User login
Finding room for hope
Dear colleagues,
I’m thrilled to introduce the August edition of The New Gastroenterologist, which features an excellent line-up of articles! Summer has been in full swing, and gradually, we eased into aspects of our prepandemic routine. The fear, caution, and isolation that characterized the last year and a half was less pervasive, and the ability to reconnect in person felt both refreshing and liberating. While new threats of variants and rising infection rates have emerged, there is hope that, with the availability of vaccines, the worst of the pandemic may still be behind us.
One of the most difficult aspects of treating patients with inflammatory bowel disease is acute pain management. Dr. Jami Kinnucan and Dr. Mehwish Ahmed (University of Michigan) outline an expert approach on differentiating between visceral and somatic pain and how to manage each accordingly.
The diagnosis of microscopic colitis can be elusive because colonic mucosa typically appears endoscopically normal and the pathognomonic findings are histologic. Management can also be challenging given the frequently relapsing and remitting nature of its clinical course. The “In Focus” feature for August, written by Dr. June Tome, Dr. Amrit Kamboj, and Dr. Darrell Pardi (Mayo Clinic), is an absolute must-read as it provides a detailed review on the diagnosis, management, and therapeutic options for microscopic colitis.
As gastroenterologists, we are often asked to place percutaneous endoscopic gastrostomy (PEG) tubes. This can be a difficult situation to navigate especially when the indication or timing of placement seems questionable. In our ethics case for this quarter, Dr. David Seres and Dr. Jane Cowan (Columbia University) unpack the ethical considerations of PEG tube placement in order to facilitate discharge to subacute nursing facilities.
Months in quarantine have incited many to crave larger living spaces, lending to a chaotic housing market. Jon Solitro (FinancialMD) offers sound financial advice for physicians interested purchasing a home – including factors to consider when choosing a home, how much to spend, and whether or not to consider a doctor’s loan.
Success in research can be particularly difficult for fellows and early career gastroenterologists as they juggle the many responsibilities inherent to busy training programs or adjust to independent practice. Dr. Dionne Rebello and Dr. Michelle Long (Boston University) compile a list of incredibly helpful tips on how to optimize productivity. For those interested in ways to harness experiences in clinical medicine into health technology, Dr. Simon Matthews (Johns Hopkins) discusses his role as chief medical officer in a health tech start-up in our postfellowship pathways section.
Lastly, our DHPA Private Practice Perspectives article, written by Dr. George Dickstein (Greater Boston Gastroenterology), nicely summarizes lessons learned from the pandemic and how a practice can be adequately prepared for a post-pandemic surge of procedures.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m thrilled to introduce the August edition of The New Gastroenterologist, which features an excellent line-up of articles! Summer has been in full swing, and gradually, we eased into aspects of our prepandemic routine. The fear, caution, and isolation that characterized the last year and a half was less pervasive, and the ability to reconnect in person felt both refreshing and liberating. While new threats of variants and rising infection rates have emerged, there is hope that, with the availability of vaccines, the worst of the pandemic may still be behind us.
One of the most difficult aspects of treating patients with inflammatory bowel disease is acute pain management. Dr. Jami Kinnucan and Dr. Mehwish Ahmed (University of Michigan) outline an expert approach on differentiating between visceral and somatic pain and how to manage each accordingly.
The diagnosis of microscopic colitis can be elusive because colonic mucosa typically appears endoscopically normal and the pathognomonic findings are histologic. Management can also be challenging given the frequently relapsing and remitting nature of its clinical course. The “In Focus” feature for August, written by Dr. June Tome, Dr. Amrit Kamboj, and Dr. Darrell Pardi (Mayo Clinic), is an absolute must-read as it provides a detailed review on the diagnosis, management, and therapeutic options for microscopic colitis.
As gastroenterologists, we are often asked to place percutaneous endoscopic gastrostomy (PEG) tubes. This can be a difficult situation to navigate especially when the indication or timing of placement seems questionable. In our ethics case for this quarter, Dr. David Seres and Dr. Jane Cowan (Columbia University) unpack the ethical considerations of PEG tube placement in order to facilitate discharge to subacute nursing facilities.
Months in quarantine have incited many to crave larger living spaces, lending to a chaotic housing market. Jon Solitro (FinancialMD) offers sound financial advice for physicians interested purchasing a home – including factors to consider when choosing a home, how much to spend, and whether or not to consider a doctor’s loan.
Success in research can be particularly difficult for fellows and early career gastroenterologists as they juggle the many responsibilities inherent to busy training programs or adjust to independent practice. Dr. Dionne Rebello and Dr. Michelle Long (Boston University) compile a list of incredibly helpful tips on how to optimize productivity. For those interested in ways to harness experiences in clinical medicine into health technology, Dr. Simon Matthews (Johns Hopkins) discusses his role as chief medical officer in a health tech start-up in our postfellowship pathways section.
Lastly, our DHPA Private Practice Perspectives article, written by Dr. George Dickstein (Greater Boston Gastroenterology), nicely summarizes lessons learned from the pandemic and how a practice can be adequately prepared for a post-pandemic surge of procedures.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m thrilled to introduce the August edition of The New Gastroenterologist, which features an excellent line-up of articles! Summer has been in full swing, and gradually, we eased into aspects of our prepandemic routine. The fear, caution, and isolation that characterized the last year and a half was less pervasive, and the ability to reconnect in person felt both refreshing and liberating. While new threats of variants and rising infection rates have emerged, there is hope that, with the availability of vaccines, the worst of the pandemic may still be behind us.
One of the most difficult aspects of treating patients with inflammatory bowel disease is acute pain management. Dr. Jami Kinnucan and Dr. Mehwish Ahmed (University of Michigan) outline an expert approach on differentiating between visceral and somatic pain and how to manage each accordingly.
The diagnosis of microscopic colitis can be elusive because colonic mucosa typically appears endoscopically normal and the pathognomonic findings are histologic. Management can also be challenging given the frequently relapsing and remitting nature of its clinical course. The “In Focus” feature for August, written by Dr. June Tome, Dr. Amrit Kamboj, and Dr. Darrell Pardi (Mayo Clinic), is an absolute must-read as it provides a detailed review on the diagnosis, management, and therapeutic options for microscopic colitis.
As gastroenterologists, we are often asked to place percutaneous endoscopic gastrostomy (PEG) tubes. This can be a difficult situation to navigate especially when the indication or timing of placement seems questionable. In our ethics case for this quarter, Dr. David Seres and Dr. Jane Cowan (Columbia University) unpack the ethical considerations of PEG tube placement in order to facilitate discharge to subacute nursing facilities.
Months in quarantine have incited many to crave larger living spaces, lending to a chaotic housing market. Jon Solitro (FinancialMD) offers sound financial advice for physicians interested purchasing a home – including factors to consider when choosing a home, how much to spend, and whether or not to consider a doctor’s loan.
Success in research can be particularly difficult for fellows and early career gastroenterologists as they juggle the many responsibilities inherent to busy training programs or adjust to independent practice. Dr. Dionne Rebello and Dr. Michelle Long (Boston University) compile a list of incredibly helpful tips on how to optimize productivity. For those interested in ways to harness experiences in clinical medicine into health technology, Dr. Simon Matthews (Johns Hopkins) discusses his role as chief medical officer in a health tech start-up in our postfellowship pathways section.
Lastly, our DHPA Private Practice Perspectives article, written by Dr. George Dickstein (Greater Boston Gastroenterology), nicely summarizes lessons learned from the pandemic and how a practice can be adequately prepared for a post-pandemic surge of procedures.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Microscopic colitis: A common, yet often overlooked, cause of chronic diarrhea
Microscopic colitis is an inflammatory disease of the colon and a frequent cause of chronic or recurrent watery diarrhea, particularly in older persons. MC consists of two subtypes, collagenous colitis (CC) and lymphocytic colitis (LC). While the primary symptom is diarrhea, other signs and symptoms such as abdominal pain, weight loss, and dehydration or electrolyte abnormalities may also be present depending on disease severity.1 In MC, the colonic mucosa usually appears normal on colonoscopy, and the diagnosis is made by histologic findings of intraepithelial lymphocytosis with (CC) or without (LC) a prominent subepithelial collagen band. The management approaches to CC and LC are similar and should be directed based on the severity of symptoms.2 We review the epidemiology, risk factors, pathophysiology, diagnosis, and clinical management for this condition, as well as novel therapeutic approaches.
Epidemiology
Although the incidence of MC increased in the late twentieth century, more recently, it has stabilized with an estimated incidence varying from 1 to 25 per 100,000 person-years.3-5 A recent meta-analysis revealed a pooled incidence of 4.85 per 100,000 persons for LC and 4.14 per 100,000 persons for CC.6 Proposed explanations for the rising incidence in the late twentieth century include improved clinical awareness of the disease, possible increased use of drugs associated with MC, and increased performance of diagnostic colonoscopies for chronic diarrhea. Since MC is now well-recognized, the recent plateau in incidence rates may reflect decreased detection bias.
The prevalence of MC ranges from 10%-20% in patients undergoing colonoscopy for chronic watery diarrhea.6,7 The prevalence of LC is approximately 63.1 cases per 100,000 person-years and, for CC, is 49.2 cases per 100,000 person-years.6-8 Recent studies have demonstrated increasing prevalence of MC likely resulting from an aging population.9,10
Risk stratification
Female gender, increasing age, concomitant autoimmune disease, and the use of certain drugs, including NSAIDs, proton pump inhibitors (PPIs), statins, and selective serotonin reuptake inhibitors (SSRIs), have been associated with an increased risk of MC.11,12 Autoimmune disorders, including celiac disease (CD), rheumatoid arthritis, hypothyroidism, and hyperthyroidism, are more common in patients with MC. The association with CD, in particular, is clinically important, as CD is associated with a 50-70 times greater risk of MC, and 2%-9% of patients with MC have CD.13,14
Several medications have been associated with MC. In a British multicenter prospective study, MC was associated with the use of NSAIDs, PPIs, and SSRIs;15 however, recent studies have questioned the association of MC with some of these medications, which might worsen diarrhea but not actually cause MC.16
An additional risk factor for MC is smoking. A recent meta-analysis demonstrated that current and former smokers had an increased risk of MC (odds ratio, 2.99; 95% confidence interval, 2.15-4.15 and OR, 1.63; 95% CI, 1.37-1.94, respectively), compared with nonsmokers.17 Smokers develop MC at a younger age, and smoking is associated with increased disease severity and decreased likelihood of attaining remission.18,19
Pathogenesis
The pathogenesis of MC remains largely unknown, although there are several hypotheses. The leading proposed mechanisms include reaction to luminal antigens, dysregulated collagen metabolism, genetic predisposition, autoimmunity, and bile acid malabsorption.
MC may be caused by abnormal epithelial barrier function, leading to increased permeability and reaction to luminal antigens, including dietary antigens, certain drugs, and bacterial products, 20,21 which themselves lead to the immune dysregulation and intestinal inflammation seen in MC. This mechanism may explain the association of several drugs with MC. Histological changes resembling LC are reported in patients with CD who consume gluten; however, large population-based studies have not found specific dietary associations with the development of MC.22
Another potential mechanism of MC is dysregulated collagen deposition. Collagen accumulation in the subepithelial layer in CC may result from increased levels of fibroblast growth factor, transforming growth factor–beta and vascular endothelial growth factor.23 Nonetheless, studies have not found an association between the severity of diarrhea in patients with CC and the thickness of the subepithelial collagen band.
Thirdly, autoimmunity and genetic predisposition have been postulated in the pathogenesis of MC. As previously discussed, MC is associated with several autoimmune diseases and predominantly occurs in women, a distinctive feature of autoimmune disorders. Several studies have demonstrated an association between MC and HLA-DQ2 and -DQ3 haplotypes,24 as well as potential polymorphisms in the serotonin transporter gene promoter.25 It is important to note, however, that only a few familial cases of MC have been reported to date.26
Lastly, bile acid malabsorption may play a role in the etiology of MC. Histologic findings of inflammation, along with villous atrophy and collagen deposition, have been reported in the ileum of patients with MC;27,28 however, because patients with MC without bile acid malabsorption may also respond to bile acid binders such as cholestyramine, these findings unlikely to be the sole mechanism explaining the development of the disease.
Despite the different proposed mechanisms for the pathogenesis of MC, no definite conclusions can be drawn because of the limited size of these studies and their often conflicting results.

Clinical features
Clinicians should suspect MC in patients with chronic or recurrent watery diarrhea, particularly in older persons. Other risk factors include female gender, use of certain culprit medications, smoking, and presence of other autoimmune diseases. The clinical manifestations of MC subtypes LC and CC are similar with no significant clinical differences.1,2 In addition to diarrhea, patients with MC may have abdominal pain, fatigue, and dehydration or electrolyte abnormalities depending on disease severity. Patients may also present with fecal urgency, incontinence, and nocturnal stools. Quality of life is often reduced in these patients, predominantly in those with severe or refractory symptoms.29,30 The natural course of MC is highly variable, with some patients achieving spontaneous resolution after one episode and others developing chronic symptoms.
Diagnosis
The differential diagnosis of chronic watery diarrhea is broad and includes malabsorption/maldigestion, inflammatory bowel disease (IBD), irritable bowel syndrome, and medication side effects. In addition, although gastrointestinal infections typically cause acute or subacute diarrhea, some can present with chronic diarrhea. Malabsorption/maldigestion may occur because of CD, lactose intolerance, and pancreatic insufficiency, among other conditions. A thorough history, regarding recent antibiotic and medication use, travel, and immunosuppression, should be obtained in patients with chronic diarrhea. Additionally, laboratory and endoscopic evaluation with random biopsies of the colon can further help differentiate these diseases from MC. A few studies suggest fecal calprotectin may be used to differentiate MC from other noninflammatory conditions such as irritable bowel syndrome, as well as to monitor disease activity. This test is not expected to distinguish MC from other inflammatory causes of diarrhea, such as IBD, and therefore, its role in clinical practice is uncertain.31
The diagnosis of MC is made by biopsy of the colonic mucosa demonstrating characteristic pathologic features.32 Unlike in diseases such as Crohn’s disease or ulcerative colitis, the colon usually appears normal in MC, although mild nonspecific changes, such as erythema or edema, may be visualized. There is no consensus on the ideal location to obtain biopsies for MC or whether biopsies from both the left and the right colon are required.2,33 The procedure of choice for the diagnosis of MC is colonoscopy with random biopsies taken throughout the colon. More limited evaluation by flexible sigmoidoscopy with biopsies may miss cases of MC as inflammation and collagen thickening are not necessarily uniform throughout the colon; however, in a patient that has undergone a recent colonoscopy for colon cancer screening without colon biopsies, a flexible sigmoidoscopy may be a reasonable next test for evaluation of MC, provided biopsies are obtained above the rectosigmoid colon.34
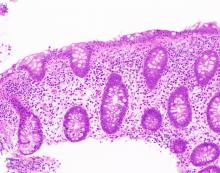
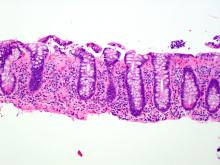
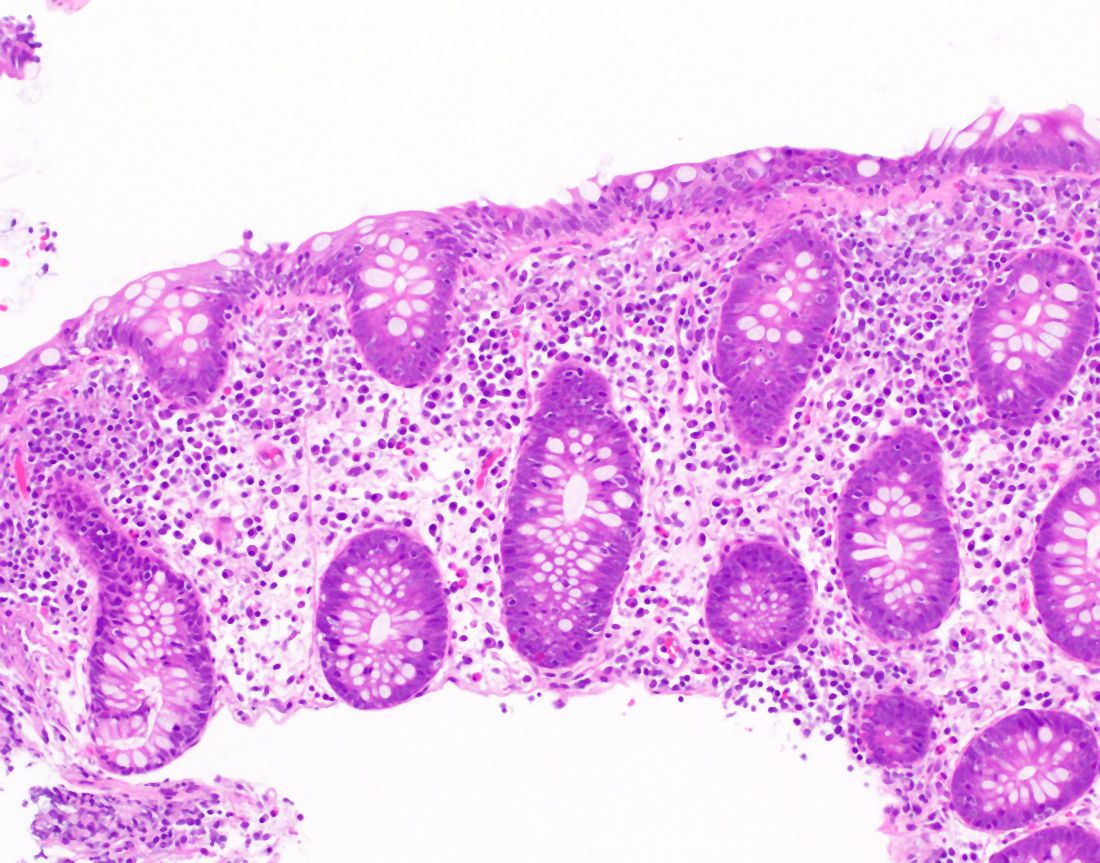
The MC subtypes are differentiated based on histology. The hallmark of LC is less than 20 intraepithelial lymphocytes per 100 surface epithelial cells (normal, less than 5) (Figure 1A). CC is characterized by a thickened subepithelial collagen band greater than 7-10 micrometers (normal, less than 5) (Figure 1B). For a subgroup of patients with milder abnormalities that do not meet these histological criteria, the terms “microscopic colitis, not otherwise specified” or “microscopic colitis, incomplete” may be used.35 These patients often respond to standard treatments for MC. There is an additional subset of patients with biopsy demonstrating features of both CC and LC simultaneously, as well as patients transitioning from one MC subtype to another over time.32,35
Management approach
The first step in management of patients with MC includes stopping culprit medications if there is a temporal relationship between the initiation of the medication and the onset of diarrhea, as well as encouraging smoking cessation. These steps alone, however, are unlikely to achieve clinical remission in most patients. A stepwise pharmacological approach is used in the management of MC based on disease severity (Figure 2). For patients with mild symptoms, antidiarrheal medications, such as loperamide, may be helpful.36 Long-term use of loperamide at therapeutic doses no greater than 16 mg daily appears to be safe if required to maintain symptom response. For those with persistent symptoms despite antidiarrheal medications, bismuth subsalicylate at three 262 mg tablets three times daily for 6-8 weeks can be considered. Long-term use of bismuth subsalicylate is not advised, especially at this dose, because of possible neurotoxicity.37
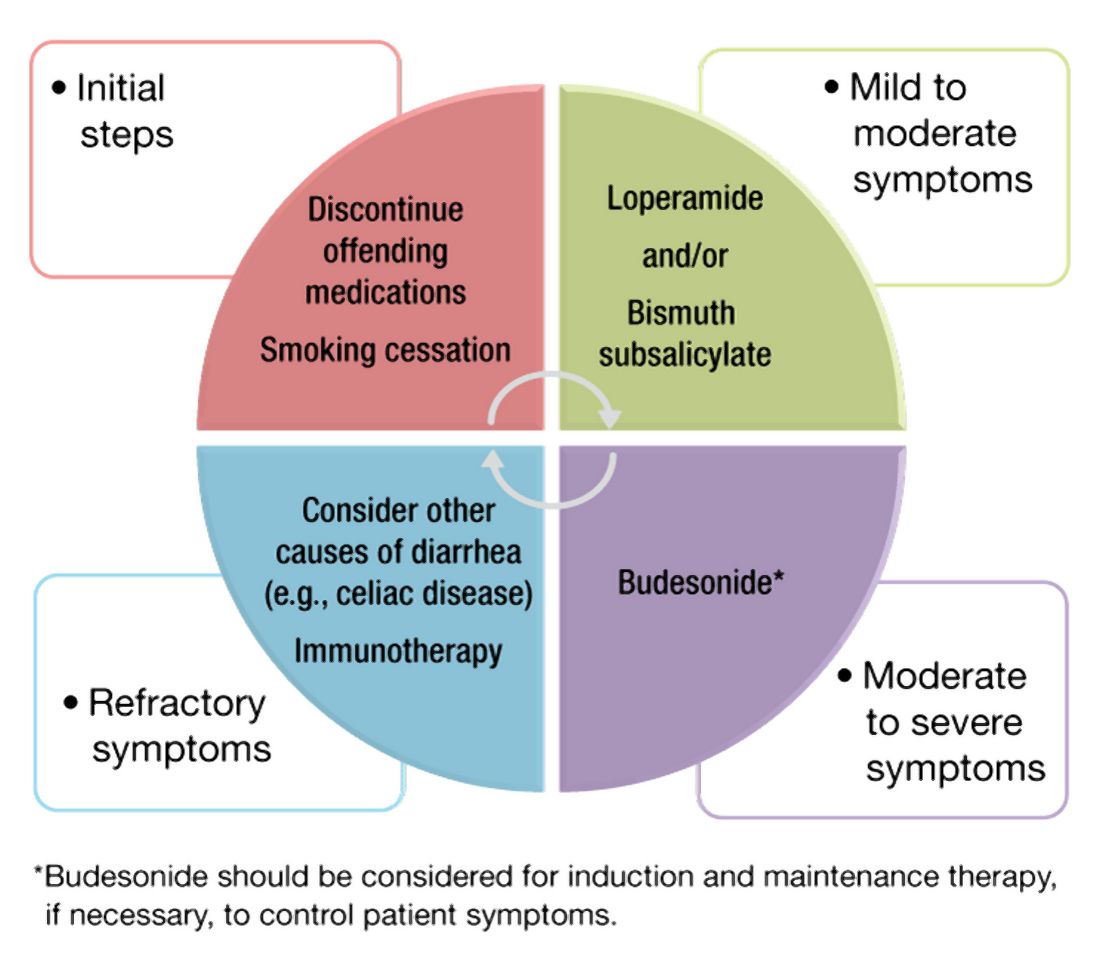
For patients refractory to the above treatments or those with moderate-to-severe symptoms, an 8-week course of budesonide at 9 mg daily is the first-line treatment.38 The dose was tapered before discontinuation in some studies but not in others. Both strategies appear effective. A recent meta-analysis of nine randomized trials demonstrated pooled ORs of 7.34 (95% CI, 4.08-13.19) and 8.35 (95% CI, 4.14-16.85) for response to budesonide induction and maintenance, respectively.39
Cholestyramine is another medication considered in the management of MC and warrants further investigation. To date, no randomized clinical trials have been conducted to evaluate bile acid sequestrants in MC, but they should be considered before placing patients on immunosuppressive medications. Some providers use mesalamine in this setting, although mesalamine is inferior to budesonide in the induction of clinical remission in MC.40
Despite high rates of response to budesonide, relapse after discontinuation is frequent (60%-80%), and time to relapse is variable41,42 The American Gastroenterological Association recommends budesonide for maintenance of remission in patients with recurrence following discontinuation of induction therapy. The lowest effective dose that maintains resolution of symptoms should be prescribed, ideally at 6 mg daily or lower.38 Although budesonide has a greater first-pass metabolism, compared with other glucocorticoids, patients should be monitored for possible side effects including hypertension, diabetes, and osteoporosis, as well as ophthalmologic disease, including cataracts and glaucoma.
For those who are intolerant to budesonide or have refractory symptoms, concomitant disorders such as CD that may be contributing to symptoms must be excluded. Immunosuppressive medications – such as thiopurines and biologic agents, including tumor necrosis factor–alpha inhibitors or vedolizumab – may be considered in refractory cases.43,44 Of note, there are limited studies evaluating the use of these medications for MC. Lastly, surgeries including ileostomy with or without colectomy have been performed in the most severe cases for resistant disease that has failed numerous pharmacological therapies.45
Patients should be counseled that, while symptoms from MC can be quite bothersome and disabling, there appears to be a normal life expectancy and no association between MC and colon cancer, unlike with other inflammatory conditions of the colon such as IBD.46,47
Conclusion and future outlook
As a common cause of chronic watery diarrhea, MC will be commonly encountered in primary care and gastroenterology practices. The diagnosis should be suspected in patients presenting with chronic or recurrent watery diarrhea, especially with female gender, autoimmune disease, and increasing age. The management of MC requires an algorithmic approach directed by symptom severity, with a subgroup of patients requiring maintenance therapy for relapsing symptoms. The care of patients with MC will continue to evolve in the future. Further work is needed to explore long-term safety outcomes with budesonide and the role of immunomodulators and newer biologic agents for patients with complex, refractory disease.
Dr. Tome is with the department of internal medicine at the Mayo Clinic, Rochester, Minn. Dr. Kamboj, and Dr. Pardi are with the division of gastroenterology and hepatology at the Mayo Clinic. Dr. Pardi has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda and has consulted for Vedanta and Otsuka. The other authors have no conflicts of interest to report.
References
1. Nyhlin N et al. Aliment Pharmacol Ther. 2014;39:963-72.
2. Miehlke S et al. United European Gastroenterol J. 2020;20-8.
3. Pardi DS et al. Gut. 2007;56:504-8.
4. Fernández-Bañares F et al. J Crohn’s Colitis.2016;10(7):805-11.
5. Gentile NM et al. Clin Gastroenterol Hepatol. 2014;12(5):838-42.
6. Tong J et al. Am J Gastroenterol. 2015;110:265-76.
7. Olesen M et al. Gut. 2004;53(3):346-50.
8. Bergman D et al. Aliment Pharmacol Ther. 2019;49(11):1395-400.
9. Guagnozzi D et al. Dig Liver Dis. 2012;44(5):384-8.
10. Münch A et al. J Crohns Colitis. 2012;6(9):932-45.
11. Macaigne G et al. Am J Gastroenterol. 2014; 09(9):1461-70.
12. Verhaegh BP et al. Aliment Pharmacol Ther. 2016;43(9):1004-13.
13. Stewart M et al. Aliment Pharmacol Ther. 2011;33(12):1340-9.
14. Green PHR et al. Clin Gastroenterol Hepatol. 2009;7(11):1210-6.
15. Masclee GM et al. Am J Gastroenterol. 2015;110:749-59.
16. Zylberberg H et al. Ailment Pharmacol Ther. 2021 Jun;53(11)1209-15.
17. Jaruvongvanich V et al. Inflamm Bowel Dis. 2019;25(4):672-8.
18. Fernández-Bañares F et al. Inflamm Bowel Dis. 2013; 19(7):1470-6.
19. Yen EF et al. Inflamm Bowel Dis. 2012;18(10):1835-41.
20. Barmeyer C et al. J Gastroenterol. 2017;52(10):1090-100.
21. Morgan DM et al. Clin Gastroenterol Hepatol. 2020;18(4):984-6.
22. Larsson JK et al. Eur J Clin Nutr. 2016;70:1309-17.
23. Madisch A et al.. Inflamm Bowel Dis. 2011;17(11):2295-8.
24. Stahl E et al. Gastroenterology. 2020;159(2):549-61.
25. Sikander A et al. Dig Dis Sci. 2015; 60:887-94.
26. Abdo AA et al. Can J Gastroenterol. 2001;15(5):341-3.
27. Fernandez-Bañares F et al. Dig Dis Sci.2001;46(10):2231-8.
28. Lyutakov I et al. Eur J Gastroenterol Hepatol. 2021;1;33(3):380-7.
29. Hjortswang H et al. Dig Liver Dis. 2011 Feb;43(2):102-9.
30. Cotter TG= et al. Gut. 2018;67(3):441-6.
31. Von Arnim U et al. Clin Exp Gastroenterol. 2016;9:97-103.
32. Langner C et al. Histopathology. 2015;66:613-26.
33. ASGE Standards of Practice Committee and Sharaf RN et al. Gastrointest Endosc. 2013;78:216-24.
34. Macaigne G et al. Clin Res Hepatol Gastroenterol. 2017;41(3):333-40.
35. Bjørnbak C et al. Aliment Pharmacol Ther. 2011;34(10):1225-34.
36. Pardi DS et al. Gastroenterology. 2016;150(1):247-74.
37. Masannat Y and Nazer E. West Virginia Med J. 2013;109(3):32-4.
38. Nguyen GC et al. Gastroenterology. 2016; 150(1):242-6.
39. Sebastian S et al. Eur J Gastroenterol Hepatol. 2019 Aug;31(8):919-27.
40. Miehlke S et al. Gastroenterology. 2014;146(5):1222-30.
41. Gentile NM et al. Am J Gastroenterol. 2013;108:256-9.
42. Münch A et al. Gut. 2016; 65(1):47-56.
43. Cotter TG et al. Aliment Pharmacol Ther. 2017; 46(2):169-74.
44. Esteve M et al. J Crohns Colitis. 2011;5(6):612-8.
45. Cottreau J et al. Clin J Gastroenterol. 2016;9:140-4.
46. Kamboj AK et al. Program No. P1876. ACG 2018 Annual Scientific Meeting Abstracts. Philadelphia, Pennsylvania: American College of Gastroenterology.
47. Yen EF et al. Dig Dis Sci. 2012;57:161-9.
Microscopic colitis is an inflammatory disease of the colon and a frequent cause of chronic or recurrent watery diarrhea, particularly in older persons. MC consists of two subtypes, collagenous colitis (CC) and lymphocytic colitis (LC). While the primary symptom is diarrhea, other signs and symptoms such as abdominal pain, weight loss, and dehydration or electrolyte abnormalities may also be present depending on disease severity.1 In MC, the colonic mucosa usually appears normal on colonoscopy, and the diagnosis is made by histologic findings of intraepithelial lymphocytosis with (CC) or without (LC) a prominent subepithelial collagen band. The management approaches to CC and LC are similar and should be directed based on the severity of symptoms.2 We review the epidemiology, risk factors, pathophysiology, diagnosis, and clinical management for this condition, as well as novel therapeutic approaches.
Epidemiology
Although the incidence of MC increased in the late twentieth century, more recently, it has stabilized with an estimated incidence varying from 1 to 25 per 100,000 person-years.3-5 A recent meta-analysis revealed a pooled incidence of 4.85 per 100,000 persons for LC and 4.14 per 100,000 persons for CC.6 Proposed explanations for the rising incidence in the late twentieth century include improved clinical awareness of the disease, possible increased use of drugs associated with MC, and increased performance of diagnostic colonoscopies for chronic diarrhea. Since MC is now well-recognized, the recent plateau in incidence rates may reflect decreased detection bias.
The prevalence of MC ranges from 10%-20% in patients undergoing colonoscopy for chronic watery diarrhea.6,7 The prevalence of LC is approximately 63.1 cases per 100,000 person-years and, for CC, is 49.2 cases per 100,000 person-years.6-8 Recent studies have demonstrated increasing prevalence of MC likely resulting from an aging population.9,10
Risk stratification
Female gender, increasing age, concomitant autoimmune disease, and the use of certain drugs, including NSAIDs, proton pump inhibitors (PPIs), statins, and selective serotonin reuptake inhibitors (SSRIs), have been associated with an increased risk of MC.11,12 Autoimmune disorders, including celiac disease (CD), rheumatoid arthritis, hypothyroidism, and hyperthyroidism, are more common in patients with MC. The association with CD, in particular, is clinically important, as CD is associated with a 50-70 times greater risk of MC, and 2%-9% of patients with MC have CD.13,14
Several medications have been associated with MC. In a British multicenter prospective study, MC was associated with the use of NSAIDs, PPIs, and SSRIs;15 however, recent studies have questioned the association of MC with some of these medications, which might worsen diarrhea but not actually cause MC.16
An additional risk factor for MC is smoking. A recent meta-analysis demonstrated that current and former smokers had an increased risk of MC (odds ratio, 2.99; 95% confidence interval, 2.15-4.15 and OR, 1.63; 95% CI, 1.37-1.94, respectively), compared with nonsmokers.17 Smokers develop MC at a younger age, and smoking is associated with increased disease severity and decreased likelihood of attaining remission.18,19
Pathogenesis
The pathogenesis of MC remains largely unknown, although there are several hypotheses. The leading proposed mechanisms include reaction to luminal antigens, dysregulated collagen metabolism, genetic predisposition, autoimmunity, and bile acid malabsorption.
MC may be caused by abnormal epithelial barrier function, leading to increased permeability and reaction to luminal antigens, including dietary antigens, certain drugs, and bacterial products, 20,21 which themselves lead to the immune dysregulation and intestinal inflammation seen in MC. This mechanism may explain the association of several drugs with MC. Histological changes resembling LC are reported in patients with CD who consume gluten; however, large population-based studies have not found specific dietary associations with the development of MC.22
Another potential mechanism of MC is dysregulated collagen deposition. Collagen accumulation in the subepithelial layer in CC may result from increased levels of fibroblast growth factor, transforming growth factor–beta and vascular endothelial growth factor.23 Nonetheless, studies have not found an association between the severity of diarrhea in patients with CC and the thickness of the subepithelial collagen band.
Thirdly, autoimmunity and genetic predisposition have been postulated in the pathogenesis of MC. As previously discussed, MC is associated with several autoimmune diseases and predominantly occurs in women, a distinctive feature of autoimmune disorders. Several studies have demonstrated an association between MC and HLA-DQ2 and -DQ3 haplotypes,24 as well as potential polymorphisms in the serotonin transporter gene promoter.25 It is important to note, however, that only a few familial cases of MC have been reported to date.26
Lastly, bile acid malabsorption may play a role in the etiology of MC. Histologic findings of inflammation, along with villous atrophy and collagen deposition, have been reported in the ileum of patients with MC;27,28 however, because patients with MC without bile acid malabsorption may also respond to bile acid binders such as cholestyramine, these findings unlikely to be the sole mechanism explaining the development of the disease.
Despite the different proposed mechanisms for the pathogenesis of MC, no definite conclusions can be drawn because of the limited size of these studies and their often conflicting results.

Clinical features
Clinicians should suspect MC in patients with chronic or recurrent watery diarrhea, particularly in older persons. Other risk factors include female gender, use of certain culprit medications, smoking, and presence of other autoimmune diseases. The clinical manifestations of MC subtypes LC and CC are similar with no significant clinical differences.1,2 In addition to diarrhea, patients with MC may have abdominal pain, fatigue, and dehydration or electrolyte abnormalities depending on disease severity. Patients may also present with fecal urgency, incontinence, and nocturnal stools. Quality of life is often reduced in these patients, predominantly in those with severe or refractory symptoms.29,30 The natural course of MC is highly variable, with some patients achieving spontaneous resolution after one episode and others developing chronic symptoms.
Diagnosis
The differential diagnosis of chronic watery diarrhea is broad and includes malabsorption/maldigestion, inflammatory bowel disease (IBD), irritable bowel syndrome, and medication side effects. In addition, although gastrointestinal infections typically cause acute or subacute diarrhea, some can present with chronic diarrhea. Malabsorption/maldigestion may occur because of CD, lactose intolerance, and pancreatic insufficiency, among other conditions. A thorough history, regarding recent antibiotic and medication use, travel, and immunosuppression, should be obtained in patients with chronic diarrhea. Additionally, laboratory and endoscopic evaluation with random biopsies of the colon can further help differentiate these diseases from MC. A few studies suggest fecal calprotectin may be used to differentiate MC from other noninflammatory conditions such as irritable bowel syndrome, as well as to monitor disease activity. This test is not expected to distinguish MC from other inflammatory causes of diarrhea, such as IBD, and therefore, its role in clinical practice is uncertain.31
The diagnosis of MC is made by biopsy of the colonic mucosa demonstrating characteristic pathologic features.32 Unlike in diseases such as Crohn’s disease or ulcerative colitis, the colon usually appears normal in MC, although mild nonspecific changes, such as erythema or edema, may be visualized. There is no consensus on the ideal location to obtain biopsies for MC or whether biopsies from both the left and the right colon are required.2,33 The procedure of choice for the diagnosis of MC is colonoscopy with random biopsies taken throughout the colon. More limited evaluation by flexible sigmoidoscopy with biopsies may miss cases of MC as inflammation and collagen thickening are not necessarily uniform throughout the colon; however, in a patient that has undergone a recent colonoscopy for colon cancer screening without colon biopsies, a flexible sigmoidoscopy may be a reasonable next test for evaluation of MC, provided biopsies are obtained above the rectosigmoid colon.34
The MC subtypes are differentiated based on histology. The hallmark of LC is less than 20 intraepithelial lymphocytes per 100 surface epithelial cells (normal, less than 5) (Figure 1A). CC is characterized by a thickened subepithelial collagen band greater than 7-10 micrometers (normal, less than 5) (Figure 1B). For a subgroup of patients with milder abnormalities that do not meet these histological criteria, the terms “microscopic colitis, not otherwise specified” or “microscopic colitis, incomplete” may be used.35 These patients often respond to standard treatments for MC. There is an additional subset of patients with biopsy demonstrating features of both CC and LC simultaneously, as well as patients transitioning from one MC subtype to another over time.32,35
Management approach
The first step in management of patients with MC includes stopping culprit medications if there is a temporal relationship between the initiation of the medication and the onset of diarrhea, as well as encouraging smoking cessation. These steps alone, however, are unlikely to achieve clinical remission in most patients. A stepwise pharmacological approach is used in the management of MC based on disease severity (Figure 2). For patients with mild symptoms, antidiarrheal medications, such as loperamide, may be helpful.36 Long-term use of loperamide at therapeutic doses no greater than 16 mg daily appears to be safe if required to maintain symptom response. For those with persistent symptoms despite antidiarrheal medications, bismuth subsalicylate at three 262 mg tablets three times daily for 6-8 weeks can be considered. Long-term use of bismuth subsalicylate is not advised, especially at this dose, because of possible neurotoxicity.37

For patients refractory to the above treatments or those with moderate-to-severe symptoms, an 8-week course of budesonide at 9 mg daily is the first-line treatment.38 The dose was tapered before discontinuation in some studies but not in others. Both strategies appear effective. A recent meta-analysis of nine randomized trials demonstrated pooled ORs of 7.34 (95% CI, 4.08-13.19) and 8.35 (95% CI, 4.14-16.85) for response to budesonide induction and maintenance, respectively.39
Cholestyramine is another medication considered in the management of MC and warrants further investigation. To date, no randomized clinical trials have been conducted to evaluate bile acid sequestrants in MC, but they should be considered before placing patients on immunosuppressive medications. Some providers use mesalamine in this setting, although mesalamine is inferior to budesonide in the induction of clinical remission in MC.40
Despite high rates of response to budesonide, relapse after discontinuation is frequent (60%-80%), and time to relapse is variable41,42 The American Gastroenterological Association recommends budesonide for maintenance of remission in patients with recurrence following discontinuation of induction therapy. The lowest effective dose that maintains resolution of symptoms should be prescribed, ideally at 6 mg daily or lower.38 Although budesonide has a greater first-pass metabolism, compared with other glucocorticoids, patients should be monitored for possible side effects including hypertension, diabetes, and osteoporosis, as well as ophthalmologic disease, including cataracts and glaucoma.
For those who are intolerant to budesonide or have refractory symptoms, concomitant disorders such as CD that may be contributing to symptoms must be excluded. Immunosuppressive medications – such as thiopurines and biologic agents, including tumor necrosis factor–alpha inhibitors or vedolizumab – may be considered in refractory cases.43,44 Of note, there are limited studies evaluating the use of these medications for MC. Lastly, surgeries including ileostomy with or without colectomy have been performed in the most severe cases for resistant disease that has failed numerous pharmacological therapies.45
Patients should be counseled that, while symptoms from MC can be quite bothersome and disabling, there appears to be a normal life expectancy and no association between MC and colon cancer, unlike with other inflammatory conditions of the colon such as IBD.46,47
Conclusion and future outlook
As a common cause of chronic watery diarrhea, MC will be commonly encountered in primary care and gastroenterology practices. The diagnosis should be suspected in patients presenting with chronic or recurrent watery diarrhea, especially with female gender, autoimmune disease, and increasing age. The management of MC requires an algorithmic approach directed by symptom severity, with a subgroup of patients requiring maintenance therapy for relapsing symptoms. The care of patients with MC will continue to evolve in the future. Further work is needed to explore long-term safety outcomes with budesonide and the role of immunomodulators and newer biologic agents for patients with complex, refractory disease.
Dr. Tome is with the department of internal medicine at the Mayo Clinic, Rochester, Minn. Dr. Kamboj, and Dr. Pardi are with the division of gastroenterology and hepatology at the Mayo Clinic. Dr. Pardi has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda and has consulted for Vedanta and Otsuka. The other authors have no conflicts of interest to report.
References
1. Nyhlin N et al. Aliment Pharmacol Ther. 2014;39:963-72.
2. Miehlke S et al. United European Gastroenterol J. 2020;20-8.
3. Pardi DS et al. Gut. 2007;56:504-8.
4. Fernández-Bañares F et al. J Crohn’s Colitis.2016;10(7):805-11.
5. Gentile NM et al. Clin Gastroenterol Hepatol. 2014;12(5):838-42.
6. Tong J et al. Am J Gastroenterol. 2015;110:265-76.
7. Olesen M et al. Gut. 2004;53(3):346-50.
8. Bergman D et al. Aliment Pharmacol Ther. 2019;49(11):1395-400.
9. Guagnozzi D et al. Dig Liver Dis. 2012;44(5):384-8.
10. Münch A et al. J Crohns Colitis. 2012;6(9):932-45.
11. Macaigne G et al. Am J Gastroenterol. 2014; 09(9):1461-70.
12. Verhaegh BP et al. Aliment Pharmacol Ther. 2016;43(9):1004-13.
13. Stewart M et al. Aliment Pharmacol Ther. 2011;33(12):1340-9.
14. Green PHR et al. Clin Gastroenterol Hepatol. 2009;7(11):1210-6.
15. Masclee GM et al. Am J Gastroenterol. 2015;110:749-59.
16. Zylberberg H et al. Ailment Pharmacol Ther. 2021 Jun;53(11)1209-15.
17. Jaruvongvanich V et al. Inflamm Bowel Dis. 2019;25(4):672-8.
18. Fernández-Bañares F et al. Inflamm Bowel Dis. 2013; 19(7):1470-6.
19. Yen EF et al. Inflamm Bowel Dis. 2012;18(10):1835-41.
20. Barmeyer C et al. J Gastroenterol. 2017;52(10):1090-100.
21. Morgan DM et al. Clin Gastroenterol Hepatol. 2020;18(4):984-6.
22. Larsson JK et al. Eur J Clin Nutr. 2016;70:1309-17.
23. Madisch A et al.. Inflamm Bowel Dis. 2011;17(11):2295-8.
24. Stahl E et al. Gastroenterology. 2020;159(2):549-61.
25. Sikander A et al. Dig Dis Sci. 2015; 60:887-94.
26. Abdo AA et al. Can J Gastroenterol. 2001;15(5):341-3.
27. Fernandez-Bañares F et al. Dig Dis Sci.2001;46(10):2231-8.
28. Lyutakov I et al. Eur J Gastroenterol Hepatol. 2021;1;33(3):380-7.
29. Hjortswang H et al. Dig Liver Dis. 2011 Feb;43(2):102-9.
30. Cotter TG= et al. Gut. 2018;67(3):441-6.
31. Von Arnim U et al. Clin Exp Gastroenterol. 2016;9:97-103.
32. Langner C et al. Histopathology. 2015;66:613-26.
33. ASGE Standards of Practice Committee and Sharaf RN et al. Gastrointest Endosc. 2013;78:216-24.
34. Macaigne G et al. Clin Res Hepatol Gastroenterol. 2017;41(3):333-40.
35. Bjørnbak C et al. Aliment Pharmacol Ther. 2011;34(10):1225-34.
36. Pardi DS et al. Gastroenterology. 2016;150(1):247-74.
37. Masannat Y and Nazer E. West Virginia Med J. 2013;109(3):32-4.
38. Nguyen GC et al. Gastroenterology. 2016; 150(1):242-6.
39. Sebastian S et al. Eur J Gastroenterol Hepatol. 2019 Aug;31(8):919-27.
40. Miehlke S et al. Gastroenterology. 2014;146(5):1222-30.
41. Gentile NM et al. Am J Gastroenterol. 2013;108:256-9.
42. Münch A et al. Gut. 2016; 65(1):47-56.
43. Cotter TG et al. Aliment Pharmacol Ther. 2017; 46(2):169-74.
44. Esteve M et al. J Crohns Colitis. 2011;5(6):612-8.
45. Cottreau J et al. Clin J Gastroenterol. 2016;9:140-4.
46. Kamboj AK et al. Program No. P1876. ACG 2018 Annual Scientific Meeting Abstracts. Philadelphia, Pennsylvania: American College of Gastroenterology.
47. Yen EF et al. Dig Dis Sci. 2012;57:161-9.
Microscopic colitis is an inflammatory disease of the colon and a frequent cause of chronic or recurrent watery diarrhea, particularly in older persons. MC consists of two subtypes, collagenous colitis (CC) and lymphocytic colitis (LC). While the primary symptom is diarrhea, other signs and symptoms such as abdominal pain, weight loss, and dehydration or electrolyte abnormalities may also be present depending on disease severity.1 In MC, the colonic mucosa usually appears normal on colonoscopy, and the diagnosis is made by histologic findings of intraepithelial lymphocytosis with (CC) or without (LC) a prominent subepithelial collagen band. The management approaches to CC and LC are similar and should be directed based on the severity of symptoms.2 We review the epidemiology, risk factors, pathophysiology, diagnosis, and clinical management for this condition, as well as novel therapeutic approaches.
Epidemiology
Although the incidence of MC increased in the late twentieth century, more recently, it has stabilized with an estimated incidence varying from 1 to 25 per 100,000 person-years.3-5 A recent meta-analysis revealed a pooled incidence of 4.85 per 100,000 persons for LC and 4.14 per 100,000 persons for CC.6 Proposed explanations for the rising incidence in the late twentieth century include improved clinical awareness of the disease, possible increased use of drugs associated with MC, and increased performance of diagnostic colonoscopies for chronic diarrhea. Since MC is now well-recognized, the recent plateau in incidence rates may reflect decreased detection bias.
The prevalence of MC ranges from 10%-20% in patients undergoing colonoscopy for chronic watery diarrhea.6,7 The prevalence of LC is approximately 63.1 cases per 100,000 person-years and, for CC, is 49.2 cases per 100,000 person-years.6-8 Recent studies have demonstrated increasing prevalence of MC likely resulting from an aging population.9,10
Risk stratification
Female gender, increasing age, concomitant autoimmune disease, and the use of certain drugs, including NSAIDs, proton pump inhibitors (PPIs), statins, and selective serotonin reuptake inhibitors (SSRIs), have been associated with an increased risk of MC.11,12 Autoimmune disorders, including celiac disease (CD), rheumatoid arthritis, hypothyroidism, and hyperthyroidism, are more common in patients with MC. The association with CD, in particular, is clinically important, as CD is associated with a 50-70 times greater risk of MC, and 2%-9% of patients with MC have CD.13,14
Several medications have been associated with MC. In a British multicenter prospective study, MC was associated with the use of NSAIDs, PPIs, and SSRIs;15 however, recent studies have questioned the association of MC with some of these medications, which might worsen diarrhea but not actually cause MC.16
An additional risk factor for MC is smoking. A recent meta-analysis demonstrated that current and former smokers had an increased risk of MC (odds ratio, 2.99; 95% confidence interval, 2.15-4.15 and OR, 1.63; 95% CI, 1.37-1.94, respectively), compared with nonsmokers.17 Smokers develop MC at a younger age, and smoking is associated with increased disease severity and decreased likelihood of attaining remission.18,19
Pathogenesis
The pathogenesis of MC remains largely unknown, although there are several hypotheses. The leading proposed mechanisms include reaction to luminal antigens, dysregulated collagen metabolism, genetic predisposition, autoimmunity, and bile acid malabsorption.
MC may be caused by abnormal epithelial barrier function, leading to increased permeability and reaction to luminal antigens, including dietary antigens, certain drugs, and bacterial products, 20,21 which themselves lead to the immune dysregulation and intestinal inflammation seen in MC. This mechanism may explain the association of several drugs with MC. Histological changes resembling LC are reported in patients with CD who consume gluten; however, large population-based studies have not found specific dietary associations with the development of MC.22
Another potential mechanism of MC is dysregulated collagen deposition. Collagen accumulation in the subepithelial layer in CC may result from increased levels of fibroblast growth factor, transforming growth factor–beta and vascular endothelial growth factor.23 Nonetheless, studies have not found an association between the severity of diarrhea in patients with CC and the thickness of the subepithelial collagen band.
Thirdly, autoimmunity and genetic predisposition have been postulated in the pathogenesis of MC. As previously discussed, MC is associated with several autoimmune diseases and predominantly occurs in women, a distinctive feature of autoimmune disorders. Several studies have demonstrated an association between MC and HLA-DQ2 and -DQ3 haplotypes,24 as well as potential polymorphisms in the serotonin transporter gene promoter.25 It is important to note, however, that only a few familial cases of MC have been reported to date.26
Lastly, bile acid malabsorption may play a role in the etiology of MC. Histologic findings of inflammation, along with villous atrophy and collagen deposition, have been reported in the ileum of patients with MC;27,28 however, because patients with MC without bile acid malabsorption may also respond to bile acid binders such as cholestyramine, these findings unlikely to be the sole mechanism explaining the development of the disease.
Despite the different proposed mechanisms for the pathogenesis of MC, no definite conclusions can be drawn because of the limited size of these studies and their often conflicting results.

Clinical features
Clinicians should suspect MC in patients with chronic or recurrent watery diarrhea, particularly in older persons. Other risk factors include female gender, use of certain culprit medications, smoking, and presence of other autoimmune diseases. The clinical manifestations of MC subtypes LC and CC are similar with no significant clinical differences.1,2 In addition to diarrhea, patients with MC may have abdominal pain, fatigue, and dehydration or electrolyte abnormalities depending on disease severity. Patients may also present with fecal urgency, incontinence, and nocturnal stools. Quality of life is often reduced in these patients, predominantly in those with severe or refractory symptoms.29,30 The natural course of MC is highly variable, with some patients achieving spontaneous resolution after one episode and others developing chronic symptoms.
Diagnosis
The differential diagnosis of chronic watery diarrhea is broad and includes malabsorption/maldigestion, inflammatory bowel disease (IBD), irritable bowel syndrome, and medication side effects. In addition, although gastrointestinal infections typically cause acute or subacute diarrhea, some can present with chronic diarrhea. Malabsorption/maldigestion may occur because of CD, lactose intolerance, and pancreatic insufficiency, among other conditions. A thorough history, regarding recent antibiotic and medication use, travel, and immunosuppression, should be obtained in patients with chronic diarrhea. Additionally, laboratory and endoscopic evaluation with random biopsies of the colon can further help differentiate these diseases from MC. A few studies suggest fecal calprotectin may be used to differentiate MC from other noninflammatory conditions such as irritable bowel syndrome, as well as to monitor disease activity. This test is not expected to distinguish MC from other inflammatory causes of diarrhea, such as IBD, and therefore, its role in clinical practice is uncertain.31
The diagnosis of MC is made by biopsy of the colonic mucosa demonstrating characteristic pathologic features.32 Unlike in diseases such as Crohn’s disease or ulcerative colitis, the colon usually appears normal in MC, although mild nonspecific changes, such as erythema or edema, may be visualized. There is no consensus on the ideal location to obtain biopsies for MC or whether biopsies from both the left and the right colon are required.2,33 The procedure of choice for the diagnosis of MC is colonoscopy with random biopsies taken throughout the colon. More limited evaluation by flexible sigmoidoscopy with biopsies may miss cases of MC as inflammation and collagen thickening are not necessarily uniform throughout the colon; however, in a patient that has undergone a recent colonoscopy for colon cancer screening without colon biopsies, a flexible sigmoidoscopy may be a reasonable next test for evaluation of MC, provided biopsies are obtained above the rectosigmoid colon.34
The MC subtypes are differentiated based on histology. The hallmark of LC is less than 20 intraepithelial lymphocytes per 100 surface epithelial cells (normal, less than 5) (Figure 1A). CC is characterized by a thickened subepithelial collagen band greater than 7-10 micrometers (normal, less than 5) (Figure 1B). For a subgroup of patients with milder abnormalities that do not meet these histological criteria, the terms “microscopic colitis, not otherwise specified” or “microscopic colitis, incomplete” may be used.35 These patients often respond to standard treatments for MC. There is an additional subset of patients with biopsy demonstrating features of both CC and LC simultaneously, as well as patients transitioning from one MC subtype to another over time.32,35
Management approach
The first step in management of patients with MC includes stopping culprit medications if there is a temporal relationship between the initiation of the medication and the onset of diarrhea, as well as encouraging smoking cessation. These steps alone, however, are unlikely to achieve clinical remission in most patients. A stepwise pharmacological approach is used in the management of MC based on disease severity (Figure 2). For patients with mild symptoms, antidiarrheal medications, such as loperamide, may be helpful.36 Long-term use of loperamide at therapeutic doses no greater than 16 mg daily appears to be safe if required to maintain symptom response. For those with persistent symptoms despite antidiarrheal medications, bismuth subsalicylate at three 262 mg tablets three times daily for 6-8 weeks can be considered. Long-term use of bismuth subsalicylate is not advised, especially at this dose, because of possible neurotoxicity.37

For patients refractory to the above treatments or those with moderate-to-severe symptoms, an 8-week course of budesonide at 9 mg daily is the first-line treatment.38 The dose was tapered before discontinuation in some studies but not in others. Both strategies appear effective. A recent meta-analysis of nine randomized trials demonstrated pooled ORs of 7.34 (95% CI, 4.08-13.19) and 8.35 (95% CI, 4.14-16.85) for response to budesonide induction and maintenance, respectively.39
Cholestyramine is another medication considered in the management of MC and warrants further investigation. To date, no randomized clinical trials have been conducted to evaluate bile acid sequestrants in MC, but they should be considered before placing patients on immunosuppressive medications. Some providers use mesalamine in this setting, although mesalamine is inferior to budesonide in the induction of clinical remission in MC.40
Despite high rates of response to budesonide, relapse after discontinuation is frequent (60%-80%), and time to relapse is variable41,42 The American Gastroenterological Association recommends budesonide for maintenance of remission in patients with recurrence following discontinuation of induction therapy. The lowest effective dose that maintains resolution of symptoms should be prescribed, ideally at 6 mg daily or lower.38 Although budesonide has a greater first-pass metabolism, compared with other glucocorticoids, patients should be monitored for possible side effects including hypertension, diabetes, and osteoporosis, as well as ophthalmologic disease, including cataracts and glaucoma.
For those who are intolerant to budesonide or have refractory symptoms, concomitant disorders such as CD that may be contributing to symptoms must be excluded. Immunosuppressive medications – such as thiopurines and biologic agents, including tumor necrosis factor–alpha inhibitors or vedolizumab – may be considered in refractory cases.43,44 Of note, there are limited studies evaluating the use of these medications for MC. Lastly, surgeries including ileostomy with or without colectomy have been performed in the most severe cases for resistant disease that has failed numerous pharmacological therapies.45
Patients should be counseled that, while symptoms from MC can be quite bothersome and disabling, there appears to be a normal life expectancy and no association between MC and colon cancer, unlike with other inflammatory conditions of the colon such as IBD.46,47
Conclusion and future outlook
As a common cause of chronic watery diarrhea, MC will be commonly encountered in primary care and gastroenterology practices. The diagnosis should be suspected in patients presenting with chronic or recurrent watery diarrhea, especially with female gender, autoimmune disease, and increasing age. The management of MC requires an algorithmic approach directed by symptom severity, with a subgroup of patients requiring maintenance therapy for relapsing symptoms. The care of patients with MC will continue to evolve in the future. Further work is needed to explore long-term safety outcomes with budesonide and the role of immunomodulators and newer biologic agents for patients with complex, refractory disease.
Dr. Tome is with the department of internal medicine at the Mayo Clinic, Rochester, Minn. Dr. Kamboj, and Dr. Pardi are with the division of gastroenterology and hepatology at the Mayo Clinic. Dr. Pardi has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda and has consulted for Vedanta and Otsuka. The other authors have no conflicts of interest to report.
References
1. Nyhlin N et al. Aliment Pharmacol Ther. 2014;39:963-72.
2. Miehlke S et al. United European Gastroenterol J. 2020;20-8.
3. Pardi DS et al. Gut. 2007;56:504-8.
4. Fernández-Bañares F et al. J Crohn’s Colitis.2016;10(7):805-11.
5. Gentile NM et al. Clin Gastroenterol Hepatol. 2014;12(5):838-42.
6. Tong J et al. Am J Gastroenterol. 2015;110:265-76.
7. Olesen M et al. Gut. 2004;53(3):346-50.
8. Bergman D et al. Aliment Pharmacol Ther. 2019;49(11):1395-400.
9. Guagnozzi D et al. Dig Liver Dis. 2012;44(5):384-8.
10. Münch A et al. J Crohns Colitis. 2012;6(9):932-45.
11. Macaigne G et al. Am J Gastroenterol. 2014; 09(9):1461-70.
12. Verhaegh BP et al. Aliment Pharmacol Ther. 2016;43(9):1004-13.
13. Stewart M et al. Aliment Pharmacol Ther. 2011;33(12):1340-9.
14. Green PHR et al. Clin Gastroenterol Hepatol. 2009;7(11):1210-6.
15. Masclee GM et al. Am J Gastroenterol. 2015;110:749-59.
16. Zylberberg H et al. Ailment Pharmacol Ther. 2021 Jun;53(11)1209-15.
17. Jaruvongvanich V et al. Inflamm Bowel Dis. 2019;25(4):672-8.
18. Fernández-Bañares F et al. Inflamm Bowel Dis. 2013; 19(7):1470-6.
19. Yen EF et al. Inflamm Bowel Dis. 2012;18(10):1835-41.
20. Barmeyer C et al. J Gastroenterol. 2017;52(10):1090-100.
21. Morgan DM et al. Clin Gastroenterol Hepatol. 2020;18(4):984-6.
22. Larsson JK et al. Eur J Clin Nutr. 2016;70:1309-17.
23. Madisch A et al.. Inflamm Bowel Dis. 2011;17(11):2295-8.
24. Stahl E et al. Gastroenterology. 2020;159(2):549-61.
25. Sikander A et al. Dig Dis Sci. 2015; 60:887-94.
26. Abdo AA et al. Can J Gastroenterol. 2001;15(5):341-3.
27. Fernandez-Bañares F et al. Dig Dis Sci.2001;46(10):2231-8.
28. Lyutakov I et al. Eur J Gastroenterol Hepatol. 2021;1;33(3):380-7.
29. Hjortswang H et al. Dig Liver Dis. 2011 Feb;43(2):102-9.
30. Cotter TG= et al. Gut. 2018;67(3):441-6.
31. Von Arnim U et al. Clin Exp Gastroenterol. 2016;9:97-103.
32. Langner C et al. Histopathology. 2015;66:613-26.
33. ASGE Standards of Practice Committee and Sharaf RN et al. Gastrointest Endosc. 2013;78:216-24.
34. Macaigne G et al. Clin Res Hepatol Gastroenterol. 2017;41(3):333-40.
35. Bjørnbak C et al. Aliment Pharmacol Ther. 2011;34(10):1225-34.
36. Pardi DS et al. Gastroenterology. 2016;150(1):247-74.
37. Masannat Y and Nazer E. West Virginia Med J. 2013;109(3):32-4.
38. Nguyen GC et al. Gastroenterology. 2016; 150(1):242-6.
39. Sebastian S et al. Eur J Gastroenterol Hepatol. 2019 Aug;31(8):919-27.
40. Miehlke S et al. Gastroenterology. 2014;146(5):1222-30.
41. Gentile NM et al. Am J Gastroenterol. 2013;108:256-9.
42. Münch A et al. Gut. 2016; 65(1):47-56.
43. Cotter TG et al. Aliment Pharmacol Ther. 2017; 46(2):169-74.
44. Esteve M et al. J Crohns Colitis. 2011;5(6):612-8.
45. Cottreau J et al. Clin J Gastroenterol. 2016;9:140-4.
46. Kamboj AK et al. Program No. P1876. ACG 2018 Annual Scientific Meeting Abstracts. Philadelphia, Pennsylvania: American College of Gastroenterology.
47. Yen EF et al. Dig Dis Sci. 2012;57:161-9.
August 2021 – ICYMI
Gastroenterology
May 2021
Understanding GI Twitter and its major contributors. Elfanagely Y et al. Gastroenterology. 2021 May;160(6):1917-21. doi: 10.1053/j.gastro.2021.01.232.
Long-term safety of fecal microbiota transplantation for recurrent Clostridioides difficile infection. Saha S et al. Gastroenterology. 2021 May;160(6):1961-9.e3. doi: 10.1053/j.gastro.2021.01.010.
How to incorporate health equity training into gastroenterology and hepatology fellowships. Lee-Allen J, Shah BJ. Gastroenterology. 2021 May;160(6):1924-8. doi: 10.1053/j.gastro.2021.03.018.
Functional dyspepsia and gastroparesis in tertiary care are interchangeable syndromes with common clinical and pathologic features. Pasricha PJ et al. Gastroenterology. 2021 May;160(6):2006-17. doi: 10.1053/j.gastro.2021.01.230.
June 2021
How to manage the large nonpedunculated colorectal polyp. Shahidi N, Bourke MJ. Gastroenterology. 2021 Jun;160(7):2239-43.e1. doi: 10.1053/j.gastro.2021.04.029.
Mortality, reoperation, and hospital stay within 90 days of primary and secondary antireflux surgery in a population-based multinational study. Yanes M et al. Gastroenterology. 2021 Jun;160(7):2283-90. doi: 10.1053/j.gastro.2021.02.022.
Endoscopic submucosal dissection in north america: A large prospective multicenter study. Draganov PV et al. Gastroenterology. 2021 Jun;160(7):2317-27.e2. doi: 10.1053/j.gastro.2021.02.036.
July 2021
Gluten degradation, pharmacokinetics, safety, and tolerability of TAK-062, an engineered enzyme to treat celiac disease. Pultz IG et al. Gastroenterology. 2021 Jul;161(1):81-93.e3. doi: 10.1053/j.gastro.2021.03.019.
Paternal exposure to immunosuppressive and/or biologic agents and birth outcomes in patients with immune-mediated inflammatory diseases. Meserve J et al. Gastroenterology. 2021 Jul;161(1):107-115.e3. doi: 10.1053/j.gastro.2021.03.020.
Ethnicity associations with food sensitization are mediated by gut microbiota development in the first year of life. Tun Hm et al. Gastroenterology. 2021 Jul;161(1):94-106. doi: 10.1053/j.gastro.2021.03.016.
How to incorporate bariatric training into your fellowship program. Jirapinyo P, Thompson CC. Gastroenterology. 2019 Jul;157(1):9-13. doi: 10.1053/j.gastro.2019.05.034.
CGH
May 2021
Intestinal failure: What all gastroenterologists should know. Jansson-Knodell CL et al. Clin Gastroenterol Hepatol. 2021 May;19(5):885-8. doi: 10.1016/j.cgh.2021.01.038.
When and how to use endoscopic tattooing in the colon: An international Delphi agreement. Medina-Prado L et al. Clin Gastroenterol Hepatol. 2021 May;19(5):1038-50. doi: 10.1016/j.cgh.2021.01.024.
Five-year outcomes of endoscopic sleeve gastroplasty for the treatment of obesity. Sharaiha RZ et al. Clin Gastroenterol Hepatol. 2021 May;19(5):1051-57.e2. doi: 10.1016/j.cgh.2020.09.055.
June 2021
GA Clinical Practice Update on management of bleeding gastric varices: Expert review. Henry Z et al. Clin Gastroenterol Hepatol. 2021 Jun;19(6):1098-107.e1. doi: 10.1016/j.cgh.2021.01.027.
Inter- and intra-individual variation, and limited prognostic utility, of serum alkaline phosphatase in a trial of patients with primary sclerosing cholangitis
Palak J. Trivedi PJ et al. Clin Gastroenterol Hepatol. 2021 Jun;19(6):1248-57. doi: 10.1016/j.cgh.2020.07.032.
Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Julia Fritsch J et al. Clin Gastroenterol Hepatol. 2021 Jun;19(6):1189-99.e30. doi: 10.1016/j.cgh.2020.05.026.
July 2021
Scoping out a better parental leave policy for gastroenterology fellows. Wegermann K. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1307-9. doi: 10.1016/j.cgh.2021.01.040.
An impetus for change: How COVID-19 will transform the delivery of GI health care. Leiman DA et al. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1310-1313. doi: 10.1016/j.cgh.2021.03.042.
Untangling nonerosive reflux disease from functional heartburn. Patel D et al. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1314-26. doi: 10.1016/j.cgh.2020.03.057.
AGA Clinical Practice Update on chemoprevention for colorectal neoplasia: Expert review. Liang PS et al. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1327-36. doi: 10.1016/j.cgh.2021.02.014.
CMGH
Drug inhibition of SARS-CoV-2 replication in human pluripotent stem cell–derived intestinal organoids. Krüger J et al. Cell Mol Gastroenterol Hepatol. 2021;11(4):935-48. doi: 10.1016/j.jcmgh.2020.11.003.
TIGE
Virtual interviews during the COVID-19 Pandemic: A survey of advanced endoscopy fellowship applicants and programs. Amrit K. Kamboj AK et al. Tech Innov Gastrointest Endosc. 2021;23(2):159-68. doi: 10.1016/j.tige.2021.02.001.
Triage of general gastrointestinal endoscopic procedures during the COVID-19 pandemic: Results from a national Delphi consensus panel. Feuerstein JD et al. Tech Innov Gastrointest Endosc. 2021;23(2):113-21. doi: 10.1016/j.tige.2020.12.005.
Development of a scoring system to predict a positive diagnosis on video capsule endoscopy for suspected small bowel bleeding. Marya NB et al. Tech Innov Gastrointest Endosc. 2021;22(4):178-84. doi: 10.1016/j.tige.2020.06.001.
Gastroenterology
May 2021
Understanding GI Twitter and its major contributors. Elfanagely Y et al. Gastroenterology. 2021 May;160(6):1917-21. doi: 10.1053/j.gastro.2021.01.232.
Long-term safety of fecal microbiota transplantation for recurrent Clostridioides difficile infection. Saha S et al. Gastroenterology. 2021 May;160(6):1961-9.e3. doi: 10.1053/j.gastro.2021.01.010.
How to incorporate health equity training into gastroenterology and hepatology fellowships. Lee-Allen J, Shah BJ. Gastroenterology. 2021 May;160(6):1924-8. doi: 10.1053/j.gastro.2021.03.018.
Functional dyspepsia and gastroparesis in tertiary care are interchangeable syndromes with common clinical and pathologic features. Pasricha PJ et al. Gastroenterology. 2021 May;160(6):2006-17. doi: 10.1053/j.gastro.2021.01.230.
June 2021
How to manage the large nonpedunculated colorectal polyp. Shahidi N, Bourke MJ. Gastroenterology. 2021 Jun;160(7):2239-43.e1. doi: 10.1053/j.gastro.2021.04.029.
Mortality, reoperation, and hospital stay within 90 days of primary and secondary antireflux surgery in a population-based multinational study. Yanes M et al. Gastroenterology. 2021 Jun;160(7):2283-90. doi: 10.1053/j.gastro.2021.02.022.
Endoscopic submucosal dissection in north america: A large prospective multicenter study. Draganov PV et al. Gastroenterology. 2021 Jun;160(7):2317-27.e2. doi: 10.1053/j.gastro.2021.02.036.
July 2021
Gluten degradation, pharmacokinetics, safety, and tolerability of TAK-062, an engineered enzyme to treat celiac disease. Pultz IG et al. Gastroenterology. 2021 Jul;161(1):81-93.e3. doi: 10.1053/j.gastro.2021.03.019.
Paternal exposure to immunosuppressive and/or biologic agents and birth outcomes in patients with immune-mediated inflammatory diseases. Meserve J et al. Gastroenterology. 2021 Jul;161(1):107-115.e3. doi: 10.1053/j.gastro.2021.03.020.
Ethnicity associations with food sensitization are mediated by gut microbiota development in the first year of life. Tun Hm et al. Gastroenterology. 2021 Jul;161(1):94-106. doi: 10.1053/j.gastro.2021.03.016.
How to incorporate bariatric training into your fellowship program. Jirapinyo P, Thompson CC. Gastroenterology. 2019 Jul;157(1):9-13. doi: 10.1053/j.gastro.2019.05.034.
CGH
May 2021
Intestinal failure: What all gastroenterologists should know. Jansson-Knodell CL et al. Clin Gastroenterol Hepatol. 2021 May;19(5):885-8. doi: 10.1016/j.cgh.2021.01.038.
When and how to use endoscopic tattooing in the colon: An international Delphi agreement. Medina-Prado L et al. Clin Gastroenterol Hepatol. 2021 May;19(5):1038-50. doi: 10.1016/j.cgh.2021.01.024.
Five-year outcomes of endoscopic sleeve gastroplasty for the treatment of obesity. Sharaiha RZ et al. Clin Gastroenterol Hepatol. 2021 May;19(5):1051-57.e2. doi: 10.1016/j.cgh.2020.09.055.
June 2021
GA Clinical Practice Update on management of bleeding gastric varices: Expert review. Henry Z et al. Clin Gastroenterol Hepatol. 2021 Jun;19(6):1098-107.e1. doi: 10.1016/j.cgh.2021.01.027.
Inter- and intra-individual variation, and limited prognostic utility, of serum alkaline phosphatase in a trial of patients with primary sclerosing cholangitis
Palak J. Trivedi PJ et al. Clin Gastroenterol Hepatol. 2021 Jun;19(6):1248-57. doi: 10.1016/j.cgh.2020.07.032.
Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Julia Fritsch J et al. Clin Gastroenterol Hepatol. 2021 Jun;19(6):1189-99.e30. doi: 10.1016/j.cgh.2020.05.026.
July 2021
Scoping out a better parental leave policy for gastroenterology fellows. Wegermann K. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1307-9. doi: 10.1016/j.cgh.2021.01.040.
An impetus for change: How COVID-19 will transform the delivery of GI health care. Leiman DA et al. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1310-1313. doi: 10.1016/j.cgh.2021.03.042.
Untangling nonerosive reflux disease from functional heartburn. Patel D et al. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1314-26. doi: 10.1016/j.cgh.2020.03.057.
AGA Clinical Practice Update on chemoprevention for colorectal neoplasia: Expert review. Liang PS et al. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1327-36. doi: 10.1016/j.cgh.2021.02.014.
CMGH
Drug inhibition of SARS-CoV-2 replication in human pluripotent stem cell–derived intestinal organoids. Krüger J et al. Cell Mol Gastroenterol Hepatol. 2021;11(4):935-48. doi: 10.1016/j.jcmgh.2020.11.003.
TIGE
Virtual interviews during the COVID-19 Pandemic: A survey of advanced endoscopy fellowship applicants and programs. Amrit K. Kamboj AK et al. Tech Innov Gastrointest Endosc. 2021;23(2):159-68. doi: 10.1016/j.tige.2021.02.001.
Triage of general gastrointestinal endoscopic procedures during the COVID-19 pandemic: Results from a national Delphi consensus panel. Feuerstein JD et al. Tech Innov Gastrointest Endosc. 2021;23(2):113-21. doi: 10.1016/j.tige.2020.12.005.
Development of a scoring system to predict a positive diagnosis on video capsule endoscopy for suspected small bowel bleeding. Marya NB et al. Tech Innov Gastrointest Endosc. 2021;22(4):178-84. doi: 10.1016/j.tige.2020.06.001.
Gastroenterology
May 2021
Understanding GI Twitter and its major contributors. Elfanagely Y et al. Gastroenterology. 2021 May;160(6):1917-21. doi: 10.1053/j.gastro.2021.01.232.
Long-term safety of fecal microbiota transplantation for recurrent Clostridioides difficile infection. Saha S et al. Gastroenterology. 2021 May;160(6):1961-9.e3. doi: 10.1053/j.gastro.2021.01.010.
How to incorporate health equity training into gastroenterology and hepatology fellowships. Lee-Allen J, Shah BJ. Gastroenterology. 2021 May;160(6):1924-8. doi: 10.1053/j.gastro.2021.03.018.
Functional dyspepsia and gastroparesis in tertiary care are interchangeable syndromes with common clinical and pathologic features. Pasricha PJ et al. Gastroenterology. 2021 May;160(6):2006-17. doi: 10.1053/j.gastro.2021.01.230.
June 2021
How to manage the large nonpedunculated colorectal polyp. Shahidi N, Bourke MJ. Gastroenterology. 2021 Jun;160(7):2239-43.e1. doi: 10.1053/j.gastro.2021.04.029.
Mortality, reoperation, and hospital stay within 90 days of primary and secondary antireflux surgery in a population-based multinational study. Yanes M et al. Gastroenterology. 2021 Jun;160(7):2283-90. doi: 10.1053/j.gastro.2021.02.022.
Endoscopic submucosal dissection in north america: A large prospective multicenter study. Draganov PV et al. Gastroenterology. 2021 Jun;160(7):2317-27.e2. doi: 10.1053/j.gastro.2021.02.036.
July 2021
Gluten degradation, pharmacokinetics, safety, and tolerability of TAK-062, an engineered enzyme to treat celiac disease. Pultz IG et al. Gastroenterology. 2021 Jul;161(1):81-93.e3. doi: 10.1053/j.gastro.2021.03.019.
Paternal exposure to immunosuppressive and/or biologic agents and birth outcomes in patients with immune-mediated inflammatory diseases. Meserve J et al. Gastroenterology. 2021 Jul;161(1):107-115.e3. doi: 10.1053/j.gastro.2021.03.020.
Ethnicity associations with food sensitization are mediated by gut microbiota development in the first year of life. Tun Hm et al. Gastroenterology. 2021 Jul;161(1):94-106. doi: 10.1053/j.gastro.2021.03.016.
How to incorporate bariatric training into your fellowship program. Jirapinyo P, Thompson CC. Gastroenterology. 2019 Jul;157(1):9-13. doi: 10.1053/j.gastro.2019.05.034.
CGH
May 2021
Intestinal failure: What all gastroenterologists should know. Jansson-Knodell CL et al. Clin Gastroenterol Hepatol. 2021 May;19(5):885-8. doi: 10.1016/j.cgh.2021.01.038.
When and how to use endoscopic tattooing in the colon: An international Delphi agreement. Medina-Prado L et al. Clin Gastroenterol Hepatol. 2021 May;19(5):1038-50. doi: 10.1016/j.cgh.2021.01.024.
Five-year outcomes of endoscopic sleeve gastroplasty for the treatment of obesity. Sharaiha RZ et al. Clin Gastroenterol Hepatol. 2021 May;19(5):1051-57.e2. doi: 10.1016/j.cgh.2020.09.055.
June 2021
GA Clinical Practice Update on management of bleeding gastric varices: Expert review. Henry Z et al. Clin Gastroenterol Hepatol. 2021 Jun;19(6):1098-107.e1. doi: 10.1016/j.cgh.2021.01.027.
Inter- and intra-individual variation, and limited prognostic utility, of serum alkaline phosphatase in a trial of patients with primary sclerosing cholangitis
Palak J. Trivedi PJ et al. Clin Gastroenterol Hepatol. 2021 Jun;19(6):1248-57. doi: 10.1016/j.cgh.2020.07.032.
Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Julia Fritsch J et al. Clin Gastroenterol Hepatol. 2021 Jun;19(6):1189-99.e30. doi: 10.1016/j.cgh.2020.05.026.
July 2021
Scoping out a better parental leave policy for gastroenterology fellows. Wegermann K. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1307-9. doi: 10.1016/j.cgh.2021.01.040.
An impetus for change: How COVID-19 will transform the delivery of GI health care. Leiman DA et al. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1310-1313. doi: 10.1016/j.cgh.2021.03.042.
Untangling nonerosive reflux disease from functional heartburn. Patel D et al. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1314-26. doi: 10.1016/j.cgh.2020.03.057.
AGA Clinical Practice Update on chemoprevention for colorectal neoplasia: Expert review. Liang PS et al. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1327-36. doi: 10.1016/j.cgh.2021.02.014.
CMGH
Drug inhibition of SARS-CoV-2 replication in human pluripotent stem cell–derived intestinal organoids. Krüger J et al. Cell Mol Gastroenterol Hepatol. 2021;11(4):935-48. doi: 10.1016/j.jcmgh.2020.11.003.
TIGE
Virtual interviews during the COVID-19 Pandemic: A survey of advanced endoscopy fellowship applicants and programs. Amrit K. Kamboj AK et al. Tech Innov Gastrointest Endosc. 2021;23(2):159-68. doi: 10.1016/j.tige.2021.02.001.
Triage of general gastrointestinal endoscopic procedures during the COVID-19 pandemic: Results from a national Delphi consensus panel. Feuerstein JD et al. Tech Innov Gastrointest Endosc. 2021;23(2):113-21. doi: 10.1016/j.tige.2020.12.005.
Development of a scoring system to predict a positive diagnosis on video capsule endoscopy for suspected small bowel bleeding. Marya NB et al. Tech Innov Gastrointest Endosc. 2021;22(4):178-84. doi: 10.1016/j.tige.2020.06.001.
Top 12 tips for research success in fellowship and early academic faculty years
Congratulations! You have matched in a competitive medical subspecialty or you have secured your first faculty position. But what do you do now? Success in your early career – as a new fellow or a new attending – requires both hard work and perseverance. We present our top 12 tips for how to be successful as you transition into your new position.
Tip #1: Be kind to yourself
As you transition from medical resident to GI fellow or from GI fellow to first-time attending, it is important to recognize that you are going through a major career transition (not as major as fourth year to intern, but probably a close second). First and foremost, remember to be kind to yourself and set reasonable expectations. You need to allow yourself time to transition to a new role which may also be in a new city or state. Take care of yourself – don’t forget to exercise, eat well, and sleep. You are in the long game now. Work to get yourself in a routine that is sustainable. Block out time to exercise, explore your new city, meal plan, and pursue your interests outside of medicine.
Tip #2: Set up for success
Since you are going through this major life/career transition, it is really helpful if you can set yourself up for success by having some projects that are easily completed during this challenging time so that you can demonstrate success. If you have projects in different stages of development, you will always have something you can work on when some projects are delayed for reasons outside of your control. In particular, it is great to have a few papers ready to go during late fellowship so they are published during your first year as an academic attending! This will allow you to continue your research trajectory as you learn the ropes of your new position.
Tip #3: Ask for help
It turns out you cannot do everything on your own! Make sure you are getting help professionally and personally so that you are set up for success. It’s okay to feel overwhelmed or confused; we all do at some point or another. Fellowship and early academic faculty years are stressful and nobody expects you to do it alone. Chances are your mentors or cofellows have had similar struggles, and in opening up, this dialogue may help you both.
Tip #4: Write out your 5-year plan
You need to know where you are going before you can figure out how to get there. Take some time for “soul searching”: Think about where you would like to be in 5 years and work backward (along with help from your mentors; see Tip #5) to determine how best to get there. If you think a career in academia might be for you, it’s never too soon to start networking and involving yourself in research. If a specific institution or clinical position draws your attention, check out the current faculty. You can use their CVs as a roadmap of types of experiences and honors that should be on your radar throughout these 5 years. Remember that your 5-year plan is not written in stone – this is something that you should re-evaluate as your interests and priorities change throughout your career.
Tip #5: Develop your personal ‘Board of Directors’
Instead of trying to find the perfect mentor, we suggest you seek out a personal “Board of Directors” who can serve as your mentoring team. There will never be a single perfect mentor for you and it is likely that you will need separate mentors to help guide you on different aspects of your career. I personally have separate individuals serving as my clinical mentor, my research content mentor, my research methods mentor, my career mentor, and my personal/life mentor. Having multiple mentors allows you to maximize the impact of your different mentors’ strengths across each component of your career. Further, your mentors themselves may have past histories of collaboration that you may then leverage to buoy your own fledgling career. When deciding on who to choose as a mentor, it is important to talk to prior mentees about their experiences with a mentor to help you decide if you may be a good match.
Tip #6: Master the art of “Menteering”
Now that you have identified mentors, you need to do your part in nurturing this mentee-mentor relationship. Be an excellent mentee: Show up, stick to a timeline, bring ideas and enthusiasm, and make it easy for your mentor. Your mentors want to see you succeed and sometimes this requires you to help them help you. If you know your own learning style and how you like to interact, have that conversation with your mentor upfront (for example, you may need strict deadlines or you may prefer having more time to develop ideas). Having these conversations before you start a project or a relationship will help set the expectations and ensure effective communication with your mentor. If you find that your mentor is doing something that hinders your progress, such as asking for updates too often or not checking in enough, have a constructive conversation with them about how you feel. Come prepared for meetings with your mentor with an agenda and timeline. Be specific if there is something you need from your mentor and be respectful of their other commitments. For example, if you would like your mentor to review your grant application, let them know the grant deadline and find out when you need to get them a draft so that they will have time to provide meaningful feedback.
Tip #7: Identify sponsors
Equally, if not more important than your mentoring team, are sponsors. These are people in positions of power who will promote you and help push your career forward. Sponsors can be people more senior to you, cofellows, or even acquaintances in industry or pharmaceuticals. Your mentor may also be your sponsor, but not always. As early academic faculty, it is important to get your name out there with speaking engagements related to your clinical and research niche, and that is one way a sponsor can help bolster your career.
Tip #8: Develop your personal brand – what is on your T-shirt?
As medicine becomes more and more subspecialized, finding your brand is becoming increasingly important. A brand could be anything from your academic niche to social justice, or even social media utilization. Your brand should encompass what you are naturally excited by within your field. Finding your brand will not only distinguish you from your peers but will also provide you with expertise which you can then offer to your colleagues, near and far. Practice the “elevator pitch” of your personal brand so that you can effectively (and efficiently) describe yourself and your interests when meeting new people and networking.
Tip #9: Meet thought leaders in your field
Think of the top five or six most prominent and influential people in your area of clinical or research interest and introduce yourself. This can be done at a national meeting or simply over email, though in person is always best if possible. Although thought leaders are busy, in my experience, if you are persistent, you can always find a few minutes to make an introduction. I’ve shared cab rides just to get a few minutes of someone’s time. In my first few years on faculty, I met with most of the thought leaders in my field; some of these meetings led to fruitful collaborations and important introductions (see tip #7). Meet others at your career level too. They can be great to bounce ideas off, and they will be future leaders in the field. Inviting thought leaders to come to your institution to give talks (in-person or virtually) is another great way to show your interest in their work and also find time to introduce yourself.
Tip #10: Apply, apply, apply
Remember that feedback is a gift and the best way to receive feedback is to apply to as many opportunities as you can. Any successful person in GI will have a ‘CV of failures’ far longer than their actual CV documenting their successes. I applied to 8 grants before landing my first one, but I received invaluable feedback and improved my writing skills in the process. Success in fellowship and early faculty takes immense grit – work on building a thick skin and finding the learning opportunity within any outcome.
Tip #11: Don’t get sucked into the email abyss
It is easy to fill your time completing low priority, but easy to complete, tasks such as responding to emails. Time management is key and you need to make sure that you dedicate time to more time-consuming tasks – such as writing and developing projects/grants – that have a high reward. Dedicate time on your calendar for high-priority tasks and make sure you don’t open your email during this time. Turn off the email pop-up window and do emails at the end of the day (or whenever you are done writing and thinking). Limiting distractions will help get your creative juices flowing.
Tip #12: Don’t always say yes
In fact, don’t ever say yes to a career or research opportunity within the first 24 hours to allow yourself time to weigh the pros and cons of the commitment, to assess the timeline feasibility, and to decide it fits into your 5-year plan. You can say you need to talk to your mentor about it first. If you decide you cannot accept an opportunity, a great way to mitigate that is to simply say “I’d love to, but my mentor says no.” Act as a sponsor to someone else by suggesting a potential colleague who might be interested in the opportunity. As you accept more responsibilities, think about what you might be able to give up to give yourself time to be successful in this new opportunity (and not distract from yourself or your 5-year plan).
Conclusion
Success in research and early academic faculty years takes planning and determination. We hope these tips provide a broad outline for what to think about and how to approach planning your future career. First and foremost, you must put in the time to think about what you really want and what will make you happy in the long run. Academic success is a broad term that each of us defines differently. What does it mean to you? Once you figure that out, make your 5-year plan and run with it!
Dr. Rebello and Dr. Long are with section of gastroenterology at Boston Medical Center and Boston University. They have no conflicts to report.
Congratulations! You have matched in a competitive medical subspecialty or you have secured your first faculty position. But what do you do now? Success in your early career – as a new fellow or a new attending – requires both hard work and perseverance. We present our top 12 tips for how to be successful as you transition into your new position.
Tip #1: Be kind to yourself
As you transition from medical resident to GI fellow or from GI fellow to first-time attending, it is important to recognize that you are going through a major career transition (not as major as fourth year to intern, but probably a close second). First and foremost, remember to be kind to yourself and set reasonable expectations. You need to allow yourself time to transition to a new role which may also be in a new city or state. Take care of yourself – don’t forget to exercise, eat well, and sleep. You are in the long game now. Work to get yourself in a routine that is sustainable. Block out time to exercise, explore your new city, meal plan, and pursue your interests outside of medicine.
Tip #2: Set up for success
Since you are going through this major life/career transition, it is really helpful if you can set yourself up for success by having some projects that are easily completed during this challenging time so that you can demonstrate success. If you have projects in different stages of development, you will always have something you can work on when some projects are delayed for reasons outside of your control. In particular, it is great to have a few papers ready to go during late fellowship so they are published during your first year as an academic attending! This will allow you to continue your research trajectory as you learn the ropes of your new position.
Tip #3: Ask for help
It turns out you cannot do everything on your own! Make sure you are getting help professionally and personally so that you are set up for success. It’s okay to feel overwhelmed or confused; we all do at some point or another. Fellowship and early academic faculty years are stressful and nobody expects you to do it alone. Chances are your mentors or cofellows have had similar struggles, and in opening up, this dialogue may help you both.
Tip #4: Write out your 5-year plan
You need to know where you are going before you can figure out how to get there. Take some time for “soul searching”: Think about where you would like to be in 5 years and work backward (along with help from your mentors; see Tip #5) to determine how best to get there. If you think a career in academia might be for you, it’s never too soon to start networking and involving yourself in research. If a specific institution or clinical position draws your attention, check out the current faculty. You can use their CVs as a roadmap of types of experiences and honors that should be on your radar throughout these 5 years. Remember that your 5-year plan is not written in stone – this is something that you should re-evaluate as your interests and priorities change throughout your career.
Tip #5: Develop your personal ‘Board of Directors’
Instead of trying to find the perfect mentor, we suggest you seek out a personal “Board of Directors” who can serve as your mentoring team. There will never be a single perfect mentor for you and it is likely that you will need separate mentors to help guide you on different aspects of your career. I personally have separate individuals serving as my clinical mentor, my research content mentor, my research methods mentor, my career mentor, and my personal/life mentor. Having multiple mentors allows you to maximize the impact of your different mentors’ strengths across each component of your career. Further, your mentors themselves may have past histories of collaboration that you may then leverage to buoy your own fledgling career. When deciding on who to choose as a mentor, it is important to talk to prior mentees about their experiences with a mentor to help you decide if you may be a good match.
Tip #6: Master the art of “Menteering”
Now that you have identified mentors, you need to do your part in nurturing this mentee-mentor relationship. Be an excellent mentee: Show up, stick to a timeline, bring ideas and enthusiasm, and make it easy for your mentor. Your mentors want to see you succeed and sometimes this requires you to help them help you. If you know your own learning style and how you like to interact, have that conversation with your mentor upfront (for example, you may need strict deadlines or you may prefer having more time to develop ideas). Having these conversations before you start a project or a relationship will help set the expectations and ensure effective communication with your mentor. If you find that your mentor is doing something that hinders your progress, such as asking for updates too often or not checking in enough, have a constructive conversation with them about how you feel. Come prepared for meetings with your mentor with an agenda and timeline. Be specific if there is something you need from your mentor and be respectful of their other commitments. For example, if you would like your mentor to review your grant application, let them know the grant deadline and find out when you need to get them a draft so that they will have time to provide meaningful feedback.
Tip #7: Identify sponsors
Equally, if not more important than your mentoring team, are sponsors. These are people in positions of power who will promote you and help push your career forward. Sponsors can be people more senior to you, cofellows, or even acquaintances in industry or pharmaceuticals. Your mentor may also be your sponsor, but not always. As early academic faculty, it is important to get your name out there with speaking engagements related to your clinical and research niche, and that is one way a sponsor can help bolster your career.
Tip #8: Develop your personal brand – what is on your T-shirt?
As medicine becomes more and more subspecialized, finding your brand is becoming increasingly important. A brand could be anything from your academic niche to social justice, or even social media utilization. Your brand should encompass what you are naturally excited by within your field. Finding your brand will not only distinguish you from your peers but will also provide you with expertise which you can then offer to your colleagues, near and far. Practice the “elevator pitch” of your personal brand so that you can effectively (and efficiently) describe yourself and your interests when meeting new people and networking.
Tip #9: Meet thought leaders in your field
Think of the top five or six most prominent and influential people in your area of clinical or research interest and introduce yourself. This can be done at a national meeting or simply over email, though in person is always best if possible. Although thought leaders are busy, in my experience, if you are persistent, you can always find a few minutes to make an introduction. I’ve shared cab rides just to get a few minutes of someone’s time. In my first few years on faculty, I met with most of the thought leaders in my field; some of these meetings led to fruitful collaborations and important introductions (see tip #7). Meet others at your career level too. They can be great to bounce ideas off, and they will be future leaders in the field. Inviting thought leaders to come to your institution to give talks (in-person or virtually) is another great way to show your interest in their work and also find time to introduce yourself.
Tip #10: Apply, apply, apply
Remember that feedback is a gift and the best way to receive feedback is to apply to as many opportunities as you can. Any successful person in GI will have a ‘CV of failures’ far longer than their actual CV documenting their successes. I applied to 8 grants before landing my first one, but I received invaluable feedback and improved my writing skills in the process. Success in fellowship and early faculty takes immense grit – work on building a thick skin and finding the learning opportunity within any outcome.
Tip #11: Don’t get sucked into the email abyss
It is easy to fill your time completing low priority, but easy to complete, tasks such as responding to emails. Time management is key and you need to make sure that you dedicate time to more time-consuming tasks – such as writing and developing projects/grants – that have a high reward. Dedicate time on your calendar for high-priority tasks and make sure you don’t open your email during this time. Turn off the email pop-up window and do emails at the end of the day (or whenever you are done writing and thinking). Limiting distractions will help get your creative juices flowing.
Tip #12: Don’t always say yes
In fact, don’t ever say yes to a career or research opportunity within the first 24 hours to allow yourself time to weigh the pros and cons of the commitment, to assess the timeline feasibility, and to decide it fits into your 5-year plan. You can say you need to talk to your mentor about it first. If you decide you cannot accept an opportunity, a great way to mitigate that is to simply say “I’d love to, but my mentor says no.” Act as a sponsor to someone else by suggesting a potential colleague who might be interested in the opportunity. As you accept more responsibilities, think about what you might be able to give up to give yourself time to be successful in this new opportunity (and not distract from yourself or your 5-year plan).
Conclusion
Success in research and early academic faculty years takes planning and determination. We hope these tips provide a broad outline for what to think about and how to approach planning your future career. First and foremost, you must put in the time to think about what you really want and what will make you happy in the long run. Academic success is a broad term that each of us defines differently. What does it mean to you? Once you figure that out, make your 5-year plan and run with it!
Dr. Rebello and Dr. Long are with section of gastroenterology at Boston Medical Center and Boston University. They have no conflicts to report.
Congratulations! You have matched in a competitive medical subspecialty or you have secured your first faculty position. But what do you do now? Success in your early career – as a new fellow or a new attending – requires both hard work and perseverance. We present our top 12 tips for how to be successful as you transition into your new position.
Tip #1: Be kind to yourself
As you transition from medical resident to GI fellow or from GI fellow to first-time attending, it is important to recognize that you are going through a major career transition (not as major as fourth year to intern, but probably a close second). First and foremost, remember to be kind to yourself and set reasonable expectations. You need to allow yourself time to transition to a new role which may also be in a new city or state. Take care of yourself – don’t forget to exercise, eat well, and sleep. You are in the long game now. Work to get yourself in a routine that is sustainable. Block out time to exercise, explore your new city, meal plan, and pursue your interests outside of medicine.
Tip #2: Set up for success
Since you are going through this major life/career transition, it is really helpful if you can set yourself up for success by having some projects that are easily completed during this challenging time so that you can demonstrate success. If you have projects in different stages of development, you will always have something you can work on when some projects are delayed for reasons outside of your control. In particular, it is great to have a few papers ready to go during late fellowship so they are published during your first year as an academic attending! This will allow you to continue your research trajectory as you learn the ropes of your new position.
Tip #3: Ask for help
It turns out you cannot do everything on your own! Make sure you are getting help professionally and personally so that you are set up for success. It’s okay to feel overwhelmed or confused; we all do at some point or another. Fellowship and early academic faculty years are stressful and nobody expects you to do it alone. Chances are your mentors or cofellows have had similar struggles, and in opening up, this dialogue may help you both.
Tip #4: Write out your 5-year plan
You need to know where you are going before you can figure out how to get there. Take some time for “soul searching”: Think about where you would like to be in 5 years and work backward (along with help from your mentors; see Tip #5) to determine how best to get there. If you think a career in academia might be for you, it’s never too soon to start networking and involving yourself in research. If a specific institution or clinical position draws your attention, check out the current faculty. You can use their CVs as a roadmap of types of experiences and honors that should be on your radar throughout these 5 years. Remember that your 5-year plan is not written in stone – this is something that you should re-evaluate as your interests and priorities change throughout your career.
Tip #5: Develop your personal ‘Board of Directors’
Instead of trying to find the perfect mentor, we suggest you seek out a personal “Board of Directors” who can serve as your mentoring team. There will never be a single perfect mentor for you and it is likely that you will need separate mentors to help guide you on different aspects of your career. I personally have separate individuals serving as my clinical mentor, my research content mentor, my research methods mentor, my career mentor, and my personal/life mentor. Having multiple mentors allows you to maximize the impact of your different mentors’ strengths across each component of your career. Further, your mentors themselves may have past histories of collaboration that you may then leverage to buoy your own fledgling career. When deciding on who to choose as a mentor, it is important to talk to prior mentees about their experiences with a mentor to help you decide if you may be a good match.
Tip #6: Master the art of “Menteering”
Now that you have identified mentors, you need to do your part in nurturing this mentee-mentor relationship. Be an excellent mentee: Show up, stick to a timeline, bring ideas and enthusiasm, and make it easy for your mentor. Your mentors want to see you succeed and sometimes this requires you to help them help you. If you know your own learning style and how you like to interact, have that conversation with your mentor upfront (for example, you may need strict deadlines or you may prefer having more time to develop ideas). Having these conversations before you start a project or a relationship will help set the expectations and ensure effective communication with your mentor. If you find that your mentor is doing something that hinders your progress, such as asking for updates too often or not checking in enough, have a constructive conversation with them about how you feel. Come prepared for meetings with your mentor with an agenda and timeline. Be specific if there is something you need from your mentor and be respectful of their other commitments. For example, if you would like your mentor to review your grant application, let them know the grant deadline and find out when you need to get them a draft so that they will have time to provide meaningful feedback.
Tip #7: Identify sponsors
Equally, if not more important than your mentoring team, are sponsors. These are people in positions of power who will promote you and help push your career forward. Sponsors can be people more senior to you, cofellows, or even acquaintances in industry or pharmaceuticals. Your mentor may also be your sponsor, but not always. As early academic faculty, it is important to get your name out there with speaking engagements related to your clinical and research niche, and that is one way a sponsor can help bolster your career.
Tip #8: Develop your personal brand – what is on your T-shirt?
As medicine becomes more and more subspecialized, finding your brand is becoming increasingly important. A brand could be anything from your academic niche to social justice, or even social media utilization. Your brand should encompass what you are naturally excited by within your field. Finding your brand will not only distinguish you from your peers but will also provide you with expertise which you can then offer to your colleagues, near and far. Practice the “elevator pitch” of your personal brand so that you can effectively (and efficiently) describe yourself and your interests when meeting new people and networking.
Tip #9: Meet thought leaders in your field
Think of the top five or six most prominent and influential people in your area of clinical or research interest and introduce yourself. This can be done at a national meeting or simply over email, though in person is always best if possible. Although thought leaders are busy, in my experience, if you are persistent, you can always find a few minutes to make an introduction. I’ve shared cab rides just to get a few minutes of someone’s time. In my first few years on faculty, I met with most of the thought leaders in my field; some of these meetings led to fruitful collaborations and important introductions (see tip #7). Meet others at your career level too. They can be great to bounce ideas off, and they will be future leaders in the field. Inviting thought leaders to come to your institution to give talks (in-person or virtually) is another great way to show your interest in their work and also find time to introduce yourself.
Tip #10: Apply, apply, apply
Remember that feedback is a gift and the best way to receive feedback is to apply to as many opportunities as you can. Any successful person in GI will have a ‘CV of failures’ far longer than their actual CV documenting their successes. I applied to 8 grants before landing my first one, but I received invaluable feedback and improved my writing skills in the process. Success in fellowship and early faculty takes immense grit – work on building a thick skin and finding the learning opportunity within any outcome.
Tip #11: Don’t get sucked into the email abyss
It is easy to fill your time completing low priority, but easy to complete, tasks such as responding to emails. Time management is key and you need to make sure that you dedicate time to more time-consuming tasks – such as writing and developing projects/grants – that have a high reward. Dedicate time on your calendar for high-priority tasks and make sure you don’t open your email during this time. Turn off the email pop-up window and do emails at the end of the day (or whenever you are done writing and thinking). Limiting distractions will help get your creative juices flowing.
Tip #12: Don’t always say yes
In fact, don’t ever say yes to a career or research opportunity within the first 24 hours to allow yourself time to weigh the pros and cons of the commitment, to assess the timeline feasibility, and to decide it fits into your 5-year plan. You can say you need to talk to your mentor about it first. If you decide you cannot accept an opportunity, a great way to mitigate that is to simply say “I’d love to, but my mentor says no.” Act as a sponsor to someone else by suggesting a potential colleague who might be interested in the opportunity. As you accept more responsibilities, think about what you might be able to give up to give yourself time to be successful in this new opportunity (and not distract from yourself or your 5-year plan).
Conclusion
Success in research and early academic faculty years takes planning and determination. We hope these tips provide a broad outline for what to think about and how to approach planning your future career. First and foremost, you must put in the time to think about what you really want and what will make you happy in the long run. Academic success is a broad term that each of us defines differently. What does it mean to you? Once you figure that out, make your 5-year plan and run with it!
Dr. Rebello and Dr. Long are with section of gastroenterology at Boston Medical Center and Boston University. They have no conflicts to report.
Lessons from COVID-19 and planning for a postpandemic screening surge
It is not an exaggeration to say that everything in my gastroenterology practice changed in response to COVID-19.
Due to the overwhelming surge that Massachusetts saw in the early days of the pandemic, the Department of Public Health issued a moratorium on elective procedures in mid-March of 2020, for both hospitals and ambulatory surgery centers. The moratorium included colorectal cancer (CRC) screenings and other procedures that make up a significant portion of the services we provide to our community. Greater Boston Gastroenterology treats patients in and around the area of Framingham, Mass. – not too far outside of Boston. In our practice, we have seven physicians and three nurse practitioners, with one main office and two satellite offices. By national standards, our practice would be considered small, but it is on the larger side of independent GI physician practices in the commonwealth.
Nationally, moratoria on elective procedures led to one of the steepest drop-offs in screenings for cancers, including colorectal cancer. In late summer of 2020, it was estimated that CRC screenings dropped by 86 percent. Two-thirds of independent GI practices saw a significant decline in patient volume, and many believe that they may not get it back.
However, I’m an optimist in this situation, and I believe that as life gets more normal, people will get back to screenings. With the recommendation by the U.S. Preventative Services Task Force that CRC screening should begin at age 45, I expect that there will be an additional increase in screening soon.
Pivoting and developing a reopening plan
Almost immediately after the Department of Public Health issued the moratorium, Greater Boston Gastroenterology began putting together a reopening plan that would allow us to continue treating some patients and prepare for a surge once restrictions were lifted.
Part of our plan was to stay informed by talking with other practices about what they were doing and to stay abreast of policy changes at the local, state, and federal levels.
We also needed to keep our patients informed to alleviate safety concerns. Just prior to our reopening, we developed videos of the precautions that we were taking in all our facilities to assure our patients that we were doing everything possible to keep them safe. We also put information on our website through every stage of reopening so patients could know what to expect at their visits.
Helping our staff feel safe as they returned to work was also an important focus of our reopening plan. We prepared for our eventual reopening by installing safety measures such as plexiglass barriers and HEPA filter machines for our common areas and exam rooms. We also procured access to rapid turnaround polymerase chain reaction (PCR) testing that allowed us to regularly test all patients seeking elective procedures. Additionally, we invested in point-of-care antigen tests for the office, and we regularly test all our patient-facing staff.
We had corralled enough personal protective equipment to keep our office infusion services operating with our nurses and patients feeling safe. The preparation allowed us to resume in-person visits almost immediately after the Department of Public Health allowed us to reopen.
Once we reopened, we concentrated on in-office visits for patients who were under 65 and at lower risk for COVID-19, while focusing our telemedicine efforts on patients who were older and at higher risk. We’re now back to seeing all patients who want to have in-office visits and are actually above par for our visits. The number of procedures we have performed in the last 3 months is similar to the 3 months before the pandemic.
During the pandemic, Massachusetts had the best conversion to telehealth in the nation, and it worked well for patients and providers. The key was to use several telehealth apps, as using only one may not work for everyone. Having several options made it likely that we would be able to do complete visits and connect with patients. When we needed to, we switched to telephone visits.
All the physicians and staff in our practice are telemedicine enthusiasts, and it will remain a significant part of our practices as long as Medicare, the state health plans, and commercial payers remain supportive.
Planning for a surge in screenings
There may be a surge in screenings once more people are vaccinated and comfortable getting back into the office, and we’re planning for this as well. We’ve recruited new physicians and have expanded our available hours for procedures at our ambulatory surgery centers (ASCs). Surprisingly, we have found that there is a lot of interest from physicians for weekend shifts at the ASC, and we now have a physician waiting list for Saturday procedure time.
With the new lower age for recommended screening, there will be a lag with primary care physicians referring their younger patients. This may provide some time to prepare for an increase in screenings resulting from this new policy.
Another strategy that has worked well for us is to train and develop our advanced practitioners into nonphysician experts in GI and liver disease. Greater Boston Gastroenterology has used this strategy since its founding, and we think our most experienced nurse practitioners could rival any office-based gastroenterologist in their acumen and capabilities.
Over the last 3 years we have transitioned our nonphysician practitioners into the inpatient setting. As a result, consults are completed earlier in the day, and we are better able to help coordinate inpatient procedure scheduling, discharge planning, and outpatient follow-up.
The time we spend on training is worth it. It improves customer service, allows us to book appointments with shorter notice, and overall has a positive effect on our bottom line. Utilizing our advanced providers in this capacity will help us manage any volume increases we see in the near future. In addition, most patients in our community are used to seeing advanced providers in their physician’s office, so the acceptance among our patients is high.
Being flexible and favoring strategic planning
Overall, I think the greatest thing we learned during the pandemic is that we need to be flexible. It was a helpful reminder that, in medicine, things are constantly changing. I remember when passing the GI boards seemed like my final step, but everyone comes to realize it is just the first step in the journey.
As an early-career physician, you should remember the hard work that helped you get to medical school, land a good residency, stand out to get a fellowship, and master your specialty. Harness that personal drive and energy and keep moving forward. Remember that your first job is unlikely to be your last. Try not to see your choices as either/or – either academic or private practice, hospital-employed or self-employed. The boundaries are blurring. We have long careers and face myriad opportunities for professional advancement.
Be patient. Some goals take time to achieve. At each stage be prepared to work hard, use your time wisely, and try not to lose sight of maximizing your professional happiness.
Dr. Dickstein is a practicing gastroenterologist at Greater Boston Gastroenterology in Framingham, Mass., and serves on the executive committee of the Digestive Health Physicians Association. He has no conflicts to declare.
It is not an exaggeration to say that everything in my gastroenterology practice changed in response to COVID-19.
Due to the overwhelming surge that Massachusetts saw in the early days of the pandemic, the Department of Public Health issued a moratorium on elective procedures in mid-March of 2020, for both hospitals and ambulatory surgery centers. The moratorium included colorectal cancer (CRC) screenings and other procedures that make up a significant portion of the services we provide to our community. Greater Boston Gastroenterology treats patients in and around the area of Framingham, Mass. – not too far outside of Boston. In our practice, we have seven physicians and three nurse practitioners, with one main office and two satellite offices. By national standards, our practice would be considered small, but it is on the larger side of independent GI physician practices in the commonwealth.
Nationally, moratoria on elective procedures led to one of the steepest drop-offs in screenings for cancers, including colorectal cancer. In late summer of 2020, it was estimated that CRC screenings dropped by 86 percent. Two-thirds of independent GI practices saw a significant decline in patient volume, and many believe that they may not get it back.
However, I’m an optimist in this situation, and I believe that as life gets more normal, people will get back to screenings. With the recommendation by the U.S. Preventative Services Task Force that CRC screening should begin at age 45, I expect that there will be an additional increase in screening soon.
Pivoting and developing a reopening plan
Almost immediately after the Department of Public Health issued the moratorium, Greater Boston Gastroenterology began putting together a reopening plan that would allow us to continue treating some patients and prepare for a surge once restrictions were lifted.
Part of our plan was to stay informed by talking with other practices about what they were doing and to stay abreast of policy changes at the local, state, and federal levels.
We also needed to keep our patients informed to alleviate safety concerns. Just prior to our reopening, we developed videos of the precautions that we were taking in all our facilities to assure our patients that we were doing everything possible to keep them safe. We also put information on our website through every stage of reopening so patients could know what to expect at their visits.
Helping our staff feel safe as they returned to work was also an important focus of our reopening plan. We prepared for our eventual reopening by installing safety measures such as plexiglass barriers and HEPA filter machines for our common areas and exam rooms. We also procured access to rapid turnaround polymerase chain reaction (PCR) testing that allowed us to regularly test all patients seeking elective procedures. Additionally, we invested in point-of-care antigen tests for the office, and we regularly test all our patient-facing staff.
We had corralled enough personal protective equipment to keep our office infusion services operating with our nurses and patients feeling safe. The preparation allowed us to resume in-person visits almost immediately after the Department of Public Health allowed us to reopen.
Once we reopened, we concentrated on in-office visits for patients who were under 65 and at lower risk for COVID-19, while focusing our telemedicine efforts on patients who were older and at higher risk. We’re now back to seeing all patients who want to have in-office visits and are actually above par for our visits. The number of procedures we have performed in the last 3 months is similar to the 3 months before the pandemic.
During the pandemic, Massachusetts had the best conversion to telehealth in the nation, and it worked well for patients and providers. The key was to use several telehealth apps, as using only one may not work for everyone. Having several options made it likely that we would be able to do complete visits and connect with patients. When we needed to, we switched to telephone visits.
All the physicians and staff in our practice are telemedicine enthusiasts, and it will remain a significant part of our practices as long as Medicare, the state health plans, and commercial payers remain supportive.
Planning for a surge in screenings
There may be a surge in screenings once more people are vaccinated and comfortable getting back into the office, and we’re planning for this as well. We’ve recruited new physicians and have expanded our available hours for procedures at our ambulatory surgery centers (ASCs). Surprisingly, we have found that there is a lot of interest from physicians for weekend shifts at the ASC, and we now have a physician waiting list for Saturday procedure time.
With the new lower age for recommended screening, there will be a lag with primary care physicians referring their younger patients. This may provide some time to prepare for an increase in screenings resulting from this new policy.
Another strategy that has worked well for us is to train and develop our advanced practitioners into nonphysician experts in GI and liver disease. Greater Boston Gastroenterology has used this strategy since its founding, and we think our most experienced nurse practitioners could rival any office-based gastroenterologist in their acumen and capabilities.
Over the last 3 years we have transitioned our nonphysician practitioners into the inpatient setting. As a result, consults are completed earlier in the day, and we are better able to help coordinate inpatient procedure scheduling, discharge planning, and outpatient follow-up.
The time we spend on training is worth it. It improves customer service, allows us to book appointments with shorter notice, and overall has a positive effect on our bottom line. Utilizing our advanced providers in this capacity will help us manage any volume increases we see in the near future. In addition, most patients in our community are used to seeing advanced providers in their physician’s office, so the acceptance among our patients is high.
Being flexible and favoring strategic planning
Overall, I think the greatest thing we learned during the pandemic is that we need to be flexible. It was a helpful reminder that, in medicine, things are constantly changing. I remember when passing the GI boards seemed like my final step, but everyone comes to realize it is just the first step in the journey.
As an early-career physician, you should remember the hard work that helped you get to medical school, land a good residency, stand out to get a fellowship, and master your specialty. Harness that personal drive and energy and keep moving forward. Remember that your first job is unlikely to be your last. Try not to see your choices as either/or – either academic or private practice, hospital-employed or self-employed. The boundaries are blurring. We have long careers and face myriad opportunities for professional advancement.
Be patient. Some goals take time to achieve. At each stage be prepared to work hard, use your time wisely, and try not to lose sight of maximizing your professional happiness.
Dr. Dickstein is a practicing gastroenterologist at Greater Boston Gastroenterology in Framingham, Mass., and serves on the executive committee of the Digestive Health Physicians Association. He has no conflicts to declare.
It is not an exaggeration to say that everything in my gastroenterology practice changed in response to COVID-19.
Due to the overwhelming surge that Massachusetts saw in the early days of the pandemic, the Department of Public Health issued a moratorium on elective procedures in mid-March of 2020, for both hospitals and ambulatory surgery centers. The moratorium included colorectal cancer (CRC) screenings and other procedures that make up a significant portion of the services we provide to our community. Greater Boston Gastroenterology treats patients in and around the area of Framingham, Mass. – not too far outside of Boston. In our practice, we have seven physicians and three nurse practitioners, with one main office and two satellite offices. By national standards, our practice would be considered small, but it is on the larger side of independent GI physician practices in the commonwealth.
Nationally, moratoria on elective procedures led to one of the steepest drop-offs in screenings for cancers, including colorectal cancer. In late summer of 2020, it was estimated that CRC screenings dropped by 86 percent. Two-thirds of independent GI practices saw a significant decline in patient volume, and many believe that they may not get it back.
However, I’m an optimist in this situation, and I believe that as life gets more normal, people will get back to screenings. With the recommendation by the U.S. Preventative Services Task Force that CRC screening should begin at age 45, I expect that there will be an additional increase in screening soon.
Pivoting and developing a reopening plan
Almost immediately after the Department of Public Health issued the moratorium, Greater Boston Gastroenterology began putting together a reopening plan that would allow us to continue treating some patients and prepare for a surge once restrictions were lifted.
Part of our plan was to stay informed by talking with other practices about what they were doing and to stay abreast of policy changes at the local, state, and federal levels.
We also needed to keep our patients informed to alleviate safety concerns. Just prior to our reopening, we developed videos of the precautions that we were taking in all our facilities to assure our patients that we were doing everything possible to keep them safe. We also put information on our website through every stage of reopening so patients could know what to expect at their visits.
Helping our staff feel safe as they returned to work was also an important focus of our reopening plan. We prepared for our eventual reopening by installing safety measures such as plexiglass barriers and HEPA filter machines for our common areas and exam rooms. We also procured access to rapid turnaround polymerase chain reaction (PCR) testing that allowed us to regularly test all patients seeking elective procedures. Additionally, we invested in point-of-care antigen tests for the office, and we regularly test all our patient-facing staff.
We had corralled enough personal protective equipment to keep our office infusion services operating with our nurses and patients feeling safe. The preparation allowed us to resume in-person visits almost immediately after the Department of Public Health allowed us to reopen.
Once we reopened, we concentrated on in-office visits for patients who were under 65 and at lower risk for COVID-19, while focusing our telemedicine efforts on patients who were older and at higher risk. We’re now back to seeing all patients who want to have in-office visits and are actually above par for our visits. The number of procedures we have performed in the last 3 months is similar to the 3 months before the pandemic.
During the pandemic, Massachusetts had the best conversion to telehealth in the nation, and it worked well for patients and providers. The key was to use several telehealth apps, as using only one may not work for everyone. Having several options made it likely that we would be able to do complete visits and connect with patients. When we needed to, we switched to telephone visits.
All the physicians and staff in our practice are telemedicine enthusiasts, and it will remain a significant part of our practices as long as Medicare, the state health plans, and commercial payers remain supportive.
Planning for a surge in screenings
There may be a surge in screenings once more people are vaccinated and comfortable getting back into the office, and we’re planning for this as well. We’ve recruited new physicians and have expanded our available hours for procedures at our ambulatory surgery centers (ASCs). Surprisingly, we have found that there is a lot of interest from physicians for weekend shifts at the ASC, and we now have a physician waiting list for Saturday procedure time.
With the new lower age for recommended screening, there will be a lag with primary care physicians referring their younger patients. This may provide some time to prepare for an increase in screenings resulting from this new policy.
Another strategy that has worked well for us is to train and develop our advanced practitioners into nonphysician experts in GI and liver disease. Greater Boston Gastroenterology has used this strategy since its founding, and we think our most experienced nurse practitioners could rival any office-based gastroenterologist in their acumen and capabilities.
Over the last 3 years we have transitioned our nonphysician practitioners into the inpatient setting. As a result, consults are completed earlier in the day, and we are better able to help coordinate inpatient procedure scheduling, discharge planning, and outpatient follow-up.
The time we spend on training is worth it. It improves customer service, allows us to book appointments with shorter notice, and overall has a positive effect on our bottom line. Utilizing our advanced providers in this capacity will help us manage any volume increases we see in the near future. In addition, most patients in our community are used to seeing advanced providers in their physician’s office, so the acceptance among our patients is high.
Being flexible and favoring strategic planning
Overall, I think the greatest thing we learned during the pandemic is that we need to be flexible. It was a helpful reminder that, in medicine, things are constantly changing. I remember when passing the GI boards seemed like my final step, but everyone comes to realize it is just the first step in the journey.
As an early-career physician, you should remember the hard work that helped you get to medical school, land a good residency, stand out to get a fellowship, and master your specialty. Harness that personal drive and energy and keep moving forward. Remember that your first job is unlikely to be your last. Try not to see your choices as either/or – either academic or private practice, hospital-employed or self-employed. The boundaries are blurring. We have long careers and face myriad opportunities for professional advancement.
Be patient. Some goals take time to achieve. At each stage be prepared to work hard, use your time wisely, and try not to lose sight of maximizing your professional happiness.
Dr. Dickstein is a practicing gastroenterologist at Greater Boston Gastroenterology in Framingham, Mass., and serves on the executive committee of the Digestive Health Physicians Association. He has no conflicts to declare.
Choosing a career as chief medical officer at a health technology startup
How did your career pathway lead you to working at a health tech startup?
I’ve always had an interest in technology – in fact, it was part of the reason I chose gastroenterology. When I finished GI fellowship, I decided to stay in academics because of an opportunity to lead clinical innovation efforts at my institution’s patient safety institute. This role provided protected time to foster external and internal partnerships around technology. It also gave me an opportunity to pursue clinical research and administrative experiences. While I enjoyed all three paths, it became clear that health technology was my passion. While the opportunity to join a startup was largely serendipitous – I met the founder of the company after presenting at a digital medicine conference – it also happened as a result of the steps outlined in a subsequent question. Not long after learning about the company, I made the transition to part-time faculty/clinical status and full-time chief medical officer (CMO).
What do you do as CMO?
There is no one answer to this question. It will depend on a number of variables, especially the type of business (for example, diagnostic, drug, digital, direct care management, and so on), stage of company (for example, concept, seed, series A/B/C, public), and the existing background of company founders (for example, technical, clinical, operations, and so on). Generally speaking, the earlier the stage of the company, the more hats you’ll wear (though this also means more risk; more on that later). An early-stage company was appealing to me because it gave me an opportunity to apply many of the same critical-thinking and problem-solving skills in clinical medicine to a host of other challenges. For example, as a practicing gastroenterologist, I know the pain points in the delivery of GI care and the challenges that my patients encounter. I then ask how can I develop our technology and product platform to address these issues. Also understanding how value and quality are measured in GI practice makes it easier to convey the effect of the solutions that are built and prioritize their development. In my current role I contribute to the following areas:
- Clinical strategy and vision. This means understanding the clinical need the company is trying to address at a fundamental level and designing how the technology or solution can address that need in a meaningful way. This includes working directly with technology and product teams to create a roadmap for how the technology/solution will continue to drive impact.
- Clinical care leadership. If the company employs or works with health professionals in any capacity, this usually involves developing clinical protocols and providing clinical direction.
- Clinical outcomes. This means being responsible for understanding and/or developing the metrics that will be used to demonstrate impact of the technology/solution. This includes designing clinical studies and being responsible for their execution.
- Stakeholder engagement. This means interfacing internally with nearly every aspect of the company and interacting externally with customers (usually medical peers and executives), investors, other companies, and key opinion leaders in the field.
- Regulatory. For companies pursuing Food and Drug Administration clearance or approval for their product, this entails developing a strategy and executing it.
- Research & development. This involves creating and executing a roadmap for integrating new technologies/ideas that generally complement the initial problem you are trying to solve.
What do you enjoy most about working at a startup?
The variety of experience, the flexibility, the fast pace, the ability to work creatively, and the potential to make a large-scale impact are all aspects of the job that I enjoy. The ability to continue clinical practice is important to me and is a major plus.
What do you find most challenging about working at a startup?
One of the biggest differences between a startup and a traditional clinical role is the degree of uncertainty that permeates the entire experience. It took some time for me to adjust to the relative volatility/risk associated with this type of work. Unlike an academic, administrative, or private practice job, things can change very quickly (as in a 24-hour period or less!). This can encompass a number of changes, such as funding, leadership, strategic direction, business model, and staffing, to name a few. What I’ve learned is that this doesn’t always mean changing for the worse, but it does mean things changing near constantly. Being mentally prepared to adapt quickly and frequently to big changes is part of the experience.
What are the ways that GIs can get involved in startups?
Gastroenterologists have more opportunities than most physicians due to the diversity of conditions we treat and the large corresponding number of unmet needs we encounter. There is also the inherent innovation potential associated with new applications in endoscopy, diagnostics, and drug therapies. As a result, there are a number of ways to get involved:
- This often takes the form of “spinning out” research from an academic institution but can also be done successfully from private practice, particularly in the context of new devices/services. Another related option is to license your technology to a company, which offloads the operational aspects of running a business.
- Provide consulting/advisory support. Many early-stage companies cannot afford to hire a full-time physician, but they are open to consulting arrangements (and of course volunteer work). Don’t hesitate to directly contact companies that are interesting to you. These opportunities are possible even while in clinical training.
- Work part time or full time. The majority of startups are supportive of physicians continuing to practice clinically. This makes engaging in a part-time position financially feasible for both parties. Given the relatively high remuneration for gastroenterologists working clinically, a full-time position at a startup may require a financial tradeoff (that is, lower short-term salary for a potential larger long-term gain – note the emphasis on “potential”).
- Invest in early-stage companies. Physicians can become angel investors for early-stage companies. Given the relatively time-intensive process of finding new opportunities and conducting due diligence, this often takes the form of pooling funds into angel networks that can distribute the execution of investments more efficiently.
How would a fellow or early-career GI who is interested in startups pursue this career pathway?
The first step I recommend is self-reflection – what about the startup experience is interesting to you? Not all aspects appeal to everyone, and not all options provide the same opportunities. Spending time deciding which specific aspects of the startup experience appeal to you will make it easier to find the right opportunity. A concurrent step is to build expertise. This can take many forms, including traditional basic science or clinical research, but also includes implementation, evaluation/analysis, design, education, regulation, policy, and so on. The next step is to proactively meet people who are doing what you are interested in doing. Reach out to mentors, alumni, faculty, and friends. Conferences and social media are also great places to network. Other potential paths can include developing expertise in an allied functional area that can be later leveraged into a startup role (for example, experience at pharma, payer, regulatory, and so on). Many of these organizations have programs specifically geared toward physicians making a transition. In addition, another potential option is to seek additional education through an MBA where internships, recruitment programs, and robust alumni networks can be helpful in finding placement.
What if I want to learn more about the health technology startup experience?
The AGA Center for GI Innovation and Technology (CGIT) has a number of programs throughout the year, including the annual Tech Summit where you can learn about new companies, ideas, and technologies from like-minded individuals. I also invite you to reach out to me directly via Twitter, LinkedIn, or email with specific questions. As gastroenterologists, we are fortunate to work in a field full of innovation and new ideas. As a result, there are many meaningful career paths available to those interested in gastroenterology and technology. Whether providing direct clinical care with the latest endoscopic techniques or developing the next digital therapy, the opportunities for gastroenterologists will only continue to grow.
Dr. Mathews is chief medical officer at Vivante Health and assistant professor of medicine at Johns Hopkins Medicine in Baltimore. He is an officer at Vivante Health with stock options, but he reports having nothing else to disclose.
How did your career pathway lead you to working at a health tech startup?
I’ve always had an interest in technology – in fact, it was part of the reason I chose gastroenterology. When I finished GI fellowship, I decided to stay in academics because of an opportunity to lead clinical innovation efforts at my institution’s patient safety institute. This role provided protected time to foster external and internal partnerships around technology. It also gave me an opportunity to pursue clinical research and administrative experiences. While I enjoyed all three paths, it became clear that health technology was my passion. While the opportunity to join a startup was largely serendipitous – I met the founder of the company after presenting at a digital medicine conference – it also happened as a result of the steps outlined in a subsequent question. Not long after learning about the company, I made the transition to part-time faculty/clinical status and full-time chief medical officer (CMO).
What do you do as CMO?
There is no one answer to this question. It will depend on a number of variables, especially the type of business (for example, diagnostic, drug, digital, direct care management, and so on), stage of company (for example, concept, seed, series A/B/C, public), and the existing background of company founders (for example, technical, clinical, operations, and so on). Generally speaking, the earlier the stage of the company, the more hats you’ll wear (though this also means more risk; more on that later). An early-stage company was appealing to me because it gave me an opportunity to apply many of the same critical-thinking and problem-solving skills in clinical medicine to a host of other challenges. For example, as a practicing gastroenterologist, I know the pain points in the delivery of GI care and the challenges that my patients encounter. I then ask how can I develop our technology and product platform to address these issues. Also understanding how value and quality are measured in GI practice makes it easier to convey the effect of the solutions that are built and prioritize their development. In my current role I contribute to the following areas:
- Clinical strategy and vision. This means understanding the clinical need the company is trying to address at a fundamental level and designing how the technology or solution can address that need in a meaningful way. This includes working directly with technology and product teams to create a roadmap for how the technology/solution will continue to drive impact.
- Clinical care leadership. If the company employs or works with health professionals in any capacity, this usually involves developing clinical protocols and providing clinical direction.
- Clinical outcomes. This means being responsible for understanding and/or developing the metrics that will be used to demonstrate impact of the technology/solution. This includes designing clinical studies and being responsible for their execution.
- Stakeholder engagement. This means interfacing internally with nearly every aspect of the company and interacting externally with customers (usually medical peers and executives), investors, other companies, and key opinion leaders in the field.
- Regulatory. For companies pursuing Food and Drug Administration clearance or approval for their product, this entails developing a strategy and executing it.
- Research & development. This involves creating and executing a roadmap for integrating new technologies/ideas that generally complement the initial problem you are trying to solve.
What do you enjoy most about working at a startup?
The variety of experience, the flexibility, the fast pace, the ability to work creatively, and the potential to make a large-scale impact are all aspects of the job that I enjoy. The ability to continue clinical practice is important to me and is a major plus.
What do you find most challenging about working at a startup?
One of the biggest differences between a startup and a traditional clinical role is the degree of uncertainty that permeates the entire experience. It took some time for me to adjust to the relative volatility/risk associated with this type of work. Unlike an academic, administrative, or private practice job, things can change very quickly (as in a 24-hour period or less!). This can encompass a number of changes, such as funding, leadership, strategic direction, business model, and staffing, to name a few. What I’ve learned is that this doesn’t always mean changing for the worse, but it does mean things changing near constantly. Being mentally prepared to adapt quickly and frequently to big changes is part of the experience.
What are the ways that GIs can get involved in startups?
Gastroenterologists have more opportunities than most physicians due to the diversity of conditions we treat and the large corresponding number of unmet needs we encounter. There is also the inherent innovation potential associated with new applications in endoscopy, diagnostics, and drug therapies. As a result, there are a number of ways to get involved:
- This often takes the form of “spinning out” research from an academic institution but can also be done successfully from private practice, particularly in the context of new devices/services. Another related option is to license your technology to a company, which offloads the operational aspects of running a business.
- Provide consulting/advisory support. Many early-stage companies cannot afford to hire a full-time physician, but they are open to consulting arrangements (and of course volunteer work). Don’t hesitate to directly contact companies that are interesting to you. These opportunities are possible even while in clinical training.
- Work part time or full time. The majority of startups are supportive of physicians continuing to practice clinically. This makes engaging in a part-time position financially feasible for both parties. Given the relatively high remuneration for gastroenterologists working clinically, a full-time position at a startup may require a financial tradeoff (that is, lower short-term salary for a potential larger long-term gain – note the emphasis on “potential”).
- Invest in early-stage companies. Physicians can become angel investors for early-stage companies. Given the relatively time-intensive process of finding new opportunities and conducting due diligence, this often takes the form of pooling funds into angel networks that can distribute the execution of investments more efficiently.
How would a fellow or early-career GI who is interested in startups pursue this career pathway?
The first step I recommend is self-reflection – what about the startup experience is interesting to you? Not all aspects appeal to everyone, and not all options provide the same opportunities. Spending time deciding which specific aspects of the startup experience appeal to you will make it easier to find the right opportunity. A concurrent step is to build expertise. This can take many forms, including traditional basic science or clinical research, but also includes implementation, evaluation/analysis, design, education, regulation, policy, and so on. The next step is to proactively meet people who are doing what you are interested in doing. Reach out to mentors, alumni, faculty, and friends. Conferences and social media are also great places to network. Other potential paths can include developing expertise in an allied functional area that can be later leveraged into a startup role (for example, experience at pharma, payer, regulatory, and so on). Many of these organizations have programs specifically geared toward physicians making a transition. In addition, another potential option is to seek additional education through an MBA where internships, recruitment programs, and robust alumni networks can be helpful in finding placement.
What if I want to learn more about the health technology startup experience?
The AGA Center for GI Innovation and Technology (CGIT) has a number of programs throughout the year, including the annual Tech Summit where you can learn about new companies, ideas, and technologies from like-minded individuals. I also invite you to reach out to me directly via Twitter, LinkedIn, or email with specific questions. As gastroenterologists, we are fortunate to work in a field full of innovation and new ideas. As a result, there are many meaningful career paths available to those interested in gastroenterology and technology. Whether providing direct clinical care with the latest endoscopic techniques or developing the next digital therapy, the opportunities for gastroenterologists will only continue to grow.
Dr. Mathews is chief medical officer at Vivante Health and assistant professor of medicine at Johns Hopkins Medicine in Baltimore. He is an officer at Vivante Health with stock options, but he reports having nothing else to disclose.
How did your career pathway lead you to working at a health tech startup?
I’ve always had an interest in technology – in fact, it was part of the reason I chose gastroenterology. When I finished GI fellowship, I decided to stay in academics because of an opportunity to lead clinical innovation efforts at my institution’s patient safety institute. This role provided protected time to foster external and internal partnerships around technology. It also gave me an opportunity to pursue clinical research and administrative experiences. While I enjoyed all three paths, it became clear that health technology was my passion. While the opportunity to join a startup was largely serendipitous – I met the founder of the company after presenting at a digital medicine conference – it also happened as a result of the steps outlined in a subsequent question. Not long after learning about the company, I made the transition to part-time faculty/clinical status and full-time chief medical officer (CMO).
What do you do as CMO?
There is no one answer to this question. It will depend on a number of variables, especially the type of business (for example, diagnostic, drug, digital, direct care management, and so on), stage of company (for example, concept, seed, series A/B/C, public), and the existing background of company founders (for example, technical, clinical, operations, and so on). Generally speaking, the earlier the stage of the company, the more hats you’ll wear (though this also means more risk; more on that later). An early-stage company was appealing to me because it gave me an opportunity to apply many of the same critical-thinking and problem-solving skills in clinical medicine to a host of other challenges. For example, as a practicing gastroenterologist, I know the pain points in the delivery of GI care and the challenges that my patients encounter. I then ask how can I develop our technology and product platform to address these issues. Also understanding how value and quality are measured in GI practice makes it easier to convey the effect of the solutions that are built and prioritize their development. In my current role I contribute to the following areas:
- Clinical strategy and vision. This means understanding the clinical need the company is trying to address at a fundamental level and designing how the technology or solution can address that need in a meaningful way. This includes working directly with technology and product teams to create a roadmap for how the technology/solution will continue to drive impact.
- Clinical care leadership. If the company employs or works with health professionals in any capacity, this usually involves developing clinical protocols and providing clinical direction.
- Clinical outcomes. This means being responsible for understanding and/or developing the metrics that will be used to demonstrate impact of the technology/solution. This includes designing clinical studies and being responsible for their execution.
- Stakeholder engagement. This means interfacing internally with nearly every aspect of the company and interacting externally with customers (usually medical peers and executives), investors, other companies, and key opinion leaders in the field.
- Regulatory. For companies pursuing Food and Drug Administration clearance or approval for their product, this entails developing a strategy and executing it.
- Research & development. This involves creating and executing a roadmap for integrating new technologies/ideas that generally complement the initial problem you are trying to solve.
What do you enjoy most about working at a startup?
The variety of experience, the flexibility, the fast pace, the ability to work creatively, and the potential to make a large-scale impact are all aspects of the job that I enjoy. The ability to continue clinical practice is important to me and is a major plus.
What do you find most challenging about working at a startup?
One of the biggest differences between a startup and a traditional clinical role is the degree of uncertainty that permeates the entire experience. It took some time for me to adjust to the relative volatility/risk associated with this type of work. Unlike an academic, administrative, or private practice job, things can change very quickly (as in a 24-hour period or less!). This can encompass a number of changes, such as funding, leadership, strategic direction, business model, and staffing, to name a few. What I’ve learned is that this doesn’t always mean changing for the worse, but it does mean things changing near constantly. Being mentally prepared to adapt quickly and frequently to big changes is part of the experience.
What are the ways that GIs can get involved in startups?
Gastroenterologists have more opportunities than most physicians due to the diversity of conditions we treat and the large corresponding number of unmet needs we encounter. There is also the inherent innovation potential associated with new applications in endoscopy, diagnostics, and drug therapies. As a result, there are a number of ways to get involved:
- This often takes the form of “spinning out” research from an academic institution but can also be done successfully from private practice, particularly in the context of new devices/services. Another related option is to license your technology to a company, which offloads the operational aspects of running a business.
- Provide consulting/advisory support. Many early-stage companies cannot afford to hire a full-time physician, but they are open to consulting arrangements (and of course volunteer work). Don’t hesitate to directly contact companies that are interesting to you. These opportunities are possible even while in clinical training.
- Work part time or full time. The majority of startups are supportive of physicians continuing to practice clinically. This makes engaging in a part-time position financially feasible for both parties. Given the relatively high remuneration for gastroenterologists working clinically, a full-time position at a startup may require a financial tradeoff (that is, lower short-term salary for a potential larger long-term gain – note the emphasis on “potential”).
- Invest in early-stage companies. Physicians can become angel investors for early-stage companies. Given the relatively time-intensive process of finding new opportunities and conducting due diligence, this often takes the form of pooling funds into angel networks that can distribute the execution of investments more efficiently.
How would a fellow or early-career GI who is interested in startups pursue this career pathway?
The first step I recommend is self-reflection – what about the startup experience is interesting to you? Not all aspects appeal to everyone, and not all options provide the same opportunities. Spending time deciding which specific aspects of the startup experience appeal to you will make it easier to find the right opportunity. A concurrent step is to build expertise. This can take many forms, including traditional basic science or clinical research, but also includes implementation, evaluation/analysis, design, education, regulation, policy, and so on. The next step is to proactively meet people who are doing what you are interested in doing. Reach out to mentors, alumni, faculty, and friends. Conferences and social media are also great places to network. Other potential paths can include developing expertise in an allied functional area that can be later leveraged into a startup role (for example, experience at pharma, payer, regulatory, and so on). Many of these organizations have programs specifically geared toward physicians making a transition. In addition, another potential option is to seek additional education through an MBA where internships, recruitment programs, and robust alumni networks can be helpful in finding placement.
What if I want to learn more about the health technology startup experience?
The AGA Center for GI Innovation and Technology (CGIT) has a number of programs throughout the year, including the annual Tech Summit where you can learn about new companies, ideas, and technologies from like-minded individuals. I also invite you to reach out to me directly via Twitter, LinkedIn, or email with specific questions. As gastroenterologists, we are fortunate to work in a field full of innovation and new ideas. As a result, there are many meaningful career paths available to those interested in gastroenterology and technology. Whether providing direct clinical care with the latest endoscopic techniques or developing the next digital therapy, the opportunities for gastroenterologists will only continue to grow.
Dr. Mathews is chief medical officer at Vivante Health and assistant professor of medicine at Johns Hopkins Medicine in Baltimore. He is an officer at Vivante Health with stock options, but he reports having nothing else to disclose.
Addressing an unmet need in IBD patients: Treatment of acute abdominal pain
In the acute care setting, providers of care for inflammatory bowel disease (IBD) patients are often faced with the dilemma of providing effective abdominal pain management in a population that has worse outcomes with both opioid and NSAID therapy. There is increased mortality associated with opioid use and risk of disease relapse with NSAID use in IBD patients.1,2 Due to this, patients often feel that their pain is inadequately addressed.3,4 There are multiple sources of abdominal pain in IBD, and understanding the mechanisms and presentations can help identify effective treatments. We will review pharmacologic and supportive therapies to optimize pain management in IBD.
Common pain presentations in IBD
Visceral pain is a dull, poorly localized, cramping pain from intestinal distension. It is associated with inflammation, dysmotility, obstruction, and visceral hypersensitivity. Somatic and parietal pain is sharp, intense, and often localizable. Somatic pain originates from surrounding skin or muscles, and parietal pain arises from irritation of the peritoneum.5 We will review two common pain presentations in IBD.
Case 1: Mr. A is a 32-year-old male with stricturing small bowel Crohn’s disease s/p small bowel resection, who presents to the ED with 3 days of abdominal pain, nausea, and vomiting. C-reactive protein is elevated to 6.8 mg/dL (normal 0.0 – 0.6 mg/dL), and CT is consistent with active small bowel inflammation, intraabdominal abscess at the anastomosis, and associated partial small bowel obstruction. He describes a sharp, intense abdominal pain with cramping. His exam is significant for diffuse abdominal tenderness and distension.
Case 2: Ms. B is a 28-year-old female with ulcerative colitis on mesalamine monotherapy who presents to the hospital for rectal bleeding and cramping abdominal pain. After 3 days of IV steroids her rectal bleeding has resolved, and CRP has normalized. However, she continues to have dull, cramping abdominal pain. Ibuprofen has improved this pain in the past.
Mr. A is having somatic pain from inflammation, abscess, and partial bowel obstruction. He also has visceral pain from luminal distension proximal to the obstruction. Ms. B is having visceral pain despite resolution of inflammation, which may be from postinflammatory visceral hypersensitivity.
Etiologies of pain
It’s best to group pain etiologies into inflammatory and noninflammatory causes. Inflammatory pain can be secondary to infection, such as abscess or enteric infection, active bowel inflammation, or disease complications (that is, enteric fistula). It is important to recognize that patients with active inflammation may also have noninflammatory pain. These include small bowel obstruction, strictures, adhesions, narcotic bowel syndrome, bacterial overgrowth, and visceral hypersensitivity. See figure 1.
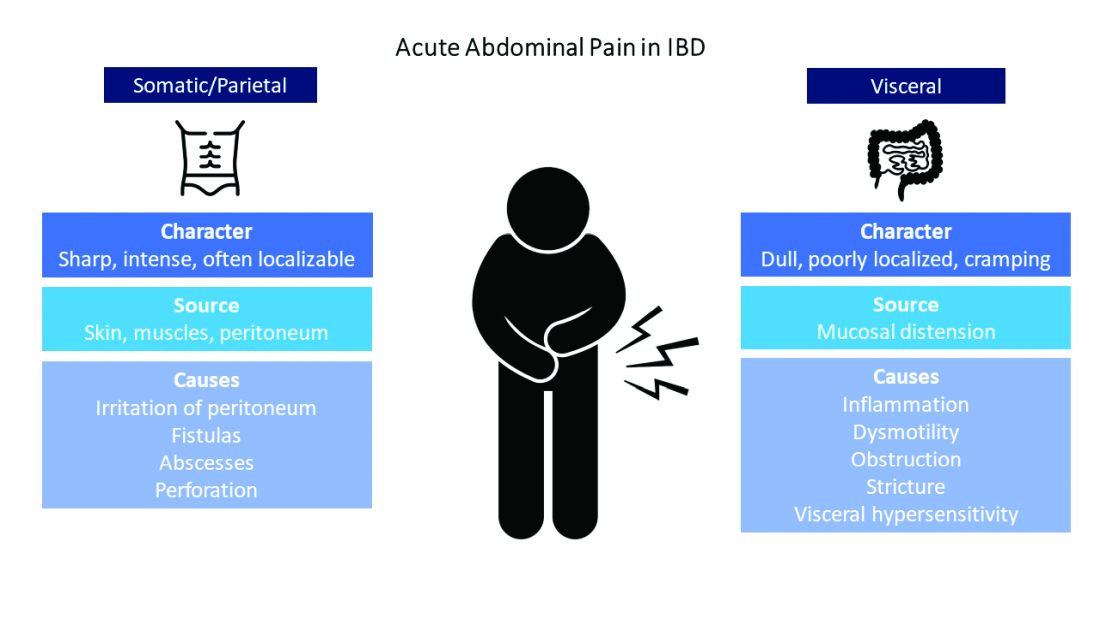
The brain-gut connection matters
Abdominal pain in IBD patients starts from painful stimuli in the gut. In addition to direct pain pathways, multiple areas of the brain modulate perception of pain.6 Patients with psychiatric comorbidities have increased perception of abdominal pain.7 In fact, high perceived stress is associated with disease relapse.8 Treatment of psychiatric disorders improves these symptoms with lasting effects.9 Addressing psychological and psychosocial needs is essential to successful pain management with long-term effect on quality of life and pain perception in IBD patients.
What are my options?
When IBD patients present with acute abdominal pain, it is important to directly address their pain as one of your primary concerns and provide them with a management plan. While this seems obvious, it is not routinely done.3-4
Next, it is important to identify the cause, whether it be infection, obstruction, active inflammation, or functional abdominal pain. In the case of active disease, in addition to steroids and optimization of IBD therapies, acetaminophen and antispasmodics can be used for initial pain management. Supportive therapies include sleep hygiene, physical activity, and psychotherapy. If initial treatments are unsuccessful in the acute setting, and presentation is consistent with somatic pain, it may be necessary to escalate to tramadol, opioid, or NSAID therapy. For visceral pain, a neuromodulator, such as a tricyclic antidepressant or gabapentin, may have greater effect. Bupropion, SNRIs, and SSRIs are options; however, they may not be effective in the acute setting. More recent focus in the IBD community has questioned the role of cannabinoids on pain in IBD patients. Cannabis has been shown in a few small studies to provide pain relief in IBD patients with active inflammation.10-11 In patients with mechanical causes for pain, management of obstruction is an important part of the treatment plan.
Let’s talk about opioids in IBD patients
Chronic narcotic use in IBD is associated with worse outcomes. So when is it okay to use opioid therapies in IBD patients? Postoperative patients, patients with severe perianal disease, or those who fail alternative pain management strategies may require opioid medications. The association with mortality and opioids in IBD is with patients who require moderate to heavy use, which is defined as being prescribed opioids more than once a year. Opioid use in IBD patients is also associated with increased risk of readmissions and poor surgical outcomes.12-13 Tramadol does not have increased mortality risk.1 If selecting opioid therapy in managing pain in IBD, it is important to define the course of therapy, with a clear goal of discontinuation after the acute episode. Opioids should be used in tandem with alternative strategies. Patients should be counseled on the synergistic effect of acetaminophen with opioids, which may allow lower effective doses of opioids.
What about NSAID use in IBD patients?
NSAIDs have negative effects in the gastrointestinal tract due to inhibition of protective prostaglandins. They also alter the gut microbiome, although clinical implications of this are unknown.14 A small study showed that IBD patients who used NSAIDs had increased risk of disease relapse.2 Symptoms of relapse would present within 2-9 days of exposure; however, most had resolution of symptoms within 2-11 days of discontinuation.2 Follow-up studies have not reliably found that NSAIDs are associated with disease relapse.8 and thus NSAIDs may be used sparingly if needed in the acute setting.
Case Review: How do we approach Mr. A and Ms. B?
Mr. A presented with a partial small bowel obstruction and abscess. His pain presentation was consistent with both visceral and somatic pain etiologies. In addition to treating active inflammation and infection, bowel rest, acetaminophen, and antispasmodics can be initiated for pain control. Concomitantly, gabapentin, TCA, or SNRI can be initiated for neurobiological pain but may have limited benefit in the acute hospitalized setting. Social work may identify needs that affect pain perception and assist in addressing those needs. If abdominal pain persists, tramadol or hydrocodone-acetaminophen can be considered.
Ms. B presented with disease relapse, but despite improving inflammatory markers she had continued cramping abdominal pain, which can be consistent with visceral hypersensitivity. Antispasmodic and neuromodulating agents, such as a TCA, could be effective. We can recommend discontinuation of chronic ibuprofen due to risk of intestinal inflammation. Patients may inquire about adjuvant cannabis in pain management. While cannabis can be considered, further research is needed to recommend its regular use.
Conclusion
Acute abdominal pain management in IBD can be challenging for providers when typical options are limited in this population. Addressing inflammatory, mechanical, neurobiological, and psychological influences is vital to appropriately address pain. Having a structured plan for pain management in IBD can improve outcomes by decreasing recurrent hospitalizations and use of opioids.15 Figure 2 presents an overview.
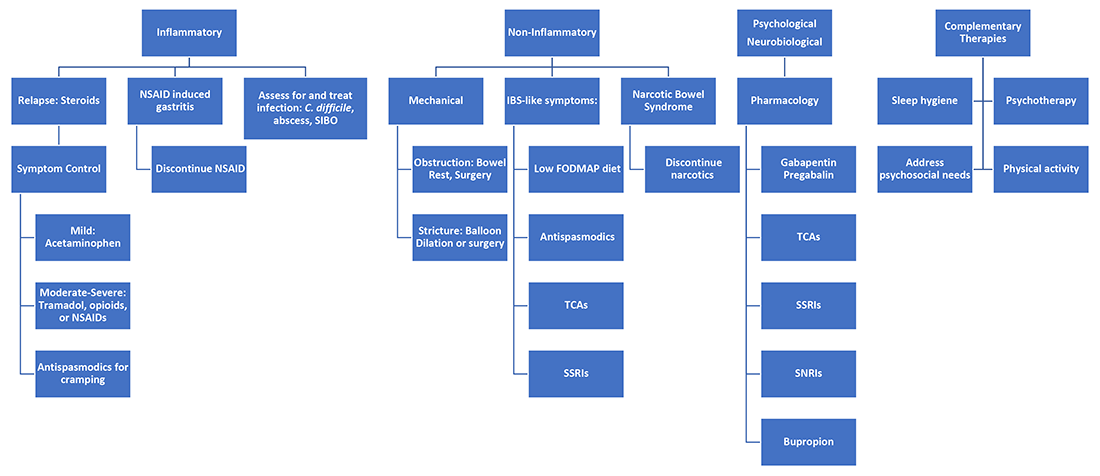
Dr. Ahmed is a second-year internal medicine resident at the University of Michigan, Ann Arbor. Dr. Kinnucan is with the department of internal medicine and the division of gastroenterology and hepatology and is an assistant professor of medicine in the division of gastroenterology, both at the University of Michigan. They have no conflicts of interest.
References
1. Burr NE et al. Clin Gastroenterol Hepatol. 2018 Apr;16(4):534-41.e6.
2. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
3. Bernhofer EI et al. Gastroenterol Nurs. 2017 May/Jun;40(3):200-7.
4. Zeitz J et al. PLoS One. 2016 Jun 22;11(6):e0156666.
5. Srinath A et al. Inflamm Bowel Dis. 2014 Dec;20(12):2433-49.
6. Docherty MJ et al. Gastroenterol Hepatol (N Y). 2011 Sep;7(9):592-601.
7. Elsenbruch S et al. Gut. 2010 Apr;59(4):489-95.
8. Bernstein CN et al. Am J Gastroenterol. 2010 Sep;105(9):1994-2002.
9. Palsson OS and Whitehead WE. Clin Gastroenterol Hepatol. 2013 Mar;11(3):208-16; quiz e22-3.
10. Swaminath A et al. Inflamm Bowel Dis. 2019 Mar; 25(3):427-35.
11. Naftali T et al. Clin Gastroenterol Hepatol. 2013 Oct;11(10):1276-80.e1.
12. Sultan K and Swaminath A. J Crohns Colitis. 2020 Sep 16;14(9):1188-89.
13. Hirsch A et al. J Gastrointest Surg. 2015 Oct;19(10):1852-61.
14. Rogers MAM and Aronoff DM. Clin Microbiol Infect. 2016;22(2):178.e1-178.e9.
15. Kaimakliotis P et al. Int J Colorectal Dis. 2021 Jun;36(6):1193-200.
In the acute care setting, providers of care for inflammatory bowel disease (IBD) patients are often faced with the dilemma of providing effective abdominal pain management in a population that has worse outcomes with both opioid and NSAID therapy. There is increased mortality associated with opioid use and risk of disease relapse with NSAID use in IBD patients.1,2 Due to this, patients often feel that their pain is inadequately addressed.3,4 There are multiple sources of abdominal pain in IBD, and understanding the mechanisms and presentations can help identify effective treatments. We will review pharmacologic and supportive therapies to optimize pain management in IBD.
Common pain presentations in IBD
Visceral pain is a dull, poorly localized, cramping pain from intestinal distension. It is associated with inflammation, dysmotility, obstruction, and visceral hypersensitivity. Somatic and parietal pain is sharp, intense, and often localizable. Somatic pain originates from surrounding skin or muscles, and parietal pain arises from irritation of the peritoneum.5 We will review two common pain presentations in IBD.
Case 1: Mr. A is a 32-year-old male with stricturing small bowel Crohn’s disease s/p small bowel resection, who presents to the ED with 3 days of abdominal pain, nausea, and vomiting. C-reactive protein is elevated to 6.8 mg/dL (normal 0.0 – 0.6 mg/dL), and CT is consistent with active small bowel inflammation, intraabdominal abscess at the anastomosis, and associated partial small bowel obstruction. He describes a sharp, intense abdominal pain with cramping. His exam is significant for diffuse abdominal tenderness and distension.
Case 2: Ms. B is a 28-year-old female with ulcerative colitis on mesalamine monotherapy who presents to the hospital for rectal bleeding and cramping abdominal pain. After 3 days of IV steroids her rectal bleeding has resolved, and CRP has normalized. However, she continues to have dull, cramping abdominal pain. Ibuprofen has improved this pain in the past.
Mr. A is having somatic pain from inflammation, abscess, and partial bowel obstruction. He also has visceral pain from luminal distension proximal to the obstruction. Ms. B is having visceral pain despite resolution of inflammation, which may be from postinflammatory visceral hypersensitivity.
Etiologies of pain
It’s best to group pain etiologies into inflammatory and noninflammatory causes. Inflammatory pain can be secondary to infection, such as abscess or enteric infection, active bowel inflammation, or disease complications (that is, enteric fistula). It is important to recognize that patients with active inflammation may also have noninflammatory pain. These include small bowel obstruction, strictures, adhesions, narcotic bowel syndrome, bacterial overgrowth, and visceral hypersensitivity. See figure 1.

The brain-gut connection matters
Abdominal pain in IBD patients starts from painful stimuli in the gut. In addition to direct pain pathways, multiple areas of the brain modulate perception of pain.6 Patients with psychiatric comorbidities have increased perception of abdominal pain.7 In fact, high perceived stress is associated with disease relapse.8 Treatment of psychiatric disorders improves these symptoms with lasting effects.9 Addressing psychological and psychosocial needs is essential to successful pain management with long-term effect on quality of life and pain perception in IBD patients.
What are my options?
When IBD patients present with acute abdominal pain, it is important to directly address their pain as one of your primary concerns and provide them with a management plan. While this seems obvious, it is not routinely done.3-4
Next, it is important to identify the cause, whether it be infection, obstruction, active inflammation, or functional abdominal pain. In the case of active disease, in addition to steroids and optimization of IBD therapies, acetaminophen and antispasmodics can be used for initial pain management. Supportive therapies include sleep hygiene, physical activity, and psychotherapy. If initial treatments are unsuccessful in the acute setting, and presentation is consistent with somatic pain, it may be necessary to escalate to tramadol, opioid, or NSAID therapy. For visceral pain, a neuromodulator, such as a tricyclic antidepressant or gabapentin, may have greater effect. Bupropion, SNRIs, and SSRIs are options; however, they may not be effective in the acute setting. More recent focus in the IBD community has questioned the role of cannabinoids on pain in IBD patients. Cannabis has been shown in a few small studies to provide pain relief in IBD patients with active inflammation.10-11 In patients with mechanical causes for pain, management of obstruction is an important part of the treatment plan.
Let’s talk about opioids in IBD patients
Chronic narcotic use in IBD is associated with worse outcomes. So when is it okay to use opioid therapies in IBD patients? Postoperative patients, patients with severe perianal disease, or those who fail alternative pain management strategies may require opioid medications. The association with mortality and opioids in IBD is with patients who require moderate to heavy use, which is defined as being prescribed opioids more than once a year. Opioid use in IBD patients is also associated with increased risk of readmissions and poor surgical outcomes.12-13 Tramadol does not have increased mortality risk.1 If selecting opioid therapy in managing pain in IBD, it is important to define the course of therapy, with a clear goal of discontinuation after the acute episode. Opioids should be used in tandem with alternative strategies. Patients should be counseled on the synergistic effect of acetaminophen with opioids, which may allow lower effective doses of opioids.
What about NSAID use in IBD patients?
NSAIDs have negative effects in the gastrointestinal tract due to inhibition of protective prostaglandins. They also alter the gut microbiome, although clinical implications of this are unknown.14 A small study showed that IBD patients who used NSAIDs had increased risk of disease relapse.2 Symptoms of relapse would present within 2-9 days of exposure; however, most had resolution of symptoms within 2-11 days of discontinuation.2 Follow-up studies have not reliably found that NSAIDs are associated with disease relapse.8 and thus NSAIDs may be used sparingly if needed in the acute setting.
Case Review: How do we approach Mr. A and Ms. B?
Mr. A presented with a partial small bowel obstruction and abscess. His pain presentation was consistent with both visceral and somatic pain etiologies. In addition to treating active inflammation and infection, bowel rest, acetaminophen, and antispasmodics can be initiated for pain control. Concomitantly, gabapentin, TCA, or SNRI can be initiated for neurobiological pain but may have limited benefit in the acute hospitalized setting. Social work may identify needs that affect pain perception and assist in addressing those needs. If abdominal pain persists, tramadol or hydrocodone-acetaminophen can be considered.
Ms. B presented with disease relapse, but despite improving inflammatory markers she had continued cramping abdominal pain, which can be consistent with visceral hypersensitivity. Antispasmodic and neuromodulating agents, such as a TCA, could be effective. We can recommend discontinuation of chronic ibuprofen due to risk of intestinal inflammation. Patients may inquire about adjuvant cannabis in pain management. While cannabis can be considered, further research is needed to recommend its regular use.
Conclusion
Acute abdominal pain management in IBD can be challenging for providers when typical options are limited in this population. Addressing inflammatory, mechanical, neurobiological, and psychological influences is vital to appropriately address pain. Having a structured plan for pain management in IBD can improve outcomes by decreasing recurrent hospitalizations and use of opioids.15 Figure 2 presents an overview.

Dr. Ahmed is a second-year internal medicine resident at the University of Michigan, Ann Arbor. Dr. Kinnucan is with the department of internal medicine and the division of gastroenterology and hepatology and is an assistant professor of medicine in the division of gastroenterology, both at the University of Michigan. They have no conflicts of interest.
References
1. Burr NE et al. Clin Gastroenterol Hepatol. 2018 Apr;16(4):534-41.e6.
2. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
3. Bernhofer EI et al. Gastroenterol Nurs. 2017 May/Jun;40(3):200-7.
4. Zeitz J et al. PLoS One. 2016 Jun 22;11(6):e0156666.
5. Srinath A et al. Inflamm Bowel Dis. 2014 Dec;20(12):2433-49.
6. Docherty MJ et al. Gastroenterol Hepatol (N Y). 2011 Sep;7(9):592-601.
7. Elsenbruch S et al. Gut. 2010 Apr;59(4):489-95.
8. Bernstein CN et al. Am J Gastroenterol. 2010 Sep;105(9):1994-2002.
9. Palsson OS and Whitehead WE. Clin Gastroenterol Hepatol. 2013 Mar;11(3):208-16; quiz e22-3.
10. Swaminath A et al. Inflamm Bowel Dis. 2019 Mar; 25(3):427-35.
11. Naftali T et al. Clin Gastroenterol Hepatol. 2013 Oct;11(10):1276-80.e1.
12. Sultan K and Swaminath A. J Crohns Colitis. 2020 Sep 16;14(9):1188-89.
13. Hirsch A et al. J Gastrointest Surg. 2015 Oct;19(10):1852-61.
14. Rogers MAM and Aronoff DM. Clin Microbiol Infect. 2016;22(2):178.e1-178.e9.
15. Kaimakliotis P et al. Int J Colorectal Dis. 2021 Jun;36(6):1193-200.
In the acute care setting, providers of care for inflammatory bowel disease (IBD) patients are often faced with the dilemma of providing effective abdominal pain management in a population that has worse outcomes with both opioid and NSAID therapy. There is increased mortality associated with opioid use and risk of disease relapse with NSAID use in IBD patients.1,2 Due to this, patients often feel that their pain is inadequately addressed.3,4 There are multiple sources of abdominal pain in IBD, and understanding the mechanisms and presentations can help identify effective treatments. We will review pharmacologic and supportive therapies to optimize pain management in IBD.
Common pain presentations in IBD
Visceral pain is a dull, poorly localized, cramping pain from intestinal distension. It is associated with inflammation, dysmotility, obstruction, and visceral hypersensitivity. Somatic and parietal pain is sharp, intense, and often localizable. Somatic pain originates from surrounding skin or muscles, and parietal pain arises from irritation of the peritoneum.5 We will review two common pain presentations in IBD.
Case 1: Mr. A is a 32-year-old male with stricturing small bowel Crohn’s disease s/p small bowel resection, who presents to the ED with 3 days of abdominal pain, nausea, and vomiting. C-reactive protein is elevated to 6.8 mg/dL (normal 0.0 – 0.6 mg/dL), and CT is consistent with active small bowel inflammation, intraabdominal abscess at the anastomosis, and associated partial small bowel obstruction. He describes a sharp, intense abdominal pain with cramping. His exam is significant for diffuse abdominal tenderness and distension.
Case 2: Ms. B is a 28-year-old female with ulcerative colitis on mesalamine monotherapy who presents to the hospital for rectal bleeding and cramping abdominal pain. After 3 days of IV steroids her rectal bleeding has resolved, and CRP has normalized. However, she continues to have dull, cramping abdominal pain. Ibuprofen has improved this pain in the past.
Mr. A is having somatic pain from inflammation, abscess, and partial bowel obstruction. He also has visceral pain from luminal distension proximal to the obstruction. Ms. B is having visceral pain despite resolution of inflammation, which may be from postinflammatory visceral hypersensitivity.
Etiologies of pain
It’s best to group pain etiologies into inflammatory and noninflammatory causes. Inflammatory pain can be secondary to infection, such as abscess or enteric infection, active bowel inflammation, or disease complications (that is, enteric fistula). It is important to recognize that patients with active inflammation may also have noninflammatory pain. These include small bowel obstruction, strictures, adhesions, narcotic bowel syndrome, bacterial overgrowth, and visceral hypersensitivity. See figure 1.

The brain-gut connection matters
Abdominal pain in IBD patients starts from painful stimuli in the gut. In addition to direct pain pathways, multiple areas of the brain modulate perception of pain.6 Patients with psychiatric comorbidities have increased perception of abdominal pain.7 In fact, high perceived stress is associated with disease relapse.8 Treatment of psychiatric disorders improves these symptoms with lasting effects.9 Addressing psychological and psychosocial needs is essential to successful pain management with long-term effect on quality of life and pain perception in IBD patients.
What are my options?
When IBD patients present with acute abdominal pain, it is important to directly address their pain as one of your primary concerns and provide them with a management plan. While this seems obvious, it is not routinely done.3-4
Next, it is important to identify the cause, whether it be infection, obstruction, active inflammation, or functional abdominal pain. In the case of active disease, in addition to steroids and optimization of IBD therapies, acetaminophen and antispasmodics can be used for initial pain management. Supportive therapies include sleep hygiene, physical activity, and psychotherapy. If initial treatments are unsuccessful in the acute setting, and presentation is consistent with somatic pain, it may be necessary to escalate to tramadol, opioid, or NSAID therapy. For visceral pain, a neuromodulator, such as a tricyclic antidepressant or gabapentin, may have greater effect. Bupropion, SNRIs, and SSRIs are options; however, they may not be effective in the acute setting. More recent focus in the IBD community has questioned the role of cannabinoids on pain in IBD patients. Cannabis has been shown in a few small studies to provide pain relief in IBD patients with active inflammation.10-11 In patients with mechanical causes for pain, management of obstruction is an important part of the treatment plan.
Let’s talk about opioids in IBD patients
Chronic narcotic use in IBD is associated with worse outcomes. So when is it okay to use opioid therapies in IBD patients? Postoperative patients, patients with severe perianal disease, or those who fail alternative pain management strategies may require opioid medications. The association with mortality and opioids in IBD is with patients who require moderate to heavy use, which is defined as being prescribed opioids more than once a year. Opioid use in IBD patients is also associated with increased risk of readmissions and poor surgical outcomes.12-13 Tramadol does not have increased mortality risk.1 If selecting opioid therapy in managing pain in IBD, it is important to define the course of therapy, with a clear goal of discontinuation after the acute episode. Opioids should be used in tandem with alternative strategies. Patients should be counseled on the synergistic effect of acetaminophen with opioids, which may allow lower effective doses of opioids.
What about NSAID use in IBD patients?
NSAIDs have negative effects in the gastrointestinal tract due to inhibition of protective prostaglandins. They also alter the gut microbiome, although clinical implications of this are unknown.14 A small study showed that IBD patients who used NSAIDs had increased risk of disease relapse.2 Symptoms of relapse would present within 2-9 days of exposure; however, most had resolution of symptoms within 2-11 days of discontinuation.2 Follow-up studies have not reliably found that NSAIDs are associated with disease relapse.8 and thus NSAIDs may be used sparingly if needed in the acute setting.
Case Review: How do we approach Mr. A and Ms. B?
Mr. A presented with a partial small bowel obstruction and abscess. His pain presentation was consistent with both visceral and somatic pain etiologies. In addition to treating active inflammation and infection, bowel rest, acetaminophen, and antispasmodics can be initiated for pain control. Concomitantly, gabapentin, TCA, or SNRI can be initiated for neurobiological pain but may have limited benefit in the acute hospitalized setting. Social work may identify needs that affect pain perception and assist in addressing those needs. If abdominal pain persists, tramadol or hydrocodone-acetaminophen can be considered.
Ms. B presented with disease relapse, but despite improving inflammatory markers she had continued cramping abdominal pain, which can be consistent with visceral hypersensitivity. Antispasmodic and neuromodulating agents, such as a TCA, could be effective. We can recommend discontinuation of chronic ibuprofen due to risk of intestinal inflammation. Patients may inquire about adjuvant cannabis in pain management. While cannabis can be considered, further research is needed to recommend its regular use.
Conclusion
Acute abdominal pain management in IBD can be challenging for providers when typical options are limited in this population. Addressing inflammatory, mechanical, neurobiological, and psychological influences is vital to appropriately address pain. Having a structured plan for pain management in IBD can improve outcomes by decreasing recurrent hospitalizations and use of opioids.15 Figure 2 presents an overview.

Dr. Ahmed is a second-year internal medicine resident at the University of Michigan, Ann Arbor. Dr. Kinnucan is with the department of internal medicine and the division of gastroenterology and hepatology and is an assistant professor of medicine in the division of gastroenterology, both at the University of Michigan. They have no conflicts of interest.
References
1. Burr NE et al. Clin Gastroenterol Hepatol. 2018 Apr;16(4):534-41.e6.
2. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
3. Bernhofer EI et al. Gastroenterol Nurs. 2017 May/Jun;40(3):200-7.
4. Zeitz J et al. PLoS One. 2016 Jun 22;11(6):e0156666.
5. Srinath A et al. Inflamm Bowel Dis. 2014 Dec;20(12):2433-49.
6. Docherty MJ et al. Gastroenterol Hepatol (N Y). 2011 Sep;7(9):592-601.
7. Elsenbruch S et al. Gut. 2010 Apr;59(4):489-95.
8. Bernstein CN et al. Am J Gastroenterol. 2010 Sep;105(9):1994-2002.
9. Palsson OS and Whitehead WE. Clin Gastroenterol Hepatol. 2013 Mar;11(3):208-16; quiz e22-3.
10. Swaminath A et al. Inflamm Bowel Dis. 2019 Mar; 25(3):427-35.
11. Naftali T et al. Clin Gastroenterol Hepatol. 2013 Oct;11(10):1276-80.e1.
12. Sultan K and Swaminath A. J Crohns Colitis. 2020 Sep 16;14(9):1188-89.
13. Hirsch A et al. J Gastrointest Surg. 2015 Oct;19(10):1852-61.
14. Rogers MAM and Aronoff DM. Clin Microbiol Infect. 2016;22(2):178.e1-178.e9.
15. Kaimakliotis P et al. Int J Colorectal Dis. 2021 Jun;36(6):1193-200.
Coerced invasive procedures: Policy overriding indication in gastrostomy tube placement
Clinical scenario
An 83-year-old man is admitted with a hemiplegic cerebrovascular accident. He is found to have dysphagia, and a nasogastric feeding tube is placed. Over the next several days, his strength begins to recover, and he tolerates his tube feeding well. Discharge to a skilled nursing facility (SNF) for subacute rehabilitation is planned. His swallowing is showing signs of recovery; it has not recovered adequately but is expected to continue to improve such that he is predicted to be independent of tube feeding within 7-14 days. None of the facilities in the region are willing to admit a patient with a nasal feeding tube, despite the anticipated short duration. The patient is medically ready for discharge but is refusing the feeding gastrostomy. “Why would I want a hole in my stomach, if I’m only going to need it for 1-2 weeks and this tube in my nose is working fine and is comfortable?” he pleads with tears in his eyes.
Feeding dysphagic patients after stroke
Dysphagia, potentially leading to aspiration and/or pneumonia, is a common sequela of stroke – up to half of hospitalized patients are affected.1 When oral intake is contraindicated, patients are often fed by nasogastric tube (NGT) or by surgically or endoscopically placed gastrostomy tube (GT). Without good justification based on outcomes, NGTs are traditionally used when the need for feeding is thought to be short term (<4 weeks) and GTs are used for long term (>4 weeks). However, in 2005, a large multicenter randomized control trial found that the majority of stroke patients with dysphagia that would resolve had resolution within 2-3 weeks. Moreover, outcomes were equivalent or better for patients fed with an NGT versus GT.
The authors concluded by recommending feeding via NGT for 2-3 weeks, after which conversion to GT can be considered if dysphagia persists.1 Notably, the recommendation allows consideration, and no evidence-based guideline requires or recommends GT be placed based on duration of tube feed dependence. Currently, while nutrition and neurology authorities have adopted these recommendations,2,3 many authors have noted poor adherence to this guideline, and many find that the median period between stroke and GT placement is 7 days rather than the recommended minimum of 14.4,5,6 While ignorance can partially explain the lack of widespread compliance,6 the policies of posthospital facilities are another culprit. Increasingly, and for a variety of reasons unsupported by the literature, SNFs refuse NGT and require GT.4,7,8,9
Ethical considerations
The four principles of medical ethics – autonomy, beneficence, nonmaleficence, and justice – can guide clinicians, patients, and family members in decision-making. In our case, by withholding needed and desired treatment (discharge to and treatment by a rehabilitation facility) the patient is being coerced to undergo a procedure he does not want, and clinicians participate in denying him autonomy. Further, given that the evidence, national guidelines, and in fact federal regulations indicate that his preferences are congruent with best practices, pressuring him to accept gastrostomy placement runs afoul of the principles of beneficence and nonmaleficence. Though the mechanism is unclear, early gastrostomy (<14-21 days) is associated with increased risk of death, worse functional outcomes, and a lower rate of return to oral feeding, as well as a significant procedure-specific complication rate.1,10 By insisting on gastrostomy, we neither act in this patient’s best interests nor “do no harm.”
However, the medical system is complex. The clinician at the bedside can evaluate this scenario, review the national guidelines, discuss the procedure and risks with the patient and family, and conclude that the patient should be discharged with a nasal feeding tube. Nevertheless, if no facility is willing to accept him without a gastrostomy, our decision-making model – previously limited to our patient’s best interests alone – is forced to change. Despite our misgivings, we often conclude that the harm done by an early gastrostomy is outweighed by the harm of remaining unnecessarily in the acute hospital setting. We further worry about other patients lingering in the emergency department for lack of an inpatient bed and the possible – though unknowable – harm done to them.
Looking forward
It is an unfortunate fact that medical decision-making must often include factors unrelated to the patient’s best interests, with financial considerations and structural barriers frequently driving deviation from ideal care. Providers and patients navigate these decisions to their best abilities, making compromises when forced. However, with education and professional activism, providers can advocate for the elimination of barriers to providing medically sound and ethically appropriate care. In our experience, delay of gastrostomy placement, until discharge is imminent and planning for postdischarge care is initiated, has resulted in a decrease by half the fraction of patients with tracheostomies who had gastrostomies placed prior to discharge.11 With aggressive outreach and education, we now have nursing homes willing to accept patients with NGTs.
Criteria for admission to discharge facilities can drive medical decision-making that is unethical and unsupported by evidence. Continued efforts to eliminate barriers to appropriate and ethical care have been successful and are encouraged.
Dr. Cowan is administrative chief resident in the department of surgery at Columbia University Irving Medical Center, New York. Dr. Seres is professor of medicine in the Institute of Human Nutrition and associate clinical ethicist at Columbia University Irving Medical Center. The authors have no conflicts of interest to disclose.
References
1. Dennis MS et al. Lancet. 2005 Feb 26-Mar 4;365(9461):764-72.
2. Powers W. et al. Stroke. 2018 Mar;49(3):e46-e110.
3. Burgos R et al. Clin Nutr. 2018 Feb;37(1):354-96.
4. Wilmskoetter J et al. J Stroke Cerebrovasc Dis. 2016 Nov;25(11):2694-700.
5. George BP et al. Stroke. 2017 Feb;48(2):420-7.
6. Fessler TA. et al. Surg Endosc. 2019 Dec;33(12):4089-97.
7. Burgermaster M et al. Nutr Clin Pract. 2016 Jun;31(3):342-8.
8. Moran C and O’Mahoney S. Curr Opin Gastroenterol. 2015 Mar;31(2):137-42.
9. Gomes CA et al. Cochrane Database Syst Rev. 2010 Nov 10;(11):CD008096.
10. Joundi RA et al. Neurology. 2018 Feb 13;90(7):e544-52.
11. Bothra A et al. J Parenter Enteral Nutr. 2018 Feb;42(2):491.
Clinical scenario
An 83-year-old man is admitted with a hemiplegic cerebrovascular accident. He is found to have dysphagia, and a nasogastric feeding tube is placed. Over the next several days, his strength begins to recover, and he tolerates his tube feeding well. Discharge to a skilled nursing facility (SNF) for subacute rehabilitation is planned. His swallowing is showing signs of recovery; it has not recovered adequately but is expected to continue to improve such that he is predicted to be independent of tube feeding within 7-14 days. None of the facilities in the region are willing to admit a patient with a nasal feeding tube, despite the anticipated short duration. The patient is medically ready for discharge but is refusing the feeding gastrostomy. “Why would I want a hole in my stomach, if I’m only going to need it for 1-2 weeks and this tube in my nose is working fine and is comfortable?” he pleads with tears in his eyes.
Feeding dysphagic patients after stroke
Dysphagia, potentially leading to aspiration and/or pneumonia, is a common sequela of stroke – up to half of hospitalized patients are affected.1 When oral intake is contraindicated, patients are often fed by nasogastric tube (NGT) or by surgically or endoscopically placed gastrostomy tube (GT). Without good justification based on outcomes, NGTs are traditionally used when the need for feeding is thought to be short term (<4 weeks) and GTs are used for long term (>4 weeks). However, in 2005, a large multicenter randomized control trial found that the majority of stroke patients with dysphagia that would resolve had resolution within 2-3 weeks. Moreover, outcomes were equivalent or better for patients fed with an NGT versus GT.
The authors concluded by recommending feeding via NGT for 2-3 weeks, after which conversion to GT can be considered if dysphagia persists.1 Notably, the recommendation allows consideration, and no evidence-based guideline requires or recommends GT be placed based on duration of tube feed dependence. Currently, while nutrition and neurology authorities have adopted these recommendations,2,3 many authors have noted poor adherence to this guideline, and many find that the median period between stroke and GT placement is 7 days rather than the recommended minimum of 14.4,5,6 While ignorance can partially explain the lack of widespread compliance,6 the policies of posthospital facilities are another culprit. Increasingly, and for a variety of reasons unsupported by the literature, SNFs refuse NGT and require GT.4,7,8,9
Ethical considerations
The four principles of medical ethics – autonomy, beneficence, nonmaleficence, and justice – can guide clinicians, patients, and family members in decision-making. In our case, by withholding needed and desired treatment (discharge to and treatment by a rehabilitation facility) the patient is being coerced to undergo a procedure he does not want, and clinicians participate in denying him autonomy. Further, given that the evidence, national guidelines, and in fact federal regulations indicate that his preferences are congruent with best practices, pressuring him to accept gastrostomy placement runs afoul of the principles of beneficence and nonmaleficence. Though the mechanism is unclear, early gastrostomy (<14-21 days) is associated with increased risk of death, worse functional outcomes, and a lower rate of return to oral feeding, as well as a significant procedure-specific complication rate.1,10 By insisting on gastrostomy, we neither act in this patient’s best interests nor “do no harm.”
However, the medical system is complex. The clinician at the bedside can evaluate this scenario, review the national guidelines, discuss the procedure and risks with the patient and family, and conclude that the patient should be discharged with a nasal feeding tube. Nevertheless, if no facility is willing to accept him without a gastrostomy, our decision-making model – previously limited to our patient’s best interests alone – is forced to change. Despite our misgivings, we often conclude that the harm done by an early gastrostomy is outweighed by the harm of remaining unnecessarily in the acute hospital setting. We further worry about other patients lingering in the emergency department for lack of an inpatient bed and the possible – though unknowable – harm done to them.
Looking forward
It is an unfortunate fact that medical decision-making must often include factors unrelated to the patient’s best interests, with financial considerations and structural barriers frequently driving deviation from ideal care. Providers and patients navigate these decisions to their best abilities, making compromises when forced. However, with education and professional activism, providers can advocate for the elimination of barriers to providing medically sound and ethically appropriate care. In our experience, delay of gastrostomy placement, until discharge is imminent and planning for postdischarge care is initiated, has resulted in a decrease by half the fraction of patients with tracheostomies who had gastrostomies placed prior to discharge.11 With aggressive outreach and education, we now have nursing homes willing to accept patients with NGTs.
Criteria for admission to discharge facilities can drive medical decision-making that is unethical and unsupported by evidence. Continued efforts to eliminate barriers to appropriate and ethical care have been successful and are encouraged.
Dr. Cowan is administrative chief resident in the department of surgery at Columbia University Irving Medical Center, New York. Dr. Seres is professor of medicine in the Institute of Human Nutrition and associate clinical ethicist at Columbia University Irving Medical Center. The authors have no conflicts of interest to disclose.
References
1. Dennis MS et al. Lancet. 2005 Feb 26-Mar 4;365(9461):764-72.
2. Powers W. et al. Stroke. 2018 Mar;49(3):e46-e110.
3. Burgos R et al. Clin Nutr. 2018 Feb;37(1):354-96.
4. Wilmskoetter J et al. J Stroke Cerebrovasc Dis. 2016 Nov;25(11):2694-700.
5. George BP et al. Stroke. 2017 Feb;48(2):420-7.
6. Fessler TA. et al. Surg Endosc. 2019 Dec;33(12):4089-97.
7. Burgermaster M et al. Nutr Clin Pract. 2016 Jun;31(3):342-8.
8. Moran C and O’Mahoney S. Curr Opin Gastroenterol. 2015 Mar;31(2):137-42.
9. Gomes CA et al. Cochrane Database Syst Rev. 2010 Nov 10;(11):CD008096.
10. Joundi RA et al. Neurology. 2018 Feb 13;90(7):e544-52.
11. Bothra A et al. J Parenter Enteral Nutr. 2018 Feb;42(2):491.
Clinical scenario
An 83-year-old man is admitted with a hemiplegic cerebrovascular accident. He is found to have dysphagia, and a nasogastric feeding tube is placed. Over the next several days, his strength begins to recover, and he tolerates his tube feeding well. Discharge to a skilled nursing facility (SNF) for subacute rehabilitation is planned. His swallowing is showing signs of recovery; it has not recovered adequately but is expected to continue to improve such that he is predicted to be independent of tube feeding within 7-14 days. None of the facilities in the region are willing to admit a patient with a nasal feeding tube, despite the anticipated short duration. The patient is medically ready for discharge but is refusing the feeding gastrostomy. “Why would I want a hole in my stomach, if I’m only going to need it for 1-2 weeks and this tube in my nose is working fine and is comfortable?” he pleads with tears in his eyes.
Feeding dysphagic patients after stroke
Dysphagia, potentially leading to aspiration and/or pneumonia, is a common sequela of stroke – up to half of hospitalized patients are affected.1 When oral intake is contraindicated, patients are often fed by nasogastric tube (NGT) or by surgically or endoscopically placed gastrostomy tube (GT). Without good justification based on outcomes, NGTs are traditionally used when the need for feeding is thought to be short term (<4 weeks) and GTs are used for long term (>4 weeks). However, in 2005, a large multicenter randomized control trial found that the majority of stroke patients with dysphagia that would resolve had resolution within 2-3 weeks. Moreover, outcomes were equivalent or better for patients fed with an NGT versus GT.
The authors concluded by recommending feeding via NGT for 2-3 weeks, after which conversion to GT can be considered if dysphagia persists.1 Notably, the recommendation allows consideration, and no evidence-based guideline requires or recommends GT be placed based on duration of tube feed dependence. Currently, while nutrition and neurology authorities have adopted these recommendations,2,3 many authors have noted poor adherence to this guideline, and many find that the median period between stroke and GT placement is 7 days rather than the recommended minimum of 14.4,5,6 While ignorance can partially explain the lack of widespread compliance,6 the policies of posthospital facilities are another culprit. Increasingly, and for a variety of reasons unsupported by the literature, SNFs refuse NGT and require GT.4,7,8,9
Ethical considerations
The four principles of medical ethics – autonomy, beneficence, nonmaleficence, and justice – can guide clinicians, patients, and family members in decision-making. In our case, by withholding needed and desired treatment (discharge to and treatment by a rehabilitation facility) the patient is being coerced to undergo a procedure he does not want, and clinicians participate in denying him autonomy. Further, given that the evidence, national guidelines, and in fact federal regulations indicate that his preferences are congruent with best practices, pressuring him to accept gastrostomy placement runs afoul of the principles of beneficence and nonmaleficence. Though the mechanism is unclear, early gastrostomy (<14-21 days) is associated with increased risk of death, worse functional outcomes, and a lower rate of return to oral feeding, as well as a significant procedure-specific complication rate.1,10 By insisting on gastrostomy, we neither act in this patient’s best interests nor “do no harm.”
However, the medical system is complex. The clinician at the bedside can evaluate this scenario, review the national guidelines, discuss the procedure and risks with the patient and family, and conclude that the patient should be discharged with a nasal feeding tube. Nevertheless, if no facility is willing to accept him without a gastrostomy, our decision-making model – previously limited to our patient’s best interests alone – is forced to change. Despite our misgivings, we often conclude that the harm done by an early gastrostomy is outweighed by the harm of remaining unnecessarily in the acute hospital setting. We further worry about other patients lingering in the emergency department for lack of an inpatient bed and the possible – though unknowable – harm done to them.
Looking forward
It is an unfortunate fact that medical decision-making must often include factors unrelated to the patient’s best interests, with financial considerations and structural barriers frequently driving deviation from ideal care. Providers and patients navigate these decisions to their best abilities, making compromises when forced. However, with education and professional activism, providers can advocate for the elimination of barriers to providing medically sound and ethically appropriate care. In our experience, delay of gastrostomy placement, until discharge is imminent and planning for postdischarge care is initiated, has resulted in a decrease by half the fraction of patients with tracheostomies who had gastrostomies placed prior to discharge.11 With aggressive outreach and education, we now have nursing homes willing to accept patients with NGTs.
Criteria for admission to discharge facilities can drive medical decision-making that is unethical and unsupported by evidence. Continued efforts to eliminate barriers to appropriate and ethical care have been successful and are encouraged.
Dr. Cowan is administrative chief resident in the department of surgery at Columbia University Irving Medical Center, New York. Dr. Seres is professor of medicine in the Institute of Human Nutrition and associate clinical ethicist at Columbia University Irving Medical Center. The authors have no conflicts of interest to disclose.
References
1. Dennis MS et al. Lancet. 2005 Feb 26-Mar 4;365(9461):764-72.
2. Powers W. et al. Stroke. 2018 Mar;49(3):e46-e110.
3. Burgos R et al. Clin Nutr. 2018 Feb;37(1):354-96.
4. Wilmskoetter J et al. J Stroke Cerebrovasc Dis. 2016 Nov;25(11):2694-700.
5. George BP et al. Stroke. 2017 Feb;48(2):420-7.
6. Fessler TA. et al. Surg Endosc. 2019 Dec;33(12):4089-97.
7. Burgermaster M et al. Nutr Clin Pract. 2016 Jun;31(3):342-8.
8. Moran C and O’Mahoney S. Curr Opin Gastroenterol. 2015 Mar;31(2):137-42.
9. Gomes CA et al. Cochrane Database Syst Rev. 2010 Nov 10;(11):CD008096.
10. Joundi RA et al. Neurology. 2018 Feb 13;90(7):e544-52.
11. Bothra A et al. J Parenter Enteral Nutr. 2018 Feb;42(2):491.
The doctor house: What to know in 2021
The concept of home ownership has changed for this generation, not only in the logistics of the best way to do it, but also in the desire and demand for owning versus renting. According to the Penn Institute for Urban Research, the U.S. homeownership rate is now at 63.7%, the lowest in 48 years. “Homeownership rates have declined for all demographic age groups. Since 2006, the number of households who own their home in the United States has decreased by 674,000 while the number of renters has increased by over 8 million.”1
But isn’t owning your own home the American dream? Isn’t it a great investment? If we’re looking strictly at the monthly payment numbers, traditionally paying a mortgage is cheaper than paying rent for the same-size home. That became even more true as home prices dropped in 2007-2008 and as interest rates also dropped over the last several years. Then there’s the age-old concept of “building equity” or “throwing away money on rent.” As a financial planner, I get asked all the time about a home as an investment. It certainly can be, like any investment, if you buy and sell at the right time. But something is only an investment if it grows, and you can’t predict if you’re going to buy and sell your house at the right time. The median price of an existing home sold in March 2021 was $329,100. That’s a 17.2% increase from March 2020. Times are certainly crazy right now in the real estate market.
What many people don’t think about are the other costs, and not just financial costs. Today’s generation is more aware of the added stress and burden of maintaining a home and the money you can sink into it for maintenance, let alone remodeling. Then of course there’s the mortgage interest, property taxes, and insurance that you won’t have with a rental. Lastly, the biggest reason today’s generation is choosing to rent more and more is the flexibility. They want to be more mobile, able to get up and move without having to worry about listing, selling, and possibly owing more than the house is worth, and being stuck. We saw this with many residents that bought a home during residency and then tried to sell when they landed their first job, only to find out they owed more than it was worth because of the real estate market collapse of 2008. Approximately 50% of physicians will leave their first job within 1-3 years, so it’s a gamble that you’ll be able to sell your house when you want to for the price you need.
But what are the benefits of buying? First, there is the age-old argument of building equity. If you stay in your house long enough, and plan to sell it when you retire and downsize, it can definitely be an investment. If you keep a mortgage long enough, eventually you’ll pay it off, and have no payment. Mortgage interest is also tax-deductible. Lastly, there are the intangibles – like being able to put down roots, build a community, and have the ability to remodel and customize your home, a feature not usually available when renting.
Now that I’ve effectively talked you out and back in again, what’s the best way to go about buying a house in today’s crazy market? We’ve seen houses list and sell within a day and get multiple offers well over asking price. It’s a very difficult time to buy, but there are ways to make it easier. First, be clear on what you want in a house, especially location. There’s nothing worse than buyer’s remorse in your primary residence, and that happens more these days when people have to quickly make a decision. Find a good, fee-based financial planner to help you decide how much house fits in your budget and your long-term goals. You’d be surprised how easily we can talk ourselves into paying much more than we had originally decided. Use your financial planner to help avoid emotions getting involved and creeping up your price (especially when multiple offers are involved). A good rule of thumb is for a housing payment to be no more than 33% of your take-home income. This includes principal, interest, and taxes. Or, many planners will use the “2x income” rule of thumb. So if you make $300,000, don’t go over $600,000. Although with today’s low interest rates, there is some more wiggle room on that. Next, get a good referral from someone you trust in looking for a realtor. Real estate is a commission-based job and can have some potential conflicts of interest, so getting a recommendation can help. Or, there are realtors who will work for a flat fee, regardless of how much you pay for a house. That can help reduce the conflict.
You made the offer, you beat out the other bids, and you’re getting a house! How are you paying for it? Hopefully, you already got preapproved. This means finding a good, reputable mortgage professional. Get referrals, shop around, and take your time. Get multiple quotes before you start the underwriting process. They shouldn’t have to pull your credit to give you a fairly accurate estimate of what interest rate you’ll qualify for. Usually, they will give you fixed and variable interest quotes. I normally recommend fixed interest. Because interest rates are so low right now, they’re only going to go up in the future, and if you have a variable rate loan, your payment will go up as interest rates rise.
Is a physician loan a good idea? Depending on your circumstance, it can often be a good deal. Most new attendings don’t have cash saved up for a down payment, and these often don’t require one. And the other big benefit is that they won’t consider your student loan payments when calculating your debt-to-income ratio. Mortgage lenders will look at how much other debt you have when determining an approval. And a conventional mortgage will take your student loan payments into consideration, which means you’ll qualify for a much lower payment and purchase price. In my experience, you want a credit score of around 700 or higher to qualify for these types of loans. The only downside of a physician mortgage is the rates are slightly higher. I encourage you to start the application process early; they’re taking upward of 90 days lately.
As the mortgage is being processed, you’ll have an inspection and appraisal, and as long as those are all favorable, you’re in! Now, should you take any surplus income each month and pay extra on your mortgage to pay it down sooner, or invest it? Everyone’s situation is different, but a good rule of thumb is to look at interest rates on the debt you want to pay off versus expected rate of return on the potential investment. For example, if you have extra money, should you invest it in an SP500 index fund that historically gets 8%-12% per year, or put it on the principal of your mortgage that has an interest rate of 3%? Assuming no other factors or goals, and you just want the best bang for your buck, my money would go toward the investment getting 8%-12% over saving 3% on my mortgage.
Take your time, do your research, and find good professionals. There’s no right answer for everyone, but there are certainly some good practices when walking through the first home decision. Here at FinancialMD, we only work with physicians, and we’re happy to chat if you want some guidance on this. Send me an email and subscribe to our weekly Didactic Minute videos on YouTube for more financial tips for young physicians. Good luck!
Mr. Solitro is a financial planner and CEO of FinancialMD. He has no other conflicts of interest.
Investment advisory services offered through FinancialMD, a registered investment adviser. Registration as an investment adviser does not imply a certain level of skill or training. This article is provided for informational purposes only and nothing contained herein should be construed as a solicitation to buy or sell any products. Advisory services are offered only to clients and prospective clients in places where FinancialMD and its investment adviser representatives are registered or exempt from registration. Investing involves the risk of loss of principal. Past performance is no guarantee of future performance and no investment strategy can guarantee a profit or protect against loss.
This article was updated June 8, 2021.
Reference
1. Wachter S and Acolin A. Owning or Renting in the US: Shifting Dynamics of the Housing Market. Penn Institute for Urban Research. 2016 May.
The concept of home ownership has changed for this generation, not only in the logistics of the best way to do it, but also in the desire and demand for owning versus renting. According to the Penn Institute for Urban Research, the U.S. homeownership rate is now at 63.7%, the lowest in 48 years. “Homeownership rates have declined for all demographic age groups. Since 2006, the number of households who own their home in the United States has decreased by 674,000 while the number of renters has increased by over 8 million.”1
But isn’t owning your own home the American dream? Isn’t it a great investment? If we’re looking strictly at the monthly payment numbers, traditionally paying a mortgage is cheaper than paying rent for the same-size home. That became even more true as home prices dropped in 2007-2008 and as interest rates also dropped over the last several years. Then there’s the age-old concept of “building equity” or “throwing away money on rent.” As a financial planner, I get asked all the time about a home as an investment. It certainly can be, like any investment, if you buy and sell at the right time. But something is only an investment if it grows, and you can’t predict if you’re going to buy and sell your house at the right time. The median price of an existing home sold in March 2021 was $329,100. That’s a 17.2% increase from March 2020. Times are certainly crazy right now in the real estate market.
What many people don’t think about are the other costs, and not just financial costs. Today’s generation is more aware of the added stress and burden of maintaining a home and the money you can sink into it for maintenance, let alone remodeling. Then of course there’s the mortgage interest, property taxes, and insurance that you won’t have with a rental. Lastly, the biggest reason today’s generation is choosing to rent more and more is the flexibility. They want to be more mobile, able to get up and move without having to worry about listing, selling, and possibly owing more than the house is worth, and being stuck. We saw this with many residents that bought a home during residency and then tried to sell when they landed their first job, only to find out they owed more than it was worth because of the real estate market collapse of 2008. Approximately 50% of physicians will leave their first job within 1-3 years, so it’s a gamble that you’ll be able to sell your house when you want to for the price you need.
But what are the benefits of buying? First, there is the age-old argument of building equity. If you stay in your house long enough, and plan to sell it when you retire and downsize, it can definitely be an investment. If you keep a mortgage long enough, eventually you’ll pay it off, and have no payment. Mortgage interest is also tax-deductible. Lastly, there are the intangibles – like being able to put down roots, build a community, and have the ability to remodel and customize your home, a feature not usually available when renting.
Now that I’ve effectively talked you out and back in again, what’s the best way to go about buying a house in today’s crazy market? We’ve seen houses list and sell within a day and get multiple offers well over asking price. It’s a very difficult time to buy, but there are ways to make it easier. First, be clear on what you want in a house, especially location. There’s nothing worse than buyer’s remorse in your primary residence, and that happens more these days when people have to quickly make a decision. Find a good, fee-based financial planner to help you decide how much house fits in your budget and your long-term goals. You’d be surprised how easily we can talk ourselves into paying much more than we had originally decided. Use your financial planner to help avoid emotions getting involved and creeping up your price (especially when multiple offers are involved). A good rule of thumb is for a housing payment to be no more than 33% of your take-home income. This includes principal, interest, and taxes. Or, many planners will use the “2x income” rule of thumb. So if you make $300,000, don’t go over $600,000. Although with today’s low interest rates, there is some more wiggle room on that. Next, get a good referral from someone you trust in looking for a realtor. Real estate is a commission-based job and can have some potential conflicts of interest, so getting a recommendation can help. Or, there are realtors who will work for a flat fee, regardless of how much you pay for a house. That can help reduce the conflict.
You made the offer, you beat out the other bids, and you’re getting a house! How are you paying for it? Hopefully, you already got preapproved. This means finding a good, reputable mortgage professional. Get referrals, shop around, and take your time. Get multiple quotes before you start the underwriting process. They shouldn’t have to pull your credit to give you a fairly accurate estimate of what interest rate you’ll qualify for. Usually, they will give you fixed and variable interest quotes. I normally recommend fixed interest. Because interest rates are so low right now, they’re only going to go up in the future, and if you have a variable rate loan, your payment will go up as interest rates rise.
Is a physician loan a good idea? Depending on your circumstance, it can often be a good deal. Most new attendings don’t have cash saved up for a down payment, and these often don’t require one. And the other big benefit is that they won’t consider your student loan payments when calculating your debt-to-income ratio. Mortgage lenders will look at how much other debt you have when determining an approval. And a conventional mortgage will take your student loan payments into consideration, which means you’ll qualify for a much lower payment and purchase price. In my experience, you want a credit score of around 700 or higher to qualify for these types of loans. The only downside of a physician mortgage is the rates are slightly higher. I encourage you to start the application process early; they’re taking upward of 90 days lately.
As the mortgage is being processed, you’ll have an inspection and appraisal, and as long as those are all favorable, you’re in! Now, should you take any surplus income each month and pay extra on your mortgage to pay it down sooner, or invest it? Everyone’s situation is different, but a good rule of thumb is to look at interest rates on the debt you want to pay off versus expected rate of return on the potential investment. For example, if you have extra money, should you invest it in an SP500 index fund that historically gets 8%-12% per year, or put it on the principal of your mortgage that has an interest rate of 3%? Assuming no other factors or goals, and you just want the best bang for your buck, my money would go toward the investment getting 8%-12% over saving 3% on my mortgage.
Take your time, do your research, and find good professionals. There’s no right answer for everyone, but there are certainly some good practices when walking through the first home decision. Here at FinancialMD, we only work with physicians, and we’re happy to chat if you want some guidance on this. Send me an email and subscribe to our weekly Didactic Minute videos on YouTube for more financial tips for young physicians. Good luck!
Mr. Solitro is a financial planner and CEO of FinancialMD. He has no other conflicts of interest.
Investment advisory services offered through FinancialMD, a registered investment adviser. Registration as an investment adviser does not imply a certain level of skill or training. This article is provided for informational purposes only and nothing contained herein should be construed as a solicitation to buy or sell any products. Advisory services are offered only to clients and prospective clients in places where FinancialMD and its investment adviser representatives are registered or exempt from registration. Investing involves the risk of loss of principal. Past performance is no guarantee of future performance and no investment strategy can guarantee a profit or protect against loss.
This article was updated June 8, 2021.
Reference
1. Wachter S and Acolin A. Owning or Renting in the US: Shifting Dynamics of the Housing Market. Penn Institute for Urban Research. 2016 May.
The concept of home ownership has changed for this generation, not only in the logistics of the best way to do it, but also in the desire and demand for owning versus renting. According to the Penn Institute for Urban Research, the U.S. homeownership rate is now at 63.7%, the lowest in 48 years. “Homeownership rates have declined for all demographic age groups. Since 2006, the number of households who own their home in the United States has decreased by 674,000 while the number of renters has increased by over 8 million.”1
But isn’t owning your own home the American dream? Isn’t it a great investment? If we’re looking strictly at the monthly payment numbers, traditionally paying a mortgage is cheaper than paying rent for the same-size home. That became even more true as home prices dropped in 2007-2008 and as interest rates also dropped over the last several years. Then there’s the age-old concept of “building equity” or “throwing away money on rent.” As a financial planner, I get asked all the time about a home as an investment. It certainly can be, like any investment, if you buy and sell at the right time. But something is only an investment if it grows, and you can’t predict if you’re going to buy and sell your house at the right time. The median price of an existing home sold in March 2021 was $329,100. That’s a 17.2% increase from March 2020. Times are certainly crazy right now in the real estate market.
What many people don’t think about are the other costs, and not just financial costs. Today’s generation is more aware of the added stress and burden of maintaining a home and the money you can sink into it for maintenance, let alone remodeling. Then of course there’s the mortgage interest, property taxes, and insurance that you won’t have with a rental. Lastly, the biggest reason today’s generation is choosing to rent more and more is the flexibility. They want to be more mobile, able to get up and move without having to worry about listing, selling, and possibly owing more than the house is worth, and being stuck. We saw this with many residents that bought a home during residency and then tried to sell when they landed their first job, only to find out they owed more than it was worth because of the real estate market collapse of 2008. Approximately 50% of physicians will leave their first job within 1-3 years, so it’s a gamble that you’ll be able to sell your house when you want to for the price you need.
But what are the benefits of buying? First, there is the age-old argument of building equity. If you stay in your house long enough, and plan to sell it when you retire and downsize, it can definitely be an investment. If you keep a mortgage long enough, eventually you’ll pay it off, and have no payment. Mortgage interest is also tax-deductible. Lastly, there are the intangibles – like being able to put down roots, build a community, and have the ability to remodel and customize your home, a feature not usually available when renting.
Now that I’ve effectively talked you out and back in again, what’s the best way to go about buying a house in today’s crazy market? We’ve seen houses list and sell within a day and get multiple offers well over asking price. It’s a very difficult time to buy, but there are ways to make it easier. First, be clear on what you want in a house, especially location. There’s nothing worse than buyer’s remorse in your primary residence, and that happens more these days when people have to quickly make a decision. Find a good, fee-based financial planner to help you decide how much house fits in your budget and your long-term goals. You’d be surprised how easily we can talk ourselves into paying much more than we had originally decided. Use your financial planner to help avoid emotions getting involved and creeping up your price (especially when multiple offers are involved). A good rule of thumb is for a housing payment to be no more than 33% of your take-home income. This includes principal, interest, and taxes. Or, many planners will use the “2x income” rule of thumb. So if you make $300,000, don’t go over $600,000. Although with today’s low interest rates, there is some more wiggle room on that. Next, get a good referral from someone you trust in looking for a realtor. Real estate is a commission-based job and can have some potential conflicts of interest, so getting a recommendation can help. Or, there are realtors who will work for a flat fee, regardless of how much you pay for a house. That can help reduce the conflict.
You made the offer, you beat out the other bids, and you’re getting a house! How are you paying for it? Hopefully, you already got preapproved. This means finding a good, reputable mortgage professional. Get referrals, shop around, and take your time. Get multiple quotes before you start the underwriting process. They shouldn’t have to pull your credit to give you a fairly accurate estimate of what interest rate you’ll qualify for. Usually, they will give you fixed and variable interest quotes. I normally recommend fixed interest. Because interest rates are so low right now, they’re only going to go up in the future, and if you have a variable rate loan, your payment will go up as interest rates rise.
Is a physician loan a good idea? Depending on your circumstance, it can often be a good deal. Most new attendings don’t have cash saved up for a down payment, and these often don’t require one. And the other big benefit is that they won’t consider your student loan payments when calculating your debt-to-income ratio. Mortgage lenders will look at how much other debt you have when determining an approval. And a conventional mortgage will take your student loan payments into consideration, which means you’ll qualify for a much lower payment and purchase price. In my experience, you want a credit score of around 700 or higher to qualify for these types of loans. The only downside of a physician mortgage is the rates are slightly higher. I encourage you to start the application process early; they’re taking upward of 90 days lately.
As the mortgage is being processed, you’ll have an inspection and appraisal, and as long as those are all favorable, you’re in! Now, should you take any surplus income each month and pay extra on your mortgage to pay it down sooner, or invest it? Everyone’s situation is different, but a good rule of thumb is to look at interest rates on the debt you want to pay off versus expected rate of return on the potential investment. For example, if you have extra money, should you invest it in an SP500 index fund that historically gets 8%-12% per year, or put it on the principal of your mortgage that has an interest rate of 3%? Assuming no other factors or goals, and you just want the best bang for your buck, my money would go toward the investment getting 8%-12% over saving 3% on my mortgage.
Take your time, do your research, and find good professionals. There’s no right answer for everyone, but there are certainly some good practices when walking through the first home decision. Here at FinancialMD, we only work with physicians, and we’re happy to chat if you want some guidance on this. Send me an email and subscribe to our weekly Didactic Minute videos on YouTube for more financial tips for young physicians. Good luck!
Mr. Solitro is a financial planner and CEO of FinancialMD. He has no other conflicts of interest.
Investment advisory services offered through FinancialMD, a registered investment adviser. Registration as an investment adviser does not imply a certain level of skill or training. This article is provided for informational purposes only and nothing contained herein should be construed as a solicitation to buy or sell any products. Advisory services are offered only to clients and prospective clients in places where FinancialMD and its investment adviser representatives are registered or exempt from registration. Investing involves the risk of loss of principal. Past performance is no guarantee of future performance and no investment strategy can guarantee a profit or protect against loss.
This article was updated June 8, 2021.
Reference
1. Wachter S and Acolin A. Owning or Renting in the US: Shifting Dynamics of the Housing Market. Penn Institute for Urban Research. 2016 May.
MPL, microaggressions, and more
Dear colleagues,
Welcome to the May edition of The New Gastroenterologist, which is packed with a fantastic line-up of articles! The 1-year mark of the pandemic recently passed, and we now are gearing up for our second virtual Digestive Disease Week (DDW®). While we are all anxious to return to lives that have some semblance of normalcy, we continue to endure the ebbs and flows that characterize life in a pandemic. For over a year now, we spend our days caught in a constant battle between the risk and reward of activities we previously took for granted or considered mundane. Our moods vacillate with the continued rise and fall of COVID-19 cases, but the advent and distribution of vaccines have offered palpable hope for better outcomes.
I’m pleased to introduce this quarter’s content – beginning with our legal section. Dr. John Azizian (UCLA-Olive-View), Dr. James Tabibian (UCLA-Olive-View), Dr. Camellia Dalai (UCLA-Olive-View/University of New Mexico), and Dr. Megan Adams (University of Michigan) contribute a comprehensive piece on medical professional liability (MPL), a topic that is seldom discussed in training but has important implications in clinical practice. This article reviews basic legal concepts, recent trends and details on gastroenterology specific claims, and most importantly, advice on how to mitigate MPL risk as gastroenterologists.
Many trainees and early career gastroenterologists face microaggressions for a variety of different reasons. Dr. Oveia Aktopaire and Dr. Rachel Issaka (University of Washington) present a thought-provoking piece as they delve into structural racism in medicine and how microaggressions are a proxy for bias.
Dyssynergic defecation (DD) affects up to one-half of patients with chronic constipation. The “In Focus” feature for May provides an excellent review of DD written by international expert Dr. Satish Rao and Dr. Asad Jehangir (both, Medical College of Georgia/Augusta University). The article provides guidance on the diagnosis of DD, including high-yield features of physical and digital rectal exams, guidance on interpretation of anorectal manometry testing, and how these can dictate an effective therapeutic plan.
Meaningful mentorship is crucial for young gastroenterologists but at times the nature of the mentor-mentee relationship can be difficult to navigate. Dr. David Fessell and Bridger Rodoni (University of Michigan) explore this dynamic and discuss the notion of mentorship malpractice in a compelling addition to our ethics case series.
Abdominal wall pain is common yet often overlooked diagnosis and a great teaching point for trainees. Dr. Manish Singla (Uniformed Services University/Capital Digestive Care) and Dr. Brian Park (Naval Medical Center) discuss the diagnosis and management and how the early recognition of abdominal wall pain can save both patients and clinicians from a battery of unnecessary diagnostic testing.
The DHPA Private Practice Perspectives article this quarter, written by Dr. Aja McCutchen (Atlanta Gastroenterology Associates), addresses colorectal cancer screening, the disparities that exist, and the important role we have as gastroenterologists in reducing barriers to screening. Lastly, Dr. Bilal Asif (University of Maryland/National Institutes of Health) walks us through a fellow’s perspective on the AGA’s first virtual Advocacy Day – demonstrating that advocacy is still possible even as a trainee and in the setting of a pandemic.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
Welcome to the May edition of The New Gastroenterologist, which is packed with a fantastic line-up of articles! The 1-year mark of the pandemic recently passed, and we now are gearing up for our second virtual Digestive Disease Week (DDW®). While we are all anxious to return to lives that have some semblance of normalcy, we continue to endure the ebbs and flows that characterize life in a pandemic. For over a year now, we spend our days caught in a constant battle between the risk and reward of activities we previously took for granted or considered mundane. Our moods vacillate with the continued rise and fall of COVID-19 cases, but the advent and distribution of vaccines have offered palpable hope for better outcomes.
I’m pleased to introduce this quarter’s content – beginning with our legal section. Dr. John Azizian (UCLA-Olive-View), Dr. James Tabibian (UCLA-Olive-View), Dr. Camellia Dalai (UCLA-Olive-View/University of New Mexico), and Dr. Megan Adams (University of Michigan) contribute a comprehensive piece on medical professional liability (MPL), a topic that is seldom discussed in training but has important implications in clinical practice. This article reviews basic legal concepts, recent trends and details on gastroenterology specific claims, and most importantly, advice on how to mitigate MPL risk as gastroenterologists.
Many trainees and early career gastroenterologists face microaggressions for a variety of different reasons. Dr. Oveia Aktopaire and Dr. Rachel Issaka (University of Washington) present a thought-provoking piece as they delve into structural racism in medicine and how microaggressions are a proxy for bias.
Dyssynergic defecation (DD) affects up to one-half of patients with chronic constipation. The “In Focus” feature for May provides an excellent review of DD written by international expert Dr. Satish Rao and Dr. Asad Jehangir (both, Medical College of Georgia/Augusta University). The article provides guidance on the diagnosis of DD, including high-yield features of physical and digital rectal exams, guidance on interpretation of anorectal manometry testing, and how these can dictate an effective therapeutic plan.
Meaningful mentorship is crucial for young gastroenterologists but at times the nature of the mentor-mentee relationship can be difficult to navigate. Dr. David Fessell and Bridger Rodoni (University of Michigan) explore this dynamic and discuss the notion of mentorship malpractice in a compelling addition to our ethics case series.
Abdominal wall pain is common yet often overlooked diagnosis and a great teaching point for trainees. Dr. Manish Singla (Uniformed Services University/Capital Digestive Care) and Dr. Brian Park (Naval Medical Center) discuss the diagnosis and management and how the early recognition of abdominal wall pain can save both patients and clinicians from a battery of unnecessary diagnostic testing.
The DHPA Private Practice Perspectives article this quarter, written by Dr. Aja McCutchen (Atlanta Gastroenterology Associates), addresses colorectal cancer screening, the disparities that exist, and the important role we have as gastroenterologists in reducing barriers to screening. Lastly, Dr. Bilal Asif (University of Maryland/National Institutes of Health) walks us through a fellow’s perspective on the AGA’s first virtual Advocacy Day – demonstrating that advocacy is still possible even as a trainee and in the setting of a pandemic.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
Welcome to the May edition of The New Gastroenterologist, which is packed with a fantastic line-up of articles! The 1-year mark of the pandemic recently passed, and we now are gearing up for our second virtual Digestive Disease Week (DDW®). While we are all anxious to return to lives that have some semblance of normalcy, we continue to endure the ebbs and flows that characterize life in a pandemic. For over a year now, we spend our days caught in a constant battle between the risk and reward of activities we previously took for granted or considered mundane. Our moods vacillate with the continued rise and fall of COVID-19 cases, but the advent and distribution of vaccines have offered palpable hope for better outcomes.
I’m pleased to introduce this quarter’s content – beginning with our legal section. Dr. John Azizian (UCLA-Olive-View), Dr. James Tabibian (UCLA-Olive-View), Dr. Camellia Dalai (UCLA-Olive-View/University of New Mexico), and Dr. Megan Adams (University of Michigan) contribute a comprehensive piece on medical professional liability (MPL), a topic that is seldom discussed in training but has important implications in clinical practice. This article reviews basic legal concepts, recent trends and details on gastroenterology specific claims, and most importantly, advice on how to mitigate MPL risk as gastroenterologists.
Many trainees and early career gastroenterologists face microaggressions for a variety of different reasons. Dr. Oveia Aktopaire and Dr. Rachel Issaka (University of Washington) present a thought-provoking piece as they delve into structural racism in medicine and how microaggressions are a proxy for bias.
Dyssynergic defecation (DD) affects up to one-half of patients with chronic constipation. The “In Focus” feature for May provides an excellent review of DD written by international expert Dr. Satish Rao and Dr. Asad Jehangir (both, Medical College of Georgia/Augusta University). The article provides guidance on the diagnosis of DD, including high-yield features of physical and digital rectal exams, guidance on interpretation of anorectal manometry testing, and how these can dictate an effective therapeutic plan.
Meaningful mentorship is crucial for young gastroenterologists but at times the nature of the mentor-mentee relationship can be difficult to navigate. Dr. David Fessell and Bridger Rodoni (University of Michigan) explore this dynamic and discuss the notion of mentorship malpractice in a compelling addition to our ethics case series.
Abdominal wall pain is common yet often overlooked diagnosis and a great teaching point for trainees. Dr. Manish Singla (Uniformed Services University/Capital Digestive Care) and Dr. Brian Park (Naval Medical Center) discuss the diagnosis and management and how the early recognition of abdominal wall pain can save both patients and clinicians from a battery of unnecessary diagnostic testing.
The DHPA Private Practice Perspectives article this quarter, written by Dr. Aja McCutchen (Atlanta Gastroenterology Associates), addresses colorectal cancer screening, the disparities that exist, and the important role we have as gastroenterologists in reducing barriers to screening. Lastly, Dr. Bilal Asif (University of Maryland/National Institutes of Health) walks us through a fellow’s perspective on the AGA’s first virtual Advocacy Day – demonstrating that advocacy is still possible even as a trainee and in the setting of a pandemic.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition