User login
Familiarizing yourself with Alcoholics Anonymous dictums
From “90 minutes in 90 days,” to “people, places, and things,” to “cucumbers and pickles,” Alcoholics Anonymous (AA) slogans have been influencing the public’s understanding of the addictive process for almost a century. Regrettably, these terms have, inadvertently, alienated the scientific community. The translation and subsequent use of AA slogans has been a valuable tool in engaging science experts with mutual-help fellowships such as AA.
Recent advances in the neurobiology and neurochemistry of addiction have validated several of the memorable sayings of AA.1 As a result, physicians and scientists are now more willing to explore AA’s mottos.
Here are five well-known AA slogans that we have translated into medical terms and then briefly assessed in terms of their validity and relevance in today’s treatment of alcohol addiction:
1. “90 meetings in 90 days”
TRUE! Clinically, the first three months of sobriety constitute the most severe part of prolonged withdrawal syndrome and pose the most dangerous opportunities for a relapse.
2. “Keep it simple”
NOT TRUE! Clinical research and everyday practice of addiction treatment show that combination approaches—with medications, group psychotherapy, individual psychotherapy, involvement in mutual-help groups, family therapy, primary care, and treatment of psychiatric comorbidities—typically result in better outcomes than singular approaches.2
3. “Denial is not just a river in Egypt”
NOT TRUE! Since motivational inter-viewing was introduced in the treatment of addiction, we have learned how to effectively work with patients who are in complete denial and have absolutely no interest in changing anything about their life.3
4. “Beware of people, places, and things”
TRUE! Otherwise known as “cues” in psychology literature, triggers of relapse have been implicated in both the basic understanding of the addictive process and its treatment. “Classical conditioning” and “operant conditioning” models of behavior incorporate triggers. Additionally, cognitive behavior therapy helps extensively with maintaining sobriety. Even the DSM-5 gives a nod to “people, places, and things” by introducing “cravings” as a bona fide criterion of a substance use disorder.
5. “A cucumber that has become a pickle cannot become a cucumber again”
EQUIVOCAL. It is not clear, and highly debatable, whether an alcoholic who has been sober for more than 20 years still has a heightened vulnerability to reverting to alcoholism after consumption of alcohol. What is evident is that, even if the neuroadaptations responsible for hijacking the pleasure-reward pathways of the brain one day return to a normal, pre-addiction state, this healing process takes a long time—probably measured in decades, not years.
Click here for another Pearl on alternatives to 12-step groups.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Volkow ND, Baler RD. Addiction science: Uncovering neurobiological complexity. Neuropharmacology. 2013; (13)217-7.
2. Nunes EV, Selzer J, Levounis P, et al. Substance dependence and co-occurring psychiatric disorders: Best practices for diagnosis and clinical treatment. New York, NY: Civic Research Institute, 2010.
3. Levounis, P, Arnaout B. Handbook of motivation and change: A practical guide for clinicians. Arlington, VA: American Psychiatric Publishing, Inc.; 2010.
From “90 minutes in 90 days,” to “people, places, and things,” to “cucumbers and pickles,” Alcoholics Anonymous (AA) slogans have been influencing the public’s understanding of the addictive process for almost a century. Regrettably, these terms have, inadvertently, alienated the scientific community. The translation and subsequent use of AA slogans has been a valuable tool in engaging science experts with mutual-help fellowships such as AA.
Recent advances in the neurobiology and neurochemistry of addiction have validated several of the memorable sayings of AA.1 As a result, physicians and scientists are now more willing to explore AA’s mottos.
Here are five well-known AA slogans that we have translated into medical terms and then briefly assessed in terms of their validity and relevance in today’s treatment of alcohol addiction:
1. “90 meetings in 90 days”
TRUE! Clinically, the first three months of sobriety constitute the most severe part of prolonged withdrawal syndrome and pose the most dangerous opportunities for a relapse.
2. “Keep it simple”
NOT TRUE! Clinical research and everyday practice of addiction treatment show that combination approaches—with medications, group psychotherapy, individual psychotherapy, involvement in mutual-help groups, family therapy, primary care, and treatment of psychiatric comorbidities—typically result in better outcomes than singular approaches.2
3. “Denial is not just a river in Egypt”
NOT TRUE! Since motivational inter-viewing was introduced in the treatment of addiction, we have learned how to effectively work with patients who are in complete denial and have absolutely no interest in changing anything about their life.3
4. “Beware of people, places, and things”
TRUE! Otherwise known as “cues” in psychology literature, triggers of relapse have been implicated in both the basic understanding of the addictive process and its treatment. “Classical conditioning” and “operant conditioning” models of behavior incorporate triggers. Additionally, cognitive behavior therapy helps extensively with maintaining sobriety. Even the DSM-5 gives a nod to “people, places, and things” by introducing “cravings” as a bona fide criterion of a substance use disorder.
5. “A cucumber that has become a pickle cannot become a cucumber again”
EQUIVOCAL. It is not clear, and highly debatable, whether an alcoholic who has been sober for more than 20 years still has a heightened vulnerability to reverting to alcoholism after consumption of alcohol. What is evident is that, even if the neuroadaptations responsible for hijacking the pleasure-reward pathways of the brain one day return to a normal, pre-addiction state, this healing process takes a long time—probably measured in decades, not years.
Click here for another Pearl on alternatives to 12-step groups.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
From “90 minutes in 90 days,” to “people, places, and things,” to “cucumbers and pickles,” Alcoholics Anonymous (AA) slogans have been influencing the public’s understanding of the addictive process for almost a century. Regrettably, these terms have, inadvertently, alienated the scientific community. The translation and subsequent use of AA slogans has been a valuable tool in engaging science experts with mutual-help fellowships such as AA.
Recent advances in the neurobiology and neurochemistry of addiction have validated several of the memorable sayings of AA.1 As a result, physicians and scientists are now more willing to explore AA’s mottos.
Here are five well-known AA slogans that we have translated into medical terms and then briefly assessed in terms of their validity and relevance in today’s treatment of alcohol addiction:
1. “90 meetings in 90 days”
TRUE! Clinically, the first three months of sobriety constitute the most severe part of prolonged withdrawal syndrome and pose the most dangerous opportunities for a relapse.
2. “Keep it simple”
NOT TRUE! Clinical research and everyday practice of addiction treatment show that combination approaches—with medications, group psychotherapy, individual psychotherapy, involvement in mutual-help groups, family therapy, primary care, and treatment of psychiatric comorbidities—typically result in better outcomes than singular approaches.2
3. “Denial is not just a river in Egypt”
NOT TRUE! Since motivational inter-viewing was introduced in the treatment of addiction, we have learned how to effectively work with patients who are in complete denial and have absolutely no interest in changing anything about their life.3
4. “Beware of people, places, and things”
TRUE! Otherwise known as “cues” in psychology literature, triggers of relapse have been implicated in both the basic understanding of the addictive process and its treatment. “Classical conditioning” and “operant conditioning” models of behavior incorporate triggers. Additionally, cognitive behavior therapy helps extensively with maintaining sobriety. Even the DSM-5 gives a nod to “people, places, and things” by introducing “cravings” as a bona fide criterion of a substance use disorder.
5. “A cucumber that has become a pickle cannot become a cucumber again”
EQUIVOCAL. It is not clear, and highly debatable, whether an alcoholic who has been sober for more than 20 years still has a heightened vulnerability to reverting to alcoholism after consumption of alcohol. What is evident is that, even if the neuroadaptations responsible for hijacking the pleasure-reward pathways of the brain one day return to a normal, pre-addiction state, this healing process takes a long time—probably measured in decades, not years.
Click here for another Pearl on alternatives to 12-step groups.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Volkow ND, Baler RD. Addiction science: Uncovering neurobiological complexity. Neuropharmacology. 2013; (13)217-7.
2. Nunes EV, Selzer J, Levounis P, et al. Substance dependence and co-occurring psychiatric disorders: Best practices for diagnosis and clinical treatment. New York, NY: Civic Research Institute, 2010.
3. Levounis, P, Arnaout B. Handbook of motivation and change: A practical guide for clinicians. Arlington, VA: American Psychiatric Publishing, Inc.; 2010.
1. Volkow ND, Baler RD. Addiction science: Uncovering neurobiological complexity. Neuropharmacology. 2013; (13)217-7.
2. Nunes EV, Selzer J, Levounis P, et al. Substance dependence and co-occurring psychiatric disorders: Best practices for diagnosis and clinical treatment. New York, NY: Civic Research Institute, 2010.
3. Levounis, P, Arnaout B. Handbook of motivation and change: A practical guide for clinicians. Arlington, VA: American Psychiatric Publishing, Inc.; 2010.
Investigational treatments for cognitive impairment in schizophrenia
Available treatments for schizophrenia (eg, antipsychotics) are primarily effective on positive symptoms (hallucinations, delusions, etc.). It is, however, increasingly clear that schizophrenia also is a severe neuropsychiatric illness associated with deficits in cognitive function. These deficits represent a core feature of the disorder, and are a major determinant of long-term disability.1 Cognitive dysfunction is among the earliest signs of illness that, typically, presents in the prodromal phase.
Since the formulation of the dopaminergic model of schizophrenia, cognitive studies of the disease primarily have examined dysfunction in dopaminergic-rich regions of the brain, such as the prefrontal cortex, and, therefore, have focused largely on executive functioning. But neurocognitive deficits in schizophrenia are not limited to executive functioning; comparable deficits have been observed across multiple areas of cognition.2
More recent formulations of cognitive dysfunction in schizophrenia divide deficits into multiple domains. These include verbal, visual, and working memory; attention and vigilance; speed of processing, reasoning, and problem solving; and social cognition (Table). Neurocognitive impairments often are closely associated with deficits in early sensory processing and basic neurophysiology.3
The prevalence of cognitive dysfunction also can be estimated using baseline data from the large-scale Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial.4 Although cognitive dysfunction was not one of the inclusion criteria in CATIE, most patients who were enrolled had profound cognitive deficits.5 Furthermore, meta-analyses6 suggest that composite neurocognitive measures can explain as much as 60% of the variance of overall functioning in schizophrenia.
Antipsychotics aren’t the answer
The cognitive-enhancing benefits of antipsychotic medications are minimal.7 As evidence of a direct relationship between cognitive dysfunction and long-term functional outcome in schizophrenia becomes established, the need for safe and effective treatment for these symptoms becomes more urgent. Given the mechanistic complexity of the potential cause of poor cognitive performance, the search for an effective treatment is ongoing—but that search has not been successful.
Despite mixed results for recent novel mechanism trials (http://newsroom.lilly.com/releasedetail.cfm?releaseid=703018) and a number of companies ceasing drug development, the work to develop safe and effective treatments for cognitive dysfunction in schizophrenia continues, as exemplified by National Institute of Mental Health-initiated programs to spur development of drugs that work by a novel mechanism. Rather than simply assessing novel compounds with paper-and-pencil cognitive scales, such programs seek to assess the ability of the compound to engage with the intended receptor (target),9 using imaging or electrophysiological tools. Without utilization of a target engagement biomarker, there is no way to know whether 1) the drug simply does not get into the brain in sufficient concentration to be effective in humans or 2) the overall mechanism is wrong.
In this article, we review several promising targets and techniques that are the subject of active research on the treatment of cognitive disorders in schizophrenia. This list isn’t exhaustive; our aim is to highlight a few of the promising treatments now being studied in clinical trials.
Acetylcholine receptors
Acetylcholine receptors comprise two major families, nicotinic and muscarinic receptors; evidence implicates deficits of both families in schizophrenia.10 Following up on epidemiological studies11 of the high percentage of schizophrenia patients who smoke tobacco (60% to 90%), the role of alpha-7 nicotinic acetylcholine receptors (á7 nAchR) has been explored. Nicotine itself might normalize some disrupted auditory processes, as measured by electroencephalography.12
Several clinical trials of partial á7 nAchR agonists have been conducted, with EVP-6124 and TC-5619 furthest along in development.
EVP-6124. Information is unavailable publicly on EVP-6124, except for an abstract presented in 2011 at the 51st Annual Meeting of the American College of Neuropsychopharmacology.13 In that study, 319 patients with schizophrenia were randomized to EVP-6124 (0.3 mg/d or 1 mg/d [n = 213]) or placebo (n = 106) adjunctive to at least 4 weeks of non-clozapine antipsychotics. Efficacy was shown up to 1 mg, in a dose-responsive manner. Modest, but significant, improvements in cognition, clinical function, and negative symptoms were seen. The most commonly reported side effects were headache (3.8%), nausea (3.2%), and nasopharyngitis (2.5%). Phase III studies are underway.
TC-5619. This partial á7 nAchR also showed positive results recently in a Phase II trial. Significant (P < .05) improvement was demonstrated in executive function in the Groton Maze Learning Task of the CogState Schizophrenia Battery and the Scale for Assessment of Negative Symptoms.14
Strong anatomic links also exist between muscarinic acetylcholine receptors and the brain dopaminergic system, especially muscarinic type-1 and type-4 (M1 and M4) receptors. The potential utility of an M1, M4, or combined M/M4 agonist is also supported by studies of M1 and M4 knockout mice, with particular evidence of cognitive enhancement with the use of M1 agonists.15
GSK1034702. Administration of the M1 allosteric agonist GSK1034702 to healthy human smokers, using the nicotine abstinence model of cognitive dysfunction, resulted in improvements in immediate recall.16
Xanomeline. In a small pilot study of 20 schizophrenia patients, xanomeline, a mixed M1/M4 agonist, demonstrated significant improvements in verbal learning, short-term memory, and overall symptoms.17
Dopamine receptors
All marketed antipsychotics block the dopamine type-2 (D2) receptor18; they are primarily effective on positive symptoms.4 In contrast, a role for the dopamine type-1 (D1) receptor in cognition is suggested by studies that demonstrate reduced D1 and N-methyl-d-aspartate (NMDA) glutamate receptor function in the prefrontal cortex.19-22
In a model of cognitive impairment in non-human primates, low-dose intermittent dosing of D1-receptor agonists produced improvements in cognitive function.23 This strategy aims to sensitize, rather than induce tolerance, to the effects of the D1-receptor agonist. Benefits were primarily seen in working memory. Phase II trials of a potent D1-receptor agonist, DAR-100A, the active enantiomer of dihydrexidine24 are ongoing (www.clinicaltrials.gov/ct2/show/NCT01519557).
Glutamatergic receptors
Intoxication with NMDA antagonists (such as phencyclidine and ketamine) yields a phenotype with similarity to schizophrenia.25 More than 20 years of research has provided evidence for the role of glutamatergic NMDA receptors in the pathophysiology of schizophrenia.26,27
NMDA receptors are distributed widely in the brain, but specific glutamatergic processes are localized to areas that are associated with cognition. This relative distribution provides a convenient framework from which to view the pattern of cognitive dysfunction associated with schizophrenia:
• NMDA receptors in the hippocampus are involved in learning and memory acquisition
• NMDA receptors in the visual cortex and auditory cortex are fundamental for auditory and visual sensory memory.
Previous reviews of ketamine administration have described cognitive deficits in healthy control subjects, comparable to what is seen in schizophrenia.28 The deficits are noted primarily in measures of executive functioning, attention/vigilance, verbal fluency, and visual and verbal working memory.
Most treatment studies of glutamatergic-based drugs have focused on positive and negative symptoms. Two recent comprehensive meta-analyses29,30 of NMDA-based treatments support small-to-moderate effect size improvement in total symptoms and in negative symptoms, in patients with chronic schizophrenia, when the drugs are used in combination with non-clozapine antipsychotics.
Bitopertin. A novel glycine-transport inhibitor, bitopertin, showed significant improvement in negative symptoms as an adjunctive treatment in a large Phase II trial.31,32 In the “per protocol” population (ie, patients who completed 8 weeks of treatment without any major protocol violations [n = 231]), negative symptoms diminished to a significantly (P < .05) greater degree from baseline in the 10 mg/d and 30 mg/d dosage groups, compared with placebo. Phase III studies of bitopertin are ongoing (www.clinicaltrials.gov/ct2/show/NCT01192906).
Direct evidence of a cognitive benefit of glutamatergic-based drugs is limited. In a recent large, multicenter study, low dosage D-serine (~30 mg/kg/d) did not separate from placebo,33 but an open-label study suggests increased efficacy with dosages >30 mg/kg/d.34 In addition to symptomatic improvements, a highly significant, large effect-size improvement was seen for overall cognition for dosages ≥60 mg/kg/d, leading to a significant dose-by-time interaction (P < .01).
Combination approaches. The value of combining glutamatergic medication and a cognitive training program is supported by the role of NMDA receptors in learning. For example, D-cycloserine, a glycine-site partial agonist, has been shown in several studies to enhance learning and behavioral therapies in anxiety disorders.35 Although an initial study in schizophrenia was negative for the effectiveness of D-serine (a glycine-site full agonist) and combined cognitive training,36 further research is ongoing to evaluate a role for such combined therapy.37,38
Brain stimulation
Two nonpharmacotherapeutic brain stimulation techniques, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), have been applied in the study of schizophrenia symptoms, particularly for enhancing cognition.39 Both techniques use electric stimulation to influence activity of underlying brain regions: rTMS utilizes a magnetic coil and electromagnetic induction; tDCS, in contrast, utilizes constant low (<2 mA) direct current to specific regions of the scalp.
Cortical neuronal excitability is increased by anodal tDCS and high-frequency rTMS and reduced by cathodal tDCS and low-frequency rTMS. Both tDCS and rTMS appear to be NMDA receptor-dependent. tDCS is relatively inexpensive and requires less expertise to administer than rTMS does.
Both techniques might be efficacious for treating resistant auditory hallucinations.40,41 Applying rTMS over the left dorsolateral prefrontal cortex has led to improvement in verbal learning and visuomotor tracking in patients with schizophrenia.39 Stimulation of both sides of the prefrontal cortex with rTMS has brought improvement in visual memory, executive function, spatial working memory, and attention. Few papers have been published so far regarding enhancement of cognition with tDCS in schizophrenia,42 but beneficial effects of this technique have been seen across several disorders.43
Cognitive remediation techniques
A fundamental starting point for cognitive remediation is the idea that there is plasticity in the brain and that repetitive practice can lead to cognitive improvement. Cognitive remediation therapy often adopts computerized programs and exercises that attempt to improve psychosocial function by targeting structures of the brain that are involved in cognitive function, such as attention, working memory, executive functioning, planning, and cognitive flexibility.
In schizophrenia, cognitive remediation studies have traditionally targeted higher-order processes, such as attention and higher level processes, that might lead to improvement in overall cognition and function.44 Cognitive remediation typically is utilized complementary to pharmacotherapy, with some studies supporting the use of combined use of cognition-enhancing drugs and remediation programs.
A 2007 meta-analysis showed a medium-size but significant improvement in cognition through the use of cognitive remediation therapy45—especially when it is combined with psychiatric rehabilitation. More recent studies utilizing techniques that focus on bottom-up (auditory and visual processing) techniques has shown significant improvements.46-48 Several multicenter studies utilizing Posit Science programs combined with antipsychotic medication are ongoing (www.clinicaltrials.gov/ct2/show/NCT01173874 and www.clinicaltrials.gov/ct2/show/NCT01422902).
Bottom Line
Although cognitive dysfunction is a leading cause of disability in schizophrenia, no treatments are approved for this condition. Numerous novel-mechanism and nonpharmaceutical modalities are actively being studied for this difficult-to-treat problem, however—offering hope to patients.
Related Resources
Javitt DC, Zukin SR, Heresco-Levy U, et al. Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Has an angel shown the way? Schizophr Bull. 2012; 38(5):958-966.
Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;(213):11-37.
Millan MJ, Agid Y, Brune M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012; 11(2):141-168.
Drug Brand Names
D-cycloserine • Seromycin Ketamine • Ketalar
Xanomeline • Lumeron, Memcor
Disclosures
Dr. Kantrowitz receives grant or research support from EnVivo, the National Institute of Mental Health, Novartis, Pfizer, Roche-Genentech, the Stanley Foundation, and Sunovion; is a consultant to Health Advances, LLC, the Healthcare Advisory Board, Otsuka Pharmaceuticals, Strategic Edge Communications, and Vindico Medical Education; and owns a small number of shares of common stock in GlaxoSmithKline. Ms. Levy and Dr. Ballon report no financial relationships with manufacturers of any products mentioned in this article or with manufacturers of competing products.
1. Bowie CR, Reichenberg A, Patterson TL, et al. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418-425.
2. Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1-3):124-131.
3. Javitt DC, Spencer KM, Thaker GK, et al. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68-83.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1253.
5. Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033-2046.
6. Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119-136.
7. Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633-647.
8. Yan J. NIMH tries to jumpstart drug innovations. Psychiatric News. 2013;48(1):8-10.
9. Javitt DC, Schoepp D, Kalivas PW, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3(102):102mr2.
10. Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov Med. 2012;14(79):413-420.
11. D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62(3):1564-1573.
12. Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998; 24(2):189-202.
13. Meltzer HY, Gawryl M, Ward S, et al. EVP-6124, an alpha-7 nicotinic partial agonist, reduces positive effects on cognition, clinical function, and negative symptoms in patients with chronic schizophrenia on stable antipsychotic therapy. Neuropsychopharmacology. 2011;36:S170-S171.
14. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
15. Digby GJ, Noetzel MJ, Bubser M, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32(25):8532-8544.
16. Nathan PJ, Watson J, Lund J, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16(4):721-731.
17. Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033-1039.
18. Di Forti M, Lappin LM, Murray RM. Risk factors for schizophrenia—all roads lead to dopamine. Eur Neuropsychopharmacol. 2007;17(suppl 2):S101-S107.
19. Krystal JH, D’Souza DC, Mathalon D, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 2003;169(3-4): 215-233.
20. Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9(5):404-416.
21. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708-3719.
22. Martinez A, Ramanathan DS, Foxe JJ, et al. The role of spatial attention in the selection of real and illusory objects. J Neurosci. 2007;27(30):7963-7973.
23. Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287(5460):2020-2022.
24. Slifstein M, Suckow RF, Javitch JA, et al. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J Cereb Blood Flow Metab. 2011;31(1):293-304.
25. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301-1308.
26. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010; 83(3-4):108-121.
27. Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4(3):189-200.
28. Kantrowitz JT, Javitt DC. Glutamatergic approaches to the conceptualization and treatment of schizophrenia. In: Javitt DC, Kantrowitz JT, eds. Handbook of neurochemistry and molecular neurobiology. New York, NY: Springer; 2009:3-36.
29. Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16(5):522-537.
30. Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25(10):859-868.
31. Umbricht D, Yoo K, Youssef E, et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Neuropharmacology. 2010;35:S320-S321.
32. Pinard E, Alanine A, Alberati D, et al. Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl-2-(( S)-2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia. J Med Chem. 2010;53(12):4603-4614.
33. Weiser M, Heresco-Levy U, Davidson M, et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry. 2012;73(6):e728-e734.
34. Kantrowitz JT, Malhotra AK, Cornblatt B, et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121(1-3):125-130.
35. Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118-1126.
36. D’Souza DC, Radhakrishnan R, Perry E, et al. Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology. 2013;38(3):492-503.
37. Gottlieb JD, Cather C, Shanahan M, et al. D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011;131(1-3):69-74.
38. Kantrowitz J, Sehatpour P, Oakman E, et al. D-Serine and NMDA based sensory modulation. Poster presented at: 3rd Biennial Schizophrenia International Research Conference; April 14-18, 2012; Florence, Italy.
39. Demirtas-Tatlidede, A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566-578.
40. Brunelin J, Mondino M, Gassab L, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169(7):719-724.
41. Matheson SL, Green MJ, Loo C, et al. Quality assessment and comparison of evidence for electroconvulsive therapy and repetitive transcranial magnetic stimulation for schizophrenia: a systematic meta-review. Schizophr Res. 2012;118(1-3):201-210.
42. Vercammen A, Rushby JA, Loo C, et al. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophr Res. 2011;131(1-3):198-205.
43. Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011; 29(6):463-492.
44. Keefe RS, Vinogradov S, Medalia A, et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. 2011;37(5):1057-1065.
45. McGurk SR, Twamley EW, Sitzer DI, et al. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791-1802.
46. Fisher M, Holland C, Merzenich MM, et al. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805-811.
47. Norton DJ, McBain RK, Ongür D, et al. Perceptual training strongly improves visual motion perception in schizophrenia. Brain Cogn. 2011;77(2):248-256.
48. Kantrowitz JT, Revheim N, Pasternak R, et al. It’s all in the cards: effect of stimulus manipulation on Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res. 2009;168(3):198-204.
Available treatments for schizophrenia (eg, antipsychotics) are primarily effective on positive symptoms (hallucinations, delusions, etc.). It is, however, increasingly clear that schizophrenia also is a severe neuropsychiatric illness associated with deficits in cognitive function. These deficits represent a core feature of the disorder, and are a major determinant of long-term disability.1 Cognitive dysfunction is among the earliest signs of illness that, typically, presents in the prodromal phase.
Since the formulation of the dopaminergic model of schizophrenia, cognitive studies of the disease primarily have examined dysfunction in dopaminergic-rich regions of the brain, such as the prefrontal cortex, and, therefore, have focused largely on executive functioning. But neurocognitive deficits in schizophrenia are not limited to executive functioning; comparable deficits have been observed across multiple areas of cognition.2
More recent formulations of cognitive dysfunction in schizophrenia divide deficits into multiple domains. These include verbal, visual, and working memory; attention and vigilance; speed of processing, reasoning, and problem solving; and social cognition (Table). Neurocognitive impairments often are closely associated with deficits in early sensory processing and basic neurophysiology.3
The prevalence of cognitive dysfunction also can be estimated using baseline data from the large-scale Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial.4 Although cognitive dysfunction was not one of the inclusion criteria in CATIE, most patients who were enrolled had profound cognitive deficits.5 Furthermore, meta-analyses6 suggest that composite neurocognitive measures can explain as much as 60% of the variance of overall functioning in schizophrenia.
Antipsychotics aren’t the answer
The cognitive-enhancing benefits of antipsychotic medications are minimal.7 As evidence of a direct relationship between cognitive dysfunction and long-term functional outcome in schizophrenia becomes established, the need for safe and effective treatment for these symptoms becomes more urgent. Given the mechanistic complexity of the potential cause of poor cognitive performance, the search for an effective treatment is ongoing—but that search has not been successful.
Despite mixed results for recent novel mechanism trials (http://newsroom.lilly.com/releasedetail.cfm?releaseid=703018) and a number of companies ceasing drug development, the work to develop safe and effective treatments for cognitive dysfunction in schizophrenia continues, as exemplified by National Institute of Mental Health-initiated programs to spur development of drugs that work by a novel mechanism. Rather than simply assessing novel compounds with paper-and-pencil cognitive scales, such programs seek to assess the ability of the compound to engage with the intended receptor (target),9 using imaging or electrophysiological tools. Without utilization of a target engagement biomarker, there is no way to know whether 1) the drug simply does not get into the brain in sufficient concentration to be effective in humans or 2) the overall mechanism is wrong.
In this article, we review several promising targets and techniques that are the subject of active research on the treatment of cognitive disorders in schizophrenia. This list isn’t exhaustive; our aim is to highlight a few of the promising treatments now being studied in clinical trials.
Acetylcholine receptors
Acetylcholine receptors comprise two major families, nicotinic and muscarinic receptors; evidence implicates deficits of both families in schizophrenia.10 Following up on epidemiological studies11 of the high percentage of schizophrenia patients who smoke tobacco (60% to 90%), the role of alpha-7 nicotinic acetylcholine receptors (á7 nAchR) has been explored. Nicotine itself might normalize some disrupted auditory processes, as measured by electroencephalography.12
Several clinical trials of partial á7 nAchR agonists have been conducted, with EVP-6124 and TC-5619 furthest along in development.
EVP-6124. Information is unavailable publicly on EVP-6124, except for an abstract presented in 2011 at the 51st Annual Meeting of the American College of Neuropsychopharmacology.13 In that study, 319 patients with schizophrenia were randomized to EVP-6124 (0.3 mg/d or 1 mg/d [n = 213]) or placebo (n = 106) adjunctive to at least 4 weeks of non-clozapine antipsychotics. Efficacy was shown up to 1 mg, in a dose-responsive manner. Modest, but significant, improvements in cognition, clinical function, and negative symptoms were seen. The most commonly reported side effects were headache (3.8%), nausea (3.2%), and nasopharyngitis (2.5%). Phase III studies are underway.
TC-5619. This partial á7 nAchR also showed positive results recently in a Phase II trial. Significant (P < .05) improvement was demonstrated in executive function in the Groton Maze Learning Task of the CogState Schizophrenia Battery and the Scale for Assessment of Negative Symptoms.14
Strong anatomic links also exist between muscarinic acetylcholine receptors and the brain dopaminergic system, especially muscarinic type-1 and type-4 (M1 and M4) receptors. The potential utility of an M1, M4, or combined M/M4 agonist is also supported by studies of M1 and M4 knockout mice, with particular evidence of cognitive enhancement with the use of M1 agonists.15
GSK1034702. Administration of the M1 allosteric agonist GSK1034702 to healthy human smokers, using the nicotine abstinence model of cognitive dysfunction, resulted in improvements in immediate recall.16
Xanomeline. In a small pilot study of 20 schizophrenia patients, xanomeline, a mixed M1/M4 agonist, demonstrated significant improvements in verbal learning, short-term memory, and overall symptoms.17
Dopamine receptors
All marketed antipsychotics block the dopamine type-2 (D2) receptor18; they are primarily effective on positive symptoms.4 In contrast, a role for the dopamine type-1 (D1) receptor in cognition is suggested by studies that demonstrate reduced D1 and N-methyl-d-aspartate (NMDA) glutamate receptor function in the prefrontal cortex.19-22
In a model of cognitive impairment in non-human primates, low-dose intermittent dosing of D1-receptor agonists produced improvements in cognitive function.23 This strategy aims to sensitize, rather than induce tolerance, to the effects of the D1-receptor agonist. Benefits were primarily seen in working memory. Phase II trials of a potent D1-receptor agonist, DAR-100A, the active enantiomer of dihydrexidine24 are ongoing (www.clinicaltrials.gov/ct2/show/NCT01519557).
Glutamatergic receptors
Intoxication with NMDA antagonists (such as phencyclidine and ketamine) yields a phenotype with similarity to schizophrenia.25 More than 20 years of research has provided evidence for the role of glutamatergic NMDA receptors in the pathophysiology of schizophrenia.26,27
NMDA receptors are distributed widely in the brain, but specific glutamatergic processes are localized to areas that are associated with cognition. This relative distribution provides a convenient framework from which to view the pattern of cognitive dysfunction associated with schizophrenia:
• NMDA receptors in the hippocampus are involved in learning and memory acquisition
• NMDA receptors in the visual cortex and auditory cortex are fundamental for auditory and visual sensory memory.
Previous reviews of ketamine administration have described cognitive deficits in healthy control subjects, comparable to what is seen in schizophrenia.28 The deficits are noted primarily in measures of executive functioning, attention/vigilance, verbal fluency, and visual and verbal working memory.
Most treatment studies of glutamatergic-based drugs have focused on positive and negative symptoms. Two recent comprehensive meta-analyses29,30 of NMDA-based treatments support small-to-moderate effect size improvement in total symptoms and in negative symptoms, in patients with chronic schizophrenia, when the drugs are used in combination with non-clozapine antipsychotics.
Bitopertin. A novel glycine-transport inhibitor, bitopertin, showed significant improvement in negative symptoms as an adjunctive treatment in a large Phase II trial.31,32 In the “per protocol” population (ie, patients who completed 8 weeks of treatment without any major protocol violations [n = 231]), negative symptoms diminished to a significantly (P < .05) greater degree from baseline in the 10 mg/d and 30 mg/d dosage groups, compared with placebo. Phase III studies of bitopertin are ongoing (www.clinicaltrials.gov/ct2/show/NCT01192906).
Direct evidence of a cognitive benefit of glutamatergic-based drugs is limited. In a recent large, multicenter study, low dosage D-serine (~30 mg/kg/d) did not separate from placebo,33 but an open-label study suggests increased efficacy with dosages >30 mg/kg/d.34 In addition to symptomatic improvements, a highly significant, large effect-size improvement was seen for overall cognition for dosages ≥60 mg/kg/d, leading to a significant dose-by-time interaction (P < .01).
Combination approaches. The value of combining glutamatergic medication and a cognitive training program is supported by the role of NMDA receptors in learning. For example, D-cycloserine, a glycine-site partial agonist, has been shown in several studies to enhance learning and behavioral therapies in anxiety disorders.35 Although an initial study in schizophrenia was negative for the effectiveness of D-serine (a glycine-site full agonist) and combined cognitive training,36 further research is ongoing to evaluate a role for such combined therapy.37,38
Brain stimulation
Two nonpharmacotherapeutic brain stimulation techniques, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), have been applied in the study of schizophrenia symptoms, particularly for enhancing cognition.39 Both techniques use electric stimulation to influence activity of underlying brain regions: rTMS utilizes a magnetic coil and electromagnetic induction; tDCS, in contrast, utilizes constant low (<2 mA) direct current to specific regions of the scalp.
Cortical neuronal excitability is increased by anodal tDCS and high-frequency rTMS and reduced by cathodal tDCS and low-frequency rTMS. Both tDCS and rTMS appear to be NMDA receptor-dependent. tDCS is relatively inexpensive and requires less expertise to administer than rTMS does.
Both techniques might be efficacious for treating resistant auditory hallucinations.40,41 Applying rTMS over the left dorsolateral prefrontal cortex has led to improvement in verbal learning and visuomotor tracking in patients with schizophrenia.39 Stimulation of both sides of the prefrontal cortex with rTMS has brought improvement in visual memory, executive function, spatial working memory, and attention. Few papers have been published so far regarding enhancement of cognition with tDCS in schizophrenia,42 but beneficial effects of this technique have been seen across several disorders.43
Cognitive remediation techniques
A fundamental starting point for cognitive remediation is the idea that there is plasticity in the brain and that repetitive practice can lead to cognitive improvement. Cognitive remediation therapy often adopts computerized programs and exercises that attempt to improve psychosocial function by targeting structures of the brain that are involved in cognitive function, such as attention, working memory, executive functioning, planning, and cognitive flexibility.
In schizophrenia, cognitive remediation studies have traditionally targeted higher-order processes, such as attention and higher level processes, that might lead to improvement in overall cognition and function.44 Cognitive remediation typically is utilized complementary to pharmacotherapy, with some studies supporting the use of combined use of cognition-enhancing drugs and remediation programs.
A 2007 meta-analysis showed a medium-size but significant improvement in cognition through the use of cognitive remediation therapy45—especially when it is combined with psychiatric rehabilitation. More recent studies utilizing techniques that focus on bottom-up (auditory and visual processing) techniques has shown significant improvements.46-48 Several multicenter studies utilizing Posit Science programs combined with antipsychotic medication are ongoing (www.clinicaltrials.gov/ct2/show/NCT01173874 and www.clinicaltrials.gov/ct2/show/NCT01422902).
Bottom Line
Although cognitive dysfunction is a leading cause of disability in schizophrenia, no treatments are approved for this condition. Numerous novel-mechanism and nonpharmaceutical modalities are actively being studied for this difficult-to-treat problem, however—offering hope to patients.
Related Resources
Javitt DC, Zukin SR, Heresco-Levy U, et al. Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Has an angel shown the way? Schizophr Bull. 2012; 38(5):958-966.
Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;(213):11-37.
Millan MJ, Agid Y, Brune M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012; 11(2):141-168.
Drug Brand Names
D-cycloserine • Seromycin Ketamine • Ketalar
Xanomeline • Lumeron, Memcor
Disclosures
Dr. Kantrowitz receives grant or research support from EnVivo, the National Institute of Mental Health, Novartis, Pfizer, Roche-Genentech, the Stanley Foundation, and Sunovion; is a consultant to Health Advances, LLC, the Healthcare Advisory Board, Otsuka Pharmaceuticals, Strategic Edge Communications, and Vindico Medical Education; and owns a small number of shares of common stock in GlaxoSmithKline. Ms. Levy and Dr. Ballon report no financial relationships with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Available treatments for schizophrenia (eg, antipsychotics) are primarily effective on positive symptoms (hallucinations, delusions, etc.). It is, however, increasingly clear that schizophrenia also is a severe neuropsychiatric illness associated with deficits in cognitive function. These deficits represent a core feature of the disorder, and are a major determinant of long-term disability.1 Cognitive dysfunction is among the earliest signs of illness that, typically, presents in the prodromal phase.
Since the formulation of the dopaminergic model of schizophrenia, cognitive studies of the disease primarily have examined dysfunction in dopaminergic-rich regions of the brain, such as the prefrontal cortex, and, therefore, have focused largely on executive functioning. But neurocognitive deficits in schizophrenia are not limited to executive functioning; comparable deficits have been observed across multiple areas of cognition.2
More recent formulations of cognitive dysfunction in schizophrenia divide deficits into multiple domains. These include verbal, visual, and working memory; attention and vigilance; speed of processing, reasoning, and problem solving; and social cognition (Table). Neurocognitive impairments often are closely associated with deficits in early sensory processing and basic neurophysiology.3
The prevalence of cognitive dysfunction also can be estimated using baseline data from the large-scale Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial.4 Although cognitive dysfunction was not one of the inclusion criteria in CATIE, most patients who were enrolled had profound cognitive deficits.5 Furthermore, meta-analyses6 suggest that composite neurocognitive measures can explain as much as 60% of the variance of overall functioning in schizophrenia.
Antipsychotics aren’t the answer
The cognitive-enhancing benefits of antipsychotic medications are minimal.7 As evidence of a direct relationship between cognitive dysfunction and long-term functional outcome in schizophrenia becomes established, the need for safe and effective treatment for these symptoms becomes more urgent. Given the mechanistic complexity of the potential cause of poor cognitive performance, the search for an effective treatment is ongoing—but that search has not been successful.
Despite mixed results for recent novel mechanism trials (http://newsroom.lilly.com/releasedetail.cfm?releaseid=703018) and a number of companies ceasing drug development, the work to develop safe and effective treatments for cognitive dysfunction in schizophrenia continues, as exemplified by National Institute of Mental Health-initiated programs to spur development of drugs that work by a novel mechanism. Rather than simply assessing novel compounds with paper-and-pencil cognitive scales, such programs seek to assess the ability of the compound to engage with the intended receptor (target),9 using imaging or electrophysiological tools. Without utilization of a target engagement biomarker, there is no way to know whether 1) the drug simply does not get into the brain in sufficient concentration to be effective in humans or 2) the overall mechanism is wrong.
In this article, we review several promising targets and techniques that are the subject of active research on the treatment of cognitive disorders in schizophrenia. This list isn’t exhaustive; our aim is to highlight a few of the promising treatments now being studied in clinical trials.
Acetylcholine receptors
Acetylcholine receptors comprise two major families, nicotinic and muscarinic receptors; evidence implicates deficits of both families in schizophrenia.10 Following up on epidemiological studies11 of the high percentage of schizophrenia patients who smoke tobacco (60% to 90%), the role of alpha-7 nicotinic acetylcholine receptors (á7 nAchR) has been explored. Nicotine itself might normalize some disrupted auditory processes, as measured by electroencephalography.12
Several clinical trials of partial á7 nAchR agonists have been conducted, with EVP-6124 and TC-5619 furthest along in development.
EVP-6124. Information is unavailable publicly on EVP-6124, except for an abstract presented in 2011 at the 51st Annual Meeting of the American College of Neuropsychopharmacology.13 In that study, 319 patients with schizophrenia were randomized to EVP-6124 (0.3 mg/d or 1 mg/d [n = 213]) or placebo (n = 106) adjunctive to at least 4 weeks of non-clozapine antipsychotics. Efficacy was shown up to 1 mg, in a dose-responsive manner. Modest, but significant, improvements in cognition, clinical function, and negative symptoms were seen. The most commonly reported side effects were headache (3.8%), nausea (3.2%), and nasopharyngitis (2.5%). Phase III studies are underway.
TC-5619. This partial á7 nAchR also showed positive results recently in a Phase II trial. Significant (P < .05) improvement was demonstrated in executive function in the Groton Maze Learning Task of the CogState Schizophrenia Battery and the Scale for Assessment of Negative Symptoms.14
Strong anatomic links also exist between muscarinic acetylcholine receptors and the brain dopaminergic system, especially muscarinic type-1 and type-4 (M1 and M4) receptors. The potential utility of an M1, M4, or combined M/M4 agonist is also supported by studies of M1 and M4 knockout mice, with particular evidence of cognitive enhancement with the use of M1 agonists.15
GSK1034702. Administration of the M1 allosteric agonist GSK1034702 to healthy human smokers, using the nicotine abstinence model of cognitive dysfunction, resulted in improvements in immediate recall.16
Xanomeline. In a small pilot study of 20 schizophrenia patients, xanomeline, a mixed M1/M4 agonist, demonstrated significant improvements in verbal learning, short-term memory, and overall symptoms.17
Dopamine receptors
All marketed antipsychotics block the dopamine type-2 (D2) receptor18; they are primarily effective on positive symptoms.4 In contrast, a role for the dopamine type-1 (D1) receptor in cognition is suggested by studies that demonstrate reduced D1 and N-methyl-d-aspartate (NMDA) glutamate receptor function in the prefrontal cortex.19-22
In a model of cognitive impairment in non-human primates, low-dose intermittent dosing of D1-receptor agonists produced improvements in cognitive function.23 This strategy aims to sensitize, rather than induce tolerance, to the effects of the D1-receptor agonist. Benefits were primarily seen in working memory. Phase II trials of a potent D1-receptor agonist, DAR-100A, the active enantiomer of dihydrexidine24 are ongoing (www.clinicaltrials.gov/ct2/show/NCT01519557).
Glutamatergic receptors
Intoxication with NMDA antagonists (such as phencyclidine and ketamine) yields a phenotype with similarity to schizophrenia.25 More than 20 years of research has provided evidence for the role of glutamatergic NMDA receptors in the pathophysiology of schizophrenia.26,27
NMDA receptors are distributed widely in the brain, but specific glutamatergic processes are localized to areas that are associated with cognition. This relative distribution provides a convenient framework from which to view the pattern of cognitive dysfunction associated with schizophrenia:
• NMDA receptors in the hippocampus are involved in learning and memory acquisition
• NMDA receptors in the visual cortex and auditory cortex are fundamental for auditory and visual sensory memory.
Previous reviews of ketamine administration have described cognitive deficits in healthy control subjects, comparable to what is seen in schizophrenia.28 The deficits are noted primarily in measures of executive functioning, attention/vigilance, verbal fluency, and visual and verbal working memory.
Most treatment studies of glutamatergic-based drugs have focused on positive and negative symptoms. Two recent comprehensive meta-analyses29,30 of NMDA-based treatments support small-to-moderate effect size improvement in total symptoms and in negative symptoms, in patients with chronic schizophrenia, when the drugs are used in combination with non-clozapine antipsychotics.
Bitopertin. A novel glycine-transport inhibitor, bitopertin, showed significant improvement in negative symptoms as an adjunctive treatment in a large Phase II trial.31,32 In the “per protocol” population (ie, patients who completed 8 weeks of treatment without any major protocol violations [n = 231]), negative symptoms diminished to a significantly (P < .05) greater degree from baseline in the 10 mg/d and 30 mg/d dosage groups, compared with placebo. Phase III studies of bitopertin are ongoing (www.clinicaltrials.gov/ct2/show/NCT01192906).
Direct evidence of a cognitive benefit of glutamatergic-based drugs is limited. In a recent large, multicenter study, low dosage D-serine (~30 mg/kg/d) did not separate from placebo,33 but an open-label study suggests increased efficacy with dosages >30 mg/kg/d.34 In addition to symptomatic improvements, a highly significant, large effect-size improvement was seen for overall cognition for dosages ≥60 mg/kg/d, leading to a significant dose-by-time interaction (P < .01).
Combination approaches. The value of combining glutamatergic medication and a cognitive training program is supported by the role of NMDA receptors in learning. For example, D-cycloserine, a glycine-site partial agonist, has been shown in several studies to enhance learning and behavioral therapies in anxiety disorders.35 Although an initial study in schizophrenia was negative for the effectiveness of D-serine (a glycine-site full agonist) and combined cognitive training,36 further research is ongoing to evaluate a role for such combined therapy.37,38
Brain stimulation
Two nonpharmacotherapeutic brain stimulation techniques, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), have been applied in the study of schizophrenia symptoms, particularly for enhancing cognition.39 Both techniques use electric stimulation to influence activity of underlying brain regions: rTMS utilizes a magnetic coil and electromagnetic induction; tDCS, in contrast, utilizes constant low (<2 mA) direct current to specific regions of the scalp.
Cortical neuronal excitability is increased by anodal tDCS and high-frequency rTMS and reduced by cathodal tDCS and low-frequency rTMS. Both tDCS and rTMS appear to be NMDA receptor-dependent. tDCS is relatively inexpensive and requires less expertise to administer than rTMS does.
Both techniques might be efficacious for treating resistant auditory hallucinations.40,41 Applying rTMS over the left dorsolateral prefrontal cortex has led to improvement in verbal learning and visuomotor tracking in patients with schizophrenia.39 Stimulation of both sides of the prefrontal cortex with rTMS has brought improvement in visual memory, executive function, spatial working memory, and attention. Few papers have been published so far regarding enhancement of cognition with tDCS in schizophrenia,42 but beneficial effects of this technique have been seen across several disorders.43
Cognitive remediation techniques
A fundamental starting point for cognitive remediation is the idea that there is plasticity in the brain and that repetitive practice can lead to cognitive improvement. Cognitive remediation therapy often adopts computerized programs and exercises that attempt to improve psychosocial function by targeting structures of the brain that are involved in cognitive function, such as attention, working memory, executive functioning, planning, and cognitive flexibility.
In schizophrenia, cognitive remediation studies have traditionally targeted higher-order processes, such as attention and higher level processes, that might lead to improvement in overall cognition and function.44 Cognitive remediation typically is utilized complementary to pharmacotherapy, with some studies supporting the use of combined use of cognition-enhancing drugs and remediation programs.
A 2007 meta-analysis showed a medium-size but significant improvement in cognition through the use of cognitive remediation therapy45—especially when it is combined with psychiatric rehabilitation. More recent studies utilizing techniques that focus on bottom-up (auditory and visual processing) techniques has shown significant improvements.46-48 Several multicenter studies utilizing Posit Science programs combined with antipsychotic medication are ongoing (www.clinicaltrials.gov/ct2/show/NCT01173874 and www.clinicaltrials.gov/ct2/show/NCT01422902).
Bottom Line
Although cognitive dysfunction is a leading cause of disability in schizophrenia, no treatments are approved for this condition. Numerous novel-mechanism and nonpharmaceutical modalities are actively being studied for this difficult-to-treat problem, however—offering hope to patients.
Related Resources
Javitt DC, Zukin SR, Heresco-Levy U, et al. Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Has an angel shown the way? Schizophr Bull. 2012; 38(5):958-966.
Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;(213):11-37.
Millan MJ, Agid Y, Brune M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012; 11(2):141-168.
Drug Brand Names
D-cycloserine • Seromycin Ketamine • Ketalar
Xanomeline • Lumeron, Memcor
Disclosures
Dr. Kantrowitz receives grant or research support from EnVivo, the National Institute of Mental Health, Novartis, Pfizer, Roche-Genentech, the Stanley Foundation, and Sunovion; is a consultant to Health Advances, LLC, the Healthcare Advisory Board, Otsuka Pharmaceuticals, Strategic Edge Communications, and Vindico Medical Education; and owns a small number of shares of common stock in GlaxoSmithKline. Ms. Levy and Dr. Ballon report no financial relationships with manufacturers of any products mentioned in this article or with manufacturers of competing products.
1. Bowie CR, Reichenberg A, Patterson TL, et al. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418-425.
2. Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1-3):124-131.
3. Javitt DC, Spencer KM, Thaker GK, et al. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68-83.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1253.
5. Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033-2046.
6. Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119-136.
7. Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633-647.
8. Yan J. NIMH tries to jumpstart drug innovations. Psychiatric News. 2013;48(1):8-10.
9. Javitt DC, Schoepp D, Kalivas PW, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3(102):102mr2.
10. Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov Med. 2012;14(79):413-420.
11. D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62(3):1564-1573.
12. Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998; 24(2):189-202.
13. Meltzer HY, Gawryl M, Ward S, et al. EVP-6124, an alpha-7 nicotinic partial agonist, reduces positive effects on cognition, clinical function, and negative symptoms in patients with chronic schizophrenia on stable antipsychotic therapy. Neuropsychopharmacology. 2011;36:S170-S171.
14. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
15. Digby GJ, Noetzel MJ, Bubser M, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32(25):8532-8544.
16. Nathan PJ, Watson J, Lund J, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16(4):721-731.
17. Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033-1039.
18. Di Forti M, Lappin LM, Murray RM. Risk factors for schizophrenia—all roads lead to dopamine. Eur Neuropsychopharmacol. 2007;17(suppl 2):S101-S107.
19. Krystal JH, D’Souza DC, Mathalon D, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 2003;169(3-4): 215-233.
20. Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9(5):404-416.
21. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708-3719.
22. Martinez A, Ramanathan DS, Foxe JJ, et al. The role of spatial attention in the selection of real and illusory objects. J Neurosci. 2007;27(30):7963-7973.
23. Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287(5460):2020-2022.
24. Slifstein M, Suckow RF, Javitch JA, et al. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J Cereb Blood Flow Metab. 2011;31(1):293-304.
25. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301-1308.
26. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010; 83(3-4):108-121.
27. Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4(3):189-200.
28. Kantrowitz JT, Javitt DC. Glutamatergic approaches to the conceptualization and treatment of schizophrenia. In: Javitt DC, Kantrowitz JT, eds. Handbook of neurochemistry and molecular neurobiology. New York, NY: Springer; 2009:3-36.
29. Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16(5):522-537.
30. Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25(10):859-868.
31. Umbricht D, Yoo K, Youssef E, et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Neuropharmacology. 2010;35:S320-S321.
32. Pinard E, Alanine A, Alberati D, et al. Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl-2-(( S)-2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia. J Med Chem. 2010;53(12):4603-4614.
33. Weiser M, Heresco-Levy U, Davidson M, et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry. 2012;73(6):e728-e734.
34. Kantrowitz JT, Malhotra AK, Cornblatt B, et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121(1-3):125-130.
35. Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118-1126.
36. D’Souza DC, Radhakrishnan R, Perry E, et al. Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology. 2013;38(3):492-503.
37. Gottlieb JD, Cather C, Shanahan M, et al. D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011;131(1-3):69-74.
38. Kantrowitz J, Sehatpour P, Oakman E, et al. D-Serine and NMDA based sensory modulation. Poster presented at: 3rd Biennial Schizophrenia International Research Conference; April 14-18, 2012; Florence, Italy.
39. Demirtas-Tatlidede, A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566-578.
40. Brunelin J, Mondino M, Gassab L, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169(7):719-724.
41. Matheson SL, Green MJ, Loo C, et al. Quality assessment and comparison of evidence for electroconvulsive therapy and repetitive transcranial magnetic stimulation for schizophrenia: a systematic meta-review. Schizophr Res. 2012;118(1-3):201-210.
42. Vercammen A, Rushby JA, Loo C, et al. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophr Res. 2011;131(1-3):198-205.
43. Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011; 29(6):463-492.
44. Keefe RS, Vinogradov S, Medalia A, et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. 2011;37(5):1057-1065.
45. McGurk SR, Twamley EW, Sitzer DI, et al. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791-1802.
46. Fisher M, Holland C, Merzenich MM, et al. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805-811.
47. Norton DJ, McBain RK, Ongür D, et al. Perceptual training strongly improves visual motion perception in schizophrenia. Brain Cogn. 2011;77(2):248-256.
48. Kantrowitz JT, Revheim N, Pasternak R, et al. It’s all in the cards: effect of stimulus manipulation on Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res. 2009;168(3):198-204.
1. Bowie CR, Reichenberg A, Patterson TL, et al. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418-425.
2. Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1-3):124-131.
3. Javitt DC, Spencer KM, Thaker GK, et al. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68-83.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1253.
5. Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033-2046.
6. Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119-136.
7. Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633-647.
8. Yan J. NIMH tries to jumpstart drug innovations. Psychiatric News. 2013;48(1):8-10.
9. Javitt DC, Schoepp D, Kalivas PW, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3(102):102mr2.
10. Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov Med. 2012;14(79):413-420.
11. D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62(3):1564-1573.
12. Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998; 24(2):189-202.
13. Meltzer HY, Gawryl M, Ward S, et al. EVP-6124, an alpha-7 nicotinic partial agonist, reduces positive effects on cognition, clinical function, and negative symptoms in patients with chronic schizophrenia on stable antipsychotic therapy. Neuropsychopharmacology. 2011;36:S170-S171.
14. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
15. Digby GJ, Noetzel MJ, Bubser M, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32(25):8532-8544.
16. Nathan PJ, Watson J, Lund J, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16(4):721-731.
17. Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033-1039.
18. Di Forti M, Lappin LM, Murray RM. Risk factors for schizophrenia—all roads lead to dopamine. Eur Neuropsychopharmacol. 2007;17(suppl 2):S101-S107.
19. Krystal JH, D’Souza DC, Mathalon D, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 2003;169(3-4): 215-233.
20. Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9(5):404-416.
21. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708-3719.
22. Martinez A, Ramanathan DS, Foxe JJ, et al. The role of spatial attention in the selection of real and illusory objects. J Neurosci. 2007;27(30):7963-7973.
23. Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287(5460):2020-2022.
24. Slifstein M, Suckow RF, Javitch JA, et al. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J Cereb Blood Flow Metab. 2011;31(1):293-304.
25. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301-1308.
26. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010; 83(3-4):108-121.
27. Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4(3):189-200.
28. Kantrowitz JT, Javitt DC. Glutamatergic approaches to the conceptualization and treatment of schizophrenia. In: Javitt DC, Kantrowitz JT, eds. Handbook of neurochemistry and molecular neurobiology. New York, NY: Springer; 2009:3-36.
29. Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16(5):522-537.
30. Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25(10):859-868.
31. Umbricht D, Yoo K, Youssef E, et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Neuropharmacology. 2010;35:S320-S321.
32. Pinard E, Alanine A, Alberati D, et al. Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl-2-(( S)-2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia. J Med Chem. 2010;53(12):4603-4614.
33. Weiser M, Heresco-Levy U, Davidson M, et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry. 2012;73(6):e728-e734.
34. Kantrowitz JT, Malhotra AK, Cornblatt B, et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121(1-3):125-130.
35. Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118-1126.
36. D’Souza DC, Radhakrishnan R, Perry E, et al. Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology. 2013;38(3):492-503.
37. Gottlieb JD, Cather C, Shanahan M, et al. D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011;131(1-3):69-74.
38. Kantrowitz J, Sehatpour P, Oakman E, et al. D-Serine and NMDA based sensory modulation. Poster presented at: 3rd Biennial Schizophrenia International Research Conference; April 14-18, 2012; Florence, Italy.
39. Demirtas-Tatlidede, A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566-578.
40. Brunelin J, Mondino M, Gassab L, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169(7):719-724.
41. Matheson SL, Green MJ, Loo C, et al. Quality assessment and comparison of evidence for electroconvulsive therapy and repetitive transcranial magnetic stimulation for schizophrenia: a systematic meta-review. Schizophr Res. 2012;118(1-3):201-210.
42. Vercammen A, Rushby JA, Loo C, et al. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophr Res. 2011;131(1-3):198-205.
43. Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011; 29(6):463-492.
44. Keefe RS, Vinogradov S, Medalia A, et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. 2011;37(5):1057-1065.
45. McGurk SR, Twamley EW, Sitzer DI, et al. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791-1802.
46. Fisher M, Holland C, Merzenich MM, et al. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805-811.
47. Norton DJ, McBain RK, Ongür D, et al. Perceptual training strongly improves visual motion perception in schizophrenia. Brain Cogn. 2011;77(2):248-256.
48. Kantrowitz JT, Revheim N, Pasternak R, et al. It’s all in the cards: effect of stimulus manipulation on Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res. 2009;168(3):198-204.
Alternatives to 12-step groups
Persons addicted to drugs often are among the most marginalized psychiatric patients, but are in need of the most support.1 Many of these patients have comorbid medical and psychiatric problems, including difficult-to-treat pathologies that may have developed because of a traumatic experience or an attachment disorder that dominates their emotional lives.2 These patients value clinicians who engage them in an open, nonjudgmental, and empathetic way.
Eliciting a patient’s reasons for change and introducing him (her) to a variety of peer-led recovery group options that complement and support psychotherapy and pharmacotherapy can be valuable. Although most clinicians are aware of the traditional 12-step group model that embraces spirituality, many might know less about other groups that can play an instrumental role in engaging patients and placing them on the path to recovery.
SMART (Self-Management and Recovery Training) Recovery5 is a nonprofit organization that does not employ the 12-step model; instead, it uses evidence-based, non-confrontational, motivational, behavioral, and cognitive approaches to achieve abstinence.
Women for Sobriety6 helps women achieve abstinence.
LifeRing Secular Recovery7 works on empowering the “sober self” through groups that de-emphasize drug and alcohol use in personal histories.
Rational Recovery8 uses the Addictive Voice Recognition Technique to empower people overcoming addictions. This technique trains individuals to recognize the “addictive voice.” It does not support the theory of continuous recovery, or even recovery groups, but enables the user to achieve sobriety independently. This program greatly limits interaction between people overcoming addiction and physicians and counselors—save for periods of serious withdrawal.
The Community Reinforcement Approach (CRA)9 is an evidence-based program that focuses primarily on environmental and social factors influencing sobriety. This behavioral approach emphasizes the role of contingencies that can encourage or discourage sobriety. CRA has been studied in outpatients—predominantly homeless persons—and inpatients, and in a range of abused substances.
Click here for another Pearl on familiarizing yourself with Alcoholics Anonymous dictums.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kreek MJ. Extreme marginalization: addiction and other mental health disorders, stigma, and imprisonment. Ann N Y Acad Sci. 2011;1231:65-72.
2. Wu NS, Schairer LC, Dellor E, et al. Childhood trauma and health outcomes in adults with comorbid substance abuse and mental health disorders. Addict Behav. 2010;35(1):68-71.
3. Moderation Management. http://www.moderation.org. Accessed April 12, 2013.
4. Moderation Management. What is moderation management? http://www.moderation.org/whatisMM.shtml. Accessed August 6, 2013.
5. SMART (Self Management and Recovery Training) Recovery. http://www.smartrecovery.org. Accessed April 12, 2013.
6. Women for Sobriety. http://www.womenforsobriety.org. Accessed April 12, 2013.
7. LifeRing. http://lifering.org. Accessed April 12, 2013.
8. Rational Recovery. http://www.rational.org. Published October 25, 1995. Accessed April 12, 2013.
9. Miller WR, Meyers RJ, Hiller-Sturmhofel S. The community-reinforcement approach. http://pubs.niaaa.nih.gov/publications/arh23-2/116-121.pdf. Accessed August 6, 2013.
Persons addicted to drugs often are among the most marginalized psychiatric patients, but are in need of the most support.1 Many of these patients have comorbid medical and psychiatric problems, including difficult-to-treat pathologies that may have developed because of a traumatic experience or an attachment disorder that dominates their emotional lives.2 These patients value clinicians who engage them in an open, nonjudgmental, and empathetic way.
Eliciting a patient’s reasons for change and introducing him (her) to a variety of peer-led recovery group options that complement and support psychotherapy and pharmacotherapy can be valuable. Although most clinicians are aware of the traditional 12-step group model that embraces spirituality, many might know less about other groups that can play an instrumental role in engaging patients and placing them on the path to recovery.
SMART (Self-Management and Recovery Training) Recovery5 is a nonprofit organization that does not employ the 12-step model; instead, it uses evidence-based, non-confrontational, motivational, behavioral, and cognitive approaches to achieve abstinence.
Women for Sobriety6 helps women achieve abstinence.
LifeRing Secular Recovery7 works on empowering the “sober self” through groups that de-emphasize drug and alcohol use in personal histories.
Rational Recovery8 uses the Addictive Voice Recognition Technique to empower people overcoming addictions. This technique trains individuals to recognize the “addictive voice.” It does not support the theory of continuous recovery, or even recovery groups, but enables the user to achieve sobriety independently. This program greatly limits interaction between people overcoming addiction and physicians and counselors—save for periods of serious withdrawal.
The Community Reinforcement Approach (CRA)9 is an evidence-based program that focuses primarily on environmental and social factors influencing sobriety. This behavioral approach emphasizes the role of contingencies that can encourage or discourage sobriety. CRA has been studied in outpatients—predominantly homeless persons—and inpatients, and in a range of abused substances.
Click here for another Pearl on familiarizing yourself with Alcoholics Anonymous dictums.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Persons addicted to drugs often are among the most marginalized psychiatric patients, but are in need of the most support.1 Many of these patients have comorbid medical and psychiatric problems, including difficult-to-treat pathologies that may have developed because of a traumatic experience or an attachment disorder that dominates their emotional lives.2 These patients value clinicians who engage them in an open, nonjudgmental, and empathetic way.
Eliciting a patient’s reasons for change and introducing him (her) to a variety of peer-led recovery group options that complement and support psychotherapy and pharmacotherapy can be valuable. Although most clinicians are aware of the traditional 12-step group model that embraces spirituality, many might know less about other groups that can play an instrumental role in engaging patients and placing them on the path to recovery.
SMART (Self-Management and Recovery Training) Recovery5 is a nonprofit organization that does not employ the 12-step model; instead, it uses evidence-based, non-confrontational, motivational, behavioral, and cognitive approaches to achieve abstinence.
Women for Sobriety6 helps women achieve abstinence.
LifeRing Secular Recovery7 works on empowering the “sober self” through groups that de-emphasize drug and alcohol use in personal histories.
Rational Recovery8 uses the Addictive Voice Recognition Technique to empower people overcoming addictions. This technique trains individuals to recognize the “addictive voice.” It does not support the theory of continuous recovery, or even recovery groups, but enables the user to achieve sobriety independently. This program greatly limits interaction between people overcoming addiction and physicians and counselors—save for periods of serious withdrawal.
The Community Reinforcement Approach (CRA)9 is an evidence-based program that focuses primarily on environmental and social factors influencing sobriety. This behavioral approach emphasizes the role of contingencies that can encourage or discourage sobriety. CRA has been studied in outpatients—predominantly homeless persons—and inpatients, and in a range of abused substances.
Click here for another Pearl on familiarizing yourself with Alcoholics Anonymous dictums.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kreek MJ. Extreme marginalization: addiction and other mental health disorders, stigma, and imprisonment. Ann N Y Acad Sci. 2011;1231:65-72.
2. Wu NS, Schairer LC, Dellor E, et al. Childhood trauma and health outcomes in adults with comorbid substance abuse and mental health disorders. Addict Behav. 2010;35(1):68-71.
3. Moderation Management. http://www.moderation.org. Accessed April 12, 2013.
4. Moderation Management. What is moderation management? http://www.moderation.org/whatisMM.shtml. Accessed August 6, 2013.
5. SMART (Self Management and Recovery Training) Recovery. http://www.smartrecovery.org. Accessed April 12, 2013.
6. Women for Sobriety. http://www.womenforsobriety.org. Accessed April 12, 2013.
7. LifeRing. http://lifering.org. Accessed April 12, 2013.
8. Rational Recovery. http://www.rational.org. Published October 25, 1995. Accessed April 12, 2013.
9. Miller WR, Meyers RJ, Hiller-Sturmhofel S. The community-reinforcement approach. http://pubs.niaaa.nih.gov/publications/arh23-2/116-121.pdf. Accessed August 6, 2013.
1. Kreek MJ. Extreme marginalization: addiction and other mental health disorders, stigma, and imprisonment. Ann N Y Acad Sci. 2011;1231:65-72.
2. Wu NS, Schairer LC, Dellor E, et al. Childhood trauma and health outcomes in adults with comorbid substance abuse and mental health disorders. Addict Behav. 2010;35(1):68-71.
3. Moderation Management. http://www.moderation.org. Accessed April 12, 2013.
4. Moderation Management. What is moderation management? http://www.moderation.org/whatisMM.shtml. Accessed August 6, 2013.
5. SMART (Self Management and Recovery Training) Recovery. http://www.smartrecovery.org. Accessed April 12, 2013.
6. Women for Sobriety. http://www.womenforsobriety.org. Accessed April 12, 2013.
7. LifeRing. http://lifering.org. Accessed April 12, 2013.
8. Rational Recovery. http://www.rational.org. Published October 25, 1995. Accessed April 12, 2013.
9. Miller WR, Meyers RJ, Hiller-Sturmhofel S. The community-reinforcement approach. http://pubs.niaaa.nih.gov/publications/arh23-2/116-121.pdf. Accessed August 6, 2013.
Do glucocorticoids hold promise as a treatment for PTSD?
As symptoms of posttraumatic stress disorder (PTSD) progress, the involved person’s physical and mental health deteriorates.1 This sparks lifestyle changes that allow them to avoid re-exposure to triggering stimuli; however, it also increases their risk of social isolation. Early clinical investigation has found that patients who experience hyperarousal symptoms of overt PTSD—difficulty sleeping, emotional dyscontrol, hypervigilance, and an enhanced startle response—could benefit from the stress-reducing capacity of glucocorticoids.
Decreased glucocorticoids
After a distressing situation, norepinephrine levels rise acutely.2,3 This contributes to a protective retention of potentially threatening memories, which is how people learn to avoid danger.
Glucocorticoid secretion enhances a patient’s coping mechanisms by helping them process information in a way that diminishes retrieval of fear-evoking memories.2,3 Glucocorticoid, also called cortisol, is referred to as a “stress hormone.” Cortisol promotes emotional adaptability following a traumatic event; this action diminishes future, inappropriate retrieval of frightening memories as a physiologic mechanism to help people cope with upsetting situations.3
PTSD pathogenesis involves altered hypothalamic-pituitary-adrenal axis function; sustained stress results in decreased levels of circulating glucocorticoid. This is a consequence of enhanced negative feedback and increased glucocorticoid receptor sensitivity, which is evidenced by results of abnormal dexamethasone suppression tests.1 Downregulation of corticotropin-releasing hormone (CRH) receptors in the pituitary glands and increased CRH levels have been documented in PTSD patients.1,4 An association between high CRH levels and an increase in startle response explains the exaggerated startle response observed in patients with PTSD. Higher circulating glucocorticoid has the opposite effect4; there is an inverse relationship between the daily level of glucocorticoid and startle amplitude. A low level of circulating glucocorticoid promotes recall of frightening events that results in persistent re-experiencing of traumatic memories.2,3
Glucocorticoids in PTSD
Glucocorticoid administration reduces psychological and physiological responses to stress.3 Exogenous glucocorticoid administration affects cognition by interacting with serotonin, dopamine, and ã-aminobutyric acid by actions on the amygdala, medial prefrontal cortex, and hippocampus.2,3 Research among veterans with and without PTSD recorded a decrease in startle response after administration of a single dose of 20 mg of hydrocortisone.4 Results of a large study documented that one dose of hydrocortisone administered at >35 mg can inhibit threatening memories and improve social function.3 Hydrocortisone is linked to anxiolytic effects in healthy persons and patients with social phobia or panic disorder.3,4 Because treatment of PTSD with antidepressants and benzodiazepines often is ineffective,5 glucocorticoids may offer a new pharmacotherapy option. Glucocorticoids have been prescribed as prophylactic agents shortly after an acutely stressful event to prevent development of PTSD.4 Hydrocortisone is not FDA-approved to treat PTSD; informed consent, physician discretion, and close monitoring are emphasized.
Glucocorticoid use in mitigating PTSD symptom emergence is under investigation. Research suggests that just one acute dose of hydrocortisone might benefit patients prone to PTSD.3,4 Further study is needed to establish whether prescribing hydrocortisone is efficacious.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jones T, Moller MD. Implications of hypothalamic-pituitary-adrenal axis functioning in posttraumatic stress disorder. J Am Psychiatr Nurses Assoc. 2011;17(6):393-403.
2. Blundell J, Blaiss CA, Lagace DC, et al. Block of glucocorticoid synthesis during re-activation inhibits extinction of an established fear memory. Neurobiol Learn Mem. 2011;95(4):453-460.
3. Putman P, Roelofs K. Effects of single cortisol administrations on human affect reviewed: coping with stress through adaptive regulation of automatic cognitive processing. Psychoneuroendocrinology. 2011;36(4):439-448.
4. Miller MW, McKinney AE, Kanter FS, et al. Hydrocortisone suppression of the fear-potentiated startle response and posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36(7):970-980.
5. Nin MS, Martinez LA, Pibiri F, et al. Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front Endocrinol (Lausanne). 2011;2(73):1-12.
As symptoms of posttraumatic stress disorder (PTSD) progress, the involved person’s physical and mental health deteriorates.1 This sparks lifestyle changes that allow them to avoid re-exposure to triggering stimuli; however, it also increases their risk of social isolation. Early clinical investigation has found that patients who experience hyperarousal symptoms of overt PTSD—difficulty sleeping, emotional dyscontrol, hypervigilance, and an enhanced startle response—could benefit from the stress-reducing capacity of glucocorticoids.
Decreased glucocorticoids
After a distressing situation, norepinephrine levels rise acutely.2,3 This contributes to a protective retention of potentially threatening memories, which is how people learn to avoid danger.
Glucocorticoid secretion enhances a patient’s coping mechanisms by helping them process information in a way that diminishes retrieval of fear-evoking memories.2,3 Glucocorticoid, also called cortisol, is referred to as a “stress hormone.” Cortisol promotes emotional adaptability following a traumatic event; this action diminishes future, inappropriate retrieval of frightening memories as a physiologic mechanism to help people cope with upsetting situations.3
PTSD pathogenesis involves altered hypothalamic-pituitary-adrenal axis function; sustained stress results in decreased levels of circulating glucocorticoid. This is a consequence of enhanced negative feedback and increased glucocorticoid receptor sensitivity, which is evidenced by results of abnormal dexamethasone suppression tests.1 Downregulation of corticotropin-releasing hormone (CRH) receptors in the pituitary glands and increased CRH levels have been documented in PTSD patients.1,4 An association between high CRH levels and an increase in startle response explains the exaggerated startle response observed in patients with PTSD. Higher circulating glucocorticoid has the opposite effect4; there is an inverse relationship between the daily level of glucocorticoid and startle amplitude. A low level of circulating glucocorticoid promotes recall of frightening events that results in persistent re-experiencing of traumatic memories.2,3
Glucocorticoids in PTSD
Glucocorticoid administration reduces psychological and physiological responses to stress.3 Exogenous glucocorticoid administration affects cognition by interacting with serotonin, dopamine, and ã-aminobutyric acid by actions on the amygdala, medial prefrontal cortex, and hippocampus.2,3 Research among veterans with and without PTSD recorded a decrease in startle response after administration of a single dose of 20 mg of hydrocortisone.4 Results of a large study documented that one dose of hydrocortisone administered at >35 mg can inhibit threatening memories and improve social function.3 Hydrocortisone is linked to anxiolytic effects in healthy persons and patients with social phobia or panic disorder.3,4 Because treatment of PTSD with antidepressants and benzodiazepines often is ineffective,5 glucocorticoids may offer a new pharmacotherapy option. Glucocorticoids have been prescribed as prophylactic agents shortly after an acutely stressful event to prevent development of PTSD.4 Hydrocortisone is not FDA-approved to treat PTSD; informed consent, physician discretion, and close monitoring are emphasized.
Glucocorticoid use in mitigating PTSD symptom emergence is under investigation. Research suggests that just one acute dose of hydrocortisone might benefit patients prone to PTSD.3,4 Further study is needed to establish whether prescribing hydrocortisone is efficacious.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
As symptoms of posttraumatic stress disorder (PTSD) progress, the involved person’s physical and mental health deteriorates.1 This sparks lifestyle changes that allow them to avoid re-exposure to triggering stimuli; however, it also increases their risk of social isolation. Early clinical investigation has found that patients who experience hyperarousal symptoms of overt PTSD—difficulty sleeping, emotional dyscontrol, hypervigilance, and an enhanced startle response—could benefit from the stress-reducing capacity of glucocorticoids.
Decreased glucocorticoids
After a distressing situation, norepinephrine levels rise acutely.2,3 This contributes to a protective retention of potentially threatening memories, which is how people learn to avoid danger.
Glucocorticoid secretion enhances a patient’s coping mechanisms by helping them process information in a way that diminishes retrieval of fear-evoking memories.2,3 Glucocorticoid, also called cortisol, is referred to as a “stress hormone.” Cortisol promotes emotional adaptability following a traumatic event; this action diminishes future, inappropriate retrieval of frightening memories as a physiologic mechanism to help people cope with upsetting situations.3
PTSD pathogenesis involves altered hypothalamic-pituitary-adrenal axis function; sustained stress results in decreased levels of circulating glucocorticoid. This is a consequence of enhanced negative feedback and increased glucocorticoid receptor sensitivity, which is evidenced by results of abnormal dexamethasone suppression tests.1 Downregulation of corticotropin-releasing hormone (CRH) receptors in the pituitary glands and increased CRH levels have been documented in PTSD patients.1,4 An association between high CRH levels and an increase in startle response explains the exaggerated startle response observed in patients with PTSD. Higher circulating glucocorticoid has the opposite effect4; there is an inverse relationship between the daily level of glucocorticoid and startle amplitude. A low level of circulating glucocorticoid promotes recall of frightening events that results in persistent re-experiencing of traumatic memories.2,3
Glucocorticoids in PTSD
Glucocorticoid administration reduces psychological and physiological responses to stress.3 Exogenous glucocorticoid administration affects cognition by interacting with serotonin, dopamine, and ã-aminobutyric acid by actions on the amygdala, medial prefrontal cortex, and hippocampus.2,3 Research among veterans with and without PTSD recorded a decrease in startle response after administration of a single dose of 20 mg of hydrocortisone.4 Results of a large study documented that one dose of hydrocortisone administered at >35 mg can inhibit threatening memories and improve social function.3 Hydrocortisone is linked to anxiolytic effects in healthy persons and patients with social phobia or panic disorder.3,4 Because treatment of PTSD with antidepressants and benzodiazepines often is ineffective,5 glucocorticoids may offer a new pharmacotherapy option. Glucocorticoids have been prescribed as prophylactic agents shortly after an acutely stressful event to prevent development of PTSD.4 Hydrocortisone is not FDA-approved to treat PTSD; informed consent, physician discretion, and close monitoring are emphasized.
Glucocorticoid use in mitigating PTSD symptom emergence is under investigation. Research suggests that just one acute dose of hydrocortisone might benefit patients prone to PTSD.3,4 Further study is needed to establish whether prescribing hydrocortisone is efficacious.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jones T, Moller MD. Implications of hypothalamic-pituitary-adrenal axis functioning in posttraumatic stress disorder. J Am Psychiatr Nurses Assoc. 2011;17(6):393-403.
2. Blundell J, Blaiss CA, Lagace DC, et al. Block of glucocorticoid synthesis during re-activation inhibits extinction of an established fear memory. Neurobiol Learn Mem. 2011;95(4):453-460.
3. Putman P, Roelofs K. Effects of single cortisol administrations on human affect reviewed: coping with stress through adaptive regulation of automatic cognitive processing. Psychoneuroendocrinology. 2011;36(4):439-448.
4. Miller MW, McKinney AE, Kanter FS, et al. Hydrocortisone suppression of the fear-potentiated startle response and posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36(7):970-980.
5. Nin MS, Martinez LA, Pibiri F, et al. Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front Endocrinol (Lausanne). 2011;2(73):1-12.
1. Jones T, Moller MD. Implications of hypothalamic-pituitary-adrenal axis functioning in posttraumatic stress disorder. J Am Psychiatr Nurses Assoc. 2011;17(6):393-403.
2. Blundell J, Blaiss CA, Lagace DC, et al. Block of glucocorticoid synthesis during re-activation inhibits extinction of an established fear memory. Neurobiol Learn Mem. 2011;95(4):453-460.
3. Putman P, Roelofs K. Effects of single cortisol administrations on human affect reviewed: coping with stress through adaptive regulation of automatic cognitive processing. Psychoneuroendocrinology. 2011;36(4):439-448.
4. Miller MW, McKinney AE, Kanter FS, et al. Hydrocortisone suppression of the fear-potentiated startle response and posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36(7):970-980.
5. Nin MS, Martinez LA, Pibiri F, et al. Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front Endocrinol (Lausanne). 2011;2(73):1-12.
How best to engage patients in their psychiatric care
Providing patients and their families with information and education about their psychiatric illness is a central tenet of mental health care. Discussions about diagnostic impressions, treatment options, and the risks and benefits of interventions are customary. Additionally, patients and families often receive written material or referral to other information sources, including self-help books and a growing number of online resources. Although patient education remains a useful and expected element of good care, there is evidence that, alone, it is insufficient to change health behaviors.1
A growing body of literature and clinical experience suggests that self-management strategies complement patient education and improve treatment outcomes for patients with chronic illnesses, including psychiatric conditions.2,3 The Cochrane Collaboration describes patient education as “teaching or training of patients concerning their own health needs,” and self-management as “the individual’s ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a long-term disorder.”4
In this article, we review:
• characteristics of long-term care models
• literature supporting the benefits of self-management programs
• clinical initiatives illustrating important elements of self-management support
• opportunities and challenges faced by clinicians, patients, families, clinics, and healthcare systems implementing self-management programs.
Principles of self-management
Chronic care models must account for conditions in which the clinical course can be variable, that are not amenable to a cure, and that demand long-term treatment. Optimal health outcomes rely on patients accurately monitoring, reporting, and responding to their symptoms, while engaging in critical health-related behaviors. In addition, clinicians must teach, partner with, and motivate patients to engage in crucial disease-management activities. Although typically not considered in this light, we believe most psychiatric disorders are best approached through a long-term care model, and benefit from self-management principles.
Basics of self-management include:
• patients must formulate goals and learn skills relevant to their disease
• problems are patient-selected and targeted with individualized, flexible treatment plans.
Corbin and Strauss believe effective “self-managers” achieve competency in three areas:
• role management, which entails healthy adjustments to changes in role
responsibilities, expectations, and self-identity
• emotional management, which often is particularly challenging in psychiatric conditions because of the emotional disruption inherent in living with a psychiatric illness.6
Proficiency in these three “self-manager” domains is enhanced by mastering five key self-management skills outlined in Table 1.7
Successful intervention programs vary widely with regard to individual vs group formats, communication interface, and involved health professionals. However, evidence indicates that problem solving, decision making, and action planning are key components.7 Successful planning includes:
• detailed descriptions of what, how, when, and where the activity will be accomplished
• assessment of patient confidence and adjustment of plans if confidence is limited
• continuous monitoring and self-tailoring of plans through collaborative discussions with providers, fostering a spirit of partnership and ownership.
Table 2 illustrates elements of successful action plans in our Action Planning Worksheet. Adapted from the work of Scharzer,8 Prochaska,9 and Clark,10 we developed this self-management tool for individual and group treatment settings. It serves as a vehicle for collaborative patient-provider discussion and planning. The nuts-and-bolts nature of the discussions inevitably leads to learning new, important, and often unexpected information about our patients’ daily lives and the challenges they face as they share their dominant priorities, fears, and insecurities.
Patients provide consistently positive feedback about action planning, and clinicians often find that the process reveals fruitful areas for further psychotherapeutic intervention. Examples include identifying a range of negative automatic thoughts or catastrophic thinking impeding initiation of important activation, and exposure activities for depressed or anxious patients.
Knowing what to do is different than actually doing it. Changing behavior is difficult in the best circumstances, let alone with the strain of a chronic illness. It is critical to recognize that the presence of depressive symptoms significantly reduces the likelihood that patients will employ self-management practices.11 When combined with anxiety and the impairment of motivation and executive functioning that is common in psychiatric conditions, it is not surprising that patients with a mental health condition struggle to embrace ownership of their illness and engage in critical health behaviors—which may include adhering to medication regimens; maintaining a healthy sleep cycle, nutrition, and exercise routines; vigilant symptom surveillance; and carrying out an agreed-upon action plan.
Interventions
Professionally-guided “light-touch” interventions and technology-assisted self-management interventions also can improve patient engagement in activities through individual encounters, group forums, and technology-mediated exchanges, including telephone, email, text message, tele-health, and web-based interventions.13 The DE-STRESS (Delivery of Self Training and Education for Stressful Situations) model illustrates these principles. This 8-week program combines elements of face-to-face, email, telephone, and web-based assignments and exchanges, and demonstrates a decline in posttraumatic stress disorder, depression, and anxiety scores.14 Our Michigan Depression Outreach and Collaborative Care program is another example of a self-management intervention (Box 1).
• modeling and social persuasion through having patients observe and engage others as they struggle to overcome similar obstacles
• re-interpretation of symptoms aimed at fostering the belief that symptoms generally are multi-determined with several potential explanations, and vary with daily routines.
Helping patients understand when common symptoms such as impaired concentration or dizziness should be “watched”—rather than responded to aggressively—is crucial for effective long-term management.
Challenges
Health care delivery and medical educational models have been slow to embrace this change to long-term care models because doing so involves what might be uncomfortable shifts in roles and responsibilities. Effective care for long-term illness necessitates that the patient become an expert on his (her) illness, and be an active participant and partner in their treatment. Preparing health professionals for this new role as teacher, mentor, and collaborator presents a challenge to health care systems and educational programs across disciplines.
When trying to facilitate effective patient-provider partnerships, it is important to recognize the variability in patient preference for what and how information is shared, how decisions are made, and the role patients are asked to play in their care. Patients differ in their desire for an active or collaborative shared-decision model; some prefer more directive provider communication and a passive role.18 Preferences are influenced by variables such as age, sex, race, anxiety level, and education.19 Open discussion of these matters between caregivers and patients is important; studies have shown that failure to address these issues of “fit” can
impede communication, healthy behavior, and positive outcomes.
Bottom Line
Emerging care models demand that health care providers become teachers and motivators to help patients develop and implement patterns of health surveillance and intervention that will optimize their well-being and functionality. As active collaborators in their care, patients form a partnership with their care teams, allowing for regular, reciprocal exchange of information and shared decision-making. This shift to a partnership creates new, exciting roles and responsibilities for all parties.
Related Resources
- Improving Chronic Illness Care. www.improvingchroniccare.org.
- Chronic disease self-management program (Better Choices, Better Health workshop). Stanford School of Medicine. http://patienteducation.stanford.edu/programs/cdsmp.html.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Thomas E. Fluent, MD, talks about addressing patient resistance to a self-management model of care. Dr. Fluent is Clinical Assistant Professor, Department of Psychiatry, University of Michigan, Ann Arbor, Michigan.
1. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469-2475.
2. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909-1914.
3. Druss BG, Zhao L, von Esenwein SA, et al. The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophr Res. 2010;118(1-3):264-270.
4. Tomkins S, Collins A. Promoting optimal self-care: consultation techniques that improve quality of life for patients and clinicians. London, United Kingdom: National Health Service; 2005.
5. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511-544.
6. Corbin JM, Strauss AL. Unending work and care : managing chronic illness at home. 1st ed. San Francisco, CA: Jossey-Bass Publishers; 1988.
7. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1-7.
8. Schwarzer R. Social-cognitive factors in changing health-related behaviors. Current Directions in Psychological Science. 2001;10(2):47-51.
9. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. Am Psycholt. 1992;47(9):1102-1114.
10. Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav. 2001; 28(6):769-782.
11. Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42(4):1443-1463.
12. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65(11):
1232-1245.
13. Tumur I, Kaltenthaler E, Ferriter M, et al. Computerised cognitive behaviour therapy for obsessive-compulsive disorder: a systematic review. Psychother Psychosom. 2007; 76(4):196-202.
14. Litz BT, Engel CC, Bryant RA, et al. A randomized, controlled proof-of-concept trial of an Internet-based, therapist-assisted self-management treatment for posttraumatic stress disorder. Am J Psychiatry. 2007;164(11):1676-1683.
15. Holman H, Lorig K. Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public Health Rep. 2004;119(3):239-243.
16. Williams A, Hagerty BM, Brasington SJ, et al. Stress Gym: feasibility of deploying a web-enhanced behavioral self-management program for stress in a military setting. Mil Med. 2010;175(7):487-493.
17. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790-804.
18. Davison BJ, Breckon E. Factors influencing treatment decision making and information p of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87(3):369-374.
19. Chewning B, Bylund CL, Shah B, et al. Patient p for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18.
Providing patients and their families with information and education about their psychiatric illness is a central tenet of mental health care. Discussions about diagnostic impressions, treatment options, and the risks and benefits of interventions are customary. Additionally, patients and families often receive written material or referral to other information sources, including self-help books and a growing number of online resources. Although patient education remains a useful and expected element of good care, there is evidence that, alone, it is insufficient to change health behaviors.1
A growing body of literature and clinical experience suggests that self-management strategies complement patient education and improve treatment outcomes for patients with chronic illnesses, including psychiatric conditions.2,3 The Cochrane Collaboration describes patient education as “teaching or training of patients concerning their own health needs,” and self-management as “the individual’s ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a long-term disorder.”4
In this article, we review:
• characteristics of long-term care models
• literature supporting the benefits of self-management programs
• clinical initiatives illustrating important elements of self-management support
• opportunities and challenges faced by clinicians, patients, families, clinics, and healthcare systems implementing self-management programs.
Principles of self-management
Chronic care models must account for conditions in which the clinical course can be variable, that are not amenable to a cure, and that demand long-term treatment. Optimal health outcomes rely on patients accurately monitoring, reporting, and responding to their symptoms, while engaging in critical health-related behaviors. In addition, clinicians must teach, partner with, and motivate patients to engage in crucial disease-management activities. Although typically not considered in this light, we believe most psychiatric disorders are best approached through a long-term care model, and benefit from self-management principles.
Basics of self-management include:
• patients must formulate goals and learn skills relevant to their disease
• problems are patient-selected and targeted with individualized, flexible treatment plans.
Corbin and Strauss believe effective “self-managers” achieve competency in three areas:
• role management, which entails healthy adjustments to changes in role
responsibilities, expectations, and self-identity
• emotional management, which often is particularly challenging in psychiatric conditions because of the emotional disruption inherent in living with a psychiatric illness.6
Proficiency in these three “self-manager” domains is enhanced by mastering five key self-management skills outlined in Table 1.7
Successful intervention programs vary widely with regard to individual vs group formats, communication interface, and involved health professionals. However, evidence indicates that problem solving, decision making, and action planning are key components.7 Successful planning includes:
• detailed descriptions of what, how, when, and where the activity will be accomplished
• assessment of patient confidence and adjustment of plans if confidence is limited
• continuous monitoring and self-tailoring of plans through collaborative discussions with providers, fostering a spirit of partnership and ownership.
Table 2 illustrates elements of successful action plans in our Action Planning Worksheet. Adapted from the work of Scharzer,8 Prochaska,9 and Clark,10 we developed this self-management tool for individual and group treatment settings. It serves as a vehicle for collaborative patient-provider discussion and planning. The nuts-and-bolts nature of the discussions inevitably leads to learning new, important, and often unexpected information about our patients’ daily lives and the challenges they face as they share their dominant priorities, fears, and insecurities.
Patients provide consistently positive feedback about action planning, and clinicians often find that the process reveals fruitful areas for further psychotherapeutic intervention. Examples include identifying a range of negative automatic thoughts or catastrophic thinking impeding initiation of important activation, and exposure activities for depressed or anxious patients.
Knowing what to do is different than actually doing it. Changing behavior is difficult in the best circumstances, let alone with the strain of a chronic illness. It is critical to recognize that the presence of depressive symptoms significantly reduces the likelihood that patients will employ self-management practices.11 When combined with anxiety and the impairment of motivation and executive functioning that is common in psychiatric conditions, it is not surprising that patients with a mental health condition struggle to embrace ownership of their illness and engage in critical health behaviors—which may include adhering to medication regimens; maintaining a healthy sleep cycle, nutrition, and exercise routines; vigilant symptom surveillance; and carrying out an agreed-upon action plan.
Interventions
Professionally-guided “light-touch” interventions and technology-assisted self-management interventions also can improve patient engagement in activities through individual encounters, group forums, and technology-mediated exchanges, including telephone, email, text message, tele-health, and web-based interventions.13 The DE-STRESS (Delivery of Self Training and Education for Stressful Situations) model illustrates these principles. This 8-week program combines elements of face-to-face, email, telephone, and web-based assignments and exchanges, and demonstrates a decline in posttraumatic stress disorder, depression, and anxiety scores.14 Our Michigan Depression Outreach and Collaborative Care program is another example of a self-management intervention (Box 1).
• modeling and social persuasion through having patients observe and engage others as they struggle to overcome similar obstacles
• re-interpretation of symptoms aimed at fostering the belief that symptoms generally are multi-determined with several potential explanations, and vary with daily routines.
Helping patients understand when common symptoms such as impaired concentration or dizziness should be “watched”—rather than responded to aggressively—is crucial for effective long-term management.
Challenges
Health care delivery and medical educational models have been slow to embrace this change to long-term care models because doing so involves what might be uncomfortable shifts in roles and responsibilities. Effective care for long-term illness necessitates that the patient become an expert on his (her) illness, and be an active participant and partner in their treatment. Preparing health professionals for this new role as teacher, mentor, and collaborator presents a challenge to health care systems and educational programs across disciplines.
When trying to facilitate effective patient-provider partnerships, it is important to recognize the variability in patient preference for what and how information is shared, how decisions are made, and the role patients are asked to play in their care. Patients differ in their desire for an active or collaborative shared-decision model; some prefer more directive provider communication and a passive role.18 Preferences are influenced by variables such as age, sex, race, anxiety level, and education.19 Open discussion of these matters between caregivers and patients is important; studies have shown that failure to address these issues of “fit” can
impede communication, healthy behavior, and positive outcomes.
Bottom Line
Emerging care models demand that health care providers become teachers and motivators to help patients develop and implement patterns of health surveillance and intervention that will optimize their well-being and functionality. As active collaborators in their care, patients form a partnership with their care teams, allowing for regular, reciprocal exchange of information and shared decision-making. This shift to a partnership creates new, exciting roles and responsibilities for all parties.
Related Resources
- Improving Chronic Illness Care. www.improvingchroniccare.org.
- Chronic disease self-management program (Better Choices, Better Health workshop). Stanford School of Medicine. http://patienteducation.stanford.edu/programs/cdsmp.html.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Thomas E. Fluent, MD, talks about addressing patient resistance to a self-management model of care. Dr. Fluent is Clinical Assistant Professor, Department of Psychiatry, University of Michigan, Ann Arbor, Michigan.
Providing patients and their families with information and education about their psychiatric illness is a central tenet of mental health care. Discussions about diagnostic impressions, treatment options, and the risks and benefits of interventions are customary. Additionally, patients and families often receive written material or referral to other information sources, including self-help books and a growing number of online resources. Although patient education remains a useful and expected element of good care, there is evidence that, alone, it is insufficient to change health behaviors.1
A growing body of literature and clinical experience suggests that self-management strategies complement patient education and improve treatment outcomes for patients with chronic illnesses, including psychiatric conditions.2,3 The Cochrane Collaboration describes patient education as “teaching or training of patients concerning their own health needs,” and self-management as “the individual’s ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a long-term disorder.”4
In this article, we review:
• characteristics of long-term care models
• literature supporting the benefits of self-management programs
• clinical initiatives illustrating important elements of self-management support
• opportunities and challenges faced by clinicians, patients, families, clinics, and healthcare systems implementing self-management programs.
Principles of self-management
Chronic care models must account for conditions in which the clinical course can be variable, that are not amenable to a cure, and that demand long-term treatment. Optimal health outcomes rely on patients accurately monitoring, reporting, and responding to their symptoms, while engaging in critical health-related behaviors. In addition, clinicians must teach, partner with, and motivate patients to engage in crucial disease-management activities. Although typically not considered in this light, we believe most psychiatric disorders are best approached through a long-term care model, and benefit from self-management principles.
Basics of self-management include:
• patients must formulate goals and learn skills relevant to their disease
• problems are patient-selected and targeted with individualized, flexible treatment plans.
Corbin and Strauss believe effective “self-managers” achieve competency in three areas:
• role management, which entails healthy adjustments to changes in role
responsibilities, expectations, and self-identity
• emotional management, which often is particularly challenging in psychiatric conditions because of the emotional disruption inherent in living with a psychiatric illness.6
Proficiency in these three “self-manager” domains is enhanced by mastering five key self-management skills outlined in Table 1.7
Successful intervention programs vary widely with regard to individual vs group formats, communication interface, and involved health professionals. However, evidence indicates that problem solving, decision making, and action planning are key components.7 Successful planning includes:
• detailed descriptions of what, how, when, and where the activity will be accomplished
• assessment of patient confidence and adjustment of plans if confidence is limited
• continuous monitoring and self-tailoring of plans through collaborative discussions with providers, fostering a spirit of partnership and ownership.
Table 2 illustrates elements of successful action plans in our Action Planning Worksheet. Adapted from the work of Scharzer,8 Prochaska,9 and Clark,10 we developed this self-management tool for individual and group treatment settings. It serves as a vehicle for collaborative patient-provider discussion and planning. The nuts-and-bolts nature of the discussions inevitably leads to learning new, important, and often unexpected information about our patients’ daily lives and the challenges they face as they share their dominant priorities, fears, and insecurities.
Patients provide consistently positive feedback about action planning, and clinicians often find that the process reveals fruitful areas for further psychotherapeutic intervention. Examples include identifying a range of negative automatic thoughts or catastrophic thinking impeding initiation of important activation, and exposure activities for depressed or anxious patients.
Knowing what to do is different than actually doing it. Changing behavior is difficult in the best circumstances, let alone with the strain of a chronic illness. It is critical to recognize that the presence of depressive symptoms significantly reduces the likelihood that patients will employ self-management practices.11 When combined with anxiety and the impairment of motivation and executive functioning that is common in psychiatric conditions, it is not surprising that patients with a mental health condition struggle to embrace ownership of their illness and engage in critical health behaviors—which may include adhering to medication regimens; maintaining a healthy sleep cycle, nutrition, and exercise routines; vigilant symptom surveillance; and carrying out an agreed-upon action plan.
Interventions
Professionally-guided “light-touch” interventions and technology-assisted self-management interventions also can improve patient engagement in activities through individual encounters, group forums, and technology-mediated exchanges, including telephone, email, text message, tele-health, and web-based interventions.13 The DE-STRESS (Delivery of Self Training and Education for Stressful Situations) model illustrates these principles. This 8-week program combines elements of face-to-face, email, telephone, and web-based assignments and exchanges, and demonstrates a decline in posttraumatic stress disorder, depression, and anxiety scores.14 Our Michigan Depression Outreach and Collaborative Care program is another example of a self-management intervention (Box 1).
• modeling and social persuasion through having patients observe and engage others as they struggle to overcome similar obstacles
• re-interpretation of symptoms aimed at fostering the belief that symptoms generally are multi-determined with several potential explanations, and vary with daily routines.
Helping patients understand when common symptoms such as impaired concentration or dizziness should be “watched”—rather than responded to aggressively—is crucial for effective long-term management.
Challenges
Health care delivery and medical educational models have been slow to embrace this change to long-term care models because doing so involves what might be uncomfortable shifts in roles and responsibilities. Effective care for long-term illness necessitates that the patient become an expert on his (her) illness, and be an active participant and partner in their treatment. Preparing health professionals for this new role as teacher, mentor, and collaborator presents a challenge to health care systems and educational programs across disciplines.
When trying to facilitate effective patient-provider partnerships, it is important to recognize the variability in patient preference for what and how information is shared, how decisions are made, and the role patients are asked to play in their care. Patients differ in their desire for an active or collaborative shared-decision model; some prefer more directive provider communication and a passive role.18 Preferences are influenced by variables such as age, sex, race, anxiety level, and education.19 Open discussion of these matters between caregivers and patients is important; studies have shown that failure to address these issues of “fit” can
impede communication, healthy behavior, and positive outcomes.
Bottom Line
Emerging care models demand that health care providers become teachers and motivators to help patients develop and implement patterns of health surveillance and intervention that will optimize their well-being and functionality. As active collaborators in their care, patients form a partnership with their care teams, allowing for regular, reciprocal exchange of information and shared decision-making. This shift to a partnership creates new, exciting roles and responsibilities for all parties.
Related Resources
- Improving Chronic Illness Care. www.improvingchroniccare.org.
- Chronic disease self-management program (Better Choices, Better Health workshop). Stanford School of Medicine. http://patienteducation.stanford.edu/programs/cdsmp.html.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Thomas E. Fluent, MD, talks about addressing patient resistance to a self-management model of care. Dr. Fluent is Clinical Assistant Professor, Department of Psychiatry, University of Michigan, Ann Arbor, Michigan.
1. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469-2475.
2. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909-1914.
3. Druss BG, Zhao L, von Esenwein SA, et al. The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophr Res. 2010;118(1-3):264-270.
4. Tomkins S, Collins A. Promoting optimal self-care: consultation techniques that improve quality of life for patients and clinicians. London, United Kingdom: National Health Service; 2005.
5. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511-544.
6. Corbin JM, Strauss AL. Unending work and care : managing chronic illness at home. 1st ed. San Francisco, CA: Jossey-Bass Publishers; 1988.
7. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1-7.
8. Schwarzer R. Social-cognitive factors in changing health-related behaviors. Current Directions in Psychological Science. 2001;10(2):47-51.
9. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. Am Psycholt. 1992;47(9):1102-1114.
10. Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav. 2001; 28(6):769-782.
11. Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42(4):1443-1463.
12. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65(11):
1232-1245.
13. Tumur I, Kaltenthaler E, Ferriter M, et al. Computerised cognitive behaviour therapy for obsessive-compulsive disorder: a systematic review. Psychother Psychosom. 2007; 76(4):196-202.
14. Litz BT, Engel CC, Bryant RA, et al. A randomized, controlled proof-of-concept trial of an Internet-based, therapist-assisted self-management treatment for posttraumatic stress disorder. Am J Psychiatry. 2007;164(11):1676-1683.
15. Holman H, Lorig K. Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public Health Rep. 2004;119(3):239-243.
16. Williams A, Hagerty BM, Brasington SJ, et al. Stress Gym: feasibility of deploying a web-enhanced behavioral self-management program for stress in a military setting. Mil Med. 2010;175(7):487-493.
17. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790-804.
18. Davison BJ, Breckon E. Factors influencing treatment decision making and information p of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87(3):369-374.
19. Chewning B, Bylund CL, Shah B, et al. Patient p for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18.
1. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469-2475.
2. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909-1914.
3. Druss BG, Zhao L, von Esenwein SA, et al. The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophr Res. 2010;118(1-3):264-270.
4. Tomkins S, Collins A. Promoting optimal self-care: consultation techniques that improve quality of life for patients and clinicians. London, United Kingdom: National Health Service; 2005.
5. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511-544.
6. Corbin JM, Strauss AL. Unending work and care : managing chronic illness at home. 1st ed. San Francisco, CA: Jossey-Bass Publishers; 1988.
7. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1-7.
8. Schwarzer R. Social-cognitive factors in changing health-related behaviors. Current Directions in Psychological Science. 2001;10(2):47-51.
9. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. Am Psycholt. 1992;47(9):1102-1114.
10. Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav. 2001; 28(6):769-782.
11. Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42(4):1443-1463.
12. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65(11):
1232-1245.
13. Tumur I, Kaltenthaler E, Ferriter M, et al. Computerised cognitive behaviour therapy for obsessive-compulsive disorder: a systematic review. Psychother Psychosom. 2007; 76(4):196-202.
14. Litz BT, Engel CC, Bryant RA, et al. A randomized, controlled proof-of-concept trial of an Internet-based, therapist-assisted self-management treatment for posttraumatic stress disorder. Am J Psychiatry. 2007;164(11):1676-1683.
15. Holman H, Lorig K. Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public Health Rep. 2004;119(3):239-243.
16. Williams A, Hagerty BM, Brasington SJ, et al. Stress Gym: feasibility of deploying a web-enhanced behavioral self-management program for stress in a military setting. Mil Med. 2010;175(7):487-493.
17. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790-804.
18. Davison BJ, Breckon E. Factors influencing treatment decision making and information p of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87(3):369-374.
19. Chewning B, Bylund CL, Shah B, et al. Patient p for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18.
VTE Prevention Guidelines for Inpatients
Patients hospitalized for acute medical illness have more than a 10‐fold increased risk for venous thromboembolism (VTE),[1] with an undeniably dramatic, negative impact on the lives of those afflicted, including fatal pulmonary embolism (PE), which most commonly affects patients on the medical service.[2, 3, 4] Yet estimates for the overall rate of VTE in this population are relatively low, raising questions about which subsets of medical patients warrant the risk and cost of prophylaxis.
Recently, the American College of Physicians published guidelines (ACP‐1)[5] and a supporting review[6] addressing VTE prophylaxis in nonsurgical inpatients, followed by publication of the American College of Chest Physicians (ACCP) 9th Edition of the Chest Guidelines on Antithrombotic Therapy and Prevention of Thrombosis (AT9),[7] which divides VTE prevention into 3 articles,[8, 9, 10] including 1 on nonsurgical patients.[8] Both ACP‐1 and AT9 differ significantly from the 2008 ACCP guidelines (AT8),[11] but took different approaches to methodology, risk assessment, and several other aspects of thromboprophylaxis (Table 1). This narrative review summarizes and compares these recommendations and the methods used to arrive at them, with a final section focusing on implications for improvement teams designing order sets and system changes to address VTE prophylaxis.
| 2008 ACCP VTE Guideline AT8 | 2012 ACCP VTE Guideline AT9 | 2011 ACP Guideline | |
|---|---|---|---|
| |||
| Stance on asymptomatic VTE end points | Because of the strong concordance between asymptomatic DVT and clinically important VTE, we believe that DVT detected by a sensitive screening tesis an appropriate outcome in the early assessment of new thromboprophylaxis interventions. | Use of this surrogate (asymptomatic, screening‐detected thrombosis) creates major problems in making the trade‐off between patient‐important outcomes (thrombosis and serious bleeding). | Surrogate outcomes of asymptomatic screening detected‐thrombosis should not be used. |
| Who should be prophylaxed? | 6.0.0: For acutely ill medical patients admitted to hospital with congestive heart failure or severe respiratory disease, or who are confined to bed and have one or more additional risk factors, including active cancer, previous VTE, sepsis, neurologic disease, or inflammatory bowel disease, we recommend thromboprophylaxis with LMWH (1A), UFH (1A), or fondaparinux (1A). | 2.3: For acutely ill hospitalized medical patients at increased risk for thrombosis, we recommend anticoagulant thromboprophylaxis, with LMWH, UFH bid, UFH tid, or fondaparinux (1B). | ACP recommends pharmacologic prophylaxis with heparin or a related drug for venous thromboembolism in medical (including stroke) patients unless the assessed risk for bleeding outweighs the likely benefits (grade: strong recommendation, moderate‐quality evidence). |
| 2.4: For acutely ill hospitalized medical patients at low risk of thrombosis, we recommend against the use of pharmacologic or mechanical prophylaxis. (1B) | |||
| Choice of anticoagulant prophylaxis | There is no compelling evidence that UFH should be administered three times daily in preference to twice daily in medical patients, although these two regimens have never been directly compared. | In choosing the specific anticoagulant drug to be used for pharmacoprophylaxis, choices should be based on patient preference, compliance, and ease of administration (eg, daily vs bid vs tid dosing), as well as on local factors affecting acquisition costs. | [T]he choice of agent for prophylaxis of VTE should be based on ease of use, adverse effect profile, and cost of medication. |
| No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | |
| Mechanical prophylaxis | 1.4.3.1: We recommend that mechanical methods of prophylaxis be used primarily in patients at high risk of bleeding (grade 1A), or possibly as an adjunct to anticoagulant‐based thromboprophylaxis (grade 2A). | 2.7.2: For acutely ill hospitalized medical patients at increased risk of thrombosis who are bleeding or at high risk for major bleeding, we suggest the optimal use of mechanical thromboprophylaxis with GCS (grade 2C) or IPC (grade 2C). | ACP recommends against the use of mechanical prophylaxis with graduated compression stockings for prevention of venous thromboembolism (grade: strong recommendation, moderate‐quality evidence). |
| No strong evidence for IPC vs GCS | |||
| Duration | [T]he optimal duration of thromboprophylaxis remains unclear. To the end of hospitalization for most patients. | 2.8: [W]e suggest against extending the duration of thromboprophylaxis beyond the period of patient immobilization or acute hospital stay (2B). | The optimal duration of heparin prophylaxis is uncertain. |
| Risk Stratification | The approach of individual prophylaxis prescribing based on formal RAMs is not used routinely by most clinicians because it has not been adequately validated and is cumbersome. Individual RAMs may not be worth the effort, because there are only a limited number of thromboprophylaxis options, and one of the principles of effective thromboprophylaxis is to reduce complexity in decision making. | (Noncritical care) No formal risk assessment recommendation. Padua point‐based model is inherent in definitions of baseline VTE risk. | ACP does not support the application of performance measures in medical (including stroke) patients that promotes universal venous thromboembolism prophylaxis regardless of risk. |
| Another approach involves implementation of group‐specific thromboprophylaxis routinely for all patients who belong to each of the major target groups. We support this approach. | There are no validated risk assessment models to stratify VTE risk in critically ill patients. | Many risk assessment tools are available for estimating thromboembolism risk, but the current evidence is insufficient to recommend a validated tool. | |
| [T]he decision is best left to physician judgment, and performance measures targeting all patients are inappropriate. | |||
WHY ARE THE NEW GUIDELINES DIFFERENT?
Major randomized controlled trials (RCTs)[12, 13, 14] of thromboprophylaxis used routine deep vein thrombosis (DVT) surveillance and included both symptomatic (S‐VTE) and asymptomatic VTE (A‐VTE) end points. These studies consistently demonstrated 44% to 63% reductions in VTE without increases in major bleeding.[11] Because of the strong relationship between A‐VTE and S‐VTE outcomes, and a paucity of studies using only S‐VTE outcomes, AT8 judged that A‐VTE outcomes were valid to include, whereas the new guidelines reject the use of asymptomatic VTE end points.[5, 8, 15] To minimize financial and intellectual conflicts of interest, AT9 also used methodologists rather than VTE experts as topic editors, excluded conflicted experts from voting on recommendations, and attempted to estimate patient values and preferences.[15] As a result, AT9 makes fewer strong recommendations (182 1A recommendations in 2008, but only 29 in 2012), replacing them with weak suggestions.
WHAT DO THE NEW GUIDELINES RECOMMEND?
AT8 recommended anticoagulant prophylaxis for acutely ill medical inpatients with known risk factors, but did not recommend routine thromboprophylaxis. However, because of well‐known problems with underprophylaxis,[16, 17, 18, 19] particularly in medical patients, the low risk of bleeding, and difficulties with explicitly defining low‐risk patients, many discounted the need for VTE risk stratification.
Both new guidelines recommend prophylaxis for many nonsurgical patients, but discourage routine thromboprophylaxis for nonsurgical inpatients. AT9 specifically recommends against any thromboprophylaxis for low‐risk medical inpatients, implying that many nonsurgical, non‐critical care patients belong in this category, citing lower estimates of benefit, lower estimates of VTE risk, and potential bleeding risks.
The guidelines[5, 8] agree that, when indicated and absent contraindications, anticoagulant prophylaxis is preferred over mechanical prophylaxis, and agree there is insufficient evidence to recommend 1 anticoagulant over another.
For patients at risk of both VTE and bleeding, ACP‐1 states that intermittent pneumatic compression (IPC) devices are a reasonable option, given the evidence showing benefit in surgical patients. However, ACP‐1 recommends against graduated compression stockings (GCS) in nonsurgical patients based on a meta‐analysis dominated by the CLOTS‐1 (Clots in Legs Or sTockings after Stroke) trial, which found that thigh‐high GCS increased the risk of skin breakdown without reducing VTE[20] in immobilized stroke patients. AT9 does not recommend against GCS for patients facing bleeding and VTE risk. AT9 notes the hazards of generalizing results from stroke patients, and also considers the somewhat contradictory results from the CLOTS‐2 trial in stroke patients, which found a lower rate of VTE with thigh‐high GCS than with knee‐high GCS.[21] AT9 designates a recommendation of 2C for either IPC devices or thigh‐high GCS for those at VTE risk when anticoagulants are contraindicated.
Combination mechanical‐pharmacologic prophylaxis has proven superior in some surgical populations, and many hospitals use combined prophylaxis in high‐risk medical patients. However, combination prophylaxis has not been studied in this population. ACP‐1 does not comment on the practice; AT9 does not recommend for or against it. Institutions that use combination prophylaxis should be aware that although it may seem logical to extrapolate estimates of benefit seen in selected surgical patients, this is not a recommended practice.
RCTs for thromboprophylaxis in nonsurgical inpatients provided prophylaxis for 6 to 21 days. Neither ACP‐1 or AT9 recommend routinely extending prophylaxis beyond the hospital stay, citing an RCT[22] in which the benefit of extended duration low molecular weight heparin was limited to selected subsets of patients and offset by bleeding complications. AT9 suggests prophylaxis for 6 to 21 days, until full mobility is restored, or until dischargewhichever comes first.[8] However, we know of no study that establishes a mobility level at which prophylaxis can be safely discontinued, especially in inpatients with multiple risk factors.
ESTIMATING RISK AND BENEFIT OF PROPHYLAXIS AND LIMITATIONS OF METHODS
Calculating risk/benefit ratios for thromboprophylaxis requires estimates of baseline VTE and bleeding risks, and estimates of the impact of prophylaxis on those baseline risks. Methods to estimate the impact of prophylaxis on S‐VTE from studies relying on A‐VTE all have limitations, as acknowledged by the AT9 introduction.[15]
The ACP‐1 review found the only significant effect of prophylaxis on medical inpatients was a modest reduction in PE and a modest increase in total bleeding events, without effects on major bleeding, DVT, or mortality.[6] The authors summarized the findings as indicative of little or no net benefit for the medical population as a whole. The ACP‐1 review derives estimates of S‐VTE risk, bleeding, and mortality from control (baseline) and interventional arms of RCTs that used routine VTE screening, and included A‐VTE end points. The baseline risk of VTE could potentially be overestimated, because the populations in the trials are not representative of the entire medical population.
On the other hand, pooling trials with screening‐detected VTE to estimate S‐VTE outcomes is a questionable practice that may falsely lower estimates of VTE prophylaxis benefit. Screening‐detected VTE may be treated or declared a study end point before it becomes symptomatic. MEDENOX (Medical Patients With Enoxaparin) is an illustrative example.[12] The 263 placebo recipients suffered 37 A‐VTEs and 4 S‐VTEs. The 272 enoxaparin recipients suffered 17 A‐VTEs and 3 S‐VTEs. Patients at the highest risk of S‐VTE were counted as reaching an end point before they could develop symptoms; this happened more than twice as often in the placebo arm. This decreases both estimates of baseline VTE risk and the measured benefit of prophylaxis for S‐VTE. Screening could conceivably reduce measured effects on mortality as well, because patients begin VTE therapy earlier. Per ACP‐1, the estimated risk for DVT is lower than for PE, running counter to literature experience[8, 16] and raising issues of face validity. The ACP‐1 review accepts all original definitions of major bleeding, including a 2 g/dL drop in hemoglobin,[12] which commonly occurs without any bleeding or clinical consequence, and bleeding events were ascribed to heparins up to 120 days after randomization, long after they could have been responsible.
Previous meta‐analyses of thromboprophylaxis studies[23, 24] shared many of these same limitations, but did not ascribe bleeding complications to heparins for this extended duration, and had point estimates that suggested a larger impact from prophylaxis than ACP‐1. Dentali et al., for example, showed statistically significant impact on PE (relative risk [RR] 0.43), fatal PE (RR 0.38), and a nearly statistically significant large impact on DVT (RR 0.47, 95% confidence interval [CI]: 0.22‐1.00),[24] whereas ACP‐1 estimated a smaller significant impact on PE (RR 0.69), no significant difference in fatal PE, and a much smaller estimate of the impact on DVT (RR 0.78, 95% CI: 0.45‐1.35) (Table 2).
| Baseline Risk | Relative Effect (95% CI) | Absolute Effect per 1000 Patients Treated (95% CI) | |
|---|---|---|---|
| |||
| ACP guideline review (Lederle), UFH or LMWH vs placebo/no treatment, medical patients | |||
| Mortality | 6.6 | OR 0.94 (0.84‐1.04) | 4 fewer (11 fewer to 3 more) |
| Major bleeding | 0.25 | OR 1.49 (0.91‐2.43) | 1 more (no effect to 3 more) |
| Symptomatic DVT | 0.96 | OR 0.78 (0.45‐1.35) | 2 fewer (6 fewer to 4 more) |
| PE | 1.2 | OR 0.69 (0.52‐0.90) | 4 fewer (6 fewer to 1 fewer) |
| Fatal PE | 0.30 | OR 0.77 (0.43‐1.37) | 1 fewer (2 fewer to 1 more) |
| ACCP AT9 (Kahn), non‐critical care medical inpatients, anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Mortality | 4.5 | OR 0.97 (0.79‐1.19) | 1 fewer (9 fewer to 8 more) |
| Major bleeding | 0.40 | OR 1.32 (0.73‐2.37) | 1 more (1 fewer to 6 more) |
| Thrombocytopenia | 0.13 | OR 0.92 (0.54‐1.53) | 1 fewer (6 fewer to 7 more) |
| Symptomatic DVT | |||
| Padua score <4 | 0.2 | RR 0.47 (0.22‐1) | 1 fewer (1 fewer to no effect) |
| Padua score 4 | 6.7 | 34 fewer (51 fewer to no effect) | |
| ACCP AT9 (Kahn) non‐critical care medical inpatients, Anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Nonfatal PE | |||
| Padua score 4 | 0.2 | RR 0.61 (0.23‐1.67) | 1 fewer (1 fewer to 1 more) |
| Padua score 4 | 3.9 | 15 fewer (30 fewer to 36 more) | |
| Fatal PE | 0.4 | RR 0.41 (0.22‐0.76) | 2 fewer (1 fewer to 3 fewer) |
| ACCP AT9 (Kahn), critical care medical inpatients, any heparin (LMWH, UFH) vs placebo/no treatment) | |||
| Mortality | 9.4 | RR 1.01 (0.04‐2.57) | 1 more (56 fewer to 148 more) |
| Major bleeding | 2.7 | RR 2.09 (0.54‐8.16) | 29 more (12 fewer to 190 more) |
| Symptomatic DVT | 5.8 | RR 0.86 (0.59‐1.25) | 4 fewer (12 fewer to 8 more) |
| Pulmonary embolus | 4.2 | RR, 0.73 (0.26‐2.11) | 11 fewer (31 fewer to 47 more) |
AT9 used a variety of methods to estimate each component of the risk/benefit equation. Critical care and non‐critical care estimates were generated independently, but because of limited data, the critical care estimates were highly imprecise. In non‐critical care patients, as in ACP‐1, treatment effects were estimated from RCTs that routinely screened for A‐VTE, and they adapted the Dentali et al. estimate of DVT risk reduction. The baseline risk for bleeding and mortality were derived from the control population of the same meta‐analysis.[24]
Using a novel approach, AT9 estimated baseline nonsurgical VTE risk from a prospective observational cohort study of 1180 medical inpatients divided into high‐ and low‐risk groups by a point‐scoring system.[25] Deriving risk estimates from an observational cohort has theoretical advantages. Many patients did not receive prophylaxis, allowing for unadjusted risk estimates; they represented a cross‐section of medical inpatients rather than a selected trial population, and risk estimates were not reduced by the culling of screen‐detected A‐VTE.
The Padua risk‐assessment model (RAM) (Table 3) defines high VTE risk as a cumulative score 4. There were 60.3% of patients at low risk and 39.7% at high risk using this threshold. Among unprophylaxed patients, VTE occurred in 11% of high‐risk patients versus 0.3% of low‐risk patients (hazard ratio 32.0, 95% CI: 4.1251.0).
| Baseline Features | Score |
|---|---|
| |
| Active cancer* | 3 |
| Previous VTE (excluding superficial thrombosis) | 3 |
| Reduced mobility | 3 |
| Already known thrombophilic condition | 3 |
| Recent (1 month) trauma and/or surgery | 2 |
| Elderly age (70 years) | 1 |
| Heart and/or respiratory failure | 1 |
| Acute myocardial infarction or stroke | 1 |
| Acute infection and/or rheumatologic disorder | 1 |
| Obesity (BMI 30) | 1 |
| Ongoing hormonal treatment | 1 |
PADUA: A CLOSER LOOK
In the Padua study, 60% of the population appeared to be at such low risk for VTE that prophylaxis would seem unnecessary, but closer scrutiny should raise concern about generalizing these results. Of the 711 low‐risk patients, <1% were immobile, only 6% had cancer, 6% were obese, and only 12% had any acute infection or inflammatory condition, yet their mean length of stay was 7.9 days. These characteristics do not apply to 60% of American inpatients. Furthermore, 964 of 2208 eligible patients (44%) were excluded because they required therapeutic anticoagulation.[25]
Correspondence with the authors revealed that the 2 PEs in patients with Padua scores 4 occurred among 192 patients with a risk score of 3 (Figure 1), a 1% (2/192) risk of PE. This is a very small sample, and the true risk of VTE for medical inpatients with a risk score of 3 may be lower or significantly higher. In the Padua population, a risk score of 3 equated to a VTE risk of 6.9%, whereas those with a score of 0 to 2 had no VTE. For those adapting the Padua model, careful consideration of using a cutoff of 3, versus 4, is warranted.
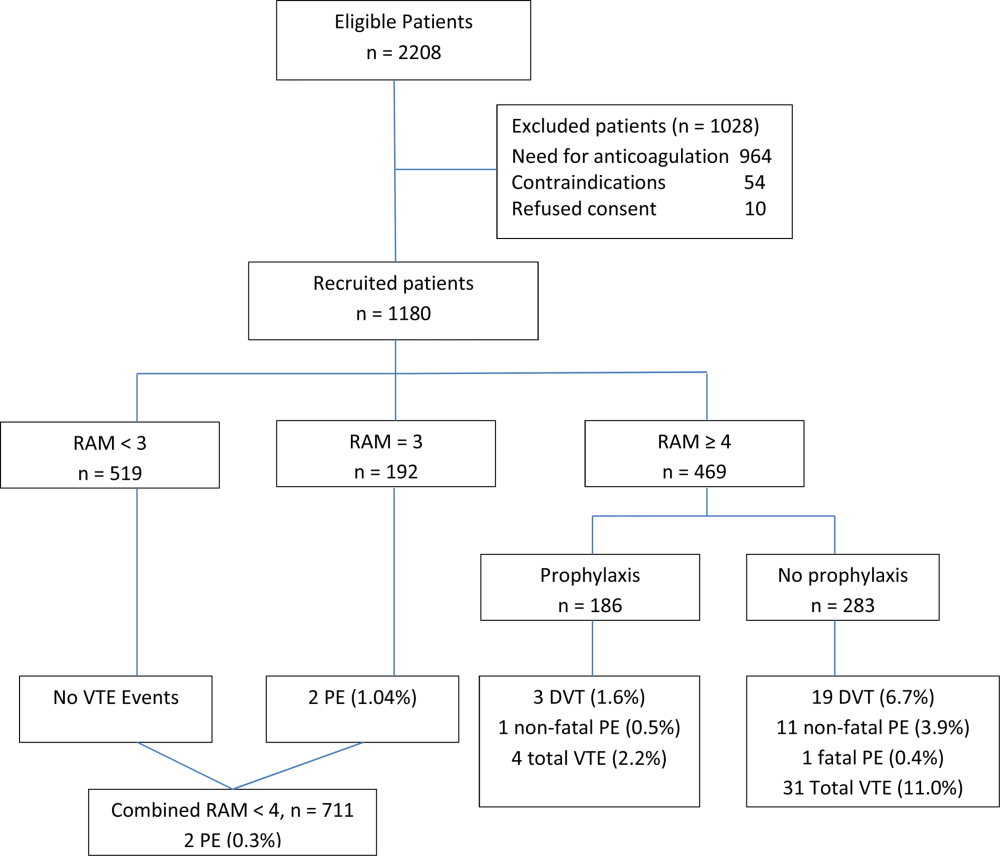
IMPLICATIONS FOR VTE PROTOCOL IMPLEMENTATION AND IMPROVEMENT TEAMS
AT9 and ACP‐1 sought to focus on S‐VTE, remove bias from recommendations, and highlight potential risks of unnecessary prophylaxis in low‐risk patients. They have largely succeeded in these important goals. However, the complexity of the new guidelines and lack of consensus about VTE risk assessment pose significant challenges to improvement teams tasked with implementing the guidelines in real‐world settings.
CHOOSING A VTE RAM
The fundamental question is: How can hospitals assess VTE risk, assure adequate prophylaxis for patients who need it, while minimizing excess prophylaxis, in a practical, efficient way?
Approach 1: Opt Out Approach
Both guidelines discourage universal prophylaxis for inpatients without contraindications unless the physician opts out. Although the simplicity of this approach is appealing, the low rate of VTE in a substantial segment of the medical inpatient population and known risks of thromboprophylaxis make this strategy suboptimal.
Approach 2: No VTE RAM
ACP‐1 notes that evidence is not sufficient to recommend 1 RAM over another, and essentially advises leaving prophylaxis decisions up to an individual physician's judgment. Although the evidence may not prove which system is best, prophylaxis reliability is dismal when there is no system or when hospitals offer prophylaxis options without guidance.[26, 27] Widespread, well‐documented underprophylaxis[16, 17, 18, 19] is largely the result of relying on unguided physician judgment and relatively passive interventions like educational sessions and pocket cards.[8] This approach also deprives improvement teams of standard definitions of VTE risk, bleeding risk, and adequate prophylaxis necessary to measure and improve VTE prophylaxis. Because of significant gray areas in the literature and varied infrastructure, institutions will not implement identical VTE prevention programs, but institutional standardization remains a cornerstone of improvement.
Approach 3: Buckets of Risk
The AT8 approach to risk assessment was to place patients into VTE risk groups described in the text, rather than have an individualized point‐scoring system.[11] These assessments can be made in seconds with high levels of interobserver agreement, implemented without undue effort, and spur high levels of compliance.[28, 29] Most importantly, implementation was associated with a 40% reduction in hospital‐associated VTE (RR 0.69, 95% CI: 0.470.79) without detectable increases in bleeding or heparin‐induced thrombocytopenia. Although this strategy has not been tested in randomized trials, it has been replicated in multiple real‐world settings that avoid concerns about generalizability due to imperfect trial populations.[28, 30]
The most popular bucket model in common use, derived from a table in the AT8 guidelines, is similar to models presented in UK National Institute for Health and Care Excellence guidelines for medical inpatients.[31] These models are potentially less precise than point‐based systems, but offer simplicity, ease of use, and improved physician acceptance, and thus may be more effective than point‐based models in settings without advanced clinical decision support. The models are flexible to reflect greater or lesser degrees of aggressiveness in defining risk categories, and can be used to approximate some point‐based systems.
Approach 4: Individualized Point‐Based RAM
AT9 authors used the Padua VTE RAM to define low‐ and high‐risk patients for VTE in their recommendation for medical inpatients. The Padua model appears relatively simple, but it does require calculations, and there is a paucity of data for implementation experience with it. As mentioned above, if teams use the Padua model, the optimal cutoff (3 vs 4) for recommending prophylaxis is uncertain, and both should be considered.
The Caprini point‐based system is not mentioned in the guideline for thromboprophylaxis in medical inpatients, but in our collaborative improvement experience, it is perhaps the most commonly used point‐based model for medical inpatients.[28, 30] It is also embedded in AT9 recommendations for prophylaxis in the nonorthopedic surgical population,[9] and thus is tempting to use for both medical and surgical patients. There are several caveats to those considering the use of these more complex point‐based models. Complex point‐based RAM suffer from poor interobserver agreement.[32] They have also had limited ability to exclude low‐risk patients from prophylaxis in validation studies,[33] and have not been tested extensively in medical populations. Although AT9 considers the Caprini RAM relatively easy to use,[9] our experience in collaboratives suggests that for many hospitals, the model is too complex to be used reliably.[28, 30] Clinicians often simply bypass the clinical decision support offered in the tool, rather than checking off all risk factors, adding up the point total, and identifying the appropriate prophylaxis choices based on the point total.[28] Other point‐based RAM (reviewed elsewhere[34, 35, 36]) pose similar implementation challenges.
On the other hand, centers with more sophisticated clinical decision support and a strong improvement framework can overcome some of these challenges to get good results with complex point‐based models. A forcing function can ensure that practitioners complete all risk‐assessment tasks. Providers can check off the VTE risk factors and bleeding risk factors on 1 screen, and several factors like age, body mass index, and renal function can be autopopulated. Instead of asking the provider to add up points, the combination of answers checked off on the first screen can drive behind‐the‐scenes calculations and seamlessly lead providers to prophylaxis choices appropriate for that combination of VTE and bleeding risks. Customized models can be designed for a wide variety of services. Similar strategies can ease adaption with more complex qualitative models as well.[37]
BOTTOM LINE IN CHOOSING A VTE RAM
Many medical inpatients are at high risk for VTE, but others are not at sufficient risk to warrant prophylaxis. VTE risk assessment should be embedded in admission, transfer, and perioperative order sets and may need a hard stop to insure completion. There is a trend to favor individualized point‐based models over models that place patients in groups of risk, but evidence is insufficient to recommend 1 type of RAM over another, and more complex point‐based models often require extensive local customization and algorithmic clinical decision support to effectively implement them. Centers without advanced capability may find the bucket models more effective. We urge improvement teams to trial their RAM with common patient case scenarios, and to make a choice based on an effort‐benefit analysis, feedback from their clinicians, and the level of customization in clinical decision support available to them.
OTHER IMPLEMENTATION STRATEGIES
VTE and bleeding risk change during hospitalization. We have used ongoing daily surveillance and measurement of patients on no prophylaxis to prompt concurrent intervention (ie, measure‐vention) to increase prophylaxis for patients at risk.[28] Improvement teams should focus not only on increasing prophylaxis for those at risk, but should also use measure‐vention, checklists, or other techniques to identify low‐risk (eg, ambulating) patients for cessation of overly aggressive prophylaxis. Efforts to improve early progressive ambulation, limit central venous catheters to those who truly need them, and improve adherence to mechanical prophylaxis can also reduce VTE, as well as benefitting patient populations in other ways.
We recognize there are several approaches to close the implementation gap in delivering thromboprophylaxis judiciously but reliably, and encourage research and publication of varied strategies. Last, we hope efforts to limit unnecessary prophylaxis and challenges inherent in implementing new and complex guidelines do not increase the morbidity and mortality of hospital‐acquired VTE, by derailing the delivery of prophylaxis to those in whom the benefits outweigh the risks.
Disclosures: Dr. Merli has conducted research for Johnson & Johnson, Bristol Myers Squibb, and Portala Scientific and has been a consultant for Johnson & Johnson and Bristol Myers Squibb.
- US Department of Health and Human Services. Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. 2008. Available at: http://www.surgeongeneral.gov/topics/deepvein/index.html. Accessed January 29, 2013.
- , , , et al. Incidence of venous thromboembolism in hospitalized patients vs. community residents. Mayo Clin Proc. 2001;76:1102–1110.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815.
- , , . New onset of venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118(6):1680–1684.
- , , , , . venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625–632.
- , , , . Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , et al. Prevention of VTE in nonsurgical patients. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Prevention of VTE in nonorthopedic surgical patients. Chest. 2012;141(2 suppl):e227S–e277S.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S–453S.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- , , , et al. The outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registry. J Thromb Haemost. 2004;2:1892–1898.
- , , , et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936–945.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387–394.
- , , , et al.; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost. 2010;103(4):736–748.
- , , , et al; CLOTS Trials Collaboration. Effectiveness of thigh‐length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomized controlled trial. Lancet. 2009;373(9679):1958–1965.
- CLOTS (Clots in Legs Or sTockings after Stroke) Trial Collaboration. Thigh‐length versus below‐knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med. 2010;153(9):553–562.
- , , , et al. Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
- , , , , . Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2007;167(1)476–486.
- , , , , . Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007; 46(4):278–288.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , . Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes. J Hosp Med. 2009;4(2):81–89.
- . Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? J Hosp Med. 2009;4(2):77–80.
- , . Designing and implementing effective VTE prevention protocols: lessons from collaboratives. J Thromb Thrombolysis. 2010;29(2):159–166.
- , , , et al. Optimizing prevention of hospital acquired venous thromboembolism: prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10–18.
- , , , et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38(7):301–310.
- NHS National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. NICE Clinical Guideline 92. 2010. Available at: http://www.nice.org.uk/guidance/CG92. Accessed April 18, 2013.
- , , , , . Reliability of a point‐based VTE risk assessment tool in the hands of medical residents. J Hosp Med. 2011;6:195–201.
- , , , , , . A validation of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251(2):344–350.
- , , , . Risk assessment models for thromboprophylaxis of medical patients. Thromb Res. 2012;129:127–132.
- , , , , . Risk‐assessment models for predicting venous thromboembolism among hospitalized non‐surgical patients: a systematic review. J Thromb Thrombolysis. 2013;35:67–80.
- , , . The use of weighted and scored risk assessment models for venous thromboembolism. Thromb Haemost. 2012;108(6):1072–1076.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
Patients hospitalized for acute medical illness have more than a 10‐fold increased risk for venous thromboembolism (VTE),[1] with an undeniably dramatic, negative impact on the lives of those afflicted, including fatal pulmonary embolism (PE), which most commonly affects patients on the medical service.[2, 3, 4] Yet estimates for the overall rate of VTE in this population are relatively low, raising questions about which subsets of medical patients warrant the risk and cost of prophylaxis.
Recently, the American College of Physicians published guidelines (ACP‐1)[5] and a supporting review[6] addressing VTE prophylaxis in nonsurgical inpatients, followed by publication of the American College of Chest Physicians (ACCP) 9th Edition of the Chest Guidelines on Antithrombotic Therapy and Prevention of Thrombosis (AT9),[7] which divides VTE prevention into 3 articles,[8, 9, 10] including 1 on nonsurgical patients.[8] Both ACP‐1 and AT9 differ significantly from the 2008 ACCP guidelines (AT8),[11] but took different approaches to methodology, risk assessment, and several other aspects of thromboprophylaxis (Table 1). This narrative review summarizes and compares these recommendations and the methods used to arrive at them, with a final section focusing on implications for improvement teams designing order sets and system changes to address VTE prophylaxis.
| 2008 ACCP VTE Guideline AT8 | 2012 ACCP VTE Guideline AT9 | 2011 ACP Guideline | |
|---|---|---|---|
| |||
| Stance on asymptomatic VTE end points | Because of the strong concordance between asymptomatic DVT and clinically important VTE, we believe that DVT detected by a sensitive screening tesis an appropriate outcome in the early assessment of new thromboprophylaxis interventions. | Use of this surrogate (asymptomatic, screening‐detected thrombosis) creates major problems in making the trade‐off between patient‐important outcomes (thrombosis and serious bleeding). | Surrogate outcomes of asymptomatic screening detected‐thrombosis should not be used. |
| Who should be prophylaxed? | 6.0.0: For acutely ill medical patients admitted to hospital with congestive heart failure or severe respiratory disease, or who are confined to bed and have one or more additional risk factors, including active cancer, previous VTE, sepsis, neurologic disease, or inflammatory bowel disease, we recommend thromboprophylaxis with LMWH (1A), UFH (1A), or fondaparinux (1A). | 2.3: For acutely ill hospitalized medical patients at increased risk for thrombosis, we recommend anticoagulant thromboprophylaxis, with LMWH, UFH bid, UFH tid, or fondaparinux (1B). | ACP recommends pharmacologic prophylaxis with heparin or a related drug for venous thromboembolism in medical (including stroke) patients unless the assessed risk for bleeding outweighs the likely benefits (grade: strong recommendation, moderate‐quality evidence). |
| 2.4: For acutely ill hospitalized medical patients at low risk of thrombosis, we recommend against the use of pharmacologic or mechanical prophylaxis. (1B) | |||
| Choice of anticoagulant prophylaxis | There is no compelling evidence that UFH should be administered three times daily in preference to twice daily in medical patients, although these two regimens have never been directly compared. | In choosing the specific anticoagulant drug to be used for pharmacoprophylaxis, choices should be based on patient preference, compliance, and ease of administration (eg, daily vs bid vs tid dosing), as well as on local factors affecting acquisition costs. | [T]he choice of agent for prophylaxis of VTE should be based on ease of use, adverse effect profile, and cost of medication. |
| No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | |
| Mechanical prophylaxis | 1.4.3.1: We recommend that mechanical methods of prophylaxis be used primarily in patients at high risk of bleeding (grade 1A), or possibly as an adjunct to anticoagulant‐based thromboprophylaxis (grade 2A). | 2.7.2: For acutely ill hospitalized medical patients at increased risk of thrombosis who are bleeding or at high risk for major bleeding, we suggest the optimal use of mechanical thromboprophylaxis with GCS (grade 2C) or IPC (grade 2C). | ACP recommends against the use of mechanical prophylaxis with graduated compression stockings for prevention of venous thromboembolism (grade: strong recommendation, moderate‐quality evidence). |
| No strong evidence for IPC vs GCS | |||
| Duration | [T]he optimal duration of thromboprophylaxis remains unclear. To the end of hospitalization for most patients. | 2.8: [W]e suggest against extending the duration of thromboprophylaxis beyond the period of patient immobilization or acute hospital stay (2B). | The optimal duration of heparin prophylaxis is uncertain. |
| Risk Stratification | The approach of individual prophylaxis prescribing based on formal RAMs is not used routinely by most clinicians because it has not been adequately validated and is cumbersome. Individual RAMs may not be worth the effort, because there are only a limited number of thromboprophylaxis options, and one of the principles of effective thromboprophylaxis is to reduce complexity in decision making. | (Noncritical care) No formal risk assessment recommendation. Padua point‐based model is inherent in definitions of baseline VTE risk. | ACP does not support the application of performance measures in medical (including stroke) patients that promotes universal venous thromboembolism prophylaxis regardless of risk. |
| Another approach involves implementation of group‐specific thromboprophylaxis routinely for all patients who belong to each of the major target groups. We support this approach. | There are no validated risk assessment models to stratify VTE risk in critically ill patients. | Many risk assessment tools are available for estimating thromboembolism risk, but the current evidence is insufficient to recommend a validated tool. | |
| [T]he decision is best left to physician judgment, and performance measures targeting all patients are inappropriate. | |||
WHY ARE THE NEW GUIDELINES DIFFERENT?
Major randomized controlled trials (RCTs)[12, 13, 14] of thromboprophylaxis used routine deep vein thrombosis (DVT) surveillance and included both symptomatic (S‐VTE) and asymptomatic VTE (A‐VTE) end points. These studies consistently demonstrated 44% to 63% reductions in VTE without increases in major bleeding.[11] Because of the strong relationship between A‐VTE and S‐VTE outcomes, and a paucity of studies using only S‐VTE outcomes, AT8 judged that A‐VTE outcomes were valid to include, whereas the new guidelines reject the use of asymptomatic VTE end points.[5, 8, 15] To minimize financial and intellectual conflicts of interest, AT9 also used methodologists rather than VTE experts as topic editors, excluded conflicted experts from voting on recommendations, and attempted to estimate patient values and preferences.[15] As a result, AT9 makes fewer strong recommendations (182 1A recommendations in 2008, but only 29 in 2012), replacing them with weak suggestions.
WHAT DO THE NEW GUIDELINES RECOMMEND?
AT8 recommended anticoagulant prophylaxis for acutely ill medical inpatients with known risk factors, but did not recommend routine thromboprophylaxis. However, because of well‐known problems with underprophylaxis,[16, 17, 18, 19] particularly in medical patients, the low risk of bleeding, and difficulties with explicitly defining low‐risk patients, many discounted the need for VTE risk stratification.
Both new guidelines recommend prophylaxis for many nonsurgical patients, but discourage routine thromboprophylaxis for nonsurgical inpatients. AT9 specifically recommends against any thromboprophylaxis for low‐risk medical inpatients, implying that many nonsurgical, non‐critical care patients belong in this category, citing lower estimates of benefit, lower estimates of VTE risk, and potential bleeding risks.
The guidelines[5, 8] agree that, when indicated and absent contraindications, anticoagulant prophylaxis is preferred over mechanical prophylaxis, and agree there is insufficient evidence to recommend 1 anticoagulant over another.
For patients at risk of both VTE and bleeding, ACP‐1 states that intermittent pneumatic compression (IPC) devices are a reasonable option, given the evidence showing benefit in surgical patients. However, ACP‐1 recommends against graduated compression stockings (GCS) in nonsurgical patients based on a meta‐analysis dominated by the CLOTS‐1 (Clots in Legs Or sTockings after Stroke) trial, which found that thigh‐high GCS increased the risk of skin breakdown without reducing VTE[20] in immobilized stroke patients. AT9 does not recommend against GCS for patients facing bleeding and VTE risk. AT9 notes the hazards of generalizing results from stroke patients, and also considers the somewhat contradictory results from the CLOTS‐2 trial in stroke patients, which found a lower rate of VTE with thigh‐high GCS than with knee‐high GCS.[21] AT9 designates a recommendation of 2C for either IPC devices or thigh‐high GCS for those at VTE risk when anticoagulants are contraindicated.
Combination mechanical‐pharmacologic prophylaxis has proven superior in some surgical populations, and many hospitals use combined prophylaxis in high‐risk medical patients. However, combination prophylaxis has not been studied in this population. ACP‐1 does not comment on the practice; AT9 does not recommend for or against it. Institutions that use combination prophylaxis should be aware that although it may seem logical to extrapolate estimates of benefit seen in selected surgical patients, this is not a recommended practice.
RCTs for thromboprophylaxis in nonsurgical inpatients provided prophylaxis for 6 to 21 days. Neither ACP‐1 or AT9 recommend routinely extending prophylaxis beyond the hospital stay, citing an RCT[22] in which the benefit of extended duration low molecular weight heparin was limited to selected subsets of patients and offset by bleeding complications. AT9 suggests prophylaxis for 6 to 21 days, until full mobility is restored, or until dischargewhichever comes first.[8] However, we know of no study that establishes a mobility level at which prophylaxis can be safely discontinued, especially in inpatients with multiple risk factors.
ESTIMATING RISK AND BENEFIT OF PROPHYLAXIS AND LIMITATIONS OF METHODS
Calculating risk/benefit ratios for thromboprophylaxis requires estimates of baseline VTE and bleeding risks, and estimates of the impact of prophylaxis on those baseline risks. Methods to estimate the impact of prophylaxis on S‐VTE from studies relying on A‐VTE all have limitations, as acknowledged by the AT9 introduction.[15]
The ACP‐1 review found the only significant effect of prophylaxis on medical inpatients was a modest reduction in PE and a modest increase in total bleeding events, without effects on major bleeding, DVT, or mortality.[6] The authors summarized the findings as indicative of little or no net benefit for the medical population as a whole. The ACP‐1 review derives estimates of S‐VTE risk, bleeding, and mortality from control (baseline) and interventional arms of RCTs that used routine VTE screening, and included A‐VTE end points. The baseline risk of VTE could potentially be overestimated, because the populations in the trials are not representative of the entire medical population.
On the other hand, pooling trials with screening‐detected VTE to estimate S‐VTE outcomes is a questionable practice that may falsely lower estimates of VTE prophylaxis benefit. Screening‐detected VTE may be treated or declared a study end point before it becomes symptomatic. MEDENOX (Medical Patients With Enoxaparin) is an illustrative example.[12] The 263 placebo recipients suffered 37 A‐VTEs and 4 S‐VTEs. The 272 enoxaparin recipients suffered 17 A‐VTEs and 3 S‐VTEs. Patients at the highest risk of S‐VTE were counted as reaching an end point before they could develop symptoms; this happened more than twice as often in the placebo arm. This decreases both estimates of baseline VTE risk and the measured benefit of prophylaxis for S‐VTE. Screening could conceivably reduce measured effects on mortality as well, because patients begin VTE therapy earlier. Per ACP‐1, the estimated risk for DVT is lower than for PE, running counter to literature experience[8, 16] and raising issues of face validity. The ACP‐1 review accepts all original definitions of major bleeding, including a 2 g/dL drop in hemoglobin,[12] which commonly occurs without any bleeding or clinical consequence, and bleeding events were ascribed to heparins up to 120 days after randomization, long after they could have been responsible.
Previous meta‐analyses of thromboprophylaxis studies[23, 24] shared many of these same limitations, but did not ascribe bleeding complications to heparins for this extended duration, and had point estimates that suggested a larger impact from prophylaxis than ACP‐1. Dentali et al., for example, showed statistically significant impact on PE (relative risk [RR] 0.43), fatal PE (RR 0.38), and a nearly statistically significant large impact on DVT (RR 0.47, 95% confidence interval [CI]: 0.22‐1.00),[24] whereas ACP‐1 estimated a smaller significant impact on PE (RR 0.69), no significant difference in fatal PE, and a much smaller estimate of the impact on DVT (RR 0.78, 95% CI: 0.45‐1.35) (Table 2).
| Baseline Risk | Relative Effect (95% CI) | Absolute Effect per 1000 Patients Treated (95% CI) | |
|---|---|---|---|
| |||
| ACP guideline review (Lederle), UFH or LMWH vs placebo/no treatment, medical patients | |||
| Mortality | 6.6 | OR 0.94 (0.84‐1.04) | 4 fewer (11 fewer to 3 more) |
| Major bleeding | 0.25 | OR 1.49 (0.91‐2.43) | 1 more (no effect to 3 more) |
| Symptomatic DVT | 0.96 | OR 0.78 (0.45‐1.35) | 2 fewer (6 fewer to 4 more) |
| PE | 1.2 | OR 0.69 (0.52‐0.90) | 4 fewer (6 fewer to 1 fewer) |
| Fatal PE | 0.30 | OR 0.77 (0.43‐1.37) | 1 fewer (2 fewer to 1 more) |
| ACCP AT9 (Kahn), non‐critical care medical inpatients, anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Mortality | 4.5 | OR 0.97 (0.79‐1.19) | 1 fewer (9 fewer to 8 more) |
| Major bleeding | 0.40 | OR 1.32 (0.73‐2.37) | 1 more (1 fewer to 6 more) |
| Thrombocytopenia | 0.13 | OR 0.92 (0.54‐1.53) | 1 fewer (6 fewer to 7 more) |
| Symptomatic DVT | |||
| Padua score <4 | 0.2 | RR 0.47 (0.22‐1) | 1 fewer (1 fewer to no effect) |
| Padua score 4 | 6.7 | 34 fewer (51 fewer to no effect) | |
| ACCP AT9 (Kahn) non‐critical care medical inpatients, Anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Nonfatal PE | |||
| Padua score 4 | 0.2 | RR 0.61 (0.23‐1.67) | 1 fewer (1 fewer to 1 more) |
| Padua score 4 | 3.9 | 15 fewer (30 fewer to 36 more) | |
| Fatal PE | 0.4 | RR 0.41 (0.22‐0.76) | 2 fewer (1 fewer to 3 fewer) |
| ACCP AT9 (Kahn), critical care medical inpatients, any heparin (LMWH, UFH) vs placebo/no treatment) | |||
| Mortality | 9.4 | RR 1.01 (0.04‐2.57) | 1 more (56 fewer to 148 more) |
| Major bleeding | 2.7 | RR 2.09 (0.54‐8.16) | 29 more (12 fewer to 190 more) |
| Symptomatic DVT | 5.8 | RR 0.86 (0.59‐1.25) | 4 fewer (12 fewer to 8 more) |
| Pulmonary embolus | 4.2 | RR, 0.73 (0.26‐2.11) | 11 fewer (31 fewer to 47 more) |
AT9 used a variety of methods to estimate each component of the risk/benefit equation. Critical care and non‐critical care estimates were generated independently, but because of limited data, the critical care estimates were highly imprecise. In non‐critical care patients, as in ACP‐1, treatment effects were estimated from RCTs that routinely screened for A‐VTE, and they adapted the Dentali et al. estimate of DVT risk reduction. The baseline risk for bleeding and mortality were derived from the control population of the same meta‐analysis.[24]
Using a novel approach, AT9 estimated baseline nonsurgical VTE risk from a prospective observational cohort study of 1180 medical inpatients divided into high‐ and low‐risk groups by a point‐scoring system.[25] Deriving risk estimates from an observational cohort has theoretical advantages. Many patients did not receive prophylaxis, allowing for unadjusted risk estimates; they represented a cross‐section of medical inpatients rather than a selected trial population, and risk estimates were not reduced by the culling of screen‐detected A‐VTE.
The Padua risk‐assessment model (RAM) (Table 3) defines high VTE risk as a cumulative score 4. There were 60.3% of patients at low risk and 39.7% at high risk using this threshold. Among unprophylaxed patients, VTE occurred in 11% of high‐risk patients versus 0.3% of low‐risk patients (hazard ratio 32.0, 95% CI: 4.1251.0).
| Baseline Features | Score |
|---|---|
| |
| Active cancer* | 3 |
| Previous VTE (excluding superficial thrombosis) | 3 |
| Reduced mobility | 3 |
| Already known thrombophilic condition | 3 |
| Recent (1 month) trauma and/or surgery | 2 |
| Elderly age (70 years) | 1 |
| Heart and/or respiratory failure | 1 |
| Acute myocardial infarction or stroke | 1 |
| Acute infection and/or rheumatologic disorder | 1 |
| Obesity (BMI 30) | 1 |
| Ongoing hormonal treatment | 1 |
PADUA: A CLOSER LOOK
In the Padua study, 60% of the population appeared to be at such low risk for VTE that prophylaxis would seem unnecessary, but closer scrutiny should raise concern about generalizing these results. Of the 711 low‐risk patients, <1% were immobile, only 6% had cancer, 6% were obese, and only 12% had any acute infection or inflammatory condition, yet their mean length of stay was 7.9 days. These characteristics do not apply to 60% of American inpatients. Furthermore, 964 of 2208 eligible patients (44%) were excluded because they required therapeutic anticoagulation.[25]
Correspondence with the authors revealed that the 2 PEs in patients with Padua scores 4 occurred among 192 patients with a risk score of 3 (Figure 1), a 1% (2/192) risk of PE. This is a very small sample, and the true risk of VTE for medical inpatients with a risk score of 3 may be lower or significantly higher. In the Padua population, a risk score of 3 equated to a VTE risk of 6.9%, whereas those with a score of 0 to 2 had no VTE. For those adapting the Padua model, careful consideration of using a cutoff of 3, versus 4, is warranted.

IMPLICATIONS FOR VTE PROTOCOL IMPLEMENTATION AND IMPROVEMENT TEAMS
AT9 and ACP‐1 sought to focus on S‐VTE, remove bias from recommendations, and highlight potential risks of unnecessary prophylaxis in low‐risk patients. They have largely succeeded in these important goals. However, the complexity of the new guidelines and lack of consensus about VTE risk assessment pose significant challenges to improvement teams tasked with implementing the guidelines in real‐world settings.
CHOOSING A VTE RAM
The fundamental question is: How can hospitals assess VTE risk, assure adequate prophylaxis for patients who need it, while minimizing excess prophylaxis, in a practical, efficient way?
Approach 1: Opt Out Approach
Both guidelines discourage universal prophylaxis for inpatients without contraindications unless the physician opts out. Although the simplicity of this approach is appealing, the low rate of VTE in a substantial segment of the medical inpatient population and known risks of thromboprophylaxis make this strategy suboptimal.
Approach 2: No VTE RAM
ACP‐1 notes that evidence is not sufficient to recommend 1 RAM over another, and essentially advises leaving prophylaxis decisions up to an individual physician's judgment. Although the evidence may not prove which system is best, prophylaxis reliability is dismal when there is no system or when hospitals offer prophylaxis options without guidance.[26, 27] Widespread, well‐documented underprophylaxis[16, 17, 18, 19] is largely the result of relying on unguided physician judgment and relatively passive interventions like educational sessions and pocket cards.[8] This approach also deprives improvement teams of standard definitions of VTE risk, bleeding risk, and adequate prophylaxis necessary to measure and improve VTE prophylaxis. Because of significant gray areas in the literature and varied infrastructure, institutions will not implement identical VTE prevention programs, but institutional standardization remains a cornerstone of improvement.
Approach 3: Buckets of Risk
The AT8 approach to risk assessment was to place patients into VTE risk groups described in the text, rather than have an individualized point‐scoring system.[11] These assessments can be made in seconds with high levels of interobserver agreement, implemented without undue effort, and spur high levels of compliance.[28, 29] Most importantly, implementation was associated with a 40% reduction in hospital‐associated VTE (RR 0.69, 95% CI: 0.470.79) without detectable increases in bleeding or heparin‐induced thrombocytopenia. Although this strategy has not been tested in randomized trials, it has been replicated in multiple real‐world settings that avoid concerns about generalizability due to imperfect trial populations.[28, 30]
The most popular bucket model in common use, derived from a table in the AT8 guidelines, is similar to models presented in UK National Institute for Health and Care Excellence guidelines for medical inpatients.[31] These models are potentially less precise than point‐based systems, but offer simplicity, ease of use, and improved physician acceptance, and thus may be more effective than point‐based models in settings without advanced clinical decision support. The models are flexible to reflect greater or lesser degrees of aggressiveness in defining risk categories, and can be used to approximate some point‐based systems.
Approach 4: Individualized Point‐Based RAM
AT9 authors used the Padua VTE RAM to define low‐ and high‐risk patients for VTE in their recommendation for medical inpatients. The Padua model appears relatively simple, but it does require calculations, and there is a paucity of data for implementation experience with it. As mentioned above, if teams use the Padua model, the optimal cutoff (3 vs 4) for recommending prophylaxis is uncertain, and both should be considered.
The Caprini point‐based system is not mentioned in the guideline for thromboprophylaxis in medical inpatients, but in our collaborative improvement experience, it is perhaps the most commonly used point‐based model for medical inpatients.[28, 30] It is also embedded in AT9 recommendations for prophylaxis in the nonorthopedic surgical population,[9] and thus is tempting to use for both medical and surgical patients. There are several caveats to those considering the use of these more complex point‐based models. Complex point‐based RAM suffer from poor interobserver agreement.[32] They have also had limited ability to exclude low‐risk patients from prophylaxis in validation studies,[33] and have not been tested extensively in medical populations. Although AT9 considers the Caprini RAM relatively easy to use,[9] our experience in collaboratives suggests that for many hospitals, the model is too complex to be used reliably.[28, 30] Clinicians often simply bypass the clinical decision support offered in the tool, rather than checking off all risk factors, adding up the point total, and identifying the appropriate prophylaxis choices based on the point total.[28] Other point‐based RAM (reviewed elsewhere[34, 35, 36]) pose similar implementation challenges.
On the other hand, centers with more sophisticated clinical decision support and a strong improvement framework can overcome some of these challenges to get good results with complex point‐based models. A forcing function can ensure that practitioners complete all risk‐assessment tasks. Providers can check off the VTE risk factors and bleeding risk factors on 1 screen, and several factors like age, body mass index, and renal function can be autopopulated. Instead of asking the provider to add up points, the combination of answers checked off on the first screen can drive behind‐the‐scenes calculations and seamlessly lead providers to prophylaxis choices appropriate for that combination of VTE and bleeding risks. Customized models can be designed for a wide variety of services. Similar strategies can ease adaption with more complex qualitative models as well.[37]
BOTTOM LINE IN CHOOSING A VTE RAM
Many medical inpatients are at high risk for VTE, but others are not at sufficient risk to warrant prophylaxis. VTE risk assessment should be embedded in admission, transfer, and perioperative order sets and may need a hard stop to insure completion. There is a trend to favor individualized point‐based models over models that place patients in groups of risk, but evidence is insufficient to recommend 1 type of RAM over another, and more complex point‐based models often require extensive local customization and algorithmic clinical decision support to effectively implement them. Centers without advanced capability may find the bucket models more effective. We urge improvement teams to trial their RAM with common patient case scenarios, and to make a choice based on an effort‐benefit analysis, feedback from their clinicians, and the level of customization in clinical decision support available to them.
OTHER IMPLEMENTATION STRATEGIES
VTE and bleeding risk change during hospitalization. We have used ongoing daily surveillance and measurement of patients on no prophylaxis to prompt concurrent intervention (ie, measure‐vention) to increase prophylaxis for patients at risk.[28] Improvement teams should focus not only on increasing prophylaxis for those at risk, but should also use measure‐vention, checklists, or other techniques to identify low‐risk (eg, ambulating) patients for cessation of overly aggressive prophylaxis. Efforts to improve early progressive ambulation, limit central venous catheters to those who truly need them, and improve adherence to mechanical prophylaxis can also reduce VTE, as well as benefitting patient populations in other ways.
We recognize there are several approaches to close the implementation gap in delivering thromboprophylaxis judiciously but reliably, and encourage research and publication of varied strategies. Last, we hope efforts to limit unnecessary prophylaxis and challenges inherent in implementing new and complex guidelines do not increase the morbidity and mortality of hospital‐acquired VTE, by derailing the delivery of prophylaxis to those in whom the benefits outweigh the risks.
Disclosures: Dr. Merli has conducted research for Johnson & Johnson, Bristol Myers Squibb, and Portala Scientific and has been a consultant for Johnson & Johnson and Bristol Myers Squibb.
Patients hospitalized for acute medical illness have more than a 10‐fold increased risk for venous thromboembolism (VTE),[1] with an undeniably dramatic, negative impact on the lives of those afflicted, including fatal pulmonary embolism (PE), which most commonly affects patients on the medical service.[2, 3, 4] Yet estimates for the overall rate of VTE in this population are relatively low, raising questions about which subsets of medical patients warrant the risk and cost of prophylaxis.
Recently, the American College of Physicians published guidelines (ACP‐1)[5] and a supporting review[6] addressing VTE prophylaxis in nonsurgical inpatients, followed by publication of the American College of Chest Physicians (ACCP) 9th Edition of the Chest Guidelines on Antithrombotic Therapy and Prevention of Thrombosis (AT9),[7] which divides VTE prevention into 3 articles,[8, 9, 10] including 1 on nonsurgical patients.[8] Both ACP‐1 and AT9 differ significantly from the 2008 ACCP guidelines (AT8),[11] but took different approaches to methodology, risk assessment, and several other aspects of thromboprophylaxis (Table 1). This narrative review summarizes and compares these recommendations and the methods used to arrive at them, with a final section focusing on implications for improvement teams designing order sets and system changes to address VTE prophylaxis.
| 2008 ACCP VTE Guideline AT8 | 2012 ACCP VTE Guideline AT9 | 2011 ACP Guideline | |
|---|---|---|---|
| |||
| Stance on asymptomatic VTE end points | Because of the strong concordance between asymptomatic DVT and clinically important VTE, we believe that DVT detected by a sensitive screening tesis an appropriate outcome in the early assessment of new thromboprophylaxis interventions. | Use of this surrogate (asymptomatic, screening‐detected thrombosis) creates major problems in making the trade‐off between patient‐important outcomes (thrombosis and serious bleeding). | Surrogate outcomes of asymptomatic screening detected‐thrombosis should not be used. |
| Who should be prophylaxed? | 6.0.0: For acutely ill medical patients admitted to hospital with congestive heart failure or severe respiratory disease, or who are confined to bed and have one or more additional risk factors, including active cancer, previous VTE, sepsis, neurologic disease, or inflammatory bowel disease, we recommend thromboprophylaxis with LMWH (1A), UFH (1A), or fondaparinux (1A). | 2.3: For acutely ill hospitalized medical patients at increased risk for thrombosis, we recommend anticoagulant thromboprophylaxis, with LMWH, UFH bid, UFH tid, or fondaparinux (1B). | ACP recommends pharmacologic prophylaxis with heparin or a related drug for venous thromboembolism in medical (including stroke) patients unless the assessed risk for bleeding outweighs the likely benefits (grade: strong recommendation, moderate‐quality evidence). |
| 2.4: For acutely ill hospitalized medical patients at low risk of thrombosis, we recommend against the use of pharmacologic or mechanical prophylaxis. (1B) | |||
| Choice of anticoagulant prophylaxis | There is no compelling evidence that UFH should be administered three times daily in preference to twice daily in medical patients, although these two regimens have never been directly compared. | In choosing the specific anticoagulant drug to be used for pharmacoprophylaxis, choices should be based on patient preference, compliance, and ease of administration (eg, daily vs bid vs tid dosing), as well as on local factors affecting acquisition costs. | [T]he choice of agent for prophylaxis of VTE should be based on ease of use, adverse effect profile, and cost of medication. |
| No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | |
| Mechanical prophylaxis | 1.4.3.1: We recommend that mechanical methods of prophylaxis be used primarily in patients at high risk of bleeding (grade 1A), or possibly as an adjunct to anticoagulant‐based thromboprophylaxis (grade 2A). | 2.7.2: For acutely ill hospitalized medical patients at increased risk of thrombosis who are bleeding or at high risk for major bleeding, we suggest the optimal use of mechanical thromboprophylaxis with GCS (grade 2C) or IPC (grade 2C). | ACP recommends against the use of mechanical prophylaxis with graduated compression stockings for prevention of venous thromboembolism (grade: strong recommendation, moderate‐quality evidence). |
| No strong evidence for IPC vs GCS | |||
| Duration | [T]he optimal duration of thromboprophylaxis remains unclear. To the end of hospitalization for most patients. | 2.8: [W]e suggest against extending the duration of thromboprophylaxis beyond the period of patient immobilization or acute hospital stay (2B). | The optimal duration of heparin prophylaxis is uncertain. |
| Risk Stratification | The approach of individual prophylaxis prescribing based on formal RAMs is not used routinely by most clinicians because it has not been adequately validated and is cumbersome. Individual RAMs may not be worth the effort, because there are only a limited number of thromboprophylaxis options, and one of the principles of effective thromboprophylaxis is to reduce complexity in decision making. | (Noncritical care) No formal risk assessment recommendation. Padua point‐based model is inherent in definitions of baseline VTE risk. | ACP does not support the application of performance measures in medical (including stroke) patients that promotes universal venous thromboembolism prophylaxis regardless of risk. |
| Another approach involves implementation of group‐specific thromboprophylaxis routinely for all patients who belong to each of the major target groups. We support this approach. | There are no validated risk assessment models to stratify VTE risk in critically ill patients. | Many risk assessment tools are available for estimating thromboembolism risk, but the current evidence is insufficient to recommend a validated tool. | |
| [T]he decision is best left to physician judgment, and performance measures targeting all patients are inappropriate. | |||
WHY ARE THE NEW GUIDELINES DIFFERENT?
Major randomized controlled trials (RCTs)[12, 13, 14] of thromboprophylaxis used routine deep vein thrombosis (DVT) surveillance and included both symptomatic (S‐VTE) and asymptomatic VTE (A‐VTE) end points. These studies consistently demonstrated 44% to 63% reductions in VTE without increases in major bleeding.[11] Because of the strong relationship between A‐VTE and S‐VTE outcomes, and a paucity of studies using only S‐VTE outcomes, AT8 judged that A‐VTE outcomes were valid to include, whereas the new guidelines reject the use of asymptomatic VTE end points.[5, 8, 15] To minimize financial and intellectual conflicts of interest, AT9 also used methodologists rather than VTE experts as topic editors, excluded conflicted experts from voting on recommendations, and attempted to estimate patient values and preferences.[15] As a result, AT9 makes fewer strong recommendations (182 1A recommendations in 2008, but only 29 in 2012), replacing them with weak suggestions.
WHAT DO THE NEW GUIDELINES RECOMMEND?
AT8 recommended anticoagulant prophylaxis for acutely ill medical inpatients with known risk factors, but did not recommend routine thromboprophylaxis. However, because of well‐known problems with underprophylaxis,[16, 17, 18, 19] particularly in medical patients, the low risk of bleeding, and difficulties with explicitly defining low‐risk patients, many discounted the need for VTE risk stratification.
Both new guidelines recommend prophylaxis for many nonsurgical patients, but discourage routine thromboprophylaxis for nonsurgical inpatients. AT9 specifically recommends against any thromboprophylaxis for low‐risk medical inpatients, implying that many nonsurgical, non‐critical care patients belong in this category, citing lower estimates of benefit, lower estimates of VTE risk, and potential bleeding risks.
The guidelines[5, 8] agree that, when indicated and absent contraindications, anticoagulant prophylaxis is preferred over mechanical prophylaxis, and agree there is insufficient evidence to recommend 1 anticoagulant over another.
For patients at risk of both VTE and bleeding, ACP‐1 states that intermittent pneumatic compression (IPC) devices are a reasonable option, given the evidence showing benefit in surgical patients. However, ACP‐1 recommends against graduated compression stockings (GCS) in nonsurgical patients based on a meta‐analysis dominated by the CLOTS‐1 (Clots in Legs Or sTockings after Stroke) trial, which found that thigh‐high GCS increased the risk of skin breakdown without reducing VTE[20] in immobilized stroke patients. AT9 does not recommend against GCS for patients facing bleeding and VTE risk. AT9 notes the hazards of generalizing results from stroke patients, and also considers the somewhat contradictory results from the CLOTS‐2 trial in stroke patients, which found a lower rate of VTE with thigh‐high GCS than with knee‐high GCS.[21] AT9 designates a recommendation of 2C for either IPC devices or thigh‐high GCS for those at VTE risk when anticoagulants are contraindicated.
Combination mechanical‐pharmacologic prophylaxis has proven superior in some surgical populations, and many hospitals use combined prophylaxis in high‐risk medical patients. However, combination prophylaxis has not been studied in this population. ACP‐1 does not comment on the practice; AT9 does not recommend for or against it. Institutions that use combination prophylaxis should be aware that although it may seem logical to extrapolate estimates of benefit seen in selected surgical patients, this is not a recommended practice.
RCTs for thromboprophylaxis in nonsurgical inpatients provided prophylaxis for 6 to 21 days. Neither ACP‐1 or AT9 recommend routinely extending prophylaxis beyond the hospital stay, citing an RCT[22] in which the benefit of extended duration low molecular weight heparin was limited to selected subsets of patients and offset by bleeding complications. AT9 suggests prophylaxis for 6 to 21 days, until full mobility is restored, or until dischargewhichever comes first.[8] However, we know of no study that establishes a mobility level at which prophylaxis can be safely discontinued, especially in inpatients with multiple risk factors.
ESTIMATING RISK AND BENEFIT OF PROPHYLAXIS AND LIMITATIONS OF METHODS
Calculating risk/benefit ratios for thromboprophylaxis requires estimates of baseline VTE and bleeding risks, and estimates of the impact of prophylaxis on those baseline risks. Methods to estimate the impact of prophylaxis on S‐VTE from studies relying on A‐VTE all have limitations, as acknowledged by the AT9 introduction.[15]
The ACP‐1 review found the only significant effect of prophylaxis on medical inpatients was a modest reduction in PE and a modest increase in total bleeding events, without effects on major bleeding, DVT, or mortality.[6] The authors summarized the findings as indicative of little or no net benefit for the medical population as a whole. The ACP‐1 review derives estimates of S‐VTE risk, bleeding, and mortality from control (baseline) and interventional arms of RCTs that used routine VTE screening, and included A‐VTE end points. The baseline risk of VTE could potentially be overestimated, because the populations in the trials are not representative of the entire medical population.
On the other hand, pooling trials with screening‐detected VTE to estimate S‐VTE outcomes is a questionable practice that may falsely lower estimates of VTE prophylaxis benefit. Screening‐detected VTE may be treated or declared a study end point before it becomes symptomatic. MEDENOX (Medical Patients With Enoxaparin) is an illustrative example.[12] The 263 placebo recipients suffered 37 A‐VTEs and 4 S‐VTEs. The 272 enoxaparin recipients suffered 17 A‐VTEs and 3 S‐VTEs. Patients at the highest risk of S‐VTE were counted as reaching an end point before they could develop symptoms; this happened more than twice as often in the placebo arm. This decreases both estimates of baseline VTE risk and the measured benefit of prophylaxis for S‐VTE. Screening could conceivably reduce measured effects on mortality as well, because patients begin VTE therapy earlier. Per ACP‐1, the estimated risk for DVT is lower than for PE, running counter to literature experience[8, 16] and raising issues of face validity. The ACP‐1 review accepts all original definitions of major bleeding, including a 2 g/dL drop in hemoglobin,[12] which commonly occurs without any bleeding or clinical consequence, and bleeding events were ascribed to heparins up to 120 days after randomization, long after they could have been responsible.
Previous meta‐analyses of thromboprophylaxis studies[23, 24] shared many of these same limitations, but did not ascribe bleeding complications to heparins for this extended duration, and had point estimates that suggested a larger impact from prophylaxis than ACP‐1. Dentali et al., for example, showed statistically significant impact on PE (relative risk [RR] 0.43), fatal PE (RR 0.38), and a nearly statistically significant large impact on DVT (RR 0.47, 95% confidence interval [CI]: 0.22‐1.00),[24] whereas ACP‐1 estimated a smaller significant impact on PE (RR 0.69), no significant difference in fatal PE, and a much smaller estimate of the impact on DVT (RR 0.78, 95% CI: 0.45‐1.35) (Table 2).
| Baseline Risk | Relative Effect (95% CI) | Absolute Effect per 1000 Patients Treated (95% CI) | |
|---|---|---|---|
| |||
| ACP guideline review (Lederle), UFH or LMWH vs placebo/no treatment, medical patients | |||
| Mortality | 6.6 | OR 0.94 (0.84‐1.04) | 4 fewer (11 fewer to 3 more) |
| Major bleeding | 0.25 | OR 1.49 (0.91‐2.43) | 1 more (no effect to 3 more) |
| Symptomatic DVT | 0.96 | OR 0.78 (0.45‐1.35) | 2 fewer (6 fewer to 4 more) |
| PE | 1.2 | OR 0.69 (0.52‐0.90) | 4 fewer (6 fewer to 1 fewer) |
| Fatal PE | 0.30 | OR 0.77 (0.43‐1.37) | 1 fewer (2 fewer to 1 more) |
| ACCP AT9 (Kahn), non‐critical care medical inpatients, anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Mortality | 4.5 | OR 0.97 (0.79‐1.19) | 1 fewer (9 fewer to 8 more) |
| Major bleeding | 0.40 | OR 1.32 (0.73‐2.37) | 1 more (1 fewer to 6 more) |
| Thrombocytopenia | 0.13 | OR 0.92 (0.54‐1.53) | 1 fewer (6 fewer to 7 more) |
| Symptomatic DVT | |||
| Padua score <4 | 0.2 | RR 0.47 (0.22‐1) | 1 fewer (1 fewer to no effect) |
| Padua score 4 | 6.7 | 34 fewer (51 fewer to no effect) | |
| ACCP AT9 (Kahn) non‐critical care medical inpatients, Anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Nonfatal PE | |||
| Padua score 4 | 0.2 | RR 0.61 (0.23‐1.67) | 1 fewer (1 fewer to 1 more) |
| Padua score 4 | 3.9 | 15 fewer (30 fewer to 36 more) | |
| Fatal PE | 0.4 | RR 0.41 (0.22‐0.76) | 2 fewer (1 fewer to 3 fewer) |
| ACCP AT9 (Kahn), critical care medical inpatients, any heparin (LMWH, UFH) vs placebo/no treatment) | |||
| Mortality | 9.4 | RR 1.01 (0.04‐2.57) | 1 more (56 fewer to 148 more) |
| Major bleeding | 2.7 | RR 2.09 (0.54‐8.16) | 29 more (12 fewer to 190 more) |
| Symptomatic DVT | 5.8 | RR 0.86 (0.59‐1.25) | 4 fewer (12 fewer to 8 more) |
| Pulmonary embolus | 4.2 | RR, 0.73 (0.26‐2.11) | 11 fewer (31 fewer to 47 more) |
AT9 used a variety of methods to estimate each component of the risk/benefit equation. Critical care and non‐critical care estimates were generated independently, but because of limited data, the critical care estimates were highly imprecise. In non‐critical care patients, as in ACP‐1, treatment effects were estimated from RCTs that routinely screened for A‐VTE, and they adapted the Dentali et al. estimate of DVT risk reduction. The baseline risk for bleeding and mortality were derived from the control population of the same meta‐analysis.[24]
Using a novel approach, AT9 estimated baseline nonsurgical VTE risk from a prospective observational cohort study of 1180 medical inpatients divided into high‐ and low‐risk groups by a point‐scoring system.[25] Deriving risk estimates from an observational cohort has theoretical advantages. Many patients did not receive prophylaxis, allowing for unadjusted risk estimates; they represented a cross‐section of medical inpatients rather than a selected trial population, and risk estimates were not reduced by the culling of screen‐detected A‐VTE.
The Padua risk‐assessment model (RAM) (Table 3) defines high VTE risk as a cumulative score 4. There were 60.3% of patients at low risk and 39.7% at high risk using this threshold. Among unprophylaxed patients, VTE occurred in 11% of high‐risk patients versus 0.3% of low‐risk patients (hazard ratio 32.0, 95% CI: 4.1251.0).
| Baseline Features | Score |
|---|---|
| |
| Active cancer* | 3 |
| Previous VTE (excluding superficial thrombosis) | 3 |
| Reduced mobility | 3 |
| Already known thrombophilic condition | 3 |
| Recent (1 month) trauma and/or surgery | 2 |
| Elderly age (70 years) | 1 |
| Heart and/or respiratory failure | 1 |
| Acute myocardial infarction or stroke | 1 |
| Acute infection and/or rheumatologic disorder | 1 |
| Obesity (BMI 30) | 1 |
| Ongoing hormonal treatment | 1 |
PADUA: A CLOSER LOOK
In the Padua study, 60% of the population appeared to be at such low risk for VTE that prophylaxis would seem unnecessary, but closer scrutiny should raise concern about generalizing these results. Of the 711 low‐risk patients, <1% were immobile, only 6% had cancer, 6% were obese, and only 12% had any acute infection or inflammatory condition, yet their mean length of stay was 7.9 days. These characteristics do not apply to 60% of American inpatients. Furthermore, 964 of 2208 eligible patients (44%) were excluded because they required therapeutic anticoagulation.[25]
Correspondence with the authors revealed that the 2 PEs in patients with Padua scores 4 occurred among 192 patients with a risk score of 3 (Figure 1), a 1% (2/192) risk of PE. This is a very small sample, and the true risk of VTE for medical inpatients with a risk score of 3 may be lower or significantly higher. In the Padua population, a risk score of 3 equated to a VTE risk of 6.9%, whereas those with a score of 0 to 2 had no VTE. For those adapting the Padua model, careful consideration of using a cutoff of 3, versus 4, is warranted.

IMPLICATIONS FOR VTE PROTOCOL IMPLEMENTATION AND IMPROVEMENT TEAMS
AT9 and ACP‐1 sought to focus on S‐VTE, remove bias from recommendations, and highlight potential risks of unnecessary prophylaxis in low‐risk patients. They have largely succeeded in these important goals. However, the complexity of the new guidelines and lack of consensus about VTE risk assessment pose significant challenges to improvement teams tasked with implementing the guidelines in real‐world settings.
CHOOSING A VTE RAM
The fundamental question is: How can hospitals assess VTE risk, assure adequate prophylaxis for patients who need it, while minimizing excess prophylaxis, in a practical, efficient way?
Approach 1: Opt Out Approach
Both guidelines discourage universal prophylaxis for inpatients without contraindications unless the physician opts out. Although the simplicity of this approach is appealing, the low rate of VTE in a substantial segment of the medical inpatient population and known risks of thromboprophylaxis make this strategy suboptimal.
Approach 2: No VTE RAM
ACP‐1 notes that evidence is not sufficient to recommend 1 RAM over another, and essentially advises leaving prophylaxis decisions up to an individual physician's judgment. Although the evidence may not prove which system is best, prophylaxis reliability is dismal when there is no system or when hospitals offer prophylaxis options without guidance.[26, 27] Widespread, well‐documented underprophylaxis[16, 17, 18, 19] is largely the result of relying on unguided physician judgment and relatively passive interventions like educational sessions and pocket cards.[8] This approach also deprives improvement teams of standard definitions of VTE risk, bleeding risk, and adequate prophylaxis necessary to measure and improve VTE prophylaxis. Because of significant gray areas in the literature and varied infrastructure, institutions will not implement identical VTE prevention programs, but institutional standardization remains a cornerstone of improvement.
Approach 3: Buckets of Risk
The AT8 approach to risk assessment was to place patients into VTE risk groups described in the text, rather than have an individualized point‐scoring system.[11] These assessments can be made in seconds with high levels of interobserver agreement, implemented without undue effort, and spur high levels of compliance.[28, 29] Most importantly, implementation was associated with a 40% reduction in hospital‐associated VTE (RR 0.69, 95% CI: 0.470.79) without detectable increases in bleeding or heparin‐induced thrombocytopenia. Although this strategy has not been tested in randomized trials, it has been replicated in multiple real‐world settings that avoid concerns about generalizability due to imperfect trial populations.[28, 30]
The most popular bucket model in common use, derived from a table in the AT8 guidelines, is similar to models presented in UK National Institute for Health and Care Excellence guidelines for medical inpatients.[31] These models are potentially less precise than point‐based systems, but offer simplicity, ease of use, and improved physician acceptance, and thus may be more effective than point‐based models in settings without advanced clinical decision support. The models are flexible to reflect greater or lesser degrees of aggressiveness in defining risk categories, and can be used to approximate some point‐based systems.
Approach 4: Individualized Point‐Based RAM
AT9 authors used the Padua VTE RAM to define low‐ and high‐risk patients for VTE in their recommendation for medical inpatients. The Padua model appears relatively simple, but it does require calculations, and there is a paucity of data for implementation experience with it. As mentioned above, if teams use the Padua model, the optimal cutoff (3 vs 4) for recommending prophylaxis is uncertain, and both should be considered.
The Caprini point‐based system is not mentioned in the guideline for thromboprophylaxis in medical inpatients, but in our collaborative improvement experience, it is perhaps the most commonly used point‐based model for medical inpatients.[28, 30] It is also embedded in AT9 recommendations for prophylaxis in the nonorthopedic surgical population,[9] and thus is tempting to use for both medical and surgical patients. There are several caveats to those considering the use of these more complex point‐based models. Complex point‐based RAM suffer from poor interobserver agreement.[32] They have also had limited ability to exclude low‐risk patients from prophylaxis in validation studies,[33] and have not been tested extensively in medical populations. Although AT9 considers the Caprini RAM relatively easy to use,[9] our experience in collaboratives suggests that for many hospitals, the model is too complex to be used reliably.[28, 30] Clinicians often simply bypass the clinical decision support offered in the tool, rather than checking off all risk factors, adding up the point total, and identifying the appropriate prophylaxis choices based on the point total.[28] Other point‐based RAM (reviewed elsewhere[34, 35, 36]) pose similar implementation challenges.
On the other hand, centers with more sophisticated clinical decision support and a strong improvement framework can overcome some of these challenges to get good results with complex point‐based models. A forcing function can ensure that practitioners complete all risk‐assessment tasks. Providers can check off the VTE risk factors and bleeding risk factors on 1 screen, and several factors like age, body mass index, and renal function can be autopopulated. Instead of asking the provider to add up points, the combination of answers checked off on the first screen can drive behind‐the‐scenes calculations and seamlessly lead providers to prophylaxis choices appropriate for that combination of VTE and bleeding risks. Customized models can be designed for a wide variety of services. Similar strategies can ease adaption with more complex qualitative models as well.[37]
BOTTOM LINE IN CHOOSING A VTE RAM
Many medical inpatients are at high risk for VTE, but others are not at sufficient risk to warrant prophylaxis. VTE risk assessment should be embedded in admission, transfer, and perioperative order sets and may need a hard stop to insure completion. There is a trend to favor individualized point‐based models over models that place patients in groups of risk, but evidence is insufficient to recommend 1 type of RAM over another, and more complex point‐based models often require extensive local customization and algorithmic clinical decision support to effectively implement them. Centers without advanced capability may find the bucket models more effective. We urge improvement teams to trial their RAM with common patient case scenarios, and to make a choice based on an effort‐benefit analysis, feedback from their clinicians, and the level of customization in clinical decision support available to them.
OTHER IMPLEMENTATION STRATEGIES
VTE and bleeding risk change during hospitalization. We have used ongoing daily surveillance and measurement of patients on no prophylaxis to prompt concurrent intervention (ie, measure‐vention) to increase prophylaxis for patients at risk.[28] Improvement teams should focus not only on increasing prophylaxis for those at risk, but should also use measure‐vention, checklists, or other techniques to identify low‐risk (eg, ambulating) patients for cessation of overly aggressive prophylaxis. Efforts to improve early progressive ambulation, limit central venous catheters to those who truly need them, and improve adherence to mechanical prophylaxis can also reduce VTE, as well as benefitting patient populations in other ways.
We recognize there are several approaches to close the implementation gap in delivering thromboprophylaxis judiciously but reliably, and encourage research and publication of varied strategies. Last, we hope efforts to limit unnecessary prophylaxis and challenges inherent in implementing new and complex guidelines do not increase the morbidity and mortality of hospital‐acquired VTE, by derailing the delivery of prophylaxis to those in whom the benefits outweigh the risks.
Disclosures: Dr. Merli has conducted research for Johnson & Johnson, Bristol Myers Squibb, and Portala Scientific and has been a consultant for Johnson & Johnson and Bristol Myers Squibb.
- US Department of Health and Human Services. Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. 2008. Available at: http://www.surgeongeneral.gov/topics/deepvein/index.html. Accessed January 29, 2013.
- , , , et al. Incidence of venous thromboembolism in hospitalized patients vs. community residents. Mayo Clin Proc. 2001;76:1102–1110.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815.
- , , . New onset of venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118(6):1680–1684.
- , , , , . venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625–632.
- , , , . Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , et al. Prevention of VTE in nonsurgical patients. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Prevention of VTE in nonorthopedic surgical patients. Chest. 2012;141(2 suppl):e227S–e277S.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S–453S.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- , , , et al. The outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registry. J Thromb Haemost. 2004;2:1892–1898.
- , , , et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936–945.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387–394.
- , , , et al.; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost. 2010;103(4):736–748.
- , , , et al; CLOTS Trials Collaboration. Effectiveness of thigh‐length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomized controlled trial. Lancet. 2009;373(9679):1958–1965.
- CLOTS (Clots in Legs Or sTockings after Stroke) Trial Collaboration. Thigh‐length versus below‐knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med. 2010;153(9):553–562.
- , , , et al. Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
- , , , , . Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2007;167(1)476–486.
- , , , , . Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007; 46(4):278–288.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , . Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes. J Hosp Med. 2009;4(2):81–89.
- . Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? J Hosp Med. 2009;4(2):77–80.
- , . Designing and implementing effective VTE prevention protocols: lessons from collaboratives. J Thromb Thrombolysis. 2010;29(2):159–166.
- , , , et al. Optimizing prevention of hospital acquired venous thromboembolism: prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10–18.
- , , , et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38(7):301–310.
- NHS National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. NICE Clinical Guideline 92. 2010. Available at: http://www.nice.org.uk/guidance/CG92. Accessed April 18, 2013.
- , , , , . Reliability of a point‐based VTE risk assessment tool in the hands of medical residents. J Hosp Med. 2011;6:195–201.
- , , , , , . A validation of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251(2):344–350.
- , , , . Risk assessment models for thromboprophylaxis of medical patients. Thromb Res. 2012;129:127–132.
- , , , , . Risk‐assessment models for predicting venous thromboembolism among hospitalized non‐surgical patients: a systematic review. J Thromb Thrombolysis. 2013;35:67–80.
- , , . The use of weighted and scored risk assessment models for venous thromboembolism. Thromb Haemost. 2012;108(6):1072–1076.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- US Department of Health and Human Services. Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. 2008. Available at: http://www.surgeongeneral.gov/topics/deepvein/index.html. Accessed January 29, 2013.
- , , , et al. Incidence of venous thromboembolism in hospitalized patients vs. community residents. Mayo Clin Proc. 2001;76:1102–1110.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815.
- , , . New onset of venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118(6):1680–1684.
- , , , , . venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625–632.
- , , , . Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , et al. Prevention of VTE in nonsurgical patients. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Prevention of VTE in nonorthopedic surgical patients. Chest. 2012;141(2 suppl):e227S–e277S.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S–453S.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- , , , et al. The outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registry. J Thromb Haemost. 2004;2:1892–1898.
- , , , et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936–945.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387–394.
- , , , et al.; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost. 2010;103(4):736–748.
- , , , et al; CLOTS Trials Collaboration. Effectiveness of thigh‐length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomized controlled trial. Lancet. 2009;373(9679):1958–1965.
- CLOTS (Clots in Legs Or sTockings after Stroke) Trial Collaboration. Thigh‐length versus below‐knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med. 2010;153(9):553–562.
- , , , et al. Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
- , , , , . Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2007;167(1)476–486.
- , , , , . Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007; 46(4):278–288.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , . Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes. J Hosp Med. 2009;4(2):81–89.
- . Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? J Hosp Med. 2009;4(2):77–80.
- , . Designing and implementing effective VTE prevention protocols: lessons from collaboratives. J Thromb Thrombolysis. 2010;29(2):159–166.
- , , , et al. Optimizing prevention of hospital acquired venous thromboembolism: prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10–18.
- , , , et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38(7):301–310.
- NHS National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. NICE Clinical Guideline 92. 2010. Available at: http://www.nice.org.uk/guidance/CG92. Accessed April 18, 2013.
- , , , , . Reliability of a point‐based VTE risk assessment tool in the hands of medical residents. J Hosp Med. 2011;6:195–201.
- , , , , , . A validation of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251(2):344–350.
- , , , . Risk assessment models for thromboprophylaxis of medical patients. Thromb Res. 2012;129:127–132.
- , , , , . Risk‐assessment models for predicting venous thromboembolism among hospitalized non‐surgical patients: a systematic review. J Thromb Thrombolysis. 2013;35:67–80.
- , , . The use of weighted and scored risk assessment models for venous thromboembolism. Thromb Haemost. 2012;108(6):1072–1076.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
Complementary and alternative medicine (CAM) use in advanced cancer: a systematic review
This systematic review synthesizes knowledge about the use of complementary and alternative medicine (CAM) among advanced cancer patients. EBSCO and Ovid databases were searched using core concepts, including advanced cancer, CAM, integrative medicine, and decision-making. Articles included in the final review were analyzed using narrative synthesis methods, including thematic analysis, concept mapping, and critical reflection on the synthesis process. Results demonstrate that advanced cancer patients who are younger, female, more educated, have longer duration of disease, and have previously used CAM are more likely to use CAM during this stage of illness. Key themes identified include patterns of and reasons for use; and barriers and facilitators to informed CAM decision-making. Knowledge regarding the use of CAM in advanced cancer remains in its nascent stages. Findings suggest a need for more research on understanding the dynamic process of CAM decision-making in the advanced cancer population from the patients’ perspective.
*For a PDF of the full article, click on the link to the left of this introduction.
This systematic review synthesizes knowledge about the use of complementary and alternative medicine (CAM) among advanced cancer patients. EBSCO and Ovid databases were searched using core concepts, including advanced cancer, CAM, integrative medicine, and decision-making. Articles included in the final review were analyzed using narrative synthesis methods, including thematic analysis, concept mapping, and critical reflection on the synthesis process. Results demonstrate that advanced cancer patients who are younger, female, more educated, have longer duration of disease, and have previously used CAM are more likely to use CAM during this stage of illness. Key themes identified include patterns of and reasons for use; and barriers and facilitators to informed CAM decision-making. Knowledge regarding the use of CAM in advanced cancer remains in its nascent stages. Findings suggest a need for more research on understanding the dynamic process of CAM decision-making in the advanced cancer population from the patients’ perspective.
*For a PDF of the full article, click on the link to the left of this introduction.
This systematic review synthesizes knowledge about the use of complementary and alternative medicine (CAM) among advanced cancer patients. EBSCO and Ovid databases were searched using core concepts, including advanced cancer, CAM, integrative medicine, and decision-making. Articles included in the final review were analyzed using narrative synthesis methods, including thematic analysis, concept mapping, and critical reflection on the synthesis process. Results demonstrate that advanced cancer patients who are younger, female, more educated, have longer duration of disease, and have previously used CAM are more likely to use CAM during this stage of illness. Key themes identified include patterns of and reasons for use; and barriers and facilitators to informed CAM decision-making. Knowledge regarding the use of CAM in advanced cancer remains in its nascent stages. Findings suggest a need for more research on understanding the dynamic process of CAM decision-making in the advanced cancer population from the patients’ perspective.
*For a PDF of the full article, click on the link to the left of this introduction.
Procalcitonin‐Guided Antibiotic Therapy
Many serum biomarkers have been identified in recent years with a wide range of potential applications, including diagnosis of local and systemic infections, differentiation of bacterial and fungal infections from viral syndromes or noninfectious conditions, prognostic stratification of patients, and enhanced management of antibiotic therapy. Currently, there are at least 178 serum biomarkers that have potential roles to guide antibiotic therapy, and among these, procalcitonin has been the most extensively studied biomarker.[1, 2]
Procalcitonin is the prohormone precursor of calcitonin that is expressed primarily in C cells of the thyroid gland. Conversion of procalcitonin to calcitonin is inhibited by various cytokines and bacterial endotoxins. Procalcitonin's primary diagnostic utility is thought to be in establishing the presence of bacterial infections, because serum procalcitonin levels rise and fall rapidly in bacterial infections.[3, 4, 5] In healthy individuals, procalcitonin levels are very low. In systemic infections, including sepsis, procalcitonin levels are generally greater than 0.5 to 2 ng/mL, but often reach levels 10 ng/mL, which correlates with severity of illness and a poor prognosis. In patients with respiratory tract infections, procalcitonin levels are less elevated, and a cutoff of 0.25 ng/mL seems to be most predictive of a bacterial respiratory tract infection requiring antibiotic therapy.[6, 7, 8] Procalcitonin levels decrease to <0.25 ng/mL as infection resolves, and a decline in procalcitonin level may guide decisions about discontinuation of antibiotic therapy.[5]
The purpose of this systematic review was to synthesize comparative studies examining the use of procalcitonin to guide antibiotic therapy in patients with suspected local or systemic infections in different patient populations. We are aware of 6 previously published systematic reviews evaluating the utility of procalcitonin guidance in the management of infections.[9, 10, 11, 12, 13, 14] Our systematic review included more studies and pooled patients into the most clinically similar groups compared to other systematic reviews.
METHODS
This review is based on a comparative effectiveness review prepared for the Agency for Healthcare Research and Quality's Effective Health Care Program.[15] A standard protocol consistent with the Methods Guide for Effectiveness and Comparative Effectiveness Reviews[16] was followed. A detailed description of the methods is available online (
Study Question
In selected populations of patients with suspected local or systemic infection, what are the effects of using procalcitonin measurement plus clinical criteria for infection to guide initiation, intensification, and/or discontinuation of antibiotic therapy when compared to clinical criteria for infection alone?
Search Strategy
MEDLINE and EMBASE were searched from January 1, 1990 through December 16, 2011, and the Cochrane Controlled Trials register was searched with no date restriction for randomized and nonrandomized comparative studies using the following search terms: procalcitonin AND chronic obstructive pulmonary disease; COPD; critical illness; critically ill; febrile neutropenia; ICU; intensive care; intensive care unit; postoperative complication(s); postoperative infection(s); postsurgical infection(s); sepsis; septic; surgical wound infection; systemic inflammatory response syndrome OR postoperative infection. In addition, a search for systematic reviews was conducted in MEDLINE, the Cochrane Database of Systematic Reviews, and Web sites of the National Institute for Clinical Excellence, the National Guideline Clearinghouse, and the Health Technology Assessment Programme. Gray literature, including databases with regulatory information, clinical trial registries, abstracts and conference papers, grants and federally funded research, and manufacturing information was searched from January 1, 2006 to June 28, 2011.
Study Selection
A single reviewer screened abstracts and selected studies looking at procalcitonin‐guided antibiotic therapy. Second and third reviewers were consulted to screen articles when needed. Studies were included if they fulfilled all of the following criteria: (1) randomized, controlled trial or nonrandomized comparative study; (2) adult and/or pediatric patients with known or suspected local or systemic infection, including critically ill patients with sepsis syndrome or ventilator‐associated pneumonia, adults with respiratory tract infections, neonates with sepsis, children with fever of unknown source, and postoperative patients at risk of infection; (3) interventions included initiation, intensification, and/or discontinuation of antibiotic therapy guided by procalcitonin plus clinical criteria; (4) primary outcomes included antibiotic usage (antibiotic prescription rate, total antibiotic exposure, duration of antibiotic therapy, and days without antibiotic therapy); and (5) secondary outcomes included morbidity (antibiotic adverse events, hospital and/or intensive care unit length of stay), mortality, and quality of life.
Studies with any of the following criteria were excluded: published in non‐English language, not reporting primary data from original research, not a randomized, controlled trial or nonrandomized comparative study, not reporting relevant outcomes.
Data Extraction and Quality Assessment
A single reviewer abstracted data and a second reviewer confirmed accuracy. Disagreements between reviewers were resolved by group discussion among the research team and final quality rating was assigned by consensus adjudication. Data elements were abstracted into the following categories: quality assessment, applicability and clinical diversity assessment, and outcome assessment. Quality of included studies was assessed using the US Preventive Services Task Force framework[17] by at least 2 independent reviewers. Three quality categories were used: good, fair, and poor.
Data Synthesis and Analysis
The decision to incorporate formal data synthesis in this review was made after completing the formal literature search, and the decision to pool studies was based on the specific number of studies with similar questions and outcomes. If a meta‐analysis could be performed, subgroup and sensitivity analyses were based on clinical similarity of available studies and reporting of mean and standard deviation. The pooling method involved inverse variance weighting and a random effects model.
The strength of evidence was graded using the Methods Guide,[16] a system based on the Grading of Recommendations Assessment, Development and Evaluation Working Group.[18] The following domains were addressed: risk of bias, consistency, directness, and precision. The overall strength of evidence was graded as high, moderate, low, or insufficient. The final strength of evidence grading was made by consensus adjudication among the authors.
RESULTS
Of the 2000 studies identified through the literature search, 1986 were excluded and 14 studies[19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32] were included. Search of gray literature yielded 4 published studies.[33, 34, 35, 36] A total of 18 randomized, controlled trials comparing procalcitonin guidance to use of clinical criteria alone to manage antibiotic therapy in patients with infections were included. The PRISMA diagram (Figure 1) depicts the flow of search screening and study selection. We sought, but did not find, nonrandomized comparative studies of populations, comparisons, interventions, and outcomes that were not adequately studied in randomized, controlled trials.
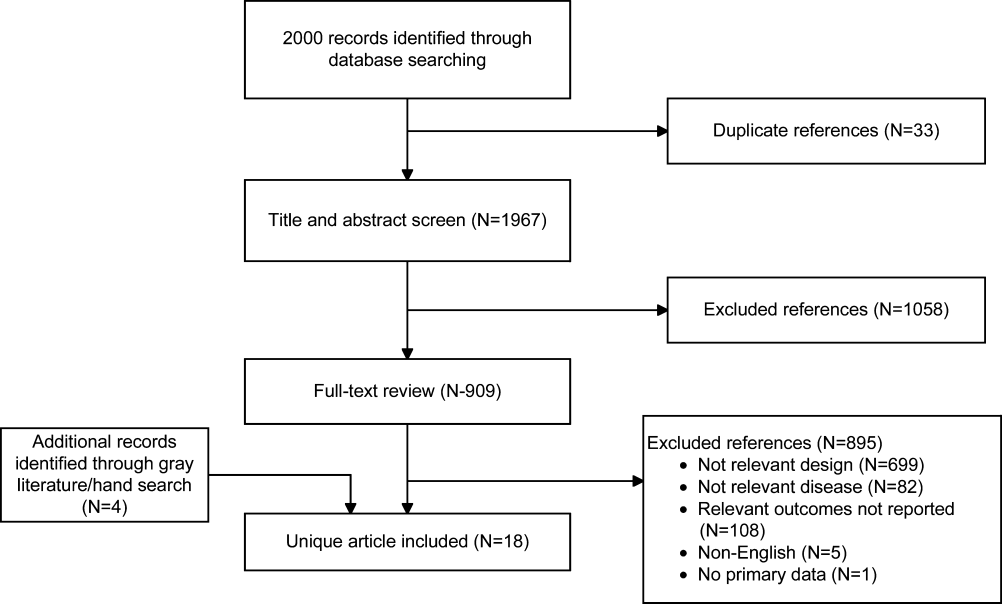
Data were pooled into clinically similar groups that were reviewed separately: (1) adult intensive care unit (ICU) patients, including patients with ventilator‐associated pneumonia; (2) adult patients with respiratory tract infections; (3) neonates with suspected sepsis; (4) children between 1 to 36 months of age with fever of unknown source; and (5) postoperative patients at risk of infection. Tables summarizing study quality and outcome measures with strength of evidence are available online (
| Outcome | Author, Year | N | PCT‐Guided Therapya | Controla | Difference PCT‐CTRL (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| ABT Duration, d | Hochreiter, 2009[22] | 110 | 5.9 | 7.9 | 2.0 (2.5 to 1.5) | <0.001 |
| Nobre, 2008[19] | 79 | 66 | 9.5 (ITT), 10 (PP) | 2.6 (5.5 to 0.3), 3.2 (1.1 to5.4) | 0.15, 0.003 | |
| Schroeder, 2009[20] | 27 | 6.6 | 8.3 | 1.7 (2.4 to 1.0) | <0.001 | |
| Stolz, 2009[21] | 101 | 10 (616)b | 15 (1023)b | 5 | 0.049 | |
| Bouadma, 2010[23] | 621 | 10.3 | 13.3 | 3.0 (4.20 to 1.80) | <0.0001 | |
| Days without ABTs, day 28 | Nobre, 2008[19] | 79 | 15.3, 17.4 | 13, 13.6 | 2.3 (5.9 to 1.8), 3.8 (0.1 to 7.5)c | 0.28, 0.04 |
| Stolz, 2009[21] | 101 | 13 (221)b | 9.5 (1.517)b | 3.5 | 0.049 | |
| Bouadma, 2010[23] | 621 | 14.3 | 11.6 | 2.7 (1.4 to 4.1) | <0.001 | |
| Total ABT exposured | Nobre, 2008[19] | 79 | 541 | 644 | 1.1e (0.9 to 1.3), 1.3e (1.1 to 1,5)c | 0.07, 0.0002 |
| 504 | 655 | |||||
| Stolz, 2009[21] | 101 | 1077 | 1341 | |||
| Bouadma, 2010[23]d | 621 | 653 | 812 | 159 (185 to 131) | <0.001 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| ABT duration, days | Jensen, 2011[33] | 1200 | 6 (311)b | 4 (310)b | NR | NR |
| Days spent in ICU on 3 ABTs | Jensen, 2011[33] | 1200 | 3570/5447 (65.5%) | 2721/4717 (57.7%) | 7.9% (6.0 to 9.7) | 0.002 |
| Adult patients with respiratory tract infections | ||||||
| ABT duration, da | Schuetz, 2009[2][5] | 1359 | 5.7 | 8.7 | 3.0 | |
| Christ‐Crain, 2004[30] | 243 | 10.9 | 12.8 | 1.9 (3.1 to 0.7) | 0.002 | |
| Kristoffersen, 2009[26] | 210 | 5.1 | 6.8 | 1.7 | ||
| Briel, 2008[27] | 458 | 6.2 | 7.1 | 1.0 (1.7 to 0.4) | <0.05 | |
| Long, 20113[5] | 162 | 5 (36)f | 7 (59)f | 2.0 | <0.001 | |
| Burkhardt, 2010[34] | 550 | 7.8 | 7.7 | 0.1 (0.7 to 0.9) | 0.8 | |
| Christ‐Crain, 2006[29] | 302 | 5.8 | 12.9 | 7.1(8.4 to 5.8) | <0.0001 | |
| Antibiotic prescription rate, % | Schuetz, 2009[2][5] | 1359 | 506/671 (75.4%) | 603/688 (87.6%) | 12.2% (16.3 to 8.1) | <0.05 |
| Christ‐Crain, 2004[30] | 243 | 55/124 (44.4%) | 99/119 (83.2%) | 38.8% (49.9 to 27.8) | <0.0001 | |
| Kristoffersen, 2009[26] | 210 | 88/103 (85.4%) | 85/107 (79.4%) | 6.0% (4.3 to 16.2) | 0.25 | |
| Briel, 2008[27] | 458 | 58/232 (25.0%) | 219/226 (96.9%) | 72% (78 to 66) | <0.05 | |
| Long, 20113[5] | 162 | NR (84.4%) | NR (97.5%) | 13.1% | 0.004 | |
| Stolz, 2007[28] | 208 | 41/102 (40.2%) | 76/106 (71.7%) | 31.5% (44.3 to 18.7) | <0.0001 | |
| Christ‐Crain, 2006[29] | 302 | 128/151 (84.8%) | 149/151 (98.79%) | 13.9% (19.9 to 7.9) | <0.0001 | |
| Burkhardt, 2010[34] | 550 | 84/275 (30.5%) | 89/275 (32.4%) | 1.8% (9.6 to 5.9) | 0.701 | |
| Total ABT exposure | Stolz, 2007[28] | 208 | NR | NR | 31.5% (18.7 to 44.3) | <0.0001 |
| Long, 20113[5] | 162 | NR | NR | NR | ||
| Christ‐Crain, 2006[29] | 302 | 136g | 323g | |||
| Christ‐Crain, 2004[30] | 243 | 332g | 661g | |||
| Neonates with sepsis | ||||||
| ABTs 72 hours, % | Stocker, 2010[31] | All neonates (N=121) | 33/60 (55%) | 50/61 (82%) | 27.0 (42.8 to 11.1) | 0.002 |
| Infection proven/probably (N=21) | 9/9 (100%) | 12/12 (100%) | 0% (0 to 0) | NA | ||
| Infection possible (N=40) | 13/21 (61.9%) | 19/19 (100%) | 38.1 (58.9 to 17.3) | 0.003 | ||
| Infection unlikely (N=60) | 11/30 (36.7%) | 19/30 (63.3%) | 26.6 (51.1 to 2.3) | 0.038 | ||
| ABT duration, h | Stocker, 2010[31] | All neonates (N=121) | 79.1 | 101.5 | 22.4 | 0.012 |
| Infection proven/probably (N=21) | 177.8 | 170.8 | 7 | NSS | ||
| Infection possible (N=40) | 83.4 | 111.5 | 28.1 | <0.001 | ||
| Infection unlikely (N=60) | 46.5 | 67.4 | 20.9 | 0.001 | ||
| Children ages 136 months with fever of unknown source | ||||||
| Antibiotic prescription rate, % | Manzano, 2010[36] | All children (N=384) | 48/192 (25%) | 54/192 (28.0%) | 3.1 (12.0 to 5.7) | 0.49 |
| No SBI or neutropenia (N=312) | 14/158 (9%) | 16/154 (10%) | 1.5 (8.1 to 5.0) | 0.65 | ||
| Adult postoperative patients at risk of infection | ||||||
| ABT duration, d | Chromik, 2006[32] | All patients (N=20) | 5.5 | 9 | 3.5 | 0.27 |
| Outcome | Author, Year | N | PCTa | Controla | Difference, PCT‐CTRL (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| ICU LOS, days | Hochreiter, 2009[22] | 110 | 15.5 | 17.7 | 2.2 | 0.046 |
| Nobre, 2008[19] | 79 | 4 | 7 | 4.6 (8.2 to 1.0) | 0.02 | |
| Schroeder, 2009[20] | 27 | 16.4 | 16.7 | 0.3 (5.6 to 5.0) | NSS | |
| Bouadma, 2010[23] | 621 | 15.9 | 14.4 | 1.5 (0.9 to 3.1) | 0.23 | |
| Hospital LOS, days | Nobre, 2008[19] | 79 | 17 | 23.5 | 2.5 (6.5 to 1.5) | 0.85 |
| Stolz, 2009[21] | 101 | 26 (721)b | 26 (16.822.3)b | 0 | 0.15 | |
| Bouadma, 2010[23] | 621 | 26.1 | 26.4 | 0.3 (3.2 to 2.7) | 0.87 | |
| ICU‐free days alive, 128 | Stolz, 2009[21] | 101 | 10 (018)b | 8.5 (018)c | 1.5 | 0.53 |
| SOFA day 28 | Bouadma, 2010[23] | 621 | 1.5 | 0.9 | 0.6 (0.0, 1.1) | 0.037 |
| SOFA score max | Schroeder, 2009[20] | 27 | 7.3 | 8.3 | 8.1 (4.1 to 1.7) | NSS |
| SAPS II score | Hochreiter, 2009[22] | 110 | 40.1 | 40.5 | 0.4 (6.4 to 5.6) | >0.05 |
| Days without MV | Stolz, 2009[21] | 101 | 21 (224)b | 19 (8.522.5)b | 2.0 | 0.46 |
| Bouadma, 2010[23] | 621 | 16.2 | 16.9 | 0.7 (2.4 to 1.1) | 0.47 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| ICU LOS, da | Svoboda, 2007[24] | 72 | 16.1 | 19.4 | 3.3 (7.0 to 0.4) | 0.09 |
| Jensen, 2011[33] | 1200 | 6 (312)b | 5 (311)b | 1 | 0.004 | |
| SOFA scorea | Svoboda, 2007[24] | 72 | 7.9 | 9.3 | 1.4 (2.8 to 0.0) | 0.06 |
| Days on MVa | Svoboda, 2007[24] | 72 | 10.3 | 13.9 | 3.6 (7.6 to 0.4) | 0.08 |
| Jensen, 2011[33] | 1200 | 3569 (65.5%) | 2861 (60.7%) | 4.9% (3 to 6.7) | <0.0001 | |
| Percent days in ICU with GFR <60 | Jensen, 2011[33] | 1200 | 2796 (51.3%) | 2187 (46.4%) | 5.0 % (3.0 to 6.9) | <0.0001 |
| Adult patients with respiratory tract infections | ||||||
| Hospital LOS, da | Schuetz, 2009[2][5] | 1359 | 9.4 | 9.2 | 0.2 | |
| Christ‐Crain, 2004[30] | 224 | 10.78.9 | 11.210.6 | 0.5 (3.0 to 2.0) | 0.69 | |
| Kristoffersen, 2009[26] | 210 | 5.9 | 6.7 | 0.8 | 0.22 | |
| Stolz, 2007[28] | 208 | 9 (115)b | 10 (115)b | 1 | 0.96 | |
| Christ‐Crain, 2006[29] | 302 | 12.09.1 | 13.09.0 | 1 (3.0 to 1.0) | 0.34 | |
| ICU admission, % | Schuetz, 2009[2][5] | 1359 | 43/671 (6.4%) | 60/688 (8.7%) | 2.3% (5.2 to 0.4) | 0.12 |
| Christ‐Crain, 2004[30] | 224 | 5/124 (4.0%) | 6/119 (5.0%) | 1.0% (6.2 to 4.2) | 0.71 | |
| Kristoffersen, 2009[26] | 210 | 7/103 (6.8%) | 5/107 (4.7%) | 2.1% (4.2 to 8.4) | 0.51 | |
| Stolz, 2007[28] | 208 | 8/102 (7.8%) | 11/106 (10.4%) | 2.5% (10.3 to 5.3) | 0.53 | |
| Christ‐Crain, 2006[29] | 302 | 20/151 (13.2%) | 21/151 (13.94%) | 0.7% (8.4 to 7.1) | 0.87 | |
| Antibiotic adverse events | Schuetz, 2009[2][5]c | 1359 | 133/671 (19.8%) | 193/688 (28.1%) | 8.2% (12.7 to 3.7) | |
| Briel, 2008[27]d | 458 | 2.34.6 days | 3.66.1 days | 1.1 days (2.1 to 0.1) | <0.05 | |
| Burkhardt, 2010[34]e | 550 | 11 /59 (18.6%) | 16/101 (15.8%) | 2.8% (9.4 to 15.0) | 0.65 | |
| Restricted activity, df | Briel, 2008[27] | 458 | 8.73.9 | 8.63.9 | 0.2 (0.4 to 0.9) | >0.05 |
| Burkhardt, 2010[34] | 550 | 9.1 | 8.8 | 0.25 (0.52 to 1.03) | >0.05 | |
| Neonates with sepsis | ||||||
| Recurrence of infection | Stocker, 2010[31] | 121 | 32% | 39% | 7 | 0.45 |
| Children ages 136 months with fever of unknown source | ||||||
| Hospitalization rate | Manzano, 2010[36] | All children (N=384) | 50/192 (26%) | 48/192 (25%) | 1 (8 to 10) | 0.81 |
| No SBI or neutropenia (N=312) | 16/158 (10%) | 11/154 (7%) | 3 (3 to 10) | 0.34 | ||
| Adult postoperative patients at risk of infection | ||||||
| Hospital LOS, days | Chromik, 2006[32] | 20 | 18 | 30 | 12 | 0.057 |
| Local wound infection, % | Chromik, 2006[32] | 20 | 1/10 | 2/10 | 10 (41.0 to 21.0) | 0.53 |
| Systemic infection, % | Chromik, 2006[32] | 20 | 3/10 | 7/10 | 40.0 (80.2 to 0.2) | 0.07 |
| Sepsis/SIRS, % | Chromik, 2006[32] | 20 | 2/10 | 8/10 | 60.0 (95.1 to 24.9) | 0.007 |
| Mortality | Mortality | Difference | ||||
|---|---|---|---|---|---|---|
| Outcome | Author, Year | N | PCT‐Guided Therapy | Control | PCT‐CTRL (95% CI) | P Value |
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| 28‐day mortality | Nobre, 2008[19] | 79 | 8/39 (20.5%) | 8/40 (20.0%) | 0.5 (17.2 to 18.2), | 0.95 |
| 5/31 (16.1%) | 6/37 (16.2%) | 0.1 (17.7 to 17.5)a | 0.99 | |||
| Stolz, 2009[21] | 101 | 8/51 (15.7%) | 12/50 (24.0%) | 8.3 (23.8 to 7.2) | 0.29 | |
| Bouadma, 2010[23] | 621 | 65/307 (21.2%) | 64/314 (20.4%) | 0.8 (5.6 to 7.2) | 0.81 | |
| 60‐day mortality | Bouadma, 2010[23] | 621 | 92/307 (30.0%) | 82/314 (26.1%) | 3.9 (3.2 to 10.9) | 0.29 |
| In‐hospital mortality | Nobre, 2008[19] | 79 | 9/39 (23.1%) | 9/40 (22.5%) | 0.6 (17.9 to 19.1) | 0.95 |
| 6/31 (19.4%) | 7/37 (18.9%) | 0.4+ (18.3 to 19.2) | 0.96 | |||
| Stolz, 2009[21] | 101 | 10/51 (19.6%) | 14/50 (28.0%) | 8.4, (24.9 to 8.1) | 0.32 | |
| Hochreiter, 2009[22] | 110 | 15/57 (26.3%) | 14/53 (26.4%) | 0.1, (16.6 to 16.4) | 0.99 | |
| Schroeder, 2009[20] | 27 | 3/14 (21.4%) | 3/13 (23.1%) | 1.7, (33.1 to 29.8) | 0.92 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| 28‐day mortality | Svoboda, 2007[24] | 72 | 10/38 (26.3%) | 13/34 (38.2%) | 11.9 (33.4 to 9.6) | 0.28 |
| 28‐day mortality | Jensen, 2011[33] | 1200 | 190/604 (31.5%) | 191/596 (32.0%) | 0.6 (4.7 to 5.9) | 0.83 |
| Adult patients with respiratory tract infections | ||||||
| 6‐month mortality | Stolz, 2007[28] | 208 | 5/102 (4.9%) | 9/106 (8.5%) | 3.6% (10.3 to 3.2%) | 0.30 |
| 6‐week mortality | Christ‐Crain, 2006[29] | 302 | 18/151 (11.9%) | 20/151 (13.2%) | 1.3% (8.8 to 6.2) | 0.73 |
| 28‐day mortality | Christ‐Crain, 2004[30] | 243 | 4/124(3.2%) | 4/119 (3.4%) | 0.1% (4.6 to 4.4) | 0.95 |
| Schuetz, 2009 (30‐day)[25] | 1359 | 34/671(5.1%) | 33/688(4.8%) | 0.3% (2.1 to 2.5) | 0.82 | |
| Briel, 2008[27] | 458 | 0/231(0%) | 1/224 (0.4%) | 0.4% (1.3 to 0.4) | 0.31 | |
| Burkhardt, 2010[34] | 550 | 0/275(0%) | 0/275 (0%) | 0 | ||
| Kristoffersen, 2009[26] | 210 | 2/103(1.9%) | 1/107 (0.9%) | 1.0% (2.2 to 4.2) | 0.54 | |
| Long, 20113[5] | 162 | 0/81 (0%) | 0/81 (0%) | 0 | ||
| Neonates with sepsis | ||||||
| Mortality (in‐hospital) | Stocker, 2010[31] | 121 | 0% | 0% | 0 (0 to 0) | NA |
| Children ages 136 months with fever of unknown source | ||||||
| Mortality | Manzano, 2010[36] | 384 | All children | 0% | 0% | 0 (0 to 0) |
| Adult postoperative patients at risk of infection | ||||||
| Mortality | Chromik, 2006[32] | 20 | 1/10 (10%) | 3/10 (30%) | 20 (54.0 to 14.0) | 0.07 |
Adult ICU Patients: Procalcitonin‐Guided Antibiotic Discontinuation
Five studies[19, 20, 21, 22, 23] (N=938) addressed procalcitonin‐guided discontinuation of antibiotic therapy in adult ICU patients. Four studies conducted superiority analyses for mortality with procalcitonin‐guided therapy, whereas 1 study conducted a noninferiority analysis. Absolute procalcitonin values for discontinuation of antibiotics ranged from 0.25 to 1 ng/mL. Physicians in control groups administered antibiotics according to their standard practice.
Antibiotic Usage
The absolute reduction in duration of antibiotic usage with procalcitonin guidance in these studies ranged from 1.7 to 5 days, and the relative reduction ranged from 21% to 38%. Meta‐analysis of antibiotic duration in adult ICU patients was performed (Figure 2A).
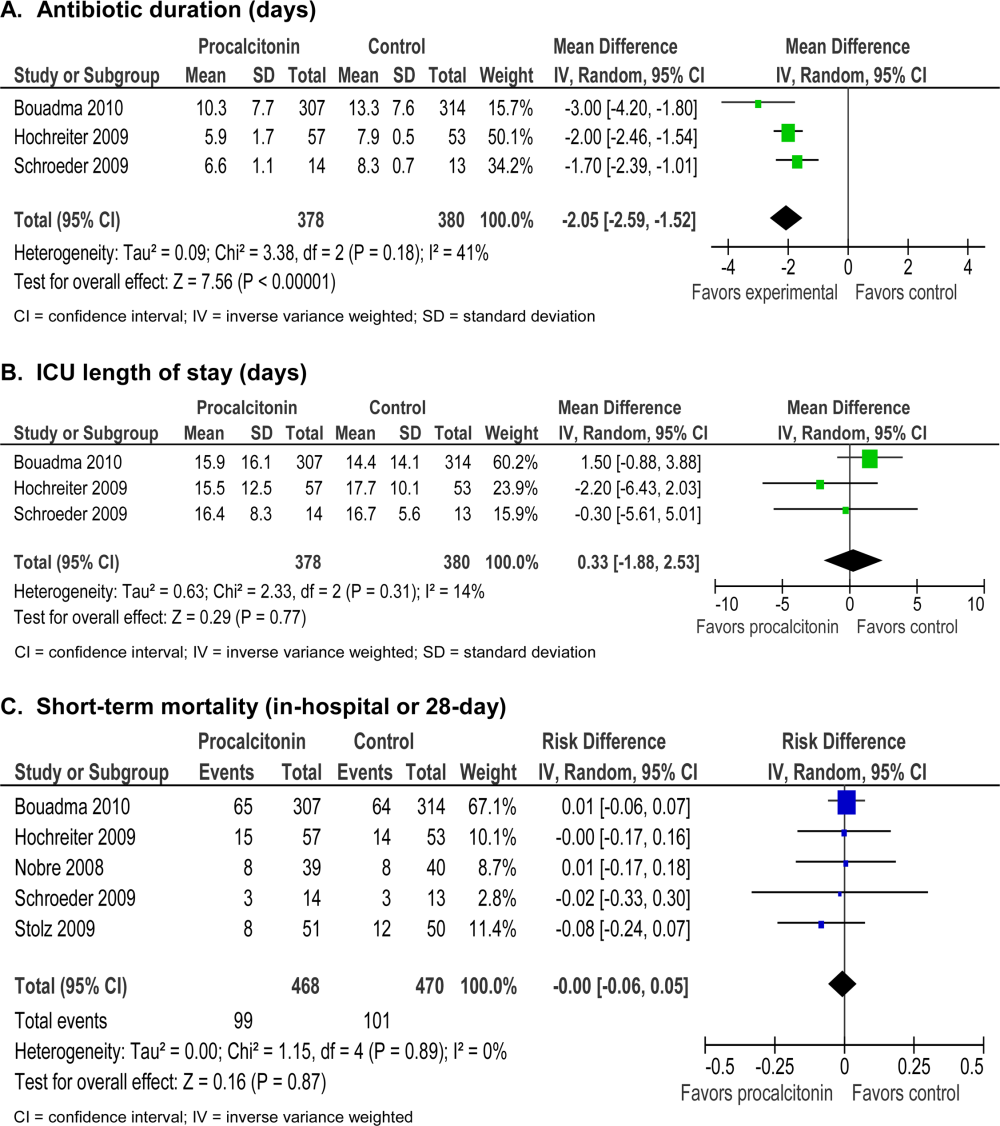
Morbidity
Procalcitonin‐guided antibiotic discontinuation did not increase morbidity, including ICU length of stay (LOS). Meta‐analysis of ICU LOS is displayed in Figure 2B. Limited data on adverse antibiotic events were reported (Table 2).
Mortality
There was no increase in mortality as a result of shorter duration of antibiotic therapy. Meta‐analysis of short‐term mortality (28‐day or in‐hospital mortality) showed a mortality difference of 0.43% favoring procalcitonin‐guided therapy, and a 95% confidence interval (CI) of 6% to 5% (Figure 2C).
Adult ICU Patients: Procalcitonin‐Guided Antibiotic Intensification
Two studies[24, 33] (N=1272) addressed procalcitonin‐guided intensification of antibiotic therapy in adult ICU patients. The Jensen et al. study[33] was a large (N=1200), high‐quality study that used a detailed algorithm for broadening antibiotic therapy in patients with elevated procalcitonin. The Jensen et al. study also educated physicians about empiric therapy and intensification of antibiotic therapy. A second study[24] was too small (N=72) and lacked sufficient details to be informative.
Antibiotic Usage
The Jensen et al. study found a 2‐day increase, or 50% relative increase, in the duration of antibiotic therapy and a 7.9% absolute increase (P=0.002) in the number of days on 3 antibiotics with procalcitonin‐guided intensification.
Morbidity
The Jensen et al. study showed a significant 1‐day increase in ICU LOS (P=0.004) and a significant increase in organ dysfunction. Specifically, patients had a highly statistically significant 5% increase in days on mechanical ventilation (P<0.0001) and 5% increase in days with abnormal renal function (P<0.0001).
Mortality
The Jensen et al. study was a superiority trial powered to test a 7.5% decrease in 28‐day mortality, but no significant difference in mortality was observed with procalcitonin‐guided intensification (31.5% vs 32.0, P=0.83).
Adult Patients With Respiratory Tract Infections
Eight studies[25, 26, 27, 28, 29, 30, 34, 35] (N=3492) addressed initiation and/or discontinuation of antibiotics in adult patients with acute upper and lower respiratory tract infections, including community‐acquired pneumonia, acute exacerbation of chronic obstructive pulmonary disease, and acute bronchitis. Settings included primary care clinics, emergency departments, and hospital wards. Physicians in control groups administered antibiotics according to their own standard practices and/or evidence‐based guidelines. All studies encouraged initiation of antibiotics with procalcitonin levels >0.25 ng/mL, and 4 studies strongly encouraged antibiotics with procalcitonin levels >0.5 ng/mL.
Antibiotic Usage
Procalcitonin guidance reduced antibiotic duration, antibiotic prescription rate, and total antibiotic exposure. Absolute reduction in antibiotic duration ranged from 1 to 7 days, and relative reductions ranged from 13% to 55%. Four of the 8 studies reported sufficient details to be pooled into a meta‐analysis (Figure 3A) with a statistically significant pooled mean difference of 2.35 days favoring procalcitonin (95% CI: 4.38 to 0.33). Procalcitonin guidance also reduced antibiotic prescription rate with absolute reductions ranging from 2% to 7% and relative reductions ranging from 1.8% to 72%. Meta‐analysis of prescription rates from 8 studies (Figure 3B) yielded a statistically significant pooled risk difference of 22% (95% CI: 41% to 4%). Total antibiotic exposure was consistently reduced in the 4 studies reporting this outcome.
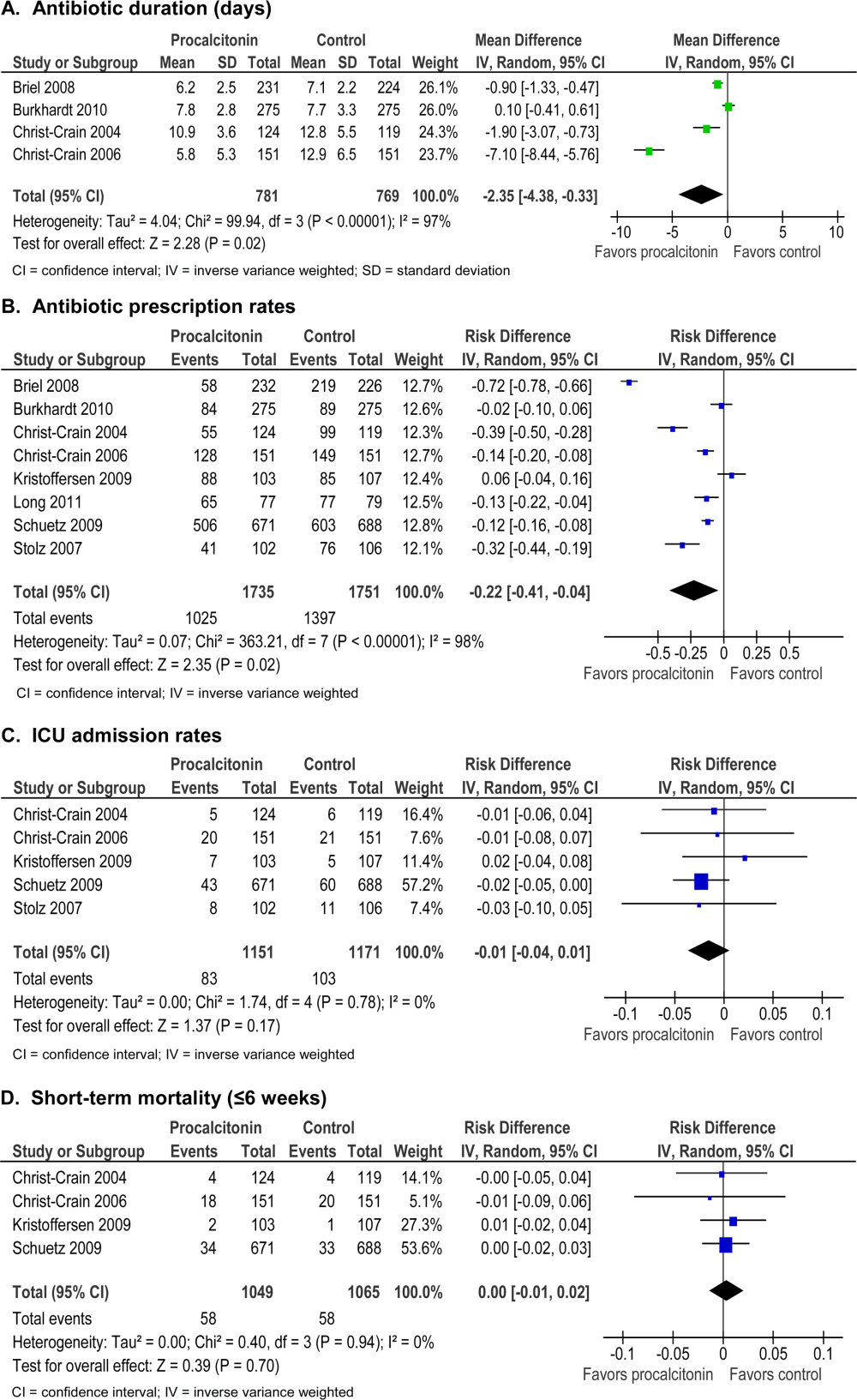
Morbidity
Procalcitonin guidance did not increase hospital LOS or ICU admission rates. Meta‐analysis of ICU admission rates from 5 studies (Figure 3C) produced a risk difference of 1%, with a narrow 95% CI (4% to 1%). There was insufficient evidence to judge the effect on days of restricted activity or antibiotic adverse events.
Mortality
Procalcitonin guidance did not increase mortality, and meta‐analysis of 4 studies (Figure 3D) produced a risk difference of 0.3% with a narrow 95% CI (1% to 2%), with no statistical heterogeneity (I2=0%).
Neonates With Sepsis
One study[31] (N=121) evaluated procalcitonin‐guided antibiotic therapy for suspected neonatal sepsis. Neonatal sepsis was suspected on the basis of risk factors and clinical signs and symptoms. Antibiotic initiation or discontinuation was based on a procalcitonin nomogram. Antibiotic therapy in the control group was based on the physician's assessment. The quality of this study was rated good, and strength of evidence was rated moderate for antibiotic usage and insufficient for morbidity and mortality outcomes.
Antibiotic Usage
Duration of antibiotic therapy was decreased by 22.4 hours (P=0.012), a 24% relative reduction, and the proportion of neonates on antibiotics 72 hours was reduced by 27% (P=0.002). The largest reduction in antibiotic duration was seen in the 80% to 85% of neonates who were categorized as having possible or infection or unlikely to have infection.
Morbidity
A statistically insignificant 7% reduction in rate of recurrence of infection was seen with procalcitonin‐guided antibiotic therapy (P=0.45).
Mortality
No in‐hospital deaths occurred in either the procalcitonin or control group.
Children Ages 1 to 36 Months With Fever of Unknown Source
One study[36] (N=384) evaluated procalcitonin‐guided antibiotic therapy for fever of unknown source in children 1 to 36 months of age, but the overall strength of evidence was judged insufficient to draw conclusions.
Antibiotic Usage
A statistically insignificant reduction of 3.1% in antibiotic prescription rate was seen with procalcitonin‐guided antibiotic therapy (P=0.49).
Morbidity
Rate of hospitalization was relatively low, and no significant difference was seen between procalcitonin and control groups.
Mortality
In‐hospital mortality was reported as 0% in both arms.
Adult Postoperative Patients at Risk of Infection
One study[32] (N =250) monitored procalcitonin in consecutive patients after colorectal surgery to identify patients at risk of infection who might benefit from prophylactic antibiotic therapy. Two hundred thirty patients had normal procalcitonin levels. Twenty patients with elevated procalcitonin levels (>1.5 ng/mL) were randomized to receive prophylactic antibiotic therapy with ceftriaxone or no antibiotics. The strength of evidence was judged insufficient to draw conclusions from this study.
Antibiotic Usage
Duration of antibiotic therapy was reduced by 3.5% but was not statistically insignificant (P=0.27).
Morbidity
Procalcitonin guidance reduced the incidence of sepsis/systemic inflammatory response syndrome by 60% (p=0.007). The incidences of local and systemic infection were reduced with procalcitonin guidance but were not statistically significant (10%, P=0.53; and 40%, P=0.07, respectively).
Mortality
Mortality was 20% higher in the control arm but was not statistically significant (P=0.07).
DISCUSSION
Summary of the Main Findings
Diagnosis of sepsis or other serious infections in critically ill patients is challenging because clinical criteria for diagnosis overlap with noninfectious causes of the systemic inflammatory response syndrome. Initiation of antibiotic therapy for presumed sepsis is necessary while diagnostic evaluation is ongoing, because delaying antibiotic therapy is associated with increased mortality.[37, 38, 39] Our review found that procalcitonin guidance significantly reduced antibiotic usage in adult ICU patients by reducing the duration of antibiotic therapy, rather than decreasing the initiation of antibiotics, without increasing morbidity or mortality.
In contrast, the use of procalcitonin as an indicator of need for intensification of antibiotic therapy in adult ICU patients should be discouraged because this approach was associated with increased morbidity. The large, well‐designed study by Jensen[33] showed that antibiotic intensification in response to elevated procalcitonin measurement was associated with increased morbidity: a longer ICU LOS, an increase in days on mechanical ventilation, and an increase in days with abnormal renal function. The authors concluded that the increased morbidity could only be explained by clinical harms of increased exposure to broad‐spectrum antibiotics.
Clinical and microbiological evaluations are neither sensitive nor specific for differentiating bacterial from viral respiratory tract infections. Procalcitonin can guide initiation of antibiotic therapy in adults with suspected bacterial respiratory tract infection. Our review showed that procalcitonin guidance significantly reduced antibiotic usage with respect to antibiotic prescription rate, duration of antibiotic therapy, and total exposure to antibiotic therapy in adult patients with respiratory tract infections.
The role of procalcitonin‐guided therapy in other populations is less clear. One study in postoperative colorectal surgery patients reported that elevated procalcitonin levels may identify patients at risk for infection who benefit from prophylactic antibiotic therapy.[32] Patients with elevated procalcitonin levels who received prophylactic antibiotic therapy had a significant decrease in the incidence and severity of systemic infections, whereas patients with normal procalcitonin levels did not require any additional surgical or medical therapy. Although these findings are promising, more data in postoperative patients are needed.
The utility of procalcitonin in pediatric settings is a significant gap in the present literature. One study[31] in neonates with suspected sepsis showed a significant decrease in the proportion of neonates started on empiric antibiotic therapy and a decrease in the duration of antibiotic therapy with procalcitonin guidance. However, there was insufficient evidence that procalcitonin guidance does not increase morbidity or mortality.
Comparison to Other Systematic Reviews
Six systematic reviews of procalcitonin guidance in the management of patients with infections were published prior to our review.[9, 10, 11, 12, 13, 14] Our systematic review differs from past reviews in the number of studies included and the pooling of studies according to patient population, type and severity of infection, and different uses of procalcitonin measurements, either for initiation, discontinuation, or intensification of antibiotic therapy. Previous systematic reviews included 7 to 14 studies, whereas ours included 18 randomized, controlled trials. One previous review[13] included and pooled the Jensen et al. study[33] with other studies of adult ICU patients. We evaluated the Jensen et al. study separately because it uniquely looked at procalcitonin‐guided antibiotic intensification in adult ICU patients, in contrast to other studies that looked at procalcitonin‐guided antibiotic discontinuation. We addressed pediatric populations separately from adult patients, and recognizing that there are distinct groups within the pediatric population, we separately grouped neonates and children ages 1 to 36 months. Despite these differences, our review and other systematic reviews, we came to similar conclusions: procalcitonin‐guided antibiotic decision making compared to clinical criteria‐guided antibiotic decision making reduces antibiotic usage without increasing morbidity or mortality.
Limitations
An important limitation of this review was the uncertainty about the noninferiority margin for morbidity and mortality in adult ICU patients. Only the Bouadma et al. study[23] did a power analysis and predefined a margin for noninferiority for 28‐ and 60‐day mortality. Meta‐analysis of all 5 ICU studies showed a pooled point estimate of 0.43% in mortality and a 95% CI of 6% to 5% for difference in mortality between procalcitonin‐guided therapy versus standard care. A 10% noninferiority margin for mortality has been recommended by the Infectious Diseases Society of America and American College of Chest Physicians, but there is concern that a 10% margin for mortality may be too high. Presently, 2 large trials are in progress that may yield more precise estimates of mortality in the future.
Differences in reporting of total antibiotic exposure and morbidity outcomes limited our ability to pool data. Total antibiotic exposure is conventionally reported as mean days per 1000 days of follow‐up, but some studies only reported relative or absolute differences. Likewise, morbidity was reported with different severity of illness scales, including Sepsis‐Related Organ Failure Assessment, Simplified Acute Physiology (SAP) II, SAP III, and Acute Physiology and Chronic Health Evaluation II, which limited comparisons across studies.
Research Gaps
We identified gaps in the available literature and opportunities for future research. First, the safety and efficacy of procalcitonin‐guided antibiotic therapy needs to be studied in patient populations excluded from current randomized controlled studies, such as immunocompromised patients and pregnant women. Patients who are immunocompromised or have chronic conditions, such as cystic fibrosis, account for a significant percentage of community‐acquired respiratory tract infections and are often treated empirically.[29, 30] Second, standardized reporting of antibiotic adverse events and emergence of antibiotic resistance is needed. Strategies to reduce antibiotic usage have been associated with reductions in antibiotic adverse events, such as Clostridium difficile colitis and superinfection with multi‐drug resistant Gram‐negative bacteria.[37, 40, 41] Few studies in our review reported allergic reactions or adverse events of antibiotic therapy, [25, 27, 34] and only 1 reported antibiotic resistance.[19] Third, procalcitonin guidance should be compared to other strategies to reduce antibiotic usage, such as structured implementation of practice guidelines and antibiotic stewardship programs.[42] One single‐arm study describes how procalcitonin can be used in antibiotic stewardship programs to decrease the duration of antibiotic therapy,[43] but additional studies are needed. Finally, generalizing results from those studies that were conducted primarily in Europe would depend on similar use of and adherence to study‐based algorithms. Newer observational studies have demonstrated reduced antibiotic usage with implementation of procalcitonin algorithms in real‐life settings in Europe, but algorithm adherence was significantly less in the United States.[44, 45]
In summary, our systematic review found that procalcitonin‐guided antibiotic therapy can significantly reduce antibiotic usage in adult ICU patients without affecting morbidity or mortality. Procalcitonin should not be used to guide intensification of antibiotic therapy in adult ICU patients because this approach may increase morbidity. In adults with respiratory infections, procalcitonin guidance can significantly reduce antibiotic usage without adversely affecting morbidity or mortality. There is insufficient evidence to recommend procalcitonin‐guided antibiotic therapy in neonates with sepsis, children with fever of unknown source, or postoperative patients at risk for infection.
Acknowledgments
Disclosures: This project was funded under contract HHSA 2902007‐10058 from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services. The authors of this article are responsible for its content, including any clinical treatment recommendations. No statement in this article should be construed as an official position of AHRQ or of the US Department of Health and Human Services. There are no conflicts of interest reported by any of the authors.
- , . Sepsis biomarkers: a review. Crit Care. 2010;14(1):R15.
- , . Biomarkers of sepsis. Crit Care Med. 2009;37(7):2290–2298.
- , , . Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888–889.
- , , , et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605–1608.
- , , , et al. Procalcitonin kinetics as a prognostic marker of ventilator‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(1):48–53.
- , , , , . Serum procalcitonin and C‐reactive protein levels as markers of bacterial infection: a systematic review and meta‐analysis. Clin Infect Dis. 2004;39(2):206–217.
- , . Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007;30(3):556–573.
- , , , et al. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis. 1998;26(3):664–672.
- , , , , . Effect of procalcitonin‐guided treatment in patients with infections: a systematic review and meta‐analysis. Infection. 2009;37(6):497–507.
- , . Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53(4):379–387.
- , , , , . Procalcitonin‐guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta‐analysis of randomized controlled trials. Crit Care Med. 2010;38(11):2229–2241.
- , , , . Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322–1331.
- , , , , , . An ESCIM systematic review and meta‐analysis of procalcitonin‐guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Med. 2012;38:940–949.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
- , , , , , . Prepared by the Blue Cross and Blue Shield Association Technology Evaluation Center Evidence‐based Practice Center under contract no. 290–2007‐10058‐I. Procalcitonin‐guided antibiotic therapy. Comparative effectiveness review No. 78. AHRQ publication no. 12(13)‐EHC124‐EF. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Published Accessed October 2012.
- Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ publication no. 10(11)‐EHC063‐EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 suppl):21–35.
- , , , et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—agency for healthcare research and quality and the effective health‐care program. J Clin Epidemiol. 2010;63(5):513–523.
- , , , , . Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177(5):498–505.
- , , , et al. Procalcitonin (PCT)‐guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009;394(2):221–226.
- , , , et al. Procalcitonin for reduced antibiotic exposure in ventilator‐associated pneumonia: a randomised study. Eur Respir J. 2009;34(6):1364–1375.
- , , , et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13(3):R83.
- , , , et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–474.
- , , , , . Can procalcitonin help us in timing of re‐intervention in septic patients after multiple trauma or major surgery? Hepatogastroenterology. 2007;54(74):359–363.
- , , , et al. Effect of procalcitonin‐based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066.
- , , , et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission—a randomized trial. Clin Microbiol Infect. 2009;15(5):481–487.
- , , , et al. Procalcitonin‐guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168(18):2000–2007; discussion 2007–2008.
- , , , et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest. 2007;131(1):9–19.
- , , , et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84–93.
- , , , et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363(9409):600–607.
- , , , , . Use of procalcitonin‐guided decision‐making to shorten antibiotic therapy in suspected neonatal early‐onset sepsis: prospective randomized intervention trial. Neonatology. 2010;97(2):165–174.
- , , , , , . Pre‐emptive antibiotic treatment vs “standard” treatment in patients with elevated serum procalcitonin levels after elective colorectal surgery: a prospective randomised pilot study. Langenbecks Arch Surg. 2006;391(3):187–194.
- , , , et al. Procalcitonin‐guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39(9):2048–2058.
- , , , et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J. 2010;36(3):601–607.
- , , , , , . Procalcitonin guidance for reduction of antibiotic use in low‐risk outpatients with community‐acquired pneumonia. Respirology. 2011;16(5):819–824.
- , , , , , . Impact of procalcitonin on the management of children aged 1 to 36 months presenting with fever without source: a randomized controlled trial. Am J Emerg Med. 2010;28(6):647–653.
- , , , , , . Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia. Crit Care Med. 2001;29(6):1109–1115.
- , , , et al. Diagnostic value of procalcitonin, interleukin‐6, and interleukin‐8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164(3):396–402.
- , , , . Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462–474.
- , , , , . Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol. 2003;24(9):699–706.
- , , , et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- , , , , . Use of procalcitonin (PCT) to guide discontinuation of antibiotic use in an unspecified sepsis is an antimicrobial stewardship program (ASP). Eur J Clin Microbiol Infect Dis. 2011;30(7):853–855.
- , , , et al. Effectiveness and safety of procalcitonin‐guided antibiotic therapy in lower respiratory tract infections in “real life.” Arch Intern Med. 2012;172(9):715–722.
- , , , et al. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: a post‐study survey. Eur J Clin Microbiol Infect Dis. 2012;29(3):269–277.
Many serum biomarkers have been identified in recent years with a wide range of potential applications, including diagnosis of local and systemic infections, differentiation of bacterial and fungal infections from viral syndromes or noninfectious conditions, prognostic stratification of patients, and enhanced management of antibiotic therapy. Currently, there are at least 178 serum biomarkers that have potential roles to guide antibiotic therapy, and among these, procalcitonin has been the most extensively studied biomarker.[1, 2]
Procalcitonin is the prohormone precursor of calcitonin that is expressed primarily in C cells of the thyroid gland. Conversion of procalcitonin to calcitonin is inhibited by various cytokines and bacterial endotoxins. Procalcitonin's primary diagnostic utility is thought to be in establishing the presence of bacterial infections, because serum procalcitonin levels rise and fall rapidly in bacterial infections.[3, 4, 5] In healthy individuals, procalcitonin levels are very low. In systemic infections, including sepsis, procalcitonin levels are generally greater than 0.5 to 2 ng/mL, but often reach levels 10 ng/mL, which correlates with severity of illness and a poor prognosis. In patients with respiratory tract infections, procalcitonin levels are less elevated, and a cutoff of 0.25 ng/mL seems to be most predictive of a bacterial respiratory tract infection requiring antibiotic therapy.[6, 7, 8] Procalcitonin levels decrease to <0.25 ng/mL as infection resolves, and a decline in procalcitonin level may guide decisions about discontinuation of antibiotic therapy.[5]
The purpose of this systematic review was to synthesize comparative studies examining the use of procalcitonin to guide antibiotic therapy in patients with suspected local or systemic infections in different patient populations. We are aware of 6 previously published systematic reviews evaluating the utility of procalcitonin guidance in the management of infections.[9, 10, 11, 12, 13, 14] Our systematic review included more studies and pooled patients into the most clinically similar groups compared to other systematic reviews.
METHODS
This review is based on a comparative effectiveness review prepared for the Agency for Healthcare Research and Quality's Effective Health Care Program.[15] A standard protocol consistent with the Methods Guide for Effectiveness and Comparative Effectiveness Reviews[16] was followed. A detailed description of the methods is available online (
Study Question
In selected populations of patients with suspected local or systemic infection, what are the effects of using procalcitonin measurement plus clinical criteria for infection to guide initiation, intensification, and/or discontinuation of antibiotic therapy when compared to clinical criteria for infection alone?
Search Strategy
MEDLINE and EMBASE were searched from January 1, 1990 through December 16, 2011, and the Cochrane Controlled Trials register was searched with no date restriction for randomized and nonrandomized comparative studies using the following search terms: procalcitonin AND chronic obstructive pulmonary disease; COPD; critical illness; critically ill; febrile neutropenia; ICU; intensive care; intensive care unit; postoperative complication(s); postoperative infection(s); postsurgical infection(s); sepsis; septic; surgical wound infection; systemic inflammatory response syndrome OR postoperative infection. In addition, a search for systematic reviews was conducted in MEDLINE, the Cochrane Database of Systematic Reviews, and Web sites of the National Institute for Clinical Excellence, the National Guideline Clearinghouse, and the Health Technology Assessment Programme. Gray literature, including databases with regulatory information, clinical trial registries, abstracts and conference papers, grants and federally funded research, and manufacturing information was searched from January 1, 2006 to June 28, 2011.
Study Selection
A single reviewer screened abstracts and selected studies looking at procalcitonin‐guided antibiotic therapy. Second and third reviewers were consulted to screen articles when needed. Studies were included if they fulfilled all of the following criteria: (1) randomized, controlled trial or nonrandomized comparative study; (2) adult and/or pediatric patients with known or suspected local or systemic infection, including critically ill patients with sepsis syndrome or ventilator‐associated pneumonia, adults with respiratory tract infections, neonates with sepsis, children with fever of unknown source, and postoperative patients at risk of infection; (3) interventions included initiation, intensification, and/or discontinuation of antibiotic therapy guided by procalcitonin plus clinical criteria; (4) primary outcomes included antibiotic usage (antibiotic prescription rate, total antibiotic exposure, duration of antibiotic therapy, and days without antibiotic therapy); and (5) secondary outcomes included morbidity (antibiotic adverse events, hospital and/or intensive care unit length of stay), mortality, and quality of life.
Studies with any of the following criteria were excluded: published in non‐English language, not reporting primary data from original research, not a randomized, controlled trial or nonrandomized comparative study, not reporting relevant outcomes.
Data Extraction and Quality Assessment
A single reviewer abstracted data and a second reviewer confirmed accuracy. Disagreements between reviewers were resolved by group discussion among the research team and final quality rating was assigned by consensus adjudication. Data elements were abstracted into the following categories: quality assessment, applicability and clinical diversity assessment, and outcome assessment. Quality of included studies was assessed using the US Preventive Services Task Force framework[17] by at least 2 independent reviewers. Three quality categories were used: good, fair, and poor.
Data Synthesis and Analysis
The decision to incorporate formal data synthesis in this review was made after completing the formal literature search, and the decision to pool studies was based on the specific number of studies with similar questions and outcomes. If a meta‐analysis could be performed, subgroup and sensitivity analyses were based on clinical similarity of available studies and reporting of mean and standard deviation. The pooling method involved inverse variance weighting and a random effects model.
The strength of evidence was graded using the Methods Guide,[16] a system based on the Grading of Recommendations Assessment, Development and Evaluation Working Group.[18] The following domains were addressed: risk of bias, consistency, directness, and precision. The overall strength of evidence was graded as high, moderate, low, or insufficient. The final strength of evidence grading was made by consensus adjudication among the authors.
RESULTS
Of the 2000 studies identified through the literature search, 1986 were excluded and 14 studies[19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32] were included. Search of gray literature yielded 4 published studies.[33, 34, 35, 36] A total of 18 randomized, controlled trials comparing procalcitonin guidance to use of clinical criteria alone to manage antibiotic therapy in patients with infections were included. The PRISMA diagram (Figure 1) depicts the flow of search screening and study selection. We sought, but did not find, nonrandomized comparative studies of populations, comparisons, interventions, and outcomes that were not adequately studied in randomized, controlled trials.

Data were pooled into clinically similar groups that were reviewed separately: (1) adult intensive care unit (ICU) patients, including patients with ventilator‐associated pneumonia; (2) adult patients with respiratory tract infections; (3) neonates with suspected sepsis; (4) children between 1 to 36 months of age with fever of unknown source; and (5) postoperative patients at risk of infection. Tables summarizing study quality and outcome measures with strength of evidence are available online (
| Outcome | Author, Year | N | PCT‐Guided Therapya | Controla | Difference PCT‐CTRL (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| ABT Duration, d | Hochreiter, 2009[22] | 110 | 5.9 | 7.9 | 2.0 (2.5 to 1.5) | <0.001 |
| Nobre, 2008[19] | 79 | 66 | 9.5 (ITT), 10 (PP) | 2.6 (5.5 to 0.3), 3.2 (1.1 to5.4) | 0.15, 0.003 | |
| Schroeder, 2009[20] | 27 | 6.6 | 8.3 | 1.7 (2.4 to 1.0) | <0.001 | |
| Stolz, 2009[21] | 101 | 10 (616)b | 15 (1023)b | 5 | 0.049 | |
| Bouadma, 2010[23] | 621 | 10.3 | 13.3 | 3.0 (4.20 to 1.80) | <0.0001 | |
| Days without ABTs, day 28 | Nobre, 2008[19] | 79 | 15.3, 17.4 | 13, 13.6 | 2.3 (5.9 to 1.8), 3.8 (0.1 to 7.5)c | 0.28, 0.04 |
| Stolz, 2009[21] | 101 | 13 (221)b | 9.5 (1.517)b | 3.5 | 0.049 | |
| Bouadma, 2010[23] | 621 | 14.3 | 11.6 | 2.7 (1.4 to 4.1) | <0.001 | |
| Total ABT exposured | Nobre, 2008[19] | 79 | 541 | 644 | 1.1e (0.9 to 1.3), 1.3e (1.1 to 1,5)c | 0.07, 0.0002 |
| 504 | 655 | |||||
| Stolz, 2009[21] | 101 | 1077 | 1341 | |||
| Bouadma, 2010[23]d | 621 | 653 | 812 | 159 (185 to 131) | <0.001 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| ABT duration, days | Jensen, 2011[33] | 1200 | 6 (311)b | 4 (310)b | NR | NR |
| Days spent in ICU on 3 ABTs | Jensen, 2011[33] | 1200 | 3570/5447 (65.5%) | 2721/4717 (57.7%) | 7.9% (6.0 to 9.7) | 0.002 |
| Adult patients with respiratory tract infections | ||||||
| ABT duration, da | Schuetz, 2009[2][5] | 1359 | 5.7 | 8.7 | 3.0 | |
| Christ‐Crain, 2004[30] | 243 | 10.9 | 12.8 | 1.9 (3.1 to 0.7) | 0.002 | |
| Kristoffersen, 2009[26] | 210 | 5.1 | 6.8 | 1.7 | ||
| Briel, 2008[27] | 458 | 6.2 | 7.1 | 1.0 (1.7 to 0.4) | <0.05 | |
| Long, 20113[5] | 162 | 5 (36)f | 7 (59)f | 2.0 | <0.001 | |
| Burkhardt, 2010[34] | 550 | 7.8 | 7.7 | 0.1 (0.7 to 0.9) | 0.8 | |
| Christ‐Crain, 2006[29] | 302 | 5.8 | 12.9 | 7.1(8.4 to 5.8) | <0.0001 | |
| Antibiotic prescription rate, % | Schuetz, 2009[2][5] | 1359 | 506/671 (75.4%) | 603/688 (87.6%) | 12.2% (16.3 to 8.1) | <0.05 |
| Christ‐Crain, 2004[30] | 243 | 55/124 (44.4%) | 99/119 (83.2%) | 38.8% (49.9 to 27.8) | <0.0001 | |
| Kristoffersen, 2009[26] | 210 | 88/103 (85.4%) | 85/107 (79.4%) | 6.0% (4.3 to 16.2) | 0.25 | |
| Briel, 2008[27] | 458 | 58/232 (25.0%) | 219/226 (96.9%) | 72% (78 to 66) | <0.05 | |
| Long, 20113[5] | 162 | NR (84.4%) | NR (97.5%) | 13.1% | 0.004 | |
| Stolz, 2007[28] | 208 | 41/102 (40.2%) | 76/106 (71.7%) | 31.5% (44.3 to 18.7) | <0.0001 | |
| Christ‐Crain, 2006[29] | 302 | 128/151 (84.8%) | 149/151 (98.79%) | 13.9% (19.9 to 7.9) | <0.0001 | |
| Burkhardt, 2010[34] | 550 | 84/275 (30.5%) | 89/275 (32.4%) | 1.8% (9.6 to 5.9) | 0.701 | |
| Total ABT exposure | Stolz, 2007[28] | 208 | NR | NR | 31.5% (18.7 to 44.3) | <0.0001 |
| Long, 20113[5] | 162 | NR | NR | NR | ||
| Christ‐Crain, 2006[29] | 302 | 136g | 323g | |||
| Christ‐Crain, 2004[30] | 243 | 332g | 661g | |||
| Neonates with sepsis | ||||||
| ABTs 72 hours, % | Stocker, 2010[31] | All neonates (N=121) | 33/60 (55%) | 50/61 (82%) | 27.0 (42.8 to 11.1) | 0.002 |
| Infection proven/probably (N=21) | 9/9 (100%) | 12/12 (100%) | 0% (0 to 0) | NA | ||
| Infection possible (N=40) | 13/21 (61.9%) | 19/19 (100%) | 38.1 (58.9 to 17.3) | 0.003 | ||
| Infection unlikely (N=60) | 11/30 (36.7%) | 19/30 (63.3%) | 26.6 (51.1 to 2.3) | 0.038 | ||
| ABT duration, h | Stocker, 2010[31] | All neonates (N=121) | 79.1 | 101.5 | 22.4 | 0.012 |
| Infection proven/probably (N=21) | 177.8 | 170.8 | 7 | NSS | ||
| Infection possible (N=40) | 83.4 | 111.5 | 28.1 | <0.001 | ||
| Infection unlikely (N=60) | 46.5 | 67.4 | 20.9 | 0.001 | ||
| Children ages 136 months with fever of unknown source | ||||||
| Antibiotic prescription rate, % | Manzano, 2010[36] | All children (N=384) | 48/192 (25%) | 54/192 (28.0%) | 3.1 (12.0 to 5.7) | 0.49 |
| No SBI or neutropenia (N=312) | 14/158 (9%) | 16/154 (10%) | 1.5 (8.1 to 5.0) | 0.65 | ||
| Adult postoperative patients at risk of infection | ||||||
| ABT duration, d | Chromik, 2006[32] | All patients (N=20) | 5.5 | 9 | 3.5 | 0.27 |
| Outcome | Author, Year | N | PCTa | Controla | Difference, PCT‐CTRL (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| ICU LOS, days | Hochreiter, 2009[22] | 110 | 15.5 | 17.7 | 2.2 | 0.046 |
| Nobre, 2008[19] | 79 | 4 | 7 | 4.6 (8.2 to 1.0) | 0.02 | |
| Schroeder, 2009[20] | 27 | 16.4 | 16.7 | 0.3 (5.6 to 5.0) | NSS | |
| Bouadma, 2010[23] | 621 | 15.9 | 14.4 | 1.5 (0.9 to 3.1) | 0.23 | |
| Hospital LOS, days | Nobre, 2008[19] | 79 | 17 | 23.5 | 2.5 (6.5 to 1.5) | 0.85 |
| Stolz, 2009[21] | 101 | 26 (721)b | 26 (16.822.3)b | 0 | 0.15 | |
| Bouadma, 2010[23] | 621 | 26.1 | 26.4 | 0.3 (3.2 to 2.7) | 0.87 | |
| ICU‐free days alive, 128 | Stolz, 2009[21] | 101 | 10 (018)b | 8.5 (018)c | 1.5 | 0.53 |
| SOFA day 28 | Bouadma, 2010[23] | 621 | 1.5 | 0.9 | 0.6 (0.0, 1.1) | 0.037 |
| SOFA score max | Schroeder, 2009[20] | 27 | 7.3 | 8.3 | 8.1 (4.1 to 1.7) | NSS |
| SAPS II score | Hochreiter, 2009[22] | 110 | 40.1 | 40.5 | 0.4 (6.4 to 5.6) | >0.05 |
| Days without MV | Stolz, 2009[21] | 101 | 21 (224)b | 19 (8.522.5)b | 2.0 | 0.46 |
| Bouadma, 2010[23] | 621 | 16.2 | 16.9 | 0.7 (2.4 to 1.1) | 0.47 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| ICU LOS, da | Svoboda, 2007[24] | 72 | 16.1 | 19.4 | 3.3 (7.0 to 0.4) | 0.09 |
| Jensen, 2011[33] | 1200 | 6 (312)b | 5 (311)b | 1 | 0.004 | |
| SOFA scorea | Svoboda, 2007[24] | 72 | 7.9 | 9.3 | 1.4 (2.8 to 0.0) | 0.06 |
| Days on MVa | Svoboda, 2007[24] | 72 | 10.3 | 13.9 | 3.6 (7.6 to 0.4) | 0.08 |
| Jensen, 2011[33] | 1200 | 3569 (65.5%) | 2861 (60.7%) | 4.9% (3 to 6.7) | <0.0001 | |
| Percent days in ICU with GFR <60 | Jensen, 2011[33] | 1200 | 2796 (51.3%) | 2187 (46.4%) | 5.0 % (3.0 to 6.9) | <0.0001 |
| Adult patients with respiratory tract infections | ||||||
| Hospital LOS, da | Schuetz, 2009[2][5] | 1359 | 9.4 | 9.2 | 0.2 | |
| Christ‐Crain, 2004[30] | 224 | 10.78.9 | 11.210.6 | 0.5 (3.0 to 2.0) | 0.69 | |
| Kristoffersen, 2009[26] | 210 | 5.9 | 6.7 | 0.8 | 0.22 | |
| Stolz, 2007[28] | 208 | 9 (115)b | 10 (115)b | 1 | 0.96 | |
| Christ‐Crain, 2006[29] | 302 | 12.09.1 | 13.09.0 | 1 (3.0 to 1.0) | 0.34 | |
| ICU admission, % | Schuetz, 2009[2][5] | 1359 | 43/671 (6.4%) | 60/688 (8.7%) | 2.3% (5.2 to 0.4) | 0.12 |
| Christ‐Crain, 2004[30] | 224 | 5/124 (4.0%) | 6/119 (5.0%) | 1.0% (6.2 to 4.2) | 0.71 | |
| Kristoffersen, 2009[26] | 210 | 7/103 (6.8%) | 5/107 (4.7%) | 2.1% (4.2 to 8.4) | 0.51 | |
| Stolz, 2007[28] | 208 | 8/102 (7.8%) | 11/106 (10.4%) | 2.5% (10.3 to 5.3) | 0.53 | |
| Christ‐Crain, 2006[29] | 302 | 20/151 (13.2%) | 21/151 (13.94%) | 0.7% (8.4 to 7.1) | 0.87 | |
| Antibiotic adverse events | Schuetz, 2009[2][5]c | 1359 | 133/671 (19.8%) | 193/688 (28.1%) | 8.2% (12.7 to 3.7) | |
| Briel, 2008[27]d | 458 | 2.34.6 days | 3.66.1 days | 1.1 days (2.1 to 0.1) | <0.05 | |
| Burkhardt, 2010[34]e | 550 | 11 /59 (18.6%) | 16/101 (15.8%) | 2.8% (9.4 to 15.0) | 0.65 | |
| Restricted activity, df | Briel, 2008[27] | 458 | 8.73.9 | 8.63.9 | 0.2 (0.4 to 0.9) | >0.05 |
| Burkhardt, 2010[34] | 550 | 9.1 | 8.8 | 0.25 (0.52 to 1.03) | >0.05 | |
| Neonates with sepsis | ||||||
| Recurrence of infection | Stocker, 2010[31] | 121 | 32% | 39% | 7 | 0.45 |
| Children ages 136 months with fever of unknown source | ||||||
| Hospitalization rate | Manzano, 2010[36] | All children (N=384) | 50/192 (26%) | 48/192 (25%) | 1 (8 to 10) | 0.81 |
| No SBI or neutropenia (N=312) | 16/158 (10%) | 11/154 (7%) | 3 (3 to 10) | 0.34 | ||
| Adult postoperative patients at risk of infection | ||||||
| Hospital LOS, days | Chromik, 2006[32] | 20 | 18 | 30 | 12 | 0.057 |
| Local wound infection, % | Chromik, 2006[32] | 20 | 1/10 | 2/10 | 10 (41.0 to 21.0) | 0.53 |
| Systemic infection, % | Chromik, 2006[32] | 20 | 3/10 | 7/10 | 40.0 (80.2 to 0.2) | 0.07 |
| Sepsis/SIRS, % | Chromik, 2006[32] | 20 | 2/10 | 8/10 | 60.0 (95.1 to 24.9) | 0.007 |
| Mortality | Mortality | Difference | ||||
|---|---|---|---|---|---|---|
| Outcome | Author, Year | N | PCT‐Guided Therapy | Control | PCT‐CTRL (95% CI) | P Value |
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| 28‐day mortality | Nobre, 2008[19] | 79 | 8/39 (20.5%) | 8/40 (20.0%) | 0.5 (17.2 to 18.2), | 0.95 |
| 5/31 (16.1%) | 6/37 (16.2%) | 0.1 (17.7 to 17.5)a | 0.99 | |||
| Stolz, 2009[21] | 101 | 8/51 (15.7%) | 12/50 (24.0%) | 8.3 (23.8 to 7.2) | 0.29 | |
| Bouadma, 2010[23] | 621 | 65/307 (21.2%) | 64/314 (20.4%) | 0.8 (5.6 to 7.2) | 0.81 | |
| 60‐day mortality | Bouadma, 2010[23] | 621 | 92/307 (30.0%) | 82/314 (26.1%) | 3.9 (3.2 to 10.9) | 0.29 |
| In‐hospital mortality | Nobre, 2008[19] | 79 | 9/39 (23.1%) | 9/40 (22.5%) | 0.6 (17.9 to 19.1) | 0.95 |
| 6/31 (19.4%) | 7/37 (18.9%) | 0.4+ (18.3 to 19.2) | 0.96 | |||
| Stolz, 2009[21] | 101 | 10/51 (19.6%) | 14/50 (28.0%) | 8.4, (24.9 to 8.1) | 0.32 | |
| Hochreiter, 2009[22] | 110 | 15/57 (26.3%) | 14/53 (26.4%) | 0.1, (16.6 to 16.4) | 0.99 | |
| Schroeder, 2009[20] | 27 | 3/14 (21.4%) | 3/13 (23.1%) | 1.7, (33.1 to 29.8) | 0.92 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| 28‐day mortality | Svoboda, 2007[24] | 72 | 10/38 (26.3%) | 13/34 (38.2%) | 11.9 (33.4 to 9.6) | 0.28 |
| 28‐day mortality | Jensen, 2011[33] | 1200 | 190/604 (31.5%) | 191/596 (32.0%) | 0.6 (4.7 to 5.9) | 0.83 |
| Adult patients with respiratory tract infections | ||||||
| 6‐month mortality | Stolz, 2007[28] | 208 | 5/102 (4.9%) | 9/106 (8.5%) | 3.6% (10.3 to 3.2%) | 0.30 |
| 6‐week mortality | Christ‐Crain, 2006[29] | 302 | 18/151 (11.9%) | 20/151 (13.2%) | 1.3% (8.8 to 6.2) | 0.73 |
| 28‐day mortality | Christ‐Crain, 2004[30] | 243 | 4/124(3.2%) | 4/119 (3.4%) | 0.1% (4.6 to 4.4) | 0.95 |
| Schuetz, 2009 (30‐day)[25] | 1359 | 34/671(5.1%) | 33/688(4.8%) | 0.3% (2.1 to 2.5) | 0.82 | |
| Briel, 2008[27] | 458 | 0/231(0%) | 1/224 (0.4%) | 0.4% (1.3 to 0.4) | 0.31 | |
| Burkhardt, 2010[34] | 550 | 0/275(0%) | 0/275 (0%) | 0 | ||
| Kristoffersen, 2009[26] | 210 | 2/103(1.9%) | 1/107 (0.9%) | 1.0% (2.2 to 4.2) | 0.54 | |
| Long, 20113[5] | 162 | 0/81 (0%) | 0/81 (0%) | 0 | ||
| Neonates with sepsis | ||||||
| Mortality (in‐hospital) | Stocker, 2010[31] | 121 | 0% | 0% | 0 (0 to 0) | NA |
| Children ages 136 months with fever of unknown source | ||||||
| Mortality | Manzano, 2010[36] | 384 | All children | 0% | 0% | 0 (0 to 0) |
| Adult postoperative patients at risk of infection | ||||||
| Mortality | Chromik, 2006[32] | 20 | 1/10 (10%) | 3/10 (30%) | 20 (54.0 to 14.0) | 0.07 |
Adult ICU Patients: Procalcitonin‐Guided Antibiotic Discontinuation
Five studies[19, 20, 21, 22, 23] (N=938) addressed procalcitonin‐guided discontinuation of antibiotic therapy in adult ICU patients. Four studies conducted superiority analyses for mortality with procalcitonin‐guided therapy, whereas 1 study conducted a noninferiority analysis. Absolute procalcitonin values for discontinuation of antibiotics ranged from 0.25 to 1 ng/mL. Physicians in control groups administered antibiotics according to their standard practice.
Antibiotic Usage
The absolute reduction in duration of antibiotic usage with procalcitonin guidance in these studies ranged from 1.7 to 5 days, and the relative reduction ranged from 21% to 38%. Meta‐analysis of antibiotic duration in adult ICU patients was performed (Figure 2A).

Morbidity
Procalcitonin‐guided antibiotic discontinuation did not increase morbidity, including ICU length of stay (LOS). Meta‐analysis of ICU LOS is displayed in Figure 2B. Limited data on adverse antibiotic events were reported (Table 2).
Mortality
There was no increase in mortality as a result of shorter duration of antibiotic therapy. Meta‐analysis of short‐term mortality (28‐day or in‐hospital mortality) showed a mortality difference of 0.43% favoring procalcitonin‐guided therapy, and a 95% confidence interval (CI) of 6% to 5% (Figure 2C).
Adult ICU Patients: Procalcitonin‐Guided Antibiotic Intensification
Two studies[24, 33] (N=1272) addressed procalcitonin‐guided intensification of antibiotic therapy in adult ICU patients. The Jensen et al. study[33] was a large (N=1200), high‐quality study that used a detailed algorithm for broadening antibiotic therapy in patients with elevated procalcitonin. The Jensen et al. study also educated physicians about empiric therapy and intensification of antibiotic therapy. A second study[24] was too small (N=72) and lacked sufficient details to be informative.
Antibiotic Usage
The Jensen et al. study found a 2‐day increase, or 50% relative increase, in the duration of antibiotic therapy and a 7.9% absolute increase (P=0.002) in the number of days on 3 antibiotics with procalcitonin‐guided intensification.
Morbidity
The Jensen et al. study showed a significant 1‐day increase in ICU LOS (P=0.004) and a significant increase in organ dysfunction. Specifically, patients had a highly statistically significant 5% increase in days on mechanical ventilation (P<0.0001) and 5% increase in days with abnormal renal function (P<0.0001).
Mortality
The Jensen et al. study was a superiority trial powered to test a 7.5% decrease in 28‐day mortality, but no significant difference in mortality was observed with procalcitonin‐guided intensification (31.5% vs 32.0, P=0.83).
Adult Patients With Respiratory Tract Infections
Eight studies[25, 26, 27, 28, 29, 30, 34, 35] (N=3492) addressed initiation and/or discontinuation of antibiotics in adult patients with acute upper and lower respiratory tract infections, including community‐acquired pneumonia, acute exacerbation of chronic obstructive pulmonary disease, and acute bronchitis. Settings included primary care clinics, emergency departments, and hospital wards. Physicians in control groups administered antibiotics according to their own standard practices and/or evidence‐based guidelines. All studies encouraged initiation of antibiotics with procalcitonin levels >0.25 ng/mL, and 4 studies strongly encouraged antibiotics with procalcitonin levels >0.5 ng/mL.
Antibiotic Usage
Procalcitonin guidance reduced antibiotic duration, antibiotic prescription rate, and total antibiotic exposure. Absolute reduction in antibiotic duration ranged from 1 to 7 days, and relative reductions ranged from 13% to 55%. Four of the 8 studies reported sufficient details to be pooled into a meta‐analysis (Figure 3A) with a statistically significant pooled mean difference of 2.35 days favoring procalcitonin (95% CI: 4.38 to 0.33). Procalcitonin guidance also reduced antibiotic prescription rate with absolute reductions ranging from 2% to 7% and relative reductions ranging from 1.8% to 72%. Meta‐analysis of prescription rates from 8 studies (Figure 3B) yielded a statistically significant pooled risk difference of 22% (95% CI: 41% to 4%). Total antibiotic exposure was consistently reduced in the 4 studies reporting this outcome.

Morbidity
Procalcitonin guidance did not increase hospital LOS or ICU admission rates. Meta‐analysis of ICU admission rates from 5 studies (Figure 3C) produced a risk difference of 1%, with a narrow 95% CI (4% to 1%). There was insufficient evidence to judge the effect on days of restricted activity or antibiotic adverse events.
Mortality
Procalcitonin guidance did not increase mortality, and meta‐analysis of 4 studies (Figure 3D) produced a risk difference of 0.3% with a narrow 95% CI (1% to 2%), with no statistical heterogeneity (I2=0%).
Neonates With Sepsis
One study[31] (N=121) evaluated procalcitonin‐guided antibiotic therapy for suspected neonatal sepsis. Neonatal sepsis was suspected on the basis of risk factors and clinical signs and symptoms. Antibiotic initiation or discontinuation was based on a procalcitonin nomogram. Antibiotic therapy in the control group was based on the physician's assessment. The quality of this study was rated good, and strength of evidence was rated moderate for antibiotic usage and insufficient for morbidity and mortality outcomes.
Antibiotic Usage
Duration of antibiotic therapy was decreased by 22.4 hours (P=0.012), a 24% relative reduction, and the proportion of neonates on antibiotics 72 hours was reduced by 27% (P=0.002). The largest reduction in antibiotic duration was seen in the 80% to 85% of neonates who were categorized as having possible or infection or unlikely to have infection.
Morbidity
A statistically insignificant 7% reduction in rate of recurrence of infection was seen with procalcitonin‐guided antibiotic therapy (P=0.45).
Mortality
No in‐hospital deaths occurred in either the procalcitonin or control group.
Children Ages 1 to 36 Months With Fever of Unknown Source
One study[36] (N=384) evaluated procalcitonin‐guided antibiotic therapy for fever of unknown source in children 1 to 36 months of age, but the overall strength of evidence was judged insufficient to draw conclusions.
Antibiotic Usage
A statistically insignificant reduction of 3.1% in antibiotic prescription rate was seen with procalcitonin‐guided antibiotic therapy (P=0.49).
Morbidity
Rate of hospitalization was relatively low, and no significant difference was seen between procalcitonin and control groups.
Mortality
In‐hospital mortality was reported as 0% in both arms.
Adult Postoperative Patients at Risk of Infection
One study[32] (N =250) monitored procalcitonin in consecutive patients after colorectal surgery to identify patients at risk of infection who might benefit from prophylactic antibiotic therapy. Two hundred thirty patients had normal procalcitonin levels. Twenty patients with elevated procalcitonin levels (>1.5 ng/mL) were randomized to receive prophylactic antibiotic therapy with ceftriaxone or no antibiotics. The strength of evidence was judged insufficient to draw conclusions from this study.
Antibiotic Usage
Duration of antibiotic therapy was reduced by 3.5% but was not statistically insignificant (P=0.27).
Morbidity
Procalcitonin guidance reduced the incidence of sepsis/systemic inflammatory response syndrome by 60% (p=0.007). The incidences of local and systemic infection were reduced with procalcitonin guidance but were not statistically significant (10%, P=0.53; and 40%, P=0.07, respectively).
Mortality
Mortality was 20% higher in the control arm but was not statistically significant (P=0.07).
DISCUSSION
Summary of the Main Findings
Diagnosis of sepsis or other serious infections in critically ill patients is challenging because clinical criteria for diagnosis overlap with noninfectious causes of the systemic inflammatory response syndrome. Initiation of antibiotic therapy for presumed sepsis is necessary while diagnostic evaluation is ongoing, because delaying antibiotic therapy is associated with increased mortality.[37, 38, 39] Our review found that procalcitonin guidance significantly reduced antibiotic usage in adult ICU patients by reducing the duration of antibiotic therapy, rather than decreasing the initiation of antibiotics, without increasing morbidity or mortality.
In contrast, the use of procalcitonin as an indicator of need for intensification of antibiotic therapy in adult ICU patients should be discouraged because this approach was associated with increased morbidity. The large, well‐designed study by Jensen[33] showed that antibiotic intensification in response to elevated procalcitonin measurement was associated with increased morbidity: a longer ICU LOS, an increase in days on mechanical ventilation, and an increase in days with abnormal renal function. The authors concluded that the increased morbidity could only be explained by clinical harms of increased exposure to broad‐spectrum antibiotics.
Clinical and microbiological evaluations are neither sensitive nor specific for differentiating bacterial from viral respiratory tract infections. Procalcitonin can guide initiation of antibiotic therapy in adults with suspected bacterial respiratory tract infection. Our review showed that procalcitonin guidance significantly reduced antibiotic usage with respect to antibiotic prescription rate, duration of antibiotic therapy, and total exposure to antibiotic therapy in adult patients with respiratory tract infections.
The role of procalcitonin‐guided therapy in other populations is less clear. One study in postoperative colorectal surgery patients reported that elevated procalcitonin levels may identify patients at risk for infection who benefit from prophylactic antibiotic therapy.[32] Patients with elevated procalcitonin levels who received prophylactic antibiotic therapy had a significant decrease in the incidence and severity of systemic infections, whereas patients with normal procalcitonin levels did not require any additional surgical or medical therapy. Although these findings are promising, more data in postoperative patients are needed.
The utility of procalcitonin in pediatric settings is a significant gap in the present literature. One study[31] in neonates with suspected sepsis showed a significant decrease in the proportion of neonates started on empiric antibiotic therapy and a decrease in the duration of antibiotic therapy with procalcitonin guidance. However, there was insufficient evidence that procalcitonin guidance does not increase morbidity or mortality.
Comparison to Other Systematic Reviews
Six systematic reviews of procalcitonin guidance in the management of patients with infections were published prior to our review.[9, 10, 11, 12, 13, 14] Our systematic review differs from past reviews in the number of studies included and the pooling of studies according to patient population, type and severity of infection, and different uses of procalcitonin measurements, either for initiation, discontinuation, or intensification of antibiotic therapy. Previous systematic reviews included 7 to 14 studies, whereas ours included 18 randomized, controlled trials. One previous review[13] included and pooled the Jensen et al. study[33] with other studies of adult ICU patients. We evaluated the Jensen et al. study separately because it uniquely looked at procalcitonin‐guided antibiotic intensification in adult ICU patients, in contrast to other studies that looked at procalcitonin‐guided antibiotic discontinuation. We addressed pediatric populations separately from adult patients, and recognizing that there are distinct groups within the pediatric population, we separately grouped neonates and children ages 1 to 36 months. Despite these differences, our review and other systematic reviews, we came to similar conclusions: procalcitonin‐guided antibiotic decision making compared to clinical criteria‐guided antibiotic decision making reduces antibiotic usage without increasing morbidity or mortality.
Limitations
An important limitation of this review was the uncertainty about the noninferiority margin for morbidity and mortality in adult ICU patients. Only the Bouadma et al. study[23] did a power analysis and predefined a margin for noninferiority for 28‐ and 60‐day mortality. Meta‐analysis of all 5 ICU studies showed a pooled point estimate of 0.43% in mortality and a 95% CI of 6% to 5% for difference in mortality between procalcitonin‐guided therapy versus standard care. A 10% noninferiority margin for mortality has been recommended by the Infectious Diseases Society of America and American College of Chest Physicians, but there is concern that a 10% margin for mortality may be too high. Presently, 2 large trials are in progress that may yield more precise estimates of mortality in the future.
Differences in reporting of total antibiotic exposure and morbidity outcomes limited our ability to pool data. Total antibiotic exposure is conventionally reported as mean days per 1000 days of follow‐up, but some studies only reported relative or absolute differences. Likewise, morbidity was reported with different severity of illness scales, including Sepsis‐Related Organ Failure Assessment, Simplified Acute Physiology (SAP) II, SAP III, and Acute Physiology and Chronic Health Evaluation II, which limited comparisons across studies.
Research Gaps
We identified gaps in the available literature and opportunities for future research. First, the safety and efficacy of procalcitonin‐guided antibiotic therapy needs to be studied in patient populations excluded from current randomized controlled studies, such as immunocompromised patients and pregnant women. Patients who are immunocompromised or have chronic conditions, such as cystic fibrosis, account for a significant percentage of community‐acquired respiratory tract infections and are often treated empirically.[29, 30] Second, standardized reporting of antibiotic adverse events and emergence of antibiotic resistance is needed. Strategies to reduce antibiotic usage have been associated with reductions in antibiotic adverse events, such as Clostridium difficile colitis and superinfection with multi‐drug resistant Gram‐negative bacteria.[37, 40, 41] Few studies in our review reported allergic reactions or adverse events of antibiotic therapy, [25, 27, 34] and only 1 reported antibiotic resistance.[19] Third, procalcitonin guidance should be compared to other strategies to reduce antibiotic usage, such as structured implementation of practice guidelines and antibiotic stewardship programs.[42] One single‐arm study describes how procalcitonin can be used in antibiotic stewardship programs to decrease the duration of antibiotic therapy,[43] but additional studies are needed. Finally, generalizing results from those studies that were conducted primarily in Europe would depend on similar use of and adherence to study‐based algorithms. Newer observational studies have demonstrated reduced antibiotic usage with implementation of procalcitonin algorithms in real‐life settings in Europe, but algorithm adherence was significantly less in the United States.[44, 45]
In summary, our systematic review found that procalcitonin‐guided antibiotic therapy can significantly reduce antibiotic usage in adult ICU patients without affecting morbidity or mortality. Procalcitonin should not be used to guide intensification of antibiotic therapy in adult ICU patients because this approach may increase morbidity. In adults with respiratory infections, procalcitonin guidance can significantly reduce antibiotic usage without adversely affecting morbidity or mortality. There is insufficient evidence to recommend procalcitonin‐guided antibiotic therapy in neonates with sepsis, children with fever of unknown source, or postoperative patients at risk for infection.
Acknowledgments
Disclosures: This project was funded under contract HHSA 2902007‐10058 from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services. The authors of this article are responsible for its content, including any clinical treatment recommendations. No statement in this article should be construed as an official position of AHRQ or of the US Department of Health and Human Services. There are no conflicts of interest reported by any of the authors.
Many serum biomarkers have been identified in recent years with a wide range of potential applications, including diagnosis of local and systemic infections, differentiation of bacterial and fungal infections from viral syndromes or noninfectious conditions, prognostic stratification of patients, and enhanced management of antibiotic therapy. Currently, there are at least 178 serum biomarkers that have potential roles to guide antibiotic therapy, and among these, procalcitonin has been the most extensively studied biomarker.[1, 2]
Procalcitonin is the prohormone precursor of calcitonin that is expressed primarily in C cells of the thyroid gland. Conversion of procalcitonin to calcitonin is inhibited by various cytokines and bacterial endotoxins. Procalcitonin's primary diagnostic utility is thought to be in establishing the presence of bacterial infections, because serum procalcitonin levels rise and fall rapidly in bacterial infections.[3, 4, 5] In healthy individuals, procalcitonin levels are very low. In systemic infections, including sepsis, procalcitonin levels are generally greater than 0.5 to 2 ng/mL, but often reach levels 10 ng/mL, which correlates with severity of illness and a poor prognosis. In patients with respiratory tract infections, procalcitonin levels are less elevated, and a cutoff of 0.25 ng/mL seems to be most predictive of a bacterial respiratory tract infection requiring antibiotic therapy.[6, 7, 8] Procalcitonin levels decrease to <0.25 ng/mL as infection resolves, and a decline in procalcitonin level may guide decisions about discontinuation of antibiotic therapy.[5]
The purpose of this systematic review was to synthesize comparative studies examining the use of procalcitonin to guide antibiotic therapy in patients with suspected local or systemic infections in different patient populations. We are aware of 6 previously published systematic reviews evaluating the utility of procalcitonin guidance in the management of infections.[9, 10, 11, 12, 13, 14] Our systematic review included more studies and pooled patients into the most clinically similar groups compared to other systematic reviews.
METHODS
This review is based on a comparative effectiveness review prepared for the Agency for Healthcare Research and Quality's Effective Health Care Program.[15] A standard protocol consistent with the Methods Guide for Effectiveness and Comparative Effectiveness Reviews[16] was followed. A detailed description of the methods is available online (
Study Question
In selected populations of patients with suspected local or systemic infection, what are the effects of using procalcitonin measurement plus clinical criteria for infection to guide initiation, intensification, and/or discontinuation of antibiotic therapy when compared to clinical criteria for infection alone?
Search Strategy
MEDLINE and EMBASE were searched from January 1, 1990 through December 16, 2011, and the Cochrane Controlled Trials register was searched with no date restriction for randomized and nonrandomized comparative studies using the following search terms: procalcitonin AND chronic obstructive pulmonary disease; COPD; critical illness; critically ill; febrile neutropenia; ICU; intensive care; intensive care unit; postoperative complication(s); postoperative infection(s); postsurgical infection(s); sepsis; septic; surgical wound infection; systemic inflammatory response syndrome OR postoperative infection. In addition, a search for systematic reviews was conducted in MEDLINE, the Cochrane Database of Systematic Reviews, and Web sites of the National Institute for Clinical Excellence, the National Guideline Clearinghouse, and the Health Technology Assessment Programme. Gray literature, including databases with regulatory information, clinical trial registries, abstracts and conference papers, grants and federally funded research, and manufacturing information was searched from January 1, 2006 to June 28, 2011.
Study Selection
A single reviewer screened abstracts and selected studies looking at procalcitonin‐guided antibiotic therapy. Second and third reviewers were consulted to screen articles when needed. Studies were included if they fulfilled all of the following criteria: (1) randomized, controlled trial or nonrandomized comparative study; (2) adult and/or pediatric patients with known or suspected local or systemic infection, including critically ill patients with sepsis syndrome or ventilator‐associated pneumonia, adults with respiratory tract infections, neonates with sepsis, children with fever of unknown source, and postoperative patients at risk of infection; (3) interventions included initiation, intensification, and/or discontinuation of antibiotic therapy guided by procalcitonin plus clinical criteria; (4) primary outcomes included antibiotic usage (antibiotic prescription rate, total antibiotic exposure, duration of antibiotic therapy, and days without antibiotic therapy); and (5) secondary outcomes included morbidity (antibiotic adverse events, hospital and/or intensive care unit length of stay), mortality, and quality of life.
Studies with any of the following criteria were excluded: published in non‐English language, not reporting primary data from original research, not a randomized, controlled trial or nonrandomized comparative study, not reporting relevant outcomes.
Data Extraction and Quality Assessment
A single reviewer abstracted data and a second reviewer confirmed accuracy. Disagreements between reviewers were resolved by group discussion among the research team and final quality rating was assigned by consensus adjudication. Data elements were abstracted into the following categories: quality assessment, applicability and clinical diversity assessment, and outcome assessment. Quality of included studies was assessed using the US Preventive Services Task Force framework[17] by at least 2 independent reviewers. Three quality categories were used: good, fair, and poor.
Data Synthesis and Analysis
The decision to incorporate formal data synthesis in this review was made after completing the formal literature search, and the decision to pool studies was based on the specific number of studies with similar questions and outcomes. If a meta‐analysis could be performed, subgroup and sensitivity analyses were based on clinical similarity of available studies and reporting of mean and standard deviation. The pooling method involved inverse variance weighting and a random effects model.
The strength of evidence was graded using the Methods Guide,[16] a system based on the Grading of Recommendations Assessment, Development and Evaluation Working Group.[18] The following domains were addressed: risk of bias, consistency, directness, and precision. The overall strength of evidence was graded as high, moderate, low, or insufficient. The final strength of evidence grading was made by consensus adjudication among the authors.
RESULTS
Of the 2000 studies identified through the literature search, 1986 were excluded and 14 studies[19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32] were included. Search of gray literature yielded 4 published studies.[33, 34, 35, 36] A total of 18 randomized, controlled trials comparing procalcitonin guidance to use of clinical criteria alone to manage antibiotic therapy in patients with infections were included. The PRISMA diagram (Figure 1) depicts the flow of search screening and study selection. We sought, but did not find, nonrandomized comparative studies of populations, comparisons, interventions, and outcomes that were not adequately studied in randomized, controlled trials.

Data were pooled into clinically similar groups that were reviewed separately: (1) adult intensive care unit (ICU) patients, including patients with ventilator‐associated pneumonia; (2) adult patients with respiratory tract infections; (3) neonates with suspected sepsis; (4) children between 1 to 36 months of age with fever of unknown source; and (5) postoperative patients at risk of infection. Tables summarizing study quality and outcome measures with strength of evidence are available online (
| Outcome | Author, Year | N | PCT‐Guided Therapya | Controla | Difference PCT‐CTRL (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| ABT Duration, d | Hochreiter, 2009[22] | 110 | 5.9 | 7.9 | 2.0 (2.5 to 1.5) | <0.001 |
| Nobre, 2008[19] | 79 | 66 | 9.5 (ITT), 10 (PP) | 2.6 (5.5 to 0.3), 3.2 (1.1 to5.4) | 0.15, 0.003 | |
| Schroeder, 2009[20] | 27 | 6.6 | 8.3 | 1.7 (2.4 to 1.0) | <0.001 | |
| Stolz, 2009[21] | 101 | 10 (616)b | 15 (1023)b | 5 | 0.049 | |
| Bouadma, 2010[23] | 621 | 10.3 | 13.3 | 3.0 (4.20 to 1.80) | <0.0001 | |
| Days without ABTs, day 28 | Nobre, 2008[19] | 79 | 15.3, 17.4 | 13, 13.6 | 2.3 (5.9 to 1.8), 3.8 (0.1 to 7.5)c | 0.28, 0.04 |
| Stolz, 2009[21] | 101 | 13 (221)b | 9.5 (1.517)b | 3.5 | 0.049 | |
| Bouadma, 2010[23] | 621 | 14.3 | 11.6 | 2.7 (1.4 to 4.1) | <0.001 | |
| Total ABT exposured | Nobre, 2008[19] | 79 | 541 | 644 | 1.1e (0.9 to 1.3), 1.3e (1.1 to 1,5)c | 0.07, 0.0002 |
| 504 | 655 | |||||
| Stolz, 2009[21] | 101 | 1077 | 1341 | |||
| Bouadma, 2010[23]d | 621 | 653 | 812 | 159 (185 to 131) | <0.001 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| ABT duration, days | Jensen, 2011[33] | 1200 | 6 (311)b | 4 (310)b | NR | NR |
| Days spent in ICU on 3 ABTs | Jensen, 2011[33] | 1200 | 3570/5447 (65.5%) | 2721/4717 (57.7%) | 7.9% (6.0 to 9.7) | 0.002 |
| Adult patients with respiratory tract infections | ||||||
| ABT duration, da | Schuetz, 2009[2][5] | 1359 | 5.7 | 8.7 | 3.0 | |
| Christ‐Crain, 2004[30] | 243 | 10.9 | 12.8 | 1.9 (3.1 to 0.7) | 0.002 | |
| Kristoffersen, 2009[26] | 210 | 5.1 | 6.8 | 1.7 | ||
| Briel, 2008[27] | 458 | 6.2 | 7.1 | 1.0 (1.7 to 0.4) | <0.05 | |
| Long, 20113[5] | 162 | 5 (36)f | 7 (59)f | 2.0 | <0.001 | |
| Burkhardt, 2010[34] | 550 | 7.8 | 7.7 | 0.1 (0.7 to 0.9) | 0.8 | |
| Christ‐Crain, 2006[29] | 302 | 5.8 | 12.9 | 7.1(8.4 to 5.8) | <0.0001 | |
| Antibiotic prescription rate, % | Schuetz, 2009[2][5] | 1359 | 506/671 (75.4%) | 603/688 (87.6%) | 12.2% (16.3 to 8.1) | <0.05 |
| Christ‐Crain, 2004[30] | 243 | 55/124 (44.4%) | 99/119 (83.2%) | 38.8% (49.9 to 27.8) | <0.0001 | |
| Kristoffersen, 2009[26] | 210 | 88/103 (85.4%) | 85/107 (79.4%) | 6.0% (4.3 to 16.2) | 0.25 | |
| Briel, 2008[27] | 458 | 58/232 (25.0%) | 219/226 (96.9%) | 72% (78 to 66) | <0.05 | |
| Long, 20113[5] | 162 | NR (84.4%) | NR (97.5%) | 13.1% | 0.004 | |
| Stolz, 2007[28] | 208 | 41/102 (40.2%) | 76/106 (71.7%) | 31.5% (44.3 to 18.7) | <0.0001 | |
| Christ‐Crain, 2006[29] | 302 | 128/151 (84.8%) | 149/151 (98.79%) | 13.9% (19.9 to 7.9) | <0.0001 | |
| Burkhardt, 2010[34] | 550 | 84/275 (30.5%) | 89/275 (32.4%) | 1.8% (9.6 to 5.9) | 0.701 | |
| Total ABT exposure | Stolz, 2007[28] | 208 | NR | NR | 31.5% (18.7 to 44.3) | <0.0001 |
| Long, 20113[5] | 162 | NR | NR | NR | ||
| Christ‐Crain, 2006[29] | 302 | 136g | 323g | |||
| Christ‐Crain, 2004[30] | 243 | 332g | 661g | |||
| Neonates with sepsis | ||||||
| ABTs 72 hours, % | Stocker, 2010[31] | All neonates (N=121) | 33/60 (55%) | 50/61 (82%) | 27.0 (42.8 to 11.1) | 0.002 |
| Infection proven/probably (N=21) | 9/9 (100%) | 12/12 (100%) | 0% (0 to 0) | NA | ||
| Infection possible (N=40) | 13/21 (61.9%) | 19/19 (100%) | 38.1 (58.9 to 17.3) | 0.003 | ||
| Infection unlikely (N=60) | 11/30 (36.7%) | 19/30 (63.3%) | 26.6 (51.1 to 2.3) | 0.038 | ||
| ABT duration, h | Stocker, 2010[31] | All neonates (N=121) | 79.1 | 101.5 | 22.4 | 0.012 |
| Infection proven/probably (N=21) | 177.8 | 170.8 | 7 | NSS | ||
| Infection possible (N=40) | 83.4 | 111.5 | 28.1 | <0.001 | ||
| Infection unlikely (N=60) | 46.5 | 67.4 | 20.9 | 0.001 | ||
| Children ages 136 months with fever of unknown source | ||||||
| Antibiotic prescription rate, % | Manzano, 2010[36] | All children (N=384) | 48/192 (25%) | 54/192 (28.0%) | 3.1 (12.0 to 5.7) | 0.49 |
| No SBI or neutropenia (N=312) | 14/158 (9%) | 16/154 (10%) | 1.5 (8.1 to 5.0) | 0.65 | ||
| Adult postoperative patients at risk of infection | ||||||
| ABT duration, d | Chromik, 2006[32] | All patients (N=20) | 5.5 | 9 | 3.5 | 0.27 |
| Outcome | Author, Year | N | PCTa | Controla | Difference, PCT‐CTRL (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| ICU LOS, days | Hochreiter, 2009[22] | 110 | 15.5 | 17.7 | 2.2 | 0.046 |
| Nobre, 2008[19] | 79 | 4 | 7 | 4.6 (8.2 to 1.0) | 0.02 | |
| Schroeder, 2009[20] | 27 | 16.4 | 16.7 | 0.3 (5.6 to 5.0) | NSS | |
| Bouadma, 2010[23] | 621 | 15.9 | 14.4 | 1.5 (0.9 to 3.1) | 0.23 | |
| Hospital LOS, days | Nobre, 2008[19] | 79 | 17 | 23.5 | 2.5 (6.5 to 1.5) | 0.85 |
| Stolz, 2009[21] | 101 | 26 (721)b | 26 (16.822.3)b | 0 | 0.15 | |
| Bouadma, 2010[23] | 621 | 26.1 | 26.4 | 0.3 (3.2 to 2.7) | 0.87 | |
| ICU‐free days alive, 128 | Stolz, 2009[21] | 101 | 10 (018)b | 8.5 (018)c | 1.5 | 0.53 |
| SOFA day 28 | Bouadma, 2010[23] | 621 | 1.5 | 0.9 | 0.6 (0.0, 1.1) | 0.037 |
| SOFA score max | Schroeder, 2009[20] | 27 | 7.3 | 8.3 | 8.1 (4.1 to 1.7) | NSS |
| SAPS II score | Hochreiter, 2009[22] | 110 | 40.1 | 40.5 | 0.4 (6.4 to 5.6) | >0.05 |
| Days without MV | Stolz, 2009[21] | 101 | 21 (224)b | 19 (8.522.5)b | 2.0 | 0.46 |
| Bouadma, 2010[23] | 621 | 16.2 | 16.9 | 0.7 (2.4 to 1.1) | 0.47 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| ICU LOS, da | Svoboda, 2007[24] | 72 | 16.1 | 19.4 | 3.3 (7.0 to 0.4) | 0.09 |
| Jensen, 2011[33] | 1200 | 6 (312)b | 5 (311)b | 1 | 0.004 | |
| SOFA scorea | Svoboda, 2007[24] | 72 | 7.9 | 9.3 | 1.4 (2.8 to 0.0) | 0.06 |
| Days on MVa | Svoboda, 2007[24] | 72 | 10.3 | 13.9 | 3.6 (7.6 to 0.4) | 0.08 |
| Jensen, 2011[33] | 1200 | 3569 (65.5%) | 2861 (60.7%) | 4.9% (3 to 6.7) | <0.0001 | |
| Percent days in ICU with GFR <60 | Jensen, 2011[33] | 1200 | 2796 (51.3%) | 2187 (46.4%) | 5.0 % (3.0 to 6.9) | <0.0001 |
| Adult patients with respiratory tract infections | ||||||
| Hospital LOS, da | Schuetz, 2009[2][5] | 1359 | 9.4 | 9.2 | 0.2 | |
| Christ‐Crain, 2004[30] | 224 | 10.78.9 | 11.210.6 | 0.5 (3.0 to 2.0) | 0.69 | |
| Kristoffersen, 2009[26] | 210 | 5.9 | 6.7 | 0.8 | 0.22 | |
| Stolz, 2007[28] | 208 | 9 (115)b | 10 (115)b | 1 | 0.96 | |
| Christ‐Crain, 2006[29] | 302 | 12.09.1 | 13.09.0 | 1 (3.0 to 1.0) | 0.34 | |
| ICU admission, % | Schuetz, 2009[2][5] | 1359 | 43/671 (6.4%) | 60/688 (8.7%) | 2.3% (5.2 to 0.4) | 0.12 |
| Christ‐Crain, 2004[30] | 224 | 5/124 (4.0%) | 6/119 (5.0%) | 1.0% (6.2 to 4.2) | 0.71 | |
| Kristoffersen, 2009[26] | 210 | 7/103 (6.8%) | 5/107 (4.7%) | 2.1% (4.2 to 8.4) | 0.51 | |
| Stolz, 2007[28] | 208 | 8/102 (7.8%) | 11/106 (10.4%) | 2.5% (10.3 to 5.3) | 0.53 | |
| Christ‐Crain, 2006[29] | 302 | 20/151 (13.2%) | 21/151 (13.94%) | 0.7% (8.4 to 7.1) | 0.87 | |
| Antibiotic adverse events | Schuetz, 2009[2][5]c | 1359 | 133/671 (19.8%) | 193/688 (28.1%) | 8.2% (12.7 to 3.7) | |
| Briel, 2008[27]d | 458 | 2.34.6 days | 3.66.1 days | 1.1 days (2.1 to 0.1) | <0.05 | |
| Burkhardt, 2010[34]e | 550 | 11 /59 (18.6%) | 16/101 (15.8%) | 2.8% (9.4 to 15.0) | 0.65 | |
| Restricted activity, df | Briel, 2008[27] | 458 | 8.73.9 | 8.63.9 | 0.2 (0.4 to 0.9) | >0.05 |
| Burkhardt, 2010[34] | 550 | 9.1 | 8.8 | 0.25 (0.52 to 1.03) | >0.05 | |
| Neonates with sepsis | ||||||
| Recurrence of infection | Stocker, 2010[31] | 121 | 32% | 39% | 7 | 0.45 |
| Children ages 136 months with fever of unknown source | ||||||
| Hospitalization rate | Manzano, 2010[36] | All children (N=384) | 50/192 (26%) | 48/192 (25%) | 1 (8 to 10) | 0.81 |
| No SBI or neutropenia (N=312) | 16/158 (10%) | 11/154 (7%) | 3 (3 to 10) | 0.34 | ||
| Adult postoperative patients at risk of infection | ||||||
| Hospital LOS, days | Chromik, 2006[32] | 20 | 18 | 30 | 12 | 0.057 |
| Local wound infection, % | Chromik, 2006[32] | 20 | 1/10 | 2/10 | 10 (41.0 to 21.0) | 0.53 |
| Systemic infection, % | Chromik, 2006[32] | 20 | 3/10 | 7/10 | 40.0 (80.2 to 0.2) | 0.07 |
| Sepsis/SIRS, % | Chromik, 2006[32] | 20 | 2/10 | 8/10 | 60.0 (95.1 to 24.9) | 0.007 |
| Mortality | Mortality | Difference | ||||
|---|---|---|---|---|---|---|
| Outcome | Author, Year | N | PCT‐Guided Therapy | Control | PCT‐CTRL (95% CI) | P Value |
| ||||||
| Critically ill adult patients: procalcitonin‐guided antibiotic discontinuation | ||||||
| 28‐day mortality | Nobre, 2008[19] | 79 | 8/39 (20.5%) | 8/40 (20.0%) | 0.5 (17.2 to 18.2), | 0.95 |
| 5/31 (16.1%) | 6/37 (16.2%) | 0.1 (17.7 to 17.5)a | 0.99 | |||
| Stolz, 2009[21] | 101 | 8/51 (15.7%) | 12/50 (24.0%) | 8.3 (23.8 to 7.2) | 0.29 | |
| Bouadma, 2010[23] | 621 | 65/307 (21.2%) | 64/314 (20.4%) | 0.8 (5.6 to 7.2) | 0.81 | |
| 60‐day mortality | Bouadma, 2010[23] | 621 | 92/307 (30.0%) | 82/314 (26.1%) | 3.9 (3.2 to 10.9) | 0.29 |
| In‐hospital mortality | Nobre, 2008[19] | 79 | 9/39 (23.1%) | 9/40 (22.5%) | 0.6 (17.9 to 19.1) | 0.95 |
| 6/31 (19.4%) | 7/37 (18.9%) | 0.4+ (18.3 to 19.2) | 0.96 | |||
| Stolz, 2009[21] | 101 | 10/51 (19.6%) | 14/50 (28.0%) | 8.4, (24.9 to 8.1) | 0.32 | |
| Hochreiter, 2009[22] | 110 | 15/57 (26.3%) | 14/53 (26.4%) | 0.1, (16.6 to 16.4) | 0.99 | |
| Schroeder, 2009[20] | 27 | 3/14 (21.4%) | 3/13 (23.1%) | 1.7, (33.1 to 29.8) | 0.92 | |
| Critically ill adult patients: procalcitonin‐guided antibiotic intensification | ||||||
| 28‐day mortality | Svoboda, 2007[24] | 72 | 10/38 (26.3%) | 13/34 (38.2%) | 11.9 (33.4 to 9.6) | 0.28 |
| 28‐day mortality | Jensen, 2011[33] | 1200 | 190/604 (31.5%) | 191/596 (32.0%) | 0.6 (4.7 to 5.9) | 0.83 |
| Adult patients with respiratory tract infections | ||||||
| 6‐month mortality | Stolz, 2007[28] | 208 | 5/102 (4.9%) | 9/106 (8.5%) | 3.6% (10.3 to 3.2%) | 0.30 |
| 6‐week mortality | Christ‐Crain, 2006[29] | 302 | 18/151 (11.9%) | 20/151 (13.2%) | 1.3% (8.8 to 6.2) | 0.73 |
| 28‐day mortality | Christ‐Crain, 2004[30] | 243 | 4/124(3.2%) | 4/119 (3.4%) | 0.1% (4.6 to 4.4) | 0.95 |
| Schuetz, 2009 (30‐day)[25] | 1359 | 34/671(5.1%) | 33/688(4.8%) | 0.3% (2.1 to 2.5) | 0.82 | |
| Briel, 2008[27] | 458 | 0/231(0%) | 1/224 (0.4%) | 0.4% (1.3 to 0.4) | 0.31 | |
| Burkhardt, 2010[34] | 550 | 0/275(0%) | 0/275 (0%) | 0 | ||
| Kristoffersen, 2009[26] | 210 | 2/103(1.9%) | 1/107 (0.9%) | 1.0% (2.2 to 4.2) | 0.54 | |
| Long, 20113[5] | 162 | 0/81 (0%) | 0/81 (0%) | 0 | ||
| Neonates with sepsis | ||||||
| Mortality (in‐hospital) | Stocker, 2010[31] | 121 | 0% | 0% | 0 (0 to 0) | NA |
| Children ages 136 months with fever of unknown source | ||||||
| Mortality | Manzano, 2010[36] | 384 | All children | 0% | 0% | 0 (0 to 0) |
| Adult postoperative patients at risk of infection | ||||||
| Mortality | Chromik, 2006[32] | 20 | 1/10 (10%) | 3/10 (30%) | 20 (54.0 to 14.0) | 0.07 |
Adult ICU Patients: Procalcitonin‐Guided Antibiotic Discontinuation
Five studies[19, 20, 21, 22, 23] (N=938) addressed procalcitonin‐guided discontinuation of antibiotic therapy in adult ICU patients. Four studies conducted superiority analyses for mortality with procalcitonin‐guided therapy, whereas 1 study conducted a noninferiority analysis. Absolute procalcitonin values for discontinuation of antibiotics ranged from 0.25 to 1 ng/mL. Physicians in control groups administered antibiotics according to their standard practice.
Antibiotic Usage
The absolute reduction in duration of antibiotic usage with procalcitonin guidance in these studies ranged from 1.7 to 5 days, and the relative reduction ranged from 21% to 38%. Meta‐analysis of antibiotic duration in adult ICU patients was performed (Figure 2A).

Morbidity
Procalcitonin‐guided antibiotic discontinuation did not increase morbidity, including ICU length of stay (LOS). Meta‐analysis of ICU LOS is displayed in Figure 2B. Limited data on adverse antibiotic events were reported (Table 2).
Mortality
There was no increase in mortality as a result of shorter duration of antibiotic therapy. Meta‐analysis of short‐term mortality (28‐day or in‐hospital mortality) showed a mortality difference of 0.43% favoring procalcitonin‐guided therapy, and a 95% confidence interval (CI) of 6% to 5% (Figure 2C).
Adult ICU Patients: Procalcitonin‐Guided Antibiotic Intensification
Two studies[24, 33] (N=1272) addressed procalcitonin‐guided intensification of antibiotic therapy in adult ICU patients. The Jensen et al. study[33] was a large (N=1200), high‐quality study that used a detailed algorithm for broadening antibiotic therapy in patients with elevated procalcitonin. The Jensen et al. study also educated physicians about empiric therapy and intensification of antibiotic therapy. A second study[24] was too small (N=72) and lacked sufficient details to be informative.
Antibiotic Usage
The Jensen et al. study found a 2‐day increase, or 50% relative increase, in the duration of antibiotic therapy and a 7.9% absolute increase (P=0.002) in the number of days on 3 antibiotics with procalcitonin‐guided intensification.
Morbidity
The Jensen et al. study showed a significant 1‐day increase in ICU LOS (P=0.004) and a significant increase in organ dysfunction. Specifically, patients had a highly statistically significant 5% increase in days on mechanical ventilation (P<0.0001) and 5% increase in days with abnormal renal function (P<0.0001).
Mortality
The Jensen et al. study was a superiority trial powered to test a 7.5% decrease in 28‐day mortality, but no significant difference in mortality was observed with procalcitonin‐guided intensification (31.5% vs 32.0, P=0.83).
Adult Patients With Respiratory Tract Infections
Eight studies[25, 26, 27, 28, 29, 30, 34, 35] (N=3492) addressed initiation and/or discontinuation of antibiotics in adult patients with acute upper and lower respiratory tract infections, including community‐acquired pneumonia, acute exacerbation of chronic obstructive pulmonary disease, and acute bronchitis. Settings included primary care clinics, emergency departments, and hospital wards. Physicians in control groups administered antibiotics according to their own standard practices and/or evidence‐based guidelines. All studies encouraged initiation of antibiotics with procalcitonin levels >0.25 ng/mL, and 4 studies strongly encouraged antibiotics with procalcitonin levels >0.5 ng/mL.
Antibiotic Usage
Procalcitonin guidance reduced antibiotic duration, antibiotic prescription rate, and total antibiotic exposure. Absolute reduction in antibiotic duration ranged from 1 to 7 days, and relative reductions ranged from 13% to 55%. Four of the 8 studies reported sufficient details to be pooled into a meta‐analysis (Figure 3A) with a statistically significant pooled mean difference of 2.35 days favoring procalcitonin (95% CI: 4.38 to 0.33). Procalcitonin guidance also reduced antibiotic prescription rate with absolute reductions ranging from 2% to 7% and relative reductions ranging from 1.8% to 72%. Meta‐analysis of prescription rates from 8 studies (Figure 3B) yielded a statistically significant pooled risk difference of 22% (95% CI: 41% to 4%). Total antibiotic exposure was consistently reduced in the 4 studies reporting this outcome.

Morbidity
Procalcitonin guidance did not increase hospital LOS or ICU admission rates. Meta‐analysis of ICU admission rates from 5 studies (Figure 3C) produced a risk difference of 1%, with a narrow 95% CI (4% to 1%). There was insufficient evidence to judge the effect on days of restricted activity or antibiotic adverse events.
Mortality
Procalcitonin guidance did not increase mortality, and meta‐analysis of 4 studies (Figure 3D) produced a risk difference of 0.3% with a narrow 95% CI (1% to 2%), with no statistical heterogeneity (I2=0%).
Neonates With Sepsis
One study[31] (N=121) evaluated procalcitonin‐guided antibiotic therapy for suspected neonatal sepsis. Neonatal sepsis was suspected on the basis of risk factors and clinical signs and symptoms. Antibiotic initiation or discontinuation was based on a procalcitonin nomogram. Antibiotic therapy in the control group was based on the physician's assessment. The quality of this study was rated good, and strength of evidence was rated moderate for antibiotic usage and insufficient for morbidity and mortality outcomes.
Antibiotic Usage
Duration of antibiotic therapy was decreased by 22.4 hours (P=0.012), a 24% relative reduction, and the proportion of neonates on antibiotics 72 hours was reduced by 27% (P=0.002). The largest reduction in antibiotic duration was seen in the 80% to 85% of neonates who were categorized as having possible or infection or unlikely to have infection.
Morbidity
A statistically insignificant 7% reduction in rate of recurrence of infection was seen with procalcitonin‐guided antibiotic therapy (P=0.45).
Mortality
No in‐hospital deaths occurred in either the procalcitonin or control group.
Children Ages 1 to 36 Months With Fever of Unknown Source
One study[36] (N=384) evaluated procalcitonin‐guided antibiotic therapy for fever of unknown source in children 1 to 36 months of age, but the overall strength of evidence was judged insufficient to draw conclusions.
Antibiotic Usage
A statistically insignificant reduction of 3.1% in antibiotic prescription rate was seen with procalcitonin‐guided antibiotic therapy (P=0.49).
Morbidity
Rate of hospitalization was relatively low, and no significant difference was seen between procalcitonin and control groups.
Mortality
In‐hospital mortality was reported as 0% in both arms.
Adult Postoperative Patients at Risk of Infection
One study[32] (N =250) monitored procalcitonin in consecutive patients after colorectal surgery to identify patients at risk of infection who might benefit from prophylactic antibiotic therapy. Two hundred thirty patients had normal procalcitonin levels. Twenty patients with elevated procalcitonin levels (>1.5 ng/mL) were randomized to receive prophylactic antibiotic therapy with ceftriaxone or no antibiotics. The strength of evidence was judged insufficient to draw conclusions from this study.
Antibiotic Usage
Duration of antibiotic therapy was reduced by 3.5% but was not statistically insignificant (P=0.27).
Morbidity
Procalcitonin guidance reduced the incidence of sepsis/systemic inflammatory response syndrome by 60% (p=0.007). The incidences of local and systemic infection were reduced with procalcitonin guidance but were not statistically significant (10%, P=0.53; and 40%, P=0.07, respectively).
Mortality
Mortality was 20% higher in the control arm but was not statistically significant (P=0.07).
DISCUSSION
Summary of the Main Findings
Diagnosis of sepsis or other serious infections in critically ill patients is challenging because clinical criteria for diagnosis overlap with noninfectious causes of the systemic inflammatory response syndrome. Initiation of antibiotic therapy for presumed sepsis is necessary while diagnostic evaluation is ongoing, because delaying antibiotic therapy is associated with increased mortality.[37, 38, 39] Our review found that procalcitonin guidance significantly reduced antibiotic usage in adult ICU patients by reducing the duration of antibiotic therapy, rather than decreasing the initiation of antibiotics, without increasing morbidity or mortality.
In contrast, the use of procalcitonin as an indicator of need for intensification of antibiotic therapy in adult ICU patients should be discouraged because this approach was associated with increased morbidity. The large, well‐designed study by Jensen[33] showed that antibiotic intensification in response to elevated procalcitonin measurement was associated with increased morbidity: a longer ICU LOS, an increase in days on mechanical ventilation, and an increase in days with abnormal renal function. The authors concluded that the increased morbidity could only be explained by clinical harms of increased exposure to broad‐spectrum antibiotics.
Clinical and microbiological evaluations are neither sensitive nor specific for differentiating bacterial from viral respiratory tract infections. Procalcitonin can guide initiation of antibiotic therapy in adults with suspected bacterial respiratory tract infection. Our review showed that procalcitonin guidance significantly reduced antibiotic usage with respect to antibiotic prescription rate, duration of antibiotic therapy, and total exposure to antibiotic therapy in adult patients with respiratory tract infections.
The role of procalcitonin‐guided therapy in other populations is less clear. One study in postoperative colorectal surgery patients reported that elevated procalcitonin levels may identify patients at risk for infection who benefit from prophylactic antibiotic therapy.[32] Patients with elevated procalcitonin levels who received prophylactic antibiotic therapy had a significant decrease in the incidence and severity of systemic infections, whereas patients with normal procalcitonin levels did not require any additional surgical or medical therapy. Although these findings are promising, more data in postoperative patients are needed.
The utility of procalcitonin in pediatric settings is a significant gap in the present literature. One study[31] in neonates with suspected sepsis showed a significant decrease in the proportion of neonates started on empiric antibiotic therapy and a decrease in the duration of antibiotic therapy with procalcitonin guidance. However, there was insufficient evidence that procalcitonin guidance does not increase morbidity or mortality.
Comparison to Other Systematic Reviews
Six systematic reviews of procalcitonin guidance in the management of patients with infections were published prior to our review.[9, 10, 11, 12, 13, 14] Our systematic review differs from past reviews in the number of studies included and the pooling of studies according to patient population, type and severity of infection, and different uses of procalcitonin measurements, either for initiation, discontinuation, or intensification of antibiotic therapy. Previous systematic reviews included 7 to 14 studies, whereas ours included 18 randomized, controlled trials. One previous review[13] included and pooled the Jensen et al. study[33] with other studies of adult ICU patients. We evaluated the Jensen et al. study separately because it uniquely looked at procalcitonin‐guided antibiotic intensification in adult ICU patients, in contrast to other studies that looked at procalcitonin‐guided antibiotic discontinuation. We addressed pediatric populations separately from adult patients, and recognizing that there are distinct groups within the pediatric population, we separately grouped neonates and children ages 1 to 36 months. Despite these differences, our review and other systematic reviews, we came to similar conclusions: procalcitonin‐guided antibiotic decision making compared to clinical criteria‐guided antibiotic decision making reduces antibiotic usage without increasing morbidity or mortality.
Limitations
An important limitation of this review was the uncertainty about the noninferiority margin for morbidity and mortality in adult ICU patients. Only the Bouadma et al. study[23] did a power analysis and predefined a margin for noninferiority for 28‐ and 60‐day mortality. Meta‐analysis of all 5 ICU studies showed a pooled point estimate of 0.43% in mortality and a 95% CI of 6% to 5% for difference in mortality between procalcitonin‐guided therapy versus standard care. A 10% noninferiority margin for mortality has been recommended by the Infectious Diseases Society of America and American College of Chest Physicians, but there is concern that a 10% margin for mortality may be too high. Presently, 2 large trials are in progress that may yield more precise estimates of mortality in the future.
Differences in reporting of total antibiotic exposure and morbidity outcomes limited our ability to pool data. Total antibiotic exposure is conventionally reported as mean days per 1000 days of follow‐up, but some studies only reported relative or absolute differences. Likewise, morbidity was reported with different severity of illness scales, including Sepsis‐Related Organ Failure Assessment, Simplified Acute Physiology (SAP) II, SAP III, and Acute Physiology and Chronic Health Evaluation II, which limited comparisons across studies.
Research Gaps
We identified gaps in the available literature and opportunities for future research. First, the safety and efficacy of procalcitonin‐guided antibiotic therapy needs to be studied in patient populations excluded from current randomized controlled studies, such as immunocompromised patients and pregnant women. Patients who are immunocompromised or have chronic conditions, such as cystic fibrosis, account for a significant percentage of community‐acquired respiratory tract infections and are often treated empirically.[29, 30] Second, standardized reporting of antibiotic adverse events and emergence of antibiotic resistance is needed. Strategies to reduce antibiotic usage have been associated with reductions in antibiotic adverse events, such as Clostridium difficile colitis and superinfection with multi‐drug resistant Gram‐negative bacteria.[37, 40, 41] Few studies in our review reported allergic reactions or adverse events of antibiotic therapy, [25, 27, 34] and only 1 reported antibiotic resistance.[19] Third, procalcitonin guidance should be compared to other strategies to reduce antibiotic usage, such as structured implementation of practice guidelines and antibiotic stewardship programs.[42] One single‐arm study describes how procalcitonin can be used in antibiotic stewardship programs to decrease the duration of antibiotic therapy,[43] but additional studies are needed. Finally, generalizing results from those studies that were conducted primarily in Europe would depend on similar use of and adherence to study‐based algorithms. Newer observational studies have demonstrated reduced antibiotic usage with implementation of procalcitonin algorithms in real‐life settings in Europe, but algorithm adherence was significantly less in the United States.[44, 45]
In summary, our systematic review found that procalcitonin‐guided antibiotic therapy can significantly reduce antibiotic usage in adult ICU patients without affecting morbidity or mortality. Procalcitonin should not be used to guide intensification of antibiotic therapy in adult ICU patients because this approach may increase morbidity. In adults with respiratory infections, procalcitonin guidance can significantly reduce antibiotic usage without adversely affecting morbidity or mortality. There is insufficient evidence to recommend procalcitonin‐guided antibiotic therapy in neonates with sepsis, children with fever of unknown source, or postoperative patients at risk for infection.
Acknowledgments
Disclosures: This project was funded under contract HHSA 2902007‐10058 from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services. The authors of this article are responsible for its content, including any clinical treatment recommendations. No statement in this article should be construed as an official position of AHRQ or of the US Department of Health and Human Services. There are no conflicts of interest reported by any of the authors.
- , . Sepsis biomarkers: a review. Crit Care. 2010;14(1):R15.
- , . Biomarkers of sepsis. Crit Care Med. 2009;37(7):2290–2298.
- , , . Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888–889.
- , , , et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605–1608.
- , , , et al. Procalcitonin kinetics as a prognostic marker of ventilator‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(1):48–53.
- , , , , . Serum procalcitonin and C‐reactive protein levels as markers of bacterial infection: a systematic review and meta‐analysis. Clin Infect Dis. 2004;39(2):206–217.
- , . Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007;30(3):556–573.
- , , , et al. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis. 1998;26(3):664–672.
- , , , , . Effect of procalcitonin‐guided treatment in patients with infections: a systematic review and meta‐analysis. Infection. 2009;37(6):497–507.
- , . Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53(4):379–387.
- , , , , . Procalcitonin‐guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta‐analysis of randomized controlled trials. Crit Care Med. 2010;38(11):2229–2241.
- , , , . Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322–1331.
- , , , , , . An ESCIM systematic review and meta‐analysis of procalcitonin‐guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Med. 2012;38:940–949.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
- , , , , , . Prepared by the Blue Cross and Blue Shield Association Technology Evaluation Center Evidence‐based Practice Center under contract no. 290–2007‐10058‐I. Procalcitonin‐guided antibiotic therapy. Comparative effectiveness review No. 78. AHRQ publication no. 12(13)‐EHC124‐EF. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Published Accessed October 2012.
- Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ publication no. 10(11)‐EHC063‐EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 suppl):21–35.
- , , , et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—agency for healthcare research and quality and the effective health‐care program. J Clin Epidemiol. 2010;63(5):513–523.
- , , , , . Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177(5):498–505.
- , , , et al. Procalcitonin (PCT)‐guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009;394(2):221–226.
- , , , et al. Procalcitonin for reduced antibiotic exposure in ventilator‐associated pneumonia: a randomised study. Eur Respir J. 2009;34(6):1364–1375.
- , , , et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13(3):R83.
- , , , et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–474.
- , , , , . Can procalcitonin help us in timing of re‐intervention in septic patients after multiple trauma or major surgery? Hepatogastroenterology. 2007;54(74):359–363.
- , , , et al. Effect of procalcitonin‐based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066.
- , , , et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission—a randomized trial. Clin Microbiol Infect. 2009;15(5):481–487.
- , , , et al. Procalcitonin‐guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168(18):2000–2007; discussion 2007–2008.
- , , , et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest. 2007;131(1):9–19.
- , , , et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84–93.
- , , , et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363(9409):600–607.
- , , , , . Use of procalcitonin‐guided decision‐making to shorten antibiotic therapy in suspected neonatal early‐onset sepsis: prospective randomized intervention trial. Neonatology. 2010;97(2):165–174.
- , , , , , . Pre‐emptive antibiotic treatment vs “standard” treatment in patients with elevated serum procalcitonin levels after elective colorectal surgery: a prospective randomised pilot study. Langenbecks Arch Surg. 2006;391(3):187–194.
- , , , et al. Procalcitonin‐guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39(9):2048–2058.
- , , , et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J. 2010;36(3):601–607.
- , , , , , . Procalcitonin guidance for reduction of antibiotic use in low‐risk outpatients with community‐acquired pneumonia. Respirology. 2011;16(5):819–824.
- , , , , , . Impact of procalcitonin on the management of children aged 1 to 36 months presenting with fever without source: a randomized controlled trial. Am J Emerg Med. 2010;28(6):647–653.
- , , , , , . Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia. Crit Care Med. 2001;29(6):1109–1115.
- , , , et al. Diagnostic value of procalcitonin, interleukin‐6, and interleukin‐8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164(3):396–402.
- , , , . Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462–474.
- , , , , . Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol. 2003;24(9):699–706.
- , , , et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- , , , , . Use of procalcitonin (PCT) to guide discontinuation of antibiotic use in an unspecified sepsis is an antimicrobial stewardship program (ASP). Eur J Clin Microbiol Infect Dis. 2011;30(7):853–855.
- , , , et al. Effectiveness and safety of procalcitonin‐guided antibiotic therapy in lower respiratory tract infections in “real life.” Arch Intern Med. 2012;172(9):715–722.
- , , , et al. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: a post‐study survey. Eur J Clin Microbiol Infect Dis. 2012;29(3):269–277.
- , . Sepsis biomarkers: a review. Crit Care. 2010;14(1):R15.
- , . Biomarkers of sepsis. Crit Care Med. 2009;37(7):2290–2298.
- , , . Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888–889.
- , , , et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605–1608.
- , , , et al. Procalcitonin kinetics as a prognostic marker of ventilator‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(1):48–53.
- , , , , . Serum procalcitonin and C‐reactive protein levels as markers of bacterial infection: a systematic review and meta‐analysis. Clin Infect Dis. 2004;39(2):206–217.
- , . Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007;30(3):556–573.
- , , , et al. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis. 1998;26(3):664–672.
- , , , , . Effect of procalcitonin‐guided treatment in patients with infections: a systematic review and meta‐analysis. Infection. 2009;37(6):497–507.
- , . Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53(4):379–387.
- , , , , . Procalcitonin‐guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta‐analysis of randomized controlled trials. Crit Care Med. 2010;38(11):2229–2241.
- , , , . Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322–1331.
- , , , , , . An ESCIM systematic review and meta‐analysis of procalcitonin‐guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Med. 2012;38:940–949.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
- , , , , , . Prepared by the Blue Cross and Blue Shield Association Technology Evaluation Center Evidence‐based Practice Center under contract no. 290–2007‐10058‐I. Procalcitonin‐guided antibiotic therapy. Comparative effectiveness review No. 78. AHRQ publication no. 12(13)‐EHC124‐EF. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Published Accessed October 2012.
- Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ publication no. 10(11)‐EHC063‐EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- , , , et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 suppl):21–35.
- , , , et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—agency for healthcare research and quality and the effective health‐care program. J Clin Epidemiol. 2010;63(5):513–523.
- , , , , . Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177(5):498–505.
- , , , et al. Procalcitonin (PCT)‐guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009;394(2):221–226.
- , , , et al. Procalcitonin for reduced antibiotic exposure in ventilator‐associated pneumonia: a randomised study. Eur Respir J. 2009;34(6):1364–1375.
- , , , et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13(3):R83.
- , , , et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–474.
- , , , , . Can procalcitonin help us in timing of re‐intervention in septic patients after multiple trauma or major surgery? Hepatogastroenterology. 2007;54(74):359–363.
- , , , et al. Effect of procalcitonin‐based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066.
- , , , et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission—a randomized trial. Clin Microbiol Infect. 2009;15(5):481–487.
- , , , et al. Procalcitonin‐guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168(18):2000–2007; discussion 2007–2008.
- , , , et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest. 2007;131(1):9–19.
- , , , et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84–93.
- , , , et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363(9409):600–607.
- , , , , . Use of procalcitonin‐guided decision‐making to shorten antibiotic therapy in suspected neonatal early‐onset sepsis: prospective randomized intervention trial. Neonatology. 2010;97(2):165–174.
- , , , , , . Pre‐emptive antibiotic treatment vs “standard” treatment in patients with elevated serum procalcitonin levels after elective colorectal surgery: a prospective randomised pilot study. Langenbecks Arch Surg. 2006;391(3):187–194.
- , , , et al. Procalcitonin‐guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39(9):2048–2058.
- , , , et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J. 2010;36(3):601–607.
- , , , , , . Procalcitonin guidance for reduction of antibiotic use in low‐risk outpatients with community‐acquired pneumonia. Respirology. 2011;16(5):819–824.
- , , , , , . Impact of procalcitonin on the management of children aged 1 to 36 months presenting with fever without source: a randomized controlled trial. Am J Emerg Med. 2010;28(6):647–653.
- , , , , , . Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia. Crit Care Med. 2001;29(6):1109–1115.
- , , , et al. Diagnostic value of procalcitonin, interleukin‐6, and interleukin‐8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164(3):396–402.
- , , , . Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462–474.
- , , , , . Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol. 2003;24(9):699–706.
- , , , et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- , , , , . Use of procalcitonin (PCT) to guide discontinuation of antibiotic use in an unspecified sepsis is an antimicrobial stewardship program (ASP). Eur J Clin Microbiol Infect Dis. 2011;30(7):853–855.
- , , , et al. Effectiveness and safety of procalcitonin‐guided antibiotic therapy in lower respiratory tract infections in “real life.” Arch Intern Med. 2012;172(9):715–722.
- , , , et al. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: a post‐study survey. Eur J Clin Microbiol Infect Dis. 2012;29(3):269–277.
Continuous imatinib therapy in patients with gastrointestinal stromal tumors
Patients with gastrointestinal stromal tumors (GIST) used to have a poor prognosis due to the very low response rate of these tumors to conventional chemotherapy and radiation therapy. However, following the introduction of imatinib as a targeted therapeutic agent with efficacy in GIST, survival outcomes have improved remarkably for patients in the advanced/metastatic and adjuvant settings. Imatinib is now approved for both indications and has become the standard of care for patients with GIST. Despite the mounting evidence demonstrating the clinical benefits of extending imatinib treatment beyond 1 year, the optimal duration of imatinib therapy has not yet been determined. Similarly, whether chronic or extended adjuvant imatinib therapy can further improve clinical outcomes in patients with GIST remains to be determined. In this review, we present recent findings from various clinical trials which indicate that prolonged, uninterrupted imatinib treatment can have durable clinical benefits in patients who underwent resection of primary, operable GIST, as well as patients with advanced, unresectable, or metastatic GIST. We also summarize data showing that treatment interruption can result in disease progression in both the adjuvant and advanced/metastatic settings. Finally, we present evidence from different trials that long-term imatinib therapy is feasible and safe (ie, without cumulative toxicities) in patients with GIST.
*Click on the link to the left for a PDF of the full article.
Patients with gastrointestinal stromal tumors (GIST) used to have a poor prognosis due to the very low response rate of these tumors to conventional chemotherapy and radiation therapy. However, following the introduction of imatinib as a targeted therapeutic agent with efficacy in GIST, survival outcomes have improved remarkably for patients in the advanced/metastatic and adjuvant settings. Imatinib is now approved for both indications and has become the standard of care for patients with GIST. Despite the mounting evidence demonstrating the clinical benefits of extending imatinib treatment beyond 1 year, the optimal duration of imatinib therapy has not yet been determined. Similarly, whether chronic or extended adjuvant imatinib therapy can further improve clinical outcomes in patients with GIST remains to be determined. In this review, we present recent findings from various clinical trials which indicate that prolonged, uninterrupted imatinib treatment can have durable clinical benefits in patients who underwent resection of primary, operable GIST, as well as patients with advanced, unresectable, or metastatic GIST. We also summarize data showing that treatment interruption can result in disease progression in both the adjuvant and advanced/metastatic settings. Finally, we present evidence from different trials that long-term imatinib therapy is feasible and safe (ie, without cumulative toxicities) in patients with GIST.
*Click on the link to the left for a PDF of the full article.
Patients with gastrointestinal stromal tumors (GIST) used to have a poor prognosis due to the very low response rate of these tumors to conventional chemotherapy and radiation therapy. However, following the introduction of imatinib as a targeted therapeutic agent with efficacy in GIST, survival outcomes have improved remarkably for patients in the advanced/metastatic and adjuvant settings. Imatinib is now approved for both indications and has become the standard of care for patients with GIST. Despite the mounting evidence demonstrating the clinical benefits of extending imatinib treatment beyond 1 year, the optimal duration of imatinib therapy has not yet been determined. Similarly, whether chronic or extended adjuvant imatinib therapy can further improve clinical outcomes in patients with GIST remains to be determined. In this review, we present recent findings from various clinical trials which indicate that prolonged, uninterrupted imatinib treatment can have durable clinical benefits in patients who underwent resection of primary, operable GIST, as well as patients with advanced, unresectable, or metastatic GIST. We also summarize data showing that treatment interruption can result in disease progression in both the adjuvant and advanced/metastatic settings. Finally, we present evidence from different trials that long-term imatinib therapy is feasible and safe (ie, without cumulative toxicities) in patients with GIST.
*Click on the link to the left for a PDF of the full article.
Dihydropyridine calcium channel blockers in dementia and hypertension
Dementia affects 34 million people globally, with the most common cause of dementia, Alzheimer’s disease (AD), affecting 5.5 million Americans.1,2 The connection between cerebrovascular disorders and AD means that antihypertensive agents may play a role in dementia prophylaxis and management.1,2
Hypertension increases the risk of intellectual dysfunction by increasing susceptibility to heart disease, ischemic brain injury, and cerebrovascular pathology.1 In addition to senile plaques, ischemic brain lesions are observed in autopsies of AD patients,1 and brain infarctions are more common among AD patients than among controls.2 Brain pathology suggestive of AD was found in 30% to 50% of postmortem examinations of patients with vascular dementia.1
It is useful to note that dihydropyridines, a subgroup of calcium channel blockers, may inhibit amyloidogenesis.3
Hypertension and cognition
Hypertension-induced hyperdense lesions in cerebral white matter reflect pathology in small vessels, inflammatory change, and disruption of the blood-brain barrier, which may precede cognitive decline.1 Even subclinical ischemic changes may increase the probability of developing dementia.2 Hypertension also reduces cerebral perfusion, especially in the hippocampus, which may promote degeneration of memory function.1 Prolonged cerebral hypoxia increases amyloid precursor protein production and β-secretase activity.1,2 Patients who died of brain ischemia show prominent β-amyloid protein and apolipoprotein E in histopathologic analysis of the hippocampus.1 Compression of vessels by â-amyloid protein further augments this degenerative process.1
Inhibition of amyloidogenesis
Long-term administration of antihypertensive medications in patients age <75 decreases the probability of dementia by 8% each year.1 Calcium channel blockers protect neurons by lowering blood pressure and reversing cellular-level calcium channel dysfunction that occurs with age, cerebral infarction, and AD.
Select dihydropyridines may inhibit amyloidogenesis in apolipoprotein E carriers:
• amlodipine and nilvadipine reduce β-secretase activity and amyloid precursor protein-β production3
• nilvadipine and nitrendipine limit β-amyloid protein synthesis in the brain and promote their clearance through the blood-brain barrier3
• nilvadipine-treated apolipoprotein E carriers experience cognitive stabilization compared with cognitive decreases seen in non-treated subjects.
Dihydropyridines can produce therapeutic effects for both AD and cerebrovascular dementia patients, indicating the potential that certain agents in this class have for treating both conditions.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Valenzuela M, Esler M, Ritchie K, et al. Antihypertensives for combating dementia? A perspective on candidate molecular mechanisms and population-based prevention. Transl Psychiatry. 2012;2:e107.
2. Pimentel-Coelho PM, Rivest S. The early contribution of cerebrovascular factors to the pathogenesis of Alzheimer’s disease. Eur J Neurosci. 2012;35(12):1917-1937.
3. Paris D, Bachmeier C, Patel N, et al. Selective antihypertensive dihydropyridines lower Aβ accumulation by targeting both the production and the clearance of Aβ across the blood-brain barrier. Mol Med. 2011;17(3-4):149-162.
Dementia affects 34 million people globally, with the most common cause of dementia, Alzheimer’s disease (AD), affecting 5.5 million Americans.1,2 The connection between cerebrovascular disorders and AD means that antihypertensive agents may play a role in dementia prophylaxis and management.1,2
Hypertension increases the risk of intellectual dysfunction by increasing susceptibility to heart disease, ischemic brain injury, and cerebrovascular pathology.1 In addition to senile plaques, ischemic brain lesions are observed in autopsies of AD patients,1 and brain infarctions are more common among AD patients than among controls.2 Brain pathology suggestive of AD was found in 30% to 50% of postmortem examinations of patients with vascular dementia.1
It is useful to note that dihydropyridines, a subgroup of calcium channel blockers, may inhibit amyloidogenesis.3
Hypertension and cognition
Hypertension-induced hyperdense lesions in cerebral white matter reflect pathology in small vessels, inflammatory change, and disruption of the blood-brain barrier, which may precede cognitive decline.1 Even subclinical ischemic changes may increase the probability of developing dementia.2 Hypertension also reduces cerebral perfusion, especially in the hippocampus, which may promote degeneration of memory function.1 Prolonged cerebral hypoxia increases amyloid precursor protein production and β-secretase activity.1,2 Patients who died of brain ischemia show prominent β-amyloid protein and apolipoprotein E in histopathologic analysis of the hippocampus.1 Compression of vessels by â-amyloid protein further augments this degenerative process.1
Inhibition of amyloidogenesis
Long-term administration of antihypertensive medications in patients age <75 decreases the probability of dementia by 8% each year.1 Calcium channel blockers protect neurons by lowering blood pressure and reversing cellular-level calcium channel dysfunction that occurs with age, cerebral infarction, and AD.
Select dihydropyridines may inhibit amyloidogenesis in apolipoprotein E carriers:
• amlodipine and nilvadipine reduce β-secretase activity and amyloid precursor protein-β production3
• nilvadipine and nitrendipine limit β-amyloid protein synthesis in the brain and promote their clearance through the blood-brain barrier3
• nilvadipine-treated apolipoprotein E carriers experience cognitive stabilization compared with cognitive decreases seen in non-treated subjects.
Dihydropyridines can produce therapeutic effects for both AD and cerebrovascular dementia patients, indicating the potential that certain agents in this class have for treating both conditions.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dementia affects 34 million people globally, with the most common cause of dementia, Alzheimer’s disease (AD), affecting 5.5 million Americans.1,2 The connection between cerebrovascular disorders and AD means that antihypertensive agents may play a role in dementia prophylaxis and management.1,2
Hypertension increases the risk of intellectual dysfunction by increasing susceptibility to heart disease, ischemic brain injury, and cerebrovascular pathology.1 In addition to senile plaques, ischemic brain lesions are observed in autopsies of AD patients,1 and brain infarctions are more common among AD patients than among controls.2 Brain pathology suggestive of AD was found in 30% to 50% of postmortem examinations of patients with vascular dementia.1
It is useful to note that dihydropyridines, a subgroup of calcium channel blockers, may inhibit amyloidogenesis.3
Hypertension and cognition
Hypertension-induced hyperdense lesions in cerebral white matter reflect pathology in small vessels, inflammatory change, and disruption of the blood-brain barrier, which may precede cognitive decline.1 Even subclinical ischemic changes may increase the probability of developing dementia.2 Hypertension also reduces cerebral perfusion, especially in the hippocampus, which may promote degeneration of memory function.1 Prolonged cerebral hypoxia increases amyloid precursor protein production and β-secretase activity.1,2 Patients who died of brain ischemia show prominent β-amyloid protein and apolipoprotein E in histopathologic analysis of the hippocampus.1 Compression of vessels by â-amyloid protein further augments this degenerative process.1
Inhibition of amyloidogenesis
Long-term administration of antihypertensive medications in patients age <75 decreases the probability of dementia by 8% each year.1 Calcium channel blockers protect neurons by lowering blood pressure and reversing cellular-level calcium channel dysfunction that occurs with age, cerebral infarction, and AD.
Select dihydropyridines may inhibit amyloidogenesis in apolipoprotein E carriers:
• amlodipine and nilvadipine reduce β-secretase activity and amyloid precursor protein-β production3
• nilvadipine and nitrendipine limit β-amyloid protein synthesis in the brain and promote their clearance through the blood-brain barrier3
• nilvadipine-treated apolipoprotein E carriers experience cognitive stabilization compared with cognitive decreases seen in non-treated subjects.
Dihydropyridines can produce therapeutic effects for both AD and cerebrovascular dementia patients, indicating the potential that certain agents in this class have for treating both conditions.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Valenzuela M, Esler M, Ritchie K, et al. Antihypertensives for combating dementia? A perspective on candidate molecular mechanisms and population-based prevention. Transl Psychiatry. 2012;2:e107.
2. Pimentel-Coelho PM, Rivest S. The early contribution of cerebrovascular factors to the pathogenesis of Alzheimer’s disease. Eur J Neurosci. 2012;35(12):1917-1937.
3. Paris D, Bachmeier C, Patel N, et al. Selective antihypertensive dihydropyridines lower Aβ accumulation by targeting both the production and the clearance of Aβ across the blood-brain barrier. Mol Med. 2011;17(3-4):149-162.
1. Valenzuela M, Esler M, Ritchie K, et al. Antihypertensives for combating dementia? A perspective on candidate molecular mechanisms and population-based prevention. Transl Psychiatry. 2012;2:e107.
2. Pimentel-Coelho PM, Rivest S. The early contribution of cerebrovascular factors to the pathogenesis of Alzheimer’s disease. Eur J Neurosci. 2012;35(12):1917-1937.
3. Paris D, Bachmeier C, Patel N, et al. Selective antihypertensive dihydropyridines lower Aβ accumulation by targeting both the production and the clearance of Aβ across the blood-brain barrier. Mol Med. 2011;17(3-4):149-162.