User login
New documentary highlights human toll of high insulin cost
A new documentary premiering at the 2023 South by Southwest (SXSW) Festival illustrates the human consequences of insulin’s high cost in the United States. Its creators hope that it will help spur action toward overall prescription pricing reform.
Pay or Die: A Documentary is scheduled to premiere March 11. It will be shown twice more during the festival, which runs from March 10 to 19 in Austin, Texas. The documentary was co-created and directed by filmmaker and cinematographer Scott Alexander Ruderman, who has type 1 diabetes, and his partner, producer and journalist Rachael Dyer. One of the executive producers is Sarah Silverman, a comic, actor, producer, and health care reform advocate.
The 90-minute film follows three human stories: A mother and young daughter who both have type 1 diabetes and become homeless after spending their rent money on insulin, a young adult diagnosed during the COVID-19 pandemic, and a mother whose 26-year-old son died from diabetic ketoacidosis (DKA) after his insulin was rationed.
“As an Australian now living in the U.S. and seeing how the health care system works here, especially for people with type 1 diabetes like Scott, and how access to insulin is a life-or-death situation, has been very eye-opening for me. I’m also half Canadian, and both are countries where access to health care is a human right, not a business,” Ms. Dyer said in an interview.
In response to the March 1 announcement from Eli Lilly about its insulin price cut, the film’s team told this news organization: “While we commend Eli Lilly in taking this first step and hope that Novo Nordisk and Sanofi [the two other major insulin manufacturers] follow suit, it is important to remember that the key issue is not about these companies voluntarily slashing prices; it’s about changing laws so the insulin manufacturers do not have the ability to raise the prices again.
“This is the life-or-death issue that we focus on in our documentary Pay or Die. It’s also important to note that insulin is just one of the many expensive prescription drugs in the U.S., which is why we need to call for reform. Affordable medication needs to be a basic human right within reach for all Americans.”
Physician perspective: Good news on insulin, but broader issues
The film features four physicians. One, Mayo Clinic oncologist/hematologist S. Vincent Rajkumar, MD, has spoken and published widely on insulin prices specifically and U.S. drug costs more broadly.
The other three are Joslin Clinic endocrinologist Elizabeth Halprin, MD, Massachusetts General Hospital internist Leigh Simmons, MD, and New York University physician and essayist Danielle Ofri, MD, PhD.
In an interview after the Lilly announcement, Dr. Rajkumar said, “I think this is very, very good news for patients. ... The fact that they’re doing it means they’re listening to us and listening to patients, which is good. And I do hope that other insulin manufacturers do the same shortly.”
However, he added, “for prescription drug prices and particularly cancer drug prices, there’s more reform that’s needed, and that’s at the policy level. ... The goal of the film was to use insulin to highlight the prescription drug price problem in the U.S.”
‘Then life changed’
The filmmaker, Mr. Ruderman, was diagnosed at age 19, during his freshman year in college. He spent several days hospitalized with DKA, and “then life changed,” he said in an interview. He went into photography first and later filmmaking, always with the uneasy knowledge that he could lose access to insulin at any time.
The impetus for the film came after he and Ms. Dyer walked into a pharmacy while visiting Canada in 2018 and discovered how much cheaper insulin was compared to the United States – roughly $20 per vial, compared to $300 in the U.S.
“When Rachael [Dyer] and I came back to the U.S., we were actually quite shocked about how many people are struggling to afford their medication ... the uninsured, those aging off their parents’ health insurance. So that was really the kickoff to us going into the field for the last 4 years making this documentary.”
As a freelancer, Mr. Ruderman has been personally paying for expensive “premium” health insurance that covers the pump and glucose monitors he uses. He buys insulin overseas as often as possible.
“Fortunately, I haven’t been in a situation where I’ve had to ration my insulin, but the fear is instilled in me. What if there’s a month when I can’t afford it? What am I going to do?” (Note: The writer of this article is in the same situation, which could be alleviated by Lilly’s action.)
Timing is everything
To be sure, even before Lilly’s announcement, some progress had been made since work on the film began.
The issue of insulin pricing has received wide media attention. More than 20 states have passed copay caps on insulin, and a new law capping the cost of insulin for Medicare beneficiaries at $35/month went into effect in January 2023. President Biden mentioned insulin during his State of the Union address, and Georgia Senator Raphael Warnock made the issue a centerpiece of his campaign.
But there have also been losses, including the failure thus far to pass a nationwide copay cap.
These recent developments make this a good time for the film’s debut, producer Yael Melamede said in an interview. “There’s a lot happening in the space, but also a lot of incredible disappointments along the way, so we are really interested in getting this film out now.”
Ms. Melamede, who owns a film production company, said, “I’ve done a lot of films that have some issue advocacy side to them. I love this film because it’s grounded in the stories of real people. ... We feel this is a perfect catalyst to keep the energy going and for people to say this is super-important and not get distracted.”
While the film doesn’t advocate for specific policies, there is a “call to action” at the end that points viewers to resources on the website for writing to their members of Congress along with additional ways to become personally involved.
Ms. Dyer told this news organization, “This film is not only focusing on type 1 diabetes. That is obviously the crux of the issue, but it is a broader health care message for everyone wanting to make a change for health care in this country, the richest country in the world.”
At SXSW, Pay or Die will be competing with seven other films in the documentary feature competition, and it is eligible to win other awards.
Several other activities at the festival will address the topics of diabetes and U.S. health care costs, including a panel discussion titled Crushing: The Burden of Diabetes on Patients, featuring musician and actor Nick Jonas, who has type 1 diabetes, and a representative from the continuous glucose monitor manufacturer Dexcom.
Another panel, Young and Uninsured: Pay or Die, will include Dr. Rajkumar, Mr. Ruderman, Texas Representative James Talarico, who is advancing an insulin cap bill in that state, and Nicole Smith-Holt, the Minnesota mother of the young man who died because he couldn’t afford his insulin.
Mr. Ruderman, Ms. Dyer, Ms. Melamede, and Dr. Rajkumar have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new documentary premiering at the 2023 South by Southwest (SXSW) Festival illustrates the human consequences of insulin’s high cost in the United States. Its creators hope that it will help spur action toward overall prescription pricing reform.
Pay or Die: A Documentary is scheduled to premiere March 11. It will be shown twice more during the festival, which runs from March 10 to 19 in Austin, Texas. The documentary was co-created and directed by filmmaker and cinematographer Scott Alexander Ruderman, who has type 1 diabetes, and his partner, producer and journalist Rachael Dyer. One of the executive producers is Sarah Silverman, a comic, actor, producer, and health care reform advocate.
The 90-minute film follows three human stories: A mother and young daughter who both have type 1 diabetes and become homeless after spending their rent money on insulin, a young adult diagnosed during the COVID-19 pandemic, and a mother whose 26-year-old son died from diabetic ketoacidosis (DKA) after his insulin was rationed.
“As an Australian now living in the U.S. and seeing how the health care system works here, especially for people with type 1 diabetes like Scott, and how access to insulin is a life-or-death situation, has been very eye-opening for me. I’m also half Canadian, and both are countries where access to health care is a human right, not a business,” Ms. Dyer said in an interview.
In response to the March 1 announcement from Eli Lilly about its insulin price cut, the film’s team told this news organization: “While we commend Eli Lilly in taking this first step and hope that Novo Nordisk and Sanofi [the two other major insulin manufacturers] follow suit, it is important to remember that the key issue is not about these companies voluntarily slashing prices; it’s about changing laws so the insulin manufacturers do not have the ability to raise the prices again.
“This is the life-or-death issue that we focus on in our documentary Pay or Die. It’s also important to note that insulin is just one of the many expensive prescription drugs in the U.S., which is why we need to call for reform. Affordable medication needs to be a basic human right within reach for all Americans.”
Physician perspective: Good news on insulin, but broader issues
The film features four physicians. One, Mayo Clinic oncologist/hematologist S. Vincent Rajkumar, MD, has spoken and published widely on insulin prices specifically and U.S. drug costs more broadly.
The other three are Joslin Clinic endocrinologist Elizabeth Halprin, MD, Massachusetts General Hospital internist Leigh Simmons, MD, and New York University physician and essayist Danielle Ofri, MD, PhD.
In an interview after the Lilly announcement, Dr. Rajkumar said, “I think this is very, very good news for patients. ... The fact that they’re doing it means they’re listening to us and listening to patients, which is good. And I do hope that other insulin manufacturers do the same shortly.”
However, he added, “for prescription drug prices and particularly cancer drug prices, there’s more reform that’s needed, and that’s at the policy level. ... The goal of the film was to use insulin to highlight the prescription drug price problem in the U.S.”
‘Then life changed’
The filmmaker, Mr. Ruderman, was diagnosed at age 19, during his freshman year in college. He spent several days hospitalized with DKA, and “then life changed,” he said in an interview. He went into photography first and later filmmaking, always with the uneasy knowledge that he could lose access to insulin at any time.
The impetus for the film came after he and Ms. Dyer walked into a pharmacy while visiting Canada in 2018 and discovered how much cheaper insulin was compared to the United States – roughly $20 per vial, compared to $300 in the U.S.
“When Rachael [Dyer] and I came back to the U.S., we were actually quite shocked about how many people are struggling to afford their medication ... the uninsured, those aging off their parents’ health insurance. So that was really the kickoff to us going into the field for the last 4 years making this documentary.”
As a freelancer, Mr. Ruderman has been personally paying for expensive “premium” health insurance that covers the pump and glucose monitors he uses. He buys insulin overseas as often as possible.
“Fortunately, I haven’t been in a situation where I’ve had to ration my insulin, but the fear is instilled in me. What if there’s a month when I can’t afford it? What am I going to do?” (Note: The writer of this article is in the same situation, which could be alleviated by Lilly’s action.)
Timing is everything
To be sure, even before Lilly’s announcement, some progress had been made since work on the film began.
The issue of insulin pricing has received wide media attention. More than 20 states have passed copay caps on insulin, and a new law capping the cost of insulin for Medicare beneficiaries at $35/month went into effect in January 2023. President Biden mentioned insulin during his State of the Union address, and Georgia Senator Raphael Warnock made the issue a centerpiece of his campaign.
But there have also been losses, including the failure thus far to pass a nationwide copay cap.
These recent developments make this a good time for the film’s debut, producer Yael Melamede said in an interview. “There’s a lot happening in the space, but also a lot of incredible disappointments along the way, so we are really interested in getting this film out now.”
Ms. Melamede, who owns a film production company, said, “I’ve done a lot of films that have some issue advocacy side to them. I love this film because it’s grounded in the stories of real people. ... We feel this is a perfect catalyst to keep the energy going and for people to say this is super-important and not get distracted.”
While the film doesn’t advocate for specific policies, there is a “call to action” at the end that points viewers to resources on the website for writing to their members of Congress along with additional ways to become personally involved.
Ms. Dyer told this news organization, “This film is not only focusing on type 1 diabetes. That is obviously the crux of the issue, but it is a broader health care message for everyone wanting to make a change for health care in this country, the richest country in the world.”
At SXSW, Pay or Die will be competing with seven other films in the documentary feature competition, and it is eligible to win other awards.
Several other activities at the festival will address the topics of diabetes and U.S. health care costs, including a panel discussion titled Crushing: The Burden of Diabetes on Patients, featuring musician and actor Nick Jonas, who has type 1 diabetes, and a representative from the continuous glucose monitor manufacturer Dexcom.
Another panel, Young and Uninsured: Pay or Die, will include Dr. Rajkumar, Mr. Ruderman, Texas Representative James Talarico, who is advancing an insulin cap bill in that state, and Nicole Smith-Holt, the Minnesota mother of the young man who died because he couldn’t afford his insulin.
Mr. Ruderman, Ms. Dyer, Ms. Melamede, and Dr. Rajkumar have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new documentary premiering at the 2023 South by Southwest (SXSW) Festival illustrates the human consequences of insulin’s high cost in the United States. Its creators hope that it will help spur action toward overall prescription pricing reform.
Pay or Die: A Documentary is scheduled to premiere March 11. It will be shown twice more during the festival, which runs from March 10 to 19 in Austin, Texas. The documentary was co-created and directed by filmmaker and cinematographer Scott Alexander Ruderman, who has type 1 diabetes, and his partner, producer and journalist Rachael Dyer. One of the executive producers is Sarah Silverman, a comic, actor, producer, and health care reform advocate.
The 90-minute film follows three human stories: A mother and young daughter who both have type 1 diabetes and become homeless after spending their rent money on insulin, a young adult diagnosed during the COVID-19 pandemic, and a mother whose 26-year-old son died from diabetic ketoacidosis (DKA) after his insulin was rationed.
“As an Australian now living in the U.S. and seeing how the health care system works here, especially for people with type 1 diabetes like Scott, and how access to insulin is a life-or-death situation, has been very eye-opening for me. I’m also half Canadian, and both are countries where access to health care is a human right, not a business,” Ms. Dyer said in an interview.
In response to the March 1 announcement from Eli Lilly about its insulin price cut, the film’s team told this news organization: “While we commend Eli Lilly in taking this first step and hope that Novo Nordisk and Sanofi [the two other major insulin manufacturers] follow suit, it is important to remember that the key issue is not about these companies voluntarily slashing prices; it’s about changing laws so the insulin manufacturers do not have the ability to raise the prices again.
“This is the life-or-death issue that we focus on in our documentary Pay or Die. It’s also important to note that insulin is just one of the many expensive prescription drugs in the U.S., which is why we need to call for reform. Affordable medication needs to be a basic human right within reach for all Americans.”
Physician perspective: Good news on insulin, but broader issues
The film features four physicians. One, Mayo Clinic oncologist/hematologist S. Vincent Rajkumar, MD, has spoken and published widely on insulin prices specifically and U.S. drug costs more broadly.
The other three are Joslin Clinic endocrinologist Elizabeth Halprin, MD, Massachusetts General Hospital internist Leigh Simmons, MD, and New York University physician and essayist Danielle Ofri, MD, PhD.
In an interview after the Lilly announcement, Dr. Rajkumar said, “I think this is very, very good news for patients. ... The fact that they’re doing it means they’re listening to us and listening to patients, which is good. And I do hope that other insulin manufacturers do the same shortly.”
However, he added, “for prescription drug prices and particularly cancer drug prices, there’s more reform that’s needed, and that’s at the policy level. ... The goal of the film was to use insulin to highlight the prescription drug price problem in the U.S.”
‘Then life changed’
The filmmaker, Mr. Ruderman, was diagnosed at age 19, during his freshman year in college. He spent several days hospitalized with DKA, and “then life changed,” he said in an interview. He went into photography first and later filmmaking, always with the uneasy knowledge that he could lose access to insulin at any time.
The impetus for the film came after he and Ms. Dyer walked into a pharmacy while visiting Canada in 2018 and discovered how much cheaper insulin was compared to the United States – roughly $20 per vial, compared to $300 in the U.S.
“When Rachael [Dyer] and I came back to the U.S., we were actually quite shocked about how many people are struggling to afford their medication ... the uninsured, those aging off their parents’ health insurance. So that was really the kickoff to us going into the field for the last 4 years making this documentary.”
As a freelancer, Mr. Ruderman has been personally paying for expensive “premium” health insurance that covers the pump and glucose monitors he uses. He buys insulin overseas as often as possible.
“Fortunately, I haven’t been in a situation where I’ve had to ration my insulin, but the fear is instilled in me. What if there’s a month when I can’t afford it? What am I going to do?” (Note: The writer of this article is in the same situation, which could be alleviated by Lilly’s action.)
Timing is everything
To be sure, even before Lilly’s announcement, some progress had been made since work on the film began.
The issue of insulin pricing has received wide media attention. More than 20 states have passed copay caps on insulin, and a new law capping the cost of insulin for Medicare beneficiaries at $35/month went into effect in January 2023. President Biden mentioned insulin during his State of the Union address, and Georgia Senator Raphael Warnock made the issue a centerpiece of his campaign.
But there have also been losses, including the failure thus far to pass a nationwide copay cap.
These recent developments make this a good time for the film’s debut, producer Yael Melamede said in an interview. “There’s a lot happening in the space, but also a lot of incredible disappointments along the way, so we are really interested in getting this film out now.”
Ms. Melamede, who owns a film production company, said, “I’ve done a lot of films that have some issue advocacy side to them. I love this film because it’s grounded in the stories of real people. ... We feel this is a perfect catalyst to keep the energy going and for people to say this is super-important and not get distracted.”
While the film doesn’t advocate for specific policies, there is a “call to action” at the end that points viewers to resources on the website for writing to their members of Congress along with additional ways to become personally involved.
Ms. Dyer told this news organization, “This film is not only focusing on type 1 diabetes. That is obviously the crux of the issue, but it is a broader health care message for everyone wanting to make a change for health care in this country, the richest country in the world.”
At SXSW, Pay or Die will be competing with seven other films in the documentary feature competition, and it is eligible to win other awards.
Several other activities at the festival will address the topics of diabetes and U.S. health care costs, including a panel discussion titled Crushing: The Burden of Diabetes on Patients, featuring musician and actor Nick Jonas, who has type 1 diabetes, and a representative from the continuous glucose monitor manufacturer Dexcom.
Another panel, Young and Uninsured: Pay or Die, will include Dr. Rajkumar, Mr. Ruderman, Texas Representative James Talarico, who is advancing an insulin cap bill in that state, and Nicole Smith-Holt, the Minnesota mother of the young man who died because he couldn’t afford his insulin.
Mr. Ruderman, Ms. Dyer, Ms. Melamede, and Dr. Rajkumar have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Causal link found between childhood obesity and adult-onset diabetes
Childhood obesity is a risk factor for four of the five subtypes of adult-onset diabetes, emphasizing the importance of childhood weight control, according to a collaborative study from the Karolinska Institutet in Stockholm, the University of Bristol (England), and Sun Yat-Sen University in China.
“Our finding is that children who have a bigger body size than the average have increased risks of developing almost all subtypes of adult-onset diabetes, except for the mild age-related subtype,” lead author Yuxia Wei, a PhD student from the Karolinska Institutet, said in an interview. “This tells us that it is important to prevent overweight/obesity in children and important for pediatric patients to lose weight if they have already been overweight/obese,” she added, while acknowledging that the study did not examine whether childhood weight loss would prevent adult-onset diabetes.
The study, published online in Diabetologia, used Mendelian randomization (MR), with data from genome-wide association studies (GWAS) of childhood obesity and the five subtypes of adult-onset diabetes: latent autoimmune diabetes in adults (LADA, proxy for severe autoimmune diabetes), severe insulin-deficient diabetes (SIDD), severe insulin-resistant diabetes (SIRD), mild obesity-related diabetes (MOD), and mild age-related diabetes (MARD). MR is “a rather new but commonly used and established technique that uses genetic information to study the causal link between an environmental risk factor and a disease, while accounting for the influence of other risk factors,” Ms. Wei explained.
To identify genetic variations associated with obesity, the study used statistics from a GWAS of 453,169 Europeans who self-reported body size at age 10 years in the UK Biobank study. After adjustment for sex, age at baseline, type of genotyping array, and month of birth, they identified 295 independent single nucleotide polymorphisms (SNPs) for childhood body size.
The researchers also used data from two GWAS of European adults with newly diagnosed diabetes, or without diabetes, to identify SNPs in 8,581 individuals with LADA, 3,937 with SIDD, 3,874 with SIRD, 4,118 with MOD, and 5,605 with MARD.
They then used MR to assess the association of genetically predicted childhood body size with the different diabetes subtypes.
The analysis showed that, with the exception of MARD, all other adult-onset diabetes subtypes were causally associated with childhood obesity, with odds ratio of 1.62 for LADA, 2.11 for SIDD, 2.76 for SIRD, and 7.30 for MOD. However, a genetic correlation between childhood obesity and adult-onset diabetes was found only for MOD, and no other subtypes. “The weak genetic correlation between childhood obesity and adult diabetes indicates that the genes promoting childhood adiposity are largely distinct from those promoting diabetes during adulthood,” noted the authors.
The findings indicate that “childhood body size and MOD may share some genetic mutations,” added Ms. Wei. “That is to say, some genes may affect childhood body size and MOD simultaneously.” But the shared genes do demonstrate the causal effect of childhood obesity on MOD, she explained. The causal effect is demonstrated through the MR analysis.
Additionally, they noted that while “the link between childhood body size and SIRD is expected, given the adverse effects of adiposity on insulin sensitivity ... the smaller OR for SIRD than for MOD suggests that non–obesity-related and/or nongenetic effects may be the main factors underlying the development of SIRD.” Asked for her theory on how childhood body size could affect diabetes subtypes characterized by autoimmunity (LADA) or impaired insulin secretion (SIDD), Ms. Wei speculated that “excess fat around the pancreas can affect insulin secretion and that impaired insulin secretion is also an important problem for LADA.”
Another theory is that it might be “metabolic memory,” suggested Jordi Merino, PhD, of the University of Copenhagen and Harvard University, Boston, who was not involved in the research. “Being exposed to obesity during childhood will tell the body to produce more insulin/aberrant immunity responses later in life.”
Dr. Merino said that, overall, the study’s findings “highlight the long and lasting effect of early-life adiposity and metabolic alterations on different forms of adult-onset diabetes,” adding that this is the first evidence “that childhood adiposity is not only linked to the more traditional diabetes subtype consequence of increased insulin resistance but also subtypes driven by autoimmunity or impaired insulin secretion.” He explained that genetics is “only part of the story” driving increased diabetes risk and “we do not know much about other factors interacting with genetics, but the results from this Mendelian randomization analysis suggest that childhood obesity is a causal factor for all adult-onset diabetes subtypes. Identifying causal factors instead of associative factors is critical to implement more targeted preventive and therapeutic strategies.”
He acknowledged, “There is a long path for these results to be eventually implemented in clinical practice, but they can support early weight control strategies for preventing different diabetes subtypes.”
The study was supported by the Swedish Research Council, Research Council for Health, Working Life and Welfare, and Novo Nordisk Foundation. Ms. Wei received a scholarship from the China Scholarship Council. One coauthor is an employee of GlaxoSmithKline. Dr. Merino reported no conflicts of interest.
Childhood obesity is a risk factor for four of the five subtypes of adult-onset diabetes, emphasizing the importance of childhood weight control, according to a collaborative study from the Karolinska Institutet in Stockholm, the University of Bristol (England), and Sun Yat-Sen University in China.
“Our finding is that children who have a bigger body size than the average have increased risks of developing almost all subtypes of adult-onset diabetes, except for the mild age-related subtype,” lead author Yuxia Wei, a PhD student from the Karolinska Institutet, said in an interview. “This tells us that it is important to prevent overweight/obesity in children and important for pediatric patients to lose weight if they have already been overweight/obese,” she added, while acknowledging that the study did not examine whether childhood weight loss would prevent adult-onset diabetes.
The study, published online in Diabetologia, used Mendelian randomization (MR), with data from genome-wide association studies (GWAS) of childhood obesity and the five subtypes of adult-onset diabetes: latent autoimmune diabetes in adults (LADA, proxy for severe autoimmune diabetes), severe insulin-deficient diabetes (SIDD), severe insulin-resistant diabetes (SIRD), mild obesity-related diabetes (MOD), and mild age-related diabetes (MARD). MR is “a rather new but commonly used and established technique that uses genetic information to study the causal link between an environmental risk factor and a disease, while accounting for the influence of other risk factors,” Ms. Wei explained.
To identify genetic variations associated with obesity, the study used statistics from a GWAS of 453,169 Europeans who self-reported body size at age 10 years in the UK Biobank study. After adjustment for sex, age at baseline, type of genotyping array, and month of birth, they identified 295 independent single nucleotide polymorphisms (SNPs) for childhood body size.
The researchers also used data from two GWAS of European adults with newly diagnosed diabetes, or without diabetes, to identify SNPs in 8,581 individuals with LADA, 3,937 with SIDD, 3,874 with SIRD, 4,118 with MOD, and 5,605 with MARD.
They then used MR to assess the association of genetically predicted childhood body size with the different diabetes subtypes.
The analysis showed that, with the exception of MARD, all other adult-onset diabetes subtypes were causally associated with childhood obesity, with odds ratio of 1.62 for LADA, 2.11 for SIDD, 2.76 for SIRD, and 7.30 for MOD. However, a genetic correlation between childhood obesity and adult-onset diabetes was found only for MOD, and no other subtypes. “The weak genetic correlation between childhood obesity and adult diabetes indicates that the genes promoting childhood adiposity are largely distinct from those promoting diabetes during adulthood,” noted the authors.
The findings indicate that “childhood body size and MOD may share some genetic mutations,” added Ms. Wei. “That is to say, some genes may affect childhood body size and MOD simultaneously.” But the shared genes do demonstrate the causal effect of childhood obesity on MOD, she explained. The causal effect is demonstrated through the MR analysis.
Additionally, they noted that while “the link between childhood body size and SIRD is expected, given the adverse effects of adiposity on insulin sensitivity ... the smaller OR for SIRD than for MOD suggests that non–obesity-related and/or nongenetic effects may be the main factors underlying the development of SIRD.” Asked for her theory on how childhood body size could affect diabetes subtypes characterized by autoimmunity (LADA) or impaired insulin secretion (SIDD), Ms. Wei speculated that “excess fat around the pancreas can affect insulin secretion and that impaired insulin secretion is also an important problem for LADA.”
Another theory is that it might be “metabolic memory,” suggested Jordi Merino, PhD, of the University of Copenhagen and Harvard University, Boston, who was not involved in the research. “Being exposed to obesity during childhood will tell the body to produce more insulin/aberrant immunity responses later in life.”
Dr. Merino said that, overall, the study’s findings “highlight the long and lasting effect of early-life adiposity and metabolic alterations on different forms of adult-onset diabetes,” adding that this is the first evidence “that childhood adiposity is not only linked to the more traditional diabetes subtype consequence of increased insulin resistance but also subtypes driven by autoimmunity or impaired insulin secretion.” He explained that genetics is “only part of the story” driving increased diabetes risk and “we do not know much about other factors interacting with genetics, but the results from this Mendelian randomization analysis suggest that childhood obesity is a causal factor for all adult-onset diabetes subtypes. Identifying causal factors instead of associative factors is critical to implement more targeted preventive and therapeutic strategies.”
He acknowledged, “There is a long path for these results to be eventually implemented in clinical practice, but they can support early weight control strategies for preventing different diabetes subtypes.”
The study was supported by the Swedish Research Council, Research Council for Health, Working Life and Welfare, and Novo Nordisk Foundation. Ms. Wei received a scholarship from the China Scholarship Council. One coauthor is an employee of GlaxoSmithKline. Dr. Merino reported no conflicts of interest.
Childhood obesity is a risk factor for four of the five subtypes of adult-onset diabetes, emphasizing the importance of childhood weight control, according to a collaborative study from the Karolinska Institutet in Stockholm, the University of Bristol (England), and Sun Yat-Sen University in China.
“Our finding is that children who have a bigger body size than the average have increased risks of developing almost all subtypes of adult-onset diabetes, except for the mild age-related subtype,” lead author Yuxia Wei, a PhD student from the Karolinska Institutet, said in an interview. “This tells us that it is important to prevent overweight/obesity in children and important for pediatric patients to lose weight if they have already been overweight/obese,” she added, while acknowledging that the study did not examine whether childhood weight loss would prevent adult-onset diabetes.
The study, published online in Diabetologia, used Mendelian randomization (MR), with data from genome-wide association studies (GWAS) of childhood obesity and the five subtypes of adult-onset diabetes: latent autoimmune diabetes in adults (LADA, proxy for severe autoimmune diabetes), severe insulin-deficient diabetes (SIDD), severe insulin-resistant diabetes (SIRD), mild obesity-related diabetes (MOD), and mild age-related diabetes (MARD). MR is “a rather new but commonly used and established technique that uses genetic information to study the causal link between an environmental risk factor and a disease, while accounting for the influence of other risk factors,” Ms. Wei explained.
To identify genetic variations associated with obesity, the study used statistics from a GWAS of 453,169 Europeans who self-reported body size at age 10 years in the UK Biobank study. After adjustment for sex, age at baseline, type of genotyping array, and month of birth, they identified 295 independent single nucleotide polymorphisms (SNPs) for childhood body size.
The researchers also used data from two GWAS of European adults with newly diagnosed diabetes, or without diabetes, to identify SNPs in 8,581 individuals with LADA, 3,937 with SIDD, 3,874 with SIRD, 4,118 with MOD, and 5,605 with MARD.
They then used MR to assess the association of genetically predicted childhood body size with the different diabetes subtypes.
The analysis showed that, with the exception of MARD, all other adult-onset diabetes subtypes were causally associated with childhood obesity, with odds ratio of 1.62 for LADA, 2.11 for SIDD, 2.76 for SIRD, and 7.30 for MOD. However, a genetic correlation between childhood obesity and adult-onset diabetes was found only for MOD, and no other subtypes. “The weak genetic correlation between childhood obesity and adult diabetes indicates that the genes promoting childhood adiposity are largely distinct from those promoting diabetes during adulthood,” noted the authors.
The findings indicate that “childhood body size and MOD may share some genetic mutations,” added Ms. Wei. “That is to say, some genes may affect childhood body size and MOD simultaneously.” But the shared genes do demonstrate the causal effect of childhood obesity on MOD, she explained. The causal effect is demonstrated through the MR analysis.
Additionally, they noted that while “the link between childhood body size and SIRD is expected, given the adverse effects of adiposity on insulin sensitivity ... the smaller OR for SIRD than for MOD suggests that non–obesity-related and/or nongenetic effects may be the main factors underlying the development of SIRD.” Asked for her theory on how childhood body size could affect diabetes subtypes characterized by autoimmunity (LADA) or impaired insulin secretion (SIDD), Ms. Wei speculated that “excess fat around the pancreas can affect insulin secretion and that impaired insulin secretion is also an important problem for LADA.”
Another theory is that it might be “metabolic memory,” suggested Jordi Merino, PhD, of the University of Copenhagen and Harvard University, Boston, who was not involved in the research. “Being exposed to obesity during childhood will tell the body to produce more insulin/aberrant immunity responses later in life.”
Dr. Merino said that, overall, the study’s findings “highlight the long and lasting effect of early-life adiposity and metabolic alterations on different forms of adult-onset diabetes,” adding that this is the first evidence “that childhood adiposity is not only linked to the more traditional diabetes subtype consequence of increased insulin resistance but also subtypes driven by autoimmunity or impaired insulin secretion.” He explained that genetics is “only part of the story” driving increased diabetes risk and “we do not know much about other factors interacting with genetics, but the results from this Mendelian randomization analysis suggest that childhood obesity is a causal factor for all adult-onset diabetes subtypes. Identifying causal factors instead of associative factors is critical to implement more targeted preventive and therapeutic strategies.”
He acknowledged, “There is a long path for these results to be eventually implemented in clinical practice, but they can support early weight control strategies for preventing different diabetes subtypes.”
The study was supported by the Swedish Research Council, Research Council for Health, Working Life and Welfare, and Novo Nordisk Foundation. Ms. Wei received a scholarship from the China Scholarship Council. One coauthor is an employee of GlaxoSmithKline. Dr. Merino reported no conflicts of interest.
FROM DIABETOLOGIA
Modified ECT lowers dental, skeletal fracture risk
“ECT is associated with a very low risk of skeletal fractures, even in high-risk patients, and is also associated with a low risk of dental fractures,” said study investigator Chittaranjan Andrade, MD, noting that preexisting bone and dental disease increase this risk.
Overall, clinicians who provide ECT “need to be aware of rare adverse effects, as well as the common ones,” Dr. Andrade, senior professor of clinical psychopharmacology and neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India, told this news organization. He added they also “need data to be able to provide reassurance.”
The findings were published online in The Journal of Clinical Psychiatry.
Avoid unmodified ECT
Dr. Andrade conducted the study because the risk of skeletal and dental fractures associated with ECT is “not commonly discussed.”
Although ECT is perhaps the most effective available treatment for major mental illness, it is associated with several adverse effects, including those associated with delivery of an electrical stimulus to the brain, which results in central and peripheral seizure, he noted.
“The central seizure is essential for the efficacy of ECT,” said Dr. Andrade. In contrast, “the motor seizure has no therapeutic value, is cosmetically displeasing, and may rarely be associated with peripheral adverse effects affecting muscles, joints, teeth, and bones,” he added.
The musculoskeletal and dental injuries are caused by stretching, twisting, compression, or direct injury. Particularly during the motor seizure, the “sudden jerk” associated with the tonic contraction of muscles as well as the repeated jerks associated with each clonic contraction can result in injuries, including skeletal and dental fractures.
To address this concern, the motor seizure is “modified” or attenuated through use of an intravenous muscle relaxant administered with other ECT premedication.
“How effectively the musculoskeletal and dental adverse effects are minimized depends on how well the motor seizure is modified,” Dr. Andrade said. He emphasized that the “use of unmodified ECT is strongly discouraged.”
Dr. Andrade reviewed prior research into the skeletal and dental risks of ECT. The infrequency of cases and ethical difficulties in conducting randomized clinical trials with such patients require reliance on anecdotal reports, he said.
Bite blocks, seizure modifiers
Population-based data showed that the fracture risk with modified ECT is two events per 100,000 ECTs. However, the risk may be as low as 0.36 events per 100,000 ECTs if calculated only with recent data, Dr. Andrade noted.
Population-based studies also suggest that the dental fracture risk with modified ECT is .02% per ECT and .17% per ECT course.
Although fractures have been reported under “unusual circumstances” among patients receiving modified ECT, many other reports point to the safety of this treatment, even in ultrahigh-risk patients.
Such patients include those with severe osteoporosis, metastatic bone disease, osteogenesis imperfecta, Ehlers-Danlos syndrome, Harrington rod implants, recent long bone fractures, multiple bone fractures, surgical repair of hip fracture, vertebroplasty, and maxillofacial repair.
Dr. Andrade noted that oral health is “poor” among patients with major mental illness for multiple reasons, including poor nutrition, self-neglect, and decreased salivation caused by the anticholinergic effects of medications.
This places these patients at increased risk for dental adverse effects during ECT because the muscles of the jaw contract forcefully during the motor seizure, causing sudden impact and, subsequently, sustained pressure on the teeth, Dr. Andrade said.
Moreover, because ECT is typically administered through repeated sessions, dental injuries may accumulate over the course of treatment.
ECT-associated skeletal risks arise from the tonic-clonic contractions of the muscles of the trunk and limbs, which need to be addressed via use of succinylcholine or other muscle relaxants included in ECT premedication.
Dr. Andrade noted that succinylcholine is effective at modifying the motor seizure at the common dose of 0.5-1.0 mg/kg. However, about 5% of patients require a higher dose (>1.5 mg/kg). If the dose is 1-2 mg/kg for patients at high risk for orthopedic complications, “muscle relaxation during ECT could be expected to be reasonably complete,” he said.
“Because of wide interpersonal variation, a neurostimulator may need to be used to identify the ideal dose for an individual patient,” he added.
In addition, use of bite blocks and effective jaw immobilization during ECT can reduce the risk. “Careful assessment of preexisting risk and good ECT practice can minimize the risk of skeletal and dental complications during ECT,” Dr. Andrade said.
Risks vs. benefits
Commenting on the study, Mark S. George, MD, distinguished professor of psychiatry, radiology, and neurology, and director of the brain stimulation division, Medical University of South Carolina, Charleston, said this was a “well-written review of how frequently patients who are undergoing modern ECT have bone fractures or dental fractures during the procedure.”
Dr. George, who was not involved with the research, added that modern medications and management “make ECT a truly safe procedure.”
“It is not without some risk, but these risks are low, especially when compared to the risks of untreated or undertreated depression or catatonia, like suicide,” he said.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made directly to registered charities, but does not benefit financially from the relationship. His travel expenses for delivering lectures and workshops have been supported by the organizers themselves or pharmaceutical companies at the behest of the organizers. He has provided advice to various pharmaceutical companies and has received “nominal compensation.” He has also received payments for developing educational materials for scientific initiatives and programs, such as for the Behavioral and Neurosciences Foundation of India, PsyBase India, Texas Tech University USA, the Nordic Association for Convulsive Therapy, and the American Society of Clinical Psychopharmacology. Dr. George reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“ECT is associated with a very low risk of skeletal fractures, even in high-risk patients, and is also associated with a low risk of dental fractures,” said study investigator Chittaranjan Andrade, MD, noting that preexisting bone and dental disease increase this risk.
Overall, clinicians who provide ECT “need to be aware of rare adverse effects, as well as the common ones,” Dr. Andrade, senior professor of clinical psychopharmacology and neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India, told this news organization. He added they also “need data to be able to provide reassurance.”
The findings were published online in The Journal of Clinical Psychiatry.
Avoid unmodified ECT
Dr. Andrade conducted the study because the risk of skeletal and dental fractures associated with ECT is “not commonly discussed.”
Although ECT is perhaps the most effective available treatment for major mental illness, it is associated with several adverse effects, including those associated with delivery of an electrical stimulus to the brain, which results in central and peripheral seizure, he noted.
“The central seizure is essential for the efficacy of ECT,” said Dr. Andrade. In contrast, “the motor seizure has no therapeutic value, is cosmetically displeasing, and may rarely be associated with peripheral adverse effects affecting muscles, joints, teeth, and bones,” he added.
The musculoskeletal and dental injuries are caused by stretching, twisting, compression, or direct injury. Particularly during the motor seizure, the “sudden jerk” associated with the tonic contraction of muscles as well as the repeated jerks associated with each clonic contraction can result in injuries, including skeletal and dental fractures.
To address this concern, the motor seizure is “modified” or attenuated through use of an intravenous muscle relaxant administered with other ECT premedication.
“How effectively the musculoskeletal and dental adverse effects are minimized depends on how well the motor seizure is modified,” Dr. Andrade said. He emphasized that the “use of unmodified ECT is strongly discouraged.”
Dr. Andrade reviewed prior research into the skeletal and dental risks of ECT. The infrequency of cases and ethical difficulties in conducting randomized clinical trials with such patients require reliance on anecdotal reports, he said.
Bite blocks, seizure modifiers
Population-based data showed that the fracture risk with modified ECT is two events per 100,000 ECTs. However, the risk may be as low as 0.36 events per 100,000 ECTs if calculated only with recent data, Dr. Andrade noted.
Population-based studies also suggest that the dental fracture risk with modified ECT is .02% per ECT and .17% per ECT course.
Although fractures have been reported under “unusual circumstances” among patients receiving modified ECT, many other reports point to the safety of this treatment, even in ultrahigh-risk patients.
Such patients include those with severe osteoporosis, metastatic bone disease, osteogenesis imperfecta, Ehlers-Danlos syndrome, Harrington rod implants, recent long bone fractures, multiple bone fractures, surgical repair of hip fracture, vertebroplasty, and maxillofacial repair.
Dr. Andrade noted that oral health is “poor” among patients with major mental illness for multiple reasons, including poor nutrition, self-neglect, and decreased salivation caused by the anticholinergic effects of medications.
This places these patients at increased risk for dental adverse effects during ECT because the muscles of the jaw contract forcefully during the motor seizure, causing sudden impact and, subsequently, sustained pressure on the teeth, Dr. Andrade said.
Moreover, because ECT is typically administered through repeated sessions, dental injuries may accumulate over the course of treatment.
ECT-associated skeletal risks arise from the tonic-clonic contractions of the muscles of the trunk and limbs, which need to be addressed via use of succinylcholine or other muscle relaxants included in ECT premedication.
Dr. Andrade noted that succinylcholine is effective at modifying the motor seizure at the common dose of 0.5-1.0 mg/kg. However, about 5% of patients require a higher dose (>1.5 mg/kg). If the dose is 1-2 mg/kg for patients at high risk for orthopedic complications, “muscle relaxation during ECT could be expected to be reasonably complete,” he said.
“Because of wide interpersonal variation, a neurostimulator may need to be used to identify the ideal dose for an individual patient,” he added.
In addition, use of bite blocks and effective jaw immobilization during ECT can reduce the risk. “Careful assessment of preexisting risk and good ECT practice can minimize the risk of skeletal and dental complications during ECT,” Dr. Andrade said.
Risks vs. benefits
Commenting on the study, Mark S. George, MD, distinguished professor of psychiatry, radiology, and neurology, and director of the brain stimulation division, Medical University of South Carolina, Charleston, said this was a “well-written review of how frequently patients who are undergoing modern ECT have bone fractures or dental fractures during the procedure.”
Dr. George, who was not involved with the research, added that modern medications and management “make ECT a truly safe procedure.”
“It is not without some risk, but these risks are low, especially when compared to the risks of untreated or undertreated depression or catatonia, like suicide,” he said.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made directly to registered charities, but does not benefit financially from the relationship. His travel expenses for delivering lectures and workshops have been supported by the organizers themselves or pharmaceutical companies at the behest of the organizers. He has provided advice to various pharmaceutical companies and has received “nominal compensation.” He has also received payments for developing educational materials for scientific initiatives and programs, such as for the Behavioral and Neurosciences Foundation of India, PsyBase India, Texas Tech University USA, the Nordic Association for Convulsive Therapy, and the American Society of Clinical Psychopharmacology. Dr. George reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“ECT is associated with a very low risk of skeletal fractures, even in high-risk patients, and is also associated with a low risk of dental fractures,” said study investigator Chittaranjan Andrade, MD, noting that preexisting bone and dental disease increase this risk.
Overall, clinicians who provide ECT “need to be aware of rare adverse effects, as well as the common ones,” Dr. Andrade, senior professor of clinical psychopharmacology and neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India, told this news organization. He added they also “need data to be able to provide reassurance.”
The findings were published online in The Journal of Clinical Psychiatry.
Avoid unmodified ECT
Dr. Andrade conducted the study because the risk of skeletal and dental fractures associated with ECT is “not commonly discussed.”
Although ECT is perhaps the most effective available treatment for major mental illness, it is associated with several adverse effects, including those associated with delivery of an electrical stimulus to the brain, which results in central and peripheral seizure, he noted.
“The central seizure is essential for the efficacy of ECT,” said Dr. Andrade. In contrast, “the motor seizure has no therapeutic value, is cosmetically displeasing, and may rarely be associated with peripheral adverse effects affecting muscles, joints, teeth, and bones,” he added.
The musculoskeletal and dental injuries are caused by stretching, twisting, compression, or direct injury. Particularly during the motor seizure, the “sudden jerk” associated with the tonic contraction of muscles as well as the repeated jerks associated with each clonic contraction can result in injuries, including skeletal and dental fractures.
To address this concern, the motor seizure is “modified” or attenuated through use of an intravenous muscle relaxant administered with other ECT premedication.
“How effectively the musculoskeletal and dental adverse effects are minimized depends on how well the motor seizure is modified,” Dr. Andrade said. He emphasized that the “use of unmodified ECT is strongly discouraged.”
Dr. Andrade reviewed prior research into the skeletal and dental risks of ECT. The infrequency of cases and ethical difficulties in conducting randomized clinical trials with such patients require reliance on anecdotal reports, he said.
Bite blocks, seizure modifiers
Population-based data showed that the fracture risk with modified ECT is two events per 100,000 ECTs. However, the risk may be as low as 0.36 events per 100,000 ECTs if calculated only with recent data, Dr. Andrade noted.
Population-based studies also suggest that the dental fracture risk with modified ECT is .02% per ECT and .17% per ECT course.
Although fractures have been reported under “unusual circumstances” among patients receiving modified ECT, many other reports point to the safety of this treatment, even in ultrahigh-risk patients.
Such patients include those with severe osteoporosis, metastatic bone disease, osteogenesis imperfecta, Ehlers-Danlos syndrome, Harrington rod implants, recent long bone fractures, multiple bone fractures, surgical repair of hip fracture, vertebroplasty, and maxillofacial repair.
Dr. Andrade noted that oral health is “poor” among patients with major mental illness for multiple reasons, including poor nutrition, self-neglect, and decreased salivation caused by the anticholinergic effects of medications.
This places these patients at increased risk for dental adverse effects during ECT because the muscles of the jaw contract forcefully during the motor seizure, causing sudden impact and, subsequently, sustained pressure on the teeth, Dr. Andrade said.
Moreover, because ECT is typically administered through repeated sessions, dental injuries may accumulate over the course of treatment.
ECT-associated skeletal risks arise from the tonic-clonic contractions of the muscles of the trunk and limbs, which need to be addressed via use of succinylcholine or other muscle relaxants included in ECT premedication.
Dr. Andrade noted that succinylcholine is effective at modifying the motor seizure at the common dose of 0.5-1.0 mg/kg. However, about 5% of patients require a higher dose (>1.5 mg/kg). If the dose is 1-2 mg/kg for patients at high risk for orthopedic complications, “muscle relaxation during ECT could be expected to be reasonably complete,” he said.
“Because of wide interpersonal variation, a neurostimulator may need to be used to identify the ideal dose for an individual patient,” he added.
In addition, use of bite blocks and effective jaw immobilization during ECT can reduce the risk. “Careful assessment of preexisting risk and good ECT practice can minimize the risk of skeletal and dental complications during ECT,” Dr. Andrade said.
Risks vs. benefits
Commenting on the study, Mark S. George, MD, distinguished professor of psychiatry, radiology, and neurology, and director of the brain stimulation division, Medical University of South Carolina, Charleston, said this was a “well-written review of how frequently patients who are undergoing modern ECT have bone fractures or dental fractures during the procedure.”
Dr. George, who was not involved with the research, added that modern medications and management “make ECT a truly safe procedure.”
“It is not without some risk, but these risks are low, especially when compared to the risks of untreated or undertreated depression or catatonia, like suicide,” he said.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made directly to registered charities, but does not benefit financially from the relationship. His travel expenses for delivering lectures and workshops have been supported by the organizers themselves or pharmaceutical companies at the behest of the organizers. He has provided advice to various pharmaceutical companies and has received “nominal compensation.” He has also received payments for developing educational materials for scientific initiatives and programs, such as for the Behavioral and Neurosciences Foundation of India, PsyBase India, Texas Tech University USA, the Nordic Association for Convulsive Therapy, and the American Society of Clinical Psychopharmacology. Dr. George reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Troubling trend as both diabetes types rise among U.S. youth
The incidence of type 1 and type 2 diabetes continues to rise among children and adolescents in the United States, new data from the SEARCH for Diabetes in Youth study show.
The SEARCH data demonstrate an increase in the youth population aged 0-19 diagnosed with type 1 or type 2 diabetes in five representative U.S. centers. Between 2002 and 2018, the annual incidence rose by about 2% per year for type 1 diabetes and 5% per year for type 2 diabetes. The rates of increase for both types were greater among non-White than White youth.
These increases “will result in an expanding population of young adults at risk of developing early complications of diabetes whose health care needs will exceed those of their peers,” write Lynne E. Wagenknecht, DrPH, of Wake Forest University School of Medicine, Winston-Salem, N.C., and colleagues in their article, recently published in The Lancet Diabetes & Endocrinology.
In an accompanying editorial, Jonathan E. Shaw, MD, and Dianna J. Magliano, PhD, both at the Baker Heart and Diabetes Institute, Melbourne, write that one of the most “concerning findings” was a 7%-9% annual increase in the incidence of type 2 diabetes among Hispanic, Asian, and Pacific Islander populations.
“This is a health care crisis in the making. ...Youth and young-adult-onset type 2 diabetes are growing problems leading to poor outcomes and to widening social inequality, adversely affecting a population that might already be disadvantaged. Better information about its natural history, prevention, and management is urgently needed,” they write.
Upward trends in both diabetes types
Overall, 18,169 children and adolescents with type 1 diabetes and 5,293 with type 2 diabetes were identified over the 17-year study period in SEARCH. After adjustment for age, sex, and race/ethnicity, there was a significant increase in type 1 diabetes incidence from 19.5 cases/100,000 population in 2002-2003 to 22.2/100,000 in 2017-2018, a 2.02% annual increase.
The upward trend was even greater for type 2 diabetes, from 9.0/100,000 in 2002-2003 to 17.9/100,000 in 2017-2018, a 5.31% annual increase.
The annual rate of increase in type 1 diabetes was highest among Asian/Pacific Islander youth (4.84%), followed by Hispanic (4.14%) and Black youth (2.93%): All significantly rose over the 17 years.
For type 2 diabetes, significant annual rates of increase were also highest for Asian/Pacific Islanders (8.92%), followed by Hispanic (7.17%) and Black youth (5.99%).
Among youth aged 15-19 years, the overall incidence of type 2 diabetes exceeded that of type 1 diabetes (19.7 vs. 14.6/100,000).
The incidence of type 2 diabetes may be rising because of increased rates of obesity, as well as increased screening of at-risk youth, the authors say.
And, the editorialists note, obesity is also a risk factor for type 1 diabetes.
Peak incidence of type 1 diabetes occurred at age 10 years, while for type 2 diabetes, the peak was at 16 years. There were also seasonal peaks, occurring in January for type 1 diabetes and in August for type 2 diabetes. Those seasonal patterns have been previously reported; they are possibly because of increased viral infections and decreased sun exposure for the former, and increased physical exams in preparation for school in the latter, the authors speculate.
Dr. Shaw and Dr. Magliano note that the reduced incidence after age 16 years “might simply reflect a failure to diagnose,” suggesting that there will likely be an upturn in incidence in the subsequent decade.
The editorialists also point out: “Not only does the long duration of diabetes that youth-onset leads to cause a large burden of fatal and nonfatal complications, but it magnifies intergenerational effects.”
“When type 2 diabetes is already present before pregnancy, birth outcomes are worse, and the long-term metabolic health of the offspring is adversely affected. This does not bode well for the epidemic of diabetes and its complications.”
The study was funded by the Centers for Disease Control and Prevention and National Institutes of Health. The authors and Dr. Magliano have reported no relevant financial relationships. Dr. Shaw has reported receiving honoraria for lectures and for advisory boards and grants from AstraZeneca, Boehringer Ingelheim, Pfizer, Eli Lilly, Sanofi, Roche, Mylan, and Zuellig Pharma.
A version of this article originally appeared on Medscape.com.
The incidence of type 1 and type 2 diabetes continues to rise among children and adolescents in the United States, new data from the SEARCH for Diabetes in Youth study show.
The SEARCH data demonstrate an increase in the youth population aged 0-19 diagnosed with type 1 or type 2 diabetes in five representative U.S. centers. Between 2002 and 2018, the annual incidence rose by about 2% per year for type 1 diabetes and 5% per year for type 2 diabetes. The rates of increase for both types were greater among non-White than White youth.
These increases “will result in an expanding population of young adults at risk of developing early complications of diabetes whose health care needs will exceed those of their peers,” write Lynne E. Wagenknecht, DrPH, of Wake Forest University School of Medicine, Winston-Salem, N.C., and colleagues in their article, recently published in The Lancet Diabetes & Endocrinology.
In an accompanying editorial, Jonathan E. Shaw, MD, and Dianna J. Magliano, PhD, both at the Baker Heart and Diabetes Institute, Melbourne, write that one of the most “concerning findings” was a 7%-9% annual increase in the incidence of type 2 diabetes among Hispanic, Asian, and Pacific Islander populations.
“This is a health care crisis in the making. ...Youth and young-adult-onset type 2 diabetes are growing problems leading to poor outcomes and to widening social inequality, adversely affecting a population that might already be disadvantaged. Better information about its natural history, prevention, and management is urgently needed,” they write.
Upward trends in both diabetes types
Overall, 18,169 children and adolescents with type 1 diabetes and 5,293 with type 2 diabetes were identified over the 17-year study period in SEARCH. After adjustment for age, sex, and race/ethnicity, there was a significant increase in type 1 diabetes incidence from 19.5 cases/100,000 population in 2002-2003 to 22.2/100,000 in 2017-2018, a 2.02% annual increase.
The upward trend was even greater for type 2 diabetes, from 9.0/100,000 in 2002-2003 to 17.9/100,000 in 2017-2018, a 5.31% annual increase.
The annual rate of increase in type 1 diabetes was highest among Asian/Pacific Islander youth (4.84%), followed by Hispanic (4.14%) and Black youth (2.93%): All significantly rose over the 17 years.
For type 2 diabetes, significant annual rates of increase were also highest for Asian/Pacific Islanders (8.92%), followed by Hispanic (7.17%) and Black youth (5.99%).
Among youth aged 15-19 years, the overall incidence of type 2 diabetes exceeded that of type 1 diabetes (19.7 vs. 14.6/100,000).
The incidence of type 2 diabetes may be rising because of increased rates of obesity, as well as increased screening of at-risk youth, the authors say.
And, the editorialists note, obesity is also a risk factor for type 1 diabetes.
Peak incidence of type 1 diabetes occurred at age 10 years, while for type 2 diabetes, the peak was at 16 years. There were also seasonal peaks, occurring in January for type 1 diabetes and in August for type 2 diabetes. Those seasonal patterns have been previously reported; they are possibly because of increased viral infections and decreased sun exposure for the former, and increased physical exams in preparation for school in the latter, the authors speculate.
Dr. Shaw and Dr. Magliano note that the reduced incidence after age 16 years “might simply reflect a failure to diagnose,” suggesting that there will likely be an upturn in incidence in the subsequent decade.
The editorialists also point out: “Not only does the long duration of diabetes that youth-onset leads to cause a large burden of fatal and nonfatal complications, but it magnifies intergenerational effects.”
“When type 2 diabetes is already present before pregnancy, birth outcomes are worse, and the long-term metabolic health of the offspring is adversely affected. This does not bode well for the epidemic of diabetes and its complications.”
The study was funded by the Centers for Disease Control and Prevention and National Institutes of Health. The authors and Dr. Magliano have reported no relevant financial relationships. Dr. Shaw has reported receiving honoraria for lectures and for advisory boards and grants from AstraZeneca, Boehringer Ingelheim, Pfizer, Eli Lilly, Sanofi, Roche, Mylan, and Zuellig Pharma.
A version of this article originally appeared on Medscape.com.
The incidence of type 1 and type 2 diabetes continues to rise among children and adolescents in the United States, new data from the SEARCH for Diabetes in Youth study show.
The SEARCH data demonstrate an increase in the youth population aged 0-19 diagnosed with type 1 or type 2 diabetes in five representative U.S. centers. Between 2002 and 2018, the annual incidence rose by about 2% per year for type 1 diabetes and 5% per year for type 2 diabetes. The rates of increase for both types were greater among non-White than White youth.
These increases “will result in an expanding population of young adults at risk of developing early complications of diabetes whose health care needs will exceed those of their peers,” write Lynne E. Wagenknecht, DrPH, of Wake Forest University School of Medicine, Winston-Salem, N.C., and colleagues in their article, recently published in The Lancet Diabetes & Endocrinology.
In an accompanying editorial, Jonathan E. Shaw, MD, and Dianna J. Magliano, PhD, both at the Baker Heart and Diabetes Institute, Melbourne, write that one of the most “concerning findings” was a 7%-9% annual increase in the incidence of type 2 diabetes among Hispanic, Asian, and Pacific Islander populations.
“This is a health care crisis in the making. ...Youth and young-adult-onset type 2 diabetes are growing problems leading to poor outcomes and to widening social inequality, adversely affecting a population that might already be disadvantaged. Better information about its natural history, prevention, and management is urgently needed,” they write.
Upward trends in both diabetes types
Overall, 18,169 children and adolescents with type 1 diabetes and 5,293 with type 2 diabetes were identified over the 17-year study period in SEARCH. After adjustment for age, sex, and race/ethnicity, there was a significant increase in type 1 diabetes incidence from 19.5 cases/100,000 population in 2002-2003 to 22.2/100,000 in 2017-2018, a 2.02% annual increase.
The upward trend was even greater for type 2 diabetes, from 9.0/100,000 in 2002-2003 to 17.9/100,000 in 2017-2018, a 5.31% annual increase.
The annual rate of increase in type 1 diabetes was highest among Asian/Pacific Islander youth (4.84%), followed by Hispanic (4.14%) and Black youth (2.93%): All significantly rose over the 17 years.
For type 2 diabetes, significant annual rates of increase were also highest for Asian/Pacific Islanders (8.92%), followed by Hispanic (7.17%) and Black youth (5.99%).
Among youth aged 15-19 years, the overall incidence of type 2 diabetes exceeded that of type 1 diabetes (19.7 vs. 14.6/100,000).
The incidence of type 2 diabetes may be rising because of increased rates of obesity, as well as increased screening of at-risk youth, the authors say.
And, the editorialists note, obesity is also a risk factor for type 1 diabetes.
Peak incidence of type 1 diabetes occurred at age 10 years, while for type 2 diabetes, the peak was at 16 years. There were also seasonal peaks, occurring in January for type 1 diabetes and in August for type 2 diabetes. Those seasonal patterns have been previously reported; they are possibly because of increased viral infections and decreased sun exposure for the former, and increased physical exams in preparation for school in the latter, the authors speculate.
Dr. Shaw and Dr. Magliano note that the reduced incidence after age 16 years “might simply reflect a failure to diagnose,” suggesting that there will likely be an upturn in incidence in the subsequent decade.
The editorialists also point out: “Not only does the long duration of diabetes that youth-onset leads to cause a large burden of fatal and nonfatal complications, but it magnifies intergenerational effects.”
“When type 2 diabetes is already present before pregnancy, birth outcomes are worse, and the long-term metabolic health of the offspring is adversely affected. This does not bode well for the epidemic of diabetes and its complications.”
The study was funded by the Centers for Disease Control and Prevention and National Institutes of Health. The authors and Dr. Magliano have reported no relevant financial relationships. Dr. Shaw has reported receiving honoraria for lectures and for advisory boards and grants from AstraZeneca, Boehringer Ingelheim, Pfizer, Eli Lilly, Sanofi, Roche, Mylan, and Zuellig Pharma.
A version of this article originally appeared on Medscape.com.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
An earlier hep B biomarker for clinical outcomes?
Low serum levels of the hepatitis B core-related antigen (HBcrAg) could be an early biomarker of a functional cure of a hepatitis B infection, according to new findings from a retrospective study.
A drop in HBcrAg predicted the seroclearance of hepatitis B surface antigen (HBsAg), the widely accepted measure of optimal liver-related outcomes in patient care and clinical trials, long before HBsAg levels actually fell.
“In a large retrospective cohort study of chronic hepatitis B patients, we found lower levels of HBcrAg were associated with higher probability of clearing HBsAg,” wrote Tai-Chung Tseng and coauthors at National Taiwan University Hospital in Taipei. “Reduction of HBcrAg developed 10 years before decline of HBsAg in patients with high HBsAg levels at baseline.”
Nearly 300 million people worldwide are estimated to be positive for the HBsAg antigen, a marker of active hepatitis B virus (HBV) infection. Chronic HBV puts individuals at greater risk of cirrhosis, hepatocellular carcinoma (HCC), and other liver complications.
Seroclearance of HBsAg is generally regarded as signaling a functional cure, because it is associated with low viral activity and good clinical outcomes. Patients with low HBsAg levels may transition to complete clearance, while those with levels of 1,000 IU/mL or higher rarely achieve clearance either spontaneously or through treatment.
As with HBsAg, higher serum levels of HBcrAg have been linked to a raised risk of adverse events, including increased viral activity and heightened risk of developing hepatitis B e antigen-negative hepatitis, cirrhosis, and HCC. Lower HBcrAg levels are associated with a greater likelihood of HBsAg seroclearance in chronic hepatitis B patients who discontinued antiviral therapy.
In a study published in Gastroenterology, researchers conducted a retrospective Taiwanese cohort study of 2,614 untreated patients with hepatitis B who underwent long-term follow-up at National Taiwan University Hospital. The median age was 38.2 years, and 60.6% were men. At baseline, 14.8% had HBsAg levels of less than 100 IU/mL, and 47.7% had HBcrAg levels less than 10,000 IU/mL. Most (77.5%) were infected with HBV genotype B. From stored serum samples, the researchers quantified levels of HBV DNA, HBsAg, and HBcrAg and evaluated the relationships with spontaneous HBsAg seroclearance.
Over an average follow-up of about 12 years, 465 patients cleared HBsAg, an incidence of 1.43% per year. Researchers stratified patients by levels of viral markers. Compared to those with the highest HBcrAg levels (> 100,000 IU/mL), lower levels of HBcrAg were associated with greater likelihood of HBsAg clearance.
Specifically, intermediate levels (10,000-99,999 IU/mL) were associated with nearly double the chance of HBsAg clearance (hazard ratio [HR], 1.95; 95% confidence interval [CI], 1.44-2.65), and the lowest levels (< 10,000 IU/mL) were associated with just over triple the chance of clearance (HR, 3.15; 95% CI, 2.45-4.05). These associations held up with multivariable analyses, and HBV DNA levels were not significantly associated with HBsAg clearance.
“Not surprisingly, HBsAg levels still serve as a better predictor than the other two biomarkers,” the authors wrote. “Notably, the HBsAg levels are more like a short-term predictor” (within 5 years).
For patients with higher HBsAg levels (> 1,000 IU/mL), it took a median of 16 years to achieve HBsAg clearance. A subanalysis of the 1,539 patients with HbsAg levels > 1,000 IU/mL found that only HBcrAg levels below 10,000 IU/mL predicted HBsAg seroclearance versus 100,000 U/mL or higher (adjusted HR, 1.95; 95% CI, 1.16-3.27).
HBsAg levels began to decline later, often between 5 and 9 years before HBsAg seroclearance occurs. However, HBcrAg levels became undetectable 10-14 years before HBsAg seroclearance. Among patients achieving undetectable levels of HBcrAg, the annual HBsAg seroclearance rate was higher in the second decade of follow-up than in the first decade (3.75% versus 0.97%).
HBcrAg levels reflect the transcriptional activity of covalently closed circular DNA (cccDNA), the authors noted, while HBsAg can come from cccDNA and HBV-DNA integrated into the host genome. Several novel hepatitis B therapies in development target cccDNA transcription, but it isn’t known if the strategy will result in HBsAg clearance.
In the discussion section, the authors speculated about the possible pathology and treatment implications for several chronic hepatitis B scenarios. For example, the finding that HBcrAg clearance usually precedes HBsAg clearance suggests that reduction of cccDNA transcription is a requirement for curing hepatitis B, the authors speculate, but it also suggests that add-on treatment may need to target HBsAg transcribed from the integrated viral genome for a functional cure.
The researchers noted several study limitations, including that the cohort included only Asians largely with HBV genotypes B or C and that “further validation from Caucasian patients infected with genotypes types A or D is mandatory.”
Tai-Chung Tseng disclosed financial conflicts with Fujirebio, Bristol-Myers Squibb, and Gilead Sciences. The remaining authors had no conflicts of interest. The study received grant support from several institutions, including National Taiwan University Hospital.
Current hepatitis B virus (HBV) therapies do not eliminate the covalently closed circular DNA (cccDNA), and a single cccDNA can cause a infection. Hepatitis B core-related antigen (HBcrAg) has shown positive correlation with serum and hepatic HBV-DNA levels and cccDNA even in patients receiving antivirals for HBV. This is demonstrated by Tseng et al., where undetectable levels of HBcrAg predicted seroclearance of HBsAg by 10-14 years. This and past studies have shown HBcrAg to be a good predictor for cccDNA transcriptional activity, allowing health care providers to predict functional loss of HBsAg, flare-ups, treatment response, and when to conclude treatment.
Clinically, HBcrAg could be monitored in chronic HBV infection while patients are receiving treatment. A rise in HBcrAg has the ability to predict HBV flares, while a decrease in HBcrAg can forecast seroclearance of HBsAg. If there is undetectable level of HBsAg with detectable HBcrAg, it can mean the relapse of HBsAg+, and oral treatment could be continued. HBsAg and HBcrAg also can be used to determine when to stop treatment, especially with nucleos(t)ide analogs (NAs). The Mayo Clinic laboratories recently opened HBcrAg testing for patients with chronic HBV.
With emerging medications, HBV cure may be possible with multiple therapies. Hepatic cccDNA turnover may be halted by inhibiting capsid assembly and secretion, relaxed-circular DNA (rcDNA) nuclear delivery or conversion to cccDNA, and formation of viral RNAs. Since HBcrAg is a good indicator of cccDNA transcriptional activity, it should be used to determine the effectiveness of these new therapies in clinical trials.
Katerina Roma, DO, is with the department of internal medicine, Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas. Robert Gish, MD, is medical director of the Hepatitis B Foundation in Doylestown, Pa. They have no financial conflicts.
Current hepatitis B virus (HBV) therapies do not eliminate the covalently closed circular DNA (cccDNA), and a single cccDNA can cause a infection. Hepatitis B core-related antigen (HBcrAg) has shown positive correlation with serum and hepatic HBV-DNA levels and cccDNA even in patients receiving antivirals for HBV. This is demonstrated by Tseng et al., where undetectable levels of HBcrAg predicted seroclearance of HBsAg by 10-14 years. This and past studies have shown HBcrAg to be a good predictor for cccDNA transcriptional activity, allowing health care providers to predict functional loss of HBsAg, flare-ups, treatment response, and when to conclude treatment.
Clinically, HBcrAg could be monitored in chronic HBV infection while patients are receiving treatment. A rise in HBcrAg has the ability to predict HBV flares, while a decrease in HBcrAg can forecast seroclearance of HBsAg. If there is undetectable level of HBsAg with detectable HBcrAg, it can mean the relapse of HBsAg+, and oral treatment could be continued. HBsAg and HBcrAg also can be used to determine when to stop treatment, especially with nucleos(t)ide analogs (NAs). The Mayo Clinic laboratories recently opened HBcrAg testing for patients with chronic HBV.
With emerging medications, HBV cure may be possible with multiple therapies. Hepatic cccDNA turnover may be halted by inhibiting capsid assembly and secretion, relaxed-circular DNA (rcDNA) nuclear delivery or conversion to cccDNA, and formation of viral RNAs. Since HBcrAg is a good indicator of cccDNA transcriptional activity, it should be used to determine the effectiveness of these new therapies in clinical trials.
Katerina Roma, DO, is with the department of internal medicine, Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas. Robert Gish, MD, is medical director of the Hepatitis B Foundation in Doylestown, Pa. They have no financial conflicts.
Current hepatitis B virus (HBV) therapies do not eliminate the covalently closed circular DNA (cccDNA), and a single cccDNA can cause a infection. Hepatitis B core-related antigen (HBcrAg) has shown positive correlation with serum and hepatic HBV-DNA levels and cccDNA even in patients receiving antivirals for HBV. This is demonstrated by Tseng et al., where undetectable levels of HBcrAg predicted seroclearance of HBsAg by 10-14 years. This and past studies have shown HBcrAg to be a good predictor for cccDNA transcriptional activity, allowing health care providers to predict functional loss of HBsAg, flare-ups, treatment response, and when to conclude treatment.
Clinically, HBcrAg could be monitored in chronic HBV infection while patients are receiving treatment. A rise in HBcrAg has the ability to predict HBV flares, while a decrease in HBcrAg can forecast seroclearance of HBsAg. If there is undetectable level of HBsAg with detectable HBcrAg, it can mean the relapse of HBsAg+, and oral treatment could be continued. HBsAg and HBcrAg also can be used to determine when to stop treatment, especially with nucleos(t)ide analogs (NAs). The Mayo Clinic laboratories recently opened HBcrAg testing for patients with chronic HBV.
With emerging medications, HBV cure may be possible with multiple therapies. Hepatic cccDNA turnover may be halted by inhibiting capsid assembly and secretion, relaxed-circular DNA (rcDNA) nuclear delivery or conversion to cccDNA, and formation of viral RNAs. Since HBcrAg is a good indicator of cccDNA transcriptional activity, it should be used to determine the effectiveness of these new therapies in clinical trials.
Katerina Roma, DO, is with the department of internal medicine, Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas. Robert Gish, MD, is medical director of the Hepatitis B Foundation in Doylestown, Pa. They have no financial conflicts.
Low serum levels of the hepatitis B core-related antigen (HBcrAg) could be an early biomarker of a functional cure of a hepatitis B infection, according to new findings from a retrospective study.
A drop in HBcrAg predicted the seroclearance of hepatitis B surface antigen (HBsAg), the widely accepted measure of optimal liver-related outcomes in patient care and clinical trials, long before HBsAg levels actually fell.
“In a large retrospective cohort study of chronic hepatitis B patients, we found lower levels of HBcrAg were associated with higher probability of clearing HBsAg,” wrote Tai-Chung Tseng and coauthors at National Taiwan University Hospital in Taipei. “Reduction of HBcrAg developed 10 years before decline of HBsAg in patients with high HBsAg levels at baseline.”
Nearly 300 million people worldwide are estimated to be positive for the HBsAg antigen, a marker of active hepatitis B virus (HBV) infection. Chronic HBV puts individuals at greater risk of cirrhosis, hepatocellular carcinoma (HCC), and other liver complications.
Seroclearance of HBsAg is generally regarded as signaling a functional cure, because it is associated with low viral activity and good clinical outcomes. Patients with low HBsAg levels may transition to complete clearance, while those with levels of 1,000 IU/mL or higher rarely achieve clearance either spontaneously or through treatment.
As with HBsAg, higher serum levels of HBcrAg have been linked to a raised risk of adverse events, including increased viral activity and heightened risk of developing hepatitis B e antigen-negative hepatitis, cirrhosis, and HCC. Lower HBcrAg levels are associated with a greater likelihood of HBsAg seroclearance in chronic hepatitis B patients who discontinued antiviral therapy.
In a study published in Gastroenterology, researchers conducted a retrospective Taiwanese cohort study of 2,614 untreated patients with hepatitis B who underwent long-term follow-up at National Taiwan University Hospital. The median age was 38.2 years, and 60.6% were men. At baseline, 14.8% had HBsAg levels of less than 100 IU/mL, and 47.7% had HBcrAg levels less than 10,000 IU/mL. Most (77.5%) were infected with HBV genotype B. From stored serum samples, the researchers quantified levels of HBV DNA, HBsAg, and HBcrAg and evaluated the relationships with spontaneous HBsAg seroclearance.
Over an average follow-up of about 12 years, 465 patients cleared HBsAg, an incidence of 1.43% per year. Researchers stratified patients by levels of viral markers. Compared to those with the highest HBcrAg levels (> 100,000 IU/mL), lower levels of HBcrAg were associated with greater likelihood of HBsAg clearance.
Specifically, intermediate levels (10,000-99,999 IU/mL) were associated with nearly double the chance of HBsAg clearance (hazard ratio [HR], 1.95; 95% confidence interval [CI], 1.44-2.65), and the lowest levels (< 10,000 IU/mL) were associated with just over triple the chance of clearance (HR, 3.15; 95% CI, 2.45-4.05). These associations held up with multivariable analyses, and HBV DNA levels were not significantly associated with HBsAg clearance.
“Not surprisingly, HBsAg levels still serve as a better predictor than the other two biomarkers,” the authors wrote. “Notably, the HBsAg levels are more like a short-term predictor” (within 5 years).
For patients with higher HBsAg levels (> 1,000 IU/mL), it took a median of 16 years to achieve HBsAg clearance. A subanalysis of the 1,539 patients with HbsAg levels > 1,000 IU/mL found that only HBcrAg levels below 10,000 IU/mL predicted HBsAg seroclearance versus 100,000 U/mL or higher (adjusted HR, 1.95; 95% CI, 1.16-3.27).
HBsAg levels began to decline later, often between 5 and 9 years before HBsAg seroclearance occurs. However, HBcrAg levels became undetectable 10-14 years before HBsAg seroclearance. Among patients achieving undetectable levels of HBcrAg, the annual HBsAg seroclearance rate was higher in the second decade of follow-up than in the first decade (3.75% versus 0.97%).
HBcrAg levels reflect the transcriptional activity of covalently closed circular DNA (cccDNA), the authors noted, while HBsAg can come from cccDNA and HBV-DNA integrated into the host genome. Several novel hepatitis B therapies in development target cccDNA transcription, but it isn’t known if the strategy will result in HBsAg clearance.
In the discussion section, the authors speculated about the possible pathology and treatment implications for several chronic hepatitis B scenarios. For example, the finding that HBcrAg clearance usually precedes HBsAg clearance suggests that reduction of cccDNA transcription is a requirement for curing hepatitis B, the authors speculate, but it also suggests that add-on treatment may need to target HBsAg transcribed from the integrated viral genome for a functional cure.
The researchers noted several study limitations, including that the cohort included only Asians largely with HBV genotypes B or C and that “further validation from Caucasian patients infected with genotypes types A or D is mandatory.”
Tai-Chung Tseng disclosed financial conflicts with Fujirebio, Bristol-Myers Squibb, and Gilead Sciences. The remaining authors had no conflicts of interest. The study received grant support from several institutions, including National Taiwan University Hospital.
Low serum levels of the hepatitis B core-related antigen (HBcrAg) could be an early biomarker of a functional cure of a hepatitis B infection, according to new findings from a retrospective study.
A drop in HBcrAg predicted the seroclearance of hepatitis B surface antigen (HBsAg), the widely accepted measure of optimal liver-related outcomes in patient care and clinical trials, long before HBsAg levels actually fell.
“In a large retrospective cohort study of chronic hepatitis B patients, we found lower levels of HBcrAg were associated with higher probability of clearing HBsAg,” wrote Tai-Chung Tseng and coauthors at National Taiwan University Hospital in Taipei. “Reduction of HBcrAg developed 10 years before decline of HBsAg in patients with high HBsAg levels at baseline.”
Nearly 300 million people worldwide are estimated to be positive for the HBsAg antigen, a marker of active hepatitis B virus (HBV) infection. Chronic HBV puts individuals at greater risk of cirrhosis, hepatocellular carcinoma (HCC), and other liver complications.
Seroclearance of HBsAg is generally regarded as signaling a functional cure, because it is associated with low viral activity and good clinical outcomes. Patients with low HBsAg levels may transition to complete clearance, while those with levels of 1,000 IU/mL or higher rarely achieve clearance either spontaneously or through treatment.
As with HBsAg, higher serum levels of HBcrAg have been linked to a raised risk of adverse events, including increased viral activity and heightened risk of developing hepatitis B e antigen-negative hepatitis, cirrhosis, and HCC. Lower HBcrAg levels are associated with a greater likelihood of HBsAg seroclearance in chronic hepatitis B patients who discontinued antiviral therapy.
In a study published in Gastroenterology, researchers conducted a retrospective Taiwanese cohort study of 2,614 untreated patients with hepatitis B who underwent long-term follow-up at National Taiwan University Hospital. The median age was 38.2 years, and 60.6% were men. At baseline, 14.8% had HBsAg levels of less than 100 IU/mL, and 47.7% had HBcrAg levels less than 10,000 IU/mL. Most (77.5%) were infected with HBV genotype B. From stored serum samples, the researchers quantified levels of HBV DNA, HBsAg, and HBcrAg and evaluated the relationships with spontaneous HBsAg seroclearance.
Over an average follow-up of about 12 years, 465 patients cleared HBsAg, an incidence of 1.43% per year. Researchers stratified patients by levels of viral markers. Compared to those with the highest HBcrAg levels (> 100,000 IU/mL), lower levels of HBcrAg were associated with greater likelihood of HBsAg clearance.
Specifically, intermediate levels (10,000-99,999 IU/mL) were associated with nearly double the chance of HBsAg clearance (hazard ratio [HR], 1.95; 95% confidence interval [CI], 1.44-2.65), and the lowest levels (< 10,000 IU/mL) were associated with just over triple the chance of clearance (HR, 3.15; 95% CI, 2.45-4.05). These associations held up with multivariable analyses, and HBV DNA levels were not significantly associated with HBsAg clearance.
“Not surprisingly, HBsAg levels still serve as a better predictor than the other two biomarkers,” the authors wrote. “Notably, the HBsAg levels are more like a short-term predictor” (within 5 years).
For patients with higher HBsAg levels (> 1,000 IU/mL), it took a median of 16 years to achieve HBsAg clearance. A subanalysis of the 1,539 patients with HbsAg levels > 1,000 IU/mL found that only HBcrAg levels below 10,000 IU/mL predicted HBsAg seroclearance versus 100,000 U/mL or higher (adjusted HR, 1.95; 95% CI, 1.16-3.27).
HBsAg levels began to decline later, often between 5 and 9 years before HBsAg seroclearance occurs. However, HBcrAg levels became undetectable 10-14 years before HBsAg seroclearance. Among patients achieving undetectable levels of HBcrAg, the annual HBsAg seroclearance rate was higher in the second decade of follow-up than in the first decade (3.75% versus 0.97%).
HBcrAg levels reflect the transcriptional activity of covalently closed circular DNA (cccDNA), the authors noted, while HBsAg can come from cccDNA and HBV-DNA integrated into the host genome. Several novel hepatitis B therapies in development target cccDNA transcription, but it isn’t known if the strategy will result in HBsAg clearance.
In the discussion section, the authors speculated about the possible pathology and treatment implications for several chronic hepatitis B scenarios. For example, the finding that HBcrAg clearance usually precedes HBsAg clearance suggests that reduction of cccDNA transcription is a requirement for curing hepatitis B, the authors speculate, but it also suggests that add-on treatment may need to target HBsAg transcribed from the integrated viral genome for a functional cure.
The researchers noted several study limitations, including that the cohort included only Asians largely with HBV genotypes B or C and that “further validation from Caucasian patients infected with genotypes types A or D is mandatory.”
Tai-Chung Tseng disclosed financial conflicts with Fujirebio, Bristol-Myers Squibb, and Gilead Sciences. The remaining authors had no conflicts of interest. The study received grant support from several institutions, including National Taiwan University Hospital.
FROM GASTROENTEROLOGY
Physical activity is a growing priority for patients with MS
SAN DIEGO – As , researchers have developed a mobile app to encourage young patients with the disease to become more active. The smartphone-based app provides tailored physical activity information, coaching advice, and tools to increase social connectedness.
A pilot study examining whether the intervention changes activity, depression, and fatigue levels should be wrapped up later this year, but it looks as though the app is succeeding.
“The feedback we’ve gotten so far from our coaches is that the kids seem highly motivated,” said one of the creators, E. Ann Yeh, MD, professor in the faculty of medicine at the University of Toronto and director of the pediatric MS and neuroinflammatory disorders program at the Hospital for Sick Children.
Preliminary work showed that use of the app was associated with a 31% increase in physical activity.
They discussed this and other studies of the role of exercise in MS at the annual meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
Higher levels of depression and fatigue
Studies show that youths with MS who are less physically active are more likely to experience higher levels of fatigue and depression. Evidence suggests just 15-30 more minutes of moderate to vigorous physical activity (MVPA) makes a clinical difference in terms of improved depression and fatigue scores, said Dr. Yeh.
With moderate physical activity (for example, a brisk walk or raking the yard), the maximal heart rate (HRmax) reaches 64%-76%, while with vigorous physical activity (which includes jogging/running or participating in a strenuous fitness class), the HRmax reaches 77%-93%.
Dr. Yeh described vigorous physical activity as “the stuff that makes you sweat, makes your heart rate go up, and makes you not be able to talk when you’re moving.”
As it stands, kids get very little MVPA – 9.5 min/day, which is well below the recommended 60 min/day. Adults do a bit better – 18.7 min/day of MVPA – but this is still below the recommended 30 min/day.
Being physically active improves fatigue for adults as well as kids, said Dr. Yeh. She referred to a network meta-analysis of 27 studies involving 1,470 participants that evaluated 10 types of exercise interventions, including yoga, resistance training, dance, and aquatic activities. It found that exercise “does move the needle,” she said. “Regardless of the kind of activity that was studied, fatigue seemed to improve.”
The authors of that study ranked aquatic exercise as the most effective intervention. “It’s possible that aquatics worked better because people who can’t move well feel more comfortable in the water,” Dr. Yeh said.
But she cautioned that the one study in the meta-analysis that found a “quite strong” effect of aquatic exercise was “very small.”
With regard to depression, which affects about 30% of people with MS, Dr. Yeh told meeting attendees, “unfortunately, the data are less clear” when it comes to physical activity for adults. One meta-analysis of 15 randomized controlled trials involving 331 exercising participants and 260 control persons found that only a few studies showed positive effects of exercise on depressive symptoms.
However, Dr. Yeh noted that in this review, the baseline depressive symptoms of participants were “above the cutoff level,” which makes it more difficult to demonstrate change in depression levels.
Clear structural effects
Researchers have also described clear brain structural and functional effects from being physically active. For example, MVPA has been shown to affect brain volume, and it has been associated with better optical coherence tomography (OCT) metrics, which measures retinal thinning.
As for the impact of exercise on memory deficits, which is of interest, given the current focus on Alzheimer’s disease, “the jury is still out,” said Dr. Yeh. One 24-week randomized controlled trial found no difference in results on the Brief Repeatable Battery of Neuropsychological tests between participants who engaged in progressive aerobic exercise and control persons.
However, said Dr. Yeh, “the problem may not be with the intervention but with the outcome measures” and potentially with the populations studied.
It might be a different story for high-intensity exercise, though. A study by Danish researchers assessed the effects of a 24-week high-intensity intervention among 84 adult patients with mild-severe impairment.
The primary outcome of that study, which was the percentage of brain volume change, was not met, possibly because the study was too short. There were significant results for some secondary endpoints, including improved cardiorespiratory fitness and lower relapse rate.
“Even though on the face of it, it sounds like a negative study, there were important outcomes,” said Dr. Yeh.
Research into the possible mechanisms behind positive effects of physical activity is limited with regard to patients with MS, said Dr. Yeh. Some studies have implicated certain circulating factors, such as the cytokine irisin and brain-derived neurotrophic factor, but more work is needed, she said.
“There is need for further mechanistic knowledge related to exercise in MS, and this must be accomplished through prospective, randomized studies.”
While exercise likely makes some difference for MS patients, the problem is in getting them to be more active. “You can’t just write a prescription,” said Dr. Yeh.
“Patients should be doing whatever they can, but gradually, and should not go crazy at the beginning because they’ll just burn out,” she said.
She stressed that patients need to find what works for them personally. It’s also important for them to find ways to be active with a friend who can be “a motivator” to help sustain physical activity goals, said Dr. Yeh.
Patients can also look online for remote physical activity programs geared to people with MS, which popped up during the pandemic.
Improved mood, cognition, pain, sleep
In a comment, Marwa Kaisey, MD, assistant professor of neurology at Cedars-Sinai Medical Center, in Los Angeles, who cochaired the session highlighting the presentation, praised Dr. Yeh’s “excellent talk,” which highlighted the “strong benefit” of exercise for patients with MS.
“As a clinician, I often talk to my patients about the importance of physical exercise and have heard countless anecdotes of how their workout programs helped improve mood, cognition, pain, or sleep.”
However, she agreed there are several areas “where we need more data-driven solutions and a mechanistic understanding of the benefits of physical exercise.”
The pilot study was funded by the Consortium of Multiple Sclerosis Centers. The MS Society of Canada funded early work on the app, and the National MS Society is funding the trial of the app. Dr. Yeh receives support from the MS Society of Canada. Dr. Kaisey reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – As , researchers have developed a mobile app to encourage young patients with the disease to become more active. The smartphone-based app provides tailored physical activity information, coaching advice, and tools to increase social connectedness.
A pilot study examining whether the intervention changes activity, depression, and fatigue levels should be wrapped up later this year, but it looks as though the app is succeeding.
“The feedback we’ve gotten so far from our coaches is that the kids seem highly motivated,” said one of the creators, E. Ann Yeh, MD, professor in the faculty of medicine at the University of Toronto and director of the pediatric MS and neuroinflammatory disorders program at the Hospital for Sick Children.
Preliminary work showed that use of the app was associated with a 31% increase in physical activity.
They discussed this and other studies of the role of exercise in MS at the annual meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
Higher levels of depression and fatigue
Studies show that youths with MS who are less physically active are more likely to experience higher levels of fatigue and depression. Evidence suggests just 15-30 more minutes of moderate to vigorous physical activity (MVPA) makes a clinical difference in terms of improved depression and fatigue scores, said Dr. Yeh.
With moderate physical activity (for example, a brisk walk or raking the yard), the maximal heart rate (HRmax) reaches 64%-76%, while with vigorous physical activity (which includes jogging/running or participating in a strenuous fitness class), the HRmax reaches 77%-93%.
Dr. Yeh described vigorous physical activity as “the stuff that makes you sweat, makes your heart rate go up, and makes you not be able to talk when you’re moving.”
As it stands, kids get very little MVPA – 9.5 min/day, which is well below the recommended 60 min/day. Adults do a bit better – 18.7 min/day of MVPA – but this is still below the recommended 30 min/day.
Being physically active improves fatigue for adults as well as kids, said Dr. Yeh. She referred to a network meta-analysis of 27 studies involving 1,470 participants that evaluated 10 types of exercise interventions, including yoga, resistance training, dance, and aquatic activities. It found that exercise “does move the needle,” she said. “Regardless of the kind of activity that was studied, fatigue seemed to improve.”
The authors of that study ranked aquatic exercise as the most effective intervention. “It’s possible that aquatics worked better because people who can’t move well feel more comfortable in the water,” Dr. Yeh said.
But she cautioned that the one study in the meta-analysis that found a “quite strong” effect of aquatic exercise was “very small.”
With regard to depression, which affects about 30% of people with MS, Dr. Yeh told meeting attendees, “unfortunately, the data are less clear” when it comes to physical activity for adults. One meta-analysis of 15 randomized controlled trials involving 331 exercising participants and 260 control persons found that only a few studies showed positive effects of exercise on depressive symptoms.
However, Dr. Yeh noted that in this review, the baseline depressive symptoms of participants were “above the cutoff level,” which makes it more difficult to demonstrate change in depression levels.
Clear structural effects
Researchers have also described clear brain structural and functional effects from being physically active. For example, MVPA has been shown to affect brain volume, and it has been associated with better optical coherence tomography (OCT) metrics, which measures retinal thinning.
As for the impact of exercise on memory deficits, which is of interest, given the current focus on Alzheimer’s disease, “the jury is still out,” said Dr. Yeh. One 24-week randomized controlled trial found no difference in results on the Brief Repeatable Battery of Neuropsychological tests between participants who engaged in progressive aerobic exercise and control persons.
However, said Dr. Yeh, “the problem may not be with the intervention but with the outcome measures” and potentially with the populations studied.
It might be a different story for high-intensity exercise, though. A study by Danish researchers assessed the effects of a 24-week high-intensity intervention among 84 adult patients with mild-severe impairment.
The primary outcome of that study, which was the percentage of brain volume change, was not met, possibly because the study was too short. There were significant results for some secondary endpoints, including improved cardiorespiratory fitness and lower relapse rate.
“Even though on the face of it, it sounds like a negative study, there were important outcomes,” said Dr. Yeh.
Research into the possible mechanisms behind positive effects of physical activity is limited with regard to patients with MS, said Dr. Yeh. Some studies have implicated certain circulating factors, such as the cytokine irisin and brain-derived neurotrophic factor, but more work is needed, she said.
“There is need for further mechanistic knowledge related to exercise in MS, and this must be accomplished through prospective, randomized studies.”
While exercise likely makes some difference for MS patients, the problem is in getting them to be more active. “You can’t just write a prescription,” said Dr. Yeh.
“Patients should be doing whatever they can, but gradually, and should not go crazy at the beginning because they’ll just burn out,” she said.
She stressed that patients need to find what works for them personally. It’s also important for them to find ways to be active with a friend who can be “a motivator” to help sustain physical activity goals, said Dr. Yeh.
Patients can also look online for remote physical activity programs geared to people with MS, which popped up during the pandemic.
Improved mood, cognition, pain, sleep
In a comment, Marwa Kaisey, MD, assistant professor of neurology at Cedars-Sinai Medical Center, in Los Angeles, who cochaired the session highlighting the presentation, praised Dr. Yeh’s “excellent talk,” which highlighted the “strong benefit” of exercise for patients with MS.
“As a clinician, I often talk to my patients about the importance of physical exercise and have heard countless anecdotes of how their workout programs helped improve mood, cognition, pain, or sleep.”
However, she agreed there are several areas “where we need more data-driven solutions and a mechanistic understanding of the benefits of physical exercise.”
The pilot study was funded by the Consortium of Multiple Sclerosis Centers. The MS Society of Canada funded early work on the app, and the National MS Society is funding the trial of the app. Dr. Yeh receives support from the MS Society of Canada. Dr. Kaisey reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – As , researchers have developed a mobile app to encourage young patients with the disease to become more active. The smartphone-based app provides tailored physical activity information, coaching advice, and tools to increase social connectedness.
A pilot study examining whether the intervention changes activity, depression, and fatigue levels should be wrapped up later this year, but it looks as though the app is succeeding.
“The feedback we’ve gotten so far from our coaches is that the kids seem highly motivated,” said one of the creators, E. Ann Yeh, MD, professor in the faculty of medicine at the University of Toronto and director of the pediatric MS and neuroinflammatory disorders program at the Hospital for Sick Children.
Preliminary work showed that use of the app was associated with a 31% increase in physical activity.
They discussed this and other studies of the role of exercise in MS at the annual meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
Higher levels of depression and fatigue
Studies show that youths with MS who are less physically active are more likely to experience higher levels of fatigue and depression. Evidence suggests just 15-30 more minutes of moderate to vigorous physical activity (MVPA) makes a clinical difference in terms of improved depression and fatigue scores, said Dr. Yeh.
With moderate physical activity (for example, a brisk walk or raking the yard), the maximal heart rate (HRmax) reaches 64%-76%, while with vigorous physical activity (which includes jogging/running or participating in a strenuous fitness class), the HRmax reaches 77%-93%.
Dr. Yeh described vigorous physical activity as “the stuff that makes you sweat, makes your heart rate go up, and makes you not be able to talk when you’re moving.”
As it stands, kids get very little MVPA – 9.5 min/day, which is well below the recommended 60 min/day. Adults do a bit better – 18.7 min/day of MVPA – but this is still below the recommended 30 min/day.
Being physically active improves fatigue for adults as well as kids, said Dr. Yeh. She referred to a network meta-analysis of 27 studies involving 1,470 participants that evaluated 10 types of exercise interventions, including yoga, resistance training, dance, and aquatic activities. It found that exercise “does move the needle,” she said. “Regardless of the kind of activity that was studied, fatigue seemed to improve.”
The authors of that study ranked aquatic exercise as the most effective intervention. “It’s possible that aquatics worked better because people who can’t move well feel more comfortable in the water,” Dr. Yeh said.
But she cautioned that the one study in the meta-analysis that found a “quite strong” effect of aquatic exercise was “very small.”
With regard to depression, which affects about 30% of people with MS, Dr. Yeh told meeting attendees, “unfortunately, the data are less clear” when it comes to physical activity for adults. One meta-analysis of 15 randomized controlled trials involving 331 exercising participants and 260 control persons found that only a few studies showed positive effects of exercise on depressive symptoms.
However, Dr. Yeh noted that in this review, the baseline depressive symptoms of participants were “above the cutoff level,” which makes it more difficult to demonstrate change in depression levels.
Clear structural effects
Researchers have also described clear brain structural and functional effects from being physically active. For example, MVPA has been shown to affect brain volume, and it has been associated with better optical coherence tomography (OCT) metrics, which measures retinal thinning.
As for the impact of exercise on memory deficits, which is of interest, given the current focus on Alzheimer’s disease, “the jury is still out,” said Dr. Yeh. One 24-week randomized controlled trial found no difference in results on the Brief Repeatable Battery of Neuropsychological tests between participants who engaged in progressive aerobic exercise and control persons.
However, said Dr. Yeh, “the problem may not be with the intervention but with the outcome measures” and potentially with the populations studied.
It might be a different story for high-intensity exercise, though. A study by Danish researchers assessed the effects of a 24-week high-intensity intervention among 84 adult patients with mild-severe impairment.
The primary outcome of that study, which was the percentage of brain volume change, was not met, possibly because the study was too short. There were significant results for some secondary endpoints, including improved cardiorespiratory fitness and lower relapse rate.
“Even though on the face of it, it sounds like a negative study, there were important outcomes,” said Dr. Yeh.
Research into the possible mechanisms behind positive effects of physical activity is limited with regard to patients with MS, said Dr. Yeh. Some studies have implicated certain circulating factors, such as the cytokine irisin and brain-derived neurotrophic factor, but more work is needed, she said.
“There is need for further mechanistic knowledge related to exercise in MS, and this must be accomplished through prospective, randomized studies.”
While exercise likely makes some difference for MS patients, the problem is in getting them to be more active. “You can’t just write a prescription,” said Dr. Yeh.
“Patients should be doing whatever they can, but gradually, and should not go crazy at the beginning because they’ll just burn out,” she said.
She stressed that patients need to find what works for them personally. It’s also important for them to find ways to be active with a friend who can be “a motivator” to help sustain physical activity goals, said Dr. Yeh.
Patients can also look online for remote physical activity programs geared to people with MS, which popped up during the pandemic.
Improved mood, cognition, pain, sleep
In a comment, Marwa Kaisey, MD, assistant professor of neurology at Cedars-Sinai Medical Center, in Los Angeles, who cochaired the session highlighting the presentation, praised Dr. Yeh’s “excellent talk,” which highlighted the “strong benefit” of exercise for patients with MS.
“As a clinician, I often talk to my patients about the importance of physical exercise and have heard countless anecdotes of how their workout programs helped improve mood, cognition, pain, or sleep.”
However, she agreed there are several areas “where we need more data-driven solutions and a mechanistic understanding of the benefits of physical exercise.”
The pilot study was funded by the Consortium of Multiple Sclerosis Centers. The MS Society of Canada funded early work on the app, and the National MS Society is funding the trial of the app. Dr. Yeh receives support from the MS Society of Canada. Dr. Kaisey reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACTRIMS FORUM 2023
New insight into preventing antipsychotic-induced weight gain
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.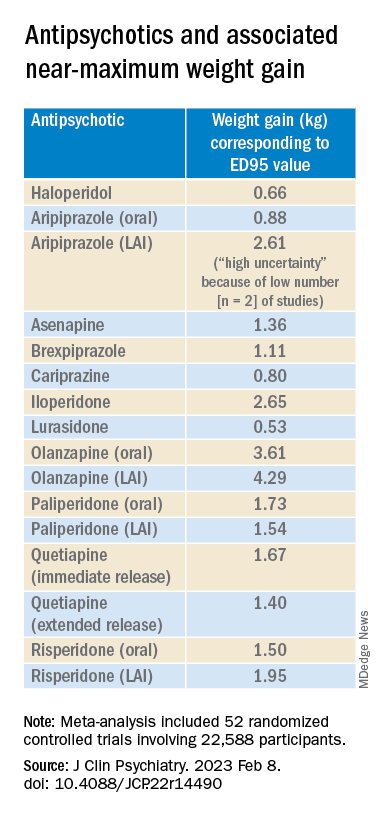
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Encouraging 3-year data for TAVR in low-risk patients: EVOLUT
Three-year results from the Evolut trial seem to provide more reassurance on the use of transcatheter aortic valve replacement (TAVR) in low-surgical-risk patients.
The 3-year results show that low-surgical-risk patients undergoing aortic valve replacement continue to show lower rates of all-cause mortality and disabling stroke with TAVR, compared with surgery.
The rates of all-cause mortality or disabling stroke (the primary endpoint) at 3 years were 7.4% with TAVR and 10.4% with surgery.
Rates of new pacemaker implantation continued to be higher after TAVR and the frequency of new onset atrial fibrillation was more common after surgery.
“At 3 years, the rate of all-cause mortality or disabling stroke after TAVR with the Evolut valve compared very favorably to surgery. The absolute difference between treatment arms remained consistent with a 30% relative reduction in the hazard of death or disabling stroke, with a P value that just missed statistical significance,” said Evolut investigator John Forrest, MD, Yale University School of Medicine, New Haven, Conn.
“The Kaplan-Meier curves show what we’ve come to expect – an early separation of the curves – but what’s unique here, and seen for the first time, is that the early separation is maintained at year 1 and year 2, and between years 2 and 3 the curve didn’t start to come together, but, if anything, separated a little,” Dr. Forrest commented.
“Both components of the primary endpoint – all cause mortality and disabling stroke – numerically favor TAVR. The separation of the curves for stroke are maintained, and if anything, we see a further slight separation of the curves as we go forward out to 3 years in terms of all-cause mortality,” he added.
Dr. Forrest presented the 3-year results from the Evolut trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. They were simultaneously published online in the Journal of the American College of Cardiology.
Dr. Forrest also reported that TAVR patients continued to have better valve hemodynamics at 3 years and very low rates of valve thrombosis; moreover, rates of moderate or greater paravalvular regurgitation and paravalvular leak (factors that can affect valve durability) were also low, although mild paravalvular regurgitation was higher with TAVR.
“In these low-risk patients, the durability of the valve is going to be critically important,” Dr. Forrest commented. “The excellent valve performance and durable outcomes out to 3 years in low-risk patients affirms the role of TAVR in this population,” he concluded.
On how these results may affect clinical practice, Dr. Forrest said: “I think in the U.S. these results reaffirm what we are doing. It gives us confidence to continue treating low-risk patients and being comfortable with that.”
He added: “Outside the U.S., the guidelines are a little different. Maybe we should reconsider some of these guidelines based on these data.”
David Moliterno, MD, Gill Heart and Vascular Institute, Lexington, Ky., who is not involved in the TAVR studies, said: “The results provide a little more reassurance ... that will go a little way further.”
“Uncertainty remains regarding long-term durability of the transcatheter valve in low-risk patients who are generally younger and likely more active than higher-risk cohorts,” he added. “The current 3-year results provide more confidence as the outcome curves for death and disabling stroke are trending in the right direction for TAVR versus surgery.”
Dr. Moliterno pointed out that while rates of paravalvular regurgitation and permanent pacemaker placement are decreasing with newer generation Evolut devices and implantation techniques, he noted that according to the U.S. Social Security Administration, patients aged 74 years as enrolled in this low-risk cohort have an additional life expectancy of approximately 12 years. “So, we have more device durability (and coronary access feasibility) to prove.”
In his presentation, Dr. Forrest explained that TAVR is now approved in the United States for all patients with aortic stenosis regardless of surgical risk and has become the dominant form of aortic valve replacement. Current ACC/AHA guidelines recommend that heart teams utilize a shared decision-making process when discussing aortic valve replacement with patients aged 65-80 years. In younger, lower-risk patients, the faster recovery and short-term benefits after TAVR must be balanced with long-term durability; however, only limited intermediate and long-term data exist to guide such discussions in this patient population.
The Evolut Low Risk trial randomly assigned 1,414 patients in need of aortic valve replacement to TAVR with a self-expanding, supra-annular valve or surgery. Results at 1 and 2 years have shown a similar benefit in the primary endpoint of all-cause mortality/disabling stroke for the less invasive TAVR procedure.
The current 3-year results suggest the benefit appears to be maintained out for another year.
The main results show that the rate of death or disabling stroke was 7.4% in the TAVR group versus 10.4% in the surgery group, giving a hazard ratio of 0.70 (P = .051).
In the JACC paper, the authors report that the absolute difference between treatment arms for all-cause mortality or disabling stroke remained broadly consistent over time: –1.8% at year 1; –2.0% at year 2; and –2.9% at year 3.
Other key results on valve durability show that mild paravalvular regurgitation was increased in the TAVR group (20.3%) versus 2.5% with surgery. However, rates of moderate or greater paravalvular regurgitation for both groups were below 1% and not significantly different between groups.
Patients who underwent TAVR had significantly improved valve hemodynamics (mean gradient 9.1 mm Hg TAVR vs. 12.1 mm Hg surgery; P < .001) at 3 years.
However, pacemaker placement was much higher in the TAVR group (23.2%), compared with 9.1% in the surgery group.
On the other hand, the surgery group had a greater incidence of atrial fibrillation (40%) versus 13% with TAVR.
Quality-of-life results looked good in both groups.
“As we’ve come to expect, patients recover more quickly after TAVR, so at 30 days their quality of life is better than those who have undergone surgery,” Dr. Forrest commented. “But by 1 year, both groups are doing exceptionally well and, remarkably, here by 3 years both groups have greater than a 20-point increase in their KCCQ score, showing a very large improvement in quality of life.”
Discussant of these latest results at the ACC late-breaking trials session, James Hermiller, MD, St. Vincent Ascension Heart Center, Indianapolis, said: “This 3-year data continues to demonstrate that the gift of TAVR keeps giving.”
Noting that the divergence in the effect curves was primarily driven by mortality rather than stroke, he asked whether this was cardiac or noncardiac mortality that was reduced.
Dr. Forrest responded: “It was a fairly equal contribution – a little bit more cardiac death. We have to remember that although the average age in this study was 74, there were some patients over 80 who were still low-surgical-risk included so we are going to see noncardiac death as well.”
Dr. Hermiller drew attention to the high pacemaker rate in the TAVR group and asked how these patients fared in comparison to those who didn’t need a pacemaker.
Dr. Forrest replied: “I think it’s fair to say that putting in a pacemaker is not a benign procedure. Patients who got a pacemaker did slightly worse than those who didn’t get a pacemaker, so we need to try to drive that rate down.”
He added that the number of patients needing a pacemaker after TAVR has come down with new implantation techniques and new generation valves.
“We realize that using a cusp overlap technique can significantly reduce the need for a pacemaker, and we see from registry data that with the use of this new technique the need for a pacemaker has dropped down to 8%-9%, significantly less than seen in this study,” Dr. Forrest commented.
Dr. Hermiller also asked about how TAVR affects future access for catheterization or percutaneous coronary intervention.
Dr. Forrest noted that 24 patients in the TAVR group required PCI in first 3 years, and all the PCI procedures had been successful. He noted that operators reported the procedure to be easy or moderately easy in about 75%-80% of cases and difficult in about 20% of patients. “So, it is slightly more challenging to engage the coronaries and have to go through the frame, but it is very feasible.”
Dr. Forrest concluded that: “These results provide patients and heart teams important data to aid in the shared decision-making process.”
But he acknowledged that longer term data are still needed. “And the potential impact that hemodynamics, valve design, new pacemakers, and other secondary endpoints have on long-term outcomes will be important to follow in this group of low-risk patients.”
The Evolut Low Risk trial was funded by Medtronic. Dr. Forrest has received grant support/research contracts and consultant fees/honoraria/speakers bureau fees from Edwards Lifesciences and Medtronic.
A version of this article first appeared on Medscape.com.
Three-year results from the Evolut trial seem to provide more reassurance on the use of transcatheter aortic valve replacement (TAVR) in low-surgical-risk patients.
The 3-year results show that low-surgical-risk patients undergoing aortic valve replacement continue to show lower rates of all-cause mortality and disabling stroke with TAVR, compared with surgery.
The rates of all-cause mortality or disabling stroke (the primary endpoint) at 3 years were 7.4% with TAVR and 10.4% with surgery.
Rates of new pacemaker implantation continued to be higher after TAVR and the frequency of new onset atrial fibrillation was more common after surgery.
“At 3 years, the rate of all-cause mortality or disabling stroke after TAVR with the Evolut valve compared very favorably to surgery. The absolute difference between treatment arms remained consistent with a 30% relative reduction in the hazard of death or disabling stroke, with a P value that just missed statistical significance,” said Evolut investigator John Forrest, MD, Yale University School of Medicine, New Haven, Conn.
“The Kaplan-Meier curves show what we’ve come to expect – an early separation of the curves – but what’s unique here, and seen for the first time, is that the early separation is maintained at year 1 and year 2, and between years 2 and 3 the curve didn’t start to come together, but, if anything, separated a little,” Dr. Forrest commented.
“Both components of the primary endpoint – all cause mortality and disabling stroke – numerically favor TAVR. The separation of the curves for stroke are maintained, and if anything, we see a further slight separation of the curves as we go forward out to 3 years in terms of all-cause mortality,” he added.
Dr. Forrest presented the 3-year results from the Evolut trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. They were simultaneously published online in the Journal of the American College of Cardiology.
Dr. Forrest also reported that TAVR patients continued to have better valve hemodynamics at 3 years and very low rates of valve thrombosis; moreover, rates of moderate or greater paravalvular regurgitation and paravalvular leak (factors that can affect valve durability) were also low, although mild paravalvular regurgitation was higher with TAVR.
“In these low-risk patients, the durability of the valve is going to be critically important,” Dr. Forrest commented. “The excellent valve performance and durable outcomes out to 3 years in low-risk patients affirms the role of TAVR in this population,” he concluded.
On how these results may affect clinical practice, Dr. Forrest said: “I think in the U.S. these results reaffirm what we are doing. It gives us confidence to continue treating low-risk patients and being comfortable with that.”
He added: “Outside the U.S., the guidelines are a little different. Maybe we should reconsider some of these guidelines based on these data.”
David Moliterno, MD, Gill Heart and Vascular Institute, Lexington, Ky., who is not involved in the TAVR studies, said: “The results provide a little more reassurance ... that will go a little way further.”
“Uncertainty remains regarding long-term durability of the transcatheter valve in low-risk patients who are generally younger and likely more active than higher-risk cohorts,” he added. “The current 3-year results provide more confidence as the outcome curves for death and disabling stroke are trending in the right direction for TAVR versus surgery.”
Dr. Moliterno pointed out that while rates of paravalvular regurgitation and permanent pacemaker placement are decreasing with newer generation Evolut devices and implantation techniques, he noted that according to the U.S. Social Security Administration, patients aged 74 years as enrolled in this low-risk cohort have an additional life expectancy of approximately 12 years. “So, we have more device durability (and coronary access feasibility) to prove.”
In his presentation, Dr. Forrest explained that TAVR is now approved in the United States for all patients with aortic stenosis regardless of surgical risk and has become the dominant form of aortic valve replacement. Current ACC/AHA guidelines recommend that heart teams utilize a shared decision-making process when discussing aortic valve replacement with patients aged 65-80 years. In younger, lower-risk patients, the faster recovery and short-term benefits after TAVR must be balanced with long-term durability; however, only limited intermediate and long-term data exist to guide such discussions in this patient population.
The Evolut Low Risk trial randomly assigned 1,414 patients in need of aortic valve replacement to TAVR with a self-expanding, supra-annular valve or surgery. Results at 1 and 2 years have shown a similar benefit in the primary endpoint of all-cause mortality/disabling stroke for the less invasive TAVR procedure.
The current 3-year results suggest the benefit appears to be maintained out for another year.
The main results show that the rate of death or disabling stroke was 7.4% in the TAVR group versus 10.4% in the surgery group, giving a hazard ratio of 0.70 (P = .051).
In the JACC paper, the authors report that the absolute difference between treatment arms for all-cause mortality or disabling stroke remained broadly consistent over time: –1.8% at year 1; –2.0% at year 2; and –2.9% at year 3.
Other key results on valve durability show that mild paravalvular regurgitation was increased in the TAVR group (20.3%) versus 2.5% with surgery. However, rates of moderate or greater paravalvular regurgitation for both groups were below 1% and not significantly different between groups.
Patients who underwent TAVR had significantly improved valve hemodynamics (mean gradient 9.1 mm Hg TAVR vs. 12.1 mm Hg surgery; P < .001) at 3 years.
However, pacemaker placement was much higher in the TAVR group (23.2%), compared with 9.1% in the surgery group.
On the other hand, the surgery group had a greater incidence of atrial fibrillation (40%) versus 13% with TAVR.
Quality-of-life results looked good in both groups.
“As we’ve come to expect, patients recover more quickly after TAVR, so at 30 days their quality of life is better than those who have undergone surgery,” Dr. Forrest commented. “But by 1 year, both groups are doing exceptionally well and, remarkably, here by 3 years both groups have greater than a 20-point increase in their KCCQ score, showing a very large improvement in quality of life.”
Discussant of these latest results at the ACC late-breaking trials session, James Hermiller, MD, St. Vincent Ascension Heart Center, Indianapolis, said: “This 3-year data continues to demonstrate that the gift of TAVR keeps giving.”
Noting that the divergence in the effect curves was primarily driven by mortality rather than stroke, he asked whether this was cardiac or noncardiac mortality that was reduced.
Dr. Forrest responded: “It was a fairly equal contribution – a little bit more cardiac death. We have to remember that although the average age in this study was 74, there were some patients over 80 who were still low-surgical-risk included so we are going to see noncardiac death as well.”
Dr. Hermiller drew attention to the high pacemaker rate in the TAVR group and asked how these patients fared in comparison to those who didn’t need a pacemaker.
Dr. Forrest replied: “I think it’s fair to say that putting in a pacemaker is not a benign procedure. Patients who got a pacemaker did slightly worse than those who didn’t get a pacemaker, so we need to try to drive that rate down.”
He added that the number of patients needing a pacemaker after TAVR has come down with new implantation techniques and new generation valves.
“We realize that using a cusp overlap technique can significantly reduce the need for a pacemaker, and we see from registry data that with the use of this new technique the need for a pacemaker has dropped down to 8%-9%, significantly less than seen in this study,” Dr. Forrest commented.
Dr. Hermiller also asked about how TAVR affects future access for catheterization or percutaneous coronary intervention.
Dr. Forrest noted that 24 patients in the TAVR group required PCI in first 3 years, and all the PCI procedures had been successful. He noted that operators reported the procedure to be easy or moderately easy in about 75%-80% of cases and difficult in about 20% of patients. “So, it is slightly more challenging to engage the coronaries and have to go through the frame, but it is very feasible.”
Dr. Forrest concluded that: “These results provide patients and heart teams important data to aid in the shared decision-making process.”
But he acknowledged that longer term data are still needed. “And the potential impact that hemodynamics, valve design, new pacemakers, and other secondary endpoints have on long-term outcomes will be important to follow in this group of low-risk patients.”
The Evolut Low Risk trial was funded by Medtronic. Dr. Forrest has received grant support/research contracts and consultant fees/honoraria/speakers bureau fees from Edwards Lifesciences and Medtronic.
A version of this article first appeared on Medscape.com.
Three-year results from the Evolut trial seem to provide more reassurance on the use of transcatheter aortic valve replacement (TAVR) in low-surgical-risk patients.
The 3-year results show that low-surgical-risk patients undergoing aortic valve replacement continue to show lower rates of all-cause mortality and disabling stroke with TAVR, compared with surgery.
The rates of all-cause mortality or disabling stroke (the primary endpoint) at 3 years were 7.4% with TAVR and 10.4% with surgery.
Rates of new pacemaker implantation continued to be higher after TAVR and the frequency of new onset atrial fibrillation was more common after surgery.
“At 3 years, the rate of all-cause mortality or disabling stroke after TAVR with the Evolut valve compared very favorably to surgery. The absolute difference between treatment arms remained consistent with a 30% relative reduction in the hazard of death or disabling stroke, with a P value that just missed statistical significance,” said Evolut investigator John Forrest, MD, Yale University School of Medicine, New Haven, Conn.
“The Kaplan-Meier curves show what we’ve come to expect – an early separation of the curves – but what’s unique here, and seen for the first time, is that the early separation is maintained at year 1 and year 2, and between years 2 and 3 the curve didn’t start to come together, but, if anything, separated a little,” Dr. Forrest commented.
“Both components of the primary endpoint – all cause mortality and disabling stroke – numerically favor TAVR. The separation of the curves for stroke are maintained, and if anything, we see a further slight separation of the curves as we go forward out to 3 years in terms of all-cause mortality,” he added.
Dr. Forrest presented the 3-year results from the Evolut trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. They were simultaneously published online in the Journal of the American College of Cardiology.
Dr. Forrest also reported that TAVR patients continued to have better valve hemodynamics at 3 years and very low rates of valve thrombosis; moreover, rates of moderate or greater paravalvular regurgitation and paravalvular leak (factors that can affect valve durability) were also low, although mild paravalvular regurgitation was higher with TAVR.
“In these low-risk patients, the durability of the valve is going to be critically important,” Dr. Forrest commented. “The excellent valve performance and durable outcomes out to 3 years in low-risk patients affirms the role of TAVR in this population,” he concluded.
On how these results may affect clinical practice, Dr. Forrest said: “I think in the U.S. these results reaffirm what we are doing. It gives us confidence to continue treating low-risk patients and being comfortable with that.”
He added: “Outside the U.S., the guidelines are a little different. Maybe we should reconsider some of these guidelines based on these data.”
David Moliterno, MD, Gill Heart and Vascular Institute, Lexington, Ky., who is not involved in the TAVR studies, said: “The results provide a little more reassurance ... that will go a little way further.”
“Uncertainty remains regarding long-term durability of the transcatheter valve in low-risk patients who are generally younger and likely more active than higher-risk cohorts,” he added. “The current 3-year results provide more confidence as the outcome curves for death and disabling stroke are trending in the right direction for TAVR versus surgery.”
Dr. Moliterno pointed out that while rates of paravalvular regurgitation and permanent pacemaker placement are decreasing with newer generation Evolut devices and implantation techniques, he noted that according to the U.S. Social Security Administration, patients aged 74 years as enrolled in this low-risk cohort have an additional life expectancy of approximately 12 years. “So, we have more device durability (and coronary access feasibility) to prove.”
In his presentation, Dr. Forrest explained that TAVR is now approved in the United States for all patients with aortic stenosis regardless of surgical risk and has become the dominant form of aortic valve replacement. Current ACC/AHA guidelines recommend that heart teams utilize a shared decision-making process when discussing aortic valve replacement with patients aged 65-80 years. In younger, lower-risk patients, the faster recovery and short-term benefits after TAVR must be balanced with long-term durability; however, only limited intermediate and long-term data exist to guide such discussions in this patient population.
The Evolut Low Risk trial randomly assigned 1,414 patients in need of aortic valve replacement to TAVR with a self-expanding, supra-annular valve or surgery. Results at 1 and 2 years have shown a similar benefit in the primary endpoint of all-cause mortality/disabling stroke for the less invasive TAVR procedure.
The current 3-year results suggest the benefit appears to be maintained out for another year.
The main results show that the rate of death or disabling stroke was 7.4% in the TAVR group versus 10.4% in the surgery group, giving a hazard ratio of 0.70 (P = .051).
In the JACC paper, the authors report that the absolute difference between treatment arms for all-cause mortality or disabling stroke remained broadly consistent over time: –1.8% at year 1; –2.0% at year 2; and –2.9% at year 3.
Other key results on valve durability show that mild paravalvular regurgitation was increased in the TAVR group (20.3%) versus 2.5% with surgery. However, rates of moderate or greater paravalvular regurgitation for both groups were below 1% and not significantly different between groups.
Patients who underwent TAVR had significantly improved valve hemodynamics (mean gradient 9.1 mm Hg TAVR vs. 12.1 mm Hg surgery; P < .001) at 3 years.
However, pacemaker placement was much higher in the TAVR group (23.2%), compared with 9.1% in the surgery group.
On the other hand, the surgery group had a greater incidence of atrial fibrillation (40%) versus 13% with TAVR.
Quality-of-life results looked good in both groups.
“As we’ve come to expect, patients recover more quickly after TAVR, so at 30 days their quality of life is better than those who have undergone surgery,” Dr. Forrest commented. “But by 1 year, both groups are doing exceptionally well and, remarkably, here by 3 years both groups have greater than a 20-point increase in their KCCQ score, showing a very large improvement in quality of life.”
Discussant of these latest results at the ACC late-breaking trials session, James Hermiller, MD, St. Vincent Ascension Heart Center, Indianapolis, said: “This 3-year data continues to demonstrate that the gift of TAVR keeps giving.”
Noting that the divergence in the effect curves was primarily driven by mortality rather than stroke, he asked whether this was cardiac or noncardiac mortality that was reduced.
Dr. Forrest responded: “It was a fairly equal contribution – a little bit more cardiac death. We have to remember that although the average age in this study was 74, there were some patients over 80 who were still low-surgical-risk included so we are going to see noncardiac death as well.”
Dr. Hermiller drew attention to the high pacemaker rate in the TAVR group and asked how these patients fared in comparison to those who didn’t need a pacemaker.
Dr. Forrest replied: “I think it’s fair to say that putting in a pacemaker is not a benign procedure. Patients who got a pacemaker did slightly worse than those who didn’t get a pacemaker, so we need to try to drive that rate down.”
He added that the number of patients needing a pacemaker after TAVR has come down with new implantation techniques and new generation valves.
“We realize that using a cusp overlap technique can significantly reduce the need for a pacemaker, and we see from registry data that with the use of this new technique the need for a pacemaker has dropped down to 8%-9%, significantly less than seen in this study,” Dr. Forrest commented.
Dr. Hermiller also asked about how TAVR affects future access for catheterization or percutaneous coronary intervention.
Dr. Forrest noted that 24 patients in the TAVR group required PCI in first 3 years, and all the PCI procedures had been successful. He noted that operators reported the procedure to be easy or moderately easy in about 75%-80% of cases and difficult in about 20% of patients. “So, it is slightly more challenging to engage the coronaries and have to go through the frame, but it is very feasible.”
Dr. Forrest concluded that: “These results provide patients and heart teams important data to aid in the shared decision-making process.”
But he acknowledged that longer term data are still needed. “And the potential impact that hemodynamics, valve design, new pacemakers, and other secondary endpoints have on long-term outcomes will be important to follow in this group of low-risk patients.”
The Evolut Low Risk trial was funded by Medtronic. Dr. Forrest has received grant support/research contracts and consultant fees/honoraria/speakers bureau fees from Edwards Lifesciences and Medtronic.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Two FDA clearances add diabetes technology options
Two diabetes management devices that aid in the precision of insulin delivery have been recently cleared by the Food and Drug Administration.
On March 2, the FDA cleared the Android version of Bigfoot Biomedical’s Unity Mobile App for use with its system of smart pen caps that are compatible with different disposable insulin pens for administering both long-acting and rapid-acting insulin.
The system, which has been compatible with iOS devices since May 2021, is “the first and only FDA-cleared smart injection system that turns CGM [continuous glucose monitoring] data into dosing recommendations displayed right on the pen cap for people using multiple daily [insulin] injection therapy,” according to a company statement.
The Bigfoot app allows users to input and review provider treatment recommendations, displays current glucose ranges, and delivers real-time alerts.
Once it is commercially launched, the Android phone application will be available via the Google Play Store. “Given that 41% of U.S. smartphone users choose Android devices, this clearance enables expanded access to a large group of people with diabetes,” the company said.
On March 6, the FDA cleared the Abbott FreeStyle Libre 2 and FreeStyle Libre 3 devices as “integrated” CGM sensors. This means that they can now be used as components in automated insulin delivery systems, along with insulin pumps and connectivity software.
Abbott is working with insulin pump manufacturers Insulet and Tandem in the United States for integration with the FreeStyle Libre versions 2 and 3. Outside the United States, the Libre 3 is already authorized to work with mylife Loop from Ypsomed and CamDiab in Germany. Further launches are expected in the United Kingdom, Switzerland, and the Netherlands later this year.
The modified FreeStyle Libre 2 and FreeStyle Libre 3 sensors have been cleared for use by patients as young as age 2 years and for up to 15 days, in contrast to the previous versions, which were available for patients as young as 4 years for use up to 14 days. The FDA has cleared all Libre sensors – 2 and 3, current and future versions – for use by pregnant women with any type of diabetes.
The modified sensors will be available in the United States later this year and will eventually replace the Libre sensors in current use, the company said in a statement.
“The FreeStyle Libre portfolio is still the most affordable CGM on the market,” an Abbott representative said in an interview.
A version of this article first appeared on Medscape.com.
Two diabetes management devices that aid in the precision of insulin delivery have been recently cleared by the Food and Drug Administration.
On March 2, the FDA cleared the Android version of Bigfoot Biomedical’s Unity Mobile App for use with its system of smart pen caps that are compatible with different disposable insulin pens for administering both long-acting and rapid-acting insulin.
The system, which has been compatible with iOS devices since May 2021, is “the first and only FDA-cleared smart injection system that turns CGM [continuous glucose monitoring] data into dosing recommendations displayed right on the pen cap for people using multiple daily [insulin] injection therapy,” according to a company statement.
The Bigfoot app allows users to input and review provider treatment recommendations, displays current glucose ranges, and delivers real-time alerts.
Once it is commercially launched, the Android phone application will be available via the Google Play Store. “Given that 41% of U.S. smartphone users choose Android devices, this clearance enables expanded access to a large group of people with diabetes,” the company said.
On March 6, the FDA cleared the Abbott FreeStyle Libre 2 and FreeStyle Libre 3 devices as “integrated” CGM sensors. This means that they can now be used as components in automated insulin delivery systems, along with insulin pumps and connectivity software.
Abbott is working with insulin pump manufacturers Insulet and Tandem in the United States for integration with the FreeStyle Libre versions 2 and 3. Outside the United States, the Libre 3 is already authorized to work with mylife Loop from Ypsomed and CamDiab in Germany. Further launches are expected in the United Kingdom, Switzerland, and the Netherlands later this year.
The modified FreeStyle Libre 2 and FreeStyle Libre 3 sensors have been cleared for use by patients as young as age 2 years and for up to 15 days, in contrast to the previous versions, which were available for patients as young as 4 years for use up to 14 days. The FDA has cleared all Libre sensors – 2 and 3, current and future versions – for use by pregnant women with any type of diabetes.
The modified sensors will be available in the United States later this year and will eventually replace the Libre sensors in current use, the company said in a statement.
“The FreeStyle Libre portfolio is still the most affordable CGM on the market,” an Abbott representative said in an interview.
A version of this article first appeared on Medscape.com.
Two diabetes management devices that aid in the precision of insulin delivery have been recently cleared by the Food and Drug Administration.
On March 2, the FDA cleared the Android version of Bigfoot Biomedical’s Unity Mobile App for use with its system of smart pen caps that are compatible with different disposable insulin pens for administering both long-acting and rapid-acting insulin.
The system, which has been compatible with iOS devices since May 2021, is “the first and only FDA-cleared smart injection system that turns CGM [continuous glucose monitoring] data into dosing recommendations displayed right on the pen cap for people using multiple daily [insulin] injection therapy,” according to a company statement.
The Bigfoot app allows users to input and review provider treatment recommendations, displays current glucose ranges, and delivers real-time alerts.
Once it is commercially launched, the Android phone application will be available via the Google Play Store. “Given that 41% of U.S. smartphone users choose Android devices, this clearance enables expanded access to a large group of people with diabetes,” the company said.
On March 6, the FDA cleared the Abbott FreeStyle Libre 2 and FreeStyle Libre 3 devices as “integrated” CGM sensors. This means that they can now be used as components in automated insulin delivery systems, along with insulin pumps and connectivity software.
Abbott is working with insulin pump manufacturers Insulet and Tandem in the United States for integration with the FreeStyle Libre versions 2 and 3. Outside the United States, the Libre 3 is already authorized to work with mylife Loop from Ypsomed and CamDiab in Germany. Further launches are expected in the United Kingdom, Switzerland, and the Netherlands later this year.
The modified FreeStyle Libre 2 and FreeStyle Libre 3 sensors have been cleared for use by patients as young as age 2 years and for up to 15 days, in contrast to the previous versions, which were available for patients as young as 4 years for use up to 14 days. The FDA has cleared all Libre sensors – 2 and 3, current and future versions – for use by pregnant women with any type of diabetes.
The modified sensors will be available in the United States later this year and will eventually replace the Libre sensors in current use, the company said in a statement.
“The FreeStyle Libre portfolio is still the most affordable CGM on the market,” an Abbott representative said in an interview.
A version of this article first appeared on Medscape.com.
APA releases updated eating disorder guidelines
The updated guidelines focus primarily on anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED) and include recommendations for screening and treatment.
“Eating disorders often are unrecognized and untreated,” Catherine Crone, MD, chair of the guideline writing group, said in a statement from APA. “This guideline and supplementary resources are intended to serve as a practical tool for clinicians, to help with screening, diagnosis, and providing evidence-based treatment for eating disorders.”
Approximately one in five children worldwide are at risk for developing an eating disorder and U.S. medical admissions for adolescents with restrictive eating disorders more than doubled during the pandemic.
The economic cost of eating disorders in the United States from 2018 to 2019 was an estimated $64.7 billion, the report notes, with an additional $326.5 billion attributable to reductions in well-being associated with eating disorders.
The executive summary of the updated guidelines was published online in The American Journal of Psychiatry.
The practice guideline, which was approved at the 2021 APA annual meeting, features 16 recommendations for clinicians, including screening patients for eating disorders as part of an initial psychiatric evaluation and conducting comprehensive patient evaluations that incorporate laboratory tests and electrocardiograms.
Recommendations also include setting individualized weight goals for patients with anorexia and incorporating family-based therapy as part of a treatment plan for adolescents with anorexia or bulimia.
“This practice guideline aims to help clinicians improve care for their patients by reviewing current evidence and providing evidence-based statements that are intended to enhance knowledge, increase assessment, and optimize treatment of eating disorders,” the authors wrote.
A range of other resources were released with the new guidelines to provide clinicians with support to implement the recommendations, including a pocket guide for clinicians, continuing medical education activities, and slides. The association is also launching a pocket guide for patients and families and an interactive tool kit with a screening assessment calculator.
The APA guidelines follow the 2021 release by the American Academy of Pediatrics on diagnosing and managing eating disorders in children and adolescents.
The development of the guidelines was supported by a grant from the Council of Medical Specialty Societies.
A version of this article first appeared on Medscape.com.
The updated guidelines focus primarily on anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED) and include recommendations for screening and treatment.
“Eating disorders often are unrecognized and untreated,” Catherine Crone, MD, chair of the guideline writing group, said in a statement from APA. “This guideline and supplementary resources are intended to serve as a practical tool for clinicians, to help with screening, diagnosis, and providing evidence-based treatment for eating disorders.”
Approximately one in five children worldwide are at risk for developing an eating disorder and U.S. medical admissions for adolescents with restrictive eating disorders more than doubled during the pandemic.
The economic cost of eating disorders in the United States from 2018 to 2019 was an estimated $64.7 billion, the report notes, with an additional $326.5 billion attributable to reductions in well-being associated with eating disorders.
The executive summary of the updated guidelines was published online in The American Journal of Psychiatry.
The practice guideline, which was approved at the 2021 APA annual meeting, features 16 recommendations for clinicians, including screening patients for eating disorders as part of an initial psychiatric evaluation and conducting comprehensive patient evaluations that incorporate laboratory tests and electrocardiograms.
Recommendations also include setting individualized weight goals for patients with anorexia and incorporating family-based therapy as part of a treatment plan for adolescents with anorexia or bulimia.
“This practice guideline aims to help clinicians improve care for their patients by reviewing current evidence and providing evidence-based statements that are intended to enhance knowledge, increase assessment, and optimize treatment of eating disorders,” the authors wrote.
A range of other resources were released with the new guidelines to provide clinicians with support to implement the recommendations, including a pocket guide for clinicians, continuing medical education activities, and slides. The association is also launching a pocket guide for patients and families and an interactive tool kit with a screening assessment calculator.
The APA guidelines follow the 2021 release by the American Academy of Pediatrics on diagnosing and managing eating disorders in children and adolescents.
The development of the guidelines was supported by a grant from the Council of Medical Specialty Societies.
A version of this article first appeared on Medscape.com.
The updated guidelines focus primarily on anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED) and include recommendations for screening and treatment.
“Eating disorders often are unrecognized and untreated,” Catherine Crone, MD, chair of the guideline writing group, said in a statement from APA. “This guideline and supplementary resources are intended to serve as a practical tool for clinicians, to help with screening, diagnosis, and providing evidence-based treatment for eating disorders.”
Approximately one in five children worldwide are at risk for developing an eating disorder and U.S. medical admissions for adolescents with restrictive eating disorders more than doubled during the pandemic.
The economic cost of eating disorders in the United States from 2018 to 2019 was an estimated $64.7 billion, the report notes, with an additional $326.5 billion attributable to reductions in well-being associated with eating disorders.
The executive summary of the updated guidelines was published online in The American Journal of Psychiatry.
The practice guideline, which was approved at the 2021 APA annual meeting, features 16 recommendations for clinicians, including screening patients for eating disorders as part of an initial psychiatric evaluation and conducting comprehensive patient evaluations that incorporate laboratory tests and electrocardiograms.
Recommendations also include setting individualized weight goals for patients with anorexia and incorporating family-based therapy as part of a treatment plan for adolescents with anorexia or bulimia.
“This practice guideline aims to help clinicians improve care for their patients by reviewing current evidence and providing evidence-based statements that are intended to enhance knowledge, increase assessment, and optimize treatment of eating disorders,” the authors wrote.
A range of other resources were released with the new guidelines to provide clinicians with support to implement the recommendations, including a pocket guide for clinicians, continuing medical education activities, and slides. The association is also launching a pocket guide for patients and families and an interactive tool kit with a screening assessment calculator.
The APA guidelines follow the 2021 release by the American Academy of Pediatrics on diagnosing and managing eating disorders in children and adolescents.
The development of the guidelines was supported by a grant from the Council of Medical Specialty Societies.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY






