User login
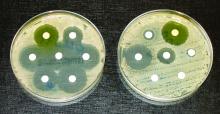
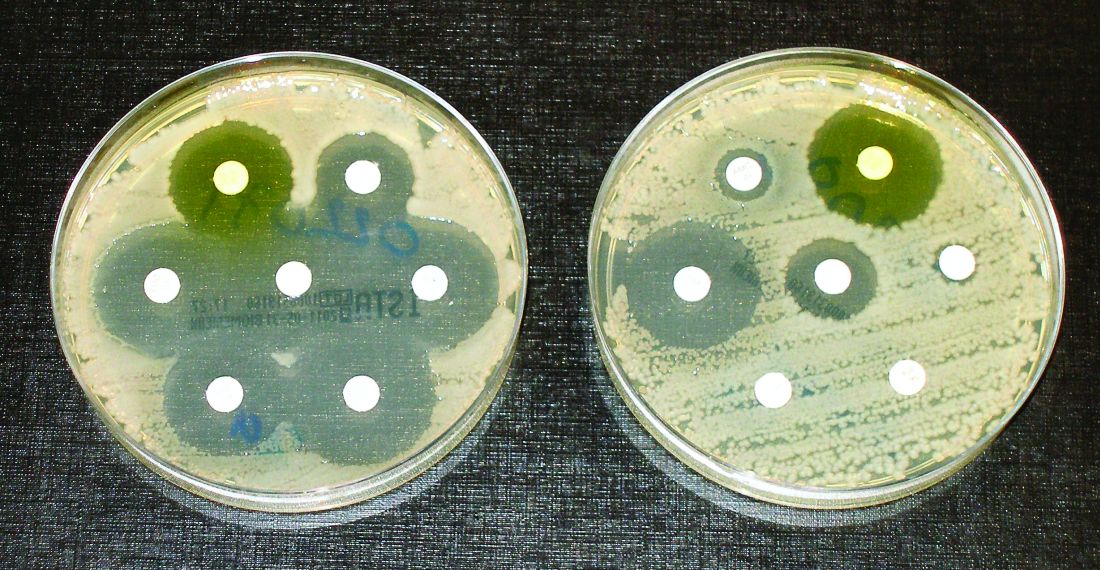
Elimination of urine culture screening prior to elective joint arthroplasty
Clinical question: What is the clinical impact of implementing a policy to no longer process urine specimens for perioperative screening in patients undergoing elective joint arthroplasty (EJA)?
Background: Despite prior studies indicating the lack of clinical benefit, preoperative urine cultures are still frequently obtained in patients undergoing EJA in attempts to reduce the risk of periprosthetic joint infections (PJI).
Study Design: Time series analysis.
Setting: Holland Orthopedic and Arthritic Center (HOAC) of Sunnybrook Health Sciences Centre.
Synopsis: After a multidisciplinary meeting, obtaining routine urine culture screening was removed from the preoperative order set. A time series analysis was performed to review the frequency of screening urine cultures obtained and processed, the number of patients treated for asymptomatic bacteriuria (ASB), and the incidence of PJI before and after the new policy was implemented. After the policy change, only 129 screening urine cultures were obtained prior to 1,891 EJAs (7 per 100 EJA; 95% CI 6-8; P less than .0001) which is a drastic decrease from the 3,069 screening urine cultures obtained prior to 3,523 EJAs (87 per 100 EJA; 95% CI, 86-88) before the policy change. Prior to the policy change, of the 352 positive urine cultures, 43 received perioperative treatment for ASB, and PJI incidence was 1/3523 (0.03%; 95% CI, 0.001-02). After the policy change, no perioperative antibiotics were prescribed for ASB, and PJI rate did not significantly change at 3/1891 (0.2%; 95% CI, 0.05-0.5; P = .1).
The study was limited by its low power to detect for small differences in rates because of its small PJI rate occurrence.
Bottom Line: A multidisciplinary approach in eliminating routine urine screening prior to EJA resulted in a decrease of urine cultures obtained and a decrease in treatment for asymptomatic bacteriuria, with no significant change in PJI rate. This change in clinical practice is supported by current evidence and has a significant impact on cost savings.
References: Lamb MJ, Baillie L, Pajak D, et al. “Elimination of Screening Urine Cultures Prior to Elective Joint Arthroplasty.”
Dr. Libot is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
Clinical question: What is the clinical impact of implementing a policy to no longer process urine specimens for perioperative screening in patients undergoing elective joint arthroplasty (EJA)?
Background: Despite prior studies indicating the lack of clinical benefit, preoperative urine cultures are still frequently obtained in patients undergoing EJA in attempts to reduce the risk of periprosthetic joint infections (PJI).
Study Design: Time series analysis.
Setting: Holland Orthopedic and Arthritic Center (HOAC) of Sunnybrook Health Sciences Centre.
Synopsis: After a multidisciplinary meeting, obtaining routine urine culture screening was removed from the preoperative order set. A time series analysis was performed to review the frequency of screening urine cultures obtained and processed, the number of patients treated for asymptomatic bacteriuria (ASB), and the incidence of PJI before and after the new policy was implemented. After the policy change, only 129 screening urine cultures were obtained prior to 1,891 EJAs (7 per 100 EJA; 95% CI 6-8; P less than .0001) which is a drastic decrease from the 3,069 screening urine cultures obtained prior to 3,523 EJAs (87 per 100 EJA; 95% CI, 86-88) before the policy change. Prior to the policy change, of the 352 positive urine cultures, 43 received perioperative treatment for ASB, and PJI incidence was 1/3523 (0.03%; 95% CI, 0.001-02). After the policy change, no perioperative antibiotics were prescribed for ASB, and PJI rate did not significantly change at 3/1891 (0.2%; 95% CI, 0.05-0.5; P = .1).
The study was limited by its low power to detect for small differences in rates because of its small PJI rate occurrence.
Bottom Line: A multidisciplinary approach in eliminating routine urine screening prior to EJA resulted in a decrease of urine cultures obtained and a decrease in treatment for asymptomatic bacteriuria, with no significant change in PJI rate. This change in clinical practice is supported by current evidence and has a significant impact on cost savings.
References: Lamb MJ, Baillie L, Pajak D, et al. “Elimination of Screening Urine Cultures Prior to Elective Joint Arthroplasty.”
Dr. Libot is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
Clinical question: What is the clinical impact of implementing a policy to no longer process urine specimens for perioperative screening in patients undergoing elective joint arthroplasty (EJA)?
Background: Despite prior studies indicating the lack of clinical benefit, preoperative urine cultures are still frequently obtained in patients undergoing EJA in attempts to reduce the risk of periprosthetic joint infections (PJI).
Study Design: Time series analysis.
Setting: Holland Orthopedic and Arthritic Center (HOAC) of Sunnybrook Health Sciences Centre.
Synopsis: After a multidisciplinary meeting, obtaining routine urine culture screening was removed from the preoperative order set. A time series analysis was performed to review the frequency of screening urine cultures obtained and processed, the number of patients treated for asymptomatic bacteriuria (ASB), and the incidence of PJI before and after the new policy was implemented. After the policy change, only 129 screening urine cultures were obtained prior to 1,891 EJAs (7 per 100 EJA; 95% CI 6-8; P less than .0001) which is a drastic decrease from the 3,069 screening urine cultures obtained prior to 3,523 EJAs (87 per 100 EJA; 95% CI, 86-88) before the policy change. Prior to the policy change, of the 352 positive urine cultures, 43 received perioperative treatment for ASB, and PJI incidence was 1/3523 (0.03%; 95% CI, 0.001-02). After the policy change, no perioperative antibiotics were prescribed for ASB, and PJI rate did not significantly change at 3/1891 (0.2%; 95% CI, 0.05-0.5; P = .1).
The study was limited by its low power to detect for small differences in rates because of its small PJI rate occurrence.
Bottom Line: A multidisciplinary approach in eliminating routine urine screening prior to EJA resulted in a decrease of urine cultures obtained and a decrease in treatment for asymptomatic bacteriuria, with no significant change in PJI rate. This change in clinical practice is supported by current evidence and has a significant impact on cost savings.
References: Lamb MJ, Baillie L, Pajak D, et al. “Elimination of Screening Urine Cultures Prior to Elective Joint Arthroplasty.”
Dr. Libot is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
In the lit: Short takes
Efficacy of ketorolac is similar at all the most commonly administered doses
A randomized, double-blind trial of IV ketorolac dosing found similar analgesic efficacy in patients aged 18-65 years with moderate to severe pain at the commonly ordered doses of 10 mg, 15 mg, and 30 mg with no increase in adverse effects.
Citation: Motov S, Yasavolian M, Likourezos A, et al. “Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: A randomized controlled trial.” Ann Emerg Med. 2016 Dec 16. doi: org/10.1016/j.annemergmed.2016.10.014.
-- Paula Marfia, MD, is assistant professor in the Division of Hospital Medicine, Loyola University Chicago, Maywood, Ill.
Viruses are common cause of nonventilated, hospital-acquired pneumonia
Retrospective analysis demonstrates that viruses are common etiology for nonventilated hospital-acquired pneumonia (NVHAP), as common as bacterial organisms. Clinicians should consider testing for viral etiologies in NVHAP in an effort to improve antibiotic stewardship.
Citation: Shorr AF, Zilberberg MD, Micek ST, Kollef MH. “Viruses are prevalent in non-ventilated hospital-acquired pneumonia.” Respir Med. 2017;122:76-80.
-- Anar Mashruwala, MD, FACP, is assistant professor in the Department of Medicine, Division of Hospital Medicine at Loyola University Medical Center, Maywood, Ill.
FDA issues important safety precautions for use of implantable infusions pumps in MRI
Serious adverse events, including patient injury and death, have been reported with the use of implantable infusion pumps in the MRI environment. The Food and Drug Administration has issued a safety communication for patients, caregivers, health care providers, and MRI technologists, outlining safety precautions and recommendations.
Citation: “Implantable Infusion Pumps in the Magnetic Resonance (MR) Environment: FDA Safety Communication – Important Safety Precautions.” FDA.gov. 2017 Jan 11.
-- Anar Mashruwala, MD, FACP, is assistant professor in the Department of Medicine, Division of Hospital Medicine at Loyola University Medical Center, Maywood, Ill.
Efficacy of ketorolac is similar at all the most commonly administered doses
A randomized, double-blind trial of IV ketorolac dosing found similar analgesic efficacy in patients aged 18-65 years with moderate to severe pain at the commonly ordered doses of 10 mg, 15 mg, and 30 mg with no increase in adverse effects.
Citation: Motov S, Yasavolian M, Likourezos A, et al. “Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: A randomized controlled trial.” Ann Emerg Med. 2016 Dec 16. doi: org/10.1016/j.annemergmed.2016.10.014.
-- Paula Marfia, MD, is assistant professor in the Division of Hospital Medicine, Loyola University Chicago, Maywood, Ill.
Viruses are common cause of nonventilated, hospital-acquired pneumonia
Retrospective analysis demonstrates that viruses are common etiology for nonventilated hospital-acquired pneumonia (NVHAP), as common as bacterial organisms. Clinicians should consider testing for viral etiologies in NVHAP in an effort to improve antibiotic stewardship.
Citation: Shorr AF, Zilberberg MD, Micek ST, Kollef MH. “Viruses are prevalent in non-ventilated hospital-acquired pneumonia.” Respir Med. 2017;122:76-80.
-- Anar Mashruwala, MD, FACP, is assistant professor in the Department of Medicine, Division of Hospital Medicine at Loyola University Medical Center, Maywood, Ill.
FDA issues important safety precautions for use of implantable infusions pumps in MRI
Serious adverse events, including patient injury and death, have been reported with the use of implantable infusion pumps in the MRI environment. The Food and Drug Administration has issued a safety communication for patients, caregivers, health care providers, and MRI technologists, outlining safety precautions and recommendations.
Citation: “Implantable Infusion Pumps in the Magnetic Resonance (MR) Environment: FDA Safety Communication – Important Safety Precautions.” FDA.gov. 2017 Jan 11.
-- Anar Mashruwala, MD, FACP, is assistant professor in the Department of Medicine, Division of Hospital Medicine at Loyola University Medical Center, Maywood, Ill.
Efficacy of ketorolac is similar at all the most commonly administered doses
A randomized, double-blind trial of IV ketorolac dosing found similar analgesic efficacy in patients aged 18-65 years with moderate to severe pain at the commonly ordered doses of 10 mg, 15 mg, and 30 mg with no increase in adverse effects.
Citation: Motov S, Yasavolian M, Likourezos A, et al. “Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: A randomized controlled trial.” Ann Emerg Med. 2016 Dec 16. doi: org/10.1016/j.annemergmed.2016.10.014.
-- Paula Marfia, MD, is assistant professor in the Division of Hospital Medicine, Loyola University Chicago, Maywood, Ill.
Viruses are common cause of nonventilated, hospital-acquired pneumonia
Retrospective analysis demonstrates that viruses are common etiology for nonventilated hospital-acquired pneumonia (NVHAP), as common as bacterial organisms. Clinicians should consider testing for viral etiologies in NVHAP in an effort to improve antibiotic stewardship.
Citation: Shorr AF, Zilberberg MD, Micek ST, Kollef MH. “Viruses are prevalent in non-ventilated hospital-acquired pneumonia.” Respir Med. 2017;122:76-80.
-- Anar Mashruwala, MD, FACP, is assistant professor in the Department of Medicine, Division of Hospital Medicine at Loyola University Medical Center, Maywood, Ill.
FDA issues important safety precautions for use of implantable infusions pumps in MRI
Serious adverse events, including patient injury and death, have been reported with the use of implantable infusion pumps in the MRI environment. The Food and Drug Administration has issued a safety communication for patients, caregivers, health care providers, and MRI technologists, outlining safety precautions and recommendations.
Citation: “Implantable Infusion Pumps in the Magnetic Resonance (MR) Environment: FDA Safety Communication – Important Safety Precautions.” FDA.gov. 2017 Jan 11.
-- Anar Mashruwala, MD, FACP, is assistant professor in the Department of Medicine, Division of Hospital Medicine at Loyola University Medical Center, Maywood, Ill.
Risks are reduced when angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers are held before noncardiac surgery
Clinical question: Is withholding angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARBs) prior to noncardiac surgery associated with a lower risk of a 30-day composite outcome of all-cause death, myocardial injury after noncardiac surgery, and stroke when compared with continuing them on the day of surgery?
Background: The current American College of Cardiology/American Heart Association guidelines recommend continuing ACEI and ARBs for noncardiac surgery. However, many clinicians, including anesthesiologists, withhold these medications to prevent intraoperative hypotension. Because of the lack of strong evidence regarding clinical outcomes, the decision to withhold ACEI and ARBs prior to noncardiac surgery is currently dictated by physician preference and local policy.
Study Design: Prospective cohort study.
Setting: Analysis sample from the VISION study (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation), which included 12 centers in eight countries.
Synopsis: A sample analysis was performed on 14,687 patients from the VISION study, who were at least 45 years old and undergoing noncardiac surgery and who required an overnight hospital admission. A total of 4,802 patients were taking ACEI/ARBs at baseline, and, for 1,245 (25.9%) of those patients, ACEI/ARBs were withheld at least 24 hours before surgery. Using multivariable regression models, the authors found that patients for whom ACEI/ARBs were withheld were less likely to suffer from the primary composite outcome of 30-day all-cause death, myocardial injury after noncardiac surgery, and stroke (12% vs 12.9%; adjusted relative risk, 0.82; 95% confidence interval, 0.7-0.96; P = .01). Withholding ACEI/ARBs prior to surgery was also associated with less risk of clinically important intraoperative hypotension, while the risk of postoperative hypotension was similar between the two groups.
Given that this was an observational study, analysis is limited because of the inability to account for every potential confounding factor.
Bottom Line: The study suggests a lower risk of postoperative death, stroke, and myocardial injury in patients for whom ACEI/ARBs were withheld prior to noncardiac surgery. A large randomized trial is needed to confirm the findings suggested by this analysis.
Citation: Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery.” Anesthesiology. 2017 Jan;126(1):16-27.
Dr. Libot is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
Clinical question: Is withholding angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARBs) prior to noncardiac surgery associated with a lower risk of a 30-day composite outcome of all-cause death, myocardial injury after noncardiac surgery, and stroke when compared with continuing them on the day of surgery?
Background: The current American College of Cardiology/American Heart Association guidelines recommend continuing ACEI and ARBs for noncardiac surgery. However, many clinicians, including anesthesiologists, withhold these medications to prevent intraoperative hypotension. Because of the lack of strong evidence regarding clinical outcomes, the decision to withhold ACEI and ARBs prior to noncardiac surgery is currently dictated by physician preference and local policy.
Study Design: Prospective cohort study.
Setting: Analysis sample from the VISION study (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation), which included 12 centers in eight countries.
Synopsis: A sample analysis was performed on 14,687 patients from the VISION study, who were at least 45 years old and undergoing noncardiac surgery and who required an overnight hospital admission. A total of 4,802 patients were taking ACEI/ARBs at baseline, and, for 1,245 (25.9%) of those patients, ACEI/ARBs were withheld at least 24 hours before surgery. Using multivariable regression models, the authors found that patients for whom ACEI/ARBs were withheld were less likely to suffer from the primary composite outcome of 30-day all-cause death, myocardial injury after noncardiac surgery, and stroke (12% vs 12.9%; adjusted relative risk, 0.82; 95% confidence interval, 0.7-0.96; P = .01). Withholding ACEI/ARBs prior to surgery was also associated with less risk of clinically important intraoperative hypotension, while the risk of postoperative hypotension was similar between the two groups.
Given that this was an observational study, analysis is limited because of the inability to account for every potential confounding factor.
Bottom Line: The study suggests a lower risk of postoperative death, stroke, and myocardial injury in patients for whom ACEI/ARBs were withheld prior to noncardiac surgery. A large randomized trial is needed to confirm the findings suggested by this analysis.
Citation: Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery.” Anesthesiology. 2017 Jan;126(1):16-27.
Dr. Libot is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
Clinical question: Is withholding angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARBs) prior to noncardiac surgery associated with a lower risk of a 30-day composite outcome of all-cause death, myocardial injury after noncardiac surgery, and stroke when compared with continuing them on the day of surgery?
Background: The current American College of Cardiology/American Heart Association guidelines recommend continuing ACEI and ARBs for noncardiac surgery. However, many clinicians, including anesthesiologists, withhold these medications to prevent intraoperative hypotension. Because of the lack of strong evidence regarding clinical outcomes, the decision to withhold ACEI and ARBs prior to noncardiac surgery is currently dictated by physician preference and local policy.
Study Design: Prospective cohort study.
Setting: Analysis sample from the VISION study (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation), which included 12 centers in eight countries.
Synopsis: A sample analysis was performed on 14,687 patients from the VISION study, who were at least 45 years old and undergoing noncardiac surgery and who required an overnight hospital admission. A total of 4,802 patients were taking ACEI/ARBs at baseline, and, for 1,245 (25.9%) of those patients, ACEI/ARBs were withheld at least 24 hours before surgery. Using multivariable regression models, the authors found that patients for whom ACEI/ARBs were withheld were less likely to suffer from the primary composite outcome of 30-day all-cause death, myocardial injury after noncardiac surgery, and stroke (12% vs 12.9%; adjusted relative risk, 0.82; 95% confidence interval, 0.7-0.96; P = .01). Withholding ACEI/ARBs prior to surgery was also associated with less risk of clinically important intraoperative hypotension, while the risk of postoperative hypotension was similar between the two groups.
Given that this was an observational study, analysis is limited because of the inability to account for every potential confounding factor.
Bottom Line: The study suggests a lower risk of postoperative death, stroke, and myocardial injury in patients for whom ACEI/ARBs were withheld prior to noncardiac surgery. A large randomized trial is needed to confirm the findings suggested by this analysis.
Citation: Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery.” Anesthesiology. 2017 Jan;126(1):16-27.
Dr. Libot is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
Patient-to-intensivist ratios can influence patient mortality
CLINICAL QUESTION: Is there variation in patient-to-intensivist ratios (PIR) across ICUs, and does that ratio affect hospital mortality?
BACKGROUND: Most studies show that intensivists improve ICU patient outcomes. With increasing ICU patients but stable intensivist staffing, patient-to-intensivist ratios are increasing. It is unclear if that rising ratio is adversely affecting patient mortality.
STUDY DESIGN: Multicenter retrospective cohort analysis.
SETTING: ICUs in the United Kingdom from 2010 to 2013.
The median PIR was 8.5 but varied substantially – PIRs were often larger. The association between PIR and mortality was U shaped. There was a decrease in mortality as the PIR reached 7.5, after which the mortality increased again. The higher mortality with very low PIRs could reflect a volume-outcome relationship. Less patients could mean less experience, different levels of ancillary staff, and so on.
This study did not take into account the possible differences in the multidisciplinary makeup of the ICU teams that would affect the intensivist’s level of responsibility.
BOTTOM LINE: There seems to be an optimal PIR for mortality, though that optimal number would likely depend on the ancillary staff, level of trainees, and patient acuity.
CITATIONS: Gershengorn HB, Harrison DA, Garland A, et al. “Association of Intensive Care Unit Patient-to-Intensivist Ratios With Hospital Mortality.” JAMA Intern Med. 2017 Mar 1;177(3):388-96.
Dr. Tsien is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
CLINICAL QUESTION: Is there variation in patient-to-intensivist ratios (PIR) across ICUs, and does that ratio affect hospital mortality?
BACKGROUND: Most studies show that intensivists improve ICU patient outcomes. With increasing ICU patients but stable intensivist staffing, patient-to-intensivist ratios are increasing. It is unclear if that rising ratio is adversely affecting patient mortality.
STUDY DESIGN: Multicenter retrospective cohort analysis.
SETTING: ICUs in the United Kingdom from 2010 to 2013.
The median PIR was 8.5 but varied substantially – PIRs were often larger. The association between PIR and mortality was U shaped. There was a decrease in mortality as the PIR reached 7.5, after which the mortality increased again. The higher mortality with very low PIRs could reflect a volume-outcome relationship. Less patients could mean less experience, different levels of ancillary staff, and so on.
This study did not take into account the possible differences in the multidisciplinary makeup of the ICU teams that would affect the intensivist’s level of responsibility.
BOTTOM LINE: There seems to be an optimal PIR for mortality, though that optimal number would likely depend on the ancillary staff, level of trainees, and patient acuity.
CITATIONS: Gershengorn HB, Harrison DA, Garland A, et al. “Association of Intensive Care Unit Patient-to-Intensivist Ratios With Hospital Mortality.” JAMA Intern Med. 2017 Mar 1;177(3):388-96.
Dr. Tsien is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
CLINICAL QUESTION: Is there variation in patient-to-intensivist ratios (PIR) across ICUs, and does that ratio affect hospital mortality?
BACKGROUND: Most studies show that intensivists improve ICU patient outcomes. With increasing ICU patients but stable intensivist staffing, patient-to-intensivist ratios are increasing. It is unclear if that rising ratio is adversely affecting patient mortality.
STUDY DESIGN: Multicenter retrospective cohort analysis.
SETTING: ICUs in the United Kingdom from 2010 to 2013.
The median PIR was 8.5 but varied substantially – PIRs were often larger. The association between PIR and mortality was U shaped. There was a decrease in mortality as the PIR reached 7.5, after which the mortality increased again. The higher mortality with very low PIRs could reflect a volume-outcome relationship. Less patients could mean less experience, different levels of ancillary staff, and so on.
This study did not take into account the possible differences in the multidisciplinary makeup of the ICU teams that would affect the intensivist’s level of responsibility.
BOTTOM LINE: There seems to be an optimal PIR for mortality, though that optimal number would likely depend on the ancillary staff, level of trainees, and patient acuity.
CITATIONS: Gershengorn HB, Harrison DA, Garland A, et al. “Association of Intensive Care Unit Patient-to-Intensivist Ratios With Hospital Mortality.” JAMA Intern Med. 2017 Mar 1;177(3):388-96.
Dr. Tsien is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding
CLINICAL QUESTION: What are the accuracy and clinical utility of risk scoring systems in the assessment of patients with upper gastrointestinal (GI) bleeding?
BACKGROUND: There are several pre- and postendoscopy risk scores to predict clinically relevant outcomes such as transfusion, mortality, endoscopy treatment, surgery, and length of hospital stay for upper GI bleeding. The accuracy and applicability of these risk scores has not been well established.
STUDY DESIGN: International multicenter prospective study.
SETTING: Six large hospitals in Europe, North America, Asia, and Oceania.
SYNOPSIS: This is a prospective study comparing three pre-endoscopy scoring systems (Rockall, AIMS65, Glasgow Blatchford) and two postendoscopy scoring systems (PNED, full Rockall) in 3,012 patients with upper GI bleeding over six hospitals. It examined clinically relevant outcomes: intervention (transfusion, endoscopy, interventional radiology, surgery), mortality, rebleeding, and length of hospital stay.
The Glasgow Blatchford risk score was the most accurate at predicting the need for hospitalization and death across all hospitals, compared with the other scoring systems. It was determined that a Glasgow Blatchford score of less than 1 is an optimal threshold for outpatient management, with a 98.6% sensitivity in identifying those who would not require intervention or die. The utility of these scores to direct management in high-risk patients is limited and needs further studies. No scoring system predicted rebleeding or length of hospital stay.
A weakness of the study is that patients who bled while already inpatients were excluded.
BOTTOM LINE: The Glasgow Blatchford risk score can help direct care of very low risk patients (score, less than 1) with upper GI bleeding toward outpatient management.
CITATIONS: Stanley AJ, Laine L, Dalton HR, et al. “Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: International multicenter prospective study.” BMJ. 2017 Jan 4;356:i6432.
Dr. Tsien is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
CLINICAL QUESTION: What are the accuracy and clinical utility of risk scoring systems in the assessment of patients with upper gastrointestinal (GI) bleeding?
BACKGROUND: There are several pre- and postendoscopy risk scores to predict clinically relevant outcomes such as transfusion, mortality, endoscopy treatment, surgery, and length of hospital stay for upper GI bleeding. The accuracy and applicability of these risk scores has not been well established.
STUDY DESIGN: International multicenter prospective study.
SETTING: Six large hospitals in Europe, North America, Asia, and Oceania.
SYNOPSIS: This is a prospective study comparing three pre-endoscopy scoring systems (Rockall, AIMS65, Glasgow Blatchford) and two postendoscopy scoring systems (PNED, full Rockall) in 3,012 patients with upper GI bleeding over six hospitals. It examined clinically relevant outcomes: intervention (transfusion, endoscopy, interventional radiology, surgery), mortality, rebleeding, and length of hospital stay.
The Glasgow Blatchford risk score was the most accurate at predicting the need for hospitalization and death across all hospitals, compared with the other scoring systems. It was determined that a Glasgow Blatchford score of less than 1 is an optimal threshold for outpatient management, with a 98.6% sensitivity in identifying those who would not require intervention or die. The utility of these scores to direct management in high-risk patients is limited and needs further studies. No scoring system predicted rebleeding or length of hospital stay.
A weakness of the study is that patients who bled while already inpatients were excluded.
BOTTOM LINE: The Glasgow Blatchford risk score can help direct care of very low risk patients (score, less than 1) with upper GI bleeding toward outpatient management.
CITATIONS: Stanley AJ, Laine L, Dalton HR, et al. “Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: International multicenter prospective study.” BMJ. 2017 Jan 4;356:i6432.
Dr. Tsien is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
CLINICAL QUESTION: What are the accuracy and clinical utility of risk scoring systems in the assessment of patients with upper gastrointestinal (GI) bleeding?
BACKGROUND: There are several pre- and postendoscopy risk scores to predict clinically relevant outcomes such as transfusion, mortality, endoscopy treatment, surgery, and length of hospital stay for upper GI bleeding. The accuracy and applicability of these risk scores has not been well established.
STUDY DESIGN: International multicenter prospective study.
SETTING: Six large hospitals in Europe, North America, Asia, and Oceania.
SYNOPSIS: This is a prospective study comparing three pre-endoscopy scoring systems (Rockall, AIMS65, Glasgow Blatchford) and two postendoscopy scoring systems (PNED, full Rockall) in 3,012 patients with upper GI bleeding over six hospitals. It examined clinically relevant outcomes: intervention (transfusion, endoscopy, interventional radiology, surgery), mortality, rebleeding, and length of hospital stay.
The Glasgow Blatchford risk score was the most accurate at predicting the need for hospitalization and death across all hospitals, compared with the other scoring systems. It was determined that a Glasgow Blatchford score of less than 1 is an optimal threshold for outpatient management, with a 98.6% sensitivity in identifying those who would not require intervention or die. The utility of these scores to direct management in high-risk patients is limited and needs further studies. No scoring system predicted rebleeding or length of hospital stay.
A weakness of the study is that patients who bled while already inpatients were excluded.
BOTTOM LINE: The Glasgow Blatchford risk score can help direct care of very low risk patients (score, less than 1) with upper GI bleeding toward outpatient management.
CITATIONS: Stanley AJ, Laine L, Dalton HR, et al. “Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: International multicenter prospective study.” BMJ. 2017 Jan 4;356:i6432.
Dr. Tsien is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
It’s been a good year for heart failure research ... mostly
WASHINGTON – It’s been a “relatively positive” year for heart failure research and advances in patient care, Christopher M. O’Connor, MD, said at the annual meeting of the American College of Cardiology.
“Having been in this field for 30 years looking at clinical trials, generally for every 10 important trials done in a year, 1 has been positive and 9 have been negative; if we look at the past year, it’s more like 5 and 6. So, not a bad year for cardiomyopathy,” declared Dr. O’Connor, CEO and executive director of the Inova Heart and Vascular Institute in Falls Church, Va., and president-elect of the Heart Failure Society of America.
The good news
• Empagliflozin (Jardiance) earns FDA approval for reduction in risk of cardiovascular death in type 2 diabetes patients. “This is one of the most amazing stories in heart failure,” said Dr. O’Connor, who is also professor of medicine at Duke University in Durham, N.C.
The pivotal EMPA-REG OUTCOME study showed a highly significant 35% reduction in the secondary endpoint of risk of hospitalization for heart failure, as well the decrease in cardiovascular mortality which was the primary endpoint and proved persuasive to the FDA (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
“It was a remarkable development. Because of this trial, there are now a number of ongoing phase III clinical trials looking at this class of drugs in heart failure patients with and without diabetes, which makes this a very important research movement. We are now looking deeper at phenotypes and trying to get more specific with these drug therapies,” he said.
• A new and improved LVAD. This fully magnetically levitated centrifugal-flow pump type of left ventricular assist device for advanced heart failure showed superior event-free survival, compared with a commercially available axial continuous-flow pump LVAD in the randomized MOMENTUM-3 trial (N Engl J Med. 2017 Feb 2;376[5]:440-50).
The novel pump was designed to overcome a significant problem with axial continuous-flow LVADs: a proclivity for pump thrombosis. The magnetically levitated centrifugal-flow pump proved a smashing success in this regard, with zero cases of pump thrombosis occurring during the 6-month study.
“This may be the first time in the history of heart failure research that the engineers have beaten the biologists in important clinical outcomes,” the cardiologist quipped.
• Omecamtiv mecarbil successfully addresses impaired contractility in heart failure with reduced ejection fraction (HFrEF). This drug, a selective cardiac myosin activator, resulted in increased duration of systole and improved stroke volume accompanied by reductions in heart rate, left ventricular end-diastolic and -systolic dimensions, and NT-proBNP in the 87-site, 13-country, phase II COSMIC-HF study (Lancet. 2016 Dec 10;388[10062]:2895-903).
“This is probably the most novel new drug mechanism out there in clinical trials,” according to Dr. O’Connor.
On the basis of the highly encouraging results for the surrogate endpoints assessed in COSMIC-HF, a large phase III clinical trial known as GALACTIC is underway.
• Palliative care gets a welcome boost. Dr. O’Connor was a coinvestigator in PAL-HF, a single-center study presented at the 2016 annual meeting of the Heart Failure Society of America.
“This is a very important trial of palliative care in advanced heart failure. We probably don’t have as much evidence in this space as we should,” he observed. “This was a multidisciplinary intervention in which we gave the patients a medical tool kit to alleviate pain, dyspnea, and discomfort. The tool kit included benzodiazepines, sleep medications, sublingual nitroglycerin, and morphinelike products, all very carefully monitored by staff coordinators.”
The primary outcome was change in two validated heart failure quality of life measures. Both instruments documented significant improvement compared with usual care.
“There was no decrease in mortality, which wasn’t a goal in this advanced heart failure population, and no reduction in heart failure hospitalizations, but there were significant reductions in depression and anxiety,” Dr. O’Connor said.
• Vericiguat. This oral soluble cyclic guanylate cyclase stimulator missed its primary endpoint in the phase II dose-escalation SOCRATES-REDUCED trial in patients with HFrEF (JAMA. 2015 Dec 1;314[21]:2251-62), but showed an impressive improvement in quality of life. It is now the subject of the ongoing, randomized, phase III VICTORIA trial involving a planned 4,000 patients with HFrEF with the composite primary endpoint of cardiovascular death or heart failure hospitalization.
The phase II SOCRATES-PRESERVED trial also missed its primary endpoint but showed a clinically meaningful improvement in quality of life in patients with heart failure with preserved ejection fraction (HFpEF) (Eur Heart J. 2017 Mar 22. doi: 10.1093/eurheartj/ehw593). Discussions are ongoing as to whether the next step should be a confirmatory phase II study or a move straight to phase III.
The bad news
• NSAIDs linked to increased risk of heart failure. European investigators analyzed five population-based databases totaling more than 8.3 million individuals and determined that current use of any of more than two dozen NSAIDs was associated with significantly increased risk of hospital admission for heart failure. The risk appeared to be dose dependent and varied between individual agents, ranging from a 16% increased risk with naproxen to an 83% increase with ketorolac (Toradol) (BMJ. 2016 Sep 28. doi: 10.1136/bmj.4857).
• Therapeutic natriuretic peptides hit bottom. The negative results for the investigational agent ularitide in patients with acute decompensated heart failure in the large phase III TRUE-AHF trial presented at the 2016 meeting of the American Heart Association, following upon an earlier negative study of the related drug nesiritide (Natrecor) in more than 7,100 acute heart failure patients (N Engl J Med. 2011 Jul 7; 365:32-43), probably spells the end of the line for this strategy of boosting outcomes in acute heart failure, according to Dr. O’Connor.
Moreover, Novartis has announced that the phase III RELAX-AHF-2 trial of serelaxin in 6,600 patients with acute heart failure failed to meet its primary endpoints of reduced cardiovascular deaths or reduced worsening of heart failure. The trial will be formally presented later this year.
“Ularitide seemed to show an early improvement in heart failure events that was not sustained in-hospital, and there was absolutely no difference in mortality. The drug probably acts like a pharmacologic tourniquet, in my view. So I think this field of therapeutic natriuretic peptides is probably closed,” he said.
• ICDs don’t reduce mortality in patients with nonischemic heart failure. This was the conclusion reached in the DANISH trial, in which more than 1,100 patients with symptomatic systolic heart failure were randomized to an ICD or usual care (N Engl J Med. 2016 Sep 29;375[13]:1221-30).
“This study really shook up the field, raising the question, ‘Are we using defibrillators too frequently in this population?’ It has stimulated a lot of discussion, including within the guidelines committee,” according to Dr. O’Connor.
• Tolvaptan nixed for acute decompensated heart failure. The TACTICS-HF trial studied the use of tolvaptan (Samsca), an oral vasopressin-2 receptor antagonist, to reduce dyspnea in patients hospitalized with acute decompensated heart failure. Dr. O’Connor was a coinvestigator in the study, which showed that tolvaptan was no better than placebo at 8 and 24 hours (J Am Coll Cardiol. 2017 Mar 21;69[11]:1399-406).
“For now, the routine use of vasopressin antagonists in acute heart failure is not to be encouraged, although there may still be subsets where it’s worth trying – certainly in severe hyponatremia,” the cardiologist said.
• GUIDE-IT gets lost. This was a roughly 1,000-patient randomized trial of a treatment strategy aimed at improving clinical outcomes by aggressively titrating evidence-based heart failure therapies in order to suppress natriuretic peptide biomarkers. GUIDE-IT was stopped early by the data safety monitoring board due to a lack of discernible difference in outcomes, compared with usual care. Dr. O’Connor, a coinvestigator, termed the soon-to-be-published study “a disappointment.”
Sleep apnea studies show silver lining
Sleep apnea is common in patients with heart failure and is associated with worse clinical heart failure outcomes. But in the large, randomized SERVE-HF trial, investigators showed that treatment of central sleep apnea with adaptive servo-ventilation in patients with HFrEF unexpectedly increased mortality, compared with optimal medical therapy (N Engl J Med. 2015 Sep 17;373:1095-105). In a more recent secondary analysis, however, the SERVE-HF investigators found that the increased risk for cardiovascular death associated with adaptive servo-ventilation was actually restricted to patients with a very poor left ventricular ejection fraction of 30% or less, and there was a signal of possible benefit in patients in the top tier of LVEF, at 36%-45% (Lancet Respir Med. 2016 Nov; 4[11]:873-81).
This observation dovetails nicely with the findings of the CAT-HF trial presented by Dr. O’Connor at the 2016 World Congress on Heart Failure. In this phase II study, 215 patients with acute decompensated heart failure and either obstructive or central sleep apnea were randomized to optimal medical therapy alone or in combination with adaptive servo-ventilation. There was no overall difference in outcome between the two groups; however, in the subgroup of patients with HFpEF, the risk of the primary composite endpoint was reduced by 62% with adaptive servo-ventilation.
As a result of these intriguing findings from SERVE-HF and CAT-HF, a registry and/or randomized clinical trial of adaptive servo-ventilation in patients with HFpEF is now being considered under NIH sponsorship, according to Dr. O’Connor.
He reported having no financial conflicts.
WASHINGTON – It’s been a “relatively positive” year for heart failure research and advances in patient care, Christopher M. O’Connor, MD, said at the annual meeting of the American College of Cardiology.
“Having been in this field for 30 years looking at clinical trials, generally for every 10 important trials done in a year, 1 has been positive and 9 have been negative; if we look at the past year, it’s more like 5 and 6. So, not a bad year for cardiomyopathy,” declared Dr. O’Connor, CEO and executive director of the Inova Heart and Vascular Institute in Falls Church, Va., and president-elect of the Heart Failure Society of America.
The good news
• Empagliflozin (Jardiance) earns FDA approval for reduction in risk of cardiovascular death in type 2 diabetes patients. “This is one of the most amazing stories in heart failure,” said Dr. O’Connor, who is also professor of medicine at Duke University in Durham, N.C.
The pivotal EMPA-REG OUTCOME study showed a highly significant 35% reduction in the secondary endpoint of risk of hospitalization for heart failure, as well the decrease in cardiovascular mortality which was the primary endpoint and proved persuasive to the FDA (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
“It was a remarkable development. Because of this trial, there are now a number of ongoing phase III clinical trials looking at this class of drugs in heart failure patients with and without diabetes, which makes this a very important research movement. We are now looking deeper at phenotypes and trying to get more specific with these drug therapies,” he said.
• A new and improved LVAD. This fully magnetically levitated centrifugal-flow pump type of left ventricular assist device for advanced heart failure showed superior event-free survival, compared with a commercially available axial continuous-flow pump LVAD in the randomized MOMENTUM-3 trial (N Engl J Med. 2017 Feb 2;376[5]:440-50).
The novel pump was designed to overcome a significant problem with axial continuous-flow LVADs: a proclivity for pump thrombosis. The magnetically levitated centrifugal-flow pump proved a smashing success in this regard, with zero cases of pump thrombosis occurring during the 6-month study.
“This may be the first time in the history of heart failure research that the engineers have beaten the biologists in important clinical outcomes,” the cardiologist quipped.
• Omecamtiv mecarbil successfully addresses impaired contractility in heart failure with reduced ejection fraction (HFrEF). This drug, a selective cardiac myosin activator, resulted in increased duration of systole and improved stroke volume accompanied by reductions in heart rate, left ventricular end-diastolic and -systolic dimensions, and NT-proBNP in the 87-site, 13-country, phase II COSMIC-HF study (Lancet. 2016 Dec 10;388[10062]:2895-903).
“This is probably the most novel new drug mechanism out there in clinical trials,” according to Dr. O’Connor.
On the basis of the highly encouraging results for the surrogate endpoints assessed in COSMIC-HF, a large phase III clinical trial known as GALACTIC is underway.
• Palliative care gets a welcome boost. Dr. O’Connor was a coinvestigator in PAL-HF, a single-center study presented at the 2016 annual meeting of the Heart Failure Society of America.
“This is a very important trial of palliative care in advanced heart failure. We probably don’t have as much evidence in this space as we should,” he observed. “This was a multidisciplinary intervention in which we gave the patients a medical tool kit to alleviate pain, dyspnea, and discomfort. The tool kit included benzodiazepines, sleep medications, sublingual nitroglycerin, and morphinelike products, all very carefully monitored by staff coordinators.”
The primary outcome was change in two validated heart failure quality of life measures. Both instruments documented significant improvement compared with usual care.
“There was no decrease in mortality, which wasn’t a goal in this advanced heart failure population, and no reduction in heart failure hospitalizations, but there were significant reductions in depression and anxiety,” Dr. O’Connor said.
• Vericiguat. This oral soluble cyclic guanylate cyclase stimulator missed its primary endpoint in the phase II dose-escalation SOCRATES-REDUCED trial in patients with HFrEF (JAMA. 2015 Dec 1;314[21]:2251-62), but showed an impressive improvement in quality of life. It is now the subject of the ongoing, randomized, phase III VICTORIA trial involving a planned 4,000 patients with HFrEF with the composite primary endpoint of cardiovascular death or heart failure hospitalization.
The phase II SOCRATES-PRESERVED trial also missed its primary endpoint but showed a clinically meaningful improvement in quality of life in patients with heart failure with preserved ejection fraction (HFpEF) (Eur Heart J. 2017 Mar 22. doi: 10.1093/eurheartj/ehw593). Discussions are ongoing as to whether the next step should be a confirmatory phase II study or a move straight to phase III.
The bad news
• NSAIDs linked to increased risk of heart failure. European investigators analyzed five population-based databases totaling more than 8.3 million individuals and determined that current use of any of more than two dozen NSAIDs was associated with significantly increased risk of hospital admission for heart failure. The risk appeared to be dose dependent and varied between individual agents, ranging from a 16% increased risk with naproxen to an 83% increase with ketorolac (Toradol) (BMJ. 2016 Sep 28. doi: 10.1136/bmj.4857).
• Therapeutic natriuretic peptides hit bottom. The negative results for the investigational agent ularitide in patients with acute decompensated heart failure in the large phase III TRUE-AHF trial presented at the 2016 meeting of the American Heart Association, following upon an earlier negative study of the related drug nesiritide (Natrecor) in more than 7,100 acute heart failure patients (N Engl J Med. 2011 Jul 7; 365:32-43), probably spells the end of the line for this strategy of boosting outcomes in acute heart failure, according to Dr. O’Connor.
Moreover, Novartis has announced that the phase III RELAX-AHF-2 trial of serelaxin in 6,600 patients with acute heart failure failed to meet its primary endpoints of reduced cardiovascular deaths or reduced worsening of heart failure. The trial will be formally presented later this year.
“Ularitide seemed to show an early improvement in heart failure events that was not sustained in-hospital, and there was absolutely no difference in mortality. The drug probably acts like a pharmacologic tourniquet, in my view. So I think this field of therapeutic natriuretic peptides is probably closed,” he said.
• ICDs don’t reduce mortality in patients with nonischemic heart failure. This was the conclusion reached in the DANISH trial, in which more than 1,100 patients with symptomatic systolic heart failure were randomized to an ICD or usual care (N Engl J Med. 2016 Sep 29;375[13]:1221-30).
“This study really shook up the field, raising the question, ‘Are we using defibrillators too frequently in this population?’ It has stimulated a lot of discussion, including within the guidelines committee,” according to Dr. O’Connor.
• Tolvaptan nixed for acute decompensated heart failure. The TACTICS-HF trial studied the use of tolvaptan (Samsca), an oral vasopressin-2 receptor antagonist, to reduce dyspnea in patients hospitalized with acute decompensated heart failure. Dr. O’Connor was a coinvestigator in the study, which showed that tolvaptan was no better than placebo at 8 and 24 hours (J Am Coll Cardiol. 2017 Mar 21;69[11]:1399-406).
“For now, the routine use of vasopressin antagonists in acute heart failure is not to be encouraged, although there may still be subsets where it’s worth trying – certainly in severe hyponatremia,” the cardiologist said.
• GUIDE-IT gets lost. This was a roughly 1,000-patient randomized trial of a treatment strategy aimed at improving clinical outcomes by aggressively titrating evidence-based heart failure therapies in order to suppress natriuretic peptide biomarkers. GUIDE-IT was stopped early by the data safety monitoring board due to a lack of discernible difference in outcomes, compared with usual care. Dr. O’Connor, a coinvestigator, termed the soon-to-be-published study “a disappointment.”
Sleep apnea studies show silver lining
Sleep apnea is common in patients with heart failure and is associated with worse clinical heart failure outcomes. But in the large, randomized SERVE-HF trial, investigators showed that treatment of central sleep apnea with adaptive servo-ventilation in patients with HFrEF unexpectedly increased mortality, compared with optimal medical therapy (N Engl J Med. 2015 Sep 17;373:1095-105). In a more recent secondary analysis, however, the SERVE-HF investigators found that the increased risk for cardiovascular death associated with adaptive servo-ventilation was actually restricted to patients with a very poor left ventricular ejection fraction of 30% or less, and there was a signal of possible benefit in patients in the top tier of LVEF, at 36%-45% (Lancet Respir Med. 2016 Nov; 4[11]:873-81).
This observation dovetails nicely with the findings of the CAT-HF trial presented by Dr. O’Connor at the 2016 World Congress on Heart Failure. In this phase II study, 215 patients with acute decompensated heart failure and either obstructive or central sleep apnea were randomized to optimal medical therapy alone or in combination with adaptive servo-ventilation. There was no overall difference in outcome between the two groups; however, in the subgroup of patients with HFpEF, the risk of the primary composite endpoint was reduced by 62% with adaptive servo-ventilation.
As a result of these intriguing findings from SERVE-HF and CAT-HF, a registry and/or randomized clinical trial of adaptive servo-ventilation in patients with HFpEF is now being considered under NIH sponsorship, according to Dr. O’Connor.
He reported having no financial conflicts.
WASHINGTON – It’s been a “relatively positive” year for heart failure research and advances in patient care, Christopher M. O’Connor, MD, said at the annual meeting of the American College of Cardiology.
“Having been in this field for 30 years looking at clinical trials, generally for every 10 important trials done in a year, 1 has been positive and 9 have been negative; if we look at the past year, it’s more like 5 and 6. So, not a bad year for cardiomyopathy,” declared Dr. O’Connor, CEO and executive director of the Inova Heart and Vascular Institute in Falls Church, Va., and president-elect of the Heart Failure Society of America.
The good news
• Empagliflozin (Jardiance) earns FDA approval for reduction in risk of cardiovascular death in type 2 diabetes patients. “This is one of the most amazing stories in heart failure,” said Dr. O’Connor, who is also professor of medicine at Duke University in Durham, N.C.
The pivotal EMPA-REG OUTCOME study showed a highly significant 35% reduction in the secondary endpoint of risk of hospitalization for heart failure, as well the decrease in cardiovascular mortality which was the primary endpoint and proved persuasive to the FDA (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
“It was a remarkable development. Because of this trial, there are now a number of ongoing phase III clinical trials looking at this class of drugs in heart failure patients with and without diabetes, which makes this a very important research movement. We are now looking deeper at phenotypes and trying to get more specific with these drug therapies,” he said.
• A new and improved LVAD. This fully magnetically levitated centrifugal-flow pump type of left ventricular assist device for advanced heart failure showed superior event-free survival, compared with a commercially available axial continuous-flow pump LVAD in the randomized MOMENTUM-3 trial (N Engl J Med. 2017 Feb 2;376[5]:440-50).
The novel pump was designed to overcome a significant problem with axial continuous-flow LVADs: a proclivity for pump thrombosis. The magnetically levitated centrifugal-flow pump proved a smashing success in this regard, with zero cases of pump thrombosis occurring during the 6-month study.
“This may be the first time in the history of heart failure research that the engineers have beaten the biologists in important clinical outcomes,” the cardiologist quipped.
• Omecamtiv mecarbil successfully addresses impaired contractility in heart failure with reduced ejection fraction (HFrEF). This drug, a selective cardiac myosin activator, resulted in increased duration of systole and improved stroke volume accompanied by reductions in heart rate, left ventricular end-diastolic and -systolic dimensions, and NT-proBNP in the 87-site, 13-country, phase II COSMIC-HF study (Lancet. 2016 Dec 10;388[10062]:2895-903).
“This is probably the most novel new drug mechanism out there in clinical trials,” according to Dr. O’Connor.
On the basis of the highly encouraging results for the surrogate endpoints assessed in COSMIC-HF, a large phase III clinical trial known as GALACTIC is underway.
• Palliative care gets a welcome boost. Dr. O’Connor was a coinvestigator in PAL-HF, a single-center study presented at the 2016 annual meeting of the Heart Failure Society of America.
“This is a very important trial of palliative care in advanced heart failure. We probably don’t have as much evidence in this space as we should,” he observed. “This was a multidisciplinary intervention in which we gave the patients a medical tool kit to alleviate pain, dyspnea, and discomfort. The tool kit included benzodiazepines, sleep medications, sublingual nitroglycerin, and morphinelike products, all very carefully monitored by staff coordinators.”
The primary outcome was change in two validated heart failure quality of life measures. Both instruments documented significant improvement compared with usual care.
“There was no decrease in mortality, which wasn’t a goal in this advanced heart failure population, and no reduction in heart failure hospitalizations, but there were significant reductions in depression and anxiety,” Dr. O’Connor said.
• Vericiguat. This oral soluble cyclic guanylate cyclase stimulator missed its primary endpoint in the phase II dose-escalation SOCRATES-REDUCED trial in patients with HFrEF (JAMA. 2015 Dec 1;314[21]:2251-62), but showed an impressive improvement in quality of life. It is now the subject of the ongoing, randomized, phase III VICTORIA trial involving a planned 4,000 patients with HFrEF with the composite primary endpoint of cardiovascular death or heart failure hospitalization.
The phase II SOCRATES-PRESERVED trial also missed its primary endpoint but showed a clinically meaningful improvement in quality of life in patients with heart failure with preserved ejection fraction (HFpEF) (Eur Heart J. 2017 Mar 22. doi: 10.1093/eurheartj/ehw593). Discussions are ongoing as to whether the next step should be a confirmatory phase II study or a move straight to phase III.
The bad news
• NSAIDs linked to increased risk of heart failure. European investigators analyzed five population-based databases totaling more than 8.3 million individuals and determined that current use of any of more than two dozen NSAIDs was associated with significantly increased risk of hospital admission for heart failure. The risk appeared to be dose dependent and varied between individual agents, ranging from a 16% increased risk with naproxen to an 83% increase with ketorolac (Toradol) (BMJ. 2016 Sep 28. doi: 10.1136/bmj.4857).
• Therapeutic natriuretic peptides hit bottom. The negative results for the investigational agent ularitide in patients with acute decompensated heart failure in the large phase III TRUE-AHF trial presented at the 2016 meeting of the American Heart Association, following upon an earlier negative study of the related drug nesiritide (Natrecor) in more than 7,100 acute heart failure patients (N Engl J Med. 2011 Jul 7; 365:32-43), probably spells the end of the line for this strategy of boosting outcomes in acute heart failure, according to Dr. O’Connor.
Moreover, Novartis has announced that the phase III RELAX-AHF-2 trial of serelaxin in 6,600 patients with acute heart failure failed to meet its primary endpoints of reduced cardiovascular deaths or reduced worsening of heart failure. The trial will be formally presented later this year.
“Ularitide seemed to show an early improvement in heart failure events that was not sustained in-hospital, and there was absolutely no difference in mortality. The drug probably acts like a pharmacologic tourniquet, in my view. So I think this field of therapeutic natriuretic peptides is probably closed,” he said.
• ICDs don’t reduce mortality in patients with nonischemic heart failure. This was the conclusion reached in the DANISH trial, in which more than 1,100 patients with symptomatic systolic heart failure were randomized to an ICD or usual care (N Engl J Med. 2016 Sep 29;375[13]:1221-30).
“This study really shook up the field, raising the question, ‘Are we using defibrillators too frequently in this population?’ It has stimulated a lot of discussion, including within the guidelines committee,” according to Dr. O’Connor.
• Tolvaptan nixed for acute decompensated heart failure. The TACTICS-HF trial studied the use of tolvaptan (Samsca), an oral vasopressin-2 receptor antagonist, to reduce dyspnea in patients hospitalized with acute decompensated heart failure. Dr. O’Connor was a coinvestigator in the study, which showed that tolvaptan was no better than placebo at 8 and 24 hours (J Am Coll Cardiol. 2017 Mar 21;69[11]:1399-406).
“For now, the routine use of vasopressin antagonists in acute heart failure is not to be encouraged, although there may still be subsets where it’s worth trying – certainly in severe hyponatremia,” the cardiologist said.
• GUIDE-IT gets lost. This was a roughly 1,000-patient randomized trial of a treatment strategy aimed at improving clinical outcomes by aggressively titrating evidence-based heart failure therapies in order to suppress natriuretic peptide biomarkers. GUIDE-IT was stopped early by the data safety monitoring board due to a lack of discernible difference in outcomes, compared with usual care. Dr. O’Connor, a coinvestigator, termed the soon-to-be-published study “a disappointment.”
Sleep apnea studies show silver lining
Sleep apnea is common in patients with heart failure and is associated with worse clinical heart failure outcomes. But in the large, randomized SERVE-HF trial, investigators showed that treatment of central sleep apnea with adaptive servo-ventilation in patients with HFrEF unexpectedly increased mortality, compared with optimal medical therapy (N Engl J Med. 2015 Sep 17;373:1095-105). In a more recent secondary analysis, however, the SERVE-HF investigators found that the increased risk for cardiovascular death associated with adaptive servo-ventilation was actually restricted to patients with a very poor left ventricular ejection fraction of 30% or less, and there was a signal of possible benefit in patients in the top tier of LVEF, at 36%-45% (Lancet Respir Med. 2016 Nov; 4[11]:873-81).
This observation dovetails nicely with the findings of the CAT-HF trial presented by Dr. O’Connor at the 2016 World Congress on Heart Failure. In this phase II study, 215 patients with acute decompensated heart failure and either obstructive or central sleep apnea were randomized to optimal medical therapy alone or in combination with adaptive servo-ventilation. There was no overall difference in outcome between the two groups; however, in the subgroup of patients with HFpEF, the risk of the primary composite endpoint was reduced by 62% with adaptive servo-ventilation.
As a result of these intriguing findings from SERVE-HF and CAT-HF, a registry and/or randomized clinical trial of adaptive servo-ventilation in patients with HFpEF is now being considered under NIH sponsorship, according to Dr. O’Connor.
He reported having no financial conflicts.
EXPERT ANALYSIS FROM ACC 17
Procalcitonin guidance improves antibiotic stewardship
The case
A 72-year-old male with COPD presents to the emergency department with increased dyspnea and cough. He is afebrile, and oxygen saturation is 87% on room air. WBC count is 9.5 with a normal differential, and chest x-ray is read by the radiologist as atelectasis versus early consolidation in the left lower lobe. Should antibiotics be initiated?
Background
The problem: Antibiotic overuse
With the increasing prevalence of antibiotic resistance in our nation’s hospitals, the need for robust antibiotic stewardship programs has continued to rise in importance. In 2016, the CDC reported a fatal case of septic shock due to a carbapenem-resistant strain of Klebsiella resistant to all tested antibiotics.1 This case received much media coverage; moreover, this patient represented only one of the approximately 23,000 patients infected with antibiotic-resistant bacteria in the United States who die each year. Although various approaches to curbing antibiotic resistance are being pursued, judicious antibiotic use is central to success. Current evidence suggests that up to 30% of antibiotics are not optimally prescribed,2 leaving a significant opportunity for improvement.
Lower respiratory infections account for a substantial proportion of antibiotic utilization in the United States. In a recent study, acute respiratory conditions generated 221 antibiotic prescriptions per 1,000 population, but only half of these were deemed appropriate.2 The inability to reliably discern viral from bacterial etiology is a driver of excess antibiotic use.
The procalcitonin assay has been touted as a possible solution to this problem. Multiple studies have evaluated its utility as a tool to help discriminate between bacterial infection and viral or noninfectious etiologies.
What is procalcitonin?
Thyroidal c-cells convert the prohormone procalcitonin to calcitonin, which is stored in secretory granules for release in response to fluctuations in calcium levels via a classical neuroendocrine feedback loop. Alternatively, procalcitonin can be synthesized in nonthyroidal parenchymal cells, and high levels of proinflammatory mediators secreted in response to bacterial endotoxin drive increased procalcitonin production. Interestingly, interferon gamma, up-regulated in viral infections, reduces procalcitonin production. Nonthyroidal parenchymal cells lack mechanisms for efficient conversion of procalcitonin to calcitonin and do not contain secretory granules to facilitate its regulated release. Hence bacterial infections correlate with higher serum procalcitonin levels.3
Evidence
Can procalcitonin guide antibiotic therapy in patients with acute respiratory illness while reducing antibiotic utilization?
The ability of procalcitonin to selectively identify bacterial infection makes it a potentially promising tool to advance the antibiotic stewardship agenda. Multiple randomized controlled trials have explored the use of procalcitonin-guided antibiotic therapy for treatment of lower respiratory tract infections such as acute bronchitis, exacerbations of COPD, and pneumonia. Each study discussed below was done in Switzerland, involved the same key investigator (Mirjam Christ-Crain, MD, PhD), and shared a similar design in which a threshold for low procalcitonin values (less than 0.1 mcg/L) and high procalcitonin values (greater than 0.25 mcg/L) was prespecified. Antibiotic therapy was strongly discouraged for patients with low procalcitonin and encouraged for those with high procalcitonin; antibiotics were not recommended for patients with intermediate values, but the treating physician was allowed ultimate discretion (Figure 1). All studies compared a procalcitonin-guided treatment group to a standard care group, in which antibiotics were prescribed by the treating physician based on established clinical guidelines.
Figure 1. Procalcitonin treatment algorithm
Procalcitonin Level (mcg/L) | Likelihood of bacterial infection | Antibiotic treatment |
less than 0.1 | Absent | Strongly discouraged |
0.1-0.25 | Unlikely | Discouraged |
0.25-0.5 | Possible | Encouraged |
greater than 0.5 | Present | Strongly encouraged |
Figure 1. Procalcitonin treatment algorithm
In a study focusing on outpatients presenting to their primary care physicians with acute respiratory tract infection, 53 primary care physicians in Switzerland recruited 458 patients. There was no significant difference in time to symptom resolution, as determined by patient report during an interview 14 days after initial presentation; however, 97% of patients in the standard-care group received antibiotics, compared with 25% in the procalcitonin-guided group. Equal numbers of patients (30% in each group) reported persistent symptoms at 28-day follow-up. Among the cohort of patients with upper respiratory infections or acute bronchitis, procalcitonin guidance reduced antibiotic prescriptions by 80%.4
In a blinded, single-center, randomized, controlled trial of 226 patients presenting to a university hospital with a COPD exacerbation severe enough to require a change in the baseline medication regimen, procalcitonin-guided therapy allowed for an absolute reduction of antibiotic use by 32% without an impact on outcomes. Rates of clinical improvement, ICU utilization, recurrent exacerbations, hospital length of stay, and mortality did not differ between the groups.5
Limitations include the possible impact of the Hawthorne effect, as physicians knew their antibiotic usage patterns were being monitored, which may impact generalizability of the findings to a real-world setting. Similarly, it is difficult to control for a spillover effect as providers exposed to the procalcitonin-guided algorithm became more comfortable with a restrictive prescribing approach. The costs of the additional procalcitonin assay must be weighed against the benefits. Incidence and cost of other adverse effects of antibiotic use (rates of Clostridium difficile, renal insufficiency, urticarial drug eruptions, etc.) were not addressed. The rapid assay currently has limited availability in the United States, though that is changing. Finally, recent additional studies (unrelated to procalcitonin) have suggested shorter antibiotic treatment durations for patients with pneumonia.8
Is there evidence for using procalcitonin to guide treatment in the broader population of ICU patients?
While there is good evidence for using procalcitonin to guide antibiotic use in patients with acute respiratory illness, the evidence for using procalcitonin in the broader cohort of critically-ill patients with sepsis is less well established.
The most promising results were reported by the Stop Antibiotics on Procalcitonin guidance Study (SAPS). Published in July 2016, this was a prospective, multicenter, randomized, controlled, open-label study of patients admitted to the ICU (not limited to respiratory illness) in the Netherlands. A total of 1,575 patients were assigned to the procalcitonin-guided group or the standard-of-care group. In the procalcitonin-guided group, procalcitonin levels were checked daily, and physicians were given nonbinding advice to discontinue antibiotics if procalcitonin levels decreased by greater than 80% from peak levels or to below 0.5 mcg/L.
Patients received an average of 7.5 daily defined antibiotic doses in the procalcitonin-guided group versus 9.3 daily defined doses in the standard-of-care group (P less than .0001). The median duration of antibiotic treatment in the procalcitonin arm was 5 days versus 7 days in the control group (P less than .0001). Mortality at 28 days was 20% in the procalcitonin group and 25% in the control group (P = .0122). At 1 year, mortality was 36% in the procalcitonin group and 43% in the control group (P = .0188). The authors hypothesized that the unexpected decrease in mortality in the procalcitonin group may have been due to earlier consideration of alternate illness etiologies in patients with a low procalcitonin level or decreased antibiotic side effects.9While the SAPS trial supports decreased antibiotic usage in ICU patients with the use of the procalcitonin assay, there are some important limitations. First, the trial was done in the Netherlands, where baseline antibiotic usage was comparatively low. Second, daily procalcitonin level monitoring was not continued for patients transferred out of the ICU while still on antibiotics. Further, guidelines for antibiotic discontinuation were nonbinding, and in many cases physicians did not stop antibiotics based on procalcitonin guidelines suggested by the study authors.
Earlier trials regarding the procalcitonin assay in the critical care setting similarly showed some promise but also concerns. One trial reported a 25% reduction in antibiotic exposure and noninferiority of 28-day mortality, but there was a nonsignificant 3.8% absolute increase in mortality at 60 days.10 Another trial reported similar survival in the procalcitonin group but more side effects and longer ICU stays.11Ultimately, while the SAPS trial supports the potential use of procalcitonin in critically-ill patients, these patients likely have complex sepsis physiology that requires clinicians to consider a number of clinical factors when making antibiotic decisions.
Back to the case
The case illustrates a common emergency department presentation where clinical and radiographic features are not convincing for bacterial infection. This patient has an acute respiratory illness, but is afebrile and lacks leukocytosis with left shift, and x-rays are indeterminate for pneumonia. The differential diagnosis also includes COPD exacerbation, viral infection, or noninfectious triggers of dyspnea.
In this scenario, obtaining procalcitonin levels is useful in the decision to initiate or withhold antibiotic treatment. An elevated procalcitonin level suggests a bacterial infection and would favor initiation of antibiotics for pneumonia. A low procalcitonin level makes a bacterial infection less likely, and a clinician may consider withholding antibiotics and consider alternate etiologies for the patient’s presentation.
Bottom line
Procalcitonin can be safely used to guide the decision to initiate antibiotics in patients presenting with acute respiratory illness. Use of the procalcitonin assay has been shown to reduce antibiotic utilization without an increase in adverse outcomes. There is potential but less conclusive evidence for procalcitonin usage in the broader population of ICU patients with sepsis.
Bryan J. Huang, MD, FHM, and Gregory B. Seymann, MD, SFHM, are in the division of hospital medicine, University of California, San Diego.
- Key Points
- Elevated procalcitonin levels suggest the presence of bacterial infection.
- In patients presenting with acute respiratory illness, procalcitonin levels can be used to guide the decision to initiate or withhold antibiotics, improving antibiotic stewardship.
- Sequential monitoring of procalcitonin levels may help guide duration of antibiotic therapy.
- There is potential but less conclusive evidence for procalcitonin usage in the broader population of ICU patients with sepsis.
References
1. Chen L, Todd R, Kiehlbauch J, Walters M, Kallen A. Notes from the field: pan-resistant New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae – Washoe County, Nevada, 2016. MMWR Morb Mortal Wkly Rep 2017; 66(1):33.
2. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016;315(17):1864-73.
3. Christ-Crain M, Muller B. Procalcitonin in bacterial infections – hype, hope, more or less? Swiss Med Wkly. 2005;135(31-32):451-60.
4. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs. a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008; 168(18): 2000-7.
5. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007;131(1): 9-19.
6. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84-93.
7. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302(10): 1059-66.
8. Uranga A, Espana PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-65.
9. de Jong E, van Oers JA, Beishiozen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27.
10. Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375(9713):463-74.
11. Jensen J-U, Lundgren B, Hein L, et al. The procalcitonin and survival study (PASS) – a randomised multicenter investigator-initiated trial to investigate whether daily measurements biomarker procalcitonin and proactive diagnostic and therapeutic responses to abnormal procalcitonin levels, can improve survival in intensive care unit patients. BMC infectious diseases. 2008;8:91-100.
1. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009;302(10):1059-66.
2. de Jong E, van Oers JA, Beishiozen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27.
3. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
The case
A 72-year-old male with COPD presents to the emergency department with increased dyspnea and cough. He is afebrile, and oxygen saturation is 87% on room air. WBC count is 9.5 with a normal differential, and chest x-ray is read by the radiologist as atelectasis versus early consolidation in the left lower lobe. Should antibiotics be initiated?
Background
The problem: Antibiotic overuse
With the increasing prevalence of antibiotic resistance in our nation’s hospitals, the need for robust antibiotic stewardship programs has continued to rise in importance. In 2016, the CDC reported a fatal case of septic shock due to a carbapenem-resistant strain of Klebsiella resistant to all tested antibiotics.1 This case received much media coverage; moreover, this patient represented only one of the approximately 23,000 patients infected with antibiotic-resistant bacteria in the United States who die each year. Although various approaches to curbing antibiotic resistance are being pursued, judicious antibiotic use is central to success. Current evidence suggests that up to 30% of antibiotics are not optimally prescribed,2 leaving a significant opportunity for improvement.
Lower respiratory infections account for a substantial proportion of antibiotic utilization in the United States. In a recent study, acute respiratory conditions generated 221 antibiotic prescriptions per 1,000 population, but only half of these were deemed appropriate.2 The inability to reliably discern viral from bacterial etiology is a driver of excess antibiotic use.
The procalcitonin assay has been touted as a possible solution to this problem. Multiple studies have evaluated its utility as a tool to help discriminate between bacterial infection and viral or noninfectious etiologies.
What is procalcitonin?
Thyroidal c-cells convert the prohormone procalcitonin to calcitonin, which is stored in secretory granules for release in response to fluctuations in calcium levels via a classical neuroendocrine feedback loop. Alternatively, procalcitonin can be synthesized in nonthyroidal parenchymal cells, and high levels of proinflammatory mediators secreted in response to bacterial endotoxin drive increased procalcitonin production. Interestingly, interferon gamma, up-regulated in viral infections, reduces procalcitonin production. Nonthyroidal parenchymal cells lack mechanisms for efficient conversion of procalcitonin to calcitonin and do not contain secretory granules to facilitate its regulated release. Hence bacterial infections correlate with higher serum procalcitonin levels.3
Evidence
Can procalcitonin guide antibiotic therapy in patients with acute respiratory illness while reducing antibiotic utilization?
The ability of procalcitonin to selectively identify bacterial infection makes it a potentially promising tool to advance the antibiotic stewardship agenda. Multiple randomized controlled trials have explored the use of procalcitonin-guided antibiotic therapy for treatment of lower respiratory tract infections such as acute bronchitis, exacerbations of COPD, and pneumonia. Each study discussed below was done in Switzerland, involved the same key investigator (Mirjam Christ-Crain, MD, PhD), and shared a similar design in which a threshold for low procalcitonin values (less than 0.1 mcg/L) and high procalcitonin values (greater than 0.25 mcg/L) was prespecified. Antibiotic therapy was strongly discouraged for patients with low procalcitonin and encouraged for those with high procalcitonin; antibiotics were not recommended for patients with intermediate values, but the treating physician was allowed ultimate discretion (Figure 1). All studies compared a procalcitonin-guided treatment group to a standard care group, in which antibiotics were prescribed by the treating physician based on established clinical guidelines.
Figure 1. Procalcitonin treatment algorithm
Procalcitonin Level (mcg/L) | Likelihood of bacterial infection | Antibiotic treatment |
less than 0.1 | Absent | Strongly discouraged |
0.1-0.25 | Unlikely | Discouraged |
0.25-0.5 | Possible | Encouraged |
greater than 0.5 | Present | Strongly encouraged |
Figure 1. Procalcitonin treatment algorithm
In a study focusing on outpatients presenting to their primary care physicians with acute respiratory tract infection, 53 primary care physicians in Switzerland recruited 458 patients. There was no significant difference in time to symptom resolution, as determined by patient report during an interview 14 days after initial presentation; however, 97% of patients in the standard-care group received antibiotics, compared with 25% in the procalcitonin-guided group. Equal numbers of patients (30% in each group) reported persistent symptoms at 28-day follow-up. Among the cohort of patients with upper respiratory infections or acute bronchitis, procalcitonin guidance reduced antibiotic prescriptions by 80%.4
In a blinded, single-center, randomized, controlled trial of 226 patients presenting to a university hospital with a COPD exacerbation severe enough to require a change in the baseline medication regimen, procalcitonin-guided therapy allowed for an absolute reduction of antibiotic use by 32% without an impact on outcomes. Rates of clinical improvement, ICU utilization, recurrent exacerbations, hospital length of stay, and mortality did not differ between the groups.5
Limitations include the possible impact of the Hawthorne effect, as physicians knew their antibiotic usage patterns were being monitored, which may impact generalizability of the findings to a real-world setting. Similarly, it is difficult to control for a spillover effect as providers exposed to the procalcitonin-guided algorithm became more comfortable with a restrictive prescribing approach. The costs of the additional procalcitonin assay must be weighed against the benefits. Incidence and cost of other adverse effects of antibiotic use (rates of Clostridium difficile, renal insufficiency, urticarial drug eruptions, etc.) were not addressed. The rapid assay currently has limited availability in the United States, though that is changing. Finally, recent additional studies (unrelated to procalcitonin) have suggested shorter antibiotic treatment durations for patients with pneumonia.8
Is there evidence for using procalcitonin to guide treatment in the broader population of ICU patients?
While there is good evidence for using procalcitonin to guide antibiotic use in patients with acute respiratory illness, the evidence for using procalcitonin in the broader cohort of critically-ill patients with sepsis is less well established.
The most promising results were reported by the Stop Antibiotics on Procalcitonin guidance Study (SAPS). Published in July 2016, this was a prospective, multicenter, randomized, controlled, open-label study of patients admitted to the ICU (not limited to respiratory illness) in the Netherlands. A total of 1,575 patients were assigned to the procalcitonin-guided group or the standard-of-care group. In the procalcitonin-guided group, procalcitonin levels were checked daily, and physicians were given nonbinding advice to discontinue antibiotics if procalcitonin levels decreased by greater than 80% from peak levels or to below 0.5 mcg/L.
Patients received an average of 7.5 daily defined antibiotic doses in the procalcitonin-guided group versus 9.3 daily defined doses in the standard-of-care group (P less than .0001). The median duration of antibiotic treatment in the procalcitonin arm was 5 days versus 7 days in the control group (P less than .0001). Mortality at 28 days was 20% in the procalcitonin group and 25% in the control group (P = .0122). At 1 year, mortality was 36% in the procalcitonin group and 43% in the control group (P = .0188). The authors hypothesized that the unexpected decrease in mortality in the procalcitonin group may have been due to earlier consideration of alternate illness etiologies in patients with a low procalcitonin level or decreased antibiotic side effects.9While the SAPS trial supports decreased antibiotic usage in ICU patients with the use of the procalcitonin assay, there are some important limitations. First, the trial was done in the Netherlands, where baseline antibiotic usage was comparatively low. Second, daily procalcitonin level monitoring was not continued for patients transferred out of the ICU while still on antibiotics. Further, guidelines for antibiotic discontinuation were nonbinding, and in many cases physicians did not stop antibiotics based on procalcitonin guidelines suggested by the study authors.
Earlier trials regarding the procalcitonin assay in the critical care setting similarly showed some promise but also concerns. One trial reported a 25% reduction in antibiotic exposure and noninferiority of 28-day mortality, but there was a nonsignificant 3.8% absolute increase in mortality at 60 days.10 Another trial reported similar survival in the procalcitonin group but more side effects and longer ICU stays.11Ultimately, while the SAPS trial supports the potential use of procalcitonin in critically-ill patients, these patients likely have complex sepsis physiology that requires clinicians to consider a number of clinical factors when making antibiotic decisions.
Back to the case
The case illustrates a common emergency department presentation where clinical and radiographic features are not convincing for bacterial infection. This patient has an acute respiratory illness, but is afebrile and lacks leukocytosis with left shift, and x-rays are indeterminate for pneumonia. The differential diagnosis also includes COPD exacerbation, viral infection, or noninfectious triggers of dyspnea.
In this scenario, obtaining procalcitonin levels is useful in the decision to initiate or withhold antibiotic treatment. An elevated procalcitonin level suggests a bacterial infection and would favor initiation of antibiotics for pneumonia. A low procalcitonin level makes a bacterial infection less likely, and a clinician may consider withholding antibiotics and consider alternate etiologies for the patient’s presentation.
Bottom line
Procalcitonin can be safely used to guide the decision to initiate antibiotics in patients presenting with acute respiratory illness. Use of the procalcitonin assay has been shown to reduce antibiotic utilization without an increase in adverse outcomes. There is potential but less conclusive evidence for procalcitonin usage in the broader population of ICU patients with sepsis.
Bryan J. Huang, MD, FHM, and Gregory B. Seymann, MD, SFHM, are in the division of hospital medicine, University of California, San Diego.
- Key Points
- Elevated procalcitonin levels suggest the presence of bacterial infection.
- In patients presenting with acute respiratory illness, procalcitonin levels can be used to guide the decision to initiate or withhold antibiotics, improving antibiotic stewardship.
- Sequential monitoring of procalcitonin levels may help guide duration of antibiotic therapy.
- There is potential but less conclusive evidence for procalcitonin usage in the broader population of ICU patients with sepsis.
References
1. Chen L, Todd R, Kiehlbauch J, Walters M, Kallen A. Notes from the field: pan-resistant New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae – Washoe County, Nevada, 2016. MMWR Morb Mortal Wkly Rep 2017; 66(1):33.
2. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016;315(17):1864-73.
3. Christ-Crain M, Muller B. Procalcitonin in bacterial infections – hype, hope, more or less? Swiss Med Wkly. 2005;135(31-32):451-60.
4. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs. a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008; 168(18): 2000-7.
5. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007;131(1): 9-19.
6. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84-93.
7. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302(10): 1059-66.
8. Uranga A, Espana PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-65.
9. de Jong E, van Oers JA, Beishiozen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27.
10. Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375(9713):463-74.
11. Jensen J-U, Lundgren B, Hein L, et al. The procalcitonin and survival study (PASS) – a randomised multicenter investigator-initiated trial to investigate whether daily measurements biomarker procalcitonin and proactive diagnostic and therapeutic responses to abnormal procalcitonin levels, can improve survival in intensive care unit patients. BMC infectious diseases. 2008;8:91-100.
1. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009;302(10):1059-66.
2. de Jong E, van Oers JA, Beishiozen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27.
3. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
The case
A 72-year-old male with COPD presents to the emergency department with increased dyspnea and cough. He is afebrile, and oxygen saturation is 87% on room air. WBC count is 9.5 with a normal differential, and chest x-ray is read by the radiologist as atelectasis versus early consolidation in the left lower lobe. Should antibiotics be initiated?
Background
The problem: Antibiotic overuse
With the increasing prevalence of antibiotic resistance in our nation’s hospitals, the need for robust antibiotic stewardship programs has continued to rise in importance. In 2016, the CDC reported a fatal case of septic shock due to a carbapenem-resistant strain of Klebsiella resistant to all tested antibiotics.1 This case received much media coverage; moreover, this patient represented only one of the approximately 23,000 patients infected with antibiotic-resistant bacteria in the United States who die each year. Although various approaches to curbing antibiotic resistance are being pursued, judicious antibiotic use is central to success. Current evidence suggests that up to 30% of antibiotics are not optimally prescribed,2 leaving a significant opportunity for improvement.
Lower respiratory infections account for a substantial proportion of antibiotic utilization in the United States. In a recent study, acute respiratory conditions generated 221 antibiotic prescriptions per 1,000 population, but only half of these were deemed appropriate.2 The inability to reliably discern viral from bacterial etiology is a driver of excess antibiotic use.
The procalcitonin assay has been touted as a possible solution to this problem. Multiple studies have evaluated its utility as a tool to help discriminate between bacterial infection and viral or noninfectious etiologies.
What is procalcitonin?
Thyroidal c-cells convert the prohormone procalcitonin to calcitonin, which is stored in secretory granules for release in response to fluctuations in calcium levels via a classical neuroendocrine feedback loop. Alternatively, procalcitonin can be synthesized in nonthyroidal parenchymal cells, and high levels of proinflammatory mediators secreted in response to bacterial endotoxin drive increased procalcitonin production. Interestingly, interferon gamma, up-regulated in viral infections, reduces procalcitonin production. Nonthyroidal parenchymal cells lack mechanisms for efficient conversion of procalcitonin to calcitonin and do not contain secretory granules to facilitate its regulated release. Hence bacterial infections correlate with higher serum procalcitonin levels.3
Evidence
Can procalcitonin guide antibiotic therapy in patients with acute respiratory illness while reducing antibiotic utilization?
The ability of procalcitonin to selectively identify bacterial infection makes it a potentially promising tool to advance the antibiotic stewardship agenda. Multiple randomized controlled trials have explored the use of procalcitonin-guided antibiotic therapy for treatment of lower respiratory tract infections such as acute bronchitis, exacerbations of COPD, and pneumonia. Each study discussed below was done in Switzerland, involved the same key investigator (Mirjam Christ-Crain, MD, PhD), and shared a similar design in which a threshold for low procalcitonin values (less than 0.1 mcg/L) and high procalcitonin values (greater than 0.25 mcg/L) was prespecified. Antibiotic therapy was strongly discouraged for patients with low procalcitonin and encouraged for those with high procalcitonin; antibiotics were not recommended for patients with intermediate values, but the treating physician was allowed ultimate discretion (Figure 1). All studies compared a procalcitonin-guided treatment group to a standard care group, in which antibiotics were prescribed by the treating physician based on established clinical guidelines.
Figure 1. Procalcitonin treatment algorithm
Procalcitonin Level (mcg/L) | Likelihood of bacterial infection | Antibiotic treatment |
less than 0.1 | Absent | Strongly discouraged |
0.1-0.25 | Unlikely | Discouraged |
0.25-0.5 | Possible | Encouraged |
greater than 0.5 | Present | Strongly encouraged |
Figure 1. Procalcitonin treatment algorithm
In a study focusing on outpatients presenting to their primary care physicians with acute respiratory tract infection, 53 primary care physicians in Switzerland recruited 458 patients. There was no significant difference in time to symptom resolution, as determined by patient report during an interview 14 days after initial presentation; however, 97% of patients in the standard-care group received antibiotics, compared with 25% in the procalcitonin-guided group. Equal numbers of patients (30% in each group) reported persistent symptoms at 28-day follow-up. Among the cohort of patients with upper respiratory infections or acute bronchitis, procalcitonin guidance reduced antibiotic prescriptions by 80%.4
In a blinded, single-center, randomized, controlled trial of 226 patients presenting to a university hospital with a COPD exacerbation severe enough to require a change in the baseline medication regimen, procalcitonin-guided therapy allowed for an absolute reduction of antibiotic use by 32% without an impact on outcomes. Rates of clinical improvement, ICU utilization, recurrent exacerbations, hospital length of stay, and mortality did not differ between the groups.5
Limitations include the possible impact of the Hawthorne effect, as physicians knew their antibiotic usage patterns were being monitored, which may impact generalizability of the findings to a real-world setting. Similarly, it is difficult to control for a spillover effect as providers exposed to the procalcitonin-guided algorithm became more comfortable with a restrictive prescribing approach. The costs of the additional procalcitonin assay must be weighed against the benefits. Incidence and cost of other adverse effects of antibiotic use (rates of Clostridium difficile, renal insufficiency, urticarial drug eruptions, etc.) were not addressed. The rapid assay currently has limited availability in the United States, though that is changing. Finally, recent additional studies (unrelated to procalcitonin) have suggested shorter antibiotic treatment durations for patients with pneumonia.8
Is there evidence for using procalcitonin to guide treatment in the broader population of ICU patients?
While there is good evidence for using procalcitonin to guide antibiotic use in patients with acute respiratory illness, the evidence for using procalcitonin in the broader cohort of critically-ill patients with sepsis is less well established.
The most promising results were reported by the Stop Antibiotics on Procalcitonin guidance Study (SAPS). Published in July 2016, this was a prospective, multicenter, randomized, controlled, open-label study of patients admitted to the ICU (not limited to respiratory illness) in the Netherlands. A total of 1,575 patients were assigned to the procalcitonin-guided group or the standard-of-care group. In the procalcitonin-guided group, procalcitonin levels were checked daily, and physicians were given nonbinding advice to discontinue antibiotics if procalcitonin levels decreased by greater than 80% from peak levels or to below 0.5 mcg/L.
Patients received an average of 7.5 daily defined antibiotic doses in the procalcitonin-guided group versus 9.3 daily defined doses in the standard-of-care group (P less than .0001). The median duration of antibiotic treatment in the procalcitonin arm was 5 days versus 7 days in the control group (P less than .0001). Mortality at 28 days was 20% in the procalcitonin group and 25% in the control group (P = .0122). At 1 year, mortality was 36% in the procalcitonin group and 43% in the control group (P = .0188). The authors hypothesized that the unexpected decrease in mortality in the procalcitonin group may have been due to earlier consideration of alternate illness etiologies in patients with a low procalcitonin level or decreased antibiotic side effects.9While the SAPS trial supports decreased antibiotic usage in ICU patients with the use of the procalcitonin assay, there are some important limitations. First, the trial was done in the Netherlands, where baseline antibiotic usage was comparatively low. Second, daily procalcitonin level monitoring was not continued for patients transferred out of the ICU while still on antibiotics. Further, guidelines for antibiotic discontinuation were nonbinding, and in many cases physicians did not stop antibiotics based on procalcitonin guidelines suggested by the study authors.
Earlier trials regarding the procalcitonin assay in the critical care setting similarly showed some promise but also concerns. One trial reported a 25% reduction in antibiotic exposure and noninferiority of 28-day mortality, but there was a nonsignificant 3.8% absolute increase in mortality at 60 days.10 Another trial reported similar survival in the procalcitonin group but more side effects and longer ICU stays.11Ultimately, while the SAPS trial supports the potential use of procalcitonin in critically-ill patients, these patients likely have complex sepsis physiology that requires clinicians to consider a number of clinical factors when making antibiotic decisions.
Back to the case
The case illustrates a common emergency department presentation where clinical and radiographic features are not convincing for bacterial infection. This patient has an acute respiratory illness, but is afebrile and lacks leukocytosis with left shift, and x-rays are indeterminate for pneumonia. The differential diagnosis also includes COPD exacerbation, viral infection, or noninfectious triggers of dyspnea.
In this scenario, obtaining procalcitonin levels is useful in the decision to initiate or withhold antibiotic treatment. An elevated procalcitonin level suggests a bacterial infection and would favor initiation of antibiotics for pneumonia. A low procalcitonin level makes a bacterial infection less likely, and a clinician may consider withholding antibiotics and consider alternate etiologies for the patient’s presentation.
Bottom line
Procalcitonin can be safely used to guide the decision to initiate antibiotics in patients presenting with acute respiratory illness. Use of the procalcitonin assay has been shown to reduce antibiotic utilization without an increase in adverse outcomes. There is potential but less conclusive evidence for procalcitonin usage in the broader population of ICU patients with sepsis.
Bryan J. Huang, MD, FHM, and Gregory B. Seymann, MD, SFHM, are in the division of hospital medicine, University of California, San Diego.
- Key Points
- Elevated procalcitonin levels suggest the presence of bacterial infection.
- In patients presenting with acute respiratory illness, procalcitonin levels can be used to guide the decision to initiate or withhold antibiotics, improving antibiotic stewardship.
- Sequential monitoring of procalcitonin levels may help guide duration of antibiotic therapy.
- There is potential but less conclusive evidence for procalcitonin usage in the broader population of ICU patients with sepsis.
References
1. Chen L, Todd R, Kiehlbauch J, Walters M, Kallen A. Notes from the field: pan-resistant New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae – Washoe County, Nevada, 2016. MMWR Morb Mortal Wkly Rep 2017; 66(1):33.
2. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016;315(17):1864-73.
3. Christ-Crain M, Muller B. Procalcitonin in bacterial infections – hype, hope, more or less? Swiss Med Wkly. 2005;135(31-32):451-60.
4. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs. a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008; 168(18): 2000-7.
5. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007;131(1): 9-19.
6. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84-93.
7. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302(10): 1059-66.
8. Uranga A, Espana PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-65.
9. de Jong E, van Oers JA, Beishiozen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27.
10. Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375(9713):463-74.
11. Jensen J-U, Lundgren B, Hein L, et al. The procalcitonin and survival study (PASS) – a randomised multicenter investigator-initiated trial to investigate whether daily measurements biomarker procalcitonin and proactive diagnostic and therapeutic responses to abnormal procalcitonin levels, can improve survival in intensive care unit patients. BMC infectious diseases. 2008;8:91-100.
1. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009;302(10):1059-66.
2. de Jong E, van Oers JA, Beishiozen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27.
3. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
Uptake of new heart failure drugs slow despite guidelines
SNOWMASS, COLO. – As William T. Abraham, MD, speaks to colleagues around the country about heart failure therapy, he has noticed that the first-in-class drug ivabradine remains below the radar of most physicians.
“I’ve found that this is an agent that very few people know about, even though it’s been FDA [Food and Drug Administration] approved for about 3 years. It’s used fairly extensively in Europe because that’s where the pivotal SHIFT trial was done, but not very much in the United States,” according to Dr. Abraham, professor of medicine, physiology, and cell biology and director of the division of cardiovascular medicine at Ohio State University in Columbus.
That’s likely to change as word spreads about the May 2016 update of the American College of Cardiology/American Heart Association Guideline for the Management of Heart Failure. The update incorporated evidence-based recommendations on the use of two important new heart failure medications: ivabradine (Corlanor), which received a moderate class IIa recommendation, meaning the drug “should be considered,” and sacubitril/valsartan (Entresto), which received the strongest class I recommendation.
In the right patients, these two oral medications improve heart failure morbidity and mortality significantly beyond what’s achievable with what has been the gold standard, guideline-directed medical therapy. Dr. Abraham described how to get started using the two medications at the Annual Cardiovascular Conference at Snowmass.
Ivabradine
Ivabradine is a selective inhibitor of the sinoatrial pacemaker modulating I(f) current. It acts by slowing the sinus rate without reducing myocardial contractility.
“This agent does one thing and one thing alone: It lowers heart rate,” the cardiologist explained.
And that, he added, was sufficient to significantly reduce the risks of death due to heart failure and recurrent hospitalization for worsening heart failure in the pivotal SHIFT trial.
SHIFT included 6,505 patients with moderate to severe heart failure with reduced left ventricular ejection fraction (LVEF) and a resting heart rate above 70 bpm despite background guideline-directed medical therapy. Participants were randomized double blind to ivabradine titrated to a maximum of 7.5 mg twice daily or placebo and followed for a median of about 23 months. The rate of death due to heart failure was 3% with ivabradine and 5% with placebo, for a statistically significant 26% relative risk reduction favoring ivabradine.
But the drug’s main benefit was in reducing recurrent hospitalizations for heart failure, an endpoint of particular interest to health policy officials given that heart failure hospitalizations chew up a substantial proportion of the Medicare budget. Ivabradine reduced first hospitalizations for heart failure during the study period by 25%, second hospitalizations by 34%, and third hospitalizations by 29% (Eur Heart J. 2012 Nov;33[22]:2813-20).
The ACC/AHA guideline update stresses the importance of reserving ivabradine for heart failure patients whose resting heart rate exceeds 70 bpm, despite being on their maximum tolerated dose of a beta-blocker, Dr. Abraham noted.
Ivabradine is contraindicated in the setting of acute decompensated heart failure, severe liver disease, or hypotension, in patients on any of the numerous agents that strongly inhibit the enzyme cytochrome P450 3A4, and in those who have sick sinus syndrome, have sinoatrial block, or are pacemaker dependent.
Sacubitril/valsartan
Sacubitril inhibits neprilysin, an enzyme that blocks the action of endogenous vasoactive peptides including bradykinin, substance P, and natriuretic peptides, all of which counter important maladaptive mechanisms in heart failure. Sacubitril has been combined with the angiotensin receptor blocker valsartan to form the first-in-class angiotensin receptor neprilysin inhibitor, or ARNI, formerly known as LCZ696 and now marketed as Entresto.
In the pivotal double-blind PARADIGM-HF trial, 8,442 patients with heart failure with reduced ejection fraction were randomized to the ARNI at 200 mg b.i.d. or to enalapril at 10 mg b.i.d. on top of background guideline-directed medical therapy. The trial was stopped early because of evidence of overwhelming benefit: a 20% relative risk reduction in cardiovascular death and a 21% decrease in the risk of heart failure hospitalizations in the sacubitril/valsartan group, as well as significant reductions in heart failure symptoms and physical limitations (N Engl J Med. 2014 Sep 11;371[11]:993-1004).
The updated heart failure guidelines strongly recommend that patients with heart failure should be treated with either an ACE inhibitor, an angiotensin receptor blocker, or an ARNI. Further, patients who remain symptomatic on an ACE inhibitor or angiotensin receptor blocker should be switched to an ARNI; that’s a class Ib recommendation based upon the results of PARADIGM-HF.
In getting started using the ARNI, Dr. Abraham said it’s important to understand as background the selective nature of the PARADIGM-HF study design. During the single-blind run-in period of 5-8 weeks, roughly 10% of patients dropped out because they couldn’t tolerate enalapril at 10 mg b.i.d., and a similar percentage dropped out during the ARNI run-in. Thus, patients who couldn’t tolerate a low dose of an ACE inhibitor weren’t in the study. And patients capable of tolerating guideline-recommended full-dose ACE inhibitor therapy were not specifically sought for participation.
“So there are some unanswered questions about the ARNI. If you’re just getting started with this compound in treating your heart failure patients, my own feeling is you should maybe aim for the type of patient that was included in this trial: patients who could tolerate a moderate dose of an ACE inhibitor and had generally good blood pressure. That’s a great way to begin to get experience with this agent in heart failure,” the cardiologist advised.
He reported serving as a consultant to Abbott Vascular, Medtronic, Novartis, and St. Jude Medical.
SNOWMASS, COLO. – As William T. Abraham, MD, speaks to colleagues around the country about heart failure therapy, he has noticed that the first-in-class drug ivabradine remains below the radar of most physicians.
“I’ve found that this is an agent that very few people know about, even though it’s been FDA [Food and Drug Administration] approved for about 3 years. It’s used fairly extensively in Europe because that’s where the pivotal SHIFT trial was done, but not very much in the United States,” according to Dr. Abraham, professor of medicine, physiology, and cell biology and director of the division of cardiovascular medicine at Ohio State University in Columbus.
That’s likely to change as word spreads about the May 2016 update of the American College of Cardiology/American Heart Association Guideline for the Management of Heart Failure. The update incorporated evidence-based recommendations on the use of two important new heart failure medications: ivabradine (Corlanor), which received a moderate class IIa recommendation, meaning the drug “should be considered,” and sacubitril/valsartan (Entresto), which received the strongest class I recommendation.
In the right patients, these two oral medications improve heart failure morbidity and mortality significantly beyond what’s achievable with what has been the gold standard, guideline-directed medical therapy. Dr. Abraham described how to get started using the two medications at the Annual Cardiovascular Conference at Snowmass.
Ivabradine
Ivabradine is a selective inhibitor of the sinoatrial pacemaker modulating I(f) current. It acts by slowing the sinus rate without reducing myocardial contractility.
“This agent does one thing and one thing alone: It lowers heart rate,” the cardiologist explained.
And that, he added, was sufficient to significantly reduce the risks of death due to heart failure and recurrent hospitalization for worsening heart failure in the pivotal SHIFT trial.
SHIFT included 6,505 patients with moderate to severe heart failure with reduced left ventricular ejection fraction (LVEF) and a resting heart rate above 70 bpm despite background guideline-directed medical therapy. Participants were randomized double blind to ivabradine titrated to a maximum of 7.5 mg twice daily or placebo and followed for a median of about 23 months. The rate of death due to heart failure was 3% with ivabradine and 5% with placebo, for a statistically significant 26% relative risk reduction favoring ivabradine.
But the drug’s main benefit was in reducing recurrent hospitalizations for heart failure, an endpoint of particular interest to health policy officials given that heart failure hospitalizations chew up a substantial proportion of the Medicare budget. Ivabradine reduced first hospitalizations for heart failure during the study period by 25%, second hospitalizations by 34%, and third hospitalizations by 29% (Eur Heart J. 2012 Nov;33[22]:2813-20).
The ACC/AHA guideline update stresses the importance of reserving ivabradine for heart failure patients whose resting heart rate exceeds 70 bpm, despite being on their maximum tolerated dose of a beta-blocker, Dr. Abraham noted.
Ivabradine is contraindicated in the setting of acute decompensated heart failure, severe liver disease, or hypotension, in patients on any of the numerous agents that strongly inhibit the enzyme cytochrome P450 3A4, and in those who have sick sinus syndrome, have sinoatrial block, or are pacemaker dependent.
Sacubitril/valsartan
Sacubitril inhibits neprilysin, an enzyme that blocks the action of endogenous vasoactive peptides including bradykinin, substance P, and natriuretic peptides, all of which counter important maladaptive mechanisms in heart failure. Sacubitril has been combined with the angiotensin receptor blocker valsartan to form the first-in-class angiotensin receptor neprilysin inhibitor, or ARNI, formerly known as LCZ696 and now marketed as Entresto.
In the pivotal double-blind PARADIGM-HF trial, 8,442 patients with heart failure with reduced ejection fraction were randomized to the ARNI at 200 mg b.i.d. or to enalapril at 10 mg b.i.d. on top of background guideline-directed medical therapy. The trial was stopped early because of evidence of overwhelming benefit: a 20% relative risk reduction in cardiovascular death and a 21% decrease in the risk of heart failure hospitalizations in the sacubitril/valsartan group, as well as significant reductions in heart failure symptoms and physical limitations (N Engl J Med. 2014 Sep 11;371[11]:993-1004).
The updated heart failure guidelines strongly recommend that patients with heart failure should be treated with either an ACE inhibitor, an angiotensin receptor blocker, or an ARNI. Further, patients who remain symptomatic on an ACE inhibitor or angiotensin receptor blocker should be switched to an ARNI; that’s a class Ib recommendation based upon the results of PARADIGM-HF.
In getting started using the ARNI, Dr. Abraham said it’s important to understand as background the selective nature of the PARADIGM-HF study design. During the single-blind run-in period of 5-8 weeks, roughly 10% of patients dropped out because they couldn’t tolerate enalapril at 10 mg b.i.d., and a similar percentage dropped out during the ARNI run-in. Thus, patients who couldn’t tolerate a low dose of an ACE inhibitor weren’t in the study. And patients capable of tolerating guideline-recommended full-dose ACE inhibitor therapy were not specifically sought for participation.
“So there are some unanswered questions about the ARNI. If you’re just getting started with this compound in treating your heart failure patients, my own feeling is you should maybe aim for the type of patient that was included in this trial: patients who could tolerate a moderate dose of an ACE inhibitor and had generally good blood pressure. That’s a great way to begin to get experience with this agent in heart failure,” the cardiologist advised.
He reported serving as a consultant to Abbott Vascular, Medtronic, Novartis, and St. Jude Medical.
SNOWMASS, COLO. – As William T. Abraham, MD, speaks to colleagues around the country about heart failure therapy, he has noticed that the first-in-class drug ivabradine remains below the radar of most physicians.
“I’ve found that this is an agent that very few people know about, even though it’s been FDA [Food and Drug Administration] approved for about 3 years. It’s used fairly extensively in Europe because that’s where the pivotal SHIFT trial was done, but not very much in the United States,” according to Dr. Abraham, professor of medicine, physiology, and cell biology and director of the division of cardiovascular medicine at Ohio State University in Columbus.
That’s likely to change as word spreads about the May 2016 update of the American College of Cardiology/American Heart Association Guideline for the Management of Heart Failure. The update incorporated evidence-based recommendations on the use of two important new heart failure medications: ivabradine (Corlanor), which received a moderate class IIa recommendation, meaning the drug “should be considered,” and sacubitril/valsartan (Entresto), which received the strongest class I recommendation.
In the right patients, these two oral medications improve heart failure morbidity and mortality significantly beyond what’s achievable with what has been the gold standard, guideline-directed medical therapy. Dr. Abraham described how to get started using the two medications at the Annual Cardiovascular Conference at Snowmass.
Ivabradine
Ivabradine is a selective inhibitor of the sinoatrial pacemaker modulating I(f) current. It acts by slowing the sinus rate without reducing myocardial contractility.
“This agent does one thing and one thing alone: It lowers heart rate,” the cardiologist explained.
And that, he added, was sufficient to significantly reduce the risks of death due to heart failure and recurrent hospitalization for worsening heart failure in the pivotal SHIFT trial.
SHIFT included 6,505 patients with moderate to severe heart failure with reduced left ventricular ejection fraction (LVEF) and a resting heart rate above 70 bpm despite background guideline-directed medical therapy. Participants were randomized double blind to ivabradine titrated to a maximum of 7.5 mg twice daily or placebo and followed for a median of about 23 months. The rate of death due to heart failure was 3% with ivabradine and 5% with placebo, for a statistically significant 26% relative risk reduction favoring ivabradine.
But the drug’s main benefit was in reducing recurrent hospitalizations for heart failure, an endpoint of particular interest to health policy officials given that heart failure hospitalizations chew up a substantial proportion of the Medicare budget. Ivabradine reduced first hospitalizations for heart failure during the study period by 25%, second hospitalizations by 34%, and third hospitalizations by 29% (Eur Heart J. 2012 Nov;33[22]:2813-20).
The ACC/AHA guideline update stresses the importance of reserving ivabradine for heart failure patients whose resting heart rate exceeds 70 bpm, despite being on their maximum tolerated dose of a beta-blocker, Dr. Abraham noted.
Ivabradine is contraindicated in the setting of acute decompensated heart failure, severe liver disease, or hypotension, in patients on any of the numerous agents that strongly inhibit the enzyme cytochrome P450 3A4, and in those who have sick sinus syndrome, have sinoatrial block, or are pacemaker dependent.
Sacubitril/valsartan
Sacubitril inhibits neprilysin, an enzyme that blocks the action of endogenous vasoactive peptides including bradykinin, substance P, and natriuretic peptides, all of which counter important maladaptive mechanisms in heart failure. Sacubitril has been combined with the angiotensin receptor blocker valsartan to form the first-in-class angiotensin receptor neprilysin inhibitor, or ARNI, formerly known as LCZ696 and now marketed as Entresto.
In the pivotal double-blind PARADIGM-HF trial, 8,442 patients with heart failure with reduced ejection fraction were randomized to the ARNI at 200 mg b.i.d. or to enalapril at 10 mg b.i.d. on top of background guideline-directed medical therapy. The trial was stopped early because of evidence of overwhelming benefit: a 20% relative risk reduction in cardiovascular death and a 21% decrease in the risk of heart failure hospitalizations in the sacubitril/valsartan group, as well as significant reductions in heart failure symptoms and physical limitations (N Engl J Med. 2014 Sep 11;371[11]:993-1004).
The updated heart failure guidelines strongly recommend that patients with heart failure should be treated with either an ACE inhibitor, an angiotensin receptor blocker, or an ARNI. Further, patients who remain symptomatic on an ACE inhibitor or angiotensin receptor blocker should be switched to an ARNI; that’s a class Ib recommendation based upon the results of PARADIGM-HF.
In getting started using the ARNI, Dr. Abraham said it’s important to understand as background the selective nature of the PARADIGM-HF study design. During the single-blind run-in period of 5-8 weeks, roughly 10% of patients dropped out because they couldn’t tolerate enalapril at 10 mg b.i.d., and a similar percentage dropped out during the ARNI run-in. Thus, patients who couldn’t tolerate a low dose of an ACE inhibitor weren’t in the study. And patients capable of tolerating guideline-recommended full-dose ACE inhibitor therapy were not specifically sought for participation.
“So there are some unanswered questions about the ARNI. If you’re just getting started with this compound in treating your heart failure patients, my own feeling is you should maybe aim for the type of patient that was included in this trial: patients who could tolerate a moderate dose of an ACE inhibitor and had generally good blood pressure. That’s a great way to begin to get experience with this agent in heart failure,” the cardiologist advised.
He reported serving as a consultant to Abbott Vascular, Medtronic, Novartis, and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
New-onset AF boosts bad HFrEF outcomes
WASHINGTON – New onset atrial fibrillation more than doubled the rate of adverse outcomes in patients with heart failure with reduced ejection fraction in a review of more than 15,000 such patients.
In 9,934 patients with heart failure with reduced ejection fraction (HFrEF) and no history of atrial fibrillation (AF), development of new-onset AF was linked with a greater than twofold increased risk of cardiovascular disease death or hospitalization for heart failure during follow-up, compared with HFrEF patients who did not initially have or later develop heart failure, after adjustment for several demographic and clinical variables, John J.V. McMurray, MD, said at the annual meeting of the American College of Cardiology. This difference for the primary endpoint of his analysis was statistically significant.
The 1,645 patients with paroxysmal AF at the start of their follow-up also had a significantly increased rate of cardiovascular death or heart failure hospitalization, but their increased risk when compared with HFrEF patients who didn’t develop AF was a much more modest 20% in his fully adjusted analysis. The patients who began follow-up with paroxysmal AF also had a relatively increased relative stroke rate of 33% when compared with HFrEF patients without AF at baseline who remained AF free, but the all-cause mortality rate among those with paroxysmal AF wasn’t significantly elevated, compared with the comparator group.
The 3,770 patients with persistent or permanent AF at baseline showed no statistically significant spike in their adverse event rates, compared with patients without AF, for any of the examined endpoints. The study group also included 66 patients with an undefined form of AF who weren’t included in these analyses.
“It’s the first episodes and paroxysmal episodes that cause trouble, and the trouble they cause is stroke,” Dr. McMurray said in an interview. Their stroke risk gets exacerbated in clinical practice, because these patients often don’t receive the stroke prevention they need in the form of anticoagulation treatment.
“We find over and over that patients with paroxysmal AF are not anticoagulated as frequently as they should be,” Dr. McMurray said. And HFrEF patients with a first AF episode need anticoagulation, too, as soon as AF is diagnosed, he advised.
He went a step further and speculated that the reason why HFrEF patients with new onset AF did so poorly in his analysis was because they already had several prior, brief AF episodes that had gone undetected. “Many of these patients probably had undiagnosed, clinically unapparent AF episodes” that then resulted in strokes, he suggested.
The upshot is that patients with HFrEF may need more aggressive monitoring for new-onset AF, possibly in the form of small, implanted arrhythmia-detection devices. Dr. McMurray said that he and other researchers are currently testing whether this hypothesis is correct. “We and others are now looking at this because these new data are convincing that new-onset AF is bad news [for HFrEF patients].”
In the analysis, “we looked only at clinically recognized and adjudicated new-onset AF. Goodness knows how many HFrEF patients are having unrecognized paroxysmal AF. Almost certainly there is a lot that is unrecognized” that potentially could be detected using a small implanted arrhythmia monitor, which could then lead to earlier anticoagulant treatment as well as possible treatment with antiarrhythmic drugs or with catheter ablation, Dr. McMurray said. Looking for undetected AF in HFrEF patients “is where the science is moving.”
The findings that Dr. McMurray reported are “something we should act on,” commented Adrian F. Hernandez, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C. The comorbidity of AF in HFrEF patients requires “aggressive anticoagulation, and also a review of their heart failure medical treatment to be sure that is optimized, because AF could be a sign of worsening heart failure,” Dr. Hernandez said in an interview. “We may need to more aggressively get HFrEF patients with AF into normal sinus rhythm.”
When Mikhail Kosiborod, MD, treats HFrEF patients with a high risk for AF, such as patients with lower ejection fractions, a dilated left ventricle, or a dilated atrium, “I frequently do 30-day loop recordings in these patients because of their risk for incident AF,” Dr. Kosiborod said in an interview. “We don’t yet have convincing evidence for this, but it makes sense.”
Another finding from his analysis was that the HFrEF patients enrolled in these two trials did not get treatment with a mineralocorticoid receptor antagonist – spironolactone or eplerenone – “as often as they should,” with treatment rates of 44%-48%, compared with use of a beta-blocker in 92%-95% of patients. Treatment with eplerenone (Inspra) “has been shown to reduce the risk for new onset AF, so adding eplerenone or spironolactone is an important step that could be taken to try to prevent AF as well as treat the HFrEF and reduce mortality,” Dr. McMurray said.
PARADIGM-HF and ATMOSPHERE were funded by Novartis. Dr. McMurray has been a consultant to and has received travel and research support from Novartis, and he has received research and travel support from Amgen. Dr. Hernandez has received honoraria from Amgen, AstraZeneca, Janssen, Merck, and Novartis, and has received research support from Amgen, Bayer, Merck, and Portola. Dr. Kosiborod has been a consultant to several drug companies, and he has received research funding from AstraZeneca, Boehringer Ingelheim, Gilead, and Sanofi-Aventis.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – New onset atrial fibrillation more than doubled the rate of adverse outcomes in patients with heart failure with reduced ejection fraction in a review of more than 15,000 such patients.
In 9,934 patients with heart failure with reduced ejection fraction (HFrEF) and no history of atrial fibrillation (AF), development of new-onset AF was linked with a greater than twofold increased risk of cardiovascular disease death or hospitalization for heart failure during follow-up, compared with HFrEF patients who did not initially have or later develop heart failure, after adjustment for several demographic and clinical variables, John J.V. McMurray, MD, said at the annual meeting of the American College of Cardiology. This difference for the primary endpoint of his analysis was statistically significant.
The 1,645 patients with paroxysmal AF at the start of their follow-up also had a significantly increased rate of cardiovascular death or heart failure hospitalization, but their increased risk when compared with HFrEF patients who didn’t develop AF was a much more modest 20% in his fully adjusted analysis. The patients who began follow-up with paroxysmal AF also had a relatively increased relative stroke rate of 33% when compared with HFrEF patients without AF at baseline who remained AF free, but the all-cause mortality rate among those with paroxysmal AF wasn’t significantly elevated, compared with the comparator group.
The 3,770 patients with persistent or permanent AF at baseline showed no statistically significant spike in their adverse event rates, compared with patients without AF, for any of the examined endpoints. The study group also included 66 patients with an undefined form of AF who weren’t included in these analyses.
“It’s the first episodes and paroxysmal episodes that cause trouble, and the trouble they cause is stroke,” Dr. McMurray said in an interview. Their stroke risk gets exacerbated in clinical practice, because these patients often don’t receive the stroke prevention they need in the form of anticoagulation treatment.
“We find over and over that patients with paroxysmal AF are not anticoagulated as frequently as they should be,” Dr. McMurray said. And HFrEF patients with a first AF episode need anticoagulation, too, as soon as AF is diagnosed, he advised.
He went a step further and speculated that the reason why HFrEF patients with new onset AF did so poorly in his analysis was because they already had several prior, brief AF episodes that had gone undetected. “Many of these patients probably had undiagnosed, clinically unapparent AF episodes” that then resulted in strokes, he suggested.
The upshot is that patients with HFrEF may need more aggressive monitoring for new-onset AF, possibly in the form of small, implanted arrhythmia-detection devices. Dr. McMurray said that he and other researchers are currently testing whether this hypothesis is correct. “We and others are now looking at this because these new data are convincing that new-onset AF is bad news [for HFrEF patients].”
In the analysis, “we looked only at clinically recognized and adjudicated new-onset AF. Goodness knows how many HFrEF patients are having unrecognized paroxysmal AF. Almost certainly there is a lot that is unrecognized” that potentially could be detected using a small implanted arrhythmia monitor, which could then lead to earlier anticoagulant treatment as well as possible treatment with antiarrhythmic drugs or with catheter ablation, Dr. McMurray said. Looking for undetected AF in HFrEF patients “is where the science is moving.”
The findings that Dr. McMurray reported are “something we should act on,” commented Adrian F. Hernandez, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C. The comorbidity of AF in HFrEF patients requires “aggressive anticoagulation, and also a review of their heart failure medical treatment to be sure that is optimized, because AF could be a sign of worsening heart failure,” Dr. Hernandez said in an interview. “We may need to more aggressively get HFrEF patients with AF into normal sinus rhythm.”
When Mikhail Kosiborod, MD, treats HFrEF patients with a high risk for AF, such as patients with lower ejection fractions, a dilated left ventricle, or a dilated atrium, “I frequently do 30-day loop recordings in these patients because of their risk for incident AF,” Dr. Kosiborod said in an interview. “We don’t yet have convincing evidence for this, but it makes sense.”
Another finding from his analysis was that the HFrEF patients enrolled in these two trials did not get treatment with a mineralocorticoid receptor antagonist – spironolactone or eplerenone – “as often as they should,” with treatment rates of 44%-48%, compared with use of a beta-blocker in 92%-95% of patients. Treatment with eplerenone (Inspra) “has been shown to reduce the risk for new onset AF, so adding eplerenone or spironolactone is an important step that could be taken to try to prevent AF as well as treat the HFrEF and reduce mortality,” Dr. McMurray said.
PARADIGM-HF and ATMOSPHERE were funded by Novartis. Dr. McMurray has been a consultant to and has received travel and research support from Novartis, and he has received research and travel support from Amgen. Dr. Hernandez has received honoraria from Amgen, AstraZeneca, Janssen, Merck, and Novartis, and has received research support from Amgen, Bayer, Merck, and Portola. Dr. Kosiborod has been a consultant to several drug companies, and he has received research funding from AstraZeneca, Boehringer Ingelheim, Gilead, and Sanofi-Aventis.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – New onset atrial fibrillation more than doubled the rate of adverse outcomes in patients with heart failure with reduced ejection fraction in a review of more than 15,000 such patients.
In 9,934 patients with heart failure with reduced ejection fraction (HFrEF) and no history of atrial fibrillation (AF), development of new-onset AF was linked with a greater than twofold increased risk of cardiovascular disease death or hospitalization for heart failure during follow-up, compared with HFrEF patients who did not initially have or later develop heart failure, after adjustment for several demographic and clinical variables, John J.V. McMurray, MD, said at the annual meeting of the American College of Cardiology. This difference for the primary endpoint of his analysis was statistically significant.
The 1,645 patients with paroxysmal AF at the start of their follow-up also had a significantly increased rate of cardiovascular death or heart failure hospitalization, but their increased risk when compared with HFrEF patients who didn’t develop AF was a much more modest 20% in his fully adjusted analysis. The patients who began follow-up with paroxysmal AF also had a relatively increased relative stroke rate of 33% when compared with HFrEF patients without AF at baseline who remained AF free, but the all-cause mortality rate among those with paroxysmal AF wasn’t significantly elevated, compared with the comparator group.
The 3,770 patients with persistent or permanent AF at baseline showed no statistically significant spike in their adverse event rates, compared with patients without AF, for any of the examined endpoints. The study group also included 66 patients with an undefined form of AF who weren’t included in these analyses.
“It’s the first episodes and paroxysmal episodes that cause trouble, and the trouble they cause is stroke,” Dr. McMurray said in an interview. Their stroke risk gets exacerbated in clinical practice, because these patients often don’t receive the stroke prevention they need in the form of anticoagulation treatment.
“We find over and over that patients with paroxysmal AF are not anticoagulated as frequently as they should be,” Dr. McMurray said. And HFrEF patients with a first AF episode need anticoagulation, too, as soon as AF is diagnosed, he advised.
He went a step further and speculated that the reason why HFrEF patients with new onset AF did so poorly in his analysis was because they already had several prior, brief AF episodes that had gone undetected. “Many of these patients probably had undiagnosed, clinically unapparent AF episodes” that then resulted in strokes, he suggested.
The upshot is that patients with HFrEF may need more aggressive monitoring for new-onset AF, possibly in the form of small, implanted arrhythmia-detection devices. Dr. McMurray said that he and other researchers are currently testing whether this hypothesis is correct. “We and others are now looking at this because these new data are convincing that new-onset AF is bad news [for HFrEF patients].”
In the analysis, “we looked only at clinically recognized and adjudicated new-onset AF. Goodness knows how many HFrEF patients are having unrecognized paroxysmal AF. Almost certainly there is a lot that is unrecognized” that potentially could be detected using a small implanted arrhythmia monitor, which could then lead to earlier anticoagulant treatment as well as possible treatment with antiarrhythmic drugs or with catheter ablation, Dr. McMurray said. Looking for undetected AF in HFrEF patients “is where the science is moving.”
The findings that Dr. McMurray reported are “something we should act on,” commented Adrian F. Hernandez, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C. The comorbidity of AF in HFrEF patients requires “aggressive anticoagulation, and also a review of their heart failure medical treatment to be sure that is optimized, because AF could be a sign of worsening heart failure,” Dr. Hernandez said in an interview. “We may need to more aggressively get HFrEF patients with AF into normal sinus rhythm.”
When Mikhail Kosiborod, MD, treats HFrEF patients with a high risk for AF, such as patients with lower ejection fractions, a dilated left ventricle, or a dilated atrium, “I frequently do 30-day loop recordings in these patients because of their risk for incident AF,” Dr. Kosiborod said in an interview. “We don’t yet have convincing evidence for this, but it makes sense.”
Another finding from his analysis was that the HFrEF patients enrolled in these two trials did not get treatment with a mineralocorticoid receptor antagonist – spironolactone or eplerenone – “as often as they should,” with treatment rates of 44%-48%, compared with use of a beta-blocker in 92%-95% of patients. Treatment with eplerenone (Inspra) “has been shown to reduce the risk for new onset AF, so adding eplerenone or spironolactone is an important step that could be taken to try to prevent AF as well as treat the HFrEF and reduce mortality,” Dr. McMurray said.
PARADIGM-HF and ATMOSPHERE were funded by Novartis. Dr. McMurray has been a consultant to and has received travel and research support from Novartis, and he has received research and travel support from Amgen. Dr. Hernandez has received honoraria from Amgen, AstraZeneca, Janssen, Merck, and Novartis, and has received research support from Amgen, Bayer, Merck, and Portola. Dr. Kosiborod has been a consultant to several drug companies, and he has received research funding from AstraZeneca, Boehringer Ingelheim, Gilead, and Sanofi-Aventis.
[email protected]
On Twitter @mitchelzoler
AT ACC 17
Key clinical point:
Major finding: Adverse outcomes were more than twice as frequent in HFrEF patients with incident atrial fibrillation, compared with those without AF.
Data source: Post hoc analysis of 15,415 heart failure patients enrolled in the PARADIGM-HF and ATMOSPHERE trials.
Disclosures: PARADIGM-HF and ATMOSPHERE were funded by Novartis. Dr. McMurray has been a consultant to and has received travel and research support from Novartis, and he has received research and travel support from Amgen.
Data-driven HR, RR parameters might help reduce alarm fatigue
Clinical question: Can alarm fatigue in pediatric inpatient settings be safely mitigated by modifying alarm limits with data-driven vital sign reference ranges?
Background: The management of patient alarms in the hospital is a significant safety issue, with the large majority of alarms (85%-99%) either false or not clinically significant. This leads to provider desensitization or alarm fatigue, which has been shown to contribute to adverse events.
In 2014, the Joint Commission made the issue of alarm system safety and alarm fatigue a priority for hospitals.1 Multiple studies have been published addressing alarm fatigue in hospitalized adult patients, but this issue is less well studied in pediatrics, including little guidance on optimizing alarm parameters. Widely used reference ranges and guides are based on limited evidence, primarily based on observational data in healthy outpatients or consensus data.
A 2013 study used vital sign data from hospitalized children to develop percentile curves for heart rate (HR) and respiratory rate (RR) and estimated that 54% of vital sign measurements in hospitalized children are out of range using currently accepted reference ranges.2
To safely decrease the number of out-of-range vital sign measurements resulting from current reference ranges, this study used data from non–critically ill hospitalized children to develop HR and RR percentile charts, and then performed retrospective safety analysis by evaluating effects of modifying the alarm limits on identification of cardiorespiratory arrests (CRA) and rapid response team (RRT) activations.
Study Design: Retrospective, cross-sectional study.
Setting: Single-site, 311-bed quaternary-care academic hospital, both general medical and surgical units.
Synopsis: Vital signs were extracted from the institution’s electronic health record (EHR) for all general medical and surgical patients discharged between Jan. 1, 3013, and May 3, 2014, excluding critically ill children and physiologically implausible vital signs. Two different sets were used, a training set (patients discharged between Jan. 1, 2013, and Dec. 31, 2013) and a validation set (Jan. 1, 2014-May 3, 2014). One HR and RR pair was randomly selected for each 4-hour interval during hospitalization, with a maximum of 10 HR and RR pairs per patient. Age-stratified percentiles were calculated using this data. The 5th and 95th percentile limits using the study data were compared with the 5th and 95th percentile values in the 2013 study, and the reference ranges currently in use at the institution (2004 National Institutes of Health ranges).2
The training set used 62,508 vital sign measurements for 7,202 patients to calculate percentiles for HR and RR among 14 different age groups. The validation set consisted of 82,993 vital sign measurements for 2,287 patients. Using the 5th and 95th percentiles for HR and RR resulted in 24,045 (55.6%) fewer out-of-range measurements in the validation set compared to NIH reference ranges (45% fewer HR values, 61% fewer RR values). This finding, as well as the vital sign percentile ranges, was consistent with the data published in the 2013 study.2
Data for all 148 out-of-ICU RRT and CRA events during the same time period were reviewed using manual chart review. Evaluating vital signs within the 12 hours preceding the events, 144 patients had out-of-range HR or RR measurements using NIH ranges. One hundred thirty-six (94.4%) of these 144 patients also had out-of-range measurements using the study-derived 5th and 95th percentile values.
Manual chart review of the remaining eight patients who had normal HR or RR demonstrated that the RRT or CRA interventions occurred for clinical indications that did no rely on HR or RR measurement (for example, desaturations, difficulty breathing, hematemesis), so the data-driven parameters did not miss any of these events.
Bottom line: In this retrospective study, using data-driven HR and RR parameters was at least as safe as the NIH-published reference ranges currently in use in this hospital. In addition to maintaining safety related to RRT and CRA events, use of the data-driven parameters resulted in 55.6% fewer out-of-range vital sign measurements in the studied population. This may reduce the frequency of false alarms and improve alarm fatigue, and should be studied prospectively in the future.
Citation: Goel VV, Poole SF, Longhurst CA, et al. Safety analysis of proposed data-driven physiologic alarm parameters for hospitalized children. J Hosp Med. 2016;11(12):817-823.
Dr. Galloway is a pediatric hospitalist at Sanford Children’s Hospital in Sioux Falls, S.D., assistant professor of pediatrics at the University of South Dakota Sanford School of Medicine, and vice chief of the division of hospital pediatrics at USD SSOM and Sanford Children’s Hospital.
References
1. The Joint Commission. Alarm system safety. Available at: https://www.jointcommission.org/assets/1/18/R3_Report_Issue_5_12_2_13_Final.pdf. Published December 11, 2013.
2. Bonafide CP, Brady PW, Keren R, Conway PH, Marsolo K, Daymont C. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131(4):e1150-1157.
Clinical question: Can alarm fatigue in pediatric inpatient settings be safely mitigated by modifying alarm limits with data-driven vital sign reference ranges?
Background: The management of patient alarms in the hospital is a significant safety issue, with the large majority of alarms (85%-99%) either false or not clinically significant. This leads to provider desensitization or alarm fatigue, which has been shown to contribute to adverse events.
In 2014, the Joint Commission made the issue of alarm system safety and alarm fatigue a priority for hospitals.1 Multiple studies have been published addressing alarm fatigue in hospitalized adult patients, but this issue is less well studied in pediatrics, including little guidance on optimizing alarm parameters. Widely used reference ranges and guides are based on limited evidence, primarily based on observational data in healthy outpatients or consensus data.
A 2013 study used vital sign data from hospitalized children to develop percentile curves for heart rate (HR) and respiratory rate (RR) and estimated that 54% of vital sign measurements in hospitalized children are out of range using currently accepted reference ranges.2
To safely decrease the number of out-of-range vital sign measurements resulting from current reference ranges, this study used data from non–critically ill hospitalized children to develop HR and RR percentile charts, and then performed retrospective safety analysis by evaluating effects of modifying the alarm limits on identification of cardiorespiratory arrests (CRA) and rapid response team (RRT) activations.
Study Design: Retrospective, cross-sectional study.
Setting: Single-site, 311-bed quaternary-care academic hospital, both general medical and surgical units.
Synopsis: Vital signs were extracted from the institution’s electronic health record (EHR) for all general medical and surgical patients discharged between Jan. 1, 3013, and May 3, 2014, excluding critically ill children and physiologically implausible vital signs. Two different sets were used, a training set (patients discharged between Jan. 1, 2013, and Dec. 31, 2013) and a validation set (Jan. 1, 2014-May 3, 2014). One HR and RR pair was randomly selected for each 4-hour interval during hospitalization, with a maximum of 10 HR and RR pairs per patient. Age-stratified percentiles were calculated using this data. The 5th and 95th percentile limits using the study data were compared with the 5th and 95th percentile values in the 2013 study, and the reference ranges currently in use at the institution (2004 National Institutes of Health ranges).2
The training set used 62,508 vital sign measurements for 7,202 patients to calculate percentiles for HR and RR among 14 different age groups. The validation set consisted of 82,993 vital sign measurements for 2,287 patients. Using the 5th and 95th percentiles for HR and RR resulted in 24,045 (55.6%) fewer out-of-range measurements in the validation set compared to NIH reference ranges (45% fewer HR values, 61% fewer RR values). This finding, as well as the vital sign percentile ranges, was consistent with the data published in the 2013 study.2
Data for all 148 out-of-ICU RRT and CRA events during the same time period were reviewed using manual chart review. Evaluating vital signs within the 12 hours preceding the events, 144 patients had out-of-range HR or RR measurements using NIH ranges. One hundred thirty-six (94.4%) of these 144 patients also had out-of-range measurements using the study-derived 5th and 95th percentile values.
Manual chart review of the remaining eight patients who had normal HR or RR demonstrated that the RRT or CRA interventions occurred for clinical indications that did no rely on HR or RR measurement (for example, desaturations, difficulty breathing, hematemesis), so the data-driven parameters did not miss any of these events.
Bottom line: In this retrospective study, using data-driven HR and RR parameters was at least as safe as the NIH-published reference ranges currently in use in this hospital. In addition to maintaining safety related to RRT and CRA events, use of the data-driven parameters resulted in 55.6% fewer out-of-range vital sign measurements in the studied population. This may reduce the frequency of false alarms and improve alarm fatigue, and should be studied prospectively in the future.
Citation: Goel VV, Poole SF, Longhurst CA, et al. Safety analysis of proposed data-driven physiologic alarm parameters for hospitalized children. J Hosp Med. 2016;11(12):817-823.
Dr. Galloway is a pediatric hospitalist at Sanford Children’s Hospital in Sioux Falls, S.D., assistant professor of pediatrics at the University of South Dakota Sanford School of Medicine, and vice chief of the division of hospital pediatrics at USD SSOM and Sanford Children’s Hospital.
References
1. The Joint Commission. Alarm system safety. Available at: https://www.jointcommission.org/assets/1/18/R3_Report_Issue_5_12_2_13_Final.pdf. Published December 11, 2013.
2. Bonafide CP, Brady PW, Keren R, Conway PH, Marsolo K, Daymont C. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131(4):e1150-1157.
Clinical question: Can alarm fatigue in pediatric inpatient settings be safely mitigated by modifying alarm limits with data-driven vital sign reference ranges?
Background: The management of patient alarms in the hospital is a significant safety issue, with the large majority of alarms (85%-99%) either false or not clinically significant. This leads to provider desensitization or alarm fatigue, which has been shown to contribute to adverse events.
In 2014, the Joint Commission made the issue of alarm system safety and alarm fatigue a priority for hospitals.1 Multiple studies have been published addressing alarm fatigue in hospitalized adult patients, but this issue is less well studied in pediatrics, including little guidance on optimizing alarm parameters. Widely used reference ranges and guides are based on limited evidence, primarily based on observational data in healthy outpatients or consensus data.
A 2013 study used vital sign data from hospitalized children to develop percentile curves for heart rate (HR) and respiratory rate (RR) and estimated that 54% of vital sign measurements in hospitalized children are out of range using currently accepted reference ranges.2
To safely decrease the number of out-of-range vital sign measurements resulting from current reference ranges, this study used data from non–critically ill hospitalized children to develop HR and RR percentile charts, and then performed retrospective safety analysis by evaluating effects of modifying the alarm limits on identification of cardiorespiratory arrests (CRA) and rapid response team (RRT) activations.
Study Design: Retrospective, cross-sectional study.
Setting: Single-site, 311-bed quaternary-care academic hospital, both general medical and surgical units.
Synopsis: Vital signs were extracted from the institution’s electronic health record (EHR) for all general medical and surgical patients discharged between Jan. 1, 3013, and May 3, 2014, excluding critically ill children and physiologically implausible vital signs. Two different sets were used, a training set (patients discharged between Jan. 1, 2013, and Dec. 31, 2013) and a validation set (Jan. 1, 2014-May 3, 2014). One HR and RR pair was randomly selected for each 4-hour interval during hospitalization, with a maximum of 10 HR and RR pairs per patient. Age-stratified percentiles were calculated using this data. The 5th and 95th percentile limits using the study data were compared with the 5th and 95th percentile values in the 2013 study, and the reference ranges currently in use at the institution (2004 National Institutes of Health ranges).2
The training set used 62,508 vital sign measurements for 7,202 patients to calculate percentiles for HR and RR among 14 different age groups. The validation set consisted of 82,993 vital sign measurements for 2,287 patients. Using the 5th and 95th percentiles for HR and RR resulted in 24,045 (55.6%) fewer out-of-range measurements in the validation set compared to NIH reference ranges (45% fewer HR values, 61% fewer RR values). This finding, as well as the vital sign percentile ranges, was consistent with the data published in the 2013 study.2
Data for all 148 out-of-ICU RRT and CRA events during the same time period were reviewed using manual chart review. Evaluating vital signs within the 12 hours preceding the events, 144 patients had out-of-range HR or RR measurements using NIH ranges. One hundred thirty-six (94.4%) of these 144 patients also had out-of-range measurements using the study-derived 5th and 95th percentile values.
Manual chart review of the remaining eight patients who had normal HR or RR demonstrated that the RRT or CRA interventions occurred for clinical indications that did no rely on HR or RR measurement (for example, desaturations, difficulty breathing, hematemesis), so the data-driven parameters did not miss any of these events.
Bottom line: In this retrospective study, using data-driven HR and RR parameters was at least as safe as the NIH-published reference ranges currently in use in this hospital. In addition to maintaining safety related to RRT and CRA events, use of the data-driven parameters resulted in 55.6% fewer out-of-range vital sign measurements in the studied population. This may reduce the frequency of false alarms and improve alarm fatigue, and should be studied prospectively in the future.
Citation: Goel VV, Poole SF, Longhurst CA, et al. Safety analysis of proposed data-driven physiologic alarm parameters for hospitalized children. J Hosp Med. 2016;11(12):817-823.
Dr. Galloway is a pediatric hospitalist at Sanford Children’s Hospital in Sioux Falls, S.D., assistant professor of pediatrics at the University of South Dakota Sanford School of Medicine, and vice chief of the division of hospital pediatrics at USD SSOM and Sanford Children’s Hospital.
References
1. The Joint Commission. Alarm system safety. Available at: https://www.jointcommission.org/assets/1/18/R3_Report_Issue_5_12_2_13_Final.pdf. Published December 11, 2013.
2. Bonafide CP, Brady PW, Keren R, Conway PH, Marsolo K, Daymont C. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131(4):e1150-1157.