient with excessive daytime sleepiness. http://www.uptodate.com/contents/approach-to-the-patient-with-excessive-daytime-sleepiness. Updated January 2016. Accessed June 5, 2017.
2. Lee CH, Ng WY, Hau W, et al. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9(12):1267-1272.
3. Hawley CJ, Gale TM, Sivakumaran T, et al. Excessive daytime sleepiness in psychiatric disorders: prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1-2):138-141.
4. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54(1):61-69.
5. Krahn LE. Excessive daytime sleepiness: diagnosing the causes. Current Psychiatry. 2002;1(1):49-57.
6. Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17-25.
7. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
8. Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124(pt 8):1482-1491.
9. Cheng P, Casement M, Chen CF, et al. Sleep disordered breathing in major depressive disorder. J Sleep Res. 2013;22(4):459-462.
10. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.
11. Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47(6):583-596.
12. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638.
13. Chellappa SL, Araújo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28(2):126-129.
14. Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11(6):552-557.
15. Lundt L. Use of the Epworth Sleepiness Scale to evaluate the symptom of excessive sleepiness in major depressive disorder. Gen Hosp Psychiatry. 2005;27(2):146-148.
16. Hawley CJ. Excessive daytime sleepiness in psychiatry: a relevant focus for clinical attention and treatment? Int J Psychiatry Clin Pract. 2006;10(2):117-123.
17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701-715.
18. Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711.
19. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9(4):687-701.
20. National Institute of Neurological Disorders and Stroke. Restless legs syndrome information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Restless-Legs-Syndrome-Information-Page. Accessed June 2, 2017.
21. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381.
1. Chervin RD. Approach to the patient with excessive daytime sleepiness. http://www.uptodate.com/contents/approach-to-the-patient-with-excessive-daytime-sleepiness. Updated January 2016. Accessed June 5, 2017.
2. Lee CH, Ng WY, Hau W, et al. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9(12):1267-1272.
3. Hawley CJ, Gale TM, Sivakumaran T, et al. Excessive daytime sleepiness in psychiatric disorders: prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1-2):138-141.
4. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54(1):61-69.
5. Krahn LE. Excessive daytime sleepiness: diagnosing the causes. Current Psychiatry. 2002;1(1):49-57.
6. Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17-25.
7. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
8. Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124(pt 8):1482-1491.
9. Cheng P, Casement M, Chen CF, et al. Sleep disordered breathing in major depressive disorder. J Sleep Res. 2013;22(4):459-462.
10. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.
11. Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47(6):583-596.
12. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638.
13. Chellappa SL, Araújo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28(2):126-129.
14. Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11(6):552-557.
15. Lundt L. Use of the Epworth Sleepiness Scale to evaluate the symptom of excessive sleepiness in major depressive disorder. Gen Hosp Psychiatry. 2005;27(2):146-148.
16. Hawley CJ. Excessive daytime sleepiness in psychiatry: a relevant focus for clinical attention and treatment? Int J Psychiatry Clin Pract. 2006;10(2):117-123.
17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701-715.
18. Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711.
19. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9(4):687-701.
20. National Institute of Neurological Disorders and Stroke. Restless legs syndrome information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Restless-Legs-Syndrome-Information-Page. Accessed June 2, 2017.
21. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381.
Nutraceuticals for traumatic brain injury: Should you recommend their use?
Traumatic brain injury (TBI) affects more than 2 million people in the United States each year.1 TBI can trigger a cascade of secondary injury mechanisms, such as inflammation, hypoxic/ischemic injury, excitotoxicity, and oxidative stress,2 that could contribute to cognitive and behavioral changes. Although neuropsychiatric symptoms might not be obvious after a TBI, they have a high prevalence in these patients, can last long term, and may be difficult to treat.3 Despite research advances in understanding the biological basis of TBI and identifying potential therapeutic targets, treatment options for individuals with TBI remain limited.
As a result, clinicians have turned to alternative treatments for TBI, including nutraceuticals. In this article, we will:
- provide an overview of nutraceuticals used in treating TBI, first exploring outcomes soon after TBI, then concentrating on neuropsychiatric outcomes
- evaluate the existing evidence, including recommended dietary allowances (Table 1)4,5 and side effects (Table 2)
- review recommendations for their clinical use.
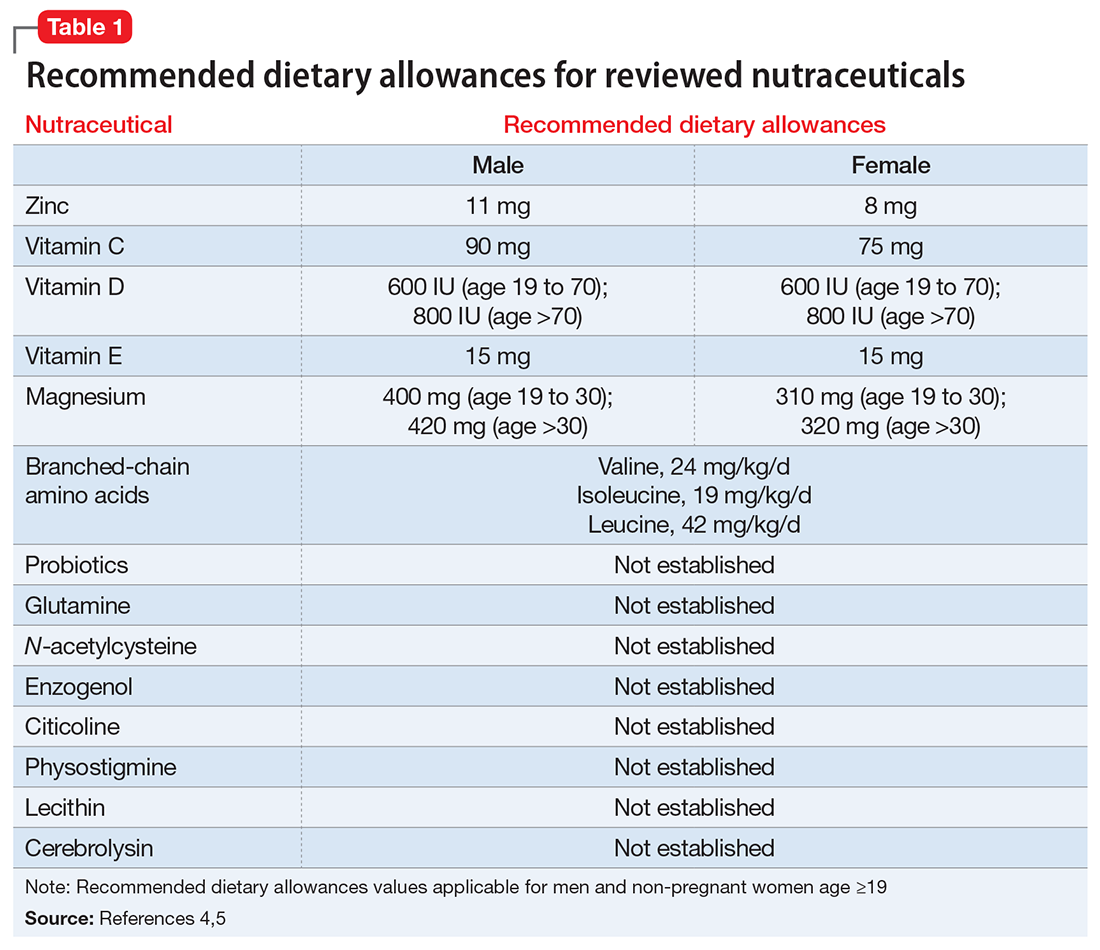
Pharmacologic approaches are limited
Nutraceuticals have gained attention for managing TBI-associated neuropsychiatric disorders because of the limited evidence supporting current approaches. Existing strategies encompass pharmacologic and non-pharmacologic interventions, psychoeducation, supportive and behavioral psychotherapies, and cognitive rehabilitation.6
Many pharmacologic options exist for specific neurobehavioral symptoms, but the evidence for their use is based on small studies, case reports, and knowledge extrapolated from their use in idiopathic psychiatric disorders.7,8 No FDA-approved drugs have been effective for treating neuropsychiatric disturbances after a TBI. Off-label use of antidepressants, anticonvulsants, dopaminergic agents, and cholinesterase inhibitors in TBI has been associated with inadequate clinical response and/or intolerable side effects.9,10
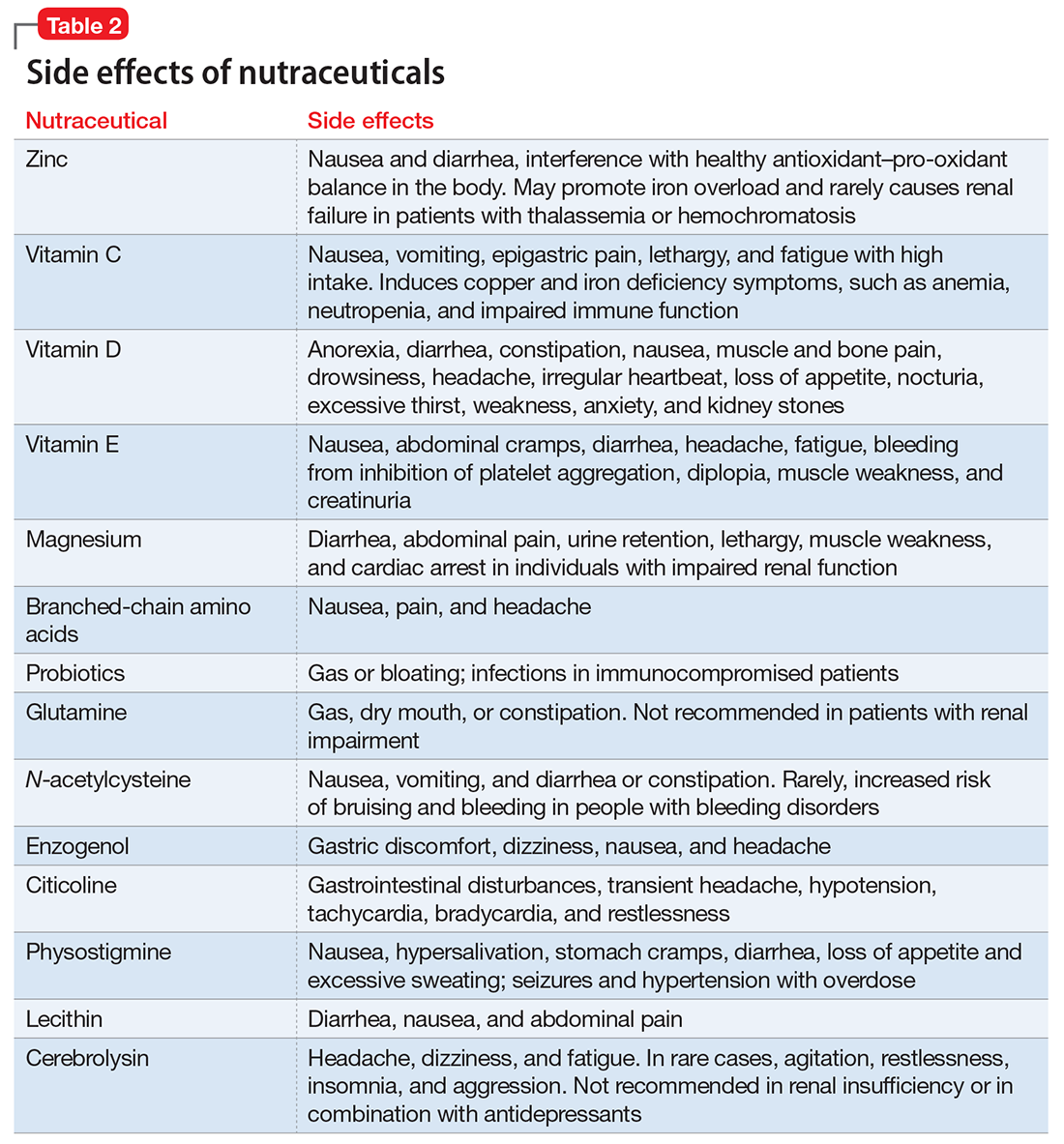
What are nutraceuticals?
DeFelice11 introduced the term “nutraceutical” to refer to “any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease.” The term has been expanded to include dietary supplements, such as vitamins, minerals, amino acids, herbal or other botanicals, and food products that provide health benefits beyond what they normally provide in food form. The FDA does not regulate the marketing or manufacturing of nutraceuticals; therefore, their bioavailability and metabolism can vary.
Despite their widespread use, the evidence supporting the efficacy of nutraceuticals for patients with TBI is limited. Their effects might vary by population and depend on dose, timing, TBI severity, and whether taken alone or in combination with other nutraceutical or pharmaceutical agents. Fourteen randomized controlled trials (RCTs) have addressed the use of nutraceuticals in TBI (Table 3), but further research is needed to clarify for which conditions they provide maximum benefit.
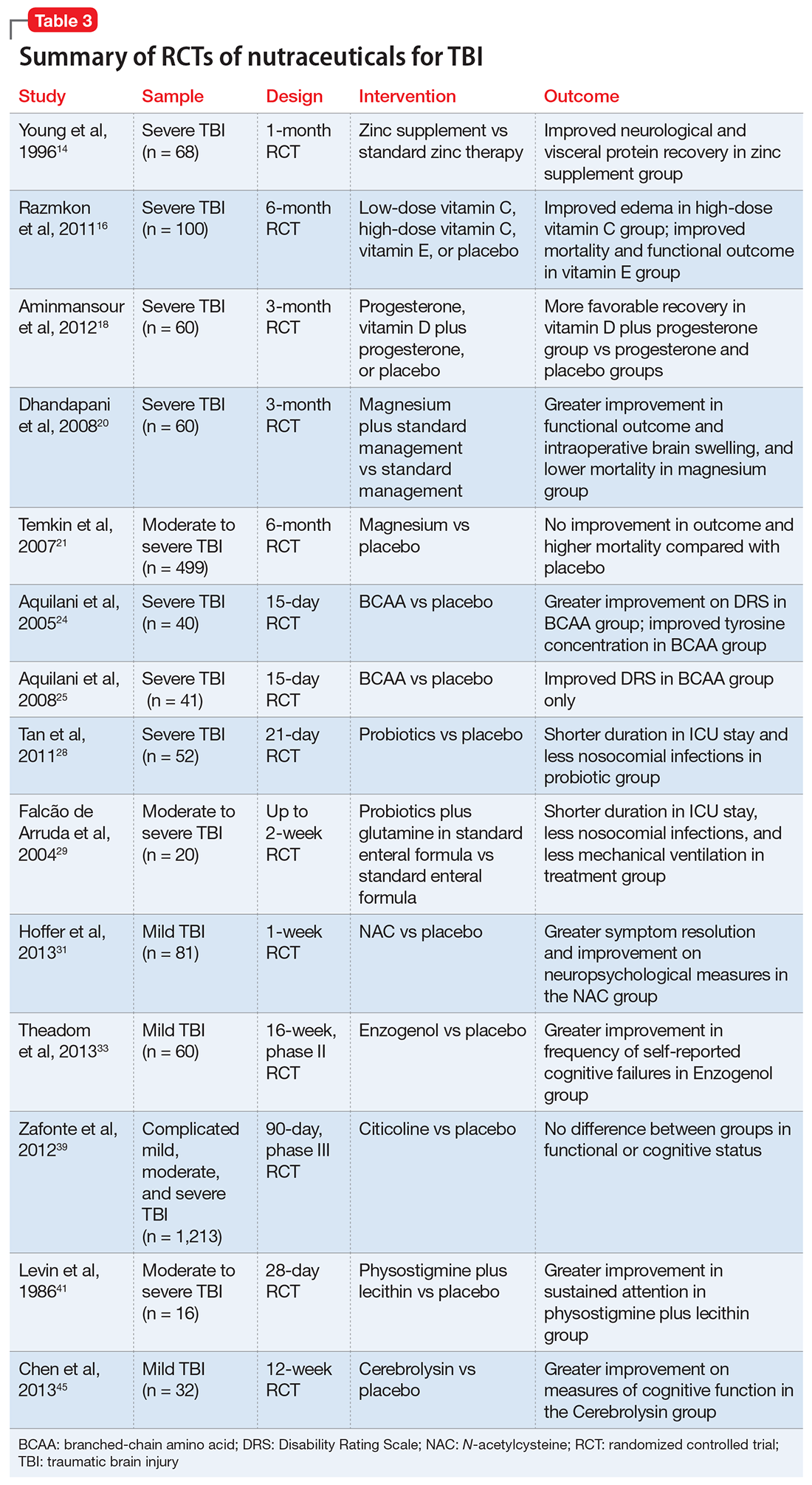
Nutraceuticals and their potential use in TBI
Zinc is considered essential for optimal CNS functioning. Patients with TBI might be at risk for zinc deficiency, which has been associated with increased cell death and behavioral deficits.12,13 A randomized, prospective, double-blinded controlled trial examined the effects of supplemental zinc administration (12 mg for 15 days) compared with standard zinc therapy (2.5 mg for 15 days) over 1 month in 68 adults with acute severe closed head injury.14 The supplemental zinc group showed improved visceral protein levels, lower mortality, and more favorable neurologic recovery based on higher adjusted mean Glasgow Coma Scale score on day 28 and mean motor score on days 15 and 21.
Rodent studies have shown that zinc supplementation could reduce deficits in spatial learning and memory and depression-like behaviors and help decrease stress and anxiety,12 although no human clinical trials have been conducted. Despite the potential neuroprotective effects of zinc supplementation, evidence exists that endogenous zinc release and accumulation following TBI can trigger cellular changes that result in neuronal death.13
Vitamins C and E. Oxidative damage is believed to play a significant role in secondary injury in TBI, so research has focused on the role of antioxidants, such as vitamins C and E, to promote post-TBI recovery.15 One RCT16 of 100 adults with acute severe head injury reported that vitamin E administration was associated with reduced mortality and lower Glasgow Outcome Scale (GOS) scores, and vitamin C was associated with stabilized or reduced perilesional edema/infarct on CT scan.
Vitamin D. An animal study reported that vitamin D supplementation can help reduce inflammation, oxidative stress, and cell death in TBI, and that vitamin D deficiency has been associated with increased inflammation and behavioral deficits.17 Further evidence suggests that vitamin D may have a synergistic effect when used in combination with the hormone progesterone. A RCT of 60 patients with severe TBI reported that 60% of those who received progesterone plus vitamin D had GOS scores of 4 (good recovery) or 5 (moderate disability) vs 45% receiving progesterone alone or 25% receiving placebo.18
Magnesium, one of the most widely used nutraceuticals, is considered essential for CNS functioning, including the regulation of N-methyl-
A RCT evaluated the safety and efficacy of magnesium supplementation in 60 patients with severe closed TBI, with one-half randomized to standard care and the other also receiving magnesium sulfate (MgSO4; initiation dose of 4 g IV and 10 g IM, continuation dose of 5 g IM every 4 hours for 24 hours).20 After 3 months, more patients in the MgSO4 group had higher GOS scores than controls (73.3% vs 40%), lower 1-month mortality rates (13.3% vs 43.3%), and lower rates of intraoperative brain swelling (29.4% vs 73.3%).
However, a larger RCT of 499 patients with moderate or severe TBI randomized to high-dose (1.25 to 2.5 mmol/L) or low-dose (1.0 to 1.85 mmol/L) IV MgSO4 or placebo provided conflicting results.21 Participants received MgSO4 8 hours after injury and continued for 5 days. After 6 months, patients in the high-dose MgSO4 and placebo groups had similar composite primary outcome measures (eg, seizures, neuropsychological measures, functional status measures), although the high-dose group had a higher mortality rate than the placebo group. Patients who received low-dose MgSO4 showed worse outcomes than those assigned to placebo.
Amino acids. Branched-chain amino acids (BCAAs), including valine, isoleucine, and leucine, are essential in protein and neurotransmitter synthesis. Reduced levels of endogenous BCAAs have been reported in patients with mild or severe TBI.22 Preclinical studies suggest that BCAAs can improve hippocampal-dependent cognitive functioning following TBI.23
Two RCTs of BCAAs have been conducted in humans. One study randomized 40 men with severe TBI to IV BCAAs or placebo.24 After 15 days, the BCAA group showed greater improvement in Disability Rating Scale scores. The study also found that supplementation increased total BCAA levels without negatively affecting plasma levels of neurotransmitter precursors tyrosine and tryptophan. A second study found that 41 patients in a vegetative or minimally conscious state who received BCAA supplementation for 15 days had higher Disability Rating Scale scores than those receiving placebo.25
Probiotics and glutamine. Probiotics are non-pathogenic microorganisms that have been shown to modulate the host’s immune system.26 TBI is associated with immunological changes, including a shift from T-helper type 1 (TH1) cells to T-helper type 2 (TH2) cells that increase susceptibility to infection.27
A RCT of 52 patients with severe TBI suggested a correlation between probiotic administration-modulated cytokine levels and TH1/TH2 balance.28 A 3-times daily probiotic mix of Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for 21 days led to shorter average ICU stays (6.8 vs 10.7 days, P = .034) and a decrease in nosocomial infections (34.6% vs 57.7%, P = .095) vs placebo, although the latter difference was not statistically significant.28
A prospective RCT of 20 patients with brain injury29 found a similar impact of early enteral nutrition supplemented with Lactobacillus johnsonii and glutamine, 30 g, vs a standard enteral nutrition formula. The treatment group experienced fewer nosocomial infections (50% vs 100%, P = .03), shorter ICU stays (10 vs 22 days, P < .01), and fewer days on mechanical ventilation (7 vs 14, P = .04). Despite these studies, evidence for the use of glutamine in patients with TBI is scarce and inconclusive.
N-acetylcysteine (NAC) comes from the amino acid L-cysteine. NAC is an effective scavenger of free radicals and improves cerebral microcirculatory blood flow and tissue oxygenation.30 A randomized, double-blind, placebo-controlled study of oral NAC supplementation in 81 active duty service members with mild TBI found NAC had a significant effect on outcomes.31 Oral NAC supplementation led to improved neuropsychological test results, number of mild TBI symptoms, complete symptom resolution by day 7 of treatment compared with placebo, and NAC was well tolerated. Lack of replication studies and generalizability of findings to civilian, moderate, or chronic TBI populations are key limitations of this study.
Proposed mechanisms for the neuroprotective benefit of NAC include its antioxidant and inflammatory activation of cysteine/glutamate exchange, metabotropic glutamate receptor modulation, and glutathione synthesis.32 NAC has poor blood–brain permeability, but the vascular disruption seen in acute TBI might facilitate its delivery to affected neural sites.31 As such, the benefits of NAC in subacute or chronic TBI are questionable.
Neuropsychiatric outcomes of nutraceuticals
Enzogenol. This flavonoid-rich extract from the bark of the Monterey pine tree (Pinus radiata), known by the trade name Enzogenol, reportedly has antioxidant and anti-inflammatory properties that may counter oxidative damage and neuroinflammation following TBI. A phase II trial randomized participants to Enzogenol, 1,000 mg/d, or placebo for 6 weeks, then all participants received Enzogenol for 6 weeks followed by placebo for 4 weeks.33 Enzogenol was well tolerated with few side effects.
Compared with placebo, participants receiving Enzogenol showed no significant change in mood, as measured by the Hospital Anxiety and Depression Scale, and greater improvement in overall cognition as assessed by the Cognitive Failures Questionnaire. However, measures of working memory (digit span, arithmetic, and letter–number sequencing subtests of the Wechsler Adult Intelligence Scale) and episodic memory (California Verbal Learning Test) showed no benefit from Enzogenol.
Citicoline (CDP-choline) is an endogenous compound widely available as a nutraceutical that has been approved for TBI therapy in 59 countries.34 Animal studies indicate that it could possess neuroprotective properties. Proposed mechanisms for such effects have included stabilizing cell membranes, reducing inflammation, reducing the presence of free radicals, or stimulating production of acetylcholine.35,36 A study in rats found that CDP-choline was associated with increased levels of acetylcholine in the hippocampus and neocortex, which may help reduce neurobehavioral deficits.37
A study of 14 adults with mild to moderate closed head injury38 found that patients who received CDP-choline showed a greater reduction in post-concussion symptoms and improvement in recognition memory than controls who received placebo. However, the Citicoline Brain Injury Treatment Trial, a large randomized trial of 1,213 adults with complicated mild, moderate, or severe TBI, reported that CDP-choline did not improve functional and cognitive status.39
Physostigmine and lecithin. The cholinergic system is a key modulatory neurotransmitter system of the brain that mediates conscious awareness, attention, learning, and working memory.40 A double-blind, placebo-controlled study of 16 patients with moderate to severe closed head injury provided inconsistent evidence for the efficacy of physostigmine and lecithin in the treatment of memory and attention disturbances.41The results showed no differences between the physostigmine–lecithin combination vs lecithin alone, although sustained attention on the Continuous Performance Test was more efficient with physostigmine than placebo when the drug condition occurred first in the crossover design. The lack of encouraging data and concerns about its cardiovascular and proconvulsant properties in patients with TBI may explain the dearth of studies with physostigmine.
Cerebrolysin. A peptide preparation produced from purified pig brain proteins, known by the trade name Cerebrolysin, is popular in Asia and Europe for its nootropic properties. Cerebrolysin may activate cerebral mechanisms related to attention and memory processes,42 and some data have shown efficacy in improving cognitive symptoms and daily activities in patients with Alzheimer’s disease43 and TBI.44
A blinded 12-week study of 32 participants with acute mild TBI reported that those randomized to Cerebrolysin showed improvement in cognitive functioning vs the placebo group.45 The authors concluded that Cerebrolysin provides an advantage for patients with mild TBI and brain contusion if treatment starts within 24 hours of mild TBI onset. Cerebrolysin was well tolerated. Major limitations of this study were small sample size, lack of information regarding comorbid neuropsychiatric conditions and treatments, and short treatment duration.
A recent Cochrane review of 6 RCTs with 1,501 participants found no clinical benefit of Cerebrolysin for treating acute ischemic stroke, and found moderate-quality evidence of an increase with non-fatal serious adverse events but not in total serious adverse events.46 We do not recommend Cerebrolysin use in patients with TBI at this time until additional efficacy and safety data are available.
Nutraceuticals used in other populations
Other nutraceuticals with preclinical evidence of possible benefit in TBI but lacking evidence from human clinical trials include omega-3 fatty acids,47 curcumin,48 and resveratrol,49 providing further proof that results from experimental studies do not necessarily extend to clinical trials.50
Studies of nutraceuticals in other neurological and psychiatric populations have yielded some promising results. Significant interest has focused on the association between vitamin D deficiency, dementia, and neurodegenerative conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease.51 The role of vitamin D in regulation of calcium-mediated neuronal excitotoxicity and oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors, and deficient neurotransmitters makes it an attractive candidate as a neuroprotective agent.52
RCTs of nutraceuticals also have reported positive findings for a variety of mood and anxiety disorders, such as St. John’s wort, S-adenosylmethionine, omega-3 fatty acids for major depression53 and bipolar depression,54 and kava for generalized anxiety disorder.55 More research, however, is needed in these areas.
The use of nonpharmacologic agents in TBI often relies on similar neuropsychiatric symptom profiles of idiopathic psychiatric disorders. Attention-deficit/hyperactivity disorder (ADHD) closely resembles TBI, but systemic reviews of studies of zinc, magnesium, and polyunsaturated fatty acids supplementation in ADHD provide no evidence of therapeutic benefit.56-58
Educate patients in role of nutraceuticals
Despite lack of FDA oversight and limited empirical support, nutraceuticals continue to be widely marketed and used for their putative health benefits59 and have gained increased attention among clinicians.60 Because nutritional deficiency may make the brain less able than other organs to recover from injury,61 supplementation is an option, especially in individuals who could be at greater risk of TBI (eg, athletes and military personnel).
Lacking robust scientific evidence to support the use of nutraceuticals either for enhancing TBI recovery or treating neuropsychiatric disturbances, clinicians must educate patients that these agents are not completely benign and can have significant side effects and drug interactions.62,63 Nutraceuticals may contain multiple ingredients, some of which can be toxic, particularly at higher doses. Many patients may not volunteer information about their nutraceutical use to their health care providers,64 so we must ask them about that and inform them of the potential for adverse events and drug interactions.
1. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Updated January 22, 2016. Accessed June 5, 2017.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
3. Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198-205.
4. National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheets. https://ods.od.nih.gov/factsheets/list-all. Accessed June 5, 2017.
5. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2002.
6. Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64-82.
7. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
8. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533; discussion 1533.
9. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1-5.
10. Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
11. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6(2):59-61.
12. Cope EC, Morris DR, Levenson CW. Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr Rev. 2012;70(7):410-413.
13. Morris DR, Levenson CW. Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care. 2013;16(6):708-711.
14. Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13(1):25-34.
15. Fernández-Gajardo R, Matamala JM, Carrasco R, et al. Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs. 2014;28(3):229-248.
16. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133-137.
17. Cekic M, Cutler SM, VanLandingham JW, et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864-874.
18. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58.
19. Cernak I, Savic VJ, Kotur J, et al. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000;17(1):53-68.
20. Dhandapani SS, Gupta A, Vivekanandhan S, et al. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. The Indian Journal of Neurotrauma. 2008;5(1):27-33.
21. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29-38.
22. Jeter CB, Hergenroeder GW, Ward NH 3rd, et al. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671-679.
23. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366-371.
24. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(9):1729-1735.
25. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642-1647.
26. Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61(suppl):S103-S105.
27. DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130(11):1159-1162; discussion 1162-1163.
28. Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
29. Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106(3):287-292.
30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130(6):1219-1226.
31. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
32. Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617.
33. Theadom A, Mahon S, Barker-Collo S, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135-1144.
34. Arenth PM, Russell KC, Ricker JH, et al. CDP-choline as a biological supplement during neurorecovery: a focused review. PM R. 2011;3(6 suppl 1):S123-S131.
35. Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10(5):839-846.
36. Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
37. Dixon CE, Ma X, Marion DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma. 1997;14(3):161-169.
38. Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(suppl):S39-S42.
39. Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993-2000.
40. Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273-280.
41. Levin HS, Peters BH, Kalisky Z, et al. Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent Nerv Syst Trauma. 1986;3(4):333-342.
42. Alvarez XA, Lombardi VR, Corzo L, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315-328.
43. Ruether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol. 2001;16(5):253-263.
44. Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl. 2005;95:59-60.
45. Chen CC, Wei ST, Tsaia SC, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br J Neurosurg. 2013;27(6):803-807.
46. Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2016;12:CD007026.
47. Barrett EC, McBurney MI, Ciappio ED. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv Nutr. 2014;5(3):268-277.
48. Sharma S, Zhuang Y, Ying Z, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161(4):1037-1044.
49. Gatson JW, Liu MM, Abdelfattah K, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470-475; discussion 474-475.
50. Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460-462.
51. Mpandzou G, Aït Ben Haddou E, Regragui W, et al. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172(2):109-122.
52. Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451-461.
53. Sarris J, Papakostas GI, Vitolo O, et al. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76-81.
54. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
55. Sarris J, Stough C, Bousman C, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643-648.
56. Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med. 2015;6:83.
57. Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986.
58. Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr. 2013;67(1):122-124.
59. U.S. Food and Drug Administration. Can a dietary supplement treat a concussion? No! http://www.fda.gov/forconsumers/consumerupdates/ucm378845.htm. Updated February 13, 2015. Accessed June 5, 2017.
60. Sarris J, Logan AC, Akbaraly TN, et al; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
61. Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472.
62. Edie CF, Dewan N. Which psychotropics interact with four common supplements. Current Psychiatry. 2005;4(1):16-30.
63. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578-592.
64. National Center for Complementary and Integrative Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers. https://nccih.nih.gov/research/statistics/2010. Published 2011. Accessed June 5, 2017.
Traumatic brain injury (TBI) affects more than 2 million people in the United States each year.1 TBI can trigger a cascade of secondary injury mechanisms, such as inflammation, hypoxic/ischemic injury, excitotoxicity, and oxidative stress,2 that could contribute to cognitive and behavioral changes. Although neuropsychiatric symptoms might not be obvious after a TBI, they have a high prevalence in these patients, can last long term, and may be difficult to treat.3 Despite research advances in understanding the biological basis of TBI and identifying potential therapeutic targets, treatment options for individuals with TBI remain limited.
As a result, clinicians have turned to alternative treatments for TBI, including nutraceuticals. In this article, we will:
- provide an overview of nutraceuticals used in treating TBI, first exploring outcomes soon after TBI, then concentrating on neuropsychiatric outcomes
- evaluate the existing evidence, including recommended dietary allowances (Table 1)4,5 and side effects (Table 2)
- review recommendations for their clinical use.

Pharmacologic approaches are limited
Nutraceuticals have gained attention for managing TBI-associated neuropsychiatric disorders because of the limited evidence supporting current approaches. Existing strategies encompass pharmacologic and non-pharmacologic interventions, psychoeducation, supportive and behavioral psychotherapies, and cognitive rehabilitation.6
Many pharmacologic options exist for specific neurobehavioral symptoms, but the evidence for their use is based on small studies, case reports, and knowledge extrapolated from their use in idiopathic psychiatric disorders.7,8 No FDA-approved drugs have been effective for treating neuropsychiatric disturbances after a TBI. Off-label use of antidepressants, anticonvulsants, dopaminergic agents, and cholinesterase inhibitors in TBI has been associated with inadequate clinical response and/or intolerable side effects.9,10

What are nutraceuticals?
DeFelice11 introduced the term “nutraceutical” to refer to “any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease.” The term has been expanded to include dietary supplements, such as vitamins, minerals, amino acids, herbal or other botanicals, and food products that provide health benefits beyond what they normally provide in food form. The FDA does not regulate the marketing or manufacturing of nutraceuticals; therefore, their bioavailability and metabolism can vary.
Despite their widespread use, the evidence supporting the efficacy of nutraceuticals for patients with TBI is limited. Their effects might vary by population and depend on dose, timing, TBI severity, and whether taken alone or in combination with other nutraceutical or pharmaceutical agents. Fourteen randomized controlled trials (RCTs) have addressed the use of nutraceuticals in TBI (Table 3), but further research is needed to clarify for which conditions they provide maximum benefit.

Nutraceuticals and their potential use in TBI
Zinc is considered essential for optimal CNS functioning. Patients with TBI might be at risk for zinc deficiency, which has been associated with increased cell death and behavioral deficits.12,13 A randomized, prospective, double-blinded controlled trial examined the effects of supplemental zinc administration (12 mg for 15 days) compared with standard zinc therapy (2.5 mg for 15 days) over 1 month in 68 adults with acute severe closed head injury.14 The supplemental zinc group showed improved visceral protein levels, lower mortality, and more favorable neurologic recovery based on higher adjusted mean Glasgow Coma Scale score on day 28 and mean motor score on days 15 and 21.
Rodent studies have shown that zinc supplementation could reduce deficits in spatial learning and memory and depression-like behaviors and help decrease stress and anxiety,12 although no human clinical trials have been conducted. Despite the potential neuroprotective effects of zinc supplementation, evidence exists that endogenous zinc release and accumulation following TBI can trigger cellular changes that result in neuronal death.13
Vitamins C and E. Oxidative damage is believed to play a significant role in secondary injury in TBI, so research has focused on the role of antioxidants, such as vitamins C and E, to promote post-TBI recovery.15 One RCT16 of 100 adults with acute severe head injury reported that vitamin E administration was associated with reduced mortality and lower Glasgow Outcome Scale (GOS) scores, and vitamin C was associated with stabilized or reduced perilesional edema/infarct on CT scan.
Vitamin D. An animal study reported that vitamin D supplementation can help reduce inflammation, oxidative stress, and cell death in TBI, and that vitamin D deficiency has been associated with increased inflammation and behavioral deficits.17 Further evidence suggests that vitamin D may have a synergistic effect when used in combination with the hormone progesterone. A RCT of 60 patients with severe TBI reported that 60% of those who received progesterone plus vitamin D had GOS scores of 4 (good recovery) or 5 (moderate disability) vs 45% receiving progesterone alone or 25% receiving placebo.18
Magnesium, one of the most widely used nutraceuticals, is considered essential for CNS functioning, including the regulation of N-methyl-
A RCT evaluated the safety and efficacy of magnesium supplementation in 60 patients with severe closed TBI, with one-half randomized to standard care and the other also receiving magnesium sulfate (MgSO4; initiation dose of 4 g IV and 10 g IM, continuation dose of 5 g IM every 4 hours for 24 hours).20 After 3 months, more patients in the MgSO4 group had higher GOS scores than controls (73.3% vs 40%), lower 1-month mortality rates (13.3% vs 43.3%), and lower rates of intraoperative brain swelling (29.4% vs 73.3%).
However, a larger RCT of 499 patients with moderate or severe TBI randomized to high-dose (1.25 to 2.5 mmol/L) or low-dose (1.0 to 1.85 mmol/L) IV MgSO4 or placebo provided conflicting results.21 Participants received MgSO4 8 hours after injury and continued for 5 days. After 6 months, patients in the high-dose MgSO4 and placebo groups had similar composite primary outcome measures (eg, seizures, neuropsychological measures, functional status measures), although the high-dose group had a higher mortality rate than the placebo group. Patients who received low-dose MgSO4 showed worse outcomes than those assigned to placebo.
Amino acids. Branched-chain amino acids (BCAAs), including valine, isoleucine, and leucine, are essential in protein and neurotransmitter synthesis. Reduced levels of endogenous BCAAs have been reported in patients with mild or severe TBI.22 Preclinical studies suggest that BCAAs can improve hippocampal-dependent cognitive functioning following TBI.23
Two RCTs of BCAAs have been conducted in humans. One study randomized 40 men with severe TBI to IV BCAAs or placebo.24 After 15 days, the BCAA group showed greater improvement in Disability Rating Scale scores. The study also found that supplementation increased total BCAA levels without negatively affecting plasma levels of neurotransmitter precursors tyrosine and tryptophan. A second study found that 41 patients in a vegetative or minimally conscious state who received BCAA supplementation for 15 days had higher Disability Rating Scale scores than those receiving placebo.25
Probiotics and glutamine. Probiotics are non-pathogenic microorganisms that have been shown to modulate the host’s immune system.26 TBI is associated with immunological changes, including a shift from T-helper type 1 (TH1) cells to T-helper type 2 (TH2) cells that increase susceptibility to infection.27
A RCT of 52 patients with severe TBI suggested a correlation between probiotic administration-modulated cytokine levels and TH1/TH2 balance.28 A 3-times daily probiotic mix of Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for 21 days led to shorter average ICU stays (6.8 vs 10.7 days, P = .034) and a decrease in nosocomial infections (34.6% vs 57.7%, P = .095) vs placebo, although the latter difference was not statistically significant.28
A prospective RCT of 20 patients with brain injury29 found a similar impact of early enteral nutrition supplemented with Lactobacillus johnsonii and glutamine, 30 g, vs a standard enteral nutrition formula. The treatment group experienced fewer nosocomial infections (50% vs 100%, P = .03), shorter ICU stays (10 vs 22 days, P < .01), and fewer days on mechanical ventilation (7 vs 14, P = .04). Despite these studies, evidence for the use of glutamine in patients with TBI is scarce and inconclusive.
N-acetylcysteine (NAC) comes from the amino acid L-cysteine. NAC is an effective scavenger of free radicals and improves cerebral microcirculatory blood flow and tissue oxygenation.30 A randomized, double-blind, placebo-controlled study of oral NAC supplementation in 81 active duty service members with mild TBI found NAC had a significant effect on outcomes.31 Oral NAC supplementation led to improved neuropsychological test results, number of mild TBI symptoms, complete symptom resolution by day 7 of treatment compared with placebo, and NAC was well tolerated. Lack of replication studies and generalizability of findings to civilian, moderate, or chronic TBI populations are key limitations of this study.
Proposed mechanisms for the neuroprotective benefit of NAC include its antioxidant and inflammatory activation of cysteine/glutamate exchange, metabotropic glutamate receptor modulation, and glutathione synthesis.32 NAC has poor blood–brain permeability, but the vascular disruption seen in acute TBI might facilitate its delivery to affected neural sites.31 As such, the benefits of NAC in subacute or chronic TBI are questionable.
Neuropsychiatric outcomes of nutraceuticals
Enzogenol. This flavonoid-rich extract from the bark of the Monterey pine tree (Pinus radiata), known by the trade name Enzogenol, reportedly has antioxidant and anti-inflammatory properties that may counter oxidative damage and neuroinflammation following TBI. A phase II trial randomized participants to Enzogenol, 1,000 mg/d, or placebo for 6 weeks, then all participants received Enzogenol for 6 weeks followed by placebo for 4 weeks.33 Enzogenol was well tolerated with few side effects.
Compared with placebo, participants receiving Enzogenol showed no significant change in mood, as measured by the Hospital Anxiety and Depression Scale, and greater improvement in overall cognition as assessed by the Cognitive Failures Questionnaire. However, measures of working memory (digit span, arithmetic, and letter–number sequencing subtests of the Wechsler Adult Intelligence Scale) and episodic memory (California Verbal Learning Test) showed no benefit from Enzogenol.
Citicoline (CDP-choline) is an endogenous compound widely available as a nutraceutical that has been approved for TBI therapy in 59 countries.34 Animal studies indicate that it could possess neuroprotective properties. Proposed mechanisms for such effects have included stabilizing cell membranes, reducing inflammation, reducing the presence of free radicals, or stimulating production of acetylcholine.35,36 A study in rats found that CDP-choline was associated with increased levels of acetylcholine in the hippocampus and neocortex, which may help reduce neurobehavioral deficits.37
A study of 14 adults with mild to moderate closed head injury38 found that patients who received CDP-choline showed a greater reduction in post-concussion symptoms and improvement in recognition memory than controls who received placebo. However, the Citicoline Brain Injury Treatment Trial, a large randomized trial of 1,213 adults with complicated mild, moderate, or severe TBI, reported that CDP-choline did not improve functional and cognitive status.39
Physostigmine and lecithin. The cholinergic system is a key modulatory neurotransmitter system of the brain that mediates conscious awareness, attention, learning, and working memory.40 A double-blind, placebo-controlled study of 16 patients with moderate to severe closed head injury provided inconsistent evidence for the efficacy of physostigmine and lecithin in the treatment of memory and attention disturbances.41The results showed no differences between the physostigmine–lecithin combination vs lecithin alone, although sustained attention on the Continuous Performance Test was more efficient with physostigmine than placebo when the drug condition occurred first in the crossover design. The lack of encouraging data and concerns about its cardiovascular and proconvulsant properties in patients with TBI may explain the dearth of studies with physostigmine.
Cerebrolysin. A peptide preparation produced from purified pig brain proteins, known by the trade name Cerebrolysin, is popular in Asia and Europe for its nootropic properties. Cerebrolysin may activate cerebral mechanisms related to attention and memory processes,42 and some data have shown efficacy in improving cognitive symptoms and daily activities in patients with Alzheimer’s disease43 and TBI.44
A blinded 12-week study of 32 participants with acute mild TBI reported that those randomized to Cerebrolysin showed improvement in cognitive functioning vs the placebo group.45 The authors concluded that Cerebrolysin provides an advantage for patients with mild TBI and brain contusion if treatment starts within 24 hours of mild TBI onset. Cerebrolysin was well tolerated. Major limitations of this study were small sample size, lack of information regarding comorbid neuropsychiatric conditions and treatments, and short treatment duration.
A recent Cochrane review of 6 RCTs with 1,501 participants found no clinical benefit of Cerebrolysin for treating acute ischemic stroke, and found moderate-quality evidence of an increase with non-fatal serious adverse events but not in total serious adverse events.46 We do not recommend Cerebrolysin use in patients with TBI at this time until additional efficacy and safety data are available.
Nutraceuticals used in other populations
Other nutraceuticals with preclinical evidence of possible benefit in TBI but lacking evidence from human clinical trials include omega-3 fatty acids,47 curcumin,48 and resveratrol,49 providing further proof that results from experimental studies do not necessarily extend to clinical trials.50
Studies of nutraceuticals in other neurological and psychiatric populations have yielded some promising results. Significant interest has focused on the association between vitamin D deficiency, dementia, and neurodegenerative conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease.51 The role of vitamin D in regulation of calcium-mediated neuronal excitotoxicity and oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors, and deficient neurotransmitters makes it an attractive candidate as a neuroprotective agent.52
RCTs of nutraceuticals also have reported positive findings for a variety of mood and anxiety disorders, such as St. John’s wort, S-adenosylmethionine, omega-3 fatty acids for major depression53 and bipolar depression,54 and kava for generalized anxiety disorder.55 More research, however, is needed in these areas.
The use of nonpharmacologic agents in TBI often relies on similar neuropsychiatric symptom profiles of idiopathic psychiatric disorders. Attention-deficit/hyperactivity disorder (ADHD) closely resembles TBI, but systemic reviews of studies of zinc, magnesium, and polyunsaturated fatty acids supplementation in ADHD provide no evidence of therapeutic benefit.56-58
Educate patients in role of nutraceuticals
Despite lack of FDA oversight and limited empirical support, nutraceuticals continue to be widely marketed and used for their putative health benefits59 and have gained increased attention among clinicians.60 Because nutritional deficiency may make the brain less able than other organs to recover from injury,61 supplementation is an option, especially in individuals who could be at greater risk of TBI (eg, athletes and military personnel).
Lacking robust scientific evidence to support the use of nutraceuticals either for enhancing TBI recovery or treating neuropsychiatric disturbances, clinicians must educate patients that these agents are not completely benign and can have significant side effects and drug interactions.62,63 Nutraceuticals may contain multiple ingredients, some of which can be toxic, particularly at higher doses. Many patients may not volunteer information about their nutraceutical use to their health care providers,64 so we must ask them about that and inform them of the potential for adverse events and drug interactions.
Traumatic brain injury (TBI) affects more than 2 million people in the United States each year.1 TBI can trigger a cascade of secondary injury mechanisms, such as inflammation, hypoxic/ischemic injury, excitotoxicity, and oxidative stress,2 that could contribute to cognitive and behavioral changes. Although neuropsychiatric symptoms might not be obvious after a TBI, they have a high prevalence in these patients, can last long term, and may be difficult to treat.3 Despite research advances in understanding the biological basis of TBI and identifying potential therapeutic targets, treatment options for individuals with TBI remain limited.
As a result, clinicians have turned to alternative treatments for TBI, including nutraceuticals. In this article, we will:
- provide an overview of nutraceuticals used in treating TBI, first exploring outcomes soon after TBI, then concentrating on neuropsychiatric outcomes
- evaluate the existing evidence, including recommended dietary allowances (Table 1)4,5 and side effects (Table 2)
- review recommendations for their clinical use.

Pharmacologic approaches are limited
Nutraceuticals have gained attention for managing TBI-associated neuropsychiatric disorders because of the limited evidence supporting current approaches. Existing strategies encompass pharmacologic and non-pharmacologic interventions, psychoeducation, supportive and behavioral psychotherapies, and cognitive rehabilitation.6
Many pharmacologic options exist for specific neurobehavioral symptoms, but the evidence for their use is based on small studies, case reports, and knowledge extrapolated from their use in idiopathic psychiatric disorders.7,8 No FDA-approved drugs have been effective for treating neuropsychiatric disturbances after a TBI. Off-label use of antidepressants, anticonvulsants, dopaminergic agents, and cholinesterase inhibitors in TBI has been associated with inadequate clinical response and/or intolerable side effects.9,10

What are nutraceuticals?
DeFelice11 introduced the term “nutraceutical” to refer to “any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease.” The term has been expanded to include dietary supplements, such as vitamins, minerals, amino acids, herbal or other botanicals, and food products that provide health benefits beyond what they normally provide in food form. The FDA does not regulate the marketing or manufacturing of nutraceuticals; therefore, their bioavailability and metabolism can vary.
Despite their widespread use, the evidence supporting the efficacy of nutraceuticals for patients with TBI is limited. Their effects might vary by population and depend on dose, timing, TBI severity, and whether taken alone or in combination with other nutraceutical or pharmaceutical agents. Fourteen randomized controlled trials (RCTs) have addressed the use of nutraceuticals in TBI (Table 3), but further research is needed to clarify for which conditions they provide maximum benefit.

Nutraceuticals and their potential use in TBI
Zinc is considered essential for optimal CNS functioning. Patients with TBI might be at risk for zinc deficiency, which has been associated with increased cell death and behavioral deficits.12,13 A randomized, prospective, double-blinded controlled trial examined the effects of supplemental zinc administration (12 mg for 15 days) compared with standard zinc therapy (2.5 mg for 15 days) over 1 month in 68 adults with acute severe closed head injury.14 The supplemental zinc group showed improved visceral protein levels, lower mortality, and more favorable neurologic recovery based on higher adjusted mean Glasgow Coma Scale score on day 28 and mean motor score on days 15 and 21.
Rodent studies have shown that zinc supplementation could reduce deficits in spatial learning and memory and depression-like behaviors and help decrease stress and anxiety,12 although no human clinical trials have been conducted. Despite the potential neuroprotective effects of zinc supplementation, evidence exists that endogenous zinc release and accumulation following TBI can trigger cellular changes that result in neuronal death.13
Vitamins C and E. Oxidative damage is believed to play a significant role in secondary injury in TBI, so research has focused on the role of antioxidants, such as vitamins C and E, to promote post-TBI recovery.15 One RCT16 of 100 adults with acute severe head injury reported that vitamin E administration was associated with reduced mortality and lower Glasgow Outcome Scale (GOS) scores, and vitamin C was associated with stabilized or reduced perilesional edema/infarct on CT scan.
Vitamin D. An animal study reported that vitamin D supplementation can help reduce inflammation, oxidative stress, and cell death in TBI, and that vitamin D deficiency has been associated with increased inflammation and behavioral deficits.17 Further evidence suggests that vitamin D may have a synergistic effect when used in combination with the hormone progesterone. A RCT of 60 patients with severe TBI reported that 60% of those who received progesterone plus vitamin D had GOS scores of 4 (good recovery) or 5 (moderate disability) vs 45% receiving progesterone alone or 25% receiving placebo.18
Magnesium, one of the most widely used nutraceuticals, is considered essential for CNS functioning, including the regulation of N-methyl-
A RCT evaluated the safety and efficacy of magnesium supplementation in 60 patients with severe closed TBI, with one-half randomized to standard care and the other also receiving magnesium sulfate (MgSO4; initiation dose of 4 g IV and 10 g IM, continuation dose of 5 g IM every 4 hours for 24 hours).20 After 3 months, more patients in the MgSO4 group had higher GOS scores than controls (73.3% vs 40%), lower 1-month mortality rates (13.3% vs 43.3%), and lower rates of intraoperative brain swelling (29.4% vs 73.3%).
However, a larger RCT of 499 patients with moderate or severe TBI randomized to high-dose (1.25 to 2.5 mmol/L) or low-dose (1.0 to 1.85 mmol/L) IV MgSO4 or placebo provided conflicting results.21 Participants received MgSO4 8 hours after injury and continued for 5 days. After 6 months, patients in the high-dose MgSO4 and placebo groups had similar composite primary outcome measures (eg, seizures, neuropsychological measures, functional status measures), although the high-dose group had a higher mortality rate than the placebo group. Patients who received low-dose MgSO4 showed worse outcomes than those assigned to placebo.
Amino acids. Branched-chain amino acids (BCAAs), including valine, isoleucine, and leucine, are essential in protein and neurotransmitter synthesis. Reduced levels of endogenous BCAAs have been reported in patients with mild or severe TBI.22 Preclinical studies suggest that BCAAs can improve hippocampal-dependent cognitive functioning following TBI.23
Two RCTs of BCAAs have been conducted in humans. One study randomized 40 men with severe TBI to IV BCAAs or placebo.24 After 15 days, the BCAA group showed greater improvement in Disability Rating Scale scores. The study also found that supplementation increased total BCAA levels without negatively affecting plasma levels of neurotransmitter precursors tyrosine and tryptophan. A second study found that 41 patients in a vegetative or minimally conscious state who received BCAA supplementation for 15 days had higher Disability Rating Scale scores than those receiving placebo.25
Probiotics and glutamine. Probiotics are non-pathogenic microorganisms that have been shown to modulate the host’s immune system.26 TBI is associated with immunological changes, including a shift from T-helper type 1 (TH1) cells to T-helper type 2 (TH2) cells that increase susceptibility to infection.27
A RCT of 52 patients with severe TBI suggested a correlation between probiotic administration-modulated cytokine levels and TH1/TH2 balance.28 A 3-times daily probiotic mix of Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for 21 days led to shorter average ICU stays (6.8 vs 10.7 days, P = .034) and a decrease in nosocomial infections (34.6% vs 57.7%, P = .095) vs placebo, although the latter difference was not statistically significant.28
A prospective RCT of 20 patients with brain injury29 found a similar impact of early enteral nutrition supplemented with Lactobacillus johnsonii and glutamine, 30 g, vs a standard enteral nutrition formula. The treatment group experienced fewer nosocomial infections (50% vs 100%, P = .03), shorter ICU stays (10 vs 22 days, P < .01), and fewer days on mechanical ventilation (7 vs 14, P = .04). Despite these studies, evidence for the use of glutamine in patients with TBI is scarce and inconclusive.
N-acetylcysteine (NAC) comes from the amino acid L-cysteine. NAC is an effective scavenger of free radicals and improves cerebral microcirculatory blood flow and tissue oxygenation.30 A randomized, double-blind, placebo-controlled study of oral NAC supplementation in 81 active duty service members with mild TBI found NAC had a significant effect on outcomes.31 Oral NAC supplementation led to improved neuropsychological test results, number of mild TBI symptoms, complete symptom resolution by day 7 of treatment compared with placebo, and NAC was well tolerated. Lack of replication studies and generalizability of findings to civilian, moderate, or chronic TBI populations are key limitations of this study.
Proposed mechanisms for the neuroprotective benefit of NAC include its antioxidant and inflammatory activation of cysteine/glutamate exchange, metabotropic glutamate receptor modulation, and glutathione synthesis.32 NAC has poor blood–brain permeability, but the vascular disruption seen in acute TBI might facilitate its delivery to affected neural sites.31 As such, the benefits of NAC in subacute or chronic TBI are questionable.
Neuropsychiatric outcomes of nutraceuticals
Enzogenol. This flavonoid-rich extract from the bark of the Monterey pine tree (Pinus radiata), known by the trade name Enzogenol, reportedly has antioxidant and anti-inflammatory properties that may counter oxidative damage and neuroinflammation following TBI. A phase II trial randomized participants to Enzogenol, 1,000 mg/d, or placebo for 6 weeks, then all participants received Enzogenol for 6 weeks followed by placebo for 4 weeks.33 Enzogenol was well tolerated with few side effects.
Compared with placebo, participants receiving Enzogenol showed no significant change in mood, as measured by the Hospital Anxiety and Depression Scale, and greater improvement in overall cognition as assessed by the Cognitive Failures Questionnaire. However, measures of working memory (digit span, arithmetic, and letter–number sequencing subtests of the Wechsler Adult Intelligence Scale) and episodic memory (California Verbal Learning Test) showed no benefit from Enzogenol.
Citicoline (CDP-choline) is an endogenous compound widely available as a nutraceutical that has been approved for TBI therapy in 59 countries.34 Animal studies indicate that it could possess neuroprotective properties. Proposed mechanisms for such effects have included stabilizing cell membranes, reducing inflammation, reducing the presence of free radicals, or stimulating production of acetylcholine.35,36 A study in rats found that CDP-choline was associated with increased levels of acetylcholine in the hippocampus and neocortex, which may help reduce neurobehavioral deficits.37
A study of 14 adults with mild to moderate closed head injury38 found that patients who received CDP-choline showed a greater reduction in post-concussion symptoms and improvement in recognition memory than controls who received placebo. However, the Citicoline Brain Injury Treatment Trial, a large randomized trial of 1,213 adults with complicated mild, moderate, or severe TBI, reported that CDP-choline did not improve functional and cognitive status.39
Physostigmine and lecithin. The cholinergic system is a key modulatory neurotransmitter system of the brain that mediates conscious awareness, attention, learning, and working memory.40 A double-blind, placebo-controlled study of 16 patients with moderate to severe closed head injury provided inconsistent evidence for the efficacy of physostigmine and lecithin in the treatment of memory and attention disturbances.41The results showed no differences between the physostigmine–lecithin combination vs lecithin alone, although sustained attention on the Continuous Performance Test was more efficient with physostigmine than placebo when the drug condition occurred first in the crossover design. The lack of encouraging data and concerns about its cardiovascular and proconvulsant properties in patients with TBI may explain the dearth of studies with physostigmine.
Cerebrolysin. A peptide preparation produced from purified pig brain proteins, known by the trade name Cerebrolysin, is popular in Asia and Europe for its nootropic properties. Cerebrolysin may activate cerebral mechanisms related to attention and memory processes,42 and some data have shown efficacy in improving cognitive symptoms and daily activities in patients with Alzheimer’s disease43 and TBI.44
A blinded 12-week study of 32 participants with acute mild TBI reported that those randomized to Cerebrolysin showed improvement in cognitive functioning vs the placebo group.45 The authors concluded that Cerebrolysin provides an advantage for patients with mild TBI and brain contusion if treatment starts within 24 hours of mild TBI onset. Cerebrolysin was well tolerated. Major limitations of this study were small sample size, lack of information regarding comorbid neuropsychiatric conditions and treatments, and short treatment duration.
A recent Cochrane review of 6 RCTs with 1,501 participants found no clinical benefit of Cerebrolysin for treating acute ischemic stroke, and found moderate-quality evidence of an increase with non-fatal serious adverse events but not in total serious adverse events.46 We do not recommend Cerebrolysin use in patients with TBI at this time until additional efficacy and safety data are available.
Nutraceuticals used in other populations
Other nutraceuticals with preclinical evidence of possible benefit in TBI but lacking evidence from human clinical trials include omega-3 fatty acids,47 curcumin,48 and resveratrol,49 providing further proof that results from experimental studies do not necessarily extend to clinical trials.50
Studies of nutraceuticals in other neurological and psychiatric populations have yielded some promising results. Significant interest has focused on the association between vitamin D deficiency, dementia, and neurodegenerative conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease.51 The role of vitamin D in regulation of calcium-mediated neuronal excitotoxicity and oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors, and deficient neurotransmitters makes it an attractive candidate as a neuroprotective agent.52
RCTs of nutraceuticals also have reported positive findings for a variety of mood and anxiety disorders, such as St. John’s wort, S-adenosylmethionine, omega-3 fatty acids for major depression53 and bipolar depression,54 and kava for generalized anxiety disorder.55 More research, however, is needed in these areas.
The use of nonpharmacologic agents in TBI often relies on similar neuropsychiatric symptom profiles of idiopathic psychiatric disorders. Attention-deficit/hyperactivity disorder (ADHD) closely resembles TBI, but systemic reviews of studies of zinc, magnesium, and polyunsaturated fatty acids supplementation in ADHD provide no evidence of therapeutic benefit.56-58
Educate patients in role of nutraceuticals
Despite lack of FDA oversight and limited empirical support, nutraceuticals continue to be widely marketed and used for their putative health benefits59 and have gained increased attention among clinicians.60 Because nutritional deficiency may make the brain less able than other organs to recover from injury,61 supplementation is an option, especially in individuals who could be at greater risk of TBI (eg, athletes and military personnel).
Lacking robust scientific evidence to support the use of nutraceuticals either for enhancing TBI recovery or treating neuropsychiatric disturbances, clinicians must educate patients that these agents are not completely benign and can have significant side effects and drug interactions.62,63 Nutraceuticals may contain multiple ingredients, some of which can be toxic, particularly at higher doses. Many patients may not volunteer information about their nutraceutical use to their health care providers,64 so we must ask them about that and inform them of the potential for adverse events and drug interactions.
1. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Updated January 22, 2016. Accessed June 5, 2017.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
3. Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198-205.
4. National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheets. https://ods.od.nih.gov/factsheets/list-all. Accessed June 5, 2017.
5. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2002.
6. Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64-82.
7. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
8. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533; discussion 1533.
9. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1-5.
10. Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
11. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6(2):59-61.
12. Cope EC, Morris DR, Levenson CW. Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr Rev. 2012;70(7):410-413.
13. Morris DR, Levenson CW. Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care. 2013;16(6):708-711.
14. Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13(1):25-34.
15. Fernández-Gajardo R, Matamala JM, Carrasco R, et al. Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs. 2014;28(3):229-248.
16. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133-137.
17. Cekic M, Cutler SM, VanLandingham JW, et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864-874.
18. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58.
19. Cernak I, Savic VJ, Kotur J, et al. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000;17(1):53-68.
20. Dhandapani SS, Gupta A, Vivekanandhan S, et al. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. The Indian Journal of Neurotrauma. 2008;5(1):27-33.
21. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29-38.
22. Jeter CB, Hergenroeder GW, Ward NH 3rd, et al. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671-679.
23. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366-371.
24. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(9):1729-1735.
25. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642-1647.
26. Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61(suppl):S103-S105.
27. DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130(11):1159-1162; discussion 1162-1163.
28. Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
29. Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106(3):287-292.
30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130(6):1219-1226.
31. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
32. Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617.
33. Theadom A, Mahon S, Barker-Collo S, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135-1144.
34. Arenth PM, Russell KC, Ricker JH, et al. CDP-choline as a biological supplement during neurorecovery: a focused review. PM R. 2011;3(6 suppl 1):S123-S131.
35. Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10(5):839-846.
36. Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
37. Dixon CE, Ma X, Marion DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma. 1997;14(3):161-169.
38. Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(suppl):S39-S42.
39. Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993-2000.
40. Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273-280.
41. Levin HS, Peters BH, Kalisky Z, et al. Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent Nerv Syst Trauma. 1986;3(4):333-342.
42. Alvarez XA, Lombardi VR, Corzo L, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315-328.
43. Ruether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol. 2001;16(5):253-263.
44. Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl. 2005;95:59-60.
45. Chen CC, Wei ST, Tsaia SC, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br J Neurosurg. 2013;27(6):803-807.
46. Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2016;12:CD007026.
47. Barrett EC, McBurney MI, Ciappio ED. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv Nutr. 2014;5(3):268-277.
48. Sharma S, Zhuang Y, Ying Z, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161(4):1037-1044.
49. Gatson JW, Liu MM, Abdelfattah K, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470-475; discussion 474-475.
50. Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460-462.
51. Mpandzou G, Aït Ben Haddou E, Regragui W, et al. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172(2):109-122.
52. Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451-461.
53. Sarris J, Papakostas GI, Vitolo O, et al. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76-81.
54. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
55. Sarris J, Stough C, Bousman C, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643-648.
56. Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med. 2015;6:83.
57. Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986.
58. Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr. 2013;67(1):122-124.
59. U.S. Food and Drug Administration. Can a dietary supplement treat a concussion? No! http://www.fda.gov/forconsumers/consumerupdates/ucm378845.htm. Updated February 13, 2015. Accessed June 5, 2017.
60. Sarris J, Logan AC, Akbaraly TN, et al; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
61. Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472.
62. Edie CF, Dewan N. Which psychotropics interact with four common supplements. Current Psychiatry. 2005;4(1):16-30.
63. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578-592.
64. National Center for Complementary and Integrative Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers. https://nccih.nih.gov/research/statistics/2010. Published 2011. Accessed June 5, 2017.
1. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Updated January 22, 2016. Accessed June 5, 2017.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
3. Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198-205.
4. National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheets. https://ods.od.nih.gov/factsheets/list-all. Accessed June 5, 2017.
5. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2002.
6. Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64-82.
7. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
8. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533; discussion 1533.
9. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1-5.
10. Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
11. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6(2):59-61.
12. Cope EC, Morris DR, Levenson CW. Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr Rev. 2012;70(7):410-413.
13. Morris DR, Levenson CW. Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care. 2013;16(6):708-711.
14. Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13(1):25-34.
15. Fernández-Gajardo R, Matamala JM, Carrasco R, et al. Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs. 2014;28(3):229-248.
16. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133-137.
17. Cekic M, Cutler SM, VanLandingham JW, et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864-874.
18. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58.
19. Cernak I, Savic VJ, Kotur J, et al. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000;17(1):53-68.
20. Dhandapani SS, Gupta A, Vivekanandhan S, et al. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. The Indian Journal of Neurotrauma. 2008;5(1):27-33.
21. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29-38.
22. Jeter CB, Hergenroeder GW, Ward NH 3rd, et al. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671-679.
23. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366-371.
24. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(9):1729-1735.
25. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642-1647.
26. Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61(suppl):S103-S105.
27. DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130(11):1159-1162; discussion 1162-1163.
28. Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
29. Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106(3):287-292.
30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130(6):1219-1226.
31. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
32. Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617.
33. Theadom A, Mahon S, Barker-Collo S, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135-1144.
34. Arenth PM, Russell KC, Ricker JH, et al. CDP-choline as a biological supplement during neurorecovery: a focused review. PM R. 2011;3(6 suppl 1):S123-S131.
35. Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10(5):839-846.
36. Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
37. Dixon CE, Ma X, Marion DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma. 1997;14(3):161-169.
38. Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(suppl):S39-S42.
39. Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993-2000.
40. Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273-280.
41. Levin HS, Peters BH, Kalisky Z, et al. Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent Nerv Syst Trauma. 1986;3(4):333-342.
42. Alvarez XA, Lombardi VR, Corzo L, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315-328.
43. Ruether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol. 2001;16(5):253-263.
44. Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl. 2005;95:59-60.
45. Chen CC, Wei ST, Tsaia SC, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br J Neurosurg. 2013;27(6):803-807.
46. Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2016;12:CD007026.
47. Barrett EC, McBurney MI, Ciappio ED. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv Nutr. 2014;5(3):268-277.
48. Sharma S, Zhuang Y, Ying Z, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161(4):1037-1044.
49. Gatson JW, Liu MM, Abdelfattah K, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470-475; discussion 474-475.
50. Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460-462.
51. Mpandzou G, Aït Ben Haddou E, Regragui W, et al. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172(2):109-122.
52. Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451-461.
53. Sarris J, Papakostas GI, Vitolo O, et al. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76-81.
54. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
55. Sarris J, Stough C, Bousman C, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643-648.
56. Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med. 2015;6:83.
57. Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986.
58. Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr. 2013;67(1):122-124.
59. U.S. Food and Drug Administration. Can a dietary supplement treat a concussion? No! http://www.fda.gov/forconsumers/consumerupdates/ucm378845.htm. Updated February 13, 2015. Accessed June 5, 2017.
60. Sarris J, Logan AC, Akbaraly TN, et al; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
61. Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472.
62. Edie CF, Dewan N. Which psychotropics interact with four common supplements. Current Psychiatry. 2005;4(1):16-30.
63. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578-592.
64. National Center for Complementary and Integrative Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers. https://nccih.nih.gov/research/statistics/2010. Published 2011. Accessed June 5, 2017.
Eating disorders: Are they age-restricted?
Eating disorders are thought to affect only the young. Although the mean age of presentation is 17 years for anorexia nervosa and 18 to 25 years for bulimia nervosa, many women >65 years suffer from these disorders.1 Often, geriatric patients with a history of eating disorders during their youth that partially remitted have the same disorders re-emerge during their golden years. Because many practitioners think of eating disorders as a younger person’s illness, we could miss an opportunity to help these individuals when screening our geriatric patients.
DSM-52 categorizes feeding and eating disorders as:
- binge eating disorder
- anorexia nervosa
- bulimia nervosa
- other specified feeding and eating disorders
- pica
- avoidant/restrictive food intake disorder.
Binge eating disorder’s main feature is recurrent binge eating, which is the sense that one has lost control when consuming a larger amount of food within a discrete time period than what most people might eat in the same time period. Binge eating may include eating rapidly, feeling uncomfortably
Anorexia nervosa is defined as the restriction of energy intake relative to necessary energy requirements, leading to significantly low body weight in the context of age, sex, developmental trajectory, and physical health, as well as an intense fear of gaining weight or persistent behaviors interfering with weight gain.
Bulimia nervosa is repetitive loss of control when eating large amounts of food (more than most would eat in a period), with compensatory behaviors to prevent weight gain. It is possible that the value attached to youthful slenderness leads to dissatisfaction among older women as their bodies change; binging might provide a sense of control during a time of uncertainty.
Body mass index typically is highest at middle age and slowly declines. In part, this decline is caused by a reduction in energy intake because of modifications in eating habits and lowered appetite often seen during aging. Older women eat 30% fewer calories than younger women.3,4 Social isolation, chronic disease, and depression also contribute to diminished food intake. It is important to remember that distorted body image can occur in older individuals as well. Anorexia nervosa has the highest fatality rate among psychiatric conditions,5 and geriatric patients could be at particularly high risk.
Assessment
Assess for eating disorders in a geriatric patient by exploring the patient’s perception of body image and ruling out underlying causes of weight loss and medical comorbidities. Take a detailed history, including:
- body image and disordered thinking about food
- abnormal behaviors or rituals surrounding food
- history of eating disorders, psychiatric illness, or hospitalization
- medical history
- current and past medications
- illicit drug use or addiction to prescription medications.
Collateral informants, such as partners and adult children of the patient, may yield important information. Because geriatric patients often take several medications, contacting the primary care physician is important in the integrated care of the patient.
A thorough physical and mental status examination will provide information about the patient’s physical appearance. For example, if the patient appears emaciated or weak, the content and process of thoughts related to food will help rule out other etiologies, such as psychosis, depressive disorders, or anxiety. Vital signs and a full physical examination are needed when caring for patients with an eating disorder, regardless of age, but particularly in medically fragile geriatric patients. Because osteoporosis and osteopenia are concerns for many older patients, it’s important to collaborate with the primary care physician early to help minimize bone loss.
Treatment
While ensuring medical stability of the patient, psychotherapy is the treatment of choice for eating disorders in geriatric patients. Moderate to severe binge eating disorder can be treated with lisdexamfetamine. For bulimia nervosa, consider a combination of SSRI and psychotherapy. There is no FDA-approved medication for treating anorexia nervosa; therefore identifying and treating underlying medical causes and/or psychiatric comorbidities can help improve prognosis. Despite this, 1 study showed 20% of geriatric patients with an eating disorder die of complications from eating disorders.6
1. Currin L, Schmidt U, Treasure J, et al. Time trends in eating disorder incidence. Br J Psychiatry. 2005;186(2):132-135.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Morley JE, Thomas DR. Anorexia and aging: pathophysiology. Nutrition. 1999;15(6):499-503.
4. Morley JE. Peptides and aging: their role in anorexia and memory. Peptides. 2015;72(10):112-118.
5. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. Arch Gen Psychiatry. 2011;68(7):724-731.
6. Lapid MI, Prom MC, Burton MC, et al. Eating disorders in the elderly. Int Psychogeriatr. 2010;22(4):523-536.
Eating disorders are thought to affect only the young. Although the mean age of presentation is 17 years for anorexia nervosa and 18 to 25 years for bulimia nervosa, many women >65 years suffer from these disorders.1 Often, geriatric patients with a history of eating disorders during their youth that partially remitted have the same disorders re-emerge during their golden years. Because many practitioners think of eating disorders as a younger person’s illness, we could miss an opportunity to help these individuals when screening our geriatric patients.
DSM-52 categorizes feeding and eating disorders as:
- binge eating disorder
- anorexia nervosa
- bulimia nervosa
- other specified feeding and eating disorders
- pica
- avoidant/restrictive food intake disorder.
Binge eating disorder’s main feature is recurrent binge eating, which is the sense that one has lost control when consuming a larger amount of food within a discrete time period than what most people might eat in the same time period. Binge eating may include eating rapidly, feeling uncomfortably
Anorexia nervosa is defined as the restriction of energy intake relative to necessary energy requirements, leading to significantly low body weight in the context of age, sex, developmental trajectory, and physical health, as well as an intense fear of gaining weight or persistent behaviors interfering with weight gain.
Bulimia nervosa is repetitive loss of control when eating large amounts of food (more than most would eat in a period), with compensatory behaviors to prevent weight gain. It is possible that the value attached to youthful slenderness leads to dissatisfaction among older women as their bodies change; binging might provide a sense of control during a time of uncertainty.
Body mass index typically is highest at middle age and slowly declines. In part, this decline is caused by a reduction in energy intake because of modifications in eating habits and lowered appetite often seen during aging. Older women eat 30% fewer calories than younger women.3,4 Social isolation, chronic disease, and depression also contribute to diminished food intake. It is important to remember that distorted body image can occur in older individuals as well. Anorexia nervosa has the highest fatality rate among psychiatric conditions,5 and geriatric patients could be at particularly high risk.
Assessment
Assess for eating disorders in a geriatric patient by exploring the patient’s perception of body image and ruling out underlying causes of weight loss and medical comorbidities. Take a detailed history, including:
- body image and disordered thinking about food
- abnormal behaviors or rituals surrounding food
- history of eating disorders, psychiatric illness, or hospitalization
- medical history
- current and past medications
- illicit drug use or addiction to prescription medications.
Collateral informants, such as partners and adult children of the patient, may yield important information. Because geriatric patients often take several medications, contacting the primary care physician is important in the integrated care of the patient.
A thorough physical and mental status examination will provide information about the patient’s physical appearance. For example, if the patient appears emaciated or weak, the content and process of thoughts related to food will help rule out other etiologies, such as psychosis, depressive disorders, or anxiety. Vital signs and a full physical examination are needed when caring for patients with an eating disorder, regardless of age, but particularly in medically fragile geriatric patients. Because osteoporosis and osteopenia are concerns for many older patients, it’s important to collaborate with the primary care physician early to help minimize bone loss.
Treatment
While ensuring medical stability of the patient, psychotherapy is the treatment of choice for eating disorders in geriatric patients. Moderate to severe binge eating disorder can be treated with lisdexamfetamine. For bulimia nervosa, consider a combination of SSRI and psychotherapy. There is no FDA-approved medication for treating anorexia nervosa; therefore identifying and treating underlying medical causes and/or psychiatric comorbidities can help improve prognosis. Despite this, 1 study showed 20% of geriatric patients with an eating disorder die of complications from eating disorders.6
Eating disorders are thought to affect only the young. Although the mean age of presentation is 17 years for anorexia nervosa and 18 to 25 years for bulimia nervosa, many women >65 years suffer from these disorders.1 Often, geriatric patients with a history of eating disorders during their youth that partially remitted have the same disorders re-emerge during their golden years. Because many practitioners think of eating disorders as a younger person’s illness, we could miss an opportunity to help these individuals when screening our geriatric patients.
DSM-52 categorizes feeding and eating disorders as:
- binge eating disorder
- anorexia nervosa
- bulimia nervosa
- other specified feeding and eating disorders
- pica
- avoidant/restrictive food intake disorder.
Binge eating disorder’s main feature is recurrent binge eating, which is the sense that one has lost control when consuming a larger amount of food within a discrete time period than what most people might eat in the same time period. Binge eating may include eating rapidly, feeling uncomfortably
Anorexia nervosa is defined as the restriction of energy intake relative to necessary energy requirements, leading to significantly low body weight in the context of age, sex, developmental trajectory, and physical health, as well as an intense fear of gaining weight or persistent behaviors interfering with weight gain.
Bulimia nervosa is repetitive loss of control when eating large amounts of food (more than most would eat in a period), with compensatory behaviors to prevent weight gain. It is possible that the value attached to youthful slenderness leads to dissatisfaction among older women as their bodies change; binging might provide a sense of control during a time of uncertainty.
Body mass index typically is highest at middle age and slowly declines. In part, this decline is caused by a reduction in energy intake because of modifications in eating habits and lowered appetite often seen during aging. Older women eat 30% fewer calories than younger women.3,4 Social isolation, chronic disease, and depression also contribute to diminished food intake. It is important to remember that distorted body image can occur in older individuals as well. Anorexia nervosa has the highest fatality rate among psychiatric conditions,5 and geriatric patients could be at particularly high risk.
Assessment
Assess for eating disorders in a geriatric patient by exploring the patient’s perception of body image and ruling out underlying causes of weight loss and medical comorbidities. Take a detailed history, including:
- body image and disordered thinking about food
- abnormal behaviors or rituals surrounding food
- history of eating disorders, psychiatric illness, or hospitalization
- medical history
- current and past medications
- illicit drug use or addiction to prescription medications.
Collateral informants, such as partners and adult children of the patient, may yield important information. Because geriatric patients often take several medications, contacting the primary care physician is important in the integrated care of the patient.
A thorough physical and mental status examination will provide information about the patient’s physical appearance. For example, if the patient appears emaciated or weak, the content and process of thoughts related to food will help rule out other etiologies, such as psychosis, depressive disorders, or anxiety. Vital signs and a full physical examination are needed when caring for patients with an eating disorder, regardless of age, but particularly in medically fragile geriatric patients. Because osteoporosis and osteopenia are concerns for many older patients, it’s important to collaborate with the primary care physician early to help minimize bone loss.
Treatment
While ensuring medical stability of the patient, psychotherapy is the treatment of choice for eating disorders in geriatric patients. Moderate to severe binge eating disorder can be treated with lisdexamfetamine. For bulimia nervosa, consider a combination of SSRI and psychotherapy. There is no FDA-approved medication for treating anorexia nervosa; therefore identifying and treating underlying medical causes and/or psychiatric comorbidities can help improve prognosis. Despite this, 1 study showed 20% of geriatric patients with an eating disorder die of complications from eating disorders.6
1. Currin L, Schmidt U, Treasure J, et al. Time trends in eating disorder incidence. Br J Psychiatry. 2005;186(2):132-135.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Morley JE, Thomas DR. Anorexia and aging: pathophysiology. Nutrition. 1999;15(6):499-503.
4. Morley JE. Peptides and aging: their role in anorexia and memory. Peptides. 2015;72(10):112-118.
5. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. Arch Gen Psychiatry. 2011;68(7):724-731.
6. Lapid MI, Prom MC, Burton MC, et al. Eating disorders in the elderly. Int Psychogeriatr. 2010;22(4):523-536.
1. Currin L, Schmidt U, Treasure J, et al. Time trends in eating disorder incidence. Br J Psychiatry. 2005;186(2):132-135.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Morley JE, Thomas DR. Anorexia and aging: pathophysiology. Nutrition. 1999;15(6):499-503.
4. Morley JE. Peptides and aging: their role in anorexia and memory. Peptides. 2015;72(10):112-118.
5. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. Arch Gen Psychiatry. 2011;68(7):724-731.
6. Lapid MI, Prom MC, Burton MC, et al. Eating disorders in the elderly. Int Psychogeriatr. 2010;22(4):523-536.
A practical approach to interviewing a somatizing patient
Somatization is the experience of psychological distress in the form of bodil
Collecting a detailed history of physical symptoms can help the patient feel that you are listening to him (her) and that the chief concern is important. A detailed review of psychiatric symptoms (eg, hallucinations, paranoia, suicidality, etc.) should be deferred until later in the examination. Asking questions relating to psychiatric symptoms early could lead to further resistance by reinforcing negative preconceptions that the patient might have regarding mental illness.
Explicitly express empathy regarding physical symptoms throughout the interview to acknowledge any real suffering the patient is experiencing and to contradict any notion that psychiatric evaluation implies that the suffering could be imaginary.
Ask, “How has this illness affected your life?” This question helps make the connection between the patient’s physical state and social milieu. If somatization is confirmed, then the provider should assist the patient in reversing the arrow of causation. Although the ultimate goal is for the patient to understand how his (her) life has affected the symptoms, simply understanding that there are connections between the two is a start toward this goal.1
Explore the response to the previous question. Expand upon it to elicit a detailed social history, listening for any social stressors.
Obtain family and personal histories of allergies, substance abuse, and medical or psychiatric illness.
Review psychiatric symptoms. Make questions less jarring2 by adapting them to the patient’s situation, such as “Has your illness become so painful that at times you don’t even want to live?”
Perform cognitive and physical examinations. Conducting a physical examination could further reassure the patient that you are not ignoring physical complaints.
Educate the patient that the mind and body are connected and emotions affect how one feels physically. Use examples, such as “When I feel anxious, my heart beats faster” or “A headache might hurt more at work than at the beach.”
Elicit feedback and questions from the patient.
Discuss your treatment plan with the patient. Resistant patients with confirmed somatization disorders might accept psychiatric care as a means of dealing with the stress or pain of their physical symptoms.
Consider asking:
- What would you be doing if you weren’t in the hospital right now?
- Aside from your health, what’s the biggest challenge in your life?
- Everything has a good side and a bad side. Is there anything positive about dealing with your illness? Providing the patient with an example of negative aspects of a good thing (such as the calories in ice cream, the high cost of gold, etc.) can help make this point.
- What would your life look like if you didn’t have these symptoms?
1. Creed F, Guthrie E. Techniques for interviewing the somatising patient. Br J Psychiatry. 1993;162:467-471.
2. Carlat DJ. The psychiatric interview: a practical guide. 2nd ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2005.
Somatization is the experience of psychological distress in the form of bodil
Collecting a detailed history of physical symptoms can help the patient feel that you are listening to him (her) and that the chief concern is important. A detailed review of psychiatric symptoms (eg, hallucinations, paranoia, suicidality, etc.) should be deferred until later in the examination. Asking questions relating to psychiatric symptoms early could lead to further resistance by reinforcing negative preconceptions that the patient might have regarding mental illness.
Explicitly express empathy regarding physical symptoms throughout the interview to acknowledge any real suffering the patient is experiencing and to contradict any notion that psychiatric evaluation implies that the suffering could be imaginary.
Ask, “How has this illness affected your life?” This question helps make the connection between the patient’s physical state and social milieu. If somatization is confirmed, then the provider should assist the patient in reversing the arrow of causation. Although the ultimate goal is for the patient to understand how his (her) life has affected the symptoms, simply understanding that there are connections between the two is a start toward this goal.1
Explore the response to the previous question. Expand upon it to elicit a detailed social history, listening for any social stressors.
Obtain family and personal histories of allergies, substance abuse, and medical or psychiatric illness.
Review psychiatric symptoms. Make questions less jarring2 by adapting them to the patient’s situation, such as “Has your illness become so painful that at times you don’t even want to live?”
Perform cognitive and physical examinations. Conducting a physical examination could further reassure the patient that you are not ignoring physical complaints.
Educate the patient that the mind and body are connected and emotions affect how one feels physically. Use examples, such as “When I feel anxious, my heart beats faster” or “A headache might hurt more at work than at the beach.”
Elicit feedback and questions from the patient.
Discuss your treatment plan with the patient. Resistant patients with confirmed somatization disorders might accept psychiatric care as a means of dealing with the stress or pain of their physical symptoms.
Consider asking:
- What would you be doing if you weren’t in the hospital right now?
- Aside from your health, what’s the biggest challenge in your life?
- Everything has a good side and a bad side. Is there anything positive about dealing with your illness? Providing the patient with an example of negative aspects of a good thing (such as the calories in ice cream, the high cost of gold, etc.) can help make this point.
- What would your life look like if you didn’t have these symptoms?
Somatization is the experience of psychological distress in the form of bodil
Collecting a detailed history of physical symptoms can help the patient feel that you are listening to him (her) and that the chief concern is important. A detailed review of psychiatric symptoms (eg, hallucinations, paranoia, suicidality, etc.) should be deferred until later in the examination. Asking questions relating to psychiatric symptoms early could lead to further resistance by reinforcing negative preconceptions that the patient might have regarding mental illness.
Explicitly express empathy regarding physical symptoms throughout the interview to acknowledge any real suffering the patient is experiencing and to contradict any notion that psychiatric evaluation implies that the suffering could be imaginary.
Ask, “How has this illness affected your life?” This question helps make the connection between the patient’s physical state and social milieu. If somatization is confirmed, then the provider should assist the patient in reversing the arrow of causation. Although the ultimate goal is for the patient to understand how his (her) life has affected the symptoms, simply understanding that there are connections between the two is a start toward this goal.1
Explore the response to the previous question. Expand upon it to elicit a detailed social history, listening for any social stressors.
Obtain family and personal histories of allergies, substance abuse, and medical or psychiatric illness.
Review psychiatric symptoms. Make questions less jarring2 by adapting them to the patient’s situation, such as “Has your illness become so painful that at times you don’t even want to live?”
Perform cognitive and physical examinations. Conducting a physical examination could further reassure the patient that you are not ignoring physical complaints.
Educate the patient that the mind and body are connected and emotions affect how one feels physically. Use examples, such as “When I feel anxious, my heart beats faster” or “A headache might hurt more at work than at the beach.”
Elicit feedback and questions from the patient.
Discuss your treatment plan with the patient. Resistant patients with confirmed somatization disorders might accept psychiatric care as a means of dealing with the stress or pain of their physical symptoms.
Consider asking:
- What would you be doing if you weren’t in the hospital right now?
- Aside from your health, what’s the biggest challenge in your life?
- Everything has a good side and a bad side. Is there anything positive about dealing with your illness? Providing the patient with an example of negative aspects of a good thing (such as the calories in ice cream, the high cost of gold, etc.) can help make this point.
- What would your life look like if you didn’t have these symptoms?
1. Creed F, Guthrie E. Techniques for interviewing the somatising patient. Br J Psychiatry. 1993;162:467-471.
2. Carlat DJ. The psychiatric interview: a practical guide. 2nd ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2005.
1. Creed F, Guthrie E. Techniques for interviewing the somatising patient. Br J Psychiatry. 1993;162:467-471.
2. Carlat DJ. The psychiatric interview: a practical guide. 2nd ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2005.
Should you recommend acupuncture to patients with substance use disorders?
Acupuncture is an ancient therapeutic tool known to be the core of traditional Chinese medicine. Two theories suggest positive outcomes in patients treated with acupuncture:
- The oxidative stress reduction theory states that a “large body of evidences demonstrated that acupuncture has [an] antioxidative effect in various diseases, but the exact mechanism remains unclear.”1
- The neurophysiological theory states that “acupuncture stimulation can facilitate the release of certain neuropeptides in the CNS, eliciting profound physiological effects and even activating self-healing mechanisms.”2
For decades, acupuncture has been used for addiction management. Here we provide information on its utility for patients with substance use disorders.
Opioid use disorder. Multiple studies have looked at withdrawal, comorbid mood disorders, and its management with acupuncture alone or in combination with psychotherapy and/or opioid agonists. Studies from Asia reported good treatment outcomes but had low-method quality.3 Western studies had superior method quality but found that acupuncture was no better than placebo as monotherapy. When acupuncture is combined with psychotherapy and an opioid agonist, treatment results are promising, showing faster taper of medications (methadone and buprenorphine/naloxone) with fewer adverse effects.
Cocaine use disorder. Most studies had poor treatment outcomes of acupuncture over placebo and were of low quality. A number of small studies were promising and found that patients treated with acupuncture were most likely to have a negative urine drug screen.3 Although acupuncture is widely used in the United States to treat cocaine dependence, evidence does not confirm its efficacy.
Tobacco use disorder. A small group of studies favored acupuncture for smoking cessation.3 Other studies reported no benefit compared with placebo or neutral results. Some studies agreed that any intervention (acupuncture or sham acupuncture) with good results is better than no intervention at all.
Alcohol use disorder. Almost no advantage over placebo was found. Studies with significant findings were in small populations.3
Amphetamine, Cannabis, and other hallucinogen use disorders. Available data on stimulants were too limited to be relevant. No studies were found on Cannabis and hallucinogens.
Further studies are needed
There is a lack of conclusive, good quality studies supporting acupuncture’s benefits in treating substance abuse. Acupuncture has been known to lack adverse effects other than those related to needle manipulation, which is dependent on the methods (depth of needle insertion, accurate anatomical location, angle, etc.). Because this treatment option is virtually side-effect free, inexpensive, with positive synergistic results, more high-method quality studies are needed to consider it for our patients.
1. Zeng XH, Li QQ, Xu Q, et al. Acupuncture mechanism and redox equilibrium. Evid Based Complement and Alternat Med. 2014;2014:483294. doi: 10.1155/2014/483294
2. Bai L, Lao L. Neurobiological foundations of acupuncture: the relevance and future prospect based on neuroimaging evidence. Evid Based Complement and Alternat Med. 2013;2013:812568. doi: 10.1155/2013/812568.
3. Boyuan Z, Yang C, Ke C, et al. Efficacy of acupuncture for psychological symptoms associated with opioid addiction: a systematic review and meta-analysis. Evid Based Complement and Alternat Med. 2014;2014:313549. doi: 10.1155/2014/313549.
Acupuncture is an ancient therapeutic tool known to be the core of traditional Chinese medicine. Two theories suggest positive outcomes in patients treated with acupuncture:
- The oxidative stress reduction theory states that a “large body of evidences demonstrated that acupuncture has [an] antioxidative effect in various diseases, but the exact mechanism remains unclear.”1
- The neurophysiological theory states that “acupuncture stimulation can facilitate the release of certain neuropeptides in the CNS, eliciting profound physiological effects and even activating self-healing mechanisms.”2
For decades, acupuncture has been used for addiction management. Here we provide information on its utility for patients with substance use disorders.
Opioid use disorder. Multiple studies have looked at withdrawal, comorbid mood disorders, and its management with acupuncture alone or in combination with psychotherapy and/or opioid agonists. Studies from Asia reported good treatment outcomes but had low-method quality.3 Western studies had superior method quality but found that acupuncture was no better than placebo as monotherapy. When acupuncture is combined with psychotherapy and an opioid agonist, treatment results are promising, showing faster taper of medications (methadone and buprenorphine/naloxone) with fewer adverse effects.
Cocaine use disorder. Most studies had poor treatment outcomes of acupuncture over placebo and were of low quality. A number of small studies were promising and found that patients treated with acupuncture were most likely to have a negative urine drug screen.3 Although acupuncture is widely used in the United States to treat cocaine dependence, evidence does not confirm its efficacy.
Tobacco use disorder. A small group of studies favored acupuncture for smoking cessation.3 Other studies reported no benefit compared with placebo or neutral results. Some studies agreed that any intervention (acupuncture or sham acupuncture) with good results is better than no intervention at all.
Alcohol use disorder. Almost no advantage over placebo was found. Studies with significant findings were in small populations.3
Amphetamine, Cannabis, and other hallucinogen use disorders. Available data on stimulants were too limited to be relevant. No studies were found on Cannabis and hallucinogens.
Further studies are needed
There is a lack of conclusive, good quality studies supporting acupuncture’s benefits in treating substance abuse. Acupuncture has been known to lack adverse effects other than those related to needle manipulation, which is dependent on the methods (depth of needle insertion, accurate anatomical location, angle, etc.). Because this treatment option is virtually side-effect free, inexpensive, with positive synergistic results, more high-method quality studies are needed to consider it for our patients.
Acupuncture is an ancient therapeutic tool known to be the core of traditional Chinese medicine. Two theories suggest positive outcomes in patients treated with acupuncture:
- The oxidative stress reduction theory states that a “large body of evidences demonstrated that acupuncture has [an] antioxidative effect in various diseases, but the exact mechanism remains unclear.”1
- The neurophysiological theory states that “acupuncture stimulation can facilitate the release of certain neuropeptides in the CNS, eliciting profound physiological effects and even activating self-healing mechanisms.”2
For decades, acupuncture has been used for addiction management. Here we provide information on its utility for patients with substance use disorders.
Opioid use disorder. Multiple studies have looked at withdrawal, comorbid mood disorders, and its management with acupuncture alone or in combination with psychotherapy and/or opioid agonists. Studies from Asia reported good treatment outcomes but had low-method quality.3 Western studies had superior method quality but found that acupuncture was no better than placebo as monotherapy. When acupuncture is combined with psychotherapy and an opioid agonist, treatment results are promising, showing faster taper of medications (methadone and buprenorphine/naloxone) with fewer adverse effects.
Cocaine use disorder. Most studies had poor treatment outcomes of acupuncture over placebo and were of low quality. A number of small studies were promising and found that patients treated with acupuncture were most likely to have a negative urine drug screen.3 Although acupuncture is widely used in the United States to treat cocaine dependence, evidence does not confirm its efficacy.
Tobacco use disorder. A small group of studies favored acupuncture for smoking cessation.3 Other studies reported no benefit compared with placebo or neutral results. Some studies agreed that any intervention (acupuncture or sham acupuncture) with good results is better than no intervention at all.
Alcohol use disorder. Almost no advantage over placebo was found. Studies with significant findings were in small populations.3
Amphetamine, Cannabis, and other hallucinogen use disorders. Available data on stimulants were too limited to be relevant. No studies were found on Cannabis and hallucinogens.
Further studies are needed
There is a lack of conclusive, good quality studies supporting acupuncture’s benefits in treating substance abuse. Acupuncture has been known to lack adverse effects other than those related to needle manipulation, which is dependent on the methods (depth of needle insertion, accurate anatomical location, angle, etc.). Because this treatment option is virtually side-effect free, inexpensive, with positive synergistic results, more high-method quality studies are needed to consider it for our patients.
1. Zeng XH, Li QQ, Xu Q, et al. Acupuncture mechanism and redox equilibrium. Evid Based Complement and Alternat Med. 2014;2014:483294. doi: 10.1155/2014/483294
2. Bai L, Lao L. Neurobiological foundations of acupuncture: the relevance and future prospect based on neuroimaging evidence. Evid Based Complement and Alternat Med. 2013;2013:812568. doi: 10.1155/2013/812568.
3. Boyuan Z, Yang C, Ke C, et al. Efficacy of acupuncture for psychological symptoms associated with opioid addiction: a systematic review and meta-analysis. Evid Based Complement and Alternat Med. 2014;2014:313549. doi: 10.1155/2014/313549.
1. Zeng XH, Li QQ, Xu Q, et al. Acupuncture mechanism and redox equilibrium. Evid Based Complement and Alternat Med. 2014;2014:483294. doi: 10.1155/2014/483294
2. Bai L, Lao L. Neurobiological foundations of acupuncture: the relevance and future prospect based on neuroimaging evidence. Evid Based Complement and Alternat Med. 2013;2013:812568. doi: 10.1155/2013/812568.
3. Boyuan Z, Yang C, Ke C, et al. Efficacy of acupuncture for psychological symptoms associated with opioid addiction: a systematic review and meta-analysis. Evid Based Complement and Alternat Med. 2014;2014:313549. doi: 10.1155/2014/313549.