User login
Improving nonverbal communication during telepsychiatry sessions
Telepsychiatry appointments (eg, video conferencing) initially replaced face-to-face outpatient encounters during the first phase of the COVID-19 pandemic. However, as offices reopened for in-person appointments, many patients still prefer “virtual” appointments. Telepsychiatry allows for easier delivery of mental health services, including psychotherapy, and may become the new normal.
Although therapy conducted via video conferencing allows you to connect with patients at a safe distance, it alters the basic conditions under which therapy occurs, such as being in the same room.1 While focusing on preserving the verbal elements of communication, you might inadvertently forget the nonverbal elements, which at times might render your words ineffective.1 The main elements of nonverbal communication are facial expression, gaze, posture, gesture, and proxemics (ie, how much space you take up, and your distance from others).2 The following tips can help you preserve the nonverbal elements of communication when conducting telepsychiatry sessions.
Reduce gaze error. Gaze error is the deviation from direct eye contact that occurs during video conferencing. It results from the distance between the image of the person on your screen and the camera above it.1 Gaze error can muddy intended cues and communicate unintended cues.2 Examples of gaze errors include downcast eyes (the most common gaze error), sideways gaze, or gazing over the person’s head.2 These errors can communicate social deference, evasion, insincerity, or even boredom.2 To lessen gaze error, move the patient’s image as close as possible to your camera.1 In addition, avoid looking at yourself on the screen; some video conferencing platforms allow users to hide their self-view.
Create distance and incorporate upper body language. In the office, sitting very close to your patient and staring directly at their face for an hour would be awkward and intrusive.1 Doing so online is no different. While you may be tempted to move close to the screen to compensate for feeling distant or having difficulty hearing or seeing your patient, you should back away from the camera. Doing so will help both parties feel less self-conscious, more at ease, and more focused on the session.1 Backing up from the camera will allow patients to see your upper body language (eg, hand gestures, posture) as well as your facial expressions.1 Empathy improves when patients can see your upper-body cues.2 Keep in mind that the angle of your camera is just as important as the distance. For example, if your camera is positioned so that it is looking up toward your eyes, patients may perceive that you are looking down at them.1 This problem can be remedied by stacking books under the monitor to raise the camera.
Be aware of your facial expressions, posture, gestures, and proxemics. Ensure that your face does not go slack when you are listening to patients talk.3 Just as you would do in person, a slight head tilt and occasional head nod lets patients know that you are engaged and actively listening.3 Maintain an open body posture by keeping your feet firmly on the ground and putting your hands on the table in front of you.3 Lean in when patients share intimate information, just as you would in person. Avoid hunching over the laptop/keyboard because this could make you seem tired or tense.3 Pay attention to your arm and hand movements so that you do not exaggerate them.
Maintain office professionalism. The office setting conveys a therapeutic formality that can get lost online.1 As tempting as it may be to conduct online sessions in pajamas or sweatpants, continue to dress as if you were in the office. Be mindful of your backdrop, set all cell phones to silent, turn off your email alerts, and lock the room.1,3 Stick to the clock as you would in the office, and encourage patients to do the same.
Minor technological improvements—such as headphones with a built-in microphone, a high-definition camera, a larger monitor, or a faster internet connection—might be needed to improve your nonverbal communication during telepsychiatry sessions.1 Although this is not an exhaustive list, these tips can serve as a starting point to ensure effective communication while you are physically distanced from your patients.
1. Arnold C, Franklin T. Seven tips for maintaining the frame in online therapy. Psychiatric News. Published June 25, 2020. Accessed May 26, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.7a21
2. Nguyen DT, Canny J. More than face-to-face: empathy effects of video framing. CHI 2009: Proceedings of the SGCHI Conference on Human Factors in Computing Systems. Published April 6, 2009. Accessed July 31, 2020. https://dl.acm.org/doi/10.1145/1518701.1518770
3. Cossar R, Navarro J. Tips for improving communication during video conferencing: do’s and don’ts for a more professional video-conference. Published March 31, 2020. Accessed July 31, 2020. https://www.psychologytoday.com/us/blog/spycatcher/202003/tips-improving-communication-during-video-conferencing
Telepsychiatry appointments (eg, video conferencing) initially replaced face-to-face outpatient encounters during the first phase of the COVID-19 pandemic. However, as offices reopened for in-person appointments, many patients still prefer “virtual” appointments. Telepsychiatry allows for easier delivery of mental health services, including psychotherapy, and may become the new normal.
Although therapy conducted via video conferencing allows you to connect with patients at a safe distance, it alters the basic conditions under which therapy occurs, such as being in the same room.1 While focusing on preserving the verbal elements of communication, you might inadvertently forget the nonverbal elements, which at times might render your words ineffective.1 The main elements of nonverbal communication are facial expression, gaze, posture, gesture, and proxemics (ie, how much space you take up, and your distance from others).2 The following tips can help you preserve the nonverbal elements of communication when conducting telepsychiatry sessions.
Reduce gaze error. Gaze error is the deviation from direct eye contact that occurs during video conferencing. It results from the distance between the image of the person on your screen and the camera above it.1 Gaze error can muddy intended cues and communicate unintended cues.2 Examples of gaze errors include downcast eyes (the most common gaze error), sideways gaze, or gazing over the person’s head.2 These errors can communicate social deference, evasion, insincerity, or even boredom.2 To lessen gaze error, move the patient’s image as close as possible to your camera.1 In addition, avoid looking at yourself on the screen; some video conferencing platforms allow users to hide their self-view.
Create distance and incorporate upper body language. In the office, sitting very close to your patient and staring directly at their face for an hour would be awkward and intrusive.1 Doing so online is no different. While you may be tempted to move close to the screen to compensate for feeling distant or having difficulty hearing or seeing your patient, you should back away from the camera. Doing so will help both parties feel less self-conscious, more at ease, and more focused on the session.1 Backing up from the camera will allow patients to see your upper body language (eg, hand gestures, posture) as well as your facial expressions.1 Empathy improves when patients can see your upper-body cues.2 Keep in mind that the angle of your camera is just as important as the distance. For example, if your camera is positioned so that it is looking up toward your eyes, patients may perceive that you are looking down at them.1 This problem can be remedied by stacking books under the monitor to raise the camera.
Be aware of your facial expressions, posture, gestures, and proxemics. Ensure that your face does not go slack when you are listening to patients talk.3 Just as you would do in person, a slight head tilt and occasional head nod lets patients know that you are engaged and actively listening.3 Maintain an open body posture by keeping your feet firmly on the ground and putting your hands on the table in front of you.3 Lean in when patients share intimate information, just as you would in person. Avoid hunching over the laptop/keyboard because this could make you seem tired or tense.3 Pay attention to your arm and hand movements so that you do not exaggerate them.
Maintain office professionalism. The office setting conveys a therapeutic formality that can get lost online.1 As tempting as it may be to conduct online sessions in pajamas or sweatpants, continue to dress as if you were in the office. Be mindful of your backdrop, set all cell phones to silent, turn off your email alerts, and lock the room.1,3 Stick to the clock as you would in the office, and encourage patients to do the same.
Minor technological improvements—such as headphones with a built-in microphone, a high-definition camera, a larger monitor, or a faster internet connection—might be needed to improve your nonverbal communication during telepsychiatry sessions.1 Although this is not an exhaustive list, these tips can serve as a starting point to ensure effective communication while you are physically distanced from your patients.
Telepsychiatry appointments (eg, video conferencing) initially replaced face-to-face outpatient encounters during the first phase of the COVID-19 pandemic. However, as offices reopened for in-person appointments, many patients still prefer “virtual” appointments. Telepsychiatry allows for easier delivery of mental health services, including psychotherapy, and may become the new normal.
Although therapy conducted via video conferencing allows you to connect with patients at a safe distance, it alters the basic conditions under which therapy occurs, such as being in the same room.1 While focusing on preserving the verbal elements of communication, you might inadvertently forget the nonverbal elements, which at times might render your words ineffective.1 The main elements of nonverbal communication are facial expression, gaze, posture, gesture, and proxemics (ie, how much space you take up, and your distance from others).2 The following tips can help you preserve the nonverbal elements of communication when conducting telepsychiatry sessions.
Reduce gaze error. Gaze error is the deviation from direct eye contact that occurs during video conferencing. It results from the distance between the image of the person on your screen and the camera above it.1 Gaze error can muddy intended cues and communicate unintended cues.2 Examples of gaze errors include downcast eyes (the most common gaze error), sideways gaze, or gazing over the person’s head.2 These errors can communicate social deference, evasion, insincerity, or even boredom.2 To lessen gaze error, move the patient’s image as close as possible to your camera.1 In addition, avoid looking at yourself on the screen; some video conferencing platforms allow users to hide their self-view.
Create distance and incorporate upper body language. In the office, sitting very close to your patient and staring directly at their face for an hour would be awkward and intrusive.1 Doing so online is no different. While you may be tempted to move close to the screen to compensate for feeling distant or having difficulty hearing or seeing your patient, you should back away from the camera. Doing so will help both parties feel less self-conscious, more at ease, and more focused on the session.1 Backing up from the camera will allow patients to see your upper body language (eg, hand gestures, posture) as well as your facial expressions.1 Empathy improves when patients can see your upper-body cues.2 Keep in mind that the angle of your camera is just as important as the distance. For example, if your camera is positioned so that it is looking up toward your eyes, patients may perceive that you are looking down at them.1 This problem can be remedied by stacking books under the monitor to raise the camera.
Be aware of your facial expressions, posture, gestures, and proxemics. Ensure that your face does not go slack when you are listening to patients talk.3 Just as you would do in person, a slight head tilt and occasional head nod lets patients know that you are engaged and actively listening.3 Maintain an open body posture by keeping your feet firmly on the ground and putting your hands on the table in front of you.3 Lean in when patients share intimate information, just as you would in person. Avoid hunching over the laptop/keyboard because this could make you seem tired or tense.3 Pay attention to your arm and hand movements so that you do not exaggerate them.
Maintain office professionalism. The office setting conveys a therapeutic formality that can get lost online.1 As tempting as it may be to conduct online sessions in pajamas or sweatpants, continue to dress as if you were in the office. Be mindful of your backdrop, set all cell phones to silent, turn off your email alerts, and lock the room.1,3 Stick to the clock as you would in the office, and encourage patients to do the same.
Minor technological improvements—such as headphones with a built-in microphone, a high-definition camera, a larger monitor, or a faster internet connection—might be needed to improve your nonverbal communication during telepsychiatry sessions.1 Although this is not an exhaustive list, these tips can serve as a starting point to ensure effective communication while you are physically distanced from your patients.
1. Arnold C, Franklin T. Seven tips for maintaining the frame in online therapy. Psychiatric News. Published June 25, 2020. Accessed May 26, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.7a21
2. Nguyen DT, Canny J. More than face-to-face: empathy effects of video framing. CHI 2009: Proceedings of the SGCHI Conference on Human Factors in Computing Systems. Published April 6, 2009. Accessed July 31, 2020. https://dl.acm.org/doi/10.1145/1518701.1518770
3. Cossar R, Navarro J. Tips for improving communication during video conferencing: do’s and don’ts for a more professional video-conference. Published March 31, 2020. Accessed July 31, 2020. https://www.psychologytoday.com/us/blog/spycatcher/202003/tips-improving-communication-during-video-conferencing
1. Arnold C, Franklin T. Seven tips for maintaining the frame in online therapy. Psychiatric News. Published June 25, 2020. Accessed May 26, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.7a21
2. Nguyen DT, Canny J. More than face-to-face: empathy effects of video framing. CHI 2009: Proceedings of the SGCHI Conference on Human Factors in Computing Systems. Published April 6, 2009. Accessed July 31, 2020. https://dl.acm.org/doi/10.1145/1518701.1518770
3. Cossar R, Navarro J. Tips for improving communication during video conferencing: do’s and don’ts for a more professional video-conference. Published March 31, 2020. Accessed July 31, 2020. https://www.psychologytoday.com/us/blog/spycatcher/202003/tips-improving-communication-during-video-conferencing
Treating psychosis in pregnant women: A measured approach
The peak age of onset of schizophrenia coincides with the peak childbearing age of 25 to 35 years.1 So it would not be unusual for your patient with schizophrenia to tell you she is trying to get pregnant, or thinks she might be pregnant. In these situations, you must carefully weigh the risks to the mother (eg, relapse, complications) and to the fetus (eg, possible miscarriage, teratogenesis) when deciding whether to continue or change her treatment regimen. When faced with making these decisions, keep the following factors in mind.
1. Most importantly: Do not make knee-jerk changes. Do not suddenly stop medications. Proceed in a thoughtful and measured way.
2. Discuss the risks with your patient. There is no such thing as a risk-free decision. There are potential risks from untreated psychosis as well as from medications. Mothers with untreated psychosis have an increased risk of suicide and violence, as well as poor self-care. Schizophrenia may be associated with an increased risk of poor birth outcomes, including preterm delivery, low birthweight, and neonatal complications.2 Avoid making absolute statements about specific medications during pregnancy; there needs to be an individualized risk-benefit discussion for each patient, and for each medication.
3. Involve the patient’s partner and family in treatment planning if possible. The patient’s family can be important in promoting mental health during pregnancy and the postpartum. Educating the family as well as the patient regarding medications and the risks of untreated mental illness can go a long way toward compliance.
4. Do not rely on what pregnancy category a medication was. There are multiple dimensions to evaluate when considering the use of an antipsychotic agent during pregnancy. Does it increase the risk of miscarriage? Malformations? Preterm birth? Perinatal toxicity? Behavioral teratogenesis (neurodevelopmental sequelae)? Looking for a simple summary or single letter grade minimizes the understanding of the specific outcome of concern in the specific mother. Instead, look at the Pregnancy section under Use in Specific Populations on the medication’s package insert (prescribing information), consult a web site such as MotherToBaby (mothertobaby.org/healthcare-professionals/), and/or search for the latest research on PubMed.
5. Collaborate with the patient’s obstetrician or family medicine physician. Make sure that you are on the same page regarding treating the patient’s psychosis. Other clinicians often will agree with your treatment plan because they understand the risks of untreated psychosis compared with other risks the patient is facing. However, if you don’t communicate with your patient’s other health care professionals, she might receive mixed messages.
6. As for medication choice, pregnancy is the most important time to conduct a careful medication history to inform your choice of medication. Was Medication X ineffective, or did the patient not pick it up from the pharmacy? Did she really have a trial of 3 months, or did she only take it for a week before she decided to stop?
Continue to: Determine which medication has worked for the patient in the past
7. Determine which medication has worked for the patient in the past. If Medication Y worked before she was pregnant, it is likely to still work during pregnancy. If it is a relatively safe option, it may be the best choice.
8. Avoid multiple medication exposures wherever possible. If a patient is taking Medication Z, it is working, and she tells you she is 3 months pregnant, it is often better to continue it (assuming it is a relatively safe medication) than to switch to Medication A, which has slightly better “safety data.” By switching to a different antipsychotic, you would be exposing the fetus to a second agent that may not even work for the mother.
9. Focus on treating the patient’s present symptoms. Medication doses may need to change due to pregnancy-related changes in symptoms, drug distribution, and/or metabolism.
10. Remain vigilant for other risks. Keep in mind that pregnant women with psychosis often face risks other than psychiatric medications and psychosis. Comorbidities such as substance use disorders, obesity, and poor prenatal care must also be addressed.3
11. Follow your patient more closely during pregnancy. Pregnancy is an uncertain time for any new mother. Be sure to have an open line of communication with the patient, and be responsive to her concerns.
Continue to: Provide psychoeducation about the postpartum period
12. Provide psychoeducation about the postpartum period. Pregnancy is the time to educate your patient about the importance of sleep, warning signs of exacerbation of psychosis, and breastfeeding safety.
13. Be proactive with future female patients of childbearing age, regardless of whether they tell you they are sexually active. Women with psychosis have higher rates of unplanned pregnancy.3,4 When initiating treatment of psychosis in a woman of childbearing age, rather than treating her with the newest available medication that does not yet have safety data in pregnancy, it is best to start with a medication already known to be relatively safe in pregnancy. This way, if she were to become pregnant, your treatment plan would already be safe and appropriate.
14. Consult a reproductive psychiatrist if needed.
1. Einarson A, Boskovic R. Use and safety of antipsychotic drugs during pregnancy. J Psychiatr Pract. 2009;15(3):183-192.
2. Galbally M, Crabb C. Schizophrenia and psychotic disorders. O&G. 2018;20(3). https://www.ogmagazine.org.au/20/3-20/schizophrenia-and-psychotic-disorders/
3. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635.
4. Friedman SH, Hall RCW, Sorrentino RM. Involuntary treatment of psychosis in pregnancy. J Am Acad Psychiatry Law. 2018;46(2):217-223.
The peak age of onset of schizophrenia coincides with the peak childbearing age of 25 to 35 years.1 So it would not be unusual for your patient with schizophrenia to tell you she is trying to get pregnant, or thinks she might be pregnant. In these situations, you must carefully weigh the risks to the mother (eg, relapse, complications) and to the fetus (eg, possible miscarriage, teratogenesis) when deciding whether to continue or change her treatment regimen. When faced with making these decisions, keep the following factors in mind.
1. Most importantly: Do not make knee-jerk changes. Do not suddenly stop medications. Proceed in a thoughtful and measured way.
2. Discuss the risks with your patient. There is no such thing as a risk-free decision. There are potential risks from untreated psychosis as well as from medications. Mothers with untreated psychosis have an increased risk of suicide and violence, as well as poor self-care. Schizophrenia may be associated with an increased risk of poor birth outcomes, including preterm delivery, low birthweight, and neonatal complications.2 Avoid making absolute statements about specific medications during pregnancy; there needs to be an individualized risk-benefit discussion for each patient, and for each medication.
3. Involve the patient’s partner and family in treatment planning if possible. The patient’s family can be important in promoting mental health during pregnancy and the postpartum. Educating the family as well as the patient regarding medications and the risks of untreated mental illness can go a long way toward compliance.
4. Do not rely on what pregnancy category a medication was. There are multiple dimensions to evaluate when considering the use of an antipsychotic agent during pregnancy. Does it increase the risk of miscarriage? Malformations? Preterm birth? Perinatal toxicity? Behavioral teratogenesis (neurodevelopmental sequelae)? Looking for a simple summary or single letter grade minimizes the understanding of the specific outcome of concern in the specific mother. Instead, look at the Pregnancy section under Use in Specific Populations on the medication’s package insert (prescribing information), consult a web site such as MotherToBaby (mothertobaby.org/healthcare-professionals/), and/or search for the latest research on PubMed.
5. Collaborate with the patient’s obstetrician or family medicine physician. Make sure that you are on the same page regarding treating the patient’s psychosis. Other clinicians often will agree with your treatment plan because they understand the risks of untreated psychosis compared with other risks the patient is facing. However, if you don’t communicate with your patient’s other health care professionals, she might receive mixed messages.
6. As for medication choice, pregnancy is the most important time to conduct a careful medication history to inform your choice of medication. Was Medication X ineffective, or did the patient not pick it up from the pharmacy? Did she really have a trial of 3 months, or did she only take it for a week before she decided to stop?
Continue to: Determine which medication has worked for the patient in the past
7. Determine which medication has worked for the patient in the past. If Medication Y worked before she was pregnant, it is likely to still work during pregnancy. If it is a relatively safe option, it may be the best choice.
8. Avoid multiple medication exposures wherever possible. If a patient is taking Medication Z, it is working, and she tells you she is 3 months pregnant, it is often better to continue it (assuming it is a relatively safe medication) than to switch to Medication A, which has slightly better “safety data.” By switching to a different antipsychotic, you would be exposing the fetus to a second agent that may not even work for the mother.
9. Focus on treating the patient’s present symptoms. Medication doses may need to change due to pregnancy-related changes in symptoms, drug distribution, and/or metabolism.
10. Remain vigilant for other risks. Keep in mind that pregnant women with psychosis often face risks other than psychiatric medications and psychosis. Comorbidities such as substance use disorders, obesity, and poor prenatal care must also be addressed.3
11. Follow your patient more closely during pregnancy. Pregnancy is an uncertain time for any new mother. Be sure to have an open line of communication with the patient, and be responsive to her concerns.
Continue to: Provide psychoeducation about the postpartum period
12. Provide psychoeducation about the postpartum period. Pregnancy is the time to educate your patient about the importance of sleep, warning signs of exacerbation of psychosis, and breastfeeding safety.
13. Be proactive with future female patients of childbearing age, regardless of whether they tell you they are sexually active. Women with psychosis have higher rates of unplanned pregnancy.3,4 When initiating treatment of psychosis in a woman of childbearing age, rather than treating her with the newest available medication that does not yet have safety data in pregnancy, it is best to start with a medication already known to be relatively safe in pregnancy. This way, if she were to become pregnant, your treatment plan would already be safe and appropriate.
14. Consult a reproductive psychiatrist if needed.
The peak age of onset of schizophrenia coincides with the peak childbearing age of 25 to 35 years.1 So it would not be unusual for your patient with schizophrenia to tell you she is trying to get pregnant, or thinks she might be pregnant. In these situations, you must carefully weigh the risks to the mother (eg, relapse, complications) and to the fetus (eg, possible miscarriage, teratogenesis) when deciding whether to continue or change her treatment regimen. When faced with making these decisions, keep the following factors in mind.
1. Most importantly: Do not make knee-jerk changes. Do not suddenly stop medications. Proceed in a thoughtful and measured way.
2. Discuss the risks with your patient. There is no such thing as a risk-free decision. There are potential risks from untreated psychosis as well as from medications. Mothers with untreated psychosis have an increased risk of suicide and violence, as well as poor self-care. Schizophrenia may be associated with an increased risk of poor birth outcomes, including preterm delivery, low birthweight, and neonatal complications.2 Avoid making absolute statements about specific medications during pregnancy; there needs to be an individualized risk-benefit discussion for each patient, and for each medication.
3. Involve the patient’s partner and family in treatment planning if possible. The patient’s family can be important in promoting mental health during pregnancy and the postpartum. Educating the family as well as the patient regarding medications and the risks of untreated mental illness can go a long way toward compliance.
4. Do not rely on what pregnancy category a medication was. There are multiple dimensions to evaluate when considering the use of an antipsychotic agent during pregnancy. Does it increase the risk of miscarriage? Malformations? Preterm birth? Perinatal toxicity? Behavioral teratogenesis (neurodevelopmental sequelae)? Looking for a simple summary or single letter grade minimizes the understanding of the specific outcome of concern in the specific mother. Instead, look at the Pregnancy section under Use in Specific Populations on the medication’s package insert (prescribing information), consult a web site such as MotherToBaby (mothertobaby.org/healthcare-professionals/), and/or search for the latest research on PubMed.
5. Collaborate with the patient’s obstetrician or family medicine physician. Make sure that you are on the same page regarding treating the patient’s psychosis. Other clinicians often will agree with your treatment plan because they understand the risks of untreated psychosis compared with other risks the patient is facing. However, if you don’t communicate with your patient’s other health care professionals, she might receive mixed messages.
6. As for medication choice, pregnancy is the most important time to conduct a careful medication history to inform your choice of medication. Was Medication X ineffective, or did the patient not pick it up from the pharmacy? Did she really have a trial of 3 months, or did she only take it for a week before she decided to stop?
Continue to: Determine which medication has worked for the patient in the past
7. Determine which medication has worked for the patient in the past. If Medication Y worked before she was pregnant, it is likely to still work during pregnancy. If it is a relatively safe option, it may be the best choice.
8. Avoid multiple medication exposures wherever possible. If a patient is taking Medication Z, it is working, and she tells you she is 3 months pregnant, it is often better to continue it (assuming it is a relatively safe medication) than to switch to Medication A, which has slightly better “safety data.” By switching to a different antipsychotic, you would be exposing the fetus to a second agent that may not even work for the mother.
9. Focus on treating the patient’s present symptoms. Medication doses may need to change due to pregnancy-related changes in symptoms, drug distribution, and/or metabolism.
10. Remain vigilant for other risks. Keep in mind that pregnant women with psychosis often face risks other than psychiatric medications and psychosis. Comorbidities such as substance use disorders, obesity, and poor prenatal care must also be addressed.3
11. Follow your patient more closely during pregnancy. Pregnancy is an uncertain time for any new mother. Be sure to have an open line of communication with the patient, and be responsive to her concerns.
Continue to: Provide psychoeducation about the postpartum period
12. Provide psychoeducation about the postpartum period. Pregnancy is the time to educate your patient about the importance of sleep, warning signs of exacerbation of psychosis, and breastfeeding safety.
13. Be proactive with future female patients of childbearing age, regardless of whether they tell you they are sexually active. Women with psychosis have higher rates of unplanned pregnancy.3,4 When initiating treatment of psychosis in a woman of childbearing age, rather than treating her with the newest available medication that does not yet have safety data in pregnancy, it is best to start with a medication already known to be relatively safe in pregnancy. This way, if she were to become pregnant, your treatment plan would already be safe and appropriate.
14. Consult a reproductive psychiatrist if needed.
1. Einarson A, Boskovic R. Use and safety of antipsychotic drugs during pregnancy. J Psychiatr Pract. 2009;15(3):183-192.
2. Galbally M, Crabb C. Schizophrenia and psychotic disorders. O&G. 2018;20(3). https://www.ogmagazine.org.au/20/3-20/schizophrenia-and-psychotic-disorders/
3. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635.
4. Friedman SH, Hall RCW, Sorrentino RM. Involuntary treatment of psychosis in pregnancy. J Am Acad Psychiatry Law. 2018;46(2):217-223.
1. Einarson A, Boskovic R. Use and safety of antipsychotic drugs during pregnancy. J Psychiatr Pract. 2009;15(3):183-192.
2. Galbally M, Crabb C. Schizophrenia and psychotic disorders. O&G. 2018;20(3). https://www.ogmagazine.org.au/20/3-20/schizophrenia-and-psychotic-disorders/
3. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635.
4. Friedman SH, Hall RCW, Sorrentino RM. Involuntary treatment of psychosis in pregnancy. J Am Acad Psychiatry Law. 2018;46(2):217-223.
Recommending esketamine? 4 factors to consider
Since receiving FDA approval in March 2019, intranasal esketamine—the S-enantiomer of ketamine—has become a valuable treatment option for adults with treatment-resistant depression (TRD), owing to its limited adverse effects profile, rapid onset, and potential for significant improvement in depressive symptoms. In August 2020, the FDA expanded esketamine’s indication to include treatment of depressive symptoms in adults with acute suicidal ideation or behavior, thus providing psychiatrists with an additional option for improving the care of their most seriously ill patients. In this article, we review 4 factors to consider before recommending esketamine.
1. Confirm that the patient’s depression qualifies as treatment-resistant. A patient is considered to have TRD if they have long-standing depression that meets DSM-5 criteria for major depressive disorder, and have not adequately responded to at least 2 antidepressant trials of adequate dose and duration.
2. Confirm that the patient’s suicidal ideation and/or suicidal behavior does not require acute hospitalization. The time between the onset of suicidal ideation and a suicide attempt typically is short, which highlights the need to intervene quickly in these patients.1 Being able to provide a treatment that works quickly and effectively may be lifesaving. However, to receive esketamine, patients must be enrolled in the Risk Evaluation Mitigation Strategy (REMS) patient registry through a certified treatment center, and prior authorization from insurance generally is required. These steps take time, so patients at high or imminent risk for suicide may initially require psychiatric hospitalization before they are able to begin esketamine treatment. Parsing out whether the suicidal ideation is chronic or acute can help clinicians assess current dangerousness and determine if esketamine treatment might be appropriate. If a patient with chronic suicidal ideation is stable for outpatient treatment with close monitoring, esketamine might provide an effective treatment option for treating both depression and suicidality. Esketamine’s rapid effect may be an integral part of the treatment for a suicidal patient by bridging the gap caused by the delayed onset of action in typical antidepressants.2
3. Identify a local certified treatment center. Use the online database at www.spravato.com/find-a-center to locate a nearby certified esketamine treatment center. Choosing a center that you can collaborate with regularly is important to determine if the treatment is effective, to provide updates on the treatment course, and to consider tailoring of ongoing treatment.
4. Ensure the patient is also treated with an oral antidepressant. Esketamine should be administered in conjunction with an oral antidepressant. As such, patients must be willing and able to tolerate treatment with a medication that can be construed as an antidepressant while undergoing esketamine treatment. A long-term maintenance trial found that patients with TRD who experienced remission or response after esketamine treatment had a delayed relapse of symptoms when they continued esketamine in addition to an oral antidepressant.3
Considering its rapid onset of action and low adverse effects profile with manageable tolerability, esketamine adjunctive to an oral antidepressant is a reasonable option to consider for patients with TRD, including those with suicidality.
1. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24.
2. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
3. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatry. 2019;76(9):893-903.
Since receiving FDA approval in March 2019, intranasal esketamine—the S-enantiomer of ketamine—has become a valuable treatment option for adults with treatment-resistant depression (TRD), owing to its limited adverse effects profile, rapid onset, and potential for significant improvement in depressive symptoms. In August 2020, the FDA expanded esketamine’s indication to include treatment of depressive symptoms in adults with acute suicidal ideation or behavior, thus providing psychiatrists with an additional option for improving the care of their most seriously ill patients. In this article, we review 4 factors to consider before recommending esketamine.
1. Confirm that the patient’s depression qualifies as treatment-resistant. A patient is considered to have TRD if they have long-standing depression that meets DSM-5 criteria for major depressive disorder, and have not adequately responded to at least 2 antidepressant trials of adequate dose and duration.
2. Confirm that the patient’s suicidal ideation and/or suicidal behavior does not require acute hospitalization. The time between the onset of suicidal ideation and a suicide attempt typically is short, which highlights the need to intervene quickly in these patients.1 Being able to provide a treatment that works quickly and effectively may be lifesaving. However, to receive esketamine, patients must be enrolled in the Risk Evaluation Mitigation Strategy (REMS) patient registry through a certified treatment center, and prior authorization from insurance generally is required. These steps take time, so patients at high or imminent risk for suicide may initially require psychiatric hospitalization before they are able to begin esketamine treatment. Parsing out whether the suicidal ideation is chronic or acute can help clinicians assess current dangerousness and determine if esketamine treatment might be appropriate. If a patient with chronic suicidal ideation is stable for outpatient treatment with close monitoring, esketamine might provide an effective treatment option for treating both depression and suicidality. Esketamine’s rapid effect may be an integral part of the treatment for a suicidal patient by bridging the gap caused by the delayed onset of action in typical antidepressants.2
3. Identify a local certified treatment center. Use the online database at www.spravato.com/find-a-center to locate a nearby certified esketamine treatment center. Choosing a center that you can collaborate with regularly is important to determine if the treatment is effective, to provide updates on the treatment course, and to consider tailoring of ongoing treatment.
4. Ensure the patient is also treated with an oral antidepressant. Esketamine should be administered in conjunction with an oral antidepressant. As such, patients must be willing and able to tolerate treatment with a medication that can be construed as an antidepressant while undergoing esketamine treatment. A long-term maintenance trial found that patients with TRD who experienced remission or response after esketamine treatment had a delayed relapse of symptoms when they continued esketamine in addition to an oral antidepressant.3
Considering its rapid onset of action and low adverse effects profile with manageable tolerability, esketamine adjunctive to an oral antidepressant is a reasonable option to consider for patients with TRD, including those with suicidality.
Since receiving FDA approval in March 2019, intranasal esketamine—the S-enantiomer of ketamine—has become a valuable treatment option for adults with treatment-resistant depression (TRD), owing to its limited adverse effects profile, rapid onset, and potential for significant improvement in depressive symptoms. In August 2020, the FDA expanded esketamine’s indication to include treatment of depressive symptoms in adults with acute suicidal ideation or behavior, thus providing psychiatrists with an additional option for improving the care of their most seriously ill patients. In this article, we review 4 factors to consider before recommending esketamine.
1. Confirm that the patient’s depression qualifies as treatment-resistant. A patient is considered to have TRD if they have long-standing depression that meets DSM-5 criteria for major depressive disorder, and have not adequately responded to at least 2 antidepressant trials of adequate dose and duration.
2. Confirm that the patient’s suicidal ideation and/or suicidal behavior does not require acute hospitalization. The time between the onset of suicidal ideation and a suicide attempt typically is short, which highlights the need to intervene quickly in these patients.1 Being able to provide a treatment that works quickly and effectively may be lifesaving. However, to receive esketamine, patients must be enrolled in the Risk Evaluation Mitigation Strategy (REMS) patient registry through a certified treatment center, and prior authorization from insurance generally is required. These steps take time, so patients at high or imminent risk for suicide may initially require psychiatric hospitalization before they are able to begin esketamine treatment. Parsing out whether the suicidal ideation is chronic or acute can help clinicians assess current dangerousness and determine if esketamine treatment might be appropriate. If a patient with chronic suicidal ideation is stable for outpatient treatment with close monitoring, esketamine might provide an effective treatment option for treating both depression and suicidality. Esketamine’s rapid effect may be an integral part of the treatment for a suicidal patient by bridging the gap caused by the delayed onset of action in typical antidepressants.2
3. Identify a local certified treatment center. Use the online database at www.spravato.com/find-a-center to locate a nearby certified esketamine treatment center. Choosing a center that you can collaborate with regularly is important to determine if the treatment is effective, to provide updates on the treatment course, and to consider tailoring of ongoing treatment.
4. Ensure the patient is also treated with an oral antidepressant. Esketamine should be administered in conjunction with an oral antidepressant. As such, patients must be willing and able to tolerate treatment with a medication that can be construed as an antidepressant while undergoing esketamine treatment. A long-term maintenance trial found that patients with TRD who experienced remission or response after esketamine treatment had a delayed relapse of symptoms when they continued esketamine in addition to an oral antidepressant.3
Considering its rapid onset of action and low adverse effects profile with manageable tolerability, esketamine adjunctive to an oral antidepressant is a reasonable option to consider for patients with TRD, including those with suicidality.
1. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24.
2. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
3. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatry. 2019;76(9):893-903.
1. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24.
2. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
3. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatry. 2019;76(9):893-903.
4 tips for working with caregivers of children with somatic disorders
Somatic symptom and related disorders—physical complaints that may or may not be medically explained that are associated with significant distress and impairment—are common in children and adolescents, and are often accompanied by anxiety and depression.1 Clinicians are likely to see children with these disorders in emergency departments, consultation services, or outpatient clinics. Common presenting symptoms include abdominal pain, headache, nausea, vomiting, dizziness, and seizures.1 Talking to the caregivers of these children can be challenging due to the subjective nature of the illness. In this article, I offer 4 tips for mental health practitioners to consider when working with caregivers of children with somatic disorders.
1. Support. Talk to the child and caregiver individually, and then together. Try to understand the caregiver’s concerns and express empathy to establish rapport. Being dismissive of their concerns is not going to help the child. Acknowledge the caregiver’s complaints and ask how seriously they feel other clinicians regard their concerns. Ask the caregiver about their perception of their child’s health, how frequently they worry about their child’s health, and the impact their worries have on their lives and their child’s life. Often the caregiver and child must miss out on obligations (eg, work, school, extracurricular activities) due to the child’s care and medical appointments.
2. Educate. This may be difficult, particularly when interacting with a caregiver who is convinced that their child is seriously physically sick. The caregiver may feel that involving psychiatry services is discrediting their concerns. Your initial interaction may be to allow the caregiver to express their frustrations toward the primary service. When talking with caregivers, avoid using medical jargon; in some instances, however, it may be necessary to use medical terminology to reassure the caregiver that you know what you are talking about. Be direct, and do not give false hope. These children often undergo extensive medical workup before psychiatry services are involved. To minimize conflicting messages from multiple clinicians who are caring for the same child, review the patient’s chart in advance, and maintain constant communication with other clinicians involved in the patient’s care.
3. Reassure. When the caregiver finally begins to acknowledge the psychological nature of their child’s illness, provide them with reassurance, but avoid emphasizing that the child is medically healthy because any relief caregivers gain from this can quickly fade and worsen their anxiety. Discuss the importance of treating underlying anxiety or depression with medication and psychotherapy where necessary. Assess the child for substance use disorders, personality disorders, and psychosocial stressors, and if present, target treatment accordingly. Discuss the potential long-term outcomes with and without treatment. Share examples of success stories from your past experiences. Emphasize the importance of noticing even slight improvements. Encourage the child to focus on goals such as attending school or passing online tests, etc.
4. Refer. Connecting the child with a therapist can significantly improve long-term outcomes, especially if coordinated well.2 This becomes more crucial in cases where caregivers are opposed to pharmacotherapy for their child. Whenever possible, communicate with the therapist before the child’s initial appointment to formulate a plan of action. The best approach is integrated care characterized by close collaboration of primary care, a somatic specialist, and mental health care professionals operating on a biopsychosocial model of distress and therapeutic factors.3
The ultimate goal is to help the child and caregiver achieve some level of relief by acknowledgment and support. Utilizing some of these tips can make our work even more meaningful for ourselves and our patients.
1. Malas N, Ortiz-Aguayo R, Giles L, et al. Pediatric somatic symptom disorders. Curr Psychiatry Rep. 2017;19(2):11. doi: 10.1007/s11920-017-0760-3
2. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93(1):49-54.
3. Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-31. doi: 10.31887/DCNS.2018.20.1/phenningsen
Somatic symptom and related disorders—physical complaints that may or may not be medically explained that are associated with significant distress and impairment—are common in children and adolescents, and are often accompanied by anxiety and depression.1 Clinicians are likely to see children with these disorders in emergency departments, consultation services, or outpatient clinics. Common presenting symptoms include abdominal pain, headache, nausea, vomiting, dizziness, and seizures.1 Talking to the caregivers of these children can be challenging due to the subjective nature of the illness. In this article, I offer 4 tips for mental health practitioners to consider when working with caregivers of children with somatic disorders.
1. Support. Talk to the child and caregiver individually, and then together. Try to understand the caregiver’s concerns and express empathy to establish rapport. Being dismissive of their concerns is not going to help the child. Acknowledge the caregiver’s complaints and ask how seriously they feel other clinicians regard their concerns. Ask the caregiver about their perception of their child’s health, how frequently they worry about their child’s health, and the impact their worries have on their lives and their child’s life. Often the caregiver and child must miss out on obligations (eg, work, school, extracurricular activities) due to the child’s care and medical appointments.
2. Educate. This may be difficult, particularly when interacting with a caregiver who is convinced that their child is seriously physically sick. The caregiver may feel that involving psychiatry services is discrediting their concerns. Your initial interaction may be to allow the caregiver to express their frustrations toward the primary service. When talking with caregivers, avoid using medical jargon; in some instances, however, it may be necessary to use medical terminology to reassure the caregiver that you know what you are talking about. Be direct, and do not give false hope. These children often undergo extensive medical workup before psychiatry services are involved. To minimize conflicting messages from multiple clinicians who are caring for the same child, review the patient’s chart in advance, and maintain constant communication with other clinicians involved in the patient’s care.
3. Reassure. When the caregiver finally begins to acknowledge the psychological nature of their child’s illness, provide them with reassurance, but avoid emphasizing that the child is medically healthy because any relief caregivers gain from this can quickly fade and worsen their anxiety. Discuss the importance of treating underlying anxiety or depression with medication and psychotherapy where necessary. Assess the child for substance use disorders, personality disorders, and psychosocial stressors, and if present, target treatment accordingly. Discuss the potential long-term outcomes with and without treatment. Share examples of success stories from your past experiences. Emphasize the importance of noticing even slight improvements. Encourage the child to focus on goals such as attending school or passing online tests, etc.
4. Refer. Connecting the child with a therapist can significantly improve long-term outcomes, especially if coordinated well.2 This becomes more crucial in cases where caregivers are opposed to pharmacotherapy for their child. Whenever possible, communicate with the therapist before the child’s initial appointment to formulate a plan of action. The best approach is integrated care characterized by close collaboration of primary care, a somatic specialist, and mental health care professionals operating on a biopsychosocial model of distress and therapeutic factors.3
The ultimate goal is to help the child and caregiver achieve some level of relief by acknowledgment and support. Utilizing some of these tips can make our work even more meaningful for ourselves and our patients.
Somatic symptom and related disorders—physical complaints that may or may not be medically explained that are associated with significant distress and impairment—are common in children and adolescents, and are often accompanied by anxiety and depression.1 Clinicians are likely to see children with these disorders in emergency departments, consultation services, or outpatient clinics. Common presenting symptoms include abdominal pain, headache, nausea, vomiting, dizziness, and seizures.1 Talking to the caregivers of these children can be challenging due to the subjective nature of the illness. In this article, I offer 4 tips for mental health practitioners to consider when working with caregivers of children with somatic disorders.
1. Support. Talk to the child and caregiver individually, and then together. Try to understand the caregiver’s concerns and express empathy to establish rapport. Being dismissive of their concerns is not going to help the child. Acknowledge the caregiver’s complaints and ask how seriously they feel other clinicians regard their concerns. Ask the caregiver about their perception of their child’s health, how frequently they worry about their child’s health, and the impact their worries have on their lives and their child’s life. Often the caregiver and child must miss out on obligations (eg, work, school, extracurricular activities) due to the child’s care and medical appointments.
2. Educate. This may be difficult, particularly when interacting with a caregiver who is convinced that their child is seriously physically sick. The caregiver may feel that involving psychiatry services is discrediting their concerns. Your initial interaction may be to allow the caregiver to express their frustrations toward the primary service. When talking with caregivers, avoid using medical jargon; in some instances, however, it may be necessary to use medical terminology to reassure the caregiver that you know what you are talking about. Be direct, and do not give false hope. These children often undergo extensive medical workup before psychiatry services are involved. To minimize conflicting messages from multiple clinicians who are caring for the same child, review the patient’s chart in advance, and maintain constant communication with other clinicians involved in the patient’s care.
3. Reassure. When the caregiver finally begins to acknowledge the psychological nature of their child’s illness, provide them with reassurance, but avoid emphasizing that the child is medically healthy because any relief caregivers gain from this can quickly fade and worsen their anxiety. Discuss the importance of treating underlying anxiety or depression with medication and psychotherapy where necessary. Assess the child for substance use disorders, personality disorders, and psychosocial stressors, and if present, target treatment accordingly. Discuss the potential long-term outcomes with and without treatment. Share examples of success stories from your past experiences. Emphasize the importance of noticing even slight improvements. Encourage the child to focus on goals such as attending school or passing online tests, etc.
4. Refer. Connecting the child with a therapist can significantly improve long-term outcomes, especially if coordinated well.2 This becomes more crucial in cases where caregivers are opposed to pharmacotherapy for their child. Whenever possible, communicate with the therapist before the child’s initial appointment to formulate a plan of action. The best approach is integrated care characterized by close collaboration of primary care, a somatic specialist, and mental health care professionals operating on a biopsychosocial model of distress and therapeutic factors.3
The ultimate goal is to help the child and caregiver achieve some level of relief by acknowledgment and support. Utilizing some of these tips can make our work even more meaningful for ourselves and our patients.
1. Malas N, Ortiz-Aguayo R, Giles L, et al. Pediatric somatic symptom disorders. Curr Psychiatry Rep. 2017;19(2):11. doi: 10.1007/s11920-017-0760-3
2. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93(1):49-54.
3. Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-31. doi: 10.31887/DCNS.2018.20.1/phenningsen
1. Malas N, Ortiz-Aguayo R, Giles L, et al. Pediatric somatic symptom disorders. Curr Psychiatry Rep. 2017;19(2):11. doi: 10.1007/s11920-017-0760-3
2. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93(1):49-54.
3. Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-31. doi: 10.31887/DCNS.2018.20.1/phenningsen
Storing patients’ credit card information: Keep it safe
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
Assessing perinatal anxiety: What to ask
Emerging data demonstrate that untreated perinatal anxiety is associated with negative outcomes, including an increased risk for suicide.1 A 2017 systematic review and meta-analysis that included 102 studies with a total of 221,974 women from 34 countries found that the prevalence of self-reported anxiety symptoms and any anxiety disorder was 22.9% and 15.2%, respectively, across the 3 trimesters.1 During pregnancy, anxiety disorders (eg, generalized anxiety disorder) and anxiety-related disorders (eg, obsessive-compulsive disorder [OCD] and posttraumatic stress disorder [PTSD]) can present as new illnesses or as a reoccurrence of an existing illness. Patients with pre-existing OCD may notice that the nature of their obsessions is changing. Women with pre-existing PTSD may have their symptoms triggered by pregnancy or delivery or may develop PTSD as a result of a traumatic delivery. Anxiety is frequently comorbid with depression, and high anxiety during pregnancy is one of the strongest risk factors for depression.1,2
In light of this data, awareness and recognition of perinatal anxiety is critical. In this article, we describe how to accurately assess perinatal anxiety by avoiding assumptions and asking key questions during the clinical interview.
Avoid these common assumptions
Assessment begins with avoiding assumptions typically associated with maternal mental health. One common assumption is that pregnancy is a joyous occasion for all women. Pregnancy can be a stressful time that has its own unique difficulties, including the potential to develop or have a relapse of a mental illness. Another assumption is that the only concern is “postpartum depression.” In actuality, a significant percentage of women will experience depression during their pregnancy (not just in the postpartum period), and many other psychiatric illnesses are common during the perinatal period, including anxiety disorders.
Conduct a focused interview
Risk factors associated with antenatal anxiety include2:
- previous history of mental illness (particularly a history of anxiety and depression and a history of psychiatric treatment)
- lack of partner or social support
- history of abuse or domestic violence
- unplanned or unwanted pregnancy
- adverse events in life and high perceived stress
- present/past pregnancy complications
- pregnancy loss.
Symptoms of anxiety. The presence of anxiety or worrying does not necessarily mean a mother has an anxiety disorder. Using the DSM-5 as a guide, we should use the questions outlined in the following sections to inquire about all of the symptoms related to a particular illness, the pervasiveness of these symptoms, and to what extent these symptoms impair a woman’s ability to function and carry out her usual activities.3
Past psychiatric history. Ask your patient the following: Have you previously experienced anxiety and/or depressive symptoms? Were those symptoms limited only to times when you were pregnant or postpartum? Were your symptoms severe enough to disrupt your life (job, school, relationships, ability to complete daily tasks)? What treatments were effective for your symptoms? What treatments were ineffective?3
Social factors. Learn more about your patient’s support systems by asking: Who do you consider to be part of your social support? How is your relationship with your social support? Are there challenges in your relationship with your friends, family, or partner? If yes, what are those challenges? Are there other children in the home, and do you have support for them? Is your home environment safe? Do you feel that you have what you need for the baby? What stressors are you currently experiencing? Do you attend support groups for expectant mothers? Are you engaged in perinatal care?3
Continue to: Given the high prevalence...
Given the high prevalence of interpersonal violence in women of reproductive age, all patients should be screened for this. The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women recommends screening for interpersonal violence at the first visit during the perinatal period, during each trimester, and at the postpartum visit (at minimum).4 Potential screening questions include (but are not limited to): Have you and/or your children ever been threatened by or felt afraid of your partner? When you argue with your partner, do either of you get physical? Has your partner ever physically hurt you (eg, hit, choked)? Do you feel safe at home? Do you have a safe place to go with resources you and your children will need in case of an emergency?4-6
Feelings toward pregnancy, past/current pregnancy complications, and pregnancy loss. Ask your patient: Was this pregnancy planned? How do you feel about your pregnancy? How do you see yourself as a mother? Do you currently have pregnancy complications and/or have had them in the past, and, if so, what are/were they? Have you lost a pregnancy? If so, what was that like? Do you have fears related to childbirth, and, if so, what are they?3
Intrusive thoughts about harming the baby. Intrusive thoughts are common in postpartum women with anxiety disorders, including OCD.7 Merely asking patients if they’ve had thoughts of harming their baby is incomplete and insufficient to assess for intrusive thoughts. This question does not distinguish between intrusive thoughts and homicidal ideation; this distinction is absolutely necessary given the difference in potential risk to the infant.
Intrusive thoughts are generally associated with a low risk of mothers acting on their thoughts. These thoughts are typically ego dystonic and, in the most severe form, can be distressing to an extent that they cause behavioral changes, such as avoiding bathing the infant, avoiding diaper changes, avoiding knives, or separating themselves from the infant.7 On the contrary, having homicidal ideation carries a higher risk for harm to the infant. Homicidal ideation may be seen in patients with co-occurring psychosis, poor reality testing, and delusions.5,7
Questions such as “Do you worry about harm coming to your baby?” “Do you worry about you causing harm to your baby?” and “Have you had an upsetting thought about harming your baby?” are more likely to reveal intrusive thoughts and prompt further exploration. Statements such as “Some people tell me that they have distressing thoughts about harm coming to their baby” can gently open the door to a having a dialogue about such thoughts. This dialogue is significantly important in making informed assessments as we develop comprehensive treatment plans.
1. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. B J Psychiatry. 2017;210(5):315-323.
2. Biaggi A, Conroy S, Pawlby S, et al. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62-77.
3. Kirby N, Kilsby A, Walker R. Assessing low mood during pregnancy. BMJ. 2019;366:I4584. doi: 10.1136/bmj.I4584
4. American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee opinion: Intimate partner violence. Number 518. February 2012. Accessed March 23, 2020. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2012/02/intimate-partner-violence
5. Massachusetts Child Psychiatry Access Program for Moms Provider Toolkit. Accessed March 18, 2020. https://www.mcpapformoms.org/Docs/AdultProviderToolkit12.09.2019.pdf
6. Ashur ML. Asking about domestic violence: SAFE questions. JAMA. 1993;269(18):2367.
7. Brandes M, Soares CN, Cohen LS. Postpartum onset obsessive-compulsive disorder: diagnosis and management. Arch Womens Ment Health. 2004;7(2):99-110.
Emerging data demonstrate that untreated perinatal anxiety is associated with negative outcomes, including an increased risk for suicide.1 A 2017 systematic review and meta-analysis that included 102 studies with a total of 221,974 women from 34 countries found that the prevalence of self-reported anxiety symptoms and any anxiety disorder was 22.9% and 15.2%, respectively, across the 3 trimesters.1 During pregnancy, anxiety disorders (eg, generalized anxiety disorder) and anxiety-related disorders (eg, obsessive-compulsive disorder [OCD] and posttraumatic stress disorder [PTSD]) can present as new illnesses or as a reoccurrence of an existing illness. Patients with pre-existing OCD may notice that the nature of their obsessions is changing. Women with pre-existing PTSD may have their symptoms triggered by pregnancy or delivery or may develop PTSD as a result of a traumatic delivery. Anxiety is frequently comorbid with depression, and high anxiety during pregnancy is one of the strongest risk factors for depression.1,2
In light of this data, awareness and recognition of perinatal anxiety is critical. In this article, we describe how to accurately assess perinatal anxiety by avoiding assumptions and asking key questions during the clinical interview.
Avoid these common assumptions
Assessment begins with avoiding assumptions typically associated with maternal mental health. One common assumption is that pregnancy is a joyous occasion for all women. Pregnancy can be a stressful time that has its own unique difficulties, including the potential to develop or have a relapse of a mental illness. Another assumption is that the only concern is “postpartum depression.” In actuality, a significant percentage of women will experience depression during their pregnancy (not just in the postpartum period), and many other psychiatric illnesses are common during the perinatal period, including anxiety disorders.
Conduct a focused interview
Risk factors associated with antenatal anxiety include2:
- previous history of mental illness (particularly a history of anxiety and depression and a history of psychiatric treatment)
- lack of partner or social support
- history of abuse or domestic violence
- unplanned or unwanted pregnancy
- adverse events in life and high perceived stress
- present/past pregnancy complications
- pregnancy loss.
Symptoms of anxiety. The presence of anxiety or worrying does not necessarily mean a mother has an anxiety disorder. Using the DSM-5 as a guide, we should use the questions outlined in the following sections to inquire about all of the symptoms related to a particular illness, the pervasiveness of these symptoms, and to what extent these symptoms impair a woman’s ability to function and carry out her usual activities.3
Past psychiatric history. Ask your patient the following: Have you previously experienced anxiety and/or depressive symptoms? Were those symptoms limited only to times when you were pregnant or postpartum? Were your symptoms severe enough to disrupt your life (job, school, relationships, ability to complete daily tasks)? What treatments were effective for your symptoms? What treatments were ineffective?3
Social factors. Learn more about your patient’s support systems by asking: Who do you consider to be part of your social support? How is your relationship with your social support? Are there challenges in your relationship with your friends, family, or partner? If yes, what are those challenges? Are there other children in the home, and do you have support for them? Is your home environment safe? Do you feel that you have what you need for the baby? What stressors are you currently experiencing? Do you attend support groups for expectant mothers? Are you engaged in perinatal care?3
Continue to: Given the high prevalence...
Given the high prevalence of interpersonal violence in women of reproductive age, all patients should be screened for this. The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women recommends screening for interpersonal violence at the first visit during the perinatal period, during each trimester, and at the postpartum visit (at minimum).4 Potential screening questions include (but are not limited to): Have you and/or your children ever been threatened by or felt afraid of your partner? When you argue with your partner, do either of you get physical? Has your partner ever physically hurt you (eg, hit, choked)? Do you feel safe at home? Do you have a safe place to go with resources you and your children will need in case of an emergency?4-6
Feelings toward pregnancy, past/current pregnancy complications, and pregnancy loss. Ask your patient: Was this pregnancy planned? How do you feel about your pregnancy? How do you see yourself as a mother? Do you currently have pregnancy complications and/or have had them in the past, and, if so, what are/were they? Have you lost a pregnancy? If so, what was that like? Do you have fears related to childbirth, and, if so, what are they?3
Intrusive thoughts about harming the baby. Intrusive thoughts are common in postpartum women with anxiety disorders, including OCD.7 Merely asking patients if they’ve had thoughts of harming their baby is incomplete and insufficient to assess for intrusive thoughts. This question does not distinguish between intrusive thoughts and homicidal ideation; this distinction is absolutely necessary given the difference in potential risk to the infant.
Intrusive thoughts are generally associated with a low risk of mothers acting on their thoughts. These thoughts are typically ego dystonic and, in the most severe form, can be distressing to an extent that they cause behavioral changes, such as avoiding bathing the infant, avoiding diaper changes, avoiding knives, or separating themselves from the infant.7 On the contrary, having homicidal ideation carries a higher risk for harm to the infant. Homicidal ideation may be seen in patients with co-occurring psychosis, poor reality testing, and delusions.5,7
Questions such as “Do you worry about harm coming to your baby?” “Do you worry about you causing harm to your baby?” and “Have you had an upsetting thought about harming your baby?” are more likely to reveal intrusive thoughts and prompt further exploration. Statements such as “Some people tell me that they have distressing thoughts about harm coming to their baby” can gently open the door to a having a dialogue about such thoughts. This dialogue is significantly important in making informed assessments as we develop comprehensive treatment plans.
Emerging data demonstrate that untreated perinatal anxiety is associated with negative outcomes, including an increased risk for suicide.1 A 2017 systematic review and meta-analysis that included 102 studies with a total of 221,974 women from 34 countries found that the prevalence of self-reported anxiety symptoms and any anxiety disorder was 22.9% and 15.2%, respectively, across the 3 trimesters.1 During pregnancy, anxiety disorders (eg, generalized anxiety disorder) and anxiety-related disorders (eg, obsessive-compulsive disorder [OCD] and posttraumatic stress disorder [PTSD]) can present as new illnesses or as a reoccurrence of an existing illness. Patients with pre-existing OCD may notice that the nature of their obsessions is changing. Women with pre-existing PTSD may have their symptoms triggered by pregnancy or delivery or may develop PTSD as a result of a traumatic delivery. Anxiety is frequently comorbid with depression, and high anxiety during pregnancy is one of the strongest risk factors for depression.1,2
In light of this data, awareness and recognition of perinatal anxiety is critical. In this article, we describe how to accurately assess perinatal anxiety by avoiding assumptions and asking key questions during the clinical interview.
Avoid these common assumptions
Assessment begins with avoiding assumptions typically associated with maternal mental health. One common assumption is that pregnancy is a joyous occasion for all women. Pregnancy can be a stressful time that has its own unique difficulties, including the potential to develop or have a relapse of a mental illness. Another assumption is that the only concern is “postpartum depression.” In actuality, a significant percentage of women will experience depression during their pregnancy (not just in the postpartum period), and many other psychiatric illnesses are common during the perinatal period, including anxiety disorders.
Conduct a focused interview
Risk factors associated with antenatal anxiety include2:
- previous history of mental illness (particularly a history of anxiety and depression and a history of psychiatric treatment)
- lack of partner or social support
- history of abuse or domestic violence
- unplanned or unwanted pregnancy
- adverse events in life and high perceived stress
- present/past pregnancy complications
- pregnancy loss.
Symptoms of anxiety. The presence of anxiety or worrying does not necessarily mean a mother has an anxiety disorder. Using the DSM-5 as a guide, we should use the questions outlined in the following sections to inquire about all of the symptoms related to a particular illness, the pervasiveness of these symptoms, and to what extent these symptoms impair a woman’s ability to function and carry out her usual activities.3
Past psychiatric history. Ask your patient the following: Have you previously experienced anxiety and/or depressive symptoms? Were those symptoms limited only to times when you were pregnant or postpartum? Were your symptoms severe enough to disrupt your life (job, school, relationships, ability to complete daily tasks)? What treatments were effective for your symptoms? What treatments were ineffective?3
Social factors. Learn more about your patient’s support systems by asking: Who do you consider to be part of your social support? How is your relationship with your social support? Are there challenges in your relationship with your friends, family, or partner? If yes, what are those challenges? Are there other children in the home, and do you have support for them? Is your home environment safe? Do you feel that you have what you need for the baby? What stressors are you currently experiencing? Do you attend support groups for expectant mothers? Are you engaged in perinatal care?3
Continue to: Given the high prevalence...
Given the high prevalence of interpersonal violence in women of reproductive age, all patients should be screened for this. The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women recommends screening for interpersonal violence at the first visit during the perinatal period, during each trimester, and at the postpartum visit (at minimum).4 Potential screening questions include (but are not limited to): Have you and/or your children ever been threatened by or felt afraid of your partner? When you argue with your partner, do either of you get physical? Has your partner ever physically hurt you (eg, hit, choked)? Do you feel safe at home? Do you have a safe place to go with resources you and your children will need in case of an emergency?4-6
Feelings toward pregnancy, past/current pregnancy complications, and pregnancy loss. Ask your patient: Was this pregnancy planned? How do you feel about your pregnancy? How do you see yourself as a mother? Do you currently have pregnancy complications and/or have had them in the past, and, if so, what are/were they? Have you lost a pregnancy? If so, what was that like? Do you have fears related to childbirth, and, if so, what are they?3
Intrusive thoughts about harming the baby. Intrusive thoughts are common in postpartum women with anxiety disorders, including OCD.7 Merely asking patients if they’ve had thoughts of harming their baby is incomplete and insufficient to assess for intrusive thoughts. This question does not distinguish between intrusive thoughts and homicidal ideation; this distinction is absolutely necessary given the difference in potential risk to the infant.
Intrusive thoughts are generally associated with a low risk of mothers acting on their thoughts. These thoughts are typically ego dystonic and, in the most severe form, can be distressing to an extent that they cause behavioral changes, such as avoiding bathing the infant, avoiding diaper changes, avoiding knives, or separating themselves from the infant.7 On the contrary, having homicidal ideation carries a higher risk for harm to the infant. Homicidal ideation may be seen in patients with co-occurring psychosis, poor reality testing, and delusions.5,7
Questions such as “Do you worry about harm coming to your baby?” “Do you worry about you causing harm to your baby?” and “Have you had an upsetting thought about harming your baby?” are more likely to reveal intrusive thoughts and prompt further exploration. Statements such as “Some people tell me that they have distressing thoughts about harm coming to their baby” can gently open the door to a having a dialogue about such thoughts. This dialogue is significantly important in making informed assessments as we develop comprehensive treatment plans.
1. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. B J Psychiatry. 2017;210(5):315-323.
2. Biaggi A, Conroy S, Pawlby S, et al. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62-77.
3. Kirby N, Kilsby A, Walker R. Assessing low mood during pregnancy. BMJ. 2019;366:I4584. doi: 10.1136/bmj.I4584
4. American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee opinion: Intimate partner violence. Number 518. February 2012. Accessed March 23, 2020. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2012/02/intimate-partner-violence
5. Massachusetts Child Psychiatry Access Program for Moms Provider Toolkit. Accessed March 18, 2020. https://www.mcpapformoms.org/Docs/AdultProviderToolkit12.09.2019.pdf
6. Ashur ML. Asking about domestic violence: SAFE questions. JAMA. 1993;269(18):2367.
7. Brandes M, Soares CN, Cohen LS. Postpartum onset obsessive-compulsive disorder: diagnosis and management. Arch Womens Ment Health. 2004;7(2):99-110.
1. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. B J Psychiatry. 2017;210(5):315-323.
2. Biaggi A, Conroy S, Pawlby S, et al. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62-77.
3. Kirby N, Kilsby A, Walker R. Assessing low mood during pregnancy. BMJ. 2019;366:I4584. doi: 10.1136/bmj.I4584
4. American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee opinion: Intimate partner violence. Number 518. February 2012. Accessed March 23, 2020. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2012/02/intimate-partner-violence
5. Massachusetts Child Psychiatry Access Program for Moms Provider Toolkit. Accessed March 18, 2020. https://www.mcpapformoms.org/Docs/AdultProviderToolkit12.09.2019.pdf
6. Ashur ML. Asking about domestic violence: SAFE questions. JAMA. 1993;269(18):2367.
7. Brandes M, Soares CN, Cohen LS. Postpartum onset obsessive-compulsive disorder: diagnosis and management. Arch Womens Ment Health. 2004;7(2):99-110.
ARISE to supportive psychotherapy
Supportive psychotherapy is a common type of therapy that often is used in combination with other modalities. By focusing on improving symptoms and accepting the patient’s limitations, it is particularly helpful for individuals who might have difficulty engaging in insight-oriented psychotherapies, such as those struggling with external stressors, including exposure to trauma, bereavement, physical disabilities, or socioeconomic challenges. Personal limitations, including severe personality disorder or intellectual disabilities, might also limit a patient’s ability to self-reflect on subconscious issues, which can lead to choosing a supportive modality.
While being supportive in the vernacular sense can be helpful, formal supportive psychotherapy employs well-defined goals and techniques.1 A therapist can facilitate progress by explicitly referring to these goals and techniques. The acronym ARISE can help therapists and other clinicians to use and appraise therapeutic progress toward these goals.
Alliance-building. The therapeutic alliance is an important predictor of the success of psychotherapy.2 Warmly encourage positive transference toward the therapist. The patient’s appreciation of the therapist’s empathic interactions can further the alliance. Paraphrasing the patient’s words can demonstrate and enhance empathy. Doing so allows clarification of the patient’s thoughts and helps the patient feel understood. Formulate and partner around shared therapeutic goals. Monitor the strength of the alliance and intervene if it is threatened. For example, if you misunderstand your patient and inadvertently offend them, apologizing may be helpful. In the face of disagreement between the patient and therapist, reorienting back to shared goals reinforces common ground.
Reduce anxiety and negative affect. In contrast to the caricature of the stiff psychoanalyst, the supportive therapist adopts an engaged conversational style to help the patient feel relaxed and to diminish the power differential between therapist and patient. If the patient appears uncomfortable with silence, maintaining the flow of conversation may reduce discomfort.1 Minimize your patient’s discomfort by approaching uncomfortable topics in manageable portions. Seek permission before introducing a subject that induces anxiety. Explain the reasoning behind approaching such topics.3 Reassurance and encouragement can further reduce anxiety.4 When not incongruous to the discussion, appropriate use of warm affect (eg, a smile) or even humor can elicit positive affect.
Increase awareness. Use psychoeducation and psychological interpretation (whether cognitive-behavioral or psychodynamic) to expand your patient’s awareness and help them understand their social contacts’ point of view. Clarification, gentle confrontation, and interpretation can make patients aware of biopsychosocial precipitants of distress.4
Strengthen coping mechanisms. Reinforce adaptive defense mechanisms, such as mature humor or suppression. Educating patients on practical organizational skills, problem-solving, relaxation techniques, and other relevant skills, can help them cope more effectively. Give advice only in limited circumstances, and when doing so, back up your advice with a rationale derived from your professional expertise. Because it is important for patients to realize that their life choices are their own, usually it is best to help the patient understand how they might come to their own decisions rather than to prescribe life choices in the form of advice.
Enhance self-esteem. Many patients in distress suffer from low self-esteem.5,6 Active encouragement and honest praise can nurture your patient’s ability to correct a distorted self-image and challenge self-reproach. Praise should not be false but reality-based. Praise can address preexisting strengths, highlight the patient’s willingness to express challenging material, or provide reinforcement on progress made toward treatment goals.
1. Rothe, EM. Supportive psychotherapy in everyday clinical practice: it’s like riding a bicycle. Psychiatric Times. Published May 24, 2017. Accessed April 12, 2021. https://www.psychiatrictimes.com/view/supportive-psychotherapy-everyday-clinical-practice-its-riding-bicycle
2. Flückiger C, Del Re AC, Wampold BE, et al. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic). 2018;55(4):316-340.
3. Pine F. The interpretive moment. Variations on classical themes. Bull Menninger Clin. 1984;48(1), 54-71.
4. Grover S, Avasthi A, Jagiwala M. Clinical practice guidelines for practice of supportive psychotherapy. Indian J Psychiatry. 2020;62(Suppl 2):S173-S182.
5. Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14(3):297-314.
6. Zahn R, Lythe KE, Gethin JA, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. 2015;186:337-341.
Supportive psychotherapy is a common type of therapy that often is used in combination with other modalities. By focusing on improving symptoms and accepting the patient’s limitations, it is particularly helpful for individuals who might have difficulty engaging in insight-oriented psychotherapies, such as those struggling with external stressors, including exposure to trauma, bereavement, physical disabilities, or socioeconomic challenges. Personal limitations, including severe personality disorder or intellectual disabilities, might also limit a patient’s ability to self-reflect on subconscious issues, which can lead to choosing a supportive modality.
While being supportive in the vernacular sense can be helpful, formal supportive psychotherapy employs well-defined goals and techniques.1 A therapist can facilitate progress by explicitly referring to these goals and techniques. The acronym ARISE can help therapists and other clinicians to use and appraise therapeutic progress toward these goals.
Alliance-building. The therapeutic alliance is an important predictor of the success of psychotherapy.2 Warmly encourage positive transference toward the therapist. The patient’s appreciation of the therapist’s empathic interactions can further the alliance. Paraphrasing the patient’s words can demonstrate and enhance empathy. Doing so allows clarification of the patient’s thoughts and helps the patient feel understood. Formulate and partner around shared therapeutic goals. Monitor the strength of the alliance and intervene if it is threatened. For example, if you misunderstand your patient and inadvertently offend them, apologizing may be helpful. In the face of disagreement between the patient and therapist, reorienting back to shared goals reinforces common ground.
Reduce anxiety and negative affect. In contrast to the caricature of the stiff psychoanalyst, the supportive therapist adopts an engaged conversational style to help the patient feel relaxed and to diminish the power differential between therapist and patient. If the patient appears uncomfortable with silence, maintaining the flow of conversation may reduce discomfort.1 Minimize your patient’s discomfort by approaching uncomfortable topics in manageable portions. Seek permission before introducing a subject that induces anxiety. Explain the reasoning behind approaching such topics.3 Reassurance and encouragement can further reduce anxiety.4 When not incongruous to the discussion, appropriate use of warm affect (eg, a smile) or even humor can elicit positive affect.
Increase awareness. Use psychoeducation and psychological interpretation (whether cognitive-behavioral or psychodynamic) to expand your patient’s awareness and help them understand their social contacts’ point of view. Clarification, gentle confrontation, and interpretation can make patients aware of biopsychosocial precipitants of distress.4
Strengthen coping mechanisms. Reinforce adaptive defense mechanisms, such as mature humor or suppression. Educating patients on practical organizational skills, problem-solving, relaxation techniques, and other relevant skills, can help them cope more effectively. Give advice only in limited circumstances, and when doing so, back up your advice with a rationale derived from your professional expertise. Because it is important for patients to realize that their life choices are their own, usually it is best to help the patient understand how they might come to their own decisions rather than to prescribe life choices in the form of advice.
Enhance self-esteem. Many patients in distress suffer from low self-esteem.5,6 Active encouragement and honest praise can nurture your patient’s ability to correct a distorted self-image and challenge self-reproach. Praise should not be false but reality-based. Praise can address preexisting strengths, highlight the patient’s willingness to express challenging material, or provide reinforcement on progress made toward treatment goals.
Supportive psychotherapy is a common type of therapy that often is used in combination with other modalities. By focusing on improving symptoms and accepting the patient’s limitations, it is particularly helpful for individuals who might have difficulty engaging in insight-oriented psychotherapies, such as those struggling with external stressors, including exposure to trauma, bereavement, physical disabilities, or socioeconomic challenges. Personal limitations, including severe personality disorder or intellectual disabilities, might also limit a patient’s ability to self-reflect on subconscious issues, which can lead to choosing a supportive modality.
While being supportive in the vernacular sense can be helpful, formal supportive psychotherapy employs well-defined goals and techniques.1 A therapist can facilitate progress by explicitly referring to these goals and techniques. The acronym ARISE can help therapists and other clinicians to use and appraise therapeutic progress toward these goals.
Alliance-building. The therapeutic alliance is an important predictor of the success of psychotherapy.2 Warmly encourage positive transference toward the therapist. The patient’s appreciation of the therapist’s empathic interactions can further the alliance. Paraphrasing the patient’s words can demonstrate and enhance empathy. Doing so allows clarification of the patient’s thoughts and helps the patient feel understood. Formulate and partner around shared therapeutic goals. Monitor the strength of the alliance and intervene if it is threatened. For example, if you misunderstand your patient and inadvertently offend them, apologizing may be helpful. In the face of disagreement between the patient and therapist, reorienting back to shared goals reinforces common ground.
Reduce anxiety and negative affect. In contrast to the caricature of the stiff psychoanalyst, the supportive therapist adopts an engaged conversational style to help the patient feel relaxed and to diminish the power differential between therapist and patient. If the patient appears uncomfortable with silence, maintaining the flow of conversation may reduce discomfort.1 Minimize your patient’s discomfort by approaching uncomfortable topics in manageable portions. Seek permission before introducing a subject that induces anxiety. Explain the reasoning behind approaching such topics.3 Reassurance and encouragement can further reduce anxiety.4 When not incongruous to the discussion, appropriate use of warm affect (eg, a smile) or even humor can elicit positive affect.
Increase awareness. Use psychoeducation and psychological interpretation (whether cognitive-behavioral or psychodynamic) to expand your patient’s awareness and help them understand their social contacts’ point of view. Clarification, gentle confrontation, and interpretation can make patients aware of biopsychosocial precipitants of distress.4
Strengthen coping mechanisms. Reinforce adaptive defense mechanisms, such as mature humor or suppression. Educating patients on practical organizational skills, problem-solving, relaxation techniques, and other relevant skills, can help them cope more effectively. Give advice only in limited circumstances, and when doing so, back up your advice with a rationale derived from your professional expertise. Because it is important for patients to realize that their life choices are their own, usually it is best to help the patient understand how they might come to their own decisions rather than to prescribe life choices in the form of advice.
Enhance self-esteem. Many patients in distress suffer from low self-esteem.5,6 Active encouragement and honest praise can nurture your patient’s ability to correct a distorted self-image and challenge self-reproach. Praise should not be false but reality-based. Praise can address preexisting strengths, highlight the patient’s willingness to express challenging material, or provide reinforcement on progress made toward treatment goals.
1. Rothe, EM. Supportive psychotherapy in everyday clinical practice: it’s like riding a bicycle. Psychiatric Times. Published May 24, 2017. Accessed April 12, 2021. https://www.psychiatrictimes.com/view/supportive-psychotherapy-everyday-clinical-practice-its-riding-bicycle
2. Flückiger C, Del Re AC, Wampold BE, et al. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic). 2018;55(4):316-340.
3. Pine F. The interpretive moment. Variations on classical themes. Bull Menninger Clin. 1984;48(1), 54-71.
4. Grover S, Avasthi A, Jagiwala M. Clinical practice guidelines for practice of supportive psychotherapy. Indian J Psychiatry. 2020;62(Suppl 2):S173-S182.
5. Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14(3):297-314.
6. Zahn R, Lythe KE, Gethin JA, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. 2015;186:337-341.
1. Rothe, EM. Supportive psychotherapy in everyday clinical practice: it’s like riding a bicycle. Psychiatric Times. Published May 24, 2017. Accessed April 12, 2021. https://www.psychiatrictimes.com/view/supportive-psychotherapy-everyday-clinical-practice-its-riding-bicycle
2. Flückiger C, Del Re AC, Wampold BE, et al. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic). 2018;55(4):316-340.
3. Pine F. The interpretive moment. Variations on classical themes. Bull Menninger Clin. 1984;48(1), 54-71.
4. Grover S, Avasthi A, Jagiwala M. Clinical practice guidelines for practice of supportive psychotherapy. Indian J Psychiatry. 2020;62(Suppl 2):S173-S182.
5. Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14(3):297-314.
6. Zahn R, Lythe KE, Gethin JA, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. 2015;186:337-341.
Switching antipsychotics: A guide to dose equivalents
Chlorpromazine (CPZ), a low-potency first-generation antipsychotic (FGA), was the first medication approved for the management of schizophrenia. Since its approval, some psychiatrists have prescribed subsequent antipsychotics based on CPZ’s efficacy and dosing. Comparing dosages of newer antipsychotics using a CPZ equivalent as a baseline remains a relevant method of determining which agent to prescribe, and at what dose.1,2
Psychiatrists frequently care for patients who are treatment-refractory or older adults with poor medication tolerance and age-related medical illness. Quick access to the comparative potency of different antipsychotics can help guide titration to the approximate equivalent dose of CPZ when initiating a medication, switching from 1 antipsychotic to another, or augmenting or combining antipsychotics. Fortunately, many authors, such as Woods2and Davis,3 have codified the dosing ratio equivalences of FGAs and second-generation antipsychotics (SGAs) using CPZ, 100 mg. To help psychiatrists use CPZ dosages as a point of comparison for prescribing other antipsychotics, the Table1,2,4 (page 14) lists dose equivalents for oral FGAs and SGAs based on CPZ, 100 mg. (For information on dose equivalents for injectable antipsychotics, see “Second-generation long-acting injectable antipsychotics: A practical guide,”
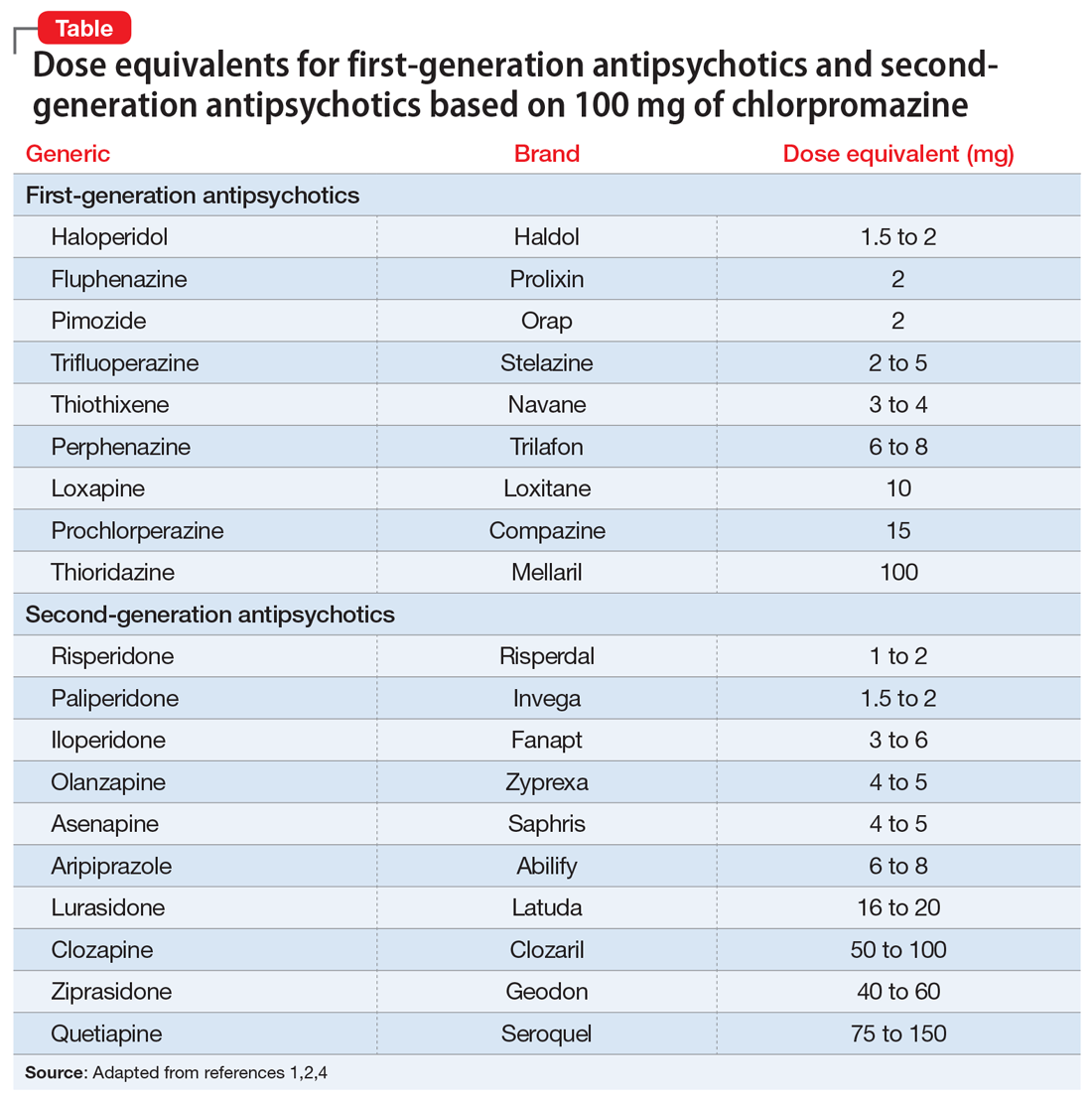
While this information cannot replace a psychiatrist’s clinical judgment, it can serve as a clinically useful prescribing tool. In addition to providing this Table, we discuss what you should consider when using these equivalents to switch antipsychotics and estimate the ultimate dose target for effective management of psychotic disorders.
A few caveats
Bioactive equivalent dosages should be targeted as a rough guide when switching from one FGA or SGA to another. Common indications for switching antipsychotics include an inadequate therapeutic response after a medication trial of an adequate dose and duration; relapse of psychosis despite medication adherence; intolerable adverse effects; cost; a new-onset, contraindicating medical illness; and lapses in medication compliance that necessitate a change to IM formulations.5 Keep in mind that medication changes should be tailored to the patient’s specific clinical characteristics.
Several other clinical and pharmacologic variabilities should be kept in mind when switching antipsychotics using CPZ dosage equivalents5,6:
- The therapeutic CPZ equivalent doses may be less precise for SGAs than for FGAs because the equivalents are largely based on dopaminergic blockade instead of cholinergic, serotonergic, or histaminergic systems
- For some antipsychotics, the relationship between dose and potency is nonlinear. For example, as the dosage of haloperidol increases, its relative antipsychotic potency decreases
- Differences in half-lives between 2 agents can add complexity to calculating the dosage equivalent
- Regardless of comparative dosing, before initiating a new antipsychotic, psychiatrists should read the dosing instructions in the FDA-approved package insert, and exercise caution before titrating a new medication to the maximum recommended dose.
1. Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: comparing apples and oranges! Indian J Psychiatry. 2013;55(2):207-208.
2. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
3. Davis JM. Dose equivalence of the anti-psychotic drugs. J Psych Res. 1974;11:65-69.
4. Psychiatric pharmacy essentials: antipsychotic dose equivalents. College of Psychiatric and Neurologic Pharmacists. Accessed February 2, 2021. https://cpnp.org/guideline/essentials/antipsychotic-dose-equivalents
5. Guidelines for antipsychotic medication switches. Humber NHS. Last Reviewed September 2012. Accessed February 2, 2021. https://www.psychdb.com/_media/meds/antipsychotics/nhs_guidelines_antipsychotic_switch.pdf
6. Bobo WV. Switching antipsychotics: why, when, and how? Psychiatric Times. Published March 14, 2013. Accessed February 2, 2021. https://www.psychiatrictimes.com/view/switching-antipsychotics-why-when-and-how
Chlorpromazine (CPZ), a low-potency first-generation antipsychotic (FGA), was the first medication approved for the management of schizophrenia. Since its approval, some psychiatrists have prescribed subsequent antipsychotics based on CPZ’s efficacy and dosing. Comparing dosages of newer antipsychotics using a CPZ equivalent as a baseline remains a relevant method of determining which agent to prescribe, and at what dose.1,2
Psychiatrists frequently care for patients who are treatment-refractory or older adults with poor medication tolerance and age-related medical illness. Quick access to the comparative potency of different antipsychotics can help guide titration to the approximate equivalent dose of CPZ when initiating a medication, switching from 1 antipsychotic to another, or augmenting or combining antipsychotics. Fortunately, many authors, such as Woods2and Davis,3 have codified the dosing ratio equivalences of FGAs and second-generation antipsychotics (SGAs) using CPZ, 100 mg. To help psychiatrists use CPZ dosages as a point of comparison for prescribing other antipsychotics, the Table1,2,4 (page 14) lists dose equivalents for oral FGAs and SGAs based on CPZ, 100 mg. (For information on dose equivalents for injectable antipsychotics, see “Second-generation long-acting injectable antipsychotics: A practical guide,”

While this information cannot replace a psychiatrist’s clinical judgment, it can serve as a clinically useful prescribing tool. In addition to providing this Table, we discuss what you should consider when using these equivalents to switch antipsychotics and estimate the ultimate dose target for effective management of psychotic disorders.
A few caveats
Bioactive equivalent dosages should be targeted as a rough guide when switching from one FGA or SGA to another. Common indications for switching antipsychotics include an inadequate therapeutic response after a medication trial of an adequate dose and duration; relapse of psychosis despite medication adherence; intolerable adverse effects; cost; a new-onset, contraindicating medical illness; and lapses in medication compliance that necessitate a change to IM formulations.5 Keep in mind that medication changes should be tailored to the patient’s specific clinical characteristics.
Several other clinical and pharmacologic variabilities should be kept in mind when switching antipsychotics using CPZ dosage equivalents5,6:
- The therapeutic CPZ equivalent doses may be less precise for SGAs than for FGAs because the equivalents are largely based on dopaminergic blockade instead of cholinergic, serotonergic, or histaminergic systems
- For some antipsychotics, the relationship between dose and potency is nonlinear. For example, as the dosage of haloperidol increases, its relative antipsychotic potency decreases
- Differences in half-lives between 2 agents can add complexity to calculating the dosage equivalent
- Regardless of comparative dosing, before initiating a new antipsychotic, psychiatrists should read the dosing instructions in the FDA-approved package insert, and exercise caution before titrating a new medication to the maximum recommended dose.
Chlorpromazine (CPZ), a low-potency first-generation antipsychotic (FGA), was the first medication approved for the management of schizophrenia. Since its approval, some psychiatrists have prescribed subsequent antipsychotics based on CPZ’s efficacy and dosing. Comparing dosages of newer antipsychotics using a CPZ equivalent as a baseline remains a relevant method of determining which agent to prescribe, and at what dose.1,2
Psychiatrists frequently care for patients who are treatment-refractory or older adults with poor medication tolerance and age-related medical illness. Quick access to the comparative potency of different antipsychotics can help guide titration to the approximate equivalent dose of CPZ when initiating a medication, switching from 1 antipsychotic to another, or augmenting or combining antipsychotics. Fortunately, many authors, such as Woods2and Davis,3 have codified the dosing ratio equivalences of FGAs and second-generation antipsychotics (SGAs) using CPZ, 100 mg. To help psychiatrists use CPZ dosages as a point of comparison for prescribing other antipsychotics, the Table1,2,4 (page 14) lists dose equivalents for oral FGAs and SGAs based on CPZ, 100 mg. (For information on dose equivalents for injectable antipsychotics, see “Second-generation long-acting injectable antipsychotics: A practical guide,”

While this information cannot replace a psychiatrist’s clinical judgment, it can serve as a clinically useful prescribing tool. In addition to providing this Table, we discuss what you should consider when using these equivalents to switch antipsychotics and estimate the ultimate dose target for effective management of psychotic disorders.
A few caveats
Bioactive equivalent dosages should be targeted as a rough guide when switching from one FGA or SGA to another. Common indications for switching antipsychotics include an inadequate therapeutic response after a medication trial of an adequate dose and duration; relapse of psychosis despite medication adherence; intolerable adverse effects; cost; a new-onset, contraindicating medical illness; and lapses in medication compliance that necessitate a change to IM formulations.5 Keep in mind that medication changes should be tailored to the patient’s specific clinical characteristics.
Several other clinical and pharmacologic variabilities should be kept in mind when switching antipsychotics using CPZ dosage equivalents5,6:
- The therapeutic CPZ equivalent doses may be less precise for SGAs than for FGAs because the equivalents are largely based on dopaminergic blockade instead of cholinergic, serotonergic, or histaminergic systems
- For some antipsychotics, the relationship between dose and potency is nonlinear. For example, as the dosage of haloperidol increases, its relative antipsychotic potency decreases
- Differences in half-lives between 2 agents can add complexity to calculating the dosage equivalent
- Regardless of comparative dosing, before initiating a new antipsychotic, psychiatrists should read the dosing instructions in the FDA-approved package insert, and exercise caution before titrating a new medication to the maximum recommended dose.
1. Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: comparing apples and oranges! Indian J Psychiatry. 2013;55(2):207-208.
2. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
3. Davis JM. Dose equivalence of the anti-psychotic drugs. J Psych Res. 1974;11:65-69.
4. Psychiatric pharmacy essentials: antipsychotic dose equivalents. College of Psychiatric and Neurologic Pharmacists. Accessed February 2, 2021. https://cpnp.org/guideline/essentials/antipsychotic-dose-equivalents
5. Guidelines for antipsychotic medication switches. Humber NHS. Last Reviewed September 2012. Accessed February 2, 2021. https://www.psychdb.com/_media/meds/antipsychotics/nhs_guidelines_antipsychotic_switch.pdf
6. Bobo WV. Switching antipsychotics: why, when, and how? Psychiatric Times. Published March 14, 2013. Accessed February 2, 2021. https://www.psychiatrictimes.com/view/switching-antipsychotics-why-when-and-how
1. Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: comparing apples and oranges! Indian J Psychiatry. 2013;55(2):207-208.
2. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
3. Davis JM. Dose equivalence of the anti-psychotic drugs. J Psych Res. 1974;11:65-69.
4. Psychiatric pharmacy essentials: antipsychotic dose equivalents. College of Psychiatric and Neurologic Pharmacists. Accessed February 2, 2021. https://cpnp.org/guideline/essentials/antipsychotic-dose-equivalents
5. Guidelines for antipsychotic medication switches. Humber NHS. Last Reviewed September 2012. Accessed February 2, 2021. https://www.psychdb.com/_media/meds/antipsychotics/nhs_guidelines_antipsychotic_switch.pdf
6. Bobo WV. Switching antipsychotics: why, when, and how? Psychiatric Times. Published March 14, 2013. Accessed February 2, 2021. https://www.psychiatrictimes.com/view/switching-antipsychotics-why-when-and-how