User login
Finding and Following Your Passion
Dear Friends,
Over the last year, I have been reading more about professional identity and professional branding, all of which have evolved in the setting of social media. However, the root of it remains constant — finding the intersection(s) of what you love. A common problem, especially as a trainee and early-career gastroenterologist, is that you may have many interests: various disease processes, innovation, medical education, leadership development, and much more. Since becoming faculty, I continue to define and refine my professional niche, trying to distinguish my “interests” from “passions.” It is a journey that my mentors advise me not to rush through and I am enjoying every moment of it!
In this issue’s “In Focus,” Dr. Hamza Salim, Dr. Anni Chowdhury, and Dr. Lavanya Viswanathan provide a practical guide for the clinical evaluation of chronic constipation and a systematic approach to treatment.
In the first of a two-part series in the “Short Clinical Review” section, Dr. Christopher Velez and Dr. Kara J. Jencks discuss the health inequities among sexual and gender minority (SGM) patients, particularly with disorders of brain-gut interaction (DBGI). They review common SGM terminology, sample verbiage for trauma-informed care, and case presentations to help guide our approach to providing care for SGM patients with DGBI.
The transition from trainee to early faculty may be difficult for those who are interested in research but struggle with the change from being a part of a research team to running one. In the “Early Career” section, Dr. Lauren Feld and colleagues describes her experience establishing a research lab as an early-career academic, from creating a niche to time management and mentorship.
The Federal Trade Commission’s noncompete ban made big news in April 2024 but there is still a lot of gray area for physicians. Dr. Timothy Craig Allen explains the ruling, what it means to physicians, the status of it today, and what the future may hold. Lastly, for “Private Practice Perspectives” in collaboration with Digestive Health Physicians Alliance, I interview Dr. Vasu Appalaneni on her use of artificial intelligence in private practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Polyethylene glycol was first used in the 1940s and 1950s to understand the physiology of the intestines, and first published as a compound for colonoscopy bowel preparation in 1981.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
Over the last year, I have been reading more about professional identity and professional branding, all of which have evolved in the setting of social media. However, the root of it remains constant — finding the intersection(s) of what you love. A common problem, especially as a trainee and early-career gastroenterologist, is that you may have many interests: various disease processes, innovation, medical education, leadership development, and much more. Since becoming faculty, I continue to define and refine my professional niche, trying to distinguish my “interests” from “passions.” It is a journey that my mentors advise me not to rush through and I am enjoying every moment of it!
In this issue’s “In Focus,” Dr. Hamza Salim, Dr. Anni Chowdhury, and Dr. Lavanya Viswanathan provide a practical guide for the clinical evaluation of chronic constipation and a systematic approach to treatment.
In the first of a two-part series in the “Short Clinical Review” section, Dr. Christopher Velez and Dr. Kara J. Jencks discuss the health inequities among sexual and gender minority (SGM) patients, particularly with disorders of brain-gut interaction (DBGI). They review common SGM terminology, sample verbiage for trauma-informed care, and case presentations to help guide our approach to providing care for SGM patients with DGBI.
The transition from trainee to early faculty may be difficult for those who are interested in research but struggle with the change from being a part of a research team to running one. In the “Early Career” section, Dr. Lauren Feld and colleagues describes her experience establishing a research lab as an early-career academic, from creating a niche to time management and mentorship.
The Federal Trade Commission’s noncompete ban made big news in April 2024 but there is still a lot of gray area for physicians. Dr. Timothy Craig Allen explains the ruling, what it means to physicians, the status of it today, and what the future may hold. Lastly, for “Private Practice Perspectives” in collaboration with Digestive Health Physicians Alliance, I interview Dr. Vasu Appalaneni on her use of artificial intelligence in private practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Polyethylene glycol was first used in the 1940s and 1950s to understand the physiology of the intestines, and first published as a compound for colonoscopy bowel preparation in 1981.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
Over the last year, I have been reading more about professional identity and professional branding, all of which have evolved in the setting of social media. However, the root of it remains constant — finding the intersection(s) of what you love. A common problem, especially as a trainee and early-career gastroenterologist, is that you may have many interests: various disease processes, innovation, medical education, leadership development, and much more. Since becoming faculty, I continue to define and refine my professional niche, trying to distinguish my “interests” from “passions.” It is a journey that my mentors advise me not to rush through and I am enjoying every moment of it!
In this issue’s “In Focus,” Dr. Hamza Salim, Dr. Anni Chowdhury, and Dr. Lavanya Viswanathan provide a practical guide for the clinical evaluation of chronic constipation and a systematic approach to treatment.
In the first of a two-part series in the “Short Clinical Review” section, Dr. Christopher Velez and Dr. Kara J. Jencks discuss the health inequities among sexual and gender minority (SGM) patients, particularly with disorders of brain-gut interaction (DBGI). They review common SGM terminology, sample verbiage for trauma-informed care, and case presentations to help guide our approach to providing care for SGM patients with DGBI.
The transition from trainee to early faculty may be difficult for those who are interested in research but struggle with the change from being a part of a research team to running one. In the “Early Career” section, Dr. Lauren Feld and colleagues describes her experience establishing a research lab as an early-career academic, from creating a niche to time management and mentorship.
The Federal Trade Commission’s noncompete ban made big news in April 2024 but there is still a lot of gray area for physicians. Dr. Timothy Craig Allen explains the ruling, what it means to physicians, the status of it today, and what the future may hold. Lastly, for “Private Practice Perspectives” in collaboration with Digestive Health Physicians Alliance, I interview Dr. Vasu Appalaneni on her use of artificial intelligence in private practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Polyethylene glycol was first used in the 1940s and 1950s to understand the physiology of the intestines, and first published as a compound for colonoscopy bowel preparation in 1981.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Journal Highlights: October-December 2024
Esophagus
Reed CC et al. Daily or Twice Daily Treatment with Topical Steroids Results in Similar Responses in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Nov. doi: 10.1016/j.cgh.2024.10.016.
Patel RV et al. Functional Lumen Imaging Probe Provides an Accurate Assessment of Esophageal Diameter in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.032.
Stomach
Shah SC et al. AGA Clinical Practice Update on Screening and Surveillance in Individuals at Increased Risk for Gastric Cancer in the United States: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.001.
IBD
Griffiths BJ et al. Hypercoagulation after Hospital Discharge in Acute Severe Ulcerative Colitis: A Prospective Study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.031.
Liver
Lassailly G et al. Resolution of MASH with no worsening of fibrosis after bariatric surgery improves 15-year survival: a prospective cohort study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.025.
Norman JS et al. Model for Urgency for Liver Transplantation in Hepatocellular Carcinoma: A Practical Model to Prioritize Patients With Hepatocellular Carcinoma on the Liver Transplant Waiting List. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.11.015.
Davis JPE et al. AGA Clinical Practice Update on Management of Portal Vein Thrombosis in Patients With Cirrhosis: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.10.038.
Pancreas
Drewes AM et al. Pain in Chronic Pancreatitis: Navigating the Maze of Blocked Tubes and Tangled Wires. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.026.
Endoscopy
Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
Schmidt KA et al. Understanding Patients’ Current Acceptability of Artificial Intelligence During Colonoscopy for Polyp Detection: A Single-Center Study. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250905.
Chandramouli S et al. Endoscopic Surveillance Patterns and Management of Helicobacter pylori in Newly Diagnosed Gastric Intestinal Metaplasia. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250904.
Practice Management
Tsai C et al. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.09.015.
Mintz KM et al. Incorporating a GI Dietitian into Your GI Practice. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.10.022.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus
Reed CC et al. Daily or Twice Daily Treatment with Topical Steroids Results in Similar Responses in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Nov. doi: 10.1016/j.cgh.2024.10.016.
Patel RV et al. Functional Lumen Imaging Probe Provides an Accurate Assessment of Esophageal Diameter in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.032.
Stomach
Shah SC et al. AGA Clinical Practice Update on Screening and Surveillance in Individuals at Increased Risk for Gastric Cancer in the United States: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.001.
IBD
Griffiths BJ et al. Hypercoagulation after Hospital Discharge in Acute Severe Ulcerative Colitis: A Prospective Study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.031.
Liver
Lassailly G et al. Resolution of MASH with no worsening of fibrosis after bariatric surgery improves 15-year survival: a prospective cohort study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.025.
Norman JS et al. Model for Urgency for Liver Transplantation in Hepatocellular Carcinoma: A Practical Model to Prioritize Patients With Hepatocellular Carcinoma on the Liver Transplant Waiting List. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.11.015.
Davis JPE et al. AGA Clinical Practice Update on Management of Portal Vein Thrombosis in Patients With Cirrhosis: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.10.038.
Pancreas
Drewes AM et al. Pain in Chronic Pancreatitis: Navigating the Maze of Blocked Tubes and Tangled Wires. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.026.
Endoscopy
Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
Schmidt KA et al. Understanding Patients’ Current Acceptability of Artificial Intelligence During Colonoscopy for Polyp Detection: A Single-Center Study. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250905.
Chandramouli S et al. Endoscopic Surveillance Patterns and Management of Helicobacter pylori in Newly Diagnosed Gastric Intestinal Metaplasia. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250904.
Practice Management
Tsai C et al. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.09.015.
Mintz KM et al. Incorporating a GI Dietitian into Your GI Practice. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.10.022.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus
Reed CC et al. Daily or Twice Daily Treatment with Topical Steroids Results in Similar Responses in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Nov. doi: 10.1016/j.cgh.2024.10.016.
Patel RV et al. Functional Lumen Imaging Probe Provides an Accurate Assessment of Esophageal Diameter in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.032.
Stomach
Shah SC et al. AGA Clinical Practice Update on Screening and Surveillance in Individuals at Increased Risk for Gastric Cancer in the United States: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.001.
IBD
Griffiths BJ et al. Hypercoagulation after Hospital Discharge in Acute Severe Ulcerative Colitis: A Prospective Study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.031.
Liver
Lassailly G et al. Resolution of MASH with no worsening of fibrosis after bariatric surgery improves 15-year survival: a prospective cohort study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.025.
Norman JS et al. Model for Urgency for Liver Transplantation in Hepatocellular Carcinoma: A Practical Model to Prioritize Patients With Hepatocellular Carcinoma on the Liver Transplant Waiting List. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.11.015.
Davis JPE et al. AGA Clinical Practice Update on Management of Portal Vein Thrombosis in Patients With Cirrhosis: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.10.038.
Pancreas
Drewes AM et al. Pain in Chronic Pancreatitis: Navigating the Maze of Blocked Tubes and Tangled Wires. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.026.
Endoscopy
Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
Schmidt KA et al. Understanding Patients’ Current Acceptability of Artificial Intelligence During Colonoscopy for Polyp Detection: A Single-Center Study. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250905.
Chandramouli S et al. Endoscopic Surveillance Patterns and Management of Helicobacter pylori in Newly Diagnosed Gastric Intestinal Metaplasia. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250904.
Practice Management
Tsai C et al. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.09.015.
Mintz KM et al. Incorporating a GI Dietitian into Your GI Practice. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.10.022.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Improving Care for Sexual and Gender Minority Patients with Disorders of Gut-Brain Interaction
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.
Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
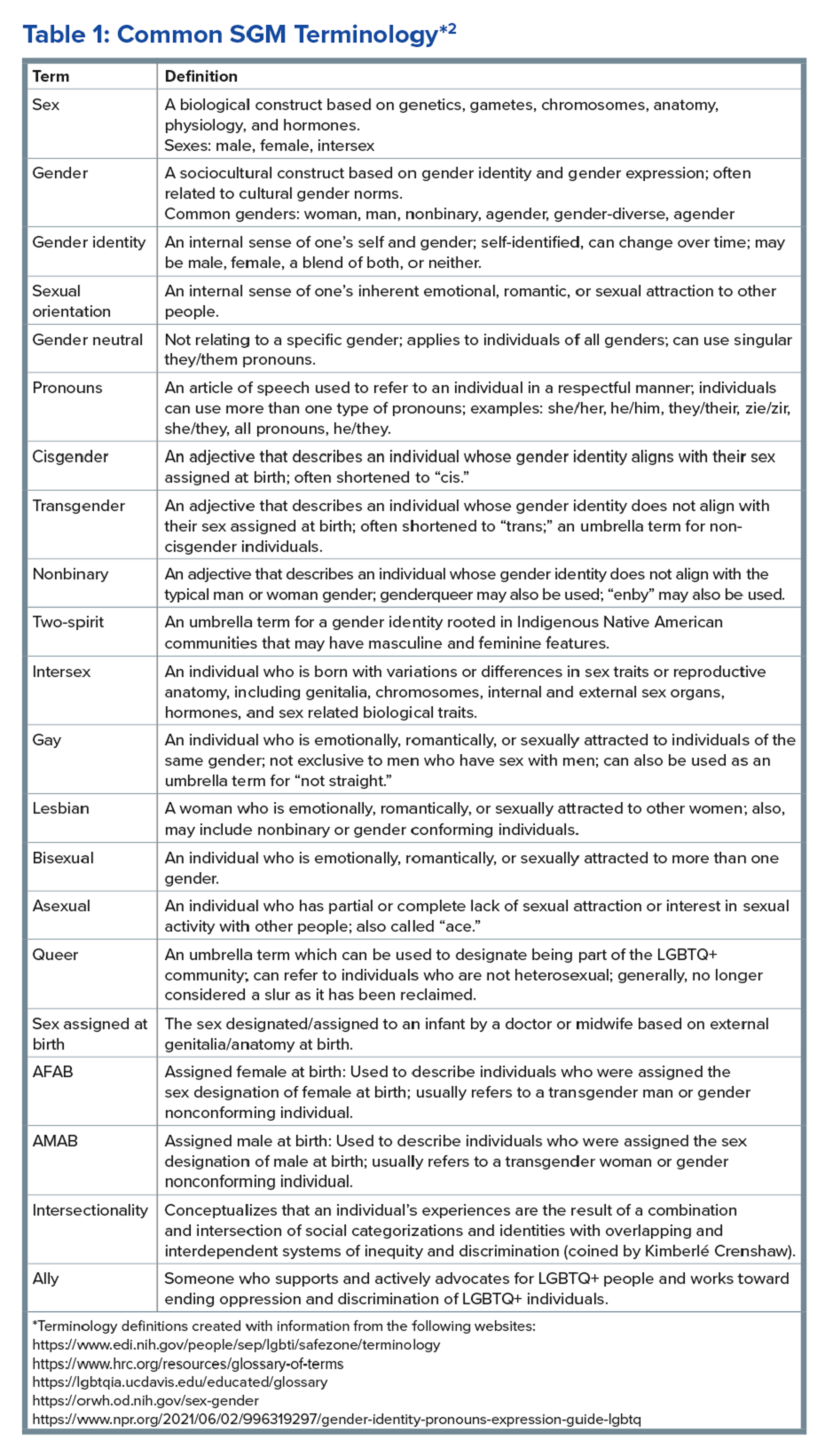
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.
Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Clinical Research in Early Career Academic Medicine
Conducting clinical research as an early career gastroenterologist can take on many forms and has varying definitions of success. This article focuses on key factors to consider and should be supplemented with mentorship tailored to personal interests, goals, and institutional criteria for success. In this article, we will discuss selected high-yield topics that assist in early-career research. We will briefly discuss 1. Defining your niche, 2. Collaboration, 3. Visibility, 4. Time management, 5. Funding, 6. Receiving mentorship, and 7. Providing mentorship. We will conclude with discussing several authors’ experience in the research lab of the first author (FELD Lab – Fostering Equity in Liver and Digestive disease).
Defining Your Niche
Defining your niche is an essential component of an early career, as when academicians must transition from a trainee, who is supporting the research of an established mentor, to defining their own subspeciality area of investigation. Early-career academics should build on their prior work, but should also explore their own passions and skill set to define what will be unique about their research program and contributions to the field. Of course, positioning oneself at the intersection of two or more seemingly unrelated fields opens much opportunity for large impact but comes at a cost of identifying mentorship and justifying the niche to funders.
Collaboration
Fostering a collaborative environment is essential for early-career physician-researchers. One effective approach is to establish collaboration circles with other early career academics. Expanding research endeavors beyond a single institution to a multi-center framework enriches both scope and impact. This collaborative approach not only amplifies the depth of research but also facilitates peer mentorship and sponsorship. Participation in such networks can significantly enhance scholarly output and broaden professional reach during this critical phase of academic progression. Furthermore, prioritizing the promotion of colleagues within these networks is crucial. Proactive sponsorship opportunities, such as inviting peers to present at institutional seminars, strengthen both individual and collective academic visibility.
Collaboration is also essential to foster between trainees involved in early-career investigators’ work. An interconnected lab environment ensures that trainees remain informed about concurrent projects, thereby fostering a culture of shared knowledge and optimized productivity. Encouraging trainees to spearhead research aligned with their interests, under mentor guidance, nurtures independent inquiry and leadership. By establishing explicit roles, responsibilities, and authorship agreements at the outset of collaborative projects, early career mentors can avoid future conflicts and preserve a collaborative culture within the lab. This structured approach cultivates a supportive ecosystem, advancing both individual and collective research achievements.
Visibility
Establishing visibility and developing name recognition are crucial components of career advancement for early-career academic physicians. By clearly defining their areas of expertise, faculty can position themselves as leaders within their discipline. Active participation in professional societies, both at the local and national level, engagement with interest groups, and frequent contributions to educational events can be effective strategies for gaining recognition. Leveraging social media platforms can be helpful in enhancing visibility by facilitating connections and promoting research to a broader audience.
Moreover, research visibility plays a vital role in academic promotion. A strong publication record, reflected by an increasing h-index, demonstrates the impact and relevance of one’s research. Self-citation, when appropriate, can reinforce the continuity and progression of scholarly contributions. While publishing in high-impact journals is desirable, adaptability in resubmitting to other journals following rejections ensures that research remains visible and accessible. It also clearly establishes by whom the work was first done, before someone else investigates the line of inquiry. Through a combination of strategic engagement and publication efforts, early-career physicians can effectively build their professional reputation and advance their academic careers.
Time Management
Time management is essential for any research, and particularly in early career when efficiency in clinical care duties is still being gained. Securing protected time for research is essential to develop a niche, build connections (both institutionally and beyond their institutions), and demonstrate productivity that can be utilized to support future grant efforts.
Similarly, using protected time efficiently is required. Without organization and planning, research time can be spent with scattered meetings and responding to various tasks that do not directly support your research. It is helpful to be introspective about the time of the day you are most productive in your research efforts and blocking off that time to focus on research tasks and minimizing distractions. Blocking monthly time for larger scale thinking and planning is also important. Weekly lab and individual one-on-one meetings also support time management for trainees and lab members, to ensure efficiency and progress. Additionally, robust clinical support is essential to ensure that research time remains protected and patient care moves forward. When negotiating for positions, and in regular meetings thereafter, it is important to advocate for sufficient clinical staffing such that non-physician tasks can be appropriately delegated to another member of the care team.
Funding
Securing adequate funding poses a significant challenge for all early-career physician-scientists, particularly because of the discrepancy between National Institutes of Health salary caps and the higher average salaries in academic gastroenterology. This financial gap can deter physicians from pursuing research-intensive careers altogether and can derail early investigators who do not obtain funding rapidly. To overcome this, early-career investigators may need to adopt flexible strategies, such as accepting a lower salary that aligns with grant funding limits or funneling incentive or bonus pay to research accounts. Alternatively, they can advocate for institutional support to bridge the salary gap, ensuring their research efforts remain financially viable.
Institutions committed to fostering research excellence may offer supplemental funding or bridge programs to retain talented physician-scientists, thereby mitigating the financial strain and encouraging long-term engagement in research. Regular meetings to review salary and support sources, including philanthropy, foundation grants, and other streams, should be undertaken with leadership to align the researcher’s timeline and available funding. If career development funding appears untenable, consideration of multi–principal investigator R01s or equivalent with senior established investigators can be a promising path.
Receiving Mentorship
Effective mentorship for early-career physician-scientists should be approached through a team-based model that leverages both internal and external mentors. Internal mentors, familiar with the specific culture, expectations, and advancement pathways of the institution, can provide invaluable guidance on navigating institutional metrics for success, such as promotion criteria, grant acquisition, and clinical-research balance. External mentors, on the other hand, bring a broader perspective by offering innovative career development strategies and solutions derived from experiences at their home institutions. This multimodal mentorship model ensures a well-rounded approach to professional growth.
All national gastroenterology societies, including the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, offer structured early-career mentorship programs designed to connect emerging researchers with experienced leaders in the field (see below). These programs typically require a formal application process and are highly regarded for their exceptional quality and impact. Participation in such initiatives can significantly enhance career development by expanding networks, fostering interdisciplinary collaboration, and providing tailored guidance that complements institutional support. Integrating both internal and external mentorship opportunities ensures a robust and dynamic foundation for long-term success in academic medicine.
- AGA Career Compass (https://gastro.org/fellows-and-early-career/mentoring/)
- ACG Early Career Leadership Program (https://gi.org/acg-institute/early-career-leadership-program/)
- AGA-AASLD Academic Skills Workshop (https://gastro.org/news/applications-now-being-accepted-for-the-2024-aga-aasld-academic-skills-workshop/)
- AASLD Women’s Initiative Committee Leadership Program (https://www.aasld.org/promoting-leadership-potential-women-hepatology)
- Scrubs n Heels Mentorship Matrix (https://scrubsandheels.com/matrix/)
Providing Mentorship
The trainee authors on this manuscript describe in this section what has been helpful for them as mentees in the FELD research lab.
Student doctor Nguyen describes her experience as a lab member and things she finds most helpful as a medical student in the lab:
- Upon joining the team, a one-to-one meeting to discuss trainee’s personal and professional goals, and availability, was crucial to building the mentor-mentee relationship. Establishing this meaningful mentorship early on clarified expectations on both sides, built trust, and increased motivation. As a trainee, it is essential for me to see how my work aligns with a long-term goal and to receive ample guidance throughout the process.
- One of the most impactful experiences has been joining informal lunch sessions where trainees discussed data collection protocols and exchanged insights. In doing so, Dr. Feld has cultivated a lab culture that encourages curiosity, constructive feedback, and collaborative learning.
- To increase productivity, our team of trainees created a useful group message thread where we coordinated more sessions to collaborate. This coordination formed stronger relationships between team members and fostered a sense of shared purpose.
Dr. Cooper, a third year internal medicine resident, describes her experience as both a research mentee and a mentor to the junior trainees: “As a resident pursuing a career in academic gastroenterology and hepatology, I have found three key elements to be most helpful: intentional mentorship, structured meetings, and leadership development.”
- Intentional mentorship: Prior to joining the lab, I met with Dr. Feld to discuss my research experience and my goals. She took the time to understand these within the context of my training timeline and tailored project opportunities that aligned with my interests and were both feasible and impactful for my next steps. This intentional approach not only fostered a productive research experience but also established a mentor-mentee relationship built on genuine care for my growth and development.
- Regular meetings: Frequent lab meetings promote accountability, teamwork, and shared problem-solving skills. The open exchange of ideas fosters collaboration and joint problem solving to elevate the quality of our research. They are also an opportunity to observe key decision-making points during the research process and have been a great way to learn more about solid methodology.
- Supervised leadership: I have had ample time to lead discussions and coordinate projects among the junior trainees. These monitored leadership experiences promote project management skills, mentorship, and team dynamic awareness while maintaining the safety net of senior guidance. This model helped me transition from a trainee supporting others’ research to a more independent role, contributing to multi-disciplinary projects while mentoring junior members.
Conclusion
In conclusion, many exciting opportunities and notable barriers exist to establishing a clinical research laboratory in the early career. While excellence in each of the areas outlined may evolve, some aspects will come easier than others and with time, persistence, and a bit of luck, the research world will be a better place because of your contributions!
Dr. Feld is assistant professor of gastroenterology and hepatology and physician executive of Diversity, Equity, Inclusion and Belonging for the department of medicine at the University of Massachusetts (UMass) Chan Medical School, Worcester. Ms. Nguyen is a medical student at UMass Chan Medical School. Dr. Cooper is a resident physician at UMass Chan Medical School. Dr. Rabinowitz is an attending physician in the Inflammatory Bowel Disease Center at Beth Israel Deaconess Medical Center, Boston, Mass. Dr. Uchida is codirector of the Multidisciplinary Eosinophilic Gastrointestinal Disease Clinic at the University of Utah School of Medicine, Salt Lake City.
Conducting clinical research as an early career gastroenterologist can take on many forms and has varying definitions of success. This article focuses on key factors to consider and should be supplemented with mentorship tailored to personal interests, goals, and institutional criteria for success. In this article, we will discuss selected high-yield topics that assist in early-career research. We will briefly discuss 1. Defining your niche, 2. Collaboration, 3. Visibility, 4. Time management, 5. Funding, 6. Receiving mentorship, and 7. Providing mentorship. We will conclude with discussing several authors’ experience in the research lab of the first author (FELD Lab – Fostering Equity in Liver and Digestive disease).
Defining Your Niche
Defining your niche is an essential component of an early career, as when academicians must transition from a trainee, who is supporting the research of an established mentor, to defining their own subspeciality area of investigation. Early-career academics should build on their prior work, but should also explore their own passions and skill set to define what will be unique about their research program and contributions to the field. Of course, positioning oneself at the intersection of two or more seemingly unrelated fields opens much opportunity for large impact but comes at a cost of identifying mentorship and justifying the niche to funders.
Collaboration
Fostering a collaborative environment is essential for early-career physician-researchers. One effective approach is to establish collaboration circles with other early career academics. Expanding research endeavors beyond a single institution to a multi-center framework enriches both scope and impact. This collaborative approach not only amplifies the depth of research but also facilitates peer mentorship and sponsorship. Participation in such networks can significantly enhance scholarly output and broaden professional reach during this critical phase of academic progression. Furthermore, prioritizing the promotion of colleagues within these networks is crucial. Proactive sponsorship opportunities, such as inviting peers to present at institutional seminars, strengthen both individual and collective academic visibility.
Collaboration is also essential to foster between trainees involved in early-career investigators’ work. An interconnected lab environment ensures that trainees remain informed about concurrent projects, thereby fostering a culture of shared knowledge and optimized productivity. Encouraging trainees to spearhead research aligned with their interests, under mentor guidance, nurtures independent inquiry and leadership. By establishing explicit roles, responsibilities, and authorship agreements at the outset of collaborative projects, early career mentors can avoid future conflicts and preserve a collaborative culture within the lab. This structured approach cultivates a supportive ecosystem, advancing both individual and collective research achievements.
Visibility
Establishing visibility and developing name recognition are crucial components of career advancement for early-career academic physicians. By clearly defining their areas of expertise, faculty can position themselves as leaders within their discipline. Active participation in professional societies, both at the local and national level, engagement with interest groups, and frequent contributions to educational events can be effective strategies for gaining recognition. Leveraging social media platforms can be helpful in enhancing visibility by facilitating connections and promoting research to a broader audience.
Moreover, research visibility plays a vital role in academic promotion. A strong publication record, reflected by an increasing h-index, demonstrates the impact and relevance of one’s research. Self-citation, when appropriate, can reinforce the continuity and progression of scholarly contributions. While publishing in high-impact journals is desirable, adaptability in resubmitting to other journals following rejections ensures that research remains visible and accessible. It also clearly establishes by whom the work was first done, before someone else investigates the line of inquiry. Through a combination of strategic engagement and publication efforts, early-career physicians can effectively build their professional reputation and advance their academic careers.
Time Management
Time management is essential for any research, and particularly in early career when efficiency in clinical care duties is still being gained. Securing protected time for research is essential to develop a niche, build connections (both institutionally and beyond their institutions), and demonstrate productivity that can be utilized to support future grant efforts.
Similarly, using protected time efficiently is required. Without organization and planning, research time can be spent with scattered meetings and responding to various tasks that do not directly support your research. It is helpful to be introspective about the time of the day you are most productive in your research efforts and blocking off that time to focus on research tasks and minimizing distractions. Blocking monthly time for larger scale thinking and planning is also important. Weekly lab and individual one-on-one meetings also support time management for trainees and lab members, to ensure efficiency and progress. Additionally, robust clinical support is essential to ensure that research time remains protected and patient care moves forward. When negotiating for positions, and in regular meetings thereafter, it is important to advocate for sufficient clinical staffing such that non-physician tasks can be appropriately delegated to another member of the care team.
Funding
Securing adequate funding poses a significant challenge for all early-career physician-scientists, particularly because of the discrepancy between National Institutes of Health salary caps and the higher average salaries in academic gastroenterology. This financial gap can deter physicians from pursuing research-intensive careers altogether and can derail early investigators who do not obtain funding rapidly. To overcome this, early-career investigators may need to adopt flexible strategies, such as accepting a lower salary that aligns with grant funding limits or funneling incentive or bonus pay to research accounts. Alternatively, they can advocate for institutional support to bridge the salary gap, ensuring their research efforts remain financially viable.
Institutions committed to fostering research excellence may offer supplemental funding or bridge programs to retain talented physician-scientists, thereby mitigating the financial strain and encouraging long-term engagement in research. Regular meetings to review salary and support sources, including philanthropy, foundation grants, and other streams, should be undertaken with leadership to align the researcher’s timeline and available funding. If career development funding appears untenable, consideration of multi–principal investigator R01s or equivalent with senior established investigators can be a promising path.
Receiving Mentorship
Effective mentorship for early-career physician-scientists should be approached through a team-based model that leverages both internal and external mentors. Internal mentors, familiar with the specific culture, expectations, and advancement pathways of the institution, can provide invaluable guidance on navigating institutional metrics for success, such as promotion criteria, grant acquisition, and clinical-research balance. External mentors, on the other hand, bring a broader perspective by offering innovative career development strategies and solutions derived from experiences at their home institutions. This multimodal mentorship model ensures a well-rounded approach to professional growth.
All national gastroenterology societies, including the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, offer structured early-career mentorship programs designed to connect emerging researchers with experienced leaders in the field (see below). These programs typically require a formal application process and are highly regarded for their exceptional quality and impact. Participation in such initiatives can significantly enhance career development by expanding networks, fostering interdisciplinary collaboration, and providing tailored guidance that complements institutional support. Integrating both internal and external mentorship opportunities ensures a robust and dynamic foundation for long-term success in academic medicine.
- AGA Career Compass (https://gastro.org/fellows-and-early-career/mentoring/)
- ACG Early Career Leadership Program (https://gi.org/acg-institute/early-career-leadership-program/)
- AGA-AASLD Academic Skills Workshop (https://gastro.org/news/applications-now-being-accepted-for-the-2024-aga-aasld-academic-skills-workshop/)
- AASLD Women’s Initiative Committee Leadership Program (https://www.aasld.org/promoting-leadership-potential-women-hepatology)
- Scrubs n Heels Mentorship Matrix (https://scrubsandheels.com/matrix/)
Providing Mentorship
The trainee authors on this manuscript describe in this section what has been helpful for them as mentees in the FELD research lab.
Student doctor Nguyen describes her experience as a lab member and things she finds most helpful as a medical student in the lab:
- Upon joining the team, a one-to-one meeting to discuss trainee’s personal and professional goals, and availability, was crucial to building the mentor-mentee relationship. Establishing this meaningful mentorship early on clarified expectations on both sides, built trust, and increased motivation. As a trainee, it is essential for me to see how my work aligns with a long-term goal and to receive ample guidance throughout the process.
- One of the most impactful experiences has been joining informal lunch sessions where trainees discussed data collection protocols and exchanged insights. In doing so, Dr. Feld has cultivated a lab culture that encourages curiosity, constructive feedback, and collaborative learning.
- To increase productivity, our team of trainees created a useful group message thread where we coordinated more sessions to collaborate. This coordination formed stronger relationships between team members and fostered a sense of shared purpose.
Dr. Cooper, a third year internal medicine resident, describes her experience as both a research mentee and a mentor to the junior trainees: “As a resident pursuing a career in academic gastroenterology and hepatology, I have found three key elements to be most helpful: intentional mentorship, structured meetings, and leadership development.”
- Intentional mentorship: Prior to joining the lab, I met with Dr. Feld to discuss my research experience and my goals. She took the time to understand these within the context of my training timeline and tailored project opportunities that aligned with my interests and were both feasible and impactful for my next steps. This intentional approach not only fostered a productive research experience but also established a mentor-mentee relationship built on genuine care for my growth and development.
- Regular meetings: Frequent lab meetings promote accountability, teamwork, and shared problem-solving skills. The open exchange of ideas fosters collaboration and joint problem solving to elevate the quality of our research. They are also an opportunity to observe key decision-making points during the research process and have been a great way to learn more about solid methodology.
- Supervised leadership: I have had ample time to lead discussions and coordinate projects among the junior trainees. These monitored leadership experiences promote project management skills, mentorship, and team dynamic awareness while maintaining the safety net of senior guidance. This model helped me transition from a trainee supporting others’ research to a more independent role, contributing to multi-disciplinary projects while mentoring junior members.
Conclusion
In conclusion, many exciting opportunities and notable barriers exist to establishing a clinical research laboratory in the early career. While excellence in each of the areas outlined may evolve, some aspects will come easier than others and with time, persistence, and a bit of luck, the research world will be a better place because of your contributions!
Dr. Feld is assistant professor of gastroenterology and hepatology and physician executive of Diversity, Equity, Inclusion and Belonging for the department of medicine at the University of Massachusetts (UMass) Chan Medical School, Worcester. Ms. Nguyen is a medical student at UMass Chan Medical School. Dr. Cooper is a resident physician at UMass Chan Medical School. Dr. Rabinowitz is an attending physician in the Inflammatory Bowel Disease Center at Beth Israel Deaconess Medical Center, Boston, Mass. Dr. Uchida is codirector of the Multidisciplinary Eosinophilic Gastrointestinal Disease Clinic at the University of Utah School of Medicine, Salt Lake City.
Conducting clinical research as an early career gastroenterologist can take on many forms and has varying definitions of success. This article focuses on key factors to consider and should be supplemented with mentorship tailored to personal interests, goals, and institutional criteria for success. In this article, we will discuss selected high-yield topics that assist in early-career research. We will briefly discuss 1. Defining your niche, 2. Collaboration, 3. Visibility, 4. Time management, 5. Funding, 6. Receiving mentorship, and 7. Providing mentorship. We will conclude with discussing several authors’ experience in the research lab of the first author (FELD Lab – Fostering Equity in Liver and Digestive disease).
Defining Your Niche
Defining your niche is an essential component of an early career, as when academicians must transition from a trainee, who is supporting the research of an established mentor, to defining their own subspeciality area of investigation. Early-career academics should build on their prior work, but should also explore their own passions and skill set to define what will be unique about their research program and contributions to the field. Of course, positioning oneself at the intersection of two or more seemingly unrelated fields opens much opportunity for large impact but comes at a cost of identifying mentorship and justifying the niche to funders.
Collaboration
Fostering a collaborative environment is essential for early-career physician-researchers. One effective approach is to establish collaboration circles with other early career academics. Expanding research endeavors beyond a single institution to a multi-center framework enriches both scope and impact. This collaborative approach not only amplifies the depth of research but also facilitates peer mentorship and sponsorship. Participation in such networks can significantly enhance scholarly output and broaden professional reach during this critical phase of academic progression. Furthermore, prioritizing the promotion of colleagues within these networks is crucial. Proactive sponsorship opportunities, such as inviting peers to present at institutional seminars, strengthen both individual and collective academic visibility.
Collaboration is also essential to foster between trainees involved in early-career investigators’ work. An interconnected lab environment ensures that trainees remain informed about concurrent projects, thereby fostering a culture of shared knowledge and optimized productivity. Encouraging trainees to spearhead research aligned with their interests, under mentor guidance, nurtures independent inquiry and leadership. By establishing explicit roles, responsibilities, and authorship agreements at the outset of collaborative projects, early career mentors can avoid future conflicts and preserve a collaborative culture within the lab. This structured approach cultivates a supportive ecosystem, advancing both individual and collective research achievements.
Visibility
Establishing visibility and developing name recognition are crucial components of career advancement for early-career academic physicians. By clearly defining their areas of expertise, faculty can position themselves as leaders within their discipline. Active participation in professional societies, both at the local and national level, engagement with interest groups, and frequent contributions to educational events can be effective strategies for gaining recognition. Leveraging social media platforms can be helpful in enhancing visibility by facilitating connections and promoting research to a broader audience.
Moreover, research visibility plays a vital role in academic promotion. A strong publication record, reflected by an increasing h-index, demonstrates the impact and relevance of one’s research. Self-citation, when appropriate, can reinforce the continuity and progression of scholarly contributions. While publishing in high-impact journals is desirable, adaptability in resubmitting to other journals following rejections ensures that research remains visible and accessible. It also clearly establishes by whom the work was first done, before someone else investigates the line of inquiry. Through a combination of strategic engagement and publication efforts, early-career physicians can effectively build their professional reputation and advance their academic careers.
Time Management
Time management is essential for any research, and particularly in early career when efficiency in clinical care duties is still being gained. Securing protected time for research is essential to develop a niche, build connections (both institutionally and beyond their institutions), and demonstrate productivity that can be utilized to support future grant efforts.
Similarly, using protected time efficiently is required. Without organization and planning, research time can be spent with scattered meetings and responding to various tasks that do not directly support your research. It is helpful to be introspective about the time of the day you are most productive in your research efforts and blocking off that time to focus on research tasks and minimizing distractions. Blocking monthly time for larger scale thinking and planning is also important. Weekly lab and individual one-on-one meetings also support time management for trainees and lab members, to ensure efficiency and progress. Additionally, robust clinical support is essential to ensure that research time remains protected and patient care moves forward. When negotiating for positions, and in regular meetings thereafter, it is important to advocate for sufficient clinical staffing such that non-physician tasks can be appropriately delegated to another member of the care team.
Funding
Securing adequate funding poses a significant challenge for all early-career physician-scientists, particularly because of the discrepancy between National Institutes of Health salary caps and the higher average salaries in academic gastroenterology. This financial gap can deter physicians from pursuing research-intensive careers altogether and can derail early investigators who do not obtain funding rapidly. To overcome this, early-career investigators may need to adopt flexible strategies, such as accepting a lower salary that aligns with grant funding limits or funneling incentive or bonus pay to research accounts. Alternatively, they can advocate for institutional support to bridge the salary gap, ensuring their research efforts remain financially viable.
Institutions committed to fostering research excellence may offer supplemental funding or bridge programs to retain talented physician-scientists, thereby mitigating the financial strain and encouraging long-term engagement in research. Regular meetings to review salary and support sources, including philanthropy, foundation grants, and other streams, should be undertaken with leadership to align the researcher’s timeline and available funding. If career development funding appears untenable, consideration of multi–principal investigator R01s or equivalent with senior established investigators can be a promising path.
Receiving Mentorship
Effective mentorship for early-career physician-scientists should be approached through a team-based model that leverages both internal and external mentors. Internal mentors, familiar with the specific culture, expectations, and advancement pathways of the institution, can provide invaluable guidance on navigating institutional metrics for success, such as promotion criteria, grant acquisition, and clinical-research balance. External mentors, on the other hand, bring a broader perspective by offering innovative career development strategies and solutions derived from experiences at their home institutions. This multimodal mentorship model ensures a well-rounded approach to professional growth.
All national gastroenterology societies, including the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, offer structured early-career mentorship programs designed to connect emerging researchers with experienced leaders in the field (see below). These programs typically require a formal application process and are highly regarded for their exceptional quality and impact. Participation in such initiatives can significantly enhance career development by expanding networks, fostering interdisciplinary collaboration, and providing tailored guidance that complements institutional support. Integrating both internal and external mentorship opportunities ensures a robust and dynamic foundation for long-term success in academic medicine.
- AGA Career Compass (https://gastro.org/fellows-and-early-career/mentoring/)
- ACG Early Career Leadership Program (https://gi.org/acg-institute/early-career-leadership-program/)
- AGA-AASLD Academic Skills Workshop (https://gastro.org/news/applications-now-being-accepted-for-the-2024-aga-aasld-academic-skills-workshop/)
- AASLD Women’s Initiative Committee Leadership Program (https://www.aasld.org/promoting-leadership-potential-women-hepatology)
- Scrubs n Heels Mentorship Matrix (https://scrubsandheels.com/matrix/)
Providing Mentorship
The trainee authors on this manuscript describe in this section what has been helpful for them as mentees in the FELD research lab.
Student doctor Nguyen describes her experience as a lab member and things she finds most helpful as a medical student in the lab:
- Upon joining the team, a one-to-one meeting to discuss trainee’s personal and professional goals, and availability, was crucial to building the mentor-mentee relationship. Establishing this meaningful mentorship early on clarified expectations on both sides, built trust, and increased motivation. As a trainee, it is essential for me to see how my work aligns with a long-term goal and to receive ample guidance throughout the process.
- One of the most impactful experiences has been joining informal lunch sessions where trainees discussed data collection protocols and exchanged insights. In doing so, Dr. Feld has cultivated a lab culture that encourages curiosity, constructive feedback, and collaborative learning.
- To increase productivity, our team of trainees created a useful group message thread where we coordinated more sessions to collaborate. This coordination formed stronger relationships between team members and fostered a sense of shared purpose.
Dr. Cooper, a third year internal medicine resident, describes her experience as both a research mentee and a mentor to the junior trainees: “As a resident pursuing a career in academic gastroenterology and hepatology, I have found three key elements to be most helpful: intentional mentorship, structured meetings, and leadership development.”
- Intentional mentorship: Prior to joining the lab, I met with Dr. Feld to discuss my research experience and my goals. She took the time to understand these within the context of my training timeline and tailored project opportunities that aligned with my interests and were both feasible and impactful for my next steps. This intentional approach not only fostered a productive research experience but also established a mentor-mentee relationship built on genuine care for my growth and development.
- Regular meetings: Frequent lab meetings promote accountability, teamwork, and shared problem-solving skills. The open exchange of ideas fosters collaboration and joint problem solving to elevate the quality of our research. They are also an opportunity to observe key decision-making points during the research process and have been a great way to learn more about solid methodology.
- Supervised leadership: I have had ample time to lead discussions and coordinate projects among the junior trainees. These monitored leadership experiences promote project management skills, mentorship, and team dynamic awareness while maintaining the safety net of senior guidance. This model helped me transition from a trainee supporting others’ research to a more independent role, contributing to multi-disciplinary projects while mentoring junior members.
Conclusion
In conclusion, many exciting opportunities and notable barriers exist to establishing a clinical research laboratory in the early career. While excellence in each of the areas outlined may evolve, some aspects will come easier than others and with time, persistence, and a bit of luck, the research world will be a better place because of your contributions!
Dr. Feld is assistant professor of gastroenterology and hepatology and physician executive of Diversity, Equity, Inclusion and Belonging for the department of medicine at the University of Massachusetts (UMass) Chan Medical School, Worcester. Ms. Nguyen is a medical student at UMass Chan Medical School. Dr. Cooper is a resident physician at UMass Chan Medical School. Dr. Rabinowitz is an attending physician in the Inflammatory Bowel Disease Center at Beth Israel Deaconess Medical Center, Boston, Mass. Dr. Uchida is codirector of the Multidisciplinary Eosinophilic Gastrointestinal Disease Clinic at the University of Utah School of Medicine, Salt Lake City.
The Federal Trade Commission’s Non-Compete Ban
Non-compete agreements (NCAs) in physician contracts, also termed “restrictive covenants” or “covenants not to compete,” have become a hot topic recently because of the Federal Trade Commission’s (FTC’s) April 2024 ruling invalidating almost all NCAs. But in fact, NCAs have long been controversial, and no more so than in the realm of physician NCAs, which involve substantial policy concerns.
how it relates to a physician employment contract currently, and its possible evolution.
What is It?
Generally speaking, an NCA, usually in the form of an employment contract clause, is an agreement between the employer and the employee that the employee will not enter into post-contract competition with that employer within the limitations of a specific duration, scope of practice, and/or geography. NCAs have traditionally been regulated under state statutory law and common law and have been permitted based on policy considerations that attempt to balance competing employee and employer interests. Physicians should understand their states’ statutory treatment of an NCA.
NCAs protect important employer business interests, including the protection of proprietary information, safeguarding trade secrets, reducing employee turnover, and protecting patient lists. Employees, though, have limited mobility in changing professional positions, have less bargaining power with the employer, and may find themselves with limited options for comparable professional positions.
The NCA ostensibly appears to greatly benefit the employer’s interests over the employee’s; however, NCA protection of employer interests may also substantially benefit employees by encouraging substantial employer investment in employees whom the employer recognizes as a stable and likely long-term human resource, ultimately fostering increased employee satisfaction and innovation. Indeed, one concern with the FTC’s non-compete ban is the potential for significant underinvestment in information sharing and employee training, because employers would, without a NCA, be less likely to recoup those employee investments and would have limited ability to keep competitors from free-riding on investments in employees who leave and join competitors. Ultimately, this would lead to decreased market efficiency.
What is Its Status Today?
Regulation of NCAs, including physician NCAs, has traditionally been based on state statutory law and by common law. Perhaps because of the increasing use of the NCA in professional settings, the NCA has been increasingly scrutinized by courts and state legislatures in the last few decades, with an overall increasing focus on NCA reasonableness and appropriate fit in individual employment settings, and with an emphasis on employer demonstration of legitimate and significant business interests for using a NCA.
States have evolved differently in their treatment of NCAs; some states ban NCAs altogether while others allow them with varying interpretation and enforceability, frequently focused upon the NCA’s duration, scope, and geography. Similarly, in common law, courts will frequently invalidate NCAs that are found to be unreasonably overbroad, either geographically, temporally, and/or in regard to scope.
On April 23, 2024, however, the FTC altered this existing state of affairs by issuing a rule banning new NCAs in all employment situations after September 3, 2024. The rule also holds that existing NCAs are not enforceable, with a small carve-out for some senior executives. It applies to for-profit businesses, and some, but not all, non-profit organizations. The FTC’s stated intent is to reduce healthcare spending by increasing employee compensation and mobility. The FTC’s ban is likely meant to reduce transaction costs by increasing physician mobility.
There have been several lawsuits regarding the FTC ruling, challenging it on different grounds. The US District Court for the Northern District of Texas in Ryan LLC v. FTC issued first a preliminary injunction, then a final decision overturning the FTC’s rule. The Court held that the FTC had exceeded its statutory authority, and further, that the rule was arbitrary and capricious. It noted that the rule’s “categorical ban” has no equivalent in state law, is “unreasonably overbroad without a reasonable explanation,” “provides no evidence or reasoned basis,” does not “consider the positive benefits of non-compete agreements,” and does not “address alternatives to the Rule.” The Ryan Court reasoned that as an administrative agency, the FTC can only act as Congress authorizes by statute. On Oct. 18, 2024, the FTC appealed the Court’s decision to the Fifth Circuit Court of Appeals, seeking to reverse the holding setting aside its NCA ban.
The United States District Court for the Eastern District of Pennsylvania in ATS Tree Services LLC v. FTC denied the plaintiff’s motion to stay enforcement of the rule, refusing to issue a preliminary injunction preventing its implementation. As in Ryan, the ATS Tree Services LLC v. FTC plaintiffs argued that the FTC had exceeded its statutory authority in issuing the rule. However, the Plaintiff did not appeal the holding.
The US District Court for the Middle District of Florida in Properties of the Villages, Inc. v. FTCheld, like Ryan, that the rule exceeds the FTC’s statutory authority, noting the FTC’s prior lack of any NCA enforcement actions; however, its reasoning differed from Ryan. The Florida Court held that the FTC in fact has statutory authority to issue such rules; however, the Court held that the FTC could not enforce its rule because it violates the “major questions doctrine.” The “major questions doctrine” requires an agency such as the FTC to “point to clear congressional authorization” for any rule it issues that has “extraordinary ... economic and political significance,” as the NCA ban rule certainly does.
What is Its Future?
The FTC’s NCA ban remains unsettled. State legislatures, in response to the recent court holdings, are reassessing their statutory law regarding NCAs. The Ryan Court’s holding prevented the FTC’s rule from going into effect on September 4, 2024. The Texas and Florida court decisions are awaiting 5th and 11th Circuit Court of Appeals review, respectively. Assuming affirmation of either of the cases on appeal, a circuit split regarding the NCA ban may occur. The US Supreme Court may be called upon to determine the validity of the FTC rule banning NCAs. The Circuit Court decisions are likely to occur in 2025, and any Supreme Court decision would not likely occur until 2026. Meanwhile, state statutory law and common law still apply to NCAs, and the FTC may challenge the validity of NCAs on a case-by-case basis.
US antitrust law remains a potential remedy to scrutinize and restrain inappropriate business practices, including NCA-related abuses. The Sherman Act allows federal and state actors and private citizens, to sue for redress. Antitrust cases are typically considered using the “rule of reason” formulated by the Supreme Court in 1911, which requires plaintiffs show that defendant businesses possessing market power did in fact undertake anticompetitive conduct that had or likely had anticompetitive effects. In other words, the court in an antitrust case will require that the plaintiff show that the business actually had a significant controlling market presence in the geographic area; and further, that the plaintiff show that the business’ actions in fact had an anticompetitive effect, or likely had one. The latter can be found by showing an anticompetitive effect such as abusive pricing
The FTC’s ruling is legally and academically controversial and in fact may not withstand court scrutiny. The rule was put forth by the FTC as an ambitious rule to reduce healthcare spending. But businesses survive only if their revenue surpasses their costs, including personnel costs. Further, maximization of capitalization is attained when businesses require NCAs. Businesses invest heavily in recruiting, hiring, and training personnel, and increased personnel turnover increases these expenditures. NCAs arguably provide a collective benefit by ensuring force continuity, mitigating the risk of the loss of highly trained personnel with proprietary knowledge. NCAs also help a business maintain a skilled workforce, helping maximize business valuation. If FTC’s NCA ban rule were ultimately upheld, businesses would likely respond by instituting longer-term employee contracts, extended termination notice periods, and disincentives for employees who do not fully serve their contract length, including substantial financial disincentives. Business valuation might decrease, reducing investment incentives.
NCAs have long been a method of balancing the interests of employees and employers. They protect businesses’ confidential information, trade secrets, and patient lists, at some cost to employees pursuing new opportunities. The employee, though, is also provided with some benefit from the NCA, albeit indirect. State statutory law and courts have traditionally worked to ensure an appropriate delicate balance between interests, with courts generally finding unbalanced NCAs unenforceable.
For now, physicians should understand the policy considerations of and recognize the uncertainty surrounding NCAs, become familiar with their state’s statutory NCA law, review employment contracts carefully for NCA reasonableness, and seek legal advice if necessary.
Perhaps the FTC’s approach is the correct one for our future. Or perhaps the appropriate future of NCA interpretation and enforcement should continue to rest on state statutory law and common law, where antitrust enforcement is on a case-by-case basis, rather than FTC rulemaking. The results of high court decisions, state statutory law changes in response to the FTC rule, and perhaps US congressional action will provide the final answer.
Dr. Allen is based at the University of Oklahoma Health Sciences Center in Oklahoma City. He has declared no conflicts of interest in relation to this article.
Non-compete agreements (NCAs) in physician contracts, also termed “restrictive covenants” or “covenants not to compete,” have become a hot topic recently because of the Federal Trade Commission’s (FTC’s) April 2024 ruling invalidating almost all NCAs. But in fact, NCAs have long been controversial, and no more so than in the realm of physician NCAs, which involve substantial policy concerns.
how it relates to a physician employment contract currently, and its possible evolution.
What is It?
Generally speaking, an NCA, usually in the form of an employment contract clause, is an agreement between the employer and the employee that the employee will not enter into post-contract competition with that employer within the limitations of a specific duration, scope of practice, and/or geography. NCAs have traditionally been regulated under state statutory law and common law and have been permitted based on policy considerations that attempt to balance competing employee and employer interests. Physicians should understand their states’ statutory treatment of an NCA.
NCAs protect important employer business interests, including the protection of proprietary information, safeguarding trade secrets, reducing employee turnover, and protecting patient lists. Employees, though, have limited mobility in changing professional positions, have less bargaining power with the employer, and may find themselves with limited options for comparable professional positions.
The NCA ostensibly appears to greatly benefit the employer’s interests over the employee’s; however, NCA protection of employer interests may also substantially benefit employees by encouraging substantial employer investment in employees whom the employer recognizes as a stable and likely long-term human resource, ultimately fostering increased employee satisfaction and innovation. Indeed, one concern with the FTC’s non-compete ban is the potential for significant underinvestment in information sharing and employee training, because employers would, without a NCA, be less likely to recoup those employee investments and would have limited ability to keep competitors from free-riding on investments in employees who leave and join competitors. Ultimately, this would lead to decreased market efficiency.
What is Its Status Today?
Regulation of NCAs, including physician NCAs, has traditionally been based on state statutory law and by common law. Perhaps because of the increasing use of the NCA in professional settings, the NCA has been increasingly scrutinized by courts and state legislatures in the last few decades, with an overall increasing focus on NCA reasonableness and appropriate fit in individual employment settings, and with an emphasis on employer demonstration of legitimate and significant business interests for using a NCA.
States have evolved differently in their treatment of NCAs; some states ban NCAs altogether while others allow them with varying interpretation and enforceability, frequently focused upon the NCA’s duration, scope, and geography. Similarly, in common law, courts will frequently invalidate NCAs that are found to be unreasonably overbroad, either geographically, temporally, and/or in regard to scope.
On April 23, 2024, however, the FTC altered this existing state of affairs by issuing a rule banning new NCAs in all employment situations after September 3, 2024. The rule also holds that existing NCAs are not enforceable, with a small carve-out for some senior executives. It applies to for-profit businesses, and some, but not all, non-profit organizations. The FTC’s stated intent is to reduce healthcare spending by increasing employee compensation and mobility. The FTC’s ban is likely meant to reduce transaction costs by increasing physician mobility.
There have been several lawsuits regarding the FTC ruling, challenging it on different grounds. The US District Court for the Northern District of Texas in Ryan LLC v. FTC issued first a preliminary injunction, then a final decision overturning the FTC’s rule. The Court held that the FTC had exceeded its statutory authority, and further, that the rule was arbitrary and capricious. It noted that the rule’s “categorical ban” has no equivalent in state law, is “unreasonably overbroad without a reasonable explanation,” “provides no evidence or reasoned basis,” does not “consider the positive benefits of non-compete agreements,” and does not “address alternatives to the Rule.” The Ryan Court reasoned that as an administrative agency, the FTC can only act as Congress authorizes by statute. On Oct. 18, 2024, the FTC appealed the Court’s decision to the Fifth Circuit Court of Appeals, seeking to reverse the holding setting aside its NCA ban.
The United States District Court for the Eastern District of Pennsylvania in ATS Tree Services LLC v. FTC denied the plaintiff’s motion to stay enforcement of the rule, refusing to issue a preliminary injunction preventing its implementation. As in Ryan, the ATS Tree Services LLC v. FTC plaintiffs argued that the FTC had exceeded its statutory authority in issuing the rule. However, the Plaintiff did not appeal the holding.
The US District Court for the Middle District of Florida in Properties of the Villages, Inc. v. FTCheld, like Ryan, that the rule exceeds the FTC’s statutory authority, noting the FTC’s prior lack of any NCA enforcement actions; however, its reasoning differed from Ryan. The Florida Court held that the FTC in fact has statutory authority to issue such rules; however, the Court held that the FTC could not enforce its rule because it violates the “major questions doctrine.” The “major questions doctrine” requires an agency such as the FTC to “point to clear congressional authorization” for any rule it issues that has “extraordinary ... economic and political significance,” as the NCA ban rule certainly does.
What is Its Future?
The FTC’s NCA ban remains unsettled. State legislatures, in response to the recent court holdings, are reassessing their statutory law regarding NCAs. The Ryan Court’s holding prevented the FTC’s rule from going into effect on September 4, 2024. The Texas and Florida court decisions are awaiting 5th and 11th Circuit Court of Appeals review, respectively. Assuming affirmation of either of the cases on appeal, a circuit split regarding the NCA ban may occur. The US Supreme Court may be called upon to determine the validity of the FTC rule banning NCAs. The Circuit Court decisions are likely to occur in 2025, and any Supreme Court decision would not likely occur until 2026. Meanwhile, state statutory law and common law still apply to NCAs, and the FTC may challenge the validity of NCAs on a case-by-case basis.
US antitrust law remains a potential remedy to scrutinize and restrain inappropriate business practices, including NCA-related abuses. The Sherman Act allows federal and state actors and private citizens, to sue for redress. Antitrust cases are typically considered using the “rule of reason” formulated by the Supreme Court in 1911, which requires plaintiffs show that defendant businesses possessing market power did in fact undertake anticompetitive conduct that had or likely had anticompetitive effects. In other words, the court in an antitrust case will require that the plaintiff show that the business actually had a significant controlling market presence in the geographic area; and further, that the plaintiff show that the business’ actions in fact had an anticompetitive effect, or likely had one. The latter can be found by showing an anticompetitive effect such as abusive pricing
The FTC’s ruling is legally and academically controversial and in fact may not withstand court scrutiny. The rule was put forth by the FTC as an ambitious rule to reduce healthcare spending. But businesses survive only if their revenue surpasses their costs, including personnel costs. Further, maximization of capitalization is attained when businesses require NCAs. Businesses invest heavily in recruiting, hiring, and training personnel, and increased personnel turnover increases these expenditures. NCAs arguably provide a collective benefit by ensuring force continuity, mitigating the risk of the loss of highly trained personnel with proprietary knowledge. NCAs also help a business maintain a skilled workforce, helping maximize business valuation. If FTC’s NCA ban rule were ultimately upheld, businesses would likely respond by instituting longer-term employee contracts, extended termination notice periods, and disincentives for employees who do not fully serve their contract length, including substantial financial disincentives. Business valuation might decrease, reducing investment incentives.
NCAs have long been a method of balancing the interests of employees and employers. They protect businesses’ confidential information, trade secrets, and patient lists, at some cost to employees pursuing new opportunities. The employee, though, is also provided with some benefit from the NCA, albeit indirect. State statutory law and courts have traditionally worked to ensure an appropriate delicate balance between interests, with courts generally finding unbalanced NCAs unenforceable.
For now, physicians should understand the policy considerations of and recognize the uncertainty surrounding NCAs, become familiar with their state’s statutory NCA law, review employment contracts carefully for NCA reasonableness, and seek legal advice if necessary.
Perhaps the FTC’s approach is the correct one for our future. Or perhaps the appropriate future of NCA interpretation and enforcement should continue to rest on state statutory law and common law, where antitrust enforcement is on a case-by-case basis, rather than FTC rulemaking. The results of high court decisions, state statutory law changes in response to the FTC rule, and perhaps US congressional action will provide the final answer.
Dr. Allen is based at the University of Oklahoma Health Sciences Center in Oklahoma City. He has declared no conflicts of interest in relation to this article.
Non-compete agreements (NCAs) in physician contracts, also termed “restrictive covenants” or “covenants not to compete,” have become a hot topic recently because of the Federal Trade Commission’s (FTC’s) April 2024 ruling invalidating almost all NCAs. But in fact, NCAs have long been controversial, and no more so than in the realm of physician NCAs, which involve substantial policy concerns.
how it relates to a physician employment contract currently, and its possible evolution.
What is It?
Generally speaking, an NCA, usually in the form of an employment contract clause, is an agreement between the employer and the employee that the employee will not enter into post-contract competition with that employer within the limitations of a specific duration, scope of practice, and/or geography. NCAs have traditionally been regulated under state statutory law and common law and have been permitted based on policy considerations that attempt to balance competing employee and employer interests. Physicians should understand their states’ statutory treatment of an NCA.
NCAs protect important employer business interests, including the protection of proprietary information, safeguarding trade secrets, reducing employee turnover, and protecting patient lists. Employees, though, have limited mobility in changing professional positions, have less bargaining power with the employer, and may find themselves with limited options for comparable professional positions.
The NCA ostensibly appears to greatly benefit the employer’s interests over the employee’s; however, NCA protection of employer interests may also substantially benefit employees by encouraging substantial employer investment in employees whom the employer recognizes as a stable and likely long-term human resource, ultimately fostering increased employee satisfaction and innovation. Indeed, one concern with the FTC’s non-compete ban is the potential for significant underinvestment in information sharing and employee training, because employers would, without a NCA, be less likely to recoup those employee investments and would have limited ability to keep competitors from free-riding on investments in employees who leave and join competitors. Ultimately, this would lead to decreased market efficiency.
What is Its Status Today?
Regulation of NCAs, including physician NCAs, has traditionally been based on state statutory law and by common law. Perhaps because of the increasing use of the NCA in professional settings, the NCA has been increasingly scrutinized by courts and state legislatures in the last few decades, with an overall increasing focus on NCA reasonableness and appropriate fit in individual employment settings, and with an emphasis on employer demonstration of legitimate and significant business interests for using a NCA.
States have evolved differently in their treatment of NCAs; some states ban NCAs altogether while others allow them with varying interpretation and enforceability, frequently focused upon the NCA’s duration, scope, and geography. Similarly, in common law, courts will frequently invalidate NCAs that are found to be unreasonably overbroad, either geographically, temporally, and/or in regard to scope.
On April 23, 2024, however, the FTC altered this existing state of affairs by issuing a rule banning new NCAs in all employment situations after September 3, 2024. The rule also holds that existing NCAs are not enforceable, with a small carve-out for some senior executives. It applies to for-profit businesses, and some, but not all, non-profit organizations. The FTC’s stated intent is to reduce healthcare spending by increasing employee compensation and mobility. The FTC’s ban is likely meant to reduce transaction costs by increasing physician mobility.
There have been several lawsuits regarding the FTC ruling, challenging it on different grounds. The US District Court for the Northern District of Texas in Ryan LLC v. FTC issued first a preliminary injunction, then a final decision overturning the FTC’s rule. The Court held that the FTC had exceeded its statutory authority, and further, that the rule was arbitrary and capricious. It noted that the rule’s “categorical ban” has no equivalent in state law, is “unreasonably overbroad without a reasonable explanation,” “provides no evidence or reasoned basis,” does not “consider the positive benefits of non-compete agreements,” and does not “address alternatives to the Rule.” The Ryan Court reasoned that as an administrative agency, the FTC can only act as Congress authorizes by statute. On Oct. 18, 2024, the FTC appealed the Court’s decision to the Fifth Circuit Court of Appeals, seeking to reverse the holding setting aside its NCA ban.
The United States District Court for the Eastern District of Pennsylvania in ATS Tree Services LLC v. FTC denied the plaintiff’s motion to stay enforcement of the rule, refusing to issue a preliminary injunction preventing its implementation. As in Ryan, the ATS Tree Services LLC v. FTC plaintiffs argued that the FTC had exceeded its statutory authority in issuing the rule. However, the Plaintiff did not appeal the holding.
The US District Court for the Middle District of Florida in Properties of the Villages, Inc. v. FTCheld, like Ryan, that the rule exceeds the FTC’s statutory authority, noting the FTC’s prior lack of any NCA enforcement actions; however, its reasoning differed from Ryan. The Florida Court held that the FTC in fact has statutory authority to issue such rules; however, the Court held that the FTC could not enforce its rule because it violates the “major questions doctrine.” The “major questions doctrine” requires an agency such as the FTC to “point to clear congressional authorization” for any rule it issues that has “extraordinary ... economic and political significance,” as the NCA ban rule certainly does.
What is Its Future?
The FTC’s NCA ban remains unsettled. State legislatures, in response to the recent court holdings, are reassessing their statutory law regarding NCAs. The Ryan Court’s holding prevented the FTC’s rule from going into effect on September 4, 2024. The Texas and Florida court decisions are awaiting 5th and 11th Circuit Court of Appeals review, respectively. Assuming affirmation of either of the cases on appeal, a circuit split regarding the NCA ban may occur. The US Supreme Court may be called upon to determine the validity of the FTC rule banning NCAs. The Circuit Court decisions are likely to occur in 2025, and any Supreme Court decision would not likely occur until 2026. Meanwhile, state statutory law and common law still apply to NCAs, and the FTC may challenge the validity of NCAs on a case-by-case basis.
US antitrust law remains a potential remedy to scrutinize and restrain inappropriate business practices, including NCA-related abuses. The Sherman Act allows federal and state actors and private citizens, to sue for redress. Antitrust cases are typically considered using the “rule of reason” formulated by the Supreme Court in 1911, which requires plaintiffs show that defendant businesses possessing market power did in fact undertake anticompetitive conduct that had or likely had anticompetitive effects. In other words, the court in an antitrust case will require that the plaintiff show that the business actually had a significant controlling market presence in the geographic area; and further, that the plaintiff show that the business’ actions in fact had an anticompetitive effect, or likely had one. The latter can be found by showing an anticompetitive effect such as abusive pricing
The FTC’s ruling is legally and academically controversial and in fact may not withstand court scrutiny. The rule was put forth by the FTC as an ambitious rule to reduce healthcare spending. But businesses survive only if their revenue surpasses their costs, including personnel costs. Further, maximization of capitalization is attained when businesses require NCAs. Businesses invest heavily in recruiting, hiring, and training personnel, and increased personnel turnover increases these expenditures. NCAs arguably provide a collective benefit by ensuring force continuity, mitigating the risk of the loss of highly trained personnel with proprietary knowledge. NCAs also help a business maintain a skilled workforce, helping maximize business valuation. If FTC’s NCA ban rule were ultimately upheld, businesses would likely respond by instituting longer-term employee contracts, extended termination notice periods, and disincentives for employees who do not fully serve their contract length, including substantial financial disincentives. Business valuation might decrease, reducing investment incentives.
NCAs have long been a method of balancing the interests of employees and employers. They protect businesses’ confidential information, trade secrets, and patient lists, at some cost to employees pursuing new opportunities. The employee, though, is also provided with some benefit from the NCA, albeit indirect. State statutory law and courts have traditionally worked to ensure an appropriate delicate balance between interests, with courts generally finding unbalanced NCAs unenforceable.
For now, physicians should understand the policy considerations of and recognize the uncertainty surrounding NCAs, become familiar with their state’s statutory NCA law, review employment contracts carefully for NCA reasonableness, and seek legal advice if necessary.
Perhaps the FTC’s approach is the correct one for our future. Or perhaps the appropriate future of NCA interpretation and enforcement should continue to rest on state statutory law and common law, where antitrust enforcement is on a case-by-case basis, rather than FTC rulemaking. The results of high court decisions, state statutory law changes in response to the FTC rule, and perhaps US congressional action will provide the final answer.
Dr. Allen is based at the University of Oklahoma Health Sciences Center in Oklahoma City. He has declared no conflicts of interest in relation to this article.
Avoid Getting Stuck: A Practical Guide to Managing Chronic Constipation
Introduction
Constipation affects one in six people worldwide and accounts for one third of outpatient visits.1 Chronic constipation is defined by difficult, infrequent, and/or incomplete defecation, quantified by less than three spontaneous bowel movements per week, persisting for at least 3 months. Patients may complain of straining during defecation, incomplete evacuation, hard stools (Bristol stool scale [BSS] type 1-2), and fullness or bloating. Chronic constipation can be subclassified as either a primary or secondary disorder.1,2
Primary Constipation Disorders
Primary constipation includes disorders of the colon or anorectum. This includes irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC), slow transit constipation (STC), dyssynergic defecation, and pelvic floor disorders (see Figure 1).
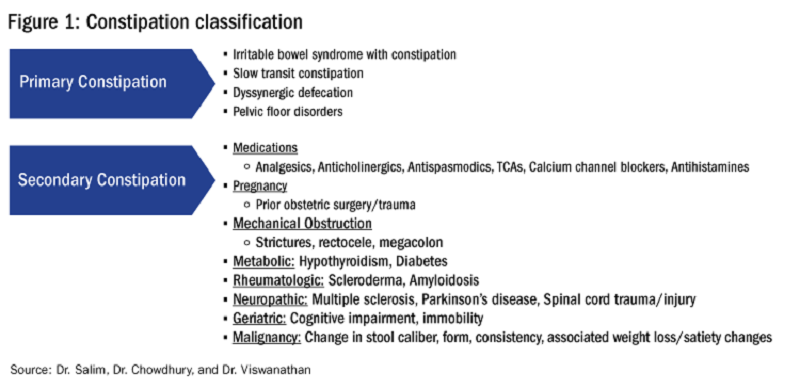
IBS-C
IBS-C is a chronic disorder of the gut-brain axis with a worldwide prevalence of 1.3% and a prevalence of 6%-16% in the United States, United Kingdom, and Canada, with females more likely to seek care than males.2 The economic impact of IBS-C is estimated to be $1.5 billion–$10 billion per year in the United States alone.3 The distinguishing characteristic is abdominal pain, however IBS-C can present with a constellation of symptoms. The diagnostic paradigm has shifted from IBS being a diagnosis of exclusion to now using a positive diagnostic strategy.2 Using this Rome IV criteria, one can make the diagnosis with > 95% accuracy.2,4
CIC
CIC, previously defined as functional constipation, is a disorder defined by incomplete defecation and difficult or infrequent stool. CIC is diagnosed in patients without an underlying anatomic or structural abnormality. Rome IV Criteria helps further classify the defining characteristics of chronic idiopathic constipation.2
Slow Transit Constipation
STC is characterized by impaired colonic transit time in the absence of pelvic floor dysfunction. It presents with infrequent bowel movements, diminished urgency, and/or straining with defecation.
Defecatory Disorders: Dyssynergic Defecation and Pelvic Floor Dysfunction
Defecatory disorders (DDs) result from alterations in the colonic-neural pathway with an unclear pathogenesis. A firm understanding of colonic physiology is necessary to identify DDs. The right colon helps to store and mix stool contents, the left colon helps add water to the stool, and the anal canal and rectum enable defecation and maintain continence. Any alteration along this physiologic pathway results in DDs.5
DDs primarily develop via maladaptive pelvic floor contraction during defecation or from muscle or nerve injury and include functional outlet obstruction, anorectal dyssynergia, and pelvic floor dysfunction. Increased resistance to defecation results from anismus, paradoxical anal sphincter contraction, or incomplete relaxation of the pelvic floor and external anal sphincter. This muscle incoordination is described as dyssynergia. DDs can involve either muscle or nerve dysfunction or a combination of the two. Reduced rectal sensation caused by reduced sensory triggers can cause stasis of stool, thus propagating the cycle of constipation. Over time, excessive straining can weaken the pelvic floor, increasing the risk of excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy.5 Thus, identification of DDs is crucial in patients with chronic constipation.
Secondary Constipation Disorders
Secondary constipation disorders are a result of an alternate process and warrant a thorough review of outpatient medications and past medical history. Figure 1 outlines the most common causes of secondary constipation, which span a wide differential.
Clinical Evaluation
The evaluation of constipation begins with a thorough history. Description of bowel habits should include frequency, duration, straining, stool consistency using a Bristol stool chart, complete vs incomplete evacuation, pain, bloating, and use of digital maneuvers (vaginal splinting or digital stool removal). One should inquire about back trauma/surgeries and obstetric history to include vaginal forceps injury or episiotomy.
With increased smartphone use, toilet time on average has increased and can contribute to maladaptive bowel habits.6 Patients may not realize they are constipated, so patient education is critical. A patient with daily bowel movements ranging between BSS type 1-6 with incomplete evacuation might complain of diarrhea but may in fact have constipation with overflow diarrhea, for example. Past medical history is also clinically relevant, as systemic conditions can cause secondary constipation. A constipated patient should also be asked what therapies he/she has tried prior to gastroenterology referral as primary care referrals for constipation account for 8 million visits to gastroenterology per year.7
While a sensitive topic, inquire about abuse history, especially in those with childhood constipation symptoms. There is a positive correlation between childhood constipation and physical, emotional, and sexual abuse and, for any number of reasons, your patient may be reluctant to share this or undergo a digital rectal exam (DRE).8 In such cases, be sensitive in asking for this history in private rather than with other family members around and always perform this exam with a chaperone present.
A detailed physical exam is an indispensable tool all gastroenterologists must master when evaluating a constipated patient. Some key exam findings include abdominal distention, high-pitched bowel sounds, and presence of a succussion splash indicating obstructive pathology. Dry skin and brittle hair indicate hypothyroidism while hypermobile joints and skin laxity suggest connective tissue disease. Finally, a physical examination is incomplete without a DRE.
DRE
DRE is an often-overlooked physical exam component which provides helpful insight that can guide management. An informed DRE can help identify structural disorders such as fissure, hemorrhoids, anorectal mass, fecal impaction, rectal prolapse, and excessive perineal descent syndrome.9 Unless contraindicated, DRE should be a standard part of the workup of a patient with chronic constipation.
Workup
Colonoscopy
The role of colonoscopy in chronic constipation is low yield and only indicated if alarm signs are present.2 When no organic causes can be identified, the patient is deemed to have a functional bowel or motility disorder leading to constipation.
Colonic Transit Time
Colonic transit time (CTT) can be evaluated by assessing the presence of radio-opaque sitz markers in the colon with an abdominal x-ray 5 days after ingestion. The presence of five or more sitz markers may indicate STC. However, this can also signal an obstructive defecatory disorder. Colon scintigraphy can determine whether there is diffuse colonic dysmotility or dysfunction in a specific segment of the colon.10
Anorectal Function Testing (AFT)
AFT can evaluate DDs, such as fecal incontinence, dyssynergic defecation, rectal sensory disorders, anorectal pain, and rectal prolapse. AFT comprises three tests: anorectal manometry (ARM), balloon expulsion test (BET), and rectal sensory testing. These assess the defecation, continence, and sensory mechanisms of the rectum, respectively.
ARM testing employs a thin, flexible probe with an attached sensor that is inserted into the rectum to measure internal and external sphincter pressures while at rest, squeezing, and bearing down to give a functional assessment of sphincter tone.11 Cough or party balloon test assesses continence and sphincter strength. Rectal sensation is assessed by inflating a balloon incrementally and asking the patient to indicate first sensation, urgency to defecate, and discomfort. If both ARM and BET are abnormal, the patient meets diagnostic criteria for dyssynergic defecation.12
Pelvic floor disorders can be further assessed by MR defecography or barium defecography. Barium defecography is the more widely available of the two. MR defecography is a dynamic study that directly assesses pelvic floor muscles and endopelvic fascia during various stages of defecation and considered superior. This testing modality can distinguish between functional causes such as dyssynergia or pelvic floor dysfunction and structural causes of obstruction such as rectocele, rectal prolapse, or rectal intussusception. MR defecography is helpful when dyssynergia is suggested by ARM with a normal BET or if there is an absent recto-anal inhibitory reflex on ARM, which may suggest rectal intussusception.
Management
CIC
Incorporating 20-30 g of total soluble fiber, such as psyllium in individuals with low dietary fiber intake is the first-line recommendation for CIC.13 If response to a trial of fiber supplementation is inadequate, over-the-counter (OTC) osmotic laxatives such as polyethylene glycol and magnesium oxide can be incorporated. In the event of failure of OTC osmotic laxatives, lactulose can be considered. Stimulant laxatives such as senna, bisacodyl, or sodium picosulfate can be added as an adjunctive measure for short periods of time, defined as daily for 4 weeks or less.
If these measures are inadequate, pharmacological therapy with secretagogues and 5HT agonists can be considered. Prucalopride, a selective agonist of serotonin 5-HT4 receptors, is approved for CIC, prescribed 2 mg daily.14 It can also be used in patients with global motility delays, such as gastroparetics with constipation. The mechanism of action of secretagogues and specific dosing of these medications are discussed in Figure 2.15 Vibrant is a non-pharmacologic, orally ingested, vibrating, and programmable capsule device that has recently received Food and Drug Administration approval for treatment of chronic constipation by stimulating the intestinal wall, thereby promoting colonic contractile activity to achieve more spontaneous bowel movements. Further studies are required to assess its efficacy.16 Additionally, if there is inadequate response to all the above, it would be prudent to evaluate for the presence of pelvic floor dysfunction as well.
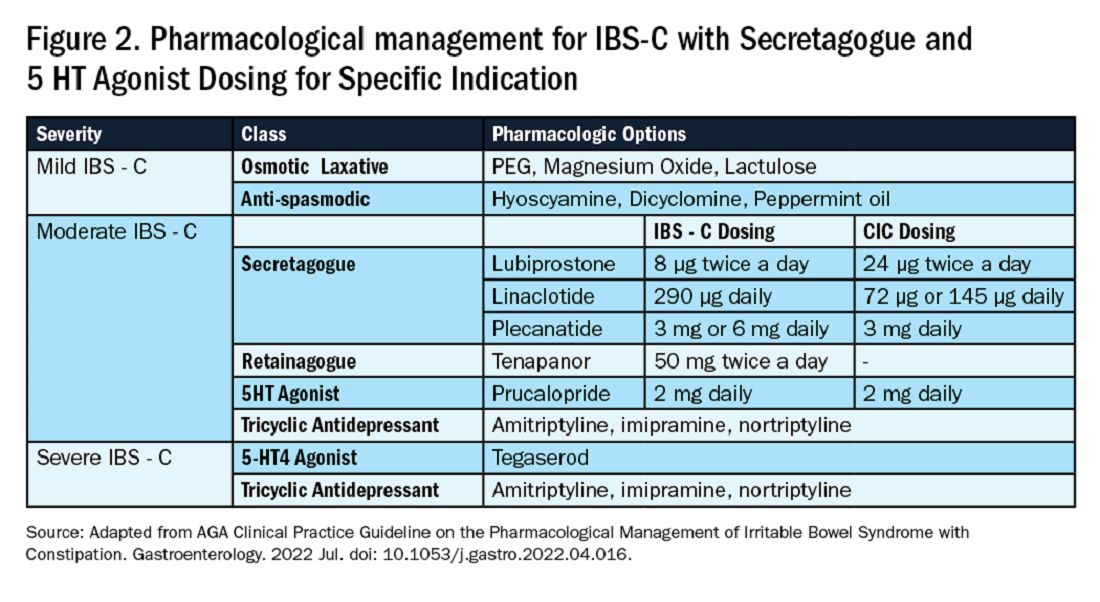
IBS-C
Similar to CIC, treatment for mild IBS-C starts with osmotic laxatives with the additional component of pain control. Antispasmodics can be used to manage the abdominal pain, cramping, and spasms associated with IBS-C. Antispasmodics available in the United States include anticholinergic agents that cause smooth muscle relaxation, such as dicyclomine or hyoscyamine or direct smooth muscle relaxants such as peppermint oil.17 IBS-C patients with moderate symptoms may need escalation of therapy to secretagogues or 5HT agonists (see Figure 2). Secretagogues increase fluid retention in the colonic lumen to promote bowel movements and improve visceral hypersensitivity. Lubiprostone is an intestinal chloride channel activator, indicated only for adult women with IBS-C. Linaclotide and plecanatide are guanylate cyclase-C activators which increase intestinal chloride and bicarbonate secretion, and both are indicated in IBS-C and CIC. Tenapanor inhibits the sodium/hydrogen exchanger in the GI tract, leading to increased water secretion, and is recommended for IBS-C in adults who have failed secretagogues.
All four of these drugs can be considered for moderate to severe IBS-C symptoms. In the case of severe IBS-C symptoms, Tegaserod, a 5-HT4 receptor partial agonist has been approved in women under 65 without significant cardiovascular or cerebrovascular disease.18 Regardless of IBS-C symptom severity, persistent visceral hypersensitivity can be treated with low-dose neuromodulators.19 Figure 2 provides treatment recommendations for IBS-C based on symptom severity.
Opioid-Induced Constipation (OIC)
In patients with OIC, peripherally acting mu-opioid receptor antagonists such as methylnaltrexone and naloxegol can be beneficial where stimulant laxatives are insufficient. Additionally, lubiprostone is indicated in OIC in non-cancer patients. At present, there are no head-to-head trials comparing efficacy of these medications.
Defecatory Disorders
Biofeedback therapy is the cornerstone of treatment for dyssynergic defecation, focusing on neuromuscular training to restore a normal pattern of defecation by teaching patients to tense the abdomen and relax the pelvic floor muscles and anal sphincter. It retrains the body to coordinate abdominal, rectal, and anal muscles to achieve synchronous contraction to achieve complete evacuation. It also increases awareness and response to rectal fullness or the need to defecate.
Biofeedback makes patients aware of counterproductive subconscious actions such as contracting of their anal sphincter during defecation followed by simulated defecation training with focus on how to tighten abdominal muscles and relax pelvic floor muscles to initiate and complete defecation.20 This is performed in the office with a physiotherapist or trained nurse for at least six sessions or at home where patients are encouraged to perform the exercises for 20 minutes, twice a day. These sessions utilize tools such as manometry probes, electromyography probes, simulated balloon, or home biofeedback training devices to provide visual feedback while practicing abdominophrenic breathing. Biofeedback is particularly helpful in patients suffering from constipation. Patients with defecatory disorders can also benefit from pelvic floor physical therapy which focuses on strengthening the pelvic and puborectal muscles, external anal sphincter, and pelvic muscles. This is more useful in patients with fecal incontinence. Despite all these treatments, a subset of patients may still not respond and may qualify for surgical evaluation.
Conclusion
While constipation is seldom life-threatening, it has a negative impact on patient quality of life and poses a significant financial burden on our overall healthcare system. The complexity of this condition should be appreciated and understood in order for a complete and thorough evaluation. We trust that our practical guide should serve as a useful tool in the evaluation of a chronically constipated patient.
Dr. Salim (@hamsalim07 on X) is based in the Department of Internal Medicine, University of Texas Medical Branch, Galveston. Dr. Chowdhury (annicho.med on Instagram) is a fellow in the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas MD Anderson Cancer Center, Houston. Dr. Viswanathan (@LavanyaMD on X) is Associate Professor, University of Texas MD Anderson Cancer Center. The authors declare no conflict of interest.
References
1. Mugie S et al. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2010.12.010.
2. Almario CV et al. Gastroenterology. 2023 Dec. doi: 10.1053/j.gastro.2023.08.010.
3. Canavan C et al. Clin Epidemiol. 2014 Feb. doi: 10.2147/CLEP.S40245.
4. Rao SSC. Gastroenterol Clin North Am. 2007 Sep. doi: 10.1016/j.gtc.2007.07.013.
5. Bharucha AE et al. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
6. Cinquetti M et al. Clin Exp Pediatr. 2021 Sep. doi: 10.3345/cep.2020.01326.
7. Shah ND et al. Am J Gastroenterol. 2008 Jul. doi: 10.1111/j.1572-0241.2008.01910.x.
8. Rajindrajith S et al. J Pediatr Gastroenterol Nutr. 2014 Apr. doi: 10.1097/MPG.0000000000000249.
9. Talley NJ. Am J Gastroenterol. 2008 Apr. doi: 10.1111/j.1572-0241.2008.01832.x.
10. Maurer AH. J Nucl Med. 2015 Sep. doi: 10.2967/jnumed.113.134551.
11. Frye J et al. Am J Gastroenterol. 2024 Aug. doi: 10.14309/ajg.0000000000002670.
12. Rao SSC et al. J Neurogastroenterol Motil. 2016 Jun. doi: 10.5056/jnm16060.
13. Chang L et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.03.214.
14. Brenner DM et al. Am J Gastroenterol. 2021 Aug. doi: 10.14309/ajg.0000000000001266.
15. Chang L et al. Gastroenterology. 2022 Jul. doi: 10.1053/j.gastro.2022.04.016.
16. Rao SSC et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.02.013.
17. Lacy BE et al. Am J Gastroenterol. 2021 Jan. doi: 10.14309/ajg.0000000000001036.
18. Anderson JL et al. J Cardiovasc Pharmacol Ther. 2009 Sep. doi: 10.1177/1074248409340158.
19. Rahimi R et al. World J Gastroenterol. 2009 Apr. doi: 10.3748/wjg.15.1548.
20. Rao SSC. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2011.01.004.
Introduction
Constipation affects one in six people worldwide and accounts for one third of outpatient visits.1 Chronic constipation is defined by difficult, infrequent, and/or incomplete defecation, quantified by less than three spontaneous bowel movements per week, persisting for at least 3 months. Patients may complain of straining during defecation, incomplete evacuation, hard stools (Bristol stool scale [BSS] type 1-2), and fullness or bloating. Chronic constipation can be subclassified as either a primary or secondary disorder.1,2
Primary Constipation Disorders
Primary constipation includes disorders of the colon or anorectum. This includes irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC), slow transit constipation (STC), dyssynergic defecation, and pelvic floor disorders (see Figure 1).

IBS-C
IBS-C is a chronic disorder of the gut-brain axis with a worldwide prevalence of 1.3% and a prevalence of 6%-16% in the United States, United Kingdom, and Canada, with females more likely to seek care than males.2 The economic impact of IBS-C is estimated to be $1.5 billion–$10 billion per year in the United States alone.3 The distinguishing characteristic is abdominal pain, however IBS-C can present with a constellation of symptoms. The diagnostic paradigm has shifted from IBS being a diagnosis of exclusion to now using a positive diagnostic strategy.2 Using this Rome IV criteria, one can make the diagnosis with > 95% accuracy.2,4
CIC
CIC, previously defined as functional constipation, is a disorder defined by incomplete defecation and difficult or infrequent stool. CIC is diagnosed in patients without an underlying anatomic or structural abnormality. Rome IV Criteria helps further classify the defining characteristics of chronic idiopathic constipation.2
Slow Transit Constipation
STC is characterized by impaired colonic transit time in the absence of pelvic floor dysfunction. It presents with infrequent bowel movements, diminished urgency, and/or straining with defecation.
Defecatory Disorders: Dyssynergic Defecation and Pelvic Floor Dysfunction
Defecatory disorders (DDs) result from alterations in the colonic-neural pathway with an unclear pathogenesis. A firm understanding of colonic physiology is necessary to identify DDs. The right colon helps to store and mix stool contents, the left colon helps add water to the stool, and the anal canal and rectum enable defecation and maintain continence. Any alteration along this physiologic pathway results in DDs.5
DDs primarily develop via maladaptive pelvic floor contraction during defecation or from muscle or nerve injury and include functional outlet obstruction, anorectal dyssynergia, and pelvic floor dysfunction. Increased resistance to defecation results from anismus, paradoxical anal sphincter contraction, or incomplete relaxation of the pelvic floor and external anal sphincter. This muscle incoordination is described as dyssynergia. DDs can involve either muscle or nerve dysfunction or a combination of the two. Reduced rectal sensation caused by reduced sensory triggers can cause stasis of stool, thus propagating the cycle of constipation. Over time, excessive straining can weaken the pelvic floor, increasing the risk of excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy.5 Thus, identification of DDs is crucial in patients with chronic constipation.
Secondary Constipation Disorders
Secondary constipation disorders are a result of an alternate process and warrant a thorough review of outpatient medications and past medical history. Figure 1 outlines the most common causes of secondary constipation, which span a wide differential.
Clinical Evaluation
The evaluation of constipation begins with a thorough history. Description of bowel habits should include frequency, duration, straining, stool consistency using a Bristol stool chart, complete vs incomplete evacuation, pain, bloating, and use of digital maneuvers (vaginal splinting or digital stool removal). One should inquire about back trauma/surgeries and obstetric history to include vaginal forceps injury or episiotomy.
With increased smartphone use, toilet time on average has increased and can contribute to maladaptive bowel habits.6 Patients may not realize they are constipated, so patient education is critical. A patient with daily bowel movements ranging between BSS type 1-6 with incomplete evacuation might complain of diarrhea but may in fact have constipation with overflow diarrhea, for example. Past medical history is also clinically relevant, as systemic conditions can cause secondary constipation. A constipated patient should also be asked what therapies he/she has tried prior to gastroenterology referral as primary care referrals for constipation account for 8 million visits to gastroenterology per year.7
While a sensitive topic, inquire about abuse history, especially in those with childhood constipation symptoms. There is a positive correlation between childhood constipation and physical, emotional, and sexual abuse and, for any number of reasons, your patient may be reluctant to share this or undergo a digital rectal exam (DRE).8 In such cases, be sensitive in asking for this history in private rather than with other family members around and always perform this exam with a chaperone present.
A detailed physical exam is an indispensable tool all gastroenterologists must master when evaluating a constipated patient. Some key exam findings include abdominal distention, high-pitched bowel sounds, and presence of a succussion splash indicating obstructive pathology. Dry skin and brittle hair indicate hypothyroidism while hypermobile joints and skin laxity suggest connective tissue disease. Finally, a physical examination is incomplete without a DRE.
DRE
DRE is an often-overlooked physical exam component which provides helpful insight that can guide management. An informed DRE can help identify structural disorders such as fissure, hemorrhoids, anorectal mass, fecal impaction, rectal prolapse, and excessive perineal descent syndrome.9 Unless contraindicated, DRE should be a standard part of the workup of a patient with chronic constipation.
Workup
Colonoscopy
The role of colonoscopy in chronic constipation is low yield and only indicated if alarm signs are present.2 When no organic causes can be identified, the patient is deemed to have a functional bowel or motility disorder leading to constipation.
Colonic Transit Time
Colonic transit time (CTT) can be evaluated by assessing the presence of radio-opaque sitz markers in the colon with an abdominal x-ray 5 days after ingestion. The presence of five or more sitz markers may indicate STC. However, this can also signal an obstructive defecatory disorder. Colon scintigraphy can determine whether there is diffuse colonic dysmotility or dysfunction in a specific segment of the colon.10
Anorectal Function Testing (AFT)
AFT can evaluate DDs, such as fecal incontinence, dyssynergic defecation, rectal sensory disorders, anorectal pain, and rectal prolapse. AFT comprises three tests: anorectal manometry (ARM), balloon expulsion test (BET), and rectal sensory testing. These assess the defecation, continence, and sensory mechanisms of the rectum, respectively.
ARM testing employs a thin, flexible probe with an attached sensor that is inserted into the rectum to measure internal and external sphincter pressures while at rest, squeezing, and bearing down to give a functional assessment of sphincter tone.11 Cough or party balloon test assesses continence and sphincter strength. Rectal sensation is assessed by inflating a balloon incrementally and asking the patient to indicate first sensation, urgency to defecate, and discomfort. If both ARM and BET are abnormal, the patient meets diagnostic criteria for dyssynergic defecation.12
Pelvic floor disorders can be further assessed by MR defecography or barium defecography. Barium defecography is the more widely available of the two. MR defecography is a dynamic study that directly assesses pelvic floor muscles and endopelvic fascia during various stages of defecation and considered superior. This testing modality can distinguish between functional causes such as dyssynergia or pelvic floor dysfunction and structural causes of obstruction such as rectocele, rectal prolapse, or rectal intussusception. MR defecography is helpful when dyssynergia is suggested by ARM with a normal BET or if there is an absent recto-anal inhibitory reflex on ARM, which may suggest rectal intussusception.
Management
CIC
Incorporating 20-30 g of total soluble fiber, such as psyllium in individuals with low dietary fiber intake is the first-line recommendation for CIC.13 If response to a trial of fiber supplementation is inadequate, over-the-counter (OTC) osmotic laxatives such as polyethylene glycol and magnesium oxide can be incorporated. In the event of failure of OTC osmotic laxatives, lactulose can be considered. Stimulant laxatives such as senna, bisacodyl, or sodium picosulfate can be added as an adjunctive measure for short periods of time, defined as daily for 4 weeks or less.
If these measures are inadequate, pharmacological therapy with secretagogues and 5HT agonists can be considered. Prucalopride, a selective agonist of serotonin 5-HT4 receptors, is approved for CIC, prescribed 2 mg daily.14 It can also be used in patients with global motility delays, such as gastroparetics with constipation. The mechanism of action of secretagogues and specific dosing of these medications are discussed in Figure 2.15 Vibrant is a non-pharmacologic, orally ingested, vibrating, and programmable capsule device that has recently received Food and Drug Administration approval for treatment of chronic constipation by stimulating the intestinal wall, thereby promoting colonic contractile activity to achieve more spontaneous bowel movements. Further studies are required to assess its efficacy.16 Additionally, if there is inadequate response to all the above, it would be prudent to evaluate for the presence of pelvic floor dysfunction as well.

IBS-C
Similar to CIC, treatment for mild IBS-C starts with osmotic laxatives with the additional component of pain control. Antispasmodics can be used to manage the abdominal pain, cramping, and spasms associated with IBS-C. Antispasmodics available in the United States include anticholinergic agents that cause smooth muscle relaxation, such as dicyclomine or hyoscyamine or direct smooth muscle relaxants such as peppermint oil.17 IBS-C patients with moderate symptoms may need escalation of therapy to secretagogues or 5HT agonists (see Figure 2). Secretagogues increase fluid retention in the colonic lumen to promote bowel movements and improve visceral hypersensitivity. Lubiprostone is an intestinal chloride channel activator, indicated only for adult women with IBS-C. Linaclotide and plecanatide are guanylate cyclase-C activators which increase intestinal chloride and bicarbonate secretion, and both are indicated in IBS-C and CIC. Tenapanor inhibits the sodium/hydrogen exchanger in the GI tract, leading to increased water secretion, and is recommended for IBS-C in adults who have failed secretagogues.
All four of these drugs can be considered for moderate to severe IBS-C symptoms. In the case of severe IBS-C symptoms, Tegaserod, a 5-HT4 receptor partial agonist has been approved in women under 65 without significant cardiovascular or cerebrovascular disease.18 Regardless of IBS-C symptom severity, persistent visceral hypersensitivity can be treated with low-dose neuromodulators.19 Figure 2 provides treatment recommendations for IBS-C based on symptom severity.
Opioid-Induced Constipation (OIC)
In patients with OIC, peripherally acting mu-opioid receptor antagonists such as methylnaltrexone and naloxegol can be beneficial where stimulant laxatives are insufficient. Additionally, lubiprostone is indicated in OIC in non-cancer patients. At present, there are no head-to-head trials comparing efficacy of these medications.
Defecatory Disorders
Biofeedback therapy is the cornerstone of treatment for dyssynergic defecation, focusing on neuromuscular training to restore a normal pattern of defecation by teaching patients to tense the abdomen and relax the pelvic floor muscles and anal sphincter. It retrains the body to coordinate abdominal, rectal, and anal muscles to achieve synchronous contraction to achieve complete evacuation. It also increases awareness and response to rectal fullness or the need to defecate.
Biofeedback makes patients aware of counterproductive subconscious actions such as contracting of their anal sphincter during defecation followed by simulated defecation training with focus on how to tighten abdominal muscles and relax pelvic floor muscles to initiate and complete defecation.20 This is performed in the office with a physiotherapist or trained nurse for at least six sessions or at home where patients are encouraged to perform the exercises for 20 minutes, twice a day. These sessions utilize tools such as manometry probes, electromyography probes, simulated balloon, or home biofeedback training devices to provide visual feedback while practicing abdominophrenic breathing. Biofeedback is particularly helpful in patients suffering from constipation. Patients with defecatory disorders can also benefit from pelvic floor physical therapy which focuses on strengthening the pelvic and puborectal muscles, external anal sphincter, and pelvic muscles. This is more useful in patients with fecal incontinence. Despite all these treatments, a subset of patients may still not respond and may qualify for surgical evaluation.
Conclusion
While constipation is seldom life-threatening, it has a negative impact on patient quality of life and poses a significant financial burden on our overall healthcare system. The complexity of this condition should be appreciated and understood in order for a complete and thorough evaluation. We trust that our practical guide should serve as a useful tool in the evaluation of a chronically constipated patient.
Dr. Salim (@hamsalim07 on X) is based in the Department of Internal Medicine, University of Texas Medical Branch, Galveston. Dr. Chowdhury (annicho.med on Instagram) is a fellow in the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas MD Anderson Cancer Center, Houston. Dr. Viswanathan (@LavanyaMD on X) is Associate Professor, University of Texas MD Anderson Cancer Center. The authors declare no conflict of interest.
References
1. Mugie S et al. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2010.12.010.
2. Almario CV et al. Gastroenterology. 2023 Dec. doi: 10.1053/j.gastro.2023.08.010.
3. Canavan C et al. Clin Epidemiol. 2014 Feb. doi: 10.2147/CLEP.S40245.
4. Rao SSC. Gastroenterol Clin North Am. 2007 Sep. doi: 10.1016/j.gtc.2007.07.013.
5. Bharucha AE et al. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
6. Cinquetti M et al. Clin Exp Pediatr. 2021 Sep. doi: 10.3345/cep.2020.01326.
7. Shah ND et al. Am J Gastroenterol. 2008 Jul. doi: 10.1111/j.1572-0241.2008.01910.x.
8. Rajindrajith S et al. J Pediatr Gastroenterol Nutr. 2014 Apr. doi: 10.1097/MPG.0000000000000249.
9. Talley NJ. Am J Gastroenterol. 2008 Apr. doi: 10.1111/j.1572-0241.2008.01832.x.
10. Maurer AH. J Nucl Med. 2015 Sep. doi: 10.2967/jnumed.113.134551.
11. Frye J et al. Am J Gastroenterol. 2024 Aug. doi: 10.14309/ajg.0000000000002670.
12. Rao SSC et al. J Neurogastroenterol Motil. 2016 Jun. doi: 10.5056/jnm16060.
13. Chang L et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.03.214.
14. Brenner DM et al. Am J Gastroenterol. 2021 Aug. doi: 10.14309/ajg.0000000000001266.
15. Chang L et al. Gastroenterology. 2022 Jul. doi: 10.1053/j.gastro.2022.04.016.
16. Rao SSC et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.02.013.
17. Lacy BE et al. Am J Gastroenterol. 2021 Jan. doi: 10.14309/ajg.0000000000001036.
18. Anderson JL et al. J Cardiovasc Pharmacol Ther. 2009 Sep. doi: 10.1177/1074248409340158.
19. Rahimi R et al. World J Gastroenterol. 2009 Apr. doi: 10.3748/wjg.15.1548.
20. Rao SSC. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2011.01.004.
Introduction
Constipation affects one in six people worldwide and accounts for one third of outpatient visits.1 Chronic constipation is defined by difficult, infrequent, and/or incomplete defecation, quantified by less than three spontaneous bowel movements per week, persisting for at least 3 months. Patients may complain of straining during defecation, incomplete evacuation, hard stools (Bristol stool scale [BSS] type 1-2), and fullness or bloating. Chronic constipation can be subclassified as either a primary or secondary disorder.1,2
Primary Constipation Disorders
Primary constipation includes disorders of the colon or anorectum. This includes irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC), slow transit constipation (STC), dyssynergic defecation, and pelvic floor disorders (see Figure 1).

IBS-C
IBS-C is a chronic disorder of the gut-brain axis with a worldwide prevalence of 1.3% and a prevalence of 6%-16% in the United States, United Kingdom, and Canada, with females more likely to seek care than males.2 The economic impact of IBS-C is estimated to be $1.5 billion–$10 billion per year in the United States alone.3 The distinguishing characteristic is abdominal pain, however IBS-C can present with a constellation of symptoms. The diagnostic paradigm has shifted from IBS being a diagnosis of exclusion to now using a positive diagnostic strategy.2 Using this Rome IV criteria, one can make the diagnosis with > 95% accuracy.2,4
CIC
CIC, previously defined as functional constipation, is a disorder defined by incomplete defecation and difficult or infrequent stool. CIC is diagnosed in patients without an underlying anatomic or structural abnormality. Rome IV Criteria helps further classify the defining characteristics of chronic idiopathic constipation.2
Slow Transit Constipation
STC is characterized by impaired colonic transit time in the absence of pelvic floor dysfunction. It presents with infrequent bowel movements, diminished urgency, and/or straining with defecation.
Defecatory Disorders: Dyssynergic Defecation and Pelvic Floor Dysfunction
Defecatory disorders (DDs) result from alterations in the colonic-neural pathway with an unclear pathogenesis. A firm understanding of colonic physiology is necessary to identify DDs. The right colon helps to store and mix stool contents, the left colon helps add water to the stool, and the anal canal and rectum enable defecation and maintain continence. Any alteration along this physiologic pathway results in DDs.5
DDs primarily develop via maladaptive pelvic floor contraction during defecation or from muscle or nerve injury and include functional outlet obstruction, anorectal dyssynergia, and pelvic floor dysfunction. Increased resistance to defecation results from anismus, paradoxical anal sphincter contraction, or incomplete relaxation of the pelvic floor and external anal sphincter. This muscle incoordination is described as dyssynergia. DDs can involve either muscle or nerve dysfunction or a combination of the two. Reduced rectal sensation caused by reduced sensory triggers can cause stasis of stool, thus propagating the cycle of constipation. Over time, excessive straining can weaken the pelvic floor, increasing the risk of excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy.5 Thus, identification of DDs is crucial in patients with chronic constipation.
Secondary Constipation Disorders
Secondary constipation disorders are a result of an alternate process and warrant a thorough review of outpatient medications and past medical history. Figure 1 outlines the most common causes of secondary constipation, which span a wide differential.
Clinical Evaluation
The evaluation of constipation begins with a thorough history. Description of bowel habits should include frequency, duration, straining, stool consistency using a Bristol stool chart, complete vs incomplete evacuation, pain, bloating, and use of digital maneuvers (vaginal splinting or digital stool removal). One should inquire about back trauma/surgeries and obstetric history to include vaginal forceps injury or episiotomy.
With increased smartphone use, toilet time on average has increased and can contribute to maladaptive bowel habits.6 Patients may not realize they are constipated, so patient education is critical. A patient with daily bowel movements ranging between BSS type 1-6 with incomplete evacuation might complain of diarrhea but may in fact have constipation with overflow diarrhea, for example. Past medical history is also clinically relevant, as systemic conditions can cause secondary constipation. A constipated patient should also be asked what therapies he/she has tried prior to gastroenterology referral as primary care referrals for constipation account for 8 million visits to gastroenterology per year.7
While a sensitive topic, inquire about abuse history, especially in those with childhood constipation symptoms. There is a positive correlation between childhood constipation and physical, emotional, and sexual abuse and, for any number of reasons, your patient may be reluctant to share this or undergo a digital rectal exam (DRE).8 In such cases, be sensitive in asking for this history in private rather than with other family members around and always perform this exam with a chaperone present.
A detailed physical exam is an indispensable tool all gastroenterologists must master when evaluating a constipated patient. Some key exam findings include abdominal distention, high-pitched bowel sounds, and presence of a succussion splash indicating obstructive pathology. Dry skin and brittle hair indicate hypothyroidism while hypermobile joints and skin laxity suggest connective tissue disease. Finally, a physical examination is incomplete without a DRE.
DRE
DRE is an often-overlooked physical exam component which provides helpful insight that can guide management. An informed DRE can help identify structural disorders such as fissure, hemorrhoids, anorectal mass, fecal impaction, rectal prolapse, and excessive perineal descent syndrome.9 Unless contraindicated, DRE should be a standard part of the workup of a patient with chronic constipation.
Workup
Colonoscopy
The role of colonoscopy in chronic constipation is low yield and only indicated if alarm signs are present.2 When no organic causes can be identified, the patient is deemed to have a functional bowel or motility disorder leading to constipation.
Colonic Transit Time
Colonic transit time (CTT) can be evaluated by assessing the presence of radio-opaque sitz markers in the colon with an abdominal x-ray 5 days after ingestion. The presence of five or more sitz markers may indicate STC. However, this can also signal an obstructive defecatory disorder. Colon scintigraphy can determine whether there is diffuse colonic dysmotility or dysfunction in a specific segment of the colon.10
Anorectal Function Testing (AFT)
AFT can evaluate DDs, such as fecal incontinence, dyssynergic defecation, rectal sensory disorders, anorectal pain, and rectal prolapse. AFT comprises three tests: anorectal manometry (ARM), balloon expulsion test (BET), and rectal sensory testing. These assess the defecation, continence, and sensory mechanisms of the rectum, respectively.
ARM testing employs a thin, flexible probe with an attached sensor that is inserted into the rectum to measure internal and external sphincter pressures while at rest, squeezing, and bearing down to give a functional assessment of sphincter tone.11 Cough or party balloon test assesses continence and sphincter strength. Rectal sensation is assessed by inflating a balloon incrementally and asking the patient to indicate first sensation, urgency to defecate, and discomfort. If both ARM and BET are abnormal, the patient meets diagnostic criteria for dyssynergic defecation.12
Pelvic floor disorders can be further assessed by MR defecography or barium defecography. Barium defecography is the more widely available of the two. MR defecography is a dynamic study that directly assesses pelvic floor muscles and endopelvic fascia during various stages of defecation and considered superior. This testing modality can distinguish between functional causes such as dyssynergia or pelvic floor dysfunction and structural causes of obstruction such as rectocele, rectal prolapse, or rectal intussusception. MR defecography is helpful when dyssynergia is suggested by ARM with a normal BET or if there is an absent recto-anal inhibitory reflex on ARM, which may suggest rectal intussusception.
Management
CIC
Incorporating 20-30 g of total soluble fiber, such as psyllium in individuals with low dietary fiber intake is the first-line recommendation for CIC.13 If response to a trial of fiber supplementation is inadequate, over-the-counter (OTC) osmotic laxatives such as polyethylene glycol and magnesium oxide can be incorporated. In the event of failure of OTC osmotic laxatives, lactulose can be considered. Stimulant laxatives such as senna, bisacodyl, or sodium picosulfate can be added as an adjunctive measure for short periods of time, defined as daily for 4 weeks or less.
If these measures are inadequate, pharmacological therapy with secretagogues and 5HT agonists can be considered. Prucalopride, a selective agonist of serotonin 5-HT4 receptors, is approved for CIC, prescribed 2 mg daily.14 It can also be used in patients with global motility delays, such as gastroparetics with constipation. The mechanism of action of secretagogues and specific dosing of these medications are discussed in Figure 2.15 Vibrant is a non-pharmacologic, orally ingested, vibrating, and programmable capsule device that has recently received Food and Drug Administration approval for treatment of chronic constipation by stimulating the intestinal wall, thereby promoting colonic contractile activity to achieve more spontaneous bowel movements. Further studies are required to assess its efficacy.16 Additionally, if there is inadequate response to all the above, it would be prudent to evaluate for the presence of pelvic floor dysfunction as well.

IBS-C
Similar to CIC, treatment for mild IBS-C starts with osmotic laxatives with the additional component of pain control. Antispasmodics can be used to manage the abdominal pain, cramping, and spasms associated with IBS-C. Antispasmodics available in the United States include anticholinergic agents that cause smooth muscle relaxation, such as dicyclomine or hyoscyamine or direct smooth muscle relaxants such as peppermint oil.17 IBS-C patients with moderate symptoms may need escalation of therapy to secretagogues or 5HT agonists (see Figure 2). Secretagogues increase fluid retention in the colonic lumen to promote bowel movements and improve visceral hypersensitivity. Lubiprostone is an intestinal chloride channel activator, indicated only for adult women with IBS-C. Linaclotide and plecanatide are guanylate cyclase-C activators which increase intestinal chloride and bicarbonate secretion, and both are indicated in IBS-C and CIC. Tenapanor inhibits the sodium/hydrogen exchanger in the GI tract, leading to increased water secretion, and is recommended for IBS-C in adults who have failed secretagogues.
All four of these drugs can be considered for moderate to severe IBS-C symptoms. In the case of severe IBS-C symptoms, Tegaserod, a 5-HT4 receptor partial agonist has been approved in women under 65 without significant cardiovascular or cerebrovascular disease.18 Regardless of IBS-C symptom severity, persistent visceral hypersensitivity can be treated with low-dose neuromodulators.19 Figure 2 provides treatment recommendations for IBS-C based on symptom severity.
Opioid-Induced Constipation (OIC)
In patients with OIC, peripherally acting mu-opioid receptor antagonists such as methylnaltrexone and naloxegol can be beneficial where stimulant laxatives are insufficient. Additionally, lubiprostone is indicated in OIC in non-cancer patients. At present, there are no head-to-head trials comparing efficacy of these medications.
Defecatory Disorders
Biofeedback therapy is the cornerstone of treatment for dyssynergic defecation, focusing on neuromuscular training to restore a normal pattern of defecation by teaching patients to tense the abdomen and relax the pelvic floor muscles and anal sphincter. It retrains the body to coordinate abdominal, rectal, and anal muscles to achieve synchronous contraction to achieve complete evacuation. It also increases awareness and response to rectal fullness or the need to defecate.
Biofeedback makes patients aware of counterproductive subconscious actions such as contracting of their anal sphincter during defecation followed by simulated defecation training with focus on how to tighten abdominal muscles and relax pelvic floor muscles to initiate and complete defecation.20 This is performed in the office with a physiotherapist or trained nurse for at least six sessions or at home where patients are encouraged to perform the exercises for 20 minutes, twice a day. These sessions utilize tools such as manometry probes, electromyography probes, simulated balloon, or home biofeedback training devices to provide visual feedback while practicing abdominophrenic breathing. Biofeedback is particularly helpful in patients suffering from constipation. Patients with defecatory disorders can also benefit from pelvic floor physical therapy which focuses on strengthening the pelvic and puborectal muscles, external anal sphincter, and pelvic muscles. This is more useful in patients with fecal incontinence. Despite all these treatments, a subset of patients may still not respond and may qualify for surgical evaluation.
Conclusion
While constipation is seldom life-threatening, it has a negative impact on patient quality of life and poses a significant financial burden on our overall healthcare system. The complexity of this condition should be appreciated and understood in order for a complete and thorough evaluation. We trust that our practical guide should serve as a useful tool in the evaluation of a chronically constipated patient.
Dr. Salim (@hamsalim07 on X) is based in the Department of Internal Medicine, University of Texas Medical Branch, Galveston. Dr. Chowdhury (annicho.med on Instagram) is a fellow in the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas MD Anderson Cancer Center, Houston. Dr. Viswanathan (@LavanyaMD on X) is Associate Professor, University of Texas MD Anderson Cancer Center. The authors declare no conflict of interest.
References
1. Mugie S et al. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2010.12.010.
2. Almario CV et al. Gastroenterology. 2023 Dec. doi: 10.1053/j.gastro.2023.08.010.
3. Canavan C et al. Clin Epidemiol. 2014 Feb. doi: 10.2147/CLEP.S40245.
4. Rao SSC. Gastroenterol Clin North Am. 2007 Sep. doi: 10.1016/j.gtc.2007.07.013.
5. Bharucha AE et al. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
6. Cinquetti M et al. Clin Exp Pediatr. 2021 Sep. doi: 10.3345/cep.2020.01326.
7. Shah ND et al. Am J Gastroenterol. 2008 Jul. doi: 10.1111/j.1572-0241.2008.01910.x.
8. Rajindrajith S et al. J Pediatr Gastroenterol Nutr. 2014 Apr. doi: 10.1097/MPG.0000000000000249.
9. Talley NJ. Am J Gastroenterol. 2008 Apr. doi: 10.1111/j.1572-0241.2008.01832.x.
10. Maurer AH. J Nucl Med. 2015 Sep. doi: 10.2967/jnumed.113.134551.
11. Frye J et al. Am J Gastroenterol. 2024 Aug. doi: 10.14309/ajg.0000000000002670.
12. Rao SSC et al. J Neurogastroenterol Motil. 2016 Jun. doi: 10.5056/jnm16060.
13. Chang L et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.03.214.
14. Brenner DM et al. Am J Gastroenterol. 2021 Aug. doi: 10.14309/ajg.0000000000001266.
15. Chang L et al. Gastroenterology. 2022 Jul. doi: 10.1053/j.gastro.2022.04.016.
16. Rao SSC et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.02.013.
17. Lacy BE et al. Am J Gastroenterol. 2021 Jan. doi: 10.14309/ajg.0000000000001036.
18. Anderson JL et al. J Cardiovasc Pharmacol Ther. 2009 Sep. doi: 10.1177/1074248409340158.
19. Rahimi R et al. World J Gastroenterol. 2009 Apr. doi: 10.3748/wjg.15.1548.
20. Rao SSC. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2011.01.004.
Integrating Artificial Intelligence Into Private Practice
In this video, Vasu Appalaneni, MD, a gastroenterologist at Dayton Gastroenterology in Beavercreek, Ohio,
In addition to her work at Dayton Gastroenterology, Dr. Appalaneni is executive vice president of clinical innovation at One GI, a gastroenterology management services organization that partners with gastroenterologists to help them manage and grow their independent gastroenterology practices. One GI is Dayton Gastroenterology’s parent company.

In this video, Vasu Appalaneni, MD, a gastroenterologist at Dayton Gastroenterology in Beavercreek, Ohio,
In addition to her work at Dayton Gastroenterology, Dr. Appalaneni is executive vice president of clinical innovation at One GI, a gastroenterology management services organization that partners with gastroenterologists to help them manage and grow their independent gastroenterology practices. One GI is Dayton Gastroenterology’s parent company.

In this video, Vasu Appalaneni, MD, a gastroenterologist at Dayton Gastroenterology in Beavercreek, Ohio,
In addition to her work at Dayton Gastroenterology, Dr. Appalaneni is executive vice president of clinical innovation at One GI, a gastroenterology management services organization that partners with gastroenterologists to help them manage and grow their independent gastroenterology practices. One GI is Dayton Gastroenterology’s parent company.

Negotiating for a Successful Career in Private Practice Gastroenterology
In this video, Aja McCutchen, MD, of Atlanta Gastroenterology Associates in Georgia, discusses why she chose to enter private practice gastroenterology, and identifies some key considerations on the road to a successful career.
Dr. McCutchen is vice chair of the AGA Research Foundation. She has no financial conflicts relative to the topics in this video.

In this video, Aja McCutchen, MD, of Atlanta Gastroenterology Associates in Georgia, discusses why she chose to enter private practice gastroenterology, and identifies some key considerations on the road to a successful career.
Dr. McCutchen is vice chair of the AGA Research Foundation. She has no financial conflicts relative to the topics in this video.

In this video, Aja McCutchen, MD, of Atlanta Gastroenterology Associates in Georgia, discusses why she chose to enter private practice gastroenterology, and identifies some key considerations on the road to a successful career.
Dr. McCutchen is vice chair of the AGA Research Foundation. She has no financial conflicts relative to the topics in this video.

How to Discuss Lifestyle Modifications in MASLD
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).
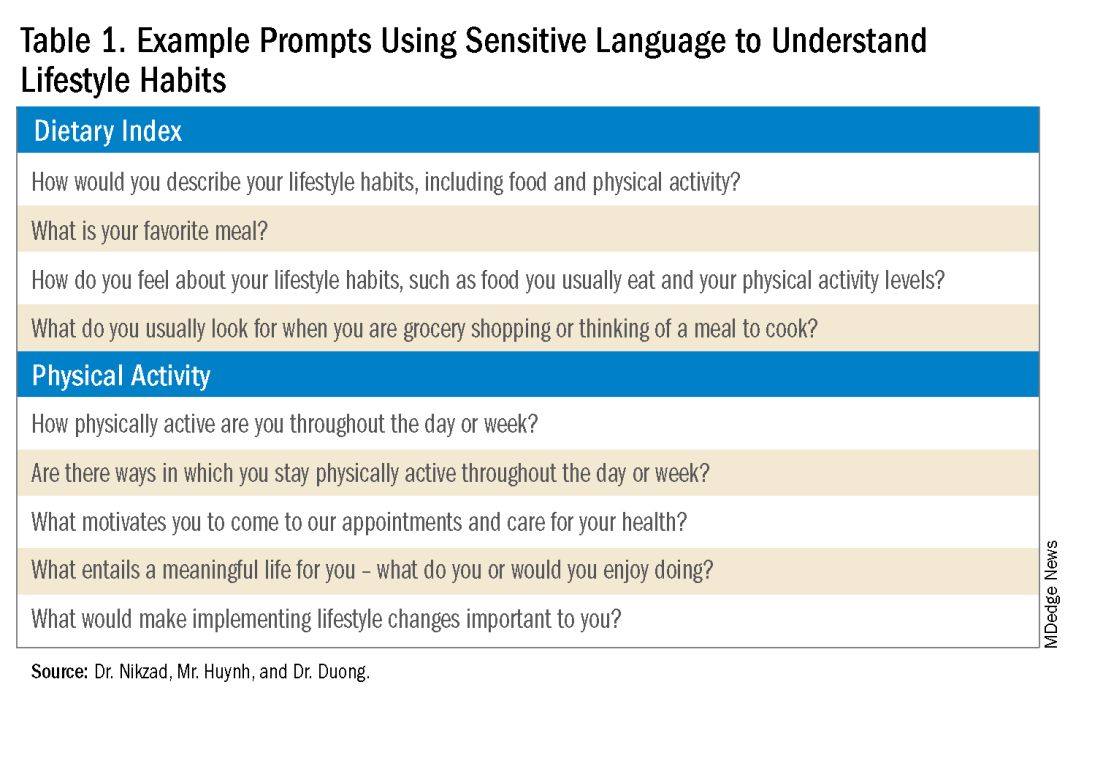
Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.
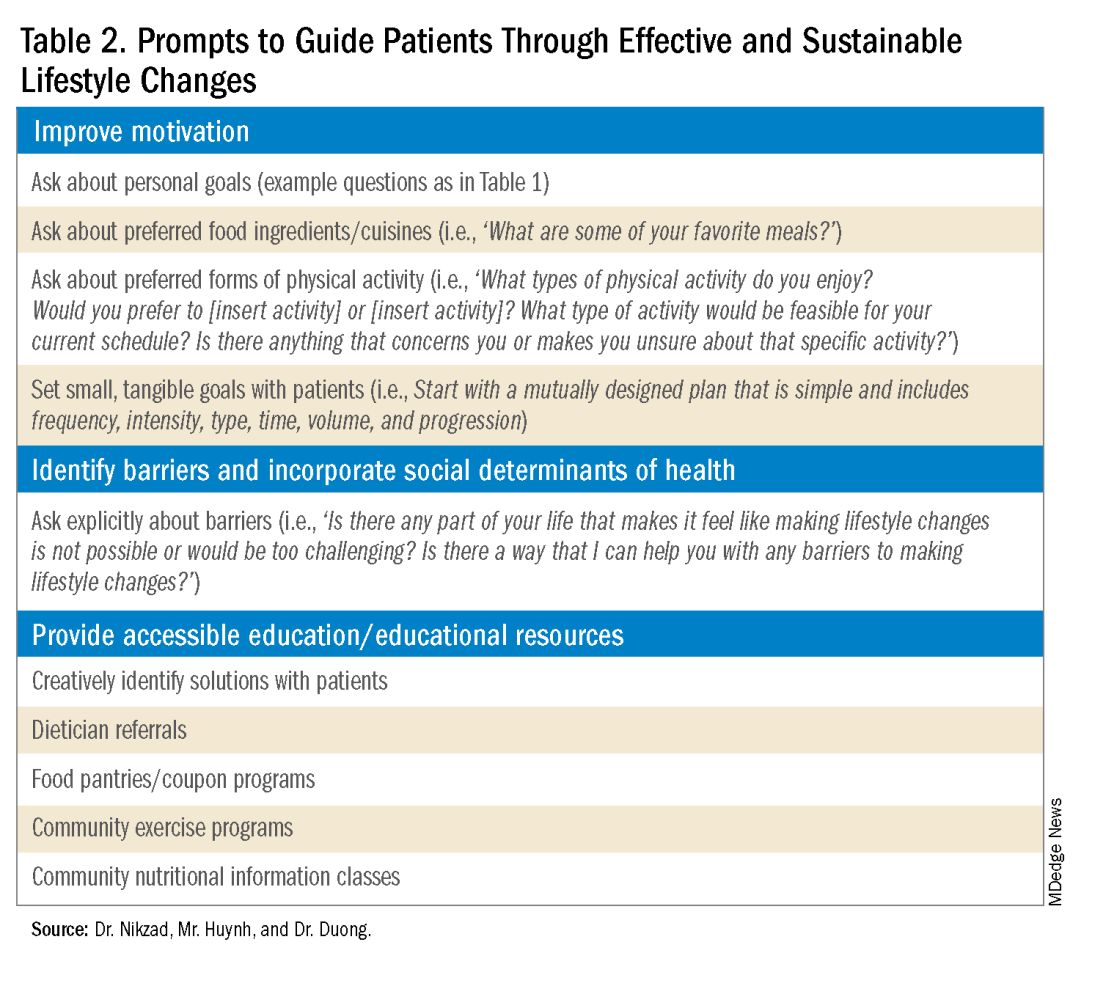
Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).

Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.

Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).

Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.

Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Financial Empowerment Journey
Dear Friends,
One of the challenges I faced during training was managing my life outside of work. Many astute trainees started their financial empowerment journey early. However, I was too overwhelmed with what I did not know (the financial world) and just avoided it. Over the last year, I finally decided to embrace my lack of knowledge and find the support of experts, just as we would in medicine. A lot of questions from my journey translated into several articles in the “Finance” section of The New Gastroenterologist, so I encourage those who need guidance on embarking on their financial journeys to explore that section!
In the “In Focus” section, Dr. Patrick Chang, Dr. Supisara Tintara, and Dr. Jennifer Phan – all from the University of Southern California – review diagnostic modalities to assess gastroesophageal reflux disease with an emphasis on medical, endoscopic, and surgical managements.
With the rise in metabolic dysfunction–associated steatotic liver disease (MASLD), patient education is starting in the primary care and gastroenterologist’s office. Dr. Newsha Nikzad, medical student Daniel Huynh, and Dr. Nikki Duong share their approach to ask effectively about and communicate lifestyle modifications, with examples of using sensitive language and prompts to help guide patients, in the “Short Clinical Review” section.
The “Finance” section highlights the ins and outs of a physician mortgage loan and additional information for first time home buyers, reviewed by John G. Kelley II, a physician mortgage specialist and vice president of mortgage lending at Arvest Bank.
Lastly, in the “Early Career” section, Dr. Neil Gupta shares his experiences of transitioning from academic medicine to building a private practice group. He reflects on lessons learned from the first year after establishing his practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The first proton pump inhibitor was omeprazole, discovered 45 years ago in 1979 in Sweden, and clinically available in the United States only 36 years ago in 1988.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
One of the challenges I faced during training was managing my life outside of work. Many astute trainees started their financial empowerment journey early. However, I was too overwhelmed with what I did not know (the financial world) and just avoided it. Over the last year, I finally decided to embrace my lack of knowledge and find the support of experts, just as we would in medicine. A lot of questions from my journey translated into several articles in the “Finance” section of The New Gastroenterologist, so I encourage those who need guidance on embarking on their financial journeys to explore that section!
In the “In Focus” section, Dr. Patrick Chang, Dr. Supisara Tintara, and Dr. Jennifer Phan – all from the University of Southern California – review diagnostic modalities to assess gastroesophageal reflux disease with an emphasis on medical, endoscopic, and surgical managements.
With the rise in metabolic dysfunction–associated steatotic liver disease (MASLD), patient education is starting in the primary care and gastroenterologist’s office. Dr. Newsha Nikzad, medical student Daniel Huynh, and Dr. Nikki Duong share their approach to ask effectively about and communicate lifestyle modifications, with examples of using sensitive language and prompts to help guide patients, in the “Short Clinical Review” section.
The “Finance” section highlights the ins and outs of a physician mortgage loan and additional information for first time home buyers, reviewed by John G. Kelley II, a physician mortgage specialist and vice president of mortgage lending at Arvest Bank.
Lastly, in the “Early Career” section, Dr. Neil Gupta shares his experiences of transitioning from academic medicine to building a private practice group. He reflects on lessons learned from the first year after establishing his practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The first proton pump inhibitor was omeprazole, discovered 45 years ago in 1979 in Sweden, and clinically available in the United States only 36 years ago in 1988.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
One of the challenges I faced during training was managing my life outside of work. Many astute trainees started their financial empowerment journey early. However, I was too overwhelmed with what I did not know (the financial world) and just avoided it. Over the last year, I finally decided to embrace my lack of knowledge and find the support of experts, just as we would in medicine. A lot of questions from my journey translated into several articles in the “Finance” section of The New Gastroenterologist, so I encourage those who need guidance on embarking on their financial journeys to explore that section!
In the “In Focus” section, Dr. Patrick Chang, Dr. Supisara Tintara, and Dr. Jennifer Phan – all from the University of Southern California – review diagnostic modalities to assess gastroesophageal reflux disease with an emphasis on medical, endoscopic, and surgical managements.
With the rise in metabolic dysfunction–associated steatotic liver disease (MASLD), patient education is starting in the primary care and gastroenterologist’s office. Dr. Newsha Nikzad, medical student Daniel Huynh, and Dr. Nikki Duong share their approach to ask effectively about and communicate lifestyle modifications, with examples of using sensitive language and prompts to help guide patients, in the “Short Clinical Review” section.
The “Finance” section highlights the ins and outs of a physician mortgage loan and additional information for first time home buyers, reviewed by John G. Kelley II, a physician mortgage specialist and vice president of mortgage lending at Arvest Bank.
Lastly, in the “Early Career” section, Dr. Neil Gupta shares his experiences of transitioning from academic medicine to building a private practice group. He reflects on lessons learned from the first year after establishing his practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The first proton pump inhibitor was omeprazole, discovered 45 years ago in 1979 in Sweden, and clinically available in the United States only 36 years ago in 1988.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis














