User login
How to have a safer and more joyful holiday season
This holiday season, I am looking forward to spending some time with family, as I have in the past. As I have chatted with others, many friends are looking forward to events that are potentially larger and potentially returning to prepandemic type gatherings.
Gathering is important and can bring joy, sense of community, and love to the lives of many. Unfortunately, the risks associated with gathering are not over. as our country faces many cases of respiratory syncytial virus (RSV), COVID-19, and influenza at the same time.
During the first week of December, cases of influenza were rising across the country1 and were rising faster than in previous years. Although getting the vaccine is an important method of influenza prevention and is recommended for everyone over the age of 6 months with rare exception, many have not gotten their vaccine this year.
Influenza
Thus far, “nearly 50% of reported flu-associated hospitalizations in women of childbearing age have been in women who are pregnant.” We are seeing this at a time with lower-than-average uptake of influenza vaccine leaving both the pregnant persons and their babies unprotected. In addition to utilizing vaccines as prevention, isolating when ill, cleaning surfaces, and practicing good hand hygiene can all decrease transmission.
RSV
In addition to rises of influenza, there are currently high rates of RSV in various parts of the country. Prior to 2020, RSV typically started in the fall and peaked in the winter months. However, since the pandemic, the typical seasonal pattern has not returned, and it is unclear when it will. Although RSV hits the very young, the old, and the immunocompromised the most, RSV can infect anyone. Unfortunately, we do not currently have a vaccine for everyone against this virus. Prevention of transmission includes, as with flu, isolating when ill, cleaning surfaces, and washing hands.2
COVID-19
Of course, the effects of the COVID-19 pandemic are also still here as well. During the first week of December, the CDC reported rising cases of COVID across the country. Within the past few months, there have been several developments, though, for protection. There are now bivalent vaccines available as either third doses or booster doses approved for all persons over 6 months of age. As of the first week of December, only 13.5% of those aged 5 and over had received an updated booster.
There is currently wider access to rapid testing, including at-home testing, which can allow individuals to identify if COVID positive. Additionally, there is access to medication to decrease the likelihood of severe disease – though this does not take the place of vaccinations.
If anyone does test positive for COVID, they should follow the most recent quarantine guidelines including wearing a well-fitted mask when they do begin returning to activities.3
With rising cases of all three of these viruses, some may be asking how we can safely gather. There are several things to consider and do to enjoy our events. The first thing everyone can do is to receive updated vaccinations for both influenza and COVID-19 if eligible. Although it may take some time to be effective, vaccination is still one of our most effective methods of disease prevention and is important this winter season. Vaccinations can also help decrease the risk of severe disease.
Although many have stopped masking, as cases rise, it is time to consider masking particularly when community levels of any of these viruses are high. Masks help with preventing and spreading more than just COVID-19. Using them can be especially important for those going places such as stores and to large public gatherings and when riding on buses, planes, or trains.
In summary
Preventing exposure by masking can help keep individuals healthy prior to celebrating the holidays with others. With access to rapid testing, it makes sense to consider testing prior to gathering with friends and family. Most importantly, although we all are looking forward to spending time with our loved ones, it is important to stay home if not feeling well. Following these recommendations will allow us to have a safer and more joyful holiday season.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Influenza (flu). [Online] Dec. 1, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/flu/index.htm.
2. Respiratory syncytial virus. Respiratory syncytial virus infection (RSV). [Online] Oct. 28, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/rsv/index.html.
3. COVID-19. [Online] Dec. 7, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/coronavirus/2019-ncov/index.html.
This holiday season, I am looking forward to spending some time with family, as I have in the past. As I have chatted with others, many friends are looking forward to events that are potentially larger and potentially returning to prepandemic type gatherings.
Gathering is important and can bring joy, sense of community, and love to the lives of many. Unfortunately, the risks associated with gathering are not over. as our country faces many cases of respiratory syncytial virus (RSV), COVID-19, and influenza at the same time.
During the first week of December, cases of influenza were rising across the country1 and were rising faster than in previous years. Although getting the vaccine is an important method of influenza prevention and is recommended for everyone over the age of 6 months with rare exception, many have not gotten their vaccine this year.
Influenza
Thus far, “nearly 50% of reported flu-associated hospitalizations in women of childbearing age have been in women who are pregnant.” We are seeing this at a time with lower-than-average uptake of influenza vaccine leaving both the pregnant persons and their babies unprotected. In addition to utilizing vaccines as prevention, isolating when ill, cleaning surfaces, and practicing good hand hygiene can all decrease transmission.
RSV
In addition to rises of influenza, there are currently high rates of RSV in various parts of the country. Prior to 2020, RSV typically started in the fall and peaked in the winter months. However, since the pandemic, the typical seasonal pattern has not returned, and it is unclear when it will. Although RSV hits the very young, the old, and the immunocompromised the most, RSV can infect anyone. Unfortunately, we do not currently have a vaccine for everyone against this virus. Prevention of transmission includes, as with flu, isolating when ill, cleaning surfaces, and washing hands.2
COVID-19
Of course, the effects of the COVID-19 pandemic are also still here as well. During the first week of December, the CDC reported rising cases of COVID across the country. Within the past few months, there have been several developments, though, for protection. There are now bivalent vaccines available as either third doses or booster doses approved for all persons over 6 months of age. As of the first week of December, only 13.5% of those aged 5 and over had received an updated booster.
There is currently wider access to rapid testing, including at-home testing, which can allow individuals to identify if COVID positive. Additionally, there is access to medication to decrease the likelihood of severe disease – though this does not take the place of vaccinations.
If anyone does test positive for COVID, they should follow the most recent quarantine guidelines including wearing a well-fitted mask when they do begin returning to activities.3
With rising cases of all three of these viruses, some may be asking how we can safely gather. There are several things to consider and do to enjoy our events. The first thing everyone can do is to receive updated vaccinations for both influenza and COVID-19 if eligible. Although it may take some time to be effective, vaccination is still one of our most effective methods of disease prevention and is important this winter season. Vaccinations can also help decrease the risk of severe disease.
Although many have stopped masking, as cases rise, it is time to consider masking particularly when community levels of any of these viruses are high. Masks help with preventing and spreading more than just COVID-19. Using them can be especially important for those going places such as stores and to large public gatherings and when riding on buses, planes, or trains.
In summary
Preventing exposure by masking can help keep individuals healthy prior to celebrating the holidays with others. With access to rapid testing, it makes sense to consider testing prior to gathering with friends and family. Most importantly, although we all are looking forward to spending time with our loved ones, it is important to stay home if not feeling well. Following these recommendations will allow us to have a safer and more joyful holiday season.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Influenza (flu). [Online] Dec. 1, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/flu/index.htm.
2. Respiratory syncytial virus. Respiratory syncytial virus infection (RSV). [Online] Oct. 28, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/rsv/index.html.
3. COVID-19. [Online] Dec. 7, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/coronavirus/2019-ncov/index.html.
This holiday season, I am looking forward to spending some time with family, as I have in the past. As I have chatted with others, many friends are looking forward to events that are potentially larger and potentially returning to prepandemic type gatherings.
Gathering is important and can bring joy, sense of community, and love to the lives of many. Unfortunately, the risks associated with gathering are not over. as our country faces many cases of respiratory syncytial virus (RSV), COVID-19, and influenza at the same time.
During the first week of December, cases of influenza were rising across the country1 and were rising faster than in previous years. Although getting the vaccine is an important method of influenza prevention and is recommended for everyone over the age of 6 months with rare exception, many have not gotten their vaccine this year.
Influenza
Thus far, “nearly 50% of reported flu-associated hospitalizations in women of childbearing age have been in women who are pregnant.” We are seeing this at a time with lower-than-average uptake of influenza vaccine leaving both the pregnant persons and their babies unprotected. In addition to utilizing vaccines as prevention, isolating when ill, cleaning surfaces, and practicing good hand hygiene can all decrease transmission.
RSV
In addition to rises of influenza, there are currently high rates of RSV in various parts of the country. Prior to 2020, RSV typically started in the fall and peaked in the winter months. However, since the pandemic, the typical seasonal pattern has not returned, and it is unclear when it will. Although RSV hits the very young, the old, and the immunocompromised the most, RSV can infect anyone. Unfortunately, we do not currently have a vaccine for everyone against this virus. Prevention of transmission includes, as with flu, isolating when ill, cleaning surfaces, and washing hands.2
COVID-19
Of course, the effects of the COVID-19 pandemic are also still here as well. During the first week of December, the CDC reported rising cases of COVID across the country. Within the past few months, there have been several developments, though, for protection. There are now bivalent vaccines available as either third doses or booster doses approved for all persons over 6 months of age. As of the first week of December, only 13.5% of those aged 5 and over had received an updated booster.
There is currently wider access to rapid testing, including at-home testing, which can allow individuals to identify if COVID positive. Additionally, there is access to medication to decrease the likelihood of severe disease – though this does not take the place of vaccinations.
If anyone does test positive for COVID, they should follow the most recent quarantine guidelines including wearing a well-fitted mask when they do begin returning to activities.3
With rising cases of all three of these viruses, some may be asking how we can safely gather. There are several things to consider and do to enjoy our events. The first thing everyone can do is to receive updated vaccinations for both influenza and COVID-19 if eligible. Although it may take some time to be effective, vaccination is still one of our most effective methods of disease prevention and is important this winter season. Vaccinations can also help decrease the risk of severe disease.
Although many have stopped masking, as cases rise, it is time to consider masking particularly when community levels of any of these viruses are high. Masks help with preventing and spreading more than just COVID-19. Using them can be especially important for those going places such as stores and to large public gatherings and when riding on buses, planes, or trains.
In summary
Preventing exposure by masking can help keep individuals healthy prior to celebrating the holidays with others. With access to rapid testing, it makes sense to consider testing prior to gathering with friends and family. Most importantly, although we all are looking forward to spending time with our loved ones, it is important to stay home if not feeling well. Following these recommendations will allow us to have a safer and more joyful holiday season.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Influenza (flu). [Online] Dec. 1, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/flu/index.htm.
2. Respiratory syncytial virus. Respiratory syncytial virus infection (RSV). [Online] Oct. 28, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/rsv/index.html.
3. COVID-19. [Online] Dec. 7, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/coronavirus/2019-ncov/index.html.
The dark side of online mom groups
I have assumed that being a parent has always been an anxiety-producing experience. Even back when the neonatal mortality rate was orders of magnitude greater than we are experiencing now, I suspect that each birth was still accompanied by a period of angst. However, as families no longer felt the need to produce more children to replace those lost to illness, each surviving child fell under the glare of an ever brightening spotlight.
Raising a child no longer became just something that came naturally, learned from one’s parents. Philosophers and eventually physicians felt obligated to advise parents on the best practices. My parents turned to Dr. Benjamin Spock’s classic work when they had a question, but I never got the feeling that they took his words as gospel.
By the time I started in practice the condition of being a parent was morphing into a verb. Books on “parenting” were beginning to fill the shelves of libraries and bookstores. Frustrated by what I saw as poorly conceived instruction manuals I succumbed to the temptation to spread my “better” advice for anxiety-tormented parents by writing books on how to feed picky eaters, or how to get erratic sleepers to sleep, or how to get a misbehaving child to understand the simple concept of “No!”
Back in the pre-Internet days I was competing for the attention of anxiety-driven parents not just with other self-described experts sitting at word processors, but with grandmothers, aunts, and the ladies next door. The book publishing market has cooled but the demand for advice on how to be the best parent has heated up. Into the void, enabled by the Internet, has erupted the phenomenon of social-media mom groups.
The lady next door and the mothers with strollers meeting informally at the playground are a tiny blip on the radar screen compared with the abundance of other mothers eager to listen and comment on social media–based mom groups unlimited by either geographic or temporal time restraints.
Unfortunately, as a recent article in the Wall Street Journal suggests, these support groups can often have a dark side. Researchers from Pepperdine University found in a small survey of a homogenous population of women that stress, as measured by saliva cortisol levels, increased with increasing use of “mom-centric social media” sites.
Citing anecdotal observations by mothers who did not participate in the study, the WSJ article describes episodes of shaming over topics such as steroid use in eczema and vaccine hesitancy. One mother described how she found group discussions about breastfeeding “particularly anxiety-producing.”
I have limited experience with online support groups but I have been surprised by how rude and condescending some of the contributors can be to what I could consider to be emotionally neutral subjects such as outboard motor oil pressure. I can imagine that when it comes to subjects in which there is no one best answer, the relative anonymity of the Internet provides cover for language that can be hurtful and stress inducing for someone already feeling isolated and anxious about being a parent.
Although this Pepperdine study is small, I suspect that a larger study would support the authors’ observations. For us as providers, it suggests that we need to find where parents are getting their information when we are trying to help those who seem particularly distressed. We should caution them that, while sharing information with peers can be reassuring and helpful at times, mom groups can be toxic as well. It also means that we should be careful in recommending social media sites – even those for which we have had good feedback.
And, most importantly, we must continue to work hard to make ourselves available to provide sensible and sensitive answers to those questions that are anxiety-producing for new parents.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I have assumed that being a parent has always been an anxiety-producing experience. Even back when the neonatal mortality rate was orders of magnitude greater than we are experiencing now, I suspect that each birth was still accompanied by a period of angst. However, as families no longer felt the need to produce more children to replace those lost to illness, each surviving child fell under the glare of an ever brightening spotlight.
Raising a child no longer became just something that came naturally, learned from one’s parents. Philosophers and eventually physicians felt obligated to advise parents on the best practices. My parents turned to Dr. Benjamin Spock’s classic work when they had a question, but I never got the feeling that they took his words as gospel.
By the time I started in practice the condition of being a parent was morphing into a verb. Books on “parenting” were beginning to fill the shelves of libraries and bookstores. Frustrated by what I saw as poorly conceived instruction manuals I succumbed to the temptation to spread my “better” advice for anxiety-tormented parents by writing books on how to feed picky eaters, or how to get erratic sleepers to sleep, or how to get a misbehaving child to understand the simple concept of “No!”
Back in the pre-Internet days I was competing for the attention of anxiety-driven parents not just with other self-described experts sitting at word processors, but with grandmothers, aunts, and the ladies next door. The book publishing market has cooled but the demand for advice on how to be the best parent has heated up. Into the void, enabled by the Internet, has erupted the phenomenon of social-media mom groups.
The lady next door and the mothers with strollers meeting informally at the playground are a tiny blip on the radar screen compared with the abundance of other mothers eager to listen and comment on social media–based mom groups unlimited by either geographic or temporal time restraints.
Unfortunately, as a recent article in the Wall Street Journal suggests, these support groups can often have a dark side. Researchers from Pepperdine University found in a small survey of a homogenous population of women that stress, as measured by saliva cortisol levels, increased with increasing use of “mom-centric social media” sites.
Citing anecdotal observations by mothers who did not participate in the study, the WSJ article describes episodes of shaming over topics such as steroid use in eczema and vaccine hesitancy. One mother described how she found group discussions about breastfeeding “particularly anxiety-producing.”
I have limited experience with online support groups but I have been surprised by how rude and condescending some of the contributors can be to what I could consider to be emotionally neutral subjects such as outboard motor oil pressure. I can imagine that when it comes to subjects in which there is no one best answer, the relative anonymity of the Internet provides cover for language that can be hurtful and stress inducing for someone already feeling isolated and anxious about being a parent.
Although this Pepperdine study is small, I suspect that a larger study would support the authors’ observations. For us as providers, it suggests that we need to find where parents are getting their information when we are trying to help those who seem particularly distressed. We should caution them that, while sharing information with peers can be reassuring and helpful at times, mom groups can be toxic as well. It also means that we should be careful in recommending social media sites – even those for which we have had good feedback.
And, most importantly, we must continue to work hard to make ourselves available to provide sensible and sensitive answers to those questions that are anxiety-producing for new parents.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I have assumed that being a parent has always been an anxiety-producing experience. Even back when the neonatal mortality rate was orders of magnitude greater than we are experiencing now, I suspect that each birth was still accompanied by a period of angst. However, as families no longer felt the need to produce more children to replace those lost to illness, each surviving child fell under the glare of an ever brightening spotlight.
Raising a child no longer became just something that came naturally, learned from one’s parents. Philosophers and eventually physicians felt obligated to advise parents on the best practices. My parents turned to Dr. Benjamin Spock’s classic work when they had a question, but I never got the feeling that they took his words as gospel.
By the time I started in practice the condition of being a parent was morphing into a verb. Books on “parenting” were beginning to fill the shelves of libraries and bookstores. Frustrated by what I saw as poorly conceived instruction manuals I succumbed to the temptation to spread my “better” advice for anxiety-tormented parents by writing books on how to feed picky eaters, or how to get erratic sleepers to sleep, or how to get a misbehaving child to understand the simple concept of “No!”
Back in the pre-Internet days I was competing for the attention of anxiety-driven parents not just with other self-described experts sitting at word processors, but with grandmothers, aunts, and the ladies next door. The book publishing market has cooled but the demand for advice on how to be the best parent has heated up. Into the void, enabled by the Internet, has erupted the phenomenon of social-media mom groups.
The lady next door and the mothers with strollers meeting informally at the playground are a tiny blip on the radar screen compared with the abundance of other mothers eager to listen and comment on social media–based mom groups unlimited by either geographic or temporal time restraints.
Unfortunately, as a recent article in the Wall Street Journal suggests, these support groups can often have a dark side. Researchers from Pepperdine University found in a small survey of a homogenous population of women that stress, as measured by saliva cortisol levels, increased with increasing use of “mom-centric social media” sites.
Citing anecdotal observations by mothers who did not participate in the study, the WSJ article describes episodes of shaming over topics such as steroid use in eczema and vaccine hesitancy. One mother described how she found group discussions about breastfeeding “particularly anxiety-producing.”
I have limited experience with online support groups but I have been surprised by how rude and condescending some of the contributors can be to what I could consider to be emotionally neutral subjects such as outboard motor oil pressure. I can imagine that when it comes to subjects in which there is no one best answer, the relative anonymity of the Internet provides cover for language that can be hurtful and stress inducing for someone already feeling isolated and anxious about being a parent.
Although this Pepperdine study is small, I suspect that a larger study would support the authors’ observations. For us as providers, it suggests that we need to find where parents are getting their information when we are trying to help those who seem particularly distressed. We should caution them that, while sharing information with peers can be reassuring and helpful at times, mom groups can be toxic as well. It also means that we should be careful in recommending social media sites – even those for which we have had good feedback.
And, most importantly, we must continue to work hard to make ourselves available to provide sensible and sensitive answers to those questions that are anxiety-producing for new parents.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Grateful and hopeful
My year is now over. My staff and I started the habit of closing down mid-December in 2013, when we realized that patients generally didn’t want to come in then, either.
To me a year really ends the day we close up for the holidays. I put away the season’s decorations, send the final batch to my billing company, and lock the door. Not much of a New Year’s, but at my age it’s not a holiday I mark, anyway. It’s more a relief that my office year, at least, is done.
So it’s always a time for reflection, between the more mundane work of returning calls, reviewing the tests that come in, and getting taxes ready. I try to relax as much as I can (given the weird state of our times, I haven’t left town since November 2019, so this is my vacation for now).
Plus, my kids all come home. I have no idea how much longer that’s going to happen, so I’ll enjoy it while I can.
It’s now almost 3 years since I last rounded at a hospital, and I can’t say I miss it. While I usually have plenty to do on my breaks and weekends, and the occasional patient call to return, it’s nice to know that I can stay in my robe, PJs, and slippers through it all.
2022 certainly wasn’t bad for my family and me, though not as good as any of us hoped. The world, already battered by the pandemic, was thrown into greater uncertainty by the war in Europe and its ramifications across the globe. In comparison, I’m very grateful that higher prices are the extent of my suffering as compared with what the people of Ukraine are going through.
But, at the end of it all, my little practice and two wonderful staff are still here, just as we’ve been since 2000. My kids will (hopefully) all be through college by the end of 2023 and moving on with their lives. I love them, and will miss them if they move away, but part of being a parent is accepting that your kids are only visitors and have their own paths to follow.
For my staff I’m glad they’ve stuck with me through good and bad times, and that we still have fun together – even when we haven’t worked under the same roof in a while.
For my patients and their families we’ve seen a few glimmers of optimism in treatments and hopefully they’ll continue to grow and be built upon. Heaven knows my field – and many others – can use them.
And so,
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My year is now over. My staff and I started the habit of closing down mid-December in 2013, when we realized that patients generally didn’t want to come in then, either.
To me a year really ends the day we close up for the holidays. I put away the season’s decorations, send the final batch to my billing company, and lock the door. Not much of a New Year’s, but at my age it’s not a holiday I mark, anyway. It’s more a relief that my office year, at least, is done.
So it’s always a time for reflection, between the more mundane work of returning calls, reviewing the tests that come in, and getting taxes ready. I try to relax as much as I can (given the weird state of our times, I haven’t left town since November 2019, so this is my vacation for now).
Plus, my kids all come home. I have no idea how much longer that’s going to happen, so I’ll enjoy it while I can.
It’s now almost 3 years since I last rounded at a hospital, and I can’t say I miss it. While I usually have plenty to do on my breaks and weekends, and the occasional patient call to return, it’s nice to know that I can stay in my robe, PJs, and slippers through it all.
2022 certainly wasn’t bad for my family and me, though not as good as any of us hoped. The world, already battered by the pandemic, was thrown into greater uncertainty by the war in Europe and its ramifications across the globe. In comparison, I’m very grateful that higher prices are the extent of my suffering as compared with what the people of Ukraine are going through.
But, at the end of it all, my little practice and two wonderful staff are still here, just as we’ve been since 2000. My kids will (hopefully) all be through college by the end of 2023 and moving on with their lives. I love them, and will miss them if they move away, but part of being a parent is accepting that your kids are only visitors and have their own paths to follow.
For my staff I’m glad they’ve stuck with me through good and bad times, and that we still have fun together – even when we haven’t worked under the same roof in a while.
For my patients and their families we’ve seen a few glimmers of optimism in treatments and hopefully they’ll continue to grow and be built upon. Heaven knows my field – and many others – can use them.
And so,
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My year is now over. My staff and I started the habit of closing down mid-December in 2013, when we realized that patients generally didn’t want to come in then, either.
To me a year really ends the day we close up for the holidays. I put away the season’s decorations, send the final batch to my billing company, and lock the door. Not much of a New Year’s, but at my age it’s not a holiday I mark, anyway. It’s more a relief that my office year, at least, is done.
So it’s always a time for reflection, between the more mundane work of returning calls, reviewing the tests that come in, and getting taxes ready. I try to relax as much as I can (given the weird state of our times, I haven’t left town since November 2019, so this is my vacation for now).
Plus, my kids all come home. I have no idea how much longer that’s going to happen, so I’ll enjoy it while I can.
It’s now almost 3 years since I last rounded at a hospital, and I can’t say I miss it. While I usually have plenty to do on my breaks and weekends, and the occasional patient call to return, it’s nice to know that I can stay in my robe, PJs, and slippers through it all.
2022 certainly wasn’t bad for my family and me, though not as good as any of us hoped. The world, already battered by the pandemic, was thrown into greater uncertainty by the war in Europe and its ramifications across the globe. In comparison, I’m very grateful that higher prices are the extent of my suffering as compared with what the people of Ukraine are going through.
But, at the end of it all, my little practice and two wonderful staff are still here, just as we’ve been since 2000. My kids will (hopefully) all be through college by the end of 2023 and moving on with their lives. I love them, and will miss them if they move away, but part of being a parent is accepting that your kids are only visitors and have their own paths to follow.
For my staff I’m glad they’ve stuck with me through good and bad times, and that we still have fun together – even when we haven’t worked under the same roof in a while.
For my patients and their families we’ve seen a few glimmers of optimism in treatments and hopefully they’ll continue to grow and be built upon. Heaven knows my field – and many others – can use them.
And so,
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Is there hope in the fight against aging?
For many years, it has been believed that the aging process is inevitable and that age-related diseases cannot be prevented or reversed. For example, the U.S. Food and Drug Administration does not recognize aging as an indication for drug approval because there are no markers to determine whether possible treatments have a significant impact on the hallmarks of aging.
The field of geroscience aims to find ways to change this by delaying the onset of age-related diseases or by extending the life span. Those mechanisms contribute to the vulnerability of older adults. The presentations focused on identifying biomarkers of aging and on the search for interventions to prevent and treat age-related diseases.
Perspectives from this meeting were published in a report.
An abridged glossary
- Senescent cells: These are old cells with irreversibly damaged DNA; they strongly resist apoptosis. Thus, they are not eliminated and continue to secrete pathogenic proinflammatory molecules.
- Senolytics: This is a class of compounds that promote the removal of senescent cells from the body.
- Autophagy: This is a process that promotes protein degradation, which is attenuated with aging and that impedes the aggregation of proteins harmful to cell function, particularly those of the central nervous system.
- Proteostasis: This is the dynamic regulation of protein homeostasis.
- Epigenetics: This is the field of biology that studies phenotype changes that are not caused by changes in DNA sequencing and that continue to affect cellular division.
- Metabolome: This refers to small molecules that make up the building blocks of all organismal features, from cell membranes to metabolic cycles to genes and proteins.
- Translational research: This involves applying primary research results to clinical research and vice versa.
Possible research topics
Senescence not only occurs with age but also drives aging. At the meeting, evidence was provided that senescent cells may exacerbate the clinical course of older adults in cases of infections (for example, COVID-19) as they lead to cytokine storms.
Experiments on old mice that have undergone genetic modification of senescent cells or the administration of “senolytic cocktails” composed of dasatinib plus quercetin protected the animals from the effects of viral infections. This finding corroborates the idea that factors involved in biological aging increase vulnerability and could be modified through treatment.
Alzheimer’s disease is an example of the effects of cellular senescence. Senescent cells develop a senescence-associated secretory phenotype that can be toxic to neighboring healthy cells and can allow senescence to propagate within tissues. This effect makes Alzheimer’s disease an essential focal point when studying the use of senolytics. In addition, agents that stimulate autophagy may be of interest for treating degenerative diseases.
Assessing therapeutic effects
It may be possible to assess the therapeutic effects of drug candidates using the following biomarkers.
- Growth hormone and type 1 insulin-like growth factor (IGF-1): Older adults are often prescribed growth hormone. However, recent data suggest that doing so is not advantageous to this patient population, because it antagonizes proteostasis and other cell maintenance mechanisms in older age. Experimental studies and studies conducted on centenarians suggest that low growth hormone and IGF-1 levels contribute to longevity and may be therapeutic biomarkers.
- Epigenetics: DNA methylation is a method that offers an “epigenetic clock” to compare biological age with chronologic age. Higher epigenetic age was associated with increased mortality risk, breast cancer, and nonalcoholic fatty liver disease. Therefore, it could also be a therapeutic biomarker.
- Metabolomics: Studying metabolomes facilitates the identification of the link between genetic polymorphisms and longevity, as most polymorphisms explain less than 0.5% of longevity variations.
- New translational strategy: It is common practice to treat each age-related disease individually. An alternative strategy would be to target the hallmarks of biological aging to prevent these diseases from developing. The rate of biological aging correlates with the speed of damage accumulation at the macromolecular, organelle, and cellular levels. It also affects the capacity of the body to repair this damage. The assessment of biomarkers would make possibile research into the effects of short- and long-term treatments that minimize damage and enhance resilience related to diseases common with aging.
New translational research
The report highlights two translational research models: the in-depth study of centenarians and the analysis of how immune aging makes older adults vulnerable to COVID-19. The impact of impaired immunity on aging became particularly evident during the pandemic. However, to home in on immunity as a therapeutic target and to better understand immune resilience, the specific nature of immune and biological deficits still need to be defined.
Metformin is among the therapeutic agents under investigation in cutting-edge clinical research. Its effect on aging will be studied in the Targeting Aging with Metformin (TAME) clinical trial. This trial is the first to study aging outcomes. The goal is to create a regulatory framework that future therapies can follow to achieve FDA approval.
There are three promising therapeutic platforms among the cutting-edge research studies. The first aims to produce adenosine triphosphate, levels of which decline dramatically with aging. The second aims to promote autophagy to remove cellular waste to treat neurodegenerative diseases. The third reprograms the epigenome to a younger state.
Research on mitochondrial dysfunction is relevant because it is highly involved in age-related diseases. Mitochondrial-derived peptides could potentially serve as biomarkers of mitochondrial function in aging studies and become promising therapeutic targets in age-related diseases. One of these peptides, humanin, has been demonstrated to exert protective effects on the heart, brain, and liver. Researchers observed that mitochondrial proteins are age-dependent and are suppressed by growth hormone and IGF-1. They also found that humanin levels are correlated with endothelial function. Data from animal studies have shown that sustained humanin levels are positively linked to longevity; these findings are mirrored in data from centenarians and their offspring, who have higher levels of humanin.
The formation of a Translational Geroscience Network composed of several scientists from various institutions should accelerate the application of this understanding. Despite the ongoing investigational and clinical studies, senolytics should not be regarded as extending life span or treating certain conditions, because their full safety profiles have not yet been elucidated.
Conclusion
Geroscience faces challenges in dealing with age-related problems. It is hoped that these challenges will be overcome through investigational and clinical studies on the mechanisms involved in aging. In-depth study of the interactions of underlying mechanisms of aging are needed to answer the following questions:
- Is there a hierarchical relationship among these mechanisms?
- Are there organ or cell-type differences in the interactions among these mechanisms?
- Is it possible to achieve a synergistic effect through combined interventions targeting several of the processes that drive aging?
It is complicated, but researchers are starting to see the light at the end of the tunnel.
This article was translated from the Medscape Portuguese edition and a version appeared on Medscape.com.
For many years, it has been believed that the aging process is inevitable and that age-related diseases cannot be prevented or reversed. For example, the U.S. Food and Drug Administration does not recognize aging as an indication for drug approval because there are no markers to determine whether possible treatments have a significant impact on the hallmarks of aging.
The field of geroscience aims to find ways to change this by delaying the onset of age-related diseases or by extending the life span. Those mechanisms contribute to the vulnerability of older adults. The presentations focused on identifying biomarkers of aging and on the search for interventions to prevent and treat age-related diseases.
Perspectives from this meeting were published in a report.
An abridged glossary
- Senescent cells: These are old cells with irreversibly damaged DNA; they strongly resist apoptosis. Thus, they are not eliminated and continue to secrete pathogenic proinflammatory molecules.
- Senolytics: This is a class of compounds that promote the removal of senescent cells from the body.
- Autophagy: This is a process that promotes protein degradation, which is attenuated with aging and that impedes the aggregation of proteins harmful to cell function, particularly those of the central nervous system.
- Proteostasis: This is the dynamic regulation of protein homeostasis.
- Epigenetics: This is the field of biology that studies phenotype changes that are not caused by changes in DNA sequencing and that continue to affect cellular division.
- Metabolome: This refers to small molecules that make up the building blocks of all organismal features, from cell membranes to metabolic cycles to genes and proteins.
- Translational research: This involves applying primary research results to clinical research and vice versa.
Possible research topics
Senescence not only occurs with age but also drives aging. At the meeting, evidence was provided that senescent cells may exacerbate the clinical course of older adults in cases of infections (for example, COVID-19) as they lead to cytokine storms.
Experiments on old mice that have undergone genetic modification of senescent cells or the administration of “senolytic cocktails” composed of dasatinib plus quercetin protected the animals from the effects of viral infections. This finding corroborates the idea that factors involved in biological aging increase vulnerability and could be modified through treatment.
Alzheimer’s disease is an example of the effects of cellular senescence. Senescent cells develop a senescence-associated secretory phenotype that can be toxic to neighboring healthy cells and can allow senescence to propagate within tissues. This effect makes Alzheimer’s disease an essential focal point when studying the use of senolytics. In addition, agents that stimulate autophagy may be of interest for treating degenerative diseases.
Assessing therapeutic effects
It may be possible to assess the therapeutic effects of drug candidates using the following biomarkers.
- Growth hormone and type 1 insulin-like growth factor (IGF-1): Older adults are often prescribed growth hormone. However, recent data suggest that doing so is not advantageous to this patient population, because it antagonizes proteostasis and other cell maintenance mechanisms in older age. Experimental studies and studies conducted on centenarians suggest that low growth hormone and IGF-1 levels contribute to longevity and may be therapeutic biomarkers.
- Epigenetics: DNA methylation is a method that offers an “epigenetic clock” to compare biological age with chronologic age. Higher epigenetic age was associated with increased mortality risk, breast cancer, and nonalcoholic fatty liver disease. Therefore, it could also be a therapeutic biomarker.
- Metabolomics: Studying metabolomes facilitates the identification of the link between genetic polymorphisms and longevity, as most polymorphisms explain less than 0.5% of longevity variations.
- New translational strategy: It is common practice to treat each age-related disease individually. An alternative strategy would be to target the hallmarks of biological aging to prevent these diseases from developing. The rate of biological aging correlates with the speed of damage accumulation at the macromolecular, organelle, and cellular levels. It also affects the capacity of the body to repair this damage. The assessment of biomarkers would make possibile research into the effects of short- and long-term treatments that minimize damage and enhance resilience related to diseases common with aging.
New translational research
The report highlights two translational research models: the in-depth study of centenarians and the analysis of how immune aging makes older adults vulnerable to COVID-19. The impact of impaired immunity on aging became particularly evident during the pandemic. However, to home in on immunity as a therapeutic target and to better understand immune resilience, the specific nature of immune and biological deficits still need to be defined.
Metformin is among the therapeutic agents under investigation in cutting-edge clinical research. Its effect on aging will be studied in the Targeting Aging with Metformin (TAME) clinical trial. This trial is the first to study aging outcomes. The goal is to create a regulatory framework that future therapies can follow to achieve FDA approval.
There are three promising therapeutic platforms among the cutting-edge research studies. The first aims to produce adenosine triphosphate, levels of which decline dramatically with aging. The second aims to promote autophagy to remove cellular waste to treat neurodegenerative diseases. The third reprograms the epigenome to a younger state.
Research on mitochondrial dysfunction is relevant because it is highly involved in age-related diseases. Mitochondrial-derived peptides could potentially serve as biomarkers of mitochondrial function in aging studies and become promising therapeutic targets in age-related diseases. One of these peptides, humanin, has been demonstrated to exert protective effects on the heart, brain, and liver. Researchers observed that mitochondrial proteins are age-dependent and are suppressed by growth hormone and IGF-1. They also found that humanin levels are correlated with endothelial function. Data from animal studies have shown that sustained humanin levels are positively linked to longevity; these findings are mirrored in data from centenarians and their offspring, who have higher levels of humanin.
The formation of a Translational Geroscience Network composed of several scientists from various institutions should accelerate the application of this understanding. Despite the ongoing investigational and clinical studies, senolytics should not be regarded as extending life span or treating certain conditions, because their full safety profiles have not yet been elucidated.
Conclusion
Geroscience faces challenges in dealing with age-related problems. It is hoped that these challenges will be overcome through investigational and clinical studies on the mechanisms involved in aging. In-depth study of the interactions of underlying mechanisms of aging are needed to answer the following questions:
- Is there a hierarchical relationship among these mechanisms?
- Are there organ or cell-type differences in the interactions among these mechanisms?
- Is it possible to achieve a synergistic effect through combined interventions targeting several of the processes that drive aging?
It is complicated, but researchers are starting to see the light at the end of the tunnel.
This article was translated from the Medscape Portuguese edition and a version appeared on Medscape.com.
For many years, it has been believed that the aging process is inevitable and that age-related diseases cannot be prevented or reversed. For example, the U.S. Food and Drug Administration does not recognize aging as an indication for drug approval because there are no markers to determine whether possible treatments have a significant impact on the hallmarks of aging.
The field of geroscience aims to find ways to change this by delaying the onset of age-related diseases or by extending the life span. Those mechanisms contribute to the vulnerability of older adults. The presentations focused on identifying biomarkers of aging and on the search for interventions to prevent and treat age-related diseases.
Perspectives from this meeting were published in a report.
An abridged glossary
- Senescent cells: These are old cells with irreversibly damaged DNA; they strongly resist apoptosis. Thus, they are not eliminated and continue to secrete pathogenic proinflammatory molecules.
- Senolytics: This is a class of compounds that promote the removal of senescent cells from the body.
- Autophagy: This is a process that promotes protein degradation, which is attenuated with aging and that impedes the aggregation of proteins harmful to cell function, particularly those of the central nervous system.
- Proteostasis: This is the dynamic regulation of protein homeostasis.
- Epigenetics: This is the field of biology that studies phenotype changes that are not caused by changes in DNA sequencing and that continue to affect cellular division.
- Metabolome: This refers to small molecules that make up the building blocks of all organismal features, from cell membranes to metabolic cycles to genes and proteins.
- Translational research: This involves applying primary research results to clinical research and vice versa.
Possible research topics
Senescence not only occurs with age but also drives aging. At the meeting, evidence was provided that senescent cells may exacerbate the clinical course of older adults in cases of infections (for example, COVID-19) as they lead to cytokine storms.
Experiments on old mice that have undergone genetic modification of senescent cells or the administration of “senolytic cocktails” composed of dasatinib plus quercetin protected the animals from the effects of viral infections. This finding corroborates the idea that factors involved in biological aging increase vulnerability and could be modified through treatment.
Alzheimer’s disease is an example of the effects of cellular senescence. Senescent cells develop a senescence-associated secretory phenotype that can be toxic to neighboring healthy cells and can allow senescence to propagate within tissues. This effect makes Alzheimer’s disease an essential focal point when studying the use of senolytics. In addition, agents that stimulate autophagy may be of interest for treating degenerative diseases.
Assessing therapeutic effects
It may be possible to assess the therapeutic effects of drug candidates using the following biomarkers.
- Growth hormone and type 1 insulin-like growth factor (IGF-1): Older adults are often prescribed growth hormone. However, recent data suggest that doing so is not advantageous to this patient population, because it antagonizes proteostasis and other cell maintenance mechanisms in older age. Experimental studies and studies conducted on centenarians suggest that low growth hormone and IGF-1 levels contribute to longevity and may be therapeutic biomarkers.
- Epigenetics: DNA methylation is a method that offers an “epigenetic clock” to compare biological age with chronologic age. Higher epigenetic age was associated with increased mortality risk, breast cancer, and nonalcoholic fatty liver disease. Therefore, it could also be a therapeutic biomarker.
- Metabolomics: Studying metabolomes facilitates the identification of the link between genetic polymorphisms and longevity, as most polymorphisms explain less than 0.5% of longevity variations.
- New translational strategy: It is common practice to treat each age-related disease individually. An alternative strategy would be to target the hallmarks of biological aging to prevent these diseases from developing. The rate of biological aging correlates with the speed of damage accumulation at the macromolecular, organelle, and cellular levels. It also affects the capacity of the body to repair this damage. The assessment of biomarkers would make possibile research into the effects of short- and long-term treatments that minimize damage and enhance resilience related to diseases common with aging.
New translational research
The report highlights two translational research models: the in-depth study of centenarians and the analysis of how immune aging makes older adults vulnerable to COVID-19. The impact of impaired immunity on aging became particularly evident during the pandemic. However, to home in on immunity as a therapeutic target and to better understand immune resilience, the specific nature of immune and biological deficits still need to be defined.
Metformin is among the therapeutic agents under investigation in cutting-edge clinical research. Its effect on aging will be studied in the Targeting Aging with Metformin (TAME) clinical trial. This trial is the first to study aging outcomes. The goal is to create a regulatory framework that future therapies can follow to achieve FDA approval.
There are three promising therapeutic platforms among the cutting-edge research studies. The first aims to produce adenosine triphosphate, levels of which decline dramatically with aging. The second aims to promote autophagy to remove cellular waste to treat neurodegenerative diseases. The third reprograms the epigenome to a younger state.
Research on mitochondrial dysfunction is relevant because it is highly involved in age-related diseases. Mitochondrial-derived peptides could potentially serve as biomarkers of mitochondrial function in aging studies and become promising therapeutic targets in age-related diseases. One of these peptides, humanin, has been demonstrated to exert protective effects on the heart, brain, and liver. Researchers observed that mitochondrial proteins are age-dependent and are suppressed by growth hormone and IGF-1. They also found that humanin levels are correlated with endothelial function. Data from animal studies have shown that sustained humanin levels are positively linked to longevity; these findings are mirrored in data from centenarians and their offspring, who have higher levels of humanin.
The formation of a Translational Geroscience Network composed of several scientists from various institutions should accelerate the application of this understanding. Despite the ongoing investigational and clinical studies, senolytics should not be regarded as extending life span or treating certain conditions, because their full safety profiles have not yet been elucidated.
Conclusion
Geroscience faces challenges in dealing with age-related problems. It is hoped that these challenges will be overcome through investigational and clinical studies on the mechanisms involved in aging. In-depth study of the interactions of underlying mechanisms of aging are needed to answer the following questions:
- Is there a hierarchical relationship among these mechanisms?
- Are there organ or cell-type differences in the interactions among these mechanisms?
- Is it possible to achieve a synergistic effect through combined interventions targeting several of the processes that drive aging?
It is complicated, but researchers are starting to see the light at the end of the tunnel.
This article was translated from the Medscape Portuguese edition and a version appeared on Medscape.com.
A reason for hope in the face of long COVID
In this issue, Mayo and colleagues1 summarize what we know about patients with long COVID. The report made me pause and realize that it has been 3 years since we heard the very first reports of patients infected with SARS-CoV-2, which would eventually cause the COVID-19 pandemic. I suspect that I am not alone in having been fascinated by the rapid communication of information (of variable quality and veracity) via peer-reviewed papers, pre-print servers, the media, and social media.
The early studies were largely descriptive, focusing on symptom constellations and outbreak data. Much of what we had by way of treatment was supportive and “let’s try anything”—whether reasonable or, in some cases, not. In relatively short order, though, we developed effective vaccines to help protect people from getting seriously ill, being hospitalized, and dying; we also identified targeted therapies for those who became ill.2 But variants continued—or rather, continue—to emerge, and we remain committed to meeting the demands of the day.
The Centers for Disease Control and Prevention reports that more than 98 million Americans have contracted COVID, and more than 1 million have died.3 Besides the astonishingly high total mortality, the ravages of COVID-19 include new-onset respiratory, cardiovascular, neurologic, and psychiatric illnesses.4,5 As many as half of adults hospitalized for COVID report having persistent symptoms.6
As described in this issue, what we know about long COVID appears to be following the early course of its parent illness. As was true then, we are learning about the symptoms, etiology, and best ways to manage our patients. As in the early days of the pandemic, treatment is supportive, and we await definitive therapies.
I am optimistic, though. Why? Because shortly after the first reports of COVID-19, the virus’ DNA sequence was shared online. Based on that information, diagnostic assays were developed. Within 2 years of the outbreak, we had effective vaccines and specific therapies.
Another call to action. If 5% of patients contracting COVID (a very low estimate) develop long COVID, that would translate to 4.9 million people with long COVID in the United States. That is an astounding burden of suffering that I have no doubt will motivate innovation.
Innovation is a strength of the US health care system. I believe we will rise to the next challenge that COVID-19 has put before us. We have reason to be hopeful.
1. Mayo NL, Ellenbogen RL, Mendoza MD, et al. The family physician’s role in long COVID management. J Fam Pract. 2022;71:426-431. doi: 10.12788/jfp.0517
2. Kulshreshtha A, Sizemore S, Barry HC. COVID-19 therapy: What works? What doesn’t? And what’s on the horizon? J Fam Pract. 2022;71:E3-E16. doi: 10.12788/jfp.0474
3. CDC. COVID data tracker. Accessed December 5, 2022. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
4. Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416-427. doi: 10.1016/s2215-0366(21) 00084-5
5. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693
6. Writing Committee for the Comebac Study Group, Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525-1534. doi: 10.1001/jama.2021.3331
In this issue, Mayo and colleagues1 summarize what we know about patients with long COVID. The report made me pause and realize that it has been 3 years since we heard the very first reports of patients infected with SARS-CoV-2, which would eventually cause the COVID-19 pandemic. I suspect that I am not alone in having been fascinated by the rapid communication of information (of variable quality and veracity) via peer-reviewed papers, pre-print servers, the media, and social media.
The early studies were largely descriptive, focusing on symptom constellations and outbreak data. Much of what we had by way of treatment was supportive and “let’s try anything”—whether reasonable or, in some cases, not. In relatively short order, though, we developed effective vaccines to help protect people from getting seriously ill, being hospitalized, and dying; we also identified targeted therapies for those who became ill.2 But variants continued—or rather, continue—to emerge, and we remain committed to meeting the demands of the day.
The Centers for Disease Control and Prevention reports that more than 98 million Americans have contracted COVID, and more than 1 million have died.3 Besides the astonishingly high total mortality, the ravages of COVID-19 include new-onset respiratory, cardiovascular, neurologic, and psychiatric illnesses.4,5 As many as half of adults hospitalized for COVID report having persistent symptoms.6
As described in this issue, what we know about long COVID appears to be following the early course of its parent illness. As was true then, we are learning about the symptoms, etiology, and best ways to manage our patients. As in the early days of the pandemic, treatment is supportive, and we await definitive therapies.
I am optimistic, though. Why? Because shortly after the first reports of COVID-19, the virus’ DNA sequence was shared online. Based on that information, diagnostic assays were developed. Within 2 years of the outbreak, we had effective vaccines and specific therapies.
Another call to action. If 5% of patients contracting COVID (a very low estimate) develop long COVID, that would translate to 4.9 million people with long COVID in the United States. That is an astounding burden of suffering that I have no doubt will motivate innovation.
Innovation is a strength of the US health care system. I believe we will rise to the next challenge that COVID-19 has put before us. We have reason to be hopeful.
In this issue, Mayo and colleagues1 summarize what we know about patients with long COVID. The report made me pause and realize that it has been 3 years since we heard the very first reports of patients infected with SARS-CoV-2, which would eventually cause the COVID-19 pandemic. I suspect that I am not alone in having been fascinated by the rapid communication of information (of variable quality and veracity) via peer-reviewed papers, pre-print servers, the media, and social media.
The early studies were largely descriptive, focusing on symptom constellations and outbreak data. Much of what we had by way of treatment was supportive and “let’s try anything”—whether reasonable or, in some cases, not. In relatively short order, though, we developed effective vaccines to help protect people from getting seriously ill, being hospitalized, and dying; we also identified targeted therapies for those who became ill.2 But variants continued—or rather, continue—to emerge, and we remain committed to meeting the demands of the day.
The Centers for Disease Control and Prevention reports that more than 98 million Americans have contracted COVID, and more than 1 million have died.3 Besides the astonishingly high total mortality, the ravages of COVID-19 include new-onset respiratory, cardiovascular, neurologic, and psychiatric illnesses.4,5 As many as half of adults hospitalized for COVID report having persistent symptoms.6
As described in this issue, what we know about long COVID appears to be following the early course of its parent illness. As was true then, we are learning about the symptoms, etiology, and best ways to manage our patients. As in the early days of the pandemic, treatment is supportive, and we await definitive therapies.
I am optimistic, though. Why? Because shortly after the first reports of COVID-19, the virus’ DNA sequence was shared online. Based on that information, diagnostic assays were developed. Within 2 years of the outbreak, we had effective vaccines and specific therapies.
Another call to action. If 5% of patients contracting COVID (a very low estimate) develop long COVID, that would translate to 4.9 million people with long COVID in the United States. That is an astounding burden of suffering that I have no doubt will motivate innovation.
Innovation is a strength of the US health care system. I believe we will rise to the next challenge that COVID-19 has put before us. We have reason to be hopeful.
1. Mayo NL, Ellenbogen RL, Mendoza MD, et al. The family physician’s role in long COVID management. J Fam Pract. 2022;71:426-431. doi: 10.12788/jfp.0517
2. Kulshreshtha A, Sizemore S, Barry HC. COVID-19 therapy: What works? What doesn’t? And what’s on the horizon? J Fam Pract. 2022;71:E3-E16. doi: 10.12788/jfp.0474
3. CDC. COVID data tracker. Accessed December 5, 2022. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
4. Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416-427. doi: 10.1016/s2215-0366(21) 00084-5
5. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693
6. Writing Committee for the Comebac Study Group, Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525-1534. doi: 10.1001/jama.2021.3331
1. Mayo NL, Ellenbogen RL, Mendoza MD, et al. The family physician’s role in long COVID management. J Fam Pract. 2022;71:426-431. doi: 10.12788/jfp.0517
2. Kulshreshtha A, Sizemore S, Barry HC. COVID-19 therapy: What works? What doesn’t? And what’s on the horizon? J Fam Pract. 2022;71:E3-E16. doi: 10.12788/jfp.0474
3. CDC. COVID data tracker. Accessed December 5, 2022. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
4. Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416-427. doi: 10.1016/s2215-0366(21) 00084-5
5. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693
6. Writing Committee for the Comebac Study Group, Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525-1534. doi: 10.1001/jama.2021.3331
Mindfulness, exercise strike out in memory trial
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.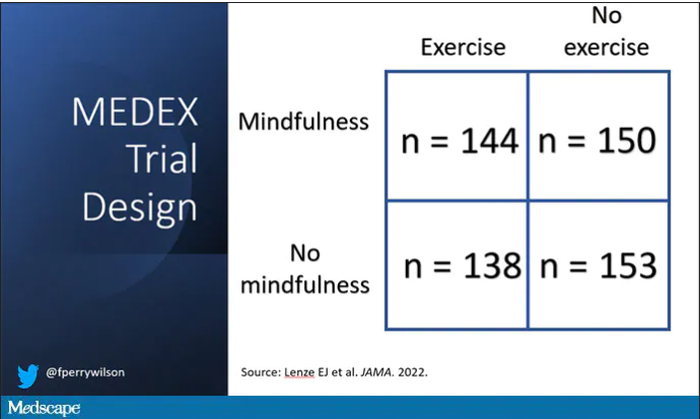
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Nitroglycerin’s safety and value examined
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
The new obesity breakthrough drugs
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.
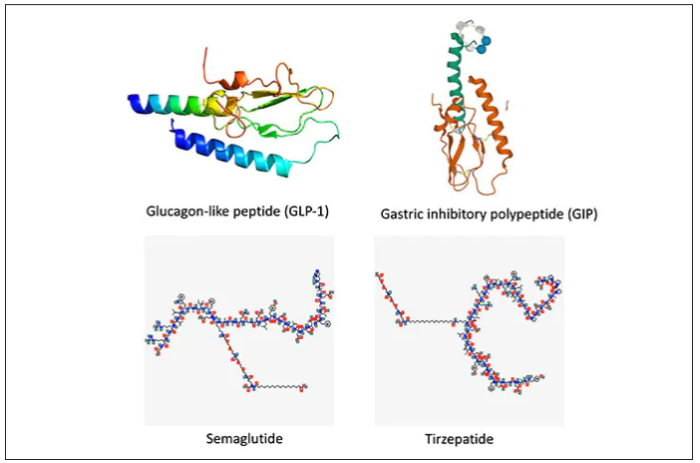
These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.
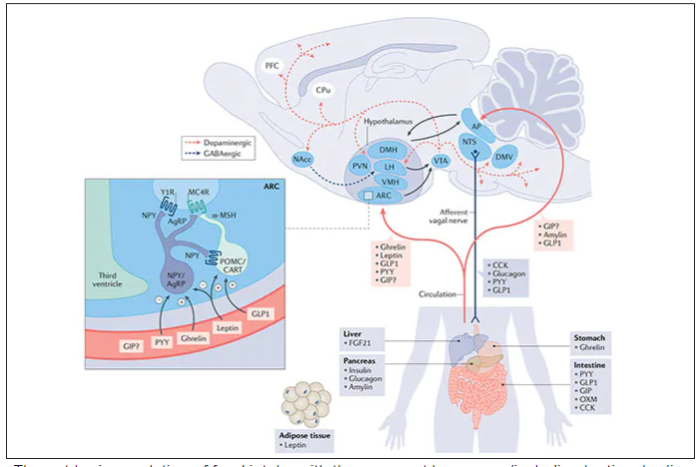
Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.
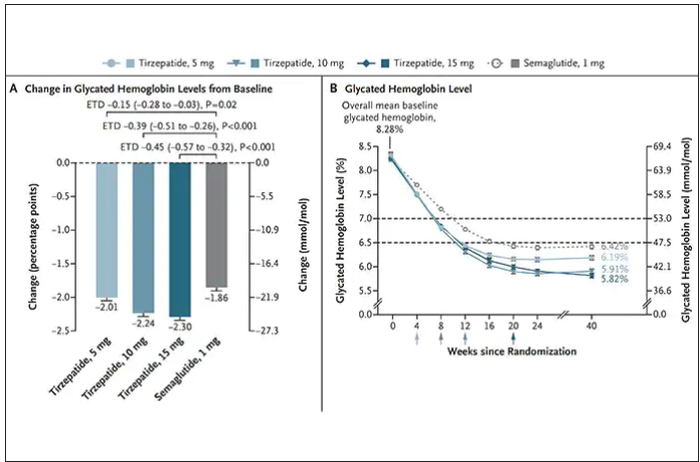
While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.
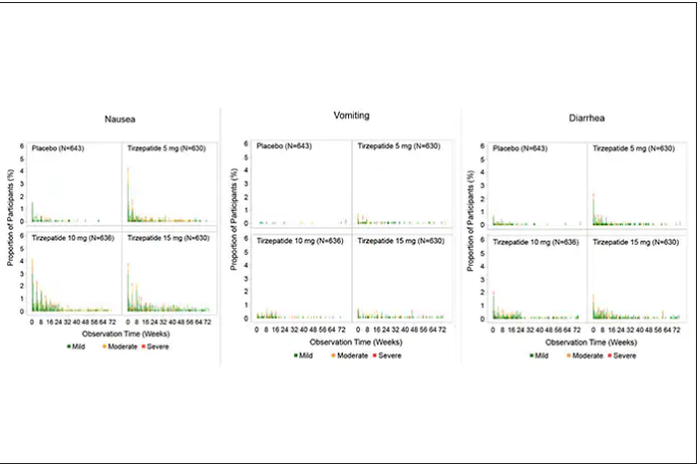
Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.

These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.

Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.

While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.

Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.

These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.

Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.

While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.

Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
Can a Mediterranean diet ease depression in young men?
This transcript has been edited for clarity.
Drew Ramsey, MD: Welcome back, everyone. I’m Dr. Drew Ramsey. I’m on the editorial board with Medscape Psychiatry and I’m an assistant clinical professor of psychiatry at Columbia University. We have a special guest today.
I’m here with nutritionist Jessica Bayes, who’s at the University of Technology Sydney, and she’s the lead author of the AMMEND trial. [Editor’s note: Since completing her PhD, Bayes is now at Southern Cross University.]
Jessica, welcome to Medscape.
Jessica Bayes, PhD: Thank you for having me.
The AMMEND Trial
Dr. Ramsey: Thank you for coming on board and helping all of us as clinicians understand some of your research and some of what is suggested by your research – that young men can change their diet and it helped their depression. Tell us a little bit about the AMMEND trial.
Dr. Bayes: The AMMEND trial was a 12-week randomized controlled trial in young men, 18-25 years old, who had diagnosed moderate to severe clinical depression. They had a poor baseline diet and we got them to eat a healthy Mediterranean diet, which improved their symptoms of depression.
Dr. Ramsey: It was a remarkable trial. Jessica, if I recall, you helped individuals improve the Mediterranean dietary pattern score by 8 points on a 14-point scale. That led to a 20-point reduction in their Beck Depression Inventory. Tell us what that looked like on the ground.
Dr. Bayes: It’s a huge improvement. Obviously, they were feeling much better in the end in terms of their depressive symptoms, but we also measured their energy, sleep, and quality of life. Many of them at the end were at a score cutoff that suggests no depression or in remission.
Dr. Ramsey: There were 72 people in your total trial, so 36% in your intervention arm went into full remission.
Dr. Bayes: Which is just amazing.
Dr. Ramsey: It also follows up the SMILES trial, which was a little bit of a different trial. You had two nutritional counseling sessions and the SMILES trial had seven, but in the SMILES trial, 32.3% of the patients went into full remission when they adopted a Mediterranean-style diet.
Jessica, what is the secret that you and your team know? I think many clinicians, especially clinicians who are parents and have teens, are kind of shaking their heads in disbelief. They’ve been telling their kids to eat healthy. What do you guys know about how to help young men change their diet?
How to Aid Adherence to Mediterranean Diet
Dr. Bayes: Prior to starting this, when I would say this idea to people, everyone would say, “Great idea. There’s no way you’re going to get depressed young men to change their diet. Not going to happen.” We went to them and we asked them. We said, “We’re going to do this study. What do you want from us? What resources would you need? How many appointments would you like? What’s too little or too many?”
We really got their feedback on board when we designed the study, and that obviously paid off. We had a personalized approach and we met them where they were at. We gave them the skills, resources, recipes, meal ideas – all those things – so we could really set them up to succeed.
Dr. Ramsey: You were telling me earlier about a few of the dietary changes that you felt made a big difference for these young men. What were those?
Dr. Bayes: Increasing the vegetables, olive oil, and legumes are probably the big ones that most of them were really not doing beforehand. They were really able to take that on board and make significant improvements in those areas.
Dr. Ramsey: These are really some of the top food categories in nutritional psychiatry as we think about how we help our efforts to improve mental health by thinking about nutrition, nutritional quality, and nutritional density. Certainly, those food categories – nuts and legumes, plants, and olive oil – are really what help get us there.
You also gave the students a food hamper. If you were going to be in charge of mental health in Australia and America and you got to give every college freshman a little box with a note, what would be in that box?
Dr. Bayes: I’d want to put everything in that box! It would be full of brightly colored fruits and vegetables, different nuts and seeds, and legumes. It would be full of recipes and ideas of how to cook things and how to prepare really delicious things. It would be full of different herbs and spices and all of those things to get people really excited about food.
Dr. Ramsey: Did the young men pick up on your enthusiasm and excitement around food? Did they begin to adopt some of that, shifting their view of how they saw the food and how they saw that it is related to their depression?
Dr. Bayes: Hopefully. I do think energy is infectious. I’m sure that played a role somewhat, but trying to get them excited about food can be really quite daunting, thinking, I’ve got to change my entire diet and I’ve got to learn to cook and go out and buy groceries. I don’t even know what to do with a piece of salmon. Trying to get them curious, interested, and just reminding them that it’s not all-or-nothing. Make small changes, give it a go, and have fun.
Dr. Ramsey: You also have a unique aspect of your research that you’re interested in male mental health, and that’s not something that’s been widely researched. Can you tell us a little bit about what these men were like in terms of coming into your trial as depressed young men?
Dr. Bayes: In the context of the COVID-19 pandemic, mental health was at the forefront of many people’s minds. They joined the study saying, “I’ve never seen anything like this before. I’ve never seen myself represented in research. I wanted to contribute. I want to add to that conversation because I feel like we are overlooked.”
Dr. Ramsey: I love hearing this notion that maybe young men aren’t quite who we think they are. They are wanting to be seen around their mental health. They can learn to use olive oil and to cook, and they can engage in mental health interventions that work. We just need to ask, give them some food, encourage them, and it makes a big difference.
Jessica Bayes, thank you so much for joining us and sharing some of your research. Everyone, it’s the AMMEND trial. We will drop a link to the trial below so you can take a peek and tell us what you think.
Please, in the comments, let us know what you think about this notion of helping young men with depression through nutritional interventions. Take a peek at the great work that Jessica and Professor Sibbritt from the University of Technology Sydney have published and put out into the scientific literature for us all.
Thanks so much, Jessica. I look forward to seeing you soon.
Dr. Bayes: Thank you.
Dr. Ramsey is assistant clinical professor, department of psychiatry, Columbia University, New York. He has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for InterContinental Hotels Group; National Kale Day 501(c)3. Received income in an amount equal to or greater than $250 from: Sharecare. Dr. Bayes is a postdoctoral research fellow; clinical nutritionist, Southern Cross University, National Center for Naturopathic Medicine, Lismore, New South Wales, Australia. She has disclosed the following relevant financial relationships: Received research grant from Endeavour College. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Drew Ramsey, MD: Welcome back, everyone. I’m Dr. Drew Ramsey. I’m on the editorial board with Medscape Psychiatry and I’m an assistant clinical professor of psychiatry at Columbia University. We have a special guest today.
I’m here with nutritionist Jessica Bayes, who’s at the University of Technology Sydney, and she’s the lead author of the AMMEND trial. [Editor’s note: Since completing her PhD, Bayes is now at Southern Cross University.]
Jessica, welcome to Medscape.
Jessica Bayes, PhD: Thank you for having me.
The AMMEND Trial
Dr. Ramsey: Thank you for coming on board and helping all of us as clinicians understand some of your research and some of what is suggested by your research – that young men can change their diet and it helped their depression. Tell us a little bit about the AMMEND trial.
Dr. Bayes: The AMMEND trial was a 12-week randomized controlled trial in young men, 18-25 years old, who had diagnosed moderate to severe clinical depression. They had a poor baseline diet and we got them to eat a healthy Mediterranean diet, which improved their symptoms of depression.
Dr. Ramsey: It was a remarkable trial. Jessica, if I recall, you helped individuals improve the Mediterranean dietary pattern score by 8 points on a 14-point scale. That led to a 20-point reduction in their Beck Depression Inventory. Tell us what that looked like on the ground.
Dr. Bayes: It’s a huge improvement. Obviously, they were feeling much better in the end in terms of their depressive symptoms, but we also measured their energy, sleep, and quality of life. Many of them at the end were at a score cutoff that suggests no depression or in remission.
Dr. Ramsey: There were 72 people in your total trial, so 36% in your intervention arm went into full remission.
Dr. Bayes: Which is just amazing.
Dr. Ramsey: It also follows up the SMILES trial, which was a little bit of a different trial. You had two nutritional counseling sessions and the SMILES trial had seven, but in the SMILES trial, 32.3% of the patients went into full remission when they adopted a Mediterranean-style diet.
Jessica, what is the secret that you and your team know? I think many clinicians, especially clinicians who are parents and have teens, are kind of shaking their heads in disbelief. They’ve been telling their kids to eat healthy. What do you guys know about how to help young men change their diet?
How to Aid Adherence to Mediterranean Diet
Dr. Bayes: Prior to starting this, when I would say this idea to people, everyone would say, “Great idea. There’s no way you’re going to get depressed young men to change their diet. Not going to happen.” We went to them and we asked them. We said, “We’re going to do this study. What do you want from us? What resources would you need? How many appointments would you like? What’s too little or too many?”
We really got their feedback on board when we designed the study, and that obviously paid off. We had a personalized approach and we met them where they were at. We gave them the skills, resources, recipes, meal ideas – all those things – so we could really set them up to succeed.
Dr. Ramsey: You were telling me earlier about a few of the dietary changes that you felt made a big difference for these young men. What were those?
Dr. Bayes: Increasing the vegetables, olive oil, and legumes are probably the big ones that most of them were really not doing beforehand. They were really able to take that on board and make significant improvements in those areas.
Dr. Ramsey: These are really some of the top food categories in nutritional psychiatry as we think about how we help our efforts to improve mental health by thinking about nutrition, nutritional quality, and nutritional density. Certainly, those food categories – nuts and legumes, plants, and olive oil – are really what help get us there.
You also gave the students a food hamper. If you were going to be in charge of mental health in Australia and America and you got to give every college freshman a little box with a note, what would be in that box?
Dr. Bayes: I’d want to put everything in that box! It would be full of brightly colored fruits and vegetables, different nuts and seeds, and legumes. It would be full of recipes and ideas of how to cook things and how to prepare really delicious things. It would be full of different herbs and spices and all of those things to get people really excited about food.
Dr. Ramsey: Did the young men pick up on your enthusiasm and excitement around food? Did they begin to adopt some of that, shifting their view of how they saw the food and how they saw that it is related to their depression?
Dr. Bayes: Hopefully. I do think energy is infectious. I’m sure that played a role somewhat, but trying to get them excited about food can be really quite daunting, thinking, I’ve got to change my entire diet and I’ve got to learn to cook and go out and buy groceries. I don’t even know what to do with a piece of salmon. Trying to get them curious, interested, and just reminding them that it’s not all-or-nothing. Make small changes, give it a go, and have fun.
Dr. Ramsey: You also have a unique aspect of your research that you’re interested in male mental health, and that’s not something that’s been widely researched. Can you tell us a little bit about what these men were like in terms of coming into your trial as depressed young men?
Dr. Bayes: In the context of the COVID-19 pandemic, mental health was at the forefront of many people’s minds. They joined the study saying, “I’ve never seen anything like this before. I’ve never seen myself represented in research. I wanted to contribute. I want to add to that conversation because I feel like we are overlooked.”
Dr. Ramsey: I love hearing this notion that maybe young men aren’t quite who we think they are. They are wanting to be seen around their mental health. They can learn to use olive oil and to cook, and they can engage in mental health interventions that work. We just need to ask, give them some food, encourage them, and it makes a big difference.
Jessica Bayes, thank you so much for joining us and sharing some of your research. Everyone, it’s the AMMEND trial. We will drop a link to the trial below so you can take a peek and tell us what you think.
Please, in the comments, let us know what you think about this notion of helping young men with depression through nutritional interventions. Take a peek at the great work that Jessica and Professor Sibbritt from the University of Technology Sydney have published and put out into the scientific literature for us all.
Thanks so much, Jessica. I look forward to seeing you soon.
Dr. Bayes: Thank you.
Dr. Ramsey is assistant clinical professor, department of psychiatry, Columbia University, New York. He has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for InterContinental Hotels Group; National Kale Day 501(c)3. Received income in an amount equal to or greater than $250 from: Sharecare. Dr. Bayes is a postdoctoral research fellow; clinical nutritionist, Southern Cross University, National Center for Naturopathic Medicine, Lismore, New South Wales, Australia. She has disclosed the following relevant financial relationships: Received research grant from Endeavour College. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Drew Ramsey, MD: Welcome back, everyone. I’m Dr. Drew Ramsey. I’m on the editorial board with Medscape Psychiatry and I’m an assistant clinical professor of psychiatry at Columbia University. We have a special guest today.
I’m here with nutritionist Jessica Bayes, who’s at the University of Technology Sydney, and she’s the lead author of the AMMEND trial. [Editor’s note: Since completing her PhD, Bayes is now at Southern Cross University.]
Jessica, welcome to Medscape.
Jessica Bayes, PhD: Thank you for having me.
The AMMEND Trial
Dr. Ramsey: Thank you for coming on board and helping all of us as clinicians understand some of your research and some of what is suggested by your research – that young men can change their diet and it helped their depression. Tell us a little bit about the AMMEND trial.
Dr. Bayes: The AMMEND trial was a 12-week randomized controlled trial in young men, 18-25 years old, who had diagnosed moderate to severe clinical depression. They had a poor baseline diet and we got them to eat a healthy Mediterranean diet, which improved their symptoms of depression.
Dr. Ramsey: It was a remarkable trial. Jessica, if I recall, you helped individuals improve the Mediterranean dietary pattern score by 8 points on a 14-point scale. That led to a 20-point reduction in their Beck Depression Inventory. Tell us what that looked like on the ground.
Dr. Bayes: It’s a huge improvement. Obviously, they were feeling much better in the end in terms of their depressive symptoms, but we also measured their energy, sleep, and quality of life. Many of them at the end were at a score cutoff that suggests no depression or in remission.
Dr. Ramsey: There were 72 people in your total trial, so 36% in your intervention arm went into full remission.
Dr. Bayes: Which is just amazing.
Dr. Ramsey: It also follows up the SMILES trial, which was a little bit of a different trial. You had two nutritional counseling sessions and the SMILES trial had seven, but in the SMILES trial, 32.3% of the patients went into full remission when they adopted a Mediterranean-style diet.
Jessica, what is the secret that you and your team know? I think many clinicians, especially clinicians who are parents and have teens, are kind of shaking their heads in disbelief. They’ve been telling their kids to eat healthy. What do you guys know about how to help young men change their diet?
How to Aid Adherence to Mediterranean Diet
Dr. Bayes: Prior to starting this, when I would say this idea to people, everyone would say, “Great idea. There’s no way you’re going to get depressed young men to change their diet. Not going to happen.” We went to them and we asked them. We said, “We’re going to do this study. What do you want from us? What resources would you need? How many appointments would you like? What’s too little or too many?”
We really got their feedback on board when we designed the study, and that obviously paid off. We had a personalized approach and we met them where they were at. We gave them the skills, resources, recipes, meal ideas – all those things – so we could really set them up to succeed.
Dr. Ramsey: You were telling me earlier about a few of the dietary changes that you felt made a big difference for these young men. What were those?
Dr. Bayes: Increasing the vegetables, olive oil, and legumes are probably the big ones that most of them were really not doing beforehand. They were really able to take that on board and make significant improvements in those areas.
Dr. Ramsey: These are really some of the top food categories in nutritional psychiatry as we think about how we help our efforts to improve mental health by thinking about nutrition, nutritional quality, and nutritional density. Certainly, those food categories – nuts and legumes, plants, and olive oil – are really what help get us there.
You also gave the students a food hamper. If you were going to be in charge of mental health in Australia and America and you got to give every college freshman a little box with a note, what would be in that box?
Dr. Bayes: I’d want to put everything in that box! It would be full of brightly colored fruits and vegetables, different nuts and seeds, and legumes. It would be full of recipes and ideas of how to cook things and how to prepare really delicious things. It would be full of different herbs and spices and all of those things to get people really excited about food.
Dr. Ramsey: Did the young men pick up on your enthusiasm and excitement around food? Did they begin to adopt some of that, shifting their view of how they saw the food and how they saw that it is related to their depression?
Dr. Bayes: Hopefully. I do think energy is infectious. I’m sure that played a role somewhat, but trying to get them excited about food can be really quite daunting, thinking, I’ve got to change my entire diet and I’ve got to learn to cook and go out and buy groceries. I don’t even know what to do with a piece of salmon. Trying to get them curious, interested, and just reminding them that it’s not all-or-nothing. Make small changes, give it a go, and have fun.
Dr. Ramsey: You also have a unique aspect of your research that you’re interested in male mental health, and that’s not something that’s been widely researched. Can you tell us a little bit about what these men were like in terms of coming into your trial as depressed young men?
Dr. Bayes: In the context of the COVID-19 pandemic, mental health was at the forefront of many people’s minds. They joined the study saying, “I’ve never seen anything like this before. I’ve never seen myself represented in research. I wanted to contribute. I want to add to that conversation because I feel like we are overlooked.”
Dr. Ramsey: I love hearing this notion that maybe young men aren’t quite who we think they are. They are wanting to be seen around their mental health. They can learn to use olive oil and to cook, and they can engage in mental health interventions that work. We just need to ask, give them some food, encourage them, and it makes a big difference.
Jessica Bayes, thank you so much for joining us and sharing some of your research. Everyone, it’s the AMMEND trial. We will drop a link to the trial below so you can take a peek and tell us what you think.
Please, in the comments, let us know what you think about this notion of helping young men with depression through nutritional interventions. Take a peek at the great work that Jessica and Professor Sibbritt from the University of Technology Sydney have published and put out into the scientific literature for us all.
Thanks so much, Jessica. I look forward to seeing you soon.
Dr. Bayes: Thank you.
Dr. Ramsey is assistant clinical professor, department of psychiatry, Columbia University, New York. He has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for InterContinental Hotels Group; National Kale Day 501(c)3. Received income in an amount equal to or greater than $250 from: Sharecare. Dr. Bayes is a postdoctoral research fellow; clinical nutritionist, Southern Cross University, National Center for Naturopathic Medicine, Lismore, New South Wales, Australia. She has disclosed the following relevant financial relationships: Received research grant from Endeavour College. A version of this article first appeared on Medscape.com.
There are new things we can do to improve early autism detection
We are all seeing more children on the autism spectrum than we ever expected. With a Centers for Disease Control–estimated prevalence of 1 in 44, the average pediatrician will be caring for 45 children with autism. It may feel like even more as parents bring in their children with related concerns or fears. Early entry into services has been shown to improve functioning, making early identification important. However, screening at the youngest ages has important limitations.
Sharing a concern about possible autism with parents is a painful aspect of primary care practice. We want to get it right, not frighten parents unnecessarily, nor miss children and delay intervention.
Autism screening is recommended by the American Academy of Pediatrics at 18- and 24-month pediatric well-child visits. There are several reasons for screening repeatedly: Autism symptoms emerge gradually in the toddler period; about 32% of children later found to have autism were developing in a typical pattern and appeared normal at 18 months only to regress by age 24 months; children may miss the 18 month screen; and all screens have false negatives as well as false positives. But even screening at these two ages is not enough.
One criticism of current screening tests pointed out by the U.S. Preventive Services Task Force has been a problem with the sample used to develop or validate the tool. Many test development studies included only children at risk by being in early intervention, siblings of children with diagnosed autism, or children only failing the screening tests rather than a community sample that the screen in actually used for.
Another obstacle to prediction of autism diagnoses made years later is that some children may not have had any clinical manifestations at the younger age even as judged by the best gold standard testing and, thus, negative screens were ambiguous. Additionally, data from prospective studies of high-risk infant siblings reveal that only 18% of children diagnosed with autism at 36 months were given that diagnosis at 18 months of age despite use of comprehensive diagnostic assessments.
Prevalence is also reported as 30% higher at age 8-12 years as at 3-7 years on gold-standard tests. Children identified later with autism tend to have milder symptoms and higher cognitive functioning. Therefore, we need some humility in thinking we can identify children as early as 18 months; rather, we need to use the best available methods at all ages and remain vigilant to symptoms as they evolve as well as to new screening and testing measures.
The most commonly used parent report screen is the 20-item Modified Checklist for Autism in Toddlers–Revised (M-CHAT-R), a modification of the original CHAT screen. To have reasonable positive predictive value, the M-CHAT-R authors recommend a clinician or trained staff member conduct a structured follow-up interview with the parent when the M-CHAT-R has a score of 3-7. Scores of 8 or more reflect enough symptoms to more strongly predict an autism diagnosis and thus the interview may be skipped in those cases. The recommended two-step process is called M-CHAT-R/F. At 18 months without the R/F, a positive M-CHAT-R only is associated with an autism diagnosis 27% of the time (PPV, 0.27); which is unacceptable for primary care use.
Unfortunately, the M-CHAT-R/F appears to be less accurate for 18-month-olds than 24-month-olds, in part because its yes/no response options are harder for a caregiver to answer, especially for behaviors just developing, or because of lack of experience with toddlers.
An alternative modification of the original CHAT called the Quantitative CHAT or Q-CHAT-10 has a range of response options for the caregiver; for example, always/usually/sometimes/rarely/never or many times a day/a few times a day/a few times a week/less than once a week/never. The authors of the Q-CHAT-10, however, recommend a summary pass/fail result for ease of use rather than using the range of response option values in the score. We recently published a study testing accuracy using add-up scoring that utilized the entire range of response option values, called Q-CHAT-10-O (O for ordinal), for children 16-20 months old as well as cartoon depictions of the behaviors. Our study also included diagnostic testing of screen-negative as well as screen-positive children to accurately calculate sensitivity and specificity for this method. In our study, Q-CHAT-10-O with a cutoff score greater than 11 showed higher sensitivity (0.63) than either M-CHAT-R/F (0.34) or Q-CHAT-10 (0.31) for this age range although the PPV (0.35) and negative predictive value (0.92) were comparable with M-CHAT R/F. Although Q-CHAT-10-O sensitivity (0.63) is less than M-CHAT-R (without follow-up; 0.73) and specificity (0.79) is less than the two-stage R/F procedure (0.90), on balance, it is more accurate and more practical for a primary care population. After 20 months of age, the M-CHAT-R/F has adequate accuracy to rescreen, if indicated, and for the subsequent 24 month screening. Language items are often of highest value in predicting outcomes in several tools including in the screen we are now validating for 18 month olds.
The Q-CHAT-10-O with ordinal scoring and pictures can also be recommended because it shows advantages over M-CHAT-R/F with half the number of items (10 vs. 20), no requirement for a follow-up interview, and improved sensitivity. Unlike M-CHAT-R, it also contributes to equity in screening because results did not differ depending on race or socioeconomic background.
Is there an even better way to detect autism in primary care? In 2022 an article was published regarding an exciting method of early autism detection called the Social Attention and Communication Surveillance–Revised (SACS-R), an eight-item observation checklist completed at public health nurse check-ups in Australia. The observers had 4 years of nursing degree education and a 3.5-hour training session.
The SACS-R and the preschool version (for older children) had significant associations with diagnostic testing at 12, 18, 24, and 42 months. The SACS-R had excellent PPV (82.6%), NPV (98.7%), and specificity (99.6%) and moderate sensitivity (61.5%) when used between 12 and 24 months of age. Pointing, eye contact, waving “bye, bye,” social communication by showing, and pretend play were the key indicators for observations at 18 months, with absence of three or more indicating risk for autism. Different key indicators were used at the other ages, reflecting the evolution of autism symptoms. This hybrid (observation and scoring) surveillance method by professionals shows hopeful data for the critical ability to identify children at risk for autism in primary care very early but requires more than parent report, that is, new levels of autism-specific clinician training and direct observations at multiple visits over time.
The takeaway is to remember that we should all watch closely for early signs of autism, informed by research on the key findings that a professional might observe, as well as by using the best screens available. We should remember that both false positives and false negatives are inherent in screening, especially at the youngest ages. We need to combine our concern with the parent’s concern as well as screen results and be sure to follow-up closely as symptoms can change in even a few months. Many factors may prevent a family from returning to see us or following our advice to go for testing or intervention, so tracking the child and their service use is an important part of the good care we strive to provide children with autism.
Other screening tools
You may have heard of other parent-report screens for autism. It is important to compare their accuracy specifically for 18-month-olds in a community setting.
- The Infant Toddler Checklist (https://psychology-tools.com/test/infant-toddler-checklist) has moderate overall psychometrics with sensitivity ranging from 0.55 to 0.77; specificity from 0.42 to 0.85; PPV from 0.20 to 0.55; and NPV from 0.83 to 0.94. However, the data were based on a sample including both community-dwelling toddlers and those with a family history of autism.
- The Brief Infant-Toddler Social and Emotional Assessment (https://eprovide.mapi-trust.org/instruments/brief-infant-toddler-social-emotional-assessment/) – the screen’s four autism-specific scales had high specificity (84%-90%) but low sensitivity (40%-52%).
- Canvas Dx (https://canvasdx.com/) from the Cognoa company is not a parent-report measure but rather a three-part evaluation including an app-based parent questionnaire, parent uploads of home videos analyzed by a specialist, and a 13- to 15-item primary care physician observational checklist. There were 56 diagnosed of the 426 children in the 18- to 24-month-old range from a sample of children presenting with parent or clinician concerns rather than from a community sample.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
References
Sturner R et al. Autism screening at 18 months of age: A comparison of the Q-CHAT-10 and M-CHAT screeners. Molecular Autism. Jan 3;13(1):2.
Barbaro J et al. Diagnostic accuracy of the Social Attention and Communication Surveillance–Revised with preschool tool for early autism detection in very young children. JAMA Netw Open. 2022;5(3):e2146415.
We are all seeing more children on the autism spectrum than we ever expected. With a Centers for Disease Control–estimated prevalence of 1 in 44, the average pediatrician will be caring for 45 children with autism. It may feel like even more as parents bring in their children with related concerns or fears. Early entry into services has been shown to improve functioning, making early identification important. However, screening at the youngest ages has important limitations.
Sharing a concern about possible autism with parents is a painful aspect of primary care practice. We want to get it right, not frighten parents unnecessarily, nor miss children and delay intervention.
Autism screening is recommended by the American Academy of Pediatrics at 18- and 24-month pediatric well-child visits. There are several reasons for screening repeatedly: Autism symptoms emerge gradually in the toddler period; about 32% of children later found to have autism were developing in a typical pattern and appeared normal at 18 months only to regress by age 24 months; children may miss the 18 month screen; and all screens have false negatives as well as false positives. But even screening at these two ages is not enough.
One criticism of current screening tests pointed out by the U.S. Preventive Services Task Force has been a problem with the sample used to develop or validate the tool. Many test development studies included only children at risk by being in early intervention, siblings of children with diagnosed autism, or children only failing the screening tests rather than a community sample that the screen in actually used for.
Another obstacle to prediction of autism diagnoses made years later is that some children may not have had any clinical manifestations at the younger age even as judged by the best gold standard testing and, thus, negative screens were ambiguous. Additionally, data from prospective studies of high-risk infant siblings reveal that only 18% of children diagnosed with autism at 36 months were given that diagnosis at 18 months of age despite use of comprehensive diagnostic assessments.
Prevalence is also reported as 30% higher at age 8-12 years as at 3-7 years on gold-standard tests. Children identified later with autism tend to have milder symptoms and higher cognitive functioning. Therefore, we need some humility in thinking we can identify children as early as 18 months; rather, we need to use the best available methods at all ages and remain vigilant to symptoms as they evolve as well as to new screening and testing measures.
The most commonly used parent report screen is the 20-item Modified Checklist for Autism in Toddlers–Revised (M-CHAT-R), a modification of the original CHAT screen. To have reasonable positive predictive value, the M-CHAT-R authors recommend a clinician or trained staff member conduct a structured follow-up interview with the parent when the M-CHAT-R has a score of 3-7. Scores of 8 or more reflect enough symptoms to more strongly predict an autism diagnosis and thus the interview may be skipped in those cases. The recommended two-step process is called M-CHAT-R/F. At 18 months without the R/F, a positive M-CHAT-R only is associated with an autism diagnosis 27% of the time (PPV, 0.27); which is unacceptable for primary care use.
Unfortunately, the M-CHAT-R/F appears to be less accurate for 18-month-olds than 24-month-olds, in part because its yes/no response options are harder for a caregiver to answer, especially for behaviors just developing, or because of lack of experience with toddlers.
An alternative modification of the original CHAT called the Quantitative CHAT or Q-CHAT-10 has a range of response options for the caregiver; for example, always/usually/sometimes/rarely/never or many times a day/a few times a day/a few times a week/less than once a week/never. The authors of the Q-CHAT-10, however, recommend a summary pass/fail result for ease of use rather than using the range of response option values in the score. We recently published a study testing accuracy using add-up scoring that utilized the entire range of response option values, called Q-CHAT-10-O (O for ordinal), for children 16-20 months old as well as cartoon depictions of the behaviors. Our study also included diagnostic testing of screen-negative as well as screen-positive children to accurately calculate sensitivity and specificity for this method. In our study, Q-CHAT-10-O with a cutoff score greater than 11 showed higher sensitivity (0.63) than either M-CHAT-R/F (0.34) or Q-CHAT-10 (0.31) for this age range although the PPV (0.35) and negative predictive value (0.92) were comparable with M-CHAT R/F. Although Q-CHAT-10-O sensitivity (0.63) is less than M-CHAT-R (without follow-up; 0.73) and specificity (0.79) is less than the two-stage R/F procedure (0.90), on balance, it is more accurate and more practical for a primary care population. After 20 months of age, the M-CHAT-R/F has adequate accuracy to rescreen, if indicated, and for the subsequent 24 month screening. Language items are often of highest value in predicting outcomes in several tools including in the screen we are now validating for 18 month olds.
The Q-CHAT-10-O with ordinal scoring and pictures can also be recommended because it shows advantages over M-CHAT-R/F with half the number of items (10 vs. 20), no requirement for a follow-up interview, and improved sensitivity. Unlike M-CHAT-R, it also contributes to equity in screening because results did not differ depending on race or socioeconomic background.
Is there an even better way to detect autism in primary care? In 2022 an article was published regarding an exciting method of early autism detection called the Social Attention and Communication Surveillance–Revised (SACS-R), an eight-item observation checklist completed at public health nurse check-ups in Australia. The observers had 4 years of nursing degree education and a 3.5-hour training session.
The SACS-R and the preschool version (for older children) had significant associations with diagnostic testing at 12, 18, 24, and 42 months. The SACS-R had excellent PPV (82.6%), NPV (98.7%), and specificity (99.6%) and moderate sensitivity (61.5%) when used between 12 and 24 months of age. Pointing, eye contact, waving “bye, bye,” social communication by showing, and pretend play were the key indicators for observations at 18 months, with absence of three or more indicating risk for autism. Different key indicators were used at the other ages, reflecting the evolution of autism symptoms. This hybrid (observation and scoring) surveillance method by professionals shows hopeful data for the critical ability to identify children at risk for autism in primary care very early but requires more than parent report, that is, new levels of autism-specific clinician training and direct observations at multiple visits over time.
The takeaway is to remember that we should all watch closely for early signs of autism, informed by research on the key findings that a professional might observe, as well as by using the best screens available. We should remember that both false positives and false negatives are inherent in screening, especially at the youngest ages. We need to combine our concern with the parent’s concern as well as screen results and be sure to follow-up closely as symptoms can change in even a few months. Many factors may prevent a family from returning to see us or following our advice to go for testing or intervention, so tracking the child and their service use is an important part of the good care we strive to provide children with autism.
Other screening tools
You may have heard of other parent-report screens for autism. It is important to compare their accuracy specifically for 18-month-olds in a community setting.
- The Infant Toddler Checklist (https://psychology-tools.com/test/infant-toddler-checklist) has moderate overall psychometrics with sensitivity ranging from 0.55 to 0.77; specificity from 0.42 to 0.85; PPV from 0.20 to 0.55; and NPV from 0.83 to 0.94. However, the data were based on a sample including both community-dwelling toddlers and those with a family history of autism.
- The Brief Infant-Toddler Social and Emotional Assessment (https://eprovide.mapi-trust.org/instruments/brief-infant-toddler-social-emotional-assessment/) – the screen’s four autism-specific scales had high specificity (84%-90%) but low sensitivity (40%-52%).
- Canvas Dx (https://canvasdx.com/) from the Cognoa company is not a parent-report measure but rather a three-part evaluation including an app-based parent questionnaire, parent uploads of home videos analyzed by a specialist, and a 13- to 15-item primary care physician observational checklist. There were 56 diagnosed of the 426 children in the 18- to 24-month-old range from a sample of children presenting with parent or clinician concerns rather than from a community sample.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
References
Sturner R et al. Autism screening at 18 months of age: A comparison of the Q-CHAT-10 and M-CHAT screeners. Molecular Autism. Jan 3;13(1):2.
Barbaro J et al. Diagnostic accuracy of the Social Attention and Communication Surveillance–Revised with preschool tool for early autism detection in very young children. JAMA Netw Open. 2022;5(3):e2146415.
We are all seeing more children on the autism spectrum than we ever expected. With a Centers for Disease Control–estimated prevalence of 1 in 44, the average pediatrician will be caring for 45 children with autism. It may feel like even more as parents bring in their children with related concerns or fears. Early entry into services has been shown to improve functioning, making early identification important. However, screening at the youngest ages has important limitations.
Sharing a concern about possible autism with parents is a painful aspect of primary care practice. We want to get it right, not frighten parents unnecessarily, nor miss children and delay intervention.
Autism screening is recommended by the American Academy of Pediatrics at 18- and 24-month pediatric well-child visits. There are several reasons for screening repeatedly: Autism symptoms emerge gradually in the toddler period; about 32% of children later found to have autism were developing in a typical pattern and appeared normal at 18 months only to regress by age 24 months; children may miss the 18 month screen; and all screens have false negatives as well as false positives. But even screening at these two ages is not enough.
One criticism of current screening tests pointed out by the U.S. Preventive Services Task Force has been a problem with the sample used to develop or validate the tool. Many test development studies included only children at risk by being in early intervention, siblings of children with diagnosed autism, or children only failing the screening tests rather than a community sample that the screen in actually used for.
Another obstacle to prediction of autism diagnoses made years later is that some children may not have had any clinical manifestations at the younger age even as judged by the best gold standard testing and, thus, negative screens were ambiguous. Additionally, data from prospective studies of high-risk infant siblings reveal that only 18% of children diagnosed with autism at 36 months were given that diagnosis at 18 months of age despite use of comprehensive diagnostic assessments.
Prevalence is also reported as 30% higher at age 8-12 years as at 3-7 years on gold-standard tests. Children identified later with autism tend to have milder symptoms and higher cognitive functioning. Therefore, we need some humility in thinking we can identify children as early as 18 months; rather, we need to use the best available methods at all ages and remain vigilant to symptoms as they evolve as well as to new screening and testing measures.
The most commonly used parent report screen is the 20-item Modified Checklist for Autism in Toddlers–Revised (M-CHAT-R), a modification of the original CHAT screen. To have reasonable positive predictive value, the M-CHAT-R authors recommend a clinician or trained staff member conduct a structured follow-up interview with the parent when the M-CHAT-R has a score of 3-7. Scores of 8 or more reflect enough symptoms to more strongly predict an autism diagnosis and thus the interview may be skipped in those cases. The recommended two-step process is called M-CHAT-R/F. At 18 months without the R/F, a positive M-CHAT-R only is associated with an autism diagnosis 27% of the time (PPV, 0.27); which is unacceptable for primary care use.
Unfortunately, the M-CHAT-R/F appears to be less accurate for 18-month-olds than 24-month-olds, in part because its yes/no response options are harder for a caregiver to answer, especially for behaviors just developing, or because of lack of experience with toddlers.
An alternative modification of the original CHAT called the Quantitative CHAT or Q-CHAT-10 has a range of response options for the caregiver; for example, always/usually/sometimes/rarely/never or many times a day/a few times a day/a few times a week/less than once a week/never. The authors of the Q-CHAT-10, however, recommend a summary pass/fail result for ease of use rather than using the range of response option values in the score. We recently published a study testing accuracy using add-up scoring that utilized the entire range of response option values, called Q-CHAT-10-O (O for ordinal), for children 16-20 months old as well as cartoon depictions of the behaviors. Our study also included diagnostic testing of screen-negative as well as screen-positive children to accurately calculate sensitivity and specificity for this method. In our study, Q-CHAT-10-O with a cutoff score greater than 11 showed higher sensitivity (0.63) than either M-CHAT-R/F (0.34) or Q-CHAT-10 (0.31) for this age range although the PPV (0.35) and negative predictive value (0.92) were comparable with M-CHAT R/F. Although Q-CHAT-10-O sensitivity (0.63) is less than M-CHAT-R (without follow-up; 0.73) and specificity (0.79) is less than the two-stage R/F procedure (0.90), on balance, it is more accurate and more practical for a primary care population. After 20 months of age, the M-CHAT-R/F has adequate accuracy to rescreen, if indicated, and for the subsequent 24 month screening. Language items are often of highest value in predicting outcomes in several tools including in the screen we are now validating for 18 month olds.
The Q-CHAT-10-O with ordinal scoring and pictures can also be recommended because it shows advantages over M-CHAT-R/F with half the number of items (10 vs. 20), no requirement for a follow-up interview, and improved sensitivity. Unlike M-CHAT-R, it also contributes to equity in screening because results did not differ depending on race or socioeconomic background.
Is there an even better way to detect autism in primary care? In 2022 an article was published regarding an exciting method of early autism detection called the Social Attention and Communication Surveillance–Revised (SACS-R), an eight-item observation checklist completed at public health nurse check-ups in Australia. The observers had 4 years of nursing degree education and a 3.5-hour training session.
The SACS-R and the preschool version (for older children) had significant associations with diagnostic testing at 12, 18, 24, and 42 months. The SACS-R had excellent PPV (82.6%), NPV (98.7%), and specificity (99.6%) and moderate sensitivity (61.5%) when used between 12 and 24 months of age. Pointing, eye contact, waving “bye, bye,” social communication by showing, and pretend play were the key indicators for observations at 18 months, with absence of three or more indicating risk for autism. Different key indicators were used at the other ages, reflecting the evolution of autism symptoms. This hybrid (observation and scoring) surveillance method by professionals shows hopeful data for the critical ability to identify children at risk for autism in primary care very early but requires more than parent report, that is, new levels of autism-specific clinician training and direct observations at multiple visits over time.
The takeaway is to remember that we should all watch closely for early signs of autism, informed by research on the key findings that a professional might observe, as well as by using the best screens available. We should remember that both false positives and false negatives are inherent in screening, especially at the youngest ages. We need to combine our concern with the parent’s concern as well as screen results and be sure to follow-up closely as symptoms can change in even a few months. Many factors may prevent a family from returning to see us or following our advice to go for testing or intervention, so tracking the child and their service use is an important part of the good care we strive to provide children with autism.
Other screening tools
You may have heard of other parent-report screens for autism. It is important to compare their accuracy specifically for 18-month-olds in a community setting.
- The Infant Toddler Checklist (https://psychology-tools.com/test/infant-toddler-checklist) has moderate overall psychometrics with sensitivity ranging from 0.55 to 0.77; specificity from 0.42 to 0.85; PPV from 0.20 to 0.55; and NPV from 0.83 to 0.94. However, the data were based on a sample including both community-dwelling toddlers and those with a family history of autism.
- The Brief Infant-Toddler Social and Emotional Assessment (https://eprovide.mapi-trust.org/instruments/brief-infant-toddler-social-emotional-assessment/) – the screen’s four autism-specific scales had high specificity (84%-90%) but low sensitivity (40%-52%).
- Canvas Dx (https://canvasdx.com/) from the Cognoa company is not a parent-report measure but rather a three-part evaluation including an app-based parent questionnaire, parent uploads of home videos analyzed by a specialist, and a 13- to 15-item primary care physician observational checklist. There were 56 diagnosed of the 426 children in the 18- to 24-month-old range from a sample of children presenting with parent or clinician concerns rather than from a community sample.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
References
Sturner R et al. Autism screening at 18 months of age: A comparison of the Q-CHAT-10 and M-CHAT screeners. Molecular Autism. Jan 3;13(1):2.
Barbaro J et al. Diagnostic accuracy of the Social Attention and Communication Surveillance–Revised with preschool tool for early autism detection in very young children. JAMA Netw Open. 2022;5(3):e2146415.







