User login
Psychiatric emergency? What to consider before prescribing
Psychiatric emergencies—such as a patient who is agitated, self-destructive, or suicidal—may arise in a variety of settings, including emergency departments and inpatient units.1 Before emergently prescribing psychotropic medications to address acute psychiatric symptoms, there are numerous factors a clinician needs to consider.1-3 Asking the following questions may help you quickly obtain important clinical information to determine which medication to use during a psychiatric emergency:
Age. Is the patient a child, adolescent, adult, or older adult?
Allergies. Does the patient have any medication allergies or sensitivities?
Behaviors. What are the imminent dangerous behaviors that warrant emergent medication use
Collateral information. If the patient was brought by police or family, how was he/she behaving in the community or at home? If brought from a correctional facility or other institution, how did he/she behave in that setting?
Concurrent diagnoses/interventions. Does the patient have a psychiatric or medical diagnosis? Is the patient receiving any pharmacologic or nonpharmacologic treatments?
First visit. Is this the patient’s first visit to your facility? Or has the patient been to the facility previously and/or repeatedly? Has the patient ever been prescribed psychotropic medications? If the patient has received emergent medications before, which medications were used, and were they helpful?
Continue to: Legal status
Legal status. Is the patient voluntary for treatment or involuntary for treatment? If voluntary, is involuntary treatment needed?
Street. Was this patient evaluated in a medical setting before presenting to your facility? Or did this patient arrive directly from the community/street?
Substance use. Has the patient been using any licit and/or illicit substances?
In my experience with psychiatric emergencies, asking these questions has helped guide my decision-making during these situations. They have helped me to determine the appropriate medication, route of administration, dose, and monitoring requirements. Although other factors can impact clinicians’ decision-making in these situations, I have found these questions to be a good starting point.
1. Mavrogiorgou P, Brüne M, Juckel G. The management of psychiatric emergencies. Dtsch Arztebl Int. 2011;108(13):222-230.
2. Glick RL, Berlin JS, Fishkind AB, et al (eds). Emergency psychiatry: principles and practice. 2nd ed. Philadelphia, PA: Wolter Kluwer; 2020.
3. Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatry. 2016;17(2):86-128.
Psychiatric emergencies—such as a patient who is agitated, self-destructive, or suicidal—may arise in a variety of settings, including emergency departments and inpatient units.1 Before emergently prescribing psychotropic medications to address acute psychiatric symptoms, there are numerous factors a clinician needs to consider.1-3 Asking the following questions may help you quickly obtain important clinical information to determine which medication to use during a psychiatric emergency:
Age. Is the patient a child, adolescent, adult, or older adult?
Allergies. Does the patient have any medication allergies or sensitivities?
Behaviors. What are the imminent dangerous behaviors that warrant emergent medication use
Collateral information. If the patient was brought by police or family, how was he/she behaving in the community or at home? If brought from a correctional facility or other institution, how did he/she behave in that setting?
Concurrent diagnoses/interventions. Does the patient have a psychiatric or medical diagnosis? Is the patient receiving any pharmacologic or nonpharmacologic treatments?
First visit. Is this the patient’s first visit to your facility? Or has the patient been to the facility previously and/or repeatedly? Has the patient ever been prescribed psychotropic medications? If the patient has received emergent medications before, which medications were used, and were they helpful?
Continue to: Legal status
Legal status. Is the patient voluntary for treatment or involuntary for treatment? If voluntary, is involuntary treatment needed?
Street. Was this patient evaluated in a medical setting before presenting to your facility? Or did this patient arrive directly from the community/street?
Substance use. Has the patient been using any licit and/or illicit substances?
In my experience with psychiatric emergencies, asking these questions has helped guide my decision-making during these situations. They have helped me to determine the appropriate medication, route of administration, dose, and monitoring requirements. Although other factors can impact clinicians’ decision-making in these situations, I have found these questions to be a good starting point.
Psychiatric emergencies—such as a patient who is agitated, self-destructive, or suicidal—may arise in a variety of settings, including emergency departments and inpatient units.1 Before emergently prescribing psychotropic medications to address acute psychiatric symptoms, there are numerous factors a clinician needs to consider.1-3 Asking the following questions may help you quickly obtain important clinical information to determine which medication to use during a psychiatric emergency:
Age. Is the patient a child, adolescent, adult, or older adult?
Allergies. Does the patient have any medication allergies or sensitivities?
Behaviors. What are the imminent dangerous behaviors that warrant emergent medication use
Collateral information. If the patient was brought by police or family, how was he/she behaving in the community or at home? If brought from a correctional facility or other institution, how did he/she behave in that setting?
Concurrent diagnoses/interventions. Does the patient have a psychiatric or medical diagnosis? Is the patient receiving any pharmacologic or nonpharmacologic treatments?
First visit. Is this the patient’s first visit to your facility? Or has the patient been to the facility previously and/or repeatedly? Has the patient ever been prescribed psychotropic medications? If the patient has received emergent medications before, which medications were used, and were they helpful?
Continue to: Legal status
Legal status. Is the patient voluntary for treatment or involuntary for treatment? If voluntary, is involuntary treatment needed?
Street. Was this patient evaluated in a medical setting before presenting to your facility? Or did this patient arrive directly from the community/street?
Substance use. Has the patient been using any licit and/or illicit substances?
In my experience with psychiatric emergencies, asking these questions has helped guide my decision-making during these situations. They have helped me to determine the appropriate medication, route of administration, dose, and monitoring requirements. Although other factors can impact clinicians’ decision-making in these situations, I have found these questions to be a good starting point.
1. Mavrogiorgou P, Brüne M, Juckel G. The management of psychiatric emergencies. Dtsch Arztebl Int. 2011;108(13):222-230.
2. Glick RL, Berlin JS, Fishkind AB, et al (eds). Emergency psychiatry: principles and practice. 2nd ed. Philadelphia, PA: Wolter Kluwer; 2020.
3. Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatry. 2016;17(2):86-128.
1. Mavrogiorgou P, Brüne M, Juckel G. The management of psychiatric emergencies. Dtsch Arztebl Int. 2011;108(13):222-230.
2. Glick RL, Berlin JS, Fishkind AB, et al (eds). Emergency psychiatry: principles and practice. 2nd ed. Philadelphia, PA: Wolter Kluwer; 2020.
3. Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatry. 2016;17(2):86-128.
The Changing Landscape of Uncomplicated Gram-Negative Bacteremia: A Narrative Review to Guide Inpatient Management
Uncomplicated bacteremia, while not precisely defined in the literature, generally implies bacteremia in the absence of a persistent or difficult-to-eradicate infectious source. Bacteremia secondary to focal infections such as skin and soft-tissue infection, pneumonia, pyelonephritis, or urinary tract infection (UTI) accounts for up to 25% of bloodstream infections (BSIs) and usually resolves with prompt and appropriate antimicrobial therapy.1,2 Current practice guidelines do not adequately address key aspects of the optimal management of gram-negative (GN)–BSI commonly encountered in hospital care.3-7 Notably, antimicrobial duration, criteria to transition from intravenous (IV) to oral step-down therapy, choice of oral antimicrobials, and reassessment of follow-up blood cultures have not been addressed. In the absence of consensus guidelines, clinicians rely on “conventional wisdom” and clinical experience, which may not be supported by scientific rigor. A growing body of research now challenges some long-standing practices once thought to be standard of care.
In this narrative review, we aim to examine and synthesize emerging information to provide an evidence-based framework in the management of hospitalized patients with GN-BSI. We highlight the unintended consequences and potential harms of excessive antimicrobial exposure and focus on areas in the fundamental approach to duration of therapy, the role of oral antimicrobials, and usefulness of follow-up blood cultures. A comprehensive search of the published literature was performed in PubMed with an emphasis on articles published during 2015-2019 with use of search terms including gram-negative bacteremia, duration, antibiotics, adverse effects, intravascular catheter, and follow-up blood cultures.
ANTIMICROBIAL RISKS: ‘PRIMUM NON NOCERE’
Antimicrobial overuse is common and may be driven by concerns for undertreatment. Clinicians may believe that prolonged antimicrobial therapy maximizes cure rates, with treatment duration often defined arbitrarily by a fixed number of “Constantine-units” (dating back to the ancient Roman emperor’s decree of 7 days in a week).8-10 Recent publications refute this notion and point out that the harms of overprescribing outweigh the perceived benefits of longer treatment duration.
Antimicrobials are lifesaving but not benign; adverse effects are common and costly to our patients and healthcare system. Among 1,488 hospitalized adults who received at least 24 hours of systemic antimicrobials, 20% had an antimicrobial-associated adverse event, mostly gastrointestinal, renal, or hematologic in nature.11 Prolonged duration of antimicrobials is further associated with adverse effects such as antimicrobial-associated diarrhea, increased rates of Clostridioides difficile infection (CDI), emergence of antimicrobial resistance, and longer hospital length of stay (LOS).11-15 Vaughn and colleagues conducted the largest observational study to date, evaluating antimicrobial prescriptions for the treatment of nearly 6,500 adults with community-acquired pneumonia in a 43-hospital consortium in Michigan.14 More than two-thirds of patients received antimicrobial courses (median 8 days) that exceeded guideline-recommended duration. Patients who received longer antimicrobial courses did not have reduced mortality, readmission, or emergency department visits. More importantly, each excess day of treatment was associated with a relative 5% increase in the odds of antimicrobial-associated adverse effects reported by patients. This is further supported by national and state hospital data that antimicrobial-associated adverse events are an independent predictor of longer LOS.12
CDI is commonly linked to destructive changes to the indigenous microbiota of the intestinal flora caused by antimicrobial administration. Stevens and colleagues identified 7,792 hospitalized patients who received at least 2 consecutive days of antimicrobial therapy13; comparing 241 cases of CDI with the control group, they observed a dose-dependent risk of CDI associated with increasing cumulative dose, number of antimicrobials, and days of antimicrobial exposure. Compared with patients who received fewer than 4 days of antimicrobials, the adjusted hazard ratios (aHR) for those who received 4-7 days or 8-18 days of therapy were 1.4 (95% CI, 0.8-2.4) and 3.0 (95% CI, 1.9-5.0), respectively. This correlates to a threefold increase in CDI risk for patients who received more than 7 days of antimicrobials. More specifically, the empiric use of antipseudomonal ß-lactams (APBL) for more than 48 hours was also found to be an independent risk factor for CDI among 808 patients with Enterobacteriaceae BSI.16 The risk of CDI within 90 days of BSI was higher among those who received >48 hours of APBL than it was among those who received ≤48 hours (HR, 3.6; 95% CI, 1.5-9.9).
While C difficile may be the most well-known pathogen implicated in antimicrobial usage, the incidence of multidrug-resistant (MDR) organisms, either as infectious or colonizing pathogens, is also tied to antimicrobial exposure. Among patients receiving systemic antimicrobials, 6% developed an MDR infection within 90 days.11 Over a 5-year period, Teshome and colleagues evaluated 7,118 critically ill patients and demonstrated that prolonged exposures to APBLs increased the risk of new antimicrobial resistance within 60 days.15 This resistance pattern was not an institutional or environmental finding but a patient-level finding. For each additional day of cefepime or piperacillin/tazobactam received, the risk of new antimicrobial resistance was increased by 8%. The authors concluded that defining a piperacillin/tazobactam course as 10 vs 7 days would result in a 24% higher relative risk of resistance per patient related to those 3 additional days of antimicrobial exposure.
Catheter complications including thrombophlebitis, infiltration, and infection are serious and frequent problems associated with IV medication administration.17 Even with short-term use, peripherally inserted central catheters (PICCs) carry a substantial risk of venous thrombosis (superficial and deep veins). The incidence of deep vein thrombosis (DVT) for PICCs is estimated between 5% and 15% for hospitalized patients and 2% and 5% for ambulatory patients.18 A recent randomized controlled trial (RCT) of oral vs IV antimicrobials for bone and joint infections reported that, compared with patients randomized to oral antimicrobials, those randomized to IV antimicrobials were more likely to have catheter complications (9.4% vs 1.0%; P < .001) and to discontinue therapy earlier (18.9% vs 12.8%; P = .006).19 Median hospital stay was also significantly longer in the IV group (14 days vs 11 days; P < .001).
SHORTEST EFFECTIVE DURATION: LESS MAY BE MORE
Optimization of antimicrobial duration has long been recognized as one of the key strategies in reducing unnecessary antimicrobial exposure, yet high-quality evidence on comparative effectiveness of duration in the setting of bacteremia has been limited until recently.20 The presence of bacteremia is often used as a justification for prolonged courses of antimicrobial regardless of infection source or clinical response. The Infectious Diseases Society of America guidelines suggest 7 to 14 days of treatment for intravascular catheter-associated gram-negative bacteremia, but the optimal duration for non–catheter-related gram-negative bacteremia is not addressed.21 This lack of clear guidance and the historical scarcity of robust data make it difficult to inform best practices, which leads to wide variability in clinical practice and 14 days being the most prescribed duration.22,23
Pooled clinical trials’ data from subsets of patients with bacteremia and those from observational studies have been the best available evidence for the treatment duration of GN-BSI until recently (Table 1).24-32 Two meta-analyses evaluating RCTs of adult and pediatric patients with pyelonephritis, UTI, peritonitis, and pneumonia found no differences in clinical failure, microbiologic cure, or survival between short and long courses of therapy in the subset of patient with associated bacteremia.24,25 Six heterogeneous RCTs of short vs long courses of therapy for complicated UTI or pyelonephritis reported no differences in clinical cure rates in the subset of patients with associated GN-BSI.2 The observational studies outlined in Table 1 are also consistent with RCT results supporting noninferiority in clinical cure and mortality outcomes between short and long courses of therapy.26-32 These findings may also be extrapolated to immunocompromised hosts given a considerable representation of 10% to 47% of the study population with immunosuppressive conditions.
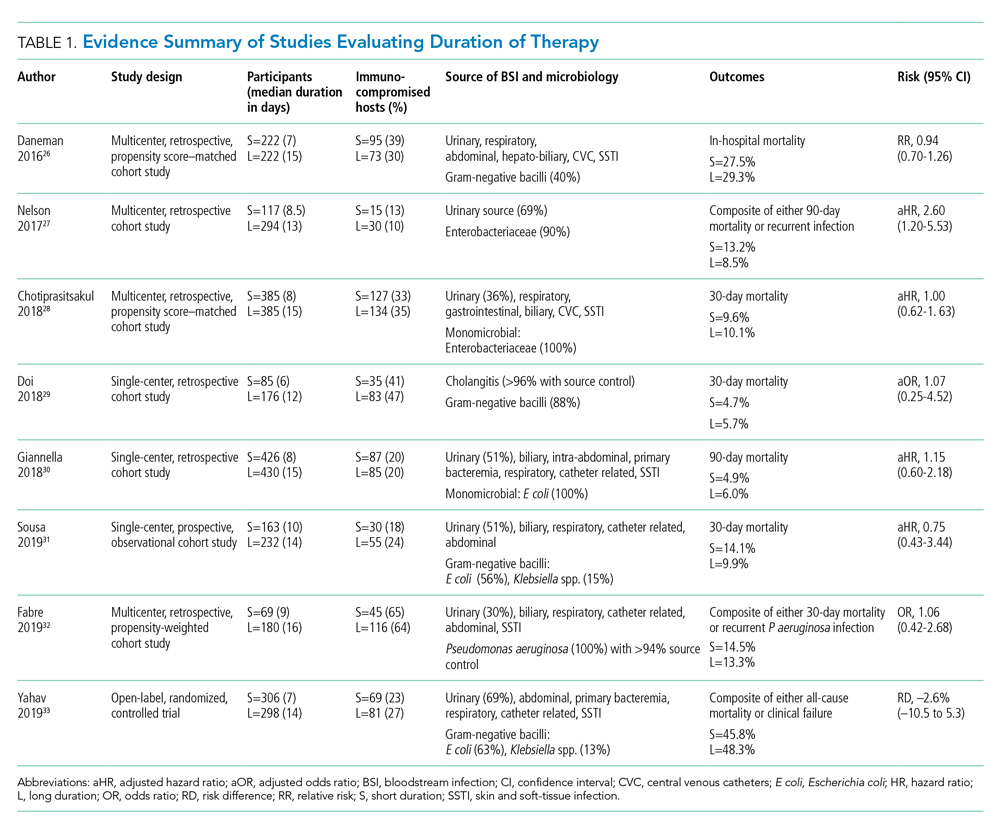
Nelson and colleagues conducted the only retrospective study to date reporting conflicting results of higher risk of treatment failure (defined as composite endpoint of mortality or recurrent infection within 90 days of index BSI) in patients receiving a short course of therapy.27 However, the difference was driven by 90-day mortality (8.2% vs 3.3%; P = .04) not recurrent infection (6.7% vs 6.5%; P = 0.93). Giannella and colleagues also evaluated 90-day mortality as a primary endpoint in a much larger cohort of over 850 patients in Italy and found no difference in mortality rates between short and long courses of antimicrobials.30
Yahav and colleagues conducted the first well-designed open-label RCT comparing short and long courses of antimicrobials in uncomplicated GN-BSI.33 This noninferiority study randomized more than 600 hospitalized patients with adequate source control who were afebrile and hemodynamically stable for ≥48 hours to receive either 7 days or 14 days of therapy. The source of infections was predominantly urinary (68%), and the causative pathogens were 90% Enterobacteriacae, including 20% MDR strains. The primary outcome was a composite of 90-day all-cause mortality or clinical failure defined as either relapse of bacteremia, local or distant complications, readmission, or extended hospital stay >14 days. The authors reported no statistically significant differences in the primary outcome between short (45.8%) and long (48.3%) courses of treatment. In the prespecified post hoc analysis designed to evaluate infection-related outcomes at an earlier time frame, there were no observed differences in complications, relapses, or mortality between study groups at 14 and 28 days. Further subgroup analysis demonstrated similar results among patients with MDR pathogens, primarily extended-spectrum ß-lactamases (ESBL). Interestingly, there was a more rapid return to baseline activity and functional capacity among patients randomized to a short course of therapy. The authors acknowledged that the patients’ perception of illness while taking antimicrobials may have influenced self-reported well-being and functional performance. In exploratory analysis, prolonged hospitalization and readmission were excluded from the primary study endpoint to mirror outcomes assessed by Nelson and colleagues. There were no statistically significant differences in death, relapses, or complications between groups randomized to short (18.6%) or long (15.1%) courses of therapy, with a risk difference of 3.5% (95% CI, –2.5% to 9.5%) in this study population.
Patients with Pseudomonas aeruginosa BSI often have more chronic medical comorbidities, immunocompromised conditions, higher severity of illness, and more indwelling catheters than do patients with Enterobacteriaceae BSI.32 It is uncertain whether shorter duration of therapy is generalizable to this population, given that Pseudomonas accounted for a relatively low number (8%) of infections in the published RCT.33 Fabre and colleagues included high-risk patients with >65% of the cohort with severe immunocompromised conditions consisting of stem cell transplantation, recent chemotherapy, or neutropenia, and they reported no difference in 30-day mortality or recurrent infections among patients with pseudomonal BSI regardless of duration of therapy.32
ORAL TREATMENT: CHALLENGING TRADITIONAL DOGMA
It is a well-accepted standard of practice that BSI are treated with upfront IV antimicrobials that can rapidly achieve therapeutic serum concentration. Whether IV administration is warranted for the entire duration of therapy, though, remains controversial. Even in an era of highly bioavailable oral antimicrobials, clinicians often assume that IV antimicrobials are more potent and efficacious than oral antimicrobials.8,9 This belief has contributed to the dogma that IV therapy is necessary irrespective of the associated risks and costs. Oral antimicrobials are often overlooked as alternatives despite established benefits in avoiding complications associated with IV catheters, decreasing hospital LOS, and improving quality of life.34 There are promising clinical data in support of the efficacy and safety of transitioning from sequential-IV to highly bioavailable oral agents for the treatment of uncomplicated bacteremia caused by both gram-positive and gram-negative pathogens.2,35 Highly bioavailable oral antimicrobials are also increasingly integrated as sequential therapy for deep-seated infections in bone and joint infections, such as vertebral osteomyelitis.19,36 These findings have been confirmed in a recent RCT demonstrating noninferiority of oral antimicrobial combinations after satisfactory clinical responses to at least 10 days of IV therapy, compared with continued IV regimens, in left-sided infective endocarditis.36 While not a prespecified endpoint, hospital LOS was shorter among patients randomized to oral antimicrobials.
Although there are no large-scale RCTs sufficiently powered to address the role of oral antimicrobials in the treatment of uncomplicated GN-BSI, some insights can be gleaned from the existing literature (Table 2). In the RCT establishing noninferiority of short vs long courses of antimicrobials for uncomplicated GN-BSI, the majority of patients randomized to 7 days vs 14 days of therapy, 64% and 81%, respectively, were de-escalated to oral antimicrobials, with fluoroquinolones (FQs) being the predominant (>70%) oral regimen, followed by trimethoprim/sulfamethoxazole (T/S) and oral ß-lactams.33
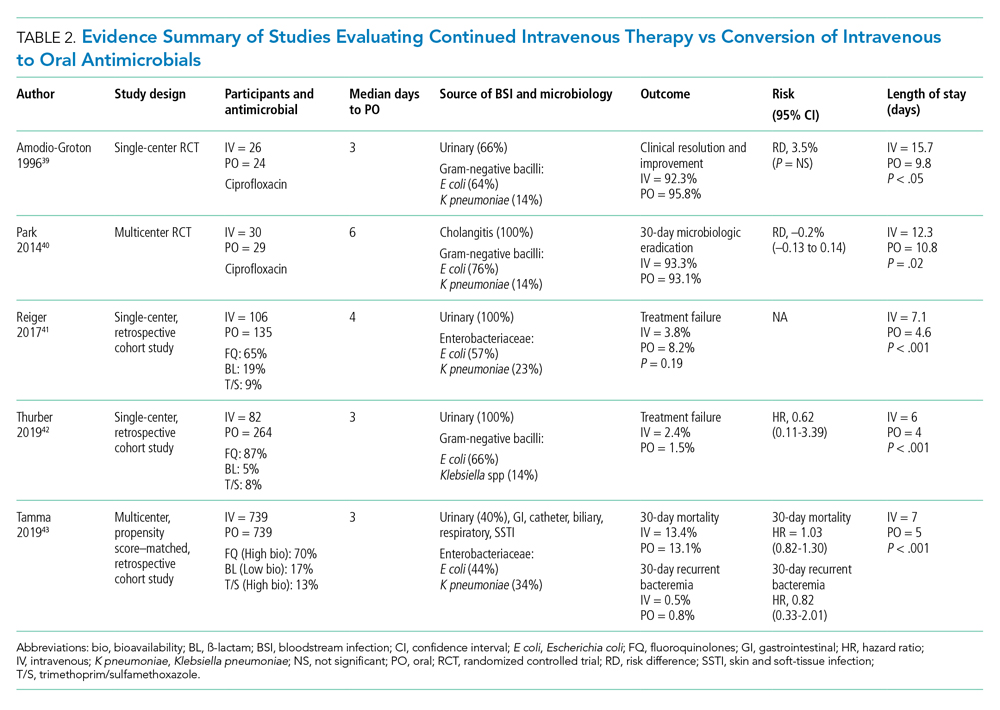
Despite the Food and Drug Administration warnings of the potentially permanent adverse effects involving tendons, muscles, joints, nerves, and most recently, aortic aneurysms and ruptures,37 FQs remain a unique class of drugs with favorable pharmacodynamic and pharmacokinetic properties that achieve approximately equivalent serum and tissue concentration when administered either intravenously or orally. This advantage was recognized early on as a potential IV-sparing therapeutic option. A prospective RCT that evaluated oral vs IV ciprofloxacin as initial empiric therapy among 141 patients with pyelonephritis or complicated UTI (38% with secondary BSI) reported no significant differences in microbiological failure or clinical response between the two treatment groups.38 Two small RCTs have also demonstrated the safety and effectiveness of sequential-IV antimicrobial to oral FQs in the setting of GN-BSI secondary to urinary source and cholangitis.39,40 Oral ß-lactams, however, achieve substantially lower serum concentration than do their IV counterparts and, accordingly, may be less reliably effective.2
Five retrospective cohort studies have more directly investigated the role of oral antimicrobials in the setting of GN-BSI secondary to common focal infections (Table 2 and Table 3).41-45 Two observational studies reported no difference of treatment failure among patients who received IV-only therapy vs those who were switched to oral therapy in bacteremia secondary to UTIs.41,42 Catheter-associated complications were higher in the IV cohort (6.1% vs 0.4%; P = .03).42 In the largest multicenter cohort study to date, which included 1,478 patients with Enterobacteriaceae bacteremia, there was no difference in 30-day mortality or recurrent bacteremia between patients converted to oral step-down therapy and patients who received the full course of IV antimicrobials.43 Furthermore, the median hospital LOS was shorter (5 days vs 7 days; P < .001) among patients who were transitioned to oral therapy, a finding that is consistent with other studies.39-42 In their analysis, the oral antimicrobials were categorized as low-bioavailability (ß-lactams) or high-bioavailability (FQ and T/S), and there was no difference in outcomes when results were stratified by bioavailability. Mercuro and colleagues reported similar clinical success among patients who received oral ß-lactams and those who received FQs as step-down therapy.44 Notably, patients were more likely to tolerate ß-lactams without experiencing adverse effects than were those who received FQs (91.7% vs 82.1%; P = .049). In contrast, Kutob and colleagues compared step-down oral antimicrobials categorized as low bioavailability (ß-lactams), moderate bioavailability (ciprofloxacin and T/S), and high bioavailability (levofloxacin). They reported that treatment failures were significantly higher among patients who received low-bioavailability (14%) and moderate-bioavailability (12%) antimicrobials, compared with those who received the high-bioavailability agent (2%; P = .02).45 Interestingly, the bioavailability of ciprofloxacin reaches 85% and T/S approaches 90%, and they are often categorized as highly bioavailable agents in other studies.43,46 If they were reclassified as highly bioavailable agents, the study conclusions might differ. Nevertheless, the reported success with oral step-down therapy exceeded 85% in all five studies.41-45
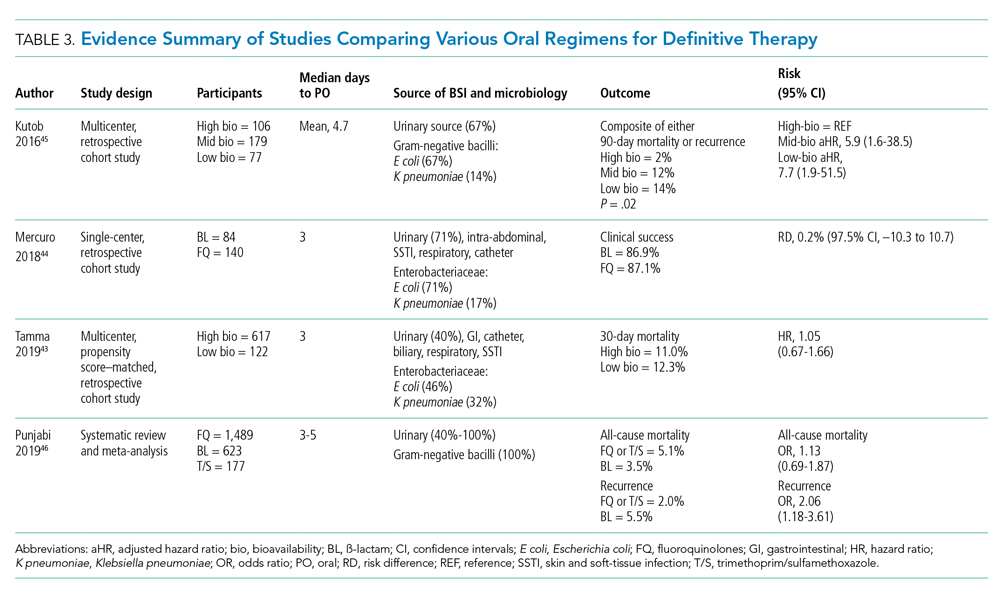
It is important to acknowledge the possibility of unmeasured confounders in these retrospective, observational studies despite statistical adjustments and that they are likely underpowered to determine the clinical significance of oral bioavailability of antimicrobials. In a meta-analysis of published studies and abstracts that included 2,289 patients with Enterobacteriaceae bacteremia, all-cause mortality was similar between patients de-escalated to an oral FQ, T/S, or ß-lactam.46 Overall recurrence of infection (bacteremia or primary site) occurred more frequently in patients transitioned to oral ß-lactams than FQs, but relapse of bacteremia was not statistically different between comparator groups. Bioavailability of the oral agents may not be the sole determinant of higher recurrence; adherence may be poor because of the more frequent dosing required for oral ß-lactams to achieve targeted pharmacokinetics. Additionally, suboptimal dosing of oral ß-lactams noted in the studies may have also contributed to the increased recurrences.
After source control has been achieved and bacterial inoculum burden is sufficiently reduced with appropriate upfront IV therapy, the bioavailability of oral antimicrobials may become less important. However, existing observational data indicate clinical experience is most established with highly bioavailable oral agents, particularly FQs, though the risks vs benefits require careful consideration. For now, the preferred oral agent remains uncertain and selections should be individualized based on susceptibility, patient factors, and other clinical considerations. More importantly, if there are no contraindications or concerns of malabsorption, oral step-down therapy should be initiated as soon as source control and good clinical responses have been achieved.
TEST OF CURE: RECONSIDERING FOLLOW-UP BLOOD CULTURES
Routine follow-up blood cultures (FUBCs) are strongly recommended in Staphylococcus aureus bacteremia because of the propensity for endovascular and metastatic infection, which dictates clinical decision-making regarding duration of therapy. In contrast, GN-BSI secondary to focal infections is usually transient, and the need for confirmation of blood culture clearance is less clear. The low yield of FUBC in many clinical settings suggests that it may not be helpful.47-50 Despite the questionable impact on the clinical management of GN-BSI, FUBCs are routinely ordered in the hospital.1,47 Although there is no high-quality evidence addressing the utility of FUBC, several observational studies suggest clinicians should reconsider routinely ordering FUBC.
Canzoneri and colleagues retrospectively evaluated 383 episodes of bacteremia with at least one FUBC drawn after the initial blood culture.48 On average, 2.32 FUBC were performed per patient for GN-BSI episode, and only 8 patients (5.7%) had persistent bacteremia. Specifically, only 3% had documented positive FUBC among patients with urinary tract source of infection. It was estimated that 17 FUBCs are needed to yield one positive result for GN-BSI. This finding is consistent with results from another study that examined 1,801 episodes of bacteremia, 901 of which were gram-negative organisms, predominantly (67%) Escherichia coli and Klebsiella spp.49 Among GN-BSI episodes, FUBCs were performed in 247 cases, with 27 (10.9%) cases demonstrating persistent bacteremia. A nested case-control analysis between patients with cleared or persistent bacteremia found a lower yield in FUBC with gram-negative organisms and a genitourinary source of infection. Moreover, persistent bacteremia did not influence a change in antimicrobial regimen. Kang and colleagues investigated 1,068 episodes of Klebsiella pneumoniae bacteremia, with FUBCs performed in 862 (80.7%) cases despite only a 7.2% incidence of persistent bacteremia.50 The independent risk factors associated with persistent bacteremia were intra-abdominal infection, solid organ transplantation, high Charlson comorbidity index score, and unfavorable treatment responses, which suggests the need for FUBC may be individualized rather than routine.
In the setting of GN-BSI in which the probability of persistent bacteremia is relatively low, especially in genitourinary sources of infection, FUBCs are not warranted. It is uncomfortable for patients and exposes them to harms of false-positive results, leading to antimicrobial administration with possible adverse effects, which can be further compounded by unnecessary testing, potentially missed alternative diagnosis, and increasing hospital LOS.1,51 Given the low yield of FUBC in GN-BSI, and the lack of association of persistent bacteremia with change in antimicrobial therapy or clinical outcomes, we recommend avoiding FUBC as a test of cure. Documentation of gram-negative blood culture clearance should be reserved for situations in which there is concern for deeper or otherwise uncontrolled source of infection.
CONCLUSION
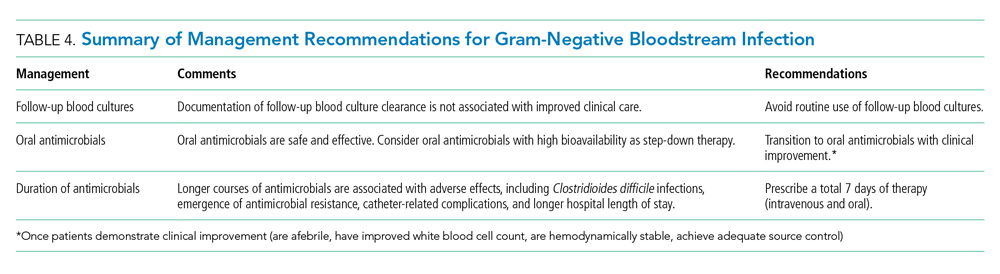
The optimal management of gram-negative bacteremia in hospitalized patients is evolving. There is a growing body of evidence supporting shorter duration for a total of 7 days with oral step-down therapy as safe and effective for patients with uncomplicated Enterobacteriaceae bacteremia who have achieved adequate source control and demonstrated clinical stability and improvement. Although comparative data regarding the optimal duration of therapy in the setting of MDR strains such as ESBL Enterobacteriaceae and pseudomonal BSI are limited, available data appear promising in favor of shorter treatment duration with oral step-down therapy. Routine follow-up blood culture is not cost-effective and may result in unnecessary healthcare resource utilization and inappropriate use of antimicrobials. Table 4 provides a framework for the clinical management of GN-BSI in the hospital. Taken together, these steps will facilitate antimicrobial stewardship, limit unnecessary antimicrobial exposure, and improve quality of patient care.
Disclosures
The authors have no conflicts of interest to disclose.
1. Cobrun B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood culture? JAMA . 2012;308(5):502-511. https://doi.org/10.1001/jama.2012.8262
2. Sutton JD, Sayood S, Spivak ES. Top questions in uncomplicated, non- Staphylococcus aureus bacteremia. Open Forum Infect Dis . 2018;5(5):ofy087. https://doi.org/10.1093/ofid/ofy087
3. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med . 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581ST
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis . 2016;63(4):e61-e111. https://doi.org/10.1093/cid/ciw353
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis . 2014;59(2):e10-e52. https://doi.org/10.1093/cid/ciu296
6. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infections in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis . 2010;50(2):133-164. https://doi.org/10.1086/649554
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of American and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis . 2011;52(5):e103-e120. https://doi.org/10.1093/cid/ciq257
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: a concise overview. J Pharmacol Pharmacother . 2014;5(2):83-87. https://doi.org/10.4103/0976-500X.130042
9. Broom J, Broom A, Adams K, Plage S. What prevents the intravenous to oral antibiotic switch? A qualitative study of hospital doctors’ accounts of what influences their clinical practice. J Antimicrob Chemother . 2016;71(8):2295-2299. https://doi.org/10.1093/jac/dkw129
10. Spellberg B. The maturing antibiotic mantra: “shorter is still better.” J Hosp Med. 2018;13(5):361-362. https://doi.org/10.12788/jhm.2904
11. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med . 2017;177(9):1308-1315. https://doi.org/10.1001/jamainternmed.2017.1938
12. Lin RY, Nuruzzaman F, Shah SN. Incidence and impact of adverse effects to antibiotics in hospitalized adults with pneumonia. J Hosp Med . 2009;4(2):E7-E15. https://doi.org/10.1002/jhm.414
13. Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis . 2011;53(1):42-48. https://doi.org/10.1093/cid/cir301.
14. Vaughn VM, Scott FA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients with hospitalized pneumonia – a multihospital cohort study. Ann Intern Med . 2019;171(3):153-163. https://doi.org/10.7326/M18-3640
15. Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of exposure to antipseudomonal ß -lactam antibiotics in the critically ill and development of new resistance. Pharmacotherapy . 2019;39(3):261-270. https://doi.org/10.1002/phar.2201
16. Dychter SS, Gold DA, Carson D, Haller M. Intravenous therapy: a review of complications and economic considerations of peripheral access. J Infus Nurs . 2012;35(2):84-91. https://doi.org/10.1097/NAN.0b013e31824237ce
17. Seddon MM, Bookstaver PB, Justo JA, et al. Role of early de-escalation of antimicrobial therapy on risk of Clostridioides difficile infection following Enterobacteriaceae bloodstream infections. Clin Infect Dis . 2019;69(3):414-420. https://doi.org/10.1093/cid/ciy863
18. Fallouh N, McGurik HM, Falnders SA, Chopra V. Peripherally inserted central catheter-associated deep vein thrombosis: a narrative review. Am J Med . 2015;128(7):722-738. https://doi.org/10.1016/j.amjmed.2015.01.027
19. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med . 2019;380:425-436. https://doi.org/10.1056/NEJMoa1710926
20. Hayashi Y, Peterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis . 2011;52(10):1232-1240. https://doi.org/10.1093/cid/cir063
21. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis . 2009;49(1):1-45. https://doi.org/10.1086/599376
22. Corona A, Bertolini G, Ricotta AM, Wilson A, Singer M. Variability of treatment duration for bacteraemia in the critically ill: a multinational survey. J Antimicrob Chemother . 2003;52(5):849-852. https://doi.org/10.1093/jac/dkg447
23. Diallo K, Thilly N, Luc A, et al. Management of bloodstream infections by infection specialists: an international ESCMID cross-sectional survey. Int J Antimicrob Agents . 2018;51(5):794-798. https://doi.org/10.1016/j.ijantimicag.2017.12.010
24. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection – 7 day or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother . 2013;68(10):2183-2191. https://doi.org/10.1093/jac/dkt177
25. Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care . 2011;15(6):R267. https://doi.org/10.1186/cc10545
26. Daneman N, Rishu AH, Xiong W, et al. Duration of antimicrobial treatment for bacteremia in Canadian critically ill patients. Crit Care Med . 2016;44(2):256-264. https://doi.org/10.1097/CCM.0000000000001393
27. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection . 2017;45(5):613-620. https://doi.org/10.1007/s15010-017-1020-5
28. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis . 2018;66:172-177. https://doi.org/10.1093/cid/cix767
29. Doi A, Morimoto T, Iwata K. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect . 2018;24(11):1184-1189. https://doi.org/10.1016/j.cmi.2018.01.021
30. Giannella M, Pascale R, Toschi A, et al. Treatment duration of Escherichia coli bloodstream infection and outcomes: retrospective single-center study. Clin Microbiol Infect . 2018;24(10):1077-1083. https://doi.org/10.1016/j.cmi.2018.01.013
31. Sousa A, Perez-Rodriguez MT, Suarez M, et al. Short- versus long-course therapy in gram-negative bacilli bloodstream infections. Eur J Clin Microbiol Infect Dis . 2019;38(5):851-857. https://doi.org/10.1007/s10096-019-03467-5.
32. Fabre V, Amoah J, Cosgrove SE, Tamma PD. Antibiotic therapy for Pseudomonas aeruginosa bloodstream infections: how long is long enough? Clin Infect Dis . 2019;69(11):2011-2014. https://doi.org/10.1093/cid/ciz223
33. Yahav D, Franceschini E, Koppel F, et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis . 2019;69(7):1091-1098. https://doi.org/10.1093/cid/ciy1054
34. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? an evidence-based narrative review. J Hosp Med . 2018;13(5):328-335. https://doi.org/10.12788/jhm.2949
35. Al-Hasan MN, Rac H. Transition from intravenous to oral antimicrobial therapy in patients with uncomplicated and complicated bloodstream infections. Clin Microbiol Infect. 2020;26(3):299-306. https://doi.org/10.1016/j.cmi.2019.05.012
36. Iversen K, Ihlemann N, Gill SU. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
37. Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Accessed October 30, 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics
38. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M. Oral vs intravenous ciprofloxacin in the initial empirical management of severe pyelonephritis or complicated urinary tract infections: a prospective randomized clinical trial. Arch Intern Med. 1999;159(1):53-58. https://doi.org/10.1001/archinte.159.1.53
39. Amodio-Groton M, Madu A, Madu CN, et al. Sequential parenteral and oral ciprofloxacin regimen versus parenteral therapy for bacteremia: a pharmacoeconomic analysis. Ann Pharmacother. 1996;30(6):596-602. http://doi.org/10.1177/106002809603000605
40. Park TY, Choi JS, Song TJ, Do JH, Choi SH, Oh HC. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59(11):2790-2796. https://doi.org/10.1007/s10620-014-3233-0
41. Rieger KL, Bosso JA, MacVane SH, Temple Z, Wahlquist A, Bohm N. Intravenous-only or intravenous transitioned to oral antimicrobials for Enterobacteriaceae-associated bacteremic urinary tract infection. Pharmacotherapy 2017;37(11):1479-1483. https://doi.org/10.1002/phar.2024
42. Thurber KM, Arnold JR, Narayanan PP, Dierkhising RA, Sampathkumar P. Comparison of intravenous and oral definitive antibiotic regimens in hospitalized patients with gram-negative bacteremia from a urinary tract infection. J Glob Antimicrob Resist. 2019;18:243-248. https://doi.org/10.1016/j.jgar.2019.03.013
43. Tamma PD, Conley AT, Cosgrove SE, et al. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019;179(3):316-323. https://doi.org/10.1001/jamainternmed.2018.6226
44. Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdwon therapy for Enterobacteriaceae bloodstream infections: fluoroquinolones versus ß-lactams. Int J Antimicrob Agents. 2018;51(5):687-692. https://doi.org/10.1016/j.ijantimicag.2017.12.007
45. Kutob LF, Justo JA, Bookstaver PB, et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. https//doi.org/10.1016/j.ijantimicag.2016.07.013
46. Punjabi C, Tien V, Meng L, et al. Oral fluoroquinolone or trimethoprim-sulfamethoxazole vs ß-lactams as step-down therapy for Enterobacteriaceae bacteremia: systematic review and meta-analysis. Open Forum Infect Dis. 2019;6(10):ofz364. https://doi.org/10.1093/ofid/ofz364
47. Chen AI, Bilker WB, Hamilton KW. Blood culture utilization at an academic hospital: addressing a gap in benchmarking. Infect Control Hosp Epidemiol. 2018:39(11):1353-1359. http://doi.org/10.1017/ice.2018.231
48. Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis. 2017;65(11):1776-1779. https://doi:10.1093/cid/cix648
49. Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis. 2016:16:286-295. https://doi.org/10.1186/s12879-016-1622-z
50. Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? a retrospective case-control study. BMC Infect Dis. 2013;13(1):365-372. https://doi.org/10.1186/1471-2334-13-365
51. Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. JAMA. 1991;265(3):365-369. https://doi:10.1001/jama.1991.03460030071031
Uncomplicated bacteremia, while not precisely defined in the literature, generally implies bacteremia in the absence of a persistent or difficult-to-eradicate infectious source. Bacteremia secondary to focal infections such as skin and soft-tissue infection, pneumonia, pyelonephritis, or urinary tract infection (UTI) accounts for up to 25% of bloodstream infections (BSIs) and usually resolves with prompt and appropriate antimicrobial therapy.1,2 Current practice guidelines do not adequately address key aspects of the optimal management of gram-negative (GN)–BSI commonly encountered in hospital care.3-7 Notably, antimicrobial duration, criteria to transition from intravenous (IV) to oral step-down therapy, choice of oral antimicrobials, and reassessment of follow-up blood cultures have not been addressed. In the absence of consensus guidelines, clinicians rely on “conventional wisdom” and clinical experience, which may not be supported by scientific rigor. A growing body of research now challenges some long-standing practices once thought to be standard of care.
In this narrative review, we aim to examine and synthesize emerging information to provide an evidence-based framework in the management of hospitalized patients with GN-BSI. We highlight the unintended consequences and potential harms of excessive antimicrobial exposure and focus on areas in the fundamental approach to duration of therapy, the role of oral antimicrobials, and usefulness of follow-up blood cultures. A comprehensive search of the published literature was performed in PubMed with an emphasis on articles published during 2015-2019 with use of search terms including gram-negative bacteremia, duration, antibiotics, adverse effects, intravascular catheter, and follow-up blood cultures.
ANTIMICROBIAL RISKS: ‘PRIMUM NON NOCERE’
Antimicrobial overuse is common and may be driven by concerns for undertreatment. Clinicians may believe that prolonged antimicrobial therapy maximizes cure rates, with treatment duration often defined arbitrarily by a fixed number of “Constantine-units” (dating back to the ancient Roman emperor’s decree of 7 days in a week).8-10 Recent publications refute this notion and point out that the harms of overprescribing outweigh the perceived benefits of longer treatment duration.
Antimicrobials are lifesaving but not benign; adverse effects are common and costly to our patients and healthcare system. Among 1,488 hospitalized adults who received at least 24 hours of systemic antimicrobials, 20% had an antimicrobial-associated adverse event, mostly gastrointestinal, renal, or hematologic in nature.11 Prolonged duration of antimicrobials is further associated with adverse effects such as antimicrobial-associated diarrhea, increased rates of Clostridioides difficile infection (CDI), emergence of antimicrobial resistance, and longer hospital length of stay (LOS).11-15 Vaughn and colleagues conducted the largest observational study to date, evaluating antimicrobial prescriptions for the treatment of nearly 6,500 adults with community-acquired pneumonia in a 43-hospital consortium in Michigan.14 More than two-thirds of patients received antimicrobial courses (median 8 days) that exceeded guideline-recommended duration. Patients who received longer antimicrobial courses did not have reduced mortality, readmission, or emergency department visits. More importantly, each excess day of treatment was associated with a relative 5% increase in the odds of antimicrobial-associated adverse effects reported by patients. This is further supported by national and state hospital data that antimicrobial-associated adverse events are an independent predictor of longer LOS.12
CDI is commonly linked to destructive changes to the indigenous microbiota of the intestinal flora caused by antimicrobial administration. Stevens and colleagues identified 7,792 hospitalized patients who received at least 2 consecutive days of antimicrobial therapy13; comparing 241 cases of CDI with the control group, they observed a dose-dependent risk of CDI associated with increasing cumulative dose, number of antimicrobials, and days of antimicrobial exposure. Compared with patients who received fewer than 4 days of antimicrobials, the adjusted hazard ratios (aHR) for those who received 4-7 days or 8-18 days of therapy were 1.4 (95% CI, 0.8-2.4) and 3.0 (95% CI, 1.9-5.0), respectively. This correlates to a threefold increase in CDI risk for patients who received more than 7 days of antimicrobials. More specifically, the empiric use of antipseudomonal ß-lactams (APBL) for more than 48 hours was also found to be an independent risk factor for CDI among 808 patients with Enterobacteriaceae BSI.16 The risk of CDI within 90 days of BSI was higher among those who received >48 hours of APBL than it was among those who received ≤48 hours (HR, 3.6; 95% CI, 1.5-9.9).
While C difficile may be the most well-known pathogen implicated in antimicrobial usage, the incidence of multidrug-resistant (MDR) organisms, either as infectious or colonizing pathogens, is also tied to antimicrobial exposure. Among patients receiving systemic antimicrobials, 6% developed an MDR infection within 90 days.11 Over a 5-year period, Teshome and colleagues evaluated 7,118 critically ill patients and demonstrated that prolonged exposures to APBLs increased the risk of new antimicrobial resistance within 60 days.15 This resistance pattern was not an institutional or environmental finding but a patient-level finding. For each additional day of cefepime or piperacillin/tazobactam received, the risk of new antimicrobial resistance was increased by 8%. The authors concluded that defining a piperacillin/tazobactam course as 10 vs 7 days would result in a 24% higher relative risk of resistance per patient related to those 3 additional days of antimicrobial exposure.
Catheter complications including thrombophlebitis, infiltration, and infection are serious and frequent problems associated with IV medication administration.17 Even with short-term use, peripherally inserted central catheters (PICCs) carry a substantial risk of venous thrombosis (superficial and deep veins). The incidence of deep vein thrombosis (DVT) for PICCs is estimated between 5% and 15% for hospitalized patients and 2% and 5% for ambulatory patients.18 A recent randomized controlled trial (RCT) of oral vs IV antimicrobials for bone and joint infections reported that, compared with patients randomized to oral antimicrobials, those randomized to IV antimicrobials were more likely to have catheter complications (9.4% vs 1.0%; P < .001) and to discontinue therapy earlier (18.9% vs 12.8%; P = .006).19 Median hospital stay was also significantly longer in the IV group (14 days vs 11 days; P < .001).
SHORTEST EFFECTIVE DURATION: LESS MAY BE MORE
Optimization of antimicrobial duration has long been recognized as one of the key strategies in reducing unnecessary antimicrobial exposure, yet high-quality evidence on comparative effectiveness of duration in the setting of bacteremia has been limited until recently.20 The presence of bacteremia is often used as a justification for prolonged courses of antimicrobial regardless of infection source or clinical response. The Infectious Diseases Society of America guidelines suggest 7 to 14 days of treatment for intravascular catheter-associated gram-negative bacteremia, but the optimal duration for non–catheter-related gram-negative bacteremia is not addressed.21 This lack of clear guidance and the historical scarcity of robust data make it difficult to inform best practices, which leads to wide variability in clinical practice and 14 days being the most prescribed duration.22,23
Pooled clinical trials’ data from subsets of patients with bacteremia and those from observational studies have been the best available evidence for the treatment duration of GN-BSI until recently (Table 1).24-32 Two meta-analyses evaluating RCTs of adult and pediatric patients with pyelonephritis, UTI, peritonitis, and pneumonia found no differences in clinical failure, microbiologic cure, or survival between short and long courses of therapy in the subset of patient with associated bacteremia.24,25 Six heterogeneous RCTs of short vs long courses of therapy for complicated UTI or pyelonephritis reported no differences in clinical cure rates in the subset of patients with associated GN-BSI.2 The observational studies outlined in Table 1 are also consistent with RCT results supporting noninferiority in clinical cure and mortality outcomes between short and long courses of therapy.26-32 These findings may also be extrapolated to immunocompromised hosts given a considerable representation of 10% to 47% of the study population with immunosuppressive conditions.

Nelson and colleagues conducted the only retrospective study to date reporting conflicting results of higher risk of treatment failure (defined as composite endpoint of mortality or recurrent infection within 90 days of index BSI) in patients receiving a short course of therapy.27 However, the difference was driven by 90-day mortality (8.2% vs 3.3%; P = .04) not recurrent infection (6.7% vs 6.5%; P = 0.93). Giannella and colleagues also evaluated 90-day mortality as a primary endpoint in a much larger cohort of over 850 patients in Italy and found no difference in mortality rates between short and long courses of antimicrobials.30
Yahav and colleagues conducted the first well-designed open-label RCT comparing short and long courses of antimicrobials in uncomplicated GN-BSI.33 This noninferiority study randomized more than 600 hospitalized patients with adequate source control who were afebrile and hemodynamically stable for ≥48 hours to receive either 7 days or 14 days of therapy. The source of infections was predominantly urinary (68%), and the causative pathogens were 90% Enterobacteriacae, including 20% MDR strains. The primary outcome was a composite of 90-day all-cause mortality or clinical failure defined as either relapse of bacteremia, local or distant complications, readmission, or extended hospital stay >14 days. The authors reported no statistically significant differences in the primary outcome between short (45.8%) and long (48.3%) courses of treatment. In the prespecified post hoc analysis designed to evaluate infection-related outcomes at an earlier time frame, there were no observed differences in complications, relapses, or mortality between study groups at 14 and 28 days. Further subgroup analysis demonstrated similar results among patients with MDR pathogens, primarily extended-spectrum ß-lactamases (ESBL). Interestingly, there was a more rapid return to baseline activity and functional capacity among patients randomized to a short course of therapy. The authors acknowledged that the patients’ perception of illness while taking antimicrobials may have influenced self-reported well-being and functional performance. In exploratory analysis, prolonged hospitalization and readmission were excluded from the primary study endpoint to mirror outcomes assessed by Nelson and colleagues. There were no statistically significant differences in death, relapses, or complications between groups randomized to short (18.6%) or long (15.1%) courses of therapy, with a risk difference of 3.5% (95% CI, –2.5% to 9.5%) in this study population.
Patients with Pseudomonas aeruginosa BSI often have more chronic medical comorbidities, immunocompromised conditions, higher severity of illness, and more indwelling catheters than do patients with Enterobacteriaceae BSI.32 It is uncertain whether shorter duration of therapy is generalizable to this population, given that Pseudomonas accounted for a relatively low number (8%) of infections in the published RCT.33 Fabre and colleagues included high-risk patients with >65% of the cohort with severe immunocompromised conditions consisting of stem cell transplantation, recent chemotherapy, or neutropenia, and they reported no difference in 30-day mortality or recurrent infections among patients with pseudomonal BSI regardless of duration of therapy.32
ORAL TREATMENT: CHALLENGING TRADITIONAL DOGMA
It is a well-accepted standard of practice that BSI are treated with upfront IV antimicrobials that can rapidly achieve therapeutic serum concentration. Whether IV administration is warranted for the entire duration of therapy, though, remains controversial. Even in an era of highly bioavailable oral antimicrobials, clinicians often assume that IV antimicrobials are more potent and efficacious than oral antimicrobials.8,9 This belief has contributed to the dogma that IV therapy is necessary irrespective of the associated risks and costs. Oral antimicrobials are often overlooked as alternatives despite established benefits in avoiding complications associated with IV catheters, decreasing hospital LOS, and improving quality of life.34 There are promising clinical data in support of the efficacy and safety of transitioning from sequential-IV to highly bioavailable oral agents for the treatment of uncomplicated bacteremia caused by both gram-positive and gram-negative pathogens.2,35 Highly bioavailable oral antimicrobials are also increasingly integrated as sequential therapy for deep-seated infections in bone and joint infections, such as vertebral osteomyelitis.19,36 These findings have been confirmed in a recent RCT demonstrating noninferiority of oral antimicrobial combinations after satisfactory clinical responses to at least 10 days of IV therapy, compared with continued IV regimens, in left-sided infective endocarditis.36 While not a prespecified endpoint, hospital LOS was shorter among patients randomized to oral antimicrobials.
Although there are no large-scale RCTs sufficiently powered to address the role of oral antimicrobials in the treatment of uncomplicated GN-BSI, some insights can be gleaned from the existing literature (Table 2). In the RCT establishing noninferiority of short vs long courses of antimicrobials for uncomplicated GN-BSI, the majority of patients randomized to 7 days vs 14 days of therapy, 64% and 81%, respectively, were de-escalated to oral antimicrobials, with fluoroquinolones (FQs) being the predominant (>70%) oral regimen, followed by trimethoprim/sulfamethoxazole (T/S) and oral ß-lactams.33

Despite the Food and Drug Administration warnings of the potentially permanent adverse effects involving tendons, muscles, joints, nerves, and most recently, aortic aneurysms and ruptures,37 FQs remain a unique class of drugs with favorable pharmacodynamic and pharmacokinetic properties that achieve approximately equivalent serum and tissue concentration when administered either intravenously or orally. This advantage was recognized early on as a potential IV-sparing therapeutic option. A prospective RCT that evaluated oral vs IV ciprofloxacin as initial empiric therapy among 141 patients with pyelonephritis or complicated UTI (38% with secondary BSI) reported no significant differences in microbiological failure or clinical response between the two treatment groups.38 Two small RCTs have also demonstrated the safety and effectiveness of sequential-IV antimicrobial to oral FQs in the setting of GN-BSI secondary to urinary source and cholangitis.39,40 Oral ß-lactams, however, achieve substantially lower serum concentration than do their IV counterparts and, accordingly, may be less reliably effective.2
Five retrospective cohort studies have more directly investigated the role of oral antimicrobials in the setting of GN-BSI secondary to common focal infections (Table 2 and Table 3).41-45 Two observational studies reported no difference of treatment failure among patients who received IV-only therapy vs those who were switched to oral therapy in bacteremia secondary to UTIs.41,42 Catheter-associated complications were higher in the IV cohort (6.1% vs 0.4%; P = .03).42 In the largest multicenter cohort study to date, which included 1,478 patients with Enterobacteriaceae bacteremia, there was no difference in 30-day mortality or recurrent bacteremia between patients converted to oral step-down therapy and patients who received the full course of IV antimicrobials.43 Furthermore, the median hospital LOS was shorter (5 days vs 7 days; P < .001) among patients who were transitioned to oral therapy, a finding that is consistent with other studies.39-42 In their analysis, the oral antimicrobials were categorized as low-bioavailability (ß-lactams) or high-bioavailability (FQ and T/S), and there was no difference in outcomes when results were stratified by bioavailability. Mercuro and colleagues reported similar clinical success among patients who received oral ß-lactams and those who received FQs as step-down therapy.44 Notably, patients were more likely to tolerate ß-lactams without experiencing adverse effects than were those who received FQs (91.7% vs 82.1%; P = .049). In contrast, Kutob and colleagues compared step-down oral antimicrobials categorized as low bioavailability (ß-lactams), moderate bioavailability (ciprofloxacin and T/S), and high bioavailability (levofloxacin). They reported that treatment failures were significantly higher among patients who received low-bioavailability (14%) and moderate-bioavailability (12%) antimicrobials, compared with those who received the high-bioavailability agent (2%; P = .02).45 Interestingly, the bioavailability of ciprofloxacin reaches 85% and T/S approaches 90%, and they are often categorized as highly bioavailable agents in other studies.43,46 If they were reclassified as highly bioavailable agents, the study conclusions might differ. Nevertheless, the reported success with oral step-down therapy exceeded 85% in all five studies.41-45

It is important to acknowledge the possibility of unmeasured confounders in these retrospective, observational studies despite statistical adjustments and that they are likely underpowered to determine the clinical significance of oral bioavailability of antimicrobials. In a meta-analysis of published studies and abstracts that included 2,289 patients with Enterobacteriaceae bacteremia, all-cause mortality was similar between patients de-escalated to an oral FQ, T/S, or ß-lactam.46 Overall recurrence of infection (bacteremia or primary site) occurred more frequently in patients transitioned to oral ß-lactams than FQs, but relapse of bacteremia was not statistically different between comparator groups. Bioavailability of the oral agents may not be the sole determinant of higher recurrence; adherence may be poor because of the more frequent dosing required for oral ß-lactams to achieve targeted pharmacokinetics. Additionally, suboptimal dosing of oral ß-lactams noted in the studies may have also contributed to the increased recurrences.
After source control has been achieved and bacterial inoculum burden is sufficiently reduced with appropriate upfront IV therapy, the bioavailability of oral antimicrobials may become less important. However, existing observational data indicate clinical experience is most established with highly bioavailable oral agents, particularly FQs, though the risks vs benefits require careful consideration. For now, the preferred oral agent remains uncertain and selections should be individualized based on susceptibility, patient factors, and other clinical considerations. More importantly, if there are no contraindications or concerns of malabsorption, oral step-down therapy should be initiated as soon as source control and good clinical responses have been achieved.
TEST OF CURE: RECONSIDERING FOLLOW-UP BLOOD CULTURES
Routine follow-up blood cultures (FUBCs) are strongly recommended in Staphylococcus aureus bacteremia because of the propensity for endovascular and metastatic infection, which dictates clinical decision-making regarding duration of therapy. In contrast, GN-BSI secondary to focal infections is usually transient, and the need for confirmation of blood culture clearance is less clear. The low yield of FUBC in many clinical settings suggests that it may not be helpful.47-50 Despite the questionable impact on the clinical management of GN-BSI, FUBCs are routinely ordered in the hospital.1,47 Although there is no high-quality evidence addressing the utility of FUBC, several observational studies suggest clinicians should reconsider routinely ordering FUBC.
Canzoneri and colleagues retrospectively evaluated 383 episodes of bacteremia with at least one FUBC drawn after the initial blood culture.48 On average, 2.32 FUBC were performed per patient for GN-BSI episode, and only 8 patients (5.7%) had persistent bacteremia. Specifically, only 3% had documented positive FUBC among patients with urinary tract source of infection. It was estimated that 17 FUBCs are needed to yield one positive result for GN-BSI. This finding is consistent with results from another study that examined 1,801 episodes of bacteremia, 901 of which were gram-negative organisms, predominantly (67%) Escherichia coli and Klebsiella spp.49 Among GN-BSI episodes, FUBCs were performed in 247 cases, with 27 (10.9%) cases demonstrating persistent bacteremia. A nested case-control analysis between patients with cleared or persistent bacteremia found a lower yield in FUBC with gram-negative organisms and a genitourinary source of infection. Moreover, persistent bacteremia did not influence a change in antimicrobial regimen. Kang and colleagues investigated 1,068 episodes of Klebsiella pneumoniae bacteremia, with FUBCs performed in 862 (80.7%) cases despite only a 7.2% incidence of persistent bacteremia.50 The independent risk factors associated with persistent bacteremia were intra-abdominal infection, solid organ transplantation, high Charlson comorbidity index score, and unfavorable treatment responses, which suggests the need for FUBC may be individualized rather than routine.
In the setting of GN-BSI in which the probability of persistent bacteremia is relatively low, especially in genitourinary sources of infection, FUBCs are not warranted. It is uncomfortable for patients and exposes them to harms of false-positive results, leading to antimicrobial administration with possible adverse effects, which can be further compounded by unnecessary testing, potentially missed alternative diagnosis, and increasing hospital LOS.1,51 Given the low yield of FUBC in GN-BSI, and the lack of association of persistent bacteremia with change in antimicrobial therapy or clinical outcomes, we recommend avoiding FUBC as a test of cure. Documentation of gram-negative blood culture clearance should be reserved for situations in which there is concern for deeper or otherwise uncontrolled source of infection.
CONCLUSION
The optimal management of gram-negative bacteremia in hospitalized patients is evolving. There is a growing body of evidence supporting shorter duration for a total of 7 days with oral step-down therapy as safe and effective for patients with uncomplicated Enterobacteriaceae bacteremia who have achieved adequate source control and demonstrated clinical stability and improvement. Although comparative data regarding the optimal duration of therapy in the setting of MDR strains such as ESBL Enterobacteriaceae and pseudomonal BSI are limited, available data appear promising in favor of shorter treatment duration with oral step-down therapy. Routine follow-up blood culture is not cost-effective and may result in unnecessary healthcare resource utilization and inappropriate use of antimicrobials. Table 4 provides a framework for the clinical management of GN-BSI in the hospital. Taken together, these steps will facilitate antimicrobial stewardship, limit unnecessary antimicrobial exposure, and improve quality of patient care.

Disclosures
The authors have no conflicts of interest to disclose.
Uncomplicated bacteremia, while not precisely defined in the literature, generally implies bacteremia in the absence of a persistent or difficult-to-eradicate infectious source. Bacteremia secondary to focal infections such as skin and soft-tissue infection, pneumonia, pyelonephritis, or urinary tract infection (UTI) accounts for up to 25% of bloodstream infections (BSIs) and usually resolves with prompt and appropriate antimicrobial therapy.1,2 Current practice guidelines do not adequately address key aspects of the optimal management of gram-negative (GN)–BSI commonly encountered in hospital care.3-7 Notably, antimicrobial duration, criteria to transition from intravenous (IV) to oral step-down therapy, choice of oral antimicrobials, and reassessment of follow-up blood cultures have not been addressed. In the absence of consensus guidelines, clinicians rely on “conventional wisdom” and clinical experience, which may not be supported by scientific rigor. A growing body of research now challenges some long-standing practices once thought to be standard of care.
In this narrative review, we aim to examine and synthesize emerging information to provide an evidence-based framework in the management of hospitalized patients with GN-BSI. We highlight the unintended consequences and potential harms of excessive antimicrobial exposure and focus on areas in the fundamental approach to duration of therapy, the role of oral antimicrobials, and usefulness of follow-up blood cultures. A comprehensive search of the published literature was performed in PubMed with an emphasis on articles published during 2015-2019 with use of search terms including gram-negative bacteremia, duration, antibiotics, adverse effects, intravascular catheter, and follow-up blood cultures.
ANTIMICROBIAL RISKS: ‘PRIMUM NON NOCERE’
Antimicrobial overuse is common and may be driven by concerns for undertreatment. Clinicians may believe that prolonged antimicrobial therapy maximizes cure rates, with treatment duration often defined arbitrarily by a fixed number of “Constantine-units” (dating back to the ancient Roman emperor’s decree of 7 days in a week).8-10 Recent publications refute this notion and point out that the harms of overprescribing outweigh the perceived benefits of longer treatment duration.
Antimicrobials are lifesaving but not benign; adverse effects are common and costly to our patients and healthcare system. Among 1,488 hospitalized adults who received at least 24 hours of systemic antimicrobials, 20% had an antimicrobial-associated adverse event, mostly gastrointestinal, renal, or hematologic in nature.11 Prolonged duration of antimicrobials is further associated with adverse effects such as antimicrobial-associated diarrhea, increased rates of Clostridioides difficile infection (CDI), emergence of antimicrobial resistance, and longer hospital length of stay (LOS).11-15 Vaughn and colleagues conducted the largest observational study to date, evaluating antimicrobial prescriptions for the treatment of nearly 6,500 adults with community-acquired pneumonia in a 43-hospital consortium in Michigan.14 More than two-thirds of patients received antimicrobial courses (median 8 days) that exceeded guideline-recommended duration. Patients who received longer antimicrobial courses did not have reduced mortality, readmission, or emergency department visits. More importantly, each excess day of treatment was associated with a relative 5% increase in the odds of antimicrobial-associated adverse effects reported by patients. This is further supported by national and state hospital data that antimicrobial-associated adverse events are an independent predictor of longer LOS.12
CDI is commonly linked to destructive changes to the indigenous microbiota of the intestinal flora caused by antimicrobial administration. Stevens and colleagues identified 7,792 hospitalized patients who received at least 2 consecutive days of antimicrobial therapy13; comparing 241 cases of CDI with the control group, they observed a dose-dependent risk of CDI associated with increasing cumulative dose, number of antimicrobials, and days of antimicrobial exposure. Compared with patients who received fewer than 4 days of antimicrobials, the adjusted hazard ratios (aHR) for those who received 4-7 days or 8-18 days of therapy were 1.4 (95% CI, 0.8-2.4) and 3.0 (95% CI, 1.9-5.0), respectively. This correlates to a threefold increase in CDI risk for patients who received more than 7 days of antimicrobials. More specifically, the empiric use of antipseudomonal ß-lactams (APBL) for more than 48 hours was also found to be an independent risk factor for CDI among 808 patients with Enterobacteriaceae BSI.16 The risk of CDI within 90 days of BSI was higher among those who received >48 hours of APBL than it was among those who received ≤48 hours (HR, 3.6; 95% CI, 1.5-9.9).
While C difficile may be the most well-known pathogen implicated in antimicrobial usage, the incidence of multidrug-resistant (MDR) organisms, either as infectious or colonizing pathogens, is also tied to antimicrobial exposure. Among patients receiving systemic antimicrobials, 6% developed an MDR infection within 90 days.11 Over a 5-year period, Teshome and colleagues evaluated 7,118 critically ill patients and demonstrated that prolonged exposures to APBLs increased the risk of new antimicrobial resistance within 60 days.15 This resistance pattern was not an institutional or environmental finding but a patient-level finding. For each additional day of cefepime or piperacillin/tazobactam received, the risk of new antimicrobial resistance was increased by 8%. The authors concluded that defining a piperacillin/tazobactam course as 10 vs 7 days would result in a 24% higher relative risk of resistance per patient related to those 3 additional days of antimicrobial exposure.
Catheter complications including thrombophlebitis, infiltration, and infection are serious and frequent problems associated with IV medication administration.17 Even with short-term use, peripherally inserted central catheters (PICCs) carry a substantial risk of venous thrombosis (superficial and deep veins). The incidence of deep vein thrombosis (DVT) for PICCs is estimated between 5% and 15% for hospitalized patients and 2% and 5% for ambulatory patients.18 A recent randomized controlled trial (RCT) of oral vs IV antimicrobials for bone and joint infections reported that, compared with patients randomized to oral antimicrobials, those randomized to IV antimicrobials were more likely to have catheter complications (9.4% vs 1.0%; P < .001) and to discontinue therapy earlier (18.9% vs 12.8%; P = .006).19 Median hospital stay was also significantly longer in the IV group (14 days vs 11 days; P < .001).
SHORTEST EFFECTIVE DURATION: LESS MAY BE MORE
Optimization of antimicrobial duration has long been recognized as one of the key strategies in reducing unnecessary antimicrobial exposure, yet high-quality evidence on comparative effectiveness of duration in the setting of bacteremia has been limited until recently.20 The presence of bacteremia is often used as a justification for prolonged courses of antimicrobial regardless of infection source or clinical response. The Infectious Diseases Society of America guidelines suggest 7 to 14 days of treatment for intravascular catheter-associated gram-negative bacteremia, but the optimal duration for non–catheter-related gram-negative bacteremia is not addressed.21 This lack of clear guidance and the historical scarcity of robust data make it difficult to inform best practices, which leads to wide variability in clinical practice and 14 days being the most prescribed duration.22,23
Pooled clinical trials’ data from subsets of patients with bacteremia and those from observational studies have been the best available evidence for the treatment duration of GN-BSI until recently (Table 1).24-32 Two meta-analyses evaluating RCTs of adult and pediatric patients with pyelonephritis, UTI, peritonitis, and pneumonia found no differences in clinical failure, microbiologic cure, or survival between short and long courses of therapy in the subset of patient with associated bacteremia.24,25 Six heterogeneous RCTs of short vs long courses of therapy for complicated UTI or pyelonephritis reported no differences in clinical cure rates in the subset of patients with associated GN-BSI.2 The observational studies outlined in Table 1 are also consistent with RCT results supporting noninferiority in clinical cure and mortality outcomes between short and long courses of therapy.26-32 These findings may also be extrapolated to immunocompromised hosts given a considerable representation of 10% to 47% of the study population with immunosuppressive conditions.

Nelson and colleagues conducted the only retrospective study to date reporting conflicting results of higher risk of treatment failure (defined as composite endpoint of mortality or recurrent infection within 90 days of index BSI) in patients receiving a short course of therapy.27 However, the difference was driven by 90-day mortality (8.2% vs 3.3%; P = .04) not recurrent infection (6.7% vs 6.5%; P = 0.93). Giannella and colleagues also evaluated 90-day mortality as a primary endpoint in a much larger cohort of over 850 patients in Italy and found no difference in mortality rates between short and long courses of antimicrobials.30
Yahav and colleagues conducted the first well-designed open-label RCT comparing short and long courses of antimicrobials in uncomplicated GN-BSI.33 This noninferiority study randomized more than 600 hospitalized patients with adequate source control who were afebrile and hemodynamically stable for ≥48 hours to receive either 7 days or 14 days of therapy. The source of infections was predominantly urinary (68%), and the causative pathogens were 90% Enterobacteriacae, including 20% MDR strains. The primary outcome was a composite of 90-day all-cause mortality or clinical failure defined as either relapse of bacteremia, local or distant complications, readmission, or extended hospital stay >14 days. The authors reported no statistically significant differences in the primary outcome between short (45.8%) and long (48.3%) courses of treatment. In the prespecified post hoc analysis designed to evaluate infection-related outcomes at an earlier time frame, there were no observed differences in complications, relapses, or mortality between study groups at 14 and 28 days. Further subgroup analysis demonstrated similar results among patients with MDR pathogens, primarily extended-spectrum ß-lactamases (ESBL). Interestingly, there was a more rapid return to baseline activity and functional capacity among patients randomized to a short course of therapy. The authors acknowledged that the patients’ perception of illness while taking antimicrobials may have influenced self-reported well-being and functional performance. In exploratory analysis, prolonged hospitalization and readmission were excluded from the primary study endpoint to mirror outcomes assessed by Nelson and colleagues. There were no statistically significant differences in death, relapses, or complications between groups randomized to short (18.6%) or long (15.1%) courses of therapy, with a risk difference of 3.5% (95% CI, –2.5% to 9.5%) in this study population.
Patients with Pseudomonas aeruginosa BSI often have more chronic medical comorbidities, immunocompromised conditions, higher severity of illness, and more indwelling catheters than do patients with Enterobacteriaceae BSI.32 It is uncertain whether shorter duration of therapy is generalizable to this population, given that Pseudomonas accounted for a relatively low number (8%) of infections in the published RCT.33 Fabre and colleagues included high-risk patients with >65% of the cohort with severe immunocompromised conditions consisting of stem cell transplantation, recent chemotherapy, or neutropenia, and they reported no difference in 30-day mortality or recurrent infections among patients with pseudomonal BSI regardless of duration of therapy.32
ORAL TREATMENT: CHALLENGING TRADITIONAL DOGMA
It is a well-accepted standard of practice that BSI are treated with upfront IV antimicrobials that can rapidly achieve therapeutic serum concentration. Whether IV administration is warranted for the entire duration of therapy, though, remains controversial. Even in an era of highly bioavailable oral antimicrobials, clinicians often assume that IV antimicrobials are more potent and efficacious than oral antimicrobials.8,9 This belief has contributed to the dogma that IV therapy is necessary irrespective of the associated risks and costs. Oral antimicrobials are often overlooked as alternatives despite established benefits in avoiding complications associated with IV catheters, decreasing hospital LOS, and improving quality of life.34 There are promising clinical data in support of the efficacy and safety of transitioning from sequential-IV to highly bioavailable oral agents for the treatment of uncomplicated bacteremia caused by both gram-positive and gram-negative pathogens.2,35 Highly bioavailable oral antimicrobials are also increasingly integrated as sequential therapy for deep-seated infections in bone and joint infections, such as vertebral osteomyelitis.19,36 These findings have been confirmed in a recent RCT demonstrating noninferiority of oral antimicrobial combinations after satisfactory clinical responses to at least 10 days of IV therapy, compared with continued IV regimens, in left-sided infective endocarditis.36 While not a prespecified endpoint, hospital LOS was shorter among patients randomized to oral antimicrobials.
Although there are no large-scale RCTs sufficiently powered to address the role of oral antimicrobials in the treatment of uncomplicated GN-BSI, some insights can be gleaned from the existing literature (Table 2). In the RCT establishing noninferiority of short vs long courses of antimicrobials for uncomplicated GN-BSI, the majority of patients randomized to 7 days vs 14 days of therapy, 64% and 81%, respectively, were de-escalated to oral antimicrobials, with fluoroquinolones (FQs) being the predominant (>70%) oral regimen, followed by trimethoprim/sulfamethoxazole (T/S) and oral ß-lactams.33

Despite the Food and Drug Administration warnings of the potentially permanent adverse effects involving tendons, muscles, joints, nerves, and most recently, aortic aneurysms and ruptures,37 FQs remain a unique class of drugs with favorable pharmacodynamic and pharmacokinetic properties that achieve approximately equivalent serum and tissue concentration when administered either intravenously or orally. This advantage was recognized early on as a potential IV-sparing therapeutic option. A prospective RCT that evaluated oral vs IV ciprofloxacin as initial empiric therapy among 141 patients with pyelonephritis or complicated UTI (38% with secondary BSI) reported no significant differences in microbiological failure or clinical response between the two treatment groups.38 Two small RCTs have also demonstrated the safety and effectiveness of sequential-IV antimicrobial to oral FQs in the setting of GN-BSI secondary to urinary source and cholangitis.39,40 Oral ß-lactams, however, achieve substantially lower serum concentration than do their IV counterparts and, accordingly, may be less reliably effective.2
Five retrospective cohort studies have more directly investigated the role of oral antimicrobials in the setting of GN-BSI secondary to common focal infections (Table 2 and Table 3).41-45 Two observational studies reported no difference of treatment failure among patients who received IV-only therapy vs those who were switched to oral therapy in bacteremia secondary to UTIs.41,42 Catheter-associated complications were higher in the IV cohort (6.1% vs 0.4%; P = .03).42 In the largest multicenter cohort study to date, which included 1,478 patients with Enterobacteriaceae bacteremia, there was no difference in 30-day mortality or recurrent bacteremia between patients converted to oral step-down therapy and patients who received the full course of IV antimicrobials.43 Furthermore, the median hospital LOS was shorter (5 days vs 7 days; P < .001) among patients who were transitioned to oral therapy, a finding that is consistent with other studies.39-42 In their analysis, the oral antimicrobials were categorized as low-bioavailability (ß-lactams) or high-bioavailability (FQ and T/S), and there was no difference in outcomes when results were stratified by bioavailability. Mercuro and colleagues reported similar clinical success among patients who received oral ß-lactams and those who received FQs as step-down therapy.44 Notably, patients were more likely to tolerate ß-lactams without experiencing adverse effects than were those who received FQs (91.7% vs 82.1%; P = .049). In contrast, Kutob and colleagues compared step-down oral antimicrobials categorized as low bioavailability (ß-lactams), moderate bioavailability (ciprofloxacin and T/S), and high bioavailability (levofloxacin). They reported that treatment failures were significantly higher among patients who received low-bioavailability (14%) and moderate-bioavailability (12%) antimicrobials, compared with those who received the high-bioavailability agent (2%; P = .02).45 Interestingly, the bioavailability of ciprofloxacin reaches 85% and T/S approaches 90%, and they are often categorized as highly bioavailable agents in other studies.43,46 If they were reclassified as highly bioavailable agents, the study conclusions might differ. Nevertheless, the reported success with oral step-down therapy exceeded 85% in all five studies.41-45

It is important to acknowledge the possibility of unmeasured confounders in these retrospective, observational studies despite statistical adjustments and that they are likely underpowered to determine the clinical significance of oral bioavailability of antimicrobials. In a meta-analysis of published studies and abstracts that included 2,289 patients with Enterobacteriaceae bacteremia, all-cause mortality was similar between patients de-escalated to an oral FQ, T/S, or ß-lactam.46 Overall recurrence of infection (bacteremia or primary site) occurred more frequently in patients transitioned to oral ß-lactams than FQs, but relapse of bacteremia was not statistically different between comparator groups. Bioavailability of the oral agents may not be the sole determinant of higher recurrence; adherence may be poor because of the more frequent dosing required for oral ß-lactams to achieve targeted pharmacokinetics. Additionally, suboptimal dosing of oral ß-lactams noted in the studies may have also contributed to the increased recurrences.
After source control has been achieved and bacterial inoculum burden is sufficiently reduced with appropriate upfront IV therapy, the bioavailability of oral antimicrobials may become less important. However, existing observational data indicate clinical experience is most established with highly bioavailable oral agents, particularly FQs, though the risks vs benefits require careful consideration. For now, the preferred oral agent remains uncertain and selections should be individualized based on susceptibility, patient factors, and other clinical considerations. More importantly, if there are no contraindications or concerns of malabsorption, oral step-down therapy should be initiated as soon as source control and good clinical responses have been achieved.
TEST OF CURE: RECONSIDERING FOLLOW-UP BLOOD CULTURES
Routine follow-up blood cultures (FUBCs) are strongly recommended in Staphylococcus aureus bacteremia because of the propensity for endovascular and metastatic infection, which dictates clinical decision-making regarding duration of therapy. In contrast, GN-BSI secondary to focal infections is usually transient, and the need for confirmation of blood culture clearance is less clear. The low yield of FUBC in many clinical settings suggests that it may not be helpful.47-50 Despite the questionable impact on the clinical management of GN-BSI, FUBCs are routinely ordered in the hospital.1,47 Although there is no high-quality evidence addressing the utility of FUBC, several observational studies suggest clinicians should reconsider routinely ordering FUBC.
Canzoneri and colleagues retrospectively evaluated 383 episodes of bacteremia with at least one FUBC drawn after the initial blood culture.48 On average, 2.32 FUBC were performed per patient for GN-BSI episode, and only 8 patients (5.7%) had persistent bacteremia. Specifically, only 3% had documented positive FUBC among patients with urinary tract source of infection. It was estimated that 17 FUBCs are needed to yield one positive result for GN-BSI. This finding is consistent with results from another study that examined 1,801 episodes of bacteremia, 901 of which were gram-negative organisms, predominantly (67%) Escherichia coli and Klebsiella spp.49 Among GN-BSI episodes, FUBCs were performed in 247 cases, with 27 (10.9%) cases demonstrating persistent bacteremia. A nested case-control analysis between patients with cleared or persistent bacteremia found a lower yield in FUBC with gram-negative organisms and a genitourinary source of infection. Moreover, persistent bacteremia did not influence a change in antimicrobial regimen. Kang and colleagues investigated 1,068 episodes of Klebsiella pneumoniae bacteremia, with FUBCs performed in 862 (80.7%) cases despite only a 7.2% incidence of persistent bacteremia.50 The independent risk factors associated with persistent bacteremia were intra-abdominal infection, solid organ transplantation, high Charlson comorbidity index score, and unfavorable treatment responses, which suggests the need for FUBC may be individualized rather than routine.
In the setting of GN-BSI in which the probability of persistent bacteremia is relatively low, especially in genitourinary sources of infection, FUBCs are not warranted. It is uncomfortable for patients and exposes them to harms of false-positive results, leading to antimicrobial administration with possible adverse effects, which can be further compounded by unnecessary testing, potentially missed alternative diagnosis, and increasing hospital LOS.1,51 Given the low yield of FUBC in GN-BSI, and the lack of association of persistent bacteremia with change in antimicrobial therapy or clinical outcomes, we recommend avoiding FUBC as a test of cure. Documentation of gram-negative blood culture clearance should be reserved for situations in which there is concern for deeper or otherwise uncontrolled source of infection.
CONCLUSION
The optimal management of gram-negative bacteremia in hospitalized patients is evolving. There is a growing body of evidence supporting shorter duration for a total of 7 days with oral step-down therapy as safe and effective for patients with uncomplicated Enterobacteriaceae bacteremia who have achieved adequate source control and demonstrated clinical stability and improvement. Although comparative data regarding the optimal duration of therapy in the setting of MDR strains such as ESBL Enterobacteriaceae and pseudomonal BSI are limited, available data appear promising in favor of shorter treatment duration with oral step-down therapy. Routine follow-up blood culture is not cost-effective and may result in unnecessary healthcare resource utilization and inappropriate use of antimicrobials. Table 4 provides a framework for the clinical management of GN-BSI in the hospital. Taken together, these steps will facilitate antimicrobial stewardship, limit unnecessary antimicrobial exposure, and improve quality of patient care.

Disclosures
The authors have no conflicts of interest to disclose.
1. Cobrun B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood culture? JAMA . 2012;308(5):502-511. https://doi.org/10.1001/jama.2012.8262
2. Sutton JD, Sayood S, Spivak ES. Top questions in uncomplicated, non- Staphylococcus aureus bacteremia. Open Forum Infect Dis . 2018;5(5):ofy087. https://doi.org/10.1093/ofid/ofy087
3. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med . 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581ST
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis . 2016;63(4):e61-e111. https://doi.org/10.1093/cid/ciw353
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis . 2014;59(2):e10-e52. https://doi.org/10.1093/cid/ciu296
6. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infections in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis . 2010;50(2):133-164. https://doi.org/10.1086/649554
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of American and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis . 2011;52(5):e103-e120. https://doi.org/10.1093/cid/ciq257
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: a concise overview. J Pharmacol Pharmacother . 2014;5(2):83-87. https://doi.org/10.4103/0976-500X.130042
9. Broom J, Broom A, Adams K, Plage S. What prevents the intravenous to oral antibiotic switch? A qualitative study of hospital doctors’ accounts of what influences their clinical practice. J Antimicrob Chemother . 2016;71(8):2295-2299. https://doi.org/10.1093/jac/dkw129
10. Spellberg B. The maturing antibiotic mantra: “shorter is still better.” J Hosp Med. 2018;13(5):361-362. https://doi.org/10.12788/jhm.2904
11. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med . 2017;177(9):1308-1315. https://doi.org/10.1001/jamainternmed.2017.1938
12. Lin RY, Nuruzzaman F, Shah SN. Incidence and impact of adverse effects to antibiotics in hospitalized adults with pneumonia. J Hosp Med . 2009;4(2):E7-E15. https://doi.org/10.1002/jhm.414
13. Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis . 2011;53(1):42-48. https://doi.org/10.1093/cid/cir301.
14. Vaughn VM, Scott FA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients with hospitalized pneumonia – a multihospital cohort study. Ann Intern Med . 2019;171(3):153-163. https://doi.org/10.7326/M18-3640
15. Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of exposure to antipseudomonal ß -lactam antibiotics in the critically ill and development of new resistance. Pharmacotherapy . 2019;39(3):261-270. https://doi.org/10.1002/phar.2201
16. Dychter SS, Gold DA, Carson D, Haller M. Intravenous therapy: a review of complications and economic considerations of peripheral access. J Infus Nurs . 2012;35(2):84-91. https://doi.org/10.1097/NAN.0b013e31824237ce
17. Seddon MM, Bookstaver PB, Justo JA, et al. Role of early de-escalation of antimicrobial therapy on risk of Clostridioides difficile infection following Enterobacteriaceae bloodstream infections. Clin Infect Dis . 2019;69(3):414-420. https://doi.org/10.1093/cid/ciy863
18. Fallouh N, McGurik HM, Falnders SA, Chopra V. Peripherally inserted central catheter-associated deep vein thrombosis: a narrative review. Am J Med . 2015;128(7):722-738. https://doi.org/10.1016/j.amjmed.2015.01.027
19. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med . 2019;380:425-436. https://doi.org/10.1056/NEJMoa1710926
20. Hayashi Y, Peterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis . 2011;52(10):1232-1240. https://doi.org/10.1093/cid/cir063
21. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis . 2009;49(1):1-45. https://doi.org/10.1086/599376
22. Corona A, Bertolini G, Ricotta AM, Wilson A, Singer M. Variability of treatment duration for bacteraemia in the critically ill: a multinational survey. J Antimicrob Chemother . 2003;52(5):849-852. https://doi.org/10.1093/jac/dkg447
23. Diallo K, Thilly N, Luc A, et al. Management of bloodstream infections by infection specialists: an international ESCMID cross-sectional survey. Int J Antimicrob Agents . 2018;51(5):794-798. https://doi.org/10.1016/j.ijantimicag.2017.12.010
24. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection – 7 day or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother . 2013;68(10):2183-2191. https://doi.org/10.1093/jac/dkt177
25. Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care . 2011;15(6):R267. https://doi.org/10.1186/cc10545
26. Daneman N, Rishu AH, Xiong W, et al. Duration of antimicrobial treatment for bacteremia in Canadian critically ill patients. Crit Care Med . 2016;44(2):256-264. https://doi.org/10.1097/CCM.0000000000001393
27. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection . 2017;45(5):613-620. https://doi.org/10.1007/s15010-017-1020-5
28. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis . 2018;66:172-177. https://doi.org/10.1093/cid/cix767
29. Doi A, Morimoto T, Iwata K. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect . 2018;24(11):1184-1189. https://doi.org/10.1016/j.cmi.2018.01.021
30. Giannella M, Pascale R, Toschi A, et al. Treatment duration of Escherichia coli bloodstream infection and outcomes: retrospective single-center study. Clin Microbiol Infect . 2018;24(10):1077-1083. https://doi.org/10.1016/j.cmi.2018.01.013
31. Sousa A, Perez-Rodriguez MT, Suarez M, et al. Short- versus long-course therapy in gram-negative bacilli bloodstream infections. Eur J Clin Microbiol Infect Dis . 2019;38(5):851-857. https://doi.org/10.1007/s10096-019-03467-5.
32. Fabre V, Amoah J, Cosgrove SE, Tamma PD. Antibiotic therapy for Pseudomonas aeruginosa bloodstream infections: how long is long enough? Clin Infect Dis . 2019;69(11):2011-2014. https://doi.org/10.1093/cid/ciz223
33. Yahav D, Franceschini E, Koppel F, et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis . 2019;69(7):1091-1098. https://doi.org/10.1093/cid/ciy1054
34. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? an evidence-based narrative review. J Hosp Med . 2018;13(5):328-335. https://doi.org/10.12788/jhm.2949
35. Al-Hasan MN, Rac H. Transition from intravenous to oral antimicrobial therapy in patients with uncomplicated and complicated bloodstream infections. Clin Microbiol Infect. 2020;26(3):299-306. https://doi.org/10.1016/j.cmi.2019.05.012
36. Iversen K, Ihlemann N, Gill SU. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
37. Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Accessed October 30, 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics
38. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M. Oral vs intravenous ciprofloxacin in the initial empirical management of severe pyelonephritis or complicated urinary tract infections: a prospective randomized clinical trial. Arch Intern Med. 1999;159(1):53-58. https://doi.org/10.1001/archinte.159.1.53
39. Amodio-Groton M, Madu A, Madu CN, et al. Sequential parenteral and oral ciprofloxacin regimen versus parenteral therapy for bacteremia: a pharmacoeconomic analysis. Ann Pharmacother. 1996;30(6):596-602. http://doi.org/10.1177/106002809603000605
40. Park TY, Choi JS, Song TJ, Do JH, Choi SH, Oh HC. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59(11):2790-2796. https://doi.org/10.1007/s10620-014-3233-0
41. Rieger KL, Bosso JA, MacVane SH, Temple Z, Wahlquist A, Bohm N. Intravenous-only or intravenous transitioned to oral antimicrobials for Enterobacteriaceae-associated bacteremic urinary tract infection. Pharmacotherapy 2017;37(11):1479-1483. https://doi.org/10.1002/phar.2024
42. Thurber KM, Arnold JR, Narayanan PP, Dierkhising RA, Sampathkumar P. Comparison of intravenous and oral definitive antibiotic regimens in hospitalized patients with gram-negative bacteremia from a urinary tract infection. J Glob Antimicrob Resist. 2019;18:243-248. https://doi.org/10.1016/j.jgar.2019.03.013
43. Tamma PD, Conley AT, Cosgrove SE, et al. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019;179(3):316-323. https://doi.org/10.1001/jamainternmed.2018.6226
44. Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdwon therapy for Enterobacteriaceae bloodstream infections: fluoroquinolones versus ß-lactams. Int J Antimicrob Agents. 2018;51(5):687-692. https://doi.org/10.1016/j.ijantimicag.2017.12.007
45. Kutob LF, Justo JA, Bookstaver PB, et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. https//doi.org/10.1016/j.ijantimicag.2016.07.013
46. Punjabi C, Tien V, Meng L, et al. Oral fluoroquinolone or trimethoprim-sulfamethoxazole vs ß-lactams as step-down therapy for Enterobacteriaceae bacteremia: systematic review and meta-analysis. Open Forum Infect Dis. 2019;6(10):ofz364. https://doi.org/10.1093/ofid/ofz364
47. Chen AI, Bilker WB, Hamilton KW. Blood culture utilization at an academic hospital: addressing a gap in benchmarking. Infect Control Hosp Epidemiol. 2018:39(11):1353-1359. http://doi.org/10.1017/ice.2018.231
48. Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis. 2017;65(11):1776-1779. https://doi:10.1093/cid/cix648
49. Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis. 2016:16:286-295. https://doi.org/10.1186/s12879-016-1622-z
50. Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? a retrospective case-control study. BMC Infect Dis. 2013;13(1):365-372. https://doi.org/10.1186/1471-2334-13-365
51. Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. JAMA. 1991;265(3):365-369. https://doi:10.1001/jama.1991.03460030071031
1. Cobrun B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood culture? JAMA . 2012;308(5):502-511. https://doi.org/10.1001/jama.2012.8262
2. Sutton JD, Sayood S, Spivak ES. Top questions in uncomplicated, non- Staphylococcus aureus bacteremia. Open Forum Infect Dis . 2018;5(5):ofy087. https://doi.org/10.1093/ofid/ofy087
3. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med . 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581ST
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis . 2016;63(4):e61-e111. https://doi.org/10.1093/cid/ciw353
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis . 2014;59(2):e10-e52. https://doi.org/10.1093/cid/ciu296
6. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infections in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis . 2010;50(2):133-164. https://doi.org/10.1086/649554
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of American and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis . 2011;52(5):e103-e120. https://doi.org/10.1093/cid/ciq257
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: a concise overview. J Pharmacol Pharmacother . 2014;5(2):83-87. https://doi.org/10.4103/0976-500X.130042
9. Broom J, Broom A, Adams K, Plage S. What prevents the intravenous to oral antibiotic switch? A qualitative study of hospital doctors’ accounts of what influences their clinical practice. J Antimicrob Chemother . 2016;71(8):2295-2299. https://doi.org/10.1093/jac/dkw129
10. Spellberg B. The maturing antibiotic mantra: “shorter is still better.” J Hosp Med. 2018;13(5):361-362. https://doi.org/10.12788/jhm.2904
11. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med . 2017;177(9):1308-1315. https://doi.org/10.1001/jamainternmed.2017.1938
12. Lin RY, Nuruzzaman F, Shah SN. Incidence and impact of adverse effects to antibiotics in hospitalized adults with pneumonia. J Hosp Med . 2009;4(2):E7-E15. https://doi.org/10.1002/jhm.414
13. Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis . 2011;53(1):42-48. https://doi.org/10.1093/cid/cir301.
14. Vaughn VM, Scott FA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients with hospitalized pneumonia – a multihospital cohort study. Ann Intern Med . 2019;171(3):153-163. https://doi.org/10.7326/M18-3640
15. Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of exposure to antipseudomonal ß -lactam antibiotics in the critically ill and development of new resistance. Pharmacotherapy . 2019;39(3):261-270. https://doi.org/10.1002/phar.2201
16. Dychter SS, Gold DA, Carson D, Haller M. Intravenous therapy: a review of complications and economic considerations of peripheral access. J Infus Nurs . 2012;35(2):84-91. https://doi.org/10.1097/NAN.0b013e31824237ce
17. Seddon MM, Bookstaver PB, Justo JA, et al. Role of early de-escalation of antimicrobial therapy on risk of Clostridioides difficile infection following Enterobacteriaceae bloodstream infections. Clin Infect Dis . 2019;69(3):414-420. https://doi.org/10.1093/cid/ciy863
18. Fallouh N, McGurik HM, Falnders SA, Chopra V. Peripherally inserted central catheter-associated deep vein thrombosis: a narrative review. Am J Med . 2015;128(7):722-738. https://doi.org/10.1016/j.amjmed.2015.01.027
19. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med . 2019;380:425-436. https://doi.org/10.1056/NEJMoa1710926
20. Hayashi Y, Peterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis . 2011;52(10):1232-1240. https://doi.org/10.1093/cid/cir063
21. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis . 2009;49(1):1-45. https://doi.org/10.1086/599376
22. Corona A, Bertolini G, Ricotta AM, Wilson A, Singer M. Variability of treatment duration for bacteraemia in the critically ill: a multinational survey. J Antimicrob Chemother . 2003;52(5):849-852. https://doi.org/10.1093/jac/dkg447
23. Diallo K, Thilly N, Luc A, et al. Management of bloodstream infections by infection specialists: an international ESCMID cross-sectional survey. Int J Antimicrob Agents . 2018;51(5):794-798. https://doi.org/10.1016/j.ijantimicag.2017.12.010
24. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection – 7 day or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother . 2013;68(10):2183-2191. https://doi.org/10.1093/jac/dkt177
25. Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care . 2011;15(6):R267. https://doi.org/10.1186/cc10545
26. Daneman N, Rishu AH, Xiong W, et al. Duration of antimicrobial treatment for bacteremia in Canadian critically ill patients. Crit Care Med . 2016;44(2):256-264. https://doi.org/10.1097/CCM.0000000000001393
27. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection . 2017;45(5):613-620. https://doi.org/10.1007/s15010-017-1020-5
28. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis . 2018;66:172-177. https://doi.org/10.1093/cid/cix767
29. Doi A, Morimoto T, Iwata K. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect . 2018;24(11):1184-1189. https://doi.org/10.1016/j.cmi.2018.01.021
30. Giannella M, Pascale R, Toschi A, et al. Treatment duration of Escherichia coli bloodstream infection and outcomes: retrospective single-center study. Clin Microbiol Infect . 2018;24(10):1077-1083. https://doi.org/10.1016/j.cmi.2018.01.013
31. Sousa A, Perez-Rodriguez MT, Suarez M, et al. Short- versus long-course therapy in gram-negative bacilli bloodstream infections. Eur J Clin Microbiol Infect Dis . 2019;38(5):851-857. https://doi.org/10.1007/s10096-019-03467-5.
32. Fabre V, Amoah J, Cosgrove SE, Tamma PD. Antibiotic therapy for Pseudomonas aeruginosa bloodstream infections: how long is long enough? Clin Infect Dis . 2019;69(11):2011-2014. https://doi.org/10.1093/cid/ciz223
33. Yahav D, Franceschini E, Koppel F, et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis . 2019;69(7):1091-1098. https://doi.org/10.1093/cid/ciy1054
34. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? an evidence-based narrative review. J Hosp Med . 2018;13(5):328-335. https://doi.org/10.12788/jhm.2949
35. Al-Hasan MN, Rac H. Transition from intravenous to oral antimicrobial therapy in patients with uncomplicated and complicated bloodstream infections. Clin Microbiol Infect. 2020;26(3):299-306. https://doi.org/10.1016/j.cmi.2019.05.012
36. Iversen K, Ihlemann N, Gill SU. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
37. Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Accessed October 30, 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics
38. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M. Oral vs intravenous ciprofloxacin in the initial empirical management of severe pyelonephritis or complicated urinary tract infections: a prospective randomized clinical trial. Arch Intern Med. 1999;159(1):53-58. https://doi.org/10.1001/archinte.159.1.53
39. Amodio-Groton M, Madu A, Madu CN, et al. Sequential parenteral and oral ciprofloxacin regimen versus parenteral therapy for bacteremia: a pharmacoeconomic analysis. Ann Pharmacother. 1996;30(6):596-602. http://doi.org/10.1177/106002809603000605
40. Park TY, Choi JS, Song TJ, Do JH, Choi SH, Oh HC. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59(11):2790-2796. https://doi.org/10.1007/s10620-014-3233-0
41. Rieger KL, Bosso JA, MacVane SH, Temple Z, Wahlquist A, Bohm N. Intravenous-only or intravenous transitioned to oral antimicrobials for Enterobacteriaceae-associated bacteremic urinary tract infection. Pharmacotherapy 2017;37(11):1479-1483. https://doi.org/10.1002/phar.2024
42. Thurber KM, Arnold JR, Narayanan PP, Dierkhising RA, Sampathkumar P. Comparison of intravenous and oral definitive antibiotic regimens in hospitalized patients with gram-negative bacteremia from a urinary tract infection. J Glob Antimicrob Resist. 2019;18:243-248. https://doi.org/10.1016/j.jgar.2019.03.013
43. Tamma PD, Conley AT, Cosgrove SE, et al. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019;179(3):316-323. https://doi.org/10.1001/jamainternmed.2018.6226
44. Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdwon therapy for Enterobacteriaceae bloodstream infections: fluoroquinolones versus ß-lactams. Int J Antimicrob Agents. 2018;51(5):687-692. https://doi.org/10.1016/j.ijantimicag.2017.12.007
45. Kutob LF, Justo JA, Bookstaver PB, et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. https//doi.org/10.1016/j.ijantimicag.2016.07.013
46. Punjabi C, Tien V, Meng L, et al. Oral fluoroquinolone or trimethoprim-sulfamethoxazole vs ß-lactams as step-down therapy for Enterobacteriaceae bacteremia: systematic review and meta-analysis. Open Forum Infect Dis. 2019;6(10):ofz364. https://doi.org/10.1093/ofid/ofz364
47. Chen AI, Bilker WB, Hamilton KW. Blood culture utilization at an academic hospital: addressing a gap in benchmarking. Infect Control Hosp Epidemiol. 2018:39(11):1353-1359. http://doi.org/10.1017/ice.2018.231
48. Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis. 2017;65(11):1776-1779. https://doi:10.1093/cid/cix648
49. Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis. 2016:16:286-295. https://doi.org/10.1186/s12879-016-1622-z
50. Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? a retrospective case-control study. BMC Infect Dis. 2013;13(1):365-372. https://doi.org/10.1186/1471-2334-13-365
51. Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. JAMA. 1991;265(3):365-369. https://doi:10.1001/jama.1991.03460030071031
© 2020 Society of Hospital Medicine
Hospitalized Medical Patients with Posttraumatic Stress Disorder (PTSD): Review of the Literature and a Roadmap for Improved Care
Posttraumatic stress disorder (PTSD) is a syndrome that occurs after exposure to a significant traumatic event and is characterized by persistent, debilitating symptoms that fall into four “diagnostic clusters” as outlined in the Diagnostic and Statistical Manual of Mental Disorders-Version V (DSM-V). Patients may experience intrusive thoughts, avoidance of distressing stimuli, persistent negative mood, and hypervigilance, all of which last longer than 1 month.1
A national survey of United States households conducted during 2001-2003 estimated the 12-month prevalence of PTSD among adults to be 3.5%.2 Lifetime prevalence has been found to be between 6.8%3 and 7.8%.4 PTSD is more common in veterans. The prevalence of PTSD in veterans differs depending on the conflict in which the veteran participated. Vietnam veterans have an estimated lifetime prevalence of approximately 30%,5,6 Gulf War veterans approximately 15%,7 and veterans of more recent conflicts in Afghanistan and Iraq of approximately 21%.8 With the MISSION Act moving more veteran care into the private sector, non-VA inpatient providers will need to become better versed in PTSD.9
Patients with PTSD have more contact with the healthcare system, even for non–mental health problems,8,10-13 and a significantly higher burden of medical comorbities,14 such as diabetes mellitus, liver disease, gastritis and gastric ulcers, HIV, arthritis,15 and coronary heart disease.16 Veterans with PTSD are hospitalized three times more often than are those with no mental health diagnoses,8 and patients with psychiatric comorbidities have higher lengths of stay.17 More than 1.4 million hospitalizations occurring during 2002-2011 had either a primary or secondary associated diagnosis of PTSD, with total inflation-adjusted charges of 34.9 billion dollars.18 In the inpatient sample from this study, greater than half were admitted for a primary diagnosis of mental diseases and disorders (Major Diagnostic Category [MDC] 19). Following mental illness, the most common primary diagnoses for men were MDC 5 (Circulatory System, 12.1%), MDC 20 (Alcohol/Drug Use or Induced Mental Disorder, 9.2%), and MDC 4 (Respiratory System, 7.4%), while the most common categories for women were MDC 20 (Alcohol/Drug Use or Induced Mental Disorder, 5.8%), MDC 21 (Injuries, Poison, and Toxic Effect of Drugs, 4.9%), and MDC 6 (Digestive System, 4.5%).18
In both the inpatient and outpatient settings, a fundamental challenge to comprehensive PTSD management is correctly diagnosing this condition.19 Confounding the difficulties in diagnosis are numerous comorbidities. In addition to the physical comorbidities described above, more than 70% of patients with PTSD have another psychological comorbidity such as affective disorders, anxiety disorders, or substance use disorder/dependency.20
Given that PTSD may be an underrecognized burden on the healthcare system, we sought to better understand how PTSD could affect hospitalized patients admitted for medical problems by conducting this narrative review. Additionally, three of the authors collaborated with the VA Employee Education Service to conduct a needs assessment of VA hospitalists in 2013. Respondents identified managing and educating patients and families about PTSD as a major educational need (unpublished data available upon request from the corresponding author). Therefore, our aims were to present a synthesis of existing literature, familiarize readers with the tenets of trauma-informed care as a framework to guide care for these patients, and generate ideas for changes that inpatient providers could implement now. We began by consulting a research librarian at the Clement J. Zablocki VA Medical Center in Milwaukee, Wisconsin, who searched the following databases: PsycInfo, CINAHL, MEDLINE, and PILOTS (a PTSD/trauma specific database). Search terms included hospital, hospitalized, and hospitalization, as well as traumatic stress, posttraumatic stress, and PTSD. Pertinent guidelines and the reference lists from included papers were examined. We focused on papers that described patients admitted for medical problems other than PTSD because those patients who are admitted for PTSD-related problems should be primarily managed by psychiatry (not hospitalists) with the primary focus of their hospitalization being their PTSD. We also excluded papers about patients developing PTSD secondary to hospitalization, which already has a well-developed literature.21-23
THE LITERATURE ABOUT PTSD IN HOSPITALIZED PATIENTS
The literature is sparse describing frequency or type of problems encountered by hospitalized medical patients with PTSD. A recent small survey study reported that 40% of patients anticipated triggers for their PTSD symptoms in the hospital; such triggers included loud noises and being shaken awake.24 Two papers describe case vignettes of patients who had exacerbations of their PTSD while in the Intensive Care Unit (ICU), although neither contain frequency or severity data.25,26 Approximately 8% of patients in VA ICUs have PTSD,27 and a published abstract suggests that they appear to require more sedation than do patients without PTSD.28 Another published case report describes a patient with recurrent PTSD symptoms (nightmares) after moving into a nursing home.29 These papers suggest other providers have recognized and are concerned about hospitalized patients with PTSD. At present, there are no data to quantify how often hospitalized patients have PTSD exacerbations or how troublesome such exacerbations are to these patients.
Given that there is little empiric literature to guide inpatient management of PTSD as a comorbidity in hospitalized medical patients, we extrapolate some information from the outpatient setting. PTSD is often underdiagnosed and underreported by individual patients in the outpatient setting.30 Failure to have an associated diagnosis of PTSD may lead to underrecognition and undertreatment of these patients by inpatient providers in the hospital setting. Additionally, the numerous psychological and physical comorbidities in PTSD can create unique challenges in properly managing any single problem in these patients.20 Armed with this knowledge, providers should be vigilant in the recognition, assessment, and treatment of PTSD.
INPATIENT MANAGEMENT OF PTSD
Trauma-Informed Care: A Conceptual Model
Trauma-informed care is a mindful and sensitive approach to caring for patients who have suffered trauma.31 It requires understanding that many people have suffered trauma in their lives and that the trauma continues to impact many aspects of their lives.32 Trauma-informed care has many advocates and has been implemented across myriad health and social services settings.33 Its principles can be applied in both the inpatient and outpatient hospital settings. While it is an appropriate approach to patients with PTSD, it is not specific to PTSD. People who have suffered sexual trauma, intimate partner violence, child abuse, or other exposures would also be included in the group of people for whom trauma-informed care is a suitable approach. There are four key assumptions to a trauma-informed approach to care (the 4 R’s): (1) realization that trauma affects an individual’s coping strategies, relationships, and health; (2) recognition of the signs of trauma; (3) having an appropriate, planned response to patients identified as having suffered a trauma; and (4) resisting retraumatization in the care setting.31,32
General Approach to Treating Medical Patients With PTSD in the Inpatient Setting
Recognition
Consistent with a trauma-informed care approach, inpatient providers should be able to recognize patients who may have PTSD. First, careful review of the past medical history may show some patients already carry this diagnosis. Second, patients with PTSD often have other comorbidities that could offer a clue that PTSD could be present as well; for example, risk for PTSD is increased when mood, anxiety, or substance use disorders are present.20 When PTSD is suspected, screening is a reasonable next step.
The Primary Care-PTSD-5 (PC-PTSD-5) is a validated screening tool used in the outpatient setting.34 It is easily administered and has good predictive validity (positive likelihood ratio [LR+] of 6.33 and LR– of 0.06). It begins with a question of whether the patient has ever experienced a trauma. A positive initial response triggers a series of five yes/no questions. Answering “yes” to three or more questions is a positive screen. A positive screen should result in consultation to psychiatry to conduct more formal evaluation and guide longer-term management.
Collaboration
Individual trauma-focused psychotherapy is the primary treatment of choice for PTSD with strong evidence supporting its practice.35 This treatment is administered by a psychiatrist or psychologist and will be limited in the inpatient medical setting. Current recommendations suggest pharmacotherapy only when individualized trauma-focused psychotherapy is not available, the patient declines it, or as an adjunct when psychotherapy alone is not effective.36 Therefore, inpatient providers may see patients who are prescribed selective serotonin reuptake inhibitors (eg, paroxetine, fluoxetine) or serotonin and norepinephrine reuptake inhibitors (eg, venlafaxine).36 In the past, PTSD-related nightmares were often treated with prazosin.37 However, a recent randomized controlled trial of prazosin in veterans with PTSD failed to show significant improvement in nightmares.38 Hence, current guidelines do not recommend prazosin as a first-line therapy.39 For hospitalized patients with PTSD symptoms refractory to the interventions outlined herein, particularly those patients with possible borderline personality traits (as suggested by severe anger and impulsivity), we strongly recommend partnering with psychiatry. Finally, given the high prevalence of substance use disorders (SUDs) in PTSD patients, awareness and treatment of comorbidities such as opioid and alcohol dependence must be concurrently addressed.
Individualizing Care
It is essential for the healthcare team to identify ways to meet each patient’s immediate needs. Many of the ideas proposed below are not specific to PTSD; many require an interprofessional approach to care.40 From a trauma-informed care standpoint, this is akin to having a planned response for patients who have suffered trauma. Assessing the individual’s needs and incorporating therapeutic modalities such as reflective listening, broadening safe opportunities for control, and providing complementary and integrative medicine (IM) therapies may help manage symptoms and establish rapport.41 Through reflective listening, a collaborative approach can be established to identify background, triggers, and a safe approach for managing PTSD and its comorbid conditions. Ensuring frequent communication and allowing the patient to be at the center of decision-making establishes a safe environment and promotes positive rapport between the patient and healthcare team.36 Providing a sense of control by involving the patients in their healthcare decisions and in the structure of care delivery may benefit the patients’ well-being. Furthermore, incorporating IM encourages rest and relaxation in the chaotic hospital environment. Suggested IM interventions include deep breathing, aromatherapy, guided imagery, muscle relaxation, and music therapy.42,43
Key Inpatient Issues Affecting PTSD
In the following sections, we outline common clinical situations that may exacerbate PTSD symptoms and propose some evidence-based responses (Table). In general, nonpharmacologic approaches are favored over pharmacologic approaches for patients with PTSD.
Sleep Hygiene
Sleep problems are very common in patients with PTSD, with nightmares occurring in more than 70% of patients and insomnia in 80%.44 In PTSD, sleep problems are linked to poor physical health and other health outcomes45,46 and may exacerbate other PTSD symptoms.4
Treating the sleep problems that occur with PTSD is an important aspect of PTSD care. Usually administered in the outpatient setting, the treatment of choice is cognitive-behavioral therapy (CBT).48 Sleep-specific CBT focuses, among other things, on strategies that encourage good sleep hygiene,49 which includes promoting regular sleep/wake-up times and specific bedtime routines, avoiding stimulation (eg, light, noise, TV) or excessive liquids before bed, refraining from daytime naps, and using relaxation techniques. Many of these recommendations seem at odds with hospital routines, which may contribute to decompensation of hospitalized patients with PTSD.
While starting sleep-specific CBT in the hospital may not be realistic, we suggest the following goals and strategies as a starting place for promoting healthy sleep for hospitalized patients with PTSD. To begin, factors affecting sleep hygiene should be addressed. Inpatient providers could pay more attention to intravenous (IV) fluid orders, perhaps adjusting them to run only during the daytime hours. Medications can be scheduled at times conducive to maintaining home routines. Avoiding the administration of diuretics close to bedtime may decrease the likelihood of frequent nighttime wakening. Grouping patient care activities, such as bathing or wound care, during daytime hours may allow more opportunities for rest at night. Incorporating uninterrupted sleep protocols, such as quiet hours between 10
Second, providers need to ask about established home bedtime routines and facilitate implementation in the hospital. Through collaboration with patients, providers can incorporate an individualized plan of care for sleep early in hospitalization.50 Partnering with nurses is also essential to creating a sleep-friendly environment that can improve patient experiences.51 Breathing exercises, meditating, listening to music and praying are all examples of “bedtime wind down” strategies recommended in sleep-specific CBT.49 Many of these could be successfully implemented in the hospital and may benefit other hospitalized patients too.52 In patients with PTSD and obstructive sleep apnea, continuous positive airway pressure (CPAP) reduces nightmares, and if inpatients are on CPAP at home, it should be continued in the hospital.53
Pain
If sleep disturbance is the hallmark of PTSD,47 chronic pain is its coconspirator.15 Uncontrolled pain can make it much more difficult to treat patients with PTSD, which in turn may lead to further decompensation from a mental health standpoint.54 SUDs such as alcohol or opioid dependencies are highly comorbid with PTSD45 and introduce a layer of complexity when managing painin these patients. Providers should be thoughtful when electing to treat acute or chronic pain with opioids and take particular care to establish realistic therapeutic goals if doing so. While patients with PTSD have a greater likelihood of having an SUD, undertreating pain risks exacerbating underlying PTSD symptoms.
Nonpharmacologic therapies, which include communicating, listening, and expressing compassion and understanding, should be utilized by inpatient providers as a first-line treatment in patients with PTSD who suffer from pain. Additionally, relaxation techniques, physical therapy, and physical activity55 can be offered. Pharmacologically, nonopioid medications such as acetaminophen or NSAIDs should always be considered first. Should the use of opioids be deemed necessary, inpatient providers should preferentially use oral over intravenous medications and consider establishing a fixed timeframe for short-term opioids, which should be limited to a few days. Providers should communicate clear expectations with their patients to maximize the desired effect of any specific treatment while minimizing the risk of medication side effects with the goal of agreeing on a short yet effective treatment course.
Anxiety and Anger
One of the most challenging situations for the inpatient provider is encountering a patient who is anxious, angry, or hypervigilant. Mismatch between actual and expected communication between the provider and the patient can lead to frustration and anxiety. A trauma-informed care approach would suggest that frequent and thorough communication with patients may prevent or ameliorate the stresses and anxieties of hospitalization that may manifest as anger because of retraumatization. Hospitalizations usually lead to disruption of normal routine (eg, unpredictable meal times or medication administration), interrupted sleep (eg, woken up for blood draws or provider evaluation), and lack of control of schedule (eg, unsure of exact time when a procedure may be occurring), any of which may trigger symptoms of anxiety and anger in patients with PTSD and lead to hypervigilance.
If situations involving patient anxiety do arise, employ compassion and communication. Extra time spent with the patient, while challenging in the hectic hospital environment, is critical, and nonpharmacological treatments should be the priority. Engaging patients by asking about their PTSD triggers24 may help prevent exacerbations. For example, some patients may specify how they prefer to be woken up to prevent startle reactions. PTSD triggers can be reduced via effective communication with the entire healthcare team. Some immediate yet effective strategies are listening, validation, and negotiation. Benzodiazepine or antipsychotic usage should be avoided.36 Inpatient social work and comanagement with psychiatry involvement may be helpful in more severe exacerbations. A small observational study of patients hospitalized for severe PTSD found an association between walking more during hospitalization and fewer PTSD symptoms,56 suggesting that staying active could be helpful for inpatients with PTSD who are able to safely ambulate.
SUMMARY
PTSD is a common comorbidity among hospitalized patients in the United States. Typical hospital routines may exacerbate symptoms of PTSD such as anxiety and anger. Inpatient providers can play an important role in making hospitalizations go more smoothly for these patients by using principles consistent with trauma-informed care. Specifically, partnering with patients to construct a plan that preserves their sleep routines and accounts for potential triggers for decompensation can improve the hospital experience for patients with PTSD. Some PTSD interventions require additional investment from the healthcare system to deploy, such as staff training in trauma-informed care and reflective listening techniques. Electronic health record–based protocols and order sets for patients with PTSD can leverage available resources. Further research should evaluate hospital outcomes that result from a more tailored approach to the care of patients with PTSD. More effective, patient-centered PTSD care could lower rates of leaving against medical advice and improve the inpatient experience for patients and providers alike.
1. DSM-5 Fact Sheet: Posttraumatic Stress Disorder. American Psychological Association. 2013. Accessed 30 July 2019. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/DSM/APA_DSM-5-PTSD.pdf
2. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. https://doi.org/10.1001/archpsyc.62.6.617
3. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. https://doi.org/10.1001/archpsyc.62.6.593
4. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060. https://doi.org/10.1001/archpsyc.1995.03950240066012
5. Weiss DS, Marmar CR, Schlenger WE, et al. The prevalence of lifetime and partial post-traumatic stress disorder in Vietnam theater veterans. J Trauma Stress. 1992;5(3):365-376. https://doi.org/10.1002/jts.2490050304
6. Kulka RA, Schlenger WE, Fairbank JA, et al. Trauma And the Vietnam War Generation: Report of findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; 1990.
7. Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. J Occup Environ Med. 2009;51(4):401-410. https://doi.org/10.1097/JOM.0b013e3181a2feeb
8. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24. https://doi.org/10.1007/s11606-009-1117-3
9. VA MISSION Act. Department of Veterans Affairs. 2019. Accessed February 2, 2020. https://missionact.va.gov/
10. Fogarty CT, Sharma S, Chetty VK, Culpepper L. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21(5):398-407. https://doi.org/10.3122/jabfm.2008.05.070082
11. Kartha A, Brower V, Saitz R, Samet JH, Keane TM, Liebschutz J. The impact of trauma exposure and post-traumatic stress disorder on healthcare utilization among primary care patients. Med Care. 2008;46(4):388-393. https://doi.org/10.1097/MLR.0b013e31815dc5d2
12. Dobie DJ, Maynard C, Kivlahan DR, et al. Posttraumatic stress disorder screening status is associated with increased VA medical and surgical utilization in women. J Gen Intern Med. 2006;21(Suppl 3):S58-S64. https://doi.org/10.1111/j.1525-1497.2006.00376.x
13. Calhoun PS, Bosworth HB, Grambow SC, Dudley TK, Beckham JC. Medical service utilization by veterans seeking help for posttraumatic stress disorder. Am J Psychiatry. 2002;159(12):2081-2086. https://doi.org/10.1176/appi.ajp.159.12.2081
14. Frayne SM, Chiu VY, Iqbal S, et al. Medical care needs of returning veterans with PTSD: their other burden. J Gen Intern Med. 2011;26(1):33-39. https://doi.org/10.1007/s11606-010-1497-4
15. Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Medical comorbidity of full and partial posttraumatic stress disorder in US adults: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2011;73(8):697-707. https://doi.org/10.1097/PSY.0b013e3182303775
16. Vaccarino V, Goldberg J, Rooks C, et al. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62(11):970-978. https://doi.org/10.1016/j.jacc.2013.04.085
17. Bressi SK, Marcus SC, Solomon PL. The impact of psychiatric comorbidity on general hospital length of stay. Psychiatr Q. 2006;77(3):203-209. https://doi.org/10.1007/s11126-006-9007-x
18. Haviland MG, Banta JE, Sonne JL, Przekop P. Posttraumatic stress disorder-related hospitalizations in the United States (2002-2011): Rates, co-occurring illnesses, suicidal ideation/self-harm, and hospital charges. J Nerv Men Dis. 2016;204(2):78-86. https://doi.org/10.1097/NMD.0000000000000432
19. Frommberger U, Angenendt J, Berger M. Post-traumatic stress disorder--a diagnostic and therapeutic challenge. Dtsch Arztebl Int. 2014;111(5):59-65. https://doi.com/10.3238/arztebl.2014.0059
20. Sareen J. Posttraumatic stress disorder in adults: impact, comorbidity, risk factors, and treatment. Can J Psychiatry. 2014;59(9):460-467. https://doi.org/10.1177/070674371405900902
21. Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30(5):421-434. https://doi.org/10.1016/j.genhosppsych.2008.05.006
22. Griffiths J, Fortune G, Barber V, Young JD. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33(9):1506-1518. https://doi.org/10.1007/s00134-007-0730-z
23. Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121-1129. https://doi.org/10.1097/CCM.0000000000000882
24. Fletcher KE, Collins J, Holzhauer B, Lewis F, Hendricks M. Medical patients with PTSD identify issues with hospitalization. J Gen Intern Med. 2020;35(6):1906-1907. https://doi.org/10.1007/s11606-019-05480-y
25. Struble LM, Sullivan BJ, Hartman LS. Psychiatric disorders impacting critical illness. Crit Care Nurs Clin North Am. 2014;26(1):115-138. https://doi.org/10.1016/j.ccell.2013.10.002
26. Baxter A. Posttraumatic stress disorder and the intensive care unit patient: implications for staff and advanced practice critical care nurses. Dimens Crit Care Nurs. 2004;23(4):145-150. http://doi.org/10.1097/00003465-200407000-00001
27. Abrams TE, Vaughan-Sarrazin M, Rosenthal GE. Preexisting comorbid psychiatric conditions and mortality in nonsurgical intensive care patients. Am J Crit Care. 2010;19(3):241-249. https://doi.org/10.4037/ajcc2010967
28. Kebbe J, Lal A, El-Solh A, Jaoude P. Effects of PTSD on patient outcomes in the intensive care unit. Chest. 2015;148(4 Suppl):220A. https://doi.org/10.1378/chest.2274366
29. Johnson KG, Rosen J. Re-emergence of posttraumatic stress disorder nightmares with nursing home admission: treatment with prazosin. J Am Med Dir Assoc. 2013;14(2):130-131. https://doi.org/10.1016/j.jamda.2012.10.007
30. Zimmerman M, Mattia JI. Is posttraumatic stress disorder underdiagnosed in routine clinical settings? J Nerv Ment Dis. 1999;187(7):420-428. https://doi.org/10.1097/00005053-199907000-00005
31. Trauma-informed care. Agency for Healthcare Research and Quality. 2015. Accessed July 30, 2019. http://www.ahrq.gov/professionals/prevention-chronic-care/healthier-pregnancy/preventive/trauma.html
32. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Substance Abuse and Mental Health Administration, Department of Health & Human Services; 2014. HHS Publication No. SMA 14-4884. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf
33. DeCandia CJ, Guarino K. Trauma-informed care: an ecological response. J Child Youth Care Work. 2015;24:7-32.
34. Prins A, Bovin MJ, Smolenski DJ, et al. The PRIMARY CARE PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. https://doi.org/10.1007/s11606-016-3703-5
35. Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systematic review and meta-analysis to determine first-line treatments. Depress Anxiety. 2016;33(9):792-806. https://doi.org/10.1002/da.22511
36. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Department of Veterans Affairs/Department of Defense. 2017. Accessed July 22, 2019. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGClinicianSummaryFinal.pdf
37. Singh B, Hughes AJ, Mehta G, Erwin PJ, Parsaik AK. Efficacy of prazosin in posttraumatic stress disorder: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(4). https://doi.org/10.4088/PCC.16r01943
38. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517. https://doi.org/10.1056/NEJMoa1507598
39. El-Solh AA. Management of nightmares in patients with posttraumatic stress disorder: current perspectives. Nat Sci Sleep. 2018;10:409-420. https://doi.org/10.2147/NSS.S166089
40. What is ROVER? Treatment Services. VA. 2018. Accessed February 14, 2020. https://www.houston.va.gov/docs/ROVERBrochure.pdf
41. Moser DK, Chung ML, McKinley S, et al. Critical care nursing practice regarding patient anxiety assessment and management. Intensive Crit Care Nurs. 2003;19(5):276-288. https://doi.org/10.1016/s0964-3397(03)00061-2
42. Bulechek G, Butcher H, Dochterman JM, Wagner C. Nursing Interventions Classification (NIC), 6th Ed. Elsevier; 2013.
43. Blanaru M, Bloch B, Vadas L, et al. The effects of music relaxation and muscle relaxation techniques on sleep quality and emotional measures among individuals with posttraumatic stress disorder. Ment Illn. 2012;4(2):e13. https://doi.org/10.4081/mi.2012.e13
44. Leskin GA, Woodward SH, Young HE, Sheikh JI. Effects of comorbid diagnoses on sleep disturbance in PTSD. J Psychiatr Res. 2002;36(6):449-452. https://doi.org/10.1016/s0022-3956(02)00025-0
45. Vandrey R, Babson KA, Herrmann ES, Bonn-Miller MO. Interactions between disordered sleep, post-traumatic stress disorder, and substance use disorders. Int Rev Psychiatry. 2014;26(2):237-247. https://doi.org/10.3109/09540261.2014.901300
46. Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618-622. https://doi.org/10.1097/00005053-200109000-00008
47. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372-382. https://doi.org/10.1176/appi.ajp.2012.12040432
48. Ho FYY, Chan CS, Tang KNS. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2016;43:90-102. https://doi.org/10.1016/j.cpr.2015.09.005
49. Thompson KE, Franklin CL, Hubbard K. PTSD sleep therapy group: training your mind and body for better sleep: Therapist Manual. A product of the Department of Veterans Affairs South Central (VISN 16) Mental Illness Research, Education, and Clinical Center (MIRECC). Accessed July 22, 2019. https://www.mirecc.va.gov/VISN16/docs/Sleep_Therapy_Group_Therapist_Manual.pdf
50. Ye L, Keane K, Hutton Johnson S, Dykes PC. How do clinicians assess, communicate about, and manage patient sleep in the hospital? J Nurs Adm. 2013;43(6):342-347. https://doi.org/10.1097/NNA.0b013e3182942c8a
51. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
52. Gagner-Tjellesen D, Yurkovich EE, Gragert M. Use of music therapy and other ITNIs in acute care. J Psychosoc Nurs Ment Health Serv. 2001;39(10):26-37.
53. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636. https://doi.org/10.5664/jcsm.3786
54. Brennstuhl MJ, Tarquinio C, Montel S. Chronic pain and PTSD: evolving views on their comorbidity. Perspect Psychiatr Care. 2015;51(4):295-304. https://doi.org/10.1111/ppc.12093
55. Bosch J, Weaver TL, Neylan TC, Herbst E, McCaslin SE. Impact of engagement in exercise on sleep quality among veterans with posttraumatic stress disorder symptoms. Mil Med. 2017;182(9):e1745-e1750. https://doi.org/10.7205/MILMED-D-16-00385
56. Rosenbaum S, Vancampfort D, Tiedemann A, et al. Among inpatients, posttraumatic stress disorder symptom severity is negatively associated with time spent walking. J Nerv Ment Dis. 2016;204(1):15-19. https://doi.org/10.1097/NMD.0000000000000415
Posttraumatic stress disorder (PTSD) is a syndrome that occurs after exposure to a significant traumatic event and is characterized by persistent, debilitating symptoms that fall into four “diagnostic clusters” as outlined in the Diagnostic and Statistical Manual of Mental Disorders-Version V (DSM-V). Patients may experience intrusive thoughts, avoidance of distressing stimuli, persistent negative mood, and hypervigilance, all of which last longer than 1 month.1
A national survey of United States households conducted during 2001-2003 estimated the 12-month prevalence of PTSD among adults to be 3.5%.2 Lifetime prevalence has been found to be between 6.8%3 and 7.8%.4 PTSD is more common in veterans. The prevalence of PTSD in veterans differs depending on the conflict in which the veteran participated. Vietnam veterans have an estimated lifetime prevalence of approximately 30%,5,6 Gulf War veterans approximately 15%,7 and veterans of more recent conflicts in Afghanistan and Iraq of approximately 21%.8 With the MISSION Act moving more veteran care into the private sector, non-VA inpatient providers will need to become better versed in PTSD.9
Patients with PTSD have more contact with the healthcare system, even for non–mental health problems,8,10-13 and a significantly higher burden of medical comorbities,14 such as diabetes mellitus, liver disease, gastritis and gastric ulcers, HIV, arthritis,15 and coronary heart disease.16 Veterans with PTSD are hospitalized three times more often than are those with no mental health diagnoses,8 and patients with psychiatric comorbidities have higher lengths of stay.17 More than 1.4 million hospitalizations occurring during 2002-2011 had either a primary or secondary associated diagnosis of PTSD, with total inflation-adjusted charges of 34.9 billion dollars.18 In the inpatient sample from this study, greater than half were admitted for a primary diagnosis of mental diseases and disorders (Major Diagnostic Category [MDC] 19). Following mental illness, the most common primary diagnoses for men were MDC 5 (Circulatory System, 12.1%), MDC 20 (Alcohol/Drug Use or Induced Mental Disorder, 9.2%), and MDC 4 (Respiratory System, 7.4%), while the most common categories for women were MDC 20 (Alcohol/Drug Use or Induced Mental Disorder, 5.8%), MDC 21 (Injuries, Poison, and Toxic Effect of Drugs, 4.9%), and MDC 6 (Digestive System, 4.5%).18
In both the inpatient and outpatient settings, a fundamental challenge to comprehensive PTSD management is correctly diagnosing this condition.19 Confounding the difficulties in diagnosis are numerous comorbidities. In addition to the physical comorbidities described above, more than 70% of patients with PTSD have another psychological comorbidity such as affective disorders, anxiety disorders, or substance use disorder/dependency.20
Given that PTSD may be an underrecognized burden on the healthcare system, we sought to better understand how PTSD could affect hospitalized patients admitted for medical problems by conducting this narrative review. Additionally, three of the authors collaborated with the VA Employee Education Service to conduct a needs assessment of VA hospitalists in 2013. Respondents identified managing and educating patients and families about PTSD as a major educational need (unpublished data available upon request from the corresponding author). Therefore, our aims were to present a synthesis of existing literature, familiarize readers with the tenets of trauma-informed care as a framework to guide care for these patients, and generate ideas for changes that inpatient providers could implement now. We began by consulting a research librarian at the Clement J. Zablocki VA Medical Center in Milwaukee, Wisconsin, who searched the following databases: PsycInfo, CINAHL, MEDLINE, and PILOTS (a PTSD/trauma specific database). Search terms included hospital, hospitalized, and hospitalization, as well as traumatic stress, posttraumatic stress, and PTSD. Pertinent guidelines and the reference lists from included papers were examined. We focused on papers that described patients admitted for medical problems other than PTSD because those patients who are admitted for PTSD-related problems should be primarily managed by psychiatry (not hospitalists) with the primary focus of their hospitalization being their PTSD. We also excluded papers about patients developing PTSD secondary to hospitalization, which already has a well-developed literature.21-23
THE LITERATURE ABOUT PTSD IN HOSPITALIZED PATIENTS
The literature is sparse describing frequency or type of problems encountered by hospitalized medical patients with PTSD. A recent small survey study reported that 40% of patients anticipated triggers for their PTSD symptoms in the hospital; such triggers included loud noises and being shaken awake.24 Two papers describe case vignettes of patients who had exacerbations of their PTSD while in the Intensive Care Unit (ICU), although neither contain frequency or severity data.25,26 Approximately 8% of patients in VA ICUs have PTSD,27 and a published abstract suggests that they appear to require more sedation than do patients without PTSD.28 Another published case report describes a patient with recurrent PTSD symptoms (nightmares) after moving into a nursing home.29 These papers suggest other providers have recognized and are concerned about hospitalized patients with PTSD. At present, there are no data to quantify how often hospitalized patients have PTSD exacerbations or how troublesome such exacerbations are to these patients.
Given that there is little empiric literature to guide inpatient management of PTSD as a comorbidity in hospitalized medical patients, we extrapolate some information from the outpatient setting. PTSD is often underdiagnosed and underreported by individual patients in the outpatient setting.30 Failure to have an associated diagnosis of PTSD may lead to underrecognition and undertreatment of these patients by inpatient providers in the hospital setting. Additionally, the numerous psychological and physical comorbidities in PTSD can create unique challenges in properly managing any single problem in these patients.20 Armed with this knowledge, providers should be vigilant in the recognition, assessment, and treatment of PTSD.
INPATIENT MANAGEMENT OF PTSD
Trauma-Informed Care: A Conceptual Model
Trauma-informed care is a mindful and sensitive approach to caring for patients who have suffered trauma.31 It requires understanding that many people have suffered trauma in their lives and that the trauma continues to impact many aspects of their lives.32 Trauma-informed care has many advocates and has been implemented across myriad health and social services settings.33 Its principles can be applied in both the inpatient and outpatient hospital settings. While it is an appropriate approach to patients with PTSD, it is not specific to PTSD. People who have suffered sexual trauma, intimate partner violence, child abuse, or other exposures would also be included in the group of people for whom trauma-informed care is a suitable approach. There are four key assumptions to a trauma-informed approach to care (the 4 R’s): (1) realization that trauma affects an individual’s coping strategies, relationships, and health; (2) recognition of the signs of trauma; (3) having an appropriate, planned response to patients identified as having suffered a trauma; and (4) resisting retraumatization in the care setting.31,32
General Approach to Treating Medical Patients With PTSD in the Inpatient Setting
Recognition
Consistent with a trauma-informed care approach, inpatient providers should be able to recognize patients who may have PTSD. First, careful review of the past medical history may show some patients already carry this diagnosis. Second, patients with PTSD often have other comorbidities that could offer a clue that PTSD could be present as well; for example, risk for PTSD is increased when mood, anxiety, or substance use disorders are present.20 When PTSD is suspected, screening is a reasonable next step.
The Primary Care-PTSD-5 (PC-PTSD-5) is a validated screening tool used in the outpatient setting.34 It is easily administered and has good predictive validity (positive likelihood ratio [LR+] of 6.33 and LR– of 0.06). It begins with a question of whether the patient has ever experienced a trauma. A positive initial response triggers a series of five yes/no questions. Answering “yes” to three or more questions is a positive screen. A positive screen should result in consultation to psychiatry to conduct more formal evaluation and guide longer-term management.
Collaboration
Individual trauma-focused psychotherapy is the primary treatment of choice for PTSD with strong evidence supporting its practice.35 This treatment is administered by a psychiatrist or psychologist and will be limited in the inpatient medical setting. Current recommendations suggest pharmacotherapy only when individualized trauma-focused psychotherapy is not available, the patient declines it, or as an adjunct when psychotherapy alone is not effective.36 Therefore, inpatient providers may see patients who are prescribed selective serotonin reuptake inhibitors (eg, paroxetine, fluoxetine) or serotonin and norepinephrine reuptake inhibitors (eg, venlafaxine).36 In the past, PTSD-related nightmares were often treated with prazosin.37 However, a recent randomized controlled trial of prazosin in veterans with PTSD failed to show significant improvement in nightmares.38 Hence, current guidelines do not recommend prazosin as a first-line therapy.39 For hospitalized patients with PTSD symptoms refractory to the interventions outlined herein, particularly those patients with possible borderline personality traits (as suggested by severe anger and impulsivity), we strongly recommend partnering with psychiatry. Finally, given the high prevalence of substance use disorders (SUDs) in PTSD patients, awareness and treatment of comorbidities such as opioid and alcohol dependence must be concurrently addressed.
Individualizing Care
It is essential for the healthcare team to identify ways to meet each patient’s immediate needs. Many of the ideas proposed below are not specific to PTSD; many require an interprofessional approach to care.40 From a trauma-informed care standpoint, this is akin to having a planned response for patients who have suffered trauma. Assessing the individual’s needs and incorporating therapeutic modalities such as reflective listening, broadening safe opportunities for control, and providing complementary and integrative medicine (IM) therapies may help manage symptoms and establish rapport.41 Through reflective listening, a collaborative approach can be established to identify background, triggers, and a safe approach for managing PTSD and its comorbid conditions. Ensuring frequent communication and allowing the patient to be at the center of decision-making establishes a safe environment and promotes positive rapport between the patient and healthcare team.36 Providing a sense of control by involving the patients in their healthcare decisions and in the structure of care delivery may benefit the patients’ well-being. Furthermore, incorporating IM encourages rest and relaxation in the chaotic hospital environment. Suggested IM interventions include deep breathing, aromatherapy, guided imagery, muscle relaxation, and music therapy.42,43
Key Inpatient Issues Affecting PTSD
In the following sections, we outline common clinical situations that may exacerbate PTSD symptoms and propose some evidence-based responses (Table). In general, nonpharmacologic approaches are favored over pharmacologic approaches for patients with PTSD.

Sleep Hygiene
Sleep problems are very common in patients with PTSD, with nightmares occurring in more than 70% of patients and insomnia in 80%.44 In PTSD, sleep problems are linked to poor physical health and other health outcomes45,46 and may exacerbate other PTSD symptoms.4
Treating the sleep problems that occur with PTSD is an important aspect of PTSD care. Usually administered in the outpatient setting, the treatment of choice is cognitive-behavioral therapy (CBT).48 Sleep-specific CBT focuses, among other things, on strategies that encourage good sleep hygiene,49 which includes promoting regular sleep/wake-up times and specific bedtime routines, avoiding stimulation (eg, light, noise, TV) or excessive liquids before bed, refraining from daytime naps, and using relaxation techniques. Many of these recommendations seem at odds with hospital routines, which may contribute to decompensation of hospitalized patients with PTSD.
While starting sleep-specific CBT in the hospital may not be realistic, we suggest the following goals and strategies as a starting place for promoting healthy sleep for hospitalized patients with PTSD. To begin, factors affecting sleep hygiene should be addressed. Inpatient providers could pay more attention to intravenous (IV) fluid orders, perhaps adjusting them to run only during the daytime hours. Medications can be scheduled at times conducive to maintaining home routines. Avoiding the administration of diuretics close to bedtime may decrease the likelihood of frequent nighttime wakening. Grouping patient care activities, such as bathing or wound care, during daytime hours may allow more opportunities for rest at night. Incorporating uninterrupted sleep protocols, such as quiet hours between 10
Second, providers need to ask about established home bedtime routines and facilitate implementation in the hospital. Through collaboration with patients, providers can incorporate an individualized plan of care for sleep early in hospitalization.50 Partnering with nurses is also essential to creating a sleep-friendly environment that can improve patient experiences.51 Breathing exercises, meditating, listening to music and praying are all examples of “bedtime wind down” strategies recommended in sleep-specific CBT.49 Many of these could be successfully implemented in the hospital and may benefit other hospitalized patients too.52 In patients with PTSD and obstructive sleep apnea, continuous positive airway pressure (CPAP) reduces nightmares, and if inpatients are on CPAP at home, it should be continued in the hospital.53
Pain
If sleep disturbance is the hallmark of PTSD,47 chronic pain is its coconspirator.15 Uncontrolled pain can make it much more difficult to treat patients with PTSD, which in turn may lead to further decompensation from a mental health standpoint.54 SUDs such as alcohol or opioid dependencies are highly comorbid with PTSD45 and introduce a layer of complexity when managing painin these patients. Providers should be thoughtful when electing to treat acute or chronic pain with opioids and take particular care to establish realistic therapeutic goals if doing so. While patients with PTSD have a greater likelihood of having an SUD, undertreating pain risks exacerbating underlying PTSD symptoms.
Nonpharmacologic therapies, which include communicating, listening, and expressing compassion and understanding, should be utilized by inpatient providers as a first-line treatment in patients with PTSD who suffer from pain. Additionally, relaxation techniques, physical therapy, and physical activity55 can be offered. Pharmacologically, nonopioid medications such as acetaminophen or NSAIDs should always be considered first. Should the use of opioids be deemed necessary, inpatient providers should preferentially use oral over intravenous medications and consider establishing a fixed timeframe for short-term opioids, which should be limited to a few days. Providers should communicate clear expectations with their patients to maximize the desired effect of any specific treatment while minimizing the risk of medication side effects with the goal of agreeing on a short yet effective treatment course.
Anxiety and Anger
One of the most challenging situations for the inpatient provider is encountering a patient who is anxious, angry, or hypervigilant. Mismatch between actual and expected communication between the provider and the patient can lead to frustration and anxiety. A trauma-informed care approach would suggest that frequent and thorough communication with patients may prevent or ameliorate the stresses and anxieties of hospitalization that may manifest as anger because of retraumatization. Hospitalizations usually lead to disruption of normal routine (eg, unpredictable meal times or medication administration), interrupted sleep (eg, woken up for blood draws or provider evaluation), and lack of control of schedule (eg, unsure of exact time when a procedure may be occurring), any of which may trigger symptoms of anxiety and anger in patients with PTSD and lead to hypervigilance.
If situations involving patient anxiety do arise, employ compassion and communication. Extra time spent with the patient, while challenging in the hectic hospital environment, is critical, and nonpharmacological treatments should be the priority. Engaging patients by asking about their PTSD triggers24 may help prevent exacerbations. For example, some patients may specify how they prefer to be woken up to prevent startle reactions. PTSD triggers can be reduced via effective communication with the entire healthcare team. Some immediate yet effective strategies are listening, validation, and negotiation. Benzodiazepine or antipsychotic usage should be avoided.36 Inpatient social work and comanagement with psychiatry involvement may be helpful in more severe exacerbations. A small observational study of patients hospitalized for severe PTSD found an association between walking more during hospitalization and fewer PTSD symptoms,56 suggesting that staying active could be helpful for inpatients with PTSD who are able to safely ambulate.
SUMMARY
PTSD is a common comorbidity among hospitalized patients in the United States. Typical hospital routines may exacerbate symptoms of PTSD such as anxiety and anger. Inpatient providers can play an important role in making hospitalizations go more smoothly for these patients by using principles consistent with trauma-informed care. Specifically, partnering with patients to construct a plan that preserves their sleep routines and accounts for potential triggers for decompensation can improve the hospital experience for patients with PTSD. Some PTSD interventions require additional investment from the healthcare system to deploy, such as staff training in trauma-informed care and reflective listening techniques. Electronic health record–based protocols and order sets for patients with PTSD can leverage available resources. Further research should evaluate hospital outcomes that result from a more tailored approach to the care of patients with PTSD. More effective, patient-centered PTSD care could lower rates of leaving against medical advice and improve the inpatient experience for patients and providers alike.
Posttraumatic stress disorder (PTSD) is a syndrome that occurs after exposure to a significant traumatic event and is characterized by persistent, debilitating symptoms that fall into four “diagnostic clusters” as outlined in the Diagnostic and Statistical Manual of Mental Disorders-Version V (DSM-V). Patients may experience intrusive thoughts, avoidance of distressing stimuli, persistent negative mood, and hypervigilance, all of which last longer than 1 month.1
A national survey of United States households conducted during 2001-2003 estimated the 12-month prevalence of PTSD among adults to be 3.5%.2 Lifetime prevalence has been found to be between 6.8%3 and 7.8%.4 PTSD is more common in veterans. The prevalence of PTSD in veterans differs depending on the conflict in which the veteran participated. Vietnam veterans have an estimated lifetime prevalence of approximately 30%,5,6 Gulf War veterans approximately 15%,7 and veterans of more recent conflicts in Afghanistan and Iraq of approximately 21%.8 With the MISSION Act moving more veteran care into the private sector, non-VA inpatient providers will need to become better versed in PTSD.9
Patients with PTSD have more contact with the healthcare system, even for non–mental health problems,8,10-13 and a significantly higher burden of medical comorbities,14 such as diabetes mellitus, liver disease, gastritis and gastric ulcers, HIV, arthritis,15 and coronary heart disease.16 Veterans with PTSD are hospitalized three times more often than are those with no mental health diagnoses,8 and patients with psychiatric comorbidities have higher lengths of stay.17 More than 1.4 million hospitalizations occurring during 2002-2011 had either a primary or secondary associated diagnosis of PTSD, with total inflation-adjusted charges of 34.9 billion dollars.18 In the inpatient sample from this study, greater than half were admitted for a primary diagnosis of mental diseases and disorders (Major Diagnostic Category [MDC] 19). Following mental illness, the most common primary diagnoses for men were MDC 5 (Circulatory System, 12.1%), MDC 20 (Alcohol/Drug Use or Induced Mental Disorder, 9.2%), and MDC 4 (Respiratory System, 7.4%), while the most common categories for women were MDC 20 (Alcohol/Drug Use or Induced Mental Disorder, 5.8%), MDC 21 (Injuries, Poison, and Toxic Effect of Drugs, 4.9%), and MDC 6 (Digestive System, 4.5%).18
In both the inpatient and outpatient settings, a fundamental challenge to comprehensive PTSD management is correctly diagnosing this condition.19 Confounding the difficulties in diagnosis are numerous comorbidities. In addition to the physical comorbidities described above, more than 70% of patients with PTSD have another psychological comorbidity such as affective disorders, anxiety disorders, or substance use disorder/dependency.20
Given that PTSD may be an underrecognized burden on the healthcare system, we sought to better understand how PTSD could affect hospitalized patients admitted for medical problems by conducting this narrative review. Additionally, three of the authors collaborated with the VA Employee Education Service to conduct a needs assessment of VA hospitalists in 2013. Respondents identified managing and educating patients and families about PTSD as a major educational need (unpublished data available upon request from the corresponding author). Therefore, our aims were to present a synthesis of existing literature, familiarize readers with the tenets of trauma-informed care as a framework to guide care for these patients, and generate ideas for changes that inpatient providers could implement now. We began by consulting a research librarian at the Clement J. Zablocki VA Medical Center in Milwaukee, Wisconsin, who searched the following databases: PsycInfo, CINAHL, MEDLINE, and PILOTS (a PTSD/trauma specific database). Search terms included hospital, hospitalized, and hospitalization, as well as traumatic stress, posttraumatic stress, and PTSD. Pertinent guidelines and the reference lists from included papers were examined. We focused on papers that described patients admitted for medical problems other than PTSD because those patients who are admitted for PTSD-related problems should be primarily managed by psychiatry (not hospitalists) with the primary focus of their hospitalization being their PTSD. We also excluded papers about patients developing PTSD secondary to hospitalization, which already has a well-developed literature.21-23
THE LITERATURE ABOUT PTSD IN HOSPITALIZED PATIENTS
The literature is sparse describing frequency or type of problems encountered by hospitalized medical patients with PTSD. A recent small survey study reported that 40% of patients anticipated triggers for their PTSD symptoms in the hospital; such triggers included loud noises and being shaken awake.24 Two papers describe case vignettes of patients who had exacerbations of their PTSD while in the Intensive Care Unit (ICU), although neither contain frequency or severity data.25,26 Approximately 8% of patients in VA ICUs have PTSD,27 and a published abstract suggests that they appear to require more sedation than do patients without PTSD.28 Another published case report describes a patient with recurrent PTSD symptoms (nightmares) after moving into a nursing home.29 These papers suggest other providers have recognized and are concerned about hospitalized patients with PTSD. At present, there are no data to quantify how often hospitalized patients have PTSD exacerbations or how troublesome such exacerbations are to these patients.
Given that there is little empiric literature to guide inpatient management of PTSD as a comorbidity in hospitalized medical patients, we extrapolate some information from the outpatient setting. PTSD is often underdiagnosed and underreported by individual patients in the outpatient setting.30 Failure to have an associated diagnosis of PTSD may lead to underrecognition and undertreatment of these patients by inpatient providers in the hospital setting. Additionally, the numerous psychological and physical comorbidities in PTSD can create unique challenges in properly managing any single problem in these patients.20 Armed with this knowledge, providers should be vigilant in the recognition, assessment, and treatment of PTSD.
INPATIENT MANAGEMENT OF PTSD
Trauma-Informed Care: A Conceptual Model
Trauma-informed care is a mindful and sensitive approach to caring for patients who have suffered trauma.31 It requires understanding that many people have suffered trauma in their lives and that the trauma continues to impact many aspects of their lives.32 Trauma-informed care has many advocates and has been implemented across myriad health and social services settings.33 Its principles can be applied in both the inpatient and outpatient hospital settings. While it is an appropriate approach to patients with PTSD, it is not specific to PTSD. People who have suffered sexual trauma, intimate partner violence, child abuse, or other exposures would also be included in the group of people for whom trauma-informed care is a suitable approach. There are four key assumptions to a trauma-informed approach to care (the 4 R’s): (1) realization that trauma affects an individual’s coping strategies, relationships, and health; (2) recognition of the signs of trauma; (3) having an appropriate, planned response to patients identified as having suffered a trauma; and (4) resisting retraumatization in the care setting.31,32
General Approach to Treating Medical Patients With PTSD in the Inpatient Setting
Recognition
Consistent with a trauma-informed care approach, inpatient providers should be able to recognize patients who may have PTSD. First, careful review of the past medical history may show some patients already carry this diagnosis. Second, patients with PTSD often have other comorbidities that could offer a clue that PTSD could be present as well; for example, risk for PTSD is increased when mood, anxiety, or substance use disorders are present.20 When PTSD is suspected, screening is a reasonable next step.
The Primary Care-PTSD-5 (PC-PTSD-5) is a validated screening tool used in the outpatient setting.34 It is easily administered and has good predictive validity (positive likelihood ratio [LR+] of 6.33 and LR– of 0.06). It begins with a question of whether the patient has ever experienced a trauma. A positive initial response triggers a series of five yes/no questions. Answering “yes” to three or more questions is a positive screen. A positive screen should result in consultation to psychiatry to conduct more formal evaluation and guide longer-term management.
Collaboration
Individual trauma-focused psychotherapy is the primary treatment of choice for PTSD with strong evidence supporting its practice.35 This treatment is administered by a psychiatrist or psychologist and will be limited in the inpatient medical setting. Current recommendations suggest pharmacotherapy only when individualized trauma-focused psychotherapy is not available, the patient declines it, or as an adjunct when psychotherapy alone is not effective.36 Therefore, inpatient providers may see patients who are prescribed selective serotonin reuptake inhibitors (eg, paroxetine, fluoxetine) or serotonin and norepinephrine reuptake inhibitors (eg, venlafaxine).36 In the past, PTSD-related nightmares were often treated with prazosin.37 However, a recent randomized controlled trial of prazosin in veterans with PTSD failed to show significant improvement in nightmares.38 Hence, current guidelines do not recommend prazosin as a first-line therapy.39 For hospitalized patients with PTSD symptoms refractory to the interventions outlined herein, particularly those patients with possible borderline personality traits (as suggested by severe anger and impulsivity), we strongly recommend partnering with psychiatry. Finally, given the high prevalence of substance use disorders (SUDs) in PTSD patients, awareness and treatment of comorbidities such as opioid and alcohol dependence must be concurrently addressed.
Individualizing Care
It is essential for the healthcare team to identify ways to meet each patient’s immediate needs. Many of the ideas proposed below are not specific to PTSD; many require an interprofessional approach to care.40 From a trauma-informed care standpoint, this is akin to having a planned response for patients who have suffered trauma. Assessing the individual’s needs and incorporating therapeutic modalities such as reflective listening, broadening safe opportunities for control, and providing complementary and integrative medicine (IM) therapies may help manage symptoms and establish rapport.41 Through reflective listening, a collaborative approach can be established to identify background, triggers, and a safe approach for managing PTSD and its comorbid conditions. Ensuring frequent communication and allowing the patient to be at the center of decision-making establishes a safe environment and promotes positive rapport between the patient and healthcare team.36 Providing a sense of control by involving the patients in their healthcare decisions and in the structure of care delivery may benefit the patients’ well-being. Furthermore, incorporating IM encourages rest and relaxation in the chaotic hospital environment. Suggested IM interventions include deep breathing, aromatherapy, guided imagery, muscle relaxation, and music therapy.42,43
Key Inpatient Issues Affecting PTSD
In the following sections, we outline common clinical situations that may exacerbate PTSD symptoms and propose some evidence-based responses (Table). In general, nonpharmacologic approaches are favored over pharmacologic approaches for patients with PTSD.

Sleep Hygiene
Sleep problems are very common in patients with PTSD, with nightmares occurring in more than 70% of patients and insomnia in 80%.44 In PTSD, sleep problems are linked to poor physical health and other health outcomes45,46 and may exacerbate other PTSD symptoms.4
Treating the sleep problems that occur with PTSD is an important aspect of PTSD care. Usually administered in the outpatient setting, the treatment of choice is cognitive-behavioral therapy (CBT).48 Sleep-specific CBT focuses, among other things, on strategies that encourage good sleep hygiene,49 which includes promoting regular sleep/wake-up times and specific bedtime routines, avoiding stimulation (eg, light, noise, TV) or excessive liquids before bed, refraining from daytime naps, and using relaxation techniques. Many of these recommendations seem at odds with hospital routines, which may contribute to decompensation of hospitalized patients with PTSD.
While starting sleep-specific CBT in the hospital may not be realistic, we suggest the following goals and strategies as a starting place for promoting healthy sleep for hospitalized patients with PTSD. To begin, factors affecting sleep hygiene should be addressed. Inpatient providers could pay more attention to intravenous (IV) fluid orders, perhaps adjusting them to run only during the daytime hours. Medications can be scheduled at times conducive to maintaining home routines. Avoiding the administration of diuretics close to bedtime may decrease the likelihood of frequent nighttime wakening. Grouping patient care activities, such as bathing or wound care, during daytime hours may allow more opportunities for rest at night. Incorporating uninterrupted sleep protocols, such as quiet hours between 10
Second, providers need to ask about established home bedtime routines and facilitate implementation in the hospital. Through collaboration with patients, providers can incorporate an individualized plan of care for sleep early in hospitalization.50 Partnering with nurses is also essential to creating a sleep-friendly environment that can improve patient experiences.51 Breathing exercises, meditating, listening to music and praying are all examples of “bedtime wind down” strategies recommended in sleep-specific CBT.49 Many of these could be successfully implemented in the hospital and may benefit other hospitalized patients too.52 In patients with PTSD and obstructive sleep apnea, continuous positive airway pressure (CPAP) reduces nightmares, and if inpatients are on CPAP at home, it should be continued in the hospital.53
Pain
If sleep disturbance is the hallmark of PTSD,47 chronic pain is its coconspirator.15 Uncontrolled pain can make it much more difficult to treat patients with PTSD, which in turn may lead to further decompensation from a mental health standpoint.54 SUDs such as alcohol or opioid dependencies are highly comorbid with PTSD45 and introduce a layer of complexity when managing painin these patients. Providers should be thoughtful when electing to treat acute or chronic pain with opioids and take particular care to establish realistic therapeutic goals if doing so. While patients with PTSD have a greater likelihood of having an SUD, undertreating pain risks exacerbating underlying PTSD symptoms.
Nonpharmacologic therapies, which include communicating, listening, and expressing compassion and understanding, should be utilized by inpatient providers as a first-line treatment in patients with PTSD who suffer from pain. Additionally, relaxation techniques, physical therapy, and physical activity55 can be offered. Pharmacologically, nonopioid medications such as acetaminophen or NSAIDs should always be considered first. Should the use of opioids be deemed necessary, inpatient providers should preferentially use oral over intravenous medications and consider establishing a fixed timeframe for short-term opioids, which should be limited to a few days. Providers should communicate clear expectations with their patients to maximize the desired effect of any specific treatment while minimizing the risk of medication side effects with the goal of agreeing on a short yet effective treatment course.
Anxiety and Anger
One of the most challenging situations for the inpatient provider is encountering a patient who is anxious, angry, or hypervigilant. Mismatch between actual and expected communication between the provider and the patient can lead to frustration and anxiety. A trauma-informed care approach would suggest that frequent and thorough communication with patients may prevent or ameliorate the stresses and anxieties of hospitalization that may manifest as anger because of retraumatization. Hospitalizations usually lead to disruption of normal routine (eg, unpredictable meal times or medication administration), interrupted sleep (eg, woken up for blood draws or provider evaluation), and lack of control of schedule (eg, unsure of exact time when a procedure may be occurring), any of which may trigger symptoms of anxiety and anger in patients with PTSD and lead to hypervigilance.
If situations involving patient anxiety do arise, employ compassion and communication. Extra time spent with the patient, while challenging in the hectic hospital environment, is critical, and nonpharmacological treatments should be the priority. Engaging patients by asking about their PTSD triggers24 may help prevent exacerbations. For example, some patients may specify how they prefer to be woken up to prevent startle reactions. PTSD triggers can be reduced via effective communication with the entire healthcare team. Some immediate yet effective strategies are listening, validation, and negotiation. Benzodiazepine or antipsychotic usage should be avoided.36 Inpatient social work and comanagement with psychiatry involvement may be helpful in more severe exacerbations. A small observational study of patients hospitalized for severe PTSD found an association between walking more during hospitalization and fewer PTSD symptoms,56 suggesting that staying active could be helpful for inpatients with PTSD who are able to safely ambulate.
SUMMARY
PTSD is a common comorbidity among hospitalized patients in the United States. Typical hospital routines may exacerbate symptoms of PTSD such as anxiety and anger. Inpatient providers can play an important role in making hospitalizations go more smoothly for these patients by using principles consistent with trauma-informed care. Specifically, partnering with patients to construct a plan that preserves their sleep routines and accounts for potential triggers for decompensation can improve the hospital experience for patients with PTSD. Some PTSD interventions require additional investment from the healthcare system to deploy, such as staff training in trauma-informed care and reflective listening techniques. Electronic health record–based protocols and order sets for patients with PTSD can leverage available resources. Further research should evaluate hospital outcomes that result from a more tailored approach to the care of patients with PTSD. More effective, patient-centered PTSD care could lower rates of leaving against medical advice and improve the inpatient experience for patients and providers alike.
1. DSM-5 Fact Sheet: Posttraumatic Stress Disorder. American Psychological Association. 2013. Accessed 30 July 2019. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/DSM/APA_DSM-5-PTSD.pdf
2. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. https://doi.org/10.1001/archpsyc.62.6.617
3. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. https://doi.org/10.1001/archpsyc.62.6.593
4. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060. https://doi.org/10.1001/archpsyc.1995.03950240066012
5. Weiss DS, Marmar CR, Schlenger WE, et al. The prevalence of lifetime and partial post-traumatic stress disorder in Vietnam theater veterans. J Trauma Stress. 1992;5(3):365-376. https://doi.org/10.1002/jts.2490050304
6. Kulka RA, Schlenger WE, Fairbank JA, et al. Trauma And the Vietnam War Generation: Report of findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; 1990.
7. Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. J Occup Environ Med. 2009;51(4):401-410. https://doi.org/10.1097/JOM.0b013e3181a2feeb
8. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24. https://doi.org/10.1007/s11606-009-1117-3
9. VA MISSION Act. Department of Veterans Affairs. 2019. Accessed February 2, 2020. https://missionact.va.gov/
10. Fogarty CT, Sharma S, Chetty VK, Culpepper L. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21(5):398-407. https://doi.org/10.3122/jabfm.2008.05.070082
11. Kartha A, Brower V, Saitz R, Samet JH, Keane TM, Liebschutz J. The impact of trauma exposure and post-traumatic stress disorder on healthcare utilization among primary care patients. Med Care. 2008;46(4):388-393. https://doi.org/10.1097/MLR.0b013e31815dc5d2
12. Dobie DJ, Maynard C, Kivlahan DR, et al. Posttraumatic stress disorder screening status is associated with increased VA medical and surgical utilization in women. J Gen Intern Med. 2006;21(Suppl 3):S58-S64. https://doi.org/10.1111/j.1525-1497.2006.00376.x
13. Calhoun PS, Bosworth HB, Grambow SC, Dudley TK, Beckham JC. Medical service utilization by veterans seeking help for posttraumatic stress disorder. Am J Psychiatry. 2002;159(12):2081-2086. https://doi.org/10.1176/appi.ajp.159.12.2081
14. Frayne SM, Chiu VY, Iqbal S, et al. Medical care needs of returning veterans with PTSD: their other burden. J Gen Intern Med. 2011;26(1):33-39. https://doi.org/10.1007/s11606-010-1497-4
15. Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Medical comorbidity of full and partial posttraumatic stress disorder in US adults: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2011;73(8):697-707. https://doi.org/10.1097/PSY.0b013e3182303775
16. Vaccarino V, Goldberg J, Rooks C, et al. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62(11):970-978. https://doi.org/10.1016/j.jacc.2013.04.085
17. Bressi SK, Marcus SC, Solomon PL. The impact of psychiatric comorbidity on general hospital length of stay. Psychiatr Q. 2006;77(3):203-209. https://doi.org/10.1007/s11126-006-9007-x
18. Haviland MG, Banta JE, Sonne JL, Przekop P. Posttraumatic stress disorder-related hospitalizations in the United States (2002-2011): Rates, co-occurring illnesses, suicidal ideation/self-harm, and hospital charges. J Nerv Men Dis. 2016;204(2):78-86. https://doi.org/10.1097/NMD.0000000000000432
19. Frommberger U, Angenendt J, Berger M. Post-traumatic stress disorder--a diagnostic and therapeutic challenge. Dtsch Arztebl Int. 2014;111(5):59-65. https://doi.com/10.3238/arztebl.2014.0059
20. Sareen J. Posttraumatic stress disorder in adults: impact, comorbidity, risk factors, and treatment. Can J Psychiatry. 2014;59(9):460-467. https://doi.org/10.1177/070674371405900902
21. Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30(5):421-434. https://doi.org/10.1016/j.genhosppsych.2008.05.006
22. Griffiths J, Fortune G, Barber V, Young JD. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33(9):1506-1518. https://doi.org/10.1007/s00134-007-0730-z
23. Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121-1129. https://doi.org/10.1097/CCM.0000000000000882
24. Fletcher KE, Collins J, Holzhauer B, Lewis F, Hendricks M. Medical patients with PTSD identify issues with hospitalization. J Gen Intern Med. 2020;35(6):1906-1907. https://doi.org/10.1007/s11606-019-05480-y
25. Struble LM, Sullivan BJ, Hartman LS. Psychiatric disorders impacting critical illness. Crit Care Nurs Clin North Am. 2014;26(1):115-138. https://doi.org/10.1016/j.ccell.2013.10.002
26. Baxter A. Posttraumatic stress disorder and the intensive care unit patient: implications for staff and advanced practice critical care nurses. Dimens Crit Care Nurs. 2004;23(4):145-150. http://doi.org/10.1097/00003465-200407000-00001
27. Abrams TE, Vaughan-Sarrazin M, Rosenthal GE. Preexisting comorbid psychiatric conditions and mortality in nonsurgical intensive care patients. Am J Crit Care. 2010;19(3):241-249. https://doi.org/10.4037/ajcc2010967
28. Kebbe J, Lal A, El-Solh A, Jaoude P. Effects of PTSD on patient outcomes in the intensive care unit. Chest. 2015;148(4 Suppl):220A. https://doi.org/10.1378/chest.2274366
29. Johnson KG, Rosen J. Re-emergence of posttraumatic stress disorder nightmares with nursing home admission: treatment with prazosin. J Am Med Dir Assoc. 2013;14(2):130-131. https://doi.org/10.1016/j.jamda.2012.10.007
30. Zimmerman M, Mattia JI. Is posttraumatic stress disorder underdiagnosed in routine clinical settings? J Nerv Ment Dis. 1999;187(7):420-428. https://doi.org/10.1097/00005053-199907000-00005
31. Trauma-informed care. Agency for Healthcare Research and Quality. 2015. Accessed July 30, 2019. http://www.ahrq.gov/professionals/prevention-chronic-care/healthier-pregnancy/preventive/trauma.html
32. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Substance Abuse and Mental Health Administration, Department of Health & Human Services; 2014. HHS Publication No. SMA 14-4884. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf
33. DeCandia CJ, Guarino K. Trauma-informed care: an ecological response. J Child Youth Care Work. 2015;24:7-32.
34. Prins A, Bovin MJ, Smolenski DJ, et al. The PRIMARY CARE PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. https://doi.org/10.1007/s11606-016-3703-5
35. Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systematic review and meta-analysis to determine first-line treatments. Depress Anxiety. 2016;33(9):792-806. https://doi.org/10.1002/da.22511
36. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Department of Veterans Affairs/Department of Defense. 2017. Accessed July 22, 2019. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGClinicianSummaryFinal.pdf
37. Singh B, Hughes AJ, Mehta G, Erwin PJ, Parsaik AK. Efficacy of prazosin in posttraumatic stress disorder: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(4). https://doi.org/10.4088/PCC.16r01943
38. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517. https://doi.org/10.1056/NEJMoa1507598
39. El-Solh AA. Management of nightmares in patients with posttraumatic stress disorder: current perspectives. Nat Sci Sleep. 2018;10:409-420. https://doi.org/10.2147/NSS.S166089
40. What is ROVER? Treatment Services. VA. 2018. Accessed February 14, 2020. https://www.houston.va.gov/docs/ROVERBrochure.pdf
41. Moser DK, Chung ML, McKinley S, et al. Critical care nursing practice regarding patient anxiety assessment and management. Intensive Crit Care Nurs. 2003;19(5):276-288. https://doi.org/10.1016/s0964-3397(03)00061-2
42. Bulechek G, Butcher H, Dochterman JM, Wagner C. Nursing Interventions Classification (NIC), 6th Ed. Elsevier; 2013.
43. Blanaru M, Bloch B, Vadas L, et al. The effects of music relaxation and muscle relaxation techniques on sleep quality and emotional measures among individuals with posttraumatic stress disorder. Ment Illn. 2012;4(2):e13. https://doi.org/10.4081/mi.2012.e13
44. Leskin GA, Woodward SH, Young HE, Sheikh JI. Effects of comorbid diagnoses on sleep disturbance in PTSD. J Psychiatr Res. 2002;36(6):449-452. https://doi.org/10.1016/s0022-3956(02)00025-0
45. Vandrey R, Babson KA, Herrmann ES, Bonn-Miller MO. Interactions between disordered sleep, post-traumatic stress disorder, and substance use disorders. Int Rev Psychiatry. 2014;26(2):237-247. https://doi.org/10.3109/09540261.2014.901300
46. Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618-622. https://doi.org/10.1097/00005053-200109000-00008
47. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372-382. https://doi.org/10.1176/appi.ajp.2012.12040432
48. Ho FYY, Chan CS, Tang KNS. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2016;43:90-102. https://doi.org/10.1016/j.cpr.2015.09.005
49. Thompson KE, Franklin CL, Hubbard K. PTSD sleep therapy group: training your mind and body for better sleep: Therapist Manual. A product of the Department of Veterans Affairs South Central (VISN 16) Mental Illness Research, Education, and Clinical Center (MIRECC). Accessed July 22, 2019. https://www.mirecc.va.gov/VISN16/docs/Sleep_Therapy_Group_Therapist_Manual.pdf
50. Ye L, Keane K, Hutton Johnson S, Dykes PC. How do clinicians assess, communicate about, and manage patient sleep in the hospital? J Nurs Adm. 2013;43(6):342-347. https://doi.org/10.1097/NNA.0b013e3182942c8a
51. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
52. Gagner-Tjellesen D, Yurkovich EE, Gragert M. Use of music therapy and other ITNIs in acute care. J Psychosoc Nurs Ment Health Serv. 2001;39(10):26-37.
53. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636. https://doi.org/10.5664/jcsm.3786
54. Brennstuhl MJ, Tarquinio C, Montel S. Chronic pain and PTSD: evolving views on their comorbidity. Perspect Psychiatr Care. 2015;51(4):295-304. https://doi.org/10.1111/ppc.12093
55. Bosch J, Weaver TL, Neylan TC, Herbst E, McCaslin SE. Impact of engagement in exercise on sleep quality among veterans with posttraumatic stress disorder symptoms. Mil Med. 2017;182(9):e1745-e1750. https://doi.org/10.7205/MILMED-D-16-00385
56. Rosenbaum S, Vancampfort D, Tiedemann A, et al. Among inpatients, posttraumatic stress disorder symptom severity is negatively associated with time spent walking. J Nerv Ment Dis. 2016;204(1):15-19. https://doi.org/10.1097/NMD.0000000000000415
1. DSM-5 Fact Sheet: Posttraumatic Stress Disorder. American Psychological Association. 2013. Accessed 30 July 2019. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/DSM/APA_DSM-5-PTSD.pdf
2. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. https://doi.org/10.1001/archpsyc.62.6.617
3. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. https://doi.org/10.1001/archpsyc.62.6.593
4. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060. https://doi.org/10.1001/archpsyc.1995.03950240066012
5. Weiss DS, Marmar CR, Schlenger WE, et al. The prevalence of lifetime and partial post-traumatic stress disorder in Vietnam theater veterans. J Trauma Stress. 1992;5(3):365-376. https://doi.org/10.1002/jts.2490050304
6. Kulka RA, Schlenger WE, Fairbank JA, et al. Trauma And the Vietnam War Generation: Report of findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; 1990.
7. Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. J Occup Environ Med. 2009;51(4):401-410. https://doi.org/10.1097/JOM.0b013e3181a2feeb
8. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24. https://doi.org/10.1007/s11606-009-1117-3
9. VA MISSION Act. Department of Veterans Affairs. 2019. Accessed February 2, 2020. https://missionact.va.gov/
10. Fogarty CT, Sharma S, Chetty VK, Culpepper L. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21(5):398-407. https://doi.org/10.3122/jabfm.2008.05.070082
11. Kartha A, Brower V, Saitz R, Samet JH, Keane TM, Liebschutz J. The impact of trauma exposure and post-traumatic stress disorder on healthcare utilization among primary care patients. Med Care. 2008;46(4):388-393. https://doi.org/10.1097/MLR.0b013e31815dc5d2
12. Dobie DJ, Maynard C, Kivlahan DR, et al. Posttraumatic stress disorder screening status is associated with increased VA medical and surgical utilization in women. J Gen Intern Med. 2006;21(Suppl 3):S58-S64. https://doi.org/10.1111/j.1525-1497.2006.00376.x
13. Calhoun PS, Bosworth HB, Grambow SC, Dudley TK, Beckham JC. Medical service utilization by veterans seeking help for posttraumatic stress disorder. Am J Psychiatry. 2002;159(12):2081-2086. https://doi.org/10.1176/appi.ajp.159.12.2081
14. Frayne SM, Chiu VY, Iqbal S, et al. Medical care needs of returning veterans with PTSD: their other burden. J Gen Intern Med. 2011;26(1):33-39. https://doi.org/10.1007/s11606-010-1497-4
15. Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Medical comorbidity of full and partial posttraumatic stress disorder in US adults: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2011;73(8):697-707. https://doi.org/10.1097/PSY.0b013e3182303775
16. Vaccarino V, Goldberg J, Rooks C, et al. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62(11):970-978. https://doi.org/10.1016/j.jacc.2013.04.085
17. Bressi SK, Marcus SC, Solomon PL. The impact of psychiatric comorbidity on general hospital length of stay. Psychiatr Q. 2006;77(3):203-209. https://doi.org/10.1007/s11126-006-9007-x
18. Haviland MG, Banta JE, Sonne JL, Przekop P. Posttraumatic stress disorder-related hospitalizations in the United States (2002-2011): Rates, co-occurring illnesses, suicidal ideation/self-harm, and hospital charges. J Nerv Men Dis. 2016;204(2):78-86. https://doi.org/10.1097/NMD.0000000000000432
19. Frommberger U, Angenendt J, Berger M. Post-traumatic stress disorder--a diagnostic and therapeutic challenge. Dtsch Arztebl Int. 2014;111(5):59-65. https://doi.com/10.3238/arztebl.2014.0059
20. Sareen J. Posttraumatic stress disorder in adults: impact, comorbidity, risk factors, and treatment. Can J Psychiatry. 2014;59(9):460-467. https://doi.org/10.1177/070674371405900902
21. Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30(5):421-434. https://doi.org/10.1016/j.genhosppsych.2008.05.006
22. Griffiths J, Fortune G, Barber V, Young JD. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33(9):1506-1518. https://doi.org/10.1007/s00134-007-0730-z
23. Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121-1129. https://doi.org/10.1097/CCM.0000000000000882
24. Fletcher KE, Collins J, Holzhauer B, Lewis F, Hendricks M. Medical patients with PTSD identify issues with hospitalization. J Gen Intern Med. 2020;35(6):1906-1907. https://doi.org/10.1007/s11606-019-05480-y
25. Struble LM, Sullivan BJ, Hartman LS. Psychiatric disorders impacting critical illness. Crit Care Nurs Clin North Am. 2014;26(1):115-138. https://doi.org/10.1016/j.ccell.2013.10.002
26. Baxter A. Posttraumatic stress disorder and the intensive care unit patient: implications for staff and advanced practice critical care nurses. Dimens Crit Care Nurs. 2004;23(4):145-150. http://doi.org/10.1097/00003465-200407000-00001
27. Abrams TE, Vaughan-Sarrazin M, Rosenthal GE. Preexisting comorbid psychiatric conditions and mortality in nonsurgical intensive care patients. Am J Crit Care. 2010;19(3):241-249. https://doi.org/10.4037/ajcc2010967
28. Kebbe J, Lal A, El-Solh A, Jaoude P. Effects of PTSD on patient outcomes in the intensive care unit. Chest. 2015;148(4 Suppl):220A. https://doi.org/10.1378/chest.2274366
29. Johnson KG, Rosen J. Re-emergence of posttraumatic stress disorder nightmares with nursing home admission: treatment with prazosin. J Am Med Dir Assoc. 2013;14(2):130-131. https://doi.org/10.1016/j.jamda.2012.10.007
30. Zimmerman M, Mattia JI. Is posttraumatic stress disorder underdiagnosed in routine clinical settings? J Nerv Ment Dis. 1999;187(7):420-428. https://doi.org/10.1097/00005053-199907000-00005
31. Trauma-informed care. Agency for Healthcare Research and Quality. 2015. Accessed July 30, 2019. http://www.ahrq.gov/professionals/prevention-chronic-care/healthier-pregnancy/preventive/trauma.html
32. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Substance Abuse and Mental Health Administration, Department of Health & Human Services; 2014. HHS Publication No. SMA 14-4884. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf
33. DeCandia CJ, Guarino K. Trauma-informed care: an ecological response. J Child Youth Care Work. 2015;24:7-32.
34. Prins A, Bovin MJ, Smolenski DJ, et al. The PRIMARY CARE PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. https://doi.org/10.1007/s11606-016-3703-5
35. Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systematic review and meta-analysis to determine first-line treatments. Depress Anxiety. 2016;33(9):792-806. https://doi.org/10.1002/da.22511
36. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Department of Veterans Affairs/Department of Defense. 2017. Accessed July 22, 2019. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGClinicianSummaryFinal.pdf
37. Singh B, Hughes AJ, Mehta G, Erwin PJ, Parsaik AK. Efficacy of prazosin in posttraumatic stress disorder: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(4). https://doi.org/10.4088/PCC.16r01943
38. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517. https://doi.org/10.1056/NEJMoa1507598
39. El-Solh AA. Management of nightmares in patients with posttraumatic stress disorder: current perspectives. Nat Sci Sleep. 2018;10:409-420. https://doi.org/10.2147/NSS.S166089
40. What is ROVER? Treatment Services. VA. 2018. Accessed February 14, 2020. https://www.houston.va.gov/docs/ROVERBrochure.pdf
41. Moser DK, Chung ML, McKinley S, et al. Critical care nursing practice regarding patient anxiety assessment and management. Intensive Crit Care Nurs. 2003;19(5):276-288. https://doi.org/10.1016/s0964-3397(03)00061-2
42. Bulechek G, Butcher H, Dochterman JM, Wagner C. Nursing Interventions Classification (NIC), 6th Ed. Elsevier; 2013.
43. Blanaru M, Bloch B, Vadas L, et al. The effects of music relaxation and muscle relaxation techniques on sleep quality and emotional measures among individuals with posttraumatic stress disorder. Ment Illn. 2012;4(2):e13. https://doi.org/10.4081/mi.2012.e13
44. Leskin GA, Woodward SH, Young HE, Sheikh JI. Effects of comorbid diagnoses on sleep disturbance in PTSD. J Psychiatr Res. 2002;36(6):449-452. https://doi.org/10.1016/s0022-3956(02)00025-0
45. Vandrey R, Babson KA, Herrmann ES, Bonn-Miller MO. Interactions between disordered sleep, post-traumatic stress disorder, and substance use disorders. Int Rev Psychiatry. 2014;26(2):237-247. https://doi.org/10.3109/09540261.2014.901300
46. Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618-622. https://doi.org/10.1097/00005053-200109000-00008
47. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372-382. https://doi.org/10.1176/appi.ajp.2012.12040432
48. Ho FYY, Chan CS, Tang KNS. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2016;43:90-102. https://doi.org/10.1016/j.cpr.2015.09.005
49. Thompson KE, Franklin CL, Hubbard K. PTSD sleep therapy group: training your mind and body for better sleep: Therapist Manual. A product of the Department of Veterans Affairs South Central (VISN 16) Mental Illness Research, Education, and Clinical Center (MIRECC). Accessed July 22, 2019. https://www.mirecc.va.gov/VISN16/docs/Sleep_Therapy_Group_Therapist_Manual.pdf
50. Ye L, Keane K, Hutton Johnson S, Dykes PC. How do clinicians assess, communicate about, and manage patient sleep in the hospital? J Nurs Adm. 2013;43(6):342-347. https://doi.org/10.1097/NNA.0b013e3182942c8a
51. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
52. Gagner-Tjellesen D, Yurkovich EE, Gragert M. Use of music therapy and other ITNIs in acute care. J Psychosoc Nurs Ment Health Serv. 2001;39(10):26-37.
53. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636. https://doi.org/10.5664/jcsm.3786
54. Brennstuhl MJ, Tarquinio C, Montel S. Chronic pain and PTSD: evolving views on their comorbidity. Perspect Psychiatr Care. 2015;51(4):295-304. https://doi.org/10.1111/ppc.12093
55. Bosch J, Weaver TL, Neylan TC, Herbst E, McCaslin SE. Impact of engagement in exercise on sleep quality among veterans with posttraumatic stress disorder symptoms. Mil Med. 2017;182(9):e1745-e1750. https://doi.org/10.7205/MILMED-D-16-00385
56. Rosenbaum S, Vancampfort D, Tiedemann A, et al. Among inpatients, posttraumatic stress disorder symptom severity is negatively associated with time spent walking. J Nerv Ment Dis. 2016;204(1):15-19. https://doi.org/10.1097/NMD.0000000000000415
© 2021 Society of Hospital Medicine
Schizoaffective disorder: A challenging diagnosis
Mr. C, age 34, presented to the emergency department with his wife because of increasingly bizarre behavior. He reported auditory and visual hallucinations, and believed that the “mob had ordered a hit” against him. He had threatened to shoot his wife and children, which led to his arrest and being briefly jailed. In jail, he was agitated, defecated on the floor, and disrobed. His wife reported that Mr. C had a long history of bipolar disorder and had experienced his first manic episode and hospitalization at age 17. Since then, he had been treated with many different antidepressants, antipsychotics, and mood stabilizers.
Mr. C was admitted to the hospital, where he developed a catatonic syndrome that was treated with a course of electroconvulsive therapy. He was eventually stabilized with
Over the next 8 years, Mr. C was often noncompliant with medication and frequently was hospitalized for mania. His symptoms included poor sleep, grandiosity, pressured speech, racing and disorganized thoughts, increased risk-taking behavior (ie, driving at excessive speeds), and hyperreligiosity (ie, speaking with God). Mr. C also occasionally used methamphetamine, cannabis, and cocaine. Although he had responded well to treatment early in the course of his illness, as he entered his late 30s, his response was less complete, and by his 40s, Mr. C was no longer able to function independently. He eventually was prescribed a long-acting injectable antipsychotic, paliperidone palmitate, 156 mg monthly. Eventually, his family was no longer able to care for him at home, so he was admitted to a residential care facility.
In this facility, based on the long-standing nature of Mr. C’s psychotic disorder and frequency with which he presented with mania, his clinicians changed his diagnosis to schizoaffective disorder, bipolar type. It had become clear that mood symptoms comprised >50% of the total duration of his illness.
Schizoaffective disorder (SAD) often has been used as a diagnosis for patients who have an admixture of mood and psychotic symptoms whose diagnosis is uncertain. Its hallmark is the presence of symptoms of a major mood episode (either a depressive or manic episode) concurrent with symptoms characteristic of schizophrenia, such as delusions, hallucinations, or disorganized speech.1
SAD is a controversial diagnosis. There has been inadequate research regarding the epidemiology, course, etiologic factors, and treatment of this disorder. Debate continues to swirl around its conceptualization; some experts view SAD as an independent disorder, while others see SAD as either a form of schizophrenia or a mood disorder.1 In this review, we describe the classification of SAD and its features, diagnosis, and treatment.
An evolving diagnosis
The term schizoaffective was first used by Jacob Kasanin, MD, in 1933.2 He described 9 patients with “acute schizoaffective psychoses,” each of whom had an abrupt onset. The term was used in the first edition of the DSM as a subtype of schizophrenia.3 In DSM-I, the “schizo-affective type” was defined as a diagnosis for patients with a “significant admixture of schizophrenic and affective reactions.”3 Diagnostic criteria for SAD were developed for DSM-III-R, published in 1987.4 These criteria continued to evolve with subsequent editions of the DSM.
Continue to: DSM-5 provides...
DSM-5 provides a clearer separation between schizophrenia with mood symptoms, bipolar disorder, and SAD (Table5). In addition, DSM-5 shifts away from the DSM-IV diagnosis of SAD as an episode, and instead focuses more on the longitudinal course of the illness. It has been suggested that this change will likely lead to reduced rates of diagnosis of SAD.6 Despite improvements in classification, the diagnosis remains controversial (Box7-11).
Box 1
Despite improvements in classification, controversy continues to swirl around the question of whether schizoaffective disorder (SAD) represents an independent disorder that stands apart from schizophrenia and bipolar disorder, whether it is a form of schizophrenia, or whether it is a form of bipolar disorder or a depressive disorder.7,8 Other possibilities are that SAD is heterogeneous or that it represents a middle point on a spectrum that bridges mood and psychotic disorders. While the merits of each possibility are beyond the scope of this review, it is safe to say that each possibility has its proponents. For these reasons, some argue that the concept itself lacks validity and shows the pitfalls of our classification system.7
Poor diagnostic reliability is one reason for concerns about validity. Most recently, a field trial using DSM-5 criteria produced a kappa of 0.50, which is moderate,9 but earlier definitions produced relatively poor results. Wilson et al10 point out that Criterion C, which concerns duration of mood symptoms, produces a particularly low kappa. Another reason is diagnostic switching, whereby patients initially diagnosed with 1 disorder receive a different diagnosis at followup. Diagnostic switching is especially problematic for SAD. In a large meta-analysis by Santelmann et al,11 36% of patients initially diagnosed with SAD had their diagnosis changed when reassessed. This diagnostic shift tended more toward schizophrenia than bipolar disorder. In addition, more than one-half of all patients initially diagnosed with schizophrenia, bipolar disorder, or major depressive disorder were re-diagnosed with SAD when reassessed.
DSM-5 subtypes and specifiers
In DSM-5,SAD has 2 subtypes5:
- Bipolar type. The bipolar type is marked by the presence of a manic episode (major depressive episodes may also occur)
- Depressive type. The depressive type is marked by the presence of only major depressive episodes.
SAD also includes several specifiers, with the express purpose of giving clinicians greater descriptive ability. The course of SAD can be described as either “first episode,” defined as the first manifestation of the disorder, or as having “multiple episodes,” defined as a minimum of 2 episodes with 1 relapse. In addition, SAD can be described as an acute episode, in partial remission, or in full remission. The course can be described as “continuous” if it is clear that symptoms have been present for the majority of the illness with very brief subthreshold periods. The course is designated as “unspecified” when information is unavailable or lacking. The 5-point Clinician-Rated Dimensions of Psychosis Symptoms was introduced to enable clinicians to make a quantitative assessment of the psychotic symptoms, although its use is not required.
Epidemiology and gender ratio
The epidemiology of SAD has not been well studied. DSM-5 estimates that SAD is approximately one-third as common as schizophrenia, which has a lifetime prevalence of 0.5% to 0.8%.5 This is similar to an estimate by Perälä et al12 of a 0.32% lifetime prevalence based on a nationally representative sample of persons in Finland age ≥30. Scully et al13 calculated a prevalence estimate of 1.1% in a representative sample of adults in rural Ireland. Based on pooled clinical data, Keck et al14 estimated the prevalence in clinical settings at 16%, similar to the figure of 19% reported by Levinson et al15 based on data from New York State psychiatric hospitals. In clinical practice, the diagnosis of SAD is used frequently when there is diagnostic uncertainty, which potentially inflates estimates of lifetime prevalence.
The prevalence of SAD is higher in women than men, with a sex ratio of about 2:1, similar to that seen in mood disorders.13,16-19 There are an equal number of men and women with the bipolar subtype, but a female preponderance with the depressive subtype.5 The bipolar subtype is more common in younger patients, while the depressive subtype is more common in older patients. SAD is a rare diagnosis in children.20
Continue to: Course and outcome
Course and outcome
The onset of SAD typically occurs in early adulthood, but can range from childhood to senescence. Approximately one-third of patients are diagnosed before age 25, one-third between age 25 and 35, and one-third after age 35.21-23 Based on a literature review, Cheniaux et al7 concluded that that age at onset for patients with SAD is between those with schizophrenia and those with mood disorders.
The course of SAD is variable but represents a middle ground between that of schizophrenia and the mood disorders. In a 4- to 5-year follow-up,24 patients with SAD had a better overall course than patients with schizophrenia but had poorer functioning than those with bipolar mania, and much poorer than those with unipolar depression. Mood-incongruent psychotic features predict a particularly worse outcome. These findings were reaffirmed at a 10-year follow-up.25 Mood symptoms portend a better outcome than do symptoms of schizophrenia.
The lifetime suicide risk for patients with SAD is estimated at 5%, with a higher risk associated with the presence of depressive symptoms.26 One study found that women with SAD had a 17.5-year reduced life expectancy (64.1 years) compared with a reduction of 8.0 years for men (69.4 years).27
Comorbidity
Patients with SAD are commonly diagnosed with other psychiatric disorders, including anxiety disorders, obsessive-compulsive disorder, posttraumatic stress disorder, and substance use disorders.21,28,29 When compared with the general population, patients with SAD are at higher risk for coronary heart disease, stroke, obesity, and smoking, likely contributing to their decreased life expectancy.27,30 Because second-generation antipsychotics (SGAs) are often used to treat SAD, patients with SAD are at risk for metabolic syndrome and diabetes mellitus.30
Clinical assessment
Because there are no diagnostic, laboratory, or neuroimaging tests for SAD, the most important basis for making the diagnosis is the patient’s history, supplemented by collateral history from family members or friends, and medical records. Determining the percentage of time spent in a mood episode (DSM-5 Criterion C) is especially important.31 This requires the clinician to pay close attention to the temporal relationship of psychotic and mood symptoms.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis for SAD is broad because it includes all of the possibilities usually considered for major mood disorders and for psychotic disorders5:
- schizophrenia
- bipolar disorder with psychotic features
- major depressive disorder with psychotic features
- depressive or bipolar disorders with catatonic features
- personality disorders (especially the schizotypal, paranoid, and borderline types)
- major neurocognitive disorders in which there are mood and psychotic symptoms
- substance/medication-induced psychotic disorder
- disorders induced by medical conditions.
With schizophrenia, the duration of all episodes of a mood syndrome is brief (<50% of the total duration of the illness) relative to the duration of the psychotic symptoms. Although psychotic symptoms may occur in persons with mood disorders, they are generally not present in the absence of depression or mania, helping to set the boundary between SAD and psychotic mania or depression. As for personality disorders, the individual will not have a true psychosis, although some symptoms, such as feelings of unreality, paranoia, or magical thinking, may cause diagnostic confusion.
Medical conditions also can present with psychotic and mood symptoms and need to be ruled out. These include psychotic disorder due to another medical condition, and delirium. A thorough medical workup should be performed to rule out any possible medical causes for the symptoms.
Substance use should also be ruled out as the cause of the symptoms because many substances are associated with mood and psychotic symptoms. It is usually clear from the history, physical examination, or laboratory tests when a medication/illicit substance has initiated and maintained the disorder.
Neurologic conditions. If a neurologic condition is suspected, a neurologic evaluation may be warranted, including laboratory tests, brain imaging to identify specific anatomical abnormalities, lumbar puncture with cerebrospinal fluid analysis, and an electroencephalogram to rule out a convulsive disorder.
Continue to: Clinical symptoms
Clinical symptoms
The signs and symptoms of SAD include those typically seen in schizophrenia and the mood disorders. Thus, the patient may exhibit elated mood and/or grandiosity, or severe depression, combined with mood-incongruent psychotic features such as paranoid delusions. The symptoms may present together or in an alternating fashion, and psychotic symptoms may be mood-congruent or mood-incongruent. Mr. C’s case illustrates some of the symptoms of the disorder.
Brain imaging
Significant changes have been reported to occur in the brain structure and function in persons with SAD. Neuroimaging studies using voxel-based morphometry have shown significant reductions in gray matter volume in several areas of the brain, including the medial prefrontal cortex, insula, Rolandic operculum, parts of the temporal lobe, and the hippocampus.32-35 Amann et al32 found that patients with SAD and schizophrenia had widespread and overlapping areas of significant volume reduction, but patients with bipolar disorder did not. These studies suggest that at least from a neuroimaging standpoint, SAD is more closely related to schizophrenia than bipolar disorder, and could represent a variant of schizophrenia.
Treatment of SAD
The pharmacotherapy of SAD is mostly empirical because of the lack of randomized controlled trials. Clinicians have traditionally prescribed an antipsychotic agent along with either a mood stabilizer (eg,
Since that exhaustive review,
Patients with SAD will require maintenance treatment for ongoing symptom control. Medication that is effective for treatment of an acute episode should be considered for maintenance treatment. Both the extended-release and long-acting injectable (LAI) formulations of paliperidone have been shown to be efficacious in the maintenance treatment of patients with SAD.40 The LAI form of paliperidone significantly delayed psychotic, depressive, and manic relapses, improved clinical rating scale scores, and increased medication adherence.41,42 In an open-label study, olanzapine LAI was effective in long-term maintenance treatment, although approximately 40% of patients experienced significant weight gain.43 One concern with olanzapine is the possible occurrence of a post-injection delirium/sedation syndrome. For that reason, patients receiving olanzapine must be monitored for at least 3 hours post-injection. The paliperidone LAI does not require monitoring after injection.
Continue to: There is a single clinical trial...
There is a single clinical trial showing that patients with SAD can be successfully switched from other antipsychotics to
Other approaches
Electroconvulsive therapy (ECT) should be considered for patients with SAD who are acutely ill and have failed to respond adequately to medication. ECT is especially relevant in the setting of acute mood symptoms (ie, depressive or manic symptoms co-occurring with psychosis or in the absence of psychosis).45
As currently conceptualized, the diagnosis of SAD is made in persons having an admixture of mood and psychotic symptoms, although by definition mood symptoms must take up the majority (≥50%) of the total duration of the illness. Unfortunately, SAD has been inadequately researched due to the unreliability of its definition and concerns about its validity. The long-term course of SAD is midway between mood and psychotic disorders, and the disorder can cause significant disability.
Bottom Line
Schizoaffective disorder (SAD) is characterized by the presence of symptoms of a major mood episode (a depressive or manic episode) concurrent with symptoms of schizophrenia. The most important basis for establishing the diagnosis is the patient’s history. Determining the percentage of time spent in a mood episode is especially important. Treatment usually consists of an antipsychotic plus a mood stabilizer or antidepressant. Electroconvulsive therapy is an option for patients with SAD who do not respond well to medication.
Related Resources
- Wy TJP, Saadabadi A. Schizoaffective disorder. NCBI Bookshelf: StatPearls Publishing. Published January 2020. https://www.ncbi.nlm.nih.gov/books/NBK541012/. Updated April 15, 2020.
- Parker G. How well does the DSM-5 capture schizoaffective disorder? Can J Psychiatry. 2019;64(9):607-610.
Drug Brand Names
Aripiprazole • Abilify
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Olanzapine long-acting injectable • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate • Invega sustenna
Valproate • Depacon
1. Miller JN, Black DW. Schizoaffective disorder: a review. Ann Clin Psychiatry. 2019;31(1):47-53.
2. Kasanin J. The acute schizoaffective psychoses. Am J Psychiatry. 1933;90:97-126.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington, DC: American Psychiatric Association; 1952.
4. Diagnostic and statistical manual of mental disorders, 3rd ed, revision. Washington, DC: American Psychiatric Association; 1987.
5. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
6. Malaspina D, Owen M, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150:21-25.
7. Cheniaux E, Landeria-Fernandez J, Telles LL, et al. Does schizoaffective disorder really exist? A systematic review of the studies that compared schizoaffective disorder with schizophrenia or mood disorders. J Affect Disord. 2008;106:209-217.
8. Kantrowitz JT, Citrome L. Schizoaffective disorder: a review of current research themes and pharmacologic management. CNS Drugs. 2011;25:317-331.
9. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170:59-70.
10. Wilson JE, Nian H, Heckers S. The schizoaffective disorder diagnosis: a conundrum in the clinical setting. Eur Arch Psychiatry Clin Neurosci. 2014;264:29-34.
11. Santelmann H, Franklin J, Bußhoff J, Baethge C. Test-retest reliability of schizoaffective disorder compared with schizophrenia, bipolar disorder, and unipolar depression--a systematic review and meta-analysis. Bipolar Disord. 2015;17:753-768.
12. Perälä J, Suvisaari J, Saarni SI, et al. Lifetime Prevalence of psychotic and bipolar I disorders in a general population. JAMA Psychiatry. 2007;64:19-28.
13. Scully PJ, Owens JM, Kinsella A, et al. Schizophrenia, schizoaffective and bipolar disorder within an epidemiologically complete, homogeneous population in rural Ireland: small area variation in rate. Schizophr Res. 2004;67:143-155.
14. Keck PE Jr, McElroy SE, Strakowski SM, et al. Pharmacologic treatment of schizoaffective disorder. Psychopharmacol. 1994;114:529-538.
15. Levinson DF, Umapathy C, Musthaq M. Treatment of schizoaffective disorder and schizophrenia with mood symptoms. Am J Psychiatry. 1999;156:1138-1148.
16. Angst J, Felder W, Lohmeyer B. Course of schizoaffective psychoses: results of a follow-up study. Schizophr Bull. 1980;6:579-585.
17. Lenz G, Simhandl C, Thau K, et al. Temporal stability of diagnostic criteria for functional psychoses. Psychopathol. 1991;24:328-335.
18. Malhi GS, Green M, Fagiolini A, et al. Schizoaffective disorder: diagnostic issues and future recommendations. Bipolar Disord. 2008;10:215-230.
19. Marneros A, Deister A, Rohde A. Psychopathological and social status of patients with affective, schizophrenic and schizoaffective disorders after long‐term course. Acta Psychiatr Scand. 1990;82:352-358.
20. Werry JS, McClellan JM, Chard L. Childhood and adolescent schizophrenic, bipolar, and schizoaffective disorders: a clinical and outcome study. J Am Acad Child Adolesc Psychiatry. 1991;30:457-465.
21. Abrams DJ, Rojas DC, Arciniegas DB. Is schizoaffective disorder a distinct categorical diagnosis? A critical review of the literature. Neuropsychiatr Dis Treat. 2008;4:1089-1109.
22. Bromet EJ, Kotov R, Fochtmann LJ, et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168:1186-1194.
23. Salvatore P, Baldessarini RJ, Tohen M, et al. The McLean-Harvard First Episode Project: two-year stability of DSM-IV diagnoses in 500 first-episode psychotic disorder patients. J Clin Psychiatry. 2009;70:458-466.
24. Grossman LS, Harrow M, Goldberg JF, et al. Outcome of schizoaffective disorder at two long term follow-ups: comparisons with outcome of schizophrenia and affective disorders. Am J Psychiatry. 1991;148:1359-1365.
25. Harrow M, Grossman L, Herbener E, et al. Ten-year outcome: patients with schizoaffective disorders, schizophrenia, affective disorders and mood-incongruent psychotic symptoms. Br J Psychiatry. 2000;177:421-426.
26. Hor K, Taylor M. Review: suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24:81-90.
27. Chang CK, Hayes RD, Perera G, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS ONE. 2011;6:e19590.
28. Byerly M, Goodman W, Acholonu W, et al. Obsessive compulsive symptoms in schizophrenia: frequency and clinical features. Schizophr Res. 2005;76:309-316.
29. Strauss JL, Calhoun PS, Marx CE, et al. Comorbid posttraumatic stress disorder is associated with suicidality in male veterans with schizophrenia or schizoaffective disorder. Schizophr Res. 2006;84:165-169.
30. Fagiolini A, Goracci A. The effects of undertreated chronic medical illnesses in patients with severe mental disorders. J Clin Psychiatry. 2009;70:22-29.
31. Black DW, Grant JE. DSM-5 guidebook: the essential companion to the diagnostic and statistical manual of mental disorders, 5th edition. Washington, DC: American Psychiatric Publishing; 2014.
32. Amann BL, Canales-Rodríguez EJ, Madre M, et al. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2016;133:23-33.
33. Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1285-1296.
34. Ivleva EI, Bidesi AS, Thomas BP, et al. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res. 2012;204:13-24.
35. Radonic
36. Jäger M, Becker T, Weinmann S, et al. Treatment of schizoaffective disorder - a challenge for evidence-based psychiatry. Acta Psychiatr Scand. 2010;121:22-32.
37. Glick ID, Mankosli R, Eudicone JM, et al. The efficacy, safety, and tolerability of aripiprazole for the treatment of schizoaffective disorder: results from a pooled analysis of a sub-population of subjects from two randomized, double-blind, placebo controlled, pivotal trials. J Affect Disord. 2009;115:18-26.
38. Canuso CM, Lindenmayer JP, Kosik-Gonzalez C, et al. A randomized, double-blind, placebo controlled study of 2 dose ranges of paliperidone extended-release in the treatment of subjects with schizoaffective disorder. J Clin Psychiatry. 2010;71:587-598.
39. Canuso CM, Schooler NR, Carothers J, et al. Paliperidone extended-release in schizoaffective disorder: a randomized controlled trial comparing a flexible-dose with placebo in patients treated with and without antidepressants and/or mood stabilizers. J Clin Psychopharmacol. 2010;30:487-495.
40. Lindenmayer JP, Kaur A. Antipsychotic management of schizoaffective disorder: a review. Drugs. 2016;76:589-604.
41. Alphs L, Fu DJ, Turkoz I. Paliperidone for the treatment of schizoaffective disorder. Expert Opin Pharmacother. 2016;176:871-883.
42. Bossie CA, Turkoz I, Alphs L, et al. Paliperidone palmitate once-monthly treatment in recent onset and chronic illness patients with schizoaffective disorder. J Nerv Ment Dis. 2017;205:324-328.
43. McDonnell DP, Landry J, Detke HC. Long-term safety and efficacy of olanzapine long-acting injection in patients with schizophrenia or schizoaffective disorder: a 6-year, multinational, single-arm, open-label study. Int Clin Psychopharmacol. 2014;29:322-331.
44. McEvoy JP, Citrome L, Hernandez D, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74:170-179.
45. Mankad MV, Beyer JL, Wiener RD, et al. Manual of electroconvulsive therapy. Washington, DC: American Psychiatric Publishing; 2010.
Mr. C, age 34, presented to the emergency department with his wife because of increasingly bizarre behavior. He reported auditory and visual hallucinations, and believed that the “mob had ordered a hit” against him. He had threatened to shoot his wife and children, which led to his arrest and being briefly jailed. In jail, he was agitated, defecated on the floor, and disrobed. His wife reported that Mr. C had a long history of bipolar disorder and had experienced his first manic episode and hospitalization at age 17. Since then, he had been treated with many different antidepressants, antipsychotics, and mood stabilizers.
Mr. C was admitted to the hospital, where he developed a catatonic syndrome that was treated with a course of electroconvulsive therapy. He was eventually stabilized with
Over the next 8 years, Mr. C was often noncompliant with medication and frequently was hospitalized for mania. His symptoms included poor sleep, grandiosity, pressured speech, racing and disorganized thoughts, increased risk-taking behavior (ie, driving at excessive speeds), and hyperreligiosity (ie, speaking with God). Mr. C also occasionally used methamphetamine, cannabis, and cocaine. Although he had responded well to treatment early in the course of his illness, as he entered his late 30s, his response was less complete, and by his 40s, Mr. C was no longer able to function independently. He eventually was prescribed a long-acting injectable antipsychotic, paliperidone palmitate, 156 mg monthly. Eventually, his family was no longer able to care for him at home, so he was admitted to a residential care facility.
In this facility, based on the long-standing nature of Mr. C’s psychotic disorder and frequency with which he presented with mania, his clinicians changed his diagnosis to schizoaffective disorder, bipolar type. It had become clear that mood symptoms comprised >50% of the total duration of his illness.
Schizoaffective disorder (SAD) often has been used as a diagnosis for patients who have an admixture of mood and psychotic symptoms whose diagnosis is uncertain. Its hallmark is the presence of symptoms of a major mood episode (either a depressive or manic episode) concurrent with symptoms characteristic of schizophrenia, such as delusions, hallucinations, or disorganized speech.1
SAD is a controversial diagnosis. There has been inadequate research regarding the epidemiology, course, etiologic factors, and treatment of this disorder. Debate continues to swirl around its conceptualization; some experts view SAD as an independent disorder, while others see SAD as either a form of schizophrenia or a mood disorder.1 In this review, we describe the classification of SAD and its features, diagnosis, and treatment.
An evolving diagnosis
The term schizoaffective was first used by Jacob Kasanin, MD, in 1933.2 He described 9 patients with “acute schizoaffective psychoses,” each of whom had an abrupt onset. The term was used in the first edition of the DSM as a subtype of schizophrenia.3 In DSM-I, the “schizo-affective type” was defined as a diagnosis for patients with a “significant admixture of schizophrenic and affective reactions.”3 Diagnostic criteria for SAD were developed for DSM-III-R, published in 1987.4 These criteria continued to evolve with subsequent editions of the DSM.
Continue to: DSM-5 provides...

DSM-5 provides a clearer separation between schizophrenia with mood symptoms, bipolar disorder, and SAD (Table5). In addition, DSM-5 shifts away from the DSM-IV diagnosis of SAD as an episode, and instead focuses more on the longitudinal course of the illness. It has been suggested that this change will likely lead to reduced rates of diagnosis of SAD.6 Despite improvements in classification, the diagnosis remains controversial (Box7-11).
Box 1
Despite improvements in classification, controversy continues to swirl around the question of whether schizoaffective disorder (SAD) represents an independent disorder that stands apart from schizophrenia and bipolar disorder, whether it is a form of schizophrenia, or whether it is a form of bipolar disorder or a depressive disorder.7,8 Other possibilities are that SAD is heterogeneous or that it represents a middle point on a spectrum that bridges mood and psychotic disorders. While the merits of each possibility are beyond the scope of this review, it is safe to say that each possibility has its proponents. For these reasons, some argue that the concept itself lacks validity and shows the pitfalls of our classification system.7
Poor diagnostic reliability is one reason for concerns about validity. Most recently, a field trial using DSM-5 criteria produced a kappa of 0.50, which is moderate,9 but earlier definitions produced relatively poor results. Wilson et al10 point out that Criterion C, which concerns duration of mood symptoms, produces a particularly low kappa. Another reason is diagnostic switching, whereby patients initially diagnosed with 1 disorder receive a different diagnosis at followup. Diagnostic switching is especially problematic for SAD. In a large meta-analysis by Santelmann et al,11 36% of patients initially diagnosed with SAD had their diagnosis changed when reassessed. This diagnostic shift tended more toward schizophrenia than bipolar disorder. In addition, more than one-half of all patients initially diagnosed with schizophrenia, bipolar disorder, or major depressive disorder were re-diagnosed with SAD when reassessed.
DSM-5 subtypes and specifiers
In DSM-5,SAD has 2 subtypes5:
- Bipolar type. The bipolar type is marked by the presence of a manic episode (major depressive episodes may also occur)
- Depressive type. The depressive type is marked by the presence of only major depressive episodes.
SAD also includes several specifiers, with the express purpose of giving clinicians greater descriptive ability. The course of SAD can be described as either “first episode,” defined as the first manifestation of the disorder, or as having “multiple episodes,” defined as a minimum of 2 episodes with 1 relapse. In addition, SAD can be described as an acute episode, in partial remission, or in full remission. The course can be described as “continuous” if it is clear that symptoms have been present for the majority of the illness with very brief subthreshold periods. The course is designated as “unspecified” when information is unavailable or lacking. The 5-point Clinician-Rated Dimensions of Psychosis Symptoms was introduced to enable clinicians to make a quantitative assessment of the psychotic symptoms, although its use is not required.
Epidemiology and gender ratio
The epidemiology of SAD has not been well studied. DSM-5 estimates that SAD is approximately one-third as common as schizophrenia, which has a lifetime prevalence of 0.5% to 0.8%.5 This is similar to an estimate by Perälä et al12 of a 0.32% lifetime prevalence based on a nationally representative sample of persons in Finland age ≥30. Scully et al13 calculated a prevalence estimate of 1.1% in a representative sample of adults in rural Ireland. Based on pooled clinical data, Keck et al14 estimated the prevalence in clinical settings at 16%, similar to the figure of 19% reported by Levinson et al15 based on data from New York State psychiatric hospitals. In clinical practice, the diagnosis of SAD is used frequently when there is diagnostic uncertainty, which potentially inflates estimates of lifetime prevalence.
The prevalence of SAD is higher in women than men, with a sex ratio of about 2:1, similar to that seen in mood disorders.13,16-19 There are an equal number of men and women with the bipolar subtype, but a female preponderance with the depressive subtype.5 The bipolar subtype is more common in younger patients, while the depressive subtype is more common in older patients. SAD is a rare diagnosis in children.20
Continue to: Course and outcome
Course and outcome
The onset of SAD typically occurs in early adulthood, but can range from childhood to senescence. Approximately one-third of patients are diagnosed before age 25, one-third between age 25 and 35, and one-third after age 35.21-23 Based on a literature review, Cheniaux et al7 concluded that that age at onset for patients with SAD is between those with schizophrenia and those with mood disorders.
The course of SAD is variable but represents a middle ground between that of schizophrenia and the mood disorders. In a 4- to 5-year follow-up,24 patients with SAD had a better overall course than patients with schizophrenia but had poorer functioning than those with bipolar mania, and much poorer than those with unipolar depression. Mood-incongruent psychotic features predict a particularly worse outcome. These findings were reaffirmed at a 10-year follow-up.25 Mood symptoms portend a better outcome than do symptoms of schizophrenia.
The lifetime suicide risk for patients with SAD is estimated at 5%, with a higher risk associated with the presence of depressive symptoms.26 One study found that women with SAD had a 17.5-year reduced life expectancy (64.1 years) compared with a reduction of 8.0 years for men (69.4 years).27
Comorbidity
Patients with SAD are commonly diagnosed with other psychiatric disorders, including anxiety disorders, obsessive-compulsive disorder, posttraumatic stress disorder, and substance use disorders.21,28,29 When compared with the general population, patients with SAD are at higher risk for coronary heart disease, stroke, obesity, and smoking, likely contributing to their decreased life expectancy.27,30 Because second-generation antipsychotics (SGAs) are often used to treat SAD, patients with SAD are at risk for metabolic syndrome and diabetes mellitus.30
Clinical assessment
Because there are no diagnostic, laboratory, or neuroimaging tests for SAD, the most important basis for making the diagnosis is the patient’s history, supplemented by collateral history from family members or friends, and medical records. Determining the percentage of time spent in a mood episode (DSM-5 Criterion C) is especially important.31 This requires the clinician to pay close attention to the temporal relationship of psychotic and mood symptoms.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis for SAD is broad because it includes all of the possibilities usually considered for major mood disorders and for psychotic disorders5:
- schizophrenia
- bipolar disorder with psychotic features
- major depressive disorder with psychotic features
- depressive or bipolar disorders with catatonic features
- personality disorders (especially the schizotypal, paranoid, and borderline types)
- major neurocognitive disorders in which there are mood and psychotic symptoms
- substance/medication-induced psychotic disorder
- disorders induced by medical conditions.
With schizophrenia, the duration of all episodes of a mood syndrome is brief (<50% of the total duration of the illness) relative to the duration of the psychotic symptoms. Although psychotic symptoms may occur in persons with mood disorders, they are generally not present in the absence of depression or mania, helping to set the boundary between SAD and psychotic mania or depression. As for personality disorders, the individual will not have a true psychosis, although some symptoms, such as feelings of unreality, paranoia, or magical thinking, may cause diagnostic confusion.
Medical conditions also can present with psychotic and mood symptoms and need to be ruled out. These include psychotic disorder due to another medical condition, and delirium. A thorough medical workup should be performed to rule out any possible medical causes for the symptoms.
Substance use should also be ruled out as the cause of the symptoms because many substances are associated with mood and psychotic symptoms. It is usually clear from the history, physical examination, or laboratory tests when a medication/illicit substance has initiated and maintained the disorder.
Neurologic conditions. If a neurologic condition is suspected, a neurologic evaluation may be warranted, including laboratory tests, brain imaging to identify specific anatomical abnormalities, lumbar puncture with cerebrospinal fluid analysis, and an electroencephalogram to rule out a convulsive disorder.
Continue to: Clinical symptoms
Clinical symptoms
The signs and symptoms of SAD include those typically seen in schizophrenia and the mood disorders. Thus, the patient may exhibit elated mood and/or grandiosity, or severe depression, combined with mood-incongruent psychotic features such as paranoid delusions. The symptoms may present together or in an alternating fashion, and psychotic symptoms may be mood-congruent or mood-incongruent. Mr. C’s case illustrates some of the symptoms of the disorder.
Brain imaging
Significant changes have been reported to occur in the brain structure and function in persons with SAD. Neuroimaging studies using voxel-based morphometry have shown significant reductions in gray matter volume in several areas of the brain, including the medial prefrontal cortex, insula, Rolandic operculum, parts of the temporal lobe, and the hippocampus.32-35 Amann et al32 found that patients with SAD and schizophrenia had widespread and overlapping areas of significant volume reduction, but patients with bipolar disorder did not. These studies suggest that at least from a neuroimaging standpoint, SAD is more closely related to schizophrenia than bipolar disorder, and could represent a variant of schizophrenia.
Treatment of SAD
The pharmacotherapy of SAD is mostly empirical because of the lack of randomized controlled trials. Clinicians have traditionally prescribed an antipsychotic agent along with either a mood stabilizer (eg,
Since that exhaustive review,
Patients with SAD will require maintenance treatment for ongoing symptom control. Medication that is effective for treatment of an acute episode should be considered for maintenance treatment. Both the extended-release and long-acting injectable (LAI) formulations of paliperidone have been shown to be efficacious in the maintenance treatment of patients with SAD.40 The LAI form of paliperidone significantly delayed psychotic, depressive, and manic relapses, improved clinical rating scale scores, and increased medication adherence.41,42 In an open-label study, olanzapine LAI was effective in long-term maintenance treatment, although approximately 40% of patients experienced significant weight gain.43 One concern with olanzapine is the possible occurrence of a post-injection delirium/sedation syndrome. For that reason, patients receiving olanzapine must be monitored for at least 3 hours post-injection. The paliperidone LAI does not require monitoring after injection.
Continue to: There is a single clinical trial...
There is a single clinical trial showing that patients with SAD can be successfully switched from other antipsychotics to
Other approaches
Electroconvulsive therapy (ECT) should be considered for patients with SAD who are acutely ill and have failed to respond adequately to medication. ECT is especially relevant in the setting of acute mood symptoms (ie, depressive or manic symptoms co-occurring with psychosis or in the absence of psychosis).45
As currently conceptualized, the diagnosis of SAD is made in persons having an admixture of mood and psychotic symptoms, although by definition mood symptoms must take up the majority (≥50%) of the total duration of the illness. Unfortunately, SAD has been inadequately researched due to the unreliability of its definition and concerns about its validity. The long-term course of SAD is midway between mood and psychotic disorders, and the disorder can cause significant disability.
Bottom Line
Schizoaffective disorder (SAD) is characterized by the presence of symptoms of a major mood episode (a depressive or manic episode) concurrent with symptoms of schizophrenia. The most important basis for establishing the diagnosis is the patient’s history. Determining the percentage of time spent in a mood episode is especially important. Treatment usually consists of an antipsychotic plus a mood stabilizer or antidepressant. Electroconvulsive therapy is an option for patients with SAD who do not respond well to medication.
Related Resources
- Wy TJP, Saadabadi A. Schizoaffective disorder. NCBI Bookshelf: StatPearls Publishing. Published January 2020. https://www.ncbi.nlm.nih.gov/books/NBK541012/. Updated April 15, 2020.
- Parker G. How well does the DSM-5 capture schizoaffective disorder? Can J Psychiatry. 2019;64(9):607-610.
Drug Brand Names
Aripiprazole • Abilify
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Olanzapine long-acting injectable • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate • Invega sustenna
Valproate • Depacon
Mr. C, age 34, presented to the emergency department with his wife because of increasingly bizarre behavior. He reported auditory and visual hallucinations, and believed that the “mob had ordered a hit” against him. He had threatened to shoot his wife and children, which led to his arrest and being briefly jailed. In jail, he was agitated, defecated on the floor, and disrobed. His wife reported that Mr. C had a long history of bipolar disorder and had experienced his first manic episode and hospitalization at age 17. Since then, he had been treated with many different antidepressants, antipsychotics, and mood stabilizers.
Mr. C was admitted to the hospital, where he developed a catatonic syndrome that was treated with a course of electroconvulsive therapy. He was eventually stabilized with
Over the next 8 years, Mr. C was often noncompliant with medication and frequently was hospitalized for mania. His symptoms included poor sleep, grandiosity, pressured speech, racing and disorganized thoughts, increased risk-taking behavior (ie, driving at excessive speeds), and hyperreligiosity (ie, speaking with God). Mr. C also occasionally used methamphetamine, cannabis, and cocaine. Although he had responded well to treatment early in the course of his illness, as he entered his late 30s, his response was less complete, and by his 40s, Mr. C was no longer able to function independently. He eventually was prescribed a long-acting injectable antipsychotic, paliperidone palmitate, 156 mg monthly. Eventually, his family was no longer able to care for him at home, so he was admitted to a residential care facility.
In this facility, based on the long-standing nature of Mr. C’s psychotic disorder and frequency with which he presented with mania, his clinicians changed his diagnosis to schizoaffective disorder, bipolar type. It had become clear that mood symptoms comprised >50% of the total duration of his illness.
Schizoaffective disorder (SAD) often has been used as a diagnosis for patients who have an admixture of mood and psychotic symptoms whose diagnosis is uncertain. Its hallmark is the presence of symptoms of a major mood episode (either a depressive or manic episode) concurrent with symptoms characteristic of schizophrenia, such as delusions, hallucinations, or disorganized speech.1
SAD is a controversial diagnosis. There has been inadequate research regarding the epidemiology, course, etiologic factors, and treatment of this disorder. Debate continues to swirl around its conceptualization; some experts view SAD as an independent disorder, while others see SAD as either a form of schizophrenia or a mood disorder.1 In this review, we describe the classification of SAD and its features, diagnosis, and treatment.
An evolving diagnosis
The term schizoaffective was first used by Jacob Kasanin, MD, in 1933.2 He described 9 patients with “acute schizoaffective psychoses,” each of whom had an abrupt onset. The term was used in the first edition of the DSM as a subtype of schizophrenia.3 In DSM-I, the “schizo-affective type” was defined as a diagnosis for patients with a “significant admixture of schizophrenic and affective reactions.”3 Diagnostic criteria for SAD were developed for DSM-III-R, published in 1987.4 These criteria continued to evolve with subsequent editions of the DSM.
Continue to: DSM-5 provides...

DSM-5 provides a clearer separation between schizophrenia with mood symptoms, bipolar disorder, and SAD (Table5). In addition, DSM-5 shifts away from the DSM-IV diagnosis of SAD as an episode, and instead focuses more on the longitudinal course of the illness. It has been suggested that this change will likely lead to reduced rates of diagnosis of SAD.6 Despite improvements in classification, the diagnosis remains controversial (Box7-11).
Box 1
Despite improvements in classification, controversy continues to swirl around the question of whether schizoaffective disorder (SAD) represents an independent disorder that stands apart from schizophrenia and bipolar disorder, whether it is a form of schizophrenia, or whether it is a form of bipolar disorder or a depressive disorder.7,8 Other possibilities are that SAD is heterogeneous or that it represents a middle point on a spectrum that bridges mood and psychotic disorders. While the merits of each possibility are beyond the scope of this review, it is safe to say that each possibility has its proponents. For these reasons, some argue that the concept itself lacks validity and shows the pitfalls of our classification system.7
Poor diagnostic reliability is one reason for concerns about validity. Most recently, a field trial using DSM-5 criteria produced a kappa of 0.50, which is moderate,9 but earlier definitions produced relatively poor results. Wilson et al10 point out that Criterion C, which concerns duration of mood symptoms, produces a particularly low kappa. Another reason is diagnostic switching, whereby patients initially diagnosed with 1 disorder receive a different diagnosis at followup. Diagnostic switching is especially problematic for SAD. In a large meta-analysis by Santelmann et al,11 36% of patients initially diagnosed with SAD had their diagnosis changed when reassessed. This diagnostic shift tended more toward schizophrenia than bipolar disorder. In addition, more than one-half of all patients initially diagnosed with schizophrenia, bipolar disorder, or major depressive disorder were re-diagnosed with SAD when reassessed.
DSM-5 subtypes and specifiers
In DSM-5,SAD has 2 subtypes5:
- Bipolar type. The bipolar type is marked by the presence of a manic episode (major depressive episodes may also occur)
- Depressive type. The depressive type is marked by the presence of only major depressive episodes.
SAD also includes several specifiers, with the express purpose of giving clinicians greater descriptive ability. The course of SAD can be described as either “first episode,” defined as the first manifestation of the disorder, or as having “multiple episodes,” defined as a minimum of 2 episodes with 1 relapse. In addition, SAD can be described as an acute episode, in partial remission, or in full remission. The course can be described as “continuous” if it is clear that symptoms have been present for the majority of the illness with very brief subthreshold periods. The course is designated as “unspecified” when information is unavailable or lacking. The 5-point Clinician-Rated Dimensions of Psychosis Symptoms was introduced to enable clinicians to make a quantitative assessment of the psychotic symptoms, although its use is not required.
Epidemiology and gender ratio
The epidemiology of SAD has not been well studied. DSM-5 estimates that SAD is approximately one-third as common as schizophrenia, which has a lifetime prevalence of 0.5% to 0.8%.5 This is similar to an estimate by Perälä et al12 of a 0.32% lifetime prevalence based on a nationally representative sample of persons in Finland age ≥30. Scully et al13 calculated a prevalence estimate of 1.1% in a representative sample of adults in rural Ireland. Based on pooled clinical data, Keck et al14 estimated the prevalence in clinical settings at 16%, similar to the figure of 19% reported by Levinson et al15 based on data from New York State psychiatric hospitals. In clinical practice, the diagnosis of SAD is used frequently when there is diagnostic uncertainty, which potentially inflates estimates of lifetime prevalence.
The prevalence of SAD is higher in women than men, with a sex ratio of about 2:1, similar to that seen in mood disorders.13,16-19 There are an equal number of men and women with the bipolar subtype, but a female preponderance with the depressive subtype.5 The bipolar subtype is more common in younger patients, while the depressive subtype is more common in older patients. SAD is a rare diagnosis in children.20
Continue to: Course and outcome
Course and outcome
The onset of SAD typically occurs in early adulthood, but can range from childhood to senescence. Approximately one-third of patients are diagnosed before age 25, one-third between age 25 and 35, and one-third after age 35.21-23 Based on a literature review, Cheniaux et al7 concluded that that age at onset for patients with SAD is between those with schizophrenia and those with mood disorders.
The course of SAD is variable but represents a middle ground between that of schizophrenia and the mood disorders. In a 4- to 5-year follow-up,24 patients with SAD had a better overall course than patients with schizophrenia but had poorer functioning than those with bipolar mania, and much poorer than those with unipolar depression. Mood-incongruent psychotic features predict a particularly worse outcome. These findings were reaffirmed at a 10-year follow-up.25 Mood symptoms portend a better outcome than do symptoms of schizophrenia.
The lifetime suicide risk for patients with SAD is estimated at 5%, with a higher risk associated with the presence of depressive symptoms.26 One study found that women with SAD had a 17.5-year reduced life expectancy (64.1 years) compared with a reduction of 8.0 years for men (69.4 years).27
Comorbidity
Patients with SAD are commonly diagnosed with other psychiatric disorders, including anxiety disorders, obsessive-compulsive disorder, posttraumatic stress disorder, and substance use disorders.21,28,29 When compared with the general population, patients with SAD are at higher risk for coronary heart disease, stroke, obesity, and smoking, likely contributing to their decreased life expectancy.27,30 Because second-generation antipsychotics (SGAs) are often used to treat SAD, patients with SAD are at risk for metabolic syndrome and diabetes mellitus.30
Clinical assessment
Because there are no diagnostic, laboratory, or neuroimaging tests for SAD, the most important basis for making the diagnosis is the patient’s history, supplemented by collateral history from family members or friends, and medical records. Determining the percentage of time spent in a mood episode (DSM-5 Criterion C) is especially important.31 This requires the clinician to pay close attention to the temporal relationship of psychotic and mood symptoms.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis for SAD is broad because it includes all of the possibilities usually considered for major mood disorders and for psychotic disorders5:
- schizophrenia
- bipolar disorder with psychotic features
- major depressive disorder with psychotic features
- depressive or bipolar disorders with catatonic features
- personality disorders (especially the schizotypal, paranoid, and borderline types)
- major neurocognitive disorders in which there are mood and psychotic symptoms
- substance/medication-induced psychotic disorder
- disorders induced by medical conditions.
With schizophrenia, the duration of all episodes of a mood syndrome is brief (<50% of the total duration of the illness) relative to the duration of the psychotic symptoms. Although psychotic symptoms may occur in persons with mood disorders, they are generally not present in the absence of depression or mania, helping to set the boundary between SAD and psychotic mania or depression. As for personality disorders, the individual will not have a true psychosis, although some symptoms, such as feelings of unreality, paranoia, or magical thinking, may cause diagnostic confusion.
Medical conditions also can present with psychotic and mood symptoms and need to be ruled out. These include psychotic disorder due to another medical condition, and delirium. A thorough medical workup should be performed to rule out any possible medical causes for the symptoms.
Substance use should also be ruled out as the cause of the symptoms because many substances are associated with mood and psychotic symptoms. It is usually clear from the history, physical examination, or laboratory tests when a medication/illicit substance has initiated and maintained the disorder.
Neurologic conditions. If a neurologic condition is suspected, a neurologic evaluation may be warranted, including laboratory tests, brain imaging to identify specific anatomical abnormalities, lumbar puncture with cerebrospinal fluid analysis, and an electroencephalogram to rule out a convulsive disorder.
Continue to: Clinical symptoms
Clinical symptoms
The signs and symptoms of SAD include those typically seen in schizophrenia and the mood disorders. Thus, the patient may exhibit elated mood and/or grandiosity, or severe depression, combined with mood-incongruent psychotic features such as paranoid delusions. The symptoms may present together or in an alternating fashion, and psychotic symptoms may be mood-congruent or mood-incongruent. Mr. C’s case illustrates some of the symptoms of the disorder.
Brain imaging
Significant changes have been reported to occur in the brain structure and function in persons with SAD. Neuroimaging studies using voxel-based morphometry have shown significant reductions in gray matter volume in several areas of the brain, including the medial prefrontal cortex, insula, Rolandic operculum, parts of the temporal lobe, and the hippocampus.32-35 Amann et al32 found that patients with SAD and schizophrenia had widespread and overlapping areas of significant volume reduction, but patients with bipolar disorder did not. These studies suggest that at least from a neuroimaging standpoint, SAD is more closely related to schizophrenia than bipolar disorder, and could represent a variant of schizophrenia.
Treatment of SAD
The pharmacotherapy of SAD is mostly empirical because of the lack of randomized controlled trials. Clinicians have traditionally prescribed an antipsychotic agent along with either a mood stabilizer (eg,
Since that exhaustive review,
Patients with SAD will require maintenance treatment for ongoing symptom control. Medication that is effective for treatment of an acute episode should be considered for maintenance treatment. Both the extended-release and long-acting injectable (LAI) formulations of paliperidone have been shown to be efficacious in the maintenance treatment of patients with SAD.40 The LAI form of paliperidone significantly delayed psychotic, depressive, and manic relapses, improved clinical rating scale scores, and increased medication adherence.41,42 In an open-label study, olanzapine LAI was effective in long-term maintenance treatment, although approximately 40% of patients experienced significant weight gain.43 One concern with olanzapine is the possible occurrence of a post-injection delirium/sedation syndrome. For that reason, patients receiving olanzapine must be monitored for at least 3 hours post-injection. The paliperidone LAI does not require monitoring after injection.
Continue to: There is a single clinical trial...
There is a single clinical trial showing that patients with SAD can be successfully switched from other antipsychotics to
Other approaches
Electroconvulsive therapy (ECT) should be considered for patients with SAD who are acutely ill and have failed to respond adequately to medication. ECT is especially relevant in the setting of acute mood symptoms (ie, depressive or manic symptoms co-occurring with psychosis or in the absence of psychosis).45
As currently conceptualized, the diagnosis of SAD is made in persons having an admixture of mood and psychotic symptoms, although by definition mood symptoms must take up the majority (≥50%) of the total duration of the illness. Unfortunately, SAD has been inadequately researched due to the unreliability of its definition and concerns about its validity. The long-term course of SAD is midway between mood and psychotic disorders, and the disorder can cause significant disability.
Bottom Line
Schizoaffective disorder (SAD) is characterized by the presence of symptoms of a major mood episode (a depressive or manic episode) concurrent with symptoms of schizophrenia. The most important basis for establishing the diagnosis is the patient’s history. Determining the percentage of time spent in a mood episode is especially important. Treatment usually consists of an antipsychotic plus a mood stabilizer or antidepressant. Electroconvulsive therapy is an option for patients with SAD who do not respond well to medication.
Related Resources
- Wy TJP, Saadabadi A. Schizoaffective disorder. NCBI Bookshelf: StatPearls Publishing. Published January 2020. https://www.ncbi.nlm.nih.gov/books/NBK541012/. Updated April 15, 2020.
- Parker G. How well does the DSM-5 capture schizoaffective disorder? Can J Psychiatry. 2019;64(9):607-610.
Drug Brand Names
Aripiprazole • Abilify
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Olanzapine long-acting injectable • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate • Invega sustenna
Valproate • Depacon
1. Miller JN, Black DW. Schizoaffective disorder: a review. Ann Clin Psychiatry. 2019;31(1):47-53.
2. Kasanin J. The acute schizoaffective psychoses. Am J Psychiatry. 1933;90:97-126.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington, DC: American Psychiatric Association; 1952.
4. Diagnostic and statistical manual of mental disorders, 3rd ed, revision. Washington, DC: American Psychiatric Association; 1987.
5. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
6. Malaspina D, Owen M, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150:21-25.
7. Cheniaux E, Landeria-Fernandez J, Telles LL, et al. Does schizoaffective disorder really exist? A systematic review of the studies that compared schizoaffective disorder with schizophrenia or mood disorders. J Affect Disord. 2008;106:209-217.
8. Kantrowitz JT, Citrome L. Schizoaffective disorder: a review of current research themes and pharmacologic management. CNS Drugs. 2011;25:317-331.
9. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170:59-70.
10. Wilson JE, Nian H, Heckers S. The schizoaffective disorder diagnosis: a conundrum in the clinical setting. Eur Arch Psychiatry Clin Neurosci. 2014;264:29-34.
11. Santelmann H, Franklin J, Bußhoff J, Baethge C. Test-retest reliability of schizoaffective disorder compared with schizophrenia, bipolar disorder, and unipolar depression--a systematic review and meta-analysis. Bipolar Disord. 2015;17:753-768.
12. Perälä J, Suvisaari J, Saarni SI, et al. Lifetime Prevalence of psychotic and bipolar I disorders in a general population. JAMA Psychiatry. 2007;64:19-28.
13. Scully PJ, Owens JM, Kinsella A, et al. Schizophrenia, schizoaffective and bipolar disorder within an epidemiologically complete, homogeneous population in rural Ireland: small area variation in rate. Schizophr Res. 2004;67:143-155.
14. Keck PE Jr, McElroy SE, Strakowski SM, et al. Pharmacologic treatment of schizoaffective disorder. Psychopharmacol. 1994;114:529-538.
15. Levinson DF, Umapathy C, Musthaq M. Treatment of schizoaffective disorder and schizophrenia with mood symptoms. Am J Psychiatry. 1999;156:1138-1148.
16. Angst J, Felder W, Lohmeyer B. Course of schizoaffective psychoses: results of a follow-up study. Schizophr Bull. 1980;6:579-585.
17. Lenz G, Simhandl C, Thau K, et al. Temporal stability of diagnostic criteria for functional psychoses. Psychopathol. 1991;24:328-335.
18. Malhi GS, Green M, Fagiolini A, et al. Schizoaffective disorder: diagnostic issues and future recommendations. Bipolar Disord. 2008;10:215-230.
19. Marneros A, Deister A, Rohde A. Psychopathological and social status of patients with affective, schizophrenic and schizoaffective disorders after long‐term course. Acta Psychiatr Scand. 1990;82:352-358.
20. Werry JS, McClellan JM, Chard L. Childhood and adolescent schizophrenic, bipolar, and schizoaffective disorders: a clinical and outcome study. J Am Acad Child Adolesc Psychiatry. 1991;30:457-465.
21. Abrams DJ, Rojas DC, Arciniegas DB. Is schizoaffective disorder a distinct categorical diagnosis? A critical review of the literature. Neuropsychiatr Dis Treat. 2008;4:1089-1109.
22. Bromet EJ, Kotov R, Fochtmann LJ, et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168:1186-1194.
23. Salvatore P, Baldessarini RJ, Tohen M, et al. The McLean-Harvard First Episode Project: two-year stability of DSM-IV diagnoses in 500 first-episode psychotic disorder patients. J Clin Psychiatry. 2009;70:458-466.
24. Grossman LS, Harrow M, Goldberg JF, et al. Outcome of schizoaffective disorder at two long term follow-ups: comparisons with outcome of schizophrenia and affective disorders. Am J Psychiatry. 1991;148:1359-1365.
25. Harrow M, Grossman L, Herbener E, et al. Ten-year outcome: patients with schizoaffective disorders, schizophrenia, affective disorders and mood-incongruent psychotic symptoms. Br J Psychiatry. 2000;177:421-426.
26. Hor K, Taylor M. Review: suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24:81-90.
27. Chang CK, Hayes RD, Perera G, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS ONE. 2011;6:e19590.
28. Byerly M, Goodman W, Acholonu W, et al. Obsessive compulsive symptoms in schizophrenia: frequency and clinical features. Schizophr Res. 2005;76:309-316.
29. Strauss JL, Calhoun PS, Marx CE, et al. Comorbid posttraumatic stress disorder is associated with suicidality in male veterans with schizophrenia or schizoaffective disorder. Schizophr Res. 2006;84:165-169.
30. Fagiolini A, Goracci A. The effects of undertreated chronic medical illnesses in patients with severe mental disorders. J Clin Psychiatry. 2009;70:22-29.
31. Black DW, Grant JE. DSM-5 guidebook: the essential companion to the diagnostic and statistical manual of mental disorders, 5th edition. Washington, DC: American Psychiatric Publishing; 2014.
32. Amann BL, Canales-Rodríguez EJ, Madre M, et al. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2016;133:23-33.
33. Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1285-1296.
34. Ivleva EI, Bidesi AS, Thomas BP, et al. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res. 2012;204:13-24.
35. Radonic
36. Jäger M, Becker T, Weinmann S, et al. Treatment of schizoaffective disorder - a challenge for evidence-based psychiatry. Acta Psychiatr Scand. 2010;121:22-32.
37. Glick ID, Mankosli R, Eudicone JM, et al. The efficacy, safety, and tolerability of aripiprazole for the treatment of schizoaffective disorder: results from a pooled analysis of a sub-population of subjects from two randomized, double-blind, placebo controlled, pivotal trials. J Affect Disord. 2009;115:18-26.
38. Canuso CM, Lindenmayer JP, Kosik-Gonzalez C, et al. A randomized, double-blind, placebo controlled study of 2 dose ranges of paliperidone extended-release in the treatment of subjects with schizoaffective disorder. J Clin Psychiatry. 2010;71:587-598.
39. Canuso CM, Schooler NR, Carothers J, et al. Paliperidone extended-release in schizoaffective disorder: a randomized controlled trial comparing a flexible-dose with placebo in patients treated with and without antidepressants and/or mood stabilizers. J Clin Psychopharmacol. 2010;30:487-495.
40. Lindenmayer JP, Kaur A. Antipsychotic management of schizoaffective disorder: a review. Drugs. 2016;76:589-604.
41. Alphs L, Fu DJ, Turkoz I. Paliperidone for the treatment of schizoaffective disorder. Expert Opin Pharmacother. 2016;176:871-883.
42. Bossie CA, Turkoz I, Alphs L, et al. Paliperidone palmitate once-monthly treatment in recent onset and chronic illness patients with schizoaffective disorder. J Nerv Ment Dis. 2017;205:324-328.
43. McDonnell DP, Landry J, Detke HC. Long-term safety and efficacy of olanzapine long-acting injection in patients with schizophrenia or schizoaffective disorder: a 6-year, multinational, single-arm, open-label study. Int Clin Psychopharmacol. 2014;29:322-331.
44. McEvoy JP, Citrome L, Hernandez D, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74:170-179.
45. Mankad MV, Beyer JL, Wiener RD, et al. Manual of electroconvulsive therapy. Washington, DC: American Psychiatric Publishing; 2010.
1. Miller JN, Black DW. Schizoaffective disorder: a review. Ann Clin Psychiatry. 2019;31(1):47-53.
2. Kasanin J. The acute schizoaffective psychoses. Am J Psychiatry. 1933;90:97-126.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington, DC: American Psychiatric Association; 1952.
4. Diagnostic and statistical manual of mental disorders, 3rd ed, revision. Washington, DC: American Psychiatric Association; 1987.
5. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
6. Malaspina D, Owen M, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150:21-25.
7. Cheniaux E, Landeria-Fernandez J, Telles LL, et al. Does schizoaffective disorder really exist? A systematic review of the studies that compared schizoaffective disorder with schizophrenia or mood disorders. J Affect Disord. 2008;106:209-217.
8. Kantrowitz JT, Citrome L. Schizoaffective disorder: a review of current research themes and pharmacologic management. CNS Drugs. 2011;25:317-331.
9. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170:59-70.
10. Wilson JE, Nian H, Heckers S. The schizoaffective disorder diagnosis: a conundrum in the clinical setting. Eur Arch Psychiatry Clin Neurosci. 2014;264:29-34.
11. Santelmann H, Franklin J, Bußhoff J, Baethge C. Test-retest reliability of schizoaffective disorder compared with schizophrenia, bipolar disorder, and unipolar depression--a systematic review and meta-analysis. Bipolar Disord. 2015;17:753-768.
12. Perälä J, Suvisaari J, Saarni SI, et al. Lifetime Prevalence of psychotic and bipolar I disorders in a general population. JAMA Psychiatry. 2007;64:19-28.
13. Scully PJ, Owens JM, Kinsella A, et al. Schizophrenia, schizoaffective and bipolar disorder within an epidemiologically complete, homogeneous population in rural Ireland: small area variation in rate. Schizophr Res. 2004;67:143-155.
14. Keck PE Jr, McElroy SE, Strakowski SM, et al. Pharmacologic treatment of schizoaffective disorder. Psychopharmacol. 1994;114:529-538.
15. Levinson DF, Umapathy C, Musthaq M. Treatment of schizoaffective disorder and schizophrenia with mood symptoms. Am J Psychiatry. 1999;156:1138-1148.
16. Angst J, Felder W, Lohmeyer B. Course of schizoaffective psychoses: results of a follow-up study. Schizophr Bull. 1980;6:579-585.
17. Lenz G, Simhandl C, Thau K, et al. Temporal stability of diagnostic criteria for functional psychoses. Psychopathol. 1991;24:328-335.
18. Malhi GS, Green M, Fagiolini A, et al. Schizoaffective disorder: diagnostic issues and future recommendations. Bipolar Disord. 2008;10:215-230.
19. Marneros A, Deister A, Rohde A. Psychopathological and social status of patients with affective, schizophrenic and schizoaffective disorders after long‐term course. Acta Psychiatr Scand. 1990;82:352-358.
20. Werry JS, McClellan JM, Chard L. Childhood and adolescent schizophrenic, bipolar, and schizoaffective disorders: a clinical and outcome study. J Am Acad Child Adolesc Psychiatry. 1991;30:457-465.
21. Abrams DJ, Rojas DC, Arciniegas DB. Is schizoaffective disorder a distinct categorical diagnosis? A critical review of the literature. Neuropsychiatr Dis Treat. 2008;4:1089-1109.
22. Bromet EJ, Kotov R, Fochtmann LJ, et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168:1186-1194.
23. Salvatore P, Baldessarini RJ, Tohen M, et al. The McLean-Harvard First Episode Project: two-year stability of DSM-IV diagnoses in 500 first-episode psychotic disorder patients. J Clin Psychiatry. 2009;70:458-466.
24. Grossman LS, Harrow M, Goldberg JF, et al. Outcome of schizoaffective disorder at two long term follow-ups: comparisons with outcome of schizophrenia and affective disorders. Am J Psychiatry. 1991;148:1359-1365.
25. Harrow M, Grossman L, Herbener E, et al. Ten-year outcome: patients with schizoaffective disorders, schizophrenia, affective disorders and mood-incongruent psychotic symptoms. Br J Psychiatry. 2000;177:421-426.
26. Hor K, Taylor M. Review: suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24:81-90.
27. Chang CK, Hayes RD, Perera G, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS ONE. 2011;6:e19590.
28. Byerly M, Goodman W, Acholonu W, et al. Obsessive compulsive symptoms in schizophrenia: frequency and clinical features. Schizophr Res. 2005;76:309-316.
29. Strauss JL, Calhoun PS, Marx CE, et al. Comorbid posttraumatic stress disorder is associated with suicidality in male veterans with schizophrenia or schizoaffective disorder. Schizophr Res. 2006;84:165-169.
30. Fagiolini A, Goracci A. The effects of undertreated chronic medical illnesses in patients with severe mental disorders. J Clin Psychiatry. 2009;70:22-29.
31. Black DW, Grant JE. DSM-5 guidebook: the essential companion to the diagnostic and statistical manual of mental disorders, 5th edition. Washington, DC: American Psychiatric Publishing; 2014.
32. Amann BL, Canales-Rodríguez EJ, Madre M, et al. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2016;133:23-33.
33. Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1285-1296.
34. Ivleva EI, Bidesi AS, Thomas BP, et al. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res. 2012;204:13-24.
35. Radonic
36. Jäger M, Becker T, Weinmann S, et al. Treatment of schizoaffective disorder - a challenge for evidence-based psychiatry. Acta Psychiatr Scand. 2010;121:22-32.
37. Glick ID, Mankosli R, Eudicone JM, et al. The efficacy, safety, and tolerability of aripiprazole for the treatment of schizoaffective disorder: results from a pooled analysis of a sub-population of subjects from two randomized, double-blind, placebo controlled, pivotal trials. J Affect Disord. 2009;115:18-26.
38. Canuso CM, Lindenmayer JP, Kosik-Gonzalez C, et al. A randomized, double-blind, placebo controlled study of 2 dose ranges of paliperidone extended-release in the treatment of subjects with schizoaffective disorder. J Clin Psychiatry. 2010;71:587-598.
39. Canuso CM, Schooler NR, Carothers J, et al. Paliperidone extended-release in schizoaffective disorder: a randomized controlled trial comparing a flexible-dose with placebo in patients treated with and without antidepressants and/or mood stabilizers. J Clin Psychopharmacol. 2010;30:487-495.
40. Lindenmayer JP, Kaur A. Antipsychotic management of schizoaffective disorder: a review. Drugs. 2016;76:589-604.
41. Alphs L, Fu DJ, Turkoz I. Paliperidone for the treatment of schizoaffective disorder. Expert Opin Pharmacother. 2016;176:871-883.
42. Bossie CA, Turkoz I, Alphs L, et al. Paliperidone palmitate once-monthly treatment in recent onset and chronic illness patients with schizoaffective disorder. J Nerv Ment Dis. 2017;205:324-328.
43. McDonnell DP, Landry J, Detke HC. Long-term safety and efficacy of olanzapine long-acting injection in patients with schizophrenia or schizoaffective disorder: a 6-year, multinational, single-arm, open-label study. Int Clin Psychopharmacol. 2014;29:322-331.
44. McEvoy JP, Citrome L, Hernandez D, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74:170-179.
45. Mankad MV, Beyer JL, Wiener RD, et al. Manual of electroconvulsive therapy. Washington, DC: American Psychiatric Publishing; 2010.
What to tell parents whose child saw them having sex
Many parents find themselves in a difficult situation when their child accidentally sees them having sex. These patients may ask us, as their clinicians, if they should discuss the incident with their child, and if so, what to say. If parents do not address the subject appropriately, their child might be confused and uncomfortable with his/her interpretation of the encounter.1 Some older research suggests that after witnessing their parents in a sexual encounter, children may have difficulty with affectional love, fears of being alone, or feelings of vulnerability.2,3 Clinicians may find themselves at a loss when parents ask them how to handle these situations. Although there are no evidence-based guidelines to consult, consider the following suggestions:
Relax. For patients who have not yet experienced this situation, tell them it is important not to panic if their child witnesses them having sex. They should cover their bodies and calmly respond to their child’s presence. Calm responsiveness is a key to diffusing this awkward situation. Otherwise, children may sense their parents’ embarrassment and conclude that sex is shameful. Parents should gently explain to their child that they are having a private, adult moment. They should ask their child if something is needed immediately, or if it could wait.
Accept that it happened. Parents should not avoid discussing the incident, but should promptly follow up with their child at an appropriate time and place. Waiting for a child to raise the topic puts the responsibility on him/her, instead of on the parent. Although some forthright children may ask questions, others may feel too ashamed or nervous to broach the topic and will prefer their parents to take the lead.
Discuss what happened. Tell parents to explore their child’s impression of what he/she saw. Tailoring the discussion to the child’s age is important. For example, a 3-year-old might wonder if anyone was harmed, and might need reassurance, whereas a 12-year-old is likely to have a better understanding of sex but still feel uncomfortable. Educational conversations about sexuality might be appropriate for children age 8 to 12. The parents’ goal should be to answer questions honestly without oversharing, and to leave the door open—so to speak—for future conversations.
Recommend that parents use plain, factual language to answer any questions their child asks. Statements such as “We were having a private, adult moment” can be helpful. Parents can categorize sex as a universal activity that is not harmful or scary by telling their child something such as, “This is what all parents do.” Parents should avoid providing unnecessary information or answering questions their child is not asking. The Table4 offers guidance on how parents might handle such conversations.

Consider potentially positive outcomes. Although parents may feel guilty or describe this as a terrible situation, remind them that there are some potentially healthy outcomes. For example, such incidents may help reassure the child that their parents love each other, which might give him/her a sense of happiness and security.
Take steps to prevent this from happening again. Advise parents to lock the door when having sex. Remind them to consider the proximity of rooms because their child might hear noises and become curious.
1. Blum HP. On the concept and consequences of the primal scene. Psychoanal Q. 1979;48(1):27-47.
2. Hoyt MF. On the psychology and psychopathology of primal-scene experience. J Am Acad Psychoanal. 1980;8(3):311-335.
3. Ikonen P, Rechardt E. On the universal nature of primal scene fantasies. Int J Psychoanal. 1984;65(pt 1):63-72.
4. Pelly J. Four things my four-year-old already knows about sex. Today’s Parent. https://www.todaysparent.com/family/parenting/4-things-my-4-year-old-already-knows-about-sex/. Published February 1, 2020. Accessed June 20, 2020.
Many parents find themselves in a difficult situation when their child accidentally sees them having sex. These patients may ask us, as their clinicians, if they should discuss the incident with their child, and if so, what to say. If parents do not address the subject appropriately, their child might be confused and uncomfortable with his/her interpretation of the encounter.1 Some older research suggests that after witnessing their parents in a sexual encounter, children may have difficulty with affectional love, fears of being alone, or feelings of vulnerability.2,3 Clinicians may find themselves at a loss when parents ask them how to handle these situations. Although there are no evidence-based guidelines to consult, consider the following suggestions:
Relax. For patients who have not yet experienced this situation, tell them it is important not to panic if their child witnesses them having sex. They should cover their bodies and calmly respond to their child’s presence. Calm responsiveness is a key to diffusing this awkward situation. Otherwise, children may sense their parents’ embarrassment and conclude that sex is shameful. Parents should gently explain to their child that they are having a private, adult moment. They should ask their child if something is needed immediately, or if it could wait.
Accept that it happened. Parents should not avoid discussing the incident, but should promptly follow up with their child at an appropriate time and place. Waiting for a child to raise the topic puts the responsibility on him/her, instead of on the parent. Although some forthright children may ask questions, others may feel too ashamed or nervous to broach the topic and will prefer their parents to take the lead.
Discuss what happened. Tell parents to explore their child’s impression of what he/she saw. Tailoring the discussion to the child’s age is important. For example, a 3-year-old might wonder if anyone was harmed, and might need reassurance, whereas a 12-year-old is likely to have a better understanding of sex but still feel uncomfortable. Educational conversations about sexuality might be appropriate for children age 8 to 12. The parents’ goal should be to answer questions honestly without oversharing, and to leave the door open—so to speak—for future conversations.
Recommend that parents use plain, factual language to answer any questions their child asks. Statements such as “We were having a private, adult moment” can be helpful. Parents can categorize sex as a universal activity that is not harmful or scary by telling their child something such as, “This is what all parents do.” Parents should avoid providing unnecessary information or answering questions their child is not asking. The Table4 offers guidance on how parents might handle such conversations.

Consider potentially positive outcomes. Although parents may feel guilty or describe this as a terrible situation, remind them that there are some potentially healthy outcomes. For example, such incidents may help reassure the child that their parents love each other, which might give him/her a sense of happiness and security.
Take steps to prevent this from happening again. Advise parents to lock the door when having sex. Remind them to consider the proximity of rooms because their child might hear noises and become curious.
Many parents find themselves in a difficult situation when their child accidentally sees them having sex. These patients may ask us, as their clinicians, if they should discuss the incident with their child, and if so, what to say. If parents do not address the subject appropriately, their child might be confused and uncomfortable with his/her interpretation of the encounter.1 Some older research suggests that after witnessing their parents in a sexual encounter, children may have difficulty with affectional love, fears of being alone, or feelings of vulnerability.2,3 Clinicians may find themselves at a loss when parents ask them how to handle these situations. Although there are no evidence-based guidelines to consult, consider the following suggestions:
Relax. For patients who have not yet experienced this situation, tell them it is important not to panic if their child witnesses them having sex. They should cover their bodies and calmly respond to their child’s presence. Calm responsiveness is a key to diffusing this awkward situation. Otherwise, children may sense their parents’ embarrassment and conclude that sex is shameful. Parents should gently explain to their child that they are having a private, adult moment. They should ask their child if something is needed immediately, or if it could wait.
Accept that it happened. Parents should not avoid discussing the incident, but should promptly follow up with their child at an appropriate time and place. Waiting for a child to raise the topic puts the responsibility on him/her, instead of on the parent. Although some forthright children may ask questions, others may feel too ashamed or nervous to broach the topic and will prefer their parents to take the lead.
Discuss what happened. Tell parents to explore their child’s impression of what he/she saw. Tailoring the discussion to the child’s age is important. For example, a 3-year-old might wonder if anyone was harmed, and might need reassurance, whereas a 12-year-old is likely to have a better understanding of sex but still feel uncomfortable. Educational conversations about sexuality might be appropriate for children age 8 to 12. The parents’ goal should be to answer questions honestly without oversharing, and to leave the door open—so to speak—for future conversations.
Recommend that parents use plain, factual language to answer any questions their child asks. Statements such as “We were having a private, adult moment” can be helpful. Parents can categorize sex as a universal activity that is not harmful or scary by telling their child something such as, “This is what all parents do.” Parents should avoid providing unnecessary information or answering questions their child is not asking. The Table4 offers guidance on how parents might handle such conversations.

Consider potentially positive outcomes. Although parents may feel guilty or describe this as a terrible situation, remind them that there are some potentially healthy outcomes. For example, such incidents may help reassure the child that their parents love each other, which might give him/her a sense of happiness and security.
Take steps to prevent this from happening again. Advise parents to lock the door when having sex. Remind them to consider the proximity of rooms because their child might hear noises and become curious.
1. Blum HP. On the concept and consequences of the primal scene. Psychoanal Q. 1979;48(1):27-47.
2. Hoyt MF. On the psychology and psychopathology of primal-scene experience. J Am Acad Psychoanal. 1980;8(3):311-335.
3. Ikonen P, Rechardt E. On the universal nature of primal scene fantasies. Int J Psychoanal. 1984;65(pt 1):63-72.
4. Pelly J. Four things my four-year-old already knows about sex. Today’s Parent. https://www.todaysparent.com/family/parenting/4-things-my-4-year-old-already-knows-about-sex/. Published February 1, 2020. Accessed June 20, 2020.
1. Blum HP. On the concept and consequences of the primal scene. Psychoanal Q. 1979;48(1):27-47.
2. Hoyt MF. On the psychology and psychopathology of primal-scene experience. J Am Acad Psychoanal. 1980;8(3):311-335.
3. Ikonen P, Rechardt E. On the universal nature of primal scene fantasies. Int J Psychoanal. 1984;65(pt 1):63-72.
4. Pelly J. Four things my four-year-old already knows about sex. Today’s Parent. https://www.todaysparent.com/family/parenting/4-things-my-4-year-old-already-knows-about-sex/. Published February 1, 2020. Accessed June 20, 2020.
Kleptomania: 4 Tips for better diagnosis and treatment
Kleptomania is characterized by a recurrent failure to resist impulses to steal objects that are not needed for personal use or their monetary value.1 It is a rare disorder; an estimated 0.3% to 0.6% of the general population meet DSM-5 criteria for kleptomania (Table 1).1 Kleptomania usually begins in early adolescence and is more common among females than males (3:1).1 Although DSM-5 does not outline how long symptoms need to be present for patients to meet the diagnostic criteria, the disorder may persist for years, even when patients face legal consequences.1

Due to the clinical ambiguities surrounding kleptomania, it remains one of psychiatry’s most poorly understood diagnoses2 and regularly goes undiagnosed and untreated. Here we provide 4 tips for better diagnosis and treatment of this condition.
1. Screen for kleptomania in patients with other psychiatric disorders because kleptomania often is comorbid with other mental illnesses. Patients who present for evaluation of a mood disorder, substance use, anxiety disorders, eating disorders, impulse control disorders, conduct disorder, and obsessive-compulsive disorder should be screened for kleptomania.1,3,4 Patients with kleptomania often are reluctant to discuss their stealing because they may experience humiliation and guilt related to theft.1,4 Undiagnosed kleptomania can be fatal; a study of suicide attempts in 107 individuals with kleptomania found that 92% of the patients attributed their attempt specifically to kleptomania.5 Table 21 offers screening questions based on the DSM-5 criteria for kleptomania.

2. Distinguish kleptomania from other diagnoses that can include stealing. Because stealing can be a symptom of several other psychiatric disorders, misdiagnosis is fairly common.1 The differential can include bipolar disorder, borderline personality disorder, antisocial personality disorder, and eating disorder. Table 31,3 describes how to differentiate these diagnoses from kleptomania.

3. Select an appropriate treatment. There are no FDA-approved medications for kleptomania, but some agents may help. In an 8-week, double-blind, placebo-controlled trial, 25 patients with kleptomania who received naltrexone (50 to 150 mg/d) demonstrated significant reductions in stealing urges and behavior.6 Some evidence suggests a combination of pharmacologic and behavioral therapy (cognitive-behavioral therapy, covert sensitization, and systemic desensitization) may be the optimal treatment strategy for kleptomania.4
4. Monitor progress. After initiating treatment, use the Kleptomania Symptom Assessment Scale7 (K-SAS) to determine treatment efficacy. The K-SAS is an 11-item self-report questionnaire that assesses the severity of kleptomania symptoms during the past week.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry. 1991;148:986-996.
3. Yao S, Kuja‐Halkola R, Thornton LM, et al. Risk of being convicted of theft and other crimes in anorexia nervosa and bulimia nervosa: a prospective cohort study in a Swedish female population. Int J Eat Disord. 2017;50(9):1095-1103.
4. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients of kleptomania. Compr Psychiatry. 2002;43(5):378-384.
5. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
6. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7):600-606.
7. Grant JE, Odlaug BL, Kim SW. Kleptomania: clinical characteristics and relationship to substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):291-295.
Kleptomania is characterized by a recurrent failure to resist impulses to steal objects that are not needed for personal use or their monetary value.1 It is a rare disorder; an estimated 0.3% to 0.6% of the general population meet DSM-5 criteria for kleptomania (Table 1).1 Kleptomania usually begins in early adolescence and is more common among females than males (3:1).1 Although DSM-5 does not outline how long symptoms need to be present for patients to meet the diagnostic criteria, the disorder may persist for years, even when patients face legal consequences.1

Due to the clinical ambiguities surrounding kleptomania, it remains one of psychiatry’s most poorly understood diagnoses2 and regularly goes undiagnosed and untreated. Here we provide 4 tips for better diagnosis and treatment of this condition.
1. Screen for kleptomania in patients with other psychiatric disorders because kleptomania often is comorbid with other mental illnesses. Patients who present for evaluation of a mood disorder, substance use, anxiety disorders, eating disorders, impulse control disorders, conduct disorder, and obsessive-compulsive disorder should be screened for kleptomania.1,3,4 Patients with kleptomania often are reluctant to discuss their stealing because they may experience humiliation and guilt related to theft.1,4 Undiagnosed kleptomania can be fatal; a study of suicide attempts in 107 individuals with kleptomania found that 92% of the patients attributed their attempt specifically to kleptomania.5 Table 21 offers screening questions based on the DSM-5 criteria for kleptomania.

2. Distinguish kleptomania from other diagnoses that can include stealing. Because stealing can be a symptom of several other psychiatric disorders, misdiagnosis is fairly common.1 The differential can include bipolar disorder, borderline personality disorder, antisocial personality disorder, and eating disorder. Table 31,3 describes how to differentiate these diagnoses from kleptomania.

3. Select an appropriate treatment. There are no FDA-approved medications for kleptomania, but some agents may help. In an 8-week, double-blind, placebo-controlled trial, 25 patients with kleptomania who received naltrexone (50 to 150 mg/d) demonstrated significant reductions in stealing urges and behavior.6 Some evidence suggests a combination of pharmacologic and behavioral therapy (cognitive-behavioral therapy, covert sensitization, and systemic desensitization) may be the optimal treatment strategy for kleptomania.4
4. Monitor progress. After initiating treatment, use the Kleptomania Symptom Assessment Scale7 (K-SAS) to determine treatment efficacy. The K-SAS is an 11-item self-report questionnaire that assesses the severity of kleptomania symptoms during the past week.
Kleptomania is characterized by a recurrent failure to resist impulses to steal objects that are not needed for personal use or their monetary value.1 It is a rare disorder; an estimated 0.3% to 0.6% of the general population meet DSM-5 criteria for kleptomania (Table 1).1 Kleptomania usually begins in early adolescence and is more common among females than males (3:1).1 Although DSM-5 does not outline how long symptoms need to be present for patients to meet the diagnostic criteria, the disorder may persist for years, even when patients face legal consequences.1

Due to the clinical ambiguities surrounding kleptomania, it remains one of psychiatry’s most poorly understood diagnoses2 and regularly goes undiagnosed and untreated. Here we provide 4 tips for better diagnosis and treatment of this condition.
1. Screen for kleptomania in patients with other psychiatric disorders because kleptomania often is comorbid with other mental illnesses. Patients who present for evaluation of a mood disorder, substance use, anxiety disorders, eating disorders, impulse control disorders, conduct disorder, and obsessive-compulsive disorder should be screened for kleptomania.1,3,4 Patients with kleptomania often are reluctant to discuss their stealing because they may experience humiliation and guilt related to theft.1,4 Undiagnosed kleptomania can be fatal; a study of suicide attempts in 107 individuals with kleptomania found that 92% of the patients attributed their attempt specifically to kleptomania.5 Table 21 offers screening questions based on the DSM-5 criteria for kleptomania.

2. Distinguish kleptomania from other diagnoses that can include stealing. Because stealing can be a symptom of several other psychiatric disorders, misdiagnosis is fairly common.1 The differential can include bipolar disorder, borderline personality disorder, antisocial personality disorder, and eating disorder. Table 31,3 describes how to differentiate these diagnoses from kleptomania.

3. Select an appropriate treatment. There are no FDA-approved medications for kleptomania, but some agents may help. In an 8-week, double-blind, placebo-controlled trial, 25 patients with kleptomania who received naltrexone (50 to 150 mg/d) demonstrated significant reductions in stealing urges and behavior.6 Some evidence suggests a combination of pharmacologic and behavioral therapy (cognitive-behavioral therapy, covert sensitization, and systemic desensitization) may be the optimal treatment strategy for kleptomania.4
4. Monitor progress. After initiating treatment, use the Kleptomania Symptom Assessment Scale7 (K-SAS) to determine treatment efficacy. The K-SAS is an 11-item self-report questionnaire that assesses the severity of kleptomania symptoms during the past week.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry. 1991;148:986-996.
3. Yao S, Kuja‐Halkola R, Thornton LM, et al. Risk of being convicted of theft and other crimes in anorexia nervosa and bulimia nervosa: a prospective cohort study in a Swedish female population. Int J Eat Disord. 2017;50(9):1095-1103.
4. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients of kleptomania. Compr Psychiatry. 2002;43(5):378-384.
5. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
6. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7):600-606.
7. Grant JE, Odlaug BL, Kim SW. Kleptomania: clinical characteristics and relationship to substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):291-295.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry. 1991;148:986-996.
3. Yao S, Kuja‐Halkola R, Thornton LM, et al. Risk of being convicted of theft and other crimes in anorexia nervosa and bulimia nervosa: a prospective cohort study in a Swedish female population. Int J Eat Disord. 2017;50(9):1095-1103.
4. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients of kleptomania. Compr Psychiatry. 2002;43(5):378-384.
5. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
6. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7):600-606.
7. Grant JE, Odlaug BL, Kim SW. Kleptomania: clinical characteristics and relationship to substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):291-295.
Improving your experience with electronic health records
The electronic health record (EHR) was introduced to improve how clinicians document patient information, contribute to medical research, and allow for medical records to be universally transferable.1 However, many clinicians find EHRs to be burdensome, time-consuming, and inefficient. Clinicians often spend multiple hours each day navigating their EHR system, which reduces the amount of time they spend interacting with patients and contributes to physician burnout.1-3 For example, in a study of 142 family medicine physicians, clinicians reported that they spent approximately 6 hours per work day interacting with their EHR.3
Clearly, the EHR needs a fundamental revision. In the meantime, how can we adapt to improve the situation? Here I suggest practical steps clinicians can take to improve their experience with their EHR system.4-8
Steps to take during patient visits
Because entering information into the EHR can be distracting, be prepared to multitask during each clinical encounter.1-7 Be ready to address pertinent inquiries and issues your patient raises, and provide instructions on therapies and interventions. Because interpersonal relations are important during clinical encounters, establish interaction with your patient by acknowledging them and maintaining frequent eye contact.7 Consider allowing your patient to view the EHR screen because doing so might increase his/her involvement in the visit.
So that you can pay closer attention to your patient, consider taking notes during the visit and entering the information into the EHR later. Consider improving your typing skills to increase the speed of your note-taking. Alternatively, using a voice-recognition recording tool to transcribe your notes via speech might help you spend less time on note-taking.3 Whenever possible, finish charting for one patient before meeting with the next because doing so will save time and help you to better remember details.7
In addition, lowering your overall stress might help reduce the burden of using the EHR.3-5 Adopt healthy behaviors, including good sleep, nutrition, exercise, and hobbies, and strive for balance in your routines. Attend to any personal medical or psychiatric conditions, and avoid misusing alcohol, medications, or other substances.
Optimize how your practice functions
With your clinical group and colleagues, create a comfortable environment, good patient-to-doctor interactions, and a smooth flow within the practice. Simplify registration. Ask patients to complete screening forms before an appointment; this information could be entered directly into their EHR.3 Consider using physician-extender staff and other personnel, such as scribes, to complete documentation into the EHR.3,8 This may help reduce burnout, create more time for clinical care, and improve face-to-face patient interactions.8 Employing scribes can allow doctors to be better able to directly attend to their patients while complying with record-keeping needs. Although scribes make charting easier, they are an additional expense, and must be trained.
Consider EHR training
EHR training sessions can teach you how to use your EHR system more efficiently.6 Such education may help boost confidence, aid documentation, and reduce the amount of time spent correcting coding errors. In a study of 3,500 physicians who underwent a 3-day intensive EHR training course, 85% to 98% reported having improved the quality, readability, and clinical accuracy of their documentation.6
Help shape future EHRs
Individual doctors and professional groups can promote EHR improvements through their state, regional, and/or national organizations and medical societies. These bodies should deliver EHR revision recommendations to government officials, who can craft laws and regulations, and can influence regulators and/or insurance companies. Clinicians also can communicate with EHR developers on ways to simplify the usability of these tools, such as reducing the amount of steps the EHR’s interface requires.5 With a more efficient EHR, we can better concentrate on patient care, which will reduce expenses and should yield better outcomes.
1. Ehrenfeld JM, Wonderer JP. Technology as friend or foe? Do electronic health records increase burnout? Curr Opin Anesthesiol. 2018;31(3):357-360.
2. Meigs SL, Solomon M. Electronic health record use a bitter pill for many physicians. Perspect Health Inf Manag. 2016;13:1d.
3. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15(5):419-426.
4. Fogarty CT, Winters P, Farah S. Improving patient-centered communication while using an electronic health record: report from a curriculum evaluation. Int J Psych Med. 2016;51(4):379-389.
5. Guo U, Chen L, Mehta PH. Electronic health record innovations: helping physicians - one less click at a time. Health Inf Manag. 2017;46(3):140-144.
6. Robinson KE, Kersey JA. Novel electronic health record (EHR) education intervention in large healthcare organization improves quality, efficiency, time, and impact on burnout. Medicine. 2018;91(38):e123419. doi: 10.1097/MD.0000000000012319.
7. Fogarty CT. Getting your notes done on time. Fam Pract Manag. 2016;23(2):40.
8. DeChant PF, Acs A, Rhee KB, et al. Effect of organization-directed workplace interventions on physician burnout: a systematic review. Mayo Clin Proc Innov Qual Outcomes. 2019;3(4):384-408.
The electronic health record (EHR) was introduced to improve how clinicians document patient information, contribute to medical research, and allow for medical records to be universally transferable.1 However, many clinicians find EHRs to be burdensome, time-consuming, and inefficient. Clinicians often spend multiple hours each day navigating their EHR system, which reduces the amount of time they spend interacting with patients and contributes to physician burnout.1-3 For example, in a study of 142 family medicine physicians, clinicians reported that they spent approximately 6 hours per work day interacting with their EHR.3
Clearly, the EHR needs a fundamental revision. In the meantime, how can we adapt to improve the situation? Here I suggest practical steps clinicians can take to improve their experience with their EHR system.4-8
Steps to take during patient visits
Because entering information into the EHR can be distracting, be prepared to multitask during each clinical encounter.1-7 Be ready to address pertinent inquiries and issues your patient raises, and provide instructions on therapies and interventions. Because interpersonal relations are important during clinical encounters, establish interaction with your patient by acknowledging them and maintaining frequent eye contact.7 Consider allowing your patient to view the EHR screen because doing so might increase his/her involvement in the visit.
So that you can pay closer attention to your patient, consider taking notes during the visit and entering the information into the EHR later. Consider improving your typing skills to increase the speed of your note-taking. Alternatively, using a voice-recognition recording tool to transcribe your notes via speech might help you spend less time on note-taking.3 Whenever possible, finish charting for one patient before meeting with the next because doing so will save time and help you to better remember details.7
In addition, lowering your overall stress might help reduce the burden of using the EHR.3-5 Adopt healthy behaviors, including good sleep, nutrition, exercise, and hobbies, and strive for balance in your routines. Attend to any personal medical or psychiatric conditions, and avoid misusing alcohol, medications, or other substances.
Optimize how your practice functions
With your clinical group and colleagues, create a comfortable environment, good patient-to-doctor interactions, and a smooth flow within the practice. Simplify registration. Ask patients to complete screening forms before an appointment; this information could be entered directly into their EHR.3 Consider using physician-extender staff and other personnel, such as scribes, to complete documentation into the EHR.3,8 This may help reduce burnout, create more time for clinical care, and improve face-to-face patient interactions.8 Employing scribes can allow doctors to be better able to directly attend to their patients while complying with record-keeping needs. Although scribes make charting easier, they are an additional expense, and must be trained.
Consider EHR training
EHR training sessions can teach you how to use your EHR system more efficiently.6 Such education may help boost confidence, aid documentation, and reduce the amount of time spent correcting coding errors. In a study of 3,500 physicians who underwent a 3-day intensive EHR training course, 85% to 98% reported having improved the quality, readability, and clinical accuracy of their documentation.6
Help shape future EHRs
Individual doctors and professional groups can promote EHR improvements through their state, regional, and/or national organizations and medical societies. These bodies should deliver EHR revision recommendations to government officials, who can craft laws and regulations, and can influence regulators and/or insurance companies. Clinicians also can communicate with EHR developers on ways to simplify the usability of these tools, such as reducing the amount of steps the EHR’s interface requires.5 With a more efficient EHR, we can better concentrate on patient care, which will reduce expenses and should yield better outcomes.
The electronic health record (EHR) was introduced to improve how clinicians document patient information, contribute to medical research, and allow for medical records to be universally transferable.1 However, many clinicians find EHRs to be burdensome, time-consuming, and inefficient. Clinicians often spend multiple hours each day navigating their EHR system, which reduces the amount of time they spend interacting with patients and contributes to physician burnout.1-3 For example, in a study of 142 family medicine physicians, clinicians reported that they spent approximately 6 hours per work day interacting with their EHR.3
Clearly, the EHR needs a fundamental revision. In the meantime, how can we adapt to improve the situation? Here I suggest practical steps clinicians can take to improve their experience with their EHR system.4-8
Steps to take during patient visits
Because entering information into the EHR can be distracting, be prepared to multitask during each clinical encounter.1-7 Be ready to address pertinent inquiries and issues your patient raises, and provide instructions on therapies and interventions. Because interpersonal relations are important during clinical encounters, establish interaction with your patient by acknowledging them and maintaining frequent eye contact.7 Consider allowing your patient to view the EHR screen because doing so might increase his/her involvement in the visit.
So that you can pay closer attention to your patient, consider taking notes during the visit and entering the information into the EHR later. Consider improving your typing skills to increase the speed of your note-taking. Alternatively, using a voice-recognition recording tool to transcribe your notes via speech might help you spend less time on note-taking.3 Whenever possible, finish charting for one patient before meeting with the next because doing so will save time and help you to better remember details.7
In addition, lowering your overall stress might help reduce the burden of using the EHR.3-5 Adopt healthy behaviors, including good sleep, nutrition, exercise, and hobbies, and strive for balance in your routines. Attend to any personal medical or psychiatric conditions, and avoid misusing alcohol, medications, or other substances.
Optimize how your practice functions
With your clinical group and colleagues, create a comfortable environment, good patient-to-doctor interactions, and a smooth flow within the practice. Simplify registration. Ask patients to complete screening forms before an appointment; this information could be entered directly into their EHR.3 Consider using physician-extender staff and other personnel, such as scribes, to complete documentation into the EHR.3,8 This may help reduce burnout, create more time for clinical care, and improve face-to-face patient interactions.8 Employing scribes can allow doctors to be better able to directly attend to their patients while complying with record-keeping needs. Although scribes make charting easier, they are an additional expense, and must be trained.
Consider EHR training
EHR training sessions can teach you how to use your EHR system more efficiently.6 Such education may help boost confidence, aid documentation, and reduce the amount of time spent correcting coding errors. In a study of 3,500 physicians who underwent a 3-day intensive EHR training course, 85% to 98% reported having improved the quality, readability, and clinical accuracy of their documentation.6
Help shape future EHRs
Individual doctors and professional groups can promote EHR improvements through their state, regional, and/or national organizations and medical societies. These bodies should deliver EHR revision recommendations to government officials, who can craft laws and regulations, and can influence regulators and/or insurance companies. Clinicians also can communicate with EHR developers on ways to simplify the usability of these tools, such as reducing the amount of steps the EHR’s interface requires.5 With a more efficient EHR, we can better concentrate on patient care, which will reduce expenses and should yield better outcomes.
1. Ehrenfeld JM, Wonderer JP. Technology as friend or foe? Do electronic health records increase burnout? Curr Opin Anesthesiol. 2018;31(3):357-360.
2. Meigs SL, Solomon M. Electronic health record use a bitter pill for many physicians. Perspect Health Inf Manag. 2016;13:1d.
3. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15(5):419-426.
4. Fogarty CT, Winters P, Farah S. Improving patient-centered communication while using an electronic health record: report from a curriculum evaluation. Int J Psych Med. 2016;51(4):379-389.
5. Guo U, Chen L, Mehta PH. Electronic health record innovations: helping physicians - one less click at a time. Health Inf Manag. 2017;46(3):140-144.
6. Robinson KE, Kersey JA. Novel electronic health record (EHR) education intervention in large healthcare organization improves quality, efficiency, time, and impact on burnout. Medicine. 2018;91(38):e123419. doi: 10.1097/MD.0000000000012319.
7. Fogarty CT. Getting your notes done on time. Fam Pract Manag. 2016;23(2):40.
8. DeChant PF, Acs A, Rhee KB, et al. Effect of organization-directed workplace interventions on physician burnout: a systematic review. Mayo Clin Proc Innov Qual Outcomes. 2019;3(4):384-408.
1. Ehrenfeld JM, Wonderer JP. Technology as friend or foe? Do electronic health records increase burnout? Curr Opin Anesthesiol. 2018;31(3):357-360.
2. Meigs SL, Solomon M. Electronic health record use a bitter pill for many physicians. Perspect Health Inf Manag. 2016;13:1d.
3. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15(5):419-426.
4. Fogarty CT, Winters P, Farah S. Improving patient-centered communication while using an electronic health record: report from a curriculum evaluation. Int J Psych Med. 2016;51(4):379-389.
5. Guo U, Chen L, Mehta PH. Electronic health record innovations: helping physicians - one less click at a time. Health Inf Manag. 2017;46(3):140-144.
6. Robinson KE, Kersey JA. Novel electronic health record (EHR) education intervention in large healthcare organization improves quality, efficiency, time, and impact on burnout. Medicine. 2018;91(38):e123419. doi: 10.1097/MD.0000000000012319.
7. Fogarty CT. Getting your notes done on time. Fam Pract Manag. 2016;23(2):40.
8. DeChant PF, Acs A, Rhee KB, et al. Effect of organization-directed workplace interventions on physician burnout: a systematic review. Mayo Clin Proc Innov Qual Outcomes. 2019;3(4):384-408.
Psychiatric manifestations of sport-related concussion
Ms. J, age 19, is a Division I collegiate volleyball player who recently sustained her third sport-related concussion (SRC). She has no psychiatric history but does have a history of migraine, and her headaches have worsened since the most recent SRC. She has a family history of depression (mother and her sole sibling). Ms. J recently experienced the loss of her coach, someone she greatly admired, in a motor vehicle accident. She is referred to outpatient psychiatry for assessment of mood symptoms that are persisting 1 month after the SRC. Upon assessment, she is found to meet 8 of the 9 criteria for a major depressive episode, including suicidality with vague plans but no intent to end her life.
Although Ms. J does not have a history of psychiatric illness, her psychiatrist recognizes that she has factors that increase her risk of developing depression post-SRC, and of poor recovery from SRC. These include pre-existing symptoms, such as her history of migraine, which is common in patients after SRC. Additionally, a family history of psychiatric disorders and high life stressors (eg, recent loss of her coach) are risk factors for a poor SRC recovery.1 Due to these risk factors and the severity of Ms. J’s symptoms—which include suicidal ideation—the psychiatrist believes that her depressive symptoms might be unlikely to improve in the coming weeks, so he establishes a diagnosis of “depressive disorder due to another medical condition (concussion)” because the development of her depressive symptoms coincided with the SRC. If Ms. J had a pre-existing mood disorder, or if her depression had not developed until later in the post-injury period, it would have been more difficult to establish confidently that the depressive episode was a direct physiologic consequence of the SRC; if that had been the case, the diagnosis probably would have been unspecified or other specified depressive disorder.2
SRC is a traumatic brain injury (TBI) induced by biomechanical forces, typically resulting in short-lived impairment of neurologic function, although signs and symptoms may evolve over minutes to hours.3 It largely reflects functional, rather than structural, brain disturbances.3 SRC has been deemed a “neuropsychiatric syndrome” because psychiatric manifestations are common.4 There may be a myriad of biopsychosocial factors involved in the etiology of psychiatric symptoms in an individual who sustains an SRC. For example, SRC may have a direct physiologic cause of psychiatric symptoms based on the location and degree of injury to the brain. Additionally, pre-existing psychiatric symptoms might increase the likelihood of sustaining an SRC. Finally, as with any major injury, illness, or event, stressors associated with SRC may cause psychiatric symptoms.
Regardless of causal factors, psychiatrists should be comfortable with managing psychiatric symptoms that commonly accompany this condition. This article highlights possible psychiatric manifestations of SRC and delineates high-yield management considerations. Although it focuses on concussions that occur in the context of sport, much of the information applies to patients who experience concussions from other causes.
SRC and depression
Changes in mood, emotion, and behavior are common following SRC. On the Sport Concussion Assessment Tool 5 (SCAT5),5 which is a standardized tool used to evaluate athletes suspected of having sustained a concussion, most symptoms overlap with those attributable to anxiety and depression.4,6 These include5:
- feeling slowed down
- “not feeling right”
- difficulty concentrating
- fatigue or loss of energy
- feeling more emotional
- irritability
- sadness
- feeling nervous or anxious
- difficulty falling asleep.
A recent systematic review of mental health outcomes of SRC in athletes found that the most commonly described and studied psychiatric symptoms following SRC were depression, anxiety, and impulsivity.7 The most rigorous study included in this review found depressive symptoms in 20% of collegiate athletes following SRC (all tested within 41 days of the SRC) vs 5% in the control group.8 These researchers delineated factors that predicted depressive symptoms after SRC (Box 18). Data were insufficient to draw conclusions about the association between SRC and other psychiatric symptoms, such as anxiety.8
Box 1
- Baseline depressive symptoms
- Baseline “post-concussion” symptoms
- Lower estimated premorbid intelligence
- Nonwhite ethnicity
- Increased number of games missed following injury
- Age of first participation in organized sport (more depression in athletes with fewer years of experience)
Source: Reference 8
Psychiatric manifestations of concussion in retired athletes may shed light on the long-term impact of SRC on psychiatric disorders, particularly depression. Hutchison et al9 conducted a systematic review of mental health outcomes of SRC in retired athletes.Two of the included studies that measured clinically diagnosed disorders found positive associations between self-reported concussion and clinically diagnosed depression.10,11 Hutchison et al9 found insufficient data to draw conclusions about depression and a lifetime history of subconcussive impacts—a topic that is receiving growing attention.
Continue to: Regarding a dose-response relationship...
Regarding a dose-response relationship in retired athletes, Guskiewicz et al11 reported a 3-fold increased risk of depression among retired professional football players who had experienced ≥3 SRCs. Five years later, the same research group reported a 5.8-fold increased risk of depression in retired professional football players after 5 to 9 concussions.10 In sum, there is evidence to suggest that the more SRCs an athlete sustains, the more likely they are to develop depression. Moreover, depression may persist or develop long after an SRC occurs.
Suicide risk
While suicide among athletes, especially football players, who have experienced concussion has received relatively widespread media attention, the risk of suicide in former professional football players appears to be significantly lower than in the general population.12 A recent large systematic review and meta-analysis reported on 713,706 individuals diagnosed with concussion and/or mild TBI and 6,236,010 individuals with no such diagnoses.13 It found a 2-fold higher risk of suicide in individuals who experienced concussion and/or mild TBI, but because participants were not necessarily athletes, it is difficult to extrapolate these findings to the athlete population.
Other psychiatric symptoms associated with SRC
Posttraumatic stress disorder (PTSD). Some athletes experience PTSD symptoms shortly after SRC, and these can be missed if clinicians do not specifically ask about them.14 For example, substantial proportions of athletes who have had an SRC report making efforts to avoid sport situations that are similar to how and where their SRC occurred (19%), having trouble keeping thoughts about sustaining the SRC out of their heads (18%), experiencing flashbacks of sustaining the SRC (13%), and having nightmares about sustaining the SRC (8%).14 Posttraumatic stress disorder may have a negative impact on an athlete’s performance because a fear of re-injury might lead them to avoid rehabilitation exercises and inhibit their effort.15-18
Attention-deficit/hyperactivity disorder (ADHD) is commonly comorbid with SRC.19,20 It is not known if pre-existing ADHD makes sustaining a concussion more likely (eg, because the athlete is distractible and thus does not notice when an opponent is about to hit them hard) and/or if a history of concussion makes ADHD more likely to develop (eg, because something about the concussed brain is changed in a way that leads to ADHD). Additionally, in some cases, ADHD has been associated with prolonged recovery from SRC.3,21
Immediate medical evaluation and cognitive assessment
Any patient in whom an SRC is suspected should undergo a medical evaluation immediately, whether in a physician’s office, emergency department, or on the sideline of a sports event. This medical evaluation should incorporate a clinical neurologic assessment, including evaluation of mental status/cognition, oculomotor function, gross sensorimotor, coordination, gait, vestibular function, and balance.3
Continue to: There is no single guideline...
There is no single guideline on how and when a neuropsychology referral is warranted.22 Insurance coverage for neurocognitive testing varies. Regardless of formal referral to neuropsychology, assessment of cognitive function is an important aspect of SRC management and is a factor in return-to-school and return-to-play decisions.3,22 Screening tools, such as the SCAT5, are useful in acute and subacute settings (ie, up to 3 to 5 days after injury); clinicians often use serial monitoring to track the resolution of symptoms.3 If pre-season baseline cognitive test results are available, clinicians may compare them to post-SRC results, but this should not be the sole basis of management decisions.3,22
Diagnosing psychiatric disorders in patients with SRC
Diagnosis of psychiatric symptoms and disorders associated with SRC can be challenging.7 There are no concussion-specific rating scales or diagnostic criteria for psychiatric disorders unique to patients who have sustained SRC. As a result, clinicians are left to use standard DSM-5 criteria for the diagnosis of psychiatric disorders in patients with SRC. Importantly, psychiatric symptoms must be distinguished from disorders. For example, Kontos et al23 reported significantly worse depressive symptoms following SRC, but not at the level to meet the criteria for major depressive disorder. This is an important distinction, because a psychiatrist might be less likely to initiate pharmacotherapy for a patient with SRC who has only a few depressive symptoms and is only 1 week post-SRC, vs for one who has had most symptoms of a major depressive episode for several weeks.
The American Medical Society for Sports Medicine has proposed 6 overlapping clinical profiles in patients with SRC (see the Table).24 Most patients with SRC have features of multiple clinical profiles.24 Anxiety/mood is one of these profiles. The impetus for developing these profiles was the recognition of heterogeneity among concussion presentations. Identification of the clinical profile(s) into which a patient’s symptoms fall might allow for more specific prognostication and targeted treatment.24 For example, referral to a psychiatrist obviously would be appropriate for a patient for whom anxiety/mood symptoms are prominent.
Treatment options for psychiatric sequelae of SRC
Both psychosocial and medical principles of management of psychiatric manifestations of SRC are important. Psychosocially, clinicians should address factors that may contribute to delayed SRC recovery (Box 225-30).
Box 2
- Recommend a progressive increase in exercise after a brief period of rest (often ameliorates psychiatric symptoms, as opposed to the historical approach of “cocoon therapy” in which the patient was to rest for prolonged periods of time in a darkened room so as to minimize brain stimulation)25
- Allow social activities, including team meetings (restriction of such activities has been associated with increased post-SRC depression)26
- Encourage members of the athlete’s “entourage” (team physicians, athletic trainers, coaches, teammates, and parents) to provide support27
- Educate coaches and teammates about how to make supportive statements because they often have trouble knowing how to do so27
- Recommend psychotherapy for mental and other physical symptoms of SRC that are moderate to severe or that persist longer than 4 weeks after the SRC28
- Recommend minimization of use of alcohol and other substances29,30
SRC: sport-related concussion
No medications are FDA-approved for SRC or associated psychiatric symptoms, and there is minimal evidence to support the use of specific medications.31 Most athletes with SRC recover quickly—typically within 2 weeks—and do not need medication.4,32 When medications are needed, start with low dosing and titrate slowly.33,34
Continue to: For patients with SRC who experience insomnia...
For patients with SRC who experience insomnia, clinicians should focus on sleep hygiene and, if needed, cognitive-behavioral therapy for insomnia (CBT-I).31 If medication is needed, melatonin may be a first-line agent.31,35,36 Trazodone may be a second option.32 Benzodiazepines typically are avoided because of their negative impact on cognition.31
For patients with SRC who have depression, selective serotonin reuptake inhibitors (SSRIs) may simultaneously improve depressed mood31 and cognition.37 Tricyclic antidepressants (TCAs) are sometimes used to treat headaches, depression, anxiety, and/or insomnia after SRC,32 but adverse effects such as sedation and weight gain may limit their use in athletes. Theoretically, serotonin-norepinephrine reuptake inhibitors might have some of the same benefits as TCAs with fewer adverse effects, but they have not been well studied in patients with SRC.
For patients with SRC who have cognitive dysfunction (eg, deficits in attention and processing speed), there is some evidence for treatment with stimulants.31,37 However, these medications are prohibited by many athletic governing organizations, including professional sports leagues, the National Collegiate Athletic Association (NCAA), and the World Anti-Doping Agency.4 If an athlete was receiving stimulants for ADHD before sustaining an SRC, there is no evidence that these medications should be stopped.
Consider interdisciplinary collaboration
Throughout the course of management, psychiatrists should consider if and when it is necessary to consult with other specialties such as primary care, sports medicine, neurology, and neuropsychology. As with many psychiatric symptoms and disorders, collaboration with an interdisciplinary team is recommended. Primary care, sports medicine, or neurology should be involved in the management of patients with SRC. Choice of which of those 3 specialties in particular will depend on comfort level and experience with managing SRC of the individual providers in question as well as availability of each provider type in a given community.
Additionally, psychiatrists may wonder if and when they should refer patients with SRC for neuroimaging. Because SRC is a functional, rather than structural, brain disturbance, neuroimaging is not typically pursued because results would be expected to be normal.3 However, when in doubt, consultation with the interdisciplinary team can guide this decision. Factors that may lead to a decision to obtain neuroimaging include:
- an abnormal neurologic examination
- prolonged loss of consciousness
- unexpected persistence of symptoms (eg, 6 to 12 weeks)
- worsening symptoms.22
Continue to: If imaging is deemed necessary...
If imaging is deemed necessary for a patient with an acute SRC, brain CT is typically the imaging modality of choice; however, if imaging is deemed necessary due to the persistence of symptoms, then MRI is often the preferred test because it provides more detailed information and does not expose the patient to ionizing radiation.22 While results are often normal, the ordering clinician should be prepared for the possibility of incidental findings, such as cysts or aneurysms, and the need for further consultation with other clinicians to weigh in on such findings.22
CASE CONTINUED
Ms. J is prescribed extended-release venlafaxine, 37.5 mg every morning for 5 days, and then is switched to 75 mg every morning. The psychiatrist hopes that venlafaxine might simultaneously offer benefit for Ms. J’s depression and migraine headaches. Venlafaxine is not FDA-approved for migraine, and there is more evidence supporting TCAs for preventing migraine. However, Ms. J is adamant that she does not want to take a medication, such as a TCA, that could cause weight gain or sedation, which could be problematic in her sport. The psychiatrist also tells Ms. J to avoid substances of abuse, and emphasizes the importance of good sleep hygiene. Finally, the psychiatrist communicates with the interdisciplinary medical team, which is helping Ms. J with gradual return-to-school and return-to-sport strategies and ensuring continued social involvement with the team even as she is held out from sport.
Ultimately, Ms. J’s extended-release venlafaxine is titrated to 150 mg every morning. After 2 months on this dose, her depressive symptoms remit. After her other symptoms remit, Ms. J has difficulty returning to certain practice drills that remind her of what she was doing when she sustained the SRC. She says that while participating in these drills, she has intrusive thoughts and images of the experience of her most recent concussion. She works with her psychiatrist on a gradual program of exposure therapy so she can return to all types of practice. Ms. J says she wishes to continue playing volleyball; however, together with her parents and treatment team, she decides that any additional SRCs might lead her to retire from the sport.
Bottom Line
Psychiatric symptoms are common after sport-related concussion (SRC). The nature of the relationship between concussion and mental health is not firmly established. Post-SRC psychiatric symptoms need to be carefully managed to avoid unnecessary treatment or restrictions.
Related Resources
- National Collegiate Athletic Association. Concussion. www.ncaa.org/sport-science-institute/concussion.
- American Academy of Neurology. Sports concussion resources. www.aan.com/tools-and-resources/practicing-neurologists-administrators/patient-resources/sports-concussion-resources. Published 2020.
Drug Brand Names
Trazodone • Desyrel
Venlafaxine • Effexor
1. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589-598.
2. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13-29.
3. McCrory P, Meeuwisse W, Dvor˘ák J, et al. Consensus statement on concussion in sport—the 5th International Conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847.
4. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667-699.
5. Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5): background and rationale. Br J Sports Med. 2017;51:848-850.
6. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65(7):601.
7. Rice SM, Parker AG, Rosenbaum S, et al. Sport-related concussion outcomes in elite athletes: a systematic review. Sports Med. 2018;48(2):447-465.
8. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-255.
9. Hutchison MG, Di Battista AP, McCoskey J, et al. Systematic review of mental health measures associated with concussive and subconcussive head trauma in former athletes. Int J Psychophysiol. 2018;132(Pt A):55-61.
10. Kerr GA, Stirling AE. Parents’ reflections on their child’s experiences of emotionally abusive coaching practices. J Appl Sport Psychol. 2012;24(2):191-206.
11. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909.
12. Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. Am J Sports Med. 2016;44(10):2486-2491.
13. Fralick M, Sy E, Hassan A, et al. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2018;76(2):144-151.
14. Brassil HE, Salvatore AP. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin Transl Med. 2018;7:25.
15. Bateman A, Morgan KAD. The postinjury psychological sequelae of high-level Jamaican athletes: exploration of a posttraumatic stress disorder-self-efficacy conceptualization. J Sport Rehabil. 2019;28(2):144-152.
16. Brewer BW, Van Raalte JL, Cornelius AE, et al. Psychological factors, rehabilitation adherence, and rehabilitation outcome after anterior cruciate ligament reconstruction. Rehabil Psychol. 2000;45(1):20-37.
17. Putukian M, Echemendia RJ. Psychological aspects of serious head injury in the competitive athlete. Clin Sports Med. 2003;22(33):617-630.
18. James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among Veterans with and without PTSD and mild TBI. Mil Med. 2014;179(4):357-363.
19. Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;47(1):15-26.
20. Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med. 2016;26(2):120-127.
21. Esfandiari A, Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2011;30(3):611-627.
22. Mahooti N. Sport-related concussion: acute management and chronic postconcussive issues. Chld Adolesc Psychiatric Clin N Am. 2018;27(1):93-108.
23. Kontos AP, Covassin T, Elbin RJ, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
24. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Clin J Sport Med. 2019;29(2):87-100.
25. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current Sports Medicine Reports. 2013;12(6):370-376.
26. Schneider KJ, Iverson GL, Emery CA, et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304-307.
27. Wayment HA, Huffman AH. Psychosocial experiences of concussed collegiate athletes: the role of emotional support in the recovery process. J Am Coll Health. 2020;68(4):438-443.
28. Todd R, Bhalerao S, Vu MT, et al. Understanding the psychiatric effects of concussion on constructed identity in hockey players: implications for health professionals. PLoS ONE. 2018;13(2):e0192125.
29. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140.
30. Gaetz M. The multi-factorial origins of chronic traumatic encephalopathy (CTE) symptomatology in post-career athletes: the athlete post-career adjustment (AP-CA) model. Med Hypotheses. 2017;102:130-143.
31. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124.
32. Broglio SP, Collins MW, Williams RM, et al. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
33. Arciniegas DB, Silver JM, McAllister TW. Stimulants and acetylcholinesterase inhibitors for the treatment of cognitive impairment after traumatic brain injury. Psychopharm Review. 2008;43(12):91-97.
34. Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
35. Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity/mortality after craniocerebral trauma. J Pineal Res. 2007;42(1):1-11.
36. Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47(2):134-142.
37. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
Ms. J, age 19, is a Division I collegiate volleyball player who recently sustained her third sport-related concussion (SRC). She has no psychiatric history but does have a history of migraine, and her headaches have worsened since the most recent SRC. She has a family history of depression (mother and her sole sibling). Ms. J recently experienced the loss of her coach, someone she greatly admired, in a motor vehicle accident. She is referred to outpatient psychiatry for assessment of mood symptoms that are persisting 1 month after the SRC. Upon assessment, she is found to meet 8 of the 9 criteria for a major depressive episode, including suicidality with vague plans but no intent to end her life.
Although Ms. J does not have a history of psychiatric illness, her psychiatrist recognizes that she has factors that increase her risk of developing depression post-SRC, and of poor recovery from SRC. These include pre-existing symptoms, such as her history of migraine, which is common in patients after SRC. Additionally, a family history of psychiatric disorders and high life stressors (eg, recent loss of her coach) are risk factors for a poor SRC recovery.1 Due to these risk factors and the severity of Ms. J’s symptoms—which include suicidal ideation—the psychiatrist believes that her depressive symptoms might be unlikely to improve in the coming weeks, so he establishes a diagnosis of “depressive disorder due to another medical condition (concussion)” because the development of her depressive symptoms coincided with the SRC. If Ms. J had a pre-existing mood disorder, or if her depression had not developed until later in the post-injury period, it would have been more difficult to establish confidently that the depressive episode was a direct physiologic consequence of the SRC; if that had been the case, the diagnosis probably would have been unspecified or other specified depressive disorder.2
SRC is a traumatic brain injury (TBI) induced by biomechanical forces, typically resulting in short-lived impairment of neurologic function, although signs and symptoms may evolve over minutes to hours.3 It largely reflects functional, rather than structural, brain disturbances.3 SRC has been deemed a “neuropsychiatric syndrome” because psychiatric manifestations are common.4 There may be a myriad of biopsychosocial factors involved in the etiology of psychiatric symptoms in an individual who sustains an SRC. For example, SRC may have a direct physiologic cause of psychiatric symptoms based on the location and degree of injury to the brain. Additionally, pre-existing psychiatric symptoms might increase the likelihood of sustaining an SRC. Finally, as with any major injury, illness, or event, stressors associated with SRC may cause psychiatric symptoms.
Regardless of causal factors, psychiatrists should be comfortable with managing psychiatric symptoms that commonly accompany this condition. This article highlights possible psychiatric manifestations of SRC and delineates high-yield management considerations. Although it focuses on concussions that occur in the context of sport, much of the information applies to patients who experience concussions from other causes.
SRC and depression
Changes in mood, emotion, and behavior are common following SRC. On the Sport Concussion Assessment Tool 5 (SCAT5),5 which is a standardized tool used to evaluate athletes suspected of having sustained a concussion, most symptoms overlap with those attributable to anxiety and depression.4,6 These include5:
- feeling slowed down
- “not feeling right”
- difficulty concentrating
- fatigue or loss of energy
- feeling more emotional
- irritability
- sadness
- feeling nervous or anxious
- difficulty falling asleep.
A recent systematic review of mental health outcomes of SRC in athletes found that the most commonly described and studied psychiatric symptoms following SRC were depression, anxiety, and impulsivity.7 The most rigorous study included in this review found depressive symptoms in 20% of collegiate athletes following SRC (all tested within 41 days of the SRC) vs 5% in the control group.8 These researchers delineated factors that predicted depressive symptoms after SRC (Box 18). Data were insufficient to draw conclusions about the association between SRC and other psychiatric symptoms, such as anxiety.8
Box 1
- Baseline depressive symptoms
- Baseline “post-concussion” symptoms
- Lower estimated premorbid intelligence
- Nonwhite ethnicity
- Increased number of games missed following injury
- Age of first participation in organized sport (more depression in athletes with fewer years of experience)
Source: Reference 8
Psychiatric manifestations of concussion in retired athletes may shed light on the long-term impact of SRC on psychiatric disorders, particularly depression. Hutchison et al9 conducted a systematic review of mental health outcomes of SRC in retired athletes.Two of the included studies that measured clinically diagnosed disorders found positive associations between self-reported concussion and clinically diagnosed depression.10,11 Hutchison et al9 found insufficient data to draw conclusions about depression and a lifetime history of subconcussive impacts—a topic that is receiving growing attention.
Continue to: Regarding a dose-response relationship...
Regarding a dose-response relationship in retired athletes, Guskiewicz et al11 reported a 3-fold increased risk of depression among retired professional football players who had experienced ≥3 SRCs. Five years later, the same research group reported a 5.8-fold increased risk of depression in retired professional football players after 5 to 9 concussions.10 In sum, there is evidence to suggest that the more SRCs an athlete sustains, the more likely they are to develop depression. Moreover, depression may persist or develop long after an SRC occurs.
Suicide risk
While suicide among athletes, especially football players, who have experienced concussion has received relatively widespread media attention, the risk of suicide in former professional football players appears to be significantly lower than in the general population.12 A recent large systematic review and meta-analysis reported on 713,706 individuals diagnosed with concussion and/or mild TBI and 6,236,010 individuals with no such diagnoses.13 It found a 2-fold higher risk of suicide in individuals who experienced concussion and/or mild TBI, but because participants were not necessarily athletes, it is difficult to extrapolate these findings to the athlete population.
Other psychiatric symptoms associated with SRC
Posttraumatic stress disorder (PTSD). Some athletes experience PTSD symptoms shortly after SRC, and these can be missed if clinicians do not specifically ask about them.14 For example, substantial proportions of athletes who have had an SRC report making efforts to avoid sport situations that are similar to how and where their SRC occurred (19%), having trouble keeping thoughts about sustaining the SRC out of their heads (18%), experiencing flashbacks of sustaining the SRC (13%), and having nightmares about sustaining the SRC (8%).14 Posttraumatic stress disorder may have a negative impact on an athlete’s performance because a fear of re-injury might lead them to avoid rehabilitation exercises and inhibit their effort.15-18
Attention-deficit/hyperactivity disorder (ADHD) is commonly comorbid with SRC.19,20 It is not known if pre-existing ADHD makes sustaining a concussion more likely (eg, because the athlete is distractible and thus does not notice when an opponent is about to hit them hard) and/or if a history of concussion makes ADHD more likely to develop (eg, because something about the concussed brain is changed in a way that leads to ADHD). Additionally, in some cases, ADHD has been associated with prolonged recovery from SRC.3,21
Immediate medical evaluation and cognitive assessment
Any patient in whom an SRC is suspected should undergo a medical evaluation immediately, whether in a physician’s office, emergency department, or on the sideline of a sports event. This medical evaluation should incorporate a clinical neurologic assessment, including evaluation of mental status/cognition, oculomotor function, gross sensorimotor, coordination, gait, vestibular function, and balance.3
Continue to: There is no single guideline...
There is no single guideline on how and when a neuropsychology referral is warranted.22 Insurance coverage for neurocognitive testing varies. Regardless of formal referral to neuropsychology, assessment of cognitive function is an important aspect of SRC management and is a factor in return-to-school and return-to-play decisions.3,22 Screening tools, such as the SCAT5, are useful in acute and subacute settings (ie, up to 3 to 5 days after injury); clinicians often use serial monitoring to track the resolution of symptoms.3 If pre-season baseline cognitive test results are available, clinicians may compare them to post-SRC results, but this should not be the sole basis of management decisions.3,22
Diagnosing psychiatric disorders in patients with SRC
Diagnosis of psychiatric symptoms and disorders associated with SRC can be challenging.7 There are no concussion-specific rating scales or diagnostic criteria for psychiatric disorders unique to patients who have sustained SRC. As a result, clinicians are left to use standard DSM-5 criteria for the diagnosis of psychiatric disorders in patients with SRC. Importantly, psychiatric symptoms must be distinguished from disorders. For example, Kontos et al23 reported significantly worse depressive symptoms following SRC, but not at the level to meet the criteria for major depressive disorder. This is an important distinction, because a psychiatrist might be less likely to initiate pharmacotherapy for a patient with SRC who has only a few depressive symptoms and is only 1 week post-SRC, vs for one who has had most symptoms of a major depressive episode for several weeks.
The American Medical Society for Sports Medicine has proposed 6 overlapping clinical profiles in patients with SRC (see the Table).24 Most patients with SRC have features of multiple clinical profiles.24 Anxiety/mood is one of these profiles. The impetus for developing these profiles was the recognition of heterogeneity among concussion presentations. Identification of the clinical profile(s) into which a patient’s symptoms fall might allow for more specific prognostication and targeted treatment.24 For example, referral to a psychiatrist obviously would be appropriate for a patient for whom anxiety/mood symptoms are prominent.

Treatment options for psychiatric sequelae of SRC
Both psychosocial and medical principles of management of psychiatric manifestations of SRC are important. Psychosocially, clinicians should address factors that may contribute to delayed SRC recovery (Box 225-30).
Box 2
- Recommend a progressive increase in exercise after a brief period of rest (often ameliorates psychiatric symptoms, as opposed to the historical approach of “cocoon therapy” in which the patient was to rest for prolonged periods of time in a darkened room so as to minimize brain stimulation)25
- Allow social activities, including team meetings (restriction of such activities has been associated with increased post-SRC depression)26
- Encourage members of the athlete’s “entourage” (team physicians, athletic trainers, coaches, teammates, and parents) to provide support27
- Educate coaches and teammates about how to make supportive statements because they often have trouble knowing how to do so27
- Recommend psychotherapy for mental and other physical symptoms of SRC that are moderate to severe or that persist longer than 4 weeks after the SRC28
- Recommend minimization of use of alcohol and other substances29,30
SRC: sport-related concussion
No medications are FDA-approved for SRC or associated psychiatric symptoms, and there is minimal evidence to support the use of specific medications.31 Most athletes with SRC recover quickly—typically within 2 weeks—and do not need medication.4,32 When medications are needed, start with low dosing and titrate slowly.33,34
Continue to: For patients with SRC who experience insomnia...
For patients with SRC who experience insomnia, clinicians should focus on sleep hygiene and, if needed, cognitive-behavioral therapy for insomnia (CBT-I).31 If medication is needed, melatonin may be a first-line agent.31,35,36 Trazodone may be a second option.32 Benzodiazepines typically are avoided because of their negative impact on cognition.31
For patients with SRC who have depression, selective serotonin reuptake inhibitors (SSRIs) may simultaneously improve depressed mood31 and cognition.37 Tricyclic antidepressants (TCAs) are sometimes used to treat headaches, depression, anxiety, and/or insomnia after SRC,32 but adverse effects such as sedation and weight gain may limit their use in athletes. Theoretically, serotonin-norepinephrine reuptake inhibitors might have some of the same benefits as TCAs with fewer adverse effects, but they have not been well studied in patients with SRC.
For patients with SRC who have cognitive dysfunction (eg, deficits in attention and processing speed), there is some evidence for treatment with stimulants.31,37 However, these medications are prohibited by many athletic governing organizations, including professional sports leagues, the National Collegiate Athletic Association (NCAA), and the World Anti-Doping Agency.4 If an athlete was receiving stimulants for ADHD before sustaining an SRC, there is no evidence that these medications should be stopped.
Consider interdisciplinary collaboration
Throughout the course of management, psychiatrists should consider if and when it is necessary to consult with other specialties such as primary care, sports medicine, neurology, and neuropsychology. As with many psychiatric symptoms and disorders, collaboration with an interdisciplinary team is recommended. Primary care, sports medicine, or neurology should be involved in the management of patients with SRC. Choice of which of those 3 specialties in particular will depend on comfort level and experience with managing SRC of the individual providers in question as well as availability of each provider type in a given community.
Additionally, psychiatrists may wonder if and when they should refer patients with SRC for neuroimaging. Because SRC is a functional, rather than structural, brain disturbance, neuroimaging is not typically pursued because results would be expected to be normal.3 However, when in doubt, consultation with the interdisciplinary team can guide this decision. Factors that may lead to a decision to obtain neuroimaging include:
- an abnormal neurologic examination
- prolonged loss of consciousness
- unexpected persistence of symptoms (eg, 6 to 12 weeks)
- worsening symptoms.22
Continue to: If imaging is deemed necessary...
If imaging is deemed necessary for a patient with an acute SRC, brain CT is typically the imaging modality of choice; however, if imaging is deemed necessary due to the persistence of symptoms, then MRI is often the preferred test because it provides more detailed information and does not expose the patient to ionizing radiation.22 While results are often normal, the ordering clinician should be prepared for the possibility of incidental findings, such as cysts or aneurysms, and the need for further consultation with other clinicians to weigh in on such findings.22
CASE CONTINUED
Ms. J is prescribed extended-release venlafaxine, 37.5 mg every morning for 5 days, and then is switched to 75 mg every morning. The psychiatrist hopes that venlafaxine might simultaneously offer benefit for Ms. J’s depression and migraine headaches. Venlafaxine is not FDA-approved for migraine, and there is more evidence supporting TCAs for preventing migraine. However, Ms. J is adamant that she does not want to take a medication, such as a TCA, that could cause weight gain or sedation, which could be problematic in her sport. The psychiatrist also tells Ms. J to avoid substances of abuse, and emphasizes the importance of good sleep hygiene. Finally, the psychiatrist communicates with the interdisciplinary medical team, which is helping Ms. J with gradual return-to-school and return-to-sport strategies and ensuring continued social involvement with the team even as she is held out from sport.
Ultimately, Ms. J’s extended-release venlafaxine is titrated to 150 mg every morning. After 2 months on this dose, her depressive symptoms remit. After her other symptoms remit, Ms. J has difficulty returning to certain practice drills that remind her of what she was doing when she sustained the SRC. She says that while participating in these drills, she has intrusive thoughts and images of the experience of her most recent concussion. She works with her psychiatrist on a gradual program of exposure therapy so she can return to all types of practice. Ms. J says she wishes to continue playing volleyball; however, together with her parents and treatment team, she decides that any additional SRCs might lead her to retire from the sport.
Bottom Line
Psychiatric symptoms are common after sport-related concussion (SRC). The nature of the relationship between concussion and mental health is not firmly established. Post-SRC psychiatric symptoms need to be carefully managed to avoid unnecessary treatment or restrictions.
Related Resources
- National Collegiate Athletic Association. Concussion. www.ncaa.org/sport-science-institute/concussion.
- American Academy of Neurology. Sports concussion resources. www.aan.com/tools-and-resources/practicing-neurologists-administrators/patient-resources/sports-concussion-resources. Published 2020.
Drug Brand Names
Trazodone • Desyrel
Venlafaxine • Effexor
Ms. J, age 19, is a Division I collegiate volleyball player who recently sustained her third sport-related concussion (SRC). She has no psychiatric history but does have a history of migraine, and her headaches have worsened since the most recent SRC. She has a family history of depression (mother and her sole sibling). Ms. J recently experienced the loss of her coach, someone she greatly admired, in a motor vehicle accident. She is referred to outpatient psychiatry for assessment of mood symptoms that are persisting 1 month after the SRC. Upon assessment, she is found to meet 8 of the 9 criteria for a major depressive episode, including suicidality with vague plans but no intent to end her life.
Although Ms. J does not have a history of psychiatric illness, her psychiatrist recognizes that she has factors that increase her risk of developing depression post-SRC, and of poor recovery from SRC. These include pre-existing symptoms, such as her history of migraine, which is common in patients after SRC. Additionally, a family history of psychiatric disorders and high life stressors (eg, recent loss of her coach) are risk factors for a poor SRC recovery.1 Due to these risk factors and the severity of Ms. J’s symptoms—which include suicidal ideation—the psychiatrist believes that her depressive symptoms might be unlikely to improve in the coming weeks, so he establishes a diagnosis of “depressive disorder due to another medical condition (concussion)” because the development of her depressive symptoms coincided with the SRC. If Ms. J had a pre-existing mood disorder, or if her depression had not developed until later in the post-injury period, it would have been more difficult to establish confidently that the depressive episode was a direct physiologic consequence of the SRC; if that had been the case, the diagnosis probably would have been unspecified or other specified depressive disorder.2
SRC is a traumatic brain injury (TBI) induced by biomechanical forces, typically resulting in short-lived impairment of neurologic function, although signs and symptoms may evolve over minutes to hours.3 It largely reflects functional, rather than structural, brain disturbances.3 SRC has been deemed a “neuropsychiatric syndrome” because psychiatric manifestations are common.4 There may be a myriad of biopsychosocial factors involved in the etiology of psychiatric symptoms in an individual who sustains an SRC. For example, SRC may have a direct physiologic cause of psychiatric symptoms based on the location and degree of injury to the brain. Additionally, pre-existing psychiatric symptoms might increase the likelihood of sustaining an SRC. Finally, as with any major injury, illness, or event, stressors associated with SRC may cause psychiatric symptoms.
Regardless of causal factors, psychiatrists should be comfortable with managing psychiatric symptoms that commonly accompany this condition. This article highlights possible psychiatric manifestations of SRC and delineates high-yield management considerations. Although it focuses on concussions that occur in the context of sport, much of the information applies to patients who experience concussions from other causes.
SRC and depression
Changes in mood, emotion, and behavior are common following SRC. On the Sport Concussion Assessment Tool 5 (SCAT5),5 which is a standardized tool used to evaluate athletes suspected of having sustained a concussion, most symptoms overlap with those attributable to anxiety and depression.4,6 These include5:
- feeling slowed down
- “not feeling right”
- difficulty concentrating
- fatigue or loss of energy
- feeling more emotional
- irritability
- sadness
- feeling nervous or anxious
- difficulty falling asleep.
A recent systematic review of mental health outcomes of SRC in athletes found that the most commonly described and studied psychiatric symptoms following SRC were depression, anxiety, and impulsivity.7 The most rigorous study included in this review found depressive symptoms in 20% of collegiate athletes following SRC (all tested within 41 days of the SRC) vs 5% in the control group.8 These researchers delineated factors that predicted depressive symptoms after SRC (Box 18). Data were insufficient to draw conclusions about the association between SRC and other psychiatric symptoms, such as anxiety.8
Box 1
- Baseline depressive symptoms
- Baseline “post-concussion” symptoms
- Lower estimated premorbid intelligence
- Nonwhite ethnicity
- Increased number of games missed following injury
- Age of first participation in organized sport (more depression in athletes with fewer years of experience)
Source: Reference 8
Psychiatric manifestations of concussion in retired athletes may shed light on the long-term impact of SRC on psychiatric disorders, particularly depression. Hutchison et al9 conducted a systematic review of mental health outcomes of SRC in retired athletes.Two of the included studies that measured clinically diagnosed disorders found positive associations between self-reported concussion and clinically diagnosed depression.10,11 Hutchison et al9 found insufficient data to draw conclusions about depression and a lifetime history of subconcussive impacts—a topic that is receiving growing attention.
Continue to: Regarding a dose-response relationship...
Regarding a dose-response relationship in retired athletes, Guskiewicz et al11 reported a 3-fold increased risk of depression among retired professional football players who had experienced ≥3 SRCs. Five years later, the same research group reported a 5.8-fold increased risk of depression in retired professional football players after 5 to 9 concussions.10 In sum, there is evidence to suggest that the more SRCs an athlete sustains, the more likely they are to develop depression. Moreover, depression may persist or develop long after an SRC occurs.
Suicide risk
While suicide among athletes, especially football players, who have experienced concussion has received relatively widespread media attention, the risk of suicide in former professional football players appears to be significantly lower than in the general population.12 A recent large systematic review and meta-analysis reported on 713,706 individuals diagnosed with concussion and/or mild TBI and 6,236,010 individuals with no such diagnoses.13 It found a 2-fold higher risk of suicide in individuals who experienced concussion and/or mild TBI, but because participants were not necessarily athletes, it is difficult to extrapolate these findings to the athlete population.
Other psychiatric symptoms associated with SRC
Posttraumatic stress disorder (PTSD). Some athletes experience PTSD symptoms shortly after SRC, and these can be missed if clinicians do not specifically ask about them.14 For example, substantial proportions of athletes who have had an SRC report making efforts to avoid sport situations that are similar to how and where their SRC occurred (19%), having trouble keeping thoughts about sustaining the SRC out of their heads (18%), experiencing flashbacks of sustaining the SRC (13%), and having nightmares about sustaining the SRC (8%).14 Posttraumatic stress disorder may have a negative impact on an athlete’s performance because a fear of re-injury might lead them to avoid rehabilitation exercises and inhibit their effort.15-18
Attention-deficit/hyperactivity disorder (ADHD) is commonly comorbid with SRC.19,20 It is not known if pre-existing ADHD makes sustaining a concussion more likely (eg, because the athlete is distractible and thus does not notice when an opponent is about to hit them hard) and/or if a history of concussion makes ADHD more likely to develop (eg, because something about the concussed brain is changed in a way that leads to ADHD). Additionally, in some cases, ADHD has been associated with prolonged recovery from SRC.3,21
Immediate medical evaluation and cognitive assessment
Any patient in whom an SRC is suspected should undergo a medical evaluation immediately, whether in a physician’s office, emergency department, or on the sideline of a sports event. This medical evaluation should incorporate a clinical neurologic assessment, including evaluation of mental status/cognition, oculomotor function, gross sensorimotor, coordination, gait, vestibular function, and balance.3
Continue to: There is no single guideline...
There is no single guideline on how and when a neuropsychology referral is warranted.22 Insurance coverage for neurocognitive testing varies. Regardless of formal referral to neuropsychology, assessment of cognitive function is an important aspect of SRC management and is a factor in return-to-school and return-to-play decisions.3,22 Screening tools, such as the SCAT5, are useful in acute and subacute settings (ie, up to 3 to 5 days after injury); clinicians often use serial monitoring to track the resolution of symptoms.3 If pre-season baseline cognitive test results are available, clinicians may compare them to post-SRC results, but this should not be the sole basis of management decisions.3,22
Diagnosing psychiatric disorders in patients with SRC
Diagnosis of psychiatric symptoms and disorders associated with SRC can be challenging.7 There are no concussion-specific rating scales or diagnostic criteria for psychiatric disorders unique to patients who have sustained SRC. As a result, clinicians are left to use standard DSM-5 criteria for the diagnosis of psychiatric disorders in patients with SRC. Importantly, psychiatric symptoms must be distinguished from disorders. For example, Kontos et al23 reported significantly worse depressive symptoms following SRC, but not at the level to meet the criteria for major depressive disorder. This is an important distinction, because a psychiatrist might be less likely to initiate pharmacotherapy for a patient with SRC who has only a few depressive symptoms and is only 1 week post-SRC, vs for one who has had most symptoms of a major depressive episode for several weeks.
The American Medical Society for Sports Medicine has proposed 6 overlapping clinical profiles in patients with SRC (see the Table).24 Most patients with SRC have features of multiple clinical profiles.24 Anxiety/mood is one of these profiles. The impetus for developing these profiles was the recognition of heterogeneity among concussion presentations. Identification of the clinical profile(s) into which a patient’s symptoms fall might allow for more specific prognostication and targeted treatment.24 For example, referral to a psychiatrist obviously would be appropriate for a patient for whom anxiety/mood symptoms are prominent.

Treatment options for psychiatric sequelae of SRC
Both psychosocial and medical principles of management of psychiatric manifestations of SRC are important. Psychosocially, clinicians should address factors that may contribute to delayed SRC recovery (Box 225-30).
Box 2
- Recommend a progressive increase in exercise after a brief period of rest (often ameliorates psychiatric symptoms, as opposed to the historical approach of “cocoon therapy” in which the patient was to rest for prolonged periods of time in a darkened room so as to minimize brain stimulation)25
- Allow social activities, including team meetings (restriction of such activities has been associated with increased post-SRC depression)26
- Encourage members of the athlete’s “entourage” (team physicians, athletic trainers, coaches, teammates, and parents) to provide support27
- Educate coaches and teammates about how to make supportive statements because they often have trouble knowing how to do so27
- Recommend psychotherapy for mental and other physical symptoms of SRC that are moderate to severe or that persist longer than 4 weeks after the SRC28
- Recommend minimization of use of alcohol and other substances29,30
SRC: sport-related concussion
No medications are FDA-approved for SRC or associated psychiatric symptoms, and there is minimal evidence to support the use of specific medications.31 Most athletes with SRC recover quickly—typically within 2 weeks—and do not need medication.4,32 When medications are needed, start with low dosing and titrate slowly.33,34
Continue to: For patients with SRC who experience insomnia...
For patients with SRC who experience insomnia, clinicians should focus on sleep hygiene and, if needed, cognitive-behavioral therapy for insomnia (CBT-I).31 If medication is needed, melatonin may be a first-line agent.31,35,36 Trazodone may be a second option.32 Benzodiazepines typically are avoided because of their negative impact on cognition.31
For patients with SRC who have depression, selective serotonin reuptake inhibitors (SSRIs) may simultaneously improve depressed mood31 and cognition.37 Tricyclic antidepressants (TCAs) are sometimes used to treat headaches, depression, anxiety, and/or insomnia after SRC,32 but adverse effects such as sedation and weight gain may limit their use in athletes. Theoretically, serotonin-norepinephrine reuptake inhibitors might have some of the same benefits as TCAs with fewer adverse effects, but they have not been well studied in patients with SRC.
For patients with SRC who have cognitive dysfunction (eg, deficits in attention and processing speed), there is some evidence for treatment with stimulants.31,37 However, these medications are prohibited by many athletic governing organizations, including professional sports leagues, the National Collegiate Athletic Association (NCAA), and the World Anti-Doping Agency.4 If an athlete was receiving stimulants for ADHD before sustaining an SRC, there is no evidence that these medications should be stopped.
Consider interdisciplinary collaboration
Throughout the course of management, psychiatrists should consider if and when it is necessary to consult with other specialties such as primary care, sports medicine, neurology, and neuropsychology. As with many psychiatric symptoms and disorders, collaboration with an interdisciplinary team is recommended. Primary care, sports medicine, or neurology should be involved in the management of patients with SRC. Choice of which of those 3 specialties in particular will depend on comfort level and experience with managing SRC of the individual providers in question as well as availability of each provider type in a given community.
Additionally, psychiatrists may wonder if and when they should refer patients with SRC for neuroimaging. Because SRC is a functional, rather than structural, brain disturbance, neuroimaging is not typically pursued because results would be expected to be normal.3 However, when in doubt, consultation with the interdisciplinary team can guide this decision. Factors that may lead to a decision to obtain neuroimaging include:
- an abnormal neurologic examination
- prolonged loss of consciousness
- unexpected persistence of symptoms (eg, 6 to 12 weeks)
- worsening symptoms.22
Continue to: If imaging is deemed necessary...
If imaging is deemed necessary for a patient with an acute SRC, brain CT is typically the imaging modality of choice; however, if imaging is deemed necessary due to the persistence of symptoms, then MRI is often the preferred test because it provides more detailed information and does not expose the patient to ionizing radiation.22 While results are often normal, the ordering clinician should be prepared for the possibility of incidental findings, such as cysts or aneurysms, and the need for further consultation with other clinicians to weigh in on such findings.22
CASE CONTINUED
Ms. J is prescribed extended-release venlafaxine, 37.5 mg every morning for 5 days, and then is switched to 75 mg every morning. The psychiatrist hopes that venlafaxine might simultaneously offer benefit for Ms. J’s depression and migraine headaches. Venlafaxine is not FDA-approved for migraine, and there is more evidence supporting TCAs for preventing migraine. However, Ms. J is adamant that she does not want to take a medication, such as a TCA, that could cause weight gain or sedation, which could be problematic in her sport. The psychiatrist also tells Ms. J to avoid substances of abuse, and emphasizes the importance of good sleep hygiene. Finally, the psychiatrist communicates with the interdisciplinary medical team, which is helping Ms. J with gradual return-to-school and return-to-sport strategies and ensuring continued social involvement with the team even as she is held out from sport.
Ultimately, Ms. J’s extended-release venlafaxine is titrated to 150 mg every morning. After 2 months on this dose, her depressive symptoms remit. After her other symptoms remit, Ms. J has difficulty returning to certain practice drills that remind her of what she was doing when she sustained the SRC. She says that while participating in these drills, she has intrusive thoughts and images of the experience of her most recent concussion. She works with her psychiatrist on a gradual program of exposure therapy so she can return to all types of practice. Ms. J says she wishes to continue playing volleyball; however, together with her parents and treatment team, she decides that any additional SRCs might lead her to retire from the sport.
Bottom Line
Psychiatric symptoms are common after sport-related concussion (SRC). The nature of the relationship between concussion and mental health is not firmly established. Post-SRC psychiatric symptoms need to be carefully managed to avoid unnecessary treatment or restrictions.
Related Resources
- National Collegiate Athletic Association. Concussion. www.ncaa.org/sport-science-institute/concussion.
- American Academy of Neurology. Sports concussion resources. www.aan.com/tools-and-resources/practicing-neurologists-administrators/patient-resources/sports-concussion-resources. Published 2020.
Drug Brand Names
Trazodone • Desyrel
Venlafaxine • Effexor
1. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589-598.
2. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13-29.
3. McCrory P, Meeuwisse W, Dvor˘ák J, et al. Consensus statement on concussion in sport—the 5th International Conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847.
4. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667-699.
5. Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5): background and rationale. Br J Sports Med. 2017;51:848-850.
6. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65(7):601.
7. Rice SM, Parker AG, Rosenbaum S, et al. Sport-related concussion outcomes in elite athletes: a systematic review. Sports Med. 2018;48(2):447-465.
8. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-255.
9. Hutchison MG, Di Battista AP, McCoskey J, et al. Systematic review of mental health measures associated with concussive and subconcussive head trauma in former athletes. Int J Psychophysiol. 2018;132(Pt A):55-61.
10. Kerr GA, Stirling AE. Parents’ reflections on their child’s experiences of emotionally abusive coaching practices. J Appl Sport Psychol. 2012;24(2):191-206.
11. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909.
12. Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. Am J Sports Med. 2016;44(10):2486-2491.
13. Fralick M, Sy E, Hassan A, et al. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2018;76(2):144-151.
14. Brassil HE, Salvatore AP. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin Transl Med. 2018;7:25.
15. Bateman A, Morgan KAD. The postinjury psychological sequelae of high-level Jamaican athletes: exploration of a posttraumatic stress disorder-self-efficacy conceptualization. J Sport Rehabil. 2019;28(2):144-152.
16. Brewer BW, Van Raalte JL, Cornelius AE, et al. Psychological factors, rehabilitation adherence, and rehabilitation outcome after anterior cruciate ligament reconstruction. Rehabil Psychol. 2000;45(1):20-37.
17. Putukian M, Echemendia RJ. Psychological aspects of serious head injury in the competitive athlete. Clin Sports Med. 2003;22(33):617-630.
18. James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among Veterans with and without PTSD and mild TBI. Mil Med. 2014;179(4):357-363.
19. Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;47(1):15-26.
20. Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med. 2016;26(2):120-127.
21. Esfandiari A, Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2011;30(3):611-627.
22. Mahooti N. Sport-related concussion: acute management and chronic postconcussive issues. Chld Adolesc Psychiatric Clin N Am. 2018;27(1):93-108.
23. Kontos AP, Covassin T, Elbin RJ, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
24. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Clin J Sport Med. 2019;29(2):87-100.
25. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current Sports Medicine Reports. 2013;12(6):370-376.
26. Schneider KJ, Iverson GL, Emery CA, et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304-307.
27. Wayment HA, Huffman AH. Psychosocial experiences of concussed collegiate athletes: the role of emotional support in the recovery process. J Am Coll Health. 2020;68(4):438-443.
28. Todd R, Bhalerao S, Vu MT, et al. Understanding the psychiatric effects of concussion on constructed identity in hockey players: implications for health professionals. PLoS ONE. 2018;13(2):e0192125.
29. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140.
30. Gaetz M. The multi-factorial origins of chronic traumatic encephalopathy (CTE) symptomatology in post-career athletes: the athlete post-career adjustment (AP-CA) model. Med Hypotheses. 2017;102:130-143.
31. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124.
32. Broglio SP, Collins MW, Williams RM, et al. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
33. Arciniegas DB, Silver JM, McAllister TW. Stimulants and acetylcholinesterase inhibitors for the treatment of cognitive impairment after traumatic brain injury. Psychopharm Review. 2008;43(12):91-97.
34. Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
35. Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity/mortality after craniocerebral trauma. J Pineal Res. 2007;42(1):1-11.
36. Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47(2):134-142.
37. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
1. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589-598.
2. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13-29.
3. McCrory P, Meeuwisse W, Dvor˘ák J, et al. Consensus statement on concussion in sport—the 5th International Conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847.
4. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667-699.
5. Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5): background and rationale. Br J Sports Med. 2017;51:848-850.
6. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65(7):601.
7. Rice SM, Parker AG, Rosenbaum S, et al. Sport-related concussion outcomes in elite athletes: a systematic review. Sports Med. 2018;48(2):447-465.
8. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-255.
9. Hutchison MG, Di Battista AP, McCoskey J, et al. Systematic review of mental health measures associated with concussive and subconcussive head trauma in former athletes. Int J Psychophysiol. 2018;132(Pt A):55-61.
10. Kerr GA, Stirling AE. Parents’ reflections on their child’s experiences of emotionally abusive coaching practices. J Appl Sport Psychol. 2012;24(2):191-206.
11. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909.
12. Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. Am J Sports Med. 2016;44(10):2486-2491.
13. Fralick M, Sy E, Hassan A, et al. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2018;76(2):144-151.
14. Brassil HE, Salvatore AP. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin Transl Med. 2018;7:25.
15. Bateman A, Morgan KAD. The postinjury psychological sequelae of high-level Jamaican athletes: exploration of a posttraumatic stress disorder-self-efficacy conceptualization. J Sport Rehabil. 2019;28(2):144-152.
16. Brewer BW, Van Raalte JL, Cornelius AE, et al. Psychological factors, rehabilitation adherence, and rehabilitation outcome after anterior cruciate ligament reconstruction. Rehabil Psychol. 2000;45(1):20-37.
17. Putukian M, Echemendia RJ. Psychological aspects of serious head injury in the competitive athlete. Clin Sports Med. 2003;22(33):617-630.
18. James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among Veterans with and without PTSD and mild TBI. Mil Med. 2014;179(4):357-363.
19. Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;47(1):15-26.
20. Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med. 2016;26(2):120-127.
21. Esfandiari A, Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2011;30(3):611-627.
22. Mahooti N. Sport-related concussion: acute management and chronic postconcussive issues. Chld Adolesc Psychiatric Clin N Am. 2018;27(1):93-108.
23. Kontos AP, Covassin T, Elbin RJ, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
24. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Clin J Sport Med. 2019;29(2):87-100.
25. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current Sports Medicine Reports. 2013;12(6):370-376.
26. Schneider KJ, Iverson GL, Emery CA, et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304-307.
27. Wayment HA, Huffman AH. Psychosocial experiences of concussed collegiate athletes: the role of emotional support in the recovery process. J Am Coll Health. 2020;68(4):438-443.
28. Todd R, Bhalerao S, Vu MT, et al. Understanding the psychiatric effects of concussion on constructed identity in hockey players: implications for health professionals. PLoS ONE. 2018;13(2):e0192125.
29. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140.
30. Gaetz M. The multi-factorial origins of chronic traumatic encephalopathy (CTE) symptomatology in post-career athletes: the athlete post-career adjustment (AP-CA) model. Med Hypotheses. 2017;102:130-143.
31. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124.
32. Broglio SP, Collins MW, Williams RM, et al. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
33. Arciniegas DB, Silver JM, McAllister TW. Stimulants and acetylcholinesterase inhibitors for the treatment of cognitive impairment after traumatic brain injury. Psychopharm Review. 2008;43(12):91-97.
34. Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
35. Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity/mortality after craniocerebral trauma. J Pineal Res. 2007;42(1):1-11.
36. Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47(2):134-142.
37. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
How to best use digital technology to help your patients
As psychiatrists, we are increasingly using digital technology, such as e-mail, video conferencing, social media, and text messaging, to communicate with and even treat our patients.1 The benefits of using digital technology for treating patients include, but are not limited to, enhancing access to psychiatric services that are unavailable due to a patient’s geographical location and/or physical disability; providing more cost‐effective delivery of services; and creating more ways for patients to communicate with their physicians.1 While there are benefits to using digital technology, there are also possible repercussions, such as breaches of confidentiality or boundary violations.2 Although there is no evidence-based guidance about how to best use digital technology in patient care,3 the following approaches can help you protect your patients and minimize your liability.
Assess competence. Determine how familiar and comfortable both you and your patient are with the specific software and/or devices you intend to use. Confirm that your patient can access the technology, and inform them of the benefits and risks of using digital technology in their care.1
Create a written policy about your use of digital technology, and review it with all patients to explain how it will be used in their treatment.1 This policy should include a back-up plan in the event of technology failures.1 It should clearly explain that the information gathered with this technology can become part of the patient’s medical record. It should also prohibit patients from using their devices to record other patients in the waiting room or other areas. Such a policy could enhance the protection of private information and help maintain clear boundaries.1 Review and update your policy as often as needed.
Obtain your patients’ written consent to use digital technology. If you want to post information about your patients on social media, obtain their written consent to do so, and mutually agree as to what information would be posted. This should not include their identity or confidential information.1
Do not accept friend requests or contact requests from current or former patients on any social networking platform. Do not follow your patients’ blogs, Twitter accounts, or any other accounts. Be aware that if you and your patients share the same “friend” network on social media, this may create boundary confusion, inappropriate dual relationships, and potential conflicts of interest.1 Keep personal and professional accounts separate to maintain appropriate boundaries and minimize compromising patient confidentiality. Do not post private information on professional practice accounts, and do not link/sync your personal accounts with professional accounts.
Do not store patient information on your personal electronic devices because these devices could be lost or hacked. Avoid contacting your patients via non-secured platforms because doing so could compromise patient confidentiality. Use encrypted software and firewalls for communicating with your patients and storing their information.1 Also, periodically assess your confidentiality policies and procedures to ensure compliance with appropriate statutes and laws.1
1. Reamer FG. Evolving standards of care in the age of cybertechnology. Behav Sci Law. 2018;36(2):257-269.
2. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499, 520.
3. Logghe HJ, Boeck MA, Gusani NJ, et al. Best practices for surgeons’ social media use: statement of the Resident and Associate Society of the American College of Surgeons. J Am Coll Surg. 2018;226(3):317-327.
As psychiatrists, we are increasingly using digital technology, such as e-mail, video conferencing, social media, and text messaging, to communicate with and even treat our patients.1 The benefits of using digital technology for treating patients include, but are not limited to, enhancing access to psychiatric services that are unavailable due to a patient’s geographical location and/or physical disability; providing more cost‐effective delivery of services; and creating more ways for patients to communicate with their physicians.1 While there are benefits to using digital technology, there are also possible repercussions, such as breaches of confidentiality or boundary violations.2 Although there is no evidence-based guidance about how to best use digital technology in patient care,3 the following approaches can help you protect your patients and minimize your liability.
Assess competence. Determine how familiar and comfortable both you and your patient are with the specific software and/or devices you intend to use. Confirm that your patient can access the technology, and inform them of the benefits and risks of using digital technology in their care.1
Create a written policy about your use of digital technology, and review it with all patients to explain how it will be used in their treatment.1 This policy should include a back-up plan in the event of technology failures.1 It should clearly explain that the information gathered with this technology can become part of the patient’s medical record. It should also prohibit patients from using their devices to record other patients in the waiting room or other areas. Such a policy could enhance the protection of private information and help maintain clear boundaries.1 Review and update your policy as often as needed.
Obtain your patients’ written consent to use digital technology. If you want to post information about your patients on social media, obtain their written consent to do so, and mutually agree as to what information would be posted. This should not include their identity or confidential information.1
Do not accept friend requests or contact requests from current or former patients on any social networking platform. Do not follow your patients’ blogs, Twitter accounts, or any other accounts. Be aware that if you and your patients share the same “friend” network on social media, this may create boundary confusion, inappropriate dual relationships, and potential conflicts of interest.1 Keep personal and professional accounts separate to maintain appropriate boundaries and minimize compromising patient confidentiality. Do not post private information on professional practice accounts, and do not link/sync your personal accounts with professional accounts.
Do not store patient information on your personal electronic devices because these devices could be lost or hacked. Avoid contacting your patients via non-secured platforms because doing so could compromise patient confidentiality. Use encrypted software and firewalls for communicating with your patients and storing their information.1 Also, periodically assess your confidentiality policies and procedures to ensure compliance with appropriate statutes and laws.1
As psychiatrists, we are increasingly using digital technology, such as e-mail, video conferencing, social media, and text messaging, to communicate with and even treat our patients.1 The benefits of using digital technology for treating patients include, but are not limited to, enhancing access to psychiatric services that are unavailable due to a patient’s geographical location and/or physical disability; providing more cost‐effective delivery of services; and creating more ways for patients to communicate with their physicians.1 While there are benefits to using digital technology, there are also possible repercussions, such as breaches of confidentiality or boundary violations.2 Although there is no evidence-based guidance about how to best use digital technology in patient care,3 the following approaches can help you protect your patients and minimize your liability.
Assess competence. Determine how familiar and comfortable both you and your patient are with the specific software and/or devices you intend to use. Confirm that your patient can access the technology, and inform them of the benefits and risks of using digital technology in their care.1
Create a written policy about your use of digital technology, and review it with all patients to explain how it will be used in their treatment.1 This policy should include a back-up plan in the event of technology failures.1 It should clearly explain that the information gathered with this technology can become part of the patient’s medical record. It should also prohibit patients from using their devices to record other patients in the waiting room or other areas. Such a policy could enhance the protection of private information and help maintain clear boundaries.1 Review and update your policy as often as needed.
Obtain your patients’ written consent to use digital technology. If you want to post information about your patients on social media, obtain their written consent to do so, and mutually agree as to what information would be posted. This should not include their identity or confidential information.1
Do not accept friend requests or contact requests from current or former patients on any social networking platform. Do not follow your patients’ blogs, Twitter accounts, or any other accounts. Be aware that if you and your patients share the same “friend” network on social media, this may create boundary confusion, inappropriate dual relationships, and potential conflicts of interest.1 Keep personal and professional accounts separate to maintain appropriate boundaries and minimize compromising patient confidentiality. Do not post private information on professional practice accounts, and do not link/sync your personal accounts with professional accounts.
Do not store patient information on your personal electronic devices because these devices could be lost or hacked. Avoid contacting your patients via non-secured platforms because doing so could compromise patient confidentiality. Use encrypted software and firewalls for communicating with your patients and storing their information.1 Also, periodically assess your confidentiality policies and procedures to ensure compliance with appropriate statutes and laws.1
1. Reamer FG. Evolving standards of care in the age of cybertechnology. Behav Sci Law. 2018;36(2):257-269.
2. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499, 520.
3. Logghe HJ, Boeck MA, Gusani NJ, et al. Best practices for surgeons’ social media use: statement of the Resident and Associate Society of the American College of Surgeons. J Am Coll Surg. 2018;226(3):317-327.
1. Reamer FG. Evolving standards of care in the age of cybertechnology. Behav Sci Law. 2018;36(2):257-269.
2. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499, 520.
3. Logghe HJ, Boeck MA, Gusani NJ, et al. Best practices for surgeons’ social media use: statement of the Resident and Associate Society of the American College of Surgeons. J Am Coll Surg. 2018;226(3):317-327.