User login
The benefits of first-trimester fetal heart evaluation
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
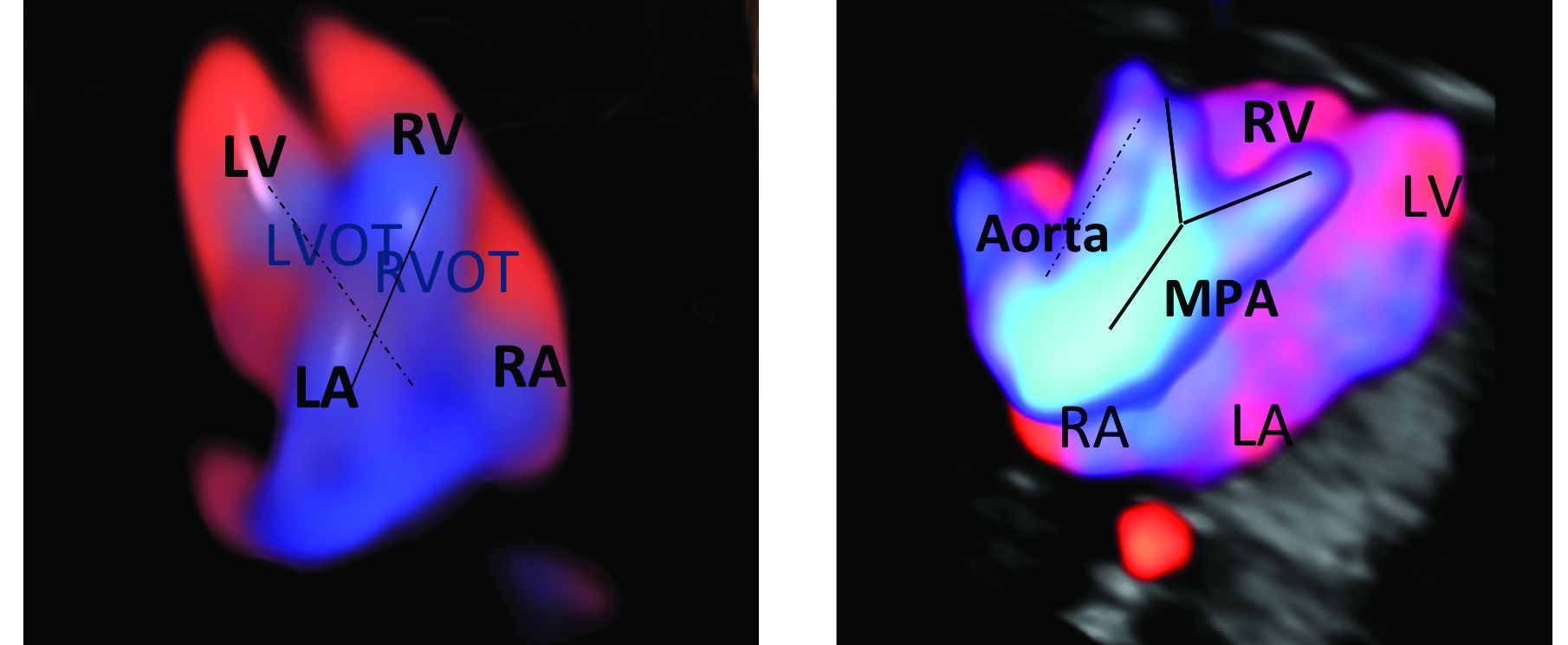
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.
Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.
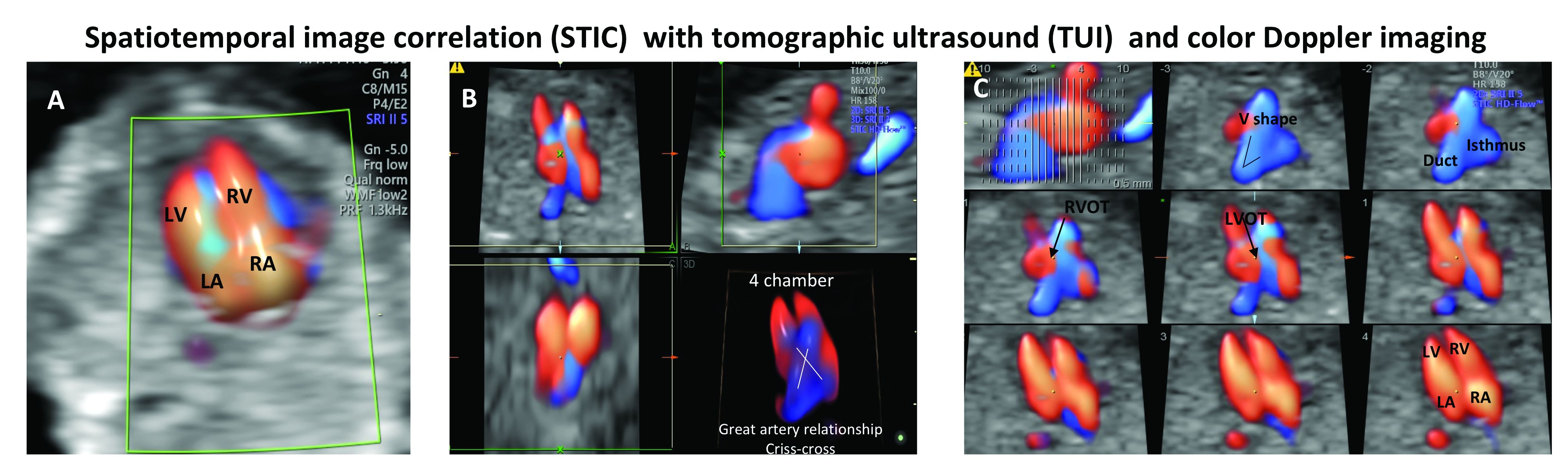
In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.

Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.

In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.

Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.

In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
Considering congenital heart defects early
Regardless of political or ideological views, detecting the embryonic heartbeat in the first trimester is a major milestone for a patient. Measured via ultrasound, normal beating of 90-110 bpm around 6 weeks’ gestation indicates a high probability of a successful pregnancy. Once the embryo becomes a fetus, around gestational weeks 8-9, a strong fetal heartbeat of 140-170 bpm should be detected. Finding a heartbeat is a reassuring sign. However, simply seeing and/or hearing the heart is not enough to ensure that the fetus will develop without problems.
Congenital heart defects (CHDs) are the most common birth defects worldwide and, although many CHDs can be mild forms, approximately 25% are severe forms requiring early detection and intervention.1 In addition, CHDs in the fetus can cause miscarriage, stillbirth, and infant deaths.
A 2014 analysis of data from the Wisconsin Stillbirth Service Program revealed that 2 An analysis of the Active Malformations Surveillance Program at Brigham and Women’s Hospital also revealed CHDs as a major cause of stillbirths.3 In addition, a retrospective study of the Metropolitan Atlanta Congenital Defects program showed that, although 1-year survival of infants with severe CHDs has improved over the last 4 decades, mortality remains high.1
Because advances in medicine and surgical procedures have significantly reduced deaths attributable to CHDs, more women with a preexisting heart condition are becoming pregnant. Women who have a CHD, even if corrected, can experience pregnancy complications such as arrhythmias, thrombosis, and cardiac dysfunction. In addition, babies of women with CHDs have a higher risk of developing cardiac defects as well.
Therefore, it is critical that we closely monitor our patients – both the mother and her baby – to ensure that the fetal heart is present, functional, and developing normally. We have invited Dr. Shifa Turan, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland and director of the Fetal Heart Program at the University of Maryland Medical Center, both in Baltimore, to discuss the fetal heart. In this first section of a two-part series, Dr. Turan addresses how we can and should monitor fetal heart development.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
References
1. Pediatrics. 2013 May. doi: 10.1542/peds.2012-3435).
2. Am J Med Genet A. 2014 Mar. doi: 10.1002/ajmg.a.36366.
3. Birth Defects Res. 2018 Jan. 29. doi: 10.1002/bdr2.1097.
Regardless of political or ideological views, detecting the embryonic heartbeat in the first trimester is a major milestone for a patient. Measured via ultrasound, normal beating of 90-110 bpm around 6 weeks’ gestation indicates a high probability of a successful pregnancy. Once the embryo becomes a fetus, around gestational weeks 8-9, a strong fetal heartbeat of 140-170 bpm should be detected. Finding a heartbeat is a reassuring sign. However, simply seeing and/or hearing the heart is not enough to ensure that the fetus will develop without problems.
Congenital heart defects (CHDs) are the most common birth defects worldwide and, although many CHDs can be mild forms, approximately 25% are severe forms requiring early detection and intervention.1 In addition, CHDs in the fetus can cause miscarriage, stillbirth, and infant deaths.
A 2014 analysis of data from the Wisconsin Stillbirth Service Program revealed that 2 An analysis of the Active Malformations Surveillance Program at Brigham and Women’s Hospital also revealed CHDs as a major cause of stillbirths.3 In addition, a retrospective study of the Metropolitan Atlanta Congenital Defects program showed that, although 1-year survival of infants with severe CHDs has improved over the last 4 decades, mortality remains high.1
Because advances in medicine and surgical procedures have significantly reduced deaths attributable to CHDs, more women with a preexisting heart condition are becoming pregnant. Women who have a CHD, even if corrected, can experience pregnancy complications such as arrhythmias, thrombosis, and cardiac dysfunction. In addition, babies of women with CHDs have a higher risk of developing cardiac defects as well.
Therefore, it is critical that we closely monitor our patients – both the mother and her baby – to ensure that the fetal heart is present, functional, and developing normally. We have invited Dr. Shifa Turan, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland and director of the Fetal Heart Program at the University of Maryland Medical Center, both in Baltimore, to discuss the fetal heart. In this first section of a two-part series, Dr. Turan addresses how we can and should monitor fetal heart development.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
References
1. Pediatrics. 2013 May. doi: 10.1542/peds.2012-3435).
2. Am J Med Genet A. 2014 Mar. doi: 10.1002/ajmg.a.36366.
3. Birth Defects Res. 2018 Jan. 29. doi: 10.1002/bdr2.1097.
Regardless of political or ideological views, detecting the embryonic heartbeat in the first trimester is a major milestone for a patient. Measured via ultrasound, normal beating of 90-110 bpm around 6 weeks’ gestation indicates a high probability of a successful pregnancy. Once the embryo becomes a fetus, around gestational weeks 8-9, a strong fetal heartbeat of 140-170 bpm should be detected. Finding a heartbeat is a reassuring sign. However, simply seeing and/or hearing the heart is not enough to ensure that the fetus will develop without problems.
Congenital heart defects (CHDs) are the most common birth defects worldwide and, although many CHDs can be mild forms, approximately 25% are severe forms requiring early detection and intervention.1 In addition, CHDs in the fetus can cause miscarriage, stillbirth, and infant deaths.
A 2014 analysis of data from the Wisconsin Stillbirth Service Program revealed that 2 An analysis of the Active Malformations Surveillance Program at Brigham and Women’s Hospital also revealed CHDs as a major cause of stillbirths.3 In addition, a retrospective study of the Metropolitan Atlanta Congenital Defects program showed that, although 1-year survival of infants with severe CHDs has improved over the last 4 decades, mortality remains high.1
Because advances in medicine and surgical procedures have significantly reduced deaths attributable to CHDs, more women with a preexisting heart condition are becoming pregnant. Women who have a CHD, even if corrected, can experience pregnancy complications such as arrhythmias, thrombosis, and cardiac dysfunction. In addition, babies of women with CHDs have a higher risk of developing cardiac defects as well.
Therefore, it is critical that we closely monitor our patients – both the mother and her baby – to ensure that the fetal heart is present, functional, and developing normally. We have invited Dr. Shifa Turan, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland and director of the Fetal Heart Program at the University of Maryland Medical Center, both in Baltimore, to discuss the fetal heart. In this first section of a two-part series, Dr. Turan addresses how we can and should monitor fetal heart development.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
References
1. Pediatrics. 2013 May. doi: 10.1542/peds.2012-3435).
2. Am J Med Genet A. 2014 Mar. doi: 10.1002/ajmg.a.36366.
3. Birth Defects Res. 2018 Jan. 29. doi: 10.1002/bdr2.1097.
The urge to move
When you have a few spare minutes on your lunch break, walk by the grade school playground in your neighborhood. Even at a quick glance you will notice that almost all the children are in motion – running, chasing, or being chased. Don’t linger too long or make repeat visits because unfortunately your presence may raise suspicions about your motives. But, even on your brief visit, you will also notice that there are a few children who are sitting down either chatting with a classmate or playing by themselves. If despite my caution you returned several days in a row, you would have noticed that the sedentary outliers tend to be the same children.
Some of the children playing alone simply may be shy loners or socially inept. But I’ve always suspected that there are some people who come in the world genetically predisposed to being sedentary. You can try to make the environment more enticing and stimulating, but the children predestined to be inactive will choose to sit and watch. Not surprisingly, most of those less active children are predestined to be overweight and obese.
At least as young children we seem to be driven to be active, and it is the few outliers who are sedentary. A recent investigation from the department of health and kinesiology at Texas A&M University at College Station is beginning to shed some light on when in our evolutionary history the urge to be active was incorporated into our genome (PLOS ONE. 2019 Apr 29. doi: 10.1371/journal.pone.0216155). The researchers found that snippets of DNA already known to be associated with levels of activity emerged in our ancestors before we were Homo sapiens about 500,000 years ago. This finding surprised the investigators who had suspected that this incorporation of a gene sequence driving activity was more likely to have occurred ten thousand years ago when subsistence farming and its physical demands first appeared.
The authors now postulate that the drive to be active coincided as pre–Homo sapiens grew larger and moved from a treed landscape into the open savanna (“To Move Is to Thrive. It’s in Our Genes” by Gretchen Reynolds. The New York Times, May 15, 2019). As J. Timothy Lightfoot, the senior investigator, observed, “If you were lazy then, you did not survive.”
Our observation of a playground in contact motion is probably evidence that those snippets of DNA still are buried in our genome. However, it is abundantly clear that in North America one doesn’t need to be active to survive, at least in the sense of being reproductively fit. It only takes a few us who must be physically active to grow and build things that we in the sedentary majority can buy or trade for.
There are some of us who have inherited some DNA snippets that drive us to be active post early childhood. My father walked two or three times a day until a few months before his death at 92, and not because someone told him it do it for his health. Like him, I just feel better if I have spent a couple of hours being active every day.
The challenge for us as pediatricians is to help families create environments that foster continued activity by discouraging sedentary entertainments and modeling active lifestyles. For example, simple things like choosing a spot at the periphery of the parking lot instead of close to the store. Choosing stairs instead of the elevator. Of course, anything you will be doing is artificial because the truth is we don’t need to be active to survive even though the urge to move is deeply rooted in our genes.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When you have a few spare minutes on your lunch break, walk by the grade school playground in your neighborhood. Even at a quick glance you will notice that almost all the children are in motion – running, chasing, or being chased. Don’t linger too long or make repeat visits because unfortunately your presence may raise suspicions about your motives. But, even on your brief visit, you will also notice that there are a few children who are sitting down either chatting with a classmate or playing by themselves. If despite my caution you returned several days in a row, you would have noticed that the sedentary outliers tend to be the same children.
Some of the children playing alone simply may be shy loners or socially inept. But I’ve always suspected that there are some people who come in the world genetically predisposed to being sedentary. You can try to make the environment more enticing and stimulating, but the children predestined to be inactive will choose to sit and watch. Not surprisingly, most of those less active children are predestined to be overweight and obese.
At least as young children we seem to be driven to be active, and it is the few outliers who are sedentary. A recent investigation from the department of health and kinesiology at Texas A&M University at College Station is beginning to shed some light on when in our evolutionary history the urge to be active was incorporated into our genome (PLOS ONE. 2019 Apr 29. doi: 10.1371/journal.pone.0216155). The researchers found that snippets of DNA already known to be associated with levels of activity emerged in our ancestors before we were Homo sapiens about 500,000 years ago. This finding surprised the investigators who had suspected that this incorporation of a gene sequence driving activity was more likely to have occurred ten thousand years ago when subsistence farming and its physical demands first appeared.
The authors now postulate that the drive to be active coincided as pre–Homo sapiens grew larger and moved from a treed landscape into the open savanna (“To Move Is to Thrive. It’s in Our Genes” by Gretchen Reynolds. The New York Times, May 15, 2019). As J. Timothy Lightfoot, the senior investigator, observed, “If you were lazy then, you did not survive.”
Our observation of a playground in contact motion is probably evidence that those snippets of DNA still are buried in our genome. However, it is abundantly clear that in North America one doesn’t need to be active to survive, at least in the sense of being reproductively fit. It only takes a few us who must be physically active to grow and build things that we in the sedentary majority can buy or trade for.
There are some of us who have inherited some DNA snippets that drive us to be active post early childhood. My father walked two or three times a day until a few months before his death at 92, and not because someone told him it do it for his health. Like him, I just feel better if I have spent a couple of hours being active every day.
The challenge for us as pediatricians is to help families create environments that foster continued activity by discouraging sedentary entertainments and modeling active lifestyles. For example, simple things like choosing a spot at the periphery of the parking lot instead of close to the store. Choosing stairs instead of the elevator. Of course, anything you will be doing is artificial because the truth is we don’t need to be active to survive even though the urge to move is deeply rooted in our genes.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When you have a few spare minutes on your lunch break, walk by the grade school playground in your neighborhood. Even at a quick glance you will notice that almost all the children are in motion – running, chasing, or being chased. Don’t linger too long or make repeat visits because unfortunately your presence may raise suspicions about your motives. But, even on your brief visit, you will also notice that there are a few children who are sitting down either chatting with a classmate or playing by themselves. If despite my caution you returned several days in a row, you would have noticed that the sedentary outliers tend to be the same children.
Some of the children playing alone simply may be shy loners or socially inept. But I’ve always suspected that there are some people who come in the world genetically predisposed to being sedentary. You can try to make the environment more enticing and stimulating, but the children predestined to be inactive will choose to sit and watch. Not surprisingly, most of those less active children are predestined to be overweight and obese.
At least as young children we seem to be driven to be active, and it is the few outliers who are sedentary. A recent investigation from the department of health and kinesiology at Texas A&M University at College Station is beginning to shed some light on when in our evolutionary history the urge to be active was incorporated into our genome (PLOS ONE. 2019 Apr 29. doi: 10.1371/journal.pone.0216155). The researchers found that snippets of DNA already known to be associated with levels of activity emerged in our ancestors before we were Homo sapiens about 500,000 years ago. This finding surprised the investigators who had suspected that this incorporation of a gene sequence driving activity was more likely to have occurred ten thousand years ago when subsistence farming and its physical demands first appeared.
The authors now postulate that the drive to be active coincided as pre–Homo sapiens grew larger and moved from a treed landscape into the open savanna (“To Move Is to Thrive. It’s in Our Genes” by Gretchen Reynolds. The New York Times, May 15, 2019). As J. Timothy Lightfoot, the senior investigator, observed, “If you were lazy then, you did not survive.”
Our observation of a playground in contact motion is probably evidence that those snippets of DNA still are buried in our genome. However, it is abundantly clear that in North America one doesn’t need to be active to survive, at least in the sense of being reproductively fit. It only takes a few us who must be physically active to grow and build things that we in the sedentary majority can buy or trade for.
There are some of us who have inherited some DNA snippets that drive us to be active post early childhood. My father walked two or three times a day until a few months before his death at 92, and not because someone told him it do it for his health. Like him, I just feel better if I have spent a couple of hours being active every day.
The challenge for us as pediatricians is to help families create environments that foster continued activity by discouraging sedentary entertainments and modeling active lifestyles. For example, simple things like choosing a spot at the periphery of the parking lot instead of close to the store. Choosing stairs instead of the elevator. Of course, anything you will be doing is artificial because the truth is we don’t need to be active to survive even though the urge to move is deeply rooted in our genes.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Structured Approach to Venous Access Associated with Zero Risk of Pneumothorax During Cardiac Device Implant Procedures
Iatrogenic pneumothorax, an acute serious complication, is reported to occur in 0.1% to 2% of permanent trans-venous cardiac device implant procedures. 1,2 A National Cardiovascular Data Registry analysis of data between January 2006 and December 2008 found that pneumothorax incidence after a new defibrillator implant was 0.5%. 1 Among 4355 Danish patients undergoing a new device implant, 0.9% experienced pneumothorax requiring drainage and 0.7% had pneumothorax treated conservatively. 2 Studies have shown a higher risk of complications when procedures were performed at low-volume centers compared with the highest volume quartile (odds ratio, 1.26; 95% confidence interval, 1.05-1.52) or when procedures were performed by low-volume operators. 1
Methods. At 2 community hospitals, a project to reduce pneumothorax risk related to new device implants was implemented. This project consisted of obtaining a pre-procedure venogram (right anterior oblique [RAO] view, 12–18 degrees, 42 cm magnification), creating a subcutaneous pocket first and then obtaining axillary venous access with a 4Fr micro-puncture needle, and obtaining a post-procedure chest radiograph. During venous access, the needle was never advanced beyond the inner border of the first rib. This new process was fully implemented by January 2015. A chart review of all patients who underwent a new device implant between January 2015 and July 2017 at the 2 community medical centers was performed.
Results. Seventy patients received new implants during the review period (31 female, 39 male). The median age was 78 years (range, 34–94 years), median body mass index was 29.05 (range, 17.3–67.9), median procedural time was 70 minutes (range, 26–146 minutes), and median fluoroscopic time was 6.4 minutes (range, 0.5–35.7 minutes). A total of 131 independent venous accesses were obtained to implant 42 pacemakers and 28 defibrillators (10 single, 54 dual, and 6 biventricular devices). Of these accesses, 127 were axillary and the remainder were cephalic. There was no incidence of pneumothorax reported during these venous accesses.
Discussion. A structured approach to venous access during device implants was associated with zero incidence of pneumothorax in a low-volume center where implants were performed by a low-volume trained operator. The venogram eliminates “blind attempts,” and the RAO view reduces the likelihood of going too posterior. Using caudal fluoroscopy and targeting the axillary vein, other groups have reported a 0% to 0.2% risk for acute pneumothorax in larger patient groups. 3,4 Creating a subcutaneous pocket first allows the needle to be aligned more longitudinally along the course of the vein. The 4Fr needle increases the ratio of vein-to-needle surface area, reducing risk for pneumothorax.
Standardization of venous access can potentially reduce iatrogenic pneumothorax risk to a never event, similar to the approach used to prevent central line–associated blood stream infections. 5
Benjamin Carmel
Lake Erie College of Osteopathic Medicine
Bradenton, FL
Indiresha R. Iyer, MD
Case Western Reserve University
Cleveland, OH
Corresponding author: Indiresha R. Iyer, MD, Indiresha.iyer@ uhhospitals.org.
Financial disclosures: None.
1. Freeman JV, Wang Y, Curtis JP, et al. The relation between hospital procedure volume and complications of cardioverter-defibrillator implantation from the implantable cardioverter-defibrillator registry. J Am Coll Cardiol . 2010; 56:1133-1139.
2. Kirkfeldt RE, Johansen JB, Nohr, EA, et al. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark, Eur Heart J . 2014;35:1186–1194.
3. Yang F, Kulbak GA. New trick to a routine procedure: taking the fear out of the axillary vein stick using the 35° caudal view. Europace . 2015;17:1157-1160.
4. Hettiarachchi EMS, Arsene C, Fares S, et al. Fluoroscopy-guided axillary vein puncture, a reliable method to prevent acute complications associated with pacemaker, defibrillator, and cardiac resynchronization therapy leads insertion. J Cardiovasc Dis Diagn. 2014;2:136.
5. Chu H, Cosgrove S, Sexton B, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl
Iatrogenic pneumothorax, an acute serious complication, is reported to occur in 0.1% to 2% of permanent trans-venous cardiac device implant procedures. 1,2 A National Cardiovascular Data Registry analysis of data between January 2006 and December 2008 found that pneumothorax incidence after a new defibrillator implant was 0.5%. 1 Among 4355 Danish patients undergoing a new device implant, 0.9% experienced pneumothorax requiring drainage and 0.7% had pneumothorax treated conservatively. 2 Studies have shown a higher risk of complications when procedures were performed at low-volume centers compared with the highest volume quartile (odds ratio, 1.26; 95% confidence interval, 1.05-1.52) or when procedures were performed by low-volume operators. 1
Methods. At 2 community hospitals, a project to reduce pneumothorax risk related to new device implants was implemented. This project consisted of obtaining a pre-procedure venogram (right anterior oblique [RAO] view, 12–18 degrees, 42 cm magnification), creating a subcutaneous pocket first and then obtaining axillary venous access with a 4Fr micro-puncture needle, and obtaining a post-procedure chest radiograph. During venous access, the needle was never advanced beyond the inner border of the first rib. This new process was fully implemented by January 2015. A chart review of all patients who underwent a new device implant between January 2015 and July 2017 at the 2 community medical centers was performed.
Results. Seventy patients received new implants during the review period (31 female, 39 male). The median age was 78 years (range, 34–94 years), median body mass index was 29.05 (range, 17.3–67.9), median procedural time was 70 minutes (range, 26–146 minutes), and median fluoroscopic time was 6.4 minutes (range, 0.5–35.7 minutes). A total of 131 independent venous accesses were obtained to implant 42 pacemakers and 28 defibrillators (10 single, 54 dual, and 6 biventricular devices). Of these accesses, 127 were axillary and the remainder were cephalic. There was no incidence of pneumothorax reported during these venous accesses.
Discussion. A structured approach to venous access during device implants was associated with zero incidence of pneumothorax in a low-volume center where implants were performed by a low-volume trained operator. The venogram eliminates “blind attempts,” and the RAO view reduces the likelihood of going too posterior. Using caudal fluoroscopy and targeting the axillary vein, other groups have reported a 0% to 0.2% risk for acute pneumothorax in larger patient groups. 3,4 Creating a subcutaneous pocket first allows the needle to be aligned more longitudinally along the course of the vein. The 4Fr needle increases the ratio of vein-to-needle surface area, reducing risk for pneumothorax.
Standardization of venous access can potentially reduce iatrogenic pneumothorax risk to a never event, similar to the approach used to prevent central line–associated blood stream infections. 5
Benjamin Carmel
Lake Erie College of Osteopathic Medicine
Bradenton, FL
Indiresha R. Iyer, MD
Case Western Reserve University
Cleveland, OH
Corresponding author: Indiresha R. Iyer, MD, Indiresha.iyer@ uhhospitals.org.
Financial disclosures: None.
Iatrogenic pneumothorax, an acute serious complication, is reported to occur in 0.1% to 2% of permanent trans-venous cardiac device implant procedures. 1,2 A National Cardiovascular Data Registry analysis of data between January 2006 and December 2008 found that pneumothorax incidence after a new defibrillator implant was 0.5%. 1 Among 4355 Danish patients undergoing a new device implant, 0.9% experienced pneumothorax requiring drainage and 0.7% had pneumothorax treated conservatively. 2 Studies have shown a higher risk of complications when procedures were performed at low-volume centers compared with the highest volume quartile (odds ratio, 1.26; 95% confidence interval, 1.05-1.52) or when procedures were performed by low-volume operators. 1
Methods. At 2 community hospitals, a project to reduce pneumothorax risk related to new device implants was implemented. This project consisted of obtaining a pre-procedure venogram (right anterior oblique [RAO] view, 12–18 degrees, 42 cm magnification), creating a subcutaneous pocket first and then obtaining axillary venous access with a 4Fr micro-puncture needle, and obtaining a post-procedure chest radiograph. During venous access, the needle was never advanced beyond the inner border of the first rib. This new process was fully implemented by January 2015. A chart review of all patients who underwent a new device implant between January 2015 and July 2017 at the 2 community medical centers was performed.
Results. Seventy patients received new implants during the review period (31 female, 39 male). The median age was 78 years (range, 34–94 years), median body mass index was 29.05 (range, 17.3–67.9), median procedural time was 70 minutes (range, 26–146 minutes), and median fluoroscopic time was 6.4 minutes (range, 0.5–35.7 minutes). A total of 131 independent venous accesses were obtained to implant 42 pacemakers and 28 defibrillators (10 single, 54 dual, and 6 biventricular devices). Of these accesses, 127 were axillary and the remainder were cephalic. There was no incidence of pneumothorax reported during these venous accesses.
Discussion. A structured approach to venous access during device implants was associated with zero incidence of pneumothorax in a low-volume center where implants were performed by a low-volume trained operator. The venogram eliminates “blind attempts,” and the RAO view reduces the likelihood of going too posterior. Using caudal fluoroscopy and targeting the axillary vein, other groups have reported a 0% to 0.2% risk for acute pneumothorax in larger patient groups. 3,4 Creating a subcutaneous pocket first allows the needle to be aligned more longitudinally along the course of the vein. The 4Fr needle increases the ratio of vein-to-needle surface area, reducing risk for pneumothorax.
Standardization of venous access can potentially reduce iatrogenic pneumothorax risk to a never event, similar to the approach used to prevent central line–associated blood stream infections. 5
Benjamin Carmel
Lake Erie College of Osteopathic Medicine
Bradenton, FL
Indiresha R. Iyer, MD
Case Western Reserve University
Cleveland, OH
Corresponding author: Indiresha R. Iyer, MD, Indiresha.iyer@ uhhospitals.org.
Financial disclosures: None.
1. Freeman JV, Wang Y, Curtis JP, et al. The relation between hospital procedure volume and complications of cardioverter-defibrillator implantation from the implantable cardioverter-defibrillator registry. J Am Coll Cardiol . 2010; 56:1133-1139.
2. Kirkfeldt RE, Johansen JB, Nohr, EA, et al. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark, Eur Heart J . 2014;35:1186–1194.
3. Yang F, Kulbak GA. New trick to a routine procedure: taking the fear out of the axillary vein stick using the 35° caudal view. Europace . 2015;17:1157-1160.
4. Hettiarachchi EMS, Arsene C, Fares S, et al. Fluoroscopy-guided axillary vein puncture, a reliable method to prevent acute complications associated with pacemaker, defibrillator, and cardiac resynchronization therapy leads insertion. J Cardiovasc Dis Diagn. 2014;2:136.
5. Chu H, Cosgrove S, Sexton B, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl
1. Freeman JV, Wang Y, Curtis JP, et al. The relation between hospital procedure volume and complications of cardioverter-defibrillator implantation from the implantable cardioverter-defibrillator registry. J Am Coll Cardiol . 2010; 56:1133-1139.
2. Kirkfeldt RE, Johansen JB, Nohr, EA, et al. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark, Eur Heart J . 2014;35:1186–1194.
3. Yang F, Kulbak GA. New trick to a routine procedure: taking the fear out of the axillary vein stick using the 35° caudal view. Europace . 2015;17:1157-1160.
4. Hettiarachchi EMS, Arsene C, Fares S, et al. Fluoroscopy-guided axillary vein puncture, a reliable method to prevent acute complications associated with pacemaker, defibrillator, and cardiac resynchronization therapy leads insertion. J Cardiovasc Dis Diagn. 2014;2:136.
5. Chu H, Cosgrove S, Sexton B, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl
Pediatric gastroesophageal reflux
In a 2018 guideline, the writing committee defined GER as reflux of stomach contents to the esophagus. GER is considered pathologic and, therefore, gastroesophageal reflux disease (GERD) when it is associated with troublesome symptoms and/or complications that can include esophagitis and aspiration.
Infants
GERD is difficult to diagnose in infants. The symptoms of GERD, such as crying after feeds, regurgitation, and irritability, occur commonly in all infants and in any individual infant may not be reflective of GERD. Regurgitation is common, frequent and normal in infants up to 6 months of age. A common challenge occurs when families request treatment for infants with irritability, back arching, and/or regurgitation who are otherwise doing well. In this group of infants it is important to recognize that neither testing nor therapy is indicated unless there is difficulty with feeding, growth, acquisition of milestones, or red flag signs.
In infants with recurrent regurgitation history, physical exam is usually sufficient to distinguish uncomplicated GER from GERD and other more worrisome diagnoses. Red flag symptoms raise the possibility of a different diagnosis. Red flag symptoms include weight loss; lethargy; excessive irritability/pain; onset of vomiting for more than 6 months or persisting past 12-18 months of age; rapidly increasing head circumference; persistent forceful, nocturnal, bloody, or bilious vomiting; abdominal distention; rectal bleeding; and chronic diarrhea. GERD that starts after 6 months of age or which persists after 12 months of age warrants further evaluation, often with referral to a pediatric gastroenterologist.
When GERD is suspected, the first therapeutic steps are to institute behavioral changes. Caregivers should avoid overfeeding and modify the feeding pattern to more frequent feedings consisting of less volume at each feed. The addition of thickeners to feeds does reduce regurgitation, although it may not affect other GERD signs and symptoms. Formula can be thickened with rice cereal, which tends to be an affordable choice that doesn’t clog nipples. Enzymes present in breast milk digest cereal thickeners, so breast milk can be thickened with xanthum gum (after 1 year of age) or carob bean–based products (after 42 weeks gestation).
If these modifications do not improve symptoms, the next step is to change the type of feeds. Some infants in whom GERD is suspected actually have cow’s milk protein allergy (CMPA), so a trial of cow’s milk elimination is warranted. A breastfeeding mother can eliminate all dairy from her diet including casein and whey. Caregivers can switch to an extensively hydrolyzed formula or an amino acid–based formula. The guideline do not recommend soy-based formulas because they are not available in Europe and because a significant percentage of infants with CMPA also develop allergy to soy, and they do not recommend rice hydrolysate formula because of a lack of evidence. Dairy can be reintroduced at a later point. While positional changes including elevating the head of the crib or placing the infant in the left lateral position can help decrease GERD, the American Academy of Pediatrics strongly discourages these positions because of safety concerns, so the guidelines do not recommend positional change.
If a 2-4 week trial of nonpharmacologic interventions fails, the next step is referral to a pediatric gastroenterologist. If a pediatric gastroenterologist is not available, a 4-8 week trial of acid suppressive medication may be given. No trial has shown utility of a trial of acid suppression as a diagnostic test for GERD. Medication should only be used in infants with strongly suspected GERD and, per the guidelines, “should not be used for the treatment of visible regurgitation in otherwise healthy infants.” Medications to treat GER do not have evidence of efficacy, and there is evidence of an increased risk of infection with use of acid suppression, including an increased risk of necrotizing enterocolitis, pneumonia, upper respiratory tract infections, sepsis, urinary tract infections, and Clostridium difficile. If used, proton-pump inhibitors are preferred over histamine-2 receptor blockers. Antacids and alginates are not recommended.
Older children
In children with heartburn or regurgitation without red flag symptoms, a trial of lifestyle changes and dietary education may be initiated. If a child is overweight, it is important to inform the patient and parents that excess body weight is associated with GERD. The head of the bed can be elevated along with left lateral positioning. The guidelines do not support any probiotics or herbal medicines.
If bothersome symptoms persist, a trial of acid-suppressing medication for 4-8 weeks is reasonable. A PPI is preferred to a histamine-2 receptor blocker. PPI safety studies are lacking, but case studies suggest an increase in infections in children taking acid-suppressing medications. Therefore, as with infants, if medications are used they should be prescribed at the lowest dose and for the shortest period of time possible. If medications are not helping, or need to be used long term, referral to a pediatric gastroenterologist can be considered. Of note, the guidelines do support a 4-8 week trial of PPIs in older children as a diagnostic test; this differs from the recommendations for infants, in whom a trial for diagnostic purposes is discouraged.
Diagnostic testing
Refer to a gastroenterologist for endoscopy in cases of persistent symptoms despite PPI use or failure to wean off medication. If there are no erosions, pH monitoring with pH-impedance monitoring or pH-metry can help distinguish between nonerosive reflux disease (NERD), reflux hypersensitivity, and functional heartburn. If it is performed when a child is off of PPIs, endoscopy can also diagnose PPI-responsive eosinophilic esophagitis. Barium contrast, abdominal ultrasonography, and manometry may be considered during the course of a search for an alternative diagnosis, but they should not be used to diagnose or confirm GERD.
The bottom line
Most GER is physiologic and does not need treatment. First-line treatment for GERD in infants and children is nonpharmacologic intervention.
Reference
Rosen R et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-554.
Dr. Oh is a third year resident in the Family Medicine Residency at Abington-Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
In a 2018 guideline, the writing committee defined GER as reflux of stomach contents to the esophagus. GER is considered pathologic and, therefore, gastroesophageal reflux disease (GERD) when it is associated with troublesome symptoms and/or complications that can include esophagitis and aspiration.
Infants
GERD is difficult to diagnose in infants. The symptoms of GERD, such as crying after feeds, regurgitation, and irritability, occur commonly in all infants and in any individual infant may not be reflective of GERD. Regurgitation is common, frequent and normal in infants up to 6 months of age. A common challenge occurs when families request treatment for infants with irritability, back arching, and/or regurgitation who are otherwise doing well. In this group of infants it is important to recognize that neither testing nor therapy is indicated unless there is difficulty with feeding, growth, acquisition of milestones, or red flag signs.
In infants with recurrent regurgitation history, physical exam is usually sufficient to distinguish uncomplicated GER from GERD and other more worrisome diagnoses. Red flag symptoms raise the possibility of a different diagnosis. Red flag symptoms include weight loss; lethargy; excessive irritability/pain; onset of vomiting for more than 6 months or persisting past 12-18 months of age; rapidly increasing head circumference; persistent forceful, nocturnal, bloody, or bilious vomiting; abdominal distention; rectal bleeding; and chronic diarrhea. GERD that starts after 6 months of age or which persists after 12 months of age warrants further evaluation, often with referral to a pediatric gastroenterologist.
When GERD is suspected, the first therapeutic steps are to institute behavioral changes. Caregivers should avoid overfeeding and modify the feeding pattern to more frequent feedings consisting of less volume at each feed. The addition of thickeners to feeds does reduce regurgitation, although it may not affect other GERD signs and symptoms. Formula can be thickened with rice cereal, which tends to be an affordable choice that doesn’t clog nipples. Enzymes present in breast milk digest cereal thickeners, so breast milk can be thickened with xanthum gum (after 1 year of age) or carob bean–based products (after 42 weeks gestation).
If these modifications do not improve symptoms, the next step is to change the type of feeds. Some infants in whom GERD is suspected actually have cow’s milk protein allergy (CMPA), so a trial of cow’s milk elimination is warranted. A breastfeeding mother can eliminate all dairy from her diet including casein and whey. Caregivers can switch to an extensively hydrolyzed formula or an amino acid–based formula. The guideline do not recommend soy-based formulas because they are not available in Europe and because a significant percentage of infants with CMPA also develop allergy to soy, and they do not recommend rice hydrolysate formula because of a lack of evidence. Dairy can be reintroduced at a later point. While positional changes including elevating the head of the crib or placing the infant in the left lateral position can help decrease GERD, the American Academy of Pediatrics strongly discourages these positions because of safety concerns, so the guidelines do not recommend positional change.
If a 2-4 week trial of nonpharmacologic interventions fails, the next step is referral to a pediatric gastroenterologist. If a pediatric gastroenterologist is not available, a 4-8 week trial of acid suppressive medication may be given. No trial has shown utility of a trial of acid suppression as a diagnostic test for GERD. Medication should only be used in infants with strongly suspected GERD and, per the guidelines, “should not be used for the treatment of visible regurgitation in otherwise healthy infants.” Medications to treat GER do not have evidence of efficacy, and there is evidence of an increased risk of infection with use of acid suppression, including an increased risk of necrotizing enterocolitis, pneumonia, upper respiratory tract infections, sepsis, urinary tract infections, and Clostridium difficile. If used, proton-pump inhibitors are preferred over histamine-2 receptor blockers. Antacids and alginates are not recommended.
Older children
In children with heartburn or regurgitation without red flag symptoms, a trial of lifestyle changes and dietary education may be initiated. If a child is overweight, it is important to inform the patient and parents that excess body weight is associated with GERD. The head of the bed can be elevated along with left lateral positioning. The guidelines do not support any probiotics or herbal medicines.
If bothersome symptoms persist, a trial of acid-suppressing medication for 4-8 weeks is reasonable. A PPI is preferred to a histamine-2 receptor blocker. PPI safety studies are lacking, but case studies suggest an increase in infections in children taking acid-suppressing medications. Therefore, as with infants, if medications are used they should be prescribed at the lowest dose and for the shortest period of time possible. If medications are not helping, or need to be used long term, referral to a pediatric gastroenterologist can be considered. Of note, the guidelines do support a 4-8 week trial of PPIs in older children as a diagnostic test; this differs from the recommendations for infants, in whom a trial for diagnostic purposes is discouraged.
Diagnostic testing
Refer to a gastroenterologist for endoscopy in cases of persistent symptoms despite PPI use or failure to wean off medication. If there are no erosions, pH monitoring with pH-impedance monitoring or pH-metry can help distinguish between nonerosive reflux disease (NERD), reflux hypersensitivity, and functional heartburn. If it is performed when a child is off of PPIs, endoscopy can also diagnose PPI-responsive eosinophilic esophagitis. Barium contrast, abdominal ultrasonography, and manometry may be considered during the course of a search for an alternative diagnosis, but they should not be used to diagnose or confirm GERD.
The bottom line
Most GER is physiologic and does not need treatment. First-line treatment for GERD in infants and children is nonpharmacologic intervention.
Reference
Rosen R et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-554.
Dr. Oh is a third year resident in the Family Medicine Residency at Abington-Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
In a 2018 guideline, the writing committee defined GER as reflux of stomach contents to the esophagus. GER is considered pathologic and, therefore, gastroesophageal reflux disease (GERD) when it is associated with troublesome symptoms and/or complications that can include esophagitis and aspiration.
Infants
GERD is difficult to diagnose in infants. The symptoms of GERD, such as crying after feeds, regurgitation, and irritability, occur commonly in all infants and in any individual infant may not be reflective of GERD. Regurgitation is common, frequent and normal in infants up to 6 months of age. A common challenge occurs when families request treatment for infants with irritability, back arching, and/or regurgitation who are otherwise doing well. In this group of infants it is important to recognize that neither testing nor therapy is indicated unless there is difficulty with feeding, growth, acquisition of milestones, or red flag signs.
In infants with recurrent regurgitation history, physical exam is usually sufficient to distinguish uncomplicated GER from GERD and other more worrisome diagnoses. Red flag symptoms raise the possibility of a different diagnosis. Red flag symptoms include weight loss; lethargy; excessive irritability/pain; onset of vomiting for more than 6 months or persisting past 12-18 months of age; rapidly increasing head circumference; persistent forceful, nocturnal, bloody, or bilious vomiting; abdominal distention; rectal bleeding; and chronic diarrhea. GERD that starts after 6 months of age or which persists after 12 months of age warrants further evaluation, often with referral to a pediatric gastroenterologist.
When GERD is suspected, the first therapeutic steps are to institute behavioral changes. Caregivers should avoid overfeeding and modify the feeding pattern to more frequent feedings consisting of less volume at each feed. The addition of thickeners to feeds does reduce regurgitation, although it may not affect other GERD signs and symptoms. Formula can be thickened with rice cereal, which tends to be an affordable choice that doesn’t clog nipples. Enzymes present in breast milk digest cereal thickeners, so breast milk can be thickened with xanthum gum (after 1 year of age) or carob bean–based products (after 42 weeks gestation).
If these modifications do not improve symptoms, the next step is to change the type of feeds. Some infants in whom GERD is suspected actually have cow’s milk protein allergy (CMPA), so a trial of cow’s milk elimination is warranted. A breastfeeding mother can eliminate all dairy from her diet including casein and whey. Caregivers can switch to an extensively hydrolyzed formula or an amino acid–based formula. The guideline do not recommend soy-based formulas because they are not available in Europe and because a significant percentage of infants with CMPA also develop allergy to soy, and they do not recommend rice hydrolysate formula because of a lack of evidence. Dairy can be reintroduced at a later point. While positional changes including elevating the head of the crib or placing the infant in the left lateral position can help decrease GERD, the American Academy of Pediatrics strongly discourages these positions because of safety concerns, so the guidelines do not recommend positional change.
If a 2-4 week trial of nonpharmacologic interventions fails, the next step is referral to a pediatric gastroenterologist. If a pediatric gastroenterologist is not available, a 4-8 week trial of acid suppressive medication may be given. No trial has shown utility of a trial of acid suppression as a diagnostic test for GERD. Medication should only be used in infants with strongly suspected GERD and, per the guidelines, “should not be used for the treatment of visible regurgitation in otherwise healthy infants.” Medications to treat GER do not have evidence of efficacy, and there is evidence of an increased risk of infection with use of acid suppression, including an increased risk of necrotizing enterocolitis, pneumonia, upper respiratory tract infections, sepsis, urinary tract infections, and Clostridium difficile. If used, proton-pump inhibitors are preferred over histamine-2 receptor blockers. Antacids and alginates are not recommended.
Older children
In children with heartburn or regurgitation without red flag symptoms, a trial of lifestyle changes and dietary education may be initiated. If a child is overweight, it is important to inform the patient and parents that excess body weight is associated with GERD. The head of the bed can be elevated along with left lateral positioning. The guidelines do not support any probiotics or herbal medicines.
If bothersome symptoms persist, a trial of acid-suppressing medication for 4-8 weeks is reasonable. A PPI is preferred to a histamine-2 receptor blocker. PPI safety studies are lacking, but case studies suggest an increase in infections in children taking acid-suppressing medications. Therefore, as with infants, if medications are used they should be prescribed at the lowest dose and for the shortest period of time possible. If medications are not helping, or need to be used long term, referral to a pediatric gastroenterologist can be considered. Of note, the guidelines do support a 4-8 week trial of PPIs in older children as a diagnostic test; this differs from the recommendations for infants, in whom a trial for diagnostic purposes is discouraged.
Diagnostic testing
Refer to a gastroenterologist for endoscopy in cases of persistent symptoms despite PPI use or failure to wean off medication. If there are no erosions, pH monitoring with pH-impedance monitoring or pH-metry can help distinguish between nonerosive reflux disease (NERD), reflux hypersensitivity, and functional heartburn. If it is performed when a child is off of PPIs, endoscopy can also diagnose PPI-responsive eosinophilic esophagitis. Barium contrast, abdominal ultrasonography, and manometry may be considered during the course of a search for an alternative diagnosis, but they should not be used to diagnose or confirm GERD.
The bottom line
Most GER is physiologic and does not need treatment. First-line treatment for GERD in infants and children is nonpharmacologic intervention.
Reference
Rosen R et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-554.
Dr. Oh is a third year resident in the Family Medicine Residency at Abington-Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
Dear Marisol
I know you don’t remember me. We met when you were just a baby. Now that you’re older, I want to tell you a story about your mom you may not know.
When your mom became pregnant with you, it was a joyous occasion. But strange things started to happen. Your mom noticed that she was bleeding into the toilet bowl. She was told pregnancy would make her gain weight, but the opposite was happening. By her second trimester, her maternity clothes were so baggy she had to exchange them.
She went to see a gastroenterologist and told him about the bleeding. He looked at her pregnant belly, ordered no additional tests, and said he would look into the bleeding if it persisted after her pregnancy.
But that was 5 months away. In the meantime, her symptoms got worse. As you probably know by now, your mom is proactive. She sought a second opinion and then a third. Three different gastroenterologists dismissed your mom. The visits were brief; as soon as each doctor noticed she was pregnant, each deferred dealing with her – even though rectal bleeding and weight loss are in no way explained by pregnancy. Even though a colonoscopy could absolutely be safely performed during pregnancy.
A few months later, she gave birth to you. You were a healthy baby and she and your dad cried. They were so happy to meet you.
The next day, your dad stayed with you while the doctors performed a colonoscopy on your mom. To everyone’s horror, the camera saw a huge colon tumor. While the gastroenterologists had been reassuring her during her pregnancy, the tumor was gnawing through the wall of her colon and invading nearby organs.
She was wheeled on a gurney from the maternity unit to oncology. That’s where I met her.
She asked a lot of good questions, none of which we could answer. Her heart rate was in the 140s, and she developed fevers. Her cancer put her at risk for a serious infection called an abscess, and it took the option of chemotherapy off the table.
We went back and forth on what to do. We got lots of experts involved, and we went through the possibilities. We realized something terrible. There was no cure anymore. There were only trade-offs.
I will never forget the meeting between all of these doctors, your mom, and a Spanish interpreter. We gave your mom a best case scenario: 1 year.
Holding her necklace cross in one hand and your dad’s hand in the other, she repeated something over and over. The interpreter couldn’t hold back tears as she translated in a soft voice: “Please don’t let me die. Please don’t let me die. Please don’t let me die.”
We were all working so hard, doing our best to find a way out for her. Meanwhile, you stayed in the newborn nursery near the maternity ward. Every day your mom would go back and forth between oncology and the nursery to hold you.
Finally, we proceeded with surgery. It was an enormous, delicate, risky operation that took more than 10 hours. There were colorectal surgeons, urologists, and gynecologic oncologists. They scooped out not only the tumor but also your mom’s uterus, her ovaries, her bladder, and part of the abdominal wall. There was just so much cancer.
But your mom made it through the operation. Two days later, she married your dad in her hospital room while a nurse held you. She called it the best day of her life.
But we were still so worried. Everyone in the oncology unit had grown to love your mom, and we knew this was not a permanent fix. She was discharged from the hospital to rehab to get stronger. The plan was to see her in clinic and consider chemotherapy.
I usually keep a list of patients I want to follow even after I’m no longer their doctor. With your mom, I couldn’t put her on any list. It was too personal; I was too invested. I knew what the outcome would be, and I couldn’t bear to see it.
I never forgot your mom though. I decided to become an oncologist, and I thought about her when I met patients, especially young women, who had been dismissed by other doctors. I vowed to be the change as I listened to them and diagnosed them and treated them. I vowed to be a part of the system that would do better.
One day, 3 years after I met your mom, I was rotating in a colon cancer clinic and looked at the schedule. I recognized a name. Was it possible? It had to be someone with the same name. Could it really be your mom?
It was. By this time, she had finished chemotherapy. The goal was to keep the cancer from growing, but it somehow did more than that. Throughout your mom’s entire body, the cancer was gone. Her stoma was reversed, and she had gained back all the weight she lost. You were there too, defying instructions telling you not to touch the medical equipment. You hugged your mom’s leg as she made plans for a routine follow-up in 6 months.
I want you to know this story about your mom because, for the rest of your life, people will tell you what to think and how to feel. They will think it’s their business to tell you when to be worried, they will talk like they know better, and they will try to make you feel small for speaking up. I don’t have a perfect solution for all of this, except to say: Don’t let them.
But I’m not worried about you. If you grow up to be anything like your mom, you will be okay.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
I know you don’t remember me. We met when you were just a baby. Now that you’re older, I want to tell you a story about your mom you may not know.
When your mom became pregnant with you, it was a joyous occasion. But strange things started to happen. Your mom noticed that she was bleeding into the toilet bowl. She was told pregnancy would make her gain weight, but the opposite was happening. By her second trimester, her maternity clothes were so baggy she had to exchange them.
She went to see a gastroenterologist and told him about the bleeding. He looked at her pregnant belly, ordered no additional tests, and said he would look into the bleeding if it persisted after her pregnancy.
But that was 5 months away. In the meantime, her symptoms got worse. As you probably know by now, your mom is proactive. She sought a second opinion and then a third. Three different gastroenterologists dismissed your mom. The visits were brief; as soon as each doctor noticed she was pregnant, each deferred dealing with her – even though rectal bleeding and weight loss are in no way explained by pregnancy. Even though a colonoscopy could absolutely be safely performed during pregnancy.
A few months later, she gave birth to you. You were a healthy baby and she and your dad cried. They were so happy to meet you.
The next day, your dad stayed with you while the doctors performed a colonoscopy on your mom. To everyone’s horror, the camera saw a huge colon tumor. While the gastroenterologists had been reassuring her during her pregnancy, the tumor was gnawing through the wall of her colon and invading nearby organs.
She was wheeled on a gurney from the maternity unit to oncology. That’s where I met her.
She asked a lot of good questions, none of which we could answer. Her heart rate was in the 140s, and she developed fevers. Her cancer put her at risk for a serious infection called an abscess, and it took the option of chemotherapy off the table.
We went back and forth on what to do. We got lots of experts involved, and we went through the possibilities. We realized something terrible. There was no cure anymore. There were only trade-offs.
I will never forget the meeting between all of these doctors, your mom, and a Spanish interpreter. We gave your mom a best case scenario: 1 year.
Holding her necklace cross in one hand and your dad’s hand in the other, she repeated something over and over. The interpreter couldn’t hold back tears as she translated in a soft voice: “Please don’t let me die. Please don’t let me die. Please don’t let me die.”
We were all working so hard, doing our best to find a way out for her. Meanwhile, you stayed in the newborn nursery near the maternity ward. Every day your mom would go back and forth between oncology and the nursery to hold you.
Finally, we proceeded with surgery. It was an enormous, delicate, risky operation that took more than 10 hours. There were colorectal surgeons, urologists, and gynecologic oncologists. They scooped out not only the tumor but also your mom’s uterus, her ovaries, her bladder, and part of the abdominal wall. There was just so much cancer.
But your mom made it through the operation. Two days later, she married your dad in her hospital room while a nurse held you. She called it the best day of her life.
But we were still so worried. Everyone in the oncology unit had grown to love your mom, and we knew this was not a permanent fix. She was discharged from the hospital to rehab to get stronger. The plan was to see her in clinic and consider chemotherapy.
I usually keep a list of patients I want to follow even after I’m no longer their doctor. With your mom, I couldn’t put her on any list. It was too personal; I was too invested. I knew what the outcome would be, and I couldn’t bear to see it.
I never forgot your mom though. I decided to become an oncologist, and I thought about her when I met patients, especially young women, who had been dismissed by other doctors. I vowed to be the change as I listened to them and diagnosed them and treated them. I vowed to be a part of the system that would do better.
One day, 3 years after I met your mom, I was rotating in a colon cancer clinic and looked at the schedule. I recognized a name. Was it possible? It had to be someone with the same name. Could it really be your mom?
It was. By this time, she had finished chemotherapy. The goal was to keep the cancer from growing, but it somehow did more than that. Throughout your mom’s entire body, the cancer was gone. Her stoma was reversed, and she had gained back all the weight she lost. You were there too, defying instructions telling you not to touch the medical equipment. You hugged your mom’s leg as she made plans for a routine follow-up in 6 months.
I want you to know this story about your mom because, for the rest of your life, people will tell you what to think and how to feel. They will think it’s their business to tell you when to be worried, they will talk like they know better, and they will try to make you feel small for speaking up. I don’t have a perfect solution for all of this, except to say: Don’t let them.
But I’m not worried about you. If you grow up to be anything like your mom, you will be okay.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
I know you don’t remember me. We met when you were just a baby. Now that you’re older, I want to tell you a story about your mom you may not know.
When your mom became pregnant with you, it was a joyous occasion. But strange things started to happen. Your mom noticed that she was bleeding into the toilet bowl. She was told pregnancy would make her gain weight, but the opposite was happening. By her second trimester, her maternity clothes were so baggy she had to exchange them.
She went to see a gastroenterologist and told him about the bleeding. He looked at her pregnant belly, ordered no additional tests, and said he would look into the bleeding if it persisted after her pregnancy.
But that was 5 months away. In the meantime, her symptoms got worse. As you probably know by now, your mom is proactive. She sought a second opinion and then a third. Three different gastroenterologists dismissed your mom. The visits were brief; as soon as each doctor noticed she was pregnant, each deferred dealing with her – even though rectal bleeding and weight loss are in no way explained by pregnancy. Even though a colonoscopy could absolutely be safely performed during pregnancy.
A few months later, she gave birth to you. You were a healthy baby and she and your dad cried. They were so happy to meet you.
The next day, your dad stayed with you while the doctors performed a colonoscopy on your mom. To everyone’s horror, the camera saw a huge colon tumor. While the gastroenterologists had been reassuring her during her pregnancy, the tumor was gnawing through the wall of her colon and invading nearby organs.
She was wheeled on a gurney from the maternity unit to oncology. That’s where I met her.
She asked a lot of good questions, none of which we could answer. Her heart rate was in the 140s, and she developed fevers. Her cancer put her at risk for a serious infection called an abscess, and it took the option of chemotherapy off the table.
We went back and forth on what to do. We got lots of experts involved, and we went through the possibilities. We realized something terrible. There was no cure anymore. There were only trade-offs.
I will never forget the meeting between all of these doctors, your mom, and a Spanish interpreter. We gave your mom a best case scenario: 1 year.
Holding her necklace cross in one hand and your dad’s hand in the other, she repeated something over and over. The interpreter couldn’t hold back tears as she translated in a soft voice: “Please don’t let me die. Please don’t let me die. Please don’t let me die.”
We were all working so hard, doing our best to find a way out for her. Meanwhile, you stayed in the newborn nursery near the maternity ward. Every day your mom would go back and forth between oncology and the nursery to hold you.
Finally, we proceeded with surgery. It was an enormous, delicate, risky operation that took more than 10 hours. There were colorectal surgeons, urologists, and gynecologic oncologists. They scooped out not only the tumor but also your mom’s uterus, her ovaries, her bladder, and part of the abdominal wall. There was just so much cancer.
But your mom made it through the operation. Two days later, she married your dad in her hospital room while a nurse held you. She called it the best day of her life.
But we were still so worried. Everyone in the oncology unit had grown to love your mom, and we knew this was not a permanent fix. She was discharged from the hospital to rehab to get stronger. The plan was to see her in clinic and consider chemotherapy.
I usually keep a list of patients I want to follow even after I’m no longer their doctor. With your mom, I couldn’t put her on any list. It was too personal; I was too invested. I knew what the outcome would be, and I couldn’t bear to see it.
I never forgot your mom though. I decided to become an oncologist, and I thought about her when I met patients, especially young women, who had been dismissed by other doctors. I vowed to be the change as I listened to them and diagnosed them and treated them. I vowed to be a part of the system that would do better.
One day, 3 years after I met your mom, I was rotating in a colon cancer clinic and looked at the schedule. I recognized a name. Was it possible? It had to be someone with the same name. Could it really be your mom?
It was. By this time, she had finished chemotherapy. The goal was to keep the cancer from growing, but it somehow did more than that. Throughout your mom’s entire body, the cancer was gone. Her stoma was reversed, and she had gained back all the weight she lost. You were there too, defying instructions telling you not to touch the medical equipment. You hugged your mom’s leg as she made plans for a routine follow-up in 6 months.
I want you to know this story about your mom because, for the rest of your life, people will tell you what to think and how to feel. They will think it’s their business to tell you when to be worried, they will talk like they know better, and they will try to make you feel small for speaking up. I don’t have a perfect solution for all of this, except to say: Don’t let them.
But I’m not worried about you. If you grow up to be anything like your mom, you will be okay.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Timed perfectly
When I entered the examination room, I saw his alma mater’s logo on his wristwatch. He was a retired physician with a new diagnosis of leukemia who drove to see me, even though he lived closer to his beloved medical school where he had practiced his entire career.
As is frequently the case, he came to see me because he could not get the appointment he wanted in his university’s clinic for another 6 months. He called us on Friday, and 3 days later, he and I were meeting. He is still an ardent supporter of his institution, but I am now his hematologist.
As it turned out, his leukemia was asymptomatic, indolent, and required no treatment. He could have waited 6 months to be seen. But, no; he couldn’t.
This story repeats itself over and over again. A sick patient calls to be seen and is told there is no availability for weeks or months. I do not understand how health care facilities, my own included, find this acceptable.
My father was very proud of his policy to see every patient in his waiting room no matter how long his office needed to stay open. He felt that access was of primary importance to his patients and to his practice. If he didn’t see them, somebody else would. Those of us working in large academic centers do not always feel the financial consequences of patients lost because of poor service.
Luckily, I work in a large cancer center that values access as much as a small practice would. When a patient calls us with a hematologic problem, we see them in less than 7 days, unless the patient prefers a different time frame. We monitor the time it takes to see patients and proactively assess upcoming appointments to ensure insurance coverage and the availability of records. If an obstruction is identified, the case is escalated to administrative leadership to be addressed and resolved. We are very proud of this work.
However, our focus on access does not end there. Once seen, we expedite patient evaluation by assessing workflows to obtain all necessary testing as quickly as possible. By doing so, we accelerate the time it takes from diagnosis to the time we start treating (time to treat). We have always tried to reduce time to treat for acute leukemia and we have applied those lessons to patients with lymphoma and solid tumors, resulting in a 33% improvement over the last 5 years.
We not only lessen the anxiety that comes with a scary diagnosis, emerging data indicate outcomes are improved with faster treatment, too (PLoS One. 2019 Mar 1;14(3):e0213209. doi: 10.1371/journal.pone.0213209).
These efforts will be criticized by those who feel the delivery of medical care should be structured more around the physician than the patient. Certainly, the system has developed to support a mindset of “physician first.” Not only do patients have to make an appointment for the privilege of seeing us, they have to navigate significant geographic and financial hurdles for that privilege.
Once at the appointment, physicians have historically been the provider giving the “orders” while others correct them, carry them out, follow-up on the results, manage phone calls, and schedule follow-up. This hierarchy has served physicians very well, but the pyramidal structure of health care is on the verge of being upended.
Too few physicians for an increasing demand for medical attention has led to the rise of advanced practice providers (APPs), who often serve as the only provider a patient may have, particularly in rural areas. In our center, we evolved from thinking of APPs as similar to house-staff who saw patients with us and did most of the work, but could not bill, to independent providers who work with us, do most of the work, and bill for their efforts. This slow transformation of our practice will soon seem quaint as we face the rapid disruption coming to our current conception of the health care delivery system.
Technologically savvy patients already demand immediate access to unlimited supplies of consumer goods, video, audio, books, magazines, and just about anything else you can think of. Immediate access to health care at a time convenient to the patient also will become an expectation because plenty of health care delivery models already are providing it. The local pharmacy or retail store may have a physician or APP right there ready to see a patient at any time. Some physicians are already online ready for an electronic interaction. See MDLIVE and Teladoc as examples.
The nimble cancer center that embraces these trends to become more patient-centric will be the center that captures national – if not international – market share, as insurance companies and governments adjust their reimbursement models to include these services. With blood work obtained just about anywhere, what would keep a patient with immune thrombocytopenic purpura from consulting with any online hematologist she chooses, whenever she chooses?
If first impressions are important, then patient access is important. Refrains of “I don’t have clinic that day,” “the pathology has not yet been reviewed,” and “that is not a disease I take care of,” ring as hollow to me as I suspect they do to our patients. When someone in my family has a significant illness, I want them to be seen now, not later. I believe we all would want prompt, efficient service.
We should strive to provide the same level of care to our patients as we expect for our family. Patients do not know that chronic leukemia is not an emergency. Time may not be critical to us, but it is to them. The perfect time to meet their needs is now.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematology and medical oncology at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
When I entered the examination room, I saw his alma mater’s logo on his wristwatch. He was a retired physician with a new diagnosis of leukemia who drove to see me, even though he lived closer to his beloved medical school where he had practiced his entire career.
As is frequently the case, he came to see me because he could not get the appointment he wanted in his university’s clinic for another 6 months. He called us on Friday, and 3 days later, he and I were meeting. He is still an ardent supporter of his institution, but I am now his hematologist.
As it turned out, his leukemia was asymptomatic, indolent, and required no treatment. He could have waited 6 months to be seen. But, no; he couldn’t.
This story repeats itself over and over again. A sick patient calls to be seen and is told there is no availability for weeks or months. I do not understand how health care facilities, my own included, find this acceptable.
My father was very proud of his policy to see every patient in his waiting room no matter how long his office needed to stay open. He felt that access was of primary importance to his patients and to his practice. If he didn’t see them, somebody else would. Those of us working in large academic centers do not always feel the financial consequences of patients lost because of poor service.
Luckily, I work in a large cancer center that values access as much as a small practice would. When a patient calls us with a hematologic problem, we see them in less than 7 days, unless the patient prefers a different time frame. We monitor the time it takes to see patients and proactively assess upcoming appointments to ensure insurance coverage and the availability of records. If an obstruction is identified, the case is escalated to administrative leadership to be addressed and resolved. We are very proud of this work.
However, our focus on access does not end there. Once seen, we expedite patient evaluation by assessing workflows to obtain all necessary testing as quickly as possible. By doing so, we accelerate the time it takes from diagnosis to the time we start treating (time to treat). We have always tried to reduce time to treat for acute leukemia and we have applied those lessons to patients with lymphoma and solid tumors, resulting in a 33% improvement over the last 5 years.
We not only lessen the anxiety that comes with a scary diagnosis, emerging data indicate outcomes are improved with faster treatment, too (PLoS One. 2019 Mar 1;14(3):e0213209. doi: 10.1371/journal.pone.0213209).
These efforts will be criticized by those who feel the delivery of medical care should be structured more around the physician than the patient. Certainly, the system has developed to support a mindset of “physician first.” Not only do patients have to make an appointment for the privilege of seeing us, they have to navigate significant geographic and financial hurdles for that privilege.
Once at the appointment, physicians have historically been the provider giving the “orders” while others correct them, carry them out, follow-up on the results, manage phone calls, and schedule follow-up. This hierarchy has served physicians very well, but the pyramidal structure of health care is on the verge of being upended.
Too few physicians for an increasing demand for medical attention has led to the rise of advanced practice providers (APPs), who often serve as the only provider a patient may have, particularly in rural areas. In our center, we evolved from thinking of APPs as similar to house-staff who saw patients with us and did most of the work, but could not bill, to independent providers who work with us, do most of the work, and bill for their efforts. This slow transformation of our practice will soon seem quaint as we face the rapid disruption coming to our current conception of the health care delivery system.
Technologically savvy patients already demand immediate access to unlimited supplies of consumer goods, video, audio, books, magazines, and just about anything else you can think of. Immediate access to health care at a time convenient to the patient also will become an expectation because plenty of health care delivery models already are providing it. The local pharmacy or retail store may have a physician or APP right there ready to see a patient at any time. Some physicians are already online ready for an electronic interaction. See MDLIVE and Teladoc as examples.
The nimble cancer center that embraces these trends to become more patient-centric will be the center that captures national – if not international – market share, as insurance companies and governments adjust their reimbursement models to include these services. With blood work obtained just about anywhere, what would keep a patient with immune thrombocytopenic purpura from consulting with any online hematologist she chooses, whenever she chooses?
If first impressions are important, then patient access is important. Refrains of “I don’t have clinic that day,” “the pathology has not yet been reviewed,” and “that is not a disease I take care of,” ring as hollow to me as I suspect they do to our patients. When someone in my family has a significant illness, I want them to be seen now, not later. I believe we all would want prompt, efficient service.
We should strive to provide the same level of care to our patients as we expect for our family. Patients do not know that chronic leukemia is not an emergency. Time may not be critical to us, but it is to them. The perfect time to meet their needs is now.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematology and medical oncology at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
When I entered the examination room, I saw his alma mater’s logo on his wristwatch. He was a retired physician with a new diagnosis of leukemia who drove to see me, even though he lived closer to his beloved medical school where he had practiced his entire career.
As is frequently the case, he came to see me because he could not get the appointment he wanted in his university’s clinic for another 6 months. He called us on Friday, and 3 days later, he and I were meeting. He is still an ardent supporter of his institution, but I am now his hematologist.
As it turned out, his leukemia was asymptomatic, indolent, and required no treatment. He could have waited 6 months to be seen. But, no; he couldn’t.
This story repeats itself over and over again. A sick patient calls to be seen and is told there is no availability for weeks or months. I do not understand how health care facilities, my own included, find this acceptable.
My father was very proud of his policy to see every patient in his waiting room no matter how long his office needed to stay open. He felt that access was of primary importance to his patients and to his practice. If he didn’t see them, somebody else would. Those of us working in large academic centers do not always feel the financial consequences of patients lost because of poor service.
Luckily, I work in a large cancer center that values access as much as a small practice would. When a patient calls us with a hematologic problem, we see them in less than 7 days, unless the patient prefers a different time frame. We monitor the time it takes to see patients and proactively assess upcoming appointments to ensure insurance coverage and the availability of records. If an obstruction is identified, the case is escalated to administrative leadership to be addressed and resolved. We are very proud of this work.
However, our focus on access does not end there. Once seen, we expedite patient evaluation by assessing workflows to obtain all necessary testing as quickly as possible. By doing so, we accelerate the time it takes from diagnosis to the time we start treating (time to treat). We have always tried to reduce time to treat for acute leukemia and we have applied those lessons to patients with lymphoma and solid tumors, resulting in a 33% improvement over the last 5 years.
We not only lessen the anxiety that comes with a scary diagnosis, emerging data indicate outcomes are improved with faster treatment, too (PLoS One. 2019 Mar 1;14(3):e0213209. doi: 10.1371/journal.pone.0213209).
These efforts will be criticized by those who feel the delivery of medical care should be structured more around the physician than the patient. Certainly, the system has developed to support a mindset of “physician first.” Not only do patients have to make an appointment for the privilege of seeing us, they have to navigate significant geographic and financial hurdles for that privilege.
Once at the appointment, physicians have historically been the provider giving the “orders” while others correct them, carry them out, follow-up on the results, manage phone calls, and schedule follow-up. This hierarchy has served physicians very well, but the pyramidal structure of health care is on the verge of being upended.
Too few physicians for an increasing demand for medical attention has led to the rise of advanced practice providers (APPs), who often serve as the only provider a patient may have, particularly in rural areas. In our center, we evolved from thinking of APPs as similar to house-staff who saw patients with us and did most of the work, but could not bill, to independent providers who work with us, do most of the work, and bill for their efforts. This slow transformation of our practice will soon seem quaint as we face the rapid disruption coming to our current conception of the health care delivery system.
Technologically savvy patients already demand immediate access to unlimited supplies of consumer goods, video, audio, books, magazines, and just about anything else you can think of. Immediate access to health care at a time convenient to the patient also will become an expectation because plenty of health care delivery models already are providing it. The local pharmacy or retail store may have a physician or APP right there ready to see a patient at any time. Some physicians are already online ready for an electronic interaction. See MDLIVE and Teladoc as examples.
The nimble cancer center that embraces these trends to become more patient-centric will be the center that captures national – if not international – market share, as insurance companies and governments adjust their reimbursement models to include these services. With blood work obtained just about anywhere, what would keep a patient with immune thrombocytopenic purpura from consulting with any online hematologist she chooses, whenever she chooses?
If first impressions are important, then patient access is important. Refrains of “I don’t have clinic that day,” “the pathology has not yet been reviewed,” and “that is not a disease I take care of,” ring as hollow to me as I suspect they do to our patients. When someone in my family has a significant illness, I want them to be seen now, not later. I believe we all would want prompt, efficient service.
We should strive to provide the same level of care to our patients as we expect for our family. Patients do not know that chronic leukemia is not an emergency. Time may not be critical to us, but it is to them. The perfect time to meet their needs is now.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematology and medical oncology at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Psychiatry and neurology, more
Dr. Nasrallah’s “Psychiatry and neurology: Sister neuroscience specialties with different approaches to the brain” (From the Editor,
In mathematics, chaos theory deals with the impossible complexity of simplicity. From primitive initial states, self-interacting systems give rise to short-term predictability, but an unpredictable long-term. Classically, this is illustrated as a hurricane born from the flapping of a butterfly’s wings. Neurology has found great clinical utility in understanding butterfly wings. However, psychiatry forsakes simplicity for complexity: it dives into the emergent systems that arise from self-interacting neurons, asking us to stand within the eye of the hurricane and understand it in its entirety. Psychiatry asks us to transcend the traditional medical focus of discrete physiological mechanisms, and ask—from the standpoint of biologic, social, and spiritual well-being—how can we calm the hurricane?
Psychiatry once had a widely-encompassing understanding of its remit: to appreciate the multifaceted experience of the human life and grant succor to the fractured or anguished soul. In such times, psychiatry was a popular destination for seniors graduating in the United States. Annually, 7% to 10% of US graduates chose psychiatry as a career, and continued to do so until the late 1970s.1 In the 1970s, the reductive understanding of the mind increased in prominence, and the role of psychiatry transitioned to one similar to that of other medical specialties: putting patients in boxes, and chronically titrating their medications. The interest of graduating seniors waned alongside the scope of our interest: in 1977, only 4.4% of US graduates pursued psychiatry.2 In 2019, 4.06% of graduating senior applications were to the field of psychiatry.3 (This is not meant to undervalue the quality of international medical graduates, but to focus on local trends in cultural values.)
Psychiatry offers diagnostic and therapeutic avenues that are traditionally undervalued in other fields of medicine. Nephrosis may not care if a patient feels that his or her life is spiritually satisfying and their actions meaningful. However, a patient’s anguish at his reduced functional status does not care for whether his albumin level is normalized—he requires that his suffering be recognized, and that we make an earnest effort to cloak “the shameful nakedness of pain.”4
Psychiatry also makes unique demands of, and offers benefits to, the practitioner. Neurologists complete their residencies feeling that their clinical acumen has increased: “I can formulate a thorough differential now.” Psychiatry asks us not only to cultivate technical proficiency, but also wisdom. The prolonged reflection on the quality and nature of human experience, and the need to guide such patients in a manner far wider and more meaningful in scope than their serotonin pathways, offers the opportunity to emerge from residency a more mindful and grateful human being.
Ultimately, the loss of this sense of scope has not been a failure of medical education. It has been a surrender of the current generation of psychiatry attendings. We have ceded responsibility for the social and spiritual care of our patients to other fields, or to no one at all. If we give up on understanding the hurricane, how can we be surprised that students prefer to chase butterflies?
James Steinberg, MPH, OMS-IV
New York Institute of Technology
College of Osteopathic Medicine
Old Westbury, New York
Robert Barris, MD
Director
Inpatient Psychiatric Services
Nassau University Medical Center
East Meadow, New York
References
1. Sierles FS, Taylor MA. Decline of U.S. medical student career choice of psychiatry and what to do about it. Am J Psychiatry. 1995;152(10):1416-1426.
2. Results and data: main residency match. NRMP data. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2013/08/resultsanddata1984.pdf. Published May 1984. Accessed May 8, 2019.
3. Advanced Data Tables. The Match 2019. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2019/03/Advance-Data-Tables-2019_WWW.pdf. Published March 2019. Accessed May 8, 2019.
4. Kipling R. Doctors. In: Kipling: poems (Everyman’s Library Pocket Poets Series). New York, NY: Random House. 2007:234.
Dr. Nasrallah responds
Thank you, Mr. Steinberg and Dr. Barris, for your comments about my editorial. I genuinely enjoyed the eloquence of your letter. In computers, which we all own and use, hardware is indispensable because it enables us to exploit the software, but the richness of the software is far more interesting than the hardware for the creative productivity of humans. So what you say is correct: T
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Continue to: Perspectives on motherhood and psychiatry
Perspectives on motherhood and psychiatry
I very much enjoyed Drs. Helen M. Farrell’s and Katherine A. Kosman’s recent article “Motherhood and the working psychiatrist” (Psychiatry 2.0,
Christina Ford, MD
Private psychiatric practice
Los Angeles, California
I doubt that anyone—male or female—would argue against the points made by Drs. Farrell and Kosman’s “Motherhood and the working psychiatrist,” which emphasized the need for breaking down the barriers that continue to exist for female physicians who choose to balance their careers with motherhood. As a female psychiatrist who has known since high school that I would choose to remain child-free, I would like to add a different perspective to this discussion and possibly help represent the 20% of women, age 40 to 44, with an MD or PhD who are also child-free.1
While Drs. Farrell and Kosman referenced many assumptions made about working physician mothers, I have not been able to move through medical school, residency, and my career without battling certain assumptions as well. Although every mother is a woman, logic dictates that the converse—every woman is a mother—is certainly not true. However, when interviewing for residency, I was paired specifically with a female attending who had children, and I was told that I could ask her questions about how to balance work-life and raising a family, despite the fact that I did not say or indicate that I had any interest in having such a conversation. There is also the assumption (sometimes more explicit than others) that those of us without children are missing out on something—that we are not included in the “having it all” category. However, in my mind, “having it all” means having the choice to remain child-free, to focus more intensely on my career, to travel when I want, and to own a white couch—without feeling the social obligation to fulfill a role in which I really have no interest.
Cherishing that ability to focus more on my career, however, does not imply that I am boundlessly able and willing to take extra calls, work holidays, or cover for all my colleagues with children (which is also a common assumption). And while I may not be a caregiver to children, that should not detract from the devotion and time I want to spend helping my parents, relatives, and friends.
The article also made the case that facilities, medical schools, and residency programs need to implement policies and procedures that guide the development of accommodations, such as flexible scheduling and lactation rooms, to meet the needs of trainees and physicians without having to jump through hoops or rely on colleagues for coverage and other assistance. Having been in situations where such policies and procedures were not in place, I can affirm that the absence of such guidelines leads not only parents but also child-free physicians to feeling unnecessarily stressed. There was no clear coverage in place when fellow classmates in my residency program went on maternity leave. Essentially, everyone else was expected to step up and take on the additional caseloads, leading the pregnant classmates to try to time things around rotations where there were lighter demands or more residents assigned—not a simple task by any means.
Post-residency, there have been continued challenges. At one point, I was working in a clinic with 2 other female psychiatrists, one of whom was making plans to take maternity leave. During a meeting with our supervisors, the other physician and I were told that we were taking on the third doctor’s patients (without any extension of our own hours or reimbursement) while she was on leave. In addition to disgruntlement over the extra work being sprung on us, I pointed out that this would, in effect, make the third physician’s role obsolete. If 2 of us were able to do the work of 3, what would be the point in keeping her position when she returned? I was assured that this wouldn’t be the case. We dealt with the weeks of covering additional patients, and when she returned from leave, she was asked to shift some of her hours to a different (and, in my opinion, less desirable) clinic.
So, yes, it is incumbent upon facilities and training programs to take responsibility and to remove the barriers that make the jobs of female physicians with children even more challenging than they need to be. This can benefit not only those physicians and their children, but also their colleagues and, ultimately, the patients, who often bear the brunt of stressed, burnt-out physicians and disorganized programs. While I am not going to take a stance on whether it truly takes a village to raise a child, I certainly do not think that it should take a village to organize maternity leave and lactation rooms.
Jessica L. Langenhan, MD, MBA, CHCQM
Medical DirectorBeacon Health Options
Cypress, California
Reference
1. Livingston G. Childlessness. Pew Research Center. https://www.pewsocialtrends.org/2015/05/07/childlessness/. Published May 7, 2015. Accessed May 9, 2019.
Dr. Nasrallah’s “Psychiatry and neurology: Sister neuroscience specialties with different approaches to the brain” (From the Editor,
In mathematics, chaos theory deals with the impossible complexity of simplicity. From primitive initial states, self-interacting systems give rise to short-term predictability, but an unpredictable long-term. Classically, this is illustrated as a hurricane born from the flapping of a butterfly’s wings. Neurology has found great clinical utility in understanding butterfly wings. However, psychiatry forsakes simplicity for complexity: it dives into the emergent systems that arise from self-interacting neurons, asking us to stand within the eye of the hurricane and understand it in its entirety. Psychiatry asks us to transcend the traditional medical focus of discrete physiological mechanisms, and ask—from the standpoint of biologic, social, and spiritual well-being—how can we calm the hurricane?
Psychiatry once had a widely-encompassing understanding of its remit: to appreciate the multifaceted experience of the human life and grant succor to the fractured or anguished soul. In such times, psychiatry was a popular destination for seniors graduating in the United States. Annually, 7% to 10% of US graduates chose psychiatry as a career, and continued to do so until the late 1970s.1 In the 1970s, the reductive understanding of the mind increased in prominence, and the role of psychiatry transitioned to one similar to that of other medical specialties: putting patients in boxes, and chronically titrating their medications. The interest of graduating seniors waned alongside the scope of our interest: in 1977, only 4.4% of US graduates pursued psychiatry.2 In 2019, 4.06% of graduating senior applications were to the field of psychiatry.3 (This is not meant to undervalue the quality of international medical graduates, but to focus on local trends in cultural values.)
Psychiatry offers diagnostic and therapeutic avenues that are traditionally undervalued in other fields of medicine. Nephrosis may not care if a patient feels that his or her life is spiritually satisfying and their actions meaningful. However, a patient’s anguish at his reduced functional status does not care for whether his albumin level is normalized—he requires that his suffering be recognized, and that we make an earnest effort to cloak “the shameful nakedness of pain.”4
Psychiatry also makes unique demands of, and offers benefits to, the practitioner. Neurologists complete their residencies feeling that their clinical acumen has increased: “I can formulate a thorough differential now.” Psychiatry asks us not only to cultivate technical proficiency, but also wisdom. The prolonged reflection on the quality and nature of human experience, and the need to guide such patients in a manner far wider and more meaningful in scope than their serotonin pathways, offers the opportunity to emerge from residency a more mindful and grateful human being.
Ultimately, the loss of this sense of scope has not been a failure of medical education. It has been a surrender of the current generation of psychiatry attendings. We have ceded responsibility for the social and spiritual care of our patients to other fields, or to no one at all. If we give up on understanding the hurricane, how can we be surprised that students prefer to chase butterflies?
James Steinberg, MPH, OMS-IV
New York Institute of Technology
College of Osteopathic Medicine
Old Westbury, New York
Robert Barris, MD
Director
Inpatient Psychiatric Services
Nassau University Medical Center
East Meadow, New York
References
1. Sierles FS, Taylor MA. Decline of U.S. medical student career choice of psychiatry and what to do about it. Am J Psychiatry. 1995;152(10):1416-1426.
2. Results and data: main residency match. NRMP data. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2013/08/resultsanddata1984.pdf. Published May 1984. Accessed May 8, 2019.
3. Advanced Data Tables. The Match 2019. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2019/03/Advance-Data-Tables-2019_WWW.pdf. Published March 2019. Accessed May 8, 2019.
4. Kipling R. Doctors. In: Kipling: poems (Everyman’s Library Pocket Poets Series). New York, NY: Random House. 2007:234.
Dr. Nasrallah responds
Thank you, Mr. Steinberg and Dr. Barris, for your comments about my editorial. I genuinely enjoyed the eloquence of your letter. In computers, which we all own and use, hardware is indispensable because it enables us to exploit the software, but the richness of the software is far more interesting than the hardware for the creative productivity of humans. So what you say is correct: T
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Continue to: Perspectives on motherhood and psychiatry
Perspectives on motherhood and psychiatry
I very much enjoyed Drs. Helen M. Farrell’s and Katherine A. Kosman’s recent article “Motherhood and the working psychiatrist” (Psychiatry 2.0,
Christina Ford, MD
Private psychiatric practice
Los Angeles, California
I doubt that anyone—male or female—would argue against the points made by Drs. Farrell and Kosman’s “Motherhood and the working psychiatrist,” which emphasized the need for breaking down the barriers that continue to exist for female physicians who choose to balance their careers with motherhood. As a female psychiatrist who has known since high school that I would choose to remain child-free, I would like to add a different perspective to this discussion and possibly help represent the 20% of women, age 40 to 44, with an MD or PhD who are also child-free.1
While Drs. Farrell and Kosman referenced many assumptions made about working physician mothers, I have not been able to move through medical school, residency, and my career without battling certain assumptions as well. Although every mother is a woman, logic dictates that the converse—every woman is a mother—is certainly not true. However, when interviewing for residency, I was paired specifically with a female attending who had children, and I was told that I could ask her questions about how to balance work-life and raising a family, despite the fact that I did not say or indicate that I had any interest in having such a conversation. There is also the assumption (sometimes more explicit than others) that those of us without children are missing out on something—that we are not included in the “having it all” category. However, in my mind, “having it all” means having the choice to remain child-free, to focus more intensely on my career, to travel when I want, and to own a white couch—without feeling the social obligation to fulfill a role in which I really have no interest.
Cherishing that ability to focus more on my career, however, does not imply that I am boundlessly able and willing to take extra calls, work holidays, or cover for all my colleagues with children (which is also a common assumption). And while I may not be a caregiver to children, that should not detract from the devotion and time I want to spend helping my parents, relatives, and friends.
The article also made the case that facilities, medical schools, and residency programs need to implement policies and procedures that guide the development of accommodations, such as flexible scheduling and lactation rooms, to meet the needs of trainees and physicians without having to jump through hoops or rely on colleagues for coverage and other assistance. Having been in situations where such policies and procedures were not in place, I can affirm that the absence of such guidelines leads not only parents but also child-free physicians to feeling unnecessarily stressed. There was no clear coverage in place when fellow classmates in my residency program went on maternity leave. Essentially, everyone else was expected to step up and take on the additional caseloads, leading the pregnant classmates to try to time things around rotations where there were lighter demands or more residents assigned—not a simple task by any means.
Post-residency, there have been continued challenges. At one point, I was working in a clinic with 2 other female psychiatrists, one of whom was making plans to take maternity leave. During a meeting with our supervisors, the other physician and I were told that we were taking on the third doctor’s patients (without any extension of our own hours or reimbursement) while she was on leave. In addition to disgruntlement over the extra work being sprung on us, I pointed out that this would, in effect, make the third physician’s role obsolete. If 2 of us were able to do the work of 3, what would be the point in keeping her position when she returned? I was assured that this wouldn’t be the case. We dealt with the weeks of covering additional patients, and when she returned from leave, she was asked to shift some of her hours to a different (and, in my opinion, less desirable) clinic.
So, yes, it is incumbent upon facilities and training programs to take responsibility and to remove the barriers that make the jobs of female physicians with children even more challenging than they need to be. This can benefit not only those physicians and their children, but also their colleagues and, ultimately, the patients, who often bear the brunt of stressed, burnt-out physicians and disorganized programs. While I am not going to take a stance on whether it truly takes a village to raise a child, I certainly do not think that it should take a village to organize maternity leave and lactation rooms.
Jessica L. Langenhan, MD, MBA, CHCQM
Medical DirectorBeacon Health Options
Cypress, California
Reference
1. Livingston G. Childlessness. Pew Research Center. https://www.pewsocialtrends.org/2015/05/07/childlessness/. Published May 7, 2015. Accessed May 9, 2019.
Dr. Nasrallah’s “Psychiatry and neurology: Sister neuroscience specialties with different approaches to the brain” (From the Editor,
In mathematics, chaos theory deals with the impossible complexity of simplicity. From primitive initial states, self-interacting systems give rise to short-term predictability, but an unpredictable long-term. Classically, this is illustrated as a hurricane born from the flapping of a butterfly’s wings. Neurology has found great clinical utility in understanding butterfly wings. However, psychiatry forsakes simplicity for complexity: it dives into the emergent systems that arise from self-interacting neurons, asking us to stand within the eye of the hurricane and understand it in its entirety. Psychiatry asks us to transcend the traditional medical focus of discrete physiological mechanisms, and ask—from the standpoint of biologic, social, and spiritual well-being—how can we calm the hurricane?
Psychiatry once had a widely-encompassing understanding of its remit: to appreciate the multifaceted experience of the human life and grant succor to the fractured or anguished soul. In such times, psychiatry was a popular destination for seniors graduating in the United States. Annually, 7% to 10% of US graduates chose psychiatry as a career, and continued to do so until the late 1970s.1 In the 1970s, the reductive understanding of the mind increased in prominence, and the role of psychiatry transitioned to one similar to that of other medical specialties: putting patients in boxes, and chronically titrating their medications. The interest of graduating seniors waned alongside the scope of our interest: in 1977, only 4.4% of US graduates pursued psychiatry.2 In 2019, 4.06% of graduating senior applications were to the field of psychiatry.3 (This is not meant to undervalue the quality of international medical graduates, but to focus on local trends in cultural values.)
Psychiatry offers diagnostic and therapeutic avenues that are traditionally undervalued in other fields of medicine. Nephrosis may not care if a patient feels that his or her life is spiritually satisfying and their actions meaningful. However, a patient’s anguish at his reduced functional status does not care for whether his albumin level is normalized—he requires that his suffering be recognized, and that we make an earnest effort to cloak “the shameful nakedness of pain.”4
Psychiatry also makes unique demands of, and offers benefits to, the practitioner. Neurologists complete their residencies feeling that their clinical acumen has increased: “I can formulate a thorough differential now.” Psychiatry asks us not only to cultivate technical proficiency, but also wisdom. The prolonged reflection on the quality and nature of human experience, and the need to guide such patients in a manner far wider and more meaningful in scope than their serotonin pathways, offers the opportunity to emerge from residency a more mindful and grateful human being.
Ultimately, the loss of this sense of scope has not been a failure of medical education. It has been a surrender of the current generation of psychiatry attendings. We have ceded responsibility for the social and spiritual care of our patients to other fields, or to no one at all. If we give up on understanding the hurricane, how can we be surprised that students prefer to chase butterflies?
James Steinberg, MPH, OMS-IV
New York Institute of Technology
College of Osteopathic Medicine
Old Westbury, New York
Robert Barris, MD
Director
Inpatient Psychiatric Services
Nassau University Medical Center
East Meadow, New York
References
1. Sierles FS, Taylor MA. Decline of U.S. medical student career choice of psychiatry and what to do about it. Am J Psychiatry. 1995;152(10):1416-1426.
2. Results and data: main residency match. NRMP data. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2013/08/resultsanddata1984.pdf. Published May 1984. Accessed May 8, 2019.
3. Advanced Data Tables. The Match 2019. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2019/03/Advance-Data-Tables-2019_WWW.pdf. Published March 2019. Accessed May 8, 2019.
4. Kipling R. Doctors. In: Kipling: poems (Everyman’s Library Pocket Poets Series). New York, NY: Random House. 2007:234.
Dr. Nasrallah responds
Thank you, Mr. Steinberg and Dr. Barris, for your comments about my editorial. I genuinely enjoyed the eloquence of your letter. In computers, which we all own and use, hardware is indispensable because it enables us to exploit the software, but the richness of the software is far more interesting than the hardware for the creative productivity of humans. So what you say is correct: T
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Continue to: Perspectives on motherhood and psychiatry
Perspectives on motherhood and psychiatry
I very much enjoyed Drs. Helen M. Farrell’s and Katherine A. Kosman’s recent article “Motherhood and the working psychiatrist” (Psychiatry 2.0,
Christina Ford, MD
Private psychiatric practice
Los Angeles, California
I doubt that anyone—male or female—would argue against the points made by Drs. Farrell and Kosman’s “Motherhood and the working psychiatrist,” which emphasized the need for breaking down the barriers that continue to exist for female physicians who choose to balance their careers with motherhood. As a female psychiatrist who has known since high school that I would choose to remain child-free, I would like to add a different perspective to this discussion and possibly help represent the 20% of women, age 40 to 44, with an MD or PhD who are also child-free.1
While Drs. Farrell and Kosman referenced many assumptions made about working physician mothers, I have not been able to move through medical school, residency, and my career without battling certain assumptions as well. Although every mother is a woman, logic dictates that the converse—every woman is a mother—is certainly not true. However, when interviewing for residency, I was paired specifically with a female attending who had children, and I was told that I could ask her questions about how to balance work-life and raising a family, despite the fact that I did not say or indicate that I had any interest in having such a conversation. There is also the assumption (sometimes more explicit than others) that those of us without children are missing out on something—that we are not included in the “having it all” category. However, in my mind, “having it all” means having the choice to remain child-free, to focus more intensely on my career, to travel when I want, and to own a white couch—without feeling the social obligation to fulfill a role in which I really have no interest.
Cherishing that ability to focus more on my career, however, does not imply that I am boundlessly able and willing to take extra calls, work holidays, or cover for all my colleagues with children (which is also a common assumption). And while I may not be a caregiver to children, that should not detract from the devotion and time I want to spend helping my parents, relatives, and friends.
The article also made the case that facilities, medical schools, and residency programs need to implement policies and procedures that guide the development of accommodations, such as flexible scheduling and lactation rooms, to meet the needs of trainees and physicians without having to jump through hoops or rely on colleagues for coverage and other assistance. Having been in situations where such policies and procedures were not in place, I can affirm that the absence of such guidelines leads not only parents but also child-free physicians to feeling unnecessarily stressed. There was no clear coverage in place when fellow classmates in my residency program went on maternity leave. Essentially, everyone else was expected to step up and take on the additional caseloads, leading the pregnant classmates to try to time things around rotations where there were lighter demands or more residents assigned—not a simple task by any means.
Post-residency, there have been continued challenges. At one point, I was working in a clinic with 2 other female psychiatrists, one of whom was making plans to take maternity leave. During a meeting with our supervisors, the other physician and I were told that we were taking on the third doctor’s patients (without any extension of our own hours or reimbursement) while she was on leave. In addition to disgruntlement over the extra work being sprung on us, I pointed out that this would, in effect, make the third physician’s role obsolete. If 2 of us were able to do the work of 3, what would be the point in keeping her position when she returned? I was assured that this wouldn’t be the case. We dealt with the weeks of covering additional patients, and when she returned from leave, she was asked to shift some of her hours to a different (and, in my opinion, less desirable) clinic.
So, yes, it is incumbent upon facilities and training programs to take responsibility and to remove the barriers that make the jobs of female physicians with children even more challenging than they need to be. This can benefit not only those physicians and their children, but also their colleagues and, ultimately, the patients, who often bear the brunt of stressed, burnt-out physicians and disorganized programs. While I am not going to take a stance on whether it truly takes a village to raise a child, I certainly do not think that it should take a village to organize maternity leave and lactation rooms.
Jessica L. Langenhan, MD, MBA, CHCQM
Medical DirectorBeacon Health Options
Cypress, California
Reference
1. Livingston G. Childlessness. Pew Research Center. https://www.pewsocialtrends.org/2015/05/07/childlessness/. Published May 7, 2015. Accessed May 9, 2019.
Mobile apps and mental health: Using technology to quantify real-time clinical risk
In today’s global society, smartphones are ubiquitous, used by >2.5 billion people.1 They provide limitless availability of on-demand services and resources, unparalleled computing power by size, and the ability to connect with anyone in the world.
Digital applications and new mobile technologies can be used to change the nature of the psychiatrist–patient relationship. The future of clinical practice is changing with the help of smartphones and apps. Diagnosis, follow-up, and treatment will never look the same as we come to better understand and apply emerging technologies.2
Both Android and iOS—the 2 largest mobile operating systems by market share3—provide outlets for the dissemination of mobile applications. There are currently >10,000 mental health–related apps available for download.4 One particular use case of mental health–related apps is digital phenotyping.
In this article, we aim to:
- define digital phenotyping
- explore the potential advances in patient care afforded by emerging technology
- discuss the ethical dilemmas and future of mental health apps.
The possibilities of digital phenotyping
Digital phenotyping is capturing a patient’s real-time clinical state using digital technology to better understand the patient’s state outside of the clinic. While digital phenotyping may seem new, the concepts behind it are grounded in good clinical care.
For example, it is important to assess sleep and physical activity for nearly all patients, regardless of diagnosis. However, the patient’s retrospective recollection of sleep, mood, and other clinically relevant metrics is often unreliable, especially when visits are months apart. With smartphones, it is possible to automatically collect metrics for sleep, activity, mood, and much more in real time from the convenience of our patients’ personal devices (Figure 1).
Smartphones can capture a seemingly endless number of data streams, from patient-interfacing active data, such as journal entries, messaging, and games, to data that is captured passively, such as screen time, Global Positioning System information, and step count. Clinicians can work with patients to customize which digital phenotyping data they would like to capture. In one study, researchers worked with 17 patients with schizophrenia by capturing self-reported surveys, anonymized phone call logs, and location data to see if they could predict relapse by observing variations in how patients interact with their smartphones.5 They observed that the rate of behavioral anomalies was 71% higher in the 2 weeks prior to relapse than during other periods. The data captured by the smartphone will depend on the patient and the clinical needs. Some clinicians may only want to collect data on step count and screen time to learn if a patient is overusing his or her smartphone, which might be related to becoming less physically active.
Continue to: One novel data stream...
One novel data stream offered by smartphone digital phenotyping is cognition. While we know that impaired cognition is a core symptom of schizophrenia, and that cognition is affected by depression and anxiety, cognitive symptoms are clinically challenging to quantify. Thus, the cognitive burden of mental illness and the cognitive effects of treatment are often overlooked. However, smartphones are beginning to offer a novel means of capturing a patient’s cognitive state through the use of common clinical tests. For example, the Trail Making Test measures visual attention and executive function by having participants connect dots that differ in number, color, or shape in an ascending pattern.6 By having patients perform this test on a smartphone, clinicians can utilize the touchscreen to capture the user’s discrete actions, such as time to completion and misclicks. These data can be used to build novel measures of cognitive performance that can account for learning bias and other confounding variables.7 While these digital cognitive biomarkers are still in active research, it is likely that they will quickly be developed for broad clinical use.
In addition to the novel data offered by digital phenotyping, another benefit is the low cost and ease of use. Unlike wearable devices such as smartwatches, which can also offer data on steps and sleep, smartphone-based digital phenotyping does not require patients to purchase or use additional devices. Running on patients’ smartphones, digital phenotyping offers the ability to capture rich and continuous health data without added effort or cost. Given that the average person interacts with their phone more than 2,600 times per day,8 smartphones are well suited for capturing large amounts of information that may provide insights into patients’ mental health.
For illnesses such as depression and anxiety, the clinical relevance of digital phenotyping is in the ability to capture symptoms as they occur in context. Figure 2 provides a simplified example of how we can learn that for this fictitious patient, exercise greatly improves anxiety, whereas being in a certain environment worsens it. Other insights about sleep and social settings could also provide further information about the context of the patient’s symptoms. While these correlations alone will not lead to better clinical outcomes, it is easy to imagine how such data could help a patient and clinician start a conversation about making impactful changes.
Continue to: Case report...
Case report: Digital phenotyping
To illustrate how digital phenotyping could be put to clinical use, we created the following case report of a fictional patient who agrees to be monitored via her smartphone.
Consider a hypothetical patient we will call Ms. T who is in her mid-20s and has been diagnosed with schizophrenia. On a follow-up visit, she says she has insomnia. She also reports having a recent loss of appetite and higher levels of anxiety. After reviewing her smartphone data (Figure 3), the clinician sees an inversely proportional relationship between her sleep quality and symptoms of anxiety, psychosis, and depression, which suggests that these symptoms might be due to poor sleep. Her step count has been fairly stable, indicating that there is no significant correlation between physical activity and her other symptoms.
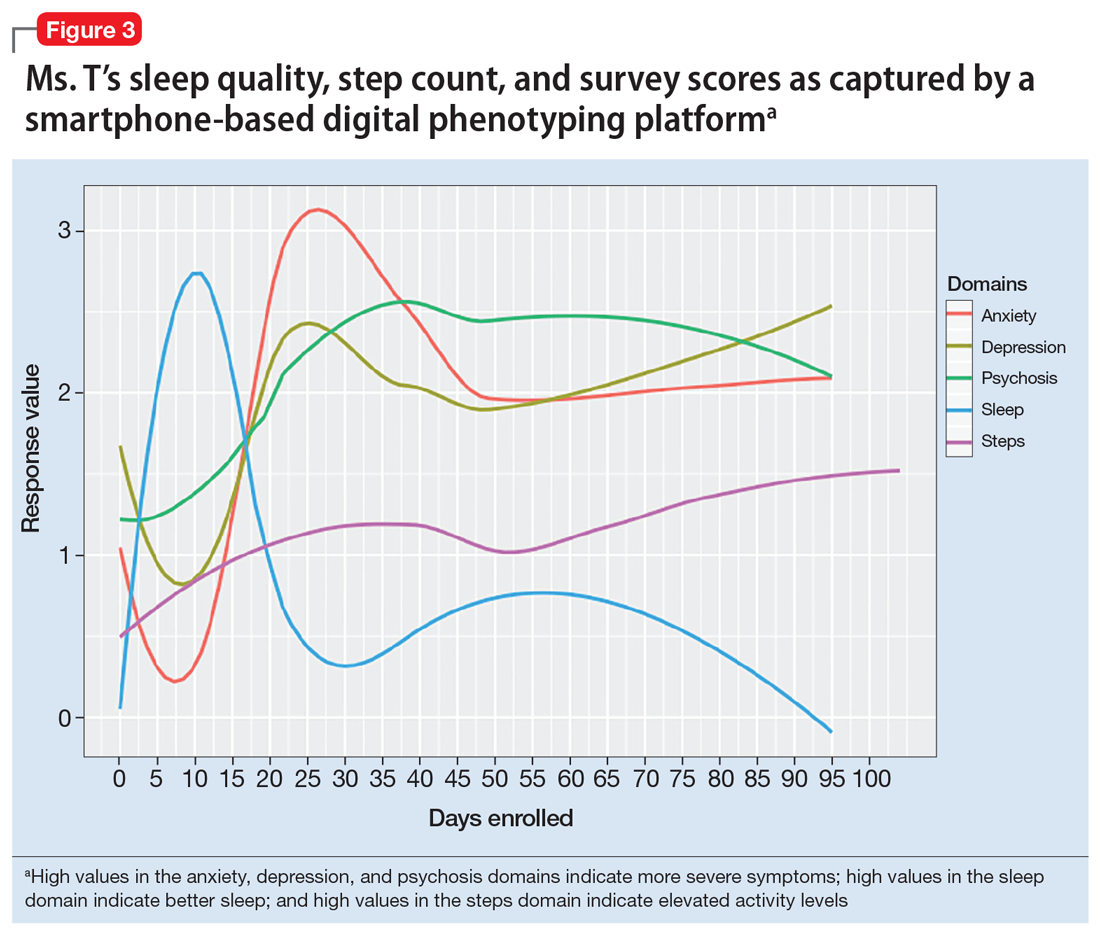
Continue to: The clinician shows...
The clinician shows Ms. T the data to help her understand why a trial of cognitive-behavioral therapy for insomnia, or at least improving sleep hygiene, may offer several benefits. The clinician advises her to continue to use the app to help assess her response to these interventions and monitor her progress in real time.
Dilemma: The ethics of continuous observation
The rich data captured by digital phenotyping afford many clinical opportunities, but also raise concerns. Among these are 3 significant ethical implications.
Firstly, the same data that may help a clinician learn about what environments are associated with less anxiety for the patient may also reveal personal details about where that patient has been or with whom they have interacted. In the wrong hands, such personal data could cause harm. And even in the hands of a trusted clinician, a breach in the patient’s privacy begs the question: “Should such information be anyone’s business at all?”
Secondly, many apps that offer digital phenotyping could also store patient data—something that currently pervades social media and causes reasonable discomfort for many people. You might have personally encountered this with social media platforms such as Facebook. When it comes to mobile mental health apps, clinicians should carefully understand the data usage agreement of any digital phenotyping app they wish to use and then share this information with their patients.
Finally, while it is possible to collect the types of data outlined in this article, less is known about how to use it directly in clinical care. Understanding for each patient which data streams are most meaningful and which data streams are noise that should be ignored is an area of ongoing research. A good first step may be to begin with data streams that are known to be clinically relevant and valuable, such as sleep and physical activity.9-11
Continue to: Discussion...
Discussion: Genomic sequencing and digital phenotyping
Although smartphones can gather a wide range of active and passive data, other data streams hold potential for predicting relapse and performing other clinically relevant actions. One data stream that could be of clinical use is genomic sequencing.12 The genotyping of patients provides a wealth of information about the underlying biology, and genomic sequencing has never been cheaper.13
Combining the data gathered via digital phenotyping with that of genotyping could help elucidate the mechanisms by which specific diseases and symptoms occur. This could be very promising to better understand and treat our patients. However, as is the case with genomics, digital phenotyping has important ethical implications. If used in the proper way to benefit our patients, the future for this new method is bright.
1. Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions). https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/. Accessed April 29, 2019.
2. Thibaut F. Digital applications: the future in psychiatry? Dialogues Clin Neurosci. 2016;18(2):123.
3. Statista. Global market share held by the leading smartphone operating systems in sales to end users from 1st quarter 2009 to 2nd quarter 2018. https://www.statista.com/statistics/266136/global-market-share-held-by-smartphone-operating-systems/. Accessed April 19, 2019.
4. Torous J, Roberts L. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
5. Barnett I, Torous J, Staples P, et al. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43(8):1660-1666.
6. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220-221.
7. Brouillette RM, Foil H, Fontenot S, et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PloS One. 2013;8(6):e65925. doi: 10.1371/journal.pone.0065925.
8. Winnick W. Putting a finger on our phone obsession. dscout. https://blog.dscout.com/mobile-touches. Published June 16, 2016. Accessed April 29, 2019.
9. Waite F, Myers E, Harvey AG, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273-287.
10. Mcgurk SR, Mueser KT, Xie H, et al. (2015). Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852-861.
11. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546-556.
12. Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258-267.
13. National Human Genome Research Institute. The cost of sequencing a human genome. https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/. Updated July 6, 2016. Accessed April 29, 2019.
In today’s global society, smartphones are ubiquitous, used by >2.5 billion people.1 They provide limitless availability of on-demand services and resources, unparalleled computing power by size, and the ability to connect with anyone in the world.
Digital applications and new mobile technologies can be used to change the nature of the psychiatrist–patient relationship. The future of clinical practice is changing with the help of smartphones and apps. Diagnosis, follow-up, and treatment will never look the same as we come to better understand and apply emerging technologies.2
Both Android and iOS—the 2 largest mobile operating systems by market share3—provide outlets for the dissemination of mobile applications. There are currently >10,000 mental health–related apps available for download.4 One particular use case of mental health–related apps is digital phenotyping.
In this article, we aim to:
- define digital phenotyping
- explore the potential advances in patient care afforded by emerging technology
- discuss the ethical dilemmas and future of mental health apps.
The possibilities of digital phenotyping
Digital phenotyping is capturing a patient’s real-time clinical state using digital technology to better understand the patient’s state outside of the clinic. While digital phenotyping may seem new, the concepts behind it are grounded in good clinical care.
For example, it is important to assess sleep and physical activity for nearly all patients, regardless of diagnosis. However, the patient’s retrospective recollection of sleep, mood, and other clinically relevant metrics is often unreliable, especially when visits are months apart. With smartphones, it is possible to automatically collect metrics for sleep, activity, mood, and much more in real time from the convenience of our patients’ personal devices (Figure 1).

Smartphones can capture a seemingly endless number of data streams, from patient-interfacing active data, such as journal entries, messaging, and games, to data that is captured passively, such as screen time, Global Positioning System information, and step count. Clinicians can work with patients to customize which digital phenotyping data they would like to capture. In one study, researchers worked with 17 patients with schizophrenia by capturing self-reported surveys, anonymized phone call logs, and location data to see if they could predict relapse by observing variations in how patients interact with their smartphones.5 They observed that the rate of behavioral anomalies was 71% higher in the 2 weeks prior to relapse than during other periods. The data captured by the smartphone will depend on the patient and the clinical needs. Some clinicians may only want to collect data on step count and screen time to learn if a patient is overusing his or her smartphone, which might be related to becoming less physically active.
Continue to: One novel data stream...
One novel data stream offered by smartphone digital phenotyping is cognition. While we know that impaired cognition is a core symptom of schizophrenia, and that cognition is affected by depression and anxiety, cognitive symptoms are clinically challenging to quantify. Thus, the cognitive burden of mental illness and the cognitive effects of treatment are often overlooked. However, smartphones are beginning to offer a novel means of capturing a patient’s cognitive state through the use of common clinical tests. For example, the Trail Making Test measures visual attention and executive function by having participants connect dots that differ in number, color, or shape in an ascending pattern.6 By having patients perform this test on a smartphone, clinicians can utilize the touchscreen to capture the user’s discrete actions, such as time to completion and misclicks. These data can be used to build novel measures of cognitive performance that can account for learning bias and other confounding variables.7 While these digital cognitive biomarkers are still in active research, it is likely that they will quickly be developed for broad clinical use.
In addition to the novel data offered by digital phenotyping, another benefit is the low cost and ease of use. Unlike wearable devices such as smartwatches, which can also offer data on steps and sleep, smartphone-based digital phenotyping does not require patients to purchase or use additional devices. Running on patients’ smartphones, digital phenotyping offers the ability to capture rich and continuous health data without added effort or cost. Given that the average person interacts with their phone more than 2,600 times per day,8 smartphones are well suited for capturing large amounts of information that may provide insights into patients’ mental health.
For illnesses such as depression and anxiety, the clinical relevance of digital phenotyping is in the ability to capture symptoms as they occur in context. Figure 2 provides a simplified example of how we can learn that for this fictitious patient, exercise greatly improves anxiety, whereas being in a certain environment worsens it. Other insights about sleep and social settings could also provide further information about the context of the patient’s symptoms. While these correlations alone will not lead to better clinical outcomes, it is easy to imagine how such data could help a patient and clinician start a conversation about making impactful changes.

Continue to: Case report...
Case report: Digital phenotyping
To illustrate how digital phenotyping could be put to clinical use, we created the following case report of a fictional patient who agrees to be monitored via her smartphone.
Consider a hypothetical patient we will call Ms. T who is in her mid-20s and has been diagnosed with schizophrenia. On a follow-up visit, she says she has insomnia. She also reports having a recent loss of appetite and higher levels of anxiety. After reviewing her smartphone data (Figure 3), the clinician sees an inversely proportional relationship between her sleep quality and symptoms of anxiety, psychosis, and depression, which suggests that these symptoms might be due to poor sleep. Her step count has been fairly stable, indicating that there is no significant correlation between physical activity and her other symptoms.

Continue to: The clinician shows...
The clinician shows Ms. T the data to help her understand why a trial of cognitive-behavioral therapy for insomnia, or at least improving sleep hygiene, may offer several benefits. The clinician advises her to continue to use the app to help assess her response to these interventions and monitor her progress in real time.
Dilemma: The ethics of continuous observation
The rich data captured by digital phenotyping afford many clinical opportunities, but also raise concerns. Among these are 3 significant ethical implications.
Firstly, the same data that may help a clinician learn about what environments are associated with less anxiety for the patient may also reveal personal details about where that patient has been or with whom they have interacted. In the wrong hands, such personal data could cause harm. And even in the hands of a trusted clinician, a breach in the patient’s privacy begs the question: “Should such information be anyone’s business at all?”
Secondly, many apps that offer digital phenotyping could also store patient data—something that currently pervades social media and causes reasonable discomfort for many people. You might have personally encountered this with social media platforms such as Facebook. When it comes to mobile mental health apps, clinicians should carefully understand the data usage agreement of any digital phenotyping app they wish to use and then share this information with their patients.
Finally, while it is possible to collect the types of data outlined in this article, less is known about how to use it directly in clinical care. Understanding for each patient which data streams are most meaningful and which data streams are noise that should be ignored is an area of ongoing research. A good first step may be to begin with data streams that are known to be clinically relevant and valuable, such as sleep and physical activity.9-11
Continue to: Discussion...
Discussion: Genomic sequencing and digital phenotyping
Although smartphones can gather a wide range of active and passive data, other data streams hold potential for predicting relapse and performing other clinically relevant actions. One data stream that could be of clinical use is genomic sequencing.12 The genotyping of patients provides a wealth of information about the underlying biology, and genomic sequencing has never been cheaper.13
Combining the data gathered via digital phenotyping with that of genotyping could help elucidate the mechanisms by which specific diseases and symptoms occur. This could be very promising to better understand and treat our patients. However, as is the case with genomics, digital phenotyping has important ethical implications. If used in the proper way to benefit our patients, the future for this new method is bright.
In today’s global society, smartphones are ubiquitous, used by >2.5 billion people.1 They provide limitless availability of on-demand services and resources, unparalleled computing power by size, and the ability to connect with anyone in the world.
Digital applications and new mobile technologies can be used to change the nature of the psychiatrist–patient relationship. The future of clinical practice is changing with the help of smartphones and apps. Diagnosis, follow-up, and treatment will never look the same as we come to better understand and apply emerging technologies.2
Both Android and iOS—the 2 largest mobile operating systems by market share3—provide outlets for the dissemination of mobile applications. There are currently >10,000 mental health–related apps available for download.4 One particular use case of mental health–related apps is digital phenotyping.
In this article, we aim to:
- define digital phenotyping
- explore the potential advances in patient care afforded by emerging technology
- discuss the ethical dilemmas and future of mental health apps.
The possibilities of digital phenotyping
Digital phenotyping is capturing a patient’s real-time clinical state using digital technology to better understand the patient’s state outside of the clinic. While digital phenotyping may seem new, the concepts behind it are grounded in good clinical care.
For example, it is important to assess sleep and physical activity for nearly all patients, regardless of diagnosis. However, the patient’s retrospective recollection of sleep, mood, and other clinically relevant metrics is often unreliable, especially when visits are months apart. With smartphones, it is possible to automatically collect metrics for sleep, activity, mood, and much more in real time from the convenience of our patients’ personal devices (Figure 1).

Smartphones can capture a seemingly endless number of data streams, from patient-interfacing active data, such as journal entries, messaging, and games, to data that is captured passively, such as screen time, Global Positioning System information, and step count. Clinicians can work with patients to customize which digital phenotyping data they would like to capture. In one study, researchers worked with 17 patients with schizophrenia by capturing self-reported surveys, anonymized phone call logs, and location data to see if they could predict relapse by observing variations in how patients interact with their smartphones.5 They observed that the rate of behavioral anomalies was 71% higher in the 2 weeks prior to relapse than during other periods. The data captured by the smartphone will depend on the patient and the clinical needs. Some clinicians may only want to collect data on step count and screen time to learn if a patient is overusing his or her smartphone, which might be related to becoming less physically active.
Continue to: One novel data stream...
One novel data stream offered by smartphone digital phenotyping is cognition. While we know that impaired cognition is a core symptom of schizophrenia, and that cognition is affected by depression and anxiety, cognitive symptoms are clinically challenging to quantify. Thus, the cognitive burden of mental illness and the cognitive effects of treatment are often overlooked. However, smartphones are beginning to offer a novel means of capturing a patient’s cognitive state through the use of common clinical tests. For example, the Trail Making Test measures visual attention and executive function by having participants connect dots that differ in number, color, or shape in an ascending pattern.6 By having patients perform this test on a smartphone, clinicians can utilize the touchscreen to capture the user’s discrete actions, such as time to completion and misclicks. These data can be used to build novel measures of cognitive performance that can account for learning bias and other confounding variables.7 While these digital cognitive biomarkers are still in active research, it is likely that they will quickly be developed for broad clinical use.
In addition to the novel data offered by digital phenotyping, another benefit is the low cost and ease of use. Unlike wearable devices such as smartwatches, which can also offer data on steps and sleep, smartphone-based digital phenotyping does not require patients to purchase or use additional devices. Running on patients’ smartphones, digital phenotyping offers the ability to capture rich and continuous health data without added effort or cost. Given that the average person interacts with their phone more than 2,600 times per day,8 smartphones are well suited for capturing large amounts of information that may provide insights into patients’ mental health.
For illnesses such as depression and anxiety, the clinical relevance of digital phenotyping is in the ability to capture symptoms as they occur in context. Figure 2 provides a simplified example of how we can learn that for this fictitious patient, exercise greatly improves anxiety, whereas being in a certain environment worsens it. Other insights about sleep and social settings could also provide further information about the context of the patient’s symptoms. While these correlations alone will not lead to better clinical outcomes, it is easy to imagine how such data could help a patient and clinician start a conversation about making impactful changes.

Continue to: Case report...
Case report: Digital phenotyping
To illustrate how digital phenotyping could be put to clinical use, we created the following case report of a fictional patient who agrees to be monitored via her smartphone.
Consider a hypothetical patient we will call Ms. T who is in her mid-20s and has been diagnosed with schizophrenia. On a follow-up visit, she says she has insomnia. She also reports having a recent loss of appetite and higher levels of anxiety. After reviewing her smartphone data (Figure 3), the clinician sees an inversely proportional relationship between her sleep quality and symptoms of anxiety, psychosis, and depression, which suggests that these symptoms might be due to poor sleep. Her step count has been fairly stable, indicating that there is no significant correlation between physical activity and her other symptoms.

Continue to: The clinician shows...
The clinician shows Ms. T the data to help her understand why a trial of cognitive-behavioral therapy for insomnia, or at least improving sleep hygiene, may offer several benefits. The clinician advises her to continue to use the app to help assess her response to these interventions and monitor her progress in real time.
Dilemma: The ethics of continuous observation
The rich data captured by digital phenotyping afford many clinical opportunities, but also raise concerns. Among these are 3 significant ethical implications.
Firstly, the same data that may help a clinician learn about what environments are associated with less anxiety for the patient may also reveal personal details about where that patient has been or with whom they have interacted. In the wrong hands, such personal data could cause harm. And even in the hands of a trusted clinician, a breach in the patient’s privacy begs the question: “Should such information be anyone’s business at all?”
Secondly, many apps that offer digital phenotyping could also store patient data—something that currently pervades social media and causes reasonable discomfort for many people. You might have personally encountered this with social media platforms such as Facebook. When it comes to mobile mental health apps, clinicians should carefully understand the data usage agreement of any digital phenotyping app they wish to use and then share this information with their patients.
Finally, while it is possible to collect the types of data outlined in this article, less is known about how to use it directly in clinical care. Understanding for each patient which data streams are most meaningful and which data streams are noise that should be ignored is an area of ongoing research. A good first step may be to begin with data streams that are known to be clinically relevant and valuable, such as sleep and physical activity.9-11
Continue to: Discussion...
Discussion: Genomic sequencing and digital phenotyping
Although smartphones can gather a wide range of active and passive data, other data streams hold potential for predicting relapse and performing other clinically relevant actions. One data stream that could be of clinical use is genomic sequencing.12 The genotyping of patients provides a wealth of information about the underlying biology, and genomic sequencing has never been cheaper.13
Combining the data gathered via digital phenotyping with that of genotyping could help elucidate the mechanisms by which specific diseases and symptoms occur. This could be very promising to better understand and treat our patients. However, as is the case with genomics, digital phenotyping has important ethical implications. If used in the proper way to benefit our patients, the future for this new method is bright.
1. Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions). https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/. Accessed April 29, 2019.
2. Thibaut F. Digital applications: the future in psychiatry? Dialogues Clin Neurosci. 2016;18(2):123.
3. Statista. Global market share held by the leading smartphone operating systems in sales to end users from 1st quarter 2009 to 2nd quarter 2018. https://www.statista.com/statistics/266136/global-market-share-held-by-smartphone-operating-systems/. Accessed April 19, 2019.
4. Torous J, Roberts L. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
5. Barnett I, Torous J, Staples P, et al. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43(8):1660-1666.
6. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220-221.
7. Brouillette RM, Foil H, Fontenot S, et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PloS One. 2013;8(6):e65925. doi: 10.1371/journal.pone.0065925.
8. Winnick W. Putting a finger on our phone obsession. dscout. https://blog.dscout.com/mobile-touches. Published June 16, 2016. Accessed April 29, 2019.
9. Waite F, Myers E, Harvey AG, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273-287.
10. Mcgurk SR, Mueser KT, Xie H, et al. (2015). Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852-861.
11. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546-556.
12. Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258-267.
13. National Human Genome Research Institute. The cost of sequencing a human genome. https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/. Updated July 6, 2016. Accessed April 29, 2019.
1. Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions). https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/. Accessed April 29, 2019.
2. Thibaut F. Digital applications: the future in psychiatry? Dialogues Clin Neurosci. 2016;18(2):123.
3. Statista. Global market share held by the leading smartphone operating systems in sales to end users from 1st quarter 2009 to 2nd quarter 2018. https://www.statista.com/statistics/266136/global-market-share-held-by-smartphone-operating-systems/. Accessed April 19, 2019.
4. Torous J, Roberts L. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
5. Barnett I, Torous J, Staples P, et al. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43(8):1660-1666.
6. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220-221.
7. Brouillette RM, Foil H, Fontenot S, et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PloS One. 2013;8(6):e65925. doi: 10.1371/journal.pone.0065925.
8. Winnick W. Putting a finger on our phone obsession. dscout. https://blog.dscout.com/mobile-touches. Published June 16, 2016. Accessed April 29, 2019.
9. Waite F, Myers E, Harvey AG, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273-287.
10. Mcgurk SR, Mueser KT, Xie H, et al. (2015). Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852-861.
11. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546-556.
12. Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258-267.
13. National Human Genome Research Institute. The cost of sequencing a human genome. https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/. Updated July 6, 2016. Accessed April 29, 2019.
It’s time to implement measurement-based care in psychiatric practice
In an editorial published in Current Psychiatry 10 years ago, I cited a stunning fact based on a readers’ survey: 98% of psychiatrists did not use any of the 4 clinical rating scales that are routinely used in the clinical trials required for FDA approval of medications for psychotic, mood, and anxiety disorders.1
As a follow-up, Ahmed Aboraya, MD, DrPH, and I would like to report on the state of measurement-based care (MBC), a term coined by Trivedi in 2006 and defined by Fortney as “the systematic administration of symptom rating scales and use of the results to drive clinical decision making at the level of the individual patient.”2
We will start with the creator of modern rating scales, Father Thomas Verner Moore (1877-1969), who is considered one of the most underrecognized legends in the history of modern psychiatry. Moore was a psychologist and psychiatrist who can lay claim to 3 major achievements in psychiatry: the creation of rating scales in psychiatry, the use of factor analysis to deconstruct psychosis, and the formulation of specific definitions for symptoms and signs of psychopathology. Moore’s 1933 book described the rating scales used in his research.3
Since that time, researchers have continued to invent clinician-rated scales, self-report scales, and other measures in psychiatry. The Handbook of Psychiatric Measures, which was published in 2000 by the American Psychiatric Association Task Force chaired by AJ Rush Jr., includes >240 measures covering adult and child psychiatric disorders.4
Recent research has shown the superiority of MBC compared with usual standard care (USC) in improving patient outcomes.2,5-7 A recent well-designed, blind-rater, randomized trial by Guo et al8 showed that MBC is more effective than USC both in achieving response and remission, and reducing the time to response and remission. Given the evidence of the benefits of MBC in improving patient outcomes, and the plethora of reliable and validated rating scales, an important question arises: Why has MBC not yet been established as the standard of care in psychiatric clinical practice? There are many barriers to implementing MBC,9 including:
- time constraints (most commonly cited reason by psychiatrists)
- mismatch between clinical needs and the content of the measure (ie, rating scales are designed for research and not for clinicians’ use)
- measurements produced by rating scales may not always be clinically relevant
- administering rating scales may interfere with establishing rapport with patients
- some measures, such as standardized diagnostic interviews, can be cumbersome, unwieldy, and complicated
- the lack of formal training for most clinicians (among the top barriers for residents and faculty)
- lack of availability of training manuals and protocols.
Clinician researchers have started to adapt and invent instruments that can be used in clinical settings. For more than 20 years, Mark Zimmerman, MD, has been the principal investigator of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project, aimed at integrating the assessment methods of researchers into routine clinical practice.10 Zimmerman has developed self-report scales and outcome measures such as the Psychiatric Diagnostic Screening Questionnaire (PDSQ), the Clinically Useful Depression Outcome Scale (CUDOS), the Standardized Clinical Outcome Rating for Depression (SCOR-D), the Clinically Useful Anxiety Outcome Scale (CUXOS), the Remission from Depression Questionnaire (RDQ), and the Clinically Useful Patient Satisfaction Scale (CUPSS).11-18
We have been critical of the utility of the existing diagnostic interviews and rating scales. I (AA) developed the Standard for Clinicians’ Interview in Psychiatry (SCIP) as a MBC tool that addresses the most common barriers that clinicians face.9,19-23 The SCIP includes 18 clinician-rated scales for the following symptom domains: generalized anxiety, obsessions, compulsions, posttraumatic stress, depression, mania, delusions, hallucinations, disorganized thoughts, aggression, negative symptoms, alcohol use, drug use, attention deficit, hyperactivity, anorexia, binge-eating, and bulimia. The SCIP rating scales meet the criteria for MBC because they are efficient, reliable, and valid. They reflect how clinicians assess psychiatric disorders, and are relevant to decision-making. Both self-report and clinician-rated scales are important MBC tools and complementary to each other. The choice to use self-report scales, clinician-rated scales, or both depends on several factors, including the clinical setting (inpatient or outpatient), psychiatric diagnoses, and patient characteristics. No measure or scale will ever replace a seasoned and experienced clinician who has been evaluating and treating real-world patients for years. Just as thermometers, stethoscopes, and laboratories help other types of physicians to reach accurate diagnoses and provide appropriate management, the use of MBC by psychiatrists will enhance the accuracy of diagnoses and improve the outcomes of care.
Continue to: On a positive note...
On a positive note, I (AA) have completed a MBC curriculum for training psychiatry residents that includes 11 videotaped interviews with actual patients covering the major adult psychiatric disorders: generalized anxiety, panic, depressive, posttraumatic stress, bipolar, psychotic, eating, and attention-deficit/hyperactivity. The interviews show and teach how to rate psychopathology items, how to score the dimensions, and how to evaluate the severity of the disorder(s). All of the SCIP’s 18 scales have been uploaded into the Epic electronic health record (EHR) system at West Virginia University hospitals. A pilot project for implementing MBC in the treatment of adult psychiatric disorders at the West Virginia University residency program and other programs is underway. If we instruct residents in MBC during their psychiatric training, they will likely practice it for the rest of their clinical careers. Except for a minority of clinicians who are involved in clinical trials and who use rating scales in practice, most practicing clinicians were never trained to use scales. For more information about the MBC curriculum and videotapes, contact Dr. Aboraya at [email protected] or visit www.scip-psychiatry.com.
Today, some of the barriers that impede the implementation of MBC in psychiatric practice have been resolved, but much more work remains. Now is the time to implement MBC and provide an answer to AJ Rush, who asked, “Isn’t it about time to employ measurement-based care in practice?”24 The 3 main ingredients for MBC implementation—useful measures, integration of EHR, and health information technologies—exist today. We strongly encourage psychiatrists, nurse practitioners, and other mental health professionals to adopt MBC in their daily practice.
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
2. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2016;68(2):179-188.
3. Moore TV. The essential psychoses and their fundamental syndromes. Baltimore, MD: Williams & Wilkins; 1933.
4. Rush AJ. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000.
5. Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cogn Behav Pract. 2015;22(1):49-59.
6. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61-S71.
7. Harding KJ, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143.
8. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013.
9. Aboraya A, Nasrallah HA, Elswick D, et al. Measurement-based care in psychiatry: past, present and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
10. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
11. Zimmerman M, Mattia JI. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60(10):677-683.
12. Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42(3):175-189.
13. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140.
14. Zimmerman M, Posternak MA, Chelminski I, et al. Standardized clinical outcome rating scale for depression for use in clinical practice. Depress Anxiety. 2005;22(1):36-40.
15. Zimmerman M, Chelminski I, Young D, et al. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71(5):534-542.
16. Zimmerman M, Galione JN, Attiullah N, et al. Depressed patients’ perspectives of 2 measures of outcome: the Quick Inventory of Depressive Symptomatology (QIDS) and the Remission from Depression Questionnaire (RDQ). Ann Clin Psychiatry. 2011;23(3):208-212.
17. Zimmerman M, Martinez JH, Attiullah N, et al. The remission from depression questionnaire as an outcome measure in the treatment of depression. Depress Anxiety. 2014;31(6):533-538.
18. Zimmerman M, Gazarian D, Multach M, et al. A clinically useful self-report measure of psychiatric patients’ satisfaction with the initial evaluation. Psychiatry Res. 2017;252:38-44.
19. Aboraya A. The validity results of the Standard for Clinicians’ Interview in Psychiatry (SCIP). Schizophrenia Bulletin. 2015;41(Suppl 1):S103-S104.
20. Aboraya A. Instruction manual for the Standard for Clinicians’ Interview in Psychiatry (SCIP). http://innovationscns.com/wp-content/uploads/SCIP_Instruction_Manual.pdf. Accessed April 29, 2019.
21. Aboraya A, El-Missiry A, Barlowe J, et al. The reliability of the Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional and numeric output. Schizophr Res. 2014;156(2-3):174-183.
22. Aboraya A, Nasrallah HA, Muvvala S, et al. The Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional, and numeric output-conceptual development, design, and description of the SCIP. Innov Clin Neurosci. 2016;13(5-6):31-77.
23. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann Clin Psychiatry. 2016;28(2):125-131.
24. Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936.
In an editorial published in Current Psychiatry 10 years ago, I cited a stunning fact based on a readers’ survey: 98% of psychiatrists did not use any of the 4 clinical rating scales that are routinely used in the clinical trials required for FDA approval of medications for psychotic, mood, and anxiety disorders.1
As a follow-up, Ahmed Aboraya, MD, DrPH, and I would like to report on the state of measurement-based care (MBC), a term coined by Trivedi in 2006 and defined by Fortney as “the systematic administration of symptom rating scales and use of the results to drive clinical decision making at the level of the individual patient.”2
We will start with the creator of modern rating scales, Father Thomas Verner Moore (1877-1969), who is considered one of the most underrecognized legends in the history of modern psychiatry. Moore was a psychologist and psychiatrist who can lay claim to 3 major achievements in psychiatry: the creation of rating scales in psychiatry, the use of factor analysis to deconstruct psychosis, and the formulation of specific definitions for symptoms and signs of psychopathology. Moore’s 1933 book described the rating scales used in his research.3
Since that time, researchers have continued to invent clinician-rated scales, self-report scales, and other measures in psychiatry. The Handbook of Psychiatric Measures, which was published in 2000 by the American Psychiatric Association Task Force chaired by AJ Rush Jr., includes >240 measures covering adult and child psychiatric disorders.4
Recent research has shown the superiority of MBC compared with usual standard care (USC) in improving patient outcomes.2,5-7 A recent well-designed, blind-rater, randomized trial by Guo et al8 showed that MBC is more effective than USC both in achieving response and remission, and reducing the time to response and remission. Given the evidence of the benefits of MBC in improving patient outcomes, and the plethora of reliable and validated rating scales, an important question arises: Why has MBC not yet been established as the standard of care in psychiatric clinical practice? There are many barriers to implementing MBC,9 including:
- time constraints (most commonly cited reason by psychiatrists)
- mismatch between clinical needs and the content of the measure (ie, rating scales are designed for research and not for clinicians’ use)
- measurements produced by rating scales may not always be clinically relevant
- administering rating scales may interfere with establishing rapport with patients
- some measures, such as standardized diagnostic interviews, can be cumbersome, unwieldy, and complicated
- the lack of formal training for most clinicians (among the top barriers for residents and faculty)
- lack of availability of training manuals and protocols.
Clinician researchers have started to adapt and invent instruments that can be used in clinical settings. For more than 20 years, Mark Zimmerman, MD, has been the principal investigator of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project, aimed at integrating the assessment methods of researchers into routine clinical practice.10 Zimmerman has developed self-report scales and outcome measures such as the Psychiatric Diagnostic Screening Questionnaire (PDSQ), the Clinically Useful Depression Outcome Scale (CUDOS), the Standardized Clinical Outcome Rating for Depression (SCOR-D), the Clinically Useful Anxiety Outcome Scale (CUXOS), the Remission from Depression Questionnaire (RDQ), and the Clinically Useful Patient Satisfaction Scale (CUPSS).11-18
We have been critical of the utility of the existing diagnostic interviews and rating scales. I (AA) developed the Standard for Clinicians’ Interview in Psychiatry (SCIP) as a MBC tool that addresses the most common barriers that clinicians face.9,19-23 The SCIP includes 18 clinician-rated scales for the following symptom domains: generalized anxiety, obsessions, compulsions, posttraumatic stress, depression, mania, delusions, hallucinations, disorganized thoughts, aggression, negative symptoms, alcohol use, drug use, attention deficit, hyperactivity, anorexia, binge-eating, and bulimia. The SCIP rating scales meet the criteria for MBC because they are efficient, reliable, and valid. They reflect how clinicians assess psychiatric disorders, and are relevant to decision-making. Both self-report and clinician-rated scales are important MBC tools and complementary to each other. The choice to use self-report scales, clinician-rated scales, or both depends on several factors, including the clinical setting (inpatient or outpatient), psychiatric diagnoses, and patient characteristics. No measure or scale will ever replace a seasoned and experienced clinician who has been evaluating and treating real-world patients for years. Just as thermometers, stethoscopes, and laboratories help other types of physicians to reach accurate diagnoses and provide appropriate management, the use of MBC by psychiatrists will enhance the accuracy of diagnoses and improve the outcomes of care.
Continue to: On a positive note...
On a positive note, I (AA) have completed a MBC curriculum for training psychiatry residents that includes 11 videotaped interviews with actual patients covering the major adult psychiatric disorders: generalized anxiety, panic, depressive, posttraumatic stress, bipolar, psychotic, eating, and attention-deficit/hyperactivity. The interviews show and teach how to rate psychopathology items, how to score the dimensions, and how to evaluate the severity of the disorder(s). All of the SCIP’s 18 scales have been uploaded into the Epic electronic health record (EHR) system at West Virginia University hospitals. A pilot project for implementing MBC in the treatment of adult psychiatric disorders at the West Virginia University residency program and other programs is underway. If we instruct residents in MBC during their psychiatric training, they will likely practice it for the rest of their clinical careers. Except for a minority of clinicians who are involved in clinical trials and who use rating scales in practice, most practicing clinicians were never trained to use scales. For more information about the MBC curriculum and videotapes, contact Dr. Aboraya at [email protected] or visit www.scip-psychiatry.com.
Today, some of the barriers that impede the implementation of MBC in psychiatric practice have been resolved, but much more work remains. Now is the time to implement MBC and provide an answer to AJ Rush, who asked, “Isn’t it about time to employ measurement-based care in practice?”24 The 3 main ingredients for MBC implementation—useful measures, integration of EHR, and health information technologies—exist today. We strongly encourage psychiatrists, nurse practitioners, and other mental health professionals to adopt MBC in their daily practice.
To comment on this editorial or other topics of interest: [email protected].
In an editorial published in Current Psychiatry 10 years ago, I cited a stunning fact based on a readers’ survey: 98% of psychiatrists did not use any of the 4 clinical rating scales that are routinely used in the clinical trials required for FDA approval of medications for psychotic, mood, and anxiety disorders.1
As a follow-up, Ahmed Aboraya, MD, DrPH, and I would like to report on the state of measurement-based care (MBC), a term coined by Trivedi in 2006 and defined by Fortney as “the systematic administration of symptom rating scales and use of the results to drive clinical decision making at the level of the individual patient.”2
We will start with the creator of modern rating scales, Father Thomas Verner Moore (1877-1969), who is considered one of the most underrecognized legends in the history of modern psychiatry. Moore was a psychologist and psychiatrist who can lay claim to 3 major achievements in psychiatry: the creation of rating scales in psychiatry, the use of factor analysis to deconstruct psychosis, and the formulation of specific definitions for symptoms and signs of psychopathology. Moore’s 1933 book described the rating scales used in his research.3
Since that time, researchers have continued to invent clinician-rated scales, self-report scales, and other measures in psychiatry. The Handbook of Psychiatric Measures, which was published in 2000 by the American Psychiatric Association Task Force chaired by AJ Rush Jr., includes >240 measures covering adult and child psychiatric disorders.4
Recent research has shown the superiority of MBC compared with usual standard care (USC) in improving patient outcomes.2,5-7 A recent well-designed, blind-rater, randomized trial by Guo et al8 showed that MBC is more effective than USC both in achieving response and remission, and reducing the time to response and remission. Given the evidence of the benefits of MBC in improving patient outcomes, and the plethora of reliable and validated rating scales, an important question arises: Why has MBC not yet been established as the standard of care in psychiatric clinical practice? There are many barriers to implementing MBC,9 including:
- time constraints (most commonly cited reason by psychiatrists)
- mismatch between clinical needs and the content of the measure (ie, rating scales are designed for research and not for clinicians’ use)
- measurements produced by rating scales may not always be clinically relevant
- administering rating scales may interfere with establishing rapport with patients
- some measures, such as standardized diagnostic interviews, can be cumbersome, unwieldy, and complicated
- the lack of formal training for most clinicians (among the top barriers for residents and faculty)
- lack of availability of training manuals and protocols.
Clinician researchers have started to adapt and invent instruments that can be used in clinical settings. For more than 20 years, Mark Zimmerman, MD, has been the principal investigator of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project, aimed at integrating the assessment methods of researchers into routine clinical practice.10 Zimmerman has developed self-report scales and outcome measures such as the Psychiatric Diagnostic Screening Questionnaire (PDSQ), the Clinically Useful Depression Outcome Scale (CUDOS), the Standardized Clinical Outcome Rating for Depression (SCOR-D), the Clinically Useful Anxiety Outcome Scale (CUXOS), the Remission from Depression Questionnaire (RDQ), and the Clinically Useful Patient Satisfaction Scale (CUPSS).11-18
We have been critical of the utility of the existing diagnostic interviews and rating scales. I (AA) developed the Standard for Clinicians’ Interview in Psychiatry (SCIP) as a MBC tool that addresses the most common barriers that clinicians face.9,19-23 The SCIP includes 18 clinician-rated scales for the following symptom domains: generalized anxiety, obsessions, compulsions, posttraumatic stress, depression, mania, delusions, hallucinations, disorganized thoughts, aggression, negative symptoms, alcohol use, drug use, attention deficit, hyperactivity, anorexia, binge-eating, and bulimia. The SCIP rating scales meet the criteria for MBC because they are efficient, reliable, and valid. They reflect how clinicians assess psychiatric disorders, and are relevant to decision-making. Both self-report and clinician-rated scales are important MBC tools and complementary to each other. The choice to use self-report scales, clinician-rated scales, or both depends on several factors, including the clinical setting (inpatient or outpatient), psychiatric diagnoses, and patient characteristics. No measure or scale will ever replace a seasoned and experienced clinician who has been evaluating and treating real-world patients for years. Just as thermometers, stethoscopes, and laboratories help other types of physicians to reach accurate diagnoses and provide appropriate management, the use of MBC by psychiatrists will enhance the accuracy of diagnoses and improve the outcomes of care.
Continue to: On a positive note...
On a positive note, I (AA) have completed a MBC curriculum for training psychiatry residents that includes 11 videotaped interviews with actual patients covering the major adult psychiatric disorders: generalized anxiety, panic, depressive, posttraumatic stress, bipolar, psychotic, eating, and attention-deficit/hyperactivity. The interviews show and teach how to rate psychopathology items, how to score the dimensions, and how to evaluate the severity of the disorder(s). All of the SCIP’s 18 scales have been uploaded into the Epic electronic health record (EHR) system at West Virginia University hospitals. A pilot project for implementing MBC in the treatment of adult psychiatric disorders at the West Virginia University residency program and other programs is underway. If we instruct residents in MBC during their psychiatric training, they will likely practice it for the rest of their clinical careers. Except for a minority of clinicians who are involved in clinical trials and who use rating scales in practice, most practicing clinicians were never trained to use scales. For more information about the MBC curriculum and videotapes, contact Dr. Aboraya at [email protected] or visit www.scip-psychiatry.com.
Today, some of the barriers that impede the implementation of MBC in psychiatric practice have been resolved, but much more work remains. Now is the time to implement MBC and provide an answer to AJ Rush, who asked, “Isn’t it about time to employ measurement-based care in practice?”24 The 3 main ingredients for MBC implementation—useful measures, integration of EHR, and health information technologies—exist today. We strongly encourage psychiatrists, nurse practitioners, and other mental health professionals to adopt MBC in their daily practice.
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
2. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2016;68(2):179-188.
3. Moore TV. The essential psychoses and their fundamental syndromes. Baltimore, MD: Williams & Wilkins; 1933.
4. Rush AJ. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000.
5. Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cogn Behav Pract. 2015;22(1):49-59.
6. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61-S71.
7. Harding KJ, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143.
8. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013.
9. Aboraya A, Nasrallah HA, Elswick D, et al. Measurement-based care in psychiatry: past, present and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
10. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
11. Zimmerman M, Mattia JI. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60(10):677-683.
12. Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42(3):175-189.
13. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140.
14. Zimmerman M, Posternak MA, Chelminski I, et al. Standardized clinical outcome rating scale for depression for use in clinical practice. Depress Anxiety. 2005;22(1):36-40.
15. Zimmerman M, Chelminski I, Young D, et al. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71(5):534-542.
16. Zimmerman M, Galione JN, Attiullah N, et al. Depressed patients’ perspectives of 2 measures of outcome: the Quick Inventory of Depressive Symptomatology (QIDS) and the Remission from Depression Questionnaire (RDQ). Ann Clin Psychiatry. 2011;23(3):208-212.
17. Zimmerman M, Martinez JH, Attiullah N, et al. The remission from depression questionnaire as an outcome measure in the treatment of depression. Depress Anxiety. 2014;31(6):533-538.
18. Zimmerman M, Gazarian D, Multach M, et al. A clinically useful self-report measure of psychiatric patients’ satisfaction with the initial evaluation. Psychiatry Res. 2017;252:38-44.
19. Aboraya A. The validity results of the Standard for Clinicians’ Interview in Psychiatry (SCIP). Schizophrenia Bulletin. 2015;41(Suppl 1):S103-S104.
20. Aboraya A. Instruction manual for the Standard for Clinicians’ Interview in Psychiatry (SCIP). http://innovationscns.com/wp-content/uploads/SCIP_Instruction_Manual.pdf. Accessed April 29, 2019.
21. Aboraya A, El-Missiry A, Barlowe J, et al. The reliability of the Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional and numeric output. Schizophr Res. 2014;156(2-3):174-183.
22. Aboraya A, Nasrallah HA, Muvvala S, et al. The Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional, and numeric output-conceptual development, design, and description of the SCIP. Innov Clin Neurosci. 2016;13(5-6):31-77.
23. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann Clin Psychiatry. 2016;28(2):125-131.
24. Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936.
1. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
2. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2016;68(2):179-188.
3. Moore TV. The essential psychoses and their fundamental syndromes. Baltimore, MD: Williams & Wilkins; 1933.
4. Rush AJ. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000.
5. Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cogn Behav Pract. 2015;22(1):49-59.
6. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61-S71.
7. Harding KJ, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143.
8. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013.
9. Aboraya A, Nasrallah HA, Elswick D, et al. Measurement-based care in psychiatry: past, present and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
10. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
11. Zimmerman M, Mattia JI. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60(10):677-683.
12. Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42(3):175-189.
13. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140.
14. Zimmerman M, Posternak MA, Chelminski I, et al. Standardized clinical outcome rating scale for depression for use in clinical practice. Depress Anxiety. 2005;22(1):36-40.
15. Zimmerman M, Chelminski I, Young D, et al. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71(5):534-542.
16. Zimmerman M, Galione JN, Attiullah N, et al. Depressed patients’ perspectives of 2 measures of outcome: the Quick Inventory of Depressive Symptomatology (QIDS) and the Remission from Depression Questionnaire (RDQ). Ann Clin Psychiatry. 2011;23(3):208-212.
17. Zimmerman M, Martinez JH, Attiullah N, et al. The remission from depression questionnaire as an outcome measure in the treatment of depression. Depress Anxiety. 2014;31(6):533-538.
18. Zimmerman M, Gazarian D, Multach M, et al. A clinically useful self-report measure of psychiatric patients’ satisfaction with the initial evaluation. Psychiatry Res. 2017;252:38-44.
19. Aboraya A. The validity results of the Standard for Clinicians’ Interview in Psychiatry (SCIP). Schizophrenia Bulletin. 2015;41(Suppl 1):S103-S104.
20. Aboraya A. Instruction manual for the Standard for Clinicians’ Interview in Psychiatry (SCIP). http://innovationscns.com/wp-content/uploads/SCIP_Instruction_Manual.pdf. Accessed April 29, 2019.
21. Aboraya A, El-Missiry A, Barlowe J, et al. The reliability of the Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional and numeric output. Schizophr Res. 2014;156(2-3):174-183.
22. Aboraya A, Nasrallah HA, Muvvala S, et al. The Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional, and numeric output-conceptual development, design, and description of the SCIP. Innov Clin Neurosci. 2016;13(5-6):31-77.
23. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann Clin Psychiatry. 2016;28(2):125-131.
24. Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936.















