User login
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
The Patient Protection and Affordable Care Act of 2010 (ACA) intended that women have access to critical preventive health services without a copay or deductible. The Institute of Medicine (IOM) was asked to help identify those critical preventive women’s health services. In 2011, the IOM Committee on Preventive Services for Women recommended that all women have access to 9 preventive services, among them1:
- screening for gestational diabetes mellitus (GDM)
- human papilloma virus testing
- contraceptive methods and counseling
- well-woman visits.
The Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services agreed to update the recommended preventive services every 5 years.
In March 2016, HRSA entered into a 5-year cooperative agreement with the American College of Obstetricians and Gynecologists (ACOG) to update the guidelines and to develop additional recommendations to enhance women’s health.2 ACOG launched the Women’s Preventive Services Initiative (WPSI) to develop the 2016 update.
The 5-year grant with HRSA will address many more preventive health services for women across their lifespan as well as implementation strategies so that women receive consistent and appropriate care, regardless of the health care provider’s specialty. The WPSI recognizes that the selection of a provider for well-woman care will be determined as much by a woman’s needs and preferences as by her access to health care services and health plan availability.
The WPSI draft recommendations were released for public comment in September 2016,2 and HRSA approved the recommendations in December 2016.3 In this editorial, I provide a look at which organizations comprise the WPSI and a summary of the 9 recommended preventive health services.
Who makes up the Women’s Preventive Services Initiative?
The WPSI is a collaboration between professional societies and consumer organizations. The goal of the WPSI is “to promote health over the course of a woman’s lifetime through disease prevention and preventive healthcare.” The WPSI advisory panel provides oversight to the effort and the multidisciplinary steering committee develops the recommendations. The WPSI advisory panel includes leaders and experts from 4 major professional organizations, whose members provide the majority of women’s health care in the United States:
- ACOG
- American College of Physicians (ACP)
- American Academy of Family Physicians (AAFP)
- National Association of Nurse Practitioners in Women’s Health (NPWH).
The multidisciplinary steering committee includes the members of the advisory panel, representatives from 17 professional and consumer organizations, a patient representative, and representatives from 6 federal agencies. The WPSI is currently chaired by Jeanne Conry, MD, PhD, past president of ACOG. The steering committee used evidence-based best practices to develop the guidelines and relied heavily on the foundation provided by the 2011 IOM report.1

The 9 WPSI recommendations
Much of the text below is directly quoted from the final recommendations. When a recommendation is paraphrased it is not placed in quotations.
Recommendation 1: Breast cancer screening for average-risk women
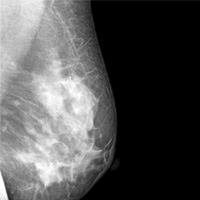
“Average-risk women should initiate mammography screening for breast cancer no earlier than age 40 and no later than age 50 years. Screeningmammography should occur at least biennially and as frequently as annually. Screening should continue through at least age 74 years and age alone should not be the basis to stop screening.”
Decisions about when to initiate screening for women between 40 and 50 years of age, how often to screen, and when to stop screening should be based on shared decision making involving the woman and her clinician.
Recommendation 2: Breastfeeding services and supplies
Women should be provided “comprehensive lactation support services including counseling, education and breast feeding equipment and supplies during the antenatal, perinatal, and postpartum periods.” These services will support the successful initiation and maintenance of breastfeeding. Women should have access to double electric breast pumps.
Recommendation 3: Screening for cervical cancer
Average-risk women should initiate cervical cancer screening with cervical cytology at age 21 years and have cervical cytology testing every 3 years from 21 to 29 years of age. “Cotesting with cytology and human papillomavirus (HPV) testing is not recommended for women younger than 30 years. Women aged 30 to 65 years should be screened with cytology and HPV testing every 5 years or cytology alone every 3 years.” Women who have received the HPV vaccine should be screened using these guidelines. Cervical cancer screening is not recommended for women younger than 21 years or older than 65 years who have had adequate prior screening and are not at high risk for cervical cancer. Cervical cancer screening is also not recommended for women who have had a hysterectomy with removal of the cervix and no personal history of cervical intraepithelial neoplasia grade 2 or 3 within the past 20 years.
Recommendation 4: Contraception
Adolescent and adult women should have access to the full range of US Food and Drug Administration–approved female-controlled contraceptives to prevent unintended pregnancy and improve birth outcomes. Multiple visits with a clinician may be needed to select an optimal contraceptive.
Recommendation 5: Screening for gestational diabetes mellitus
Pregnant women should be screened for GDM between 24 and 28 weeks’ gestation to prevent adverse birth outcomes. Screening should be performed with a “50 gm oral glucose challenge test followed by a 3-hour 100 gm oral glucose tolerance test” if the results on the initial oral glucose tolerance test are abnormal. This testing sequence has high sensitivity and specificity. Women with risk factors for diabetes mellitus should be screened for diabetes at the first prenatal visit using current best clinical practice.
Recommendation 6: Screening for human immunodeficiency virus (HIV) infection
Adolescents and women should receive education and risk assessment for HIV annually and should be tested for HIV at least once during their lifetime. Based on assessed risk, screening annually may be appropriate. “Screening for HIV is recommended for all pregnant women upon initiation of prenatal care with retesting during pregnancy based on risk factors. Rapid HIV testing is recommended for pregnant women who present in active labor with an undocumented HIV status.” Risk-based screening does not identify approximately 20% of HIV-infected people. Hence screening annually may be reasonable.
Recommendation 7: Screening for interpersonal and domestic violence
All adolescents and women should be screened annually for both interpersonal violence (IPV) and domestic violence (DV). Intervention services should be available to all adolescents and women. IPV and DV are prevalent problems, and they are often undetected by clinicians. Hence annual screening is recommended.
Recommendation 8: Counseling for sexually transmitted infections
Adolescents and women should be assessed for sexually transmittedinfection (STI) risk. Risk factors include:
- “age younger than 25 years,
- a recent STI,
- a new sex partner,
- multiple partners,
- a partner with concurrent partners,
- a partner with an STI, and
- a lack of or inconsistent condom use.”
Women at increased risk for an STI should receive behavioral counseling.
Recommendation 9: Well-woman preventive visits
Women should “receive at least one preventive care visit per year beginning in adolescence and continuing across the lifespan to ensure that the recommended preventive services including preconception and many services necessary for prenatal and interconception care are obtained. The primary purpose of these visits is the delivery and coordination of recommended preventive services as determined by age and risk factors.”
- Abridged guidelines for the Women's Preventive Services Initiative can be found here: http://www.womenspreventivehealth.org/wp-content/uploads/2017/01/WPSI_2016AbridgedReport.pdf.
- Evidence-based summaries and appendices are available at this link: http://www.womenspreventivehealth.org/wp-content/uploads/2016/12/Evidence-Summaries-and-Appendices.pdf.
I plan on using these recommendations to guide my practice
Historically, many high-profile expert professional groups have developed their own women’s health services guidelines. The proliferation of conflicting guidelines confused both patients and clinicians. Dueling guidelines likely undermine public health because they result in confusion among patients and inconsistent care across the many disciplines that provide medical services to women.
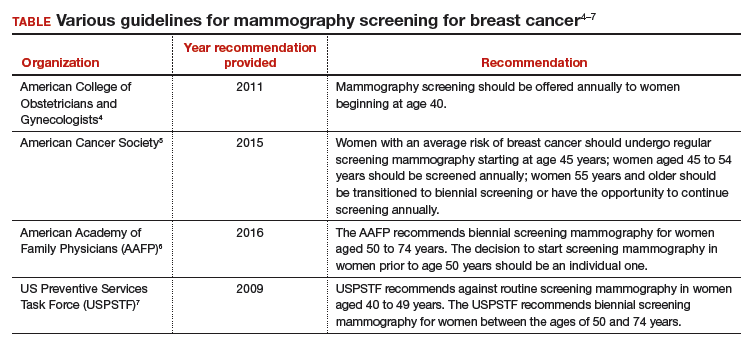
The proliferation of conflicting guidelines for mammography screening for breast cancer is a good example of how dueling guidelines can undermine public health (TABLE).4−7 The WPSI has done a great service to women and clinicians by creating a shared framework for consistently providing critical services across a woman’s entire life. I plan on using these recommendations to guide my practice. Patients and clinicians will greatly benefit from the exceptionally thoughtful women’s preventive services guidelines provided by the WPSI.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Institute of Medicine. Clinical preventive services for women: closing the gaps. Washington DC: The National Academies Press; 2011. http://nap.edu/13181. Accessed January 16, 2017.
- American Congress of Obstetricians and Gynecologists (ACOG). Women's Preventive Services Initiative (WPSI). http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Womens-Preventive-Services-Initiative. Accessed January 16, 2017.
- Health Resources and Services Administration website. Women's preventive services guidelines. https://www.hrsa.gov/womensguidelines/. Accessed January 16, 2017.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
- American Academy of Family Physicians website. Clinical preventive service recommendation: breast cancer. http://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html. Accessed January 16, 2017.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716-726.
The Patient Protection and Affordable Care Act of 2010 (ACA) intended that women have access to critical preventive health services without a copay or deductible. The Institute of Medicine (IOM) was asked to help identify those critical preventive women’s health services. In 2011, the IOM Committee on Preventive Services for Women recommended that all women have access to 9 preventive services, among them1:
- screening for gestational diabetes mellitus (GDM)
- human papilloma virus testing
- contraceptive methods and counseling
- well-woman visits.
The Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services agreed to update the recommended preventive services every 5 years.
In March 2016, HRSA entered into a 5-year cooperative agreement with the American College of Obstetricians and Gynecologists (ACOG) to update the guidelines and to develop additional recommendations to enhance women’s health.2 ACOG launched the Women’s Preventive Services Initiative (WPSI) to develop the 2016 update.
The 5-year grant with HRSA will address many more preventive health services for women across their lifespan as well as implementation strategies so that women receive consistent and appropriate care, regardless of the health care provider’s specialty. The WPSI recognizes that the selection of a provider for well-woman care will be determined as much by a woman’s needs and preferences as by her access to health care services and health plan availability.
The WPSI draft recommendations were released for public comment in September 2016,2 and HRSA approved the recommendations in December 2016.3 In this editorial, I provide a look at which organizations comprise the WPSI and a summary of the 9 recommended preventive health services.
Who makes up the Women’s Preventive Services Initiative?
The WPSI is a collaboration between professional societies and consumer organizations. The goal of the WPSI is “to promote health over the course of a woman’s lifetime through disease prevention and preventive healthcare.” The WPSI advisory panel provides oversight to the effort and the multidisciplinary steering committee develops the recommendations. The WPSI advisory panel includes leaders and experts from 4 major professional organizations, whose members provide the majority of women’s health care in the United States:
- ACOG
- American College of Physicians (ACP)
- American Academy of Family Physicians (AAFP)
- National Association of Nurse Practitioners in Women’s Health (NPWH).
The multidisciplinary steering committee includes the members of the advisory panel, representatives from 17 professional and consumer organizations, a patient representative, and representatives from 6 federal agencies. The WPSI is currently chaired by Jeanne Conry, MD, PhD, past president of ACOG. The steering committee used evidence-based best practices to develop the guidelines and relied heavily on the foundation provided by the 2011 IOM report.1

The 9 WPSI recommendations
Much of the text below is directly quoted from the final recommendations. When a recommendation is paraphrased it is not placed in quotations.
Recommendation 1: Breast cancer screening for average-risk women
“Average-risk women should initiate mammography screening for breast cancer no earlier than age 40 and no later than age 50 years. Screeningmammography should occur at least biennially and as frequently as annually. Screening should continue through at least age 74 years and age alone should not be the basis to stop screening.”
Decisions about when to initiate screening for women between 40 and 50 years of age, how often to screen, and when to stop screening should be based on shared decision making involving the woman and her clinician.
Recommendation 2: Breastfeeding services and supplies
Women should be provided “comprehensive lactation support services including counseling, education and breast feeding equipment and supplies during the antenatal, perinatal, and postpartum periods.” These services will support the successful initiation and maintenance of breastfeeding. Women should have access to double electric breast pumps.
Recommendation 3: Screening for cervical cancer
Average-risk women should initiate cervical cancer screening with cervical cytology at age 21 years and have cervical cytology testing every 3 years from 21 to 29 years of age. “Cotesting with cytology and human papillomavirus (HPV) testing is not recommended for women younger than 30 years. Women aged 30 to 65 years should be screened with cytology and HPV testing every 5 years or cytology alone every 3 years.” Women who have received the HPV vaccine should be screened using these guidelines. Cervical cancer screening is not recommended for women younger than 21 years or older than 65 years who have had adequate prior screening and are not at high risk for cervical cancer. Cervical cancer screening is also not recommended for women who have had a hysterectomy with removal of the cervix and no personal history of cervical intraepithelial neoplasia grade 2 or 3 within the past 20 years.
Recommendation 4: Contraception
Adolescent and adult women should have access to the full range of US Food and Drug Administration–approved female-controlled contraceptives to prevent unintended pregnancy and improve birth outcomes. Multiple visits with a clinician may be needed to select an optimal contraceptive.
Recommendation 5: Screening for gestational diabetes mellitus
Pregnant women should be screened for GDM between 24 and 28 weeks’ gestation to prevent adverse birth outcomes. Screening should be performed with a “50 gm oral glucose challenge test followed by a 3-hour 100 gm oral glucose tolerance test” if the results on the initial oral glucose tolerance test are abnormal. This testing sequence has high sensitivity and specificity. Women with risk factors for diabetes mellitus should be screened for diabetes at the first prenatal visit using current best clinical practice.
Recommendation 6: Screening for human immunodeficiency virus (HIV) infection
Adolescents and women should receive education and risk assessment for HIV annually and should be tested for HIV at least once during their lifetime. Based on assessed risk, screening annually may be appropriate. “Screening for HIV is recommended for all pregnant women upon initiation of prenatal care with retesting during pregnancy based on risk factors. Rapid HIV testing is recommended for pregnant women who present in active labor with an undocumented HIV status.” Risk-based screening does not identify approximately 20% of HIV-infected people. Hence screening annually may be reasonable.
Recommendation 7: Screening for interpersonal and domestic violence
All adolescents and women should be screened annually for both interpersonal violence (IPV) and domestic violence (DV). Intervention services should be available to all adolescents and women. IPV and DV are prevalent problems, and they are often undetected by clinicians. Hence annual screening is recommended.
Recommendation 8: Counseling for sexually transmitted infections
Adolescents and women should be assessed for sexually transmittedinfection (STI) risk. Risk factors include:
- “age younger than 25 years,
- a recent STI,
- a new sex partner,
- multiple partners,
- a partner with concurrent partners,
- a partner with an STI, and
- a lack of or inconsistent condom use.”
Women at increased risk for an STI should receive behavioral counseling.
Recommendation 9: Well-woman preventive visits
Women should “receive at least one preventive care visit per year beginning in adolescence and continuing across the lifespan to ensure that the recommended preventive services including preconception and many services necessary for prenatal and interconception care are obtained. The primary purpose of these visits is the delivery and coordination of recommended preventive services as determined by age and risk factors.”
- Abridged guidelines for the Women's Preventive Services Initiative can be found here: http://www.womenspreventivehealth.org/wp-content/uploads/2017/01/WPSI_2016AbridgedReport.pdf.
- Evidence-based summaries and appendices are available at this link: http://www.womenspreventivehealth.org/wp-content/uploads/2016/12/Evidence-Summaries-and-Appendices.pdf.
I plan on using these recommendations to guide my practice
Historically, many high-profile expert professional groups have developed their own women’s health services guidelines. The proliferation of conflicting guidelines confused both patients and clinicians. Dueling guidelines likely undermine public health because they result in confusion among patients and inconsistent care across the many disciplines that provide medical services to women.
The proliferation of conflicting guidelines for mammography screening for breast cancer is a good example of how dueling guidelines can undermine public health (TABLE).4−7 The WPSI has done a great service to women and clinicians by creating a shared framework for consistently providing critical services across a woman’s entire life. I plan on using these recommendations to guide my practice. Patients and clinicians will greatly benefit from the exceptionally thoughtful women’s preventive services guidelines provided by the WPSI.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The Patient Protection and Affordable Care Act of 2010 (ACA) intended that women have access to critical preventive health services without a copay or deductible. The Institute of Medicine (IOM) was asked to help identify those critical preventive women’s health services. In 2011, the IOM Committee on Preventive Services for Women recommended that all women have access to 9 preventive services, among them1:
- screening for gestational diabetes mellitus (GDM)
- human papilloma virus testing
- contraceptive methods and counseling
- well-woman visits.
The Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services agreed to update the recommended preventive services every 5 years.
In March 2016, HRSA entered into a 5-year cooperative agreement with the American College of Obstetricians and Gynecologists (ACOG) to update the guidelines and to develop additional recommendations to enhance women’s health.2 ACOG launched the Women’s Preventive Services Initiative (WPSI) to develop the 2016 update.
The 5-year grant with HRSA will address many more preventive health services for women across their lifespan as well as implementation strategies so that women receive consistent and appropriate care, regardless of the health care provider’s specialty. The WPSI recognizes that the selection of a provider for well-woman care will be determined as much by a woman’s needs and preferences as by her access to health care services and health plan availability.
The WPSI draft recommendations were released for public comment in September 2016,2 and HRSA approved the recommendations in December 2016.3 In this editorial, I provide a look at which organizations comprise the WPSI and a summary of the 9 recommended preventive health services.
Who makes up the Women’s Preventive Services Initiative?
The WPSI is a collaboration between professional societies and consumer organizations. The goal of the WPSI is “to promote health over the course of a woman’s lifetime through disease prevention and preventive healthcare.” The WPSI advisory panel provides oversight to the effort and the multidisciplinary steering committee develops the recommendations. The WPSI advisory panel includes leaders and experts from 4 major professional organizations, whose members provide the majority of women’s health care in the United States:
- ACOG
- American College of Physicians (ACP)
- American Academy of Family Physicians (AAFP)
- National Association of Nurse Practitioners in Women’s Health (NPWH).
The multidisciplinary steering committee includes the members of the advisory panel, representatives from 17 professional and consumer organizations, a patient representative, and representatives from 6 federal agencies. The WPSI is currently chaired by Jeanne Conry, MD, PhD, past president of ACOG. The steering committee used evidence-based best practices to develop the guidelines and relied heavily on the foundation provided by the 2011 IOM report.1

The 9 WPSI recommendations
Much of the text below is directly quoted from the final recommendations. When a recommendation is paraphrased it is not placed in quotations.
Recommendation 1: Breast cancer screening for average-risk women
“Average-risk women should initiate mammography screening for breast cancer no earlier than age 40 and no later than age 50 years. Screeningmammography should occur at least biennially and as frequently as annually. Screening should continue through at least age 74 years and age alone should not be the basis to stop screening.”
Decisions about when to initiate screening for women between 40 and 50 years of age, how often to screen, and when to stop screening should be based on shared decision making involving the woman and her clinician.
Recommendation 2: Breastfeeding services and supplies
Women should be provided “comprehensive lactation support services including counseling, education and breast feeding equipment and supplies during the antenatal, perinatal, and postpartum periods.” These services will support the successful initiation and maintenance of breastfeeding. Women should have access to double electric breast pumps.
Recommendation 3: Screening for cervical cancer
Average-risk women should initiate cervical cancer screening with cervical cytology at age 21 years and have cervical cytology testing every 3 years from 21 to 29 years of age. “Cotesting with cytology and human papillomavirus (HPV) testing is not recommended for women younger than 30 years. Women aged 30 to 65 years should be screened with cytology and HPV testing every 5 years or cytology alone every 3 years.” Women who have received the HPV vaccine should be screened using these guidelines. Cervical cancer screening is not recommended for women younger than 21 years or older than 65 years who have had adequate prior screening and are not at high risk for cervical cancer. Cervical cancer screening is also not recommended for women who have had a hysterectomy with removal of the cervix and no personal history of cervical intraepithelial neoplasia grade 2 or 3 within the past 20 years.
Recommendation 4: Contraception
Adolescent and adult women should have access to the full range of US Food and Drug Administration–approved female-controlled contraceptives to prevent unintended pregnancy and improve birth outcomes. Multiple visits with a clinician may be needed to select an optimal contraceptive.
Recommendation 5: Screening for gestational diabetes mellitus
Pregnant women should be screened for GDM between 24 and 28 weeks’ gestation to prevent adverse birth outcomes. Screening should be performed with a “50 gm oral glucose challenge test followed by a 3-hour 100 gm oral glucose tolerance test” if the results on the initial oral glucose tolerance test are abnormal. This testing sequence has high sensitivity and specificity. Women with risk factors for diabetes mellitus should be screened for diabetes at the first prenatal visit using current best clinical practice.
Recommendation 6: Screening for human immunodeficiency virus (HIV) infection
Adolescents and women should receive education and risk assessment for HIV annually and should be tested for HIV at least once during their lifetime. Based on assessed risk, screening annually may be appropriate. “Screening for HIV is recommended for all pregnant women upon initiation of prenatal care with retesting during pregnancy based on risk factors. Rapid HIV testing is recommended for pregnant women who present in active labor with an undocumented HIV status.” Risk-based screening does not identify approximately 20% of HIV-infected people. Hence screening annually may be reasonable.
Recommendation 7: Screening for interpersonal and domestic violence
All adolescents and women should be screened annually for both interpersonal violence (IPV) and domestic violence (DV). Intervention services should be available to all adolescents and women. IPV and DV are prevalent problems, and they are often undetected by clinicians. Hence annual screening is recommended.
Recommendation 8: Counseling for sexually transmitted infections
Adolescents and women should be assessed for sexually transmittedinfection (STI) risk. Risk factors include:
- “age younger than 25 years,
- a recent STI,
- a new sex partner,
- multiple partners,
- a partner with concurrent partners,
- a partner with an STI, and
- a lack of or inconsistent condom use.”
Women at increased risk for an STI should receive behavioral counseling.
Recommendation 9: Well-woman preventive visits
Women should “receive at least one preventive care visit per year beginning in adolescence and continuing across the lifespan to ensure that the recommended preventive services including preconception and many services necessary for prenatal and interconception care are obtained. The primary purpose of these visits is the delivery and coordination of recommended preventive services as determined by age and risk factors.”
- Abridged guidelines for the Women's Preventive Services Initiative can be found here: http://www.womenspreventivehealth.org/wp-content/uploads/2017/01/WPSI_2016AbridgedReport.pdf.
- Evidence-based summaries and appendices are available at this link: http://www.womenspreventivehealth.org/wp-content/uploads/2016/12/Evidence-Summaries-and-Appendices.pdf.
I plan on using these recommendations to guide my practice
Historically, many high-profile expert professional groups have developed their own women’s health services guidelines. The proliferation of conflicting guidelines confused both patients and clinicians. Dueling guidelines likely undermine public health because they result in confusion among patients and inconsistent care across the many disciplines that provide medical services to women.
The proliferation of conflicting guidelines for mammography screening for breast cancer is a good example of how dueling guidelines can undermine public health (TABLE).4−7 The WPSI has done a great service to women and clinicians by creating a shared framework for consistently providing critical services across a woman’s entire life. I plan on using these recommendations to guide my practice. Patients and clinicians will greatly benefit from the exceptionally thoughtful women’s preventive services guidelines provided by the WPSI.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Institute of Medicine. Clinical preventive services for women: closing the gaps. Washington DC: The National Academies Press; 2011. http://nap.edu/13181. Accessed January 16, 2017.
- American Congress of Obstetricians and Gynecologists (ACOG). Women's Preventive Services Initiative (WPSI). http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Womens-Preventive-Services-Initiative. Accessed January 16, 2017.
- Health Resources and Services Administration website. Women's preventive services guidelines. https://www.hrsa.gov/womensguidelines/. Accessed January 16, 2017.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
- American Academy of Family Physicians website. Clinical preventive service recommendation: breast cancer. http://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html. Accessed January 16, 2017.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716-726.
- Institute of Medicine. Clinical preventive services for women: closing the gaps. Washington DC: The National Academies Press; 2011. http://nap.edu/13181. Accessed January 16, 2017.
- American Congress of Obstetricians and Gynecologists (ACOG). Women's Preventive Services Initiative (WPSI). http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Womens-Preventive-Services-Initiative. Accessed January 16, 2017.
- Health Resources and Services Administration website. Women's preventive services guidelines. https://www.hrsa.gov/womensguidelines/. Accessed January 16, 2017.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
- American Academy of Family Physicians website. Clinical preventive service recommendation: breast cancer. http://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html. Accessed January 16, 2017.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716-726.
From the Editors: One pebble at a time
This is a story about Sarah Prince, FRCS, and thousands of others here and abroad who are surgeons. Only a few of you may have heard of Miss Prince, consultant surgeon from Fort William, Scotland; but she represents to me one of thousands of stories that make surgery such a rich subject that spans more than pure science. Sarah achieved immortality in what she accomplished in 43 short years.
Sarah was trained in the United Kingdom system, attaining specialty training in hepatobiliary disease. While she loved that sort of work she decided, with her internist husband Patrick Byrne, to work in a rural town in northern Scotland. In nine years she built up the hospital there and its training paradigm. She went on to work toward creating a better rural surgical system in Scotland, eventually becoming an expert who spoke all over the world about rural surgery and allocating resources to build surgical capacity in rural areas. She understood the volume debate and the need for rural surgeons to have a connection that was substantive with a larger center in a collaborative way benefiting both locales.
I bring her up because she represents something we all can do. A few surgeons become academic giants known far and wide, but all surgeons have the ability to be local giants, unknown but immortal and essential in their own way. Sarah’s accomplishments confirm that.
Unlike surgery in the United States, the U.K. system is more regimented in many ways and even more political than what the average U.S. surgeon experiences. It is a single-payer system that was there long before Sarah became a surgeon and will be there long after. The fact that the system into which she was born was not of her making did not deter Sarah from taking on that very system to make her corner of the world a better place. I was always surprised when speaking with her that the problems she faced in Scotland were much the same as what I’ve seen in rural surgery in the United States and in other countries. She didn’t bend the whole system but she made a significant dent in how things were done. Isn’t that the challenge for us all?
Recently on the ACS Communities and elsewhere, the debate on single-payer, multitier, and market-driven health care is being argued. In light of the current political environment, the path forward seems bewilderingly tangled. Most surgeons just want to operate. The OR may be the last bastion of control we surgeons have in our professional lives. There may be a barrage of obstacles getting to the OR and hordes of explanations and details postoperatively, but in the OR we still get to do what we think is best at the moment using all those skills we so painfully acquired during a career of learning and practice. To despair is easy until one takes a look at what so many surgeons achieve in their lives.
Like Sarah, most of us try to make the profession a little better. In small town Iowa, that may be getting sonography privileges for FAST exams that improves the lot of trauma patients in that town. In an exburbia hospital, the surgeon may bring new expertise not previously available. It goes on and on with each of us contributing one pebble at a time to a mountain of effort. Any one pebble seems so insignificant in itself and sometimes just placing it on the mountain takes enormous effort, but each is worth the toil to put it there.
Which brings us back to Miss Prince (it is a faux pas to call a consultant surgeon in the U.K. by the honorific doctor). Sarah faced just as many challenges and perhaps more than surgeons elsewhere. Yet she brought her best every day to her hospital until cruel fate delivered her a fatal blow at a young age. Even then facing her imminent death, Sarah made sure that her patients and trainees would be well cared for after her passing. Her indomitable approach to surgical life shows that no matter what the opposition, a surgeon can with grit and wit make life better in his or her town, region, and maybe even the world.
As we face 2017 with all its potential for defeat or victory for our patients, let us remember surgeons like Sarah Prince who made a difference and commit ourselves to the same goal. We can do it one pebble at a time until we’ve created a mountain of accomplishment.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
This is a story about Sarah Prince, FRCS, and thousands of others here and abroad who are surgeons. Only a few of you may have heard of Miss Prince, consultant surgeon from Fort William, Scotland; but she represents to me one of thousands of stories that make surgery such a rich subject that spans more than pure science. Sarah achieved immortality in what she accomplished in 43 short years.
Sarah was trained in the United Kingdom system, attaining specialty training in hepatobiliary disease. While she loved that sort of work she decided, with her internist husband Patrick Byrne, to work in a rural town in northern Scotland. In nine years she built up the hospital there and its training paradigm. She went on to work toward creating a better rural surgical system in Scotland, eventually becoming an expert who spoke all over the world about rural surgery and allocating resources to build surgical capacity in rural areas. She understood the volume debate and the need for rural surgeons to have a connection that was substantive with a larger center in a collaborative way benefiting both locales.
I bring her up because she represents something we all can do. A few surgeons become academic giants known far and wide, but all surgeons have the ability to be local giants, unknown but immortal and essential in their own way. Sarah’s accomplishments confirm that.
Unlike surgery in the United States, the U.K. system is more regimented in many ways and even more political than what the average U.S. surgeon experiences. It is a single-payer system that was there long before Sarah became a surgeon and will be there long after. The fact that the system into which she was born was not of her making did not deter Sarah from taking on that very system to make her corner of the world a better place. I was always surprised when speaking with her that the problems she faced in Scotland were much the same as what I’ve seen in rural surgery in the United States and in other countries. She didn’t bend the whole system but she made a significant dent in how things were done. Isn’t that the challenge for us all?
Recently on the ACS Communities and elsewhere, the debate on single-payer, multitier, and market-driven health care is being argued. In light of the current political environment, the path forward seems bewilderingly tangled. Most surgeons just want to operate. The OR may be the last bastion of control we surgeons have in our professional lives. There may be a barrage of obstacles getting to the OR and hordes of explanations and details postoperatively, but in the OR we still get to do what we think is best at the moment using all those skills we so painfully acquired during a career of learning and practice. To despair is easy until one takes a look at what so many surgeons achieve in their lives.
Like Sarah, most of us try to make the profession a little better. In small town Iowa, that may be getting sonography privileges for FAST exams that improves the lot of trauma patients in that town. In an exburbia hospital, the surgeon may bring new expertise not previously available. It goes on and on with each of us contributing one pebble at a time to a mountain of effort. Any one pebble seems so insignificant in itself and sometimes just placing it on the mountain takes enormous effort, but each is worth the toil to put it there.
Which brings us back to Miss Prince (it is a faux pas to call a consultant surgeon in the U.K. by the honorific doctor). Sarah faced just as many challenges and perhaps more than surgeons elsewhere. Yet she brought her best every day to her hospital until cruel fate delivered her a fatal blow at a young age. Even then facing her imminent death, Sarah made sure that her patients and trainees would be well cared for after her passing. Her indomitable approach to surgical life shows that no matter what the opposition, a surgeon can with grit and wit make life better in his or her town, region, and maybe even the world.
As we face 2017 with all its potential for defeat or victory for our patients, let us remember surgeons like Sarah Prince who made a difference and commit ourselves to the same goal. We can do it one pebble at a time until we’ve created a mountain of accomplishment.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
This is a story about Sarah Prince, FRCS, and thousands of others here and abroad who are surgeons. Only a few of you may have heard of Miss Prince, consultant surgeon from Fort William, Scotland; but she represents to me one of thousands of stories that make surgery such a rich subject that spans more than pure science. Sarah achieved immortality in what she accomplished in 43 short years.
Sarah was trained in the United Kingdom system, attaining specialty training in hepatobiliary disease. While she loved that sort of work she decided, with her internist husband Patrick Byrne, to work in a rural town in northern Scotland. In nine years she built up the hospital there and its training paradigm. She went on to work toward creating a better rural surgical system in Scotland, eventually becoming an expert who spoke all over the world about rural surgery and allocating resources to build surgical capacity in rural areas. She understood the volume debate and the need for rural surgeons to have a connection that was substantive with a larger center in a collaborative way benefiting both locales.
I bring her up because she represents something we all can do. A few surgeons become academic giants known far and wide, but all surgeons have the ability to be local giants, unknown but immortal and essential in their own way. Sarah’s accomplishments confirm that.
Unlike surgery in the United States, the U.K. system is more regimented in many ways and even more political than what the average U.S. surgeon experiences. It is a single-payer system that was there long before Sarah became a surgeon and will be there long after. The fact that the system into which she was born was not of her making did not deter Sarah from taking on that very system to make her corner of the world a better place. I was always surprised when speaking with her that the problems she faced in Scotland were much the same as what I’ve seen in rural surgery in the United States and in other countries. She didn’t bend the whole system but she made a significant dent in how things were done. Isn’t that the challenge for us all?
Recently on the ACS Communities and elsewhere, the debate on single-payer, multitier, and market-driven health care is being argued. In light of the current political environment, the path forward seems bewilderingly tangled. Most surgeons just want to operate. The OR may be the last bastion of control we surgeons have in our professional lives. There may be a barrage of obstacles getting to the OR and hordes of explanations and details postoperatively, but in the OR we still get to do what we think is best at the moment using all those skills we so painfully acquired during a career of learning and practice. To despair is easy until one takes a look at what so many surgeons achieve in their lives.
Like Sarah, most of us try to make the profession a little better. In small town Iowa, that may be getting sonography privileges for FAST exams that improves the lot of trauma patients in that town. In an exburbia hospital, the surgeon may bring new expertise not previously available. It goes on and on with each of us contributing one pebble at a time to a mountain of effort. Any one pebble seems so insignificant in itself and sometimes just placing it on the mountain takes enormous effort, but each is worth the toil to put it there.
Which brings us back to Miss Prince (it is a faux pas to call a consultant surgeon in the U.K. by the honorific doctor). Sarah faced just as many challenges and perhaps more than surgeons elsewhere. Yet she brought her best every day to her hospital until cruel fate delivered her a fatal blow at a young age. Even then facing her imminent death, Sarah made sure that her patients and trainees would be well cared for after her passing. Her indomitable approach to surgical life shows that no matter what the opposition, a surgeon can with grit and wit make life better in his or her town, region, and maybe even the world.
As we face 2017 with all its potential for defeat or victory for our patients, let us remember surgeons like Sarah Prince who made a difference and commit ourselves to the same goal. We can do it one pebble at a time until we’ve created a mountain of accomplishment.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
Long-acting reversible contraceptives and acne in adolescents
Examining the impact of contraception on acne in adolescents is clinically important because acne affects about 85% of adolescents, and contraceptives may influence the course of acne disease. Estrogen-progestin contraceptives cause a significant improvement in acne.1,2 By contrast, the levonorgestrel-releasing intrauterine device and the etonogestrel contraceptive implant may exacerbate acne. In this editorial we review the hormonal contraception−acne relationship, available acne treatments, and appropriate management.
Related article:
Your teenage patient and contraception: Think “long-acting” first
Combination oral contraception and acne
As noted, combination oral contraceptives generally result in acne improvement.1,2 Estrogen-progestin contraceptives improve the condition through two mechanisms. Primarily, estrogen-progestin contraceptives suppress pituitary luteinizing hormone secretion, thereby decreasing ovarian testosterone production. These contraceptives also increase liver production of sex hormone-binding globulin (SHBG), thereby increasing bound testosterone and decreasing free testosterone. The decrease in ovarian testosterone production and the increase in SHBG-bound testosterone reduce sebum production, resulting in acne improvement.
The US Food and Drug Administration has approved 4 estrogen-progestin contraceptives for acne treatment:
- Estrostep (norethindrone acetate-ethinyl estradiol plus ferrous fumarate)
- Ortho Tri-Cyclen (norgestimate-ethinyl estradiol)
- Yaz (drospirenone-ethinyl estradiol)
- BeYaz (drospirenone-ethinyl estradiol plus levomefolate).
LARC and acne
The levonorgestrel intrauterine devices (LNG-IUDs), including the levonorgestrel intrauterine systems Mirena, Liletta, Skyla, and Kyleena, and the etonogestrel implant (Nexplanon) are among the most effective contraceptives available for women. Over the last decade there has been a marked increase in the use of LARC. In 2002, 1.3% of women aged 15 to 24 years used an IUD or progestin implant, and this percentage increased to 10% by 2013.3
Progestin-containing LARC may cause acne to worsen. In a large 3-year prospective study of more than 2,900 women using the progestin implant or the copper IUD (ParaGard), use of the progestin implant was associated with a higher rate of reported acne than the copper IUD (18% vs 13%, respectively; relative risk, 1.4; 95% confidence interval, 1.20−1.56; P<.0001).4 In a retrospective review of 991 women who used the etonogestrel implant, 24% of the women requested that the implant be removed; the 3 most common reasons for removal were: bleeding disturbances (45%), worsening acne, (12%) and desire to conceive (12%).5
Similar differences in reported acne are seen between the LNG-IUD and the copper IUD. In a study of 320 women using the LNG-IUD and the copper IUD, an increase in acne was reported by 17% and 7%, respectively (P<.025).6 In a small prospective study of the LNG-IUD versus the copper IUD over the first 12 months of use, use of the LNG-IUD was associated with a statistically significant worsening of acne scores while use of the copper IUD had no impact on acne scores.7
Related article:
Overcoming LARC complications: 7 case challenges
In a study of 2,147 consecutive women using a hormonal contraceptive who presented to a dermatologist for the treatment of acne, patients were asked to assess how the contraceptive affected their acne. By type of contraceptive, the percent of women who reported that the contraceptive made their acne worse was: LNG-IUD, 36%; progestin implant, 33%; depot medroxyprogesterone acetate (MPA), 27%; levonorgestrel-ethinyl estradiol oral contraceptive, 10%; norgestimate-ethinyl estradiol (EE), 6%; etonogestrel-EE vaginal ring, 4%; drospirenone-EE, 3%; and desogestrel-EE, 2%. The percent of women who reported that the contraceptive significantly improved their acne was: drospirenone-EE, 26%; norgestimate-EE, 17%; desogestrel-EE, 15%; etonogestrel-EE vaginal ring, 14%; norethindrone-EE, 8%; levonorgestrel-EE, 6%; depot MPA, 5%; LNG-IUD, 3%; and progestin implant, 1%.8
In adolescents with acne, switching from an estrogen-progestin contraceptive to a LNG-IUD or an etonogestrel implant may cause the patient to report that her acne has worsened. As mentioned, combination estrogen-progestin contraceptives reduce free testosterone, thereby improving acne. When an estrogen-progestin contraceptive is discontinued, free testosterone levels will increase. If a LARC method is initiated and the patient’s acne worsens, the patient may attribute this change to the LARC. For clinicians planning on switching a patient from an estrogen-progestin contraceptive to a LNG-IUD or etonogestrel implant, evaluation of current acne symptoms and acne history may be particularly important.
Acne treatment
Acne is caused by follicular hyperproliferation and abnormal desquamation, excess sebum production, proliferation of Propionibacterium acnes, and inflammation.
First-line agents. An expert guideline developed under the auspices of the American Academy of Dermatology recommends that topical agents including retinoids and antimicrobials be first-line treatments for acne.9,10
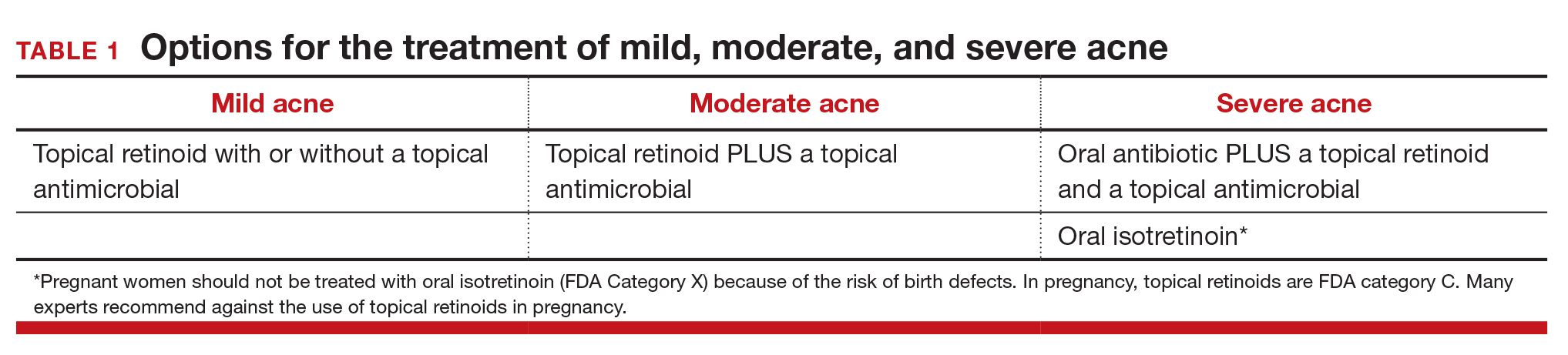
Topical retinoids are the primary component of topical acne treatment and can be used as monotherapy or in combination with topical antimicrobials (TABLE 1). Three topical retinoids are approved for use in the United States: tretinoin, adapalene, and tazarotene. Adapalene is available by prescription, 0.1% and 0.3% gel, and over the counter, 0.1% gel (Differin Gel) (TABLE 2). The topical retinoids are applied once daily at bedtime and can cause local skin irritation and dryness. Pregnant women should not be treated with topical retinoids.
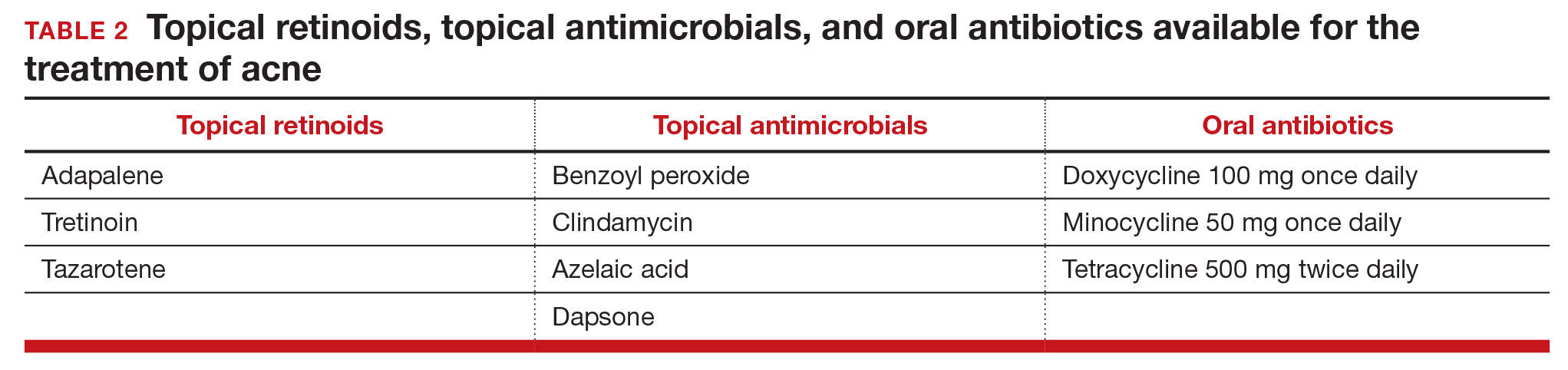
Topical antimicrobials for the treatment of acne include: benzoyl peroxide, clindamycin, azelaic acid, and dapsone. Clindamycin is only recommended for use in combination with benzoyl peroxide in order to reduce the development of bacterial resistance to the antibiotic.
Related article:
Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception?
Approach to mild, moderate, and severe acne. In adolescents with mild acne a topical retinoid or benzoyl peroxide can be used as monotherapy or used together. Referral to a dermatologist is recommended for moderate to severe acne. Moderate acne is treated with combination topical therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). Severe acne is treated with 3 months of oral antibiotics plus topical combination therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). In cases of severe nodular acne or acne that produces scarring the patient may require oral isotretinoin treatment.
Acne management for adolescents seeking LARC
Given the data that the LNG-IUD and the etonogestrel implant may worsen acne, it may be wise to preemptively ensure that adolescents with acne who are initiating these contraceptives are also being adequately treated for their acne. Gynecologists should provide anticipatory guidance for adolescents with mild acne who initiate progestin-based LARC. Topical benzoyl peroxide is available over-the-counter and can be recommended to these patients. Follow-up in clinic a few months after initiation also may be helpful to assess side effects.
In moderate and severe cases, coordination with dermatology is recommended. For these patients, gynecologists could consider prescribing a topical retinoid or antibiotic medication in conjunction with a new progestin-based LARC method. Those with severe acne also may benefit from concurrent use of oral contraceptives. In adolescents who do not tolerate progestin-based LARC, the copper IUD is a highly effective alternative and can be paired with estrogen-progestin contraception for acne treatment.
Related article:
With no budge in more than 20 years, are US unintended pregnancy rates finally on the decline?
Acne is but one consideration for contraceptive choice
With the above methods, acne can be managed in adolescents seeking a LNG-IUD or implant and should not be considered a contraindication or reason to avoid progestin-based LARC. Adolescents are more likely to continue LARC than estrogen-progestin contraceptives and LARC methods are associated with substantially lower pregnancy rates in this patient population.11 LARC is recommended as first-line contraception for adolescents by both the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.12,13
In choosing contraception with your adolescent patient, the risk of unintended pregnancy should be weighed against the risk of acne and other potential side effects. Do not select a contraceptive based on the presence or absence of acne disease. However, be aware that contraceptives can either improve or worsen acne. Patients with mild and moderate acne disease should be considered for treatment with topical retinoids and/or antimicrobial agents.

Dr. Barbieri reports no financial relationships relevant to this article.
Dr. Roe reports receiving grant or research support from the Society of Family Planning.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;(7):CD004425.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71(3):450-459.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15 to 44: United States 2011-2013. Natl Health Stat Report. 2015;(86):1-14.
- Bahamondes L, Brache V, Meirik O, Ali M, Habib N, Landoulsi S; WHO Study Group on Contraceptive Implants for Women. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum Reprod. 2015;30(11):2527-2538.
- Bitzer J, Tschudin S, Adler J; Swiss Implanon Study Group. Acceptability and side-effects of Implanon in Switzerland: a retrospective study by the Implanon Swiss Study Group. Eur J Contracept Reprod Health Care. 2004;9(4):278-284.
- Nilsson CG, Luukkainen T, Diaz J, Allonen H. Clinical performance of a new levonorgestrel-releasing intrauterine device. A randomized comparison with a Nova-T-copper device. Contraception. 1982;25(4):345-356.
- Kelekci S, Kelecki KH, Yilmaz B. Effects of levonorgestrel-releasing intrauterine system and T380A intrauterine copper device on dysmenorrhea and days of bleeding in women with and without adenomyosis. Contraception. 2012;86(5):458-463.
- Lortscher D, Admani S, Satur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
- Roman CJ, Cifu AD, Stein SL. Management of acne vulgaris. JAMA. 2016;316(13):1402-1403.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998-2007.
- American Academy of Pediatrics Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014;134(4):e1244-e1256.
- American College of Obstetricians and Gynecologists Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group. Committee Opinion No. 539. Adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012;120(4):983-988.
Examining the impact of contraception on acne in adolescents is clinically important because acne affects about 85% of adolescents, and contraceptives may influence the course of acne disease. Estrogen-progestin contraceptives cause a significant improvement in acne.1,2 By contrast, the levonorgestrel-releasing intrauterine device and the etonogestrel contraceptive implant may exacerbate acne. In this editorial we review the hormonal contraception−acne relationship, available acne treatments, and appropriate management.
Related article:
Your teenage patient and contraception: Think “long-acting” first
Combination oral contraception and acne
As noted, combination oral contraceptives generally result in acne improvement.1,2 Estrogen-progestin contraceptives improve the condition through two mechanisms. Primarily, estrogen-progestin contraceptives suppress pituitary luteinizing hormone secretion, thereby decreasing ovarian testosterone production. These contraceptives also increase liver production of sex hormone-binding globulin (SHBG), thereby increasing bound testosterone and decreasing free testosterone. The decrease in ovarian testosterone production and the increase in SHBG-bound testosterone reduce sebum production, resulting in acne improvement.
The US Food and Drug Administration has approved 4 estrogen-progestin contraceptives for acne treatment:
- Estrostep (norethindrone acetate-ethinyl estradiol plus ferrous fumarate)
- Ortho Tri-Cyclen (norgestimate-ethinyl estradiol)
- Yaz (drospirenone-ethinyl estradiol)
- BeYaz (drospirenone-ethinyl estradiol plus levomefolate).
LARC and acne
The levonorgestrel intrauterine devices (LNG-IUDs), including the levonorgestrel intrauterine systems Mirena, Liletta, Skyla, and Kyleena, and the etonogestrel implant (Nexplanon) are among the most effective contraceptives available for women. Over the last decade there has been a marked increase in the use of LARC. In 2002, 1.3% of women aged 15 to 24 years used an IUD or progestin implant, and this percentage increased to 10% by 2013.3
Progestin-containing LARC may cause acne to worsen. In a large 3-year prospective study of more than 2,900 women using the progestin implant or the copper IUD (ParaGard), use of the progestin implant was associated with a higher rate of reported acne than the copper IUD (18% vs 13%, respectively; relative risk, 1.4; 95% confidence interval, 1.20−1.56; P<.0001).4 In a retrospective review of 991 women who used the etonogestrel implant, 24% of the women requested that the implant be removed; the 3 most common reasons for removal were: bleeding disturbances (45%), worsening acne, (12%) and desire to conceive (12%).5
Similar differences in reported acne are seen between the LNG-IUD and the copper IUD. In a study of 320 women using the LNG-IUD and the copper IUD, an increase in acne was reported by 17% and 7%, respectively (P<.025).6 In a small prospective study of the LNG-IUD versus the copper IUD over the first 12 months of use, use of the LNG-IUD was associated with a statistically significant worsening of acne scores while use of the copper IUD had no impact on acne scores.7
Related article:
Overcoming LARC complications: 7 case challenges
In a study of 2,147 consecutive women using a hormonal contraceptive who presented to a dermatologist for the treatment of acne, patients were asked to assess how the contraceptive affected their acne. By type of contraceptive, the percent of women who reported that the contraceptive made their acne worse was: LNG-IUD, 36%; progestin implant, 33%; depot medroxyprogesterone acetate (MPA), 27%; levonorgestrel-ethinyl estradiol oral contraceptive, 10%; norgestimate-ethinyl estradiol (EE), 6%; etonogestrel-EE vaginal ring, 4%; drospirenone-EE, 3%; and desogestrel-EE, 2%. The percent of women who reported that the contraceptive significantly improved their acne was: drospirenone-EE, 26%; norgestimate-EE, 17%; desogestrel-EE, 15%; etonogestrel-EE vaginal ring, 14%; norethindrone-EE, 8%; levonorgestrel-EE, 6%; depot MPA, 5%; LNG-IUD, 3%; and progestin implant, 1%.8
In adolescents with acne, switching from an estrogen-progestin contraceptive to a LNG-IUD or an etonogestrel implant may cause the patient to report that her acne has worsened. As mentioned, combination estrogen-progestin contraceptives reduce free testosterone, thereby improving acne. When an estrogen-progestin contraceptive is discontinued, free testosterone levels will increase. If a LARC method is initiated and the patient’s acne worsens, the patient may attribute this change to the LARC. For clinicians planning on switching a patient from an estrogen-progestin contraceptive to a LNG-IUD or etonogestrel implant, evaluation of current acne symptoms and acne history may be particularly important.
Acne treatment
Acne is caused by follicular hyperproliferation and abnormal desquamation, excess sebum production, proliferation of Propionibacterium acnes, and inflammation.
First-line agents. An expert guideline developed under the auspices of the American Academy of Dermatology recommends that topical agents including retinoids and antimicrobials be first-line treatments for acne.9,10
Topical retinoids are the primary component of topical acne treatment and can be used as monotherapy or in combination with topical antimicrobials (TABLE 1). Three topical retinoids are approved for use in the United States: tretinoin, adapalene, and tazarotene. Adapalene is available by prescription, 0.1% and 0.3% gel, and over the counter, 0.1% gel (Differin Gel) (TABLE 2). The topical retinoids are applied once daily at bedtime and can cause local skin irritation and dryness. Pregnant women should not be treated with topical retinoids.


Topical antimicrobials for the treatment of acne include: benzoyl peroxide, clindamycin, azelaic acid, and dapsone. Clindamycin is only recommended for use in combination with benzoyl peroxide in order to reduce the development of bacterial resistance to the antibiotic.
Related article:
Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception?
Approach to mild, moderate, and severe acne. In adolescents with mild acne a topical retinoid or benzoyl peroxide can be used as monotherapy or used together. Referral to a dermatologist is recommended for moderate to severe acne. Moderate acne is treated with combination topical therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). Severe acne is treated with 3 months of oral antibiotics plus topical combination therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). In cases of severe nodular acne or acne that produces scarring the patient may require oral isotretinoin treatment.
Acne management for adolescents seeking LARC
Given the data that the LNG-IUD and the etonogestrel implant may worsen acne, it may be wise to preemptively ensure that adolescents with acne who are initiating these contraceptives are also being adequately treated for their acne. Gynecologists should provide anticipatory guidance for adolescents with mild acne who initiate progestin-based LARC. Topical benzoyl peroxide is available over-the-counter and can be recommended to these patients. Follow-up in clinic a few months after initiation also may be helpful to assess side effects.
In moderate and severe cases, coordination with dermatology is recommended. For these patients, gynecologists could consider prescribing a topical retinoid or antibiotic medication in conjunction with a new progestin-based LARC method. Those with severe acne also may benefit from concurrent use of oral contraceptives. In adolescents who do not tolerate progestin-based LARC, the copper IUD is a highly effective alternative and can be paired with estrogen-progestin contraception for acne treatment.
Related article:
With no budge in more than 20 years, are US unintended pregnancy rates finally on the decline?
Acne is but one consideration for contraceptive choice
With the above methods, acne can be managed in adolescents seeking a LNG-IUD or implant and should not be considered a contraindication or reason to avoid progestin-based LARC. Adolescents are more likely to continue LARC than estrogen-progestin contraceptives and LARC methods are associated with substantially lower pregnancy rates in this patient population.11 LARC is recommended as first-line contraception for adolescents by both the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.12,13
In choosing contraception with your adolescent patient, the risk of unintended pregnancy should be weighed against the risk of acne and other potential side effects. Do not select a contraceptive based on the presence or absence of acne disease. However, be aware that contraceptives can either improve or worsen acne. Patients with mild and moderate acne disease should be considered for treatment with topical retinoids and/or antimicrobial agents.

Dr. Barbieri reports no financial relationships relevant to this article.
Dr. Roe reports receiving grant or research support from the Society of Family Planning.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Examining the impact of contraception on acne in adolescents is clinically important because acne affects about 85% of adolescents, and contraceptives may influence the course of acne disease. Estrogen-progestin contraceptives cause a significant improvement in acne.1,2 By contrast, the levonorgestrel-releasing intrauterine device and the etonogestrel contraceptive implant may exacerbate acne. In this editorial we review the hormonal contraception−acne relationship, available acne treatments, and appropriate management.
Related article:
Your teenage patient and contraception: Think “long-acting” first
Combination oral contraception and acne
As noted, combination oral contraceptives generally result in acne improvement.1,2 Estrogen-progestin contraceptives improve the condition through two mechanisms. Primarily, estrogen-progestin contraceptives suppress pituitary luteinizing hormone secretion, thereby decreasing ovarian testosterone production. These contraceptives also increase liver production of sex hormone-binding globulin (SHBG), thereby increasing bound testosterone and decreasing free testosterone. The decrease in ovarian testosterone production and the increase in SHBG-bound testosterone reduce sebum production, resulting in acne improvement.
The US Food and Drug Administration has approved 4 estrogen-progestin contraceptives for acne treatment:
- Estrostep (norethindrone acetate-ethinyl estradiol plus ferrous fumarate)
- Ortho Tri-Cyclen (norgestimate-ethinyl estradiol)
- Yaz (drospirenone-ethinyl estradiol)
- BeYaz (drospirenone-ethinyl estradiol plus levomefolate).
LARC and acne
The levonorgestrel intrauterine devices (LNG-IUDs), including the levonorgestrel intrauterine systems Mirena, Liletta, Skyla, and Kyleena, and the etonogestrel implant (Nexplanon) are among the most effective contraceptives available for women. Over the last decade there has been a marked increase in the use of LARC. In 2002, 1.3% of women aged 15 to 24 years used an IUD or progestin implant, and this percentage increased to 10% by 2013.3
Progestin-containing LARC may cause acne to worsen. In a large 3-year prospective study of more than 2,900 women using the progestin implant or the copper IUD (ParaGard), use of the progestin implant was associated with a higher rate of reported acne than the copper IUD (18% vs 13%, respectively; relative risk, 1.4; 95% confidence interval, 1.20−1.56; P<.0001).4 In a retrospective review of 991 women who used the etonogestrel implant, 24% of the women requested that the implant be removed; the 3 most common reasons for removal were: bleeding disturbances (45%), worsening acne, (12%) and desire to conceive (12%).5
Similar differences in reported acne are seen between the LNG-IUD and the copper IUD. In a study of 320 women using the LNG-IUD and the copper IUD, an increase in acne was reported by 17% and 7%, respectively (P<.025).6 In a small prospective study of the LNG-IUD versus the copper IUD over the first 12 months of use, use of the LNG-IUD was associated with a statistically significant worsening of acne scores while use of the copper IUD had no impact on acne scores.7
Related article:
Overcoming LARC complications: 7 case challenges
In a study of 2,147 consecutive women using a hormonal contraceptive who presented to a dermatologist for the treatment of acne, patients were asked to assess how the contraceptive affected their acne. By type of contraceptive, the percent of women who reported that the contraceptive made their acne worse was: LNG-IUD, 36%; progestin implant, 33%; depot medroxyprogesterone acetate (MPA), 27%; levonorgestrel-ethinyl estradiol oral contraceptive, 10%; norgestimate-ethinyl estradiol (EE), 6%; etonogestrel-EE vaginal ring, 4%; drospirenone-EE, 3%; and desogestrel-EE, 2%. The percent of women who reported that the contraceptive significantly improved their acne was: drospirenone-EE, 26%; norgestimate-EE, 17%; desogestrel-EE, 15%; etonogestrel-EE vaginal ring, 14%; norethindrone-EE, 8%; levonorgestrel-EE, 6%; depot MPA, 5%; LNG-IUD, 3%; and progestin implant, 1%.8
In adolescents with acne, switching from an estrogen-progestin contraceptive to a LNG-IUD or an etonogestrel implant may cause the patient to report that her acne has worsened. As mentioned, combination estrogen-progestin contraceptives reduce free testosterone, thereby improving acne. When an estrogen-progestin contraceptive is discontinued, free testosterone levels will increase. If a LARC method is initiated and the patient’s acne worsens, the patient may attribute this change to the LARC. For clinicians planning on switching a patient from an estrogen-progestin contraceptive to a LNG-IUD or etonogestrel implant, evaluation of current acne symptoms and acne history may be particularly important.
Acne treatment
Acne is caused by follicular hyperproliferation and abnormal desquamation, excess sebum production, proliferation of Propionibacterium acnes, and inflammation.
First-line agents. An expert guideline developed under the auspices of the American Academy of Dermatology recommends that topical agents including retinoids and antimicrobials be first-line treatments for acne.9,10
Topical retinoids are the primary component of topical acne treatment and can be used as monotherapy or in combination with topical antimicrobials (TABLE 1). Three topical retinoids are approved for use in the United States: tretinoin, adapalene, and tazarotene. Adapalene is available by prescription, 0.1% and 0.3% gel, and over the counter, 0.1% gel (Differin Gel) (TABLE 2). The topical retinoids are applied once daily at bedtime and can cause local skin irritation and dryness. Pregnant women should not be treated with topical retinoids.


Topical antimicrobials for the treatment of acne include: benzoyl peroxide, clindamycin, azelaic acid, and dapsone. Clindamycin is only recommended for use in combination with benzoyl peroxide in order to reduce the development of bacterial resistance to the antibiotic.
Related article:
Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception?
Approach to mild, moderate, and severe acne. In adolescents with mild acne a topical retinoid or benzoyl peroxide can be used as monotherapy or used together. Referral to a dermatologist is recommended for moderate to severe acne. Moderate acne is treated with combination topical therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). Severe acne is treated with 3 months of oral antibiotics plus topical combination therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). In cases of severe nodular acne or acne that produces scarring the patient may require oral isotretinoin treatment.
Acne management for adolescents seeking LARC
Given the data that the LNG-IUD and the etonogestrel implant may worsen acne, it may be wise to preemptively ensure that adolescents with acne who are initiating these contraceptives are also being adequately treated for their acne. Gynecologists should provide anticipatory guidance for adolescents with mild acne who initiate progestin-based LARC. Topical benzoyl peroxide is available over-the-counter and can be recommended to these patients. Follow-up in clinic a few months after initiation also may be helpful to assess side effects.
In moderate and severe cases, coordination with dermatology is recommended. For these patients, gynecologists could consider prescribing a topical retinoid or antibiotic medication in conjunction with a new progestin-based LARC method. Those with severe acne also may benefit from concurrent use of oral contraceptives. In adolescents who do not tolerate progestin-based LARC, the copper IUD is a highly effective alternative and can be paired with estrogen-progestin contraception for acne treatment.
Related article:
With no budge in more than 20 years, are US unintended pregnancy rates finally on the decline?
Acne is but one consideration for contraceptive choice
With the above methods, acne can be managed in adolescents seeking a LNG-IUD or implant and should not be considered a contraindication or reason to avoid progestin-based LARC. Adolescents are more likely to continue LARC than estrogen-progestin contraceptives and LARC methods are associated with substantially lower pregnancy rates in this patient population.11 LARC is recommended as first-line contraception for adolescents by both the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.12,13
In choosing contraception with your adolescent patient, the risk of unintended pregnancy should be weighed against the risk of acne and other potential side effects. Do not select a contraceptive based on the presence or absence of acne disease. However, be aware that contraceptives can either improve or worsen acne. Patients with mild and moderate acne disease should be considered for treatment with topical retinoids and/or antimicrobial agents.

Dr. Barbieri reports no financial relationships relevant to this article.
Dr. Roe reports receiving grant or research support from the Society of Family Planning.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;(7):CD004425.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71(3):450-459.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15 to 44: United States 2011-2013. Natl Health Stat Report. 2015;(86):1-14.
- Bahamondes L, Brache V, Meirik O, Ali M, Habib N, Landoulsi S; WHO Study Group on Contraceptive Implants for Women. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum Reprod. 2015;30(11):2527-2538.
- Bitzer J, Tschudin S, Adler J; Swiss Implanon Study Group. Acceptability and side-effects of Implanon in Switzerland: a retrospective study by the Implanon Swiss Study Group. Eur J Contracept Reprod Health Care. 2004;9(4):278-284.
- Nilsson CG, Luukkainen T, Diaz J, Allonen H. Clinical performance of a new levonorgestrel-releasing intrauterine device. A randomized comparison with a Nova-T-copper device. Contraception. 1982;25(4):345-356.
- Kelekci S, Kelecki KH, Yilmaz B. Effects of levonorgestrel-releasing intrauterine system and T380A intrauterine copper device on dysmenorrhea and days of bleeding in women with and without adenomyosis. Contraception. 2012;86(5):458-463.
- Lortscher D, Admani S, Satur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
- Roman CJ, Cifu AD, Stein SL. Management of acne vulgaris. JAMA. 2016;316(13):1402-1403.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998-2007.
- American Academy of Pediatrics Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014;134(4):e1244-e1256.
- American College of Obstetricians and Gynecologists Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group. Committee Opinion No. 539. Adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012;120(4):983-988.
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;(7):CD004425.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71(3):450-459.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15 to 44: United States 2011-2013. Natl Health Stat Report. 2015;(86):1-14.
- Bahamondes L, Brache V, Meirik O, Ali M, Habib N, Landoulsi S; WHO Study Group on Contraceptive Implants for Women. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum Reprod. 2015;30(11):2527-2538.
- Bitzer J, Tschudin S, Adler J; Swiss Implanon Study Group. Acceptability and side-effects of Implanon in Switzerland: a retrospective study by the Implanon Swiss Study Group. Eur J Contracept Reprod Health Care. 2004;9(4):278-284.
- Nilsson CG, Luukkainen T, Diaz J, Allonen H. Clinical performance of a new levonorgestrel-releasing intrauterine device. A randomized comparison with a Nova-T-copper device. Contraception. 1982;25(4):345-356.
- Kelekci S, Kelecki KH, Yilmaz B. Effects of levonorgestrel-releasing intrauterine system and T380A intrauterine copper device on dysmenorrhea and days of bleeding in women with and without adenomyosis. Contraception. 2012;86(5):458-463.
- Lortscher D, Admani S, Satur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
- Roman CJ, Cifu AD, Stein SL. Management of acne vulgaris. JAMA. 2016;316(13):1402-1403.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998-2007.
- American Academy of Pediatrics Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014;134(4):e1244-e1256.
- American College of Obstetricians and Gynecologists Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group. Committee Opinion No. 539. Adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012;120(4):983-988.
A rose by any other name is still a rose—but why a rose?
Christopher Columbus, age 41, returned to Europe from his first voyage to the New World suffering from what would turn out to be a chronic illness that included recurrent febrile bouts of severe lower-extremity arthritis. For many years he was thought to have had gout, but careful reanalysis led Allison1 and then Arnett et al2 to propose an alternative diagnosis. Based on the pattern of Columbus’s arthritis and the accompanying symptoms, which these clinicians linked to the arthritis, they proposed the diagnosis of reactive arthritis—actually a subset of reactive arthritis, which at the time of their publications was called Reiter’s syndrome. Arnett discussed this extensively at a historic clinicopathologic conference, noting that the bouts of arthritis were prolonged (too long for typical gout) and that the historically described recurrent episodes of red (“bleeding”) eyes with intermittent “blindness” and ultimate immobility due to spine pain (arthritis) would not be typical of gout. Arnett et al recognized the pattern of this arthritis—lower-extremity-predominant with prolonged episodes, associated inflammatory eye disease, spine involvement, and the protracted course of nearly a decade—as far more typical of a reactive arthritis than gout (or ongoing bacterial infection), occurring in a likely HLA-B27-positive individual. They proposed that the probable trigger for the arthritis was an episode of food-borne bacterial infection acquired during the extensive transatlantic journey.
Fast forward about 470 years to an epidemic aboard a US Navy ship of Shigella-associated dysentery that was accompanied by readily diagnosed reactive arthritis.3 Several diarrheal epidemics with associated reactive arthritis aboard cruise ships have been described, and the association is well documented following Shigella, Salmonella, Campylobacter, and nondiarrheal chlamydial infections.
In this issue of the Journal, Gómez-Moyano et al present images of a patient with dermatologic features of reactive arthritis. I have two points to make in introducing this historical background. First, I want to highlight some features of this syndrome that are distinctive enough as a clinical pattern to enable a diagnosis by appearance alone or, as in the case of Columbus, by historical description alone. But second and more important, I wonder, as perhaps you do, why similar inflammatory features occur in patients of seemingly diverse backgrounds, triggered by different organisms that may have infected different mucosal areas. Why does this rose look like a rose and not a gardenia?
Reactive arthritis is classically thought of as self-limited, lasting less than 6 months. But a significant minority of patients may have chronic disease, particularly with spondylitis, which may be more common in patients who have the HLA-B27 haplotype. The peripheral arthritis generally affects relatively few joints, with the larger lower-extremity joints a common target. Enthesitis, especially at the Achilles tendon heel insertion, is common, and dactylitis or “sausage digits” can occur. These features may also be seen in patients with psoriatic or enteropathic arthritis. Inflammation of the skin (as shown by Gómez-Moyano et al), the eye (conjunctivitis, uveitis, episcleritis), oral mucosa, and the genitourinary tract (urethritis, prostatitis) can also occur. Urethritis can occur in patients with enteric diarrhea as the trigger; thus, it is not just associated with genitourinary infections like chlamydia. What all these sites have in common to be targeted following an infection is not known with certainty; relevant bacterial antigens cannot always be detected in the involved tissues. It is intriguing that transgenic rats engineered to contain many copies of the human HLA-B27 gene develop arthritis along with mucosal, eye, and skin inflammation, which can be avoided if the animals are raised in a germ-free environment. But probably fewer than 50% of patients with reactive arthritis have the B27 gene, so other factors must contribute to this uncommon syndrome. B27 is thus not a generally useful diagnostic test.
When a patient presents with acute inflammatory asymmetric oligoarthritis (affecting < 4 joints), infectious arthritis and crystal-induced arthritis need to be excluded. But looking for and finding other features more typical of reactive arthritis may spare the patient a more extensive and expensive inpatient evaluation.
Recognizing a rose can provide an aesthetic moment, even if we can’t define exactly what makes it a rose. Pattern recognition matters in clinical practice, even without full understanding of the molecular backdrop.
- Allison DJ. Christopher Columbus: first case of Reiter’s disease in the Old World? Lancet 1980; 2:1309.
- Arnett FC, Merrill C, Albardaner F, Mackowiak PA. A mariner with crippling arthritis and bleeding eyes. Am J Med Sci 2006; 332:123–130.
- Noer HR. An “experimental” epidemic of Reiter’s syndrome. JAMA 1966; 198:693–698.
Christopher Columbus, age 41, returned to Europe from his first voyage to the New World suffering from what would turn out to be a chronic illness that included recurrent febrile bouts of severe lower-extremity arthritis. For many years he was thought to have had gout, but careful reanalysis led Allison1 and then Arnett et al2 to propose an alternative diagnosis. Based on the pattern of Columbus’s arthritis and the accompanying symptoms, which these clinicians linked to the arthritis, they proposed the diagnosis of reactive arthritis—actually a subset of reactive arthritis, which at the time of their publications was called Reiter’s syndrome. Arnett discussed this extensively at a historic clinicopathologic conference, noting that the bouts of arthritis were prolonged (too long for typical gout) and that the historically described recurrent episodes of red (“bleeding”) eyes with intermittent “blindness” and ultimate immobility due to spine pain (arthritis) would not be typical of gout. Arnett et al recognized the pattern of this arthritis—lower-extremity-predominant with prolonged episodes, associated inflammatory eye disease, spine involvement, and the protracted course of nearly a decade—as far more typical of a reactive arthritis than gout (or ongoing bacterial infection), occurring in a likely HLA-B27-positive individual. They proposed that the probable trigger for the arthritis was an episode of food-borne bacterial infection acquired during the extensive transatlantic journey.
Fast forward about 470 years to an epidemic aboard a US Navy ship of Shigella-associated dysentery that was accompanied by readily diagnosed reactive arthritis.3 Several diarrheal epidemics with associated reactive arthritis aboard cruise ships have been described, and the association is well documented following Shigella, Salmonella, Campylobacter, and nondiarrheal chlamydial infections.
In this issue of the Journal, Gómez-Moyano et al present images of a patient with dermatologic features of reactive arthritis. I have two points to make in introducing this historical background. First, I want to highlight some features of this syndrome that are distinctive enough as a clinical pattern to enable a diagnosis by appearance alone or, as in the case of Columbus, by historical description alone. But second and more important, I wonder, as perhaps you do, why similar inflammatory features occur in patients of seemingly diverse backgrounds, triggered by different organisms that may have infected different mucosal areas. Why does this rose look like a rose and not a gardenia?
Reactive arthritis is classically thought of as self-limited, lasting less than 6 months. But a significant minority of patients may have chronic disease, particularly with spondylitis, which may be more common in patients who have the HLA-B27 haplotype. The peripheral arthritis generally affects relatively few joints, with the larger lower-extremity joints a common target. Enthesitis, especially at the Achilles tendon heel insertion, is common, and dactylitis or “sausage digits” can occur. These features may also be seen in patients with psoriatic or enteropathic arthritis. Inflammation of the skin (as shown by Gómez-Moyano et al), the eye (conjunctivitis, uveitis, episcleritis), oral mucosa, and the genitourinary tract (urethritis, prostatitis) can also occur. Urethritis can occur in patients with enteric diarrhea as the trigger; thus, it is not just associated with genitourinary infections like chlamydia. What all these sites have in common to be targeted following an infection is not known with certainty; relevant bacterial antigens cannot always be detected in the involved tissues. It is intriguing that transgenic rats engineered to contain many copies of the human HLA-B27 gene develop arthritis along with mucosal, eye, and skin inflammation, which can be avoided if the animals are raised in a germ-free environment. But probably fewer than 50% of patients with reactive arthritis have the B27 gene, so other factors must contribute to this uncommon syndrome. B27 is thus not a generally useful diagnostic test.
When a patient presents with acute inflammatory asymmetric oligoarthritis (affecting < 4 joints), infectious arthritis and crystal-induced arthritis need to be excluded. But looking for and finding other features more typical of reactive arthritis may spare the patient a more extensive and expensive inpatient evaluation.
Recognizing a rose can provide an aesthetic moment, even if we can’t define exactly what makes it a rose. Pattern recognition matters in clinical practice, even without full understanding of the molecular backdrop.
Christopher Columbus, age 41, returned to Europe from his first voyage to the New World suffering from what would turn out to be a chronic illness that included recurrent febrile bouts of severe lower-extremity arthritis. For many years he was thought to have had gout, but careful reanalysis led Allison1 and then Arnett et al2 to propose an alternative diagnosis. Based on the pattern of Columbus’s arthritis and the accompanying symptoms, which these clinicians linked to the arthritis, they proposed the diagnosis of reactive arthritis—actually a subset of reactive arthritis, which at the time of their publications was called Reiter’s syndrome. Arnett discussed this extensively at a historic clinicopathologic conference, noting that the bouts of arthritis were prolonged (too long for typical gout) and that the historically described recurrent episodes of red (“bleeding”) eyes with intermittent “blindness” and ultimate immobility due to spine pain (arthritis) would not be typical of gout. Arnett et al recognized the pattern of this arthritis—lower-extremity-predominant with prolonged episodes, associated inflammatory eye disease, spine involvement, and the protracted course of nearly a decade—as far more typical of a reactive arthritis than gout (or ongoing bacterial infection), occurring in a likely HLA-B27-positive individual. They proposed that the probable trigger for the arthritis was an episode of food-borne bacterial infection acquired during the extensive transatlantic journey.
Fast forward about 470 years to an epidemic aboard a US Navy ship of Shigella-associated dysentery that was accompanied by readily diagnosed reactive arthritis.3 Several diarrheal epidemics with associated reactive arthritis aboard cruise ships have been described, and the association is well documented following Shigella, Salmonella, Campylobacter, and nondiarrheal chlamydial infections.
In this issue of the Journal, Gómez-Moyano et al present images of a patient with dermatologic features of reactive arthritis. I have two points to make in introducing this historical background. First, I want to highlight some features of this syndrome that are distinctive enough as a clinical pattern to enable a diagnosis by appearance alone or, as in the case of Columbus, by historical description alone. But second and more important, I wonder, as perhaps you do, why similar inflammatory features occur in patients of seemingly diverse backgrounds, triggered by different organisms that may have infected different mucosal areas. Why does this rose look like a rose and not a gardenia?
Reactive arthritis is classically thought of as self-limited, lasting less than 6 months. But a significant minority of patients may have chronic disease, particularly with spondylitis, which may be more common in patients who have the HLA-B27 haplotype. The peripheral arthritis generally affects relatively few joints, with the larger lower-extremity joints a common target. Enthesitis, especially at the Achilles tendon heel insertion, is common, and dactylitis or “sausage digits” can occur. These features may also be seen in patients with psoriatic or enteropathic arthritis. Inflammation of the skin (as shown by Gómez-Moyano et al), the eye (conjunctivitis, uveitis, episcleritis), oral mucosa, and the genitourinary tract (urethritis, prostatitis) can also occur. Urethritis can occur in patients with enteric diarrhea as the trigger; thus, it is not just associated with genitourinary infections like chlamydia. What all these sites have in common to be targeted following an infection is not known with certainty; relevant bacterial antigens cannot always be detected in the involved tissues. It is intriguing that transgenic rats engineered to contain many copies of the human HLA-B27 gene develop arthritis along with mucosal, eye, and skin inflammation, which can be avoided if the animals are raised in a germ-free environment. But probably fewer than 50% of patients with reactive arthritis have the B27 gene, so other factors must contribute to this uncommon syndrome. B27 is thus not a generally useful diagnostic test.
When a patient presents with acute inflammatory asymmetric oligoarthritis (affecting < 4 joints), infectious arthritis and crystal-induced arthritis need to be excluded. But looking for and finding other features more typical of reactive arthritis may spare the patient a more extensive and expensive inpatient evaluation.
Recognizing a rose can provide an aesthetic moment, even if we can’t define exactly what makes it a rose. Pattern recognition matters in clinical practice, even without full understanding of the molecular backdrop.
- Allison DJ. Christopher Columbus: first case of Reiter’s disease in the Old World? Lancet 1980; 2:1309.
- Arnett FC, Merrill C, Albardaner F, Mackowiak PA. A mariner with crippling arthritis and bleeding eyes. Am J Med Sci 2006; 332:123–130.
- Noer HR. An “experimental” epidemic of Reiter’s syndrome. JAMA 1966; 198:693–698.
- Allison DJ. Christopher Columbus: first case of Reiter’s disease in the Old World? Lancet 1980; 2:1309.
- Arnett FC, Merrill C, Albardaner F, Mackowiak PA. A mariner with crippling arthritis and bleeding eyes. Am J Med Sci 2006; 332:123–130.
- Noer HR. An “experimental” epidemic of Reiter’s syndrome. JAMA 1966; 198:693–698.
Maddening therapies: How hallucinogens morphed into novel treatments
Snake venom is deadly but is being used to treat some cancers,1 because it produces contortrostatin, a protein that “paralyzes” cancer cells and prevents them from migrating. Venoms from spiders are being investigated as a treatment to slow the progression of muscular dystrophy by preventing muscle cells from deteriorating. Venom from tarantulas can relieve chronic pain, and those from centipedes help rodents tolerate thermal, chemical, or acid pain. Scorpion venom can cause cancer cells to glow under a flashlight, enabling surgeons to locate and remove them. Anemones toxin could be used to treat autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, and lupus.
Vaccines are an excellent example of how deadly pathogens can be transformed into life-saving therapies. Billions of people have been protected from polio, smallpox, tetanus, diphtheria, measles, mumps, rubella, influenza, pneumococcus, hepatitis A and B, rabies, shingles, typhoid, meningitis, or cholera. Turning killers into saviors is one of the most remarkable miracles of medical research.2
The mind-boggling transformation of mind-altering drugs
In psychiatry, psychedelic drugs have been repurposed into useful therapies for mental illness. As recently as a decade ago, psychiatric practitioners—physicians and nurse practitioners—regarded hallucinogens as dangerous, “must-avoid” drugs of abuse that could trigger or exacerbate serious psychiatric disorders. Then, thanks to ongoing research, the psychedelic “caterpillars” transformed into therapeutic “butterflies,” and the despised drugs of abuse became welcome adjuncts for treating some stubborn psychopathologies. Such paradoxical developments are emblematic of how one can always find a silver lining.
Consider the following transformations of various psychedelics and hallucinogens—also called “entheogens”—into novel pharmacotherapies. Note that in most cases, the application of these mind-altering drugs into useful medications is still a work in progress.
LSD
Lysergic acid diethylamide (LSD) was used extensively for treating mood disorders in the pre-antidepressant era, before it was prohibited in the late 1960s. A review of 19 studies—many uncontrolled—concluded that approximately 80% of patients improved, according to the treating physicians.3 However, research on LSD was halted for several decades after it became illegal, and resumed in 2010. Neuropsychiatrists and neuroscience researchers are now employing advanced techniques, such as neuroimaging, molecular pharmacology, and connectomics, to study its therapeutic effects.4 LSD is not only being used for treatment-resistant depression but also anxiety, alcoholism, autism, and even schizophrenia. However, despite its potential uses for treating alcoholism and anxiety, enhancing creativity, or caring for terminally ill patients, using LSD requires expertise, caution, and adherence to ethical standards.5
In healthy individuals, the effects of LSD include visual hallucinations, audiovisual synesthesia, depersonalization and derealization, and a sense of well-being, happiness, closeness to others, and trust.
Biologic effects include increased heart rate and blood pressure, elevated temperature, dilated pupils, and increased serum cortisol, prolactin, oxytocin, and epinephrine. All effects subside within 3 days.6
Psilocybin
Psilocybin, a component of some mushrooms that is known for its use during rituals in some cultures, has been discovered to have antidepressant, anxiolytic, and anti-addictive effects.7 Recent controlled studies at Johns Hopkins University reported that a single dose of psilocybin can relieve anxiety or depression for up to 6 months, which, if replicated, could lead to a remarkable paradigm shift in treating mood and anxiety disorders, especially if patients do not respond to standard antidepressants.3 Other emerging uses of both psilocybin and LSD are in treating addictions8 where psychiatry is desperately looking for innovative new therapies.
Ecstasy
MDMA (3,4-methylenedioxymethamphetamine), also known as ecstasy, is widely regarded as a harmful party drug that produces euphoria, but not hallucinations. However, it has emerged as a useful treatment for posttraumatic stress disorder (PTSD). In one study of female sexual abuse victims, 80% of the patients who received MDMA with psychotherapy no longer met diagnostic criteria for PTSD after 2 months.9 Other studies showed no effects. Despite persistent skepticisms by many, the Multidisciplinary Association for Psychedelics Studies organization is investing millions of dollars into studying MDMA for PTSD in several countries.9,10 One hurdle is that it is difficult to conduct truly blind studies with psychedelic drugs because of their profound effects. MDMA releases cortisol, oxytocin—which are known to facilitate psychotherapy—and testosterone, but the debate about the risk–benefit ratio will continue.11 MDMA also is being studied for treating social anxiety in adults with autism.12
Ketamine
Ketamine is a weaker cousin of the potent psychotogenic phencyclidine (approximately one-fiftieth the potency) and is a well-known drug of abuse that causes dissociation and hallucinations. It is used as an anesthetic in veterinary medicine and in children undergoing surgical procedures. Until recently, its only use in psychiatry has been as an anesthetic during electroconvulsive therapy. However, over the past few years, IV ketamine has been in the spotlight as a breakthrough, rapid-onset antidepressant and anti-suicidal agent in several controlled studies.13 This drug is revolutionizing the management of treatment-resistant depression and suicidal ideation and generating new insights into the neurobiology of depression.
Cannabis
Last, but certainly not least, is marijuana, which is more widely used than all the other psychedelics combined, and is currently at the center of a national debate about its legalization. Although the director of the National Institute on Drug Abuse highlighted the many risk of marijuana,14 studies have pointed to the myriad medical uses of Cannabis.15,16 An editorial in Nature Medicine recently urged that regulators reconsider the tight constraints on marijuana research.17 Some of the medical applications of marijuana include:
- psychiatry (anxiety, PTSD)
- neurology (severe epilepsy, tremors in Parkinson’s disease, traumatic brain injury, pain of multiple sclerosis, muscle spasms, and progression of Alzheimer’s disease)
- oncology (nausea and pain of chemotherapy, reduction of metastasis)
- ophthalmology (decrease of intraocular pressure in glaucoma)
- autoimmune disorders (rheumatoid arthritis, Crohn’s disease, lupus).
However, as a schizophrenia researcher, I am wary about marijuana’s high risk of triggering psychosis in young adults with a family history of schizophrenia spectrum disorders.18
The above are examples of how psychiatry is finally recognizing the therapeutic value inherent in traditionally “evil” street drugs that we euphemistically refer to as “recreational drugs.” Even methamphetamine, the universally condemned and clearly harmful drug, was recently reported to be neuroprotective at low dosages!19 Could our field have suffered from a blind eye to the benefits of these hallucinogens and ignored the possibility that some persons with addiction who use these “recreational drugs” may have been self-medicating to alleviate their un-diagnosed psychiatric disorder? We need to reconceptualize the pejorative term “mind-altering drug” because of its implicitly negative connotation. After all, alteration may indicate a favorable, not just a deleterious, outcome.
1. Vyas VK, Brahmbhatt K, Bhatt H, et al. Therapeutic potential of snake venom in cancer therapy: current perspectives. Asian Pac J Trop Biomed. 2013;3(2):156-162.
2. Loehr J. The vaccine answer book: 200 essential answers to help you make the right decisions for your child. Naperville, IL: Sourcebooks Inc; 2009.
3. Rucker JJ, Jelen LA, Flynn S, et al. Psychedelics in the treatment of unipolar mood disorders: a systematic review. J Psychopharmacol. 2016;30(12):1220-1229.
4. Mucke HA. From psychiatry to flower power and back again: the amazing story of lysergic acid diethylamide [published online July 8, 2016]. Assay Drug Dev Technol. doi: 10.1089/adt.2016.747.
5. Das S, Barnwal P, Ramasamy A, et al. Lysergic acid diethylamide: a drug of ‘use’? Ther Advances Pychopharmacol. 2016;6(3):214-228.
6. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
7. Dos Santos RG, Osório FL, Crippa JA, et al. Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years. Ther Adv Psychopharmacol. 2016;6(3):193-213.
8. Bogenschutz MP. Studying the effects of classic hallucinogens in the treatment of alcoholism: rationale, methodology, and current research with psilocybin. Curr Drug Abuse Rev. 2013;6(1):17-29.
9. Kupferschmidt K. Can ecstasy treat the agony of PTSD? Science. 2014;345:22-23.
10. Sessa B. MDMA and PTSD treatment: PTSD: from novel pathophysiology to innovative therapeutics [published online July 6, 2016]. Neurosci Lett. doi: 10.1016/j.neulet.2016.07.004.
11. Parrott AC. The potential dangers of using MDMA for psychotherapy. J Psychoactive Drugs. 2014;46(1):37-43.
12. Danforth AL, Struble CM, Yazar-Klosinski B, et al. MDMA-assisted therapy: a new treatment model for social anxiety in autistic adults. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:237-249.
13. Feifel D. Breaking sad: unleashing the breakthrough potential of ketamine’s rapid antidepressant effects [published online November 26, 2016]. Drug Dev Res. doi: 10.1002/ddr.21347.
14. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227.
15. Murnion B. Medicinal cannabis. Aust Prescr. 2015;38(6):212-215.
16. Borgelt LM, Franson KL, Nussbaum AM, et al. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33(2):195-209.
17. Release the strains. Nat Med. 2015;21(9):963.
18. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319-328.
19. Rau T, Ziemniak J, Poulsen D, et al. The neuroprotective potential of low-dose methamphetamine in preclinical models of stroke and traumatic brain injury. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:231-236.
Snake venom is deadly but is being used to treat some cancers,1 because it produces contortrostatin, a protein that “paralyzes” cancer cells and prevents them from migrating. Venoms from spiders are being investigated as a treatment to slow the progression of muscular dystrophy by preventing muscle cells from deteriorating. Venom from tarantulas can relieve chronic pain, and those from centipedes help rodents tolerate thermal, chemical, or acid pain. Scorpion venom can cause cancer cells to glow under a flashlight, enabling surgeons to locate and remove them. Anemones toxin could be used to treat autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, and lupus.
Vaccines are an excellent example of how deadly pathogens can be transformed into life-saving therapies. Billions of people have been protected from polio, smallpox, tetanus, diphtheria, measles, mumps, rubella, influenza, pneumococcus, hepatitis A and B, rabies, shingles, typhoid, meningitis, or cholera. Turning killers into saviors is one of the most remarkable miracles of medical research.2
The mind-boggling transformation of mind-altering drugs
In psychiatry, psychedelic drugs have been repurposed into useful therapies for mental illness. As recently as a decade ago, psychiatric practitioners—physicians and nurse practitioners—regarded hallucinogens as dangerous, “must-avoid” drugs of abuse that could trigger or exacerbate serious psychiatric disorders. Then, thanks to ongoing research, the psychedelic “caterpillars” transformed into therapeutic “butterflies,” and the despised drugs of abuse became welcome adjuncts for treating some stubborn psychopathologies. Such paradoxical developments are emblematic of how one can always find a silver lining.
Consider the following transformations of various psychedelics and hallucinogens—also called “entheogens”—into novel pharmacotherapies. Note that in most cases, the application of these mind-altering drugs into useful medications is still a work in progress.
LSD
Lysergic acid diethylamide (LSD) was used extensively for treating mood disorders in the pre-antidepressant era, before it was prohibited in the late 1960s. A review of 19 studies—many uncontrolled—concluded that approximately 80% of patients improved, according to the treating physicians.3 However, research on LSD was halted for several decades after it became illegal, and resumed in 2010. Neuropsychiatrists and neuroscience researchers are now employing advanced techniques, such as neuroimaging, molecular pharmacology, and connectomics, to study its therapeutic effects.4 LSD is not only being used for treatment-resistant depression but also anxiety, alcoholism, autism, and even schizophrenia. However, despite its potential uses for treating alcoholism and anxiety, enhancing creativity, or caring for terminally ill patients, using LSD requires expertise, caution, and adherence to ethical standards.5
In healthy individuals, the effects of LSD include visual hallucinations, audiovisual synesthesia, depersonalization and derealization, and a sense of well-being, happiness, closeness to others, and trust.
Biologic effects include increased heart rate and blood pressure, elevated temperature, dilated pupils, and increased serum cortisol, prolactin, oxytocin, and epinephrine. All effects subside within 3 days.6
Psilocybin
Psilocybin, a component of some mushrooms that is known for its use during rituals in some cultures, has been discovered to have antidepressant, anxiolytic, and anti-addictive effects.7 Recent controlled studies at Johns Hopkins University reported that a single dose of psilocybin can relieve anxiety or depression for up to 6 months, which, if replicated, could lead to a remarkable paradigm shift in treating mood and anxiety disorders, especially if patients do not respond to standard antidepressants.3 Other emerging uses of both psilocybin and LSD are in treating addictions8 where psychiatry is desperately looking for innovative new therapies.
Ecstasy
MDMA (3,4-methylenedioxymethamphetamine), also known as ecstasy, is widely regarded as a harmful party drug that produces euphoria, but not hallucinations. However, it has emerged as a useful treatment for posttraumatic stress disorder (PTSD). In one study of female sexual abuse victims, 80% of the patients who received MDMA with psychotherapy no longer met diagnostic criteria for PTSD after 2 months.9 Other studies showed no effects. Despite persistent skepticisms by many, the Multidisciplinary Association for Psychedelics Studies organization is investing millions of dollars into studying MDMA for PTSD in several countries.9,10 One hurdle is that it is difficult to conduct truly blind studies with psychedelic drugs because of their profound effects. MDMA releases cortisol, oxytocin—which are known to facilitate psychotherapy—and testosterone, but the debate about the risk–benefit ratio will continue.11 MDMA also is being studied for treating social anxiety in adults with autism.12
Ketamine
Ketamine is a weaker cousin of the potent psychotogenic phencyclidine (approximately one-fiftieth the potency) and is a well-known drug of abuse that causes dissociation and hallucinations. It is used as an anesthetic in veterinary medicine and in children undergoing surgical procedures. Until recently, its only use in psychiatry has been as an anesthetic during electroconvulsive therapy. However, over the past few years, IV ketamine has been in the spotlight as a breakthrough, rapid-onset antidepressant and anti-suicidal agent in several controlled studies.13 This drug is revolutionizing the management of treatment-resistant depression and suicidal ideation and generating new insights into the neurobiology of depression.
Cannabis
Last, but certainly not least, is marijuana, which is more widely used than all the other psychedelics combined, and is currently at the center of a national debate about its legalization. Although the director of the National Institute on Drug Abuse highlighted the many risk of marijuana,14 studies have pointed to the myriad medical uses of Cannabis.15,16 An editorial in Nature Medicine recently urged that regulators reconsider the tight constraints on marijuana research.17 Some of the medical applications of marijuana include:
- psychiatry (anxiety, PTSD)
- neurology (severe epilepsy, tremors in Parkinson’s disease, traumatic brain injury, pain of multiple sclerosis, muscle spasms, and progression of Alzheimer’s disease)
- oncology (nausea and pain of chemotherapy, reduction of metastasis)
- ophthalmology (decrease of intraocular pressure in glaucoma)
- autoimmune disorders (rheumatoid arthritis, Crohn’s disease, lupus).
However, as a schizophrenia researcher, I am wary about marijuana’s high risk of triggering psychosis in young adults with a family history of schizophrenia spectrum disorders.18
The above are examples of how psychiatry is finally recognizing the therapeutic value inherent in traditionally “evil” street drugs that we euphemistically refer to as “recreational drugs.” Even methamphetamine, the universally condemned and clearly harmful drug, was recently reported to be neuroprotective at low dosages!19 Could our field have suffered from a blind eye to the benefits of these hallucinogens and ignored the possibility that some persons with addiction who use these “recreational drugs” may have been self-medicating to alleviate their un-diagnosed psychiatric disorder? We need to reconceptualize the pejorative term “mind-altering drug” because of its implicitly negative connotation. After all, alteration may indicate a favorable, not just a deleterious, outcome.
Snake venom is deadly but is being used to treat some cancers,1 because it produces contortrostatin, a protein that “paralyzes” cancer cells and prevents them from migrating. Venoms from spiders are being investigated as a treatment to slow the progression of muscular dystrophy by preventing muscle cells from deteriorating. Venom from tarantulas can relieve chronic pain, and those from centipedes help rodents tolerate thermal, chemical, or acid pain. Scorpion venom can cause cancer cells to glow under a flashlight, enabling surgeons to locate and remove them. Anemones toxin could be used to treat autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, and lupus.
Vaccines are an excellent example of how deadly pathogens can be transformed into life-saving therapies. Billions of people have been protected from polio, smallpox, tetanus, diphtheria, measles, mumps, rubella, influenza, pneumococcus, hepatitis A and B, rabies, shingles, typhoid, meningitis, or cholera. Turning killers into saviors is one of the most remarkable miracles of medical research.2
The mind-boggling transformation of mind-altering drugs
In psychiatry, psychedelic drugs have been repurposed into useful therapies for mental illness. As recently as a decade ago, psychiatric practitioners—physicians and nurse practitioners—regarded hallucinogens as dangerous, “must-avoid” drugs of abuse that could trigger or exacerbate serious psychiatric disorders. Then, thanks to ongoing research, the psychedelic “caterpillars” transformed into therapeutic “butterflies,” and the despised drugs of abuse became welcome adjuncts for treating some stubborn psychopathologies. Such paradoxical developments are emblematic of how one can always find a silver lining.
Consider the following transformations of various psychedelics and hallucinogens—also called “entheogens”—into novel pharmacotherapies. Note that in most cases, the application of these mind-altering drugs into useful medications is still a work in progress.
LSD
Lysergic acid diethylamide (LSD) was used extensively for treating mood disorders in the pre-antidepressant era, before it was prohibited in the late 1960s. A review of 19 studies—many uncontrolled—concluded that approximately 80% of patients improved, according to the treating physicians.3 However, research on LSD was halted for several decades after it became illegal, and resumed in 2010. Neuropsychiatrists and neuroscience researchers are now employing advanced techniques, such as neuroimaging, molecular pharmacology, and connectomics, to study its therapeutic effects.4 LSD is not only being used for treatment-resistant depression but also anxiety, alcoholism, autism, and even schizophrenia. However, despite its potential uses for treating alcoholism and anxiety, enhancing creativity, or caring for terminally ill patients, using LSD requires expertise, caution, and adherence to ethical standards.5
In healthy individuals, the effects of LSD include visual hallucinations, audiovisual synesthesia, depersonalization and derealization, and a sense of well-being, happiness, closeness to others, and trust.
Biologic effects include increased heart rate and blood pressure, elevated temperature, dilated pupils, and increased serum cortisol, prolactin, oxytocin, and epinephrine. All effects subside within 3 days.6
Psilocybin
Psilocybin, a component of some mushrooms that is known for its use during rituals in some cultures, has been discovered to have antidepressant, anxiolytic, and anti-addictive effects.7 Recent controlled studies at Johns Hopkins University reported that a single dose of psilocybin can relieve anxiety or depression for up to 6 months, which, if replicated, could lead to a remarkable paradigm shift in treating mood and anxiety disorders, especially if patients do not respond to standard antidepressants.3 Other emerging uses of both psilocybin and LSD are in treating addictions8 where psychiatry is desperately looking for innovative new therapies.
Ecstasy
MDMA (3,4-methylenedioxymethamphetamine), also known as ecstasy, is widely regarded as a harmful party drug that produces euphoria, but not hallucinations. However, it has emerged as a useful treatment for posttraumatic stress disorder (PTSD). In one study of female sexual abuse victims, 80% of the patients who received MDMA with psychotherapy no longer met diagnostic criteria for PTSD after 2 months.9 Other studies showed no effects. Despite persistent skepticisms by many, the Multidisciplinary Association for Psychedelics Studies organization is investing millions of dollars into studying MDMA for PTSD in several countries.9,10 One hurdle is that it is difficult to conduct truly blind studies with psychedelic drugs because of their profound effects. MDMA releases cortisol, oxytocin—which are known to facilitate psychotherapy—and testosterone, but the debate about the risk–benefit ratio will continue.11 MDMA also is being studied for treating social anxiety in adults with autism.12
Ketamine
Ketamine is a weaker cousin of the potent psychotogenic phencyclidine (approximately one-fiftieth the potency) and is a well-known drug of abuse that causes dissociation and hallucinations. It is used as an anesthetic in veterinary medicine and in children undergoing surgical procedures. Until recently, its only use in psychiatry has been as an anesthetic during electroconvulsive therapy. However, over the past few years, IV ketamine has been in the spotlight as a breakthrough, rapid-onset antidepressant and anti-suicidal agent in several controlled studies.13 This drug is revolutionizing the management of treatment-resistant depression and suicidal ideation and generating new insights into the neurobiology of depression.
Cannabis
Last, but certainly not least, is marijuana, which is more widely used than all the other psychedelics combined, and is currently at the center of a national debate about its legalization. Although the director of the National Institute on Drug Abuse highlighted the many risk of marijuana,14 studies have pointed to the myriad medical uses of Cannabis.15,16 An editorial in Nature Medicine recently urged that regulators reconsider the tight constraints on marijuana research.17 Some of the medical applications of marijuana include:
- psychiatry (anxiety, PTSD)
- neurology (severe epilepsy, tremors in Parkinson’s disease, traumatic brain injury, pain of multiple sclerosis, muscle spasms, and progression of Alzheimer’s disease)
- oncology (nausea and pain of chemotherapy, reduction of metastasis)
- ophthalmology (decrease of intraocular pressure in glaucoma)
- autoimmune disorders (rheumatoid arthritis, Crohn’s disease, lupus).
However, as a schizophrenia researcher, I am wary about marijuana’s high risk of triggering psychosis in young adults with a family history of schizophrenia spectrum disorders.18
The above are examples of how psychiatry is finally recognizing the therapeutic value inherent in traditionally “evil” street drugs that we euphemistically refer to as “recreational drugs.” Even methamphetamine, the universally condemned and clearly harmful drug, was recently reported to be neuroprotective at low dosages!19 Could our field have suffered from a blind eye to the benefits of these hallucinogens and ignored the possibility that some persons with addiction who use these “recreational drugs” may have been self-medicating to alleviate their un-diagnosed psychiatric disorder? We need to reconceptualize the pejorative term “mind-altering drug” because of its implicitly negative connotation. After all, alteration may indicate a favorable, not just a deleterious, outcome.
1. Vyas VK, Brahmbhatt K, Bhatt H, et al. Therapeutic potential of snake venom in cancer therapy: current perspectives. Asian Pac J Trop Biomed. 2013;3(2):156-162.
2. Loehr J. The vaccine answer book: 200 essential answers to help you make the right decisions for your child. Naperville, IL: Sourcebooks Inc; 2009.
3. Rucker JJ, Jelen LA, Flynn S, et al. Psychedelics in the treatment of unipolar mood disorders: a systematic review. J Psychopharmacol. 2016;30(12):1220-1229.
4. Mucke HA. From psychiatry to flower power and back again: the amazing story of lysergic acid diethylamide [published online July 8, 2016]. Assay Drug Dev Technol. doi: 10.1089/adt.2016.747.
5. Das S, Barnwal P, Ramasamy A, et al. Lysergic acid diethylamide: a drug of ‘use’? Ther Advances Pychopharmacol. 2016;6(3):214-228.
6. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
7. Dos Santos RG, Osório FL, Crippa JA, et al. Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years. Ther Adv Psychopharmacol. 2016;6(3):193-213.
8. Bogenschutz MP. Studying the effects of classic hallucinogens in the treatment of alcoholism: rationale, methodology, and current research with psilocybin. Curr Drug Abuse Rev. 2013;6(1):17-29.
9. Kupferschmidt K. Can ecstasy treat the agony of PTSD? Science. 2014;345:22-23.
10. Sessa B. MDMA and PTSD treatment: PTSD: from novel pathophysiology to innovative therapeutics [published online July 6, 2016]. Neurosci Lett. doi: 10.1016/j.neulet.2016.07.004.
11. Parrott AC. The potential dangers of using MDMA for psychotherapy. J Psychoactive Drugs. 2014;46(1):37-43.
12. Danforth AL, Struble CM, Yazar-Klosinski B, et al. MDMA-assisted therapy: a new treatment model for social anxiety in autistic adults. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:237-249.
13. Feifel D. Breaking sad: unleashing the breakthrough potential of ketamine’s rapid antidepressant effects [published online November 26, 2016]. Drug Dev Res. doi: 10.1002/ddr.21347.
14. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227.
15. Murnion B. Medicinal cannabis. Aust Prescr. 2015;38(6):212-215.
16. Borgelt LM, Franson KL, Nussbaum AM, et al. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33(2):195-209.
17. Release the strains. Nat Med. 2015;21(9):963.
18. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319-328.
19. Rau T, Ziemniak J, Poulsen D, et al. The neuroprotective potential of low-dose methamphetamine in preclinical models of stroke and traumatic brain injury. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:231-236.
1. Vyas VK, Brahmbhatt K, Bhatt H, et al. Therapeutic potential of snake venom in cancer therapy: current perspectives. Asian Pac J Trop Biomed. 2013;3(2):156-162.
2. Loehr J. The vaccine answer book: 200 essential answers to help you make the right decisions for your child. Naperville, IL: Sourcebooks Inc; 2009.
3. Rucker JJ, Jelen LA, Flynn S, et al. Psychedelics in the treatment of unipolar mood disorders: a systematic review. J Psychopharmacol. 2016;30(12):1220-1229.
4. Mucke HA. From psychiatry to flower power and back again: the amazing story of lysergic acid diethylamide [published online July 8, 2016]. Assay Drug Dev Technol. doi: 10.1089/adt.2016.747.
5. Das S, Barnwal P, Ramasamy A, et al. Lysergic acid diethylamide: a drug of ‘use’? Ther Advances Pychopharmacol. 2016;6(3):214-228.
6. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
7. Dos Santos RG, Osório FL, Crippa JA, et al. Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years. Ther Adv Psychopharmacol. 2016;6(3):193-213.
8. Bogenschutz MP. Studying the effects of classic hallucinogens in the treatment of alcoholism: rationale, methodology, and current research with psilocybin. Curr Drug Abuse Rev. 2013;6(1):17-29.
9. Kupferschmidt K. Can ecstasy treat the agony of PTSD? Science. 2014;345:22-23.
10. Sessa B. MDMA and PTSD treatment: PTSD: from novel pathophysiology to innovative therapeutics [published online July 6, 2016]. Neurosci Lett. doi: 10.1016/j.neulet.2016.07.004.
11. Parrott AC. The potential dangers of using MDMA for psychotherapy. J Psychoactive Drugs. 2014;46(1):37-43.
12. Danforth AL, Struble CM, Yazar-Klosinski B, et al. MDMA-assisted therapy: a new treatment model for social anxiety in autistic adults. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:237-249.
13. Feifel D. Breaking sad: unleashing the breakthrough potential of ketamine’s rapid antidepressant effects [published online November 26, 2016]. Drug Dev Res. doi: 10.1002/ddr.21347.
14. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227.
15. Murnion B. Medicinal cannabis. Aust Prescr. 2015;38(6):212-215.
16. Borgelt LM, Franson KL, Nussbaum AM, et al. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33(2):195-209.
17. Release the strains. Nat Med. 2015;21(9):963.
18. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319-328.
19. Rau T, Ziemniak J, Poulsen D, et al. The neuroprotective potential of low-dose methamphetamine in preclinical models of stroke and traumatic brain injury. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:231-236.
Clinical, practice, and policy trends: a round-up and review of the 2016 oncology landscape
We end this year with yet another encouraging list from the US Food and Drug Administration (FDA) of new drugs or expanded uses for some previously approved drugs for patients with life-threatening cancers. As clinicians focused on delivering quality, cost-effective care to our patients, that is exciting, but the overarching issues of dosing specificity, increasingly specific gene mutation testing, and complex therapy sequencing requirements explain another major trend of 2016: the increasing adoption of standardized pathways. In addition, given the continued explosion in drug pricing and the expanding use of high-cost drugs in more common diseases and in more lines of therapy, payers and providers are working to incorporate expanded decision support tools such as pathways to guide and optimally monitor therapies for patients.
Click on the PDF icon below to read the full article.
We end this year with yet another encouraging list from the US Food and Drug Administration (FDA) of new drugs or expanded uses for some previously approved drugs for patients with life-threatening cancers. As clinicians focused on delivering quality, cost-effective care to our patients, that is exciting, but the overarching issues of dosing specificity, increasingly specific gene mutation testing, and complex therapy sequencing requirements explain another major trend of 2016: the increasing adoption of standardized pathways. In addition, given the continued explosion in drug pricing and the expanding use of high-cost drugs in more common diseases and in more lines of therapy, payers and providers are working to incorporate expanded decision support tools such as pathways to guide and optimally monitor therapies for patients.
Click on the PDF icon below to read the full article.
We end this year with yet another encouraging list from the US Food and Drug Administration (FDA) of new drugs or expanded uses for some previously approved drugs for patients with life-threatening cancers. As clinicians focused on delivering quality, cost-effective care to our patients, that is exciting, but the overarching issues of dosing specificity, increasingly specific gene mutation testing, and complex therapy sequencing requirements explain another major trend of 2016: the increasing adoption of standardized pathways. In addition, given the continued explosion in drug pricing and the expanding use of high-cost drugs in more common diseases and in more lines of therapy, payers and providers are working to incorporate expanded decision support tools such as pathways to guide and optimally monitor therapies for patients.
Click on the PDF icon below to read the full article.
Professional time
As I write this article, the snow is piling up outside. While Cleveland’s west side citizens are raking up the last of fallen leaves, its east siders will dig out of 2 feet of snow. The lake effect is affecting us. The snow plow trucks vainly clear a path only for it to disappear in minutes. There seems to be no end to the torrents of white flakes that are each unique and tiny, but in aggregate uniform and overwhelming.
A blizzard of patients awaits my return from the annual meeting of the American Society of Hematology in San Diego. Like snowflakes, they are each unique, but in aggregate can be overwhelming. Plowing through a clinic, we go from patient to patient knowing that we will eventually see them all, then return to our offices or home to finish the labor of charting.
For some physicians, this is a daily reality. Whether patients in the clinic, or cases in the queue, some hematologists revisit the storm every day. Most, however, are engaged in an academic practice where at least some respite from direct patient care is offered. Whether teaching medical students, analyzing data, participating in administrative meetings, or writing manuscripts, most of us do something more beyond the clinic. We do this during our “protected time.”
But what are we protected from? Patients and their concerns? Really, this is what we want to be protected from?
“Protected” is the wrong word. The time we spend pursuing academics is really “professional” time. Some centers call it administrative time, but this also falls short. Time allotted to nonclinical activities keeps us fresh, sharpens our intellect, and ultimately helps our patients. Professional time helps prevent burnout by making us more present when we are in clinic. Professional time allows for scientific inquiry to advance treatments, and encourages continuing education to remain at the cutting edge of technology. Professional time, though, competes with patient time and that tension can drive disengagement.
Patients, and their problems, do not operate according to half-day clinic schedules. When there exists any professional time, patient time is always interfering. The interference becomes more acute as academic success increases and the allotted professional time seems inadequate. Hematologists then start to blame patients for interfering with their careers. A pernicious disdain for patient care may develop because it interrupts the academic motivations that drive many physicians once they get a taste of success. Manifestations of this attitude include dread of inpatient service, negotiations to reduce clinic time for research, and refusal to see or sometimes even talk to patients when not assigned to clinic. The more successful the academic hematologist becomes, the less he or she wants to be troubled with patients without whom professional success could not have been achieved.
The professional and patient time balance is as important to recognize as work and life balance, as one tension directly impacts the other. When nature sends a snowstorm, a warm home allows survival, but if one never ventures from home, the beauty and grandeur of nature is lost. True satisfaction comes from a balance of the two and no one person knows how best to accomplish it. I believe we can learn to manage our professional and patient time better by exchanging ideas and best practices. Please email me at [email protected] with your ideas and we will post as many as we can on the Hematology News website for all to learn from.
Dr. Kalaycio is Editor in Chief of Hematology News. Dr. Kalaycio chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
As I write this article, the snow is piling up outside. While Cleveland’s west side citizens are raking up the last of fallen leaves, its east siders will dig out of 2 feet of snow. The lake effect is affecting us. The snow plow trucks vainly clear a path only for it to disappear in minutes. There seems to be no end to the torrents of white flakes that are each unique and tiny, but in aggregate uniform and overwhelming.
A blizzard of patients awaits my return from the annual meeting of the American Society of Hematology in San Diego. Like snowflakes, they are each unique, but in aggregate can be overwhelming. Plowing through a clinic, we go from patient to patient knowing that we will eventually see them all, then return to our offices or home to finish the labor of charting.
For some physicians, this is a daily reality. Whether patients in the clinic, or cases in the queue, some hematologists revisit the storm every day. Most, however, are engaged in an academic practice where at least some respite from direct patient care is offered. Whether teaching medical students, analyzing data, participating in administrative meetings, or writing manuscripts, most of us do something more beyond the clinic. We do this during our “protected time.”
But what are we protected from? Patients and their concerns? Really, this is what we want to be protected from?
“Protected” is the wrong word. The time we spend pursuing academics is really “professional” time. Some centers call it administrative time, but this also falls short. Time allotted to nonclinical activities keeps us fresh, sharpens our intellect, and ultimately helps our patients. Professional time helps prevent burnout by making us more present when we are in clinic. Professional time allows for scientific inquiry to advance treatments, and encourages continuing education to remain at the cutting edge of technology. Professional time, though, competes with patient time and that tension can drive disengagement.
Patients, and their problems, do not operate according to half-day clinic schedules. When there exists any professional time, patient time is always interfering. The interference becomes more acute as academic success increases and the allotted professional time seems inadequate. Hematologists then start to blame patients for interfering with their careers. A pernicious disdain for patient care may develop because it interrupts the academic motivations that drive many physicians once they get a taste of success. Manifestations of this attitude include dread of inpatient service, negotiations to reduce clinic time for research, and refusal to see or sometimes even talk to patients when not assigned to clinic. The more successful the academic hematologist becomes, the less he or she wants to be troubled with patients without whom professional success could not have been achieved.
The professional and patient time balance is as important to recognize as work and life balance, as one tension directly impacts the other. When nature sends a snowstorm, a warm home allows survival, but if one never ventures from home, the beauty and grandeur of nature is lost. True satisfaction comes from a balance of the two and no one person knows how best to accomplish it. I believe we can learn to manage our professional and patient time better by exchanging ideas and best practices. Please email me at [email protected] with your ideas and we will post as many as we can on the Hematology News website for all to learn from.
Dr. Kalaycio is Editor in Chief of Hematology News. Dr. Kalaycio chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
As I write this article, the snow is piling up outside. While Cleveland’s west side citizens are raking up the last of fallen leaves, its east siders will dig out of 2 feet of snow. The lake effect is affecting us. The snow plow trucks vainly clear a path only for it to disappear in minutes. There seems to be no end to the torrents of white flakes that are each unique and tiny, but in aggregate uniform and overwhelming.
A blizzard of patients awaits my return from the annual meeting of the American Society of Hematology in San Diego. Like snowflakes, they are each unique, but in aggregate can be overwhelming. Plowing through a clinic, we go from patient to patient knowing that we will eventually see them all, then return to our offices or home to finish the labor of charting.
For some physicians, this is a daily reality. Whether patients in the clinic, or cases in the queue, some hematologists revisit the storm every day. Most, however, are engaged in an academic practice where at least some respite from direct patient care is offered. Whether teaching medical students, analyzing data, participating in administrative meetings, or writing manuscripts, most of us do something more beyond the clinic. We do this during our “protected time.”
But what are we protected from? Patients and their concerns? Really, this is what we want to be protected from?
“Protected” is the wrong word. The time we spend pursuing academics is really “professional” time. Some centers call it administrative time, but this also falls short. Time allotted to nonclinical activities keeps us fresh, sharpens our intellect, and ultimately helps our patients. Professional time helps prevent burnout by making us more present when we are in clinic. Professional time allows for scientific inquiry to advance treatments, and encourages continuing education to remain at the cutting edge of technology. Professional time, though, competes with patient time and that tension can drive disengagement.
Patients, and their problems, do not operate according to half-day clinic schedules. When there exists any professional time, patient time is always interfering. The interference becomes more acute as academic success increases and the allotted professional time seems inadequate. Hematologists then start to blame patients for interfering with their careers. A pernicious disdain for patient care may develop because it interrupts the academic motivations that drive many physicians once they get a taste of success. Manifestations of this attitude include dread of inpatient service, negotiations to reduce clinic time for research, and refusal to see or sometimes even talk to patients when not assigned to clinic. The more successful the academic hematologist becomes, the less he or she wants to be troubled with patients without whom professional success could not have been achieved.
The professional and patient time balance is as important to recognize as work and life balance, as one tension directly impacts the other. When nature sends a snowstorm, a warm home allows survival, but if one never ventures from home, the beauty and grandeur of nature is lost. True satisfaction comes from a balance of the two and no one person knows how best to accomplish it. I believe we can learn to manage our professional and patient time better by exchanging ideas and best practices. Please email me at [email protected] with your ideas and we will post as many as we can on the Hematology News website for all to learn from.
Dr. Kalaycio is Editor in Chief of Hematology News. Dr. Kalaycio chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].