User login
AGA News
New patient care resource: NASH Clinical Care Pathway
The American Gastroenterological Association – in collaboration with seven professional associations – assembled a multidisciplinary taskforce of 15 experts to develop an action plan to develop a nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) Clinical Care Pathway providing practical guidance across multiple disciplines of care. The guidance ranges from screening and diagnosis to management of individuals with NAFLD and NASH, as well as facilitating value-based, efficient, and safe care that is consistent with evidence-based guidelines.
This clinical care pathway is intended to be applicable in any setting in which care for patients with NAFLD is provided, including primary care, endocrine, obesity medicine, and gastroenterology practices.
Read the special report: Clinical Care Pathway for the Risk Stratification and Management of Patients with Nonalcoholic Fatty Liver Disease.
To learn more about the development of this publication, visit NASH.gastro.org.
GI societies push CMS for payment rules favorable for practices
As part of our longstanding collaboration and ongoing efforts on critical policy and payment issues impacting GI clinicians, AGA, the American College of Gastroenterology, and American Society for Gastrointestinal Endoscopy submitted comments on proposed 2022 Medicare payments to physicians, ambulatory surgery centers (ASCs), and hospital outpatient departments to the CMS. We advocated for the following:
Increased and more accurate valuation for peroral endoscopic myotomy (POEM) and capsule endoscopy services.
Continued flexibility and payment parity for telehealth and telephone services.
Elimination of the secondary scalar for ASCs, which contributes to the widening differential in payments to ASCs compared to the hospital outpatient department.
You can access our letter here.
New patient care resource: NASH Clinical Care Pathway
The American Gastroenterological Association – in collaboration with seven professional associations – assembled a multidisciplinary taskforce of 15 experts to develop an action plan to develop a nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) Clinical Care Pathway providing practical guidance across multiple disciplines of care. The guidance ranges from screening and diagnosis to management of individuals with NAFLD and NASH, as well as facilitating value-based, efficient, and safe care that is consistent with evidence-based guidelines.
This clinical care pathway is intended to be applicable in any setting in which care for patients with NAFLD is provided, including primary care, endocrine, obesity medicine, and gastroenterology practices.
Read the special report: Clinical Care Pathway for the Risk Stratification and Management of Patients with Nonalcoholic Fatty Liver Disease.
To learn more about the development of this publication, visit NASH.gastro.org.
GI societies push CMS for payment rules favorable for practices
As part of our longstanding collaboration and ongoing efforts on critical policy and payment issues impacting GI clinicians, AGA, the American College of Gastroenterology, and American Society for Gastrointestinal Endoscopy submitted comments on proposed 2022 Medicare payments to physicians, ambulatory surgery centers (ASCs), and hospital outpatient departments to the CMS. We advocated for the following:
Increased and more accurate valuation for peroral endoscopic myotomy (POEM) and capsule endoscopy services.
Continued flexibility and payment parity for telehealth and telephone services.
Elimination of the secondary scalar for ASCs, which contributes to the widening differential in payments to ASCs compared to the hospital outpatient department.
You can access our letter here.
New patient care resource: NASH Clinical Care Pathway
The American Gastroenterological Association – in collaboration with seven professional associations – assembled a multidisciplinary taskforce of 15 experts to develop an action plan to develop a nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) Clinical Care Pathway providing practical guidance across multiple disciplines of care. The guidance ranges from screening and diagnosis to management of individuals with NAFLD and NASH, as well as facilitating value-based, efficient, and safe care that is consistent with evidence-based guidelines.
This clinical care pathway is intended to be applicable in any setting in which care for patients with NAFLD is provided, including primary care, endocrine, obesity medicine, and gastroenterology practices.
Read the special report: Clinical Care Pathway for the Risk Stratification and Management of Patients with Nonalcoholic Fatty Liver Disease.
To learn more about the development of this publication, visit NASH.gastro.org.
GI societies push CMS for payment rules favorable for practices
As part of our longstanding collaboration and ongoing efforts on critical policy and payment issues impacting GI clinicians, AGA, the American College of Gastroenterology, and American Society for Gastrointestinal Endoscopy submitted comments on proposed 2022 Medicare payments to physicians, ambulatory surgery centers (ASCs), and hospital outpatient departments to the CMS. We advocated for the following:
Increased and more accurate valuation for peroral endoscopic myotomy (POEM) and capsule endoscopy services.
Continued flexibility and payment parity for telehealth and telephone services.
Elimination of the secondary scalar for ASCs, which contributes to the widening differential in payments to ASCs compared to the hospital outpatient department.
You can access our letter here.
November 2021 – ICYMI
Gastroenterology
August 2021
How to perform a high-quality endoscopic submucosal dissection
Saito Y et al. Gastroenterology. 2021 Aug;161(2):405-10. doi: 10.1053/j.gastro.2021.05.051.
Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori infection: A network meta-analysis
Rokkas T et al. Gastroenterology. 2021 Aug;161(2):495-507.e4. doi: 10.1053/j.gastro.2021.04.012.
The optimal age to stop endoscopic surveillance of patients with Barrett’s esophagus based on sex and comorbidity: A comparative cost-effectiveness analysis
Omidvari AH et al. Gastroenterology. 2021 Aug;161(2):487-94.e4. doi: 10.1053/j.gastro.2021.05.003.
Development and validation of test for “leaky gut” small intestinal and colonic permeability using sugars in healthy adults
Khoshbin K et al. Gastroenterology. 2021 Aug;161(2):463-75.e13. doi: 10.1053/j.gastro.2021.04.020.
September 2021
Pregnancy and the working gastroenterologist: Perceptions, realities, and systemic challenges
David YN et al. Gastroenterology. 2021 Sep;161(3):756-60. doi: 10.1053/j.gastro.2021.05.053.
New drugs on the horizon for functional and motility gastrointestinal disorders
Camilleri M. Gastroenterology. 2021 Sep;161(3):761-4. doi: 10.1053/j.gastro.2021.04.079.
A randomized trial comparing the specific carbohydrate diet to a Mediterranean diet in adults with Crohn’s disease
Lewis JD et al. Gastroenterology. 2021 Sep;161(3):837-52.e9. doi: 10.1053/j.gastro.2021.05.047.
How to promote career advancement and gender equity for women in gastroenterology: a multifaceted approach
Chua SG et al. Gastroenterology. 2021 Sep;161(3):792-7. doi: 10.1053/j.gastro.2021.06.057.
October 2021
How to approach a patient with difficult-to-treat IBS
Chang L. Gastroenterology. 2021 Oct;161(4):1092-8.e3. doi: 10.1053/j.gastro.2021.07.034.
Early-age onset colorectal neoplasia in average-risk individuals undergoing screening colonoscopy: A systematic review and meta-analysis
Kolb JM et al. Gastroenterology. 2021 Oct;161(4):1145-55.e12. doi: 10.1053/j.gastro.2021.06.006.
Adalimumab subcutaneous in participants with ulcerative colitis (VARSITY)
Peyrin-Biroulet L et al. Gastroenterology. 2021 Oct;161(4):1156-67.e3. doi: 10.1053/j.gastro.2021.06.015.
Extraintestinal manifestations of inflammatory bowel disease: Current concepts, treatment, and implications for disease management
Rogler G et al. Gastroenterology. 2021 Oct;161(4):1118-32. doi: 10.1053/j.gastro.2021.07.042.
Clinical Gastroenterology and Hepatology
August 2021
Health equity and telemedicine in gastroenterology and hepatology
Wegermann K et al. Clin Gastroenterol Hepatol. 2021 Aug;19(8):1516-9. doi: 10.1016/j.cgh.2021.04.024.
AGA Clinical Practice Update on evaluation and management of early complications after bariatric/metabolic surgery: Expert review
Kumbhari V et al. Clin Gastroenterol Hepatol. 2021 Aug;19(8):1531-7. doi: 10.1016/j.cgh.2021.03.020.
Clinical, pathology, genetic, and molecular features of colorectal tumors in adolescents and adults 25 years or younger
de Voer RM et al. Clin Gastroenterol Hepatol. 2021 Aug;19(8):1642-51.e8. doi: 10.1016/j.cgh.2020.06.034.
Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis
Deepak P et al. Clin Gastroenterol Hepatol. 2021 Aug;19(8):1592-601.e3. doi: 10.1016/j.cgh.2020.06.050.
September 2021
Association of adenoma detection rate and adenoma characteristics with colorectal cancer mortality after screening colonoscopy
Waldmann E et al. Clin Gastroenterol Hepatol. 2021 Sep;19(9):1890-8. doi: 10.1016/j.cgh.2021.04.023.
Prevalence and characteristics of abdominal pain in the United States
Lakhoo K et al. Clin Gastroenterol Hepatol. 2021 Sep;19(9):1864-72.e5. doi: 10.1016/j.cgh.2020.06.065.
Model using clinical and endoscopic characteristics identifies patients at risk for eosinophilic esophagitis according to updated diagnostic guidelines
Cotton CC et al. Clin Gastroenterol Hepatol. 2021 Sep;19(9):1824-34.e2. doi: 10.1016/j.cgh.2020.06.068.
October 2021
A high-yield approach to effective endoscopy teaching and assessment
Huang HZ et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):1999-2001. doi: 10.1016/j.cgh.2021.07.013.
2021 E/M code changes: Forecasted impacts to gastroenterology practices
Francis DL et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):2002-5. doi: 10.1016/j.cgh.2021.07.008.
You can’t have one without the other: Innovation and ethical dilemmas in gastroenterology and hepatology
Couri T et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):2015-9. doi: 10.1016/j.cgh.2020.05.024.
Psychiatric disorders in patients with a diagnosis of celiac disease during childhood from 1973 to 2016
Lebwohl B et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):2093-101.e13. doi: 10.1016/j.cgh.2020.08.018.
Mast cell and eosinophil counts in gastric and duodenal biopsy specimens from patients with and without eosinophilic gastroenteritis
Reed CC et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):2102-2111. doi: 10.1016/j.cgh.2020.08.013.
Cellular and Molecular Gastroenterology and Hepatology
Sex differences in the exocrine pancreas and associated diseases
Wang M et al. Cell Mol Gastroenterol Hepatol. 2021;12(2):427-41. doi: 10.1016/j.jcmgh.2021.04.005.
Mesenteric neural crest cells are the embryological basis of skip segment Hirschsprung’s disease
Yu Q et al. Cell Mol Gastroenterol Hepatol. 2021;12(1):1-24. doi: 10.1016/j.jcmgh.2020.12.010.
Helicobacter pylori–induced rev-erbα fosters gastric bacteria colonization by impairing host innate and adaptive defense
Mao MY et al. Cell Mol Gastroenterol Hepatol. 2021;12(2):395-425. doi: 10.1016/j.jcmgh.2021.02.013.
Techniques and Innovations in Gastrointestinal Endoscopy
Staying (mentally) healthy: The impact of COVID-19 on personal and professional lives
Alkandari A et al. Tech Innov Gastrointest Endosc. 2021;23(2):199-206. doi: 10.1016/j.tige.2021.01.003.
Establishing new endoscopic programs in the unit pitfalls and tips for success
Siddiqui UD. Tech Innov Gastrointest Endosc. 2021;23(3):263-7. doi: 10.1016/j.tige.2021.03.002.
Chief of endoscopy: Specific challenges to leading the team and running the unit
Michelle A. Anderson MA et al. Tech Innov Gastrointest Endosc. 2021;23(3):249-55. doi: 10.1016/j.tige.2021.03.004.
Safety in endoscopy for patients and healthcare workers During the COVID-19 pandemic
Lui RN. Tech Innov Gastrointest Endosc. 2021;23(2):170-178. doi: 10.1016/j.tige.2020.10.004.
Gastroenterology
August 2021
How to perform a high-quality endoscopic submucosal dissection
Saito Y et al. Gastroenterology. 2021 Aug;161(2):405-10. doi: 10.1053/j.gastro.2021.05.051.
Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori infection: A network meta-analysis
Rokkas T et al. Gastroenterology. 2021 Aug;161(2):495-507.e4. doi: 10.1053/j.gastro.2021.04.012.
The optimal age to stop endoscopic surveillance of patients with Barrett’s esophagus based on sex and comorbidity: A comparative cost-effectiveness analysis
Omidvari AH et al. Gastroenterology. 2021 Aug;161(2):487-94.e4. doi: 10.1053/j.gastro.2021.05.003.
Development and validation of test for “leaky gut” small intestinal and colonic permeability using sugars in healthy adults
Khoshbin K et al. Gastroenterology. 2021 Aug;161(2):463-75.e13. doi: 10.1053/j.gastro.2021.04.020.
September 2021
Pregnancy and the working gastroenterologist: Perceptions, realities, and systemic challenges
David YN et al. Gastroenterology. 2021 Sep;161(3):756-60. doi: 10.1053/j.gastro.2021.05.053.
New drugs on the horizon for functional and motility gastrointestinal disorders
Camilleri M. Gastroenterology. 2021 Sep;161(3):761-4. doi: 10.1053/j.gastro.2021.04.079.
A randomized trial comparing the specific carbohydrate diet to a Mediterranean diet in adults with Crohn’s disease
Lewis JD et al. Gastroenterology. 2021 Sep;161(3):837-52.e9. doi: 10.1053/j.gastro.2021.05.047.
How to promote career advancement and gender equity for women in gastroenterology: a multifaceted approach
Chua SG et al. Gastroenterology. 2021 Sep;161(3):792-7. doi: 10.1053/j.gastro.2021.06.057.
October 2021
How to approach a patient with difficult-to-treat IBS
Chang L. Gastroenterology. 2021 Oct;161(4):1092-8.e3. doi: 10.1053/j.gastro.2021.07.034.
Early-age onset colorectal neoplasia in average-risk individuals undergoing screening colonoscopy: A systematic review and meta-analysis
Kolb JM et al. Gastroenterology. 2021 Oct;161(4):1145-55.e12. doi: 10.1053/j.gastro.2021.06.006.
Adalimumab subcutaneous in participants with ulcerative colitis (VARSITY)
Peyrin-Biroulet L et al. Gastroenterology. 2021 Oct;161(4):1156-67.e3. doi: 10.1053/j.gastro.2021.06.015.
Extraintestinal manifestations of inflammatory bowel disease: Current concepts, treatment, and implications for disease management
Rogler G et al. Gastroenterology. 2021 Oct;161(4):1118-32. doi: 10.1053/j.gastro.2021.07.042.
Clinical Gastroenterology and Hepatology
August 2021
Health equity and telemedicine in gastroenterology and hepatology
Wegermann K et al. Clin Gastroenterol Hepatol. 2021 Aug;19(8):1516-9. doi: 10.1016/j.cgh.2021.04.024.
AGA Clinical Practice Update on evaluation and management of early complications after bariatric/metabolic surgery: Expert review
Kumbhari V et al. Clin Gastroenterol Hepatol. 2021 Aug;19(8):1531-7. doi: 10.1016/j.cgh.2021.03.020.
Clinical, pathology, genetic, and molecular features of colorectal tumors in adolescents and adults 25 years or younger
de Voer RM et al. Clin Gastroenterol Hepatol. 2021 Aug;19(8):1642-51.e8. doi: 10.1016/j.cgh.2020.06.034.
Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis
Deepak P et al. Clin Gastroenterol Hepatol. 2021 Aug;19(8):1592-601.e3. doi: 10.1016/j.cgh.2020.06.050.
September 2021
Association of adenoma detection rate and adenoma characteristics with colorectal cancer mortality after screening colonoscopy
Waldmann E et al. Clin Gastroenterol Hepatol. 2021 Sep;19(9):1890-8. doi: 10.1016/j.cgh.2021.04.023.
Prevalence and characteristics of abdominal pain in the United States
Lakhoo K et al. Clin Gastroenterol Hepatol. 2021 Sep;19(9):1864-72.e5. doi: 10.1016/j.cgh.2020.06.065.
Model using clinical and endoscopic characteristics identifies patients at risk for eosinophilic esophagitis according to updated diagnostic guidelines
Cotton CC et al. Clin Gastroenterol Hepatol. 2021 Sep;19(9):1824-34.e2. doi: 10.1016/j.cgh.2020.06.068.
October 2021
A high-yield approach to effective endoscopy teaching and assessment
Huang HZ et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):1999-2001. doi: 10.1016/j.cgh.2021.07.013.
2021 E/M code changes: Forecasted impacts to gastroenterology practices
Francis DL et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):2002-5. doi: 10.1016/j.cgh.2021.07.008.
You can’t have one without the other: Innovation and ethical dilemmas in gastroenterology and hepatology
Couri T et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):2015-9. doi: 10.1016/j.cgh.2020.05.024.
Psychiatric disorders in patients with a diagnosis of celiac disease during childhood from 1973 to 2016
Lebwohl B et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):2093-101.e13. doi: 10.1016/j.cgh.2020.08.018.
Mast cell and eosinophil counts in gastric and duodenal biopsy specimens from patients with and without eosinophilic gastroenteritis
Reed CC et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):2102-2111. doi: 10.1016/j.cgh.2020.08.013.
Cellular and Molecular Gastroenterology and Hepatology
Sex differences in the exocrine pancreas and associated diseases
Wang M et al. Cell Mol Gastroenterol Hepatol. 2021;12(2):427-41. doi: 10.1016/j.jcmgh.2021.04.005.
Mesenteric neural crest cells are the embryological basis of skip segment Hirschsprung’s disease
Yu Q et al. Cell Mol Gastroenterol Hepatol. 2021;12(1):1-24. doi: 10.1016/j.jcmgh.2020.12.010.
Helicobacter pylori–induced rev-erbα fosters gastric bacteria colonization by impairing host innate and adaptive defense
Mao MY et al. Cell Mol Gastroenterol Hepatol. 2021;12(2):395-425. doi: 10.1016/j.jcmgh.2021.02.013.
Techniques and Innovations in Gastrointestinal Endoscopy
Staying (mentally) healthy: The impact of COVID-19 on personal and professional lives
Alkandari A et al. Tech Innov Gastrointest Endosc. 2021;23(2):199-206. doi: 10.1016/j.tige.2021.01.003.
Establishing new endoscopic programs in the unit pitfalls and tips for success
Siddiqui UD. Tech Innov Gastrointest Endosc. 2021;23(3):263-7. doi: 10.1016/j.tige.2021.03.002.
Chief of endoscopy: Specific challenges to leading the team and running the unit
Michelle A. Anderson MA et al. Tech Innov Gastrointest Endosc. 2021;23(3):249-55. doi: 10.1016/j.tige.2021.03.004.
Safety in endoscopy for patients and healthcare workers During the COVID-19 pandemic
Lui RN. Tech Innov Gastrointest Endosc. 2021;23(2):170-178. doi: 10.1016/j.tige.2020.10.004.
Gastroenterology
August 2021
How to perform a high-quality endoscopic submucosal dissection
Saito Y et al. Gastroenterology. 2021 Aug;161(2):405-10. doi: 10.1053/j.gastro.2021.05.051.
Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori infection: A network meta-analysis
Rokkas T et al. Gastroenterology. 2021 Aug;161(2):495-507.e4. doi: 10.1053/j.gastro.2021.04.012.
The optimal age to stop endoscopic surveillance of patients with Barrett’s esophagus based on sex and comorbidity: A comparative cost-effectiveness analysis
Omidvari AH et al. Gastroenterology. 2021 Aug;161(2):487-94.e4. doi: 10.1053/j.gastro.2021.05.003.
Development and validation of test for “leaky gut” small intestinal and colonic permeability using sugars in healthy adults
Khoshbin K et al. Gastroenterology. 2021 Aug;161(2):463-75.e13. doi: 10.1053/j.gastro.2021.04.020.
September 2021
Pregnancy and the working gastroenterologist: Perceptions, realities, and systemic challenges
David YN et al. Gastroenterology. 2021 Sep;161(3):756-60. doi: 10.1053/j.gastro.2021.05.053.
New drugs on the horizon for functional and motility gastrointestinal disorders
Camilleri M. Gastroenterology. 2021 Sep;161(3):761-4. doi: 10.1053/j.gastro.2021.04.079.
A randomized trial comparing the specific carbohydrate diet to a Mediterranean diet in adults with Crohn’s disease
Lewis JD et al. Gastroenterology. 2021 Sep;161(3):837-52.e9. doi: 10.1053/j.gastro.2021.05.047.
How to promote career advancement and gender equity for women in gastroenterology: a multifaceted approach
Chua SG et al. Gastroenterology. 2021 Sep;161(3):792-7. doi: 10.1053/j.gastro.2021.06.057.
October 2021
How to approach a patient with difficult-to-treat IBS
Chang L. Gastroenterology. 2021 Oct;161(4):1092-8.e3. doi: 10.1053/j.gastro.2021.07.034.
Early-age onset colorectal neoplasia in average-risk individuals undergoing screening colonoscopy: A systematic review and meta-analysis
Kolb JM et al. Gastroenterology. 2021 Oct;161(4):1145-55.e12. doi: 10.1053/j.gastro.2021.06.006.
Adalimumab subcutaneous in participants with ulcerative colitis (VARSITY)
Peyrin-Biroulet L et al. Gastroenterology. 2021 Oct;161(4):1156-67.e3. doi: 10.1053/j.gastro.2021.06.015.
Extraintestinal manifestations of inflammatory bowel disease: Current concepts, treatment, and implications for disease management
Rogler G et al. Gastroenterology. 2021 Oct;161(4):1118-32. doi: 10.1053/j.gastro.2021.07.042.
Clinical Gastroenterology and Hepatology
August 2021
Health equity and telemedicine in gastroenterology and hepatology
Wegermann K et al. Clin Gastroenterol Hepatol. 2021 Aug;19(8):1516-9. doi: 10.1016/j.cgh.2021.04.024.
AGA Clinical Practice Update on evaluation and management of early complications after bariatric/metabolic surgery: Expert review
Kumbhari V et al. Clin Gastroenterol Hepatol. 2021 Aug;19(8):1531-7. doi: 10.1016/j.cgh.2021.03.020.
Clinical, pathology, genetic, and molecular features of colorectal tumors in adolescents and adults 25 years or younger
de Voer RM et al. Clin Gastroenterol Hepatol. 2021 Aug;19(8):1642-51.e8. doi: 10.1016/j.cgh.2020.06.034.
Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis
Deepak P et al. Clin Gastroenterol Hepatol. 2021 Aug;19(8):1592-601.e3. doi: 10.1016/j.cgh.2020.06.050.
September 2021
Association of adenoma detection rate and adenoma characteristics with colorectal cancer mortality after screening colonoscopy
Waldmann E et al. Clin Gastroenterol Hepatol. 2021 Sep;19(9):1890-8. doi: 10.1016/j.cgh.2021.04.023.
Prevalence and characteristics of abdominal pain in the United States
Lakhoo K et al. Clin Gastroenterol Hepatol. 2021 Sep;19(9):1864-72.e5. doi: 10.1016/j.cgh.2020.06.065.
Model using clinical and endoscopic characteristics identifies patients at risk for eosinophilic esophagitis according to updated diagnostic guidelines
Cotton CC et al. Clin Gastroenterol Hepatol. 2021 Sep;19(9):1824-34.e2. doi: 10.1016/j.cgh.2020.06.068.
October 2021
A high-yield approach to effective endoscopy teaching and assessment
Huang HZ et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):1999-2001. doi: 10.1016/j.cgh.2021.07.013.
2021 E/M code changes: Forecasted impacts to gastroenterology practices
Francis DL et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):2002-5. doi: 10.1016/j.cgh.2021.07.008.
You can’t have one without the other: Innovation and ethical dilemmas in gastroenterology and hepatology
Couri T et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):2015-9. doi: 10.1016/j.cgh.2020.05.024.
Psychiatric disorders in patients with a diagnosis of celiac disease during childhood from 1973 to 2016
Lebwohl B et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):2093-101.e13. doi: 10.1016/j.cgh.2020.08.018.
Mast cell and eosinophil counts in gastric and duodenal biopsy specimens from patients with and without eosinophilic gastroenteritis
Reed CC et al. Clin Gastroenterol Hepatol. 2021 Oct;19(10):2102-2111. doi: 10.1016/j.cgh.2020.08.013.
Cellular and Molecular Gastroenterology and Hepatology
Sex differences in the exocrine pancreas and associated diseases
Wang M et al. Cell Mol Gastroenterol Hepatol. 2021;12(2):427-41. doi: 10.1016/j.jcmgh.2021.04.005.
Mesenteric neural crest cells are the embryological basis of skip segment Hirschsprung’s disease
Yu Q et al. Cell Mol Gastroenterol Hepatol. 2021;12(1):1-24. doi: 10.1016/j.jcmgh.2020.12.010.
Helicobacter pylori–induced rev-erbα fosters gastric bacteria colonization by impairing host innate and adaptive defense
Mao MY et al. Cell Mol Gastroenterol Hepatol. 2021;12(2):395-425. doi: 10.1016/j.jcmgh.2021.02.013.
Techniques and Innovations in Gastrointestinal Endoscopy
Staying (mentally) healthy: The impact of COVID-19 on personal and professional lives
Alkandari A et al. Tech Innov Gastrointest Endosc. 2021;23(2):199-206. doi: 10.1016/j.tige.2021.01.003.
Establishing new endoscopic programs in the unit pitfalls and tips for success
Siddiqui UD. Tech Innov Gastrointest Endosc. 2021;23(3):263-7. doi: 10.1016/j.tige.2021.03.002.
Chief of endoscopy: Specific challenges to leading the team and running the unit
Michelle A. Anderson MA et al. Tech Innov Gastrointest Endosc. 2021;23(3):249-55. doi: 10.1016/j.tige.2021.03.004.
Safety in endoscopy for patients and healthcare workers During the COVID-19 pandemic
Lui RN. Tech Innov Gastrointest Endosc. 2021;23(2):170-178. doi: 10.1016/j.tige.2020.10.004.
Developing a career in medical pancreatology: An emerging postfellowship career path
Although described by the Greek physician Herophilos around 300 B.C., it was not until the 19th century that enzymes began to be isolated from pancreatic secretions and their digestive action described, and not until early in the 20th century that Banting, Macleod, and Best received the Nobel prize for purifying insulin from the pancreata of dogs. For centuries in between, the pancreas was considered to be just a ‘beautiful piece of flesh’ (kallikreas), the main role of which was to protect the blood vessels in the abdomen and to serve as a cushion to the stomach.1 Certainly, the pancreas has come a long way since then but, like most other organs in the body, is oft ignored until it develops issues.
Like many other disorders in gastroenterology, pancreatic disorders were historically approached as mechanical or “plumbing” issues. As modern technology and innovation percolated through the world of endoscopy, a wide array of state-of-the-art tools were devised. Availability of newer “toys” and development of newer techniques also means that an ever-increasing curriculum has been squeezed into a generally single year of therapeutic endoscopy training, such that trainees can no longer limit themselves to learning only endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) or intervening on pancreatic disease alone. Modern, subspecialized approaches to disease and economic considerations often dictate that the therapeutic endoscopist of today must perform a wide range of procedures besides ERCP and EUS, such as advanced resection using endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), per-oral endoscopic myotomy (POEM), endoscopic bariatric procedures, and newer techniques and acronyms that continue to evolve on a regular basis. This leaves the therapeutic endoscopist with little time for outpatient management of many patients that don’t need interventional procedures but are often very complex and need ongoing, long-term follow-up. In addition, any clinic slots available for interventional endoscopists may be utilized by patients coming in to discuss complex procedures or for postprocedure follow-up. Endoscopic management is not the definitive treatment for most pancreatic disorders. In fact, as our knowledge of pancreatic disease has continued to evolve, endoscopic intervention is now required in a minority of cases.
Role of the medical pancreatologist
Patient Care
As part of a comprehensive, multidisciplinary team that also includes an interventional gastroenterologist, pancreatic surgeon, transplant surgeon (in centers offering islet autotransplantation with total pancreatectomy), radiology, endocrinology, and GI pathologist, the medical pancreatologist helps lead the care of patients with pancreatic disorders, such as pancreatic cysts, acute and chronic pancreatitis (especially in cases where there is no role for active endoscopic intervention), autoimmune pancreatitis, indeterminate pancreatic masses, as well as screens high-risk patients for pancreatic cancer in conjunction with a genetic counselor. The medical pancreatologist often also serves as a bridge between various members of a large multidisciplinary team that, formally in the form of conferences or informally, discusses the management of complex patients, with each member available to help the other based on the patient’s most immediate clinical need at that time. A schematic showing how the medical pancreatologist collaborates with the therapeutic endoscopist is provided in Figure 1.
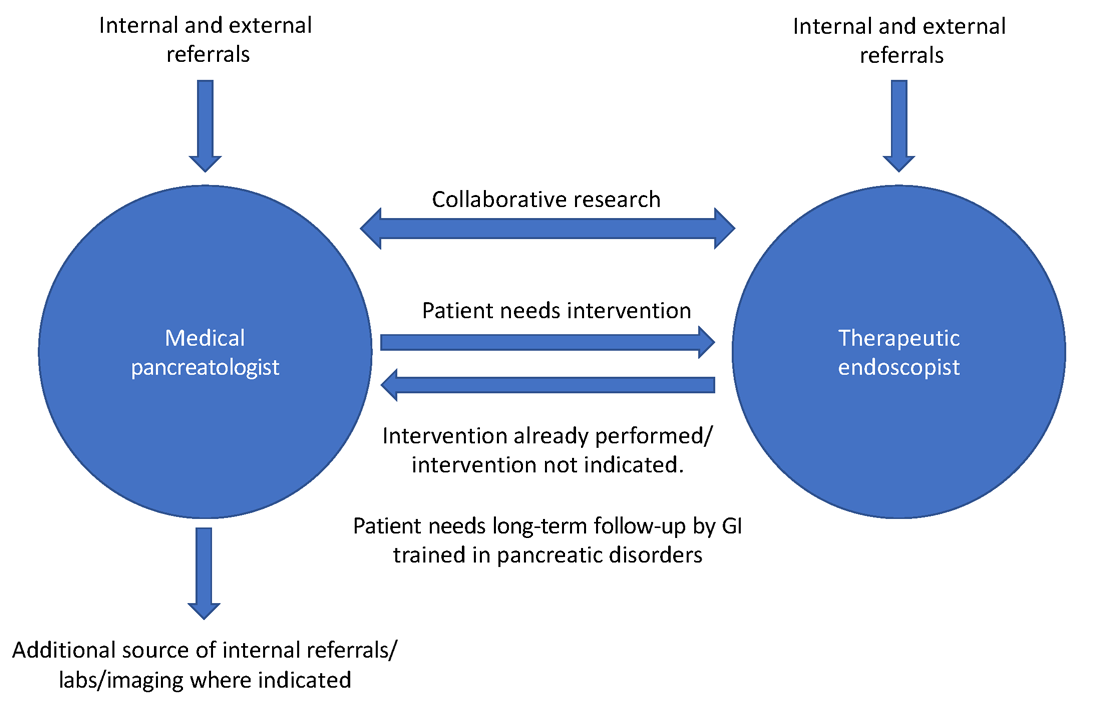
Uzma Siddiqui, MD, director for the Center for Endoscopic Research and Technology (CERT) at the University of Chicago said, “The management of pancreatic diseases is often challenging. Surgeons and endoscopists can offer some treatments that focus on one aspect or symptom, but the medical pancreatologist brings focus to the patient as a whole and helps organize care. It is only with everyone’s combined efforts and the added perspective of the medical pancreatologist that we can provide the best care for our shared patients.”
David Xin, MD, MPH, a medical pancreatologist at Brigham and Women’s Hospital, Boston, added, “I am often asked what it means to be a medical pancreatologist. What do I do if not EUS and ERCP? I provide longitudinal care, coordinate multidisciplinary management, assess nutritional status, optimize quality of life, and manage pain. But perhaps most importantly, I make myself available for patients who seek understanding and sympathy regarding their complex disease. I became a medical pancreatologist because my mentors during training helped me recognize how rewarding this career would be.”
Insights from other medical pancreatologists and therapeutic endoscopists are provided in Figure 2.

Education
Having a dedicated medical pancreatology clinic has the potential to add a unique element to the training of gastroenterology fellows. In my own experience, besides fellows interested in medical pancreatology, even those interested in therapeutic endoscopy find it useful to rotate through the pancreas clinic and follow patients after or leading to their procedures, becoming comfortable with noninterventional pain management of patients with pancreatic disorders and risk stratification of pancreatic cystic lesions, and learning about the management of rare disorders such as autoimmune pancreatitis. Most importantly, this allows trainees to identify cases where endoscopic intervention may not offer definitive treatment for complex conditions such as pancreatic pain. Trainee-centered organizations such as the Collaborative Alliance for Pancreatic Education and Research (CAPER) enable trainees and young investigators to network with other physicians who are passionate about the pancreas and establish early research collaborations for current and future research endeavors that will help advance this field.
Research
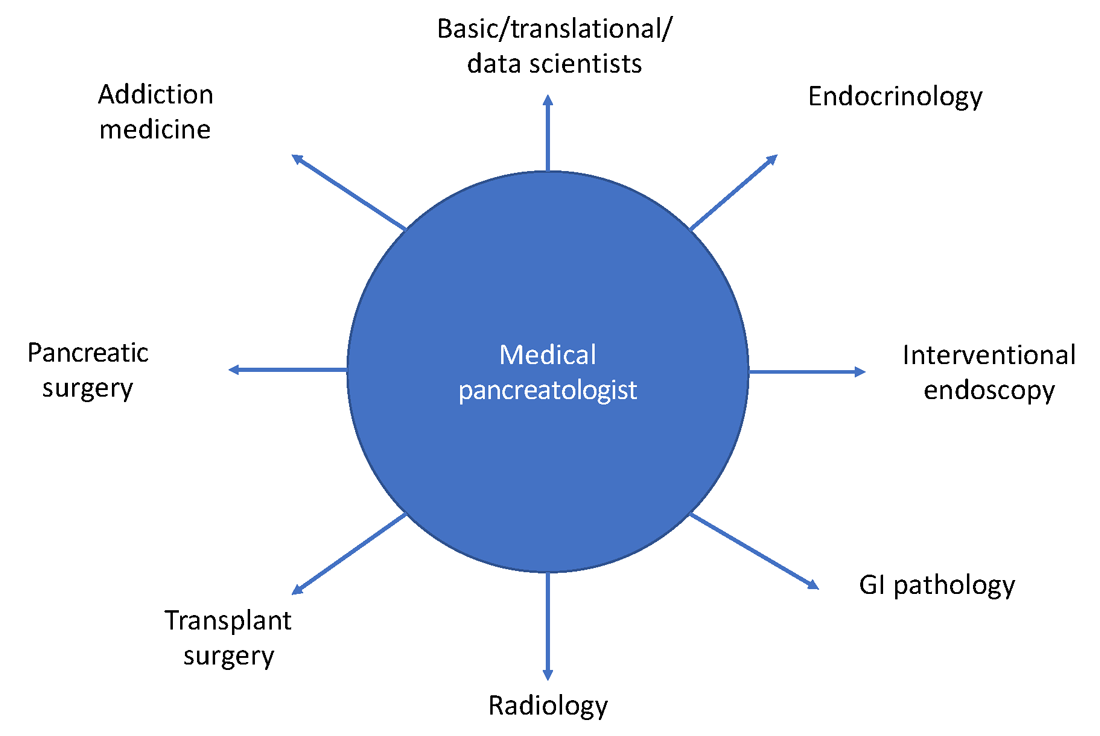
Having a trained medical pancreatologist adds the possibility of adding a unique angle to ongoing research within a gastroenterology division, especially in collaboration with others. For example, during my fellowship training I was able to focus on histological changes in pancreatic islets of patients with pancreatic cancer that develop diabetes, compared with those that do not, in collaboration with a pathologist who focused on studying islet pathology and under the guidance of my mentor, Dr. Suresh Chari, a medical pancreatologist.2 I was also part of other studies within the GI division with other medical pancreatologists, such as Dr. Santhi Vege and Dr. Shounak Majumder, who have continued to serve as career and research mentors.3 Collaborative, multicenter studies on pancreatic disease are also conducted by CAPER, the organization mentioned above. A list of potential collaborations for the fellow interested
in medical pancreatology is provided in Figure 3.
Marketing considerations for the gastroenterology division
Having a medical pancreatologist in the team is not only attractive for referring physicians within an institution but is often a great asset from a marketing standpoint, especially for tertiary care academic centers and large community practices with a broad referral base. Given that there are a limited number of medical pancreatologists in the country, having one as part of the faculty can certainly provide a competitive edge to that center within the area, especially with an ever-increasing preference of patients for hyperspecialized care.
How to develop a career in medical pancreatology
Gastroenterology fellows often start their fellowships “undifferentiated” and try to get exposed to a wide variety of GI pathology, either through general GI clinics or as part of subspecialized clinics, as they attempt to decide how they want their careers to look down the line. Similar to other subspecialities, if a trainee has already decided to pursue medical pancreatology (as happened in my case), they should strongly consider ranking programs with available opportunities for research/clinic in medical pancreatology and ideally undergo an additional year of training. Fellows who decide during the course of their fellowship that they want to pursue a career in medical pancreatology should consider applying for a 4th year in the subject to not only obtain further training in the field but to also conduct research in the area and become more “marketable” as a person that could start a medical pancreatology program at their future academic or community position. Trainees interested in medical pancreatology should try to focus their time on long-term, clinical management of patients with pancreatic disorders, engaging a multidisciplinary team composed of interventional endoscopists, pancreatic surgeons, transplant surgeons (if total pancreatectomy and islet autotransplantation is available), radiology, addiction medicine (if available), endocrinology, and pathology. The list of places that offer a 4th year in medical pancreatology is increasing every year, and as of the writing of this article there are six programs that have this opportunity, which include:
- Mayo Clinic, Rochester, Minn.
- Beth Israel Deaconess Medical Center, Boston
- Brigham and Women’s Hospital, Boston
- Johns Hopkins Hospital, Baltimore
- University of Pittsburgh Medical Center, Pittsburgh, Penn.
The CAPER website is also a great resource for education as well as for identifying potential medical pancreatology programs.
In summary, medical pancreatology is an evolving and rapidly growing career path for gastroenterology fellows interested in providing care to patients with pancreatic disease in close collaboration with multiple other subspecialties, especially therapeutic endoscopy and pancreatic surgery. The field is also ripe for fellows interested in clinical, translational, and basic science research related to pancreatic disorders.
Dr. Nagpal is assistant professor of medicine, director, pancreas clinic, University of Chicago. He had no conflicts to disclose.
References
1. Feldman M et al. “Sleisenger and Fordtran’s Gastrointestinal and Liver Disease,” 11th ed. (Philadelphia: Elsevier, 2021).
2. Nagpal SJS et al. Pancreatology. 2020 Jul;20(5):929-35.
3. Nagpal SJS et al. Pancreatology. 2019 Mar;19(2):290-5.
Although described by the Greek physician Herophilos around 300 B.C., it was not until the 19th century that enzymes began to be isolated from pancreatic secretions and their digestive action described, and not until early in the 20th century that Banting, Macleod, and Best received the Nobel prize for purifying insulin from the pancreata of dogs. For centuries in between, the pancreas was considered to be just a ‘beautiful piece of flesh’ (kallikreas), the main role of which was to protect the blood vessels in the abdomen and to serve as a cushion to the stomach.1 Certainly, the pancreas has come a long way since then but, like most other organs in the body, is oft ignored until it develops issues.
Like many other disorders in gastroenterology, pancreatic disorders were historically approached as mechanical or “plumbing” issues. As modern technology and innovation percolated through the world of endoscopy, a wide array of state-of-the-art tools were devised. Availability of newer “toys” and development of newer techniques also means that an ever-increasing curriculum has been squeezed into a generally single year of therapeutic endoscopy training, such that trainees can no longer limit themselves to learning only endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) or intervening on pancreatic disease alone. Modern, subspecialized approaches to disease and economic considerations often dictate that the therapeutic endoscopist of today must perform a wide range of procedures besides ERCP and EUS, such as advanced resection using endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), per-oral endoscopic myotomy (POEM), endoscopic bariatric procedures, and newer techniques and acronyms that continue to evolve on a regular basis. This leaves the therapeutic endoscopist with little time for outpatient management of many patients that don’t need interventional procedures but are often very complex and need ongoing, long-term follow-up. In addition, any clinic slots available for interventional endoscopists may be utilized by patients coming in to discuss complex procedures or for postprocedure follow-up. Endoscopic management is not the definitive treatment for most pancreatic disorders. In fact, as our knowledge of pancreatic disease has continued to evolve, endoscopic intervention is now required in a minority of cases.
Role of the medical pancreatologist
Patient Care
As part of a comprehensive, multidisciplinary team that also includes an interventional gastroenterologist, pancreatic surgeon, transplant surgeon (in centers offering islet autotransplantation with total pancreatectomy), radiology, endocrinology, and GI pathologist, the medical pancreatologist helps lead the care of patients with pancreatic disorders, such as pancreatic cysts, acute and chronic pancreatitis (especially in cases where there is no role for active endoscopic intervention), autoimmune pancreatitis, indeterminate pancreatic masses, as well as screens high-risk patients for pancreatic cancer in conjunction with a genetic counselor. The medical pancreatologist often also serves as a bridge between various members of a large multidisciplinary team that, formally in the form of conferences or informally, discusses the management of complex patients, with each member available to help the other based on the patient’s most immediate clinical need at that time. A schematic showing how the medical pancreatologist collaborates with the therapeutic endoscopist is provided in Figure 1.

Uzma Siddiqui, MD, director for the Center for Endoscopic Research and Technology (CERT) at the University of Chicago said, “The management of pancreatic diseases is often challenging. Surgeons and endoscopists can offer some treatments that focus on one aspect or symptom, but the medical pancreatologist brings focus to the patient as a whole and helps organize care. It is only with everyone’s combined efforts and the added perspective of the medical pancreatologist that we can provide the best care for our shared patients.”
David Xin, MD, MPH, a medical pancreatologist at Brigham and Women’s Hospital, Boston, added, “I am often asked what it means to be a medical pancreatologist. What do I do if not EUS and ERCP? I provide longitudinal care, coordinate multidisciplinary management, assess nutritional status, optimize quality of life, and manage pain. But perhaps most importantly, I make myself available for patients who seek understanding and sympathy regarding their complex disease. I became a medical pancreatologist because my mentors during training helped me recognize how rewarding this career would be.”
Insights from other medical pancreatologists and therapeutic endoscopists are provided in Figure 2.

Education
Having a dedicated medical pancreatology clinic has the potential to add a unique element to the training of gastroenterology fellows. In my own experience, besides fellows interested in medical pancreatology, even those interested in therapeutic endoscopy find it useful to rotate through the pancreas clinic and follow patients after or leading to their procedures, becoming comfortable with noninterventional pain management of patients with pancreatic disorders and risk stratification of pancreatic cystic lesions, and learning about the management of rare disorders such as autoimmune pancreatitis. Most importantly, this allows trainees to identify cases where endoscopic intervention may not offer definitive treatment for complex conditions such as pancreatic pain. Trainee-centered organizations such as the Collaborative Alliance for Pancreatic Education and Research (CAPER) enable trainees and young investigators to network with other physicians who are passionate about the pancreas and establish early research collaborations for current and future research endeavors that will help advance this field.
Research
Having a trained medical pancreatologist adds the possibility of adding a unique angle to ongoing research within a gastroenterology division, especially in collaboration with others. For example, during my fellowship training I was able to focus on histological changes in pancreatic islets of patients with pancreatic cancer that develop diabetes, compared with those that do not, in collaboration with a pathologist who focused on studying islet pathology and under the guidance of my mentor, Dr. Suresh Chari, a medical pancreatologist.2 I was also part of other studies within the GI division with other medical pancreatologists, such as Dr. Santhi Vege and Dr. Shounak Majumder, who have continued to serve as career and research mentors.3 Collaborative, multicenter studies on pancreatic disease are also conducted by CAPER, the organization mentioned above. A list of potential collaborations for the fellow interested
in medical pancreatology is provided in Figure 3.

Marketing considerations for the gastroenterology division
Having a medical pancreatologist in the team is not only attractive for referring physicians within an institution but is often a great asset from a marketing standpoint, especially for tertiary care academic centers and large community practices with a broad referral base. Given that there are a limited number of medical pancreatologists in the country, having one as part of the faculty can certainly provide a competitive edge to that center within the area, especially with an ever-increasing preference of patients for hyperspecialized care.
How to develop a career in medical pancreatology
Gastroenterology fellows often start their fellowships “undifferentiated” and try to get exposed to a wide variety of GI pathology, either through general GI clinics or as part of subspecialized clinics, as they attempt to decide how they want their careers to look down the line. Similar to other subspecialities, if a trainee has already decided to pursue medical pancreatology (as happened in my case), they should strongly consider ranking programs with available opportunities for research/clinic in medical pancreatology and ideally undergo an additional year of training. Fellows who decide during the course of their fellowship that they want to pursue a career in medical pancreatology should consider applying for a 4th year in the subject to not only obtain further training in the field but to also conduct research in the area and become more “marketable” as a person that could start a medical pancreatology program at their future academic or community position. Trainees interested in medical pancreatology should try to focus their time on long-term, clinical management of patients with pancreatic disorders, engaging a multidisciplinary team composed of interventional endoscopists, pancreatic surgeons, transplant surgeons (if total pancreatectomy and islet autotransplantation is available), radiology, addiction medicine (if available), endocrinology, and pathology. The list of places that offer a 4th year in medical pancreatology is increasing every year, and as of the writing of this article there are six programs that have this opportunity, which include:
- Mayo Clinic, Rochester, Minn.
- Beth Israel Deaconess Medical Center, Boston
- Brigham and Women’s Hospital, Boston
- Johns Hopkins Hospital, Baltimore
- University of Pittsburgh Medical Center, Pittsburgh, Penn.
The CAPER website is also a great resource for education as well as for identifying potential medical pancreatology programs.
In summary, medical pancreatology is an evolving and rapidly growing career path for gastroenterology fellows interested in providing care to patients with pancreatic disease in close collaboration with multiple other subspecialties, especially therapeutic endoscopy and pancreatic surgery. The field is also ripe for fellows interested in clinical, translational, and basic science research related to pancreatic disorders.
Dr. Nagpal is assistant professor of medicine, director, pancreas clinic, University of Chicago. He had no conflicts to disclose.
References
1. Feldman M et al. “Sleisenger and Fordtran’s Gastrointestinal and Liver Disease,” 11th ed. (Philadelphia: Elsevier, 2021).
2. Nagpal SJS et al. Pancreatology. 2020 Jul;20(5):929-35.
3. Nagpal SJS et al. Pancreatology. 2019 Mar;19(2):290-5.
Although described by the Greek physician Herophilos around 300 B.C., it was not until the 19th century that enzymes began to be isolated from pancreatic secretions and their digestive action described, and not until early in the 20th century that Banting, Macleod, and Best received the Nobel prize for purifying insulin from the pancreata of dogs. For centuries in between, the pancreas was considered to be just a ‘beautiful piece of flesh’ (kallikreas), the main role of which was to protect the blood vessels in the abdomen and to serve as a cushion to the stomach.1 Certainly, the pancreas has come a long way since then but, like most other organs in the body, is oft ignored until it develops issues.
Like many other disorders in gastroenterology, pancreatic disorders were historically approached as mechanical or “plumbing” issues. As modern technology and innovation percolated through the world of endoscopy, a wide array of state-of-the-art tools were devised. Availability of newer “toys” and development of newer techniques also means that an ever-increasing curriculum has been squeezed into a generally single year of therapeutic endoscopy training, such that trainees can no longer limit themselves to learning only endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) or intervening on pancreatic disease alone. Modern, subspecialized approaches to disease and economic considerations often dictate that the therapeutic endoscopist of today must perform a wide range of procedures besides ERCP and EUS, such as advanced resection using endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), per-oral endoscopic myotomy (POEM), endoscopic bariatric procedures, and newer techniques and acronyms that continue to evolve on a regular basis. This leaves the therapeutic endoscopist with little time for outpatient management of many patients that don’t need interventional procedures but are often very complex and need ongoing, long-term follow-up. In addition, any clinic slots available for interventional endoscopists may be utilized by patients coming in to discuss complex procedures or for postprocedure follow-up. Endoscopic management is not the definitive treatment for most pancreatic disorders. In fact, as our knowledge of pancreatic disease has continued to evolve, endoscopic intervention is now required in a minority of cases.
Role of the medical pancreatologist
Patient Care
As part of a comprehensive, multidisciplinary team that also includes an interventional gastroenterologist, pancreatic surgeon, transplant surgeon (in centers offering islet autotransplantation with total pancreatectomy), radiology, endocrinology, and GI pathologist, the medical pancreatologist helps lead the care of patients with pancreatic disorders, such as pancreatic cysts, acute and chronic pancreatitis (especially in cases where there is no role for active endoscopic intervention), autoimmune pancreatitis, indeterminate pancreatic masses, as well as screens high-risk patients for pancreatic cancer in conjunction with a genetic counselor. The medical pancreatologist often also serves as a bridge between various members of a large multidisciplinary team that, formally in the form of conferences or informally, discusses the management of complex patients, with each member available to help the other based on the patient’s most immediate clinical need at that time. A schematic showing how the medical pancreatologist collaborates with the therapeutic endoscopist is provided in Figure 1.

Uzma Siddiqui, MD, director for the Center for Endoscopic Research and Technology (CERT) at the University of Chicago said, “The management of pancreatic diseases is often challenging. Surgeons and endoscopists can offer some treatments that focus on one aspect or symptom, but the medical pancreatologist brings focus to the patient as a whole and helps organize care. It is only with everyone’s combined efforts and the added perspective of the medical pancreatologist that we can provide the best care for our shared patients.”
David Xin, MD, MPH, a medical pancreatologist at Brigham and Women’s Hospital, Boston, added, “I am often asked what it means to be a medical pancreatologist. What do I do if not EUS and ERCP? I provide longitudinal care, coordinate multidisciplinary management, assess nutritional status, optimize quality of life, and manage pain. But perhaps most importantly, I make myself available for patients who seek understanding and sympathy regarding their complex disease. I became a medical pancreatologist because my mentors during training helped me recognize how rewarding this career would be.”
Insights from other medical pancreatologists and therapeutic endoscopists are provided in Figure 2.

Education
Having a dedicated medical pancreatology clinic has the potential to add a unique element to the training of gastroenterology fellows. In my own experience, besides fellows interested in medical pancreatology, even those interested in therapeutic endoscopy find it useful to rotate through the pancreas clinic and follow patients after or leading to their procedures, becoming comfortable with noninterventional pain management of patients with pancreatic disorders and risk stratification of pancreatic cystic lesions, and learning about the management of rare disorders such as autoimmune pancreatitis. Most importantly, this allows trainees to identify cases where endoscopic intervention may not offer definitive treatment for complex conditions such as pancreatic pain. Trainee-centered organizations such as the Collaborative Alliance for Pancreatic Education and Research (CAPER) enable trainees and young investigators to network with other physicians who are passionate about the pancreas and establish early research collaborations for current and future research endeavors that will help advance this field.
Research
Having a trained medical pancreatologist adds the possibility of adding a unique angle to ongoing research within a gastroenterology division, especially in collaboration with others. For example, during my fellowship training I was able to focus on histological changes in pancreatic islets of patients with pancreatic cancer that develop diabetes, compared with those that do not, in collaboration with a pathologist who focused on studying islet pathology and under the guidance of my mentor, Dr. Suresh Chari, a medical pancreatologist.2 I was also part of other studies within the GI division with other medical pancreatologists, such as Dr. Santhi Vege and Dr. Shounak Majumder, who have continued to serve as career and research mentors.3 Collaborative, multicenter studies on pancreatic disease are also conducted by CAPER, the organization mentioned above. A list of potential collaborations for the fellow interested
in medical pancreatology is provided in Figure 3.

Marketing considerations for the gastroenterology division
Having a medical pancreatologist in the team is not only attractive for referring physicians within an institution but is often a great asset from a marketing standpoint, especially for tertiary care academic centers and large community practices with a broad referral base. Given that there are a limited number of medical pancreatologists in the country, having one as part of the faculty can certainly provide a competitive edge to that center within the area, especially with an ever-increasing preference of patients for hyperspecialized care.
How to develop a career in medical pancreatology
Gastroenterology fellows often start their fellowships “undifferentiated” and try to get exposed to a wide variety of GI pathology, either through general GI clinics or as part of subspecialized clinics, as they attempt to decide how they want their careers to look down the line. Similar to other subspecialities, if a trainee has already decided to pursue medical pancreatology (as happened in my case), they should strongly consider ranking programs with available opportunities for research/clinic in medical pancreatology and ideally undergo an additional year of training. Fellows who decide during the course of their fellowship that they want to pursue a career in medical pancreatology should consider applying for a 4th year in the subject to not only obtain further training in the field but to also conduct research in the area and become more “marketable” as a person that could start a medical pancreatology program at their future academic or community position. Trainees interested in medical pancreatology should try to focus their time on long-term, clinical management of patients with pancreatic disorders, engaging a multidisciplinary team composed of interventional endoscopists, pancreatic surgeons, transplant surgeons (if total pancreatectomy and islet autotransplantation is available), radiology, addiction medicine (if available), endocrinology, and pathology. The list of places that offer a 4th year in medical pancreatology is increasing every year, and as of the writing of this article there are six programs that have this opportunity, which include:
- Mayo Clinic, Rochester, Minn.
- Beth Israel Deaconess Medical Center, Boston
- Brigham and Women’s Hospital, Boston
- Johns Hopkins Hospital, Baltimore
- University of Pittsburgh Medical Center, Pittsburgh, Penn.
The CAPER website is also a great resource for education as well as for identifying potential medical pancreatology programs.
In summary, medical pancreatology is an evolving and rapidly growing career path for gastroenterology fellows interested in providing care to patients with pancreatic disease in close collaboration with multiple other subspecialties, especially therapeutic endoscopy and pancreatic surgery. The field is also ripe for fellows interested in clinical, translational, and basic science research related to pancreatic disorders.
Dr. Nagpal is assistant professor of medicine, director, pancreas clinic, University of Chicago. He had no conflicts to disclose.
References
1. Feldman M et al. “Sleisenger and Fordtran’s Gastrointestinal and Liver Disease,” 11th ed. (Philadelphia: Elsevier, 2021).
2. Nagpal SJS et al. Pancreatology. 2020 Jul;20(5):929-35.
3. Nagpal SJS et al. Pancreatology. 2019 Mar;19(2):290-5.
The importance of education and screening for nonalcoholic fatty liver disease
For the past 18 months, we’ve all been focused on defeating the COVID-19 pandemic and preparing for the effects of cancer screenings that were delayed or put off entirely. But COVID isn’t the only epidemic we’re facing in the United States. Obesity is the second leading cause of preventable death in the United States. and its related diseases account for $480.7 billion in direct health care costs, with an additional $1.24 trillion in indirect costs from lost economic productivity.
More than two in five Americans are obese and that number is predicted to grow to more than half of the U.S. population by 2030. Obesity is a risk factor for nonalcoholic fatty liver disease (NAFLD), a buildup of fat in the liver with little or no inflammation or cell damage that affects one in three (30%-37%) of adults in the U.S.
NAFLD can progress to nonalcoholic steatohepatitis (NASH), which affects about 1 in 10 (8%-12%) of adults in the U.S. NASH is fat in the liver with inflammation and cell damage, and it can lead to fibrosis and liver failure. The number of patients we see with NALFD and NASH continues to rise and it’s taking its toll. One in five people who have NASH will have the disease progress to liver cirrhosis. NASH is expected to be the leading cause of liver transplant in the U.S. for the next 5 years.
Stemming the tide of NAFLD and NASH
In terms of diet, limiting sugar and eating a diet rich in vegetables, whole grains, and healthy fats can prevent the factors that lead to liver disease.
If this were easy, we wouldn’t be facing the obesity epidemic that is plaguing the United States. One of the issues is that medicine has only recognized obesity as a disease for less than 10 years. We aren’t trained in medical school, residencies, or fellowships in managing obesity, beyond advising people to exercise and eat right. We know this doesn’t work.
That’s why many independent GI groups are exploring comprehensive weight management programs that take a holistic approach to weight management involving a team of health care providers and educators helping patients gradually exercise more and eat healthy while providing a social support system to lose weight and keep it off.
The best way to educate is to listen first
As gastroenterologists, we see many obesity-related issues and have an opportunity to intervene before other more serious issues show up – like cancer, hypertension, and stroke. And educating the public and primary care physicians is key to ensuring that patients who are high risk are screened for liver disease.
Some GI practices leverage awareness events such as International NASH Day in June, or National Liver Cancer Awareness Month in October, to provide primary care physicians and patients with educational materials about making healthier choices and what options are available to screen for NAFLD and NASH.
While the awareness events offer a ready-made context for outreach, the physicians in my practice work year-round to provide information on liver disease. When patients are brought in for issues that may indicate future problems, we look for signs of chronic liver disease and educate them and their family members about liver disease and cirrhosis.
Discussions of weight are very personal, and it’s important to approach the conversation with sensitivity. It’s also good to understand as best as possible any cultural implications of discussing a person’s weight to ensure that the patient or their family members are not embarrassed by the discussion. I find that oftentimes the best approach is to listen to the patient and hear what factors are influencing their ability to exercise and eat healthy foods so that you can work together to find the best solution.
It’s also important to recognize that racial disparities exist in many aspects of NAFLD, including prevalence, severity, genetic predisposition, and overall chance of recovery. For instance, Hispanics and Asian Americans have a higher prevalence of NAFLD, compared with other ethnic and racial groups.
Early detection is key
Screenings have become a lot simpler and more convenient. There are alternatives to the painful, expensive liver biopsy. There are blood biomarker tests designed to assess liver fibrosis in patients. Specialized vibration-controlled transient elastography, such as Fibroscan, can measure scarring and fat buildup in the liver. And because it’s noninvasive, it doesn’t come with the same risks as a traditional liver biopsy. It also costs about four or five times less, which is important in this era of value-based care.
These simple tests can be reassuring, or they can lead down another path of treating the disease, but not being screened at all can come at a steep price. Severe fibrosis can lead to cirrhosis, a dangerous condition where the liver can no longer function correctly. NAFLD and NASH can also lead to liver cancer.
There are some medications that are in phase 2 and some in phase 3 clinical trials that aim to reduce fatty liver by cutting down fibrosis and steatosis, and there are other medications that can be used to help with weight loss. But the reality is that lifestyle changes are currently the best way to reverse NAFLD or stop it from progressing to NASH or cirrhosis.
Join an innovative practice
For the next 20 years, the obesity epidemic will be the biggest issue facing our society and a major focus of our cancer prevention efforts. Early-career physicians who are looking to join an independent GI practice should ask questions to determine whether the partners in the practice are taking a comprehensive approach to treating issues of obesity, NAFLD, NASH, and liver disease. Discuss what steps the practice takes to educate primary care physicians and their patients about the dangers of NAFLD and NASH.
We’re looking for early-career physicians who are entrepreneurial, not just for the sake of the practice, but because the future is in digital technologies and chronic care management, such as Chronwell, that help people maintain health through remote care and coaching. We want people who are thinking about fixing the problems of today and tomorrow with new technologies and scalable solutions. Through education and new screening and treatment options, we can ensure that fewer people develop serious liver disease or cancer.
Dr. Sanjay Sandhir is a practicing gastroenterologist at Dayton Gastroenterology, One GI in Ohio and is an executive committee member of the Digestive Health Physicians Association. He has no conflicts to declare.
For the past 18 months, we’ve all been focused on defeating the COVID-19 pandemic and preparing for the effects of cancer screenings that were delayed or put off entirely. But COVID isn’t the only epidemic we’re facing in the United States. Obesity is the second leading cause of preventable death in the United States. and its related diseases account for $480.7 billion in direct health care costs, with an additional $1.24 trillion in indirect costs from lost economic productivity.
More than two in five Americans are obese and that number is predicted to grow to more than half of the U.S. population by 2030. Obesity is a risk factor for nonalcoholic fatty liver disease (NAFLD), a buildup of fat in the liver with little or no inflammation or cell damage that affects one in three (30%-37%) of adults in the U.S.
NAFLD can progress to nonalcoholic steatohepatitis (NASH), which affects about 1 in 10 (8%-12%) of adults in the U.S. NASH is fat in the liver with inflammation and cell damage, and it can lead to fibrosis and liver failure. The number of patients we see with NALFD and NASH continues to rise and it’s taking its toll. One in five people who have NASH will have the disease progress to liver cirrhosis. NASH is expected to be the leading cause of liver transplant in the U.S. for the next 5 years.
Stemming the tide of NAFLD and NASH
In terms of diet, limiting sugar and eating a diet rich in vegetables, whole grains, and healthy fats can prevent the factors that lead to liver disease.
If this were easy, we wouldn’t be facing the obesity epidemic that is plaguing the United States. One of the issues is that medicine has only recognized obesity as a disease for less than 10 years. We aren’t trained in medical school, residencies, or fellowships in managing obesity, beyond advising people to exercise and eat right. We know this doesn’t work.
That’s why many independent GI groups are exploring comprehensive weight management programs that take a holistic approach to weight management involving a team of health care providers and educators helping patients gradually exercise more and eat healthy while providing a social support system to lose weight and keep it off.
The best way to educate is to listen first
As gastroenterologists, we see many obesity-related issues and have an opportunity to intervene before other more serious issues show up – like cancer, hypertension, and stroke. And educating the public and primary care physicians is key to ensuring that patients who are high risk are screened for liver disease.
Some GI practices leverage awareness events such as International NASH Day in June, or National Liver Cancer Awareness Month in October, to provide primary care physicians and patients with educational materials about making healthier choices and what options are available to screen for NAFLD and NASH.
While the awareness events offer a ready-made context for outreach, the physicians in my practice work year-round to provide information on liver disease. When patients are brought in for issues that may indicate future problems, we look for signs of chronic liver disease and educate them and their family members about liver disease and cirrhosis.
Discussions of weight are very personal, and it’s important to approach the conversation with sensitivity. It’s also good to understand as best as possible any cultural implications of discussing a person’s weight to ensure that the patient or their family members are not embarrassed by the discussion. I find that oftentimes the best approach is to listen to the patient and hear what factors are influencing their ability to exercise and eat healthy foods so that you can work together to find the best solution.
It’s also important to recognize that racial disparities exist in many aspects of NAFLD, including prevalence, severity, genetic predisposition, and overall chance of recovery. For instance, Hispanics and Asian Americans have a higher prevalence of NAFLD, compared with other ethnic and racial groups.
Early detection is key
Screenings have become a lot simpler and more convenient. There are alternatives to the painful, expensive liver biopsy. There are blood biomarker tests designed to assess liver fibrosis in patients. Specialized vibration-controlled transient elastography, such as Fibroscan, can measure scarring and fat buildup in the liver. And because it’s noninvasive, it doesn’t come with the same risks as a traditional liver biopsy. It also costs about four or five times less, which is important in this era of value-based care.
These simple tests can be reassuring, or they can lead down another path of treating the disease, but not being screened at all can come at a steep price. Severe fibrosis can lead to cirrhosis, a dangerous condition where the liver can no longer function correctly. NAFLD and NASH can also lead to liver cancer.
There are some medications that are in phase 2 and some in phase 3 clinical trials that aim to reduce fatty liver by cutting down fibrosis and steatosis, and there are other medications that can be used to help with weight loss. But the reality is that lifestyle changes are currently the best way to reverse NAFLD or stop it from progressing to NASH or cirrhosis.
Join an innovative practice
For the next 20 years, the obesity epidemic will be the biggest issue facing our society and a major focus of our cancer prevention efforts. Early-career physicians who are looking to join an independent GI practice should ask questions to determine whether the partners in the practice are taking a comprehensive approach to treating issues of obesity, NAFLD, NASH, and liver disease. Discuss what steps the practice takes to educate primary care physicians and their patients about the dangers of NAFLD and NASH.
We’re looking for early-career physicians who are entrepreneurial, not just for the sake of the practice, but because the future is in digital technologies and chronic care management, such as Chronwell, that help people maintain health through remote care and coaching. We want people who are thinking about fixing the problems of today and tomorrow with new technologies and scalable solutions. Through education and new screening and treatment options, we can ensure that fewer people develop serious liver disease or cancer.
Dr. Sanjay Sandhir is a practicing gastroenterologist at Dayton Gastroenterology, One GI in Ohio and is an executive committee member of the Digestive Health Physicians Association. He has no conflicts to declare.
For the past 18 months, we’ve all been focused on defeating the COVID-19 pandemic and preparing for the effects of cancer screenings that were delayed or put off entirely. But COVID isn’t the only epidemic we’re facing in the United States. Obesity is the second leading cause of preventable death in the United States. and its related diseases account for $480.7 billion in direct health care costs, with an additional $1.24 trillion in indirect costs from lost economic productivity.
More than two in five Americans are obese and that number is predicted to grow to more than half of the U.S. population by 2030. Obesity is a risk factor for nonalcoholic fatty liver disease (NAFLD), a buildup of fat in the liver with little or no inflammation or cell damage that affects one in three (30%-37%) of adults in the U.S.
NAFLD can progress to nonalcoholic steatohepatitis (NASH), which affects about 1 in 10 (8%-12%) of adults in the U.S. NASH is fat in the liver with inflammation and cell damage, and it can lead to fibrosis and liver failure. The number of patients we see with NALFD and NASH continues to rise and it’s taking its toll. One in five people who have NASH will have the disease progress to liver cirrhosis. NASH is expected to be the leading cause of liver transplant in the U.S. for the next 5 years.
Stemming the tide of NAFLD and NASH
In terms of diet, limiting sugar and eating a diet rich in vegetables, whole grains, and healthy fats can prevent the factors that lead to liver disease.
If this were easy, we wouldn’t be facing the obesity epidemic that is plaguing the United States. One of the issues is that medicine has only recognized obesity as a disease for less than 10 years. We aren’t trained in medical school, residencies, or fellowships in managing obesity, beyond advising people to exercise and eat right. We know this doesn’t work.
That’s why many independent GI groups are exploring comprehensive weight management programs that take a holistic approach to weight management involving a team of health care providers and educators helping patients gradually exercise more and eat healthy while providing a social support system to lose weight and keep it off.
The best way to educate is to listen first
As gastroenterologists, we see many obesity-related issues and have an opportunity to intervene before other more serious issues show up – like cancer, hypertension, and stroke. And educating the public and primary care physicians is key to ensuring that patients who are high risk are screened for liver disease.
Some GI practices leverage awareness events such as International NASH Day in June, or National Liver Cancer Awareness Month in October, to provide primary care physicians and patients with educational materials about making healthier choices and what options are available to screen for NAFLD and NASH.
While the awareness events offer a ready-made context for outreach, the physicians in my practice work year-round to provide information on liver disease. When patients are brought in for issues that may indicate future problems, we look for signs of chronic liver disease and educate them and their family members about liver disease and cirrhosis.
Discussions of weight are very personal, and it’s important to approach the conversation with sensitivity. It’s also good to understand as best as possible any cultural implications of discussing a person’s weight to ensure that the patient or their family members are not embarrassed by the discussion. I find that oftentimes the best approach is to listen to the patient and hear what factors are influencing their ability to exercise and eat healthy foods so that you can work together to find the best solution.
It’s also important to recognize that racial disparities exist in many aspects of NAFLD, including prevalence, severity, genetic predisposition, and overall chance of recovery. For instance, Hispanics and Asian Americans have a higher prevalence of NAFLD, compared with other ethnic and racial groups.
Early detection is key
Screenings have become a lot simpler and more convenient. There are alternatives to the painful, expensive liver biopsy. There are blood biomarker tests designed to assess liver fibrosis in patients. Specialized vibration-controlled transient elastography, such as Fibroscan, can measure scarring and fat buildup in the liver. And because it’s noninvasive, it doesn’t come with the same risks as a traditional liver biopsy. It also costs about four or five times less, which is important in this era of value-based care.
These simple tests can be reassuring, or they can lead down another path of treating the disease, but not being screened at all can come at a steep price. Severe fibrosis can lead to cirrhosis, a dangerous condition where the liver can no longer function correctly. NAFLD and NASH can also lead to liver cancer.
There are some medications that are in phase 2 and some in phase 3 clinical trials that aim to reduce fatty liver by cutting down fibrosis and steatosis, and there are other medications that can be used to help with weight loss. But the reality is that lifestyle changes are currently the best way to reverse NAFLD or stop it from progressing to NASH or cirrhosis.
Join an innovative practice
For the next 20 years, the obesity epidemic will be the biggest issue facing our society and a major focus of our cancer prevention efforts. Early-career physicians who are looking to join an independent GI practice should ask questions to determine whether the partners in the practice are taking a comprehensive approach to treating issues of obesity, NAFLD, NASH, and liver disease. Discuss what steps the practice takes to educate primary care physicians and their patients about the dangers of NAFLD and NASH.
We’re looking for early-career physicians who are entrepreneurial, not just for the sake of the practice, but because the future is in digital technologies and chronic care management, such as Chronwell, that help people maintain health through remote care and coaching. We want people who are thinking about fixing the problems of today and tomorrow with new technologies and scalable solutions. Through education and new screening and treatment options, we can ensure that fewer people develop serious liver disease or cancer.
Dr. Sanjay Sandhir is a practicing gastroenterologist at Dayton Gastroenterology, One GI in Ohio and is an executive committee member of the Digestive Health Physicians Association. He has no conflicts to declare.
Sharing notes with our patients: Ethical considerations
Even a decade ago, the idea of providers sharing clinical notes with patients was almost unfathomable to most in medicine. We have since seen a sea change regarding the need for transparency in health care, leading to dramatic legislative and policy shifts in recent years.
On April 5, 2021, the federal program rule on Interoperability, Information Blocking, and ONC Health IT Certification took effect, which implemented a part of the bipartisan 21st Century Cures Act of 2016 requiring most of a patient’s electronic health information (EHI) be made easily accessible free of charge and “without delay.”1
Included in this defined set of EHI, known as the United States Core Data for Interoperability, are eight types of clinical notes that must be shared with patients, including: progress notes, history and physical notes, consultation notes, discharge summary notes, procedure notes, laboratory report narratives, imaging narratives, and pathology report narratives. Many clinicians viewed this federally mandated transition to note sharing with patients with concern, fearing increased documentation burdens, needless patient anxiety, and inevitable deluge of follow-up questions and requests for chart corrections.
In reality, the Health Insurance Portability and Accountability Act (HIPAA) granted virtually all patients the right to review a paper copy of their medical records, including all clinical notes, way back in 1996. Practically speaking, though, the multiple steps required to formally make these requests kept most patients from regularly accessing their health information.
The 21st Century Cures Act streamlines and modernizes this process by requiring electronic access. Certain note types, including psychotherapy notes, are exempt from this requirement. As has always been true since HIPAA was enacted, exceptions may be used for circumstances in which a clinician holds a reasonable belief that blocking information is necessary to prevent harm to a patient or another person or to protect an individual’s privacy. By continuing to allow for these exceptions, clinicians maintain the autonomy to block sharing of notes in the rare, complex situations in which doing so may truly be harmful.
And while the legal requirement to share most clinical notes is new, there is already a wealth of evidence from the earliest adopters (part of the OpenNotes movement) affirming the significant benefits from this practice – for patients and providers – with few negative effects on workflows or documentation patterns.2 Findings published as early as 2012, and regularly since then, among OpenNotes adopters from a diverse set of health care institutions have shown access to notes improves patient engagement, activation, and communication, as well as patient and clinician satisfaction.3
Still, providers may argue, shouldn’t clinical notes be a space where providers are free to articulate uncertainties, work through clinical reasoning, and share subtle observations about a patient’s presentation and findings with colleagues without having to worry about alarming patients who may lack the background to understand medical nuances?
It’s a fine balance in certain situations since we want to document our objective clinical assessments and prognoses without needlessly upsetting our patients, especially when considering a potentially life-changing diagnosis. How do we continue offering hope to our patients while still respecting their autonomy and sharing their health information with them? There is no uniform approach or standard playbook to follow since each patient and clinical circumstance is unique.
Fundamentally, sharing clinical notes is about granting access to one’s own health information, promoting patient activation and engagement, and making health care more patient centered. As a clinician, it’s important to frame the conversations we have with our patients so they are not surprised or caught off guard by what we have written in our notes. If you had a difficult or contentious conversation, document it objectively and without bias. If you are discussing obesity, substance abuse, or mental health, do so respectfully, supportively, and without judgment. If one of the reasons you are doing a CT scan is to rule out pancreatic cancer, it’s hard to argue that the patient does not deserve to know that beforehand.
The OpenNotes experience to date has consistently shown that patients benefit from direct discussions and transparency, which can even motivate difficult behavior changes.4 As clinicians, we may have to make minor changes in how we document, such as using less medical jargon and fewer abbreviations, but based on data from the longest tenured participants in OpenNotes, these adjustments do not add to documentation burdens.5 An activated patient who is reading their notes is an engaged patient, one who will often collaborate more in their own care, offer additional insights, and feel more empowered to take responsibility for their own health.6
When surveyed, patients report that access to their clinical notes helps them feel more in control of their health by understanding their medical conditions better, which makes them feel more prepared for their visits.4 Studies have shown that patients forget between 40%-80% of the information communicated during a visit, making clinical notes a valuable reminder and reference. Over 75% of patients in one study reported that reading notes helped them better understand the meaning of results and the rationale for referrals and tests, which led to greater follow-through with their treatment plans and follow-up appointments.3 A remarkable 99% of patients in the same study reported feeling the same or better about their physician after reading their notes.
Sharing notes with patients also makes care safer and more equitable. A written record of a visit serves as an important source of information about why a medicine is prescribed, a reminder about additions or changes to a regimen, and potential adverse effects of medications. In the first OpenNotes study, which had more than 100 primary care physicians and 20,000 patients, 60%-78% of patients with access to their notes reported improved medication adherence.2 A later study reported similar benefits, particularly among patients who identify as racial or ethnic minorities, non-native English speakers, and those with a high school education or less. These findings may reflect increased trust that comes with a more collaborative relationship between providers and patients. Patients who can read their notes also show a willingness to review their medication lists and report discrepancies and errors, making their care safer still.7
Conclusion
The move to widespread shared notes, though prompted by a federal mandate, is a critical step forward in patient activation, engagement, and satisfaction. Importantly, there is a large body of evidence showing multiple benefits, including better communication and safer and more equitable care at sites that have already been sharing notes for over a decade. When surveyed, both patients and providers who have been participating in shared notes believe the practice should continue.
In April 2021, we began a massive natural experiment in the U.S. with ubiquitous sharing of clinical notes, one that will help us learn more about how best to make our patients’ health information accessible, meaningful, and most meaningful in improving their overall health and well-being. Sharing notes with our patients is at once relatively easy to implement but complex in its implications and represents a significant paradigm shift in medicine toward a safer, more patient-centered approach. The evidence to date has shown that embracing shared notes promotes greater patient activation and engagement, and with it a more transparent and collaborative relationship between providers and patients that could lead to transformative benefits to the quality of the care we can achieve together.
Dr. Shah is an associate professor of medicine and pediatrics and associate chief medical information officer at University of Chicago Medicine. He has no disclosures
References
1. 21st Century Cures Act, HR 34, 114th Congress (2015). Accessed 2021 Sep 23. https://www.congress.gov/bill/114th-congress/house-bill/34.
2. Delbanco T et al. Ann Intern Med. 2012 Oct;157(7):461-70.
3. Bell S et al. BMJ Qual Saf. 2017 Apr;26(4):262-70.
4. Walker J et al. J Med Internet Res. 2019 May. doi: 10.2196/13876.
5. DesRoches C et al. JAMA Netw Open. 2020 Mar. doi: 10.1001/jamanetworkopen.2020.1753.
6. Blease C et al. J Med Ethics. 2021 May. doi: 10.1136/medethics-2021-107275.
7. DesRoches C et al. Ann Intern Med. 2019 Jul 2;171(1):69-71.
Even a decade ago, the idea of providers sharing clinical notes with patients was almost unfathomable to most in medicine. We have since seen a sea change regarding the need for transparency in health care, leading to dramatic legislative and policy shifts in recent years.
On April 5, 2021, the federal program rule on Interoperability, Information Blocking, and ONC Health IT Certification took effect, which implemented a part of the bipartisan 21st Century Cures Act of 2016 requiring most of a patient’s electronic health information (EHI) be made easily accessible free of charge and “without delay.”1
Included in this defined set of EHI, known as the United States Core Data for Interoperability, are eight types of clinical notes that must be shared with patients, including: progress notes, history and physical notes, consultation notes, discharge summary notes, procedure notes, laboratory report narratives, imaging narratives, and pathology report narratives. Many clinicians viewed this federally mandated transition to note sharing with patients with concern, fearing increased documentation burdens, needless patient anxiety, and inevitable deluge of follow-up questions and requests for chart corrections.
In reality, the Health Insurance Portability and Accountability Act (HIPAA) granted virtually all patients the right to review a paper copy of their medical records, including all clinical notes, way back in 1996. Practically speaking, though, the multiple steps required to formally make these requests kept most patients from regularly accessing their health information.
The 21st Century Cures Act streamlines and modernizes this process by requiring electronic access. Certain note types, including psychotherapy notes, are exempt from this requirement. As has always been true since HIPAA was enacted, exceptions may be used for circumstances in which a clinician holds a reasonable belief that blocking information is necessary to prevent harm to a patient or another person or to protect an individual’s privacy. By continuing to allow for these exceptions, clinicians maintain the autonomy to block sharing of notes in the rare, complex situations in which doing so may truly be harmful.
And while the legal requirement to share most clinical notes is new, there is already a wealth of evidence from the earliest adopters (part of the OpenNotes movement) affirming the significant benefits from this practice – for patients and providers – with few negative effects on workflows or documentation patterns.2 Findings published as early as 2012, and regularly since then, among OpenNotes adopters from a diverse set of health care institutions have shown access to notes improves patient engagement, activation, and communication, as well as patient and clinician satisfaction.3
Still, providers may argue, shouldn’t clinical notes be a space where providers are free to articulate uncertainties, work through clinical reasoning, and share subtle observations about a patient’s presentation and findings with colleagues without having to worry about alarming patients who may lack the background to understand medical nuances?
It’s a fine balance in certain situations since we want to document our objective clinical assessments and prognoses without needlessly upsetting our patients, especially when considering a potentially life-changing diagnosis. How do we continue offering hope to our patients while still respecting their autonomy and sharing their health information with them? There is no uniform approach or standard playbook to follow since each patient and clinical circumstance is unique.
Fundamentally, sharing clinical notes is about granting access to one’s own health information, promoting patient activation and engagement, and making health care more patient centered. As a clinician, it’s important to frame the conversations we have with our patients so they are not surprised or caught off guard by what we have written in our notes. If you had a difficult or contentious conversation, document it objectively and without bias. If you are discussing obesity, substance abuse, or mental health, do so respectfully, supportively, and without judgment. If one of the reasons you are doing a CT scan is to rule out pancreatic cancer, it’s hard to argue that the patient does not deserve to know that beforehand.
The OpenNotes experience to date has consistently shown that patients benefit from direct discussions and transparency, which can even motivate difficult behavior changes.4 As clinicians, we may have to make minor changes in how we document, such as using less medical jargon and fewer abbreviations, but based on data from the longest tenured participants in OpenNotes, these adjustments do not add to documentation burdens.5 An activated patient who is reading their notes is an engaged patient, one who will often collaborate more in their own care, offer additional insights, and feel more empowered to take responsibility for their own health.6
When surveyed, patients report that access to their clinical notes helps them feel more in control of their health by understanding their medical conditions better, which makes them feel more prepared for their visits.4 Studies have shown that patients forget between 40%-80% of the information communicated during a visit, making clinical notes a valuable reminder and reference. Over 75% of patients in one study reported that reading notes helped them better understand the meaning of results and the rationale for referrals and tests, which led to greater follow-through with their treatment plans and follow-up appointments.3 A remarkable 99% of patients in the same study reported feeling the same or better about their physician after reading their notes.
Sharing notes with patients also makes care safer and more equitable. A written record of a visit serves as an important source of information about why a medicine is prescribed, a reminder about additions or changes to a regimen, and potential adverse effects of medications. In the first OpenNotes study, which had more than 100 primary care physicians and 20,000 patients, 60%-78% of patients with access to their notes reported improved medication adherence.2 A later study reported similar benefits, particularly among patients who identify as racial or ethnic minorities, non-native English speakers, and those with a high school education or less. These findings may reflect increased trust that comes with a more collaborative relationship between providers and patients. Patients who can read their notes also show a willingness to review their medication lists and report discrepancies and errors, making their care safer still.7
Conclusion
The move to widespread shared notes, though prompted by a federal mandate, is a critical step forward in patient activation, engagement, and satisfaction. Importantly, there is a large body of evidence showing multiple benefits, including better communication and safer and more equitable care at sites that have already been sharing notes for over a decade. When surveyed, both patients and providers who have been participating in shared notes believe the practice should continue.
In April 2021, we began a massive natural experiment in the U.S. with ubiquitous sharing of clinical notes, one that will help us learn more about how best to make our patients’ health information accessible, meaningful, and most meaningful in improving their overall health and well-being. Sharing notes with our patients is at once relatively easy to implement but complex in its implications and represents a significant paradigm shift in medicine toward a safer, more patient-centered approach. The evidence to date has shown that embracing shared notes promotes greater patient activation and engagement, and with it a more transparent and collaborative relationship between providers and patients that could lead to transformative benefits to the quality of the care we can achieve together.
Dr. Shah is an associate professor of medicine and pediatrics and associate chief medical information officer at University of Chicago Medicine. He has no disclosures
References
1. 21st Century Cures Act, HR 34, 114th Congress (2015). Accessed 2021 Sep 23. https://www.congress.gov/bill/114th-congress/house-bill/34.
2. Delbanco T et al. Ann Intern Med. 2012 Oct;157(7):461-70.
3. Bell S et al. BMJ Qual Saf. 2017 Apr;26(4):262-70.
4. Walker J et al. J Med Internet Res. 2019 May. doi: 10.2196/13876.
5. DesRoches C et al. JAMA Netw Open. 2020 Mar. doi: 10.1001/jamanetworkopen.2020.1753.
6. Blease C et al. J Med Ethics. 2021 May. doi: 10.1136/medethics-2021-107275.
7. DesRoches C et al. Ann Intern Med. 2019 Jul 2;171(1):69-71.
Even a decade ago, the idea of providers sharing clinical notes with patients was almost unfathomable to most in medicine. We have since seen a sea change regarding the need for transparency in health care, leading to dramatic legislative and policy shifts in recent years.
On April 5, 2021, the federal program rule on Interoperability, Information Blocking, and ONC Health IT Certification took effect, which implemented a part of the bipartisan 21st Century Cures Act of 2016 requiring most of a patient’s electronic health information (EHI) be made easily accessible free of charge and “without delay.”1
Included in this defined set of EHI, known as the United States Core Data for Interoperability, are eight types of clinical notes that must be shared with patients, including: progress notes, history and physical notes, consultation notes, discharge summary notes, procedure notes, laboratory report narratives, imaging narratives, and pathology report narratives. Many clinicians viewed this federally mandated transition to note sharing with patients with concern, fearing increased documentation burdens, needless patient anxiety, and inevitable deluge of follow-up questions and requests for chart corrections.
In reality, the Health Insurance Portability and Accountability Act (HIPAA) granted virtually all patients the right to review a paper copy of their medical records, including all clinical notes, way back in 1996. Practically speaking, though, the multiple steps required to formally make these requests kept most patients from regularly accessing their health information.
The 21st Century Cures Act streamlines and modernizes this process by requiring electronic access. Certain note types, including psychotherapy notes, are exempt from this requirement. As has always been true since HIPAA was enacted, exceptions may be used for circumstances in which a clinician holds a reasonable belief that blocking information is necessary to prevent harm to a patient or another person or to protect an individual’s privacy. By continuing to allow for these exceptions, clinicians maintain the autonomy to block sharing of notes in the rare, complex situations in which doing so may truly be harmful.
And while the legal requirement to share most clinical notes is new, there is already a wealth of evidence from the earliest adopters (part of the OpenNotes movement) affirming the significant benefits from this practice – for patients and providers – with few negative effects on workflows or documentation patterns.2 Findings published as early as 2012, and regularly since then, among OpenNotes adopters from a diverse set of health care institutions have shown access to notes improves patient engagement, activation, and communication, as well as patient and clinician satisfaction.3
Still, providers may argue, shouldn’t clinical notes be a space where providers are free to articulate uncertainties, work through clinical reasoning, and share subtle observations about a patient’s presentation and findings with colleagues without having to worry about alarming patients who may lack the background to understand medical nuances?
It’s a fine balance in certain situations since we want to document our objective clinical assessments and prognoses without needlessly upsetting our patients, especially when considering a potentially life-changing diagnosis. How do we continue offering hope to our patients while still respecting their autonomy and sharing their health information with them? There is no uniform approach or standard playbook to follow since each patient and clinical circumstance is unique.
Fundamentally, sharing clinical notes is about granting access to one’s own health information, promoting patient activation and engagement, and making health care more patient centered. As a clinician, it’s important to frame the conversations we have with our patients so they are not surprised or caught off guard by what we have written in our notes. If you had a difficult or contentious conversation, document it objectively and without bias. If you are discussing obesity, substance abuse, or mental health, do so respectfully, supportively, and without judgment. If one of the reasons you are doing a CT scan is to rule out pancreatic cancer, it’s hard to argue that the patient does not deserve to know that beforehand.
The OpenNotes experience to date has consistently shown that patients benefit from direct discussions and transparency, which can even motivate difficult behavior changes.4 As clinicians, we may have to make minor changes in how we document, such as using less medical jargon and fewer abbreviations, but based on data from the longest tenured participants in OpenNotes, these adjustments do not add to documentation burdens.5 An activated patient who is reading their notes is an engaged patient, one who will often collaborate more in their own care, offer additional insights, and feel more empowered to take responsibility for their own health.6
When surveyed, patients report that access to their clinical notes helps them feel more in control of their health by understanding their medical conditions better, which makes them feel more prepared for their visits.4 Studies have shown that patients forget between 40%-80% of the information communicated during a visit, making clinical notes a valuable reminder and reference. Over 75% of patients in one study reported that reading notes helped them better understand the meaning of results and the rationale for referrals and tests, which led to greater follow-through with their treatment plans and follow-up appointments.3 A remarkable 99% of patients in the same study reported feeling the same or better about their physician after reading their notes.
Sharing notes with patients also makes care safer and more equitable. A written record of a visit serves as an important source of information about why a medicine is prescribed, a reminder about additions or changes to a regimen, and potential adverse effects of medications. In the first OpenNotes study, which had more than 100 primary care physicians and 20,000 patients, 60%-78% of patients with access to their notes reported improved medication adherence.2 A later study reported similar benefits, particularly among patients who identify as racial or ethnic minorities, non-native English speakers, and those with a high school education or less. These findings may reflect increased trust that comes with a more collaborative relationship between providers and patients. Patients who can read their notes also show a willingness to review their medication lists and report discrepancies and errors, making their care safer still.7
Conclusion
The move to widespread shared notes, though prompted by a federal mandate, is a critical step forward in patient activation, engagement, and satisfaction. Importantly, there is a large body of evidence showing multiple benefits, including better communication and safer and more equitable care at sites that have already been sharing notes for over a decade. When surveyed, both patients and providers who have been participating in shared notes believe the practice should continue.
In April 2021, we began a massive natural experiment in the U.S. with ubiquitous sharing of clinical notes, one that will help us learn more about how best to make our patients’ health information accessible, meaningful, and most meaningful in improving their overall health and well-being. Sharing notes with our patients is at once relatively easy to implement but complex in its implications and represents a significant paradigm shift in medicine toward a safer, more patient-centered approach. The evidence to date has shown that embracing shared notes promotes greater patient activation and engagement, and with it a more transparent and collaborative relationship between providers and patients that could lead to transformative benefits to the quality of the care we can achieve together.
Dr. Shah is an associate professor of medicine and pediatrics and associate chief medical information officer at University of Chicago Medicine. He has no disclosures
References
1. 21st Century Cures Act, HR 34, 114th Congress (2015). Accessed 2021 Sep 23. https://www.congress.gov/bill/114th-congress/house-bill/34.
2. Delbanco T et al. Ann Intern Med. 2012 Oct;157(7):461-70.
3. Bell S et al. BMJ Qual Saf. 2017 Apr;26(4):262-70.
4. Walker J et al. J Med Internet Res. 2019 May. doi: 10.2196/13876.
5. DesRoches C et al. JAMA Netw Open. 2020 Mar. doi: 10.1001/jamanetworkopen.2020.1753.
6. Blease C et al. J Med Ethics. 2021 May. doi: 10.1136/medethics-2021-107275.
7. DesRoches C et al. Ann Intern Med. 2019 Jul 2;171(1):69-71.
Standing up to ‘injustice in health’: The Association of Black Gastroenterologists and Hepatologists
“Of all the forms of inequality, injustice in health is the most shocking and inhuman.” – Martin Luther King Jr., March 25, 1966. 1
This single disparity – health care injustice – too often results in needless mental anguish, physical suffering, or death. In the spring of 2020, at the peak of the COVID-19 pandemic, the convergence of injustices in health care and policing led to the disproportionate preventable physical deaths of Black men and women. This became the watershed moment for 11 gastroenterologists and hepatologists who collectively grieved but heeded the call of social responsibility to form the Association of Black Gastroenterologists and Hepatologists.
The mission of ABGH is laser focused. It is to promote health equity in Black communities, advance science, and develop the careers of Black gastroenterologists, hepatologists, and scientists. The vision is to improve gastrointestinal health outcomes in Black communities; to develop the pipeline of Black gastroenterologists and hepatologists given that currently only 4% in the United States identify as Black; to foster networking, mentoring, and sponsorship among Black students, clinical trainees, gastroenterologists, and hepatologists; and to promote the scholarship of Black gastroenterologists and hepatologists.
Through community engagement, ABGH stands to empower the Black community with knowledge and choices, which inherently strengthens the physician-patient relationship. ABGH also exists to implement positive change in long term outcome statistics in Black communities. Black Americans are 20% more likely to be diagnosed with colorectal cancer and 40% more likely to die from the disease. In addition to colorectal cancer, rates of esophageal squamous cancer, as well as cancer of the small bowel and pancreas, are highest in Black people.2 Through scientific research and clinical care, we aim to eradicate digestive health disparities.
Yet in this space, we know first-hand that, in the United States, the wellness of a community is not measured by the medical fitness of its members alone but also by the availability of equitable opportunities for fulfillment of nonmedical but health-impacting social needs. These needs, also known as social determinants of health, are made inaccessible to vulnerable populations because of systemic racism. Importantly, we recognize that dismantling racist systems is not a singular effort, nor are we pioneers in this work, but we look forward to executing health equity goals collaboratively with our fellow gastrointestinal national societies and other leading community and grassroots organizations.
The founders of ABGH are a distinctive group of practicing gastroenterologists and hepatologists from across the United States with a strong track record in DEI work through their community, clinical, and research activities. The board of directors reflects only the depth of talent shared throughout the ABGH membership. The strength of the organization lies in its diverse and energetic constituents who all exemplify outstanding training and the readiness to redefine the standard of health care delivery to the Black community. From medical students to senior level gastroenterologists, we collectively embody a considerable momentum for formation of this organization at this point in our history.
ABGH fulfills a professional career development need for budding gastroenterologists not so readily available from other organizations. The compelling impact of representing the embodiment of what many of us were told we could not become is limitless. The personal and professional growth enabled by our networking and learning from each other is both motivating and empowering since, even after overcoming the obstacles needed to become a medical provider, Black professionals are often not afforded the bandwidth, range of emotion, and protection to reveal their specific needs. For this author personally, the ABGH provides a psychological safety that allows authentic self-identity without code-switching.3 Through this authenticity has arisen formidable strength, creativity, and productivity. The leadership and innovation cultivated in ABGH stands to benefit many generations to come, both within and outside the organization.
Reflections from a junior member of ABGH: Dr. Kafayat Busari
My desire to pursue gastroenterology was bolstered by determination, curiosity, and passion, yet ironically was often met with skepticism by many in position to help advance this goal. Although projections of incertitude on members of a community that are often made to feel inadequate can diminish even the brightest of lights, conversely it can fuel the creation of an organization emboldened to specifically address GI-related health disparities. When I was a second-year internal medicine resident, I encountered a GI physician who told me GI “wasn’t something I wanted to do”—despite me expressing my interest.
Confused by the statement, I reached out to Renee L. Williams, MD, of NYU Langone Health, who I had met during my medical school training. She suggested I join a conference call later that week. On that call and the many that took place thereafter, I was introduced to Black gastroenterologists who are luminal disease experts, chair members, journal editors, transplant hepatologists, interventional endoscopists, researchers, and professors (in other words, GI professional leaders). My time on the initial call lasted perhaps less than 20 minutes, but the impact has been immeasurable.
I was provided the emotional reassurance that GI was indeed for me and told “there’s always a seat, and if it feels like there’s not, we just need to get more chairs.” Little did I know, but those metaphorical chairs were being gathered so that I and other aspiring gastroenterologists will be able to sit comfortably at these tables one day. I was witnessing these GI professional leaders set in motion the beginning of what will undoubtedly be a pivotal component in the way I approach my career as a gastroenterologist. The experience reignited my mental determination to one day attain the level of success represented by the ABGH board members and to persevere in my quest to help redefine how Black medical students and residents serve their communities as physicians.
The creation of the ABGH could not have come at a better time in my training. In the wake of recent public protests for equity of African Americans within every institution (academia, housing, banks, policing, health care, and beyond), which were fundamentally built on racism, being a junior member of ABGH has not only given me a platform to speak my truth but has also provided me with tools to help others do so as well. As someone very passionate about research, primarily in colorectal cancer, I have been given an opportunity to connect with a dream team of mentors who have taken research ideas to new levels and have challenged me to dig deeper and expand my curiosity to investigate what still needs to be uncovered. It has created opportunity after opportunity for actively building relationships, leading to meaningful collaborations and the sharing of innovative ideas and discoveries.
It is important to emphasize that ABGH is not an organization wanting to exclude themselves on the basis of ethnicity. ABGH is an example of how shared health goals within a medical discipline can be achieved when inclusion and equity is at the helm. ABGH led and represented events that raise awareness of diseases affecting all patients and aim to make the GI community more culturally competent. ABGH is future-oriented and embraces all members who align with the mission regardless of ethnicity, gender, orientation, or disability. The institution that is and will be the ABGH impresses upon me a feeling of excitement, gratitude, and humility. I look forward to continuing the mission created by the founding members and being to others what ABGH is to me.
For more information on this organization, please visit blackingastro.org.
Dr. Busari is a resident physician at Florida State University-SMH and a junior member of ABGH. Dr. Guillaume director of the Gastrointestinal Motility Center at Stony Brook (New York) University Hospital and an assistant professor of medicine at the Renaissance School of Medicine at Stony Brook University. They have no disclosures.
References
1. Galarneau C. J Health Care Poor Underserved. 2018;29(1):5-8.
2. Ashktorab H et al. Gastroenterology. 2017 Oct;153(4):910-923.
3. Blanchard AK. N Engl J Med. 2021 Jun 10;384(23):e87.
“Of all the forms of inequality, injustice in health is the most shocking and inhuman.” – Martin Luther King Jr., March 25, 1966. 1
This single disparity – health care injustice – too often results in needless mental anguish, physical suffering, or death. In the spring of 2020, at the peak of the COVID-19 pandemic, the convergence of injustices in health care and policing led to the disproportionate preventable physical deaths of Black men and women. This became the watershed moment for 11 gastroenterologists and hepatologists who collectively grieved but heeded the call of social responsibility to form the Association of Black Gastroenterologists and Hepatologists.
The mission of ABGH is laser focused. It is to promote health equity in Black communities, advance science, and develop the careers of Black gastroenterologists, hepatologists, and scientists. The vision is to improve gastrointestinal health outcomes in Black communities; to develop the pipeline of Black gastroenterologists and hepatologists given that currently only 4% in the United States identify as Black; to foster networking, mentoring, and sponsorship among Black students, clinical trainees, gastroenterologists, and hepatologists; and to promote the scholarship of Black gastroenterologists and hepatologists.
Through community engagement, ABGH stands to empower the Black community with knowledge and choices, which inherently strengthens the physician-patient relationship. ABGH also exists to implement positive change in long term outcome statistics in Black communities. Black Americans are 20% more likely to be diagnosed with colorectal cancer and 40% more likely to die from the disease. In addition to colorectal cancer, rates of esophageal squamous cancer, as well as cancer of the small bowel and pancreas, are highest in Black people.2 Through scientific research and clinical care, we aim to eradicate digestive health disparities.
Yet in this space, we know first-hand that, in the United States, the wellness of a community is not measured by the medical fitness of its members alone but also by the availability of equitable opportunities for fulfillment of nonmedical but health-impacting social needs. These needs, also known as social determinants of health, are made inaccessible to vulnerable populations because of systemic racism. Importantly, we recognize that dismantling racist systems is not a singular effort, nor are we pioneers in this work, but we look forward to executing health equity goals collaboratively with our fellow gastrointestinal national societies and other leading community and grassroots organizations.
The founders of ABGH are a distinctive group of practicing gastroenterologists and hepatologists from across the United States with a strong track record in DEI work through their community, clinical, and research activities. The board of directors reflects only the depth of talent shared throughout the ABGH membership. The strength of the organization lies in its diverse and energetic constituents who all exemplify outstanding training and the readiness to redefine the standard of health care delivery to the Black community. From medical students to senior level gastroenterologists, we collectively embody a considerable momentum for formation of this organization at this point in our history.
ABGH fulfills a professional career development need for budding gastroenterologists not so readily available from other organizations. The compelling impact of representing the embodiment of what many of us were told we could not become is limitless. The personal and professional growth enabled by our networking and learning from each other is both motivating and empowering since, even after overcoming the obstacles needed to become a medical provider, Black professionals are often not afforded the bandwidth, range of emotion, and protection to reveal their specific needs. For this author personally, the ABGH provides a psychological safety that allows authentic self-identity without code-switching.3 Through this authenticity has arisen formidable strength, creativity, and productivity. The leadership and innovation cultivated in ABGH stands to benefit many generations to come, both within and outside the organization.
Reflections from a junior member of ABGH: Dr. Kafayat Busari
My desire to pursue gastroenterology was bolstered by determination, curiosity, and passion, yet ironically was often met with skepticism by many in position to help advance this goal. Although projections of incertitude on members of a community that are often made to feel inadequate can diminish even the brightest of lights, conversely it can fuel the creation of an organization emboldened to specifically address GI-related health disparities. When I was a second-year internal medicine resident, I encountered a GI physician who told me GI “wasn’t something I wanted to do”—despite me expressing my interest.
Confused by the statement, I reached out to Renee L. Williams, MD, of NYU Langone Health, who I had met during my medical school training. She suggested I join a conference call later that week. On that call and the many that took place thereafter, I was introduced to Black gastroenterologists who are luminal disease experts, chair members, journal editors, transplant hepatologists, interventional endoscopists, researchers, and professors (in other words, GI professional leaders). My time on the initial call lasted perhaps less than 20 minutes, but the impact has been immeasurable.
I was provided the emotional reassurance that GI was indeed for me and told “there’s always a seat, and if it feels like there’s not, we just need to get more chairs.” Little did I know, but those metaphorical chairs were being gathered so that I and other aspiring gastroenterologists will be able to sit comfortably at these tables one day. I was witnessing these GI professional leaders set in motion the beginning of what will undoubtedly be a pivotal component in the way I approach my career as a gastroenterologist. The experience reignited my mental determination to one day attain the level of success represented by the ABGH board members and to persevere in my quest to help redefine how Black medical students and residents serve their communities as physicians.
The creation of the ABGH could not have come at a better time in my training. In the wake of recent public protests for equity of African Americans within every institution (academia, housing, banks, policing, health care, and beyond), which were fundamentally built on racism, being a junior member of ABGH has not only given me a platform to speak my truth but has also provided me with tools to help others do so as well. As someone very passionate about research, primarily in colorectal cancer, I have been given an opportunity to connect with a dream team of mentors who have taken research ideas to new levels and have challenged me to dig deeper and expand my curiosity to investigate what still needs to be uncovered. It has created opportunity after opportunity for actively building relationships, leading to meaningful collaborations and the sharing of innovative ideas and discoveries.
It is important to emphasize that ABGH is not an organization wanting to exclude themselves on the basis of ethnicity. ABGH is an example of how shared health goals within a medical discipline can be achieved when inclusion and equity is at the helm. ABGH led and represented events that raise awareness of diseases affecting all patients and aim to make the GI community more culturally competent. ABGH is future-oriented and embraces all members who align with the mission regardless of ethnicity, gender, orientation, or disability. The institution that is and will be the ABGH impresses upon me a feeling of excitement, gratitude, and humility. I look forward to continuing the mission created by the founding members and being to others what ABGH is to me.
For more information on this organization, please visit blackingastro.org.
Dr. Busari is a resident physician at Florida State University-SMH and a junior member of ABGH. Dr. Guillaume director of the Gastrointestinal Motility Center at Stony Brook (New York) University Hospital and an assistant professor of medicine at the Renaissance School of Medicine at Stony Brook University. They have no disclosures.
References
1. Galarneau C. J Health Care Poor Underserved. 2018;29(1):5-8.
2. Ashktorab H et al. Gastroenterology. 2017 Oct;153(4):910-923.
3. Blanchard AK. N Engl J Med. 2021 Jun 10;384(23):e87.
“Of all the forms of inequality, injustice in health is the most shocking and inhuman.” – Martin Luther King Jr., March 25, 1966. 1
This single disparity – health care injustice – too often results in needless mental anguish, physical suffering, or death. In the spring of 2020, at the peak of the COVID-19 pandemic, the convergence of injustices in health care and policing led to the disproportionate preventable physical deaths of Black men and women. This became the watershed moment for 11 gastroenterologists and hepatologists who collectively grieved but heeded the call of social responsibility to form the Association of Black Gastroenterologists and Hepatologists.
The mission of ABGH is laser focused. It is to promote health equity in Black communities, advance science, and develop the careers of Black gastroenterologists, hepatologists, and scientists. The vision is to improve gastrointestinal health outcomes in Black communities; to develop the pipeline of Black gastroenterologists and hepatologists given that currently only 4% in the United States identify as Black; to foster networking, mentoring, and sponsorship among Black students, clinical trainees, gastroenterologists, and hepatologists; and to promote the scholarship of Black gastroenterologists and hepatologists.
Through community engagement, ABGH stands to empower the Black community with knowledge and choices, which inherently strengthens the physician-patient relationship. ABGH also exists to implement positive change in long term outcome statistics in Black communities. Black Americans are 20% more likely to be diagnosed with colorectal cancer and 40% more likely to die from the disease. In addition to colorectal cancer, rates of esophageal squamous cancer, as well as cancer of the small bowel and pancreas, are highest in Black people.2 Through scientific research and clinical care, we aim to eradicate digestive health disparities.
Yet in this space, we know first-hand that, in the United States, the wellness of a community is not measured by the medical fitness of its members alone but also by the availability of equitable opportunities for fulfillment of nonmedical but health-impacting social needs. These needs, also known as social determinants of health, are made inaccessible to vulnerable populations because of systemic racism. Importantly, we recognize that dismantling racist systems is not a singular effort, nor are we pioneers in this work, but we look forward to executing health equity goals collaboratively with our fellow gastrointestinal national societies and other leading community and grassroots organizations.
The founders of ABGH are a distinctive group of practicing gastroenterologists and hepatologists from across the United States with a strong track record in DEI work through their community, clinical, and research activities. The board of directors reflects only the depth of talent shared throughout the ABGH membership. The strength of the organization lies in its diverse and energetic constituents who all exemplify outstanding training and the readiness to redefine the standard of health care delivery to the Black community. From medical students to senior level gastroenterologists, we collectively embody a considerable momentum for formation of this organization at this point in our history.
ABGH fulfills a professional career development need for budding gastroenterologists not so readily available from other organizations. The compelling impact of representing the embodiment of what many of us were told we could not become is limitless. The personal and professional growth enabled by our networking and learning from each other is both motivating and empowering since, even after overcoming the obstacles needed to become a medical provider, Black professionals are often not afforded the bandwidth, range of emotion, and protection to reveal their specific needs. For this author personally, the ABGH provides a psychological safety that allows authentic self-identity without code-switching.3 Through this authenticity has arisen formidable strength, creativity, and productivity. The leadership and innovation cultivated in ABGH stands to benefit many generations to come, both within and outside the organization.
Reflections from a junior member of ABGH: Dr. Kafayat Busari
My desire to pursue gastroenterology was bolstered by determination, curiosity, and passion, yet ironically was often met with skepticism by many in position to help advance this goal. Although projections of incertitude on members of a community that are often made to feel inadequate can diminish even the brightest of lights, conversely it can fuel the creation of an organization emboldened to specifically address GI-related health disparities. When I was a second-year internal medicine resident, I encountered a GI physician who told me GI “wasn’t something I wanted to do”—despite me expressing my interest.
Confused by the statement, I reached out to Renee L. Williams, MD, of NYU Langone Health, who I had met during my medical school training. She suggested I join a conference call later that week. On that call and the many that took place thereafter, I was introduced to Black gastroenterologists who are luminal disease experts, chair members, journal editors, transplant hepatologists, interventional endoscopists, researchers, and professors (in other words, GI professional leaders). My time on the initial call lasted perhaps less than 20 minutes, but the impact has been immeasurable.
I was provided the emotional reassurance that GI was indeed for me and told “there’s always a seat, and if it feels like there’s not, we just need to get more chairs.” Little did I know, but those metaphorical chairs were being gathered so that I and other aspiring gastroenterologists will be able to sit comfortably at these tables one day. I was witnessing these GI professional leaders set in motion the beginning of what will undoubtedly be a pivotal component in the way I approach my career as a gastroenterologist. The experience reignited my mental determination to one day attain the level of success represented by the ABGH board members and to persevere in my quest to help redefine how Black medical students and residents serve their communities as physicians.
The creation of the ABGH could not have come at a better time in my training. In the wake of recent public protests for equity of African Americans within every institution (academia, housing, banks, policing, health care, and beyond), which were fundamentally built on racism, being a junior member of ABGH has not only given me a platform to speak my truth but has also provided me with tools to help others do so as well. As someone very passionate about research, primarily in colorectal cancer, I have been given an opportunity to connect with a dream team of mentors who have taken research ideas to new levels and have challenged me to dig deeper and expand my curiosity to investigate what still needs to be uncovered. It has created opportunity after opportunity for actively building relationships, leading to meaningful collaborations and the sharing of innovative ideas and discoveries.
It is important to emphasize that ABGH is not an organization wanting to exclude themselves on the basis of ethnicity. ABGH is an example of how shared health goals within a medical discipline can be achieved when inclusion and equity is at the helm. ABGH led and represented events that raise awareness of diseases affecting all patients and aim to make the GI community more culturally competent. ABGH is future-oriented and embraces all members who align with the mission regardless of ethnicity, gender, orientation, or disability. The institution that is and will be the ABGH impresses upon me a feeling of excitement, gratitude, and humility. I look forward to continuing the mission created by the founding members and being to others what ABGH is to me.
For more information on this organization, please visit blackingastro.org.
Dr. Busari is a resident physician at Florida State University-SMH and a junior member of ABGH. Dr. Guillaume director of the Gastrointestinal Motility Center at Stony Brook (New York) University Hospital and an assistant professor of medicine at the Renaissance School of Medicine at Stony Brook University. They have no disclosures.
References
1. Galarneau C. J Health Care Poor Underserved. 2018;29(1):5-8.
2. Ashktorab H et al. Gastroenterology. 2017 Oct;153(4):910-923.
3. Blanchard AK. N Engl J Med. 2021 Jun 10;384(23):e87.
An update on COVID-19 vaccine recommendations for patients with IBD
In December 2019, cases of pulmonary infection secondary to a novel coronavirus, known as severe acute respiratory syndrome coronavirus 2, were first identified in the city of Wuhan, China.
The clinical disease caused by the virus, COVID-19, has resulted in a worldwide pandemic that has portended significant morbidity and mortality throughout the United States. Three highly efficacious COVID-19 vaccines have received emergency use authorization (EUA) by the Food and Drug Administration to help prevent COVID-19, all of which are effective at preventing severe COVID-19.1-3 The Pfizer vaccine was given full FDA approval on Aug. 23, 2021.4
Patients with inflammatory bowel disease (IBD) are commonly treated with immune-modifying therapies that may increase their risk for serious and opportunistic infections. As such, there was concern at the beginning of the pandemic that patients with IBD may be at increased risk of contracting COVID-19 and/or developing severe disease (that is, ICU-level care, mechanical ventilation, and/or death). There is evidence that the incidence of COVID-19 in the IBD population is similar to that of the general population.5-7 Furthermore, most patients with IBD are not at increased risk of severe disease, including those on biologic therapies. Several studies demonstrated that those on corticosteroids are at increased risk of severe COVID-19, while those on other immune-modifying therapies such as tumor necrosis factor inhibitors (anti-TNFs) are not at increased risk.5,7-10 Patients with IBD with other well-known risk factors for severe disease include comorbidities such as diabetes and obesity.
It is known that patients with IBD on certain immune-modifying therapies such as anti-TNFs, especially those on combination therapy, may have a blunted immune response to certain vaccines.11 Neither patients with IBD nor patients on immunosuppressive therapy were included in phase 3 clinical trials for COVID-19 vaccine development, contributing to uncertainty regarding the safety and efficacy in our patient population. The risk of adverse events following COVID-19 vaccination in the IBD population has been found to be similar to that of the general population.12 It has also been reported that those who have had reactions to injectable therapies in the past may safely be vaccinated against COVID-19.13,14 With regard to vaccine efficacy, initial studies, including ICARUS, PREVENT-COVID, and CORALE-IBD, have demonstrated that patients with IBD do indeed mount a humoral immune response to the vaccine, including those on immune-modifying therapies.15-17 Nonhumoral aspects of immunity, such as cell-mediated immunity, have not yet been thoroughly evaluated. In addition, the risk of breakthrough COVID-19 infection after vaccination is low in patients with IBD, including those on immune-modifying therapy.14-18 While initial studies are reassuring that the vast majority of patients with IBD are able to mount a vaccine response, future studies are needed to determine the effects of immune-modifying therapy on sustained antibody concentrations and other correlates of immunity.
For those who received the Pfizer or Moderna vaccines, on Aug. 12, 2021, the FDA amended their EUA to allow for an additional dose in the initial vaccination series for certain immunocompromised individuals, specifically solid organ transplant recipients or those with conditions that make them equally immunocompromised.19 Based on evidence suggesting that certain solid organ transplant recipients do not mount an immune response after completing a two-dose series, the Advisory Committee on Immunization Practices, which advises the Centers for Disease Control and Prevention on how to use vaccines, recommended that moderate to severely immunocompromised individuals should complete a three-dose series, with the third dose being given at least 28 days after the second dose.20 This recommendation included those on high-dose corticosteroids defined as oral prednisone at least 20 mg/day, anti-TNFs and biosimilars, and antimetabolites such as azathioprine, mercaptopurine, and methotrexate.
It is worth noting that the role of the ACIP here was to consider the available evidence supporting the use of an additional dose and then make recommendations on which conditions may qualify; the ACIP was not able to provide recommendations for every disease state. At the time of writing this article, no recommendations have been made with regards to an additional dose of the Janssen vaccine. Likewise, in response to the ACIP recommendations, the Crohn’s & Colitis Foundation recommended an additional dose for patients with IBD on immune-modifying therapies.21,22
Less than one week after the EUA amendment for an additional dose, the Department of Health & Human Services announced that booster shots would likely become available to the general population as early as the week of Sept. 20, 2021 and starting 8 months after an individual’s second dose.23 Here, it is worth noting that an additional dose is distinct from a booster. An additional dose (or third dose here) refers to the initial vaccination series and is given when the standard schedule is thought to be insufficient in a certain patient population. In contrast, a booster dose is administered when the initial and sufficient immunity gained from a primary vaccination series has likely dissipated. The HHS acknowledged that boosters would likely be needed for those who received the Janssen vaccine but noted that further data and recommendations would be forthcoming.
To summarize, COVID-19 vaccines are safe and effective in the IBD population, and patients should be vaccinated at the earliest opportunity regardless of concurrent therapies. For those that received the Pfizer or Moderna vaccine, the ACIP recommended an additional dose in the initial vaccination series to be given at least 28 days after the second dose for those that are immunosuppressed. This recommendation was largely based off of transplant data. Reassuringly, the available data demonstrates a humoral immune response to a two-dose vaccination series in patients with IBD, including those on immune-modifying therapies. The Crohn’s & Colitis Foundation recommends that patients with IBD on immune-modifying therapy receive an additional dose (i.e., a three-dose series), which should be from the same manufacturer as the first two doses. In addition, at press time, HHS indicated that there will be a movement toward a booster dose for the general population in late September, which would also apply to patients with IBD. The ACIP has yet to comment on this change at the time of preparing this article, but the announcement indicated that a booster could be given “8 months after an individual’s second dose.” It is unclear how those who may receive a three-dose vaccination series will factor in, but it is possible that they would be eligible for a booster 8 months after their most recent dose. Gastroenterologists should also be aware that there is no role for serologic testing in the clinical setting because it has not been validated for such purposes and is primarily used in the research setting. Finally, it is paramount to emphasize that patients with IBD have historically had lower vaccination rates than the general population,24 and we must take an active role in ensuring that our patients are immunized by addressing their concerns, communicating the risks of COVID-19 and the benefits of vaccination, providing information on how to get vaccinated, and strongly recommending vaccination.
The following list also summarizes the recommendations:
- Patients with IBD should be vaccinated against COVID-19 regardless of concurrent therapies.
- Patients with IBD are not at increased risk of severe COVID-19.
- Patients with IBD, including those on immune-modifying therapies, mount a humoral immune response to the vaccine.
- Patients with IBD on immune-modifying therapies, who received either the Pfizer or Moderna vaccine, should receive a three-dose vaccination series, with the third dose at least 28 days after the second dose.
- Patients with IBD on biologic therapy can receive the third dose of the vaccine at any time point and should not interrupt biologic therapy.
- Boosters are likely to become available to the general public in September and would be given at least 8 months after an individual’s second dose.
- Recommendations regarding boosters for those who received a three-dose vaccination series are forthcoming.
- Recommendations regarding boosters and additional doses for those that received the Janssen vaccine are forthcoming.
- Gastroenterologists should take an active role in ensuring that their patients are vaccinated.
Dr. Schell is a second-year graduate student in the division of internal medicine at the University of Wisconsin–Madison. Dr. Caldera is an associate professor of medicine in the division of gastroenterology & hepatology at the University of Wisconsin–Madison. Dr. Schell has no conflicts of interest to disclose. Dr. Caldera has received research support from Takeda Pharmaceuticals and Sanofi. He has been a consultant for Takeda, Arena Pharmaceuticals, GSK, and Celgene.
References
1. Sadoff J et al. N Engl J Med. 2021;384(23):2187-201.
2. Baden LR et al. N Engl J Med. 2021;384(5):403-16.
3. Polack FP et al. N Engl J Med. 2020;383:2603-15.
4. Johnson K et al. U.S. FDA aims to give full approval to Pfizer vaccine on Monday – NYT. Reuters. 2021 Aug 20. https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-aims-give-full-nod-pfizers-covid-19-vaccine-monday-new-york-times-2021-08-20/.
5. Allocca M et al. J Clin Med. 2020 Oct;9(11):3533.
6. Monteleone G and Ardizzone S. J Crohns Colitis. 2020 Sep;14(9):1334-6.
7. Papa A et al. Am J Gastroenterol. 2020;115(10):1722-4.
8. Derikx LAAP et al. J Crohn’s Colitis. 2021 Apr 6;15(4):529-39.
9. Brenner EJ et al. Gastroenterology. 2020;159(2):481-91.
10. Ungaro RC et al. Gut. 2021;70(4):725-32.
11. Caldera F et al. Inflamm Bowel Dis. 2020;26(4):593-602.
12. Botwin GJ et al. Am J Gastroenterol. 2021. doi: 10.14309/ajg.0000000000001342.
13. Squire JD et al. Inflamm Bowel Dis. 2021 Jul 27;27(8):1358-60.
14. Hadi YB et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.014.
15. Wong S-Y et al. Gastroenterology. 2021;161:715-8.
16. Kappelman MD et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.016.
17. Pozdnyakova V et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.08.014.
18. Ben-Tov A et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.076.
19. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. FDA News Release. 2021. Accessed 2021 Aug 18. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised.
20. Centers for Disease Control and Prevention. COVID-19 Vaccines for Moderately to Severely Immunocompromised People. 2021. Accessed 2021 Aug 18. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.
21. Allocca M et al. J Clin Med. 2020 Oct 31;9(11):3533.
22. Crohn’s & Colitis Foundation. COVID-19 Vaccines: Position Statements. IBD & Coronavirus. 2021. Accessed 2021 Aug 20. https://www.crohnscolitisfoundation.org/coronavirus/vaccine-position-statements.
23. Centers for Disease Control and Prevention. Joint Statement from HHS Public Health and Medical Experts on COVID-19 Booster Shots. https://www.cdc.gov/media/releases/2021/s0818-covid-19-booster-shots.html.
24. Caldera F et al. Inflamm Bowel Dis. 2021;27(1):123-133.
In December 2019, cases of pulmonary infection secondary to a novel coronavirus, known as severe acute respiratory syndrome coronavirus 2, were first identified in the city of Wuhan, China.
The clinical disease caused by the virus, COVID-19, has resulted in a worldwide pandemic that has portended significant morbidity and mortality throughout the United States. Three highly efficacious COVID-19 vaccines have received emergency use authorization (EUA) by the Food and Drug Administration to help prevent COVID-19, all of which are effective at preventing severe COVID-19.1-3 The Pfizer vaccine was given full FDA approval on Aug. 23, 2021.4
Patients with inflammatory bowel disease (IBD) are commonly treated with immune-modifying therapies that may increase their risk for serious and opportunistic infections. As such, there was concern at the beginning of the pandemic that patients with IBD may be at increased risk of contracting COVID-19 and/or developing severe disease (that is, ICU-level care, mechanical ventilation, and/or death). There is evidence that the incidence of COVID-19 in the IBD population is similar to that of the general population.5-7 Furthermore, most patients with IBD are not at increased risk of severe disease, including those on biologic therapies. Several studies demonstrated that those on corticosteroids are at increased risk of severe COVID-19, while those on other immune-modifying therapies such as tumor necrosis factor inhibitors (anti-TNFs) are not at increased risk.5,7-10 Patients with IBD with other well-known risk factors for severe disease include comorbidities such as diabetes and obesity.
It is known that patients with IBD on certain immune-modifying therapies such as anti-TNFs, especially those on combination therapy, may have a blunted immune response to certain vaccines.11 Neither patients with IBD nor patients on immunosuppressive therapy were included in phase 3 clinical trials for COVID-19 vaccine development, contributing to uncertainty regarding the safety and efficacy in our patient population. The risk of adverse events following COVID-19 vaccination in the IBD population has been found to be similar to that of the general population.12 It has also been reported that those who have had reactions to injectable therapies in the past may safely be vaccinated against COVID-19.13,14 With regard to vaccine efficacy, initial studies, including ICARUS, PREVENT-COVID, and CORALE-IBD, have demonstrated that patients with IBD do indeed mount a humoral immune response to the vaccine, including those on immune-modifying therapies.15-17 Nonhumoral aspects of immunity, such as cell-mediated immunity, have not yet been thoroughly evaluated. In addition, the risk of breakthrough COVID-19 infection after vaccination is low in patients with IBD, including those on immune-modifying therapy.14-18 While initial studies are reassuring that the vast majority of patients with IBD are able to mount a vaccine response, future studies are needed to determine the effects of immune-modifying therapy on sustained antibody concentrations and other correlates of immunity.
For those who received the Pfizer or Moderna vaccines, on Aug. 12, 2021, the FDA amended their EUA to allow for an additional dose in the initial vaccination series for certain immunocompromised individuals, specifically solid organ transplant recipients or those with conditions that make them equally immunocompromised.19 Based on evidence suggesting that certain solid organ transplant recipients do not mount an immune response after completing a two-dose series, the Advisory Committee on Immunization Practices, which advises the Centers for Disease Control and Prevention on how to use vaccines, recommended that moderate to severely immunocompromised individuals should complete a three-dose series, with the third dose being given at least 28 days after the second dose.20 This recommendation included those on high-dose corticosteroids defined as oral prednisone at least 20 mg/day, anti-TNFs and biosimilars, and antimetabolites such as azathioprine, mercaptopurine, and methotrexate.
It is worth noting that the role of the ACIP here was to consider the available evidence supporting the use of an additional dose and then make recommendations on which conditions may qualify; the ACIP was not able to provide recommendations for every disease state. At the time of writing this article, no recommendations have been made with regards to an additional dose of the Janssen vaccine. Likewise, in response to the ACIP recommendations, the Crohn’s & Colitis Foundation recommended an additional dose for patients with IBD on immune-modifying therapies.21,22
Less than one week after the EUA amendment for an additional dose, the Department of Health & Human Services announced that booster shots would likely become available to the general population as early as the week of Sept. 20, 2021 and starting 8 months after an individual’s second dose.23 Here, it is worth noting that an additional dose is distinct from a booster. An additional dose (or third dose here) refers to the initial vaccination series and is given when the standard schedule is thought to be insufficient in a certain patient population. In contrast, a booster dose is administered when the initial and sufficient immunity gained from a primary vaccination series has likely dissipated. The HHS acknowledged that boosters would likely be needed for those who received the Janssen vaccine but noted that further data and recommendations would be forthcoming.
To summarize, COVID-19 vaccines are safe and effective in the IBD population, and patients should be vaccinated at the earliest opportunity regardless of concurrent therapies. For those that received the Pfizer or Moderna vaccine, the ACIP recommended an additional dose in the initial vaccination series to be given at least 28 days after the second dose for those that are immunosuppressed. This recommendation was largely based off of transplant data. Reassuringly, the available data demonstrates a humoral immune response to a two-dose vaccination series in patients with IBD, including those on immune-modifying therapies. The Crohn’s & Colitis Foundation recommends that patients with IBD on immune-modifying therapy receive an additional dose (i.e., a three-dose series), which should be from the same manufacturer as the first two doses. In addition, at press time, HHS indicated that there will be a movement toward a booster dose for the general population in late September, which would also apply to patients with IBD. The ACIP has yet to comment on this change at the time of preparing this article, but the announcement indicated that a booster could be given “8 months after an individual’s second dose.” It is unclear how those who may receive a three-dose vaccination series will factor in, but it is possible that they would be eligible for a booster 8 months after their most recent dose. Gastroenterologists should also be aware that there is no role for serologic testing in the clinical setting because it has not been validated for such purposes and is primarily used in the research setting. Finally, it is paramount to emphasize that patients with IBD have historically had lower vaccination rates than the general population,24 and we must take an active role in ensuring that our patients are immunized by addressing their concerns, communicating the risks of COVID-19 and the benefits of vaccination, providing information on how to get vaccinated, and strongly recommending vaccination.
The following list also summarizes the recommendations:
- Patients with IBD should be vaccinated against COVID-19 regardless of concurrent therapies.
- Patients with IBD are not at increased risk of severe COVID-19.
- Patients with IBD, including those on immune-modifying therapies, mount a humoral immune response to the vaccine.
- Patients with IBD on immune-modifying therapies, who received either the Pfizer or Moderna vaccine, should receive a three-dose vaccination series, with the third dose at least 28 days after the second dose.
- Patients with IBD on biologic therapy can receive the third dose of the vaccine at any time point and should not interrupt biologic therapy.
- Boosters are likely to become available to the general public in September and would be given at least 8 months after an individual’s second dose.
- Recommendations regarding boosters for those who received a three-dose vaccination series are forthcoming.
- Recommendations regarding boosters and additional doses for those that received the Janssen vaccine are forthcoming.
- Gastroenterologists should take an active role in ensuring that their patients are vaccinated.
Dr. Schell is a second-year graduate student in the division of internal medicine at the University of Wisconsin–Madison. Dr. Caldera is an associate professor of medicine in the division of gastroenterology & hepatology at the University of Wisconsin–Madison. Dr. Schell has no conflicts of interest to disclose. Dr. Caldera has received research support from Takeda Pharmaceuticals and Sanofi. He has been a consultant for Takeda, Arena Pharmaceuticals, GSK, and Celgene.
References
1. Sadoff J et al. N Engl J Med. 2021;384(23):2187-201.
2. Baden LR et al. N Engl J Med. 2021;384(5):403-16.
3. Polack FP et al. N Engl J Med. 2020;383:2603-15.
4. Johnson K et al. U.S. FDA aims to give full approval to Pfizer vaccine on Monday – NYT. Reuters. 2021 Aug 20. https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-aims-give-full-nod-pfizers-covid-19-vaccine-monday-new-york-times-2021-08-20/.
5. Allocca M et al. J Clin Med. 2020 Oct;9(11):3533.
6. Monteleone G and Ardizzone S. J Crohns Colitis. 2020 Sep;14(9):1334-6.
7. Papa A et al. Am J Gastroenterol. 2020;115(10):1722-4.
8. Derikx LAAP et al. J Crohn’s Colitis. 2021 Apr 6;15(4):529-39.
9. Brenner EJ et al. Gastroenterology. 2020;159(2):481-91.
10. Ungaro RC et al. Gut. 2021;70(4):725-32.
11. Caldera F et al. Inflamm Bowel Dis. 2020;26(4):593-602.
12. Botwin GJ et al. Am J Gastroenterol. 2021. doi: 10.14309/ajg.0000000000001342.
13. Squire JD et al. Inflamm Bowel Dis. 2021 Jul 27;27(8):1358-60.
14. Hadi YB et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.014.
15. Wong S-Y et al. Gastroenterology. 2021;161:715-8.
16. Kappelman MD et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.016.
17. Pozdnyakova V et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.08.014.
18. Ben-Tov A et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.076.
19. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. FDA News Release. 2021. Accessed 2021 Aug 18. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised.
20. Centers for Disease Control and Prevention. COVID-19 Vaccines for Moderately to Severely Immunocompromised People. 2021. Accessed 2021 Aug 18. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.
21. Allocca M et al. J Clin Med. 2020 Oct 31;9(11):3533.
22. Crohn’s & Colitis Foundation. COVID-19 Vaccines: Position Statements. IBD & Coronavirus. 2021. Accessed 2021 Aug 20. https://www.crohnscolitisfoundation.org/coronavirus/vaccine-position-statements.
23. Centers for Disease Control and Prevention. Joint Statement from HHS Public Health and Medical Experts on COVID-19 Booster Shots. https://www.cdc.gov/media/releases/2021/s0818-covid-19-booster-shots.html.
24. Caldera F et al. Inflamm Bowel Dis. 2021;27(1):123-133.
In December 2019, cases of pulmonary infection secondary to a novel coronavirus, known as severe acute respiratory syndrome coronavirus 2, were first identified in the city of Wuhan, China.
The clinical disease caused by the virus, COVID-19, has resulted in a worldwide pandemic that has portended significant morbidity and mortality throughout the United States. Three highly efficacious COVID-19 vaccines have received emergency use authorization (EUA) by the Food and Drug Administration to help prevent COVID-19, all of which are effective at preventing severe COVID-19.1-3 The Pfizer vaccine was given full FDA approval on Aug. 23, 2021.4
Patients with inflammatory bowel disease (IBD) are commonly treated with immune-modifying therapies that may increase their risk for serious and opportunistic infections. As such, there was concern at the beginning of the pandemic that patients with IBD may be at increased risk of contracting COVID-19 and/or developing severe disease (that is, ICU-level care, mechanical ventilation, and/or death). There is evidence that the incidence of COVID-19 in the IBD population is similar to that of the general population.5-7 Furthermore, most patients with IBD are not at increased risk of severe disease, including those on biologic therapies. Several studies demonstrated that those on corticosteroids are at increased risk of severe COVID-19, while those on other immune-modifying therapies such as tumor necrosis factor inhibitors (anti-TNFs) are not at increased risk.5,7-10 Patients with IBD with other well-known risk factors for severe disease include comorbidities such as diabetes and obesity.
It is known that patients with IBD on certain immune-modifying therapies such as anti-TNFs, especially those on combination therapy, may have a blunted immune response to certain vaccines.11 Neither patients with IBD nor patients on immunosuppressive therapy were included in phase 3 clinical trials for COVID-19 vaccine development, contributing to uncertainty regarding the safety and efficacy in our patient population. The risk of adverse events following COVID-19 vaccination in the IBD population has been found to be similar to that of the general population.12 It has also been reported that those who have had reactions to injectable therapies in the past may safely be vaccinated against COVID-19.13,14 With regard to vaccine efficacy, initial studies, including ICARUS, PREVENT-COVID, and CORALE-IBD, have demonstrated that patients with IBD do indeed mount a humoral immune response to the vaccine, including those on immune-modifying therapies.15-17 Nonhumoral aspects of immunity, such as cell-mediated immunity, have not yet been thoroughly evaluated. In addition, the risk of breakthrough COVID-19 infection after vaccination is low in patients with IBD, including those on immune-modifying therapy.14-18 While initial studies are reassuring that the vast majority of patients with IBD are able to mount a vaccine response, future studies are needed to determine the effects of immune-modifying therapy on sustained antibody concentrations and other correlates of immunity.
For those who received the Pfizer or Moderna vaccines, on Aug. 12, 2021, the FDA amended their EUA to allow for an additional dose in the initial vaccination series for certain immunocompromised individuals, specifically solid organ transplant recipients or those with conditions that make them equally immunocompromised.19 Based on evidence suggesting that certain solid organ transplant recipients do not mount an immune response after completing a two-dose series, the Advisory Committee on Immunization Practices, which advises the Centers for Disease Control and Prevention on how to use vaccines, recommended that moderate to severely immunocompromised individuals should complete a three-dose series, with the third dose being given at least 28 days after the second dose.20 This recommendation included those on high-dose corticosteroids defined as oral prednisone at least 20 mg/day, anti-TNFs and biosimilars, and antimetabolites such as azathioprine, mercaptopurine, and methotrexate.
It is worth noting that the role of the ACIP here was to consider the available evidence supporting the use of an additional dose and then make recommendations on which conditions may qualify; the ACIP was not able to provide recommendations for every disease state. At the time of writing this article, no recommendations have been made with regards to an additional dose of the Janssen vaccine. Likewise, in response to the ACIP recommendations, the Crohn’s & Colitis Foundation recommended an additional dose for patients with IBD on immune-modifying therapies.21,22
Less than one week after the EUA amendment for an additional dose, the Department of Health & Human Services announced that booster shots would likely become available to the general population as early as the week of Sept. 20, 2021 and starting 8 months after an individual’s second dose.23 Here, it is worth noting that an additional dose is distinct from a booster. An additional dose (or third dose here) refers to the initial vaccination series and is given when the standard schedule is thought to be insufficient in a certain patient population. In contrast, a booster dose is administered when the initial and sufficient immunity gained from a primary vaccination series has likely dissipated. The HHS acknowledged that boosters would likely be needed for those who received the Janssen vaccine but noted that further data and recommendations would be forthcoming.
To summarize, COVID-19 vaccines are safe and effective in the IBD population, and patients should be vaccinated at the earliest opportunity regardless of concurrent therapies. For those that received the Pfizer or Moderna vaccine, the ACIP recommended an additional dose in the initial vaccination series to be given at least 28 days after the second dose for those that are immunosuppressed. This recommendation was largely based off of transplant data. Reassuringly, the available data demonstrates a humoral immune response to a two-dose vaccination series in patients with IBD, including those on immune-modifying therapies. The Crohn’s & Colitis Foundation recommends that patients with IBD on immune-modifying therapy receive an additional dose (i.e., a three-dose series), which should be from the same manufacturer as the first two doses. In addition, at press time, HHS indicated that there will be a movement toward a booster dose for the general population in late September, which would also apply to patients with IBD. The ACIP has yet to comment on this change at the time of preparing this article, but the announcement indicated that a booster could be given “8 months after an individual’s second dose.” It is unclear how those who may receive a three-dose vaccination series will factor in, but it is possible that they would be eligible for a booster 8 months after their most recent dose. Gastroenterologists should also be aware that there is no role for serologic testing in the clinical setting because it has not been validated for such purposes and is primarily used in the research setting. Finally, it is paramount to emphasize that patients with IBD have historically had lower vaccination rates than the general population,24 and we must take an active role in ensuring that our patients are immunized by addressing their concerns, communicating the risks of COVID-19 and the benefits of vaccination, providing information on how to get vaccinated, and strongly recommending vaccination.
The following list also summarizes the recommendations:
- Patients with IBD should be vaccinated against COVID-19 regardless of concurrent therapies.
- Patients with IBD are not at increased risk of severe COVID-19.
- Patients with IBD, including those on immune-modifying therapies, mount a humoral immune response to the vaccine.
- Patients with IBD on immune-modifying therapies, who received either the Pfizer or Moderna vaccine, should receive a three-dose vaccination series, with the third dose at least 28 days after the second dose.
- Patients with IBD on biologic therapy can receive the third dose of the vaccine at any time point and should not interrupt biologic therapy.
- Boosters are likely to become available to the general public in September and would be given at least 8 months after an individual’s second dose.
- Recommendations regarding boosters for those who received a three-dose vaccination series are forthcoming.
- Recommendations regarding boosters and additional doses for those that received the Janssen vaccine are forthcoming.
- Gastroenterologists should take an active role in ensuring that their patients are vaccinated.
Dr. Schell is a second-year graduate student in the division of internal medicine at the University of Wisconsin–Madison. Dr. Caldera is an associate professor of medicine in the division of gastroenterology & hepatology at the University of Wisconsin–Madison. Dr. Schell has no conflicts of interest to disclose. Dr. Caldera has received research support from Takeda Pharmaceuticals and Sanofi. He has been a consultant for Takeda, Arena Pharmaceuticals, GSK, and Celgene.
References
1. Sadoff J et al. N Engl J Med. 2021;384(23):2187-201.
2. Baden LR et al. N Engl J Med. 2021;384(5):403-16.
3. Polack FP et al. N Engl J Med. 2020;383:2603-15.
4. Johnson K et al. U.S. FDA aims to give full approval to Pfizer vaccine on Monday – NYT. Reuters. 2021 Aug 20. https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-aims-give-full-nod-pfizers-covid-19-vaccine-monday-new-york-times-2021-08-20/.
5. Allocca M et al. J Clin Med. 2020 Oct;9(11):3533.
6. Monteleone G and Ardizzone S. J Crohns Colitis. 2020 Sep;14(9):1334-6.
7. Papa A et al. Am J Gastroenterol. 2020;115(10):1722-4.
8. Derikx LAAP et al. J Crohn’s Colitis. 2021 Apr 6;15(4):529-39.
9. Brenner EJ et al. Gastroenterology. 2020;159(2):481-91.
10. Ungaro RC et al. Gut. 2021;70(4):725-32.
11. Caldera F et al. Inflamm Bowel Dis. 2020;26(4):593-602.
12. Botwin GJ et al. Am J Gastroenterol. 2021. doi: 10.14309/ajg.0000000000001342.
13. Squire JD et al. Inflamm Bowel Dis. 2021 Jul 27;27(8):1358-60.
14. Hadi YB et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.014.
15. Wong S-Y et al. Gastroenterology. 2021;161:715-8.
16. Kappelman MD et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.016.
17. Pozdnyakova V et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.08.014.
18. Ben-Tov A et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.076.
19. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. FDA News Release. 2021. Accessed 2021 Aug 18. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised.
20. Centers for Disease Control and Prevention. COVID-19 Vaccines for Moderately to Severely Immunocompromised People. 2021. Accessed 2021 Aug 18. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.
21. Allocca M et al. J Clin Med. 2020 Oct 31;9(11):3533.
22. Crohn’s & Colitis Foundation. COVID-19 Vaccines: Position Statements. IBD & Coronavirus. 2021. Accessed 2021 Aug 20. https://www.crohnscolitisfoundation.org/coronavirus/vaccine-position-statements.
23. Centers for Disease Control and Prevention. Joint Statement from HHS Public Health and Medical Experts on COVID-19 Booster Shots. https://www.cdc.gov/media/releases/2021/s0818-covid-19-booster-shots.html.
24. Caldera F et al. Inflamm Bowel Dis. 2021;27(1):123-133.
Open notes: Legal issues
In July, I had my annual physical with my primary care physician, whose practice is based out of a large urban academic medical center. As she concluded my visit and directed me to the lab to have my blood work done, she said, “You’ll be receiving an automatic notice from MyChart by 9 am tomorrow that your medical records from today’s visit are available. I apologize if I have not yet had the opportunity to review them and enter my note, but you’ll get access to all of that, as well, as soon as it is in the system.”
This sort of interaction is increasingly common across the United States as health care institutions implement policies and procedures to comply with new regulations promulgated by the Office of the National Coordinator for Health Information Technology (ONC), which went into effect on April 5, 2021. These rules were promulgated in accordance with the 21st Century Cures Act of 2016 (Cures Act).1 The regulations, known as the Interoperability, Information Blocking, and the ONC Health IT Certification Program, implement provisions of the Cures Act intended to “support the access, exchange, and use of electronic health information.” The rule is considered a significant step in the “open notes” movement, which is intended to make health care more transparent by enabling patients to access their medical records. The drafters of the ONC regulations have carved out certain exceptions to the information blocking rule. For example, one exception allows some patient information to be withheld where making that information available might cause physical harm to the patient or another person.
Thus far, few patients have been informed about the new regulation.2 By forbidding “information blocking,” the rule enables patients to more easily access and control their health information. Records must be provided “without delay,” or at least as soon as the physician’s office receives an electronic copy. In 2022, it will be required that access to even more of a patient’s personal electronic health record be provided in real-time through a patient portal and that electronic health information be shareable across third-party apps.
The Cures Act and the regulations governing its implementation highlight the inherent tension between two core principles of bioethical inquiry: autonomy and beneficence. The first principle, autonomy, champions allowing patient access and control over their own personal information. Beneficence, which is often expressed as paternalism, ensures that the experts are able to analyze and interpret data so that patients are in the best position to then make informed decisions.
With these principles in mind, arguments against open notes have generally fallen into three related categories. First, critics worry that immediate access to one’s medical record will increase patient anxiety caused by feelings of being inundated with complex medical information that patients may be ill-equipped to analyze and understand. This is a common refrain any time policies are implemented to improve medical information sharing. For example, critics of direct-to-consumer genetic testing caution that permitting unfettered access to complex information, particularly without an intermediary to interpret the data, could lead to confusion and poor medical choices.
There may be validity to this claim. One study found that 3% of patients reported feeling very confused when granted access to their medical notes.3 Another study concluded that direct release of medical test results “sometimes leads to unnecessary anxiety.”4 While the drafters of the ONC regulations have carved out certain exceptions to the information blocking rule, those exceptions do not allow for withholding of information because of concerns about patient anxiety or psychological harms.
The second common critique of open notes is that requiring release of all clinical notes will lead to clinician self-censorship, effectively muzzling or silencing the experts whose responsibility it is to objectively interpret results in order to provide the best care for their patients. Some have expressed concern that clinicians will be forced to “code” their records to avoid addressing “sensitive” subjects that might make patients feel offended or judged. This, in turn, might lead to less complete, reliable, or useful clinician communication.3
In fact, open notes has led to changes in the documentation process for some clinicians. They have reported modifying the way they document patient visits by changing their use of critical language and sensitive information.5 One study found that open notes led physicians to adjust “their language to avoid being perceived as critical of patients; omitting certain terms, such as ‘noncompliant’ and ‘patient denies’; and modifying how they document sensitive information.”3
In response, experts recommend focusing on precise and empathetic patient notes; in other words, the clinician should not write something in the note that they would not say directly to the patient. For example, they recommend that clinicians use precise language (for example, identifying the patient’s BMI) rather than using terms that could be offensive (for example, labeling the patient as “obese”).6 The shift to more empathetic note-taking could be seen less as a burden and more as a valuable tool in the shared decision-making endeavor: It could allow physicians to document both their clinical judgments and the patient’s values and preferences, which could lead to better medical decision-making.
Third, critics of open notes point to concerns about the burden it places on clinicians’ already limited time. The ONC rule requires automatic release of test results regardless of whether the clinician has had the opportunity to review them and offer their interpretation and insight. Because physician interpretation of results has known benefits,4 this puts additional pressure on clinicians to review results and enter notes in a timely manner. But physicians have reported that often open notes necessitates that they spend more time on documentation than they would otherwise.5
Despite critiques of open notes, the benefits of allowing patients access to their medical records have been repeatedly demonstrated. And research has shown that patients benefit from accessing open notes by allowing them to access and control their own personal medical information.5 Patients report that they understand and value the information provided to them in their medical records,7 and they feel empowered to participate in their medical decision-making. In surveys, patients report that reading their doctors’ notes is useful for taking care of their health and for remembering their care plans, understanding why a medication was prescribed, and reinforcing the need to take their medications and adhere to treatment plans.8
Importantly, open notes can increase patient engagement and patients’ trust in their physicians,9 thereby improving the doctor-patient relationship.3 And allowing patients to share their medical records with care partners enables supported decision-making, particularly for older and chronically ill individuals.3 Additionally, it is predicted that open notes may, in fact, decrease legal liability.9 By improving both trust in the doctor-patient relationship and safety, some experts expect that legal claims against clinicians will, in turn, decrease.10
The modern practice of medicine necessitates a more empathetic approach to clinical note-taking, even in the absence of regulation requiring it. As the regulations implementing the Cures Act roll out, patients will have easier, and more immediate, access to their medical records. Despite earlier hesitancy, clinicians are steadily beginning to support sharing access to notes with patients.5 Change can be hard. But the change expected of clinicians because of these new regulations appears to be less onerous than originally anticipated.
Prof. Koch is codirector of Health Law & Policy Institute and assistant professor at the University of Houston Law Center, as well as director of law and ethics at the MacLean Center for Clinical Medical Ethics at the University of Chicago. She has no disclosures.
This article was updated Sept. 9, 2021.
References
1. Fed Regist. 2020 May;85(85):25642-961.
2. The Petrie-Flom Center Staff. “New Rule Puts Medical Data in Patients’ Hands.” Bill of Health. July 12, 2021. Accessed August 30, 2021. https://blog.petrieflom.law.harvard.edu/2021/07/12/new-rule-puts-medical-data-in-patients-hands/.
3. Blease C et al. Ann Intern Med. 2021 Jan;174(1):101-2.
4. Pillemer F et al. PLoS One. 2016 Jun. doi: 10.1371/journal.pone.0154743.
5. DesRoches CM et al. JAMA Netw Open. 2020 Mar. doi: 10.1001/jamanetworkopen.2020.1753.
6. Heath S. “Most Patients Understand Clinical Notes, Patient Data Access.” Patient Engagement HIT. July 29, 2020. Accessed August 30, 2021. https://patientengagementhit.com/news/most-patients-understand-clinical-notes-patient-data-access
7. Leveille SG et al. J Gen Intern Med. 2020 Dec;35(12):3510-6.
8. Walker J et al. J Med Internet Res. 2019 May. doi: 10.2196/13876.
9. Bell SK et al. BMJ Qual Saf. 2017 Apr;26(4):262-70.
10. Kachalia A, Mello MM. N Engl J Med. 2011 Apr;364(16):1564-72.
In July, I had my annual physical with my primary care physician, whose practice is based out of a large urban academic medical center. As she concluded my visit and directed me to the lab to have my blood work done, she said, “You’ll be receiving an automatic notice from MyChart by 9 am tomorrow that your medical records from today’s visit are available. I apologize if I have not yet had the opportunity to review them and enter my note, but you’ll get access to all of that, as well, as soon as it is in the system.”
This sort of interaction is increasingly common across the United States as health care institutions implement policies and procedures to comply with new regulations promulgated by the Office of the National Coordinator for Health Information Technology (ONC), which went into effect on April 5, 2021. These rules were promulgated in accordance with the 21st Century Cures Act of 2016 (Cures Act).1 The regulations, known as the Interoperability, Information Blocking, and the ONC Health IT Certification Program, implement provisions of the Cures Act intended to “support the access, exchange, and use of electronic health information.” The rule is considered a significant step in the “open notes” movement, which is intended to make health care more transparent by enabling patients to access their medical records. The drafters of the ONC regulations have carved out certain exceptions to the information blocking rule. For example, one exception allows some patient information to be withheld where making that information available might cause physical harm to the patient or another person.
Thus far, few patients have been informed about the new regulation.2 By forbidding “information blocking,” the rule enables patients to more easily access and control their health information. Records must be provided “without delay,” or at least as soon as the physician’s office receives an electronic copy. In 2022, it will be required that access to even more of a patient’s personal electronic health record be provided in real-time through a patient portal and that electronic health information be shareable across third-party apps.
The Cures Act and the regulations governing its implementation highlight the inherent tension between two core principles of bioethical inquiry: autonomy and beneficence. The first principle, autonomy, champions allowing patient access and control over their own personal information. Beneficence, which is often expressed as paternalism, ensures that the experts are able to analyze and interpret data so that patients are in the best position to then make informed decisions.
With these principles in mind, arguments against open notes have generally fallen into three related categories. First, critics worry that immediate access to one’s medical record will increase patient anxiety caused by feelings of being inundated with complex medical information that patients may be ill-equipped to analyze and understand. This is a common refrain any time policies are implemented to improve medical information sharing. For example, critics of direct-to-consumer genetic testing caution that permitting unfettered access to complex information, particularly without an intermediary to interpret the data, could lead to confusion and poor medical choices.
There may be validity to this claim. One study found that 3% of patients reported feeling very confused when granted access to their medical notes.3 Another study concluded that direct release of medical test results “sometimes leads to unnecessary anxiety.”4 While the drafters of the ONC regulations have carved out certain exceptions to the information blocking rule, those exceptions do not allow for withholding of information because of concerns about patient anxiety or psychological harms.
The second common critique of open notes is that requiring release of all clinical notes will lead to clinician self-censorship, effectively muzzling or silencing the experts whose responsibility it is to objectively interpret results in order to provide the best care for their patients. Some have expressed concern that clinicians will be forced to “code” their records to avoid addressing “sensitive” subjects that might make patients feel offended or judged. This, in turn, might lead to less complete, reliable, or useful clinician communication.3
In fact, open notes has led to changes in the documentation process for some clinicians. They have reported modifying the way they document patient visits by changing their use of critical language and sensitive information.5 One study found that open notes led physicians to adjust “their language to avoid being perceived as critical of patients; omitting certain terms, such as ‘noncompliant’ and ‘patient denies’; and modifying how they document sensitive information.”3
In response, experts recommend focusing on precise and empathetic patient notes; in other words, the clinician should not write something in the note that they would not say directly to the patient. For example, they recommend that clinicians use precise language (for example, identifying the patient’s BMI) rather than using terms that could be offensive (for example, labeling the patient as “obese”).6 The shift to more empathetic note-taking could be seen less as a burden and more as a valuable tool in the shared decision-making endeavor: It could allow physicians to document both their clinical judgments and the patient’s values and preferences, which could lead to better medical decision-making.
Third, critics of open notes point to concerns about the burden it places on clinicians’ already limited time. The ONC rule requires automatic release of test results regardless of whether the clinician has had the opportunity to review them and offer their interpretation and insight. Because physician interpretation of results has known benefits,4 this puts additional pressure on clinicians to review results and enter notes in a timely manner. But physicians have reported that often open notes necessitates that they spend more time on documentation than they would otherwise.5
Despite critiques of open notes, the benefits of allowing patients access to their medical records have been repeatedly demonstrated. And research has shown that patients benefit from accessing open notes by allowing them to access and control their own personal medical information.5 Patients report that they understand and value the information provided to them in their medical records,7 and they feel empowered to participate in their medical decision-making. In surveys, patients report that reading their doctors’ notes is useful for taking care of their health and for remembering their care plans, understanding why a medication was prescribed, and reinforcing the need to take their medications and adhere to treatment plans.8
Importantly, open notes can increase patient engagement and patients’ trust in their physicians,9 thereby improving the doctor-patient relationship.3 And allowing patients to share their medical records with care partners enables supported decision-making, particularly for older and chronically ill individuals.3 Additionally, it is predicted that open notes may, in fact, decrease legal liability.9 By improving both trust in the doctor-patient relationship and safety, some experts expect that legal claims against clinicians will, in turn, decrease.10
The modern practice of medicine necessitates a more empathetic approach to clinical note-taking, even in the absence of regulation requiring it. As the regulations implementing the Cures Act roll out, patients will have easier, and more immediate, access to their medical records. Despite earlier hesitancy, clinicians are steadily beginning to support sharing access to notes with patients.5 Change can be hard. But the change expected of clinicians because of these new regulations appears to be less onerous than originally anticipated.
Prof. Koch is codirector of Health Law & Policy Institute and assistant professor at the University of Houston Law Center, as well as director of law and ethics at the MacLean Center for Clinical Medical Ethics at the University of Chicago. She has no disclosures.
This article was updated Sept. 9, 2021.
References
1. Fed Regist. 2020 May;85(85):25642-961.
2. The Petrie-Flom Center Staff. “New Rule Puts Medical Data in Patients’ Hands.” Bill of Health. July 12, 2021. Accessed August 30, 2021. https://blog.petrieflom.law.harvard.edu/2021/07/12/new-rule-puts-medical-data-in-patients-hands/.
3. Blease C et al. Ann Intern Med. 2021 Jan;174(1):101-2.
4. Pillemer F et al. PLoS One. 2016 Jun. doi: 10.1371/journal.pone.0154743.
5. DesRoches CM et al. JAMA Netw Open. 2020 Mar. doi: 10.1001/jamanetworkopen.2020.1753.
6. Heath S. “Most Patients Understand Clinical Notes, Patient Data Access.” Patient Engagement HIT. July 29, 2020. Accessed August 30, 2021. https://patientengagementhit.com/news/most-patients-understand-clinical-notes-patient-data-access
7. Leveille SG et al. J Gen Intern Med. 2020 Dec;35(12):3510-6.
8. Walker J et al. J Med Internet Res. 2019 May. doi: 10.2196/13876.
9. Bell SK et al. BMJ Qual Saf. 2017 Apr;26(4):262-70.
10. Kachalia A, Mello MM. N Engl J Med. 2011 Apr;364(16):1564-72.
In July, I had my annual physical with my primary care physician, whose practice is based out of a large urban academic medical center. As she concluded my visit and directed me to the lab to have my blood work done, she said, “You’ll be receiving an automatic notice from MyChart by 9 am tomorrow that your medical records from today’s visit are available. I apologize if I have not yet had the opportunity to review them and enter my note, but you’ll get access to all of that, as well, as soon as it is in the system.”
This sort of interaction is increasingly common across the United States as health care institutions implement policies and procedures to comply with new regulations promulgated by the Office of the National Coordinator for Health Information Technology (ONC), which went into effect on April 5, 2021. These rules were promulgated in accordance with the 21st Century Cures Act of 2016 (Cures Act).1 The regulations, known as the Interoperability, Information Blocking, and the ONC Health IT Certification Program, implement provisions of the Cures Act intended to “support the access, exchange, and use of electronic health information.” The rule is considered a significant step in the “open notes” movement, which is intended to make health care more transparent by enabling patients to access their medical records. The drafters of the ONC regulations have carved out certain exceptions to the information blocking rule. For example, one exception allows some patient information to be withheld where making that information available might cause physical harm to the patient or another person.
Thus far, few patients have been informed about the new regulation.2 By forbidding “information blocking,” the rule enables patients to more easily access and control their health information. Records must be provided “without delay,” or at least as soon as the physician’s office receives an electronic copy. In 2022, it will be required that access to even more of a patient’s personal electronic health record be provided in real-time through a patient portal and that electronic health information be shareable across third-party apps.
The Cures Act and the regulations governing its implementation highlight the inherent tension between two core principles of bioethical inquiry: autonomy and beneficence. The first principle, autonomy, champions allowing patient access and control over their own personal information. Beneficence, which is often expressed as paternalism, ensures that the experts are able to analyze and interpret data so that patients are in the best position to then make informed decisions.
With these principles in mind, arguments against open notes have generally fallen into three related categories. First, critics worry that immediate access to one’s medical record will increase patient anxiety caused by feelings of being inundated with complex medical information that patients may be ill-equipped to analyze and understand. This is a common refrain any time policies are implemented to improve medical information sharing. For example, critics of direct-to-consumer genetic testing caution that permitting unfettered access to complex information, particularly without an intermediary to interpret the data, could lead to confusion and poor medical choices.
There may be validity to this claim. One study found that 3% of patients reported feeling very confused when granted access to their medical notes.3 Another study concluded that direct release of medical test results “sometimes leads to unnecessary anxiety.”4 While the drafters of the ONC regulations have carved out certain exceptions to the information blocking rule, those exceptions do not allow for withholding of information because of concerns about patient anxiety or psychological harms.
The second common critique of open notes is that requiring release of all clinical notes will lead to clinician self-censorship, effectively muzzling or silencing the experts whose responsibility it is to objectively interpret results in order to provide the best care for their patients. Some have expressed concern that clinicians will be forced to “code” their records to avoid addressing “sensitive” subjects that might make patients feel offended or judged. This, in turn, might lead to less complete, reliable, or useful clinician communication.3
In fact, open notes has led to changes in the documentation process for some clinicians. They have reported modifying the way they document patient visits by changing their use of critical language and sensitive information.5 One study found that open notes led physicians to adjust “their language to avoid being perceived as critical of patients; omitting certain terms, such as ‘noncompliant’ and ‘patient denies’; and modifying how they document sensitive information.”3
In response, experts recommend focusing on precise and empathetic patient notes; in other words, the clinician should not write something in the note that they would not say directly to the patient. For example, they recommend that clinicians use precise language (for example, identifying the patient’s BMI) rather than using terms that could be offensive (for example, labeling the patient as “obese”).6 The shift to more empathetic note-taking could be seen less as a burden and more as a valuable tool in the shared decision-making endeavor: It could allow physicians to document both their clinical judgments and the patient’s values and preferences, which could lead to better medical decision-making.
Third, critics of open notes point to concerns about the burden it places on clinicians’ already limited time. The ONC rule requires automatic release of test results regardless of whether the clinician has had the opportunity to review them and offer their interpretation and insight. Because physician interpretation of results has known benefits,4 this puts additional pressure on clinicians to review results and enter notes in a timely manner. But physicians have reported that often open notes necessitates that they spend more time on documentation than they would otherwise.5
Despite critiques of open notes, the benefits of allowing patients access to their medical records have been repeatedly demonstrated. And research has shown that patients benefit from accessing open notes by allowing them to access and control their own personal medical information.5 Patients report that they understand and value the information provided to them in their medical records,7 and they feel empowered to participate in their medical decision-making. In surveys, patients report that reading their doctors’ notes is useful for taking care of their health and for remembering their care plans, understanding why a medication was prescribed, and reinforcing the need to take their medications and adhere to treatment plans.8
Importantly, open notes can increase patient engagement and patients’ trust in their physicians,9 thereby improving the doctor-patient relationship.3 And allowing patients to share their medical records with care partners enables supported decision-making, particularly for older and chronically ill individuals.3 Additionally, it is predicted that open notes may, in fact, decrease legal liability.9 By improving both trust in the doctor-patient relationship and safety, some experts expect that legal claims against clinicians will, in turn, decrease.10
The modern practice of medicine necessitates a more empathetic approach to clinical note-taking, even in the absence of regulation requiring it. As the regulations implementing the Cures Act roll out, patients will have easier, and more immediate, access to their medical records. Despite earlier hesitancy, clinicians are steadily beginning to support sharing access to notes with patients.5 Change can be hard. But the change expected of clinicians because of these new regulations appears to be less onerous than originally anticipated.
Prof. Koch is codirector of Health Law & Policy Institute and assistant professor at the University of Houston Law Center, as well as director of law and ethics at the MacLean Center for Clinical Medical Ethics at the University of Chicago. She has no disclosures.
This article was updated Sept. 9, 2021.
References
1. Fed Regist. 2020 May;85(85):25642-961.
2. The Petrie-Flom Center Staff. “New Rule Puts Medical Data in Patients’ Hands.” Bill of Health. July 12, 2021. Accessed August 30, 2021. https://blog.petrieflom.law.harvard.edu/2021/07/12/new-rule-puts-medical-data-in-patients-hands/.
3. Blease C et al. Ann Intern Med. 2021 Jan;174(1):101-2.
4. Pillemer F et al. PLoS One. 2016 Jun. doi: 10.1371/journal.pone.0154743.
5. DesRoches CM et al. JAMA Netw Open. 2020 Mar. doi: 10.1001/jamanetworkopen.2020.1753.
6. Heath S. “Most Patients Understand Clinical Notes, Patient Data Access.” Patient Engagement HIT. July 29, 2020. Accessed August 30, 2021. https://patientengagementhit.com/news/most-patients-understand-clinical-notes-patient-data-access
7. Leveille SG et al. J Gen Intern Med. 2020 Dec;35(12):3510-6.
8. Walker J et al. J Med Internet Res. 2019 May. doi: 10.2196/13876.
9. Bell SK et al. BMJ Qual Saf. 2017 Apr;26(4):262-70.
10. Kachalia A, Mello MM. N Engl J Med. 2011 Apr;364(16):1564-72.
Pregnancy and parental leave during gastroenterology fellowship training: A program perspective
Due to broad social changes and efforts from leaders in GI, there is renewed interest in family planning and parental leave policies for GI trainees. The American Board of Medical Specialties now permits trainees a minimum of 6 weeks away during training, without automatically requiring an extension of training time or completely depleting vacation time, for boards eligibility.1,2 However, national and institutional guidance for family planning and pregnancy during GI fellowship is lacking. How can gastroenterology fellowship programs support fellows taking parental leave and enact fair policies? We review the scope of the problem, describe our experience in developing resources within our GI fellowship program, and highlight areas that require further development.
The scope of the issue
There is no national data yet on the number of GI fellows that are parents prior to starting fellowship or who become parents during fellowship. We estimate that approximately 25% of fellows enter training as parents or become parents during fellowship, although 40%-50% may have an intention to have children.3,4 Fellows may be worried that they will “fall behind” or be perceived as less committed if they devote time to childrearing or take parental leave.5-7 Indeed, worries about discrimination based on pregnancy and parental leave are borne out by the experiences of older physicians (in particular, female physicians).8,9
State- and institution-specific benefits vary from program to program. Nationally, the Family and Medical Leave Act provides only unpaid leave and applies only to trainees who have been employed for greater than 12 months.10 Benefits may not always be well advertised, and even when they are, trainees (and attendings) may feel uncomfortable taking full advantage. One survey of GIs revealed that, although two-thirds believed that 6-8 weeks of maternity leave was inadequate, half took less than that amount due to fears about financial and professional consequences.8
Pregnancy during GI fellowship is a special concern. GI fellowship consists of long work hours, includes night call, and can be physically demanding. All three of these factors have been associated with preterm delivery, infertility, and miscarriage.11,12 In addition, there are no guidelines for ergonomic adjustments or infection precautions for pregnant endoscopists. We have compiled information about infection prevention guidance in pregnancy (available from the authors on request) derived from guidance from the National Institute for Occupational Safety and Health, which recommends the same precautions for pregnant health care workers as for nonpregnant health care workers.13 In regards to SARS-CoV-2, we believe that the decision to perform procedures on patients with COVID-19 infection should be individualized, although vaccinated endoscopists should be reassured by the exceedingly low rates of infection after vaccination and with appropriate personal protective equipment. Radiation is yet another concern. There are limited data on radiation dosages incurred by workers in the endoscopy suite and no pregnancy-specific data, which may lead trainees to avoid fluoroscopic procedures and unnecessarily double up on lead gowns.8,11,14-17
Breastfeeding accommodations, and access to lactation rooms for trainees, are required by federal law for a minimum of 12 months.18 Should a trainee choose to breastfeed, education of staff and attendings is critical because many may be unaware of the specific needs pertaining to lactation. Staff should be aware that 30-45 minutes are needed to prepare, pump, and store milk. Trainees should not be solely responsible for educating their attendings and staff.
Our Experience in Creating a Policy
We developed a formal fellowship program policy on parental leave and pregnancy in the setting of a broader discussion about fellow workload and wellness. We agreed that trainees should be allowed to make changes to their schedule with co-fellows as needed for medical appointments or procedures and that our backup policy should be flexible enough to provide spot coverage for unexpected complications and family emergencies. We also incorporated a GI psychologist to provide wellness resources and suggestions for reducing burnout for our fellows.
We strove to follow certain principles in creating this policy. Trainees who are parents should have a comparable clinical experience to their nonparenting colleagues and should take the lead in rearranging their own schedule. Nonbirthing parents, adopting parents, and parents using surrogacy should be included in any parental leave policy. Fellowship leaders have an important responsibility in helping fellows proactively plan to meet requirements for graduation and maximize learning and exposure (Figure). We also recognized the importance of equitable coverage. For example, there is sometimes a perception that fellows with children “burden” fellows who are not parents.3,19 On the other hand, fellows without children may feel that they are called on more than their colleagues with children to cover those with childcare issues. In addition, as a recent study of general surgery residency program directors indicates, there are complex interpersonal issues that play into a colleague’s willingness to provide coverage.20 It behooves program leadership to be cognizant of group dynamics that might cause conflict over what should be a straightforward coverage situation.
We first researched national and societal guidelines if available, as well as our institution’s graduate medical education (GME) website. We categorized benefits by whether they were federal, state-mandated, or institutional. It is important to note that any concerns about trainee salaries should be discussed with one’s GME office to ensure the leave policy is in accordance with federal funding policies.21 We solicited experiences and advice from former and current fellows who had gone through, or were planning, pregnancy and parental leave. A few faculty members volunteered to serve as a resource for fellows; these “ambassadors” discussed their experiences during a lunchtime panel, as well as offered to provide one-on-one advice and participate in future panels. We also reached out to our infection control experts to review the literature and federal policies on infections of special consideration during pregnancy and endoscopy. As for radiation safety, given the importance of education and active monitoring, we offer the option of reaching out to our radiation safety officer for individualized counseling.22
Based on these efforts, we drafted a written policy designed for pregnant fellows and fellows planning parental leave on expectations for the program and fellows, benefits, and advice, including childcare options, lactation room locations, and financial planning tips. We shared this document with fellows and incorporated feedback. As a “living document” it is subject to change and will be updated as needed (at least annually).
Additional planning considerations for fellows
Research childcare options (ideally 6 months or more before leave).
- Start to explore your institution’s resources for leave and childcare options (daycare waitlists may be greater than 1 year in some cities).
Inform your program director (4-5 months before leave).
- Consider informing your program director about pregnancy at the beginning of the second trimester.
- Discuss Accreditation Council for Graduate Medical Education requirements and scheduling responsibilities.
- Explicitly discuss whether you plan to graduate on time or extend fellowship.
Inform your colleagues and patients (at least 3-4 months before leave).
- If comfortable, consider getting advice from a co-fellow and/or faculty mentor parent to facilitate transition to parenthood.
- When you feel ready, begin trading rotations and calls with co-fellows. If you have a results inbox or pager, discuss who can help cover those during your leave.
- Inform research collaborators about your leave and make preparations to keep projects progressing during your leave.
- If you have “active” clinic patients, when appropriate, begin to inform them that you will be away and provide reassurance that a colleague will be covering you. Leave clear plans with contingencies for these patients in your last progress notes.
Complete institutional paperwork and map out facilities (at least 2-3 months before leave).
- Review your options for using time toward leave, including vacation, research, or Family and Medical Leave Act–provided leave (unpaid), and what paperwork you need to fill out.
- Contact your payroll and/or human resources office to inform them of birth/adoption.
- Research potential program parental benefits, such as dependent daycare and/or health care flexible spending accounts.
- If choosing to breastfeed, explore the lactation rooms that are closest to your workroom and endoscopy suite and determine how much time you will need to set aside for pumping.
Be prepared to make adjustments as needed.
- Endoscopy-heavy rotations may be more difficult in the third trimester of pregnancy.
- Make contingency plans for early or late delivery dates, as well as if you undergo a cesarean that requires additional recovery time.
- Consider scheduling elective rotations (research, clinic) toward the end of leave and for the first month of “return to work.”
- If you plan to join limited clinic or outpatient endoscopy blocks later in your leave, make early arrangements to work regularly with these attendings.
Conclusion
Trainee needs assessments in gastroenterology fellowship similar to those in other specialties should be performed, and are in fact underway.19,23,24 There is a lack of data regarding the availability of fellowship program guidance and, specifically, adherence to required policies, and more data from program directors at a national level need to be collected.20 We recommend that programs engage in identifying specific needs at their institutions with the goal of eventually sharing this knowledge with other programs. Gastroenterology society recommendations for performing endoscopy while pregnant, with regard to ergonomics, infection control, and radiation exposure, would be instrumental. GI fellowships should consolidate institutional knowledge and engage key stakeholders – including trainees, prior trainees, occupational safety experts, radiation safety offices, wellness experts, and GME – to create program-specific policies that are flexible but rigorous and generous but equitable.
Dr. Liu and Dr. Summa are gastroenterology fellows at Northwestern University, Chicago. Dr. Patel is an assistant professor of medicine and a gastroenterology fellowship assistant program director at Northwestern University, Chicago. Dr. Donnan and Dr. Guentner are assistant professors of medicine at Northwestern University. Dr. Kia is an assistant professor of medicine and the gastroenterology fellowship program director at Northwestern University. They have no conflicts of interest to disclose. The authors would like to thank Michelle Clermont, MD, and Maureen K. Bolon, MD, for their discussion and assistance during the drafting of this article.
References
1. Section on Medical Students, Residents, and Fellowship Trainees; Committee on Early Childhood. Pediatrics. 2013;131(2):387-90.
2. American Board of Medical Specialties. ABMS Announces Progressive Leave Policy for Residents and Fellows. July 13, 2020. Accessed May 1, 2021. https://www.abms.org/news-events/abms-announces-progressive-leave-policy-for-residents-and-fellows/.
3. Magudia K et al. J Grad Med Educ. 2020;12(2):162-7.
4. Blair JE et al. Acad Med. 2016;91(7):972-8.
5. Feld LD. Am J Gastroenterol. 2021;116(3):505-8.
6. Roubaud MS. Plast Reconstr Surg Glob Open. 2019. doi: 10.1097/GOX.0000000000002104.
7. Price J, Dunbar K. Gastrointest Endosc. 2009;69(1):121-3.
8. David YN et al. Gastrointest Endosc. 2020;91(6):AB75-AB76.
9. Webb AMB et al. Acad Med J Assoc Am Med Coll. 2019;94(11):1631-4.
10. Weinstein DF et al. N Engl J Med. 2019 Sep 12;381(11):995-7.
11. Anderson M, Goldman RH. JAMA Surg. 2020;155(3):243-9.
12. Palmer KT et al. Occup Environ Med. 2013;70(4):213-22.
13. Siegel JD et al; Healthcare Infection, Control Practices Advisory Committee. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings (2007). 2007. Last reviewed July 22, 2019. Accessed April 28, 2021. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html
14. David YN et al. Am J Gastroenterol. 2021;116(3):539-50.
15. Sethi S et al. Dig Dis Sci. 2019;64(9):2455-66.
16. Alzimami K et al. Gastroenterol Res Pract. 2013;2013:587574.
17. Hayashi S et al. World J Clin Cases. 2018;6(16):1087-93.
18. U.S. Department of Labor, Wage and Hour Division. “Frequently Asked Questions – Break Time for Nursing Mothers.” Accessed May 1, 2021. https://www.dol.gov/agencies/whd/nursing-mothers/faq.
19. Mwakyanjala EJ et al. J Am Heart Assoc. 2019. doi: 10.1161/JAHA.119.012137.
20. Castillo-Angeles M et al. JAMA Surg. 2021 Jul 1;156(7):647-53.
21. Prasad S et al. J Grad Med Educ. 2021 Jun;13(3):349-54.
22. Ho IKH et al. Am J Gastroenterol. 2014;109(8):1180-94.
23. Sherbaf FG et al. AJNR Am J Neuroradiol. 2020;41(8):1348-54.
24. Altieri MS et al. JAMA Surg. 2019;154(10):952-58.
Due to broad social changes and efforts from leaders in GI, there is renewed interest in family planning and parental leave policies for GI trainees. The American Board of Medical Specialties now permits trainees a minimum of 6 weeks away during training, without automatically requiring an extension of training time or completely depleting vacation time, for boards eligibility.1,2 However, national and institutional guidance for family planning and pregnancy during GI fellowship is lacking. How can gastroenterology fellowship programs support fellows taking parental leave and enact fair policies? We review the scope of the problem, describe our experience in developing resources within our GI fellowship program, and highlight areas that require further development.
The scope of the issue
There is no national data yet on the number of GI fellows that are parents prior to starting fellowship or who become parents during fellowship. We estimate that approximately 25% of fellows enter training as parents or become parents during fellowship, although 40%-50% may have an intention to have children.3,4 Fellows may be worried that they will “fall behind” or be perceived as less committed if they devote time to childrearing or take parental leave.5-7 Indeed, worries about discrimination based on pregnancy and parental leave are borne out by the experiences of older physicians (in particular, female physicians).8,9
State- and institution-specific benefits vary from program to program. Nationally, the Family and Medical Leave Act provides only unpaid leave and applies only to trainees who have been employed for greater than 12 months.10 Benefits may not always be well advertised, and even when they are, trainees (and attendings) may feel uncomfortable taking full advantage. One survey of GIs revealed that, although two-thirds believed that 6-8 weeks of maternity leave was inadequate, half took less than that amount due to fears about financial and professional consequences.8
Pregnancy during GI fellowship is a special concern. GI fellowship consists of long work hours, includes night call, and can be physically demanding. All three of these factors have been associated with preterm delivery, infertility, and miscarriage.11,12 In addition, there are no guidelines for ergonomic adjustments or infection precautions for pregnant endoscopists. We have compiled information about infection prevention guidance in pregnancy (available from the authors on request) derived from guidance from the National Institute for Occupational Safety and Health, which recommends the same precautions for pregnant health care workers as for nonpregnant health care workers.13 In regards to SARS-CoV-2, we believe that the decision to perform procedures on patients with COVID-19 infection should be individualized, although vaccinated endoscopists should be reassured by the exceedingly low rates of infection after vaccination and with appropriate personal protective equipment. Radiation is yet another concern. There are limited data on radiation dosages incurred by workers in the endoscopy suite and no pregnancy-specific data, which may lead trainees to avoid fluoroscopic procedures and unnecessarily double up on lead gowns.8,11,14-17
Breastfeeding accommodations, and access to lactation rooms for trainees, are required by federal law for a minimum of 12 months.18 Should a trainee choose to breastfeed, education of staff and attendings is critical because many may be unaware of the specific needs pertaining to lactation. Staff should be aware that 30-45 minutes are needed to prepare, pump, and store milk. Trainees should not be solely responsible for educating their attendings and staff.
Our Experience in Creating a Policy
We developed a formal fellowship program policy on parental leave and pregnancy in the setting of a broader discussion about fellow workload and wellness. We agreed that trainees should be allowed to make changes to their schedule with co-fellows as needed for medical appointments or procedures and that our backup policy should be flexible enough to provide spot coverage for unexpected complications and family emergencies. We also incorporated a GI psychologist to provide wellness resources and suggestions for reducing burnout for our fellows.
We strove to follow certain principles in creating this policy. Trainees who are parents should have a comparable clinical experience to their nonparenting colleagues and should take the lead in rearranging their own schedule. Nonbirthing parents, adopting parents, and parents using surrogacy should be included in any parental leave policy. Fellowship leaders have an important responsibility in helping fellows proactively plan to meet requirements for graduation and maximize learning and exposure (Figure). We also recognized the importance of equitable coverage. For example, there is sometimes a perception that fellows with children “burden” fellows who are not parents.3,19 On the other hand, fellows without children may feel that they are called on more than their colleagues with children to cover those with childcare issues. In addition, as a recent study of general surgery residency program directors indicates, there are complex interpersonal issues that play into a colleague’s willingness to provide coverage.20 It behooves program leadership to be cognizant of group dynamics that might cause conflict over what should be a straightforward coverage situation.

We first researched national and societal guidelines if available, as well as our institution’s graduate medical education (GME) website. We categorized benefits by whether they were federal, state-mandated, or institutional. It is important to note that any concerns about trainee salaries should be discussed with one’s GME office to ensure the leave policy is in accordance with federal funding policies.21 We solicited experiences and advice from former and current fellows who had gone through, or were planning, pregnancy and parental leave. A few faculty members volunteered to serve as a resource for fellows; these “ambassadors” discussed their experiences during a lunchtime panel, as well as offered to provide one-on-one advice and participate in future panels. We also reached out to our infection control experts to review the literature and federal policies on infections of special consideration during pregnancy and endoscopy. As for radiation safety, given the importance of education and active monitoring, we offer the option of reaching out to our radiation safety officer for individualized counseling.22
Based on these efforts, we drafted a written policy designed for pregnant fellows and fellows planning parental leave on expectations for the program and fellows, benefits, and advice, including childcare options, lactation room locations, and financial planning tips. We shared this document with fellows and incorporated feedback. As a “living document” it is subject to change and will be updated as needed (at least annually).
Additional planning considerations for fellows
Research childcare options (ideally 6 months or more before leave).
- Start to explore your institution’s resources for leave and childcare options (daycare waitlists may be greater than 1 year in some cities).
Inform your program director (4-5 months before leave).
- Consider informing your program director about pregnancy at the beginning of the second trimester.
- Discuss Accreditation Council for Graduate Medical Education requirements and scheduling responsibilities.
- Explicitly discuss whether you plan to graduate on time or extend fellowship.
Inform your colleagues and patients (at least 3-4 months before leave).
- If comfortable, consider getting advice from a co-fellow and/or faculty mentor parent to facilitate transition to parenthood.
- When you feel ready, begin trading rotations and calls with co-fellows. If you have a results inbox or pager, discuss who can help cover those during your leave.
- Inform research collaborators about your leave and make preparations to keep projects progressing during your leave.
- If you have “active” clinic patients, when appropriate, begin to inform them that you will be away and provide reassurance that a colleague will be covering you. Leave clear plans with contingencies for these patients in your last progress notes.
Complete institutional paperwork and map out facilities (at least 2-3 months before leave).
- Review your options for using time toward leave, including vacation, research, or Family and Medical Leave Act–provided leave (unpaid), and what paperwork you need to fill out.
- Contact your payroll and/or human resources office to inform them of birth/adoption.
- Research potential program parental benefits, such as dependent daycare and/or health care flexible spending accounts.
- If choosing to breastfeed, explore the lactation rooms that are closest to your workroom and endoscopy suite and determine how much time you will need to set aside for pumping.
Be prepared to make adjustments as needed.
- Endoscopy-heavy rotations may be more difficult in the third trimester of pregnancy.
- Make contingency plans for early or late delivery dates, as well as if you undergo a cesarean that requires additional recovery time.
- Consider scheduling elective rotations (research, clinic) toward the end of leave and for the first month of “return to work.”
- If you plan to join limited clinic or outpatient endoscopy blocks later in your leave, make early arrangements to work regularly with these attendings.
Conclusion
Trainee needs assessments in gastroenterology fellowship similar to those in other specialties should be performed, and are in fact underway.19,23,24 There is a lack of data regarding the availability of fellowship program guidance and, specifically, adherence to required policies, and more data from program directors at a national level need to be collected.20 We recommend that programs engage in identifying specific needs at their institutions with the goal of eventually sharing this knowledge with other programs. Gastroenterology society recommendations for performing endoscopy while pregnant, with regard to ergonomics, infection control, and radiation exposure, would be instrumental. GI fellowships should consolidate institutional knowledge and engage key stakeholders – including trainees, prior trainees, occupational safety experts, radiation safety offices, wellness experts, and GME – to create program-specific policies that are flexible but rigorous and generous but equitable.
Dr. Liu and Dr. Summa are gastroenterology fellows at Northwestern University, Chicago. Dr. Patel is an assistant professor of medicine and a gastroenterology fellowship assistant program director at Northwestern University, Chicago. Dr. Donnan and Dr. Guentner are assistant professors of medicine at Northwestern University. Dr. Kia is an assistant professor of medicine and the gastroenterology fellowship program director at Northwestern University. They have no conflicts of interest to disclose. The authors would like to thank Michelle Clermont, MD, and Maureen K. Bolon, MD, for their discussion and assistance during the drafting of this article.
References
1. Section on Medical Students, Residents, and Fellowship Trainees; Committee on Early Childhood. Pediatrics. 2013;131(2):387-90.
2. American Board of Medical Specialties. ABMS Announces Progressive Leave Policy for Residents and Fellows. July 13, 2020. Accessed May 1, 2021. https://www.abms.org/news-events/abms-announces-progressive-leave-policy-for-residents-and-fellows/.
3. Magudia K et al. J Grad Med Educ. 2020;12(2):162-7.
4. Blair JE et al. Acad Med. 2016;91(7):972-8.
5. Feld LD. Am J Gastroenterol. 2021;116(3):505-8.
6. Roubaud MS. Plast Reconstr Surg Glob Open. 2019. doi: 10.1097/GOX.0000000000002104.
7. Price J, Dunbar K. Gastrointest Endosc. 2009;69(1):121-3.
8. David YN et al. Gastrointest Endosc. 2020;91(6):AB75-AB76.
9. Webb AMB et al. Acad Med J Assoc Am Med Coll. 2019;94(11):1631-4.
10. Weinstein DF et al. N Engl J Med. 2019 Sep 12;381(11):995-7.
11. Anderson M, Goldman RH. JAMA Surg. 2020;155(3):243-9.
12. Palmer KT et al. Occup Environ Med. 2013;70(4):213-22.
13. Siegel JD et al; Healthcare Infection, Control Practices Advisory Committee. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings (2007). 2007. Last reviewed July 22, 2019. Accessed April 28, 2021. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html
14. David YN et al. Am J Gastroenterol. 2021;116(3):539-50.
15. Sethi S et al. Dig Dis Sci. 2019;64(9):2455-66.
16. Alzimami K et al. Gastroenterol Res Pract. 2013;2013:587574.
17. Hayashi S et al. World J Clin Cases. 2018;6(16):1087-93.
18. U.S. Department of Labor, Wage and Hour Division. “Frequently Asked Questions – Break Time for Nursing Mothers.” Accessed May 1, 2021. https://www.dol.gov/agencies/whd/nursing-mothers/faq.
19. Mwakyanjala EJ et al. J Am Heart Assoc. 2019. doi: 10.1161/JAHA.119.012137.
20. Castillo-Angeles M et al. JAMA Surg. 2021 Jul 1;156(7):647-53.
21. Prasad S et al. J Grad Med Educ. 2021 Jun;13(3):349-54.
22. Ho IKH et al. Am J Gastroenterol. 2014;109(8):1180-94.
23. Sherbaf FG et al. AJNR Am J Neuroradiol. 2020;41(8):1348-54.
24. Altieri MS et al. JAMA Surg. 2019;154(10):952-58.
Due to broad social changes and efforts from leaders in GI, there is renewed interest in family planning and parental leave policies for GI trainees. The American Board of Medical Specialties now permits trainees a minimum of 6 weeks away during training, without automatically requiring an extension of training time or completely depleting vacation time, for boards eligibility.1,2 However, national and institutional guidance for family planning and pregnancy during GI fellowship is lacking. How can gastroenterology fellowship programs support fellows taking parental leave and enact fair policies? We review the scope of the problem, describe our experience in developing resources within our GI fellowship program, and highlight areas that require further development.
The scope of the issue
There is no national data yet on the number of GI fellows that are parents prior to starting fellowship or who become parents during fellowship. We estimate that approximately 25% of fellows enter training as parents or become parents during fellowship, although 40%-50% may have an intention to have children.3,4 Fellows may be worried that they will “fall behind” or be perceived as less committed if they devote time to childrearing or take parental leave.5-7 Indeed, worries about discrimination based on pregnancy and parental leave are borne out by the experiences of older physicians (in particular, female physicians).8,9
State- and institution-specific benefits vary from program to program. Nationally, the Family and Medical Leave Act provides only unpaid leave and applies only to trainees who have been employed for greater than 12 months.10 Benefits may not always be well advertised, and even when they are, trainees (and attendings) may feel uncomfortable taking full advantage. One survey of GIs revealed that, although two-thirds believed that 6-8 weeks of maternity leave was inadequate, half took less than that amount due to fears about financial and professional consequences.8
Pregnancy during GI fellowship is a special concern. GI fellowship consists of long work hours, includes night call, and can be physically demanding. All three of these factors have been associated with preterm delivery, infertility, and miscarriage.11,12 In addition, there are no guidelines for ergonomic adjustments or infection precautions for pregnant endoscopists. We have compiled information about infection prevention guidance in pregnancy (available from the authors on request) derived from guidance from the National Institute for Occupational Safety and Health, which recommends the same precautions for pregnant health care workers as for nonpregnant health care workers.13 In regards to SARS-CoV-2, we believe that the decision to perform procedures on patients with COVID-19 infection should be individualized, although vaccinated endoscopists should be reassured by the exceedingly low rates of infection after vaccination and with appropriate personal protective equipment. Radiation is yet another concern. There are limited data on radiation dosages incurred by workers in the endoscopy suite and no pregnancy-specific data, which may lead trainees to avoid fluoroscopic procedures and unnecessarily double up on lead gowns.8,11,14-17
Breastfeeding accommodations, and access to lactation rooms for trainees, are required by federal law for a minimum of 12 months.18 Should a trainee choose to breastfeed, education of staff and attendings is critical because many may be unaware of the specific needs pertaining to lactation. Staff should be aware that 30-45 minutes are needed to prepare, pump, and store milk. Trainees should not be solely responsible for educating their attendings and staff.
Our Experience in Creating a Policy
We developed a formal fellowship program policy on parental leave and pregnancy in the setting of a broader discussion about fellow workload and wellness. We agreed that trainees should be allowed to make changes to their schedule with co-fellows as needed for medical appointments or procedures and that our backup policy should be flexible enough to provide spot coverage for unexpected complications and family emergencies. We also incorporated a GI psychologist to provide wellness resources and suggestions for reducing burnout for our fellows.
We strove to follow certain principles in creating this policy. Trainees who are parents should have a comparable clinical experience to their nonparenting colleagues and should take the lead in rearranging their own schedule. Nonbirthing parents, adopting parents, and parents using surrogacy should be included in any parental leave policy. Fellowship leaders have an important responsibility in helping fellows proactively plan to meet requirements for graduation and maximize learning and exposure (Figure). We also recognized the importance of equitable coverage. For example, there is sometimes a perception that fellows with children “burden” fellows who are not parents.3,19 On the other hand, fellows without children may feel that they are called on more than their colleagues with children to cover those with childcare issues. In addition, as a recent study of general surgery residency program directors indicates, there are complex interpersonal issues that play into a colleague’s willingness to provide coverage.20 It behooves program leadership to be cognizant of group dynamics that might cause conflict over what should be a straightforward coverage situation.

We first researched national and societal guidelines if available, as well as our institution’s graduate medical education (GME) website. We categorized benefits by whether they were federal, state-mandated, or institutional. It is important to note that any concerns about trainee salaries should be discussed with one’s GME office to ensure the leave policy is in accordance with federal funding policies.21 We solicited experiences and advice from former and current fellows who had gone through, or were planning, pregnancy and parental leave. A few faculty members volunteered to serve as a resource for fellows; these “ambassadors” discussed their experiences during a lunchtime panel, as well as offered to provide one-on-one advice and participate in future panels. We also reached out to our infection control experts to review the literature and federal policies on infections of special consideration during pregnancy and endoscopy. As for radiation safety, given the importance of education and active monitoring, we offer the option of reaching out to our radiation safety officer for individualized counseling.22
Based on these efforts, we drafted a written policy designed for pregnant fellows and fellows planning parental leave on expectations for the program and fellows, benefits, and advice, including childcare options, lactation room locations, and financial planning tips. We shared this document with fellows and incorporated feedback. As a “living document” it is subject to change and will be updated as needed (at least annually).
Additional planning considerations for fellows
Research childcare options (ideally 6 months or more before leave).
- Start to explore your institution’s resources for leave and childcare options (daycare waitlists may be greater than 1 year in some cities).
Inform your program director (4-5 months before leave).
- Consider informing your program director about pregnancy at the beginning of the second trimester.
- Discuss Accreditation Council for Graduate Medical Education requirements and scheduling responsibilities.
- Explicitly discuss whether you plan to graduate on time or extend fellowship.
Inform your colleagues and patients (at least 3-4 months before leave).
- If comfortable, consider getting advice from a co-fellow and/or faculty mentor parent to facilitate transition to parenthood.
- When you feel ready, begin trading rotations and calls with co-fellows. If you have a results inbox or pager, discuss who can help cover those during your leave.
- Inform research collaborators about your leave and make preparations to keep projects progressing during your leave.
- If you have “active” clinic patients, when appropriate, begin to inform them that you will be away and provide reassurance that a colleague will be covering you. Leave clear plans with contingencies for these patients in your last progress notes.
Complete institutional paperwork and map out facilities (at least 2-3 months before leave).
- Review your options for using time toward leave, including vacation, research, or Family and Medical Leave Act–provided leave (unpaid), and what paperwork you need to fill out.
- Contact your payroll and/or human resources office to inform them of birth/adoption.
- Research potential program parental benefits, such as dependent daycare and/or health care flexible spending accounts.
- If choosing to breastfeed, explore the lactation rooms that are closest to your workroom and endoscopy suite and determine how much time you will need to set aside for pumping.
Be prepared to make adjustments as needed.
- Endoscopy-heavy rotations may be more difficult in the third trimester of pregnancy.
- Make contingency plans for early or late delivery dates, as well as if you undergo a cesarean that requires additional recovery time.
- Consider scheduling elective rotations (research, clinic) toward the end of leave and for the first month of “return to work.”
- If you plan to join limited clinic or outpatient endoscopy blocks later in your leave, make early arrangements to work regularly with these attendings.
Conclusion
Trainee needs assessments in gastroenterology fellowship similar to those in other specialties should be performed, and are in fact underway.19,23,24 There is a lack of data regarding the availability of fellowship program guidance and, specifically, adherence to required policies, and more data from program directors at a national level need to be collected.20 We recommend that programs engage in identifying specific needs at their institutions with the goal of eventually sharing this knowledge with other programs. Gastroenterology society recommendations for performing endoscopy while pregnant, with regard to ergonomics, infection control, and radiation exposure, would be instrumental. GI fellowships should consolidate institutional knowledge and engage key stakeholders – including trainees, prior trainees, occupational safety experts, radiation safety offices, wellness experts, and GME – to create program-specific policies that are flexible but rigorous and generous but equitable.
Dr. Liu and Dr. Summa are gastroenterology fellows at Northwestern University, Chicago. Dr. Patel is an assistant professor of medicine and a gastroenterology fellowship assistant program director at Northwestern University, Chicago. Dr. Donnan and Dr. Guentner are assistant professors of medicine at Northwestern University. Dr. Kia is an assistant professor of medicine and the gastroenterology fellowship program director at Northwestern University. They have no conflicts of interest to disclose. The authors would like to thank Michelle Clermont, MD, and Maureen K. Bolon, MD, for their discussion and assistance during the drafting of this article.
References
1. Section on Medical Students, Residents, and Fellowship Trainees; Committee on Early Childhood. Pediatrics. 2013;131(2):387-90.
2. American Board of Medical Specialties. ABMS Announces Progressive Leave Policy for Residents and Fellows. July 13, 2020. Accessed May 1, 2021. https://www.abms.org/news-events/abms-announces-progressive-leave-policy-for-residents-and-fellows/.
3. Magudia K et al. J Grad Med Educ. 2020;12(2):162-7.
4. Blair JE et al. Acad Med. 2016;91(7):972-8.
5. Feld LD. Am J Gastroenterol. 2021;116(3):505-8.
6. Roubaud MS. Plast Reconstr Surg Glob Open. 2019. doi: 10.1097/GOX.0000000000002104.
7. Price J, Dunbar K. Gastrointest Endosc. 2009;69(1):121-3.
8. David YN et al. Gastrointest Endosc. 2020;91(6):AB75-AB76.
9. Webb AMB et al. Acad Med J Assoc Am Med Coll. 2019;94(11):1631-4.
10. Weinstein DF et al. N Engl J Med. 2019 Sep 12;381(11):995-7.
11. Anderson M, Goldman RH. JAMA Surg. 2020;155(3):243-9.
12. Palmer KT et al. Occup Environ Med. 2013;70(4):213-22.
13. Siegel JD et al; Healthcare Infection, Control Practices Advisory Committee. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings (2007). 2007. Last reviewed July 22, 2019. Accessed April 28, 2021. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html
14. David YN et al. Am J Gastroenterol. 2021;116(3):539-50.
15. Sethi S et al. Dig Dis Sci. 2019;64(9):2455-66.
16. Alzimami K et al. Gastroenterol Res Pract. 2013;2013:587574.
17. Hayashi S et al. World J Clin Cases. 2018;6(16):1087-93.
18. U.S. Department of Labor, Wage and Hour Division. “Frequently Asked Questions – Break Time for Nursing Mothers.” Accessed May 1, 2021. https://www.dol.gov/agencies/whd/nursing-mothers/faq.
19. Mwakyanjala EJ et al. J Am Heart Assoc. 2019. doi: 10.1161/JAHA.119.012137.
20. Castillo-Angeles M et al. JAMA Surg. 2021 Jul 1;156(7):647-53.
21. Prasad S et al. J Grad Med Educ. 2021 Jun;13(3):349-54.
22. Ho IKH et al. Am J Gastroenterol. 2014;109(8):1180-94.
23. Sherbaf FG et al. AJNR Am J Neuroradiol. 2020;41(8):1348-54.
24. Altieri MS et al. JAMA Surg. 2019;154(10):952-58.
AGA News
AGA journals’ reach record-high Impact Factors
AGA is proud to announce that its journals have maintained their exceptional standing in the field of gastroenterology and hepatology, based on Impact Factor. The Impact Factor is a measure of the frequency with which articles published in the previous 2 years are cited and is commonly used to rank the significance of journals within their fields.
Gastroenterology, AGA’s flagship journal, received a record-high Impact Factor of 22.682, a substantial increase from its 2019 Impact Factor of 17.37. Gastroenterology maintains its position among an elite group of journals focused on publishing original research, spanning basic to clinical, in our field. Co–Editors in Chief (EICs) Richard M. Peek Jr., MD, and Douglas A. Corley, MD, PhD, remarked, “We would like to thank our entire board of editors and reviewers, as well as the incredible AGA editorial staff, for their exceptional work in this challenging pandemic year as we continue to publish articles and reviews of outstanding quality that are widely used by our readership. It is an honor to be part of such a remarkable team.”
Clinical Gastroenterology and Hepatology (CGH), AGA’s clinically focused journal, also reached a record high with an Impact Factor of 11.382, pulling ahead as the field’s top exclusively clinically oriented journal. This puts CGH at a rank of 8th among 92 journals in the field. Fasiha Kanwal, MD, MSHS, EIC of CGH, noted, “We are delighted that CGH remains in a strong position in the top 10 GI journals in terms of Impact Factor. On behalf of the CGH board of editors, I want to extend a warm and most heartfelt thanks to our authors, reviewers, and readers! We would not have been able to achieve this milestone without your support, contributions, and the faith that you place in us.”
To round things out, Cellular and Molecular Gastroenterology and Hepatology (CMGH), AGA’s basic and translational open-access journal also reached a record high with an Impact Factor of 9.225, placing it 13th, and second among nonclinical journals in that topic area. EICs Klaus Kaestner, PhD, and Michael Pack, MD, stated, “As co-EICs of CMGH, we send congratulations to the journal’s board of editors, editorial board, reviewers, and superb editorial staff on this year’s Impact Factor. We are honored to work with these outstanding colleagues and to provide our readership with highly impactful and cutting-edge research and review articles.”
In its online announcement, AGA congratulates and thanks the boards of all three journals for their editorial leadership. We also thank our authors, readers, and reviewers for their continued support of AGA’s journals. We look forward to continuing to push the envelope in scientific publishing in the upcoming year.
AGA journals select new editorial fellows
The AGA journals Gastroenterology, Clinical Gastroenterology and Hepatology (CGH), Cellular and Molecular Gastroenterology and Hepatology (CMGH), and Techniques and Innovations in Gastrointestinal Endoscopy (TIGE) recently selected the recipients of their editorial fellowships, which will run from July 2021 through June 2022. The AGA editorial fellowship program is in its 4th year.
- Amisha Ahuja, MD (Gastroenterology)
- Helenie Kefalalkes, MD (Gastroenterology)
- Katherine Falloon, MD (CGH)
- Judy Trieu, MD, MPH (CGH)
- Lindsey Kennedy, PhD (CMGH)
- Vivian Ortiz, MD (CMGH)
- Sagarika Satyavada, MD (TIGE)
- Eric Swei, MD (TIGE)
Gain perspectives, insights, and experience in diagnostic and therapeutic GI care
Accurately diagnosing and treating GI disorders such as irritable bowel syndrome, inflammatory bowel disease, or eosinophilic esophagitis are challenging for any health care practitioners. Why not be the advanced practice provider (APP) in your practice that others look to for providing the best course of action for patients? The all-virtual 2021 Principles of GI for the NP and PA, Aug. 14-15, 2021, explores these GI conditions in detail, as well as colorectal cancer and disorders of the liver and pancreas, to give you a foundation in which to provide superlative patient care.
The virtual format also offers a safe and affordable forum for learning from your home or office as the impact of the COVID-19 pandemic continues to be felt throughout 2021. You’ll also benefit from on-demand access for 2 years after the live course so you can reference and refresh what you learned.
Take the opportunity to refine your skills and improve your patient care outcomes.
Honor your peers with an AGA Recognition Award
When you think about outstanding GI educators, clinicians, investigators, and mentors, who comes to mind?
Share your appreciation by nominating your colleagues for a prestigious 2022 AGA Recognition Award!
Make your nominee stand out by sharing specific examples of how they have devoted themselves to eradicating the world of digestive disease, demonstrated innovation in bettering our community, and made a lasting impact, all of which exemplifies an outstanding AGA member.
Need some inspiration? Read about our 2021 winners before submitting your nomination.
AGA journals’ reach record-high Impact Factors
AGA is proud to announce that its journals have maintained their exceptional standing in the field of gastroenterology and hepatology, based on Impact Factor. The Impact Factor is a measure of the frequency with which articles published in the previous 2 years are cited and is commonly used to rank the significance of journals within their fields.
Gastroenterology, AGA’s flagship journal, received a record-high Impact Factor of 22.682, a substantial increase from its 2019 Impact Factor of 17.37. Gastroenterology maintains its position among an elite group of journals focused on publishing original research, spanning basic to clinical, in our field. Co–Editors in Chief (EICs) Richard M. Peek Jr., MD, and Douglas A. Corley, MD, PhD, remarked, “We would like to thank our entire board of editors and reviewers, as well as the incredible AGA editorial staff, for their exceptional work in this challenging pandemic year as we continue to publish articles and reviews of outstanding quality that are widely used by our readership. It is an honor to be part of such a remarkable team.”
Clinical Gastroenterology and Hepatology (CGH), AGA’s clinically focused journal, also reached a record high with an Impact Factor of 11.382, pulling ahead as the field’s top exclusively clinically oriented journal. This puts CGH at a rank of 8th among 92 journals in the field. Fasiha Kanwal, MD, MSHS, EIC of CGH, noted, “We are delighted that CGH remains in a strong position in the top 10 GI journals in terms of Impact Factor. On behalf of the CGH board of editors, I want to extend a warm and most heartfelt thanks to our authors, reviewers, and readers! We would not have been able to achieve this milestone without your support, contributions, and the faith that you place in us.”
To round things out, Cellular and Molecular Gastroenterology and Hepatology (CMGH), AGA’s basic and translational open-access journal also reached a record high with an Impact Factor of 9.225, placing it 13th, and second among nonclinical journals in that topic area. EICs Klaus Kaestner, PhD, and Michael Pack, MD, stated, “As co-EICs of CMGH, we send congratulations to the journal’s board of editors, editorial board, reviewers, and superb editorial staff on this year’s Impact Factor. We are honored to work with these outstanding colleagues and to provide our readership with highly impactful and cutting-edge research and review articles.”
In its online announcement, AGA congratulates and thanks the boards of all three journals for their editorial leadership. We also thank our authors, readers, and reviewers for their continued support of AGA’s journals. We look forward to continuing to push the envelope in scientific publishing in the upcoming year.
AGA journals select new editorial fellows
The AGA journals Gastroenterology, Clinical Gastroenterology and Hepatology (CGH), Cellular and Molecular Gastroenterology and Hepatology (CMGH), and Techniques and Innovations in Gastrointestinal Endoscopy (TIGE) recently selected the recipients of their editorial fellowships, which will run from July 2021 through June 2022. The AGA editorial fellowship program is in its 4th year.
- Amisha Ahuja, MD (Gastroenterology)
- Helenie Kefalalkes, MD (Gastroenterology)
- Katherine Falloon, MD (CGH)
- Judy Trieu, MD, MPH (CGH)
- Lindsey Kennedy, PhD (CMGH)
- Vivian Ortiz, MD (CMGH)
- Sagarika Satyavada, MD (TIGE)
- Eric Swei, MD (TIGE)
Gain perspectives, insights, and experience in diagnostic and therapeutic GI care
Accurately diagnosing and treating GI disorders such as irritable bowel syndrome, inflammatory bowel disease, or eosinophilic esophagitis are challenging for any health care practitioners. Why not be the advanced practice provider (APP) in your practice that others look to for providing the best course of action for patients? The all-virtual 2021 Principles of GI for the NP and PA, Aug. 14-15, 2021, explores these GI conditions in detail, as well as colorectal cancer and disorders of the liver and pancreas, to give you a foundation in which to provide superlative patient care.
The virtual format also offers a safe and affordable forum for learning from your home or office as the impact of the COVID-19 pandemic continues to be felt throughout 2021. You’ll also benefit from on-demand access for 2 years after the live course so you can reference and refresh what you learned.
Take the opportunity to refine your skills and improve your patient care outcomes.
Honor your peers with an AGA Recognition Award
When you think about outstanding GI educators, clinicians, investigators, and mentors, who comes to mind?
Share your appreciation by nominating your colleagues for a prestigious 2022 AGA Recognition Award!
Make your nominee stand out by sharing specific examples of how they have devoted themselves to eradicating the world of digestive disease, demonstrated innovation in bettering our community, and made a lasting impact, all of which exemplifies an outstanding AGA member.
Need some inspiration? Read about our 2021 winners before submitting your nomination.
AGA journals’ reach record-high Impact Factors
AGA is proud to announce that its journals have maintained their exceptional standing in the field of gastroenterology and hepatology, based on Impact Factor. The Impact Factor is a measure of the frequency with which articles published in the previous 2 years are cited and is commonly used to rank the significance of journals within their fields.
Gastroenterology, AGA’s flagship journal, received a record-high Impact Factor of 22.682, a substantial increase from its 2019 Impact Factor of 17.37. Gastroenterology maintains its position among an elite group of journals focused on publishing original research, spanning basic to clinical, in our field. Co–Editors in Chief (EICs) Richard M. Peek Jr., MD, and Douglas A. Corley, MD, PhD, remarked, “We would like to thank our entire board of editors and reviewers, as well as the incredible AGA editorial staff, for their exceptional work in this challenging pandemic year as we continue to publish articles and reviews of outstanding quality that are widely used by our readership. It is an honor to be part of such a remarkable team.”
Clinical Gastroenterology and Hepatology (CGH), AGA’s clinically focused journal, also reached a record high with an Impact Factor of 11.382, pulling ahead as the field’s top exclusively clinically oriented journal. This puts CGH at a rank of 8th among 92 journals in the field. Fasiha Kanwal, MD, MSHS, EIC of CGH, noted, “We are delighted that CGH remains in a strong position in the top 10 GI journals in terms of Impact Factor. On behalf of the CGH board of editors, I want to extend a warm and most heartfelt thanks to our authors, reviewers, and readers! We would not have been able to achieve this milestone without your support, contributions, and the faith that you place in us.”
To round things out, Cellular and Molecular Gastroenterology and Hepatology (CMGH), AGA’s basic and translational open-access journal also reached a record high with an Impact Factor of 9.225, placing it 13th, and second among nonclinical journals in that topic area. EICs Klaus Kaestner, PhD, and Michael Pack, MD, stated, “As co-EICs of CMGH, we send congratulations to the journal’s board of editors, editorial board, reviewers, and superb editorial staff on this year’s Impact Factor. We are honored to work with these outstanding colleagues and to provide our readership with highly impactful and cutting-edge research and review articles.”
In its online announcement, AGA congratulates and thanks the boards of all three journals for their editorial leadership. We also thank our authors, readers, and reviewers for their continued support of AGA’s journals. We look forward to continuing to push the envelope in scientific publishing in the upcoming year.
AGA journals select new editorial fellows
The AGA journals Gastroenterology, Clinical Gastroenterology and Hepatology (CGH), Cellular and Molecular Gastroenterology and Hepatology (CMGH), and Techniques and Innovations in Gastrointestinal Endoscopy (TIGE) recently selected the recipients of their editorial fellowships, which will run from July 2021 through June 2022. The AGA editorial fellowship program is in its 4th year.
- Amisha Ahuja, MD (Gastroenterology)
- Helenie Kefalalkes, MD (Gastroenterology)
- Katherine Falloon, MD (CGH)
- Judy Trieu, MD, MPH (CGH)
- Lindsey Kennedy, PhD (CMGH)
- Vivian Ortiz, MD (CMGH)
- Sagarika Satyavada, MD (TIGE)
- Eric Swei, MD (TIGE)
Gain perspectives, insights, and experience in diagnostic and therapeutic GI care
Accurately diagnosing and treating GI disorders such as irritable bowel syndrome, inflammatory bowel disease, or eosinophilic esophagitis are challenging for any health care practitioners. Why not be the advanced practice provider (APP) in your practice that others look to for providing the best course of action for patients? The all-virtual 2021 Principles of GI for the NP and PA, Aug. 14-15, 2021, explores these GI conditions in detail, as well as colorectal cancer and disorders of the liver and pancreas, to give you a foundation in which to provide superlative patient care.
The virtual format also offers a safe and affordable forum for learning from your home or office as the impact of the COVID-19 pandemic continues to be felt throughout 2021. You’ll also benefit from on-demand access for 2 years after the live course so you can reference and refresh what you learned.
Take the opportunity to refine your skills and improve your patient care outcomes.
Honor your peers with an AGA Recognition Award
When you think about outstanding GI educators, clinicians, investigators, and mentors, who comes to mind?
Share your appreciation by nominating your colleagues for a prestigious 2022 AGA Recognition Award!
Make your nominee stand out by sharing specific examples of how they have devoted themselves to eradicating the world of digestive disease, demonstrated innovation in bettering our community, and made a lasting impact, all of which exemplifies an outstanding AGA member.
Need some inspiration? Read about our 2021 winners before submitting your nomination.




















