User login
New ‘legal’ highs: Kratom and methoxetamine
The demand for “legal highs”—intoxicating natural or synthetic substances that are not prohibited by law—continues to increase. Young adults may use these substances, which are widely available on the internet, at “head shops,” and at gas stations. Such substances frequently cause adverse medical and psychiatric effects, exemplified by recent reports concerning the dangers of using synthetic cannabinoids (eg, “Spice,” “K2”) and synthetic cathinones (“bath salts”). Although these 2 substances now are illegal in many jurisdictions, other novel substances of misuse remain legal and widely available, including Kratom and methoxetamine.
Because these substances usually are not detectable on standard urine toxicology screens, clinicians need to be aware of them to be able to take an accurate substance use history, consider possible dangerous interactions with prescribed psychotropics, and address medical and psychiatric complications.
Kratom is an herbal product derived from Mitragyna speciosa, a plant native to Southeast Asia. Traditionally used as a medicinal herb, it increasingly is being used for recreational purposes and remains legal and widely available in the United States. Kratom’s leaves contain multiple alkaloids, including mitragynine and 7-hydroxymitragynine, which are believed to act as agonists at the μ-opioid receptor. Mitragynine also may have agonist activity at post-synaptic
α2-adrenergic receptors, as well as antagonist activity at 5-HT2A receptors.1 Mitragynine is 13 times more potent than morphine, and 7-hydroxymitragynine is 4 times more potent than mitragynine.2
Kratom is available as leaves, powdered leaves, or gum. It can be smoked, brewed into tea, or mixed with liquid and ingested. Effects are dose-dependent; lower doses tend to produce a stimulant effect and higher doses produce an opioid effect. A typical dose is 1 to 8 g.3 Users may take Kratom to experience euphoria or analgesia, or to self-treat opioid withdrawal symptoms.3 Kratom withdrawal syndrome shares many features of classic opioid withdrawal—diarrhea, rhinorrhea, cravings, anxiety, tremor, myalgia, sweating, and irritability—but has been reported to be less severe and shorter-lasting.1 Kratom withdrawal, like opioid withdrawal, may respond to supportive care in combination with opioid-replacement therapy. Airway management and naloxone treatment may be needed on an emergent basis if a user develops respiratory depression.2 There have been case reports of seizures occurring following Kratom use.2
Methoxetamine is a ketamine analog originally developed as an alternative to ketamine. It isn’t classified as a controlled substance in the United States and is available on the internet.2 Methoxetamine is a white powder typically snorted or taken sublingually, although it can be injected intramuscularly. Because methoxetamine’s structure is similar to ketamine, its mechanism of action is assumed to involve glutamate N-methyl-d-aspartate receptor antagonism and dopamine reuptake inhibition. Doses range from 20 to 100 mg orally and 10 to 50 mg when injected. Effects may not be apparent for 30 to 90 minutes after the drug is snorted, which may cause users to take another dose or ingest a different substance, possibly leading to synergistic adverse effects. Effects may emerge within 5 minutes when injected. The duration of effect generally is 5 to 7 hours—notably longer than ketamine—but as little as 1 hour when injected.
No clinical human or animal studies have been conducted on methoxetamine, which makes it difficult to ascertain the drug’s true clinical and toxic effects; instead, these effects must be surmised from user reports and case studies. Desired effects described by users are similar to those of ketamine: dissociation, short-term mood elevation, visual hallucinations, and alteration of sensory experiences. Reported adverse effects include catatonia, confusion, agitation, and depression.4 In addition, methoxetamine may induce sympathomimetic toxicity as evidenced by tachycardia and hypertension. Researchers have suggested that patients who experience methoxetamine toxicity and require emergency treatment be managed with supportive care and benzodiazepines.5
Staying current is key
New and potentially dangerous substances are being produced so quickly distributors are able to stay ahead of regulatory efforts. When one substance is declared illegal, another related substance quickly is available to take its place. To provide the best care for our patients, it is essential for psychiatrists to stay up-to-date about these novel substances.
Disclosure
Dr. Troy reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
2. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), Kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
3. Boyer EW, Babu KM, Macalino GE. Self-treatment of opioid withdrawal with a dietary supplement, Kratom. Am J Addict. 2007;16(5):352-356.
4. Corazza O, Schifano F, Simonato P, et al. Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine. Hum Psychopharmacol. 2012;27(2):
145-149.
5. Wood DM, Davies S, Puchnarewicz M, et al. Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur J Clin Pharmacol. 2012; 68(5):853-856.
The demand for “legal highs”—intoxicating natural or synthetic substances that are not prohibited by law—continues to increase. Young adults may use these substances, which are widely available on the internet, at “head shops,” and at gas stations. Such substances frequently cause adverse medical and psychiatric effects, exemplified by recent reports concerning the dangers of using synthetic cannabinoids (eg, “Spice,” “K2”) and synthetic cathinones (“bath salts”). Although these 2 substances now are illegal in many jurisdictions, other novel substances of misuse remain legal and widely available, including Kratom and methoxetamine.
Because these substances usually are not detectable on standard urine toxicology screens, clinicians need to be aware of them to be able to take an accurate substance use history, consider possible dangerous interactions with prescribed psychotropics, and address medical and psychiatric complications.
Kratom is an herbal product derived from Mitragyna speciosa, a plant native to Southeast Asia. Traditionally used as a medicinal herb, it increasingly is being used for recreational purposes and remains legal and widely available in the United States. Kratom’s leaves contain multiple alkaloids, including mitragynine and 7-hydroxymitragynine, which are believed to act as agonists at the μ-opioid receptor. Mitragynine also may have agonist activity at post-synaptic
α2-adrenergic receptors, as well as antagonist activity at 5-HT2A receptors.1 Mitragynine is 13 times more potent than morphine, and 7-hydroxymitragynine is 4 times more potent than mitragynine.2
Kratom is available as leaves, powdered leaves, or gum. It can be smoked, brewed into tea, or mixed with liquid and ingested. Effects are dose-dependent; lower doses tend to produce a stimulant effect and higher doses produce an opioid effect. A typical dose is 1 to 8 g.3 Users may take Kratom to experience euphoria or analgesia, or to self-treat opioid withdrawal symptoms.3 Kratom withdrawal syndrome shares many features of classic opioid withdrawal—diarrhea, rhinorrhea, cravings, anxiety, tremor, myalgia, sweating, and irritability—but has been reported to be less severe and shorter-lasting.1 Kratom withdrawal, like opioid withdrawal, may respond to supportive care in combination with opioid-replacement therapy. Airway management and naloxone treatment may be needed on an emergent basis if a user develops respiratory depression.2 There have been case reports of seizures occurring following Kratom use.2
Methoxetamine is a ketamine analog originally developed as an alternative to ketamine. It isn’t classified as a controlled substance in the United States and is available on the internet.2 Methoxetamine is a white powder typically snorted or taken sublingually, although it can be injected intramuscularly. Because methoxetamine’s structure is similar to ketamine, its mechanism of action is assumed to involve glutamate N-methyl-d-aspartate receptor antagonism and dopamine reuptake inhibition. Doses range from 20 to 100 mg orally and 10 to 50 mg when injected. Effects may not be apparent for 30 to 90 minutes after the drug is snorted, which may cause users to take another dose or ingest a different substance, possibly leading to synergistic adverse effects. Effects may emerge within 5 minutes when injected. The duration of effect generally is 5 to 7 hours—notably longer than ketamine—but as little as 1 hour when injected.
No clinical human or animal studies have been conducted on methoxetamine, which makes it difficult to ascertain the drug’s true clinical and toxic effects; instead, these effects must be surmised from user reports and case studies. Desired effects described by users are similar to those of ketamine: dissociation, short-term mood elevation, visual hallucinations, and alteration of sensory experiences. Reported adverse effects include catatonia, confusion, agitation, and depression.4 In addition, methoxetamine may induce sympathomimetic toxicity as evidenced by tachycardia and hypertension. Researchers have suggested that patients who experience methoxetamine toxicity and require emergency treatment be managed with supportive care and benzodiazepines.5
Staying current is key
New and potentially dangerous substances are being produced so quickly distributors are able to stay ahead of regulatory efforts. When one substance is declared illegal, another related substance quickly is available to take its place. To provide the best care for our patients, it is essential for psychiatrists to stay up-to-date about these novel substances.
Disclosure
Dr. Troy reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
The demand for “legal highs”—intoxicating natural or synthetic substances that are not prohibited by law—continues to increase. Young adults may use these substances, which are widely available on the internet, at “head shops,” and at gas stations. Such substances frequently cause adverse medical and psychiatric effects, exemplified by recent reports concerning the dangers of using synthetic cannabinoids (eg, “Spice,” “K2”) and synthetic cathinones (“bath salts”). Although these 2 substances now are illegal in many jurisdictions, other novel substances of misuse remain legal and widely available, including Kratom and methoxetamine.
Because these substances usually are not detectable on standard urine toxicology screens, clinicians need to be aware of them to be able to take an accurate substance use history, consider possible dangerous interactions with prescribed psychotropics, and address medical and psychiatric complications.
Kratom is an herbal product derived from Mitragyna speciosa, a plant native to Southeast Asia. Traditionally used as a medicinal herb, it increasingly is being used for recreational purposes and remains legal and widely available in the United States. Kratom’s leaves contain multiple alkaloids, including mitragynine and 7-hydroxymitragynine, which are believed to act as agonists at the μ-opioid receptor. Mitragynine also may have agonist activity at post-synaptic
α2-adrenergic receptors, as well as antagonist activity at 5-HT2A receptors.1 Mitragynine is 13 times more potent than morphine, and 7-hydroxymitragynine is 4 times more potent than mitragynine.2
Kratom is available as leaves, powdered leaves, or gum. It can be smoked, brewed into tea, or mixed with liquid and ingested. Effects are dose-dependent; lower doses tend to produce a stimulant effect and higher doses produce an opioid effect. A typical dose is 1 to 8 g.3 Users may take Kratom to experience euphoria or analgesia, or to self-treat opioid withdrawal symptoms.3 Kratom withdrawal syndrome shares many features of classic opioid withdrawal—diarrhea, rhinorrhea, cravings, anxiety, tremor, myalgia, sweating, and irritability—but has been reported to be less severe and shorter-lasting.1 Kratom withdrawal, like opioid withdrawal, may respond to supportive care in combination with opioid-replacement therapy. Airway management and naloxone treatment may be needed on an emergent basis if a user develops respiratory depression.2 There have been case reports of seizures occurring following Kratom use.2
Methoxetamine is a ketamine analog originally developed as an alternative to ketamine. It isn’t classified as a controlled substance in the United States and is available on the internet.2 Methoxetamine is a white powder typically snorted or taken sublingually, although it can be injected intramuscularly. Because methoxetamine’s structure is similar to ketamine, its mechanism of action is assumed to involve glutamate N-methyl-d-aspartate receptor antagonism and dopamine reuptake inhibition. Doses range from 20 to 100 mg orally and 10 to 50 mg when injected. Effects may not be apparent for 30 to 90 minutes after the drug is snorted, which may cause users to take another dose or ingest a different substance, possibly leading to synergistic adverse effects. Effects may emerge within 5 minutes when injected. The duration of effect generally is 5 to 7 hours—notably longer than ketamine—but as little as 1 hour when injected.
No clinical human or animal studies have been conducted on methoxetamine, which makes it difficult to ascertain the drug’s true clinical and toxic effects; instead, these effects must be surmised from user reports and case studies. Desired effects described by users are similar to those of ketamine: dissociation, short-term mood elevation, visual hallucinations, and alteration of sensory experiences. Reported adverse effects include catatonia, confusion, agitation, and depression.4 In addition, methoxetamine may induce sympathomimetic toxicity as evidenced by tachycardia and hypertension. Researchers have suggested that patients who experience methoxetamine toxicity and require emergency treatment be managed with supportive care and benzodiazepines.5
Staying current is key
New and potentially dangerous substances are being produced so quickly distributors are able to stay ahead of regulatory efforts. When one substance is declared illegal, another related substance quickly is available to take its place. To provide the best care for our patients, it is essential for psychiatrists to stay up-to-date about these novel substances.
Disclosure
Dr. Troy reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
2. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), Kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
3. Boyer EW, Babu KM, Macalino GE. Self-treatment of opioid withdrawal with a dietary supplement, Kratom. Am J Addict. 2007;16(5):352-356.
4. Corazza O, Schifano F, Simonato P, et al. Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine. Hum Psychopharmacol. 2012;27(2):
145-149.
5. Wood DM, Davies S, Puchnarewicz M, et al. Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur J Clin Pharmacol. 2012; 68(5):853-856.
1. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
2. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), Kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
3. Boyer EW, Babu KM, Macalino GE. Self-treatment of opioid withdrawal with a dietary supplement, Kratom. Am J Addict. 2007;16(5):352-356.
4. Corazza O, Schifano F, Simonato P, et al. Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine. Hum Psychopharmacol. 2012;27(2):
145-149.
5. Wood DM, Davies S, Puchnarewicz M, et al. Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur J Clin Pharmacol. 2012; 68(5):853-856.
Bowel perforation causes woman’s death: $1.5M verdict
A 46-year-old woman underwent laparoscopic supracervical hysterectomy to remove her uterus but preserve her cervix. Postsurgically, she had difficulty breathing deeply and reported abdominal pain. The nurses and on-call physician reassured her that she was experiencing “gas pains” due to insufflation. After same-day discharge, she stayed in a motel room to avoid a second-floor bedroom at home.
She called the gynecologist’s office the following day to report continued pain and severe hot flashes and sweats. The gynecologist instructed his nurse to advise the patient to stop taking her birth control pill (ethinyl estradiol/norethindrone, Microgestin) and “to ride out” the hot flashes.
The woman was found dead in her motel room the next morning. An autopsy revealed a perforated small intestine with leakage into the abdominal cavity causing sepsis, multi-organ failure, and death.
ESTATE’S CLAIM The gynecologist reviewed the medical records and found an error in the operative report, but he made no addendum or late entry to correct the operative report. His defense counsel instructed him to draft a letter clarifying the surgery; this clarification was given to defense experts. The description of the procedure in the clarification was different from what was described in the medical records. For example, the clarification reported making 4 incisions for 4 trocars; the operative report indicated using 3 trocars. The pathologist and 2 nurses who treated the patient after surgery confirmed that there were 3 trocar incisions. The pathologist found no tissue necrosis at or around the perforation site, indicating that the perforation likely occurred during surgery.
PHYSICIAN’S DEFENSE Bowel perforation is a known complication of the procedure. The perforation was not present at the time of surgery because leakage of bowel content would have been obvious.
VERDICT A $1.5 million Virginia settlement was reached.
Retained products of conception after D&C
When sonography indicated that a 30-year-old woman was pregnant, she decided to abort the pregnancy and was given mifepristone.
Another sonogram 5 weeks later showed retained products of conception within the uterus. An ObGyn performed dilation and curettage (D&C) at an outpatient clinic. Because he believed the cannula did not remove everything, he used a curette to scrape the uterus. After the patient was dizzy, hypotensive, and in pain for 4 hours, an ambulance transported her to a hospital. Perforations of the uterus and sigmoid colon were discovered and repaired during emergency surgery. The patient has a large scar on her abdomen.
PATIENT'S CLAIM The ObGyn did not perform the D&C properly and perforated the uterus and colon. An earlier response to symptoms could have prevented repair surgery. Damage to the uterus may now preclude her from having a successful pregnancy.
DEFENDANTS’ DEFENSE The ObGyn argued that the aborted pregnancy was ectopic; spontaneous rupture caused the perforations.
VERDICT A $340,000 New York settlement was reached with the ObGyn. By the time of trial, the clinic had closed.
Wrong-site biopsy; records altered
A 40-year-old woman underwent excisional breast biopsy. The wrong lump was removed and the woman had to have another procedure.
PATIENT'S CLAIM The hospital’s nursing staff failed to properly mark the operative site. The breast surgeon did not confirm that the markings were correct. The surgeon altered the written operative report after the surgery to conceal negligence.
DEFENDANTS’ DEFENSE The nurses properly marked the biopsy site, but the surgeon chose another route. The surgeon edited the original report to reflect events that occurred during surgery that had not been included in the original dictation. The added material gave justification for performing the procedure at a different site than originally intended.
VERDICT A $15,500 Connecticut verdict was returned.
Second twin has CP and brain damage: $10M settlement
A woman gave birth to twins at an Army hospital. The first twin was delivered without complications. The second twin developed a prolapsed cord during delivery of the first twin. A resident and the attending physician allowed the mother to continue with vaginal delivery. The heart-rate monitor showed fetal distress, but the medical staff did not respond. After an hour, another physician was consulted, and he ordered immediate delivery. The attending physician decided to continue with vaginal delivery using forceps, but it took 15 minutes to locate forceps in the hospital. The infant suffered severe brain damage and cerebral palsy. She will require 24-hour nursing care for life, including treatment of a tracheostomy.
PARENTS' CLAIM The physicians were negligent for not reacting to non-reassuring monitor strips and for allowing the vaginal delivery to continue. An emergency cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $10 million North Carolina settlement was reached for past medical bills and future care.
Faulty biopsies: breast cancer diagnosis missed
In September 2006, a 40-year-old woman underwent breast sonography. A radiologist, Dr. A, reported finding a mass and a smaller nodule in the right breast, and recommended a biopsy of each area. Two weeks later, a second radiologist, Dr. B, biopsied the larger of the two areas and diagnosed a hyalinized fibroadenoma. He did not biopsy the smaller growth, but reported it as a benign nodule. He recommended more frequent screenings. The patient was referred to a surgeon, who determined that she should be seen in 6 months.
In June 2007, the patient underwent right-breast sonography that revealed cysts and three nodules. The surgeon recommended a biopsy, but the biopsy was performed on only two of three nodules. A third radiologist, Dr. C, determined that the nodules were all benign.
In November 2007, when the patient reported a painful lump in her right breast, her gynecologist ordered mammography, which revealed lesions. A biopsy revealed that one lesion was stage III invasive ductal carcinoma. The patient underwent extensive treatment, including a mastectomy, lymphadenectomy, chemotherapy, and radiation therapy, and prophylactic surgical reduction of the left breast.
PATIENT'S CLAIM The cancer should have been diagnosed in September 2006. Prompt treatment would have decreased the progression of the disease. The September 2006 biopsy should have included both lumps, as recommended by Dr. A.
DEFENDANTS’ DEFENSE There was no indication of cancer in September 2006. Reasonable follow-up care was given.
VERDICT A New York defense verdict was returned.
Tumor not found during surgery; BSO performed
A 41-year-old woman underwent surgery to remove a pelvic tumor in November 2004. The gynecologist was unable to locate the tumor during surgery. He performed bilateral salpingo-oophorectomy (BSO) because of a visual diagnosis of endometriosis. In August 2005, the patient underwent surgical removal of the tumor by another surgeon. She was hospitalized for several weeks and suffered a large scar that required additional surgery.
PATIENT'S CLAIM BSO was unnecessary, and caused early menopause, with vaginal atrophy and dryness, depression, fatigue, insomnia, loss of hair, and other symptoms.
The patient claimed lack of informed consent. From Ecuador, the patient’s command of English was not sufficient for her to completely understand the consent form; an interpreter should have been provided.
DEFENDANTS’ DEFENSE BSO did not cause a significant acceleration of the onset of menopause. It was necessary to treat the endometriosis.
The patient signed a consent form that included BSO. The patient did not indicate that she did not understand the language on the form; had she asked, an interpreter would have been provided.
VERDICT A $750,000 New York settlement was reached with the gynecologist and medical center.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
A 46-year-old woman underwent laparoscopic supracervical hysterectomy to remove her uterus but preserve her cervix. Postsurgically, she had difficulty breathing deeply and reported abdominal pain. The nurses and on-call physician reassured her that she was experiencing “gas pains” due to insufflation. After same-day discharge, she stayed in a motel room to avoid a second-floor bedroom at home.
She called the gynecologist’s office the following day to report continued pain and severe hot flashes and sweats. The gynecologist instructed his nurse to advise the patient to stop taking her birth control pill (ethinyl estradiol/norethindrone, Microgestin) and “to ride out” the hot flashes.
The woman was found dead in her motel room the next morning. An autopsy revealed a perforated small intestine with leakage into the abdominal cavity causing sepsis, multi-organ failure, and death.
ESTATE’S CLAIM The gynecologist reviewed the medical records and found an error in the operative report, but he made no addendum or late entry to correct the operative report. His defense counsel instructed him to draft a letter clarifying the surgery; this clarification was given to defense experts. The description of the procedure in the clarification was different from what was described in the medical records. For example, the clarification reported making 4 incisions for 4 trocars; the operative report indicated using 3 trocars. The pathologist and 2 nurses who treated the patient after surgery confirmed that there were 3 trocar incisions. The pathologist found no tissue necrosis at or around the perforation site, indicating that the perforation likely occurred during surgery.
PHYSICIAN’S DEFENSE Bowel perforation is a known complication of the procedure. The perforation was not present at the time of surgery because leakage of bowel content would have been obvious.
VERDICT A $1.5 million Virginia settlement was reached.
Retained products of conception after D&C
When sonography indicated that a 30-year-old woman was pregnant, she decided to abort the pregnancy and was given mifepristone.
Another sonogram 5 weeks later showed retained products of conception within the uterus. An ObGyn performed dilation and curettage (D&C) at an outpatient clinic. Because he believed the cannula did not remove everything, he used a curette to scrape the uterus. After the patient was dizzy, hypotensive, and in pain for 4 hours, an ambulance transported her to a hospital. Perforations of the uterus and sigmoid colon were discovered and repaired during emergency surgery. The patient has a large scar on her abdomen.
PATIENT'S CLAIM The ObGyn did not perform the D&C properly and perforated the uterus and colon. An earlier response to symptoms could have prevented repair surgery. Damage to the uterus may now preclude her from having a successful pregnancy.
DEFENDANTS’ DEFENSE The ObGyn argued that the aborted pregnancy was ectopic; spontaneous rupture caused the perforations.
VERDICT A $340,000 New York settlement was reached with the ObGyn. By the time of trial, the clinic had closed.
Wrong-site biopsy; records altered
A 40-year-old woman underwent excisional breast biopsy. The wrong lump was removed and the woman had to have another procedure.
PATIENT'S CLAIM The hospital’s nursing staff failed to properly mark the operative site. The breast surgeon did not confirm that the markings were correct. The surgeon altered the written operative report after the surgery to conceal negligence.
DEFENDANTS’ DEFENSE The nurses properly marked the biopsy site, but the surgeon chose another route. The surgeon edited the original report to reflect events that occurred during surgery that had not been included in the original dictation. The added material gave justification for performing the procedure at a different site than originally intended.
VERDICT A $15,500 Connecticut verdict was returned.
Second twin has CP and brain damage: $10M settlement
A woman gave birth to twins at an Army hospital. The first twin was delivered without complications. The second twin developed a prolapsed cord during delivery of the first twin. A resident and the attending physician allowed the mother to continue with vaginal delivery. The heart-rate monitor showed fetal distress, but the medical staff did not respond. After an hour, another physician was consulted, and he ordered immediate delivery. The attending physician decided to continue with vaginal delivery using forceps, but it took 15 minutes to locate forceps in the hospital. The infant suffered severe brain damage and cerebral palsy. She will require 24-hour nursing care for life, including treatment of a tracheostomy.
PARENTS' CLAIM The physicians were negligent for not reacting to non-reassuring monitor strips and for allowing the vaginal delivery to continue. An emergency cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $10 million North Carolina settlement was reached for past medical bills and future care.
Faulty biopsies: breast cancer diagnosis missed
In September 2006, a 40-year-old woman underwent breast sonography. A radiologist, Dr. A, reported finding a mass and a smaller nodule in the right breast, and recommended a biopsy of each area. Two weeks later, a second radiologist, Dr. B, biopsied the larger of the two areas and diagnosed a hyalinized fibroadenoma. He did not biopsy the smaller growth, but reported it as a benign nodule. He recommended more frequent screenings. The patient was referred to a surgeon, who determined that she should be seen in 6 months.
In June 2007, the patient underwent right-breast sonography that revealed cysts and three nodules. The surgeon recommended a biopsy, but the biopsy was performed on only two of three nodules. A third radiologist, Dr. C, determined that the nodules were all benign.
In November 2007, when the patient reported a painful lump in her right breast, her gynecologist ordered mammography, which revealed lesions. A biopsy revealed that one lesion was stage III invasive ductal carcinoma. The patient underwent extensive treatment, including a mastectomy, lymphadenectomy, chemotherapy, and radiation therapy, and prophylactic surgical reduction of the left breast.
PATIENT'S CLAIM The cancer should have been diagnosed in September 2006. Prompt treatment would have decreased the progression of the disease. The September 2006 biopsy should have included both lumps, as recommended by Dr. A.
DEFENDANTS’ DEFENSE There was no indication of cancer in September 2006. Reasonable follow-up care was given.
VERDICT A New York defense verdict was returned.
Tumor not found during surgery; BSO performed
A 41-year-old woman underwent surgery to remove a pelvic tumor in November 2004. The gynecologist was unable to locate the tumor during surgery. He performed bilateral salpingo-oophorectomy (BSO) because of a visual diagnosis of endometriosis. In August 2005, the patient underwent surgical removal of the tumor by another surgeon. She was hospitalized for several weeks and suffered a large scar that required additional surgery.
PATIENT'S CLAIM BSO was unnecessary, and caused early menopause, with vaginal atrophy and dryness, depression, fatigue, insomnia, loss of hair, and other symptoms.
The patient claimed lack of informed consent. From Ecuador, the patient’s command of English was not sufficient for her to completely understand the consent form; an interpreter should have been provided.
DEFENDANTS’ DEFENSE BSO did not cause a significant acceleration of the onset of menopause. It was necessary to treat the endometriosis.
The patient signed a consent form that included BSO. The patient did not indicate that she did not understand the language on the form; had she asked, an interpreter would have been provided.
VERDICT A $750,000 New York settlement was reached with the gynecologist and medical center.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
A 46-year-old woman underwent laparoscopic supracervical hysterectomy to remove her uterus but preserve her cervix. Postsurgically, she had difficulty breathing deeply and reported abdominal pain. The nurses and on-call physician reassured her that she was experiencing “gas pains” due to insufflation. After same-day discharge, she stayed in a motel room to avoid a second-floor bedroom at home.
She called the gynecologist’s office the following day to report continued pain and severe hot flashes and sweats. The gynecologist instructed his nurse to advise the patient to stop taking her birth control pill (ethinyl estradiol/norethindrone, Microgestin) and “to ride out” the hot flashes.
The woman was found dead in her motel room the next morning. An autopsy revealed a perforated small intestine with leakage into the abdominal cavity causing sepsis, multi-organ failure, and death.
ESTATE’S CLAIM The gynecologist reviewed the medical records and found an error in the operative report, but he made no addendum or late entry to correct the operative report. His defense counsel instructed him to draft a letter clarifying the surgery; this clarification was given to defense experts. The description of the procedure in the clarification was different from what was described in the medical records. For example, the clarification reported making 4 incisions for 4 trocars; the operative report indicated using 3 trocars. The pathologist and 2 nurses who treated the patient after surgery confirmed that there were 3 trocar incisions. The pathologist found no tissue necrosis at or around the perforation site, indicating that the perforation likely occurred during surgery.
PHYSICIAN’S DEFENSE Bowel perforation is a known complication of the procedure. The perforation was not present at the time of surgery because leakage of bowel content would have been obvious.
VERDICT A $1.5 million Virginia settlement was reached.
Retained products of conception after D&C
When sonography indicated that a 30-year-old woman was pregnant, she decided to abort the pregnancy and was given mifepristone.
Another sonogram 5 weeks later showed retained products of conception within the uterus. An ObGyn performed dilation and curettage (D&C) at an outpatient clinic. Because he believed the cannula did not remove everything, he used a curette to scrape the uterus. After the patient was dizzy, hypotensive, and in pain for 4 hours, an ambulance transported her to a hospital. Perforations of the uterus and sigmoid colon were discovered and repaired during emergency surgery. The patient has a large scar on her abdomen.
PATIENT'S CLAIM The ObGyn did not perform the D&C properly and perforated the uterus and colon. An earlier response to symptoms could have prevented repair surgery. Damage to the uterus may now preclude her from having a successful pregnancy.
DEFENDANTS’ DEFENSE The ObGyn argued that the aborted pregnancy was ectopic; spontaneous rupture caused the perforations.
VERDICT A $340,000 New York settlement was reached with the ObGyn. By the time of trial, the clinic had closed.
Wrong-site biopsy; records altered
A 40-year-old woman underwent excisional breast biopsy. The wrong lump was removed and the woman had to have another procedure.
PATIENT'S CLAIM The hospital’s nursing staff failed to properly mark the operative site. The breast surgeon did not confirm that the markings were correct. The surgeon altered the written operative report after the surgery to conceal negligence.
DEFENDANTS’ DEFENSE The nurses properly marked the biopsy site, but the surgeon chose another route. The surgeon edited the original report to reflect events that occurred during surgery that had not been included in the original dictation. The added material gave justification for performing the procedure at a different site than originally intended.
VERDICT A $15,500 Connecticut verdict was returned.
Second twin has CP and brain damage: $10M settlement
A woman gave birth to twins at an Army hospital. The first twin was delivered without complications. The second twin developed a prolapsed cord during delivery of the first twin. A resident and the attending physician allowed the mother to continue with vaginal delivery. The heart-rate monitor showed fetal distress, but the medical staff did not respond. After an hour, another physician was consulted, and he ordered immediate delivery. The attending physician decided to continue with vaginal delivery using forceps, but it took 15 minutes to locate forceps in the hospital. The infant suffered severe brain damage and cerebral palsy. She will require 24-hour nursing care for life, including treatment of a tracheostomy.
PARENTS' CLAIM The physicians were negligent for not reacting to non-reassuring monitor strips and for allowing the vaginal delivery to continue. An emergency cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $10 million North Carolina settlement was reached for past medical bills and future care.
Faulty biopsies: breast cancer diagnosis missed
In September 2006, a 40-year-old woman underwent breast sonography. A radiologist, Dr. A, reported finding a mass and a smaller nodule in the right breast, and recommended a biopsy of each area. Two weeks later, a second radiologist, Dr. B, biopsied the larger of the two areas and diagnosed a hyalinized fibroadenoma. He did not biopsy the smaller growth, but reported it as a benign nodule. He recommended more frequent screenings. The patient was referred to a surgeon, who determined that she should be seen in 6 months.
In June 2007, the patient underwent right-breast sonography that revealed cysts and three nodules. The surgeon recommended a biopsy, but the biopsy was performed on only two of three nodules. A third radiologist, Dr. C, determined that the nodules were all benign.
In November 2007, when the patient reported a painful lump in her right breast, her gynecologist ordered mammography, which revealed lesions. A biopsy revealed that one lesion was stage III invasive ductal carcinoma. The patient underwent extensive treatment, including a mastectomy, lymphadenectomy, chemotherapy, and radiation therapy, and prophylactic surgical reduction of the left breast.
PATIENT'S CLAIM The cancer should have been diagnosed in September 2006. Prompt treatment would have decreased the progression of the disease. The September 2006 biopsy should have included both lumps, as recommended by Dr. A.
DEFENDANTS’ DEFENSE There was no indication of cancer in September 2006. Reasonable follow-up care was given.
VERDICT A New York defense verdict was returned.
Tumor not found during surgery; BSO performed
A 41-year-old woman underwent surgery to remove a pelvic tumor in November 2004. The gynecologist was unable to locate the tumor during surgery. He performed bilateral salpingo-oophorectomy (BSO) because of a visual diagnosis of endometriosis. In August 2005, the patient underwent surgical removal of the tumor by another surgeon. She was hospitalized for several weeks and suffered a large scar that required additional surgery.
PATIENT'S CLAIM BSO was unnecessary, and caused early menopause, with vaginal atrophy and dryness, depression, fatigue, insomnia, loss of hair, and other symptoms.
The patient claimed lack of informed consent. From Ecuador, the patient’s command of English was not sufficient for her to completely understand the consent form; an interpreter should have been provided.
DEFENDANTS’ DEFENSE BSO did not cause a significant acceleration of the onset of menopause. It was necessary to treat the endometriosis.
The patient signed a consent form that included BSO. The patient did not indicate that she did not understand the language on the form; had she asked, an interpreter would have been provided.
VERDICT A $750,000 New York settlement was reached with the gynecologist and medical center.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Tips for discussing sexual dysfunction with oncology patients
Cancer therapy presents unique challenges to health care providers because of the evolving nature of managing a patient’s diagnosis, treatment, and recovery. Be conscientious about a patient’s mental health and physical health when considering treatment and support.
Sexual health is important
Sexual dysfunction is one of many variables a patient considers when deciding on a cancer treatment plan—particularly those who have a gynecological, gastrointestinal, or reproductive-tract cancer. Additionally, sexual dysfunction remains one of the major health complaints after many cancer therapies, which may be overlooked because of patients’ hesitancy to initiate discussion.
Many oncologic treatment options—surgery, chemotherapy, radiotherapy, and hormone therapy—are associated with sexual side effects, including radiation sequelae, erectile dysfunction, decreased lubrication, and vaginal atrophy.1 Because sexual dysfunction often is multifactorial, an approach that involves psychological assessment and treatment usually is optimal. A mental health provider can explore the interactions of such factors as decreased self-esteem, negative body image, altered interpersonal relationships, and change or loss of libido when assessing reported sexual dysfunction.2
The mnemonic SEMEN can help you address sexual health topics with oncology patients:
Take a Sexual history at diagnosis and before treatment begins.
Provide Educational materials to warn of potential adverse sexual side effects of various treatments.
Maintain an open dialogue during cancer therapy. Discuss any adverse sexual side effects the patient may be experiencing.
Educate and treat your patient. Offer information on medications, devices, and techniques that target sexual dysfunction.
For men with erectile dysfunction, recommend a phosphodiesterase type 5 (PDE5) inhibitor (sildenafil citrate, tadalafil, vardenafil), a vacuum pump, or intracavernosal penile injection, such as synthetic prostaglandin E1.
For men experiencing premature ejaculation, consider providing instruction on the “squeeze-pause” technique or prescribing a topical anesthetic cream such as lidocaine/prilocaine (available under the brand name EMLA), which is applied to the head of the penis and wiped off before intercourse. Some selective serotonin reuptake inhibitors, including fluoxetine, paroxetine, and sertraline, have been used off-label to treat premature ejaculation.
Women experiencing vaginal dryness or vaginal atrophy might benefit from vaginal estrogen (such as conjugated or estradiol estrogen tablets), an estradiol cream, or the estradiol vaginal ring. Other options include a vaginal moisturizing agent or lubricant.
Additional sexual education topics include:
• adjusting sexual positions
• enhancing foreplay
• seeking help from support organizations
• engaging a sexual therapist (recommend one who specializes in treating oncology patients).
Make Normality the goal after treatment or recovery. Encourage your patient to maintain a healthy sexual lifestyle by continuing discussions about sexual health, supporting healthy self-perception, and addressing possible future sexual dysfunction.
Being given a diagnosis of cancer, undergoing treatment, and surviving the experience are life-altering. Healthcare providers should be open to discussing patients’ past and current sexual practices; along with treating physical illness, you should attempt to maintain a sense of normality, which includes maintaining healthy sexuality.
1. Levenson JL. Textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005.
2. National Institutes of Health. National Cancer Institute. Treatment of sexual problems in people with cancer. http://www.cancer.gov/cancertopics/pdq/supportivecare/sexuality/HealthProfessional/page5. Accessed March 26, 2013.
Cancer therapy presents unique challenges to health care providers because of the evolving nature of managing a patient’s diagnosis, treatment, and recovery. Be conscientious about a patient’s mental health and physical health when considering treatment and support.
Sexual health is important
Sexual dysfunction is one of many variables a patient considers when deciding on a cancer treatment plan—particularly those who have a gynecological, gastrointestinal, or reproductive-tract cancer. Additionally, sexual dysfunction remains one of the major health complaints after many cancer therapies, which may be overlooked because of patients’ hesitancy to initiate discussion.
Many oncologic treatment options—surgery, chemotherapy, radiotherapy, and hormone therapy—are associated with sexual side effects, including radiation sequelae, erectile dysfunction, decreased lubrication, and vaginal atrophy.1 Because sexual dysfunction often is multifactorial, an approach that involves psychological assessment and treatment usually is optimal. A mental health provider can explore the interactions of such factors as decreased self-esteem, negative body image, altered interpersonal relationships, and change or loss of libido when assessing reported sexual dysfunction.2
The mnemonic SEMEN can help you address sexual health topics with oncology patients:
Take a Sexual history at diagnosis and before treatment begins.
Provide Educational materials to warn of potential adverse sexual side effects of various treatments.
Maintain an open dialogue during cancer therapy. Discuss any adverse sexual side effects the patient may be experiencing.
Educate and treat your patient. Offer information on medications, devices, and techniques that target sexual dysfunction.
For men with erectile dysfunction, recommend a phosphodiesterase type 5 (PDE5) inhibitor (sildenafil citrate, tadalafil, vardenafil), a vacuum pump, or intracavernosal penile injection, such as synthetic prostaglandin E1.
For men experiencing premature ejaculation, consider providing instruction on the “squeeze-pause” technique or prescribing a topical anesthetic cream such as lidocaine/prilocaine (available under the brand name EMLA), which is applied to the head of the penis and wiped off before intercourse. Some selective serotonin reuptake inhibitors, including fluoxetine, paroxetine, and sertraline, have been used off-label to treat premature ejaculation.
Women experiencing vaginal dryness or vaginal atrophy might benefit from vaginal estrogen (such as conjugated or estradiol estrogen tablets), an estradiol cream, or the estradiol vaginal ring. Other options include a vaginal moisturizing agent or lubricant.
Additional sexual education topics include:
• adjusting sexual positions
• enhancing foreplay
• seeking help from support organizations
• engaging a sexual therapist (recommend one who specializes in treating oncology patients).
Make Normality the goal after treatment or recovery. Encourage your patient to maintain a healthy sexual lifestyle by continuing discussions about sexual health, supporting healthy self-perception, and addressing possible future sexual dysfunction.
Being given a diagnosis of cancer, undergoing treatment, and surviving the experience are life-altering. Healthcare providers should be open to discussing patients’ past and current sexual practices; along with treating physical illness, you should attempt to maintain a sense of normality, which includes maintaining healthy sexuality.
Cancer therapy presents unique challenges to health care providers because of the evolving nature of managing a patient’s diagnosis, treatment, and recovery. Be conscientious about a patient’s mental health and physical health when considering treatment and support.
Sexual health is important
Sexual dysfunction is one of many variables a patient considers when deciding on a cancer treatment plan—particularly those who have a gynecological, gastrointestinal, or reproductive-tract cancer. Additionally, sexual dysfunction remains one of the major health complaints after many cancer therapies, which may be overlooked because of patients’ hesitancy to initiate discussion.
Many oncologic treatment options—surgery, chemotherapy, radiotherapy, and hormone therapy—are associated with sexual side effects, including radiation sequelae, erectile dysfunction, decreased lubrication, and vaginal atrophy.1 Because sexual dysfunction often is multifactorial, an approach that involves psychological assessment and treatment usually is optimal. A mental health provider can explore the interactions of such factors as decreased self-esteem, negative body image, altered interpersonal relationships, and change or loss of libido when assessing reported sexual dysfunction.2
The mnemonic SEMEN can help you address sexual health topics with oncology patients:
Take a Sexual history at diagnosis and before treatment begins.
Provide Educational materials to warn of potential adverse sexual side effects of various treatments.
Maintain an open dialogue during cancer therapy. Discuss any adverse sexual side effects the patient may be experiencing.
Educate and treat your patient. Offer information on medications, devices, and techniques that target sexual dysfunction.
For men with erectile dysfunction, recommend a phosphodiesterase type 5 (PDE5) inhibitor (sildenafil citrate, tadalafil, vardenafil), a vacuum pump, or intracavernosal penile injection, such as synthetic prostaglandin E1.
For men experiencing premature ejaculation, consider providing instruction on the “squeeze-pause” technique or prescribing a topical anesthetic cream such as lidocaine/prilocaine (available under the brand name EMLA), which is applied to the head of the penis and wiped off before intercourse. Some selective serotonin reuptake inhibitors, including fluoxetine, paroxetine, and sertraline, have been used off-label to treat premature ejaculation.
Women experiencing vaginal dryness or vaginal atrophy might benefit from vaginal estrogen (such as conjugated or estradiol estrogen tablets), an estradiol cream, or the estradiol vaginal ring. Other options include a vaginal moisturizing agent or lubricant.
Additional sexual education topics include:
• adjusting sexual positions
• enhancing foreplay
• seeking help from support organizations
• engaging a sexual therapist (recommend one who specializes in treating oncology patients).
Make Normality the goal after treatment or recovery. Encourage your patient to maintain a healthy sexual lifestyle by continuing discussions about sexual health, supporting healthy self-perception, and addressing possible future sexual dysfunction.
Being given a diagnosis of cancer, undergoing treatment, and surviving the experience are life-altering. Healthcare providers should be open to discussing patients’ past and current sexual practices; along with treating physical illness, you should attempt to maintain a sense of normality, which includes maintaining healthy sexuality.
1. Levenson JL. Textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005.
2. National Institutes of Health. National Cancer Institute. Treatment of sexual problems in people with cancer. http://www.cancer.gov/cancertopics/pdq/supportivecare/sexuality/HealthProfessional/page5. Accessed March 26, 2013.
1. Levenson JL. Textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005.
2. National Institutes of Health. National Cancer Institute. Treatment of sexual problems in people with cancer. http://www.cancer.gov/cancertopics/pdq/supportivecare/sexuality/HealthProfessional/page5. Accessed March 26, 2013.
Tips for making the transition from inpatient to outpatient practice
Years of outpatient practice and supervising residents as they move from inpatient to outpatient rotations prompted me to examine the advice I give to clinicians transitioning to outpatient care.
1. Slow down! Keep in mind that your full assessment may take more than
1 session. Take advantage of follow-up appointments to add details or round out your sense of what is going on with your patient.
2. You don’t always have to ‘do something.’ We often feel that we need to “do something.” Perhaps it’s the difficulty of sitting with someone who’s suffering, or our own feelings of helplessness. Recognize this urge and evaluate whether your findings are something you must act on or if it’s your anxiety that is driving you.
3. Know the particulars of outpatient prescribing. Keep in mind that you should treat the whole person, not just her (his) symptoms. Sometimes it’s appropriate to treat individual symptoms but the justification for this and any other medical decisions needs to be documented.
- Be methodical. Often, this means making one medication change at a time. Although the urgency of inpatient hospitalization sometimes necessitates starting several medications simultaneously, outpatient psychiatry rarely requires that step. Most illnesses in outpatients are chronic;clinicians need to balance the need for treatment with the understanding that the patient may require psychiatric medication indefinitely. Starting several medications at once often leaves the patient and psychiatrist wondering which medications are helping and which may be causing adverse effects.
- Practice educated polypharmacy. Be careful and deliberate; maximize dosages before adding adjunctive therapy. Consider interactions with other medications (such as warfarin or omeprazole), their side effects, and alternative psychosocial treatments.
- Know the cost of medication. Consider generic drugs or medications on the $4 list available at some pharmacies. Be cognizant of less expensive dosing options and combinations. For example, one month of duloxetine, 90 mg/d, costs $587 if prescribed as 30-mg pills; the same dosage costs $390 when prescribed as 30-mg pills and 60-mg pills.1 Advise patients to shop around when purchasing prescriptions because cost can vary significantly among pharmacies.
- Often, patients should be weaned off medications.2 Most selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can cause a discontinuation syndrome. Fluoxetine can be tapered faster; paroxetine and venlafaxine are notorious for causing issues. Abrupt discontinuation of mood stabilizers—especially lithium3—can cause rebound mania, and should be tapered cautiously.
Disclosure
Dr. Pheister reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. DRX. Drug compare. https://drugcompare.destinationrx.com/Home.aspx. Accessed October 17, 2012.
2. Baldessarini RJ, Viguera AC, Tondo L. Discontinuing psychotropic agents. J Psychopharmacol. 1999;13(3):292-293; discussion 299.
3. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
Years of outpatient practice and supervising residents as they move from inpatient to outpatient rotations prompted me to examine the advice I give to clinicians transitioning to outpatient care.
1. Slow down! Keep in mind that your full assessment may take more than
1 session. Take advantage of follow-up appointments to add details or round out your sense of what is going on with your patient.
2. You don’t always have to ‘do something.’ We often feel that we need to “do something.” Perhaps it’s the difficulty of sitting with someone who’s suffering, or our own feelings of helplessness. Recognize this urge and evaluate whether your findings are something you must act on or if it’s your anxiety that is driving you.
3. Know the particulars of outpatient prescribing. Keep in mind that you should treat the whole person, not just her (his) symptoms. Sometimes it’s appropriate to treat individual symptoms but the justification for this and any other medical decisions needs to be documented.
- Be methodical. Often, this means making one medication change at a time. Although the urgency of inpatient hospitalization sometimes necessitates starting several medications simultaneously, outpatient psychiatry rarely requires that step. Most illnesses in outpatients are chronic;clinicians need to balance the need for treatment with the understanding that the patient may require psychiatric medication indefinitely. Starting several medications at once often leaves the patient and psychiatrist wondering which medications are helping and which may be causing adverse effects.
- Practice educated polypharmacy. Be careful and deliberate; maximize dosages before adding adjunctive therapy. Consider interactions with other medications (such as warfarin or omeprazole), their side effects, and alternative psychosocial treatments.
- Know the cost of medication. Consider generic drugs or medications on the $4 list available at some pharmacies. Be cognizant of less expensive dosing options and combinations. For example, one month of duloxetine, 90 mg/d, costs $587 if prescribed as 30-mg pills; the same dosage costs $390 when prescribed as 30-mg pills and 60-mg pills.1 Advise patients to shop around when purchasing prescriptions because cost can vary significantly among pharmacies.
- Often, patients should be weaned off medications.2 Most selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can cause a discontinuation syndrome. Fluoxetine can be tapered faster; paroxetine and venlafaxine are notorious for causing issues. Abrupt discontinuation of mood stabilizers—especially lithium3—can cause rebound mania, and should be tapered cautiously.
Disclosure
Dr. Pheister reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. DRX. Drug compare. https://drugcompare.destinationrx.com/Home.aspx. Accessed October 17, 2012.
2. Baldessarini RJ, Viguera AC, Tondo L. Discontinuing psychotropic agents. J Psychopharmacol. 1999;13(3):292-293; discussion 299.
3. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
Years of outpatient practice and supervising residents as they move from inpatient to outpatient rotations prompted me to examine the advice I give to clinicians transitioning to outpatient care.
1. Slow down! Keep in mind that your full assessment may take more than
1 session. Take advantage of follow-up appointments to add details or round out your sense of what is going on with your patient.
2. You don’t always have to ‘do something.’ We often feel that we need to “do something.” Perhaps it’s the difficulty of sitting with someone who’s suffering, or our own feelings of helplessness. Recognize this urge and evaluate whether your findings are something you must act on or if it’s your anxiety that is driving you.
3. Know the particulars of outpatient prescribing. Keep in mind that you should treat the whole person, not just her (his) symptoms. Sometimes it’s appropriate to treat individual symptoms but the justification for this and any other medical decisions needs to be documented.
- Be methodical. Often, this means making one medication change at a time. Although the urgency of inpatient hospitalization sometimes necessitates starting several medications simultaneously, outpatient psychiatry rarely requires that step. Most illnesses in outpatients are chronic;clinicians need to balance the need for treatment with the understanding that the patient may require psychiatric medication indefinitely. Starting several medications at once often leaves the patient and psychiatrist wondering which medications are helping and which may be causing adverse effects.
- Practice educated polypharmacy. Be careful and deliberate; maximize dosages before adding adjunctive therapy. Consider interactions with other medications (such as warfarin or omeprazole), their side effects, and alternative psychosocial treatments.
- Know the cost of medication. Consider generic drugs or medications on the $4 list available at some pharmacies. Be cognizant of less expensive dosing options and combinations. For example, one month of duloxetine, 90 mg/d, costs $587 if prescribed as 30-mg pills; the same dosage costs $390 when prescribed as 30-mg pills and 60-mg pills.1 Advise patients to shop around when purchasing prescriptions because cost can vary significantly among pharmacies.
- Often, patients should be weaned off medications.2 Most selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can cause a discontinuation syndrome. Fluoxetine can be tapered faster; paroxetine and venlafaxine are notorious for causing issues. Abrupt discontinuation of mood stabilizers—especially lithium3—can cause rebound mania, and should be tapered cautiously.
Disclosure
Dr. Pheister reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. DRX. Drug compare. https://drugcompare.destinationrx.com/Home.aspx. Accessed October 17, 2012.
2. Baldessarini RJ, Viguera AC, Tondo L. Discontinuing psychotropic agents. J Psychopharmacol. 1999;13(3):292-293; discussion 299.
3. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
Hospitalized, elderly, and delirious: What should you do for these patients?
Delirium is a common condition in hospitalized older patients. Often, a report of a “change in mental status” is the reason geriatric patients are sent to the emergency room for evaluation, although delirium also can develop after admission.
Delirium is a marker of underlying medical illness that needs careful workup and treatment. The condition can be iatrogenic, resulting from prescribed medication or a surgical procedure; most often, it is the consequence of multiple factors. Delirium can be expensive, because it increases hospital length of stay and overall costs—particularly if the patient is discharged to a nursing facility, not to home. Patients with delirium are at higher risk of death.
Delirium often goes unrecognized by physicians and nursing staff, and is not documented in medical records. Educating the medical staff on the identification and management of delirium is a key role for consulting psychiatrists.
CASE: Confused and agitated
Ms. T, a 93-year-old resident of an assisted living facility with a history of three
cerebral vascular accidents, atrial fibrillation, hypertension, multiple deep venous thromboses, blindness in her right eye, and deafness in her right ear without a hearing aid, is brought to the hospital after a syncopal episode lasting 10 minutes that was followed by slurred speech, confusion, and transient hypotension. Her dentist recently started her on azithromycin.
In the emergency room, Ms. T’s elevated blood pressure is managed with hydralazine and diltiazem. A CT scan of the head rules out hemorrhagic stroke. Complete blood count and tests of electrolytes, vitamin B12, and thyroid-stimulating hormone are within normal limits; urinalysis is negative for urinary tract infection.
Ms. T is noted to be in and out of sleep, with some confusion. She is maintained without oral food or fluids because of concerns about her ability to swallow. After 5 or 6 hours in the ER, Ms. T is transferred to a medical unit, where she becomes agitated and paranoid, with the delusion that her daughter is an impostor. She yells, is combative, and refuses medication.
Her confusion and behaviors become worse at night: She pulls out her IV line and telemetry leads. Blood pressure remains elevated, for which she receives additional doses of hydralazine.
For behavioral management, the medical team orders a one-time IM dose of haloperidol and starts her on risperidone, 0.5 mg every 4 hours as needed, which Ms. T refuses to take. She is incontinent and has foul-smelling urine.
Ms. T’s family is shocked at her condition; nursing staff is frustrated. With her worsening paranoia, delusions, and combative behaviors towards the nursing staff, psychiatry is consulted.
How to recognize and diagnose
The Box lists DSM-5 criteria for delirium.1 The key feature is a disturbance in attention—what was referred to in DSM-IV-TR as “disturbance in consciousness.” That finding contrasts with what is seen in dementia, with its hallmark memory impairment and chronic deterioration.
In a hospital setting, the question is often asked: Does this patient have dementia or delirium? In many cases, the answer is both, because preexisting cognitive impairment is an important risk factor for delirium.
In addition to the standard clinical interview, several screening instruments or delirium rating scales have been developed. The most commonly used (Table 1) is the Confusion Assessment Method developed by Inouye and colleagues.2
Subtypes of delirium have been described, largely based on motor activity. Patients can present as hyperactive, hypoactive, mixed, or neither.3 Psychiatrists are more likely to be consulted regarding patients with hyperactive delirium, because they are the ones who scream, pull out their IV line, hallucinate, and are delusional, insisting they “have to go home”—such as the patient described in the case above.
Patients with hypoactive delirium often, on the other hand, are difficult to recognize; they present with lethargy, drowsiness, apathy, and confusion. They become withdrawn and answer slowly4; often, psychiatry is consulted to assess them for depression.
Delirium can be difficult to diagnose in patients with underlying dementia, who are not able to provide information. In such cases, obtaining collateral information from a family member or primary caretaker is crucial. Knowing the patient’s baseline helps to determine whether there has been an acute change in mental status.
CASE CONTINUED: Acute mental status changes
Ms. T’s daughter reports that her mother has not been in this condition before. At baseline, Ms. T has had memory problems but no paranoia, delusions, or agitated behaviors. Her daughter also reports that Ms. T has visual and hearing impairments and is not wearing her hearing aid.
The acute change in mental status and the perceptual disturbances indicate that Ms. T has delirium, not dementia.
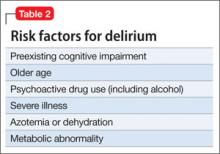
Who is likely to develop delirium?
Risk factors for delirium (Table 2) include preexisting cognitive impairment, older age, vision and hearing impairment, use of psychoactive drugs, severe illness, azotemia and dehydration, a metabolic abnormality, and infection. Male sex also seems to be a risk factor, perhaps because men are more likely to abuse alcohol before admission.
Many patients become delirious after starting a new medication. An experienced geriatrician teaches that the main causes of delirium are “drugs, drugs, drugs, infections, and everything else” (Kenneth Rockwood, MD, personal communication, 2012). At admission, urinary tract infection and pneumonia are common causes of delirium, especially in geriatric patients.
What is the clinical course?
The clinical course varies widely. Delirium often is the reason that a patient is brought to the hospital, presenting with the condition at admission or early in hospitalization. The highest incidence among surgical patients appears to be on the third postoperative day—in some cases because of alcohol or drug withdrawal.
As noted in the DSM-5 criteria, delirium often comes on acutely, over hours or days. Symptoms can persist for weeks after initial onset of episodes of delirium.5 Symptoms fluctuate over the course of the day; at times, they can be missed if a provider sees the patient only while she (he) is clearer and doesn’t review nursing notes from other shifts.
How does delirium affect outcome?
Delirium has been shown to be associated with prolonged hospital stay (21 days, compared with 11 days in the absence of delirium), functional decline during hospitalization, and increased admission to long-term care (36% compared with 13%).6 In a study by O’Keefe and Lavan,6 delirious patients were more likely to sustain falls and to develop urinary incontinence, pressure sores, and other complications during hospitalization.
Older patients with delirium superimposed on dementia had a more than twofold increased risk of mortality compared with patients with dementia alone or with neither dementia nor delirium.7 Rockwood found that an episode of delirium was associated with a much higher rate of subsequent dementia.8
Think of an acute medical illness as a “stress test” for the brain, such that, if the patient develops delirium, it suggests an underlying brain disease that was not evident before the acute episode. After hip fracture, for example, delirium was independently associated with poor functional recovery at 1 month9 and at 2 years.10
Older patients admitted to a skilled nursing facility with delirium are more likely to experience one or more complications (73% compared with 41%).11 In the study by Marcantonio and colleagues, patients with delirium were more than twice as likely to be hospitalized again within 30 days (30% and 13%), and less than half as likely to be discharged to the community (30% and 73%). Table 3 summarizes the impact of delirium on outcomes.
Appropriate management steps
Identifying and treating underlying medical illness is the definitive treatment for delirium; in a geriatric patient with multiple medical comorbidities the pathogenesis often is multifactorial or a definitive precipitant cannot always be identified.12
Managing a patient with delirium includes both non-pharmacotherapeutic interventions, which should be considered first-line, and pharmacotherapeutic interventions. Non-pharmacotherapeutic interventions include, but are not limited to:
• support and close observation by nursing staff
• placing a clock or calendar in the room
• frequent reorientation and reminders
• placing familiar possessions in the room
• putting the patient in an isolated room with a window
• regulating the sleep-wake cycle.4
Pharmacotherapeutic intervention in delirium should be used for behavioral symptoms, but only for the minimum duration necessary4 and preferably oral or IV. No drugs are FDA-approved for delirium, which means that use of any agent is off-label.13
Antipsychotics are the mainstay of pharmacotherapy for delirium in most settings. The use of antipsychotics relates to the dopamine excess-acetylcholine deficiency hypothesis of delirium pathophysiology.12 Haloperidol remains the first-line agent because it is available in multiple dosages and can be given by various routes. IV haloperidol appears to carry less risk of extrapyramidal symptoms than oral haloperidol does but, as with all antipsychotics, its use warrants monitoring for QTc prolongation.12
Studies have not shown that atypical antipsychotics are superior to typical antipsychotics for delirium. Multiple studies have shown that atypicals are as efficacious as haloperidol.
Benzodiazepines are the treatment of choice for delirium caused by alcohol withdrawal. A Cochrane review found no evidence that benzodiazepines were helpful in treating delirium unrelated to alcohol withdrawal.14 In some studies, benzodiazepines were associated with an increased risk of delirium, especially in patients in the intensive care unit.15
More recently, cholinesterase inhibitors have been used to treat delirium. The reasoning behind their use is the hypothesis of a central cholinergic deficiency in delirium.12 Regrettably, there have been few well-conducted studies of these agents in delirium, and a Cochrane review found no significant benefit for cholinesterase inhibitors.16 With the same hypothesis in mind, anticholinergic medications in patients with delirium should be avoided because these agents could exacerbate delirium by further decreasing the acetylcholine level.
Because delirium is common in the hospitalized population (especially older patients), a number of studies have examined strategies to prevent or reduce its development. Inouye and colleagues conducted a controlled clinical trial, in which they intervened to reduce six risk factors for delirium: cognitive impairment, sleep deprivation, immobility, visual and hearing impairment, and dehydration in hospitalized geriatric patients. The number and duration of events of delirium were significantly lower in the intervention group.17
Brummel et al reported that reducing modifiable risk factors in intensive care unit patients—including sedation management, minimizing deliriogenic medications (anticholinergics, antihistamines), minimizing sleep disruption, and encouraging early mobility—could prevent or reduce the incidence of delirium.15
CASE CONCLUDED: Return to baseline
Ms. T’s medications are minimized or discontinued, including azithromycin, based on case reports in the literature. She is stabilized hemodynamically.
Clinicians educate Ms. T’s family about delirium. To address Ms. T’s aggressive and paranoid behaviors, clinicians request that a family member is present to reassure Ms. T. She is continued on low-dose haloperidol. The family also is asked to bring Ms. T’s hearing aid and eyeglasses.
MRI is performed after Ms. T’s behavior is under control. The scan is negative for a new stroke.
Repeat blood tests the following day show an elevated white blood cell count; urinalysis is positive for a urinary tract infection. Ms. T is started on antibiotics. Subsequent urine culture shows no bacterial growth; the antibiotics are stopped after 3 days.
Ms. T slowly improves. According to her family, she is back at baseline in 3 or 4 days.
This case illustrates the complexity of trying to identify the precise cause of delirium among the many that could be involved. Often, no single cause can be found.18
Bottom Line
Delirium is a common and potentially life-threatening condition in hospitalized geriatric patients. General hospital psychiatrists should know how to recognize and treat the condition in collaboration with their medical colleagues.
Related Resources
- Treating delirium: a quick reference guide. Arlington, VA: American Psychiatric Association. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1662986.
- Cook IA. Guideline watch: practice guidelines for the treatment of patients with delirium. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1681952.
- Fearing MA, Inouye SK. Delirium. In: Blazer DG, Steffens D, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:229-241.
- Ghandour A, Saab R, Mehr D. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60(12):726-734.
- Leentjens AF, Rundell J, Rummans T, et al. Delirium: an evidence-based medicine (EBM) monograph for psychosomatic medicine practice. J Psychosom Res. 2012;73:149-152.
- Liptzin B, Jacobson SA. Delirium. In: Sadock BJ, Sadock VA, Ruiz P, eds. Comprehensive textbook of psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:4066-4073.
Drug Brand Names
Azithromycin • Zithromax Hydralazine • Apresoline
Diltiazem • Cardizem Risperidone • Risperdal
Haloperidol • Haldo
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Benjamin Liptzin, MD, describes the distinction between dementia and delirium. Dr. Liptzin is Chair of Psychiatry, Baystate Medical Center, Springfield, Massachusetts, and Professor and Deputy Chair, Department of Psychiatry, Tufts University School of Medicine, Boston, Massachusetts.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
3. Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843-845.
4. Martins S, Fernandes L. Delirium in elderly people: a review. Front Neurol. 2012;3:101.
5. Levkoff SE, Liptzin B, Evans D, et al. Progression and resolution of delirium in elderly patients hospitalized for acute care. Am J Geriatr Psychiatry. 1994;2:230-238.
6. O’Keefe S, Lavan J. The prognostic significance of delirium in older hospitalized patients. J Am Geriatr Soc. 1997;45:247-248.
7. Tsai MC, Weng HH, Chou SY, et al. One-year mortality of elderly inpatients with delirium, dementia or depression seen by a consultation-liaison service. Psychosomatics. 2012;53:433-438.
8. Rockwood K, Cosway S, Carver D, et al. The risk of dementia and death after delirium. Age Ageing. 1999;28:551-556.
9. Marcantonio E, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618-624.
10. Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M27-M34.
11. Marcantonio ER, Kiely DK, Simon SE, et al. Outcomes of elders admitted to post-acute facilities with delirium. J Am Geriatr Soc. 2005;53:963-969.
12. Bledowski J, Trutia A. A review of pharmacologic management and prevention strategies of delirium in the intensive care unit. Psychosomatics. 2012;53:203-211.
13. Breitbart W, Alici-Evcimen Y. Why off-label antipsychotics remain first-choice drugs for delirium. Current Psychiatry. 2007;6(9):49-63.
14. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. doi: 10.1002/14651858.CD006379.pub3.
15. Brummel NE, Girard TD. Preventing delirium in the ICU. Crit Care Clin. 2013;(29):51-65.
16. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008(1):CD005317. doi: 10.1002/14651858.CD005317.
17. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669-676.
18. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097-1101.
Delirium is a common condition in hospitalized older patients. Often, a report of a “change in mental status” is the reason geriatric patients are sent to the emergency room for evaluation, although delirium also can develop after admission.
Delirium is a marker of underlying medical illness that needs careful workup and treatment. The condition can be iatrogenic, resulting from prescribed medication or a surgical procedure; most often, it is the consequence of multiple factors. Delirium can be expensive, because it increases hospital length of stay and overall costs—particularly if the patient is discharged to a nursing facility, not to home. Patients with delirium are at higher risk of death.
Delirium often goes unrecognized by physicians and nursing staff, and is not documented in medical records. Educating the medical staff on the identification and management of delirium is a key role for consulting psychiatrists.
CASE: Confused and agitated
Ms. T, a 93-year-old resident of an assisted living facility with a history of three
cerebral vascular accidents, atrial fibrillation, hypertension, multiple deep venous thromboses, blindness in her right eye, and deafness in her right ear without a hearing aid, is brought to the hospital after a syncopal episode lasting 10 minutes that was followed by slurred speech, confusion, and transient hypotension. Her dentist recently started her on azithromycin.
In the emergency room, Ms. T’s elevated blood pressure is managed with hydralazine and diltiazem. A CT scan of the head rules out hemorrhagic stroke. Complete blood count and tests of electrolytes, vitamin B12, and thyroid-stimulating hormone are within normal limits; urinalysis is negative for urinary tract infection.
Ms. T is noted to be in and out of sleep, with some confusion. She is maintained without oral food or fluids because of concerns about her ability to swallow. After 5 or 6 hours in the ER, Ms. T is transferred to a medical unit, where she becomes agitated and paranoid, with the delusion that her daughter is an impostor. She yells, is combative, and refuses medication.
Her confusion and behaviors become worse at night: She pulls out her IV line and telemetry leads. Blood pressure remains elevated, for which she receives additional doses of hydralazine.
For behavioral management, the medical team orders a one-time IM dose of haloperidol and starts her on risperidone, 0.5 mg every 4 hours as needed, which Ms. T refuses to take. She is incontinent and has foul-smelling urine.
Ms. T’s family is shocked at her condition; nursing staff is frustrated. With her worsening paranoia, delusions, and combative behaviors towards the nursing staff, psychiatry is consulted.
How to recognize and diagnose
The Box lists DSM-5 criteria for delirium.1 The key feature is a disturbance in attention—what was referred to in DSM-IV-TR as “disturbance in consciousness.” That finding contrasts with what is seen in dementia, with its hallmark memory impairment and chronic deterioration.
In a hospital setting, the question is often asked: Does this patient have dementia or delirium? In many cases, the answer is both, because preexisting cognitive impairment is an important risk factor for delirium.
In addition to the standard clinical interview, several screening instruments or delirium rating scales have been developed. The most commonly used (Table 1) is the Confusion Assessment Method developed by Inouye and colleagues.2
Subtypes of delirium have been described, largely based on motor activity. Patients can present as hyperactive, hypoactive, mixed, or neither.3 Psychiatrists are more likely to be consulted regarding patients with hyperactive delirium, because they are the ones who scream, pull out their IV line, hallucinate, and are delusional, insisting they “have to go home”—such as the patient described in the case above.
Patients with hypoactive delirium often, on the other hand, are difficult to recognize; they present with lethargy, drowsiness, apathy, and confusion. They become withdrawn and answer slowly4; often, psychiatry is consulted to assess them for depression.
Delirium can be difficult to diagnose in patients with underlying dementia, who are not able to provide information. In such cases, obtaining collateral information from a family member or primary caretaker is crucial. Knowing the patient’s baseline helps to determine whether there has been an acute change in mental status.
CASE CONTINUED: Acute mental status changes
Ms. T’s daughter reports that her mother has not been in this condition before. At baseline, Ms. T has had memory problems but no paranoia, delusions, or agitated behaviors. Her daughter also reports that Ms. T has visual and hearing impairments and is not wearing her hearing aid.
The acute change in mental status and the perceptual disturbances indicate that Ms. T has delirium, not dementia.
Who is likely to develop delirium?
Risk factors for delirium (Table 2) include preexisting cognitive impairment, older age, vision and hearing impairment, use of psychoactive drugs, severe illness, azotemia and dehydration, a metabolic abnormality, and infection. Male sex also seems to be a risk factor, perhaps because men are more likely to abuse alcohol before admission.
Many patients become delirious after starting a new medication. An experienced geriatrician teaches that the main causes of delirium are “drugs, drugs, drugs, infections, and everything else” (Kenneth Rockwood, MD, personal communication, 2012). At admission, urinary tract infection and pneumonia are common causes of delirium, especially in geriatric patients.
What is the clinical course?
The clinical course varies widely. Delirium often is the reason that a patient is brought to the hospital, presenting with the condition at admission or early in hospitalization. The highest incidence among surgical patients appears to be on the third postoperative day—in some cases because of alcohol or drug withdrawal.
As noted in the DSM-5 criteria, delirium often comes on acutely, over hours or days. Symptoms can persist for weeks after initial onset of episodes of delirium.5 Symptoms fluctuate over the course of the day; at times, they can be missed if a provider sees the patient only while she (he) is clearer and doesn’t review nursing notes from other shifts.
How does delirium affect outcome?
Delirium has been shown to be associated with prolonged hospital stay (21 days, compared with 11 days in the absence of delirium), functional decline during hospitalization, and increased admission to long-term care (36% compared with 13%).6 In a study by O’Keefe and Lavan,6 delirious patients were more likely to sustain falls and to develop urinary incontinence, pressure sores, and other complications during hospitalization.
Older patients with delirium superimposed on dementia had a more than twofold increased risk of mortality compared with patients with dementia alone or with neither dementia nor delirium.7 Rockwood found that an episode of delirium was associated with a much higher rate of subsequent dementia.8
Think of an acute medical illness as a “stress test” for the brain, such that, if the patient develops delirium, it suggests an underlying brain disease that was not evident before the acute episode. After hip fracture, for example, delirium was independently associated with poor functional recovery at 1 month9 and at 2 years.10
Older patients admitted to a skilled nursing facility with delirium are more likely to experience one or more complications (73% compared with 41%).11 In the study by Marcantonio and colleagues, patients with delirium were more than twice as likely to be hospitalized again within 30 days (30% and 13%), and less than half as likely to be discharged to the community (30% and 73%). Table 3 summarizes the impact of delirium on outcomes.
Appropriate management steps
Identifying and treating underlying medical illness is the definitive treatment for delirium; in a geriatric patient with multiple medical comorbidities the pathogenesis often is multifactorial or a definitive precipitant cannot always be identified.12
Managing a patient with delirium includes both non-pharmacotherapeutic interventions, which should be considered first-line, and pharmacotherapeutic interventions. Non-pharmacotherapeutic interventions include, but are not limited to:
• support and close observation by nursing staff
• placing a clock or calendar in the room
• frequent reorientation and reminders
• placing familiar possessions in the room
• putting the patient in an isolated room with a window
• regulating the sleep-wake cycle.4
Pharmacotherapeutic intervention in delirium should be used for behavioral symptoms, but only for the minimum duration necessary4 and preferably oral or IV. No drugs are FDA-approved for delirium, which means that use of any agent is off-label.13
Antipsychotics are the mainstay of pharmacotherapy for delirium in most settings. The use of antipsychotics relates to the dopamine excess-acetylcholine deficiency hypothesis of delirium pathophysiology.12 Haloperidol remains the first-line agent because it is available in multiple dosages and can be given by various routes. IV haloperidol appears to carry less risk of extrapyramidal symptoms than oral haloperidol does but, as with all antipsychotics, its use warrants monitoring for QTc prolongation.12
Studies have not shown that atypical antipsychotics are superior to typical antipsychotics for delirium. Multiple studies have shown that atypicals are as efficacious as haloperidol.
Benzodiazepines are the treatment of choice for delirium caused by alcohol withdrawal. A Cochrane review found no evidence that benzodiazepines were helpful in treating delirium unrelated to alcohol withdrawal.14 In some studies, benzodiazepines were associated with an increased risk of delirium, especially in patients in the intensive care unit.15
More recently, cholinesterase inhibitors have been used to treat delirium. The reasoning behind their use is the hypothesis of a central cholinergic deficiency in delirium.12 Regrettably, there have been few well-conducted studies of these agents in delirium, and a Cochrane review found no significant benefit for cholinesterase inhibitors.16 With the same hypothesis in mind, anticholinergic medications in patients with delirium should be avoided because these agents could exacerbate delirium by further decreasing the acetylcholine level.
Because delirium is common in the hospitalized population (especially older patients), a number of studies have examined strategies to prevent or reduce its development. Inouye and colleagues conducted a controlled clinical trial, in which they intervened to reduce six risk factors for delirium: cognitive impairment, sleep deprivation, immobility, visual and hearing impairment, and dehydration in hospitalized geriatric patients. The number and duration of events of delirium were significantly lower in the intervention group.17
Brummel et al reported that reducing modifiable risk factors in intensive care unit patients—including sedation management, minimizing deliriogenic medications (anticholinergics, antihistamines), minimizing sleep disruption, and encouraging early mobility—could prevent or reduce the incidence of delirium.15
CASE CONCLUDED: Return to baseline
Ms. T’s medications are minimized or discontinued, including azithromycin, based on case reports in the literature. She is stabilized hemodynamically.
Clinicians educate Ms. T’s family about delirium. To address Ms. T’s aggressive and paranoid behaviors, clinicians request that a family member is present to reassure Ms. T. She is continued on low-dose haloperidol. The family also is asked to bring Ms. T’s hearing aid and eyeglasses.
MRI is performed after Ms. T’s behavior is under control. The scan is negative for a new stroke.
Repeat blood tests the following day show an elevated white blood cell count; urinalysis is positive for a urinary tract infection. Ms. T is started on antibiotics. Subsequent urine culture shows no bacterial growth; the antibiotics are stopped after 3 days.
Ms. T slowly improves. According to her family, she is back at baseline in 3 or 4 days.
This case illustrates the complexity of trying to identify the precise cause of delirium among the many that could be involved. Often, no single cause can be found.18
Bottom Line
Delirium is a common and potentially life-threatening condition in hospitalized geriatric patients. General hospital psychiatrists should know how to recognize and treat the condition in collaboration with their medical colleagues.
Related Resources
- Treating delirium: a quick reference guide. Arlington, VA: American Psychiatric Association. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1662986.
- Cook IA. Guideline watch: practice guidelines for the treatment of patients with delirium. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1681952.
- Fearing MA, Inouye SK. Delirium. In: Blazer DG, Steffens D, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:229-241.
- Ghandour A, Saab R, Mehr D. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60(12):726-734.
- Leentjens AF, Rundell J, Rummans T, et al. Delirium: an evidence-based medicine (EBM) monograph for psychosomatic medicine practice. J Psychosom Res. 2012;73:149-152.
- Liptzin B, Jacobson SA. Delirium. In: Sadock BJ, Sadock VA, Ruiz P, eds. Comprehensive textbook of psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:4066-4073.
Drug Brand Names
Azithromycin • Zithromax Hydralazine • Apresoline
Diltiazem • Cardizem Risperidone • Risperdal
Haloperidol • Haldo
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Benjamin Liptzin, MD, describes the distinction between dementia and delirium. Dr. Liptzin is Chair of Psychiatry, Baystate Medical Center, Springfield, Massachusetts, and Professor and Deputy Chair, Department of Psychiatry, Tufts University School of Medicine, Boston, Massachusetts.
Delirium is a common condition in hospitalized older patients. Often, a report of a “change in mental status” is the reason geriatric patients are sent to the emergency room for evaluation, although delirium also can develop after admission.
Delirium is a marker of underlying medical illness that needs careful workup and treatment. The condition can be iatrogenic, resulting from prescribed medication or a surgical procedure; most often, it is the consequence of multiple factors. Delirium can be expensive, because it increases hospital length of stay and overall costs—particularly if the patient is discharged to a nursing facility, not to home. Patients with delirium are at higher risk of death.
Delirium often goes unrecognized by physicians and nursing staff, and is not documented in medical records. Educating the medical staff on the identification and management of delirium is a key role for consulting psychiatrists.
CASE: Confused and agitated
Ms. T, a 93-year-old resident of an assisted living facility with a history of three
cerebral vascular accidents, atrial fibrillation, hypertension, multiple deep venous thromboses, blindness in her right eye, and deafness in her right ear without a hearing aid, is brought to the hospital after a syncopal episode lasting 10 minutes that was followed by slurred speech, confusion, and transient hypotension. Her dentist recently started her on azithromycin.
In the emergency room, Ms. T’s elevated blood pressure is managed with hydralazine and diltiazem. A CT scan of the head rules out hemorrhagic stroke. Complete blood count and tests of electrolytes, vitamin B12, and thyroid-stimulating hormone are within normal limits; urinalysis is negative for urinary tract infection.
Ms. T is noted to be in and out of sleep, with some confusion. She is maintained without oral food or fluids because of concerns about her ability to swallow. After 5 or 6 hours in the ER, Ms. T is transferred to a medical unit, where she becomes agitated and paranoid, with the delusion that her daughter is an impostor. She yells, is combative, and refuses medication.
Her confusion and behaviors become worse at night: She pulls out her IV line and telemetry leads. Blood pressure remains elevated, for which she receives additional doses of hydralazine.
For behavioral management, the medical team orders a one-time IM dose of haloperidol and starts her on risperidone, 0.5 mg every 4 hours as needed, which Ms. T refuses to take. She is incontinent and has foul-smelling urine.
Ms. T’s family is shocked at her condition; nursing staff is frustrated. With her worsening paranoia, delusions, and combative behaviors towards the nursing staff, psychiatry is consulted.
How to recognize and diagnose
The Box lists DSM-5 criteria for delirium.1 The key feature is a disturbance in attention—what was referred to in DSM-IV-TR as “disturbance in consciousness.” That finding contrasts with what is seen in dementia, with its hallmark memory impairment and chronic deterioration.
In a hospital setting, the question is often asked: Does this patient have dementia or delirium? In many cases, the answer is both, because preexisting cognitive impairment is an important risk factor for delirium.
In addition to the standard clinical interview, several screening instruments or delirium rating scales have been developed. The most commonly used (Table 1) is the Confusion Assessment Method developed by Inouye and colleagues.2
Subtypes of delirium have been described, largely based on motor activity. Patients can present as hyperactive, hypoactive, mixed, or neither.3 Psychiatrists are more likely to be consulted regarding patients with hyperactive delirium, because they are the ones who scream, pull out their IV line, hallucinate, and are delusional, insisting they “have to go home”—such as the patient described in the case above.
Patients with hypoactive delirium often, on the other hand, are difficult to recognize; they present with lethargy, drowsiness, apathy, and confusion. They become withdrawn and answer slowly4; often, psychiatry is consulted to assess them for depression.
Delirium can be difficult to diagnose in patients with underlying dementia, who are not able to provide information. In such cases, obtaining collateral information from a family member or primary caretaker is crucial. Knowing the patient’s baseline helps to determine whether there has been an acute change in mental status.
CASE CONTINUED: Acute mental status changes
Ms. T’s daughter reports that her mother has not been in this condition before. At baseline, Ms. T has had memory problems but no paranoia, delusions, or agitated behaviors. Her daughter also reports that Ms. T has visual and hearing impairments and is not wearing her hearing aid.
The acute change in mental status and the perceptual disturbances indicate that Ms. T has delirium, not dementia.
Who is likely to develop delirium?
Risk factors for delirium (Table 2) include preexisting cognitive impairment, older age, vision and hearing impairment, use of psychoactive drugs, severe illness, azotemia and dehydration, a metabolic abnormality, and infection. Male sex also seems to be a risk factor, perhaps because men are more likely to abuse alcohol before admission.
Many patients become delirious after starting a new medication. An experienced geriatrician teaches that the main causes of delirium are “drugs, drugs, drugs, infections, and everything else” (Kenneth Rockwood, MD, personal communication, 2012). At admission, urinary tract infection and pneumonia are common causes of delirium, especially in geriatric patients.
What is the clinical course?
The clinical course varies widely. Delirium often is the reason that a patient is brought to the hospital, presenting with the condition at admission or early in hospitalization. The highest incidence among surgical patients appears to be on the third postoperative day—in some cases because of alcohol or drug withdrawal.
As noted in the DSM-5 criteria, delirium often comes on acutely, over hours or days. Symptoms can persist for weeks after initial onset of episodes of delirium.5 Symptoms fluctuate over the course of the day; at times, they can be missed if a provider sees the patient only while she (he) is clearer and doesn’t review nursing notes from other shifts.
How does delirium affect outcome?
Delirium has been shown to be associated with prolonged hospital stay (21 days, compared with 11 days in the absence of delirium), functional decline during hospitalization, and increased admission to long-term care (36% compared with 13%).6 In a study by O’Keefe and Lavan,6 delirious patients were more likely to sustain falls and to develop urinary incontinence, pressure sores, and other complications during hospitalization.
Older patients with delirium superimposed on dementia had a more than twofold increased risk of mortality compared with patients with dementia alone or with neither dementia nor delirium.7 Rockwood found that an episode of delirium was associated with a much higher rate of subsequent dementia.8
Think of an acute medical illness as a “stress test” for the brain, such that, if the patient develops delirium, it suggests an underlying brain disease that was not evident before the acute episode. After hip fracture, for example, delirium was independently associated with poor functional recovery at 1 month9 and at 2 years.10
Older patients admitted to a skilled nursing facility with delirium are more likely to experience one or more complications (73% compared with 41%).11 In the study by Marcantonio and colleagues, patients with delirium were more than twice as likely to be hospitalized again within 30 days (30% and 13%), and less than half as likely to be discharged to the community (30% and 73%). Table 3 summarizes the impact of delirium on outcomes.
Appropriate management steps
Identifying and treating underlying medical illness is the definitive treatment for delirium; in a geriatric patient with multiple medical comorbidities the pathogenesis often is multifactorial or a definitive precipitant cannot always be identified.12
Managing a patient with delirium includes both non-pharmacotherapeutic interventions, which should be considered first-line, and pharmacotherapeutic interventions. Non-pharmacotherapeutic interventions include, but are not limited to:
• support and close observation by nursing staff
• placing a clock or calendar in the room
• frequent reorientation and reminders
• placing familiar possessions in the room
• putting the patient in an isolated room with a window
• regulating the sleep-wake cycle.4
Pharmacotherapeutic intervention in delirium should be used for behavioral symptoms, but only for the minimum duration necessary4 and preferably oral or IV. No drugs are FDA-approved for delirium, which means that use of any agent is off-label.13
Antipsychotics are the mainstay of pharmacotherapy for delirium in most settings. The use of antipsychotics relates to the dopamine excess-acetylcholine deficiency hypothesis of delirium pathophysiology.12 Haloperidol remains the first-line agent because it is available in multiple dosages and can be given by various routes. IV haloperidol appears to carry less risk of extrapyramidal symptoms than oral haloperidol does but, as with all antipsychotics, its use warrants monitoring for QTc prolongation.12
Studies have not shown that atypical antipsychotics are superior to typical antipsychotics for delirium. Multiple studies have shown that atypicals are as efficacious as haloperidol.
Benzodiazepines are the treatment of choice for delirium caused by alcohol withdrawal. A Cochrane review found no evidence that benzodiazepines were helpful in treating delirium unrelated to alcohol withdrawal.14 In some studies, benzodiazepines were associated with an increased risk of delirium, especially in patients in the intensive care unit.15
More recently, cholinesterase inhibitors have been used to treat delirium. The reasoning behind their use is the hypothesis of a central cholinergic deficiency in delirium.12 Regrettably, there have been few well-conducted studies of these agents in delirium, and a Cochrane review found no significant benefit for cholinesterase inhibitors.16 With the same hypothesis in mind, anticholinergic medications in patients with delirium should be avoided because these agents could exacerbate delirium by further decreasing the acetylcholine level.
Because delirium is common in the hospitalized population (especially older patients), a number of studies have examined strategies to prevent or reduce its development. Inouye and colleagues conducted a controlled clinical trial, in which they intervened to reduce six risk factors for delirium: cognitive impairment, sleep deprivation, immobility, visual and hearing impairment, and dehydration in hospitalized geriatric patients. The number and duration of events of delirium were significantly lower in the intervention group.17
Brummel et al reported that reducing modifiable risk factors in intensive care unit patients—including sedation management, minimizing deliriogenic medications (anticholinergics, antihistamines), minimizing sleep disruption, and encouraging early mobility—could prevent or reduce the incidence of delirium.15
CASE CONCLUDED: Return to baseline
Ms. T’s medications are minimized or discontinued, including azithromycin, based on case reports in the literature. She is stabilized hemodynamically.
Clinicians educate Ms. T’s family about delirium. To address Ms. T’s aggressive and paranoid behaviors, clinicians request that a family member is present to reassure Ms. T. She is continued on low-dose haloperidol. The family also is asked to bring Ms. T’s hearing aid and eyeglasses.
MRI is performed after Ms. T’s behavior is under control. The scan is negative for a new stroke.
Repeat blood tests the following day show an elevated white blood cell count; urinalysis is positive for a urinary tract infection. Ms. T is started on antibiotics. Subsequent urine culture shows no bacterial growth; the antibiotics are stopped after 3 days.
Ms. T slowly improves. According to her family, she is back at baseline in 3 or 4 days.
This case illustrates the complexity of trying to identify the precise cause of delirium among the many that could be involved. Often, no single cause can be found.18
Bottom Line
Delirium is a common and potentially life-threatening condition in hospitalized geriatric patients. General hospital psychiatrists should know how to recognize and treat the condition in collaboration with their medical colleagues.
Related Resources
- Treating delirium: a quick reference guide. Arlington, VA: American Psychiatric Association. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1662986.
- Cook IA. Guideline watch: practice guidelines for the treatment of patients with delirium. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1681952.
- Fearing MA, Inouye SK. Delirium. In: Blazer DG, Steffens D, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:229-241.
- Ghandour A, Saab R, Mehr D. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60(12):726-734.
- Leentjens AF, Rundell J, Rummans T, et al. Delirium: an evidence-based medicine (EBM) monograph for psychosomatic medicine practice. J Psychosom Res. 2012;73:149-152.
- Liptzin B, Jacobson SA. Delirium. In: Sadock BJ, Sadock VA, Ruiz P, eds. Comprehensive textbook of psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:4066-4073.
Drug Brand Names
Azithromycin • Zithromax Hydralazine • Apresoline
Diltiazem • Cardizem Risperidone • Risperdal
Haloperidol • Haldo
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Benjamin Liptzin, MD, describes the distinction between dementia and delirium. Dr. Liptzin is Chair of Psychiatry, Baystate Medical Center, Springfield, Massachusetts, and Professor and Deputy Chair, Department of Psychiatry, Tufts University School of Medicine, Boston, Massachusetts.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
3. Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843-845.
4. Martins S, Fernandes L. Delirium in elderly people: a review. Front Neurol. 2012;3:101.
5. Levkoff SE, Liptzin B, Evans D, et al. Progression and resolution of delirium in elderly patients hospitalized for acute care. Am J Geriatr Psychiatry. 1994;2:230-238.
6. O’Keefe S, Lavan J. The prognostic significance of delirium in older hospitalized patients. J Am Geriatr Soc. 1997;45:247-248.
7. Tsai MC, Weng HH, Chou SY, et al. One-year mortality of elderly inpatients with delirium, dementia or depression seen by a consultation-liaison service. Psychosomatics. 2012;53:433-438.
8. Rockwood K, Cosway S, Carver D, et al. The risk of dementia and death after delirium. Age Ageing. 1999;28:551-556.
9. Marcantonio E, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618-624.
10. Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M27-M34.
11. Marcantonio ER, Kiely DK, Simon SE, et al. Outcomes of elders admitted to post-acute facilities with delirium. J Am Geriatr Soc. 2005;53:963-969.
12. Bledowski J, Trutia A. A review of pharmacologic management and prevention strategies of delirium in the intensive care unit. Psychosomatics. 2012;53:203-211.
13. Breitbart W, Alici-Evcimen Y. Why off-label antipsychotics remain first-choice drugs for delirium. Current Psychiatry. 2007;6(9):49-63.
14. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. doi: 10.1002/14651858.CD006379.pub3.
15. Brummel NE, Girard TD. Preventing delirium in the ICU. Crit Care Clin. 2013;(29):51-65.
16. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008(1):CD005317. doi: 10.1002/14651858.CD005317.
17. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669-676.
18. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097-1101.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
3. Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843-845.
4. Martins S, Fernandes L. Delirium in elderly people: a review. Front Neurol. 2012;3:101.
5. Levkoff SE, Liptzin B, Evans D, et al. Progression and resolution of delirium in elderly patients hospitalized for acute care. Am J Geriatr Psychiatry. 1994;2:230-238.
6. O’Keefe S, Lavan J. The prognostic significance of delirium in older hospitalized patients. J Am Geriatr Soc. 1997;45:247-248.
7. Tsai MC, Weng HH, Chou SY, et al. One-year mortality of elderly inpatients with delirium, dementia or depression seen by a consultation-liaison service. Psychosomatics. 2012;53:433-438.
8. Rockwood K, Cosway S, Carver D, et al. The risk of dementia and death after delirium. Age Ageing. 1999;28:551-556.
9. Marcantonio E, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618-624.
10. Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M27-M34.
11. Marcantonio ER, Kiely DK, Simon SE, et al. Outcomes of elders admitted to post-acute facilities with delirium. J Am Geriatr Soc. 2005;53:963-969.
12. Bledowski J, Trutia A. A review of pharmacologic management and prevention strategies of delirium in the intensive care unit. Psychosomatics. 2012;53:203-211.
13. Breitbart W, Alici-Evcimen Y. Why off-label antipsychotics remain first-choice drugs for delirium. Current Psychiatry. 2007;6(9):49-63.
14. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. doi: 10.1002/14651858.CD006379.pub3.
15. Brummel NE, Girard TD. Preventing delirium in the ICU. Crit Care Clin. 2013;(29):51-65.
16. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008(1):CD005317. doi: 10.1002/14651858.CD005317.
17. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669-676.
18. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097-1101.
No laughing matter: Laughter is good psychiatric medicine
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
Incidental ovarian cysts: When to reassure, when to reassess, when to refer
Ovarian cysts, sometimes reported as ovarian masses or adnexal masses, are frequently found incidentally in women who have no symptoms. These cysts can be physiologic (having to do with ovulation) or neoplastic—either benign, borderline (having low malignant potential), or frankly malignant. Thus, these incidental lesions pose many diagnostic challenges to the clinician.
The vast majority of cysts are benign, but a few are malignant, and ovarian malignancies have a notoriously poor survival rate. The diagnosis can only be obtained surgically, as aspiration and biopsy are not definitive and may be harmful. Therefore, the clinician must try to balance the risks of surgery for what may be a benign lesion with the risk of delaying diagnosis of a malignancy.
In this article we provide an approach to evaluating these cysts, with guidance on when the patient can be reassured and when referral is needed.
THE DILEMMA OF OVARIAN CYSTS
Ovarian cysts are common
Premenopausal women can be expected to make at least a small cyst or follicle almost every month. The point prevalence for significant cysts has been reported to be almost 8% in premenopausal women.1
Surprisingly, the prevalence in postmenopausal women is as high as 14% to 18%, with a yearly incidence of 8%. From 30% to 54% of postmenopausal ovarian cysts persist for years.2,3
Little is known about the cause of most cysts
Little is known about the cause of most ovarian cysts. Functional or physiologic cysts are thought to be variations in the ovulatory process. They do not seem to be precursors to ovarian cancer.
Most benign neoplastic cysts are also not thought to be precancerous, with the possible exception of the mucinous kind.4 Ovarian cysts do not increase the risk of ovarian cancer later in life,3,9 and removing benign cysts has not been shown to decrease the risk of death from ovarian cancer.10
Most ovarian cysts and masses are benign
Simple ovarian cysts are much more likely to be benign than malignant. Complex and solid ovarian masses are also more likely to be benign, regardless of menopausal status, but more malignancies are found in this group.
With any kind of mass, the chances of malignancy increase with age. Children and adolescents are not discussed in this article; they should be referred to a specialist.
Ovarian cancer often has a poor prognosis
This “silent” cancer is most often discovered and treated when it has already spread, contributing to a reported 5-year survival rate of only 33% to 46%.11–13 Ideally, ovarian cancer would be found and removed while still confined to the ovary, when the 5-year survival rate is greater than 90%.
Unfortunately, there does not seem to be a precursor lesion for most ovarian cancers, and there is no good way of finding it in the stage 1 phase, so detecting this cancer before it spreads remains an elusive goal.11,14
Surgery is required to diagnose difficult cases
There is no perfect test for the preoperative assessment of a cystic ovarian mass. Every method has drawbacks (Table 1).15–18 Therefore, the National Institutes of Health estimates that 5% to 10% of women in the United States will undergo surgical exploration for an ovarian cyst in their lifetime. Only 13% to 21% of these cysts will be malignant.5
ASSESSING AN INCIDENTALLY DISCOVERED OVARIAN MASS
Certain factors in the history, physical examination, and blood work may suggest the cyst is either benign or malignant and may influence the subsequent assessment. However, in most cases, the best next step is to perform transvaginal ultrasonography, which we will discuss later in this paper.
History
Age is a major risk factor for ovarian cancer; the median age at diagnosis is 63 years.9 In the reproductive-age group, ovarian cysts are much more likely to be functional than neoplastic. Epithelial cancers are rare before the age of 40, but other cancer types such as borderline, germ cell, and sex cord stromal tumors may occur.19
In every age group a cyst is more likely to be benign than malignant, although, as noted above, the probability of malignancy increases with age.
Symptoms. Most ovarian cysts, benign or malignant, are asymptomatic and are found only incidentally.
The most commonly reported symptoms are pelvic or lower-abdominal pressure or pain. Acutely painful conditions include ovarian torsion, hemorrhage into the cyst, cyst rupture with or without intra-abdominal hemorrhage, ectopic pregnancy, and pelvic inflammatory disease with tubo-ovarian abscess.
Some patients who have ovarian cancer report vague symptoms such as urinary urgency or frequency, abdominal distention or bloating, and difficulty eating or early satiety.20 Although the positive predictive value of this symptom constellation is only about 1%, its usefulness increases if these symptoms arose recently (within the past year) and occur than 12 days a month.21
Family history of ovarian, breast, endometrial, or colon cancer is of particular interest. The greater the number of affected relatives and the closer the degree of relation, the greater the risk; in some cases the relative risk is 40 times greater.22 Breast-ovarian cancer syndromes, hereditary nonpolyposis colorectal cancer syndrome, and family cancer syndrome, as well as extremely high-risk pedigrees such as BRCA1, BRCA2, and Lynch syndrome, all place women at significantly higher risk. Daughters tend to develop cancer at a younger age than their affected mothers.
However, only 10% of ovarian cancers occur in patients who have a family history of it, leaving 90% as sporadic occurrences.
Other history. Factors protective against ovarian cancer include use of oral contraceptives at any time, tubal ligation, hysterectomy, having had children, breastfeeding, a low-fat diet, and possibly use of aspirin and acetaminophen.23,24
Risk factors for malignancy include advanced age; nulliparity; family history of ovarian or breast cancer; personal history of breast cancer; talc use; asbestos exposure; white ethnicity; pelvic irradiation; smoking; alcohol use; possibly the previous use of fertility drugs, estrogen, or androgen; history of mumps; urban location; early menarche; and late menopause.24
Physical examination
Vital signs. Fever can indicate an infectious process or torsion of the ovary. A sudden onset of low blood pressure or rapid pulse can indicate a hemorrhagic condition such as ectopic pregnancy or ruptured hemorrhagic cyst.
Bimanual pelvic examination is notoriously inaccurate for detecting and characterizing ovarian cysts. In one prospective study, examiners blinded to the reason for surgery evaluated women under anesthesia. The authors concluded that bimanual examination was of limited value even under the best circumstances.15 Pelvic examination can be even more difficult in patients who are obese, are virginal, have vaginal atrophy, or are in pain.
Useful information that can be obtained through the bimanual examination includes the exact location of pelvic tenderness, the relative firmness of an identified mass, and the existence of nodularity in the posterior cul-de-sac, suggesting advanced ovarian cancer.
Tumor markers
Cancer antigen 125 (CA125) is the most studied and widely used of the ovarian cancer tumor markers. When advanced epithelial ovarian cancer is associated with a markedly elevated level, the value correlates with tumor burden.25
Unfortunately, only about half of early-stage ovarian cancers and 75% to 80% of advanced ovarian cancers express this marker.26 Especially in premenopausal women, there are many pelvic conditions that can falsely elevate CA125. Therefore, its sensitivity and specificity for predicting ovarian cancer are suboptimal. Nevertheless, CA125 is often used to help stratify risk when assessing known ovarian cysts and masses.
The value considered abnormal in postmenopausal women is 35 U/mL or greater, while in premenopausal women the cutoff is less well defined. The lower the cutoff level is set, the more sensitive the test. Recent recommendations advise 50 U/mL or 67 U/mL, rather than the 200 U/mL recommended in the 2002 joint guidelines of the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology.27,28
However, specificity is likely to be lower with these lower cutoff values. Conditions that can elevate CA125 levels include almost anything that irritates the peritoneum, including pregnancy, menstruation, fibroids, endometriosis, infection, and ovarian hyperstimulation, as well as medical conditions such as liver or renal disease, colitis, diverticulitis, congestive heart failure, diabetes, autoimmune diseases, and ascites.
Following serial CA125 levels may be more sensitive than trying to establish a single cutoff value.29 CA125 should not be used as a screening tool in average-risk women.26
OVA1. Several biomarker panels have been developed and evaluated for risk assessment in women with pelvic masses. OVA1, a proprietary panel of tests (Vermillion; Austin, TX) received US Food and Drug Administration approval in 2009. It includes CA125 and four other proteins, from which it calculates a probability score (high or low) using a proprietary formula.
In prospective studies, OVA1 was more sensitive than clinical assessment or CA125 alone.30 The higher sensitivity and negative predictive value were counterbalanced by a lower specificity and positive predictive value.31 Its cost ($650) is not always covered by insurance. OVA1 is not a screening tool.
EVALUATION WITH ULTRASONOGRAPHY
Ultrasonography is the imaging test of choice in assessing adnexal cysts and masses, and therefore it is the best next step after taking a history, performing a physical examination, and obtaining blood work.32 In cases in which an incidental ovarian mass is discovered on computed tomography (CT), further characterization by ultrasonography will likely yield helpful information.
Pelvic ultrasonography can be performed transabdominally or transvaginally. Vaginal ultrasonography gives the clearest images in most patients. Abdominal scanning is indicated for large masses, when vaginal access is difficult (as in virginal patients or those with vaginal atrophy) or when the mass is out of the focal length of the vaginal probe. A full bladder is usually required for the best transabdominal images.
The value of the images obtained depends on the experience of the ultrasonographer and reader and on the equipment. Also, there is currently no widely used standard for reporting the findings33—descriptions are individualized, leading some authors to recommend that the clinician personally review the films to get the most accurate picture.19
Size
Size alone cannot be used to distinguish between benign and malignant lesions. Simple cysts up to 10 cm are most likely benign regardless of menopausal status.2,34 However, in a complex or solid mass, size correlates somewhat with the chance of malignancy, with notable exceptions, such as the famously large sizes of some solid fibromas or mucinous cystadenomas. Also, size may correlate with risk of other complications such as torsion or symptomatic rupture.
Complexity
Simple cysts have clear fluid, thin smooth walls, no loculations or septae, and enhanced through-transmission of echo waves.32,33
Complexity is described in terms of septations, wall thickness, internal echoes, and solid nodules. Increasing complexity does correlate with increased risk of malignancy.
Worrisome findings
The most worrisome findings are:
- Solid areas that are not hyperechoic, especially when there is blood flow to them
- Thick septations, more than 2 or 3 mm wide, especially if there is blood flow within them
- Excrescences on the inner or outer aspect of a cystic area
- Ascites
- Other pelvic or omental masses.
Benign conditions
Several benign conditions have characteristic complex findings on ultrasonography (Table 2), whereas other findings can be indeterminate (Table 3) or worrisome for malignancy (Table 4).
Hemorrhagic corpus luteum cysts can be complex with an internal reticular pattern due to organizing clot and fibrin strands. A “ring of fire” vascular pattern is often seen around the cyst bed.
Dermoids (mature cystic teratomas) may have hyperechoic elements with acoustic shadowing and no internal Doppler flow. They can have a complex appearance due to fat, hair, and sebum within the cyst. Dermoid cysts have a pathognomonic appearance on CT with a clear fat-fluid level.
Endometriomas classically have a homogeneous “ground-glass” appearance or low-level echoes, without internal color Doppler flow, wall nodules, or other malignant features.
Fibroids may be pedunculated and may appear to be complex or solid adnexal masses.
Hydrosalpinges may present as tortuous tubular-shaped cystic masses. There may be incomplete septations or indentations seen on opposite sides (the “waist” sign).
Paratubal and paraovarian cysts are usually simple round cysts that can be demonstrated as separate from the ovary. Sometimes these appear complex as well.
Peritoneal inclusion cysts, also known as pseudocysts, are seen in patients with intra-abdominal adhesions. Often multiple septations are seen through clear fluid, with the cyst conforming to the shape of other pelvic structures.
Torsion of the ovary may occur with either benign or malignant masses. Torsion can be diagnosed when venous flow is absent on Doppler. The presence of flow, however, doesn’t rule out torsion, as torsion is often intermittent. The twisted ovary is most often enlarged and can have an edematous appearance. Although typically benign, these should be referred for urgent surgical treatment.
Vascularity
Doppler imaging is being extensively studied. The general principle is that malignant masses will be more vascular, with a high-volume, low-resistance pattern of flow. This can result in a pulsatility index of less than 1 or a resistive index of less than 0.4. In practice, however, there is significant overlap between high and low pulsatility indices and resistive indices in benign and malignant cysts. Low resistance can also be found in endometriomas, corpus luteum cysts, inflammatory masses, and vascular benign neoplasms. A normal (high) resistive index does not rule out malignancy.32,33
One Doppler finding that does seem to correlate with malignancy is the presence of any flow within a solid nodule or wall excrescence.
3D ultrasonography
As the use of 3D ultrasonography increases, studies are yielding different results as to its utility in describing ovarian masses. 3D ultrasonography may be useful in finding centrally located vessels so that Doppler can be applied.32
OTHER IMAGING
Although ultrasonography is the initial imaging study of choice in the evaluation of adnexal masses owing to its high sensitivity, availability, and low cost, studies have shown that up to 20% of adnexal masses can be reported as indeterminate by ultrasonography (Table 1).
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is emerging as a very valuable tool when ultrasonography is inconclusive or limited.35 Although MRI is very accurate (Table 1), it is not considered a first-line imaging test because it is more expensive, less available, and more inconvenient for the patient than ultrasonography.
MRI provides additional information on the composition of soft-tissue tumors. Usually, MRI is ordered with contrast, unless there are contraindications to it. The radiologist will evaluate morphologic features, signal intensity, and enhancement of solid areas. Techniques such as dynamic contrast-enhanced MRI (following the distribution of contrast material over time), in- and out-of-phase T1 imaging (looking for fat, such as in dermoids), and the newer diffusion-weighted imaging may further improve characterization.
In one study of MRI as second-line imaging, contrast-enhanced MRI contributed to a greater change in the probability of ovarian cancer than did CT, Doppler ultrasonography, or MRI without contrast.36 This may result in a reduction in unnecessary surgeries and in an increase in proper referrals in cases of suspected malignancy.
Computed tomography
Disadvantages of CT include radiation exposure and poor discrimination of soft tissue. It can, however, differentiate fat or calcifications that may be found in dermoids. While CT is not often used to describe an ovarian lesion, it may be used preoperatively to stage an ovarian cancer or to look for a primary intra-abdominal cancer when an ovarian mass may represent metastasis.32
MANAGING AN INCIDENTAL OVARIAN CYST OR CYSTIC MASS
Combining information from the history, physical examination, imaging, and blood work to assign a level of risk of malignancy is not straightforward. The clinician must weigh several imperfect tests, each with its own sensitivity and specificity, against the background of the individual patient’s likelihood of malignancy. Whereas a 4-cm simple cyst in a premenopausal woman can be assigned to a low-risk category and a complex mass with flow to a solid component in a postmenopausal woman can be assigned to a high-risk category, many lesions are more difficult to assess.
Several systems have been proposed for analyzing data and standardizing risk assessment. There are a number of scoring systems based on ultrasonographic morphology and several mathematical regression models that include menopausal status and tumor markers. But each has drawbacks, and none is definitively superior to expert opinion.16,17,37,38
A 2012 systematic review and meta-analysis39 calculated sensitivity and specificity for several imaging tests, scoring systems, and blood tumor markers. Some results are presented in Table 1.
The management of an ovarian cyst depends on symptoms, likelihood of torsion or rupture, and the level of concern for malignancy. At the lower-risk end of the spectrum, reassurance or observation over time may be appropriate. A general gynecologist can evaluate indeterminate or symptomatic ovarian cysts. Patients with masses frankly suspicious for malignancy are best referred to a gynecologic oncologist.
Expectant management for low-risk lesions
Low-risk lesions such as simple cysts, endometriomas, and dermoids have a less than 1% chance of malignancy. Most patients who have them require only reassurance or follow-up with serial ultrasonography. Oral contraceptives may prevent new cysts from forming. Aspiration is not recommended.
In 2010, the Society of Radiologists in Ultrasound issued a consensus statement regarding re-imaging of simple ovarian cysts.33
In premenopausal women, they recommend no further testing for cysts 5 cm or smaller, yearly follow-up for cysts larger than 5 cm and up to and including 7 cm, and MRI or surgical evaluation for cysts larger than 7 cm, as it is difficult to completely image a large cyst with ultrasonography.
In postmenopausal women, if the cyst is 1 cm in diameter or smaller, no further studies need to be done. For simple cysts larger than 1 cm and up to and including 7 cm, yearly re-imaging is recommended. And for cysts larger than 7 cm, MRI or surgery is indicated. The American College of Radiology recommends repeat ultrasonography and CA125 testing for cysts 3 cm and larger but doesn’t specify an interval.32
A cyst that is otherwise simple but has a single thin septation (< 3 mm) or a small calcification in the wall is almost always benign. Such a cyst should be followed as if it were a simple cyst, as indicated by patient age and cyst size.
There are no official guidelines as to when to stop serial imaging,22,32 but a recent paper suggested one or two ultrasonographic examinations to confirm size and morphologic stability.19 Once a lesion has resolved, there is no need for further imaging (Figures 1–3).
Birth control pills for suppression of new cysts. Oral contraceptives do not hasten the resolution of ovarian cysts, according to a 2011 Cochrane review.40 Some practitioners will, nevertheless, prescribe them in an attempt to prevent new cysts from confusing the picture.
Aspiration is not recommended for either diagnosis or treatment. It can only be considered in patients at high risk who are not surgical candidates. Results of cytologic study of specimens obtained by fine-needle aspiration cannot reliably determine the presence or absence of malignancy.41 There is also a theoretical risk of spreading cancer from an early-stage lesion. A retrospective study has suggested that spillage of cyst contents during surgery in early ovarian cancer is associated with a worse prognosis.42
From a therapeutic point of view, studies have shown the same resolution rate at 6 months for aspirated cysts vs those followed expectantly.43 Another study found a recurrence rate of 25% within 1 year of aspiration.44
Referral for medium-risk or indeterminant-risk ovarian masses
Patients who have medium- or indeterminaterisk ovarian masses (Table 3) should be referred to a gynecologist. Further testing will help stratify the risk of malignancy. This can include tumor marker blood tests, MRI, or CT, the addition of Doppler or 3D ultrasonography, serial ultrasonography, or surgical exploration.
If repeat ultrasonography is chosen, the interval will likely be 6 to 12 weeks. Surgery may consist of removing only the cyst itself, or the whole ovary with or without the tube, or sometimes both ovaries. Purely diagnostic laparoscopy is rarely performed, as direct visualization of a lesion is rarely helpful. Frozen section should be employed, and the operating gynecologist should have oncologic backup, since the surgery is performed to rule out malignancy.
In the case of a benign-appearing cyst larger than 6 cm, thought must be given as to whether it is likely to rupture or twist. Rupture of a large cyst can lead to pain and in some cases to hemorrhage. Contents of a ruptured dermoid cyst can cause chemical peritonitis. Torsion of an ovary can result in loss of the ovary through compromised perfusion. A general gynecologist can decide with the patient whether preemptive surgery is indicated.
Operative evaluation for high-risk masses
Patients with high-risk ovarian masses (Table 4) are best referred to a gynecologic oncologist for operative evaluation. If features are seen that indicate malignancy, such as thick septations, solid areas with blood flow, ascites, or other pelvic masses, surgery is indicated. The surgical approach may be through laparoscopy or laparotomy.45 It should be noted that even in the face of worrisome features on ultrasonography, many masses turn out to be benign.
In 2011, the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology issued new guidelines recommending oncologic referral of patients with high-risk masses. Elevated CA125, ascites, a nodular or fixed pelvic mass, or evidence of metastasis in postmenopausal women requires oncologic evaluation.26 For premenopausal women, a very elevated CA125, ascites, or metastasis requires referral (Table 4).26
Direct referral to a gynecologic oncologist is underutilized. A recent study found that fewer than half of primary care physicians said that they would refer a classic suspicious case directly to a subspecialist.46 It is estimated that only 33% of ovarian cancers are first operated on by a gynecologic oncologist.47
A 2011 Cochrane review confirmed a survival benefit for women with cancer who are operated on by gynecologic oncologists primarily, rather than by a general gynecologist and then referred.48 A gynecologic oncologist is most likely to perform proper staging and debulking at the time of initial diagnosis.49
Special situations require consultation
Ovarian cysts in pregnancy are most often benign,50 but malignancy is always a possibility. Functional cysts and dermoids are the most common. These may remain asymptomatic or may rupture or twist or cause difficulty with delivering the baby. Surgical intervention, if needed, should be performed in the second trimester if possible. A multidisciplinary approach and referral to a perinatologist and gynecologic oncologist are advised.
Symptomatic ovarian cysts that may need surgical intervention are the purview of the general gynecologist. If the risk of a surgical emergency is judged to be low, a symptomatic patient may be supported with pain medication and may be managed on an outpatient basis. Immediate surgical consultation is appropriate if the patient appears toxic or in shock. Depending on the clinical picture, there may be a ruptured tubo-ovarian abscess, ruptured ectopic pregnancy, ruptured hemorrhagic cyst, or ovarian torsion, any of which may need immediate surgical intervention.
If a symptomatic mass is highly suspicious for cancer, a gynecologic oncologist should be consulted directly.
WHEN TO REASSURE, REASSESS, REFER
Ovarian masses often pose diagnostic and management dilemmas. Reassurance can be offered to women with small simple cysts. Interval follow-up with ultrasonography is appropriate for cysts that are most likely to be benign. If malignancy is suspected based on ultrasonography, other imaging, blood testing, or expert opinion, referral to a surgical gynecologist or gynecologic oncologist is recommended. If malignancy is strongly suspected, direct referral to a gynecologic oncologist offers the best chance of survival if cancer is actually present.
Reassure
- When simple cysts are less than 1 cm in postmenopausal women
- When simple cysts are less than 5 cm in premenopausal patients.
Reassess
- With yearly ultrasonography in cases of very low risk
- With repeat ultrasonography in 6 to 12 weeks when the diagnosis is not clear but the cyst is likely benign.
Refer
- To a gynecologist for symptomatic cysts, cysts larger than 6 cm, and cysts that require ancillary testing
- To a gynecologic oncologist for findings worrisome for cancer, such as thick septations, solid areas with flow, ascites, evidence of metastasis, or high cancer antigen 125 levels.
- Borgfeldt C, Andolf E. Transvaginal sonographic ovarian findings in a random sample of women 25–40 years old. Ultrasound Obstet Gynecol 1999; 13:345–350.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol 2003; 102:594–599.
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol 2010; 202:373.e1–373.e9.
- Jordan SJ, Green AC, Whiteman DC, Webb PM; Australian Ovarian Cancer Study Group. Risk factors for benign, borderline and invasive mucinous ovarian tumors: epidemiological evidence of a neoplastic continuum? Gynecol Oncol 2007; 107:223–230.
- NIH consensus conference. Ovarian cancer. Screening, treatment, and follow-up. NIH Consensus Development Panel on Ovarian Cancer. JAMA 1995; 273:491–497.
- The reduction in risk of ovarian cancer associated with oral-contraceptive use. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. N Engl J Med 1987; 316:650–655.
- Young RL, Snabes MC, Frank ML, Reilly M. A randomized, double-blind, placebo-controlled comparison of the impact of low-dose and triphasic oral contraceptives on follicular development. Am J Obstet Gynecol 1992; 167:678–682.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol 2009; 113:1027–1037.
- Sharma A, Gentry-Maharaj A, Burnell M, et al; UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Assessing the malignant potential of ovarian inclusion cysts in postmenopausal women within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a prospective cohort study. BJOG 2012; 119:207–219.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011; 305:2295–2303.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009; 361:170–177.
- National Cancer Institute. Surveillance Epidemiology and End Results (SEER). Cancer statistics on ovarian cancer. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed May 9, 2013.
- American Cancer Society. Survival by ovarian cancer stage. www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-survival-rates. Accessed May 9, 2013.
- Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med 2009; 6:e1000114.
- Padilla LA, Radosevich DM, Milad MP. Limitations of the pelvic examination for evaluation of the female pelvic organs. Int J Gynaecol Obstet 2005; 88:84–88.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of Adnexal Mass. Evidence Report/Technology Assessment No.130 (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-02-0025.) AHRQ Publication No. 06-E004. Rockville, MD: Agency for Healthcare Research and Quality. February 2006.
- Ameye L, Timmerman D, Valentin L, et al. Clinically oriented three-step strategy for assessment of adnexal pathology. Ultrasound Obstet Gynecol 2012; 40:582–591.
- Covens AL, Dodge JE, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Surgical management of a suspicious adnexal mass: a systematic review. Gynecol Oncol 2012; 126:149–156.
- Liu JH, Zanotti KM. Management of the adnexal mass. Obstet Gynecol 2011; 117:1413–1428.
- Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer 2007; 109:221–227.
- Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst 2010; 102:222–229.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Management of adnexal masses. Obstet Gynecol 2007; 110:201–214.
- BestPractice BMJ Evidence Centre. Ovarian cysts-Diagnosis-History & examination—Risk factors. http://bestpractice.bmj.com/best-practice/monograph/660/diagnosis.html. Accessed June 2, 2013.
- American Cancer Society. Ovarian-cancer risk factors. www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-survival-rates. Accessed May 9, 2013.
- Bast RC, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983; 309:883–887.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 477: the role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer. Obstet Gynecol 2011; 117:742–746.
- Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol 2005; 105:35–41.
- Dearking AC, Aletti GD, McGree ME, Weaver AL, Sommerfield MK, Cliby WA. How relevant are ACOG and SGO guidelines for referral of adnexal mass? Obstet Gynecol 2007; 110:841–848.
- Skates SJ, Menon U, MacDonald N, et al. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J Clin Oncol 2003; 21(suppl 10):206s–210s.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol 2011; 117:1289–1297.
- Ware Miller R, Smith A, DeSimone CP, et al. Performance of the American College of Obstetricians and Gynecologists’ ovarian tumor referral guidelines with a multivariate index assay. Obstet Gynecol 2011; 117:1298–1306.
- Lev-Toaff AS, Horrow MM, Andreotti RF, et al. Expert Panel on Women’s Imaging. ACR Appropriateness Criteria clinically suspected adnexal mass. Reston, VA: American College of Radiology (ACR), 2009. www.guidelines.gov/content.aspx?id=15780&search=adnexal+mass. Accessed May 9, 2013.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256:943–954.
- Alcázar JL, Castillo G, Jurado M, García GL. Is expectant management of sonographically benign adnexal cysts an option in selected asymptomatic premenopausal women? Hum Reprod 2005; 20:3231–3234.
- Medeiros LR, Freitas LB, Rosa DD, et al. Accuracy of magnetic resonance imaging in ovarian tumor: a systematic quantitative review. Am J Obstet Gynecol 2011; 204:67.e1–67.e10.
- Kinkel K, Lu Y, Mehdizade A, Pelte MF, Hricak H. Indeterminate ovarian mass at US: incremental value of second imaging test for characterization—meta-analysis and Bayesian analysis. Radiology 2005; 236:85–94.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ 2010; 341:c6839.
- Valentin L, Ameye L, Savelli L, et al. Adnexal masses difficult to classify as benign or malignant using subjective assessment of gray-scale and Doppler ultrasound findings: logistic regression models do not help. Ultrasound Obstet Gynecol 2011; 38:456–465.
- Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol 2012; 126:157–166.
- Grimes DA, Jones LB, Lopez LM, Schulz KF. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev 2011; 9:CD006134.
- Higgins RV, Matkins JF, Marroum MC. Comparison of fine-needle aspiration cytologic findings of ovarian cysts with ovarian histologic findings. Am J Obstet Gynecol 1999; 180:550–553.
- Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 2001; 357:176–182.
- Zanetta G, Lissoni A, Torri V, et al. Role of puncture and aspiration in expectant management of simple ovarian cysts: a randomised study. BMJ 1996; 313:1110–1113.
- Bonilla-Musoles F, Ballester MJ, Simon C, Serra V, Raga F. Is avoidance of surgery possible in patients with perimenopausal ovarian tumors using transvaginal ultrasound and duplex color Doppler sonography? J Ultrasound Med 1993; 12:33–39.
- Medeiros LR, Rosa DD, Bozzetti MC, et al. Laparoscopy versus laparotomy for FIGO Stage I ovarian cancer. Cochrane Database Syst Rev 2008; 4:CD005344.
- Goff BA, Miller JW, Matthews B, et al. Involvement of gynecologic oncologists in the treatment of patients with a suspicious ovarian mass. Obstet Gynecol 2011; 118:854–862.
- Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst 2006; 98:172–180.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol 2005; 99:447–461.
- Cress RD, Bauer K, O’Malley CD, et al. Surgical staging of early stage epithelial ovarian cancer: results from the CDC-NPCR ovarian patterns of care study. Gynecol Oncol 2011; 121:94–99.
- Horowitz NS. Management of adnexal masses in pregnancy. Clin Obstet Gynecol 2011; 54:519–527.
Ovarian cysts, sometimes reported as ovarian masses or adnexal masses, are frequently found incidentally in women who have no symptoms. These cysts can be physiologic (having to do with ovulation) or neoplastic—either benign, borderline (having low malignant potential), or frankly malignant. Thus, these incidental lesions pose many diagnostic challenges to the clinician.
The vast majority of cysts are benign, but a few are malignant, and ovarian malignancies have a notoriously poor survival rate. The diagnosis can only be obtained surgically, as aspiration and biopsy are not definitive and may be harmful. Therefore, the clinician must try to balance the risks of surgery for what may be a benign lesion with the risk of delaying diagnosis of a malignancy.
In this article we provide an approach to evaluating these cysts, with guidance on when the patient can be reassured and when referral is needed.
THE DILEMMA OF OVARIAN CYSTS
Ovarian cysts are common
Premenopausal women can be expected to make at least a small cyst or follicle almost every month. The point prevalence for significant cysts has been reported to be almost 8% in premenopausal women.1
Surprisingly, the prevalence in postmenopausal women is as high as 14% to 18%, with a yearly incidence of 8%. From 30% to 54% of postmenopausal ovarian cysts persist for years.2,3
Little is known about the cause of most cysts
Little is known about the cause of most ovarian cysts. Functional or physiologic cysts are thought to be variations in the ovulatory process. They do not seem to be precursors to ovarian cancer.
Most benign neoplastic cysts are also not thought to be precancerous, with the possible exception of the mucinous kind.4 Ovarian cysts do not increase the risk of ovarian cancer later in life,3,9 and removing benign cysts has not been shown to decrease the risk of death from ovarian cancer.10
Most ovarian cysts and masses are benign
Simple ovarian cysts are much more likely to be benign than malignant. Complex and solid ovarian masses are also more likely to be benign, regardless of menopausal status, but more malignancies are found in this group.
With any kind of mass, the chances of malignancy increase with age. Children and adolescents are not discussed in this article; they should be referred to a specialist.
Ovarian cancer often has a poor prognosis
This “silent” cancer is most often discovered and treated when it has already spread, contributing to a reported 5-year survival rate of only 33% to 46%.11–13 Ideally, ovarian cancer would be found and removed while still confined to the ovary, when the 5-year survival rate is greater than 90%.
Unfortunately, there does not seem to be a precursor lesion for most ovarian cancers, and there is no good way of finding it in the stage 1 phase, so detecting this cancer before it spreads remains an elusive goal.11,14
Surgery is required to diagnose difficult cases
There is no perfect test for the preoperative assessment of a cystic ovarian mass. Every method has drawbacks (Table 1).15–18 Therefore, the National Institutes of Health estimates that 5% to 10% of women in the United States will undergo surgical exploration for an ovarian cyst in their lifetime. Only 13% to 21% of these cysts will be malignant.5
ASSESSING AN INCIDENTALLY DISCOVERED OVARIAN MASS
Certain factors in the history, physical examination, and blood work may suggest the cyst is either benign or malignant and may influence the subsequent assessment. However, in most cases, the best next step is to perform transvaginal ultrasonography, which we will discuss later in this paper.
History
Age is a major risk factor for ovarian cancer; the median age at diagnosis is 63 years.9 In the reproductive-age group, ovarian cysts are much more likely to be functional than neoplastic. Epithelial cancers are rare before the age of 40, but other cancer types such as borderline, germ cell, and sex cord stromal tumors may occur.19
In every age group a cyst is more likely to be benign than malignant, although, as noted above, the probability of malignancy increases with age.
Symptoms. Most ovarian cysts, benign or malignant, are asymptomatic and are found only incidentally.
The most commonly reported symptoms are pelvic or lower-abdominal pressure or pain. Acutely painful conditions include ovarian torsion, hemorrhage into the cyst, cyst rupture with or without intra-abdominal hemorrhage, ectopic pregnancy, and pelvic inflammatory disease with tubo-ovarian abscess.
Some patients who have ovarian cancer report vague symptoms such as urinary urgency or frequency, abdominal distention or bloating, and difficulty eating or early satiety.20 Although the positive predictive value of this symptom constellation is only about 1%, its usefulness increases if these symptoms arose recently (within the past year) and occur than 12 days a month.21
Family history of ovarian, breast, endometrial, or colon cancer is of particular interest. The greater the number of affected relatives and the closer the degree of relation, the greater the risk; in some cases the relative risk is 40 times greater.22 Breast-ovarian cancer syndromes, hereditary nonpolyposis colorectal cancer syndrome, and family cancer syndrome, as well as extremely high-risk pedigrees such as BRCA1, BRCA2, and Lynch syndrome, all place women at significantly higher risk. Daughters tend to develop cancer at a younger age than their affected mothers.
However, only 10% of ovarian cancers occur in patients who have a family history of it, leaving 90% as sporadic occurrences.
Other history. Factors protective against ovarian cancer include use of oral contraceptives at any time, tubal ligation, hysterectomy, having had children, breastfeeding, a low-fat diet, and possibly use of aspirin and acetaminophen.23,24
Risk factors for malignancy include advanced age; nulliparity; family history of ovarian or breast cancer; personal history of breast cancer; talc use; asbestos exposure; white ethnicity; pelvic irradiation; smoking; alcohol use; possibly the previous use of fertility drugs, estrogen, or androgen; history of mumps; urban location; early menarche; and late menopause.24
Physical examination
Vital signs. Fever can indicate an infectious process or torsion of the ovary. A sudden onset of low blood pressure or rapid pulse can indicate a hemorrhagic condition such as ectopic pregnancy or ruptured hemorrhagic cyst.
Bimanual pelvic examination is notoriously inaccurate for detecting and characterizing ovarian cysts. In one prospective study, examiners blinded to the reason for surgery evaluated women under anesthesia. The authors concluded that bimanual examination was of limited value even under the best circumstances.15 Pelvic examination can be even more difficult in patients who are obese, are virginal, have vaginal atrophy, or are in pain.
Useful information that can be obtained through the bimanual examination includes the exact location of pelvic tenderness, the relative firmness of an identified mass, and the existence of nodularity in the posterior cul-de-sac, suggesting advanced ovarian cancer.
Tumor markers
Cancer antigen 125 (CA125) is the most studied and widely used of the ovarian cancer tumor markers. When advanced epithelial ovarian cancer is associated with a markedly elevated level, the value correlates with tumor burden.25
Unfortunately, only about half of early-stage ovarian cancers and 75% to 80% of advanced ovarian cancers express this marker.26 Especially in premenopausal women, there are many pelvic conditions that can falsely elevate CA125. Therefore, its sensitivity and specificity for predicting ovarian cancer are suboptimal. Nevertheless, CA125 is often used to help stratify risk when assessing known ovarian cysts and masses.
The value considered abnormal in postmenopausal women is 35 U/mL or greater, while in premenopausal women the cutoff is less well defined. The lower the cutoff level is set, the more sensitive the test. Recent recommendations advise 50 U/mL or 67 U/mL, rather than the 200 U/mL recommended in the 2002 joint guidelines of the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology.27,28
However, specificity is likely to be lower with these lower cutoff values. Conditions that can elevate CA125 levels include almost anything that irritates the peritoneum, including pregnancy, menstruation, fibroids, endometriosis, infection, and ovarian hyperstimulation, as well as medical conditions such as liver or renal disease, colitis, diverticulitis, congestive heart failure, diabetes, autoimmune diseases, and ascites.
Following serial CA125 levels may be more sensitive than trying to establish a single cutoff value.29 CA125 should not be used as a screening tool in average-risk women.26
OVA1. Several biomarker panels have been developed and evaluated for risk assessment in women with pelvic masses. OVA1, a proprietary panel of tests (Vermillion; Austin, TX) received US Food and Drug Administration approval in 2009. It includes CA125 and four other proteins, from which it calculates a probability score (high or low) using a proprietary formula.
In prospective studies, OVA1 was more sensitive than clinical assessment or CA125 alone.30 The higher sensitivity and negative predictive value were counterbalanced by a lower specificity and positive predictive value.31 Its cost ($650) is not always covered by insurance. OVA1 is not a screening tool.
EVALUATION WITH ULTRASONOGRAPHY
Ultrasonography is the imaging test of choice in assessing adnexal cysts and masses, and therefore it is the best next step after taking a history, performing a physical examination, and obtaining blood work.32 In cases in which an incidental ovarian mass is discovered on computed tomography (CT), further characterization by ultrasonography will likely yield helpful information.
Pelvic ultrasonography can be performed transabdominally or transvaginally. Vaginal ultrasonography gives the clearest images in most patients. Abdominal scanning is indicated for large masses, when vaginal access is difficult (as in virginal patients or those with vaginal atrophy) or when the mass is out of the focal length of the vaginal probe. A full bladder is usually required for the best transabdominal images.
The value of the images obtained depends on the experience of the ultrasonographer and reader and on the equipment. Also, there is currently no widely used standard for reporting the findings33—descriptions are individualized, leading some authors to recommend that the clinician personally review the films to get the most accurate picture.19
Size
Size alone cannot be used to distinguish between benign and malignant lesions. Simple cysts up to 10 cm are most likely benign regardless of menopausal status.2,34 However, in a complex or solid mass, size correlates somewhat with the chance of malignancy, with notable exceptions, such as the famously large sizes of some solid fibromas or mucinous cystadenomas. Also, size may correlate with risk of other complications such as torsion or symptomatic rupture.
Complexity
Simple cysts have clear fluid, thin smooth walls, no loculations or septae, and enhanced through-transmission of echo waves.32,33
Complexity is described in terms of septations, wall thickness, internal echoes, and solid nodules. Increasing complexity does correlate with increased risk of malignancy.
Worrisome findings
The most worrisome findings are:
- Solid areas that are not hyperechoic, especially when there is blood flow to them
- Thick septations, more than 2 or 3 mm wide, especially if there is blood flow within them
- Excrescences on the inner or outer aspect of a cystic area
- Ascites
- Other pelvic or omental masses.
Benign conditions
Several benign conditions have characteristic complex findings on ultrasonography (Table 2), whereas other findings can be indeterminate (Table 3) or worrisome for malignancy (Table 4).
Hemorrhagic corpus luteum cysts can be complex with an internal reticular pattern due to organizing clot and fibrin strands. A “ring of fire” vascular pattern is often seen around the cyst bed.
Dermoids (mature cystic teratomas) may have hyperechoic elements with acoustic shadowing and no internal Doppler flow. They can have a complex appearance due to fat, hair, and sebum within the cyst. Dermoid cysts have a pathognomonic appearance on CT with a clear fat-fluid level.
Endometriomas classically have a homogeneous “ground-glass” appearance or low-level echoes, without internal color Doppler flow, wall nodules, or other malignant features.
Fibroids may be pedunculated and may appear to be complex or solid adnexal masses.
Hydrosalpinges may present as tortuous tubular-shaped cystic masses. There may be incomplete septations or indentations seen on opposite sides (the “waist” sign).
Paratubal and paraovarian cysts are usually simple round cysts that can be demonstrated as separate from the ovary. Sometimes these appear complex as well.
Peritoneal inclusion cysts, also known as pseudocysts, are seen in patients with intra-abdominal adhesions. Often multiple septations are seen through clear fluid, with the cyst conforming to the shape of other pelvic structures.
Torsion of the ovary may occur with either benign or malignant masses. Torsion can be diagnosed when venous flow is absent on Doppler. The presence of flow, however, doesn’t rule out torsion, as torsion is often intermittent. The twisted ovary is most often enlarged and can have an edematous appearance. Although typically benign, these should be referred for urgent surgical treatment.
Vascularity
Doppler imaging is being extensively studied. The general principle is that malignant masses will be more vascular, with a high-volume, low-resistance pattern of flow. This can result in a pulsatility index of less than 1 or a resistive index of less than 0.4. In practice, however, there is significant overlap between high and low pulsatility indices and resistive indices in benign and malignant cysts. Low resistance can also be found in endometriomas, corpus luteum cysts, inflammatory masses, and vascular benign neoplasms. A normal (high) resistive index does not rule out malignancy.32,33
One Doppler finding that does seem to correlate with malignancy is the presence of any flow within a solid nodule or wall excrescence.
3D ultrasonography
As the use of 3D ultrasonography increases, studies are yielding different results as to its utility in describing ovarian masses. 3D ultrasonography may be useful in finding centrally located vessels so that Doppler can be applied.32
OTHER IMAGING
Although ultrasonography is the initial imaging study of choice in the evaluation of adnexal masses owing to its high sensitivity, availability, and low cost, studies have shown that up to 20% of adnexal masses can be reported as indeterminate by ultrasonography (Table 1).
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is emerging as a very valuable tool when ultrasonography is inconclusive or limited.35 Although MRI is very accurate (Table 1), it is not considered a first-line imaging test because it is more expensive, less available, and more inconvenient for the patient than ultrasonography.
MRI provides additional information on the composition of soft-tissue tumors. Usually, MRI is ordered with contrast, unless there are contraindications to it. The radiologist will evaluate morphologic features, signal intensity, and enhancement of solid areas. Techniques such as dynamic contrast-enhanced MRI (following the distribution of contrast material over time), in- and out-of-phase T1 imaging (looking for fat, such as in dermoids), and the newer diffusion-weighted imaging may further improve characterization.
In one study of MRI as second-line imaging, contrast-enhanced MRI contributed to a greater change in the probability of ovarian cancer than did CT, Doppler ultrasonography, or MRI without contrast.36 This may result in a reduction in unnecessary surgeries and in an increase in proper referrals in cases of suspected malignancy.
Computed tomography
Disadvantages of CT include radiation exposure and poor discrimination of soft tissue. It can, however, differentiate fat or calcifications that may be found in dermoids. While CT is not often used to describe an ovarian lesion, it may be used preoperatively to stage an ovarian cancer or to look for a primary intra-abdominal cancer when an ovarian mass may represent metastasis.32
MANAGING AN INCIDENTAL OVARIAN CYST OR CYSTIC MASS
Combining information from the history, physical examination, imaging, and blood work to assign a level of risk of malignancy is not straightforward. The clinician must weigh several imperfect tests, each with its own sensitivity and specificity, against the background of the individual patient’s likelihood of malignancy. Whereas a 4-cm simple cyst in a premenopausal woman can be assigned to a low-risk category and a complex mass with flow to a solid component in a postmenopausal woman can be assigned to a high-risk category, many lesions are more difficult to assess.
Several systems have been proposed for analyzing data and standardizing risk assessment. There are a number of scoring systems based on ultrasonographic morphology and several mathematical regression models that include menopausal status and tumor markers. But each has drawbacks, and none is definitively superior to expert opinion.16,17,37,38
A 2012 systematic review and meta-analysis39 calculated sensitivity and specificity for several imaging tests, scoring systems, and blood tumor markers. Some results are presented in Table 1.
The management of an ovarian cyst depends on symptoms, likelihood of torsion or rupture, and the level of concern for malignancy. At the lower-risk end of the spectrum, reassurance or observation over time may be appropriate. A general gynecologist can evaluate indeterminate or symptomatic ovarian cysts. Patients with masses frankly suspicious for malignancy are best referred to a gynecologic oncologist.
Expectant management for low-risk lesions
Low-risk lesions such as simple cysts, endometriomas, and dermoids have a less than 1% chance of malignancy. Most patients who have them require only reassurance or follow-up with serial ultrasonography. Oral contraceptives may prevent new cysts from forming. Aspiration is not recommended.
In 2010, the Society of Radiologists in Ultrasound issued a consensus statement regarding re-imaging of simple ovarian cysts.33
In premenopausal women, they recommend no further testing for cysts 5 cm or smaller, yearly follow-up for cysts larger than 5 cm and up to and including 7 cm, and MRI or surgical evaluation for cysts larger than 7 cm, as it is difficult to completely image a large cyst with ultrasonography.
In postmenopausal women, if the cyst is 1 cm in diameter or smaller, no further studies need to be done. For simple cysts larger than 1 cm and up to and including 7 cm, yearly re-imaging is recommended. And for cysts larger than 7 cm, MRI or surgery is indicated. The American College of Radiology recommends repeat ultrasonography and CA125 testing for cysts 3 cm and larger but doesn’t specify an interval.32
A cyst that is otherwise simple but has a single thin septation (< 3 mm) or a small calcification in the wall is almost always benign. Such a cyst should be followed as if it were a simple cyst, as indicated by patient age and cyst size.
There are no official guidelines as to when to stop serial imaging,22,32 but a recent paper suggested one or two ultrasonographic examinations to confirm size and morphologic stability.19 Once a lesion has resolved, there is no need for further imaging (Figures 1–3).
Birth control pills for suppression of new cysts. Oral contraceptives do not hasten the resolution of ovarian cysts, according to a 2011 Cochrane review.40 Some practitioners will, nevertheless, prescribe them in an attempt to prevent new cysts from confusing the picture.
Aspiration is not recommended for either diagnosis or treatment. It can only be considered in patients at high risk who are not surgical candidates. Results of cytologic study of specimens obtained by fine-needle aspiration cannot reliably determine the presence or absence of malignancy.41 There is also a theoretical risk of spreading cancer from an early-stage lesion. A retrospective study has suggested that spillage of cyst contents during surgery in early ovarian cancer is associated with a worse prognosis.42
From a therapeutic point of view, studies have shown the same resolution rate at 6 months for aspirated cysts vs those followed expectantly.43 Another study found a recurrence rate of 25% within 1 year of aspiration.44
Referral for medium-risk or indeterminant-risk ovarian masses
Patients who have medium- or indeterminaterisk ovarian masses (Table 3) should be referred to a gynecologist. Further testing will help stratify the risk of malignancy. This can include tumor marker blood tests, MRI, or CT, the addition of Doppler or 3D ultrasonography, serial ultrasonography, or surgical exploration.
If repeat ultrasonography is chosen, the interval will likely be 6 to 12 weeks. Surgery may consist of removing only the cyst itself, or the whole ovary with or without the tube, or sometimes both ovaries. Purely diagnostic laparoscopy is rarely performed, as direct visualization of a lesion is rarely helpful. Frozen section should be employed, and the operating gynecologist should have oncologic backup, since the surgery is performed to rule out malignancy.
In the case of a benign-appearing cyst larger than 6 cm, thought must be given as to whether it is likely to rupture or twist. Rupture of a large cyst can lead to pain and in some cases to hemorrhage. Contents of a ruptured dermoid cyst can cause chemical peritonitis. Torsion of an ovary can result in loss of the ovary through compromised perfusion. A general gynecologist can decide with the patient whether preemptive surgery is indicated.
Operative evaluation for high-risk masses
Patients with high-risk ovarian masses (Table 4) are best referred to a gynecologic oncologist for operative evaluation. If features are seen that indicate malignancy, such as thick septations, solid areas with blood flow, ascites, or other pelvic masses, surgery is indicated. The surgical approach may be through laparoscopy or laparotomy.45 It should be noted that even in the face of worrisome features on ultrasonography, many masses turn out to be benign.
In 2011, the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology issued new guidelines recommending oncologic referral of patients with high-risk masses. Elevated CA125, ascites, a nodular or fixed pelvic mass, or evidence of metastasis in postmenopausal women requires oncologic evaluation.26 For premenopausal women, a very elevated CA125, ascites, or metastasis requires referral (Table 4).26
Direct referral to a gynecologic oncologist is underutilized. A recent study found that fewer than half of primary care physicians said that they would refer a classic suspicious case directly to a subspecialist.46 It is estimated that only 33% of ovarian cancers are first operated on by a gynecologic oncologist.47
A 2011 Cochrane review confirmed a survival benefit for women with cancer who are operated on by gynecologic oncologists primarily, rather than by a general gynecologist and then referred.48 A gynecologic oncologist is most likely to perform proper staging and debulking at the time of initial diagnosis.49
Special situations require consultation
Ovarian cysts in pregnancy are most often benign,50 but malignancy is always a possibility. Functional cysts and dermoids are the most common. These may remain asymptomatic or may rupture or twist or cause difficulty with delivering the baby. Surgical intervention, if needed, should be performed in the second trimester if possible. A multidisciplinary approach and referral to a perinatologist and gynecologic oncologist are advised.
Symptomatic ovarian cysts that may need surgical intervention are the purview of the general gynecologist. If the risk of a surgical emergency is judged to be low, a symptomatic patient may be supported with pain medication and may be managed on an outpatient basis. Immediate surgical consultation is appropriate if the patient appears toxic or in shock. Depending on the clinical picture, there may be a ruptured tubo-ovarian abscess, ruptured ectopic pregnancy, ruptured hemorrhagic cyst, or ovarian torsion, any of which may need immediate surgical intervention.
If a symptomatic mass is highly suspicious for cancer, a gynecologic oncologist should be consulted directly.
WHEN TO REASSURE, REASSESS, REFER
Ovarian masses often pose diagnostic and management dilemmas. Reassurance can be offered to women with small simple cysts. Interval follow-up with ultrasonography is appropriate for cysts that are most likely to be benign. If malignancy is suspected based on ultrasonography, other imaging, blood testing, or expert opinion, referral to a surgical gynecologist or gynecologic oncologist is recommended. If malignancy is strongly suspected, direct referral to a gynecologic oncologist offers the best chance of survival if cancer is actually present.
Reassure
- When simple cysts are less than 1 cm in postmenopausal women
- When simple cysts are less than 5 cm in premenopausal patients.
Reassess
- With yearly ultrasonography in cases of very low risk
- With repeat ultrasonography in 6 to 12 weeks when the diagnosis is not clear but the cyst is likely benign.
Refer
- To a gynecologist for symptomatic cysts, cysts larger than 6 cm, and cysts that require ancillary testing
- To a gynecologic oncologist for findings worrisome for cancer, such as thick septations, solid areas with flow, ascites, evidence of metastasis, or high cancer antigen 125 levels.
Ovarian cysts, sometimes reported as ovarian masses or adnexal masses, are frequently found incidentally in women who have no symptoms. These cysts can be physiologic (having to do with ovulation) or neoplastic—either benign, borderline (having low malignant potential), or frankly malignant. Thus, these incidental lesions pose many diagnostic challenges to the clinician.
The vast majority of cysts are benign, but a few are malignant, and ovarian malignancies have a notoriously poor survival rate. The diagnosis can only be obtained surgically, as aspiration and biopsy are not definitive and may be harmful. Therefore, the clinician must try to balance the risks of surgery for what may be a benign lesion with the risk of delaying diagnosis of a malignancy.
In this article we provide an approach to evaluating these cysts, with guidance on when the patient can be reassured and when referral is needed.
THE DILEMMA OF OVARIAN CYSTS
Ovarian cysts are common
Premenopausal women can be expected to make at least a small cyst or follicle almost every month. The point prevalence for significant cysts has been reported to be almost 8% in premenopausal women.1
Surprisingly, the prevalence in postmenopausal women is as high as 14% to 18%, with a yearly incidence of 8%. From 30% to 54% of postmenopausal ovarian cysts persist for years.2,3
Little is known about the cause of most cysts
Little is known about the cause of most ovarian cysts. Functional or physiologic cysts are thought to be variations in the ovulatory process. They do not seem to be precursors to ovarian cancer.
Most benign neoplastic cysts are also not thought to be precancerous, with the possible exception of the mucinous kind.4 Ovarian cysts do not increase the risk of ovarian cancer later in life,3,9 and removing benign cysts has not been shown to decrease the risk of death from ovarian cancer.10
Most ovarian cysts and masses are benign
Simple ovarian cysts are much more likely to be benign than malignant. Complex and solid ovarian masses are also more likely to be benign, regardless of menopausal status, but more malignancies are found in this group.
With any kind of mass, the chances of malignancy increase with age. Children and adolescents are not discussed in this article; they should be referred to a specialist.
Ovarian cancer often has a poor prognosis
This “silent” cancer is most often discovered and treated when it has already spread, contributing to a reported 5-year survival rate of only 33% to 46%.11–13 Ideally, ovarian cancer would be found and removed while still confined to the ovary, when the 5-year survival rate is greater than 90%.
Unfortunately, there does not seem to be a precursor lesion for most ovarian cancers, and there is no good way of finding it in the stage 1 phase, so detecting this cancer before it spreads remains an elusive goal.11,14
Surgery is required to diagnose difficult cases
There is no perfect test for the preoperative assessment of a cystic ovarian mass. Every method has drawbacks (Table 1).15–18 Therefore, the National Institutes of Health estimates that 5% to 10% of women in the United States will undergo surgical exploration for an ovarian cyst in their lifetime. Only 13% to 21% of these cysts will be malignant.5
ASSESSING AN INCIDENTALLY DISCOVERED OVARIAN MASS
Certain factors in the history, physical examination, and blood work may suggest the cyst is either benign or malignant and may influence the subsequent assessment. However, in most cases, the best next step is to perform transvaginal ultrasonography, which we will discuss later in this paper.
History
Age is a major risk factor for ovarian cancer; the median age at diagnosis is 63 years.9 In the reproductive-age group, ovarian cysts are much more likely to be functional than neoplastic. Epithelial cancers are rare before the age of 40, but other cancer types such as borderline, germ cell, and sex cord stromal tumors may occur.19
In every age group a cyst is more likely to be benign than malignant, although, as noted above, the probability of malignancy increases with age.
Symptoms. Most ovarian cysts, benign or malignant, are asymptomatic and are found only incidentally.
The most commonly reported symptoms are pelvic or lower-abdominal pressure or pain. Acutely painful conditions include ovarian torsion, hemorrhage into the cyst, cyst rupture with or without intra-abdominal hemorrhage, ectopic pregnancy, and pelvic inflammatory disease with tubo-ovarian abscess.
Some patients who have ovarian cancer report vague symptoms such as urinary urgency or frequency, abdominal distention or bloating, and difficulty eating or early satiety.20 Although the positive predictive value of this symptom constellation is only about 1%, its usefulness increases if these symptoms arose recently (within the past year) and occur than 12 days a month.21
Family history of ovarian, breast, endometrial, or colon cancer is of particular interest. The greater the number of affected relatives and the closer the degree of relation, the greater the risk; in some cases the relative risk is 40 times greater.22 Breast-ovarian cancer syndromes, hereditary nonpolyposis colorectal cancer syndrome, and family cancer syndrome, as well as extremely high-risk pedigrees such as BRCA1, BRCA2, and Lynch syndrome, all place women at significantly higher risk. Daughters tend to develop cancer at a younger age than their affected mothers.
However, only 10% of ovarian cancers occur in patients who have a family history of it, leaving 90% as sporadic occurrences.
Other history. Factors protective against ovarian cancer include use of oral contraceptives at any time, tubal ligation, hysterectomy, having had children, breastfeeding, a low-fat diet, and possibly use of aspirin and acetaminophen.23,24
Risk factors for malignancy include advanced age; nulliparity; family history of ovarian or breast cancer; personal history of breast cancer; talc use; asbestos exposure; white ethnicity; pelvic irradiation; smoking; alcohol use; possibly the previous use of fertility drugs, estrogen, or androgen; history of mumps; urban location; early menarche; and late menopause.24
Physical examination
Vital signs. Fever can indicate an infectious process or torsion of the ovary. A sudden onset of low blood pressure or rapid pulse can indicate a hemorrhagic condition such as ectopic pregnancy or ruptured hemorrhagic cyst.
Bimanual pelvic examination is notoriously inaccurate for detecting and characterizing ovarian cysts. In one prospective study, examiners blinded to the reason for surgery evaluated women under anesthesia. The authors concluded that bimanual examination was of limited value even under the best circumstances.15 Pelvic examination can be even more difficult in patients who are obese, are virginal, have vaginal atrophy, or are in pain.
Useful information that can be obtained through the bimanual examination includes the exact location of pelvic tenderness, the relative firmness of an identified mass, and the existence of nodularity in the posterior cul-de-sac, suggesting advanced ovarian cancer.
Tumor markers
Cancer antigen 125 (CA125) is the most studied and widely used of the ovarian cancer tumor markers. When advanced epithelial ovarian cancer is associated with a markedly elevated level, the value correlates with tumor burden.25
Unfortunately, only about half of early-stage ovarian cancers and 75% to 80% of advanced ovarian cancers express this marker.26 Especially in premenopausal women, there are many pelvic conditions that can falsely elevate CA125. Therefore, its sensitivity and specificity for predicting ovarian cancer are suboptimal. Nevertheless, CA125 is often used to help stratify risk when assessing known ovarian cysts and masses.
The value considered abnormal in postmenopausal women is 35 U/mL or greater, while in premenopausal women the cutoff is less well defined. The lower the cutoff level is set, the more sensitive the test. Recent recommendations advise 50 U/mL or 67 U/mL, rather than the 200 U/mL recommended in the 2002 joint guidelines of the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology.27,28
However, specificity is likely to be lower with these lower cutoff values. Conditions that can elevate CA125 levels include almost anything that irritates the peritoneum, including pregnancy, menstruation, fibroids, endometriosis, infection, and ovarian hyperstimulation, as well as medical conditions such as liver or renal disease, colitis, diverticulitis, congestive heart failure, diabetes, autoimmune diseases, and ascites.
Following serial CA125 levels may be more sensitive than trying to establish a single cutoff value.29 CA125 should not be used as a screening tool in average-risk women.26
OVA1. Several biomarker panels have been developed and evaluated for risk assessment in women with pelvic masses. OVA1, a proprietary panel of tests (Vermillion; Austin, TX) received US Food and Drug Administration approval in 2009. It includes CA125 and four other proteins, from which it calculates a probability score (high or low) using a proprietary formula.
In prospective studies, OVA1 was more sensitive than clinical assessment or CA125 alone.30 The higher sensitivity and negative predictive value were counterbalanced by a lower specificity and positive predictive value.31 Its cost ($650) is not always covered by insurance. OVA1 is not a screening tool.
EVALUATION WITH ULTRASONOGRAPHY
Ultrasonography is the imaging test of choice in assessing adnexal cysts and masses, and therefore it is the best next step after taking a history, performing a physical examination, and obtaining blood work.32 In cases in which an incidental ovarian mass is discovered on computed tomography (CT), further characterization by ultrasonography will likely yield helpful information.
Pelvic ultrasonography can be performed transabdominally or transvaginally. Vaginal ultrasonography gives the clearest images in most patients. Abdominal scanning is indicated for large masses, when vaginal access is difficult (as in virginal patients or those with vaginal atrophy) or when the mass is out of the focal length of the vaginal probe. A full bladder is usually required for the best transabdominal images.
The value of the images obtained depends on the experience of the ultrasonographer and reader and on the equipment. Also, there is currently no widely used standard for reporting the findings33—descriptions are individualized, leading some authors to recommend that the clinician personally review the films to get the most accurate picture.19
Size
Size alone cannot be used to distinguish between benign and malignant lesions. Simple cysts up to 10 cm are most likely benign regardless of menopausal status.2,34 However, in a complex or solid mass, size correlates somewhat with the chance of malignancy, with notable exceptions, such as the famously large sizes of some solid fibromas or mucinous cystadenomas. Also, size may correlate with risk of other complications such as torsion or symptomatic rupture.
Complexity
Simple cysts have clear fluid, thin smooth walls, no loculations or septae, and enhanced through-transmission of echo waves.32,33
Complexity is described in terms of septations, wall thickness, internal echoes, and solid nodules. Increasing complexity does correlate with increased risk of malignancy.
Worrisome findings
The most worrisome findings are:
- Solid areas that are not hyperechoic, especially when there is blood flow to them
- Thick septations, more than 2 or 3 mm wide, especially if there is blood flow within them
- Excrescences on the inner or outer aspect of a cystic area
- Ascites
- Other pelvic or omental masses.
Benign conditions
Several benign conditions have characteristic complex findings on ultrasonography (Table 2), whereas other findings can be indeterminate (Table 3) or worrisome for malignancy (Table 4).
Hemorrhagic corpus luteum cysts can be complex with an internal reticular pattern due to organizing clot and fibrin strands. A “ring of fire” vascular pattern is often seen around the cyst bed.
Dermoids (mature cystic teratomas) may have hyperechoic elements with acoustic shadowing and no internal Doppler flow. They can have a complex appearance due to fat, hair, and sebum within the cyst. Dermoid cysts have a pathognomonic appearance on CT with a clear fat-fluid level.
Endometriomas classically have a homogeneous “ground-glass” appearance or low-level echoes, without internal color Doppler flow, wall nodules, or other malignant features.
Fibroids may be pedunculated and may appear to be complex or solid adnexal masses.
Hydrosalpinges may present as tortuous tubular-shaped cystic masses. There may be incomplete septations or indentations seen on opposite sides (the “waist” sign).
Paratubal and paraovarian cysts are usually simple round cysts that can be demonstrated as separate from the ovary. Sometimes these appear complex as well.
Peritoneal inclusion cysts, also known as pseudocysts, are seen in patients with intra-abdominal adhesions. Often multiple septations are seen through clear fluid, with the cyst conforming to the shape of other pelvic structures.
Torsion of the ovary may occur with either benign or malignant masses. Torsion can be diagnosed when venous flow is absent on Doppler. The presence of flow, however, doesn’t rule out torsion, as torsion is often intermittent. The twisted ovary is most often enlarged and can have an edematous appearance. Although typically benign, these should be referred for urgent surgical treatment.
Vascularity
Doppler imaging is being extensively studied. The general principle is that malignant masses will be more vascular, with a high-volume, low-resistance pattern of flow. This can result in a pulsatility index of less than 1 or a resistive index of less than 0.4. In practice, however, there is significant overlap between high and low pulsatility indices and resistive indices in benign and malignant cysts. Low resistance can also be found in endometriomas, corpus luteum cysts, inflammatory masses, and vascular benign neoplasms. A normal (high) resistive index does not rule out malignancy.32,33
One Doppler finding that does seem to correlate with malignancy is the presence of any flow within a solid nodule or wall excrescence.
3D ultrasonography
As the use of 3D ultrasonography increases, studies are yielding different results as to its utility in describing ovarian masses. 3D ultrasonography may be useful in finding centrally located vessels so that Doppler can be applied.32
OTHER IMAGING
Although ultrasonography is the initial imaging study of choice in the evaluation of adnexal masses owing to its high sensitivity, availability, and low cost, studies have shown that up to 20% of adnexal masses can be reported as indeterminate by ultrasonography (Table 1).
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is emerging as a very valuable tool when ultrasonography is inconclusive or limited.35 Although MRI is very accurate (Table 1), it is not considered a first-line imaging test because it is more expensive, less available, and more inconvenient for the patient than ultrasonography.
MRI provides additional information on the composition of soft-tissue tumors. Usually, MRI is ordered with contrast, unless there are contraindications to it. The radiologist will evaluate morphologic features, signal intensity, and enhancement of solid areas. Techniques such as dynamic contrast-enhanced MRI (following the distribution of contrast material over time), in- and out-of-phase T1 imaging (looking for fat, such as in dermoids), and the newer diffusion-weighted imaging may further improve characterization.
In one study of MRI as second-line imaging, contrast-enhanced MRI contributed to a greater change in the probability of ovarian cancer than did CT, Doppler ultrasonography, or MRI without contrast.36 This may result in a reduction in unnecessary surgeries and in an increase in proper referrals in cases of suspected malignancy.
Computed tomography
Disadvantages of CT include radiation exposure and poor discrimination of soft tissue. It can, however, differentiate fat or calcifications that may be found in dermoids. While CT is not often used to describe an ovarian lesion, it may be used preoperatively to stage an ovarian cancer or to look for a primary intra-abdominal cancer when an ovarian mass may represent metastasis.32
MANAGING AN INCIDENTAL OVARIAN CYST OR CYSTIC MASS
Combining information from the history, physical examination, imaging, and blood work to assign a level of risk of malignancy is not straightforward. The clinician must weigh several imperfect tests, each with its own sensitivity and specificity, against the background of the individual patient’s likelihood of malignancy. Whereas a 4-cm simple cyst in a premenopausal woman can be assigned to a low-risk category and a complex mass with flow to a solid component in a postmenopausal woman can be assigned to a high-risk category, many lesions are more difficult to assess.
Several systems have been proposed for analyzing data and standardizing risk assessment. There are a number of scoring systems based on ultrasonographic morphology and several mathematical regression models that include menopausal status and tumor markers. But each has drawbacks, and none is definitively superior to expert opinion.16,17,37,38
A 2012 systematic review and meta-analysis39 calculated sensitivity and specificity for several imaging tests, scoring systems, and blood tumor markers. Some results are presented in Table 1.
The management of an ovarian cyst depends on symptoms, likelihood of torsion or rupture, and the level of concern for malignancy. At the lower-risk end of the spectrum, reassurance or observation over time may be appropriate. A general gynecologist can evaluate indeterminate or symptomatic ovarian cysts. Patients with masses frankly suspicious for malignancy are best referred to a gynecologic oncologist.
Expectant management for low-risk lesions
Low-risk lesions such as simple cysts, endometriomas, and dermoids have a less than 1% chance of malignancy. Most patients who have them require only reassurance or follow-up with serial ultrasonography. Oral contraceptives may prevent new cysts from forming. Aspiration is not recommended.
In 2010, the Society of Radiologists in Ultrasound issued a consensus statement regarding re-imaging of simple ovarian cysts.33
In premenopausal women, they recommend no further testing for cysts 5 cm or smaller, yearly follow-up for cysts larger than 5 cm and up to and including 7 cm, and MRI or surgical evaluation for cysts larger than 7 cm, as it is difficult to completely image a large cyst with ultrasonography.
In postmenopausal women, if the cyst is 1 cm in diameter or smaller, no further studies need to be done. For simple cysts larger than 1 cm and up to and including 7 cm, yearly re-imaging is recommended. And for cysts larger than 7 cm, MRI or surgery is indicated. The American College of Radiology recommends repeat ultrasonography and CA125 testing for cysts 3 cm and larger but doesn’t specify an interval.32
A cyst that is otherwise simple but has a single thin septation (< 3 mm) or a small calcification in the wall is almost always benign. Such a cyst should be followed as if it were a simple cyst, as indicated by patient age and cyst size.
There are no official guidelines as to when to stop serial imaging,22,32 but a recent paper suggested one or two ultrasonographic examinations to confirm size and morphologic stability.19 Once a lesion has resolved, there is no need for further imaging (Figures 1–3).
Birth control pills for suppression of new cysts. Oral contraceptives do not hasten the resolution of ovarian cysts, according to a 2011 Cochrane review.40 Some practitioners will, nevertheless, prescribe them in an attempt to prevent new cysts from confusing the picture.
Aspiration is not recommended for either diagnosis or treatment. It can only be considered in patients at high risk who are not surgical candidates. Results of cytologic study of specimens obtained by fine-needle aspiration cannot reliably determine the presence or absence of malignancy.41 There is also a theoretical risk of spreading cancer from an early-stage lesion. A retrospective study has suggested that spillage of cyst contents during surgery in early ovarian cancer is associated with a worse prognosis.42
From a therapeutic point of view, studies have shown the same resolution rate at 6 months for aspirated cysts vs those followed expectantly.43 Another study found a recurrence rate of 25% within 1 year of aspiration.44
Referral for medium-risk or indeterminant-risk ovarian masses
Patients who have medium- or indeterminaterisk ovarian masses (Table 3) should be referred to a gynecologist. Further testing will help stratify the risk of malignancy. This can include tumor marker blood tests, MRI, or CT, the addition of Doppler or 3D ultrasonography, serial ultrasonography, or surgical exploration.
If repeat ultrasonography is chosen, the interval will likely be 6 to 12 weeks. Surgery may consist of removing only the cyst itself, or the whole ovary with or without the tube, or sometimes both ovaries. Purely diagnostic laparoscopy is rarely performed, as direct visualization of a lesion is rarely helpful. Frozen section should be employed, and the operating gynecologist should have oncologic backup, since the surgery is performed to rule out malignancy.
In the case of a benign-appearing cyst larger than 6 cm, thought must be given as to whether it is likely to rupture or twist. Rupture of a large cyst can lead to pain and in some cases to hemorrhage. Contents of a ruptured dermoid cyst can cause chemical peritonitis. Torsion of an ovary can result in loss of the ovary through compromised perfusion. A general gynecologist can decide with the patient whether preemptive surgery is indicated.
Operative evaluation for high-risk masses
Patients with high-risk ovarian masses (Table 4) are best referred to a gynecologic oncologist for operative evaluation. If features are seen that indicate malignancy, such as thick septations, solid areas with blood flow, ascites, or other pelvic masses, surgery is indicated. The surgical approach may be through laparoscopy or laparotomy.45 It should be noted that even in the face of worrisome features on ultrasonography, many masses turn out to be benign.
In 2011, the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology issued new guidelines recommending oncologic referral of patients with high-risk masses. Elevated CA125, ascites, a nodular or fixed pelvic mass, or evidence of metastasis in postmenopausal women requires oncologic evaluation.26 For premenopausal women, a very elevated CA125, ascites, or metastasis requires referral (Table 4).26
Direct referral to a gynecologic oncologist is underutilized. A recent study found that fewer than half of primary care physicians said that they would refer a classic suspicious case directly to a subspecialist.46 It is estimated that only 33% of ovarian cancers are first operated on by a gynecologic oncologist.47
A 2011 Cochrane review confirmed a survival benefit for women with cancer who are operated on by gynecologic oncologists primarily, rather than by a general gynecologist and then referred.48 A gynecologic oncologist is most likely to perform proper staging and debulking at the time of initial diagnosis.49
Special situations require consultation
Ovarian cysts in pregnancy are most often benign,50 but malignancy is always a possibility. Functional cysts and dermoids are the most common. These may remain asymptomatic or may rupture or twist or cause difficulty with delivering the baby. Surgical intervention, if needed, should be performed in the second trimester if possible. A multidisciplinary approach and referral to a perinatologist and gynecologic oncologist are advised.
Symptomatic ovarian cysts that may need surgical intervention are the purview of the general gynecologist. If the risk of a surgical emergency is judged to be low, a symptomatic patient may be supported with pain medication and may be managed on an outpatient basis. Immediate surgical consultation is appropriate if the patient appears toxic or in shock. Depending on the clinical picture, there may be a ruptured tubo-ovarian abscess, ruptured ectopic pregnancy, ruptured hemorrhagic cyst, or ovarian torsion, any of which may need immediate surgical intervention.
If a symptomatic mass is highly suspicious for cancer, a gynecologic oncologist should be consulted directly.
WHEN TO REASSURE, REASSESS, REFER
Ovarian masses often pose diagnostic and management dilemmas. Reassurance can be offered to women with small simple cysts. Interval follow-up with ultrasonography is appropriate for cysts that are most likely to be benign. If malignancy is suspected based on ultrasonography, other imaging, blood testing, or expert opinion, referral to a surgical gynecologist or gynecologic oncologist is recommended. If malignancy is strongly suspected, direct referral to a gynecologic oncologist offers the best chance of survival if cancer is actually present.
Reassure
- When simple cysts are less than 1 cm in postmenopausal women
- When simple cysts are less than 5 cm in premenopausal patients.
Reassess
- With yearly ultrasonography in cases of very low risk
- With repeat ultrasonography in 6 to 12 weeks when the diagnosis is not clear but the cyst is likely benign.
Refer
- To a gynecologist for symptomatic cysts, cysts larger than 6 cm, and cysts that require ancillary testing
- To a gynecologic oncologist for findings worrisome for cancer, such as thick septations, solid areas with flow, ascites, evidence of metastasis, or high cancer antigen 125 levels.
- Borgfeldt C, Andolf E. Transvaginal sonographic ovarian findings in a random sample of women 25–40 years old. Ultrasound Obstet Gynecol 1999; 13:345–350.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol 2003; 102:594–599.
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol 2010; 202:373.e1–373.e9.
- Jordan SJ, Green AC, Whiteman DC, Webb PM; Australian Ovarian Cancer Study Group. Risk factors for benign, borderline and invasive mucinous ovarian tumors: epidemiological evidence of a neoplastic continuum? Gynecol Oncol 2007; 107:223–230.
- NIH consensus conference. Ovarian cancer. Screening, treatment, and follow-up. NIH Consensus Development Panel on Ovarian Cancer. JAMA 1995; 273:491–497.
- The reduction in risk of ovarian cancer associated with oral-contraceptive use. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. N Engl J Med 1987; 316:650–655.
- Young RL, Snabes MC, Frank ML, Reilly M. A randomized, double-blind, placebo-controlled comparison of the impact of low-dose and triphasic oral contraceptives on follicular development. Am J Obstet Gynecol 1992; 167:678–682.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol 2009; 113:1027–1037.
- Sharma A, Gentry-Maharaj A, Burnell M, et al; UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Assessing the malignant potential of ovarian inclusion cysts in postmenopausal women within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a prospective cohort study. BJOG 2012; 119:207–219.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011; 305:2295–2303.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009; 361:170–177.
- National Cancer Institute. Surveillance Epidemiology and End Results (SEER). Cancer statistics on ovarian cancer. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed May 9, 2013.
- American Cancer Society. Survival by ovarian cancer stage. www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-survival-rates. Accessed May 9, 2013.
- Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med 2009; 6:e1000114.
- Padilla LA, Radosevich DM, Milad MP. Limitations of the pelvic examination for evaluation of the female pelvic organs. Int J Gynaecol Obstet 2005; 88:84–88.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of Adnexal Mass. Evidence Report/Technology Assessment No.130 (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-02-0025.) AHRQ Publication No. 06-E004. Rockville, MD: Agency for Healthcare Research and Quality. February 2006.
- Ameye L, Timmerman D, Valentin L, et al. Clinically oriented three-step strategy for assessment of adnexal pathology. Ultrasound Obstet Gynecol 2012; 40:582–591.
- Covens AL, Dodge JE, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Surgical management of a suspicious adnexal mass: a systematic review. Gynecol Oncol 2012; 126:149–156.
- Liu JH, Zanotti KM. Management of the adnexal mass. Obstet Gynecol 2011; 117:1413–1428.
- Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer 2007; 109:221–227.
- Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst 2010; 102:222–229.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Management of adnexal masses. Obstet Gynecol 2007; 110:201–214.
- BestPractice BMJ Evidence Centre. Ovarian cysts-Diagnosis-History & examination—Risk factors. http://bestpractice.bmj.com/best-practice/monograph/660/diagnosis.html. Accessed June 2, 2013.
- American Cancer Society. Ovarian-cancer risk factors. www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-survival-rates. Accessed May 9, 2013.
- Bast RC, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983; 309:883–887.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 477: the role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer. Obstet Gynecol 2011; 117:742–746.
- Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol 2005; 105:35–41.
- Dearking AC, Aletti GD, McGree ME, Weaver AL, Sommerfield MK, Cliby WA. How relevant are ACOG and SGO guidelines for referral of adnexal mass? Obstet Gynecol 2007; 110:841–848.
- Skates SJ, Menon U, MacDonald N, et al. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J Clin Oncol 2003; 21(suppl 10):206s–210s.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol 2011; 117:1289–1297.
- Ware Miller R, Smith A, DeSimone CP, et al. Performance of the American College of Obstetricians and Gynecologists’ ovarian tumor referral guidelines with a multivariate index assay. Obstet Gynecol 2011; 117:1298–1306.
- Lev-Toaff AS, Horrow MM, Andreotti RF, et al. Expert Panel on Women’s Imaging. ACR Appropriateness Criteria clinically suspected adnexal mass. Reston, VA: American College of Radiology (ACR), 2009. www.guidelines.gov/content.aspx?id=15780&search=adnexal+mass. Accessed May 9, 2013.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256:943–954.
- Alcázar JL, Castillo G, Jurado M, García GL. Is expectant management of sonographically benign adnexal cysts an option in selected asymptomatic premenopausal women? Hum Reprod 2005; 20:3231–3234.
- Medeiros LR, Freitas LB, Rosa DD, et al. Accuracy of magnetic resonance imaging in ovarian tumor: a systematic quantitative review. Am J Obstet Gynecol 2011; 204:67.e1–67.e10.
- Kinkel K, Lu Y, Mehdizade A, Pelte MF, Hricak H. Indeterminate ovarian mass at US: incremental value of second imaging test for characterization—meta-analysis and Bayesian analysis. Radiology 2005; 236:85–94.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ 2010; 341:c6839.
- Valentin L, Ameye L, Savelli L, et al. Adnexal masses difficult to classify as benign or malignant using subjective assessment of gray-scale and Doppler ultrasound findings: logistic regression models do not help. Ultrasound Obstet Gynecol 2011; 38:456–465.
- Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol 2012; 126:157–166.
- Grimes DA, Jones LB, Lopez LM, Schulz KF. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev 2011; 9:CD006134.
- Higgins RV, Matkins JF, Marroum MC. Comparison of fine-needle aspiration cytologic findings of ovarian cysts with ovarian histologic findings. Am J Obstet Gynecol 1999; 180:550–553.
- Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 2001; 357:176–182.
- Zanetta G, Lissoni A, Torri V, et al. Role of puncture and aspiration in expectant management of simple ovarian cysts: a randomised study. BMJ 1996; 313:1110–1113.
- Bonilla-Musoles F, Ballester MJ, Simon C, Serra V, Raga F. Is avoidance of surgery possible in patients with perimenopausal ovarian tumors using transvaginal ultrasound and duplex color Doppler sonography? J Ultrasound Med 1993; 12:33–39.
- Medeiros LR, Rosa DD, Bozzetti MC, et al. Laparoscopy versus laparotomy for FIGO Stage I ovarian cancer. Cochrane Database Syst Rev 2008; 4:CD005344.
- Goff BA, Miller JW, Matthews B, et al. Involvement of gynecologic oncologists in the treatment of patients with a suspicious ovarian mass. Obstet Gynecol 2011; 118:854–862.
- Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst 2006; 98:172–180.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol 2005; 99:447–461.
- Cress RD, Bauer K, O’Malley CD, et al. Surgical staging of early stage epithelial ovarian cancer: results from the CDC-NPCR ovarian patterns of care study. Gynecol Oncol 2011; 121:94–99.
- Horowitz NS. Management of adnexal masses in pregnancy. Clin Obstet Gynecol 2011; 54:519–527.
- Borgfeldt C, Andolf E. Transvaginal sonographic ovarian findings in a random sample of women 25–40 years old. Ultrasound Obstet Gynecol 1999; 13:345–350.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol 2003; 102:594–599.
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol 2010; 202:373.e1–373.e9.
- Jordan SJ, Green AC, Whiteman DC, Webb PM; Australian Ovarian Cancer Study Group. Risk factors for benign, borderline and invasive mucinous ovarian tumors: epidemiological evidence of a neoplastic continuum? Gynecol Oncol 2007; 107:223–230.
- NIH consensus conference. Ovarian cancer. Screening, treatment, and follow-up. NIH Consensus Development Panel on Ovarian Cancer. JAMA 1995; 273:491–497.
- The reduction in risk of ovarian cancer associated with oral-contraceptive use. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. N Engl J Med 1987; 316:650–655.
- Young RL, Snabes MC, Frank ML, Reilly M. A randomized, double-blind, placebo-controlled comparison of the impact of low-dose and triphasic oral contraceptives on follicular development. Am J Obstet Gynecol 1992; 167:678–682.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol 2009; 113:1027–1037.
- Sharma A, Gentry-Maharaj A, Burnell M, et al; UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Assessing the malignant potential of ovarian inclusion cysts in postmenopausal women within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a prospective cohort study. BJOG 2012; 119:207–219.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011; 305:2295–2303.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009; 361:170–177.
- National Cancer Institute. Surveillance Epidemiology and End Results (SEER). Cancer statistics on ovarian cancer. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed May 9, 2013.
- American Cancer Society. Survival by ovarian cancer stage. www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-survival-rates. Accessed May 9, 2013.
- Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med 2009; 6:e1000114.
- Padilla LA, Radosevich DM, Milad MP. Limitations of the pelvic examination for evaluation of the female pelvic organs. Int J Gynaecol Obstet 2005; 88:84–88.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of Adnexal Mass. Evidence Report/Technology Assessment No.130 (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-02-0025.) AHRQ Publication No. 06-E004. Rockville, MD: Agency for Healthcare Research and Quality. February 2006.
- Ameye L, Timmerman D, Valentin L, et al. Clinically oriented three-step strategy for assessment of adnexal pathology. Ultrasound Obstet Gynecol 2012; 40:582–591.
- Covens AL, Dodge JE, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Surgical management of a suspicious adnexal mass: a systematic review. Gynecol Oncol 2012; 126:149–156.
- Liu JH, Zanotti KM. Management of the adnexal mass. Obstet Gynecol 2011; 117:1413–1428.
- Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer 2007; 109:221–227.
- Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst 2010; 102:222–229.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Management of adnexal masses. Obstet Gynecol 2007; 110:201–214.
- BestPractice BMJ Evidence Centre. Ovarian cysts-Diagnosis-History & examination—Risk factors. http://bestpractice.bmj.com/best-practice/monograph/660/diagnosis.html. Accessed June 2, 2013.
- American Cancer Society. Ovarian-cancer risk factors. www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-survival-rates. Accessed May 9, 2013.
- Bast RC, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983; 309:883–887.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 477: the role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer. Obstet Gynecol 2011; 117:742–746.
- Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol 2005; 105:35–41.
- Dearking AC, Aletti GD, McGree ME, Weaver AL, Sommerfield MK, Cliby WA. How relevant are ACOG and SGO guidelines for referral of adnexal mass? Obstet Gynecol 2007; 110:841–848.
- Skates SJ, Menon U, MacDonald N, et al. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J Clin Oncol 2003; 21(suppl 10):206s–210s.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol 2011; 117:1289–1297.
- Ware Miller R, Smith A, DeSimone CP, et al. Performance of the American College of Obstetricians and Gynecologists’ ovarian tumor referral guidelines with a multivariate index assay. Obstet Gynecol 2011; 117:1298–1306.
- Lev-Toaff AS, Horrow MM, Andreotti RF, et al. Expert Panel on Women’s Imaging. ACR Appropriateness Criteria clinically suspected adnexal mass. Reston, VA: American College of Radiology (ACR), 2009. www.guidelines.gov/content.aspx?id=15780&search=adnexal+mass. Accessed May 9, 2013.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256:943–954.
- Alcázar JL, Castillo G, Jurado M, García GL. Is expectant management of sonographically benign adnexal cysts an option in selected asymptomatic premenopausal women? Hum Reprod 2005; 20:3231–3234.
- Medeiros LR, Freitas LB, Rosa DD, et al. Accuracy of magnetic resonance imaging in ovarian tumor: a systematic quantitative review. Am J Obstet Gynecol 2011; 204:67.e1–67.e10.
- Kinkel K, Lu Y, Mehdizade A, Pelte MF, Hricak H. Indeterminate ovarian mass at US: incremental value of second imaging test for characterization—meta-analysis and Bayesian analysis. Radiology 2005; 236:85–94.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ 2010; 341:c6839.
- Valentin L, Ameye L, Savelli L, et al. Adnexal masses difficult to classify as benign or malignant using subjective assessment of gray-scale and Doppler ultrasound findings: logistic regression models do not help. Ultrasound Obstet Gynecol 2011; 38:456–465.
- Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol 2012; 126:157–166.
- Grimes DA, Jones LB, Lopez LM, Schulz KF. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev 2011; 9:CD006134.
- Higgins RV, Matkins JF, Marroum MC. Comparison of fine-needle aspiration cytologic findings of ovarian cysts with ovarian histologic findings. Am J Obstet Gynecol 1999; 180:550–553.
- Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 2001; 357:176–182.
- Zanetta G, Lissoni A, Torri V, et al. Role of puncture and aspiration in expectant management of simple ovarian cysts: a randomised study. BMJ 1996; 313:1110–1113.
- Bonilla-Musoles F, Ballester MJ, Simon C, Serra V, Raga F. Is avoidance of surgery possible in patients with perimenopausal ovarian tumors using transvaginal ultrasound and duplex color Doppler sonography? J Ultrasound Med 1993; 12:33–39.
- Medeiros LR, Rosa DD, Bozzetti MC, et al. Laparoscopy versus laparotomy for FIGO Stage I ovarian cancer. Cochrane Database Syst Rev 2008; 4:CD005344.
- Goff BA, Miller JW, Matthews B, et al. Involvement of gynecologic oncologists in the treatment of patients with a suspicious ovarian mass. Obstet Gynecol 2011; 118:854–862.
- Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst 2006; 98:172–180.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol 2005; 99:447–461.
- Cress RD, Bauer K, O’Malley CD, et al. Surgical staging of early stage epithelial ovarian cancer: results from the CDC-NPCR ovarian patterns of care study. Gynecol Oncol 2011; 121:94–99.
- Horowitz NS. Management of adnexal masses in pregnancy. Clin Obstet Gynecol 2011; 54:519–527.
KEY POINTS
- Incidentally discovered ovarian cysts are common and most are benign, but a minority can represent ovarian cancer, which is difficult to detect before it has spread and therefore often has a poor prognosis.
- Patients can be reassured if they are postmenopausal and have a simple cyst smaller than 1 cm or if they are premenopausal and have a simple cyst smaller than 5 cm.
- Reassess with yearly ultrasonography in very low-risk situations and with repeat ultrasonography in 6 to 12 weeks if the diagnosis is not clear but is likely benign.
- Refer to a gynecologist in cases of symptomatic cysts, cysts larger than 6 cm, and cysts that require ancillary testing.
- Refer to a gynecologic oncologist for findings worrisome for cancer such as thick septations, solid areas with flow, ascites, evidence of metastasis, or high cancer antigen 125 levels.
Patients with Psychiatric Comorbidity
Mental illness is highly prevalent, with approximately 30% of the US population meeting criteria for at least 1 disorder.[1] In the medically ill population, psychiatric disease is even more common; a 2005 survey showed that half of all patients visiting primary care physicians met criteria for a mental disorder.[2] Conversely, those with serious mental illness suffer greater medical morbidity than the general population, with higher rates of obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, human immunodeficiency virus, viral hepatitis, and tuberculosis.[3] When acute medical problems arise, those with mental illness endure longer hospitalizations; the presence of a psychiatric disturbance in the general medical setting has been shown to be a robust predictor of increased hospital length of stay.[4, 5]
Because of the strong correlation between medical and mental illness, hospitalists will care for patients with psychiatric disorders. Despite this, internists generally receive a paucity of formal training in the treatment of mental disturbances. One survey of university‐affiliated internal medicine residencies revealed that only 10% of programs offered any kind of modest curriculum in psychiatric education.[6] Regardless of this lack of preparation, hospitalists are called upon at each admission to make decisions that affect the psychiatric treatment of patients on psychotropic medication; namely, they must decide whether to continue or discontinue psychiatric medications. Many physicians reflexively discontinue a patient's chronic medications upon admission to the hospital; one study reported an adjusted odds ratio of between 1.18 and 1.86 for stopping a medication prescribed for a chronic condition.[7]
This review aims to assist the hospitalist in making an informed decision about the continuation of psychotropic medications in the medically ill patient. First, it examines the risks of stopping psychotropic medication, including psychiatric decompensation and discontinuation syndromes. It also explores the challenges of medication continuation in the context of changing pharmacokinetics and emerging side effects. Ultimately, physicians and patients must make collaborative decisions, weighing the risk of medication interactions against the potential adverse effects of psychiatric decompensation.
DISCONTINUATION
Decompensation of Mental Health
Approximately 10% to 15% of patients hospitalized for medical illness require reduction or discontinuation of psychotropic medications because they may be contributing to the clinical presentation.[4] The rate and method of drug discontinuation can affect the course of major psychiatric disorders.[8] A growing number of studies demonstrate high rates of relapse when medications are discontinued in patients suffering from mood disorders, schizophrenia, and anxiety disorders.[9] Abrupt cessation of psychotropics is especially dangerous, leading to a greater chance of destabilization than if medications are tapered. Episodes of active illness even appear to occur more frequently with sudden psychotropic cessation than they would in the natural course of untreated disease. This is true for several classes of psychotropics, including antidepressants, mood stabilizers, and antipsychotics. For example, in a study of pregnant women who suddenly stopped their psychotropic medication (both antidepressants and benzodiazepines), nearly one‐third experienced suicidal ideation.[10] Depression and suicidality have also been documented in bipolar patients who were abruptly taken off of lithium. More commonly, rapid lithium discontinuation in bipolar patients causes mania, with illness relapse as soon as 4 days after cessation.[10] Additionally, abrupt discontinuation of antipsychotics in patients with schizophrenia leads to early, and often severe, psychosis. One study found a relapse rate of 50% within 30 weeks of sudden oral neuroleptic cessation.[11] Furthermore, restarting medications, even at the previous effective dose, may not return the patient to their prior baseline.[12] Psychiatric decompensation in the hospitalized patient can worsen medical outcomes, with decreased adherence to treatment plans. In extreme circumstances, patients may be at risk of self‐harm or suicide.
DRUG‐SPECIFIC DISCONTINUATION SYNDROMES
Antidepressants
Discontinuation of medications presents additional problems, and sudden cessation of psychotropic medications can lead to uncomfortable or even dangerous symptoms. For example, the serotonin discontinuation syndrome has been well documented. Chronic use of serotonin re‐uptake inhibitors (generally greater than 6 to 8 weeks) leads to downregulation of postsynaptic serotonin receptors. When selective serotonin re‐uptake inhibitors (SSRIs) or serotonin‐norepinephrine re‐uptake inhibitors are abruptly stopped, the brain experiences a relative decline in serotonin. Symptoms include a flu‐like illness, nausea, imbalance, insomnia, sensory disturbances, and dysphoria. Onset may be within hours of missing a dose, but typically occurs within 3 days of medication discontinuation. The syndrome is more likely to occur with cessation of medications of shorter half‐life and less likely to occur with medications with a long half‐life, such as fluoxetine (Table 1).[13, 14] The symptoms can be ameliorated with a gradual tapering or reintroduction of the antidepressant.[15] Untreated symptoms resolve in 1 to 2 weeks. Although the syndrome in isolation is not life‐threatening, a number of the symptoms can complicate medical illness and muddle diagnosis of other diseases.[14, 16]
| Medication | Half‐Life (Hours) |
|---|---|
| |
| SSRIs | |
| Fluoxetine | 84144 |
| Paroxetine | 21 |
| Sertraline | 26 |
| Citalopram | 35 |
| Escitalopram | 2732 |
| Fluvoxamine | 15 |
| SNRIs | |
| Venlafaxine | 313 |
| Duloxetine | 1116 |
Older antidepressants, including the tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs), have serotonergic effects, and thus discontinuation may cause the symptoms described above. However, these agents also have effects on other neurotransmitters. The TCAs block muscarinic cholinergic receptors, leading to upregulation. Abrupt cessation can lead to cholinergic rebound, with parkinsonism and mania emerging. Multiple case reports document improvement in these symptoms with an anticholinergic agent, such as benztropine.[17, 18] MAOIs lead to changes in ‐2 adrenergic and dopaminergic receptors. Sudden discontinuation has been associated with agitation, delirium, and psychosis; 1 case report even documents catatonia associated with autonomic instability.[19]
In addition, sudden discontinuation of antidepressants (including the SSRIs) may provoke mania or hypomania in some patients, regardless of whether they have experienced previous spontaneous manic episodes.[8]
Neuroleptics
Data for an antipsychotic withdrawal syndrome are less convincing than those for serotonergic agents. However, certain symptoms have been associated with abrupt neuroleptic discontinuation. Most frequently, gastrointestinal distress and diaphoresis are described. Anxiety, agitation, and insomnia are also common. These symptoms are thought to be associated with cholinergic rebound, mediated by direct effects of neuroleptics on muscarinic receptors or indirectly through dopamine receptor blockade and the dopamine‐cholinergic balance. Symptoms may be more severe when antimuscarinic, antiparkinsonism drugs are simultaneously stopped. Some authors argue that the timing of symptom onset can differentiate antipsychotic withdrawal from illness relapse, with discontinuation syndrome occurring within the first 7 days of medication cessation.[20]
Additionally, abrupt cessation of antipsychotics may be associated with rapid‐onset psychosis. The data are strongest for clozapine discontinuation, where overall incidence is approximately 20%. This is hypothesized to be mediated by dopamine receptor upregulation and subsequent hypersensitivity to endogenous dopamine. The emerging psychosis is purportedly distinct from the underlying illness. Episodes have been described in patients on chronic metoclopramide who have no prior psychiatric history, as well as in patients with bipolar disorder without psychosis prior to neuroleptic discontinuation.[21]
Movement disorders may emerge during neuroleptic discontinuation. Both parkinsonism and dyskinesias have been described. In some patients, dyskinesias resolve within weeks of drug discontinuation; however, others experience permanent symptoms, termed covert dyskinesia.[22] In rare circumstances, dyskinesias may affect the respiratory muscles, causing distress. Several case reports of withdrawal‐emergent respiratory dyskinesia have been reported following risperidone cessation.[23, 24, 25] Additionally, several case reports have described catatonia occurring after abrupt discontinuation of clozapine. In all cases, symptoms promptly resolved with reinitiation of clozapine.[26]
Neuroleptic malignant syndrome (NMS) is a rare but potentially fatal complication of antipsychotic administration. Symptoms include fever, rigidity, autonomic instability, and mental status changes.[27] Though NMS is hypothesized to occur due to dopamine receptor blockade, rare cases of NMS have also been reported with abrupt cessation of neuroleptics. Of the 8 case reports in the literature, 1 resulted in death.[22, 28]
Mood Stabilizers
Though some of the atypical antipsychotics are used to treat bipolar disorder, mood stabilizers are the mainstay of pharmacotherapy. The most commonly used mood stabilizers are lithium, valproic acid, lamotrigine, topirimate, carbamezapine, and oxcarbazepine. When mood stabilizers are discontinued, patients are at risk of psychiatric relapse, as documented above. However, withdrawal symptoms have not been commonly documented upon abrupt discontinuation of lithium or the anticonvulsants used to treat bipolar disorder.[29, 30]
Benzodiazepines
Benzodiazepines are widely prescribed for insomnia and anxiety. Chronic legal use of benzodiazepines is approximately 2% in the general population.[31] Like ethanol, benzodiazepines bind nonselectively to the GABA‐A receptor, resulting in downregulation of GABA receptors and compensatory increased N‐methyl‐D‐aspartate transmission. Sudden discontinuation of benzodiazepines results in a syndrome that mirrors that of alcohol withdrawal. Symptoms range from mild (tremor, insomnia, and anxiety) to life‐threatening (seizures, delirium, and autonomic instability). Serious withdrawal is more likely with substances of shorter half‐life and with higher chronic doses. Onset often occurs between 2 and 10 days after discontinuation, depending on the half‐life of the benzodiazepine.[32] Other rare serious reactions have been documented following abrupt benzodiazepine cessation, including NMS and catatonia.[30]
CONTINUATION
Reflexive discontinuation of psychotropic medications can clearly lead to adverse outcomes. However, when hospitalists decide to continue a patient's psychotropic medications, they must also be cognizant of potential complications. Modifications may be necessary because of hepatic, renal, or cardiac disease. In addition, physicians need to be aware of drug‐drug interactions. Pharmacotherapy for medically ill elderly patients may require dose modifications to account for an increased lipophilic volume of distribution and a decreased rate of metabolism.[33] Finally, pregnancy can present additional challenges regarding dose modifications and teratogenicity.
On the other hand, hospitalists must be aware that continuing a patient's psychotropic medication may not be the cause of new psychiatric symptoms. Drugs prescribed for medical disorders (eg, corticosteroids) often cause psychiatric symptoms. In addition, psychiatric symptoms may emerge at times of nonpsychotropic medication withdrawal or due to nonpsychotropic drug‐drug interactions.[4] Groups of medications commonly associated with psychiatric disturbances include analgesics, sedatives, anesthetics, anticonvulsants, and anticholinergics.
PHARMACOKINETICS: PSYCHOTROPIC TOXICITY
Medical illness alters the body's steady state, and renal or hepatic metabolism may be impaired when a patient requires hospitalization. Additionally, new medications may increase the effects of psychotropics, whether by intrinsic augmentation of effect or decreased psychotropic clearance. Ultimately, these changes can lead to psychotropic toxicity.
Specific toxicities merit discussion. First, serotonin syndrome is a potentially fatal condition. The majority of cases occur with synergistic serotonergic medication administration, though there are case reports of the syndrome occurring with addition of inhibitors of cytochrome p450 2D6 and/or 3A4 to SSRIs. A large number of medications from different classes have been indicated (Table 2).[34] Symptoms generally occur within 24 hours of medication administration and include mental status changes, autonomic instability, and neuromuscular hyperactivity. When serotonin syndrome is suspected, the offending agent should be discontinued immediately. There is no definitive treatment, though supportive care can be lifesaving.[34]
| Amphetamines and Derivatives | Antidepressants and Mood Stabilizers | Antimigraine Drugs | Analgesics | Antiemetics | Miscellaneous |
|---|---|---|---|---|---|
| |||||
| MDMA | Buspirone | Ergot alkaloids | Cyclobenzaprine | Metoclopramide | Cocaine |
| Dextroamphetamine | Carbamazepine | Triptans | Fentanyl | Ondansetron | Dextromethorphan |
| Methamphetamine | Lithium | Meperidine | Linezolid | ||
| Sibutramine | MAOIs | Tramadol | L‐tryptophan | ||
| SSRIs | 5‐hydroxytrytophan | ||||
| SNRIs | |||||
| Serotonin 2A receptor blockers (eg, trazodone) | |||||
| St. John's Wort | |||||
| TCAs | |||||
| Valproic Acid | |||||
In addition to serotonin syndrome, hypertensive emergency may occur due to drug interactions with MAOIs. MAOIs inhibit the enzyme monoamine oxidase, resulting in elevated levels of serotonin, histamine, and catecholamines in the blood. Coadministration of MAOIs and sympathomimetic agents (such as cough suppressants and analgesics) may dangerously increase adrenergic stimulation, elevating blood pressure to the point of end‐organ damage. Please see Table 3 for a full list of drugs indicated in MAOI‐associated hypertensive crisis.[35] To ensure safety, it is recommended that MAOIs be discontinued for 14 days prior to introducing medications with sympathomimetic properties, and vice versa. Because of its longer half‐life, a 5‐week washout period is recommended for fluoxetine.[35]
|
| Amphetamines |
| Analgesics: meperedine |
| Anesthetics |
| Antidepressants: buproprion, buspirone, other MAOIs, SSRIs, SNRIs, TCAs, |
| Mirtazapine |
| Cocaine |
| Dibenzazepine‐related agents: carbamezapine, cyclobenzaprine, perphenazine |
| Female sex steroids |
| Sympathomimetics: dopamine, epinephrine, levodopa, methyldopa, methylphenidate, norepinephrine, phenylalanine, reserpine, tyrosine, tryptophan |
| Other vasoconstrictors: pseudoephedrine, phenylephrine, phenylpropanolamine, ephedrine |
Lithium toxicity may result from changing patient pharmacokinetics. Lithium is almost entirely renally excreted, and acute kidney injury may precipitously raise serum levels. Within the renal collecting system, lithium is handled similarly to sodium, with 80% reabsorbed from the proximal tubule to the collecting duct. Thus, factors that decrease glomerular filtration rate (GFR) and increase proximal tubule absorption will increase serum lithium levels. For example, decreased effective arterial volume (due to dehydration, cirrhosis, nephrotic syndrome, or heart failure) may elevate lithium levels. Additionally, medications that decrease GFR may increase lithium reabsorption. These include nonsteroidal anti‐inflammatory drugs, angiotensin‐converting enzyme inhibitors, and thiazide diuretics. Because of lithium's narrow therapeutic index, small elevations in serum levels can lead to toxicity. Severity of intoxication correlates with serum concentration. Symptoms range from lethargy, weakness, tremor, ataxia, and gastrointestinal distress to coma, seizures, renal failure, and death. Toxicity is also associated with electrocardiograph (ECG) changes, including ST‐segment depression and T‐wave inversion in the lateral precordial leads. Sinus node dysfunction can also occur. Definitive treatment for lithium toxicity is hemodialysis.[36]
Though the therapeutic index is much wider for valproic acid than for lithium, valproate toxicity may also occur in the medically ill patient with previously stable serum levels. Valproic acid is highly protein‐bound at therapeutic levels, and is metabolized largely through hepatic glucuronidation. Initiation of medications that compete for protein‐binding sites, including aspirin, has led to valproate toxicity. Moreover, acute liver failure or addition of drugs that compete with hepatic microsomal enzymes may lead to decreased excretion of valproic acid. Poisoning may result in central nervous system (CNS) and respiratory depression, hypotension, cerebral edema, and pancreatitis. True hepatoxicity is rare, though hyperammonemia is widely documented. Thrombocytopenia is the most common hematologic abnormality associated with overdose. However, thrombocytopenia may also occur without complication in patients on stable therapeutic doses. Treatment is largely supportive, though hemoperfusion and hemodialysis may be used when serum levels are >300 g/mL, as only 35% of the drug is protein‐bound at that level. Naloxone has been shown in case reports to reverse valproic acid‐induced coma, and L‐carnitine has been increasingly recommended for hyperammonemia.[37, 38]
PHARMACOKINETICS: DRUG‐DRUG INTERACTIONS
As discussed above, the addition of a new medication can increase previously stable levels of psychotropic drugs, leading to toxicity. Conversely, mental health medications can alter the expected metabolism of a drug being used to treat acute medical illness. Many psychotropics are metabolized via the cytochrome p450 enzyme, particularly the SSRIs (Table 4).[39] A number of antimicrobial and antiarrythmic medications are also cleared via this route, leading to potential toxic or subtherapeutic levels when drug‐drug interactions occur.
| CYP 1A2 | CYP 2B6 | CYP 2C9 | CYP 2C19 | CYP 2D6 | CYP 3A4/3A5/3A7 |
|---|---|---|---|---|---|
| Clozapine | Bupropion | Amitriptyline | Phenytoin | Antidepressants | Alprazolam |
| Duloxetine | Methadone | Fluoxetine | Amitriptyline | Amitriptyline | Diazepam |
| Fluvoxamine | Phenytoin | Citalopram | Clomipramine | Midazolam | |
| Haloperidol | Clomipramine | Duloxetine | Aripiprazole | ||
| Imipramine | Diazepam | Desipramine | Buspirone | ||
| Olanzapine | Imipramine | Fluoxetine | Haldol | ||
| Ramelteon | Fluvoxamine | Quetiapine | |||
| Imipramine | Ziprasidone | ||||
| Nortriptyline | Zolpidem | ||||
| Paroxetine | Dextromethorphan | ||||
| Venlafaxine | |||||
| Antipsychotics | |||||
| Aripiprazole | |||||
| Clorpromazine | |||||
| Haldol | |||||
| Perphenazine | |||||
| Risperidone |
PSYCHOTROPIC ADVERSE EFFECTS
Psychotropic medications may also cause side effects that contribute to the clinical presentation, requiring ongoing monitoring, a dose reduction, or psychotropic discontinuation. Potential adverse effects that commonly impact psychopharmacologic management include anticholinergic side effects, cardiac effects, and sedation.
ANTICHOLINERGIC EFFECTS AND TOXICITY
Newly emerging anticholinergic effects may be particularly troubling. Dry mouth may cause swallowing difficulty and aspiration. Pupillary dilatation and dry eyes can increase risk of falls. Constipation may evolve into fecal impaction, and urinary retention can contribute to increased catheter use and infection. CNS effects are perhaps the most serious, ranging from drowsiness and memory impairment to frank delirium.[40]
Many psychotropic drugs are anti cholinergic. Among the antidepressants, the TCAs and paroxetine have the highest anticholinergic activity. Anticholinergic effects have also been reported with the low potency first generation neuroleptics and with the atypical antipsychotics olanzapine and clozapine. Additionally, medications used to treat the extrapyramidal symptoms associated with antipsychotics (such as benztropine and diphenhydramine) are strongly anticholinergic.[41] Patients without previous overt anticholinergic symptoms from these medications may experience adverse effects when hospitalized. Medical illness or new medications may alter psychotropic drug metabolism and elimination, leading to accumulation of their anticholinergic effects. Many medications used in the hospital also have intrinsic anticholinergic activity. These include some antiemetics, antispasmodics, antiarrhythmics, and histamine H2 receptor blockers. Elderly patients are particularly prone to anticholinergic effects due to age‐related deficits in cholinergic transmission.[40]
QTc PROLONGATION
QTc prolongation is a potentially lethal side effect of certain medications. Prolonged QTc increases the risk of cardiac mortality and sudden death, presumably related to onset of torsades de pointe. Certain antidepressants have consistently been associated with QTc prolongation, particularly the TCAs. In addition, the US Food and Drug Administration recently issued the recommendation that the SSRI citalopram not be used at doses >40 mg (and 20 mg in those with hepatic impairment or age >60 years) due to results of a randomized controlled trial that showed a dose‐response increase in QTc. Antipsychotic medications have also been shown to increase QTc, with the greatest evidence for thioridazine and the first‐generation, low‐potency neuroleptics. Haloperidol (particularly the intravenous formulation) has also been linked to both long QTc and torsades, though data may be confounded by the degree of medical illness of patients receiving the medication.[42] Of the atypicals, ziprasidone causes the greatest QTc prolongation.[43] However, a large retrospective cohort study showed similar risk increase of sudden cardiac death (2‐fold) among all antipsychotics (including typicals and atypicals) when examined individually.[44]
Additional risk factors for QTc prolongation abound in the hospitalized patient. These include many medical problems, including electrolyte abnormalities, heart conditions, renal and hepatic dysfunction, and CNS injury. Hospitalized patients are also exposed to the cumulative effects of medications that increase duration of QTc, such as class I and class III antiarrhythmics. Additionally, certain antimicrobials, including macrolide antibiotics and antifungals, have been associated with QTc prolongation via pharmacokinetic interactions.
Because of this, QTc should be measured upon admission in patients on stable doses of psychotropics that predispose to prolongation. Addition of other medications known to contribute to increased QTc should prompt further ECGs. Electrolytes, particularly potassium and magnesium, should be aggressively repleted. When QTc extends beyond 500 ms, consideration should be given to discontinuing or changing medications that can contribute to QTc prolongation (whether psychotropics or drugs used to treat acute medical problems).[42]
SEDATION
Sedation is another potential side effect of psychotropic medications. Although sedation is beneficial for agitation and anxiety, sedated patients may be unable to participate in treatment. They are also at greater risk of aspiration and falls.
Antipsychotics cause sedation through antagonism of 1 adrenergic and H1 histaminergic receptors. Effects are most pronounced with the low‐potency, first‐generation antipsychotics and the atypicals quetiapine and clozapine. Some antidepressants, including the TCAs and mirtazapine, also cause somnolence by histamine H1‐receptor antagonism. If a patient has been psychiatrically stable on a particular antipsychotic or antidepressant, but has psychotropic‐induced sedation that interferes with treatment, hospitalists may want to consider temporarily decreasing the dose.
Benzodiazepines induce sedation by increasing ‐aminobutyric acid‐ergic transmission. There is significant overlap between anxiolytic and sedating doses of benzodiazepines. The amount of sedation is related to dose, speed of absorption, and onset of CNS penetration. Thus, sedation may be minimized by using a lower dose or switching to an equivalent dose of a slower‐onset benzodiazepine.[45, 46]
CONCLUSION
The decision to continue or discontinue psychotropic medications is often challenging. It requires the hospitalist to carefully weigh the risks and benefits of the ongoing treatment versus discontinuation, while also considering the patient's preference whenever possible. Sudden cessation of psychotropics can lead to a number of unwanted complications, from mild withdrawal to life‐threatening autonomic instability and psychiatric decompensation. Yet psychotropic continuation can also lead to unwanted drug‐drug interactions and newly emergent side effects.
To improve patient safety and outcomes, hospitalists must take a cautious and well‐thought out approach to treating patients on psychotropic drugs. Reflexive cessation of home medications must be avoided. If hepatic or renal insufficiency develops, medication doses may need to be adjusted. When available, drug levels should guide dose adjustments. If side effects occur, the patient's medication list should be carefully reviewed for potential drug‐drug interactions. Maintaining mental health may mean substituting another drug for one that interferes with home psychotropic medications. Psychotropic doses may also be minimized to decrease side effects. When a psychotropic must be discontinued, tapering is recommended over an abrupt discontinuation, except in the case of an acute toxicity. Moreover, cross‐titration to another effective agent may prevent psychiatric decompensation.
Additionally, hospitalists should use all available resources when deciding on a patient's psychotropic regimen. Mobile devices and online resources can assist with pharmacokinetics. Pharmacists can help with more complex questions or potential drug substitutions. Consultation‐liaison psychiatrists can be a valuable resource in ensuring the safety and stability of a patient with psychiatric comorbidity within the medical environment. The consultant may assist by assessing a patient's current psychiatric state and recommending psychotropic medication changes when needed.
Disclosure
Nothing to report.
- , , , et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352(24):2515–2523.
- , , , , , . Mental disorders in primary care: prevalence and co‐morbidity among disorders. results from the functional illness in primary care (FIP) study. Psychol Med. 2005;35(8):1175–1184.
- , , , et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52–77.
- , , , et al. The Academy of Psychosomatic Medicine practice guidelines for psychiatric consultation in the general medical setting. The Academy of Psychosomatic Medicine. Psychosomatics. 1998;39(4):S8–S30.
- , , , , . Impact of psychiatric comorbidity on length of hospital stay for medical/surgical patients: a preliminary report. Am J Psychiatry. 1987;144(7):878–882.
- . Need for better psychiatric training for primary care providers. Acad Med. 1996;71(6):574–575.
- , , , et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840–847.
- . Discontinuing treatment for psychiatric disorders. J Psychiatry Neurosci. 2006;31(1):11–12.
- , , , , , . Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592–606.
- , , . Abrupt discontinuation of psychotropic drugs during pregnancy: fear of teratogenic risk and impact of counselling. J Psychiatry Neurosci. 2001;26(1):44–48.
- , , , , . Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry. 1997;54(1):49–55.
- . Potential adverse effects of discontinuing psychotropic drugs. Part 3: Antipsychotic, dopaminergic, and mood‐stabilizing drugs. J Psychosoc Nurs Ment Health Serv. 2010;48(8):11–14.
- , . Fluoxetine. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. 2nd ed. Chapter 13. Washington, DC: American Psychiatric Press; 2004:235.
- , , , , . Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449–456.
- , , , , . Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77–87.
- , , , . Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255–261.
- . Antidepressant withdrawal syndromes: phenomenology and pathophysiology. Acta Psychiatr Scand. 1989;79(2):113–117.
- . Heterocyclic antidepressant, monoamine oxidase inhibitor and neuroleptic withdrawal phenomena. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14(2):137–161.
- . Monoamine oxidase inhibitor withdrawal phenomena: symptoms and pathophysiology. Acta Psychiatr Scand. 1988;78(1):1–7.
- , . Antipsychotic withdrawal symptoms: phenomenology and pathophysiology. Acta Psychiatr Scand. 1988;77(3):241–246.
- . Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal‐related relapse. Acta Psychiatr Scand. 2006;114(1):3–13.
- , . Neuroleptic malignant syndrome after neuroleptic discontinuation. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(8):1323–1334.
- , . Withdrawal‐emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179–182.
- , , . Respiratory dyskinesia as discontinuation effect of risperidone. J Clin Psychopharmacol. 2005;25(6):609.
- , . Withdrawal‐emergent respiratory dyskinesia with risperidone treated with clozapine. J Neuropsychiatry Clin Neurosci. 2010;22(2):E24.
- , . Clozapine‐withdrawal catatonia. Psychosomatics. 2010;51(4):355–355.e2.
- , , . Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870–876.
- , , . Psychotropic discontinuation symptoms: a case of withdrawal neuroleptic malignant syndrome. Gen Hosp Psychiatry. 2006;28(6):541–543.
- , . Discontinuation symptoms and psychotropic drugs. Lancet. 2000;355(9210):1184.
- . Potential adverse effects of discontinuing psychotropic drugs. J Psychosoc Nurs Ment Health Serv. 2010;48(9):11–14.
- , , , . Pharmacological interventions for benzodiazepine mono‐dependence management in outpatient settings. Cochrane Database Syst Rev. 2006(3):CD005194.
- , . Substance abuse and withdrawal in the critical care setting. Crit Care Clin. 2008;24(4):767–788, viii.
- . Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76.
- , . Prevention, recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139–1142.
- . Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73(suppl 1):17–24.
- , . Lithium intoxication. J Am Soc Nephrol. 1999;10(3):666–674.
- . Valproic acid toxicity: overview and management. J Toxicol Clin Toxicol. 2002;40(6):789–801.
- , , . Lithium. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. 2nd ed. Chapter 35. Washington, DC: American Psychiatric Press; 2004:547–549.
- , . 2010 guide to psychiatric drug interactions. Prim Psychiatry. 2009;16(12):45–74.
- , . Anticholinergic side‐effects of drugs in elderly people. J R Soc Med. 2000;93(9):457–462.
- , , . Measurement of anticholinergic effects of psychotropic drugs in humans. Pharmacopsychiatry. 2005;38(5):187–193.
- , , , , . QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54(1):1–13.
- , , , et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24(1):62–69.
- , , , , . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235.
- , . Sedation, an unpleasant, undesirable and potentially dangerous side‐effect of many psychotropic drugs. Hum Psychopharmacol. 2004;19(2):135–139.
- , . Toxicology and overdose of atypical antipsychotics. J Emerg Med. 2012;43(5):906–913.
Mental illness is highly prevalent, with approximately 30% of the US population meeting criteria for at least 1 disorder.[1] In the medically ill population, psychiatric disease is even more common; a 2005 survey showed that half of all patients visiting primary care physicians met criteria for a mental disorder.[2] Conversely, those with serious mental illness suffer greater medical morbidity than the general population, with higher rates of obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, human immunodeficiency virus, viral hepatitis, and tuberculosis.[3] When acute medical problems arise, those with mental illness endure longer hospitalizations; the presence of a psychiatric disturbance in the general medical setting has been shown to be a robust predictor of increased hospital length of stay.[4, 5]
Because of the strong correlation between medical and mental illness, hospitalists will care for patients with psychiatric disorders. Despite this, internists generally receive a paucity of formal training in the treatment of mental disturbances. One survey of university‐affiliated internal medicine residencies revealed that only 10% of programs offered any kind of modest curriculum in psychiatric education.[6] Regardless of this lack of preparation, hospitalists are called upon at each admission to make decisions that affect the psychiatric treatment of patients on psychotropic medication; namely, they must decide whether to continue or discontinue psychiatric medications. Many physicians reflexively discontinue a patient's chronic medications upon admission to the hospital; one study reported an adjusted odds ratio of between 1.18 and 1.86 for stopping a medication prescribed for a chronic condition.[7]
This review aims to assist the hospitalist in making an informed decision about the continuation of psychotropic medications in the medically ill patient. First, it examines the risks of stopping psychotropic medication, including psychiatric decompensation and discontinuation syndromes. It also explores the challenges of medication continuation in the context of changing pharmacokinetics and emerging side effects. Ultimately, physicians and patients must make collaborative decisions, weighing the risk of medication interactions against the potential adverse effects of psychiatric decompensation.
DISCONTINUATION
Decompensation of Mental Health
Approximately 10% to 15% of patients hospitalized for medical illness require reduction or discontinuation of psychotropic medications because they may be contributing to the clinical presentation.[4] The rate and method of drug discontinuation can affect the course of major psychiatric disorders.[8] A growing number of studies demonstrate high rates of relapse when medications are discontinued in patients suffering from mood disorders, schizophrenia, and anxiety disorders.[9] Abrupt cessation of psychotropics is especially dangerous, leading to a greater chance of destabilization than if medications are tapered. Episodes of active illness even appear to occur more frequently with sudden psychotropic cessation than they would in the natural course of untreated disease. This is true for several classes of psychotropics, including antidepressants, mood stabilizers, and antipsychotics. For example, in a study of pregnant women who suddenly stopped their psychotropic medication (both antidepressants and benzodiazepines), nearly one‐third experienced suicidal ideation.[10] Depression and suicidality have also been documented in bipolar patients who were abruptly taken off of lithium. More commonly, rapid lithium discontinuation in bipolar patients causes mania, with illness relapse as soon as 4 days after cessation.[10] Additionally, abrupt discontinuation of antipsychotics in patients with schizophrenia leads to early, and often severe, psychosis. One study found a relapse rate of 50% within 30 weeks of sudden oral neuroleptic cessation.[11] Furthermore, restarting medications, even at the previous effective dose, may not return the patient to their prior baseline.[12] Psychiatric decompensation in the hospitalized patient can worsen medical outcomes, with decreased adherence to treatment plans. In extreme circumstances, patients may be at risk of self‐harm or suicide.
DRUG‐SPECIFIC DISCONTINUATION SYNDROMES
Antidepressants
Discontinuation of medications presents additional problems, and sudden cessation of psychotropic medications can lead to uncomfortable or even dangerous symptoms. For example, the serotonin discontinuation syndrome has been well documented. Chronic use of serotonin re‐uptake inhibitors (generally greater than 6 to 8 weeks) leads to downregulation of postsynaptic serotonin receptors. When selective serotonin re‐uptake inhibitors (SSRIs) or serotonin‐norepinephrine re‐uptake inhibitors are abruptly stopped, the brain experiences a relative decline in serotonin. Symptoms include a flu‐like illness, nausea, imbalance, insomnia, sensory disturbances, and dysphoria. Onset may be within hours of missing a dose, but typically occurs within 3 days of medication discontinuation. The syndrome is more likely to occur with cessation of medications of shorter half‐life and less likely to occur with medications with a long half‐life, such as fluoxetine (Table 1).[13, 14] The symptoms can be ameliorated with a gradual tapering or reintroduction of the antidepressant.[15] Untreated symptoms resolve in 1 to 2 weeks. Although the syndrome in isolation is not life‐threatening, a number of the symptoms can complicate medical illness and muddle diagnosis of other diseases.[14, 16]
| Medication | Half‐Life (Hours) |
|---|---|
| |
| SSRIs | |
| Fluoxetine | 84144 |
| Paroxetine | 21 |
| Sertraline | 26 |
| Citalopram | 35 |
| Escitalopram | 2732 |
| Fluvoxamine | 15 |
| SNRIs | |
| Venlafaxine | 313 |
| Duloxetine | 1116 |
Older antidepressants, including the tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs), have serotonergic effects, and thus discontinuation may cause the symptoms described above. However, these agents also have effects on other neurotransmitters. The TCAs block muscarinic cholinergic receptors, leading to upregulation. Abrupt cessation can lead to cholinergic rebound, with parkinsonism and mania emerging. Multiple case reports document improvement in these symptoms with an anticholinergic agent, such as benztropine.[17, 18] MAOIs lead to changes in ‐2 adrenergic and dopaminergic receptors. Sudden discontinuation has been associated with agitation, delirium, and psychosis; 1 case report even documents catatonia associated with autonomic instability.[19]
In addition, sudden discontinuation of antidepressants (including the SSRIs) may provoke mania or hypomania in some patients, regardless of whether they have experienced previous spontaneous manic episodes.[8]
Neuroleptics
Data for an antipsychotic withdrawal syndrome are less convincing than those for serotonergic agents. However, certain symptoms have been associated with abrupt neuroleptic discontinuation. Most frequently, gastrointestinal distress and diaphoresis are described. Anxiety, agitation, and insomnia are also common. These symptoms are thought to be associated with cholinergic rebound, mediated by direct effects of neuroleptics on muscarinic receptors or indirectly through dopamine receptor blockade and the dopamine‐cholinergic balance. Symptoms may be more severe when antimuscarinic, antiparkinsonism drugs are simultaneously stopped. Some authors argue that the timing of symptom onset can differentiate antipsychotic withdrawal from illness relapse, with discontinuation syndrome occurring within the first 7 days of medication cessation.[20]
Additionally, abrupt cessation of antipsychotics may be associated with rapid‐onset psychosis. The data are strongest for clozapine discontinuation, where overall incidence is approximately 20%. This is hypothesized to be mediated by dopamine receptor upregulation and subsequent hypersensitivity to endogenous dopamine. The emerging psychosis is purportedly distinct from the underlying illness. Episodes have been described in patients on chronic metoclopramide who have no prior psychiatric history, as well as in patients with bipolar disorder without psychosis prior to neuroleptic discontinuation.[21]
Movement disorders may emerge during neuroleptic discontinuation. Both parkinsonism and dyskinesias have been described. In some patients, dyskinesias resolve within weeks of drug discontinuation; however, others experience permanent symptoms, termed covert dyskinesia.[22] In rare circumstances, dyskinesias may affect the respiratory muscles, causing distress. Several case reports of withdrawal‐emergent respiratory dyskinesia have been reported following risperidone cessation.[23, 24, 25] Additionally, several case reports have described catatonia occurring after abrupt discontinuation of clozapine. In all cases, symptoms promptly resolved with reinitiation of clozapine.[26]
Neuroleptic malignant syndrome (NMS) is a rare but potentially fatal complication of antipsychotic administration. Symptoms include fever, rigidity, autonomic instability, and mental status changes.[27] Though NMS is hypothesized to occur due to dopamine receptor blockade, rare cases of NMS have also been reported with abrupt cessation of neuroleptics. Of the 8 case reports in the literature, 1 resulted in death.[22, 28]
Mood Stabilizers
Though some of the atypical antipsychotics are used to treat bipolar disorder, mood stabilizers are the mainstay of pharmacotherapy. The most commonly used mood stabilizers are lithium, valproic acid, lamotrigine, topirimate, carbamezapine, and oxcarbazepine. When mood stabilizers are discontinued, patients are at risk of psychiatric relapse, as documented above. However, withdrawal symptoms have not been commonly documented upon abrupt discontinuation of lithium or the anticonvulsants used to treat bipolar disorder.[29, 30]
Benzodiazepines
Benzodiazepines are widely prescribed for insomnia and anxiety. Chronic legal use of benzodiazepines is approximately 2% in the general population.[31] Like ethanol, benzodiazepines bind nonselectively to the GABA‐A receptor, resulting in downregulation of GABA receptors and compensatory increased N‐methyl‐D‐aspartate transmission. Sudden discontinuation of benzodiazepines results in a syndrome that mirrors that of alcohol withdrawal. Symptoms range from mild (tremor, insomnia, and anxiety) to life‐threatening (seizures, delirium, and autonomic instability). Serious withdrawal is more likely with substances of shorter half‐life and with higher chronic doses. Onset often occurs between 2 and 10 days after discontinuation, depending on the half‐life of the benzodiazepine.[32] Other rare serious reactions have been documented following abrupt benzodiazepine cessation, including NMS and catatonia.[30]
CONTINUATION
Reflexive discontinuation of psychotropic medications can clearly lead to adverse outcomes. However, when hospitalists decide to continue a patient's psychotropic medications, they must also be cognizant of potential complications. Modifications may be necessary because of hepatic, renal, or cardiac disease. In addition, physicians need to be aware of drug‐drug interactions. Pharmacotherapy for medically ill elderly patients may require dose modifications to account for an increased lipophilic volume of distribution and a decreased rate of metabolism.[33] Finally, pregnancy can present additional challenges regarding dose modifications and teratogenicity.
On the other hand, hospitalists must be aware that continuing a patient's psychotropic medication may not be the cause of new psychiatric symptoms. Drugs prescribed for medical disorders (eg, corticosteroids) often cause psychiatric symptoms. In addition, psychiatric symptoms may emerge at times of nonpsychotropic medication withdrawal or due to nonpsychotropic drug‐drug interactions.[4] Groups of medications commonly associated with psychiatric disturbances include analgesics, sedatives, anesthetics, anticonvulsants, and anticholinergics.
PHARMACOKINETICS: PSYCHOTROPIC TOXICITY
Medical illness alters the body's steady state, and renal or hepatic metabolism may be impaired when a patient requires hospitalization. Additionally, new medications may increase the effects of psychotropics, whether by intrinsic augmentation of effect or decreased psychotropic clearance. Ultimately, these changes can lead to psychotropic toxicity.
Specific toxicities merit discussion. First, serotonin syndrome is a potentially fatal condition. The majority of cases occur with synergistic serotonergic medication administration, though there are case reports of the syndrome occurring with addition of inhibitors of cytochrome p450 2D6 and/or 3A4 to SSRIs. A large number of medications from different classes have been indicated (Table 2).[34] Symptoms generally occur within 24 hours of medication administration and include mental status changes, autonomic instability, and neuromuscular hyperactivity. When serotonin syndrome is suspected, the offending agent should be discontinued immediately. There is no definitive treatment, though supportive care can be lifesaving.[34]
| Amphetamines and Derivatives | Antidepressants and Mood Stabilizers | Antimigraine Drugs | Analgesics | Antiemetics | Miscellaneous |
|---|---|---|---|---|---|
| |||||
| MDMA | Buspirone | Ergot alkaloids | Cyclobenzaprine | Metoclopramide | Cocaine |
| Dextroamphetamine | Carbamazepine | Triptans | Fentanyl | Ondansetron | Dextromethorphan |
| Methamphetamine | Lithium | Meperidine | Linezolid | ||
| Sibutramine | MAOIs | Tramadol | L‐tryptophan | ||
| SSRIs | 5‐hydroxytrytophan | ||||
| SNRIs | |||||
| Serotonin 2A receptor blockers (eg, trazodone) | |||||
| St. John's Wort | |||||
| TCAs | |||||
| Valproic Acid | |||||
In addition to serotonin syndrome, hypertensive emergency may occur due to drug interactions with MAOIs. MAOIs inhibit the enzyme monoamine oxidase, resulting in elevated levels of serotonin, histamine, and catecholamines in the blood. Coadministration of MAOIs and sympathomimetic agents (such as cough suppressants and analgesics) may dangerously increase adrenergic stimulation, elevating blood pressure to the point of end‐organ damage. Please see Table 3 for a full list of drugs indicated in MAOI‐associated hypertensive crisis.[35] To ensure safety, it is recommended that MAOIs be discontinued for 14 days prior to introducing medications with sympathomimetic properties, and vice versa. Because of its longer half‐life, a 5‐week washout period is recommended for fluoxetine.[35]
|
| Amphetamines |
| Analgesics: meperedine |
| Anesthetics |
| Antidepressants: buproprion, buspirone, other MAOIs, SSRIs, SNRIs, TCAs, |
| Mirtazapine |
| Cocaine |
| Dibenzazepine‐related agents: carbamezapine, cyclobenzaprine, perphenazine |
| Female sex steroids |
| Sympathomimetics: dopamine, epinephrine, levodopa, methyldopa, methylphenidate, norepinephrine, phenylalanine, reserpine, tyrosine, tryptophan |
| Other vasoconstrictors: pseudoephedrine, phenylephrine, phenylpropanolamine, ephedrine |
Lithium toxicity may result from changing patient pharmacokinetics. Lithium is almost entirely renally excreted, and acute kidney injury may precipitously raise serum levels. Within the renal collecting system, lithium is handled similarly to sodium, with 80% reabsorbed from the proximal tubule to the collecting duct. Thus, factors that decrease glomerular filtration rate (GFR) and increase proximal tubule absorption will increase serum lithium levels. For example, decreased effective arterial volume (due to dehydration, cirrhosis, nephrotic syndrome, or heart failure) may elevate lithium levels. Additionally, medications that decrease GFR may increase lithium reabsorption. These include nonsteroidal anti‐inflammatory drugs, angiotensin‐converting enzyme inhibitors, and thiazide diuretics. Because of lithium's narrow therapeutic index, small elevations in serum levels can lead to toxicity. Severity of intoxication correlates with serum concentration. Symptoms range from lethargy, weakness, tremor, ataxia, and gastrointestinal distress to coma, seizures, renal failure, and death. Toxicity is also associated with electrocardiograph (ECG) changes, including ST‐segment depression and T‐wave inversion in the lateral precordial leads. Sinus node dysfunction can also occur. Definitive treatment for lithium toxicity is hemodialysis.[36]
Though the therapeutic index is much wider for valproic acid than for lithium, valproate toxicity may also occur in the medically ill patient with previously stable serum levels. Valproic acid is highly protein‐bound at therapeutic levels, and is metabolized largely through hepatic glucuronidation. Initiation of medications that compete for protein‐binding sites, including aspirin, has led to valproate toxicity. Moreover, acute liver failure or addition of drugs that compete with hepatic microsomal enzymes may lead to decreased excretion of valproic acid. Poisoning may result in central nervous system (CNS) and respiratory depression, hypotension, cerebral edema, and pancreatitis. True hepatoxicity is rare, though hyperammonemia is widely documented. Thrombocytopenia is the most common hematologic abnormality associated with overdose. However, thrombocytopenia may also occur without complication in patients on stable therapeutic doses. Treatment is largely supportive, though hemoperfusion and hemodialysis may be used when serum levels are >300 g/mL, as only 35% of the drug is protein‐bound at that level. Naloxone has been shown in case reports to reverse valproic acid‐induced coma, and L‐carnitine has been increasingly recommended for hyperammonemia.[37, 38]
PHARMACOKINETICS: DRUG‐DRUG INTERACTIONS
As discussed above, the addition of a new medication can increase previously stable levels of psychotropic drugs, leading to toxicity. Conversely, mental health medications can alter the expected metabolism of a drug being used to treat acute medical illness. Many psychotropics are metabolized via the cytochrome p450 enzyme, particularly the SSRIs (Table 4).[39] A number of antimicrobial and antiarrythmic medications are also cleared via this route, leading to potential toxic or subtherapeutic levels when drug‐drug interactions occur.
| CYP 1A2 | CYP 2B6 | CYP 2C9 | CYP 2C19 | CYP 2D6 | CYP 3A4/3A5/3A7 |
|---|---|---|---|---|---|
| Clozapine | Bupropion | Amitriptyline | Phenytoin | Antidepressants | Alprazolam |
| Duloxetine | Methadone | Fluoxetine | Amitriptyline | Amitriptyline | Diazepam |
| Fluvoxamine | Phenytoin | Citalopram | Clomipramine | Midazolam | |
| Haloperidol | Clomipramine | Duloxetine | Aripiprazole | ||
| Imipramine | Diazepam | Desipramine | Buspirone | ||
| Olanzapine | Imipramine | Fluoxetine | Haldol | ||
| Ramelteon | Fluvoxamine | Quetiapine | |||
| Imipramine | Ziprasidone | ||||
| Nortriptyline | Zolpidem | ||||
| Paroxetine | Dextromethorphan | ||||
| Venlafaxine | |||||
| Antipsychotics | |||||
| Aripiprazole | |||||
| Clorpromazine | |||||
| Haldol | |||||
| Perphenazine | |||||
| Risperidone |
PSYCHOTROPIC ADVERSE EFFECTS
Psychotropic medications may also cause side effects that contribute to the clinical presentation, requiring ongoing monitoring, a dose reduction, or psychotropic discontinuation. Potential adverse effects that commonly impact psychopharmacologic management include anticholinergic side effects, cardiac effects, and sedation.
ANTICHOLINERGIC EFFECTS AND TOXICITY
Newly emerging anticholinergic effects may be particularly troubling. Dry mouth may cause swallowing difficulty and aspiration. Pupillary dilatation and dry eyes can increase risk of falls. Constipation may evolve into fecal impaction, and urinary retention can contribute to increased catheter use and infection. CNS effects are perhaps the most serious, ranging from drowsiness and memory impairment to frank delirium.[40]
Many psychotropic drugs are anti cholinergic. Among the antidepressants, the TCAs and paroxetine have the highest anticholinergic activity. Anticholinergic effects have also been reported with the low potency first generation neuroleptics and with the atypical antipsychotics olanzapine and clozapine. Additionally, medications used to treat the extrapyramidal symptoms associated with antipsychotics (such as benztropine and diphenhydramine) are strongly anticholinergic.[41] Patients without previous overt anticholinergic symptoms from these medications may experience adverse effects when hospitalized. Medical illness or new medications may alter psychotropic drug metabolism and elimination, leading to accumulation of their anticholinergic effects. Many medications used in the hospital also have intrinsic anticholinergic activity. These include some antiemetics, antispasmodics, antiarrhythmics, and histamine H2 receptor blockers. Elderly patients are particularly prone to anticholinergic effects due to age‐related deficits in cholinergic transmission.[40]
QTc PROLONGATION
QTc prolongation is a potentially lethal side effect of certain medications. Prolonged QTc increases the risk of cardiac mortality and sudden death, presumably related to onset of torsades de pointe. Certain antidepressants have consistently been associated with QTc prolongation, particularly the TCAs. In addition, the US Food and Drug Administration recently issued the recommendation that the SSRI citalopram not be used at doses >40 mg (and 20 mg in those with hepatic impairment or age >60 years) due to results of a randomized controlled trial that showed a dose‐response increase in QTc. Antipsychotic medications have also been shown to increase QTc, with the greatest evidence for thioridazine and the first‐generation, low‐potency neuroleptics. Haloperidol (particularly the intravenous formulation) has also been linked to both long QTc and torsades, though data may be confounded by the degree of medical illness of patients receiving the medication.[42] Of the atypicals, ziprasidone causes the greatest QTc prolongation.[43] However, a large retrospective cohort study showed similar risk increase of sudden cardiac death (2‐fold) among all antipsychotics (including typicals and atypicals) when examined individually.[44]
Additional risk factors for QTc prolongation abound in the hospitalized patient. These include many medical problems, including electrolyte abnormalities, heart conditions, renal and hepatic dysfunction, and CNS injury. Hospitalized patients are also exposed to the cumulative effects of medications that increase duration of QTc, such as class I and class III antiarrhythmics. Additionally, certain antimicrobials, including macrolide antibiotics and antifungals, have been associated with QTc prolongation via pharmacokinetic interactions.
Because of this, QTc should be measured upon admission in patients on stable doses of psychotropics that predispose to prolongation. Addition of other medications known to contribute to increased QTc should prompt further ECGs. Electrolytes, particularly potassium and magnesium, should be aggressively repleted. When QTc extends beyond 500 ms, consideration should be given to discontinuing or changing medications that can contribute to QTc prolongation (whether psychotropics or drugs used to treat acute medical problems).[42]
SEDATION
Sedation is another potential side effect of psychotropic medications. Although sedation is beneficial for agitation and anxiety, sedated patients may be unable to participate in treatment. They are also at greater risk of aspiration and falls.
Antipsychotics cause sedation through antagonism of 1 adrenergic and H1 histaminergic receptors. Effects are most pronounced with the low‐potency, first‐generation antipsychotics and the atypicals quetiapine and clozapine. Some antidepressants, including the TCAs and mirtazapine, also cause somnolence by histamine H1‐receptor antagonism. If a patient has been psychiatrically stable on a particular antipsychotic or antidepressant, but has psychotropic‐induced sedation that interferes with treatment, hospitalists may want to consider temporarily decreasing the dose.
Benzodiazepines induce sedation by increasing ‐aminobutyric acid‐ergic transmission. There is significant overlap between anxiolytic and sedating doses of benzodiazepines. The amount of sedation is related to dose, speed of absorption, and onset of CNS penetration. Thus, sedation may be minimized by using a lower dose or switching to an equivalent dose of a slower‐onset benzodiazepine.[45, 46]
CONCLUSION
The decision to continue or discontinue psychotropic medications is often challenging. It requires the hospitalist to carefully weigh the risks and benefits of the ongoing treatment versus discontinuation, while also considering the patient's preference whenever possible. Sudden cessation of psychotropics can lead to a number of unwanted complications, from mild withdrawal to life‐threatening autonomic instability and psychiatric decompensation. Yet psychotropic continuation can also lead to unwanted drug‐drug interactions and newly emergent side effects.
To improve patient safety and outcomes, hospitalists must take a cautious and well‐thought out approach to treating patients on psychotropic drugs. Reflexive cessation of home medications must be avoided. If hepatic or renal insufficiency develops, medication doses may need to be adjusted. When available, drug levels should guide dose adjustments. If side effects occur, the patient's medication list should be carefully reviewed for potential drug‐drug interactions. Maintaining mental health may mean substituting another drug for one that interferes with home psychotropic medications. Psychotropic doses may also be minimized to decrease side effects. When a psychotropic must be discontinued, tapering is recommended over an abrupt discontinuation, except in the case of an acute toxicity. Moreover, cross‐titration to another effective agent may prevent psychiatric decompensation.
Additionally, hospitalists should use all available resources when deciding on a patient's psychotropic regimen. Mobile devices and online resources can assist with pharmacokinetics. Pharmacists can help with more complex questions or potential drug substitutions. Consultation‐liaison psychiatrists can be a valuable resource in ensuring the safety and stability of a patient with psychiatric comorbidity within the medical environment. The consultant may assist by assessing a patient's current psychiatric state and recommending psychotropic medication changes when needed.
Disclosure
Nothing to report.
Mental illness is highly prevalent, with approximately 30% of the US population meeting criteria for at least 1 disorder.[1] In the medically ill population, psychiatric disease is even more common; a 2005 survey showed that half of all patients visiting primary care physicians met criteria for a mental disorder.[2] Conversely, those with serious mental illness suffer greater medical morbidity than the general population, with higher rates of obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, human immunodeficiency virus, viral hepatitis, and tuberculosis.[3] When acute medical problems arise, those with mental illness endure longer hospitalizations; the presence of a psychiatric disturbance in the general medical setting has been shown to be a robust predictor of increased hospital length of stay.[4, 5]
Because of the strong correlation between medical and mental illness, hospitalists will care for patients with psychiatric disorders. Despite this, internists generally receive a paucity of formal training in the treatment of mental disturbances. One survey of university‐affiliated internal medicine residencies revealed that only 10% of programs offered any kind of modest curriculum in psychiatric education.[6] Regardless of this lack of preparation, hospitalists are called upon at each admission to make decisions that affect the psychiatric treatment of patients on psychotropic medication; namely, they must decide whether to continue or discontinue psychiatric medications. Many physicians reflexively discontinue a patient's chronic medications upon admission to the hospital; one study reported an adjusted odds ratio of between 1.18 and 1.86 for stopping a medication prescribed for a chronic condition.[7]
This review aims to assist the hospitalist in making an informed decision about the continuation of psychotropic medications in the medically ill patient. First, it examines the risks of stopping psychotropic medication, including psychiatric decompensation and discontinuation syndromes. It also explores the challenges of medication continuation in the context of changing pharmacokinetics and emerging side effects. Ultimately, physicians and patients must make collaborative decisions, weighing the risk of medication interactions against the potential adverse effects of psychiatric decompensation.
DISCONTINUATION
Decompensation of Mental Health
Approximately 10% to 15% of patients hospitalized for medical illness require reduction or discontinuation of psychotropic medications because they may be contributing to the clinical presentation.[4] The rate and method of drug discontinuation can affect the course of major psychiatric disorders.[8] A growing number of studies demonstrate high rates of relapse when medications are discontinued in patients suffering from mood disorders, schizophrenia, and anxiety disorders.[9] Abrupt cessation of psychotropics is especially dangerous, leading to a greater chance of destabilization than if medications are tapered. Episodes of active illness even appear to occur more frequently with sudden psychotropic cessation than they would in the natural course of untreated disease. This is true for several classes of psychotropics, including antidepressants, mood stabilizers, and antipsychotics. For example, in a study of pregnant women who suddenly stopped their psychotropic medication (both antidepressants and benzodiazepines), nearly one‐third experienced suicidal ideation.[10] Depression and suicidality have also been documented in bipolar patients who were abruptly taken off of lithium. More commonly, rapid lithium discontinuation in bipolar patients causes mania, with illness relapse as soon as 4 days after cessation.[10] Additionally, abrupt discontinuation of antipsychotics in patients with schizophrenia leads to early, and often severe, psychosis. One study found a relapse rate of 50% within 30 weeks of sudden oral neuroleptic cessation.[11] Furthermore, restarting medications, even at the previous effective dose, may not return the patient to their prior baseline.[12] Psychiatric decompensation in the hospitalized patient can worsen medical outcomes, with decreased adherence to treatment plans. In extreme circumstances, patients may be at risk of self‐harm or suicide.
DRUG‐SPECIFIC DISCONTINUATION SYNDROMES
Antidepressants
Discontinuation of medications presents additional problems, and sudden cessation of psychotropic medications can lead to uncomfortable or even dangerous symptoms. For example, the serotonin discontinuation syndrome has been well documented. Chronic use of serotonin re‐uptake inhibitors (generally greater than 6 to 8 weeks) leads to downregulation of postsynaptic serotonin receptors. When selective serotonin re‐uptake inhibitors (SSRIs) or serotonin‐norepinephrine re‐uptake inhibitors are abruptly stopped, the brain experiences a relative decline in serotonin. Symptoms include a flu‐like illness, nausea, imbalance, insomnia, sensory disturbances, and dysphoria. Onset may be within hours of missing a dose, but typically occurs within 3 days of medication discontinuation. The syndrome is more likely to occur with cessation of medications of shorter half‐life and less likely to occur with medications with a long half‐life, such as fluoxetine (Table 1).[13, 14] The symptoms can be ameliorated with a gradual tapering or reintroduction of the antidepressant.[15] Untreated symptoms resolve in 1 to 2 weeks. Although the syndrome in isolation is not life‐threatening, a number of the symptoms can complicate medical illness and muddle diagnosis of other diseases.[14, 16]
| Medication | Half‐Life (Hours) |
|---|---|
| |
| SSRIs | |
| Fluoxetine | 84144 |
| Paroxetine | 21 |
| Sertraline | 26 |
| Citalopram | 35 |
| Escitalopram | 2732 |
| Fluvoxamine | 15 |
| SNRIs | |
| Venlafaxine | 313 |
| Duloxetine | 1116 |
Older antidepressants, including the tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs), have serotonergic effects, and thus discontinuation may cause the symptoms described above. However, these agents also have effects on other neurotransmitters. The TCAs block muscarinic cholinergic receptors, leading to upregulation. Abrupt cessation can lead to cholinergic rebound, with parkinsonism and mania emerging. Multiple case reports document improvement in these symptoms with an anticholinergic agent, such as benztropine.[17, 18] MAOIs lead to changes in ‐2 adrenergic and dopaminergic receptors. Sudden discontinuation has been associated with agitation, delirium, and psychosis; 1 case report even documents catatonia associated with autonomic instability.[19]
In addition, sudden discontinuation of antidepressants (including the SSRIs) may provoke mania or hypomania in some patients, regardless of whether they have experienced previous spontaneous manic episodes.[8]
Neuroleptics
Data for an antipsychotic withdrawal syndrome are less convincing than those for serotonergic agents. However, certain symptoms have been associated with abrupt neuroleptic discontinuation. Most frequently, gastrointestinal distress and diaphoresis are described. Anxiety, agitation, and insomnia are also common. These symptoms are thought to be associated with cholinergic rebound, mediated by direct effects of neuroleptics on muscarinic receptors or indirectly through dopamine receptor blockade and the dopamine‐cholinergic balance. Symptoms may be more severe when antimuscarinic, antiparkinsonism drugs are simultaneously stopped. Some authors argue that the timing of symptom onset can differentiate antipsychotic withdrawal from illness relapse, with discontinuation syndrome occurring within the first 7 days of medication cessation.[20]
Additionally, abrupt cessation of antipsychotics may be associated with rapid‐onset psychosis. The data are strongest for clozapine discontinuation, where overall incidence is approximately 20%. This is hypothesized to be mediated by dopamine receptor upregulation and subsequent hypersensitivity to endogenous dopamine. The emerging psychosis is purportedly distinct from the underlying illness. Episodes have been described in patients on chronic metoclopramide who have no prior psychiatric history, as well as in patients with bipolar disorder without psychosis prior to neuroleptic discontinuation.[21]
Movement disorders may emerge during neuroleptic discontinuation. Both parkinsonism and dyskinesias have been described. In some patients, dyskinesias resolve within weeks of drug discontinuation; however, others experience permanent symptoms, termed covert dyskinesia.[22] In rare circumstances, dyskinesias may affect the respiratory muscles, causing distress. Several case reports of withdrawal‐emergent respiratory dyskinesia have been reported following risperidone cessation.[23, 24, 25] Additionally, several case reports have described catatonia occurring after abrupt discontinuation of clozapine. In all cases, symptoms promptly resolved with reinitiation of clozapine.[26]
Neuroleptic malignant syndrome (NMS) is a rare but potentially fatal complication of antipsychotic administration. Symptoms include fever, rigidity, autonomic instability, and mental status changes.[27] Though NMS is hypothesized to occur due to dopamine receptor blockade, rare cases of NMS have also been reported with abrupt cessation of neuroleptics. Of the 8 case reports in the literature, 1 resulted in death.[22, 28]
Mood Stabilizers
Though some of the atypical antipsychotics are used to treat bipolar disorder, mood stabilizers are the mainstay of pharmacotherapy. The most commonly used mood stabilizers are lithium, valproic acid, lamotrigine, topirimate, carbamezapine, and oxcarbazepine. When mood stabilizers are discontinued, patients are at risk of psychiatric relapse, as documented above. However, withdrawal symptoms have not been commonly documented upon abrupt discontinuation of lithium or the anticonvulsants used to treat bipolar disorder.[29, 30]
Benzodiazepines
Benzodiazepines are widely prescribed for insomnia and anxiety. Chronic legal use of benzodiazepines is approximately 2% in the general population.[31] Like ethanol, benzodiazepines bind nonselectively to the GABA‐A receptor, resulting in downregulation of GABA receptors and compensatory increased N‐methyl‐D‐aspartate transmission. Sudden discontinuation of benzodiazepines results in a syndrome that mirrors that of alcohol withdrawal. Symptoms range from mild (tremor, insomnia, and anxiety) to life‐threatening (seizures, delirium, and autonomic instability). Serious withdrawal is more likely with substances of shorter half‐life and with higher chronic doses. Onset often occurs between 2 and 10 days after discontinuation, depending on the half‐life of the benzodiazepine.[32] Other rare serious reactions have been documented following abrupt benzodiazepine cessation, including NMS and catatonia.[30]
CONTINUATION
Reflexive discontinuation of psychotropic medications can clearly lead to adverse outcomes. However, when hospitalists decide to continue a patient's psychotropic medications, they must also be cognizant of potential complications. Modifications may be necessary because of hepatic, renal, or cardiac disease. In addition, physicians need to be aware of drug‐drug interactions. Pharmacotherapy for medically ill elderly patients may require dose modifications to account for an increased lipophilic volume of distribution and a decreased rate of metabolism.[33] Finally, pregnancy can present additional challenges regarding dose modifications and teratogenicity.
On the other hand, hospitalists must be aware that continuing a patient's psychotropic medication may not be the cause of new psychiatric symptoms. Drugs prescribed for medical disorders (eg, corticosteroids) often cause psychiatric symptoms. In addition, psychiatric symptoms may emerge at times of nonpsychotropic medication withdrawal or due to nonpsychotropic drug‐drug interactions.[4] Groups of medications commonly associated with psychiatric disturbances include analgesics, sedatives, anesthetics, anticonvulsants, and anticholinergics.
PHARMACOKINETICS: PSYCHOTROPIC TOXICITY
Medical illness alters the body's steady state, and renal or hepatic metabolism may be impaired when a patient requires hospitalization. Additionally, new medications may increase the effects of psychotropics, whether by intrinsic augmentation of effect or decreased psychotropic clearance. Ultimately, these changes can lead to psychotropic toxicity.
Specific toxicities merit discussion. First, serotonin syndrome is a potentially fatal condition. The majority of cases occur with synergistic serotonergic medication administration, though there are case reports of the syndrome occurring with addition of inhibitors of cytochrome p450 2D6 and/or 3A4 to SSRIs. A large number of medications from different classes have been indicated (Table 2).[34] Symptoms generally occur within 24 hours of medication administration and include mental status changes, autonomic instability, and neuromuscular hyperactivity. When serotonin syndrome is suspected, the offending agent should be discontinued immediately. There is no definitive treatment, though supportive care can be lifesaving.[34]
| Amphetamines and Derivatives | Antidepressants and Mood Stabilizers | Antimigraine Drugs | Analgesics | Antiemetics | Miscellaneous |
|---|---|---|---|---|---|
| |||||
| MDMA | Buspirone | Ergot alkaloids | Cyclobenzaprine | Metoclopramide | Cocaine |
| Dextroamphetamine | Carbamazepine | Triptans | Fentanyl | Ondansetron | Dextromethorphan |
| Methamphetamine | Lithium | Meperidine | Linezolid | ||
| Sibutramine | MAOIs | Tramadol | L‐tryptophan | ||
| SSRIs | 5‐hydroxytrytophan | ||||
| SNRIs | |||||
| Serotonin 2A receptor blockers (eg, trazodone) | |||||
| St. John's Wort | |||||
| TCAs | |||||
| Valproic Acid | |||||
In addition to serotonin syndrome, hypertensive emergency may occur due to drug interactions with MAOIs. MAOIs inhibit the enzyme monoamine oxidase, resulting in elevated levels of serotonin, histamine, and catecholamines in the blood. Coadministration of MAOIs and sympathomimetic agents (such as cough suppressants and analgesics) may dangerously increase adrenergic stimulation, elevating blood pressure to the point of end‐organ damage. Please see Table 3 for a full list of drugs indicated in MAOI‐associated hypertensive crisis.[35] To ensure safety, it is recommended that MAOIs be discontinued for 14 days prior to introducing medications with sympathomimetic properties, and vice versa. Because of its longer half‐life, a 5‐week washout period is recommended for fluoxetine.[35]
|
| Amphetamines |
| Analgesics: meperedine |
| Anesthetics |
| Antidepressants: buproprion, buspirone, other MAOIs, SSRIs, SNRIs, TCAs, |
| Mirtazapine |
| Cocaine |
| Dibenzazepine‐related agents: carbamezapine, cyclobenzaprine, perphenazine |
| Female sex steroids |
| Sympathomimetics: dopamine, epinephrine, levodopa, methyldopa, methylphenidate, norepinephrine, phenylalanine, reserpine, tyrosine, tryptophan |
| Other vasoconstrictors: pseudoephedrine, phenylephrine, phenylpropanolamine, ephedrine |
Lithium toxicity may result from changing patient pharmacokinetics. Lithium is almost entirely renally excreted, and acute kidney injury may precipitously raise serum levels. Within the renal collecting system, lithium is handled similarly to sodium, with 80% reabsorbed from the proximal tubule to the collecting duct. Thus, factors that decrease glomerular filtration rate (GFR) and increase proximal tubule absorption will increase serum lithium levels. For example, decreased effective arterial volume (due to dehydration, cirrhosis, nephrotic syndrome, or heart failure) may elevate lithium levels. Additionally, medications that decrease GFR may increase lithium reabsorption. These include nonsteroidal anti‐inflammatory drugs, angiotensin‐converting enzyme inhibitors, and thiazide diuretics. Because of lithium's narrow therapeutic index, small elevations in serum levels can lead to toxicity. Severity of intoxication correlates with serum concentration. Symptoms range from lethargy, weakness, tremor, ataxia, and gastrointestinal distress to coma, seizures, renal failure, and death. Toxicity is also associated with electrocardiograph (ECG) changes, including ST‐segment depression and T‐wave inversion in the lateral precordial leads. Sinus node dysfunction can also occur. Definitive treatment for lithium toxicity is hemodialysis.[36]
Though the therapeutic index is much wider for valproic acid than for lithium, valproate toxicity may also occur in the medically ill patient with previously stable serum levels. Valproic acid is highly protein‐bound at therapeutic levels, and is metabolized largely through hepatic glucuronidation. Initiation of medications that compete for protein‐binding sites, including aspirin, has led to valproate toxicity. Moreover, acute liver failure or addition of drugs that compete with hepatic microsomal enzymes may lead to decreased excretion of valproic acid. Poisoning may result in central nervous system (CNS) and respiratory depression, hypotension, cerebral edema, and pancreatitis. True hepatoxicity is rare, though hyperammonemia is widely documented. Thrombocytopenia is the most common hematologic abnormality associated with overdose. However, thrombocytopenia may also occur without complication in patients on stable therapeutic doses. Treatment is largely supportive, though hemoperfusion and hemodialysis may be used when serum levels are >300 g/mL, as only 35% of the drug is protein‐bound at that level. Naloxone has been shown in case reports to reverse valproic acid‐induced coma, and L‐carnitine has been increasingly recommended for hyperammonemia.[37, 38]
PHARMACOKINETICS: DRUG‐DRUG INTERACTIONS
As discussed above, the addition of a new medication can increase previously stable levels of psychotropic drugs, leading to toxicity. Conversely, mental health medications can alter the expected metabolism of a drug being used to treat acute medical illness. Many psychotropics are metabolized via the cytochrome p450 enzyme, particularly the SSRIs (Table 4).[39] A number of antimicrobial and antiarrythmic medications are also cleared via this route, leading to potential toxic or subtherapeutic levels when drug‐drug interactions occur.
| CYP 1A2 | CYP 2B6 | CYP 2C9 | CYP 2C19 | CYP 2D6 | CYP 3A4/3A5/3A7 |
|---|---|---|---|---|---|
| Clozapine | Bupropion | Amitriptyline | Phenytoin | Antidepressants | Alprazolam |
| Duloxetine | Methadone | Fluoxetine | Amitriptyline | Amitriptyline | Diazepam |
| Fluvoxamine | Phenytoin | Citalopram | Clomipramine | Midazolam | |
| Haloperidol | Clomipramine | Duloxetine | Aripiprazole | ||
| Imipramine | Diazepam | Desipramine | Buspirone | ||
| Olanzapine | Imipramine | Fluoxetine | Haldol | ||
| Ramelteon | Fluvoxamine | Quetiapine | |||
| Imipramine | Ziprasidone | ||||
| Nortriptyline | Zolpidem | ||||
| Paroxetine | Dextromethorphan | ||||
| Venlafaxine | |||||
| Antipsychotics | |||||
| Aripiprazole | |||||
| Clorpromazine | |||||
| Haldol | |||||
| Perphenazine | |||||
| Risperidone |
PSYCHOTROPIC ADVERSE EFFECTS
Psychotropic medications may also cause side effects that contribute to the clinical presentation, requiring ongoing monitoring, a dose reduction, or psychotropic discontinuation. Potential adverse effects that commonly impact psychopharmacologic management include anticholinergic side effects, cardiac effects, and sedation.
ANTICHOLINERGIC EFFECTS AND TOXICITY
Newly emerging anticholinergic effects may be particularly troubling. Dry mouth may cause swallowing difficulty and aspiration. Pupillary dilatation and dry eyes can increase risk of falls. Constipation may evolve into fecal impaction, and urinary retention can contribute to increased catheter use and infection. CNS effects are perhaps the most serious, ranging from drowsiness and memory impairment to frank delirium.[40]
Many psychotropic drugs are anti cholinergic. Among the antidepressants, the TCAs and paroxetine have the highest anticholinergic activity. Anticholinergic effects have also been reported with the low potency first generation neuroleptics and with the atypical antipsychotics olanzapine and clozapine. Additionally, medications used to treat the extrapyramidal symptoms associated with antipsychotics (such as benztropine and diphenhydramine) are strongly anticholinergic.[41] Patients without previous overt anticholinergic symptoms from these medications may experience adverse effects when hospitalized. Medical illness or new medications may alter psychotropic drug metabolism and elimination, leading to accumulation of their anticholinergic effects. Many medications used in the hospital also have intrinsic anticholinergic activity. These include some antiemetics, antispasmodics, antiarrhythmics, and histamine H2 receptor blockers. Elderly patients are particularly prone to anticholinergic effects due to age‐related deficits in cholinergic transmission.[40]
QTc PROLONGATION
QTc prolongation is a potentially lethal side effect of certain medications. Prolonged QTc increases the risk of cardiac mortality and sudden death, presumably related to onset of torsades de pointe. Certain antidepressants have consistently been associated with QTc prolongation, particularly the TCAs. In addition, the US Food and Drug Administration recently issued the recommendation that the SSRI citalopram not be used at doses >40 mg (and 20 mg in those with hepatic impairment or age >60 years) due to results of a randomized controlled trial that showed a dose‐response increase in QTc. Antipsychotic medications have also been shown to increase QTc, with the greatest evidence for thioridazine and the first‐generation, low‐potency neuroleptics. Haloperidol (particularly the intravenous formulation) has also been linked to both long QTc and torsades, though data may be confounded by the degree of medical illness of patients receiving the medication.[42] Of the atypicals, ziprasidone causes the greatest QTc prolongation.[43] However, a large retrospective cohort study showed similar risk increase of sudden cardiac death (2‐fold) among all antipsychotics (including typicals and atypicals) when examined individually.[44]
Additional risk factors for QTc prolongation abound in the hospitalized patient. These include many medical problems, including electrolyte abnormalities, heart conditions, renal and hepatic dysfunction, and CNS injury. Hospitalized patients are also exposed to the cumulative effects of medications that increase duration of QTc, such as class I and class III antiarrhythmics. Additionally, certain antimicrobials, including macrolide antibiotics and antifungals, have been associated with QTc prolongation via pharmacokinetic interactions.
Because of this, QTc should be measured upon admission in patients on stable doses of psychotropics that predispose to prolongation. Addition of other medications known to contribute to increased QTc should prompt further ECGs. Electrolytes, particularly potassium and magnesium, should be aggressively repleted. When QTc extends beyond 500 ms, consideration should be given to discontinuing or changing medications that can contribute to QTc prolongation (whether psychotropics or drugs used to treat acute medical problems).[42]
SEDATION
Sedation is another potential side effect of psychotropic medications. Although sedation is beneficial for agitation and anxiety, sedated patients may be unable to participate in treatment. They are also at greater risk of aspiration and falls.
Antipsychotics cause sedation through antagonism of 1 adrenergic and H1 histaminergic receptors. Effects are most pronounced with the low‐potency, first‐generation antipsychotics and the atypicals quetiapine and clozapine. Some antidepressants, including the TCAs and mirtazapine, also cause somnolence by histamine H1‐receptor antagonism. If a patient has been psychiatrically stable on a particular antipsychotic or antidepressant, but has psychotropic‐induced sedation that interferes with treatment, hospitalists may want to consider temporarily decreasing the dose.
Benzodiazepines induce sedation by increasing ‐aminobutyric acid‐ergic transmission. There is significant overlap between anxiolytic and sedating doses of benzodiazepines. The amount of sedation is related to dose, speed of absorption, and onset of CNS penetration. Thus, sedation may be minimized by using a lower dose or switching to an equivalent dose of a slower‐onset benzodiazepine.[45, 46]
CONCLUSION
The decision to continue or discontinue psychotropic medications is often challenging. It requires the hospitalist to carefully weigh the risks and benefits of the ongoing treatment versus discontinuation, while also considering the patient's preference whenever possible. Sudden cessation of psychotropics can lead to a number of unwanted complications, from mild withdrawal to life‐threatening autonomic instability and psychiatric decompensation. Yet psychotropic continuation can also lead to unwanted drug‐drug interactions and newly emergent side effects.
To improve patient safety and outcomes, hospitalists must take a cautious and well‐thought out approach to treating patients on psychotropic drugs. Reflexive cessation of home medications must be avoided. If hepatic or renal insufficiency develops, medication doses may need to be adjusted. When available, drug levels should guide dose adjustments. If side effects occur, the patient's medication list should be carefully reviewed for potential drug‐drug interactions. Maintaining mental health may mean substituting another drug for one that interferes with home psychotropic medications. Psychotropic doses may also be minimized to decrease side effects. When a psychotropic must be discontinued, tapering is recommended over an abrupt discontinuation, except in the case of an acute toxicity. Moreover, cross‐titration to another effective agent may prevent psychiatric decompensation.
Additionally, hospitalists should use all available resources when deciding on a patient's psychotropic regimen. Mobile devices and online resources can assist with pharmacokinetics. Pharmacists can help with more complex questions or potential drug substitutions. Consultation‐liaison psychiatrists can be a valuable resource in ensuring the safety and stability of a patient with psychiatric comorbidity within the medical environment. The consultant may assist by assessing a patient's current psychiatric state and recommending psychotropic medication changes when needed.
Disclosure
Nothing to report.
- , , , et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352(24):2515–2523.
- , , , , , . Mental disorders in primary care: prevalence and co‐morbidity among disorders. results from the functional illness in primary care (FIP) study. Psychol Med. 2005;35(8):1175–1184.
- , , , et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52–77.
- , , , et al. The Academy of Psychosomatic Medicine practice guidelines for psychiatric consultation in the general medical setting. The Academy of Psychosomatic Medicine. Psychosomatics. 1998;39(4):S8–S30.
- , , , , . Impact of psychiatric comorbidity on length of hospital stay for medical/surgical patients: a preliminary report. Am J Psychiatry. 1987;144(7):878–882.
- . Need for better psychiatric training for primary care providers. Acad Med. 1996;71(6):574–575.
- , , , et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840–847.
- . Discontinuing treatment for psychiatric disorders. J Psychiatry Neurosci. 2006;31(1):11–12.
- , , , , , . Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592–606.
- , , . Abrupt discontinuation of psychotropic drugs during pregnancy: fear of teratogenic risk and impact of counselling. J Psychiatry Neurosci. 2001;26(1):44–48.
- , , , , . Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry. 1997;54(1):49–55.
- . Potential adverse effects of discontinuing psychotropic drugs. Part 3: Antipsychotic, dopaminergic, and mood‐stabilizing drugs. J Psychosoc Nurs Ment Health Serv. 2010;48(8):11–14.
- , . Fluoxetine. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. 2nd ed. Chapter 13. Washington, DC: American Psychiatric Press; 2004:235.
- , , , , . Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449–456.
- , , , , . Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77–87.
- , , , . Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255–261.
- . Antidepressant withdrawal syndromes: phenomenology and pathophysiology. Acta Psychiatr Scand. 1989;79(2):113–117.
- . Heterocyclic antidepressant, monoamine oxidase inhibitor and neuroleptic withdrawal phenomena. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14(2):137–161.
- . Monoamine oxidase inhibitor withdrawal phenomena: symptoms and pathophysiology. Acta Psychiatr Scand. 1988;78(1):1–7.
- , . Antipsychotic withdrawal symptoms: phenomenology and pathophysiology. Acta Psychiatr Scand. 1988;77(3):241–246.
- . Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal‐related relapse. Acta Psychiatr Scand. 2006;114(1):3–13.
- , . Neuroleptic malignant syndrome after neuroleptic discontinuation. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(8):1323–1334.
- , . Withdrawal‐emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179–182.
- , , . Respiratory dyskinesia as discontinuation effect of risperidone. J Clin Psychopharmacol. 2005;25(6):609.
- , . Withdrawal‐emergent respiratory dyskinesia with risperidone treated with clozapine. J Neuropsychiatry Clin Neurosci. 2010;22(2):E24.
- , . Clozapine‐withdrawal catatonia. Psychosomatics. 2010;51(4):355–355.e2.
- , , . Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870–876.
- , , . Psychotropic discontinuation symptoms: a case of withdrawal neuroleptic malignant syndrome. Gen Hosp Psychiatry. 2006;28(6):541–543.
- , . Discontinuation symptoms and psychotropic drugs. Lancet. 2000;355(9210):1184.
- . Potential adverse effects of discontinuing psychotropic drugs. J Psychosoc Nurs Ment Health Serv. 2010;48(9):11–14.
- , , , . Pharmacological interventions for benzodiazepine mono‐dependence management in outpatient settings. Cochrane Database Syst Rev. 2006(3):CD005194.
- , . Substance abuse and withdrawal in the critical care setting. Crit Care Clin. 2008;24(4):767–788, viii.
- . Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76.
- , . Prevention, recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139–1142.
- . Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73(suppl 1):17–24.
- , . Lithium intoxication. J Am Soc Nephrol. 1999;10(3):666–674.
- . Valproic acid toxicity: overview and management. J Toxicol Clin Toxicol. 2002;40(6):789–801.
- , , . Lithium. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. 2nd ed. Chapter 35. Washington, DC: American Psychiatric Press; 2004:547–549.
- , . 2010 guide to psychiatric drug interactions. Prim Psychiatry. 2009;16(12):45–74.
- , . Anticholinergic side‐effects of drugs in elderly people. J R Soc Med. 2000;93(9):457–462.
- , , . Measurement of anticholinergic effects of psychotropic drugs in humans. Pharmacopsychiatry. 2005;38(5):187–193.
- , , , , . QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54(1):1–13.
- , , , et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24(1):62–69.
- , , , , . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235.
- , . Sedation, an unpleasant, undesirable and potentially dangerous side‐effect of many psychotropic drugs. Hum Psychopharmacol. 2004;19(2):135–139.
- , . Toxicology and overdose of atypical antipsychotics. J Emerg Med. 2012;43(5):906–913.
- , , , et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352(24):2515–2523.
- , , , , , . Mental disorders in primary care: prevalence and co‐morbidity among disorders. results from the functional illness in primary care (FIP) study. Psychol Med. 2005;35(8):1175–1184.
- , , , et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52–77.
- , , , et al. The Academy of Psychosomatic Medicine practice guidelines for psychiatric consultation in the general medical setting. The Academy of Psychosomatic Medicine. Psychosomatics. 1998;39(4):S8–S30.
- , , , , . Impact of psychiatric comorbidity on length of hospital stay for medical/surgical patients: a preliminary report. Am J Psychiatry. 1987;144(7):878–882.
- . Need for better psychiatric training for primary care providers. Acad Med. 1996;71(6):574–575.
- , , , et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840–847.
- . Discontinuing treatment for psychiatric disorders. J Psychiatry Neurosci. 2006;31(1):11–12.
- , , , , , . Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592–606.
- , , . Abrupt discontinuation of psychotropic drugs during pregnancy: fear of teratogenic risk and impact of counselling. J Psychiatry Neurosci. 2001;26(1):44–48.
- , , , , . Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry. 1997;54(1):49–55.
- . Potential adverse effects of discontinuing psychotropic drugs. Part 3: Antipsychotic, dopaminergic, and mood‐stabilizing drugs. J Psychosoc Nurs Ment Health Serv. 2010;48(8):11–14.
- , . Fluoxetine. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. 2nd ed. Chapter 13. Washington, DC: American Psychiatric Press; 2004:235.
- , , , , . Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449–456.
- , , , , . Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77–87.
- , , , . Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255–261.
- . Antidepressant withdrawal syndromes: phenomenology and pathophysiology. Acta Psychiatr Scand. 1989;79(2):113–117.
- . Heterocyclic antidepressant, monoamine oxidase inhibitor and neuroleptic withdrawal phenomena. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14(2):137–161.
- . Monoamine oxidase inhibitor withdrawal phenomena: symptoms and pathophysiology. Acta Psychiatr Scand. 1988;78(1):1–7.
- , . Antipsychotic withdrawal symptoms: phenomenology and pathophysiology. Acta Psychiatr Scand. 1988;77(3):241–246.
- . Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal‐related relapse. Acta Psychiatr Scand. 2006;114(1):3–13.
- , . Neuroleptic malignant syndrome after neuroleptic discontinuation. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(8):1323–1334.
- , . Withdrawal‐emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179–182.
- , , . Respiratory dyskinesia as discontinuation effect of risperidone. J Clin Psychopharmacol. 2005;25(6):609.
- , . Withdrawal‐emergent respiratory dyskinesia with risperidone treated with clozapine. J Neuropsychiatry Clin Neurosci. 2010;22(2):E24.
- , . Clozapine‐withdrawal catatonia. Psychosomatics. 2010;51(4):355–355.e2.
- , , . Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870–876.
- , , . Psychotropic discontinuation symptoms: a case of withdrawal neuroleptic malignant syndrome. Gen Hosp Psychiatry. 2006;28(6):541–543.
- , . Discontinuation symptoms and psychotropic drugs. Lancet. 2000;355(9210):1184.
- . Potential adverse effects of discontinuing psychotropic drugs. J Psychosoc Nurs Ment Health Serv. 2010;48(9):11–14.
- , , , . Pharmacological interventions for benzodiazepine mono‐dependence management in outpatient settings. Cochrane Database Syst Rev. 2006(3):CD005194.
- , . Substance abuse and withdrawal in the critical care setting. Crit Care Clin. 2008;24(4):767–788, viii.
- . Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76.
- , . Prevention, recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139–1142.
- . Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73(suppl 1):17–24.
- , . Lithium intoxication. J Am Soc Nephrol. 1999;10(3):666–674.
- . Valproic acid toxicity: overview and management. J Toxicol Clin Toxicol. 2002;40(6):789–801.
- , , . Lithium. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. 2nd ed. Chapter 35. Washington, DC: American Psychiatric Press; 2004:547–549.
- , . 2010 guide to psychiatric drug interactions. Prim Psychiatry. 2009;16(12):45–74.
- , . Anticholinergic side‐effects of drugs in elderly people. J R Soc Med. 2000;93(9):457–462.
- , , . Measurement of anticholinergic effects of psychotropic drugs in humans. Pharmacopsychiatry. 2005;38(5):187–193.
- , , , , . QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54(1):1–13.
- , , , et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24(1):62–69.
- , , , , . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235.
- , . Sedation, an unpleasant, undesirable and potentially dangerous side‐effect of many psychotropic drugs. Hum Psychopharmacol. 2004;19(2):135–139.
- , . Toxicology and overdose of atypical antipsychotics. J Emerg Med. 2012;43(5):906–913.
Four pillars of a successful practice: 4. Motivate your staff
READ THE REST OF THE SERIES
Pillar 1: Keep your current patients happy (March 2013)
Dr. Baum describes his number one strategy to retain patients (Audiocast, March 2013)
Pillar 2: Attract new patients (May 2013)
Pillar 3: Obtain and maintain physician referrals (June 2013)
The success of any medical practice and any marketing program begins and ends with the staff. You can gain new patients, forge excellent relationships with referring physicians, and maintain a plentiful number of existing patients—but if you don’t have a staff that is excited, enthusiastic, and knowledgeable when answering the telephone and managing patients, your marketing plan will be ineffective, and you will be disappointed in your practice.
In this article, I review the importance of motivating employees by providing measurable, written goals in the form of a succinct, effective mission statement and policy manual. I also offer practical strategies to inspire your employees by sharing the power, vision, and rewards.
Start with your mission statement
Nearly every successful practice and every successful business has a well-defined vision, mission, goal, or objective. The mission statement should spell out the purpose of the practice and the methods of achieving it. It serves as the road map, providing direction to all members of the staff, doctors included.
The mission statement for my practice is:
We are committed to:
- excellence
- providing the best urologic health care for our patients
- persistent and consistent attention to the little details because they make a big difference.
Develop a policy manual
Every practice should have a manual that contains its rules and regulations. Ideally, this manual should also serve as a guide for any new or temporary employee who comes to work in the office.
The manual should cover job descriptions, the dress code, hours of operation, the division of office responsibilities, vacation and sick days, and emergency telephone numbers.
In my practice, we summarize our policy manual with this expectation:
Dr. Baum’s policy manual statement:
Rule #1— The patient is always right.
Rule #2— If you think the patient is wrong, reread rule #1.
ALL OTHER POLICIES ARE NULL AND VOID.
We post the mission statement in prominent places throughout the office (the reception area and most of the examination rooms, our Web site, and on a large banner in the employee lounge) to remind us and our patients of our dedication to excellent customer service.
Whenever a mistake or problem occurs, the first question we ask each other is, “Did we adhere to the mission statement and the policy statement?” Usually, we discover that we did not. We use the mission statement and the policy statement to refocus us on our number one priority: our patients.
10 LOW-COST WAYS TO MOTIVATE STAFF
A well-motivated staff creates an effective team environment. Most enlightened businesses have discovered that team management leads to increased output and productivity. Your employees want to be valued as human beings and individuals, not just as workers. The more you include them in the process of running the office, the more invested they become in helping to improve the way it works.
1. Review staff performance regularly
Employees like to know where they stand and how they can improve performance on the job. Motivated staff members appreciate feedback on their progress—or, even, their lack of it. The best way to furnish this important feedback is by conducting periodic performance reviews.
I suggest that you meet with your employees on a scheduled basis every 3 to 4 months. Give each employee a worksheet before the scheduled review (see Worksheet below), and then go over her responses during the review. You can learn a lot about what motivates her during this process.
I always end each performance review on a positive note, by telling the employee how great an asset she is to the practice. I document these meetings in the employee’s file.
2. Encourage continuing education
Just as physicians need continuing medical education to stay up to date, your staff members require continuing motivational experiences. Encourage your staff to participate in continuing education courses and support their efforts financially—you’ll get a favorable return on your investment.
I suggest that you offer to pay the fees for any seminars and classes your employees take. You may want to suggest courses in computers, social media, marketing, or any other subject area that will help the practice grow and prosper.
To make these educational experiences even more effective, ask employees to share what they have learned with other staff members. This can be done at a staff meeting. Simply ask the employee who attended a seminar or a course to share the information with the rest of the staff by briefly reviewing the course or describing what he learned and how it applies to the practice.
3. Empower your staff
Office management is complicated. Few ObGyns have a thorough understanding of all business aspects of a medical practice. Most successful ObGyns have learned to delegate the responsibility of running the office and to empower their employees to take control and assume responsibility for their decisions and actions.
In my practice, I empower any employee to make financial decisions up to a limit of $200 without consulting me. For instance, if the office needs a new telephone answering machine, I expect my employees to consider which features we need, check the machines that are available, and compare prices at the local electronics outlet, office supply store, and online retailers to find the best machine at the lowest price.
The take-home message: More than ever before, ObGyns should do what we are best trained to do—diagnose and treat diseases. Very few ObGyns are experts on fax machines. Don’t waste time on activities that your staff members can do.
4. Promote a positive mental attitude
As Ralph Waldo Emerson once said, “Nothing great was ever achieved without enthusiasm.” This is also true of the practice of medicine. When the doctor has a positive mental attitude, employees are motivated by the example. When a doctor is easily irritable and carries problems from home to the office and takes her frustration out on the staff, the employees will, in turn, take it out on the patients.
I have an attitude that employees are on stage. The moment they walk in the door in the morning, they have to leave all other problems and concerns behind them. They need to believe that they are responsible for making sure that each patient has a positive experience with the office at every contact point. That includes the telephone, the receptionist who welcomes patients to the practice, the nurse taking the patient into the exam room, the billing clerk who handles the patient’s bill, and, yes, the doctor, too! We all contribute to the patient’s experience, and we all need to have a positive attitude.
5. Recognize achievement
Nothing is more motivating for an employee than for the doctor to recognize his achievements and accomplishments. When an employee improves in job performance, tell him directly. You will satisfy that employee’s need for self-esteem, improve his confidence, and help him fulfill the need for self-esteem from fellow employees.
6. Show your staff that you care
Your employees need to know that you care about them not just as workers but as individuals with their own personal lives. When one of my employees is sick, or one of her family members is ill, I call her at home to check on her and make sure that she has access to adequate medical care. If someone gets sick in the office, I call another medical office and get the employee seen immediately.
7. Catch your employees doing things right
My philosophy is to praise in public, pan in private. When I catch an employee doing something right, I send a thank-you note to her home address, making sure that it arrives on a Saturday. I hope the employee will show my note to family and friends. I use a specially created card or a “thanks a million” check (a non-negotiable replication of a check that is made out to the employee and says, “Thanks a million,” with my name signed at the bottom).
You will be amazed at how appreciative the employee is that you not only recognized her superior service but took the time to put your recognition in writing.
8. Reward your staff for saving money
If a staff member comes up with an idea that saves the practice money, give her a bonus. For example, in my practice, the 15-year-old autoclave broke down. When I tried to get parts, I was informed that the machine is no longer made. The nurse in our office took the autoclave to the hospital’s biomedical engineering department, where workers installed a $30 part that saved me from buying a new $2,000 machine. The nurse deserved to be rewarded for that, so I gave her a $50 check on the spot.
I try to motivate my staff not just to earn more money for the practice but to reduce expenses, so I pay them when they identify and design money-saving ideas.
9. Involve your employees in decision making
Ask your employees for advice. Then make sure you follow it. Your staff members are on the front line; they want the office routine to go well. Include them in the decision-making process, whether the task is writing a mission statement or policy manual, determining a change in procedures, implementing an electronic health record, or meeting new job candidates. By including them, you make them feel like part of the team.
10. Have fun!
Surprise is the spice of life. Whenever you can provide an unexpected perk for your staff, you can be sure the gesture will be appreciated. For example, during a week in which two of my employees were unable to work (due to vacation and illness), the rest of us had to take up the slack. Despite being short-handed, we were able to function at regular speed and capacity without affecting the quality of care. I was so impressed by the extra effort that I arranged for a massage therapist to visit our practice at the end of the week and give everyone a 15- to 20-minute massage. It was my way of saying, “Thank you.”
THE BOTTOM LINE
Encourage team spirit. It makes good business sense. When your employees have a personal investment in problem-solving and decision-making, they will go the extra mile for your patients and your practice.
This is the last article in this four-part series on promoting your practice and increasing productivity. I hope you have identified the four pillars of success for your practice—and that I have helped you understand the importance of all four pillars. They represent the strength and stability of a successful ObGyn practice.
READ THE REST OF THE SERIES
Pillar 1: Keep your current patients happy (March 2013)
Dr. Baum describes his number one strategy to retain patients (Audiocast, March 2013)
Pillar 2: Attract new patients (May 2013)
Pillar 3: Obtain and maintain physician referrals (June 2013)
The success of any medical practice and any marketing program begins and ends with the staff. You can gain new patients, forge excellent relationships with referring physicians, and maintain a plentiful number of existing patients—but if you don’t have a staff that is excited, enthusiastic, and knowledgeable when answering the telephone and managing patients, your marketing plan will be ineffective, and you will be disappointed in your practice.
In this article, I review the importance of motivating employees by providing measurable, written goals in the form of a succinct, effective mission statement and policy manual. I also offer practical strategies to inspire your employees by sharing the power, vision, and rewards.
Start with your mission statement
Nearly every successful practice and every successful business has a well-defined vision, mission, goal, or objective. The mission statement should spell out the purpose of the practice and the methods of achieving it. It serves as the road map, providing direction to all members of the staff, doctors included.
The mission statement for my practice is:
We are committed to:
- excellence
- providing the best urologic health care for our patients
- persistent and consistent attention to the little details because they make a big difference.
Develop a policy manual
Every practice should have a manual that contains its rules and regulations. Ideally, this manual should also serve as a guide for any new or temporary employee who comes to work in the office.
The manual should cover job descriptions, the dress code, hours of operation, the division of office responsibilities, vacation and sick days, and emergency telephone numbers.
In my practice, we summarize our policy manual with this expectation:
Dr. Baum’s policy manual statement:
Rule #1— The patient is always right.
Rule #2— If you think the patient is wrong, reread rule #1.
ALL OTHER POLICIES ARE NULL AND VOID.
We post the mission statement in prominent places throughout the office (the reception area and most of the examination rooms, our Web site, and on a large banner in the employee lounge) to remind us and our patients of our dedication to excellent customer service.
Whenever a mistake or problem occurs, the first question we ask each other is, “Did we adhere to the mission statement and the policy statement?” Usually, we discover that we did not. We use the mission statement and the policy statement to refocus us on our number one priority: our patients.
10 LOW-COST WAYS TO MOTIVATE STAFF
A well-motivated staff creates an effective team environment. Most enlightened businesses have discovered that team management leads to increased output and productivity. Your employees want to be valued as human beings and individuals, not just as workers. The more you include them in the process of running the office, the more invested they become in helping to improve the way it works.
1. Review staff performance regularly
Employees like to know where they stand and how they can improve performance on the job. Motivated staff members appreciate feedback on their progress—or, even, their lack of it. The best way to furnish this important feedback is by conducting periodic performance reviews.
I suggest that you meet with your employees on a scheduled basis every 3 to 4 months. Give each employee a worksheet before the scheduled review (see Worksheet below), and then go over her responses during the review. You can learn a lot about what motivates her during this process.
I always end each performance review on a positive note, by telling the employee how great an asset she is to the practice. I document these meetings in the employee’s file.
2. Encourage continuing education
Just as physicians need continuing medical education to stay up to date, your staff members require continuing motivational experiences. Encourage your staff to participate in continuing education courses and support their efforts financially—you’ll get a favorable return on your investment.
I suggest that you offer to pay the fees for any seminars and classes your employees take. You may want to suggest courses in computers, social media, marketing, or any other subject area that will help the practice grow and prosper.
To make these educational experiences even more effective, ask employees to share what they have learned with other staff members. This can be done at a staff meeting. Simply ask the employee who attended a seminar or a course to share the information with the rest of the staff by briefly reviewing the course or describing what he learned and how it applies to the practice.
3. Empower your staff
Office management is complicated. Few ObGyns have a thorough understanding of all business aspects of a medical practice. Most successful ObGyns have learned to delegate the responsibility of running the office and to empower their employees to take control and assume responsibility for their decisions and actions.
In my practice, I empower any employee to make financial decisions up to a limit of $200 without consulting me. For instance, if the office needs a new telephone answering machine, I expect my employees to consider which features we need, check the machines that are available, and compare prices at the local electronics outlet, office supply store, and online retailers to find the best machine at the lowest price.
The take-home message: More than ever before, ObGyns should do what we are best trained to do—diagnose and treat diseases. Very few ObGyns are experts on fax machines. Don’t waste time on activities that your staff members can do.
4. Promote a positive mental attitude
As Ralph Waldo Emerson once said, “Nothing great was ever achieved without enthusiasm.” This is also true of the practice of medicine. When the doctor has a positive mental attitude, employees are motivated by the example. When a doctor is easily irritable and carries problems from home to the office and takes her frustration out on the staff, the employees will, in turn, take it out on the patients.
I have an attitude that employees are on stage. The moment they walk in the door in the morning, they have to leave all other problems and concerns behind them. They need to believe that they are responsible for making sure that each patient has a positive experience with the office at every contact point. That includes the telephone, the receptionist who welcomes patients to the practice, the nurse taking the patient into the exam room, the billing clerk who handles the patient’s bill, and, yes, the doctor, too! We all contribute to the patient’s experience, and we all need to have a positive attitude.
5. Recognize achievement
Nothing is more motivating for an employee than for the doctor to recognize his achievements and accomplishments. When an employee improves in job performance, tell him directly. You will satisfy that employee’s need for self-esteem, improve his confidence, and help him fulfill the need for self-esteem from fellow employees.
6. Show your staff that you care
Your employees need to know that you care about them not just as workers but as individuals with their own personal lives. When one of my employees is sick, or one of her family members is ill, I call her at home to check on her and make sure that she has access to adequate medical care. If someone gets sick in the office, I call another medical office and get the employee seen immediately.
7. Catch your employees doing things right
My philosophy is to praise in public, pan in private. When I catch an employee doing something right, I send a thank-you note to her home address, making sure that it arrives on a Saturday. I hope the employee will show my note to family and friends. I use a specially created card or a “thanks a million” check (a non-negotiable replication of a check that is made out to the employee and says, “Thanks a million,” with my name signed at the bottom).
You will be amazed at how appreciative the employee is that you not only recognized her superior service but took the time to put your recognition in writing.
8. Reward your staff for saving money
If a staff member comes up with an idea that saves the practice money, give her a bonus. For example, in my practice, the 15-year-old autoclave broke down. When I tried to get parts, I was informed that the machine is no longer made. The nurse in our office took the autoclave to the hospital’s biomedical engineering department, where workers installed a $30 part that saved me from buying a new $2,000 machine. The nurse deserved to be rewarded for that, so I gave her a $50 check on the spot.
I try to motivate my staff not just to earn more money for the practice but to reduce expenses, so I pay them when they identify and design money-saving ideas.
9. Involve your employees in decision making
Ask your employees for advice. Then make sure you follow it. Your staff members are on the front line; they want the office routine to go well. Include them in the decision-making process, whether the task is writing a mission statement or policy manual, determining a change in procedures, implementing an electronic health record, or meeting new job candidates. By including them, you make them feel like part of the team.
10. Have fun!
Surprise is the spice of life. Whenever you can provide an unexpected perk for your staff, you can be sure the gesture will be appreciated. For example, during a week in which two of my employees were unable to work (due to vacation and illness), the rest of us had to take up the slack. Despite being short-handed, we were able to function at regular speed and capacity without affecting the quality of care. I was so impressed by the extra effort that I arranged for a massage therapist to visit our practice at the end of the week and give everyone a 15- to 20-minute massage. It was my way of saying, “Thank you.”
THE BOTTOM LINE
Encourage team spirit. It makes good business sense. When your employees have a personal investment in problem-solving and decision-making, they will go the extra mile for your patients and your practice.
This is the last article in this four-part series on promoting your practice and increasing productivity. I hope you have identified the four pillars of success for your practice—and that I have helped you understand the importance of all four pillars. They represent the strength and stability of a successful ObGyn practice.
READ THE REST OF THE SERIES
Pillar 1: Keep your current patients happy (March 2013)
Dr. Baum describes his number one strategy to retain patients (Audiocast, March 2013)
Pillar 2: Attract new patients (May 2013)
Pillar 3: Obtain and maintain physician referrals (June 2013)
The success of any medical practice and any marketing program begins and ends with the staff. You can gain new patients, forge excellent relationships with referring physicians, and maintain a plentiful number of existing patients—but if you don’t have a staff that is excited, enthusiastic, and knowledgeable when answering the telephone and managing patients, your marketing plan will be ineffective, and you will be disappointed in your practice.
In this article, I review the importance of motivating employees by providing measurable, written goals in the form of a succinct, effective mission statement and policy manual. I also offer practical strategies to inspire your employees by sharing the power, vision, and rewards.
Start with your mission statement
Nearly every successful practice and every successful business has a well-defined vision, mission, goal, or objective. The mission statement should spell out the purpose of the practice and the methods of achieving it. It serves as the road map, providing direction to all members of the staff, doctors included.
The mission statement for my practice is:
We are committed to:
- excellence
- providing the best urologic health care for our patients
- persistent and consistent attention to the little details because they make a big difference.
Develop a policy manual
Every practice should have a manual that contains its rules and regulations. Ideally, this manual should also serve as a guide for any new or temporary employee who comes to work in the office.
The manual should cover job descriptions, the dress code, hours of operation, the division of office responsibilities, vacation and sick days, and emergency telephone numbers.
In my practice, we summarize our policy manual with this expectation:
Dr. Baum’s policy manual statement:
Rule #1— The patient is always right.
Rule #2— If you think the patient is wrong, reread rule #1.
ALL OTHER POLICIES ARE NULL AND VOID.
We post the mission statement in prominent places throughout the office (the reception area and most of the examination rooms, our Web site, and on a large banner in the employee lounge) to remind us and our patients of our dedication to excellent customer service.
Whenever a mistake or problem occurs, the first question we ask each other is, “Did we adhere to the mission statement and the policy statement?” Usually, we discover that we did not. We use the mission statement and the policy statement to refocus us on our number one priority: our patients.
10 LOW-COST WAYS TO MOTIVATE STAFF
A well-motivated staff creates an effective team environment. Most enlightened businesses have discovered that team management leads to increased output and productivity. Your employees want to be valued as human beings and individuals, not just as workers. The more you include them in the process of running the office, the more invested they become in helping to improve the way it works.
1. Review staff performance regularly
Employees like to know where they stand and how they can improve performance on the job. Motivated staff members appreciate feedback on their progress—or, even, their lack of it. The best way to furnish this important feedback is by conducting periodic performance reviews.
I suggest that you meet with your employees on a scheduled basis every 3 to 4 months. Give each employee a worksheet before the scheduled review (see Worksheet below), and then go over her responses during the review. You can learn a lot about what motivates her during this process.
I always end each performance review on a positive note, by telling the employee how great an asset she is to the practice. I document these meetings in the employee’s file.
2. Encourage continuing education
Just as physicians need continuing medical education to stay up to date, your staff members require continuing motivational experiences. Encourage your staff to participate in continuing education courses and support their efforts financially—you’ll get a favorable return on your investment.
I suggest that you offer to pay the fees for any seminars and classes your employees take. You may want to suggest courses in computers, social media, marketing, or any other subject area that will help the practice grow and prosper.
To make these educational experiences even more effective, ask employees to share what they have learned with other staff members. This can be done at a staff meeting. Simply ask the employee who attended a seminar or a course to share the information with the rest of the staff by briefly reviewing the course or describing what he learned and how it applies to the practice.
3. Empower your staff
Office management is complicated. Few ObGyns have a thorough understanding of all business aspects of a medical practice. Most successful ObGyns have learned to delegate the responsibility of running the office and to empower their employees to take control and assume responsibility for their decisions and actions.
In my practice, I empower any employee to make financial decisions up to a limit of $200 without consulting me. For instance, if the office needs a new telephone answering machine, I expect my employees to consider which features we need, check the machines that are available, and compare prices at the local electronics outlet, office supply store, and online retailers to find the best machine at the lowest price.
The take-home message: More than ever before, ObGyns should do what we are best trained to do—diagnose and treat diseases. Very few ObGyns are experts on fax machines. Don’t waste time on activities that your staff members can do.
4. Promote a positive mental attitude
As Ralph Waldo Emerson once said, “Nothing great was ever achieved without enthusiasm.” This is also true of the practice of medicine. When the doctor has a positive mental attitude, employees are motivated by the example. When a doctor is easily irritable and carries problems from home to the office and takes her frustration out on the staff, the employees will, in turn, take it out on the patients.
I have an attitude that employees are on stage. The moment they walk in the door in the morning, they have to leave all other problems and concerns behind them. They need to believe that they are responsible for making sure that each patient has a positive experience with the office at every contact point. That includes the telephone, the receptionist who welcomes patients to the practice, the nurse taking the patient into the exam room, the billing clerk who handles the patient’s bill, and, yes, the doctor, too! We all contribute to the patient’s experience, and we all need to have a positive attitude.
5. Recognize achievement
Nothing is more motivating for an employee than for the doctor to recognize his achievements and accomplishments. When an employee improves in job performance, tell him directly. You will satisfy that employee’s need for self-esteem, improve his confidence, and help him fulfill the need for self-esteem from fellow employees.
6. Show your staff that you care
Your employees need to know that you care about them not just as workers but as individuals with their own personal lives. When one of my employees is sick, or one of her family members is ill, I call her at home to check on her and make sure that she has access to adequate medical care. If someone gets sick in the office, I call another medical office and get the employee seen immediately.
7. Catch your employees doing things right
My philosophy is to praise in public, pan in private. When I catch an employee doing something right, I send a thank-you note to her home address, making sure that it arrives on a Saturday. I hope the employee will show my note to family and friends. I use a specially created card or a “thanks a million” check (a non-negotiable replication of a check that is made out to the employee and says, “Thanks a million,” with my name signed at the bottom).
You will be amazed at how appreciative the employee is that you not only recognized her superior service but took the time to put your recognition in writing.
8. Reward your staff for saving money
If a staff member comes up with an idea that saves the practice money, give her a bonus. For example, in my practice, the 15-year-old autoclave broke down. When I tried to get parts, I was informed that the machine is no longer made. The nurse in our office took the autoclave to the hospital’s biomedical engineering department, where workers installed a $30 part that saved me from buying a new $2,000 machine. The nurse deserved to be rewarded for that, so I gave her a $50 check on the spot.
I try to motivate my staff not just to earn more money for the practice but to reduce expenses, so I pay them when they identify and design money-saving ideas.
9. Involve your employees in decision making
Ask your employees for advice. Then make sure you follow it. Your staff members are on the front line; they want the office routine to go well. Include them in the decision-making process, whether the task is writing a mission statement or policy manual, determining a change in procedures, implementing an electronic health record, or meeting new job candidates. By including them, you make them feel like part of the team.
10. Have fun!
Surprise is the spice of life. Whenever you can provide an unexpected perk for your staff, you can be sure the gesture will be appreciated. For example, during a week in which two of my employees were unable to work (due to vacation and illness), the rest of us had to take up the slack. Despite being short-handed, we were able to function at regular speed and capacity without affecting the quality of care. I was so impressed by the extra effort that I arranged for a massage therapist to visit our practice at the end of the week and give everyone a 15- to 20-minute massage. It was my way of saying, “Thank you.”
THE BOTTOM LINE
Encourage team spirit. It makes good business sense. When your employees have a personal investment in problem-solving and decision-making, they will go the extra mile for your patients and your practice.
This is the last article in this four-part series on promoting your practice and increasing productivity. I hope you have identified the four pillars of success for your practice—and that I have helped you understand the importance of all four pillars. They represent the strength and stability of a successful ObGyn practice.
Premature baby is severely handicapped: $21M verdict
AT 31 2/7 WEEKS' GESTATION, a woman was admitted to the hospital for hypertension. A maternal-fetal medicine specialist determined that a vaginal delivery was reasonable as long as the mother and fetus remained clinically stable; a cesarean delivery would be required if the status changed. An ObGyn and nurse midwife took over the mother’s care. Before dinoprostone and oxytocin were administered the next morning, a second ObGyn conducted a vaginal exam and found the mother’s cervix to be 4-cm dilated. After noon, the fetal heart rate became nonreassuring, with late and prolonged variable decelerations. The baby was born shortly after 5:00 pm with the umbilical cord wrapped around his neck. He was pale, lifeless, and had Apgar scores of 4 and 7 at 1 and 5 minutes, respectively. He required initial positive pressure ventilation due to bradycardia and poor respiratory effort.
The boy has cerebral palsy; although not cognitively impaired, he is severely physically handicapped. He has had several operations because one leg is shorter than the other. He has 65% function of his arms, making it impossible for him to complete normal, daily tasks by himself.
PARENTS' CLAIM A cesarean delivery should have been performed 3 hours earlier.
DEFENDANT' DEFENSE Fetal heart-rate monitoring was reassuring during the last 40 minutes of labor. An Apgar score of 7 at 5 minutes is normal. Blood gases taken at birth were normal (7.3 pH). Ultrasonography of the baby’s head at age 3 days showed normal findings. Problems were not evident on the head ultrasound until the child was 2 weeks of age, showing that the injury occurred after birth and was due to prematurity. Defendants included both ObGyns, the midwife, and the hospital.
VERDICT A $21 million Maryland verdict was returned, including $1 million in noneconomic damages that was reduced to $650,000 under the state cap.
PHYSICIAN APOLOGIZED: DIDN'T READ BIOPSY REPORT BEFORE SURGERY
A 34-YEAR-OLD WOMAN with a family history of breast cancer found a lump in her left breast. After fine-needle aspiration, a general surgeon diagnosed cancer and performed a double mastectomy.
At the first postoperative visit, the surgeon told the patient that she did not have breast cancer, and that the fine-needle aspiration results were negative. The surgeon apologized for never looking at the biopsy report prior to surgery, and admitted that is she had seen the report, she would have cancelled surgery.
PATIENT'S CLAIM The surgeon was negligent in performing bilateral mastectomies without first reading biopsy results.
PHYSICIAN'S DEFENSE The case was settled before trial.
VERDICT Michigan case evaluation delivered an award of $542,000, which both parties accepted.
CYSTOSCOPY BLAMED FOR URETERAL OBSTRUCTION, POOR KIDNEY FUNCTION
WHEN A 59-YEAR-OLD WOMAN underwent gynecologic surgery that included a cystoscopy, her uterers were functioning normally. During the following month, the ObGyn performed several follow-up examinations. A year later, the patient's right ureter was completely obstructed. The obstruction was repaired, but the patient lost function in her right kidney. She must take a drug to improve kidney function for the rest of her life.
PATIENT'S CLAIM The obstruction was caused by ligation that occurred during cystoscopy. The ObGyn should have diagnosed the obstruction during the weeks following surgery.
PHYSICIAN'S DEFENSE The cystoscopy was properly performed. The patient had not reported any symptoms after the procedure that suggested the presence of an obstruction. The obstruction gradually developed and could not have been diagnosed earlier.
VERDICT A New York defense verdict was returned.
INFERIOR VENA CAVA DAMAGED DURING ROBOTIC HYSTERECTOMY
A HYSTERECTOMY AND SALPINGO-OOPHORECTOMY were performed on a 64-year-old woman using the da Vinci Surgical System. The gynecologist also removed a cancerous endometrial mass and dissected the periaortic lymph nodes. When the gynecologist used the robot to lift a lymph fat pad, the inferior vena cava was injured and the patient lost 3 L of blood. After converting the laparotomy, a vascular surgeon implanted an artificial graft to repair the inferior vena cava. The patient fully recovered.
PATIENT'S CLAIM The gynecologist did not perform robotic surgery properly, and the patient was not told of all of the risks associated with robotic surgery. Due to the uncertainty regarding the graft's effectiveness, the patient developed posttraumatic stress disorder.
PHYSICIAN'S DEFENSE The vascular injury was a known risk associated with the procedure. The vena cava was not lacerated or transected: perforator veins that joined the lymph fat pad were unintentionally pulled out. The injury was most likely due to the application of pressure, not laceration by the surgical instrument.
VERDICT A $300,000 New York settlement was reached.
READ: The robot is gaining ground in gynecologic surgery. Should you be using it? A roundtable discussion with Arnold P. Advincula, MD; Cheryl B. Iglesia, MD; Rosanne M. Kho, MD; Jamal Mourad, DO; Marie Fidela R. Paraiso, MD; and Jason D. Wright, MD (April 2013)
FETAL DISTRESS CAUSED BRAIN INJURY: $13.9M
DURING THE LAST 2 HOURS OF LABOR, the mother was febrile, the baby's heart rate rose to over 160 bpm, and fetal monitoring indicated fetal distress. Oxytocin was administered to hasten delivery, but the mother's uterus became hyperstimulated. After nearly 17 hours of labor, the child was born without respirations. A video of the vaginal birth shows that the child was blue and unresponsive. The baby was resuscitated, and was subsequently found to have cerebral palsy, epilepsy, and mental retardation. At the time of trial, the 10-year-old had the mental capacity of a 3-year-old.
PARENTS' CLAIM The child suffered brain injury due to hypoxic ischemic encephalopathy. A cesarean delivery should have been performed as soon as fetal distress was evident. The doctors and nurses misread the baseline heart rate, and did not react when the baby did not recover well from the mother's contractions. Brain imaging did not show damage caused by infection or meningitis.
PHYSICIAN'S DEFENSE The girl's condition was caused by an infection or meningitis.
VERDICT A confidential settlement was reached with the midwife before the trial. The ObGyn was dismissed because he was never alerted to any problem by the labor and delivery team. A $13.9 million Georgia verdict was returned against the hospital system.
UTERINE ARTERY INJURED DURING CESAREAN DELIVERY
AFTER A SCHEDULED CESAREAN delivery, the 29-year-old mother had low blood pressure and an altered state of consciousness When she returned to the OR several hours later, her ObGyn found a uterine artery hematoma and laceration. After the laceration was clamped and sutured, uterine atony was noted and an emergency hysterectomy was performed
PATIENT'S CLAIM The mother was no longer able to bear children. The ObGyn was negligent in lacerating the uterine artery, failing to recognize the laceration during cesarean surgery, failing to properly monitor the patient after surgery, and failing to repair the artery in a timely manner. The patient's low blood pressure and altered state of consciousness should have been an indication that she had severe blood loss. The hospital's nursing staff failed to properly check her vital signs after surgery, and failed to report the abnormalities in blood pressure and consciousness to the ObGyn.
DEFENDANTS' DEFENSE The ObGyn claimed that a uterine laceration is a known risk of cesarean delivery; it can occur in the absence of negligence. The hospital also denied negligence.
VERDICT A Texas defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.versictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
AT 31 2/7 WEEKS' GESTATION, a woman was admitted to the hospital for hypertension. A maternal-fetal medicine specialist determined that a vaginal delivery was reasonable as long as the mother and fetus remained clinically stable; a cesarean delivery would be required if the status changed. An ObGyn and nurse midwife took over the mother’s care. Before dinoprostone and oxytocin were administered the next morning, a second ObGyn conducted a vaginal exam and found the mother’s cervix to be 4-cm dilated. After noon, the fetal heart rate became nonreassuring, with late and prolonged variable decelerations. The baby was born shortly after 5:00 pm with the umbilical cord wrapped around his neck. He was pale, lifeless, and had Apgar scores of 4 and 7 at 1 and 5 minutes, respectively. He required initial positive pressure ventilation due to bradycardia and poor respiratory effort.
The boy has cerebral palsy; although not cognitively impaired, he is severely physically handicapped. He has had several operations because one leg is shorter than the other. He has 65% function of his arms, making it impossible for him to complete normal, daily tasks by himself.
PARENTS' CLAIM A cesarean delivery should have been performed 3 hours earlier.
DEFENDANT' DEFENSE Fetal heart-rate monitoring was reassuring during the last 40 minutes of labor. An Apgar score of 7 at 5 minutes is normal. Blood gases taken at birth were normal (7.3 pH). Ultrasonography of the baby’s head at age 3 days showed normal findings. Problems were not evident on the head ultrasound until the child was 2 weeks of age, showing that the injury occurred after birth and was due to prematurity. Defendants included both ObGyns, the midwife, and the hospital.
VERDICT A $21 million Maryland verdict was returned, including $1 million in noneconomic damages that was reduced to $650,000 under the state cap.
PHYSICIAN APOLOGIZED: DIDN'T READ BIOPSY REPORT BEFORE SURGERY
A 34-YEAR-OLD WOMAN with a family history of breast cancer found a lump in her left breast. After fine-needle aspiration, a general surgeon diagnosed cancer and performed a double mastectomy.
At the first postoperative visit, the surgeon told the patient that she did not have breast cancer, and that the fine-needle aspiration results were negative. The surgeon apologized for never looking at the biopsy report prior to surgery, and admitted that is she had seen the report, she would have cancelled surgery.
PATIENT'S CLAIM The surgeon was negligent in performing bilateral mastectomies without first reading biopsy results.
PHYSICIAN'S DEFENSE The case was settled before trial.
VERDICT Michigan case evaluation delivered an award of $542,000, which both parties accepted.
CYSTOSCOPY BLAMED FOR URETERAL OBSTRUCTION, POOR KIDNEY FUNCTION
WHEN A 59-YEAR-OLD WOMAN underwent gynecologic surgery that included a cystoscopy, her uterers were functioning normally. During the following month, the ObGyn performed several follow-up examinations. A year later, the patient's right ureter was completely obstructed. The obstruction was repaired, but the patient lost function in her right kidney. She must take a drug to improve kidney function for the rest of her life.
PATIENT'S CLAIM The obstruction was caused by ligation that occurred during cystoscopy. The ObGyn should have diagnosed the obstruction during the weeks following surgery.
PHYSICIAN'S DEFENSE The cystoscopy was properly performed. The patient had not reported any symptoms after the procedure that suggested the presence of an obstruction. The obstruction gradually developed and could not have been diagnosed earlier.
VERDICT A New York defense verdict was returned.
INFERIOR VENA CAVA DAMAGED DURING ROBOTIC HYSTERECTOMY
A HYSTERECTOMY AND SALPINGO-OOPHORECTOMY were performed on a 64-year-old woman using the da Vinci Surgical System. The gynecologist also removed a cancerous endometrial mass and dissected the periaortic lymph nodes. When the gynecologist used the robot to lift a lymph fat pad, the inferior vena cava was injured and the patient lost 3 L of blood. After converting the laparotomy, a vascular surgeon implanted an artificial graft to repair the inferior vena cava. The patient fully recovered.
PATIENT'S CLAIM The gynecologist did not perform robotic surgery properly, and the patient was not told of all of the risks associated with robotic surgery. Due to the uncertainty regarding the graft's effectiveness, the patient developed posttraumatic stress disorder.
PHYSICIAN'S DEFENSE The vascular injury was a known risk associated with the procedure. The vena cava was not lacerated or transected: perforator veins that joined the lymph fat pad were unintentionally pulled out. The injury was most likely due to the application of pressure, not laceration by the surgical instrument.
VERDICT A $300,000 New York settlement was reached.
READ: The robot is gaining ground in gynecologic surgery. Should you be using it? A roundtable discussion with Arnold P. Advincula, MD; Cheryl B. Iglesia, MD; Rosanne M. Kho, MD; Jamal Mourad, DO; Marie Fidela R. Paraiso, MD; and Jason D. Wright, MD (April 2013)
FETAL DISTRESS CAUSED BRAIN INJURY: $13.9M
DURING THE LAST 2 HOURS OF LABOR, the mother was febrile, the baby's heart rate rose to over 160 bpm, and fetal monitoring indicated fetal distress. Oxytocin was administered to hasten delivery, but the mother's uterus became hyperstimulated. After nearly 17 hours of labor, the child was born without respirations. A video of the vaginal birth shows that the child was blue and unresponsive. The baby was resuscitated, and was subsequently found to have cerebral palsy, epilepsy, and mental retardation. At the time of trial, the 10-year-old had the mental capacity of a 3-year-old.
PARENTS' CLAIM The child suffered brain injury due to hypoxic ischemic encephalopathy. A cesarean delivery should have been performed as soon as fetal distress was evident. The doctors and nurses misread the baseline heart rate, and did not react when the baby did not recover well from the mother's contractions. Brain imaging did not show damage caused by infection or meningitis.
PHYSICIAN'S DEFENSE The girl's condition was caused by an infection or meningitis.
VERDICT A confidential settlement was reached with the midwife before the trial. The ObGyn was dismissed because he was never alerted to any problem by the labor and delivery team. A $13.9 million Georgia verdict was returned against the hospital system.
UTERINE ARTERY INJURED DURING CESAREAN DELIVERY
AFTER A SCHEDULED CESAREAN delivery, the 29-year-old mother had low blood pressure and an altered state of consciousness When she returned to the OR several hours later, her ObGyn found a uterine artery hematoma and laceration. After the laceration was clamped and sutured, uterine atony was noted and an emergency hysterectomy was performed
PATIENT'S CLAIM The mother was no longer able to bear children. The ObGyn was negligent in lacerating the uterine artery, failing to recognize the laceration during cesarean surgery, failing to properly monitor the patient after surgery, and failing to repair the artery in a timely manner. The patient's low blood pressure and altered state of consciousness should have been an indication that she had severe blood loss. The hospital's nursing staff failed to properly check her vital signs after surgery, and failed to report the abnormalities in blood pressure and consciousness to the ObGyn.
DEFENDANTS' DEFENSE The ObGyn claimed that a uterine laceration is a known risk of cesarean delivery; it can occur in the absence of negligence. The hospital also denied negligence.
VERDICT A Texas defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.versictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
AT 31 2/7 WEEKS' GESTATION, a woman was admitted to the hospital for hypertension. A maternal-fetal medicine specialist determined that a vaginal delivery was reasonable as long as the mother and fetus remained clinically stable; a cesarean delivery would be required if the status changed. An ObGyn and nurse midwife took over the mother’s care. Before dinoprostone and oxytocin were administered the next morning, a second ObGyn conducted a vaginal exam and found the mother’s cervix to be 4-cm dilated. After noon, the fetal heart rate became nonreassuring, with late and prolonged variable decelerations. The baby was born shortly after 5:00 pm with the umbilical cord wrapped around his neck. He was pale, lifeless, and had Apgar scores of 4 and 7 at 1 and 5 minutes, respectively. He required initial positive pressure ventilation due to bradycardia and poor respiratory effort.
The boy has cerebral palsy; although not cognitively impaired, he is severely physically handicapped. He has had several operations because one leg is shorter than the other. He has 65% function of his arms, making it impossible for him to complete normal, daily tasks by himself.
PARENTS' CLAIM A cesarean delivery should have been performed 3 hours earlier.
DEFENDANT' DEFENSE Fetal heart-rate monitoring was reassuring during the last 40 minutes of labor. An Apgar score of 7 at 5 minutes is normal. Blood gases taken at birth were normal (7.3 pH). Ultrasonography of the baby’s head at age 3 days showed normal findings. Problems were not evident on the head ultrasound until the child was 2 weeks of age, showing that the injury occurred after birth and was due to prematurity. Defendants included both ObGyns, the midwife, and the hospital.
VERDICT A $21 million Maryland verdict was returned, including $1 million in noneconomic damages that was reduced to $650,000 under the state cap.
PHYSICIAN APOLOGIZED: DIDN'T READ BIOPSY REPORT BEFORE SURGERY
A 34-YEAR-OLD WOMAN with a family history of breast cancer found a lump in her left breast. After fine-needle aspiration, a general surgeon diagnosed cancer and performed a double mastectomy.
At the first postoperative visit, the surgeon told the patient that she did not have breast cancer, and that the fine-needle aspiration results were negative. The surgeon apologized for never looking at the biopsy report prior to surgery, and admitted that is she had seen the report, she would have cancelled surgery.
PATIENT'S CLAIM The surgeon was negligent in performing bilateral mastectomies without first reading biopsy results.
PHYSICIAN'S DEFENSE The case was settled before trial.
VERDICT Michigan case evaluation delivered an award of $542,000, which both parties accepted.
CYSTOSCOPY BLAMED FOR URETERAL OBSTRUCTION, POOR KIDNEY FUNCTION
WHEN A 59-YEAR-OLD WOMAN underwent gynecologic surgery that included a cystoscopy, her uterers were functioning normally. During the following month, the ObGyn performed several follow-up examinations. A year later, the patient's right ureter was completely obstructed. The obstruction was repaired, but the patient lost function in her right kidney. She must take a drug to improve kidney function for the rest of her life.
PATIENT'S CLAIM The obstruction was caused by ligation that occurred during cystoscopy. The ObGyn should have diagnosed the obstruction during the weeks following surgery.
PHYSICIAN'S DEFENSE The cystoscopy was properly performed. The patient had not reported any symptoms after the procedure that suggested the presence of an obstruction. The obstruction gradually developed and could not have been diagnosed earlier.
VERDICT A New York defense verdict was returned.
INFERIOR VENA CAVA DAMAGED DURING ROBOTIC HYSTERECTOMY
A HYSTERECTOMY AND SALPINGO-OOPHORECTOMY were performed on a 64-year-old woman using the da Vinci Surgical System. The gynecologist also removed a cancerous endometrial mass and dissected the periaortic lymph nodes. When the gynecologist used the robot to lift a lymph fat pad, the inferior vena cava was injured and the patient lost 3 L of blood. After converting the laparotomy, a vascular surgeon implanted an artificial graft to repair the inferior vena cava. The patient fully recovered.
PATIENT'S CLAIM The gynecologist did not perform robotic surgery properly, and the patient was not told of all of the risks associated with robotic surgery. Due to the uncertainty regarding the graft's effectiveness, the patient developed posttraumatic stress disorder.
PHYSICIAN'S DEFENSE The vascular injury was a known risk associated with the procedure. The vena cava was not lacerated or transected: perforator veins that joined the lymph fat pad were unintentionally pulled out. The injury was most likely due to the application of pressure, not laceration by the surgical instrument.
VERDICT A $300,000 New York settlement was reached.
READ: The robot is gaining ground in gynecologic surgery. Should you be using it? A roundtable discussion with Arnold P. Advincula, MD; Cheryl B. Iglesia, MD; Rosanne M. Kho, MD; Jamal Mourad, DO; Marie Fidela R. Paraiso, MD; and Jason D. Wright, MD (April 2013)
FETAL DISTRESS CAUSED BRAIN INJURY: $13.9M
DURING THE LAST 2 HOURS OF LABOR, the mother was febrile, the baby's heart rate rose to over 160 bpm, and fetal monitoring indicated fetal distress. Oxytocin was administered to hasten delivery, but the mother's uterus became hyperstimulated. After nearly 17 hours of labor, the child was born without respirations. A video of the vaginal birth shows that the child was blue and unresponsive. The baby was resuscitated, and was subsequently found to have cerebral palsy, epilepsy, and mental retardation. At the time of trial, the 10-year-old had the mental capacity of a 3-year-old.
PARENTS' CLAIM The child suffered brain injury due to hypoxic ischemic encephalopathy. A cesarean delivery should have been performed as soon as fetal distress was evident. The doctors and nurses misread the baseline heart rate, and did not react when the baby did not recover well from the mother's contractions. Brain imaging did not show damage caused by infection or meningitis.
PHYSICIAN'S DEFENSE The girl's condition was caused by an infection or meningitis.
VERDICT A confidential settlement was reached with the midwife before the trial. The ObGyn was dismissed because he was never alerted to any problem by the labor and delivery team. A $13.9 million Georgia verdict was returned against the hospital system.
UTERINE ARTERY INJURED DURING CESAREAN DELIVERY
AFTER A SCHEDULED CESAREAN delivery, the 29-year-old mother had low blood pressure and an altered state of consciousness When she returned to the OR several hours later, her ObGyn found a uterine artery hematoma and laceration. After the laceration was clamped and sutured, uterine atony was noted and an emergency hysterectomy was performed
PATIENT'S CLAIM The mother was no longer able to bear children. The ObGyn was negligent in lacerating the uterine artery, failing to recognize the laceration during cesarean surgery, failing to properly monitor the patient after surgery, and failing to repair the artery in a timely manner. The patient's low blood pressure and altered state of consciousness should have been an indication that she had severe blood loss. The hospital's nursing staff failed to properly check her vital signs after surgery, and failed to report the abnormalities in blood pressure and consciousness to the ObGyn.
DEFENDANTS' DEFENSE The ObGyn claimed that a uterine laceration is a known risk of cesarean delivery; it can occur in the absence of negligence. The hospital also denied negligence.
VERDICT A Texas defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.versictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.