User login
Adolescent immunizations – Focus on HPV vaccine
The U.S. immunization program has been one of the country’s most successful initiatives and best investments. Prior to 2005, vaccines were targeted for administration to infants and young children. Adolescence was a period for catch-up immunizations. All that changed in 2005 when the first meningococcal conjugate vaccine (MCV) was recommended for administration to preteens at 11-12 years and college freshmen residing in dormitories by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP). Shortly thereafter in 2006, a new tetanus toxoid, diphtheria, and acellular pertussis vaccine (Tdap) was recommended, and in March 2007 the quadrivalent human papillomavirus vaccine (HPV4: types 6, 11, 16, and 18) was recommended for use in girls, starting at age 11-12 years, and young women up to 26 years of age. In 2009, a bivalent HPV vaccine (HPV2: types 16 and 18) was licensed, and in 2010, ACIP recommendations indicated that either HPV4 or HPV2 vaccine could be administered to girls and young women. In addition, the use of HPV4 vaccine in males was permitted. In 2011, ACIP recommended routine administration of HPV4 to boys and young adult males up to 21 years of age. Adolescents were the target population for these vaccines, and administration was recommended at the 11- to 12-year wellness visit. The primary role of the adolescent encounter was no longer to provide catch-up immunizations. A definitive adolescent immunization schedule had been established.
Why introduce the HPV vaccine so early?
HPV is the most common sexually transmitted infection in both men and women. Recent data suggest that approximately 79 million individuals are infected (Sex. Transm. Dis. 2013;40:187-93). Annually, about 14 million, mostly young adults are infected. Most sexually active individuals will acquire HPV. It is most common in teens and young adults, and intercourse is not required for transmission. It can be transmitted with any type of intimate sexual contact, and it has been isolated from virgins. The majority of these infections are asymptomatic and self- limited. However, persistent infection is associated with cervical and other types of anogenital cancer, and genital warts in both men and women. Complications of these infections may take years to manifest.
HPV is categorized by its epidemiologic association with cervical cancer. High-risk types cause cervical cancer, and HPV types 16 and 18 account for the majority of cervical cancers (66%) These two types are also associated with vaginal (55%), anal (79%), and oropharyngeal (62%) cancer (MMWR 2014 Jan. 31;63;69-72). It is estimated that each year there are 26,000 HPV-related cancers including 8,800 cases in men and 17,000 in women, 4,000 of whom will die of cervical cancer, according to the CDC. Low-risk types including HPV types 6 and 11 cause benign/low-grade cervical cell changes, recurrent papillomatosis, and 90% of the cases of genital warts.
Once a person is infected, HPV usually clears. If not, cervical intraepithelial neoplasia (CIN) may occur. The infection may still resolve spontaneously. If it persists, the degree of dysplasia can progress. Several years may pass before progression to invasive cancer. HPV vaccines are prophylactic like other vaccines. They cannot prevent disease progression and need to be administered before exposure to the viruses.
Compared with the introduction of other vaccines, such as Haemophilus influenzae type b and Prevnar7, some pediatric care providers may feel we may not have the benefit of realizing our efforts as immediately as in the past. However, encouraging vaccine effectiveness data in U.S. teens has been published. In one study, the investigators compared HPV prevalence data from the pre- and postvaccine era collected during the National Health and Nutrition Examination Survey. Among females aged 14-19 years, HPV prevalence (HPV-6, -11, -16, or -18 ) decreased from 11.5% in 2003-2006 to 5.1% in 2007-2010. That is a 56% reduction in vaccine type HPV prevalence. This decrease in prevalence occurred within 4 years of vaccine introduction and low vaccine uptake. (J. Infect. Dis. 2013;208:385-93). Studies conducted in Denmark, Australia, Germany, and New Zealand also have shown significant declines in HPV4 vaccine type infection prevalence.
Vaccination coverage
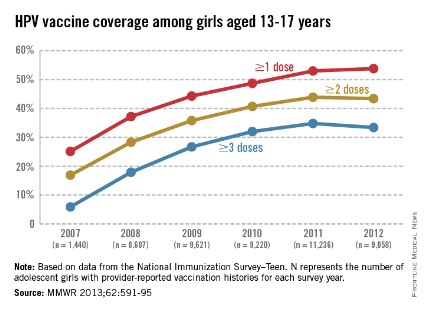
The CDC tracks vaccination coverage annually in the National Immunization Survey–Teen (NIS-Teen), with data obtained from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR 2013;62:685-93). Vaccination coverage differed significantly although each vaccine is recommended to be routinely administered at the 11- to 12-year visit. Although an increase from 25% to 53% had been noted between 2007 and 2011, in 2012, coverage for receiving at least one dose of HPV among females was almost 54%, essentially unchanged since 2011. The number who had received the recommended three doses was also essentially unchanged from 2011 to 2012 (34.8% in 2011 and 33.4% in 2012). Receipt of a single dose of HPV in boys was 8.3% in 2011 and 20.8% in 2012, the first year after the vaccine was recommended. Completion of the series in boys was 6.3%, an increase from 1.3% in 2011.
In contrast, the 2012 coverage for Tdap increased to 85% and MCV4, to 74%. It has been suggested that the higher coverage of Tdap and MCV may be due to the 40 and 13 states, respectively, that require them for middle school entry.
The disparity in coverage between Tdap and other vaccines suggests there are numerous missed opportunities to vaccinate adolescents. Data revealed that missed opportunities for girls increased from 20.8% in 2007 to 84% in 2012. If all missed opportunities had been eliminated, HPV coverage for at least one dose could have reached 92.6%.Almost 25% of parents indicated that they had no plan to immunize their daughter. The top reasons parents stated for not immunizing their daughters included: not needed or necessary, 19.1%; not recommended by provider, 14.2%; safety concerns, 13.3%; lack of knowledge, 12.6%; and not sexually active, 10.1%. (MMWR 2013;62:591-5).
Vaccine safety also was addressed. All reported adverse events were consistent with prelicensure clinical trial data. Ninety two percent of all adverse events were nonserious and included syncope, dizziness, nausea, and fever. Reports peaked in 2008 and have declined each year thereafter.
Challenges for HPV prevention
Improving immunization coverage is critical. There are numerous strategies to increase coverage including reminder recall systems, standing orders, and educating parents, patients, health care providers, and office staff who interact with parents. Education should reemphasize why immunization is initiated at 11-12 years and that completion of the series is recommended by 13 years. School requirements have always led to an increase in vaccination coverage. Only the District of Columbia has one for HPV. In this case, eliminating missed opportunities is crucial. It is estimated that for every year coverage is delayed, an additional 4,400 women will develop cervical cancer. The reality is that the burden of HPV-related cancers will persist if coverage is not increased.
As Louis Pasteur once said, "When meditating over a disease, I never think of finding a remedy for it, but, instead a means of preventing it."
For additional resources to assist with discussions about HPV, click here.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. E-mail her at [email protected]. Scan this QR code or visit pediatricnews.com.
The U.S. immunization program has been one of the country’s most successful initiatives and best investments. Prior to 2005, vaccines were targeted for administration to infants and young children. Adolescence was a period for catch-up immunizations. All that changed in 2005 when the first meningococcal conjugate vaccine (MCV) was recommended for administration to preteens at 11-12 years and college freshmen residing in dormitories by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP). Shortly thereafter in 2006, a new tetanus toxoid, diphtheria, and acellular pertussis vaccine (Tdap) was recommended, and in March 2007 the quadrivalent human papillomavirus vaccine (HPV4: types 6, 11, 16, and 18) was recommended for use in girls, starting at age 11-12 years, and young women up to 26 years of age. In 2009, a bivalent HPV vaccine (HPV2: types 16 and 18) was licensed, and in 2010, ACIP recommendations indicated that either HPV4 or HPV2 vaccine could be administered to girls and young women. In addition, the use of HPV4 vaccine in males was permitted. In 2011, ACIP recommended routine administration of HPV4 to boys and young adult males up to 21 years of age. Adolescents were the target population for these vaccines, and administration was recommended at the 11- to 12-year wellness visit. The primary role of the adolescent encounter was no longer to provide catch-up immunizations. A definitive adolescent immunization schedule had been established.
Why introduce the HPV vaccine so early?

HPV is the most common sexually transmitted infection in both men and women. Recent data suggest that approximately 79 million individuals are infected (Sex. Transm. Dis. 2013;40:187-93). Annually, about 14 million, mostly young adults are infected. Most sexually active individuals will acquire HPV. It is most common in teens and young adults, and intercourse is not required for transmission. It can be transmitted with any type of intimate sexual contact, and it has been isolated from virgins. The majority of these infections are asymptomatic and self- limited. However, persistent infection is associated with cervical and other types of anogenital cancer, and genital warts in both men and women. Complications of these infections may take years to manifest.
HPV is categorized by its epidemiologic association with cervical cancer. High-risk types cause cervical cancer, and HPV types 16 and 18 account for the majority of cervical cancers (66%) These two types are also associated with vaginal (55%), anal (79%), and oropharyngeal (62%) cancer (MMWR 2014 Jan. 31;63;69-72). It is estimated that each year there are 26,000 HPV-related cancers including 8,800 cases in men and 17,000 in women, 4,000 of whom will die of cervical cancer, according to the CDC. Low-risk types including HPV types 6 and 11 cause benign/low-grade cervical cell changes, recurrent papillomatosis, and 90% of the cases of genital warts.
Once a person is infected, HPV usually clears. If not, cervical intraepithelial neoplasia (CIN) may occur. The infection may still resolve spontaneously. If it persists, the degree of dysplasia can progress. Several years may pass before progression to invasive cancer. HPV vaccines are prophylactic like other vaccines. They cannot prevent disease progression and need to be administered before exposure to the viruses.

Compared with the introduction of other vaccines, such as Haemophilus influenzae type b and Prevnar7, some pediatric care providers may feel we may not have the benefit of realizing our efforts as immediately as in the past. However, encouraging vaccine effectiveness data in U.S. teens has been published. In one study, the investigators compared HPV prevalence data from the pre- and postvaccine era collected during the National Health and Nutrition Examination Survey. Among females aged 14-19 years, HPV prevalence (HPV-6, -11, -16, or -18 ) decreased from 11.5% in 2003-2006 to 5.1% in 2007-2010. That is a 56% reduction in vaccine type HPV prevalence. This decrease in prevalence occurred within 4 years of vaccine introduction and low vaccine uptake. (J. Infect. Dis. 2013;208:385-93). Studies conducted in Denmark, Australia, Germany, and New Zealand also have shown significant declines in HPV4 vaccine type infection prevalence.
Vaccination coverage
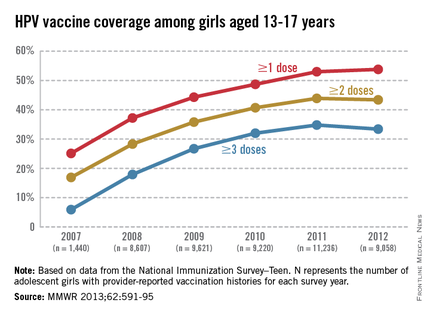
The CDC tracks vaccination coverage annually in the National Immunization Survey–Teen (NIS-Teen), with data obtained from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR 2013;62:685-93). Vaccination coverage differed significantly although each vaccine is recommended to be routinely administered at the 11- to 12-year visit. Although an increase from 25% to 53% had been noted between 2007 and 2011, in 2012, coverage for receiving at least one dose of HPV among females was almost 54%, essentially unchanged since 2011. The number who had received the recommended three doses was also essentially unchanged from 2011 to 2012 (34.8% in 2011 and 33.4% in 2012). Receipt of a single dose of HPV in boys was 8.3% in 2011 and 20.8% in 2012, the first year after the vaccine was recommended. Completion of the series in boys was 6.3%, an increase from 1.3% in 2011.
In contrast, the 2012 coverage for Tdap increased to 85% and MCV4, to 74%. It has been suggested that the higher coverage of Tdap and MCV may be due to the 40 and 13 states, respectively, that require them for middle school entry.
The disparity in coverage between Tdap and other vaccines suggests there are numerous missed opportunities to vaccinate adolescents. Data revealed that missed opportunities for girls increased from 20.8% in 2007 to 84% in 2012. If all missed opportunities had been eliminated, HPV coverage for at least one dose could have reached 92.6%.Almost 25% of parents indicated that they had no plan to immunize their daughter. The top reasons parents stated for not immunizing their daughters included: not needed or necessary, 19.1%; not recommended by provider, 14.2%; safety concerns, 13.3%; lack of knowledge, 12.6%; and not sexually active, 10.1%. (MMWR 2013;62:591-5).
Vaccine safety also was addressed. All reported adverse events were consistent with prelicensure clinical trial data. Ninety two percent of all adverse events were nonserious and included syncope, dizziness, nausea, and fever. Reports peaked in 2008 and have declined each year thereafter.
Challenges for HPV prevention
Improving immunization coverage is critical. There are numerous strategies to increase coverage including reminder recall systems, standing orders, and educating parents, patients, health care providers, and office staff who interact with parents. Education should reemphasize why immunization is initiated at 11-12 years and that completion of the series is recommended by 13 years. School requirements have always led to an increase in vaccination coverage. Only the District of Columbia has one for HPV. In this case, eliminating missed opportunities is crucial. It is estimated that for every year coverage is delayed, an additional 4,400 women will develop cervical cancer. The reality is that the burden of HPV-related cancers will persist if coverage is not increased.
As Louis Pasteur once said, "When meditating over a disease, I never think of finding a remedy for it, but, instead a means of preventing it."
For additional resources to assist with discussions about HPV, click here.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. E-mail her at [email protected]. Scan this QR code or visit pediatricnews.com.
The U.S. immunization program has been one of the country’s most successful initiatives and best investments. Prior to 2005, vaccines were targeted for administration to infants and young children. Adolescence was a period for catch-up immunizations. All that changed in 2005 when the first meningococcal conjugate vaccine (MCV) was recommended for administration to preteens at 11-12 years and college freshmen residing in dormitories by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP). Shortly thereafter in 2006, a new tetanus toxoid, diphtheria, and acellular pertussis vaccine (Tdap) was recommended, and in March 2007 the quadrivalent human papillomavirus vaccine (HPV4: types 6, 11, 16, and 18) was recommended for use in girls, starting at age 11-12 years, and young women up to 26 years of age. In 2009, a bivalent HPV vaccine (HPV2: types 16 and 18) was licensed, and in 2010, ACIP recommendations indicated that either HPV4 or HPV2 vaccine could be administered to girls and young women. In addition, the use of HPV4 vaccine in males was permitted. In 2011, ACIP recommended routine administration of HPV4 to boys and young adult males up to 21 years of age. Adolescents were the target population for these vaccines, and administration was recommended at the 11- to 12-year wellness visit. The primary role of the adolescent encounter was no longer to provide catch-up immunizations. A definitive adolescent immunization schedule had been established.
Why introduce the HPV vaccine so early?

HPV is the most common sexually transmitted infection in both men and women. Recent data suggest that approximately 79 million individuals are infected (Sex. Transm. Dis. 2013;40:187-93). Annually, about 14 million, mostly young adults are infected. Most sexually active individuals will acquire HPV. It is most common in teens and young adults, and intercourse is not required for transmission. It can be transmitted with any type of intimate sexual contact, and it has been isolated from virgins. The majority of these infections are asymptomatic and self- limited. However, persistent infection is associated with cervical and other types of anogenital cancer, and genital warts in both men and women. Complications of these infections may take years to manifest.
HPV is categorized by its epidemiologic association with cervical cancer. High-risk types cause cervical cancer, and HPV types 16 and 18 account for the majority of cervical cancers (66%) These two types are also associated with vaginal (55%), anal (79%), and oropharyngeal (62%) cancer (MMWR 2014 Jan. 31;63;69-72). It is estimated that each year there are 26,000 HPV-related cancers including 8,800 cases in men and 17,000 in women, 4,000 of whom will die of cervical cancer, according to the CDC. Low-risk types including HPV types 6 and 11 cause benign/low-grade cervical cell changes, recurrent papillomatosis, and 90% of the cases of genital warts.
Once a person is infected, HPV usually clears. If not, cervical intraepithelial neoplasia (CIN) may occur. The infection may still resolve spontaneously. If it persists, the degree of dysplasia can progress. Several years may pass before progression to invasive cancer. HPV vaccines are prophylactic like other vaccines. They cannot prevent disease progression and need to be administered before exposure to the viruses.

Compared with the introduction of other vaccines, such as Haemophilus influenzae type b and Prevnar7, some pediatric care providers may feel we may not have the benefit of realizing our efforts as immediately as in the past. However, encouraging vaccine effectiveness data in U.S. teens has been published. In one study, the investigators compared HPV prevalence data from the pre- and postvaccine era collected during the National Health and Nutrition Examination Survey. Among females aged 14-19 years, HPV prevalence (HPV-6, -11, -16, or -18 ) decreased from 11.5% in 2003-2006 to 5.1% in 2007-2010. That is a 56% reduction in vaccine type HPV prevalence. This decrease in prevalence occurred within 4 years of vaccine introduction and low vaccine uptake. (J. Infect. Dis. 2013;208:385-93). Studies conducted in Denmark, Australia, Germany, and New Zealand also have shown significant declines in HPV4 vaccine type infection prevalence.
Vaccination coverage
The CDC tracks vaccination coverage annually in the National Immunization Survey–Teen (NIS-Teen), with data obtained from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR 2013;62:685-93). Vaccination coverage differed significantly although each vaccine is recommended to be routinely administered at the 11- to 12-year visit. Although an increase from 25% to 53% had been noted between 2007 and 2011, in 2012, coverage for receiving at least one dose of HPV among females was almost 54%, essentially unchanged since 2011. The number who had received the recommended three doses was also essentially unchanged from 2011 to 2012 (34.8% in 2011 and 33.4% in 2012). Receipt of a single dose of HPV in boys was 8.3% in 2011 and 20.8% in 2012, the first year after the vaccine was recommended. Completion of the series in boys was 6.3%, an increase from 1.3% in 2011.
In contrast, the 2012 coverage for Tdap increased to 85% and MCV4, to 74%. It has been suggested that the higher coverage of Tdap and MCV may be due to the 40 and 13 states, respectively, that require them for middle school entry.
The disparity in coverage between Tdap and other vaccines suggests there are numerous missed opportunities to vaccinate adolescents. Data revealed that missed opportunities for girls increased from 20.8% in 2007 to 84% in 2012. If all missed opportunities had been eliminated, HPV coverage for at least one dose could have reached 92.6%.Almost 25% of parents indicated that they had no plan to immunize their daughter. The top reasons parents stated for not immunizing their daughters included: not needed or necessary, 19.1%; not recommended by provider, 14.2%; safety concerns, 13.3%; lack of knowledge, 12.6%; and not sexually active, 10.1%. (MMWR 2013;62:591-5).
Vaccine safety also was addressed. All reported adverse events were consistent with prelicensure clinical trial data. Ninety two percent of all adverse events were nonserious and included syncope, dizziness, nausea, and fever. Reports peaked in 2008 and have declined each year thereafter.
Challenges for HPV prevention
Improving immunization coverage is critical. There are numerous strategies to increase coverage including reminder recall systems, standing orders, and educating parents, patients, health care providers, and office staff who interact with parents. Education should reemphasize why immunization is initiated at 11-12 years and that completion of the series is recommended by 13 years. School requirements have always led to an increase in vaccination coverage. Only the District of Columbia has one for HPV. In this case, eliminating missed opportunities is crucial. It is estimated that for every year coverage is delayed, an additional 4,400 women will develop cervical cancer. The reality is that the burden of HPV-related cancers will persist if coverage is not increased.
As Louis Pasteur once said, "When meditating over a disease, I never think of finding a remedy for it, but, instead a means of preventing it."
For additional resources to assist with discussions about HPV, click here.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. E-mail her at [email protected]. Scan this QR code or visit pediatricnews.com.
Predictions for 2014
Are you prepared to manage the infectious disease challenges you’ll be facing in 2014? Here are my Top 5 predictions for what lies ahead in infectious diseases for the next year with pearls to help you in your practice. The first addresses a series of concerns around influenza. Others target diagnoses you might not have encountered or considered in the past. The last will hopefully improve HPV vaccination rates in your practice.
1. Expect an especially busy influenza season and the possibility that you may encounter patients with life-threatening influenza. We’ve already detected influenza in over 1,000 children at my institution, almost all 2009 pandemic H1N1 influenza A viruses, which is consistent with the national data from the Centers for Disease Control and Prevention. We are really just a month into influenza season, and we are seeing a significant number of children admitted to our pediatric intensive care unit with life-threatening disease presentations, and we’ve also seen unusual influenza complications. Talk to your ID colleagues about the potential for intravenous zanamivir in critically ill children who do not respond to oseltamivir. While pulmonary complications of influenza are most common, unusual presentations you may encounter include influenza encephalopathy (altered mental status, seizures, and mutism) and bacterial superinfection (when fever recurs or recrudesces after initial improvement, often 3-5 days into the course, think Staphylococcus aureus or Group A streptococcal disease). The CDC is alerting practitioners to the potential for increased morbidity and mortality in young/middle aged adults so the parents of your patients are at increased risk this year.
• False-negative testing can happen if the sensitivity of the rapid test is low, but a false-negative test can occur if the specimen is collected late in the clinical course. (This is especially true in the adult population in which testing may be negative at just 4-5 days into the course of disease.)
• Recognize that all hospitalized children should be treated with oseltamivir, as well as children who are immunocompromised; have chronic cardiopulmonary conditions, including hemodynamically significant heart disease and asthma; renal disease; metabolic disease, including diabetes; pregnant teens; morbidly obese patients; patients with neuromuscular/neurodevelopmental conditions (especially those with difficulty controlling airway secretions); and children under 2 years of age.
• I predict you may be hearing about oseltamivir shortages, but for now this relates to the sporadic difficulty in finding the oseltamivir suspension, in part, because of the lack of early season availability of this product at retail pharmacies, many of which are just getting in their stock. Prescribe the suspension for children aged younger than 1 year and be explicit about the mL dosage that should be dispensed. For children over 1 year of age, capsules can be opened and placed in pudding for those who cannot swallow capsules. Lexicomp Online offers guidelines for easy use of 30-mg, 45-mg and 75-mg capsules for different weight categories. If the suspension is necessary for an infant and is not available, the drug can be compounded by your pharmacy using capsules. You may find some pharmacies are reluctant to compound, so be prepared to contact your local children’s hospital for help. And keep offering vaccine throughout the season to healthy patients!
2. Most practitioners are aware of the importance of methicillin-resistant S. aureus (MRSA) as a pathogen that causes bacteremia and musculoskeletal and pulmonary disease in otherwise healthy children. I suspect there is less awareness that, in many locales, methicillin-sensitive S. aureus (MSSA) is being seen just as often, if not slightly more often than MRSA, as a bloodstream pathogen. The inclusion of vancomycin (which covers MRSA) with cefepime should be considered for empiric coverage in the otherwise healthy child with suspected sepsis. Cefepime is a fourth-generation cephalosporin with good gram-negative and gram-positive coverage and also has bactericidal activity against MSSA strains. Clindamycin should be considered as an adjunct to vancomycin and cefepime in those with toxin-mediated disease/toxic shock syndrome. Of course, modification of the empiric regimen should follow identification of the specific pathogen and the site(s) of infection.
3. E. coli remains the most common cause of urinary tract infections in children, but infections caused by multiple drug resistant (MDR) Escherichia coli strains are increasingly being seen. Consider infection caused by extended spectrum beta-lactamase–producing organisms in children with underlying renal anomalies, especially if they have been previously exposed to third-generation cephalosporins. Most strains are also resistant to fluoroquinolones, trimethoprim-sulfamethoxazole, and aminoglycosides as well as to non–carbapenem beta-lactams. Speaking of antibiotic resistance, look for many hospital microbiology laboratories to begin using advanced molecular detection methodology to more quickly identify bacterial and fungal isolates; such methods could reduce the time of identification from over 24 hours with conventional techniques to less than one hour. The use of newer systems to identify microbes and confirm susceptibility testing has the potential to transform care and improve outcomes.
4. Consider the diagnosis of human parechovirus (HPeV) infection in young febrile infants with sepsis/meningitis presentation but negative bacterial cultures. Detection of HPeV by polymerase chain reaction testing in serum or cerebrospinal fluid is diagnostic. Exclusion of herpes simplex virus and enterovirus disease is key, as similar clinical presentations may be seen. HPeV infections are more commonly noted in late spring and early summer in contrast to enteroviral infections, which tend to occur from July to September.
5. The strength of your vaccine recommendation continues to be the most important factor affecting the parental decision to vaccinate a child. Nowhere is this more obvious than with human papillomavirus vaccine (HPV), where practitioners often simply offer the vaccine rather than recommend it. In terms of teenage vaccines, when practitioners recommend Tdap (tetanus, diphtheria, and pertussis vaccine) and meningococcal conjugate vaccine as standard for their patients ("Today your child will receive whooping cough vaccine and the meningitis vaccine."), vaccine uptake is very high. But when it comes to the HPV vaccine, some practitioners feel they first must establish whether the parents are aware of HPV vaccine; then discuss their questions regarding the safety of the vaccine; and finally, explain that the vaccine prevents cancer. Some practitioners offer the option of "thinking about" the vaccine for the next visit, but in such cases, the patient generally leaves without receiving the vaccine. Add HPV vaccine into your standard teen vaccine recommendation and make it a goal to get the first vaccine initiated in all eligible patients. The three-dose HPV vaccine schedule is still recommended, but I predict that simplification of the schedule may occur as early as 2014 in the United States. We’ll keep you posted.
Dr. Jackson is director of the division of infectious disease and associate director of the infectious disease fellowship program at the University of Missouri, Kansas City.
Are you prepared to manage the infectious disease challenges you’ll be facing in 2014? Here are my Top 5 predictions for what lies ahead in infectious diseases for the next year with pearls to help you in your practice. The first addresses a series of concerns around influenza. Others target diagnoses you might not have encountered or considered in the past. The last will hopefully improve HPV vaccination rates in your practice.
1. Expect an especially busy influenza season and the possibility that you may encounter patients with life-threatening influenza. We’ve already detected influenza in over 1,000 children at my institution, almost all 2009 pandemic H1N1 influenza A viruses, which is consistent with the national data from the Centers for Disease Control and Prevention. We are really just a month into influenza season, and we are seeing a significant number of children admitted to our pediatric intensive care unit with life-threatening disease presentations, and we’ve also seen unusual influenza complications. Talk to your ID colleagues about the potential for intravenous zanamivir in critically ill children who do not respond to oseltamivir. While pulmonary complications of influenza are most common, unusual presentations you may encounter include influenza encephalopathy (altered mental status, seizures, and mutism) and bacterial superinfection (when fever recurs or recrudesces after initial improvement, often 3-5 days into the course, think Staphylococcus aureus or Group A streptococcal disease). The CDC is alerting practitioners to the potential for increased morbidity and mortality in young/middle aged adults so the parents of your patients are at increased risk this year.
• False-negative testing can happen if the sensitivity of the rapid test is low, but a false-negative test can occur if the specimen is collected late in the clinical course. (This is especially true in the adult population in which testing may be negative at just 4-5 days into the course of disease.)
• Recognize that all hospitalized children should be treated with oseltamivir, as well as children who are immunocompromised; have chronic cardiopulmonary conditions, including hemodynamically significant heart disease and asthma; renal disease; metabolic disease, including diabetes; pregnant teens; morbidly obese patients; patients with neuromuscular/neurodevelopmental conditions (especially those with difficulty controlling airway secretions); and children under 2 years of age.
• I predict you may be hearing about oseltamivir shortages, but for now this relates to the sporadic difficulty in finding the oseltamivir suspension, in part, because of the lack of early season availability of this product at retail pharmacies, many of which are just getting in their stock. Prescribe the suspension for children aged younger than 1 year and be explicit about the mL dosage that should be dispensed. For children over 1 year of age, capsules can be opened and placed in pudding for those who cannot swallow capsules. Lexicomp Online offers guidelines for easy use of 30-mg, 45-mg and 75-mg capsules for different weight categories. If the suspension is necessary for an infant and is not available, the drug can be compounded by your pharmacy using capsules. You may find some pharmacies are reluctant to compound, so be prepared to contact your local children’s hospital for help. And keep offering vaccine throughout the season to healthy patients!
2. Most practitioners are aware of the importance of methicillin-resistant S. aureus (MRSA) as a pathogen that causes bacteremia and musculoskeletal and pulmonary disease in otherwise healthy children. I suspect there is less awareness that, in many locales, methicillin-sensitive S. aureus (MSSA) is being seen just as often, if not slightly more often than MRSA, as a bloodstream pathogen. The inclusion of vancomycin (which covers MRSA) with cefepime should be considered for empiric coverage in the otherwise healthy child with suspected sepsis. Cefepime is a fourth-generation cephalosporin with good gram-negative and gram-positive coverage and also has bactericidal activity against MSSA strains. Clindamycin should be considered as an adjunct to vancomycin and cefepime in those with toxin-mediated disease/toxic shock syndrome. Of course, modification of the empiric regimen should follow identification of the specific pathogen and the site(s) of infection.
3. E. coli remains the most common cause of urinary tract infections in children, but infections caused by multiple drug resistant (MDR) Escherichia coli strains are increasingly being seen. Consider infection caused by extended spectrum beta-lactamase–producing organisms in children with underlying renal anomalies, especially if they have been previously exposed to third-generation cephalosporins. Most strains are also resistant to fluoroquinolones, trimethoprim-sulfamethoxazole, and aminoglycosides as well as to non–carbapenem beta-lactams. Speaking of antibiotic resistance, look for many hospital microbiology laboratories to begin using advanced molecular detection methodology to more quickly identify bacterial and fungal isolates; such methods could reduce the time of identification from over 24 hours with conventional techniques to less than one hour. The use of newer systems to identify microbes and confirm susceptibility testing has the potential to transform care and improve outcomes.
4. Consider the diagnosis of human parechovirus (HPeV) infection in young febrile infants with sepsis/meningitis presentation but negative bacterial cultures. Detection of HPeV by polymerase chain reaction testing in serum or cerebrospinal fluid is diagnostic. Exclusion of herpes simplex virus and enterovirus disease is key, as similar clinical presentations may be seen. HPeV infections are more commonly noted in late spring and early summer in contrast to enteroviral infections, which tend to occur from July to September.
5. The strength of your vaccine recommendation continues to be the most important factor affecting the parental decision to vaccinate a child. Nowhere is this more obvious than with human papillomavirus vaccine (HPV), where practitioners often simply offer the vaccine rather than recommend it. In terms of teenage vaccines, when practitioners recommend Tdap (tetanus, diphtheria, and pertussis vaccine) and meningococcal conjugate vaccine as standard for their patients ("Today your child will receive whooping cough vaccine and the meningitis vaccine."), vaccine uptake is very high. But when it comes to the HPV vaccine, some practitioners feel they first must establish whether the parents are aware of HPV vaccine; then discuss their questions regarding the safety of the vaccine; and finally, explain that the vaccine prevents cancer. Some practitioners offer the option of "thinking about" the vaccine for the next visit, but in such cases, the patient generally leaves without receiving the vaccine. Add HPV vaccine into your standard teen vaccine recommendation and make it a goal to get the first vaccine initiated in all eligible patients. The three-dose HPV vaccine schedule is still recommended, but I predict that simplification of the schedule may occur as early as 2014 in the United States. We’ll keep you posted.
Dr. Jackson is director of the division of infectious disease and associate director of the infectious disease fellowship program at the University of Missouri, Kansas City.
Are you prepared to manage the infectious disease challenges you’ll be facing in 2014? Here are my Top 5 predictions for what lies ahead in infectious diseases for the next year with pearls to help you in your practice. The first addresses a series of concerns around influenza. Others target diagnoses you might not have encountered or considered in the past. The last will hopefully improve HPV vaccination rates in your practice.
1. Expect an especially busy influenza season and the possibility that you may encounter patients with life-threatening influenza. We’ve already detected influenza in over 1,000 children at my institution, almost all 2009 pandemic H1N1 influenza A viruses, which is consistent with the national data from the Centers for Disease Control and Prevention. We are really just a month into influenza season, and we are seeing a significant number of children admitted to our pediatric intensive care unit with life-threatening disease presentations, and we’ve also seen unusual influenza complications. Talk to your ID colleagues about the potential for intravenous zanamivir in critically ill children who do not respond to oseltamivir. While pulmonary complications of influenza are most common, unusual presentations you may encounter include influenza encephalopathy (altered mental status, seizures, and mutism) and bacterial superinfection (when fever recurs or recrudesces after initial improvement, often 3-5 days into the course, think Staphylococcus aureus or Group A streptococcal disease). The CDC is alerting practitioners to the potential for increased morbidity and mortality in young/middle aged adults so the parents of your patients are at increased risk this year.
• False-negative testing can happen if the sensitivity of the rapid test is low, but a false-negative test can occur if the specimen is collected late in the clinical course. (This is especially true in the adult population in which testing may be negative at just 4-5 days into the course of disease.)
• Recognize that all hospitalized children should be treated with oseltamivir, as well as children who are immunocompromised; have chronic cardiopulmonary conditions, including hemodynamically significant heart disease and asthma; renal disease; metabolic disease, including diabetes; pregnant teens; morbidly obese patients; patients with neuromuscular/neurodevelopmental conditions (especially those with difficulty controlling airway secretions); and children under 2 years of age.
• I predict you may be hearing about oseltamivir shortages, but for now this relates to the sporadic difficulty in finding the oseltamivir suspension, in part, because of the lack of early season availability of this product at retail pharmacies, many of which are just getting in their stock. Prescribe the suspension for children aged younger than 1 year and be explicit about the mL dosage that should be dispensed. For children over 1 year of age, capsules can be opened and placed in pudding for those who cannot swallow capsules. Lexicomp Online offers guidelines for easy use of 30-mg, 45-mg and 75-mg capsules for different weight categories. If the suspension is necessary for an infant and is not available, the drug can be compounded by your pharmacy using capsules. You may find some pharmacies are reluctant to compound, so be prepared to contact your local children’s hospital for help. And keep offering vaccine throughout the season to healthy patients!
2. Most practitioners are aware of the importance of methicillin-resistant S. aureus (MRSA) as a pathogen that causes bacteremia and musculoskeletal and pulmonary disease in otherwise healthy children. I suspect there is less awareness that, in many locales, methicillin-sensitive S. aureus (MSSA) is being seen just as often, if not slightly more often than MRSA, as a bloodstream pathogen. The inclusion of vancomycin (which covers MRSA) with cefepime should be considered for empiric coverage in the otherwise healthy child with suspected sepsis. Cefepime is a fourth-generation cephalosporin with good gram-negative and gram-positive coverage and also has bactericidal activity against MSSA strains. Clindamycin should be considered as an adjunct to vancomycin and cefepime in those with toxin-mediated disease/toxic shock syndrome. Of course, modification of the empiric regimen should follow identification of the specific pathogen and the site(s) of infection.
3. E. coli remains the most common cause of urinary tract infections in children, but infections caused by multiple drug resistant (MDR) Escherichia coli strains are increasingly being seen. Consider infection caused by extended spectrum beta-lactamase–producing organisms in children with underlying renal anomalies, especially if they have been previously exposed to third-generation cephalosporins. Most strains are also resistant to fluoroquinolones, trimethoprim-sulfamethoxazole, and aminoglycosides as well as to non–carbapenem beta-lactams. Speaking of antibiotic resistance, look for many hospital microbiology laboratories to begin using advanced molecular detection methodology to more quickly identify bacterial and fungal isolates; such methods could reduce the time of identification from over 24 hours with conventional techniques to less than one hour. The use of newer systems to identify microbes and confirm susceptibility testing has the potential to transform care and improve outcomes.
4. Consider the diagnosis of human parechovirus (HPeV) infection in young febrile infants with sepsis/meningitis presentation but negative bacterial cultures. Detection of HPeV by polymerase chain reaction testing in serum or cerebrospinal fluid is diagnostic. Exclusion of herpes simplex virus and enterovirus disease is key, as similar clinical presentations may be seen. HPeV infections are more commonly noted in late spring and early summer in contrast to enteroviral infections, which tend to occur from July to September.
5. The strength of your vaccine recommendation continues to be the most important factor affecting the parental decision to vaccinate a child. Nowhere is this more obvious than with human papillomavirus vaccine (HPV), where practitioners often simply offer the vaccine rather than recommend it. In terms of teenage vaccines, when practitioners recommend Tdap (tetanus, diphtheria, and pertussis vaccine) and meningococcal conjugate vaccine as standard for their patients ("Today your child will receive whooping cough vaccine and the meningitis vaccine."), vaccine uptake is very high. But when it comes to the HPV vaccine, some practitioners feel they first must establish whether the parents are aware of HPV vaccine; then discuss their questions regarding the safety of the vaccine; and finally, explain that the vaccine prevents cancer. Some practitioners offer the option of "thinking about" the vaccine for the next visit, but in such cases, the patient generally leaves without receiving the vaccine. Add HPV vaccine into your standard teen vaccine recommendation and make it a goal to get the first vaccine initiated in all eligible patients. The three-dose HPV vaccine schedule is still recommended, but I predict that simplification of the schedule may occur as early as 2014 in the United States. We’ll keep you posted.
Dr. Jackson is director of the division of infectious disease and associate director of the infectious disease fellowship program at the University of Missouri, Kansas City.
Spectral gradient acoustic reflectometry aids diagnosis of acute otitis media and otitis media with effusion
Spectral gradient acoustic reflectometer (SGAR) is a technology to assist in the detection of middle ear fluid occurring in the context of diagnosing acute otitis media (AOM) and otitis media with effusion (OME). The technology involves sending a harmless, inaudible sonar-like sound wave from the emitter that goes through the tympanic membrane, hits the posterior wall of the middle ear space, and bounces back to the sound detector in the device. If there is only air in the middle ear space, the sound wave bounces back quickly, and you get a high reading. If the sound wave bounces back more slowly, there is middle ear effusion. The thicker the effusion, the more likely it is pus and an AOM or a chronic OME (depending on the clinical situation), causing the sound wave to bounce back more slowly and giving a low reading.
The specificity of a high reading is remarkable at around 95%, so a high reading is a big reassurance that middle ear effusion is absent. A lower reading suggests effusion and the lower it is, the greater the sensitivity. When I get an unexpected higher or lower reading, I go back and reexamine the patient.
I asked our nurses to compare the handheld tympanometer to the SGAR. They actually perform the testing, and I interpret it. The nurses said:
• The SGAR is easier to use because of how quickly a readout is obtained.
• If a child is crying or moving, they can still get a readout.
• You don’t have to change the tip of the SGAR for the size of the external ear canal.
• The SGAR is easier to read than the tympanometer.
• The SGAR is easier to interpret for the parents.
• You don’t have to get a seal with the ear canal with SGAR, as you do with a tympanometer.
• The SGAR uses a disposable tip.
I asked our office manager to look up our use of the SGAR and tympanometer during our everyday practice. We found that SGAR or tympanometry was used in 12% of patient encounters in which the diagnosis of AOM or OME was part of the chief complaint. The ratio of use was 3:1, favoring SGAR. The most frequent use was in 30% of patient encounters tied to the diagnosis of "otalgia" (388.70) because with that diagnosis, we are stating to parents and patients that there is no middle ear pathology seen on exam, and it is confirmed by a test using sonar waves with the SGAR device. Our nurse practitioners and physician assistants particularly find the use of the SGAR beneficial in helping to reassure the parents and patients that they have not missed an AOM or OME.
The billing code is the same for SGAR and tympanometry (92567), so the fee payment is the same for both tests. Our second most common use is in association with possible AOM (382.9) at 12% of visits. Third is OME (381.02) used in a follow-up visit to determine the presence and thickness of persisting effusion.
About one-quarter of children seen in our practice with a chief complaint of "earache" receive the diagnosis of otalgia, often confirmed by SGAR, and do not receive an antibiotic. Thus, they are offsetting the charge for the procedure by saving on the costs of antibiotics and the accumulation of excessive diagnoses of AOM and OME leading to ear tube surgeries and tonsillectomy/adenoidectomy. The diagnosis of AOM and OME requires a middle ear effusion to be accurate, and only SGAR measures detection of middle ear effusion. SGAR is a must own device for clinicians who exam ears. SGAR can help in conjunction with otoscopy for a difficult diagnosis of AOM. If I am having troubleremoving wax, or if the external ear canal is particularly curved, or if I’m on the fence or the parent seems to need further evidence of my diagnosis, I turn to the SGAR. If I can get a reading, then it can really help, and my nurses are successful in getting a reading about 90% of the time. The main issue is ear canal wax, because occlusion by wax of more than 50% of the external ear canal opening causes invalid readings.
We should prescribe antibiotics for AOM in my opinion, but not for otalgia and not if the diagnosis is uncertain. The SGAR device when properly used can help to reduce unnecessary use of antibiotics and their complications. In prior "ID Consult" columns, I have discussed improving the diagnostic accuracy of AOM and OME. Performing a good otoscopic exam with the best tools available and combining that exam with SGAR or tympanometry, in selected cases, is the best practice in my opinion, and what I do in my own practice.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester General Hospital, N.Y. He is also a pediatrician at Legacy Pediatrics in Rochester. E-mail him at [email protected]. Innovia Medical, the company that is bringing the SGAR EarCheck Pro back to market in 2014 after improvement and the addition of a USB port to allow the import of the data readout into the electronic medical record, asked Dr. Pichichero to assess the SGAR device.
Spectral gradient acoustic reflectometer (SGAR) is a technology to assist in the detection of middle ear fluid occurring in the context of diagnosing acute otitis media (AOM) and otitis media with effusion (OME). The technology involves sending a harmless, inaudible sonar-like sound wave from the emitter that goes through the tympanic membrane, hits the posterior wall of the middle ear space, and bounces back to the sound detector in the device. If there is only air in the middle ear space, the sound wave bounces back quickly, and you get a high reading. If the sound wave bounces back more slowly, there is middle ear effusion. The thicker the effusion, the more likely it is pus and an AOM or a chronic OME (depending on the clinical situation), causing the sound wave to bounce back more slowly and giving a low reading.
The specificity of a high reading is remarkable at around 95%, so a high reading is a big reassurance that middle ear effusion is absent. A lower reading suggests effusion and the lower it is, the greater the sensitivity. When I get an unexpected higher or lower reading, I go back and reexamine the patient.
I asked our nurses to compare the handheld tympanometer to the SGAR. They actually perform the testing, and I interpret it. The nurses said:
• The SGAR is easier to use because of how quickly a readout is obtained.
• If a child is crying or moving, they can still get a readout.
• You don’t have to change the tip of the SGAR for the size of the external ear canal.
• The SGAR is easier to read than the tympanometer.
• The SGAR is easier to interpret for the parents.
• You don’t have to get a seal with the ear canal with SGAR, as you do with a tympanometer.
• The SGAR uses a disposable tip.
I asked our office manager to look up our use of the SGAR and tympanometer during our everyday practice. We found that SGAR or tympanometry was used in 12% of patient encounters in which the diagnosis of AOM or OME was part of the chief complaint. The ratio of use was 3:1, favoring SGAR. The most frequent use was in 30% of patient encounters tied to the diagnosis of "otalgia" (388.70) because with that diagnosis, we are stating to parents and patients that there is no middle ear pathology seen on exam, and it is confirmed by a test using sonar waves with the SGAR device. Our nurse practitioners and physician assistants particularly find the use of the SGAR beneficial in helping to reassure the parents and patients that they have not missed an AOM or OME.
The billing code is the same for SGAR and tympanometry (92567), so the fee payment is the same for both tests. Our second most common use is in association with possible AOM (382.9) at 12% of visits. Third is OME (381.02) used in a follow-up visit to determine the presence and thickness of persisting effusion.
About one-quarter of children seen in our practice with a chief complaint of "earache" receive the diagnosis of otalgia, often confirmed by SGAR, and do not receive an antibiotic. Thus, they are offsetting the charge for the procedure by saving on the costs of antibiotics and the accumulation of excessive diagnoses of AOM and OME leading to ear tube surgeries and tonsillectomy/adenoidectomy. The diagnosis of AOM and OME requires a middle ear effusion to be accurate, and only SGAR measures detection of middle ear effusion. SGAR is a must own device for clinicians who exam ears. SGAR can help in conjunction with otoscopy for a difficult diagnosis of AOM. If I am having troubleremoving wax, or if the external ear canal is particularly curved, or if I’m on the fence or the parent seems to need further evidence of my diagnosis, I turn to the SGAR. If I can get a reading, then it can really help, and my nurses are successful in getting a reading about 90% of the time. The main issue is ear canal wax, because occlusion by wax of more than 50% of the external ear canal opening causes invalid readings.
We should prescribe antibiotics for AOM in my opinion, but not for otalgia and not if the diagnosis is uncertain. The SGAR device when properly used can help to reduce unnecessary use of antibiotics and their complications. In prior "ID Consult" columns, I have discussed improving the diagnostic accuracy of AOM and OME. Performing a good otoscopic exam with the best tools available and combining that exam with SGAR or tympanometry, in selected cases, is the best practice in my opinion, and what I do in my own practice.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester General Hospital, N.Y. He is also a pediatrician at Legacy Pediatrics in Rochester. E-mail him at [email protected]. Innovia Medical, the company that is bringing the SGAR EarCheck Pro back to market in 2014 after improvement and the addition of a USB port to allow the import of the data readout into the electronic medical record, asked Dr. Pichichero to assess the SGAR device.
Spectral gradient acoustic reflectometer (SGAR) is a technology to assist in the detection of middle ear fluid occurring in the context of diagnosing acute otitis media (AOM) and otitis media with effusion (OME). The technology involves sending a harmless, inaudible sonar-like sound wave from the emitter that goes through the tympanic membrane, hits the posterior wall of the middle ear space, and bounces back to the sound detector in the device. If there is only air in the middle ear space, the sound wave bounces back quickly, and you get a high reading. If the sound wave bounces back more slowly, there is middle ear effusion. The thicker the effusion, the more likely it is pus and an AOM or a chronic OME (depending on the clinical situation), causing the sound wave to bounce back more slowly and giving a low reading.
The specificity of a high reading is remarkable at around 95%, so a high reading is a big reassurance that middle ear effusion is absent. A lower reading suggests effusion and the lower it is, the greater the sensitivity. When I get an unexpected higher or lower reading, I go back and reexamine the patient.
I asked our nurses to compare the handheld tympanometer to the SGAR. They actually perform the testing, and I interpret it. The nurses said:
• The SGAR is easier to use because of how quickly a readout is obtained.
• If a child is crying or moving, they can still get a readout.
• You don’t have to change the tip of the SGAR for the size of the external ear canal.
• The SGAR is easier to read than the tympanometer.
• The SGAR is easier to interpret for the parents.
• You don’t have to get a seal with the ear canal with SGAR, as you do with a tympanometer.
• The SGAR uses a disposable tip.
I asked our office manager to look up our use of the SGAR and tympanometer during our everyday practice. We found that SGAR or tympanometry was used in 12% of patient encounters in which the diagnosis of AOM or OME was part of the chief complaint. The ratio of use was 3:1, favoring SGAR. The most frequent use was in 30% of patient encounters tied to the diagnosis of "otalgia" (388.70) because with that diagnosis, we are stating to parents and patients that there is no middle ear pathology seen on exam, and it is confirmed by a test using sonar waves with the SGAR device. Our nurse practitioners and physician assistants particularly find the use of the SGAR beneficial in helping to reassure the parents and patients that they have not missed an AOM or OME.
The billing code is the same for SGAR and tympanometry (92567), so the fee payment is the same for both tests. Our second most common use is in association with possible AOM (382.9) at 12% of visits. Third is OME (381.02) used in a follow-up visit to determine the presence and thickness of persisting effusion.
About one-quarter of children seen in our practice with a chief complaint of "earache" receive the diagnosis of otalgia, often confirmed by SGAR, and do not receive an antibiotic. Thus, they are offsetting the charge for the procedure by saving on the costs of antibiotics and the accumulation of excessive diagnoses of AOM and OME leading to ear tube surgeries and tonsillectomy/adenoidectomy. The diagnosis of AOM and OME requires a middle ear effusion to be accurate, and only SGAR measures detection of middle ear effusion. SGAR is a must own device for clinicians who exam ears. SGAR can help in conjunction with otoscopy for a difficult diagnosis of AOM. If I am having troubleremoving wax, or if the external ear canal is particularly curved, or if I’m on the fence or the parent seems to need further evidence of my diagnosis, I turn to the SGAR. If I can get a reading, then it can really help, and my nurses are successful in getting a reading about 90% of the time. The main issue is ear canal wax, because occlusion by wax of more than 50% of the external ear canal opening causes invalid readings.
We should prescribe antibiotics for AOM in my opinion, but not for otalgia and not if the diagnosis is uncertain. The SGAR device when properly used can help to reduce unnecessary use of antibiotics and their complications. In prior "ID Consult" columns, I have discussed improving the diagnostic accuracy of AOM and OME. Performing a good otoscopic exam with the best tools available and combining that exam with SGAR or tympanometry, in selected cases, is the best practice in my opinion, and what I do in my own practice.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester General Hospital, N.Y. He is also a pediatrician at Legacy Pediatrics in Rochester. E-mail him at [email protected]. Innovia Medical, the company that is bringing the SGAR EarCheck Pro back to market in 2014 after improvement and the addition of a USB port to allow the import of the data readout into the electronic medical record, asked Dr. Pichichero to assess the SGAR device.
Improving diagnosis of otitis media
The diagnosis of otitis media absolutely requires visualization of the tympanic membrane. So it may be time to upgrade your tools to do a better job in diagnosing. Think about how often you use your otoscope. Are you using the best available technology, or are you using the otoscope you got in medical school, perhaps quite a few years ago? It may be time for an upgrade. Considering how often you might use an otoscope, you can afford it. You deserve it.
The improved features of new otoscopes include remarkably better illumination. The quality of the light not only has to do with the lumens, but also the color of the light. Also there is a version of an otoscope called a Macro View (Welch Allyn, Skaneateles Falls, N.Y.). It allows you to increase the magnification on the tympanic membrane (TM) as needed. There is an option to purchase a lighter and smaller handle for the scope, and that can improve ease of use for persons with small hands.
For all otoscopes, the bulb should be replaced when illumination begins to fade and you cannot get back the intensity of light with a battery recharge. For most primary care practitioners, bulbs usually require replacement annually.
Speculum size is key to getting the most light onto the TM; the bigger the speculum, the better. Advancing the speculum as far into the external ear canal as you can without causing discomfort helps improve the intensity of the light shone on the TM. While it is convenient to use disposable specula, they are not as good as reusable ones because the finish on the inside of disposable specula is duller than on reusable specula, thus decreasing the amount of light shone on the TM. Also, disposable specula often are too short, and that too reduces the light shone on the TM.
Many clinicians have not been trained on using pneumatic otoscopy, or even if trained, they find it inconvenient and/or problematic to use because it requires a seal of the speculum against the external auditory canal; this makes children cry. The problem is that you really need to use pneumatic otoscopy in some cases to determine if the TM is retracted (no acute infection) or bulging (acute infection, or AOM). I use pneumatic otoscopy in about one-third of cases, and to this day I am surprised sometimes when the negative pressure pulls a retracted TM forward when I was pretty sure the TM more likely was bulging. There are specula with a semisoft sleeve midway down the shaft, but I have not found they are any less likely to cause the child to cry, because as anyone knows who has stuck a Q-tip swab into their ear canal, it is sensitive skin.
Then there is the wax! Clinical studies show that about half of children have wax in their external auditory canal blocking 25% of the view, and one-quarter have wax blocking 50% of the view. The best tool I have found to clear the wax is a plastic cerumen spoon (called a safe ear curette) made by Bionix Medical Technologies (Toledo, Ohio). I use the white ones as they are the most flexible. Ninety percent of the time I can scoop the wax out of the way and get a good view. For the remaining difficult cases, the ear canal needs to be irrigated with warm water (code 69210), and then the remaining wax can be scooped out.
Tympanometry (code 92567) is another tool to aid in accurate diagnosis and follow-up of otitis media. A key aspect of the diagnostic algorithm advocated by the American Academy of Pediatrics is a determination of whether the TM is bulging (AOM) or not (no AOM). A retracted TM is inconsistent with the diagnosis of AOM. Tympanometry requires a seal with the external auditory canal because a pressure is applied to the TM to determine TM movement. After positive and negative pressure are applied by the instrument, the readout will be a positive peaked curve (bulging), a negative peaked curve (retracted), a normal peaked curve (normal), or flat, no curve (stiff TM).
The first three readouts are very helpful in distinguishing AOM from no AOM. The flat curve indicates three possibilities: The TM is stiff, perhaps due to thickening; the TM is not moving because the middle ear space is filled with pus behind it, meaning it is AOM; or the TM is not moving because the middle ear space is filled with effusion fluid behind it, meaning the patient has otitis media with effusion. In the case of a flat readout, the tie breaker should come from the visual exam and/or the use of spectral gradient acoustic reflectometry (code 92567).
These better tools and techniques should improve your diagnosis of otitis media.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester General Hospital, N.Y. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no financial disclosures relevant to this article. To comment, e-mail him at [email protected].
The diagnosis of otitis media absolutely requires visualization of the tympanic membrane. So it may be time to upgrade your tools to do a better job in diagnosing. Think about how often you use your otoscope. Are you using the best available technology, or are you using the otoscope you got in medical school, perhaps quite a few years ago? It may be time for an upgrade. Considering how often you might use an otoscope, you can afford it. You deserve it.
The improved features of new otoscopes include remarkably better illumination. The quality of the light not only has to do with the lumens, but also the color of the light. Also there is a version of an otoscope called a Macro View (Welch Allyn, Skaneateles Falls, N.Y.). It allows you to increase the magnification on the tympanic membrane (TM) as needed. There is an option to purchase a lighter and smaller handle for the scope, and that can improve ease of use for persons with small hands.
For all otoscopes, the bulb should be replaced when illumination begins to fade and you cannot get back the intensity of light with a battery recharge. For most primary care practitioners, bulbs usually require replacement annually.
Speculum size is key to getting the most light onto the TM; the bigger the speculum, the better. Advancing the speculum as far into the external ear canal as you can without causing discomfort helps improve the intensity of the light shone on the TM. While it is convenient to use disposable specula, they are not as good as reusable ones because the finish on the inside of disposable specula is duller than on reusable specula, thus decreasing the amount of light shone on the TM. Also, disposable specula often are too short, and that too reduces the light shone on the TM.
Many clinicians have not been trained on using pneumatic otoscopy, or even if trained, they find it inconvenient and/or problematic to use because it requires a seal of the speculum against the external auditory canal; this makes children cry. The problem is that you really need to use pneumatic otoscopy in some cases to determine if the TM is retracted (no acute infection) or bulging (acute infection, or AOM). I use pneumatic otoscopy in about one-third of cases, and to this day I am surprised sometimes when the negative pressure pulls a retracted TM forward when I was pretty sure the TM more likely was bulging. There are specula with a semisoft sleeve midway down the shaft, but I have not found they are any less likely to cause the child to cry, because as anyone knows who has stuck a Q-tip swab into their ear canal, it is sensitive skin.
Then there is the wax! Clinical studies show that about half of children have wax in their external auditory canal blocking 25% of the view, and one-quarter have wax blocking 50% of the view. The best tool I have found to clear the wax is a plastic cerumen spoon (called a safe ear curette) made by Bionix Medical Technologies (Toledo, Ohio). I use the white ones as they are the most flexible. Ninety percent of the time I can scoop the wax out of the way and get a good view. For the remaining difficult cases, the ear canal needs to be irrigated with warm water (code 69210), and then the remaining wax can be scooped out.
Tympanometry (code 92567) is another tool to aid in accurate diagnosis and follow-up of otitis media. A key aspect of the diagnostic algorithm advocated by the American Academy of Pediatrics is a determination of whether the TM is bulging (AOM) or not (no AOM). A retracted TM is inconsistent with the diagnosis of AOM. Tympanometry requires a seal with the external auditory canal because a pressure is applied to the TM to determine TM movement. After positive and negative pressure are applied by the instrument, the readout will be a positive peaked curve (bulging), a negative peaked curve (retracted), a normal peaked curve (normal), or flat, no curve (stiff TM).
The first three readouts are very helpful in distinguishing AOM from no AOM. The flat curve indicates three possibilities: The TM is stiff, perhaps due to thickening; the TM is not moving because the middle ear space is filled with pus behind it, meaning it is AOM; or the TM is not moving because the middle ear space is filled with effusion fluid behind it, meaning the patient has otitis media with effusion. In the case of a flat readout, the tie breaker should come from the visual exam and/or the use of spectral gradient acoustic reflectometry (code 92567).
These better tools and techniques should improve your diagnosis of otitis media.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester General Hospital, N.Y. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no financial disclosures relevant to this article. To comment, e-mail him at [email protected].
The diagnosis of otitis media absolutely requires visualization of the tympanic membrane. So it may be time to upgrade your tools to do a better job in diagnosing. Think about how often you use your otoscope. Are you using the best available technology, or are you using the otoscope you got in medical school, perhaps quite a few years ago? It may be time for an upgrade. Considering how often you might use an otoscope, you can afford it. You deserve it.
The improved features of new otoscopes include remarkably better illumination. The quality of the light not only has to do with the lumens, but also the color of the light. Also there is a version of an otoscope called a Macro View (Welch Allyn, Skaneateles Falls, N.Y.). It allows you to increase the magnification on the tympanic membrane (TM) as needed. There is an option to purchase a lighter and smaller handle for the scope, and that can improve ease of use for persons with small hands.
For all otoscopes, the bulb should be replaced when illumination begins to fade and you cannot get back the intensity of light with a battery recharge. For most primary care practitioners, bulbs usually require replacement annually.
Speculum size is key to getting the most light onto the TM; the bigger the speculum, the better. Advancing the speculum as far into the external ear canal as you can without causing discomfort helps improve the intensity of the light shone on the TM. While it is convenient to use disposable specula, they are not as good as reusable ones because the finish on the inside of disposable specula is duller than on reusable specula, thus decreasing the amount of light shone on the TM. Also, disposable specula often are too short, and that too reduces the light shone on the TM.
Many clinicians have not been trained on using pneumatic otoscopy, or even if trained, they find it inconvenient and/or problematic to use because it requires a seal of the speculum against the external auditory canal; this makes children cry. The problem is that you really need to use pneumatic otoscopy in some cases to determine if the TM is retracted (no acute infection) or bulging (acute infection, or AOM). I use pneumatic otoscopy in about one-third of cases, and to this day I am surprised sometimes when the negative pressure pulls a retracted TM forward when I was pretty sure the TM more likely was bulging. There are specula with a semisoft sleeve midway down the shaft, but I have not found they are any less likely to cause the child to cry, because as anyone knows who has stuck a Q-tip swab into their ear canal, it is sensitive skin.
Then there is the wax! Clinical studies show that about half of children have wax in their external auditory canal blocking 25% of the view, and one-quarter have wax blocking 50% of the view. The best tool I have found to clear the wax is a plastic cerumen spoon (called a safe ear curette) made by Bionix Medical Technologies (Toledo, Ohio). I use the white ones as they are the most flexible. Ninety percent of the time I can scoop the wax out of the way and get a good view. For the remaining difficult cases, the ear canal needs to be irrigated with warm water (code 69210), and then the remaining wax can be scooped out.
Tympanometry (code 92567) is another tool to aid in accurate diagnosis and follow-up of otitis media. A key aspect of the diagnostic algorithm advocated by the American Academy of Pediatrics is a determination of whether the TM is bulging (AOM) or not (no AOM). A retracted TM is inconsistent with the diagnosis of AOM. Tympanometry requires a seal with the external auditory canal because a pressure is applied to the TM to determine TM movement. After positive and negative pressure are applied by the instrument, the readout will be a positive peaked curve (bulging), a negative peaked curve (retracted), a normal peaked curve (normal), or flat, no curve (stiff TM).
The first three readouts are very helpful in distinguishing AOM from no AOM. The flat curve indicates three possibilities: The TM is stiff, perhaps due to thickening; the TM is not moving because the middle ear space is filled with pus behind it, meaning it is AOM; or the TM is not moving because the middle ear space is filled with effusion fluid behind it, meaning the patient has otitis media with effusion. In the case of a flat readout, the tie breaker should come from the visual exam and/or the use of spectral gradient acoustic reflectometry (code 92567).
These better tools and techniques should improve your diagnosis of otitis media.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester General Hospital, N.Y. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no financial disclosures relevant to this article. To comment, e-mail him at [email protected].
Mycoplasma pneumoniae
Mycoplasma pneumoniae is a cell wall–deficient pleomorphic bacterium and well-reported cause of respiratory tract infection in the school-aged child. Symptoms are variable, and clinical presentations run the gamut from upper respiratory (usually self-limited) and lower respiratory tract involvement (pneumonia) to unusual manifestations including nervous system disease (encephalitis, cerebellar ataxia, transverse myelitis), hemolytic anemia, Stevens-Johnson syndrome, and myocarditis/pericarditis.
Pneumonia occurs in 10% of infected school-aged children, and cough can persist for 3-4 weeks; some children wheeze in the setting of Mycoplasma infection. Radiographic patterns of disease are variable; patchy alveolar infiltrates with small pleural effusions are often described. Consolidated pneumonia, large effusions, and hilar adenopathy are uncommonly reported, and severe disease has been described in certain patient populations, including those with sickle cell disease, children with Down syndrome, and those with immunodeficiencies. The acute chest presentation has been associated with M. pneumoniae in children with sickle cell anemia and prolonged hospitalizations (mean, 10 days), and the need for transfusion and mechanical ventilation was noted in 82% and 6%, respectively, in one study (Pediatrics 2003;112(1 Pt 1):87-95). Community clusters of pneumonia are reported in school-aged children, and in Rhode Island, an outbreak was reported in children from four schools; 76 had pneumonia and 3 had encephalitis (J. Infect. Dis. 2008;198:1365-74).
Considering this is a common pathogen, there are a number of questions regarding the scope of disease and impact of treatment that are incompletely answered. The first problem is that it is hard to confirm diagnostically. Culture is technically difficult, the organism takes up to 3 weeks to grow, and the diagnostic test is offered in very few labs. The old-fashioned cold agglutinin test has a low sensitivity and specificity; an increase in titers can be seen during a variety of viral infections. Polymerase chain reaction (PCR) on respiratory secretions is increasingly available; sensitivity and specificity are said to be 80% and 100%, respectively. The organism can persist in the respiratory tract for several weeks though, even after treatment, so PCR can remain positive for 2-3 weeks. This makes it hard to use PCR to confirm M. pneumoniae as the etiologic agent, especially in the setting of unusual clinical presentations. Serologic testing is often ordered and hard to interpret. False positive IgM antibody tests are not uncommon, and IgM antibody can persist for months. Outside of PCR and culture, acute and convalescent specimens can be used diagnostically, and a fourfold IgG antibody rise is consistent with acute infection.
Macrolides are regarded as the preferred treatment for M. pneumoniae pneumonia, but several studies question whether treatment impacts the clinical course. This may be due to the inherent difficulty of confirming M. pneumoniae as the etiologic agent, as most studies used serology to confirm the diagnosis. In countries outside the United States, macrolide resistance is well reported, and this may be underappreciated in the United States. We recently cared for a teenager with Down syndrome with pneumonia caused by M. pneumoniae who had a protracted clinical course. Fever and hypoxemia were persistent over a several-week period despite two courses of azithromycin and exclusion of virus, bacteria, and fungal pathogens. Bronchoalveolar lavage was performed, M. pneumoniae was detected by PCR, and macrolide resistance was confirmed. Levofloxacin was given, and she recovered over the next week.
Macrolide resistance is commonly reported outside the United States; rates in China are reported to be greater than 90%, in Japan 80%, and in Europe, between 15% and 25%. A recent study from Greg Storch and his colleagues (Pediatr. Infect. Dis. J. 2012;31:409-10) documented macrolide resistance in 8% of respiratory samples collected between 2007 and 2010 (49 patients; mean age, 10 years), noting the resistance rate was 3% in 2007-2008 and 15% in 2009-2010. A recently published study of data from Canada reported that 12.1% of M. pneumoniae–positive specimens collected between 2010 and January 2012 carried nucleotide mutations associated with macrolide resistance in the 23S rRNA gene (Emerg. Infect. Dis. 2013 September [doi: 10.3201/eid1909.121466]). Anecdotal studies suggest that patients with macrolide-resistant M. pneumoniae infection clinically improve when given doxycycline (or minocycline) or levofloxacin.
A number of clinical questions regarding M. pneumoniae may be answered more definitively in the future, but we need more easily available diagnostics (PCR is a good start), routinely accessible susceptibility data, and a good randomized controlled study to investigate the question of whether treatment shortens the course of disease.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri-Kansas City. She said she has no conflicts of interest to disclose. E-mail her at [email protected].
Mycoplasma pneumoniae is a cell wall–deficient pleomorphic bacterium and well-reported cause of respiratory tract infection in the school-aged child. Symptoms are variable, and clinical presentations run the gamut from upper respiratory (usually self-limited) and lower respiratory tract involvement (pneumonia) to unusual manifestations including nervous system disease (encephalitis, cerebellar ataxia, transverse myelitis), hemolytic anemia, Stevens-Johnson syndrome, and myocarditis/pericarditis.
Pneumonia occurs in 10% of infected school-aged children, and cough can persist for 3-4 weeks; some children wheeze in the setting of Mycoplasma infection. Radiographic patterns of disease are variable; patchy alveolar infiltrates with small pleural effusions are often described. Consolidated pneumonia, large effusions, and hilar adenopathy are uncommonly reported, and severe disease has been described in certain patient populations, including those with sickle cell disease, children with Down syndrome, and those with immunodeficiencies. The acute chest presentation has been associated with M. pneumoniae in children with sickle cell anemia and prolonged hospitalizations (mean, 10 days), and the need for transfusion and mechanical ventilation was noted in 82% and 6%, respectively, in one study (Pediatrics 2003;112(1 Pt 1):87-95). Community clusters of pneumonia are reported in school-aged children, and in Rhode Island, an outbreak was reported in children from four schools; 76 had pneumonia and 3 had encephalitis (J. Infect. Dis. 2008;198:1365-74).
Considering this is a common pathogen, there are a number of questions regarding the scope of disease and impact of treatment that are incompletely answered. The first problem is that it is hard to confirm diagnostically. Culture is technically difficult, the organism takes up to 3 weeks to grow, and the diagnostic test is offered in very few labs. The old-fashioned cold agglutinin test has a low sensitivity and specificity; an increase in titers can be seen during a variety of viral infections. Polymerase chain reaction (PCR) on respiratory secretions is increasingly available; sensitivity and specificity are said to be 80% and 100%, respectively. The organism can persist in the respiratory tract for several weeks though, even after treatment, so PCR can remain positive for 2-3 weeks. This makes it hard to use PCR to confirm M. pneumoniae as the etiologic agent, especially in the setting of unusual clinical presentations. Serologic testing is often ordered and hard to interpret. False positive IgM antibody tests are not uncommon, and IgM antibody can persist for months. Outside of PCR and culture, acute and convalescent specimens can be used diagnostically, and a fourfold IgG antibody rise is consistent with acute infection.
Macrolides are regarded as the preferred treatment for M. pneumoniae pneumonia, but several studies question whether treatment impacts the clinical course. This may be due to the inherent difficulty of confirming M. pneumoniae as the etiologic agent, as most studies used serology to confirm the diagnosis. In countries outside the United States, macrolide resistance is well reported, and this may be underappreciated in the United States. We recently cared for a teenager with Down syndrome with pneumonia caused by M. pneumoniae who had a protracted clinical course. Fever and hypoxemia were persistent over a several-week period despite two courses of azithromycin and exclusion of virus, bacteria, and fungal pathogens. Bronchoalveolar lavage was performed, M. pneumoniae was detected by PCR, and macrolide resistance was confirmed. Levofloxacin was given, and she recovered over the next week.
Macrolide resistance is commonly reported outside the United States; rates in China are reported to be greater than 90%, in Japan 80%, and in Europe, between 15% and 25%. A recent study from Greg Storch and his colleagues (Pediatr. Infect. Dis. J. 2012;31:409-10) documented macrolide resistance in 8% of respiratory samples collected between 2007 and 2010 (49 patients; mean age, 10 years), noting the resistance rate was 3% in 2007-2008 and 15% in 2009-2010. A recently published study of data from Canada reported that 12.1% of M. pneumoniae–positive specimens collected between 2010 and January 2012 carried nucleotide mutations associated with macrolide resistance in the 23S rRNA gene (Emerg. Infect. Dis. 2013 September [doi: 10.3201/eid1909.121466]). Anecdotal studies suggest that patients with macrolide-resistant M. pneumoniae infection clinically improve when given doxycycline (or minocycline) or levofloxacin.
A number of clinical questions regarding M. pneumoniae may be answered more definitively in the future, but we need more easily available diagnostics (PCR is a good start), routinely accessible susceptibility data, and a good randomized controlled study to investigate the question of whether treatment shortens the course of disease.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri-Kansas City. She said she has no conflicts of interest to disclose. E-mail her at [email protected].
Mycoplasma pneumoniae is a cell wall–deficient pleomorphic bacterium and well-reported cause of respiratory tract infection in the school-aged child. Symptoms are variable, and clinical presentations run the gamut from upper respiratory (usually self-limited) and lower respiratory tract involvement (pneumonia) to unusual manifestations including nervous system disease (encephalitis, cerebellar ataxia, transverse myelitis), hemolytic anemia, Stevens-Johnson syndrome, and myocarditis/pericarditis.
Pneumonia occurs in 10% of infected school-aged children, and cough can persist for 3-4 weeks; some children wheeze in the setting of Mycoplasma infection. Radiographic patterns of disease are variable; patchy alveolar infiltrates with small pleural effusions are often described. Consolidated pneumonia, large effusions, and hilar adenopathy are uncommonly reported, and severe disease has been described in certain patient populations, including those with sickle cell disease, children with Down syndrome, and those with immunodeficiencies. The acute chest presentation has been associated with M. pneumoniae in children with sickle cell anemia and prolonged hospitalizations (mean, 10 days), and the need for transfusion and mechanical ventilation was noted in 82% and 6%, respectively, in one study (Pediatrics 2003;112(1 Pt 1):87-95). Community clusters of pneumonia are reported in school-aged children, and in Rhode Island, an outbreak was reported in children from four schools; 76 had pneumonia and 3 had encephalitis (J. Infect. Dis. 2008;198:1365-74).
Considering this is a common pathogen, there are a number of questions regarding the scope of disease and impact of treatment that are incompletely answered. The first problem is that it is hard to confirm diagnostically. Culture is technically difficult, the organism takes up to 3 weeks to grow, and the diagnostic test is offered in very few labs. The old-fashioned cold agglutinin test has a low sensitivity and specificity; an increase in titers can be seen during a variety of viral infections. Polymerase chain reaction (PCR) on respiratory secretions is increasingly available; sensitivity and specificity are said to be 80% and 100%, respectively. The organism can persist in the respiratory tract for several weeks though, even after treatment, so PCR can remain positive for 2-3 weeks. This makes it hard to use PCR to confirm M. pneumoniae as the etiologic agent, especially in the setting of unusual clinical presentations. Serologic testing is often ordered and hard to interpret. False positive IgM antibody tests are not uncommon, and IgM antibody can persist for months. Outside of PCR and culture, acute and convalescent specimens can be used diagnostically, and a fourfold IgG antibody rise is consistent with acute infection.
Macrolides are regarded as the preferred treatment for M. pneumoniae pneumonia, but several studies question whether treatment impacts the clinical course. This may be due to the inherent difficulty of confirming M. pneumoniae as the etiologic agent, as most studies used serology to confirm the diagnosis. In countries outside the United States, macrolide resistance is well reported, and this may be underappreciated in the United States. We recently cared for a teenager with Down syndrome with pneumonia caused by M. pneumoniae who had a protracted clinical course. Fever and hypoxemia were persistent over a several-week period despite two courses of azithromycin and exclusion of virus, bacteria, and fungal pathogens. Bronchoalveolar lavage was performed, M. pneumoniae was detected by PCR, and macrolide resistance was confirmed. Levofloxacin was given, and she recovered over the next week.
Macrolide resistance is commonly reported outside the United States; rates in China are reported to be greater than 90%, in Japan 80%, and in Europe, between 15% and 25%. A recent study from Greg Storch and his colleagues (Pediatr. Infect. Dis. J. 2012;31:409-10) documented macrolide resistance in 8% of respiratory samples collected between 2007 and 2010 (49 patients; mean age, 10 years), noting the resistance rate was 3% in 2007-2008 and 15% in 2009-2010. A recently published study of data from Canada reported that 12.1% of M. pneumoniae–positive specimens collected between 2010 and January 2012 carried nucleotide mutations associated with macrolide resistance in the 23S rRNA gene (Emerg. Infect. Dis. 2013 September [doi: 10.3201/eid1909.121466]). Anecdotal studies suggest that patients with macrolide-resistant M. pneumoniae infection clinically improve when given doxycycline (or minocycline) or levofloxacin.
A number of clinical questions regarding M. pneumoniae may be answered more definitively in the future, but we need more easily available diagnostics (PCR is a good start), routinely accessible susceptibility data, and a good randomized controlled study to investigate the question of whether treatment shortens the course of disease.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri-Kansas City. She said she has no conflicts of interest to disclose. E-mail her at [email protected].
The human microbiome
Microbiome refers to all the microbial life that exists in a specific niche. In the case of humans that means a lot of bacteria, viruses, fungi, parasites, and a very old class of single-celled organisms called archaea. The organisms include commensals and pathogenic microorganisms. Many articles distinguish "microbiome" and "microbiota" to differentiate the collective genomes of the microorganisms or the microorganisms themselves, respectively. However, these terms are largely synonymous.
A number of advances have allowed scientists to make major advances in understanding the microbiome. Specifically, we now have the molecular tools to perform gene expression analysis for an entire microbial community in the new discipline of metagenomics and analyze the massive results with new methods of mathematical analysis.
The human body contains over 10 times more microorganisms than human cells. The existence of a remarkably diverse and enormously large microbial world on us and in us first began to come to light in the late 1990s. We are learning more and more about the individual locations of the human host that have different populations of microbes and about differences among humans that contribute to or account for susceptibility to infectious diseases as well as autoimmune diseases and even obesity and cancer.
The nasopharyngeal microbiome has become an area of research by our group led by Qingfu Xu, Ph.D., at the Rochester (N.Y.) General Hospital Research Institute in collaboration with Melinda M. Pettigrew, Ph.D., at the Yale School of Public Health, New Haven, Conn., and Dr. Janet R. Casey at Legacy Pediatrics, also in Rochester. The traditional view of the immune system is undergoing reassessment as we learn that our microbiota has coevolved with our immune system, and each exerts influence over the other. Our group has a special interest in the impact of the nasopharyngeal microbiome on the innate immune response in that physiologic niche, and the way the innate immune system modifies the microbiome. With a special interest in the bacteria that cause respiratory infections such as acute otitis media, acute sinusitis, bronchopneumonia, and pneumonia, we have identified how microbes like Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis compete and synergize in the nasopharynx to cause infections.
Also, we seek to better understand how respiratory viruses like respiratory syncytial virus (RSV), influenzae, parainfluenzae, rhinovirus, and others facilitate the overgrowth of S. pneumoniae, H. flu, and M. catarrhalis in the nose such that they convert from commensals to pathogens. But the synergy goes both ways, as we have recently found that S. pneumoniae facilitates upper respiratory viral infections.
Up to now most of the work on the human microbiome has focused on the gut, and nearly all studies have occurred in adults. Perhaps readers are aware of the use of "fecal microbiota transplantation" as a treatment/cure for Clostridium difficile infection. Unhealthy gut microbiota in premature neonates are a major contributing factor in necrotizing enterocolitis.
For decades, physicians have been taught that obesity is a problem derived from excessive caloric intake and inadequate caloric consumption through activity, plus vaguely defined differences in "metabolism." As a consequence, we checked for hypothyroidism � I never found a case. New research has shown that there is a difference in the "metabolism" of obese patients, but the difference is how the individual gut microbiota metabolizes our food. It turns out the thinner individuals have a microbiota that is less efficient in breaking down the food we ingest to allow efficient absorption into the bloodstream, whereas obese individuals have a more efficient microbiota that facilitates absorption of a greater percentage of the proteins, carbohydrates, and fats that are ingested. So the pathway to treatment of obesity may lie in the study of the microbiome!
It turns out that the microbiota of the skin is highly diverse. The microbiota colonizing the antecubital fossa is different from that of the forearm or biceps or axillae. When atopic dermatitis flares, it is often in the antecubital fossa, and it is caused by overgrowth of Staphylococcus aureus. The microbiome of a patient with atopic dermatitis is different from that of a person without atopic dermatitis, and the former microbiota is more permissive to S. aureus becoming a pathogen rather than a commensal of the skin.
Prevention of urogenital infections in girls depends on a healthy vaginal microbiota. Bacterial vaginosis requires the establishment of overgrowth by Gardnerella vaginalis and Peptostreptococcus anaerobius that can only occur if the resident microbiota is unable to control the proliferation of these bacteria. Only if the microbiota of the perineum, urethra, and bladder will allow potential urinary tract infection pathogens access to epithelial attachment sites can infection become established.
A last topic for this column is the role of the microbiota in autoimmune diseases. In particular, I find it fascinating to learn that aberrant, unstable intestinal microbiota can lead to a leaky intestinal mucosal barrier. Combined with inadequate innate immune responses in the gut, progression may occur that allows antigens from microbes that cross-react with antigens of self in the pancreas to stimulate autoimmune antibodies. Similar pathogenic mechanisms may contribute to inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and other autoimmune diseases.
I anticipate future research will establish the makeup of a healthy microbiota associated with protection from the diseases mentioned here. With that knowledge, the next efforts in research will focus on how to convert an unhealthy microbiota to a healthy one. If the efforts succeed, I see new promising treatments in the future.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. The microbiome research at the Rochester General Hospital Research Institute is supported by the National Institutes of Health and the National Institute for Deafness and Communication Disorders. To comment, e-mail him at pdnews@ frontlinemedcom.com.
Microbiome refers to all the microbial life that exists in a specific niche. In the case of humans that means a lot of bacteria, viruses, fungi, parasites, and a very old class of single-celled organisms called archaea. The organisms include commensals and pathogenic microorganisms. Many articles distinguish "microbiome" and "microbiota" to differentiate the collective genomes of the microorganisms or the microorganisms themselves, respectively. However, these terms are largely synonymous.
A number of advances have allowed scientists to make major advances in understanding the microbiome. Specifically, we now have the molecular tools to perform gene expression analysis for an entire microbial community in the new discipline of metagenomics and analyze the massive results with new methods of mathematical analysis.
The human body contains over 10 times more microorganisms than human cells. The existence of a remarkably diverse and enormously large microbial world on us and in us first began to come to light in the late 1990s. We are learning more and more about the individual locations of the human host that have different populations of microbes and about differences among humans that contribute to or account for susceptibility to infectious diseases as well as autoimmune diseases and even obesity and cancer.
The nasopharyngeal microbiome has become an area of research by our group led by Qingfu Xu, Ph.D., at the Rochester (N.Y.) General Hospital Research Institute in collaboration with Melinda M. Pettigrew, Ph.D., at the Yale School of Public Health, New Haven, Conn., and Dr. Janet R. Casey at Legacy Pediatrics, also in Rochester. The traditional view of the immune system is undergoing reassessment as we learn that our microbiota has coevolved with our immune system, and each exerts influence over the other. Our group has a special interest in the impact of the nasopharyngeal microbiome on the innate immune response in that physiologic niche, and the way the innate immune system modifies the microbiome. With a special interest in the bacteria that cause respiratory infections such as acute otitis media, acute sinusitis, bronchopneumonia, and pneumonia, we have identified how microbes like Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis compete and synergize in the nasopharynx to cause infections.
Also, we seek to better understand how respiratory viruses like respiratory syncytial virus (RSV), influenzae, parainfluenzae, rhinovirus, and others facilitate the overgrowth of S. pneumoniae, H. flu, and M. catarrhalis in the nose such that they convert from commensals to pathogens. But the synergy goes both ways, as we have recently found that S. pneumoniae facilitates upper respiratory viral infections.
Up to now most of the work on the human microbiome has focused on the gut, and nearly all studies have occurred in adults. Perhaps readers are aware of the use of "fecal microbiota transplantation" as a treatment/cure for Clostridium difficile infection. Unhealthy gut microbiota in premature neonates are a major contributing factor in necrotizing enterocolitis.
For decades, physicians have been taught that obesity is a problem derived from excessive caloric intake and inadequate caloric consumption through activity, plus vaguely defined differences in "metabolism." As a consequence, we checked for hypothyroidism � I never found a case. New research has shown that there is a difference in the "metabolism" of obese patients, but the difference is how the individual gut microbiota metabolizes our food. It turns out the thinner individuals have a microbiota that is less efficient in breaking down the food we ingest to allow efficient absorption into the bloodstream, whereas obese individuals have a more efficient microbiota that facilitates absorption of a greater percentage of the proteins, carbohydrates, and fats that are ingested. So the pathway to treatment of obesity may lie in the study of the microbiome!
It turns out that the microbiota of the skin is highly diverse. The microbiota colonizing the antecubital fossa is different from that of the forearm or biceps or axillae. When atopic dermatitis flares, it is often in the antecubital fossa, and it is caused by overgrowth of Staphylococcus aureus. The microbiome of a patient with atopic dermatitis is different from that of a person without atopic dermatitis, and the former microbiota is more permissive to S. aureus becoming a pathogen rather than a commensal of the skin.
Prevention of urogenital infections in girls depends on a healthy vaginal microbiota. Bacterial vaginosis requires the establishment of overgrowth by Gardnerella vaginalis and Peptostreptococcus anaerobius that can only occur if the resident microbiota is unable to control the proliferation of these bacteria. Only if the microbiota of the perineum, urethra, and bladder will allow potential urinary tract infection pathogens access to epithelial attachment sites can infection become established.
A last topic for this column is the role of the microbiota in autoimmune diseases. In particular, I find it fascinating to learn that aberrant, unstable intestinal microbiota can lead to a leaky intestinal mucosal barrier. Combined with inadequate innate immune responses in the gut, progression may occur that allows antigens from microbes that cross-react with antigens of self in the pancreas to stimulate autoimmune antibodies. Similar pathogenic mechanisms may contribute to inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and other autoimmune diseases.
I anticipate future research will establish the makeup of a healthy microbiota associated with protection from the diseases mentioned here. With that knowledge, the next efforts in research will focus on how to convert an unhealthy microbiota to a healthy one. If the efforts succeed, I see new promising treatments in the future.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. The microbiome research at the Rochester General Hospital Research Institute is supported by the National Institutes of Health and the National Institute for Deafness and Communication Disorders. To comment, e-mail him at pdnews@ frontlinemedcom.com.
Microbiome refers to all the microbial life that exists in a specific niche. In the case of humans that means a lot of bacteria, viruses, fungi, parasites, and a very old class of single-celled organisms called archaea. The organisms include commensals and pathogenic microorganisms. Many articles distinguish "microbiome" and "microbiota" to differentiate the collective genomes of the microorganisms or the microorganisms themselves, respectively. However, these terms are largely synonymous.
A number of advances have allowed scientists to make major advances in understanding the microbiome. Specifically, we now have the molecular tools to perform gene expression analysis for an entire microbial community in the new discipline of metagenomics and analyze the massive results with new methods of mathematical analysis.
The human body contains over 10 times more microorganisms than human cells. The existence of a remarkably diverse and enormously large microbial world on us and in us first began to come to light in the late 1990s. We are learning more and more about the individual locations of the human host that have different populations of microbes and about differences among humans that contribute to or account for susceptibility to infectious diseases as well as autoimmune diseases and even obesity and cancer.
The nasopharyngeal microbiome has become an area of research by our group led by Qingfu Xu, Ph.D., at the Rochester (N.Y.) General Hospital Research Institute in collaboration with Melinda M. Pettigrew, Ph.D., at the Yale School of Public Health, New Haven, Conn., and Dr. Janet R. Casey at Legacy Pediatrics, also in Rochester. The traditional view of the immune system is undergoing reassessment as we learn that our microbiota has coevolved with our immune system, and each exerts influence over the other. Our group has a special interest in the impact of the nasopharyngeal microbiome on the innate immune response in that physiologic niche, and the way the innate immune system modifies the microbiome. With a special interest in the bacteria that cause respiratory infections such as acute otitis media, acute sinusitis, bronchopneumonia, and pneumonia, we have identified how microbes like Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis compete and synergize in the nasopharynx to cause infections.
Also, we seek to better understand how respiratory viruses like respiratory syncytial virus (RSV), influenzae, parainfluenzae, rhinovirus, and others facilitate the overgrowth of S. pneumoniae, H. flu, and M. catarrhalis in the nose such that they convert from commensals to pathogens. But the synergy goes both ways, as we have recently found that S. pneumoniae facilitates upper respiratory viral infections.
Up to now most of the work on the human microbiome has focused on the gut, and nearly all studies have occurred in adults. Perhaps readers are aware of the use of "fecal microbiota transplantation" as a treatment/cure for Clostridium difficile infection. Unhealthy gut microbiota in premature neonates are a major contributing factor in necrotizing enterocolitis.
For decades, physicians have been taught that obesity is a problem derived from excessive caloric intake and inadequate caloric consumption through activity, plus vaguely defined differences in "metabolism." As a consequence, we checked for hypothyroidism � I never found a case. New research has shown that there is a difference in the "metabolism" of obese patients, but the difference is how the individual gut microbiota metabolizes our food. It turns out the thinner individuals have a microbiota that is less efficient in breaking down the food we ingest to allow efficient absorption into the bloodstream, whereas obese individuals have a more efficient microbiota that facilitates absorption of a greater percentage of the proteins, carbohydrates, and fats that are ingested. So the pathway to treatment of obesity may lie in the study of the microbiome!
It turns out that the microbiota of the skin is highly diverse. The microbiota colonizing the antecubital fossa is different from that of the forearm or biceps or axillae. When atopic dermatitis flares, it is often in the antecubital fossa, and it is caused by overgrowth of Staphylococcus aureus. The microbiome of a patient with atopic dermatitis is different from that of a person without atopic dermatitis, and the former microbiota is more permissive to S. aureus becoming a pathogen rather than a commensal of the skin.
Prevention of urogenital infections in girls depends on a healthy vaginal microbiota. Bacterial vaginosis requires the establishment of overgrowth by Gardnerella vaginalis and Peptostreptococcus anaerobius that can only occur if the resident microbiota is unable to control the proliferation of these bacteria. Only if the microbiota of the perineum, urethra, and bladder will allow potential urinary tract infection pathogens access to epithelial attachment sites can infection become established.
A last topic for this column is the role of the microbiota in autoimmune diseases. In particular, I find it fascinating to learn that aberrant, unstable intestinal microbiota can lead to a leaky intestinal mucosal barrier. Combined with inadequate innate immune responses in the gut, progression may occur that allows antigens from microbes that cross-react with antigens of self in the pancreas to stimulate autoimmune antibodies. Similar pathogenic mechanisms may contribute to inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and other autoimmune diseases.
I anticipate future research will establish the makeup of a healthy microbiota associated with protection from the diseases mentioned here. With that knowledge, the next efforts in research will focus on how to convert an unhealthy microbiota to a healthy one. If the efforts succeed, I see new promising treatments in the future.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. The microbiome research at the Rochester General Hospital Research Institute is supported by the National Institutes of Health and the National Institute for Deafness and Communication Disorders. To comment, e-mail him at pdnews@ frontlinemedcom.com.
Current recs for JE-VC extended
Japanese encephalitis virus is a leading cause of encephalitis in Asia. The disease is mosquito borne where humans are incidental hosts who do not develop high-enough bloodstream concentrations to infect feeding mosquitoes. Culex tritaeniorhynchus mosquitos, an evening- and nighttime-biting mosquito, is the most important vector for transmission to humans.
Japanese encephalitis (JE) occurs primarily in rural agricultural areas, specifically in areas of rice production using flood irrigation. Although primarily rural, these ecologic conditions can be found near urban areas. Virus transmission is seasonal, with peak incidence in summer and fall. JE occurs throughout most of Asia and parts of the Western Pacific. The largest numbers of cases have been among people traveling to Thailand, followed by China, Indonesia, and the Philippines.
JE is primarily a disease of children in endemic countries, with annual incidences of 5-50 cases per 100,000 children. However, as adult travelers are both greater in number and lack protective antibody, they represent the majority of travel-acquired cases. Between 1973 and 2012, 65 cases of travel-associated JE among persons from nonendemic areas were reported in the literature. There was a median of 1 case per year, with 6 (9%) in children under 17 years of age. Among the six pediatric cases, the median age was 9 years, with a range of 1-11 years. Cases occurred most commonly between June and August, although they were reported year-round.
Symptomatic disease is often severe; however, the majority of cases are asymptomatic. Current estimates are 68,000 cases annually, with case fatality rates of 20%-30%. Thirty percent to 50% of survivors have significant neurologic, cognitive, or behavioral sequelae.
JE-VC, a formalin-inactivated vaccine derived from an attenuated virus strain and propagated in Vero cells, was licensed for use in children beginning at 2 months of age in May 2013. This is the only JE vaccine currently licensed and available in the United States. The JE-VC vaccine, manufactured as IXIARO, was licensed for use in adults in the United States, Europe, and Australia in 2009. The primary immunization series is two doses administered intramuscularly at 0 and 28 days.
The Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) reviewed the relevant data for a June 2013 meeting. The working group concluded that the overall risk of JE for most travelers to Asia is very low, but that the risk varies based on destination, duration, season, and activities. Prolonged travel in rural areas with active JE virus transmission may confer risks to travelers that are similar to risks in susceptible resident populations. Shorter-term travelers may still be at risk if their itinerary includes outdoor or nighttime exposure in rural areas during periods of active transmission. Short-term travel restricted to major urban areas confers minimal risk of JE.
ACIP recommendations for adults 17 years of age and older were approved in June 2009, and a booster dose recommendation was approved in February 2011. Recommendations state that health providers who are considering the use of JE vaccines for travelers must weigh the risk of travel-associated JE with the benefits and potential risks of the JE vaccine.
JE is a severe disease with substantial morbidity and mortality, and there is no specific treatment. A safe and effective vaccine is available; however, the vaccine is relatively expensive and the possibility of rare, serious adverse events cannot be excluded. The 2009 and 2011 recommendations for adults included the following:
• Travelers to JE-endemic countries should be advised of the risks of JE disease and the importance of measures to reduce mosquito bites.
• JE vaccine is recommended for travelers who plan to spend a month or longer in endemic areas during the JE virus transmission season.
• JE vaccine should be considered for short-term travelers to endemic areas if they will travel outside of an urban area, and if their activities will increase the risk of JE virus exposure.
• JE vaccine is not recommended for short-term travelers whose visit will be restricted to urban areas or times outside of a well-defined JE virus transmission season.
• If it has been 1 year since the primary series, a booster dose may be given prior to potential JE virus exposure.
• Data on the need for and timing of additional booster doses are not available.
A recommendation to expand the recommended use of JE-VC to children aged 2 months was approved by ACIP in June 2013. Their recommendation was based on the data demonstrating a high rate of seroconversion in children following the two-dose primary series, low rates of serious or systemic adverse events, and the lack of therapy for a serious disease.
In summary, JE-VC is recommended for travelers who plan to spend a month or longer in endemic areas during the JE virus transmission season. This includes long-term travelers, recurrent travelers, or expatriates who will be based in urban areas but are likely to visit endemic rural or agricultural areas during a high-risk season; the vaccine also should be considered for short-term travelers to rural endemic areas during virus transmission season, as well when there are outbreaks.
Dr. Pelton is chief of pediatric infectious disease and also is the coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he has attended and received honoraria for Novartis advisory board meetings on vaccines, although JE-VC has not been discussed. E-mail him at [email protected].
Japanese encephalitis (JE) occurs primarily in rural agricultural areas, specifically in areas of rice production using flood irrigation. Although primarily rural, these ecologic conditions can be found near urban areas. Virus transmission is seasonal, with peak incidence in summer and fall. JE occurs throughout most of Asia and parts of the Western Pacific. The largest numbers of cases have been among people traveling to Thailand, followed by China, Indonesia, and the Philippines.
Japanese encephalitis virus is a leading cause of encephalitis in Asia. The disease is mosquito borne where humans are incidental hosts who do not develop high-enough bloodstream concentrations to infect feeding mosquitoes. Culex tritaeniorhynchus mosquitos, an evening- and nighttime-biting mosquito, is the most important vector for transmission to humans.
Japanese encephalitis (JE) occurs primarily in rural agricultural areas, specifically in areas of rice production using flood irrigation. Although primarily rural, these ecologic conditions can be found near urban areas. Virus transmission is seasonal, with peak incidence in summer and fall. JE occurs throughout most of Asia and parts of the Western Pacific. The largest numbers of cases have been among people traveling to Thailand, followed by China, Indonesia, and the Philippines.
JE is primarily a disease of children in endemic countries, with annual incidences of 5-50 cases per 100,000 children. However, as adult travelers are both greater in number and lack protective antibody, they represent the majority of travel-acquired cases. Between 1973 and 2012, 65 cases of travel-associated JE among persons from nonendemic areas were reported in the literature. There was a median of 1 case per year, with 6 (9%) in children under 17 years of age. Among the six pediatric cases, the median age was 9 years, with a range of 1-11 years. Cases occurred most commonly between June and August, although they were reported year-round.
Symptomatic disease is often severe; however, the majority of cases are asymptomatic. Current estimates are 68,000 cases annually, with case fatality rates of 20%-30%. Thirty percent to 50% of survivors have significant neurologic, cognitive, or behavioral sequelae.
JE-VC, a formalin-inactivated vaccine derived from an attenuated virus strain and propagated in Vero cells, was licensed for use in children beginning at 2 months of age in May 2013. This is the only JE vaccine currently licensed and available in the United States. The JE-VC vaccine, manufactured as IXIARO, was licensed for use in adults in the United States, Europe, and Australia in 2009. The primary immunization series is two doses administered intramuscularly at 0 and 28 days.
The Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) reviewed the relevant data for a June 2013 meeting. The working group concluded that the overall risk of JE for most travelers to Asia is very low, but that the risk varies based on destination, duration, season, and activities. Prolonged travel in rural areas with active JE virus transmission may confer risks to travelers that are similar to risks in susceptible resident populations. Shorter-term travelers may still be at risk if their itinerary includes outdoor or nighttime exposure in rural areas during periods of active transmission. Short-term travel restricted to major urban areas confers minimal risk of JE.
ACIP recommendations for adults 17 years of age and older were approved in June 2009, and a booster dose recommendation was approved in February 2011. Recommendations state that health providers who are considering the use of JE vaccines for travelers must weigh the risk of travel-associated JE with the benefits and potential risks of the JE vaccine.
JE is a severe disease with substantial morbidity and mortality, and there is no specific treatment. A safe and effective vaccine is available; however, the vaccine is relatively expensive and the possibility of rare, serious adverse events cannot be excluded. The 2009 and 2011 recommendations for adults included the following:
• Travelers to JE-endemic countries should be advised of the risks of JE disease and the importance of measures to reduce mosquito bites.
• JE vaccine is recommended for travelers who plan to spend a month or longer in endemic areas during the JE virus transmission season.
• JE vaccine should be considered for short-term travelers to endemic areas if they will travel outside of an urban area, and if their activities will increase the risk of JE virus exposure.
• JE vaccine is not recommended for short-term travelers whose visit will be restricted to urban areas or times outside of a well-defined JE virus transmission season.
• If it has been 1 year since the primary series, a booster dose may be given prior to potential JE virus exposure.
• Data on the need for and timing of additional booster doses are not available.
A recommendation to expand the recommended use of JE-VC to children aged 2 months was approved by ACIP in June 2013. Their recommendation was based on the data demonstrating a high rate of seroconversion in children following the two-dose primary series, low rates of serious or systemic adverse events, and the lack of therapy for a serious disease.
In summary, JE-VC is recommended for travelers who plan to spend a month or longer in endemic areas during the JE virus transmission season. This includes long-term travelers, recurrent travelers, or expatriates who will be based in urban areas but are likely to visit endemic rural or agricultural areas during a high-risk season; the vaccine also should be considered for short-term travelers to rural endemic areas during virus transmission season, as well when there are outbreaks.
Dr. Pelton is chief of pediatric infectious disease and also is the coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he has attended and received honoraria for Novartis advisory board meetings on vaccines, although JE-VC has not been discussed. E-mail him at [email protected].
Japanese encephalitis virus is a leading cause of encephalitis in Asia. The disease is mosquito borne where humans are incidental hosts who do not develop high-enough bloodstream concentrations to infect feeding mosquitoes. Culex tritaeniorhynchus mosquitos, an evening- and nighttime-biting mosquito, is the most important vector for transmission to humans.
Japanese encephalitis (JE) occurs primarily in rural agricultural areas, specifically in areas of rice production using flood irrigation. Although primarily rural, these ecologic conditions can be found near urban areas. Virus transmission is seasonal, with peak incidence in summer and fall. JE occurs throughout most of Asia and parts of the Western Pacific. The largest numbers of cases have been among people traveling to Thailand, followed by China, Indonesia, and the Philippines.
JE is primarily a disease of children in endemic countries, with annual incidences of 5-50 cases per 100,000 children. However, as adult travelers are both greater in number and lack protective antibody, they represent the majority of travel-acquired cases. Between 1973 and 2012, 65 cases of travel-associated JE among persons from nonendemic areas were reported in the literature. There was a median of 1 case per year, with 6 (9%) in children under 17 years of age. Among the six pediatric cases, the median age was 9 years, with a range of 1-11 years. Cases occurred most commonly between June and August, although they were reported year-round.
Symptomatic disease is often severe; however, the majority of cases are asymptomatic. Current estimates are 68,000 cases annually, with case fatality rates of 20%-30%. Thirty percent to 50% of survivors have significant neurologic, cognitive, or behavioral sequelae.
JE-VC, a formalin-inactivated vaccine derived from an attenuated virus strain and propagated in Vero cells, was licensed for use in children beginning at 2 months of age in May 2013. This is the only JE vaccine currently licensed and available in the United States. The JE-VC vaccine, manufactured as IXIARO, was licensed for use in adults in the United States, Europe, and Australia in 2009. The primary immunization series is two doses administered intramuscularly at 0 and 28 days.
The Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) reviewed the relevant data for a June 2013 meeting. The working group concluded that the overall risk of JE for most travelers to Asia is very low, but that the risk varies based on destination, duration, season, and activities. Prolonged travel in rural areas with active JE virus transmission may confer risks to travelers that are similar to risks in susceptible resident populations. Shorter-term travelers may still be at risk if their itinerary includes outdoor or nighttime exposure in rural areas during periods of active transmission. Short-term travel restricted to major urban areas confers minimal risk of JE.
ACIP recommendations for adults 17 years of age and older were approved in June 2009, and a booster dose recommendation was approved in February 2011. Recommendations state that health providers who are considering the use of JE vaccines for travelers must weigh the risk of travel-associated JE with the benefits and potential risks of the JE vaccine.
JE is a severe disease with substantial morbidity and mortality, and there is no specific treatment. A safe and effective vaccine is available; however, the vaccine is relatively expensive and the possibility of rare, serious adverse events cannot be excluded. The 2009 and 2011 recommendations for adults included the following:
• Travelers to JE-endemic countries should be advised of the risks of JE disease and the importance of measures to reduce mosquito bites.
• JE vaccine is recommended for travelers who plan to spend a month or longer in endemic areas during the JE virus transmission season.
• JE vaccine should be considered for short-term travelers to endemic areas if they will travel outside of an urban area, and if their activities will increase the risk of JE virus exposure.
• JE vaccine is not recommended for short-term travelers whose visit will be restricted to urban areas or times outside of a well-defined JE virus transmission season.
• If it has been 1 year since the primary series, a booster dose may be given prior to potential JE virus exposure.
• Data on the need for and timing of additional booster doses are not available.
A recommendation to expand the recommended use of JE-VC to children aged 2 months was approved by ACIP in June 2013. Their recommendation was based on the data demonstrating a high rate of seroconversion in children following the two-dose primary series, low rates of serious or systemic adverse events, and the lack of therapy for a serious disease.
In summary, JE-VC is recommended for travelers who plan to spend a month or longer in endemic areas during the JE virus transmission season. This includes long-term travelers, recurrent travelers, or expatriates who will be based in urban areas but are likely to visit endemic rural or agricultural areas during a high-risk season; the vaccine also should be considered for short-term travelers to rural endemic areas during virus transmission season, as well when there are outbreaks.
Dr. Pelton is chief of pediatric infectious disease and also is the coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he has attended and received honoraria for Novartis advisory board meetings on vaccines, although JE-VC has not been discussed. E-mail him at [email protected].
Japanese encephalitis (JE) occurs primarily in rural agricultural areas, specifically in areas of rice production using flood irrigation. Although primarily rural, these ecologic conditions can be found near urban areas. Virus transmission is seasonal, with peak incidence in summer and fall. JE occurs throughout most of Asia and parts of the Western Pacific. The largest numbers of cases have been among people traveling to Thailand, followed by China, Indonesia, and the Philippines.
Japanese encephalitis (JE) occurs primarily in rural agricultural areas, specifically in areas of rice production using flood irrigation. Although primarily rural, these ecologic conditions can be found near urban areas. Virus transmission is seasonal, with peak incidence in summer and fall. JE occurs throughout most of Asia and parts of the Western Pacific. The largest numbers of cases have been among people traveling to Thailand, followed by China, Indonesia, and the Philippines.
International travel - Focus on timely intervention
Many of your patients will head for international destinations this summer, where they may be exposed to infectious diseases and other health risks they normally do not encounter in the United States.
For the majority of patients, these exposures will be brief; however, several may be extended due to study abroad or parental job relocation. More and more adolescents also are traveling to resource-limited areas doing volunteer work or adventure travel, and many are residing with host families. Children with chronic diseases pose concerns directly related to their underlying conditions, susceptibility, and availability of medical care in the host country. While most international travel plans are made at least 3 months in advance, health precautions such as immunizations and preventive medication often are not considered as travel plans are being finalized. If you are lucky, your patients will have mentioned their plans to you prior to finalizing their trips. You may receive a call at the last minute for assistance in helping to prepare them for a safe and healthy journey.
The U.S. Office of Travel & Tourism reports that slightly more than 60 million Americans traveled outside of the United States in 2012, with 28.5 million of the final destinations being overseas. Children accounted for approximately 2.4 million travelers. While tourism was the most common reason for travel, children were more likely to be visiting friends and relatives (VFR). Studies have revealed significantly increased health risks among VFR travelers, who often stay in private homes and in less-developed areas, compared with vacationers or business travelers who are more likely to be staying in hotels and in urban areas (Pediatrics 2010;125:e1072-80).
Is it really necessary to seek pretravel advice? Some travelers are not convinced. To facilitate this discussion, I thought I would share a recent call.
You are informed via voicemail that a 3-year-old is traveling with his family to Madras, India, for 8 weeks. He is visiting relatives, and the family may visit rural areas. The accommodations are air conditioned and the family is departing in 5 days! They would like to schedule an appointment immediately. What can you do?
Vital information has already been provided. The destination, type of accommodations, activities, duration of stay, and that the patient is a VFR are all important details when making vaccine and other recommendations. First, determine if the child’s routine immunizations are up to date. Next, determine the potential exposures for this patient, and identify vaccine-preventable and nonpreventable diseases. If there is a travel medicine specialist in your area who also sees children, you can refer the patient. If one is not readily available or you prefer to manage the patient, a great resource is the Centers for Disease Control and Prevention Traveler's Health site.
Vaccine preventable diseases include hepatitis A, hepatitis B, Japanese encephalitis, polio, rabies, typhoid, and influenza. Nonvaccine preventable diseases include chikungunya and dengue fevers. Avian influenza, malaria, tuberculosis, and traveler’s diarrhea are also cause for concern.
If you determine the routine immunizations are up to date, remember that measles is still a concern in many countries, and current U.S. recommendations state that all children at least 12 months of age should have two doses prior to leaving the United States. Although routinely administered at 4 years of age, the second dose of MMR can be administered as early as 4 weeks after the first dose. Those aged 6-11 months should have one dose prior to leaving the country. The remaining two doses should be administered at the usual time. Therefore, a total of three doses will be required to complete the series. Since the immunizations are up to date, this patient will also be protected against hepatitis A and B in addition to polio. Hepatitis A is the most common vaccine preventable disease acquired by travelers.
Rabies is prevalent in India, and all animal bites should be taken seriously. Because the patient is in a major urban area, access to both rabies vaccine and immunoglobulin should not be a concern. Japanese encephalitis will be circulating (May-October), but is usually found in rural agricultural areas. Mosquito precautions utilizing DEET (30%) on exposed areas or Permethrine-containing sprays on clothes to repel mosquitoes and ticks should be emphasized if travel to rural areas occurs. Vaccines for rabies and Japanese encephalitis would not be recommended for this patient. If the itinerary were different, they may be considered. Ixiaro, an inactivated Japanese encephalitis (JE) vaccine was approved for use in children as young as 2 months of age in May 2013. Previously, it was approved for use only in those at least 17 years of age in the United States. Both rabies and JE require a minimum of 21 and 28 days, respectively, to complete, and JE should be completed at least 1 week prior to exposure.
Typhoid fever (enteric fever) occurs worldwide, with an estimated 22 million cases annually. In 2012, 343 cases were reported in the United States, most of which were in recent travelers. The risk for typhoid fever is highest for travelers to southern Asia (6-30 times higher) than for all other destinations (Centers for Disease Control and Prevention. CDC Health Information for International Travel 2012. New York: Oxford University Press; 2012). Two types of vaccine are available: an oral, live attenuated vaccine for those at least 6 years of age and an injectable polysaccharide vaccine for those at least 2 years of age. In this case there is only one option, the injectable vaccine. Ideally, it should be administered at least 2 weeks prior to travel. Although this patient will not have optimal benefit of vaccine for at least 2 weeks, he will be there an additional 6 weeks, staying with friends and relatives, and is traveling to a high-risk country. Vaccine administration is recommended, and the parent should be fully informed when maximum benefit will occur. Food and water precautions are essential, especially during the first 2 weeks.
Precautions such as consumption of only boiled or bottled water, avoidance of undercooked or raw meat and seafood, and avoidance of raw fruit and vegetables to minimize acquisition of traveler’s diarrhea should be discussed. Antimicrobials also can be provided.
Options for malaria prophylaxis are limited due to the ensuing departure date and the child’s age. Atovaquone-Proguanil can be prescribed because it can be initiated 1-2 days prior to departure. It is taken daily while in India and for 1 week after return. He is too young for doxycycline. Mefloquine, administered weekly, should begin at least 2 weeks prior to exposure, so it is not an option. There is no role for chloroquine because chloroquine-resistant malaria is present in this country. In contrast to malaria, where mosquitoes usually feed dusk to dawn, chikungunya and dengue fever are transmitted by mosquitoes during the daytime.
No specific prevention for tuberculosis is available. Avoidance of persons with chronic cough or known disease is recommended.
It can be challenging for a busy practitioner to stay abreast of the latest developments in non–routinely administered vaccines, disease outbreaks, or country-specific entry requirements. Many vaccines, such as those against typhoid or rabies, are not routinely available in the patient’s medical home.
Ideally, patients planning international travel should be referred to a travel medicine clinic 1 month prior to travel. Some vaccines take up to 2 weeks to become effective, while others – such as yellow fever – should be administered at least 10 days prior to travel. However, interventions are still available for the last-minute patient, as in this case. Counseling for a variety of issues is provided. It’s not just about the vaccines.
International travel among children and adolescents will continue to rise. It behooves every primary care practitioner to develop a system to determine the summertime plans/needs of their patients. Not all travel medicine clinics provide services to children. It’s a good idea to find out which ones do in your area. You can always locate a clinic through the International Society of Travel Medicine and the Centers for Disease Control and Prevention.
While this call is not the norm, it occurs frequently. In contrast, another call for a 2-month photography trip to Uganda was received the same day. Departure was 6 weeks later!
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
Many of your patients will head for international destinations this summer, where they may be exposed to infectious diseases and other health risks they normally do not encounter in the United States.
For the majority of patients, these exposures will be brief; however, several may be extended due to study abroad or parental job relocation. More and more adolescents also are traveling to resource-limited areas doing volunteer work or adventure travel, and many are residing with host families. Children with chronic diseases pose concerns directly related to their underlying conditions, susceptibility, and availability of medical care in the host country. While most international travel plans are made at least 3 months in advance, health precautions such as immunizations and preventive medication often are not considered as travel plans are being finalized. If you are lucky, your patients will have mentioned their plans to you prior to finalizing their trips. You may receive a call at the last minute for assistance in helping to prepare them for a safe and healthy journey.
The U.S. Office of Travel & Tourism reports that slightly more than 60 million Americans traveled outside of the United States in 2012, with 28.5 million of the final destinations being overseas. Children accounted for approximately 2.4 million travelers. While tourism was the most common reason for travel, children were more likely to be visiting friends and relatives (VFR). Studies have revealed significantly increased health risks among VFR travelers, who often stay in private homes and in less-developed areas, compared with vacationers or business travelers who are more likely to be staying in hotels and in urban areas (Pediatrics 2010;125:e1072-80).
Is it really necessary to seek pretravel advice? Some travelers are not convinced. To facilitate this discussion, I thought I would share a recent call.
You are informed via voicemail that a 3-year-old is traveling with his family to Madras, India, for 8 weeks. He is visiting relatives, and the family may visit rural areas. The accommodations are air conditioned and the family is departing in 5 days! They would like to schedule an appointment immediately. What can you do?
Vital information has already been provided. The destination, type of accommodations, activities, duration of stay, and that the patient is a VFR are all important details when making vaccine and other recommendations. First, determine if the child’s routine immunizations are up to date. Next, determine the potential exposures for this patient, and identify vaccine-preventable and nonpreventable diseases. If there is a travel medicine specialist in your area who also sees children, you can refer the patient. If one is not readily available or you prefer to manage the patient, a great resource is the Centers for Disease Control and Prevention Traveler's Health site.
Vaccine preventable diseases include hepatitis A, hepatitis B, Japanese encephalitis, polio, rabies, typhoid, and influenza. Nonvaccine preventable diseases include chikungunya and dengue fevers. Avian influenza, malaria, tuberculosis, and traveler’s diarrhea are also cause for concern.
If you determine the routine immunizations are up to date, remember that measles is still a concern in many countries, and current U.S. recommendations state that all children at least 12 months of age should have two doses prior to leaving the United States. Although routinely administered at 4 years of age, the second dose of MMR can be administered as early as 4 weeks after the first dose. Those aged 6-11 months should have one dose prior to leaving the country. The remaining two doses should be administered at the usual time. Therefore, a total of three doses will be required to complete the series. Since the immunizations are up to date, this patient will also be protected against hepatitis A and B in addition to polio. Hepatitis A is the most common vaccine preventable disease acquired by travelers.
Rabies is prevalent in India, and all animal bites should be taken seriously. Because the patient is in a major urban area, access to both rabies vaccine and immunoglobulin should not be a concern. Japanese encephalitis will be circulating (May-October), but is usually found in rural agricultural areas. Mosquito precautions utilizing DEET (30%) on exposed areas or Permethrine-containing sprays on clothes to repel mosquitoes and ticks should be emphasized if travel to rural areas occurs. Vaccines for rabies and Japanese encephalitis would not be recommended for this patient. If the itinerary were different, they may be considered. Ixiaro, an inactivated Japanese encephalitis (JE) vaccine was approved for use in children as young as 2 months of age in May 2013. Previously, it was approved for use only in those at least 17 years of age in the United States. Both rabies and JE require a minimum of 21 and 28 days, respectively, to complete, and JE should be completed at least 1 week prior to exposure.
Typhoid fever (enteric fever) occurs worldwide, with an estimated 22 million cases annually. In 2012, 343 cases were reported in the United States, most of which were in recent travelers. The risk for typhoid fever is highest for travelers to southern Asia (6-30 times higher) than for all other destinations (Centers for Disease Control and Prevention. CDC Health Information for International Travel 2012. New York: Oxford University Press; 2012). Two types of vaccine are available: an oral, live attenuated vaccine for those at least 6 years of age and an injectable polysaccharide vaccine for those at least 2 years of age. In this case there is only one option, the injectable vaccine. Ideally, it should be administered at least 2 weeks prior to travel. Although this patient will not have optimal benefit of vaccine for at least 2 weeks, he will be there an additional 6 weeks, staying with friends and relatives, and is traveling to a high-risk country. Vaccine administration is recommended, and the parent should be fully informed when maximum benefit will occur. Food and water precautions are essential, especially during the first 2 weeks.
Precautions such as consumption of only boiled or bottled water, avoidance of undercooked or raw meat and seafood, and avoidance of raw fruit and vegetables to minimize acquisition of traveler’s diarrhea should be discussed. Antimicrobials also can be provided.
Options for malaria prophylaxis are limited due to the ensuing departure date and the child’s age. Atovaquone-Proguanil can be prescribed because it can be initiated 1-2 days prior to departure. It is taken daily while in India and for 1 week after return. He is too young for doxycycline. Mefloquine, administered weekly, should begin at least 2 weeks prior to exposure, so it is not an option. There is no role for chloroquine because chloroquine-resistant malaria is present in this country. In contrast to malaria, where mosquitoes usually feed dusk to dawn, chikungunya and dengue fever are transmitted by mosquitoes during the daytime.
No specific prevention for tuberculosis is available. Avoidance of persons with chronic cough or known disease is recommended.
It can be challenging for a busy practitioner to stay abreast of the latest developments in non–routinely administered vaccines, disease outbreaks, or country-specific entry requirements. Many vaccines, such as those against typhoid or rabies, are not routinely available in the patient’s medical home.
Ideally, patients planning international travel should be referred to a travel medicine clinic 1 month prior to travel. Some vaccines take up to 2 weeks to become effective, while others – such as yellow fever – should be administered at least 10 days prior to travel. However, interventions are still available for the last-minute patient, as in this case. Counseling for a variety of issues is provided. It’s not just about the vaccines.
International travel among children and adolescents will continue to rise. It behooves every primary care practitioner to develop a system to determine the summertime plans/needs of their patients. Not all travel medicine clinics provide services to children. It’s a good idea to find out which ones do in your area. You can always locate a clinic through the International Society of Travel Medicine and the Centers for Disease Control and Prevention.
While this call is not the norm, it occurs frequently. In contrast, another call for a 2-month photography trip to Uganda was received the same day. Departure was 6 weeks later!
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
Many of your patients will head for international destinations this summer, where they may be exposed to infectious diseases and other health risks they normally do not encounter in the United States.
For the majority of patients, these exposures will be brief; however, several may be extended due to study abroad or parental job relocation. More and more adolescents also are traveling to resource-limited areas doing volunteer work or adventure travel, and many are residing with host families. Children with chronic diseases pose concerns directly related to their underlying conditions, susceptibility, and availability of medical care in the host country. While most international travel plans are made at least 3 months in advance, health precautions such as immunizations and preventive medication often are not considered as travel plans are being finalized. If you are lucky, your patients will have mentioned their plans to you prior to finalizing their trips. You may receive a call at the last minute for assistance in helping to prepare them for a safe and healthy journey.
The U.S. Office of Travel & Tourism reports that slightly more than 60 million Americans traveled outside of the United States in 2012, with 28.5 million of the final destinations being overseas. Children accounted for approximately 2.4 million travelers. While tourism was the most common reason for travel, children were more likely to be visiting friends and relatives (VFR). Studies have revealed significantly increased health risks among VFR travelers, who often stay in private homes and in less-developed areas, compared with vacationers or business travelers who are more likely to be staying in hotels and in urban areas (Pediatrics 2010;125:e1072-80).
Is it really necessary to seek pretravel advice? Some travelers are not convinced. To facilitate this discussion, I thought I would share a recent call.
You are informed via voicemail that a 3-year-old is traveling with his family to Madras, India, for 8 weeks. He is visiting relatives, and the family may visit rural areas. The accommodations are air conditioned and the family is departing in 5 days! They would like to schedule an appointment immediately. What can you do?
Vital information has already been provided. The destination, type of accommodations, activities, duration of stay, and that the patient is a VFR are all important details when making vaccine and other recommendations. First, determine if the child’s routine immunizations are up to date. Next, determine the potential exposures for this patient, and identify vaccine-preventable and nonpreventable diseases. If there is a travel medicine specialist in your area who also sees children, you can refer the patient. If one is not readily available or you prefer to manage the patient, a great resource is the Centers for Disease Control and Prevention Traveler's Health site.
Vaccine preventable diseases include hepatitis A, hepatitis B, Japanese encephalitis, polio, rabies, typhoid, and influenza. Nonvaccine preventable diseases include chikungunya and dengue fevers. Avian influenza, malaria, tuberculosis, and traveler’s diarrhea are also cause for concern.
If you determine the routine immunizations are up to date, remember that measles is still a concern in many countries, and current U.S. recommendations state that all children at least 12 months of age should have two doses prior to leaving the United States. Although routinely administered at 4 years of age, the second dose of MMR can be administered as early as 4 weeks after the first dose. Those aged 6-11 months should have one dose prior to leaving the country. The remaining two doses should be administered at the usual time. Therefore, a total of three doses will be required to complete the series. Since the immunizations are up to date, this patient will also be protected against hepatitis A and B in addition to polio. Hepatitis A is the most common vaccine preventable disease acquired by travelers.
Rabies is prevalent in India, and all animal bites should be taken seriously. Because the patient is in a major urban area, access to both rabies vaccine and immunoglobulin should not be a concern. Japanese encephalitis will be circulating (May-October), but is usually found in rural agricultural areas. Mosquito precautions utilizing DEET (30%) on exposed areas or Permethrine-containing sprays on clothes to repel mosquitoes and ticks should be emphasized if travel to rural areas occurs. Vaccines for rabies and Japanese encephalitis would not be recommended for this patient. If the itinerary were different, they may be considered. Ixiaro, an inactivated Japanese encephalitis (JE) vaccine was approved for use in children as young as 2 months of age in May 2013. Previously, it was approved for use only in those at least 17 years of age in the United States. Both rabies and JE require a minimum of 21 and 28 days, respectively, to complete, and JE should be completed at least 1 week prior to exposure.
Typhoid fever (enteric fever) occurs worldwide, with an estimated 22 million cases annually. In 2012, 343 cases were reported in the United States, most of which were in recent travelers. The risk for typhoid fever is highest for travelers to southern Asia (6-30 times higher) than for all other destinations (Centers for Disease Control and Prevention. CDC Health Information for International Travel 2012. New York: Oxford University Press; 2012). Two types of vaccine are available: an oral, live attenuated vaccine for those at least 6 years of age and an injectable polysaccharide vaccine for those at least 2 years of age. In this case there is only one option, the injectable vaccine. Ideally, it should be administered at least 2 weeks prior to travel. Although this patient will not have optimal benefit of vaccine for at least 2 weeks, he will be there an additional 6 weeks, staying with friends and relatives, and is traveling to a high-risk country. Vaccine administration is recommended, and the parent should be fully informed when maximum benefit will occur. Food and water precautions are essential, especially during the first 2 weeks.
Precautions such as consumption of only boiled or bottled water, avoidance of undercooked or raw meat and seafood, and avoidance of raw fruit and vegetables to minimize acquisition of traveler’s diarrhea should be discussed. Antimicrobials also can be provided.
Options for malaria prophylaxis are limited due to the ensuing departure date and the child’s age. Atovaquone-Proguanil can be prescribed because it can be initiated 1-2 days prior to departure. It is taken daily while in India and for 1 week after return. He is too young for doxycycline. Mefloquine, administered weekly, should begin at least 2 weeks prior to exposure, so it is not an option. There is no role for chloroquine because chloroquine-resistant malaria is present in this country. In contrast to malaria, where mosquitoes usually feed dusk to dawn, chikungunya and dengue fever are transmitted by mosquitoes during the daytime.
No specific prevention for tuberculosis is available. Avoidance of persons with chronic cough or known disease is recommended.
It can be challenging for a busy practitioner to stay abreast of the latest developments in non–routinely administered vaccines, disease outbreaks, or country-specific entry requirements. Many vaccines, such as those against typhoid or rabies, are not routinely available in the patient’s medical home.
Ideally, patients planning international travel should be referred to a travel medicine clinic 1 month prior to travel. Some vaccines take up to 2 weeks to become effective, while others – such as yellow fever – should be administered at least 10 days prior to travel. However, interventions are still available for the last-minute patient, as in this case. Counseling for a variety of issues is provided. It’s not just about the vaccines.
International travel among children and adolescents will continue to rise. It behooves every primary care practitioner to develop a system to determine the summertime plans/needs of their patients. Not all travel medicine clinics provide services to children. It’s a good idea to find out which ones do in your area. You can always locate a clinic through the International Society of Travel Medicine and the Centers for Disease Control and Prevention.
While this call is not the norm, it occurs frequently. In contrast, another call for a 2-month photography trip to Uganda was received the same day. Departure was 6 weeks later!
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
Sepsis-like syndrome: Enteroviruses vs. human parechovirus
Not infrequently, less than 90 day old infants have fever and irritability and are more sleepy than usual, but have no apparent focus of infection. The sepsis evaluation is usually negative. It is frustrating for parents and providers when we report that we don’t really know what caused the febrile illness.
In summer/autumn season, some infants have enterovirus, with the predominate serotypes varying year to year. The enterovirus genus has several species, i.e., polio, enterovirus, echovirus, and Coxsackie A and Coxsackie B viruses. Echovirus is an acronym for enteric, cytopathic, human, orphan virus. Coxsackie is named from the city where it was first reported. Recently discovered enteroviruses have numbers starting at 68, and include a strain causing severe disease in Asia, enterovirus 71.
Sometimes clinicians can tell that enterovirus is in the community without laboratory tests because children present with hand, foot, and mouth (and sometimes buttock) disease. or herpangina. Enteroviruses also cause pericarditis, myocarditis, or pleurodynia (a.k.a. the "devil’s grippe").
But enteroviruses also cause aseptic meningitis. A modest pleocytosis with a mononuclear predominance and near-normal CSF glucose/protein values, plus negative bacterial cultures, is commonly called "aseptic meningitis."
Enteroviruses cause modest CSF pleocytosis (50-400 WBC), usually with mononuclear predominance and relatively normal CSF chemistries. While there can initially be a CSF neutrophil predominance, the differential usually shifts to mostly mononuclear cells less than 24 hours later. In the 1970s and 1980s (before polymerase chain reaction [PCR]), we used a "double tap" strategy to allow early discontinuation of antibiotics and hospital discharge. If the second CSF obtained within 24 hours of the first CSF had reasonably normal chemistries plus fewer WBCs or shifted to almost all mononuclear cells, children were discharged before final culture results. While hypoglycorrhachia is seen rarely with enterovirus (as low as 10 mg/dL), low CSF glucose values are usually due to bacterial or tuberculous meningitis. CSF protein concentrations with enteroviral meningitis are rarely greater than 80 mg/dL, the usual values for bacterial meningitis.
But consider this caveat: When "aseptic meningitis" seems present but CSF chemistries are abnormal (elevated protein or low glucose), check for tuberculosis risk factors and/or indolent neurological findings that could indicate tuberculous meningitis. In infants less than 2 months of age, consider neonatal herpes simplex virus (HSV) disease, particularly if the CSF protein is elevated.
These days "double taps" are not routine. Instead, CSF PCR is used. HSV and enterovirus PCR on CSF is available at most institutions with results available before bacteria cultures are final. A positive CSF enterovirus PCR (J. Pediatr. 1997;131:393-7) allows discontinuing antibacterials and acyclovir, if it was started empirically, plus early discharge. A positive HSV PCR also clarifies management: Continue acyclovir but discontinue antibacterial drugs. Keep in mind that enteroviral meningitis outbreaks are quite seasonal, with the majority of disease noted in the summer and early fall.
So we know the answer if the enterovirus or HSV PCR is positive. But what if these PCRs and bacterial cultures are negative in a child not pretreated with antibiotics? Well, the new kid on the block for aseptic meningitis is human parechovirus (HPeV). The first viruses classified as HPeV (HPeV1 and HPeV2) were previously called echovirus 22 and echovirus 23. But clinical and genome differences from enteroviruses led to reclassification as HPeVs. Now there are 16 HPeV serotypes. So why do we care?
In the past 6 years, HPeV3 emerged as the most common definable cause of sepsis-like syndrome in young infants with negative bacterial cultures (J. Clin. Virol. 2011;52:187-91; Pediatr. Infect. Dis. J. 2013;32:213-6). Interestingly, HPeV3-infected infants have more frequent peripheral leukopenia and lymphopenia plus more febrile days and higher fevers than those with enteroviruses. HPeV3 has a nearly every-other-year cycle (May-November). HPeV was as frequent or more frequent than all enteroviruses combined.
HPEV is not detected by enterovirus PCR, but is confirmed by HPeV-specific PCR. Like enteroviruses, PCR of blood is usually positive in HPeV-infected infants.
An important difference from HSV or enteroviruses is that almost no HPeV3 CNS-infected infants have CSF pleocytosis. That’s right. CSF in HPeV CNS infection is like HHV-6 (minimal CSF WBCs despite CNS infection). At our institution, HPeV3 PCR is performed routinely on CSF from all infants less than 90 days of age undergoing sepsis evaluations in summer/autumn.
If cultures and PCRs are negative in young infants with sepsis-like syndrome, your laboratory can likely perform or send out HPeV3 PCR. When HPeV3 CSF PCRs are positive, antibacterials can be stopped and patients discharged. Clinicians may be reluctant to discharge before final negative bacterial cultures because these infants can still "look ill," and providers are just learning about HPeV3. But based on our multiyear experience, it appears safe. We saw only three concurrent bacterial infections when HPeV3 was detected in CSF – all urinary tract infections that were easily detected during the sepsis evaluation and treated as such.
There have been no defined sequelae of HPeV CNS infection to date in the United States, although long-term follow-up is lacking for this emerging pathogen. There have been rare CNS sequelae, including white matter changes or seizures outside the United States, but these were apparent during the acute illness. We recommend outpatient follow-up soon after discharge, particularly if fever persists at discharge.
If we add HPeV3 PCR to our testing for infant sepsis-like syndrome during summer/fall, particularly when there is no or minimal CSF pleocytosis plus peripheral leuko/lymphopenia, there will fewer times when we lack a confirmed cause.
Dr. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures.
Not infrequently, less than 90 day old infants have fever and irritability and are more sleepy than usual, but have no apparent focus of infection. The sepsis evaluation is usually negative. It is frustrating for parents and providers when we report that we don’t really know what caused the febrile illness.
In summer/autumn season, some infants have enterovirus, with the predominate serotypes varying year to year. The enterovirus genus has several species, i.e., polio, enterovirus, echovirus, and Coxsackie A and Coxsackie B viruses. Echovirus is an acronym for enteric, cytopathic, human, orphan virus. Coxsackie is named from the city where it was first reported. Recently discovered enteroviruses have numbers starting at 68, and include a strain causing severe disease in Asia, enterovirus 71.

Sometimes clinicians can tell that enterovirus is in the community without laboratory tests because children present with hand, foot, and mouth (and sometimes buttock) disease. or herpangina. Enteroviruses also cause pericarditis, myocarditis, or pleurodynia (a.k.a. the "devil’s grippe").
But enteroviruses also cause aseptic meningitis. A modest pleocytosis with a mononuclear predominance and near-normal CSF glucose/protein values, plus negative bacterial cultures, is commonly called "aseptic meningitis."
Enteroviruses cause modest CSF pleocytosis (50-400 WBC), usually with mononuclear predominance and relatively normal CSF chemistries. While there can initially be a CSF neutrophil predominance, the differential usually shifts to mostly mononuclear cells less than 24 hours later. In the 1970s and 1980s (before polymerase chain reaction [PCR]), we used a "double tap" strategy to allow early discontinuation of antibiotics and hospital discharge. If the second CSF obtained within 24 hours of the first CSF had reasonably normal chemistries plus fewer WBCs or shifted to almost all mononuclear cells, children were discharged before final culture results. While hypoglycorrhachia is seen rarely with enterovirus (as low as 10 mg/dL), low CSF glucose values are usually due to bacterial or tuberculous meningitis. CSF protein concentrations with enteroviral meningitis are rarely greater than 80 mg/dL, the usual values for bacterial meningitis.
But consider this caveat: When "aseptic meningitis" seems present but CSF chemistries are abnormal (elevated protein or low glucose), check for tuberculosis risk factors and/or indolent neurological findings that could indicate tuberculous meningitis. In infants less than 2 months of age, consider neonatal herpes simplex virus (HSV) disease, particularly if the CSF protein is elevated.
These days "double taps" are not routine. Instead, CSF PCR is used. HSV and enterovirus PCR on CSF is available at most institutions with results available before bacteria cultures are final. A positive CSF enterovirus PCR (J. Pediatr. 1997;131:393-7) allows discontinuing antibacterials and acyclovir, if it was started empirically, plus early discharge. A positive HSV PCR also clarifies management: Continue acyclovir but discontinue antibacterial drugs. Keep in mind that enteroviral meningitis outbreaks are quite seasonal, with the majority of disease noted in the summer and early fall.
So we know the answer if the enterovirus or HSV PCR is positive. But what if these PCRs and bacterial cultures are negative in a child not pretreated with antibiotics? Well, the new kid on the block for aseptic meningitis is human parechovirus (HPeV). The first viruses classified as HPeV (HPeV1 and HPeV2) were previously called echovirus 22 and echovirus 23. But clinical and genome differences from enteroviruses led to reclassification as HPeVs. Now there are 16 HPeV serotypes. So why do we care?
In the past 6 years, HPeV3 emerged as the most common definable cause of sepsis-like syndrome in young infants with negative bacterial cultures (J. Clin. Virol. 2011;52:187-91; Pediatr. Infect. Dis. J. 2013;32:213-6). Interestingly, HPeV3-infected infants have more frequent peripheral leukopenia and lymphopenia plus more febrile days and higher fevers than those with enteroviruses. HPeV3 has a nearly every-other-year cycle (May-November). HPeV was as frequent or more frequent than all enteroviruses combined.
HPEV is not detected by enterovirus PCR, but is confirmed by HPeV-specific PCR. Like enteroviruses, PCR of blood is usually positive in HPeV-infected infants.
An important difference from HSV or enteroviruses is that almost no HPeV3 CNS-infected infants have CSF pleocytosis. That’s right. CSF in HPeV CNS infection is like HHV-6 (minimal CSF WBCs despite CNS infection). At our institution, HPeV3 PCR is performed routinely on CSF from all infants less than 90 days of age undergoing sepsis evaluations in summer/autumn.
If cultures and PCRs are negative in young infants with sepsis-like syndrome, your laboratory can likely perform or send out HPeV3 PCR. When HPeV3 CSF PCRs are positive, antibacterials can be stopped and patients discharged. Clinicians may be reluctant to discharge before final negative bacterial cultures because these infants can still "look ill," and providers are just learning about HPeV3. But based on our multiyear experience, it appears safe. We saw only three concurrent bacterial infections when HPeV3 was detected in CSF – all urinary tract infections that were easily detected during the sepsis evaluation and treated as such.
There have been no defined sequelae of HPeV CNS infection to date in the United States, although long-term follow-up is lacking for this emerging pathogen. There have been rare CNS sequelae, including white matter changes or seizures outside the United States, but these were apparent during the acute illness. We recommend outpatient follow-up soon after discharge, particularly if fever persists at discharge.
If we add HPeV3 PCR to our testing for infant sepsis-like syndrome during summer/fall, particularly when there is no or minimal CSF pleocytosis plus peripheral leuko/lymphopenia, there will fewer times when we lack a confirmed cause.
Dr. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures.
Not infrequently, less than 90 day old infants have fever and irritability and are more sleepy than usual, but have no apparent focus of infection. The sepsis evaluation is usually negative. It is frustrating for parents and providers when we report that we don’t really know what caused the febrile illness.
In summer/autumn season, some infants have enterovirus, with the predominate serotypes varying year to year. The enterovirus genus has several species, i.e., polio, enterovirus, echovirus, and Coxsackie A and Coxsackie B viruses. Echovirus is an acronym for enteric, cytopathic, human, orphan virus. Coxsackie is named from the city where it was first reported. Recently discovered enteroviruses have numbers starting at 68, and include a strain causing severe disease in Asia, enterovirus 71.

Sometimes clinicians can tell that enterovirus is in the community without laboratory tests because children present with hand, foot, and mouth (and sometimes buttock) disease. or herpangina. Enteroviruses also cause pericarditis, myocarditis, or pleurodynia (a.k.a. the "devil’s grippe").
But enteroviruses also cause aseptic meningitis. A modest pleocytosis with a mononuclear predominance and near-normal CSF glucose/protein values, plus negative bacterial cultures, is commonly called "aseptic meningitis."
Enteroviruses cause modest CSF pleocytosis (50-400 WBC), usually with mononuclear predominance and relatively normal CSF chemistries. While there can initially be a CSF neutrophil predominance, the differential usually shifts to mostly mononuclear cells less than 24 hours later. In the 1970s and 1980s (before polymerase chain reaction [PCR]), we used a "double tap" strategy to allow early discontinuation of antibiotics and hospital discharge. If the second CSF obtained within 24 hours of the first CSF had reasonably normal chemistries plus fewer WBCs or shifted to almost all mononuclear cells, children were discharged before final culture results. While hypoglycorrhachia is seen rarely with enterovirus (as low as 10 mg/dL), low CSF glucose values are usually due to bacterial or tuberculous meningitis. CSF protein concentrations with enteroviral meningitis are rarely greater than 80 mg/dL, the usual values for bacterial meningitis.
But consider this caveat: When "aseptic meningitis" seems present but CSF chemistries are abnormal (elevated protein or low glucose), check for tuberculosis risk factors and/or indolent neurological findings that could indicate tuberculous meningitis. In infants less than 2 months of age, consider neonatal herpes simplex virus (HSV) disease, particularly if the CSF protein is elevated.
These days "double taps" are not routine. Instead, CSF PCR is used. HSV and enterovirus PCR on CSF is available at most institutions with results available before bacteria cultures are final. A positive CSF enterovirus PCR (J. Pediatr. 1997;131:393-7) allows discontinuing antibacterials and acyclovir, if it was started empirically, plus early discharge. A positive HSV PCR also clarifies management: Continue acyclovir but discontinue antibacterial drugs. Keep in mind that enteroviral meningitis outbreaks are quite seasonal, with the majority of disease noted in the summer and early fall.
So we know the answer if the enterovirus or HSV PCR is positive. But what if these PCRs and bacterial cultures are negative in a child not pretreated with antibiotics? Well, the new kid on the block for aseptic meningitis is human parechovirus (HPeV). The first viruses classified as HPeV (HPeV1 and HPeV2) were previously called echovirus 22 and echovirus 23. But clinical and genome differences from enteroviruses led to reclassification as HPeVs. Now there are 16 HPeV serotypes. So why do we care?
In the past 6 years, HPeV3 emerged as the most common definable cause of sepsis-like syndrome in young infants with negative bacterial cultures (J. Clin. Virol. 2011;52:187-91; Pediatr. Infect. Dis. J. 2013;32:213-6). Interestingly, HPeV3-infected infants have more frequent peripheral leukopenia and lymphopenia plus more febrile days and higher fevers than those with enteroviruses. HPeV3 has a nearly every-other-year cycle (May-November). HPeV was as frequent or more frequent than all enteroviruses combined.
HPEV is not detected by enterovirus PCR, but is confirmed by HPeV-specific PCR. Like enteroviruses, PCR of blood is usually positive in HPeV-infected infants.
An important difference from HSV or enteroviruses is that almost no HPeV3 CNS-infected infants have CSF pleocytosis. That’s right. CSF in HPeV CNS infection is like HHV-6 (minimal CSF WBCs despite CNS infection). At our institution, HPeV3 PCR is performed routinely on CSF from all infants less than 90 days of age undergoing sepsis evaluations in summer/autumn.
If cultures and PCRs are negative in young infants with sepsis-like syndrome, your laboratory can likely perform or send out HPeV3 PCR. When HPeV3 CSF PCRs are positive, antibacterials can be stopped and patients discharged. Clinicians may be reluctant to discharge before final negative bacterial cultures because these infants can still "look ill," and providers are just learning about HPeV3. But based on our multiyear experience, it appears safe. We saw only three concurrent bacterial infections when HPeV3 was detected in CSF – all urinary tract infections that were easily detected during the sepsis evaluation and treated as such.
There have been no defined sequelae of HPeV CNS infection to date in the United States, although long-term follow-up is lacking for this emerging pathogen. There have been rare CNS sequelae, including white matter changes or seizures outside the United States, but these were apparent during the acute illness. We recommend outpatient follow-up soon after discharge, particularly if fever persists at discharge.
If we add HPeV3 PCR to our testing for infant sepsis-like syndrome during summer/fall, particularly when there is no or minimal CSF pleocytosis plus peripheral leuko/lymphopenia, there will fewer times when we lack a confirmed cause.
Dr. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures.
A new way to treat ear infections
A new way to treat ear infections in children called "individualized care" is described in the May 2013 issue of Pediatric Infectious Diseases Journal. It explains how to reduce the frequency of repeated ear infections nearly 500% and how to reduce the need for ear tube surgery by 600% in your practice.
Dr. Janet Casey at Legacy Pediatrics in Rochester, N.Y.; Anthony Almudevar, Ph.D., of the University of Rochester; and I conducted the prospective, longitudinal multiyear study with the support of the National Institutes of Health’s National Institute for Deafness and Communication Disorders and the Thrasher Research Fund (Pediatr. Infect. Dis. J. 2013 Jan. 21 [Epub ahead of print]).
The study compared three groups: children who were in the Legacy Pediatrics practice and received individualized care; control children in the Legacy practice who did not participate because their parents declined participation (they did not want venipunctures or ear taps); and community controls drawn from a different pediatric practice in the suburbs of Rochester that used the diagnostic criteria of the American Academy of Pediatrics and treated all children empirically with high-dose amoxicillin as endorsed by the former and new AAP treatment guidelines (Pediatrics 2013;131:e964-99).
The new treatment paradigm of individualized care included a tympanocentesis procedure, also called an ear tap, to determine precisely the bacteria causing the ear infection. Treatment was started with high-dose amoxicillin/clavulanate. The sample of fluid then was taken to my laboratory at the Rochester General Hospital Research Institute, where the bacteria isolated were tested against a panel of antibiotics to determine whether to continue with amoxicillin/clavulanate or switch to a more effective antibiotic for the child based on culture susceptibility. By doing the ear tap and antibiotic testing, the frequency of repeated ear infections was reduced by 250%, compared with the Legacy practice controls who did not participate, and by 460%, compared with the community controls.
The most common reason for children to receive ear tubes is repeated ear infections, so when the frequency of ear infections was reduced so too was the frequency of ear tube surgery. The new treatment approach resulted in 260% fewer ear tube surgeries in the individualized care group, compared with the Legacy Pediatrics controls, and 620% fewer surgeries than the community controls.
Allowing the child to receive an ear tap was a requirement for the study. Dr. Casey and I found a way to do the procedure painlessly by instilling 8% Novocain in the ear canal as drops to anesthetize the tympanic membrane. After 15 minutes there was no pain when the tap was done. We used a papoose to hold the child still.
The ear-tap procedure not only allowed individualized care with the astonishing results reported, it also allowed more rapid healing of the ear since removal of the pus and bacteria from behind the ear allowed the antibiotics to work better and the immune system to clear the infection more effectively.
The article discusses reasons for the remarkable difference in results with the individualized care approach. First, Dr. Casey and I have undergone special training from ear, nose, and throat (ENT) doctors in the diagnosis of ear infections.
In earlier studies, a group of experts in otitis media diagnosis joined together in a continuing medical education course sponsored by Outcomes Management Education Workshops to use video exams to test whether pediatricians, family physicians, and urgent care physicians knew how to correctly distinguish true acute otitis media (AOM) from otitis media with effusion (OME) and variations of normal in the tympanic membrane exam. We found that all three specialty groups and residents in training in all three specialties and nurse practitioners and physician assistants overdiagnosed AOM about half the time.
Second, the selection of antibiotic proved to be key. Dr. Casey and I have the only otitis media research center in the United States providing tympanocentesis data at the current time. We have found that amoxicillin kills the bacteria causing AOM infections in children in the Rochester area only about 30% of the time. By knowing the bacteria, an evidence-based antibiotic can be chosen.
I expect that readers of this column will believe they diagnose AOM correctly nearly all the time and that it is the other physician who overdiagnoses. I expect that readers will be reluctant to not adhere to the AAP guideline recommendation of using amoxicillin as the treatment of first choice. Most of all, I expect readers to be reluctant to undertake training on how to do the ear tap procedure. Change is always resisted by the majority, and only with time does it occur if the evidence is strong and there is growing adoption.
Nevertheless, I encourage all to find an opportunity to attend a CME course on AOM diagnosis and I hope that resident training programs will incorporate more effective teaching on AOM diagnosis. I recommend high-dose amoxicillin/clavulanate as the treatment of choice for AOM; if it is not tolerated, then one of the preferred cephalosporins endorsed by the AAP guideline should be chosen.
I recommend that resident training programs include tympanocentesis as part of the curriculum. Why are residents taught how to do a spinal tap, arterial artery puncture, and lung tap but not an ear tap? I also recommend that practicing pediatricians gain the skill to perform tympanocentesis as well. I recognize that some just won’t have the hand/eye coordination or steady hand needed, so it’s not for everyone. However, especially in group practices, a few trained providers could become an internal referral resource for getting the procedure done.
Arguments about malpractice are a smokescreen. The risks of tympanocentesis are no greater than venipuncture in trained and skilled hands. It is included as a standard procedure for pediatricians in our state without any additional malpractice insurance costs. And Dr. Casey and I have effectively managed to get the procedure done when a patient needs it without blowing our schedules off the map and raising the ire of patients and staff. It just takes a commitment.
It would be convenient to refer to an ENT doctor for a tympanocentesis, but most ENT doctors have not been trained to do the procedure while the child is awake and prefer to have the child asleep. Also, try to get a child in for an appointment with an ENT with no notice on the same day! Moreover, ENT doctors have been trained that if an ear tap is needed then it is advisable to go ahead and put in an ear tube.
Because of the success of this research, our center received a renewal of support from NIH in 2012 to continue the study through 2017. Several pediatric practices in Rochester are part of the research – Long Pond Pediatrics, Westfall Pediatrics, Sunrise Pediatrics, Lewis Pediatrics, and Pathway Pediatrics – as well as Dr. Margo Benoit of the department of otolaryngology at the University of Rochester and Dr. Frank Salamone and Dr. Kevin Kozara of the Rochester Otolaryngology Group, which is affiliated with Rochester General Hospital.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. He said he had no relevant financial conflicts of interest to disclose.
A new way to treat ear infections in children called "individualized care" is described in the May 2013 issue of Pediatric Infectious Diseases Journal. It explains how to reduce the frequency of repeated ear infections nearly 500% and how to reduce the need for ear tube surgery by 600% in your practice.
Dr. Janet Casey at Legacy Pediatrics in Rochester, N.Y.; Anthony Almudevar, Ph.D., of the University of Rochester; and I conducted the prospective, longitudinal multiyear study with the support of the National Institutes of Health’s National Institute for Deafness and Communication Disorders and the Thrasher Research Fund (Pediatr. Infect. Dis. J. 2013 Jan. 21 [Epub ahead of print]).
The study compared three groups: children who were in the Legacy Pediatrics practice and received individualized care; control children in the Legacy practice who did not participate because their parents declined participation (they did not want venipunctures or ear taps); and community controls drawn from a different pediatric practice in the suburbs of Rochester that used the diagnostic criteria of the American Academy of Pediatrics and treated all children empirically with high-dose amoxicillin as endorsed by the former and new AAP treatment guidelines (Pediatrics 2013;131:e964-99).
The new treatment paradigm of individualized care included a tympanocentesis procedure, also called an ear tap, to determine precisely the bacteria causing the ear infection. Treatment was started with high-dose amoxicillin/clavulanate. The sample of fluid then was taken to my laboratory at the Rochester General Hospital Research Institute, where the bacteria isolated were tested against a panel of antibiotics to determine whether to continue with amoxicillin/clavulanate or switch to a more effective antibiotic for the child based on culture susceptibility. By doing the ear tap and antibiotic testing, the frequency of repeated ear infections was reduced by 250%, compared with the Legacy practice controls who did not participate, and by 460%, compared with the community controls.
The most common reason for children to receive ear tubes is repeated ear infections, so when the frequency of ear infections was reduced so too was the frequency of ear tube surgery. The new treatment approach resulted in 260% fewer ear tube surgeries in the individualized care group, compared with the Legacy Pediatrics controls, and 620% fewer surgeries than the community controls.
Allowing the child to receive an ear tap was a requirement for the study. Dr. Casey and I found a way to do the procedure painlessly by instilling 8% Novocain in the ear canal as drops to anesthetize the tympanic membrane. After 15 minutes there was no pain when the tap was done. We used a papoose to hold the child still.
The ear-tap procedure not only allowed individualized care with the astonishing results reported, it also allowed more rapid healing of the ear since removal of the pus and bacteria from behind the ear allowed the antibiotics to work better and the immune system to clear the infection more effectively.
The article discusses reasons for the remarkable difference in results with the individualized care approach. First, Dr. Casey and I have undergone special training from ear, nose, and throat (ENT) doctors in the diagnosis of ear infections.
In earlier studies, a group of experts in otitis media diagnosis joined together in a continuing medical education course sponsored by Outcomes Management Education Workshops to use video exams to test whether pediatricians, family physicians, and urgent care physicians knew how to correctly distinguish true acute otitis media (AOM) from otitis media with effusion (OME) and variations of normal in the tympanic membrane exam. We found that all three specialty groups and residents in training in all three specialties and nurse practitioners and physician assistants overdiagnosed AOM about half the time.
Second, the selection of antibiotic proved to be key. Dr. Casey and I have the only otitis media research center in the United States providing tympanocentesis data at the current time. We have found that amoxicillin kills the bacteria causing AOM infections in children in the Rochester area only about 30% of the time. By knowing the bacteria, an evidence-based antibiotic can be chosen.
I expect that readers of this column will believe they diagnose AOM correctly nearly all the time and that it is the other physician who overdiagnoses. I expect that readers will be reluctant to not adhere to the AAP guideline recommendation of using amoxicillin as the treatment of first choice. Most of all, I expect readers to be reluctant to undertake training on how to do the ear tap procedure. Change is always resisted by the majority, and only with time does it occur if the evidence is strong and there is growing adoption.
Nevertheless, I encourage all to find an opportunity to attend a CME course on AOM diagnosis and I hope that resident training programs will incorporate more effective teaching on AOM diagnosis. I recommend high-dose amoxicillin/clavulanate as the treatment of choice for AOM; if it is not tolerated, then one of the preferred cephalosporins endorsed by the AAP guideline should be chosen.
I recommend that resident training programs include tympanocentesis as part of the curriculum. Why are residents taught how to do a spinal tap, arterial artery puncture, and lung tap but not an ear tap? I also recommend that practicing pediatricians gain the skill to perform tympanocentesis as well. I recognize that some just won’t have the hand/eye coordination or steady hand needed, so it’s not for everyone. However, especially in group practices, a few trained providers could become an internal referral resource for getting the procedure done.
Arguments about malpractice are a smokescreen. The risks of tympanocentesis are no greater than venipuncture in trained and skilled hands. It is included as a standard procedure for pediatricians in our state without any additional malpractice insurance costs. And Dr. Casey and I have effectively managed to get the procedure done when a patient needs it without blowing our schedules off the map and raising the ire of patients and staff. It just takes a commitment.
It would be convenient to refer to an ENT doctor for a tympanocentesis, but most ENT doctors have not been trained to do the procedure while the child is awake and prefer to have the child asleep. Also, try to get a child in for an appointment with an ENT with no notice on the same day! Moreover, ENT doctors have been trained that if an ear tap is needed then it is advisable to go ahead and put in an ear tube.
Because of the success of this research, our center received a renewal of support from NIH in 2012 to continue the study through 2017. Several pediatric practices in Rochester are part of the research – Long Pond Pediatrics, Westfall Pediatrics, Sunrise Pediatrics, Lewis Pediatrics, and Pathway Pediatrics – as well as Dr. Margo Benoit of the department of otolaryngology at the University of Rochester and Dr. Frank Salamone and Dr. Kevin Kozara of the Rochester Otolaryngology Group, which is affiliated with Rochester General Hospital.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. He said he had no relevant financial conflicts of interest to disclose.
A new way to treat ear infections in children called "individualized care" is described in the May 2013 issue of Pediatric Infectious Diseases Journal. It explains how to reduce the frequency of repeated ear infections nearly 500% and how to reduce the need for ear tube surgery by 600% in your practice.
Dr. Janet Casey at Legacy Pediatrics in Rochester, N.Y.; Anthony Almudevar, Ph.D., of the University of Rochester; and I conducted the prospective, longitudinal multiyear study with the support of the National Institutes of Health’s National Institute for Deafness and Communication Disorders and the Thrasher Research Fund (Pediatr. Infect. Dis. J. 2013 Jan. 21 [Epub ahead of print]).
The study compared three groups: children who were in the Legacy Pediatrics practice and received individualized care; control children in the Legacy practice who did not participate because their parents declined participation (they did not want venipunctures or ear taps); and community controls drawn from a different pediatric practice in the suburbs of Rochester that used the diagnostic criteria of the American Academy of Pediatrics and treated all children empirically with high-dose amoxicillin as endorsed by the former and new AAP treatment guidelines (Pediatrics 2013;131:e964-99).
The new treatment paradigm of individualized care included a tympanocentesis procedure, also called an ear tap, to determine precisely the bacteria causing the ear infection. Treatment was started with high-dose amoxicillin/clavulanate. The sample of fluid then was taken to my laboratory at the Rochester General Hospital Research Institute, where the bacteria isolated were tested against a panel of antibiotics to determine whether to continue with amoxicillin/clavulanate or switch to a more effective antibiotic for the child based on culture susceptibility. By doing the ear tap and antibiotic testing, the frequency of repeated ear infections was reduced by 250%, compared with the Legacy practice controls who did not participate, and by 460%, compared with the community controls.
The most common reason for children to receive ear tubes is repeated ear infections, so when the frequency of ear infections was reduced so too was the frequency of ear tube surgery. The new treatment approach resulted in 260% fewer ear tube surgeries in the individualized care group, compared with the Legacy Pediatrics controls, and 620% fewer surgeries than the community controls.
Allowing the child to receive an ear tap was a requirement for the study. Dr. Casey and I found a way to do the procedure painlessly by instilling 8% Novocain in the ear canal as drops to anesthetize the tympanic membrane. After 15 minutes there was no pain when the tap was done. We used a papoose to hold the child still.
The ear-tap procedure not only allowed individualized care with the astonishing results reported, it also allowed more rapid healing of the ear since removal of the pus and bacteria from behind the ear allowed the antibiotics to work better and the immune system to clear the infection more effectively.
The article discusses reasons for the remarkable difference in results with the individualized care approach. First, Dr. Casey and I have undergone special training from ear, nose, and throat (ENT) doctors in the diagnosis of ear infections.
In earlier studies, a group of experts in otitis media diagnosis joined together in a continuing medical education course sponsored by Outcomes Management Education Workshops to use video exams to test whether pediatricians, family physicians, and urgent care physicians knew how to correctly distinguish true acute otitis media (AOM) from otitis media with effusion (OME) and variations of normal in the tympanic membrane exam. We found that all three specialty groups and residents in training in all three specialties and nurse practitioners and physician assistants overdiagnosed AOM about half the time.
Second, the selection of antibiotic proved to be key. Dr. Casey and I have the only otitis media research center in the United States providing tympanocentesis data at the current time. We have found that amoxicillin kills the bacteria causing AOM infections in children in the Rochester area only about 30% of the time. By knowing the bacteria, an evidence-based antibiotic can be chosen.
I expect that readers of this column will believe they diagnose AOM correctly nearly all the time and that it is the other physician who overdiagnoses. I expect that readers will be reluctant to not adhere to the AAP guideline recommendation of using amoxicillin as the treatment of first choice. Most of all, I expect readers to be reluctant to undertake training on how to do the ear tap procedure. Change is always resisted by the majority, and only with time does it occur if the evidence is strong and there is growing adoption.
Nevertheless, I encourage all to find an opportunity to attend a CME course on AOM diagnosis and I hope that resident training programs will incorporate more effective teaching on AOM diagnosis. I recommend high-dose amoxicillin/clavulanate as the treatment of choice for AOM; if it is not tolerated, then one of the preferred cephalosporins endorsed by the AAP guideline should be chosen.
I recommend that resident training programs include tympanocentesis as part of the curriculum. Why are residents taught how to do a spinal tap, arterial artery puncture, and lung tap but not an ear tap? I also recommend that practicing pediatricians gain the skill to perform tympanocentesis as well. I recognize that some just won’t have the hand/eye coordination or steady hand needed, so it’s not for everyone. However, especially in group practices, a few trained providers could become an internal referral resource for getting the procedure done.
Arguments about malpractice are a smokescreen. The risks of tympanocentesis are no greater than venipuncture in trained and skilled hands. It is included as a standard procedure for pediatricians in our state without any additional malpractice insurance costs. And Dr. Casey and I have effectively managed to get the procedure done when a patient needs it without blowing our schedules off the map and raising the ire of patients and staff. It just takes a commitment.
It would be convenient to refer to an ENT doctor for a tympanocentesis, but most ENT doctors have not been trained to do the procedure while the child is awake and prefer to have the child asleep. Also, try to get a child in for an appointment with an ENT with no notice on the same day! Moreover, ENT doctors have been trained that if an ear tap is needed then it is advisable to go ahead and put in an ear tube.
Because of the success of this research, our center received a renewal of support from NIH in 2012 to continue the study through 2017. Several pediatric practices in Rochester are part of the research – Long Pond Pediatrics, Westfall Pediatrics, Sunrise Pediatrics, Lewis Pediatrics, and Pathway Pediatrics – as well as Dr. Margo Benoit of the department of otolaryngology at the University of Rochester and Dr. Frank Salamone and Dr. Kevin Kozara of the Rochester Otolaryngology Group, which is affiliated with Rochester General Hospital.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. He said he had no relevant financial conflicts of interest to disclose.