User login
Research supports cannabis in MS, but legal, clinical pictures are murky
The medical marijuana landscape is changing so fast that Colorado Neurological Institute neurologist Allen C. Bowling, MD, PhD, already needs to update a presentation he gave about cannabis in multiple sclerosis in late May.
Since then, both those facts became history over a span of 2 days.
First, on June 25, the FDA announced its approval of Epidiolex (cannabidiol) for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. It’s the first time the FDA has approved a drug with a purified ingredient – cannabidiol, a nonpsychoactive substance – that’s derived from marijuana.
Then, on June 26, voters in Oklahoma approved a ballot measure that allows the possession of marijuana for medical use; users must register with the state. Thirty states and the District of Columbia have made medical marijuana legal, according to the procon.org website, although the two newest ones (Oklahoma and West Virginia) are still developing procedures.
The laws vary widely. Some states don’t allow patients to smoke medical marijuana, and some don’t allow visitors to use out-of-state registry ID cards. And certain states limit the use of medical marijuana to specific conditions. Medical marijuana use by patients with MS is specifically allowed in many states, including Alaska, Arizona, Florida, Minnesota, and several others.
There’s another complexity: According to procon.org, 17 states have laws about the use of cannabidiol. In Georgia, for instance, the use of some cannabis oil is allowed for the treatment of MS and other conditions.
In the wake of the FDA ruling, Dr. Bowling spoke in an interview about cannabis, MS, and the questions that neurologists should be asking themselves.
Q: What are studies telling us about cannabis and MS?
A: There are lots of clinical studies – 19 randomized controlled trials. A consistent finding is that there’s benefit in terms of pain and people’s subjective sense of spasticity (Neurology. 2014 Apr 29;82(17):1556-63).
Q: During your CMSC presentation, you talked about how “fidelity” has been a problem in cannabis research. Could you elaborate on what you mean?
A: The products used in these studies are generally standardized, research-grade products that you can’t buy in any U.S. dispensary.
Cannabis is complex and contains more than 100 different potentially pharmacologically active molecules. You can’t conclude that if you see a product in clinical trials, you’ll then be able to walk into a dispensary for recreational or medical cannabis and get a product that produces the same effect.
Q: What have you seen in your own patient population in terms of cannabis use?
A: I find what’s been found with the studies: It helps with pain and people’s sense of muscle stiffness.
It’s especially helpful in people with pain and spasticity that breaks through in the late afternoon or at night when they’re trying to go to sleep. Just a little bit of cannabis can get them through those difficult times and improve their quality of life.
Q: What choices do patients make regarding whether to get high from the cannabis they use?
A: Some have absolutely zero interest in getting high, and they try to avoid the THC-containing products. Other like getting high in addition to getting help with pain and spasticity.
Q: Who should not use medical marijuana in the MS community?
A: Patients who don’t have symptoms that could respond.
I’m also very concerned about patients who are 25 years and younger because of the effects that cannabis can have on brain development out to age 25 and the higher risk of addiction in people who are younger.
Q: What do you think the future will hold on the cannabis front?
A: Now that it’s less of a taboo topic, there’s an ever-growing number of trials each year, including very high-quality studies.
Pharmaceutically produced, cannabis-based medicines will be a growing area. Epidiolex is a perfect example of that.
It’s important for physicians to know that the way cannabis-based medicine is produced by a pharmaceutical company is different in so many levels than the cannabis in states with recreational and medical marijuana.
Q: What are some ways that the pharmaceutical products are different?
A: The rigor of the production process, the standardization, the purity, the correct labeling and expiration dates. Plus, the lack of the use of pesticides and other contaminants. And they’re distributed by pharmacists.
Q: What should neurologists be thinking if they’re considering whether to recommend cannabis to their patients?
A: This is a very complex topic, and it’s not something that most of us have training in. You can’t sit down for 1 or 2 hours, get up to speed, and have your own well-informed opinion on it. You really need to put more time and effort.
Q: What are some issues that neurologists should consider?
A: You really need to find out what your state is doing about it and see how you feel about that.
How is your state administering medical and/or recreational marijuana? The administration of these programs is extremely different from state to state. Do these details satisfy you, and are you content having your patients interface with these programs?
Dr. Bowling reports no relevant disclosures.
The medical marijuana landscape is changing so fast that Colorado Neurological Institute neurologist Allen C. Bowling, MD, PhD, already needs to update a presentation he gave about cannabis in multiple sclerosis in late May.
Since then, both those facts became history over a span of 2 days.
First, on June 25, the FDA announced its approval of Epidiolex (cannabidiol) for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. It’s the first time the FDA has approved a drug with a purified ingredient – cannabidiol, a nonpsychoactive substance – that’s derived from marijuana.
Then, on June 26, voters in Oklahoma approved a ballot measure that allows the possession of marijuana for medical use; users must register with the state. Thirty states and the District of Columbia have made medical marijuana legal, according to the procon.org website, although the two newest ones (Oklahoma and West Virginia) are still developing procedures.
The laws vary widely. Some states don’t allow patients to smoke medical marijuana, and some don’t allow visitors to use out-of-state registry ID cards. And certain states limit the use of medical marijuana to specific conditions. Medical marijuana use by patients with MS is specifically allowed in many states, including Alaska, Arizona, Florida, Minnesota, and several others.
There’s another complexity: According to procon.org, 17 states have laws about the use of cannabidiol. In Georgia, for instance, the use of some cannabis oil is allowed for the treatment of MS and other conditions.
In the wake of the FDA ruling, Dr. Bowling spoke in an interview about cannabis, MS, and the questions that neurologists should be asking themselves.
Q: What are studies telling us about cannabis and MS?
A: There are lots of clinical studies – 19 randomized controlled trials. A consistent finding is that there’s benefit in terms of pain and people’s subjective sense of spasticity (Neurology. 2014 Apr 29;82(17):1556-63).
Q: During your CMSC presentation, you talked about how “fidelity” has been a problem in cannabis research. Could you elaborate on what you mean?
A: The products used in these studies are generally standardized, research-grade products that you can’t buy in any U.S. dispensary.
Cannabis is complex and contains more than 100 different potentially pharmacologically active molecules. You can’t conclude that if you see a product in clinical trials, you’ll then be able to walk into a dispensary for recreational or medical cannabis and get a product that produces the same effect.
Q: What have you seen in your own patient population in terms of cannabis use?
A: I find what’s been found with the studies: It helps with pain and people’s sense of muscle stiffness.
It’s especially helpful in people with pain and spasticity that breaks through in the late afternoon or at night when they’re trying to go to sleep. Just a little bit of cannabis can get them through those difficult times and improve their quality of life.
Q: What choices do patients make regarding whether to get high from the cannabis they use?
A: Some have absolutely zero interest in getting high, and they try to avoid the THC-containing products. Other like getting high in addition to getting help with pain and spasticity.
Q: Who should not use medical marijuana in the MS community?
A: Patients who don’t have symptoms that could respond.
I’m also very concerned about patients who are 25 years and younger because of the effects that cannabis can have on brain development out to age 25 and the higher risk of addiction in people who are younger.
Q: What do you think the future will hold on the cannabis front?
A: Now that it’s less of a taboo topic, there’s an ever-growing number of trials each year, including very high-quality studies.
Pharmaceutically produced, cannabis-based medicines will be a growing area. Epidiolex is a perfect example of that.
It’s important for physicians to know that the way cannabis-based medicine is produced by a pharmaceutical company is different in so many levels than the cannabis in states with recreational and medical marijuana.
Q: What are some ways that the pharmaceutical products are different?
A: The rigor of the production process, the standardization, the purity, the correct labeling and expiration dates. Plus, the lack of the use of pesticides and other contaminants. And they’re distributed by pharmacists.
Q: What should neurologists be thinking if they’re considering whether to recommend cannabis to their patients?
A: This is a very complex topic, and it’s not something that most of us have training in. You can’t sit down for 1 or 2 hours, get up to speed, and have your own well-informed opinion on it. You really need to put more time and effort.
Q: What are some issues that neurologists should consider?
A: You really need to find out what your state is doing about it and see how you feel about that.
How is your state administering medical and/or recreational marijuana? The administration of these programs is extremely different from state to state. Do these details satisfy you, and are you content having your patients interface with these programs?
Dr. Bowling reports no relevant disclosures.
The medical marijuana landscape is changing so fast that Colorado Neurological Institute neurologist Allen C. Bowling, MD, PhD, already needs to update a presentation he gave about cannabis in multiple sclerosis in late May.
Since then, both those facts became history over a span of 2 days.
First, on June 25, the FDA announced its approval of Epidiolex (cannabidiol) for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. It’s the first time the FDA has approved a drug with a purified ingredient – cannabidiol, a nonpsychoactive substance – that’s derived from marijuana.
Then, on June 26, voters in Oklahoma approved a ballot measure that allows the possession of marijuana for medical use; users must register with the state. Thirty states and the District of Columbia have made medical marijuana legal, according to the procon.org website, although the two newest ones (Oklahoma and West Virginia) are still developing procedures.
The laws vary widely. Some states don’t allow patients to smoke medical marijuana, and some don’t allow visitors to use out-of-state registry ID cards. And certain states limit the use of medical marijuana to specific conditions. Medical marijuana use by patients with MS is specifically allowed in many states, including Alaska, Arizona, Florida, Minnesota, and several others.
There’s another complexity: According to procon.org, 17 states have laws about the use of cannabidiol. In Georgia, for instance, the use of some cannabis oil is allowed for the treatment of MS and other conditions.
In the wake of the FDA ruling, Dr. Bowling spoke in an interview about cannabis, MS, and the questions that neurologists should be asking themselves.
Q: What are studies telling us about cannabis and MS?
A: There are lots of clinical studies – 19 randomized controlled trials. A consistent finding is that there’s benefit in terms of pain and people’s subjective sense of spasticity (Neurology. 2014 Apr 29;82(17):1556-63).
Q: During your CMSC presentation, you talked about how “fidelity” has been a problem in cannabis research. Could you elaborate on what you mean?
A: The products used in these studies are generally standardized, research-grade products that you can’t buy in any U.S. dispensary.
Cannabis is complex and contains more than 100 different potentially pharmacologically active molecules. You can’t conclude that if you see a product in clinical trials, you’ll then be able to walk into a dispensary for recreational or medical cannabis and get a product that produces the same effect.
Q: What have you seen in your own patient population in terms of cannabis use?
A: I find what’s been found with the studies: It helps with pain and people’s sense of muscle stiffness.
It’s especially helpful in people with pain and spasticity that breaks through in the late afternoon or at night when they’re trying to go to sleep. Just a little bit of cannabis can get them through those difficult times and improve their quality of life.
Q: What choices do patients make regarding whether to get high from the cannabis they use?
A: Some have absolutely zero interest in getting high, and they try to avoid the THC-containing products. Other like getting high in addition to getting help with pain and spasticity.
Q: Who should not use medical marijuana in the MS community?
A: Patients who don’t have symptoms that could respond.
I’m also very concerned about patients who are 25 years and younger because of the effects that cannabis can have on brain development out to age 25 and the higher risk of addiction in people who are younger.
Q: What do you think the future will hold on the cannabis front?
A: Now that it’s less of a taboo topic, there’s an ever-growing number of trials each year, including very high-quality studies.
Pharmaceutically produced, cannabis-based medicines will be a growing area. Epidiolex is a perfect example of that.
It’s important for physicians to know that the way cannabis-based medicine is produced by a pharmaceutical company is different in so many levels than the cannabis in states with recreational and medical marijuana.
Q: What are some ways that the pharmaceutical products are different?
A: The rigor of the production process, the standardization, the purity, the correct labeling and expiration dates. Plus, the lack of the use of pesticides and other contaminants. And they’re distributed by pharmacists.
Q: What should neurologists be thinking if they’re considering whether to recommend cannabis to their patients?
A: This is a very complex topic, and it’s not something that most of us have training in. You can’t sit down for 1 or 2 hours, get up to speed, and have your own well-informed opinion on it. You really need to put more time and effort.
Q: What are some issues that neurologists should consider?
A: You really need to find out what your state is doing about it and see how you feel about that.
How is your state administering medical and/or recreational marijuana? The administration of these programs is extremely different from state to state. Do these details satisfy you, and are you content having your patients interface with these programs?
Dr. Bowling reports no relevant disclosures.
Diet and Dermatology: Google Search Results for Acne, Psoriasis, and Eczema
Researching medical information currently is the third most common use of the Internet in the United States,1 with the majority of adults using the Web as their first source for health information before seeing a physician.2 When assessing health-related information online, resources can be grouped into 4 categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational.3 Access to such a wide range of sources may give readers the opportunity to share personal anecdotes and opinions, thereby serving as a forum for information that essentially cannot be validated. Although such websites may include useful information and cite current literature, in other instances health-related information may be misleading or fabricated.3
In a study evaluating 291 skin conditions and related Google trends, acne, psoriasis, and eczema were among the most burdensome diseases, with acne yielding the highest number of search results.4 Results of the study indicated a positive correlation between disease burden and online search interest.4 The impact of these online searches and the validity of Google search results are topics worth considering, as more dermatology patients are relying on holistic and nonpharmaceutical approaches to treatment and disease management.5 The purpose of this study was to evaluate content on diet and dermatology available on the Internet for acne, psoriasis, and eczema.
Methods
Google searches were performed in December 2017 using the terms diet and acne, diet and psoriasis, and diet and eczema. The first 10 results for each respective search were reviewed for recommendations about which foods to incorporate in the diet and which to avoid. They also were classified according to the following 4 website categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational. The recommendations gathered from the 30 websites were then compared to the current literature assessing the impact of diet on these respective conditions by conducting PubMed searches of articles indexed for MEDLINE using the same terms.
Results
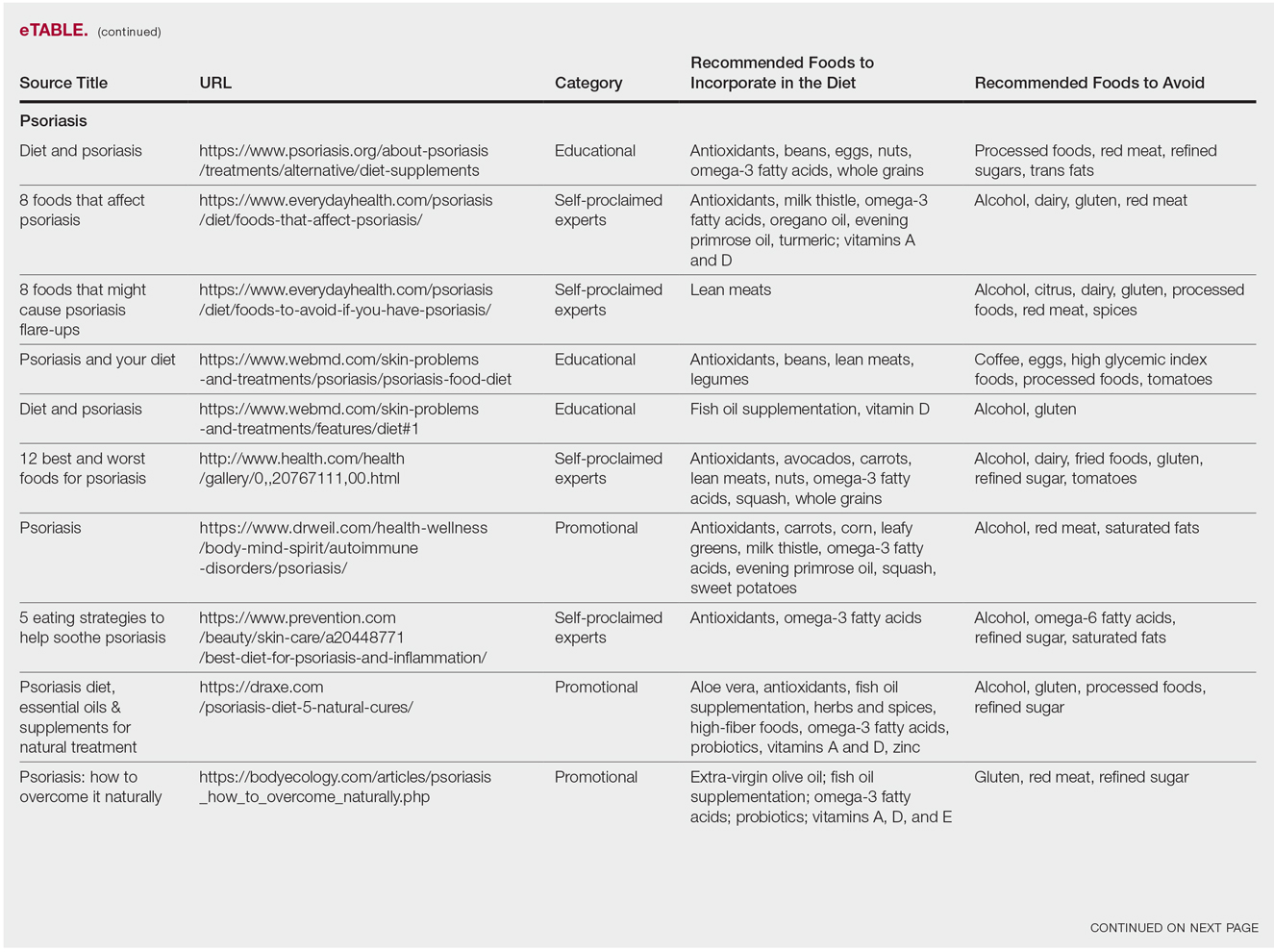
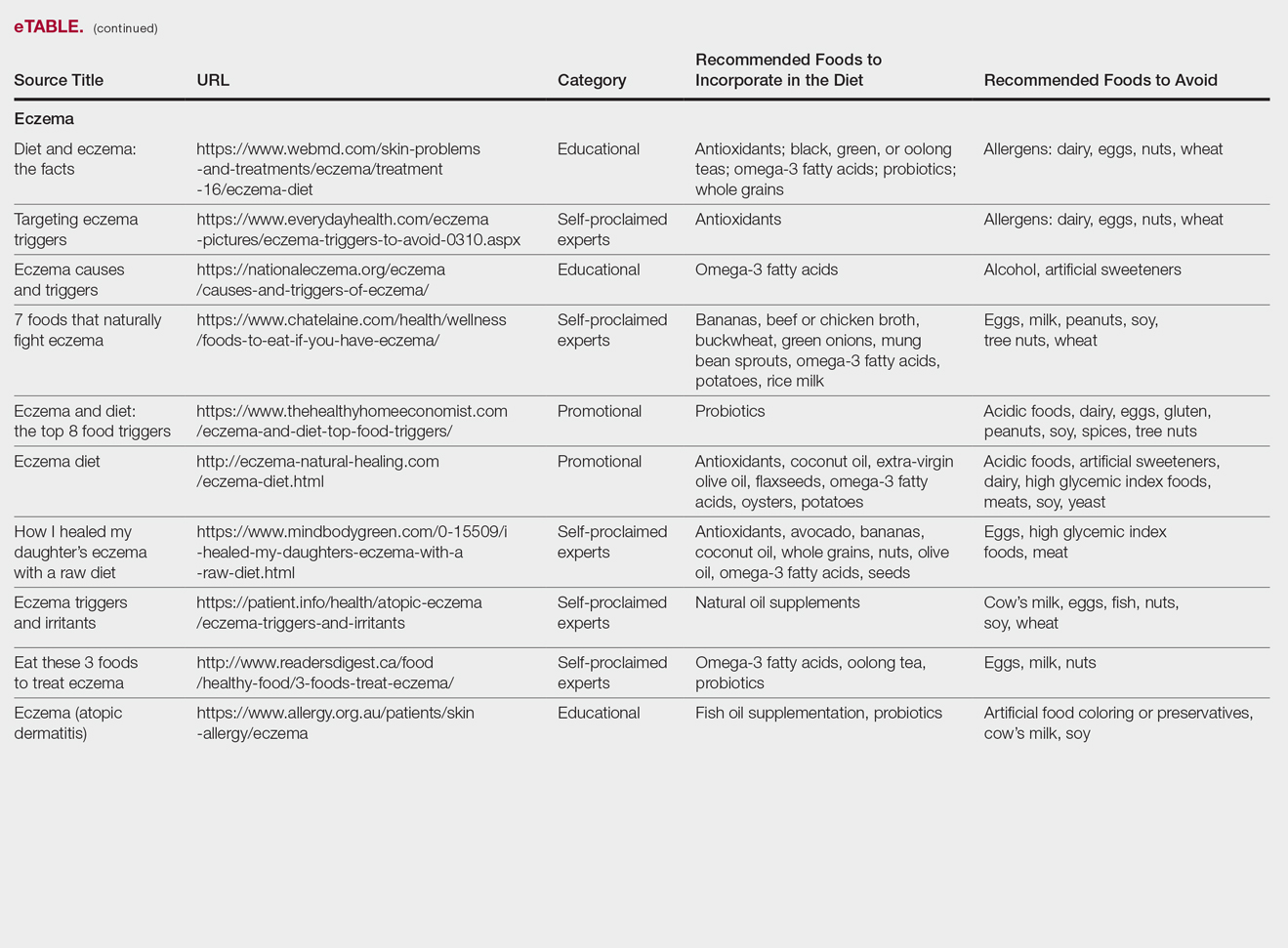
The results of this study are outlined in the eTable.

Acne
Our Google search using the term diet and acne produced 17,500,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 40% (4/10) were educational resources, and 20% (2/10) were promotional websites. Most of the websites advised acne patients to avoid high glycemic index foods (90% [9/10]) and dairy products (90% [9/10]). When discussing which foods to include in the diet, 70% (7/10) of websites recommended that patients incorporate omega-3 fatty acids and antioxidants in the diet.
Research has shown that a low glycemic index diet can lead to a decrease in patients’ acne lesion counts in some instances.6,7 In a case-controlled study of 2258 patients on a popular weight loss diet that emphasized low glycemic index foods, 87% of participants reported a reduction in acne and 91% reported a decrease in their dosage or number of acne medications.7 Still, the exact correlation between acne development and consumption of glycemic index foods has not been confirmed. However, high glycemic index diets have been linked to hyperinsulinemia, indicating that insulin levels may play a role in acne formation.8 The majority of other currently available studies evaluated the potential link between dairy consumption and acne. A retrospective analysis of 47,355 women spanning 12 weeks showed a positive link between increased dairy consumption, specifically skim milk, and acne formation. Despite the positive trend, limitations such as recall bias made it difficult to draw a conclusion based on these findings.9 However, results of a longitudinal questionnaire-based population study evaluating the impact of dairy consumption on acne in 2489 adolescent patients confirmed a positive correlation.10 Studies conducted in 2009 and 2011 concluded that milk consumption results in elevated insulinlike growth factor 1 levels, which were linked to comedogenesis.8,11
Currently, there are well-described mechanisms to explain the association of dairy consumption and glycemic index with acne. Confirming a correlation between acne development and dairy consumption suggests that a dairy-free diet may benefit acne patients.5 Other trials indicate that low glycemic index diets are beneficial in treating acne.6,7 Therefore, some of the recommendations made in our search results may be of merit; however, there is minimal evidence proving the benefits of the other dietary recommendations made in the websites we evaluated.
Psoriasis
Our Google search using the term diet and psoriasis yielded a total of 9,420,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 30% (3/10) were promotional, and 30% (3/10) were educational. Seventy percent (7/10) of websites recommended avoiding alcohol and 60% (6/10) recommended avoiding gluten, with others discouraging consumption of red meat. Most of the websites encouraged patients to consume omega-3 fatty acids and antioxidants, while a few also recommended vitamins A, D, and E, as well as evening primrose oil supplements.
Although current research indicates a positive correlation between excessive alcohol use and psoriasis severity, it is still unclear whether alcohol consumption can be directly linked to the disease.12-14 Likewise, despite belief that increased oxidative stress likely contributes to inflammation in psoriasis, there is little evidence linking antioxidants to improvement in psoriasis symptoms.12 However, the current literature is inconsistent regarding the effects of fish oil supplementation on psoriasis.12 In a randomized double-blind study of 145 patients, there was no significant difference in psoriasis area and severity index scores between a control group and a treatment group receiving fish oil supplementation.15 In another RCT of 45 participants, those given daily very long-chain omega-3 fatty acid supplements saw no difference in psoriasis symptoms.15 Despite debate, literature assessing the impact of gluten-free diets has described improvement in psoriasis lesions in patients with celiac-specific antibodies.16 Although some observational studies described vitamin D supplementation to be beneficial in the treatment of psoriatic lesions, a more recent RCT found no significant difference between control and treatment groups.17-19
Studies also have revealed that certain eating patterns, such as those associated with the Mediterranean diet that is rich in fruits, vegetables, whole grains, and omega-3 fatty acids may be linked to improved endothelial function scores and reduced C-reactive protein and IL-18levels.20,21 In a double-blind RCT of 75 patients with plaque psoriasis, mean (SD) psoriasis area and severity index scores decreased by 11.2 (9.8) in a group treated with omega-3 fatty acids compared to 7.5 (8.8) with omega-6 fatty acids (P=.048).22
Although excessive alcohol use may be linked to psoriasis, there is no conclusive evidence indicating causation, thereby discrediting online claims.12-14 Research has revealed that gluten-free diets in psoriasis patients with celiac disease may improve psoriasis treatment16; however, sufficient evidence is lacking for diets low in gluten and high in polyunsaturated fatty acids or antioxidant supplementation. Of the dietary supplements recommended in the search results we reviewed, fish oil appears to be the most promising, but no recommendations can be made based on the current research.
Eczema
Our Google search using the term diet and eczema yielded 1,160,000 results, with 50% (5/10) of websites attributed to self-proclaimed experts, 30% (3/10) to educational websites, and 20% (2/10) to promotional sites. Of the first 10 results, 80% (8/10) recommended that patients with eczema avoid milk/dairy and 50% (5/10) advised to avoid soy and wheat/gluten. Other websites indicated to avoid eggs, nuts, and artificial sweeteners. Patients were encouraged to incorporate omega-3 fatty acids in their diets, and a few sites recommended bananas, coconut oil, olive oil, and various teas.
In a review of 11 studies with a total of 596 participants, supplementation with vitamins D and E, fish oil, olive oil, and linoleic acid was evaluated for the treatment of eczema.23 Although results indicated modest improvement of eczema severity with supplementation of fish oil, evidence favoring this treatment is limited and unconvincing. Furthermore, some evidence indicates that elimination diets are only appropriate for patients with food allergies.24 In a study evaluating an egg-free and dairy-free diet for eczema patients, only participants with positive egg-specific serum IgE levels saw improvement in disease severity.23 Even though IgE-mediated food allergies have been reported in 40% of children with moderate eczema, the contribution of these allergies to eczema is questionable.25
There is little evidence in the literature to indicate a definitive correlation between the foods mentioned in the search results we evaluated and the development of eczema; however, for patients with food allergies and eczema, elimination diets may decrease disease severity.25,26 There is insufficient evidence to suggest a benefit from evening primrose oil or fish oil supplementation, thereby debunking claims found online.
Comment
Although our Google search results included a wide range of sources and information regarding diet and dermatologic conditions such as acne, psoriasis, and eczema, most of the information we found was either unfounded or misleading. Study limitations in the current literature include small sample size, potential recall bias, lack of appropriate controls, incomplete reported results, and the failure to clearly define skin changes.
When considering the accuracy and type of information regarding skin conditions that is available on the Internet, it is important to note that most of the results we reviewed were webpages attributed to self-proclaimed experts. Although educational websites also were included in the search results, whether or not patients prefer or understand the content of such websites is still unknown; therefore, health organizations should consider revising online patient education materials to allow universal comprehension.27
Furthermore, it is important to consider the impact that widespread Internet access may have on the physician-patient relationship. Having access to health-related information online and being able to potentially self-diagnose could delay or deter patients from seeking professional advice or care.3 A study evaluating the impact of online searches on the physician-patient relationship among 175 patients determined that 36.5% of patients gathered information online prior to their consultation with a physician, while 67.3% chose to complement the information given to them by their physician with online resources.28 Based on these statistics, it is important that physicians be up-to-date with Internet discourse to discredit unfounded recommendations. Ultimately, when it comes to diet and dermatology, patients ought to be skeptical of the information currently available on the Internet, given that most of it is unsubstantiated by medical research.
- Fox S. Online health search 2006. Pew Research Center website. http://www.pewinternet.org/2006/10/29/online-health-search-2006/. Published October 29, 2006. Accessed May 3, 2018.
- Prestin A, Vieux SN, Chou WY. Is online health activity alive and well or flatlining? findings from 10 years of the health information national trends survey. J Health Commun. 2015;20:790-798.
- Zeichner JA, Del Rosso JQ. Acne and the internet. Dermatol Clin. 2016;34:129-132.
- Whitsitt J, Karimkhani C, Boyers LN, et al. Comparing burden of dermatologic disease to search interest on Google trends. Dermatol Online J. 2015;21. pii:13030/qt5xg811qp.
- Shokeen D. Influence of diet in acne vulgaris and atopic dermatitis. Cutis. 2016;98:E28-E29.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
- Rouhani P. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(3, suppl 1):P706.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop Ser Pediatr Program. 2011;67:131-145.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online July 16, 2016]. J Eur Acad Dermatol Venereol. 2017;31:530-535.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: part II. melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.E1-1053.E16.
- Tobin AM, Higgins EM, Norris S, et al. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol. 2009;34:698-701.
- Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138-140.
- Søyland E, Funk J, Rajika G, et al. Effect of dietary supplementation with very long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Morimoto S, Yoshikawa K. Psoriasis and vitamin D3. a review of our experience. Arch Dermatol. 1989;125:231-234.
- Smith EL, Pincus SH, Donovan L, et al. A novel approach for the evaluation and treatment of psoriasis. oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516-528.
- Siddiqui MA, Al-Khawajah MM. Vitamin D3 and psoriasis: a randomized double-blind placebo-controlled study. J Dermatol Treat. 1990;1:243-245.
- Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067-2075.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13. pii:E743. doi:10.3390/ijerph13070743.
- Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.E1-1039.E12.
- Campbell DE. The role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012;48:1058-1064.
- Werfel T, Erdmann S, Fuchs T, et al. Approach to suspected food allergy in atopic dermatitis. guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;3:265-271.
- John AM, John ES, Hansberry DR, et al. Assessment of online patient education materials from major dermatologic associations. J Clin Aesthet Dermatol. 2016;9:23-28.
- Orgaz-Molina J, Cotugno M, Girón-Prieto MS, et al. A study of internet searches for medical information in dermatology patients: the patient-physician relationship. Actas Dermosifiliogr. 2015;106:493-499.
Researching medical information currently is the third most common use of the Internet in the United States,1 with the majority of adults using the Web as their first source for health information before seeing a physician.2 When assessing health-related information online, resources can be grouped into 4 categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational.3 Access to such a wide range of sources may give readers the opportunity to share personal anecdotes and opinions, thereby serving as a forum for information that essentially cannot be validated. Although such websites may include useful information and cite current literature, in other instances health-related information may be misleading or fabricated.3
In a study evaluating 291 skin conditions and related Google trends, acne, psoriasis, and eczema were among the most burdensome diseases, with acne yielding the highest number of search results.4 Results of the study indicated a positive correlation between disease burden and online search interest.4 The impact of these online searches and the validity of Google search results are topics worth considering, as more dermatology patients are relying on holistic and nonpharmaceutical approaches to treatment and disease management.5 The purpose of this study was to evaluate content on diet and dermatology available on the Internet for acne, psoriasis, and eczema.
Methods
Google searches were performed in December 2017 using the terms diet and acne, diet and psoriasis, and diet and eczema. The first 10 results for each respective search were reviewed for recommendations about which foods to incorporate in the diet and which to avoid. They also were classified according to the following 4 website categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational. The recommendations gathered from the 30 websites were then compared to the current literature assessing the impact of diet on these respective conditions by conducting PubMed searches of articles indexed for MEDLINE using the same terms.
Results
The results of this study are outlined in the eTable.



Acne
Our Google search using the term diet and acne produced 17,500,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 40% (4/10) were educational resources, and 20% (2/10) were promotional websites. Most of the websites advised acne patients to avoid high glycemic index foods (90% [9/10]) and dairy products (90% [9/10]). When discussing which foods to include in the diet, 70% (7/10) of websites recommended that patients incorporate omega-3 fatty acids and antioxidants in the diet.
Research has shown that a low glycemic index diet can lead to a decrease in patients’ acne lesion counts in some instances.6,7 In a case-controlled study of 2258 patients on a popular weight loss diet that emphasized low glycemic index foods, 87% of participants reported a reduction in acne and 91% reported a decrease in their dosage or number of acne medications.7 Still, the exact correlation between acne development and consumption of glycemic index foods has not been confirmed. However, high glycemic index diets have been linked to hyperinsulinemia, indicating that insulin levels may play a role in acne formation.8 The majority of other currently available studies evaluated the potential link between dairy consumption and acne. A retrospective analysis of 47,355 women spanning 12 weeks showed a positive link between increased dairy consumption, specifically skim milk, and acne formation. Despite the positive trend, limitations such as recall bias made it difficult to draw a conclusion based on these findings.9 However, results of a longitudinal questionnaire-based population study evaluating the impact of dairy consumption on acne in 2489 adolescent patients confirmed a positive correlation.10 Studies conducted in 2009 and 2011 concluded that milk consumption results in elevated insulinlike growth factor 1 levels, which were linked to comedogenesis.8,11
Currently, there are well-described mechanisms to explain the association of dairy consumption and glycemic index with acne. Confirming a correlation between acne development and dairy consumption suggests that a dairy-free diet may benefit acne patients.5 Other trials indicate that low glycemic index diets are beneficial in treating acne.6,7 Therefore, some of the recommendations made in our search results may be of merit; however, there is minimal evidence proving the benefits of the other dietary recommendations made in the websites we evaluated.
Psoriasis
Our Google search using the term diet and psoriasis yielded a total of 9,420,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 30% (3/10) were promotional, and 30% (3/10) were educational. Seventy percent (7/10) of websites recommended avoiding alcohol and 60% (6/10) recommended avoiding gluten, with others discouraging consumption of red meat. Most of the websites encouraged patients to consume omega-3 fatty acids and antioxidants, while a few also recommended vitamins A, D, and E, as well as evening primrose oil supplements.
Although current research indicates a positive correlation between excessive alcohol use and psoriasis severity, it is still unclear whether alcohol consumption can be directly linked to the disease.12-14 Likewise, despite belief that increased oxidative stress likely contributes to inflammation in psoriasis, there is little evidence linking antioxidants to improvement in psoriasis symptoms.12 However, the current literature is inconsistent regarding the effects of fish oil supplementation on psoriasis.12 In a randomized double-blind study of 145 patients, there was no significant difference in psoriasis area and severity index scores between a control group and a treatment group receiving fish oil supplementation.15 In another RCT of 45 participants, those given daily very long-chain omega-3 fatty acid supplements saw no difference in psoriasis symptoms.15 Despite debate, literature assessing the impact of gluten-free diets has described improvement in psoriasis lesions in patients with celiac-specific antibodies.16 Although some observational studies described vitamin D supplementation to be beneficial in the treatment of psoriatic lesions, a more recent RCT found no significant difference between control and treatment groups.17-19
Studies also have revealed that certain eating patterns, such as those associated with the Mediterranean diet that is rich in fruits, vegetables, whole grains, and omega-3 fatty acids may be linked to improved endothelial function scores and reduced C-reactive protein and IL-18levels.20,21 In a double-blind RCT of 75 patients with plaque psoriasis, mean (SD) psoriasis area and severity index scores decreased by 11.2 (9.8) in a group treated with omega-3 fatty acids compared to 7.5 (8.8) with omega-6 fatty acids (P=.048).22
Although excessive alcohol use may be linked to psoriasis, there is no conclusive evidence indicating causation, thereby discrediting online claims.12-14 Research has revealed that gluten-free diets in psoriasis patients with celiac disease may improve psoriasis treatment16; however, sufficient evidence is lacking for diets low in gluten and high in polyunsaturated fatty acids or antioxidant supplementation. Of the dietary supplements recommended in the search results we reviewed, fish oil appears to be the most promising, but no recommendations can be made based on the current research.
Eczema
Our Google search using the term diet and eczema yielded 1,160,000 results, with 50% (5/10) of websites attributed to self-proclaimed experts, 30% (3/10) to educational websites, and 20% (2/10) to promotional sites. Of the first 10 results, 80% (8/10) recommended that patients with eczema avoid milk/dairy and 50% (5/10) advised to avoid soy and wheat/gluten. Other websites indicated to avoid eggs, nuts, and artificial sweeteners. Patients were encouraged to incorporate omega-3 fatty acids in their diets, and a few sites recommended bananas, coconut oil, olive oil, and various teas.
In a review of 11 studies with a total of 596 participants, supplementation with vitamins D and E, fish oil, olive oil, and linoleic acid was evaluated for the treatment of eczema.23 Although results indicated modest improvement of eczema severity with supplementation of fish oil, evidence favoring this treatment is limited and unconvincing. Furthermore, some evidence indicates that elimination diets are only appropriate for patients with food allergies.24 In a study evaluating an egg-free and dairy-free diet for eczema patients, only participants with positive egg-specific serum IgE levels saw improvement in disease severity.23 Even though IgE-mediated food allergies have been reported in 40% of children with moderate eczema, the contribution of these allergies to eczema is questionable.25
There is little evidence in the literature to indicate a definitive correlation between the foods mentioned in the search results we evaluated and the development of eczema; however, for patients with food allergies and eczema, elimination diets may decrease disease severity.25,26 There is insufficient evidence to suggest a benefit from evening primrose oil or fish oil supplementation, thereby debunking claims found online.
Comment
Although our Google search results included a wide range of sources and information regarding diet and dermatologic conditions such as acne, psoriasis, and eczema, most of the information we found was either unfounded or misleading. Study limitations in the current literature include small sample size, potential recall bias, lack of appropriate controls, incomplete reported results, and the failure to clearly define skin changes.
When considering the accuracy and type of information regarding skin conditions that is available on the Internet, it is important to note that most of the results we reviewed were webpages attributed to self-proclaimed experts. Although educational websites also were included in the search results, whether or not patients prefer or understand the content of such websites is still unknown; therefore, health organizations should consider revising online patient education materials to allow universal comprehension.27
Furthermore, it is important to consider the impact that widespread Internet access may have on the physician-patient relationship. Having access to health-related information online and being able to potentially self-diagnose could delay or deter patients from seeking professional advice or care.3 A study evaluating the impact of online searches on the physician-patient relationship among 175 patients determined that 36.5% of patients gathered information online prior to their consultation with a physician, while 67.3% chose to complement the information given to them by their physician with online resources.28 Based on these statistics, it is important that physicians be up-to-date with Internet discourse to discredit unfounded recommendations. Ultimately, when it comes to diet and dermatology, patients ought to be skeptical of the information currently available on the Internet, given that most of it is unsubstantiated by medical research.
Researching medical information currently is the third most common use of the Internet in the United States,1 with the majority of adults using the Web as their first source for health information before seeing a physician.2 When assessing health-related information online, resources can be grouped into 4 categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational.3 Access to such a wide range of sources may give readers the opportunity to share personal anecdotes and opinions, thereby serving as a forum for information that essentially cannot be validated. Although such websites may include useful information and cite current literature, in other instances health-related information may be misleading or fabricated.3
In a study evaluating 291 skin conditions and related Google trends, acne, psoriasis, and eczema were among the most burdensome diseases, with acne yielding the highest number of search results.4 Results of the study indicated a positive correlation between disease burden and online search interest.4 The impact of these online searches and the validity of Google search results are topics worth considering, as more dermatology patients are relying on holistic and nonpharmaceutical approaches to treatment and disease management.5 The purpose of this study was to evaluate content on diet and dermatology available on the Internet for acne, psoriasis, and eczema.
Methods
Google searches were performed in December 2017 using the terms diet and acne, diet and psoriasis, and diet and eczema. The first 10 results for each respective search were reviewed for recommendations about which foods to incorporate in the diet and which to avoid. They also were classified according to the following 4 website categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational. The recommendations gathered from the 30 websites were then compared to the current literature assessing the impact of diet on these respective conditions by conducting PubMed searches of articles indexed for MEDLINE using the same terms.
Results
The results of this study are outlined in the eTable.



Acne
Our Google search using the term diet and acne produced 17,500,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 40% (4/10) were educational resources, and 20% (2/10) were promotional websites. Most of the websites advised acne patients to avoid high glycemic index foods (90% [9/10]) and dairy products (90% [9/10]). When discussing which foods to include in the diet, 70% (7/10) of websites recommended that patients incorporate omega-3 fatty acids and antioxidants in the diet.
Research has shown that a low glycemic index diet can lead to a decrease in patients’ acne lesion counts in some instances.6,7 In a case-controlled study of 2258 patients on a popular weight loss diet that emphasized low glycemic index foods, 87% of participants reported a reduction in acne and 91% reported a decrease in their dosage or number of acne medications.7 Still, the exact correlation between acne development and consumption of glycemic index foods has not been confirmed. However, high glycemic index diets have been linked to hyperinsulinemia, indicating that insulin levels may play a role in acne formation.8 The majority of other currently available studies evaluated the potential link between dairy consumption and acne. A retrospective analysis of 47,355 women spanning 12 weeks showed a positive link between increased dairy consumption, specifically skim milk, and acne formation. Despite the positive trend, limitations such as recall bias made it difficult to draw a conclusion based on these findings.9 However, results of a longitudinal questionnaire-based population study evaluating the impact of dairy consumption on acne in 2489 adolescent patients confirmed a positive correlation.10 Studies conducted in 2009 and 2011 concluded that milk consumption results in elevated insulinlike growth factor 1 levels, which were linked to comedogenesis.8,11
Currently, there are well-described mechanisms to explain the association of dairy consumption and glycemic index with acne. Confirming a correlation between acne development and dairy consumption suggests that a dairy-free diet may benefit acne patients.5 Other trials indicate that low glycemic index diets are beneficial in treating acne.6,7 Therefore, some of the recommendations made in our search results may be of merit; however, there is minimal evidence proving the benefits of the other dietary recommendations made in the websites we evaluated.
Psoriasis
Our Google search using the term diet and psoriasis yielded a total of 9,420,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 30% (3/10) were promotional, and 30% (3/10) were educational. Seventy percent (7/10) of websites recommended avoiding alcohol and 60% (6/10) recommended avoiding gluten, with others discouraging consumption of red meat. Most of the websites encouraged patients to consume omega-3 fatty acids and antioxidants, while a few also recommended vitamins A, D, and E, as well as evening primrose oil supplements.
Although current research indicates a positive correlation between excessive alcohol use and psoriasis severity, it is still unclear whether alcohol consumption can be directly linked to the disease.12-14 Likewise, despite belief that increased oxidative stress likely contributes to inflammation in psoriasis, there is little evidence linking antioxidants to improvement in psoriasis symptoms.12 However, the current literature is inconsistent regarding the effects of fish oil supplementation on psoriasis.12 In a randomized double-blind study of 145 patients, there was no significant difference in psoriasis area and severity index scores between a control group and a treatment group receiving fish oil supplementation.15 In another RCT of 45 participants, those given daily very long-chain omega-3 fatty acid supplements saw no difference in psoriasis symptoms.15 Despite debate, literature assessing the impact of gluten-free diets has described improvement in psoriasis lesions in patients with celiac-specific antibodies.16 Although some observational studies described vitamin D supplementation to be beneficial in the treatment of psoriatic lesions, a more recent RCT found no significant difference between control and treatment groups.17-19
Studies also have revealed that certain eating patterns, such as those associated with the Mediterranean diet that is rich in fruits, vegetables, whole grains, and omega-3 fatty acids may be linked to improved endothelial function scores and reduced C-reactive protein and IL-18levels.20,21 In a double-blind RCT of 75 patients with plaque psoriasis, mean (SD) psoriasis area and severity index scores decreased by 11.2 (9.8) in a group treated with omega-3 fatty acids compared to 7.5 (8.8) with omega-6 fatty acids (P=.048).22
Although excessive alcohol use may be linked to psoriasis, there is no conclusive evidence indicating causation, thereby discrediting online claims.12-14 Research has revealed that gluten-free diets in psoriasis patients with celiac disease may improve psoriasis treatment16; however, sufficient evidence is lacking for diets low in gluten and high in polyunsaturated fatty acids or antioxidant supplementation. Of the dietary supplements recommended in the search results we reviewed, fish oil appears to be the most promising, but no recommendations can be made based on the current research.
Eczema
Our Google search using the term diet and eczema yielded 1,160,000 results, with 50% (5/10) of websites attributed to self-proclaimed experts, 30% (3/10) to educational websites, and 20% (2/10) to promotional sites. Of the first 10 results, 80% (8/10) recommended that patients with eczema avoid milk/dairy and 50% (5/10) advised to avoid soy and wheat/gluten. Other websites indicated to avoid eggs, nuts, and artificial sweeteners. Patients were encouraged to incorporate omega-3 fatty acids in their diets, and a few sites recommended bananas, coconut oil, olive oil, and various teas.
In a review of 11 studies with a total of 596 participants, supplementation with vitamins D and E, fish oil, olive oil, and linoleic acid was evaluated for the treatment of eczema.23 Although results indicated modest improvement of eczema severity with supplementation of fish oil, evidence favoring this treatment is limited and unconvincing. Furthermore, some evidence indicates that elimination diets are only appropriate for patients with food allergies.24 In a study evaluating an egg-free and dairy-free diet for eczema patients, only participants with positive egg-specific serum IgE levels saw improvement in disease severity.23 Even though IgE-mediated food allergies have been reported in 40% of children with moderate eczema, the contribution of these allergies to eczema is questionable.25
There is little evidence in the literature to indicate a definitive correlation between the foods mentioned in the search results we evaluated and the development of eczema; however, for patients with food allergies and eczema, elimination diets may decrease disease severity.25,26 There is insufficient evidence to suggest a benefit from evening primrose oil or fish oil supplementation, thereby debunking claims found online.
Comment
Although our Google search results included a wide range of sources and information regarding diet and dermatologic conditions such as acne, psoriasis, and eczema, most of the information we found was either unfounded or misleading. Study limitations in the current literature include small sample size, potential recall bias, lack of appropriate controls, incomplete reported results, and the failure to clearly define skin changes.
When considering the accuracy and type of information regarding skin conditions that is available on the Internet, it is important to note that most of the results we reviewed were webpages attributed to self-proclaimed experts. Although educational websites also were included in the search results, whether or not patients prefer or understand the content of such websites is still unknown; therefore, health organizations should consider revising online patient education materials to allow universal comprehension.27
Furthermore, it is important to consider the impact that widespread Internet access may have on the physician-patient relationship. Having access to health-related information online and being able to potentially self-diagnose could delay or deter patients from seeking professional advice or care.3 A study evaluating the impact of online searches on the physician-patient relationship among 175 patients determined that 36.5% of patients gathered information online prior to their consultation with a physician, while 67.3% chose to complement the information given to them by their physician with online resources.28 Based on these statistics, it is important that physicians be up-to-date with Internet discourse to discredit unfounded recommendations. Ultimately, when it comes to diet and dermatology, patients ought to be skeptical of the information currently available on the Internet, given that most of it is unsubstantiated by medical research.
- Fox S. Online health search 2006. Pew Research Center website. http://www.pewinternet.org/2006/10/29/online-health-search-2006/. Published October 29, 2006. Accessed May 3, 2018.
- Prestin A, Vieux SN, Chou WY. Is online health activity alive and well or flatlining? findings from 10 years of the health information national trends survey. J Health Commun. 2015;20:790-798.
- Zeichner JA, Del Rosso JQ. Acne and the internet. Dermatol Clin. 2016;34:129-132.
- Whitsitt J, Karimkhani C, Boyers LN, et al. Comparing burden of dermatologic disease to search interest on Google trends. Dermatol Online J. 2015;21. pii:13030/qt5xg811qp.
- Shokeen D. Influence of diet in acne vulgaris and atopic dermatitis. Cutis. 2016;98:E28-E29.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
- Rouhani P. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(3, suppl 1):P706.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop Ser Pediatr Program. 2011;67:131-145.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online July 16, 2016]. J Eur Acad Dermatol Venereol. 2017;31:530-535.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: part II. melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.E1-1053.E16.
- Tobin AM, Higgins EM, Norris S, et al. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol. 2009;34:698-701.
- Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138-140.
- Søyland E, Funk J, Rajika G, et al. Effect of dietary supplementation with very long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Morimoto S, Yoshikawa K. Psoriasis and vitamin D3. a review of our experience. Arch Dermatol. 1989;125:231-234.
- Smith EL, Pincus SH, Donovan L, et al. A novel approach for the evaluation and treatment of psoriasis. oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516-528.
- Siddiqui MA, Al-Khawajah MM. Vitamin D3 and psoriasis: a randomized double-blind placebo-controlled study. J Dermatol Treat. 1990;1:243-245.
- Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067-2075.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13. pii:E743. doi:10.3390/ijerph13070743.
- Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.E1-1039.E12.
- Campbell DE. The role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012;48:1058-1064.
- Werfel T, Erdmann S, Fuchs T, et al. Approach to suspected food allergy in atopic dermatitis. guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;3:265-271.
- John AM, John ES, Hansberry DR, et al. Assessment of online patient education materials from major dermatologic associations. J Clin Aesthet Dermatol. 2016;9:23-28.
- Orgaz-Molina J, Cotugno M, Girón-Prieto MS, et al. A study of internet searches for medical information in dermatology patients: the patient-physician relationship. Actas Dermosifiliogr. 2015;106:493-499.
- Fox S. Online health search 2006. Pew Research Center website. http://www.pewinternet.org/2006/10/29/online-health-search-2006/. Published October 29, 2006. Accessed May 3, 2018.
- Prestin A, Vieux SN, Chou WY. Is online health activity alive and well or flatlining? findings from 10 years of the health information national trends survey. J Health Commun. 2015;20:790-798.
- Zeichner JA, Del Rosso JQ. Acne and the internet. Dermatol Clin. 2016;34:129-132.
- Whitsitt J, Karimkhani C, Boyers LN, et al. Comparing burden of dermatologic disease to search interest on Google trends. Dermatol Online J. 2015;21. pii:13030/qt5xg811qp.
- Shokeen D. Influence of diet in acne vulgaris and atopic dermatitis. Cutis. 2016;98:E28-E29.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
- Rouhani P. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(3, suppl 1):P706.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop Ser Pediatr Program. 2011;67:131-145.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online July 16, 2016]. J Eur Acad Dermatol Venereol. 2017;31:530-535.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: part II. melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.E1-1053.E16.
- Tobin AM, Higgins EM, Norris S, et al. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol. 2009;34:698-701.
- Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138-140.
- Søyland E, Funk J, Rajika G, et al. Effect of dietary supplementation with very long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Morimoto S, Yoshikawa K. Psoriasis and vitamin D3. a review of our experience. Arch Dermatol. 1989;125:231-234.
- Smith EL, Pincus SH, Donovan L, et al. A novel approach for the evaluation and treatment of psoriasis. oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516-528.
- Siddiqui MA, Al-Khawajah MM. Vitamin D3 and psoriasis: a randomized double-blind placebo-controlled study. J Dermatol Treat. 1990;1:243-245.
- Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067-2075.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13. pii:E743. doi:10.3390/ijerph13070743.
- Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.E1-1039.E12.
- Campbell DE. The role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012;48:1058-1064.
- Werfel T, Erdmann S, Fuchs T, et al. Approach to suspected food allergy in atopic dermatitis. guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;3:265-271.
- John AM, John ES, Hansberry DR, et al. Assessment of online patient education materials from major dermatologic associations. J Clin Aesthet Dermatol. 2016;9:23-28.
- Orgaz-Molina J, Cotugno M, Girón-Prieto MS, et al. A study of internet searches for medical information in dermatology patients: the patient-physician relationship. Actas Dermosifiliogr. 2015;106:493-499.
Practice Points
- It is important physicians be well-informed regarding Internet discourse to discredit unfounded recommendations.
- It is likely that patients seeking medical advice regarding their dermatologic condition and treatment will have done prior research on the Internet.
- Oftentimes, the information on educational health websites can be confusing to patients.
- Because of widespread Internet access to health-related information, patients may opt to self-diagnose and therefore delay seeking professional care.
FDA Approves a Cannabinoid Medicine for Two Forms of Epilepsy
Epidiolex (cannabidiol) oral solution may treat seizures in patients with Lennox-Gastaut syndrome and Dravet syndrome.
The FDA has approved Epidiolex (cannabidiol [CBD]) oral solution for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome in patients age 2 and older. Epidiolex is the first FDA-approved drug that contains a derivative of marijuana. It also is the first drug approved by the FDA for the treatment of Dravet syndrome.
The approval was based on three randomized, double-blind, placebo-controlled clinical trials that included 516 patients with Lennox-Gastaut syndrome or Dravet syndrome. Epidiolex taken with other epilepsy medications reduced the frequency of seizures, compared with placebo. The most common side effects included lethargy, elevated liver enzymes, decreased appetite, diarrhea, rash, weakness, sleep disorder, and infection.
“Because of the adequate and well-controlled clinical studies that supported this approval, prescribers can have confidence in the drug’s uniform strength and consistent delivery that support appropriate dosing needed for treating patients with these complex and serious epilepsy syndromes,” said FDA Commissioner Scott Gottlieb, MD. “We will continue to support rigorous scientific research on the potential medical uses of marijuana-derived products…. But at the same time, we are prepared to take action when we see the illegal marketing of CBD-containing products with serious, unproven medical claims.”
CBD, a component of Cannabis sativa, does not cause intoxication or euphoria, unlike tetrahydrocannabinol (THC), the plant’s primary psychoactive component. CBD currently is a Schedule I substance because it is a chemical component of the cannabis plant. The Drug Enforcement Administration (DEA) is expected reschedule CBD within 90 days.
Epidiolex will be marketed in the US by Carlsbad, California-based Greenwich Biosciences, the US subsidiary of GW Pharmaceuticals, which is headquartered in London. Access to Epidiolex is expected to be similar to that for other branded antiepileptic drugs, and the treatment is expected to be available by Fall 2018, the company said.
Epidiolex (cannabidiol) oral solution may treat seizures in patients with Lennox-Gastaut syndrome and Dravet syndrome.
Epidiolex (cannabidiol) oral solution may treat seizures in patients with Lennox-Gastaut syndrome and Dravet syndrome.
The FDA has approved Epidiolex (cannabidiol [CBD]) oral solution for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome in patients age 2 and older. Epidiolex is the first FDA-approved drug that contains a derivative of marijuana. It also is the first drug approved by the FDA for the treatment of Dravet syndrome.
The approval was based on three randomized, double-blind, placebo-controlled clinical trials that included 516 patients with Lennox-Gastaut syndrome or Dravet syndrome. Epidiolex taken with other epilepsy medications reduced the frequency of seizures, compared with placebo. The most common side effects included lethargy, elevated liver enzymes, decreased appetite, diarrhea, rash, weakness, sleep disorder, and infection.
“Because of the adequate and well-controlled clinical studies that supported this approval, prescribers can have confidence in the drug’s uniform strength and consistent delivery that support appropriate dosing needed for treating patients with these complex and serious epilepsy syndromes,” said FDA Commissioner Scott Gottlieb, MD. “We will continue to support rigorous scientific research on the potential medical uses of marijuana-derived products…. But at the same time, we are prepared to take action when we see the illegal marketing of CBD-containing products with serious, unproven medical claims.”
CBD, a component of Cannabis sativa, does not cause intoxication or euphoria, unlike tetrahydrocannabinol (THC), the plant’s primary psychoactive component. CBD currently is a Schedule I substance because it is a chemical component of the cannabis plant. The Drug Enforcement Administration (DEA) is expected reschedule CBD within 90 days.
Epidiolex will be marketed in the US by Carlsbad, California-based Greenwich Biosciences, the US subsidiary of GW Pharmaceuticals, which is headquartered in London. Access to Epidiolex is expected to be similar to that for other branded antiepileptic drugs, and the treatment is expected to be available by Fall 2018, the company said.
The FDA has approved Epidiolex (cannabidiol [CBD]) oral solution for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome in patients age 2 and older. Epidiolex is the first FDA-approved drug that contains a derivative of marijuana. It also is the first drug approved by the FDA for the treatment of Dravet syndrome.
The approval was based on three randomized, double-blind, placebo-controlled clinical trials that included 516 patients with Lennox-Gastaut syndrome or Dravet syndrome. Epidiolex taken with other epilepsy medications reduced the frequency of seizures, compared with placebo. The most common side effects included lethargy, elevated liver enzymes, decreased appetite, diarrhea, rash, weakness, sleep disorder, and infection.
“Because of the adequate and well-controlled clinical studies that supported this approval, prescribers can have confidence in the drug’s uniform strength and consistent delivery that support appropriate dosing needed for treating patients with these complex and serious epilepsy syndromes,” said FDA Commissioner Scott Gottlieb, MD. “We will continue to support rigorous scientific research on the potential medical uses of marijuana-derived products…. But at the same time, we are prepared to take action when we see the illegal marketing of CBD-containing products with serious, unproven medical claims.”
CBD, a component of Cannabis sativa, does not cause intoxication or euphoria, unlike tetrahydrocannabinol (THC), the plant’s primary psychoactive component. CBD currently is a Schedule I substance because it is a chemical component of the cannabis plant. The Drug Enforcement Administration (DEA) is expected reschedule CBD within 90 days.
Epidiolex will be marketed in the US by Carlsbad, California-based Greenwich Biosciences, the US subsidiary of GW Pharmaceuticals, which is headquartered in London. Access to Epidiolex is expected to be similar to that for other branded antiepileptic drugs, and the treatment is expected to be available by Fall 2018, the company said.
Predicting Platinum Efficacy
Platinum-based chemotherapy is effective in metastatic triple negative breast cancer (mTNBC), but predictive biomarkers would help identify the best candidates for the treatment. Two sets of parameters—neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)—have already demonstrated their prognostic prowess in many malignancies, but how well will they do in platinum-treated mTNBC patients? Researchers from Fondazione IRCCS Istituto Nazionale dei Tumori, in Milan, Italy conducted a retrospective, single-center study to evaluate the association between baseline NLR or PLR and progression-free survival (PFS) in 57 mTNBC patients treated with carboplatin-paclitaxel or carboplatin-gemcitabine between 2007 and 2017, compared with 148 patients with hormone receptor-positive HER2-negative metastatic breast cancer.
Response was assessed every 3 chemotherapy cycles. Among platinum-treated patients, high NLR and PLR were associated with significantly lower PFS. Median PFS was 304 days in patients with NLR < 2.5, and 158 days in those with NLR ≥ 2.5. Progression-free survival was longer in patients with baseline PLR < 200, compared with PLR ≥ 200. The researchers found no significant association between NLR or PLR and the PFS of control patients.
When the same parameters were evaluated before the administration of the third treatment cycle, NLR < 2.5 was still associated with reduced risk of disease progression, although PLR < 200 was not.
In patients with mTNBC, median overall survival was significantly longer in patients with NLR < 2.5 compared with NLR ≥ 2.5. Platelet-to-lymphocyte ratio values were not associated with overall survival. The ratios also appeared to have a generally prognostic role independently from tumor biology.
The hormone receptors for NLR and PLR in multivariable analysis for PFS were similar, and the parameters correlated with each other, the researchers say, suggesting that both NLR and PLR “well reflect the inflammatory/immune contexture in mTNBC, and may be redundant as predictive biomarkers.”
Source:
Vernieri C, Mennitto A, Prisciandaro M, et al. Sci Rep. 2018;8(1):8703.
Platinum-based chemotherapy is effective in metastatic triple negative breast cancer (mTNBC), but predictive biomarkers would help identify the best candidates for the treatment. Two sets of parameters—neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)—have already demonstrated their prognostic prowess in many malignancies, but how well will they do in platinum-treated mTNBC patients? Researchers from Fondazione IRCCS Istituto Nazionale dei Tumori, in Milan, Italy conducted a retrospective, single-center study to evaluate the association between baseline NLR or PLR and progression-free survival (PFS) in 57 mTNBC patients treated with carboplatin-paclitaxel or carboplatin-gemcitabine between 2007 and 2017, compared with 148 patients with hormone receptor-positive HER2-negative metastatic breast cancer.
Response was assessed every 3 chemotherapy cycles. Among platinum-treated patients, high NLR and PLR were associated with significantly lower PFS. Median PFS was 304 days in patients with NLR < 2.5, and 158 days in those with NLR ≥ 2.5. Progression-free survival was longer in patients with baseline PLR < 200, compared with PLR ≥ 200. The researchers found no significant association between NLR or PLR and the PFS of control patients.
When the same parameters were evaluated before the administration of the third treatment cycle, NLR < 2.5 was still associated with reduced risk of disease progression, although PLR < 200 was not.
In patients with mTNBC, median overall survival was significantly longer in patients with NLR < 2.5 compared with NLR ≥ 2.5. Platelet-to-lymphocyte ratio values were not associated with overall survival. The ratios also appeared to have a generally prognostic role independently from tumor biology.
The hormone receptors for NLR and PLR in multivariable analysis for PFS were similar, and the parameters correlated with each other, the researchers say, suggesting that both NLR and PLR “well reflect the inflammatory/immune contexture in mTNBC, and may be redundant as predictive biomarkers.”
Source:
Vernieri C, Mennitto A, Prisciandaro M, et al. Sci Rep. 2018;8(1):8703.
Platinum-based chemotherapy is effective in metastatic triple negative breast cancer (mTNBC), but predictive biomarkers would help identify the best candidates for the treatment. Two sets of parameters—neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)—have already demonstrated their prognostic prowess in many malignancies, but how well will they do in platinum-treated mTNBC patients? Researchers from Fondazione IRCCS Istituto Nazionale dei Tumori, in Milan, Italy conducted a retrospective, single-center study to evaluate the association between baseline NLR or PLR and progression-free survival (PFS) in 57 mTNBC patients treated with carboplatin-paclitaxel or carboplatin-gemcitabine between 2007 and 2017, compared with 148 patients with hormone receptor-positive HER2-negative metastatic breast cancer.
Response was assessed every 3 chemotherapy cycles. Among platinum-treated patients, high NLR and PLR were associated with significantly lower PFS. Median PFS was 304 days in patients with NLR < 2.5, and 158 days in those with NLR ≥ 2.5. Progression-free survival was longer in patients with baseline PLR < 200, compared with PLR ≥ 200. The researchers found no significant association between NLR or PLR and the PFS of control patients.
When the same parameters were evaluated before the administration of the third treatment cycle, NLR < 2.5 was still associated with reduced risk of disease progression, although PLR < 200 was not.
In patients with mTNBC, median overall survival was significantly longer in patients with NLR < 2.5 compared with NLR ≥ 2.5. Platelet-to-lymphocyte ratio values were not associated with overall survival. The ratios also appeared to have a generally prognostic role independently from tumor biology.
The hormone receptors for NLR and PLR in multivariable analysis for PFS were similar, and the parameters correlated with each other, the researchers say, suggesting that both NLR and PLR “well reflect the inflammatory/immune contexture in mTNBC, and may be redundant as predictive biomarkers.”
Source:
Vernieri C, Mennitto A, Prisciandaro M, et al. Sci Rep. 2018;8(1):8703.
Focus on preventing comorbidities in MS, physician urges
NASHVILLE, TENN. – Some patients use multiple sclerosis as an excuse to make poor health choices, but Allen C. Bowling, MD, PhD, of the Colorado Neurological Institute has seen another kind of story unfold. Fifteen to 20 years ago, Dr. Bowling said, he treated patients who took the development of MS in their 20s as a sign they needed to take better care of themselves. “They said MS was the best thing that happened to them ‘because it motivated me to make these healthy lifestyle changes I wouldn’t have made otherwise.’ ”
These patients have maintained their lifestyle changes, he said, lowering their risk of comorbidities and – perhaps – changing the course of their MS for the better.
“It’s all one machine, and sometimes we lose sight of that in our sub-sub-specialized world of treating MS ... You’re caring for a whole person. If you start thinking about that, it does make you think differently about how you treat the person, how you try to prevent disease in terms of certain pathways,” Dr. Bowling said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers, where he spoke to colleagues about the importance of helping patients to adopt lifestyle changes.
According to Dr. Bowling, there’s evidence linking lifestyle-related comorbidities, poorer food quality, and tobacco use to higher levels of overall MS risk, relapses, disability, and symptoms.
Researchers have also linked other life factors to higher MS risks: obesity (linked to overall MS risk, disability, symptoms); lack of physical activity (linked to relapses, disability, symptoms); emotional factors (relapses, symptoms); and alcohol overuse (linked to overall risk, disability, symptoms).
“Data is mild to moderate to strong in all those areas for lifestyle approaches like diet, physical activity, emotional health, alcohol in moderation or less, and no tobacco smoking,” Dr. Bowling said.
He said he believes physical activity leads to “much higher and earlier success than diet” in MS patients, although there’s no confirmed “best exercise.”
As for nutrition, he said vitamins D and B12 are possibly beneficial. But he cautioned against the potential for harm from supplements and added that there’s no proven best diet for MS.
As for finding time to address these issues in clinic, Dr. Bowling recommended mentioning various lifestyle issues over multiple office visits.
“Some of the effort should be switched to the primary care doctor,” he said, “but you can use a strong collection of words to convey to the person with MS that this is serious: ‘It’s not MS, but it’s a serious issue, and you must see your primary care doctor.’ ”
He believes that this approach can have a significant impact, “especially for those aged 20-40, because the doctor they pay the most attention to may be their MS clinician.”
Dr. Bowling said that he receives royalties from a book he authored, “Optimal Health With Multiple Sclerosis.”
NASHVILLE, TENN. – Some patients use multiple sclerosis as an excuse to make poor health choices, but Allen C. Bowling, MD, PhD, of the Colorado Neurological Institute has seen another kind of story unfold. Fifteen to 20 years ago, Dr. Bowling said, he treated patients who took the development of MS in their 20s as a sign they needed to take better care of themselves. “They said MS was the best thing that happened to them ‘because it motivated me to make these healthy lifestyle changes I wouldn’t have made otherwise.’ ”
These patients have maintained their lifestyle changes, he said, lowering their risk of comorbidities and – perhaps – changing the course of their MS for the better.
“It’s all one machine, and sometimes we lose sight of that in our sub-sub-specialized world of treating MS ... You’re caring for a whole person. If you start thinking about that, it does make you think differently about how you treat the person, how you try to prevent disease in terms of certain pathways,” Dr. Bowling said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers, where he spoke to colleagues about the importance of helping patients to adopt lifestyle changes.
According to Dr. Bowling, there’s evidence linking lifestyle-related comorbidities, poorer food quality, and tobacco use to higher levels of overall MS risk, relapses, disability, and symptoms.
Researchers have also linked other life factors to higher MS risks: obesity (linked to overall MS risk, disability, symptoms); lack of physical activity (linked to relapses, disability, symptoms); emotional factors (relapses, symptoms); and alcohol overuse (linked to overall risk, disability, symptoms).
“Data is mild to moderate to strong in all those areas for lifestyle approaches like diet, physical activity, emotional health, alcohol in moderation or less, and no tobacco smoking,” Dr. Bowling said.
He said he believes physical activity leads to “much higher and earlier success than diet” in MS patients, although there’s no confirmed “best exercise.”
As for nutrition, he said vitamins D and B12 are possibly beneficial. But he cautioned against the potential for harm from supplements and added that there’s no proven best diet for MS.
As for finding time to address these issues in clinic, Dr. Bowling recommended mentioning various lifestyle issues over multiple office visits.
“Some of the effort should be switched to the primary care doctor,” he said, “but you can use a strong collection of words to convey to the person with MS that this is serious: ‘It’s not MS, but it’s a serious issue, and you must see your primary care doctor.’ ”
He believes that this approach can have a significant impact, “especially for those aged 20-40, because the doctor they pay the most attention to may be their MS clinician.”
Dr. Bowling said that he receives royalties from a book he authored, “Optimal Health With Multiple Sclerosis.”
NASHVILLE, TENN. – Some patients use multiple sclerosis as an excuse to make poor health choices, but Allen C. Bowling, MD, PhD, of the Colorado Neurological Institute has seen another kind of story unfold. Fifteen to 20 years ago, Dr. Bowling said, he treated patients who took the development of MS in their 20s as a sign they needed to take better care of themselves. “They said MS was the best thing that happened to them ‘because it motivated me to make these healthy lifestyle changes I wouldn’t have made otherwise.’ ”
These patients have maintained their lifestyle changes, he said, lowering their risk of comorbidities and – perhaps – changing the course of their MS for the better.
“It’s all one machine, and sometimes we lose sight of that in our sub-sub-specialized world of treating MS ... You’re caring for a whole person. If you start thinking about that, it does make you think differently about how you treat the person, how you try to prevent disease in terms of certain pathways,” Dr. Bowling said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers, where he spoke to colleagues about the importance of helping patients to adopt lifestyle changes.
According to Dr. Bowling, there’s evidence linking lifestyle-related comorbidities, poorer food quality, and tobacco use to higher levels of overall MS risk, relapses, disability, and symptoms.
Researchers have also linked other life factors to higher MS risks: obesity (linked to overall MS risk, disability, symptoms); lack of physical activity (linked to relapses, disability, symptoms); emotional factors (relapses, symptoms); and alcohol overuse (linked to overall risk, disability, symptoms).
“Data is mild to moderate to strong in all those areas for lifestyle approaches like diet, physical activity, emotional health, alcohol in moderation or less, and no tobacco smoking,” Dr. Bowling said.
He said he believes physical activity leads to “much higher and earlier success than diet” in MS patients, although there’s no confirmed “best exercise.”
As for nutrition, he said vitamins D and B12 are possibly beneficial. But he cautioned against the potential for harm from supplements and added that there’s no proven best diet for MS.
As for finding time to address these issues in clinic, Dr. Bowling recommended mentioning various lifestyle issues over multiple office visits.
“Some of the effort should be switched to the primary care doctor,” he said, “but you can use a strong collection of words to convey to the person with MS that this is serious: ‘It’s not MS, but it’s a serious issue, and you must see your primary care doctor.’ ”
He believes that this approach can have a significant impact, “especially for those aged 20-40, because the doctor they pay the most attention to may be their MS clinician.”
Dr. Bowling said that he receives royalties from a book he authored, “Optimal Health With Multiple Sclerosis.”
REPORTING FROM THE CMSC ANNUAL MEETING
New guidelines for gadolinium-based contrast agents take conservative stance
NASHVILLE, Tenn. – Gadolinium-based contrast agents (GBCAs) are necessary for the accurate initial diagnosis of patients experiencing a first clinical attack of symptoms consistent with multiple sclerosis and for following patients with highly active disease or sudden, unexpected declines.
But according to new guidelines issued by the Consortium of Multiple Sclerosis Centers, GBCAs are optional – although helpful – in many other clinical scenarios, especially when noncontrast MRI can provide answers.
“The key is that there is an optional role for gadolinium,” David Li, MD, said at the annual meeting of the Consortium of Multiple Sclerosis Centers. Although a GBCA is still “essential” for some clinical scenarios in clinically isolated syndrome and MS, the
“But I would like to remind you that if you need to know about ongoing, current activity,” in settings of acute change, then gadolinium is still necessary, Dr. Li of the University of British Columbia, Vancouver, said in a video interview.
The guideline is an update of CMSC’s 2015 document, which endorsed a more liberal use of GBCAs. This more conservative stance reflects new research on the agents and an update in 2017 from the Food and Drug Administration that required a class-wide warning about gadolinium retention.
The agency began investigating gadolinium in 2015. In May 2017, it issued a statement confirming that gadolinium accumulates in neural tissue and can be retained for an extended period. However, in reviewing the evidence, FDA found no concerning safety signals. Despite the presumed lack of toxicity, the agency issued the warning and recommended limiting the contrast agent’s use – a move reflected in CMSC’s new MRI protocol guidelines.
“While there is no known CNS toxicity, these agents should be used judiciously, recognizing that gadolinium continues to play an invaluable role in specific circumstances related to the diagnosis and follow-up of individuals with MS,” the document notes.
Dr. Li concurred.
“It remains indispensable in patients presenting with their first clinical attack (CIS) as [its] use allows for an earlier diagnosis by demonstrating lesion dissemination in time in addition to lesion dissemination in space, the hallmarks for the diagnosis of MS. Early diagnosis leads to early treatment, which may help in preventing disease progression and improve long-term prognosis.”
Dr. Li has received multiple drug company grants and acted as a consultant to multiple pharmaceutical companies, but had no disclosures relevant to gadolinium.
NASHVILLE, Tenn. – Gadolinium-based contrast agents (GBCAs) are necessary for the accurate initial diagnosis of patients experiencing a first clinical attack of symptoms consistent with multiple sclerosis and for following patients with highly active disease or sudden, unexpected declines.
But according to new guidelines issued by the Consortium of Multiple Sclerosis Centers, GBCAs are optional – although helpful – in many other clinical scenarios, especially when noncontrast MRI can provide answers.
“The key is that there is an optional role for gadolinium,” David Li, MD, said at the annual meeting of the Consortium of Multiple Sclerosis Centers. Although a GBCA is still “essential” for some clinical scenarios in clinically isolated syndrome and MS, the
“But I would like to remind you that if you need to know about ongoing, current activity,” in settings of acute change, then gadolinium is still necessary, Dr. Li of the University of British Columbia, Vancouver, said in a video interview.
The guideline is an update of CMSC’s 2015 document, which endorsed a more liberal use of GBCAs. This more conservative stance reflects new research on the agents and an update in 2017 from the Food and Drug Administration that required a class-wide warning about gadolinium retention.
The agency began investigating gadolinium in 2015. In May 2017, it issued a statement confirming that gadolinium accumulates in neural tissue and can be retained for an extended period. However, in reviewing the evidence, FDA found no concerning safety signals. Despite the presumed lack of toxicity, the agency issued the warning and recommended limiting the contrast agent’s use – a move reflected in CMSC’s new MRI protocol guidelines.
“While there is no known CNS toxicity, these agents should be used judiciously, recognizing that gadolinium continues to play an invaluable role in specific circumstances related to the diagnosis and follow-up of individuals with MS,” the document notes.
Dr. Li concurred.
“It remains indispensable in patients presenting with their first clinical attack (CIS) as [its] use allows for an earlier diagnosis by demonstrating lesion dissemination in time in addition to lesion dissemination in space, the hallmarks for the diagnosis of MS. Early diagnosis leads to early treatment, which may help in preventing disease progression and improve long-term prognosis.”
Dr. Li has received multiple drug company grants and acted as a consultant to multiple pharmaceutical companies, but had no disclosures relevant to gadolinium.
NASHVILLE, Tenn. – Gadolinium-based contrast agents (GBCAs) are necessary for the accurate initial diagnosis of patients experiencing a first clinical attack of symptoms consistent with multiple sclerosis and for following patients with highly active disease or sudden, unexpected declines.
But according to new guidelines issued by the Consortium of Multiple Sclerosis Centers, GBCAs are optional – although helpful – in many other clinical scenarios, especially when noncontrast MRI can provide answers.
“The key is that there is an optional role for gadolinium,” David Li, MD, said at the annual meeting of the Consortium of Multiple Sclerosis Centers. Although a GBCA is still “essential” for some clinical scenarios in clinically isolated syndrome and MS, the
“But I would like to remind you that if you need to know about ongoing, current activity,” in settings of acute change, then gadolinium is still necessary, Dr. Li of the University of British Columbia, Vancouver, said in a video interview.
The guideline is an update of CMSC’s 2015 document, which endorsed a more liberal use of GBCAs. This more conservative stance reflects new research on the agents and an update in 2017 from the Food and Drug Administration that required a class-wide warning about gadolinium retention.
The agency began investigating gadolinium in 2015. In May 2017, it issued a statement confirming that gadolinium accumulates in neural tissue and can be retained for an extended period. However, in reviewing the evidence, FDA found no concerning safety signals. Despite the presumed lack of toxicity, the agency issued the warning and recommended limiting the contrast agent’s use – a move reflected in CMSC’s new MRI protocol guidelines.
“While there is no known CNS toxicity, these agents should be used judiciously, recognizing that gadolinium continues to play an invaluable role in specific circumstances related to the diagnosis and follow-up of individuals with MS,” the document notes.
Dr. Li concurred.
“It remains indispensable in patients presenting with their first clinical attack (CIS) as [its] use allows for an earlier diagnosis by demonstrating lesion dissemination in time in addition to lesion dissemination in space, the hallmarks for the diagnosis of MS. Early diagnosis leads to early treatment, which may help in preventing disease progression and improve long-term prognosis.”
Dr. Li has received multiple drug company grants and acted as a consultant to multiple pharmaceutical companies, but had no disclosures relevant to gadolinium.
REPORTING FROM THE CMSC ANNUAL MEETING
VIDEO: PML prevention is possible, even when treating patients with aggressive MS
NASHVILLE, TENN. – Armed with new statistics, neurologist Joseph R. Berger, MD, has a message for colleagues about the widely feared risk of progressive multifocal leukoencephalopathy (PML) in multiple sclerosis: It’s not as inevitable as you might think.
“You can actually prevent this disease from occurring because we have risk-limiting strategies in many circumstances,” said Dr. Berger of the University of Pennsylvania, Philadelphia, in a presentation on PML at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Unlike other conditions such as HIV, MS itself is not linked to a higher risk of PML, said Dr. Berger, a leading PML researcher. Instead, it’s the medications that spark the condition, he said, with at least three and possibly four drugs posing a risk to patients.
Natalizumab (Tysabri) is especially risky. “We know that the risk with natalizumab is incredibly high in the context of JC [John Cunningham] virus antibody positivity and prolonged therapy,” Dr. Berger said in an interview after his presentation.
Still, “you can safely give natalizumab for a short period of time when treating patients with aggressive MS,” he said. “I will frequently employ that strategy even in the context of JC virus antibody positivity.”
According to Dr. Berger, there’s no risk of PML when natalizumab is used for under 8 months (Mult Scler Relat Disord. 2017 Feb;12:59-63).
However, “if you leave people on the drug indefinitely, there is a substantial risk of developing PML,” he said. “Individuals who have been left on the drug for 2 years, who’ve seen prior immunosuppressant therapy, who are JC virus antibody positive – that group of individuals develops PML at rates of 1 in 50 to 1 in 100.”
These levels are “enormous,” he said, higher even than those in the HIV population before the rise of antiretroviral medications.
Overall, as of Nov. 30, 2017, 177,800 patients have received natalizumab in the postmarketing setting, and 756 cases of PML have been reported as of Dec. 7, 2017. All but three of those cases were in patients with MS, and the overall incidence was 4.19/1,000.
Dr. Berger recommends regular screening MRIs for PML in patients taking natalizumab, and he advised physicians to be on alert for signs of trouble like the appearance of new neurologic symptoms or a new or increasing JC virus antibody index.
Two other MS drugs, fingolimod (Gilenya) and dimethyl fumarate (Tecfidera), fall into the category of low risk, with just 19 and 5 reported cases, respectively, as of February 2018, Dr. Berger said. He added that two of the fingolimod patients had earlier exposure to natalizumab.
With dimethyl fumarate, the risk appears to disappear – although this isn’t confirmed – when JC antibody–positive patients are taken off the drug, and their lymphocyte counts fall below 500 per mcL, Dr. Berger said.
“Unfortunately for fingolimod, we don’t have a defined risk-mitigation strategy,” he said. However, researchers have noticed that the fingolimod cases have occurred more often in older people, possibly because of the aging of the immune system, he said.
Another three MS drugs – alemtuzumab (Lemtrada), ocrelizumab (Ocrevus; with rituximab as proxy), and teriflunomide (Aubagio; with leflunomide as proxy) have unknown risk, according to Dr. Berger. There have been three cases in ocrelizumab (rituximab as proxy) and one in teriflunomide (leflunomide as proxy), but all were carry-overs from natalizumab or fingolimod exposure or occurred after natalizumab exposure.
What can physicians do if a patient develops PML? Stopping the drug and restoring the immune system is crucial, he said.
While there’s evidence that plasma exchange clears natalizumab (Neurology. 2009 Feb 3;72[5]:402-9), “there’s no study that demonstrates it’s in the patient’s best interest,” Dr. Berger said during his presentation. He noted that a retrospective study found no improvement in morbidity or mortality (Neurology. 2017 Mar 21;88[12];1144-52).
Multiple strategies to treat PML – including immunizations and inhibitors of DNA replication – have failed to make an impact so far, Dr. Berger said. According to him, the reasons for the failure of PML treatment are a lack of hard evidence, apart from anecdotal, to support them, based on a history of failed clinical trials.
Dr. Berger disclosed serving as a consultant for numerous pharmaceutical companies.
NASHVILLE, TENN. – Armed with new statistics, neurologist Joseph R. Berger, MD, has a message for colleagues about the widely feared risk of progressive multifocal leukoencephalopathy (PML) in multiple sclerosis: It’s not as inevitable as you might think.
“You can actually prevent this disease from occurring because we have risk-limiting strategies in many circumstances,” said Dr. Berger of the University of Pennsylvania, Philadelphia, in a presentation on PML at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Unlike other conditions such as HIV, MS itself is not linked to a higher risk of PML, said Dr. Berger, a leading PML researcher. Instead, it’s the medications that spark the condition, he said, with at least three and possibly four drugs posing a risk to patients.
Natalizumab (Tysabri) is especially risky. “We know that the risk with natalizumab is incredibly high in the context of JC [John Cunningham] virus antibody positivity and prolonged therapy,” Dr. Berger said in an interview after his presentation.
Still, “you can safely give natalizumab for a short period of time when treating patients with aggressive MS,” he said. “I will frequently employ that strategy even in the context of JC virus antibody positivity.”
According to Dr. Berger, there’s no risk of PML when natalizumab is used for under 8 months (Mult Scler Relat Disord. 2017 Feb;12:59-63).
However, “if you leave people on the drug indefinitely, there is a substantial risk of developing PML,” he said. “Individuals who have been left on the drug for 2 years, who’ve seen prior immunosuppressant therapy, who are JC virus antibody positive – that group of individuals develops PML at rates of 1 in 50 to 1 in 100.”
These levels are “enormous,” he said, higher even than those in the HIV population before the rise of antiretroviral medications.
Overall, as of Nov. 30, 2017, 177,800 patients have received natalizumab in the postmarketing setting, and 756 cases of PML have been reported as of Dec. 7, 2017. All but three of those cases were in patients with MS, and the overall incidence was 4.19/1,000.
Dr. Berger recommends regular screening MRIs for PML in patients taking natalizumab, and he advised physicians to be on alert for signs of trouble like the appearance of new neurologic symptoms or a new or increasing JC virus antibody index.
Two other MS drugs, fingolimod (Gilenya) and dimethyl fumarate (Tecfidera), fall into the category of low risk, with just 19 and 5 reported cases, respectively, as of February 2018, Dr. Berger said. He added that two of the fingolimod patients had earlier exposure to natalizumab.
With dimethyl fumarate, the risk appears to disappear – although this isn’t confirmed – when JC antibody–positive patients are taken off the drug, and their lymphocyte counts fall below 500 per mcL, Dr. Berger said.
“Unfortunately for fingolimod, we don’t have a defined risk-mitigation strategy,” he said. However, researchers have noticed that the fingolimod cases have occurred more often in older people, possibly because of the aging of the immune system, he said.
Another three MS drugs – alemtuzumab (Lemtrada), ocrelizumab (Ocrevus; with rituximab as proxy), and teriflunomide (Aubagio; with leflunomide as proxy) have unknown risk, according to Dr. Berger. There have been three cases in ocrelizumab (rituximab as proxy) and one in teriflunomide (leflunomide as proxy), but all were carry-overs from natalizumab or fingolimod exposure or occurred after natalizumab exposure.
What can physicians do if a patient develops PML? Stopping the drug and restoring the immune system is crucial, he said.
While there’s evidence that plasma exchange clears natalizumab (Neurology. 2009 Feb 3;72[5]:402-9), “there’s no study that demonstrates it’s in the patient’s best interest,” Dr. Berger said during his presentation. He noted that a retrospective study found no improvement in morbidity or mortality (Neurology. 2017 Mar 21;88[12];1144-52).
Multiple strategies to treat PML – including immunizations and inhibitors of DNA replication – have failed to make an impact so far, Dr. Berger said. According to him, the reasons for the failure of PML treatment are a lack of hard evidence, apart from anecdotal, to support them, based on a history of failed clinical trials.
Dr. Berger disclosed serving as a consultant for numerous pharmaceutical companies.
NASHVILLE, TENN. – Armed with new statistics, neurologist Joseph R. Berger, MD, has a message for colleagues about the widely feared risk of progressive multifocal leukoencephalopathy (PML) in multiple sclerosis: It’s not as inevitable as you might think.
“You can actually prevent this disease from occurring because we have risk-limiting strategies in many circumstances,” said Dr. Berger of the University of Pennsylvania, Philadelphia, in a presentation on PML at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Unlike other conditions such as HIV, MS itself is not linked to a higher risk of PML, said Dr. Berger, a leading PML researcher. Instead, it’s the medications that spark the condition, he said, with at least three and possibly four drugs posing a risk to patients.
Natalizumab (Tysabri) is especially risky. “We know that the risk with natalizumab is incredibly high in the context of JC [John Cunningham] virus antibody positivity and prolonged therapy,” Dr. Berger said in an interview after his presentation.
Still, “you can safely give natalizumab for a short period of time when treating patients with aggressive MS,” he said. “I will frequently employ that strategy even in the context of JC virus antibody positivity.”
According to Dr. Berger, there’s no risk of PML when natalizumab is used for under 8 months (Mult Scler Relat Disord. 2017 Feb;12:59-63).
However, “if you leave people on the drug indefinitely, there is a substantial risk of developing PML,” he said. “Individuals who have been left on the drug for 2 years, who’ve seen prior immunosuppressant therapy, who are JC virus antibody positive – that group of individuals develops PML at rates of 1 in 50 to 1 in 100.”
These levels are “enormous,” he said, higher even than those in the HIV population before the rise of antiretroviral medications.
Overall, as of Nov. 30, 2017, 177,800 patients have received natalizumab in the postmarketing setting, and 756 cases of PML have been reported as of Dec. 7, 2017. All but three of those cases were in patients with MS, and the overall incidence was 4.19/1,000.
Dr. Berger recommends regular screening MRIs for PML in patients taking natalizumab, and he advised physicians to be on alert for signs of trouble like the appearance of new neurologic symptoms or a new or increasing JC virus antibody index.
Two other MS drugs, fingolimod (Gilenya) and dimethyl fumarate (Tecfidera), fall into the category of low risk, with just 19 and 5 reported cases, respectively, as of February 2018, Dr. Berger said. He added that two of the fingolimod patients had earlier exposure to natalizumab.
With dimethyl fumarate, the risk appears to disappear – although this isn’t confirmed – when JC antibody–positive patients are taken off the drug, and their lymphocyte counts fall below 500 per mcL, Dr. Berger said.
“Unfortunately for fingolimod, we don’t have a defined risk-mitigation strategy,” he said. However, researchers have noticed that the fingolimod cases have occurred more often in older people, possibly because of the aging of the immune system, he said.
Another three MS drugs – alemtuzumab (Lemtrada), ocrelizumab (Ocrevus; with rituximab as proxy), and teriflunomide (Aubagio; with leflunomide as proxy) have unknown risk, according to Dr. Berger. There have been three cases in ocrelizumab (rituximab as proxy) and one in teriflunomide (leflunomide as proxy), but all were carry-overs from natalizumab or fingolimod exposure or occurred after natalizumab exposure.
What can physicians do if a patient develops PML? Stopping the drug and restoring the immune system is crucial, he said.
While there’s evidence that plasma exchange clears natalizumab (Neurology. 2009 Feb 3;72[5]:402-9), “there’s no study that demonstrates it’s in the patient’s best interest,” Dr. Berger said during his presentation. He noted that a retrospective study found no improvement in morbidity or mortality (Neurology. 2017 Mar 21;88[12];1144-52).
Multiple strategies to treat PML – including immunizations and inhibitors of DNA replication – have failed to make an impact so far, Dr. Berger said. According to him, the reasons for the failure of PML treatment are a lack of hard evidence, apart from anecdotal, to support them, based on a history of failed clinical trials.
Dr. Berger disclosed serving as a consultant for numerous pharmaceutical companies.
REPORTING FROM THE CMSC ANNUAL MEETING
Value of alemtuzumab demonstrated in RRMS patients with prior IFNB-1a treatment
LOS ANGELES – Patients with multiple sclerosis in the CARE-MS II study who switched from interferon beta-1a therapy to the humanized monoclonal antibody alemtuzumab experienced continued reductions in brain volume loss and lesions on MRI through 5 years, according to follow-up data from the CARE-MS II extension study known as TOPAZ.
These outcomes support core findings from the CARE-MS II study, and suggest that alemtuzumab (Lemtrada) provides a unique treatment approach for patients with prior subcutaneous interferon beta-1a (IFNB-1a) treatment, Daniel Pelletier, MD, reported in a poster discussion session at the annual meeting of the American Academy of Neurology.
In CARE-MS II, relapsing-remitting multiple sclerosis patients with inadequate response to prior therapy experienced improvements in MRI lesions and brain volume loss with two courses of alemtuzumab versus IFNB-1a through 2 years, and in a 4-year extension in which participants discontinued subcutaneous IFNB-1a and initiated alemtuzumab at 12 mg/day, they experienced durable efficacy in the absence of continuous treatment, explained Dr. Pelletier, a professor of neurology at the University of Southern California, Los Angeles.
In the extension, patients could receive alemtuzumab retreatment as needed for relapse/MRI activity or receive other disease-modifying therapies at the investigator’s discretion. Patients completing the extension could enter the 5-year TOPAZ study for further evaluation, he said.
Of 119 patients who completed TOPAZ year 1, and thus had 5 years of follow-up after initiating alemtuzumab, 78% were free of new, gadolinium-enhancing lesions in IFNB-1a year 2; this increased significantly to 92% in post-alemtuzumab year 2, and remained high at 85%-89% in years 3-5. Additionally, 48% of patients were free of new/enlarging T2 lesions in IFNB-1a year 2; this increased significantly to 81% in post-alemtuzumab year 2 and remained high in years 3-5.
Further, 47% of the TOPAZ patients were MRI disease activity–free in IFNB-1a year 2; this increased significantly to 81% in post-alemtuzumab year 2, and remained high at 67%-72% in years 3-5.
“Perhaps for me what makes even more sense is looking at the yearly brain parenchymal fraction change,” Dr. Pelletier said, noting that the median annual reductions in years 1-5, respectively, were 0.02%, 0.04%, 0.15%, 0.14%, and 0.08%, compared with –0.33% for subcutaneous IFNB-1a at year 2 in CARE-MS II. The median brain parenchymal fraction change from baseline to post-alemtuzumab year 5 was –1.40%.
In 61% of patients, no further treatment was given after the second course of alemtuzumab.
The findings suggest that alemtuzumab provides durable efficacy without continuous treatment, he concluded.
This study was supported by Sanofi and Bayer Healthcare Pharmaceuticals. Dr. Pelletier has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Merck Serono, Novartis, Roche, and Sanofi, as well as research support from Biogen, Merck Serono, Novartis, Roche, and Sanofi.
SOURCE: Pelletier D et al. Neurology. 2018 Apr 10. 90(15 Suppl.):P5.031.
LOS ANGELES – Patients with multiple sclerosis in the CARE-MS II study who switched from interferon beta-1a therapy to the humanized monoclonal antibody alemtuzumab experienced continued reductions in brain volume loss and lesions on MRI through 5 years, according to follow-up data from the CARE-MS II extension study known as TOPAZ.
These outcomes support core findings from the CARE-MS II study, and suggest that alemtuzumab (Lemtrada) provides a unique treatment approach for patients with prior subcutaneous interferon beta-1a (IFNB-1a) treatment, Daniel Pelletier, MD, reported in a poster discussion session at the annual meeting of the American Academy of Neurology.
In CARE-MS II, relapsing-remitting multiple sclerosis patients with inadequate response to prior therapy experienced improvements in MRI lesions and brain volume loss with two courses of alemtuzumab versus IFNB-1a through 2 years, and in a 4-year extension in which participants discontinued subcutaneous IFNB-1a and initiated alemtuzumab at 12 mg/day, they experienced durable efficacy in the absence of continuous treatment, explained Dr. Pelletier, a professor of neurology at the University of Southern California, Los Angeles.
In the extension, patients could receive alemtuzumab retreatment as needed for relapse/MRI activity or receive other disease-modifying therapies at the investigator’s discretion. Patients completing the extension could enter the 5-year TOPAZ study for further evaluation, he said.
Of 119 patients who completed TOPAZ year 1, and thus had 5 years of follow-up after initiating alemtuzumab, 78% were free of new, gadolinium-enhancing lesions in IFNB-1a year 2; this increased significantly to 92% in post-alemtuzumab year 2, and remained high at 85%-89% in years 3-5. Additionally, 48% of patients were free of new/enlarging T2 lesions in IFNB-1a year 2; this increased significantly to 81% in post-alemtuzumab year 2 and remained high in years 3-5.
Further, 47% of the TOPAZ patients were MRI disease activity–free in IFNB-1a year 2; this increased significantly to 81% in post-alemtuzumab year 2, and remained high at 67%-72% in years 3-5.
“Perhaps for me what makes even more sense is looking at the yearly brain parenchymal fraction change,” Dr. Pelletier said, noting that the median annual reductions in years 1-5, respectively, were 0.02%, 0.04%, 0.15%, 0.14%, and 0.08%, compared with –0.33% for subcutaneous IFNB-1a at year 2 in CARE-MS II. The median brain parenchymal fraction change from baseline to post-alemtuzumab year 5 was –1.40%.
In 61% of patients, no further treatment was given after the second course of alemtuzumab.
The findings suggest that alemtuzumab provides durable efficacy without continuous treatment, he concluded.
This study was supported by Sanofi and Bayer Healthcare Pharmaceuticals. Dr. Pelletier has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Merck Serono, Novartis, Roche, and Sanofi, as well as research support from Biogen, Merck Serono, Novartis, Roche, and Sanofi.
SOURCE: Pelletier D et al. Neurology. 2018 Apr 10. 90(15 Suppl.):P5.031.
LOS ANGELES – Patients with multiple sclerosis in the CARE-MS II study who switched from interferon beta-1a therapy to the humanized monoclonal antibody alemtuzumab experienced continued reductions in brain volume loss and lesions on MRI through 5 years, according to follow-up data from the CARE-MS II extension study known as TOPAZ.
These outcomes support core findings from the CARE-MS II study, and suggest that alemtuzumab (Lemtrada) provides a unique treatment approach for patients with prior subcutaneous interferon beta-1a (IFNB-1a) treatment, Daniel Pelletier, MD, reported in a poster discussion session at the annual meeting of the American Academy of Neurology.
In CARE-MS II, relapsing-remitting multiple sclerosis patients with inadequate response to prior therapy experienced improvements in MRI lesions and brain volume loss with two courses of alemtuzumab versus IFNB-1a through 2 years, and in a 4-year extension in which participants discontinued subcutaneous IFNB-1a and initiated alemtuzumab at 12 mg/day, they experienced durable efficacy in the absence of continuous treatment, explained Dr. Pelletier, a professor of neurology at the University of Southern California, Los Angeles.
In the extension, patients could receive alemtuzumab retreatment as needed for relapse/MRI activity or receive other disease-modifying therapies at the investigator’s discretion. Patients completing the extension could enter the 5-year TOPAZ study for further evaluation, he said.
Of 119 patients who completed TOPAZ year 1, and thus had 5 years of follow-up after initiating alemtuzumab, 78% were free of new, gadolinium-enhancing lesions in IFNB-1a year 2; this increased significantly to 92% in post-alemtuzumab year 2, and remained high at 85%-89% in years 3-5. Additionally, 48% of patients were free of new/enlarging T2 lesions in IFNB-1a year 2; this increased significantly to 81% in post-alemtuzumab year 2 and remained high in years 3-5.
Further, 47% of the TOPAZ patients were MRI disease activity–free in IFNB-1a year 2; this increased significantly to 81% in post-alemtuzumab year 2, and remained high at 67%-72% in years 3-5.
“Perhaps for me what makes even more sense is looking at the yearly brain parenchymal fraction change,” Dr. Pelletier said, noting that the median annual reductions in years 1-5, respectively, were 0.02%, 0.04%, 0.15%, 0.14%, and 0.08%, compared with –0.33% for subcutaneous IFNB-1a at year 2 in CARE-MS II. The median brain parenchymal fraction change from baseline to post-alemtuzumab year 5 was –1.40%.
In 61% of patients, no further treatment was given after the second course of alemtuzumab.
The findings suggest that alemtuzumab provides durable efficacy without continuous treatment, he concluded.
This study was supported by Sanofi and Bayer Healthcare Pharmaceuticals. Dr. Pelletier has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Merck Serono, Novartis, Roche, and Sanofi, as well as research support from Biogen, Merck Serono, Novartis, Roche, and Sanofi.
SOURCE: Pelletier D et al. Neurology. 2018 Apr 10. 90(15 Suppl.):P5.031.
REPORTING FROM AAN 2018
Key clinical point: Alemtuzumab appears to provide durable efficacy without continuous treatment.
Major finding: Brain parenchymal fraction reductions with alemtuzumab in years 1-5, respectively, were 0.02%, 0.04%, 0.15%, 0.14%, and 0.08%.
Study details: A total of 119 patients who completed CARE-MS II and its 4-year extension, as well as 1 year of the TOPAZ study.
Disclosures: This study was supported by Sanofi and Bayer Healthcare Pharmaceuticals. Dr. Pelletier has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Merck Serono, Novartis, Roche, and Sanofi, as well as research support from Biogen, Merck Serono, Novartis, Roche, and Sanofi.
Source: Pelletier D et al. Neurology. 2018 Apr 10. 90(15 Suppl.):P5.031.
Pediatric MS gets a win with fingolimod
NASHVILLE, TENN. – Pediatric multiple sclerosis is a confirmed clinical entity, which virtually always presents as relapsing-remitting disease, Brenda Banwell, MD, said at the at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“MS in children is the same disease as MS in adults,” said Dr. Banwell, chief of neurology at Children’s Hospital of Philadelphia and the Grace R. Loeb Endowed Chair in Neurosciences there. And although she is unaware of a single case of childhood MS presenting as primary progressive disease, the impact of relapsing-remitting childhood MS may ultimately be progressive damage.
Pediatric MS is also quite rare, a characteristic that makes therapeutic progress challenging. Recruiting sufficient patients for a definitive phase 3 trial is incredibly difficult, especially when multiple trials are commencing simultaneously, not only in the United States but around the world.
Nevertheless, there has been excellent news, Dr. Banwell said in a video interview. Fingolimod (Gilenya), the immunomodulator approved for adult relapsing-remitting MS, gained a pediatric approval under the breakthrough therapy designation on May 11, 2018, on the basis of the successful phase 3 PARADIGMS study.
Compared with intramuscular interferon beta-1a, fingolimod cut the annualized relapse rate by 82%. It also was associated with a 53% annualized reduction in new or newly enlarged T2 lesions and 66% decrease in gadolinium-enhancing T1 lesions.
This big win is prompting researchers and clinicians to rethink the pediatric MS treatment paradigm, Dr. Banwell said. Traditional thinking falls along a dose-escalation pattern that follows relapses. However, “we may take a cue from our rheumatology colleagues, who have seen the benefit of starting with more aggressive treatment and higher doses, getting disease control, and then slowly tapering off.”
Whether this option could actually modify disease progression, as it seems to do in some other inflammatory disorders, is an intriguing – but unproven – possibility, she said.
Dr. Banwell disclosed that she received financial remuneration as a central MRI reviewer for the Novartis-sponsored PARADIGMS study.
NASHVILLE, TENN. – Pediatric multiple sclerosis is a confirmed clinical entity, which virtually always presents as relapsing-remitting disease, Brenda Banwell, MD, said at the at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“MS in children is the same disease as MS in adults,” said Dr. Banwell, chief of neurology at Children’s Hospital of Philadelphia and the Grace R. Loeb Endowed Chair in Neurosciences there. And although she is unaware of a single case of childhood MS presenting as primary progressive disease, the impact of relapsing-remitting childhood MS may ultimately be progressive damage.
Pediatric MS is also quite rare, a characteristic that makes therapeutic progress challenging. Recruiting sufficient patients for a definitive phase 3 trial is incredibly difficult, especially when multiple trials are commencing simultaneously, not only in the United States but around the world.
Nevertheless, there has been excellent news, Dr. Banwell said in a video interview. Fingolimod (Gilenya), the immunomodulator approved for adult relapsing-remitting MS, gained a pediatric approval under the breakthrough therapy designation on May 11, 2018, on the basis of the successful phase 3 PARADIGMS study.
Compared with intramuscular interferon beta-1a, fingolimod cut the annualized relapse rate by 82%. It also was associated with a 53% annualized reduction in new or newly enlarged T2 lesions and 66% decrease in gadolinium-enhancing T1 lesions.
This big win is prompting researchers and clinicians to rethink the pediatric MS treatment paradigm, Dr. Banwell said. Traditional thinking falls along a dose-escalation pattern that follows relapses. However, “we may take a cue from our rheumatology colleagues, who have seen the benefit of starting with more aggressive treatment and higher doses, getting disease control, and then slowly tapering off.”
Whether this option could actually modify disease progression, as it seems to do in some other inflammatory disorders, is an intriguing – but unproven – possibility, she said.
Dr. Banwell disclosed that she received financial remuneration as a central MRI reviewer for the Novartis-sponsored PARADIGMS study.
NASHVILLE, TENN. – Pediatric multiple sclerosis is a confirmed clinical entity, which virtually always presents as relapsing-remitting disease, Brenda Banwell, MD, said at the at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“MS in children is the same disease as MS in adults,” said Dr. Banwell, chief of neurology at Children’s Hospital of Philadelphia and the Grace R. Loeb Endowed Chair in Neurosciences there. And although she is unaware of a single case of childhood MS presenting as primary progressive disease, the impact of relapsing-remitting childhood MS may ultimately be progressive damage.
Pediatric MS is also quite rare, a characteristic that makes therapeutic progress challenging. Recruiting sufficient patients for a definitive phase 3 trial is incredibly difficult, especially when multiple trials are commencing simultaneously, not only in the United States but around the world.
Nevertheless, there has been excellent news, Dr. Banwell said in a video interview. Fingolimod (Gilenya), the immunomodulator approved for adult relapsing-remitting MS, gained a pediatric approval under the breakthrough therapy designation on May 11, 2018, on the basis of the successful phase 3 PARADIGMS study.
Compared with intramuscular interferon beta-1a, fingolimod cut the annualized relapse rate by 82%. It also was associated with a 53% annualized reduction in new or newly enlarged T2 lesions and 66% decrease in gadolinium-enhancing T1 lesions.
This big win is prompting researchers and clinicians to rethink the pediatric MS treatment paradigm, Dr. Banwell said. Traditional thinking falls along a dose-escalation pattern that follows relapses. However, “we may take a cue from our rheumatology colleagues, who have seen the benefit of starting with more aggressive treatment and higher doses, getting disease control, and then slowly tapering off.”
Whether this option could actually modify disease progression, as it seems to do in some other inflammatory disorders, is an intriguing – but unproven – possibility, she said.
Dr. Banwell disclosed that she received financial remuneration as a central MRI reviewer for the Novartis-sponsored PARADIGMS study.
REPORTING FROM THE CMSC ANNUAL MEETING
Inside the complex, surprising world of MS comorbidities
NASHVILLE, Tenn. – Recent research into comorbidities in multiple sclerosis – including head-scratching findings about lower cancer rates – is shedding light on the links between the disease and other illnesses, according to an epidemiologist specializing in MS.
“People should be mindful that if they look at having a positive impact on those comorbidities, they may have the ability to benefit patients in context of their MS,” Helen Tremlett, PhD, said in a video interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. She is the Canada Research Chair in Neuroepidemiology and Multiple Sclerosis at the University of British Columbia, Vancouver.
In recent years, research into comorbidities in MS has risen dramatically. Dr. Tremlett found that the number of papers per year in PubMed that address MS and comorbidity has risen from roughly 30 in 2007 to about 80 in 2015, although the numbers dipped to about 50 and 60, respectively, in 2016 and 2017.
A 2015 systematic review of research into MS and comorbidities reported that while “findings were inconsistent overall,” studies suggested that “meningiomas and possibly urinary system cancers, inflammatory bowel disease, irritable bowel syndrome, epilepsy, depression, anxiety, bipolar disorder, early cataracts, and restless legs syndrome were more common than expected in the MS population.” (Mult Scler. 2015 Mar;21[3]:263-81).
Notably, most cancers are missing from this list. In fact, Dr. Tremlett cowrote a 2012 study that found lower risks of all cancers and several specific types of cancer – breast, lung, colorectal, prostate, and melanoma – in MS patients, compared with age- and gender-matched controls (Brain. 2012 Oct;135[Pt 10]:2973-9).
According to Dr. Tremlett, there are several theories about the apparent lower cancer risk in patients with MS. Perhaps their immune systems are hypervigilant, or maybe MS diagnoses inspire healthier lifestyles.
Researchers have been intrigued by another possibility – that cancer diagnoses are being delayed in patients with MS. Indeed, the 2012 study found that tumor sizes at diagnosis in patients with MS were larger than expected in breast, prostate, lung, and colorectal cancer (P = .04).
“We couldn’t record why that’s the case, but there may be some so-called ‘diagnostic neglect,’ ” she said. “You could imagine a scenario where a typical person with MS goes to see their physician and says, ‘I’m tired. I have fatigue,’ and the physician says, ‘Yes, you have MS, that’s what you should expect.’ Someone in the general population might get additional investigation, get blood work done, and their cancer might be found earlier.”
It’s also possible, she said, that cancer isn’t picked up earlier because it can be difficult to screen people with disabilities. “It’s only recently that physicians can offer the Pap smear to women in a wheelchair.”
On another front, there’s evidence linking comorbidities to worsening MS. A 2018 study coauthored by Dr. Tremlett found that patients with more comorbidities had more disability. Specifically, ischemic heart disease and epilepsy were associated with greater Expanded Disability Status Scale scores (Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004885).
Other research coauthored by Dr. Tremlett has linked comorbidities in MS – specifically, hyperlipidemia, migraine, and three or more comorbidities – to higher risk of MS relapse (Neurology. 2017 Dec 12;89[24]:2455-61).
Dr. Tremlett reported having no relevant disclosures.
NASHVILLE, Tenn. – Recent research into comorbidities in multiple sclerosis – including head-scratching findings about lower cancer rates – is shedding light on the links between the disease and other illnesses, according to an epidemiologist specializing in MS.
“People should be mindful that if they look at having a positive impact on those comorbidities, they may have the ability to benefit patients in context of their MS,” Helen Tremlett, PhD, said in a video interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. She is the Canada Research Chair in Neuroepidemiology and Multiple Sclerosis at the University of British Columbia, Vancouver.
In recent years, research into comorbidities in MS has risen dramatically. Dr. Tremlett found that the number of papers per year in PubMed that address MS and comorbidity has risen from roughly 30 in 2007 to about 80 in 2015, although the numbers dipped to about 50 and 60, respectively, in 2016 and 2017.
A 2015 systematic review of research into MS and comorbidities reported that while “findings were inconsistent overall,” studies suggested that “meningiomas and possibly urinary system cancers, inflammatory bowel disease, irritable bowel syndrome, epilepsy, depression, anxiety, bipolar disorder, early cataracts, and restless legs syndrome were more common than expected in the MS population.” (Mult Scler. 2015 Mar;21[3]:263-81).
Notably, most cancers are missing from this list. In fact, Dr. Tremlett cowrote a 2012 study that found lower risks of all cancers and several specific types of cancer – breast, lung, colorectal, prostate, and melanoma – in MS patients, compared with age- and gender-matched controls (Brain. 2012 Oct;135[Pt 10]:2973-9).
According to Dr. Tremlett, there are several theories about the apparent lower cancer risk in patients with MS. Perhaps their immune systems are hypervigilant, or maybe MS diagnoses inspire healthier lifestyles.
Researchers have been intrigued by another possibility – that cancer diagnoses are being delayed in patients with MS. Indeed, the 2012 study found that tumor sizes at diagnosis in patients with MS were larger than expected in breast, prostate, lung, and colorectal cancer (P = .04).
“We couldn’t record why that’s the case, but there may be some so-called ‘diagnostic neglect,’ ” she said. “You could imagine a scenario where a typical person with MS goes to see their physician and says, ‘I’m tired. I have fatigue,’ and the physician says, ‘Yes, you have MS, that’s what you should expect.’ Someone in the general population might get additional investigation, get blood work done, and their cancer might be found earlier.”
It’s also possible, she said, that cancer isn’t picked up earlier because it can be difficult to screen people with disabilities. “It’s only recently that physicians can offer the Pap smear to women in a wheelchair.”
On another front, there’s evidence linking comorbidities to worsening MS. A 2018 study coauthored by Dr. Tremlett found that patients with more comorbidities had more disability. Specifically, ischemic heart disease and epilepsy were associated with greater Expanded Disability Status Scale scores (Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004885).
Other research coauthored by Dr. Tremlett has linked comorbidities in MS – specifically, hyperlipidemia, migraine, and three or more comorbidities – to higher risk of MS relapse (Neurology. 2017 Dec 12;89[24]:2455-61).
Dr. Tremlett reported having no relevant disclosures.
NASHVILLE, Tenn. – Recent research into comorbidities in multiple sclerosis – including head-scratching findings about lower cancer rates – is shedding light on the links between the disease and other illnesses, according to an epidemiologist specializing in MS.
“People should be mindful that if they look at having a positive impact on those comorbidities, they may have the ability to benefit patients in context of their MS,” Helen Tremlett, PhD, said in a video interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. She is the Canada Research Chair in Neuroepidemiology and Multiple Sclerosis at the University of British Columbia, Vancouver.
In recent years, research into comorbidities in MS has risen dramatically. Dr. Tremlett found that the number of papers per year in PubMed that address MS and comorbidity has risen from roughly 30 in 2007 to about 80 in 2015, although the numbers dipped to about 50 and 60, respectively, in 2016 and 2017.
A 2015 systematic review of research into MS and comorbidities reported that while “findings were inconsistent overall,” studies suggested that “meningiomas and possibly urinary system cancers, inflammatory bowel disease, irritable bowel syndrome, epilepsy, depression, anxiety, bipolar disorder, early cataracts, and restless legs syndrome were more common than expected in the MS population.” (Mult Scler. 2015 Mar;21[3]:263-81).
Notably, most cancers are missing from this list. In fact, Dr. Tremlett cowrote a 2012 study that found lower risks of all cancers and several specific types of cancer – breast, lung, colorectal, prostate, and melanoma – in MS patients, compared with age- and gender-matched controls (Brain. 2012 Oct;135[Pt 10]:2973-9).
According to Dr. Tremlett, there are several theories about the apparent lower cancer risk in patients with MS. Perhaps their immune systems are hypervigilant, or maybe MS diagnoses inspire healthier lifestyles.
Researchers have been intrigued by another possibility – that cancer diagnoses are being delayed in patients with MS. Indeed, the 2012 study found that tumor sizes at diagnosis in patients with MS were larger than expected in breast, prostate, lung, and colorectal cancer (P = .04).
“We couldn’t record why that’s the case, but there may be some so-called ‘diagnostic neglect,’ ” she said. “You could imagine a scenario where a typical person with MS goes to see their physician and says, ‘I’m tired. I have fatigue,’ and the physician says, ‘Yes, you have MS, that’s what you should expect.’ Someone in the general population might get additional investigation, get blood work done, and their cancer might be found earlier.”
It’s also possible, she said, that cancer isn’t picked up earlier because it can be difficult to screen people with disabilities. “It’s only recently that physicians can offer the Pap smear to women in a wheelchair.”
On another front, there’s evidence linking comorbidities to worsening MS. A 2018 study coauthored by Dr. Tremlett found that patients with more comorbidities had more disability. Specifically, ischemic heart disease and epilepsy were associated with greater Expanded Disability Status Scale scores (Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004885).
Other research coauthored by Dr. Tremlett has linked comorbidities in MS – specifically, hyperlipidemia, migraine, and three or more comorbidities – to higher risk of MS relapse (Neurology. 2017 Dec 12;89[24]:2455-61).
Dr. Tremlett reported having no relevant disclosures.
EXPERT ANALYSIS FROM THE CMSC ANNUAL MEETING









