User login
Multicomponent Exercise Program Can Reverse Hospitalization-Associated Functional Decline in Elderly Patients
Study Overview
Objective. To assess the effects of an individualized, multicomponent exercise intervention on the functional status of very elderly patients who were acutely hospitalized compared with those who received usual care.
Design. A single-center, single-blind randomized clinical trial comparing elderly (≥ 75 years old) hospitalized patients who received in-hospital exercise (ie, individualized low-intensity resistance, balance, and walking exercises) versus control (ie, usual care that included physical rehabilitation if needed) interventions. The exercise intervention was adapted from the multicomponent physical exercise program Vivifrail and was supervised and conducted by a fitness specialist in 2 daily (1 morning and 1 evening) sessions lasting 20 minutes for 5 to 7 consecutive days. The morning session consisted of supervised and individualized progressive resistance, balance, and walking exercises. The evening session consisted of functional unsupervised exercises including light weights, extension and flexion of knee and hip, and walking.
Setting and participants. The study was conducted in an acute care unit in a tertiary public hospital in Navarra, Spain, between 1 February 2015 and 30 August 2017. A total of 370 elderly patients undergoing acute care hospitalization were enrolled in the study and randomly assigned to receive in-hospital exercise or control intervention. Inclusion criteria were: age ≥ 75 years, Barthel Index score ≥ 60, and ambulatory with or without assistance.
Main outcome measures. The primary outcome was change in functional capacity from baseline (beginning of exercise or control intervention) to hospital discharge as assessed by the Barthel Index of independence and the Short Physical Performance Battery (SPPB). Secondary outcomes were changes in cognitive capacity (Mini-Mental State Examination [MMSE]) and mood status (Yesavage Geriatric Depression Scale [GDS]), quality of life (QoL; EuroQol-5D), handgrip strength (dominant hand), incident delirium (Confusion Assessment Method), length of stay (LOS), falls during hospitalization, transfer after discharge, and readmission rate and mortality at 3 months after discharge. Intention-to-treat analysis was conducted.
Main results. Of the 370 patients included in the study’s analyses, 209 (56.5%) were women, mean age was 87.3 ± 4.9 years (range, 75-101 years; 130 [35.1%] nonagenarians). The median LOS was 8 days in both groups (interquartile range [IQR], 4 and 4 days, respectively). The median duration of the intervention was 5 days (IQR, 0 days), with 5 ± 1 morning and 4 ± 1 evening sessions in the exercise group. Adherence to the exercise intervention was high (95.8% for morning sessions; 83.4% for evening sessions), and no adverse effects were observed with the intervention.
The in-hospital exercise intervention program yielded significant benefits over usual care in functional outcomes in elderly patients. The exercise group had an increased change in measures of functional capacity compared to the usual care group (ie, Barthel Index, 6.9 points; 95% confidence interval [CI], 4.4-9.5; SPPB score, 2.2 points; 95% CI, 1.7-2.6). Furthermore, acute hospitalization led to an impairment in functional capacity from baseline to discharge in the Barthel Index (−5.0 points; 95% CI, −6.8 to −3.2) in the usual care group. In contrast, exercise intervention reversed this decline and improved functional outcomes as assessed by Barthel Index (1.9 points; 95% CI, 0.2-3.7) and SPPB score (2.4 points; 95% CI, 2.1-2.7).
The beneficial effects of the in-hospital exercise intervention extended to secondary end points indicative of cognitive capacity (MMSE, 1.8 points; 95% CI, 1.3-2.3), mood status (GDS, −2.0 points; 95% CI, −2.5 to −1.6), QoL (EuroQol-5D, 13.2 points; 95% CI, 8.2-18.2), and handgrip strength (2.3 kg; 95% CI, 1.8-2.8) compared to those who received usual care. In contrast, no differences were observed between groups that received exercise intervention and usual care in incident delirium, LOS, falls during hospitalization, transfer after discharge, and 3-month hospital readmission rate and mortality.
Conclusion. An individualized, multicomponent physical exercise program that includes low-intensity resistance, balance, and walking exercises performed during the course of hospitalization (average of 5 days) can reverse functional decline associated with acute hospitalization in very elderly patients. Furthermore, this in-hospital exercise intervention is safe and has a high adherence rate, and thus represents an opportunity to improve quality of care in this vulnerable population.
Commentary
Frail elderly patients are highly susceptible to adverse outcomes of acute hospitalization, including functional decline, disability, nursing home placement, rehospitalization, and mortality.1 Mobility limitation, a major hazard of hospitalization, has been associated with poorer functional recovery and increased vulnerability to these major adverse events after hospital discharge.2-4 Interdisciplinary care models delivered during hospitalization (eg, Geriatric Evaluation Unit, Acute Care for Elders) that emphasize functional independence and provide protocols for exercise and rehabilitation have demonstrated reduced hospital LOS, discharge to nursing home, and mortality, and improved functional status in elderly patients.5-7 Despite this evidence, significant gaps in knowledge exist in understanding whether early implementation of an individualized, multicomponent exercise training program can benefit the oldest old patients who are acutely hospitalized.
This study reported by Martinez-Velilla and colleagues provides an important and timely investigation in examining the effects of an individualized, multicomponent (ie, low-intensity resistance, balance, and walking) in-hospital exercise intervention on functional outcomes of hospitalized octogenarians and nonagenarians. The authors reported that such an intervention, administered 2 sessions per day for 5 to 7 consecutive days, can be safely implemented and reverse functional decline (ie, improvement in Barthel Index and SPPB score over course of hospital stay) typically associated with acute hospitalization in these vulnerable individuals. These findings are particularly significant given the paucity of randomized controlled trials evaluating the impact of exercise intervention in preserving functional capacity of geriatric patients in the setting of acute hospitalization. While much more research is needed to facilitate future development of a consensus opinion in this regard, results from this study provide the rationale that implementation of an individualized multicomponent exercise program is feasible and safe and may attenuate functional decline in hospitalized older patients. Finally, the beneficial effects of in-hospital exercise intervention may extend to cognitive capacity, mood status, and QoL—domains that are essential to optimizing patient-centered care in the frailest elderly patients.
The study was well conceived with a number of strengths, including its randomized clinical trial design. In addition, the trial patients were advanced in age (35.1% were nonagenarians), which is particularly important because this is a vulnerable population that is frequently excluded from participation in trials of exercise interventions and because the evidence-base for physical activity guidelines is suboptimal. Moreover, the authors demonstrated that an individualized multicomponent exercise program could be successfully implemented in elderly patients in an acute setting via daily exercise sessions. This test of feasibility is significant in that clinical trials in exercise intervention in geriatrics are commonly performed in nonacute settings in the community, long-term care facilities, or subacute care. The major limitation in this study centers on the generalizability of its findings. It was noted that some patients were not assessed for changes from baseline to discharge on the Barthel Index (6.1%) and SPPB (2.3%) because of their poor condition. The exclusion of the most debilitated patients limits the application of the study’s key findings to the frailest elderly patients, who are most likely to require acute hospital care.
Applications for Clinical Pract
Functional decline is an exceedingly common adverse outcome associated with hospitalization in older patients. While more evidence is needed, early implementation of an individualized, multicomponent exercise regimen during hospitalization may help to prevent functional decline in vulnerable elderly patients.
—Fred Ko, MD, MS
1. Goldwater DS, Dharmarajan K, McEwan BS, Krumholz HM. Is posthospital syndrome a result of hospitalization-induced allostatic overload? J Hosp Med. 2018;13(5).doi:10.12788/jhm.2986.
2. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219-223.
3. Minnick AF, Mion LC, Johnson ME, et al. Prevalence and variation of physical restraint use in acute care settings in the US. J Nurs Scholarsh. 2007;39:30-37.
4. Zisberg A, Shadmi E, Sinoff G et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266-273.
5. Rubenstein LZ, et al. Effectiveness of a geriatric evaluation unit. A randomized clinical trial. N Engl J Med. 1984;311:1664-1670.
6. Landefeld CS, Palmer RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338-1344.
7. de Morton NA, Keating JL, Jeffs K. Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007;CD005955.
Study Overview
Objective. To assess the effects of an individualized, multicomponent exercise intervention on the functional status of very elderly patients who were acutely hospitalized compared with those who received usual care.
Design. A single-center, single-blind randomized clinical trial comparing elderly (≥ 75 years old) hospitalized patients who received in-hospital exercise (ie, individualized low-intensity resistance, balance, and walking exercises) versus control (ie, usual care that included physical rehabilitation if needed) interventions. The exercise intervention was adapted from the multicomponent physical exercise program Vivifrail and was supervised and conducted by a fitness specialist in 2 daily (1 morning and 1 evening) sessions lasting 20 minutes for 5 to 7 consecutive days. The morning session consisted of supervised and individualized progressive resistance, balance, and walking exercises. The evening session consisted of functional unsupervised exercises including light weights, extension and flexion of knee and hip, and walking.
Setting and participants. The study was conducted in an acute care unit in a tertiary public hospital in Navarra, Spain, between 1 February 2015 and 30 August 2017. A total of 370 elderly patients undergoing acute care hospitalization were enrolled in the study and randomly assigned to receive in-hospital exercise or control intervention. Inclusion criteria were: age ≥ 75 years, Barthel Index score ≥ 60, and ambulatory with or without assistance.
Main outcome measures. The primary outcome was change in functional capacity from baseline (beginning of exercise or control intervention) to hospital discharge as assessed by the Barthel Index of independence and the Short Physical Performance Battery (SPPB). Secondary outcomes were changes in cognitive capacity (Mini-Mental State Examination [MMSE]) and mood status (Yesavage Geriatric Depression Scale [GDS]), quality of life (QoL; EuroQol-5D), handgrip strength (dominant hand), incident delirium (Confusion Assessment Method), length of stay (LOS), falls during hospitalization, transfer after discharge, and readmission rate and mortality at 3 months after discharge. Intention-to-treat analysis was conducted.
Main results. Of the 370 patients included in the study’s analyses, 209 (56.5%) were women, mean age was 87.3 ± 4.9 years (range, 75-101 years; 130 [35.1%] nonagenarians). The median LOS was 8 days in both groups (interquartile range [IQR], 4 and 4 days, respectively). The median duration of the intervention was 5 days (IQR, 0 days), with 5 ± 1 morning and 4 ± 1 evening sessions in the exercise group. Adherence to the exercise intervention was high (95.8% for morning sessions; 83.4% for evening sessions), and no adverse effects were observed with the intervention.
The in-hospital exercise intervention program yielded significant benefits over usual care in functional outcomes in elderly patients. The exercise group had an increased change in measures of functional capacity compared to the usual care group (ie, Barthel Index, 6.9 points; 95% confidence interval [CI], 4.4-9.5; SPPB score, 2.2 points; 95% CI, 1.7-2.6). Furthermore, acute hospitalization led to an impairment in functional capacity from baseline to discharge in the Barthel Index (−5.0 points; 95% CI, −6.8 to −3.2) in the usual care group. In contrast, exercise intervention reversed this decline and improved functional outcomes as assessed by Barthel Index (1.9 points; 95% CI, 0.2-3.7) and SPPB score (2.4 points; 95% CI, 2.1-2.7).
The beneficial effects of the in-hospital exercise intervention extended to secondary end points indicative of cognitive capacity (MMSE, 1.8 points; 95% CI, 1.3-2.3), mood status (GDS, −2.0 points; 95% CI, −2.5 to −1.6), QoL (EuroQol-5D, 13.2 points; 95% CI, 8.2-18.2), and handgrip strength (2.3 kg; 95% CI, 1.8-2.8) compared to those who received usual care. In contrast, no differences were observed between groups that received exercise intervention and usual care in incident delirium, LOS, falls during hospitalization, transfer after discharge, and 3-month hospital readmission rate and mortality.
Conclusion. An individualized, multicomponent physical exercise program that includes low-intensity resistance, balance, and walking exercises performed during the course of hospitalization (average of 5 days) can reverse functional decline associated with acute hospitalization in very elderly patients. Furthermore, this in-hospital exercise intervention is safe and has a high adherence rate, and thus represents an opportunity to improve quality of care in this vulnerable population.
Commentary
Frail elderly patients are highly susceptible to adverse outcomes of acute hospitalization, including functional decline, disability, nursing home placement, rehospitalization, and mortality.1 Mobility limitation, a major hazard of hospitalization, has been associated with poorer functional recovery and increased vulnerability to these major adverse events after hospital discharge.2-4 Interdisciplinary care models delivered during hospitalization (eg, Geriatric Evaluation Unit, Acute Care for Elders) that emphasize functional independence and provide protocols for exercise and rehabilitation have demonstrated reduced hospital LOS, discharge to nursing home, and mortality, and improved functional status in elderly patients.5-7 Despite this evidence, significant gaps in knowledge exist in understanding whether early implementation of an individualized, multicomponent exercise training program can benefit the oldest old patients who are acutely hospitalized.
This study reported by Martinez-Velilla and colleagues provides an important and timely investigation in examining the effects of an individualized, multicomponent (ie, low-intensity resistance, balance, and walking) in-hospital exercise intervention on functional outcomes of hospitalized octogenarians and nonagenarians. The authors reported that such an intervention, administered 2 sessions per day for 5 to 7 consecutive days, can be safely implemented and reverse functional decline (ie, improvement in Barthel Index and SPPB score over course of hospital stay) typically associated with acute hospitalization in these vulnerable individuals. These findings are particularly significant given the paucity of randomized controlled trials evaluating the impact of exercise intervention in preserving functional capacity of geriatric patients in the setting of acute hospitalization. While much more research is needed to facilitate future development of a consensus opinion in this regard, results from this study provide the rationale that implementation of an individualized multicomponent exercise program is feasible and safe and may attenuate functional decline in hospitalized older patients. Finally, the beneficial effects of in-hospital exercise intervention may extend to cognitive capacity, mood status, and QoL—domains that are essential to optimizing patient-centered care in the frailest elderly patients.
The study was well conceived with a number of strengths, including its randomized clinical trial design. In addition, the trial patients were advanced in age (35.1% were nonagenarians), which is particularly important because this is a vulnerable population that is frequently excluded from participation in trials of exercise interventions and because the evidence-base for physical activity guidelines is suboptimal. Moreover, the authors demonstrated that an individualized multicomponent exercise program could be successfully implemented in elderly patients in an acute setting via daily exercise sessions. This test of feasibility is significant in that clinical trials in exercise intervention in geriatrics are commonly performed in nonacute settings in the community, long-term care facilities, or subacute care. The major limitation in this study centers on the generalizability of its findings. It was noted that some patients were not assessed for changes from baseline to discharge on the Barthel Index (6.1%) and SPPB (2.3%) because of their poor condition. The exclusion of the most debilitated patients limits the application of the study’s key findings to the frailest elderly patients, who are most likely to require acute hospital care.
Applications for Clinical Pract
Functional decline is an exceedingly common adverse outcome associated with hospitalization in older patients. While more evidence is needed, early implementation of an individualized, multicomponent exercise regimen during hospitalization may help to prevent functional decline in vulnerable elderly patients.
—Fred Ko, MD, MS
Study Overview
Objective. To assess the effects of an individualized, multicomponent exercise intervention on the functional status of very elderly patients who were acutely hospitalized compared with those who received usual care.
Design. A single-center, single-blind randomized clinical trial comparing elderly (≥ 75 years old) hospitalized patients who received in-hospital exercise (ie, individualized low-intensity resistance, balance, and walking exercises) versus control (ie, usual care that included physical rehabilitation if needed) interventions. The exercise intervention was adapted from the multicomponent physical exercise program Vivifrail and was supervised and conducted by a fitness specialist in 2 daily (1 morning and 1 evening) sessions lasting 20 minutes for 5 to 7 consecutive days. The morning session consisted of supervised and individualized progressive resistance, balance, and walking exercises. The evening session consisted of functional unsupervised exercises including light weights, extension and flexion of knee and hip, and walking.
Setting and participants. The study was conducted in an acute care unit in a tertiary public hospital in Navarra, Spain, between 1 February 2015 and 30 August 2017. A total of 370 elderly patients undergoing acute care hospitalization were enrolled in the study and randomly assigned to receive in-hospital exercise or control intervention. Inclusion criteria were: age ≥ 75 years, Barthel Index score ≥ 60, and ambulatory with or without assistance.
Main outcome measures. The primary outcome was change in functional capacity from baseline (beginning of exercise or control intervention) to hospital discharge as assessed by the Barthel Index of independence and the Short Physical Performance Battery (SPPB). Secondary outcomes were changes in cognitive capacity (Mini-Mental State Examination [MMSE]) and mood status (Yesavage Geriatric Depression Scale [GDS]), quality of life (QoL; EuroQol-5D), handgrip strength (dominant hand), incident delirium (Confusion Assessment Method), length of stay (LOS), falls during hospitalization, transfer after discharge, and readmission rate and mortality at 3 months after discharge. Intention-to-treat analysis was conducted.
Main results. Of the 370 patients included in the study’s analyses, 209 (56.5%) were women, mean age was 87.3 ± 4.9 years (range, 75-101 years; 130 [35.1%] nonagenarians). The median LOS was 8 days in both groups (interquartile range [IQR], 4 and 4 days, respectively). The median duration of the intervention was 5 days (IQR, 0 days), with 5 ± 1 morning and 4 ± 1 evening sessions in the exercise group. Adherence to the exercise intervention was high (95.8% for morning sessions; 83.4% for evening sessions), and no adverse effects were observed with the intervention.
The in-hospital exercise intervention program yielded significant benefits over usual care in functional outcomes in elderly patients. The exercise group had an increased change in measures of functional capacity compared to the usual care group (ie, Barthel Index, 6.9 points; 95% confidence interval [CI], 4.4-9.5; SPPB score, 2.2 points; 95% CI, 1.7-2.6). Furthermore, acute hospitalization led to an impairment in functional capacity from baseline to discharge in the Barthel Index (−5.0 points; 95% CI, −6.8 to −3.2) in the usual care group. In contrast, exercise intervention reversed this decline and improved functional outcomes as assessed by Barthel Index (1.9 points; 95% CI, 0.2-3.7) and SPPB score (2.4 points; 95% CI, 2.1-2.7).
The beneficial effects of the in-hospital exercise intervention extended to secondary end points indicative of cognitive capacity (MMSE, 1.8 points; 95% CI, 1.3-2.3), mood status (GDS, −2.0 points; 95% CI, −2.5 to −1.6), QoL (EuroQol-5D, 13.2 points; 95% CI, 8.2-18.2), and handgrip strength (2.3 kg; 95% CI, 1.8-2.8) compared to those who received usual care. In contrast, no differences were observed between groups that received exercise intervention and usual care in incident delirium, LOS, falls during hospitalization, transfer after discharge, and 3-month hospital readmission rate and mortality.
Conclusion. An individualized, multicomponent physical exercise program that includes low-intensity resistance, balance, and walking exercises performed during the course of hospitalization (average of 5 days) can reverse functional decline associated with acute hospitalization in very elderly patients. Furthermore, this in-hospital exercise intervention is safe and has a high adherence rate, and thus represents an opportunity to improve quality of care in this vulnerable population.
Commentary
Frail elderly patients are highly susceptible to adverse outcomes of acute hospitalization, including functional decline, disability, nursing home placement, rehospitalization, and mortality.1 Mobility limitation, a major hazard of hospitalization, has been associated with poorer functional recovery and increased vulnerability to these major adverse events after hospital discharge.2-4 Interdisciplinary care models delivered during hospitalization (eg, Geriatric Evaluation Unit, Acute Care for Elders) that emphasize functional independence and provide protocols for exercise and rehabilitation have demonstrated reduced hospital LOS, discharge to nursing home, and mortality, and improved functional status in elderly patients.5-7 Despite this evidence, significant gaps in knowledge exist in understanding whether early implementation of an individualized, multicomponent exercise training program can benefit the oldest old patients who are acutely hospitalized.
This study reported by Martinez-Velilla and colleagues provides an important and timely investigation in examining the effects of an individualized, multicomponent (ie, low-intensity resistance, balance, and walking) in-hospital exercise intervention on functional outcomes of hospitalized octogenarians and nonagenarians. The authors reported that such an intervention, administered 2 sessions per day for 5 to 7 consecutive days, can be safely implemented and reverse functional decline (ie, improvement in Barthel Index and SPPB score over course of hospital stay) typically associated with acute hospitalization in these vulnerable individuals. These findings are particularly significant given the paucity of randomized controlled trials evaluating the impact of exercise intervention in preserving functional capacity of geriatric patients in the setting of acute hospitalization. While much more research is needed to facilitate future development of a consensus opinion in this regard, results from this study provide the rationale that implementation of an individualized multicomponent exercise program is feasible and safe and may attenuate functional decline in hospitalized older patients. Finally, the beneficial effects of in-hospital exercise intervention may extend to cognitive capacity, mood status, and QoL—domains that are essential to optimizing patient-centered care in the frailest elderly patients.
The study was well conceived with a number of strengths, including its randomized clinical trial design. In addition, the trial patients were advanced in age (35.1% were nonagenarians), which is particularly important because this is a vulnerable population that is frequently excluded from participation in trials of exercise interventions and because the evidence-base for physical activity guidelines is suboptimal. Moreover, the authors demonstrated that an individualized multicomponent exercise program could be successfully implemented in elderly patients in an acute setting via daily exercise sessions. This test of feasibility is significant in that clinical trials in exercise intervention in geriatrics are commonly performed in nonacute settings in the community, long-term care facilities, or subacute care. The major limitation in this study centers on the generalizability of its findings. It was noted that some patients were not assessed for changes from baseline to discharge on the Barthel Index (6.1%) and SPPB (2.3%) because of their poor condition. The exclusion of the most debilitated patients limits the application of the study’s key findings to the frailest elderly patients, who are most likely to require acute hospital care.
Applications for Clinical Pract
Functional decline is an exceedingly common adverse outcome associated with hospitalization in older patients. While more evidence is needed, early implementation of an individualized, multicomponent exercise regimen during hospitalization may help to prevent functional decline in vulnerable elderly patients.
—Fred Ko, MD, MS
1. Goldwater DS, Dharmarajan K, McEwan BS, Krumholz HM. Is posthospital syndrome a result of hospitalization-induced allostatic overload? J Hosp Med. 2018;13(5).doi:10.12788/jhm.2986.
2. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219-223.
3. Minnick AF, Mion LC, Johnson ME, et al. Prevalence and variation of physical restraint use in acute care settings in the US. J Nurs Scholarsh. 2007;39:30-37.
4. Zisberg A, Shadmi E, Sinoff G et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266-273.
5. Rubenstein LZ, et al. Effectiveness of a geriatric evaluation unit. A randomized clinical trial. N Engl J Med. 1984;311:1664-1670.
6. Landefeld CS, Palmer RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338-1344.
7. de Morton NA, Keating JL, Jeffs K. Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007;CD005955.
1. Goldwater DS, Dharmarajan K, McEwan BS, Krumholz HM. Is posthospital syndrome a result of hospitalization-induced allostatic overload? J Hosp Med. 2018;13(5).doi:10.12788/jhm.2986.
2. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219-223.
3. Minnick AF, Mion LC, Johnson ME, et al. Prevalence and variation of physical restraint use in acute care settings in the US. J Nurs Scholarsh. 2007;39:30-37.
4. Zisberg A, Shadmi E, Sinoff G et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266-273.
5. Rubenstein LZ, et al. Effectiveness of a geriatric evaluation unit. A randomized clinical trial. N Engl J Med. 1984;311:1664-1670.
6. Landefeld CS, Palmer RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338-1344.
7. de Morton NA, Keating JL, Jeffs K. Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007;CD005955.
Androgen Deprivation Therapy Combined with Radiation in High-Risk Prostate Cancer . . . How Long Do We Go?
Study Overview
Objective. To compare the outcomes of 18 months versus 36 months of androgen deprivation therapy (ADT) combined with radiation in high-risk prostate cancer (HRPC).
Design. Phase 3 multicenter, randomized superiority trial.
Participants. This study enrolled patients aged ≤ 80 years with HRPC. All patients had no evidence of regional or distant metastasis. High-risk disease was defined as any of the following: clinical stage T3 or T4, prostate-specific antigen (PSA) level > 20 ng/mL, or Gleason score > 7.
Methods. Prior to randomization, all patients received 4 months of ADT with goserelin 10.8 mg and anti-androgen therapy with bicalutamide 50 mg daily for 30 days. Patients were then randomly assigned to 18 (short arm) or 36 (long arm) months of ADT in combination with radiation therapy (RT). The randomization was stratified by stage (T1-2 vs T3-4), Gleason score (< 7 vs > 7) and PSA level (< 20 ng/mL vs > 20 ng/mL). The standard radiation dose was 70 Gy to the prostate and 44 Gy to the pelvis. Computed tomography or magnetic resonance imaging exam of the abdomen and pelvis and a bone scan were performed to rule out regional or distant metastases. PSA level was monitored every 3 months for 18 months, every 6 months up to the third year, and yearly thereafter.
Main outcome measures. The 2 primary outcomes were overall survival (OS) and quality of life (QoL) at 5 years. The secondary end points were biochemical failure (BF)defined as PSA nadir plus 2, disease-free survival (DFS), and site(s) of tumor relapse.
Main results. The 5-year OS was 91% and 86% for the 36- and 18-month groups, respectively (P = 0.07). The 10-year OS was 62% for both groups (P = 0.7), and the global hazard ratio (HR) was 1.02 (P = 0.8). The disease-specific survival (DSS) was similar in both groups at 5 years (98% vs 97%) and at 10 years (91% vs 92%) in the long versus short arm, respectively. The rate of prostate cancer–specific death was 21% versus 23% in the long versus short arm, respectively. In a multivariate analysis for OS, only age and Gleason score > 7 were statistically significant survival predictors. BF rate at 10 years was 25% for 36 months as compared with 31% for 18 months (HR, 0.71, P = 0.02). The 10-year DFS rates were 45% and 39% for 36 and 18 months, respectively (HR, 0.68, P = 0.08). Forty patients in the long arm versus 43 in the short arm developed distant metastasis. Both groups developed similar sites of metastasis, which was predominantly osseous. Some aspects of the EORTC30 and PR25 scales were significant, mostly pertaining to sexual activity, fatigue, and hormone-related symptoms in favor of the 18-month group. The median time to testosterone recovery after completion of ADT was 2.1 years for the short arm versus 4 years in the long arm (P = 0.02). The compliance rate with ADT was 88% in the short arm versus 53% in the long arm. The main reason for nonadherence was side effects in 54% of the patients in the long arm and 31% in the short arm.
Conclusion. The results of the current study suggest that 18 months of ADT in combination with RT yields similar 10-year OS and improved QoL compared with 36 months in patients with HRPC.
Commentary
The role of ADT for HRPC in combination with RT has been well established by evidence from several trials; however, the comparator arms and patient characteristics between these studies have been quite heterogeneous. For instance, the Radiation Therapy Oncology Group (RTOG) 85-31 trial compared indefinite ADT with RT versus RT alone and showed significantly better 10-year OS in the ADT plus RT arm.1 Similarly, the European Organisation for Research and Treatment of Cancer (EROTC) 22961 trial showed an OS benefit for 36 months versus 6 months of ADT in combination with radiation.2 Additionally, the RTOG 92-02 trial, which compared 4 months versus 24 months of ADT with radiation, also found a significantly improved 10-year OS with a longer course of ADT.3 Taken together these data suggest that 4 to 6 months of ADT is inferior to 24 to 36 months of ADT in HRPC.
Several differences, however, exist in patient characteristics between the present trial and the earlier trials, justifiably reflecting the change of practice in the PSA era. For instance, the present study has a higher percentage of patients with Gleason scores 8-10 (60%) compared to the EROTC and RTOG studies (15%-35%) and a lower percentage of patients with T3 and T4 tumors. Patients with high Gleason scores are believed to have a higher risk of micro-metastasis at the time of diagnosis and higher chances of castration resistance. Therefore, inclusion of a (presumably) larger high-risk patient subgroup in the present study lends further credence to results indicating similar OS with a shorter course of ADT. A post hoc analysis including only patients with Gleason score 8-10 performed for OS, DSS, BF, and DFS showed no significant difference in any of these variables between the arms. Analysis of the interaction between ADT duration in the Gleason 8-10 subgroup versus Gleason 7 for OS, DFS, DSS and BF found no significant differences. This again suggests that 18 months of ADT may be sufficient for this high-risk group; however, it is difficult to draw definitive conclusions from this unplanned subgroup analysis.
Based on the results of the current study, it seems that 18 months of ADT is adequate for many, but not necessarily all, patients. For instance, there was a significantly higher incidence of BF in the 18-month arm. Applying this data to younger patients may require a more nuanced approach, as it is possible that with longer follow-up this higher rate of BF may translate into a difference in OS. Therefore, life expectancy and comorbid conditions always need to be incorporated into clinical decision making with regards to ADT duration. In a study by Rose et al, the risk of prostate cancer–specific mortality significantly decreased by using ADT plus RT for men with HRPC with a low, but not a high, competing mortality score.4 The clinical significance of this finding is that adding ADT to RT might significantly reduce the risk of death from prostate cancer only in the setting of low competing risks.
Another concept to ponder is the optimum duration of ADT in the era of RT dose escalation. Currently, there are emerging techniques for delivering higher radiation doses and combining brachytherapy with external beam radiotherapy for HRPC, and the role of whole pelvic radiation is being investigated. New data suggests that higher radiation doses can lead to improvement in outcomes for HRPC. The DART01/05 study compared 4 versus 24 months of ADT with 76 to 82 Gy of RT and reported improved 5-year OS, DFS, and metastasis-free survival with longer ADT duration.5 Moreover, Kishan et al reported improved prostate cancer–specific mortality when brachytherapy boost was added to radiation compared to radiation alone in patients with Gleason scores 9 and 10.6 Therefore, the optimal duration of ADT in the setting of dose-escalated radiotherapy is not yet known. Also, it is important to note that unlike the prior RTOG and EORTC studies, this study did not include patients with evidence of regional nodal disease, and thus the present data should not be applied to this patient population.
Applications to Clinical Practice
This study’s results suggesting that 18 months of ADT in combination with RT yields similar 10-year OS and improved QoL compared with 36 months of ADT in patients with HRPC should be interpreted with caution when treating very young patients, since the higher rate of BF in the short arm may impact the OS with longer follow-up. Additionally, patients’ QoL and tolerance to ADT-related adverse effects should be taken into consideration given that compliance with 36 months of ADT was only 53% in this study.
—Jailan Elayoubi, MD, Michigan State University, East Lansing, MI
1. Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys. 2005;61:1285-1290.
2. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516-2527.
3. Horwitz EM, Bae K, Hanks GE, Porter A, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504.
4. Rose BS, Chen MH, Wu J, et al. Androgen deprivation therapy use in the setting of high-dose radiation therapy and the risk of prostate cancer-specific mortality stratified by the extent of competing mortality. Int J Radiat Oncol Biol Phys. 2016;96:778-784.
5. Zapatero A, Guerrero A, Maldonado X, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:320-327.
6. Kishan, AU, Cook, RR, Ciezki, JP, et al. Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with gleason score 9-10 prostate cancer. JAMA. 2018;319:896-905.
Study Overview
Objective. To compare the outcomes of 18 months versus 36 months of androgen deprivation therapy (ADT) combined with radiation in high-risk prostate cancer (HRPC).
Design. Phase 3 multicenter, randomized superiority trial.
Participants. This study enrolled patients aged ≤ 80 years with HRPC. All patients had no evidence of regional or distant metastasis. High-risk disease was defined as any of the following: clinical stage T3 or T4, prostate-specific antigen (PSA) level > 20 ng/mL, or Gleason score > 7.
Methods. Prior to randomization, all patients received 4 months of ADT with goserelin 10.8 mg and anti-androgen therapy with bicalutamide 50 mg daily for 30 days. Patients were then randomly assigned to 18 (short arm) or 36 (long arm) months of ADT in combination with radiation therapy (RT). The randomization was stratified by stage (T1-2 vs T3-4), Gleason score (< 7 vs > 7) and PSA level (< 20 ng/mL vs > 20 ng/mL). The standard radiation dose was 70 Gy to the prostate and 44 Gy to the pelvis. Computed tomography or magnetic resonance imaging exam of the abdomen and pelvis and a bone scan were performed to rule out regional or distant metastases. PSA level was monitored every 3 months for 18 months, every 6 months up to the third year, and yearly thereafter.
Main outcome measures. The 2 primary outcomes were overall survival (OS) and quality of life (QoL) at 5 years. The secondary end points were biochemical failure (BF)defined as PSA nadir plus 2, disease-free survival (DFS), and site(s) of tumor relapse.
Main results. The 5-year OS was 91% and 86% for the 36- and 18-month groups, respectively (P = 0.07). The 10-year OS was 62% for both groups (P = 0.7), and the global hazard ratio (HR) was 1.02 (P = 0.8). The disease-specific survival (DSS) was similar in both groups at 5 years (98% vs 97%) and at 10 years (91% vs 92%) in the long versus short arm, respectively. The rate of prostate cancer–specific death was 21% versus 23% in the long versus short arm, respectively. In a multivariate analysis for OS, only age and Gleason score > 7 were statistically significant survival predictors. BF rate at 10 years was 25% for 36 months as compared with 31% for 18 months (HR, 0.71, P = 0.02). The 10-year DFS rates were 45% and 39% for 36 and 18 months, respectively (HR, 0.68, P = 0.08). Forty patients in the long arm versus 43 in the short arm developed distant metastasis. Both groups developed similar sites of metastasis, which was predominantly osseous. Some aspects of the EORTC30 and PR25 scales were significant, mostly pertaining to sexual activity, fatigue, and hormone-related symptoms in favor of the 18-month group. The median time to testosterone recovery after completion of ADT was 2.1 years for the short arm versus 4 years in the long arm (P = 0.02). The compliance rate with ADT was 88% in the short arm versus 53% in the long arm. The main reason for nonadherence was side effects in 54% of the patients in the long arm and 31% in the short arm.
Conclusion. The results of the current study suggest that 18 months of ADT in combination with RT yields similar 10-year OS and improved QoL compared with 36 months in patients with HRPC.
Commentary
The role of ADT for HRPC in combination with RT has been well established by evidence from several trials; however, the comparator arms and patient characteristics between these studies have been quite heterogeneous. For instance, the Radiation Therapy Oncology Group (RTOG) 85-31 trial compared indefinite ADT with RT versus RT alone and showed significantly better 10-year OS in the ADT plus RT arm.1 Similarly, the European Organisation for Research and Treatment of Cancer (EROTC) 22961 trial showed an OS benefit for 36 months versus 6 months of ADT in combination with radiation.2 Additionally, the RTOG 92-02 trial, which compared 4 months versus 24 months of ADT with radiation, also found a significantly improved 10-year OS with a longer course of ADT.3 Taken together these data suggest that 4 to 6 months of ADT is inferior to 24 to 36 months of ADT in HRPC.
Several differences, however, exist in patient characteristics between the present trial and the earlier trials, justifiably reflecting the change of practice in the PSA era. For instance, the present study has a higher percentage of patients with Gleason scores 8-10 (60%) compared to the EROTC and RTOG studies (15%-35%) and a lower percentage of patients with T3 and T4 tumors. Patients with high Gleason scores are believed to have a higher risk of micro-metastasis at the time of diagnosis and higher chances of castration resistance. Therefore, inclusion of a (presumably) larger high-risk patient subgroup in the present study lends further credence to results indicating similar OS with a shorter course of ADT. A post hoc analysis including only patients with Gleason score 8-10 performed for OS, DSS, BF, and DFS showed no significant difference in any of these variables between the arms. Analysis of the interaction between ADT duration in the Gleason 8-10 subgroup versus Gleason 7 for OS, DFS, DSS and BF found no significant differences. This again suggests that 18 months of ADT may be sufficient for this high-risk group; however, it is difficult to draw definitive conclusions from this unplanned subgroup analysis.
Based on the results of the current study, it seems that 18 months of ADT is adequate for many, but not necessarily all, patients. For instance, there was a significantly higher incidence of BF in the 18-month arm. Applying this data to younger patients may require a more nuanced approach, as it is possible that with longer follow-up this higher rate of BF may translate into a difference in OS. Therefore, life expectancy and comorbid conditions always need to be incorporated into clinical decision making with regards to ADT duration. In a study by Rose et al, the risk of prostate cancer–specific mortality significantly decreased by using ADT plus RT for men with HRPC with a low, but not a high, competing mortality score.4 The clinical significance of this finding is that adding ADT to RT might significantly reduce the risk of death from prostate cancer only in the setting of low competing risks.
Another concept to ponder is the optimum duration of ADT in the era of RT dose escalation. Currently, there are emerging techniques for delivering higher radiation doses and combining brachytherapy with external beam radiotherapy for HRPC, and the role of whole pelvic radiation is being investigated. New data suggests that higher radiation doses can lead to improvement in outcomes for HRPC. The DART01/05 study compared 4 versus 24 months of ADT with 76 to 82 Gy of RT and reported improved 5-year OS, DFS, and metastasis-free survival with longer ADT duration.5 Moreover, Kishan et al reported improved prostate cancer–specific mortality when brachytherapy boost was added to radiation compared to radiation alone in patients with Gleason scores 9 and 10.6 Therefore, the optimal duration of ADT in the setting of dose-escalated radiotherapy is not yet known. Also, it is important to note that unlike the prior RTOG and EORTC studies, this study did not include patients with evidence of regional nodal disease, and thus the present data should not be applied to this patient population.
Applications to Clinical Practice
This study’s results suggesting that 18 months of ADT in combination with RT yields similar 10-year OS and improved QoL compared with 36 months of ADT in patients with HRPC should be interpreted with caution when treating very young patients, since the higher rate of BF in the short arm may impact the OS with longer follow-up. Additionally, patients’ QoL and tolerance to ADT-related adverse effects should be taken into consideration given that compliance with 36 months of ADT was only 53% in this study.
—Jailan Elayoubi, MD, Michigan State University, East Lansing, MI
Study Overview
Objective. To compare the outcomes of 18 months versus 36 months of androgen deprivation therapy (ADT) combined with radiation in high-risk prostate cancer (HRPC).
Design. Phase 3 multicenter, randomized superiority trial.
Participants. This study enrolled patients aged ≤ 80 years with HRPC. All patients had no evidence of regional or distant metastasis. High-risk disease was defined as any of the following: clinical stage T3 or T4, prostate-specific antigen (PSA) level > 20 ng/mL, or Gleason score > 7.
Methods. Prior to randomization, all patients received 4 months of ADT with goserelin 10.8 mg and anti-androgen therapy with bicalutamide 50 mg daily for 30 days. Patients were then randomly assigned to 18 (short arm) or 36 (long arm) months of ADT in combination with radiation therapy (RT). The randomization was stratified by stage (T1-2 vs T3-4), Gleason score (< 7 vs > 7) and PSA level (< 20 ng/mL vs > 20 ng/mL). The standard radiation dose was 70 Gy to the prostate and 44 Gy to the pelvis. Computed tomography or magnetic resonance imaging exam of the abdomen and pelvis and a bone scan were performed to rule out regional or distant metastases. PSA level was monitored every 3 months for 18 months, every 6 months up to the third year, and yearly thereafter.
Main outcome measures. The 2 primary outcomes were overall survival (OS) and quality of life (QoL) at 5 years. The secondary end points were biochemical failure (BF)defined as PSA nadir plus 2, disease-free survival (DFS), and site(s) of tumor relapse.
Main results. The 5-year OS was 91% and 86% for the 36- and 18-month groups, respectively (P = 0.07). The 10-year OS was 62% for both groups (P = 0.7), and the global hazard ratio (HR) was 1.02 (P = 0.8). The disease-specific survival (DSS) was similar in both groups at 5 years (98% vs 97%) and at 10 years (91% vs 92%) in the long versus short arm, respectively. The rate of prostate cancer–specific death was 21% versus 23% in the long versus short arm, respectively. In a multivariate analysis for OS, only age and Gleason score > 7 were statistically significant survival predictors. BF rate at 10 years was 25% for 36 months as compared with 31% for 18 months (HR, 0.71, P = 0.02). The 10-year DFS rates were 45% and 39% for 36 and 18 months, respectively (HR, 0.68, P = 0.08). Forty patients in the long arm versus 43 in the short arm developed distant metastasis. Both groups developed similar sites of metastasis, which was predominantly osseous. Some aspects of the EORTC30 and PR25 scales were significant, mostly pertaining to sexual activity, fatigue, and hormone-related symptoms in favor of the 18-month group. The median time to testosterone recovery after completion of ADT was 2.1 years for the short arm versus 4 years in the long arm (P = 0.02). The compliance rate with ADT was 88% in the short arm versus 53% in the long arm. The main reason for nonadherence was side effects in 54% of the patients in the long arm and 31% in the short arm.
Conclusion. The results of the current study suggest that 18 months of ADT in combination with RT yields similar 10-year OS and improved QoL compared with 36 months in patients with HRPC.
Commentary
The role of ADT for HRPC in combination with RT has been well established by evidence from several trials; however, the comparator arms and patient characteristics between these studies have been quite heterogeneous. For instance, the Radiation Therapy Oncology Group (RTOG) 85-31 trial compared indefinite ADT with RT versus RT alone and showed significantly better 10-year OS in the ADT plus RT arm.1 Similarly, the European Organisation for Research and Treatment of Cancer (EROTC) 22961 trial showed an OS benefit for 36 months versus 6 months of ADT in combination with radiation.2 Additionally, the RTOG 92-02 trial, which compared 4 months versus 24 months of ADT with radiation, also found a significantly improved 10-year OS with a longer course of ADT.3 Taken together these data suggest that 4 to 6 months of ADT is inferior to 24 to 36 months of ADT in HRPC.
Several differences, however, exist in patient characteristics between the present trial and the earlier trials, justifiably reflecting the change of practice in the PSA era. For instance, the present study has a higher percentage of patients with Gleason scores 8-10 (60%) compared to the EROTC and RTOG studies (15%-35%) and a lower percentage of patients with T3 and T4 tumors. Patients with high Gleason scores are believed to have a higher risk of micro-metastasis at the time of diagnosis and higher chances of castration resistance. Therefore, inclusion of a (presumably) larger high-risk patient subgroup in the present study lends further credence to results indicating similar OS with a shorter course of ADT. A post hoc analysis including only patients with Gleason score 8-10 performed for OS, DSS, BF, and DFS showed no significant difference in any of these variables between the arms. Analysis of the interaction between ADT duration in the Gleason 8-10 subgroup versus Gleason 7 for OS, DFS, DSS and BF found no significant differences. This again suggests that 18 months of ADT may be sufficient for this high-risk group; however, it is difficult to draw definitive conclusions from this unplanned subgroup analysis.
Based on the results of the current study, it seems that 18 months of ADT is adequate for many, but not necessarily all, patients. For instance, there was a significantly higher incidence of BF in the 18-month arm. Applying this data to younger patients may require a more nuanced approach, as it is possible that with longer follow-up this higher rate of BF may translate into a difference in OS. Therefore, life expectancy and comorbid conditions always need to be incorporated into clinical decision making with regards to ADT duration. In a study by Rose et al, the risk of prostate cancer–specific mortality significantly decreased by using ADT plus RT for men with HRPC with a low, but not a high, competing mortality score.4 The clinical significance of this finding is that adding ADT to RT might significantly reduce the risk of death from prostate cancer only in the setting of low competing risks.
Another concept to ponder is the optimum duration of ADT in the era of RT dose escalation. Currently, there are emerging techniques for delivering higher radiation doses and combining brachytherapy with external beam radiotherapy for HRPC, and the role of whole pelvic radiation is being investigated. New data suggests that higher radiation doses can lead to improvement in outcomes for HRPC. The DART01/05 study compared 4 versus 24 months of ADT with 76 to 82 Gy of RT and reported improved 5-year OS, DFS, and metastasis-free survival with longer ADT duration.5 Moreover, Kishan et al reported improved prostate cancer–specific mortality when brachytherapy boost was added to radiation compared to radiation alone in patients with Gleason scores 9 and 10.6 Therefore, the optimal duration of ADT in the setting of dose-escalated radiotherapy is not yet known. Also, it is important to note that unlike the prior RTOG and EORTC studies, this study did not include patients with evidence of regional nodal disease, and thus the present data should not be applied to this patient population.
Applications to Clinical Practice
This study’s results suggesting that 18 months of ADT in combination with RT yields similar 10-year OS and improved QoL compared with 36 months of ADT in patients with HRPC should be interpreted with caution when treating very young patients, since the higher rate of BF in the short arm may impact the OS with longer follow-up. Additionally, patients’ QoL and tolerance to ADT-related adverse effects should be taken into consideration given that compliance with 36 months of ADT was only 53% in this study.
—Jailan Elayoubi, MD, Michigan State University, East Lansing, MI
1. Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys. 2005;61:1285-1290.
2. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516-2527.
3. Horwitz EM, Bae K, Hanks GE, Porter A, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504.
4. Rose BS, Chen MH, Wu J, et al. Androgen deprivation therapy use in the setting of high-dose radiation therapy and the risk of prostate cancer-specific mortality stratified by the extent of competing mortality. Int J Radiat Oncol Biol Phys. 2016;96:778-784.
5. Zapatero A, Guerrero A, Maldonado X, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:320-327.
6. Kishan, AU, Cook, RR, Ciezki, JP, et al. Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with gleason score 9-10 prostate cancer. JAMA. 2018;319:896-905.
1. Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys. 2005;61:1285-1290.
2. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516-2527.
3. Horwitz EM, Bae K, Hanks GE, Porter A, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504.
4. Rose BS, Chen MH, Wu J, et al. Androgen deprivation therapy use in the setting of high-dose radiation therapy and the risk of prostate cancer-specific mortality stratified by the extent of competing mortality. Int J Radiat Oncol Biol Phys. 2016;96:778-784.
5. Zapatero A, Guerrero A, Maldonado X, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:320-327.
6. Kishan, AU, Cook, RR, Ciezki, JP, et al. Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with gleason score 9-10 prostate cancer. JAMA. 2018;319:896-905.
Management of treatment-resistant depression: A review of 3 studies
An estimated 7.1% of the adults in United States had a major depressive episode in 2017, and this prevalence has been trending upward over the past few years.1 The prevalence is even higher in adults between age 18 and 25 (13.1%).1 Like other psychiatric diagnoses, major depressive disorder (MDD) has a significant impact on productivity as well as daily functioning. Only one-third of patients with MDD achieve remission on the first antidepressant medication.2 This leaves an estimated 11.47 million people in the United States in need of an alternate regimen for management of their depressive episode.
The data on evidence-based biologic treatments for treatment-resistant depression are limited (other than for electroconvulsive therapy). Pharmacologic options include switching to a different medication, combining medications, and augmentation strategies or novel approaches such as ketamine and related agents. Here we summarize the findings from 3 recent studies that investigate alternate management options for MDD.
Ketamine: Randomized controlled trial
Traditional antidepressants may reduce suicidal ideation by improving depressive symptoms, but this effect may take weeks. Ketamine, an N-methyl-
_
1. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
Grunebaum et al3 evaluated the acute effect of adjunctive subanesthetic IV ketamine on clinically significant suicidal ideation in patients with MDD, with a comparison arm that received an infusion of midazolam.
Study design
- 80 inpatients (age 18 to 65 years) with MDD who had a score ≥16 on the Hamilton Depression Rating Scale (HAM-D) and a score ≥4 on the Scale for Suicidal Ideation (SSI). Approximately one-half (54%) were taking an antidepressant
- Patients were randomly assigned to IV racemic ketamine hydrochloride, .5 mg/kg, or IV midazolam, .02 mg/kg, both administered in 100 mL normal saline over 40 minutes.
Outcomes
- Scale for Suicidal Ideation scores were assessed at screening, before infusion, 230 minutes after infusion, 24 hours after infusion, and after 1 to 6 weeks of follow-up. The average SSI score on Day 1 was 4.96 points lower in the ketamine group compared with the midazolam group. The proportion of responders (defined as patients who experienced a 50% reduction in SSI score) on Day 1 was 55% for patients in the ketamine group compared with 30% in the midazolam group.
Conclusion
- Compared with midazolam, ketamine produced a greater clinically meaningful reduction in suicidal ideation 24 hours after infusion.
Apart from the primary outcome of reduction in suicidal ideation, greater reductions were also found in overall mood disturbance, depression subscale, and fatigue subscale scores as assessed on the Profile of Mood States (POMS). Although the study noted improvement in depression scores, the proportion of responders on Day 1 in depression scales, including HAM-D and the self-rated Beck Depression Inventory, fell short of statistical significance. Overall, compared with the midazolam infusion, a single adjunctive subanesthetic ketamine infusion was associated with a greater clinically significant reduction in suicidal ideation on Day 1.
Continue to: Ketamine
Ketamine: Review and meta-analysis
Wilkinson et al4 conducted a systematic review and individual participant data meta-analysis of 11 similar comparison intervention studies examining the effects of ketamine in reducing suicidal thoughts.
2. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
Study design
- Review of 11 studies of a single dose of IV ketamine for treatment of any psychiatric disorder. Only comparison intervention trials using saline placebo or midazolam were included:
- Individual patient-level data of 298 patients were obtained from 10 of the 11 trials. Analysis was performed on 167 patients who had suicidal ideation at baseline.
- Results were assessed by clinician-administered rating scales.
Outcomes
- Ketamine reduced suicidal ideation more rapidly compared with control infusions as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) and HAM-D, with significant benefits appearing on Day 1 and extending up to Day 7. The mean MADRS score in the ketamine group decreased to 19.5 from 33.8 within 1 day of infusion, compared with a reduction to 29.2 from 32.9 in the control groups.
- The number needed to treat to be free of suicidal ideation for ketamine (compared with control) was 3.1 to 4.0 for all time points in the first week after infusion.
Conclusion
- This meta-analysis provided evidence from the largest sample to date (N = 298) that ketamine reduces suicidal ideation partially independently of mood symptoms.
While the anti-suicidal effects of ketamine appear to be robust in the above studies, the possibility of rebound suicidal ideation remains in the weeks or months following exposure. Also, these studies only prove a reduction in suicidal ideation; reduction in suicidal behavior was not studied. Nevertheless, ketamine holds considerable promise as a potential rapid-acting agent in patients at risk of suicide.
Continue to: Strategies for augmentation or switching
Strategies for augmentation or switching
Only one-third of the patients with depression achieve remission on the first antidepressant medication. The American Psychiatric Association’s current management guidelines2 for patients who do not respond to the first-choice antidepressant include multiple options. Switching strategies recommended in these guidelines include changing to an antidepressant of the same class, or to one from a different class (eg, from a selective serotonin reuptake inhibitor [SSRI] to a serotonin-norepinephrine reuptake inhibitor, or from an SSRI to a tricyclic antidepressant). Augmentation strategies include augmenting with a non-monoamine oxidase inhibitor antidepressant from a different class, lithium, thyroid hormone, or an atypical antipsychotic.
The VAST-D trial5 evaluated the relative effectiveness and safety of 3 common treatments for treatment-resistant MDD:
- switching to bupropion
- augmenting the current treatment with bupropion
- augmenting the current treatment with the second-generation antipsychotic aripiprazole.
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Study design
- A multi-site, randomized, single-blind, parallel-assignment trial of 1,522 patients at 35 US Veteran Health Administration medical centers with nonpsychotic MDD with a suboptimal response to at least one antidepressant (defined as a score of ≥16 on the Quick Inventory Depressive Symptomatology-Clinician Rated questionnaire [QIDS-C16]).
- Participants were randomly assigned to 1 of 3 groups: switching to bupropion (n = 511), augmenting with bupropion (n = 506), or augmenting with aripiprazole (n = 505).
- The primary outcome was remission (defined as a QIDS-C16 score ≤5 at 2 consecutively scheduled follow-up visits). Secondary outcome was a reduction in QIDS-C16 score by ≥50%, or a Clinical Global Impression (CGI) Improvement scale score of 1 (very much improved) or 2 (much improved).
Outcomes
- The aripiprazole group showed a modest, statistically significant remission rate (28.9%) compared with the bupropion switch group (22.3%), but did not show any statistically significant difference compared with the bupropion augmentation group.
- For the secondary outcome, there was a significantly higher response rate in the aripiprazole group (74.3%) compared with the bupropion switch group (62.4%) and bupropion augmentation group (65.6%). Response measured by the CGI– Improvement scale score also favored the aripiprazole group (79%) compared with the bupropion switch group (70%) and bupropion augmentation group (74%).
Continue to: Conclusion
Conclusion
- Overall, the study found a statistically significant but modest increased likelihood of remission during 12 weeks of augmentation treatment with aripiprazole, compared with switching to bupropion monotherapy.
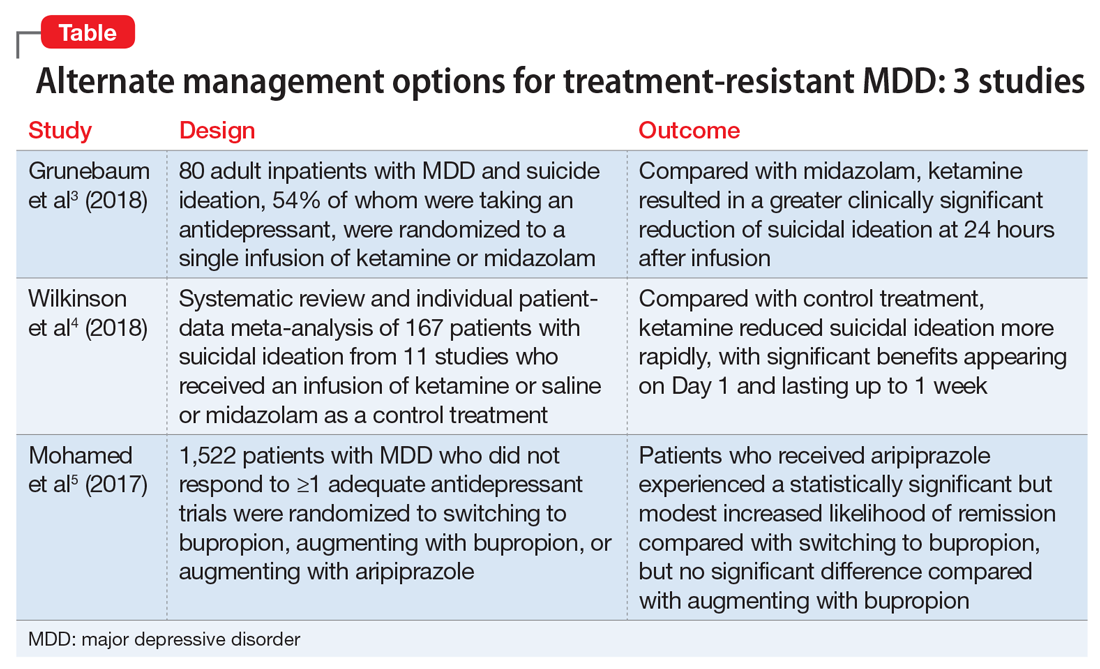
The studies discussed here, which are summarized in the Table,3-5 provide some potential avenues for research into interventions for patients who are acutely suicidal and those with treatment-resistant depression. Further research into long-term outcomes and adverse effects of ketamine use for suicidality in patients with depression is needed. The VAST-D trial suggests a need for further exploration into the efficacy of augmentation with second-generation antipsychotics for treatment-resistant depression.
1. Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed November 12, 2018.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published 2010. Accessed November 12, 2018.
3. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
4. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
5. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
An estimated 7.1% of the adults in United States had a major depressive episode in 2017, and this prevalence has been trending upward over the past few years.1 The prevalence is even higher in adults between age 18 and 25 (13.1%).1 Like other psychiatric diagnoses, major depressive disorder (MDD) has a significant impact on productivity as well as daily functioning. Only one-third of patients with MDD achieve remission on the first antidepressant medication.2 This leaves an estimated 11.47 million people in the United States in need of an alternate regimen for management of their depressive episode.
The data on evidence-based biologic treatments for treatment-resistant depression are limited (other than for electroconvulsive therapy). Pharmacologic options include switching to a different medication, combining medications, and augmentation strategies or novel approaches such as ketamine and related agents. Here we summarize the findings from 3 recent studies that investigate alternate management options for MDD.
Ketamine: Randomized controlled trial
Traditional antidepressants may reduce suicidal ideation by improving depressive symptoms, but this effect may take weeks. Ketamine, an N-methyl-
_
1. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
Grunebaum et al3 evaluated the acute effect of adjunctive subanesthetic IV ketamine on clinically significant suicidal ideation in patients with MDD, with a comparison arm that received an infusion of midazolam.
Study design
- 80 inpatients (age 18 to 65 years) with MDD who had a score ≥16 on the Hamilton Depression Rating Scale (HAM-D) and a score ≥4 on the Scale for Suicidal Ideation (SSI). Approximately one-half (54%) were taking an antidepressant
- Patients were randomly assigned to IV racemic ketamine hydrochloride, .5 mg/kg, or IV midazolam, .02 mg/kg, both administered in 100 mL normal saline over 40 minutes.
Outcomes
- Scale for Suicidal Ideation scores were assessed at screening, before infusion, 230 minutes after infusion, 24 hours after infusion, and after 1 to 6 weeks of follow-up. The average SSI score on Day 1 was 4.96 points lower in the ketamine group compared with the midazolam group. The proportion of responders (defined as patients who experienced a 50% reduction in SSI score) on Day 1 was 55% for patients in the ketamine group compared with 30% in the midazolam group.
Conclusion
- Compared with midazolam, ketamine produced a greater clinically meaningful reduction in suicidal ideation 24 hours after infusion.
Apart from the primary outcome of reduction in suicidal ideation, greater reductions were also found in overall mood disturbance, depression subscale, and fatigue subscale scores as assessed on the Profile of Mood States (POMS). Although the study noted improvement in depression scores, the proportion of responders on Day 1 in depression scales, including HAM-D and the self-rated Beck Depression Inventory, fell short of statistical significance. Overall, compared with the midazolam infusion, a single adjunctive subanesthetic ketamine infusion was associated with a greater clinically significant reduction in suicidal ideation on Day 1.
Continue to: Ketamine
Ketamine: Review and meta-analysis
Wilkinson et al4 conducted a systematic review and individual participant data meta-analysis of 11 similar comparison intervention studies examining the effects of ketamine in reducing suicidal thoughts.
2. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
Study design
- Review of 11 studies of a single dose of IV ketamine for treatment of any psychiatric disorder. Only comparison intervention trials using saline placebo or midazolam were included:
- Individual patient-level data of 298 patients were obtained from 10 of the 11 trials. Analysis was performed on 167 patients who had suicidal ideation at baseline.
- Results were assessed by clinician-administered rating scales.
Outcomes
- Ketamine reduced suicidal ideation more rapidly compared with control infusions as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) and HAM-D, with significant benefits appearing on Day 1 and extending up to Day 7. The mean MADRS score in the ketamine group decreased to 19.5 from 33.8 within 1 day of infusion, compared with a reduction to 29.2 from 32.9 in the control groups.
- The number needed to treat to be free of suicidal ideation for ketamine (compared with control) was 3.1 to 4.0 for all time points in the first week after infusion.
Conclusion
- This meta-analysis provided evidence from the largest sample to date (N = 298) that ketamine reduces suicidal ideation partially independently of mood symptoms.
While the anti-suicidal effects of ketamine appear to be robust in the above studies, the possibility of rebound suicidal ideation remains in the weeks or months following exposure. Also, these studies only prove a reduction in suicidal ideation; reduction in suicidal behavior was not studied. Nevertheless, ketamine holds considerable promise as a potential rapid-acting agent in patients at risk of suicide.
Continue to: Strategies for augmentation or switching
Strategies for augmentation or switching
Only one-third of the patients with depression achieve remission on the first antidepressant medication. The American Psychiatric Association’s current management guidelines2 for patients who do not respond to the first-choice antidepressant include multiple options. Switching strategies recommended in these guidelines include changing to an antidepressant of the same class, or to one from a different class (eg, from a selective serotonin reuptake inhibitor [SSRI] to a serotonin-norepinephrine reuptake inhibitor, or from an SSRI to a tricyclic antidepressant). Augmentation strategies include augmenting with a non-monoamine oxidase inhibitor antidepressant from a different class, lithium, thyroid hormone, or an atypical antipsychotic.
The VAST-D trial5 evaluated the relative effectiveness and safety of 3 common treatments for treatment-resistant MDD:
- switching to bupropion
- augmenting the current treatment with bupropion
- augmenting the current treatment with the second-generation antipsychotic aripiprazole.
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Study design
- A multi-site, randomized, single-blind, parallel-assignment trial of 1,522 patients at 35 US Veteran Health Administration medical centers with nonpsychotic MDD with a suboptimal response to at least one antidepressant (defined as a score of ≥16 on the Quick Inventory Depressive Symptomatology-Clinician Rated questionnaire [QIDS-C16]).
- Participants were randomly assigned to 1 of 3 groups: switching to bupropion (n = 511), augmenting with bupropion (n = 506), or augmenting with aripiprazole (n = 505).
- The primary outcome was remission (defined as a QIDS-C16 score ≤5 at 2 consecutively scheduled follow-up visits). Secondary outcome was a reduction in QIDS-C16 score by ≥50%, or a Clinical Global Impression (CGI) Improvement scale score of 1 (very much improved) or 2 (much improved).
Outcomes
- The aripiprazole group showed a modest, statistically significant remission rate (28.9%) compared with the bupropion switch group (22.3%), but did not show any statistically significant difference compared with the bupropion augmentation group.
- For the secondary outcome, there was a significantly higher response rate in the aripiprazole group (74.3%) compared with the bupropion switch group (62.4%) and bupropion augmentation group (65.6%). Response measured by the CGI– Improvement scale score also favored the aripiprazole group (79%) compared with the bupropion switch group (70%) and bupropion augmentation group (74%).
Continue to: Conclusion
Conclusion
- Overall, the study found a statistically significant but modest increased likelihood of remission during 12 weeks of augmentation treatment with aripiprazole, compared with switching to bupropion monotherapy.

The studies discussed here, which are summarized in the Table,3-5 provide some potential avenues for research into interventions for patients who are acutely suicidal and those with treatment-resistant depression. Further research into long-term outcomes and adverse effects of ketamine use for suicidality in patients with depression is needed. The VAST-D trial suggests a need for further exploration into the efficacy of augmentation with second-generation antipsychotics for treatment-resistant depression.
An estimated 7.1% of the adults in United States had a major depressive episode in 2017, and this prevalence has been trending upward over the past few years.1 The prevalence is even higher in adults between age 18 and 25 (13.1%).1 Like other psychiatric diagnoses, major depressive disorder (MDD) has a significant impact on productivity as well as daily functioning. Only one-third of patients with MDD achieve remission on the first antidepressant medication.2 This leaves an estimated 11.47 million people in the United States in need of an alternate regimen for management of their depressive episode.
The data on evidence-based biologic treatments for treatment-resistant depression are limited (other than for electroconvulsive therapy). Pharmacologic options include switching to a different medication, combining medications, and augmentation strategies or novel approaches such as ketamine and related agents. Here we summarize the findings from 3 recent studies that investigate alternate management options for MDD.
Ketamine: Randomized controlled trial
Traditional antidepressants may reduce suicidal ideation by improving depressive symptoms, but this effect may take weeks. Ketamine, an N-methyl-
_
1. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
Grunebaum et al3 evaluated the acute effect of adjunctive subanesthetic IV ketamine on clinically significant suicidal ideation in patients with MDD, with a comparison arm that received an infusion of midazolam.
Study design
- 80 inpatients (age 18 to 65 years) with MDD who had a score ≥16 on the Hamilton Depression Rating Scale (HAM-D) and a score ≥4 on the Scale for Suicidal Ideation (SSI). Approximately one-half (54%) were taking an antidepressant
- Patients were randomly assigned to IV racemic ketamine hydrochloride, .5 mg/kg, or IV midazolam, .02 mg/kg, both administered in 100 mL normal saline over 40 minutes.
Outcomes
- Scale for Suicidal Ideation scores were assessed at screening, before infusion, 230 minutes after infusion, 24 hours after infusion, and after 1 to 6 weeks of follow-up. The average SSI score on Day 1 was 4.96 points lower in the ketamine group compared with the midazolam group. The proportion of responders (defined as patients who experienced a 50% reduction in SSI score) on Day 1 was 55% for patients in the ketamine group compared with 30% in the midazolam group.
Conclusion
- Compared with midazolam, ketamine produced a greater clinically meaningful reduction in suicidal ideation 24 hours after infusion.
Apart from the primary outcome of reduction in suicidal ideation, greater reductions were also found in overall mood disturbance, depression subscale, and fatigue subscale scores as assessed on the Profile of Mood States (POMS). Although the study noted improvement in depression scores, the proportion of responders on Day 1 in depression scales, including HAM-D and the self-rated Beck Depression Inventory, fell short of statistical significance. Overall, compared with the midazolam infusion, a single adjunctive subanesthetic ketamine infusion was associated with a greater clinically significant reduction in suicidal ideation on Day 1.
Continue to: Ketamine
Ketamine: Review and meta-analysis
Wilkinson et al4 conducted a systematic review and individual participant data meta-analysis of 11 similar comparison intervention studies examining the effects of ketamine in reducing suicidal thoughts.
2. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
Study design
- Review of 11 studies of a single dose of IV ketamine for treatment of any psychiatric disorder. Only comparison intervention trials using saline placebo or midazolam were included:
- Individual patient-level data of 298 patients were obtained from 10 of the 11 trials. Analysis was performed on 167 patients who had suicidal ideation at baseline.
- Results were assessed by clinician-administered rating scales.
Outcomes
- Ketamine reduced suicidal ideation more rapidly compared with control infusions as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) and HAM-D, with significant benefits appearing on Day 1 and extending up to Day 7. The mean MADRS score in the ketamine group decreased to 19.5 from 33.8 within 1 day of infusion, compared with a reduction to 29.2 from 32.9 in the control groups.
- The number needed to treat to be free of suicidal ideation for ketamine (compared with control) was 3.1 to 4.0 for all time points in the first week after infusion.
Conclusion
- This meta-analysis provided evidence from the largest sample to date (N = 298) that ketamine reduces suicidal ideation partially independently of mood symptoms.
While the anti-suicidal effects of ketamine appear to be robust in the above studies, the possibility of rebound suicidal ideation remains in the weeks or months following exposure. Also, these studies only prove a reduction in suicidal ideation; reduction in suicidal behavior was not studied. Nevertheless, ketamine holds considerable promise as a potential rapid-acting agent in patients at risk of suicide.
Continue to: Strategies for augmentation or switching
Strategies for augmentation or switching
Only one-third of the patients with depression achieve remission on the first antidepressant medication. The American Psychiatric Association’s current management guidelines2 for patients who do not respond to the first-choice antidepressant include multiple options. Switching strategies recommended in these guidelines include changing to an antidepressant of the same class, or to one from a different class (eg, from a selective serotonin reuptake inhibitor [SSRI] to a serotonin-norepinephrine reuptake inhibitor, or from an SSRI to a tricyclic antidepressant). Augmentation strategies include augmenting with a non-monoamine oxidase inhibitor antidepressant from a different class, lithium, thyroid hormone, or an atypical antipsychotic.
The VAST-D trial5 evaluated the relative effectiveness and safety of 3 common treatments for treatment-resistant MDD:
- switching to bupropion
- augmenting the current treatment with bupropion
- augmenting the current treatment with the second-generation antipsychotic aripiprazole.
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Study design
- A multi-site, randomized, single-blind, parallel-assignment trial of 1,522 patients at 35 US Veteran Health Administration medical centers with nonpsychotic MDD with a suboptimal response to at least one antidepressant (defined as a score of ≥16 on the Quick Inventory Depressive Symptomatology-Clinician Rated questionnaire [QIDS-C16]).
- Participants were randomly assigned to 1 of 3 groups: switching to bupropion (n = 511), augmenting with bupropion (n = 506), or augmenting with aripiprazole (n = 505).
- The primary outcome was remission (defined as a QIDS-C16 score ≤5 at 2 consecutively scheduled follow-up visits). Secondary outcome was a reduction in QIDS-C16 score by ≥50%, or a Clinical Global Impression (CGI) Improvement scale score of 1 (very much improved) or 2 (much improved).
Outcomes
- The aripiprazole group showed a modest, statistically significant remission rate (28.9%) compared with the bupropion switch group (22.3%), but did not show any statistically significant difference compared with the bupropion augmentation group.
- For the secondary outcome, there was a significantly higher response rate in the aripiprazole group (74.3%) compared with the bupropion switch group (62.4%) and bupropion augmentation group (65.6%). Response measured by the CGI– Improvement scale score also favored the aripiprazole group (79%) compared with the bupropion switch group (70%) and bupropion augmentation group (74%).
Continue to: Conclusion
Conclusion
- Overall, the study found a statistically significant but modest increased likelihood of remission during 12 weeks of augmentation treatment with aripiprazole, compared with switching to bupropion monotherapy.

The studies discussed here, which are summarized in the Table,3-5 provide some potential avenues for research into interventions for patients who are acutely suicidal and those with treatment-resistant depression. Further research into long-term outcomes and adverse effects of ketamine use for suicidality in patients with depression is needed. The VAST-D trial suggests a need for further exploration into the efficacy of augmentation with second-generation antipsychotics for treatment-resistant depression.
1. Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed November 12, 2018.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published 2010. Accessed November 12, 2018.
3. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
4. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
5. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
1. Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed November 12, 2018.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published 2010. Accessed November 12, 2018.
3. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
4. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
5. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Procalcitonin, Will It Guide Us?
Study Overview
Objective. To assess whether procalcitonin-guided antibiotic usage results in less exposure to antibiotics than usual care, without a significantly higher rate of adverse events.
Design. Multi-center 1:1 randomized trial.
Setting and participants. This study was conducted at 14 academic hospitals in the United States between 2014 and 2017 in which procalcitonin assay was not routinely used. All adult patients in the emergency department with an initial diagnosis of acute lower respiratory tract infection without a decision to give or withhold antibiotics because of uncertainty regarding the need for antibiotics were included in the study. Patients were excluded if antibiotics were unlikely to be held in their case, such as if there was a need for mechanical ventilation or known severe immunosuppression, and if procalcitonin could be falsely elevated (chronic dialysis, metastatic cancer, surgery in the past 7 days).
Intervention. Patients were randomly assigned to receive guideline-based care using procalcitonin (procalcitonin group) or usual care (usual-care group). In the procalcitonin group, the procalcitonin assay results, and the procalcitonin treatment guidelines were provided to the treating physician. The guideline used previously established cutoffs (procalcitonin level of < 0.1 µg/L, antibiotics were strongly discouraged; 0.1 to 0.25 µg/L, antibiotics were discouraged; 0.25 to 0.5 µg/L, antibiotics were recommended; and > 0.5 µg/L, antibiotics were strongly recommended). Procalcitonin was measured initially in the emergency department. If the patient was hospitalized, procalcitonin was again measured 6 to 24 hours later, and on hospital days 3, 5, and 7. To implement this intervention, a multifaceted approach was used, which included sending letters to local primary care providers describing the trial, ensuring rapid delivery of procalcitonin results by tracking and coordinating blood samples with routine morning draws, and embedding the procalcitonin results and guidelines into the sites’ electronic health records. In the usual-care group, procalcitonin levels at enrollment were measured but not disclosed to clinicians. In both treatment groups, clinicians retained autonomy regarding care decisions.
Main outcome measures. The primary outcome was total antibiotic exposure, defined as the total number of antibiotic-days within 30 days after enrollment. The primary safety outcome was any adverse effects that could be attributable to withholding antibiotics in lower respiratory tract infections, within 30 days after enrollment. Secondary outcomes included admission to the intensive care unit (ICU), subsequent emergency department visits by day 30, and quality of life as assessed with the Airway Questionnaire 20.
Main results. 8360 patients with acute lower respiratory tract infection who presented to the emergency department were screened for eligibility; of these, 1664 patients underwent randomization. Ultimately, 1656 patients were included in the final analysis cohort (826 in the procalcitonin group and 830 in the usual-care group), because 8 patients withdrew. Of the cohort, 1345 (81.2%) patients completed the full 30-day follow up. Baseline characteristics were similar between the treatment groups. In the procalcitonin group, clinicians received the procalcitonin results for 95.9% of the patients. As a result of clinical care, 2.2% of the patients in the usual-care group also had procalcitonin testing. Clinicians adhered to the procalcitonin guideline recommendations for 64.8% of the procalcitonin group.
There was no significant difference in the intention-treat-treat analysis between the procalcitonin group and the usual-care group in antibiotic days during the first 30 days (mean antibiotic days, 4.2 and 4.3 days, respectively [95% confidence interval {CI}, –0.6 to 0.5; P = 0.87]). Within 30 days there was no significant difference in the proportion of patients with adverse outcomes in the procalcitonin group and usual-care group (11.7% and 13.1%, respectively [95% CI, –4.6 to 1.7]; P < 0.01 for noninferiority). There was no significant difference between the procalcitonin and usual-care groups for any of the secondary outcomes.
Conclusion. A procalcitonin-directed antibiotic administration guideline did not result in fewer antibiotic days than did usual-care among patients with suspected lower respiratory tract infection.
Commentary
Procalcitonin is a serum biomarker synthesized in thyroid neuroendocrine cells and is the precursor to calcitonin.1 It is undetectable in healthy human serum, but in the setting of systemic inflammation caused by bacterial infection, procalcitonin synthesis is induced in many tissues. Since its discovery in 1970, procalcitonin’s potential utility has been sought in various settings, such as guiding the initiation and/or discontinuation of antibiotics.2
In a prospective randomized trial in patients with an acute chronic obstructive pulmonary disease (COPD) exacerbation, treatment success was not better with antibiotics than placebo in patients with a procalcitonin level < 0.1 µg/L.3 Others replicated these results in COPD patients with acute exacerbation of COPD.4 Another small randomized trial showed that using procalcitonin in intensive care patients reduced antibiotic duration.5 Another small study found similar results in their critical care setting.6 Procalcitonin-guided antibiotic treatment produced similar results in patients with aspiration pneumonia.7 In summary, previously published studies nearly uniformly report reduced antibiotic duration or initiation using procalcitonin cutoffs without increasing adverse events.
In the current study, Huang and colleagues conducted a multi-center randomized trial in 14 academic US hospitals, while simultaneously attempting quality improvement methods for implementing and maximizing compliance with procalcitonin guidelines for local physicians. This study was able to achieve approximately 65% compliance with the guideline, which is relatively lower than in previously reported studies using procalcitonin guidelines. This study was larger and involved more hospitals than the other studies. Interestingly, this study did not find statistically significant differences in antibiotic usage or duration between the procalcitonin group compared to the usual-care group. While this result can be partially explained by the low rate of compliance with the guideline, the result may actually reflect the real-life pattern of procalcitonin guideline usage in clinicians. These results suggest that procalcitonin-based guidelines attempting to reduce antibiotic usage and exposure may be of low value, contrasting with findings from previous studies.
The Huang et al study is well-designed, had a low rate of follow-up loss and withdrawal, was conducted mostly at urban academic hospitals that had a high level of adherence to Joint Commission pneumonia core measures, and had appropriate statistical analyses; however, several factors should be considered when applying the results of this study to clinical practice. First, the large majority (80.1%) of the study cohort had final diagnoses of a COPD exacerbation, asthma exacerbation, or acute bronchitis. These patients had a moderate degree of disease (required hospitalization in 59% of patients with a mean hospital length of stay of 5 days), but their symptoms were severe enough for the patients to present to the emergency department. Patients with a suspected nonrespiratory infection or a milder degree of infection, especially in the ambulatory care setting, may have different antibiotic prescribing patterns. Also, patients in the ambulatory care setting likely have different causal organisms of their diagnosis. Second, this study excluded patients with severe disease who required ICU admission with either septic shock or respiratory failure, patients with pre-existing diseases that placed them at high risk (eg, immunosuppressed patients), and/or patients who had complications of their infection with either a lung abscess or empyema. This pattern of exclusion was widely similar to the other previous procalcitonin studies, which shows that procalcitonin guidelines should not be applied blindly in critically ill patients, even those not requiring ICU admission. Third, patients were excluded from the study if they were on chronic dialysis, had metastatic cancer, or had a recent surgery because of possible elevation of procalcitonin levels without a bacterial infection.
In conclusion, the current study did not find any difference in antibiotic exposure throughout the course of care (including discharge or hospitalization) of patients with a lower respiratory tract infection who presented to the emergency department when a procalcitonin guideline was implemented. The results of the current study raise questions regarding the new trend of widely accepting procalcitonin-based antibiotic usage.
Applications for Clinical Practice
Procalcitonin is a relatively new marker that is released during a systemic bacterial infection. While prior studies have supported systematic use of procalcitonin-based guidelines to initiate and discontinue antibiotics in order to limit antibiotic exposure, clinicians should be mindful that a procalcitonin antibiotic guideline may be useful in specific patients and should only be used in combination with usual clinical judgment. Clinicians must also recognize the medical conditions that may falsely elevate the procalcitonin level. Most important, the procalcitonin level should not be used as the sole indication to withhold antibiotics in an otherwise appropriately indicated clinical scenario.
—Minkyung Kwon, MD, Scott A. Helgeson, MD, and Vichaya Arunthari, MD
Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
1. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57-S61.
2. Deftos LJ, Roos BA, Bronzert D, Parthemore JG. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocr Metab. 1975;40:409-412.
3. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
4. Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381-1389.
5. Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266-271.
6. Najafi A, Khodadadian A, Sanatkar M, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562-567.
7. Tanaka K, Ogasawara T, Aoshima Y, et al. Procalcitonin-guided algorithm in nursing and healthcare-associated pneumonia. Respirology. 2014;19:220-220.
Study Overview
Objective. To assess whether procalcitonin-guided antibiotic usage results in less exposure to antibiotics than usual care, without a significantly higher rate of adverse events.
Design. Multi-center 1:1 randomized trial.
Setting and participants. This study was conducted at 14 academic hospitals in the United States between 2014 and 2017 in which procalcitonin assay was not routinely used. All adult patients in the emergency department with an initial diagnosis of acute lower respiratory tract infection without a decision to give or withhold antibiotics because of uncertainty regarding the need for antibiotics were included in the study. Patients were excluded if antibiotics were unlikely to be held in their case, such as if there was a need for mechanical ventilation or known severe immunosuppression, and if procalcitonin could be falsely elevated (chronic dialysis, metastatic cancer, surgery in the past 7 days).
Intervention. Patients were randomly assigned to receive guideline-based care using procalcitonin (procalcitonin group) or usual care (usual-care group). In the procalcitonin group, the procalcitonin assay results, and the procalcitonin treatment guidelines were provided to the treating physician. The guideline used previously established cutoffs (procalcitonin level of < 0.1 µg/L, antibiotics were strongly discouraged; 0.1 to 0.25 µg/L, antibiotics were discouraged; 0.25 to 0.5 µg/L, antibiotics were recommended; and > 0.5 µg/L, antibiotics were strongly recommended). Procalcitonin was measured initially in the emergency department. If the patient was hospitalized, procalcitonin was again measured 6 to 24 hours later, and on hospital days 3, 5, and 7. To implement this intervention, a multifaceted approach was used, which included sending letters to local primary care providers describing the trial, ensuring rapid delivery of procalcitonin results by tracking and coordinating blood samples with routine morning draws, and embedding the procalcitonin results and guidelines into the sites’ electronic health records. In the usual-care group, procalcitonin levels at enrollment were measured but not disclosed to clinicians. In both treatment groups, clinicians retained autonomy regarding care decisions.
Main outcome measures. The primary outcome was total antibiotic exposure, defined as the total number of antibiotic-days within 30 days after enrollment. The primary safety outcome was any adverse effects that could be attributable to withholding antibiotics in lower respiratory tract infections, within 30 days after enrollment. Secondary outcomes included admission to the intensive care unit (ICU), subsequent emergency department visits by day 30, and quality of life as assessed with the Airway Questionnaire 20.
Main results. 8360 patients with acute lower respiratory tract infection who presented to the emergency department were screened for eligibility; of these, 1664 patients underwent randomization. Ultimately, 1656 patients were included in the final analysis cohort (826 in the procalcitonin group and 830 in the usual-care group), because 8 patients withdrew. Of the cohort, 1345 (81.2%) patients completed the full 30-day follow up. Baseline characteristics were similar between the treatment groups. In the procalcitonin group, clinicians received the procalcitonin results for 95.9% of the patients. As a result of clinical care, 2.2% of the patients in the usual-care group also had procalcitonin testing. Clinicians adhered to the procalcitonin guideline recommendations for 64.8% of the procalcitonin group.
There was no significant difference in the intention-treat-treat analysis between the procalcitonin group and the usual-care group in antibiotic days during the first 30 days (mean antibiotic days, 4.2 and 4.3 days, respectively [95% confidence interval {CI}, –0.6 to 0.5; P = 0.87]). Within 30 days there was no significant difference in the proportion of patients with adverse outcomes in the procalcitonin group and usual-care group (11.7% and 13.1%, respectively [95% CI, –4.6 to 1.7]; P < 0.01 for noninferiority). There was no significant difference between the procalcitonin and usual-care groups for any of the secondary outcomes.
Conclusion. A procalcitonin-directed antibiotic administration guideline did not result in fewer antibiotic days than did usual-care among patients with suspected lower respiratory tract infection.
Commentary
Procalcitonin is a serum biomarker synthesized in thyroid neuroendocrine cells and is the precursor to calcitonin.1 It is undetectable in healthy human serum, but in the setting of systemic inflammation caused by bacterial infection, procalcitonin synthesis is induced in many tissues. Since its discovery in 1970, procalcitonin’s potential utility has been sought in various settings, such as guiding the initiation and/or discontinuation of antibiotics.2
In a prospective randomized trial in patients with an acute chronic obstructive pulmonary disease (COPD) exacerbation, treatment success was not better with antibiotics than placebo in patients with a procalcitonin level < 0.1 µg/L.3 Others replicated these results in COPD patients with acute exacerbation of COPD.4 Another small randomized trial showed that using procalcitonin in intensive care patients reduced antibiotic duration.5 Another small study found similar results in their critical care setting.6 Procalcitonin-guided antibiotic treatment produced similar results in patients with aspiration pneumonia.7 In summary, previously published studies nearly uniformly report reduced antibiotic duration or initiation using procalcitonin cutoffs without increasing adverse events.
In the current study, Huang and colleagues conducted a multi-center randomized trial in 14 academic US hospitals, while simultaneously attempting quality improvement methods for implementing and maximizing compliance with procalcitonin guidelines for local physicians. This study was able to achieve approximately 65% compliance with the guideline, which is relatively lower than in previously reported studies using procalcitonin guidelines. This study was larger and involved more hospitals than the other studies. Interestingly, this study did not find statistically significant differences in antibiotic usage or duration between the procalcitonin group compared to the usual-care group. While this result can be partially explained by the low rate of compliance with the guideline, the result may actually reflect the real-life pattern of procalcitonin guideline usage in clinicians. These results suggest that procalcitonin-based guidelines attempting to reduce antibiotic usage and exposure may be of low value, contrasting with findings from previous studies.
The Huang et al study is well-designed, had a low rate of follow-up loss and withdrawal, was conducted mostly at urban academic hospitals that had a high level of adherence to Joint Commission pneumonia core measures, and had appropriate statistical analyses; however, several factors should be considered when applying the results of this study to clinical practice. First, the large majority (80.1%) of the study cohort had final diagnoses of a COPD exacerbation, asthma exacerbation, or acute bronchitis. These patients had a moderate degree of disease (required hospitalization in 59% of patients with a mean hospital length of stay of 5 days), but their symptoms were severe enough for the patients to present to the emergency department. Patients with a suspected nonrespiratory infection or a milder degree of infection, especially in the ambulatory care setting, may have different antibiotic prescribing patterns. Also, patients in the ambulatory care setting likely have different causal organisms of their diagnosis. Second, this study excluded patients with severe disease who required ICU admission with either septic shock or respiratory failure, patients with pre-existing diseases that placed them at high risk (eg, immunosuppressed patients), and/or patients who had complications of their infection with either a lung abscess or empyema. This pattern of exclusion was widely similar to the other previous procalcitonin studies, which shows that procalcitonin guidelines should not be applied blindly in critically ill patients, even those not requiring ICU admission. Third, patients were excluded from the study if they were on chronic dialysis, had metastatic cancer, or had a recent surgery because of possible elevation of procalcitonin levels without a bacterial infection.
In conclusion, the current study did not find any difference in antibiotic exposure throughout the course of care (including discharge or hospitalization) of patients with a lower respiratory tract infection who presented to the emergency department when a procalcitonin guideline was implemented. The results of the current study raise questions regarding the new trend of widely accepting procalcitonin-based antibiotic usage.
Applications for Clinical Practice
Procalcitonin is a relatively new marker that is released during a systemic bacterial infection. While prior studies have supported systematic use of procalcitonin-based guidelines to initiate and discontinue antibiotics in order to limit antibiotic exposure, clinicians should be mindful that a procalcitonin antibiotic guideline may be useful in specific patients and should only be used in combination with usual clinical judgment. Clinicians must also recognize the medical conditions that may falsely elevate the procalcitonin level. Most important, the procalcitonin level should not be used as the sole indication to withhold antibiotics in an otherwise appropriately indicated clinical scenario.
—Minkyung Kwon, MD, Scott A. Helgeson, MD, and Vichaya Arunthari, MD
Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
Study Overview
Objective. To assess whether procalcitonin-guided antibiotic usage results in less exposure to antibiotics than usual care, without a significantly higher rate of adverse events.
Design. Multi-center 1:1 randomized trial.
Setting and participants. This study was conducted at 14 academic hospitals in the United States between 2014 and 2017 in which procalcitonin assay was not routinely used. All adult patients in the emergency department with an initial diagnosis of acute lower respiratory tract infection without a decision to give or withhold antibiotics because of uncertainty regarding the need for antibiotics were included in the study. Patients were excluded if antibiotics were unlikely to be held in their case, such as if there was a need for mechanical ventilation or known severe immunosuppression, and if procalcitonin could be falsely elevated (chronic dialysis, metastatic cancer, surgery in the past 7 days).
Intervention. Patients were randomly assigned to receive guideline-based care using procalcitonin (procalcitonin group) or usual care (usual-care group). In the procalcitonin group, the procalcitonin assay results, and the procalcitonin treatment guidelines were provided to the treating physician. The guideline used previously established cutoffs (procalcitonin level of < 0.1 µg/L, antibiotics were strongly discouraged; 0.1 to 0.25 µg/L, antibiotics were discouraged; 0.25 to 0.5 µg/L, antibiotics were recommended; and > 0.5 µg/L, antibiotics were strongly recommended). Procalcitonin was measured initially in the emergency department. If the patient was hospitalized, procalcitonin was again measured 6 to 24 hours later, and on hospital days 3, 5, and 7. To implement this intervention, a multifaceted approach was used, which included sending letters to local primary care providers describing the trial, ensuring rapid delivery of procalcitonin results by tracking and coordinating blood samples with routine morning draws, and embedding the procalcitonin results and guidelines into the sites’ electronic health records. In the usual-care group, procalcitonin levels at enrollment were measured but not disclosed to clinicians. In both treatment groups, clinicians retained autonomy regarding care decisions.
Main outcome measures. The primary outcome was total antibiotic exposure, defined as the total number of antibiotic-days within 30 days after enrollment. The primary safety outcome was any adverse effects that could be attributable to withholding antibiotics in lower respiratory tract infections, within 30 days after enrollment. Secondary outcomes included admission to the intensive care unit (ICU), subsequent emergency department visits by day 30, and quality of life as assessed with the Airway Questionnaire 20.
Main results. 8360 patients with acute lower respiratory tract infection who presented to the emergency department were screened for eligibility; of these, 1664 patients underwent randomization. Ultimately, 1656 patients were included in the final analysis cohort (826 in the procalcitonin group and 830 in the usual-care group), because 8 patients withdrew. Of the cohort, 1345 (81.2%) patients completed the full 30-day follow up. Baseline characteristics were similar between the treatment groups. In the procalcitonin group, clinicians received the procalcitonin results for 95.9% of the patients. As a result of clinical care, 2.2% of the patients in the usual-care group also had procalcitonin testing. Clinicians adhered to the procalcitonin guideline recommendations for 64.8% of the procalcitonin group.
There was no significant difference in the intention-treat-treat analysis between the procalcitonin group and the usual-care group in antibiotic days during the first 30 days (mean antibiotic days, 4.2 and 4.3 days, respectively [95% confidence interval {CI}, –0.6 to 0.5; P = 0.87]). Within 30 days there was no significant difference in the proportion of patients with adverse outcomes in the procalcitonin group and usual-care group (11.7% and 13.1%, respectively [95% CI, –4.6 to 1.7]; P < 0.01 for noninferiority). There was no significant difference between the procalcitonin and usual-care groups for any of the secondary outcomes.
Conclusion. A procalcitonin-directed antibiotic administration guideline did not result in fewer antibiotic days than did usual-care among patients with suspected lower respiratory tract infection.
Commentary
Procalcitonin is a serum biomarker synthesized in thyroid neuroendocrine cells and is the precursor to calcitonin.1 It is undetectable in healthy human serum, but in the setting of systemic inflammation caused by bacterial infection, procalcitonin synthesis is induced in many tissues. Since its discovery in 1970, procalcitonin’s potential utility has been sought in various settings, such as guiding the initiation and/or discontinuation of antibiotics.2
In a prospective randomized trial in patients with an acute chronic obstructive pulmonary disease (COPD) exacerbation, treatment success was not better with antibiotics than placebo in patients with a procalcitonin level < 0.1 µg/L.3 Others replicated these results in COPD patients with acute exacerbation of COPD.4 Another small randomized trial showed that using procalcitonin in intensive care patients reduced antibiotic duration.5 Another small study found similar results in their critical care setting.6 Procalcitonin-guided antibiotic treatment produced similar results in patients with aspiration pneumonia.7 In summary, previously published studies nearly uniformly report reduced antibiotic duration or initiation using procalcitonin cutoffs without increasing adverse events.
In the current study, Huang and colleagues conducted a multi-center randomized trial in 14 academic US hospitals, while simultaneously attempting quality improvement methods for implementing and maximizing compliance with procalcitonin guidelines for local physicians. This study was able to achieve approximately 65% compliance with the guideline, which is relatively lower than in previously reported studies using procalcitonin guidelines. This study was larger and involved more hospitals than the other studies. Interestingly, this study did not find statistically significant differences in antibiotic usage or duration between the procalcitonin group compared to the usual-care group. While this result can be partially explained by the low rate of compliance with the guideline, the result may actually reflect the real-life pattern of procalcitonin guideline usage in clinicians. These results suggest that procalcitonin-based guidelines attempting to reduce antibiotic usage and exposure may be of low value, contrasting with findings from previous studies.
The Huang et al study is well-designed, had a low rate of follow-up loss and withdrawal, was conducted mostly at urban academic hospitals that had a high level of adherence to Joint Commission pneumonia core measures, and had appropriate statistical analyses; however, several factors should be considered when applying the results of this study to clinical practice. First, the large majority (80.1%) of the study cohort had final diagnoses of a COPD exacerbation, asthma exacerbation, or acute bronchitis. These patients had a moderate degree of disease (required hospitalization in 59% of patients with a mean hospital length of stay of 5 days), but their symptoms were severe enough for the patients to present to the emergency department. Patients with a suspected nonrespiratory infection or a milder degree of infection, especially in the ambulatory care setting, may have different antibiotic prescribing patterns. Also, patients in the ambulatory care setting likely have different causal organisms of their diagnosis. Second, this study excluded patients with severe disease who required ICU admission with either septic shock or respiratory failure, patients with pre-existing diseases that placed them at high risk (eg, immunosuppressed patients), and/or patients who had complications of their infection with either a lung abscess or empyema. This pattern of exclusion was widely similar to the other previous procalcitonin studies, which shows that procalcitonin guidelines should not be applied blindly in critically ill patients, even those not requiring ICU admission. Third, patients were excluded from the study if they were on chronic dialysis, had metastatic cancer, or had a recent surgery because of possible elevation of procalcitonin levels without a bacterial infection.
In conclusion, the current study did not find any difference in antibiotic exposure throughout the course of care (including discharge or hospitalization) of patients with a lower respiratory tract infection who presented to the emergency department when a procalcitonin guideline was implemented. The results of the current study raise questions regarding the new trend of widely accepting procalcitonin-based antibiotic usage.
Applications for Clinical Practice
Procalcitonin is a relatively new marker that is released during a systemic bacterial infection. While prior studies have supported systematic use of procalcitonin-based guidelines to initiate and discontinue antibiotics in order to limit antibiotic exposure, clinicians should be mindful that a procalcitonin antibiotic guideline may be useful in specific patients and should only be used in combination with usual clinical judgment. Clinicians must also recognize the medical conditions that may falsely elevate the procalcitonin level. Most important, the procalcitonin level should not be used as the sole indication to withhold antibiotics in an otherwise appropriately indicated clinical scenario.
—Minkyung Kwon, MD, Scott A. Helgeson, MD, and Vichaya Arunthari, MD
Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
1. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57-S61.
2. Deftos LJ, Roos BA, Bronzert D, Parthemore JG. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocr Metab. 1975;40:409-412.
3. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
4. Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381-1389.
5. Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266-271.
6. Najafi A, Khodadadian A, Sanatkar M, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562-567.
7. Tanaka K, Ogasawara T, Aoshima Y, et al. Procalcitonin-guided algorithm in nursing and healthcare-associated pneumonia. Respirology. 2014;19:220-220.
1. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57-S61.
2. Deftos LJ, Roos BA, Bronzert D, Parthemore JG. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocr Metab. 1975;40:409-412.
3. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
4. Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381-1389.
5. Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266-271.
6. Najafi A, Khodadadian A, Sanatkar M, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562-567.
7. Tanaka K, Ogasawara T, Aoshima Y, et al. Procalcitonin-guided algorithm in nursing and healthcare-associated pneumonia. Respirology. 2014;19:220-220.
Hip Fracture in Nursing Home Residents with Advanced Dementia: An Opportunity for Palliative Care
Study Overview
Objective. To compare clinical outcomes (mortality, pain, physical restraint use, pressure ulcer, antipsychotic drug use) in long-term care nursing home (NH) residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management.
Design. A retrospective cohort study utilizing nationwide Medicare (Parts A, B, D and hospice) claims data linked with Centers for Medicare & Medicaid Services Minimum Data Set (MDS version 2.0) assessments.
Setting and participants. Long-stay NH residents older than 65 years with advanced dementia (defined as being assigned to Cognitive Performance Scale category 5 or 6 and a diagnosis of dementia or Alzheimer disease) and without a do not hospitalize (DNH) directive before hip fracture were identified by using MDS assessments completed from January 1, 2008 to December 31, 2013. Medicare (Part A – inpatient, or Part B – outpatient) claims data was then used to identify those residents who experienced a hip fracture within 2 years of the full MDS assessment using the International Classification of Diseases, Ninth Revision diagnostic codes. Procedure codes were used to determine whether a resident who experienced hip fracture underwent surgical repair.
Main outcome measures. The main outcome measure was all-cause mortality after hip fracture ascertained by the Medicare Enrollment File through 2013. The secondary outcome measures were documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status in NH residents who survived 6 months after hip fracture. These outcome measures were captured from the first MDS assessment completed between 120 and 240 days following the fracture or Medicare Part D claims. Documented pain was determined using a validated MDS 2.0 nursing assessment pain instrument within 7 days preceding MDS assessment. Physical restraint use was defined by the use of trunk, limb, or chair restraint within 7 days prior to MDS assessment. Pressure ulcer was defined as any stage 2 to 4 pressure ulcer. Antipsychotic drug use of any medication subclass was determined using Medicare Part D claims data and affirmative if drug was administered 180 days after hip fracture. Ambulatory status between 120 and 240 days following the fracture was determined in a subset of NH residents who were ambulatory before the hip fracture. The utilization of comfort-focused care after hip fracture was determined in NH residents who had a Medicare hospice claim or a new DNH directive in the 180 days after hip fracture.
The differences in survival among NH residents with advanced dementia and hip fracture were described by Kaplan-Meier curves. The association between surgical repair and survival was determined using multivariable Cox proportional hazards for all NH residents and stratified by pre-fracture ambulatory status. In those who survived 6 months after hip fracture, the associations between surgical repair and outcomes including documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status were determined using multivariable logistic regression models. Adjustment for differences in characteristics before hip fracture was performed using inverse probability of treatment weighting (IPTW) models.
Main results. 3083 long-stay NH residents with advanced dementia and hip fracture were included in the study. The cohort’s mean age was 84.2 ± 7.1 years, 79.2% were female (n = 2441), and 28.5% were ambulatory before hip fracture (n = 879). Of these NH residents, 84.8% (n = 2615) underwent surgical repair and 15.2% (n = 468) received nonsurgical management. At 6 months after hip fracture, mortality was 31.5% in the surgical group compared to 53.8% in the nonsurgical group. The greatest mortality difference between groups occurred in the first 30 days after hip fracture (11.5% in surgical group versus 30.6% in nonsurgical group). Surgical repair was associated with a decreased risk of death (Cox proportional hazard ratio) in the unadjusted (hazard ratio [HR], 0.55 [95% confidence interval {CI}, 0.49-0.61), multivariable adjusted (adjusted HR, 0.56 [95% CI, 0.49-0.63]), and IPTW (adjusted HR, 0.88 [95% CI, 0.79-0.98]) models. Similarly, surgically treated NH residents were less likely to die than those managed non-surgically when mortality was stratified by pre-fracture ambulatory status.
Among NH residents who survived 6 months after hip fracture, those who underwent surgical repair compared with those who received nonsurgical management had less documented pain (29.0% versus 30.9%), fewer pressure ulcers (11.2% versus 19.0%), greater physical restraint use (13.0% versus 11.1%), and greater antipsychotic drug use (29.5% versus 20.4%). In the adjusted IPTW models, surgical repair was associated with less pain (adjusted HR, 0.78 [95% CI, 0.61-0.99]) and fewer pressure ulcers (adjusted HR, 0.64 [95% CI, 0.47-0.86]).
Overall, 21.5% of NH residents utilized comfort-focused care within 6 months after hip fracture, with a mean time to utilization of hospice care of 56 ± 49 days. In those who were managed surgically, 19.3% utilized hospice care, as compared with 33.8% in those who did not receive surgical intervention. In NH residents who survived 6 months after hip fracture, only 1.1% in both groups acquired a DNH directive.
Conclusion. In older long-stay NH residents with advanced dementia and hip fracture, surgical repair was associated with lower all-cause mortality, less documented pain, and fewer pressure ulcers compared to nonsurgical management. However, adverse clinical outcomes such as pain, physical restraint use, pressure ulcers, and antipsychotic drug use were common regardless of treatment modality. The high incidence of these adverse outcomes and hazardous interventions, coupled with low utilization of comfort-focused care and DNH directive, highlight an opportunity to improve the quality of care in this vulnerable population.
Commentary
Hip fracture is very common in NH residents, with an overall incident rate of 2.3 per 100 person years and is associated with a high mortality rate of 36.2% by 6 months after fracture.1,2 Moreover, Neuman and colleagues have recently reported that among NH residents who have some degree of functional independence in locomotion prior to hip fracture, 54% either die or develop new total dependence in locomotion within 6 months of fracture and that severe cognitive impairment is a risk factor highly associated with these adverse outcomes.3 Despite this emerging knowledge, surgical repair of hip fracture remains the mainstay treatment in many NH residents in the hope of alleviating pain and improving mobility, and palliative care is considered only when patients are imminently dying or have deteriorated past the point of meaningful recovery. In cases of NH residents with advanced dementia whose life expectancy is limited and whose care goals may favor maintaining comfort, the health care proxies are frequently challenged with a difficult choice of either pursuing or foregoing surgical management—a complex medical decision to be made in the absence of sufficient evidence in this uniquely frail patient population.
The study reported by Berry and colleagues provides an important and timely investigation in examining associations of adverse clinical outcomes (mortality, pain, pressure ulcer) and hazardous interventions (physical restraint and antipsychotic drug use) in long-stay NH residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management. The authors reported a 6-month mortality rate of 31.5% in NH residents who underwent surgical repair, an event rate similar to that reported by Neuman and colleagues. While surgical repair after hip fracture was associated with a decreased risk of death compared to nonsurgical management, high incidences of pain (29.0% to 30.9%) and pressure ulcers (11.2% to 19.0%), and frequent physical restraint use (11.1% to 13.0%) and antipsychotic drug use (20.4% to 29.5%) were noted in NH residents who survived 6 months after fracture regardless of treatment modality. These findings are consistent with the high rate of post-hip fracture functional disability previously reported by Neuman and colleagues, and highlight the trajectory of decline, frequent distressing symptoms, and prevalent use of pharmacologic and nonpharmacologic restraints in long-stay NH residents after hip fracture. Taken together, the low utilization of comfort-focused care (21.5%) and DNH directive (1.1%) in NH residents who survived 6 months suggest a missed opportunity to integrate palliative care in a patient population that stands to benefit from this intervention.
This study is the first to report the associations between hip fracture surgery and a reduction in adverse outcomes such as pain and pressure ulcer that commonly affect vulnerable NH residents with advanced dementia. This study was well designed and leveraged strengths of Medicare claims data linked with MDS assessments to capture outcome measures including pain, pressure ulcer, and restraint use that would otherwise be difficult to ascertain. However, as in all retrospective cohort design, there were limitations in this study. For instance, secondary outcomes were determined from a single time point (ie, first MDS assessment completed between 120 to 240 days following hip fracture) and thus data capture may be incomplete. Additionally, other conditions important to complex decision making in the care of frail older adults including postoperative complications (eg, delirium, infections, cardiac complications) and in-hospital mortality were not examined. Despite these limitations, this study has enhanced our understanding of the clinical course of long-term care NH residents with advanced dementia who endured hip fracture.
Applications for Clinical Practice
Patients’ goals of care should guide medical decision making in the management of hip fracture in NH residents with advanced dementia. The increased survival benefit of surgical repair of hip fracture in this patient population should be considered in the medical decision-making process if life-prolongation is preferred. However, palliative and hospice care need to be an important facet of discussion given the high rates of mortality, pain, pressure ulcer, and restraint use in this vulnerable subset of older adults.
—Fred Ko, MD, MS
1. Berry SD, Lee Y, Zullo AR, et al. Incidence of hip fracture in U.S. nursing homes. J Gerontol A Biol Sci Med Sci. 2016;71:1230-1234.
2. Neuman MD, Silber JH, Magaziner JS, et al. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174:1273-1280.
3. Berry SD, Rothbaum RR, Kiel DP, et al. Association of clinical outcomes with surgical repair of hip fractures vs nonsurgical management in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178:774-780.
Study Overview
Objective. To compare clinical outcomes (mortality, pain, physical restraint use, pressure ulcer, antipsychotic drug use) in long-term care nursing home (NH) residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management.
Design. A retrospective cohort study utilizing nationwide Medicare (Parts A, B, D and hospice) claims data linked with Centers for Medicare & Medicaid Services Minimum Data Set (MDS version 2.0) assessments.
Setting and participants. Long-stay NH residents older than 65 years with advanced dementia (defined as being assigned to Cognitive Performance Scale category 5 or 6 and a diagnosis of dementia or Alzheimer disease) and without a do not hospitalize (DNH) directive before hip fracture were identified by using MDS assessments completed from January 1, 2008 to December 31, 2013. Medicare (Part A – inpatient, or Part B – outpatient) claims data was then used to identify those residents who experienced a hip fracture within 2 years of the full MDS assessment using the International Classification of Diseases, Ninth Revision diagnostic codes. Procedure codes were used to determine whether a resident who experienced hip fracture underwent surgical repair.
Main outcome measures. The main outcome measure was all-cause mortality after hip fracture ascertained by the Medicare Enrollment File through 2013. The secondary outcome measures were documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status in NH residents who survived 6 months after hip fracture. These outcome measures were captured from the first MDS assessment completed between 120 and 240 days following the fracture or Medicare Part D claims. Documented pain was determined using a validated MDS 2.0 nursing assessment pain instrument within 7 days preceding MDS assessment. Physical restraint use was defined by the use of trunk, limb, or chair restraint within 7 days prior to MDS assessment. Pressure ulcer was defined as any stage 2 to 4 pressure ulcer. Antipsychotic drug use of any medication subclass was determined using Medicare Part D claims data and affirmative if drug was administered 180 days after hip fracture. Ambulatory status between 120 and 240 days following the fracture was determined in a subset of NH residents who were ambulatory before the hip fracture. The utilization of comfort-focused care after hip fracture was determined in NH residents who had a Medicare hospice claim or a new DNH directive in the 180 days after hip fracture.
The differences in survival among NH residents with advanced dementia and hip fracture were described by Kaplan-Meier curves. The association between surgical repair and survival was determined using multivariable Cox proportional hazards for all NH residents and stratified by pre-fracture ambulatory status. In those who survived 6 months after hip fracture, the associations between surgical repair and outcomes including documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status were determined using multivariable logistic regression models. Adjustment for differences in characteristics before hip fracture was performed using inverse probability of treatment weighting (IPTW) models.
Main results. 3083 long-stay NH residents with advanced dementia and hip fracture were included in the study. The cohort’s mean age was 84.2 ± 7.1 years, 79.2% were female (n = 2441), and 28.5% were ambulatory before hip fracture (n = 879). Of these NH residents, 84.8% (n = 2615) underwent surgical repair and 15.2% (n = 468) received nonsurgical management. At 6 months after hip fracture, mortality was 31.5% in the surgical group compared to 53.8% in the nonsurgical group. The greatest mortality difference between groups occurred in the first 30 days after hip fracture (11.5% in surgical group versus 30.6% in nonsurgical group). Surgical repair was associated with a decreased risk of death (Cox proportional hazard ratio) in the unadjusted (hazard ratio [HR], 0.55 [95% confidence interval {CI}, 0.49-0.61), multivariable adjusted (adjusted HR, 0.56 [95% CI, 0.49-0.63]), and IPTW (adjusted HR, 0.88 [95% CI, 0.79-0.98]) models. Similarly, surgically treated NH residents were less likely to die than those managed non-surgically when mortality was stratified by pre-fracture ambulatory status.
Among NH residents who survived 6 months after hip fracture, those who underwent surgical repair compared with those who received nonsurgical management had less documented pain (29.0% versus 30.9%), fewer pressure ulcers (11.2% versus 19.0%), greater physical restraint use (13.0% versus 11.1%), and greater antipsychotic drug use (29.5% versus 20.4%). In the adjusted IPTW models, surgical repair was associated with less pain (adjusted HR, 0.78 [95% CI, 0.61-0.99]) and fewer pressure ulcers (adjusted HR, 0.64 [95% CI, 0.47-0.86]).
Overall, 21.5% of NH residents utilized comfort-focused care within 6 months after hip fracture, with a mean time to utilization of hospice care of 56 ± 49 days. In those who were managed surgically, 19.3% utilized hospice care, as compared with 33.8% in those who did not receive surgical intervention. In NH residents who survived 6 months after hip fracture, only 1.1% in both groups acquired a DNH directive.
Conclusion. In older long-stay NH residents with advanced dementia and hip fracture, surgical repair was associated with lower all-cause mortality, less documented pain, and fewer pressure ulcers compared to nonsurgical management. However, adverse clinical outcomes such as pain, physical restraint use, pressure ulcers, and antipsychotic drug use were common regardless of treatment modality. The high incidence of these adverse outcomes and hazardous interventions, coupled with low utilization of comfort-focused care and DNH directive, highlight an opportunity to improve the quality of care in this vulnerable population.
Commentary
Hip fracture is very common in NH residents, with an overall incident rate of 2.3 per 100 person years and is associated with a high mortality rate of 36.2% by 6 months after fracture.1,2 Moreover, Neuman and colleagues have recently reported that among NH residents who have some degree of functional independence in locomotion prior to hip fracture, 54% either die or develop new total dependence in locomotion within 6 months of fracture and that severe cognitive impairment is a risk factor highly associated with these adverse outcomes.3 Despite this emerging knowledge, surgical repair of hip fracture remains the mainstay treatment in many NH residents in the hope of alleviating pain and improving mobility, and palliative care is considered only when patients are imminently dying or have deteriorated past the point of meaningful recovery. In cases of NH residents with advanced dementia whose life expectancy is limited and whose care goals may favor maintaining comfort, the health care proxies are frequently challenged with a difficult choice of either pursuing or foregoing surgical management—a complex medical decision to be made in the absence of sufficient evidence in this uniquely frail patient population.
The study reported by Berry and colleagues provides an important and timely investigation in examining associations of adverse clinical outcomes (mortality, pain, pressure ulcer) and hazardous interventions (physical restraint and antipsychotic drug use) in long-stay NH residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management. The authors reported a 6-month mortality rate of 31.5% in NH residents who underwent surgical repair, an event rate similar to that reported by Neuman and colleagues. While surgical repair after hip fracture was associated with a decreased risk of death compared to nonsurgical management, high incidences of pain (29.0% to 30.9%) and pressure ulcers (11.2% to 19.0%), and frequent physical restraint use (11.1% to 13.0%) and antipsychotic drug use (20.4% to 29.5%) were noted in NH residents who survived 6 months after fracture regardless of treatment modality. These findings are consistent with the high rate of post-hip fracture functional disability previously reported by Neuman and colleagues, and highlight the trajectory of decline, frequent distressing symptoms, and prevalent use of pharmacologic and nonpharmacologic restraints in long-stay NH residents after hip fracture. Taken together, the low utilization of comfort-focused care (21.5%) and DNH directive (1.1%) in NH residents who survived 6 months suggest a missed opportunity to integrate palliative care in a patient population that stands to benefit from this intervention.
This study is the first to report the associations between hip fracture surgery and a reduction in adverse outcomes such as pain and pressure ulcer that commonly affect vulnerable NH residents with advanced dementia. This study was well designed and leveraged strengths of Medicare claims data linked with MDS assessments to capture outcome measures including pain, pressure ulcer, and restraint use that would otherwise be difficult to ascertain. However, as in all retrospective cohort design, there were limitations in this study. For instance, secondary outcomes were determined from a single time point (ie, first MDS assessment completed between 120 to 240 days following hip fracture) and thus data capture may be incomplete. Additionally, other conditions important to complex decision making in the care of frail older adults including postoperative complications (eg, delirium, infections, cardiac complications) and in-hospital mortality were not examined. Despite these limitations, this study has enhanced our understanding of the clinical course of long-term care NH residents with advanced dementia who endured hip fracture.
Applications for Clinical Practice
Patients’ goals of care should guide medical decision making in the management of hip fracture in NH residents with advanced dementia. The increased survival benefit of surgical repair of hip fracture in this patient population should be considered in the medical decision-making process if life-prolongation is preferred. However, palliative and hospice care need to be an important facet of discussion given the high rates of mortality, pain, pressure ulcer, and restraint use in this vulnerable subset of older adults.
—Fred Ko, MD, MS
Study Overview
Objective. To compare clinical outcomes (mortality, pain, physical restraint use, pressure ulcer, antipsychotic drug use) in long-term care nursing home (NH) residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management.
Design. A retrospective cohort study utilizing nationwide Medicare (Parts A, B, D and hospice) claims data linked with Centers for Medicare & Medicaid Services Minimum Data Set (MDS version 2.0) assessments.
Setting and participants. Long-stay NH residents older than 65 years with advanced dementia (defined as being assigned to Cognitive Performance Scale category 5 or 6 and a diagnosis of dementia or Alzheimer disease) and without a do not hospitalize (DNH) directive before hip fracture were identified by using MDS assessments completed from January 1, 2008 to December 31, 2013. Medicare (Part A – inpatient, or Part B – outpatient) claims data was then used to identify those residents who experienced a hip fracture within 2 years of the full MDS assessment using the International Classification of Diseases, Ninth Revision diagnostic codes. Procedure codes were used to determine whether a resident who experienced hip fracture underwent surgical repair.
Main outcome measures. The main outcome measure was all-cause mortality after hip fracture ascertained by the Medicare Enrollment File through 2013. The secondary outcome measures were documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status in NH residents who survived 6 months after hip fracture. These outcome measures were captured from the first MDS assessment completed between 120 and 240 days following the fracture or Medicare Part D claims. Documented pain was determined using a validated MDS 2.0 nursing assessment pain instrument within 7 days preceding MDS assessment. Physical restraint use was defined by the use of trunk, limb, or chair restraint within 7 days prior to MDS assessment. Pressure ulcer was defined as any stage 2 to 4 pressure ulcer. Antipsychotic drug use of any medication subclass was determined using Medicare Part D claims data and affirmative if drug was administered 180 days after hip fracture. Ambulatory status between 120 and 240 days following the fracture was determined in a subset of NH residents who were ambulatory before the hip fracture. The utilization of comfort-focused care after hip fracture was determined in NH residents who had a Medicare hospice claim or a new DNH directive in the 180 days after hip fracture.
The differences in survival among NH residents with advanced dementia and hip fracture were described by Kaplan-Meier curves. The association between surgical repair and survival was determined using multivariable Cox proportional hazards for all NH residents and stratified by pre-fracture ambulatory status. In those who survived 6 months after hip fracture, the associations between surgical repair and outcomes including documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status were determined using multivariable logistic regression models. Adjustment for differences in characteristics before hip fracture was performed using inverse probability of treatment weighting (IPTW) models.
Main results. 3083 long-stay NH residents with advanced dementia and hip fracture were included in the study. The cohort’s mean age was 84.2 ± 7.1 years, 79.2% were female (n = 2441), and 28.5% were ambulatory before hip fracture (n = 879). Of these NH residents, 84.8% (n = 2615) underwent surgical repair and 15.2% (n = 468) received nonsurgical management. At 6 months after hip fracture, mortality was 31.5% in the surgical group compared to 53.8% in the nonsurgical group. The greatest mortality difference between groups occurred in the first 30 days after hip fracture (11.5% in surgical group versus 30.6% in nonsurgical group). Surgical repair was associated with a decreased risk of death (Cox proportional hazard ratio) in the unadjusted (hazard ratio [HR], 0.55 [95% confidence interval {CI}, 0.49-0.61), multivariable adjusted (adjusted HR, 0.56 [95% CI, 0.49-0.63]), and IPTW (adjusted HR, 0.88 [95% CI, 0.79-0.98]) models. Similarly, surgically treated NH residents were less likely to die than those managed non-surgically when mortality was stratified by pre-fracture ambulatory status.
Among NH residents who survived 6 months after hip fracture, those who underwent surgical repair compared with those who received nonsurgical management had less documented pain (29.0% versus 30.9%), fewer pressure ulcers (11.2% versus 19.0%), greater physical restraint use (13.0% versus 11.1%), and greater antipsychotic drug use (29.5% versus 20.4%). In the adjusted IPTW models, surgical repair was associated with less pain (adjusted HR, 0.78 [95% CI, 0.61-0.99]) and fewer pressure ulcers (adjusted HR, 0.64 [95% CI, 0.47-0.86]).
Overall, 21.5% of NH residents utilized comfort-focused care within 6 months after hip fracture, with a mean time to utilization of hospice care of 56 ± 49 days. In those who were managed surgically, 19.3% utilized hospice care, as compared with 33.8% in those who did not receive surgical intervention. In NH residents who survived 6 months after hip fracture, only 1.1% in both groups acquired a DNH directive.
Conclusion. In older long-stay NH residents with advanced dementia and hip fracture, surgical repair was associated with lower all-cause mortality, less documented pain, and fewer pressure ulcers compared to nonsurgical management. However, adverse clinical outcomes such as pain, physical restraint use, pressure ulcers, and antipsychotic drug use were common regardless of treatment modality. The high incidence of these adverse outcomes and hazardous interventions, coupled with low utilization of comfort-focused care and DNH directive, highlight an opportunity to improve the quality of care in this vulnerable population.
Commentary
Hip fracture is very common in NH residents, with an overall incident rate of 2.3 per 100 person years and is associated with a high mortality rate of 36.2% by 6 months after fracture.1,2 Moreover, Neuman and colleagues have recently reported that among NH residents who have some degree of functional independence in locomotion prior to hip fracture, 54% either die or develop new total dependence in locomotion within 6 months of fracture and that severe cognitive impairment is a risk factor highly associated with these adverse outcomes.3 Despite this emerging knowledge, surgical repair of hip fracture remains the mainstay treatment in many NH residents in the hope of alleviating pain and improving mobility, and palliative care is considered only when patients are imminently dying or have deteriorated past the point of meaningful recovery. In cases of NH residents with advanced dementia whose life expectancy is limited and whose care goals may favor maintaining comfort, the health care proxies are frequently challenged with a difficult choice of either pursuing or foregoing surgical management—a complex medical decision to be made in the absence of sufficient evidence in this uniquely frail patient population.
The study reported by Berry and colleagues provides an important and timely investigation in examining associations of adverse clinical outcomes (mortality, pain, pressure ulcer) and hazardous interventions (physical restraint and antipsychotic drug use) in long-stay NH residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management. The authors reported a 6-month mortality rate of 31.5% in NH residents who underwent surgical repair, an event rate similar to that reported by Neuman and colleagues. While surgical repair after hip fracture was associated with a decreased risk of death compared to nonsurgical management, high incidences of pain (29.0% to 30.9%) and pressure ulcers (11.2% to 19.0%), and frequent physical restraint use (11.1% to 13.0%) and antipsychotic drug use (20.4% to 29.5%) were noted in NH residents who survived 6 months after fracture regardless of treatment modality. These findings are consistent with the high rate of post-hip fracture functional disability previously reported by Neuman and colleagues, and highlight the trajectory of decline, frequent distressing symptoms, and prevalent use of pharmacologic and nonpharmacologic restraints in long-stay NH residents after hip fracture. Taken together, the low utilization of comfort-focused care (21.5%) and DNH directive (1.1%) in NH residents who survived 6 months suggest a missed opportunity to integrate palliative care in a patient population that stands to benefit from this intervention.
This study is the first to report the associations between hip fracture surgery and a reduction in adverse outcomes such as pain and pressure ulcer that commonly affect vulnerable NH residents with advanced dementia. This study was well designed and leveraged strengths of Medicare claims data linked with MDS assessments to capture outcome measures including pain, pressure ulcer, and restraint use that would otherwise be difficult to ascertain. However, as in all retrospective cohort design, there were limitations in this study. For instance, secondary outcomes were determined from a single time point (ie, first MDS assessment completed between 120 to 240 days following hip fracture) and thus data capture may be incomplete. Additionally, other conditions important to complex decision making in the care of frail older adults including postoperative complications (eg, delirium, infections, cardiac complications) and in-hospital mortality were not examined. Despite these limitations, this study has enhanced our understanding of the clinical course of long-term care NH residents with advanced dementia who endured hip fracture.
Applications for Clinical Practice
Patients’ goals of care should guide medical decision making in the management of hip fracture in NH residents with advanced dementia. The increased survival benefit of surgical repair of hip fracture in this patient population should be considered in the medical decision-making process if life-prolongation is preferred. However, palliative and hospice care need to be an important facet of discussion given the high rates of mortality, pain, pressure ulcer, and restraint use in this vulnerable subset of older adults.
—Fred Ko, MD, MS
1. Berry SD, Lee Y, Zullo AR, et al. Incidence of hip fracture in U.S. nursing homes. J Gerontol A Biol Sci Med Sci. 2016;71:1230-1234.
2. Neuman MD, Silber JH, Magaziner JS, et al. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174:1273-1280.
3. Berry SD, Rothbaum RR, Kiel DP, et al. Association of clinical outcomes with surgical repair of hip fractures vs nonsurgical management in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178:774-780.
1. Berry SD, Lee Y, Zullo AR, et al. Incidence of hip fracture in U.S. nursing homes. J Gerontol A Biol Sci Med Sci. 2016;71:1230-1234.
2. Neuman MD, Silber JH, Magaziner JS, et al. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174:1273-1280.
3. Berry SD, Rothbaum RR, Kiel DP, et al. Association of clinical outcomes with surgical repair of hip fractures vs nonsurgical management in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178:774-780.
Can the Use of Siri, Alexa, and Google Assistant for Medical Information Result in Patient Harm?
Study Overview
Objective. To determine the prevalence and nature of the harm that could result from patients or consumers using conversational assistants for medical information.
Design. Observational study.
Settings and participants. Participants were recruited from an online job posting site and were eligible if they were aged ≥ 21 years and were native speakers of English. There were no other eligibility requirements. Participants contacted a research assistant by phone or email, and eligibility was confirmed before scheduling the study visit and again after arrival. However, data from 4 participants was excluded after the participants disclosed that they were not native English speakers at the end of their study sessions. Participants were compensated for their time.
Each participant took part in a single 60-minute usability session. Following informed consent and administration of baseline questionnaires, each was assigned a random selection of 2 medication tasks and 1 emergency task (provided as written scenarios) to perform with each conversational assistant—Siri, Alexa, and Google Assistant—with the order of assistants and tasks counterbalanced. Before the participants completed their first task with each conversational assistant, the research assistant demonstrated how to activate the conversational assistant using a standard weather-related question, after which the participant was asked to think of a health-related question and given 5 minutes to practice interacting with the conversational assistant with their question. Participants were then asked to complete the 3 tasks in sequence, querying the conversational assistant in their own words. Tasks were considered completed either when participants stated that they had found an answer to the question or when 5 minutes had elapsed. At task completion, the research assistant asked the participant what they would do next given the information obtained during the interaction with the conversational assistant. After the participant completed the third task with a given conversational assistant, the research assistant administered the satisfaction questionnaire. After a participant finished interacting with all 3 conversational assistants, they were interviewed about their experience.
Measures and analysis. Interactions with conversational assistants were video recorded, with the audio transcribed for analysis. Since each task typically took multiple attempts before resolution or the participant gave up, usability metrics were coded at both the task and attempt level, including time, outcomes, and error analysis. Participant-reported actions for each medical task were rated for patient harm by 2 judges (an internist and a pharmacist) using a scale adapted from those used by the Agency for Healthcare Research and Quality and the US Food and Drug Administration. Scoring was based on the following values: 0 for no harm; 1 for mild harm, resulting in bodily or psychological injury; 2 for moderate harm, resulting in bodily or psychological injury adversely affecting the functional ability or quality of life; 3 for severe harm, resulting in bodily or psychological injury, including pain or disfigurement, that interferes substantially with functional ability or quality of life; and 4 was awarded in the event of death. The 2 judges first assigned ratings independently, then met to reach consensus on cases where they disagreed. Every harmful outcome was then analyzed to determine the type of error and cause of the outcome (user error, system error, or both). The satisfaction questionnaire included 6 self-report items with response values on a 7-point scale ranging from “Not at all” to “Very satisfied.”
Main results. 54 participants completed the study, with a mean age of 42 years (SD 18) and a higher representation of individuals in the 21- to 24-year-old category than the general US adult population (30% compared to 14%). Twenty-nine (54%) were female, 31 (57%) Caucasian, and 26 (50%) college educated. Most (52 [96%]) had high levels of health literacy. Only 8 (15%) reported using a conversational assistant regularly, while 22 (41%) had never used one, and 24 (44%) had tried one “a few times.” Forty-four (82%) used computers regularly.
Of the 168 tasks completed with reported actions, 49 (29.2%) could have resulted in some degree of harm, including 27 (16.1%) that could have resulted in death. An analysis of 44 cases that potentially resulted in harm yielded several recurring error scenarios, with blame attributed solely to the conversational assistant in 13 (30%) cases, to the user in 20 (46%) cases, and to both the user and the conversational assistant in the remaining 11 (25%) cases. The most common harm scenario (9 cases, (21%) is one where the participant fails to provide all the information in the task description, and the conversational assistant responds correctly to the partial query, which the user then accepts as the recommended action to take. The next most common type of harm scenario occurs when the participant provides a complete and correct utterance describing the problem and the conversational assistant responds with partial information (7 cases, 16%). Overall self-reported satisfaction with conversational assistants was neutral, with a median rating of 4 (IQR 1-6).
Outcomes by conversational assistant were significantly different (X24 = 132.2, P < 0.001). Alexa failed for most tasks (125/394 [91.9%]), resulting in significantly more attempts made but significantly fewer instances in which responses could lead to harm. Siri had the highest task completion rate (365 [77.6%]), in part because it typically displayed a list of web pages in its response that provided at least some information to the participant. However, because of this, it had the highest likelihood of causing harm for the tasks tested (27 [20.9%]). Median user satisfaction with the 3 conversational assistants was neutral, but with significant differences among them. Participants were least satisfied with Alexa and most satisfied with Siri, and stated they were most likely to follow the recommendations provided by Siri.
Qualitatively, most participants said they would use conversational assistants for medical information, but many felt they were not quite up to the task yet. When asked about their trust in the results provided by the conversational assistants, participants said they trusted Siri the most because it provided links to multiple websites in response to their queries, allowing them to choose the response that most closely matched their assumptions. They also appreciated that Siri provided a display of its speech recognition results, giving them more confidence in its responses, and allowing them to modify their query if needed. Many participants expressed frustration with the systems, but particularly Alexa.
Conclusion. Reliance on conversational assistants for actionable medical information represents a safety risk for patients and consumers. Patients should be cautioned to not use these technologies for answers to medical questions they intend to act on without further consultation from a health care provider.
Commentary
Roughly 9 in 10 American adults use the Internet,1 with the ability to easily access information through a variety of devices including smartphones, tablets, and laptop computers. This ease of access to information has played an important role in shifting how individuals access health information and interact with their health care provider.2,3 Online health information can increase patients’ knowledge of, competence with, and engagement in health care decision-making strategies. Online health information seeking can also complement and be used in synergy with provider-patient interactions. However, online health information is difficult to regulate, complicated further by the wide range of health information literacy among patients. Inaccurate or misleading health information can lead patients to make detrimental or even dangerous health decisions. These benefits and concerns similarly apply to conversational assistants like Siri (Apple), Alexa (Amazon), and Google Assistant, which are increasingly being used by patients and consumers to access medical- and health-related information. As these technologies are voice-activated, they appear to address some health literacy limitations. However, they still pose important limitations and safety risks,4 especially as conversational assistants are being perceived as a trustworthy parallel to clinical assessment and counseling systems.5
There has been little systematic research to explore potential risks of these platforms, as well as systematically characterize error types and error rates. This study aimed to determine the capabilities of widely used, general-purpose conversational assistants in responding to a broad range of medical questions when asked by laypersons in their own words and sought to conduct a systematic evaluation of the potential harm that could result from patients or consumers acting on the resulting recommendations. The study authors found that when asked questions about situations that require medical expertise, conversational assistants failed more than half of the time and led study participants to report that they would take actions that could have resulted in harm or death. Further, the authors characterized several failure modes, including errors due to misrecognition of study participant queries, study participant misunderstanding of tasks and responses by the conversation assistant, and limited understanding of the capabilities of the assistants to understand user queries. This misalignment of expectations by users that assistants can follow conversations/discourse led to frustrating experiences by some study participants.
Not only do these findings make important contributions to the literature of health information–seeking behaviors and limitations via conversational assistants, the study design highlights relevant approaches to evaluating interactions between users and conversational assistants and other voice-activated platforms. The authors designed a range of everyday task scenarios that real-life users may be experiencing and that can lead to querying home or smartphone devices to seek health- or medical-related information. These scenarios were also written with a level of real-life complexity that incorporated multiple facts to be considered for a successful resolution and the potential of harmful consequences should the correct course of action not be taken. In addition, they allowed study participants to interpret these task scenarios and query the conversational assistants in their own words, which further aligned with how users would typically interact with their devices.
However, this study also had some limitations, which the authors highlighted. Eligibility was limited to only English-speakers and the study sample was skewed towards younger, more educated individuals with high health literacy. Combined with the small convenience sample used, findings may not be generalizable to other/broader populations and further studies are needed, especially to highlight potential differences in population subgroups (eg, race/ethnicity, age, health literacy).
Applications for Clinical Practice
Because of the increased prevalence of online health-information–seeking behaviors by patients, clinicians must be prepared to adequately address, and in some cases, educate patients on the accuracy or relevance of medical/health information they find. Conversational assistants pose an important risk in health care as they incorporate natural language interfaces that can simulate and be misinterpreted as counseling systems by patients. As the authors highlight, laypersons cannot know what the full, detailed capabilities of conversational assistants are, either concerning their medical expertise or the aspects of natural language dialogue the conversational assistants can handle. Therefore, it is critical that clinicians and other providers emphasize the limitations of these technologies to patients and that any medical recommendations should be confirmed with health care professionals before they are acted on.
— Katrina F. Mateo, MPH
1. Pew Research Center. Demographics of Internet and Home Broadband Usage in the United States [online]. Accessed at: http://www.pewinternet.org/fact-sheet/internet-broadband/.
2. Tonsaker T, Bartlett G, Trpkov C. Health information on the Internet: gold mine or minefield? Can Fam Physician. 2014;60:407-408.
3. Tan SS-L, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
4. Chung H, Iorga M, Voas J, Lee S. Alexa, can I trust you? Computer (Long Beach Calif). 2017;50:100-104.
5. Miner AS, Milstein A, Hancock JT. Talking to machines about personal mental health problems. JAMA. 2017;318:1217.
Study Overview
Objective. To determine the prevalence and nature of the harm that could result from patients or consumers using conversational assistants for medical information.
Design. Observational study.
Settings and participants. Participants were recruited from an online job posting site and were eligible if they were aged ≥ 21 years and were native speakers of English. There were no other eligibility requirements. Participants contacted a research assistant by phone or email, and eligibility was confirmed before scheduling the study visit and again after arrival. However, data from 4 participants was excluded after the participants disclosed that they were not native English speakers at the end of their study sessions. Participants were compensated for their time.
Each participant took part in a single 60-minute usability session. Following informed consent and administration of baseline questionnaires, each was assigned a random selection of 2 medication tasks and 1 emergency task (provided as written scenarios) to perform with each conversational assistant—Siri, Alexa, and Google Assistant—with the order of assistants and tasks counterbalanced. Before the participants completed their first task with each conversational assistant, the research assistant demonstrated how to activate the conversational assistant using a standard weather-related question, after which the participant was asked to think of a health-related question and given 5 minutes to practice interacting with the conversational assistant with their question. Participants were then asked to complete the 3 tasks in sequence, querying the conversational assistant in their own words. Tasks were considered completed either when participants stated that they had found an answer to the question or when 5 minutes had elapsed. At task completion, the research assistant asked the participant what they would do next given the information obtained during the interaction with the conversational assistant. After the participant completed the third task with a given conversational assistant, the research assistant administered the satisfaction questionnaire. After a participant finished interacting with all 3 conversational assistants, they were interviewed about their experience.
Measures and analysis. Interactions with conversational assistants were video recorded, with the audio transcribed for analysis. Since each task typically took multiple attempts before resolution or the participant gave up, usability metrics were coded at both the task and attempt level, including time, outcomes, and error analysis. Participant-reported actions for each medical task were rated for patient harm by 2 judges (an internist and a pharmacist) using a scale adapted from those used by the Agency for Healthcare Research and Quality and the US Food and Drug Administration. Scoring was based on the following values: 0 for no harm; 1 for mild harm, resulting in bodily or psychological injury; 2 for moderate harm, resulting in bodily or psychological injury adversely affecting the functional ability or quality of life; 3 for severe harm, resulting in bodily or psychological injury, including pain or disfigurement, that interferes substantially with functional ability or quality of life; and 4 was awarded in the event of death. The 2 judges first assigned ratings independently, then met to reach consensus on cases where they disagreed. Every harmful outcome was then analyzed to determine the type of error and cause of the outcome (user error, system error, or both). The satisfaction questionnaire included 6 self-report items with response values on a 7-point scale ranging from “Not at all” to “Very satisfied.”
Main results. 54 participants completed the study, with a mean age of 42 years (SD 18) and a higher representation of individuals in the 21- to 24-year-old category than the general US adult population (30% compared to 14%). Twenty-nine (54%) were female, 31 (57%) Caucasian, and 26 (50%) college educated. Most (52 [96%]) had high levels of health literacy. Only 8 (15%) reported using a conversational assistant regularly, while 22 (41%) had never used one, and 24 (44%) had tried one “a few times.” Forty-four (82%) used computers regularly.
Of the 168 tasks completed with reported actions, 49 (29.2%) could have resulted in some degree of harm, including 27 (16.1%) that could have resulted in death. An analysis of 44 cases that potentially resulted in harm yielded several recurring error scenarios, with blame attributed solely to the conversational assistant in 13 (30%) cases, to the user in 20 (46%) cases, and to both the user and the conversational assistant in the remaining 11 (25%) cases. The most common harm scenario (9 cases, (21%) is one where the participant fails to provide all the information in the task description, and the conversational assistant responds correctly to the partial query, which the user then accepts as the recommended action to take. The next most common type of harm scenario occurs when the participant provides a complete and correct utterance describing the problem and the conversational assistant responds with partial information (7 cases, 16%). Overall self-reported satisfaction with conversational assistants was neutral, with a median rating of 4 (IQR 1-6).
Outcomes by conversational assistant were significantly different (X24 = 132.2, P < 0.001). Alexa failed for most tasks (125/394 [91.9%]), resulting in significantly more attempts made but significantly fewer instances in which responses could lead to harm. Siri had the highest task completion rate (365 [77.6%]), in part because it typically displayed a list of web pages in its response that provided at least some information to the participant. However, because of this, it had the highest likelihood of causing harm for the tasks tested (27 [20.9%]). Median user satisfaction with the 3 conversational assistants was neutral, but with significant differences among them. Participants were least satisfied with Alexa and most satisfied with Siri, and stated they were most likely to follow the recommendations provided by Siri.
Qualitatively, most participants said they would use conversational assistants for medical information, but many felt they were not quite up to the task yet. When asked about their trust in the results provided by the conversational assistants, participants said they trusted Siri the most because it provided links to multiple websites in response to their queries, allowing them to choose the response that most closely matched their assumptions. They also appreciated that Siri provided a display of its speech recognition results, giving them more confidence in its responses, and allowing them to modify their query if needed. Many participants expressed frustration with the systems, but particularly Alexa.
Conclusion. Reliance on conversational assistants for actionable medical information represents a safety risk for patients and consumers. Patients should be cautioned to not use these technologies for answers to medical questions they intend to act on without further consultation from a health care provider.
Commentary
Roughly 9 in 10 American adults use the Internet,1 with the ability to easily access information through a variety of devices including smartphones, tablets, and laptop computers. This ease of access to information has played an important role in shifting how individuals access health information and interact with their health care provider.2,3 Online health information can increase patients’ knowledge of, competence with, and engagement in health care decision-making strategies. Online health information seeking can also complement and be used in synergy with provider-patient interactions. However, online health information is difficult to regulate, complicated further by the wide range of health information literacy among patients. Inaccurate or misleading health information can lead patients to make detrimental or even dangerous health decisions. These benefits and concerns similarly apply to conversational assistants like Siri (Apple), Alexa (Amazon), and Google Assistant, which are increasingly being used by patients and consumers to access medical- and health-related information. As these technologies are voice-activated, they appear to address some health literacy limitations. However, they still pose important limitations and safety risks,4 especially as conversational assistants are being perceived as a trustworthy parallel to clinical assessment and counseling systems.5
There has been little systematic research to explore potential risks of these platforms, as well as systematically characterize error types and error rates. This study aimed to determine the capabilities of widely used, general-purpose conversational assistants in responding to a broad range of medical questions when asked by laypersons in their own words and sought to conduct a systematic evaluation of the potential harm that could result from patients or consumers acting on the resulting recommendations. The study authors found that when asked questions about situations that require medical expertise, conversational assistants failed more than half of the time and led study participants to report that they would take actions that could have resulted in harm or death. Further, the authors characterized several failure modes, including errors due to misrecognition of study participant queries, study participant misunderstanding of tasks and responses by the conversation assistant, and limited understanding of the capabilities of the assistants to understand user queries. This misalignment of expectations by users that assistants can follow conversations/discourse led to frustrating experiences by some study participants.
Not only do these findings make important contributions to the literature of health information–seeking behaviors and limitations via conversational assistants, the study design highlights relevant approaches to evaluating interactions between users and conversational assistants and other voice-activated platforms. The authors designed a range of everyday task scenarios that real-life users may be experiencing and that can lead to querying home or smartphone devices to seek health- or medical-related information. These scenarios were also written with a level of real-life complexity that incorporated multiple facts to be considered for a successful resolution and the potential of harmful consequences should the correct course of action not be taken. In addition, they allowed study participants to interpret these task scenarios and query the conversational assistants in their own words, which further aligned with how users would typically interact with their devices.
However, this study also had some limitations, which the authors highlighted. Eligibility was limited to only English-speakers and the study sample was skewed towards younger, more educated individuals with high health literacy. Combined with the small convenience sample used, findings may not be generalizable to other/broader populations and further studies are needed, especially to highlight potential differences in population subgroups (eg, race/ethnicity, age, health literacy).
Applications for Clinical Practice
Because of the increased prevalence of online health-information–seeking behaviors by patients, clinicians must be prepared to adequately address, and in some cases, educate patients on the accuracy or relevance of medical/health information they find. Conversational assistants pose an important risk in health care as they incorporate natural language interfaces that can simulate and be misinterpreted as counseling systems by patients. As the authors highlight, laypersons cannot know what the full, detailed capabilities of conversational assistants are, either concerning their medical expertise or the aspects of natural language dialogue the conversational assistants can handle. Therefore, it is critical that clinicians and other providers emphasize the limitations of these technologies to patients and that any medical recommendations should be confirmed with health care professionals before they are acted on.
— Katrina F. Mateo, MPH
Study Overview
Objective. To determine the prevalence and nature of the harm that could result from patients or consumers using conversational assistants for medical information.
Design. Observational study.
Settings and participants. Participants were recruited from an online job posting site and were eligible if they were aged ≥ 21 years and were native speakers of English. There were no other eligibility requirements. Participants contacted a research assistant by phone or email, and eligibility was confirmed before scheduling the study visit and again after arrival. However, data from 4 participants was excluded after the participants disclosed that they were not native English speakers at the end of their study sessions. Participants were compensated for their time.
Each participant took part in a single 60-minute usability session. Following informed consent and administration of baseline questionnaires, each was assigned a random selection of 2 medication tasks and 1 emergency task (provided as written scenarios) to perform with each conversational assistant—Siri, Alexa, and Google Assistant—with the order of assistants and tasks counterbalanced. Before the participants completed their first task with each conversational assistant, the research assistant demonstrated how to activate the conversational assistant using a standard weather-related question, after which the participant was asked to think of a health-related question and given 5 minutes to practice interacting with the conversational assistant with their question. Participants were then asked to complete the 3 tasks in sequence, querying the conversational assistant in their own words. Tasks were considered completed either when participants stated that they had found an answer to the question or when 5 minutes had elapsed. At task completion, the research assistant asked the participant what they would do next given the information obtained during the interaction with the conversational assistant. After the participant completed the third task with a given conversational assistant, the research assistant administered the satisfaction questionnaire. After a participant finished interacting with all 3 conversational assistants, they were interviewed about their experience.
Measures and analysis. Interactions with conversational assistants were video recorded, with the audio transcribed for analysis. Since each task typically took multiple attempts before resolution or the participant gave up, usability metrics were coded at both the task and attempt level, including time, outcomes, and error analysis. Participant-reported actions for each medical task were rated for patient harm by 2 judges (an internist and a pharmacist) using a scale adapted from those used by the Agency for Healthcare Research and Quality and the US Food and Drug Administration. Scoring was based on the following values: 0 for no harm; 1 for mild harm, resulting in bodily or psychological injury; 2 for moderate harm, resulting in bodily or psychological injury adversely affecting the functional ability or quality of life; 3 for severe harm, resulting in bodily or psychological injury, including pain or disfigurement, that interferes substantially with functional ability or quality of life; and 4 was awarded in the event of death. The 2 judges first assigned ratings independently, then met to reach consensus on cases where they disagreed. Every harmful outcome was then analyzed to determine the type of error and cause of the outcome (user error, system error, or both). The satisfaction questionnaire included 6 self-report items with response values on a 7-point scale ranging from “Not at all” to “Very satisfied.”
Main results. 54 participants completed the study, with a mean age of 42 years (SD 18) and a higher representation of individuals in the 21- to 24-year-old category than the general US adult population (30% compared to 14%). Twenty-nine (54%) were female, 31 (57%) Caucasian, and 26 (50%) college educated. Most (52 [96%]) had high levels of health literacy. Only 8 (15%) reported using a conversational assistant regularly, while 22 (41%) had never used one, and 24 (44%) had tried one “a few times.” Forty-four (82%) used computers regularly.
Of the 168 tasks completed with reported actions, 49 (29.2%) could have resulted in some degree of harm, including 27 (16.1%) that could have resulted in death. An analysis of 44 cases that potentially resulted in harm yielded several recurring error scenarios, with blame attributed solely to the conversational assistant in 13 (30%) cases, to the user in 20 (46%) cases, and to both the user and the conversational assistant in the remaining 11 (25%) cases. The most common harm scenario (9 cases, (21%) is one where the participant fails to provide all the information in the task description, and the conversational assistant responds correctly to the partial query, which the user then accepts as the recommended action to take. The next most common type of harm scenario occurs when the participant provides a complete and correct utterance describing the problem and the conversational assistant responds with partial information (7 cases, 16%). Overall self-reported satisfaction with conversational assistants was neutral, with a median rating of 4 (IQR 1-6).
Outcomes by conversational assistant were significantly different (X24 = 132.2, P < 0.001). Alexa failed for most tasks (125/394 [91.9%]), resulting in significantly more attempts made but significantly fewer instances in which responses could lead to harm. Siri had the highest task completion rate (365 [77.6%]), in part because it typically displayed a list of web pages in its response that provided at least some information to the participant. However, because of this, it had the highest likelihood of causing harm for the tasks tested (27 [20.9%]). Median user satisfaction with the 3 conversational assistants was neutral, but with significant differences among them. Participants were least satisfied with Alexa and most satisfied with Siri, and stated they were most likely to follow the recommendations provided by Siri.
Qualitatively, most participants said they would use conversational assistants for medical information, but many felt they were not quite up to the task yet. When asked about their trust in the results provided by the conversational assistants, participants said they trusted Siri the most because it provided links to multiple websites in response to their queries, allowing them to choose the response that most closely matched their assumptions. They also appreciated that Siri provided a display of its speech recognition results, giving them more confidence in its responses, and allowing them to modify their query if needed. Many participants expressed frustration with the systems, but particularly Alexa.
Conclusion. Reliance on conversational assistants for actionable medical information represents a safety risk for patients and consumers. Patients should be cautioned to not use these technologies for answers to medical questions they intend to act on without further consultation from a health care provider.
Commentary
Roughly 9 in 10 American adults use the Internet,1 with the ability to easily access information through a variety of devices including smartphones, tablets, and laptop computers. This ease of access to information has played an important role in shifting how individuals access health information and interact with their health care provider.2,3 Online health information can increase patients’ knowledge of, competence with, and engagement in health care decision-making strategies. Online health information seeking can also complement and be used in synergy with provider-patient interactions. However, online health information is difficult to regulate, complicated further by the wide range of health information literacy among patients. Inaccurate or misleading health information can lead patients to make detrimental or even dangerous health decisions. These benefits and concerns similarly apply to conversational assistants like Siri (Apple), Alexa (Amazon), and Google Assistant, which are increasingly being used by patients and consumers to access medical- and health-related information. As these technologies are voice-activated, they appear to address some health literacy limitations. However, they still pose important limitations and safety risks,4 especially as conversational assistants are being perceived as a trustworthy parallel to clinical assessment and counseling systems.5
There has been little systematic research to explore potential risks of these platforms, as well as systematically characterize error types and error rates. This study aimed to determine the capabilities of widely used, general-purpose conversational assistants in responding to a broad range of medical questions when asked by laypersons in their own words and sought to conduct a systematic evaluation of the potential harm that could result from patients or consumers acting on the resulting recommendations. The study authors found that when asked questions about situations that require medical expertise, conversational assistants failed more than half of the time and led study participants to report that they would take actions that could have resulted in harm or death. Further, the authors characterized several failure modes, including errors due to misrecognition of study participant queries, study participant misunderstanding of tasks and responses by the conversation assistant, and limited understanding of the capabilities of the assistants to understand user queries. This misalignment of expectations by users that assistants can follow conversations/discourse led to frustrating experiences by some study participants.
Not only do these findings make important contributions to the literature of health information–seeking behaviors and limitations via conversational assistants, the study design highlights relevant approaches to evaluating interactions between users and conversational assistants and other voice-activated platforms. The authors designed a range of everyday task scenarios that real-life users may be experiencing and that can lead to querying home or smartphone devices to seek health- or medical-related information. These scenarios were also written with a level of real-life complexity that incorporated multiple facts to be considered for a successful resolution and the potential of harmful consequences should the correct course of action not be taken. In addition, they allowed study participants to interpret these task scenarios and query the conversational assistants in their own words, which further aligned with how users would typically interact with their devices.
However, this study also had some limitations, which the authors highlighted. Eligibility was limited to only English-speakers and the study sample was skewed towards younger, more educated individuals with high health literacy. Combined with the small convenience sample used, findings may not be generalizable to other/broader populations and further studies are needed, especially to highlight potential differences in population subgroups (eg, race/ethnicity, age, health literacy).
Applications for Clinical Practice
Because of the increased prevalence of online health-information–seeking behaviors by patients, clinicians must be prepared to adequately address, and in some cases, educate patients on the accuracy or relevance of medical/health information they find. Conversational assistants pose an important risk in health care as they incorporate natural language interfaces that can simulate and be misinterpreted as counseling systems by patients. As the authors highlight, laypersons cannot know what the full, detailed capabilities of conversational assistants are, either concerning their medical expertise or the aspects of natural language dialogue the conversational assistants can handle. Therefore, it is critical that clinicians and other providers emphasize the limitations of these technologies to patients and that any medical recommendations should be confirmed with health care professionals before they are acted on.
— Katrina F. Mateo, MPH
1. Pew Research Center. Demographics of Internet and Home Broadband Usage in the United States [online]. Accessed at: http://www.pewinternet.org/fact-sheet/internet-broadband/.
2. Tonsaker T, Bartlett G, Trpkov C. Health information on the Internet: gold mine or minefield? Can Fam Physician. 2014;60:407-408.
3. Tan SS-L, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
4. Chung H, Iorga M, Voas J, Lee S. Alexa, can I trust you? Computer (Long Beach Calif). 2017;50:100-104.
5. Miner AS, Milstein A, Hancock JT. Talking to machines about personal mental health problems. JAMA. 2017;318:1217.
1. Pew Research Center. Demographics of Internet and Home Broadband Usage in the United States [online]. Accessed at: http://www.pewinternet.org/fact-sheet/internet-broadband/.
2. Tonsaker T, Bartlett G, Trpkov C. Health information on the Internet: gold mine or minefield? Can Fam Physician. 2014;60:407-408.
3. Tan SS-L, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
4. Chung H, Iorga M, Voas J, Lee S. Alexa, can I trust you? Computer (Long Beach Calif). 2017;50:100-104.
5. Miner AS, Milstein A, Hancock JT. Talking to machines about personal mental health problems. JAMA. 2017;318:1217.
Bundled Hospital-at-Home and Transitional Care Program Is Associated with Reduced Rate of Hospital Readmission
Study Overview
Objective. To examine the effect of a hospital-at-home (HaH) and transitional care program on clinical outcomes and patient experiences when compared with inpatient hospitalization.
Design. Cohort study with matched controls.
Setting and participants. The study was conducted in a single center and aimed to evaluate a HaH program bundled with a 30-day postacute period of home-based transitional care. The program is funded by the Center for Medicare and Medicaid Innovation of the Centers for Medicare and Medicaid Services (CMS) with the goal of establishing a new HaH program that provides acute hospital-level care in a patient’s home as a substitute for transitional inpatient care.
Patients were eligible for the program if they were aged 18 years or older, lived in Manhattan, New York, had fee-for-service Medicare or private insurer that had contracted for HaH services, and required inpatient hospital admission for eligible conditions. Eligible conditions included acute exacerbations of asthma or chronic obstructive pulmonary disease, congestive heart failure (CHF), urinary tract infections (UTI), community-acquired pneumonia (CAP), cellulitis of lower extremities, deep venous thrombosis, pulmonary embolism, hypertensive urgency, hyperglycemia, and dehydration; this list was later expanded to 19 conditions representing 65 diagnosis-related groups. Patients were excluded if they were clinically unstable, required cardiac monitoring or intensive care, or lived in an unsafe home environment. Patients were identified in the emergency department (ED) and approached for enrollment in the program. Patients who were eligible for admission but refused HaH admission, or those who were identified as eligible for admission but for whom HaH clinicians were not available were enrolled as control patients.
Intervention. The HaH intervention included physician or nurse practitioner visits at home to provide acute care services including physical examination, illness and vital signs monitoring, intravenous infusions, wound care, and education regarding the illness. Nurses visited patients once or more a day to provide most of the care, and a physician or nurse practitioner saw patients at least daily in person or via video call facilitated by the nurse. A social worker also visited each patient at least once. Medical equipment, phlebotomy, and home radiography were also provided at home as needed. Patients were discharged from acute care when their acute illness resolved; subsequently, nurses and social workers provided self-
Main outcome measures. Main study outcome measures include duration of the acute care period (length of stay [LOS]) and 30-day all-cause hospital readmissions or ED visits, transfer to a skilled nursing facility, and referral to a certified home health care agency. LOS was defined as being from the date the patient was listed for admission by an ED physician to the date that post-acute care was initiated (for HaH) or hospital discharge (for control patients). Other measures include patient’s rating of care measured using items in 6 of the 9 domains of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey that were most salient to care at home, including communication with nurses, communication with physicians, pain management, communication about medicines, discharge information, and overall hospital rating.
Main results. The HaH clinical team approached 460 patients and enrolled 295 to the program. A total of 212 patients who were admitted to the hospital were enrolled as control patients. HaH patients were older than control patients, with an average age of 76.9 years (SD, 16.6) and 71.5 years (SD 13.8), respectively, and more likely to have at least 1 functional limitation (71.5% vs. 55.5%). The most frequent admission diagnoses to HaH were UTIs, CAP, cellulitis, and CHF. HaH patients had a shorter hospitalization LOS (3.2 days) compared with the control group (5.5 days; 95% confidence interval [CI], –1.8 to –2.7 days). HaH patients were less likely to have 30-day all-cause hospital readmissions (8.6% vs. 15.6%; 95% CI, –12.9% to –1.1%) and 30-day ED revisits (5.8% vs. 11.7%) compared to controls. Analysis adjusted for age, sex, race, ethnicity, education, insurance type, physical function, general health, and admitting diagnosis found that HaH patients had lower odds of hospital readmission (odds ratio [OR], 0.43; 95% CI, 0.36-0.52) and lower odds of ED revisits (OR, 0.39; 95% CI, 0.31-0.49). HaH patients reported higher ratings for communication with nurses and physicians and communication about medicines when compared with controls; they were also more likely to report the highest rating for overall hospital care (68.8% vs. 45.3%). Scores for pain management were lower for HaH patients when compared with controls.
Conclusions. Patients receiving care through the HaH program were less likely to be readmitted at 30 days after hospital discharge, had lower hospital LOS and reported higher ratings of care when compared to patients receiving care in the hospital. The study demonstrated the potential benefits of the HaH model of care for adults who need inpatient hospitalization.
Commentary
This study adds to the literature on outcomes associated with HaH programs. The first study of the HaH model in the United States was published in 2005,1 and despite the early demonstration of its feasibility and outcomes in this and subsequent studies,2,3 HaH models have not been widely adopted, unlike in other countries with integrated health care systems.4 One of the primary reasons this model has not been adopted is the lack of a specific payment mechanism in Medicare fee for service for HaH. Implementation of the HaH program described in the current study was an effort funded by a CMS innovation award to test the effect of models of care with the potential of developing payment mechanisms that would support further dissemination of these models. The results from the current study were encouraging and have led to the Physician-Focused Payment Model Technical Advisory Committee’s unanimous recommendation to the U.S. Department of Health and Human Services for full implementation in 2017.
The current study does have certain limitations. It is not a randomized trial, and thus control group selection could be affected by selection bias. Also, the study was conducted in a single health system and thus may have limited generalizability. Nevertheless, this study was designed based on prior studies of HaH, including randomized and non-randomized studies, that have demonstrated benefits similar to the current study. The finding that HaH patients reported worse pain control than did patients hospitalized in the inpatient setting, where staff is available 24 hours a day, may suggest differences in care that is feasible at home versus in the inpatient setting. Finally, because it is a bundled program that includes both HaH and a post-discharge care transition program, it is unclear if the effects found in this evaluation can be attributed to specific components within the bundled program.
Applications for Clinical Practice
Patients, particularly older adults, may prefer to have hospital-level care delivered at home; clinicians may consider how HaH may allow patients to avoid potential hazards of hospitalization,5 such as inpatient falls, delirium, and other iatrogenic events. The HaH program is feasible and safe, and is associated with improved outcomes of care for patients.
—William W. Hung, MD, MPH
1. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
2. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital at home”. Med J Aust. 2012;197:512-519.
3. Mader SL, Medcraft MC, Joseph C, et al. Program at home: a Veteran Affairs healthcare program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56: 2317-2322.
4. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health Review of the hospital in the home program. Med J Aust. 2010;193:598-601.
5. Creditor MC. Hazards of hospitalization. Ann Intern Med. 1993;118:219-223.
Study Overview
Objective. To examine the effect of a hospital-at-home (HaH) and transitional care program on clinical outcomes and patient experiences when compared with inpatient hospitalization.
Design. Cohort study with matched controls.
Setting and participants. The study was conducted in a single center and aimed to evaluate a HaH program bundled with a 30-day postacute period of home-based transitional care. The program is funded by the Center for Medicare and Medicaid Innovation of the Centers for Medicare and Medicaid Services (CMS) with the goal of establishing a new HaH program that provides acute hospital-level care in a patient’s home as a substitute for transitional inpatient care.
Patients were eligible for the program if they were aged 18 years or older, lived in Manhattan, New York, had fee-for-service Medicare or private insurer that had contracted for HaH services, and required inpatient hospital admission for eligible conditions. Eligible conditions included acute exacerbations of asthma or chronic obstructive pulmonary disease, congestive heart failure (CHF), urinary tract infections (UTI), community-acquired pneumonia (CAP), cellulitis of lower extremities, deep venous thrombosis, pulmonary embolism, hypertensive urgency, hyperglycemia, and dehydration; this list was later expanded to 19 conditions representing 65 diagnosis-related groups. Patients were excluded if they were clinically unstable, required cardiac monitoring or intensive care, or lived in an unsafe home environment. Patients were identified in the emergency department (ED) and approached for enrollment in the program. Patients who were eligible for admission but refused HaH admission, or those who were identified as eligible for admission but for whom HaH clinicians were not available were enrolled as control patients.
Intervention. The HaH intervention included physician or nurse practitioner visits at home to provide acute care services including physical examination, illness and vital signs monitoring, intravenous infusions, wound care, and education regarding the illness. Nurses visited patients once or more a day to provide most of the care, and a physician or nurse practitioner saw patients at least daily in person or via video call facilitated by the nurse. A social worker also visited each patient at least once. Medical equipment, phlebotomy, and home radiography were also provided at home as needed. Patients were discharged from acute care when their acute illness resolved; subsequently, nurses and social workers provided self-
Main outcome measures. Main study outcome measures include duration of the acute care period (length of stay [LOS]) and 30-day all-cause hospital readmissions or ED visits, transfer to a skilled nursing facility, and referral to a certified home health care agency. LOS was defined as being from the date the patient was listed for admission by an ED physician to the date that post-acute care was initiated (for HaH) or hospital discharge (for control patients). Other measures include patient’s rating of care measured using items in 6 of the 9 domains of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey that were most salient to care at home, including communication with nurses, communication with physicians, pain management, communication about medicines, discharge information, and overall hospital rating.
Main results. The HaH clinical team approached 460 patients and enrolled 295 to the program. A total of 212 patients who were admitted to the hospital were enrolled as control patients. HaH patients were older than control patients, with an average age of 76.9 years (SD, 16.6) and 71.5 years (SD 13.8), respectively, and more likely to have at least 1 functional limitation (71.5% vs. 55.5%). The most frequent admission diagnoses to HaH were UTIs, CAP, cellulitis, and CHF. HaH patients had a shorter hospitalization LOS (3.2 days) compared with the control group (5.5 days; 95% confidence interval [CI], –1.8 to –2.7 days). HaH patients were less likely to have 30-day all-cause hospital readmissions (8.6% vs. 15.6%; 95% CI, –12.9% to –1.1%) and 30-day ED revisits (5.8% vs. 11.7%) compared to controls. Analysis adjusted for age, sex, race, ethnicity, education, insurance type, physical function, general health, and admitting diagnosis found that HaH patients had lower odds of hospital readmission (odds ratio [OR], 0.43; 95% CI, 0.36-0.52) and lower odds of ED revisits (OR, 0.39; 95% CI, 0.31-0.49). HaH patients reported higher ratings for communication with nurses and physicians and communication about medicines when compared with controls; they were also more likely to report the highest rating for overall hospital care (68.8% vs. 45.3%). Scores for pain management were lower for HaH patients when compared with controls.
Conclusions. Patients receiving care through the HaH program were less likely to be readmitted at 30 days after hospital discharge, had lower hospital LOS and reported higher ratings of care when compared to patients receiving care in the hospital. The study demonstrated the potential benefits of the HaH model of care for adults who need inpatient hospitalization.
Commentary
This study adds to the literature on outcomes associated with HaH programs. The first study of the HaH model in the United States was published in 2005,1 and despite the early demonstration of its feasibility and outcomes in this and subsequent studies,2,3 HaH models have not been widely adopted, unlike in other countries with integrated health care systems.4 One of the primary reasons this model has not been adopted is the lack of a specific payment mechanism in Medicare fee for service for HaH. Implementation of the HaH program described in the current study was an effort funded by a CMS innovation award to test the effect of models of care with the potential of developing payment mechanisms that would support further dissemination of these models. The results from the current study were encouraging and have led to the Physician-Focused Payment Model Technical Advisory Committee’s unanimous recommendation to the U.S. Department of Health and Human Services for full implementation in 2017.
The current study does have certain limitations. It is not a randomized trial, and thus control group selection could be affected by selection bias. Also, the study was conducted in a single health system and thus may have limited generalizability. Nevertheless, this study was designed based on prior studies of HaH, including randomized and non-randomized studies, that have demonstrated benefits similar to the current study. The finding that HaH patients reported worse pain control than did patients hospitalized in the inpatient setting, where staff is available 24 hours a day, may suggest differences in care that is feasible at home versus in the inpatient setting. Finally, because it is a bundled program that includes both HaH and a post-discharge care transition program, it is unclear if the effects found in this evaluation can be attributed to specific components within the bundled program.
Applications for Clinical Practice
Patients, particularly older adults, may prefer to have hospital-level care delivered at home; clinicians may consider how HaH may allow patients to avoid potential hazards of hospitalization,5 such as inpatient falls, delirium, and other iatrogenic events. The HaH program is feasible and safe, and is associated with improved outcomes of care for patients.
—William W. Hung, MD, MPH
Study Overview
Objective. To examine the effect of a hospital-at-home (HaH) and transitional care program on clinical outcomes and patient experiences when compared with inpatient hospitalization.
Design. Cohort study with matched controls.
Setting and participants. The study was conducted in a single center and aimed to evaluate a HaH program bundled with a 30-day postacute period of home-based transitional care. The program is funded by the Center for Medicare and Medicaid Innovation of the Centers for Medicare and Medicaid Services (CMS) with the goal of establishing a new HaH program that provides acute hospital-level care in a patient’s home as a substitute for transitional inpatient care.
Patients were eligible for the program if they were aged 18 years or older, lived in Manhattan, New York, had fee-for-service Medicare or private insurer that had contracted for HaH services, and required inpatient hospital admission for eligible conditions. Eligible conditions included acute exacerbations of asthma or chronic obstructive pulmonary disease, congestive heart failure (CHF), urinary tract infections (UTI), community-acquired pneumonia (CAP), cellulitis of lower extremities, deep venous thrombosis, pulmonary embolism, hypertensive urgency, hyperglycemia, and dehydration; this list was later expanded to 19 conditions representing 65 diagnosis-related groups. Patients were excluded if they were clinically unstable, required cardiac monitoring or intensive care, or lived in an unsafe home environment. Patients were identified in the emergency department (ED) and approached for enrollment in the program. Patients who were eligible for admission but refused HaH admission, or those who were identified as eligible for admission but for whom HaH clinicians were not available were enrolled as control patients.
Intervention. The HaH intervention included physician or nurse practitioner visits at home to provide acute care services including physical examination, illness and vital signs monitoring, intravenous infusions, wound care, and education regarding the illness. Nurses visited patients once or more a day to provide most of the care, and a physician or nurse practitioner saw patients at least daily in person or via video call facilitated by the nurse. A social worker also visited each patient at least once. Medical equipment, phlebotomy, and home radiography were also provided at home as needed. Patients were discharged from acute care when their acute illness resolved; subsequently, nurses and social workers provided self-
Main outcome measures. Main study outcome measures include duration of the acute care period (length of stay [LOS]) and 30-day all-cause hospital readmissions or ED visits, transfer to a skilled nursing facility, and referral to a certified home health care agency. LOS was defined as being from the date the patient was listed for admission by an ED physician to the date that post-acute care was initiated (for HaH) or hospital discharge (for control patients). Other measures include patient’s rating of care measured using items in 6 of the 9 domains of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey that were most salient to care at home, including communication with nurses, communication with physicians, pain management, communication about medicines, discharge information, and overall hospital rating.
Main results. The HaH clinical team approached 460 patients and enrolled 295 to the program. A total of 212 patients who were admitted to the hospital were enrolled as control patients. HaH patients were older than control patients, with an average age of 76.9 years (SD, 16.6) and 71.5 years (SD 13.8), respectively, and more likely to have at least 1 functional limitation (71.5% vs. 55.5%). The most frequent admission diagnoses to HaH were UTIs, CAP, cellulitis, and CHF. HaH patients had a shorter hospitalization LOS (3.2 days) compared with the control group (5.5 days; 95% confidence interval [CI], –1.8 to –2.7 days). HaH patients were less likely to have 30-day all-cause hospital readmissions (8.6% vs. 15.6%; 95% CI, –12.9% to –1.1%) and 30-day ED revisits (5.8% vs. 11.7%) compared to controls. Analysis adjusted for age, sex, race, ethnicity, education, insurance type, physical function, general health, and admitting diagnosis found that HaH patients had lower odds of hospital readmission (odds ratio [OR], 0.43; 95% CI, 0.36-0.52) and lower odds of ED revisits (OR, 0.39; 95% CI, 0.31-0.49). HaH patients reported higher ratings for communication with nurses and physicians and communication about medicines when compared with controls; they were also more likely to report the highest rating for overall hospital care (68.8% vs. 45.3%). Scores for pain management were lower for HaH patients when compared with controls.
Conclusions. Patients receiving care through the HaH program were less likely to be readmitted at 30 days after hospital discharge, had lower hospital LOS and reported higher ratings of care when compared to patients receiving care in the hospital. The study demonstrated the potential benefits of the HaH model of care for adults who need inpatient hospitalization.
Commentary
This study adds to the literature on outcomes associated with HaH programs. The first study of the HaH model in the United States was published in 2005,1 and despite the early demonstration of its feasibility and outcomes in this and subsequent studies,2,3 HaH models have not been widely adopted, unlike in other countries with integrated health care systems.4 One of the primary reasons this model has not been adopted is the lack of a specific payment mechanism in Medicare fee for service for HaH. Implementation of the HaH program described in the current study was an effort funded by a CMS innovation award to test the effect of models of care with the potential of developing payment mechanisms that would support further dissemination of these models. The results from the current study were encouraging and have led to the Physician-Focused Payment Model Technical Advisory Committee’s unanimous recommendation to the U.S. Department of Health and Human Services for full implementation in 2017.
The current study does have certain limitations. It is not a randomized trial, and thus control group selection could be affected by selection bias. Also, the study was conducted in a single health system and thus may have limited generalizability. Nevertheless, this study was designed based on prior studies of HaH, including randomized and non-randomized studies, that have demonstrated benefits similar to the current study. The finding that HaH patients reported worse pain control than did patients hospitalized in the inpatient setting, where staff is available 24 hours a day, may suggest differences in care that is feasible at home versus in the inpatient setting. Finally, because it is a bundled program that includes both HaH and a post-discharge care transition program, it is unclear if the effects found in this evaluation can be attributed to specific components within the bundled program.
Applications for Clinical Practice
Patients, particularly older adults, may prefer to have hospital-level care delivered at home; clinicians may consider how HaH may allow patients to avoid potential hazards of hospitalization,5 such as inpatient falls, delirium, and other iatrogenic events. The HaH program is feasible and safe, and is associated with improved outcomes of care for patients.
—William W. Hung, MD, MPH
1. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
2. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital at home”. Med J Aust. 2012;197:512-519.
3. Mader SL, Medcraft MC, Joseph C, et al. Program at home: a Veteran Affairs healthcare program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56: 2317-2322.
4. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health Review of the hospital in the home program. Med J Aust. 2010;193:598-601.
5. Creditor MC. Hazards of hospitalization. Ann Intern Med. 1993;118:219-223.
1. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
2. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital at home”. Med J Aust. 2012;197:512-519.
3. Mader SL, Medcraft MC, Joseph C, et al. Program at home: a Veteran Affairs healthcare program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56: 2317-2322.
4. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health Review of the hospital in the home program. Med J Aust. 2010;193:598-601.
5. Creditor MC. Hazards of hospitalization. Ann Intern Med. 1993;118:219-223.
Effectiveness of Epinephrine in Out-of-Hospital Cardiac Arrest
Study Overview
Objective. To assess the safety and effectiveness of the use of epinephrine in out-of-hospital cardiac arrest patients.
Design. Randomized, double-blind placebo-controlled trial in the United Kingdom.
Setting and participants. Patients aged 16 years or older who had sustained an out-of-hospital cardiac arrest for which advanced life support was provided by trial-trained paramedics were eligible for inclusion. Exclusion criteria included apparent pregnancy, arrest from anaphylaxis or asthma, or the administration of epinephrine before the arrival of the trial-trained paramedic. In 1 of the 5 ambulance services, traumatic cardiac arrests were also excluded in accordance with local protocol.
Main outcome measures. The primary outcome was the rate of survival at 30 days. Secondary outcomes included rate of survival until hospital admission, length of stay in the hospital and intensive care unit (ICU), rates of survival at hospital discharge and at 3 months, and neurologic outcomes at hospital discharge and at 3 months.
Main results. Between December 2014 and October 2017, 10,623 patients were screened for eligibility in 5 National Health Service ambulance services in the United Kingdom. Of these, 8103 were eligible, and 8014 patients were assigned to either the epinephrine group (4015 patients) or the placebo group (3999 patients).
For the primary outcome, 130 patients (3.2%) in the epinephrine group were alive at 30 days in comparison to 94 patients (2.4%) in the placebo group (unadjusted odds ratio [OR] for survival, 1.39; 95% confidence interval [CI], 1.06-1.82; P = 0.02). The number needed to treat for a 30-day survival was 112 patients (95% CI, 63-500).
For the secondary outcomes, the epinephrine group had a higher survival until hospital admission: 947 patients (23.8%) as compared to 319 (8.0%) patients in the placebo group (unadjusted OR, 3.59). Otherwise, there were no difference between the 2 groups in the hospital and ICU LOS. There also was not a significant difference between the epinephrine group and the placebo group in the proportion of patients who survived until hospital discharge: 87 of 4007 patients (2.2%) in the epinephrine group and 74 of 3994 patients (1.9%) in the placebo group, with an unadjusted OR of 1.18 (95% CI, 0.85-1.61). Patients in the epinephrine group had a higher rate of severe neurologic impairment at discharge: 39 of 126 patients (31.0%) versus 16 of 90 patients (17.8%).
Conclusion. Among adults with out-of-hospital cardiac arrest, the use of epinephrine resulted in a higher rate of 30-day survival as compared with the use of placebo; however, there was no difference in the rate of a favorable neurologic outcome as more survivors in the epinephrine group had severe neurologic impairment.
Commentary
Epinephrine has been used as part of the resuscitation of patients with cardiac arrest since the 1960s. Epinephrine increases vasomotor tone during circulatory collapse, shunts more blood to the heart, and increases the likelihood of restoring spontaneous circulation.1 However, epinephrine also decreases microvascular blood flow and can result in long-term organ dysfunction or hypoperfusion of the heart and brain.2 The current study, the PARAMEDIC2 trial, by Perkins and colleagues is the largest randomized controlled trial to date to address the question of patient-centered benefit of the use of epinephrine during out-of-hospital cardiac arrest.
Similar to prior studies, patients who received epinephrine had a higher rate of 30-day survival than those who received placebo. However, there was no clear improvement in functional recovery among patients who survived, and the proportion of survivors with severe neurologic impairment was higher in the epinephrine group as compared to the placebo group. These results demonstrate that despite its ability to restore spontaneous circulation after out-of-hospital cardiac arrest, epinephrine produced only a small absolute increase in survival with worse functional recovery as compared with placebo.
One major limitation of this study is that the protocol did not control for or measure in-hospital treatments. In a prior study, the most common cause of in-hospital death was iatrogenic limitation of life support, which may result in the death of potentially viable patients.3 Another limitation of the study was the timing to administration of epinephrine. In the current study, paramedics administered the trial agent within a median of 21 minutes after the emergency call, which is a longer duration than previous out-of-hospital trials.4 In addition, this time to administration is much longer than that of in-hospital cardiac arrest, where epinephrine is administered a median of 3 minutes after resuscitation starts.5 Therefore, the results from this study cannot be extrapolated to patients with in-hospital cardiac arrest.
Applications for Clinical Practice
The current study by Perkins et al demonstrated the powerful effect of epinephrine in restoring spontaneous circulation after out-of-hospital cardiac arrest. However, epinephrine produced only a small absolute increase in survival with worse functional recovery, as compared to placebo. While further studies regarding dosage of epinephrine as well as administration based on the basis of cardiac rhythm are needed, we should question our tradition of using epinephrine in out-of-hospital cardiac arrest if meaningful neurological function is our priority.
—Ka Ming Gordon Ngai, MD, MPH, FACEP
1. Paradis NA, Martin GB, Rosenberg J, et al. The effect of standard- ad high-dose epinephrine on coronary perfusion pressure during prolonged cardiopulmonary resuscitation. JAMA. 1991;265:1139-1144.
2. Ristagno G, Sun S, Tang W, et al. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145-2149.
3. Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127-135.
4. Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711-1722.
5. Donnino MW, Salciccioli JD, Howell MD, et al. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ. 2014;348:g3028l.
Study Overview
Objective. To assess the safety and effectiveness of the use of epinephrine in out-of-hospital cardiac arrest patients.
Design. Randomized, double-blind placebo-controlled trial in the United Kingdom.
Setting and participants. Patients aged 16 years or older who had sustained an out-of-hospital cardiac arrest for which advanced life support was provided by trial-trained paramedics were eligible for inclusion. Exclusion criteria included apparent pregnancy, arrest from anaphylaxis or asthma, or the administration of epinephrine before the arrival of the trial-trained paramedic. In 1 of the 5 ambulance services, traumatic cardiac arrests were also excluded in accordance with local protocol.
Main outcome measures. The primary outcome was the rate of survival at 30 days. Secondary outcomes included rate of survival until hospital admission, length of stay in the hospital and intensive care unit (ICU), rates of survival at hospital discharge and at 3 months, and neurologic outcomes at hospital discharge and at 3 months.
Main results. Between December 2014 and October 2017, 10,623 patients were screened for eligibility in 5 National Health Service ambulance services in the United Kingdom. Of these, 8103 were eligible, and 8014 patients were assigned to either the epinephrine group (4015 patients) or the placebo group (3999 patients).
For the primary outcome, 130 patients (3.2%) in the epinephrine group were alive at 30 days in comparison to 94 patients (2.4%) in the placebo group (unadjusted odds ratio [OR] for survival, 1.39; 95% confidence interval [CI], 1.06-1.82; P = 0.02). The number needed to treat for a 30-day survival was 112 patients (95% CI, 63-500).
For the secondary outcomes, the epinephrine group had a higher survival until hospital admission: 947 patients (23.8%) as compared to 319 (8.0%) patients in the placebo group (unadjusted OR, 3.59). Otherwise, there were no difference between the 2 groups in the hospital and ICU LOS. There also was not a significant difference between the epinephrine group and the placebo group in the proportion of patients who survived until hospital discharge: 87 of 4007 patients (2.2%) in the epinephrine group and 74 of 3994 patients (1.9%) in the placebo group, with an unadjusted OR of 1.18 (95% CI, 0.85-1.61). Patients in the epinephrine group had a higher rate of severe neurologic impairment at discharge: 39 of 126 patients (31.0%) versus 16 of 90 patients (17.8%).
Conclusion. Among adults with out-of-hospital cardiac arrest, the use of epinephrine resulted in a higher rate of 30-day survival as compared with the use of placebo; however, there was no difference in the rate of a favorable neurologic outcome as more survivors in the epinephrine group had severe neurologic impairment.
Commentary
Epinephrine has been used as part of the resuscitation of patients with cardiac arrest since the 1960s. Epinephrine increases vasomotor tone during circulatory collapse, shunts more blood to the heart, and increases the likelihood of restoring spontaneous circulation.1 However, epinephrine also decreases microvascular blood flow and can result in long-term organ dysfunction or hypoperfusion of the heart and brain.2 The current study, the PARAMEDIC2 trial, by Perkins and colleagues is the largest randomized controlled trial to date to address the question of patient-centered benefit of the use of epinephrine during out-of-hospital cardiac arrest.
Similar to prior studies, patients who received epinephrine had a higher rate of 30-day survival than those who received placebo. However, there was no clear improvement in functional recovery among patients who survived, and the proportion of survivors with severe neurologic impairment was higher in the epinephrine group as compared to the placebo group. These results demonstrate that despite its ability to restore spontaneous circulation after out-of-hospital cardiac arrest, epinephrine produced only a small absolute increase in survival with worse functional recovery as compared with placebo.
One major limitation of this study is that the protocol did not control for or measure in-hospital treatments. In a prior study, the most common cause of in-hospital death was iatrogenic limitation of life support, which may result in the death of potentially viable patients.3 Another limitation of the study was the timing to administration of epinephrine. In the current study, paramedics administered the trial agent within a median of 21 minutes after the emergency call, which is a longer duration than previous out-of-hospital trials.4 In addition, this time to administration is much longer than that of in-hospital cardiac arrest, where epinephrine is administered a median of 3 minutes after resuscitation starts.5 Therefore, the results from this study cannot be extrapolated to patients with in-hospital cardiac arrest.
Applications for Clinical Practice
The current study by Perkins et al demonstrated the powerful effect of epinephrine in restoring spontaneous circulation after out-of-hospital cardiac arrest. However, epinephrine produced only a small absolute increase in survival with worse functional recovery, as compared to placebo. While further studies regarding dosage of epinephrine as well as administration based on the basis of cardiac rhythm are needed, we should question our tradition of using epinephrine in out-of-hospital cardiac arrest if meaningful neurological function is our priority.
—Ka Ming Gordon Ngai, MD, MPH, FACEP
Study Overview
Objective. To assess the safety and effectiveness of the use of epinephrine in out-of-hospital cardiac arrest patients.
Design. Randomized, double-blind placebo-controlled trial in the United Kingdom.
Setting and participants. Patients aged 16 years or older who had sustained an out-of-hospital cardiac arrest for which advanced life support was provided by trial-trained paramedics were eligible for inclusion. Exclusion criteria included apparent pregnancy, arrest from anaphylaxis or asthma, or the administration of epinephrine before the arrival of the trial-trained paramedic. In 1 of the 5 ambulance services, traumatic cardiac arrests were also excluded in accordance with local protocol.
Main outcome measures. The primary outcome was the rate of survival at 30 days. Secondary outcomes included rate of survival until hospital admission, length of stay in the hospital and intensive care unit (ICU), rates of survival at hospital discharge and at 3 months, and neurologic outcomes at hospital discharge and at 3 months.
Main results. Between December 2014 and October 2017, 10,623 patients were screened for eligibility in 5 National Health Service ambulance services in the United Kingdom. Of these, 8103 were eligible, and 8014 patients were assigned to either the epinephrine group (4015 patients) or the placebo group (3999 patients).
For the primary outcome, 130 patients (3.2%) in the epinephrine group were alive at 30 days in comparison to 94 patients (2.4%) in the placebo group (unadjusted odds ratio [OR] for survival, 1.39; 95% confidence interval [CI], 1.06-1.82; P = 0.02). The number needed to treat for a 30-day survival was 112 patients (95% CI, 63-500).
For the secondary outcomes, the epinephrine group had a higher survival until hospital admission: 947 patients (23.8%) as compared to 319 (8.0%) patients in the placebo group (unadjusted OR, 3.59). Otherwise, there were no difference between the 2 groups in the hospital and ICU LOS. There also was not a significant difference between the epinephrine group and the placebo group in the proportion of patients who survived until hospital discharge: 87 of 4007 patients (2.2%) in the epinephrine group and 74 of 3994 patients (1.9%) in the placebo group, with an unadjusted OR of 1.18 (95% CI, 0.85-1.61). Patients in the epinephrine group had a higher rate of severe neurologic impairment at discharge: 39 of 126 patients (31.0%) versus 16 of 90 patients (17.8%).
Conclusion. Among adults with out-of-hospital cardiac arrest, the use of epinephrine resulted in a higher rate of 30-day survival as compared with the use of placebo; however, there was no difference in the rate of a favorable neurologic outcome as more survivors in the epinephrine group had severe neurologic impairment.
Commentary
Epinephrine has been used as part of the resuscitation of patients with cardiac arrest since the 1960s. Epinephrine increases vasomotor tone during circulatory collapse, shunts more blood to the heart, and increases the likelihood of restoring spontaneous circulation.1 However, epinephrine also decreases microvascular blood flow and can result in long-term organ dysfunction or hypoperfusion of the heart and brain.2 The current study, the PARAMEDIC2 trial, by Perkins and colleagues is the largest randomized controlled trial to date to address the question of patient-centered benefit of the use of epinephrine during out-of-hospital cardiac arrest.
Similar to prior studies, patients who received epinephrine had a higher rate of 30-day survival than those who received placebo. However, there was no clear improvement in functional recovery among patients who survived, and the proportion of survivors with severe neurologic impairment was higher in the epinephrine group as compared to the placebo group. These results demonstrate that despite its ability to restore spontaneous circulation after out-of-hospital cardiac arrest, epinephrine produced only a small absolute increase in survival with worse functional recovery as compared with placebo.
One major limitation of this study is that the protocol did not control for or measure in-hospital treatments. In a prior study, the most common cause of in-hospital death was iatrogenic limitation of life support, which may result in the death of potentially viable patients.3 Another limitation of the study was the timing to administration of epinephrine. In the current study, paramedics administered the trial agent within a median of 21 minutes after the emergency call, which is a longer duration than previous out-of-hospital trials.4 In addition, this time to administration is much longer than that of in-hospital cardiac arrest, where epinephrine is administered a median of 3 minutes after resuscitation starts.5 Therefore, the results from this study cannot be extrapolated to patients with in-hospital cardiac arrest.
Applications for Clinical Practice
The current study by Perkins et al demonstrated the powerful effect of epinephrine in restoring spontaneous circulation after out-of-hospital cardiac arrest. However, epinephrine produced only a small absolute increase in survival with worse functional recovery, as compared to placebo. While further studies regarding dosage of epinephrine as well as administration based on the basis of cardiac rhythm are needed, we should question our tradition of using epinephrine in out-of-hospital cardiac arrest if meaningful neurological function is our priority.
—Ka Ming Gordon Ngai, MD, MPH, FACEP
1. Paradis NA, Martin GB, Rosenberg J, et al. The effect of standard- ad high-dose epinephrine on coronary perfusion pressure during prolonged cardiopulmonary resuscitation. JAMA. 1991;265:1139-1144.
2. Ristagno G, Sun S, Tang W, et al. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145-2149.
3. Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127-135.
4. Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711-1722.
5. Donnino MW, Salciccioli JD, Howell MD, et al. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ. 2014;348:g3028l.
1. Paradis NA, Martin GB, Rosenberg J, et al. The effect of standard- ad high-dose epinephrine on coronary perfusion pressure during prolonged cardiopulmonary resuscitation. JAMA. 1991;265:1139-1144.
2. Ristagno G, Sun S, Tang W, et al. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145-2149.
3. Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127-135.
4. Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711-1722.
5. Donnino MW, Salciccioli JD, Howell MD, et al. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ. 2014;348:g3028l.
Quality of Life After Treatment of Chronic Total Occlusions with Revascularization versus Optimal Medical Therapy
Study Overview
Objective. To compare the benefit of percutaneous coronary intervention (PCI) plus optimal medical therapy (OMT) versus OMT alone on the health status of patients with chronic total occlusions (CTOs).
Design. Multicenter, open-label, prospective randomized control trial.
Setting and participants. 396 patients with at least 1 CTO were assigned to PCI or OMT with a 2:1 randomization ratio.
Main outcome measures. The primary endpoint was the change in health status as assessed by the Seattle Angina Questionnaire (SAQ) between baseline and 12-month follow-up.
Main results. At 12 months, greater improvement of 3 SAQ domains was observed with PCI compared to OMT: angina frequency (5.23, 95% confidence interval [CI], 1.75-8.31, P = 0.0003), physical limitation (P = 0.02), and quality of life (6.62, 95% CI 1.78-11.46, P = 0.0007). More patients in the PCI group than in the OMT group had complete freedom from angina (71.6% vs. 57.8%, P = 0.008). There were no occurrences of periprocedural death or myocardial infarction.
Conclusion. Among patients with stable angina and CTO, PCI leads to significant health status improvement compared with OMT alone.
Commentary
CTOs are present in 15% to 25% of patients undergoing coronary angiogram1 and are associated with increased mortality.2 The benefits of successful CTO intervention observed in multiple large-scale registries include improvement in quality of life, left ventricular function, and survival as well as avoidance of coronary bypass surgery. The main indication for CTO intervention is improvement in quality of life,3 although this has not been confirmed by a randomized controlled trial comparing medical therapy to CTO-PCI.
Previous studies have assessed the health status benefits associated with CTO-PCI.4,5 Most recently, the OPEN CTO study showed significant improvement in health status in 1000 consecutive patients undergoing CTO-PCI in 12 experienced U.S. centers.6 Similarly, in a Canadian registry, revascularization of CTO was associated with greater health status benefit compared to medical therapy alone.4 However, these studies compared CTO-PCI success to failure, rather than to medical therapy.
In this context, Werner and colleagues investigated the value of PCI versus OMT for CTO by performing a well-designed randomized clinical trial in patients with CTO by assessing their health status with the SAQ.7 The SAQ is a 19-item questionnaire with a 4-week recall period that measures 5 domains of health status in patients with coronary artery disease (CAD).8,9 Scores in each domain range from 0 to 100, with higher scores indicating fewer symptoms and better quality of life. The SAQ has undergone extensive reliability and validity testing and is associated with long-term survival and health care utilization among patients with chronic CAD.10,11 At 12 months follow-up, patients who underwent CTO-PCI had greater improvement in SAQ subscales, including angina frequency and quality of life, reaching the pre-specified significance level of 0.01. There was also numerical improvement in physical limitation (P = 0.02)
The strengths of this current study include the randomized design and the careful treatment of non-CTO- PCI lesions before enrollment into the study. These non-CTO lesions were treated before the baseline health status assessment so that the additional health status benefit of non-CTO-PCI would not affect the results. This was one of multiple major limitations of the recently presented DECISION-CTO trial, as the non-CTO lesions were treated after the randomization and baseline assessment, leading to inaccurate comparison between medical therapy and CTO-PCI.12
Another interesting point of the current study is the patient selection. Since the treatment sites included were all expert centers in Europe, many patients who were referred to their institution for CTO-PCI were excluded from the study. For example, among the 1980 patients with screening log, 1381 were excluded because they were referred for CTO-PCI and 122 were excluded because they were “too symptomatic.” This suggests that the population studied were less symptomatic than the overall symptomatic CTO population from previous registries, as evidenced by about 40% of patients having Canadian Cardiovascular Society (CCS) class I/II angina at baseline. In the recent consecutively enrolled OPEN CTO registry, only 26% of patients reported CCS class I/II angina at baseline.6 These observations likely represent biases to the null, and thus one can reasonably speculate that the impact among unselected patients would be greater. Degree of baseline angina has been reported to be a predictor in patients with stable angina.13 Moreover, the degree of health status improvement is significantly larger in patients with refractory angina undergoing CTO- PCI.14
In this study, the success rate of CTO PCI was 83.1% at the initial attempt and 86.6% at the final attempt. The in-hospital complication rate was 2.9%, which included pericardial tamponade, vascular surgical repair, and need for blood transfusion. The success rate and complication rates were consistent with previous observational studies from expert centers.1,6
Applications for Clinical Practice
In patients presenting with stable angina with CTO, the health status improvement is larger with CTO-PCI plus medical therapy compared to medical therapy alone. CTO-PCI should be offered to symptomatic patients in conjunction with OMT.
—Taishi Hirai, MD, and J. Aaron Grantham, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
1. Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991-997.
2. Ramunddal T, Hoebers LP, Henriques JP, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv. 2016;9:1535-1544.
3. Grantham JA, Marso SP, Spertus J, et al. Chronic total occlusion angioplasty in the United States. JACC Cardiovasc Interv. 2009;2:479-486.
4. Wijeysundera HC, Norris C, Fefer P, et al. Relationship between initial treatment strategy and quality of life in patients with coronary chronic total occlusions. EuroIntervention. 2014;9:1165-1172.
5. Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: Results from the FlowCardia’s Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ Cardiovasc Qual Outcomes. 2010;3:284-290.
6. Sapontis J, Salisbury AC, Yeh RW, C et al. Early procedural and health status outcomes after chronic total occlusion angioplasty: a report from the OPEN-CTO registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovasc Interv. 2017;10:1523-1534.
7. Werner GS, Martin-Yuste V, Hildick-Smith D, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39:2484-2993.
8. Spertus JA, Winder JA, Dewhurst TA, et al. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240-1244.
9. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333-341.
10. Mozaffarian D, Bryson CL, Spertus JA, et al. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015-1022.
11. Spertus JA, Jones P, McDonell M, et al. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43-49.
12. Park S. Drug-eluting stent versus optimal medical therapy in patients with coronary chronic total occlusion: DECISION CTO randomized trial. Presented at the American College of Cardiology Annual Scientific Session (ACC 2017), Washington, DC, March 18, 2017.
13. Spertus JA, Salisbury AC, Jones PG, et al. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110:3789-3794.
14. Hirai T, Grantham JA, Gosch K, L et al. Quality of life in patients with refractory angina after chronic total occlusion angioplasty. J Am Coll Cardiol. 2018;72(13 supplement):TCT-79.
Study Overview
Objective. To compare the benefit of percutaneous coronary intervention (PCI) plus optimal medical therapy (OMT) versus OMT alone on the health status of patients with chronic total occlusions (CTOs).
Design. Multicenter, open-label, prospective randomized control trial.
Setting and participants. 396 patients with at least 1 CTO were assigned to PCI or OMT with a 2:1 randomization ratio.
Main outcome measures. The primary endpoint was the change in health status as assessed by the Seattle Angina Questionnaire (SAQ) between baseline and 12-month follow-up.
Main results. At 12 months, greater improvement of 3 SAQ domains was observed with PCI compared to OMT: angina frequency (5.23, 95% confidence interval [CI], 1.75-8.31, P = 0.0003), physical limitation (P = 0.02), and quality of life (6.62, 95% CI 1.78-11.46, P = 0.0007). More patients in the PCI group than in the OMT group had complete freedom from angina (71.6% vs. 57.8%, P = 0.008). There were no occurrences of periprocedural death or myocardial infarction.
Conclusion. Among patients with stable angina and CTO, PCI leads to significant health status improvement compared with OMT alone.
Commentary
CTOs are present in 15% to 25% of patients undergoing coronary angiogram1 and are associated with increased mortality.2 The benefits of successful CTO intervention observed in multiple large-scale registries include improvement in quality of life, left ventricular function, and survival as well as avoidance of coronary bypass surgery. The main indication for CTO intervention is improvement in quality of life,3 although this has not been confirmed by a randomized controlled trial comparing medical therapy to CTO-PCI.
Previous studies have assessed the health status benefits associated with CTO-PCI.4,5 Most recently, the OPEN CTO study showed significant improvement in health status in 1000 consecutive patients undergoing CTO-PCI in 12 experienced U.S. centers.6 Similarly, in a Canadian registry, revascularization of CTO was associated with greater health status benefit compared to medical therapy alone.4 However, these studies compared CTO-PCI success to failure, rather than to medical therapy.
In this context, Werner and colleagues investigated the value of PCI versus OMT for CTO by performing a well-designed randomized clinical trial in patients with CTO by assessing their health status with the SAQ.7 The SAQ is a 19-item questionnaire with a 4-week recall period that measures 5 domains of health status in patients with coronary artery disease (CAD).8,9 Scores in each domain range from 0 to 100, with higher scores indicating fewer symptoms and better quality of life. The SAQ has undergone extensive reliability and validity testing and is associated with long-term survival and health care utilization among patients with chronic CAD.10,11 At 12 months follow-up, patients who underwent CTO-PCI had greater improvement in SAQ subscales, including angina frequency and quality of life, reaching the pre-specified significance level of 0.01. There was also numerical improvement in physical limitation (P = 0.02)
The strengths of this current study include the randomized design and the careful treatment of non-CTO- PCI lesions before enrollment into the study. These non-CTO lesions were treated before the baseline health status assessment so that the additional health status benefit of non-CTO-PCI would not affect the results. This was one of multiple major limitations of the recently presented DECISION-CTO trial, as the non-CTO lesions were treated after the randomization and baseline assessment, leading to inaccurate comparison between medical therapy and CTO-PCI.12
Another interesting point of the current study is the patient selection. Since the treatment sites included were all expert centers in Europe, many patients who were referred to their institution for CTO-PCI were excluded from the study. For example, among the 1980 patients with screening log, 1381 were excluded because they were referred for CTO-PCI and 122 were excluded because they were “too symptomatic.” This suggests that the population studied were less symptomatic than the overall symptomatic CTO population from previous registries, as evidenced by about 40% of patients having Canadian Cardiovascular Society (CCS) class I/II angina at baseline. In the recent consecutively enrolled OPEN CTO registry, only 26% of patients reported CCS class I/II angina at baseline.6 These observations likely represent biases to the null, and thus one can reasonably speculate that the impact among unselected patients would be greater. Degree of baseline angina has been reported to be a predictor in patients with stable angina.13 Moreover, the degree of health status improvement is significantly larger in patients with refractory angina undergoing CTO- PCI.14
In this study, the success rate of CTO PCI was 83.1% at the initial attempt and 86.6% at the final attempt. The in-hospital complication rate was 2.9%, which included pericardial tamponade, vascular surgical repair, and need for blood transfusion. The success rate and complication rates were consistent with previous observational studies from expert centers.1,6
Applications for Clinical Practice
In patients presenting with stable angina with CTO, the health status improvement is larger with CTO-PCI plus medical therapy compared to medical therapy alone. CTO-PCI should be offered to symptomatic patients in conjunction with OMT.
—Taishi Hirai, MD, and J. Aaron Grantham, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
Study Overview
Objective. To compare the benefit of percutaneous coronary intervention (PCI) plus optimal medical therapy (OMT) versus OMT alone on the health status of patients with chronic total occlusions (CTOs).
Design. Multicenter, open-label, prospective randomized control trial.
Setting and participants. 396 patients with at least 1 CTO were assigned to PCI or OMT with a 2:1 randomization ratio.
Main outcome measures. The primary endpoint was the change in health status as assessed by the Seattle Angina Questionnaire (SAQ) between baseline and 12-month follow-up.
Main results. At 12 months, greater improvement of 3 SAQ domains was observed with PCI compared to OMT: angina frequency (5.23, 95% confidence interval [CI], 1.75-8.31, P = 0.0003), physical limitation (P = 0.02), and quality of life (6.62, 95% CI 1.78-11.46, P = 0.0007). More patients in the PCI group than in the OMT group had complete freedom from angina (71.6% vs. 57.8%, P = 0.008). There were no occurrences of periprocedural death or myocardial infarction.
Conclusion. Among patients with stable angina and CTO, PCI leads to significant health status improvement compared with OMT alone.
Commentary
CTOs are present in 15% to 25% of patients undergoing coronary angiogram1 and are associated with increased mortality.2 The benefits of successful CTO intervention observed in multiple large-scale registries include improvement in quality of life, left ventricular function, and survival as well as avoidance of coronary bypass surgery. The main indication for CTO intervention is improvement in quality of life,3 although this has not been confirmed by a randomized controlled trial comparing medical therapy to CTO-PCI.
Previous studies have assessed the health status benefits associated with CTO-PCI.4,5 Most recently, the OPEN CTO study showed significant improvement in health status in 1000 consecutive patients undergoing CTO-PCI in 12 experienced U.S. centers.6 Similarly, in a Canadian registry, revascularization of CTO was associated with greater health status benefit compared to medical therapy alone.4 However, these studies compared CTO-PCI success to failure, rather than to medical therapy.
In this context, Werner and colleagues investigated the value of PCI versus OMT for CTO by performing a well-designed randomized clinical trial in patients with CTO by assessing their health status with the SAQ.7 The SAQ is a 19-item questionnaire with a 4-week recall period that measures 5 domains of health status in patients with coronary artery disease (CAD).8,9 Scores in each domain range from 0 to 100, with higher scores indicating fewer symptoms and better quality of life. The SAQ has undergone extensive reliability and validity testing and is associated with long-term survival and health care utilization among patients with chronic CAD.10,11 At 12 months follow-up, patients who underwent CTO-PCI had greater improvement in SAQ subscales, including angina frequency and quality of life, reaching the pre-specified significance level of 0.01. There was also numerical improvement in physical limitation (P = 0.02)
The strengths of this current study include the randomized design and the careful treatment of non-CTO- PCI lesions before enrollment into the study. These non-CTO lesions were treated before the baseline health status assessment so that the additional health status benefit of non-CTO-PCI would not affect the results. This was one of multiple major limitations of the recently presented DECISION-CTO trial, as the non-CTO lesions were treated after the randomization and baseline assessment, leading to inaccurate comparison between medical therapy and CTO-PCI.12
Another interesting point of the current study is the patient selection. Since the treatment sites included were all expert centers in Europe, many patients who were referred to their institution for CTO-PCI were excluded from the study. For example, among the 1980 patients with screening log, 1381 were excluded because they were referred for CTO-PCI and 122 were excluded because they were “too symptomatic.” This suggests that the population studied were less symptomatic than the overall symptomatic CTO population from previous registries, as evidenced by about 40% of patients having Canadian Cardiovascular Society (CCS) class I/II angina at baseline. In the recent consecutively enrolled OPEN CTO registry, only 26% of patients reported CCS class I/II angina at baseline.6 These observations likely represent biases to the null, and thus one can reasonably speculate that the impact among unselected patients would be greater. Degree of baseline angina has been reported to be a predictor in patients with stable angina.13 Moreover, the degree of health status improvement is significantly larger in patients with refractory angina undergoing CTO- PCI.14
In this study, the success rate of CTO PCI was 83.1% at the initial attempt and 86.6% at the final attempt. The in-hospital complication rate was 2.9%, which included pericardial tamponade, vascular surgical repair, and need for blood transfusion. The success rate and complication rates were consistent with previous observational studies from expert centers.1,6
Applications for Clinical Practice
In patients presenting with stable angina with CTO, the health status improvement is larger with CTO-PCI plus medical therapy compared to medical therapy alone. CTO-PCI should be offered to symptomatic patients in conjunction with OMT.
—Taishi Hirai, MD, and J. Aaron Grantham, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
1. Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991-997.
2. Ramunddal T, Hoebers LP, Henriques JP, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv. 2016;9:1535-1544.
3. Grantham JA, Marso SP, Spertus J, et al. Chronic total occlusion angioplasty in the United States. JACC Cardiovasc Interv. 2009;2:479-486.
4. Wijeysundera HC, Norris C, Fefer P, et al. Relationship between initial treatment strategy and quality of life in patients with coronary chronic total occlusions. EuroIntervention. 2014;9:1165-1172.
5. Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: Results from the FlowCardia’s Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ Cardiovasc Qual Outcomes. 2010;3:284-290.
6. Sapontis J, Salisbury AC, Yeh RW, C et al. Early procedural and health status outcomes after chronic total occlusion angioplasty: a report from the OPEN-CTO registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovasc Interv. 2017;10:1523-1534.
7. Werner GS, Martin-Yuste V, Hildick-Smith D, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39:2484-2993.
8. Spertus JA, Winder JA, Dewhurst TA, et al. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240-1244.
9. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333-341.
10. Mozaffarian D, Bryson CL, Spertus JA, et al. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015-1022.
11. Spertus JA, Jones P, McDonell M, et al. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43-49.
12. Park S. Drug-eluting stent versus optimal medical therapy in patients with coronary chronic total occlusion: DECISION CTO randomized trial. Presented at the American College of Cardiology Annual Scientific Session (ACC 2017), Washington, DC, March 18, 2017.
13. Spertus JA, Salisbury AC, Jones PG, et al. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110:3789-3794.
14. Hirai T, Grantham JA, Gosch K, L et al. Quality of life in patients with refractory angina after chronic total occlusion angioplasty. J Am Coll Cardiol. 2018;72(13 supplement):TCT-79.
1. Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991-997.
2. Ramunddal T, Hoebers LP, Henriques JP, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv. 2016;9:1535-1544.
3. Grantham JA, Marso SP, Spertus J, et al. Chronic total occlusion angioplasty in the United States. JACC Cardiovasc Interv. 2009;2:479-486.
4. Wijeysundera HC, Norris C, Fefer P, et al. Relationship between initial treatment strategy and quality of life in patients with coronary chronic total occlusions. EuroIntervention. 2014;9:1165-1172.
5. Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: Results from the FlowCardia’s Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ Cardiovasc Qual Outcomes. 2010;3:284-290.
6. Sapontis J, Salisbury AC, Yeh RW, C et al. Early procedural and health status outcomes after chronic total occlusion angioplasty: a report from the OPEN-CTO registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovasc Interv. 2017;10:1523-1534.
7. Werner GS, Martin-Yuste V, Hildick-Smith D, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39:2484-2993.
8. Spertus JA, Winder JA, Dewhurst TA, et al. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240-1244.
9. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333-341.
10. Mozaffarian D, Bryson CL, Spertus JA, et al. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015-1022.
11. Spertus JA, Jones P, McDonell M, et al. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43-49.
12. Park S. Drug-eluting stent versus optimal medical therapy in patients with coronary chronic total occlusion: DECISION CTO randomized trial. Presented at the American College of Cardiology Annual Scientific Session (ACC 2017), Washington, DC, March 18, 2017.
13. Spertus JA, Salisbury AC, Jones PG, et al. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110:3789-3794.
14. Hirai T, Grantham JA, Gosch K, L et al. Quality of life in patients with refractory angina after chronic total occlusion angioplasty. J Am Coll Cardiol. 2018;72(13 supplement):TCT-79.
Prescription Drug Benefits and Survival in Myeloma Among Medicare Beneficiaries
Study Overview
Objective. To investigate the relationship between prescription drug coverage, receipt of active myeloma therapy, and overall survival (OS) among Medicare beneficiaries with multiple myeloma.
Design. Case-control and retrospective cohort archival data research.
Setting and participants. Authors examined SEER-Medicare registry and extracted patients with histologically confirmed multiple myeloma diagnosed in the period 2006 to 2011. Availability of complete Medicare part A/B claims from 1 year before diagnosis until December 2013 was required for analysis. Patients with Medicare advantage or managed care plans did not have claims data available and hence were excluded. Beneficiaries with a diagnosis of diffuse large B-cell lymphoma (DLBCL), who typically receive parenteral drugs for lymphoma therapy, were used as a control cohort.
Main outcome measures. Association between prescription drug coverage status and OS was the primary outcome measure of interest. Authors reported 3-year restricted survival time (RMST) ratios to compare OS among the beneficiaries with different prescription drug coverages. Receipt of active myeloma therapy among beneficiaries was also studied. Relative risk, adjusting for patient and disease-related characteristics, was reported to examine receipt of active myeloma therapy.
Results. Records of 9755 Medicare beneficiaries were evaluated. Of these, 1460 (15%) had no prescription coverage at diagnosis, 3283 (34%) had part D plan prescription benefits, 3607 (37%) had sponsored prescription coverage through an employer, federal employer, or veterans plan, and 1405 (14%) had a Medicaid prescription plan. Beneficiaries without coverage had fewer comorbidities, including anemia, neuropathy, or renal disease, than those with part D prescription coverage or Medicaid. Of those without any prescription drug coverage, 41% obtained prescription plan coverage after diagnosis of myeloma by the following January. Conversely, only 19% of patients with DLBCL and no coverage obtained a prescription plan.
Patients with myeloma were followed for 4.9 years and median survival was 2.3 years, with a 3-year OS rate of 43.1% (95% confidence interval [CI], 42.1%-44.1%). Relative to the group without coverage, survival was 16% longer in the Medicare part D group and sponsored plan group (RMST 1.16; 95% CI, 1.12-1.21). Medicaid/Medicare dual beneficiaries had worse OS in both myeloma and DLBCL consistent with poor performance status and unfavorable baseline comorbidities. However, among patients with myeloma, Medicaid/Medicare dual beneficiaries had better survival (RMST 1.08; 95% CI, 1.03-1.13) compared to the group without coverage. There was no difference in OS for those with or without prescription drug coverage in the DLBCL cohort.
There were significant differences in treatment of myeloma based on types of prescription drug coverage. Due to increasing use of bortezomib following its approval by the U.S. Food and Drug Administration (FDA), parenteral chemotherapy use doubled from 24% to 48% from 2006 to 2011, and utility of active myeloma care increased from 88% to 91%. Medicare part D plan enrollees were 6% more likely to receive active myeloma care, and both Medicaid group and sponsored plan group beneficiaries were equally likely to receive active myeloma care compared to beneficiaries without prescription coverage. Medicaid enrollees were less likely to receive parenteral therapy.
Conclusion. Medicare beneficiaries with prescription drug coverage and multiple myeloma are more likely to receive myeloma therapy and have longer OS compared to those without prescription drug coverage.
Commentary
First-line therapy of multiple myeloma has evolved over the past 2 decades. Parenteral agents such as vincristine, adriamycin, dexamethasone, and cyclophosphamide and oral therapy with melphalan and prednisone were the mainstay of treatment in the past. In the past decade, the arrival of oral therapy using thalidomide or lenalidomide and parenteral therapy using bortezomib has increased OS in patients with myeloma. Most recently, a combination of lenalidomide, bortezomib, and dexamethasone has emerged as one of the frontline therapies of choice.1 Incorporation of bortezomib or an oral immunomodulatory drug is almost universal in first-line therapy.
Oral antineoplastic therapy is increasingly being approved by the FDA and being utilized in the community. During the period 2016-2018, more than half the new FDA-approved oncology drugs were in oral formulation.2 As such, access to these agents is crucial in cancer therapy. The cost of oral therapy in patients without prescription drug coverage is sometimes more than $10,000 per month, which represents a significant impediment to its adoption. Forty-three states and Washington, DC, have enacted drug parity laws that require patients to pay no more for an oral cancer treatment than they would for an infusion. However, currently there is no such federal law, and Medicare beneficiaries must participate either through part D, state Medicaid, or a sponsored program to obtain prescription drug coverage. Despite being enrolled in part D, many beneficiaries fall into the “doughnut hole” (the requirement of Part D beneficiaries with high prescription drug expenses to pay more once the total cost of their medicines reaches a certain threshold) for prescription drugs at the time of need. From 2019 onward, enrollees will see significant, yet sometimes still insufficient, coverage benefits due to ending of the doughnut hole.3 Only a very limited number of oral chemotherapy agents are covered through Medicare part B, and of those covered, only oral melphalan is used for myeloma.
The authors have acknowledged multiple limitations of their investigation, including possible unobserved clinical differences between beneficiaries. SEER-Medicare registry has limitations in obtaining individual level data and may not contain specific results of cytogenetics, laboratory risk markers, and response to therapy, which are important to determine overall outcome. A prospective evaluation may be more suitable to assess these variables independently or through a multivariate analysis in determining receipt of therapy on OS, although such a study is currently not feasible.
The indicator of active myeloma care was defined as 2 or more outpatient physician visits or receipt of parenteral chemotherapy. This definition is somewhat suboptimal, as often patients with myeloma are under surveillance and may not necessarily be receiving active treatment. Moreover, the exact prescription pattern of lenalidomide, the most active first-line oral therapy, could not be captured from this retrospective registry review. Therefore, definitive conclusions regarding use of lenalidomide and thalidomide and receipt of therapy in this population cannot be made.
A significant improvement in OS has been established using maintenance lenalidomide following high-dose chemotherapy and stem cell transplantation.4 Only 5% of this study population received stem cell transplantation. This may be due to a median age of 77 years at diagnosis in the group studied, higher than the 66 to 70 years previously published.5 Stem cell transplantation is now commonly being used even in the older population. The 3-year survival of 83% following stem cell transplantation in myeloma patients aged 75 to 84 years was nearly identical to that of the younger population.6 Since stem cell transplantation is feasible in older Medicare beneficiaries and maintenance lenalidomide for 2 years following transplant improves survival, the option of providing maintenance therapy with oral lenalidomide must be made available to Medicare beneficiaries. Due to a very limited use of transplantation in this study, the impact of oral lenalidomide maintenance in OS cannot be judged.
Of the patients reviewed in this study, 6% had a listed diagnosis of plasmacytoma. These individuals typically are treated with radiation therapy only. It is unclear if these patients also received any systemic myeloma therapy or if they ever progressed to myeloma. Availability of prescription drug coverage may not be relevant to this group. Also, the authors reported that part D participants were less likely to receive classic cytotoxic chemotherapy. This may be somewhat irrelevant in Medicare beneficiaries with a median age of 77 years for current practice, as frontline induction with old classic cytotoxic chemotherapy is less commonly used in this population.
Investigators have appropriately recognized a lack of ability to discern whether inferior survival in the group without prescription drug coverage was the result of not receiving therapy at all or inability to receive oral immunomodulatory drugs. There would have been little reason for not proceeding to parenteral therapy. As noted, 41% of beneficiaries without coverage at diagnosis subsequently obtained coverage but continued to have significantly worse survival. Cause of death, including whether related to myeloma, was not reported. The authors suggest that early separation of survival curves could therefore be reflective of suboptimal first-line therapy that lacked oral immunomodulatory drugs. During the study period 2006-2011, first-line use of lenalidomide was common.
Median survival of patients with myeloma in this study was only 27 months. According to the American Cancer Society, in 2018 median survival for stage I myeloma has not been reached, stage II myeloma is 83 months, and stage III myeloma is 43 months. A robust and dynamic landscape in myeloma therapy prevents a clear attribution to individual agents, whether oral or parenteral, in improving OS. Thus, 3-year RMST, while appropriate for 2006-2011, may not be relevant today.
Applications for Clinical Practice
The oncology community routinely encounters difficulty in initiating therapy using oral agents rapidly after diagnosis of myeloma. The retrospective data analyzed in the current study suggests that delay in initiating or unavailability of oral agents may adversely impact OS. The common approach of initiating parenteral therapy while awaiting approvals from payers or charity programs and subsequently adding oral therapy when available has not been studied in assessing OS. The oncology community should initiate plans to obtain prescription drug coverage through either Medicare part D, Medicaid, a sponsored plan, or financial assistance charity programs as soon as possible after diagnosis of myeloma. Moreover, continuation of these prescription drug plans should be strongly considered throughout the course of myeloma, as subsequent lines of treatment will quite likely involve other active and approved oral agents, such as pomalidomide, ixazomib, and panobinostat, besides other supportive therapy.
One of the mechanisms to obtain prescription drug coverage includes enrollment in state Medicaid programs for those who are eligible. Currently, 17 states have not yet adopted Medicaid expansion under the Affordable Care Act. Expansion of Medicaid in these states could increase availability of prescription drug benefits. In this study, 15.8% of Medicare and Medicaid dual enrollees with access to oral agents at low or no cost did not receive myeloma care, slightly higher than the 13.1% with no prescription drug coverage. Lower utilization in this population may be explained based on differences in comorbidities or socioeconomic conditions rather than availability of a prescription plan.
The incidence of myeloma is expected to be higher in Medicare beneficiaries, and according to one estimate, in 2030 and beyond nearly 75% of diagnosed myeloma patients will be aged 64 to 84 years, an increase from nearly 66% today.7 Changing demographics, increasing oral therapy options, and patient convenience demand attention to providing prescription drug coverage to all Medicare beneficiaries. This study lends support to that demand.
—Rakesh Gaur, MD, MPH, FACP, Cancer and Blood Center at Kansas Institute of Medicine, Lenexa, KS
1. Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomized, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
2. U.S. Food and Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications. www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm. Accessed October 11, 2018.
3. Dusetzina SB, Keating NL. Mind the gap: Why closing the doughnut hole is insufficient for increasing Medicare beneficiary access to oral chemotherapy. J Clin Oncol. 2016;34:375-380.
4. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279-3289.
5. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33.
6. Dong N, McKiernan P, Samuel D, et al. Autologous stem cell transplantation in multiple myeloma patients over age 75 [abstract]. J Clin Oncol. 2018;36(suppl): 8025.
7. Rosenberg PS, Barker KA, Anderson WF. Future distribution of multiple myeloma in the United States by sex, age, and race/ethnicity. Blood. 2015;125:410–412.
Study Overview
Objective. To investigate the relationship between prescription drug coverage, receipt of active myeloma therapy, and overall survival (OS) among Medicare beneficiaries with multiple myeloma.
Design. Case-control and retrospective cohort archival data research.
Setting and participants. Authors examined SEER-Medicare registry and extracted patients with histologically confirmed multiple myeloma diagnosed in the period 2006 to 2011. Availability of complete Medicare part A/B claims from 1 year before diagnosis until December 2013 was required for analysis. Patients with Medicare advantage or managed care plans did not have claims data available and hence were excluded. Beneficiaries with a diagnosis of diffuse large B-cell lymphoma (DLBCL), who typically receive parenteral drugs for lymphoma therapy, were used as a control cohort.
Main outcome measures. Association between prescription drug coverage status and OS was the primary outcome measure of interest. Authors reported 3-year restricted survival time (RMST) ratios to compare OS among the beneficiaries with different prescription drug coverages. Receipt of active myeloma therapy among beneficiaries was also studied. Relative risk, adjusting for patient and disease-related characteristics, was reported to examine receipt of active myeloma therapy.
Results. Records of 9755 Medicare beneficiaries were evaluated. Of these, 1460 (15%) had no prescription coverage at diagnosis, 3283 (34%) had part D plan prescription benefits, 3607 (37%) had sponsored prescription coverage through an employer, federal employer, or veterans plan, and 1405 (14%) had a Medicaid prescription plan. Beneficiaries without coverage had fewer comorbidities, including anemia, neuropathy, or renal disease, than those with part D prescription coverage or Medicaid. Of those without any prescription drug coverage, 41% obtained prescription plan coverage after diagnosis of myeloma by the following January. Conversely, only 19% of patients with DLBCL and no coverage obtained a prescription plan.
Patients with myeloma were followed for 4.9 years and median survival was 2.3 years, with a 3-year OS rate of 43.1% (95% confidence interval [CI], 42.1%-44.1%). Relative to the group without coverage, survival was 16% longer in the Medicare part D group and sponsored plan group (RMST 1.16; 95% CI, 1.12-1.21). Medicaid/Medicare dual beneficiaries had worse OS in both myeloma and DLBCL consistent with poor performance status and unfavorable baseline comorbidities. However, among patients with myeloma, Medicaid/Medicare dual beneficiaries had better survival (RMST 1.08; 95% CI, 1.03-1.13) compared to the group without coverage. There was no difference in OS for those with or without prescription drug coverage in the DLBCL cohort.
There were significant differences in treatment of myeloma based on types of prescription drug coverage. Due to increasing use of bortezomib following its approval by the U.S. Food and Drug Administration (FDA), parenteral chemotherapy use doubled from 24% to 48% from 2006 to 2011, and utility of active myeloma care increased from 88% to 91%. Medicare part D plan enrollees were 6% more likely to receive active myeloma care, and both Medicaid group and sponsored plan group beneficiaries were equally likely to receive active myeloma care compared to beneficiaries without prescription coverage. Medicaid enrollees were less likely to receive parenteral therapy.
Conclusion. Medicare beneficiaries with prescription drug coverage and multiple myeloma are more likely to receive myeloma therapy and have longer OS compared to those without prescription drug coverage.
Commentary
First-line therapy of multiple myeloma has evolved over the past 2 decades. Parenteral agents such as vincristine, adriamycin, dexamethasone, and cyclophosphamide and oral therapy with melphalan and prednisone were the mainstay of treatment in the past. In the past decade, the arrival of oral therapy using thalidomide or lenalidomide and parenteral therapy using bortezomib has increased OS in patients with myeloma. Most recently, a combination of lenalidomide, bortezomib, and dexamethasone has emerged as one of the frontline therapies of choice.1 Incorporation of bortezomib or an oral immunomodulatory drug is almost universal in first-line therapy.
Oral antineoplastic therapy is increasingly being approved by the FDA and being utilized in the community. During the period 2016-2018, more than half the new FDA-approved oncology drugs were in oral formulation.2 As such, access to these agents is crucial in cancer therapy. The cost of oral therapy in patients without prescription drug coverage is sometimes more than $10,000 per month, which represents a significant impediment to its adoption. Forty-three states and Washington, DC, have enacted drug parity laws that require patients to pay no more for an oral cancer treatment than they would for an infusion. However, currently there is no such federal law, and Medicare beneficiaries must participate either through part D, state Medicaid, or a sponsored program to obtain prescription drug coverage. Despite being enrolled in part D, many beneficiaries fall into the “doughnut hole” (the requirement of Part D beneficiaries with high prescription drug expenses to pay more once the total cost of their medicines reaches a certain threshold) for prescription drugs at the time of need. From 2019 onward, enrollees will see significant, yet sometimes still insufficient, coverage benefits due to ending of the doughnut hole.3 Only a very limited number of oral chemotherapy agents are covered through Medicare part B, and of those covered, only oral melphalan is used for myeloma.
The authors have acknowledged multiple limitations of their investigation, including possible unobserved clinical differences between beneficiaries. SEER-Medicare registry has limitations in obtaining individual level data and may not contain specific results of cytogenetics, laboratory risk markers, and response to therapy, which are important to determine overall outcome. A prospective evaluation may be more suitable to assess these variables independently or through a multivariate analysis in determining receipt of therapy on OS, although such a study is currently not feasible.
The indicator of active myeloma care was defined as 2 or more outpatient physician visits or receipt of parenteral chemotherapy. This definition is somewhat suboptimal, as often patients with myeloma are under surveillance and may not necessarily be receiving active treatment. Moreover, the exact prescription pattern of lenalidomide, the most active first-line oral therapy, could not be captured from this retrospective registry review. Therefore, definitive conclusions regarding use of lenalidomide and thalidomide and receipt of therapy in this population cannot be made.
A significant improvement in OS has been established using maintenance lenalidomide following high-dose chemotherapy and stem cell transplantation.4 Only 5% of this study population received stem cell transplantation. This may be due to a median age of 77 years at diagnosis in the group studied, higher than the 66 to 70 years previously published.5 Stem cell transplantation is now commonly being used even in the older population. The 3-year survival of 83% following stem cell transplantation in myeloma patients aged 75 to 84 years was nearly identical to that of the younger population.6 Since stem cell transplantation is feasible in older Medicare beneficiaries and maintenance lenalidomide for 2 years following transplant improves survival, the option of providing maintenance therapy with oral lenalidomide must be made available to Medicare beneficiaries. Due to a very limited use of transplantation in this study, the impact of oral lenalidomide maintenance in OS cannot be judged.
Of the patients reviewed in this study, 6% had a listed diagnosis of plasmacytoma. These individuals typically are treated with radiation therapy only. It is unclear if these patients also received any systemic myeloma therapy or if they ever progressed to myeloma. Availability of prescription drug coverage may not be relevant to this group. Also, the authors reported that part D participants were less likely to receive classic cytotoxic chemotherapy. This may be somewhat irrelevant in Medicare beneficiaries with a median age of 77 years for current practice, as frontline induction with old classic cytotoxic chemotherapy is less commonly used in this population.
Investigators have appropriately recognized a lack of ability to discern whether inferior survival in the group without prescription drug coverage was the result of not receiving therapy at all or inability to receive oral immunomodulatory drugs. There would have been little reason for not proceeding to parenteral therapy. As noted, 41% of beneficiaries without coverage at diagnosis subsequently obtained coverage but continued to have significantly worse survival. Cause of death, including whether related to myeloma, was not reported. The authors suggest that early separation of survival curves could therefore be reflective of suboptimal first-line therapy that lacked oral immunomodulatory drugs. During the study period 2006-2011, first-line use of lenalidomide was common.
Median survival of patients with myeloma in this study was only 27 months. According to the American Cancer Society, in 2018 median survival for stage I myeloma has not been reached, stage II myeloma is 83 months, and stage III myeloma is 43 months. A robust and dynamic landscape in myeloma therapy prevents a clear attribution to individual agents, whether oral or parenteral, in improving OS. Thus, 3-year RMST, while appropriate for 2006-2011, may not be relevant today.
Applications for Clinical Practice
The oncology community routinely encounters difficulty in initiating therapy using oral agents rapidly after diagnosis of myeloma. The retrospective data analyzed in the current study suggests that delay in initiating or unavailability of oral agents may adversely impact OS. The common approach of initiating parenteral therapy while awaiting approvals from payers or charity programs and subsequently adding oral therapy when available has not been studied in assessing OS. The oncology community should initiate plans to obtain prescription drug coverage through either Medicare part D, Medicaid, a sponsored plan, or financial assistance charity programs as soon as possible after diagnosis of myeloma. Moreover, continuation of these prescription drug plans should be strongly considered throughout the course of myeloma, as subsequent lines of treatment will quite likely involve other active and approved oral agents, such as pomalidomide, ixazomib, and panobinostat, besides other supportive therapy.
One of the mechanisms to obtain prescription drug coverage includes enrollment in state Medicaid programs for those who are eligible. Currently, 17 states have not yet adopted Medicaid expansion under the Affordable Care Act. Expansion of Medicaid in these states could increase availability of prescription drug benefits. In this study, 15.8% of Medicare and Medicaid dual enrollees with access to oral agents at low or no cost did not receive myeloma care, slightly higher than the 13.1% with no prescription drug coverage. Lower utilization in this population may be explained based on differences in comorbidities or socioeconomic conditions rather than availability of a prescription plan.
The incidence of myeloma is expected to be higher in Medicare beneficiaries, and according to one estimate, in 2030 and beyond nearly 75% of diagnosed myeloma patients will be aged 64 to 84 years, an increase from nearly 66% today.7 Changing demographics, increasing oral therapy options, and patient convenience demand attention to providing prescription drug coverage to all Medicare beneficiaries. This study lends support to that demand.
—Rakesh Gaur, MD, MPH, FACP, Cancer and Blood Center at Kansas Institute of Medicine, Lenexa, KS
Study Overview
Objective. To investigate the relationship between prescription drug coverage, receipt of active myeloma therapy, and overall survival (OS) among Medicare beneficiaries with multiple myeloma.
Design. Case-control and retrospective cohort archival data research.
Setting and participants. Authors examined SEER-Medicare registry and extracted patients with histologically confirmed multiple myeloma diagnosed in the period 2006 to 2011. Availability of complete Medicare part A/B claims from 1 year before diagnosis until December 2013 was required for analysis. Patients with Medicare advantage or managed care plans did not have claims data available and hence were excluded. Beneficiaries with a diagnosis of diffuse large B-cell lymphoma (DLBCL), who typically receive parenteral drugs for lymphoma therapy, were used as a control cohort.
Main outcome measures. Association between prescription drug coverage status and OS was the primary outcome measure of interest. Authors reported 3-year restricted survival time (RMST) ratios to compare OS among the beneficiaries with different prescription drug coverages. Receipt of active myeloma therapy among beneficiaries was also studied. Relative risk, adjusting for patient and disease-related characteristics, was reported to examine receipt of active myeloma therapy.
Results. Records of 9755 Medicare beneficiaries were evaluated. Of these, 1460 (15%) had no prescription coverage at diagnosis, 3283 (34%) had part D plan prescription benefits, 3607 (37%) had sponsored prescription coverage through an employer, federal employer, or veterans plan, and 1405 (14%) had a Medicaid prescription plan. Beneficiaries without coverage had fewer comorbidities, including anemia, neuropathy, or renal disease, than those with part D prescription coverage or Medicaid. Of those without any prescription drug coverage, 41% obtained prescription plan coverage after diagnosis of myeloma by the following January. Conversely, only 19% of patients with DLBCL and no coverage obtained a prescription plan.
Patients with myeloma were followed for 4.9 years and median survival was 2.3 years, with a 3-year OS rate of 43.1% (95% confidence interval [CI], 42.1%-44.1%). Relative to the group without coverage, survival was 16% longer in the Medicare part D group and sponsored plan group (RMST 1.16; 95% CI, 1.12-1.21). Medicaid/Medicare dual beneficiaries had worse OS in both myeloma and DLBCL consistent with poor performance status and unfavorable baseline comorbidities. However, among patients with myeloma, Medicaid/Medicare dual beneficiaries had better survival (RMST 1.08; 95% CI, 1.03-1.13) compared to the group without coverage. There was no difference in OS for those with or without prescription drug coverage in the DLBCL cohort.
There were significant differences in treatment of myeloma based on types of prescription drug coverage. Due to increasing use of bortezomib following its approval by the U.S. Food and Drug Administration (FDA), parenteral chemotherapy use doubled from 24% to 48% from 2006 to 2011, and utility of active myeloma care increased from 88% to 91%. Medicare part D plan enrollees were 6% more likely to receive active myeloma care, and both Medicaid group and sponsored plan group beneficiaries were equally likely to receive active myeloma care compared to beneficiaries without prescription coverage. Medicaid enrollees were less likely to receive parenteral therapy.
Conclusion. Medicare beneficiaries with prescription drug coverage and multiple myeloma are more likely to receive myeloma therapy and have longer OS compared to those without prescription drug coverage.
Commentary
First-line therapy of multiple myeloma has evolved over the past 2 decades. Parenteral agents such as vincristine, adriamycin, dexamethasone, and cyclophosphamide and oral therapy with melphalan and prednisone were the mainstay of treatment in the past. In the past decade, the arrival of oral therapy using thalidomide or lenalidomide and parenteral therapy using bortezomib has increased OS in patients with myeloma. Most recently, a combination of lenalidomide, bortezomib, and dexamethasone has emerged as one of the frontline therapies of choice.1 Incorporation of bortezomib or an oral immunomodulatory drug is almost universal in first-line therapy.
Oral antineoplastic therapy is increasingly being approved by the FDA and being utilized in the community. During the period 2016-2018, more than half the new FDA-approved oncology drugs were in oral formulation.2 As such, access to these agents is crucial in cancer therapy. The cost of oral therapy in patients without prescription drug coverage is sometimes more than $10,000 per month, which represents a significant impediment to its adoption. Forty-three states and Washington, DC, have enacted drug parity laws that require patients to pay no more for an oral cancer treatment than they would for an infusion. However, currently there is no such federal law, and Medicare beneficiaries must participate either through part D, state Medicaid, or a sponsored program to obtain prescription drug coverage. Despite being enrolled in part D, many beneficiaries fall into the “doughnut hole” (the requirement of Part D beneficiaries with high prescription drug expenses to pay more once the total cost of their medicines reaches a certain threshold) for prescription drugs at the time of need. From 2019 onward, enrollees will see significant, yet sometimes still insufficient, coverage benefits due to ending of the doughnut hole.3 Only a very limited number of oral chemotherapy agents are covered through Medicare part B, and of those covered, only oral melphalan is used for myeloma.
The authors have acknowledged multiple limitations of their investigation, including possible unobserved clinical differences between beneficiaries. SEER-Medicare registry has limitations in obtaining individual level data and may not contain specific results of cytogenetics, laboratory risk markers, and response to therapy, which are important to determine overall outcome. A prospective evaluation may be more suitable to assess these variables independently or through a multivariate analysis in determining receipt of therapy on OS, although such a study is currently not feasible.
The indicator of active myeloma care was defined as 2 or more outpatient physician visits or receipt of parenteral chemotherapy. This definition is somewhat suboptimal, as often patients with myeloma are under surveillance and may not necessarily be receiving active treatment. Moreover, the exact prescription pattern of lenalidomide, the most active first-line oral therapy, could not be captured from this retrospective registry review. Therefore, definitive conclusions regarding use of lenalidomide and thalidomide and receipt of therapy in this population cannot be made.
A significant improvement in OS has been established using maintenance lenalidomide following high-dose chemotherapy and stem cell transplantation.4 Only 5% of this study population received stem cell transplantation. This may be due to a median age of 77 years at diagnosis in the group studied, higher than the 66 to 70 years previously published.5 Stem cell transplantation is now commonly being used even in the older population. The 3-year survival of 83% following stem cell transplantation in myeloma patients aged 75 to 84 years was nearly identical to that of the younger population.6 Since stem cell transplantation is feasible in older Medicare beneficiaries and maintenance lenalidomide for 2 years following transplant improves survival, the option of providing maintenance therapy with oral lenalidomide must be made available to Medicare beneficiaries. Due to a very limited use of transplantation in this study, the impact of oral lenalidomide maintenance in OS cannot be judged.
Of the patients reviewed in this study, 6% had a listed diagnosis of plasmacytoma. These individuals typically are treated with radiation therapy only. It is unclear if these patients also received any systemic myeloma therapy or if they ever progressed to myeloma. Availability of prescription drug coverage may not be relevant to this group. Also, the authors reported that part D participants were less likely to receive classic cytotoxic chemotherapy. This may be somewhat irrelevant in Medicare beneficiaries with a median age of 77 years for current practice, as frontline induction with old classic cytotoxic chemotherapy is less commonly used in this population.
Investigators have appropriately recognized a lack of ability to discern whether inferior survival in the group without prescription drug coverage was the result of not receiving therapy at all or inability to receive oral immunomodulatory drugs. There would have been little reason for not proceeding to parenteral therapy. As noted, 41% of beneficiaries without coverage at diagnosis subsequently obtained coverage but continued to have significantly worse survival. Cause of death, including whether related to myeloma, was not reported. The authors suggest that early separation of survival curves could therefore be reflective of suboptimal first-line therapy that lacked oral immunomodulatory drugs. During the study period 2006-2011, first-line use of lenalidomide was common.
Median survival of patients with myeloma in this study was only 27 months. According to the American Cancer Society, in 2018 median survival for stage I myeloma has not been reached, stage II myeloma is 83 months, and stage III myeloma is 43 months. A robust and dynamic landscape in myeloma therapy prevents a clear attribution to individual agents, whether oral or parenteral, in improving OS. Thus, 3-year RMST, while appropriate for 2006-2011, may not be relevant today.
Applications for Clinical Practice
The oncology community routinely encounters difficulty in initiating therapy using oral agents rapidly after diagnosis of myeloma. The retrospective data analyzed in the current study suggests that delay in initiating or unavailability of oral agents may adversely impact OS. The common approach of initiating parenteral therapy while awaiting approvals from payers or charity programs and subsequently adding oral therapy when available has not been studied in assessing OS. The oncology community should initiate plans to obtain prescription drug coverage through either Medicare part D, Medicaid, a sponsored plan, or financial assistance charity programs as soon as possible after diagnosis of myeloma. Moreover, continuation of these prescription drug plans should be strongly considered throughout the course of myeloma, as subsequent lines of treatment will quite likely involve other active and approved oral agents, such as pomalidomide, ixazomib, and panobinostat, besides other supportive therapy.
One of the mechanisms to obtain prescription drug coverage includes enrollment in state Medicaid programs for those who are eligible. Currently, 17 states have not yet adopted Medicaid expansion under the Affordable Care Act. Expansion of Medicaid in these states could increase availability of prescription drug benefits. In this study, 15.8% of Medicare and Medicaid dual enrollees with access to oral agents at low or no cost did not receive myeloma care, slightly higher than the 13.1% with no prescription drug coverage. Lower utilization in this population may be explained based on differences in comorbidities or socioeconomic conditions rather than availability of a prescription plan.
The incidence of myeloma is expected to be higher in Medicare beneficiaries, and according to one estimate, in 2030 and beyond nearly 75% of diagnosed myeloma patients will be aged 64 to 84 years, an increase from nearly 66% today.7 Changing demographics, increasing oral therapy options, and patient convenience demand attention to providing prescription drug coverage to all Medicare beneficiaries. This study lends support to that demand.
—Rakesh Gaur, MD, MPH, FACP, Cancer and Blood Center at Kansas Institute of Medicine, Lenexa, KS
1. Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomized, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
2. U.S. Food and Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications. www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm. Accessed October 11, 2018.
3. Dusetzina SB, Keating NL. Mind the gap: Why closing the doughnut hole is insufficient for increasing Medicare beneficiary access to oral chemotherapy. J Clin Oncol. 2016;34:375-380.
4. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279-3289.
5. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33.
6. Dong N, McKiernan P, Samuel D, et al. Autologous stem cell transplantation in multiple myeloma patients over age 75 [abstract]. J Clin Oncol. 2018;36(suppl): 8025.
7. Rosenberg PS, Barker KA, Anderson WF. Future distribution of multiple myeloma in the United States by sex, age, and race/ethnicity. Blood. 2015;125:410–412.
1. Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomized, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
2. U.S. Food and Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications. www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm. Accessed October 11, 2018.
3. Dusetzina SB, Keating NL. Mind the gap: Why closing the doughnut hole is insufficient for increasing Medicare beneficiary access to oral chemotherapy. J Clin Oncol. 2016;34:375-380.
4. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279-3289.
5. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33.
6. Dong N, McKiernan P, Samuel D, et al. Autologous stem cell transplantation in multiple myeloma patients over age 75 [abstract]. J Clin Oncol. 2018;36(suppl): 8025.
7. Rosenberg PS, Barker KA, Anderson WF. Future distribution of multiple myeloma in the United States by sex, age, and race/ethnicity. Blood. 2015;125:410–412.
