User login
NAMDRC Signals Concern Over CMS Proposed Changes in Payment Methodology
CMS has proposed a dramatic change in the methodology used to determine payment to physician offices that provide drugs covered under the Part B Medicare benefit. Under current policy, physician offices are reimbursed at a rate of the average sales price (ASP) +6%. While not exactly a “moneymaker” in the pulmonary space, this policy has been particularly attractive to oncologists who have the opportunity to select expensive medicines that, according to CMS, may overlook very similar, less expensive drugs.
The proposal, in effect a nationwide pilot, reduces the payment to ASP + 3%, plus a $16 administrative fee. The response from the oncology community has been vociferous opposition, and it has garnered significant support on Capitol Hill. But the impact on the pulmonary community has, for the most part, been muted, thanks to uncertainty about the impact such a policy change may trigger. NAMDRC submitted detailed comments to CMS, highlighting concerns that this initiative, which is mandatory and nationwide in scope, could adversely impact the medical care of seniors who suffer from COPD and other related pulmonary diseases. NAMDRC is also concerned about the precedent this policy sets by using limited demonstration authority to change statutory payment policy nationwide.
While NAMDRC supports the goals of developing new health-care delivery methods to increase quality and provide more efficient patient care, it is nevertheless troubled by the Part B drug reimbursement policy proposed by CMS, as it appears to have been created in a vacuum without any input from stakeholders involved, particularly beneficiaries and their physicians. Forcing vulnerable Medicare beneficiaries, many with potentially life-threatening conditions, including COPD, the third leading cause of death in the United States, and asthma, to be exposed to a new mandatory payment initiative that runs the notable risk of impeding access to life-saving therapies runs counter to the initiatives that Congress has put forth.
While NAMDRC understands the need to look seriously at cost issues within our core health programs, we must not subject beneficiaries and their physicians to the problematic choice between practice economics and prescribing the most medically appropriate treatment for each individual patient. As CMS knows, biologic medications for treatment of asthma are likely to take an important role in treatment protocols in the immediate future; one new biologic for asthma was approved recently, and two new biologics in the pipeline are likely to be approved by the end of the year. Beyond asthma, the development of new biologics in the pulmonary field is likely to expand in the foreseeable future.
As noted above, in the proposed rule, CMS expresses concern that the current 6% ASP add-on payment “may encourage the use of more expensive drugs because the 6% add-on generates more revenue for more expensive drugs.” In addition to lacking any data to support this premise, the reimbursement changes contemplated under this model may actually increase overall health-care spending by causing patients to receive care in more expensive settings.
Most importantly, there is no evidence indicating that the payment changes contemplated by the model will improve quality of care and may adversely impact those patients who lose access to their most appropriate treatments. Instead, NAMDRC believes that Medicare beneficiaries would be best served by a more patient-centric approach with appropriate safeguards, while also fostering physician-patient collaboration and ensuring that the unique needs of seniors are met. Therefore, NAMDRC strongly requested that CMS withdraw the proposed rule and obtain meaningful stakeholder input, including from patients and providers, before proceeding with Phase 2 of the proposed pilot.
2017 Educational Conference planning underway: NAMDRC’s 2017 Program Committee is targeting the middle of July for completion of the primary program for the 2017 conference to be held at the Meritage Resort, Napa, CA March 23-25, 2017. For information on the program, visit www.namdrc.org or call the Executive Office at 703/752-4359.
CMS has proposed a dramatic change in the methodology used to determine payment to physician offices that provide drugs covered under the Part B Medicare benefit. Under current policy, physician offices are reimbursed at a rate of the average sales price (ASP) +6%. While not exactly a “moneymaker” in the pulmonary space, this policy has been particularly attractive to oncologists who have the opportunity to select expensive medicines that, according to CMS, may overlook very similar, less expensive drugs.
The proposal, in effect a nationwide pilot, reduces the payment to ASP + 3%, plus a $16 administrative fee. The response from the oncology community has been vociferous opposition, and it has garnered significant support on Capitol Hill. But the impact on the pulmonary community has, for the most part, been muted, thanks to uncertainty about the impact such a policy change may trigger. NAMDRC submitted detailed comments to CMS, highlighting concerns that this initiative, which is mandatory and nationwide in scope, could adversely impact the medical care of seniors who suffer from COPD and other related pulmonary diseases. NAMDRC is also concerned about the precedent this policy sets by using limited demonstration authority to change statutory payment policy nationwide.
While NAMDRC supports the goals of developing new health-care delivery methods to increase quality and provide more efficient patient care, it is nevertheless troubled by the Part B drug reimbursement policy proposed by CMS, as it appears to have been created in a vacuum without any input from stakeholders involved, particularly beneficiaries and their physicians. Forcing vulnerable Medicare beneficiaries, many with potentially life-threatening conditions, including COPD, the third leading cause of death in the United States, and asthma, to be exposed to a new mandatory payment initiative that runs the notable risk of impeding access to life-saving therapies runs counter to the initiatives that Congress has put forth.
While NAMDRC understands the need to look seriously at cost issues within our core health programs, we must not subject beneficiaries and their physicians to the problematic choice between practice economics and prescribing the most medically appropriate treatment for each individual patient. As CMS knows, biologic medications for treatment of asthma are likely to take an important role in treatment protocols in the immediate future; one new biologic for asthma was approved recently, and two new biologics in the pipeline are likely to be approved by the end of the year. Beyond asthma, the development of new biologics in the pulmonary field is likely to expand in the foreseeable future.
As noted above, in the proposed rule, CMS expresses concern that the current 6% ASP add-on payment “may encourage the use of more expensive drugs because the 6% add-on generates more revenue for more expensive drugs.” In addition to lacking any data to support this premise, the reimbursement changes contemplated under this model may actually increase overall health-care spending by causing patients to receive care in more expensive settings.
Most importantly, there is no evidence indicating that the payment changes contemplated by the model will improve quality of care and may adversely impact those patients who lose access to their most appropriate treatments. Instead, NAMDRC believes that Medicare beneficiaries would be best served by a more patient-centric approach with appropriate safeguards, while also fostering physician-patient collaboration and ensuring that the unique needs of seniors are met. Therefore, NAMDRC strongly requested that CMS withdraw the proposed rule and obtain meaningful stakeholder input, including from patients and providers, before proceeding with Phase 2 of the proposed pilot.
2017 Educational Conference planning underway: NAMDRC’s 2017 Program Committee is targeting the middle of July for completion of the primary program for the 2017 conference to be held at the Meritage Resort, Napa, CA March 23-25, 2017. For information on the program, visit www.namdrc.org or call the Executive Office at 703/752-4359.
CMS has proposed a dramatic change in the methodology used to determine payment to physician offices that provide drugs covered under the Part B Medicare benefit. Under current policy, physician offices are reimbursed at a rate of the average sales price (ASP) +6%. While not exactly a “moneymaker” in the pulmonary space, this policy has been particularly attractive to oncologists who have the opportunity to select expensive medicines that, according to CMS, may overlook very similar, less expensive drugs.
The proposal, in effect a nationwide pilot, reduces the payment to ASP + 3%, plus a $16 administrative fee. The response from the oncology community has been vociferous opposition, and it has garnered significant support on Capitol Hill. But the impact on the pulmonary community has, for the most part, been muted, thanks to uncertainty about the impact such a policy change may trigger. NAMDRC submitted detailed comments to CMS, highlighting concerns that this initiative, which is mandatory and nationwide in scope, could adversely impact the medical care of seniors who suffer from COPD and other related pulmonary diseases. NAMDRC is also concerned about the precedent this policy sets by using limited demonstration authority to change statutory payment policy nationwide.
While NAMDRC supports the goals of developing new health-care delivery methods to increase quality and provide more efficient patient care, it is nevertheless troubled by the Part B drug reimbursement policy proposed by CMS, as it appears to have been created in a vacuum without any input from stakeholders involved, particularly beneficiaries and their physicians. Forcing vulnerable Medicare beneficiaries, many with potentially life-threatening conditions, including COPD, the third leading cause of death in the United States, and asthma, to be exposed to a new mandatory payment initiative that runs the notable risk of impeding access to life-saving therapies runs counter to the initiatives that Congress has put forth.
While NAMDRC understands the need to look seriously at cost issues within our core health programs, we must not subject beneficiaries and their physicians to the problematic choice between practice economics and prescribing the most medically appropriate treatment for each individual patient. As CMS knows, biologic medications for treatment of asthma are likely to take an important role in treatment protocols in the immediate future; one new biologic for asthma was approved recently, and two new biologics in the pipeline are likely to be approved by the end of the year. Beyond asthma, the development of new biologics in the pulmonary field is likely to expand in the foreseeable future.
As noted above, in the proposed rule, CMS expresses concern that the current 6% ASP add-on payment “may encourage the use of more expensive drugs because the 6% add-on generates more revenue for more expensive drugs.” In addition to lacking any data to support this premise, the reimbursement changes contemplated under this model may actually increase overall health-care spending by causing patients to receive care in more expensive settings.
Most importantly, there is no evidence indicating that the payment changes contemplated by the model will improve quality of care and may adversely impact those patients who lose access to their most appropriate treatments. Instead, NAMDRC believes that Medicare beneficiaries would be best served by a more patient-centric approach with appropriate safeguards, while also fostering physician-patient collaboration and ensuring that the unique needs of seniors are met. Therefore, NAMDRC strongly requested that CMS withdraw the proposed rule and obtain meaningful stakeholder input, including from patients and providers, before proceeding with Phase 2 of the proposed pilot.
2017 Educational Conference planning underway: NAMDRC’s 2017 Program Committee is targeting the middle of July for completion of the primary program for the 2017 conference to be held at the Meritage Resort, Napa, CA March 23-25, 2017. For information on the program, visit www.namdrc.org or call the Executive Office at 703/752-4359.
Alert - Edit Errors on EBUS
Beginning this year, the CPT® code for endobronchial ultrasound (EBUS) 31620 was replaced by three new codes that more accurately describe the procedure as it is currently performed. Codes 31652 and 31653 are reported when EBUS is used for sampling proximal lesions (mediastinal or hilar). Code 31654 is used in identifying more distal lesions. As with other bronchoscopy procedures, the diagnostic code, 31622, is included with these three new codes and the multiple endoscopy rule applies.
CPT code 31652 is utilized when one samples two or fewer proximal locations. CPT code 31653 is utilized when one samples three or more proximal locations. 31652 and 31653 may not be used together; use the code that best describes the work that was done. These two codes include the sampling procedures and, therefore, one does not use CPT codes for sampling, e.g., 31628 or 31629, with either 31652 or 31653. However, if additional procedures are performed on structures distal to the hila, then it is appropriate to use other bronchoscopy codes with 31652 and 31653.
CPT code 31654 is an “add-on” code that is used to identify more peripheral lesions for sampling. As such, it may be used with all of the other bronchoscopy codes.
Unfortunately, when CMS originally published the National Correct Coding Initiative (NCCI) edits for these new codes, there were errors present. NCCI edits are used to instruct CMS payers and clinicians when two distinct CPT codes may or may not be used together. The NCCI edits for 31652 and 31653 published on January 1, 2016, had a value of “0” for all other bronchoscopy codes; this instructed payers to reject any claims for 31652 or 31653 if any other bronchoscopy code was appended. The societies alerted CMS to these problems, and the NCCI edits were corrected. However, these corrections did not take effect until April 1, 2016. It is, therefore, quite possible that some claims will have been rejected by CMS and other carriers from January 1 until March 31. All claims for EBUS procedures during this time should be reviewed and resubmitted if rejected. You have 1 year to resubmit these claims to avoid nonpayment for untimely filing.
Beginning this year, the CPT® code for endobronchial ultrasound (EBUS) 31620 was replaced by three new codes that more accurately describe the procedure as it is currently performed. Codes 31652 and 31653 are reported when EBUS is used for sampling proximal lesions (mediastinal or hilar). Code 31654 is used in identifying more distal lesions. As with other bronchoscopy procedures, the diagnostic code, 31622, is included with these three new codes and the multiple endoscopy rule applies.
CPT code 31652 is utilized when one samples two or fewer proximal locations. CPT code 31653 is utilized when one samples three or more proximal locations. 31652 and 31653 may not be used together; use the code that best describes the work that was done. These two codes include the sampling procedures and, therefore, one does not use CPT codes for sampling, e.g., 31628 or 31629, with either 31652 or 31653. However, if additional procedures are performed on structures distal to the hila, then it is appropriate to use other bronchoscopy codes with 31652 and 31653.
CPT code 31654 is an “add-on” code that is used to identify more peripheral lesions for sampling. As such, it may be used with all of the other bronchoscopy codes.
Unfortunately, when CMS originally published the National Correct Coding Initiative (NCCI) edits for these new codes, there were errors present. NCCI edits are used to instruct CMS payers and clinicians when two distinct CPT codes may or may not be used together. The NCCI edits for 31652 and 31653 published on January 1, 2016, had a value of “0” for all other bronchoscopy codes; this instructed payers to reject any claims for 31652 or 31653 if any other bronchoscopy code was appended. The societies alerted CMS to these problems, and the NCCI edits were corrected. However, these corrections did not take effect until April 1, 2016. It is, therefore, quite possible that some claims will have been rejected by CMS and other carriers from January 1 until March 31. All claims for EBUS procedures during this time should be reviewed and resubmitted if rejected. You have 1 year to resubmit these claims to avoid nonpayment for untimely filing.
Beginning this year, the CPT® code for endobronchial ultrasound (EBUS) 31620 was replaced by three new codes that more accurately describe the procedure as it is currently performed. Codes 31652 and 31653 are reported when EBUS is used for sampling proximal lesions (mediastinal or hilar). Code 31654 is used in identifying more distal lesions. As with other bronchoscopy procedures, the diagnostic code, 31622, is included with these three new codes and the multiple endoscopy rule applies.
CPT code 31652 is utilized when one samples two or fewer proximal locations. CPT code 31653 is utilized when one samples three or more proximal locations. 31652 and 31653 may not be used together; use the code that best describes the work that was done. These two codes include the sampling procedures and, therefore, one does not use CPT codes for sampling, e.g., 31628 or 31629, with either 31652 or 31653. However, if additional procedures are performed on structures distal to the hila, then it is appropriate to use other bronchoscopy codes with 31652 and 31653.
CPT code 31654 is an “add-on” code that is used to identify more peripheral lesions for sampling. As such, it may be used with all of the other bronchoscopy codes.
Unfortunately, when CMS originally published the National Correct Coding Initiative (NCCI) edits for these new codes, there were errors present. NCCI edits are used to instruct CMS payers and clinicians when two distinct CPT codes may or may not be used together. The NCCI edits for 31652 and 31653 published on January 1, 2016, had a value of “0” for all other bronchoscopy codes; this instructed payers to reject any claims for 31652 or 31653 if any other bronchoscopy code was appended. The societies alerted CMS to these problems, and the NCCI edits were corrected. However, these corrections did not take effect until April 1, 2016. It is, therefore, quite possible that some claims will have been rejected by CMS and other carriers from January 1 until March 31. All claims for EBUS procedures during this time should be reviewed and resubmitted if rejected. You have 1 year to resubmit these claims to avoid nonpayment for untimely filing.
Like tobacco, recent marijuana use decreases exhaled nitric oxide
Recent marijuana use may have an effect similar to recent tobacco use with regard to decreased production of exhaled nitric oxide (eNO), but a very different effect on forced vital capacity (FVC), according to the results of a study published in Chest.
Dr. Stefania I. Papatheodorou from the Cyprus International Institute for Environmental and Public Health, Limassol, Cyprus, in association with the Harvard T. H. Chan School of Public Health, and her colleagues conducted a retrospective analysis of National Health and Nutrition Examination Survey (NHANES) data collected from 10,327 people aged 18-59 years between 2007 to 2012. Respondents completed a questionnaire on illicit drug use and were given a physical examination. Outcomes of interest for this study were eNO levels and pulmonary function measurements including the forced expiratory volume in 1 second (FEV1), FVC, the FEV1/FVC ratio, and the average forced expiratory flow during the mid (25%-75%) portion of the FVC (FEF25%-75%) (Chest. 2016 Jan 16. doi: 10.1016/j.chest.2015.12.033).
The population available for analysis included 4,797 people who reported never having used marijuana, and 4,084 who reported past use. The recent users were divided into two groups; 555 and 891 respondents who reported using marijuana 5-30 days and 0-4 days before their examinations, respectively. The study results from age-adjusted analyses standardized to the 2000 U.S. Census population indicated that both past and current users had significantly lower eNO levels (in parts per billion) than those that had never used (all P less than .001). The same analysis demonstrated that FEV1, FVC, and FEV1/FVC ratios were all higher in current and past users than in never users (all P less than .001).
Using a multivariable model adjusted for age, sex, race/ethnicity, height, education level, income, marital status, asthma, tobacco use in pack-years, smoking category, and body mass index, both the 0-4 day users and the 5-30 day users showed significantly decreased eNO levels, compared with never users (-7%, 95% confidence interval -12% to -2%, P = .03; -13%, CI -18% to -8%, P less than .001, respectively). Additionally, recent users in the 0-4 day group had significantly higher FEV1 measures than never users (89 mL, CI 29-150, P = 0.005), as well as lower FEV1/FVC ratios (-.02, CI –.03 to –.01, P = .003).
Regarding the broader implications of their study findings, Dr. Papatheodorou and colleagues stated, “Given that nitric oxide plays a role in inflammatory and immune defense pathways in the respiratory system and is a mediator of vasodilation in the pulmonary and systemic vasculature, it would be useful to further explore the associations between marijuana use and vascular and pulmonary function in randomized trials.”
Dr. Mary B. Rice received funding from the Institute for Environmental Sciences. The other authors reported no conflicts of interest.
Recent marijuana use may have an effect similar to recent tobacco use with regard to decreased production of exhaled nitric oxide (eNO), but a very different effect on forced vital capacity (FVC), according to the results of a study published in Chest.
Dr. Stefania I. Papatheodorou from the Cyprus International Institute for Environmental and Public Health, Limassol, Cyprus, in association with the Harvard T. H. Chan School of Public Health, and her colleagues conducted a retrospective analysis of National Health and Nutrition Examination Survey (NHANES) data collected from 10,327 people aged 18-59 years between 2007 to 2012. Respondents completed a questionnaire on illicit drug use and were given a physical examination. Outcomes of interest for this study were eNO levels and pulmonary function measurements including the forced expiratory volume in 1 second (FEV1), FVC, the FEV1/FVC ratio, and the average forced expiratory flow during the mid (25%-75%) portion of the FVC (FEF25%-75%) (Chest. 2016 Jan 16. doi: 10.1016/j.chest.2015.12.033).
The population available for analysis included 4,797 people who reported never having used marijuana, and 4,084 who reported past use. The recent users were divided into two groups; 555 and 891 respondents who reported using marijuana 5-30 days and 0-4 days before their examinations, respectively. The study results from age-adjusted analyses standardized to the 2000 U.S. Census population indicated that both past and current users had significantly lower eNO levels (in parts per billion) than those that had never used (all P less than .001). The same analysis demonstrated that FEV1, FVC, and FEV1/FVC ratios were all higher in current and past users than in never users (all P less than .001).
Using a multivariable model adjusted for age, sex, race/ethnicity, height, education level, income, marital status, asthma, tobacco use in pack-years, smoking category, and body mass index, both the 0-4 day users and the 5-30 day users showed significantly decreased eNO levels, compared with never users (-7%, 95% confidence interval -12% to -2%, P = .03; -13%, CI -18% to -8%, P less than .001, respectively). Additionally, recent users in the 0-4 day group had significantly higher FEV1 measures than never users (89 mL, CI 29-150, P = 0.005), as well as lower FEV1/FVC ratios (-.02, CI –.03 to –.01, P = .003).
Regarding the broader implications of their study findings, Dr. Papatheodorou and colleagues stated, “Given that nitric oxide plays a role in inflammatory and immune defense pathways in the respiratory system and is a mediator of vasodilation in the pulmonary and systemic vasculature, it would be useful to further explore the associations between marijuana use and vascular and pulmonary function in randomized trials.”
Dr. Mary B. Rice received funding from the Institute for Environmental Sciences. The other authors reported no conflicts of interest.
Recent marijuana use may have an effect similar to recent tobacco use with regard to decreased production of exhaled nitric oxide (eNO), but a very different effect on forced vital capacity (FVC), according to the results of a study published in Chest.
Dr. Stefania I. Papatheodorou from the Cyprus International Institute for Environmental and Public Health, Limassol, Cyprus, in association with the Harvard T. H. Chan School of Public Health, and her colleagues conducted a retrospective analysis of National Health and Nutrition Examination Survey (NHANES) data collected from 10,327 people aged 18-59 years between 2007 to 2012. Respondents completed a questionnaire on illicit drug use and were given a physical examination. Outcomes of interest for this study were eNO levels and pulmonary function measurements including the forced expiratory volume in 1 second (FEV1), FVC, the FEV1/FVC ratio, and the average forced expiratory flow during the mid (25%-75%) portion of the FVC (FEF25%-75%) (Chest. 2016 Jan 16. doi: 10.1016/j.chest.2015.12.033).
The population available for analysis included 4,797 people who reported never having used marijuana, and 4,084 who reported past use. The recent users were divided into two groups; 555 and 891 respondents who reported using marijuana 5-30 days and 0-4 days before their examinations, respectively. The study results from age-adjusted analyses standardized to the 2000 U.S. Census population indicated that both past and current users had significantly lower eNO levels (in parts per billion) than those that had never used (all P less than .001). The same analysis demonstrated that FEV1, FVC, and FEV1/FVC ratios were all higher in current and past users than in never users (all P less than .001).
Using a multivariable model adjusted for age, sex, race/ethnicity, height, education level, income, marital status, asthma, tobacco use in pack-years, smoking category, and body mass index, both the 0-4 day users and the 5-30 day users showed significantly decreased eNO levels, compared with never users (-7%, 95% confidence interval -12% to -2%, P = .03; -13%, CI -18% to -8%, P less than .001, respectively). Additionally, recent users in the 0-4 day group had significantly higher FEV1 measures than never users (89 mL, CI 29-150, P = 0.005), as well as lower FEV1/FVC ratios (-.02, CI –.03 to –.01, P = .003).
Regarding the broader implications of their study findings, Dr. Papatheodorou and colleagues stated, “Given that nitric oxide plays a role in inflammatory and immune defense pathways in the respiratory system and is a mediator of vasodilation in the pulmonary and systemic vasculature, it would be useful to further explore the associations between marijuana use and vascular and pulmonary function in randomized trials.”
Dr. Mary B. Rice received funding from the Institute for Environmental Sciences. The other authors reported no conflicts of interest.
FROM CHEST
Key clinical point: Recent marijuana use can have acute negative effects on exhaled nitric oxide levels and aspects of pulmonary functioning.
Major finding: Current marijuana users showed significantly decreased eNO levels, compared with never users (-7% and -13% for those using within 0-4 days and within 5 to 30 days of examination, respectively).
Data sources: National Health and Nutrition Examination Survey (NHANES) data from 2007 to 2012
Disclosures: Dr. Mary B. Rice received funding from the Institute for Environmental Sciences. The other authors reported no conflicts of interest.
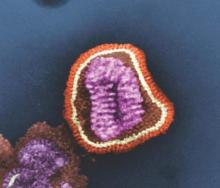
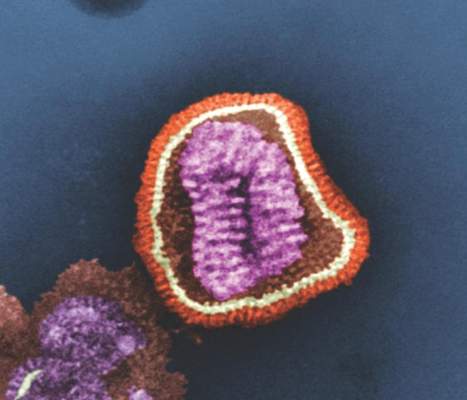
2015-2016 flu season slower and milder than past 3 years
The 2015-2016 flu season was less severe than the last three seasons, with a lower hospitalization rate and fewer pediatric deaths.
Cases of influenza appeared later in the season that typically seen, and activity didn’t peak until March, Stacy L. Davlin, Ph.D., wrote in the June 10 issue of the Morbidity and Mortality report (MMWR 2016; 22:567-75)
“During the most recent 18 influenza seasons, only two other seasons have peaked in March (2011-2012 and 2005-2006),” wrote Dr. Davlin, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta. This serves as a reminder that influenza can and does occur outside the traditionally expected season, and that clinicians shouldn’t discount the possibility of flu when a patient presents with typical symptoms.
“Although summer influenza activity in the United States typically is low, influenza cases and outbreaks have occurred during summer months, and clinicians should remain vigilant in considering influenza in the differential diagnosis of summer respiratory illnesses,” Dr. Davlin said.
The most common influenza virus of the last season was A(H1N1), which accounted for about half of cases in those aged 5-24 years, and about 70% of cases in those younger than 5 years and those 65 years and older.
Three novel viruses were seen as well: variants of A(H1N1), A(H1N2), and A(H3N2). The A(H1N1) variant occurred in a Minnesota resident who lived and worked in an area of swine farming, but who denied direct contact with pigs. The A(H3N2) variant occurred in a New Jersey resident who reported visiting a farm shortly before symptom onset. There was no evidence of human-to-human transmission. Both recovered fully without hospitalization. The A(H1N2) variant occurred in a Minnesota resident who was hospitalized but who recovered. This person was not interviewed so no possible source of infection was identified.
The CDC tested 2,408 viral specimens for susceptibility to antiviral medications. Among the 2,193 A(H1N1) specimens, less than 1% were resistant to oseltamivir and peramivir. All were susceptible to zanamivir. However, the testing found persistent high levels of resistant to amantadine and rimantadine in the A viruses. Amantadine is not effective on the B strains at all. Therefore, CDC does not recommend the use of amantadine as an anti-influenza medication.
Reports of influenza first exceeded the 2.1% baseline level in the week ending Dec. 26, 2015, according to the U.S. Outpatient Influenza-Like Illness Surveillance Network (ILINet). They remained elevated for the next 17 weeks, with a peak of 3.6% of all outpatient visits in the week ending March 12. From October 2015-April 2016, the overall hospitalization rate for influenza-like illness was 31 per 100,000. This was highest in those aged 65 years and older (85/100,000), and lowest in those aged 5-17 years (10/100,000). About 92% of adults hospitalized for flu-like illness had at least one underlying medical comorbidity, including obesity (42%), cardiovascular disease (40%), and metabolic disorders (38%). Almost half of children (48%) also had medical comorbidities, including asthma or other reactive airway disease (22%) and neurologic disorders (18%).
CDC’s National Center for Health Statistics Mortality Surveillance System found that the percentage of deaths attributed to pneumonia and influenza peaked at 8% during the week ending March 19. This is slightly lower than the death rate seen in the last 5 years, which ranged from 9% in 2011-2012 to 11% in 2012-2013.
Of this season’s deaths, 74 occurred in children. The mean and median ages of these patients were 7 years and 6 years, respectively; the range was 2 months-16 years. This total was lower than that recorded in any of the past three influenza seasons: 171 pediatric deaths in 2012-2013, 111 in 2013-2014, and 148 in 2014-2015.
Dr. Davlin also announced the Food and Drug Administration’s recommendations for composition of the 2016-2017 influenza vaccine.
Trivalent vaccines should contain an A/California/7/2009 (H1N1) pdm09-like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008-like virus (B/Victoria lineage). Quadrivalent vaccines, which have two influenza B viruses, should include the viruses recommended for the trivalent vaccines, as well as a B/Phuket/3073/2013-like virus (B/Yamagata lineage).
“The vaccine viruses recommended for inclusion in the 2016-2017 Northern Hemisphere influenza vaccines are the same vaccine viruses that were chosen for inclusion in 2016 Southern Hemisphere seasonal influenza vaccines,” Dr. Davlin noted. “These vaccine recommendations were based on a number of factors, including global influenza virologic and epidemiologic surveillance, genetic and antigenic characterization, antiviral susceptibility, and the availability of candidate vaccine viruses for production.”
As a CDC employee, Dr. Davlin had no financial disclosures.
On Twitter @Alz_Gal
The 2015-2016 flu season was less severe than the last three seasons, with a lower hospitalization rate and fewer pediatric deaths.
Cases of influenza appeared later in the season that typically seen, and activity didn’t peak until March, Stacy L. Davlin, Ph.D., wrote in the June 10 issue of the Morbidity and Mortality report (MMWR 2016; 22:567-75)
“During the most recent 18 influenza seasons, only two other seasons have peaked in March (2011-2012 and 2005-2006),” wrote Dr. Davlin, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta. This serves as a reminder that influenza can and does occur outside the traditionally expected season, and that clinicians shouldn’t discount the possibility of flu when a patient presents with typical symptoms.
“Although summer influenza activity in the United States typically is low, influenza cases and outbreaks have occurred during summer months, and clinicians should remain vigilant in considering influenza in the differential diagnosis of summer respiratory illnesses,” Dr. Davlin said.
The most common influenza virus of the last season was A(H1N1), which accounted for about half of cases in those aged 5-24 years, and about 70% of cases in those younger than 5 years and those 65 years and older.
Three novel viruses were seen as well: variants of A(H1N1), A(H1N2), and A(H3N2). The A(H1N1) variant occurred in a Minnesota resident who lived and worked in an area of swine farming, but who denied direct contact with pigs. The A(H3N2) variant occurred in a New Jersey resident who reported visiting a farm shortly before symptom onset. There was no evidence of human-to-human transmission. Both recovered fully without hospitalization. The A(H1N2) variant occurred in a Minnesota resident who was hospitalized but who recovered. This person was not interviewed so no possible source of infection was identified.
The CDC tested 2,408 viral specimens for susceptibility to antiviral medications. Among the 2,193 A(H1N1) specimens, less than 1% were resistant to oseltamivir and peramivir. All were susceptible to zanamivir. However, the testing found persistent high levels of resistant to amantadine and rimantadine in the A viruses. Amantadine is not effective on the B strains at all. Therefore, CDC does not recommend the use of amantadine as an anti-influenza medication.
Reports of influenza first exceeded the 2.1% baseline level in the week ending Dec. 26, 2015, according to the U.S. Outpatient Influenza-Like Illness Surveillance Network (ILINet). They remained elevated for the next 17 weeks, with a peak of 3.6% of all outpatient visits in the week ending March 12. From October 2015-April 2016, the overall hospitalization rate for influenza-like illness was 31 per 100,000. This was highest in those aged 65 years and older (85/100,000), and lowest in those aged 5-17 years (10/100,000). About 92% of adults hospitalized for flu-like illness had at least one underlying medical comorbidity, including obesity (42%), cardiovascular disease (40%), and metabolic disorders (38%). Almost half of children (48%) also had medical comorbidities, including asthma or other reactive airway disease (22%) and neurologic disorders (18%).
CDC’s National Center for Health Statistics Mortality Surveillance System found that the percentage of deaths attributed to pneumonia and influenza peaked at 8% during the week ending March 19. This is slightly lower than the death rate seen in the last 5 years, which ranged from 9% in 2011-2012 to 11% in 2012-2013.
Of this season’s deaths, 74 occurred in children. The mean and median ages of these patients were 7 years and 6 years, respectively; the range was 2 months-16 years. This total was lower than that recorded in any of the past three influenza seasons: 171 pediatric deaths in 2012-2013, 111 in 2013-2014, and 148 in 2014-2015.
Dr. Davlin also announced the Food and Drug Administration’s recommendations for composition of the 2016-2017 influenza vaccine.
Trivalent vaccines should contain an A/California/7/2009 (H1N1) pdm09-like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008-like virus (B/Victoria lineage). Quadrivalent vaccines, which have two influenza B viruses, should include the viruses recommended for the trivalent vaccines, as well as a B/Phuket/3073/2013-like virus (B/Yamagata lineage).
“The vaccine viruses recommended for inclusion in the 2016-2017 Northern Hemisphere influenza vaccines are the same vaccine viruses that were chosen for inclusion in 2016 Southern Hemisphere seasonal influenza vaccines,” Dr. Davlin noted. “These vaccine recommendations were based on a number of factors, including global influenza virologic and epidemiologic surveillance, genetic and antigenic characterization, antiviral susceptibility, and the availability of candidate vaccine viruses for production.”
As a CDC employee, Dr. Davlin had no financial disclosures.
On Twitter @Alz_Gal
The 2015-2016 flu season was less severe than the last three seasons, with a lower hospitalization rate and fewer pediatric deaths.
Cases of influenza appeared later in the season that typically seen, and activity didn’t peak until March, Stacy L. Davlin, Ph.D., wrote in the June 10 issue of the Morbidity and Mortality report (MMWR 2016; 22:567-75)
“During the most recent 18 influenza seasons, only two other seasons have peaked in March (2011-2012 and 2005-2006),” wrote Dr. Davlin, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta. This serves as a reminder that influenza can and does occur outside the traditionally expected season, and that clinicians shouldn’t discount the possibility of flu when a patient presents with typical symptoms.
“Although summer influenza activity in the United States typically is low, influenza cases and outbreaks have occurred during summer months, and clinicians should remain vigilant in considering influenza in the differential diagnosis of summer respiratory illnesses,” Dr. Davlin said.
The most common influenza virus of the last season was A(H1N1), which accounted for about half of cases in those aged 5-24 years, and about 70% of cases in those younger than 5 years and those 65 years and older.
Three novel viruses were seen as well: variants of A(H1N1), A(H1N2), and A(H3N2). The A(H1N1) variant occurred in a Minnesota resident who lived and worked in an area of swine farming, but who denied direct contact with pigs. The A(H3N2) variant occurred in a New Jersey resident who reported visiting a farm shortly before symptom onset. There was no evidence of human-to-human transmission. Both recovered fully without hospitalization. The A(H1N2) variant occurred in a Minnesota resident who was hospitalized but who recovered. This person was not interviewed so no possible source of infection was identified.
The CDC tested 2,408 viral specimens for susceptibility to antiviral medications. Among the 2,193 A(H1N1) specimens, less than 1% were resistant to oseltamivir and peramivir. All were susceptible to zanamivir. However, the testing found persistent high levels of resistant to amantadine and rimantadine in the A viruses. Amantadine is not effective on the B strains at all. Therefore, CDC does not recommend the use of amantadine as an anti-influenza medication.
Reports of influenza first exceeded the 2.1% baseline level in the week ending Dec. 26, 2015, according to the U.S. Outpatient Influenza-Like Illness Surveillance Network (ILINet). They remained elevated for the next 17 weeks, with a peak of 3.6% of all outpatient visits in the week ending March 12. From October 2015-April 2016, the overall hospitalization rate for influenza-like illness was 31 per 100,000. This was highest in those aged 65 years and older (85/100,000), and lowest in those aged 5-17 years (10/100,000). About 92% of adults hospitalized for flu-like illness had at least one underlying medical comorbidity, including obesity (42%), cardiovascular disease (40%), and metabolic disorders (38%). Almost half of children (48%) also had medical comorbidities, including asthma or other reactive airway disease (22%) and neurologic disorders (18%).
CDC’s National Center for Health Statistics Mortality Surveillance System found that the percentage of deaths attributed to pneumonia and influenza peaked at 8% during the week ending March 19. This is slightly lower than the death rate seen in the last 5 years, which ranged from 9% in 2011-2012 to 11% in 2012-2013.
Of this season’s deaths, 74 occurred in children. The mean and median ages of these patients were 7 years and 6 years, respectively; the range was 2 months-16 years. This total was lower than that recorded in any of the past three influenza seasons: 171 pediatric deaths in 2012-2013, 111 in 2013-2014, and 148 in 2014-2015.
Dr. Davlin also announced the Food and Drug Administration’s recommendations for composition of the 2016-2017 influenza vaccine.
Trivalent vaccines should contain an A/California/7/2009 (H1N1) pdm09-like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008-like virus (B/Victoria lineage). Quadrivalent vaccines, which have two influenza B viruses, should include the viruses recommended for the trivalent vaccines, as well as a B/Phuket/3073/2013-like virus (B/Yamagata lineage).
“The vaccine viruses recommended for inclusion in the 2016-2017 Northern Hemisphere influenza vaccines are the same vaccine viruses that were chosen for inclusion in 2016 Southern Hemisphere seasonal influenza vaccines,” Dr. Davlin noted. “These vaccine recommendations were based on a number of factors, including global influenza virologic and epidemiologic surveillance, genetic and antigenic characterization, antiviral susceptibility, and the availability of candidate vaccine viruses for production.”
As a CDC employee, Dr. Davlin had no financial disclosures.
On Twitter @Alz_Gal
FROM THE MMWR
Key clinical point: The last flu season peaked later, and killed fewer people than the last three seasons.
Major finding: The overall death rate was 31/100,000, with a peak of 8% occurring in March.
Data source: Numbers were drawn from CDC databases and other national influenza surveillance programs.
Disclosures: As a CDC employee, Dr. Davlin had no financial disclosures.
Clinical Trials Update
Are you a clinical trials investigator with unused capacity? Would you like to refer patients to participate in groundbreaking clinical trials?
The CHEST Clinical Trials Registry is a free service that connects physicians to information about clinical trials in respiratory disease conducted by participating pharmaceutical companies.
Ongoing groundbreaking research could have a measurable impact on patient care, but a lack of clinical trial participants is significantly slowing research and threatening the development of new treatments. Recruiting and retaining trial participants are the greatest challenges to developing the next generation of treatment options.
Participation in clinical trials provides an opportunity to advance and accelerate medical research and contribute to an improved health outlook for future generations. Use our registry to get immediate information on how you can be involved in a clinical trial.
Access to learn more: chestnet.org/Guidelines-and-Resources/Clinical-Trials/Clinical-Trials-Registry.
Are you a clinical trials investigator with unused capacity? Would you like to refer patients to participate in groundbreaking clinical trials?
The CHEST Clinical Trials Registry is a free service that connects physicians to information about clinical trials in respiratory disease conducted by participating pharmaceutical companies.
Ongoing groundbreaking research could have a measurable impact on patient care, but a lack of clinical trial participants is significantly slowing research and threatening the development of new treatments. Recruiting and retaining trial participants are the greatest challenges to developing the next generation of treatment options.
Participation in clinical trials provides an opportunity to advance and accelerate medical research and contribute to an improved health outlook for future generations. Use our registry to get immediate information on how you can be involved in a clinical trial.
Access to learn more: chestnet.org/Guidelines-and-Resources/Clinical-Trials/Clinical-Trials-Registry.
Are you a clinical trials investigator with unused capacity? Would you like to refer patients to participate in groundbreaking clinical trials?
The CHEST Clinical Trials Registry is a free service that connects physicians to information about clinical trials in respiratory disease conducted by participating pharmaceutical companies.
Ongoing groundbreaking research could have a measurable impact on patient care, but a lack of clinical trial participants is significantly slowing research and threatening the development of new treatments. Recruiting and retaining trial participants are the greatest challenges to developing the next generation of treatment options.
Participation in clinical trials provides an opportunity to advance and accelerate medical research and contribute to an improved health outlook for future generations. Use our registry to get immediate information on how you can be involved in a clinical trial.
Access to learn more: chestnet.org/Guidelines-and-Resources/Clinical-Trials/Clinical-Trials-Registry.
In Memoriam
Lawrence H. Cohn, MD, FCCP, a past president of the American College of Chest Physicians (1986-1987), pioneering cardiac surgeon and devoted educator, former chief of the Division of Cardiac Surgery at Brigham and Women’s Hospital, and the Virginia and James Hubbard Chair in Cardiac Surgery at Harvard Medical School, died Jan. 9, 2016. An internationally renowned surgeon, he was a pioneer in minimally invasive procedures to fix heart valves.
He aso performed more than 11,000 cardiac surgeries, including being part of the team for New England’s first heart transplant, which took place at the Brigham hospital. Dr. Cohn published more than 500 peer-reviewed publications, 105 book chapters, and 12 books, including four editions of “Cardiac Surgery in the Adult.” CHEST extends its heartfelt condolences to the entire Cohn family.
Lawrence H. Cohn, MD, FCCP, a past president of the American College of Chest Physicians (1986-1987), pioneering cardiac surgeon and devoted educator, former chief of the Division of Cardiac Surgery at Brigham and Women’s Hospital, and the Virginia and James Hubbard Chair in Cardiac Surgery at Harvard Medical School, died Jan. 9, 2016. An internationally renowned surgeon, he was a pioneer in minimally invasive procedures to fix heart valves.
He aso performed more than 11,000 cardiac surgeries, including being part of the team for New England’s first heart transplant, which took place at the Brigham hospital. Dr. Cohn published more than 500 peer-reviewed publications, 105 book chapters, and 12 books, including four editions of “Cardiac Surgery in the Adult.” CHEST extends its heartfelt condolences to the entire Cohn family.
Lawrence H. Cohn, MD, FCCP, a past president of the American College of Chest Physicians (1986-1987), pioneering cardiac surgeon and devoted educator, former chief of the Division of Cardiac Surgery at Brigham and Women’s Hospital, and the Virginia and James Hubbard Chair in Cardiac Surgery at Harvard Medical School, died Jan. 9, 2016. An internationally renowned surgeon, he was a pioneer in minimally invasive procedures to fix heart valves.
He aso performed more than 11,000 cardiac surgeries, including being part of the team for New England’s first heart transplant, which took place at the Brigham hospital. Dr. Cohn published more than 500 peer-reviewed publications, 105 book chapters, and 12 books, including four editions of “Cardiac Surgery in the Adult.” CHEST extends its heartfelt condolences to the entire Cohn family.
President’s Report Who’s Running the Show?
Dr. Barbara Phillips, MSPH, FCCP
CHEST President 2015-2016
Nancy MacRae
Senior Vice President, Governance and Operations
Jenny Nemkovich, CAE
Chief Strategy Officer, Executive Office
Ever wonder how decisions get made and work gets done at CHEST? It all starts with our strategic plan (www.chestnet.org/About/Overview/Strategic-Plan), which was developed by the Board of Regents and other key stakeholders. The development of the strategic plan was informed by our vision to be the global leader in advancing best patient outcomes through innovative chest medicine, education, clinical research, and team-based care, as well as our mission and values. As a result of our strategic planning, CHEST is all about clinical education, which is our “hedgehog,” in organization-speak (1), but we also have goals in guideline development, global impact, membership recruitment and retention, and (of course) fiscal health. We follow progress toward our goals with measurable, relevant key performance indicators (KPIs), and the board reviews progress toward KPIs and goals at nearly every meeting, making recommendations for adjustments, as needed.
But how do decisions get made? CHEST volunteer and staff leadership work together to initiate and execute projects consistent with our plan and respond to requests from others to explore collaborative opportunities to advance our goals.
An example of a process that was initiated by leadership was the development of our new membership model. The Community and Engagement Work Group, along with key staff and other stakeholders, reviewed environmental scans, their personal knowledge and situations, and data from surveys of CHEST members, as well as association trends. They then proposed a new membership model to the Board of Regents (BoR). The BoR reviewed the proposal, along with other important information, and expressed concerns about several key constituent groups, such as global members and members in training, along with several other issues. In fact, the BoR sent the proposal back to the Work Group. Twice. As with any new project, the BoR makes a concerted effort to focus on the strategic plan in these types of deliberations and was guided particularly by the strategy to “optimize new membership model to increase engagement of all clinicians on the health-care team.” The final proposal, implemented in May 2015, truly reflects input and concerns from the BoR, key staff, the Membership Committee, and those CHEST members who responded to the surveys.
An example of a request by another organization to sign on, endorse, cosponsor, or otherwise support a guideline or project is the Campaign for Tobacco-Free Kids contacting us asking us to sign on to a letter to all members of the United States Senate and House of Representatives supporting the tobacco control measure included in the Trans-Pacific Partnership (TPP) trade agreement. The provision will protect the rights of nations participating in the TPP to adopt public health measures that reduce tobacco use without fear of facing lengthy and expensive trade disputes under the TPP initiated by tobacco companies.
Our process in these situations is to gather as much input as possible from CHEST members and experts. Again, using our strategic plan as guidance, in this specific instance, we are expanding our global impact, using targeted strategic alliances, so the decision was made to support this initiative. The CHEST name and brand are valuable assets, and we take endorsement of any project or document very seriously.
Key to our organizational success is our outstanding volunteer/staff partnership that fosters teamwork in translating the strategic vision, mission, and goals of the organization, engaging in a deliberative process that considers organizational history, data, trends, and expert opinion – all to help inform leadership in its decision-making. This collaboration between our content experts and our association professionals is a huge asset to our organization and one that will continue to propel CHEST to achieve our goals.
1. Collins J. Good to Great and the Social Sectors: A Monograph to Accompany Good to Great. New York, NY: HarperCollins Publishers; 2005.
Dr. Barbara Phillips, MSPH, FCCP
CHEST President 2015-2016
Nancy MacRae
Senior Vice President, Governance and Operations
Jenny Nemkovich, CAE
Chief Strategy Officer, Executive Office
Ever wonder how decisions get made and work gets done at CHEST? It all starts with our strategic plan (www.chestnet.org/About/Overview/Strategic-Plan), which was developed by the Board of Regents and other key stakeholders. The development of the strategic plan was informed by our vision to be the global leader in advancing best patient outcomes through innovative chest medicine, education, clinical research, and team-based care, as well as our mission and values. As a result of our strategic planning, CHEST is all about clinical education, which is our “hedgehog,” in organization-speak (1), but we also have goals in guideline development, global impact, membership recruitment and retention, and (of course) fiscal health. We follow progress toward our goals with measurable, relevant key performance indicators (KPIs), and the board reviews progress toward KPIs and goals at nearly every meeting, making recommendations for adjustments, as needed.
But how do decisions get made? CHEST volunteer and staff leadership work together to initiate and execute projects consistent with our plan and respond to requests from others to explore collaborative opportunities to advance our goals.
An example of a process that was initiated by leadership was the development of our new membership model. The Community and Engagement Work Group, along with key staff and other stakeholders, reviewed environmental scans, their personal knowledge and situations, and data from surveys of CHEST members, as well as association trends. They then proposed a new membership model to the Board of Regents (BoR). The BoR reviewed the proposal, along with other important information, and expressed concerns about several key constituent groups, such as global members and members in training, along with several other issues. In fact, the BoR sent the proposal back to the Work Group. Twice. As with any new project, the BoR makes a concerted effort to focus on the strategic plan in these types of deliberations and was guided particularly by the strategy to “optimize new membership model to increase engagement of all clinicians on the health-care team.” The final proposal, implemented in May 2015, truly reflects input and concerns from the BoR, key staff, the Membership Committee, and those CHEST members who responded to the surveys.
An example of a request by another organization to sign on, endorse, cosponsor, or otherwise support a guideline or project is the Campaign for Tobacco-Free Kids contacting us asking us to sign on to a letter to all members of the United States Senate and House of Representatives supporting the tobacco control measure included in the Trans-Pacific Partnership (TPP) trade agreement. The provision will protect the rights of nations participating in the TPP to adopt public health measures that reduce tobacco use without fear of facing lengthy and expensive trade disputes under the TPP initiated by tobacco companies.
Our process in these situations is to gather as much input as possible from CHEST members and experts. Again, using our strategic plan as guidance, in this specific instance, we are expanding our global impact, using targeted strategic alliances, so the decision was made to support this initiative. The CHEST name and brand are valuable assets, and we take endorsement of any project or document very seriously.
Key to our organizational success is our outstanding volunteer/staff partnership that fosters teamwork in translating the strategic vision, mission, and goals of the organization, engaging in a deliberative process that considers organizational history, data, trends, and expert opinion – all to help inform leadership in its decision-making. This collaboration between our content experts and our association professionals is a huge asset to our organization and one that will continue to propel CHEST to achieve our goals.
1. Collins J. Good to Great and the Social Sectors: A Monograph to Accompany Good to Great. New York, NY: HarperCollins Publishers; 2005.
Dr. Barbara Phillips, MSPH, FCCP
CHEST President 2015-2016
Nancy MacRae
Senior Vice President, Governance and Operations
Jenny Nemkovich, CAE
Chief Strategy Officer, Executive Office
Ever wonder how decisions get made and work gets done at CHEST? It all starts with our strategic plan (www.chestnet.org/About/Overview/Strategic-Plan), which was developed by the Board of Regents and other key stakeholders. The development of the strategic plan was informed by our vision to be the global leader in advancing best patient outcomes through innovative chest medicine, education, clinical research, and team-based care, as well as our mission and values. As a result of our strategic planning, CHEST is all about clinical education, which is our “hedgehog,” in organization-speak (1), but we also have goals in guideline development, global impact, membership recruitment and retention, and (of course) fiscal health. We follow progress toward our goals with measurable, relevant key performance indicators (KPIs), and the board reviews progress toward KPIs and goals at nearly every meeting, making recommendations for adjustments, as needed.
But how do decisions get made? CHEST volunteer and staff leadership work together to initiate and execute projects consistent with our plan and respond to requests from others to explore collaborative opportunities to advance our goals.
An example of a process that was initiated by leadership was the development of our new membership model. The Community and Engagement Work Group, along with key staff and other stakeholders, reviewed environmental scans, their personal knowledge and situations, and data from surveys of CHEST members, as well as association trends. They then proposed a new membership model to the Board of Regents (BoR). The BoR reviewed the proposal, along with other important information, and expressed concerns about several key constituent groups, such as global members and members in training, along with several other issues. In fact, the BoR sent the proposal back to the Work Group. Twice. As with any new project, the BoR makes a concerted effort to focus on the strategic plan in these types of deliberations and was guided particularly by the strategy to “optimize new membership model to increase engagement of all clinicians on the health-care team.” The final proposal, implemented in May 2015, truly reflects input and concerns from the BoR, key staff, the Membership Committee, and those CHEST members who responded to the surveys.
An example of a request by another organization to sign on, endorse, cosponsor, or otherwise support a guideline or project is the Campaign for Tobacco-Free Kids contacting us asking us to sign on to a letter to all members of the United States Senate and House of Representatives supporting the tobacco control measure included in the Trans-Pacific Partnership (TPP) trade agreement. The provision will protect the rights of nations participating in the TPP to adopt public health measures that reduce tobacco use without fear of facing lengthy and expensive trade disputes under the TPP initiated by tobacco companies.
Our process in these situations is to gather as much input as possible from CHEST members and experts. Again, using our strategic plan as guidance, in this specific instance, we are expanding our global impact, using targeted strategic alliances, so the decision was made to support this initiative. The CHEST name and brand are valuable assets, and we take endorsement of any project or document very seriously.
Key to our organizational success is our outstanding volunteer/staff partnership that fosters teamwork in translating the strategic vision, mission, and goals of the organization, engaging in a deliberative process that considers organizational history, data, trends, and expert opinion – all to help inform leadership in its decision-making. This collaboration between our content experts and our association professionals is a huge asset to our organization and one that will continue to propel CHEST to achieve our goals.
1. Collins J. Good to Great and the Social Sectors: A Monograph to Accompany Good to Great. New York, NY: HarperCollins Publishers; 2005.
CHEST announces a historic collaboration
The American College of Chest Physicians (CHEST) and the Chinese Association of Chest Physicians (CACP), the respiratory arm of the Chinese Medical Doctor Association (CMDA), have signed an exclusive agreement to collaborate on expanding China’s first-ever fellowship training program, providing clinical education in pulmonary and critical care medicine (PCCM) for Chinese physicians.
This historic announcement came during the opening session of CHEST World Congress 2016 in Shanghai, where approximately 2,000 health care professionals gathered for a 3-day event aimed at connecting clinicians from the United States, China, and around the world for hands-on clinical education in pulmonary, critical care, and sleep medicine. Among those in attendance were fellows and faculty from the 12 institutions that participated in the inaugural offering of the China-CHEST PCCM program, which was developed and implemented over the past 4 years and is expected to grow to dozens of institutions over the next several years.
Since 2012, CHEST has worked with the Chinese Thoracic Society on the development of China’s first fellowship program, offering standardized training in PCCM for Chinese physicians. As a result of these collective efforts, PCCM has now officially earned recognition as a medical subspecialty by the Chinese Ministry of Health – the first subspecialty of its kind in a country where medical training typically ends after a physician completes residency training. Only a decade ago, physicians in China went directly into practice following medical school. The development of a PCCM subspecialty in China – made possible through the engagement of CHEST’s expert faculty and administration – parallels what has occurred over the past 3 decades in the United States, during which the fields of pulmonary and critical care medicine evolved into the combined subspecialty of PCCM.
Through their groundbreaking collaboration announced today, CHEST and the CACP have committed to working exclusively as partners in continuing to advance the PCCM subspecialty in China to improve patient care, expand in-depth clinical training for Chinese physicians, and develop a growing force of expert Chinese faculty. The goal of such training is to ensure that patients receive the best possible care from Chinese physicians who complete the China-CHEST PCCM fellowship program.
“Recognition by the Chinese Ministry of Health of PCCM as this country’s first-ever physician subspecialty is welcomed acknowledgment that we’re making tremendous headway in advancing physician fellowship training in China,” said Barbara Phillips, MD, MPH, FCCP, President of the American College of Chest Physicians. “We are proud to join collaborative partners like the CMDA in these cooperative efforts to prepare Chinese physicians in the PCCM subspecialty, partnering in this historic effort to drive immeasurable improvements in clinical training and the delivery of patient care.”
The American College of Chest Physicians (CHEST) and the Chinese Association of Chest Physicians (CACP), the respiratory arm of the Chinese Medical Doctor Association (CMDA), have signed an exclusive agreement to collaborate on expanding China’s first-ever fellowship training program, providing clinical education in pulmonary and critical care medicine (PCCM) for Chinese physicians.
This historic announcement came during the opening session of CHEST World Congress 2016 in Shanghai, where approximately 2,000 health care professionals gathered for a 3-day event aimed at connecting clinicians from the United States, China, and around the world for hands-on clinical education in pulmonary, critical care, and sleep medicine. Among those in attendance were fellows and faculty from the 12 institutions that participated in the inaugural offering of the China-CHEST PCCM program, which was developed and implemented over the past 4 years and is expected to grow to dozens of institutions over the next several years.
Since 2012, CHEST has worked with the Chinese Thoracic Society on the development of China’s first fellowship program, offering standardized training in PCCM for Chinese physicians. As a result of these collective efforts, PCCM has now officially earned recognition as a medical subspecialty by the Chinese Ministry of Health – the first subspecialty of its kind in a country where medical training typically ends after a physician completes residency training. Only a decade ago, physicians in China went directly into practice following medical school. The development of a PCCM subspecialty in China – made possible through the engagement of CHEST’s expert faculty and administration – parallels what has occurred over the past 3 decades in the United States, during which the fields of pulmonary and critical care medicine evolved into the combined subspecialty of PCCM.
Through their groundbreaking collaboration announced today, CHEST and the CACP have committed to working exclusively as partners in continuing to advance the PCCM subspecialty in China to improve patient care, expand in-depth clinical training for Chinese physicians, and develop a growing force of expert Chinese faculty. The goal of such training is to ensure that patients receive the best possible care from Chinese physicians who complete the China-CHEST PCCM fellowship program.
“Recognition by the Chinese Ministry of Health of PCCM as this country’s first-ever physician subspecialty is welcomed acknowledgment that we’re making tremendous headway in advancing physician fellowship training in China,” said Barbara Phillips, MD, MPH, FCCP, President of the American College of Chest Physicians. “We are proud to join collaborative partners like the CMDA in these cooperative efforts to prepare Chinese physicians in the PCCM subspecialty, partnering in this historic effort to drive immeasurable improvements in clinical training and the delivery of patient care.”
The American College of Chest Physicians (CHEST) and the Chinese Association of Chest Physicians (CACP), the respiratory arm of the Chinese Medical Doctor Association (CMDA), have signed an exclusive agreement to collaborate on expanding China’s first-ever fellowship training program, providing clinical education in pulmonary and critical care medicine (PCCM) for Chinese physicians.
This historic announcement came during the opening session of CHEST World Congress 2016 in Shanghai, where approximately 2,000 health care professionals gathered for a 3-day event aimed at connecting clinicians from the United States, China, and around the world for hands-on clinical education in pulmonary, critical care, and sleep medicine. Among those in attendance were fellows and faculty from the 12 institutions that participated in the inaugural offering of the China-CHEST PCCM program, which was developed and implemented over the past 4 years and is expected to grow to dozens of institutions over the next several years.
Since 2012, CHEST has worked with the Chinese Thoracic Society on the development of China’s first fellowship program, offering standardized training in PCCM for Chinese physicians. As a result of these collective efforts, PCCM has now officially earned recognition as a medical subspecialty by the Chinese Ministry of Health – the first subspecialty of its kind in a country where medical training typically ends after a physician completes residency training. Only a decade ago, physicians in China went directly into practice following medical school. The development of a PCCM subspecialty in China – made possible through the engagement of CHEST’s expert faculty and administration – parallels what has occurred over the past 3 decades in the United States, during which the fields of pulmonary and critical care medicine evolved into the combined subspecialty of PCCM.
Through their groundbreaking collaboration announced today, CHEST and the CACP have committed to working exclusively as partners in continuing to advance the PCCM subspecialty in China to improve patient care, expand in-depth clinical training for Chinese physicians, and develop a growing force of expert Chinese faculty. The goal of such training is to ensure that patients receive the best possible care from Chinese physicians who complete the China-CHEST PCCM fellowship program.
“Recognition by the Chinese Ministry of Health of PCCM as this country’s first-ever physician subspecialty is welcomed acknowledgment that we’re making tremendous headway in advancing physician fellowship training in China,” said Barbara Phillips, MD, MPH, FCCP, President of the American College of Chest Physicians. “We are proud to join collaborative partners like the CMDA in these cooperative efforts to prepare Chinese physicians in the PCCM subspecialty, partnering in this historic effort to drive immeasurable improvements in clinical training and the delivery of patient care.”
Recharge in Los Angeles
When you imagine Los Angeles, you probably envision images of glamorous Hollywood celebrities and ritzy beaches featured on television shows. While LA does, indeed, allure visitors with its high-end environment, there are also many opportunities to unwind and recharge in the Golden State. With mild temperatures and sunshine nearly 300 days a year, Los Angeles provides a haven for outdoor activities. While you’re attending CHEST 2016 from October 22 to 26, we encourage you to trade your stilettos for hiking boots or athletic shoes, and get outside during your free time.
The San Gabriel Mountains are about an hour’s drive from the Los Angeles Convention Center. Visitors can hike, picnic, and enjoy equestrian trails. Our favorite LA experts – CHEST members – have recommended renting a mountain bike and riding up to Mount Wilson. You can also hike the Eaton Canyon Trail and find the Eaton Canyon Waterfall, a 40-foot waterfall with a pool at its base. You can find more mountain ranges and hiking opportunities in the Santa Monica mountains, as well. Find more tips on trails and hiking at discoverlosangeles.com.
If you’re not interested in retreating from Los Angeles’s urban oasis, but you still want to enjoy some fresh air and exercise, you have a couple options closer to downtown. Runyon Canyon is about a 20-minute drive from the convention center. It features a gently paved path for beginners, a rugged outer path for a more intense workout, free yoga near the Fuller Avenue entrance, a great setting for watching the sunset, and strong possibilities of celebrity sightings. You can also rent a bike and cycle along the beaches. You can stop off for a bite to eat, some sand volleyball, or just to do some people watching.
And if you’re a golf enthusiast, there is a bevy of options for golfing in and around LA. The most iconic golf course is the Trump National Golf Club located on the Palos Verdes Peninsula, about a 40-minute drive from the convention center. You’ll experience uncompromising luxury overlooking the beautiful Pacific Ocean. If you’d like to stay close to the convention center but still get out to play 9 holes, you may want to check out Wilshire Country Club (20-minute drive) or Monterey Park Golf Club (12-minute drive).
While Los Angeles refreshes you with outdoor beauty and sunshine, CHEST 2016 will energize and recharge you with the latest information in chest medicine. You’ll connect with an international community of the best minds in pulmonary, critical care, and sleep medicine. Find everything you need to know to make the best clinical decisions and inspire your patient care. Learn more at chestmeeting.chestnet.org.
When you imagine Los Angeles, you probably envision images of glamorous Hollywood celebrities and ritzy beaches featured on television shows. While LA does, indeed, allure visitors with its high-end environment, there are also many opportunities to unwind and recharge in the Golden State. With mild temperatures and sunshine nearly 300 days a year, Los Angeles provides a haven for outdoor activities. While you’re attending CHEST 2016 from October 22 to 26, we encourage you to trade your stilettos for hiking boots or athletic shoes, and get outside during your free time.
The San Gabriel Mountains are about an hour’s drive from the Los Angeles Convention Center. Visitors can hike, picnic, and enjoy equestrian trails. Our favorite LA experts – CHEST members – have recommended renting a mountain bike and riding up to Mount Wilson. You can also hike the Eaton Canyon Trail and find the Eaton Canyon Waterfall, a 40-foot waterfall with a pool at its base. You can find more mountain ranges and hiking opportunities in the Santa Monica mountains, as well. Find more tips on trails and hiking at discoverlosangeles.com.
If you’re not interested in retreating from Los Angeles’s urban oasis, but you still want to enjoy some fresh air and exercise, you have a couple options closer to downtown. Runyon Canyon is about a 20-minute drive from the convention center. It features a gently paved path for beginners, a rugged outer path for a more intense workout, free yoga near the Fuller Avenue entrance, a great setting for watching the sunset, and strong possibilities of celebrity sightings. You can also rent a bike and cycle along the beaches. You can stop off for a bite to eat, some sand volleyball, or just to do some people watching.
And if you’re a golf enthusiast, there is a bevy of options for golfing in and around LA. The most iconic golf course is the Trump National Golf Club located on the Palos Verdes Peninsula, about a 40-minute drive from the convention center. You’ll experience uncompromising luxury overlooking the beautiful Pacific Ocean. If you’d like to stay close to the convention center but still get out to play 9 holes, you may want to check out Wilshire Country Club (20-minute drive) or Monterey Park Golf Club (12-minute drive).
While Los Angeles refreshes you with outdoor beauty and sunshine, CHEST 2016 will energize and recharge you with the latest information in chest medicine. You’ll connect with an international community of the best minds in pulmonary, critical care, and sleep medicine. Find everything you need to know to make the best clinical decisions and inspire your patient care. Learn more at chestmeeting.chestnet.org.
When you imagine Los Angeles, you probably envision images of glamorous Hollywood celebrities and ritzy beaches featured on television shows. While LA does, indeed, allure visitors with its high-end environment, there are also many opportunities to unwind and recharge in the Golden State. With mild temperatures and sunshine nearly 300 days a year, Los Angeles provides a haven for outdoor activities. While you’re attending CHEST 2016 from October 22 to 26, we encourage you to trade your stilettos for hiking boots or athletic shoes, and get outside during your free time.
The San Gabriel Mountains are about an hour’s drive from the Los Angeles Convention Center. Visitors can hike, picnic, and enjoy equestrian trails. Our favorite LA experts – CHEST members – have recommended renting a mountain bike and riding up to Mount Wilson. You can also hike the Eaton Canyon Trail and find the Eaton Canyon Waterfall, a 40-foot waterfall with a pool at its base. You can find more mountain ranges and hiking opportunities in the Santa Monica mountains, as well. Find more tips on trails and hiking at discoverlosangeles.com.
If you’re not interested in retreating from Los Angeles’s urban oasis, but you still want to enjoy some fresh air and exercise, you have a couple options closer to downtown. Runyon Canyon is about a 20-minute drive from the convention center. It features a gently paved path for beginners, a rugged outer path for a more intense workout, free yoga near the Fuller Avenue entrance, a great setting for watching the sunset, and strong possibilities of celebrity sightings. You can also rent a bike and cycle along the beaches. You can stop off for a bite to eat, some sand volleyball, or just to do some people watching.
And if you’re a golf enthusiast, there is a bevy of options for golfing in and around LA. The most iconic golf course is the Trump National Golf Club located on the Palos Verdes Peninsula, about a 40-minute drive from the convention center. You’ll experience uncompromising luxury overlooking the beautiful Pacific Ocean. If you’d like to stay close to the convention center but still get out to play 9 holes, you may want to check out Wilshire Country Club (20-minute drive) or Monterey Park Golf Club (12-minute drive).
While Los Angeles refreshes you with outdoor beauty and sunshine, CHEST 2016 will energize and recharge you with the latest information in chest medicine. You’ll connect with an international community of the best minds in pulmonary, critical care, and sleep medicine. Find everything you need to know to make the best clinical decisions and inspire your patient care. Learn more at chestmeeting.chestnet.org.
SLEEP STRATEGIES Treating childhood OSA
In most children, obstructive sleep apnea (OSA) is a curable disease that responds to surgical treatment with adenotonsillectomy. Do all children need surgery for their sleep apnea? Recent research suggests perhaps not. Some children with mild obstructive sleep apnea may “grow out of it” without having their tonsils and adenoids removed.
New research may identify children who can avoid surgery
The Childhood Adenotonsillectomy Trial (CHAT), first published in 2013, suggested to clinicians that a higher than realized number of children may grow out of their OSA (Marcus et al. N Engl J Med. 2013;368[25]:2366). This was the first study to examine the risks and benefits of early adenotonsillectomy vs. watchful waiting in children 5 to 9 years of age. When sleep studies were performed at baseline and at 7 months of follow-up, it was found that 46% of the children in the watchful waiting group had resolution of their OSA. In addition, it was noted that resolution of OSA after adenotonsillectomy was not absolute, either, as 21% in the early adenotonsillectomy group had residual OSA at 7 months postsurgery. This leads to the questions of which children should undergo surgery and how we are to identify them.
A more recent study by Chervin and colleagues published in CHEST identifies factors associated with resolution of sleep apnea in the children in the watchful waiting arm of the CHAT trial (Chest. 2015;148[5]:1204). The factors identified included baseline polysomnography characteristics of mild sleep apnea, such as lower baseline apnea/hypopnea index (AHI) and less oxygen desaturation. Figure 1 demonstrates the percentage of children in the watchful waiting group that had resolution of their OSA by their baseline AHI. The CHAT trial did not include many children with severe OSA, as median AHI was less than 5, and children with sustained desaturations were excluded. Chervin and colleagues noted that those children with a lower AHI and waist circumference profile were more likely to have resolution. Other factors that may also be associated with resolution without surgery suggested by these data include a lower waist circumference percentile and lower baseline questionnaire scores on the pediatric sleep questionnaire (PSQ). The PSQ has also been identified as a tool to help assess for likelihood of OSA. Some clinical factors may also be associated with resolution of OSA, such as the absence of loud or habitual snoring or observed apneas.
The identification of these factors, along with future research in pediatric OSA, may begin to help practitioners to identify children who can avoid surgery and help identify those who may have residual disease after surgery. While AHI improved in a large portion of children in this study, symptoms did not show as drastic an association. Only 15% had a symptomatic improvement in questionnaire scores of at least 25% (Chervin et al. previous mention). Researchers are also examining biomarkers associated with OSA. A recent study showed that increased C-reactive protein (CRP) levels were associated with residual OSA after adenotonsillectomy (Bhattacharjee et al. Sleep. 2016;39[2]:283).
Why is this important?
OSA in children is common and affects an estimated 1% to 5% of children (Marcus et al. Pediatrics. 2012;130[3]:576). In the majority of children, this is due to hypertrophy of their tonsils and adenoids. Other factors, such as obesity, craniofacial features, and genetic syndromes, can play a role. Many studies examining the effects of OSA in children have demonstrated a negative impact on attention, cognitive function, and behavior. There is often significant impact on other members of a household when a child has a sleep disorder. Longitudinal effects on how OSA in childhood impacts adulthood are still unknown.
The impact of surgery
Approximately half a million adenotonsillectomies are performed per year in the United States, with the indication primarily due to OSA increasing from being primarily for infection during the past several decades (Bhattacharyya et al. Otolaryngol Head Neck Surg. 2010;143[5]:680). Minor complications, such as throat pain and dehydration, are common. Primary (within 24 hours) and secondary (typically between 5 and 10 days postoperatively) bleeding can occur in 2% to 3 % of children. Respiratory compromise is a complication that is at increased risk when the indication for surgery is OSA and can occur in almost 10% of children (De Luca Canto et al. Pediatrics. 2015;136[4]:702). Cost of surgery and missed days of school (child) and work (parent) can all be factors. The decision for surgery rests on the parents, and they may have varying preferences as to how aggressively they want their child to be treated with surgery. While surgery is often curative, it is not 100%.
How is obstructive sleep apnea diagnosed in children?
Obstructive sleep apnea is diagnosed by overnight sleep study or polysomnography. Criteria for diagnosis of OSA in children can vary in the literature, but criteria used clinically are often similar to what was used in the CHAT trial: an obstructive AHI greater than 2 per hour or an obstructive apnea index (OAI) of greater than one per hour. Alternatives to an overnight sleep study have not yet been found for children. In adults, overnight oximetry, if normal, can be valuable in ruling out obstructive sleep apnea. In children, almost 50% of those screened with a normal oximetry went on to have sleep apnea during an overnight sleep study, making this an ineffective screening tool to rule out sleep apnea for most children (Brouillette et al. Pediatrics. 2000;105[2]:405). Home sleep testing has yet to be shown to be a reliable option in children but may be in the future (Tan et al. Chest;2015:148[6]:1382). Clinical history taking can be more challenging as well, as children do not typically have bed partners, and OSA is often clustered in the early morning hours in association with rapid eye movement sleep (REM), making parental observation less likely. These factors make overnight sleep studies the gold standard for diagnosis.
However, overnight sleep studies are costly, time consuming, and can be technically challenging in children depending on age and development. In certain areas, access to pediatric sleep centers can be limited. Overnight sleep studies can also be an additional economic burden to parents who are required to spend the night with their child, perhaps missing work and needing child care for other children in the family. All of this adds to the importance of identifying children who truly need to undergo a sleep study.
Can we change guidelines? Not yet.
We are not at a point where we can yet rewrite guidelines. The American Academy of Pediatrics (AAP) and the American Academy of Sleep Medicine (AASM) recommend a sleep study prior to proceeding to adenotonsillectomy in children. A repeat sleep study is recommended in children with moderate to severe OSA after adenotonsillectomy and when symptoms remain. Are there any other tools that can be used? New research may give suggestion as to which children can avoid repeating the costly and time-consuming test. In areas where sleep studies have limited availability, avoiding unnecessary testing is important.
Bottom line: More questions remain
The diagnosis and treatment of childhood OSA continue to challenge practitioners. While adenotonsillectomy is still the common treatment for OSA, it is reasonable to consider watchful waiting in some circumstances, particularly in children with mild disease. More questions still remain, however, in regards to the follow-up of these children. Practitioners still struggle with the definition of mild OSA in children (Is this an AHI less than 3, or is this an AHI less than 5? How do symptoms impact diagnosis and treatment?). Studies such as the CHAT trial are important first steps in helping to sort out the behavior of OSA in children long term. What happens to children farther out than 7 months? Additional research should help clarify these issues.
Dr. Baughn is a Consultant, Pediatric Pulmonology and Sleep Medicine, Department of Pediatrics and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota.
In most children, obstructive sleep apnea (OSA) is a curable disease that responds to surgical treatment with adenotonsillectomy. Do all children need surgery for their sleep apnea? Recent research suggests perhaps not. Some children with mild obstructive sleep apnea may “grow out of it” without having their tonsils and adenoids removed.
New research may identify children who can avoid surgery
The Childhood Adenotonsillectomy Trial (CHAT), first published in 2013, suggested to clinicians that a higher than realized number of children may grow out of their OSA (Marcus et al. N Engl J Med. 2013;368[25]:2366). This was the first study to examine the risks and benefits of early adenotonsillectomy vs. watchful waiting in children 5 to 9 years of age. When sleep studies were performed at baseline and at 7 months of follow-up, it was found that 46% of the children in the watchful waiting group had resolution of their OSA. In addition, it was noted that resolution of OSA after adenotonsillectomy was not absolute, either, as 21% in the early adenotonsillectomy group had residual OSA at 7 months postsurgery. This leads to the questions of which children should undergo surgery and how we are to identify them.
A more recent study by Chervin and colleagues published in CHEST identifies factors associated with resolution of sleep apnea in the children in the watchful waiting arm of the CHAT trial (Chest. 2015;148[5]:1204). The factors identified included baseline polysomnography characteristics of mild sleep apnea, such as lower baseline apnea/hypopnea index (AHI) and less oxygen desaturation. Figure 1 demonstrates the percentage of children in the watchful waiting group that had resolution of their OSA by their baseline AHI. The CHAT trial did not include many children with severe OSA, as median AHI was less than 5, and children with sustained desaturations were excluded. Chervin and colleagues noted that those children with a lower AHI and waist circumference profile were more likely to have resolution. Other factors that may also be associated with resolution without surgery suggested by these data include a lower waist circumference percentile and lower baseline questionnaire scores on the pediatric sleep questionnaire (PSQ). The PSQ has also been identified as a tool to help assess for likelihood of OSA. Some clinical factors may also be associated with resolution of OSA, such as the absence of loud or habitual snoring or observed apneas.
The identification of these factors, along with future research in pediatric OSA, may begin to help practitioners to identify children who can avoid surgery and help identify those who may have residual disease after surgery. While AHI improved in a large portion of children in this study, symptoms did not show as drastic an association. Only 15% had a symptomatic improvement in questionnaire scores of at least 25% (Chervin et al. previous mention). Researchers are also examining biomarkers associated with OSA. A recent study showed that increased C-reactive protein (CRP) levels were associated with residual OSA after adenotonsillectomy (Bhattacharjee et al. Sleep. 2016;39[2]:283).
Why is this important?
OSA in children is common and affects an estimated 1% to 5% of children (Marcus et al. Pediatrics. 2012;130[3]:576). In the majority of children, this is due to hypertrophy of their tonsils and adenoids. Other factors, such as obesity, craniofacial features, and genetic syndromes, can play a role. Many studies examining the effects of OSA in children have demonstrated a negative impact on attention, cognitive function, and behavior. There is often significant impact on other members of a household when a child has a sleep disorder. Longitudinal effects on how OSA in childhood impacts adulthood are still unknown.
The impact of surgery
Approximately half a million adenotonsillectomies are performed per year in the United States, with the indication primarily due to OSA increasing from being primarily for infection during the past several decades (Bhattacharyya et al. Otolaryngol Head Neck Surg. 2010;143[5]:680). Minor complications, such as throat pain and dehydration, are common. Primary (within 24 hours) and secondary (typically between 5 and 10 days postoperatively) bleeding can occur in 2% to 3 % of children. Respiratory compromise is a complication that is at increased risk when the indication for surgery is OSA and can occur in almost 10% of children (De Luca Canto et al. Pediatrics. 2015;136[4]:702). Cost of surgery and missed days of school (child) and work (parent) can all be factors. The decision for surgery rests on the parents, and they may have varying preferences as to how aggressively they want their child to be treated with surgery. While surgery is often curative, it is not 100%.
How is obstructive sleep apnea diagnosed in children?
Obstructive sleep apnea is diagnosed by overnight sleep study or polysomnography. Criteria for diagnosis of OSA in children can vary in the literature, but criteria used clinically are often similar to what was used in the CHAT trial: an obstructive AHI greater than 2 per hour or an obstructive apnea index (OAI) of greater than one per hour. Alternatives to an overnight sleep study have not yet been found for children. In adults, overnight oximetry, if normal, can be valuable in ruling out obstructive sleep apnea. In children, almost 50% of those screened with a normal oximetry went on to have sleep apnea during an overnight sleep study, making this an ineffective screening tool to rule out sleep apnea for most children (Brouillette et al. Pediatrics. 2000;105[2]:405). Home sleep testing has yet to be shown to be a reliable option in children but may be in the future (Tan et al. Chest;2015:148[6]:1382). Clinical history taking can be more challenging as well, as children do not typically have bed partners, and OSA is often clustered in the early morning hours in association with rapid eye movement sleep (REM), making parental observation less likely. These factors make overnight sleep studies the gold standard for diagnosis.
However, overnight sleep studies are costly, time consuming, and can be technically challenging in children depending on age and development. In certain areas, access to pediatric sleep centers can be limited. Overnight sleep studies can also be an additional economic burden to parents who are required to spend the night with their child, perhaps missing work and needing child care for other children in the family. All of this adds to the importance of identifying children who truly need to undergo a sleep study.
Can we change guidelines? Not yet.
We are not at a point where we can yet rewrite guidelines. The American Academy of Pediatrics (AAP) and the American Academy of Sleep Medicine (AASM) recommend a sleep study prior to proceeding to adenotonsillectomy in children. A repeat sleep study is recommended in children with moderate to severe OSA after adenotonsillectomy and when symptoms remain. Are there any other tools that can be used? New research may give suggestion as to which children can avoid repeating the costly and time-consuming test. In areas where sleep studies have limited availability, avoiding unnecessary testing is important.
Bottom line: More questions remain
The diagnosis and treatment of childhood OSA continue to challenge practitioners. While adenotonsillectomy is still the common treatment for OSA, it is reasonable to consider watchful waiting in some circumstances, particularly in children with mild disease. More questions still remain, however, in regards to the follow-up of these children. Practitioners still struggle with the definition of mild OSA in children (Is this an AHI less than 3, or is this an AHI less than 5? How do symptoms impact diagnosis and treatment?). Studies such as the CHAT trial are important first steps in helping to sort out the behavior of OSA in children long term. What happens to children farther out than 7 months? Additional research should help clarify these issues.
Dr. Baughn is a Consultant, Pediatric Pulmonology and Sleep Medicine, Department of Pediatrics and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota.
In most children, obstructive sleep apnea (OSA) is a curable disease that responds to surgical treatment with adenotonsillectomy. Do all children need surgery for their sleep apnea? Recent research suggests perhaps not. Some children with mild obstructive sleep apnea may “grow out of it” without having their tonsils and adenoids removed.
New research may identify children who can avoid surgery
The Childhood Adenotonsillectomy Trial (CHAT), first published in 2013, suggested to clinicians that a higher than realized number of children may grow out of their OSA (Marcus et al. N Engl J Med. 2013;368[25]:2366). This was the first study to examine the risks and benefits of early adenotonsillectomy vs. watchful waiting in children 5 to 9 years of age. When sleep studies were performed at baseline and at 7 months of follow-up, it was found that 46% of the children in the watchful waiting group had resolution of their OSA. In addition, it was noted that resolution of OSA after adenotonsillectomy was not absolute, either, as 21% in the early adenotonsillectomy group had residual OSA at 7 months postsurgery. This leads to the questions of which children should undergo surgery and how we are to identify them.
A more recent study by Chervin and colleagues published in CHEST identifies factors associated with resolution of sleep apnea in the children in the watchful waiting arm of the CHAT trial (Chest. 2015;148[5]:1204). The factors identified included baseline polysomnography characteristics of mild sleep apnea, such as lower baseline apnea/hypopnea index (AHI) and less oxygen desaturation. Figure 1 demonstrates the percentage of children in the watchful waiting group that had resolution of their OSA by their baseline AHI. The CHAT trial did not include many children with severe OSA, as median AHI was less than 5, and children with sustained desaturations were excluded. Chervin and colleagues noted that those children with a lower AHI and waist circumference profile were more likely to have resolution. Other factors that may also be associated with resolution without surgery suggested by these data include a lower waist circumference percentile and lower baseline questionnaire scores on the pediatric sleep questionnaire (PSQ). The PSQ has also been identified as a tool to help assess for likelihood of OSA. Some clinical factors may also be associated with resolution of OSA, such as the absence of loud or habitual snoring or observed apneas.
The identification of these factors, along with future research in pediatric OSA, may begin to help practitioners to identify children who can avoid surgery and help identify those who may have residual disease after surgery. While AHI improved in a large portion of children in this study, symptoms did not show as drastic an association. Only 15% had a symptomatic improvement in questionnaire scores of at least 25% (Chervin et al. previous mention). Researchers are also examining biomarkers associated with OSA. A recent study showed that increased C-reactive protein (CRP) levels were associated with residual OSA after adenotonsillectomy (Bhattacharjee et al. Sleep. 2016;39[2]:283).
Why is this important?
OSA in children is common and affects an estimated 1% to 5% of children (Marcus et al. Pediatrics. 2012;130[3]:576). In the majority of children, this is due to hypertrophy of their tonsils and adenoids. Other factors, such as obesity, craniofacial features, and genetic syndromes, can play a role. Many studies examining the effects of OSA in children have demonstrated a negative impact on attention, cognitive function, and behavior. There is often significant impact on other members of a household when a child has a sleep disorder. Longitudinal effects on how OSA in childhood impacts adulthood are still unknown.
The impact of surgery
Approximately half a million adenotonsillectomies are performed per year in the United States, with the indication primarily due to OSA increasing from being primarily for infection during the past several decades (Bhattacharyya et al. Otolaryngol Head Neck Surg. 2010;143[5]:680). Minor complications, such as throat pain and dehydration, are common. Primary (within 24 hours) and secondary (typically between 5 and 10 days postoperatively) bleeding can occur in 2% to 3 % of children. Respiratory compromise is a complication that is at increased risk when the indication for surgery is OSA and can occur in almost 10% of children (De Luca Canto et al. Pediatrics. 2015;136[4]:702). Cost of surgery and missed days of school (child) and work (parent) can all be factors. The decision for surgery rests on the parents, and they may have varying preferences as to how aggressively they want their child to be treated with surgery. While surgery is often curative, it is not 100%.
How is obstructive sleep apnea diagnosed in children?
Obstructive sleep apnea is diagnosed by overnight sleep study or polysomnography. Criteria for diagnosis of OSA in children can vary in the literature, but criteria used clinically are often similar to what was used in the CHAT trial: an obstructive AHI greater than 2 per hour or an obstructive apnea index (OAI) of greater than one per hour. Alternatives to an overnight sleep study have not yet been found for children. In adults, overnight oximetry, if normal, can be valuable in ruling out obstructive sleep apnea. In children, almost 50% of those screened with a normal oximetry went on to have sleep apnea during an overnight sleep study, making this an ineffective screening tool to rule out sleep apnea for most children (Brouillette et al. Pediatrics. 2000;105[2]:405). Home sleep testing has yet to be shown to be a reliable option in children but may be in the future (Tan et al. Chest;2015:148[6]:1382). Clinical history taking can be more challenging as well, as children do not typically have bed partners, and OSA is often clustered in the early morning hours in association with rapid eye movement sleep (REM), making parental observation less likely. These factors make overnight sleep studies the gold standard for diagnosis.
However, overnight sleep studies are costly, time consuming, and can be technically challenging in children depending on age and development. In certain areas, access to pediatric sleep centers can be limited. Overnight sleep studies can also be an additional economic burden to parents who are required to spend the night with their child, perhaps missing work and needing child care for other children in the family. All of this adds to the importance of identifying children who truly need to undergo a sleep study.
Can we change guidelines? Not yet.
We are not at a point where we can yet rewrite guidelines. The American Academy of Pediatrics (AAP) and the American Academy of Sleep Medicine (AASM) recommend a sleep study prior to proceeding to adenotonsillectomy in children. A repeat sleep study is recommended in children with moderate to severe OSA after adenotonsillectomy and when symptoms remain. Are there any other tools that can be used? New research may give suggestion as to which children can avoid repeating the costly and time-consuming test. In areas where sleep studies have limited availability, avoiding unnecessary testing is important.
Bottom line: More questions remain
The diagnosis and treatment of childhood OSA continue to challenge practitioners. While adenotonsillectomy is still the common treatment for OSA, it is reasonable to consider watchful waiting in some circumstances, particularly in children with mild disease. More questions still remain, however, in regards to the follow-up of these children. Practitioners still struggle with the definition of mild OSA in children (Is this an AHI less than 3, or is this an AHI less than 5? How do symptoms impact diagnosis and treatment?). Studies such as the CHAT trial are important first steps in helping to sort out the behavior of OSA in children long term. What happens to children farther out than 7 months? Additional research should help clarify these issues.
Dr. Baughn is a Consultant, Pediatric Pulmonology and Sleep Medicine, Department of Pediatrics and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota.