User login
FDA approves Xofluza for treatment of influenza
The Food and Drug Administration has approved Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for 48 hours or less.
The FDA approval is based on results from two randomized, clinical trials. In both trials, patients who received Xofluza experienced a shorter duration until alleviation of symptoms, compared with patients who received a placebo. In the second trial, patients who received Xofluza and patients who received another approved antiviral influenza medication experienced similar durations until symptom alleviation.
“When treatment is started within 48 hours of becoming sick with flu symptoms, antiviral drugs can lessen symptoms and shorten the time patients feel sick. Having more treatment options that work in different ways to attack the virus is important because flu viruses can become resistant to antiviral drugs,” Debra Birnkrant, MD, director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
The most common adverse events associated with Xofluza were diarrhea and bronchitis.
“This is the first new antiviral flu treatment with a novel mechanism of action approved by the FDA in nearly 20 years,” FDA Commissioner Scott Gottlieb, MD, added. “With thousands of people getting the flu every year, and many people becoming seriously ill, having safe and effective treatment alternatives is critical. This novel drug provides an important, additional treatment option.”
Find the full press release on the FDA website.
The Food and Drug Administration has approved Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for 48 hours or less.
The FDA approval is based on results from two randomized, clinical trials. In both trials, patients who received Xofluza experienced a shorter duration until alleviation of symptoms, compared with patients who received a placebo. In the second trial, patients who received Xofluza and patients who received another approved antiviral influenza medication experienced similar durations until symptom alleviation.
“When treatment is started within 48 hours of becoming sick with flu symptoms, antiviral drugs can lessen symptoms and shorten the time patients feel sick. Having more treatment options that work in different ways to attack the virus is important because flu viruses can become resistant to antiviral drugs,” Debra Birnkrant, MD, director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
The most common adverse events associated with Xofluza were diarrhea and bronchitis.
“This is the first new antiviral flu treatment with a novel mechanism of action approved by the FDA in nearly 20 years,” FDA Commissioner Scott Gottlieb, MD, added. “With thousands of people getting the flu every year, and many people becoming seriously ill, having safe and effective treatment alternatives is critical. This novel drug provides an important, additional treatment option.”
Find the full press release on the FDA website.
The Food and Drug Administration has approved Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for 48 hours or less.
The FDA approval is based on results from two randomized, clinical trials. In both trials, patients who received Xofluza experienced a shorter duration until alleviation of symptoms, compared with patients who received a placebo. In the second trial, patients who received Xofluza and patients who received another approved antiviral influenza medication experienced similar durations until symptom alleviation.
“When treatment is started within 48 hours of becoming sick with flu symptoms, antiviral drugs can lessen symptoms and shorten the time patients feel sick. Having more treatment options that work in different ways to attack the virus is important because flu viruses can become resistant to antiviral drugs,” Debra Birnkrant, MD, director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
The most common adverse events associated with Xofluza were diarrhea and bronchitis.
“This is the first new antiviral flu treatment with a novel mechanism of action approved by the FDA in nearly 20 years,” FDA Commissioner Scott Gottlieb, MD, added. “With thousands of people getting the flu every year, and many people becoming seriously ill, having safe and effective treatment alternatives is critical. This novel drug provides an important, additional treatment option.”
Find the full press release on the FDA website.
Stroke risk in elderly following AMI extends to 12 weeks
ATLANTA – Acute myocardial infarction is associated with a risk of stroke that extends beyond the 1-month time window currently considered the at-risk period, according to an analysis of Medicare data.
“The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiology classification systems and clinical trial selection criteria,” lead study author Alexander E. Merkler, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In an effort to better understand the duration of heightened stroke risk after acute myocardial infarction, Dr. Merkler, a neurologist at New York–based Weill Cornell Medicine, and his colleagues conducted a retrospective cohort study using inpatient and outpatient claims during 2008-2015 from a nationally representative 5% sample of Medicare beneficiaries who were at least 66 years old. They used previously validated ICD-9-CM diagnosis codes to ascertain the exposure variable of acute MI and the outcome of ischemic stroke but excluded strokes that occurred during an acute MI hospitalization.
Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or by Sept. 30, 2015. The researchers fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjusting for demographics, stroke risk factors, and Charlson comorbidities. Next, they used the corresponding survival probabilities to compute the hazard ratio (HR) in each 4-week interval after discharge, up to week 12. They also conducted a subgroup analysis to evaluate the duration of heightened ischemic stroke risk by MI type: ST-segment elevation MI (STEMI) versus non-STEMI (NSTEMI).
Dr. Merkler and his colleagues drew from data on 1.7 million eligible beneficiaries. Of these, 46,182 were hospitalized for acute MI and 80,466 for ischemic stroke. After they adjusted for demographics, stroke risk factors, and Charlson comorbidities, the researchers found that the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6). It was no longer significantly elevated afterward. The prolonged period of heightened ischemic stroke risk was evident in patients with both STEMI and NSTEMI.
“We were surprised by how long the risk of stroke lasts after MI,” Dr. Merkler said. He acknowledged certain limitations of the analysis, including the fact that patients were all over the age of 65 years. “In addition, we lack granular detail such as severity of MI [and] the extent of stroke work-up,” he said.
Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
[email protected]
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
ATLANTA – Acute myocardial infarction is associated with a risk of stroke that extends beyond the 1-month time window currently considered the at-risk period, according to an analysis of Medicare data.
“The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiology classification systems and clinical trial selection criteria,” lead study author Alexander E. Merkler, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In an effort to better understand the duration of heightened stroke risk after acute myocardial infarction, Dr. Merkler, a neurologist at New York–based Weill Cornell Medicine, and his colleagues conducted a retrospective cohort study using inpatient and outpatient claims during 2008-2015 from a nationally representative 5% sample of Medicare beneficiaries who were at least 66 years old. They used previously validated ICD-9-CM diagnosis codes to ascertain the exposure variable of acute MI and the outcome of ischemic stroke but excluded strokes that occurred during an acute MI hospitalization.
Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or by Sept. 30, 2015. The researchers fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjusting for demographics, stroke risk factors, and Charlson comorbidities. Next, they used the corresponding survival probabilities to compute the hazard ratio (HR) in each 4-week interval after discharge, up to week 12. They also conducted a subgroup analysis to evaluate the duration of heightened ischemic stroke risk by MI type: ST-segment elevation MI (STEMI) versus non-STEMI (NSTEMI).
Dr. Merkler and his colleagues drew from data on 1.7 million eligible beneficiaries. Of these, 46,182 were hospitalized for acute MI and 80,466 for ischemic stroke. After they adjusted for demographics, stroke risk factors, and Charlson comorbidities, the researchers found that the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6). It was no longer significantly elevated afterward. The prolonged period of heightened ischemic stroke risk was evident in patients with both STEMI and NSTEMI.
“We were surprised by how long the risk of stroke lasts after MI,” Dr. Merkler said. He acknowledged certain limitations of the analysis, including the fact that patients were all over the age of 65 years. “In addition, we lack granular detail such as severity of MI [and] the extent of stroke work-up,” he said.
Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
[email protected]
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
ATLANTA – Acute myocardial infarction is associated with a risk of stroke that extends beyond the 1-month time window currently considered the at-risk period, according to an analysis of Medicare data.
“The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiology classification systems and clinical trial selection criteria,” lead study author Alexander E. Merkler, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In an effort to better understand the duration of heightened stroke risk after acute myocardial infarction, Dr. Merkler, a neurologist at New York–based Weill Cornell Medicine, and his colleagues conducted a retrospective cohort study using inpatient and outpatient claims during 2008-2015 from a nationally representative 5% sample of Medicare beneficiaries who were at least 66 years old. They used previously validated ICD-9-CM diagnosis codes to ascertain the exposure variable of acute MI and the outcome of ischemic stroke but excluded strokes that occurred during an acute MI hospitalization.
Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or by Sept. 30, 2015. The researchers fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjusting for demographics, stroke risk factors, and Charlson comorbidities. Next, they used the corresponding survival probabilities to compute the hazard ratio (HR) in each 4-week interval after discharge, up to week 12. They also conducted a subgroup analysis to evaluate the duration of heightened ischemic stroke risk by MI type: ST-segment elevation MI (STEMI) versus non-STEMI (NSTEMI).
Dr. Merkler and his colleagues drew from data on 1.7 million eligible beneficiaries. Of these, 46,182 were hospitalized for acute MI and 80,466 for ischemic stroke. After they adjusted for demographics, stroke risk factors, and Charlson comorbidities, the researchers found that the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6). It was no longer significantly elevated afterward. The prolonged period of heightened ischemic stroke risk was evident in patients with both STEMI and NSTEMI.
“We were surprised by how long the risk of stroke lasts after MI,” Dr. Merkler said. He acknowledged certain limitations of the analysis, including the fact that patients were all over the age of 65 years. “In addition, we lack granular detail such as severity of MI [and] the extent of stroke work-up,” he said.
Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
[email protected]
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
AT ANA 2018
Key clinical point: .
Major finding: The risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6).
Study details: An analysis of 46,182 Medicare beneficiaries who were hospitalized for acute MI and 80,466 who were hospitalized for ischemic stroke.
Disclosures: Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
Drug overdose deaths down since late 2017
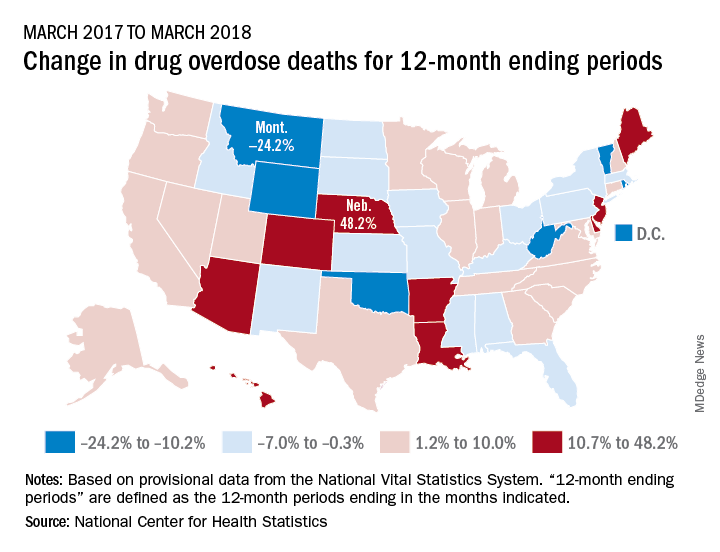
Longer-term data, however, show an increase over the year from March 2017 to March 2018, as the short-term decrease was not enough to overcome the previous year’s increase. The provisional 12-month ending count – deaths during the 12-month period ending in the month indicated – went from 66,859 in March 2017 to 68,690 in March 2018, an increase of 2.7%, the NCHS reported.
That year-long increase was not spread evenly among the states. Nebraska’s 12-month ending count jumped over 48% from March 2017 to March 2018, more than twice as much as second-place Hawaii’s 20.9%. Montana had the largest drop over that year, –24.2%, with Wyoming next at –20.7% and the District of Columbia third at –14.8%, data from the National Vital Statistics System show.
“Provisional drug overdose death data are often incomplete,” the NCHS noted, “and the degree of completeness varies by jurisdiction and 12-month ending period. Consequently, the numbers of drug overdose deaths are underestimated, based on provisional data relative to final data and are subject to random variation.”

Longer-term data, however, show an increase over the year from March 2017 to March 2018, as the short-term decrease was not enough to overcome the previous year’s increase. The provisional 12-month ending count – deaths during the 12-month period ending in the month indicated – went from 66,859 in March 2017 to 68,690 in March 2018, an increase of 2.7%, the NCHS reported.
That year-long increase was not spread evenly among the states. Nebraska’s 12-month ending count jumped over 48% from March 2017 to March 2018, more than twice as much as second-place Hawaii’s 20.9%. Montana had the largest drop over that year, –24.2%, with Wyoming next at –20.7% and the District of Columbia third at –14.8%, data from the National Vital Statistics System show.
“Provisional drug overdose death data are often incomplete,” the NCHS noted, “and the degree of completeness varies by jurisdiction and 12-month ending period. Consequently, the numbers of drug overdose deaths are underestimated, based on provisional data relative to final data and are subject to random variation.”

Longer-term data, however, show an increase over the year from March 2017 to March 2018, as the short-term decrease was not enough to overcome the previous year’s increase. The provisional 12-month ending count – deaths during the 12-month period ending in the month indicated – went from 66,859 in March 2017 to 68,690 in March 2018, an increase of 2.7%, the NCHS reported.
That year-long increase was not spread evenly among the states. Nebraska’s 12-month ending count jumped over 48% from March 2017 to March 2018, more than twice as much as second-place Hawaii’s 20.9%. Montana had the largest drop over that year, –24.2%, with Wyoming next at –20.7% and the District of Columbia third at –14.8%, data from the National Vital Statistics System show.
“Provisional drug overdose death data are often incomplete,” the NCHS noted, “and the degree of completeness varies by jurisdiction and 12-month ending period. Consequently, the numbers of drug overdose deaths are underestimated, based on provisional data relative to final data and are subject to random variation.”
Reducing alarm fatigue
Monitoring from a centralized location
Hospitalists hearing the constant noise from cardiac telemetry monitoring systems can experience alarm fatigue – a nationwide phenomenon that can lead to an increase in patient deaths.
The American Heart Association reports that fewer than one in four adults survived an in-hospital cardiac arrest in 2013; other studies showed that up to 44% of inpatient cardiac arrests were not detected appropriately, according to the Cleveland Clinic.
Clinicians at the Cleveland Clinic have tried centralized monitoring to address the problem. They’ve established a “mission control” center, where off-site personnel monitor sensors and high-definition cameras and vital signs such as blood pressure, heart rate, and respiration. On-site action is requested when appropriate; unimportant alarms are dismissed.
In August 2016, results from the first 13 months of the Cleveland Clinic program were published in JAMA. They revealed that the monitoring system could help reduce rates of unimportant alarms with no increase in cardiopulmonary arrest events. The centralized unit monitored 99,048 patient orders, and ultimately detected serious problems and accurately notified on-site staff for 79% of 3,243 events, which included a rhythm and/or rate change within 1 hour or less of the event. Accurate notification to on-site hospital staff was more than 84%.
Since then, improvements to the system have continued, according to Cleveland Clinic, and include doubling the number of monitored patients per technician and improved clinical outcomes.
Reference
Cleveland Clinic: Consult QD – An update on the centralized cardiac telemetry monitoring unit
Monitoring from a centralized location
Monitoring from a centralized location
Hospitalists hearing the constant noise from cardiac telemetry monitoring systems can experience alarm fatigue – a nationwide phenomenon that can lead to an increase in patient deaths.
The American Heart Association reports that fewer than one in four adults survived an in-hospital cardiac arrest in 2013; other studies showed that up to 44% of inpatient cardiac arrests were not detected appropriately, according to the Cleveland Clinic.
Clinicians at the Cleveland Clinic have tried centralized monitoring to address the problem. They’ve established a “mission control” center, where off-site personnel monitor sensors and high-definition cameras and vital signs such as blood pressure, heart rate, and respiration. On-site action is requested when appropriate; unimportant alarms are dismissed.
In August 2016, results from the first 13 months of the Cleveland Clinic program were published in JAMA. They revealed that the monitoring system could help reduce rates of unimportant alarms with no increase in cardiopulmonary arrest events. The centralized unit monitored 99,048 patient orders, and ultimately detected serious problems and accurately notified on-site staff for 79% of 3,243 events, which included a rhythm and/or rate change within 1 hour or less of the event. Accurate notification to on-site hospital staff was more than 84%.
Since then, improvements to the system have continued, according to Cleveland Clinic, and include doubling the number of monitored patients per technician and improved clinical outcomes.
Reference
Cleveland Clinic: Consult QD – An update on the centralized cardiac telemetry monitoring unit
Hospitalists hearing the constant noise from cardiac telemetry monitoring systems can experience alarm fatigue – a nationwide phenomenon that can lead to an increase in patient deaths.
The American Heart Association reports that fewer than one in four adults survived an in-hospital cardiac arrest in 2013; other studies showed that up to 44% of inpatient cardiac arrests were not detected appropriately, according to the Cleveland Clinic.
Clinicians at the Cleveland Clinic have tried centralized monitoring to address the problem. They’ve established a “mission control” center, where off-site personnel monitor sensors and high-definition cameras and vital signs such as blood pressure, heart rate, and respiration. On-site action is requested when appropriate; unimportant alarms are dismissed.
In August 2016, results from the first 13 months of the Cleveland Clinic program were published in JAMA. They revealed that the monitoring system could help reduce rates of unimportant alarms with no increase in cardiopulmonary arrest events. The centralized unit monitored 99,048 patient orders, and ultimately detected serious problems and accurately notified on-site staff for 79% of 3,243 events, which included a rhythm and/or rate change within 1 hour or less of the event. Accurate notification to on-site hospital staff was more than 84%.
Since then, improvements to the system have continued, according to Cleveland Clinic, and include doubling the number of monitored patients per technician and improved clinical outcomes.
Reference
Cleveland Clinic: Consult QD – An update on the centralized cardiac telemetry monitoring unit
Readmission to non-index hospital following acute stroke linked to worse outcomes
ATLANTA – Following an acute stroke, optimizing stroke secondary prevention measures, medical complications, and transitions of care is essential to reducing 30-day readmissions and improving patient outcomes, a large analysis of national data showed.
“Care that is fragmented with readmissions to other hospitals results not only in more expensive care and longer length of stay but also increased mortality for our acute stroke patients,” lead study author Laura K. Stein, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In 2017, a study of the Nationwide Readmissions Database demonstrated that 12.1% of patients with acute ischemic stroke were readmitted within 30 days (Stroke 2017;48:1386-8). It cited that 89.6% were unplanned and 12.9% were preventable. “However, this study did not examine whether patients were admitted to the discharging hospital or a different hospital,” said Dr. Stein, a neurologist at the Icahn School of Medicine at Mount Sinai, New York. “Furthermore, it did not include metrics such as cost, length of stay, and mortality with 30-day readmissions. Hospitals are increasingly held accountable and penalized for metrics such as length of stay and 30-day readmissions.”
In 2010, the Centers for Medicare & Medicaid Services introduced the Hospital Readmissions Reduction Program in an attempt to decrease readmissions following hospitalizations for acute myocardial infarction, heart failure, and pneumonia. “In 2012, CMS started reducing Medicare payments for hospitals with excess readmissions,” said Dr. Stein, who is a fellowship-trained stroke specialist. “While readmission to the same hospital has great implications for hospital systems, any readmission has great implications for patients.”
In what is believed to be the first study of its kind, Dr. Stein and her colleagues drew from the 2013 Nationwide Readmissions Database to examine in-hospital outcomes associated with 30-day readmission to a different hospital for acute ischemic stroke. They used ICD-9 codes to identify index stroke admissions and all-cause readmissions. Outcomes of interest were length of stay, total charges, and in-hospital mortality during the 30-day readmission. The main predictor was readmission to another hospital, compared with readmission to the same hospital as the index acute stroke admission. The researchers used linear regression for the outcomes of length of stay and charges, and logistic regression for in-hospital mortality. They adjusted for several variables during the index admission, including age, sex, vascular risk factors, hospital bed size, teaching hospital status, insurance status, discharge destination, National Center for Health Statistics urban-rural location classification, length of stay, and total charges.
Of 24,545 acute stroke patients readmitted within 30 days, 7,274 (30%) were readmitted to a different hospital. The top three reasons for readmission were acute cerebrovascular disease, septicemia, and renal failure. In fully adjusted models, readmission to a different hospital was associated with an increased length of stay of 0.97 days (P less than .0001) and a mean of $7,677.28 greater total charges, compared with readmission to the same hospital (P less than .0001). The fully adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
“While it is conceivable that cost and length of stay could be higher with readmission to a different hospital because of a need for additional testing with a lack of familiarity with the patient, it is concerning that mortality is higher,” Dr. Stein said. “These findings emphasize the importance of optimizing secondary stroke prevention and medical complications following acute stroke before discharge. Additionally, they emphasize the importance of good transitions of care from the inpatient to outpatient setting (whether that’s to a rehabilitation facility, skilled nursing facility, or home) and accessibility of the discharging stroke team after discharge.”
She acknowledged certain limitations of the analysis, including its reliance of administrative data, which could include misclassification of diagnoses and comorbidities based on ICD-9 codes. “However, we have chosen ICD-9 codes for stroke that have been previously validated in the literature,” Dr. Stein said. “For instance, the validated codes for stroke as the primary discharge diagnosis have a sensitivity of 74%, specificity of 95%, and positive predictive value of 88%. Second, we do not know stroke subtype or severity of stroke. Third, we do not know what the transitions of care plan were when the patients left the hospital following index acute ischemic stroke admission and why these patients ended up being readmitted to a different hospital rather than the one that treated them for their acute stroke.”
The researchers reported having no financial disclosures.
SOURCE: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
ATLANTA – Following an acute stroke, optimizing stroke secondary prevention measures, medical complications, and transitions of care is essential to reducing 30-day readmissions and improving patient outcomes, a large analysis of national data showed.
“Care that is fragmented with readmissions to other hospitals results not only in more expensive care and longer length of stay but also increased mortality for our acute stroke patients,” lead study author Laura K. Stein, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In 2017, a study of the Nationwide Readmissions Database demonstrated that 12.1% of patients with acute ischemic stroke were readmitted within 30 days (Stroke 2017;48:1386-8). It cited that 89.6% were unplanned and 12.9% were preventable. “However, this study did not examine whether patients were admitted to the discharging hospital or a different hospital,” said Dr. Stein, a neurologist at the Icahn School of Medicine at Mount Sinai, New York. “Furthermore, it did not include metrics such as cost, length of stay, and mortality with 30-day readmissions. Hospitals are increasingly held accountable and penalized for metrics such as length of stay and 30-day readmissions.”
In 2010, the Centers for Medicare & Medicaid Services introduced the Hospital Readmissions Reduction Program in an attempt to decrease readmissions following hospitalizations for acute myocardial infarction, heart failure, and pneumonia. “In 2012, CMS started reducing Medicare payments for hospitals with excess readmissions,” said Dr. Stein, who is a fellowship-trained stroke specialist. “While readmission to the same hospital has great implications for hospital systems, any readmission has great implications for patients.”
In what is believed to be the first study of its kind, Dr. Stein and her colleagues drew from the 2013 Nationwide Readmissions Database to examine in-hospital outcomes associated with 30-day readmission to a different hospital for acute ischemic stroke. They used ICD-9 codes to identify index stroke admissions and all-cause readmissions. Outcomes of interest were length of stay, total charges, and in-hospital mortality during the 30-day readmission. The main predictor was readmission to another hospital, compared with readmission to the same hospital as the index acute stroke admission. The researchers used linear regression for the outcomes of length of stay and charges, and logistic regression for in-hospital mortality. They adjusted for several variables during the index admission, including age, sex, vascular risk factors, hospital bed size, teaching hospital status, insurance status, discharge destination, National Center for Health Statistics urban-rural location classification, length of stay, and total charges.
Of 24,545 acute stroke patients readmitted within 30 days, 7,274 (30%) were readmitted to a different hospital. The top three reasons for readmission were acute cerebrovascular disease, septicemia, and renal failure. In fully adjusted models, readmission to a different hospital was associated with an increased length of stay of 0.97 days (P less than .0001) and a mean of $7,677.28 greater total charges, compared with readmission to the same hospital (P less than .0001). The fully adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
“While it is conceivable that cost and length of stay could be higher with readmission to a different hospital because of a need for additional testing with a lack of familiarity with the patient, it is concerning that mortality is higher,” Dr. Stein said. “These findings emphasize the importance of optimizing secondary stroke prevention and medical complications following acute stroke before discharge. Additionally, they emphasize the importance of good transitions of care from the inpatient to outpatient setting (whether that’s to a rehabilitation facility, skilled nursing facility, or home) and accessibility of the discharging stroke team after discharge.”
She acknowledged certain limitations of the analysis, including its reliance of administrative data, which could include misclassification of diagnoses and comorbidities based on ICD-9 codes. “However, we have chosen ICD-9 codes for stroke that have been previously validated in the literature,” Dr. Stein said. “For instance, the validated codes for stroke as the primary discharge diagnosis have a sensitivity of 74%, specificity of 95%, and positive predictive value of 88%. Second, we do not know stroke subtype or severity of stroke. Third, we do not know what the transitions of care plan were when the patients left the hospital following index acute ischemic stroke admission and why these patients ended up being readmitted to a different hospital rather than the one that treated them for their acute stroke.”
The researchers reported having no financial disclosures.
SOURCE: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
ATLANTA – Following an acute stroke, optimizing stroke secondary prevention measures, medical complications, and transitions of care is essential to reducing 30-day readmissions and improving patient outcomes, a large analysis of national data showed.
“Care that is fragmented with readmissions to other hospitals results not only in more expensive care and longer length of stay but also increased mortality for our acute stroke patients,” lead study author Laura K. Stein, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In 2017, a study of the Nationwide Readmissions Database demonstrated that 12.1% of patients with acute ischemic stroke were readmitted within 30 days (Stroke 2017;48:1386-8). It cited that 89.6% were unplanned and 12.9% were preventable. “However, this study did not examine whether patients were admitted to the discharging hospital or a different hospital,” said Dr. Stein, a neurologist at the Icahn School of Medicine at Mount Sinai, New York. “Furthermore, it did not include metrics such as cost, length of stay, and mortality with 30-day readmissions. Hospitals are increasingly held accountable and penalized for metrics such as length of stay and 30-day readmissions.”
In 2010, the Centers for Medicare & Medicaid Services introduced the Hospital Readmissions Reduction Program in an attempt to decrease readmissions following hospitalizations for acute myocardial infarction, heart failure, and pneumonia. “In 2012, CMS started reducing Medicare payments for hospitals with excess readmissions,” said Dr. Stein, who is a fellowship-trained stroke specialist. “While readmission to the same hospital has great implications for hospital systems, any readmission has great implications for patients.”
In what is believed to be the first study of its kind, Dr. Stein and her colleagues drew from the 2013 Nationwide Readmissions Database to examine in-hospital outcomes associated with 30-day readmission to a different hospital for acute ischemic stroke. They used ICD-9 codes to identify index stroke admissions and all-cause readmissions. Outcomes of interest were length of stay, total charges, and in-hospital mortality during the 30-day readmission. The main predictor was readmission to another hospital, compared with readmission to the same hospital as the index acute stroke admission. The researchers used linear regression for the outcomes of length of stay and charges, and logistic regression for in-hospital mortality. They adjusted for several variables during the index admission, including age, sex, vascular risk factors, hospital bed size, teaching hospital status, insurance status, discharge destination, National Center for Health Statistics urban-rural location classification, length of stay, and total charges.
Of 24,545 acute stroke patients readmitted within 30 days, 7,274 (30%) were readmitted to a different hospital. The top three reasons for readmission were acute cerebrovascular disease, septicemia, and renal failure. In fully adjusted models, readmission to a different hospital was associated with an increased length of stay of 0.97 days (P less than .0001) and a mean of $7,677.28 greater total charges, compared with readmission to the same hospital (P less than .0001). The fully adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
“While it is conceivable that cost and length of stay could be higher with readmission to a different hospital because of a need for additional testing with a lack of familiarity with the patient, it is concerning that mortality is higher,” Dr. Stein said. “These findings emphasize the importance of optimizing secondary stroke prevention and medical complications following acute stroke before discharge. Additionally, they emphasize the importance of good transitions of care from the inpatient to outpatient setting (whether that’s to a rehabilitation facility, skilled nursing facility, or home) and accessibility of the discharging stroke team after discharge.”
She acknowledged certain limitations of the analysis, including its reliance of administrative data, which could include misclassification of diagnoses and comorbidities based on ICD-9 codes. “However, we have chosen ICD-9 codes for stroke that have been previously validated in the literature,” Dr. Stein said. “For instance, the validated codes for stroke as the primary discharge diagnosis have a sensitivity of 74%, specificity of 95%, and positive predictive value of 88%. Second, we do not know stroke subtype or severity of stroke. Third, we do not know what the transitions of care plan were when the patients left the hospital following index acute ischemic stroke admission and why these patients ended up being readmitted to a different hospital rather than the one that treated them for their acute stroke.”
The researchers reported having no financial disclosures.
SOURCE: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
REPORTING FROM ANA 2018
Key clinical point:
Major finding: The adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
Study details: A review of 24,545 acute stroke patients 2013 from the Nationwide Readmissions Database.
Disclosures: The researchers reported having no financial disclosures.
Source: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
Blood test may obviate need for head CTs in brain trauma evaluation
SAN DIEGO – A biomarker test based on the presence of two proteins in the blood appears to be suitable for ruling out significant intracranial injuries in patients with a history of mild traumatic brain injury (TBI) without the need for a CT head scan, according to data presented at the annual meeting of the American College of Emergency Physicians.
according to Jeffrey J. Bazarian, MD, professor of emergency medicine, University of Rochester (New York).
In the ALERT-TBI study, which evaluated the biomarker test, 1,959 patients with suspected TBI at 22 participating EDs in the United States and Europe were enrolled and available for analysis. All had mild TBI as defined as a Glasgow Coma Scale (GCS) score of 13-15.
The treating ED physician’s decision to order a head CT scan was the major criterion for study entry. All enrolled patients had their blood drawn within 12 hours in order to quantify two biomarkers, C-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP).
The biomarker test for TBI was negative when the UCH-L1 value was less than 327 pg/mL and the GFAP was less than 22 pg/mL; the test was positive if either value was at this threshold or higher. To evaluate the sensitivity and specificity of this dual-biomarker test, results were correlated with head CT scans read by two neurologists blinded to the biomarker values.
The mean age of the study population was 48.8 years and slightly more than half were male. About half of the suspected TBI in these patients was attributed to falls and about one third to motor vehicle accidents.
Typical of TBI with GCS scores in the mild range, only 6% of the patients had a positive CT head scan. Of the 125 positive CT scans, the most common injury detected on CT scan was subarachnoid hemorrhage followed by subdural hematoma.
Of the 671 negative biomarker tests, 668 had normal head CT scans. Of the three false positives, one included a cavernous malformation that may have been present prior to the TBI. The others were a small subarachnoid hemorrhage and a small subdural hematoma. Overall the negative predictive value was 99.6% and the sensitivity was 97.6%.
Although the biomarker specificity was only 36% with an even-lower positive predictive value, the goal of the test was to rule out significant TBI to avoid the need for CT scan. On this basis, the biomarker test, which is being developed under the proprietary name Banyan BTI, appears to be promising. The data, according to Dr. Bazarian, have been submitted to the Food and Drug Administration.
“Head CT scans are the current standard for evaluating intracranial injuries after TBI, but they are overused, based on the high proportion that do not show an injury,” said Dr. Bazarian. Although he does not know the disposition of the FDA application, he said, based on these data, “I would definitely be using this test if it were available.”
SAN DIEGO – A biomarker test based on the presence of two proteins in the blood appears to be suitable for ruling out significant intracranial injuries in patients with a history of mild traumatic brain injury (TBI) without the need for a CT head scan, according to data presented at the annual meeting of the American College of Emergency Physicians.
according to Jeffrey J. Bazarian, MD, professor of emergency medicine, University of Rochester (New York).
In the ALERT-TBI study, which evaluated the biomarker test, 1,959 patients with suspected TBI at 22 participating EDs in the United States and Europe were enrolled and available for analysis. All had mild TBI as defined as a Glasgow Coma Scale (GCS) score of 13-15.
The treating ED physician’s decision to order a head CT scan was the major criterion for study entry. All enrolled patients had their blood drawn within 12 hours in order to quantify two biomarkers, C-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP).
The biomarker test for TBI was negative when the UCH-L1 value was less than 327 pg/mL and the GFAP was less than 22 pg/mL; the test was positive if either value was at this threshold or higher. To evaluate the sensitivity and specificity of this dual-biomarker test, results were correlated with head CT scans read by two neurologists blinded to the biomarker values.
The mean age of the study population was 48.8 years and slightly more than half were male. About half of the suspected TBI in these patients was attributed to falls and about one third to motor vehicle accidents.
Typical of TBI with GCS scores in the mild range, only 6% of the patients had a positive CT head scan. Of the 125 positive CT scans, the most common injury detected on CT scan was subarachnoid hemorrhage followed by subdural hematoma.
Of the 671 negative biomarker tests, 668 had normal head CT scans. Of the three false positives, one included a cavernous malformation that may have been present prior to the TBI. The others were a small subarachnoid hemorrhage and a small subdural hematoma. Overall the negative predictive value was 99.6% and the sensitivity was 97.6%.
Although the biomarker specificity was only 36% with an even-lower positive predictive value, the goal of the test was to rule out significant TBI to avoid the need for CT scan. On this basis, the biomarker test, which is being developed under the proprietary name Banyan BTI, appears to be promising. The data, according to Dr. Bazarian, have been submitted to the Food and Drug Administration.
“Head CT scans are the current standard for evaluating intracranial injuries after TBI, but they are overused, based on the high proportion that do not show an injury,” said Dr. Bazarian. Although he does not know the disposition of the FDA application, he said, based on these data, “I would definitely be using this test if it were available.”
SAN DIEGO – A biomarker test based on the presence of two proteins in the blood appears to be suitable for ruling out significant intracranial injuries in patients with a history of mild traumatic brain injury (TBI) without the need for a CT head scan, according to data presented at the annual meeting of the American College of Emergency Physicians.
according to Jeffrey J. Bazarian, MD, professor of emergency medicine, University of Rochester (New York).
In the ALERT-TBI study, which evaluated the biomarker test, 1,959 patients with suspected TBI at 22 participating EDs in the United States and Europe were enrolled and available for analysis. All had mild TBI as defined as a Glasgow Coma Scale (GCS) score of 13-15.
The treating ED physician’s decision to order a head CT scan was the major criterion for study entry. All enrolled patients had their blood drawn within 12 hours in order to quantify two biomarkers, C-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP).
The biomarker test for TBI was negative when the UCH-L1 value was less than 327 pg/mL and the GFAP was less than 22 pg/mL; the test was positive if either value was at this threshold or higher. To evaluate the sensitivity and specificity of this dual-biomarker test, results were correlated with head CT scans read by two neurologists blinded to the biomarker values.
The mean age of the study population was 48.8 years and slightly more than half were male. About half of the suspected TBI in these patients was attributed to falls and about one third to motor vehicle accidents.
Typical of TBI with GCS scores in the mild range, only 6% of the patients had a positive CT head scan. Of the 125 positive CT scans, the most common injury detected on CT scan was subarachnoid hemorrhage followed by subdural hematoma.
Of the 671 negative biomarker tests, 668 had normal head CT scans. Of the three false positives, one included a cavernous malformation that may have been present prior to the TBI. The others were a small subarachnoid hemorrhage and a small subdural hematoma. Overall the negative predictive value was 99.6% and the sensitivity was 97.6%.
Although the biomarker specificity was only 36% with an even-lower positive predictive value, the goal of the test was to rule out significant TBI to avoid the need for CT scan. On this basis, the biomarker test, which is being developed under the proprietary name Banyan BTI, appears to be promising. The data, according to Dr. Bazarian, have been submitted to the Food and Drug Administration.
“Head CT scans are the current standard for evaluating intracranial injuries after TBI, but they are overused, based on the high proportion that do not show an injury,” said Dr. Bazarian. Although he does not know the disposition of the FDA application, he said, based on these data, “I would definitely be using this test if it were available.”
FROM ACEP 2018
Key clinical point: In patients with mild head trauma, a simple blood test may eliminate need and cost for routine CT scans.
Major finding: In patients a history of head trauma, the biomarker test had a 99.6% negative predictive value in ruling out injury.
Study details: Prospective, controlled registration study.
Disclosures: Dr. Bazarian reported no financial relationships relevant to this study, which was in part funded by Banyan Biomarkers.
DAPT’s benefit after stroke or TIA clusters in first 21 days
MONTREAL – The optimal length for dual antiplatelet therapy in patients who have just had a mild stroke or transient ischemic attack is 21 days, a duration of combined treatment that maximized protection against major ischemic events while minimizing the extra risk for a major hemorrhage, according to a prespecified analysis of data from the POINT trial.
The POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial randomized 4,881 patients with a very recent mild stroke or transient ischemic attack and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, dual antiplatelet therapy (DAPT) cut the incidence of a major ischemic event by a relative 25% but also more than doubled the rate of major hemorrhage (New Engl J Med. 2018 Jul 19;377[3]:215-25).
The new, prespecified analysis looked at outcomes on a week-by-week basis over the course of 90 days of treatment, and showed that during the first 21 days the rate of major ischemic events was 5.6% among patients on aspirin only and 3.6% among those on DAPT, a statistically significant 35% relative cut in these adverse outcomes by using DAPT, Jordan J. Elm, PhD, reported at the World Stroke Congress. During the subsequent 69 days on treatment, the incidence of major ischemic events was roughly 1% in both arms of the study, showing that after 3 weeks the incremental benefit from DAPT disappeared, said Dr. Elm, a biostatistician at the Medical University of South Carolina, Charleston.
In contrast, the doubled rate of major hemorrhages (mostly reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment, meaning that limiting DAPT to just 21 days could prevent many of the excess hemorrhages.
“These results suggest that limiting clopidogrel plus aspirin use to 21 days may maximize benefit and reduce risk,” Dr. Elm said, especially in light of the findings confirming the efficacy of 21 days of DAPT following a minor stroke or TIA that had been reported several years ago in the CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial (New Engl J Med. 2013 Jul 4;369[1]:11-9).
Although the new finding from the POINT results came in a secondary analysis, it’s statistically legitimate and should be taken into account when writing treatment guidelines, she said, emphasizing that “this is a very important analysis that is not just hypothesis generating.”
Another finding from the new analysis was that a large number of major ischemic events, and hence a large number of the events prevented by DAPT, occurred in the first 2 days following the index event, a finding made possible because the POINT investigators enrolled patients and started treatment within 12 hours of the qualifying events.
“It’s better to start treatment early,” Dr. Elm noted, but she also highlighted that major ischemic events continued to accumulate during days 3-21, suggesting that patients could still benefit from DAPT even if treatment did not start until 24 or 48 hours after their index event.
POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm reported no disclosures.
SOURCE: Elm JJ et al. World Stroke Congress, Late-breaking session.
The new model using data from the POINT trial confirms what had been previously shown in the CHANCE trial – that 21 days is a sensible cutoff for dual antiplatelet treatment for patients immediately following a mild stroke or transient ischemic attack. Treatment with dual antiplatelet therapy for 21 days provides the same added benefit as 90 days of treatment but with less excess bleeding. The new findings confirm that the CHANCE results were not specific to a Chinese population.

For the time being, clopidogrel is the evidence-based antiplatelet drug to pair with aspirin for this indication. Clopidogrel has the advantages of being generic, cheap, available, and familiar. It’s possible that another P2Y12 inhibitor, such as ticagrelor (Brilinta), might work even better, but that needs to be proven to justify the added expense of a brand-name antiplatelet drug.
Mike Sharma, MD , is a stroke neurologist at McMaster University, Hamilton, Ont. He has been an advisor to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. He made these comments in an interview.
The new model using data from the POINT trial confirms what had been previously shown in the CHANCE trial – that 21 days is a sensible cutoff for dual antiplatelet treatment for patients immediately following a mild stroke or transient ischemic attack. Treatment with dual antiplatelet therapy for 21 days provides the same added benefit as 90 days of treatment but with less excess bleeding. The new findings confirm that the CHANCE results were not specific to a Chinese population.

For the time being, clopidogrel is the evidence-based antiplatelet drug to pair with aspirin for this indication. Clopidogrel has the advantages of being generic, cheap, available, and familiar. It’s possible that another P2Y12 inhibitor, such as ticagrelor (Brilinta), might work even better, but that needs to be proven to justify the added expense of a brand-name antiplatelet drug.
Mike Sharma, MD , is a stroke neurologist at McMaster University, Hamilton, Ont. He has been an advisor to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. He made these comments in an interview.
The new model using data from the POINT trial confirms what had been previously shown in the CHANCE trial – that 21 days is a sensible cutoff for dual antiplatelet treatment for patients immediately following a mild stroke or transient ischemic attack. Treatment with dual antiplatelet therapy for 21 days provides the same added benefit as 90 days of treatment but with less excess bleeding. The new findings confirm that the CHANCE results were not specific to a Chinese population.

For the time being, clopidogrel is the evidence-based antiplatelet drug to pair with aspirin for this indication. Clopidogrel has the advantages of being generic, cheap, available, and familiar. It’s possible that another P2Y12 inhibitor, such as ticagrelor (Brilinta), might work even better, but that needs to be proven to justify the added expense of a brand-name antiplatelet drug.
Mike Sharma, MD , is a stroke neurologist at McMaster University, Hamilton, Ont. He has been an advisor to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. He made these comments in an interview.
MONTREAL – The optimal length for dual antiplatelet therapy in patients who have just had a mild stroke or transient ischemic attack is 21 days, a duration of combined treatment that maximized protection against major ischemic events while minimizing the extra risk for a major hemorrhage, according to a prespecified analysis of data from the POINT trial.
The POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial randomized 4,881 patients with a very recent mild stroke or transient ischemic attack and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, dual antiplatelet therapy (DAPT) cut the incidence of a major ischemic event by a relative 25% but also more than doubled the rate of major hemorrhage (New Engl J Med. 2018 Jul 19;377[3]:215-25).
The new, prespecified analysis looked at outcomes on a week-by-week basis over the course of 90 days of treatment, and showed that during the first 21 days the rate of major ischemic events was 5.6% among patients on aspirin only and 3.6% among those on DAPT, a statistically significant 35% relative cut in these adverse outcomes by using DAPT, Jordan J. Elm, PhD, reported at the World Stroke Congress. During the subsequent 69 days on treatment, the incidence of major ischemic events was roughly 1% in both arms of the study, showing that after 3 weeks the incremental benefit from DAPT disappeared, said Dr. Elm, a biostatistician at the Medical University of South Carolina, Charleston.
In contrast, the doubled rate of major hemorrhages (mostly reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment, meaning that limiting DAPT to just 21 days could prevent many of the excess hemorrhages.
“These results suggest that limiting clopidogrel plus aspirin use to 21 days may maximize benefit and reduce risk,” Dr. Elm said, especially in light of the findings confirming the efficacy of 21 days of DAPT following a minor stroke or TIA that had been reported several years ago in the CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial (New Engl J Med. 2013 Jul 4;369[1]:11-9).
Although the new finding from the POINT results came in a secondary analysis, it’s statistically legitimate and should be taken into account when writing treatment guidelines, she said, emphasizing that “this is a very important analysis that is not just hypothesis generating.”
Another finding from the new analysis was that a large number of major ischemic events, and hence a large number of the events prevented by DAPT, occurred in the first 2 days following the index event, a finding made possible because the POINT investigators enrolled patients and started treatment within 12 hours of the qualifying events.
“It’s better to start treatment early,” Dr. Elm noted, but she also highlighted that major ischemic events continued to accumulate during days 3-21, suggesting that patients could still benefit from DAPT even if treatment did not start until 24 or 48 hours after their index event.
POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm reported no disclosures.
SOURCE: Elm JJ et al. World Stroke Congress, Late-breaking session.
MONTREAL – The optimal length for dual antiplatelet therapy in patients who have just had a mild stroke or transient ischemic attack is 21 days, a duration of combined treatment that maximized protection against major ischemic events while minimizing the extra risk for a major hemorrhage, according to a prespecified analysis of data from the POINT trial.
The POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial randomized 4,881 patients with a very recent mild stroke or transient ischemic attack and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, dual antiplatelet therapy (DAPT) cut the incidence of a major ischemic event by a relative 25% but also more than doubled the rate of major hemorrhage (New Engl J Med. 2018 Jul 19;377[3]:215-25).
The new, prespecified analysis looked at outcomes on a week-by-week basis over the course of 90 days of treatment, and showed that during the first 21 days the rate of major ischemic events was 5.6% among patients on aspirin only and 3.6% among those on DAPT, a statistically significant 35% relative cut in these adverse outcomes by using DAPT, Jordan J. Elm, PhD, reported at the World Stroke Congress. During the subsequent 69 days on treatment, the incidence of major ischemic events was roughly 1% in both arms of the study, showing that after 3 weeks the incremental benefit from DAPT disappeared, said Dr. Elm, a biostatistician at the Medical University of South Carolina, Charleston.
In contrast, the doubled rate of major hemorrhages (mostly reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment, meaning that limiting DAPT to just 21 days could prevent many of the excess hemorrhages.
“These results suggest that limiting clopidogrel plus aspirin use to 21 days may maximize benefit and reduce risk,” Dr. Elm said, especially in light of the findings confirming the efficacy of 21 days of DAPT following a minor stroke or TIA that had been reported several years ago in the CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial (New Engl J Med. 2013 Jul 4;369[1]:11-9).
Although the new finding from the POINT results came in a secondary analysis, it’s statistically legitimate and should be taken into account when writing treatment guidelines, she said, emphasizing that “this is a very important analysis that is not just hypothesis generating.”
Another finding from the new analysis was that a large number of major ischemic events, and hence a large number of the events prevented by DAPT, occurred in the first 2 days following the index event, a finding made possible because the POINT investigators enrolled patients and started treatment within 12 hours of the qualifying events.
“It’s better to start treatment early,” Dr. Elm noted, but she also highlighted that major ischemic events continued to accumulate during days 3-21, suggesting that patients could still benefit from DAPT even if treatment did not start until 24 or 48 hours after their index event.
POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm reported no disclosures.
SOURCE: Elm JJ et al. World Stroke Congress, Late-breaking session.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point: All of DAPT’s extra benefit over aspirin alone in recent stroke or transient ischemic attack patients happened during the first 21 days.
Major finding: During the first 21 days, DAPT cut major ischemic events by 35%, compared with aspirin only.
Study details: A prespecified, secondary analysis from POINT, a multicenter, randomized trial with 4,881 patients.
Disclosures: POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm had no disclosures.
Source: Elm JJ et al. World Stroke Congress, Late-breaking session.
Smartphone device beat Holter for post-stroke AF detection
MONTREAL – A smartphone-based method for quick and inexpensive monitoring for atrial fibrillation in patients hospitalized for a recent acute ischemic stroke or transient ischemic attack identified three times more patients with the arrhythmia than did 24-hour Holter monitoring of the same patients after their hospital discharge.
This high level of atrial fibrillation (AF) detection using a relatively cheap and noninvasive device suggests that this method is a good “complement” to conventional monitoring by a 24-hour Holter recording or an implanted loop recorder in recent stroke patients, as called for in current guidelines of the world’s cardiology societies.
In the study, 294 of 1,079 patients hospitalized for an acute ischemic stroke or transient ischemic attack (TIA) underwent Holter monitoring, which identified 8 patients (3%) with AF, compared with 25 of these 294 patients (9%) identified with AF while they were hospitalized using serial, 30-second monitoring with the AliveCor device for smartphone assessment of ECG measurement, Bernard Yan, MD, said at the World Stroke Congress. Seven of the eight patients identified with AF by Holter monitoring were also found to have AF by the AliveCor device.
Dr. Yan, an interventional neurologist at the Comprehensive Stroke Center at the Royal Melbourne Hospital, attributed the higher pick-up rate for AF by monitoring during hospitalization to the timing of screening, which was within days of the stroke or TIA, rather than waiting to run a Holter sometime after the patient left the hospital.
“I suspect the difference in timing explains the difference” in detection, he said in an interview. “The difference may be because we monitored patients [with the AliveCor device] much earlier, during their ‘hot’ period, right after their stroke.”
The SPOT-AF trial ran at several centers in Australia, China, and Hong Kong, and enrolled 1,079 patients hospitalized for acute ischemic stroke or TIA who all underwent AliveCor monitoring during their median 4-day stay in the hospital. Patients performed a 30-second heart rhythm check every time a nurse saw them for a routine vital-sign examination, usually three or four times a day. The current analysis focused on the 294 patients (27% of the 1,079 patients) who also underwent 24-hour Holter monitoring following hospital discharge when ordered by their personal physician. This 27% incidence of postdischarge Holter monitoring despite guidelines that call for AF screening in all recent ischemic stroke and TIA patients was consistent with a 2016 review of more than 17,000 stroke or TIA patients in Canada that showed 31% underwent 24-hour Holter monitoring for AF during the 30 days following their index event (Stroke. 2016 Aug;47[8]:1982-9).
Although AF screening with a smartphone-based device is inexpensive and easy, Dr. Yan stopped short of suggesting that it is time for this approach to replace a Holter monitor or an implanted loop recorder because that is what current guidelines call for. “To change the guidelines, we need a different study that compares these approaches head to head.”
SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has been a speaker on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
MONTREAL – A smartphone-based method for quick and inexpensive monitoring for atrial fibrillation in patients hospitalized for a recent acute ischemic stroke or transient ischemic attack identified three times more patients with the arrhythmia than did 24-hour Holter monitoring of the same patients after their hospital discharge.
This high level of atrial fibrillation (AF) detection using a relatively cheap and noninvasive device suggests that this method is a good “complement” to conventional monitoring by a 24-hour Holter recording or an implanted loop recorder in recent stroke patients, as called for in current guidelines of the world’s cardiology societies.
In the study, 294 of 1,079 patients hospitalized for an acute ischemic stroke or transient ischemic attack (TIA) underwent Holter monitoring, which identified 8 patients (3%) with AF, compared with 25 of these 294 patients (9%) identified with AF while they were hospitalized using serial, 30-second monitoring with the AliveCor device for smartphone assessment of ECG measurement, Bernard Yan, MD, said at the World Stroke Congress. Seven of the eight patients identified with AF by Holter monitoring were also found to have AF by the AliveCor device.
Dr. Yan, an interventional neurologist at the Comprehensive Stroke Center at the Royal Melbourne Hospital, attributed the higher pick-up rate for AF by monitoring during hospitalization to the timing of screening, which was within days of the stroke or TIA, rather than waiting to run a Holter sometime after the patient left the hospital.
“I suspect the difference in timing explains the difference” in detection, he said in an interview. “The difference may be because we monitored patients [with the AliveCor device] much earlier, during their ‘hot’ period, right after their stroke.”
The SPOT-AF trial ran at several centers in Australia, China, and Hong Kong, and enrolled 1,079 patients hospitalized for acute ischemic stroke or TIA who all underwent AliveCor monitoring during their median 4-day stay in the hospital. Patients performed a 30-second heart rhythm check every time a nurse saw them for a routine vital-sign examination, usually three or four times a day. The current analysis focused on the 294 patients (27% of the 1,079 patients) who also underwent 24-hour Holter monitoring following hospital discharge when ordered by their personal physician. This 27% incidence of postdischarge Holter monitoring despite guidelines that call for AF screening in all recent ischemic stroke and TIA patients was consistent with a 2016 review of more than 17,000 stroke or TIA patients in Canada that showed 31% underwent 24-hour Holter monitoring for AF during the 30 days following their index event (Stroke. 2016 Aug;47[8]:1982-9).
Although AF screening with a smartphone-based device is inexpensive and easy, Dr. Yan stopped short of suggesting that it is time for this approach to replace a Holter monitor or an implanted loop recorder because that is what current guidelines call for. “To change the guidelines, we need a different study that compares these approaches head to head.”
SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has been a speaker on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
MONTREAL – A smartphone-based method for quick and inexpensive monitoring for atrial fibrillation in patients hospitalized for a recent acute ischemic stroke or transient ischemic attack identified three times more patients with the arrhythmia than did 24-hour Holter monitoring of the same patients after their hospital discharge.
This high level of atrial fibrillation (AF) detection using a relatively cheap and noninvasive device suggests that this method is a good “complement” to conventional monitoring by a 24-hour Holter recording or an implanted loop recorder in recent stroke patients, as called for in current guidelines of the world’s cardiology societies.
In the study, 294 of 1,079 patients hospitalized for an acute ischemic stroke or transient ischemic attack (TIA) underwent Holter monitoring, which identified 8 patients (3%) with AF, compared with 25 of these 294 patients (9%) identified with AF while they were hospitalized using serial, 30-second monitoring with the AliveCor device for smartphone assessment of ECG measurement, Bernard Yan, MD, said at the World Stroke Congress. Seven of the eight patients identified with AF by Holter monitoring were also found to have AF by the AliveCor device.
Dr. Yan, an interventional neurologist at the Comprehensive Stroke Center at the Royal Melbourne Hospital, attributed the higher pick-up rate for AF by monitoring during hospitalization to the timing of screening, which was within days of the stroke or TIA, rather than waiting to run a Holter sometime after the patient left the hospital.
“I suspect the difference in timing explains the difference” in detection, he said in an interview. “The difference may be because we monitored patients [with the AliveCor device] much earlier, during their ‘hot’ period, right after their stroke.”
The SPOT-AF trial ran at several centers in Australia, China, and Hong Kong, and enrolled 1,079 patients hospitalized for acute ischemic stroke or TIA who all underwent AliveCor monitoring during their median 4-day stay in the hospital. Patients performed a 30-second heart rhythm check every time a nurse saw them for a routine vital-sign examination, usually three or four times a day. The current analysis focused on the 294 patients (27% of the 1,079 patients) who also underwent 24-hour Holter monitoring following hospital discharge when ordered by their personal physician. This 27% incidence of postdischarge Holter monitoring despite guidelines that call for AF screening in all recent ischemic stroke and TIA patients was consistent with a 2016 review of more than 17,000 stroke or TIA patients in Canada that showed 31% underwent 24-hour Holter monitoring for AF during the 30 days following their index event (Stroke. 2016 Aug;47[8]:1982-9).
Although AF screening with a smartphone-based device is inexpensive and easy, Dr. Yan stopped short of suggesting that it is time for this approach to replace a Holter monitor or an implanted loop recorder because that is what current guidelines call for. “To change the guidelines, we need a different study that compares these approaches head to head.”
SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has been a speaker on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point:
Major finding: Holter monitoring detected atrial fibrillation in 8 of 294 patients, while smartphone monitoring identified 25 with the arrhythmia.
Study details: SPOT-AF, a multicenter study with 1,079 total patients, including 294 who underwent Holter monitoring.
Disclosures: SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has been a speaker on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
How much more proof do you need?
One piece of wisdom I was given in medical school was to never be the first nor the last to adopt a new treatment. The history of medicine is full of new discoveries that don’t work out as well as the first report. It also is full of long standing dogmas that later were proven false. This balancing act is part of being a professional and being an advocate for your patient. There is science behind this art. Everett Rogers identified innovators, early adopters, and laggards as new ideas are diffused into practice.1
A 2007 French study2 that investigated oral amoxicillin for early-onset group B streptococcal (GBS) disease is one of the few times in the past 3 decades in which I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis like American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by full term neonates, even mildly sick ones. It does adequately cross the blood-brain barrier. The French researchers measured serum levels and proved all this using both scientific principles and through a clinical trial.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician to be early adopters. I expected the Red Book would update its recommendations. That didn’t happen.
Meanwhile, I have seen other babies kept for 10 days in the hospital for IV therapy with resultant wasted costs (about $20 million/year in the United States) and income loss for the parents. I’ve treated complications and readmissions caused by peripherally inserted central catheter (PICC) line issues. One baby at home got a syringe of gentamicin given as an IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Because late-onset GBS can be acquired environmentally, there always will be recurrences. Unless you are practicing defensive medicine, the issue isn’t the rate of recurrence; it is whether the more invasive intervention of prolonged IV therapy reduces that rate. Then balance any measured reduction (which apparently is zero) against the adverse effects of the invasive intervention, such as PICC line infections. This Bayesian decision making is hard for some risk-averse humans to assimilate. (I’m part Borg.)
Coon et al.3 have confirmed, using big data, that prolonged IV therapy of uncomplicated, late-onset GBS bacteremia does not generate a clinically significant benefit. It certainly is possible to sow doubt by asking for proof in a variety of subpopulations. Even in the era of intrapartum antibiotic prophylaxis, which has halved the incidence of GBS disease, GBS disease occurs in about 2,000 babies per year in the United States. However, most are treated in community hospitals and are not included in the database used in this new report. With fewer than 2-3 cases of GBS bacteremia per year per hospital, a multicenter, randomized controlled trial would be an unprecedented undertaking, is ethically problematic, and is not realistically happening soon. So these observational data, skillfully acquired and analyzed, are and will remain the best available data.
This new article is in the context of multiple articles over the past decade that have disproven the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Coon et al. show that, by 2015, 5 of 49 children’s hospitals (10%) were early adopters and had already made the switch to mostly using short treatment courses for uncomplicated GBS bacteremia; 14 of 49 (29%) hadn’t changed at all from the obsolete Red Book recommendation. Given this new analysis, what are you laggards4 waiting for?
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Diffusion of Innovations,” 5th ed. (New York: Free Press, 2003).
2. Eur J Clin Pharmacol. 2007 Jul;63(7):657-62.
3. Pediatrics. 2018;142(5):e20180345.
4. https://en.wikipedia.org/wiki/Diffusion_of_innovations.
One piece of wisdom I was given in medical school was to never be the first nor the last to adopt a new treatment. The history of medicine is full of new discoveries that don’t work out as well as the first report. It also is full of long standing dogmas that later were proven false. This balancing act is part of being a professional and being an advocate for your patient. There is science behind this art. Everett Rogers identified innovators, early adopters, and laggards as new ideas are diffused into practice.1
A 2007 French study2 that investigated oral amoxicillin for early-onset group B streptococcal (GBS) disease is one of the few times in the past 3 decades in which I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis like American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by full term neonates, even mildly sick ones. It does adequately cross the blood-brain barrier. The French researchers measured serum levels and proved all this using both scientific principles and through a clinical trial.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician to be early adopters. I expected the Red Book would update its recommendations. That didn’t happen.
Meanwhile, I have seen other babies kept for 10 days in the hospital for IV therapy with resultant wasted costs (about $20 million/year in the United States) and income loss for the parents. I’ve treated complications and readmissions caused by peripherally inserted central catheter (PICC) line issues. One baby at home got a syringe of gentamicin given as an IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Because late-onset GBS can be acquired environmentally, there always will be recurrences. Unless you are practicing defensive medicine, the issue isn’t the rate of recurrence; it is whether the more invasive intervention of prolonged IV therapy reduces that rate. Then balance any measured reduction (which apparently is zero) against the adverse effects of the invasive intervention, such as PICC line infections. This Bayesian decision making is hard for some risk-averse humans to assimilate. (I’m part Borg.)
Coon et al.3 have confirmed, using big data, that prolonged IV therapy of uncomplicated, late-onset GBS bacteremia does not generate a clinically significant benefit. It certainly is possible to sow doubt by asking for proof in a variety of subpopulations. Even in the era of intrapartum antibiotic prophylaxis, which has halved the incidence of GBS disease, GBS disease occurs in about 2,000 babies per year in the United States. However, most are treated in community hospitals and are not included in the database used in this new report. With fewer than 2-3 cases of GBS bacteremia per year per hospital, a multicenter, randomized controlled trial would be an unprecedented undertaking, is ethically problematic, and is not realistically happening soon. So these observational data, skillfully acquired and analyzed, are and will remain the best available data.
This new article is in the context of multiple articles over the past decade that have disproven the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Coon et al. show that, by 2015, 5 of 49 children’s hospitals (10%) were early adopters and had already made the switch to mostly using short treatment courses for uncomplicated GBS bacteremia; 14 of 49 (29%) hadn’t changed at all from the obsolete Red Book recommendation. Given this new analysis, what are you laggards4 waiting for?
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Diffusion of Innovations,” 5th ed. (New York: Free Press, 2003).
2. Eur J Clin Pharmacol. 2007 Jul;63(7):657-62.
3. Pediatrics. 2018;142(5):e20180345.
4. https://en.wikipedia.org/wiki/Diffusion_of_innovations.
One piece of wisdom I was given in medical school was to never be the first nor the last to adopt a new treatment. The history of medicine is full of new discoveries that don’t work out as well as the first report. It also is full of long standing dogmas that later were proven false. This balancing act is part of being a professional and being an advocate for your patient. There is science behind this art. Everett Rogers identified innovators, early adopters, and laggards as new ideas are diffused into practice.1
A 2007 French study2 that investigated oral amoxicillin for early-onset group B streptococcal (GBS) disease is one of the few times in the past 3 decades in which I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis like American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by full term neonates, even mildly sick ones. It does adequately cross the blood-brain barrier. The French researchers measured serum levels and proved all this using both scientific principles and through a clinical trial.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician to be early adopters. I expected the Red Book would update its recommendations. That didn’t happen.
Meanwhile, I have seen other babies kept for 10 days in the hospital for IV therapy with resultant wasted costs (about $20 million/year in the United States) and income loss for the parents. I’ve treated complications and readmissions caused by peripherally inserted central catheter (PICC) line issues. One baby at home got a syringe of gentamicin given as an IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Because late-onset GBS can be acquired environmentally, there always will be recurrences. Unless you are practicing defensive medicine, the issue isn’t the rate of recurrence; it is whether the more invasive intervention of prolonged IV therapy reduces that rate. Then balance any measured reduction (which apparently is zero) against the adverse effects of the invasive intervention, such as PICC line infections. This Bayesian decision making is hard for some risk-averse humans to assimilate. (I’m part Borg.)
Coon et al.3 have confirmed, using big data, that prolonged IV therapy of uncomplicated, late-onset GBS bacteremia does not generate a clinically significant benefit. It certainly is possible to sow doubt by asking for proof in a variety of subpopulations. Even in the era of intrapartum antibiotic prophylaxis, which has halved the incidence of GBS disease, GBS disease occurs in about 2,000 babies per year in the United States. However, most are treated in community hospitals and are not included in the database used in this new report. With fewer than 2-3 cases of GBS bacteremia per year per hospital, a multicenter, randomized controlled trial would be an unprecedented undertaking, is ethically problematic, and is not realistically happening soon. So these observational data, skillfully acquired and analyzed, are and will remain the best available data.
This new article is in the context of multiple articles over the past decade that have disproven the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Coon et al. show that, by 2015, 5 of 49 children’s hospitals (10%) were early adopters and had already made the switch to mostly using short treatment courses for uncomplicated GBS bacteremia; 14 of 49 (29%) hadn’t changed at all from the obsolete Red Book recommendation. Given this new analysis, what are you laggards4 waiting for?
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Diffusion of Innovations,” 5th ed. (New York: Free Press, 2003).
2. Eur J Clin Pharmacol. 2007 Jul;63(7):657-62.
3. Pediatrics. 2018;142(5):e20180345.
4. https://en.wikipedia.org/wiki/Diffusion_of_innovations.
Protocol violations, missed transfusions among blood delivery errors
BOSTON – Even the most vigilant hospitals experience problems with blood storage and delivery on the patient floor, particularly in pediatric units, investigators cautioned.
A review of patient safety incidents that occurred surrounding more than 1 million transfusions in U.S. hospitals showed that pediatric transfusions were associated with a higher rate of safety problems compared with adult transfusions, with errors differing by age group.
“We just looked at units transfused [and] incidents that occurred during product administration and we found that the highest incident in the pediatric population is that the protocol is not being followed, and the highest incident in the adult population is that the transfusion is not performed, in error, at all,” said Sarah Vossoughi, MD, of Columbia University and New York–Presbyterian Hospital, New York.
In both settings, the investigators observed problems with product storage on the patient floor. “It’s very common for blood banks to find platelets in the refrigerator. It doesn’t matter how old you are or what type of hospital you’re at – everyone’s putting platelets in the fridge,” she said in an interview at AABB 2018, the annual meeting of the organization formerly known as the American Association of Blood Banks.
Dr. Vossoughi and her colleagues in New York and at the University of Vermont in Burlington noted that the National Patient Safety Foundation, now a part of the Institute for Healthcare Improvement, declared preventable medical harm to be a public health crisis. In a paper published in the BMJ in 2016, researchers estimated that medical errors were the third leading cause of death in the United States, accounting for more than 250,000 fatalities annually.
Dr. Vossoughi also pointed to a study suggesting that the incidence of nonlethal medical errors may be 10- to 20-fold higher than the number of fatal errors (J Patient Saf. 2013 Sep;9[3]:122-8).
To evaluate patient safety events related to blood transfusions, Dr. Vossoughi and her colleagues drew data on events reported by three children’s hospitals and 29 adult hospitals to either the AABB Center for Patient Safety or the medical center’s own adverse event reporting system from January 2010 through September 2017.
They identified a total of 1,806 reports associated with approximately 1,088.884 transfusions. Of these reports, 249 were associated with 99,064 pediatric transfusions, and 1,577 were reported in association with 989,820 adult transfusions.
In all, 31% of the pediatric events were failure to follow the transfusion protocol.
“In a lot of the pediatric hospitals, it’s kind of like the Wild West. People say, ‘well I know it’s the hospital policy, but this child is special, so I’m going to do it this way, this time.’ That seems to be a culture in pediatrics, whereas on the adult side [clinicians] seem to be much less likely to just deviate from the protocol,” Dr. Vossoughi said.
Among adults, 43% of the errors were “transfusion not performed,” which may occur because of a bungled patient hand-off during a shift change, or when a patient is being moved from one unit to another.
“The next day, they’ll check the patient’s CBC and realize that the patient didn’t respond to the infusion that it turned out they never got, and then the product will be found on the floor, expired,” Dr. Vossoughi said.
In all, 20% of pediatric errors and 24% of adult errors were associated with incorrect storage of blood products on the patient floor.
The information they presented could help inpatient blood management programs target education and interventions to providers who commit similar errors.
“If you know that a particular provider group has problems following the protocol, maybe you can make the protocol a little simpler to follow, or make the checklist less cumbersome, and then maybe they’ll follow them more often,” she said.
The study was supported by the AABB Center for Patient Safety and University of Vermont Medical Center. The authors reported no conflicts of interest.
SOURCE: Vossoughi S et al. AABB 2018, Abstract QT4.
BOSTON – Even the most vigilant hospitals experience problems with blood storage and delivery on the patient floor, particularly in pediatric units, investigators cautioned.
A review of patient safety incidents that occurred surrounding more than 1 million transfusions in U.S. hospitals showed that pediatric transfusions were associated with a higher rate of safety problems compared with adult transfusions, with errors differing by age group.
“We just looked at units transfused [and] incidents that occurred during product administration and we found that the highest incident in the pediatric population is that the protocol is not being followed, and the highest incident in the adult population is that the transfusion is not performed, in error, at all,” said Sarah Vossoughi, MD, of Columbia University and New York–Presbyterian Hospital, New York.
In both settings, the investigators observed problems with product storage on the patient floor. “It’s very common for blood banks to find platelets in the refrigerator. It doesn’t matter how old you are or what type of hospital you’re at – everyone’s putting platelets in the fridge,” she said in an interview at AABB 2018, the annual meeting of the organization formerly known as the American Association of Blood Banks.
Dr. Vossoughi and her colleagues in New York and at the University of Vermont in Burlington noted that the National Patient Safety Foundation, now a part of the Institute for Healthcare Improvement, declared preventable medical harm to be a public health crisis. In a paper published in the BMJ in 2016, researchers estimated that medical errors were the third leading cause of death in the United States, accounting for more than 250,000 fatalities annually.
Dr. Vossoughi also pointed to a study suggesting that the incidence of nonlethal medical errors may be 10- to 20-fold higher than the number of fatal errors (J Patient Saf. 2013 Sep;9[3]:122-8).
To evaluate patient safety events related to blood transfusions, Dr. Vossoughi and her colleagues drew data on events reported by three children’s hospitals and 29 adult hospitals to either the AABB Center for Patient Safety or the medical center’s own adverse event reporting system from January 2010 through September 2017.
They identified a total of 1,806 reports associated with approximately 1,088.884 transfusions. Of these reports, 249 were associated with 99,064 pediatric transfusions, and 1,577 were reported in association with 989,820 adult transfusions.
In all, 31% of the pediatric events were failure to follow the transfusion protocol.
“In a lot of the pediatric hospitals, it’s kind of like the Wild West. People say, ‘well I know it’s the hospital policy, but this child is special, so I’m going to do it this way, this time.’ That seems to be a culture in pediatrics, whereas on the adult side [clinicians] seem to be much less likely to just deviate from the protocol,” Dr. Vossoughi said.
Among adults, 43% of the errors were “transfusion not performed,” which may occur because of a bungled patient hand-off during a shift change, or when a patient is being moved from one unit to another.
“The next day, they’ll check the patient’s CBC and realize that the patient didn’t respond to the infusion that it turned out they never got, and then the product will be found on the floor, expired,” Dr. Vossoughi said.
In all, 20% of pediatric errors and 24% of adult errors were associated with incorrect storage of blood products on the patient floor.
The information they presented could help inpatient blood management programs target education and interventions to providers who commit similar errors.
“If you know that a particular provider group has problems following the protocol, maybe you can make the protocol a little simpler to follow, or make the checklist less cumbersome, and then maybe they’ll follow them more often,” she said.
The study was supported by the AABB Center for Patient Safety and University of Vermont Medical Center. The authors reported no conflicts of interest.
SOURCE: Vossoughi S et al. AABB 2018, Abstract QT4.
BOSTON – Even the most vigilant hospitals experience problems with blood storage and delivery on the patient floor, particularly in pediatric units, investigators cautioned.
A review of patient safety incidents that occurred surrounding more than 1 million transfusions in U.S. hospitals showed that pediatric transfusions were associated with a higher rate of safety problems compared with adult transfusions, with errors differing by age group.
“We just looked at units transfused [and] incidents that occurred during product administration and we found that the highest incident in the pediatric population is that the protocol is not being followed, and the highest incident in the adult population is that the transfusion is not performed, in error, at all,” said Sarah Vossoughi, MD, of Columbia University and New York–Presbyterian Hospital, New York.
In both settings, the investigators observed problems with product storage on the patient floor. “It’s very common for blood banks to find platelets in the refrigerator. It doesn’t matter how old you are or what type of hospital you’re at – everyone’s putting platelets in the fridge,” she said in an interview at AABB 2018, the annual meeting of the organization formerly known as the American Association of Blood Banks.
Dr. Vossoughi and her colleagues in New York and at the University of Vermont in Burlington noted that the National Patient Safety Foundation, now a part of the Institute for Healthcare Improvement, declared preventable medical harm to be a public health crisis. In a paper published in the BMJ in 2016, researchers estimated that medical errors were the third leading cause of death in the United States, accounting for more than 250,000 fatalities annually.
Dr. Vossoughi also pointed to a study suggesting that the incidence of nonlethal medical errors may be 10- to 20-fold higher than the number of fatal errors (J Patient Saf. 2013 Sep;9[3]:122-8).
To evaluate patient safety events related to blood transfusions, Dr. Vossoughi and her colleagues drew data on events reported by three children’s hospitals and 29 adult hospitals to either the AABB Center for Patient Safety or the medical center’s own adverse event reporting system from January 2010 through September 2017.
They identified a total of 1,806 reports associated with approximately 1,088.884 transfusions. Of these reports, 249 were associated with 99,064 pediatric transfusions, and 1,577 were reported in association with 989,820 adult transfusions.
In all, 31% of the pediatric events were failure to follow the transfusion protocol.
“In a lot of the pediatric hospitals, it’s kind of like the Wild West. People say, ‘well I know it’s the hospital policy, but this child is special, so I’m going to do it this way, this time.’ That seems to be a culture in pediatrics, whereas on the adult side [clinicians] seem to be much less likely to just deviate from the protocol,” Dr. Vossoughi said.
Among adults, 43% of the errors were “transfusion not performed,” which may occur because of a bungled patient hand-off during a shift change, or when a patient is being moved from one unit to another.
“The next day, they’ll check the patient’s CBC and realize that the patient didn’t respond to the infusion that it turned out they never got, and then the product will be found on the floor, expired,” Dr. Vossoughi said.
In all, 20% of pediatric errors and 24% of adult errors were associated with incorrect storage of blood products on the patient floor.
The information they presented could help inpatient blood management programs target education and interventions to providers who commit similar errors.
“If you know that a particular provider group has problems following the protocol, maybe you can make the protocol a little simpler to follow, or make the checklist less cumbersome, and then maybe they’ll follow them more often,” she said.
The study was supported by the AABB Center for Patient Safety and University of Vermont Medical Center. The authors reported no conflicts of interest.
SOURCE: Vossoughi S et al. AABB 2018, Abstract QT4.
REPORTING FROM AABB 2018
Key clinical point:
Major finding: In all, 31% of pediatric errors were due to protocol violation, and 43% of adult errors were due to an ordered transfusion not being performed.
Study details: Descriptive study of data from 32 U.S. hospitals that reported transfusion safety events.
Disclosures: The study was supported by the AABB Center for Patient Safety and University of Vermont. The authors reported no conflicts of interest.
Source: Vossoughi S et al. AABB 2018, Abstract QT4.