User login
Standardized infection ratio for CLABSI almost halved since 2009
The standardized infection ratio (SIR) for central line–associated bloodstream infections dropped 42% from 2009 to 2014, according to the Agency for Healthcare Research and Quality.
For acute care hospitalizations, the SIR for central line–associated bloodstream infections (CLABSIs) fell from 0.854 in 2009 to 0.495 in 2014. Over that same time period, the SIR for surgical site infections involving Surgical Care Improvement Project procedures decreased from 0.981 to 0.827 – almost 16%, the AHRQ said in its annual National Healthcare Quality and Disparities Report.
From 2010 to 2014, the SIR for catheter-associated urinary tract infections increased 6.7% from 0.937 to 1.000, but that change was not significant. For laboratory-identified hospital-onset Clostridium difficile infection, the SIR dropped from 0.963 to 0.924 – about 4% – from 2012 to 2014, the AHRQ reported using data from the National Center for Emerging and Zoonotic Infectious Diseases and the National Healthcare Safety Network.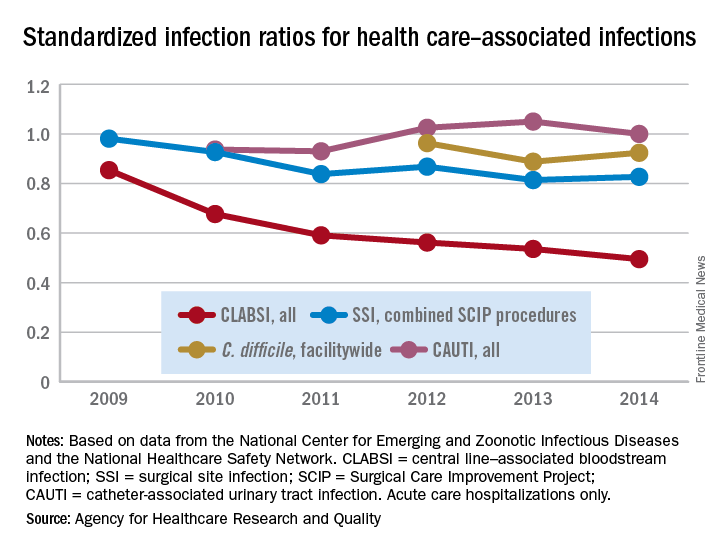
The standardized infection ratio (SIR) for central line–associated bloodstream infections dropped 42% from 2009 to 2014, according to the Agency for Healthcare Research and Quality.
For acute care hospitalizations, the SIR for central line–associated bloodstream infections (CLABSIs) fell from 0.854 in 2009 to 0.495 in 2014. Over that same time period, the SIR for surgical site infections involving Surgical Care Improvement Project procedures decreased from 0.981 to 0.827 – almost 16%, the AHRQ said in its annual National Healthcare Quality and Disparities Report.
From 2010 to 2014, the SIR for catheter-associated urinary tract infections increased 6.7% from 0.937 to 1.000, but that change was not significant. For laboratory-identified hospital-onset Clostridium difficile infection, the SIR dropped from 0.963 to 0.924 – about 4% – from 2012 to 2014, the AHRQ reported using data from the National Center for Emerging and Zoonotic Infectious Diseases and the National Healthcare Safety Network.
The standardized infection ratio (SIR) for central line–associated bloodstream infections dropped 42% from 2009 to 2014, according to the Agency for Healthcare Research and Quality.
For acute care hospitalizations, the SIR for central line–associated bloodstream infections (CLABSIs) fell from 0.854 in 2009 to 0.495 in 2014. Over that same time period, the SIR for surgical site infections involving Surgical Care Improvement Project procedures decreased from 0.981 to 0.827 – almost 16%, the AHRQ said in its annual National Healthcare Quality and Disparities Report.
From 2010 to 2014, the SIR for catheter-associated urinary tract infections increased 6.7% from 0.937 to 1.000, but that change was not significant. For laboratory-identified hospital-onset Clostridium difficile infection, the SIR dropped from 0.963 to 0.924 – about 4% – from 2012 to 2014, the AHRQ reported using data from the National Center for Emerging and Zoonotic Infectious Diseases and the National Healthcare Safety Network.
How to make the move away from opioids for chronic noncancer pain
Standard care of chronic noncancer pain should start moving away from chronic opioid treatment, which can put patients in greater danger of developing a substance use disorder, according to evidence presented at a meeting held by the American Pain Society and Global Academy for Medical Education.
As the effects of the U.S. opioid epidemic continue to gain public attention – recently spurring a declaration of a state of emergency –
Use of opioid therapy for pain conditions such as osteoarthritis, fibromyalgia, and migraine – once a common treatment approach – has been shown to be a dangerous breeding ground for opioid substance use disorders, and physicians would do well to re-evaluate their treatment methods, according to Edwin Salsitz, MD, assistant clinical professor at Mount Sinai Beth Israel Hospital, New York.
“Each prescriber is going to have to review this, digest it, reflect on it, and decide what they are going to do,” said Dr. Salsitz in an interview. “Base it on the Centers for Disease Control and Prevention’s guideline as a good starting point, and then individualize it for yourself and your patients.”
One of the major steps toward lowering the rate of opioid addiction through prescription is avoiding opioids as a treatment for acute pain.
“The first recommendation [of the CDC guideline] is nonpharmaceutical therapy, including physical therapy, massage therapy, acupuncture, and cognitive-behavioral therapy – and there’s a whole lot of evidence for these types of therapy,” said Dr. Salsitz. “The second option is that if you’re going to use medications, use those that aren’t opioids, like Tylenol, Motrin, and antidepressants.”
If opioids are necessary, said Dr. Salsitz, immediate-release opioids in limited prescriptions are a good way to lower the risk of addiction.
“The extended-release opioids have many more milligrams than the immediate-release opioids,” according to Dr. Salsitz. “For example, in New York state, we have a law now that says for acute pain, you cannot prescribe for more than a 7-day amount.”
That 7-day limit helps keep excess opioids out of households, he noted, making it harder for patients to share their medication with friends and family, which has proven to be the most common source for opioids during the onset of substance use disorders. In the first 12 months of use, friends and family members accounted for 55% of reported sources of opioids, according to the U.S. 2010 National Survey on Drug Use and Health.
Providers may also want to consider screening pain patients for psychological disorders, Dr. Salsitz said, as many psychological conditions are associated with a high risk of developing a substance use disorder. Patients with major depression, dysthymia, or panic disorder were 3.43, 6.51, and 5.37 times more likely, respectively, than those without to initiate a prescription for and regularly use opioids, according to a study cited by Dr. Salsitz (Arch Intern Med. 2006 Oct 23;166[19]:2087-93).
One of the largest barriers preventing providers from implementing these methods, however, is a lack of resources, particularly in rural areas with increasing rates of opioid substance use disorders and limited provider options.
While these limitations do pose a problem, physicians should not feel they can’t provide proper care, according to Dr. Salsitz. “I think that each individual provider, wherever they are located, can do a reasonable job.”
Global Academy and this news organization are owned by the same company.
[email protected]
On Twitter @eaztweets
Standard care of chronic noncancer pain should start moving away from chronic opioid treatment, which can put patients in greater danger of developing a substance use disorder, according to evidence presented at a meeting held by the American Pain Society and Global Academy for Medical Education.
As the effects of the U.S. opioid epidemic continue to gain public attention – recently spurring a declaration of a state of emergency –
Use of opioid therapy for pain conditions such as osteoarthritis, fibromyalgia, and migraine – once a common treatment approach – has been shown to be a dangerous breeding ground for opioid substance use disorders, and physicians would do well to re-evaluate their treatment methods, according to Edwin Salsitz, MD, assistant clinical professor at Mount Sinai Beth Israel Hospital, New York.
“Each prescriber is going to have to review this, digest it, reflect on it, and decide what they are going to do,” said Dr. Salsitz in an interview. “Base it on the Centers for Disease Control and Prevention’s guideline as a good starting point, and then individualize it for yourself and your patients.”
One of the major steps toward lowering the rate of opioid addiction through prescription is avoiding opioids as a treatment for acute pain.
“The first recommendation [of the CDC guideline] is nonpharmaceutical therapy, including physical therapy, massage therapy, acupuncture, and cognitive-behavioral therapy – and there’s a whole lot of evidence for these types of therapy,” said Dr. Salsitz. “The second option is that if you’re going to use medications, use those that aren’t opioids, like Tylenol, Motrin, and antidepressants.”
If opioids are necessary, said Dr. Salsitz, immediate-release opioids in limited prescriptions are a good way to lower the risk of addiction.
“The extended-release opioids have many more milligrams than the immediate-release opioids,” according to Dr. Salsitz. “For example, in New York state, we have a law now that says for acute pain, you cannot prescribe for more than a 7-day amount.”
That 7-day limit helps keep excess opioids out of households, he noted, making it harder for patients to share their medication with friends and family, which has proven to be the most common source for opioids during the onset of substance use disorders. In the first 12 months of use, friends and family members accounted for 55% of reported sources of opioids, according to the U.S. 2010 National Survey on Drug Use and Health.
Providers may also want to consider screening pain patients for psychological disorders, Dr. Salsitz said, as many psychological conditions are associated with a high risk of developing a substance use disorder. Patients with major depression, dysthymia, or panic disorder were 3.43, 6.51, and 5.37 times more likely, respectively, than those without to initiate a prescription for and regularly use opioids, according to a study cited by Dr. Salsitz (Arch Intern Med. 2006 Oct 23;166[19]:2087-93).
One of the largest barriers preventing providers from implementing these methods, however, is a lack of resources, particularly in rural areas with increasing rates of opioid substance use disorders and limited provider options.
While these limitations do pose a problem, physicians should not feel they can’t provide proper care, according to Dr. Salsitz. “I think that each individual provider, wherever they are located, can do a reasonable job.”
Global Academy and this news organization are owned by the same company.
[email protected]
On Twitter @eaztweets
Standard care of chronic noncancer pain should start moving away from chronic opioid treatment, which can put patients in greater danger of developing a substance use disorder, according to evidence presented at a meeting held by the American Pain Society and Global Academy for Medical Education.
As the effects of the U.S. opioid epidemic continue to gain public attention – recently spurring a declaration of a state of emergency –
Use of opioid therapy for pain conditions such as osteoarthritis, fibromyalgia, and migraine – once a common treatment approach – has been shown to be a dangerous breeding ground for opioid substance use disorders, and physicians would do well to re-evaluate their treatment methods, according to Edwin Salsitz, MD, assistant clinical professor at Mount Sinai Beth Israel Hospital, New York.
“Each prescriber is going to have to review this, digest it, reflect on it, and decide what they are going to do,” said Dr. Salsitz in an interview. “Base it on the Centers for Disease Control and Prevention’s guideline as a good starting point, and then individualize it for yourself and your patients.”
One of the major steps toward lowering the rate of opioid addiction through prescription is avoiding opioids as a treatment for acute pain.
“The first recommendation [of the CDC guideline] is nonpharmaceutical therapy, including physical therapy, massage therapy, acupuncture, and cognitive-behavioral therapy – and there’s a whole lot of evidence for these types of therapy,” said Dr. Salsitz. “The second option is that if you’re going to use medications, use those that aren’t opioids, like Tylenol, Motrin, and antidepressants.”
If opioids are necessary, said Dr. Salsitz, immediate-release opioids in limited prescriptions are a good way to lower the risk of addiction.
“The extended-release opioids have many more milligrams than the immediate-release opioids,” according to Dr. Salsitz. “For example, in New York state, we have a law now that says for acute pain, you cannot prescribe for more than a 7-day amount.”
That 7-day limit helps keep excess opioids out of households, he noted, making it harder for patients to share their medication with friends and family, which has proven to be the most common source for opioids during the onset of substance use disorders. In the first 12 months of use, friends and family members accounted for 55% of reported sources of opioids, according to the U.S. 2010 National Survey on Drug Use and Health.
Providers may also want to consider screening pain patients for psychological disorders, Dr. Salsitz said, as many psychological conditions are associated with a high risk of developing a substance use disorder. Patients with major depression, dysthymia, or panic disorder were 3.43, 6.51, and 5.37 times more likely, respectively, than those without to initiate a prescription for and regularly use opioids, according to a study cited by Dr. Salsitz (Arch Intern Med. 2006 Oct 23;166[19]:2087-93).
One of the largest barriers preventing providers from implementing these methods, however, is a lack of resources, particularly in rural areas with increasing rates of opioid substance use disorders and limited provider options.
While these limitations do pose a problem, physicians should not feel they can’t provide proper care, according to Dr. Salsitz. “I think that each individual provider, wherever they are located, can do a reasonable job.”
Global Academy and this news organization are owned by the same company.
[email protected]
On Twitter @eaztweets
FROM PAIN CARE FOR PRIMARY CARE
New findings from first all-female TAVR registry
Paris – A history of pregnancy did not protect against adverse outcomes at 1 year in the Women’s International Transcatheter Aortic Valve Implantation Registry (WIN-TAVI), even though it did within the first 30 days, Alaide Chieffo, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
One year ago, at EuroPCR 2016, she reported that in WIN-TAVI, a history of pregnancy – albeit typically more than half a century previously – was independently associated with a 43% reduction in the Valve Academic Research Consortium-2 (VARC-2) 30-day composite endpoint, including death, stroke, major vascular complications, life-threatening bleeding, stage 2 or 3 acute kidney injury, coronary artery obstruction, or repeat transcatheter aortic valve replacement (TAVR) done because of valve-related dysfunction. Those early findings, first reported in this publication, were later published (JACC Cardiovasc Interv. 2016 Aug 8;9[15]:1589-600).
At 1 year of follow-up, however, the rate of the VARC-2 composite endpoint was no longer significantly different in women with or without a history of pregnancy. Nor was a history of pregnancy associated with a significantly reduced risk of the secondary endpoint of death or stroke: The 27% reduction in risk of this secondary endpoint in women with a history of pregnancy, compared with that of nulliparous women, didn’t achieve statistical significance in multivariate analysis, according to Dr. Chieffo of the San Raffaele Scientific Institute in Milan.
She speculated that pregnancy earlier in life provided strong protection against poor 30-day outcomes and a similar trend – albeit not statistically significant – at 1 year because women without children may have less family support.
“They are old women, left alone, without the family taking care of them. This is socially important, I think, because we are investing quite a lot of money in a procedure, and then maybe we’re adding adverse events because these patients are not properly taken care of when they are out of the hospital,” the interventional cardiologist said.
Neither of the other two female-specific characteristics evaluated in WIN-TAVI – having a history of osteoporosis or age at menopause – turned out to be related to the risk of bad outcomes at 1 year, she added.
WIN-TAVI is the first all-female registry of patients undergoing TAVR for severe aortic stenosis. The prospective, observational registry includes 1,019 women treated at 19 highly experienced European and North American TAVR centers. They averaged 82.5 years of age with a mean Society of Thoracic Surgeons score of 8.3%, putting them at intermediate or high surgical risk. A percutaneous transfemoral approach was used in 91% of cases. TAVR was performed under conscious sedation in 28% of the women and under local anesthesia in another 37%. Of the women in the registry, 42% received a newer-generation device.
In addition to the lack of significant impact of prior pregnancy on 1-year outcomes, another noteworthy finding at 1 year of follow-up was that preprocedural atrial fibrillation was independently associated with a 58% increase in the risk of death or stroke (P = .02). Prior percutaneous coronary intervention and EuroSCORE (European System for Cardiac Operative Risk Evaluation) were the only other independent predictors.
This observation suggests the need for a women-only randomized trial of TAVR versus surgical aortic valve replacement in women with intermediate surgical risk, Dr. Chieffo suggested. It will be important to learn whether the ability to surgically ablate preoperative atrial fibrillation in women during surgical valve replacement results in a lower 1-year risk of death or stroke than is achieved with TAVR.
Overall, the 1-year clinical outcomes seen in WIN-TAVI are “very good,” she noted. The VARC-2 composite endpoint occurred in 16.5% of women, all-cause mortality in 12.5%, cardiovascular mortality in 10.8%, and stroke in 2.2%. Only 3.2% of women were hospitalized for heart failure or valve-related symptoms. A new pacemaker was implanted in 12.7% of participants. At baseline 74% of women were New York Heart Association functional class III or IV; at 1 year, only 8.1% were. Moderate paravalvular aortic regurgitation was present in 6% of patients at 6 months and in 9.7% at 1 year
The WIN-TAVI registry is entirely self-funded. Dr. Chieffo reported having no financial conflicts regarding her presentation.
Paris – A history of pregnancy did not protect against adverse outcomes at 1 year in the Women’s International Transcatheter Aortic Valve Implantation Registry (WIN-TAVI), even though it did within the first 30 days, Alaide Chieffo, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
One year ago, at EuroPCR 2016, she reported that in WIN-TAVI, a history of pregnancy – albeit typically more than half a century previously – was independently associated with a 43% reduction in the Valve Academic Research Consortium-2 (VARC-2) 30-day composite endpoint, including death, stroke, major vascular complications, life-threatening bleeding, stage 2 or 3 acute kidney injury, coronary artery obstruction, or repeat transcatheter aortic valve replacement (TAVR) done because of valve-related dysfunction. Those early findings, first reported in this publication, were later published (JACC Cardiovasc Interv. 2016 Aug 8;9[15]:1589-600).
At 1 year of follow-up, however, the rate of the VARC-2 composite endpoint was no longer significantly different in women with or without a history of pregnancy. Nor was a history of pregnancy associated with a significantly reduced risk of the secondary endpoint of death or stroke: The 27% reduction in risk of this secondary endpoint in women with a history of pregnancy, compared with that of nulliparous women, didn’t achieve statistical significance in multivariate analysis, according to Dr. Chieffo of the San Raffaele Scientific Institute in Milan.
She speculated that pregnancy earlier in life provided strong protection against poor 30-day outcomes and a similar trend – albeit not statistically significant – at 1 year because women without children may have less family support.
“They are old women, left alone, without the family taking care of them. This is socially important, I think, because we are investing quite a lot of money in a procedure, and then maybe we’re adding adverse events because these patients are not properly taken care of when they are out of the hospital,” the interventional cardiologist said.
Neither of the other two female-specific characteristics evaluated in WIN-TAVI – having a history of osteoporosis or age at menopause – turned out to be related to the risk of bad outcomes at 1 year, she added.
WIN-TAVI is the first all-female registry of patients undergoing TAVR for severe aortic stenosis. The prospective, observational registry includes 1,019 women treated at 19 highly experienced European and North American TAVR centers. They averaged 82.5 years of age with a mean Society of Thoracic Surgeons score of 8.3%, putting them at intermediate or high surgical risk. A percutaneous transfemoral approach was used in 91% of cases. TAVR was performed under conscious sedation in 28% of the women and under local anesthesia in another 37%. Of the women in the registry, 42% received a newer-generation device.
In addition to the lack of significant impact of prior pregnancy on 1-year outcomes, another noteworthy finding at 1 year of follow-up was that preprocedural atrial fibrillation was independently associated with a 58% increase in the risk of death or stroke (P = .02). Prior percutaneous coronary intervention and EuroSCORE (European System for Cardiac Operative Risk Evaluation) were the only other independent predictors.
This observation suggests the need for a women-only randomized trial of TAVR versus surgical aortic valve replacement in women with intermediate surgical risk, Dr. Chieffo suggested. It will be important to learn whether the ability to surgically ablate preoperative atrial fibrillation in women during surgical valve replacement results in a lower 1-year risk of death or stroke than is achieved with TAVR.
Overall, the 1-year clinical outcomes seen in WIN-TAVI are “very good,” she noted. The VARC-2 composite endpoint occurred in 16.5% of women, all-cause mortality in 12.5%, cardiovascular mortality in 10.8%, and stroke in 2.2%. Only 3.2% of women were hospitalized for heart failure or valve-related symptoms. A new pacemaker was implanted in 12.7% of participants. At baseline 74% of women were New York Heart Association functional class III or IV; at 1 year, only 8.1% were. Moderate paravalvular aortic regurgitation was present in 6% of patients at 6 months and in 9.7% at 1 year
The WIN-TAVI registry is entirely self-funded. Dr. Chieffo reported having no financial conflicts regarding her presentation.
Paris – A history of pregnancy did not protect against adverse outcomes at 1 year in the Women’s International Transcatheter Aortic Valve Implantation Registry (WIN-TAVI), even though it did within the first 30 days, Alaide Chieffo, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
One year ago, at EuroPCR 2016, she reported that in WIN-TAVI, a history of pregnancy – albeit typically more than half a century previously – was independently associated with a 43% reduction in the Valve Academic Research Consortium-2 (VARC-2) 30-day composite endpoint, including death, stroke, major vascular complications, life-threatening bleeding, stage 2 or 3 acute kidney injury, coronary artery obstruction, or repeat transcatheter aortic valve replacement (TAVR) done because of valve-related dysfunction. Those early findings, first reported in this publication, were later published (JACC Cardiovasc Interv. 2016 Aug 8;9[15]:1589-600).
At 1 year of follow-up, however, the rate of the VARC-2 composite endpoint was no longer significantly different in women with or without a history of pregnancy. Nor was a history of pregnancy associated with a significantly reduced risk of the secondary endpoint of death or stroke: The 27% reduction in risk of this secondary endpoint in women with a history of pregnancy, compared with that of nulliparous women, didn’t achieve statistical significance in multivariate analysis, according to Dr. Chieffo of the San Raffaele Scientific Institute in Milan.
She speculated that pregnancy earlier in life provided strong protection against poor 30-day outcomes and a similar trend – albeit not statistically significant – at 1 year because women without children may have less family support.
“They are old women, left alone, without the family taking care of them. This is socially important, I think, because we are investing quite a lot of money in a procedure, and then maybe we’re adding adverse events because these patients are not properly taken care of when they are out of the hospital,” the interventional cardiologist said.
Neither of the other two female-specific characteristics evaluated in WIN-TAVI – having a history of osteoporosis or age at menopause – turned out to be related to the risk of bad outcomes at 1 year, she added.
WIN-TAVI is the first all-female registry of patients undergoing TAVR for severe aortic stenosis. The prospective, observational registry includes 1,019 women treated at 19 highly experienced European and North American TAVR centers. They averaged 82.5 years of age with a mean Society of Thoracic Surgeons score of 8.3%, putting them at intermediate or high surgical risk. A percutaneous transfemoral approach was used in 91% of cases. TAVR was performed under conscious sedation in 28% of the women and under local anesthesia in another 37%. Of the women in the registry, 42% received a newer-generation device.
In addition to the lack of significant impact of prior pregnancy on 1-year outcomes, another noteworthy finding at 1 year of follow-up was that preprocedural atrial fibrillation was independently associated with a 58% increase in the risk of death or stroke (P = .02). Prior percutaneous coronary intervention and EuroSCORE (European System for Cardiac Operative Risk Evaluation) were the only other independent predictors.
This observation suggests the need for a women-only randomized trial of TAVR versus surgical aortic valve replacement in women with intermediate surgical risk, Dr. Chieffo suggested. It will be important to learn whether the ability to surgically ablate preoperative atrial fibrillation in women during surgical valve replacement results in a lower 1-year risk of death or stroke than is achieved with TAVR.
Overall, the 1-year clinical outcomes seen in WIN-TAVI are “very good,” she noted. The VARC-2 composite endpoint occurred in 16.5% of women, all-cause mortality in 12.5%, cardiovascular mortality in 10.8%, and stroke in 2.2%. Only 3.2% of women were hospitalized for heart failure or valve-related symptoms. A new pacemaker was implanted in 12.7% of participants. At baseline 74% of women were New York Heart Association functional class III or IV; at 1 year, only 8.1% were. Moderate paravalvular aortic regurgitation was present in 6% of patients at 6 months and in 9.7% at 1 year
The WIN-TAVI registry is entirely self-funded. Dr. Chieffo reported having no financial conflicts regarding her presentation.
AT EuroPCR
Key clinical point:
Major finding: Prior pregnancy didn’t protect women against death or stroke at 1 year post TAVR.
Data source: WIN-TAVI, a prospective, multicenter, observational registry includes 1,019 women who underwent TAVR.
Disclosures: WIN-TAVI is entirely self-funded. The presenter reported having no financial conflicts.
How to manage submassive pulmonary embolism
The case
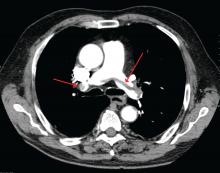
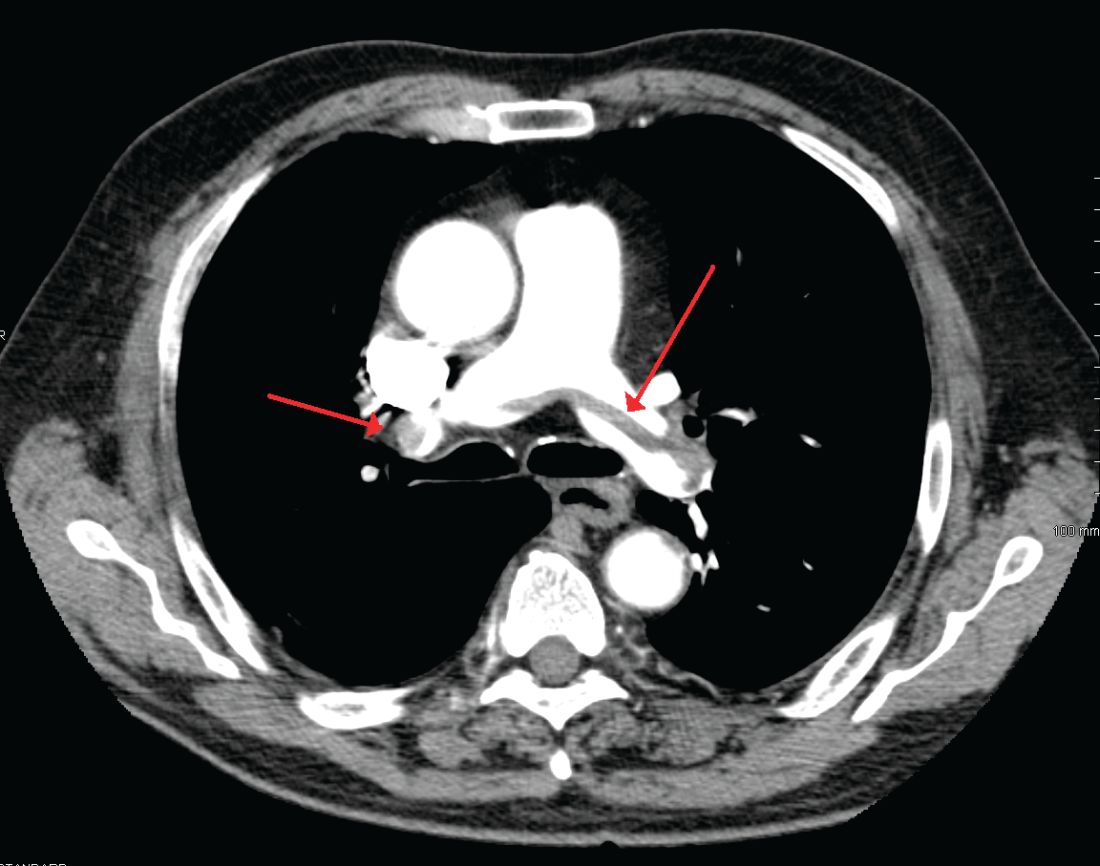
A 49-year-old morbidly obese woman presented to the emergency department with shortness of breath and abdominal distention. On presentation, her blood pressure was 100/60 mm Hg with a heart rate of 110, respiratory rate of 24, and a pulse oximetric saturation (SpO2) of 86% on room air. Troponin T was elevated at 0.3 ng/mL. Computed tomography (CT) of the chest with intravenous contrast showed saddle pulmonary embolism (PE) with dilated right ventricle (RV). CT abdomen/pelvis revealed a very large uterine mass with diffuse lymphadenopathy.
Heparin infusion was started promptly. Echocardiogram demonstrated RV strain. Findings on duplex ultrasound of the lower extremities were consistent with acute deep vein thromboses (DVT) involving the left common femoral vein and the right popliteal vein. Biopsy of a supraclavicular lymph node showed high grade undifferentiated carcinoma most likely of uterine origin.
Clinical questions
What, if any, therapeutic options should be considered beyond standard systemic anticoagulation? Is there a role for:
1. Systemic thrombolysis?
2. Catheter-directed thrombolysis (CDT)?
3. Inferior vena cava (IVC) filter placement?
What is the appropriate management of “submassive” PE?
In the case of massive PE, where the thrombus is located in the central pulmonary vasculature and associated with hypotension due to impaired cardiac output, systemic thrombolysis, embolectomy, and CDT are indicated as potentially life-saving measures. However, the evidence is less clear when the PE is large and has led to RV strain, but without overt hemodynamic instability. This is commonly known as an intermediate risk or “submassive” PE. Submassive PE based on American Heart Association (AHA) guidelines is:1
An acute PE without systemic hypotension (systolic blood pressure less than 90 mm Hg) but with either RV dysfunction or myocardial necrosis. RV dysfunction is defined by the presence of at least one of these following:
• RV dilation (apical 4-chamber RV diameter divided by LV diameter greater than 0.9) or RV systolic dysfunction on echocardiography;
• RV dilation on CT, elevation of BNP (greater than 90 pg/mL), elevation of N-terminal pro-BNP (greater than 500 pg/mL);
• Electrocardiographic changes (new complete or incomplete right bundle branch block, anteroseptal ST elevation or depression, or anteroseptal T-wave inversion).
Myocardial necrosis is defined as elevated troponin I (greater than 0.4 ng/mL) or elevated troponin T (greater than 0.1 ng/mL).
Why is submassive PE of clinical significance?
In 1999, analysis of the International Cooperative Pulmonary Embolism Registry (ICOPER) revealed that RV dysfunction in PE patients was associated with a near doubling of the 3-month mortality risk (hazard ratio 2.0, 1.3-2.9).2 Given this increased risk, one could draw the logical conclusion that we need to treat submassive PE more aggressively than PE without RV strain. But will this necessarily result in a better outcome for the patient given the 3% risk of intracranial hemorrhage associated with thrombolytic therapy?
In the clinical scenario above, the patient did meet the definition of submassive PE. While the patient did not experience systemic hypotension, she did have RV dilation on CT, RV systolic dysfunction on echo as well as an elevated Troponin T level. In addition to starting anticoagulant therapy, what more should be done to increase her probability of a good outcome?
The AHA recommends that systemic thrombolysis and CDT be considered for patients with acute submassive PE if they have clinical evidence of adverse prognosis, including worsening respiratory failure, severe RV dysfunction, or major myocardial necrosis and low risk of bleeding complications (Class IIB; Level of Evidence C).1
The 2016 American College of Chest Physicians (CHEST) guidelines update3 recommends systemically administered thrombolytic therapy over no therapy in selected patients with acute PE who deteriorate after starting anticoagulant therapy but have yet to develop hypotension and who have a low bleeding risk (Grade 2C recommendation).
Systemic thrombolysis
Systemic thrombolysis is administered as an intravenous thrombolytic infusion delivered over a period of time. The Food and Drug Administration–approved thrombolytic drugs currently include tissue plasminogen activator (tPA)/alteplase, streptokinase and urokinase.
Efficacy of low dose thrombolysis was studied in MOPETT 2013,5 a single-center, prospective, randomized, open label study, in which 126 participants found to have submassive PE based on symptoms and CT angiographic or ventilation/perfusion scan data received either 50 mg tPA plus heparin or heparin anticoagulation alone. The composite endpoint of pulmonary hypertension and recurrent PE at 28 months was 16% in the tPA group compared to 63% in the control group (P less than .001). Systemic thrombolysis was associated with lower risk of pulmonary hypertension and recurrent PE, although no mortality benefit was seen in this small study.
In the randomized, double-blind PEITHO trial (n = 1,006) of 20146 comparing tenecteplase plus heparin versus heparin in the submassive PE patients, the primary outcomes of death and hemodynamic decompensation occurred in 2.6% of the tenecteplase group, compared to 5.6% in the placebo group (P = .02). Thrombolytic therapy was associated with 2% rate of hemorrhagic stroke, whereas hemorrhagic stroke in the placebo group was 0.2% (P = .03). In this case, systemic thrombolysis was associated with a 3% lower risk of death and hemodynamic instability, but also a 1.8% increased risk of hemorrhagic stroke.
Catheter-directed thrombolysis (CDT)
CDT was originally developed to treat arterial, dialysis graft and deep vein thromboses, but is now approved by the FDA for the treatment of acute submassive or massive PE.
A wire is passed through the embolus and a multihole infusion catheter is placed, through which a thrombolytic drug is infused over 12-24 hours. The direct delivery of the drug into the thrombus is thought to be as effective as systemic therapy but with a lower risk of bleeding. If more rapid thrombus removal is indicated due to large clot burden and hemodynamic instability, mechanical therapies, such as fragmentation and aspiration, can be used as an adjunct to CDT. However, these mechanical techniques carry the risk of pulmonary artery injury, and therefore should only be used as a last resort. An ultrasound-emitting wire can be added to the multihole infusion catheter to expedite thrombolysis by ultrasonically disrupting the thrombus, a technique known as ultrasound-enhanced thrombolysis (EKOS).7,10
The ULTIMA 2014 trial,8 a small, randomized, open-label study of Ultrasound-Assisted Catheter Directed Thrombolysis (USAT, the term can be used interchangeably with EKOS) versus heparin anticoagulation alone in 59 patients, was designed to study if the former strategy was better at improving the primary outcome measure of RV/LV ratio in submassive PE patients. The mean reduction in RV/LV ratio was 0.30 +/– 0.20 in the USAT group compared to 0.03 +/– 0.16 in the heparin group (P less than .001). However, no significant difference in mortality or bleeding was observed in the groups at 90-day follow up.
The PERFECT 2015 Trial,9 a multicenter registry-based study, prospectively enrolled 101 patients who received CDT as first-line therapy for massive and submassive PE. Among patients with submassive PE, 97.3% were found to have “clinical success” with this treatment, defined as stabilization of hemodynamics, improvement in pulmonary hypertension and right heart strain, and survival to hospital discharge. There was no major bleeding or intracranial hemorrhage. Subgroup analyses in this study comparing USAT against standard CDT did not reveal significant difference in average pulmonary pressure changes, average thrombolytic doses, or average infusion times.
A prospective single-arm multicenter trial, SEATTLE II 2015,10 evaluated the efficacy of EKOS in a sample of 159 patients. Patients with both massive and submassive PE received approximately 24 mg tPA infused via a catheter over 12-24 hours. The primary efficacy outcome was the chest CT-measured RV/LV ratio decrease from the baseline compared to 48 hours post procedure. The pre- and postprocedure ratio was 1.55 versus 1.13 respectively (P less than .001), indicating that EKOS decreased RV dilation. No intracranial hemorrhage was observed and the investigators did not comment on long-term outcomes such as mortality or quality of life. The study was limited by the lack of a comparison group, such as anticoagulation with heparin as monotherapy, or systemic thrombolysis or standard CDT.
Treatment of submassive PE varies between different institutions. There simply are not adequate data comparing low dose systemic thrombolysis, CDT, EKOS, and standard heparin anticoagulation to make firm recommendations. Some investigators feel low-dose systemic thrombolysis is probably as good as the expensive catheter-based thrombolytic therapies.11,12 Low-dose thrombolytic therapy can be followed by use of oral direct factor Xa inhibitors for maintenance of antithrombotic activity.13
Bottom line
In our institution, the interventional radiology team screens patients who meet criteria for submassive PE on a case-by-case basis. We use pulmonary angiographic data (nature and extent of the thrombus), clinical stability, and analysis of other comorbid conditions to decide the best treatment modality for an individual patient. Our team prefers EKOS for submassive PE patients as well as for massive PE patients and as a rescue procedure for patients who have failed systemic thrombolysis.
Until more data are available to support firm guidelines, we feel establishing multidisciplinary teams composed of interventional radiologists, intensivists, cardiologists, and vascular surgeons is prudent to make individualized decisions and to achieve the best outcomes for our patients.14
IVC filter
Since the patient in this case already has a submassive PE, can she tolerate additional clot burden should her remaining DVT embolize again? Is there a role for IVC filter?
The implantation of IVC filters has increased significantly in the past 30 years, without quality evidence justifying their use.15
The 2016 Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report states clearly: In patients with acute DVT of the leg or PE who are treated with anticoagulants, the use of an IVC filter is not recommended (Grade 1B).3 This recommendation is based on findings of the Prevention du Risque d’Embolie Pulmonaire par Interruption Cave (PREPIC) randomized trial,16 and the recently published PREPIC 2 randomized trial,17 both showing that in anticoagulated patients with PE and DVT, concurrent placement of an IVC filter for 3 months did not reduce recurrent PE, including fatal PE.
CHEST guidelines state that an IVC filter should not be routinely placed as an adjunct in patients with PE and DVT. However, what about in the subgroup of patients with submassive or massive PE in whom another PE would be catastrophic? Clinical data are lacking in this area.
Deshpande et al. reported on a series of six patients with massive PE and cardiopulmonary instability; patients all received an IVC filter with anticoagulation. The short-term outcome was excellent, but long-term follow-up was not done.18 Kucher and colleagues reported that from the ICOPER in 2006, out of the 108 massive PE patients with systolic arterial pressure under 90 mm Hg, 11 patients received adjunctive IVC filter placement. None of these 11 patients developed recurrent PE in 90 days and 10 of them survived at least 90 days; IVC filter placement was associated with a reduction in 90-day mortality. In this study, the placement of an IVC filter was entirely decided by the physicians at different sites.19 In a 2012 study examining case fatality rates in 3,770 patients with acute PE who received pulmonary embolectomy, the data showed that in both unstable and stable patients, case fatality rates were lower in those who received an IVC filter.20
Although the above data are favorable for adjunctive IVC filter placement in massive PE patients, at least in short-term outcomes, the small size and lack of randomization preclude establishment of evidence-based guidelines. The 2016 CHEST guidelines point out that as it is uncertain if there is benefit to place an IVC filter adjunctively in anticoagulated patients with severe PE, in this specific subgroup of patients, the recommendation against insertion of an IVC filter in patients with acute PE who are anticoagulated may not apply.3
Bottom line
There is no evidence-based guideline as to whether IVC filters should be placed adjunctively in patients with submassive or massive PE; however, based on expert consensus, it may be appropriate to place an IVC filter as an adjunct to anticoagulation in patients with severe PE. The decision should be individualized based on each patient’s characteristics, preferences, and institutional expertise.
In our case, in hope of preventing further embolic burden, the patient received an IVC filter the day after presentation. Despite the initiation of anticoagulation with heparin, she remained tachycardic and tachypneic, prompting referral for CDT. The interventional radiology team did not feel that she was a good candidate, given her persistent vaginal bleeding and widely metastatic uterine carcinoma. She was switched to therapeutic enoxaparin after no further invasive intervention was deemed appropriate. Her respiratory status did not improve and bilevel positive airway pressure was initiated. Taking into consideration the terminal nature of her cancer, she ultimately elected to pursue comfort care and died shortly afterward.
Acknowledgements
The authors would like to thank Benjamin A. Hohmuth, MD, A. Joseph Layon, MD, and Luis L. Nadal, MD, for their review of the article and invaluable feedback.
Dr. Wenqian Wang, Dr. Vedamurthy, and Dr. Wang are based in the department of hospital medicine at The Medicine Institute, Geisinger Health System, Danville, Penn. Contact Dr. Wenqian Wang at [email protected].
Key Points
• Use pulmonary angiographic data, clinical stability, and analysis of other comorbid conditions to decide the best treatment modality.
• Our team prefers ultrasound-enhanced thrombolysis (EKOS) for submassive PE patients, massive PE patients, and as a rescue procedure for patients who fail systemic thrombolysis.
• Establishing multidisciplinary teams composed of interventional radiologists, intensivists, cardiologists, and vascular surgeons is prudent to make individualized decisions.
• It may be appropriate to place an IVC filter as an adjunct to anticoagulation in patients with severe PE.
References
1. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788-1830.
2. Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386-9.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-52.
4. Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W. Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143-50.
5. Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M, “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol. 2013;111:273-7.
6. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-11.
7. Kuo WT. Endovascular therapy for acute pulmonary embolism. J Vasc Interv Radiol 2012;23:167-79. e164
8. Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479-86.
9. Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results From a Prospective Multicenter Registry. Chest. 2015;148:667-73.
10. Piazza G, Hohlfelder B, Jaff MR, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv. 2015;8:1382-92.
11. Sharifi M. Systemic Full Dose, Half Dose, and Catheter Directed Thrombolysis for Pulmonary Embolism. When to Use and How to Choose? Curr Treat Options Cardiovasc Med. 2016;18:31.
12. Wang C, Zhai Z, Yang Y, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. 2010;137:254-62.
13. Sharifi M, Vajo Z, Freeman W, Bay C, Sharifi M, Schwartz F. Transforming and simplifying the treatment of pulmonary embolism: “safe dose” thrombolysis plus new oral anticoagulants. Lung. 2015;193:369-74.
14. Kabrhel C, Rosovsky R, Channick R, et al. A Multidisciplinary Pulmonary Embolism Response Team: Initial 30-Month Experience With a Novel Approach to Delivery of Care to Patients With Submassive and Massive Pulmonary Embolism. Chest. 2016;150:384-93.
15. Lessne ML, Sing RF. Counterpoint: Do the Benefits Outweigh the Risks for Most Patients Under Consideration for inferior vena cava filters? No. Chest. 2016; 150(6):1182-4.
16. The PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416-22.
17. Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs. anticoagulation alone on risk of recurrent pulmonary embolism: a randomized clinical trial. JAMA. 2015. 313(16):1627-35.
18. Deshpande KS, Hatem C, Karwa M, et al. The use of inferior vena cava filter as a treatment modality for massive pulmonary embolism. A case series and review of pathophysiology. Respir Med. 2002.96(12):984-9.
19. Kucher N, Rossi E, De Rosa M, et al. Massive Pulmonary embolism. Circulation. 2006;113(4):577-82.
20. Stein P, Matta F. Case Fatality Rate with Pulmonary Embolectomy for Acute Pulmonary Embolism. Am J Med. 2012;125:471-7.
The case
A 49-year-old morbidly obese woman presented to the emergency department with shortness of breath and abdominal distention. On presentation, her blood pressure was 100/60 mm Hg with a heart rate of 110, respiratory rate of 24, and a pulse oximetric saturation (SpO2) of 86% on room air. Troponin T was elevated at 0.3 ng/mL. Computed tomography (CT) of the chest with intravenous contrast showed saddle pulmonary embolism (PE) with dilated right ventricle (RV). CT abdomen/pelvis revealed a very large uterine mass with diffuse lymphadenopathy.
Heparin infusion was started promptly. Echocardiogram demonstrated RV strain. Findings on duplex ultrasound of the lower extremities were consistent with acute deep vein thromboses (DVT) involving the left common femoral vein and the right popliteal vein. Biopsy of a supraclavicular lymph node showed high grade undifferentiated carcinoma most likely of uterine origin.
Clinical questions
What, if any, therapeutic options should be considered beyond standard systemic anticoagulation? Is there a role for:
1. Systemic thrombolysis?
2. Catheter-directed thrombolysis (CDT)?
3. Inferior vena cava (IVC) filter placement?
What is the appropriate management of “submassive” PE?
In the case of massive PE, where the thrombus is located in the central pulmonary vasculature and associated with hypotension due to impaired cardiac output, systemic thrombolysis, embolectomy, and CDT are indicated as potentially life-saving measures. However, the evidence is less clear when the PE is large and has led to RV strain, but without overt hemodynamic instability. This is commonly known as an intermediate risk or “submassive” PE. Submassive PE based on American Heart Association (AHA) guidelines is:1
An acute PE without systemic hypotension (systolic blood pressure less than 90 mm Hg) but with either RV dysfunction or myocardial necrosis. RV dysfunction is defined by the presence of at least one of these following:
• RV dilation (apical 4-chamber RV diameter divided by LV diameter greater than 0.9) or RV systolic dysfunction on echocardiography;
• RV dilation on CT, elevation of BNP (greater than 90 pg/mL), elevation of N-terminal pro-BNP (greater than 500 pg/mL);
• Electrocardiographic changes (new complete or incomplete right bundle branch block, anteroseptal ST elevation or depression, or anteroseptal T-wave inversion).
Myocardial necrosis is defined as elevated troponin I (greater than 0.4 ng/mL) or elevated troponin T (greater than 0.1 ng/mL).
Why is submassive PE of clinical significance?
In 1999, analysis of the International Cooperative Pulmonary Embolism Registry (ICOPER) revealed that RV dysfunction in PE patients was associated with a near doubling of the 3-month mortality risk (hazard ratio 2.0, 1.3-2.9).2 Given this increased risk, one could draw the logical conclusion that we need to treat submassive PE more aggressively than PE without RV strain. But will this necessarily result in a better outcome for the patient given the 3% risk of intracranial hemorrhage associated with thrombolytic therapy?
In the clinical scenario above, the patient did meet the definition of submassive PE. While the patient did not experience systemic hypotension, she did have RV dilation on CT, RV systolic dysfunction on echo as well as an elevated Troponin T level. In addition to starting anticoagulant therapy, what more should be done to increase her probability of a good outcome?
The AHA recommends that systemic thrombolysis and CDT be considered for patients with acute submassive PE if they have clinical evidence of adverse prognosis, including worsening respiratory failure, severe RV dysfunction, or major myocardial necrosis and low risk of bleeding complications (Class IIB; Level of Evidence C).1
The 2016 American College of Chest Physicians (CHEST) guidelines update3 recommends systemically administered thrombolytic therapy over no therapy in selected patients with acute PE who deteriorate after starting anticoagulant therapy but have yet to develop hypotension and who have a low bleeding risk (Grade 2C recommendation).
Systemic thrombolysis
Systemic thrombolysis is administered as an intravenous thrombolytic infusion delivered over a period of time. The Food and Drug Administration–approved thrombolytic drugs currently include tissue plasminogen activator (tPA)/alteplase, streptokinase and urokinase.
Efficacy of low dose thrombolysis was studied in MOPETT 2013,5 a single-center, prospective, randomized, open label study, in which 126 participants found to have submassive PE based on symptoms and CT angiographic or ventilation/perfusion scan data received either 50 mg tPA plus heparin or heparin anticoagulation alone. The composite endpoint of pulmonary hypertension and recurrent PE at 28 months was 16% in the tPA group compared to 63% in the control group (P less than .001). Systemic thrombolysis was associated with lower risk of pulmonary hypertension and recurrent PE, although no mortality benefit was seen in this small study.
In the randomized, double-blind PEITHO trial (n = 1,006) of 20146 comparing tenecteplase plus heparin versus heparin in the submassive PE patients, the primary outcomes of death and hemodynamic decompensation occurred in 2.6% of the tenecteplase group, compared to 5.6% in the placebo group (P = .02). Thrombolytic therapy was associated with 2% rate of hemorrhagic stroke, whereas hemorrhagic stroke in the placebo group was 0.2% (P = .03). In this case, systemic thrombolysis was associated with a 3% lower risk of death and hemodynamic instability, but also a 1.8% increased risk of hemorrhagic stroke.
Catheter-directed thrombolysis (CDT)
CDT was originally developed to treat arterial, dialysis graft and deep vein thromboses, but is now approved by the FDA for the treatment of acute submassive or massive PE.
A wire is passed through the embolus and a multihole infusion catheter is placed, through which a thrombolytic drug is infused over 12-24 hours. The direct delivery of the drug into the thrombus is thought to be as effective as systemic therapy but with a lower risk of bleeding. If more rapid thrombus removal is indicated due to large clot burden and hemodynamic instability, mechanical therapies, such as fragmentation and aspiration, can be used as an adjunct to CDT. However, these mechanical techniques carry the risk of pulmonary artery injury, and therefore should only be used as a last resort. An ultrasound-emitting wire can be added to the multihole infusion catheter to expedite thrombolysis by ultrasonically disrupting the thrombus, a technique known as ultrasound-enhanced thrombolysis (EKOS).7,10
The ULTIMA 2014 trial,8 a small, randomized, open-label study of Ultrasound-Assisted Catheter Directed Thrombolysis (USAT, the term can be used interchangeably with EKOS) versus heparin anticoagulation alone in 59 patients, was designed to study if the former strategy was better at improving the primary outcome measure of RV/LV ratio in submassive PE patients. The mean reduction in RV/LV ratio was 0.30 +/– 0.20 in the USAT group compared to 0.03 +/– 0.16 in the heparin group (P less than .001). However, no significant difference in mortality or bleeding was observed in the groups at 90-day follow up.
The PERFECT 2015 Trial,9 a multicenter registry-based study, prospectively enrolled 101 patients who received CDT as first-line therapy for massive and submassive PE. Among patients with submassive PE, 97.3% were found to have “clinical success” with this treatment, defined as stabilization of hemodynamics, improvement in pulmonary hypertension and right heart strain, and survival to hospital discharge. There was no major bleeding or intracranial hemorrhage. Subgroup analyses in this study comparing USAT against standard CDT did not reveal significant difference in average pulmonary pressure changes, average thrombolytic doses, or average infusion times.
A prospective single-arm multicenter trial, SEATTLE II 2015,10 evaluated the efficacy of EKOS in a sample of 159 patients. Patients with both massive and submassive PE received approximately 24 mg tPA infused via a catheter over 12-24 hours. The primary efficacy outcome was the chest CT-measured RV/LV ratio decrease from the baseline compared to 48 hours post procedure. The pre- and postprocedure ratio was 1.55 versus 1.13 respectively (P less than .001), indicating that EKOS decreased RV dilation. No intracranial hemorrhage was observed and the investigators did not comment on long-term outcomes such as mortality or quality of life. The study was limited by the lack of a comparison group, such as anticoagulation with heparin as monotherapy, or systemic thrombolysis or standard CDT.
Treatment of submassive PE varies between different institutions. There simply are not adequate data comparing low dose systemic thrombolysis, CDT, EKOS, and standard heparin anticoagulation to make firm recommendations. Some investigators feel low-dose systemic thrombolysis is probably as good as the expensive catheter-based thrombolytic therapies.11,12 Low-dose thrombolytic therapy can be followed by use of oral direct factor Xa inhibitors for maintenance of antithrombotic activity.13
Bottom line
In our institution, the interventional radiology team screens patients who meet criteria for submassive PE on a case-by-case basis. We use pulmonary angiographic data (nature and extent of the thrombus), clinical stability, and analysis of other comorbid conditions to decide the best treatment modality for an individual patient. Our team prefers EKOS for submassive PE patients as well as for massive PE patients and as a rescue procedure for patients who have failed systemic thrombolysis.
Until more data are available to support firm guidelines, we feel establishing multidisciplinary teams composed of interventional radiologists, intensivists, cardiologists, and vascular surgeons is prudent to make individualized decisions and to achieve the best outcomes for our patients.14
IVC filter
Since the patient in this case already has a submassive PE, can she tolerate additional clot burden should her remaining DVT embolize again? Is there a role for IVC filter?
The implantation of IVC filters has increased significantly in the past 30 years, without quality evidence justifying their use.15
The 2016 Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report states clearly: In patients with acute DVT of the leg or PE who are treated with anticoagulants, the use of an IVC filter is not recommended (Grade 1B).3 This recommendation is based on findings of the Prevention du Risque d’Embolie Pulmonaire par Interruption Cave (PREPIC) randomized trial,16 and the recently published PREPIC 2 randomized trial,17 both showing that in anticoagulated patients with PE and DVT, concurrent placement of an IVC filter for 3 months did not reduce recurrent PE, including fatal PE.
CHEST guidelines state that an IVC filter should not be routinely placed as an adjunct in patients with PE and DVT. However, what about in the subgroup of patients with submassive or massive PE in whom another PE would be catastrophic? Clinical data are lacking in this area.
Deshpande et al. reported on a series of six patients with massive PE and cardiopulmonary instability; patients all received an IVC filter with anticoagulation. The short-term outcome was excellent, but long-term follow-up was not done.18 Kucher and colleagues reported that from the ICOPER in 2006, out of the 108 massive PE patients with systolic arterial pressure under 90 mm Hg, 11 patients received adjunctive IVC filter placement. None of these 11 patients developed recurrent PE in 90 days and 10 of them survived at least 90 days; IVC filter placement was associated with a reduction in 90-day mortality. In this study, the placement of an IVC filter was entirely decided by the physicians at different sites.19 In a 2012 study examining case fatality rates in 3,770 patients with acute PE who received pulmonary embolectomy, the data showed that in both unstable and stable patients, case fatality rates were lower in those who received an IVC filter.20
Although the above data are favorable for adjunctive IVC filter placement in massive PE patients, at least in short-term outcomes, the small size and lack of randomization preclude establishment of evidence-based guidelines. The 2016 CHEST guidelines point out that as it is uncertain if there is benefit to place an IVC filter adjunctively in anticoagulated patients with severe PE, in this specific subgroup of patients, the recommendation against insertion of an IVC filter in patients with acute PE who are anticoagulated may not apply.3
Bottom line
There is no evidence-based guideline as to whether IVC filters should be placed adjunctively in patients with submassive or massive PE; however, based on expert consensus, it may be appropriate to place an IVC filter as an adjunct to anticoagulation in patients with severe PE. The decision should be individualized based on each patient’s characteristics, preferences, and institutional expertise.
In our case, in hope of preventing further embolic burden, the patient received an IVC filter the day after presentation. Despite the initiation of anticoagulation with heparin, she remained tachycardic and tachypneic, prompting referral for CDT. The interventional radiology team did not feel that she was a good candidate, given her persistent vaginal bleeding and widely metastatic uterine carcinoma. She was switched to therapeutic enoxaparin after no further invasive intervention was deemed appropriate. Her respiratory status did not improve and bilevel positive airway pressure was initiated. Taking into consideration the terminal nature of her cancer, she ultimately elected to pursue comfort care and died shortly afterward.
Acknowledgements
The authors would like to thank Benjamin A. Hohmuth, MD, A. Joseph Layon, MD, and Luis L. Nadal, MD, for their review of the article and invaluable feedback.
Dr. Wenqian Wang, Dr. Vedamurthy, and Dr. Wang are based in the department of hospital medicine at The Medicine Institute, Geisinger Health System, Danville, Penn. Contact Dr. Wenqian Wang at [email protected].
Key Points
• Use pulmonary angiographic data, clinical stability, and analysis of other comorbid conditions to decide the best treatment modality.
• Our team prefers ultrasound-enhanced thrombolysis (EKOS) for submassive PE patients, massive PE patients, and as a rescue procedure for patients who fail systemic thrombolysis.
• Establishing multidisciplinary teams composed of interventional radiologists, intensivists, cardiologists, and vascular surgeons is prudent to make individualized decisions.
• It may be appropriate to place an IVC filter as an adjunct to anticoagulation in patients with severe PE.
References
1. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788-1830.
2. Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386-9.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-52.
4. Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W. Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143-50.
5. Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M, “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol. 2013;111:273-7.
6. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-11.
7. Kuo WT. Endovascular therapy for acute pulmonary embolism. J Vasc Interv Radiol 2012;23:167-79. e164
8. Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479-86.
9. Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results From a Prospective Multicenter Registry. Chest. 2015;148:667-73.
10. Piazza G, Hohlfelder B, Jaff MR, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv. 2015;8:1382-92.
11. Sharifi M. Systemic Full Dose, Half Dose, and Catheter Directed Thrombolysis for Pulmonary Embolism. When to Use and How to Choose? Curr Treat Options Cardiovasc Med. 2016;18:31.
12. Wang C, Zhai Z, Yang Y, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. 2010;137:254-62.
13. Sharifi M, Vajo Z, Freeman W, Bay C, Sharifi M, Schwartz F. Transforming and simplifying the treatment of pulmonary embolism: “safe dose” thrombolysis plus new oral anticoagulants. Lung. 2015;193:369-74.
14. Kabrhel C, Rosovsky R, Channick R, et al. A Multidisciplinary Pulmonary Embolism Response Team: Initial 30-Month Experience With a Novel Approach to Delivery of Care to Patients With Submassive and Massive Pulmonary Embolism. Chest. 2016;150:384-93.
15. Lessne ML, Sing RF. Counterpoint: Do the Benefits Outweigh the Risks for Most Patients Under Consideration for inferior vena cava filters? No. Chest. 2016; 150(6):1182-4.
16. The PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416-22.
17. Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs. anticoagulation alone on risk of recurrent pulmonary embolism: a randomized clinical trial. JAMA. 2015. 313(16):1627-35.
18. Deshpande KS, Hatem C, Karwa M, et al. The use of inferior vena cava filter as a treatment modality for massive pulmonary embolism. A case series and review of pathophysiology. Respir Med. 2002.96(12):984-9.
19. Kucher N, Rossi E, De Rosa M, et al. Massive Pulmonary embolism. Circulation. 2006;113(4):577-82.
20. Stein P, Matta F. Case Fatality Rate with Pulmonary Embolectomy for Acute Pulmonary Embolism. Am J Med. 2012;125:471-7.
The case
A 49-year-old morbidly obese woman presented to the emergency department with shortness of breath and abdominal distention. On presentation, her blood pressure was 100/60 mm Hg with a heart rate of 110, respiratory rate of 24, and a pulse oximetric saturation (SpO2) of 86% on room air. Troponin T was elevated at 0.3 ng/mL. Computed tomography (CT) of the chest with intravenous contrast showed saddle pulmonary embolism (PE) with dilated right ventricle (RV). CT abdomen/pelvis revealed a very large uterine mass with diffuse lymphadenopathy.
Heparin infusion was started promptly. Echocardiogram demonstrated RV strain. Findings on duplex ultrasound of the lower extremities were consistent with acute deep vein thromboses (DVT) involving the left common femoral vein and the right popliteal vein. Biopsy of a supraclavicular lymph node showed high grade undifferentiated carcinoma most likely of uterine origin.
Clinical questions
What, if any, therapeutic options should be considered beyond standard systemic anticoagulation? Is there a role for:
1. Systemic thrombolysis?
2. Catheter-directed thrombolysis (CDT)?
3. Inferior vena cava (IVC) filter placement?
What is the appropriate management of “submassive” PE?
In the case of massive PE, where the thrombus is located in the central pulmonary vasculature and associated with hypotension due to impaired cardiac output, systemic thrombolysis, embolectomy, and CDT are indicated as potentially life-saving measures. However, the evidence is less clear when the PE is large and has led to RV strain, but without overt hemodynamic instability. This is commonly known as an intermediate risk or “submassive” PE. Submassive PE based on American Heart Association (AHA) guidelines is:1
An acute PE without systemic hypotension (systolic blood pressure less than 90 mm Hg) but with either RV dysfunction or myocardial necrosis. RV dysfunction is defined by the presence of at least one of these following:
• RV dilation (apical 4-chamber RV diameter divided by LV diameter greater than 0.9) or RV systolic dysfunction on echocardiography;
• RV dilation on CT, elevation of BNP (greater than 90 pg/mL), elevation of N-terminal pro-BNP (greater than 500 pg/mL);
• Electrocardiographic changes (new complete or incomplete right bundle branch block, anteroseptal ST elevation or depression, or anteroseptal T-wave inversion).
Myocardial necrosis is defined as elevated troponin I (greater than 0.4 ng/mL) or elevated troponin T (greater than 0.1 ng/mL).
Why is submassive PE of clinical significance?
In 1999, analysis of the International Cooperative Pulmonary Embolism Registry (ICOPER) revealed that RV dysfunction in PE patients was associated with a near doubling of the 3-month mortality risk (hazard ratio 2.0, 1.3-2.9).2 Given this increased risk, one could draw the logical conclusion that we need to treat submassive PE more aggressively than PE without RV strain. But will this necessarily result in a better outcome for the patient given the 3% risk of intracranial hemorrhage associated with thrombolytic therapy?
In the clinical scenario above, the patient did meet the definition of submassive PE. While the patient did not experience systemic hypotension, she did have RV dilation on CT, RV systolic dysfunction on echo as well as an elevated Troponin T level. In addition to starting anticoagulant therapy, what more should be done to increase her probability of a good outcome?
The AHA recommends that systemic thrombolysis and CDT be considered for patients with acute submassive PE if they have clinical evidence of adverse prognosis, including worsening respiratory failure, severe RV dysfunction, or major myocardial necrosis and low risk of bleeding complications (Class IIB; Level of Evidence C).1
The 2016 American College of Chest Physicians (CHEST) guidelines update3 recommends systemically administered thrombolytic therapy over no therapy in selected patients with acute PE who deteriorate after starting anticoagulant therapy but have yet to develop hypotension and who have a low bleeding risk (Grade 2C recommendation).
Systemic thrombolysis
Systemic thrombolysis is administered as an intravenous thrombolytic infusion delivered over a period of time. The Food and Drug Administration–approved thrombolytic drugs currently include tissue plasminogen activator (tPA)/alteplase, streptokinase and urokinase.
Efficacy of low dose thrombolysis was studied in MOPETT 2013,5 a single-center, prospective, randomized, open label study, in which 126 participants found to have submassive PE based on symptoms and CT angiographic or ventilation/perfusion scan data received either 50 mg tPA plus heparin or heparin anticoagulation alone. The composite endpoint of pulmonary hypertension and recurrent PE at 28 months was 16% in the tPA group compared to 63% in the control group (P less than .001). Systemic thrombolysis was associated with lower risk of pulmonary hypertension and recurrent PE, although no mortality benefit was seen in this small study.
In the randomized, double-blind PEITHO trial (n = 1,006) of 20146 comparing tenecteplase plus heparin versus heparin in the submassive PE patients, the primary outcomes of death and hemodynamic decompensation occurred in 2.6% of the tenecteplase group, compared to 5.6% in the placebo group (P = .02). Thrombolytic therapy was associated with 2% rate of hemorrhagic stroke, whereas hemorrhagic stroke in the placebo group was 0.2% (P = .03). In this case, systemic thrombolysis was associated with a 3% lower risk of death and hemodynamic instability, but also a 1.8% increased risk of hemorrhagic stroke.
Catheter-directed thrombolysis (CDT)
CDT was originally developed to treat arterial, dialysis graft and deep vein thromboses, but is now approved by the FDA for the treatment of acute submassive or massive PE.
A wire is passed through the embolus and a multihole infusion catheter is placed, through which a thrombolytic drug is infused over 12-24 hours. The direct delivery of the drug into the thrombus is thought to be as effective as systemic therapy but with a lower risk of bleeding. If more rapid thrombus removal is indicated due to large clot burden and hemodynamic instability, mechanical therapies, such as fragmentation and aspiration, can be used as an adjunct to CDT. However, these mechanical techniques carry the risk of pulmonary artery injury, and therefore should only be used as a last resort. An ultrasound-emitting wire can be added to the multihole infusion catheter to expedite thrombolysis by ultrasonically disrupting the thrombus, a technique known as ultrasound-enhanced thrombolysis (EKOS).7,10
The ULTIMA 2014 trial,8 a small, randomized, open-label study of Ultrasound-Assisted Catheter Directed Thrombolysis (USAT, the term can be used interchangeably with EKOS) versus heparin anticoagulation alone in 59 patients, was designed to study if the former strategy was better at improving the primary outcome measure of RV/LV ratio in submassive PE patients. The mean reduction in RV/LV ratio was 0.30 +/– 0.20 in the USAT group compared to 0.03 +/– 0.16 in the heparin group (P less than .001). However, no significant difference in mortality or bleeding was observed in the groups at 90-day follow up.
The PERFECT 2015 Trial,9 a multicenter registry-based study, prospectively enrolled 101 patients who received CDT as first-line therapy for massive and submassive PE. Among patients with submassive PE, 97.3% were found to have “clinical success” with this treatment, defined as stabilization of hemodynamics, improvement in pulmonary hypertension and right heart strain, and survival to hospital discharge. There was no major bleeding or intracranial hemorrhage. Subgroup analyses in this study comparing USAT against standard CDT did not reveal significant difference in average pulmonary pressure changes, average thrombolytic doses, or average infusion times.
A prospective single-arm multicenter trial, SEATTLE II 2015,10 evaluated the efficacy of EKOS in a sample of 159 patients. Patients with both massive and submassive PE received approximately 24 mg tPA infused via a catheter over 12-24 hours. The primary efficacy outcome was the chest CT-measured RV/LV ratio decrease from the baseline compared to 48 hours post procedure. The pre- and postprocedure ratio was 1.55 versus 1.13 respectively (P less than .001), indicating that EKOS decreased RV dilation. No intracranial hemorrhage was observed and the investigators did not comment on long-term outcomes such as mortality or quality of life. The study was limited by the lack of a comparison group, such as anticoagulation with heparin as monotherapy, or systemic thrombolysis or standard CDT.
Treatment of submassive PE varies between different institutions. There simply are not adequate data comparing low dose systemic thrombolysis, CDT, EKOS, and standard heparin anticoagulation to make firm recommendations. Some investigators feel low-dose systemic thrombolysis is probably as good as the expensive catheter-based thrombolytic therapies.11,12 Low-dose thrombolytic therapy can be followed by use of oral direct factor Xa inhibitors for maintenance of antithrombotic activity.13
Bottom line
In our institution, the interventional radiology team screens patients who meet criteria for submassive PE on a case-by-case basis. We use pulmonary angiographic data (nature and extent of the thrombus), clinical stability, and analysis of other comorbid conditions to decide the best treatment modality for an individual patient. Our team prefers EKOS for submassive PE patients as well as for massive PE patients and as a rescue procedure for patients who have failed systemic thrombolysis.
Until more data are available to support firm guidelines, we feel establishing multidisciplinary teams composed of interventional radiologists, intensivists, cardiologists, and vascular surgeons is prudent to make individualized decisions and to achieve the best outcomes for our patients.14
IVC filter
Since the patient in this case already has a submassive PE, can she tolerate additional clot burden should her remaining DVT embolize again? Is there a role for IVC filter?
The implantation of IVC filters has increased significantly in the past 30 years, without quality evidence justifying their use.15
The 2016 Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report states clearly: In patients with acute DVT of the leg or PE who are treated with anticoagulants, the use of an IVC filter is not recommended (Grade 1B).3 This recommendation is based on findings of the Prevention du Risque d’Embolie Pulmonaire par Interruption Cave (PREPIC) randomized trial,16 and the recently published PREPIC 2 randomized trial,17 both showing that in anticoagulated patients with PE and DVT, concurrent placement of an IVC filter for 3 months did not reduce recurrent PE, including fatal PE.
CHEST guidelines state that an IVC filter should not be routinely placed as an adjunct in patients with PE and DVT. However, what about in the subgroup of patients with submassive or massive PE in whom another PE would be catastrophic? Clinical data are lacking in this area.
Deshpande et al. reported on a series of six patients with massive PE and cardiopulmonary instability; patients all received an IVC filter with anticoagulation. The short-term outcome was excellent, but long-term follow-up was not done.18 Kucher and colleagues reported that from the ICOPER in 2006, out of the 108 massive PE patients with systolic arterial pressure under 90 mm Hg, 11 patients received adjunctive IVC filter placement. None of these 11 patients developed recurrent PE in 90 days and 10 of them survived at least 90 days; IVC filter placement was associated with a reduction in 90-day mortality. In this study, the placement of an IVC filter was entirely decided by the physicians at different sites.19 In a 2012 study examining case fatality rates in 3,770 patients with acute PE who received pulmonary embolectomy, the data showed that in both unstable and stable patients, case fatality rates were lower in those who received an IVC filter.20
Although the above data are favorable for adjunctive IVC filter placement in massive PE patients, at least in short-term outcomes, the small size and lack of randomization preclude establishment of evidence-based guidelines. The 2016 CHEST guidelines point out that as it is uncertain if there is benefit to place an IVC filter adjunctively in anticoagulated patients with severe PE, in this specific subgroup of patients, the recommendation against insertion of an IVC filter in patients with acute PE who are anticoagulated may not apply.3
Bottom line
There is no evidence-based guideline as to whether IVC filters should be placed adjunctively in patients with submassive or massive PE; however, based on expert consensus, it may be appropriate to place an IVC filter as an adjunct to anticoagulation in patients with severe PE. The decision should be individualized based on each patient’s characteristics, preferences, and institutional expertise.
In our case, in hope of preventing further embolic burden, the patient received an IVC filter the day after presentation. Despite the initiation of anticoagulation with heparin, she remained tachycardic and tachypneic, prompting referral for CDT. The interventional radiology team did not feel that she was a good candidate, given her persistent vaginal bleeding and widely metastatic uterine carcinoma. She was switched to therapeutic enoxaparin after no further invasive intervention was deemed appropriate. Her respiratory status did not improve and bilevel positive airway pressure was initiated. Taking into consideration the terminal nature of her cancer, she ultimately elected to pursue comfort care and died shortly afterward.
Acknowledgements
The authors would like to thank Benjamin A. Hohmuth, MD, A. Joseph Layon, MD, and Luis L. Nadal, MD, for their review of the article and invaluable feedback.
Dr. Wenqian Wang, Dr. Vedamurthy, and Dr. Wang are based in the department of hospital medicine at The Medicine Institute, Geisinger Health System, Danville, Penn. Contact Dr. Wenqian Wang at [email protected].
Key Points
• Use pulmonary angiographic data, clinical stability, and analysis of other comorbid conditions to decide the best treatment modality.
• Our team prefers ultrasound-enhanced thrombolysis (EKOS) for submassive PE patients, massive PE patients, and as a rescue procedure for patients who fail systemic thrombolysis.
• Establishing multidisciplinary teams composed of interventional radiologists, intensivists, cardiologists, and vascular surgeons is prudent to make individualized decisions.
• It may be appropriate to place an IVC filter as an adjunct to anticoagulation in patients with severe PE.
References
1. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788-1830.
2. Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386-9.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-52.
4. Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W. Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143-50.
5. Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M, “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol. 2013;111:273-7.
6. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-11.
7. Kuo WT. Endovascular therapy for acute pulmonary embolism. J Vasc Interv Radiol 2012;23:167-79. e164
8. Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479-86.
9. Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results From a Prospective Multicenter Registry. Chest. 2015;148:667-73.
10. Piazza G, Hohlfelder B, Jaff MR, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv. 2015;8:1382-92.
11. Sharifi M. Systemic Full Dose, Half Dose, and Catheter Directed Thrombolysis for Pulmonary Embolism. When to Use and How to Choose? Curr Treat Options Cardiovasc Med. 2016;18:31.
12. Wang C, Zhai Z, Yang Y, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. 2010;137:254-62.
13. Sharifi M, Vajo Z, Freeman W, Bay C, Sharifi M, Schwartz F. Transforming and simplifying the treatment of pulmonary embolism: “safe dose” thrombolysis plus new oral anticoagulants. Lung. 2015;193:369-74.
14. Kabrhel C, Rosovsky R, Channick R, et al. A Multidisciplinary Pulmonary Embolism Response Team: Initial 30-Month Experience With a Novel Approach to Delivery of Care to Patients With Submassive and Massive Pulmonary Embolism. Chest. 2016;150:384-93.
15. Lessne ML, Sing RF. Counterpoint: Do the Benefits Outweigh the Risks for Most Patients Under Consideration for inferior vena cava filters? No. Chest. 2016; 150(6):1182-4.
16. The PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416-22.
17. Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs. anticoagulation alone on risk of recurrent pulmonary embolism: a randomized clinical trial. JAMA. 2015. 313(16):1627-35.
18. Deshpande KS, Hatem C, Karwa M, et al. The use of inferior vena cava filter as a treatment modality for massive pulmonary embolism. A case series and review of pathophysiology. Respir Med. 2002.96(12):984-9.
19. Kucher N, Rossi E, De Rosa M, et al. Massive Pulmonary embolism. Circulation. 2006;113(4):577-82.
20. Stein P, Matta F. Case Fatality Rate with Pulmonary Embolectomy for Acute Pulmonary Embolism. Am J Med. 2012;125:471-7.
Cancer the most common diagnosis in palliative care patients
More than a quarter of the patients in palliative care have a primary diagnosis of cancer, according to the Center to Advance Palliative Care.
A survey of 351 palliative care programs showed that 27% of their patients had been diagnosed with cancer in 2016, more than twice as many patients who had a cardiac (13%) or pulmonary (12%) diagnosis. The next most common primary diagnosis category in 2016 was neurologic at 8%, with a tie at 6% between diagnoses classified as infectious or complex chronic, followed by patients with dementia at 5%, Maggie Rogers and Tamara Dumanovsky, PhD, of the CAPC reported.
A medical/surgical unit was the referring site for 43% of palliative care referrals in 2016, with 26% of patients coming from an intensive care unit, 13% from a step-down unit, and 8% from an oncology unit, they noted.
More than a quarter of the patients in palliative care have a primary diagnosis of cancer, according to the Center to Advance Palliative Care.
A survey of 351 palliative care programs showed that 27% of their patients had been diagnosed with cancer in 2016, more than twice as many patients who had a cardiac (13%) or pulmonary (12%) diagnosis. The next most common primary diagnosis category in 2016 was neurologic at 8%, with a tie at 6% between diagnoses classified as infectious or complex chronic, followed by patients with dementia at 5%, Maggie Rogers and Tamara Dumanovsky, PhD, of the CAPC reported.
A medical/surgical unit was the referring site for 43% of palliative care referrals in 2016, with 26% of patients coming from an intensive care unit, 13% from a step-down unit, and 8% from an oncology unit, they noted.
More than a quarter of the patients in palliative care have a primary diagnosis of cancer, according to the Center to Advance Palliative Care.
A survey of 351 palliative care programs showed that 27% of their patients had been diagnosed with cancer in 2016, more than twice as many patients who had a cardiac (13%) or pulmonary (12%) diagnosis. The next most common primary diagnosis category in 2016 was neurologic at 8%, with a tie at 6% between diagnoses classified as infectious or complex chronic, followed by patients with dementia at 5%, Maggie Rogers and Tamara Dumanovsky, PhD, of the CAPC reported.
A medical/surgical unit was the referring site for 43% of palliative care referrals in 2016, with 26% of patients coming from an intensive care unit, 13% from a step-down unit, and 8% from an oncology unit, they noted.
When families participate in rounds, errors decrease
NASHVILLE, TENN. – When families are actively included in pediatric hospital rounds, preventable adverse events drop 38% and families report better hospital experiences, with no negative impact on rounds duration or teaching, according to a prospective investigation on inpatient pediatric units of seven North American hospitals.
“We always talk about how parents know their children better than anyone else; empowering the family to know what we are looking for can have downstream safety implications,” she said. In the study, families often caught problems before medical staff, such as IV infiltrations. They also reported delays in diagnoses and conflicting information, among other things, Dr. Khan explained at the Pediatric Hospital Medicine meeting.
There’s not much data on family-centered rounds in pediatric medicine, so Dr. Khan and her team decided to investigate. They modified the I-PASS resident handoff model (illness severity; patient summary; action list; situation awareness and contingency planning; and synthesis by receiver) to be more family friendly.
Families were given a short form before rounds that asked if their child was better, worse, or about the same as the day before, and what questions and items they wanted to address. There was also space for them to take notes during the presentation about what had changed overnight, what still needed to be done, and what to look out for.
Families were given the opportunity to speak first during rounds, and medical staff used plain language: “has a fever” instead of “febrile,” for instance. At the end of the presentation, families were asked to read back their take-aways.
The investigators compared baseline data from the 3 months before implementation with data for the 3 months afterward. The study included more than 1,500 patients and more than 300 rounds in both the pre- and postimplementation arms. The children were general inpatients; surgery and ICU patients were excluded.
Harmful errors/preventable AEs dropped from 20.7/1,000 patients days to 12.9/1,000 after implementation, a 38% reduction (P = .01). There was also a reduction in overall AEs from 34 to 18.5/1,000 patient-days (P = .002).
Compared with baseline data, after implementation, families were more likely to report that they understood the medical plan and what was said on rounds. They also were more likely to report that nurses had addressed their concerns and made them feel like an important member of the team.
Direct observation of pre- and postimplementation rounds showed that family and nursing engagement improved and families more often got written updates. There were no statistically significant differences in rounds duration or decreases in teaching.
“Congratulations. This is very impressive work, and also the right thing to do,” an audience member said after Dr. Khan’s presentation at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The work was funded by the Patient-Centered Outcomes Research Institute and the Agency for Healthcare Research and Quality. Dr. Khan had no disclosures.
NASHVILLE, TENN. – When families are actively included in pediatric hospital rounds, preventable adverse events drop 38% and families report better hospital experiences, with no negative impact on rounds duration or teaching, according to a prospective investigation on inpatient pediatric units of seven North American hospitals.
“We always talk about how parents know their children better than anyone else; empowering the family to know what we are looking for can have downstream safety implications,” she said. In the study, families often caught problems before medical staff, such as IV infiltrations. They also reported delays in diagnoses and conflicting information, among other things, Dr. Khan explained at the Pediatric Hospital Medicine meeting.
There’s not much data on family-centered rounds in pediatric medicine, so Dr. Khan and her team decided to investigate. They modified the I-PASS resident handoff model (illness severity; patient summary; action list; situation awareness and contingency planning; and synthesis by receiver) to be more family friendly.
Families were given a short form before rounds that asked if their child was better, worse, or about the same as the day before, and what questions and items they wanted to address. There was also space for them to take notes during the presentation about what had changed overnight, what still needed to be done, and what to look out for.
Families were given the opportunity to speak first during rounds, and medical staff used plain language: “has a fever” instead of “febrile,” for instance. At the end of the presentation, families were asked to read back their take-aways.
The investigators compared baseline data from the 3 months before implementation with data for the 3 months afterward. The study included more than 1,500 patients and more than 300 rounds in both the pre- and postimplementation arms. The children were general inpatients; surgery and ICU patients were excluded.
Harmful errors/preventable AEs dropped from 20.7/1,000 patients days to 12.9/1,000 after implementation, a 38% reduction (P = .01). There was also a reduction in overall AEs from 34 to 18.5/1,000 patient-days (P = .002).
Compared with baseline data, after implementation, families were more likely to report that they understood the medical plan and what was said on rounds. They also were more likely to report that nurses had addressed their concerns and made them feel like an important member of the team.
Direct observation of pre- and postimplementation rounds showed that family and nursing engagement improved and families more often got written updates. There were no statistically significant differences in rounds duration or decreases in teaching.
“Congratulations. This is very impressive work, and also the right thing to do,” an audience member said after Dr. Khan’s presentation at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The work was funded by the Patient-Centered Outcomes Research Institute and the Agency for Healthcare Research and Quality. Dr. Khan had no disclosures.
NASHVILLE, TENN. – When families are actively included in pediatric hospital rounds, preventable adverse events drop 38% and families report better hospital experiences, with no negative impact on rounds duration or teaching, according to a prospective investigation on inpatient pediatric units of seven North American hospitals.
“We always talk about how parents know their children better than anyone else; empowering the family to know what we are looking for can have downstream safety implications,” she said. In the study, families often caught problems before medical staff, such as IV infiltrations. They also reported delays in diagnoses and conflicting information, among other things, Dr. Khan explained at the Pediatric Hospital Medicine meeting.
There’s not much data on family-centered rounds in pediatric medicine, so Dr. Khan and her team decided to investigate. They modified the I-PASS resident handoff model (illness severity; patient summary; action list; situation awareness and contingency planning; and synthesis by receiver) to be more family friendly.
Families were given a short form before rounds that asked if their child was better, worse, or about the same as the day before, and what questions and items they wanted to address. There was also space for them to take notes during the presentation about what had changed overnight, what still needed to be done, and what to look out for.
Families were given the opportunity to speak first during rounds, and medical staff used plain language: “has a fever” instead of “febrile,” for instance. At the end of the presentation, families were asked to read back their take-aways.
The investigators compared baseline data from the 3 months before implementation with data for the 3 months afterward. The study included more than 1,500 patients and more than 300 rounds in both the pre- and postimplementation arms. The children were general inpatients; surgery and ICU patients were excluded.
Harmful errors/preventable AEs dropped from 20.7/1,000 patients days to 12.9/1,000 after implementation, a 38% reduction (P = .01). There was also a reduction in overall AEs from 34 to 18.5/1,000 patient-days (P = .002).
Compared with baseline data, after implementation, families were more likely to report that they understood the medical plan and what was said on rounds. They also were more likely to report that nurses had addressed their concerns and made them feel like an important member of the team.
Direct observation of pre- and postimplementation rounds showed that family and nursing engagement improved and families more often got written updates. There were no statistically significant differences in rounds duration or decreases in teaching.
“Congratulations. This is very impressive work, and also the right thing to do,” an audience member said after Dr. Khan’s presentation at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The work was funded by the Patient-Centered Outcomes Research Institute and the Agency for Healthcare Research and Quality. Dr. Khan had no disclosures.
AT PHM 2017
Key clinical point:
Major finding: Harmful errors/preventable AEs dropped from 20.7/1,000 patients days to 12.9 after implementation of a program to engage families in pediatric rounds, a 38% reduction (P = .01).
Data source: More than 600 pediatric inpatient rounds at seven North American hospitals.
Disclosures: The work was funded by the Patient-Centered Outcomes Research Institute and the Agency for Healthcare Research and Quality. The lead investigator had no disclosures.
FDA approves first spironolactone oral suspension
The Food and Drug Administration has approved CaroSpir, the first oral suspension form of spironolactone, the aldosterone antagonist that was first approved in 1960, according to an announcement from CMP Pharma.
CaroSpir is intended for the treatment of New York Heart Association class III-IV heart failure and reduced ejection fraction, usually in combination with other treatments. CaroSpir is also indicated as an add-on medication for the treatment of hypertension, and for the treatment of edema in cirrhotic patients who have not adequately responded to fluid and sodium restriction.
“CaroSpir provides a stable, ready to use, and consistent liquid treatment option for adult patients. Up until now, these patients have been prescribed a pharmacy-compounded liquid form of spironolactone. The dosing inconsistencies of compounded liquids have long been a persistent challenge for physicians,” Gerald Sakowski, CEO at CMP Pharma, said in the press release.
Find the full press release on the CMP Pharma website.
The Food and Drug Administration has approved CaroSpir, the first oral suspension form of spironolactone, the aldosterone antagonist that was first approved in 1960, according to an announcement from CMP Pharma.
CaroSpir is intended for the treatment of New York Heart Association class III-IV heart failure and reduced ejection fraction, usually in combination with other treatments. CaroSpir is also indicated as an add-on medication for the treatment of hypertension, and for the treatment of edema in cirrhotic patients who have not adequately responded to fluid and sodium restriction.
“CaroSpir provides a stable, ready to use, and consistent liquid treatment option for adult patients. Up until now, these patients have been prescribed a pharmacy-compounded liquid form of spironolactone. The dosing inconsistencies of compounded liquids have long been a persistent challenge for physicians,” Gerald Sakowski, CEO at CMP Pharma, said in the press release.
Find the full press release on the CMP Pharma website.
The Food and Drug Administration has approved CaroSpir, the first oral suspension form of spironolactone, the aldosterone antagonist that was first approved in 1960, according to an announcement from CMP Pharma.
CaroSpir is intended for the treatment of New York Heart Association class III-IV heart failure and reduced ejection fraction, usually in combination with other treatments. CaroSpir is also indicated as an add-on medication for the treatment of hypertension, and for the treatment of edema in cirrhotic patients who have not adequately responded to fluid and sodium restriction.
“CaroSpir provides a stable, ready to use, and consistent liquid treatment option for adult patients. Up until now, these patients have been prescribed a pharmacy-compounded liquid form of spironolactone. The dosing inconsistencies of compounded liquids have long been a persistent challenge for physicians,” Gerald Sakowski, CEO at CMP Pharma, said in the press release.
Find the full press release on the CMP Pharma website.
The Core Competencies in Hospital Medicine – 2017 revision
“You must be the change you wish to see in the world.” This famous quote from Mahatma Gandhi has inspired many to transform their work and personal space into an eternal quest for improvement. We hospitalists are now well-recognized agents of change in our work environment, improving the quality and safety of inpatient care, striving to create increased value, and promoting the delivery of cost-effective care.
Much has changed in the U.S. health care and hospital practice environment over the past decade. The 2017 revision of the Core Competencies seeks to maintain its relevance, value and more importantly, highlight areas for future growth and innovation.
What does the “Core Competencies” represent and who should use it?
It comprises a set of competency-based learning objectives that present a shared understanding of the knowledge, skills, and attitudes expected of physicians practicing hospital medicine in the United States.
A common misconception is that every hospitalist can be expected to demonstrate proficiency in all topics in the Core Competencies. While every item in the compendium is highly relevant to the field as a whole, its significance for individual hospitalists will vary depending on their practice pattern, leadership role, and local culture.
It also is noteworthy to indicate that it is not a set of practice guidelines that provide recommendations based on the latest scientific evidence, nor does it represent any legal standard of care. Rather, the Core Competencies offers an agenda for curricular training and to broadly influence the direction of the field. It also is important to realize that the Core Competencies is not an all-inclusive list that restricts a hospitalist’s scope of practice. Instead, hospitalists should use the Core Competencies as an educational and professional benchmark with the ultimate goal of providing safe, efficient, and high-value care using interdisciplinary collaboration when necessary.
As a core set of attributes, all hospitalists can use it to reflect on their knowledge, skills, and attitudes, as well as those of their group or practice collectively. The Core Competencies highlights areas within the field that are prime for further research and quality improvement initiatives on a national, regional, and local level. Thus, they also should be of interest to health care administrators and a variety of stakeholders looking to support and fund such efforts in enhancing health care value and quality for all.
It is also a framework for the development of curricula for both education and professional development purposes for use by hospitalists, hospital medicine programs, and health care institutions. Course Directors of Continuing Medical Education programs can use the Core Competencies to identify learning objectives that fulfill the goal of the educational program. Similarly, residency and fellowship program directors and medical school clerkship directors can use it to develop course syllabi targeted to the needs of their learner groups.
The structure and format of the Core Competencies in Hospital Medicine
The 53 chapters in the 2017 revision are divided into three sections – Clinical Conditions, Procedures, and Healthcare Systems, all integral to the practice of hospital medicine. Each chapter starts with an introductory paragraph that discusses the relevance and importance of the subject. Each competency-based learning objective describes a particular concept coupled with an action verb that specifies an expected level of proficiency.
For example, the action verb “explain” that requires a mere description of a subject denotes a lower competency level, compared with the verb “evaluate,” which implies not only an understanding of the matter but also the ability to assess its value for a particular purpose. These learning objectives are further categorized into knowledge, skills, and attitudes subsections to reflect the cognitive, psychomotor, and affective domains of learning.
Because hospitalists are the experts in complex hospital systems, the clinical and procedural sections have an additional subsection, “System Organization and Improvement.” The objectives in this paragraph emphasize the critical role that hospitalists can play as leaders of multidisciplinary teams to improve the quality of care of all patients with a similar condition or undergoing the same procedure.
Examples of everyday use of the Core Competencies for practicing hospitalists
A hospitalist looking to improve her performance of bedside thoracentesis reviews the chapter on Thoracentesis. She then decides to enhance her skills by attending an educational workshop on the use of point-of-care ultrasonography.
A hospital medicine group interested in improving the rate of common hospital-acquired infections reviews the Urinary Tract Infection, Hospital-Acquired and Healthcare-Associated Pneumonia, and Prevention of Healthcare-Associated Infections and Antimicrobial Resistance chapters to identify possible gaps in practice patterns. The group also goes through the chapters on Quality Improvement, Practice-based Learning and Improvement, and Hospitalist as Educator, to further reflect upon the characteristics of their practice environment. The group then adopts a separate strategy to address identified gaps by finding suitable evidence-based content in a format that best fits their need.
An attending physician leading a team of medical residents and students reviews the chapter on Syncope to identify the teaching objectives for each learner. He decides that the medical student should be able to “define syncope” and “explain the physiologic mechanisms that lead to reflex or neurally mediated syncope.” He determines that the intern on the team should be able to “differentiate syncope from other causes of loss of consciousness,” and the senior resident should be able to “formulate a logical diagnostic plan to determine the cause of syncope while avoiding rarely indicated diagnostic tests … ”
New chapters in the 2017 revision
SHM’s Core Competencies Task Force (CCTF) considered several topics as potential new chapters for the 2017 Revision. The SHM Education Committee judged each for its value as a “core” subject by its relevance, intersection with other specialties, and its scope as a stand-alone chapter.
There are two new clinical conditions – hyponatremia and syncope – mainly chosen because of their clinical importance, the risk of complications, and management inconsistencies that offer hospitalists great opportunities for quality improvement initiatives. The CCTF also identified the use of point-of-care ultrasonography as a notable advancement in the field. A separate task force is working to evaluate best practices and develop a practice guideline that hospitalists can use. The CCTF expects to add more chapters as the field of hospital medicine continues to advance and transform the delivery of health care globally.
The 2017 Revision of the Core Competencies in Hospital Medicine is located online at www.journalofhospitalmedicine.com or using the URL shortener bit.ly/corecomp17.
Dr. Nichani is assistant professor of medicine and director of education for the division of hospital medicine at Michigan Medicine, University of Michigan, Ann Arbor. He serves as the chair of the SHM Education Committee.
“You must be the change you wish to see in the world.” This famous quote from Mahatma Gandhi has inspired many to transform their work and personal space into an eternal quest for improvement. We hospitalists are now well-recognized agents of change in our work environment, improving the quality and safety of inpatient care, striving to create increased value, and promoting the delivery of cost-effective care.
Much has changed in the U.S. health care and hospital practice environment over the past decade. The 2017 revision of the Core Competencies seeks to maintain its relevance, value and more importantly, highlight areas for future growth and innovation.
What does the “Core Competencies” represent and who should use it?
It comprises a set of competency-based learning objectives that present a shared understanding of the knowledge, skills, and attitudes expected of physicians practicing hospital medicine in the United States.
A common misconception is that every hospitalist can be expected to demonstrate proficiency in all topics in the Core Competencies. While every item in the compendium is highly relevant to the field as a whole, its significance for individual hospitalists will vary depending on their practice pattern, leadership role, and local culture.
It also is noteworthy to indicate that it is not a set of practice guidelines that provide recommendations based on the latest scientific evidence, nor does it represent any legal standard of care. Rather, the Core Competencies offers an agenda for curricular training and to broadly influence the direction of the field. It also is important to realize that the Core Competencies is not an all-inclusive list that restricts a hospitalist’s scope of practice. Instead, hospitalists should use the Core Competencies as an educational and professional benchmark with the ultimate goal of providing safe, efficient, and high-value care using interdisciplinary collaboration when necessary.
As a core set of attributes, all hospitalists can use it to reflect on their knowledge, skills, and attitudes, as well as those of their group or practice collectively. The Core Competencies highlights areas within the field that are prime for further research and quality improvement initiatives on a national, regional, and local level. Thus, they also should be of interest to health care administrators and a variety of stakeholders looking to support and fund such efforts in enhancing health care value and quality for all.
It is also a framework for the development of curricula for both education and professional development purposes for use by hospitalists, hospital medicine programs, and health care institutions. Course Directors of Continuing Medical Education programs can use the Core Competencies to identify learning objectives that fulfill the goal of the educational program. Similarly, residency and fellowship program directors and medical school clerkship directors can use it to develop course syllabi targeted to the needs of their learner groups.
The structure and format of the Core Competencies in Hospital Medicine
The 53 chapters in the 2017 revision are divided into three sections – Clinical Conditions, Procedures, and Healthcare Systems, all integral to the practice of hospital medicine. Each chapter starts with an introductory paragraph that discusses the relevance and importance of the subject. Each competency-based learning objective describes a particular concept coupled with an action verb that specifies an expected level of proficiency.
For example, the action verb “explain” that requires a mere description of a subject denotes a lower competency level, compared with the verb “evaluate,” which implies not only an understanding of the matter but also the ability to assess its value for a particular purpose. These learning objectives are further categorized into knowledge, skills, and attitudes subsections to reflect the cognitive, psychomotor, and affective domains of learning.
Because hospitalists are the experts in complex hospital systems, the clinical and procedural sections have an additional subsection, “System Organization and Improvement.” The objectives in this paragraph emphasize the critical role that hospitalists can play as leaders of multidisciplinary teams to improve the quality of care of all patients with a similar condition or undergoing the same procedure.
Examples of everyday use of the Core Competencies for practicing hospitalists
A hospitalist looking to improve her performance of bedside thoracentesis reviews the chapter on Thoracentesis. She then decides to enhance her skills by attending an educational workshop on the use of point-of-care ultrasonography.
A hospital medicine group interested in improving the rate of common hospital-acquired infections reviews the Urinary Tract Infection, Hospital-Acquired and Healthcare-Associated Pneumonia, and Prevention of Healthcare-Associated Infections and Antimicrobial Resistance chapters to identify possible gaps in practice patterns. The group also goes through the chapters on Quality Improvement, Practice-based Learning and Improvement, and Hospitalist as Educator, to further reflect upon the characteristics of their practice environment. The group then adopts a separate strategy to address identified gaps by finding suitable evidence-based content in a format that best fits their need.
An attending physician leading a team of medical residents and students reviews the chapter on Syncope to identify the teaching objectives for each learner. He decides that the medical student should be able to “define syncope” and “explain the physiologic mechanisms that lead to reflex or neurally mediated syncope.” He determines that the intern on the team should be able to “differentiate syncope from other causes of loss of consciousness,” and the senior resident should be able to “formulate a logical diagnostic plan to determine the cause of syncope while avoiding rarely indicated diagnostic tests … ”
New chapters in the 2017 revision
SHM’s Core Competencies Task Force (CCTF) considered several topics as potential new chapters for the 2017 Revision. The SHM Education Committee judged each for its value as a “core” subject by its relevance, intersection with other specialties, and its scope as a stand-alone chapter.
There are two new clinical conditions – hyponatremia and syncope – mainly chosen because of their clinical importance, the risk of complications, and management inconsistencies that offer hospitalists great opportunities for quality improvement initiatives. The CCTF also identified the use of point-of-care ultrasonography as a notable advancement in the field. A separate task force is working to evaluate best practices and develop a practice guideline that hospitalists can use. The CCTF expects to add more chapters as the field of hospital medicine continues to advance and transform the delivery of health care globally.
The 2017 Revision of the Core Competencies in Hospital Medicine is located online at www.journalofhospitalmedicine.com or using the URL shortener bit.ly/corecomp17.
Dr. Nichani is assistant professor of medicine and director of education for the division of hospital medicine at Michigan Medicine, University of Michigan, Ann Arbor. He serves as the chair of the SHM Education Committee.
“You must be the change you wish to see in the world.” This famous quote from Mahatma Gandhi has inspired many to transform their work and personal space into an eternal quest for improvement. We hospitalists are now well-recognized agents of change in our work environment, improving the quality and safety of inpatient care, striving to create increased value, and promoting the delivery of cost-effective care.
Much has changed in the U.S. health care and hospital practice environment over the past decade. The 2017 revision of the Core Competencies seeks to maintain its relevance, value and more importantly, highlight areas for future growth and innovation.
What does the “Core Competencies” represent and who should use it?
It comprises a set of competency-based learning objectives that present a shared understanding of the knowledge, skills, and attitudes expected of physicians practicing hospital medicine in the United States.
A common misconception is that every hospitalist can be expected to demonstrate proficiency in all topics in the Core Competencies. While every item in the compendium is highly relevant to the field as a whole, its significance for individual hospitalists will vary depending on their practice pattern, leadership role, and local culture.
It also is noteworthy to indicate that it is not a set of practice guidelines that provide recommendations based on the latest scientific evidence, nor does it represent any legal standard of care. Rather, the Core Competencies offers an agenda for curricular training and to broadly influence the direction of the field. It also is important to realize that the Core Competencies is not an all-inclusive list that restricts a hospitalist’s scope of practice. Instead, hospitalists should use the Core Competencies as an educational and professional benchmark with the ultimate goal of providing safe, efficient, and high-value care using interdisciplinary collaboration when necessary.
As a core set of attributes, all hospitalists can use it to reflect on their knowledge, skills, and attitudes, as well as those of their group or practice collectively. The Core Competencies highlights areas within the field that are prime for further research and quality improvement initiatives on a national, regional, and local level. Thus, they also should be of interest to health care administrators and a variety of stakeholders looking to support and fund such efforts in enhancing health care value and quality for all.
It is also a framework for the development of curricula for both education and professional development purposes for use by hospitalists, hospital medicine programs, and health care institutions. Course Directors of Continuing Medical Education programs can use the Core Competencies to identify learning objectives that fulfill the goal of the educational program. Similarly, residency and fellowship program directors and medical school clerkship directors can use it to develop course syllabi targeted to the needs of their learner groups.
The structure and format of the Core Competencies in Hospital Medicine
The 53 chapters in the 2017 revision are divided into three sections – Clinical Conditions, Procedures, and Healthcare Systems, all integral to the practice of hospital medicine. Each chapter starts with an introductory paragraph that discusses the relevance and importance of the subject. Each competency-based learning objective describes a particular concept coupled with an action verb that specifies an expected level of proficiency.
For example, the action verb “explain” that requires a mere description of a subject denotes a lower competency level, compared with the verb “evaluate,” which implies not only an understanding of the matter but also the ability to assess its value for a particular purpose. These learning objectives are further categorized into knowledge, skills, and attitudes subsections to reflect the cognitive, psychomotor, and affective domains of learning.
Because hospitalists are the experts in complex hospital systems, the clinical and procedural sections have an additional subsection, “System Organization and Improvement.” The objectives in this paragraph emphasize the critical role that hospitalists can play as leaders of multidisciplinary teams to improve the quality of care of all patients with a similar condition or undergoing the same procedure.
Examples of everyday use of the Core Competencies for practicing hospitalists
A hospitalist looking to improve her performance of bedside thoracentesis reviews the chapter on Thoracentesis. She then decides to enhance her skills by attending an educational workshop on the use of point-of-care ultrasonography.
A hospital medicine group interested in improving the rate of common hospital-acquired infections reviews the Urinary Tract Infection, Hospital-Acquired and Healthcare-Associated Pneumonia, and Prevention of Healthcare-Associated Infections and Antimicrobial Resistance chapters to identify possible gaps in practice patterns. The group also goes through the chapters on Quality Improvement, Practice-based Learning and Improvement, and Hospitalist as Educator, to further reflect upon the characteristics of their practice environment. The group then adopts a separate strategy to address identified gaps by finding suitable evidence-based content in a format that best fits their need.
An attending physician leading a team of medical residents and students reviews the chapter on Syncope to identify the teaching objectives for each learner. He decides that the medical student should be able to “define syncope” and “explain the physiologic mechanisms that lead to reflex or neurally mediated syncope.” He determines that the intern on the team should be able to “differentiate syncope from other causes of loss of consciousness,” and the senior resident should be able to “formulate a logical diagnostic plan to determine the cause of syncope while avoiding rarely indicated diagnostic tests … ”
New chapters in the 2017 revision
SHM’s Core Competencies Task Force (CCTF) considered several topics as potential new chapters for the 2017 Revision. The SHM Education Committee judged each for its value as a “core” subject by its relevance, intersection with other specialties, and its scope as a stand-alone chapter.
There are two new clinical conditions – hyponatremia and syncope – mainly chosen because of their clinical importance, the risk of complications, and management inconsistencies that offer hospitalists great opportunities for quality improvement initiatives. The CCTF also identified the use of point-of-care ultrasonography as a notable advancement in the field. A separate task force is working to evaluate best practices and develop a practice guideline that hospitalists can use. The CCTF expects to add more chapters as the field of hospital medicine continues to advance and transform the delivery of health care globally.
The 2017 Revision of the Core Competencies in Hospital Medicine is located online at www.journalofhospitalmedicine.com or using the URL shortener bit.ly/corecomp17.
Dr. Nichani is assistant professor of medicine and director of education for the division of hospital medicine at Michigan Medicine, University of Michigan, Ann Arbor. He serves as the chair of the SHM Education Committee.
High-flow nasal cannula safe outside of pediatric ICU, but may up length of stay
NASHVILLE, TENN. – Young children with acute bronchiolitis do not need to be admitted to the pediatric ICU for high-flow nasal cannula treatment of up to 6 L/min and 50% oxygen; it is safe to administer it on the floor, according to a review of 6,804 acute bronchiolitis cases in children younger than 2 years treated at the University of Texas Southwestern Medical Center, Dallas.
Use of high-flow nasal cannulas (HFNC) has increased dramatically in recent years at UT Southwestern and elsewhere. It soothes children and can rapidly improve breathing without the nasal edema and nose bleeds common with cooler, drier, 100% oxygen. At Southwestern, HFNC use on the pediatric wards increased from 5% of acute bronchiolitis cases in the September 2010 to April 2011 season to 60% in the 2015-2016 season. Use for bronchiolitis in the PICU increased from 82% to 98% over the same period.
The increase correlated with a drop in intubation for acute bronchiolitis from 14% of children in 2010-2011 to just 2% in 2015-2016. The only HFNC adverse events were minor air leaks in two children.
As HFNC became more common, however, the Dallas team found that length of stay for acute bronchiolitis increased from 1.8 days in 2011-2012 to 2.4 days in 2015-2016, perhaps because the use of HFNC gives providers the impression that children are sicker than they actually are.
To counter the problem, lead investigator Vineeta Mittal, MD, associate professor of pediatrics, and her colleagues created an HFNC weaning protocol that gradually steps down treatment based on blood oxygen saturation levels and breathing effort, leading ultimately to a room-air challenge. It helped; the mean length of stay as of November 2016 was 1.7 days.
There’s been pushback in some places about giving HFNC on the floor: Intensivists sometimes consider it a form of ventilation that should be administered in the PICU. At levels up to 6 L/min and 50% oxygen, though, HFNC is “safe to give on the floor, because there’s no pneumothorax risk,” Dr. Mittal explained. HFNC “is not a ventilator; it’s an effective form of noninvasive respiratory support in children with moderate to severe respiratory distress from bronchiolitis.”
At Southwestern, “we are managing 80% of cases on the floor” with the help of HFNC, Dr. Mittal said at Pediatric Hospital Medicine.
At least for now, children at Southwestern go to the PICU if they need higher flow rates, but Dr. Mittal said it’s not clear if that’s necessary. “We said [6 L/min] is safe,” but maybe “we could even use 8 L/min or even 12 L/min” – the maximum delivered in the PICU over the study period – “because we know it’s safe,” she said. In addition, keeping kids on the floor also saves money, she noted at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Dr. Mittal is concerned HFNC might be overused. “We have gotten so used to this machine that the moment we see distress, we put the kid on high flow,” rather than observing them for a bit to see if they recover on their own. More data are needed to determine when HFNC should be initiated, and when to pull the plug on HFNC and intubate, she said.
Dr. Mittal had no disclosures.
NASHVILLE, TENN. – Young children with acute bronchiolitis do not need to be admitted to the pediatric ICU for high-flow nasal cannula treatment of up to 6 L/min and 50% oxygen; it is safe to administer it on the floor, according to a review of 6,804 acute bronchiolitis cases in children younger than 2 years treated at the University of Texas Southwestern Medical Center, Dallas.
Use of high-flow nasal cannulas (HFNC) has increased dramatically in recent years at UT Southwestern and elsewhere. It soothes children and can rapidly improve breathing without the nasal edema and nose bleeds common with cooler, drier, 100% oxygen. At Southwestern, HFNC use on the pediatric wards increased from 5% of acute bronchiolitis cases in the September 2010 to April 2011 season to 60% in the 2015-2016 season. Use for bronchiolitis in the PICU increased from 82% to 98% over the same period.
The increase correlated with a drop in intubation for acute bronchiolitis from 14% of children in 2010-2011 to just 2% in 2015-2016. The only HFNC adverse events were minor air leaks in two children.
As HFNC became more common, however, the Dallas team found that length of stay for acute bronchiolitis increased from 1.8 days in 2011-2012 to 2.4 days in 2015-2016, perhaps because the use of HFNC gives providers the impression that children are sicker than they actually are.
To counter the problem, lead investigator Vineeta Mittal, MD, associate professor of pediatrics, and her colleagues created an HFNC weaning protocol that gradually steps down treatment based on blood oxygen saturation levels and breathing effort, leading ultimately to a room-air challenge. It helped; the mean length of stay as of November 2016 was 1.7 days.
There’s been pushback in some places about giving HFNC on the floor: Intensivists sometimes consider it a form of ventilation that should be administered in the PICU. At levels up to 6 L/min and 50% oxygen, though, HFNC is “safe to give on the floor, because there’s no pneumothorax risk,” Dr. Mittal explained. HFNC “is not a ventilator; it’s an effective form of noninvasive respiratory support in children with moderate to severe respiratory distress from bronchiolitis.”
At Southwestern, “we are managing 80% of cases on the floor” with the help of HFNC, Dr. Mittal said at Pediatric Hospital Medicine.
At least for now, children at Southwestern go to the PICU if they need higher flow rates, but Dr. Mittal said it’s not clear if that’s necessary. “We said [6 L/min] is safe,” but maybe “we could even use 8 L/min or even 12 L/min” – the maximum delivered in the PICU over the study period – “because we know it’s safe,” she said. In addition, keeping kids on the floor also saves money, she noted at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Dr. Mittal is concerned HFNC might be overused. “We have gotten so used to this machine that the moment we see distress, we put the kid on high flow,” rather than observing them for a bit to see if they recover on their own. More data are needed to determine when HFNC should be initiated, and when to pull the plug on HFNC and intubate, she said.
Dr. Mittal had no disclosures.
NASHVILLE, TENN. – Young children with acute bronchiolitis do not need to be admitted to the pediatric ICU for high-flow nasal cannula treatment of up to 6 L/min and 50% oxygen; it is safe to administer it on the floor, according to a review of 6,804 acute bronchiolitis cases in children younger than 2 years treated at the University of Texas Southwestern Medical Center, Dallas.
Use of high-flow nasal cannulas (HFNC) has increased dramatically in recent years at UT Southwestern and elsewhere. It soothes children and can rapidly improve breathing without the nasal edema and nose bleeds common with cooler, drier, 100% oxygen. At Southwestern, HFNC use on the pediatric wards increased from 5% of acute bronchiolitis cases in the September 2010 to April 2011 season to 60% in the 2015-2016 season. Use for bronchiolitis in the PICU increased from 82% to 98% over the same period.
The increase correlated with a drop in intubation for acute bronchiolitis from 14% of children in 2010-2011 to just 2% in 2015-2016. The only HFNC adverse events were minor air leaks in two children.
As HFNC became more common, however, the Dallas team found that length of stay for acute bronchiolitis increased from 1.8 days in 2011-2012 to 2.4 days in 2015-2016, perhaps because the use of HFNC gives providers the impression that children are sicker than they actually are.
To counter the problem, lead investigator Vineeta Mittal, MD, associate professor of pediatrics, and her colleagues created an HFNC weaning protocol that gradually steps down treatment based on blood oxygen saturation levels and breathing effort, leading ultimately to a room-air challenge. It helped; the mean length of stay as of November 2016 was 1.7 days.
There’s been pushback in some places about giving HFNC on the floor: Intensivists sometimes consider it a form of ventilation that should be administered in the PICU. At levels up to 6 L/min and 50% oxygen, though, HFNC is “safe to give on the floor, because there’s no pneumothorax risk,” Dr. Mittal explained. HFNC “is not a ventilator; it’s an effective form of noninvasive respiratory support in children with moderate to severe respiratory distress from bronchiolitis.”
At Southwestern, “we are managing 80% of cases on the floor” with the help of HFNC, Dr. Mittal said at Pediatric Hospital Medicine.
At least for now, children at Southwestern go to the PICU if they need higher flow rates, but Dr. Mittal said it’s not clear if that’s necessary. “We said [6 L/min] is safe,” but maybe “we could even use 8 L/min or even 12 L/min” – the maximum delivered in the PICU over the study period – “because we know it’s safe,” she said. In addition, keeping kids on the floor also saves money, she noted at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Dr. Mittal is concerned HFNC might be overused. “We have gotten so used to this machine that the moment we see distress, we put the kid on high flow,” rather than observing them for a bit to see if they recover on their own. More data are needed to determine when HFNC should be initiated, and when to pull the plug on HFNC and intubate, she said.
Dr. Mittal had no disclosures.
AT PHM 2017
Key clinical point:
Major finding: The increased use of HFNC corresponded with an increase in length of stay for acute bronchiolitis, from 1.8 days in the 2011-2012 season to 2.4 days in the 2015-2016 season.
Data source: A single-center review of almost 7,000 acute bronchiolitis cases.
Disclosures: The lead investigator had no disclosures.
Short Takes
Condition Help: A patient- and family-initiated rapid response system
Implementation of a patient/family-initiated rapid response system at an academic, urban medical center resulted in 367 calls over 3½ years with 83.4% of them being for “nonsafety” issues and 11.4% being for “safety” issues.
Citation: Elizabeth L. Eden, MD, Laurie L. Rack, DNP, RN, Ling-Wan Chen, MS, Bump GM, Condition Help: A patient- and family-initiated rapid response system. J Hosp Med. 2017;3;157-161. doi: 10.12788/jhm.2697.
Association between U.S. norepinephrine shortage and mortality among patients with septic shock
Citation: Vail E, Gershengorn HB, Hua M, Walkey AJ, Rubenfeld G, Wunsch H. Association Between US Norepinephrine Shortage and Mortality Among Patients With Septic Shock. JAMA. 2017;317(14):1433-1442. doi: 10.1001/jama.2017.2841
Patient mortality during unannounced accreditation surveys at U.S. hospitals
An evaluation of quasi-randomized Medicare admissions at 1,984 hospitals demonstrated that 30-day mortality decreased by 0.18% in all hospitals and 0.48% at major teaching hospitals during The Joint Commission survey periods; both changes were greater than could be attributed to chance alone when compared to other, similar time periods.
Citation: Barnett ML, Olenski AR, Jena AB. Patient Mortality During Unannounced Accreditation Surveys at US Hospitals. JAMA Intern Med. 2017;177(5):693-700. doi: 10.1001/jamainternmed.2016.9685
Association between a virtual glucose management service and glycemic control in hospitalized adult patients
Institution of a virtual glucose management system resulted in a 39% decrease in hyperglycemic patients and a 36% decrease in hypoglycemic patients per 100 patient-days at three major teaching hospitals.
Citation: Rushakoff RJ, Sullivan MM, MacMaster HW, Shah AD, Rajkomar A, Glidden DV, et al. Association Between a Virtual Glucose Management Service and Glycemic Control in Hospitalized Adult Patients: An Observational Study. Ann Intern Med. 2017;166:621-627. doi: 10.7326/M16-1413
Dr. Imber is assistant professor in the division of hospital medicine at the University of New Mexico.
Condition Help: A patient- and family-initiated rapid response system
Implementation of a patient/family-initiated rapid response system at an academic, urban medical center resulted in 367 calls over 3½ years with 83.4% of them being for “nonsafety” issues and 11.4% being for “safety” issues.
Citation: Elizabeth L. Eden, MD, Laurie L. Rack, DNP, RN, Ling-Wan Chen, MS, Bump GM, Condition Help: A patient- and family-initiated rapid response system. J Hosp Med. 2017;3;157-161. doi: 10.12788/jhm.2697.
Association between U.S. norepinephrine shortage and mortality among patients with septic shock
Citation: Vail E, Gershengorn HB, Hua M, Walkey AJ, Rubenfeld G, Wunsch H. Association Between US Norepinephrine Shortage and Mortality Among Patients With Septic Shock. JAMA. 2017;317(14):1433-1442. doi: 10.1001/jama.2017.2841
Patient mortality during unannounced accreditation surveys at U.S. hospitals
An evaluation of quasi-randomized Medicare admissions at 1,984 hospitals demonstrated that 30-day mortality decreased by 0.18% in all hospitals and 0.48% at major teaching hospitals during The Joint Commission survey periods; both changes were greater than could be attributed to chance alone when compared to other, similar time periods.
Citation: Barnett ML, Olenski AR, Jena AB. Patient Mortality During Unannounced Accreditation Surveys at US Hospitals. JAMA Intern Med. 2017;177(5):693-700. doi: 10.1001/jamainternmed.2016.9685
Association between a virtual glucose management service and glycemic control in hospitalized adult patients
Institution of a virtual glucose management system resulted in a 39% decrease in hyperglycemic patients and a 36% decrease in hypoglycemic patients per 100 patient-days at three major teaching hospitals.
Citation: Rushakoff RJ, Sullivan MM, MacMaster HW, Shah AD, Rajkomar A, Glidden DV, et al. Association Between a Virtual Glucose Management Service and Glycemic Control in Hospitalized Adult Patients: An Observational Study. Ann Intern Med. 2017;166:621-627. doi: 10.7326/M16-1413
Dr. Imber is assistant professor in the division of hospital medicine at the University of New Mexico.
Condition Help: A patient- and family-initiated rapid response system
Implementation of a patient/family-initiated rapid response system at an academic, urban medical center resulted in 367 calls over 3½ years with 83.4% of them being for “nonsafety” issues and 11.4% being for “safety” issues.
Citation: Elizabeth L. Eden, MD, Laurie L. Rack, DNP, RN, Ling-Wan Chen, MS, Bump GM, Condition Help: A patient- and family-initiated rapid response system. J Hosp Med. 2017;3;157-161. doi: 10.12788/jhm.2697.
Association between U.S. norepinephrine shortage and mortality among patients with septic shock
Citation: Vail E, Gershengorn HB, Hua M, Walkey AJ, Rubenfeld G, Wunsch H. Association Between US Norepinephrine Shortage and Mortality Among Patients With Septic Shock. JAMA. 2017;317(14):1433-1442. doi: 10.1001/jama.2017.2841
Patient mortality during unannounced accreditation surveys at U.S. hospitals
An evaluation of quasi-randomized Medicare admissions at 1,984 hospitals demonstrated that 30-day mortality decreased by 0.18% in all hospitals and 0.48% at major teaching hospitals during The Joint Commission survey periods; both changes were greater than could be attributed to chance alone when compared to other, similar time periods.
Citation: Barnett ML, Olenski AR, Jena AB. Patient Mortality During Unannounced Accreditation Surveys at US Hospitals. JAMA Intern Med. 2017;177(5):693-700. doi: 10.1001/jamainternmed.2016.9685
Association between a virtual glucose management service and glycemic control in hospitalized adult patients
Institution of a virtual glucose management system resulted in a 39% decrease in hyperglycemic patients and a 36% decrease in hypoglycemic patients per 100 patient-days at three major teaching hospitals.
Citation: Rushakoff RJ, Sullivan MM, MacMaster HW, Shah AD, Rajkomar A, Glidden DV, et al. Association Between a Virtual Glucose Management Service and Glycemic Control in Hospitalized Adult Patients: An Observational Study. Ann Intern Med. 2017;166:621-627. doi: 10.7326/M16-1413
Dr. Imber is assistant professor in the division of hospital medicine at the University of New Mexico.