User login
Can lifestyle modifications delay or prevent Alzheimer’s disease?
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
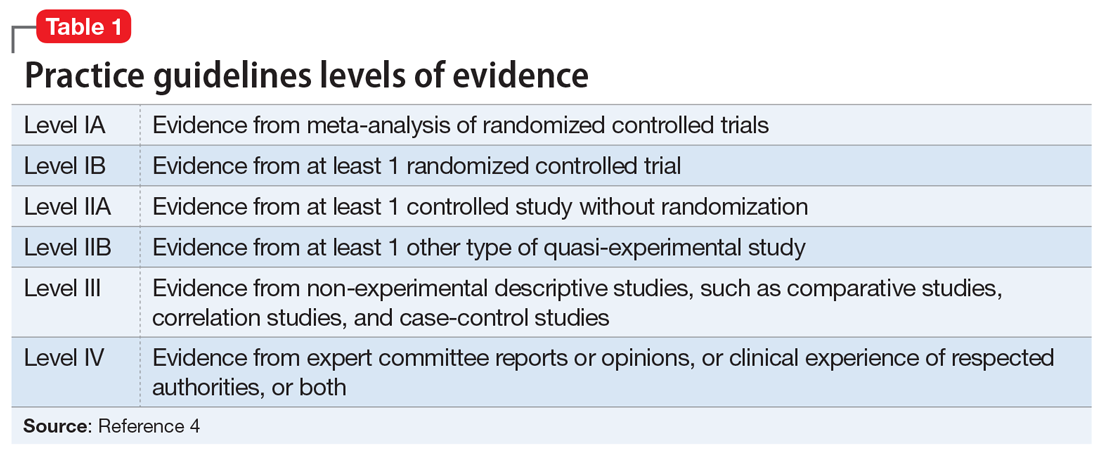
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
Abuse of psychiatric medications: Not just stimulants and benzodiazepines
While some classes of medications used to treat psychiatric disorders, such as stimulants and benzodiazepines, are well-recognized as controlled substances and drugs of abuse, clinicians may be less familiar with the potential misuse/abuse of other psychiatric medications. This article reviews the evidence related to the misuse/abuse of anticholinergics, antidepressants, antipsychotics, and gabapentinoids.
The terms “misuse,” “abuse,” and “addiction” are used variably in the literature without standardized definitions. For this review, “misuse/abuse (M/A)” will be used to collectively describe self-administration that is recreational or otherwise inconsistent with legal or medical guidelines, unless a specific distinction is made. Whether or not the medications reviewed are truly “addictive” will be briefly discussed for each drug class, but the focus will be on clinically relevant aspects of M/A, including:
- excessive self-administration
- self-administration by non-oral routes
- co-administration with other drugs of abuse
- malingering of psychiatric symptoms to obtain prescriptions
- diversion for sale to third parties
- toxicity from overdose.
Anticholinergic medications
The first case describing the deliberate M/A of an anticholinergic medication for its euphoric effects was published in 1960.Further reportsfollowed in Europe before the M/A potential of prescription anticholinergic medications among psychiatric patients with an overdose syndrome characterized by atropinism and toxic psychosis was more widely recognized in the United States in the 1970s. Most reported cases of M/A to date have occurred among patients with psychiatric illness because anticholinergic medications, including trihexyphenidyl, benztropine, biperiden, procyclidine, and orphenadrine, were commonly prescribed for the management of first-generation and high dopamine D2-affinity antipsychotic-induced extrapyramidal symptoms (EPS). For example, one study of 234 consecutively hospitalized patients with schizophrenia noted an anticholinergic M/A incidence of 6.5%.1
However, anticholinergic M/A is not limited to individuals with psychotic disorders. A UK study of 154 admissions to an inpatient unit specializing in behavioral disturbances found a 12-month trihexyphenidyl M/A incidence of 17%; the most common diagnosis among abusers was antisocial personality disorder.2 Anticholinergic M/A has also been reported among patients with a primary diagnosis of substance use disorders (SUDs)3 as well as more indiscriminately in prison settings,4 with some inmates exchanging trihexyphenidyl as currency and using it recreationally by crushing it into powder and smoking it with tobacco.5 Others have noted that abusers sometimes take anticholinergics with alcohol in order to “potentiate” the effects of each substance.6,7 Pullen et al8 described individuals with and without psychiatric illness who stole anticholinergic medications, purchased them from other patients, or bought them “on the street.” Malingering EPS in order to obtain anticholinergic medications has also been well documented.9 Clearly, anticholinergic M/A can occur in psychiatric and non-psychiatric populations, both within and outside of clinical settings. Although anticholinergic M/A appears to be less frequent in the United States now that second-generation antipsychotics (SGAs) are more frequently prescribed, M/A remains common in some settings outside of the United States.7
Among the various anticholinergic medications prescribed for EPS, trihexyphenidyl has been reported to have the greatest M/A potential, which has been attributed to its potency,10 its stimulating effects (whereas benztropine is more sedating),11 and its former popularity among prescribers.8 Marken et al11 published a review of 110 reports of M/A occurring in patients receiving anticholinergic medications as part of psychiatric treatment in which 69% of cases involved taking trihexyphenidyl 15 to 60 mg at a time (recommended dosing is 6 to 10 mg/d in divided doses).Most of these patients were prescribed anticholinergic medications for diagnostically appropriate reasons—only 7% were described as “true abusers” with no medical indication. Anticholinergic M/A was typically driven by a desire for euphoric and psychedelic/hallucinogenic effects, although in some cases, anticholinergic M/A was attributed to self-medication of EPS and depressive symptoms. These findings illustrate the blurred distinction between recreational use and perceived subjective benefit, and match those of a subsequent study of 50 psychiatric patients who reported anticholinergic M/A not only to “get high,” but to “decrease depression,” “increase energy,” and decrease antipsychotic adverse effects.12 Once again, trihexyphenidyl was the most frequently misused anticholinergic in this sample.
Table 12,3,7,8,10-15 outlines the subjective effects sought and experienced by anticholinergic abusers as well as potential toxic effects; there is the potential for overlap. Several authors have also described physiologic dependence with long-term trihexyphenidyl use, including tolerance and a withdrawal/abstinence syndrome.7,16 In addition, there have been several reports of coma13 and death in the setting of intended suicide by overdose of anticholinergic medications.14,15
Although anticholinergic M/A in the United States now appears to be less common, clinicians should remain aware of the M/A potential of anticholinergic medications prescribed for EPS. Management of M/A involves:
- detection
- reducing anticholinergic exposure by managing EPS with alternative strategies, such as switching or reducing the dose of the antipsychotic medication
- gradual tapering of anticholinergic medications to minimize withdrawal.11
Continue to: Antidepressants
Antidepressants
Haddad17 published a review of 21 English-language case reports from 1966 to 1998 describing antidepressant use in which individuals met DSM-IV criteria for substance dependence to the medication. An additional 14 cases of antidepressant M/A were excluded based on insufficient details to support a diagnosis of dependence. The 21 reported cases involved:
- tranylcypromine (a monoamine oxidase inhibitor [MAOI])
- amitriptyline (a tricyclic antidepressant [TCA])
- fluoxetine (a selective serotonin reuptake inhibitor [SSRI])
- amineptine (a TCA previously available in France but removed from the market in 1999 in part due to its abuse potential)
- nomifensine (a norepinephrine/dopamine reuptake inhibitor previously available in the United Kingdom but removed in 1986 due to hemolytic anemia).
In 95% of cases, the antidepressants were prescribed for treatment of an affective disorder but were abused for stimulant effects or the perceived ability to lift mood, cause euphoria or a “high,” or to improve functioning. Two-thirds of cases involved patients with preexisting substance misuse. Placing the case reports in the context of the millions of patients prescribed antidepressants during this period, Haddad concluded the “incidence of [antidepressant] addiction [is] so low as to be clinically irrelevant.”17
Despite this conclusion, Haddad singled out amineptine and tranylcypromine as antidepressants with some evidence of true addictive potential.17,18 A more recent case series described 14 patients who met DSM-IV criteria for substance abuse of tertiary amine TCAs (which have strong anticholinergic activity) and concluded that “misuse of [TCAs] is more common than generally appreciated.”19 In keeping with that claim, a study of 54 outpatients taking unspecified antidepressants found that up to 15% met DSM-III-R criteria for substance dependence (for the antidepressant) in the past year, although that rate was much lower than the rate of benzodiazepine dependence (47%) in a comparative sample.20 Finally, a comprehensive review by Evans and Sullivan21 found anecdotal reports published before 2014 that detailed misuse, abuse, and dependence with MAOIs, TCAs, fluoxetine, venlafaxine, bupropion, tianeptine, and amineptine. Taken together, existing evidence indicates that select individuals—typically those with other SUD comorbidity—sometimes misuse antidepressants in a way that suggests addiction.
Still, while it is well known that abrupt cessation of antidepressants can result in a discontinuation syndrome characterized by flu-like symptoms, nausea, and dizziness,22 physiologic withdrawal effects must be distinguished from historical definitions of substance “abuse” and the broader concept of psychological “addiction” or drug dependence18,23 now incorporated into the DSM-5 definition of SUDs.24 Indeed, although withdrawal symptoms were reported by more than half of those who took antidepressants and responded to a recent online survey,25 evidence to support the existence of significant antidepressant tolerance, craving, or compulsive use is lacking.17,18 Antidepressants as a class do not appear to be significantly rewarding or reinforcing and, on the contrary, discontinuation by patients is common in clinical practice.26 The popular claim that some individuals taking antidepressants “can’t quit”27 must also be disentangled from loss of therapeutic effects upon cessation.
Bupropion. A more convincing argument for antidepressant addiction can be made for bupropion, a weak norepinephrine and dopamine reuptake inhibitor with an otherwise unclear mechanism of action.28 In 2002, the first report of recreational bupropion M/A described a 13-year-old girl who took 2,400 mg orally (recommended maximum dose is 450 mg/d in divided doses) after being told it would give her “a better high than amphetamine.”29 This was followed in the same year by the first report of recreational M/A of bupropion via nasal insufflation (snorting), resulting in a seizure,30 and in 2013 by the first published case of M/A by IV self-administration.31
Continue to: The M/A potential of bupropion...
The M/A potential of bupropion, most commonly via intranasal administration, is now broadly recognized based on several case reports describing desired effects that include a euphoric high and a stimulating “buzz” similar to that of cocaine or methamphetamine but less intense.29-36 Among recreational users, bupropion tablets are referred to as “welbys,” “wellies,” “dubs,” or “barnies.”37 Media coverage of a 2013 outbreak of bupropion M/A in Toronto detailed administration by snorting, smoking, and injection, and described bupropion as “poor man’s cocaine.”38 Between 2003 and 2016, 2,232 cases of bupropion misuse/abuse/dependence adverse drug reactions were reported to the European Monitoring Agency.37 A review of intentional bupropion M/A reported to US Poison Control Centers between 2000 to 2013 found 975 such cases, with the yearly number tripling between 2000 and 2012.39 In this sample, nearly half (45%) of the users were age 13 to 19, and 76% of cases involved oral ingestion. In addition to bupropion M/A among younger people, individuals who misuse bupropion often include those with existing SUDs but limited access to illicit stimulants and those trying to evade detection by urine toxicology screening.33 For example, widespread use and diversion has been well documented within correctional settings, and as a result, many facilities have removed bupropion from their formularies.21,28,33,34,40
Beyond desired effects, the most common adverse events associated with bupropion M/A are listed in Table 2,28,30,32-34,36,39 along with their incidence based on cases brought to the attention of US Poison Control Centers.39 With relatively little evidence of a significant bupropion withdrawal syndrome,37 the argument in favor of modeling bupropion as a truly addictive drug is limited to anecdotal reports of cravings and compulsive self-administration35 and pro-dopaminergic activity (reuptake inhibition) that might provide a mechanism for potential rewarding and reinforcing effects.40 While early preclinical studies of bupropion failed to provide evidence of amphetamine-like abuse potential,41,42 non-oral administration in amounts well beyond therapeutic dosing could account for euphoric effects and a greater risk of psychological dependence and addiction.21,28,40
Bupropion also has an FDA indication as an aid to smoking cessation treatment, and the medication demonstrated early promise in the pharmacologic treatment of psychostimulant use disorders, with reported improvements in cravings and other SUD outcomes.43-45 However, subsequent randomized controlled trials (RCTs) failed to demonstrate a clear therapeutic role for bupropion in the treatment of cocaine46,47 and methamphetamine use disorders (although some secondary analyses suggest possible therapeutic effects among non-daily stimulant users who are able to maintain good adherence with bupropion).48-51 Given these overall discouraging results, the additive seizure risk of bupropion use with concomitant psychostimulant use, and the potential for M/A and diversion of bupropion (particularly among those with existing SUDs), the use of bupropion for the off-label treatment of stimulant use disorders is not advised.
Antipsychotics
As dopamine antagonists, antipsychotics are typically considered to have low potential for rewarding or reinforcing effects. Indeed, misuse of antipsychotics was a rarity in the first-generation era, with only a few published reports of haloperidol M/A within a small cluster of naïve young people who developed acute EPS,52 and a report of diversion in a prison with the “sadistic” intent of inflicting dystonic reactions on others.53 A more recent report described 2additional cases of M/A involving haloperidol and trifluoperazine.54 Some authors have described occasional drug-seeking behavior for low-potency D2 blockers such as chlorpromazine, presumably based on their M/A as anticholinergic medications.55
The potential for antipsychotic M/A has gained wider recognition since the advent of the SGAs. Three cases of prescription olanzapine M/A have been published to date. One involved a man who malingered manic symptoms to obtain olanzapine, taking ≥40 mg at a time (beyond his prescribed dose of 20 mg twice daily) to get a “buzz,” and combining it with alcohol and benzodiazepines for additive effects or to “come down” from cocaine.56 This patient noted that olanzapine was “a popular drug at parties” and was bought, sold, or traded among users, and occasionally administered intravenously. Two other cases described women who self-administered olanzapine, 40 to 50 mg/d, for euphoric and anxiolytic effects.57,58 James et al59 detailed a sample of 28 adults who reported “non-medical use” of olanzapine for anxiolytic effects, as a sleep aid, or to “escape from worries.”
Continue to: Quetiapine
Quetiapine. In contrast to some reports of olanzapine M/A in which the line between M/A and “self-medication” was blurred, quetiapine has become a more convincing example of clear recreational antipsychotic M/A. Since the first report of oral and intranasal quetiapine M/A in the Los Angeles County Jail published in 2004,55 subsequent cases have detailed other novel methods of recreational self-administration60-68 (Table 355,60-68), and additional reports have been published in non-English language journals.69,70 Collectively, these case reports have detailed that quetiapine is:
- misused for primary subjective effects as well as to mitigate the unpleasant effects of other drugs60,67
- referred to as “quell,”“Q,” “Susie-Q,” “squirrel,” and “baby heroin”55,71,72
- often obtained by malingering psychiatric symptoms55,61,63,65
- diverted/sold with “street value” both within and outside of psychiatric facilities and correctional settings.55,60-62,67,68,73
These anecdotal accounts of quetiapine M/A have since been corroborated on a larger scale based on several retrospective studies. Although early reports of quetiapine M/A occurring in correctional settings have resulted in formulary removal,71,74 quetiapine M/A is by no means limited to forensic populations and is especially common among those with comorbid SUDs. A survey of 74 patients enrolled in a Canadian methadone program reported that nearly 60% had misused quetiapine at some point.75 Among an Australian sample of 868 individuals with active IV drug abuse, 31% reported having misused quetiapine.76 Finally, within a small sample of patients with SUDs admitted to a detoxification unit in New York City, 17% reported M/A of SGAs.77 In this study, SGAs were often taken in conjunction with other drugs of abuse in order to “recover” from or “enhance” the effects of other drugs or to “experiment.” Quetiapine was by far the most frequently abused SGA, reported in 96% of the sample; the most frequently reported SGA/drug combinations were quetiapine/alcohol/opioids, quetiapine/cocaine, and quetiapine/opioids.
Looking more broadly at poison center data, reports to the US National Poison Data System (NPDS) from 2005 to 2011 included 3,116 cases of quetiapine abuse (37.5%, defined as intentional recreational use in order to obtain a “high”) or misuse (62.5%, defined as improper use or dosing for non-recreational purposes).78 A more recent analysis of NPDS reports from 2003 to 2013 found 2,118 cases of quetiapine abuse, representing 61% of all cases of reported SGA abuse.79 An analysis of the European Medicines Agency Adverse Drug Database yielded 18,112 reports of quetiapine misuse, abuse, dependence, and withdrawal for quetiapine (from 2005 to 2016) compared with 4,178 for olanzapine (from 2004 to 2016).80 These reports identified 368 fatalities associated with quetiapine.
The rate of quetiapine M/A appears to be increasing sharply. Reports of quetiapine M/A to poison centers in Australia increased nearly 7-fold from 2006 to 2016.81 Based on reports to the Drug Abuse Warning System, US emergency department visits for M/A of quetiapine increased from 19,195 in 2005 to 32,024 in 2011 (an average of 27,114 visits/year), with 75% of cases involving quetiapine taken in combination with other prescription drugs, alcohol, or illicit drugs.82 Consistent with poison center data, M/A was reported for other antipsychotics, but none nearly as frequently as for quetiapine.
With increasingly frequent quetiapine M/A, clinicians should be vigilant in monitoring for medical morbidity related to quetiapine and cumulative toxicity with other drugs. The most frequent adverse events associated with quetiapine M/A reported to US Poison Control Centers are presented in Table 4.78,79
Continue to: Unlike bupropion...
Unlike bupropion, quetiapine’s dopamine antagonism makes it unlikely to be a truly addictive drug, although this mechanism of action could mediate an increase in concurrent psychostimulant use.83 A few case reports have described a quetiapine discontinuation syndrome similar to that of antidepressants,60,65,84-88 but withdrawal symptoms suggestive of physiologic dependence may be mediated by non-dopaminergic effects through histamine and serotonin receptors.84,89 Evidence for quetiapine misuse being associated with craving and compulsive use is lacking, and true quetiapine addiction is probably rare.
Similar to bupropion, preliminary findings have suggested promise for quetiapine as a putative therapy for other SUDs.90-93 However, subsequent RCTs have failed to demonstrate a therapeutic effect for alcohol and cocaine use disorders.94-96 Given these negative results and the clear M/A potential of quetiapine, off-label use of quetiapine for the treatment of SUDs and psychiatric symptoms among those with SUDs must be considered judiciously, with an eye towards possible diversion and avoiding the substitution of one drug of abuse for another.
Gabapentinoids
In 1997, the first published case report of gabapentin M/A described a woman who self-administered her husband’s gabapentin to reduce cravings for and withdrawal from cocaine.97 The authors highlighted the possible therapeutic benefit of gabapentin in this regard rather than raising concerns about diversion and M/A. By 2004, however, reports of recreational gabapentin M/A emerged among inmates incarcerated within Florida correctional facilities who self-administered intranasal gabapentin to achieve a “high” that was “reminiscent of prior effects from intranasal ingestion of cocaine powder.”98 In 2007, a single case of gabapentin misuse up to 7,200 mg/d (recommended dosing is ≤3,600 mg/d) was reported, with documentation of both tolerance and withdrawal symptoms.99 As of 2017, a total of 36 cases of gabapentin M/A and 19 cases of pregabalin M/A have been published.100
In the past decade, anecdotal reports have given way to larger-scale epidemiologic data painting a clear picture of the now-widespread M/A of gabapentin and other gabapentinoids. For example, a study of online descriptions of gabapentin and pregabalin M/A from 2008 to 2010 documented:
- oral and IM use (gabapentin)
- IV and rectal (“plugging”) use (pregabalin)
- “parachuting” (emptying the contents of capsules for a larger dose) (pregabalin)
- euphoric, entactogenic, stimulant, calming/anxiolytic, and dissociative subjective effects (gabapentin/pregabalin)
- rapid development of tolerance to euphoric effects leading to self-administration of increasing doses (gabapentin/pregabalin)
- frequent co-administration with other drugs of abuse, including alcohol, benzodiazepines, cannabis, stimulants, opiates, hallucinogens, gamma-hydroxybutyrate, mephedrone, and Salvia divinorum (gabapentin/pregabalin)101
Several systematic reviews of both anecdotal reports and epidemiologic studies published in the past few years provide additional evidence of the above, such as:
- excessive dosing with self-administration
- intranasal and inhaled routes of administration
- diversion and “street value”
- greater M/A potential of pregabalin than gabapentin
- the presence of gabapentinoids in postmortem toxicology analyses, suggesting a role in overdose fatalities when combined with other drugs.100,102,103
Continue to: The European Medicine Agency's EudraVigilance database...
The European Medicine Agency’s EudraVigilance database included 4,301 reports of gabapentin misuse, abuse, or dependence, and 7,639 such reports for pregabalin, from 2006 to 2015 (rising sharply after 2012), with 86 gabapentin-related and 27 pregabalin-related fatalities.104 Data from the Drug Diversion Program of the Researched Abuse, Diversion, and Addiction-Related Surveillance System from 2002 to 2015 have likewise revealed that gabapentin diversion increased significantly in 2013.105
While the prevalence of gabapentinoid M/A is not known, rates appear to be significantly lower than for traditional drugs of abuse such as cannabis, cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and opioids.106,107 However, gabapentin and pregabalin M/A appears to be increasingly common among individuals with SUDs and in particular among those with opioid use disorders (OUDs). For example, a 2015 report indicated that 15% of an adult cohort in Appalachian Kentucky with nonmedical use of diverted prescription opioids reported gabapentin M/A, an increase of nearly 3,000% since 2008.108 Based on data from a US insurance enrollment and claims database, researchers found that the rate of gabapentin overuse among those also overusing opioids was 12% compared with only 2% for those using gabapentin alone.109 It has also been reported that gabapentin is sometimes used as a “cutting agent” for heroin.110
Those who use gabapentinoids together with opioids report that gabapentin and pregabalin potentiate the euphoric effects of methadone111 and endorse specific beliefs that pregabalin increases both the desired effects of heroin as well as negative effects such as “blackouts,” loss of control, and risk of overdose.112 Indeed, sustained M/A of gabapentin and opioids together has been found to increase emergency department utilization, drug-related hospitalization, and respiratory depression.113 Based on a case-control study of opioid users in Canada, co-prescription of gabapentin and opioids was associated with a 50% increase in death from opioid-related causes compared with prescription of opioids alone.114
Case reports documenting tolerance, withdrawal, craving, and loss of control suggest a true addictive potential for gabapentinoids, but Bonnet and Sherbaum100 concluded that while there is robust evidence of abusers “liking” gabapentin and pregabalin (eg, reward), evidence of “wanting” them (eg, psychological dependence) in the absence of other SUDs has been limited to only a few anecdotal reports with pregabalin. Accordingly, the risk of true addiction to gabapentinoids by those without preexisting SUDs appears to be low. Nonetheless, the M/A potential of both gabapentin and pregabalin is clear and in the context of a nationwide opioid epidemic, the increased morbidity/mortality risk related to combined use of gabapentinoids and opioids is both striking and concerning. Consequently, the state of Kentucky recently recognized the M/A potential of gabapentin by designating it a Schedule V controlled substance (pregabalin is already a Schedule V drug according to the US Drug Enforcement Agency),103,113 and several other states now mandate the reporting of gabapentin prescriptions to prescription drug monitoring programs.115
Following a similar pattern to antidepressants and antipsychotics, a potential role for gabapentin in the treatment of cocaine use disorders was supported in preliminary studies,116-118 but not in subsequent RCTs.119-121 However, there is evidence from RCTs to support the use of gabapentin and pregabalin in the treatment of alcohol use disorders.122-124 Gabapentin was also found to significantly reduce cannabis use and withdrawal symptoms in patients compared with placebo in an RCT of individuals with cannabis use disorders.125 The perceived safety of gabapentinoids by clinicians, their subjective desirability by patients with SUDs, and efficacy data supporting a therapeutic role in SUDs must be balanced with recognition that approximately 80% of gabapentin prescriptions are written for off-label indications for which there is little supporting evidence,109 such as low back pain.126 Clinicians considering prescribing gabapentinoids to manage psychiatric symptoms, such as anxiety and insomnia, should carefully consider the risk of M/A and other potential morbidities, especially in the setting of SUDs and OUD in particular.
Continue to: Problematic, even if not addictive
Problematic, even if not addictive
It is sometimes claimed that “addiction” to psychiatric medications is not limited to stimulants and benzodiazepines.27,127 Although anticholinergics, antidepressants, antipsychotics, and gabapentinoids can be drugs of abuse, with some users reporting physiologic withdrawal upon discontinuation, there is only limited evidence that the M/A of these psychiatric medications is associated with the characteristic features of a more complete definition of “addiction,” which may include:
- inability to consistently abstain
- impairment in behavioral control
- diminished recognition of significant problems associated with use
- a dysfunctional emotional response to chronic use.128
Nonetheless, the literature documenting anticholinergic, antidepressant, antipsychotic, and gabapentinoid M/A includes several common features, including:
- initial reports among those with limited access to illicit drugs (eg, young people and incarcerated individuals) and subsequent spread to a wider population with more unconventional routes of administration
- use for recreational purposes and other subjective pseudo-therapeutic effects, often in combination with alcohol and illicit drugs
- greater M/A potential of certain medications within each of these drug classes (eg, trihexyphenidyl, bupropion, quetiapine)
- malingering psychiatric symptoms in order to obtain medications from prescribers and diversion for black market sale
- observations that medications might constitute therapy for SUDs that were not supported in subsequent RCTs (with the exception of gabapentin for alcohol and cannabis use disorders)
- increasing evidence of toxicity related to M/A, which suggests that prescription by clinicians has limited benefit and high risk for patients with SUDs.
Bottom Line
Some psychiatric medications are taken as drugs of abuse. Clinicians should be particularly aware of the misuse/abuse potential of anticholinergics, antidepressants, antipsychotics, and gabapentinoids, and use them cautiously, if at all, when treating patients with existing substance use disorders.
Related Resources
- Substance Abuse and Mental Health Services Administration. Prescription drug misuse and abuse. https://www.samhsa.gov/topics/prescription-drug-misuse-abuse.
- Substance Abuse and Mental Health Services Administration. Types of commonly misused or abused drugs. https://www.samhsa.gov/prescription-drug-misuse-abuse/types.
- National Institute on Drug Abuse. Misuse of prescription drugs. https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/summary.
- National Institute on Drug Abuse. New clinician screening tool available for substance use. https://www.drugabuse.gov/news-events/news-releases/2018/06/newclinician-screening-tool-available-substance-use.
Drug Brand Names
Amitriptyline • Elavil, Endep
Benztropine • Cogentin
Biperiden • Akineton
Bupropion • Wellbutrin, Zyban
Chlorpromazine • Thorzine
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Orphenadrine • Disipal, Norflex
Pregabalin • Lyrica, Lyrica CR
Procyclidine • Kemadrin
Quetiapine • Seroquel
Tianeptine • Coaxil, Stablon
Tranylcypromine • Parnate
Trifluoperazine • Stelazine
Trihexyphenidyl • Artane, Tremin
Venlafaxine • Effexor
1. Zemishlany Z, Aizenberg D, Weiner Z, et al. Trihexyphenidyl (Artane) abuse in schizophrenic patients. Int Clin Psychopharmacol. 1996;11(3):199-202.
2. Crawshaw JA, Mullen PE. A study of benzhexol abuse. Brit J Psychiatry. 1984;145:300-303.
3. Woody GE, O’Brien CP. Anticholinergic toxic psychosis in drug abusers treated with benztropine. Comp Psychiatry. 1974;15(5):439-442.
4. Lowry TP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
5. Rouchell AM, Dixon SP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
6. Kaminer Y, Munitz H, Wijsenbeek H. Trihexyphenidyl (Artane) abuse: euphoriant and anxiolytic. Brit J Psychiatry. 1982;140(5):473-474.
7. Nappo SA, de Oliviera LG, Sanchez Zv, et al. Trihexyphenidyl (Artane): a Brazilian study of its abuse. Subst Use Misuse. 2005;40(4):473-482.
8. Pullen GP, Best NR, Macguire J. Anticholinergic drug abuse: a common problem? Brit Med J (Clin Res Ed). 1984;289(6445):612-613.
9. Rubinstein JS. Abuse of antiparkinsonian drugs: feigning of extrapyramidal symptoms to obtain trihexyphenidyl. JAMA. 1978;239(22):2365-2366.
10. Mohan D, Mohandas E, Dube S. Trihexyphenidyl abuse. Brit J Addiction. 1981:76(2);195-197.
11. Marken PA, Stoner SC, Bunker MT. Anticholinergic drug abuse and misuse. CNS Drugs. 1996;5(3):190-199.
12. Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatric Serv. 2000;51(7):928-929.
13. Goldstein MR, Kasper R. Hyperpyrexia and coma due to overdose of benztropine. South Med J. 1968;61(9):984.
14. Petkovi
15. McIntyre IM, Mallett P, Burton CG, et al. Acute benztropine intoxication and fatality. J Forensic Sci. 2014;59(6):1675-1678.
16. Dilsaver SC. Antimuscarinic agents as substances of abuse: A review. J Clin Psychopharmacol. 1988:8(1):14-22.
17. Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300-307.
18. Haddad PM. Do antidepressants cause dependence? Epidemiol Psichiatr Soc. 2005;14(2):58-62.
19. Shenouda R, Desan PH. Abuse of tricyclic antidepressant drugs: a case series. J Clin Psychopharmacol. 2013;33(3):440-442.
20. van Broekhoven F, Kan CC, Zitman FG. Dependence potential of antidepressants compared to benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):939-943.
21. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
22. Warner CH, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449-456.
23. Lichtigfeld FJ, Gillman MA. Antidepressants are not drugs of abuse or dependence. Postgrad Med J. 1998;74(875):529-532.
24. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
25. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73.
26. Haddad P, Anderson I. Antidepressants aren’t addictive: clinicians have depended on them for years. J Psychopharmacol. 1999;13(3):291-292.
27. Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html. Published April 7, 2018. Accessed December 11, 2018.
28. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158-161.
29. McCormick J. Recreational bupropion in a teenager. Br J Clin Pharmacol. 2002;53(2):214.
30. Welsh C, Doyon S. Seizure induced by insufflation of bupropion. N Engl J Med. 2002; 347(2):951.
31. Baribeau D, Araki KF. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J Addict Med. 2013;7(3):216-217.
32. Hill SH, Sikand H, Lee J. A case report of seizure induced by bupropion nasal insufflation. Prim Care Companion J Clin Psych. 2007;9(1):67-69.
33. Yoon G, Westermeyer J. Intranasal bupropion abuse. Am J Addict. 2013;22(2):180.
34. Reeves RR, Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol. 2013;33(4):584-585.
35. Oppek K, Koller G, Zwergal A, et al. Intravenous administration and abuse of bupropion: a case report and a review of the literature. J Addict Med. 2014;8(4):290-293.
36. Strike M, Hatcher S. Bupropion injection resulting in tissue necrosis and psychosis: previously undocumented complications of intravenous bupropion use disorder. J Addict Med. 2015;9(3):246-250.
37. Schifano F, Chiappini S. Is there a potential of misuse for venlafaxine and bupropion? Front Pharmacol. 2018;9:239.
38. Tryon J, Logan N. Antidepressant Wellbutrin becomes ‘poor man’s cocaine’ on Toronto streets. Global News. https://globalnews.ca/news/846576/antidepressant-wellbutrin-becomes-poor-mans-cocaine-on-toronto-streets/. Published September 18, 2013. Accessed December 11, 2018.
39. Stassinos GL, Klein-Schwartz W. Bupropion “abuse” reported to US Poison Centers. J Addict Med. 2016;10(5):357-362.
40. Hilliard WT, Barloon L, Farley P, et al. Bupropion diversion and misuse in the correctional facility. J Correct Health Care. 2013;19(3):211-217.
41. Griffith JD, Carranza J, Griffith C, et al. Bupropion clinical assay for amphetamine-like abuse potential. J Clin Psychiatry.1983;44(5 Pt 2):206-208.
42. Miller L, Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacol (Berl). 1983;80(3):199-205.
43. Berigan TR, Russell ML. Treatment of methamphetamine cravings with bupropion: A case report. Prim Care Companion J Clin Psychiatry. 2001;3(6):267-268.
44. Tardieu T, Poirier Y, Micallef J, et al. Amphetamine-like stimulant cessation in an abusing patient treated with bupropion. Acta Psychiatr Scand. 2004;109(1):75-78.
45. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced cravings. Neuropsychopharmacology. 2006;31(7):1537-1544.
46. Margolin A, Kosten TR, Avants SK, et al. A multicenter trial for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125-131.
47. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23.
48. Anderson AL, Li S, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind placebo-controlled trial. Drug Alcohol Depend. 2015;150:170-174.
49. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222-232.
50. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
51. Heinzerling KG, Swanson A, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878-1886.
52. Doenecke AL, Heuerman RC. Treatment of haloperidol abuse with diphenhydramine. Am J Psychiatry. 1980;137(4):487-488.
53. Weddington WW, Leventhal BL. Sadistic abuse of haloperidol. Am J Psychiatry. 1982;139:132-133.
54. Basu D, Marudkar M, Khurana H. Abuse of neuroleptic drugs by psychiatric patients. Indian J Med Sci. 2000;54(2):59-62.
55. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry 2004;161(9):1718.
56. Reeves RR. Abuse of olanzapine by substance abusers. J Psychoactive Drugs. 2007;39(3):297-299.
57. Kumsar NA, Erol A. Olanzapine abuse. Subst Abus. 2013;34(1):73-74.
58. Lai C. Olanzapine abuse was relieved after switching to aripiprazole in a patient with psychotic depression. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34(7):1363-1364.
59. James PD, Fida AS, Konovalov P, et al. Non-medical use of olanzapine by people on methadone treatment. BJPsych Bull. 2016;40(6):314-317.
60. Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J. 2007;100(8):834-836.
61. Murphy D, Bailey K, Stone M, et al. Addictive potential of quetiapine. Am J Psychiatry. 2008;165(7):918.
62. Paparrigopoulos T, Karaiskos D, Liappas J. Quetiapine: another drug with potential for misuse? J Clin Psychiatry. 2008;69(1):162-163.
63. Reeves RR, Burke RS. Abuse of the combination of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;16(5): doi: 10.4088/PCC.14l01660.
64. Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm. 2007;64(7):723-725.
65. Caniato RN, Gundabawady A, Baune BT, et al. Malingered psychotic symptoms and quetiapine abuse in a forensic setting. J Forens Psychiatr Psychol. 2009;20(6):928-935.
66. Hussain MZ, Waheed W, Hussain S. Intravenous quetiapine abuse. Am J Psychiatry. 2005; 162(9):1755-1756.
67. Waters BM, Joshi KG. Intravenous quetiapine-cocaine use (“Q-ball”). Am J Psychiatry. 2007;164(1):173-174.
68. Haridas A, Kushon D, Gurmu S, et al. Smoking quetiapine: a “Maq ball?” Prim Psychiatry. 2010;17:38-39.
69. Cubala WJ, Springer J. Quetiapine abuse and dependence in psychiatric patients: a systematic review of 25 case reports in the literature. J Subs Use. 2014;19(5):388-393.
70. Piróg-Balcerzak A, Habrat B, Mierzejewski P. Misuse and abuse of quetiapine [in Polish]. Psychiatr Pol. 2015;49(1):81-93.
71. Pinta ER, Taylor RE. Quetiapine addiction? Am J Psychiatry. 2007;164(1):174.
72. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Amer Acad Psychiatr Law. 2012;40(4):502-508.
73. Tarasoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164(2):350.
74. Reccoppa L. Less abuse potential with XR formulation of quetiapine. Am J Addiction. 2010;20(2):178.
75. McLarnon ME, Fulton HG, MacIsaac C, et al. Characteristics of quetiapine misuse among clients of a community-based methadone maintenance program. J Clin Psychopharmacol. 2012;32(5):721-723.
76. Reddel SE, Bruno R, Burns L, et al. Prevalence and associations of quetiapine fumarate misuse among an Australian national city sample of people who regularly inject drugs. Addiction. 2013;109(2):295-302.
77. Malekshahi T, Tioleco N, Ahmed N, et al. Misuse of atypical antipsychotics in conjunction with alcohol and other drugs of abuse. J Subs Abuse Treat. 2015;48(1):8-12.
78. Klein-Schwartz W, Schwartz EK, Anderson BD. Evaluation of quetiapine abuse and misuse reported to poison centers. J Addict Med. 2014;8(3):195-198.
79. Klein L, Bangh S, Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med. 2017;18(2):243-250.
80. Chiappini S, Schifano F. Is there a potential of misuse for quetiapine?: Literature review and analysis of the European Medicines Agency/European Medicines Agency Adverse Drug Reactions’ Database. J Clin Psychopharmacol. 2018;38(1):72-79.
81. Lee J, Pilgrim J, Gerostamoulos D, et al. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95-99.
82. Mattson ME, Albright VA, Yoon J, et al. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: Results from the
83. Brutcher RE, Nader SH, Nader MA. Evaluation of the reinforcing effect of quetiapine, alone and in combination with cocaine, in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(2):244-250.
84. Kim DR, Staab JP. Quetiapine discontinuation syndrome. Am J Psychiatry. 2005;162(5):1020.
85. Thurstone CC, Alahi P. A possible case of quetiapine withdrawal syndrome. J Clin Psychiatry. 2000;61(8):602-603.
86. Kohen I, Kremen N. A case report of quetiapine withdrawal syndrome in a geriatric patient. World J Biol Psychiatry. 2009;10(4 pt 3):985-986.
87. Yargic I, Caferov C. Quetiapine dependence and withdrawal: a case report. Subst Abus. 2011;32(3):168-169.
88. Koch HJ. Severe quetiapine withdrawal syndrome with nausea and vomiting in a 65-year-old patient with psychotic depression. Therapie. 2015;70(6):537-538.
89. Fischer BA, Boggs DL. The role of antihistaminic effects in the misuse of quetiapine: a case report and review of the literature. Neurosci Biobehav Rev. 2010;34(4):555-558.
90. Longoria J, Brown ES, Perantie DC, et al. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol. 2004;24(1):101-102.
91. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24(5):532-535.
92. Kennedy A, Wood AE, Saxon AJ, et al. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28(2):221-224.
93. Mariani JJ, Pavlicova M, Mamczur A, et al. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280-284.
94. Guardia J, Roncero C, Galan J, et al. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265-269.
95. Litten RZ, Fertig JB, Falk DE, et al; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406-416.
96. Tapp A, Wood AE, Kennedy A, et al. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24.
97. Markowitz JS, Finkenbine R, Myrick H, et al. Gabapentin abuse in a cocaine user: Implications for treatment. J Clin Psychopharmacol. 1997;17(5):423-424.
98. Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13(3):321-323.
99. Victorri-Vigneau C, Guelais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40(1):43-44.
100. Bonnet U, Sherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185-1215.
101. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118-122.
102. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
103. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
104. Chiappini S, Shifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647-654.
105. Buttram ME, Kurtz SP, Dart R, et al. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083-1086.
106. Kapil V, Green JL, Le Lait M, et al. Misuse of the y-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2013;78(1):190-191.
107. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Invest. 2017;37(8):763-773.
108. Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488.
109. Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy. 2018;38(4):436-443.
110. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):401-407.
111. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res. 2014;20(3):115-118.
112. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580-1589.
113. Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.
114. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e10022396. doi: 10.1371/journal.pmed.1002396.
115. Peckham AM, Fairman K, Sclar DA. Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed? Exp Opin Drug Saf. 2018;17(5):519-523.
116. Raby WN. Gabapentin for cocaine cravings. Am J Psychiatry. 2000;157(12):2058-2059.
117. Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J CLin Psychiatry. 2001;62(1):19-23.
118. Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84-86.
119. Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279-287.
120. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alc Depend. 2006;81(3):267-274.
121. Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2-3):274-277.
122. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
123. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
124. Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24(9):1367-1374.
125. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychpharmacology. 2012;27(7):1689-1698.
126. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793.
127. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407.
128. American Society of Addiction Medicine. Public policy statement: definition of addiction. https://www.asam.org/docs/default-source/public-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=a8f64512_4. Published August 15, 2011. Accessed July 23, 2018.
While some classes of medications used to treat psychiatric disorders, such as stimulants and benzodiazepines, are well-recognized as controlled substances and drugs of abuse, clinicians may be less familiar with the potential misuse/abuse of other psychiatric medications. This article reviews the evidence related to the misuse/abuse of anticholinergics, antidepressants, antipsychotics, and gabapentinoids.
The terms “misuse,” “abuse,” and “addiction” are used variably in the literature without standardized definitions. For this review, “misuse/abuse (M/A)” will be used to collectively describe self-administration that is recreational or otherwise inconsistent with legal or medical guidelines, unless a specific distinction is made. Whether or not the medications reviewed are truly “addictive” will be briefly discussed for each drug class, but the focus will be on clinically relevant aspects of M/A, including:
- excessive self-administration
- self-administration by non-oral routes
- co-administration with other drugs of abuse
- malingering of psychiatric symptoms to obtain prescriptions
- diversion for sale to third parties
- toxicity from overdose.
Anticholinergic medications
The first case describing the deliberate M/A of an anticholinergic medication for its euphoric effects was published in 1960.Further reportsfollowed in Europe before the M/A potential of prescription anticholinergic medications among psychiatric patients with an overdose syndrome characterized by atropinism and toxic psychosis was more widely recognized in the United States in the 1970s. Most reported cases of M/A to date have occurred among patients with psychiatric illness because anticholinergic medications, including trihexyphenidyl, benztropine, biperiden, procyclidine, and orphenadrine, were commonly prescribed for the management of first-generation and high dopamine D2-affinity antipsychotic-induced extrapyramidal symptoms (EPS). For example, one study of 234 consecutively hospitalized patients with schizophrenia noted an anticholinergic M/A incidence of 6.5%.1
However, anticholinergic M/A is not limited to individuals with psychotic disorders. A UK study of 154 admissions to an inpatient unit specializing in behavioral disturbances found a 12-month trihexyphenidyl M/A incidence of 17%; the most common diagnosis among abusers was antisocial personality disorder.2 Anticholinergic M/A has also been reported among patients with a primary diagnosis of substance use disorders (SUDs)3 as well as more indiscriminately in prison settings,4 with some inmates exchanging trihexyphenidyl as currency and using it recreationally by crushing it into powder and smoking it with tobacco.5 Others have noted that abusers sometimes take anticholinergics with alcohol in order to “potentiate” the effects of each substance.6,7 Pullen et al8 described individuals with and without psychiatric illness who stole anticholinergic medications, purchased them from other patients, or bought them “on the street.” Malingering EPS in order to obtain anticholinergic medications has also been well documented.9 Clearly, anticholinergic M/A can occur in psychiatric and non-psychiatric populations, both within and outside of clinical settings. Although anticholinergic M/A appears to be less frequent in the United States now that second-generation antipsychotics (SGAs) are more frequently prescribed, M/A remains common in some settings outside of the United States.7
Among the various anticholinergic medications prescribed for EPS, trihexyphenidyl has been reported to have the greatest M/A potential, which has been attributed to its potency,10 its stimulating effects (whereas benztropine is more sedating),11 and its former popularity among prescribers.8 Marken et al11 published a review of 110 reports of M/A occurring in patients receiving anticholinergic medications as part of psychiatric treatment in which 69% of cases involved taking trihexyphenidyl 15 to 60 mg at a time (recommended dosing is 6 to 10 mg/d in divided doses).Most of these patients were prescribed anticholinergic medications for diagnostically appropriate reasons—only 7% were described as “true abusers” with no medical indication. Anticholinergic M/A was typically driven by a desire for euphoric and psychedelic/hallucinogenic effects, although in some cases, anticholinergic M/A was attributed to self-medication of EPS and depressive symptoms. These findings illustrate the blurred distinction between recreational use and perceived subjective benefit, and match those of a subsequent study of 50 psychiatric patients who reported anticholinergic M/A not only to “get high,” but to “decrease depression,” “increase energy,” and decrease antipsychotic adverse effects.12 Once again, trihexyphenidyl was the most frequently misused anticholinergic in this sample.
Table 12,3,7,8,10-15 outlines the subjective effects sought and experienced by anticholinergic abusers as well as potential toxic effects; there is the potential for overlap. Several authors have also described physiologic dependence with long-term trihexyphenidyl use, including tolerance and a withdrawal/abstinence syndrome.7,16 In addition, there have been several reports of coma13 and death in the setting of intended suicide by overdose of anticholinergic medications.14,15
Although anticholinergic M/A in the United States now appears to be less common, clinicians should remain aware of the M/A potential of anticholinergic medications prescribed for EPS. Management of M/A involves:
- detection
- reducing anticholinergic exposure by managing EPS with alternative strategies, such as switching or reducing the dose of the antipsychotic medication
- gradual tapering of anticholinergic medications to minimize withdrawal.11
Continue to: Antidepressants
Antidepressants
Haddad17 published a review of 21 English-language case reports from 1966 to 1998 describing antidepressant use in which individuals met DSM-IV criteria for substance dependence to the medication. An additional 14 cases of antidepressant M/A were excluded based on insufficient details to support a diagnosis of dependence. The 21 reported cases involved:
- tranylcypromine (a monoamine oxidase inhibitor [MAOI])
- amitriptyline (a tricyclic antidepressant [TCA])
- fluoxetine (a selective serotonin reuptake inhibitor [SSRI])
- amineptine (a TCA previously available in France but removed from the market in 1999 in part due to its abuse potential)
- nomifensine (a norepinephrine/dopamine reuptake inhibitor previously available in the United Kingdom but removed in 1986 due to hemolytic anemia).
In 95% of cases, the antidepressants were prescribed for treatment of an affective disorder but were abused for stimulant effects or the perceived ability to lift mood, cause euphoria or a “high,” or to improve functioning. Two-thirds of cases involved patients with preexisting substance misuse. Placing the case reports in the context of the millions of patients prescribed antidepressants during this period, Haddad concluded the “incidence of [antidepressant] addiction [is] so low as to be clinically irrelevant.”17
Despite this conclusion, Haddad singled out amineptine and tranylcypromine as antidepressants with some evidence of true addictive potential.17,18 A more recent case series described 14 patients who met DSM-IV criteria for substance abuse of tertiary amine TCAs (which have strong anticholinergic activity) and concluded that “misuse of [TCAs] is more common than generally appreciated.”19 In keeping with that claim, a study of 54 outpatients taking unspecified antidepressants found that up to 15% met DSM-III-R criteria for substance dependence (for the antidepressant) in the past year, although that rate was much lower than the rate of benzodiazepine dependence (47%) in a comparative sample.20 Finally, a comprehensive review by Evans and Sullivan21 found anecdotal reports published before 2014 that detailed misuse, abuse, and dependence with MAOIs, TCAs, fluoxetine, venlafaxine, bupropion, tianeptine, and amineptine. Taken together, existing evidence indicates that select individuals—typically those with other SUD comorbidity—sometimes misuse antidepressants in a way that suggests addiction.
Still, while it is well known that abrupt cessation of antidepressants can result in a discontinuation syndrome characterized by flu-like symptoms, nausea, and dizziness,22 physiologic withdrawal effects must be distinguished from historical definitions of substance “abuse” and the broader concept of psychological “addiction” or drug dependence18,23 now incorporated into the DSM-5 definition of SUDs.24 Indeed, although withdrawal symptoms were reported by more than half of those who took antidepressants and responded to a recent online survey,25 evidence to support the existence of significant antidepressant tolerance, craving, or compulsive use is lacking.17,18 Antidepressants as a class do not appear to be significantly rewarding or reinforcing and, on the contrary, discontinuation by patients is common in clinical practice.26 The popular claim that some individuals taking antidepressants “can’t quit”27 must also be disentangled from loss of therapeutic effects upon cessation.
Bupropion. A more convincing argument for antidepressant addiction can be made for bupropion, a weak norepinephrine and dopamine reuptake inhibitor with an otherwise unclear mechanism of action.28 In 2002, the first report of recreational bupropion M/A described a 13-year-old girl who took 2,400 mg orally (recommended maximum dose is 450 mg/d in divided doses) after being told it would give her “a better high than amphetamine.”29 This was followed in the same year by the first report of recreational M/A of bupropion via nasal insufflation (snorting), resulting in a seizure,30 and in 2013 by the first published case of M/A by IV self-administration.31
Continue to: The M/A potential of bupropion...
The M/A potential of bupropion, most commonly via intranasal administration, is now broadly recognized based on several case reports describing desired effects that include a euphoric high and a stimulating “buzz” similar to that of cocaine or methamphetamine but less intense.29-36 Among recreational users, bupropion tablets are referred to as “welbys,” “wellies,” “dubs,” or “barnies.”37 Media coverage of a 2013 outbreak of bupropion M/A in Toronto detailed administration by snorting, smoking, and injection, and described bupropion as “poor man’s cocaine.”38 Between 2003 and 2016, 2,232 cases of bupropion misuse/abuse/dependence adverse drug reactions were reported to the European Monitoring Agency.37 A review of intentional bupropion M/A reported to US Poison Control Centers between 2000 to 2013 found 975 such cases, with the yearly number tripling between 2000 and 2012.39 In this sample, nearly half (45%) of the users were age 13 to 19, and 76% of cases involved oral ingestion. In addition to bupropion M/A among younger people, individuals who misuse bupropion often include those with existing SUDs but limited access to illicit stimulants and those trying to evade detection by urine toxicology screening.33 For example, widespread use and diversion has been well documented within correctional settings, and as a result, many facilities have removed bupropion from their formularies.21,28,33,34,40
Beyond desired effects, the most common adverse events associated with bupropion M/A are listed in Table 2,28,30,32-34,36,39 along with their incidence based on cases brought to the attention of US Poison Control Centers.39 With relatively little evidence of a significant bupropion withdrawal syndrome,37 the argument in favor of modeling bupropion as a truly addictive drug is limited to anecdotal reports of cravings and compulsive self-administration35 and pro-dopaminergic activity (reuptake inhibition) that might provide a mechanism for potential rewarding and reinforcing effects.40 While early preclinical studies of bupropion failed to provide evidence of amphetamine-like abuse potential,41,42 non-oral administration in amounts well beyond therapeutic dosing could account for euphoric effects and a greater risk of psychological dependence and addiction.21,28,40
Bupropion also has an FDA indication as an aid to smoking cessation treatment, and the medication demonstrated early promise in the pharmacologic treatment of psychostimulant use disorders, with reported improvements in cravings and other SUD outcomes.43-45 However, subsequent randomized controlled trials (RCTs) failed to demonstrate a clear therapeutic role for bupropion in the treatment of cocaine46,47 and methamphetamine use disorders (although some secondary analyses suggest possible therapeutic effects among non-daily stimulant users who are able to maintain good adherence with bupropion).48-51 Given these overall discouraging results, the additive seizure risk of bupropion use with concomitant psychostimulant use, and the potential for M/A and diversion of bupropion (particularly among those with existing SUDs), the use of bupropion for the off-label treatment of stimulant use disorders is not advised.
Antipsychotics
As dopamine antagonists, antipsychotics are typically considered to have low potential for rewarding or reinforcing effects. Indeed, misuse of antipsychotics was a rarity in the first-generation era, with only a few published reports of haloperidol M/A within a small cluster of naïve young people who developed acute EPS,52 and a report of diversion in a prison with the “sadistic” intent of inflicting dystonic reactions on others.53 A more recent report described 2additional cases of M/A involving haloperidol and trifluoperazine.54 Some authors have described occasional drug-seeking behavior for low-potency D2 blockers such as chlorpromazine, presumably based on their M/A as anticholinergic medications.55
The potential for antipsychotic M/A has gained wider recognition since the advent of the SGAs. Three cases of prescription olanzapine M/A have been published to date. One involved a man who malingered manic symptoms to obtain olanzapine, taking ≥40 mg at a time (beyond his prescribed dose of 20 mg twice daily) to get a “buzz,” and combining it with alcohol and benzodiazepines for additive effects or to “come down” from cocaine.56 This patient noted that olanzapine was “a popular drug at parties” and was bought, sold, or traded among users, and occasionally administered intravenously. Two other cases described women who self-administered olanzapine, 40 to 50 mg/d, for euphoric and anxiolytic effects.57,58 James et al59 detailed a sample of 28 adults who reported “non-medical use” of olanzapine for anxiolytic effects, as a sleep aid, or to “escape from worries.”
Continue to: Quetiapine
Quetiapine. In contrast to some reports of olanzapine M/A in which the line between M/A and “self-medication” was blurred, quetiapine has become a more convincing example of clear recreational antipsychotic M/A. Since the first report of oral and intranasal quetiapine M/A in the Los Angeles County Jail published in 2004,55 subsequent cases have detailed other novel methods of recreational self-administration60-68 (Table 355,60-68), and additional reports have been published in non-English language journals.69,70 Collectively, these case reports have detailed that quetiapine is:
- misused for primary subjective effects as well as to mitigate the unpleasant effects of other drugs60,67
- referred to as “quell,”“Q,” “Susie-Q,” “squirrel,” and “baby heroin”55,71,72
- often obtained by malingering psychiatric symptoms55,61,63,65
- diverted/sold with “street value” both within and outside of psychiatric facilities and correctional settings.55,60-62,67,68,73
These anecdotal accounts of quetiapine M/A have since been corroborated on a larger scale based on several retrospective studies. Although early reports of quetiapine M/A occurring in correctional settings have resulted in formulary removal,71,74 quetiapine M/A is by no means limited to forensic populations and is especially common among those with comorbid SUDs. A survey of 74 patients enrolled in a Canadian methadone program reported that nearly 60% had misused quetiapine at some point.75 Among an Australian sample of 868 individuals with active IV drug abuse, 31% reported having misused quetiapine.76 Finally, within a small sample of patients with SUDs admitted to a detoxification unit in New York City, 17% reported M/A of SGAs.77 In this study, SGAs were often taken in conjunction with other drugs of abuse in order to “recover” from or “enhance” the effects of other drugs or to “experiment.” Quetiapine was by far the most frequently abused SGA, reported in 96% of the sample; the most frequently reported SGA/drug combinations were quetiapine/alcohol/opioids, quetiapine/cocaine, and quetiapine/opioids.
Looking more broadly at poison center data, reports to the US National Poison Data System (NPDS) from 2005 to 2011 included 3,116 cases of quetiapine abuse (37.5%, defined as intentional recreational use in order to obtain a “high”) or misuse (62.5%, defined as improper use or dosing for non-recreational purposes).78 A more recent analysis of NPDS reports from 2003 to 2013 found 2,118 cases of quetiapine abuse, representing 61% of all cases of reported SGA abuse.79 An analysis of the European Medicines Agency Adverse Drug Database yielded 18,112 reports of quetiapine misuse, abuse, dependence, and withdrawal for quetiapine (from 2005 to 2016) compared with 4,178 for olanzapine (from 2004 to 2016).80 These reports identified 368 fatalities associated with quetiapine.
The rate of quetiapine M/A appears to be increasing sharply. Reports of quetiapine M/A to poison centers in Australia increased nearly 7-fold from 2006 to 2016.81 Based on reports to the Drug Abuse Warning System, US emergency department visits for M/A of quetiapine increased from 19,195 in 2005 to 32,024 in 2011 (an average of 27,114 visits/year), with 75% of cases involving quetiapine taken in combination with other prescription drugs, alcohol, or illicit drugs.82 Consistent with poison center data, M/A was reported for other antipsychotics, but none nearly as frequently as for quetiapine.
With increasingly frequent quetiapine M/A, clinicians should be vigilant in monitoring for medical morbidity related to quetiapine and cumulative toxicity with other drugs. The most frequent adverse events associated with quetiapine M/A reported to US Poison Control Centers are presented in Table 4.78,79
Continue to: Unlike bupropion...
Unlike bupropion, quetiapine’s dopamine antagonism makes it unlikely to be a truly addictive drug, although this mechanism of action could mediate an increase in concurrent psychostimulant use.83 A few case reports have described a quetiapine discontinuation syndrome similar to that of antidepressants,60,65,84-88 but withdrawal symptoms suggestive of physiologic dependence may be mediated by non-dopaminergic effects through histamine and serotonin receptors.84,89 Evidence for quetiapine misuse being associated with craving and compulsive use is lacking, and true quetiapine addiction is probably rare.
Similar to bupropion, preliminary findings have suggested promise for quetiapine as a putative therapy for other SUDs.90-93 However, subsequent RCTs have failed to demonstrate a therapeutic effect for alcohol and cocaine use disorders.94-96 Given these negative results and the clear M/A potential of quetiapine, off-label use of quetiapine for the treatment of SUDs and psychiatric symptoms among those with SUDs must be considered judiciously, with an eye towards possible diversion and avoiding the substitution of one drug of abuse for another.
Gabapentinoids
In 1997, the first published case report of gabapentin M/A described a woman who self-administered her husband’s gabapentin to reduce cravings for and withdrawal from cocaine.97 The authors highlighted the possible therapeutic benefit of gabapentin in this regard rather than raising concerns about diversion and M/A. By 2004, however, reports of recreational gabapentin M/A emerged among inmates incarcerated within Florida correctional facilities who self-administered intranasal gabapentin to achieve a “high” that was “reminiscent of prior effects from intranasal ingestion of cocaine powder.”98 In 2007, a single case of gabapentin misuse up to 7,200 mg/d (recommended dosing is ≤3,600 mg/d) was reported, with documentation of both tolerance and withdrawal symptoms.99 As of 2017, a total of 36 cases of gabapentin M/A and 19 cases of pregabalin M/A have been published.100
In the past decade, anecdotal reports have given way to larger-scale epidemiologic data painting a clear picture of the now-widespread M/A of gabapentin and other gabapentinoids. For example, a study of online descriptions of gabapentin and pregabalin M/A from 2008 to 2010 documented:
- oral and IM use (gabapentin)
- IV and rectal (“plugging”) use (pregabalin)
- “parachuting” (emptying the contents of capsules for a larger dose) (pregabalin)
- euphoric, entactogenic, stimulant, calming/anxiolytic, and dissociative subjective effects (gabapentin/pregabalin)
- rapid development of tolerance to euphoric effects leading to self-administration of increasing doses (gabapentin/pregabalin)
- frequent co-administration with other drugs of abuse, including alcohol, benzodiazepines, cannabis, stimulants, opiates, hallucinogens, gamma-hydroxybutyrate, mephedrone, and Salvia divinorum (gabapentin/pregabalin)101
Several systematic reviews of both anecdotal reports and epidemiologic studies published in the past few years provide additional evidence of the above, such as:
- excessive dosing with self-administration
- intranasal and inhaled routes of administration
- diversion and “street value”
- greater M/A potential of pregabalin than gabapentin
- the presence of gabapentinoids in postmortem toxicology analyses, suggesting a role in overdose fatalities when combined with other drugs.100,102,103
Continue to: The European Medicine Agency's EudraVigilance database...
The European Medicine Agency’s EudraVigilance database included 4,301 reports of gabapentin misuse, abuse, or dependence, and 7,639 such reports for pregabalin, from 2006 to 2015 (rising sharply after 2012), with 86 gabapentin-related and 27 pregabalin-related fatalities.104 Data from the Drug Diversion Program of the Researched Abuse, Diversion, and Addiction-Related Surveillance System from 2002 to 2015 have likewise revealed that gabapentin diversion increased significantly in 2013.105
While the prevalence of gabapentinoid M/A is not known, rates appear to be significantly lower than for traditional drugs of abuse such as cannabis, cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and opioids.106,107 However, gabapentin and pregabalin M/A appears to be increasingly common among individuals with SUDs and in particular among those with opioid use disorders (OUDs). For example, a 2015 report indicated that 15% of an adult cohort in Appalachian Kentucky with nonmedical use of diverted prescription opioids reported gabapentin M/A, an increase of nearly 3,000% since 2008.108 Based on data from a US insurance enrollment and claims database, researchers found that the rate of gabapentin overuse among those also overusing opioids was 12% compared with only 2% for those using gabapentin alone.109 It has also been reported that gabapentin is sometimes used as a “cutting agent” for heroin.110
Those who use gabapentinoids together with opioids report that gabapentin and pregabalin potentiate the euphoric effects of methadone111 and endorse specific beliefs that pregabalin increases both the desired effects of heroin as well as negative effects such as “blackouts,” loss of control, and risk of overdose.112 Indeed, sustained M/A of gabapentin and opioids together has been found to increase emergency department utilization, drug-related hospitalization, and respiratory depression.113 Based on a case-control study of opioid users in Canada, co-prescription of gabapentin and opioids was associated with a 50% increase in death from opioid-related causes compared with prescription of opioids alone.114
Case reports documenting tolerance, withdrawal, craving, and loss of control suggest a true addictive potential for gabapentinoids, but Bonnet and Sherbaum100 concluded that while there is robust evidence of abusers “liking” gabapentin and pregabalin (eg, reward), evidence of “wanting” them (eg, psychological dependence) in the absence of other SUDs has been limited to only a few anecdotal reports with pregabalin. Accordingly, the risk of true addiction to gabapentinoids by those without preexisting SUDs appears to be low. Nonetheless, the M/A potential of both gabapentin and pregabalin is clear and in the context of a nationwide opioid epidemic, the increased morbidity/mortality risk related to combined use of gabapentinoids and opioids is both striking and concerning. Consequently, the state of Kentucky recently recognized the M/A potential of gabapentin by designating it a Schedule V controlled substance (pregabalin is already a Schedule V drug according to the US Drug Enforcement Agency),103,113 and several other states now mandate the reporting of gabapentin prescriptions to prescription drug monitoring programs.115
Following a similar pattern to antidepressants and antipsychotics, a potential role for gabapentin in the treatment of cocaine use disorders was supported in preliminary studies,116-118 but not in subsequent RCTs.119-121 However, there is evidence from RCTs to support the use of gabapentin and pregabalin in the treatment of alcohol use disorders.122-124 Gabapentin was also found to significantly reduce cannabis use and withdrawal symptoms in patients compared with placebo in an RCT of individuals with cannabis use disorders.125 The perceived safety of gabapentinoids by clinicians, their subjective desirability by patients with SUDs, and efficacy data supporting a therapeutic role in SUDs must be balanced with recognition that approximately 80% of gabapentin prescriptions are written for off-label indications for which there is little supporting evidence,109 such as low back pain.126 Clinicians considering prescribing gabapentinoids to manage psychiatric symptoms, such as anxiety and insomnia, should carefully consider the risk of M/A and other potential morbidities, especially in the setting of SUDs and OUD in particular.
Continue to: Problematic, even if not addictive
Problematic, even if not addictive
It is sometimes claimed that “addiction” to psychiatric medications is not limited to stimulants and benzodiazepines.27,127 Although anticholinergics, antidepressants, antipsychotics, and gabapentinoids can be drugs of abuse, with some users reporting physiologic withdrawal upon discontinuation, there is only limited evidence that the M/A of these psychiatric medications is associated with the characteristic features of a more complete definition of “addiction,” which may include:
- inability to consistently abstain
- impairment in behavioral control
- diminished recognition of significant problems associated with use
- a dysfunctional emotional response to chronic use.128
Nonetheless, the literature documenting anticholinergic, antidepressant, antipsychotic, and gabapentinoid M/A includes several common features, including:
- initial reports among those with limited access to illicit drugs (eg, young people and incarcerated individuals) and subsequent spread to a wider population with more unconventional routes of administration
- use for recreational purposes and other subjective pseudo-therapeutic effects, often in combination with alcohol and illicit drugs
- greater M/A potential of certain medications within each of these drug classes (eg, trihexyphenidyl, bupropion, quetiapine)
- malingering psychiatric symptoms in order to obtain medications from prescribers and diversion for black market sale
- observations that medications might constitute therapy for SUDs that were not supported in subsequent RCTs (with the exception of gabapentin for alcohol and cannabis use disorders)
- increasing evidence of toxicity related to M/A, which suggests that prescription by clinicians has limited benefit and high risk for patients with SUDs.
Bottom Line
Some psychiatric medications are taken as drugs of abuse. Clinicians should be particularly aware of the misuse/abuse potential of anticholinergics, antidepressants, antipsychotics, and gabapentinoids, and use them cautiously, if at all, when treating patients with existing substance use disorders.
Related Resources
- Substance Abuse and Mental Health Services Administration. Prescription drug misuse and abuse. https://www.samhsa.gov/topics/prescription-drug-misuse-abuse.
- Substance Abuse and Mental Health Services Administration. Types of commonly misused or abused drugs. https://www.samhsa.gov/prescription-drug-misuse-abuse/types.
- National Institute on Drug Abuse. Misuse of prescription drugs. https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/summary.
- National Institute on Drug Abuse. New clinician screening tool available for substance use. https://www.drugabuse.gov/news-events/news-releases/2018/06/newclinician-screening-tool-available-substance-use.
Drug Brand Names
Amitriptyline • Elavil, Endep
Benztropine • Cogentin
Biperiden • Akineton
Bupropion • Wellbutrin, Zyban
Chlorpromazine • Thorzine
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Orphenadrine • Disipal, Norflex
Pregabalin • Lyrica, Lyrica CR
Procyclidine • Kemadrin
Quetiapine • Seroquel
Tianeptine • Coaxil, Stablon
Tranylcypromine • Parnate
Trifluoperazine • Stelazine
Trihexyphenidyl • Artane, Tremin
Venlafaxine • Effexor
While some classes of medications used to treat psychiatric disorders, such as stimulants and benzodiazepines, are well-recognized as controlled substances and drugs of abuse, clinicians may be less familiar with the potential misuse/abuse of other psychiatric medications. This article reviews the evidence related to the misuse/abuse of anticholinergics, antidepressants, antipsychotics, and gabapentinoids.
The terms “misuse,” “abuse,” and “addiction” are used variably in the literature without standardized definitions. For this review, “misuse/abuse (M/A)” will be used to collectively describe self-administration that is recreational or otherwise inconsistent with legal or medical guidelines, unless a specific distinction is made. Whether or not the medications reviewed are truly “addictive” will be briefly discussed for each drug class, but the focus will be on clinically relevant aspects of M/A, including:
- excessive self-administration
- self-administration by non-oral routes
- co-administration with other drugs of abuse
- malingering of psychiatric symptoms to obtain prescriptions
- diversion for sale to third parties
- toxicity from overdose.
Anticholinergic medications
The first case describing the deliberate M/A of an anticholinergic medication for its euphoric effects was published in 1960.Further reportsfollowed in Europe before the M/A potential of prescription anticholinergic medications among psychiatric patients with an overdose syndrome characterized by atropinism and toxic psychosis was more widely recognized in the United States in the 1970s. Most reported cases of M/A to date have occurred among patients with psychiatric illness because anticholinergic medications, including trihexyphenidyl, benztropine, biperiden, procyclidine, and orphenadrine, were commonly prescribed for the management of first-generation and high dopamine D2-affinity antipsychotic-induced extrapyramidal symptoms (EPS). For example, one study of 234 consecutively hospitalized patients with schizophrenia noted an anticholinergic M/A incidence of 6.5%.1
However, anticholinergic M/A is not limited to individuals with psychotic disorders. A UK study of 154 admissions to an inpatient unit specializing in behavioral disturbances found a 12-month trihexyphenidyl M/A incidence of 17%; the most common diagnosis among abusers was antisocial personality disorder.2 Anticholinergic M/A has also been reported among patients with a primary diagnosis of substance use disorders (SUDs)3 as well as more indiscriminately in prison settings,4 with some inmates exchanging trihexyphenidyl as currency and using it recreationally by crushing it into powder and smoking it with tobacco.5 Others have noted that abusers sometimes take anticholinergics with alcohol in order to “potentiate” the effects of each substance.6,7 Pullen et al8 described individuals with and without psychiatric illness who stole anticholinergic medications, purchased them from other patients, or bought them “on the street.” Malingering EPS in order to obtain anticholinergic medications has also been well documented.9 Clearly, anticholinergic M/A can occur in psychiatric and non-psychiatric populations, both within and outside of clinical settings. Although anticholinergic M/A appears to be less frequent in the United States now that second-generation antipsychotics (SGAs) are more frequently prescribed, M/A remains common in some settings outside of the United States.7
Among the various anticholinergic medications prescribed for EPS, trihexyphenidyl has been reported to have the greatest M/A potential, which has been attributed to its potency,10 its stimulating effects (whereas benztropine is more sedating),11 and its former popularity among prescribers.8 Marken et al11 published a review of 110 reports of M/A occurring in patients receiving anticholinergic medications as part of psychiatric treatment in which 69% of cases involved taking trihexyphenidyl 15 to 60 mg at a time (recommended dosing is 6 to 10 mg/d in divided doses).Most of these patients were prescribed anticholinergic medications for diagnostically appropriate reasons—only 7% were described as “true abusers” with no medical indication. Anticholinergic M/A was typically driven by a desire for euphoric and psychedelic/hallucinogenic effects, although in some cases, anticholinergic M/A was attributed to self-medication of EPS and depressive symptoms. These findings illustrate the blurred distinction between recreational use and perceived subjective benefit, and match those of a subsequent study of 50 psychiatric patients who reported anticholinergic M/A not only to “get high,” but to “decrease depression,” “increase energy,” and decrease antipsychotic adverse effects.12 Once again, trihexyphenidyl was the most frequently misused anticholinergic in this sample.
Table 12,3,7,8,10-15 outlines the subjective effects sought and experienced by anticholinergic abusers as well as potential toxic effects; there is the potential for overlap. Several authors have also described physiologic dependence with long-term trihexyphenidyl use, including tolerance and a withdrawal/abstinence syndrome.7,16 In addition, there have been several reports of coma13 and death in the setting of intended suicide by overdose of anticholinergic medications.14,15
Although anticholinergic M/A in the United States now appears to be less common, clinicians should remain aware of the M/A potential of anticholinergic medications prescribed for EPS. Management of M/A involves:
- detection
- reducing anticholinergic exposure by managing EPS with alternative strategies, such as switching or reducing the dose of the antipsychotic medication
- gradual tapering of anticholinergic medications to minimize withdrawal.11
Continue to: Antidepressants
Antidepressants
Haddad17 published a review of 21 English-language case reports from 1966 to 1998 describing antidepressant use in which individuals met DSM-IV criteria for substance dependence to the medication. An additional 14 cases of antidepressant M/A were excluded based on insufficient details to support a diagnosis of dependence. The 21 reported cases involved:
- tranylcypromine (a monoamine oxidase inhibitor [MAOI])
- amitriptyline (a tricyclic antidepressant [TCA])
- fluoxetine (a selective serotonin reuptake inhibitor [SSRI])
- amineptine (a TCA previously available in France but removed from the market in 1999 in part due to its abuse potential)
- nomifensine (a norepinephrine/dopamine reuptake inhibitor previously available in the United Kingdom but removed in 1986 due to hemolytic anemia).
In 95% of cases, the antidepressants were prescribed for treatment of an affective disorder but were abused for stimulant effects or the perceived ability to lift mood, cause euphoria or a “high,” or to improve functioning. Two-thirds of cases involved patients with preexisting substance misuse. Placing the case reports in the context of the millions of patients prescribed antidepressants during this period, Haddad concluded the “incidence of [antidepressant] addiction [is] so low as to be clinically irrelevant.”17
Despite this conclusion, Haddad singled out amineptine and tranylcypromine as antidepressants with some evidence of true addictive potential.17,18 A more recent case series described 14 patients who met DSM-IV criteria for substance abuse of tertiary amine TCAs (which have strong anticholinergic activity) and concluded that “misuse of [TCAs] is more common than generally appreciated.”19 In keeping with that claim, a study of 54 outpatients taking unspecified antidepressants found that up to 15% met DSM-III-R criteria for substance dependence (for the antidepressant) in the past year, although that rate was much lower than the rate of benzodiazepine dependence (47%) in a comparative sample.20 Finally, a comprehensive review by Evans and Sullivan21 found anecdotal reports published before 2014 that detailed misuse, abuse, and dependence with MAOIs, TCAs, fluoxetine, venlafaxine, bupropion, tianeptine, and amineptine. Taken together, existing evidence indicates that select individuals—typically those with other SUD comorbidity—sometimes misuse antidepressants in a way that suggests addiction.
Still, while it is well known that abrupt cessation of antidepressants can result in a discontinuation syndrome characterized by flu-like symptoms, nausea, and dizziness,22 physiologic withdrawal effects must be distinguished from historical definitions of substance “abuse” and the broader concept of psychological “addiction” or drug dependence18,23 now incorporated into the DSM-5 definition of SUDs.24 Indeed, although withdrawal symptoms were reported by more than half of those who took antidepressants and responded to a recent online survey,25 evidence to support the existence of significant antidepressant tolerance, craving, or compulsive use is lacking.17,18 Antidepressants as a class do not appear to be significantly rewarding or reinforcing and, on the contrary, discontinuation by patients is common in clinical practice.26 The popular claim that some individuals taking antidepressants “can’t quit”27 must also be disentangled from loss of therapeutic effects upon cessation.
Bupropion. A more convincing argument for antidepressant addiction can be made for bupropion, a weak norepinephrine and dopamine reuptake inhibitor with an otherwise unclear mechanism of action.28 In 2002, the first report of recreational bupropion M/A described a 13-year-old girl who took 2,400 mg orally (recommended maximum dose is 450 mg/d in divided doses) after being told it would give her “a better high than amphetamine.”29 This was followed in the same year by the first report of recreational M/A of bupropion via nasal insufflation (snorting), resulting in a seizure,30 and in 2013 by the first published case of M/A by IV self-administration.31
Continue to: The M/A potential of bupropion...
The M/A potential of bupropion, most commonly via intranasal administration, is now broadly recognized based on several case reports describing desired effects that include a euphoric high and a stimulating “buzz” similar to that of cocaine or methamphetamine but less intense.29-36 Among recreational users, bupropion tablets are referred to as “welbys,” “wellies,” “dubs,” or “barnies.”37 Media coverage of a 2013 outbreak of bupropion M/A in Toronto detailed administration by snorting, smoking, and injection, and described bupropion as “poor man’s cocaine.”38 Between 2003 and 2016, 2,232 cases of bupropion misuse/abuse/dependence adverse drug reactions were reported to the European Monitoring Agency.37 A review of intentional bupropion M/A reported to US Poison Control Centers between 2000 to 2013 found 975 such cases, with the yearly number tripling between 2000 and 2012.39 In this sample, nearly half (45%) of the users were age 13 to 19, and 76% of cases involved oral ingestion. In addition to bupropion M/A among younger people, individuals who misuse bupropion often include those with existing SUDs but limited access to illicit stimulants and those trying to evade detection by urine toxicology screening.33 For example, widespread use and diversion has been well documented within correctional settings, and as a result, many facilities have removed bupropion from their formularies.21,28,33,34,40
Beyond desired effects, the most common adverse events associated with bupropion M/A are listed in Table 2,28,30,32-34,36,39 along with their incidence based on cases brought to the attention of US Poison Control Centers.39 With relatively little evidence of a significant bupropion withdrawal syndrome,37 the argument in favor of modeling bupropion as a truly addictive drug is limited to anecdotal reports of cravings and compulsive self-administration35 and pro-dopaminergic activity (reuptake inhibition) that might provide a mechanism for potential rewarding and reinforcing effects.40 While early preclinical studies of bupropion failed to provide evidence of amphetamine-like abuse potential,41,42 non-oral administration in amounts well beyond therapeutic dosing could account for euphoric effects and a greater risk of psychological dependence and addiction.21,28,40
Bupropion also has an FDA indication as an aid to smoking cessation treatment, and the medication demonstrated early promise in the pharmacologic treatment of psychostimulant use disorders, with reported improvements in cravings and other SUD outcomes.43-45 However, subsequent randomized controlled trials (RCTs) failed to demonstrate a clear therapeutic role for bupropion in the treatment of cocaine46,47 and methamphetamine use disorders (although some secondary analyses suggest possible therapeutic effects among non-daily stimulant users who are able to maintain good adherence with bupropion).48-51 Given these overall discouraging results, the additive seizure risk of bupropion use with concomitant psychostimulant use, and the potential for M/A and diversion of bupropion (particularly among those with existing SUDs), the use of bupropion for the off-label treatment of stimulant use disorders is not advised.
Antipsychotics
As dopamine antagonists, antipsychotics are typically considered to have low potential for rewarding or reinforcing effects. Indeed, misuse of antipsychotics was a rarity in the first-generation era, with only a few published reports of haloperidol M/A within a small cluster of naïve young people who developed acute EPS,52 and a report of diversion in a prison with the “sadistic” intent of inflicting dystonic reactions on others.53 A more recent report described 2additional cases of M/A involving haloperidol and trifluoperazine.54 Some authors have described occasional drug-seeking behavior for low-potency D2 blockers such as chlorpromazine, presumably based on their M/A as anticholinergic medications.55
The potential for antipsychotic M/A has gained wider recognition since the advent of the SGAs. Three cases of prescription olanzapine M/A have been published to date. One involved a man who malingered manic symptoms to obtain olanzapine, taking ≥40 mg at a time (beyond his prescribed dose of 20 mg twice daily) to get a “buzz,” and combining it with alcohol and benzodiazepines for additive effects or to “come down” from cocaine.56 This patient noted that olanzapine was “a popular drug at parties” and was bought, sold, or traded among users, and occasionally administered intravenously. Two other cases described women who self-administered olanzapine, 40 to 50 mg/d, for euphoric and anxiolytic effects.57,58 James et al59 detailed a sample of 28 adults who reported “non-medical use” of olanzapine for anxiolytic effects, as a sleep aid, or to “escape from worries.”
Continue to: Quetiapine
Quetiapine. In contrast to some reports of olanzapine M/A in which the line between M/A and “self-medication” was blurred, quetiapine has become a more convincing example of clear recreational antipsychotic M/A. Since the first report of oral and intranasal quetiapine M/A in the Los Angeles County Jail published in 2004,55 subsequent cases have detailed other novel methods of recreational self-administration60-68 (Table 355,60-68), and additional reports have been published in non-English language journals.69,70 Collectively, these case reports have detailed that quetiapine is:
- misused for primary subjective effects as well as to mitigate the unpleasant effects of other drugs60,67
- referred to as “quell,”“Q,” “Susie-Q,” “squirrel,” and “baby heroin”55,71,72
- often obtained by malingering psychiatric symptoms55,61,63,65
- diverted/sold with “street value” both within and outside of psychiatric facilities and correctional settings.55,60-62,67,68,73
These anecdotal accounts of quetiapine M/A have since been corroborated on a larger scale based on several retrospective studies. Although early reports of quetiapine M/A occurring in correctional settings have resulted in formulary removal,71,74 quetiapine M/A is by no means limited to forensic populations and is especially common among those with comorbid SUDs. A survey of 74 patients enrolled in a Canadian methadone program reported that nearly 60% had misused quetiapine at some point.75 Among an Australian sample of 868 individuals with active IV drug abuse, 31% reported having misused quetiapine.76 Finally, within a small sample of patients with SUDs admitted to a detoxification unit in New York City, 17% reported M/A of SGAs.77 In this study, SGAs were often taken in conjunction with other drugs of abuse in order to “recover” from or “enhance” the effects of other drugs or to “experiment.” Quetiapine was by far the most frequently abused SGA, reported in 96% of the sample; the most frequently reported SGA/drug combinations were quetiapine/alcohol/opioids, quetiapine/cocaine, and quetiapine/opioids.
Looking more broadly at poison center data, reports to the US National Poison Data System (NPDS) from 2005 to 2011 included 3,116 cases of quetiapine abuse (37.5%, defined as intentional recreational use in order to obtain a “high”) or misuse (62.5%, defined as improper use or dosing for non-recreational purposes).78 A more recent analysis of NPDS reports from 2003 to 2013 found 2,118 cases of quetiapine abuse, representing 61% of all cases of reported SGA abuse.79 An analysis of the European Medicines Agency Adverse Drug Database yielded 18,112 reports of quetiapine misuse, abuse, dependence, and withdrawal for quetiapine (from 2005 to 2016) compared with 4,178 for olanzapine (from 2004 to 2016).80 These reports identified 368 fatalities associated with quetiapine.
The rate of quetiapine M/A appears to be increasing sharply. Reports of quetiapine M/A to poison centers in Australia increased nearly 7-fold from 2006 to 2016.81 Based on reports to the Drug Abuse Warning System, US emergency department visits for M/A of quetiapine increased from 19,195 in 2005 to 32,024 in 2011 (an average of 27,114 visits/year), with 75% of cases involving quetiapine taken in combination with other prescription drugs, alcohol, or illicit drugs.82 Consistent with poison center data, M/A was reported for other antipsychotics, but none nearly as frequently as for quetiapine.
With increasingly frequent quetiapine M/A, clinicians should be vigilant in monitoring for medical morbidity related to quetiapine and cumulative toxicity with other drugs. The most frequent adverse events associated with quetiapine M/A reported to US Poison Control Centers are presented in Table 4.78,79
Continue to: Unlike bupropion...
Unlike bupropion, quetiapine’s dopamine antagonism makes it unlikely to be a truly addictive drug, although this mechanism of action could mediate an increase in concurrent psychostimulant use.83 A few case reports have described a quetiapine discontinuation syndrome similar to that of antidepressants,60,65,84-88 but withdrawal symptoms suggestive of physiologic dependence may be mediated by non-dopaminergic effects through histamine and serotonin receptors.84,89 Evidence for quetiapine misuse being associated with craving and compulsive use is lacking, and true quetiapine addiction is probably rare.
Similar to bupropion, preliminary findings have suggested promise for quetiapine as a putative therapy for other SUDs.90-93 However, subsequent RCTs have failed to demonstrate a therapeutic effect for alcohol and cocaine use disorders.94-96 Given these negative results and the clear M/A potential of quetiapine, off-label use of quetiapine for the treatment of SUDs and psychiatric symptoms among those with SUDs must be considered judiciously, with an eye towards possible diversion and avoiding the substitution of one drug of abuse for another.
Gabapentinoids
In 1997, the first published case report of gabapentin M/A described a woman who self-administered her husband’s gabapentin to reduce cravings for and withdrawal from cocaine.97 The authors highlighted the possible therapeutic benefit of gabapentin in this regard rather than raising concerns about diversion and M/A. By 2004, however, reports of recreational gabapentin M/A emerged among inmates incarcerated within Florida correctional facilities who self-administered intranasal gabapentin to achieve a “high” that was “reminiscent of prior effects from intranasal ingestion of cocaine powder.”98 In 2007, a single case of gabapentin misuse up to 7,200 mg/d (recommended dosing is ≤3,600 mg/d) was reported, with documentation of both tolerance and withdrawal symptoms.99 As of 2017, a total of 36 cases of gabapentin M/A and 19 cases of pregabalin M/A have been published.100
In the past decade, anecdotal reports have given way to larger-scale epidemiologic data painting a clear picture of the now-widespread M/A of gabapentin and other gabapentinoids. For example, a study of online descriptions of gabapentin and pregabalin M/A from 2008 to 2010 documented:
- oral and IM use (gabapentin)
- IV and rectal (“plugging”) use (pregabalin)
- “parachuting” (emptying the contents of capsules for a larger dose) (pregabalin)
- euphoric, entactogenic, stimulant, calming/anxiolytic, and dissociative subjective effects (gabapentin/pregabalin)
- rapid development of tolerance to euphoric effects leading to self-administration of increasing doses (gabapentin/pregabalin)
- frequent co-administration with other drugs of abuse, including alcohol, benzodiazepines, cannabis, stimulants, opiates, hallucinogens, gamma-hydroxybutyrate, mephedrone, and Salvia divinorum (gabapentin/pregabalin)101
Several systematic reviews of both anecdotal reports and epidemiologic studies published in the past few years provide additional evidence of the above, such as:
- excessive dosing with self-administration
- intranasal and inhaled routes of administration
- diversion and “street value”
- greater M/A potential of pregabalin than gabapentin
- the presence of gabapentinoids in postmortem toxicology analyses, suggesting a role in overdose fatalities when combined with other drugs.100,102,103
Continue to: The European Medicine Agency's EudraVigilance database...
The European Medicine Agency’s EudraVigilance database included 4,301 reports of gabapentin misuse, abuse, or dependence, and 7,639 such reports for pregabalin, from 2006 to 2015 (rising sharply after 2012), with 86 gabapentin-related and 27 pregabalin-related fatalities.104 Data from the Drug Diversion Program of the Researched Abuse, Diversion, and Addiction-Related Surveillance System from 2002 to 2015 have likewise revealed that gabapentin diversion increased significantly in 2013.105
While the prevalence of gabapentinoid M/A is not known, rates appear to be significantly lower than for traditional drugs of abuse such as cannabis, cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and opioids.106,107 However, gabapentin and pregabalin M/A appears to be increasingly common among individuals with SUDs and in particular among those with opioid use disorders (OUDs). For example, a 2015 report indicated that 15% of an adult cohort in Appalachian Kentucky with nonmedical use of diverted prescription opioids reported gabapentin M/A, an increase of nearly 3,000% since 2008.108 Based on data from a US insurance enrollment and claims database, researchers found that the rate of gabapentin overuse among those also overusing opioids was 12% compared with only 2% for those using gabapentin alone.109 It has also been reported that gabapentin is sometimes used as a “cutting agent” for heroin.110
Those who use gabapentinoids together with opioids report that gabapentin and pregabalin potentiate the euphoric effects of methadone111 and endorse specific beliefs that pregabalin increases both the desired effects of heroin as well as negative effects such as “blackouts,” loss of control, and risk of overdose.112 Indeed, sustained M/A of gabapentin and opioids together has been found to increase emergency department utilization, drug-related hospitalization, and respiratory depression.113 Based on a case-control study of opioid users in Canada, co-prescription of gabapentin and opioids was associated with a 50% increase in death from opioid-related causes compared with prescription of opioids alone.114
Case reports documenting tolerance, withdrawal, craving, and loss of control suggest a true addictive potential for gabapentinoids, but Bonnet and Sherbaum100 concluded that while there is robust evidence of abusers “liking” gabapentin and pregabalin (eg, reward), evidence of “wanting” them (eg, psychological dependence) in the absence of other SUDs has been limited to only a few anecdotal reports with pregabalin. Accordingly, the risk of true addiction to gabapentinoids by those without preexisting SUDs appears to be low. Nonetheless, the M/A potential of both gabapentin and pregabalin is clear and in the context of a nationwide opioid epidemic, the increased morbidity/mortality risk related to combined use of gabapentinoids and opioids is both striking and concerning. Consequently, the state of Kentucky recently recognized the M/A potential of gabapentin by designating it a Schedule V controlled substance (pregabalin is already a Schedule V drug according to the US Drug Enforcement Agency),103,113 and several other states now mandate the reporting of gabapentin prescriptions to prescription drug monitoring programs.115
Following a similar pattern to antidepressants and antipsychotics, a potential role for gabapentin in the treatment of cocaine use disorders was supported in preliminary studies,116-118 but not in subsequent RCTs.119-121 However, there is evidence from RCTs to support the use of gabapentin and pregabalin in the treatment of alcohol use disorders.122-124 Gabapentin was also found to significantly reduce cannabis use and withdrawal symptoms in patients compared with placebo in an RCT of individuals with cannabis use disorders.125 The perceived safety of gabapentinoids by clinicians, their subjective desirability by patients with SUDs, and efficacy data supporting a therapeutic role in SUDs must be balanced with recognition that approximately 80% of gabapentin prescriptions are written for off-label indications for which there is little supporting evidence,109 such as low back pain.126 Clinicians considering prescribing gabapentinoids to manage psychiatric symptoms, such as anxiety and insomnia, should carefully consider the risk of M/A and other potential morbidities, especially in the setting of SUDs and OUD in particular.
Continue to: Problematic, even if not addictive
Problematic, even if not addictive
It is sometimes claimed that “addiction” to psychiatric medications is not limited to stimulants and benzodiazepines.27,127 Although anticholinergics, antidepressants, antipsychotics, and gabapentinoids can be drugs of abuse, with some users reporting physiologic withdrawal upon discontinuation, there is only limited evidence that the M/A of these psychiatric medications is associated with the characteristic features of a more complete definition of “addiction,” which may include:
- inability to consistently abstain
- impairment in behavioral control
- diminished recognition of significant problems associated with use
- a dysfunctional emotional response to chronic use.128
Nonetheless, the literature documenting anticholinergic, antidepressant, antipsychotic, and gabapentinoid M/A includes several common features, including:
- initial reports among those with limited access to illicit drugs (eg, young people and incarcerated individuals) and subsequent spread to a wider population with more unconventional routes of administration
- use for recreational purposes and other subjective pseudo-therapeutic effects, often in combination with alcohol and illicit drugs
- greater M/A potential of certain medications within each of these drug classes (eg, trihexyphenidyl, bupropion, quetiapine)
- malingering psychiatric symptoms in order to obtain medications from prescribers and diversion for black market sale
- observations that medications might constitute therapy for SUDs that were not supported in subsequent RCTs (with the exception of gabapentin for alcohol and cannabis use disorders)
- increasing evidence of toxicity related to M/A, which suggests that prescription by clinicians has limited benefit and high risk for patients with SUDs.
Bottom Line
Some psychiatric medications are taken as drugs of abuse. Clinicians should be particularly aware of the misuse/abuse potential of anticholinergics, antidepressants, antipsychotics, and gabapentinoids, and use them cautiously, if at all, when treating patients with existing substance use disorders.
Related Resources
- Substance Abuse and Mental Health Services Administration. Prescription drug misuse and abuse. https://www.samhsa.gov/topics/prescription-drug-misuse-abuse.
- Substance Abuse and Mental Health Services Administration. Types of commonly misused or abused drugs. https://www.samhsa.gov/prescription-drug-misuse-abuse/types.
- National Institute on Drug Abuse. Misuse of prescription drugs. https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/summary.
- National Institute on Drug Abuse. New clinician screening tool available for substance use. https://www.drugabuse.gov/news-events/news-releases/2018/06/newclinician-screening-tool-available-substance-use.
Drug Brand Names
Amitriptyline • Elavil, Endep
Benztropine • Cogentin
Biperiden • Akineton
Bupropion • Wellbutrin, Zyban
Chlorpromazine • Thorzine
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Orphenadrine • Disipal, Norflex
Pregabalin • Lyrica, Lyrica CR
Procyclidine • Kemadrin
Quetiapine • Seroquel
Tianeptine • Coaxil, Stablon
Tranylcypromine • Parnate
Trifluoperazine • Stelazine
Trihexyphenidyl • Artane, Tremin
Venlafaxine • Effexor
1. Zemishlany Z, Aizenberg D, Weiner Z, et al. Trihexyphenidyl (Artane) abuse in schizophrenic patients. Int Clin Psychopharmacol. 1996;11(3):199-202.
2. Crawshaw JA, Mullen PE. A study of benzhexol abuse. Brit J Psychiatry. 1984;145:300-303.
3. Woody GE, O’Brien CP. Anticholinergic toxic psychosis in drug abusers treated with benztropine. Comp Psychiatry. 1974;15(5):439-442.
4. Lowry TP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
5. Rouchell AM, Dixon SP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
6. Kaminer Y, Munitz H, Wijsenbeek H. Trihexyphenidyl (Artane) abuse: euphoriant and anxiolytic. Brit J Psychiatry. 1982;140(5):473-474.
7. Nappo SA, de Oliviera LG, Sanchez Zv, et al. Trihexyphenidyl (Artane): a Brazilian study of its abuse. Subst Use Misuse. 2005;40(4):473-482.
8. Pullen GP, Best NR, Macguire J. Anticholinergic drug abuse: a common problem? Brit Med J (Clin Res Ed). 1984;289(6445):612-613.
9. Rubinstein JS. Abuse of antiparkinsonian drugs: feigning of extrapyramidal symptoms to obtain trihexyphenidyl. JAMA. 1978;239(22):2365-2366.
10. Mohan D, Mohandas E, Dube S. Trihexyphenidyl abuse. Brit J Addiction. 1981:76(2);195-197.
11. Marken PA, Stoner SC, Bunker MT. Anticholinergic drug abuse and misuse. CNS Drugs. 1996;5(3):190-199.
12. Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatric Serv. 2000;51(7):928-929.
13. Goldstein MR, Kasper R. Hyperpyrexia and coma due to overdose of benztropine. South Med J. 1968;61(9):984.
14. Petkovi
15. McIntyre IM, Mallett P, Burton CG, et al. Acute benztropine intoxication and fatality. J Forensic Sci. 2014;59(6):1675-1678.
16. Dilsaver SC. Antimuscarinic agents as substances of abuse: A review. J Clin Psychopharmacol. 1988:8(1):14-22.
17. Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300-307.
18. Haddad PM. Do antidepressants cause dependence? Epidemiol Psichiatr Soc. 2005;14(2):58-62.
19. Shenouda R, Desan PH. Abuse of tricyclic antidepressant drugs: a case series. J Clin Psychopharmacol. 2013;33(3):440-442.
20. van Broekhoven F, Kan CC, Zitman FG. Dependence potential of antidepressants compared to benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):939-943.
21. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
22. Warner CH, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449-456.
23. Lichtigfeld FJ, Gillman MA. Antidepressants are not drugs of abuse or dependence. Postgrad Med J. 1998;74(875):529-532.
24. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
25. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73.
26. Haddad P, Anderson I. Antidepressants aren’t addictive: clinicians have depended on them for years. J Psychopharmacol. 1999;13(3):291-292.
27. Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html. Published April 7, 2018. Accessed December 11, 2018.
28. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158-161.
29. McCormick J. Recreational bupropion in a teenager. Br J Clin Pharmacol. 2002;53(2):214.
30. Welsh C, Doyon S. Seizure induced by insufflation of bupropion. N Engl J Med. 2002; 347(2):951.
31. Baribeau D, Araki KF. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J Addict Med. 2013;7(3):216-217.
32. Hill SH, Sikand H, Lee J. A case report of seizure induced by bupropion nasal insufflation. Prim Care Companion J Clin Psych. 2007;9(1):67-69.
33. Yoon G, Westermeyer J. Intranasal bupropion abuse. Am J Addict. 2013;22(2):180.
34. Reeves RR, Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol. 2013;33(4):584-585.
35. Oppek K, Koller G, Zwergal A, et al. Intravenous administration and abuse of bupropion: a case report and a review of the literature. J Addict Med. 2014;8(4):290-293.
36. Strike M, Hatcher S. Bupropion injection resulting in tissue necrosis and psychosis: previously undocumented complications of intravenous bupropion use disorder. J Addict Med. 2015;9(3):246-250.
37. Schifano F, Chiappini S. Is there a potential of misuse for venlafaxine and bupropion? Front Pharmacol. 2018;9:239.
38. Tryon J, Logan N. Antidepressant Wellbutrin becomes ‘poor man’s cocaine’ on Toronto streets. Global News. https://globalnews.ca/news/846576/antidepressant-wellbutrin-becomes-poor-mans-cocaine-on-toronto-streets/. Published September 18, 2013. Accessed December 11, 2018.
39. Stassinos GL, Klein-Schwartz W. Bupropion “abuse” reported to US Poison Centers. J Addict Med. 2016;10(5):357-362.
40. Hilliard WT, Barloon L, Farley P, et al. Bupropion diversion and misuse in the correctional facility. J Correct Health Care. 2013;19(3):211-217.
41. Griffith JD, Carranza J, Griffith C, et al. Bupropion clinical assay for amphetamine-like abuse potential. J Clin Psychiatry.1983;44(5 Pt 2):206-208.
42. Miller L, Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacol (Berl). 1983;80(3):199-205.
43. Berigan TR, Russell ML. Treatment of methamphetamine cravings with bupropion: A case report. Prim Care Companion J Clin Psychiatry. 2001;3(6):267-268.
44. Tardieu T, Poirier Y, Micallef J, et al. Amphetamine-like stimulant cessation in an abusing patient treated with bupropion. Acta Psychiatr Scand. 2004;109(1):75-78.
45. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced cravings. Neuropsychopharmacology. 2006;31(7):1537-1544.
46. Margolin A, Kosten TR, Avants SK, et al. A multicenter trial for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125-131.
47. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23.
48. Anderson AL, Li S, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind placebo-controlled trial. Drug Alcohol Depend. 2015;150:170-174.
49. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222-232.
50. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
51. Heinzerling KG, Swanson A, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878-1886.
52. Doenecke AL, Heuerman RC. Treatment of haloperidol abuse with diphenhydramine. Am J Psychiatry. 1980;137(4):487-488.
53. Weddington WW, Leventhal BL. Sadistic abuse of haloperidol. Am J Psychiatry. 1982;139:132-133.
54. Basu D, Marudkar M, Khurana H. Abuse of neuroleptic drugs by psychiatric patients. Indian J Med Sci. 2000;54(2):59-62.
55. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry 2004;161(9):1718.
56. Reeves RR. Abuse of olanzapine by substance abusers. J Psychoactive Drugs. 2007;39(3):297-299.
57. Kumsar NA, Erol A. Olanzapine abuse. Subst Abus. 2013;34(1):73-74.
58. Lai C. Olanzapine abuse was relieved after switching to aripiprazole in a patient with psychotic depression. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34(7):1363-1364.
59. James PD, Fida AS, Konovalov P, et al. Non-medical use of olanzapine by people on methadone treatment. BJPsych Bull. 2016;40(6):314-317.
60. Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J. 2007;100(8):834-836.
61. Murphy D, Bailey K, Stone M, et al. Addictive potential of quetiapine. Am J Psychiatry. 2008;165(7):918.
62. Paparrigopoulos T, Karaiskos D, Liappas J. Quetiapine: another drug with potential for misuse? J Clin Psychiatry. 2008;69(1):162-163.
63. Reeves RR, Burke RS. Abuse of the combination of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;16(5): doi: 10.4088/PCC.14l01660.
64. Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm. 2007;64(7):723-725.
65. Caniato RN, Gundabawady A, Baune BT, et al. Malingered psychotic symptoms and quetiapine abuse in a forensic setting. J Forens Psychiatr Psychol. 2009;20(6):928-935.
66. Hussain MZ, Waheed W, Hussain S. Intravenous quetiapine abuse. Am J Psychiatry. 2005; 162(9):1755-1756.
67. Waters BM, Joshi KG. Intravenous quetiapine-cocaine use (“Q-ball”). Am J Psychiatry. 2007;164(1):173-174.
68. Haridas A, Kushon D, Gurmu S, et al. Smoking quetiapine: a “Maq ball?” Prim Psychiatry. 2010;17:38-39.
69. Cubala WJ, Springer J. Quetiapine abuse and dependence in psychiatric patients: a systematic review of 25 case reports in the literature. J Subs Use. 2014;19(5):388-393.
70. Piróg-Balcerzak A, Habrat B, Mierzejewski P. Misuse and abuse of quetiapine [in Polish]. Psychiatr Pol. 2015;49(1):81-93.
71. Pinta ER, Taylor RE. Quetiapine addiction? Am J Psychiatry. 2007;164(1):174.
72. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Amer Acad Psychiatr Law. 2012;40(4):502-508.
73. Tarasoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164(2):350.
74. Reccoppa L. Less abuse potential with XR formulation of quetiapine. Am J Addiction. 2010;20(2):178.
75. McLarnon ME, Fulton HG, MacIsaac C, et al. Characteristics of quetiapine misuse among clients of a community-based methadone maintenance program. J Clin Psychopharmacol. 2012;32(5):721-723.
76. Reddel SE, Bruno R, Burns L, et al. Prevalence and associations of quetiapine fumarate misuse among an Australian national city sample of people who regularly inject drugs. Addiction. 2013;109(2):295-302.
77. Malekshahi T, Tioleco N, Ahmed N, et al. Misuse of atypical antipsychotics in conjunction with alcohol and other drugs of abuse. J Subs Abuse Treat. 2015;48(1):8-12.
78. Klein-Schwartz W, Schwartz EK, Anderson BD. Evaluation of quetiapine abuse and misuse reported to poison centers. J Addict Med. 2014;8(3):195-198.
79. Klein L, Bangh S, Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med. 2017;18(2):243-250.
80. Chiappini S, Schifano F. Is there a potential of misuse for quetiapine?: Literature review and analysis of the European Medicines Agency/European Medicines Agency Adverse Drug Reactions’ Database. J Clin Psychopharmacol. 2018;38(1):72-79.
81. Lee J, Pilgrim J, Gerostamoulos D, et al. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95-99.
82. Mattson ME, Albright VA, Yoon J, et al. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: Results from the
83. Brutcher RE, Nader SH, Nader MA. Evaluation of the reinforcing effect of quetiapine, alone and in combination with cocaine, in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(2):244-250.
84. Kim DR, Staab JP. Quetiapine discontinuation syndrome. Am J Psychiatry. 2005;162(5):1020.
85. Thurstone CC, Alahi P. A possible case of quetiapine withdrawal syndrome. J Clin Psychiatry. 2000;61(8):602-603.
86. Kohen I, Kremen N. A case report of quetiapine withdrawal syndrome in a geriatric patient. World J Biol Psychiatry. 2009;10(4 pt 3):985-986.
87. Yargic I, Caferov C. Quetiapine dependence and withdrawal: a case report. Subst Abus. 2011;32(3):168-169.
88. Koch HJ. Severe quetiapine withdrawal syndrome with nausea and vomiting in a 65-year-old patient with psychotic depression. Therapie. 2015;70(6):537-538.
89. Fischer BA, Boggs DL. The role of antihistaminic effects in the misuse of quetiapine: a case report and review of the literature. Neurosci Biobehav Rev. 2010;34(4):555-558.
90. Longoria J, Brown ES, Perantie DC, et al. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol. 2004;24(1):101-102.
91. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24(5):532-535.
92. Kennedy A, Wood AE, Saxon AJ, et al. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28(2):221-224.
93. Mariani JJ, Pavlicova M, Mamczur A, et al. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280-284.
94. Guardia J, Roncero C, Galan J, et al. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265-269.
95. Litten RZ, Fertig JB, Falk DE, et al; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406-416.
96. Tapp A, Wood AE, Kennedy A, et al. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24.
97. Markowitz JS, Finkenbine R, Myrick H, et al. Gabapentin abuse in a cocaine user: Implications for treatment. J Clin Psychopharmacol. 1997;17(5):423-424.
98. Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13(3):321-323.
99. Victorri-Vigneau C, Guelais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40(1):43-44.
100. Bonnet U, Sherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185-1215.
101. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118-122.
102. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
103. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
104. Chiappini S, Shifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647-654.
105. Buttram ME, Kurtz SP, Dart R, et al. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083-1086.
106. Kapil V, Green JL, Le Lait M, et al. Misuse of the y-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2013;78(1):190-191.
107. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Invest. 2017;37(8):763-773.
108. Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488.
109. Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy. 2018;38(4):436-443.
110. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):401-407.
111. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res. 2014;20(3):115-118.
112. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580-1589.
113. Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.
114. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e10022396. doi: 10.1371/journal.pmed.1002396.
115. Peckham AM, Fairman K, Sclar DA. Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed? Exp Opin Drug Saf. 2018;17(5):519-523.
116. Raby WN. Gabapentin for cocaine cravings. Am J Psychiatry. 2000;157(12):2058-2059.
117. Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J CLin Psychiatry. 2001;62(1):19-23.
118. Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84-86.
119. Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279-287.
120. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alc Depend. 2006;81(3):267-274.
121. Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2-3):274-277.
122. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
123. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
124. Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24(9):1367-1374.
125. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychpharmacology. 2012;27(7):1689-1698.
126. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793.
127. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407.
128. American Society of Addiction Medicine. Public policy statement: definition of addiction. https://www.asam.org/docs/default-source/public-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=a8f64512_4. Published August 15, 2011. Accessed July 23, 2018.
1. Zemishlany Z, Aizenberg D, Weiner Z, et al. Trihexyphenidyl (Artane) abuse in schizophrenic patients. Int Clin Psychopharmacol. 1996;11(3):199-202.
2. Crawshaw JA, Mullen PE. A study of benzhexol abuse. Brit J Psychiatry. 1984;145:300-303.
3. Woody GE, O’Brien CP. Anticholinergic toxic psychosis in drug abusers treated with benztropine. Comp Psychiatry. 1974;15(5):439-442.
4. Lowry TP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
5. Rouchell AM, Dixon SP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
6. Kaminer Y, Munitz H, Wijsenbeek H. Trihexyphenidyl (Artane) abuse: euphoriant and anxiolytic. Brit J Psychiatry. 1982;140(5):473-474.
7. Nappo SA, de Oliviera LG, Sanchez Zv, et al. Trihexyphenidyl (Artane): a Brazilian study of its abuse. Subst Use Misuse. 2005;40(4):473-482.
8. Pullen GP, Best NR, Macguire J. Anticholinergic drug abuse: a common problem? Brit Med J (Clin Res Ed). 1984;289(6445):612-613.
9. Rubinstein JS. Abuse of antiparkinsonian drugs: feigning of extrapyramidal symptoms to obtain trihexyphenidyl. JAMA. 1978;239(22):2365-2366.
10. Mohan D, Mohandas E, Dube S. Trihexyphenidyl abuse. Brit J Addiction. 1981:76(2);195-197.
11. Marken PA, Stoner SC, Bunker MT. Anticholinergic drug abuse and misuse. CNS Drugs. 1996;5(3):190-199.
12. Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatric Serv. 2000;51(7):928-929.
13. Goldstein MR, Kasper R. Hyperpyrexia and coma due to overdose of benztropine. South Med J. 1968;61(9):984.
14. Petkovi
15. McIntyre IM, Mallett P, Burton CG, et al. Acute benztropine intoxication and fatality. J Forensic Sci. 2014;59(6):1675-1678.
16. Dilsaver SC. Antimuscarinic agents as substances of abuse: A review. J Clin Psychopharmacol. 1988:8(1):14-22.
17. Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300-307.
18. Haddad PM. Do antidepressants cause dependence? Epidemiol Psichiatr Soc. 2005;14(2):58-62.
19. Shenouda R, Desan PH. Abuse of tricyclic antidepressant drugs: a case series. J Clin Psychopharmacol. 2013;33(3):440-442.
20. van Broekhoven F, Kan CC, Zitman FG. Dependence potential of antidepressants compared to benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):939-943.
21. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
22. Warner CH, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449-456.
23. Lichtigfeld FJ, Gillman MA. Antidepressants are not drugs of abuse or dependence. Postgrad Med J. 1998;74(875):529-532.
24. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
25. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73.
26. Haddad P, Anderson I. Antidepressants aren’t addictive: clinicians have depended on them for years. J Psychopharmacol. 1999;13(3):291-292.
27. Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html. Published April 7, 2018. Accessed December 11, 2018.
28. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158-161.
29. McCormick J. Recreational bupropion in a teenager. Br J Clin Pharmacol. 2002;53(2):214.
30. Welsh C, Doyon S. Seizure induced by insufflation of bupropion. N Engl J Med. 2002; 347(2):951.
31. Baribeau D, Araki KF. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J Addict Med. 2013;7(3):216-217.
32. Hill SH, Sikand H, Lee J. A case report of seizure induced by bupropion nasal insufflation. Prim Care Companion J Clin Psych. 2007;9(1):67-69.
33. Yoon G, Westermeyer J. Intranasal bupropion abuse. Am J Addict. 2013;22(2):180.
34. Reeves RR, Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol. 2013;33(4):584-585.
35. Oppek K, Koller G, Zwergal A, et al. Intravenous administration and abuse of bupropion: a case report and a review of the literature. J Addict Med. 2014;8(4):290-293.
36. Strike M, Hatcher S. Bupropion injection resulting in tissue necrosis and psychosis: previously undocumented complications of intravenous bupropion use disorder. J Addict Med. 2015;9(3):246-250.
37. Schifano F, Chiappini S. Is there a potential of misuse for venlafaxine and bupropion? Front Pharmacol. 2018;9:239.
38. Tryon J, Logan N. Antidepressant Wellbutrin becomes ‘poor man’s cocaine’ on Toronto streets. Global News. https://globalnews.ca/news/846576/antidepressant-wellbutrin-becomes-poor-mans-cocaine-on-toronto-streets/. Published September 18, 2013. Accessed December 11, 2018.
39. Stassinos GL, Klein-Schwartz W. Bupropion “abuse” reported to US Poison Centers. J Addict Med. 2016;10(5):357-362.
40. Hilliard WT, Barloon L, Farley P, et al. Bupropion diversion and misuse in the correctional facility. J Correct Health Care. 2013;19(3):211-217.
41. Griffith JD, Carranza J, Griffith C, et al. Bupropion clinical assay for amphetamine-like abuse potential. J Clin Psychiatry.1983;44(5 Pt 2):206-208.
42. Miller L, Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacol (Berl). 1983;80(3):199-205.
43. Berigan TR, Russell ML. Treatment of methamphetamine cravings with bupropion: A case report. Prim Care Companion J Clin Psychiatry. 2001;3(6):267-268.
44. Tardieu T, Poirier Y, Micallef J, et al. Amphetamine-like stimulant cessation in an abusing patient treated with bupropion. Acta Psychiatr Scand. 2004;109(1):75-78.
45. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced cravings. Neuropsychopharmacology. 2006;31(7):1537-1544.
46. Margolin A, Kosten TR, Avants SK, et al. A multicenter trial for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125-131.
47. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23.
48. Anderson AL, Li S, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind placebo-controlled trial. Drug Alcohol Depend. 2015;150:170-174.
49. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222-232.
50. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
51. Heinzerling KG, Swanson A, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878-1886.
52. Doenecke AL, Heuerman RC. Treatment of haloperidol abuse with diphenhydramine. Am J Psychiatry. 1980;137(4):487-488.
53. Weddington WW, Leventhal BL. Sadistic abuse of haloperidol. Am J Psychiatry. 1982;139:132-133.
54. Basu D, Marudkar M, Khurana H. Abuse of neuroleptic drugs by psychiatric patients. Indian J Med Sci. 2000;54(2):59-62.
55. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry 2004;161(9):1718.
56. Reeves RR. Abuse of olanzapine by substance abusers. J Psychoactive Drugs. 2007;39(3):297-299.
57. Kumsar NA, Erol A. Olanzapine abuse. Subst Abus. 2013;34(1):73-74.
58. Lai C. Olanzapine abuse was relieved after switching to aripiprazole in a patient with psychotic depression. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34(7):1363-1364.
59. James PD, Fida AS, Konovalov P, et al. Non-medical use of olanzapine by people on methadone treatment. BJPsych Bull. 2016;40(6):314-317.
60. Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J. 2007;100(8):834-836.
61. Murphy D, Bailey K, Stone M, et al. Addictive potential of quetiapine. Am J Psychiatry. 2008;165(7):918.
62. Paparrigopoulos T, Karaiskos D, Liappas J. Quetiapine: another drug with potential for misuse? J Clin Psychiatry. 2008;69(1):162-163.
63. Reeves RR, Burke RS. Abuse of the combination of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;16(5): doi: 10.4088/PCC.14l01660.
64. Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm. 2007;64(7):723-725.
65. Caniato RN, Gundabawady A, Baune BT, et al. Malingered psychotic symptoms and quetiapine abuse in a forensic setting. J Forens Psychiatr Psychol. 2009;20(6):928-935.
66. Hussain MZ, Waheed W, Hussain S. Intravenous quetiapine abuse. Am J Psychiatry. 2005; 162(9):1755-1756.
67. Waters BM, Joshi KG. Intravenous quetiapine-cocaine use (“Q-ball”). Am J Psychiatry. 2007;164(1):173-174.
68. Haridas A, Kushon D, Gurmu S, et al. Smoking quetiapine: a “Maq ball?” Prim Psychiatry. 2010;17:38-39.
69. Cubala WJ, Springer J. Quetiapine abuse and dependence in psychiatric patients: a systematic review of 25 case reports in the literature. J Subs Use. 2014;19(5):388-393.
70. Piróg-Balcerzak A, Habrat B, Mierzejewski P. Misuse and abuse of quetiapine [in Polish]. Psychiatr Pol. 2015;49(1):81-93.
71. Pinta ER, Taylor RE. Quetiapine addiction? Am J Psychiatry. 2007;164(1):174.
72. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Amer Acad Psychiatr Law. 2012;40(4):502-508.
73. Tarasoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164(2):350.
74. Reccoppa L. Less abuse potential with XR formulation of quetiapine. Am J Addiction. 2010;20(2):178.
75. McLarnon ME, Fulton HG, MacIsaac C, et al. Characteristics of quetiapine misuse among clients of a community-based methadone maintenance program. J Clin Psychopharmacol. 2012;32(5):721-723.
76. Reddel SE, Bruno R, Burns L, et al. Prevalence and associations of quetiapine fumarate misuse among an Australian national city sample of people who regularly inject drugs. Addiction. 2013;109(2):295-302.
77. Malekshahi T, Tioleco N, Ahmed N, et al. Misuse of atypical antipsychotics in conjunction with alcohol and other drugs of abuse. J Subs Abuse Treat. 2015;48(1):8-12.
78. Klein-Schwartz W, Schwartz EK, Anderson BD. Evaluation of quetiapine abuse and misuse reported to poison centers. J Addict Med. 2014;8(3):195-198.
79. Klein L, Bangh S, Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med. 2017;18(2):243-250.
80. Chiappini S, Schifano F. Is there a potential of misuse for quetiapine?: Literature review and analysis of the European Medicines Agency/European Medicines Agency Adverse Drug Reactions’ Database. J Clin Psychopharmacol. 2018;38(1):72-79.
81. Lee J, Pilgrim J, Gerostamoulos D, et al. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95-99.
82. Mattson ME, Albright VA, Yoon J, et al. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: Results from the
83. Brutcher RE, Nader SH, Nader MA. Evaluation of the reinforcing effect of quetiapine, alone and in combination with cocaine, in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(2):244-250.
84. Kim DR, Staab JP. Quetiapine discontinuation syndrome. Am J Psychiatry. 2005;162(5):1020.
85. Thurstone CC, Alahi P. A possible case of quetiapine withdrawal syndrome. J Clin Psychiatry. 2000;61(8):602-603.
86. Kohen I, Kremen N. A case report of quetiapine withdrawal syndrome in a geriatric patient. World J Biol Psychiatry. 2009;10(4 pt 3):985-986.
87. Yargic I, Caferov C. Quetiapine dependence and withdrawal: a case report. Subst Abus. 2011;32(3):168-169.
88. Koch HJ. Severe quetiapine withdrawal syndrome with nausea and vomiting in a 65-year-old patient with psychotic depression. Therapie. 2015;70(6):537-538.
89. Fischer BA, Boggs DL. The role of antihistaminic effects in the misuse of quetiapine: a case report and review of the literature. Neurosci Biobehav Rev. 2010;34(4):555-558.
90. Longoria J, Brown ES, Perantie DC, et al. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol. 2004;24(1):101-102.
91. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24(5):532-535.
92. Kennedy A, Wood AE, Saxon AJ, et al. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28(2):221-224.
93. Mariani JJ, Pavlicova M, Mamczur A, et al. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280-284.
94. Guardia J, Roncero C, Galan J, et al. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265-269.
95. Litten RZ, Fertig JB, Falk DE, et al; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406-416.
96. Tapp A, Wood AE, Kennedy A, et al. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24.
97. Markowitz JS, Finkenbine R, Myrick H, et al. Gabapentin abuse in a cocaine user: Implications for treatment. J Clin Psychopharmacol. 1997;17(5):423-424.
98. Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13(3):321-323.
99. Victorri-Vigneau C, Guelais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40(1):43-44.
100. Bonnet U, Sherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185-1215.
101. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118-122.
102. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
103. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
104. Chiappini S, Shifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647-654.
105. Buttram ME, Kurtz SP, Dart R, et al. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083-1086.
106. Kapil V, Green JL, Le Lait M, et al. Misuse of the y-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2013;78(1):190-191.
107. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Invest. 2017;37(8):763-773.
108. Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488.
109. Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy. 2018;38(4):436-443.
110. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):401-407.
111. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res. 2014;20(3):115-118.
112. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580-1589.
113. Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.
114. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e10022396. doi: 10.1371/journal.pmed.1002396.
115. Peckham AM, Fairman K, Sclar DA. Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed? Exp Opin Drug Saf. 2018;17(5):519-523.
116. Raby WN. Gabapentin for cocaine cravings. Am J Psychiatry. 2000;157(12):2058-2059.
117. Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J CLin Psychiatry. 2001;62(1):19-23.
118. Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84-86.
119. Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279-287.
120. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alc Depend. 2006;81(3):267-274.
121. Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2-3):274-277.
122. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
123. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
124. Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24(9):1367-1374.
125. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychpharmacology. 2012;27(7):1689-1698.
126. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793.
127. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407.
128. American Society of Addiction Medicine. Public policy statement: definition of addiction. https://www.asam.org/docs/default-source/public-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=a8f64512_4. Published August 15, 2011. Accessed July 23, 2018.
Effectiveness of duloxetine in treatment of painful chemotherapy-induced peripheral neuropathy: a systematic review
Chemotherapy-induced peripheral neuropathy (CIPN) is a serious side effect that can be dose limiting and affect patient quality of life for prolonged time,1 with an overall incidence of about 38% in patients who are treated with multiple chemotherapeutic agents. 2
The most common antineoplastic agents causing peripheral neuropathy are oxaliplatin, cisplatin, taxanes, Vinca alkaloids, bortezomib, and thalidomide.3,8,9
Different components of the nervous system are targets of various chemotherapeutic agents, from dorsal root ganglion (DRG) cells to the distal axon. The DRG is the most vulnerable to neurotoxicity because it is less protected by the nervous system blood barrier, hence the predominance of sensory symptoms in CIPN.10 The pathogenesis of CIPN is not fully understood, and it is most probably multifaceted and not always related to the antineoplastic mechanism. Findings from experimental studies have shown an accumulation of chemotherapeutic compounds in the cell bodies of the DRG, resulting in decreased cellular metabolism and axoplasmic transport. Another proposed mechanism is the induction of apoptosis in sensory neuron of the posterior spinal ganglion after binding to DNA strands.7,11
Opioids had been used for managing pain in patients with cancer, but their addictive side effects limit use in the treatment of chronic pain,12 so several drugs called coanalgesics have been introduced as a treatment
The imbalance of 5HT and NE in the pain inhibitory pathways may contribute to the peripheral neuropathic pain.20 Duloxetine hydrochloride is a 5HT–NE reuptake inhibitor used to treat depression and generalized anxiety disorder.21 Duloxetine effect in decreasing pain transmission through increasing synaptic concentrations of NE and 5HT, which results in blocking input signals to the dorsal horn neurons in the spinal cord.12
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA statement) guidelines during the preparation of this systematic review.22
Inclusion criteria
Trial or study type. Articles publishing findings from randomized controlled trials, nonrandomized controlled trials, retrospective studies, and single-arm studies of duloxetine in the treatment of CIPN were included in our review.
Intervention. The intervention was duloxetine with all doses, either administered alone or with other antineuropathic drugs.
Comparator. The comparator was placebo (control group) or other antineuropathic drugs or no control group.
Population. The population included cancer patients with painful CIPN.
Outcome. At least one of the following outcomes was used for pain assessment:
Exclusion criteria
Studies in a non-English language, animal studies, studies whose full-text article was not available, and thesis and conference papers were not included.
Objective and study design
The objective of this systematic review was to systematically assess the effectiveness of duloxetine in the treatment of pain in patients with CIPN.
Information sources and search
Medical electronic databases. PubMed and Scopus, from inception to January 2018, were searched using the following search queries: (((duloxetine) AND chemotherapy induced peripheral neuropathy)) OR ((((chemotherapy) AND (neuropathic pain OR peripheral neuropathy))) AND duloxetine).
Selection of studies. The authors selected eligible studies. The screening of search results was performed in the following 2 steps:
n Screen title and abstracts against the selection criteria. Articles that were unclear from their title or abstract were reviewed against the selection criteria through the full text.
n Retrieve and screen full-text articles of eligible abstracts for eligibility to systematic review.
Data extraction
Two authors extracted the following data independently: sample size, mean age, chemotherapeutic drug, duloxetine dosage, and outcomes for pain assessment using at least one score from VAS, BPI-SF, neuropathic pain score using the NCI-CTCAE v3.0 and v4.0, or FACT-Tax, and other secondary outcomes. The data was exported from the online forms as a Microsoft Excel sheet.
Statistical analysis
We calculated the mean age and associated standard deviations (SDs) for all patients by using the pooled mean and pooled SD equation, according to Cochrane handbook of systematic reviews of interventions 5.1.0 (updated March 2011).23 When data are expressed as median and interquartile range, we used Hozo and colleagues’ BMC Research Methodology equation to calculate or estimate the mean and SD.24
Data are expressed as means with SD (unless stated otherwise); statistical results were considered significant when the P-value was less than .05. Data analysis was performed using the SPSS Statistical Package, version 23 (IBM Corp., Armonk, NY).
Synthesis of data and analysis
Because of heterogeneity and low sample size of studies, no statistically justified analyses could be performed on the provided data. Instead, a descriptive analysis of published studies was performed.
Summary measures
The search strings, the list of relevant reviews, the data coding, and the quality criteria that were used can be requested from the corresponding author.
Results
Selection of articles
The
Study characteristics
Characteristics of the included studies and patient outcome are summarized in Table 1 and Table 2. A total of 5 studies from 2012 through 2017 were included in the descriptive analysis and systematic review. In all, 4 trials were prospective studies, and 1 trial was retrospective; among all trials, 2 studies were single arm and 3 were placebo-controlled and/or crossover.
There were 431 participants in the total 5 studies included in this systematic review. The number of patients per study ranged from 25 to 231. Patients were mostly older, with mean sample ages ranging from 47.9 to 63 years, and the pooled mean age for all participants in the total 5 studies was 57.7 years.
In all included studies, duloxetine was given in varying doses of 20 mg, 30 mg, 40 mg, or 60 mg. Also, different therapeutic regimens of duloxetine were used, including placebo control or crossover with vitamin B12; 80% of the studies used escalation of doses over time (only 1 trial used fixed doses for each group of patients in the study). Escalation of duloxetine by doubling the dose was done in all 4 studies, with duloxetine doses of 30 mg and 60 mg used in 75% of those studies (3 out of 4 studies).
Comparator drug was used in 4 studies (1 was single arm) in our review analysis. The comparator drug was placebo in 1 study only, and the remaining 3 studies used other antineurotoxicity or antineuropathic pain therapy, mainly vitamin B12 (as only comparator in 1 study), fish oil, pregabalin, selective 5HT reuptake inhibitors, and nonsteroidal anti-inflammatory agents.
Regarding CIPN, the chemotherapeutic agents used in the studies were as follows (after exclusion of 11 patients who never received treatment in 1 study): 224 patients (52.9%) were on paclitaxel, 168 (39.7%) on oxaliplatin, 14 (3.30%) on R-CHOP, 8 (1.89%) on combined bortezomib–dexamethasone, 5 (1.18%) on FOLFOX, and 4 (0.94%) on other taxanes.
Improvement in pain scores was the primary and/or secondary endpoint in the included studies (Table 2). Pain was assessed by 6 different scores, including the VAS, BPI-SF, neuropathic pain score using NCI-CTCAE v3.0 and v4.0, and FACT-Tax, with all reported once except the VAS score, which was reported in 2 studies. Only 1 study, by Yang and colleagues,25 measured pain by 2 scores (the VAS and NCI-CTCAE v3.0), with the rest of the studies assessing pain by just 1 of the aforementioned scores. The pretreatment pain score was reported in only 2 studies, by Smith and colleagues and Wang and colleagues, using BPI-SF and FACT-Tax scores, respectively, with total respective mean scores of 5.8 (SD, 1.7) and 11.77 (SD, 1.73).17,26
Secondary endpoints were related mainly to pain score, drug adverse effect, and assessment of quality of life (Table 2). In the study by Yang and colleagues,25 9 patients (28.1%) discontinued duloxetine because of intolerable adverse events, with dizziness or giddiness as the most common cause (44.4% of patients who discontinued treatment). Studies by Otake and colleagues12 and Hirayama and colleagues2 reported duloxetine adverse events that were very mild and usually well tolerated in collectively 22 patients, with fatigue (n = 6) and somnolence (n = 5) as the most reported adverse effects. Wang and colleagues17 reported nonneuropathic adverse events that were attributed to chemotherapy and were mild and similar in both study groups.
Discussion
To our knowledge, this is the first systematic review to discuss the effectiveness of duloxetine specifically in treatment of pain in CIPN. The administration of chemotherapeutic agents such as paclitaxel, cisplatin, oxaliplatin, and vincristine was accompanied by CIPN. The currently available treatment options for CIPN are limited. To date, no drug has been approved specifically for treatment of pain in CIPN.12
In our review, we included cancer patients with CIPN and associated pain. Several previous studies8,27,28 discussed tingling and numbness as a common adverse effect in CIPN, and usually about 20% to 42% of patients develop chronic pain.
Six different pain assessment scores were reported in the total 5 studies in our review, with VAS and NCI-CTCAE scores reported in more than 1 study. This reflects the major challenges facing the assessment of CIPN, as various scales and tools are available for pain assessment but without a standardized approach for CIPN that can be precisely implemented.8 Several other challenges regarding pain scores were observed, with Smith and colleagues as the only authors to report both pretreatment pain score and grade, while the rest of the studies failed to report either pain score or grade, or even both.
Another difficulty observed in our review was the variability in study participants in both population size and type of cancer treated. The population size in largest study included in our review was 231 patients and the smallest was 25 patients; collectively, there were only 431 patients in the included studies. Although the type of primary cancer varied in between studies, gynecologic malignancies comprised most cases (215 patients), followed by gastrointestinal tumors, and few cases of hematologic and genitourinary malignancies were reported. Similar results were observed by Geber and colleagues in their large study screening pain in cancer patients, in which gynecologic malignancies were diagnosed in 28 patients out of 61 with CIPN, representing the highest percentage (45.9%) of malignancy type in that study.26
In the study by Otake and colleagues12 examining duloxetine for CIPN in patients with gynecologic cancer, the authors concluded that duloxetine dosage either 20 mg/day or 40 mg/day was not associated with the effectiveness of duloxetine treatment by either univariate or multivariate analysis. Previous authors have provided an explanation for the difference in duloxetine response among CIPN patients and attributed those differences to the underlying pain mechanisms.14,29 In other words, pain in those patients is both peripheral nociceptive and central neuropathic, and it is likely to be caused by mixed mechanisms.
Another variation observed among CIPN patients in our review was the chemotherapeutic agents used. That was noted by Smith and colleagues,26 who reported that patients with cancer who received platinum therapies (oxaliplatin) experienced more benefit from duloxetine in terms of pain improvement than those who received taxanes (P = .13). We found no other published studies on the response to duloxetine among different chemotherapeutic agents used. However, 2 studies of duloxetine response in patients with other pain-related disorders (painful diabetic peripheral neuropathy and fibromyalgia) showed significant improvement in pain symptoms compared with placebo. In a study of pain in chemotherapy-induced neuropathy (CIN) by Geber and colleagues,29 the preexisting pain medication was not reported, but the authors concluded that treatment for CIN-related neuropathic pain differs from that for nonneuropathic (ie, musculoskeletal) pain, with the former being treated mainly with pharmacotherapy and the latter with physiotherapy and behavioral exercises. They asserted that different pain patterns could help flag neuropathic or musculoskeletal pain so that the selected treatments would be more specific. Differences in pain improvement related to duloxetine may be attributed to the underlying pain mechanism, and whether it is mixed or centrally or peripherally related was also discussed by Geber and colleagues.29
In the study by
Findings from studies on the effect of duloxetine in treatment of pain in diabetic peripheral neuropathy have shown that duloxetine at a dose of 60 mg/day effectively improves pain in 43% to 68% of patients.15,16,30 Similarly, in our review, the study by Yang and colleagues25 showed a 63% subjective reduction in pain severity by VAS score in CIPN patients but lower improvement of 47.4% by NCI-CTCAE v3.0; this can be attributed to the simplistic 4-grade rating scale of the latter.
During our analysis of studies, we noticed that no diagnostic criteria were implemented for diagnosis or inclusion of CIPN patients in any of the included studies, and this represents a major challenge in any analysis of studies with neuropathic pain patients. In 2016, Finnerup and colleagues updated the previous 2008 grading system for diagnosis of neuropathic pain, which is intended to determine the level of certainty with which the pain in question is neuropathic.31 The system defines the diagnostic certainty into 3 levels: Possible, Probable, and Definite. Although the number of studies used the grading system during the inclusion of neuropathic pain patients increased from 5% in 2009 to 30% in 2014, still more than two-thirds of studies do not use a standardized system for diagnosis and/or inclusion of neuropathic pain in patients.
Strength and limitations
The first strength of this review is that it identifies gaps in our current knowledge about duloxetine in the treatment of pain in cancer patients with CIPN. Second, we collected all available articles from inception until January 2018. Third, this review can serve as a model for future studies investigating the effectiveness of duloxetine in treatment of CIPN.
There are also limitations to this review that should be discussed. First, the studies vary greatly in samples, methodologies, and outcomes measured. Second, the diagnostic criteria for CIPN and the pain assessment tools vary among the studies. Third, there is also variability in the duloxetine doses and administration regimens among the studies, and some articles did not report the precise outcome in pain scores. Furthermore, the articles reviewed included retrospective, single-arm, or nonrandomized controlled studies with relatively small numbers of participants.
To improve the results, more placebo-controlled or head-to-head trials (with other agents used in treatment of CIPN) with large sample sizes would be needed.
Conclusions
Our purpose was to describe the effectiveness of duloxetine in improving pain scores among CIPN patients
Acknowledgments
That authors express their sincere gratitude to Nahla A Merghany and Sarah M Abd Elfadel for helping them retrieve all the relevant articles for this review.
1. Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27-46.
2. Hirayama Y, Ishitani K, Sato Y, et al. Effect of duloxetine in Japanese patients with chemotherapy-induced peripheral neuropathy: a pilot randomized trial. Int J Clin Oncol. 2015;20(5):866-871.
3. Stubblefield MD, McNeely ML, Alfano CM, Mayer DK. A prospective surveillance model for physical rehabilitation of women with breast cancer: chemotherapy-induced peripheral neuropathy. Cancer. 2012;118(suppl 8):2250-2260.
4. Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63(6):419-437.
5. Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res. 2014;6(1):135-147.
6. Bakitas MA. Background noise: the experience of chemotherapy-induced peripheral neuropathy. Nurs Res. 2007;56(5):323-331.
7. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
8. Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33(1):15-49.
9. Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15(29):3081-3094.
10. Caponero R, Montarroyos ES, Tahamtani SMM. Post-chemotherapy neuropathy. Rev Dor. Sao Paulo. 2016;17(suppl 1):S56-S58.
11. Velasco R, Bruna J. Chemotherapy-induced peripheral neuropathy: an unresolved issue. Neurologia. 2010;25(2):116-131.
12. Otake A, Yoshino K, Ueda Y, et al. Usefulness of duloxetine for paclitaxel-induced peripheral neuropathy treatment in gynecological cancer patients. Anticancer Res. 2015;35(1):359-363.
13. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941-1967.
14. Smith EM, Pang H, Ye C, et al. Predictors of duloxetine response in patients with oxaliplatin-induced painful chemotherapy-induced peripheral neuropathy (CIPN): a secondary analysis of randomised controlled trial – CALGB/alliance 170601 [published online November 25, 2015]. Eur J Cancer Care (Engl). 2017;26(2). doi:10.1111/ecc.12421
15. Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1-2):109-118.
16. Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6(5):346-356.
17. Wang J, Li Q, Xu B, Zhang T, Chen S, Luo Y. Efficacy and safety of duloxetine in Chinese breast cancer patients with paclitaxel-induced peripheral neuropathy. Chin J Cancer Res. 2017;29(5):411-418.
18. Irving G, Tanenberg RJ, Raskin J, Risser RC, Malcolm S. Comparative safety and tolerability of duloxetine vs pregabalin vs duloxetine plus gabapentin in patients with diabetic peripheral neuropathic pain. Int J Clin Pract. 2014;68(9):1130-1140.
19. Esin E, Yalcin S. Neuropathic cancer pain: what we are dealing with? How to manage it? Onco Targets Ther. 2014;7:599-618.
20. Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25(12):613-617.
21. Mancini M, Perna G, Rossi A, Petralia A. Use of duloxetine in patients with an anxiety disorder, or with comorbid anxiety and major depressive disorder: a review of the literature. Expert Opin Pharmacother. 2010;11(7):1167-1181.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269.
23. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. http://handbook-5-1.cochrane.org/. Updated March 2011. Accessed November 19, 2018.
24. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
25. Yang YH, Lin JK, Chen WS, et al. Duloxetine improves oxaliplatin-induced neuropathy in patients with colorectal cancer: an open-label pilot study. Support Care Cancer. 2012;20(7):1491-1497.
26. Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359-1367.
27. Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18(6):343-349.
28. Cavenagh J, Good P, Ravenscroft P. Neuropathic pain: are we out of the woods yet? Intern Med J. 2006;36(4):251-255.
29. Geber C, Breimhorst M, Burbach B, et al. Pain in chemotherapy-induced neuropathy—more than neuropathic? Pain. 2013;154(12):2877-2887.
30. Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–1420.
31. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. 2016;157(8):1599-1606.
Chemotherapy-induced peripheral neuropathy (CIPN) is a serious side effect that can be dose limiting and affect patient quality of life for prolonged time,1 with an overall incidence of about 38% in patients who are treated with multiple chemotherapeutic agents. 2
The most common antineoplastic agents causing peripheral neuropathy are oxaliplatin, cisplatin, taxanes, Vinca alkaloids, bortezomib, and thalidomide.3,8,9
Different components of the nervous system are targets of various chemotherapeutic agents, from dorsal root ganglion (DRG) cells to the distal axon. The DRG is the most vulnerable to neurotoxicity because it is less protected by the nervous system blood barrier, hence the predominance of sensory symptoms in CIPN.10 The pathogenesis of CIPN is not fully understood, and it is most probably multifaceted and not always related to the antineoplastic mechanism. Findings from experimental studies have shown an accumulation of chemotherapeutic compounds in the cell bodies of the DRG, resulting in decreased cellular metabolism and axoplasmic transport. Another proposed mechanism is the induction of apoptosis in sensory neuron of the posterior spinal ganglion after binding to DNA strands.7,11
Opioids had been used for managing pain in patients with cancer, but their addictive side effects limit use in the treatment of chronic pain,12 so several drugs called coanalgesics have been introduced as a treatment
The imbalance of 5HT and NE in the pain inhibitory pathways may contribute to the peripheral neuropathic pain.20 Duloxetine hydrochloride is a 5HT–NE reuptake inhibitor used to treat depression and generalized anxiety disorder.21 Duloxetine effect in decreasing pain transmission through increasing synaptic concentrations of NE and 5HT, which results in blocking input signals to the dorsal horn neurons in the spinal cord.12
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA statement) guidelines during the preparation of this systematic review.22
Inclusion criteria
Trial or study type. Articles publishing findings from randomized controlled trials, nonrandomized controlled trials, retrospective studies, and single-arm studies of duloxetine in the treatment of CIPN were included in our review.
Intervention. The intervention was duloxetine with all doses, either administered alone or with other antineuropathic drugs.
Comparator. The comparator was placebo (control group) or other antineuropathic drugs or no control group.
Population. The population included cancer patients with painful CIPN.
Outcome. At least one of the following outcomes was used for pain assessment:
Exclusion criteria
Studies in a non-English language, animal studies, studies whose full-text article was not available, and thesis and conference papers were not included.
Objective and study design
The objective of this systematic review was to systematically assess the effectiveness of duloxetine in the treatment of pain in patients with CIPN.
Information sources and search
Medical electronic databases. PubMed and Scopus, from inception to January 2018, were searched using the following search queries: (((duloxetine) AND chemotherapy induced peripheral neuropathy)) OR ((((chemotherapy) AND (neuropathic pain OR peripheral neuropathy))) AND duloxetine).
Selection of studies. The authors selected eligible studies. The screening of search results was performed in the following 2 steps:
n Screen title and abstracts against the selection criteria. Articles that were unclear from their title or abstract were reviewed against the selection criteria through the full text.
n Retrieve and screen full-text articles of eligible abstracts for eligibility to systematic review.
Data extraction
Two authors extracted the following data independently: sample size, mean age, chemotherapeutic drug, duloxetine dosage, and outcomes for pain assessment using at least one score from VAS, BPI-SF, neuropathic pain score using the NCI-CTCAE v3.0 and v4.0, or FACT-Tax, and other secondary outcomes. The data was exported from the online forms as a Microsoft Excel sheet.
Statistical analysis
We calculated the mean age and associated standard deviations (SDs) for all patients by using the pooled mean and pooled SD equation, according to Cochrane handbook of systematic reviews of interventions 5.1.0 (updated March 2011).23 When data are expressed as median and interquartile range, we used Hozo and colleagues’ BMC Research Methodology equation to calculate or estimate the mean and SD.24
Data are expressed as means with SD (unless stated otherwise); statistical results were considered significant when the P-value was less than .05. Data analysis was performed using the SPSS Statistical Package, version 23 (IBM Corp., Armonk, NY).
Synthesis of data and analysis
Because of heterogeneity and low sample size of studies, no statistically justified analyses could be performed on the provided data. Instead, a descriptive analysis of published studies was performed.
Summary measures
The search strings, the list of relevant reviews, the data coding, and the quality criteria that were used can be requested from the corresponding author.
Results
Selection of articles
The
Study characteristics
Characteristics of the included studies and patient outcome are summarized in Table 1 and Table 2. A total of 5 studies from 2012 through 2017 were included in the descriptive analysis and systematic review. In all, 4 trials were prospective studies, and 1 trial was retrospective; among all trials, 2 studies were single arm and 3 were placebo-controlled and/or crossover.
There were 431 participants in the total 5 studies included in this systematic review. The number of patients per study ranged from 25 to 231. Patients were mostly older, with mean sample ages ranging from 47.9 to 63 years, and the pooled mean age for all participants in the total 5 studies was 57.7 years.
In all included studies, duloxetine was given in varying doses of 20 mg, 30 mg, 40 mg, or 60 mg. Also, different therapeutic regimens of duloxetine were used, including placebo control or crossover with vitamin B12; 80% of the studies used escalation of doses over time (only 1 trial used fixed doses for each group of patients in the study). Escalation of duloxetine by doubling the dose was done in all 4 studies, with duloxetine doses of 30 mg and 60 mg used in 75% of those studies (3 out of 4 studies).
Comparator drug was used in 4 studies (1 was single arm) in our review analysis. The comparator drug was placebo in 1 study only, and the remaining 3 studies used other antineurotoxicity or antineuropathic pain therapy, mainly vitamin B12 (as only comparator in 1 study), fish oil, pregabalin, selective 5HT reuptake inhibitors, and nonsteroidal anti-inflammatory agents.
Regarding CIPN, the chemotherapeutic agents used in the studies were as follows (after exclusion of 11 patients who never received treatment in 1 study): 224 patients (52.9%) were on paclitaxel, 168 (39.7%) on oxaliplatin, 14 (3.30%) on R-CHOP, 8 (1.89%) on combined bortezomib–dexamethasone, 5 (1.18%) on FOLFOX, and 4 (0.94%) on other taxanes.
Improvement in pain scores was the primary and/or secondary endpoint in the included studies (Table 2). Pain was assessed by 6 different scores, including the VAS, BPI-SF, neuropathic pain score using NCI-CTCAE v3.0 and v4.0, and FACT-Tax, with all reported once except the VAS score, which was reported in 2 studies. Only 1 study, by Yang and colleagues,25 measured pain by 2 scores (the VAS and NCI-CTCAE v3.0), with the rest of the studies assessing pain by just 1 of the aforementioned scores. The pretreatment pain score was reported in only 2 studies, by Smith and colleagues and Wang and colleagues, using BPI-SF and FACT-Tax scores, respectively, with total respective mean scores of 5.8 (SD, 1.7) and 11.77 (SD, 1.73).17,26
Secondary endpoints were related mainly to pain score, drug adverse effect, and assessment of quality of life (Table 2). In the study by Yang and colleagues,25 9 patients (28.1%) discontinued duloxetine because of intolerable adverse events, with dizziness or giddiness as the most common cause (44.4% of patients who discontinued treatment). Studies by Otake and colleagues12 and Hirayama and colleagues2 reported duloxetine adverse events that were very mild and usually well tolerated in collectively 22 patients, with fatigue (n = 6) and somnolence (n = 5) as the most reported adverse effects. Wang and colleagues17 reported nonneuropathic adverse events that were attributed to chemotherapy and were mild and similar in both study groups.
Discussion
To our knowledge, this is the first systematic review to discuss the effectiveness of duloxetine specifically in treatment of pain in CIPN. The administration of chemotherapeutic agents such as paclitaxel, cisplatin, oxaliplatin, and vincristine was accompanied by CIPN. The currently available treatment options for CIPN are limited. To date, no drug has been approved specifically for treatment of pain in CIPN.12
In our review, we included cancer patients with CIPN and associated pain. Several previous studies8,27,28 discussed tingling and numbness as a common adverse effect in CIPN, and usually about 20% to 42% of patients develop chronic pain.
Six different pain assessment scores were reported in the total 5 studies in our review, with VAS and NCI-CTCAE scores reported in more than 1 study. This reflects the major challenges facing the assessment of CIPN, as various scales and tools are available for pain assessment but without a standardized approach for CIPN that can be precisely implemented.8 Several other challenges regarding pain scores were observed, with Smith and colleagues as the only authors to report both pretreatment pain score and grade, while the rest of the studies failed to report either pain score or grade, or even both.
Another difficulty observed in our review was the variability in study participants in both population size and type of cancer treated. The population size in largest study included in our review was 231 patients and the smallest was 25 patients; collectively, there were only 431 patients in the included studies. Although the type of primary cancer varied in between studies, gynecologic malignancies comprised most cases (215 patients), followed by gastrointestinal tumors, and few cases of hematologic and genitourinary malignancies were reported. Similar results were observed by Geber and colleagues in their large study screening pain in cancer patients, in which gynecologic malignancies were diagnosed in 28 patients out of 61 with CIPN, representing the highest percentage (45.9%) of malignancy type in that study.26
In the study by Otake and colleagues12 examining duloxetine for CIPN in patients with gynecologic cancer, the authors concluded that duloxetine dosage either 20 mg/day or 40 mg/day was not associated with the effectiveness of duloxetine treatment by either univariate or multivariate analysis. Previous authors have provided an explanation for the difference in duloxetine response among CIPN patients and attributed those differences to the underlying pain mechanisms.14,29 In other words, pain in those patients is both peripheral nociceptive and central neuropathic, and it is likely to be caused by mixed mechanisms.
Another variation observed among CIPN patients in our review was the chemotherapeutic agents used. That was noted by Smith and colleagues,26 who reported that patients with cancer who received platinum therapies (oxaliplatin) experienced more benefit from duloxetine in terms of pain improvement than those who received taxanes (P = .13). We found no other published studies on the response to duloxetine among different chemotherapeutic agents used. However, 2 studies of duloxetine response in patients with other pain-related disorders (painful diabetic peripheral neuropathy and fibromyalgia) showed significant improvement in pain symptoms compared with placebo. In a study of pain in chemotherapy-induced neuropathy (CIN) by Geber and colleagues,29 the preexisting pain medication was not reported, but the authors concluded that treatment for CIN-related neuropathic pain differs from that for nonneuropathic (ie, musculoskeletal) pain, with the former being treated mainly with pharmacotherapy and the latter with physiotherapy and behavioral exercises. They asserted that different pain patterns could help flag neuropathic or musculoskeletal pain so that the selected treatments would be more specific. Differences in pain improvement related to duloxetine may be attributed to the underlying pain mechanism, and whether it is mixed or centrally or peripherally related was also discussed by Geber and colleagues.29
In the study by
Findings from studies on the effect of duloxetine in treatment of pain in diabetic peripheral neuropathy have shown that duloxetine at a dose of 60 mg/day effectively improves pain in 43% to 68% of patients.15,16,30 Similarly, in our review, the study by Yang and colleagues25 showed a 63% subjective reduction in pain severity by VAS score in CIPN patients but lower improvement of 47.4% by NCI-CTCAE v3.0; this can be attributed to the simplistic 4-grade rating scale of the latter.
During our analysis of studies, we noticed that no diagnostic criteria were implemented for diagnosis or inclusion of CIPN patients in any of the included studies, and this represents a major challenge in any analysis of studies with neuropathic pain patients. In 2016, Finnerup and colleagues updated the previous 2008 grading system for diagnosis of neuropathic pain, which is intended to determine the level of certainty with which the pain in question is neuropathic.31 The system defines the diagnostic certainty into 3 levels: Possible, Probable, and Definite. Although the number of studies used the grading system during the inclusion of neuropathic pain patients increased from 5% in 2009 to 30% in 2014, still more than two-thirds of studies do not use a standardized system for diagnosis and/or inclusion of neuropathic pain in patients.
Strength and limitations
The first strength of this review is that it identifies gaps in our current knowledge about duloxetine in the treatment of pain in cancer patients with CIPN. Second, we collected all available articles from inception until January 2018. Third, this review can serve as a model for future studies investigating the effectiveness of duloxetine in treatment of CIPN.
There are also limitations to this review that should be discussed. First, the studies vary greatly in samples, methodologies, and outcomes measured. Second, the diagnostic criteria for CIPN and the pain assessment tools vary among the studies. Third, there is also variability in the duloxetine doses and administration regimens among the studies, and some articles did not report the precise outcome in pain scores. Furthermore, the articles reviewed included retrospective, single-arm, or nonrandomized controlled studies with relatively small numbers of participants.
To improve the results, more placebo-controlled or head-to-head trials (with other agents used in treatment of CIPN) with large sample sizes would be needed.
Conclusions
Our purpose was to describe the effectiveness of duloxetine in improving pain scores among CIPN patients
Acknowledgments
That authors express their sincere gratitude to Nahla A Merghany and Sarah M Abd Elfadel for helping them retrieve all the relevant articles for this review.
Chemotherapy-induced peripheral neuropathy (CIPN) is a serious side effect that can be dose limiting and affect patient quality of life for prolonged time,1 with an overall incidence of about 38% in patients who are treated with multiple chemotherapeutic agents. 2
The most common antineoplastic agents causing peripheral neuropathy are oxaliplatin, cisplatin, taxanes, Vinca alkaloids, bortezomib, and thalidomide.3,8,9
Different components of the nervous system are targets of various chemotherapeutic agents, from dorsal root ganglion (DRG) cells to the distal axon. The DRG is the most vulnerable to neurotoxicity because it is less protected by the nervous system blood barrier, hence the predominance of sensory symptoms in CIPN.10 The pathogenesis of CIPN is not fully understood, and it is most probably multifaceted and not always related to the antineoplastic mechanism. Findings from experimental studies have shown an accumulation of chemotherapeutic compounds in the cell bodies of the DRG, resulting in decreased cellular metabolism and axoplasmic transport. Another proposed mechanism is the induction of apoptosis in sensory neuron of the posterior spinal ganglion after binding to DNA strands.7,11
Opioids had been used for managing pain in patients with cancer, but their addictive side effects limit use in the treatment of chronic pain,12 so several drugs called coanalgesics have been introduced as a treatment
The imbalance of 5HT and NE in the pain inhibitory pathways may contribute to the peripheral neuropathic pain.20 Duloxetine hydrochloride is a 5HT–NE reuptake inhibitor used to treat depression and generalized anxiety disorder.21 Duloxetine effect in decreasing pain transmission through increasing synaptic concentrations of NE and 5HT, which results in blocking input signals to the dorsal horn neurons in the spinal cord.12
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA statement) guidelines during the preparation of this systematic review.22
Inclusion criteria
Trial or study type. Articles publishing findings from randomized controlled trials, nonrandomized controlled trials, retrospective studies, and single-arm studies of duloxetine in the treatment of CIPN were included in our review.
Intervention. The intervention was duloxetine with all doses, either administered alone or with other antineuropathic drugs.
Comparator. The comparator was placebo (control group) or other antineuropathic drugs or no control group.
Population. The population included cancer patients with painful CIPN.
Outcome. At least one of the following outcomes was used for pain assessment:
Exclusion criteria
Studies in a non-English language, animal studies, studies whose full-text article was not available, and thesis and conference papers were not included.
Objective and study design
The objective of this systematic review was to systematically assess the effectiveness of duloxetine in the treatment of pain in patients with CIPN.
Information sources and search
Medical electronic databases. PubMed and Scopus, from inception to January 2018, were searched using the following search queries: (((duloxetine) AND chemotherapy induced peripheral neuropathy)) OR ((((chemotherapy) AND (neuropathic pain OR peripheral neuropathy))) AND duloxetine).
Selection of studies. The authors selected eligible studies. The screening of search results was performed in the following 2 steps:
n Screen title and abstracts against the selection criteria. Articles that were unclear from their title or abstract were reviewed against the selection criteria through the full text.
n Retrieve and screen full-text articles of eligible abstracts for eligibility to systematic review.
Data extraction
Two authors extracted the following data independently: sample size, mean age, chemotherapeutic drug, duloxetine dosage, and outcomes for pain assessment using at least one score from VAS, BPI-SF, neuropathic pain score using the NCI-CTCAE v3.0 and v4.0, or FACT-Tax, and other secondary outcomes. The data was exported from the online forms as a Microsoft Excel sheet.
Statistical analysis
We calculated the mean age and associated standard deviations (SDs) for all patients by using the pooled mean and pooled SD equation, according to Cochrane handbook of systematic reviews of interventions 5.1.0 (updated March 2011).23 When data are expressed as median and interquartile range, we used Hozo and colleagues’ BMC Research Methodology equation to calculate or estimate the mean and SD.24
Data are expressed as means with SD (unless stated otherwise); statistical results were considered significant when the P-value was less than .05. Data analysis was performed using the SPSS Statistical Package, version 23 (IBM Corp., Armonk, NY).
Synthesis of data and analysis
Because of heterogeneity and low sample size of studies, no statistically justified analyses could be performed on the provided data. Instead, a descriptive analysis of published studies was performed.
Summary measures
The search strings, the list of relevant reviews, the data coding, and the quality criteria that were used can be requested from the corresponding author.
Results
Selection of articles
The
Study characteristics
Characteristics of the included studies and patient outcome are summarized in Table 1 and Table 2. A total of 5 studies from 2012 through 2017 were included in the descriptive analysis and systematic review. In all, 4 trials were prospective studies, and 1 trial was retrospective; among all trials, 2 studies were single arm and 3 were placebo-controlled and/or crossover.
There were 431 participants in the total 5 studies included in this systematic review. The number of patients per study ranged from 25 to 231. Patients were mostly older, with mean sample ages ranging from 47.9 to 63 years, and the pooled mean age for all participants in the total 5 studies was 57.7 years.
In all included studies, duloxetine was given in varying doses of 20 mg, 30 mg, 40 mg, or 60 mg. Also, different therapeutic regimens of duloxetine were used, including placebo control or crossover with vitamin B12; 80% of the studies used escalation of doses over time (only 1 trial used fixed doses for each group of patients in the study). Escalation of duloxetine by doubling the dose was done in all 4 studies, with duloxetine doses of 30 mg and 60 mg used in 75% of those studies (3 out of 4 studies).
Comparator drug was used in 4 studies (1 was single arm) in our review analysis. The comparator drug was placebo in 1 study only, and the remaining 3 studies used other antineurotoxicity or antineuropathic pain therapy, mainly vitamin B12 (as only comparator in 1 study), fish oil, pregabalin, selective 5HT reuptake inhibitors, and nonsteroidal anti-inflammatory agents.
Regarding CIPN, the chemotherapeutic agents used in the studies were as follows (after exclusion of 11 patients who never received treatment in 1 study): 224 patients (52.9%) were on paclitaxel, 168 (39.7%) on oxaliplatin, 14 (3.30%) on R-CHOP, 8 (1.89%) on combined bortezomib–dexamethasone, 5 (1.18%) on FOLFOX, and 4 (0.94%) on other taxanes.
Improvement in pain scores was the primary and/or secondary endpoint in the included studies (Table 2). Pain was assessed by 6 different scores, including the VAS, BPI-SF, neuropathic pain score using NCI-CTCAE v3.0 and v4.0, and FACT-Tax, with all reported once except the VAS score, which was reported in 2 studies. Only 1 study, by Yang and colleagues,25 measured pain by 2 scores (the VAS and NCI-CTCAE v3.0), with the rest of the studies assessing pain by just 1 of the aforementioned scores. The pretreatment pain score was reported in only 2 studies, by Smith and colleagues and Wang and colleagues, using BPI-SF and FACT-Tax scores, respectively, with total respective mean scores of 5.8 (SD, 1.7) and 11.77 (SD, 1.73).17,26
Secondary endpoints were related mainly to pain score, drug adverse effect, and assessment of quality of life (Table 2). In the study by Yang and colleagues,25 9 patients (28.1%) discontinued duloxetine because of intolerable adverse events, with dizziness or giddiness as the most common cause (44.4% of patients who discontinued treatment). Studies by Otake and colleagues12 and Hirayama and colleagues2 reported duloxetine adverse events that were very mild and usually well tolerated in collectively 22 patients, with fatigue (n = 6) and somnolence (n = 5) as the most reported adverse effects. Wang and colleagues17 reported nonneuropathic adverse events that were attributed to chemotherapy and were mild and similar in both study groups.
Discussion
To our knowledge, this is the first systematic review to discuss the effectiveness of duloxetine specifically in treatment of pain in CIPN. The administration of chemotherapeutic agents such as paclitaxel, cisplatin, oxaliplatin, and vincristine was accompanied by CIPN. The currently available treatment options for CIPN are limited. To date, no drug has been approved specifically for treatment of pain in CIPN.12
In our review, we included cancer patients with CIPN and associated pain. Several previous studies8,27,28 discussed tingling and numbness as a common adverse effect in CIPN, and usually about 20% to 42% of patients develop chronic pain.
Six different pain assessment scores were reported in the total 5 studies in our review, with VAS and NCI-CTCAE scores reported in more than 1 study. This reflects the major challenges facing the assessment of CIPN, as various scales and tools are available for pain assessment but without a standardized approach for CIPN that can be precisely implemented.8 Several other challenges regarding pain scores were observed, with Smith and colleagues as the only authors to report both pretreatment pain score and grade, while the rest of the studies failed to report either pain score or grade, or even both.
Another difficulty observed in our review was the variability in study participants in both population size and type of cancer treated. The population size in largest study included in our review was 231 patients and the smallest was 25 patients; collectively, there were only 431 patients in the included studies. Although the type of primary cancer varied in between studies, gynecologic malignancies comprised most cases (215 patients), followed by gastrointestinal tumors, and few cases of hematologic and genitourinary malignancies were reported. Similar results were observed by Geber and colleagues in their large study screening pain in cancer patients, in which gynecologic malignancies were diagnosed in 28 patients out of 61 with CIPN, representing the highest percentage (45.9%) of malignancy type in that study.26
In the study by Otake and colleagues12 examining duloxetine for CIPN in patients with gynecologic cancer, the authors concluded that duloxetine dosage either 20 mg/day or 40 mg/day was not associated with the effectiveness of duloxetine treatment by either univariate or multivariate analysis. Previous authors have provided an explanation for the difference in duloxetine response among CIPN patients and attributed those differences to the underlying pain mechanisms.14,29 In other words, pain in those patients is both peripheral nociceptive and central neuropathic, and it is likely to be caused by mixed mechanisms.
Another variation observed among CIPN patients in our review was the chemotherapeutic agents used. That was noted by Smith and colleagues,26 who reported that patients with cancer who received platinum therapies (oxaliplatin) experienced more benefit from duloxetine in terms of pain improvement than those who received taxanes (P = .13). We found no other published studies on the response to duloxetine among different chemotherapeutic agents used. However, 2 studies of duloxetine response in patients with other pain-related disorders (painful diabetic peripheral neuropathy and fibromyalgia) showed significant improvement in pain symptoms compared with placebo. In a study of pain in chemotherapy-induced neuropathy (CIN) by Geber and colleagues,29 the preexisting pain medication was not reported, but the authors concluded that treatment for CIN-related neuropathic pain differs from that for nonneuropathic (ie, musculoskeletal) pain, with the former being treated mainly with pharmacotherapy and the latter with physiotherapy and behavioral exercises. They asserted that different pain patterns could help flag neuropathic or musculoskeletal pain so that the selected treatments would be more specific. Differences in pain improvement related to duloxetine may be attributed to the underlying pain mechanism, and whether it is mixed or centrally or peripherally related was also discussed by Geber and colleagues.29
In the study by
Findings from studies on the effect of duloxetine in treatment of pain in diabetic peripheral neuropathy have shown that duloxetine at a dose of 60 mg/day effectively improves pain in 43% to 68% of patients.15,16,30 Similarly, in our review, the study by Yang and colleagues25 showed a 63% subjective reduction in pain severity by VAS score in CIPN patients but lower improvement of 47.4% by NCI-CTCAE v3.0; this can be attributed to the simplistic 4-grade rating scale of the latter.
During our analysis of studies, we noticed that no diagnostic criteria were implemented for diagnosis or inclusion of CIPN patients in any of the included studies, and this represents a major challenge in any analysis of studies with neuropathic pain patients. In 2016, Finnerup and colleagues updated the previous 2008 grading system for diagnosis of neuropathic pain, which is intended to determine the level of certainty with which the pain in question is neuropathic.31 The system defines the diagnostic certainty into 3 levels: Possible, Probable, and Definite. Although the number of studies used the grading system during the inclusion of neuropathic pain patients increased from 5% in 2009 to 30% in 2014, still more than two-thirds of studies do not use a standardized system for diagnosis and/or inclusion of neuropathic pain in patients.
Strength and limitations
The first strength of this review is that it identifies gaps in our current knowledge about duloxetine in the treatment of pain in cancer patients with CIPN. Second, we collected all available articles from inception until January 2018. Third, this review can serve as a model for future studies investigating the effectiveness of duloxetine in treatment of CIPN.
There are also limitations to this review that should be discussed. First, the studies vary greatly in samples, methodologies, and outcomes measured. Second, the diagnostic criteria for CIPN and the pain assessment tools vary among the studies. Third, there is also variability in the duloxetine doses and administration regimens among the studies, and some articles did not report the precise outcome in pain scores. Furthermore, the articles reviewed included retrospective, single-arm, or nonrandomized controlled studies with relatively small numbers of participants.
To improve the results, more placebo-controlled or head-to-head trials (with other agents used in treatment of CIPN) with large sample sizes would be needed.
Conclusions
Our purpose was to describe the effectiveness of duloxetine in improving pain scores among CIPN patients
Acknowledgments
That authors express their sincere gratitude to Nahla A Merghany and Sarah M Abd Elfadel for helping them retrieve all the relevant articles for this review.
1. Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27-46.
2. Hirayama Y, Ishitani K, Sato Y, et al. Effect of duloxetine in Japanese patients with chemotherapy-induced peripheral neuropathy: a pilot randomized trial. Int J Clin Oncol. 2015;20(5):866-871.
3. Stubblefield MD, McNeely ML, Alfano CM, Mayer DK. A prospective surveillance model for physical rehabilitation of women with breast cancer: chemotherapy-induced peripheral neuropathy. Cancer. 2012;118(suppl 8):2250-2260.
4. Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63(6):419-437.
5. Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res. 2014;6(1):135-147.
6. Bakitas MA. Background noise: the experience of chemotherapy-induced peripheral neuropathy. Nurs Res. 2007;56(5):323-331.
7. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
8. Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33(1):15-49.
9. Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15(29):3081-3094.
10. Caponero R, Montarroyos ES, Tahamtani SMM. Post-chemotherapy neuropathy. Rev Dor. Sao Paulo. 2016;17(suppl 1):S56-S58.
11. Velasco R, Bruna J. Chemotherapy-induced peripheral neuropathy: an unresolved issue. Neurologia. 2010;25(2):116-131.
12. Otake A, Yoshino K, Ueda Y, et al. Usefulness of duloxetine for paclitaxel-induced peripheral neuropathy treatment in gynecological cancer patients. Anticancer Res. 2015;35(1):359-363.
13. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941-1967.
14. Smith EM, Pang H, Ye C, et al. Predictors of duloxetine response in patients with oxaliplatin-induced painful chemotherapy-induced peripheral neuropathy (CIPN): a secondary analysis of randomised controlled trial – CALGB/alliance 170601 [published online November 25, 2015]. Eur J Cancer Care (Engl). 2017;26(2). doi:10.1111/ecc.12421
15. Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1-2):109-118.
16. Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6(5):346-356.
17. Wang J, Li Q, Xu B, Zhang T, Chen S, Luo Y. Efficacy and safety of duloxetine in Chinese breast cancer patients with paclitaxel-induced peripheral neuropathy. Chin J Cancer Res. 2017;29(5):411-418.
18. Irving G, Tanenberg RJ, Raskin J, Risser RC, Malcolm S. Comparative safety and tolerability of duloxetine vs pregabalin vs duloxetine plus gabapentin in patients with diabetic peripheral neuropathic pain. Int J Clin Pract. 2014;68(9):1130-1140.
19. Esin E, Yalcin S. Neuropathic cancer pain: what we are dealing with? How to manage it? Onco Targets Ther. 2014;7:599-618.
20. Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25(12):613-617.
21. Mancini M, Perna G, Rossi A, Petralia A. Use of duloxetine in patients with an anxiety disorder, or with comorbid anxiety and major depressive disorder: a review of the literature. Expert Opin Pharmacother. 2010;11(7):1167-1181.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269.
23. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. http://handbook-5-1.cochrane.org/. Updated March 2011. Accessed November 19, 2018.
24. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
25. Yang YH, Lin JK, Chen WS, et al. Duloxetine improves oxaliplatin-induced neuropathy in patients with colorectal cancer: an open-label pilot study. Support Care Cancer. 2012;20(7):1491-1497.
26. Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359-1367.
27. Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18(6):343-349.
28. Cavenagh J, Good P, Ravenscroft P. Neuropathic pain: are we out of the woods yet? Intern Med J. 2006;36(4):251-255.
29. Geber C, Breimhorst M, Burbach B, et al. Pain in chemotherapy-induced neuropathy—more than neuropathic? Pain. 2013;154(12):2877-2887.
30. Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–1420.
31. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. 2016;157(8):1599-1606.
1. Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27-46.
2. Hirayama Y, Ishitani K, Sato Y, et al. Effect of duloxetine in Japanese patients with chemotherapy-induced peripheral neuropathy: a pilot randomized trial. Int J Clin Oncol. 2015;20(5):866-871.
3. Stubblefield MD, McNeely ML, Alfano CM, Mayer DK. A prospective surveillance model for physical rehabilitation of women with breast cancer: chemotherapy-induced peripheral neuropathy. Cancer. 2012;118(suppl 8):2250-2260.
4. Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63(6):419-437.
5. Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res. 2014;6(1):135-147.
6. Bakitas MA. Background noise: the experience of chemotherapy-induced peripheral neuropathy. Nurs Res. 2007;56(5):323-331.
7. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
8. Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33(1):15-49.
9. Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15(29):3081-3094.
10. Caponero R, Montarroyos ES, Tahamtani SMM. Post-chemotherapy neuropathy. Rev Dor. Sao Paulo. 2016;17(suppl 1):S56-S58.
11. Velasco R, Bruna J. Chemotherapy-induced peripheral neuropathy: an unresolved issue. Neurologia. 2010;25(2):116-131.
12. Otake A, Yoshino K, Ueda Y, et al. Usefulness of duloxetine for paclitaxel-induced peripheral neuropathy treatment in gynecological cancer patients. Anticancer Res. 2015;35(1):359-363.
13. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941-1967.
14. Smith EM, Pang H, Ye C, et al. Predictors of duloxetine response in patients with oxaliplatin-induced painful chemotherapy-induced peripheral neuropathy (CIPN): a secondary analysis of randomised controlled trial – CALGB/alliance 170601 [published online November 25, 2015]. Eur J Cancer Care (Engl). 2017;26(2). doi:10.1111/ecc.12421
15. Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1-2):109-118.
16. Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6(5):346-356.
17. Wang J, Li Q, Xu B, Zhang T, Chen S, Luo Y. Efficacy and safety of duloxetine in Chinese breast cancer patients with paclitaxel-induced peripheral neuropathy. Chin J Cancer Res. 2017;29(5):411-418.
18. Irving G, Tanenberg RJ, Raskin J, Risser RC, Malcolm S. Comparative safety and tolerability of duloxetine vs pregabalin vs duloxetine plus gabapentin in patients with diabetic peripheral neuropathic pain. Int J Clin Pract. 2014;68(9):1130-1140.
19. Esin E, Yalcin S. Neuropathic cancer pain: what we are dealing with? How to manage it? Onco Targets Ther. 2014;7:599-618.
20. Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25(12):613-617.
21. Mancini M, Perna G, Rossi A, Petralia A. Use of duloxetine in patients with an anxiety disorder, or with comorbid anxiety and major depressive disorder: a review of the literature. Expert Opin Pharmacother. 2010;11(7):1167-1181.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269.
23. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. http://handbook-5-1.cochrane.org/. Updated March 2011. Accessed November 19, 2018.
24. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
25. Yang YH, Lin JK, Chen WS, et al. Duloxetine improves oxaliplatin-induced neuropathy in patients with colorectal cancer: an open-label pilot study. Support Care Cancer. 2012;20(7):1491-1497.
26. Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359-1367.
27. Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18(6):343-349.
28. Cavenagh J, Good P, Ravenscroft P. Neuropathic pain: are we out of the woods yet? Intern Med J. 2006;36(4):251-255.
29. Geber C, Breimhorst M, Burbach B, et al. Pain in chemotherapy-induced neuropathy—more than neuropathic? Pain. 2013;154(12):2877-2887.
30. Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–1420.
31. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. 2016;157(8):1599-1606.
Beta-cell therapies for type 1 diabetes: Transplants and bionics
With intensive insulin regimens and home blood glucose monitoring, patients with type 1 diabetes are controlling their blood glucose better than in the past. Nevertheless, glucose regulation is still imperfect and tedious, and striving for tight glycemic control poses the risk of hypoglycemia.
Prominent among the challenges are the sheer numbers involved. Some 1.25 million Americans have type 1 diabetes, and another 30 million have type 2, but only about 7,000 to 8,000 pancreases are available for transplant each year.1 While awaiting a breakthrough—perhaps involving stem cells, perhaps involving organs obtained from animals—an insulin pump may offer better diabetes control for many. Another possibility is a closed-loop system with a continuous glucose monitor that drives a dual-infusion pump, delivering insulin when glucose levels rise too high, and glucagon when they dip too low.
DIABETES WAS KNOWN IN ANCIENT TIMES
About 3,000 years ago, Egyptians described the syndrome of thirst, emaciation, and sweet urine that attracted ants. The term diabetes (Greek for siphon) was first recorded in 1425; mellitus (Latin for sweet with honey) was not added until 1675.
In 1857, Bernard hypothesized that diabetes was caused by overproduction of glucose in the liver. This idea was replaced in 1889, when Mering and Minkowski proposed the dysfunctional pancreas theory that eventually led to the discovery of the beta cell.2
In 1921, Banting and Best isolated insulin, and for the past 100 years subcutaneous insulin replacement has been the mainstay of treatment. But starting about 50 years ago, researchers have been looking for safe and long-lasting ways to replace beta cells and eliminate the need for exogenous insulin replacement.
TRANSPLANTING THE WHOLE PANCREAS
The first whole-pancreas transplant was performed in 1966 by Kelly et al,3 followed by 13 more by 1973.4 These first transplant grafts were short-lived, with only 1 graft surviving longer than 1 year. Since then, more than 12,000 pancreases have been transplanted worldwide, as refinements in surgical techniques and immunosuppressive therapies have improved patient and graft survival rates.4
Today, most pancreas transplants are in patients who have both type 1 diabetes and end-stage renal disease due to diabetic nephropathy, and most receive both a kidney and a pancreas at the same time. Far fewer patients receive a pancreas after previously receiving a kidney, or receive a pancreas alone.
The bile duct of the transplanted pancreas is usually routed into the patient’s small intestine, as nature intended, and less often into the bladder. Although bladder drainage is associated with urinary complications, it has the advantage of allowing measurement of pancreatic amylase levels in the urine to monitor for graft rejection. With simultaneous pancreas and kidney transplant, the serum creatinine concentration can also be monitored for rejection of the kidney graft.
Current immunosuppressive regimens vary but generally consist of anti-T-cell antibodies at the time of surgery, followed by lifelong treatment with the combination of a calcineurin inhibitor (cyclosporine or tacrolimus) and an antimetabolite (mycophenolate mofetil or azathioprine).
Outcomes are good. The rates of patient and graft survival are highest with simultaneous pancreas-kidney transplant, and somewhat lower with pancreas-after-kidney and pancreas-alone transplant.
Benefits of pancreas transplant
Most recipients can stop taking insulin immediately after the procedure, and their hemoglobin A1c levels normalize and stay low for the life of the graft. Lipid levels also decrease, although this has not been directly correlated with lower risk of vascular disease.4
Transplant also reduces or eliminates some complications of diabetes, including retinopathy, nephropathy, cardiomyopathy, and gastropathy.
For example, in patients undergoing simultaneous pancreas-kidney transplant, diabetic nephropathy does not recur in the new kidney. Fioretto et al5 reported that nephropathy lesions reversed during the 10 years after pancreas transplant.
Kennedy et al6,7 found that preexisting diabetic neuropathy improved slightly (although neurologic status did not completely return to normal) over a period of up to 42 months in a group of patients who received a pancreas transplant, whereas it tended to worsen in a control group. Both groups were assessed at baseline and at 12 and 24 months, with a subgroup followed through 42 months, and they underwent testing of motor, sensory, and autonomic function.6,7
Disadvantages of pancreas transplant
Disadvantages of whole-pancreas transplant include hypoglycemia (usually mild), adverse effects of immunosuppression, potential for surgical complications including an increased rate of death in the first 90 days after the procedure, and cost.
In an analysis comparing the 5-year estimated costs of dialysis, kidney transplant alone from cadavers or live donors, or simultaneous pancreas-kidney transplant for diabetic patients with end-stage renal disease, the least expensive option was kidney transplant from a live donor.8 The most expensive option was simultaneous pancreas-kidney transplant, but quality of life was better with this option. The analysis did not consider the potential cost of long-term treatments for complications related to diabetes that could be saved with a pancreas transplant.
Data conflict regarding the risk of death with different types of pancreas transplants. A retrospective cohort study of data from 124 US transplant centers reported in 2003 found higher mortality rates in pancreas-alone transplant recipients than in patients on a transplant waiting list receiving conventional therapy.9 In contrast, a 2004 study reported that after the first 90 days, when the risk of death was clearly higher, mortality rates were lower after simultaneous pancreas-kidney transplant and pancreas-after-kidney transplant.10 After pancreas-alone transplant, however, mortality rates were higher than with exogenous insulin therapy.
Although outcomes have improved, fewer patients with type 1 diabetes are undergoing pancreas transplant in recent years.
Interestingly, more simultaneous pancreas-kidney transplants are being successfully performed in patients with type 2 diabetes, who now account for 8% of all simultaneous pancreas-kidney transplant recipients.11 Outcomes of pancreas transplant appear to be similar regardless of diabetes type.
Bottom line
Pancreas transplant is a viable option for certain cases of complicated diabetes.
TRANSPLANTING ISLET CELLS
Despite its successes, pancreas transplant is major surgery and requires lifetime immunosuppression. Research is ongoing into a less-invasive procedure that, it is hoped, would require less immunosuppression: transplanting islets by themselves.
Islet autotransplant after pancreatectomy
For some patients with chronic pancreatitis, the only option to relieve chronic pain, narcotic dependence, and poor quality of life is to remove the pancreas. In the past, this desperate measure would instantly and inevitably cause diabetes, but not anymore.
Alpha cells and glucagon are a different story; a complication of islet transplant is hypoglycemia. In 2016, Lin et al12 reported spontaneous hypoglycemia in 6 of 12 patients who maintained insulin independence after autotransplant of islets. Although the transplanted islets had functional alpha cells that could in theory produce glucagon, as well as beta cells that produce insulin and C-peptide, apparently the alpha cells were not secreting glucagon in response to the hypoglycemia.
Location may matter. Gupta et al,13 in a 1997 study in dogs, found that more hypoglycemia occurs if islets are autotransplanted into the liver than if they are transplanted into the peritoneal cavity. A possible explanation may have to do with the glycemic environment of the liver.
Islet allotransplant
Islets can also be taken from cadaver donors and transplanted into patients with type 1 diabetes, who do not have enough working beta cells.
Success of allotransplant increased after the publication of observational data from the program in Edmonton in Canada, in which 7 consecutive patients with type 1 diabetes achieved initial insulin independence after islet allotransplant using steroid-free immunosuppression.14 Six recipients required islets from 2 donors, and 1 required islets from 4 donors, so they all received large volumes of at least 11,000 islet equivalents (IEQ) per kilogram of body weight.
In a subsequent report from the same team,15 16 (44%) of 36 patients remained insulin-free at 1 year, and C-peptide secretion was detectable in 70% at 2 years. But despite the elevated C-peptide levels, only 5 patients remained insulin-independent by 2 years. Lower hemoglobin A1c levels and decreases in hypoglycemic events from baseline also were noted.
The Clinical Islet Transplantation Consortium (CITC)16 and Collaborative Islet Transplant Registry (CITR)17 were established in 2004 to combine data and resources from centers around the world, including several that specialize in islet isolation and purification. Currently, more than 80 studies are being conducted.
The CITC and CITR now have data on more than 1,000 allogeneic islet transplant recipients (islet transplant alone, after kidney transplant, or simultaneous with it). The primary outcomes are hemoglobin A1c levels below 7% fasting C-peptide levels 0.3 ng/mL or higher, and fasting blood glucose of 60 to 140 mg/dL with no severe hypoglycemic events. The best results for islet-alone transplant have been in recipients over age 35 who received at least 325,000 IEQs with use of tumor necrosis factor antagonists for induction and calcineurin inhibitors or mammalian target of rapamycin (mTOR) inhibitors for maintenance.17
The best success for islet-after-kidney transplant was achieved with the same protocol but with insulin given to the donor during hospitalization before pancreas procurement. For participants with favorable factors, a hemoglobin A1c at or below 6.5% was achieved in about 80% at 1 year after last infusion, with more than 80% maintaining their fasting blood glucose level goals. About 70% of these patients were insulin-independent at 1 year. Hypoglycemia unawareness resolved in these patients even 5 years after infusion. Although there were no deaths or disabilities related to these transplants, bleeding occurred in 1 of 15 procedures. There was also a notable decline in estimated glomerular filtration rates with calcineurin inhibitor-based immunosuppression.17
Making islets go farther
One of the greatest challenges to islet transplant is the need for multiple donors to provide enough islet cells to overcome the loss of cells during transplant. Pancreases are already in short supply, and if each recipient needs more than 1, this makes the shortage worse. Some centers have achieved transplant with fewer donors,18,19 possibly by selecting pancreases from young donors who had a high body mass index and more islet cells, and harvesting and using them with a shorter cold ischemic time.
The number of viable, functioning islet cells drastically decreases after transplant, especially when transplanted into the portal system. This phenomenon is linked to an instant, blood-mediated inflammatory reaction involving antibody binding, complement and coagulation cascade activation, and platelet aggregation. The reaction, part of the innate immune system, damages the islet cells and leads to insulin dumping and early graft loss in studies in vitro and in vivo. Another factor affecting the survival of the graft cells is the low oxygen tension in the portal system.
For this reason, sites such as the pancreas, gastric submucosa, genitourinary tract, muscle, omentum, bone marrow, kidney capsule, peritoneum, anterior eye chamber, testis, and thymus are being explored.20
To create a more supportive environment for the transplanted cells, biotechnicians are trying to encapsulate islets in a semipermeable membrane that would protect them from the immune system while still allowing oxygen, nutrients, waste products, and, critically, insulin to diffuse in and out. Currently, no site or encapsulated product has been more successful than the current practice of implanting naked islets in the portal system.20
Bottom line
Without advances in transplant sites or increasing the yield of islet cells to allow single-donor transplants, islet cell allotransplant will not be feasible for most patients with type 1 diabetes.
Xenotransplant: Can pig cells make up the shortage?
Use of animal kidneys (xenotransplant) is a potential solution to the shortage of human organs for transplant.
In theory, pigs could be a source. Porcine insulin is similar to human insulin (differing by only 1 amino acid), and it should be possible to breed “knockout” pigs that lack the antigens responsible for acute humoral rejection.21
On the other hand, transplant of porcine islets poses several immunologic, physiologic, ethical, legal, and infectious concerns. For example, porcine tissue could carry pig viruses, such as porcine endogenous retroviruses.21 And even if the pigs are genetically modified, patients will still require immunosuppressive therapy.
A review of 17 studies of pig islet xenotransplant into nonhuman primates found that in 5 of the studies (4 using diabetic primates) the grafts survived at least 3 months.22 Of these, 1 study used encapsulation, and the rest used intensive and toxic immunosuppression.
More research is needed to make xenotransplant a clinical option.
Transplanting stem cells or beta cells grown from stem cells
Stem cells provide an exciting potential alternative to the limited donor pool. During the past decade, several studies have shown success using human pluripotent stem cells (embryonic stem cells and human-induced pluripotent stem cells), mesenchymal stem cells isolated from adult tissues, and directly programmed somatic cells. Researchers have created stable cultures of pluripotent stem cells from embryonic stem cells, which could possibly be produced on a large scale and banked.23
Human pluripotent stem cells derived from pancreatic progenitors have been shown to mature into more functional, islet-like structures in vivo. They transform into subtypes of islet cells including alpha, beta, and delta cells, ghrelin-producing cells, and pancreatic polypeptide hormone-producing cells. This process takes 2 to 6 weeks. In mice, these cells have been shown to maintain glucose homeostasis.24 Phase 1 and 2 trials in humans are now being conducted.
Pagliuca et al25 generated functional human pancreatic beta cells in vitro from embryonic stem cells. Rezania et al24 reversed diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. The techniques used in these studies contributed to the success of a study by Vegas et al,26 who achieved successful long-term glycemic control in mice using polymer-encapsulated human stem cell-derived beta cells.
Reversal of autoimmunity is an important step that needs to be overcome in stem cell transplant for type 1 diabetes. Nikolic et al27 have achieved mixed allogeneic chimerism across major histocompatibility complex barriers with nonmyeloablative conditioning in advanced-diabetic nonobese diabetic mice. However, conditioning alone (ie, without bone marrow transplant) does not permit acceptance of allogeneic islets and does not reverse autoimmunity or allow islet regeneration.28 Adding allogeneic bone marrow transplant to conditioned nonobese diabetic mice leads to tolerance to the donor and reverses autoimmunity.
THE ‘BIONIC’ PANCREAS
While we wait for advances in islet cell transplant, improved insulin pumps hold promise.
One such experimental device, the iLet (Beta Bionics, Boston, MA), designed by Damiano et al, consists of 2 infusion pumps (1 for insulin, 1 for glucagon) linked to a continuous glucose monitor via a smartphone app.
The monitor measures the glucose level every 5 minutes and transmits the information wirelessly to the phone app, which calculates the amount of insulin and glucagon required to stabilize the blood glucose: more insulin if too high, more glucagon if too low. The phone transmits this information to the pumps.
Dubbed the “bionic” pancreas, this closed-loop system frees patients from the tasks of measuring their glucose multiple times a day, calculating the appropriate dose, and giving multiple insulin injections.
The 2016 summer camp study29 followed 19 preteens wearing the bionic pancreas for 5 days. During this time, the patients had lower mean glucose levels and less hypoglycemia than during control periods. No episodes of severe hypoglycemia were recorded.
El-Khatib et al30 randomly assigned 43 patients to treatment with either the bihormonal bionic pancreas or usual care (a conventional insulin pump or a sensor-augmented insulin pump) for 11 days, followed by 11 days of the opposite treatment. All participants continued their normal activities. The bionic pancreas system was superior to the insulin pump in terms of the mean glucose concentration and mean time in the hypoglycemic range (P < .0001 for both results).
Bottom line
As the search continues for better solutions, advances in technology such as the bionic pancreas could provide a safer (ie, less hypoglycemic) and more successful alternative for insulin replacement in the near future.
- American Diabetes Association. Statistics about diabetes: overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 6, 2018.
- Ahmed AM. History of diabetes mellitus. Saudi Med J 2002; 23(4):373–378. pmid:11953758
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837. pmid: 5338113
- Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001; 233(4):463–501. pmid:11303130
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339(2):69–75. doi:10.1056/NEJM199807093390202
- Kennedy WR, Navarro X, Goetz FC, Sutherland DE, Najarian JS. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med 1990; 322(15):1031–1037. doi:10.1056/NEJM199004123221503
- Kennedy WR, Navarro X, Sutherland DER. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology 1995; 45(4):773–780. pmid:7723969
- Douzdjian V, Ferrara D, Silvestri G. Treatment strategies for insulin-dependent diabetics with ESRD: a cost-effectiveness decision analysis model. Am J Kidney Dis 1998; 31(5):794–802. pmid:9590189
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290(21):2817–2823. doi:10.1001/jama.290.21.2817
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4(12):2018–2026. doi:10.1111/j.1600-6143.2004.00667.x
- Redfield RR, Scalea JR, Odorico JS. Simultaneous pancreas and kidney transplantation: current trends and future directions. Curr Opin Organ Transplant 2015; 20(1):94-102. doi:10.1097/MOT.0000000000000146
- Lin YK, Faiman C, Johnston PC, et al. Spontaneous hypoglycemia after islet autotransplantation for chronic pancreatitis. J Clin Endocrinol Metab 2016; 101(10):3669–3675. doi:10.1210/jc.2016-2111
- Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes 1997; 46(1):28–33. pmid:8971077
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343(4):230–238. doi:10.1056/NEJM200007273430401
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355(13):1318–1330. doi:10.1056/NEJMoa061267
- Clinical Islet Transplantation (CIT) Consortium. www.citisletstudy.org. Accessed November 6, 2018.
- Collaborative Islet Transplantation Registry (CITR). CITR 10th Annual Report. https://citregistry.org/system/files/10th_AR.pdf. Accessed November 6, 2018.
- Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004; 4(3):390–401. pmid:14961992
- Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant 2010; 10(8):1870–1880. doi:10.1111/j.1600-6143.2010.03073.x
- Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep 2011; 11(5):364–374. doi:10.1007/s11892-011-0216-9
- Cooper DK, Gollackner B, Knosalla C, Teranishi K. Xenotransplantation—how far have we come? Transpl Immunol 2002; 9(2–4):251–256. pmid:12180839
- Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R. Pig-to-nonhuman primates pancreatic islet xenotransplantation: an overview. Curr Diab Rep 2011; 11(5):402–412. doi:10.1007/s11892-011-0213-z
- Bartlett ST, Markmann JF, Johnson P, et al. Report from IPITA-TTS opinion leaders meeting on the future of beta-cell replacement. Transplantation 2016; 100(suppl 2):S1–S44. doi:10.1097/TP.0000000000001055
- Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32(11):1121–1133. doi:10.1038/nbt.3033
- Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159(2):428–439. doi:10.1016/j.cell.2014.09.040
- Vegas AJ, Veiseh O, Gurtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016; 22(3):306–311. doi:10.1038/nm.4030
- Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune tolerance and reversal of autoimmunity. Diabetes 2004; 53(2):376–383. pmid:14747288
- Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol 2012; 12(6):403–416. doi:10.1038/nri3226
- Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016; 4(3):233–243. doi:10.1016/S2213-8587(15)00489-1
- El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicenter randomized crossover trial. Lancet 2017; 389(10067):369–380. doi:10.1016/S0140-6736(16)32567-3
With intensive insulin regimens and home blood glucose monitoring, patients with type 1 diabetes are controlling their blood glucose better than in the past. Nevertheless, glucose regulation is still imperfect and tedious, and striving for tight glycemic control poses the risk of hypoglycemia.
Prominent among the challenges are the sheer numbers involved. Some 1.25 million Americans have type 1 diabetes, and another 30 million have type 2, but only about 7,000 to 8,000 pancreases are available for transplant each year.1 While awaiting a breakthrough—perhaps involving stem cells, perhaps involving organs obtained from animals—an insulin pump may offer better diabetes control for many. Another possibility is a closed-loop system with a continuous glucose monitor that drives a dual-infusion pump, delivering insulin when glucose levels rise too high, and glucagon when they dip too low.
DIABETES WAS KNOWN IN ANCIENT TIMES
About 3,000 years ago, Egyptians described the syndrome of thirst, emaciation, and sweet urine that attracted ants. The term diabetes (Greek for siphon) was first recorded in 1425; mellitus (Latin for sweet with honey) was not added until 1675.
In 1857, Bernard hypothesized that diabetes was caused by overproduction of glucose in the liver. This idea was replaced in 1889, when Mering and Minkowski proposed the dysfunctional pancreas theory that eventually led to the discovery of the beta cell.2
In 1921, Banting and Best isolated insulin, and for the past 100 years subcutaneous insulin replacement has been the mainstay of treatment. But starting about 50 years ago, researchers have been looking for safe and long-lasting ways to replace beta cells and eliminate the need for exogenous insulin replacement.
TRANSPLANTING THE WHOLE PANCREAS
The first whole-pancreas transplant was performed in 1966 by Kelly et al,3 followed by 13 more by 1973.4 These first transplant grafts were short-lived, with only 1 graft surviving longer than 1 year. Since then, more than 12,000 pancreases have been transplanted worldwide, as refinements in surgical techniques and immunosuppressive therapies have improved patient and graft survival rates.4
Today, most pancreas transplants are in patients who have both type 1 diabetes and end-stage renal disease due to diabetic nephropathy, and most receive both a kidney and a pancreas at the same time. Far fewer patients receive a pancreas after previously receiving a kidney, or receive a pancreas alone.
The bile duct of the transplanted pancreas is usually routed into the patient’s small intestine, as nature intended, and less often into the bladder. Although bladder drainage is associated with urinary complications, it has the advantage of allowing measurement of pancreatic amylase levels in the urine to monitor for graft rejection. With simultaneous pancreas and kidney transplant, the serum creatinine concentration can also be monitored for rejection of the kidney graft.
Current immunosuppressive regimens vary but generally consist of anti-T-cell antibodies at the time of surgery, followed by lifelong treatment with the combination of a calcineurin inhibitor (cyclosporine or tacrolimus) and an antimetabolite (mycophenolate mofetil or azathioprine).
Outcomes are good. The rates of patient and graft survival are highest with simultaneous pancreas-kidney transplant, and somewhat lower with pancreas-after-kidney and pancreas-alone transplant.
Benefits of pancreas transplant
Most recipients can stop taking insulin immediately after the procedure, and their hemoglobin A1c levels normalize and stay low for the life of the graft. Lipid levels also decrease, although this has not been directly correlated with lower risk of vascular disease.4
Transplant also reduces or eliminates some complications of diabetes, including retinopathy, nephropathy, cardiomyopathy, and gastropathy.
For example, in patients undergoing simultaneous pancreas-kidney transplant, diabetic nephropathy does not recur in the new kidney. Fioretto et al5 reported that nephropathy lesions reversed during the 10 years after pancreas transplant.
Kennedy et al6,7 found that preexisting diabetic neuropathy improved slightly (although neurologic status did not completely return to normal) over a period of up to 42 months in a group of patients who received a pancreas transplant, whereas it tended to worsen in a control group. Both groups were assessed at baseline and at 12 and 24 months, with a subgroup followed through 42 months, and they underwent testing of motor, sensory, and autonomic function.6,7
Disadvantages of pancreas transplant
Disadvantages of whole-pancreas transplant include hypoglycemia (usually mild), adverse effects of immunosuppression, potential for surgical complications including an increased rate of death in the first 90 days after the procedure, and cost.
In an analysis comparing the 5-year estimated costs of dialysis, kidney transplant alone from cadavers or live donors, or simultaneous pancreas-kidney transplant for diabetic patients with end-stage renal disease, the least expensive option was kidney transplant from a live donor.8 The most expensive option was simultaneous pancreas-kidney transplant, but quality of life was better with this option. The analysis did not consider the potential cost of long-term treatments for complications related to diabetes that could be saved with a pancreas transplant.
Data conflict regarding the risk of death with different types of pancreas transplants. A retrospective cohort study of data from 124 US transplant centers reported in 2003 found higher mortality rates in pancreas-alone transplant recipients than in patients on a transplant waiting list receiving conventional therapy.9 In contrast, a 2004 study reported that after the first 90 days, when the risk of death was clearly higher, mortality rates were lower after simultaneous pancreas-kidney transplant and pancreas-after-kidney transplant.10 After pancreas-alone transplant, however, mortality rates were higher than with exogenous insulin therapy.
Although outcomes have improved, fewer patients with type 1 diabetes are undergoing pancreas transplant in recent years.
Interestingly, more simultaneous pancreas-kidney transplants are being successfully performed in patients with type 2 diabetes, who now account for 8% of all simultaneous pancreas-kidney transplant recipients.11 Outcomes of pancreas transplant appear to be similar regardless of diabetes type.
Bottom line
Pancreas transplant is a viable option for certain cases of complicated diabetes.
TRANSPLANTING ISLET CELLS
Despite its successes, pancreas transplant is major surgery and requires lifetime immunosuppression. Research is ongoing into a less-invasive procedure that, it is hoped, would require less immunosuppression: transplanting islets by themselves.
Islet autotransplant after pancreatectomy
For some patients with chronic pancreatitis, the only option to relieve chronic pain, narcotic dependence, and poor quality of life is to remove the pancreas. In the past, this desperate measure would instantly and inevitably cause diabetes, but not anymore.
Alpha cells and glucagon are a different story; a complication of islet transplant is hypoglycemia. In 2016, Lin et al12 reported spontaneous hypoglycemia in 6 of 12 patients who maintained insulin independence after autotransplant of islets. Although the transplanted islets had functional alpha cells that could in theory produce glucagon, as well as beta cells that produce insulin and C-peptide, apparently the alpha cells were not secreting glucagon in response to the hypoglycemia.
Location may matter. Gupta et al,13 in a 1997 study in dogs, found that more hypoglycemia occurs if islets are autotransplanted into the liver than if they are transplanted into the peritoneal cavity. A possible explanation may have to do with the glycemic environment of the liver.
Islet allotransplant
Islets can also be taken from cadaver donors and transplanted into patients with type 1 diabetes, who do not have enough working beta cells.
Success of allotransplant increased after the publication of observational data from the program in Edmonton in Canada, in which 7 consecutive patients with type 1 diabetes achieved initial insulin independence after islet allotransplant using steroid-free immunosuppression.14 Six recipients required islets from 2 donors, and 1 required islets from 4 donors, so they all received large volumes of at least 11,000 islet equivalents (IEQ) per kilogram of body weight.
In a subsequent report from the same team,15 16 (44%) of 36 patients remained insulin-free at 1 year, and C-peptide secretion was detectable in 70% at 2 years. But despite the elevated C-peptide levels, only 5 patients remained insulin-independent by 2 years. Lower hemoglobin A1c levels and decreases in hypoglycemic events from baseline also were noted.
The Clinical Islet Transplantation Consortium (CITC)16 and Collaborative Islet Transplant Registry (CITR)17 were established in 2004 to combine data and resources from centers around the world, including several that specialize in islet isolation and purification. Currently, more than 80 studies are being conducted.
The CITC and CITR now have data on more than 1,000 allogeneic islet transplant recipients (islet transplant alone, after kidney transplant, or simultaneous with it). The primary outcomes are hemoglobin A1c levels below 7% fasting C-peptide levels 0.3 ng/mL or higher, and fasting blood glucose of 60 to 140 mg/dL with no severe hypoglycemic events. The best results for islet-alone transplant have been in recipients over age 35 who received at least 325,000 IEQs with use of tumor necrosis factor antagonists for induction and calcineurin inhibitors or mammalian target of rapamycin (mTOR) inhibitors for maintenance.17
The best success for islet-after-kidney transplant was achieved with the same protocol but with insulin given to the donor during hospitalization before pancreas procurement. For participants with favorable factors, a hemoglobin A1c at or below 6.5% was achieved in about 80% at 1 year after last infusion, with more than 80% maintaining their fasting blood glucose level goals. About 70% of these patients were insulin-independent at 1 year. Hypoglycemia unawareness resolved in these patients even 5 years after infusion. Although there were no deaths or disabilities related to these transplants, bleeding occurred in 1 of 15 procedures. There was also a notable decline in estimated glomerular filtration rates with calcineurin inhibitor-based immunosuppression.17
Making islets go farther
One of the greatest challenges to islet transplant is the need for multiple donors to provide enough islet cells to overcome the loss of cells during transplant. Pancreases are already in short supply, and if each recipient needs more than 1, this makes the shortage worse. Some centers have achieved transplant with fewer donors,18,19 possibly by selecting pancreases from young donors who had a high body mass index and more islet cells, and harvesting and using them with a shorter cold ischemic time.
The number of viable, functioning islet cells drastically decreases after transplant, especially when transplanted into the portal system. This phenomenon is linked to an instant, blood-mediated inflammatory reaction involving antibody binding, complement and coagulation cascade activation, and platelet aggregation. The reaction, part of the innate immune system, damages the islet cells and leads to insulin dumping and early graft loss in studies in vitro and in vivo. Another factor affecting the survival of the graft cells is the low oxygen tension in the portal system.
For this reason, sites such as the pancreas, gastric submucosa, genitourinary tract, muscle, omentum, bone marrow, kidney capsule, peritoneum, anterior eye chamber, testis, and thymus are being explored.20
To create a more supportive environment for the transplanted cells, biotechnicians are trying to encapsulate islets in a semipermeable membrane that would protect them from the immune system while still allowing oxygen, nutrients, waste products, and, critically, insulin to diffuse in and out. Currently, no site or encapsulated product has been more successful than the current practice of implanting naked islets in the portal system.20
Bottom line
Without advances in transplant sites or increasing the yield of islet cells to allow single-donor transplants, islet cell allotransplant will not be feasible for most patients with type 1 diabetes.
Xenotransplant: Can pig cells make up the shortage?
Use of animal kidneys (xenotransplant) is a potential solution to the shortage of human organs for transplant.
In theory, pigs could be a source. Porcine insulin is similar to human insulin (differing by only 1 amino acid), and it should be possible to breed “knockout” pigs that lack the antigens responsible for acute humoral rejection.21
On the other hand, transplant of porcine islets poses several immunologic, physiologic, ethical, legal, and infectious concerns. For example, porcine tissue could carry pig viruses, such as porcine endogenous retroviruses.21 And even if the pigs are genetically modified, patients will still require immunosuppressive therapy.
A review of 17 studies of pig islet xenotransplant into nonhuman primates found that in 5 of the studies (4 using diabetic primates) the grafts survived at least 3 months.22 Of these, 1 study used encapsulation, and the rest used intensive and toxic immunosuppression.
More research is needed to make xenotransplant a clinical option.
Transplanting stem cells or beta cells grown from stem cells
Stem cells provide an exciting potential alternative to the limited donor pool. During the past decade, several studies have shown success using human pluripotent stem cells (embryonic stem cells and human-induced pluripotent stem cells), mesenchymal stem cells isolated from adult tissues, and directly programmed somatic cells. Researchers have created stable cultures of pluripotent stem cells from embryonic stem cells, which could possibly be produced on a large scale and banked.23
Human pluripotent stem cells derived from pancreatic progenitors have been shown to mature into more functional, islet-like structures in vivo. They transform into subtypes of islet cells including alpha, beta, and delta cells, ghrelin-producing cells, and pancreatic polypeptide hormone-producing cells. This process takes 2 to 6 weeks. In mice, these cells have been shown to maintain glucose homeostasis.24 Phase 1 and 2 trials in humans are now being conducted.
Pagliuca et al25 generated functional human pancreatic beta cells in vitro from embryonic stem cells. Rezania et al24 reversed diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. The techniques used in these studies contributed to the success of a study by Vegas et al,26 who achieved successful long-term glycemic control in mice using polymer-encapsulated human stem cell-derived beta cells.
Reversal of autoimmunity is an important step that needs to be overcome in stem cell transplant for type 1 diabetes. Nikolic et al27 have achieved mixed allogeneic chimerism across major histocompatibility complex barriers with nonmyeloablative conditioning in advanced-diabetic nonobese diabetic mice. However, conditioning alone (ie, without bone marrow transplant) does not permit acceptance of allogeneic islets and does not reverse autoimmunity or allow islet regeneration.28 Adding allogeneic bone marrow transplant to conditioned nonobese diabetic mice leads to tolerance to the donor and reverses autoimmunity.
THE ‘BIONIC’ PANCREAS
While we wait for advances in islet cell transplant, improved insulin pumps hold promise.
One such experimental device, the iLet (Beta Bionics, Boston, MA), designed by Damiano et al, consists of 2 infusion pumps (1 for insulin, 1 for glucagon) linked to a continuous glucose monitor via a smartphone app.
The monitor measures the glucose level every 5 minutes and transmits the information wirelessly to the phone app, which calculates the amount of insulin and glucagon required to stabilize the blood glucose: more insulin if too high, more glucagon if too low. The phone transmits this information to the pumps.
Dubbed the “bionic” pancreas, this closed-loop system frees patients from the tasks of measuring their glucose multiple times a day, calculating the appropriate dose, and giving multiple insulin injections.
The 2016 summer camp study29 followed 19 preteens wearing the bionic pancreas for 5 days. During this time, the patients had lower mean glucose levels and less hypoglycemia than during control periods. No episodes of severe hypoglycemia were recorded.
El-Khatib et al30 randomly assigned 43 patients to treatment with either the bihormonal bionic pancreas or usual care (a conventional insulin pump or a sensor-augmented insulin pump) for 11 days, followed by 11 days of the opposite treatment. All participants continued their normal activities. The bionic pancreas system was superior to the insulin pump in terms of the mean glucose concentration and mean time in the hypoglycemic range (P < .0001 for both results).
Bottom line
As the search continues for better solutions, advances in technology such as the bionic pancreas could provide a safer (ie, less hypoglycemic) and more successful alternative for insulin replacement in the near future.
With intensive insulin regimens and home blood glucose monitoring, patients with type 1 diabetes are controlling their blood glucose better than in the past. Nevertheless, glucose regulation is still imperfect and tedious, and striving for tight glycemic control poses the risk of hypoglycemia.
Prominent among the challenges are the sheer numbers involved. Some 1.25 million Americans have type 1 diabetes, and another 30 million have type 2, but only about 7,000 to 8,000 pancreases are available for transplant each year.1 While awaiting a breakthrough—perhaps involving stem cells, perhaps involving organs obtained from animals—an insulin pump may offer better diabetes control for many. Another possibility is a closed-loop system with a continuous glucose monitor that drives a dual-infusion pump, delivering insulin when glucose levels rise too high, and glucagon when they dip too low.
DIABETES WAS KNOWN IN ANCIENT TIMES
About 3,000 years ago, Egyptians described the syndrome of thirst, emaciation, and sweet urine that attracted ants. The term diabetes (Greek for siphon) was first recorded in 1425; mellitus (Latin for sweet with honey) was not added until 1675.
In 1857, Bernard hypothesized that diabetes was caused by overproduction of glucose in the liver. This idea was replaced in 1889, when Mering and Minkowski proposed the dysfunctional pancreas theory that eventually led to the discovery of the beta cell.2
In 1921, Banting and Best isolated insulin, and for the past 100 years subcutaneous insulin replacement has been the mainstay of treatment. But starting about 50 years ago, researchers have been looking for safe and long-lasting ways to replace beta cells and eliminate the need for exogenous insulin replacement.
TRANSPLANTING THE WHOLE PANCREAS
The first whole-pancreas transplant was performed in 1966 by Kelly et al,3 followed by 13 more by 1973.4 These first transplant grafts were short-lived, with only 1 graft surviving longer than 1 year. Since then, more than 12,000 pancreases have been transplanted worldwide, as refinements in surgical techniques and immunosuppressive therapies have improved patient and graft survival rates.4
Today, most pancreas transplants are in patients who have both type 1 diabetes and end-stage renal disease due to diabetic nephropathy, and most receive both a kidney and a pancreas at the same time. Far fewer patients receive a pancreas after previously receiving a kidney, or receive a pancreas alone.
The bile duct of the transplanted pancreas is usually routed into the patient’s small intestine, as nature intended, and less often into the bladder. Although bladder drainage is associated with urinary complications, it has the advantage of allowing measurement of pancreatic amylase levels in the urine to monitor for graft rejection. With simultaneous pancreas and kidney transplant, the serum creatinine concentration can also be monitored for rejection of the kidney graft.
Current immunosuppressive regimens vary but generally consist of anti-T-cell antibodies at the time of surgery, followed by lifelong treatment with the combination of a calcineurin inhibitor (cyclosporine or tacrolimus) and an antimetabolite (mycophenolate mofetil or azathioprine).
Outcomes are good. The rates of patient and graft survival are highest with simultaneous pancreas-kidney transplant, and somewhat lower with pancreas-after-kidney and pancreas-alone transplant.
Benefits of pancreas transplant
Most recipients can stop taking insulin immediately after the procedure, and their hemoglobin A1c levels normalize and stay low for the life of the graft. Lipid levels also decrease, although this has not been directly correlated with lower risk of vascular disease.4
Transplant also reduces or eliminates some complications of diabetes, including retinopathy, nephropathy, cardiomyopathy, and gastropathy.
For example, in patients undergoing simultaneous pancreas-kidney transplant, diabetic nephropathy does not recur in the new kidney. Fioretto et al5 reported that nephropathy lesions reversed during the 10 years after pancreas transplant.
Kennedy et al6,7 found that preexisting diabetic neuropathy improved slightly (although neurologic status did not completely return to normal) over a period of up to 42 months in a group of patients who received a pancreas transplant, whereas it tended to worsen in a control group. Both groups were assessed at baseline and at 12 and 24 months, with a subgroup followed through 42 months, and they underwent testing of motor, sensory, and autonomic function.6,7
Disadvantages of pancreas transplant
Disadvantages of whole-pancreas transplant include hypoglycemia (usually mild), adverse effects of immunosuppression, potential for surgical complications including an increased rate of death in the first 90 days after the procedure, and cost.
In an analysis comparing the 5-year estimated costs of dialysis, kidney transplant alone from cadavers or live donors, or simultaneous pancreas-kidney transplant for diabetic patients with end-stage renal disease, the least expensive option was kidney transplant from a live donor.8 The most expensive option was simultaneous pancreas-kidney transplant, but quality of life was better with this option. The analysis did not consider the potential cost of long-term treatments for complications related to diabetes that could be saved with a pancreas transplant.
Data conflict regarding the risk of death with different types of pancreas transplants. A retrospective cohort study of data from 124 US transplant centers reported in 2003 found higher mortality rates in pancreas-alone transplant recipients than in patients on a transplant waiting list receiving conventional therapy.9 In contrast, a 2004 study reported that after the first 90 days, when the risk of death was clearly higher, mortality rates were lower after simultaneous pancreas-kidney transplant and pancreas-after-kidney transplant.10 After pancreas-alone transplant, however, mortality rates were higher than with exogenous insulin therapy.
Although outcomes have improved, fewer patients with type 1 diabetes are undergoing pancreas transplant in recent years.
Interestingly, more simultaneous pancreas-kidney transplants are being successfully performed in patients with type 2 diabetes, who now account for 8% of all simultaneous pancreas-kidney transplant recipients.11 Outcomes of pancreas transplant appear to be similar regardless of diabetes type.
Bottom line
Pancreas transplant is a viable option for certain cases of complicated diabetes.
TRANSPLANTING ISLET CELLS
Despite its successes, pancreas transplant is major surgery and requires lifetime immunosuppression. Research is ongoing into a less-invasive procedure that, it is hoped, would require less immunosuppression: transplanting islets by themselves.
Islet autotransplant after pancreatectomy
For some patients with chronic pancreatitis, the only option to relieve chronic pain, narcotic dependence, and poor quality of life is to remove the pancreas. In the past, this desperate measure would instantly and inevitably cause diabetes, but not anymore.
Alpha cells and glucagon are a different story; a complication of islet transplant is hypoglycemia. In 2016, Lin et al12 reported spontaneous hypoglycemia in 6 of 12 patients who maintained insulin independence after autotransplant of islets. Although the transplanted islets had functional alpha cells that could in theory produce glucagon, as well as beta cells that produce insulin and C-peptide, apparently the alpha cells were not secreting glucagon in response to the hypoglycemia.
Location may matter. Gupta et al,13 in a 1997 study in dogs, found that more hypoglycemia occurs if islets are autotransplanted into the liver than if they are transplanted into the peritoneal cavity. A possible explanation may have to do with the glycemic environment of the liver.
Islet allotransplant
Islets can also be taken from cadaver donors and transplanted into patients with type 1 diabetes, who do not have enough working beta cells.
Success of allotransplant increased after the publication of observational data from the program in Edmonton in Canada, in which 7 consecutive patients with type 1 diabetes achieved initial insulin independence after islet allotransplant using steroid-free immunosuppression.14 Six recipients required islets from 2 donors, and 1 required islets from 4 donors, so they all received large volumes of at least 11,000 islet equivalents (IEQ) per kilogram of body weight.
In a subsequent report from the same team,15 16 (44%) of 36 patients remained insulin-free at 1 year, and C-peptide secretion was detectable in 70% at 2 years. But despite the elevated C-peptide levels, only 5 patients remained insulin-independent by 2 years. Lower hemoglobin A1c levels and decreases in hypoglycemic events from baseline also were noted.
The Clinical Islet Transplantation Consortium (CITC)16 and Collaborative Islet Transplant Registry (CITR)17 were established in 2004 to combine data and resources from centers around the world, including several that specialize in islet isolation and purification. Currently, more than 80 studies are being conducted.
The CITC and CITR now have data on more than 1,000 allogeneic islet transplant recipients (islet transplant alone, after kidney transplant, or simultaneous with it). The primary outcomes are hemoglobin A1c levels below 7% fasting C-peptide levels 0.3 ng/mL or higher, and fasting blood glucose of 60 to 140 mg/dL with no severe hypoglycemic events. The best results for islet-alone transplant have been in recipients over age 35 who received at least 325,000 IEQs with use of tumor necrosis factor antagonists for induction and calcineurin inhibitors or mammalian target of rapamycin (mTOR) inhibitors for maintenance.17
The best success for islet-after-kidney transplant was achieved with the same protocol but with insulin given to the donor during hospitalization before pancreas procurement. For participants with favorable factors, a hemoglobin A1c at or below 6.5% was achieved in about 80% at 1 year after last infusion, with more than 80% maintaining their fasting blood glucose level goals. About 70% of these patients were insulin-independent at 1 year. Hypoglycemia unawareness resolved in these patients even 5 years after infusion. Although there were no deaths or disabilities related to these transplants, bleeding occurred in 1 of 15 procedures. There was also a notable decline in estimated glomerular filtration rates with calcineurin inhibitor-based immunosuppression.17
Making islets go farther
One of the greatest challenges to islet transplant is the need for multiple donors to provide enough islet cells to overcome the loss of cells during transplant. Pancreases are already in short supply, and if each recipient needs more than 1, this makes the shortage worse. Some centers have achieved transplant with fewer donors,18,19 possibly by selecting pancreases from young donors who had a high body mass index and more islet cells, and harvesting and using them with a shorter cold ischemic time.
The number of viable, functioning islet cells drastically decreases after transplant, especially when transplanted into the portal system. This phenomenon is linked to an instant, blood-mediated inflammatory reaction involving antibody binding, complement and coagulation cascade activation, and platelet aggregation. The reaction, part of the innate immune system, damages the islet cells and leads to insulin dumping and early graft loss in studies in vitro and in vivo. Another factor affecting the survival of the graft cells is the low oxygen tension in the portal system.
For this reason, sites such as the pancreas, gastric submucosa, genitourinary tract, muscle, omentum, bone marrow, kidney capsule, peritoneum, anterior eye chamber, testis, and thymus are being explored.20
To create a more supportive environment for the transplanted cells, biotechnicians are trying to encapsulate islets in a semipermeable membrane that would protect them from the immune system while still allowing oxygen, nutrients, waste products, and, critically, insulin to diffuse in and out. Currently, no site or encapsulated product has been more successful than the current practice of implanting naked islets in the portal system.20
Bottom line
Without advances in transplant sites or increasing the yield of islet cells to allow single-donor transplants, islet cell allotransplant will not be feasible for most patients with type 1 diabetes.
Xenotransplant: Can pig cells make up the shortage?
Use of animal kidneys (xenotransplant) is a potential solution to the shortage of human organs for transplant.
In theory, pigs could be a source. Porcine insulin is similar to human insulin (differing by only 1 amino acid), and it should be possible to breed “knockout” pigs that lack the antigens responsible for acute humoral rejection.21
On the other hand, transplant of porcine islets poses several immunologic, physiologic, ethical, legal, and infectious concerns. For example, porcine tissue could carry pig viruses, such as porcine endogenous retroviruses.21 And even if the pigs are genetically modified, patients will still require immunosuppressive therapy.
A review of 17 studies of pig islet xenotransplant into nonhuman primates found that in 5 of the studies (4 using diabetic primates) the grafts survived at least 3 months.22 Of these, 1 study used encapsulation, and the rest used intensive and toxic immunosuppression.
More research is needed to make xenotransplant a clinical option.
Transplanting stem cells or beta cells grown from stem cells
Stem cells provide an exciting potential alternative to the limited donor pool. During the past decade, several studies have shown success using human pluripotent stem cells (embryonic stem cells and human-induced pluripotent stem cells), mesenchymal stem cells isolated from adult tissues, and directly programmed somatic cells. Researchers have created stable cultures of pluripotent stem cells from embryonic stem cells, which could possibly be produced on a large scale and banked.23
Human pluripotent stem cells derived from pancreatic progenitors have been shown to mature into more functional, islet-like structures in vivo. They transform into subtypes of islet cells including alpha, beta, and delta cells, ghrelin-producing cells, and pancreatic polypeptide hormone-producing cells. This process takes 2 to 6 weeks. In mice, these cells have been shown to maintain glucose homeostasis.24 Phase 1 and 2 trials in humans are now being conducted.
Pagliuca et al25 generated functional human pancreatic beta cells in vitro from embryonic stem cells. Rezania et al24 reversed diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. The techniques used in these studies contributed to the success of a study by Vegas et al,26 who achieved successful long-term glycemic control in mice using polymer-encapsulated human stem cell-derived beta cells.
Reversal of autoimmunity is an important step that needs to be overcome in stem cell transplant for type 1 diabetes. Nikolic et al27 have achieved mixed allogeneic chimerism across major histocompatibility complex barriers with nonmyeloablative conditioning in advanced-diabetic nonobese diabetic mice. However, conditioning alone (ie, without bone marrow transplant) does not permit acceptance of allogeneic islets and does not reverse autoimmunity or allow islet regeneration.28 Adding allogeneic bone marrow transplant to conditioned nonobese diabetic mice leads to tolerance to the donor and reverses autoimmunity.
THE ‘BIONIC’ PANCREAS
While we wait for advances in islet cell transplant, improved insulin pumps hold promise.
One such experimental device, the iLet (Beta Bionics, Boston, MA), designed by Damiano et al, consists of 2 infusion pumps (1 for insulin, 1 for glucagon) linked to a continuous glucose monitor via a smartphone app.
The monitor measures the glucose level every 5 minutes and transmits the information wirelessly to the phone app, which calculates the amount of insulin and glucagon required to stabilize the blood glucose: more insulin if too high, more glucagon if too low. The phone transmits this information to the pumps.
Dubbed the “bionic” pancreas, this closed-loop system frees patients from the tasks of measuring their glucose multiple times a day, calculating the appropriate dose, and giving multiple insulin injections.
The 2016 summer camp study29 followed 19 preteens wearing the bionic pancreas for 5 days. During this time, the patients had lower mean glucose levels and less hypoglycemia than during control periods. No episodes of severe hypoglycemia were recorded.
El-Khatib et al30 randomly assigned 43 patients to treatment with either the bihormonal bionic pancreas or usual care (a conventional insulin pump or a sensor-augmented insulin pump) for 11 days, followed by 11 days of the opposite treatment. All participants continued their normal activities. The bionic pancreas system was superior to the insulin pump in terms of the mean glucose concentration and mean time in the hypoglycemic range (P < .0001 for both results).
Bottom line
As the search continues for better solutions, advances in technology such as the bionic pancreas could provide a safer (ie, less hypoglycemic) and more successful alternative for insulin replacement in the near future.
- American Diabetes Association. Statistics about diabetes: overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 6, 2018.
- Ahmed AM. History of diabetes mellitus. Saudi Med J 2002; 23(4):373–378. pmid:11953758
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837. pmid: 5338113
- Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001; 233(4):463–501. pmid:11303130
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339(2):69–75. doi:10.1056/NEJM199807093390202
- Kennedy WR, Navarro X, Goetz FC, Sutherland DE, Najarian JS. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med 1990; 322(15):1031–1037. doi:10.1056/NEJM199004123221503
- Kennedy WR, Navarro X, Sutherland DER. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology 1995; 45(4):773–780. pmid:7723969
- Douzdjian V, Ferrara D, Silvestri G. Treatment strategies for insulin-dependent diabetics with ESRD: a cost-effectiveness decision analysis model. Am J Kidney Dis 1998; 31(5):794–802. pmid:9590189
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290(21):2817–2823. doi:10.1001/jama.290.21.2817
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4(12):2018–2026. doi:10.1111/j.1600-6143.2004.00667.x
- Redfield RR, Scalea JR, Odorico JS. Simultaneous pancreas and kidney transplantation: current trends and future directions. Curr Opin Organ Transplant 2015; 20(1):94-102. doi:10.1097/MOT.0000000000000146
- Lin YK, Faiman C, Johnston PC, et al. Spontaneous hypoglycemia after islet autotransplantation for chronic pancreatitis. J Clin Endocrinol Metab 2016; 101(10):3669–3675. doi:10.1210/jc.2016-2111
- Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes 1997; 46(1):28–33. pmid:8971077
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343(4):230–238. doi:10.1056/NEJM200007273430401
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355(13):1318–1330. doi:10.1056/NEJMoa061267
- Clinical Islet Transplantation (CIT) Consortium. www.citisletstudy.org. Accessed November 6, 2018.
- Collaborative Islet Transplantation Registry (CITR). CITR 10th Annual Report. https://citregistry.org/system/files/10th_AR.pdf. Accessed November 6, 2018.
- Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004; 4(3):390–401. pmid:14961992
- Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant 2010; 10(8):1870–1880. doi:10.1111/j.1600-6143.2010.03073.x
- Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep 2011; 11(5):364–374. doi:10.1007/s11892-011-0216-9
- Cooper DK, Gollackner B, Knosalla C, Teranishi K. Xenotransplantation—how far have we come? Transpl Immunol 2002; 9(2–4):251–256. pmid:12180839
- Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R. Pig-to-nonhuman primates pancreatic islet xenotransplantation: an overview. Curr Diab Rep 2011; 11(5):402–412. doi:10.1007/s11892-011-0213-z
- Bartlett ST, Markmann JF, Johnson P, et al. Report from IPITA-TTS opinion leaders meeting on the future of beta-cell replacement. Transplantation 2016; 100(suppl 2):S1–S44. doi:10.1097/TP.0000000000001055
- Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32(11):1121–1133. doi:10.1038/nbt.3033
- Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159(2):428–439. doi:10.1016/j.cell.2014.09.040
- Vegas AJ, Veiseh O, Gurtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016; 22(3):306–311. doi:10.1038/nm.4030
- Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune tolerance and reversal of autoimmunity. Diabetes 2004; 53(2):376–383. pmid:14747288
- Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol 2012; 12(6):403–416. doi:10.1038/nri3226
- Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016; 4(3):233–243. doi:10.1016/S2213-8587(15)00489-1
- El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicenter randomized crossover trial. Lancet 2017; 389(10067):369–380. doi:10.1016/S0140-6736(16)32567-3
- American Diabetes Association. Statistics about diabetes: overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 6, 2018.
- Ahmed AM. History of diabetes mellitus. Saudi Med J 2002; 23(4):373–378. pmid:11953758
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837. pmid: 5338113
- Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001; 233(4):463–501. pmid:11303130
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339(2):69–75. doi:10.1056/NEJM199807093390202
- Kennedy WR, Navarro X, Goetz FC, Sutherland DE, Najarian JS. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med 1990; 322(15):1031–1037. doi:10.1056/NEJM199004123221503
- Kennedy WR, Navarro X, Sutherland DER. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology 1995; 45(4):773–780. pmid:7723969
- Douzdjian V, Ferrara D, Silvestri G. Treatment strategies for insulin-dependent diabetics with ESRD: a cost-effectiveness decision analysis model. Am J Kidney Dis 1998; 31(5):794–802. pmid:9590189
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290(21):2817–2823. doi:10.1001/jama.290.21.2817
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4(12):2018–2026. doi:10.1111/j.1600-6143.2004.00667.x
- Redfield RR, Scalea JR, Odorico JS. Simultaneous pancreas and kidney transplantation: current trends and future directions. Curr Opin Organ Transplant 2015; 20(1):94-102. doi:10.1097/MOT.0000000000000146
- Lin YK, Faiman C, Johnston PC, et al. Spontaneous hypoglycemia after islet autotransplantation for chronic pancreatitis. J Clin Endocrinol Metab 2016; 101(10):3669–3675. doi:10.1210/jc.2016-2111
- Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes 1997; 46(1):28–33. pmid:8971077
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343(4):230–238. doi:10.1056/NEJM200007273430401
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355(13):1318–1330. doi:10.1056/NEJMoa061267
- Clinical Islet Transplantation (CIT) Consortium. www.citisletstudy.org. Accessed November 6, 2018.
- Collaborative Islet Transplantation Registry (CITR). CITR 10th Annual Report. https://citregistry.org/system/files/10th_AR.pdf. Accessed November 6, 2018.
- Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004; 4(3):390–401. pmid:14961992
- Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant 2010; 10(8):1870–1880. doi:10.1111/j.1600-6143.2010.03073.x
- Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep 2011; 11(5):364–374. doi:10.1007/s11892-011-0216-9
- Cooper DK, Gollackner B, Knosalla C, Teranishi K. Xenotransplantation—how far have we come? Transpl Immunol 2002; 9(2–4):251–256. pmid:12180839
- Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R. Pig-to-nonhuman primates pancreatic islet xenotransplantation: an overview. Curr Diab Rep 2011; 11(5):402–412. doi:10.1007/s11892-011-0213-z
- Bartlett ST, Markmann JF, Johnson P, et al. Report from IPITA-TTS opinion leaders meeting on the future of beta-cell replacement. Transplantation 2016; 100(suppl 2):S1–S44. doi:10.1097/TP.0000000000001055
- Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32(11):1121–1133. doi:10.1038/nbt.3033
- Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159(2):428–439. doi:10.1016/j.cell.2014.09.040
- Vegas AJ, Veiseh O, Gurtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016; 22(3):306–311. doi:10.1038/nm.4030
- Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune tolerance and reversal of autoimmunity. Diabetes 2004; 53(2):376–383. pmid:14747288
- Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol 2012; 12(6):403–416. doi:10.1038/nri3226
- Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016; 4(3):233–243. doi:10.1016/S2213-8587(15)00489-1
- El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicenter randomized crossover trial. Lancet 2017; 389(10067):369–380. doi:10.1016/S0140-6736(16)32567-3
KEY POINTS
- Most pancreas transplant recipients become insulin-independent immediately.
- A key drawback to islet transplant is the need for multiple donors to provide enough islet cells to achieve insulin independence.
- As with other organs for transplant, the need for donor pancreases far outnumbers the supply. Stem cells or beta cells grown from stem cells may avoid this problem. Another potential solution is to use organs from animals, possibly pigs, but much more work is needed to make these procedures viable.
- While we await a breakthrough in beta-cell therapy, a bionic pancreas may be the answer for a number of patients.
Cannabis for peripheral neuropathy: The good, the bad, and the unknown
Marijuana, which is still illegal under federal law but legal in 30 states for medical purposes as of this writing, has shown promising results for treating peripheral neuropathy. Studies suggest that cannabis may be an option for patients whose pain responds poorly to standard treatments; however, its use may be restricted by cognitive and psychiatric adverse effects, particularly at high doses.1
In this article, we discuss the basic pharmacology of cannabis and how it may affect neuropathic pain. We review clinical trials on its use for peripheral neuropathy and provide guidance for its use.
PERIPHERAL NEUROPATHY IS COMMON AND COMPLEX
An estimated 20 million people in the United States suffer from neuropathic pain. The prevalence is higher in certain populations, with 26% of people over age 65 and 30% of patients with diabetes mellitus affected.2–4
Peripheral neuropathy is a complex, chronic state that occurs when nerve fibers are damaged, dysfunctional, or injured, sending incorrect signals to pain centers in the central nervous system.5 It is characterized by weakness, pain, and paresthesias that typically begin in the hands or feet and progress proximally.4 Symptoms depend on the number and types of nerves affected.
In many cases, peripheral neuropathy is idiopathic, but common causes include diabetes, alcoholism, human immunodeficiency virus (HIV) infection, and autoimmune disease. Others include toxicity from chemotherapy and heavy metals.
Peripheral neuropathy significantly worsens quality of life and function. Many patients experience emotional, cognitive, and functional problems, resulting in high rates of medical and psychiatric comorbidities and occupational impairment.4,6,7 Yet despite its clinical and epidemiologic significance, it is often undertreated.8
STANDARD TREATMENTS INADEQUATE
Peripheral neuropathy occurs in patients with a wide range of comorbidities and is especially difficult to treat. Mainstays of therapy include anticonvulsants, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors.9 A more invasive option is spinal cord stimulation.
These treatments can have considerable adverse effects, and response rates remain suboptimal, with pain relief insufficient to improve quality of life for many patients.9,10 Better treatments are needed to improve clinical outcomes and patient experience.11
CANNABIS: A MIX OF COMPOUNDS
Cannabis sativa has been used as an analgesic for centuries. The plant contains more than 400 chemical compounds and is often used for its euphoric properties. Long-term use may lead to addiction and cognitive impairment.12,13
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the main components and the 2 best-studied cannabinoids with analgesic effects.
THC is the primary psychoactive component of cannabis. Its effects include relaxation, altered perception, heightened sensations, increased libido, and perceptual distortions of time and space. Temporary effects may include decreased short-term memory, dry mouth, impaired motor function, conjunctival injection, paranoia, and anxiety.
CBD is nonpsychoactive and has anti-inflammatory and antioxidant properties. It has been shown to reduce pain and inflammation without the effects of THC.14
Other compounds in the cannabis plant include phytocannabinoids, flavonoids, and tapenoids, which may produce individual, interactive, or synergistic effects.15 Different strains of cannabis have varying amounts of the individual components, making comparisons among clinical studies difficult.
THE ENDOCANNABINOID SYSTEM
The endogenous mammalian cannabinoid system plays a regulatory role in the development, homeostasis, and neuroplasticity of the central nervous system. It is also involved in modulating pain transmission in the nociceptive pathway.
Two of the most abundant cannabinoid endogenous ligands are anandamide and 2-arachidonylglycerol.9 These endocannabinoids are produced on demand in the central nervous system to reduce pain by acting as a circuit breaker.16–18 They target the G protein-coupled cannabinoid receptors CB1 and CB2, located throughout the central and peripheral nervous system and in organs and tissues.12
CB1 receptors are found primarily in the central nervous system, specifically in areas involved in movement, such as the basal ganglia and cerebellum, as well as in areas involved in memory, such as the hippocampus.12 They are also abundant in brain regions implicated in conducting and modulating pain signals, including the periaqueductal gray and the dorsal horn of the spinal cord.16–20
CB2 receptors are mostly found in peripheral tissues and organs, mainly those involved in the immune system, including splenic, tonsillar, and hematopoietic cells.12 They help regulate inflammation, allodynia, and hyperalgesia.17
Modifying response to injury
Following a nerve injury, neurons along the nociceptive pathway may become more reactive and responsive in a process known as sensitization.21 The process involves a cascade of cellular events that result in sprouting of pain-sensitive nerve endings.21,22
Cannabinoids are thought to reduce pain by modifying these cellular events. They also inhibit nociceptive conduction in the dorsal horn of the spinal cord and in the ascending spinothalamic tract.20 CB1 receptors found in nociceptive terminals along the peripheral nervous system impede pain conduction, while activation of CB2 receptors in immune cells decreases the release of nociceptive agents.
STUDIES OF CANNABIS FOR NEUROPATHIC PAIN
A number of studies have evaluated cannabis for treating neuropathic pain. Overall, available data support the efficacy of smoked or inhaled cannabis in its flower form when used as monotherapy or adjunctive therapy for relief of neuropathic pain of various etiologies. Many studies also report secondary benefits, including better sleep and functional improvement.23,24
However, adverse effects are common, especially at high doses, and include difficulty concentrating, lightheadedness, fatigue, and tachycardia. More serious reported adverse effects include anxiety, paranoia, and psychosis.
Wilsey et al, 2008: Neuropathic pain reduced
Wilsey et al25 conducted a double-blind, placebo-controlled crossover study that assessed the effects of smoking cannabis in 38 patients with central or peripheral neuropathic pain. Participants were assigned to smoke either high- or low-dose cannabis (7% or 3.5% delta-9-THC) or placebo cigarettes. Cigarettes were smoked during treatment sessions using the following regimen: 2 puffs at 60 minutes from baseline, 3 puffs at 120 minutes, and 4 puffs at 180 minutes. Patients were assessed after each set of puffs and for 2 hours afterwards. The primary outcome was spontaneous relief of pain as measured by a visual analog scale.
Pain intensity was comparable and significantly reduced in both treatment groups compared with placebo. At the high dose, some participants experienced neurocognitive impairment in attention, learning, memory, and psychomotor speed; only learning and memory declined at the low dose.
Ellis et al, 2009: Pain reduction in HIV neuropathy
Ellis et al23 conducted a double-blind, placebo-controlled crossover trial in patients with HIV neuropathy that was unresponsive to at least 2 analgesics with different modes of action. During each treatment week, participants were randomly assigned to smoke either active cannabis or placebo, while continuing their standard therapy. Titration started at 4% THC and was adjusted based on tolerability and efficacy. Twenty-eight of the 34 enrolled patients completed both cannabis and placebo treatments. The principal outcome was change in pain intensity from baseline at the end of each week, using the Descriptor Differential Scale of Pain Intensity.
Of the 28 patients, 46% achieved an average pain reduction of 3.3 points (30%). One patient experienced cannabis-induced psychosis, and another developed an intractable cough, which resolved with smoking cessation.
Ware et al, 2010: Reduced posttraumatic or postsurgical neuropathic pain
Ware et al24 performed a randomized crossover trial in 21 patients with posttraumatic or postsurgical neuropathic pain. Participants inhaled 4 different formulations of cannabis (containing 0%, 2.5%, 6.0%, and 9.4% THC) during 4 14-day periods. They inhaled a 25-mg dose through a pipe 3 times a day for the first 5 days of each cycle, followed by a 9-day washout period. Daily average pain intensity was measured using a numeric rating scale. The investigators also assessed mood, sleep, quality of life, and adverse effects.
Patients in the 9.4% THC group reported significantly less pain and better sleep, with average pain scores decreasing from 6.1 to 5.4 on an 11-point scale. Although the benefit was modest, the authors noted that the pain had been refractory to standard treatments.
The number of reported adverse events increased with greater potency and were most commonly throat irritation, burning sensation, headache, dizziness, and fatigue. This study suggests that THC potency affects tolerability, with higher doses eliciting clinically important adverse effects, some of which may reduce the ability to perform activities of daily living, such as driving.
Wilsey et al, 2013: Use in resistant neuropathic pain
Wilsey et al26 conducted another double-blind, placebo-controlled crossover study assessing the effect of vaporized cannabis on central and peripheral neuropathic pain resistant to first-line pharmacotherapies. Dose-effect relationships were explored using medium-dose (3.5%), low-dose (1.3%), and placebo cannabis. The primary outcome measure was a 30% reduction in pain intensity based on a visual analog scale.
In the placebo group, 26% of patients achieved this vs 57% of the low-dose cannabis group and 61% of those receiving the medium dose. No significant difference was found between the 2 active doses in reducing neuropathic pain, and both were more effective than placebo. The number needed to treat to achieve a 30% reduction in pain was about 3 for both cannabis groups compared with placebo. Psychoactive effects were minimal, of short duration, and reversible.
Wallace et al, 2015: Use in diabetic peripheral neuropathy
Wallace et al27 conducted a randomized, double-blind, placebo-controlled crossover study evaluating cannabis for diabetic peripheral neuropathy in 16 patients. Each had experienced at least 6 months of neuropathic pain in their feet. The participants inhaled a single dose of 1%, 4%, or 7% THC cannabis or placebo. Spontaneous pain was reported with a visual analog scale and also tested with a foam brush and von Frey filament at intervals until 4 hours after treatment.
Pain scores were lower with treatment compared with placebo, with high-dose cannabis having the greatest analgesic effect. Pain reduction lasted for the full duration of the test. Cannabis recipients had declines in attention and working memory, with the high-dose group experiencing the greatest impact 15 minutes after treatment. High-dose recipients also had poorer scores on testing of quick task-switching, with the greatest effect at 2 hours.27
Research and market cannabis are not equal
Results of US studies must be qualified. Most have used cannabis provided by the National Institute of Drug Abuse (NIDA),23–26 which differs in potency from commercially available preparations. This limits the clinical usefulness of the analysis of benefits and risks.
Vergara et al28 found that NIDA varieties contained much lower THC levels and as much as 23 times the cannabinol content as cannabis in state-legalized markets.
Studies based on NIDA varieties likely underestimate the risks of consumer-purchased cannabis, as THC is believed to be most responsible for the risk of psychosis and impaired driving and cognition.24,28
CBD MAY PROTECT AGAINST ADVERSE EFFECTS
Studies of CBD alone are limited to preclinical data.29 Evidence suggests that CBD alone or combined with THC can suppress chronic neuropathic pain, and that CBD may have a protective effect after nerve injury.30
Nabiximols, an oromucosal spray preparation with equal amounts of THC and CBD, has been approved in Canada as well as in European countries including the United Kingdom. Although its use has not been associated with many of the adverse effects of inhaled cannabis,30–32 evidence of efficacy from clinical trials has been mixed.
Lynch et al,31 in a 2014 randomized, double-blind, placebo-controlled crossover pilot study31 evaluated nabiximols in 16 patients with neuropathic pain related to chemotherapy. No statistically significant difference was found between treatment and placebo. However, the trial was underpowered.
Serpell et al,32 in a 2014 European randomized, placebo-controlled parallel-group study, evaluated 246 patients with peripheral neuropathy with allodynia, with 128 receiving active treatment (THC-CBD oromucosal spray) and 118 receiving placebo. Over the 15-week study, participants continued their current analgesic treatments.
Pain was reduced in the treatment group, but the difference from placebo was not statistically significant. However, the treatment group reported significantly better sleep quality and Patient Global Impression of Change measures (reflecting a patient’s belief of treatment efficacy).
META-ANALYSES CONFIRM EFFECT
Three meta-analyses of available studies of the effects of cannabis on neuropathic pain have been completed.
Andreae et al, 2015: 5 trials, 178 patients
Andreae et al1 evaluated 5 randomized controlled trials in 178 patients in North America. All had had neuropathy for at least 3 months, with a pain level of at least about 3 on a scale of 10. Two studies had patients with HIV-related neuropathy; the other 3 involved patients with neuropathy related to trauma, diabetes, complex regional pain syndrome, or spinal cord injury. All trials used whole cannabis plant provided by NIDA, and the main outcomes were patient-reported pain scales. No study evaluated pain beyond 2 weeks after trial termination.
They found that 1 of every 5 to 6 patients treated with cannabis had at least a 30% pain reduction.
Nugent et al, 2017: 13 trials, 246 patients
Nugent et al33 reviewed 13 trials in 246 patients that evaluated the effects of different cannabis-based preparations on either central or peripheral neuropathic pain from various conditions. Actively treated patients were more likely to report a 30% improvement in neuropathic pain. Again, studies tended to be small and brief.
Cochrane review, 2018: 16 trials, 1,750 patients
A Cochrane review34 analyzed 16 trials (in 1,750 patients) lasting 2 to 26 weeks. Treatments included an oromucosal spray with a plant-derived combination of THC and CBD, nabilone, inhaled herbal cannabis, and plant-derived THC.
With cannabis-based treatments, significantly more people achieved 50% or greater pain relief than with placebo (21% vs 17%, number needed to treat 20); 30% pain reduction was achieved in 39% of treated patients vs 33% of patients taking placebo (number needed to treat 11).
On the other hand, significantly more participants withdrew from studies because of adverse events with cannabis-based treatments than placebo (10% vs 5%), with psychiatric disorders occurring in 17% of patients receiving active treatment vs 5% of those receiving placebo (number needed to harm 10).
The primary studies suffered from methodologic limitations including small size, short duration, and inconsistency of formulations and study designs. Further evaluation of long-term efficacy, tolerability, and addiction potential is critical to determine the risk-benefit ratio.
RISKS OF CANNABIS USE
Like any drug therapy, cannabis has effects that may limit its use. Cannabis can affect a person’s psyche, physiology, and lifestyle.
Impaired attention, task speed
Neurocognitive changes associated with cannabis use—especially dizziness, fatigue, and slowed task-switching—could affect driving and other complex tasks. Evidence indicates that such activities should be avoided in the hours after treatment.26,27,32,33
Concern over brain development
Most worrisome is the effect of long-term cannabis use on brain development in young adults. Regular use of cannabis at an early age is associated with lower IQ, decline in school performance, and lower rates of high school graduation.35
Avoid in psychiatric patients
It is unlikely that cannabis can be safely used in patients with psychiatric illnesses. Anxiety, depression, and psychotic disorders can be exacerbated by the regular use of cannabis, and the risk of developing these conditions is increased while using cannabis.36,37
High concentrations of THC (the highest concentration used in the above studies was 9.5%) can cause anxiety, paranoia, and psychosis.
Respiratory effects
Long-term cannabis smoking may cause wheezing, cough, dyspnea, and exacerbations of chronic bronchitis. There is some evidence that symptoms improve after stopping smoking.33,38
SHOULD WE RECOMMEND CANNABIS?
Where cannabis can be legally used, doctors should be familiar with the literature and its limitations so that they can counsel patients on the best use and potential risks and benefits of cannabis treatment.
A recent conceptualization of pain suggests that a pain score reflects a composite of sensory factors (eg, tissue damage), cognitive factors (eg, beliefs about pain), and affective factors (eg, anxiety, depression).39 Physicians should keep this in mind when evaluating patients to better assess the risks and benefits of cannabis. While pharmacotherapy may address sensory factors, cognitive behavioral therapy may help alter beliefs about the pain as well as anxiety and depressive symptoms that might influence subjective reports.
Ideally, patients being considered for cannabis treatment would have a type of neuropathic pain proven to respond to cannabis in randomized, controlled studies, as well as evidence of failed first-line treatments.
Relative contraindications include depression, anxiety, substance use, psychotic disorders, and respiratory conditions, and these should also be considered.
Although current research shows an analgesic benefit of cannabis on neuropathic pain comparable to that of gabapentin,40 further investigation is needed to better evaluate long-term safety, efficacy, and interactions with standard therapies. Until we have a more complete picture, we should use the current literature, along with a thorough knowledge of each patient, to determine if the benefits of cannabis therapy outweigh the risks.
Acknowledgments: We thank Camillo Ferrari, BS, and Christina McMahon, BA, for their helpful comments.
- Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain 2015; 16(12):1221–1232. doi:10.1016/j.jpain.2015.07.009
- National Institute of Neurological Disorders and Stroke. Peripheral Neuropathy Fact Sheet. www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Accessed November 14, 2018.
- Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older adults. J Am Board Fam Med 2004; 17(5):308–318. doi:10.3122/jabfm.17.5.309
- Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of developing peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig 2014; 5(6):714–721. doi:10.1111/jdi.12223
- Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016; 157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
- Maldonado R, Banos JE, Cabanero D. The endocannabinoid system and neuropathic pain. Pain 2016; 157(suppl 1):S23–S32. doi:10.1097/j.pain.0000000000000428
- Zeng L, Alongkronrusmee D, van Rijn RM. An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res 2017; 10:219–228. doi:10.2147/JPR.S125987
- Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA 2015; 314(20):2172–2181. doi:10.1001/jama.2015.13611
- Adams AS, Callaghan B, Grant RW. Overcoming barriers to diabetic polyneuropathy management in primary care. Healthc (Amst) 2017; 5(4):171–173. doi:10.1016/j.hjdsi.2016.10.003
- Gwak YS, Kim HY, Lee BH, Yang CH. Combined approaches for the relief of spinal cord injury-induced neuropathic pain. Complement Ther Med 2016; 25:27–33. doi:10.1016/j.ctim.2015.12.021
- Majithia N, Loprinzi CL, Smith TJ. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology 2016; 30(11):1020–1029. pmid:27854104
- Grotenhermen F. Cannabinoids and the endocannabinoid system. Cannabinoids 2006; 1(1):10–14.
- Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA 2015; 313(24):2474–2483. doi:10.1001/jama.2015.6199
- Campos AC, Fogaça MV, Scarante FF, et al. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 2017; 8:269. doi:10.3389/fphar.2017.00269
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163(7):1344–1364. doi:10.1111/j.1476-5381.2011.01238.x
- Freitas HR, Isaac AR, Malcher-Lopes R, Diaz BL, Trevenzoli IH, De Melo Reis RA. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr Neurosci 2017; Jul 7: 1–20. doi:10.1080/1028415X.2017.1347373
- Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018; 43(1):155–172. doi:10.1038/npp.2017.130
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991; 11(2):563–583. pmid:1992016
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience1998; 83(2):393–411. pmid:9460749
- Russo EB, Hohmann AG. Role of cannabinoids in pain management. In: Deer TR, Leong MS, ed. Comprehensve Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches. New York, NY: Springer; 2013:181–193.
- Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 2012; 12(4):304–314. pmid:23033930
- Yamanaka H, Noguchi K. Pathophysiology of neuropathic pain: molecular mechanisms underlying central sensitization in the dorsal horn in neuropathic pain. Brain Nerve 2012; 64(11):1255–1265. Japanese. pmid:23131736
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010; 182(14):E694–E701. doi:10.1503/cmaj.091414
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008; 9(6):506–521. doi:10.1016/j.jpain.2007.12.010
- Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013; 14(2):136–148. doi:10.1016/j.jpain.2012.10.009
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Vergara D, Bidwell LC, Gaudino R, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Scientific Reports. 2017; 7(1):1-8. doi:10.1038/srep46528
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterized by allodynia: a randomized, double-blind, placebo-controlled clinical trial. Pain 2007; 133(1–3):210–220. doi:10.1016/j.pain.2007.08.028
- Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017; 158(12):2442–2451. doi:10.1097/j.pain.0000000000001052
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage 2014; 47(1):166–173. doi:10.1016/j.jpainsymman.2013.02.018
- Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014; 18(7):999–1012. doi:10.1002/j.1532-2149.2013.00445.x
- Nugent SM, Morasco BJ, O’Neil ME, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med 2017; 167(5):319–331. doi:10.7326/M17-0155
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. doi:10.1002/14651858.CD012182.pub2
- Castellanos-Ryan N, Pingault JB, Parent S, Vitaro F, Tremblay RE, Seguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol 2017; 29(4):1253–1266. doi:10.1017/S0954579416001280
- Karila L, Roux P, Benyamina A, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des 2014; 20(25):4112–4118. pmid:24001294
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry 2001; 178:116–122. pmid:11157424
- National Academies of Science, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academy Press; 2017. doi:10.17226/24625
- Modesto-Lowe V, Griard L, Chaplin M. Cancer pain in the opioid-addicted patient: can we treat it right? J Opioid Manag 2012; 8(3):167–175. doi:10.5055/jom.2012.0113
- Grant I. Medicinal cannabis and painful sensory neuropathy. Virtual Mentor 2013; 15(5):466–469. doi:10.1001/virtualmentor.2013.15.5.oped1-1305
Marijuana, which is still illegal under federal law but legal in 30 states for medical purposes as of this writing, has shown promising results for treating peripheral neuropathy. Studies suggest that cannabis may be an option for patients whose pain responds poorly to standard treatments; however, its use may be restricted by cognitive and psychiatric adverse effects, particularly at high doses.1
In this article, we discuss the basic pharmacology of cannabis and how it may affect neuropathic pain. We review clinical trials on its use for peripheral neuropathy and provide guidance for its use.
PERIPHERAL NEUROPATHY IS COMMON AND COMPLEX
An estimated 20 million people in the United States suffer from neuropathic pain. The prevalence is higher in certain populations, with 26% of people over age 65 and 30% of patients with diabetes mellitus affected.2–4
Peripheral neuropathy is a complex, chronic state that occurs when nerve fibers are damaged, dysfunctional, or injured, sending incorrect signals to pain centers in the central nervous system.5 It is characterized by weakness, pain, and paresthesias that typically begin in the hands or feet and progress proximally.4 Symptoms depend on the number and types of nerves affected.
In many cases, peripheral neuropathy is idiopathic, but common causes include diabetes, alcoholism, human immunodeficiency virus (HIV) infection, and autoimmune disease. Others include toxicity from chemotherapy and heavy metals.
Peripheral neuropathy significantly worsens quality of life and function. Many patients experience emotional, cognitive, and functional problems, resulting in high rates of medical and psychiatric comorbidities and occupational impairment.4,6,7 Yet despite its clinical and epidemiologic significance, it is often undertreated.8
STANDARD TREATMENTS INADEQUATE
Peripheral neuropathy occurs in patients with a wide range of comorbidities and is especially difficult to treat. Mainstays of therapy include anticonvulsants, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors.9 A more invasive option is spinal cord stimulation.
These treatments can have considerable adverse effects, and response rates remain suboptimal, with pain relief insufficient to improve quality of life for many patients.9,10 Better treatments are needed to improve clinical outcomes and patient experience.11
CANNABIS: A MIX OF COMPOUNDS
Cannabis sativa has been used as an analgesic for centuries. The plant contains more than 400 chemical compounds and is often used for its euphoric properties. Long-term use may lead to addiction and cognitive impairment.12,13
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the main components and the 2 best-studied cannabinoids with analgesic effects.
THC is the primary psychoactive component of cannabis. Its effects include relaxation, altered perception, heightened sensations, increased libido, and perceptual distortions of time and space. Temporary effects may include decreased short-term memory, dry mouth, impaired motor function, conjunctival injection, paranoia, and anxiety.
CBD is nonpsychoactive and has anti-inflammatory and antioxidant properties. It has been shown to reduce pain and inflammation without the effects of THC.14
Other compounds in the cannabis plant include phytocannabinoids, flavonoids, and tapenoids, which may produce individual, interactive, or synergistic effects.15 Different strains of cannabis have varying amounts of the individual components, making comparisons among clinical studies difficult.
THE ENDOCANNABINOID SYSTEM
The endogenous mammalian cannabinoid system plays a regulatory role in the development, homeostasis, and neuroplasticity of the central nervous system. It is also involved in modulating pain transmission in the nociceptive pathway.
Two of the most abundant cannabinoid endogenous ligands are anandamide and 2-arachidonylglycerol.9 These endocannabinoids are produced on demand in the central nervous system to reduce pain by acting as a circuit breaker.16–18 They target the G protein-coupled cannabinoid receptors CB1 and CB2, located throughout the central and peripheral nervous system and in organs and tissues.12
CB1 receptors are found primarily in the central nervous system, specifically in areas involved in movement, such as the basal ganglia and cerebellum, as well as in areas involved in memory, such as the hippocampus.12 They are also abundant in brain regions implicated in conducting and modulating pain signals, including the periaqueductal gray and the dorsal horn of the spinal cord.16–20
CB2 receptors are mostly found in peripheral tissues and organs, mainly those involved in the immune system, including splenic, tonsillar, and hematopoietic cells.12 They help regulate inflammation, allodynia, and hyperalgesia.17
Modifying response to injury
Following a nerve injury, neurons along the nociceptive pathway may become more reactive and responsive in a process known as sensitization.21 The process involves a cascade of cellular events that result in sprouting of pain-sensitive nerve endings.21,22
Cannabinoids are thought to reduce pain by modifying these cellular events. They also inhibit nociceptive conduction in the dorsal horn of the spinal cord and in the ascending spinothalamic tract.20 CB1 receptors found in nociceptive terminals along the peripheral nervous system impede pain conduction, while activation of CB2 receptors in immune cells decreases the release of nociceptive agents.
STUDIES OF CANNABIS FOR NEUROPATHIC PAIN
A number of studies have evaluated cannabis for treating neuropathic pain. Overall, available data support the efficacy of smoked or inhaled cannabis in its flower form when used as monotherapy or adjunctive therapy for relief of neuropathic pain of various etiologies. Many studies also report secondary benefits, including better sleep and functional improvement.23,24
However, adverse effects are common, especially at high doses, and include difficulty concentrating, lightheadedness, fatigue, and tachycardia. More serious reported adverse effects include anxiety, paranoia, and psychosis.
Wilsey et al, 2008: Neuropathic pain reduced
Wilsey et al25 conducted a double-blind, placebo-controlled crossover study that assessed the effects of smoking cannabis in 38 patients with central or peripheral neuropathic pain. Participants were assigned to smoke either high- or low-dose cannabis (7% or 3.5% delta-9-THC) or placebo cigarettes. Cigarettes were smoked during treatment sessions using the following regimen: 2 puffs at 60 minutes from baseline, 3 puffs at 120 minutes, and 4 puffs at 180 minutes. Patients were assessed after each set of puffs and for 2 hours afterwards. The primary outcome was spontaneous relief of pain as measured by a visual analog scale.
Pain intensity was comparable and significantly reduced in both treatment groups compared with placebo. At the high dose, some participants experienced neurocognitive impairment in attention, learning, memory, and psychomotor speed; only learning and memory declined at the low dose.
Ellis et al, 2009: Pain reduction in HIV neuropathy
Ellis et al23 conducted a double-blind, placebo-controlled crossover trial in patients with HIV neuropathy that was unresponsive to at least 2 analgesics with different modes of action. During each treatment week, participants were randomly assigned to smoke either active cannabis or placebo, while continuing their standard therapy. Titration started at 4% THC and was adjusted based on tolerability and efficacy. Twenty-eight of the 34 enrolled patients completed both cannabis and placebo treatments. The principal outcome was change in pain intensity from baseline at the end of each week, using the Descriptor Differential Scale of Pain Intensity.
Of the 28 patients, 46% achieved an average pain reduction of 3.3 points (30%). One patient experienced cannabis-induced psychosis, and another developed an intractable cough, which resolved with smoking cessation.
Ware et al, 2010: Reduced posttraumatic or postsurgical neuropathic pain
Ware et al24 performed a randomized crossover trial in 21 patients with posttraumatic or postsurgical neuropathic pain. Participants inhaled 4 different formulations of cannabis (containing 0%, 2.5%, 6.0%, and 9.4% THC) during 4 14-day periods. They inhaled a 25-mg dose through a pipe 3 times a day for the first 5 days of each cycle, followed by a 9-day washout period. Daily average pain intensity was measured using a numeric rating scale. The investigators also assessed mood, sleep, quality of life, and adverse effects.
Patients in the 9.4% THC group reported significantly less pain and better sleep, with average pain scores decreasing from 6.1 to 5.4 on an 11-point scale. Although the benefit was modest, the authors noted that the pain had been refractory to standard treatments.
The number of reported adverse events increased with greater potency and were most commonly throat irritation, burning sensation, headache, dizziness, and fatigue. This study suggests that THC potency affects tolerability, with higher doses eliciting clinically important adverse effects, some of which may reduce the ability to perform activities of daily living, such as driving.
Wilsey et al, 2013: Use in resistant neuropathic pain
Wilsey et al26 conducted another double-blind, placebo-controlled crossover study assessing the effect of vaporized cannabis on central and peripheral neuropathic pain resistant to first-line pharmacotherapies. Dose-effect relationships were explored using medium-dose (3.5%), low-dose (1.3%), and placebo cannabis. The primary outcome measure was a 30% reduction in pain intensity based on a visual analog scale.
In the placebo group, 26% of patients achieved this vs 57% of the low-dose cannabis group and 61% of those receiving the medium dose. No significant difference was found between the 2 active doses in reducing neuropathic pain, and both were more effective than placebo. The number needed to treat to achieve a 30% reduction in pain was about 3 for both cannabis groups compared with placebo. Psychoactive effects were minimal, of short duration, and reversible.
Wallace et al, 2015: Use in diabetic peripheral neuropathy
Wallace et al27 conducted a randomized, double-blind, placebo-controlled crossover study evaluating cannabis for diabetic peripheral neuropathy in 16 patients. Each had experienced at least 6 months of neuropathic pain in their feet. The participants inhaled a single dose of 1%, 4%, or 7% THC cannabis or placebo. Spontaneous pain was reported with a visual analog scale and also tested with a foam brush and von Frey filament at intervals until 4 hours after treatment.
Pain scores were lower with treatment compared with placebo, with high-dose cannabis having the greatest analgesic effect. Pain reduction lasted for the full duration of the test. Cannabis recipients had declines in attention and working memory, with the high-dose group experiencing the greatest impact 15 minutes after treatment. High-dose recipients also had poorer scores on testing of quick task-switching, with the greatest effect at 2 hours.27
Research and market cannabis are not equal
Results of US studies must be qualified. Most have used cannabis provided by the National Institute of Drug Abuse (NIDA),23–26 which differs in potency from commercially available preparations. This limits the clinical usefulness of the analysis of benefits and risks.
Vergara et al28 found that NIDA varieties contained much lower THC levels and as much as 23 times the cannabinol content as cannabis in state-legalized markets.
Studies based on NIDA varieties likely underestimate the risks of consumer-purchased cannabis, as THC is believed to be most responsible for the risk of psychosis and impaired driving and cognition.24,28
CBD MAY PROTECT AGAINST ADVERSE EFFECTS
Studies of CBD alone are limited to preclinical data.29 Evidence suggests that CBD alone or combined with THC can suppress chronic neuropathic pain, and that CBD may have a protective effect after nerve injury.30
Nabiximols, an oromucosal spray preparation with equal amounts of THC and CBD, has been approved in Canada as well as in European countries including the United Kingdom. Although its use has not been associated with many of the adverse effects of inhaled cannabis,30–32 evidence of efficacy from clinical trials has been mixed.
Lynch et al,31 in a 2014 randomized, double-blind, placebo-controlled crossover pilot study31 evaluated nabiximols in 16 patients with neuropathic pain related to chemotherapy. No statistically significant difference was found between treatment and placebo. However, the trial was underpowered.
Serpell et al,32 in a 2014 European randomized, placebo-controlled parallel-group study, evaluated 246 patients with peripheral neuropathy with allodynia, with 128 receiving active treatment (THC-CBD oromucosal spray) and 118 receiving placebo. Over the 15-week study, participants continued their current analgesic treatments.
Pain was reduced in the treatment group, but the difference from placebo was not statistically significant. However, the treatment group reported significantly better sleep quality and Patient Global Impression of Change measures (reflecting a patient’s belief of treatment efficacy).
META-ANALYSES CONFIRM EFFECT
Three meta-analyses of available studies of the effects of cannabis on neuropathic pain have been completed.
Andreae et al, 2015: 5 trials, 178 patients
Andreae et al1 evaluated 5 randomized controlled trials in 178 patients in North America. All had had neuropathy for at least 3 months, with a pain level of at least about 3 on a scale of 10. Two studies had patients with HIV-related neuropathy; the other 3 involved patients with neuropathy related to trauma, diabetes, complex regional pain syndrome, or spinal cord injury. All trials used whole cannabis plant provided by NIDA, and the main outcomes were patient-reported pain scales. No study evaluated pain beyond 2 weeks after trial termination.
They found that 1 of every 5 to 6 patients treated with cannabis had at least a 30% pain reduction.
Nugent et al, 2017: 13 trials, 246 patients
Nugent et al33 reviewed 13 trials in 246 patients that evaluated the effects of different cannabis-based preparations on either central or peripheral neuropathic pain from various conditions. Actively treated patients were more likely to report a 30% improvement in neuropathic pain. Again, studies tended to be small and brief.
Cochrane review, 2018: 16 trials, 1,750 patients
A Cochrane review34 analyzed 16 trials (in 1,750 patients) lasting 2 to 26 weeks. Treatments included an oromucosal spray with a plant-derived combination of THC and CBD, nabilone, inhaled herbal cannabis, and plant-derived THC.
With cannabis-based treatments, significantly more people achieved 50% or greater pain relief than with placebo (21% vs 17%, number needed to treat 20); 30% pain reduction was achieved in 39% of treated patients vs 33% of patients taking placebo (number needed to treat 11).
On the other hand, significantly more participants withdrew from studies because of adverse events with cannabis-based treatments than placebo (10% vs 5%), with psychiatric disorders occurring in 17% of patients receiving active treatment vs 5% of those receiving placebo (number needed to harm 10).
The primary studies suffered from methodologic limitations including small size, short duration, and inconsistency of formulations and study designs. Further evaluation of long-term efficacy, tolerability, and addiction potential is critical to determine the risk-benefit ratio.
RISKS OF CANNABIS USE
Like any drug therapy, cannabis has effects that may limit its use. Cannabis can affect a person’s psyche, physiology, and lifestyle.
Impaired attention, task speed
Neurocognitive changes associated with cannabis use—especially dizziness, fatigue, and slowed task-switching—could affect driving and other complex tasks. Evidence indicates that such activities should be avoided in the hours after treatment.26,27,32,33
Concern over brain development
Most worrisome is the effect of long-term cannabis use on brain development in young adults. Regular use of cannabis at an early age is associated with lower IQ, decline in school performance, and lower rates of high school graduation.35
Avoid in psychiatric patients
It is unlikely that cannabis can be safely used in patients with psychiatric illnesses. Anxiety, depression, and psychotic disorders can be exacerbated by the regular use of cannabis, and the risk of developing these conditions is increased while using cannabis.36,37
High concentrations of THC (the highest concentration used in the above studies was 9.5%) can cause anxiety, paranoia, and psychosis.
Respiratory effects
Long-term cannabis smoking may cause wheezing, cough, dyspnea, and exacerbations of chronic bronchitis. There is some evidence that symptoms improve after stopping smoking.33,38
SHOULD WE RECOMMEND CANNABIS?
Where cannabis can be legally used, doctors should be familiar with the literature and its limitations so that they can counsel patients on the best use and potential risks and benefits of cannabis treatment.
A recent conceptualization of pain suggests that a pain score reflects a composite of sensory factors (eg, tissue damage), cognitive factors (eg, beliefs about pain), and affective factors (eg, anxiety, depression).39 Physicians should keep this in mind when evaluating patients to better assess the risks and benefits of cannabis. While pharmacotherapy may address sensory factors, cognitive behavioral therapy may help alter beliefs about the pain as well as anxiety and depressive symptoms that might influence subjective reports.
Ideally, patients being considered for cannabis treatment would have a type of neuropathic pain proven to respond to cannabis in randomized, controlled studies, as well as evidence of failed first-line treatments.
Relative contraindications include depression, anxiety, substance use, psychotic disorders, and respiratory conditions, and these should also be considered.
Although current research shows an analgesic benefit of cannabis on neuropathic pain comparable to that of gabapentin,40 further investigation is needed to better evaluate long-term safety, efficacy, and interactions with standard therapies. Until we have a more complete picture, we should use the current literature, along with a thorough knowledge of each patient, to determine if the benefits of cannabis therapy outweigh the risks.
Acknowledgments: We thank Camillo Ferrari, BS, and Christina McMahon, BA, for their helpful comments.
Marijuana, which is still illegal under federal law but legal in 30 states for medical purposes as of this writing, has shown promising results for treating peripheral neuropathy. Studies suggest that cannabis may be an option for patients whose pain responds poorly to standard treatments; however, its use may be restricted by cognitive and psychiatric adverse effects, particularly at high doses.1
In this article, we discuss the basic pharmacology of cannabis and how it may affect neuropathic pain. We review clinical trials on its use for peripheral neuropathy and provide guidance for its use.
PERIPHERAL NEUROPATHY IS COMMON AND COMPLEX
An estimated 20 million people in the United States suffer from neuropathic pain. The prevalence is higher in certain populations, with 26% of people over age 65 and 30% of patients with diabetes mellitus affected.2–4
Peripheral neuropathy is a complex, chronic state that occurs when nerve fibers are damaged, dysfunctional, or injured, sending incorrect signals to pain centers in the central nervous system.5 It is characterized by weakness, pain, and paresthesias that typically begin in the hands or feet and progress proximally.4 Symptoms depend on the number and types of nerves affected.
In many cases, peripheral neuropathy is idiopathic, but common causes include diabetes, alcoholism, human immunodeficiency virus (HIV) infection, and autoimmune disease. Others include toxicity from chemotherapy and heavy metals.
Peripheral neuropathy significantly worsens quality of life and function. Many patients experience emotional, cognitive, and functional problems, resulting in high rates of medical and psychiatric comorbidities and occupational impairment.4,6,7 Yet despite its clinical and epidemiologic significance, it is often undertreated.8
STANDARD TREATMENTS INADEQUATE
Peripheral neuropathy occurs in patients with a wide range of comorbidities and is especially difficult to treat. Mainstays of therapy include anticonvulsants, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors.9 A more invasive option is spinal cord stimulation.
These treatments can have considerable adverse effects, and response rates remain suboptimal, with pain relief insufficient to improve quality of life for many patients.9,10 Better treatments are needed to improve clinical outcomes and patient experience.11
CANNABIS: A MIX OF COMPOUNDS
Cannabis sativa has been used as an analgesic for centuries. The plant contains more than 400 chemical compounds and is often used for its euphoric properties. Long-term use may lead to addiction and cognitive impairment.12,13
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the main components and the 2 best-studied cannabinoids with analgesic effects.
THC is the primary psychoactive component of cannabis. Its effects include relaxation, altered perception, heightened sensations, increased libido, and perceptual distortions of time and space. Temporary effects may include decreased short-term memory, dry mouth, impaired motor function, conjunctival injection, paranoia, and anxiety.
CBD is nonpsychoactive and has anti-inflammatory and antioxidant properties. It has been shown to reduce pain and inflammation without the effects of THC.14
Other compounds in the cannabis plant include phytocannabinoids, flavonoids, and tapenoids, which may produce individual, interactive, or synergistic effects.15 Different strains of cannabis have varying amounts of the individual components, making comparisons among clinical studies difficult.
THE ENDOCANNABINOID SYSTEM
The endogenous mammalian cannabinoid system plays a regulatory role in the development, homeostasis, and neuroplasticity of the central nervous system. It is also involved in modulating pain transmission in the nociceptive pathway.
Two of the most abundant cannabinoid endogenous ligands are anandamide and 2-arachidonylglycerol.9 These endocannabinoids are produced on demand in the central nervous system to reduce pain by acting as a circuit breaker.16–18 They target the G protein-coupled cannabinoid receptors CB1 and CB2, located throughout the central and peripheral nervous system and in organs and tissues.12
CB1 receptors are found primarily in the central nervous system, specifically in areas involved in movement, such as the basal ganglia and cerebellum, as well as in areas involved in memory, such as the hippocampus.12 They are also abundant in brain regions implicated in conducting and modulating pain signals, including the periaqueductal gray and the dorsal horn of the spinal cord.16–20
CB2 receptors are mostly found in peripheral tissues and organs, mainly those involved in the immune system, including splenic, tonsillar, and hematopoietic cells.12 They help regulate inflammation, allodynia, and hyperalgesia.17
Modifying response to injury
Following a nerve injury, neurons along the nociceptive pathway may become more reactive and responsive in a process known as sensitization.21 The process involves a cascade of cellular events that result in sprouting of pain-sensitive nerve endings.21,22
Cannabinoids are thought to reduce pain by modifying these cellular events. They also inhibit nociceptive conduction in the dorsal horn of the spinal cord and in the ascending spinothalamic tract.20 CB1 receptors found in nociceptive terminals along the peripheral nervous system impede pain conduction, while activation of CB2 receptors in immune cells decreases the release of nociceptive agents.
STUDIES OF CANNABIS FOR NEUROPATHIC PAIN
A number of studies have evaluated cannabis for treating neuropathic pain. Overall, available data support the efficacy of smoked or inhaled cannabis in its flower form when used as monotherapy or adjunctive therapy for relief of neuropathic pain of various etiologies. Many studies also report secondary benefits, including better sleep and functional improvement.23,24
However, adverse effects are common, especially at high doses, and include difficulty concentrating, lightheadedness, fatigue, and tachycardia. More serious reported adverse effects include anxiety, paranoia, and psychosis.
Wilsey et al, 2008: Neuropathic pain reduced
Wilsey et al25 conducted a double-blind, placebo-controlled crossover study that assessed the effects of smoking cannabis in 38 patients with central or peripheral neuropathic pain. Participants were assigned to smoke either high- or low-dose cannabis (7% or 3.5% delta-9-THC) or placebo cigarettes. Cigarettes were smoked during treatment sessions using the following regimen: 2 puffs at 60 minutes from baseline, 3 puffs at 120 minutes, and 4 puffs at 180 minutes. Patients were assessed after each set of puffs and for 2 hours afterwards. The primary outcome was spontaneous relief of pain as measured by a visual analog scale.
Pain intensity was comparable and significantly reduced in both treatment groups compared with placebo. At the high dose, some participants experienced neurocognitive impairment in attention, learning, memory, and psychomotor speed; only learning and memory declined at the low dose.
Ellis et al, 2009: Pain reduction in HIV neuropathy
Ellis et al23 conducted a double-blind, placebo-controlled crossover trial in patients with HIV neuropathy that was unresponsive to at least 2 analgesics with different modes of action. During each treatment week, participants were randomly assigned to smoke either active cannabis or placebo, while continuing their standard therapy. Titration started at 4% THC and was adjusted based on tolerability and efficacy. Twenty-eight of the 34 enrolled patients completed both cannabis and placebo treatments. The principal outcome was change in pain intensity from baseline at the end of each week, using the Descriptor Differential Scale of Pain Intensity.
Of the 28 patients, 46% achieved an average pain reduction of 3.3 points (30%). One patient experienced cannabis-induced psychosis, and another developed an intractable cough, which resolved with smoking cessation.
Ware et al, 2010: Reduced posttraumatic or postsurgical neuropathic pain
Ware et al24 performed a randomized crossover trial in 21 patients with posttraumatic or postsurgical neuropathic pain. Participants inhaled 4 different formulations of cannabis (containing 0%, 2.5%, 6.0%, and 9.4% THC) during 4 14-day periods. They inhaled a 25-mg dose through a pipe 3 times a day for the first 5 days of each cycle, followed by a 9-day washout period. Daily average pain intensity was measured using a numeric rating scale. The investigators also assessed mood, sleep, quality of life, and adverse effects.
Patients in the 9.4% THC group reported significantly less pain and better sleep, with average pain scores decreasing from 6.1 to 5.4 on an 11-point scale. Although the benefit was modest, the authors noted that the pain had been refractory to standard treatments.
The number of reported adverse events increased with greater potency and were most commonly throat irritation, burning sensation, headache, dizziness, and fatigue. This study suggests that THC potency affects tolerability, with higher doses eliciting clinically important adverse effects, some of which may reduce the ability to perform activities of daily living, such as driving.
Wilsey et al, 2013: Use in resistant neuropathic pain
Wilsey et al26 conducted another double-blind, placebo-controlled crossover study assessing the effect of vaporized cannabis on central and peripheral neuropathic pain resistant to first-line pharmacotherapies. Dose-effect relationships were explored using medium-dose (3.5%), low-dose (1.3%), and placebo cannabis. The primary outcome measure was a 30% reduction in pain intensity based on a visual analog scale.
In the placebo group, 26% of patients achieved this vs 57% of the low-dose cannabis group and 61% of those receiving the medium dose. No significant difference was found between the 2 active doses in reducing neuropathic pain, and both were more effective than placebo. The number needed to treat to achieve a 30% reduction in pain was about 3 for both cannabis groups compared with placebo. Psychoactive effects were minimal, of short duration, and reversible.
Wallace et al, 2015: Use in diabetic peripheral neuropathy
Wallace et al27 conducted a randomized, double-blind, placebo-controlled crossover study evaluating cannabis for diabetic peripheral neuropathy in 16 patients. Each had experienced at least 6 months of neuropathic pain in their feet. The participants inhaled a single dose of 1%, 4%, or 7% THC cannabis or placebo. Spontaneous pain was reported with a visual analog scale and also tested with a foam brush and von Frey filament at intervals until 4 hours after treatment.
Pain scores were lower with treatment compared with placebo, with high-dose cannabis having the greatest analgesic effect. Pain reduction lasted for the full duration of the test. Cannabis recipients had declines in attention and working memory, with the high-dose group experiencing the greatest impact 15 minutes after treatment. High-dose recipients also had poorer scores on testing of quick task-switching, with the greatest effect at 2 hours.27
Research and market cannabis are not equal
Results of US studies must be qualified. Most have used cannabis provided by the National Institute of Drug Abuse (NIDA),23–26 which differs in potency from commercially available preparations. This limits the clinical usefulness of the analysis of benefits and risks.
Vergara et al28 found that NIDA varieties contained much lower THC levels and as much as 23 times the cannabinol content as cannabis in state-legalized markets.
Studies based on NIDA varieties likely underestimate the risks of consumer-purchased cannabis, as THC is believed to be most responsible for the risk of psychosis and impaired driving and cognition.24,28
CBD MAY PROTECT AGAINST ADVERSE EFFECTS
Studies of CBD alone are limited to preclinical data.29 Evidence suggests that CBD alone or combined with THC can suppress chronic neuropathic pain, and that CBD may have a protective effect after nerve injury.30
Nabiximols, an oromucosal spray preparation with equal amounts of THC and CBD, has been approved in Canada as well as in European countries including the United Kingdom. Although its use has not been associated with many of the adverse effects of inhaled cannabis,30–32 evidence of efficacy from clinical trials has been mixed.
Lynch et al,31 in a 2014 randomized, double-blind, placebo-controlled crossover pilot study31 evaluated nabiximols in 16 patients with neuropathic pain related to chemotherapy. No statistically significant difference was found between treatment and placebo. However, the trial was underpowered.
Serpell et al,32 in a 2014 European randomized, placebo-controlled parallel-group study, evaluated 246 patients with peripheral neuropathy with allodynia, with 128 receiving active treatment (THC-CBD oromucosal spray) and 118 receiving placebo. Over the 15-week study, participants continued their current analgesic treatments.
Pain was reduced in the treatment group, but the difference from placebo was not statistically significant. However, the treatment group reported significantly better sleep quality and Patient Global Impression of Change measures (reflecting a patient’s belief of treatment efficacy).
META-ANALYSES CONFIRM EFFECT
Three meta-analyses of available studies of the effects of cannabis on neuropathic pain have been completed.
Andreae et al, 2015: 5 trials, 178 patients
Andreae et al1 evaluated 5 randomized controlled trials in 178 patients in North America. All had had neuropathy for at least 3 months, with a pain level of at least about 3 on a scale of 10. Two studies had patients with HIV-related neuropathy; the other 3 involved patients with neuropathy related to trauma, diabetes, complex regional pain syndrome, or spinal cord injury. All trials used whole cannabis plant provided by NIDA, and the main outcomes were patient-reported pain scales. No study evaluated pain beyond 2 weeks after trial termination.
They found that 1 of every 5 to 6 patients treated with cannabis had at least a 30% pain reduction.
Nugent et al, 2017: 13 trials, 246 patients
Nugent et al33 reviewed 13 trials in 246 patients that evaluated the effects of different cannabis-based preparations on either central or peripheral neuropathic pain from various conditions. Actively treated patients were more likely to report a 30% improvement in neuropathic pain. Again, studies tended to be small and brief.
Cochrane review, 2018: 16 trials, 1,750 patients
A Cochrane review34 analyzed 16 trials (in 1,750 patients) lasting 2 to 26 weeks. Treatments included an oromucosal spray with a plant-derived combination of THC and CBD, nabilone, inhaled herbal cannabis, and plant-derived THC.
With cannabis-based treatments, significantly more people achieved 50% or greater pain relief than with placebo (21% vs 17%, number needed to treat 20); 30% pain reduction was achieved in 39% of treated patients vs 33% of patients taking placebo (number needed to treat 11).
On the other hand, significantly more participants withdrew from studies because of adverse events with cannabis-based treatments than placebo (10% vs 5%), with psychiatric disorders occurring in 17% of patients receiving active treatment vs 5% of those receiving placebo (number needed to harm 10).
The primary studies suffered from methodologic limitations including small size, short duration, and inconsistency of formulations and study designs. Further evaluation of long-term efficacy, tolerability, and addiction potential is critical to determine the risk-benefit ratio.
RISKS OF CANNABIS USE
Like any drug therapy, cannabis has effects that may limit its use. Cannabis can affect a person’s psyche, physiology, and lifestyle.
Impaired attention, task speed
Neurocognitive changes associated with cannabis use—especially dizziness, fatigue, and slowed task-switching—could affect driving and other complex tasks. Evidence indicates that such activities should be avoided in the hours after treatment.26,27,32,33
Concern over brain development
Most worrisome is the effect of long-term cannabis use on brain development in young adults. Regular use of cannabis at an early age is associated with lower IQ, decline in school performance, and lower rates of high school graduation.35
Avoid in psychiatric patients
It is unlikely that cannabis can be safely used in patients with psychiatric illnesses. Anxiety, depression, and psychotic disorders can be exacerbated by the regular use of cannabis, and the risk of developing these conditions is increased while using cannabis.36,37
High concentrations of THC (the highest concentration used in the above studies was 9.5%) can cause anxiety, paranoia, and psychosis.
Respiratory effects
Long-term cannabis smoking may cause wheezing, cough, dyspnea, and exacerbations of chronic bronchitis. There is some evidence that symptoms improve after stopping smoking.33,38
SHOULD WE RECOMMEND CANNABIS?
Where cannabis can be legally used, doctors should be familiar with the literature and its limitations so that they can counsel patients on the best use and potential risks and benefits of cannabis treatment.
A recent conceptualization of pain suggests that a pain score reflects a composite of sensory factors (eg, tissue damage), cognitive factors (eg, beliefs about pain), and affective factors (eg, anxiety, depression).39 Physicians should keep this in mind when evaluating patients to better assess the risks and benefits of cannabis. While pharmacotherapy may address sensory factors, cognitive behavioral therapy may help alter beliefs about the pain as well as anxiety and depressive symptoms that might influence subjective reports.
Ideally, patients being considered for cannabis treatment would have a type of neuropathic pain proven to respond to cannabis in randomized, controlled studies, as well as evidence of failed first-line treatments.
Relative contraindications include depression, anxiety, substance use, psychotic disorders, and respiratory conditions, and these should also be considered.
Although current research shows an analgesic benefit of cannabis on neuropathic pain comparable to that of gabapentin,40 further investigation is needed to better evaluate long-term safety, efficacy, and interactions with standard therapies. Until we have a more complete picture, we should use the current literature, along with a thorough knowledge of each patient, to determine if the benefits of cannabis therapy outweigh the risks.
Acknowledgments: We thank Camillo Ferrari, BS, and Christina McMahon, BA, for their helpful comments.
- Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain 2015; 16(12):1221–1232. doi:10.1016/j.jpain.2015.07.009
- National Institute of Neurological Disorders and Stroke. Peripheral Neuropathy Fact Sheet. www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Accessed November 14, 2018.
- Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older adults. J Am Board Fam Med 2004; 17(5):308–318. doi:10.3122/jabfm.17.5.309
- Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of developing peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig 2014; 5(6):714–721. doi:10.1111/jdi.12223
- Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016; 157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
- Maldonado R, Banos JE, Cabanero D. The endocannabinoid system and neuropathic pain. Pain 2016; 157(suppl 1):S23–S32. doi:10.1097/j.pain.0000000000000428
- Zeng L, Alongkronrusmee D, van Rijn RM. An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res 2017; 10:219–228. doi:10.2147/JPR.S125987
- Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA 2015; 314(20):2172–2181. doi:10.1001/jama.2015.13611
- Adams AS, Callaghan B, Grant RW. Overcoming barriers to diabetic polyneuropathy management in primary care. Healthc (Amst) 2017; 5(4):171–173. doi:10.1016/j.hjdsi.2016.10.003
- Gwak YS, Kim HY, Lee BH, Yang CH. Combined approaches for the relief of spinal cord injury-induced neuropathic pain. Complement Ther Med 2016; 25:27–33. doi:10.1016/j.ctim.2015.12.021
- Majithia N, Loprinzi CL, Smith TJ. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology 2016; 30(11):1020–1029. pmid:27854104
- Grotenhermen F. Cannabinoids and the endocannabinoid system. Cannabinoids 2006; 1(1):10–14.
- Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA 2015; 313(24):2474–2483. doi:10.1001/jama.2015.6199
- Campos AC, Fogaça MV, Scarante FF, et al. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 2017; 8:269. doi:10.3389/fphar.2017.00269
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163(7):1344–1364. doi:10.1111/j.1476-5381.2011.01238.x
- Freitas HR, Isaac AR, Malcher-Lopes R, Diaz BL, Trevenzoli IH, De Melo Reis RA. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr Neurosci 2017; Jul 7: 1–20. doi:10.1080/1028415X.2017.1347373
- Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018; 43(1):155–172. doi:10.1038/npp.2017.130
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991; 11(2):563–583. pmid:1992016
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience1998; 83(2):393–411. pmid:9460749
- Russo EB, Hohmann AG. Role of cannabinoids in pain management. In: Deer TR, Leong MS, ed. Comprehensve Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches. New York, NY: Springer; 2013:181–193.
- Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 2012; 12(4):304–314. pmid:23033930
- Yamanaka H, Noguchi K. Pathophysiology of neuropathic pain: molecular mechanisms underlying central sensitization in the dorsal horn in neuropathic pain. Brain Nerve 2012; 64(11):1255–1265. Japanese. pmid:23131736
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010; 182(14):E694–E701. doi:10.1503/cmaj.091414
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008; 9(6):506–521. doi:10.1016/j.jpain.2007.12.010
- Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013; 14(2):136–148. doi:10.1016/j.jpain.2012.10.009
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Vergara D, Bidwell LC, Gaudino R, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Scientific Reports. 2017; 7(1):1-8. doi:10.1038/srep46528
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterized by allodynia: a randomized, double-blind, placebo-controlled clinical trial. Pain 2007; 133(1–3):210–220. doi:10.1016/j.pain.2007.08.028
- Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017; 158(12):2442–2451. doi:10.1097/j.pain.0000000000001052
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage 2014; 47(1):166–173. doi:10.1016/j.jpainsymman.2013.02.018
- Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014; 18(7):999–1012. doi:10.1002/j.1532-2149.2013.00445.x
- Nugent SM, Morasco BJ, O’Neil ME, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med 2017; 167(5):319–331. doi:10.7326/M17-0155
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. doi:10.1002/14651858.CD012182.pub2
- Castellanos-Ryan N, Pingault JB, Parent S, Vitaro F, Tremblay RE, Seguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol 2017; 29(4):1253–1266. doi:10.1017/S0954579416001280
- Karila L, Roux P, Benyamina A, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des 2014; 20(25):4112–4118. pmid:24001294
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry 2001; 178:116–122. pmid:11157424
- National Academies of Science, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academy Press; 2017. doi:10.17226/24625
- Modesto-Lowe V, Griard L, Chaplin M. Cancer pain in the opioid-addicted patient: can we treat it right? J Opioid Manag 2012; 8(3):167–175. doi:10.5055/jom.2012.0113
- Grant I. Medicinal cannabis and painful sensory neuropathy. Virtual Mentor 2013; 15(5):466–469. doi:10.1001/virtualmentor.2013.15.5.oped1-1305
- Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain 2015; 16(12):1221–1232. doi:10.1016/j.jpain.2015.07.009
- National Institute of Neurological Disorders and Stroke. Peripheral Neuropathy Fact Sheet. www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Accessed November 14, 2018.
- Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older adults. J Am Board Fam Med 2004; 17(5):308–318. doi:10.3122/jabfm.17.5.309
- Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of developing peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig 2014; 5(6):714–721. doi:10.1111/jdi.12223
- Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016; 157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
- Maldonado R, Banos JE, Cabanero D. The endocannabinoid system and neuropathic pain. Pain 2016; 157(suppl 1):S23–S32. doi:10.1097/j.pain.0000000000000428
- Zeng L, Alongkronrusmee D, van Rijn RM. An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res 2017; 10:219–228. doi:10.2147/JPR.S125987
- Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA 2015; 314(20):2172–2181. doi:10.1001/jama.2015.13611
- Adams AS, Callaghan B, Grant RW. Overcoming barriers to diabetic polyneuropathy management in primary care. Healthc (Amst) 2017; 5(4):171–173. doi:10.1016/j.hjdsi.2016.10.003
- Gwak YS, Kim HY, Lee BH, Yang CH. Combined approaches for the relief of spinal cord injury-induced neuropathic pain. Complement Ther Med 2016; 25:27–33. doi:10.1016/j.ctim.2015.12.021
- Majithia N, Loprinzi CL, Smith TJ. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology 2016; 30(11):1020–1029. pmid:27854104
- Grotenhermen F. Cannabinoids and the endocannabinoid system. Cannabinoids 2006; 1(1):10–14.
- Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA 2015; 313(24):2474–2483. doi:10.1001/jama.2015.6199
- Campos AC, Fogaça MV, Scarante FF, et al. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 2017; 8:269. doi:10.3389/fphar.2017.00269
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163(7):1344–1364. doi:10.1111/j.1476-5381.2011.01238.x
- Freitas HR, Isaac AR, Malcher-Lopes R, Diaz BL, Trevenzoli IH, De Melo Reis RA. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr Neurosci 2017; Jul 7: 1–20. doi:10.1080/1028415X.2017.1347373
- Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018; 43(1):155–172. doi:10.1038/npp.2017.130
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991; 11(2):563–583. pmid:1992016
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience1998; 83(2):393–411. pmid:9460749
- Russo EB, Hohmann AG. Role of cannabinoids in pain management. In: Deer TR, Leong MS, ed. Comprehensve Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches. New York, NY: Springer; 2013:181–193.
- Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 2012; 12(4):304–314. pmid:23033930
- Yamanaka H, Noguchi K. Pathophysiology of neuropathic pain: molecular mechanisms underlying central sensitization in the dorsal horn in neuropathic pain. Brain Nerve 2012; 64(11):1255–1265. Japanese. pmid:23131736
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010; 182(14):E694–E701. doi:10.1503/cmaj.091414
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008; 9(6):506–521. doi:10.1016/j.jpain.2007.12.010
- Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013; 14(2):136–148. doi:10.1016/j.jpain.2012.10.009
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Vergara D, Bidwell LC, Gaudino R, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Scientific Reports. 2017; 7(1):1-8. doi:10.1038/srep46528
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterized by allodynia: a randomized, double-blind, placebo-controlled clinical trial. Pain 2007; 133(1–3):210–220. doi:10.1016/j.pain.2007.08.028
- Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017; 158(12):2442–2451. doi:10.1097/j.pain.0000000000001052
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage 2014; 47(1):166–173. doi:10.1016/j.jpainsymman.2013.02.018
- Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014; 18(7):999–1012. doi:10.1002/j.1532-2149.2013.00445.x
- Nugent SM, Morasco BJ, O’Neil ME, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med 2017; 167(5):319–331. doi:10.7326/M17-0155
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. doi:10.1002/14651858.CD012182.pub2
- Castellanos-Ryan N, Pingault JB, Parent S, Vitaro F, Tremblay RE, Seguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol 2017; 29(4):1253–1266. doi:10.1017/S0954579416001280
- Karila L, Roux P, Benyamina A, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des 2014; 20(25):4112–4118. pmid:24001294
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry 2001; 178:116–122. pmid:11157424
- National Academies of Science, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academy Press; 2017. doi:10.17226/24625
- Modesto-Lowe V, Griard L, Chaplin M. Cancer pain in the opioid-addicted patient: can we treat it right? J Opioid Manag 2012; 8(3):167–175. doi:10.5055/jom.2012.0113
- Grant I. Medicinal cannabis and painful sensory neuropathy. Virtual Mentor 2013; 15(5):466–469. doi:10.1001/virtualmentor.2013.15.5.oped1-1305
KEY POINTS
- Small clinical studies have found that cannabis provides benefits for peripheral neuropathy, including pain reduction, better sleep, and improved function, even in patients with symptoms refractory to standard therapies.
- Adverse effects such as throat irritation, headache, and dizziness are common, and serious neuropsychiatric effects can occur at high doses.
- Safety may not be adequately assessed in US trials because cannabis supplied by the National Institute of Drug Abuse is less potent than commercially available products.
Geriatrics update 2018: Challenges in mental health, mobility, and postdischarge care
Unfortunately, recent research has not unveiled a breakthrough for preventing or treating cognitive impairment or Alzheimer disease. But several studies from the last 2 years are helping to drive the field of geriatrics forward, providing evidence of what does and does not help a variety of issues specific to the elderly.
Based on a search of the 2017 and 2018 literature, this article presents new evidence on preventing and treating cognitive impairment, managing dementia-associated behavioral disturbances and delirium, preventing falls, and improving inpatient mobility and posthospital care transitions.
COGNITIVE IMPAIRMENT, DEMENTIA: STILL NO SILVER BULLET
With the exception of oral anticoagulation treatment for atrial fibrillation, there is little evidence that pharmacologic or nonpharmacologic interventions slow the onset or progression of Alzheimer disease.
Nonpharmacologic interventions
Home occupational therapy. A 2-year home-based occupational therapy intervention1 showed no evidence of slowing functional decline in patients with Alzheimer disease. The randomized controlled trial involving 180 participants consisted of monthly sessions of an intensive, well-established collaborative-care management model that included fall prevention and other safety strategies, personalized training in activities of daily living, exercise, and education. Outcome measures for activities of daily living did not differ significantly between the treatment and control groups.1
Physical activity. Whether physical activity interventions slow cognitive decline and prevent dementia in cognitively intact adults was examined in a systematic review of 32 trials.2 Most of the trials followed patients for 6 months; a few stretched for 1 or 2 years.
Evidence was insufficient to prove cognitive benefit for short-term, single-component or multicomponent physical activity interventions. However, a multidomain physical activity intervention that also included dietary modifications and cognitive training did show a delay in cognitive decline, but only “low-strength” evidence.2
Nutritional supplements. The antioxidants vitamin E and selenium were studied for their possible cognitive benefit in the double-blind randomized Prevention of Alzheimer Disease by Vitamin E and Selenium trial3 in 3,786 asymptomatic men ages 60 and older. Neither supplement was found to prevent dementia over a 7-year follow-up period.
A review of 38 trials4 evaluated the effects on cognition of omega-3 fatty acids, soy, ginkgo biloba, B vitamins, vitamin D plus calcium, vitamin C, beta-carotene, and multi-ingredient supplements. It found insufficient evidence to recommend any over-the-counter supplement for cognitive protection in adults with normal cognition or mild cognitive impairment.
Pharmacologic treatments
Testosterone supplementation. The Testosterone Trials tested the effects of testosterone gel vs placebo for 1 year on 493 men over age 65 with low testosterone (< 275 ng/mL) and with subjective memory complaints and objective memory performance deficits. Treatment was not associated with improved memory or other cognitive functions compared with placebo.5
Antiamyloid drugs. A randomized, double-blind, placebo-controlled trial in nearly 2,000 patients evaluated verubecestat, an oral beta-site amyloid precursor protein-cleaving enzyme-1 inhibitor that reduces the amyloid-beta level in cerebrospinal fluid.6
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer disease, while adverse events including rashes, falls, injuries, sleep disturbances, suicidal ideation, weight loss, and hair color change were more common in the treatment groups. The trial was terminated early because of futility at 50 months.
And in a placebo-controlled trial of solanezumab, a monoclonal antibody directed against the amyloid beta peptide, no benefit was demonstrated at 80 weeks in more than 2,000 patients with Alzheimer disease.7
Multiple common agents. A well-conducted systematic review8 of 51 trials of at least a 6-month duration did not support the use of antihypertensive agents, diabetes medications, nonsteroidal anti-inflammatory drugs, aspirin, hormones, or lipid-lowering drugs for cognitive protection for people with normal cognition or mild cognitive impairment.
However, some studies found reassuring evidence that standard therapies for other conditions do not worsen cognitive decline and are protective for atrial fibrillation.8
Proton-pump inhibitors. Concern exists for a potential link between dementia risk and proton-pump inhibitors, which are widely used to treat acid-related gastrointestinal disorders.9
A prospective population-based cohort study10 of nearly 3,500 people ages 65 and older without baseline dementia screened participants for dementia every 2 years over a mean period of 7.5 years and provided further evaluation for those who screened positive. Use of proton-pump inhibitors was not found to be associated with dementia risk, even with high cumulative exposure.
Results from this study do not support avoiding proton-pump inhibitors out of concern for dementia risk, although long-term use is associated with other safety concerns.
Oral anticoagulation. The increased risk of dementia with atrial fibrillation is well documented.11
A retrospective study12 based on a Swedish health registry and using more than 444,000 patients covering more than 1.5 million years at risk found that oral anticoagulant treatment at baseline conferred a 29% lower risk of dementia in an intention-to-treat analysis and a 48% lower risk in on-treatment analysis compared with no oral anticoagulation therapy. No difference was found between new oral anticoagulants and warfarin.
Transcatheter aortic valve implantation is not associated with cognitive decline
For patients with severe aortic stenosis who are not surgical candidates, transcatheter aortic valve implantation is superior to standard medical therapy,13 but there are concerns of neurologic and cognitive changes after the procedure.14 A meta-analysis of 18 studies assessing cognitive performance in more than 1,000 patients (average age ≥ 80) after undergoing the procedure for severe aortic stenosis found no significant cognitive performance changes from baseline perioperatively or 3 or 6 months later.15
TREATING DEMENTIA-ASSOCIATED BEHAVIORAL DISTURBANCES
Behavioral and psychiatric symptoms often accompany dementia, but no drugs have yet been approved by the US Food and Drug Administration (FDA) to address them in this population. Nonpharmacologic interventions are recommended as first-line therapy.
Antipsychotics are not recommended
Antipsychotics are often prescribed,16 although they are associated with metabolic syndrome17 and increased risks of stroke and death.18 The FDA has issued black box warnings against using antipsychotics for behavioral management in patients with dementia. Further, the American Geriatrics Society and the American Psychiatric Association do not endorse using them as initial therapy for behavioral and psychological symptoms of dementia.16,19
The Centers for Medicare and Medicaid Services partnered with nursing homes to improve the quality of care for patients with dementia, with results measured as the rate of prescribing antipsychotic medications. Although the use of psychotropic medications declined after initiating the partnership, the use of mood stabilizers increased, possibly as a substitute for antipsychotics.20
Dextromethorphan-quinidine use is up, despite modest evidence of benefit
A consumer news report in 2017 stated that the use of dextromethorphan-quinidine in long-term care facilities increased by nearly 400% between 2012 and 2016.21
Evidence for its benefits comes from a 10-week, phase 2, randomized controlled trial conducted at 42 US study sites with 194 patients with probable Alzheimer disease. Compared with the placebo group, the active treatment group had mildly reduced agitation but an increased risk of falls, dizziness, and diarrhea. However, rates of adverse effects were low, and the authors concluded that treatment was generally well tolerated.22
Pimavanserin: No long-term benefit for psychosis
In a phase 2, randomized, double-blind, placebo-controlled trial in 181 patients with possible or probable Alzheimer disease and psychotic symptoms, pimavanserin was associated with improved symptoms as measured by the Neuropsychiatric Inventory–Nursing Home Version psychosis score at 6 weeks, but no difference was found compared with placebo at 12 weeks. The treatment group had more adverse events, including agitation, aggression, peripheral edema, anxiety, and symptoms of dementia, although the differences were not statistically significant.23
DELIRIUM: AVOID ANTIPSYCHOTICS
Delirium is common in hospitalized older adults, especially those who have baseline cognitive or functional impairment and are exposed to precipitating factors such as treatment with anticholinergic or narcotic medications, infection, surgery, or admission to an intensive care unit.24
Delirium at discharge predicts poor outcomes
In a prospective study of 152 hospitalized patients with delirium, those who either did not recover from delirium or had only partially recovered at discharge were more likely to visit the emergency department, be rehospitalized, or die during the subsequent 3 months than those who had fully recovered from delirium at discharge.25
Multicomponent, patient-centered approach can help
A randomized trial in 377 patients in Taiwan evaluated the use of a modified Hospital Elder Life Program, consisting of 3 protocols focused on orienting communication, oral and nutritional assistance, and early mobilization. Patients were at least 65 years old and undergoing elective abdominal surgery with expected length of hospital stay longer than 6 days. The program, administered daily during hospitalization, significantly lowered postoperative delirium by 56% and hospital stay by 2 days compared with usual care.26
Prophylactic haloperidol does not improve outcomes
In a multicenter randomized, double-blind, placebo-controlled trial, van den Boogaard et al studied prophylactic intravenous haloperidol in nearly 1,800 critically ill patients at high risk of delirium.27 Haloperidol did not improve survival at 28 days compared with placebo. For secondary outcomes, including delirium incidence, delirium-free and coma-free days, duration of mechanical ventilation, and hospital and intensive care department length of stay, treatment was not found to differ statistically from placebo.
Antipsychotics may worsen delirium
A double-blind, parallel-arm, dose-titrated randomized trial, conducted at 11 Australian hospices or hospitals with palliative care services, administered oral risperidone, haloperidol, or placebo to 247 patients with life-limiting illness and delirium. Both treatment groups had higher delirium symptom scores than the placebo group.28
In addition, a systematic review and meta-analysis of 19 studies found no benefit of antipsychotic medications for preventing or treating delirium in hospitalized adults.29
Antipsychotics are often continued indefinitely
A retrospective chart review at a US academic health system found30 that among 487 patients with a new antipsychotic medication prescribed during hospitalization, 147 (30.2%) were discharged on an antipsychotic. Of these, 121 (82.3%) had a diagnosis of delirium. Only 15 (12.4%) had discharge summaries that included instructions for discontinuing the drug.
Another US health system retrospectively reviewed antipsychotic use and found31 that out of 260 patients who were newly exposed to an antipsychotic drug during hospitalization, 146 (56.2%) were discharged on an antipsychotic drug, and 65% of these patients were still on the drug at the time of the next hospital admission.
EXERCISE, EXERCISE, EXERCISE
Exercise recommended, but not vitamin D, to prevent falls
In 2018, the US Preventive Services Task Force updated its recommendations for preventing falls in community-dwelling older adults.32 Based on the findings of several trials, the task force recommends exercise interventions for adults age 65 and older who are at increased risk for falls. Gait, balance, and functional training were studied in 17 trials, resistance training in 13, flexibility in 8, endurance training in 5, and tai chi in 3, with 5 studies including general physical activity. Exercise interventions most commonly took place for 3 sessions per week for 12 months (range 2–42 months).
The task force also recommends against vitamin D supplementation for fall prevention in community-dwelling adults age 65 or older who are not known to have osteoporosis or vitamin D deficiency.
Early mobilization helps inpatients
Hospitalized older adults usually spend most of their time in bed. Forty-five previously ambulatory patients (age ≥ 65 without dementia or delirium) in a Veterans Affairs hospital were monitored with wireless accelerometers and were found to spend, on average, 83% of the measured hospital stay in bed. Standing or walking time ranged from 0.2% to 21%, with a median of only 3% (43 minutes a day).33
Since falls with injury became a Centers for Medicare and Medicaid Services nonreimbursable hospital-acquired condition, tension has arisen between promoting mobility and preventing falls.34 Two studies evaluating the adoption of mobility-restricting approaches such as bed-alarms, “fall-alert” signs, supervision of patients in the bathroom, and ensuring patients’ walking aids are within reach, did not find a significant reduction in falls or fall-related injuries.35,36
A clinically significant loss of community mobility is common after hospitalization in older adults.37 Older adults who developed mobility impairment during hospitalization had a higher risk of death in a large, retrospective study.38 A large Canadian multisite intervention trial39 that promoted early mobilization in older patients who were admitted to general medical wards resulted in increased mobilization and significantly shorter hospital stays.
POSTHOSPITAL CARE NEEDS IMPROVEMENT
After hospitalization, older adults who have difficulty with activities of daily living or complex medical needs often require continued care.
About 20% of hospitalized Medicare beneficiaries in the United States are discharged to skilled nursing facilities.40 This is often a stressful transition, and most people have little guidance on selecting a facility and simply choose one based on its proximity to home.41
A program of frequent visits by hospital-employed physicians and advanced practice professionals at skilled nursing facilities resulted in a significantly lower 30-day readmission rate compared with nonparticipating skilled nursing facilities in the same geographic area.42
Home healthcare is recommended after hospital discharge at a rapidly increasing rate. Overall referral rates increased from 8.6% to 14.1% between 2001 and 2012, and from 14.3% to 24.0% for patients with heart failure.43 A qualitative study of home healthcare nurses found a need for improved care coordination between home healthcare agencies and discharging hospitals, including defining accountability for orders and enhancing communication.44
- Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med 2017; 166(3):164–171. doi:10.7326/M16-0830
- Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):30–38. doi:10.7326/M17-1528
- Kryscio RJ, Abner EL, Caban-Holt A, et al. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 2017; 74(5):567–573. doi:10.1001/jamaneurol.2016.5778
- Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):52–62. doi:10.7326/M17-1530
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017; 317(7):717–727. doi:10.1001/jama.2016.21044
- Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 2018; 378(18):1691–1703. doi:10.1056/NEJMoa1706441
- Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378(4):321–330. doi:10.1056/NEJMoa1705971
- Fink HA, Jutkowitz E, McCarten JR, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):39–51. doi:10.7326/M17-1529
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73(4):410–416. doi:10.1001/jamaneurol.2015.4791
- Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018; 66(2):247–253. doi:10.1111/jgs.15073
- de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72(11):1288–1294. doi:10.1001/jamaneurol.2015.2161
- Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39(6):453–460. doi:10.1093/eurheartj/ehx579
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17):1597–1607. doi:10.1056/NEJMoa1008232
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016; 316(6):592–601. doi:10.1001/jama.2016.10302
- Khan MM, Herrmann N, Gallagher D, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc 2018; 66(2):254–262. doi:10.1111/jgs.15123
- Choosing Wisely; ABIM Foundation. American Geriatrics Society: ten things physicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society. Accessed November 6, 2018.
- Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004; 6(suppl 2):8–13. pmid:16001095
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294(15):1934–1943. doi:10.1001/jama.294.15.1934
- Choosing Wisely; ABIM Foundation. American Psychiatric Association: five things physicians and patients should question. www.choosingwisely.org/societies/american-psychiatric-association. Accessed November 6, 2018.
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ National Partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018; 178(5):640–647. doi:10.1001/jamainternmed.2018.0379
- CNN. The little red pill being pushed on the elderly. www.cnn.com/2017/10/12/health/nuedexta-nursing-homes-invs/index.html. Accessed November 6, 2018.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314(12):1242–1254. doi:10.1001/jama.2015.10214
- Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 2018; 17(3):213–222. doi:10.1016/S1474-4422(18)30039-5
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165. doi:10.1056/NEJMra052321
- Cole MG, McCusker J, Bailey R, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017; 46(1):90–95. doi:10.1093/ageing/afw153
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017; 152(9):827–834. doi:10.1001/jamasurg.2017.1083
- van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319(7):680–690. doi:10.1001/jama.2018.0160
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017; 177(1):34–42. doi:10.1001/jamainternmed.2016.7491
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64(4):705–714. doi:10.1111/jgs.14076
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11(8):550–555. doi:10.1002/jhm.2585
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation statement. JAMA 2018; 319(16):1696–1704. doi:10.1001/jama.2018.3097
- Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57(9):1660–1665. doi:10.1111/j.1532-5415.2009.02393.x
- Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med 2017; 177(6):759–760. doi:10.1001/jamainternmed.2017.0840
- Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016; 352:h6781. doi:10.1136/bmj.h6781
- Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med 2012; 157(10):692–699. doi:10.7326/0003-4819-157-10-201211200-00005
- Loyd C, Beasley TM, Miltner RS, Clark D, King B, Brown CJ. Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403. doi:10.1111/jgs.15397
- Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden Activity subscale for mobility status in hospitalized older adults. J Hosp Med 2017; 12(6):396–401. doi:10.12788/jhm.2748
- Liu B, Moore JE, Almaawiy U, et al; MOVE ON Collaboration. Outcomes of mobilisation of vulnerable elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018; 47(1):112–119. doi:10.1093/ageing/afx128
- Report to Congress: Medicare Payment Policy. Medicare Payment Advisory Commission 2016. www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed November 6, 2018.
- Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc 2017; 65(11):2459–2465. doi:10.1111/jgs.14988
- Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med 2017; 12(4):238–244. doi:10.12788/jhm.2710
- Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc 2015; 63(6):1265–1266. doi:10.1111/jgs.13467
- Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med 2017; 32(10):1114–1121. doi:10.1007/s11606-017-4104-0
Unfortunately, recent research has not unveiled a breakthrough for preventing or treating cognitive impairment or Alzheimer disease. But several studies from the last 2 years are helping to drive the field of geriatrics forward, providing evidence of what does and does not help a variety of issues specific to the elderly.
Based on a search of the 2017 and 2018 literature, this article presents new evidence on preventing and treating cognitive impairment, managing dementia-associated behavioral disturbances and delirium, preventing falls, and improving inpatient mobility and posthospital care transitions.
COGNITIVE IMPAIRMENT, DEMENTIA: STILL NO SILVER BULLET
With the exception of oral anticoagulation treatment for atrial fibrillation, there is little evidence that pharmacologic or nonpharmacologic interventions slow the onset or progression of Alzheimer disease.
Nonpharmacologic interventions
Home occupational therapy. A 2-year home-based occupational therapy intervention1 showed no evidence of slowing functional decline in patients with Alzheimer disease. The randomized controlled trial involving 180 participants consisted of monthly sessions of an intensive, well-established collaborative-care management model that included fall prevention and other safety strategies, personalized training in activities of daily living, exercise, and education. Outcome measures for activities of daily living did not differ significantly between the treatment and control groups.1
Physical activity. Whether physical activity interventions slow cognitive decline and prevent dementia in cognitively intact adults was examined in a systematic review of 32 trials.2 Most of the trials followed patients for 6 months; a few stretched for 1 or 2 years.
Evidence was insufficient to prove cognitive benefit for short-term, single-component or multicomponent physical activity interventions. However, a multidomain physical activity intervention that also included dietary modifications and cognitive training did show a delay in cognitive decline, but only “low-strength” evidence.2
Nutritional supplements. The antioxidants vitamin E and selenium were studied for their possible cognitive benefit in the double-blind randomized Prevention of Alzheimer Disease by Vitamin E and Selenium trial3 in 3,786 asymptomatic men ages 60 and older. Neither supplement was found to prevent dementia over a 7-year follow-up period.
A review of 38 trials4 evaluated the effects on cognition of omega-3 fatty acids, soy, ginkgo biloba, B vitamins, vitamin D plus calcium, vitamin C, beta-carotene, and multi-ingredient supplements. It found insufficient evidence to recommend any over-the-counter supplement for cognitive protection in adults with normal cognition or mild cognitive impairment.
Pharmacologic treatments
Testosterone supplementation. The Testosterone Trials tested the effects of testosterone gel vs placebo for 1 year on 493 men over age 65 with low testosterone (< 275 ng/mL) and with subjective memory complaints and objective memory performance deficits. Treatment was not associated with improved memory or other cognitive functions compared with placebo.5
Antiamyloid drugs. A randomized, double-blind, placebo-controlled trial in nearly 2,000 patients evaluated verubecestat, an oral beta-site amyloid precursor protein-cleaving enzyme-1 inhibitor that reduces the amyloid-beta level in cerebrospinal fluid.6
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer disease, while adverse events including rashes, falls, injuries, sleep disturbances, suicidal ideation, weight loss, and hair color change were more common in the treatment groups. The trial was terminated early because of futility at 50 months.
And in a placebo-controlled trial of solanezumab, a monoclonal antibody directed against the amyloid beta peptide, no benefit was demonstrated at 80 weeks in more than 2,000 patients with Alzheimer disease.7
Multiple common agents. A well-conducted systematic review8 of 51 trials of at least a 6-month duration did not support the use of antihypertensive agents, diabetes medications, nonsteroidal anti-inflammatory drugs, aspirin, hormones, or lipid-lowering drugs for cognitive protection for people with normal cognition or mild cognitive impairment.
However, some studies found reassuring evidence that standard therapies for other conditions do not worsen cognitive decline and are protective for atrial fibrillation.8
Proton-pump inhibitors. Concern exists for a potential link between dementia risk and proton-pump inhibitors, which are widely used to treat acid-related gastrointestinal disorders.9
A prospective population-based cohort study10 of nearly 3,500 people ages 65 and older without baseline dementia screened participants for dementia every 2 years over a mean period of 7.5 years and provided further evaluation for those who screened positive. Use of proton-pump inhibitors was not found to be associated with dementia risk, even with high cumulative exposure.
Results from this study do not support avoiding proton-pump inhibitors out of concern for dementia risk, although long-term use is associated with other safety concerns.
Oral anticoagulation. The increased risk of dementia with atrial fibrillation is well documented.11
A retrospective study12 based on a Swedish health registry and using more than 444,000 patients covering more than 1.5 million years at risk found that oral anticoagulant treatment at baseline conferred a 29% lower risk of dementia in an intention-to-treat analysis and a 48% lower risk in on-treatment analysis compared with no oral anticoagulation therapy. No difference was found between new oral anticoagulants and warfarin.
Transcatheter aortic valve implantation is not associated with cognitive decline
For patients with severe aortic stenosis who are not surgical candidates, transcatheter aortic valve implantation is superior to standard medical therapy,13 but there are concerns of neurologic and cognitive changes after the procedure.14 A meta-analysis of 18 studies assessing cognitive performance in more than 1,000 patients (average age ≥ 80) after undergoing the procedure for severe aortic stenosis found no significant cognitive performance changes from baseline perioperatively or 3 or 6 months later.15
TREATING DEMENTIA-ASSOCIATED BEHAVIORAL DISTURBANCES
Behavioral and psychiatric symptoms often accompany dementia, but no drugs have yet been approved by the US Food and Drug Administration (FDA) to address them in this population. Nonpharmacologic interventions are recommended as first-line therapy.
Antipsychotics are not recommended
Antipsychotics are often prescribed,16 although they are associated with metabolic syndrome17 and increased risks of stroke and death.18 The FDA has issued black box warnings against using antipsychotics for behavioral management in patients with dementia. Further, the American Geriatrics Society and the American Psychiatric Association do not endorse using them as initial therapy for behavioral and psychological symptoms of dementia.16,19
The Centers for Medicare and Medicaid Services partnered with nursing homes to improve the quality of care for patients with dementia, with results measured as the rate of prescribing antipsychotic medications. Although the use of psychotropic medications declined after initiating the partnership, the use of mood stabilizers increased, possibly as a substitute for antipsychotics.20
Dextromethorphan-quinidine use is up, despite modest evidence of benefit
A consumer news report in 2017 stated that the use of dextromethorphan-quinidine in long-term care facilities increased by nearly 400% between 2012 and 2016.21
Evidence for its benefits comes from a 10-week, phase 2, randomized controlled trial conducted at 42 US study sites with 194 patients with probable Alzheimer disease. Compared with the placebo group, the active treatment group had mildly reduced agitation but an increased risk of falls, dizziness, and diarrhea. However, rates of adverse effects were low, and the authors concluded that treatment was generally well tolerated.22
Pimavanserin: No long-term benefit for psychosis
In a phase 2, randomized, double-blind, placebo-controlled trial in 181 patients with possible or probable Alzheimer disease and psychotic symptoms, pimavanserin was associated with improved symptoms as measured by the Neuropsychiatric Inventory–Nursing Home Version psychosis score at 6 weeks, but no difference was found compared with placebo at 12 weeks. The treatment group had more adverse events, including agitation, aggression, peripheral edema, anxiety, and symptoms of dementia, although the differences were not statistically significant.23
DELIRIUM: AVOID ANTIPSYCHOTICS
Delirium is common in hospitalized older adults, especially those who have baseline cognitive or functional impairment and are exposed to precipitating factors such as treatment with anticholinergic or narcotic medications, infection, surgery, or admission to an intensive care unit.24
Delirium at discharge predicts poor outcomes
In a prospective study of 152 hospitalized patients with delirium, those who either did not recover from delirium or had only partially recovered at discharge were more likely to visit the emergency department, be rehospitalized, or die during the subsequent 3 months than those who had fully recovered from delirium at discharge.25
Multicomponent, patient-centered approach can help
A randomized trial in 377 patients in Taiwan evaluated the use of a modified Hospital Elder Life Program, consisting of 3 protocols focused on orienting communication, oral and nutritional assistance, and early mobilization. Patients were at least 65 years old and undergoing elective abdominal surgery with expected length of hospital stay longer than 6 days. The program, administered daily during hospitalization, significantly lowered postoperative delirium by 56% and hospital stay by 2 days compared with usual care.26
Prophylactic haloperidol does not improve outcomes
In a multicenter randomized, double-blind, placebo-controlled trial, van den Boogaard et al studied prophylactic intravenous haloperidol in nearly 1,800 critically ill patients at high risk of delirium.27 Haloperidol did not improve survival at 28 days compared with placebo. For secondary outcomes, including delirium incidence, delirium-free and coma-free days, duration of mechanical ventilation, and hospital and intensive care department length of stay, treatment was not found to differ statistically from placebo.
Antipsychotics may worsen delirium
A double-blind, parallel-arm, dose-titrated randomized trial, conducted at 11 Australian hospices or hospitals with palliative care services, administered oral risperidone, haloperidol, or placebo to 247 patients with life-limiting illness and delirium. Both treatment groups had higher delirium symptom scores than the placebo group.28
In addition, a systematic review and meta-analysis of 19 studies found no benefit of antipsychotic medications for preventing or treating delirium in hospitalized adults.29
Antipsychotics are often continued indefinitely
A retrospective chart review at a US academic health system found30 that among 487 patients with a new antipsychotic medication prescribed during hospitalization, 147 (30.2%) were discharged on an antipsychotic. Of these, 121 (82.3%) had a diagnosis of delirium. Only 15 (12.4%) had discharge summaries that included instructions for discontinuing the drug.
Another US health system retrospectively reviewed antipsychotic use and found31 that out of 260 patients who were newly exposed to an antipsychotic drug during hospitalization, 146 (56.2%) were discharged on an antipsychotic drug, and 65% of these patients were still on the drug at the time of the next hospital admission.
EXERCISE, EXERCISE, EXERCISE
Exercise recommended, but not vitamin D, to prevent falls
In 2018, the US Preventive Services Task Force updated its recommendations for preventing falls in community-dwelling older adults.32 Based on the findings of several trials, the task force recommends exercise interventions for adults age 65 and older who are at increased risk for falls. Gait, balance, and functional training were studied in 17 trials, resistance training in 13, flexibility in 8, endurance training in 5, and tai chi in 3, with 5 studies including general physical activity. Exercise interventions most commonly took place for 3 sessions per week for 12 months (range 2–42 months).
The task force also recommends against vitamin D supplementation for fall prevention in community-dwelling adults age 65 or older who are not known to have osteoporosis or vitamin D deficiency.
Early mobilization helps inpatients
Hospitalized older adults usually spend most of their time in bed. Forty-five previously ambulatory patients (age ≥ 65 without dementia or delirium) in a Veterans Affairs hospital were monitored with wireless accelerometers and were found to spend, on average, 83% of the measured hospital stay in bed. Standing or walking time ranged from 0.2% to 21%, with a median of only 3% (43 minutes a day).33
Since falls with injury became a Centers for Medicare and Medicaid Services nonreimbursable hospital-acquired condition, tension has arisen between promoting mobility and preventing falls.34 Two studies evaluating the adoption of mobility-restricting approaches such as bed-alarms, “fall-alert” signs, supervision of patients in the bathroom, and ensuring patients’ walking aids are within reach, did not find a significant reduction in falls or fall-related injuries.35,36
A clinically significant loss of community mobility is common after hospitalization in older adults.37 Older adults who developed mobility impairment during hospitalization had a higher risk of death in a large, retrospective study.38 A large Canadian multisite intervention trial39 that promoted early mobilization in older patients who were admitted to general medical wards resulted in increased mobilization and significantly shorter hospital stays.
POSTHOSPITAL CARE NEEDS IMPROVEMENT
After hospitalization, older adults who have difficulty with activities of daily living or complex medical needs often require continued care.
About 20% of hospitalized Medicare beneficiaries in the United States are discharged to skilled nursing facilities.40 This is often a stressful transition, and most people have little guidance on selecting a facility and simply choose one based on its proximity to home.41
A program of frequent visits by hospital-employed physicians and advanced practice professionals at skilled nursing facilities resulted in a significantly lower 30-day readmission rate compared with nonparticipating skilled nursing facilities in the same geographic area.42
Home healthcare is recommended after hospital discharge at a rapidly increasing rate. Overall referral rates increased from 8.6% to 14.1% between 2001 and 2012, and from 14.3% to 24.0% for patients with heart failure.43 A qualitative study of home healthcare nurses found a need for improved care coordination between home healthcare agencies and discharging hospitals, including defining accountability for orders and enhancing communication.44
Unfortunately, recent research has not unveiled a breakthrough for preventing or treating cognitive impairment or Alzheimer disease. But several studies from the last 2 years are helping to drive the field of geriatrics forward, providing evidence of what does and does not help a variety of issues specific to the elderly.
Based on a search of the 2017 and 2018 literature, this article presents new evidence on preventing and treating cognitive impairment, managing dementia-associated behavioral disturbances and delirium, preventing falls, and improving inpatient mobility and posthospital care transitions.
COGNITIVE IMPAIRMENT, DEMENTIA: STILL NO SILVER BULLET
With the exception of oral anticoagulation treatment for atrial fibrillation, there is little evidence that pharmacologic or nonpharmacologic interventions slow the onset or progression of Alzheimer disease.
Nonpharmacologic interventions
Home occupational therapy. A 2-year home-based occupational therapy intervention1 showed no evidence of slowing functional decline in patients with Alzheimer disease. The randomized controlled trial involving 180 participants consisted of monthly sessions of an intensive, well-established collaborative-care management model that included fall prevention and other safety strategies, personalized training in activities of daily living, exercise, and education. Outcome measures for activities of daily living did not differ significantly between the treatment and control groups.1
Physical activity. Whether physical activity interventions slow cognitive decline and prevent dementia in cognitively intact adults was examined in a systematic review of 32 trials.2 Most of the trials followed patients for 6 months; a few stretched for 1 or 2 years.
Evidence was insufficient to prove cognitive benefit for short-term, single-component or multicomponent physical activity interventions. However, a multidomain physical activity intervention that also included dietary modifications and cognitive training did show a delay in cognitive decline, but only “low-strength” evidence.2
Nutritional supplements. The antioxidants vitamin E and selenium were studied for their possible cognitive benefit in the double-blind randomized Prevention of Alzheimer Disease by Vitamin E and Selenium trial3 in 3,786 asymptomatic men ages 60 and older. Neither supplement was found to prevent dementia over a 7-year follow-up period.
A review of 38 trials4 evaluated the effects on cognition of omega-3 fatty acids, soy, ginkgo biloba, B vitamins, vitamin D plus calcium, vitamin C, beta-carotene, and multi-ingredient supplements. It found insufficient evidence to recommend any over-the-counter supplement for cognitive protection in adults with normal cognition or mild cognitive impairment.
Pharmacologic treatments
Testosterone supplementation. The Testosterone Trials tested the effects of testosterone gel vs placebo for 1 year on 493 men over age 65 with low testosterone (< 275 ng/mL) and with subjective memory complaints and objective memory performance deficits. Treatment was not associated with improved memory or other cognitive functions compared with placebo.5
Antiamyloid drugs. A randomized, double-blind, placebo-controlled trial in nearly 2,000 patients evaluated verubecestat, an oral beta-site amyloid precursor protein-cleaving enzyme-1 inhibitor that reduces the amyloid-beta level in cerebrospinal fluid.6
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer disease, while adverse events including rashes, falls, injuries, sleep disturbances, suicidal ideation, weight loss, and hair color change were more common in the treatment groups. The trial was terminated early because of futility at 50 months.
And in a placebo-controlled trial of solanezumab, a monoclonal antibody directed against the amyloid beta peptide, no benefit was demonstrated at 80 weeks in more than 2,000 patients with Alzheimer disease.7
Multiple common agents. A well-conducted systematic review8 of 51 trials of at least a 6-month duration did not support the use of antihypertensive agents, diabetes medications, nonsteroidal anti-inflammatory drugs, aspirin, hormones, or lipid-lowering drugs for cognitive protection for people with normal cognition or mild cognitive impairment.
However, some studies found reassuring evidence that standard therapies for other conditions do not worsen cognitive decline and are protective for atrial fibrillation.8
Proton-pump inhibitors. Concern exists for a potential link between dementia risk and proton-pump inhibitors, which are widely used to treat acid-related gastrointestinal disorders.9
A prospective population-based cohort study10 of nearly 3,500 people ages 65 and older without baseline dementia screened participants for dementia every 2 years over a mean period of 7.5 years and provided further evaluation for those who screened positive. Use of proton-pump inhibitors was not found to be associated with dementia risk, even with high cumulative exposure.
Results from this study do not support avoiding proton-pump inhibitors out of concern for dementia risk, although long-term use is associated with other safety concerns.
Oral anticoagulation. The increased risk of dementia with atrial fibrillation is well documented.11
A retrospective study12 based on a Swedish health registry and using more than 444,000 patients covering more than 1.5 million years at risk found that oral anticoagulant treatment at baseline conferred a 29% lower risk of dementia in an intention-to-treat analysis and a 48% lower risk in on-treatment analysis compared with no oral anticoagulation therapy. No difference was found between new oral anticoagulants and warfarin.
Transcatheter aortic valve implantation is not associated with cognitive decline
For patients with severe aortic stenosis who are not surgical candidates, transcatheter aortic valve implantation is superior to standard medical therapy,13 but there are concerns of neurologic and cognitive changes after the procedure.14 A meta-analysis of 18 studies assessing cognitive performance in more than 1,000 patients (average age ≥ 80) after undergoing the procedure for severe aortic stenosis found no significant cognitive performance changes from baseline perioperatively or 3 or 6 months later.15
TREATING DEMENTIA-ASSOCIATED BEHAVIORAL DISTURBANCES
Behavioral and psychiatric symptoms often accompany dementia, but no drugs have yet been approved by the US Food and Drug Administration (FDA) to address them in this population. Nonpharmacologic interventions are recommended as first-line therapy.
Antipsychotics are not recommended
Antipsychotics are often prescribed,16 although they are associated with metabolic syndrome17 and increased risks of stroke and death.18 The FDA has issued black box warnings against using antipsychotics for behavioral management in patients with dementia. Further, the American Geriatrics Society and the American Psychiatric Association do not endorse using them as initial therapy for behavioral and psychological symptoms of dementia.16,19
The Centers for Medicare and Medicaid Services partnered with nursing homes to improve the quality of care for patients with dementia, with results measured as the rate of prescribing antipsychotic medications. Although the use of psychotropic medications declined after initiating the partnership, the use of mood stabilizers increased, possibly as a substitute for antipsychotics.20
Dextromethorphan-quinidine use is up, despite modest evidence of benefit
A consumer news report in 2017 stated that the use of dextromethorphan-quinidine in long-term care facilities increased by nearly 400% between 2012 and 2016.21
Evidence for its benefits comes from a 10-week, phase 2, randomized controlled trial conducted at 42 US study sites with 194 patients with probable Alzheimer disease. Compared with the placebo group, the active treatment group had mildly reduced agitation but an increased risk of falls, dizziness, and diarrhea. However, rates of adverse effects were low, and the authors concluded that treatment was generally well tolerated.22
Pimavanserin: No long-term benefit for psychosis
In a phase 2, randomized, double-blind, placebo-controlled trial in 181 patients with possible or probable Alzheimer disease and psychotic symptoms, pimavanserin was associated with improved symptoms as measured by the Neuropsychiatric Inventory–Nursing Home Version psychosis score at 6 weeks, but no difference was found compared with placebo at 12 weeks. The treatment group had more adverse events, including agitation, aggression, peripheral edema, anxiety, and symptoms of dementia, although the differences were not statistically significant.23
DELIRIUM: AVOID ANTIPSYCHOTICS
Delirium is common in hospitalized older adults, especially those who have baseline cognitive or functional impairment and are exposed to precipitating factors such as treatment with anticholinergic or narcotic medications, infection, surgery, or admission to an intensive care unit.24
Delirium at discharge predicts poor outcomes
In a prospective study of 152 hospitalized patients with delirium, those who either did not recover from delirium or had only partially recovered at discharge were more likely to visit the emergency department, be rehospitalized, or die during the subsequent 3 months than those who had fully recovered from delirium at discharge.25
Multicomponent, patient-centered approach can help
A randomized trial in 377 patients in Taiwan evaluated the use of a modified Hospital Elder Life Program, consisting of 3 protocols focused on orienting communication, oral and nutritional assistance, and early mobilization. Patients were at least 65 years old and undergoing elective abdominal surgery with expected length of hospital stay longer than 6 days. The program, administered daily during hospitalization, significantly lowered postoperative delirium by 56% and hospital stay by 2 days compared with usual care.26
Prophylactic haloperidol does not improve outcomes
In a multicenter randomized, double-blind, placebo-controlled trial, van den Boogaard et al studied prophylactic intravenous haloperidol in nearly 1,800 critically ill patients at high risk of delirium.27 Haloperidol did not improve survival at 28 days compared with placebo. For secondary outcomes, including delirium incidence, delirium-free and coma-free days, duration of mechanical ventilation, and hospital and intensive care department length of stay, treatment was not found to differ statistically from placebo.
Antipsychotics may worsen delirium
A double-blind, parallel-arm, dose-titrated randomized trial, conducted at 11 Australian hospices or hospitals with palliative care services, administered oral risperidone, haloperidol, or placebo to 247 patients with life-limiting illness and delirium. Both treatment groups had higher delirium symptom scores than the placebo group.28
In addition, a systematic review and meta-analysis of 19 studies found no benefit of antipsychotic medications for preventing or treating delirium in hospitalized adults.29
Antipsychotics are often continued indefinitely
A retrospective chart review at a US academic health system found30 that among 487 patients with a new antipsychotic medication prescribed during hospitalization, 147 (30.2%) were discharged on an antipsychotic. Of these, 121 (82.3%) had a diagnosis of delirium. Only 15 (12.4%) had discharge summaries that included instructions for discontinuing the drug.
Another US health system retrospectively reviewed antipsychotic use and found31 that out of 260 patients who were newly exposed to an antipsychotic drug during hospitalization, 146 (56.2%) were discharged on an antipsychotic drug, and 65% of these patients were still on the drug at the time of the next hospital admission.
EXERCISE, EXERCISE, EXERCISE
Exercise recommended, but not vitamin D, to prevent falls
In 2018, the US Preventive Services Task Force updated its recommendations for preventing falls in community-dwelling older adults.32 Based on the findings of several trials, the task force recommends exercise interventions for adults age 65 and older who are at increased risk for falls. Gait, balance, and functional training were studied in 17 trials, resistance training in 13, flexibility in 8, endurance training in 5, and tai chi in 3, with 5 studies including general physical activity. Exercise interventions most commonly took place for 3 sessions per week for 12 months (range 2–42 months).
The task force also recommends against vitamin D supplementation for fall prevention in community-dwelling adults age 65 or older who are not known to have osteoporosis or vitamin D deficiency.
Early mobilization helps inpatients
Hospitalized older adults usually spend most of their time in bed. Forty-five previously ambulatory patients (age ≥ 65 without dementia or delirium) in a Veterans Affairs hospital were monitored with wireless accelerometers and were found to spend, on average, 83% of the measured hospital stay in bed. Standing or walking time ranged from 0.2% to 21%, with a median of only 3% (43 minutes a day).33
Since falls with injury became a Centers for Medicare and Medicaid Services nonreimbursable hospital-acquired condition, tension has arisen between promoting mobility and preventing falls.34 Two studies evaluating the adoption of mobility-restricting approaches such as bed-alarms, “fall-alert” signs, supervision of patients in the bathroom, and ensuring patients’ walking aids are within reach, did not find a significant reduction in falls or fall-related injuries.35,36
A clinically significant loss of community mobility is common after hospitalization in older adults.37 Older adults who developed mobility impairment during hospitalization had a higher risk of death in a large, retrospective study.38 A large Canadian multisite intervention trial39 that promoted early mobilization in older patients who were admitted to general medical wards resulted in increased mobilization and significantly shorter hospital stays.
POSTHOSPITAL CARE NEEDS IMPROVEMENT
After hospitalization, older adults who have difficulty with activities of daily living or complex medical needs often require continued care.
About 20% of hospitalized Medicare beneficiaries in the United States are discharged to skilled nursing facilities.40 This is often a stressful transition, and most people have little guidance on selecting a facility and simply choose one based on its proximity to home.41
A program of frequent visits by hospital-employed physicians and advanced practice professionals at skilled nursing facilities resulted in a significantly lower 30-day readmission rate compared with nonparticipating skilled nursing facilities in the same geographic area.42
Home healthcare is recommended after hospital discharge at a rapidly increasing rate. Overall referral rates increased from 8.6% to 14.1% between 2001 and 2012, and from 14.3% to 24.0% for patients with heart failure.43 A qualitative study of home healthcare nurses found a need for improved care coordination between home healthcare agencies and discharging hospitals, including defining accountability for orders and enhancing communication.44
- Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med 2017; 166(3):164–171. doi:10.7326/M16-0830
- Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):30–38. doi:10.7326/M17-1528
- Kryscio RJ, Abner EL, Caban-Holt A, et al. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 2017; 74(5):567–573. doi:10.1001/jamaneurol.2016.5778
- Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):52–62. doi:10.7326/M17-1530
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017; 317(7):717–727. doi:10.1001/jama.2016.21044
- Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 2018; 378(18):1691–1703. doi:10.1056/NEJMoa1706441
- Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378(4):321–330. doi:10.1056/NEJMoa1705971
- Fink HA, Jutkowitz E, McCarten JR, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):39–51. doi:10.7326/M17-1529
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73(4):410–416. doi:10.1001/jamaneurol.2015.4791
- Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018; 66(2):247–253. doi:10.1111/jgs.15073
- de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72(11):1288–1294. doi:10.1001/jamaneurol.2015.2161
- Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39(6):453–460. doi:10.1093/eurheartj/ehx579
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17):1597–1607. doi:10.1056/NEJMoa1008232
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016; 316(6):592–601. doi:10.1001/jama.2016.10302
- Khan MM, Herrmann N, Gallagher D, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc 2018; 66(2):254–262. doi:10.1111/jgs.15123
- Choosing Wisely; ABIM Foundation. American Geriatrics Society: ten things physicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society. Accessed November 6, 2018.
- Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004; 6(suppl 2):8–13. pmid:16001095
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294(15):1934–1943. doi:10.1001/jama.294.15.1934
- Choosing Wisely; ABIM Foundation. American Psychiatric Association: five things physicians and patients should question. www.choosingwisely.org/societies/american-psychiatric-association. Accessed November 6, 2018.
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ National Partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018; 178(5):640–647. doi:10.1001/jamainternmed.2018.0379
- CNN. The little red pill being pushed on the elderly. www.cnn.com/2017/10/12/health/nuedexta-nursing-homes-invs/index.html. Accessed November 6, 2018.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314(12):1242–1254. doi:10.1001/jama.2015.10214
- Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 2018; 17(3):213–222. doi:10.1016/S1474-4422(18)30039-5
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165. doi:10.1056/NEJMra052321
- Cole MG, McCusker J, Bailey R, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017; 46(1):90–95. doi:10.1093/ageing/afw153
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017; 152(9):827–834. doi:10.1001/jamasurg.2017.1083
- van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319(7):680–690. doi:10.1001/jama.2018.0160
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017; 177(1):34–42. doi:10.1001/jamainternmed.2016.7491
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64(4):705–714. doi:10.1111/jgs.14076
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11(8):550–555. doi:10.1002/jhm.2585
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation statement. JAMA 2018; 319(16):1696–1704. doi:10.1001/jama.2018.3097
- Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57(9):1660–1665. doi:10.1111/j.1532-5415.2009.02393.x
- Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med 2017; 177(6):759–760. doi:10.1001/jamainternmed.2017.0840
- Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016; 352:h6781. doi:10.1136/bmj.h6781
- Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med 2012; 157(10):692–699. doi:10.7326/0003-4819-157-10-201211200-00005
- Loyd C, Beasley TM, Miltner RS, Clark D, King B, Brown CJ. Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403. doi:10.1111/jgs.15397
- Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden Activity subscale for mobility status in hospitalized older adults. J Hosp Med 2017; 12(6):396–401. doi:10.12788/jhm.2748
- Liu B, Moore JE, Almaawiy U, et al; MOVE ON Collaboration. Outcomes of mobilisation of vulnerable elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018; 47(1):112–119. doi:10.1093/ageing/afx128
- Report to Congress: Medicare Payment Policy. Medicare Payment Advisory Commission 2016. www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed November 6, 2018.
- Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc 2017; 65(11):2459–2465. doi:10.1111/jgs.14988
- Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med 2017; 12(4):238–244. doi:10.12788/jhm.2710
- Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc 2015; 63(6):1265–1266. doi:10.1111/jgs.13467
- Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med 2017; 32(10):1114–1121. doi:10.1007/s11606-017-4104-0
- Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med 2017; 166(3):164–171. doi:10.7326/M16-0830
- Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):30–38. doi:10.7326/M17-1528
- Kryscio RJ, Abner EL, Caban-Holt A, et al. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 2017; 74(5):567–573. doi:10.1001/jamaneurol.2016.5778
- Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):52–62. doi:10.7326/M17-1530
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017; 317(7):717–727. doi:10.1001/jama.2016.21044
- Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 2018; 378(18):1691–1703. doi:10.1056/NEJMoa1706441
- Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378(4):321–330. doi:10.1056/NEJMoa1705971
- Fink HA, Jutkowitz E, McCarten JR, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):39–51. doi:10.7326/M17-1529
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73(4):410–416. doi:10.1001/jamaneurol.2015.4791
- Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018; 66(2):247–253. doi:10.1111/jgs.15073
- de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72(11):1288–1294. doi:10.1001/jamaneurol.2015.2161
- Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39(6):453–460. doi:10.1093/eurheartj/ehx579
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17):1597–1607. doi:10.1056/NEJMoa1008232
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016; 316(6):592–601. doi:10.1001/jama.2016.10302
- Khan MM, Herrmann N, Gallagher D, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc 2018; 66(2):254–262. doi:10.1111/jgs.15123
- Choosing Wisely; ABIM Foundation. American Geriatrics Society: ten things physicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society. Accessed November 6, 2018.
- Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004; 6(suppl 2):8–13. pmid:16001095
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294(15):1934–1943. doi:10.1001/jama.294.15.1934
- Choosing Wisely; ABIM Foundation. American Psychiatric Association: five things physicians and patients should question. www.choosingwisely.org/societies/american-psychiatric-association. Accessed November 6, 2018.
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ National Partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018; 178(5):640–647. doi:10.1001/jamainternmed.2018.0379
- CNN. The little red pill being pushed on the elderly. www.cnn.com/2017/10/12/health/nuedexta-nursing-homes-invs/index.html. Accessed November 6, 2018.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314(12):1242–1254. doi:10.1001/jama.2015.10214
- Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 2018; 17(3):213–222. doi:10.1016/S1474-4422(18)30039-5
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165. doi:10.1056/NEJMra052321
- Cole MG, McCusker J, Bailey R, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017; 46(1):90–95. doi:10.1093/ageing/afw153
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017; 152(9):827–834. doi:10.1001/jamasurg.2017.1083
- van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319(7):680–690. doi:10.1001/jama.2018.0160
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017; 177(1):34–42. doi:10.1001/jamainternmed.2016.7491
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64(4):705–714. doi:10.1111/jgs.14076
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11(8):550–555. doi:10.1002/jhm.2585
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation statement. JAMA 2018; 319(16):1696–1704. doi:10.1001/jama.2018.3097
- Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57(9):1660–1665. doi:10.1111/j.1532-5415.2009.02393.x
- Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med 2017; 177(6):759–760. doi:10.1001/jamainternmed.2017.0840
- Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016; 352:h6781. doi:10.1136/bmj.h6781
- Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med 2012; 157(10):692–699. doi:10.7326/0003-4819-157-10-201211200-00005
- Loyd C, Beasley TM, Miltner RS, Clark D, King B, Brown CJ. Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403. doi:10.1111/jgs.15397
- Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden Activity subscale for mobility status in hospitalized older adults. J Hosp Med 2017; 12(6):396–401. doi:10.12788/jhm.2748
- Liu B, Moore JE, Almaawiy U, et al; MOVE ON Collaboration. Outcomes of mobilisation of vulnerable elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018; 47(1):112–119. doi:10.1093/ageing/afx128
- Report to Congress: Medicare Payment Policy. Medicare Payment Advisory Commission 2016. www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed November 6, 2018.
- Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc 2017; 65(11):2459–2465. doi:10.1111/jgs.14988
- Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med 2017; 12(4):238–244. doi:10.12788/jhm.2710
- Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc 2015; 63(6):1265–1266. doi:10.1111/jgs.13467
- Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med 2017; 32(10):1114–1121. doi:10.1007/s11606-017-4104-0
KEY POINTS
- Oral anticoagulant treatment for atrial fibrillation helps preserve cognitive function.
- Antipsychotics are not recommended as initial therapy for dementia-associated behavioral disturbances or for hospitalization-induced delirium.
- A multicomponent inpatient program can help prevent postoperative delirium in hospitalized patients.
- The US Preventive Services Task Force recommends exercise to prevent falls.
- Early mobility should be encouraged for hospitalized patients.
- Better continuity of care between hospitals and skilled nursing facilities can reduce hospital readmission rates.
Narcolepsy: Diagnosis and management
Narcolepsy was originally described in the late 1800s by the French physician Jean-Baptiste-Edouard Gélineau, who reported the case of a wine merchant suffering from somnolence. In this first description, he coined the term narcolepsie by joining the Greek words narke (numbness or stupor) and lepsis (attack).1
Since then, the disorder has been further characterized, and some insight into its biological underpinnings has been established. Importantly, treatments have improved and expanded, facilitating its management and thereby improving quality of life for those with the disorder.
This review focuses on clinically relevant features of the disorder and proposes management strategies.
CLINICAL FEATURES
Narcolepsy is characterized by instability of sleep-wake transitions.
Daytime sleepiness
Clinically, narcolepsy manifests with excessive daytime sleepiness that can be personally and socially disabling. Cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations can also be present,2,3 but they are not necessary for diagnosis. In fact, a minority of patients with narcolepsy have all these symptoms.4 Narcolepsy is divided into type 1 (with cataplexy) and type 2 (without cataplexy).2
Sleepiness tends to be worse with inactivity, and sleep can often be irresistible. Sleep attacks can come on suddenly and may be brief enough to manifest as a lapse in consciousness.
Short naps tend to be refreshing. Rapid eye movement (REM) latency—the interval between falling asleep and the onset of the REM sleep—is short in narcolepsy, and since the REM stage is when dreaming occurs, naps often include dreaming. Therefore, when taking a history, it is worthwhile to ask patients whether they dream during naps; a yes answer supports the diagnosis of narcolepsy.5
In children, sleepiness can manifest in reduced concentration and behavioral issues.6 Napping after age 5 or 6 is considered abnormal and may reflect pathologic sleepiness.7
Cataplexy
Cataplexy—transient muscle weakness triggered by emotion—is a specific feature of narcolepsy type 1. It often begins in the facial muscles and can manifest with slackening of the jaw or brief dropping of the head. However, episodes can be more dramatic and, if the trunk and limb muscles are affected, can result in collapsing to the ground.
Cataplexy usually has its onset at about the same time as the sleepiness associated with narcolepsy, but it can arise even years later.8 Episodes can last from a few seconds to 2 minutes. Consciousness is always preserved. A range of emotions can trigger cataplexy, but typically the emotion is a positive one such as laughter or excitement.9 Deep tendon reflexes disappear in cataplexy, so checking reflexes during a witnessed episode can be clinically valuable.2
Cataplexy can worsen with stress and insufficient sleep, occasionally with “status cataplecticus,” in which repeated, persistent episodes of cataplexy occur over several hours.8 Status cataplecticus can be spontaneous or an effect of withdrawal from anticataplectic medications.2
Cataplexy is thought to represent intrusion of REM sleep and its associated muscle atonia during wakefulness.
Sleep paralysis, hallucinations
Sleep paralysis and hallucinations are other features of narcolepsy that reflect this REM dissociation from sleep.
Sleep paralysis occurs most commonly upon awakening, but sometimes just before sleep onset. In most cases, it is manifested by inability to move the limbs or speak, lasting several seconds or, in rare cases, minutes at a time. Sleep paralysis can be associated with a sensation of fear or suffocation, especially when initially experienced.8
Hypnopompic hallucinations, occurring upon awakening, are more common than hypnagogic hallucinations, which are experienced before falling asleep. The hallucinations are often vivid and usually visual, although other types of hallucinations are possible. Unlike those that occur in psychotic disorders, the hallucinations tend to be associated with preserved insight that they are not real.10
Notably, both sleep paralysis and hallucinations are nonspecific symptoms that are common in the general population.8,11,12
Fragmented sleep
Although they are very sleepy, people with narcolepsy generally cannot stay asleep for very long. Their sleep tends to be extremely fragmented, and they often wake up several times a night.2
This sleep pattern reflects the inherent instability of sleep-wake transitions in narcolepsy. In fact, over a 24-hour period, adults with narcolepsy have a normal amount of sleep.13 In children, however, when narcolepsy first arises, the 24-hour sleep time can increase abruptly and can sometimes be associated with persistent cataplexy that can manifest as a clumsy gait.14
Weight gain, obstructive sleep apnea
Weight gain is common, particularly after symptom onset, and especially in children. As a result, obesity is a frequent comorbidity.15 Because obstructive sleep apnea can consequently develop, all patients with narcolepsy require screening for sleep-disordered breathing.
Other sleep disorders often accompany narcolepsy and are more common than in the general population.16 In a study incorporating both clinical and polysomnographic data of 100 patients with narcolepsy, insomnia was the most common comorbid sleep disorder, with a prevalence of 28%; others were REM sleep behavior disorder (24%), restless legs syndrome (24%), obstructive sleep apnea (21%), and non-REM parasomnias.17
PSYCHOSOCIAL CONSEQUENCES
Narcolepsy has significant psychosocial consequences. As a result of their symptoms, people with narcolepsy may not be able to meet academic or work-related demands.
Additionally, their risk of a motor vehicle accident is 3 to 4 times higher than in the general population, and more than one-third of patients have been in an accident due to sleepiness.18 There is some evidence to show that treatment eliminates this risk.19
Few systematic studies have examined mood disorders in narcolepsy. However, studies tend to show a higher prevalence of psychiatric disorders than in the general population, with depression and anxiety the most com-mon.20,21
DIAGNOSIS IS OFTEN DELAYED
The prevalence of narcolepsy type 1 is between 25 and 100 per 100,000 people.22 In a Mayo Clinic study,23 the incidence of narcolepsy type 1 was estimated to be 0.74 per 100,000 person-years. Epidemiologic data on narcolepsy type 2 are sparse, but patients with narcolepsy without cataplexy are thought to represent only 36% of all narcolepsy patients.23
Diagnosis is often delayed, with the average time between the onset of symptoms and the diagnosis ranging from 8 to 22 years. With increasing awareness, the efficiency of the diagnostic process is improving, and this delay is expected to lessen accordingly.24
Symptoms most commonly arise in the second decade; but the age at onset ranges significantly, between the first and fifth decades. Narcolepsy has a bimodal distribution in incidence, with the biggest peak at approximately age 15 and second smaller peak in the mid-30s. Some studies have suggested a slight male predominance.23,25
DIAGNOSIS
History is key
The history should include specific questions about the hallmark features of narcolepsy, including cataplexy, sleep paralysis, and sleep-related hallucinations. For individual assessment of subjective sleepiness, the Epworth Sleepiness Scale or Pediatric Daytime Sleepiness Scale can be administered quickly in the office setting.26,27
The Epworth score is calculated from the self-rated likelihood of falling asleep in 8 different situations, with possible scores of 0 (would never doze) to 3 (high chance of dozing) on each question, for a total possible score of 0 to 24. Normal total scores are between 0 and 10, while scores greater than 10 reflect pathologic sleepiness. Scores on the Epworth Sleepiness Scale in those with narcolepsy tend to reflect moderate to severe sleepiness, or at least 13, as opposed to patients with obstructive sleep apnea, whose scores commonly reflect milder sleepiness.28
Testing with actigraphy and polysomnography
It is imperative to rule out insufficient sleep and other sleep disorders as a cause of daytime sleepiness. This can be done with a careful clinical history, actigraphy with sleep logs, and polysomnography.
In the 2 to 4 weeks before actigraphy and subsequent testing, all medications with alerting or sedating properties (including antidepressants) should be tapered off to prevent influence on the results of the study.
Delayed sleep-phase disorder presents at a similar age as narcolepsy and can be associated with similar degrees of sleepiness. However, individuals with delayed sleep phase disorder have an inappropriately timed sleep-wake cycle so that there is a shift in their desired sleep onset and awakening times. It is common—prevalence estimates vary but average about 1% in the general population.29
Insufficient sleep syndrome is even more common, especially in teenagers and young adults, with increasing family, social, and academic demands. Sleep needs vary across the life span. A teenager needs 8 to 10 hours of sleep per night, and a young adult needs 7 to 9 hours. A study of 1,285 high school students found that 10.4% were not getting enough sleep.30
If actigraphy data suggest a circadian rhythm disorder or insufficient sleep that could explain the symptoms of sleepiness, then further testing should be halted and these specific issues should be addressed. In these cases, working with the patient toward maintaining a regular sleep-wake schedule with 7 to 8 hours of nightly sleep will often resolve symptoms.
If actigraphy demonstrates the patient is maintaining a regular sleep schedule and allowing adequate time for nightly sleep, the next step is polysomnography.
Polysomnography is performed to detect other disorders that can disrupt sleep, such as sleep-disordered breathing or periodic limb movement disorder.2,5 In addition, polysomnography can provide assurance that adequate sleep was obtained prior to the next step in testing.
Multiple sleep latency test
If sufficient sleep is obtained on polysomnograpy (at least 6 hours for an adult) and no other sleep disorder is identified, a multiple sleep latency test is performed. A urine toxicology screen is typically performed on the day of the test to ensure that drugs are not affecting the results.
The multiple sleep latency test consists of 4 to 5 nap opportunities at 2-hour intervals in a quiet dark room conducive to sleep, during which both sleep and REM latency are recorded. The sleep latency of those with narcolepsy is significantly shortened, and the diagnosis of narcolepsy requires an average sleep latency of less than 8 minutes.
Given the propensity for REM sleep in narcolepsy, another essential feature for diagnosis is the sleep-onset REM period (SOREMP). A SOREMP is defined as a REM latency of less than 15 minutes. A diagnosis of narcolepsy re-quires a SOREMP in at least 2 of the naps in a multiple sleep latency test (or 1 nap if the shortened REM latency is seen during polysomnography).31
The multiple sleep latency test has an imperfect sensitivity, though, and should be repeated when there is a high suspicion of narcolepsy.32–34 It is not completely specific either, and false-positive results occur. In fact, SOREMPs can be seen in the general population, particularly in those with a circadian rhythm disorder, insufficient sleep, or sleep-disordered breathing. Two or more SOREMPs in an multiple sleep latency test can be seen in a small proportion of the general population.35 The results of a multiple sleep latency test should be interpreted in the clinical context.
Differential diagnosis
Narcolepsy type 1 is distinguished from type 2 by the presence of cataplexy. A cerebrospinal fluid hypocretin 1 level of 110 pg/mL or less, or less than one-third of the mean value obtained in normal individuals, can substitute for the multiple sleep latency test in diagnosing narcolepsy type 1.31 Currently, hypocretin testing is generally not performed in clinical practice, although it may become a routine part of the narcolepsy evaluation in the future.
Thus, according to the International Classification of Sleep Disorders, 3rd edition,31 the diagnosis of narcolepsy type 1 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder, and at least 1 of the following:
- Cataplexy and mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing (1 of which can be on the preceding night’s polysomography)
- Cerebrospinal fluid hypocretin 1 levels less than 110 pg/mL or one-third the baseline normal levels and mean sleep latency ≤ 8 minutes with ≥ 2 SOREMPs on multiple sleep latency testing.
Similarly, the diagnosis of narcolepsy type 2 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder, plus:
- Mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing.
Idiopathic hypersomnia, another disorder of central hypersomnolence, is also characterized by disabling sleepiness. It is diagnostically differentiated from narcolepsy, as there are fewer than 2 SOREMPs. As opposed to narcolepsy, in which naps tend to be refreshing, even prolonged naps in idiopathic hypersomnia are often not helpful in restoring wakefulness. In idiopathic hypersomnia, sleep is usually not fragmented, and there are few nocturnal arousals. Sleep times can often be prolonged as well, whereas in narcolepsy total sleep time through the day may not be increased but is not consolidated.
Kleine-Levin syndrome is a rarer disorder of hypersomnia. It is episodic compared with the relatively persistent sleepiness in narcolepsy and idiopathic hypersomnia. Periods of hypersomnia occur intermittently for days to weeks and are accompanied by cognitive and behavioral changes including hyperphagia and hypersexuality.4
LINKED TO HYPOCRETIN DEFICIENCY
Over the past 2 decades, the underlying pathophysiology of narcolepsy type 1 has been better characterized.
Narcolepsy type 1 has been linked to a deficiency in hypocretin in the central nervous system.36 Hypocretin (also known as orexin) is a hormone produced in the hypothalamus that acts on multiple brain regions and maintains alertness. For unclear reasons, hypothalamic neurons producing hypocretin are selectively reduced in narcolepsy type 1. Hypocretin also stabilizes wakefulness and inhibits REM sleep; therefore, hypocretin deficiency can lead to inappropriate intrusions of REM sleep onto wakefulness, leading to the hallmark features of narcolepsy—cataplexy, sleep-related hallucinations, and sleep paralysis.37 According to one theory, cataplexy is triggered by emotional stimuli because of a pathway between the medial prefrontal cortex and the amygdala to the pons.38
Cerebrospinal fluid levels of hypocretin in patients with narcolepsy type 2 tend to be normal, and the biologic underpinnings of narcolepsy type 2 remain mysterious. However, in the subgroup of those with narcolepsy type 2 in which hypocretin is low, many individuals go on to develop cataplexy, thereby evolving to narcolepsy type 1.36
POSSIBLE AUTOIMMUNE BASIS
Narcolepsy is typically a sporadic disorder, although familial cases have been described. The risk of a parent with narcolepsy having a child who is affected is approximately 1%.5
Narcolepsy type 1 is strongly associated with HLA-DQB1*0602, with up to 95% of those affected having at least one allele.39 Having 2 copies of the allele further increases the risk of developing narcolepsy.40 However, this allele is far from specific for narcolepsy with cataplexy, as it occurs in 12% to 38% of the general population.41 Therefore, HLA typing currently has limited clinical utility. The exact cause is as yet unknown, but substantial literature proposes an autoimmune basis of the disorder, given the strong association with the HLA subtype.42–44
After the 2009 H1N1 influenza pandemic, there was a significant increase in the incidence of narcolepsy with cataplexy, which again sparked interest in an autoimmune etiology underlying the disorder. Pandemrix, an H1N1 vaccine produced as a result of the 2009 pandemic, appeared to also be associated with an increase in the incidence of narcolepsy. An association with other upper respiratory infections has also been noted, further supporting a possible autoimmune basis.
A few studies have looked for serum autoantibodies involved in the pathogenesis of narcolepsy. Thus far, only one has been identified, an antibody to Tribbles homolog 2, found in 20% to 40% of those with new onset of nar-colepsy.42–44
TREATMENTS FOR DAYTIME SLEEPINESS
As with many chronic disorders, the treatment of narcolepsy consists of symptomatic rather than curative management, which can be done through both pharmacologic and nonpharmacologic means.
Nondrug measures
Scheduled naps lasting 15 to 20 minutes can help improve alertness.45 A consistent sleep schedule with good sleep hygiene, ensuring sufficient nightly sleep, is also important. In one study, the combination of scheduled naps and regular nocturnal sleep times reduced the level of daytime sleepiness and unintentional daytime sleep. Daytime naps were most helpful for those with the highest degree of daytime sleepiness.45
Strategic use of caffeine can be helpful and can reduce dependence on pharmacologic treatment.
Screening should be performed routinely for other sleep disorders, such as sleep-disordered breathing, which should be treated if identified.5,18 When being treated for other medical conditions, individuals with narcolepsy should avoid medications that can cause sedation, such as opiates or barbiturates; alcohol should be minimized or avoided.
Networking with other individuals with narcolepsy through support groups such as Narcolepsy Network can be valuable for learning coping skills and connecting with community resources. Psychological counseling for the patient, and sometimes the family, can also be useful. School-age children may need special accommodations such as schedule adjustments to allow for scheduled naps or frequent breaks to maintain alertness.
People with narcolepsy tend to function better in careers that do not require long periods of sitting, as sleepiness tends to be worse, but instead offer flexibility and require higher levels of activity that tend to combat sleepiness. They should not work as commercial drivers.18
Medications
While behavioral interventions in narcolepsy are vital, they are rarely sufficient, and drugs that promote daytime wakefulness are used as an adjunct (Table 2).46
Realistic expectations should be established when starting, as some degree of residual sleepiness usually remains even with optimal medical therapy. Medications should be strategically scheduled to maximize alertness during necessary times such as at work or school or during driving. Patients should specifically be counseled to avoid driving if sleepy.18,47
Modafinil is often used as a first-line therapy, given its favorable side-effect profile and low potential for abuse. Its pharmacologic action has been debated but it probably acts as a selective dopamine reuptake inhibitor. It is typically taken twice daily (upon waking and early afternoon) and is usually well tolerated.
Potential side effects include headache, nausea, dry mouth, anorexia, diarrhea, and, rarely, Stevens-Johnson syndrome. Cardiovascular side effects are minimal, making it a favorable choice in older patients.18,48
A trial in 283 patients showed significantly lower levels of sleepiness in patients taking modafinil 200 mg or 400 mg than in a control group. Other trials have supported these findings and showed improved driving performance on modafinil.18
Notably, modafinil can increase the metabolism of oral contraceptives, thereby reducing their efficacy. Women of childbearing age should be warned about this interaction and should be transitioned to nonhormonal forms of contraception.2,47
Armodafinil, a purified R-isomer of modafinil, has a longer half-life and requires only once-daily dosing.5
If modafinil or armodafinil fails to optimally manage daytime sleepiness, a traditional stimulant such as methylphenidate or an amphetamine is often used.
Methylphenidate and amphetamines primarily inhibit the reuptake and increase the release of the monoamines, mainly dopamine, and to a lesser degree serotonin and norepinephrine.
These drugs have more significant adverse effects that can involve the cardiovascular system, causing hypertension and arrhythmias. Anorexia, weight loss, and, particularly with high doses, psychosis can occur.49
These drugs should be avoided in patients with a history of significant cardiovascular disease. Before starting stimulant therapy, a thorough cardiovascular examination should be done, often including electrocardiography to ensure there is no baseline arrhythmia.
Patients on these medications should be followed closely to ensure that blood pressure, pulse, and weight are not negatively affected.18,50 Addiction and tolerance can develop with these drugs, and follow-up should include assessment for dependence. Some states may require prescription drug monitoring to ensure the drugs are not being abused or diverted.
Short- and long-acting formulations of both methylphenidate and amphetamines are available, and a long-acting form is often used in conjunction with a short-acting form as needed.18
Addiction and drug-seeking behavior can develop but are unusual in those taking stimulants to treat narcolepsy.49
Follow-up
Residual daytime sleepiness can be measured subjectively through the Epworth Sleepiness Scale on follow-up. If necessary, a maintenance-of-wakefulness test can provide an objective assessment of treatment efficacy.18
As narcolepsy is a chronic disorder, treatment should evolve with time. Most medications that treat narcolepsy are categorized by the US Food and Drug Administration as pregnancy category C, as we do not have adequate studies in human pregnancies to evaluate their effects. When a patient with narcolepsy becomes pregnant, she should be counseled about the risks and benefits of remaining on therapy. Treatment should balance the risks of sleepiness with the potential risks of remaining on medications.50 In the elderly, as cardiovascular comorbidities tend to increase, the risks and benefits of therapy should be routinely reevaluated.
For cataplexy
Sodium oxybate,51–53 the most potent anticataplectic drug, is the sodium salt of gamma hydroxybutyrate, a metabolite of gamma-aminobutyric acid. Sodium oxybate can be prescribed in the United States, Canada, and Europe. The American Academy of Sleep Medicine recommends sodium oxybate for cataplexy, daytime sleepiness, and disrupted sleep based on 3 level-1 studies and 2 level-4 studies.46
Sodium oxybate increases slow-wave sleep, improves sleep continuity, and often helps to mitigate daytime sleepiness. Due to its short half-life, its administration is unusual: the first dose is taken before bedtime and the second dose 2.5 to 4 hours later. Some patients set an alarm clock to take the second dose, while others awaken spontaneously to take the second dose. Most patients find that with adherence to dosing and safety instructions, sodium oxybate can serve as a highly effective form of treatment of both excessive sleepiness and cataplexy and may reduce the need for stimulant-based therapies.
The most common adverse effects are nausea, mood swings, and enuresis. Occasionally, psychosis can result and limit use of the drug. Obstructive sleep apnea can also develop or worsen.52 Because of its high salt content, sodium oxybate should be used with caution in those with heart failure, hypertension, or renal impairment. Its relative, gamma hydroxybutyrate, causes rapid sedation and has been notorious for illegal use as a date rape drug.
In the United States, sodium oxybate is distributed only through a central pharmacy to mitigate potential abuse. Due to this system, the rates of diversion are extremely low, estimated in a postmarketing analysis to be 1 instance per 5,200 patients treated. In the same study, abuse and dependence were both rare as well, about 1 case for every 2,600 and 6,500 patients treated.6,18,52,53
Antidepressants promote the action of norepinephrine and, to a lesser degree, serotonin, thereby suppressing REM sleep.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is often used as a first-line treatment for cataplexy. Selective serotonin reuptake inhibitors such as fluoxetine are also used with success. Tricyclic antidepressants such as protriptyline or clomipramine are extremely effective for cataplexy, but are rarely used due to their adverse effects.2,47
FUTURE WORK
While our understanding of narcolepsy has advanced, there are still gaps in our knowledge of the disorder—namely, the specific trigger for the loss of hypocretin neurons in type 1 narcolepsy and the underlying pathophysiology of type 2.
A number of emerging therapies target the hypocretin system, including peptide replacement, neuronal transplant, and immunotherapy preventing hypocretin neuronal cell death.50,54,55 Additional drugs designed to improve alertness that do not involve the hypocretin system are also being developed, including a histamine inverse agonist.50,56 Sodium oxybate and modafinil, although currently approved for use in adults, are still off-label in pediatric practice. Studies of the safety and efficacy of these medications in children are needed.7,57
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux Civils et Militaires 1880; part a, 53:626–628, part b, 54:635–637.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
- Scammell TE. Clinical features and diagnosis of narcolepsy in adults. In: Eichler AF, ed. UpToDate. Waltham, MA: UpToDate; 2018. www.uptodate.com. Accessed October 31, 2018.
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004; 5(1):37–41. pmid:14725825
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373(27):2654–2662. doi:10.1056/NEJMra1500587
- Babiker MO, Prasad M. Narcolepsy in children: a diagnostic and management approach. Pediatr Neurol 2015; 52(6):557–565. doi:10.1016/j.pediatrneurol.2015.02.020
- Kotagal S. Narcolepsy in children. In: UpToDate, Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003; 53(2):154–166. doi:10.1002/ana.10444
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med 2011; 12(1):12–18. doi:10.1016/j.sleep.2010.05.010
- Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 2015; 71(4):218–224. doi:10.1159/000432400
- Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97(2-3):153–164. pmid:11166087
- Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev 2011;5(5):311–315. doi:10.1016/j.smrv.2011.01.007
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol 1988; 70(6):473–481. pmid:2461281
- Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain 2013; 136(pt 12):3787–3795. doi:10.1093/brain/awt277
- Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med 2004; 5(2):147–150. doi:10.1016/j.sleep.2003.10.006
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013; 70(1-2):22–26. doi:10.1159/000348719
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med 2013; 9(8):805–812. doi:10.5664/jcsm.2926
- Scammell TE. Treatment of narcolepsy in adults. In: Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015; 10(6):e0129386. doi:10.1371/journal.pone.0129386
- Fortuyn HD, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 2010; 32(1):49–56. doi:10.1016/j.genhosppsych.2009.08.007
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry 2017; 78(2):171–176. doi:10.4088/JCP.15m10262
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007; 30(1):13–26. pmid:17310860
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002; 25(2):197–202. pmid:11902429
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014; 15(5):502–507. doi:10.1016/j.sleep.2014.01.015
- Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57(11):2029–2033. pmid:11739821
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6):540–545. pmid:1798888
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep 2003; 26(4):455–458. pmid:12841372
- van der Heide A, van Schie MK, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 2015; 38(7):1051–1058. doi:10.5665/sleep.4810
- Nesbitt AD. Delayed sleep-wake phase disorder. J Thorac Dis 2018; 10(suppl 1):S103–S111. doi:10.21037/jtd.2018.01.11
- Pallesen S, Saxvig IW, Molde H, Sørensen E, Wilhelmsen-Langeland A, Bjorvatn B. Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc 2011; 34(2):391–395. doi:10.1016/j.adolescence.2010.02.005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Disorders; 2014.
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013; 9(8):789–795. doi:10.5664/jcsm.2922
- Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol 2013; 70(7):891–902. doi:10.1001/jamaneurol.2013.1589
- Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep 2015; 38(10):1575–1581. doi:10.5665/sleep.5050
- Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain 2006; 129(6):1609–1623. doi:10.1093/brain/awl079
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197):39–40. doi:10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6(9):991–997. doi:10.1038/79690
- Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33(23):9743–9751. doi:10.1523/JNEUROSCI.0499-13.2013
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients.. Sleep 1997; 20(11):1012–1020. pmid:9456467
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51(1):96–100. pmid:9459509
- Akintomide GS, Rickards H. Narcolepsy: a review. Neuropsychiatr Dis Treat 2011; 7(1):507–518. doi:10.2147/NDT.S23624
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol 2013; 23(5):767–773. doi:10.1016/j.conb.2013.04.013
- Degn M, Kornum BR. Type 1 narcolepsy: a CD8(+) T cell-mediated disease? Ann N Y Acad Sci 2015;1 351:80–88. doi:10.1111/nyas.12793
- Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14(3):318–328. doi:10.1016/S1474-4422(14)70218-2
- Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001; 24(4):385–391. pmid:11403522
- Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007; 30(12):1705–1711. pmid:18246980
- Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 2012; 9(4):739–752. doi:10.1007/s13311-012-0150-9
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007; 3(6):595–602. pmid:17993041
- Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep 2005; 28(6):667–672. pmid:16477952
- Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep 2017; 9:39–57. doi:10.2147/NSS.S103467
- Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med 2017; 35:80–84. doi:10.1016/j.sleep.2017.03.028
- Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy–cataplexy: a retrospective study. Sleep Med 2012; 13(6):606–610. doi:10.1016/j.sleep.2011.10.032
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 2009; 5(4):365–371. pmid:19968016
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262:8–13. doi:10.1016/j.bbr.2013.12.045
- Arias-Carrión O, Murillo-Rodriguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9(4):e95342. doi:10.1371/journal.pone.0095342
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014; 15(6):681–687. doi:10.1016/j.sleep.2014.01.021
- Lecendreux M, Bruni O, Franco P, et al. Clinical experience suggests that modafinil is an effective and safe treatment for paediatric narcolepsy. J Sleep Res 2012; 21(4):481–483. doi:10.1111/j.1365-2869.2011.00991.x
Narcolepsy was originally described in the late 1800s by the French physician Jean-Baptiste-Edouard Gélineau, who reported the case of a wine merchant suffering from somnolence. In this first description, he coined the term narcolepsie by joining the Greek words narke (numbness or stupor) and lepsis (attack).1
Since then, the disorder has been further characterized, and some insight into its biological underpinnings has been established. Importantly, treatments have improved and expanded, facilitating its management and thereby improving quality of life for those with the disorder.
This review focuses on clinically relevant features of the disorder and proposes management strategies.
CLINICAL FEATURES
Narcolepsy is characterized by instability of sleep-wake transitions.
Daytime sleepiness
Clinically, narcolepsy manifests with excessive daytime sleepiness that can be personally and socially disabling. Cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations can also be present,2,3 but they are not necessary for diagnosis. In fact, a minority of patients with narcolepsy have all these symptoms.4 Narcolepsy is divided into type 1 (with cataplexy) and type 2 (without cataplexy).2
Sleepiness tends to be worse with inactivity, and sleep can often be irresistible. Sleep attacks can come on suddenly and may be brief enough to manifest as a lapse in consciousness.
Short naps tend to be refreshing. Rapid eye movement (REM) latency—the interval between falling asleep and the onset of the REM sleep—is short in narcolepsy, and since the REM stage is when dreaming occurs, naps often include dreaming. Therefore, when taking a history, it is worthwhile to ask patients whether they dream during naps; a yes answer supports the diagnosis of narcolepsy.5
In children, sleepiness can manifest in reduced concentration and behavioral issues.6 Napping after age 5 or 6 is considered abnormal and may reflect pathologic sleepiness.7
Cataplexy
Cataplexy—transient muscle weakness triggered by emotion—is a specific feature of narcolepsy type 1. It often begins in the facial muscles and can manifest with slackening of the jaw or brief dropping of the head. However, episodes can be more dramatic and, if the trunk and limb muscles are affected, can result in collapsing to the ground.
Cataplexy usually has its onset at about the same time as the sleepiness associated with narcolepsy, but it can arise even years later.8 Episodes can last from a few seconds to 2 minutes. Consciousness is always preserved. A range of emotions can trigger cataplexy, but typically the emotion is a positive one such as laughter or excitement.9 Deep tendon reflexes disappear in cataplexy, so checking reflexes during a witnessed episode can be clinically valuable.2
Cataplexy can worsen with stress and insufficient sleep, occasionally with “status cataplecticus,” in which repeated, persistent episodes of cataplexy occur over several hours.8 Status cataplecticus can be spontaneous or an effect of withdrawal from anticataplectic medications.2
Cataplexy is thought to represent intrusion of REM sleep and its associated muscle atonia during wakefulness.
Sleep paralysis, hallucinations
Sleep paralysis and hallucinations are other features of narcolepsy that reflect this REM dissociation from sleep.
Sleep paralysis occurs most commonly upon awakening, but sometimes just before sleep onset. In most cases, it is manifested by inability to move the limbs or speak, lasting several seconds or, in rare cases, minutes at a time. Sleep paralysis can be associated with a sensation of fear or suffocation, especially when initially experienced.8
Hypnopompic hallucinations, occurring upon awakening, are more common than hypnagogic hallucinations, which are experienced before falling asleep. The hallucinations are often vivid and usually visual, although other types of hallucinations are possible. Unlike those that occur in psychotic disorders, the hallucinations tend to be associated with preserved insight that they are not real.10
Notably, both sleep paralysis and hallucinations are nonspecific symptoms that are common in the general population.8,11,12
Fragmented sleep
Although they are very sleepy, people with narcolepsy generally cannot stay asleep for very long. Their sleep tends to be extremely fragmented, and they often wake up several times a night.2
This sleep pattern reflects the inherent instability of sleep-wake transitions in narcolepsy. In fact, over a 24-hour period, adults with narcolepsy have a normal amount of sleep.13 In children, however, when narcolepsy first arises, the 24-hour sleep time can increase abruptly and can sometimes be associated with persistent cataplexy that can manifest as a clumsy gait.14
Weight gain, obstructive sleep apnea
Weight gain is common, particularly after symptom onset, and especially in children. As a result, obesity is a frequent comorbidity.15 Because obstructive sleep apnea can consequently develop, all patients with narcolepsy require screening for sleep-disordered breathing.
Other sleep disorders often accompany narcolepsy and are more common than in the general population.16 In a study incorporating both clinical and polysomnographic data of 100 patients with narcolepsy, insomnia was the most common comorbid sleep disorder, with a prevalence of 28%; others were REM sleep behavior disorder (24%), restless legs syndrome (24%), obstructive sleep apnea (21%), and non-REM parasomnias.17
PSYCHOSOCIAL CONSEQUENCES
Narcolepsy has significant psychosocial consequences. As a result of their symptoms, people with narcolepsy may not be able to meet academic or work-related demands.
Additionally, their risk of a motor vehicle accident is 3 to 4 times higher than in the general population, and more than one-third of patients have been in an accident due to sleepiness.18 There is some evidence to show that treatment eliminates this risk.19
Few systematic studies have examined mood disorders in narcolepsy. However, studies tend to show a higher prevalence of psychiatric disorders than in the general population, with depression and anxiety the most com-mon.20,21
DIAGNOSIS IS OFTEN DELAYED
The prevalence of narcolepsy type 1 is between 25 and 100 per 100,000 people.22 In a Mayo Clinic study,23 the incidence of narcolepsy type 1 was estimated to be 0.74 per 100,000 person-years. Epidemiologic data on narcolepsy type 2 are sparse, but patients with narcolepsy without cataplexy are thought to represent only 36% of all narcolepsy patients.23
Diagnosis is often delayed, with the average time between the onset of symptoms and the diagnosis ranging from 8 to 22 years. With increasing awareness, the efficiency of the diagnostic process is improving, and this delay is expected to lessen accordingly.24
Symptoms most commonly arise in the second decade; but the age at onset ranges significantly, between the first and fifth decades. Narcolepsy has a bimodal distribution in incidence, with the biggest peak at approximately age 15 and second smaller peak in the mid-30s. Some studies have suggested a slight male predominance.23,25
DIAGNOSIS
History is key
The history should include specific questions about the hallmark features of narcolepsy, including cataplexy, sleep paralysis, and sleep-related hallucinations. For individual assessment of subjective sleepiness, the Epworth Sleepiness Scale or Pediatric Daytime Sleepiness Scale can be administered quickly in the office setting.26,27
The Epworth score is calculated from the self-rated likelihood of falling asleep in 8 different situations, with possible scores of 0 (would never doze) to 3 (high chance of dozing) on each question, for a total possible score of 0 to 24. Normal total scores are between 0 and 10, while scores greater than 10 reflect pathologic sleepiness. Scores on the Epworth Sleepiness Scale in those with narcolepsy tend to reflect moderate to severe sleepiness, or at least 13, as opposed to patients with obstructive sleep apnea, whose scores commonly reflect milder sleepiness.28
Testing with actigraphy and polysomnography
It is imperative to rule out insufficient sleep and other sleep disorders as a cause of daytime sleepiness. This can be done with a careful clinical history, actigraphy with sleep logs, and polysomnography.
In the 2 to 4 weeks before actigraphy and subsequent testing, all medications with alerting or sedating properties (including antidepressants) should be tapered off to prevent influence on the results of the study.
Delayed sleep-phase disorder presents at a similar age as narcolepsy and can be associated with similar degrees of sleepiness. However, individuals with delayed sleep phase disorder have an inappropriately timed sleep-wake cycle so that there is a shift in their desired sleep onset and awakening times. It is common—prevalence estimates vary but average about 1% in the general population.29
Insufficient sleep syndrome is even more common, especially in teenagers and young adults, with increasing family, social, and academic demands. Sleep needs vary across the life span. A teenager needs 8 to 10 hours of sleep per night, and a young adult needs 7 to 9 hours. A study of 1,285 high school students found that 10.4% were not getting enough sleep.30
If actigraphy data suggest a circadian rhythm disorder or insufficient sleep that could explain the symptoms of sleepiness, then further testing should be halted and these specific issues should be addressed. In these cases, working with the patient toward maintaining a regular sleep-wake schedule with 7 to 8 hours of nightly sleep will often resolve symptoms.
If actigraphy demonstrates the patient is maintaining a regular sleep schedule and allowing adequate time for nightly sleep, the next step is polysomnography.
Polysomnography is performed to detect other disorders that can disrupt sleep, such as sleep-disordered breathing or periodic limb movement disorder.2,5 In addition, polysomnography can provide assurance that adequate sleep was obtained prior to the next step in testing.
Multiple sleep latency test
If sufficient sleep is obtained on polysomnograpy (at least 6 hours for an adult) and no other sleep disorder is identified, a multiple sleep latency test is performed. A urine toxicology screen is typically performed on the day of the test to ensure that drugs are not affecting the results.
The multiple sleep latency test consists of 4 to 5 nap opportunities at 2-hour intervals in a quiet dark room conducive to sleep, during which both sleep and REM latency are recorded. The sleep latency of those with narcolepsy is significantly shortened, and the diagnosis of narcolepsy requires an average sleep latency of less than 8 minutes.
Given the propensity for REM sleep in narcolepsy, another essential feature for diagnosis is the sleep-onset REM period (SOREMP). A SOREMP is defined as a REM latency of less than 15 minutes. A diagnosis of narcolepsy re-quires a SOREMP in at least 2 of the naps in a multiple sleep latency test (or 1 nap if the shortened REM latency is seen during polysomnography).31
The multiple sleep latency test has an imperfect sensitivity, though, and should be repeated when there is a high suspicion of narcolepsy.32–34 It is not completely specific either, and false-positive results occur. In fact, SOREMPs can be seen in the general population, particularly in those with a circadian rhythm disorder, insufficient sleep, or sleep-disordered breathing. Two or more SOREMPs in an multiple sleep latency test can be seen in a small proportion of the general population.35 The results of a multiple sleep latency test should be interpreted in the clinical context.
Differential diagnosis
Narcolepsy type 1 is distinguished from type 2 by the presence of cataplexy. A cerebrospinal fluid hypocretin 1 level of 110 pg/mL or less, or less than one-third of the mean value obtained in normal individuals, can substitute for the multiple sleep latency test in diagnosing narcolepsy type 1.31 Currently, hypocretin testing is generally not performed in clinical practice, although it may become a routine part of the narcolepsy evaluation in the future.
Thus, according to the International Classification of Sleep Disorders, 3rd edition,31 the diagnosis of narcolepsy type 1 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder, and at least 1 of the following:
- Cataplexy and mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing (1 of which can be on the preceding night’s polysomography)
- Cerebrospinal fluid hypocretin 1 levels less than 110 pg/mL or one-third the baseline normal levels and mean sleep latency ≤ 8 minutes with ≥ 2 SOREMPs on multiple sleep latency testing.
Similarly, the diagnosis of narcolepsy type 2 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder, plus:
- Mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing.
Idiopathic hypersomnia, another disorder of central hypersomnolence, is also characterized by disabling sleepiness. It is diagnostically differentiated from narcolepsy, as there are fewer than 2 SOREMPs. As opposed to narcolepsy, in which naps tend to be refreshing, even prolonged naps in idiopathic hypersomnia are often not helpful in restoring wakefulness. In idiopathic hypersomnia, sleep is usually not fragmented, and there are few nocturnal arousals. Sleep times can often be prolonged as well, whereas in narcolepsy total sleep time through the day may not be increased but is not consolidated.
Kleine-Levin syndrome is a rarer disorder of hypersomnia. It is episodic compared with the relatively persistent sleepiness in narcolepsy and idiopathic hypersomnia. Periods of hypersomnia occur intermittently for days to weeks and are accompanied by cognitive and behavioral changes including hyperphagia and hypersexuality.4
LINKED TO HYPOCRETIN DEFICIENCY
Over the past 2 decades, the underlying pathophysiology of narcolepsy type 1 has been better characterized.
Narcolepsy type 1 has been linked to a deficiency in hypocretin in the central nervous system.36 Hypocretin (also known as orexin) is a hormone produced in the hypothalamus that acts on multiple brain regions and maintains alertness. For unclear reasons, hypothalamic neurons producing hypocretin are selectively reduced in narcolepsy type 1. Hypocretin also stabilizes wakefulness and inhibits REM sleep; therefore, hypocretin deficiency can lead to inappropriate intrusions of REM sleep onto wakefulness, leading to the hallmark features of narcolepsy—cataplexy, sleep-related hallucinations, and sleep paralysis.37 According to one theory, cataplexy is triggered by emotional stimuli because of a pathway between the medial prefrontal cortex and the amygdala to the pons.38
Cerebrospinal fluid levels of hypocretin in patients with narcolepsy type 2 tend to be normal, and the biologic underpinnings of narcolepsy type 2 remain mysterious. However, in the subgroup of those with narcolepsy type 2 in which hypocretin is low, many individuals go on to develop cataplexy, thereby evolving to narcolepsy type 1.36
POSSIBLE AUTOIMMUNE BASIS
Narcolepsy is typically a sporadic disorder, although familial cases have been described. The risk of a parent with narcolepsy having a child who is affected is approximately 1%.5
Narcolepsy type 1 is strongly associated with HLA-DQB1*0602, with up to 95% of those affected having at least one allele.39 Having 2 copies of the allele further increases the risk of developing narcolepsy.40 However, this allele is far from specific for narcolepsy with cataplexy, as it occurs in 12% to 38% of the general population.41 Therefore, HLA typing currently has limited clinical utility. The exact cause is as yet unknown, but substantial literature proposes an autoimmune basis of the disorder, given the strong association with the HLA subtype.42–44
After the 2009 H1N1 influenza pandemic, there was a significant increase in the incidence of narcolepsy with cataplexy, which again sparked interest in an autoimmune etiology underlying the disorder. Pandemrix, an H1N1 vaccine produced as a result of the 2009 pandemic, appeared to also be associated with an increase in the incidence of narcolepsy. An association with other upper respiratory infections has also been noted, further supporting a possible autoimmune basis.
A few studies have looked for serum autoantibodies involved in the pathogenesis of narcolepsy. Thus far, only one has been identified, an antibody to Tribbles homolog 2, found in 20% to 40% of those with new onset of nar-colepsy.42–44
TREATMENTS FOR DAYTIME SLEEPINESS
As with many chronic disorders, the treatment of narcolepsy consists of symptomatic rather than curative management, which can be done through both pharmacologic and nonpharmacologic means.
Nondrug measures
Scheduled naps lasting 15 to 20 minutes can help improve alertness.45 A consistent sleep schedule with good sleep hygiene, ensuring sufficient nightly sleep, is also important. In one study, the combination of scheduled naps and regular nocturnal sleep times reduced the level of daytime sleepiness and unintentional daytime sleep. Daytime naps were most helpful for those with the highest degree of daytime sleepiness.45
Strategic use of caffeine can be helpful and can reduce dependence on pharmacologic treatment.
Screening should be performed routinely for other sleep disorders, such as sleep-disordered breathing, which should be treated if identified.5,18 When being treated for other medical conditions, individuals with narcolepsy should avoid medications that can cause sedation, such as opiates or barbiturates; alcohol should be minimized or avoided.
Networking with other individuals with narcolepsy through support groups such as Narcolepsy Network can be valuable for learning coping skills and connecting with community resources. Psychological counseling for the patient, and sometimes the family, can also be useful. School-age children may need special accommodations such as schedule adjustments to allow for scheduled naps or frequent breaks to maintain alertness.
People with narcolepsy tend to function better in careers that do not require long periods of sitting, as sleepiness tends to be worse, but instead offer flexibility and require higher levels of activity that tend to combat sleepiness. They should not work as commercial drivers.18
Medications
While behavioral interventions in narcolepsy are vital, they are rarely sufficient, and drugs that promote daytime wakefulness are used as an adjunct (Table 2).46
Realistic expectations should be established when starting, as some degree of residual sleepiness usually remains even with optimal medical therapy. Medications should be strategically scheduled to maximize alertness during necessary times such as at work or school or during driving. Patients should specifically be counseled to avoid driving if sleepy.18,47
Modafinil is often used as a first-line therapy, given its favorable side-effect profile and low potential for abuse. Its pharmacologic action has been debated but it probably acts as a selective dopamine reuptake inhibitor. It is typically taken twice daily (upon waking and early afternoon) and is usually well tolerated.
Potential side effects include headache, nausea, dry mouth, anorexia, diarrhea, and, rarely, Stevens-Johnson syndrome. Cardiovascular side effects are minimal, making it a favorable choice in older patients.18,48
A trial in 283 patients showed significantly lower levels of sleepiness in patients taking modafinil 200 mg or 400 mg than in a control group. Other trials have supported these findings and showed improved driving performance on modafinil.18
Notably, modafinil can increase the metabolism of oral contraceptives, thereby reducing their efficacy. Women of childbearing age should be warned about this interaction and should be transitioned to nonhormonal forms of contraception.2,47
Armodafinil, a purified R-isomer of modafinil, has a longer half-life and requires only once-daily dosing.5
If modafinil or armodafinil fails to optimally manage daytime sleepiness, a traditional stimulant such as methylphenidate or an amphetamine is often used.
Methylphenidate and amphetamines primarily inhibit the reuptake and increase the release of the monoamines, mainly dopamine, and to a lesser degree serotonin and norepinephrine.
These drugs have more significant adverse effects that can involve the cardiovascular system, causing hypertension and arrhythmias. Anorexia, weight loss, and, particularly with high doses, psychosis can occur.49
These drugs should be avoided in patients with a history of significant cardiovascular disease. Before starting stimulant therapy, a thorough cardiovascular examination should be done, often including electrocardiography to ensure there is no baseline arrhythmia.
Patients on these medications should be followed closely to ensure that blood pressure, pulse, and weight are not negatively affected.18,50 Addiction and tolerance can develop with these drugs, and follow-up should include assessment for dependence. Some states may require prescription drug monitoring to ensure the drugs are not being abused or diverted.
Short- and long-acting formulations of both methylphenidate and amphetamines are available, and a long-acting form is often used in conjunction with a short-acting form as needed.18
Addiction and drug-seeking behavior can develop but are unusual in those taking stimulants to treat narcolepsy.49
Follow-up
Residual daytime sleepiness can be measured subjectively through the Epworth Sleepiness Scale on follow-up. If necessary, a maintenance-of-wakefulness test can provide an objective assessment of treatment efficacy.18
As narcolepsy is a chronic disorder, treatment should evolve with time. Most medications that treat narcolepsy are categorized by the US Food and Drug Administration as pregnancy category C, as we do not have adequate studies in human pregnancies to evaluate their effects. When a patient with narcolepsy becomes pregnant, she should be counseled about the risks and benefits of remaining on therapy. Treatment should balance the risks of sleepiness with the potential risks of remaining on medications.50 In the elderly, as cardiovascular comorbidities tend to increase, the risks and benefits of therapy should be routinely reevaluated.
For cataplexy
Sodium oxybate,51–53 the most potent anticataplectic drug, is the sodium salt of gamma hydroxybutyrate, a metabolite of gamma-aminobutyric acid. Sodium oxybate can be prescribed in the United States, Canada, and Europe. The American Academy of Sleep Medicine recommends sodium oxybate for cataplexy, daytime sleepiness, and disrupted sleep based on 3 level-1 studies and 2 level-4 studies.46
Sodium oxybate increases slow-wave sleep, improves sleep continuity, and often helps to mitigate daytime sleepiness. Due to its short half-life, its administration is unusual: the first dose is taken before bedtime and the second dose 2.5 to 4 hours later. Some patients set an alarm clock to take the second dose, while others awaken spontaneously to take the second dose. Most patients find that with adherence to dosing and safety instructions, sodium oxybate can serve as a highly effective form of treatment of both excessive sleepiness and cataplexy and may reduce the need for stimulant-based therapies.
The most common adverse effects are nausea, mood swings, and enuresis. Occasionally, psychosis can result and limit use of the drug. Obstructive sleep apnea can also develop or worsen.52 Because of its high salt content, sodium oxybate should be used with caution in those with heart failure, hypertension, or renal impairment. Its relative, gamma hydroxybutyrate, causes rapid sedation and has been notorious for illegal use as a date rape drug.
In the United States, sodium oxybate is distributed only through a central pharmacy to mitigate potential abuse. Due to this system, the rates of diversion are extremely low, estimated in a postmarketing analysis to be 1 instance per 5,200 patients treated. In the same study, abuse and dependence were both rare as well, about 1 case for every 2,600 and 6,500 patients treated.6,18,52,53
Antidepressants promote the action of norepinephrine and, to a lesser degree, serotonin, thereby suppressing REM sleep.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is often used as a first-line treatment for cataplexy. Selective serotonin reuptake inhibitors such as fluoxetine are also used with success. Tricyclic antidepressants such as protriptyline or clomipramine are extremely effective for cataplexy, but are rarely used due to their adverse effects.2,47
FUTURE WORK
While our understanding of narcolepsy has advanced, there are still gaps in our knowledge of the disorder—namely, the specific trigger for the loss of hypocretin neurons in type 1 narcolepsy and the underlying pathophysiology of type 2.
A number of emerging therapies target the hypocretin system, including peptide replacement, neuronal transplant, and immunotherapy preventing hypocretin neuronal cell death.50,54,55 Additional drugs designed to improve alertness that do not involve the hypocretin system are also being developed, including a histamine inverse agonist.50,56 Sodium oxybate and modafinil, although currently approved for use in adults, are still off-label in pediatric practice. Studies of the safety and efficacy of these medications in children are needed.7,57
Narcolepsy was originally described in the late 1800s by the French physician Jean-Baptiste-Edouard Gélineau, who reported the case of a wine merchant suffering from somnolence. In this first description, he coined the term narcolepsie by joining the Greek words narke (numbness or stupor) and lepsis (attack).1
Since then, the disorder has been further characterized, and some insight into its biological underpinnings has been established. Importantly, treatments have improved and expanded, facilitating its management and thereby improving quality of life for those with the disorder.
This review focuses on clinically relevant features of the disorder and proposes management strategies.
CLINICAL FEATURES
Narcolepsy is characterized by instability of sleep-wake transitions.
Daytime sleepiness
Clinically, narcolepsy manifests with excessive daytime sleepiness that can be personally and socially disabling. Cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations can also be present,2,3 but they are not necessary for diagnosis. In fact, a minority of patients with narcolepsy have all these symptoms.4 Narcolepsy is divided into type 1 (with cataplexy) and type 2 (without cataplexy).2
Sleepiness tends to be worse with inactivity, and sleep can often be irresistible. Sleep attacks can come on suddenly and may be brief enough to manifest as a lapse in consciousness.
Short naps tend to be refreshing. Rapid eye movement (REM) latency—the interval between falling asleep and the onset of the REM sleep—is short in narcolepsy, and since the REM stage is when dreaming occurs, naps often include dreaming. Therefore, when taking a history, it is worthwhile to ask patients whether they dream during naps; a yes answer supports the diagnosis of narcolepsy.5
In children, sleepiness can manifest in reduced concentration and behavioral issues.6 Napping after age 5 or 6 is considered abnormal and may reflect pathologic sleepiness.7
Cataplexy
Cataplexy—transient muscle weakness triggered by emotion—is a specific feature of narcolepsy type 1. It often begins in the facial muscles and can manifest with slackening of the jaw or brief dropping of the head. However, episodes can be more dramatic and, if the trunk and limb muscles are affected, can result in collapsing to the ground.
Cataplexy usually has its onset at about the same time as the sleepiness associated with narcolepsy, but it can arise even years later.8 Episodes can last from a few seconds to 2 minutes. Consciousness is always preserved. A range of emotions can trigger cataplexy, but typically the emotion is a positive one such as laughter or excitement.9 Deep tendon reflexes disappear in cataplexy, so checking reflexes during a witnessed episode can be clinically valuable.2
Cataplexy can worsen with stress and insufficient sleep, occasionally with “status cataplecticus,” in which repeated, persistent episodes of cataplexy occur over several hours.8 Status cataplecticus can be spontaneous or an effect of withdrawal from anticataplectic medications.2
Cataplexy is thought to represent intrusion of REM sleep and its associated muscle atonia during wakefulness.
Sleep paralysis, hallucinations
Sleep paralysis and hallucinations are other features of narcolepsy that reflect this REM dissociation from sleep.
Sleep paralysis occurs most commonly upon awakening, but sometimes just before sleep onset. In most cases, it is manifested by inability to move the limbs or speak, lasting several seconds or, in rare cases, minutes at a time. Sleep paralysis can be associated with a sensation of fear or suffocation, especially when initially experienced.8
Hypnopompic hallucinations, occurring upon awakening, are more common than hypnagogic hallucinations, which are experienced before falling asleep. The hallucinations are often vivid and usually visual, although other types of hallucinations are possible. Unlike those that occur in psychotic disorders, the hallucinations tend to be associated with preserved insight that they are not real.10
Notably, both sleep paralysis and hallucinations are nonspecific symptoms that are common in the general population.8,11,12
Fragmented sleep
Although they are very sleepy, people with narcolepsy generally cannot stay asleep for very long. Their sleep tends to be extremely fragmented, and they often wake up several times a night.2
This sleep pattern reflects the inherent instability of sleep-wake transitions in narcolepsy. In fact, over a 24-hour period, adults with narcolepsy have a normal amount of sleep.13 In children, however, when narcolepsy first arises, the 24-hour sleep time can increase abruptly and can sometimes be associated with persistent cataplexy that can manifest as a clumsy gait.14
Weight gain, obstructive sleep apnea
Weight gain is common, particularly after symptom onset, and especially in children. As a result, obesity is a frequent comorbidity.15 Because obstructive sleep apnea can consequently develop, all patients with narcolepsy require screening for sleep-disordered breathing.
Other sleep disorders often accompany narcolepsy and are more common than in the general population.16 In a study incorporating both clinical and polysomnographic data of 100 patients with narcolepsy, insomnia was the most common comorbid sleep disorder, with a prevalence of 28%; others were REM sleep behavior disorder (24%), restless legs syndrome (24%), obstructive sleep apnea (21%), and non-REM parasomnias.17
PSYCHOSOCIAL CONSEQUENCES
Narcolepsy has significant psychosocial consequences. As a result of their symptoms, people with narcolepsy may not be able to meet academic or work-related demands.
Additionally, their risk of a motor vehicle accident is 3 to 4 times higher than in the general population, and more than one-third of patients have been in an accident due to sleepiness.18 There is some evidence to show that treatment eliminates this risk.19
Few systematic studies have examined mood disorders in narcolepsy. However, studies tend to show a higher prevalence of psychiatric disorders than in the general population, with depression and anxiety the most com-mon.20,21
DIAGNOSIS IS OFTEN DELAYED
The prevalence of narcolepsy type 1 is between 25 and 100 per 100,000 people.22 In a Mayo Clinic study,23 the incidence of narcolepsy type 1 was estimated to be 0.74 per 100,000 person-years. Epidemiologic data on narcolepsy type 2 are sparse, but patients with narcolepsy without cataplexy are thought to represent only 36% of all narcolepsy patients.23
Diagnosis is often delayed, with the average time between the onset of symptoms and the diagnosis ranging from 8 to 22 years. With increasing awareness, the efficiency of the diagnostic process is improving, and this delay is expected to lessen accordingly.24
Symptoms most commonly arise in the second decade; but the age at onset ranges significantly, between the first and fifth decades. Narcolepsy has a bimodal distribution in incidence, with the biggest peak at approximately age 15 and second smaller peak in the mid-30s. Some studies have suggested a slight male predominance.23,25
DIAGNOSIS
History is key
The history should include specific questions about the hallmark features of narcolepsy, including cataplexy, sleep paralysis, and sleep-related hallucinations. For individual assessment of subjective sleepiness, the Epworth Sleepiness Scale or Pediatric Daytime Sleepiness Scale can be administered quickly in the office setting.26,27
The Epworth score is calculated from the self-rated likelihood of falling asleep in 8 different situations, with possible scores of 0 (would never doze) to 3 (high chance of dozing) on each question, for a total possible score of 0 to 24. Normal total scores are between 0 and 10, while scores greater than 10 reflect pathologic sleepiness. Scores on the Epworth Sleepiness Scale in those with narcolepsy tend to reflect moderate to severe sleepiness, or at least 13, as opposed to patients with obstructive sleep apnea, whose scores commonly reflect milder sleepiness.28
Testing with actigraphy and polysomnography
It is imperative to rule out insufficient sleep and other sleep disorders as a cause of daytime sleepiness. This can be done with a careful clinical history, actigraphy with sleep logs, and polysomnography.
In the 2 to 4 weeks before actigraphy and subsequent testing, all medications with alerting or sedating properties (including antidepressants) should be tapered off to prevent influence on the results of the study.
Delayed sleep-phase disorder presents at a similar age as narcolepsy and can be associated with similar degrees of sleepiness. However, individuals with delayed sleep phase disorder have an inappropriately timed sleep-wake cycle so that there is a shift in their desired sleep onset and awakening times. It is common—prevalence estimates vary but average about 1% in the general population.29
Insufficient sleep syndrome is even more common, especially in teenagers and young adults, with increasing family, social, and academic demands. Sleep needs vary across the life span. A teenager needs 8 to 10 hours of sleep per night, and a young adult needs 7 to 9 hours. A study of 1,285 high school students found that 10.4% were not getting enough sleep.30
If actigraphy data suggest a circadian rhythm disorder or insufficient sleep that could explain the symptoms of sleepiness, then further testing should be halted and these specific issues should be addressed. In these cases, working with the patient toward maintaining a regular sleep-wake schedule with 7 to 8 hours of nightly sleep will often resolve symptoms.
If actigraphy demonstrates the patient is maintaining a regular sleep schedule and allowing adequate time for nightly sleep, the next step is polysomnography.
Polysomnography is performed to detect other disorders that can disrupt sleep, such as sleep-disordered breathing or periodic limb movement disorder.2,5 In addition, polysomnography can provide assurance that adequate sleep was obtained prior to the next step in testing.
Multiple sleep latency test
If sufficient sleep is obtained on polysomnograpy (at least 6 hours for an adult) and no other sleep disorder is identified, a multiple sleep latency test is performed. A urine toxicology screen is typically performed on the day of the test to ensure that drugs are not affecting the results.
The multiple sleep latency test consists of 4 to 5 nap opportunities at 2-hour intervals in a quiet dark room conducive to sleep, during which both sleep and REM latency are recorded. The sleep latency of those with narcolepsy is significantly shortened, and the diagnosis of narcolepsy requires an average sleep latency of less than 8 minutes.
Given the propensity for REM sleep in narcolepsy, another essential feature for diagnosis is the sleep-onset REM period (SOREMP). A SOREMP is defined as a REM latency of less than 15 minutes. A diagnosis of narcolepsy re-quires a SOREMP in at least 2 of the naps in a multiple sleep latency test (or 1 nap if the shortened REM latency is seen during polysomnography).31
The multiple sleep latency test has an imperfect sensitivity, though, and should be repeated when there is a high suspicion of narcolepsy.32–34 It is not completely specific either, and false-positive results occur. In fact, SOREMPs can be seen in the general population, particularly in those with a circadian rhythm disorder, insufficient sleep, or sleep-disordered breathing. Two or more SOREMPs in an multiple sleep latency test can be seen in a small proportion of the general population.35 The results of a multiple sleep latency test should be interpreted in the clinical context.
Differential diagnosis
Narcolepsy type 1 is distinguished from type 2 by the presence of cataplexy. A cerebrospinal fluid hypocretin 1 level of 110 pg/mL or less, or less than one-third of the mean value obtained in normal individuals, can substitute for the multiple sleep latency test in diagnosing narcolepsy type 1.31 Currently, hypocretin testing is generally not performed in clinical practice, although it may become a routine part of the narcolepsy evaluation in the future.
Thus, according to the International Classification of Sleep Disorders, 3rd edition,31 the diagnosis of narcolepsy type 1 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder, and at least 1 of the following:
- Cataplexy and mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing (1 of which can be on the preceding night’s polysomography)
- Cerebrospinal fluid hypocretin 1 levels less than 110 pg/mL or one-third the baseline normal levels and mean sleep latency ≤ 8 minutes with ≥ 2 SOREMPs on multiple sleep latency testing.
Similarly, the diagnosis of narcolepsy type 2 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder, plus:
- Mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing.
Idiopathic hypersomnia, another disorder of central hypersomnolence, is also characterized by disabling sleepiness. It is diagnostically differentiated from narcolepsy, as there are fewer than 2 SOREMPs. As opposed to narcolepsy, in which naps tend to be refreshing, even prolonged naps in idiopathic hypersomnia are often not helpful in restoring wakefulness. In idiopathic hypersomnia, sleep is usually not fragmented, and there are few nocturnal arousals. Sleep times can often be prolonged as well, whereas in narcolepsy total sleep time through the day may not be increased but is not consolidated.
Kleine-Levin syndrome is a rarer disorder of hypersomnia. It is episodic compared with the relatively persistent sleepiness in narcolepsy and idiopathic hypersomnia. Periods of hypersomnia occur intermittently for days to weeks and are accompanied by cognitive and behavioral changes including hyperphagia and hypersexuality.4
LINKED TO HYPOCRETIN DEFICIENCY
Over the past 2 decades, the underlying pathophysiology of narcolepsy type 1 has been better characterized.
Narcolepsy type 1 has been linked to a deficiency in hypocretin in the central nervous system.36 Hypocretin (also known as orexin) is a hormone produced in the hypothalamus that acts on multiple brain regions and maintains alertness. For unclear reasons, hypothalamic neurons producing hypocretin are selectively reduced in narcolepsy type 1. Hypocretin also stabilizes wakefulness and inhibits REM sleep; therefore, hypocretin deficiency can lead to inappropriate intrusions of REM sleep onto wakefulness, leading to the hallmark features of narcolepsy—cataplexy, sleep-related hallucinations, and sleep paralysis.37 According to one theory, cataplexy is triggered by emotional stimuli because of a pathway between the medial prefrontal cortex and the amygdala to the pons.38
Cerebrospinal fluid levels of hypocretin in patients with narcolepsy type 2 tend to be normal, and the biologic underpinnings of narcolepsy type 2 remain mysterious. However, in the subgroup of those with narcolepsy type 2 in which hypocretin is low, many individuals go on to develop cataplexy, thereby evolving to narcolepsy type 1.36
POSSIBLE AUTOIMMUNE BASIS
Narcolepsy is typically a sporadic disorder, although familial cases have been described. The risk of a parent with narcolepsy having a child who is affected is approximately 1%.5
Narcolepsy type 1 is strongly associated with HLA-DQB1*0602, with up to 95% of those affected having at least one allele.39 Having 2 copies of the allele further increases the risk of developing narcolepsy.40 However, this allele is far from specific for narcolepsy with cataplexy, as it occurs in 12% to 38% of the general population.41 Therefore, HLA typing currently has limited clinical utility. The exact cause is as yet unknown, but substantial literature proposes an autoimmune basis of the disorder, given the strong association with the HLA subtype.42–44
After the 2009 H1N1 influenza pandemic, there was a significant increase in the incidence of narcolepsy with cataplexy, which again sparked interest in an autoimmune etiology underlying the disorder. Pandemrix, an H1N1 vaccine produced as a result of the 2009 pandemic, appeared to also be associated with an increase in the incidence of narcolepsy. An association with other upper respiratory infections has also been noted, further supporting a possible autoimmune basis.
A few studies have looked for serum autoantibodies involved in the pathogenesis of narcolepsy. Thus far, only one has been identified, an antibody to Tribbles homolog 2, found in 20% to 40% of those with new onset of nar-colepsy.42–44
TREATMENTS FOR DAYTIME SLEEPINESS
As with many chronic disorders, the treatment of narcolepsy consists of symptomatic rather than curative management, which can be done through both pharmacologic and nonpharmacologic means.
Nondrug measures
Scheduled naps lasting 15 to 20 minutes can help improve alertness.45 A consistent sleep schedule with good sleep hygiene, ensuring sufficient nightly sleep, is also important. In one study, the combination of scheduled naps and regular nocturnal sleep times reduced the level of daytime sleepiness and unintentional daytime sleep. Daytime naps were most helpful for those with the highest degree of daytime sleepiness.45
Strategic use of caffeine can be helpful and can reduce dependence on pharmacologic treatment.
Screening should be performed routinely for other sleep disorders, such as sleep-disordered breathing, which should be treated if identified.5,18 When being treated for other medical conditions, individuals with narcolepsy should avoid medications that can cause sedation, such as opiates or barbiturates; alcohol should be minimized or avoided.
Networking with other individuals with narcolepsy through support groups such as Narcolepsy Network can be valuable for learning coping skills and connecting with community resources. Psychological counseling for the patient, and sometimes the family, can also be useful. School-age children may need special accommodations such as schedule adjustments to allow for scheduled naps or frequent breaks to maintain alertness.
People with narcolepsy tend to function better in careers that do not require long periods of sitting, as sleepiness tends to be worse, but instead offer flexibility and require higher levels of activity that tend to combat sleepiness. They should not work as commercial drivers.18
Medications
While behavioral interventions in narcolepsy are vital, they are rarely sufficient, and drugs that promote daytime wakefulness are used as an adjunct (Table 2).46
Realistic expectations should be established when starting, as some degree of residual sleepiness usually remains even with optimal medical therapy. Medications should be strategically scheduled to maximize alertness during necessary times such as at work or school or during driving. Patients should specifically be counseled to avoid driving if sleepy.18,47
Modafinil is often used as a first-line therapy, given its favorable side-effect profile and low potential for abuse. Its pharmacologic action has been debated but it probably acts as a selective dopamine reuptake inhibitor. It is typically taken twice daily (upon waking and early afternoon) and is usually well tolerated.
Potential side effects include headache, nausea, dry mouth, anorexia, diarrhea, and, rarely, Stevens-Johnson syndrome. Cardiovascular side effects are minimal, making it a favorable choice in older patients.18,48
A trial in 283 patients showed significantly lower levels of sleepiness in patients taking modafinil 200 mg or 400 mg than in a control group. Other trials have supported these findings and showed improved driving performance on modafinil.18
Notably, modafinil can increase the metabolism of oral contraceptives, thereby reducing their efficacy. Women of childbearing age should be warned about this interaction and should be transitioned to nonhormonal forms of contraception.2,47
Armodafinil, a purified R-isomer of modafinil, has a longer half-life and requires only once-daily dosing.5
If modafinil or armodafinil fails to optimally manage daytime sleepiness, a traditional stimulant such as methylphenidate or an amphetamine is often used.
Methylphenidate and amphetamines primarily inhibit the reuptake and increase the release of the monoamines, mainly dopamine, and to a lesser degree serotonin and norepinephrine.
These drugs have more significant adverse effects that can involve the cardiovascular system, causing hypertension and arrhythmias. Anorexia, weight loss, and, particularly with high doses, psychosis can occur.49
These drugs should be avoided in patients with a history of significant cardiovascular disease. Before starting stimulant therapy, a thorough cardiovascular examination should be done, often including electrocardiography to ensure there is no baseline arrhythmia.
Patients on these medications should be followed closely to ensure that blood pressure, pulse, and weight are not negatively affected.18,50 Addiction and tolerance can develop with these drugs, and follow-up should include assessment for dependence. Some states may require prescription drug monitoring to ensure the drugs are not being abused or diverted.
Short- and long-acting formulations of both methylphenidate and amphetamines are available, and a long-acting form is often used in conjunction with a short-acting form as needed.18
Addiction and drug-seeking behavior can develop but are unusual in those taking stimulants to treat narcolepsy.49
Follow-up
Residual daytime sleepiness can be measured subjectively through the Epworth Sleepiness Scale on follow-up. If necessary, a maintenance-of-wakefulness test can provide an objective assessment of treatment efficacy.18
As narcolepsy is a chronic disorder, treatment should evolve with time. Most medications that treat narcolepsy are categorized by the US Food and Drug Administration as pregnancy category C, as we do not have adequate studies in human pregnancies to evaluate their effects. When a patient with narcolepsy becomes pregnant, she should be counseled about the risks and benefits of remaining on therapy. Treatment should balance the risks of sleepiness with the potential risks of remaining on medications.50 In the elderly, as cardiovascular comorbidities tend to increase, the risks and benefits of therapy should be routinely reevaluated.
For cataplexy
Sodium oxybate,51–53 the most potent anticataplectic drug, is the sodium salt of gamma hydroxybutyrate, a metabolite of gamma-aminobutyric acid. Sodium oxybate can be prescribed in the United States, Canada, and Europe. The American Academy of Sleep Medicine recommends sodium oxybate for cataplexy, daytime sleepiness, and disrupted sleep based on 3 level-1 studies and 2 level-4 studies.46
Sodium oxybate increases slow-wave sleep, improves sleep continuity, and often helps to mitigate daytime sleepiness. Due to its short half-life, its administration is unusual: the first dose is taken before bedtime and the second dose 2.5 to 4 hours later. Some patients set an alarm clock to take the second dose, while others awaken spontaneously to take the second dose. Most patients find that with adherence to dosing and safety instructions, sodium oxybate can serve as a highly effective form of treatment of both excessive sleepiness and cataplexy and may reduce the need for stimulant-based therapies.
The most common adverse effects are nausea, mood swings, and enuresis. Occasionally, psychosis can result and limit use of the drug. Obstructive sleep apnea can also develop or worsen.52 Because of its high salt content, sodium oxybate should be used with caution in those with heart failure, hypertension, or renal impairment. Its relative, gamma hydroxybutyrate, causes rapid sedation and has been notorious for illegal use as a date rape drug.
In the United States, sodium oxybate is distributed only through a central pharmacy to mitigate potential abuse. Due to this system, the rates of diversion are extremely low, estimated in a postmarketing analysis to be 1 instance per 5,200 patients treated. In the same study, abuse and dependence were both rare as well, about 1 case for every 2,600 and 6,500 patients treated.6,18,52,53
Antidepressants promote the action of norepinephrine and, to a lesser degree, serotonin, thereby suppressing REM sleep.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is often used as a first-line treatment for cataplexy. Selective serotonin reuptake inhibitors such as fluoxetine are also used with success. Tricyclic antidepressants such as protriptyline or clomipramine are extremely effective for cataplexy, but are rarely used due to their adverse effects.2,47
FUTURE WORK
While our understanding of narcolepsy has advanced, there are still gaps in our knowledge of the disorder—namely, the specific trigger for the loss of hypocretin neurons in type 1 narcolepsy and the underlying pathophysiology of type 2.
A number of emerging therapies target the hypocretin system, including peptide replacement, neuronal transplant, and immunotherapy preventing hypocretin neuronal cell death.50,54,55 Additional drugs designed to improve alertness that do not involve the hypocretin system are also being developed, including a histamine inverse agonist.50,56 Sodium oxybate and modafinil, although currently approved for use in adults, are still off-label in pediatric practice. Studies of the safety and efficacy of these medications in children are needed.7,57
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux Civils et Militaires 1880; part a, 53:626–628, part b, 54:635–637.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
- Scammell TE. Clinical features and diagnosis of narcolepsy in adults. In: Eichler AF, ed. UpToDate. Waltham, MA: UpToDate; 2018. www.uptodate.com. Accessed October 31, 2018.
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004; 5(1):37–41. pmid:14725825
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373(27):2654–2662. doi:10.1056/NEJMra1500587
- Babiker MO, Prasad M. Narcolepsy in children: a diagnostic and management approach. Pediatr Neurol 2015; 52(6):557–565. doi:10.1016/j.pediatrneurol.2015.02.020
- Kotagal S. Narcolepsy in children. In: UpToDate, Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003; 53(2):154–166. doi:10.1002/ana.10444
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med 2011; 12(1):12–18. doi:10.1016/j.sleep.2010.05.010
- Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 2015; 71(4):218–224. doi:10.1159/000432400
- Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97(2-3):153–164. pmid:11166087
- Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev 2011;5(5):311–315. doi:10.1016/j.smrv.2011.01.007
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol 1988; 70(6):473–481. pmid:2461281
- Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain 2013; 136(pt 12):3787–3795. doi:10.1093/brain/awt277
- Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med 2004; 5(2):147–150. doi:10.1016/j.sleep.2003.10.006
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013; 70(1-2):22–26. doi:10.1159/000348719
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med 2013; 9(8):805–812. doi:10.5664/jcsm.2926
- Scammell TE. Treatment of narcolepsy in adults. In: Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015; 10(6):e0129386. doi:10.1371/journal.pone.0129386
- Fortuyn HD, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 2010; 32(1):49–56. doi:10.1016/j.genhosppsych.2009.08.007
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry 2017; 78(2):171–176. doi:10.4088/JCP.15m10262
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007; 30(1):13–26. pmid:17310860
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002; 25(2):197–202. pmid:11902429
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014; 15(5):502–507. doi:10.1016/j.sleep.2014.01.015
- Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57(11):2029–2033. pmid:11739821
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6):540–545. pmid:1798888
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep 2003; 26(4):455–458. pmid:12841372
- van der Heide A, van Schie MK, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 2015; 38(7):1051–1058. doi:10.5665/sleep.4810
- Nesbitt AD. Delayed sleep-wake phase disorder. J Thorac Dis 2018; 10(suppl 1):S103–S111. doi:10.21037/jtd.2018.01.11
- Pallesen S, Saxvig IW, Molde H, Sørensen E, Wilhelmsen-Langeland A, Bjorvatn B. Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc 2011; 34(2):391–395. doi:10.1016/j.adolescence.2010.02.005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Disorders; 2014.
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013; 9(8):789–795. doi:10.5664/jcsm.2922
- Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol 2013; 70(7):891–902. doi:10.1001/jamaneurol.2013.1589
- Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep 2015; 38(10):1575–1581. doi:10.5665/sleep.5050
- Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain 2006; 129(6):1609–1623. doi:10.1093/brain/awl079
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197):39–40. doi:10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6(9):991–997. doi:10.1038/79690
- Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33(23):9743–9751. doi:10.1523/JNEUROSCI.0499-13.2013
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients.. Sleep 1997; 20(11):1012–1020. pmid:9456467
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51(1):96–100. pmid:9459509
- Akintomide GS, Rickards H. Narcolepsy: a review. Neuropsychiatr Dis Treat 2011; 7(1):507–518. doi:10.2147/NDT.S23624
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol 2013; 23(5):767–773. doi:10.1016/j.conb.2013.04.013
- Degn M, Kornum BR. Type 1 narcolepsy: a CD8(+) T cell-mediated disease? Ann N Y Acad Sci 2015;1 351:80–88. doi:10.1111/nyas.12793
- Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14(3):318–328. doi:10.1016/S1474-4422(14)70218-2
- Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001; 24(4):385–391. pmid:11403522
- Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007; 30(12):1705–1711. pmid:18246980
- Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 2012; 9(4):739–752. doi:10.1007/s13311-012-0150-9
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007; 3(6):595–602. pmid:17993041
- Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep 2005; 28(6):667–672. pmid:16477952
- Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep 2017; 9:39–57. doi:10.2147/NSS.S103467
- Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med 2017; 35:80–84. doi:10.1016/j.sleep.2017.03.028
- Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy–cataplexy: a retrospective study. Sleep Med 2012; 13(6):606–610. doi:10.1016/j.sleep.2011.10.032
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 2009; 5(4):365–371. pmid:19968016
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262:8–13. doi:10.1016/j.bbr.2013.12.045
- Arias-Carrión O, Murillo-Rodriguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9(4):e95342. doi:10.1371/journal.pone.0095342
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014; 15(6):681–687. doi:10.1016/j.sleep.2014.01.021
- Lecendreux M, Bruni O, Franco P, et al. Clinical experience suggests that modafinil is an effective and safe treatment for paediatric narcolepsy. J Sleep Res 2012; 21(4):481–483. doi:10.1111/j.1365-2869.2011.00991.x
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux Civils et Militaires 1880; part a, 53:626–628, part b, 54:635–637.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
- Scammell TE. Clinical features and diagnosis of narcolepsy in adults. In: Eichler AF, ed. UpToDate. Waltham, MA: UpToDate; 2018. www.uptodate.com. Accessed October 31, 2018.
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004; 5(1):37–41. pmid:14725825
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373(27):2654–2662. doi:10.1056/NEJMra1500587
- Babiker MO, Prasad M. Narcolepsy in children: a diagnostic and management approach. Pediatr Neurol 2015; 52(6):557–565. doi:10.1016/j.pediatrneurol.2015.02.020
- Kotagal S. Narcolepsy in children. In: UpToDate, Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003; 53(2):154–166. doi:10.1002/ana.10444
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med 2011; 12(1):12–18. doi:10.1016/j.sleep.2010.05.010
- Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 2015; 71(4):218–224. doi:10.1159/000432400
- Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97(2-3):153–164. pmid:11166087
- Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev 2011;5(5):311–315. doi:10.1016/j.smrv.2011.01.007
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol 1988; 70(6):473–481. pmid:2461281
- Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain 2013; 136(pt 12):3787–3795. doi:10.1093/brain/awt277
- Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med 2004; 5(2):147–150. doi:10.1016/j.sleep.2003.10.006
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013; 70(1-2):22–26. doi:10.1159/000348719
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med 2013; 9(8):805–812. doi:10.5664/jcsm.2926
- Scammell TE. Treatment of narcolepsy in adults. In: Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015; 10(6):e0129386. doi:10.1371/journal.pone.0129386
- Fortuyn HD, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 2010; 32(1):49–56. doi:10.1016/j.genhosppsych.2009.08.007
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry 2017; 78(2):171–176. doi:10.4088/JCP.15m10262
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007; 30(1):13–26. pmid:17310860
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002; 25(2):197–202. pmid:11902429
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014; 15(5):502–507. doi:10.1016/j.sleep.2014.01.015
- Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57(11):2029–2033. pmid:11739821
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6):540–545. pmid:1798888
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep 2003; 26(4):455–458. pmid:12841372
- van der Heide A, van Schie MK, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 2015; 38(7):1051–1058. doi:10.5665/sleep.4810
- Nesbitt AD. Delayed sleep-wake phase disorder. J Thorac Dis 2018; 10(suppl 1):S103–S111. doi:10.21037/jtd.2018.01.11
- Pallesen S, Saxvig IW, Molde H, Sørensen E, Wilhelmsen-Langeland A, Bjorvatn B. Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc 2011; 34(2):391–395. doi:10.1016/j.adolescence.2010.02.005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Disorders; 2014.
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013; 9(8):789–795. doi:10.5664/jcsm.2922
- Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol 2013; 70(7):891–902. doi:10.1001/jamaneurol.2013.1589
- Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep 2015; 38(10):1575–1581. doi:10.5665/sleep.5050
- Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain 2006; 129(6):1609–1623. doi:10.1093/brain/awl079
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197):39–40. doi:10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6(9):991–997. doi:10.1038/79690
- Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33(23):9743–9751. doi:10.1523/JNEUROSCI.0499-13.2013
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients.. Sleep 1997; 20(11):1012–1020. pmid:9456467
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51(1):96–100. pmid:9459509
- Akintomide GS, Rickards H. Narcolepsy: a review. Neuropsychiatr Dis Treat 2011; 7(1):507–518. doi:10.2147/NDT.S23624
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol 2013; 23(5):767–773. doi:10.1016/j.conb.2013.04.013
- Degn M, Kornum BR. Type 1 narcolepsy: a CD8(+) T cell-mediated disease? Ann N Y Acad Sci 2015;1 351:80–88. doi:10.1111/nyas.12793
- Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14(3):318–328. doi:10.1016/S1474-4422(14)70218-2
- Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001; 24(4):385–391. pmid:11403522
- Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007; 30(12):1705–1711. pmid:18246980
- Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 2012; 9(4):739–752. doi:10.1007/s13311-012-0150-9
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007; 3(6):595–602. pmid:17993041
- Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep 2005; 28(6):667–672. pmid:16477952
- Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep 2017; 9:39–57. doi:10.2147/NSS.S103467
- Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med 2017; 35:80–84. doi:10.1016/j.sleep.2017.03.028
- Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy–cataplexy: a retrospective study. Sleep Med 2012; 13(6):606–610. doi:10.1016/j.sleep.2011.10.032
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 2009; 5(4):365–371. pmid:19968016
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262:8–13. doi:10.1016/j.bbr.2013.12.045
- Arias-Carrión O, Murillo-Rodriguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9(4):e95342. doi:10.1371/journal.pone.0095342
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014; 15(6):681–687. doi:10.1016/j.sleep.2014.01.021
- Lecendreux M, Bruni O, Franco P, et al. Clinical experience suggests that modafinil is an effective and safe treatment for paediatric narcolepsy. J Sleep Res 2012; 21(4):481–483. doi:10.1111/j.1365-2869.2011.00991.x
KEY POINTS
- Features of narcolepsy include daytime sleepiness, sleep attacks, cataplexy (in narcolepsy type 1), sleep paralysis, and sleep-related hallucinations.
- People with narcolepsy feel sleepy and can fall asleep quickly, but they do not stay asleep for long. They go into rapid eye movement sleep soon after falling asleep. Total sleep time is normal, but sleep is fragmented.
- Scheduled naps lasting 15 to 20 minutes can improve alertness. A consistent sleep schedule with good sleep hygiene is also important.
- Modafinil, methylphenidate, and amphetamines are used to manage daytime sleepiness, and sodium oxybate and antidepressants are used for cataplexy.
A look at new guidelines for HIV treatment and prevention
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1
An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.
Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1
An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.
Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1
An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.
Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.