User login
P3SONG: Evaluation for autism spectrum disorder
Autism spectrum disorder (ASD) is characterized by impairments in communication and social interactions, along with repetitive and perseverant behaviors.1 It has a prevalence of 0.75% to 1.1% among the general population.1 The presentation of ASD can vary, and patients may have a wide range of comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), neurologic disorders, and genetic disorders.1 Therefore, a comprehensive evaluation needs to include a multidisciplinary assessment by clinicians from several specialties, including primary care, psychiatry, psychology, and neurology. Here I offer psychiatrists 3 Ps and the mnemonic SONG to describe a multidisciplinary approach to assessing a patient with suspected or confirmed ASD.
Primary care evaluation of patients with ASD is important for the diagnosis and treatment of any co-existing medical conditions. Primary care physicians are often the source of referrals to psychiatry, although the reason for the referral may not always be suspicion of autism. In my clinical practice, almost all referrals from primary care involve a chief complaint of anger or behavioral problems, or even obsessive-compulsive behaviors.
Psychiatric evaluation should include obtaining a detailed history of the patient’s conception, birth, development, and social life, and his/her family history of genetic conditions. In my practice, ADHD and elimination disorders are common comorbidities in patients with ASD. Consider communicating with daycare staff or teachers and auxiliary staff, such as guidance counselors, because doing so can help elucidate the diagnosis. Also, ask adult family members, preferably a parent, for collateral information to help establish an accurate diagnosis in your adult patients.
Psychological evaluation should include testing to rule out intellectual disability and learning disorders, which are common in patients with ASD.2 Tests commonly used for evaluation of ASD include the Autism Diagnostic Observation Schedule (ADOS), Childhood Autism Rating Scale (CARS), and Autism Diagnostic Interview-Revised (ADI-R).
Speech evaluation. Deficits in language and communication are commonly observed in patients with ASD, especially in younger patients.3 A study of the relationship between early language skills (age of first word production) and later functioning in children with ASD indicated that earlier age of first word acquisition was associated with higher cognitive ability and adaptive skills when measured later in childhood.3 Therefore, timely intervention following speech evaluation can result in favorable outcomes.
Occupational evaluation. Approximately 69% to 93% of children and adults with ASD exhibit sensory symptoms (hyperresponsive, hyporesponsive, and sensory-seeking behaviors).4 Patients with sensory symptoms often experience limitations in multiple areas of their life. Early intervention by an occupational therapist can help improve long-term outcomes.4
Neurologic evaluation is important because ASD is a neurodevelopmental disorder. Patients with ASD often have comorbid seizure disorders.1 The estimated prevalence of epilepsy in these patients ranges from 2.7% to 44.4%.1 A baseline EEG and neuroimaging can help improve your understanding of the relationship between ASD and seizure disorders, and guide treatment.
Genetic testing. Between 10% to 15% of individuals with ASD have a medical condition, such as cytogenetic or single-gene disorder, that causes ASD.5 Fragile X syndrome, tuberous sclerosis, and Prader-Willi syndrome are a few common examples of genetic disorders associated with ASD.5 Autism spectrum disorder has also been known to have a strong genetic basis with high probability of heritability in families.5 Genetic testing can help to detect any underlying genetic disorders in your patients as well as their family members. Chromosomal microarray analysis has become more accessible due to improved insurance coverage, and is convenient to perform by collection of a buccal mucosa sample in the office setting.
1. Strasser L, Downes M, Kung J, et al. Prevalence and risk factors for autism spectrum disorder in epilepsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2018;60(1):19-29.
2. Schwatrz CE, Neri G. Autism and intellectual disability: two sides of the same coin. Am J Med Genet Part C Semin Med Genet. 2012;160C(2):89-89.
3. Mayo J, Chlebowski C, Fein DA, et al. Age of first words predicts cognitive ability and adaptive skills in children with ASD. J Autism Dev Disord. 2013;43(2):253-264.
4. McCormick C, Hepburn S, Young GS, et al. Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: a longitudinal study. Autism. 2016;20(5):572-579.
5. Balasubramanian B, Bhatt CV, Goyel NA. Genetic studies in children with intellectual disability and autistic spectrum of disorders. Indian J Hum Genet. 2009;15(3):103-107.
Autism spectrum disorder (ASD) is characterized by impairments in communication and social interactions, along with repetitive and perseverant behaviors.1 It has a prevalence of 0.75% to 1.1% among the general population.1 The presentation of ASD can vary, and patients may have a wide range of comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), neurologic disorders, and genetic disorders.1 Therefore, a comprehensive evaluation needs to include a multidisciplinary assessment by clinicians from several specialties, including primary care, psychiatry, psychology, and neurology. Here I offer psychiatrists 3 Ps and the mnemonic SONG to describe a multidisciplinary approach to assessing a patient with suspected or confirmed ASD.
Primary care evaluation of patients with ASD is important for the diagnosis and treatment of any co-existing medical conditions. Primary care physicians are often the source of referrals to psychiatry, although the reason for the referral may not always be suspicion of autism. In my clinical practice, almost all referrals from primary care involve a chief complaint of anger or behavioral problems, or even obsessive-compulsive behaviors.
Psychiatric evaluation should include obtaining a detailed history of the patient’s conception, birth, development, and social life, and his/her family history of genetic conditions. In my practice, ADHD and elimination disorders are common comorbidities in patients with ASD. Consider communicating with daycare staff or teachers and auxiliary staff, such as guidance counselors, because doing so can help elucidate the diagnosis. Also, ask adult family members, preferably a parent, for collateral information to help establish an accurate diagnosis in your adult patients.
Psychological evaluation should include testing to rule out intellectual disability and learning disorders, which are common in patients with ASD.2 Tests commonly used for evaluation of ASD include the Autism Diagnostic Observation Schedule (ADOS), Childhood Autism Rating Scale (CARS), and Autism Diagnostic Interview-Revised (ADI-R).
Speech evaluation. Deficits in language and communication are commonly observed in patients with ASD, especially in younger patients.3 A study of the relationship between early language skills (age of first word production) and later functioning in children with ASD indicated that earlier age of first word acquisition was associated with higher cognitive ability and adaptive skills when measured later in childhood.3 Therefore, timely intervention following speech evaluation can result in favorable outcomes.
Occupational evaluation. Approximately 69% to 93% of children and adults with ASD exhibit sensory symptoms (hyperresponsive, hyporesponsive, and sensory-seeking behaviors).4 Patients with sensory symptoms often experience limitations in multiple areas of their life. Early intervention by an occupational therapist can help improve long-term outcomes.4
Neurologic evaluation is important because ASD is a neurodevelopmental disorder. Patients with ASD often have comorbid seizure disorders.1 The estimated prevalence of epilepsy in these patients ranges from 2.7% to 44.4%.1 A baseline EEG and neuroimaging can help improve your understanding of the relationship between ASD and seizure disorders, and guide treatment.
Genetic testing. Between 10% to 15% of individuals with ASD have a medical condition, such as cytogenetic or single-gene disorder, that causes ASD.5 Fragile X syndrome, tuberous sclerosis, and Prader-Willi syndrome are a few common examples of genetic disorders associated with ASD.5 Autism spectrum disorder has also been known to have a strong genetic basis with high probability of heritability in families.5 Genetic testing can help to detect any underlying genetic disorders in your patients as well as their family members. Chromosomal microarray analysis has become more accessible due to improved insurance coverage, and is convenient to perform by collection of a buccal mucosa sample in the office setting.
Autism spectrum disorder (ASD) is characterized by impairments in communication and social interactions, along with repetitive and perseverant behaviors.1 It has a prevalence of 0.75% to 1.1% among the general population.1 The presentation of ASD can vary, and patients may have a wide range of comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), neurologic disorders, and genetic disorders.1 Therefore, a comprehensive evaluation needs to include a multidisciplinary assessment by clinicians from several specialties, including primary care, psychiatry, psychology, and neurology. Here I offer psychiatrists 3 Ps and the mnemonic SONG to describe a multidisciplinary approach to assessing a patient with suspected or confirmed ASD.
Primary care evaluation of patients with ASD is important for the diagnosis and treatment of any co-existing medical conditions. Primary care physicians are often the source of referrals to psychiatry, although the reason for the referral may not always be suspicion of autism. In my clinical practice, almost all referrals from primary care involve a chief complaint of anger or behavioral problems, or even obsessive-compulsive behaviors.
Psychiatric evaluation should include obtaining a detailed history of the patient’s conception, birth, development, and social life, and his/her family history of genetic conditions. In my practice, ADHD and elimination disorders are common comorbidities in patients with ASD. Consider communicating with daycare staff or teachers and auxiliary staff, such as guidance counselors, because doing so can help elucidate the diagnosis. Also, ask adult family members, preferably a parent, for collateral information to help establish an accurate diagnosis in your adult patients.
Psychological evaluation should include testing to rule out intellectual disability and learning disorders, which are common in patients with ASD.2 Tests commonly used for evaluation of ASD include the Autism Diagnostic Observation Schedule (ADOS), Childhood Autism Rating Scale (CARS), and Autism Diagnostic Interview-Revised (ADI-R).
Speech evaluation. Deficits in language and communication are commonly observed in patients with ASD, especially in younger patients.3 A study of the relationship between early language skills (age of first word production) and later functioning in children with ASD indicated that earlier age of first word acquisition was associated with higher cognitive ability and adaptive skills when measured later in childhood.3 Therefore, timely intervention following speech evaluation can result in favorable outcomes.
Occupational evaluation. Approximately 69% to 93% of children and adults with ASD exhibit sensory symptoms (hyperresponsive, hyporesponsive, and sensory-seeking behaviors).4 Patients with sensory symptoms often experience limitations in multiple areas of their life. Early intervention by an occupational therapist can help improve long-term outcomes.4
Neurologic evaluation is important because ASD is a neurodevelopmental disorder. Patients with ASD often have comorbid seizure disorders.1 The estimated prevalence of epilepsy in these patients ranges from 2.7% to 44.4%.1 A baseline EEG and neuroimaging can help improve your understanding of the relationship between ASD and seizure disorders, and guide treatment.
Genetic testing. Between 10% to 15% of individuals with ASD have a medical condition, such as cytogenetic or single-gene disorder, that causes ASD.5 Fragile X syndrome, tuberous sclerosis, and Prader-Willi syndrome are a few common examples of genetic disorders associated with ASD.5 Autism spectrum disorder has also been known to have a strong genetic basis with high probability of heritability in families.5 Genetic testing can help to detect any underlying genetic disorders in your patients as well as their family members. Chromosomal microarray analysis has become more accessible due to improved insurance coverage, and is convenient to perform by collection of a buccal mucosa sample in the office setting.
1. Strasser L, Downes M, Kung J, et al. Prevalence and risk factors for autism spectrum disorder in epilepsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2018;60(1):19-29.
2. Schwatrz CE, Neri G. Autism and intellectual disability: two sides of the same coin. Am J Med Genet Part C Semin Med Genet. 2012;160C(2):89-89.
3. Mayo J, Chlebowski C, Fein DA, et al. Age of first words predicts cognitive ability and adaptive skills in children with ASD. J Autism Dev Disord. 2013;43(2):253-264.
4. McCormick C, Hepburn S, Young GS, et al. Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: a longitudinal study. Autism. 2016;20(5):572-579.
5. Balasubramanian B, Bhatt CV, Goyel NA. Genetic studies in children with intellectual disability and autistic spectrum of disorders. Indian J Hum Genet. 2009;15(3):103-107.
1. Strasser L, Downes M, Kung J, et al. Prevalence and risk factors for autism spectrum disorder in epilepsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2018;60(1):19-29.
2. Schwatrz CE, Neri G. Autism and intellectual disability: two sides of the same coin. Am J Med Genet Part C Semin Med Genet. 2012;160C(2):89-89.
3. Mayo J, Chlebowski C, Fein DA, et al. Age of first words predicts cognitive ability and adaptive skills in children with ASD. J Autism Dev Disord. 2013;43(2):253-264.
4. McCormick C, Hepburn S, Young GS, et al. Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: a longitudinal study. Autism. 2016;20(5):572-579.
5. Balasubramanian B, Bhatt CV, Goyel NA. Genetic studies in children with intellectual disability and autistic spectrum of disorders. Indian J Hum Genet. 2009;15(3):103-107.
How to handle negative online reviews
Online reviews have become a popular method for patients to rate their psychiatrists. Patients’ online reviews can help other patients make more informed decisions about pursuing treatment, offer us valuable feedback on our performance, and help improve standards of care.1 However, during the course of our careers, we may receive negative online reviews. These reviews may range from mild dissatisfaction to abusive comments, and they could have adverse personal and professional consequences.2 For example, online discussions might make current patients question your practices or consider ending their treatment with you.2 Also, potential patients might decide to not inquire about your services.2 Here I offer suggestions for approaching negative online reviews, and point out some potential pitfalls of responding to them.
Remain professional. You might become upset or frazzled after reading online criticisms about your performance, particularly if the information is erroneous or deceptive. As much as you would like to immediately respond, a public tit-for-tat could prolong or fuel a conflict, or make you come across as angry.2
There may be occasions, however, when it would be appropriate to respond. If you choose to respond to a negative online review, you need to have a methodical plan. Avoid reacting in a knee-jerk manner because this is usually unproductive. In addition, ensure that your response is professional and polite, because an intemperate response could undermine the public’s confidence in our profession.2
Maintain patient confidentiality. Although patients are free to post anything they desire, psychiatrists must maintain confidentiality. The Health Insurance Portability and Accountability Act (HIPAA) applies to online reviews, which prevents us from disclosing information about patients without their permission, including even acknowledging that someone is our patient.3 Your patients’ disclosures are not permission to disclose their health information. Potential patients might avoid us or existing patients may end their treatment with us if they believe their personal information could be disclosed online without their consent. To avoid such concerns, reply to online reviews with generic comments about your practice’s general policies without violating confidentiality. Also, to avoid violating HIPAA rules, you may want to contact your malpractice carrier or your facility’s legal department before replying.1
Invite patients to discuss their grievances. If your patients identify themselves in a review, or if you are able to identify them, consider inviting them to discuss their concerns with you (over the phone, face-to-face, or via video conferencing). During such conversations, thank the patient for their review, and do not ask them to delete it.2 Focus on addressing their concerns and resolving any problems they experienced during treatment; doing so can help improve your practice. This approach also might lead a patient to remove their negative review or to write a review that lets other patients know that you are listening to them.
Even if you choose not to invite your patients to air their concerns, do not entirely dismiss negative reviews. Instead, try to step back from your emotions and take an objective look at such reviews so you can determine what steps to take to improve your practices. Improving your communication with patients could decrease the likelihood that they will write negative reviews in the first place.
Take action on fake reviews. If a negative review is fake (not written by one of your patients) or blatantly untrue, contact the web site administrator and provide evidence to support having the review deleted, especially if it violates the site’s terms of service.1 However, this approach may not be fruitful. Web sites can be manipulated, and many do not require users to authenticate that they are actual patients.1 Although most web sites would not want their reputation damaged by users posting fake reviews, more dramatic reviews could help lead to increased traffic, which lowers an administrator’s incentive to remove negative reviews.1
Continue to: Consider legal repercussions
Consider legal repercussions. Stay up-to-date with online reviews about you by conducting internet searches once every 3 months.1 Consider notifying your malpractice carrier or facility’s legal department if a review suggests a patient or family might initiate legal action against you or the facility.1 You might consider pursuing legal action if an online review is defamatory, but such claims often are difficult to prove in court.1 Even if you win, such a case could later be repeatedly mentioned in articles and journals, thus creating a permanent record of the negative review in the literature.1
Enlist help with your online image. If financially feasible, hire a professional service to help improve your online image or assist in responding to negative reviews.1 Build your profile on review web sites to help frame your online image, and include information that mentions the pertinent steps you are taking to address any legitimate concerns your patients raise in their reviews. Encourage your patients to post reviews because that could produce a more equitable sample and paint a more accurate picture of your practice.
Lobby professional medical organizations to take action to protect psychiatrists from negative online reviews by creating legislation that holds web sites accountable for their content.1
Stay positive. Unfounded or not, negative online reviews are an inevitable part of a psychiatrist’s professional life.2 One negative review (or even several) is not going to destroy your reputation or career. Do not feel alone if you receive a negative review. Seek advice from colleagues who have received negative reviews; in addition to offering advice, they can also provide a listening ear.2
1. Kendall L, Botello T. Internet sabotage: negative online reviews of psychiatrists. Psychiatr Ann. 2016;46(12):715-716, 718-719.
2. Rimmer A. A patient has complained about me online. What should I do? BMJ. 2019;366:I5705. doi: 10.1136/bmj.I5705.
3. Health Insurance Portability and Accountability Act of 1996 (HIPAA), S 1028, 104th Cong, Public Law No. 104-191, 110 Stat. 1936 (1996). https://www.govinfo.gov/content/pkg/PLAW-104publ191/pdf/PLAW-104publ191.pdf. Accessed Novermber 16, 2020.
Online reviews have become a popular method for patients to rate their psychiatrists. Patients’ online reviews can help other patients make more informed decisions about pursuing treatment, offer us valuable feedback on our performance, and help improve standards of care.1 However, during the course of our careers, we may receive negative online reviews. These reviews may range from mild dissatisfaction to abusive comments, and they could have adverse personal and professional consequences.2 For example, online discussions might make current patients question your practices or consider ending their treatment with you.2 Also, potential patients might decide to not inquire about your services.2 Here I offer suggestions for approaching negative online reviews, and point out some potential pitfalls of responding to them.
Remain professional. You might become upset or frazzled after reading online criticisms about your performance, particularly if the information is erroneous or deceptive. As much as you would like to immediately respond, a public tit-for-tat could prolong or fuel a conflict, or make you come across as angry.2
There may be occasions, however, when it would be appropriate to respond. If you choose to respond to a negative online review, you need to have a methodical plan. Avoid reacting in a knee-jerk manner because this is usually unproductive. In addition, ensure that your response is professional and polite, because an intemperate response could undermine the public’s confidence in our profession.2
Maintain patient confidentiality. Although patients are free to post anything they desire, psychiatrists must maintain confidentiality. The Health Insurance Portability and Accountability Act (HIPAA) applies to online reviews, which prevents us from disclosing information about patients without their permission, including even acknowledging that someone is our patient.3 Your patients’ disclosures are not permission to disclose their health information. Potential patients might avoid us or existing patients may end their treatment with us if they believe their personal information could be disclosed online without their consent. To avoid such concerns, reply to online reviews with generic comments about your practice’s general policies without violating confidentiality. Also, to avoid violating HIPAA rules, you may want to contact your malpractice carrier or your facility’s legal department before replying.1
Invite patients to discuss their grievances. If your patients identify themselves in a review, or if you are able to identify them, consider inviting them to discuss their concerns with you (over the phone, face-to-face, or via video conferencing). During such conversations, thank the patient for their review, and do not ask them to delete it.2 Focus on addressing their concerns and resolving any problems they experienced during treatment; doing so can help improve your practice. This approach also might lead a patient to remove their negative review or to write a review that lets other patients know that you are listening to them.
Even if you choose not to invite your patients to air their concerns, do not entirely dismiss negative reviews. Instead, try to step back from your emotions and take an objective look at such reviews so you can determine what steps to take to improve your practices. Improving your communication with patients could decrease the likelihood that they will write negative reviews in the first place.
Take action on fake reviews. If a negative review is fake (not written by one of your patients) or blatantly untrue, contact the web site administrator and provide evidence to support having the review deleted, especially if it violates the site’s terms of service.1 However, this approach may not be fruitful. Web sites can be manipulated, and many do not require users to authenticate that they are actual patients.1 Although most web sites would not want their reputation damaged by users posting fake reviews, more dramatic reviews could help lead to increased traffic, which lowers an administrator’s incentive to remove negative reviews.1
Continue to: Consider legal repercussions
Consider legal repercussions. Stay up-to-date with online reviews about you by conducting internet searches once every 3 months.1 Consider notifying your malpractice carrier or facility’s legal department if a review suggests a patient or family might initiate legal action against you or the facility.1 You might consider pursuing legal action if an online review is defamatory, but such claims often are difficult to prove in court.1 Even if you win, such a case could later be repeatedly mentioned in articles and journals, thus creating a permanent record of the negative review in the literature.1
Enlist help with your online image. If financially feasible, hire a professional service to help improve your online image or assist in responding to negative reviews.1 Build your profile on review web sites to help frame your online image, and include information that mentions the pertinent steps you are taking to address any legitimate concerns your patients raise in their reviews. Encourage your patients to post reviews because that could produce a more equitable sample and paint a more accurate picture of your practice.
Lobby professional medical organizations to take action to protect psychiatrists from negative online reviews by creating legislation that holds web sites accountable for their content.1
Stay positive. Unfounded or not, negative online reviews are an inevitable part of a psychiatrist’s professional life.2 One negative review (or even several) is not going to destroy your reputation or career. Do not feel alone if you receive a negative review. Seek advice from colleagues who have received negative reviews; in addition to offering advice, they can also provide a listening ear.2
Online reviews have become a popular method for patients to rate their psychiatrists. Patients’ online reviews can help other patients make more informed decisions about pursuing treatment, offer us valuable feedback on our performance, and help improve standards of care.1 However, during the course of our careers, we may receive negative online reviews. These reviews may range from mild dissatisfaction to abusive comments, and they could have adverse personal and professional consequences.2 For example, online discussions might make current patients question your practices or consider ending their treatment with you.2 Also, potential patients might decide to not inquire about your services.2 Here I offer suggestions for approaching negative online reviews, and point out some potential pitfalls of responding to them.
Remain professional. You might become upset or frazzled after reading online criticisms about your performance, particularly if the information is erroneous or deceptive. As much as you would like to immediately respond, a public tit-for-tat could prolong or fuel a conflict, or make you come across as angry.2
There may be occasions, however, when it would be appropriate to respond. If you choose to respond to a negative online review, you need to have a methodical plan. Avoid reacting in a knee-jerk manner because this is usually unproductive. In addition, ensure that your response is professional and polite, because an intemperate response could undermine the public’s confidence in our profession.2
Maintain patient confidentiality. Although patients are free to post anything they desire, psychiatrists must maintain confidentiality. The Health Insurance Portability and Accountability Act (HIPAA) applies to online reviews, which prevents us from disclosing information about patients without their permission, including even acknowledging that someone is our patient.3 Your patients’ disclosures are not permission to disclose their health information. Potential patients might avoid us or existing patients may end their treatment with us if they believe their personal information could be disclosed online without their consent. To avoid such concerns, reply to online reviews with generic comments about your practice’s general policies without violating confidentiality. Also, to avoid violating HIPAA rules, you may want to contact your malpractice carrier or your facility’s legal department before replying.1
Invite patients to discuss their grievances. If your patients identify themselves in a review, or if you are able to identify them, consider inviting them to discuss their concerns with you (over the phone, face-to-face, or via video conferencing). During such conversations, thank the patient for their review, and do not ask them to delete it.2 Focus on addressing their concerns and resolving any problems they experienced during treatment; doing so can help improve your practice. This approach also might lead a patient to remove their negative review or to write a review that lets other patients know that you are listening to them.
Even if you choose not to invite your patients to air their concerns, do not entirely dismiss negative reviews. Instead, try to step back from your emotions and take an objective look at such reviews so you can determine what steps to take to improve your practices. Improving your communication with patients could decrease the likelihood that they will write negative reviews in the first place.
Take action on fake reviews. If a negative review is fake (not written by one of your patients) or blatantly untrue, contact the web site administrator and provide evidence to support having the review deleted, especially if it violates the site’s terms of service.1 However, this approach may not be fruitful. Web sites can be manipulated, and many do not require users to authenticate that they are actual patients.1 Although most web sites would not want their reputation damaged by users posting fake reviews, more dramatic reviews could help lead to increased traffic, which lowers an administrator’s incentive to remove negative reviews.1
Continue to: Consider legal repercussions
Consider legal repercussions. Stay up-to-date with online reviews about you by conducting internet searches once every 3 months.1 Consider notifying your malpractice carrier or facility’s legal department if a review suggests a patient or family might initiate legal action against you or the facility.1 You might consider pursuing legal action if an online review is defamatory, but such claims often are difficult to prove in court.1 Even if you win, such a case could later be repeatedly mentioned in articles and journals, thus creating a permanent record of the negative review in the literature.1
Enlist help with your online image. If financially feasible, hire a professional service to help improve your online image or assist in responding to negative reviews.1 Build your profile on review web sites to help frame your online image, and include information that mentions the pertinent steps you are taking to address any legitimate concerns your patients raise in their reviews. Encourage your patients to post reviews because that could produce a more equitable sample and paint a more accurate picture of your practice.
Lobby professional medical organizations to take action to protect psychiatrists from negative online reviews by creating legislation that holds web sites accountable for their content.1
Stay positive. Unfounded or not, negative online reviews are an inevitable part of a psychiatrist’s professional life.2 One negative review (or even several) is not going to destroy your reputation or career. Do not feel alone if you receive a negative review. Seek advice from colleagues who have received negative reviews; in addition to offering advice, they can also provide a listening ear.2
1. Kendall L, Botello T. Internet sabotage: negative online reviews of psychiatrists. Psychiatr Ann. 2016;46(12):715-716, 718-719.
2. Rimmer A. A patient has complained about me online. What should I do? BMJ. 2019;366:I5705. doi: 10.1136/bmj.I5705.
3. Health Insurance Portability and Accountability Act of 1996 (HIPAA), S 1028, 104th Cong, Public Law No. 104-191, 110 Stat. 1936 (1996). https://www.govinfo.gov/content/pkg/PLAW-104publ191/pdf/PLAW-104publ191.pdf. Accessed Novermber 16, 2020.
1. Kendall L, Botello T. Internet sabotage: negative online reviews of psychiatrists. Psychiatr Ann. 2016;46(12):715-716, 718-719.
2. Rimmer A. A patient has complained about me online. What should I do? BMJ. 2019;366:I5705. doi: 10.1136/bmj.I5705.
3. Health Insurance Portability and Accountability Act of 1996 (HIPAA), S 1028, 104th Cong, Public Law No. 104-191, 110 Stat. 1936 (1996). https://www.govinfo.gov/content/pkg/PLAW-104publ191/pdf/PLAW-104publ191.pdf. Accessed Novermber 16, 2020.
Caring for outpatients during COVID-19: 4 themes
As a result of the coronavirus disease 2019 (COVID-19) pandemic, the content of outpatient psychotherapy and psychopharmacology sessions has seen significant change, with many patients focusing on how the pandemic has altered their daily lives and emotional well-being. Most patients were suddenly limited in both the amount of time they spent, and in their interactions with people, outside of their homes. Additionally, employment-related stressors such as working from home and the potential loss of a job and/or income added to pandemic stress.1 Patients simultaneously processed their experiences of the COVID-19 pandemic while often striving to adapt to new virtual modes of mental health care delivery via phone or video conferencing.
The clinic staff at our large, multidisciplinary, urban outpatient mental health practice conducts weekly case consultation meetings. In meetings held during the early stages of the COVID-19 pandemic, we noted 4 dominant clinical themes emerging across our patients’ experiences:
- isolation
- uncertainty
- household stress
- grief.
These themes occurred across many diagnostic categories, suggesting they reflect a dramatic shift brought on by the pandemic. Our group compared clinical experiences from the beginning of the pandemic through the end of May 2020. For this article, we considered several patients who expressed these 4 themes and created a “composite patient.” In the following sections, we describe the typical presentation of, and recommended interventions for, a composite patient for each of these 4 themes.
Isolation
Mr. J, a 60-year-old, single, African American man diagnosed with bipolar disorder with psychotic features, lives alone in an apartment in a densely populated area. Before COVID-19, he had been attending a day treatment program. His daily walks for coffee and cigarettes provided the scaffolding to his emotional stability and gave him a sense of belonging to a world outside of his home. Mr. J also had been able to engage in informal social activities in the common areas of his apartment complex.
The start of the COVID-19 pandemic ends his interpersonal interactions, from the passive and superficial conversations he had with strangers in coffee shops to the more intimate engagement with his peers in his treatment program. The common areas of Mr. J’s apartment building are closed, and his routine cigarette breaks with neighbors have become solitary events, with the added stress of having to schedule his use of the building’s designated smoking area. Before COVID-19, Mr. J had been regularly meeting his brother for coffee to talk about the recent death of their father, but these meetings end due to infection concerns by Mr. J and his brother, who cares for their ailing mother who is at high risk for COVID-19 infection.
Mr. J begins to report self-referential ideation when walking in public, citing his inability to see peoples’ facial expressions because they are wearing masks. As a result of the pandemic restrictions, he becomes depressed and develops increased paranoid ideation. Fortunately, Mr. J begins to participate in a virtual partial hospitalization program to address his paranoid ideation through intensive and clinically-based social interactions. He is unfamiliar with the technology used for virtual visits, but is given the necessary technical support. He is also able to begin virtual visits with his brother and mother. Mr. J soon reports his symptoms are reduced and his mood is more stable.
Engaging in interpersonal interactions can have a positive impact on mental health. Social isolation has demonstrated negative effects that are amplified in individuals with psychiatric disorders.2 Interpersonal interactions can provide a shared experience, promote positive feelings of social connection, and aid in the development of social skills.3,4 Among our patients, we have begun to see the effects of isolation manifest as loneliness and demoralization.
Continue to: Interventions
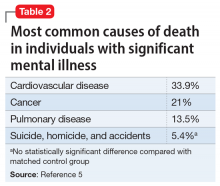
Interventions. Due to restrictions imposed to limit the spread of COVID-19, evidence-based interventions such as meeting a friend for a meal or participating in in-person support groups typically are not options, thus forcing clinicians to accommodate, adapt, and use technology to develop parallel interventions to provide the same therapeutic effects.5,6 These solutions need to be individualized to accommodate each patient’s unique social and clinical situation (Table 1). Engaging through technology can be problematic for patients with psychosis and paranoid ideation, or those with depressive symptoms. Psychopharmacology or therapy visit time has to be dedicated to helping patients become comfortable and confident when using technology to access their clinicians. Patients can use this same technology to establish virtual social connections. Providing patients with accurate, factual information about infection control during clinical visits ultimately supports their mental health. Delivering clinical care during COVID-19 has required creativity and flexibility to optimize available resources and capitalize on patients’ social supports. These strategies help decrease isolation, loneliness, and exacerbation of psychiatric symptoms.
Uncertainty
Ms. L, age 42, has a history of posttraumatic stress disorder and obstructive sleep apnea, for which she uses a continuous airway positive pressure (CPAP) device. She had been working as a part-time nanny when her employer furloughed her early in the COVID-19 pandemic. Her anxiety has gotten worse throughout the quarantine; she fears her unemployment benefits will run out and she will lose her job. Her anxiety manifests as somatic “pit-of-stomach” sensations. Her sleep has been disrupted; she reports more frequent nightmares, and her partner says that Ms. L has had apneic episodes and bruxism. The parameters of Ms. L’s CPAP device need to be adjusted, but a previously scheduled overnight polysomnography test is deemed a nonessential procedure and canceled. Ms. L has been reluctant to go to a food pantry because she is afraid of being exposed to COVID-19. In virtual sessions, Ms. L says she is uncertain if she will be able to pay her rent, buy food, or access medical care, and expresses overriding helplessness.
During COVID-19, anxiety and insomnia are driven by the sudden manifestation of uncertainty regarding being able to work, pay rent or mortgage, buy food and other provisions, or visit family and friends, including those who are hospitalized or live in nursing homes. Additional uncertainties include how long the quarantine will last, who will become ill, and when, or if, life will return to normal. Taken together, these uncertainties impart a pervasive dread to daily experience.
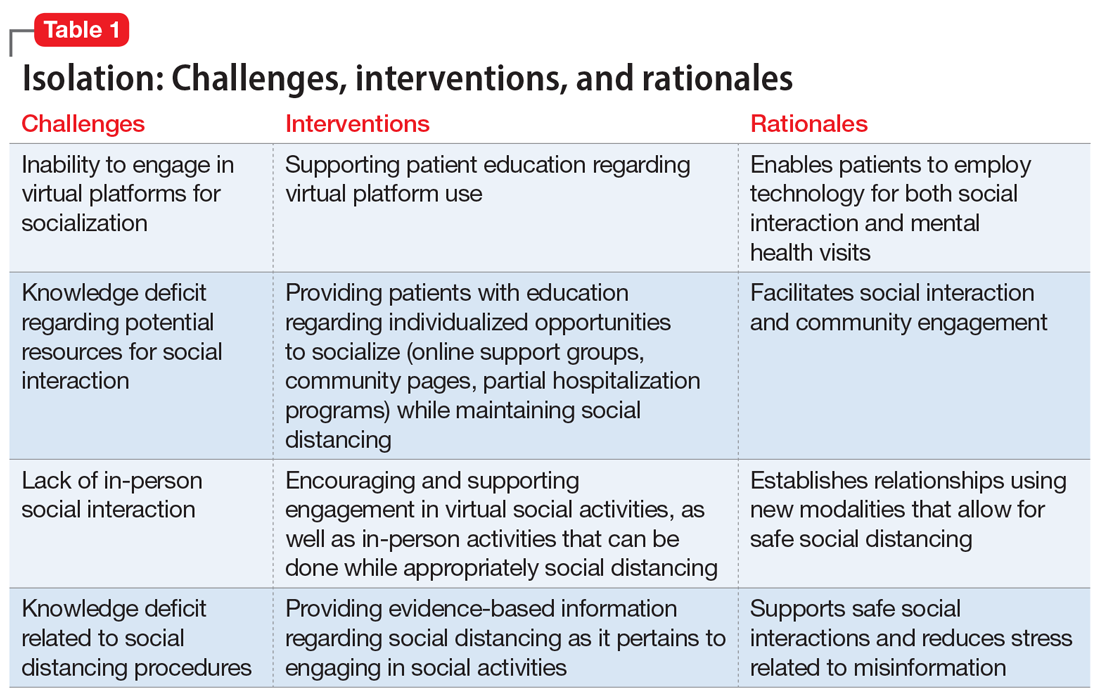
Interventions. Clinicians can facilitate access to services (eg, social services, benefits specialists) and help patients parse out what they should and can address practically, and which challenges are outside of their personal or communal control (Table 2). Patients can be encouraged to identify paralytic rumination and shift their mental focus to engage in constructive projects. They can be advised to limit their intake of media that increases their anxiety and replace it with phone calls or e-mails to family and friends. Scheduled practice of mindfulness meditation and diaphragmatic breathing can help reduce anxiety.7,8 Pharmacotherapeutic interventions should be low-risk to minimize burdening emergency departments saturated with patients who have COVID-19 and serve to reduce symptoms that interfere with behavioral activation. While the research on benzodiazepines and non-benzodiazepine receptor agonists (“Z-drugs” such as zolpidem and eszopiclone) in the setting of obstructive sleep apnea is complex, and there is some evidence that the latter may not exacerbate apnea,9 benzodiazepines and Z-drugs are associated with an array of risks, including tolerance, withdrawal, and traumatic falls, particularly in older adults.10 Sleep hygiene and cognitive-behavioral therapy are first-line therapies for insomnia.11
Household stress
Ms. M, a 45-year-old single mother with a history of generalized anxiety disorder, is suddenly thrust into homeschooling her 2 children, ages 10 and 8, while trying to remain productive at her job as a software engineer. She no longer has time for herself, and spends much of her day helping her children with schoolwork or planning activities to keep them engaged rather than arguing with each other. She feels intense pressure, heightened stress, and increased anxiety as she tries to navigate this new daily routine.
Continue to: New household dynamics...
New household dynamics abound when people are suddenly forced into atypical routines. In the context of COVID-19, working parents may be forced to balance the demands of their jobs with homeschooling their children. Couples may find themselves arguing more frequently. Adult children may find themselves needing to care for their ill parents. Limited space, a lack of leisure activities, and uncertainty about the future coalesce to increase conflict and stress. Research suggests that how people cope with a stressor is a more reliable determinant of health and well-being than the stressor itself.12
Interventions. Mental health clinicians can offer several recommendations to help patients cope with increased household stress (Table 3). We can encourage patients to have clear communication with their loved ones regarding new expectations, roles, and their feelings. Demarcating specific areas within living spaces to each person in the household can help each member feel a sense of autonomy, regardless of how small their area may be. Clinicians can help patients learn to take the time as a family to work on establishing new household routines. Telepsychiatry offers clinicians a unique window into patients’ lives and family dynamics, and we can use this perspective to deepen our understanding of the patient’s context and household relationships and help them navigate the situation thrust upon them.

Grief
Following a psychiatric hospitalization for an acute exacerbation of psychosis, Ms. S, age 79, is transferred to a rehabilitation facility, where she contracts COVID-19. Because Ms. S did not have a history of chronic medical illness, her family anticipates a full recovery. Early in the course of Ms. S’s admission, the rehabilitation facility restricts visitations, and her family is unable to see her. Ms. S dies in this facility without her family’s presence and without her family having the opportunity to say goodbye. Ms. S’s psychiatrist offers her family a virtual session to provide support. During the virtual session, the psychiatrist notes signs of complicated bereavement among Ms. S’s family members, including nonacceptance of the death, rumination about the circumstances of the death, and describing life as having no purpose.
The COVID-19 pandemic has complicated the natural process of loss and grief across multiple dimensions. Studies have shown that an inability to say goodbye before death, a lack of social support,13 and a lack of preparation for loss14 are associated with complicated bereavement and depression. Many people are experiencing the loss of loved ones without having a chance to appropriately mourn. Forbidding visits to family members who are hospitalized also prevents the practice of religious and spiritual rituals that typically occur at the end of life. This is worsened by truncated or absent funeral services. Support for those who are grieving may be offered from a distance, if at all. When surviving family members have been with the deceased prior to hospitalization, they may be required to self-quarantine, potentially exacerbating their grief and other symptoms associated with loss.
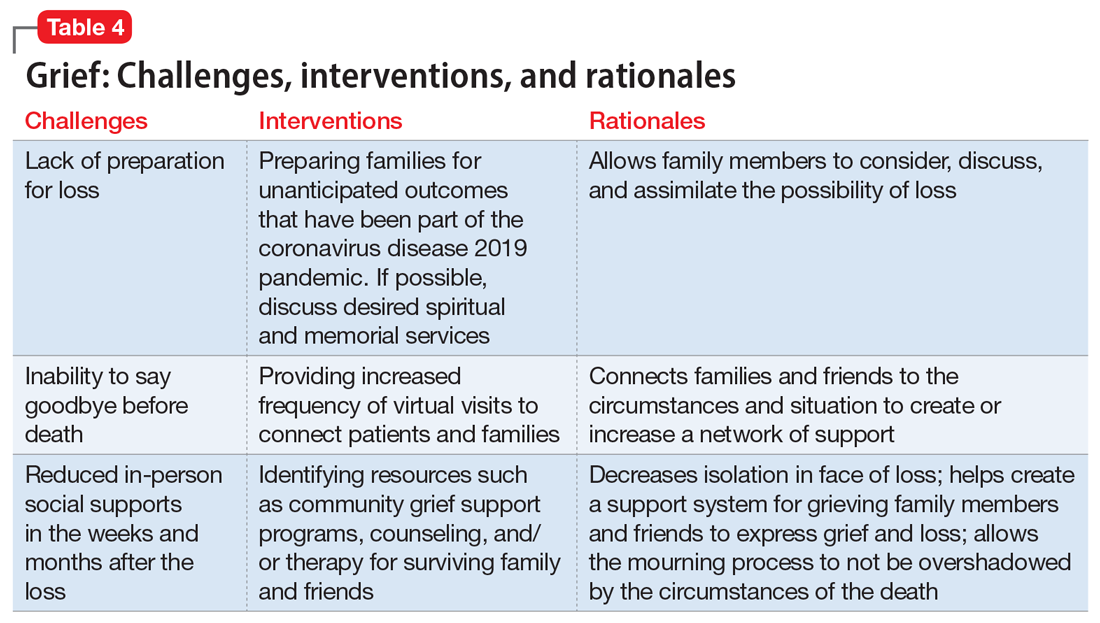
Interventions. Because social support is a protective factor against complicated grief,14 there are several recommendations for survivors as they work through the process of grief (Table 4). These include preparing families for a potential death; discussing desired spiritual and memorial services15; connecting families to resources such as community grief support programs, counseling/therapy, funeral services, video conferencing, and other communication tools; and planning for additional support for surviving family and friends, both immediately after the death and in the long term. It is also important to provide appropriate counseling and support for surviving family members to focus on their own well-being by exercising, eating nutritious meals, getting enough sleep, and abstaining from alcohol and drugs of abuse.16
Continue to: An ongoing challenge
An ongoing challenge
Our clinical team recommends further investigation to define additional psychotherapeutic themes arising from the COVID-19 pandemic and provide evidence-based interventions to address these categories, which we expect will increase in clinical salience in the months and years ahead. Close monitoring, follow-up by clinical and research staff, and evidence-based interventions will help address these dominant themes, with the goal of alleviating patient suffering.
Bottom Line
Our team identified 4 dominant clinical themes emerging across our patients’ experiences during the coronavirus disease 2019 pandemic: isolation, uncertainty, household stress, and grief. Clinicians can implement specific interventions to reduce the impact of these themes, which we expect to remain clinically relevant in the upcoming months and years.
Related Resources
- Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-32,39.
- Carr D, Boerner K, Moorman S. Bereavement in the time of coronavirus: unprecedented challenges demand novel interventions. J Aging Soc Policy. 2020;32(4-5):425-431.
Drug Brand Names
Eszopiclone • Lunesta
Zolpidem • Ambien
1. Bloom N. How working from home works out. Stanford Institute for Economic Policy Research Policy Brief. https://siepr.stanford.edu/research/publications/how-working-home-works-out. Published June 2020. Accessed October 28, 2020.
2. Linz SJ, Sturm BA. The phenomenon of social isolation in the severely mentally ill. Perspect Psychiatr Care. 2013;49(4):243-254.
3. Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405-429.
4. Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54‐S66.
5. Mann F, Bone JK, Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52(6):627-638.
6. Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthc Inform Res. 2012;18(3):191‐198.
7. Chen YF, Huang ZY, Chien CH, et al. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53(4):329-336.
8. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786‐792.
9. Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - an open-label pilot study. Sleep Res. 2019;28(6):e12853. doi: 10.1111/jsr.12853.
10. Markota M, Rummans TA, Bostwick JM, et al. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632-1639.
11. Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35.
12. Dijkstra MT, Homan AC. Engaging in rather than disengaging from stress: effective coping and perceived control. Front Psychol. 2016;7:1415.
13. Romero MM, Ott CH, Kelber ST. Predictors of grief in bereaved family caregivers of person’s with Alzheimer’s disease: a prospective study. Death Stud. 2014;38(6-10):395-403.
14. Lobb EA, Kristjanson LJ, Aoun SM, et al. Predictors of complicated grief: a systematic review of empirical studies. Death Stud. 2010;34(8):673-698.
15. Wallace CL, Wladkowski SP, Gibson A, et al. Grief during the COVID-19 pandemic: considerations for palliative care providers. J Pain Symptom Manage. 2020;60(1):e70-e76. doi: 10.1016/j.jpainsymman.2020.04.012
16. Selman LE, Chao D, Sowden R, et al. Bereavement support on the frontline of COVID-19: recommendations for hospital clinicians. J Pain Symptom Manage. 2020;60(2):e81-e86. doi: 10.1016/j.jpainsymman.2020.04.024
As a result of the coronavirus disease 2019 (COVID-19) pandemic, the content of outpatient psychotherapy and psychopharmacology sessions has seen significant change, with many patients focusing on how the pandemic has altered their daily lives and emotional well-being. Most patients were suddenly limited in both the amount of time they spent, and in their interactions with people, outside of their homes. Additionally, employment-related stressors such as working from home and the potential loss of a job and/or income added to pandemic stress.1 Patients simultaneously processed their experiences of the COVID-19 pandemic while often striving to adapt to new virtual modes of mental health care delivery via phone or video conferencing.
The clinic staff at our large, multidisciplinary, urban outpatient mental health practice conducts weekly case consultation meetings. In meetings held during the early stages of the COVID-19 pandemic, we noted 4 dominant clinical themes emerging across our patients’ experiences:
- isolation
- uncertainty
- household stress
- grief.
These themes occurred across many diagnostic categories, suggesting they reflect a dramatic shift brought on by the pandemic. Our group compared clinical experiences from the beginning of the pandemic through the end of May 2020. For this article, we considered several patients who expressed these 4 themes and created a “composite patient.” In the following sections, we describe the typical presentation of, and recommended interventions for, a composite patient for each of these 4 themes.
Isolation
Mr. J, a 60-year-old, single, African American man diagnosed with bipolar disorder with psychotic features, lives alone in an apartment in a densely populated area. Before COVID-19, he had been attending a day treatment program. His daily walks for coffee and cigarettes provided the scaffolding to his emotional stability and gave him a sense of belonging to a world outside of his home. Mr. J also had been able to engage in informal social activities in the common areas of his apartment complex.
The start of the COVID-19 pandemic ends his interpersonal interactions, from the passive and superficial conversations he had with strangers in coffee shops to the more intimate engagement with his peers in his treatment program. The common areas of Mr. J’s apartment building are closed, and his routine cigarette breaks with neighbors have become solitary events, with the added stress of having to schedule his use of the building’s designated smoking area. Before COVID-19, Mr. J had been regularly meeting his brother for coffee to talk about the recent death of their father, but these meetings end due to infection concerns by Mr. J and his brother, who cares for their ailing mother who is at high risk for COVID-19 infection.
Mr. J begins to report self-referential ideation when walking in public, citing his inability to see peoples’ facial expressions because they are wearing masks. As a result of the pandemic restrictions, he becomes depressed and develops increased paranoid ideation. Fortunately, Mr. J begins to participate in a virtual partial hospitalization program to address his paranoid ideation through intensive and clinically-based social interactions. He is unfamiliar with the technology used for virtual visits, but is given the necessary technical support. He is also able to begin virtual visits with his brother and mother. Mr. J soon reports his symptoms are reduced and his mood is more stable.
Engaging in interpersonal interactions can have a positive impact on mental health. Social isolation has demonstrated negative effects that are amplified in individuals with psychiatric disorders.2 Interpersonal interactions can provide a shared experience, promote positive feelings of social connection, and aid in the development of social skills.3,4 Among our patients, we have begun to see the effects of isolation manifest as loneliness and demoralization.
Continue to: Interventions
Interventions. Due to restrictions imposed to limit the spread of COVID-19, evidence-based interventions such as meeting a friend for a meal or participating in in-person support groups typically are not options, thus forcing clinicians to accommodate, adapt, and use technology to develop parallel interventions to provide the same therapeutic effects.5,6 These solutions need to be individualized to accommodate each patient’s unique social and clinical situation (Table 1). Engaging through technology can be problematic for patients with psychosis and paranoid ideation, or those with depressive symptoms. Psychopharmacology or therapy visit time has to be dedicated to helping patients become comfortable and confident when using technology to access their clinicians. Patients can use this same technology to establish virtual social connections. Providing patients with accurate, factual information about infection control during clinical visits ultimately supports their mental health. Delivering clinical care during COVID-19 has required creativity and flexibility to optimize available resources and capitalize on patients’ social supports. These strategies help decrease isolation, loneliness, and exacerbation of psychiatric symptoms.

Uncertainty
Ms. L, age 42, has a history of posttraumatic stress disorder and obstructive sleep apnea, for which she uses a continuous airway positive pressure (CPAP) device. She had been working as a part-time nanny when her employer furloughed her early in the COVID-19 pandemic. Her anxiety has gotten worse throughout the quarantine; she fears her unemployment benefits will run out and she will lose her job. Her anxiety manifests as somatic “pit-of-stomach” sensations. Her sleep has been disrupted; she reports more frequent nightmares, and her partner says that Ms. L has had apneic episodes and bruxism. The parameters of Ms. L’s CPAP device need to be adjusted, but a previously scheduled overnight polysomnography test is deemed a nonessential procedure and canceled. Ms. L has been reluctant to go to a food pantry because she is afraid of being exposed to COVID-19. In virtual sessions, Ms. L says she is uncertain if she will be able to pay her rent, buy food, or access medical care, and expresses overriding helplessness.
During COVID-19, anxiety and insomnia are driven by the sudden manifestation of uncertainty regarding being able to work, pay rent or mortgage, buy food and other provisions, or visit family and friends, including those who are hospitalized or live in nursing homes. Additional uncertainties include how long the quarantine will last, who will become ill, and when, or if, life will return to normal. Taken together, these uncertainties impart a pervasive dread to daily experience.
Interventions. Clinicians can facilitate access to services (eg, social services, benefits specialists) and help patients parse out what they should and can address practically, and which challenges are outside of their personal or communal control (Table 2). Patients can be encouraged to identify paralytic rumination and shift their mental focus to engage in constructive projects. They can be advised to limit their intake of media that increases their anxiety and replace it with phone calls or e-mails to family and friends. Scheduled practice of mindfulness meditation and diaphragmatic breathing can help reduce anxiety.7,8 Pharmacotherapeutic interventions should be low-risk to minimize burdening emergency departments saturated with patients who have COVID-19 and serve to reduce symptoms that interfere with behavioral activation. While the research on benzodiazepines and non-benzodiazepine receptor agonists (“Z-drugs” such as zolpidem and eszopiclone) in the setting of obstructive sleep apnea is complex, and there is some evidence that the latter may not exacerbate apnea,9 benzodiazepines and Z-drugs are associated with an array of risks, including tolerance, withdrawal, and traumatic falls, particularly in older adults.10 Sleep hygiene and cognitive-behavioral therapy are first-line therapies for insomnia.11

Household stress
Ms. M, a 45-year-old single mother with a history of generalized anxiety disorder, is suddenly thrust into homeschooling her 2 children, ages 10 and 8, while trying to remain productive at her job as a software engineer. She no longer has time for herself, and spends much of her day helping her children with schoolwork or planning activities to keep them engaged rather than arguing with each other. She feels intense pressure, heightened stress, and increased anxiety as she tries to navigate this new daily routine.
Continue to: New household dynamics...
New household dynamics abound when people are suddenly forced into atypical routines. In the context of COVID-19, working parents may be forced to balance the demands of their jobs with homeschooling their children. Couples may find themselves arguing more frequently. Adult children may find themselves needing to care for their ill parents. Limited space, a lack of leisure activities, and uncertainty about the future coalesce to increase conflict and stress. Research suggests that how people cope with a stressor is a more reliable determinant of health and well-being than the stressor itself.12
Interventions. Mental health clinicians can offer several recommendations to help patients cope with increased household stress (Table 3). We can encourage patients to have clear communication with their loved ones regarding new expectations, roles, and their feelings. Demarcating specific areas within living spaces to each person in the household can help each member feel a sense of autonomy, regardless of how small their area may be. Clinicians can help patients learn to take the time as a family to work on establishing new household routines. Telepsychiatry offers clinicians a unique window into patients’ lives and family dynamics, and we can use this perspective to deepen our understanding of the patient’s context and household relationships and help them navigate the situation thrust upon them.

Grief
Following a psychiatric hospitalization for an acute exacerbation of psychosis, Ms. S, age 79, is transferred to a rehabilitation facility, where she contracts COVID-19. Because Ms. S did not have a history of chronic medical illness, her family anticipates a full recovery. Early in the course of Ms. S’s admission, the rehabilitation facility restricts visitations, and her family is unable to see her. Ms. S dies in this facility without her family’s presence and without her family having the opportunity to say goodbye. Ms. S’s psychiatrist offers her family a virtual session to provide support. During the virtual session, the psychiatrist notes signs of complicated bereavement among Ms. S’s family members, including nonacceptance of the death, rumination about the circumstances of the death, and describing life as having no purpose.
The COVID-19 pandemic has complicated the natural process of loss and grief across multiple dimensions. Studies have shown that an inability to say goodbye before death, a lack of social support,13 and a lack of preparation for loss14 are associated with complicated bereavement and depression. Many people are experiencing the loss of loved ones without having a chance to appropriately mourn. Forbidding visits to family members who are hospitalized also prevents the practice of religious and spiritual rituals that typically occur at the end of life. This is worsened by truncated or absent funeral services. Support for those who are grieving may be offered from a distance, if at all. When surviving family members have been with the deceased prior to hospitalization, they may be required to self-quarantine, potentially exacerbating their grief and other symptoms associated with loss.
Interventions. Because social support is a protective factor against complicated grief,14 there are several recommendations for survivors as they work through the process of grief (Table 4). These include preparing families for a potential death; discussing desired spiritual and memorial services15; connecting families to resources such as community grief support programs, counseling/therapy, funeral services, video conferencing, and other communication tools; and planning for additional support for surviving family and friends, both immediately after the death and in the long term. It is also important to provide appropriate counseling and support for surviving family members to focus on their own well-being by exercising, eating nutritious meals, getting enough sleep, and abstaining from alcohol and drugs of abuse.16

Continue to: An ongoing challenge
An ongoing challenge
Our clinical team recommends further investigation to define additional psychotherapeutic themes arising from the COVID-19 pandemic and provide evidence-based interventions to address these categories, which we expect will increase in clinical salience in the months and years ahead. Close monitoring, follow-up by clinical and research staff, and evidence-based interventions will help address these dominant themes, with the goal of alleviating patient suffering.
Bottom Line
Our team identified 4 dominant clinical themes emerging across our patients’ experiences during the coronavirus disease 2019 pandemic: isolation, uncertainty, household stress, and grief. Clinicians can implement specific interventions to reduce the impact of these themes, which we expect to remain clinically relevant in the upcoming months and years.
Related Resources
- Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-32,39.
- Carr D, Boerner K, Moorman S. Bereavement in the time of coronavirus: unprecedented challenges demand novel interventions. J Aging Soc Policy. 2020;32(4-5):425-431.
Drug Brand Names
Eszopiclone • Lunesta
Zolpidem • Ambien
As a result of the coronavirus disease 2019 (COVID-19) pandemic, the content of outpatient psychotherapy and psychopharmacology sessions has seen significant change, with many patients focusing on how the pandemic has altered their daily lives and emotional well-being. Most patients were suddenly limited in both the amount of time they spent, and in their interactions with people, outside of their homes. Additionally, employment-related stressors such as working from home and the potential loss of a job and/or income added to pandemic stress.1 Patients simultaneously processed their experiences of the COVID-19 pandemic while often striving to adapt to new virtual modes of mental health care delivery via phone or video conferencing.
The clinic staff at our large, multidisciplinary, urban outpatient mental health practice conducts weekly case consultation meetings. In meetings held during the early stages of the COVID-19 pandemic, we noted 4 dominant clinical themes emerging across our patients’ experiences:
- isolation
- uncertainty
- household stress
- grief.
These themes occurred across many diagnostic categories, suggesting they reflect a dramatic shift brought on by the pandemic. Our group compared clinical experiences from the beginning of the pandemic through the end of May 2020. For this article, we considered several patients who expressed these 4 themes and created a “composite patient.” In the following sections, we describe the typical presentation of, and recommended interventions for, a composite patient for each of these 4 themes.
Isolation
Mr. J, a 60-year-old, single, African American man diagnosed with bipolar disorder with psychotic features, lives alone in an apartment in a densely populated area. Before COVID-19, he had been attending a day treatment program. His daily walks for coffee and cigarettes provided the scaffolding to his emotional stability and gave him a sense of belonging to a world outside of his home. Mr. J also had been able to engage in informal social activities in the common areas of his apartment complex.
The start of the COVID-19 pandemic ends his interpersonal interactions, from the passive and superficial conversations he had with strangers in coffee shops to the more intimate engagement with his peers in his treatment program. The common areas of Mr. J’s apartment building are closed, and his routine cigarette breaks with neighbors have become solitary events, with the added stress of having to schedule his use of the building’s designated smoking area. Before COVID-19, Mr. J had been regularly meeting his brother for coffee to talk about the recent death of their father, but these meetings end due to infection concerns by Mr. J and his brother, who cares for their ailing mother who is at high risk for COVID-19 infection.
Mr. J begins to report self-referential ideation when walking in public, citing his inability to see peoples’ facial expressions because they are wearing masks. As a result of the pandemic restrictions, he becomes depressed and develops increased paranoid ideation. Fortunately, Mr. J begins to participate in a virtual partial hospitalization program to address his paranoid ideation through intensive and clinically-based social interactions. He is unfamiliar with the technology used for virtual visits, but is given the necessary technical support. He is also able to begin virtual visits with his brother and mother. Mr. J soon reports his symptoms are reduced and his mood is more stable.
Engaging in interpersonal interactions can have a positive impact on mental health. Social isolation has demonstrated negative effects that are amplified in individuals with psychiatric disorders.2 Interpersonal interactions can provide a shared experience, promote positive feelings of social connection, and aid in the development of social skills.3,4 Among our patients, we have begun to see the effects of isolation manifest as loneliness and demoralization.
Continue to: Interventions
Interventions. Due to restrictions imposed to limit the spread of COVID-19, evidence-based interventions such as meeting a friend for a meal or participating in in-person support groups typically are not options, thus forcing clinicians to accommodate, adapt, and use technology to develop parallel interventions to provide the same therapeutic effects.5,6 These solutions need to be individualized to accommodate each patient’s unique social and clinical situation (Table 1). Engaging through technology can be problematic for patients with psychosis and paranoid ideation, or those with depressive symptoms. Psychopharmacology or therapy visit time has to be dedicated to helping patients become comfortable and confident when using technology to access their clinicians. Patients can use this same technology to establish virtual social connections. Providing patients with accurate, factual information about infection control during clinical visits ultimately supports their mental health. Delivering clinical care during COVID-19 has required creativity and flexibility to optimize available resources and capitalize on patients’ social supports. These strategies help decrease isolation, loneliness, and exacerbation of psychiatric symptoms.

Uncertainty
Ms. L, age 42, has a history of posttraumatic stress disorder and obstructive sleep apnea, for which she uses a continuous airway positive pressure (CPAP) device. She had been working as a part-time nanny when her employer furloughed her early in the COVID-19 pandemic. Her anxiety has gotten worse throughout the quarantine; she fears her unemployment benefits will run out and she will lose her job. Her anxiety manifests as somatic “pit-of-stomach” sensations. Her sleep has been disrupted; she reports more frequent nightmares, and her partner says that Ms. L has had apneic episodes and bruxism. The parameters of Ms. L’s CPAP device need to be adjusted, but a previously scheduled overnight polysomnography test is deemed a nonessential procedure and canceled. Ms. L has been reluctant to go to a food pantry because she is afraid of being exposed to COVID-19. In virtual sessions, Ms. L says she is uncertain if she will be able to pay her rent, buy food, or access medical care, and expresses overriding helplessness.
During COVID-19, anxiety and insomnia are driven by the sudden manifestation of uncertainty regarding being able to work, pay rent or mortgage, buy food and other provisions, or visit family and friends, including those who are hospitalized or live in nursing homes. Additional uncertainties include how long the quarantine will last, who will become ill, and when, or if, life will return to normal. Taken together, these uncertainties impart a pervasive dread to daily experience.
Interventions. Clinicians can facilitate access to services (eg, social services, benefits specialists) and help patients parse out what they should and can address practically, and which challenges are outside of their personal or communal control (Table 2). Patients can be encouraged to identify paralytic rumination and shift their mental focus to engage in constructive projects. They can be advised to limit their intake of media that increases their anxiety and replace it with phone calls or e-mails to family and friends. Scheduled practice of mindfulness meditation and diaphragmatic breathing can help reduce anxiety.7,8 Pharmacotherapeutic interventions should be low-risk to minimize burdening emergency departments saturated with patients who have COVID-19 and serve to reduce symptoms that interfere with behavioral activation. While the research on benzodiazepines and non-benzodiazepine receptor agonists (“Z-drugs” such as zolpidem and eszopiclone) in the setting of obstructive sleep apnea is complex, and there is some evidence that the latter may not exacerbate apnea,9 benzodiazepines and Z-drugs are associated with an array of risks, including tolerance, withdrawal, and traumatic falls, particularly in older adults.10 Sleep hygiene and cognitive-behavioral therapy are first-line therapies for insomnia.11

Household stress
Ms. M, a 45-year-old single mother with a history of generalized anxiety disorder, is suddenly thrust into homeschooling her 2 children, ages 10 and 8, while trying to remain productive at her job as a software engineer. She no longer has time for herself, and spends much of her day helping her children with schoolwork or planning activities to keep them engaged rather than arguing with each other. She feels intense pressure, heightened stress, and increased anxiety as she tries to navigate this new daily routine.
Continue to: New household dynamics...
New household dynamics abound when people are suddenly forced into atypical routines. In the context of COVID-19, working parents may be forced to balance the demands of their jobs with homeschooling their children. Couples may find themselves arguing more frequently. Adult children may find themselves needing to care for their ill parents. Limited space, a lack of leisure activities, and uncertainty about the future coalesce to increase conflict and stress. Research suggests that how people cope with a stressor is a more reliable determinant of health and well-being than the stressor itself.12
Interventions. Mental health clinicians can offer several recommendations to help patients cope with increased household stress (Table 3). We can encourage patients to have clear communication with their loved ones regarding new expectations, roles, and their feelings. Demarcating specific areas within living spaces to each person in the household can help each member feel a sense of autonomy, regardless of how small their area may be. Clinicians can help patients learn to take the time as a family to work on establishing new household routines. Telepsychiatry offers clinicians a unique window into patients’ lives and family dynamics, and we can use this perspective to deepen our understanding of the patient’s context and household relationships and help them navigate the situation thrust upon them.

Grief
Following a psychiatric hospitalization for an acute exacerbation of psychosis, Ms. S, age 79, is transferred to a rehabilitation facility, where she contracts COVID-19. Because Ms. S did not have a history of chronic medical illness, her family anticipates a full recovery. Early in the course of Ms. S’s admission, the rehabilitation facility restricts visitations, and her family is unable to see her. Ms. S dies in this facility without her family’s presence and without her family having the opportunity to say goodbye. Ms. S’s psychiatrist offers her family a virtual session to provide support. During the virtual session, the psychiatrist notes signs of complicated bereavement among Ms. S’s family members, including nonacceptance of the death, rumination about the circumstances of the death, and describing life as having no purpose.
The COVID-19 pandemic has complicated the natural process of loss and grief across multiple dimensions. Studies have shown that an inability to say goodbye before death, a lack of social support,13 and a lack of preparation for loss14 are associated with complicated bereavement and depression. Many people are experiencing the loss of loved ones without having a chance to appropriately mourn. Forbidding visits to family members who are hospitalized also prevents the practice of religious and spiritual rituals that typically occur at the end of life. This is worsened by truncated or absent funeral services. Support for those who are grieving may be offered from a distance, if at all. When surviving family members have been with the deceased prior to hospitalization, they may be required to self-quarantine, potentially exacerbating their grief and other symptoms associated with loss.
Interventions. Because social support is a protective factor against complicated grief,14 there are several recommendations for survivors as they work through the process of grief (Table 4). These include preparing families for a potential death; discussing desired spiritual and memorial services15; connecting families to resources such as community grief support programs, counseling/therapy, funeral services, video conferencing, and other communication tools; and planning for additional support for surviving family and friends, both immediately after the death and in the long term. It is also important to provide appropriate counseling and support for surviving family members to focus on their own well-being by exercising, eating nutritious meals, getting enough sleep, and abstaining from alcohol and drugs of abuse.16

Continue to: An ongoing challenge
An ongoing challenge
Our clinical team recommends further investigation to define additional psychotherapeutic themes arising from the COVID-19 pandemic and provide evidence-based interventions to address these categories, which we expect will increase in clinical salience in the months and years ahead. Close monitoring, follow-up by clinical and research staff, and evidence-based interventions will help address these dominant themes, with the goal of alleviating patient suffering.
Bottom Line
Our team identified 4 dominant clinical themes emerging across our patients’ experiences during the coronavirus disease 2019 pandemic: isolation, uncertainty, household stress, and grief. Clinicians can implement specific interventions to reduce the impact of these themes, which we expect to remain clinically relevant in the upcoming months and years.
Related Resources
- Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-32,39.
- Carr D, Boerner K, Moorman S. Bereavement in the time of coronavirus: unprecedented challenges demand novel interventions. J Aging Soc Policy. 2020;32(4-5):425-431.
Drug Brand Names
Eszopiclone • Lunesta
Zolpidem • Ambien
1. Bloom N. How working from home works out. Stanford Institute for Economic Policy Research Policy Brief. https://siepr.stanford.edu/research/publications/how-working-home-works-out. Published June 2020. Accessed October 28, 2020.
2. Linz SJ, Sturm BA. The phenomenon of social isolation in the severely mentally ill. Perspect Psychiatr Care. 2013;49(4):243-254.
3. Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405-429.
4. Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54‐S66.
5. Mann F, Bone JK, Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52(6):627-638.
6. Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthc Inform Res. 2012;18(3):191‐198.
7. Chen YF, Huang ZY, Chien CH, et al. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53(4):329-336.
8. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786‐792.
9. Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - an open-label pilot study. Sleep Res. 2019;28(6):e12853. doi: 10.1111/jsr.12853.
10. Markota M, Rummans TA, Bostwick JM, et al. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632-1639.
11. Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35.
12. Dijkstra MT, Homan AC. Engaging in rather than disengaging from stress: effective coping and perceived control. Front Psychol. 2016;7:1415.
13. Romero MM, Ott CH, Kelber ST. Predictors of grief in bereaved family caregivers of person’s with Alzheimer’s disease: a prospective study. Death Stud. 2014;38(6-10):395-403.
14. Lobb EA, Kristjanson LJ, Aoun SM, et al. Predictors of complicated grief: a systematic review of empirical studies. Death Stud. 2010;34(8):673-698.
15. Wallace CL, Wladkowski SP, Gibson A, et al. Grief during the COVID-19 pandemic: considerations for palliative care providers. J Pain Symptom Manage. 2020;60(1):e70-e76. doi: 10.1016/j.jpainsymman.2020.04.012
16. Selman LE, Chao D, Sowden R, et al. Bereavement support on the frontline of COVID-19: recommendations for hospital clinicians. J Pain Symptom Manage. 2020;60(2):e81-e86. doi: 10.1016/j.jpainsymman.2020.04.024
1. Bloom N. How working from home works out. Stanford Institute for Economic Policy Research Policy Brief. https://siepr.stanford.edu/research/publications/how-working-home-works-out. Published June 2020. Accessed October 28, 2020.
2. Linz SJ, Sturm BA. The phenomenon of social isolation in the severely mentally ill. Perspect Psychiatr Care. 2013;49(4):243-254.
3. Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405-429.
4. Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54‐S66.
5. Mann F, Bone JK, Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52(6):627-638.
6. Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthc Inform Res. 2012;18(3):191‐198.
7. Chen YF, Huang ZY, Chien CH, et al. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53(4):329-336.
8. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786‐792.
9. Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - an open-label pilot study. Sleep Res. 2019;28(6):e12853. doi: 10.1111/jsr.12853.
10. Markota M, Rummans TA, Bostwick JM, et al. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632-1639.
11. Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35.
12. Dijkstra MT, Homan AC. Engaging in rather than disengaging from stress: effective coping and perceived control. Front Psychol. 2016;7:1415.
13. Romero MM, Ott CH, Kelber ST. Predictors of grief in bereaved family caregivers of person’s with Alzheimer’s disease: a prospective study. Death Stud. 2014;38(6-10):395-403.
14. Lobb EA, Kristjanson LJ, Aoun SM, et al. Predictors of complicated grief: a systematic review of empirical studies. Death Stud. 2010;34(8):673-698.
15. Wallace CL, Wladkowski SP, Gibson A, et al. Grief during the COVID-19 pandemic: considerations for palliative care providers. J Pain Symptom Manage. 2020;60(1):e70-e76. doi: 10.1016/j.jpainsymman.2020.04.012
16. Selman LE, Chao D, Sowden R, et al. Bereavement support on the frontline of COVID-19: recommendations for hospital clinicians. J Pain Symptom Manage. 2020;60(2):e81-e86. doi: 10.1016/j.jpainsymman.2020.04.024
Managing metabolic syndrome in patients with schizophrenia
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.
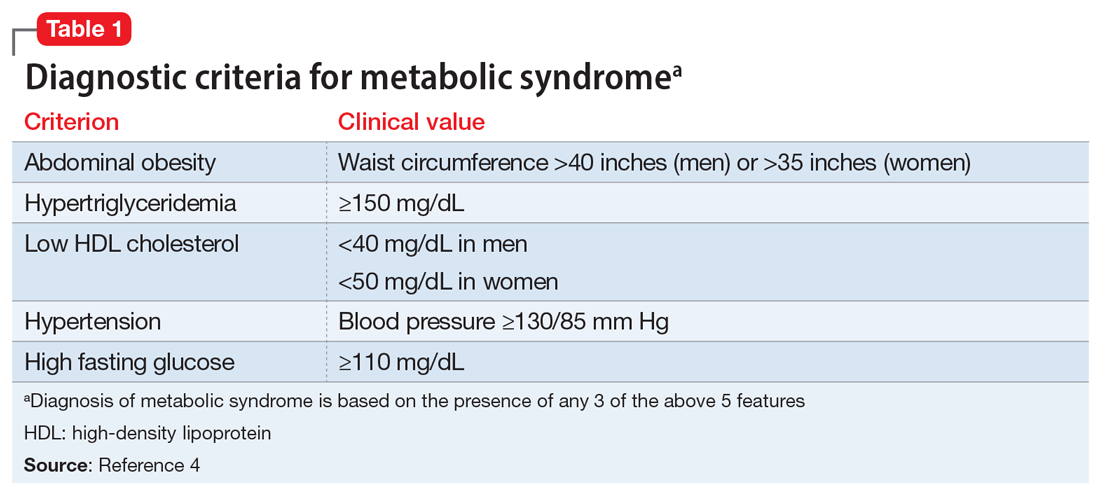
Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.

Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.

Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
The psychiatric consequences of COVID-19: 8 Studies
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.
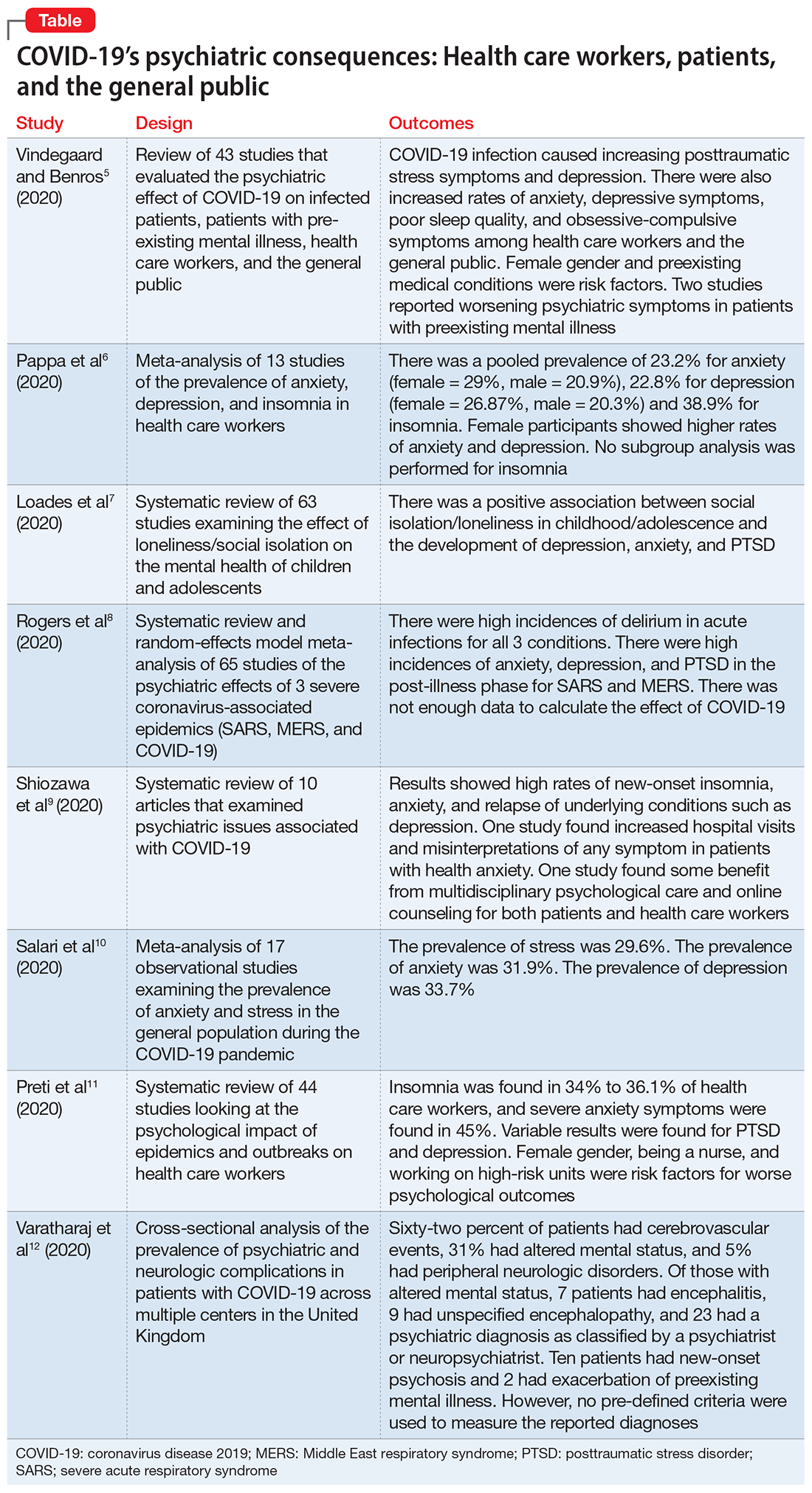
1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.

1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.

1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Using seclusion to prevent COVID-19 transmission on inpatient psychiatry units
Mr. T, age 26, presents to the psychiatric emergency department with acutely worsening symptoms of schizophrenia. The treating team decides to admit him to the inpatient psychiatry unit. The patient agrees to admission bloodwork, but adamantly refuses a coronavirus disease 2019 (COVID-19) nasal swab, stating that he does not consent to “having COVID-19 injected into his nose.” His nurse pages the psychiatry resident on call, asking her for seclusion orders to be placed for the patient in order to quarantine him.
This case illustrates a quandary that has arisen during the COVID-19 era. Traditionally, the use of seclusion in inpatient psychiatry wards has been restricted to the management of violent or self-destructive behavior. Most guidelines advise that seclusion should be used only to ensure the immediate physical safety of a patient, staff members, or other patients.1 Using seclusion for other purposes, such as to quarantine patients suspected of having an infectious disease, raises ethical questions.
What is seclusion?
To best understand the questions that arise from the above scenario, a thorough understanding of the terminology used is needed. Although the terms “isolation,” “quarantine,” and “seclusion” are often used interchangeably, each has a distinct definition and unique history.
Isolation in a medical context refers to the practice of isolating people confirmed to have a disease from the general population. The earliest description of medical isolation dates back to the 7th century BC in the Book of Leviticus, which mentions a protocol for separating individuals infected with leprosy from those who are healthy.2
Quarantine hearkens back to the most fatal pandemic recorded in human history, the Black Death. In 1377, on the advice of the city’s chief physician, the Mediterranean seaport of Ragusa passed a law establishing an isolation period for all visitors from plague-endemic lands.2 Initially a 30-day isolation period (a trentino), this was extended to 40 days (a quarantino). Distinct from isolation, quarantine is the practice of limiting movements of apparently healthy individuals who may have been exposed to a disease but do not have a confirmed diagnosis.
Seclusion, a term used most often in psychiatry, is defined as “the involuntary confinement of a patient alone in a room or area from which the patient is physically prevented from leaving.”3 The use of seclusion rooms in psychiatric facilities was originally championed by the 19th century British psychiatrist John Conolly.4 In The Treatment of the Insane without Mechanical Restraints, Conolly argued that a padded seclusion room was far more humane and effective in calming a violent patient than mechanical restraints. After exhausting less restrictive measures, seclusion is one of the most common means of restraining violent patients in inpatient psychiatric facilities.
Why consider seclusion?
The discussion of using seclusion as a means of quarantine has arisen recently due to the COVID-19 pandemic. This infectious disease was first identified in December 2019 in Wuhan, China.5 Since then, it has spread rapidly across the world. As of mid-October 2020, >39 million cases across 189 countries had been reported.6 The primary means by which the virus is spread is through respiratory droplets released from infected individuals through coughing, sneezing, or talking.7 These droplets can remain airborne or fall onto surfaces that become fomites. Transmission is possible before symptoms appear in an infected individual or even from individuals who are asymptomatic.8
Continue to: The typical layout and requirements...
The typical layout and requirements of an inpatient psychiatric ward intensify the risk of COVID-19 transmission.9 Unlike most medical specialty wards, psychiatric wards are set up with a therapeutic milieu where patients have the opportunity to mingle and interact with each other and staff members. Patients are allowed to walk around the unit, spend time in group therapy, eat meals with each other, and have visitation hours. The therapeutic benefit of such a milieu, however, must be weighed against the risks that patients pose to staff members and other patients. While many facilities have restricted some of these activities to limit COVID-19 exposure, the overall risk of transmission is still elevated. Early in course of the pandemic, the virus spread to an inpatient psychiatric ward in South Korea. Although health officials put the ward on lockdown, given the heightened risk of transmission, the virus quickly spread from patient to patient. Out of 103 inpatients, 101 contracted COVID-19.10
To mitigate this risk, many inpatient psychiatric facilities have mandated that all newly admitted patients be tested for COVID-19. By obtaining COVID-19 testing, facilities are better able to risk stratify their patient population and appropriately protect all patients. A dilemma arises, however, when a patient refuses to consent to COVID-19 testing. In such cases, the infectious risk of the patient remains unknown. Given the potentially disastrous consequences of an unchecked COVID-19 infection running rampant in an inpatient ward, some facilities have elected to use seclusion as a means of quarantining the patient.
Is seclusion justifiable?
There are legitimate objections to using seclusion as a means of quarantine. Most guidelines state that the only time seclusion is ethical is when it is used to prevent immediate physical danger, either to the patient or others.11 Involuntary confinement entails considerable restriction of a patient’s rights and thus should be used only after all other options have been exhausted. People opposed to the use of seclusion point out that outside of the hospital, people are not forcibly restrained in order to enforce social distancing,12 so by extension, those who are inside the hospital should not be forced to seclude.
Seclusion also comes with potentially harmful effects. For the 14 days that a patient is in quarantine, they are cut off from most social contact, which is the opposite of the intended purpose of the therapeutic milieu in inpatient psychiatric wards. Several quantitative studies have shown that individuals who are quarantined tend to report a high prevalence of symptoms of psychological distress, including low mood, irritability, depression, stress, anger, and posttraumatic stress disorder.13
Furthermore, there is considerable evidence that a negative test does not definitively rule out a COVID-19 infection. Nasal swabs for COVID-19 have a false-negative rate of 27%.14 In other words, patients on an inpatient psychiatry ward who are free to walk around the unit and interact with others are only probably COVID-19 free, not definitively. This fact throws into question the original justification for seclusion—to protect other patients from COVID-19.
Continue to: Support for using seclusion as quarantine
Support for using seclusion as quarantine
Despite these objections, there are clear arguments in favor of using seclusion as a means of quarantine. First, the danger posed by an unidentified COVID-19 infection to the inpatient psychiatric population is not small. As of mid-October 2020, >217,000 Americans had died of COVID-19.6 Psychiatric patients, especially those who are acutely decompensated and hospitalized, have a heightened risk.15 Those with underlying medical issues are more likely to be seriously affected by an infection. Patients with serious mental illness have higher rates of medical comorbidities16 and premature death.17 The risk of a patient contracting and then dying from COVID-19 is elevated in an inpatient psychiatric ward. Even if a test is not 100% sensitive or specific, the balance of probability it provides is sufficient to make an informed decision about transmission risk.
In choosing to seclude a patient who refuses COVID-19 testing, the treating team must weigh one person’s autonomy against the safety of every other individual on the ward. From a purely utilitarian perspective, the lives of the many outweigh the discomfort of one. Addressing this balance, the American Medical Association (AMA) Code of Ethics states “Although physicians’ primary ethical obligation is to their individual patients, they also have a long-recognized public health responsibility. In the context of infectious disease, this may include the use of quarantine and isolation to reduce the transmission of disease and protect the health of the public. In such situations, physicians have a further responsibility to protect their own health to ensure that they remain able to provide care. These responsibilities potentially conflict with patients’ rights of self-determination and with physicians’ duty to advocate for the best interests of individual patients and to provide care in emergencies.”18
The AMA Code of Ethics further mentions that physicians should “support mandatory quarantine and isolation when a patient fails to adhere voluntarily.” Medical evidence supports both quarantine19 and enacting isolation measures for COVID-19–positive hospitalized patients.20 Table 121-24 summarizes the recommendations of major medical societies regarding isolation on hospital units.
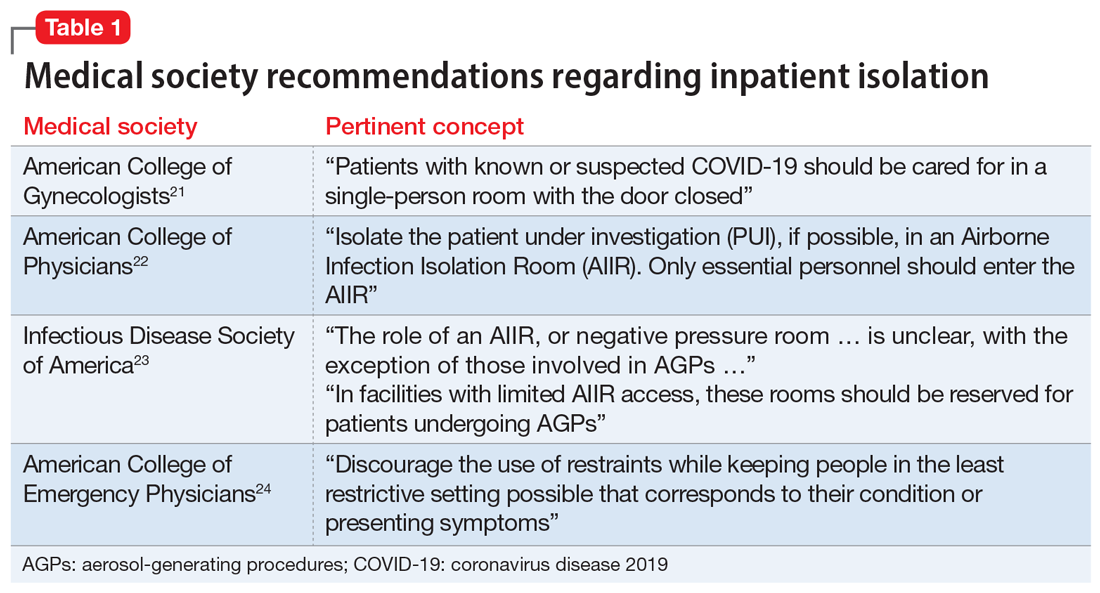
Further, public health officials and law enforcement officials do in fact have the authority25 to enforce quarantine and restrict a citizen’s movement outside a hospital setting. Recent cases have illustrated how this has been enforced, particularly with the use of electronic monitoring units and even criminal sanctions.26,27
It is also important to consider that when used as quarantine, seclusion is not an indefinite action. Current recommendations suggest the longest period of time a patient would need to be in seclusion is 14 days. A patient could potentially reduce this period by agreeing to COVID-19 testing and obtaining a negative test result.
Continue to: Enacting inpatient quarantine
Enacting inpatient quarantine
In Mr. T’s case, the resident physician was asked to make a decision regarding seclusion on the spot. Prudent facilities will set policies and educate clinicians before they need to face this conundrum. The following practical considerations may guide implementation of seclusion as a measure of quarantine on an inpatient psychiatric unit:
- given the risk of asymptomatic carriers, all admitted patients should be tested for COVID-19
- patients who refuse a test should be evaluated by the psychiatrist on duty to determine if the patient has the capacity to make this decision
- if a patient demonstrates capacity to refuse and continues to refuse testing, seclusion orders should then be placed
- the facility should create a protocol to ensure consistent application of seclusion orders.
So that they can make an informed decision, patients should be educated about the risks of not undergoing testing. It is important to correctly frame a seclusion decision to the patient. Explain that seclusion is not a punitive measure, but rather a means of respecting the patient’s right to refuse testing while ensuring other patients’ right to be protected from COVID-19 transmission.
It is crucial to not allow psychiatric care to be diminished because a patient is isolated due to COVID-19. Psychiatrists have legal duties to provide care when a patient is admitted to their unit,28-30 and state laws generally outline patients’ rights while they are hospitalized.31 The use of technology can ensure these duties are fulfilled. Patient rounds and group treatment can be conducted through telehealth.10,32 When in-person interaction is required, caretakers should don proper personal protective equipment and interact with the patient as often as they would if the patient were not in seclusion. Table 233-36 summarizes further ethical considerations when implementing quarantine measures on a psychiatry unit.
The contemporary inpatient unit
The ideal design to optimize care and safety is to create designated COVID-19 psychiatric units. Indeed, the US Substance Abuse and Mental Health Services Administration recommends segregating floors based on infection status where possible.37 This minimizes the risk of transmission to other patients while maintaining the same standards of psychiatric treatment, including milieu and group therapy (which may also require adjustments). Such a unit already has precedent.38 Although designated COVID-19 psychiatric units present clinical and administrative hurdles,39 they may become more commonplace as the number of COVID-19–positive inpatients continues to rise.
Bottom Line
The coronavirus disease 2019 (COVID-19) pandemic has created challenges for inpatient psychiatric facilities. Although seclusion is a serious decision and should not be undertaken lightly, there are clear ethical and practical justifications for using it as a means of quarantine for patients who are COVID-19–positive or refuse testing.
Related Resources
- Askew L, Fisher P, Beazley P. What are adult psychiatric inpatients’ experience of seclusion: a systematic review of qualitative studies. J Psychiatr Ment Health Nurs. 2019; 26(7-8):274-285.
- Komrad MS. Medical ethics in the time of COVID-19. Current Psychiatry. 2020;19(7):29-32,46.
1. Knox DK, Holloman GH Jr. Use and avoidance of seclusion and restraint: consensus statement of the American Association for Emergency Psychiatry Project BETA Seclusion and Restraint Workgroup. West J Emerg Med. 2012;13(1):35-40.
2. Sehdev PS. The origin of quarantine. Clin Infect Dis. 2002;35(9):1071-1072.
3. 42 CFR § 482.13. Condition of participation: patient’s rights.
4. Colaizzi J. Seclusion & restraint: a historical perspective. J Psychosoc Nurs Ment Health Serv. 2005;43(2):31-37.
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
6. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). ArcGIS. Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed October 16, 2020.
7. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11.
8. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407.
9. Li L. Challenges and priorities in responding to COVID-19 in inpatient psychiatry. Psychiatr Serv. 2020;71(6):624-626.
10. Kim MJ. ‘It was a medical disaster’: the psychiatric ward that saw 100 patients diagnosed with new coronavirus. Independent. https://www.independent.co.uk/news/world/asia/coronavirus-south-korea-outbreak-hospital-patients-lockdown-a9367486.html. Published March 1, 2020. Accessed July 12, 2020.
11. Petrini C. Ethical considerations for evaluating the issue of physical restraint in psychiatry. Ann Ist Super Sanita. 2013;49(3):281-285.
12. Gessen M. Why psychiatric wards are uniquely vulnerable to the coronavirus. https://www.newyorker.com/news/news-desk/why-psychiatric-wards-are-uniquely-vulnerable-to-the-coronavirus. Published April 21, 2020. Accessed July 12, 2020.
13. Brooks SK, Webster RK, Smith, LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
14. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383(6):e38. doi: 10.1056/NEJMp2015897.
15. Druss BG. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatry. 2020;77(9):891-892.
16. Rao S, Raney L, Xiong GL. Reducing medical comorbidity and mortality in severe mental illness. Current Psychiatry. 2015;14(7):14-20.
17. Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827-1835.
18. American Medical Association. Ethical use of quarantine and isolation. Code of Ethics Opinion 8.4. https://www.ama-assn.org/delivering-care/ethics/ethical-use-quarantine-isolation. Published November 14, 2016. Accessed July 12, 2020.
19. Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;4(4):CD013574.
20. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Duration of isolation & precautions for adults. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Updated August 16, 2020. Accessed August 21, 2020.
21. American College of Gynecologists. Novel coronavirus 2019 (COVID-19). https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019. Updated August 12, 2020. Accessed August 26, 2020.
22. American College of Physicians. COVID-19: an ACP physician’s guide + resources. Chapter 14 of 31. Infection control: advice for physicians. https://assets.acponline.org/coronavirus/scormcontent/#/. Updated September 3, 2020. Accessed September 9, 2020.
23. Infectious Disease Society of America. Infectious Diseases Society of America Guidelines on Infection Prevention in Patients with Suspected or Known COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/#toc-9-9. Updated April 20, 2020. Accessed August 26, 2020.
24. American College of Emergency Physicians. Joint Statement for Care of Patients with Behavioral Health Emergencies and Suspected or Confirmed COVID-19. https://www.acep.org/corona/covid-19-field-guide/special-populations/behavioral-health-patients/. Updated June 17, 2020. Accessed August 26, 2020.
25. Centers for Disease Control and Prevention. Quarantine and isolation. Legal authorities. https://www.cdc.gov/quarantine/aboutlawsregulationsquarantineisolation.html. Updated February 24, 2020. Accessed August 31, 2020.
26. Roberts A. Kentucky couple under house arrest after refusing to sign self-quarantine agreement. https://abcnews.go.com/US/kentucky-couple-house-arrest-refusing-sign-quarantine-agreement/story?id=71886479. Published July 20, 2020. Accessed July 24, 2020.
27. Satter R. To keep COVID-19 patients home, some U.S. states weigh house arrest tech. https://www.reuters.com/article/us-health-coronavirus-quarantine-tech/to-keep-covid-19-patients-home-some-us-states-weigh-house-arrest-tech-idUSKBN22J1U8. Published May 7, 2020. Accessed July 24, 2020.
28. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
29. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
30. Donaldson v O’Connor, 519, F2d 59 (5th Cir 1975).
31. Ohio Revised Code § 5122.290.
32. Shore JH. Telepsychiatry: videoconferencing in the delivery of psychiatric care. Am J Psychiatry. 2013;170(3):256-262.
33. Bostick NA, Levine MA, Sade RM. Ethical obligations of physicians participating in public health quarantine and isolation measures. Public Health Rep. 2008;123(1):3-8.
34. Upshur RE. Principles for the justification of public health intervention. Can J Public Health. 2002;93(2):101-103.
35. Barbera J, Macintyre A, Gostin L, et al. Large-scale quarantine following biological terrorism in the United States: scientific examination, logistic and legal limits, and possible consequences. JAMA. 2001;286(21):2711-2717.
36. Stanford Encyclopedia of Philosophy. Doctrine of double effect. https://plato.stanford.edu/entries/double-effect/. Revised December 24, 2018. Accessed July 12, 2020.
37. Substance Abuse and Mental Health Services Administration. Covid19: interim considerations for state psychiatric hospitals. https://www.samhsa.gov/sites/default/files/covid19-interim-considerations-for-state-psychiatric-hospitals.pdf. Updated May 8, 2020. Accessed July 24, 2020.
38. Augenstein TM, Pigeon WR, DiGiovanni SK, et al. Creating a novel inpatient psychiatric unit with integrated medical support for patients with COVID-19. N Engl J Med Catalyst. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0249. Published June 22, 2020. Accessed July 12, 2020.
39. Bojdani E, Rajagopalan A, Chen A, et al. COVID-19 pandemic: impact on psychiatric care in the United States. Psychiatry Research. 2020;289:113069. doi: 10.1016/j.psychres.2020.113069.
Mr. T, age 26, presents to the psychiatric emergency department with acutely worsening symptoms of schizophrenia. The treating team decides to admit him to the inpatient psychiatry unit. The patient agrees to admission bloodwork, but adamantly refuses a coronavirus disease 2019 (COVID-19) nasal swab, stating that he does not consent to “having COVID-19 injected into his nose.” His nurse pages the psychiatry resident on call, asking her for seclusion orders to be placed for the patient in order to quarantine him.
This case illustrates a quandary that has arisen during the COVID-19 era. Traditionally, the use of seclusion in inpatient psychiatry wards has been restricted to the management of violent or self-destructive behavior. Most guidelines advise that seclusion should be used only to ensure the immediate physical safety of a patient, staff members, or other patients.1 Using seclusion for other purposes, such as to quarantine patients suspected of having an infectious disease, raises ethical questions.
What is seclusion?
To best understand the questions that arise from the above scenario, a thorough understanding of the terminology used is needed. Although the terms “isolation,” “quarantine,” and “seclusion” are often used interchangeably, each has a distinct definition and unique history.
Isolation in a medical context refers to the practice of isolating people confirmed to have a disease from the general population. The earliest description of medical isolation dates back to the 7th century BC in the Book of Leviticus, which mentions a protocol for separating individuals infected with leprosy from those who are healthy.2
Quarantine hearkens back to the most fatal pandemic recorded in human history, the Black Death. In 1377, on the advice of the city’s chief physician, the Mediterranean seaport of Ragusa passed a law establishing an isolation period for all visitors from plague-endemic lands.2 Initially a 30-day isolation period (a trentino), this was extended to 40 days (a quarantino). Distinct from isolation, quarantine is the practice of limiting movements of apparently healthy individuals who may have been exposed to a disease but do not have a confirmed diagnosis.
Seclusion, a term used most often in psychiatry, is defined as “the involuntary confinement of a patient alone in a room or area from which the patient is physically prevented from leaving.”3 The use of seclusion rooms in psychiatric facilities was originally championed by the 19th century British psychiatrist John Conolly.4 In The Treatment of the Insane without Mechanical Restraints, Conolly argued that a padded seclusion room was far more humane and effective in calming a violent patient than mechanical restraints. After exhausting less restrictive measures, seclusion is one of the most common means of restraining violent patients in inpatient psychiatric facilities.
Why consider seclusion?
The discussion of using seclusion as a means of quarantine has arisen recently due to the COVID-19 pandemic. This infectious disease was first identified in December 2019 in Wuhan, China.5 Since then, it has spread rapidly across the world. As of mid-October 2020, >39 million cases across 189 countries had been reported.6 The primary means by which the virus is spread is through respiratory droplets released from infected individuals through coughing, sneezing, or talking.7 These droplets can remain airborne or fall onto surfaces that become fomites. Transmission is possible before symptoms appear in an infected individual or even from individuals who are asymptomatic.8
Continue to: The typical layout and requirements...
The typical layout and requirements of an inpatient psychiatric ward intensify the risk of COVID-19 transmission.9 Unlike most medical specialty wards, psychiatric wards are set up with a therapeutic milieu where patients have the opportunity to mingle and interact with each other and staff members. Patients are allowed to walk around the unit, spend time in group therapy, eat meals with each other, and have visitation hours. The therapeutic benefit of such a milieu, however, must be weighed against the risks that patients pose to staff members and other patients. While many facilities have restricted some of these activities to limit COVID-19 exposure, the overall risk of transmission is still elevated. Early in course of the pandemic, the virus spread to an inpatient psychiatric ward in South Korea. Although health officials put the ward on lockdown, given the heightened risk of transmission, the virus quickly spread from patient to patient. Out of 103 inpatients, 101 contracted COVID-19.10
To mitigate this risk, many inpatient psychiatric facilities have mandated that all newly admitted patients be tested for COVID-19. By obtaining COVID-19 testing, facilities are better able to risk stratify their patient population and appropriately protect all patients. A dilemma arises, however, when a patient refuses to consent to COVID-19 testing. In such cases, the infectious risk of the patient remains unknown. Given the potentially disastrous consequences of an unchecked COVID-19 infection running rampant in an inpatient ward, some facilities have elected to use seclusion as a means of quarantining the patient.
Is seclusion justifiable?
There are legitimate objections to using seclusion as a means of quarantine. Most guidelines state that the only time seclusion is ethical is when it is used to prevent immediate physical danger, either to the patient or others.11 Involuntary confinement entails considerable restriction of a patient’s rights and thus should be used only after all other options have been exhausted. People opposed to the use of seclusion point out that outside of the hospital, people are not forcibly restrained in order to enforce social distancing,12 so by extension, those who are inside the hospital should not be forced to seclude.
Seclusion also comes with potentially harmful effects. For the 14 days that a patient is in quarantine, they are cut off from most social contact, which is the opposite of the intended purpose of the therapeutic milieu in inpatient psychiatric wards. Several quantitative studies have shown that individuals who are quarantined tend to report a high prevalence of symptoms of psychological distress, including low mood, irritability, depression, stress, anger, and posttraumatic stress disorder.13
Furthermore, there is considerable evidence that a negative test does not definitively rule out a COVID-19 infection. Nasal swabs for COVID-19 have a false-negative rate of 27%.14 In other words, patients on an inpatient psychiatry ward who are free to walk around the unit and interact with others are only probably COVID-19 free, not definitively. This fact throws into question the original justification for seclusion—to protect other patients from COVID-19.
Continue to: Support for using seclusion as quarantine
Support for using seclusion as quarantine
Despite these objections, there are clear arguments in favor of using seclusion as a means of quarantine. First, the danger posed by an unidentified COVID-19 infection to the inpatient psychiatric population is not small. As of mid-October 2020, >217,000 Americans had died of COVID-19.6 Psychiatric patients, especially those who are acutely decompensated and hospitalized, have a heightened risk.15 Those with underlying medical issues are more likely to be seriously affected by an infection. Patients with serious mental illness have higher rates of medical comorbidities16 and premature death.17 The risk of a patient contracting and then dying from COVID-19 is elevated in an inpatient psychiatric ward. Even if a test is not 100% sensitive or specific, the balance of probability it provides is sufficient to make an informed decision about transmission risk.
In choosing to seclude a patient who refuses COVID-19 testing, the treating team must weigh one person’s autonomy against the safety of every other individual on the ward. From a purely utilitarian perspective, the lives of the many outweigh the discomfort of one. Addressing this balance, the American Medical Association (AMA) Code of Ethics states “Although physicians’ primary ethical obligation is to their individual patients, they also have a long-recognized public health responsibility. In the context of infectious disease, this may include the use of quarantine and isolation to reduce the transmission of disease and protect the health of the public. In such situations, physicians have a further responsibility to protect their own health to ensure that they remain able to provide care. These responsibilities potentially conflict with patients’ rights of self-determination and with physicians’ duty to advocate for the best interests of individual patients and to provide care in emergencies.”18
The AMA Code of Ethics further mentions that physicians should “support mandatory quarantine and isolation when a patient fails to adhere voluntarily.” Medical evidence supports both quarantine19 and enacting isolation measures for COVID-19–positive hospitalized patients.20 Table 121-24 summarizes the recommendations of major medical societies regarding isolation on hospital units.

Further, public health officials and law enforcement officials do in fact have the authority25 to enforce quarantine and restrict a citizen’s movement outside a hospital setting. Recent cases have illustrated how this has been enforced, particularly with the use of electronic monitoring units and even criminal sanctions.26,27
It is also important to consider that when used as quarantine, seclusion is not an indefinite action. Current recommendations suggest the longest period of time a patient would need to be in seclusion is 14 days. A patient could potentially reduce this period by agreeing to COVID-19 testing and obtaining a negative test result.
Continue to: Enacting inpatient quarantine
Enacting inpatient quarantine
In Mr. T’s case, the resident physician was asked to make a decision regarding seclusion on the spot. Prudent facilities will set policies and educate clinicians before they need to face this conundrum. The following practical considerations may guide implementation of seclusion as a measure of quarantine on an inpatient psychiatric unit:
- given the risk of asymptomatic carriers, all admitted patients should be tested for COVID-19
- patients who refuse a test should be evaluated by the psychiatrist on duty to determine if the patient has the capacity to make this decision
- if a patient demonstrates capacity to refuse and continues to refuse testing, seclusion orders should then be placed
- the facility should create a protocol to ensure consistent application of seclusion orders.
So that they can make an informed decision, patients should be educated about the risks of not undergoing testing. It is important to correctly frame a seclusion decision to the patient. Explain that seclusion is not a punitive measure, but rather a means of respecting the patient’s right to refuse testing while ensuring other patients’ right to be protected from COVID-19 transmission.
It is crucial to not allow psychiatric care to be diminished because a patient is isolated due to COVID-19. Psychiatrists have legal duties to provide care when a patient is admitted to their unit,28-30 and state laws generally outline patients’ rights while they are hospitalized.31 The use of technology can ensure these duties are fulfilled. Patient rounds and group treatment can be conducted through telehealth.10,32 When in-person interaction is required, caretakers should don proper personal protective equipment and interact with the patient as often as they would if the patient were not in seclusion. Table 233-36 summarizes further ethical considerations when implementing quarantine measures on a psychiatry unit.
The contemporary inpatient unit
The ideal design to optimize care and safety is to create designated COVID-19 psychiatric units. Indeed, the US Substance Abuse and Mental Health Services Administration recommends segregating floors based on infection status where possible.37 This minimizes the risk of transmission to other patients while maintaining the same standards of psychiatric treatment, including milieu and group therapy (which may also require adjustments). Such a unit already has precedent.38 Although designated COVID-19 psychiatric units present clinical and administrative hurdles,39 they may become more commonplace as the number of COVID-19–positive inpatients continues to rise.
Bottom Line
The coronavirus disease 2019 (COVID-19) pandemic has created challenges for inpatient psychiatric facilities. Although seclusion is a serious decision and should not be undertaken lightly, there are clear ethical and practical justifications for using it as a means of quarantine for patients who are COVID-19–positive or refuse testing.
Related Resources
- Askew L, Fisher P, Beazley P. What are adult psychiatric inpatients’ experience of seclusion: a systematic review of qualitative studies. J Psychiatr Ment Health Nurs. 2019; 26(7-8):274-285.
- Komrad MS. Medical ethics in the time of COVID-19. Current Psychiatry. 2020;19(7):29-32,46.
Mr. T, age 26, presents to the psychiatric emergency department with acutely worsening symptoms of schizophrenia. The treating team decides to admit him to the inpatient psychiatry unit. The patient agrees to admission bloodwork, but adamantly refuses a coronavirus disease 2019 (COVID-19) nasal swab, stating that he does not consent to “having COVID-19 injected into his nose.” His nurse pages the psychiatry resident on call, asking her for seclusion orders to be placed for the patient in order to quarantine him.
This case illustrates a quandary that has arisen during the COVID-19 era. Traditionally, the use of seclusion in inpatient psychiatry wards has been restricted to the management of violent or self-destructive behavior. Most guidelines advise that seclusion should be used only to ensure the immediate physical safety of a patient, staff members, or other patients.1 Using seclusion for other purposes, such as to quarantine patients suspected of having an infectious disease, raises ethical questions.
What is seclusion?
To best understand the questions that arise from the above scenario, a thorough understanding of the terminology used is needed. Although the terms “isolation,” “quarantine,” and “seclusion” are often used interchangeably, each has a distinct definition and unique history.
Isolation in a medical context refers to the practice of isolating people confirmed to have a disease from the general population. The earliest description of medical isolation dates back to the 7th century BC in the Book of Leviticus, which mentions a protocol for separating individuals infected with leprosy from those who are healthy.2
Quarantine hearkens back to the most fatal pandemic recorded in human history, the Black Death. In 1377, on the advice of the city’s chief physician, the Mediterranean seaport of Ragusa passed a law establishing an isolation period for all visitors from plague-endemic lands.2 Initially a 30-day isolation period (a trentino), this was extended to 40 days (a quarantino). Distinct from isolation, quarantine is the practice of limiting movements of apparently healthy individuals who may have been exposed to a disease but do not have a confirmed diagnosis.
Seclusion, a term used most often in psychiatry, is defined as “the involuntary confinement of a patient alone in a room or area from which the patient is physically prevented from leaving.”3 The use of seclusion rooms in psychiatric facilities was originally championed by the 19th century British psychiatrist John Conolly.4 In The Treatment of the Insane without Mechanical Restraints, Conolly argued that a padded seclusion room was far more humane and effective in calming a violent patient than mechanical restraints. After exhausting less restrictive measures, seclusion is one of the most common means of restraining violent patients in inpatient psychiatric facilities.
Why consider seclusion?
The discussion of using seclusion as a means of quarantine has arisen recently due to the COVID-19 pandemic. This infectious disease was first identified in December 2019 in Wuhan, China.5 Since then, it has spread rapidly across the world. As of mid-October 2020, >39 million cases across 189 countries had been reported.6 The primary means by which the virus is spread is through respiratory droplets released from infected individuals through coughing, sneezing, or talking.7 These droplets can remain airborne or fall onto surfaces that become fomites. Transmission is possible before symptoms appear in an infected individual or even from individuals who are asymptomatic.8
Continue to: The typical layout and requirements...
The typical layout and requirements of an inpatient psychiatric ward intensify the risk of COVID-19 transmission.9 Unlike most medical specialty wards, psychiatric wards are set up with a therapeutic milieu where patients have the opportunity to mingle and interact with each other and staff members. Patients are allowed to walk around the unit, spend time in group therapy, eat meals with each other, and have visitation hours. The therapeutic benefit of such a milieu, however, must be weighed against the risks that patients pose to staff members and other patients. While many facilities have restricted some of these activities to limit COVID-19 exposure, the overall risk of transmission is still elevated. Early in course of the pandemic, the virus spread to an inpatient psychiatric ward in South Korea. Although health officials put the ward on lockdown, given the heightened risk of transmission, the virus quickly spread from patient to patient. Out of 103 inpatients, 101 contracted COVID-19.10
To mitigate this risk, many inpatient psychiatric facilities have mandated that all newly admitted patients be tested for COVID-19. By obtaining COVID-19 testing, facilities are better able to risk stratify their patient population and appropriately protect all patients. A dilemma arises, however, when a patient refuses to consent to COVID-19 testing. In such cases, the infectious risk of the patient remains unknown. Given the potentially disastrous consequences of an unchecked COVID-19 infection running rampant in an inpatient ward, some facilities have elected to use seclusion as a means of quarantining the patient.
Is seclusion justifiable?
There are legitimate objections to using seclusion as a means of quarantine. Most guidelines state that the only time seclusion is ethical is when it is used to prevent immediate physical danger, either to the patient or others.11 Involuntary confinement entails considerable restriction of a patient’s rights and thus should be used only after all other options have been exhausted. People opposed to the use of seclusion point out that outside of the hospital, people are not forcibly restrained in order to enforce social distancing,12 so by extension, those who are inside the hospital should not be forced to seclude.
Seclusion also comes with potentially harmful effects. For the 14 days that a patient is in quarantine, they are cut off from most social contact, which is the opposite of the intended purpose of the therapeutic milieu in inpatient psychiatric wards. Several quantitative studies have shown that individuals who are quarantined tend to report a high prevalence of symptoms of psychological distress, including low mood, irritability, depression, stress, anger, and posttraumatic stress disorder.13
Furthermore, there is considerable evidence that a negative test does not definitively rule out a COVID-19 infection. Nasal swabs for COVID-19 have a false-negative rate of 27%.14 In other words, patients on an inpatient psychiatry ward who are free to walk around the unit and interact with others are only probably COVID-19 free, not definitively. This fact throws into question the original justification for seclusion—to protect other patients from COVID-19.
Continue to: Support for using seclusion as quarantine
Support for using seclusion as quarantine
Despite these objections, there are clear arguments in favor of using seclusion as a means of quarantine. First, the danger posed by an unidentified COVID-19 infection to the inpatient psychiatric population is not small. As of mid-October 2020, >217,000 Americans had died of COVID-19.6 Psychiatric patients, especially those who are acutely decompensated and hospitalized, have a heightened risk.15 Those with underlying medical issues are more likely to be seriously affected by an infection. Patients with serious mental illness have higher rates of medical comorbidities16 and premature death.17 The risk of a patient contracting and then dying from COVID-19 is elevated in an inpatient psychiatric ward. Even if a test is not 100% sensitive or specific, the balance of probability it provides is sufficient to make an informed decision about transmission risk.
In choosing to seclude a patient who refuses COVID-19 testing, the treating team must weigh one person’s autonomy against the safety of every other individual on the ward. From a purely utilitarian perspective, the lives of the many outweigh the discomfort of one. Addressing this balance, the American Medical Association (AMA) Code of Ethics states “Although physicians’ primary ethical obligation is to their individual patients, they also have a long-recognized public health responsibility. In the context of infectious disease, this may include the use of quarantine and isolation to reduce the transmission of disease and protect the health of the public. In such situations, physicians have a further responsibility to protect their own health to ensure that they remain able to provide care. These responsibilities potentially conflict with patients’ rights of self-determination and with physicians’ duty to advocate for the best interests of individual patients and to provide care in emergencies.”18
The AMA Code of Ethics further mentions that physicians should “support mandatory quarantine and isolation when a patient fails to adhere voluntarily.” Medical evidence supports both quarantine19 and enacting isolation measures for COVID-19–positive hospitalized patients.20 Table 121-24 summarizes the recommendations of major medical societies regarding isolation on hospital units.

Further, public health officials and law enforcement officials do in fact have the authority25 to enforce quarantine and restrict a citizen’s movement outside a hospital setting. Recent cases have illustrated how this has been enforced, particularly with the use of electronic monitoring units and even criminal sanctions.26,27
It is also important to consider that when used as quarantine, seclusion is not an indefinite action. Current recommendations suggest the longest period of time a patient would need to be in seclusion is 14 days. A patient could potentially reduce this period by agreeing to COVID-19 testing and obtaining a negative test result.
Continue to: Enacting inpatient quarantine
Enacting inpatient quarantine
In Mr. T’s case, the resident physician was asked to make a decision regarding seclusion on the spot. Prudent facilities will set policies and educate clinicians before they need to face this conundrum. The following practical considerations may guide implementation of seclusion as a measure of quarantine on an inpatient psychiatric unit:
- given the risk of asymptomatic carriers, all admitted patients should be tested for COVID-19
- patients who refuse a test should be evaluated by the psychiatrist on duty to determine if the patient has the capacity to make this decision
- if a patient demonstrates capacity to refuse and continues to refuse testing, seclusion orders should then be placed
- the facility should create a protocol to ensure consistent application of seclusion orders.
So that they can make an informed decision, patients should be educated about the risks of not undergoing testing. It is important to correctly frame a seclusion decision to the patient. Explain that seclusion is not a punitive measure, but rather a means of respecting the patient’s right to refuse testing while ensuring other patients’ right to be protected from COVID-19 transmission.
It is crucial to not allow psychiatric care to be diminished because a patient is isolated due to COVID-19. Psychiatrists have legal duties to provide care when a patient is admitted to their unit,28-30 and state laws generally outline patients’ rights while they are hospitalized.31 The use of technology can ensure these duties are fulfilled. Patient rounds and group treatment can be conducted through telehealth.10,32 When in-person interaction is required, caretakers should don proper personal protective equipment and interact with the patient as often as they would if the patient were not in seclusion. Table 233-36 summarizes further ethical considerations when implementing quarantine measures on a psychiatry unit.
The contemporary inpatient unit
The ideal design to optimize care and safety is to create designated COVID-19 psychiatric units. Indeed, the US Substance Abuse and Mental Health Services Administration recommends segregating floors based on infection status where possible.37 This minimizes the risk of transmission to other patients while maintaining the same standards of psychiatric treatment, including milieu and group therapy (which may also require adjustments). Such a unit already has precedent.38 Although designated COVID-19 psychiatric units present clinical and administrative hurdles,39 they may become more commonplace as the number of COVID-19–positive inpatients continues to rise.
Bottom Line
The coronavirus disease 2019 (COVID-19) pandemic has created challenges for inpatient psychiatric facilities. Although seclusion is a serious decision and should not be undertaken lightly, there are clear ethical and practical justifications for using it as a means of quarantine for patients who are COVID-19–positive or refuse testing.
Related Resources
- Askew L, Fisher P, Beazley P. What are adult psychiatric inpatients’ experience of seclusion: a systematic review of qualitative studies. J Psychiatr Ment Health Nurs. 2019; 26(7-8):274-285.
- Komrad MS. Medical ethics in the time of COVID-19. Current Psychiatry. 2020;19(7):29-32,46.
1. Knox DK, Holloman GH Jr. Use and avoidance of seclusion and restraint: consensus statement of the American Association for Emergency Psychiatry Project BETA Seclusion and Restraint Workgroup. West J Emerg Med. 2012;13(1):35-40.
2. Sehdev PS. The origin of quarantine. Clin Infect Dis. 2002;35(9):1071-1072.
3. 42 CFR § 482.13. Condition of participation: patient’s rights.
4. Colaizzi J. Seclusion & restraint: a historical perspective. J Psychosoc Nurs Ment Health Serv. 2005;43(2):31-37.
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
6. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). ArcGIS. Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed October 16, 2020.
7. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11.
8. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407.
9. Li L. Challenges and priorities in responding to COVID-19 in inpatient psychiatry. Psychiatr Serv. 2020;71(6):624-626.
10. Kim MJ. ‘It was a medical disaster’: the psychiatric ward that saw 100 patients diagnosed with new coronavirus. Independent. https://www.independent.co.uk/news/world/asia/coronavirus-south-korea-outbreak-hospital-patients-lockdown-a9367486.html. Published March 1, 2020. Accessed July 12, 2020.
11. Petrini C. Ethical considerations for evaluating the issue of physical restraint in psychiatry. Ann Ist Super Sanita. 2013;49(3):281-285.
12. Gessen M. Why psychiatric wards are uniquely vulnerable to the coronavirus. https://www.newyorker.com/news/news-desk/why-psychiatric-wards-are-uniquely-vulnerable-to-the-coronavirus. Published April 21, 2020. Accessed July 12, 2020.
13. Brooks SK, Webster RK, Smith, LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
14. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383(6):e38. doi: 10.1056/NEJMp2015897.
15. Druss BG. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatry. 2020;77(9):891-892.
16. Rao S, Raney L, Xiong GL. Reducing medical comorbidity and mortality in severe mental illness. Current Psychiatry. 2015;14(7):14-20.
17. Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827-1835.
18. American Medical Association. Ethical use of quarantine and isolation. Code of Ethics Opinion 8.4. https://www.ama-assn.org/delivering-care/ethics/ethical-use-quarantine-isolation. Published November 14, 2016. Accessed July 12, 2020.
19. Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;4(4):CD013574.
20. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Duration of isolation & precautions for adults. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Updated August 16, 2020. Accessed August 21, 2020.
21. American College of Gynecologists. Novel coronavirus 2019 (COVID-19). https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019. Updated August 12, 2020. Accessed August 26, 2020.
22. American College of Physicians. COVID-19: an ACP physician’s guide + resources. Chapter 14 of 31. Infection control: advice for physicians. https://assets.acponline.org/coronavirus/scormcontent/#/. Updated September 3, 2020. Accessed September 9, 2020.
23. Infectious Disease Society of America. Infectious Diseases Society of America Guidelines on Infection Prevention in Patients with Suspected or Known COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/#toc-9-9. Updated April 20, 2020. Accessed August 26, 2020.
24. American College of Emergency Physicians. Joint Statement for Care of Patients with Behavioral Health Emergencies and Suspected or Confirmed COVID-19. https://www.acep.org/corona/covid-19-field-guide/special-populations/behavioral-health-patients/. Updated June 17, 2020. Accessed August 26, 2020.
25. Centers for Disease Control and Prevention. Quarantine and isolation. Legal authorities. https://www.cdc.gov/quarantine/aboutlawsregulationsquarantineisolation.html. Updated February 24, 2020. Accessed August 31, 2020.
26. Roberts A. Kentucky couple under house arrest after refusing to sign self-quarantine agreement. https://abcnews.go.com/US/kentucky-couple-house-arrest-refusing-sign-quarantine-agreement/story?id=71886479. Published July 20, 2020. Accessed July 24, 2020.
27. Satter R. To keep COVID-19 patients home, some U.S. states weigh house arrest tech. https://www.reuters.com/article/us-health-coronavirus-quarantine-tech/to-keep-covid-19-patients-home-some-us-states-weigh-house-arrest-tech-idUSKBN22J1U8. Published May 7, 2020. Accessed July 24, 2020.
28. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
29. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
30. Donaldson v O’Connor, 519, F2d 59 (5th Cir 1975).
31. Ohio Revised Code § 5122.290.
32. Shore JH. Telepsychiatry: videoconferencing in the delivery of psychiatric care. Am J Psychiatry. 2013;170(3):256-262.
33. Bostick NA, Levine MA, Sade RM. Ethical obligations of physicians participating in public health quarantine and isolation measures. Public Health Rep. 2008;123(1):3-8.
34. Upshur RE. Principles for the justification of public health intervention. Can J Public Health. 2002;93(2):101-103.
35. Barbera J, Macintyre A, Gostin L, et al. Large-scale quarantine following biological terrorism in the United States: scientific examination, logistic and legal limits, and possible consequences. JAMA. 2001;286(21):2711-2717.
36. Stanford Encyclopedia of Philosophy. Doctrine of double effect. https://plato.stanford.edu/entries/double-effect/. Revised December 24, 2018. Accessed July 12, 2020.
37. Substance Abuse and Mental Health Services Administration. Covid19: interim considerations for state psychiatric hospitals. https://www.samhsa.gov/sites/default/files/covid19-interim-considerations-for-state-psychiatric-hospitals.pdf. Updated May 8, 2020. Accessed July 24, 2020.
38. Augenstein TM, Pigeon WR, DiGiovanni SK, et al. Creating a novel inpatient psychiatric unit with integrated medical support for patients with COVID-19. N Engl J Med Catalyst. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0249. Published June 22, 2020. Accessed July 12, 2020.
39. Bojdani E, Rajagopalan A, Chen A, et al. COVID-19 pandemic: impact on psychiatric care in the United States. Psychiatry Research. 2020;289:113069. doi: 10.1016/j.psychres.2020.113069.
1. Knox DK, Holloman GH Jr. Use and avoidance of seclusion and restraint: consensus statement of the American Association for Emergency Psychiatry Project BETA Seclusion and Restraint Workgroup. West J Emerg Med. 2012;13(1):35-40.
2. Sehdev PS. The origin of quarantine. Clin Infect Dis. 2002;35(9):1071-1072.
3. 42 CFR § 482.13. Condition of participation: patient’s rights.
4. Colaizzi J. Seclusion & restraint: a historical perspective. J Psychosoc Nurs Ment Health Serv. 2005;43(2):31-37.
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
6. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). ArcGIS. Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed October 16, 2020.
7. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11.
8. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407.
9. Li L. Challenges and priorities in responding to COVID-19 in inpatient psychiatry. Psychiatr Serv. 2020;71(6):624-626.
10. Kim MJ. ‘It was a medical disaster’: the psychiatric ward that saw 100 patients diagnosed with new coronavirus. Independent. https://www.independent.co.uk/news/world/asia/coronavirus-south-korea-outbreak-hospital-patients-lockdown-a9367486.html. Published March 1, 2020. Accessed July 12, 2020.
11. Petrini C. Ethical considerations for evaluating the issue of physical restraint in psychiatry. Ann Ist Super Sanita. 2013;49(3):281-285.
12. Gessen M. Why psychiatric wards are uniquely vulnerable to the coronavirus. https://www.newyorker.com/news/news-desk/why-psychiatric-wards-are-uniquely-vulnerable-to-the-coronavirus. Published April 21, 2020. Accessed July 12, 2020.
13. Brooks SK, Webster RK, Smith, LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
14. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383(6):e38. doi: 10.1056/NEJMp2015897.
15. Druss BG. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatry. 2020;77(9):891-892.
16. Rao S, Raney L, Xiong GL. Reducing medical comorbidity and mortality in severe mental illness. Current Psychiatry. 2015;14(7):14-20.
17. Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827-1835.
18. American Medical Association. Ethical use of quarantine and isolation. Code of Ethics Opinion 8.4. https://www.ama-assn.org/delivering-care/ethics/ethical-use-quarantine-isolation. Published November 14, 2016. Accessed July 12, 2020.
19. Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;4(4):CD013574.
20. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Duration of isolation & precautions for adults. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Updated August 16, 2020. Accessed August 21, 2020.
21. American College of Gynecologists. Novel coronavirus 2019 (COVID-19). https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019. Updated August 12, 2020. Accessed August 26, 2020.
22. American College of Physicians. COVID-19: an ACP physician’s guide + resources. Chapter 14 of 31. Infection control: advice for physicians. https://assets.acponline.org/coronavirus/scormcontent/#/. Updated September 3, 2020. Accessed September 9, 2020.
23. Infectious Disease Society of America. Infectious Diseases Society of America Guidelines on Infection Prevention in Patients with Suspected or Known COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/#toc-9-9. Updated April 20, 2020. Accessed August 26, 2020.
24. American College of Emergency Physicians. Joint Statement for Care of Patients with Behavioral Health Emergencies and Suspected or Confirmed COVID-19. https://www.acep.org/corona/covid-19-field-guide/special-populations/behavioral-health-patients/. Updated June 17, 2020. Accessed August 26, 2020.
25. Centers for Disease Control and Prevention. Quarantine and isolation. Legal authorities. https://www.cdc.gov/quarantine/aboutlawsregulationsquarantineisolation.html. Updated February 24, 2020. Accessed August 31, 2020.
26. Roberts A. Kentucky couple under house arrest after refusing to sign self-quarantine agreement. https://abcnews.go.com/US/kentucky-couple-house-arrest-refusing-sign-quarantine-agreement/story?id=71886479. Published July 20, 2020. Accessed July 24, 2020.
27. Satter R. To keep COVID-19 patients home, some U.S. states weigh house arrest tech. https://www.reuters.com/article/us-health-coronavirus-quarantine-tech/to-keep-covid-19-patients-home-some-us-states-weigh-house-arrest-tech-idUSKBN22J1U8. Published May 7, 2020. Accessed July 24, 2020.
28. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
29. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
30. Donaldson v O’Connor, 519, F2d 59 (5th Cir 1975).
31. Ohio Revised Code § 5122.290.
32. Shore JH. Telepsychiatry: videoconferencing in the delivery of psychiatric care. Am J Psychiatry. 2013;170(3):256-262.
33. Bostick NA, Levine MA, Sade RM. Ethical obligations of physicians participating in public health quarantine and isolation measures. Public Health Rep. 2008;123(1):3-8.
34. Upshur RE. Principles for the justification of public health intervention. Can J Public Health. 2002;93(2):101-103.
35. Barbera J, Macintyre A, Gostin L, et al. Large-scale quarantine following biological terrorism in the United States: scientific examination, logistic and legal limits, and possible consequences. JAMA. 2001;286(21):2711-2717.
36. Stanford Encyclopedia of Philosophy. Doctrine of double effect. https://plato.stanford.edu/entries/double-effect/. Revised December 24, 2018. Accessed July 12, 2020.
37. Substance Abuse and Mental Health Services Administration. Covid19: interim considerations for state psychiatric hospitals. https://www.samhsa.gov/sites/default/files/covid19-interim-considerations-for-state-psychiatric-hospitals.pdf. Updated May 8, 2020. Accessed July 24, 2020.
38. Augenstein TM, Pigeon WR, DiGiovanni SK, et al. Creating a novel inpatient psychiatric unit with integrated medical support for patients with COVID-19. N Engl J Med Catalyst. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0249. Published June 22, 2020. Accessed July 12, 2020.
39. Bojdani E, Rajagopalan A, Chen A, et al. COVID-19 pandemic: impact on psychiatric care in the United States. Psychiatry Research. 2020;289:113069. doi: 10.1016/j.psychres.2020.113069.
Pharmacogenetic testing: 5 Questions
When selecting a psychotropic medication for a patient with a challenging illness, you may want to consider ordering pharmacogenetic testing. By characterizing how a patient’s genetic profile affects their medication metabolism, pharmacogenetic testing can potentially help improve medication adherence, reduce “trial-and-error” prescribing, and target an effective treatment. Here we address 5 important questions about using pharmacogenetic testing.
1. What can pharmacogenetic testing tell you? Pharmacogenetic testing looks for variants in genes that can affect how a patient metabolizes specific medications. While the results will not inform you about a specific medication’s effectiveness, they can describe the patient’s tolerability of that medication based on his/her metabolism. Most psychotropic medications are biotransformed in the liver by cytochrome P450 (CYP) through pathway enzymes such as 2D6, 2C19, and 3A4. For example, fluoxetine and paroxetine exert their inhibition on CYP2D6, while other psychotropic medications, such as lurasidone, are metabolized at CYP3A4 and are contraindicated with potent CYP3A4 inhibitors (eg, grapefruit juice).1
In addition to CYP450 enzymes, pharmacogenetic testing can assess for the serotonin transporter gene, SLC6A4, and its sequence promoter variant, 5-HTTLPR. This sequence variation influences response to selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tertiary amine tricyclic antidepressants.2 Pharmacogenetic testing also looks for genes related to Stevens-Johnson syndrome, such as HLA-B*1502, which is associated with adverse effects of carbamazepine and lamotrigine.
2. When should you order pharmacogenetic testing? Not all patients require pharmacogenetic testing. Anxious patients who have had multiple unsuccessful medication trials may be good candidates for testing. Consider testing for patients with a history of sensitivity to medications, or whose family members have experienced unusual drug responses.2
3. What steps should you take before ordering pharmacogenetic testing? First, obtain your patient’s informed consent, because clinical testing reveals personal genetic information. Make sure your patient understands that such testing is voluntary and that he/she can opt out. Also, explain that the information obtained from pharmacogenetic testing is confidential and will become part of the patient’s medical record.
Second, choose the best test for your patient’s needs. Pharmacogenetic tests can assess for single genes encoded for selected CYP450 enzymes based on pharmacokinetics (metabolism), or for multiple genes based on pharmacodynamic (mechanism of action) factors.5 In a recent randomized controlled trial, Bradley et al6 found that testing for multiple genes improved response and remission rates in patients with depression and/or anxiety.
Third, confirm that your patient’s insurance covers pharmacogenetic testing, because this testing can be expensive, although some genetic testing companies may offer patients financial assistance.
Continue to: How are samples taken?
4. How are samples taken? Several methods are used for obtaining samples, including saliva, buccal swab, and peripheral blood. Your patient should not smoke, eat, or drink for at least 30 minutes before providing a saliva sample. For a buccal swab, a cotton swab is rubbed in a circular motion along the oral lining inside each of the patient’s cheeks. The most invasive method is peripheral blood obtained via venipuncture. The sample is sent through expedited mail to an accredited genetic processing laboratory for analysis.
5. How do you interpret the results? Pharmacogenetic testing results are provided in a confidential report. A single gene report allows you to choose psychotropic agents based on pharmacokinetics.5 Some laboratories assess multiple genes in a single report, and create categories of medications (such as “use as directed” or “use with caution”) based on the pharmacodynamic factors of each agent.5,6 Certain laboratories offer dosing guidelines for types of medications that you should use with caution.1,5,6
When interpreting such testing results, it is critical to use your clinical judgment, because pharmacogenetic testing alone does not assess whether a medication will help improve the patient’s symptoms. It is of utmost importance that you have an understanding of pharmacodynamics, knowledge of the patient’s diet and age, and a caring doctor–patient relationship.
1. Madhusoodanan S, Velama U, Parmar J, et al. A current review of cytochrome P450 interactions of psychotropic drugs. Ann Clin Psychiatry. 2014;26(2):120-138.
2. Mrazek DA. Psychiatric pharmacogenomic testing in clinical practice. Dialogues Clin Neurosci. 2010;12(1):69-76.
3. Drozda K, Müller DJ, Bishop JR. Pharmacogenomic testing for neuropsychiatric drugs: current status of drug labeling, guidelines for using genetic information, and test options. Pharmacotherapy. 2014;34(2):166-184.
4. Shelton RC, Sloan Manning J, Barrentine LW, et al. Assessing effects of l-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):PCC.13m01520. doi: 10.4088/PCC.13m01520.
5. Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded randomized, controlled study. J Psychiatr Res. 2019;111:59-67.
6. Bradley P, Shiekh M, Mehra V, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psych Res. 2018;96:100-107.
When selecting a psychotropic medication for a patient with a challenging illness, you may want to consider ordering pharmacogenetic testing. By characterizing how a patient’s genetic profile affects their medication metabolism, pharmacogenetic testing can potentially help improve medication adherence, reduce “trial-and-error” prescribing, and target an effective treatment. Here we address 5 important questions about using pharmacogenetic testing.
1. What can pharmacogenetic testing tell you? Pharmacogenetic testing looks for variants in genes that can affect how a patient metabolizes specific medications. While the results will not inform you about a specific medication’s effectiveness, they can describe the patient’s tolerability of that medication based on his/her metabolism. Most psychotropic medications are biotransformed in the liver by cytochrome P450 (CYP) through pathway enzymes such as 2D6, 2C19, and 3A4. For example, fluoxetine and paroxetine exert their inhibition on CYP2D6, while other psychotropic medications, such as lurasidone, are metabolized at CYP3A4 and are contraindicated with potent CYP3A4 inhibitors (eg, grapefruit juice).1
In addition to CYP450 enzymes, pharmacogenetic testing can assess for the serotonin transporter gene, SLC6A4, and its sequence promoter variant, 5-HTTLPR. This sequence variation influences response to selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tertiary amine tricyclic antidepressants.2 Pharmacogenetic testing also looks for genes related to Stevens-Johnson syndrome, such as HLA-B*1502, which is associated with adverse effects of carbamazepine and lamotrigine.
2. When should you order pharmacogenetic testing? Not all patients require pharmacogenetic testing. Anxious patients who have had multiple unsuccessful medication trials may be good candidates for testing. Consider testing for patients with a history of sensitivity to medications, or whose family members have experienced unusual drug responses.2
3. What steps should you take before ordering pharmacogenetic testing? First, obtain your patient’s informed consent, because clinical testing reveals personal genetic information. Make sure your patient understands that such testing is voluntary and that he/she can opt out. Also, explain that the information obtained from pharmacogenetic testing is confidential and will become part of the patient’s medical record.
Second, choose the best test for your patient’s needs. Pharmacogenetic tests can assess for single genes encoded for selected CYP450 enzymes based on pharmacokinetics (metabolism), or for multiple genes based on pharmacodynamic (mechanism of action) factors.5 In a recent randomized controlled trial, Bradley et al6 found that testing for multiple genes improved response and remission rates in patients with depression and/or anxiety.
Third, confirm that your patient’s insurance covers pharmacogenetic testing, because this testing can be expensive, although some genetic testing companies may offer patients financial assistance.
Continue to: How are samples taken?
4. How are samples taken? Several methods are used for obtaining samples, including saliva, buccal swab, and peripheral blood. Your patient should not smoke, eat, or drink for at least 30 minutes before providing a saliva sample. For a buccal swab, a cotton swab is rubbed in a circular motion along the oral lining inside each of the patient’s cheeks. The most invasive method is peripheral blood obtained via venipuncture. The sample is sent through expedited mail to an accredited genetic processing laboratory for analysis.
5. How do you interpret the results? Pharmacogenetic testing results are provided in a confidential report. A single gene report allows you to choose psychotropic agents based on pharmacokinetics.5 Some laboratories assess multiple genes in a single report, and create categories of medications (such as “use as directed” or “use with caution”) based on the pharmacodynamic factors of each agent.5,6 Certain laboratories offer dosing guidelines for types of medications that you should use with caution.1,5,6
When interpreting such testing results, it is critical to use your clinical judgment, because pharmacogenetic testing alone does not assess whether a medication will help improve the patient’s symptoms. It is of utmost importance that you have an understanding of pharmacodynamics, knowledge of the patient’s diet and age, and a caring doctor–patient relationship.
When selecting a psychotropic medication for a patient with a challenging illness, you may want to consider ordering pharmacogenetic testing. By characterizing how a patient’s genetic profile affects their medication metabolism, pharmacogenetic testing can potentially help improve medication adherence, reduce “trial-and-error” prescribing, and target an effective treatment. Here we address 5 important questions about using pharmacogenetic testing.
1. What can pharmacogenetic testing tell you? Pharmacogenetic testing looks for variants in genes that can affect how a patient metabolizes specific medications. While the results will not inform you about a specific medication’s effectiveness, they can describe the patient’s tolerability of that medication based on his/her metabolism. Most psychotropic medications are biotransformed in the liver by cytochrome P450 (CYP) through pathway enzymes such as 2D6, 2C19, and 3A4. For example, fluoxetine and paroxetine exert their inhibition on CYP2D6, while other psychotropic medications, such as lurasidone, are metabolized at CYP3A4 and are contraindicated with potent CYP3A4 inhibitors (eg, grapefruit juice).1
In addition to CYP450 enzymes, pharmacogenetic testing can assess for the serotonin transporter gene, SLC6A4, and its sequence promoter variant, 5-HTTLPR. This sequence variation influences response to selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tertiary amine tricyclic antidepressants.2 Pharmacogenetic testing also looks for genes related to Stevens-Johnson syndrome, such as HLA-B*1502, which is associated with adverse effects of carbamazepine and lamotrigine.
2. When should you order pharmacogenetic testing? Not all patients require pharmacogenetic testing. Anxious patients who have had multiple unsuccessful medication trials may be good candidates for testing. Consider testing for patients with a history of sensitivity to medications, or whose family members have experienced unusual drug responses.2
3. What steps should you take before ordering pharmacogenetic testing? First, obtain your patient’s informed consent, because clinical testing reveals personal genetic information. Make sure your patient understands that such testing is voluntary and that he/she can opt out. Also, explain that the information obtained from pharmacogenetic testing is confidential and will become part of the patient’s medical record.
Second, choose the best test for your patient’s needs. Pharmacogenetic tests can assess for single genes encoded for selected CYP450 enzymes based on pharmacokinetics (metabolism), or for multiple genes based on pharmacodynamic (mechanism of action) factors.5 In a recent randomized controlled trial, Bradley et al6 found that testing for multiple genes improved response and remission rates in patients with depression and/or anxiety.
Third, confirm that your patient’s insurance covers pharmacogenetic testing, because this testing can be expensive, although some genetic testing companies may offer patients financial assistance.
Continue to: How are samples taken?
4. How are samples taken? Several methods are used for obtaining samples, including saliva, buccal swab, and peripheral blood. Your patient should not smoke, eat, or drink for at least 30 minutes before providing a saliva sample. For a buccal swab, a cotton swab is rubbed in a circular motion along the oral lining inside each of the patient’s cheeks. The most invasive method is peripheral blood obtained via venipuncture. The sample is sent through expedited mail to an accredited genetic processing laboratory for analysis.
5. How do you interpret the results? Pharmacogenetic testing results are provided in a confidential report. A single gene report allows you to choose psychotropic agents based on pharmacokinetics.5 Some laboratories assess multiple genes in a single report, and create categories of medications (such as “use as directed” or “use with caution”) based on the pharmacodynamic factors of each agent.5,6 Certain laboratories offer dosing guidelines for types of medications that you should use with caution.1,5,6
When interpreting such testing results, it is critical to use your clinical judgment, because pharmacogenetic testing alone does not assess whether a medication will help improve the patient’s symptoms. It is of utmost importance that you have an understanding of pharmacodynamics, knowledge of the patient’s diet and age, and a caring doctor–patient relationship.
1. Madhusoodanan S, Velama U, Parmar J, et al. A current review of cytochrome P450 interactions of psychotropic drugs. Ann Clin Psychiatry. 2014;26(2):120-138.
2. Mrazek DA. Psychiatric pharmacogenomic testing in clinical practice. Dialogues Clin Neurosci. 2010;12(1):69-76.
3. Drozda K, Müller DJ, Bishop JR. Pharmacogenomic testing for neuropsychiatric drugs: current status of drug labeling, guidelines for using genetic information, and test options. Pharmacotherapy. 2014;34(2):166-184.
4. Shelton RC, Sloan Manning J, Barrentine LW, et al. Assessing effects of l-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):PCC.13m01520. doi: 10.4088/PCC.13m01520.
5. Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded randomized, controlled study. J Psychiatr Res. 2019;111:59-67.
6. Bradley P, Shiekh M, Mehra V, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psych Res. 2018;96:100-107.
1. Madhusoodanan S, Velama U, Parmar J, et al. A current review of cytochrome P450 interactions of psychotropic drugs. Ann Clin Psychiatry. 2014;26(2):120-138.
2. Mrazek DA. Psychiatric pharmacogenomic testing in clinical practice. Dialogues Clin Neurosci. 2010;12(1):69-76.
3. Drozda K, Müller DJ, Bishop JR. Pharmacogenomic testing for neuropsychiatric drugs: current status of drug labeling, guidelines for using genetic information, and test options. Pharmacotherapy. 2014;34(2):166-184.
4. Shelton RC, Sloan Manning J, Barrentine LW, et al. Assessing effects of l-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):PCC.13m01520. doi: 10.4088/PCC.13m01520.
5. Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded randomized, controlled study. J Psychiatr Res. 2019;111:59-67.
6. Bradley P, Shiekh M, Mehra V, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psych Res. 2018;96:100-107.
Lemborexant for insomnia
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.
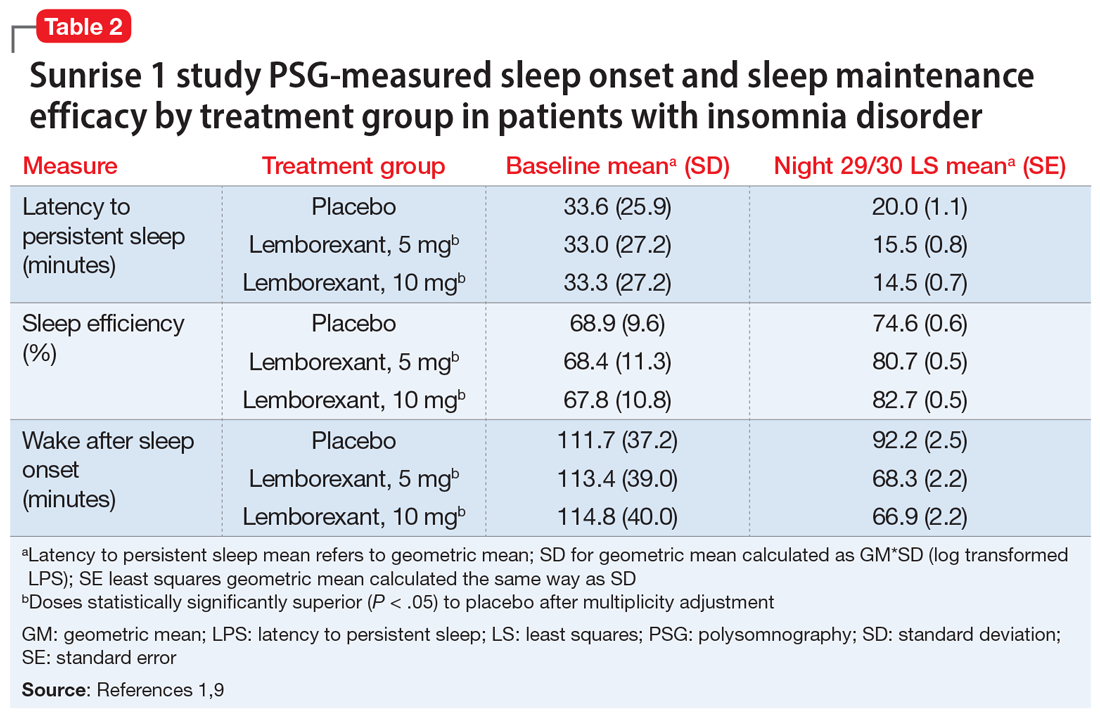
In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.
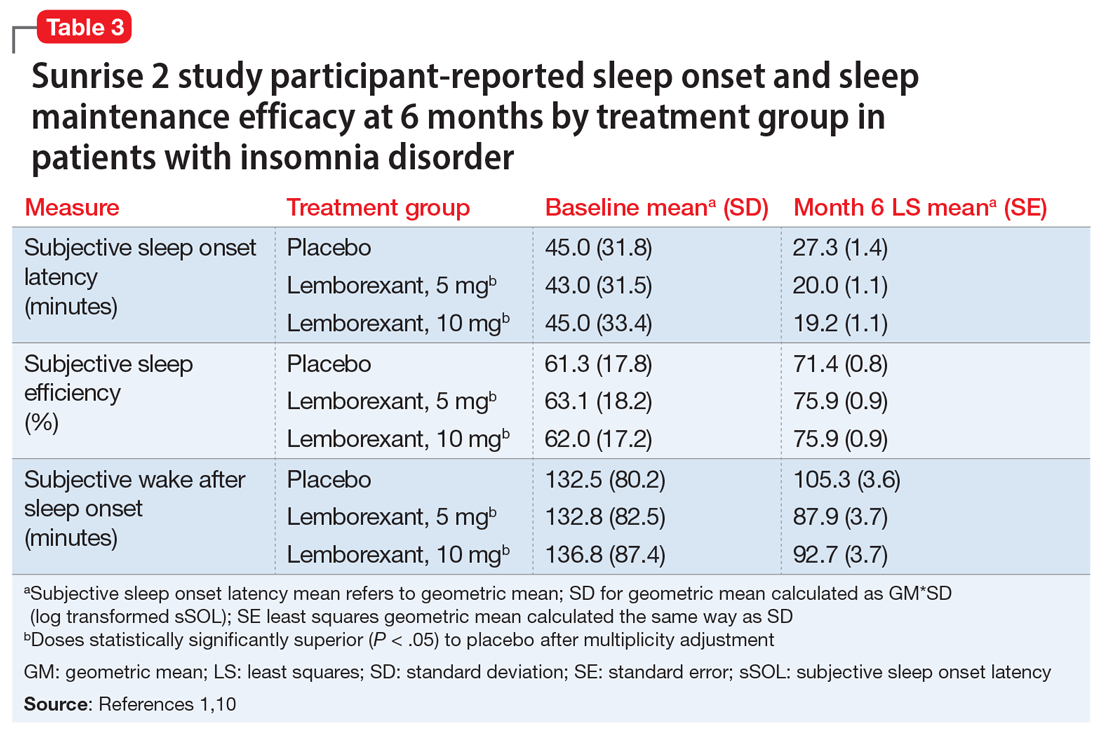
Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.

In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.

Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.

In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.

Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.