User login
New ACC guidance on heart failure with preserved ejection fraction
The American College of Cardiology has released an Expert Consensus Decision Pathway (ECDP) on the management of heart failure with preserved ejection fraction (HFpEF).
The 44-page document highlights the “critical need” to accurately diagnose HFpEF to permit timely implementation of evidence- and guideline-based therapies to improve patient outcomes.
Although the incidence of overall HF in the United States appears to be stable or declining, the incidence of HFpEF continues to rise in tandem with increasing age and burdens of obesity, sedentary lifestyle, and cardiometabolic disorders.
HFpEF now accounts for more than one half of HF cases but remains “underrecognized” in everyday clinical practice, said the writing group, led by Michelle Kittleson, MD, PhD, professor of medicine, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
HFpEF is a complex condition, often with multiple overlapping comorbidities, including hypertension, diabetes, obesity, and sleep apnea; optimal management requires a multidisciplinary approach, the writing group said.
The ECDP on HFpEF lays out a structure for diagnosis, clinical decision-making, management of comorbidities, implementation of the latest guideline-directed medical therapy (pharmacologic and nonpharmacologic), and equitable delivery of care.
The document was published online in the Journal of the American College of Cardiology.
It aligns with and builds on recommendations from the 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure.
“HFpEF is one of the most pressing diagnostic and therapeutic challenges in clinical medicine today given its increasing prevalence, under diagnosis, poor prognosis, limited therapeutic options, and substantial burden on the health care system worldwide,” wrote the authors of a companion scientific statement on HFpEF.
Despite these challenges, the success of recent sodium-glucose cotransporter 2 inhibitor trials has shown that HFpEF is treatable, Barry Borlaug, MD, department of cardiovascular medicine, Mayo Clinic, Rochester, Minn., and coauthors pointed out.
They noted that “ongoing large-scale studies of HFpEF pathobiology, an increasing number of translational studies spanning the gap between the bedside and the bench, and numerous clinical trials of novel therapeutics in HFpEF offer a glimpse of hope toward a future of reduced prevalence, morbidity, and mortality associated with HFpEF, which would be a major advance for population health.”
A version of this article originally appeared on Medscape.com.
The American College of Cardiology has released an Expert Consensus Decision Pathway (ECDP) on the management of heart failure with preserved ejection fraction (HFpEF).
The 44-page document highlights the “critical need” to accurately diagnose HFpEF to permit timely implementation of evidence- and guideline-based therapies to improve patient outcomes.
Although the incidence of overall HF in the United States appears to be stable or declining, the incidence of HFpEF continues to rise in tandem with increasing age and burdens of obesity, sedentary lifestyle, and cardiometabolic disorders.
HFpEF now accounts for more than one half of HF cases but remains “underrecognized” in everyday clinical practice, said the writing group, led by Michelle Kittleson, MD, PhD, professor of medicine, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
HFpEF is a complex condition, often with multiple overlapping comorbidities, including hypertension, diabetes, obesity, and sleep apnea; optimal management requires a multidisciplinary approach, the writing group said.
The ECDP on HFpEF lays out a structure for diagnosis, clinical decision-making, management of comorbidities, implementation of the latest guideline-directed medical therapy (pharmacologic and nonpharmacologic), and equitable delivery of care.
The document was published online in the Journal of the American College of Cardiology.
It aligns with and builds on recommendations from the 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure.
“HFpEF is one of the most pressing diagnostic and therapeutic challenges in clinical medicine today given its increasing prevalence, under diagnosis, poor prognosis, limited therapeutic options, and substantial burden on the health care system worldwide,” wrote the authors of a companion scientific statement on HFpEF.
Despite these challenges, the success of recent sodium-glucose cotransporter 2 inhibitor trials has shown that HFpEF is treatable, Barry Borlaug, MD, department of cardiovascular medicine, Mayo Clinic, Rochester, Minn., and coauthors pointed out.
They noted that “ongoing large-scale studies of HFpEF pathobiology, an increasing number of translational studies spanning the gap between the bedside and the bench, and numerous clinical trials of novel therapeutics in HFpEF offer a glimpse of hope toward a future of reduced prevalence, morbidity, and mortality associated with HFpEF, which would be a major advance for population health.”
A version of this article originally appeared on Medscape.com.
The American College of Cardiology has released an Expert Consensus Decision Pathway (ECDP) on the management of heart failure with preserved ejection fraction (HFpEF).
The 44-page document highlights the “critical need” to accurately diagnose HFpEF to permit timely implementation of evidence- and guideline-based therapies to improve patient outcomes.
Although the incidence of overall HF in the United States appears to be stable or declining, the incidence of HFpEF continues to rise in tandem with increasing age and burdens of obesity, sedentary lifestyle, and cardiometabolic disorders.
HFpEF now accounts for more than one half of HF cases but remains “underrecognized” in everyday clinical practice, said the writing group, led by Michelle Kittleson, MD, PhD, professor of medicine, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
HFpEF is a complex condition, often with multiple overlapping comorbidities, including hypertension, diabetes, obesity, and sleep apnea; optimal management requires a multidisciplinary approach, the writing group said.
The ECDP on HFpEF lays out a structure for diagnosis, clinical decision-making, management of comorbidities, implementation of the latest guideline-directed medical therapy (pharmacologic and nonpharmacologic), and equitable delivery of care.
The document was published online in the Journal of the American College of Cardiology.
It aligns with and builds on recommendations from the 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure.
“HFpEF is one of the most pressing diagnostic and therapeutic challenges in clinical medicine today given its increasing prevalence, under diagnosis, poor prognosis, limited therapeutic options, and substantial burden on the health care system worldwide,” wrote the authors of a companion scientific statement on HFpEF.
Despite these challenges, the success of recent sodium-glucose cotransporter 2 inhibitor trials has shown that HFpEF is treatable, Barry Borlaug, MD, department of cardiovascular medicine, Mayo Clinic, Rochester, Minn., and coauthors pointed out.
They noted that “ongoing large-scale studies of HFpEF pathobiology, an increasing number of translational studies spanning the gap between the bedside and the bench, and numerous clinical trials of novel therapeutics in HFpEF offer a glimpse of hope toward a future of reduced prevalence, morbidity, and mortality associated with HFpEF, which would be a major advance for population health.”
A version of this article originally appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Proposed Medicare bill would raise docs’ pay with inflation
Introduced by four physician U.S. House representatives, HR 2474 would link Medicare fee schedule updates to the Medicare Economic Index, a measure of inflation related to physicians’ practice costs and wages.
That’s a long-sought goal of the American Medical Association, which is leading 120 state medical societies and medical specialty groups in championing the bill.
The legislation is essential to enabling physician practices to better absorb payment distributions triggered by budget neutrality rules, performance adjustments, and periods of high inflation, the groups wrote in a joint letter sent to the bill’s sponsors. The sponsors say they hope the legislation will improve access to care, as low reimbursements cause some physicians to limit their number of Medicare patients.
Physicians groups for years have urged federal lawmakers to scrap short-term fixes staving off Medicare pay cuts in favor of permanent reforms. Unlike nearly all other Medicare clinicians including hospitals, physicians’ Medicare payment updates aren’t currently tied to inflation.
Adjusted for inflation, Medicare payments to physicians have declined 26% between 2001 and 2023, including a 2% payment reduction in 2023, according to the AMA. Small and rural physician practices have been disproportionately affected by these reductions, as have doctors treating low-income or uninsured patients, the AMA said.
Last month, an influential federal advisory panel recommended permanently tying Medicare physician pay increases to inflation. Clinicians’ cost of providing services, measured by the Medicare Economic Index, rose by 2.6% in 2021 and are estimated to have risen 4.7% in 2022, significantly more than in recent years, the Medicare Payment Advisory Commission said.
A version of this article originally appeared on Medscape.com.
Introduced by four physician U.S. House representatives, HR 2474 would link Medicare fee schedule updates to the Medicare Economic Index, a measure of inflation related to physicians’ practice costs and wages.
That’s a long-sought goal of the American Medical Association, which is leading 120 state medical societies and medical specialty groups in championing the bill.
The legislation is essential to enabling physician practices to better absorb payment distributions triggered by budget neutrality rules, performance adjustments, and periods of high inflation, the groups wrote in a joint letter sent to the bill’s sponsors. The sponsors say they hope the legislation will improve access to care, as low reimbursements cause some physicians to limit their number of Medicare patients.
Physicians groups for years have urged federal lawmakers to scrap short-term fixes staving off Medicare pay cuts in favor of permanent reforms. Unlike nearly all other Medicare clinicians including hospitals, physicians’ Medicare payment updates aren’t currently tied to inflation.
Adjusted for inflation, Medicare payments to physicians have declined 26% between 2001 and 2023, including a 2% payment reduction in 2023, according to the AMA. Small and rural physician practices have been disproportionately affected by these reductions, as have doctors treating low-income or uninsured patients, the AMA said.
Last month, an influential federal advisory panel recommended permanently tying Medicare physician pay increases to inflation. Clinicians’ cost of providing services, measured by the Medicare Economic Index, rose by 2.6% in 2021 and are estimated to have risen 4.7% in 2022, significantly more than in recent years, the Medicare Payment Advisory Commission said.
A version of this article originally appeared on Medscape.com.
Introduced by four physician U.S. House representatives, HR 2474 would link Medicare fee schedule updates to the Medicare Economic Index, a measure of inflation related to physicians’ practice costs and wages.
That’s a long-sought goal of the American Medical Association, which is leading 120 state medical societies and medical specialty groups in championing the bill.
The legislation is essential to enabling physician practices to better absorb payment distributions triggered by budget neutrality rules, performance adjustments, and periods of high inflation, the groups wrote in a joint letter sent to the bill’s sponsors. The sponsors say they hope the legislation will improve access to care, as low reimbursements cause some physicians to limit their number of Medicare patients.
Physicians groups for years have urged federal lawmakers to scrap short-term fixes staving off Medicare pay cuts in favor of permanent reforms. Unlike nearly all other Medicare clinicians including hospitals, physicians’ Medicare payment updates aren’t currently tied to inflation.
Adjusted for inflation, Medicare payments to physicians have declined 26% between 2001 and 2023, including a 2% payment reduction in 2023, according to the AMA. Small and rural physician practices have been disproportionately affected by these reductions, as have doctors treating low-income or uninsured patients, the AMA said.
Last month, an influential federal advisory panel recommended permanently tying Medicare physician pay increases to inflation. Clinicians’ cost of providing services, measured by the Medicare Economic Index, rose by 2.6% in 2021 and are estimated to have risen 4.7% in 2022, significantly more than in recent years, the Medicare Payment Advisory Commission said.
A version of this article originally appeared on Medscape.com.
Surgeons, intensivists earn more than do colleagues from private insurance
General and orthopedic surgeons and intensivists earn the highest net reimbursements from private U.S. insurers, a new report estimates.
On average in 2021, they were paid $5.8 million, $4.9 million, and $3.3 million, respectively, according to figures compiled by AMN Healthcare, a Dallas-based health staffing company.
None of 15 other physician specialties topped $3 million in net reimbursement on average, and three – dermatology, pediatrics, and family medicine – didn’t reach $1 million.
The report doesn’t include data about reimbursement from Medicare and Medicaid, and its numbers assume that 50% of insurance claims are denied. Denial rates differ from practice to practice.
Still, the findings offer a “benchmark tool” to help clinicians understand how they rank against their peers, Linda Murphy, president of AMN Healthcare’s Revenue Cycle Solutions division, said in an interview.
This is the first year that the company has calculated physician reimbursement levels by using claim and clearinghouse data, Ms. Murphy said. Previously, a division of the firm compiled data by surveying chief financial officers from hospitals.
The report’s estimate that insurers deny 50% of claims is “conservative,” Ms. Murphy said. Miscoding is a significant factor behind that number.
The estimated 2021 net private insurance reimbursements by specialty for direct services, assuming a 50% denial rate:
- Anesthesiology: $1,665,510
- Cardiology: $1,703,013
- Critical Care (intensivist): $3,338,656
- Dermatology: $729,107
- Family medicine: $697,094
- Gastroenterology: $2,765,110
- Internal medicine: $1,297,200
- Neurology: $1,390,181
- Obstetrician/gynecology: $1,880,888
- Otolaryngology: $2,095,277
- Pediatrics: $661,552
- Psychiatry: $1,348,730
- Pulmonology: $1,561,617
- Radiology: $1,015,750
- Rheumatology: $1,705,140
- General surgery: $5,834,508
- Orthopedic surgery: $4,904,757
- Urology: $2,943,381
Among 18 physician specialties overall, the report estimated that the average net reimbursement in 2021 was $1.9 million.
The report also estimated that the net reimbursement amounts at $875,140 for certified registered nurse anesthetists and $388,696 for nurse practitioners.
Surprisingly, Ms. Murphy said, there’s “a really large swing” among reimbursement levels for individual specialties. The quartile of cardiologists with the lowest level of reimbursement, for example, submitted $2.1 million in claims in 2021, netting about $1 million at a 50% denial rate versus the $7.3 million made by those in the highest quartile, netting about $3.6 million.
The gap seems to be due to regional variations, she said, adding that a rural cardiologist will have different billing practices than does one practicing in New York City.
The quartile of general surgeons with the highest reimbursement levels billed for $21.1 million on average in 2021, making about $10.5 million at a 50% denial rate. The lowest quartile billed for $5.5 million, making about $2.7 million at a 50% denial rate.
The report noted that primary care physicians – that is, family medicine, internal medicine, and pediatrics specialists – have much lower levels of reimbursement, compared with most other specialties. But the work of primary care physicians “may lead to considerable ‘downstream revenue’ through the hospital admissions, tests and treatment they order.”
A previous analysis by a division of AMN Healthcare found that primary care physicians, on average, generate $2,113,273 a year in net annual revenue for their affiliated hospitals, nearing the $2,446,429 in net annual hospital revenue generated by specialists.
AMN Healthcare is preparing another report that will examine Medicare reimbursements, Ms. Murphy said. According to the new report, payments by nonprivate insurers amount to about one-third of the total amount of reimbursement by commercial insurers.
A version of this article originally appeared on Medscape.com.
General and orthopedic surgeons and intensivists earn the highest net reimbursements from private U.S. insurers, a new report estimates.
On average in 2021, they were paid $5.8 million, $4.9 million, and $3.3 million, respectively, according to figures compiled by AMN Healthcare, a Dallas-based health staffing company.
None of 15 other physician specialties topped $3 million in net reimbursement on average, and three – dermatology, pediatrics, and family medicine – didn’t reach $1 million.
The report doesn’t include data about reimbursement from Medicare and Medicaid, and its numbers assume that 50% of insurance claims are denied. Denial rates differ from practice to practice.
Still, the findings offer a “benchmark tool” to help clinicians understand how they rank against their peers, Linda Murphy, president of AMN Healthcare’s Revenue Cycle Solutions division, said in an interview.
This is the first year that the company has calculated physician reimbursement levels by using claim and clearinghouse data, Ms. Murphy said. Previously, a division of the firm compiled data by surveying chief financial officers from hospitals.
The report’s estimate that insurers deny 50% of claims is “conservative,” Ms. Murphy said. Miscoding is a significant factor behind that number.
The estimated 2021 net private insurance reimbursements by specialty for direct services, assuming a 50% denial rate:
- Anesthesiology: $1,665,510
- Cardiology: $1,703,013
- Critical Care (intensivist): $3,338,656
- Dermatology: $729,107
- Family medicine: $697,094
- Gastroenterology: $2,765,110
- Internal medicine: $1,297,200
- Neurology: $1,390,181
- Obstetrician/gynecology: $1,880,888
- Otolaryngology: $2,095,277
- Pediatrics: $661,552
- Psychiatry: $1,348,730
- Pulmonology: $1,561,617
- Radiology: $1,015,750
- Rheumatology: $1,705,140
- General surgery: $5,834,508
- Orthopedic surgery: $4,904,757
- Urology: $2,943,381
Among 18 physician specialties overall, the report estimated that the average net reimbursement in 2021 was $1.9 million.
The report also estimated that the net reimbursement amounts at $875,140 for certified registered nurse anesthetists and $388,696 for nurse practitioners.
Surprisingly, Ms. Murphy said, there’s “a really large swing” among reimbursement levels for individual specialties. The quartile of cardiologists with the lowest level of reimbursement, for example, submitted $2.1 million in claims in 2021, netting about $1 million at a 50% denial rate versus the $7.3 million made by those in the highest quartile, netting about $3.6 million.
The gap seems to be due to regional variations, she said, adding that a rural cardiologist will have different billing practices than does one practicing in New York City.
The quartile of general surgeons with the highest reimbursement levels billed for $21.1 million on average in 2021, making about $10.5 million at a 50% denial rate. The lowest quartile billed for $5.5 million, making about $2.7 million at a 50% denial rate.
The report noted that primary care physicians – that is, family medicine, internal medicine, and pediatrics specialists – have much lower levels of reimbursement, compared with most other specialties. But the work of primary care physicians “may lead to considerable ‘downstream revenue’ through the hospital admissions, tests and treatment they order.”
A previous analysis by a division of AMN Healthcare found that primary care physicians, on average, generate $2,113,273 a year in net annual revenue for their affiliated hospitals, nearing the $2,446,429 in net annual hospital revenue generated by specialists.
AMN Healthcare is preparing another report that will examine Medicare reimbursements, Ms. Murphy said. According to the new report, payments by nonprivate insurers amount to about one-third of the total amount of reimbursement by commercial insurers.
A version of this article originally appeared on Medscape.com.
General and orthopedic surgeons and intensivists earn the highest net reimbursements from private U.S. insurers, a new report estimates.
On average in 2021, they were paid $5.8 million, $4.9 million, and $3.3 million, respectively, according to figures compiled by AMN Healthcare, a Dallas-based health staffing company.
None of 15 other physician specialties topped $3 million in net reimbursement on average, and three – dermatology, pediatrics, and family medicine – didn’t reach $1 million.
The report doesn’t include data about reimbursement from Medicare and Medicaid, and its numbers assume that 50% of insurance claims are denied. Denial rates differ from practice to practice.
Still, the findings offer a “benchmark tool” to help clinicians understand how they rank against their peers, Linda Murphy, president of AMN Healthcare’s Revenue Cycle Solutions division, said in an interview.
This is the first year that the company has calculated physician reimbursement levels by using claim and clearinghouse data, Ms. Murphy said. Previously, a division of the firm compiled data by surveying chief financial officers from hospitals.
The report’s estimate that insurers deny 50% of claims is “conservative,” Ms. Murphy said. Miscoding is a significant factor behind that number.
The estimated 2021 net private insurance reimbursements by specialty for direct services, assuming a 50% denial rate:
- Anesthesiology: $1,665,510
- Cardiology: $1,703,013
- Critical Care (intensivist): $3,338,656
- Dermatology: $729,107
- Family medicine: $697,094
- Gastroenterology: $2,765,110
- Internal medicine: $1,297,200
- Neurology: $1,390,181
- Obstetrician/gynecology: $1,880,888
- Otolaryngology: $2,095,277
- Pediatrics: $661,552
- Psychiatry: $1,348,730
- Pulmonology: $1,561,617
- Radiology: $1,015,750
- Rheumatology: $1,705,140
- General surgery: $5,834,508
- Orthopedic surgery: $4,904,757
- Urology: $2,943,381
Among 18 physician specialties overall, the report estimated that the average net reimbursement in 2021 was $1.9 million.
The report also estimated that the net reimbursement amounts at $875,140 for certified registered nurse anesthetists and $388,696 for nurse practitioners.
Surprisingly, Ms. Murphy said, there’s “a really large swing” among reimbursement levels for individual specialties. The quartile of cardiologists with the lowest level of reimbursement, for example, submitted $2.1 million in claims in 2021, netting about $1 million at a 50% denial rate versus the $7.3 million made by those in the highest quartile, netting about $3.6 million.
The gap seems to be due to regional variations, she said, adding that a rural cardiologist will have different billing practices than does one practicing in New York City.
The quartile of general surgeons with the highest reimbursement levels billed for $21.1 million on average in 2021, making about $10.5 million at a 50% denial rate. The lowest quartile billed for $5.5 million, making about $2.7 million at a 50% denial rate.
The report noted that primary care physicians – that is, family medicine, internal medicine, and pediatrics specialists – have much lower levels of reimbursement, compared with most other specialties. But the work of primary care physicians “may lead to considerable ‘downstream revenue’ through the hospital admissions, tests and treatment they order.”
A previous analysis by a division of AMN Healthcare found that primary care physicians, on average, generate $2,113,273 a year in net annual revenue for their affiliated hospitals, nearing the $2,446,429 in net annual hospital revenue generated by specialists.
AMN Healthcare is preparing another report that will examine Medicare reimbursements, Ms. Murphy said. According to the new report, payments by nonprivate insurers amount to about one-third of the total amount of reimbursement by commercial insurers.
A version of this article originally appeared on Medscape.com.
Frontline CLL treatment: Avoiding adverse events
NEW YORK – The Food and Drug Administration’s 2016 approval of the Bruton’s tyrosine kinase inhibitor ibrutinib (IB) as a frontline therapy for chronic lymphocytic leukemia (CLL) dramatically improved overall survival rates for patients with this condition. Follow-up data from 8 years after the RESONATE-2 trial indicated that patients with CLL (65 years or older) who remain on IB therapy can expect to live as long as someone in the general population.
Physicians now face two challenges in frontline CLL treatment: finding safe and effective drugs with fewer side effects, allowing patients to maintain therapy; and offering young or genomically high-risk patients treatments that reduce the risk of relapse.
said John N. Allan, associate professor at Weill Cornell Medicine, New York, in his presentation on frontline CLL treatments at the Great Debates and Updates Hematologic Malignancies Conference. “This is true even of older patients or those with comorbidities because this class of drug allows us to keep patients on treatment with excellent long-term outcomes.”
Results from the Alpine trial (NCT03734016), which included patients with and without high genomic risk, confirmed the superiority of the second generation Bruton’s tyrosine kinase inhibitor zanubrutinib (ZB) versus ibrutinib in terms of overall response rate 86.2% versus 75.5%, progression free survival 2-years after treatment 79.5% versus 67.3%, and adverse events (AEs) leading to discontinuation 15.4% versus 22.2% respectively.
The SEQUOIA trial (NCT03336333) demonstrated the effectiveness of ZB versus bendamustine + rituximab combination (BR) therapy in treatment-naive CLL / small lymphocytic leukemia patients with normal and high genomic risk. Overall 24-month progression free survival (PFS) was 85% in the ZB cohort vs. 69% in the BR cohort. This trend held true among high-risk subgroups like patients with an unmutated IgVH gene or 11q22.3 gene deletion.
Therapies known as “doublets” and “triplets” (which include a Bruton’s tyrosine kinase inhibitor in addition to other drugs) are not FDA approved for frontline CLL treatment. Yet studies suggest that young patients who are better able to tolerate AEs or high-risk patients with a greater risk of relapse (even on monotherapy maintenance), may derive benefits from multidrug frontline treatment.
“With doublets and triplets, doctors add treatment intensity up front so that patients can have a fixed duration of therapy versus continuous indefinite therapy,” said Vu Nguyen MD, a hematologist at Oakland (Calif.) Medical Center. “This is encouraging because if you can have a fixed duration of treatment, patients can come off treatment agents and hopefully have a prolonged remission and normal lifespan without chronic therapy and side effects.”
The CAPTIVATE study confirmed this approach with 3 cycles of IB followed by 12 cycles of IB + venetoclax leading to a 24-month PFS rate of 94% in patients with high risk or relapse. “Furthermore, 95% of study participants patients less than 70 years old completed 12 months of combination treatment without major problems,” said Dr. Allan. He concluded his remarks by noting that “we need longer term data on the use of combination therapy for frontline CLL treatment to confirm if and when it should be used.”
Dr. Allan disclosed relationships with Adaptive Biotechnologies, ADC Therapeutics, AstraZeneca, BeiGene, Epizyme, Genentech, Janssen, Lilly, Pharmacyclics, and TG Therapeutics. Dr. Nguyen reported no disclosures.
NEW YORK – The Food and Drug Administration’s 2016 approval of the Bruton’s tyrosine kinase inhibitor ibrutinib (IB) as a frontline therapy for chronic lymphocytic leukemia (CLL) dramatically improved overall survival rates for patients with this condition. Follow-up data from 8 years after the RESONATE-2 trial indicated that patients with CLL (65 years or older) who remain on IB therapy can expect to live as long as someone in the general population.
Physicians now face two challenges in frontline CLL treatment: finding safe and effective drugs with fewer side effects, allowing patients to maintain therapy; and offering young or genomically high-risk patients treatments that reduce the risk of relapse.
said John N. Allan, associate professor at Weill Cornell Medicine, New York, in his presentation on frontline CLL treatments at the Great Debates and Updates Hematologic Malignancies Conference. “This is true even of older patients or those with comorbidities because this class of drug allows us to keep patients on treatment with excellent long-term outcomes.”
Results from the Alpine trial (NCT03734016), which included patients with and without high genomic risk, confirmed the superiority of the second generation Bruton’s tyrosine kinase inhibitor zanubrutinib (ZB) versus ibrutinib in terms of overall response rate 86.2% versus 75.5%, progression free survival 2-years after treatment 79.5% versus 67.3%, and adverse events (AEs) leading to discontinuation 15.4% versus 22.2% respectively.
The SEQUOIA trial (NCT03336333) demonstrated the effectiveness of ZB versus bendamustine + rituximab combination (BR) therapy in treatment-naive CLL / small lymphocytic leukemia patients with normal and high genomic risk. Overall 24-month progression free survival (PFS) was 85% in the ZB cohort vs. 69% in the BR cohort. This trend held true among high-risk subgroups like patients with an unmutated IgVH gene or 11q22.3 gene deletion.
Therapies known as “doublets” and “triplets” (which include a Bruton’s tyrosine kinase inhibitor in addition to other drugs) are not FDA approved for frontline CLL treatment. Yet studies suggest that young patients who are better able to tolerate AEs or high-risk patients with a greater risk of relapse (even on monotherapy maintenance), may derive benefits from multidrug frontline treatment.
“With doublets and triplets, doctors add treatment intensity up front so that patients can have a fixed duration of therapy versus continuous indefinite therapy,” said Vu Nguyen MD, a hematologist at Oakland (Calif.) Medical Center. “This is encouraging because if you can have a fixed duration of treatment, patients can come off treatment agents and hopefully have a prolonged remission and normal lifespan without chronic therapy and side effects.”
The CAPTIVATE study confirmed this approach with 3 cycles of IB followed by 12 cycles of IB + venetoclax leading to a 24-month PFS rate of 94% in patients with high risk or relapse. “Furthermore, 95% of study participants patients less than 70 years old completed 12 months of combination treatment without major problems,” said Dr. Allan. He concluded his remarks by noting that “we need longer term data on the use of combination therapy for frontline CLL treatment to confirm if and when it should be used.”
Dr. Allan disclosed relationships with Adaptive Biotechnologies, ADC Therapeutics, AstraZeneca, BeiGene, Epizyme, Genentech, Janssen, Lilly, Pharmacyclics, and TG Therapeutics. Dr. Nguyen reported no disclosures.
NEW YORK – The Food and Drug Administration’s 2016 approval of the Bruton’s tyrosine kinase inhibitor ibrutinib (IB) as a frontline therapy for chronic lymphocytic leukemia (CLL) dramatically improved overall survival rates for patients with this condition. Follow-up data from 8 years after the RESONATE-2 trial indicated that patients with CLL (65 years or older) who remain on IB therapy can expect to live as long as someone in the general population.
Physicians now face two challenges in frontline CLL treatment: finding safe and effective drugs with fewer side effects, allowing patients to maintain therapy; and offering young or genomically high-risk patients treatments that reduce the risk of relapse.
said John N. Allan, associate professor at Weill Cornell Medicine, New York, in his presentation on frontline CLL treatments at the Great Debates and Updates Hematologic Malignancies Conference. “This is true even of older patients or those with comorbidities because this class of drug allows us to keep patients on treatment with excellent long-term outcomes.”
Results from the Alpine trial (NCT03734016), which included patients with and without high genomic risk, confirmed the superiority of the second generation Bruton’s tyrosine kinase inhibitor zanubrutinib (ZB) versus ibrutinib in terms of overall response rate 86.2% versus 75.5%, progression free survival 2-years after treatment 79.5% versus 67.3%, and adverse events (AEs) leading to discontinuation 15.4% versus 22.2% respectively.
The SEQUOIA trial (NCT03336333) demonstrated the effectiveness of ZB versus bendamustine + rituximab combination (BR) therapy in treatment-naive CLL / small lymphocytic leukemia patients with normal and high genomic risk. Overall 24-month progression free survival (PFS) was 85% in the ZB cohort vs. 69% in the BR cohort. This trend held true among high-risk subgroups like patients with an unmutated IgVH gene or 11q22.3 gene deletion.
Therapies known as “doublets” and “triplets” (which include a Bruton’s tyrosine kinase inhibitor in addition to other drugs) are not FDA approved for frontline CLL treatment. Yet studies suggest that young patients who are better able to tolerate AEs or high-risk patients with a greater risk of relapse (even on monotherapy maintenance), may derive benefits from multidrug frontline treatment.
“With doublets and triplets, doctors add treatment intensity up front so that patients can have a fixed duration of therapy versus continuous indefinite therapy,” said Vu Nguyen MD, a hematologist at Oakland (Calif.) Medical Center. “This is encouraging because if you can have a fixed duration of treatment, patients can come off treatment agents and hopefully have a prolonged remission and normal lifespan without chronic therapy and side effects.”
The CAPTIVATE study confirmed this approach with 3 cycles of IB followed by 12 cycles of IB + venetoclax leading to a 24-month PFS rate of 94% in patients with high risk or relapse. “Furthermore, 95% of study participants patients less than 70 years old completed 12 months of combination treatment without major problems,” said Dr. Allan. He concluded his remarks by noting that “we need longer term data on the use of combination therapy for frontline CLL treatment to confirm if and when it should be used.”
Dr. Allan disclosed relationships with Adaptive Biotechnologies, ADC Therapeutics, AstraZeneca, BeiGene, Epizyme, Genentech, Janssen, Lilly, Pharmacyclics, and TG Therapeutics. Dr. Nguyen reported no disclosures.
AT 2023 GREAT DEBATES AND UPDATES HEMATOLOGIC MALIGNANCIES CONFERENCE
NPF provides guidance for virtual psoriasis visits
.
The success of telemedicine in managing chronic inflammatory skin conditions including psoriasis during the COVID-19 pandemic “highlighted that teledermatology can be used beyond the context of a global health crisis to provide continuity of care and improve access to health care more broadly,” the task force wrote in a paper published online in JAAD International.
Co–senior author George Han, MD, PhD, said in an interview that the impetus for the guidelines came from NPF patient advocates, who realized that the organization needed something to take to payers and governmental agencies to advocate for better access to dermatologic care. He is associate professor of dermatology and director of teledermatology at the Hofstra/Northwell department of dermatology, Hyde Park, New York.
“We realized that, in many places around the country, people don’t have access to dermatology.” In upstate New York, said Dr. Han, his anecdotal research has revealed wait times of 6 months or more.
As a guiding principle, the authors pronounce teledermatology “a reasonable alternative for providing long-term management of patients with psoriasis.” Research shows that nearly all dermatologists used teledermatology during the pandemic, the authors noted, and that well-run programs improve Psoriasis Area and Severity Index (PASI) scores and other measures on par with in-person care. Telemedicine may be especially useful for initial visits, they added, particularly when distance, patient incapacity, and circumstances prevent face-to-face evaluation.
Additional position statements emphasize that teledermatology should support rather than supplant in-person visits, and that this balance may be particularly important in cases involving psoriatic arthritis (PsA). “Even though we can’t do a physical exam and palpate some of those joints in person,” said Dr. Han, “tools have been developed that, through a series of questions the patient can answer, can guide you towards whether there is a high index of suspicion for psoriatic arthritis.” Such patients require in-person evaluation with urgency, he said, because delays in PsA diagnosis and treatment can lead to irreversible joint damage and significant functional impairment.
Another motivation for producing the guidelines, said Dr. Han, was that, even when underserved patients get a dermatology appointment, some providers may not have all the latest tools or medicines available for treating psoriasis. In such cases, telemedicine may allow dermatologists specializing in psoriasis care to extend their reach in comanaging patients with primary care physicians and community dermatologists.
Before the appointment, guidelines suggest determining what form of teledermatology will best suit each patient. Authors recommended gauging patients’ savviness with computers and cameras, and counseling patients regarding available virtual evaluation tools – such as live video visits, store-and-forward photo strategies, and assessment-tool training videos.
A subsequent guideline underscores the importance of continuously improving technology to support expeditious image capture and workflows that emulate in-person practice. Dr. Han explained, “we wanted to make sure that on the back end there’s adequate support such that – if through teledermatology, we determine that the patient should get, say, a systemic treatment – the patient is able to get the appropriate lab tests, get the medicine, and know how to inject it.”
Regarding reimbursement, Dr. Han said that policies varied prepandemic, but many commercial insurers covered telemedicine at a rate 20% lower than the in-person rate. During the pandemic, he said, insurers shifted to provide the higher rate for telemedicine, consistent with policies adopted by the Centers for Medicare & Medicaid Services.
“There are differences in coverage and reimbursement from plan to plan,” Dr. Han added. “And even within the same plan, there are carve-outs so that some plans don’t allow certain services. The big picture is that for the most part these services are covered at a level comparable to an in-person visit at present.”
With the Department of Health & Human Services’ public health emergency declaration expiring in May, he said, physicians have worried that some of the allowances made by CMS – such as lifting requirements that Medicare patients in rural areas be seen at care sites – will expire. “It seems that some of those limitations have been addressed, and those allowances are going to be extended until Congress is able to pass something that gives us durable access to telemedicine care. We think that based on the current environment telemedicine is here to stay.”
The study was funded by the NPF. Dr. Han has been an investigator, adviser, speaker, or researcher for AbbVie, Amgen, Apogee Therapeutics, Arcutis, Athenex, Bausch Health, Beiersdorf, Boehringer Ingelheim, Bond Avillion, Bristol Myers Squibb, Celgene, CeraVe, Dermavant, DermTech, Eli Lilly, EPI Health, Janssen Pharmaceuticals, LEO Pharma, L’Oreal, MC2 Therapeutics, Novartis, Ortho Dermatologics, PellePharm, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, SUN Pharmaceuticals, and UCB.
.
The success of telemedicine in managing chronic inflammatory skin conditions including psoriasis during the COVID-19 pandemic “highlighted that teledermatology can be used beyond the context of a global health crisis to provide continuity of care and improve access to health care more broadly,” the task force wrote in a paper published online in JAAD International.
Co–senior author George Han, MD, PhD, said in an interview that the impetus for the guidelines came from NPF patient advocates, who realized that the organization needed something to take to payers and governmental agencies to advocate for better access to dermatologic care. He is associate professor of dermatology and director of teledermatology at the Hofstra/Northwell department of dermatology, Hyde Park, New York.
“We realized that, in many places around the country, people don’t have access to dermatology.” In upstate New York, said Dr. Han, his anecdotal research has revealed wait times of 6 months or more.
As a guiding principle, the authors pronounce teledermatology “a reasonable alternative for providing long-term management of patients with psoriasis.” Research shows that nearly all dermatologists used teledermatology during the pandemic, the authors noted, and that well-run programs improve Psoriasis Area and Severity Index (PASI) scores and other measures on par with in-person care. Telemedicine may be especially useful for initial visits, they added, particularly when distance, patient incapacity, and circumstances prevent face-to-face evaluation.
Additional position statements emphasize that teledermatology should support rather than supplant in-person visits, and that this balance may be particularly important in cases involving psoriatic arthritis (PsA). “Even though we can’t do a physical exam and palpate some of those joints in person,” said Dr. Han, “tools have been developed that, through a series of questions the patient can answer, can guide you towards whether there is a high index of suspicion for psoriatic arthritis.” Such patients require in-person evaluation with urgency, he said, because delays in PsA diagnosis and treatment can lead to irreversible joint damage and significant functional impairment.
Another motivation for producing the guidelines, said Dr. Han, was that, even when underserved patients get a dermatology appointment, some providers may not have all the latest tools or medicines available for treating psoriasis. In such cases, telemedicine may allow dermatologists specializing in psoriasis care to extend their reach in comanaging patients with primary care physicians and community dermatologists.
Before the appointment, guidelines suggest determining what form of teledermatology will best suit each patient. Authors recommended gauging patients’ savviness with computers and cameras, and counseling patients regarding available virtual evaluation tools – such as live video visits, store-and-forward photo strategies, and assessment-tool training videos.
A subsequent guideline underscores the importance of continuously improving technology to support expeditious image capture and workflows that emulate in-person practice. Dr. Han explained, “we wanted to make sure that on the back end there’s adequate support such that – if through teledermatology, we determine that the patient should get, say, a systemic treatment – the patient is able to get the appropriate lab tests, get the medicine, and know how to inject it.”
Regarding reimbursement, Dr. Han said that policies varied prepandemic, but many commercial insurers covered telemedicine at a rate 20% lower than the in-person rate. During the pandemic, he said, insurers shifted to provide the higher rate for telemedicine, consistent with policies adopted by the Centers for Medicare & Medicaid Services.
“There are differences in coverage and reimbursement from plan to plan,” Dr. Han added. “And even within the same plan, there are carve-outs so that some plans don’t allow certain services. The big picture is that for the most part these services are covered at a level comparable to an in-person visit at present.”
With the Department of Health & Human Services’ public health emergency declaration expiring in May, he said, physicians have worried that some of the allowances made by CMS – such as lifting requirements that Medicare patients in rural areas be seen at care sites – will expire. “It seems that some of those limitations have been addressed, and those allowances are going to be extended until Congress is able to pass something that gives us durable access to telemedicine care. We think that based on the current environment telemedicine is here to stay.”
The study was funded by the NPF. Dr. Han has been an investigator, adviser, speaker, or researcher for AbbVie, Amgen, Apogee Therapeutics, Arcutis, Athenex, Bausch Health, Beiersdorf, Boehringer Ingelheim, Bond Avillion, Bristol Myers Squibb, Celgene, CeraVe, Dermavant, DermTech, Eli Lilly, EPI Health, Janssen Pharmaceuticals, LEO Pharma, L’Oreal, MC2 Therapeutics, Novartis, Ortho Dermatologics, PellePharm, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, SUN Pharmaceuticals, and UCB.
.
The success of telemedicine in managing chronic inflammatory skin conditions including psoriasis during the COVID-19 pandemic “highlighted that teledermatology can be used beyond the context of a global health crisis to provide continuity of care and improve access to health care more broadly,” the task force wrote in a paper published online in JAAD International.
Co–senior author George Han, MD, PhD, said in an interview that the impetus for the guidelines came from NPF patient advocates, who realized that the organization needed something to take to payers and governmental agencies to advocate for better access to dermatologic care. He is associate professor of dermatology and director of teledermatology at the Hofstra/Northwell department of dermatology, Hyde Park, New York.
“We realized that, in many places around the country, people don’t have access to dermatology.” In upstate New York, said Dr. Han, his anecdotal research has revealed wait times of 6 months or more.
As a guiding principle, the authors pronounce teledermatology “a reasonable alternative for providing long-term management of patients with psoriasis.” Research shows that nearly all dermatologists used teledermatology during the pandemic, the authors noted, and that well-run programs improve Psoriasis Area and Severity Index (PASI) scores and other measures on par with in-person care. Telemedicine may be especially useful for initial visits, they added, particularly when distance, patient incapacity, and circumstances prevent face-to-face evaluation.
Additional position statements emphasize that teledermatology should support rather than supplant in-person visits, and that this balance may be particularly important in cases involving psoriatic arthritis (PsA). “Even though we can’t do a physical exam and palpate some of those joints in person,” said Dr. Han, “tools have been developed that, through a series of questions the patient can answer, can guide you towards whether there is a high index of suspicion for psoriatic arthritis.” Such patients require in-person evaluation with urgency, he said, because delays in PsA diagnosis and treatment can lead to irreversible joint damage and significant functional impairment.
Another motivation for producing the guidelines, said Dr. Han, was that, even when underserved patients get a dermatology appointment, some providers may not have all the latest tools or medicines available for treating psoriasis. In such cases, telemedicine may allow dermatologists specializing in psoriasis care to extend their reach in comanaging patients with primary care physicians and community dermatologists.
Before the appointment, guidelines suggest determining what form of teledermatology will best suit each patient. Authors recommended gauging patients’ savviness with computers and cameras, and counseling patients regarding available virtual evaluation tools – such as live video visits, store-and-forward photo strategies, and assessment-tool training videos.
A subsequent guideline underscores the importance of continuously improving technology to support expeditious image capture and workflows that emulate in-person practice. Dr. Han explained, “we wanted to make sure that on the back end there’s adequate support such that – if through teledermatology, we determine that the patient should get, say, a systemic treatment – the patient is able to get the appropriate lab tests, get the medicine, and know how to inject it.”
Regarding reimbursement, Dr. Han said that policies varied prepandemic, but many commercial insurers covered telemedicine at a rate 20% lower than the in-person rate. During the pandemic, he said, insurers shifted to provide the higher rate for telemedicine, consistent with policies adopted by the Centers for Medicare & Medicaid Services.
“There are differences in coverage and reimbursement from plan to plan,” Dr. Han added. “And even within the same plan, there are carve-outs so that some plans don’t allow certain services. The big picture is that for the most part these services are covered at a level comparable to an in-person visit at present.”
With the Department of Health & Human Services’ public health emergency declaration expiring in May, he said, physicians have worried that some of the allowances made by CMS – such as lifting requirements that Medicare patients in rural areas be seen at care sites – will expire. “It seems that some of those limitations have been addressed, and those allowances are going to be extended until Congress is able to pass something that gives us durable access to telemedicine care. We think that based on the current environment telemedicine is here to stay.”
The study was funded by the NPF. Dr. Han has been an investigator, adviser, speaker, or researcher for AbbVie, Amgen, Apogee Therapeutics, Arcutis, Athenex, Bausch Health, Beiersdorf, Boehringer Ingelheim, Bond Avillion, Bristol Myers Squibb, Celgene, CeraVe, Dermavant, DermTech, Eli Lilly, EPI Health, Janssen Pharmaceuticals, LEO Pharma, L’Oreal, MC2 Therapeutics, Novartis, Ortho Dermatologics, PellePharm, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, SUN Pharmaceuticals, and UCB.
FROM JAAD INTERNATIONAL
Ten-year analysis finds relatively low complication rate from fractional resurfacing lasers
PHOENIX – over a decade showed.
To investigate, Dr. Hashemi, a third-year dermatology resident at Harvard University and Massachusetts General Hospital, Boston, and Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at MGH, drew from the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database, which compiles medical device reports for suspected injuries from device use or malfunction and represents the largest repository of device adverse effects. Medical device reports are submitted by manufacturers, clinicians, patients, and others.
The researchers limited their query to MDRs related to ablative and nonablative fractional resurfacing lasers over the 10-year period from 2013 to 2022. The query was performed in January 2023 using a comprehensive list of product names and manufacturers.
The initial search yielded 240 MDRs, which were individually reviewed for duplicate records or insufficient data, and the final analysis included 165 MDRs. The 10 most reported adverse events were burns (30%), followed by dyspigmentation (14%), scarring (12%), other (11%), postoperative infection (8%), blisters (6%), pain (5%), hypertrophic scar (4%), post-treatment inflammation (4%), and textural changes (3%). Within the 10-year period analyzed, 56% of MDRs occurred between 2016 and 2019, with a disproportionately low percentage of MDRs occurring in 2022 (5%).
“Adverse events due to ablative and nonablative fractional resurfacing lasers are rare but potentially serious,” Dr. Hashemi concluded. “Care must be taken with counseling, patient selection, and treatment settings to optimize safety, informed consent, and patient satisfaction. Given the relatively low number of adverse events seen with fractional resurfacing lasers, factors driving their safety should be further explored.”
He added that he was surprised by the relatively low number of reported issues, referring to the total of 165 cases over 10 years. By comparison, he said, body contouring had 660 cases reported over a 7-year period in one recent study.
According to the MAUDE website, submitting MDRs to MAUDE is mandatory for manufacturers, importers, and device user facilities, and are voluntary for other groups, such as health care professionals, patients, and consumers.
Dr. Hashemi disclosed that he is a consultant for Castle Biosciences. He is also an entrepreneur in residence for Gore Range Capital.
PHOENIX – over a decade showed.
To investigate, Dr. Hashemi, a third-year dermatology resident at Harvard University and Massachusetts General Hospital, Boston, and Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at MGH, drew from the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database, which compiles medical device reports for suspected injuries from device use or malfunction and represents the largest repository of device adverse effects. Medical device reports are submitted by manufacturers, clinicians, patients, and others.
The researchers limited their query to MDRs related to ablative and nonablative fractional resurfacing lasers over the 10-year period from 2013 to 2022. The query was performed in January 2023 using a comprehensive list of product names and manufacturers.
The initial search yielded 240 MDRs, which were individually reviewed for duplicate records or insufficient data, and the final analysis included 165 MDRs. The 10 most reported adverse events were burns (30%), followed by dyspigmentation (14%), scarring (12%), other (11%), postoperative infection (8%), blisters (6%), pain (5%), hypertrophic scar (4%), post-treatment inflammation (4%), and textural changes (3%). Within the 10-year period analyzed, 56% of MDRs occurred between 2016 and 2019, with a disproportionately low percentage of MDRs occurring in 2022 (5%).
“Adverse events due to ablative and nonablative fractional resurfacing lasers are rare but potentially serious,” Dr. Hashemi concluded. “Care must be taken with counseling, patient selection, and treatment settings to optimize safety, informed consent, and patient satisfaction. Given the relatively low number of adverse events seen with fractional resurfacing lasers, factors driving their safety should be further explored.”
He added that he was surprised by the relatively low number of reported issues, referring to the total of 165 cases over 10 years. By comparison, he said, body contouring had 660 cases reported over a 7-year period in one recent study.
According to the MAUDE website, submitting MDRs to MAUDE is mandatory for manufacturers, importers, and device user facilities, and are voluntary for other groups, such as health care professionals, patients, and consumers.
Dr. Hashemi disclosed that he is a consultant for Castle Biosciences. He is also an entrepreneur in residence for Gore Range Capital.
PHOENIX – over a decade showed.
To investigate, Dr. Hashemi, a third-year dermatology resident at Harvard University and Massachusetts General Hospital, Boston, and Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at MGH, drew from the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database, which compiles medical device reports for suspected injuries from device use or malfunction and represents the largest repository of device adverse effects. Medical device reports are submitted by manufacturers, clinicians, patients, and others.
The researchers limited their query to MDRs related to ablative and nonablative fractional resurfacing lasers over the 10-year period from 2013 to 2022. The query was performed in January 2023 using a comprehensive list of product names and manufacturers.
The initial search yielded 240 MDRs, which were individually reviewed for duplicate records or insufficient data, and the final analysis included 165 MDRs. The 10 most reported adverse events were burns (30%), followed by dyspigmentation (14%), scarring (12%), other (11%), postoperative infection (8%), blisters (6%), pain (5%), hypertrophic scar (4%), post-treatment inflammation (4%), and textural changes (3%). Within the 10-year period analyzed, 56% of MDRs occurred between 2016 and 2019, with a disproportionately low percentage of MDRs occurring in 2022 (5%).
“Adverse events due to ablative and nonablative fractional resurfacing lasers are rare but potentially serious,” Dr. Hashemi concluded. “Care must be taken with counseling, patient selection, and treatment settings to optimize safety, informed consent, and patient satisfaction. Given the relatively low number of adverse events seen with fractional resurfacing lasers, factors driving their safety should be further explored.”
He added that he was surprised by the relatively low number of reported issues, referring to the total of 165 cases over 10 years. By comparison, he said, body contouring had 660 cases reported over a 7-year period in one recent study.
According to the MAUDE website, submitting MDRs to MAUDE is mandatory for manufacturers, importers, and device user facilities, and are voluntary for other groups, such as health care professionals, patients, and consumers.
Dr. Hashemi disclosed that he is a consultant for Castle Biosciences. He is also an entrepreneur in residence for Gore Range Capital.
AT ASLMS 2023
Poor representation of patients with darker skin phototypes in laser and light device studies
, according to a systematic review of the literature, the authors reported.
“While there broadly appears to be skin of color representation [in such studies], a more granular understanding of the data shows a large discrepancy in representation between ‘lighter’ and ‘darker’ skin of color patients,” Priya Manjaly and associates wrote in the Journal of Cosmetic Dermatology.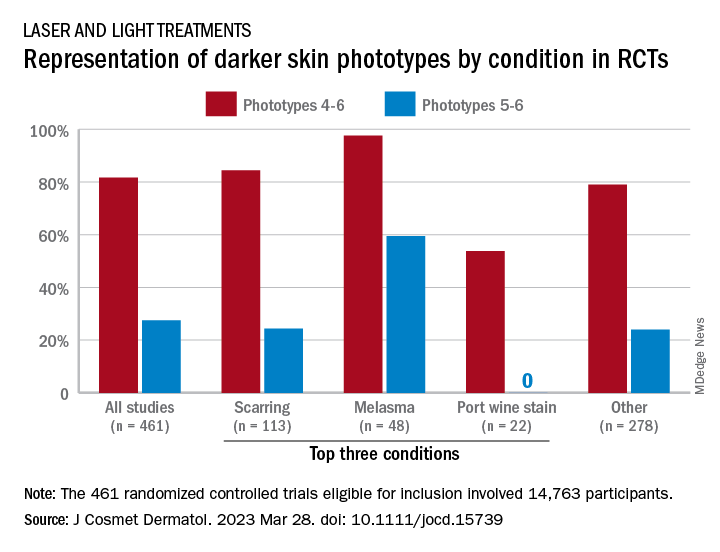
Among the 461 randomized controlled trials (RCTs) eligible for inclusion, most (81.7%) included participants with skin phototypes 4-6, which is considered skin of color. When only phototypes 5 and 6 were included, however, representation in studies involving laser and light devices was only 27.5%, said Ms. Manjaly, a research fellow in the department of dermatology at Boston University, and associates.
“This trend of excluding darker skin phototypes persisted when the results were stratified by condition, laser of study, study location, journal type, and funding source,” the investigators noted.
RCTs of laser/light devices for scarring, the most common dermatologic condition represented, included phototypes 5 and 6 in 24.4% of studies, compared with 84.4% for phototypes 4-6. The gap was smaller for melasma, but not for port wine stains. Among the devices examined, RCTs of diode lasers and intense pulsed light had the smallest gaps between inclusion of the two groups of phototypes, while pulsed-dye laser studies had the largest, they reported.
Stratification by journal showed the largest gap in studies published by Lasers in Medical Science and the smallest gap coming from Lasers in Surgery and Medicine. Funding was not specified for the majority of the eligible device RCTs, but those funded by industry had the smallest discrepancy between types 5-6 and types 4-6 and those supported by foundations/nonprofits the largest, Ms. Manjaly and associates said.
“With projections estimating that more than 50% of the U.S. population is set to identify as Hispanic or nonwhite by 2045 ... the lack of information has important consequences for clinical practice, as clinicians are unable to counsel patients on the efficacy and possible complications of various devices in patient with skin of color,” they wrote.
The investigators did not declare any conflicts of interest or funding sources.
, according to a systematic review of the literature, the authors reported.
“While there broadly appears to be skin of color representation [in such studies], a more granular understanding of the data shows a large discrepancy in representation between ‘lighter’ and ‘darker’ skin of color patients,” Priya Manjaly and associates wrote in the Journal of Cosmetic Dermatology.
Among the 461 randomized controlled trials (RCTs) eligible for inclusion, most (81.7%) included participants with skin phototypes 4-6, which is considered skin of color. When only phototypes 5 and 6 were included, however, representation in studies involving laser and light devices was only 27.5%, said Ms. Manjaly, a research fellow in the department of dermatology at Boston University, and associates.
“This trend of excluding darker skin phototypes persisted when the results were stratified by condition, laser of study, study location, journal type, and funding source,” the investigators noted.
RCTs of laser/light devices for scarring, the most common dermatologic condition represented, included phototypes 5 and 6 in 24.4% of studies, compared with 84.4% for phototypes 4-6. The gap was smaller for melasma, but not for port wine stains. Among the devices examined, RCTs of diode lasers and intense pulsed light had the smallest gaps between inclusion of the two groups of phototypes, while pulsed-dye laser studies had the largest, they reported.
Stratification by journal showed the largest gap in studies published by Lasers in Medical Science and the smallest gap coming from Lasers in Surgery and Medicine. Funding was not specified for the majority of the eligible device RCTs, but those funded by industry had the smallest discrepancy between types 5-6 and types 4-6 and those supported by foundations/nonprofits the largest, Ms. Manjaly and associates said.
“With projections estimating that more than 50% of the U.S. population is set to identify as Hispanic or nonwhite by 2045 ... the lack of information has important consequences for clinical practice, as clinicians are unable to counsel patients on the efficacy and possible complications of various devices in patient with skin of color,” they wrote.
The investigators did not declare any conflicts of interest or funding sources.
, according to a systematic review of the literature, the authors reported.
“While there broadly appears to be skin of color representation [in such studies], a more granular understanding of the data shows a large discrepancy in representation between ‘lighter’ and ‘darker’ skin of color patients,” Priya Manjaly and associates wrote in the Journal of Cosmetic Dermatology.
Among the 461 randomized controlled trials (RCTs) eligible for inclusion, most (81.7%) included participants with skin phototypes 4-6, which is considered skin of color. When only phototypes 5 and 6 were included, however, representation in studies involving laser and light devices was only 27.5%, said Ms. Manjaly, a research fellow in the department of dermatology at Boston University, and associates.
“This trend of excluding darker skin phototypes persisted when the results were stratified by condition, laser of study, study location, journal type, and funding source,” the investigators noted.
RCTs of laser/light devices for scarring, the most common dermatologic condition represented, included phototypes 5 and 6 in 24.4% of studies, compared with 84.4% for phototypes 4-6. The gap was smaller for melasma, but not for port wine stains. Among the devices examined, RCTs of diode lasers and intense pulsed light had the smallest gaps between inclusion of the two groups of phototypes, while pulsed-dye laser studies had the largest, they reported.
Stratification by journal showed the largest gap in studies published by Lasers in Medical Science and the smallest gap coming from Lasers in Surgery and Medicine. Funding was not specified for the majority of the eligible device RCTs, but those funded by industry had the smallest discrepancy between types 5-6 and types 4-6 and those supported by foundations/nonprofits the largest, Ms. Manjaly and associates said.
“With projections estimating that more than 50% of the U.S. population is set to identify as Hispanic or nonwhite by 2045 ... the lack of information has important consequences for clinical practice, as clinicians are unable to counsel patients on the efficacy and possible complications of various devices in patient with skin of color,” they wrote.
The investigators did not declare any conflicts of interest or funding sources.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
‘Exciting’ results for cancer vaccine plus pembro in melanoma
according to the latest data from the KEYNOTE-942 trial.
This recurrence-free survival benefit corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with the immunotherapy alone.
The randomized phase 2b trial is the first to show a positive result for a cancer vaccine in a randomized trial. The results, if confirmed in further studies, hold promise for treating other solid tumors with sensitivity to the programmed death-1 (PD-1) protein, investigators said.
“KEYNOTE-942 is the first randomized study to demonstrate improvement in recurrence-free survival in melanoma, or in any cancer in my view, with an individualized neoantigen vaccine approach,” trial investigator Jeffrey S. Weber, MD, PhD, of NYU Langone Perlmutter Cancer Center in New York, said during an oral abstract session at the annual meeting of the American Association for Cancer Research.
“I have every confidence that this strategy will be expanded to other histologies that are PD-1 sensitive, such as non–small cell lung cancer, renal cell cancer, hepatocellular cancer, gastroesophageal cancer, et cetera,” Dr. Weber said.
Invited discussant Margaret Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, called the results “exciting,” especially in light of previous results in cancer vaccine trials. “Despite hundreds of formulations and dozens of studies, cancer vaccines have been disappointing so far, and have largely failed to have a meaningful impact in oncology,” she said.
A promising personalized vaccine
The mRNA vaccine is individually tailored and encodes up to 34 patient-specific tumor neoantigens. The vaccine also acts as an adjuvant to strengthen the immune response.
Dr. Weber said that the “mRNA 4157 is what one would call an individualized neoantigen therapy. It will target an individual patient’s unique tumor mutations, and the revelation over the last 5-10 years, is that, for better or worse, virtually all the neoantigens are unique to an individual patient. There are very, very few true universal neoantigens, or at least universal neoantigens that could have clinical utility.”
The vaccines are developed from tumor biopsy tissues that then undergo whole exome and RNA sequencing to identify single nucleotide variants that are present in the tumor but not in normal tissue.
The findings are then fed into a computer algorithm that identifies potential neoepitope peptides that would bind well to the patient’s human leukocyte antigen (HLA) type and could evoke strong T-cell responses.
“Once they’re chosen, you concatenate the sequences together into a single-strand mRNA vaccine, it’s packaged with nanoparticles to encapsulate it, and there you have your mRNA vaccine,” Dr. Weber explained.
In the KEYNOTE-942 trial, the investigators randomly assigned patients with completely resected high-risk cutaneous melanoma on a 2:1 basis to receive mRNA-4157 via intramuscular injection every 3 weeks for a total of nine doses, plus intravenous pembrolizumab every 3 weeks for 18 cycles (107 patients) or pembrolizumab alone (50 patients). Median follow-up was 101 weeks in the combination group and 105 weeks in the pembrolizumab group.
Overall, the 18-month recurrence-free survival rates were 78.6% in the combination arm and 62.2% in the pembrolizumab arm. The recurrence-free survival rates corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with those who received only pembrolizumab (hazard ratio [HR] for recurrence, 0.561; P =.0266).
Grade 3 or greater adverse events occurred in 25% of patients in the combination group and 18% of patients in the pembrolizumab group. The most common grade 3 event associated with the vaccine was fatigue. No grade 4 adverse events or deaths were associated with the vaccine, and the addition of the vaccine to pembrolizumab did not appear to increase risk for immune-mediated adverse events.
In a subanalysis, Dr. Weber and colleagues explored the relationship between tumor mutational burden and recurrence-free survival. Higher tumor mutational burden may mean more neoepitopes to target, which is helpful when developing personalized neoantigen vaccines, explained coinvestigator Ryan Sullivan, MD, associate director of the melanoma program at Mass General Cancer Center, Boston, who presented the subanalysis results.
The investigators performed whole exome and whole transcriptome sequencing of baseline tumor biopsy samples to determine the mutational burden of tumors and defined a high mutational burden as 10 or more mutations per megabase.
Overall, in the combination group, patients with a higher tumor mutational burden at baseline showed improved outcomes (HR, 0.652; 95% confidence interval [CI], 0.284-1.494), as did patients with a lower tumor mutational burden (HR, 0.586; 95% CI, 0.243-1.415).
The authors found the same was true for patients with high vs. low tumor inflammation scores (high: HR, 0.576; 95% CI, 0.209-1.591 vs. low: HR, 0.528; 95% CI, 0.253-1.101) and higher PD-L1 expression (PD-L1 positive: HR, 0.485; 95% CI, 0.226-1.039 vs. PD-L1 negative: HR, 0.162; 95% CI, 0.038-0.685).
The hazard ratios crossed 1, which suggest that the combination was similarly effective in all patient subsets, said Dr. Sullivan.
Dr. Callahan also highlighted that the P value was based on a one-side log-rank test, “a relatively low bar to jump over” and that there were slight imbalances in both PD-1 expression status and tumor mutational burden – both of which favored the vaccine group and may be associated with better recurrence-free survival.
The 16% difference in recurrence-free survival seen with the combination vs. pembrolizumab alone, if confirmed in further studies, “is clinically meaningful for high-risk patients,” said Dr. Callahan. “The authors are to be congratulated for presenting the first randomized study of a neoantigen vaccine with a clinical efficacy primary endpoint, and this is a trial that incorporates many of the lessons we’ve learned along the years.”
Dr. Sullivan also commented on the promising results. “The field of cancer vaccines is a wasteland of failed clinical trials after some initial promising data, so to have something like this where it does appear that this vaccine strategy works is good not only for patients with melanoma but for those people who have dedicated their lives to trying to develop cancer vaccines,” he said in an interview.
KEYNOTE-942 was funded by Moderna with collaboration from Merck. Dr. Weber has financial relationships with Merck, Moderna, and other companies. Dr. Sullivan has served as a paid consultant for Merck and has received research funding from the company. Dr. Callahan disclosed a consulting/advisory role with Moderna, Merck, and others.
A version of this article first appeared on Medscape.com.
according to the latest data from the KEYNOTE-942 trial.
This recurrence-free survival benefit corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with the immunotherapy alone.
The randomized phase 2b trial is the first to show a positive result for a cancer vaccine in a randomized trial. The results, if confirmed in further studies, hold promise for treating other solid tumors with sensitivity to the programmed death-1 (PD-1) protein, investigators said.
“KEYNOTE-942 is the first randomized study to demonstrate improvement in recurrence-free survival in melanoma, or in any cancer in my view, with an individualized neoantigen vaccine approach,” trial investigator Jeffrey S. Weber, MD, PhD, of NYU Langone Perlmutter Cancer Center in New York, said during an oral abstract session at the annual meeting of the American Association for Cancer Research.
“I have every confidence that this strategy will be expanded to other histologies that are PD-1 sensitive, such as non–small cell lung cancer, renal cell cancer, hepatocellular cancer, gastroesophageal cancer, et cetera,” Dr. Weber said.
Invited discussant Margaret Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, called the results “exciting,” especially in light of previous results in cancer vaccine trials. “Despite hundreds of formulations and dozens of studies, cancer vaccines have been disappointing so far, and have largely failed to have a meaningful impact in oncology,” she said.
A promising personalized vaccine
The mRNA vaccine is individually tailored and encodes up to 34 patient-specific tumor neoantigens. The vaccine also acts as an adjuvant to strengthen the immune response.
Dr. Weber said that the “mRNA 4157 is what one would call an individualized neoantigen therapy. It will target an individual patient’s unique tumor mutations, and the revelation over the last 5-10 years, is that, for better or worse, virtually all the neoantigens are unique to an individual patient. There are very, very few true universal neoantigens, or at least universal neoantigens that could have clinical utility.”
The vaccines are developed from tumor biopsy tissues that then undergo whole exome and RNA sequencing to identify single nucleotide variants that are present in the tumor but not in normal tissue.
The findings are then fed into a computer algorithm that identifies potential neoepitope peptides that would bind well to the patient’s human leukocyte antigen (HLA) type and could evoke strong T-cell responses.
“Once they’re chosen, you concatenate the sequences together into a single-strand mRNA vaccine, it’s packaged with nanoparticles to encapsulate it, and there you have your mRNA vaccine,” Dr. Weber explained.
In the KEYNOTE-942 trial, the investigators randomly assigned patients with completely resected high-risk cutaneous melanoma on a 2:1 basis to receive mRNA-4157 via intramuscular injection every 3 weeks for a total of nine doses, plus intravenous pembrolizumab every 3 weeks for 18 cycles (107 patients) or pembrolizumab alone (50 patients). Median follow-up was 101 weeks in the combination group and 105 weeks in the pembrolizumab group.
Overall, the 18-month recurrence-free survival rates were 78.6% in the combination arm and 62.2% in the pembrolizumab arm. The recurrence-free survival rates corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with those who received only pembrolizumab (hazard ratio [HR] for recurrence, 0.561; P =.0266).
Grade 3 or greater adverse events occurred in 25% of patients in the combination group and 18% of patients in the pembrolizumab group. The most common grade 3 event associated with the vaccine was fatigue. No grade 4 adverse events or deaths were associated with the vaccine, and the addition of the vaccine to pembrolizumab did not appear to increase risk for immune-mediated adverse events.
In a subanalysis, Dr. Weber and colleagues explored the relationship between tumor mutational burden and recurrence-free survival. Higher tumor mutational burden may mean more neoepitopes to target, which is helpful when developing personalized neoantigen vaccines, explained coinvestigator Ryan Sullivan, MD, associate director of the melanoma program at Mass General Cancer Center, Boston, who presented the subanalysis results.
The investigators performed whole exome and whole transcriptome sequencing of baseline tumor biopsy samples to determine the mutational burden of tumors and defined a high mutational burden as 10 or more mutations per megabase.
Overall, in the combination group, patients with a higher tumor mutational burden at baseline showed improved outcomes (HR, 0.652; 95% confidence interval [CI], 0.284-1.494), as did patients with a lower tumor mutational burden (HR, 0.586; 95% CI, 0.243-1.415).
The authors found the same was true for patients with high vs. low tumor inflammation scores (high: HR, 0.576; 95% CI, 0.209-1.591 vs. low: HR, 0.528; 95% CI, 0.253-1.101) and higher PD-L1 expression (PD-L1 positive: HR, 0.485; 95% CI, 0.226-1.039 vs. PD-L1 negative: HR, 0.162; 95% CI, 0.038-0.685).
The hazard ratios crossed 1, which suggest that the combination was similarly effective in all patient subsets, said Dr. Sullivan.
Dr. Callahan also highlighted that the P value was based on a one-side log-rank test, “a relatively low bar to jump over” and that there were slight imbalances in both PD-1 expression status and tumor mutational burden – both of which favored the vaccine group and may be associated with better recurrence-free survival.
The 16% difference in recurrence-free survival seen with the combination vs. pembrolizumab alone, if confirmed in further studies, “is clinically meaningful for high-risk patients,” said Dr. Callahan. “The authors are to be congratulated for presenting the first randomized study of a neoantigen vaccine with a clinical efficacy primary endpoint, and this is a trial that incorporates many of the lessons we’ve learned along the years.”
Dr. Sullivan also commented on the promising results. “The field of cancer vaccines is a wasteland of failed clinical trials after some initial promising data, so to have something like this where it does appear that this vaccine strategy works is good not only for patients with melanoma but for those people who have dedicated their lives to trying to develop cancer vaccines,” he said in an interview.
KEYNOTE-942 was funded by Moderna with collaboration from Merck. Dr. Weber has financial relationships with Merck, Moderna, and other companies. Dr. Sullivan has served as a paid consultant for Merck and has received research funding from the company. Dr. Callahan disclosed a consulting/advisory role with Moderna, Merck, and others.
A version of this article first appeared on Medscape.com.
according to the latest data from the KEYNOTE-942 trial.
This recurrence-free survival benefit corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with the immunotherapy alone.
The randomized phase 2b trial is the first to show a positive result for a cancer vaccine in a randomized trial. The results, if confirmed in further studies, hold promise for treating other solid tumors with sensitivity to the programmed death-1 (PD-1) protein, investigators said.
“KEYNOTE-942 is the first randomized study to demonstrate improvement in recurrence-free survival in melanoma, or in any cancer in my view, with an individualized neoantigen vaccine approach,” trial investigator Jeffrey S. Weber, MD, PhD, of NYU Langone Perlmutter Cancer Center in New York, said during an oral abstract session at the annual meeting of the American Association for Cancer Research.
“I have every confidence that this strategy will be expanded to other histologies that are PD-1 sensitive, such as non–small cell lung cancer, renal cell cancer, hepatocellular cancer, gastroesophageal cancer, et cetera,” Dr. Weber said.
Invited discussant Margaret Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, called the results “exciting,” especially in light of previous results in cancer vaccine trials. “Despite hundreds of formulations and dozens of studies, cancer vaccines have been disappointing so far, and have largely failed to have a meaningful impact in oncology,” she said.
A promising personalized vaccine
The mRNA vaccine is individually tailored and encodes up to 34 patient-specific tumor neoantigens. The vaccine also acts as an adjuvant to strengthen the immune response.
Dr. Weber said that the “mRNA 4157 is what one would call an individualized neoantigen therapy. It will target an individual patient’s unique tumor mutations, and the revelation over the last 5-10 years, is that, for better or worse, virtually all the neoantigens are unique to an individual patient. There are very, very few true universal neoantigens, or at least universal neoantigens that could have clinical utility.”
The vaccines are developed from tumor biopsy tissues that then undergo whole exome and RNA sequencing to identify single nucleotide variants that are present in the tumor but not in normal tissue.
The findings are then fed into a computer algorithm that identifies potential neoepitope peptides that would bind well to the patient’s human leukocyte antigen (HLA) type and could evoke strong T-cell responses.
“Once they’re chosen, you concatenate the sequences together into a single-strand mRNA vaccine, it’s packaged with nanoparticles to encapsulate it, and there you have your mRNA vaccine,” Dr. Weber explained.
In the KEYNOTE-942 trial, the investigators randomly assigned patients with completely resected high-risk cutaneous melanoma on a 2:1 basis to receive mRNA-4157 via intramuscular injection every 3 weeks for a total of nine doses, plus intravenous pembrolizumab every 3 weeks for 18 cycles (107 patients) or pembrolizumab alone (50 patients). Median follow-up was 101 weeks in the combination group and 105 weeks in the pembrolizumab group.
Overall, the 18-month recurrence-free survival rates were 78.6% in the combination arm and 62.2% in the pembrolizumab arm. The recurrence-free survival rates corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with those who received only pembrolizumab (hazard ratio [HR] for recurrence, 0.561; P =.0266).
Grade 3 or greater adverse events occurred in 25% of patients in the combination group and 18% of patients in the pembrolizumab group. The most common grade 3 event associated with the vaccine was fatigue. No grade 4 adverse events or deaths were associated with the vaccine, and the addition of the vaccine to pembrolizumab did not appear to increase risk for immune-mediated adverse events.
In a subanalysis, Dr. Weber and colleagues explored the relationship between tumor mutational burden and recurrence-free survival. Higher tumor mutational burden may mean more neoepitopes to target, which is helpful when developing personalized neoantigen vaccines, explained coinvestigator Ryan Sullivan, MD, associate director of the melanoma program at Mass General Cancer Center, Boston, who presented the subanalysis results.
The investigators performed whole exome and whole transcriptome sequencing of baseline tumor biopsy samples to determine the mutational burden of tumors and defined a high mutational burden as 10 or more mutations per megabase.
Overall, in the combination group, patients with a higher tumor mutational burden at baseline showed improved outcomes (HR, 0.652; 95% confidence interval [CI], 0.284-1.494), as did patients with a lower tumor mutational burden (HR, 0.586; 95% CI, 0.243-1.415).
The authors found the same was true for patients with high vs. low tumor inflammation scores (high: HR, 0.576; 95% CI, 0.209-1.591 vs. low: HR, 0.528; 95% CI, 0.253-1.101) and higher PD-L1 expression (PD-L1 positive: HR, 0.485; 95% CI, 0.226-1.039 vs. PD-L1 negative: HR, 0.162; 95% CI, 0.038-0.685).
The hazard ratios crossed 1, which suggest that the combination was similarly effective in all patient subsets, said Dr. Sullivan.
Dr. Callahan also highlighted that the P value was based on a one-side log-rank test, “a relatively low bar to jump over” and that there were slight imbalances in both PD-1 expression status and tumor mutational burden – both of which favored the vaccine group and may be associated with better recurrence-free survival.
The 16% difference in recurrence-free survival seen with the combination vs. pembrolizumab alone, if confirmed in further studies, “is clinically meaningful for high-risk patients,” said Dr. Callahan. “The authors are to be congratulated for presenting the first randomized study of a neoantigen vaccine with a clinical efficacy primary endpoint, and this is a trial that incorporates many of the lessons we’ve learned along the years.”
Dr. Sullivan also commented on the promising results. “The field of cancer vaccines is a wasteland of failed clinical trials after some initial promising data, so to have something like this where it does appear that this vaccine strategy works is good not only for patients with melanoma but for those people who have dedicated their lives to trying to develop cancer vaccines,” he said in an interview.
KEYNOTE-942 was funded by Moderna with collaboration from Merck. Dr. Weber has financial relationships with Merck, Moderna, and other companies. Dr. Sullivan has served as a paid consultant for Merck and has received research funding from the company. Dr. Callahan disclosed a consulting/advisory role with Moderna, Merck, and others.
A version of this article first appeared on Medscape.com.
FROM AACR 2023
Racial disparities in cardiotoxicity after chemotherapy
a research review indicates.
“It’s important that both patients and clinicians be aware of these disparities so that more meaningful conversations around long-term cardiac health and cancer treatment can take place,” lead investigator Wondewossen Gebeyehu, with the University of Toronto, said in an interview.
However, patients “should not avoid chemotherapy, as the most important thing is making sure they get the best cancer treatment possible, and studies already show Black patients may get less optimal cancer treatments,” Mr. Gebeyehu added in a statement.
Ana Barac, MD, PHD, chair of cardio-oncology at Inova Schar Cancer Institute and Inova Heart and Vascular Institute, Fairfax, Va., who wasn’t involved in the study, agreed.
“The most important message is to look at preexisting cardiovascular disease, oncology diagnosis, and be aware of existing disparities in a specific cancer and CVD,” Barac said in an interview.
“What should NOT happen is to overinterpret this report of cardiotoxicity as an indication to modify/avoid planned cancer treatment to decrease cardiotoxicity. This approach could worsen oncology outcomes and lead to undertreatment of cancer, therefore posing real danger,” said Dr. Barac.
The study was presented at the American College of Cardiology Advancing the Cardiovascular Care of the Oncology Patient 2023 conference.
Causes unclear
Chemotherapy is known to increase the risk of cardiovascular heart failure and other forms of CVD, but less is known about racial disparities in the incidence of chemotherapy-induced cardiotoxicity.
Mr. Gebeyehu and colleagues conducted a systematic review and meta-analysis of the available literature to assess racial disparities in CV adverse effects among cancer patients who were treated with chemotherapeutic agents. They screened 7,057 studies, fully reviewed 57, and included 24 studies, representing 683,749 participants, in their analysis.
Breast cancer was the most commonly reported malignancy. Other common malignancies were prostate, kidney, and hematologic malignancies such as leukemia and lymphoma.
Chemotherapeutic agents included anthracyclines (doxorubicin, daunorubicin), trastuzumab, and hormonal therapies.
Black race or African ancestry was associated with increased odds of chemotherapy-associated cardiotoxicity (odds ratio, 1.71; 95% confidence interval, 1.40-2.10), as well as congestive heart failure (OR, 1.92; 95% CI, 1.68-2.19).
Mr. Gebeyehu said in an interview that it’s hard to speculate on causation with an analysis of preexisting data such as this. “Our initial analysis that we’ve reported on so far are unadjusted values, meaning they don’t adjust for those potential underlying factors,” he noted.
“However, some of the studies individually controlled for socioeconomic factors and still found increased vulnerability to chemotherapy-associated cardiotoxicity in patients of Black race or African ancestry,” Mr. Gebeyehu said.
“It’s certainly possible that a mix of both biological and socioeconomic factors are interacting to lead to these disparities. One example could be the underrepresentation of Black patients in clinical trials to develop drugs. These could lead to chemotherapeutic agents being poorly optimized in this population relative to other racial/ethnic groups,” he added.
Dr. Barac said this study adds to the growing body of evidence about the importance of racial disparities in CVD and cancer outcomes.
“It is important to note that only the unadjusted odds ratio was reported and that much more detail is needed to understand what may be underlying the disparities. It is critically important to await the adjusted analysis, as well as details of the type of cancers and treatment used, before clinical implications can be discussed,” said Dr. Barac, who served as codirector of the conference.
“The risk of cardiotoxicity needs to be presented in the context of the oncology and CV disease burden, as both can influence the risk, and there could be a synergistic effect of disparities,” Dr. Barac added.
The study had no specific funding. Mr. Gebeyehu and Dr. Barac disclosed no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
a research review indicates.
“It’s important that both patients and clinicians be aware of these disparities so that more meaningful conversations around long-term cardiac health and cancer treatment can take place,” lead investigator Wondewossen Gebeyehu, with the University of Toronto, said in an interview.
However, patients “should not avoid chemotherapy, as the most important thing is making sure they get the best cancer treatment possible, and studies already show Black patients may get less optimal cancer treatments,” Mr. Gebeyehu added in a statement.
Ana Barac, MD, PHD, chair of cardio-oncology at Inova Schar Cancer Institute and Inova Heart and Vascular Institute, Fairfax, Va., who wasn’t involved in the study, agreed.
“The most important message is to look at preexisting cardiovascular disease, oncology diagnosis, and be aware of existing disparities in a specific cancer and CVD,” Barac said in an interview.
“What should NOT happen is to overinterpret this report of cardiotoxicity as an indication to modify/avoid planned cancer treatment to decrease cardiotoxicity. This approach could worsen oncology outcomes and lead to undertreatment of cancer, therefore posing real danger,” said Dr. Barac.
The study was presented at the American College of Cardiology Advancing the Cardiovascular Care of the Oncology Patient 2023 conference.
Causes unclear
Chemotherapy is known to increase the risk of cardiovascular heart failure and other forms of CVD, but less is known about racial disparities in the incidence of chemotherapy-induced cardiotoxicity.
Mr. Gebeyehu and colleagues conducted a systematic review and meta-analysis of the available literature to assess racial disparities in CV adverse effects among cancer patients who were treated with chemotherapeutic agents. They screened 7,057 studies, fully reviewed 57, and included 24 studies, representing 683,749 participants, in their analysis.
Breast cancer was the most commonly reported malignancy. Other common malignancies were prostate, kidney, and hematologic malignancies such as leukemia and lymphoma.
Chemotherapeutic agents included anthracyclines (doxorubicin, daunorubicin), trastuzumab, and hormonal therapies.
Black race or African ancestry was associated with increased odds of chemotherapy-associated cardiotoxicity (odds ratio, 1.71; 95% confidence interval, 1.40-2.10), as well as congestive heart failure (OR, 1.92; 95% CI, 1.68-2.19).
Mr. Gebeyehu said in an interview that it’s hard to speculate on causation with an analysis of preexisting data such as this. “Our initial analysis that we’ve reported on so far are unadjusted values, meaning they don’t adjust for those potential underlying factors,” he noted.
“However, some of the studies individually controlled for socioeconomic factors and still found increased vulnerability to chemotherapy-associated cardiotoxicity in patients of Black race or African ancestry,” Mr. Gebeyehu said.
“It’s certainly possible that a mix of both biological and socioeconomic factors are interacting to lead to these disparities. One example could be the underrepresentation of Black patients in clinical trials to develop drugs. These could lead to chemotherapeutic agents being poorly optimized in this population relative to other racial/ethnic groups,” he added.
Dr. Barac said this study adds to the growing body of evidence about the importance of racial disparities in CVD and cancer outcomes.
“It is important to note that only the unadjusted odds ratio was reported and that much more detail is needed to understand what may be underlying the disparities. It is critically important to await the adjusted analysis, as well as details of the type of cancers and treatment used, before clinical implications can be discussed,” said Dr. Barac, who served as codirector of the conference.
“The risk of cardiotoxicity needs to be presented in the context of the oncology and CV disease burden, as both can influence the risk, and there could be a synergistic effect of disparities,” Dr. Barac added.
The study had no specific funding. Mr. Gebeyehu and Dr. Barac disclosed no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
a research review indicates.
“It’s important that both patients and clinicians be aware of these disparities so that more meaningful conversations around long-term cardiac health and cancer treatment can take place,” lead investigator Wondewossen Gebeyehu, with the University of Toronto, said in an interview.
However, patients “should not avoid chemotherapy, as the most important thing is making sure they get the best cancer treatment possible, and studies already show Black patients may get less optimal cancer treatments,” Mr. Gebeyehu added in a statement.
Ana Barac, MD, PHD, chair of cardio-oncology at Inova Schar Cancer Institute and Inova Heart and Vascular Institute, Fairfax, Va., who wasn’t involved in the study, agreed.
“The most important message is to look at preexisting cardiovascular disease, oncology diagnosis, and be aware of existing disparities in a specific cancer and CVD,” Barac said in an interview.
“What should NOT happen is to overinterpret this report of cardiotoxicity as an indication to modify/avoid planned cancer treatment to decrease cardiotoxicity. This approach could worsen oncology outcomes and lead to undertreatment of cancer, therefore posing real danger,” said Dr. Barac.
The study was presented at the American College of Cardiology Advancing the Cardiovascular Care of the Oncology Patient 2023 conference.
Causes unclear
Chemotherapy is known to increase the risk of cardiovascular heart failure and other forms of CVD, but less is known about racial disparities in the incidence of chemotherapy-induced cardiotoxicity.
Mr. Gebeyehu and colleagues conducted a systematic review and meta-analysis of the available literature to assess racial disparities in CV adverse effects among cancer patients who were treated with chemotherapeutic agents. They screened 7,057 studies, fully reviewed 57, and included 24 studies, representing 683,749 participants, in their analysis.
Breast cancer was the most commonly reported malignancy. Other common malignancies were prostate, kidney, and hematologic malignancies such as leukemia and lymphoma.
Chemotherapeutic agents included anthracyclines (doxorubicin, daunorubicin), trastuzumab, and hormonal therapies.
Black race or African ancestry was associated with increased odds of chemotherapy-associated cardiotoxicity (odds ratio, 1.71; 95% confidence interval, 1.40-2.10), as well as congestive heart failure (OR, 1.92; 95% CI, 1.68-2.19).
Mr. Gebeyehu said in an interview that it’s hard to speculate on causation with an analysis of preexisting data such as this. “Our initial analysis that we’ve reported on so far are unadjusted values, meaning they don’t adjust for those potential underlying factors,” he noted.
“However, some of the studies individually controlled for socioeconomic factors and still found increased vulnerability to chemotherapy-associated cardiotoxicity in patients of Black race or African ancestry,” Mr. Gebeyehu said.
“It’s certainly possible that a mix of both biological and socioeconomic factors are interacting to lead to these disparities. One example could be the underrepresentation of Black patients in clinical trials to develop drugs. These could lead to chemotherapeutic agents being poorly optimized in this population relative to other racial/ethnic groups,” he added.
Dr. Barac said this study adds to the growing body of evidence about the importance of racial disparities in CVD and cancer outcomes.
“It is important to note that only the unadjusted odds ratio was reported and that much more detail is needed to understand what may be underlying the disparities. It is critically important to await the adjusted analysis, as well as details of the type of cancers and treatment used, before clinical implications can be discussed,” said Dr. Barac, who served as codirector of the conference.
“The risk of cardiotoxicity needs to be presented in the context of the oncology and CV disease burden, as both can influence the risk, and there could be a synergistic effect of disparities,” Dr. Barac added.
The study had no specific funding. Mr. Gebeyehu and Dr. Barac disclosed no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
Breast cancer screening advice ‘dangerous’ for black women
The U.S. Preventive Services Task Force currently recommends that breast cancer screening start at age 50 years, regardless of race or ethnicity.
But
The current “one-size-fits-all” policy to screen the entire female population from a certain age may be “neither fair and equitable nor optimal,” noted the authors, led by Tianhui Chen, PhD, with Zhejiang Cancer Hospital, Hangzhou, China.
The study was published online in JAMA Network Open.
Laurie R. Margolies, MD, chief of breast imaging at the Dubin Breast Center of the Mount Sinai Tisch Cancer Center in New York City, agreed.
Black women get breast cancer at a much younger age, are less likely to be diagnosed with early breast cancer, and are more likely to die of breast cancer, explained Dr. Margolies, who was not involved in the study.
“That’s why the guidelines that say begin at age 50 are flawed and so dangerous,” she said in an interview with this news organization. “This study is really important to highlight that we’re missing an opportunity to detect and treat breast cancer early in the Black population.”
The current study explored the optimal race- and ethnicity-specific ages to initiate breast cancer screening to address racial disparities in breast cancer mortality.
Using a nationwide population-based cross-sectional study design, the team analyzed data on 415,277 women who died of breast cancer in the United States from 2011 to 2020.
The cohort was 75% White, 15% Black, 7% Hispanic, 3% Asian or Pacific Islander, and < 1% Native American or Alaska Native. A total of 115,214 women (28%) died before age 60. The team calculated the 10-year cumulative risk of breast cancer–specific death by age and by race and ethnicity.
For those aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years), followed by White women (15 deaths per 100,000 person-years) and American Indian/Alaska Native, Hispanic, and Asian/Pacific Islander women (11 deaths per 100,000 person-years).
If breast screening started at age 50 for the entire population, the mean 10-year cumulative risk of dying from breast cancer would be 0.329%. Black women reached this risk threshold level at age 42, whereas non-Hispanic White women reached the threshold at age 51, American Indian/Alaska Native and Hispanic women at age 57, and Asian/Pacific Islander women at age 61.
If screening started at age 45 for all women, the mean 10-year cumulative risk of breast cancer death would be 0.235%. Black women reached this risk threshold level at age 38, non-Hispanic White women at age 46, Hispanic women at age 49, Asian/Pacific Islander women at age 50, and American Indian/Alaska Native women at age 51.
If screening started at age 40 for all women, with a mean 10-year cumulative risk of 0.154%, Black women would reach this risk threshold at age 34, White women at age 41, Hispanic women at age 43, and American Indian/Alaska Native and Asian/Pacific Islander women at age 43.
Dr. Chen and colleagues concluded that failure to consider race and ethnicity in breast cancer screening guidelines “may pose a significant risk for greater harm to a group already at increased risk.
“Changing guidelines based on readily available risk factors, such as race and ethnicity, is possible and may be the first, yet important step toward a personalized and fair screening program,” the team explained.
Dr. Margolies said she believes individualized screening recommendations will likely come, but first, all women should start screening at age 40 instead of age 50.
“Most American women are starting in their 40s, or starting at 40, because we know what the current guidelines are,” she said. “The question that this study doesn’t answer is, is age 40 young enough for the Black population? Maybe it should be 35.”
The study was supported by grants from the National Key Research-Development Program of China and from the Ten-Thousand Talents Plan of Zhejiang Province and by Start-Up Funds for Recruited Talents in Zhejiang Cancer Hospital. Dr. Chen and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The U.S. Preventive Services Task Force currently recommends that breast cancer screening start at age 50 years, regardless of race or ethnicity.
But
The current “one-size-fits-all” policy to screen the entire female population from a certain age may be “neither fair and equitable nor optimal,” noted the authors, led by Tianhui Chen, PhD, with Zhejiang Cancer Hospital, Hangzhou, China.
The study was published online in JAMA Network Open.
Laurie R. Margolies, MD, chief of breast imaging at the Dubin Breast Center of the Mount Sinai Tisch Cancer Center in New York City, agreed.
Black women get breast cancer at a much younger age, are less likely to be diagnosed with early breast cancer, and are more likely to die of breast cancer, explained Dr. Margolies, who was not involved in the study.
“That’s why the guidelines that say begin at age 50 are flawed and so dangerous,” she said in an interview with this news organization. “This study is really important to highlight that we’re missing an opportunity to detect and treat breast cancer early in the Black population.”
The current study explored the optimal race- and ethnicity-specific ages to initiate breast cancer screening to address racial disparities in breast cancer mortality.
Using a nationwide population-based cross-sectional study design, the team analyzed data on 415,277 women who died of breast cancer in the United States from 2011 to 2020.
The cohort was 75% White, 15% Black, 7% Hispanic, 3% Asian or Pacific Islander, and < 1% Native American or Alaska Native. A total of 115,214 women (28%) died before age 60. The team calculated the 10-year cumulative risk of breast cancer–specific death by age and by race and ethnicity.
For those aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years), followed by White women (15 deaths per 100,000 person-years) and American Indian/Alaska Native, Hispanic, and Asian/Pacific Islander women (11 deaths per 100,000 person-years).
If breast screening started at age 50 for the entire population, the mean 10-year cumulative risk of dying from breast cancer would be 0.329%. Black women reached this risk threshold level at age 42, whereas non-Hispanic White women reached the threshold at age 51, American Indian/Alaska Native and Hispanic women at age 57, and Asian/Pacific Islander women at age 61.
If screening started at age 45 for all women, the mean 10-year cumulative risk of breast cancer death would be 0.235%. Black women reached this risk threshold level at age 38, non-Hispanic White women at age 46, Hispanic women at age 49, Asian/Pacific Islander women at age 50, and American Indian/Alaska Native women at age 51.
If screening started at age 40 for all women, with a mean 10-year cumulative risk of 0.154%, Black women would reach this risk threshold at age 34, White women at age 41, Hispanic women at age 43, and American Indian/Alaska Native and Asian/Pacific Islander women at age 43.
Dr. Chen and colleagues concluded that failure to consider race and ethnicity in breast cancer screening guidelines “may pose a significant risk for greater harm to a group already at increased risk.
“Changing guidelines based on readily available risk factors, such as race and ethnicity, is possible and may be the first, yet important step toward a personalized and fair screening program,” the team explained.
Dr. Margolies said she believes individualized screening recommendations will likely come, but first, all women should start screening at age 40 instead of age 50.
“Most American women are starting in their 40s, or starting at 40, because we know what the current guidelines are,” she said. “The question that this study doesn’t answer is, is age 40 young enough for the Black population? Maybe it should be 35.”
The study was supported by grants from the National Key Research-Development Program of China and from the Ten-Thousand Talents Plan of Zhejiang Province and by Start-Up Funds for Recruited Talents in Zhejiang Cancer Hospital. Dr. Chen and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The U.S. Preventive Services Task Force currently recommends that breast cancer screening start at age 50 years, regardless of race or ethnicity.
But
The current “one-size-fits-all” policy to screen the entire female population from a certain age may be “neither fair and equitable nor optimal,” noted the authors, led by Tianhui Chen, PhD, with Zhejiang Cancer Hospital, Hangzhou, China.
The study was published online in JAMA Network Open.
Laurie R. Margolies, MD, chief of breast imaging at the Dubin Breast Center of the Mount Sinai Tisch Cancer Center in New York City, agreed.
Black women get breast cancer at a much younger age, are less likely to be diagnosed with early breast cancer, and are more likely to die of breast cancer, explained Dr. Margolies, who was not involved in the study.
“That’s why the guidelines that say begin at age 50 are flawed and so dangerous,” she said in an interview with this news organization. “This study is really important to highlight that we’re missing an opportunity to detect and treat breast cancer early in the Black population.”
The current study explored the optimal race- and ethnicity-specific ages to initiate breast cancer screening to address racial disparities in breast cancer mortality.
Using a nationwide population-based cross-sectional study design, the team analyzed data on 415,277 women who died of breast cancer in the United States from 2011 to 2020.
The cohort was 75% White, 15% Black, 7% Hispanic, 3% Asian or Pacific Islander, and < 1% Native American or Alaska Native. A total of 115,214 women (28%) died before age 60. The team calculated the 10-year cumulative risk of breast cancer–specific death by age and by race and ethnicity.
For those aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years), followed by White women (15 deaths per 100,000 person-years) and American Indian/Alaska Native, Hispanic, and Asian/Pacific Islander women (11 deaths per 100,000 person-years).
If breast screening started at age 50 for the entire population, the mean 10-year cumulative risk of dying from breast cancer would be 0.329%. Black women reached this risk threshold level at age 42, whereas non-Hispanic White women reached the threshold at age 51, American Indian/Alaska Native and Hispanic women at age 57, and Asian/Pacific Islander women at age 61.
If screening started at age 45 for all women, the mean 10-year cumulative risk of breast cancer death would be 0.235%. Black women reached this risk threshold level at age 38, non-Hispanic White women at age 46, Hispanic women at age 49, Asian/Pacific Islander women at age 50, and American Indian/Alaska Native women at age 51.
If screening started at age 40 for all women, with a mean 10-year cumulative risk of 0.154%, Black women would reach this risk threshold at age 34, White women at age 41, Hispanic women at age 43, and American Indian/Alaska Native and Asian/Pacific Islander women at age 43.
Dr. Chen and colleagues concluded that failure to consider race and ethnicity in breast cancer screening guidelines “may pose a significant risk for greater harm to a group already at increased risk.
“Changing guidelines based on readily available risk factors, such as race and ethnicity, is possible and may be the first, yet important step toward a personalized and fair screening program,” the team explained.
Dr. Margolies said she believes individualized screening recommendations will likely come, but first, all women should start screening at age 40 instead of age 50.
“Most American women are starting in their 40s, or starting at 40, because we know what the current guidelines are,” she said. “The question that this study doesn’t answer is, is age 40 young enough for the Black population? Maybe it should be 35.”
The study was supported by grants from the National Key Research-Development Program of China and from the Ten-Thousand Talents Plan of Zhejiang Province and by Start-Up Funds for Recruited Talents in Zhejiang Cancer Hospital. Dr. Chen and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.






