User login
Letters to the Editor
Mohamed‐Kalib et al. illustrate 2 important caveats to penicillin skin testing (PST): (1) there is an exceptionally rare potential for resensitization, a phenomenon in which a previously reactive patient is proven tolerant, then develops sensitivity and has a positive PST; (2) consider repeating PST prior to a parenteral ‐lactam prescription in patients who previously reported severe anaphylactic reactions.
Our negative predictive value of 100% does not abate the tentative concern for resensitization.[1] Similar to the likelihood of becoming allergic initially, 0% to 3.2% of PST‐negative patients can become allergic again, more commonly with parenteral therapy and among children.[2, 3, 4]
The author describes a seemingly resensitized patient who reacted in an outpatient setting. Theoretically, anyone could resensitize, regardless of their setting or whether a single dose or full course was given after the PST. Individuals with a proven tolerance by PST and repeated courses are at a very low risk of future immunoglobulin E‐mediated reactions, a risk similar to that of the general population.
Whether previously reactive or not, patients receiving medicinal therapies should always be monitored for allergic reactions. Although PST may not be prudent in the minority of patients who report recent or severe reactions, a repeat PST prior to prescribing parenteral ‐lactam may potentially avoid instances described by Mohamed‐Kalib et al.
- , , , et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):342–345.
- , , . Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162(7):822–826.
- . Elective penicillin skin testing and amoxicillin challenge: effect on outpatient antibiotic use, cost, and clinical outcomes. J Allergy Clin Immunol. 1998;102(2):281–285.
- , . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–273.
Mohamed‐Kalib et al. illustrate 2 important caveats to penicillin skin testing (PST): (1) there is an exceptionally rare potential for resensitization, a phenomenon in which a previously reactive patient is proven tolerant, then develops sensitivity and has a positive PST; (2) consider repeating PST prior to a parenteral ‐lactam prescription in patients who previously reported severe anaphylactic reactions.
Our negative predictive value of 100% does not abate the tentative concern for resensitization.[1] Similar to the likelihood of becoming allergic initially, 0% to 3.2% of PST‐negative patients can become allergic again, more commonly with parenteral therapy and among children.[2, 3, 4]
The author describes a seemingly resensitized patient who reacted in an outpatient setting. Theoretically, anyone could resensitize, regardless of their setting or whether a single dose or full course was given after the PST. Individuals with a proven tolerance by PST and repeated courses are at a very low risk of future immunoglobulin E‐mediated reactions, a risk similar to that of the general population.
Whether previously reactive or not, patients receiving medicinal therapies should always be monitored for allergic reactions. Although PST may not be prudent in the minority of patients who report recent or severe reactions, a repeat PST prior to prescribing parenteral ‐lactam may potentially avoid instances described by Mohamed‐Kalib et al.
Mohamed‐Kalib et al. illustrate 2 important caveats to penicillin skin testing (PST): (1) there is an exceptionally rare potential for resensitization, a phenomenon in which a previously reactive patient is proven tolerant, then develops sensitivity and has a positive PST; (2) consider repeating PST prior to a parenteral ‐lactam prescription in patients who previously reported severe anaphylactic reactions.
Our negative predictive value of 100% does not abate the tentative concern for resensitization.[1] Similar to the likelihood of becoming allergic initially, 0% to 3.2% of PST‐negative patients can become allergic again, more commonly with parenteral therapy and among children.[2, 3, 4]
The author describes a seemingly resensitized patient who reacted in an outpatient setting. Theoretically, anyone could resensitize, regardless of their setting or whether a single dose or full course was given after the PST. Individuals with a proven tolerance by PST and repeated courses are at a very low risk of future immunoglobulin E‐mediated reactions, a risk similar to that of the general population.
Whether previously reactive or not, patients receiving medicinal therapies should always be monitored for allergic reactions. Although PST may not be prudent in the minority of patients who report recent or severe reactions, a repeat PST prior to prescribing parenteral ‐lactam may potentially avoid instances described by Mohamed‐Kalib et al.
- , , , et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):342–345.
- , , . Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162(7):822–826.
- . Elective penicillin skin testing and amoxicillin challenge: effect on outpatient antibiotic use, cost, and clinical outcomes. J Allergy Clin Immunol. 1998;102(2):281–285.
- , . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–273.
- , , , et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):342–345.
- , , . Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162(7):822–826.
- . Elective penicillin skin testing and amoxicillin challenge: effect on outpatient antibiotic use, cost, and clinical outcomes. J Allergy Clin Immunol. 1998;102(2):281–285.
- , . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–273.
Comment on “The impact of penicillin skin testing on clinical practice and antimicrobial stewardship”
We read with interest the report by Rimawi et al.[1] They showed convincing evidence that with a negative penicillin skin test, a course of ‐lactam is safe 2 hours after a negative challenge. However, we advise caution in generalizing these data to the outpatient setting where resensitization is a possibility. One study showed that 4.9% of patients who had negative skin tests and drug challenges reacted on rechallenges 3 weeks later.[2]
In our center, ‐lactam allergy assessment is carried out according to European Academy of Allergy and Clinical Immunology guidelines.[3] We encountered a patient who had life‐threatening anaphylaxis with co‐amoxiclav 1 month after negative allergy investigations.
A 43‐year‐old woman was referred with a history of non‐drug related urticarial episodes and urticaria and angioedema of face, neck, and arms 30 minutes after a first dose of oral co‐amoxiclav 2 years previously. Specific immunoglobulin E tests to penicillin and amoxicillin, skin tests, and oral co‐amoxiclav challenge were negative. A month later, she developed anaphylaxis (intraoral angioedema, wheeze, hypotension [70/30 mm Hg], oxygen desaturation to 60% on room air, becoming unresponsive) within minutes of an intravenous dose of co‐amoxiclav for acute cholecystitis.
Our case illustrates that despite a detailed negative allergy assessment, severe anaphylaxis can occur requiring prompt identification and appropriate treatment.
- , , , et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):342–345.
- , , , et al. J Investig Allergol Clin Immunol. 2012;22(1):41–47.
- , , , et al. Diagnosis of immediate allergic reactions to beta‐lactam antibiotics. Allergy. 2003;58:961–972.
We read with interest the report by Rimawi et al.[1] They showed convincing evidence that with a negative penicillin skin test, a course of ‐lactam is safe 2 hours after a negative challenge. However, we advise caution in generalizing these data to the outpatient setting where resensitization is a possibility. One study showed that 4.9% of patients who had negative skin tests and drug challenges reacted on rechallenges 3 weeks later.[2]
In our center, ‐lactam allergy assessment is carried out according to European Academy of Allergy and Clinical Immunology guidelines.[3] We encountered a patient who had life‐threatening anaphylaxis with co‐amoxiclav 1 month after negative allergy investigations.
A 43‐year‐old woman was referred with a history of non‐drug related urticarial episodes and urticaria and angioedema of face, neck, and arms 30 minutes after a first dose of oral co‐amoxiclav 2 years previously. Specific immunoglobulin E tests to penicillin and amoxicillin, skin tests, and oral co‐amoxiclav challenge were negative. A month later, she developed anaphylaxis (intraoral angioedema, wheeze, hypotension [70/30 mm Hg], oxygen desaturation to 60% on room air, becoming unresponsive) within minutes of an intravenous dose of co‐amoxiclav for acute cholecystitis.
Our case illustrates that despite a detailed negative allergy assessment, severe anaphylaxis can occur requiring prompt identification and appropriate treatment.
We read with interest the report by Rimawi et al.[1] They showed convincing evidence that with a negative penicillin skin test, a course of ‐lactam is safe 2 hours after a negative challenge. However, we advise caution in generalizing these data to the outpatient setting where resensitization is a possibility. One study showed that 4.9% of patients who had negative skin tests and drug challenges reacted on rechallenges 3 weeks later.[2]
In our center, ‐lactam allergy assessment is carried out according to European Academy of Allergy and Clinical Immunology guidelines.[3] We encountered a patient who had life‐threatening anaphylaxis with co‐amoxiclav 1 month after negative allergy investigations.
A 43‐year‐old woman was referred with a history of non‐drug related urticarial episodes and urticaria and angioedema of face, neck, and arms 30 minutes after a first dose of oral co‐amoxiclav 2 years previously. Specific immunoglobulin E tests to penicillin and amoxicillin, skin tests, and oral co‐amoxiclav challenge were negative. A month later, she developed anaphylaxis (intraoral angioedema, wheeze, hypotension [70/30 mm Hg], oxygen desaturation to 60% on room air, becoming unresponsive) within minutes of an intravenous dose of co‐amoxiclav for acute cholecystitis.
Our case illustrates that despite a detailed negative allergy assessment, severe anaphylaxis can occur requiring prompt identification and appropriate treatment.
- , , , et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):342–345.
- , , , et al. J Investig Allergol Clin Immunol. 2012;22(1):41–47.
- , , , et al. Diagnosis of immediate allergic reactions to beta‐lactam antibiotics. Allergy. 2003;58:961–972.
- , , , et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):342–345.
- , , , et al. J Investig Allergol Clin Immunol. 2012;22(1):41–47.
- , , , et al. Diagnosis of immediate allergic reactions to beta‐lactam antibiotics. Allergy. 2003;58:961–972.
The FREEDOM trial
To the Editor: We would like to raise the following points about the paper by Dr. Aggarwal et al1 interpreting the Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial.2
The patients enrolled in the FREEDOM trial do not in our opinion completely reflect the real patients that we meet in our daily “real-world” practice.2 The patients in the FREEDOM trial did not have a high-risk profile. Rather, the mean European System for Cardiac Operative Risk Evaluation score (EuroSCORE) was 2.7 ± 2.4 in the percutaneous coronary intervention (PCI) group and 2.8 ± 2.5 in the coronary artery bypass grafting group—whereas a score of 5 or more on the EuroSCORE is associated with decreased rates of survival.2
Furthermore, patients with left main coronary artery stenosis were completely excluded from the FREEDOM trial,2 but this type of stenosis, with different grades, is found in about 30% of diabetic patients with multivessel coronary artery disease, a fact that may significantly influence the decision regarding the revascularization strategy (bypass grafting or PCI), especially in a clinical setting such as acute coronary syndrome.3–5
In addition, the authors did not clearly highlight that diabetes mellitus is an independent risk factor for coronary lesion progression, coronary bypass graft occlusion, and cardiac mortality after bypass grafting surgery.6–8 Clinical outcomes after bypass grafting in diabetic patients are worse than in nondiabetic patients; diabetic patients have higher rates of morbidity (deep sternal instability, wound infection, stroke, renal dysfunction, and respiratory problems), longer intensive care unit and hospital stays, and poorer postoperative physical functioning and quality of life.6–8
The authors correctly explain the reasons for the superiority of coronary artery bypass grafting vs PCI in diabetic patients, either by the ability to achieve complete revascularization or by using more arterial grafts, and especially the left internal thoracic artery.1 However, clarifying details on the strategy of revascularization in the FREEDOM trial are scarcely provided.2 All we know from the provided details in this regard is that “for CABG surgery, arterial revascularization was encouraged” and 94.4% of the patients undergoing bypass grafting received left internal thoracic artery grafts.2
In addition, whereas off-pump coronary artery bypass grafting surgery is superior to conventional bypass grafting in terms of lower rates of death and major adverse cardiac and cerebrovascular events in diabetic patients with multivessel coronary artery disease,3 only 165 (18.5%) of the 893 patients who underwent bypass grafting in the FREEDOM trial underwent an off-pump procedure.2,3
Therefore, all these considerations should be taken into account as the physician team discusses the therapeutic options (PCI and bypass grafting surgery) with diabetic patients who have multivessel coronary artery disease.
- Aggarwal B, Goel S, Sabik JF, Shishehbor MH. The FREEDOM trial: in appropriate patients with diabetes and multivessel coronary artery disease, CABG beats PCI. Cleve Clin J Med 2013; 80:515–523.
- Farkouh ME, Domanski M, Sleeper LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Emmert MY, Salzberg SP, Seifert B, et al. Is off-pump superior to conventional coronary artery bypass grafting in diabetic patients with multivessel disease? Eur J Cardiothorac Surg 2011; 40:233–239.
- Perrier S, Kindo M, Gerelli S, Mazzucotelli JP. Coronary artery bypass grafting or percutaneous revascularization in acute myocardial infarction? Interact Cardiovasc Thorac Surg 2013 Aug 20 [Epub ahead of print]
- Sabik JF, Blackstone EH, Firstenberg M, Lytle BW. A benchmark for evaluating innovative treatment of left main coronary disease. Circulation 2007; 116(11 Suppl):I232–I239.
- Lu JC, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Euro J Cardiothorac Surg 2003; 23:943–949.
- Ji Q, Mei Y, Wang X, Feng J, Cai J, Sun Y. Impact of diabetes mellitus on old patients undergoing coronary artery bypass grafting. Int Heart J 2009; 50:693–700.
- Stevens LM, Carrier M, Perrault LP, et al. Influence of diabetes and bilateral internal thoracic artery grafts on long-term outcome for multivessel coronary artery bypass grafting. Eur J Cardiothorac Surg 2005; 27:281–288.
To the Editor: We would like to raise the following points about the paper by Dr. Aggarwal et al1 interpreting the Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial.2
The patients enrolled in the FREEDOM trial do not in our opinion completely reflect the real patients that we meet in our daily “real-world” practice.2 The patients in the FREEDOM trial did not have a high-risk profile. Rather, the mean European System for Cardiac Operative Risk Evaluation score (EuroSCORE) was 2.7 ± 2.4 in the percutaneous coronary intervention (PCI) group and 2.8 ± 2.5 in the coronary artery bypass grafting group—whereas a score of 5 or more on the EuroSCORE is associated with decreased rates of survival.2
Furthermore, patients with left main coronary artery stenosis were completely excluded from the FREEDOM trial,2 but this type of stenosis, with different grades, is found in about 30% of diabetic patients with multivessel coronary artery disease, a fact that may significantly influence the decision regarding the revascularization strategy (bypass grafting or PCI), especially in a clinical setting such as acute coronary syndrome.3–5
In addition, the authors did not clearly highlight that diabetes mellitus is an independent risk factor for coronary lesion progression, coronary bypass graft occlusion, and cardiac mortality after bypass grafting surgery.6–8 Clinical outcomes after bypass grafting in diabetic patients are worse than in nondiabetic patients; diabetic patients have higher rates of morbidity (deep sternal instability, wound infection, stroke, renal dysfunction, and respiratory problems), longer intensive care unit and hospital stays, and poorer postoperative physical functioning and quality of life.6–8
The authors correctly explain the reasons for the superiority of coronary artery bypass grafting vs PCI in diabetic patients, either by the ability to achieve complete revascularization or by using more arterial grafts, and especially the left internal thoracic artery.1 However, clarifying details on the strategy of revascularization in the FREEDOM trial are scarcely provided.2 All we know from the provided details in this regard is that “for CABG surgery, arterial revascularization was encouraged” and 94.4% of the patients undergoing bypass grafting received left internal thoracic artery grafts.2
In addition, whereas off-pump coronary artery bypass grafting surgery is superior to conventional bypass grafting in terms of lower rates of death and major adverse cardiac and cerebrovascular events in diabetic patients with multivessel coronary artery disease,3 only 165 (18.5%) of the 893 patients who underwent bypass grafting in the FREEDOM trial underwent an off-pump procedure.2,3
Therefore, all these considerations should be taken into account as the physician team discusses the therapeutic options (PCI and bypass grafting surgery) with diabetic patients who have multivessel coronary artery disease.
To the Editor: We would like to raise the following points about the paper by Dr. Aggarwal et al1 interpreting the Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial.2
The patients enrolled in the FREEDOM trial do not in our opinion completely reflect the real patients that we meet in our daily “real-world” practice.2 The patients in the FREEDOM trial did not have a high-risk profile. Rather, the mean European System for Cardiac Operative Risk Evaluation score (EuroSCORE) was 2.7 ± 2.4 in the percutaneous coronary intervention (PCI) group and 2.8 ± 2.5 in the coronary artery bypass grafting group—whereas a score of 5 or more on the EuroSCORE is associated with decreased rates of survival.2
Furthermore, patients with left main coronary artery stenosis were completely excluded from the FREEDOM trial,2 but this type of stenosis, with different grades, is found in about 30% of diabetic patients with multivessel coronary artery disease, a fact that may significantly influence the decision regarding the revascularization strategy (bypass grafting or PCI), especially in a clinical setting such as acute coronary syndrome.3–5
In addition, the authors did not clearly highlight that diabetes mellitus is an independent risk factor for coronary lesion progression, coronary bypass graft occlusion, and cardiac mortality after bypass grafting surgery.6–8 Clinical outcomes after bypass grafting in diabetic patients are worse than in nondiabetic patients; diabetic patients have higher rates of morbidity (deep sternal instability, wound infection, stroke, renal dysfunction, and respiratory problems), longer intensive care unit and hospital stays, and poorer postoperative physical functioning and quality of life.6–8
The authors correctly explain the reasons for the superiority of coronary artery bypass grafting vs PCI in diabetic patients, either by the ability to achieve complete revascularization or by using more arterial grafts, and especially the left internal thoracic artery.1 However, clarifying details on the strategy of revascularization in the FREEDOM trial are scarcely provided.2 All we know from the provided details in this regard is that “for CABG surgery, arterial revascularization was encouraged” and 94.4% of the patients undergoing bypass grafting received left internal thoracic artery grafts.2
In addition, whereas off-pump coronary artery bypass grafting surgery is superior to conventional bypass grafting in terms of lower rates of death and major adverse cardiac and cerebrovascular events in diabetic patients with multivessel coronary artery disease,3 only 165 (18.5%) of the 893 patients who underwent bypass grafting in the FREEDOM trial underwent an off-pump procedure.2,3
Therefore, all these considerations should be taken into account as the physician team discusses the therapeutic options (PCI and bypass grafting surgery) with diabetic patients who have multivessel coronary artery disease.
- Aggarwal B, Goel S, Sabik JF, Shishehbor MH. The FREEDOM trial: in appropriate patients with diabetes and multivessel coronary artery disease, CABG beats PCI. Cleve Clin J Med 2013; 80:515–523.
- Farkouh ME, Domanski M, Sleeper LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Emmert MY, Salzberg SP, Seifert B, et al. Is off-pump superior to conventional coronary artery bypass grafting in diabetic patients with multivessel disease? Eur J Cardiothorac Surg 2011; 40:233–239.
- Perrier S, Kindo M, Gerelli S, Mazzucotelli JP. Coronary artery bypass grafting or percutaneous revascularization in acute myocardial infarction? Interact Cardiovasc Thorac Surg 2013 Aug 20 [Epub ahead of print]
- Sabik JF, Blackstone EH, Firstenberg M, Lytle BW. A benchmark for evaluating innovative treatment of left main coronary disease. Circulation 2007; 116(11 Suppl):I232–I239.
- Lu JC, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Euro J Cardiothorac Surg 2003; 23:943–949.
- Ji Q, Mei Y, Wang X, Feng J, Cai J, Sun Y. Impact of diabetes mellitus on old patients undergoing coronary artery bypass grafting. Int Heart J 2009; 50:693–700.
- Stevens LM, Carrier M, Perrault LP, et al. Influence of diabetes and bilateral internal thoracic artery grafts on long-term outcome for multivessel coronary artery bypass grafting. Eur J Cardiothorac Surg 2005; 27:281–288.
- Aggarwal B, Goel S, Sabik JF, Shishehbor MH. The FREEDOM trial: in appropriate patients with diabetes and multivessel coronary artery disease, CABG beats PCI. Cleve Clin J Med 2013; 80:515–523.
- Farkouh ME, Domanski M, Sleeper LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Emmert MY, Salzberg SP, Seifert B, et al. Is off-pump superior to conventional coronary artery bypass grafting in diabetic patients with multivessel disease? Eur J Cardiothorac Surg 2011; 40:233–239.
- Perrier S, Kindo M, Gerelli S, Mazzucotelli JP. Coronary artery bypass grafting or percutaneous revascularization in acute myocardial infarction? Interact Cardiovasc Thorac Surg 2013 Aug 20 [Epub ahead of print]
- Sabik JF, Blackstone EH, Firstenberg M, Lytle BW. A benchmark for evaluating innovative treatment of left main coronary disease. Circulation 2007; 116(11 Suppl):I232–I239.
- Lu JC, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Euro J Cardiothorac Surg 2003; 23:943–949.
- Ji Q, Mei Y, Wang X, Feng J, Cai J, Sun Y. Impact of diabetes mellitus on old patients undergoing coronary artery bypass grafting. Int Heart J 2009; 50:693–700.
- Stevens LM, Carrier M, Perrault LP, et al. Influence of diabetes and bilateral internal thoracic artery grafts on long-term outcome for multivessel coronary artery bypass grafting. Eur J Cardiothorac Surg 2005; 27:281–288.
In reply: The FREEDOM trial
In Reply: We appreciate the comments of Dr. Saeed and colleagues. As stated in our article, given that the patients included in the FREEDOM trial represent a select group with diabetes and multivessel coronary artery disease, they may not represent all patients encountered in a real-world setting. We highlighted that only 10% of the patients screened were included for randomization, which limits the generalizability of the results. Also, the overall patient population may not be at high risk, as evidenced by low mean EuroSCORE and SYNTAX scores and by the low proportion of patients with ejection fractions less than 40%. However, patients with left main coronary artery disease (even without diabetes) have been shown to have better outcomes with coronary artery bypass grafting than with PCI, although a head-to-head trial in a diabetic subgroup is currently not available.1,2 In addition, it is important to realize that the FREEDOM trial deals with stable angina; therefore, the results may not extend to patients with acute coronary syndrome wherein primary PCI remains the most feasible option in most cases.
Diabetes mellitus is independently associated with complex, accelerated, and multifocal coronary artery disease. Therefore, outcomes after revascularization (with bypass grafting or PCI) are worse in diabetic patients than in those without diabetes. However, this association does not prove the superiority of PCI over bypass grafting.
As we stated in our paper, the FREEDOM trial did not clearly define the strategy for arterial grafts in patients undergoing bypass grafting. The mean number of coronary lesions in the bypass grafting group was high (mean = 5.74), but the average number of grafts used was only 2.9, and data were not provided on the use of sequential grafting and multiple arterial conduits. Lastly, it is true that the FREEDOM trial had relatively fewer patients (18.5%) that underwent off-pump bypass grafting surgery; however, this approach has never been shown to be superior in large randomized trials.3,4
In conclusion, no randomized trial should replace clinical judgment to define the targeted revascularization strategy for an individual patient. Rather, results from the FREEDOM trial should help support clinical decision-making in the context of the patient and the institution.
- Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Diegeler A, Börgermann J, Kappert U, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med 2013; 368:1189–1198.
- Lamy A, Devereaux PJ, Prabhakaran D, et al; CORONARY Investigators. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med 2013; 368:1179–1188.
In Reply: We appreciate the comments of Dr. Saeed and colleagues. As stated in our article, given that the patients included in the FREEDOM trial represent a select group with diabetes and multivessel coronary artery disease, they may not represent all patients encountered in a real-world setting. We highlighted that only 10% of the patients screened were included for randomization, which limits the generalizability of the results. Also, the overall patient population may not be at high risk, as evidenced by low mean EuroSCORE and SYNTAX scores and by the low proportion of patients with ejection fractions less than 40%. However, patients with left main coronary artery disease (even without diabetes) have been shown to have better outcomes with coronary artery bypass grafting than with PCI, although a head-to-head trial in a diabetic subgroup is currently not available.1,2 In addition, it is important to realize that the FREEDOM trial deals with stable angina; therefore, the results may not extend to patients with acute coronary syndrome wherein primary PCI remains the most feasible option in most cases.
Diabetes mellitus is independently associated with complex, accelerated, and multifocal coronary artery disease. Therefore, outcomes after revascularization (with bypass grafting or PCI) are worse in diabetic patients than in those without diabetes. However, this association does not prove the superiority of PCI over bypass grafting.
As we stated in our paper, the FREEDOM trial did not clearly define the strategy for arterial grafts in patients undergoing bypass grafting. The mean number of coronary lesions in the bypass grafting group was high (mean = 5.74), but the average number of grafts used was only 2.9, and data were not provided on the use of sequential grafting and multiple arterial conduits. Lastly, it is true that the FREEDOM trial had relatively fewer patients (18.5%) that underwent off-pump bypass grafting surgery; however, this approach has never been shown to be superior in large randomized trials.3,4
In conclusion, no randomized trial should replace clinical judgment to define the targeted revascularization strategy for an individual patient. Rather, results from the FREEDOM trial should help support clinical decision-making in the context of the patient and the institution.
In Reply: We appreciate the comments of Dr. Saeed and colleagues. As stated in our article, given that the patients included in the FREEDOM trial represent a select group with diabetes and multivessel coronary artery disease, they may not represent all patients encountered in a real-world setting. We highlighted that only 10% of the patients screened were included for randomization, which limits the generalizability of the results. Also, the overall patient population may not be at high risk, as evidenced by low mean EuroSCORE and SYNTAX scores and by the low proportion of patients with ejection fractions less than 40%. However, patients with left main coronary artery disease (even without diabetes) have been shown to have better outcomes with coronary artery bypass grafting than with PCI, although a head-to-head trial in a diabetic subgroup is currently not available.1,2 In addition, it is important to realize that the FREEDOM trial deals with stable angina; therefore, the results may not extend to patients with acute coronary syndrome wherein primary PCI remains the most feasible option in most cases.
Diabetes mellitus is independently associated with complex, accelerated, and multifocal coronary artery disease. Therefore, outcomes after revascularization (with bypass grafting or PCI) are worse in diabetic patients than in those without diabetes. However, this association does not prove the superiority of PCI over bypass grafting.
As we stated in our paper, the FREEDOM trial did not clearly define the strategy for arterial grafts in patients undergoing bypass grafting. The mean number of coronary lesions in the bypass grafting group was high (mean = 5.74), but the average number of grafts used was only 2.9, and data were not provided on the use of sequential grafting and multiple arterial conduits. Lastly, it is true that the FREEDOM trial had relatively fewer patients (18.5%) that underwent off-pump bypass grafting surgery; however, this approach has never been shown to be superior in large randomized trials.3,4
In conclusion, no randomized trial should replace clinical judgment to define the targeted revascularization strategy for an individual patient. Rather, results from the FREEDOM trial should help support clinical decision-making in the context of the patient and the institution.
- Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Diegeler A, Börgermann J, Kappert U, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med 2013; 368:1189–1198.
- Lamy A, Devereaux PJ, Prabhakaran D, et al; CORONARY Investigators. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med 2013; 368:1179–1188.
- Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Diegeler A, Börgermann J, Kappert U, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med 2013; 368:1189–1198.
- Lamy A, Devereaux PJ, Prabhakaran D, et al; CORONARY Investigators. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med 2013; 368:1179–1188.
Electronic health records
To the Editor: The July 2013 Cleveland Clinic Journal of Medicine includes timely articles addressing the problems of electronic health records (EHRs). At least to this reader, there is little that is surprising in the observations.
A common inside joke among programmers, sometimes displayed at one’s cubicle, is: “Fast, good, or cheap (pick two).” In other words, there is always a compromise to be had between a good product and one that is punched out on a given timetable and inexpensive. Economists call this the “second best.”
Any truly great software product accomplishes three goals. First, it allows the user to do everything previously doable at least as well or as easily as before. Second, it eliminates drudgery. And third, ideally, it provides new functionality, which previously was difficult or impossible to accomplish or to afford.
The reality is that much software is sold on the basis of the third goal, whereas goal number 1 and sometimes goal number 2 get short shrift. And for EHRs in particular, it is a fallacy for physicians to think that EHRs were brought out primarily for their benefit rather than for the benefit of the front office. This was all the more true a decade ago, when very few physicians were employed by hospitals. Thus, if the physician’s workload was increased because of the hospital’s choice of EHR, the hospital felt no financial pain. With greater reliance on an employment model, we can hope that hospitals will recognize that physicians should not be turned into very expensive secretaries.
To the Editor: The July 2013 Cleveland Clinic Journal of Medicine includes timely articles addressing the problems of electronic health records (EHRs). At least to this reader, there is little that is surprising in the observations.
A common inside joke among programmers, sometimes displayed at one’s cubicle, is: “Fast, good, or cheap (pick two).” In other words, there is always a compromise to be had between a good product and one that is punched out on a given timetable and inexpensive. Economists call this the “second best.”
Any truly great software product accomplishes three goals. First, it allows the user to do everything previously doable at least as well or as easily as before. Second, it eliminates drudgery. And third, ideally, it provides new functionality, which previously was difficult or impossible to accomplish or to afford.
The reality is that much software is sold on the basis of the third goal, whereas goal number 1 and sometimes goal number 2 get short shrift. And for EHRs in particular, it is a fallacy for physicians to think that EHRs were brought out primarily for their benefit rather than for the benefit of the front office. This was all the more true a decade ago, when very few physicians were employed by hospitals. Thus, if the physician’s workload was increased because of the hospital’s choice of EHR, the hospital felt no financial pain. With greater reliance on an employment model, we can hope that hospitals will recognize that physicians should not be turned into very expensive secretaries.
To the Editor: The July 2013 Cleveland Clinic Journal of Medicine includes timely articles addressing the problems of electronic health records (EHRs). At least to this reader, there is little that is surprising in the observations.
A common inside joke among programmers, sometimes displayed at one’s cubicle, is: “Fast, good, or cheap (pick two).” In other words, there is always a compromise to be had between a good product and one that is punched out on a given timetable and inexpensive. Economists call this the “second best.”
Any truly great software product accomplishes three goals. First, it allows the user to do everything previously doable at least as well or as easily as before. Second, it eliminates drudgery. And third, ideally, it provides new functionality, which previously was difficult or impossible to accomplish or to afford.
The reality is that much software is sold on the basis of the third goal, whereas goal number 1 and sometimes goal number 2 get short shrift. And for EHRs in particular, it is a fallacy for physicians to think that EHRs were brought out primarily for their benefit rather than for the benefit of the front office. This was all the more true a decade ago, when very few physicians were employed by hospitals. Thus, if the physician’s workload was increased because of the hospital’s choice of EHR, the hospital felt no financial pain. With greater reliance on an employment model, we can hope that hospitals will recognize that physicians should not be turned into very expensive secretaries.
Renal risk stratification with the new oral anticoagulants
To the Editor: I read with interest the review of the new oral anticoagulants by Fawole et al1 and agree with their comments on the prevention of bleeding and the importance of monitoring renal function in managing patients on the new classes of oral anticoagulants. However, no specifics were given on how to proceed. Thus, I recommend that renal risk stratification be done before and 1 week after starting these new drugs.
Originally, the US Food and Drug Administration approved dabigatran (Pradaxa) at a dose of 150 mg orally twice daily in patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2. This dosing corresponded to the estimated glomerular filtration rate (eGFR) in patients with stage 4 chronic kidney disease, but this dosing is contraindicated in other guidelines worldwide (Canada, Europe, the United Kingdom, Japan, Australia, and New Zealand).2 Not unexpectedly, 3,781 serious adverse effects were noted in the 2011 US postmarketing experience with dabigatran. These included death (542 cases), hemorrhage (2,367 cases), acute renal failure (291 cases), stroke (644 cases), and suspected liver failure (15 cases).3 Thirteen months after dabigatran’s approval in the United States, Boehringer Ingelheim changed the dosage and product guidelines.2–4 The new dosage4 is 75 mg twice daily for patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2.
Therefore, I suggest a nephrologic “way out”5 when using the new oral anticoagulants to avoid the problems with dabigatran noted above.
First, if these drugs are to be used in nonvalvular atrial fibrillation, risk factors should be determined using the CHADS2 or the CHADS2-VASc score. Special attention should be given to patients age 75 and older, women, and patients with a history of stroke, transient ischemic attack, or systemic embolism. All of these have been noted to be major risk factors.6,7
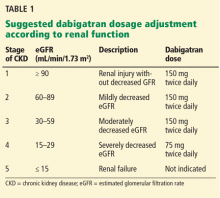
Second, renal risk stratification8 should be done using a comprehensive metabolic panel before and 1 week after starting new oral anticoagulants, or if there is a change in the patient’s clinical condition. Most US laboratories now provide an eGFR and the stage of chronic kidney disease.3,5 For example (Table 1), if dabigatran is used, one should follow current dosing guidelines for chronic kidney disease stages 1 through 3, ie, 150 mg twice daily. If stage 4 chronic kidney disease is detected (creatinine clearance 15–29 mL/min/1.73 m2), the updated recommended dosage is 75 mg twice daily. If stage 5 is noted (eGFR ≤ 15 mL/min/1.73 m2), dabigatran is not indicated. Similar steps can be done using current guidelines for the other new oral anticoagulants.
This simple renal risk stratification guideline should help avoid some of the problems noted in the dabigatran postmarketing experience, which were aggravated by the lack of approval of a 110-mg dose and by misleading advertising, claiming that no blood monitoring was required.2–5 Thus, the new oral anticoagulants should be a welcome addition to our armamentarium in patients who need them, and we hope to avoid the risks, morbidity, mortality, and expense of trying to reverse adverse effects.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants, dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013, 80:443–451.
- Pazmiño PA. Dabigatran associated acute renal failure (DAARF). El Paso Physician 2011; 34:7–9.
- Pazmiño PA. Adverse effects of dabigatran (Letter). Ann Intern Med 2012; 157:916.
- Pradaxa (prescribing information). Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals 2011.
- Pazmiño PA. Dabigatran: a nephrological way out. Am J Med 2013; 126;e21–e22.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. The Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20:705–711.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
To the Editor: I read with interest the review of the new oral anticoagulants by Fawole et al1 and agree with their comments on the prevention of bleeding and the importance of monitoring renal function in managing patients on the new classes of oral anticoagulants. However, no specifics were given on how to proceed. Thus, I recommend that renal risk stratification be done before and 1 week after starting these new drugs.
Originally, the US Food and Drug Administration approved dabigatran (Pradaxa) at a dose of 150 mg orally twice daily in patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2. This dosing corresponded to the estimated glomerular filtration rate (eGFR) in patients with stage 4 chronic kidney disease, but this dosing is contraindicated in other guidelines worldwide (Canada, Europe, the United Kingdom, Japan, Australia, and New Zealand).2 Not unexpectedly, 3,781 serious adverse effects were noted in the 2011 US postmarketing experience with dabigatran. These included death (542 cases), hemorrhage (2,367 cases), acute renal failure (291 cases), stroke (644 cases), and suspected liver failure (15 cases).3 Thirteen months after dabigatran’s approval in the United States, Boehringer Ingelheim changed the dosage and product guidelines.2–4 The new dosage4 is 75 mg twice daily for patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2.
Therefore, I suggest a nephrologic “way out”5 when using the new oral anticoagulants to avoid the problems with dabigatran noted above.
First, if these drugs are to be used in nonvalvular atrial fibrillation, risk factors should be determined using the CHADS2 or the CHADS2-VASc score. Special attention should be given to patients age 75 and older, women, and patients with a history of stroke, transient ischemic attack, or systemic embolism. All of these have been noted to be major risk factors.6,7
Second, renal risk stratification8 should be done using a comprehensive metabolic panel before and 1 week after starting new oral anticoagulants, or if there is a change in the patient’s clinical condition. Most US laboratories now provide an eGFR and the stage of chronic kidney disease.3,5 For example (Table 1), if dabigatran is used, one should follow current dosing guidelines for chronic kidney disease stages 1 through 3, ie, 150 mg twice daily. If stage 4 chronic kidney disease is detected (creatinine clearance 15–29 mL/min/1.73 m2), the updated recommended dosage is 75 mg twice daily. If stage 5 is noted (eGFR ≤ 15 mL/min/1.73 m2), dabigatran is not indicated. Similar steps can be done using current guidelines for the other new oral anticoagulants.
This simple renal risk stratification guideline should help avoid some of the problems noted in the dabigatran postmarketing experience, which were aggravated by the lack of approval of a 110-mg dose and by misleading advertising, claiming that no blood monitoring was required.2–5 Thus, the new oral anticoagulants should be a welcome addition to our armamentarium in patients who need them, and we hope to avoid the risks, morbidity, mortality, and expense of trying to reverse adverse effects.
To the Editor: I read with interest the review of the new oral anticoagulants by Fawole et al1 and agree with their comments on the prevention of bleeding and the importance of monitoring renal function in managing patients on the new classes of oral anticoagulants. However, no specifics were given on how to proceed. Thus, I recommend that renal risk stratification be done before and 1 week after starting these new drugs.
Originally, the US Food and Drug Administration approved dabigatran (Pradaxa) at a dose of 150 mg orally twice daily in patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2. This dosing corresponded to the estimated glomerular filtration rate (eGFR) in patients with stage 4 chronic kidney disease, but this dosing is contraindicated in other guidelines worldwide (Canada, Europe, the United Kingdom, Japan, Australia, and New Zealand).2 Not unexpectedly, 3,781 serious adverse effects were noted in the 2011 US postmarketing experience with dabigatran. These included death (542 cases), hemorrhage (2,367 cases), acute renal failure (291 cases), stroke (644 cases), and suspected liver failure (15 cases).3 Thirteen months after dabigatran’s approval in the United States, Boehringer Ingelheim changed the dosage and product guidelines.2–4 The new dosage4 is 75 mg twice daily for patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2.
Therefore, I suggest a nephrologic “way out”5 when using the new oral anticoagulants to avoid the problems with dabigatran noted above.
First, if these drugs are to be used in nonvalvular atrial fibrillation, risk factors should be determined using the CHADS2 or the CHADS2-VASc score. Special attention should be given to patients age 75 and older, women, and patients with a history of stroke, transient ischemic attack, or systemic embolism. All of these have been noted to be major risk factors.6,7
Second, renal risk stratification8 should be done using a comprehensive metabolic panel before and 1 week after starting new oral anticoagulants, or if there is a change in the patient’s clinical condition. Most US laboratories now provide an eGFR and the stage of chronic kidney disease.3,5 For example (Table 1), if dabigatran is used, one should follow current dosing guidelines for chronic kidney disease stages 1 through 3, ie, 150 mg twice daily. If stage 4 chronic kidney disease is detected (creatinine clearance 15–29 mL/min/1.73 m2), the updated recommended dosage is 75 mg twice daily. If stage 5 is noted (eGFR ≤ 15 mL/min/1.73 m2), dabigatran is not indicated. Similar steps can be done using current guidelines for the other new oral anticoagulants.
This simple renal risk stratification guideline should help avoid some of the problems noted in the dabigatran postmarketing experience, which were aggravated by the lack of approval of a 110-mg dose and by misleading advertising, claiming that no blood monitoring was required.2–5 Thus, the new oral anticoagulants should be a welcome addition to our armamentarium in patients who need them, and we hope to avoid the risks, morbidity, mortality, and expense of trying to reverse adverse effects.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants, dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013, 80:443–451.
- Pazmiño PA. Dabigatran associated acute renal failure (DAARF). El Paso Physician 2011; 34:7–9.
- Pazmiño PA. Adverse effects of dabigatran (Letter). Ann Intern Med 2012; 157:916.
- Pradaxa (prescribing information). Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals 2011.
- Pazmiño PA. Dabigatran: a nephrological way out. Am J Med 2013; 126;e21–e22.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. The Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20:705–711.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants, dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013, 80:443–451.
- Pazmiño PA. Dabigatran associated acute renal failure (DAARF). El Paso Physician 2011; 34:7–9.
- Pazmiño PA. Adverse effects of dabigatran (Letter). Ann Intern Med 2012; 157:916.
- Pradaxa (prescribing information). Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals 2011.
- Pazmiño PA. Dabigatran: a nephrological way out. Am J Med 2013; 126;e21–e22.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. The Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20:705–711.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
In reply: Renal risk stratification with the new oral anticoagulants
In Reply: We agree with the comments of Dr. Pazmiño regarding specifics of renal risk stratification in patients taking the new oral anticoagulants. In order to reduce the bleeding risks associated with these agents, they should be prescribed on the basis of the individual patient’s clinical characteristics. We did not discuss this since the focus of our article was management of bleeding that resulted from use of these drugs. We appreciate the recommendations of Dr. Pazmiño.
In Reply: We agree with the comments of Dr. Pazmiño regarding specifics of renal risk stratification in patients taking the new oral anticoagulants. In order to reduce the bleeding risks associated with these agents, they should be prescribed on the basis of the individual patient’s clinical characteristics. We did not discuss this since the focus of our article was management of bleeding that resulted from use of these drugs. We appreciate the recommendations of Dr. Pazmiño.
In Reply: We agree with the comments of Dr. Pazmiño regarding specifics of renal risk stratification in patients taking the new oral anticoagulants. In order to reduce the bleeding risks associated with these agents, they should be prescribed on the basis of the individual patient’s clinical characteristics. We did not discuss this since the focus of our article was management of bleeding that resulted from use of these drugs. We appreciate the recommendations of Dr. Pazmiño.
Not all joint pain is arthritis
To the Editor: I was somewhat confused by the Clinical Picture case in the May 2013 issue.1 The caption for Figure 1 stated that the MRI showed erosions and marrow edema, which were “asymmetrical compared with the other wrist, a finding highly suggestive of rheumatoid arthritis.” However, rheumatoid arthritis is generally considered to be symmetrical.2 Was this a typographical error, or did I miss a crucial concept somewhere?
- Kochhar GS, Rizk M, Patil DT. Not all joint pain is arthritis. Cleve Clin J Med 2013; 80:272–273.
- Bukhari M, Lunt M, Harrison BJ, Scott DG, Symmons DP, Silman AJ. Erosions in inflammatory polyarthritis are symmetrical regardless of rheumatoid factor status: results from a primary care-based inception cohort of patients. Rheumatology 2002; 41:246–252.
To the Editor: I was somewhat confused by the Clinical Picture case in the May 2013 issue.1 The caption for Figure 1 stated that the MRI showed erosions and marrow edema, which were “asymmetrical compared with the other wrist, a finding highly suggestive of rheumatoid arthritis.” However, rheumatoid arthritis is generally considered to be symmetrical.2 Was this a typographical error, or did I miss a crucial concept somewhere?
To the Editor: I was somewhat confused by the Clinical Picture case in the May 2013 issue.1 The caption for Figure 1 stated that the MRI showed erosions and marrow edema, which were “asymmetrical compared with the other wrist, a finding highly suggestive of rheumatoid arthritis.” However, rheumatoid arthritis is generally considered to be symmetrical.2 Was this a typographical error, or did I miss a crucial concept somewhere?
- Kochhar GS, Rizk M, Patil DT. Not all joint pain is arthritis. Cleve Clin J Med 2013; 80:272–273.
- Bukhari M, Lunt M, Harrison BJ, Scott DG, Symmons DP, Silman AJ. Erosions in inflammatory polyarthritis are symmetrical regardless of rheumatoid factor status: results from a primary care-based inception cohort of patients. Rheumatology 2002; 41:246–252.
- Kochhar GS, Rizk M, Patil DT. Not all joint pain is arthritis. Cleve Clin J Med 2013; 80:272–273.
- Bukhari M, Lunt M, Harrison BJ, Scott DG, Symmons DP, Silman AJ. Erosions in inflammatory polyarthritis are symmetrical regardless of rheumatoid factor status: results from a primary care-based inception cohort of patients. Rheumatology 2002; 41:246–252.
In reply: Not all joint pain is arthritis
In Reply: We apologize for the confusion. We wanted to convey that, in that patient at that time, synovitis with erosions and edema indicating inflammation (greater on the right than on the left left) was suggestive of rheumatoid arthritis despite the asymmetry seen (findings greater in the right wrist than in the left). Given the patient’s clinical findings at that time and the above imaging findings, the initial diagnosis of rheumatoid arthritis was correct. But since the patient was not responding to therapy and since the abdominal pain was worsening, we probed further. Subsequently, the patient was diagnosed with Whipple disease. The fact that inflammatory arthritis can occur in other conditions that are not rheumatologic is a primary reason we found this case worth sharing.
In Reply: We apologize for the confusion. We wanted to convey that, in that patient at that time, synovitis with erosions and edema indicating inflammation (greater on the right than on the left left) was suggestive of rheumatoid arthritis despite the asymmetry seen (findings greater in the right wrist than in the left). Given the patient’s clinical findings at that time and the above imaging findings, the initial diagnosis of rheumatoid arthritis was correct. But since the patient was not responding to therapy and since the abdominal pain was worsening, we probed further. Subsequently, the patient was diagnosed with Whipple disease. The fact that inflammatory arthritis can occur in other conditions that are not rheumatologic is a primary reason we found this case worth sharing.
In Reply: We apologize for the confusion. We wanted to convey that, in that patient at that time, synovitis with erosions and edema indicating inflammation (greater on the right than on the left left) was suggestive of rheumatoid arthritis despite the asymmetry seen (findings greater in the right wrist than in the left). Given the patient’s clinical findings at that time and the above imaging findings, the initial diagnosis of rheumatoid arthritis was correct. But since the patient was not responding to therapy and since the abdominal pain was worsening, we probed further. Subsequently, the patient was diagnosed with Whipple disease. The fact that inflammatory arthritis can occur in other conditions that are not rheumatologic is a primary reason we found this case worth sharing.
Sleep-disordered breathing and resistant hypertension
To the Editor: We recently read the article by Dr. Emmanuel Bravo.1 In his comprehensive paper, he defined a road map for the workup of resistant hypertension. Resistant hypertension is a challenging problem in everyday practice, with multiple pitfalls at each step from diagnosis to treatment.
Although not mentioned in the paper, obstructive sleep apnea is strongly associated with hypertension, and its prevalence in patients with resistant hypertension can be as high as 83%.2 The upper airway resistance syndrome is another form of sleep-disordered breathing in which transient increases in upper airway resistance result in repetitive electroencephalographic arousals. Unlike obstructive sleep apnea, upper airway resistance syndrome is not associated with apnea or diminished airflow, although snoring and excessive daytime somnolence are common. Repeated arousals, desaturations, or both during sleep lead to recurrent sympathetic surges with resultant nocturnal hypertension. There are a number of reports in the literature of large blood-pressure reductions after continuous positive airway pressure treatment.3
In conclusion, sleep-disordered breathing syndromes should be sought vigorously in cases of resistant hypertension, and every effort should be taken for proper management.
- Bravo E. Resistant hypertension: diagnostic strategies and management. Cleve Clin J Med 2013; 80:91–96.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J 2003; 21:241–247.
To the Editor: We recently read the article by Dr. Emmanuel Bravo.1 In his comprehensive paper, he defined a road map for the workup of resistant hypertension. Resistant hypertension is a challenging problem in everyday practice, with multiple pitfalls at each step from diagnosis to treatment.
Although not mentioned in the paper, obstructive sleep apnea is strongly associated with hypertension, and its prevalence in patients with resistant hypertension can be as high as 83%.2 The upper airway resistance syndrome is another form of sleep-disordered breathing in which transient increases in upper airway resistance result in repetitive electroencephalographic arousals. Unlike obstructive sleep apnea, upper airway resistance syndrome is not associated with apnea or diminished airflow, although snoring and excessive daytime somnolence are common. Repeated arousals, desaturations, or both during sleep lead to recurrent sympathetic surges with resultant nocturnal hypertension. There are a number of reports in the literature of large blood-pressure reductions after continuous positive airway pressure treatment.3
In conclusion, sleep-disordered breathing syndromes should be sought vigorously in cases of resistant hypertension, and every effort should be taken for proper management.
To the Editor: We recently read the article by Dr. Emmanuel Bravo.1 In his comprehensive paper, he defined a road map for the workup of resistant hypertension. Resistant hypertension is a challenging problem in everyday practice, with multiple pitfalls at each step from diagnosis to treatment.
Although not mentioned in the paper, obstructive sleep apnea is strongly associated with hypertension, and its prevalence in patients with resistant hypertension can be as high as 83%.2 The upper airway resistance syndrome is another form of sleep-disordered breathing in which transient increases in upper airway resistance result in repetitive electroencephalographic arousals. Unlike obstructive sleep apnea, upper airway resistance syndrome is not associated with apnea or diminished airflow, although snoring and excessive daytime somnolence are common. Repeated arousals, desaturations, or both during sleep lead to recurrent sympathetic surges with resultant nocturnal hypertension. There are a number of reports in the literature of large blood-pressure reductions after continuous positive airway pressure treatment.3
In conclusion, sleep-disordered breathing syndromes should be sought vigorously in cases of resistant hypertension, and every effort should be taken for proper management.
- Bravo E. Resistant hypertension: diagnostic strategies and management. Cleve Clin J Med 2013; 80:91–96.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J 2003; 21:241–247.
- Bravo E. Resistant hypertension: diagnostic strategies and management. Cleve Clin J Med 2013; 80:91–96.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J 2003; 21:241–247.