User login
What is the best approach to goiter for euthyroid patients?
A detailed history and exam, confirmation of euthyroid status, and imaging when appropriate is the best approach to euthyroid patients with thyroid enlargement in regions where goiters are not endemic. Ultrasound imaging is recommended in any case of diagnostic uncertainty. Evaluate dominant or suspicious nodules further, while diffuse goiters without symptoms require no further evaluation and can be followed clinically (strength of recommendation [SOR]: C, expert opinion).
Suppressive therapy with levothyroxine can be used to decrease thyroid size for cosmetic reasons or in the case of mild local symptoms, although response is variable (SOR: B, based on small placebo-controlled trials). Patients with severe local symptoms should receive further evaluation and possible surgical management.
A patient with a euthyroid goiter; an opportunity to do no harm
John P. Langlois, MD
University of North Carolina School of Medicine, Chapel Hill
One of my patients is a 95-year-old woman who has spent most of the last 95 years taking very good care of herself. She has a small, slightly asymmetrical goiter that is probably only noticeable to her and to me (when she regularly calls my attention to it). One of the precepts of medicine is to “first do no harm,” and care of the patient with goiter is an excellent opportunity to practice this approach.
An initial ultrasound can give you good reassurance that the goiter is benign. Treatment of a goiter with thyroxine has questionable positive value and puts the patient at considerable risk for the problems of hyperthyroidism such as cardiac dysrhythmias and especially osteoporosis. So I keep tabs on her thyroid with a periodic thyroid-stimulating hormone (TSH) test and regular exams, and we spend a few moments at nearly every visit talking about how the best treatment can often be no treatment at all.
Evidence summary
Prevalence and types
In areas where iodine supplementation is routine, the prevalence of goiter is estimated to be 4% to 7%; however, autopsy studies show a 50% prevalence of nodules, most of which are multinodular goiter.1 Multinodular goiter is diagnosed in up to 5% of the general population and can be classified as euthyroid (“nontoxic”), hypothyroid, or hyperthyroid (“toxic”).1 Multinodular goiter is the most common diagnosis in cases of euthyroid goiter, but other conditions such as diffuse goiter (often idiopathic), thyroiditis, and neoplasms can also present in a euthyroid state.
Diagnosis: Exam and imaging
Studies show variable correlation between physical exam findings and findings on imaging studies. In a retrospective chart review, ultrasound findings differed from clinical exam findings in 63% of cases.2 Thyroid ultrasound is less expensive and less invasive than other imaging modalities, provides excellent visualization of thyroid structure and the nature of cysts and nodules, and allows for estimation of thyroid size.
Computed tomography and magnetic resonance imaging perform better for visualization of extension of thyroid tissue substernally and are also preferred for evaluation of the neck in cases of severe local complications of goiter, such as compressive symptoms.3
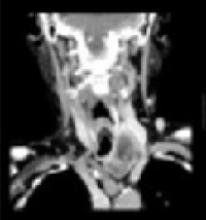
Abnormal left lobe of the thyroid gland
The malignant potential in multinodular goiter (2%–4%) is similar to that of solitary nodules (4%–6%).3 Therefore, any dominant nodule in a multinodular goiter should be evaluated the same way one would evaluate a solitary nodule.
Treatment and follow-up
Patients with a reassuring initial work-up can be followed clinically and should be assessed with serial clinical evaluations. No evidence could be found regarding optimal intervals for examination and testing; yearly exams and TSH testing are considered adequate by some experts.4
Because a few non-benign conditions such as thyroiditis and neoplasm can sometimes present in a euthyroid state, the clinician should be alert for any physical exam or laboratory changes. If any changes occur, then further workup is indicated.
Suppressive therapy with thyroxine is an option for decreasing thyroid size in euthyroid goiter, but this therapy remains controversial. One placebo-controlled trial of thyroid suppression in nontoxic multinodular goiter showed regression of thyroid size with suppressive therapy (58% reduction in size in treatment group vs 5% reduction in control group). However, not all goiters responded to this therapy, and the thyroid size returned to pretreatment size within 9 months of discontinuation of suppressive therapy.5
Many experts argue against the use of suppressive therapy in long-standing goiters, citing less response from these patients, along with concern about side effects and possible oversuppression, but the evidence in this area is limited. Patients who are treated with thyroxine should be followed for possible side effects of the medication, including arrhythmia and osteopenia, particularly in elderly patients and those who take the medication for long periods.
Recommendations of others
Guidelines from the American Association of Clinical Endocrinology’s Task Force on Thyroid Nodules, released in 2006, recommends ultrasound be used routinely in the case of multinodular goiter to assist with diagnosis, detect suspicious nodules that may require biopsy, and to serve as an objective baseline measure. This group recommends against use of suppression therapy in long-standing goiters.6
1. Day T, Chu A, Hoang K. Multinodular goiter. Otolaryngol Clin N Am 2003;36:35-54.
2. Marqusee E, Benson C, Frates M, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Int Med 2000;133:691-700.
3. Hurley D, Gharib H. Evaluation and management of multinodular goiter. Otolaryngol Clin N Am 1996;29:527-540.
4. Supit E, Peiris A. Cost-effective management of thyroid nodules and nodular thyroid goiters. Southern Med J 2002;95:514-519.
5. Berghout A, et al. Comparison of placebo with L-thyroxine alone or carbimazole for treatment of sporadic non-toxic goiter. Lancet 1990;336:193-197.
6. AACE/AME Task Force on Thyroid Nodules. AACE/AME medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract 2006;12:53-102.
A detailed history and exam, confirmation of euthyroid status, and imaging when appropriate is the best approach to euthyroid patients with thyroid enlargement in regions where goiters are not endemic. Ultrasound imaging is recommended in any case of diagnostic uncertainty. Evaluate dominant or suspicious nodules further, while diffuse goiters without symptoms require no further evaluation and can be followed clinically (strength of recommendation [SOR]: C, expert opinion).
Suppressive therapy with levothyroxine can be used to decrease thyroid size for cosmetic reasons or in the case of mild local symptoms, although response is variable (SOR: B, based on small placebo-controlled trials). Patients with severe local symptoms should receive further evaluation and possible surgical management.
A patient with a euthyroid goiter; an opportunity to do no harm
John P. Langlois, MD
University of North Carolina School of Medicine, Chapel Hill
One of my patients is a 95-year-old woman who has spent most of the last 95 years taking very good care of herself. She has a small, slightly asymmetrical goiter that is probably only noticeable to her and to me (when she regularly calls my attention to it). One of the precepts of medicine is to “first do no harm,” and care of the patient with goiter is an excellent opportunity to practice this approach.
An initial ultrasound can give you good reassurance that the goiter is benign. Treatment of a goiter with thyroxine has questionable positive value and puts the patient at considerable risk for the problems of hyperthyroidism such as cardiac dysrhythmias and especially osteoporosis. So I keep tabs on her thyroid with a periodic thyroid-stimulating hormone (TSH) test and regular exams, and we spend a few moments at nearly every visit talking about how the best treatment can often be no treatment at all.
Evidence summary
Prevalence and types
In areas where iodine supplementation is routine, the prevalence of goiter is estimated to be 4% to 7%; however, autopsy studies show a 50% prevalence of nodules, most of which are multinodular goiter.1 Multinodular goiter is diagnosed in up to 5% of the general population and can be classified as euthyroid (“nontoxic”), hypothyroid, or hyperthyroid (“toxic”).1 Multinodular goiter is the most common diagnosis in cases of euthyroid goiter, but other conditions such as diffuse goiter (often idiopathic), thyroiditis, and neoplasms can also present in a euthyroid state.
Diagnosis: Exam and imaging
Studies show variable correlation between physical exam findings and findings on imaging studies. In a retrospective chart review, ultrasound findings differed from clinical exam findings in 63% of cases.2 Thyroid ultrasound is less expensive and less invasive than other imaging modalities, provides excellent visualization of thyroid structure and the nature of cysts and nodules, and allows for estimation of thyroid size.
Computed tomography and magnetic resonance imaging perform better for visualization of extension of thyroid tissue substernally and are also preferred for evaluation of the neck in cases of severe local complications of goiter, such as compressive symptoms.3
Abnormal left lobe of the thyroid gland
The malignant potential in multinodular goiter (2%–4%) is similar to that of solitary nodules (4%–6%).3 Therefore, any dominant nodule in a multinodular goiter should be evaluated the same way one would evaluate a solitary nodule.
Treatment and follow-up
Patients with a reassuring initial work-up can be followed clinically and should be assessed with serial clinical evaluations. No evidence could be found regarding optimal intervals for examination and testing; yearly exams and TSH testing are considered adequate by some experts.4
Because a few non-benign conditions such as thyroiditis and neoplasm can sometimes present in a euthyroid state, the clinician should be alert for any physical exam or laboratory changes. If any changes occur, then further workup is indicated.
Suppressive therapy with thyroxine is an option for decreasing thyroid size in euthyroid goiter, but this therapy remains controversial. One placebo-controlled trial of thyroid suppression in nontoxic multinodular goiter showed regression of thyroid size with suppressive therapy (58% reduction in size in treatment group vs 5% reduction in control group). However, not all goiters responded to this therapy, and the thyroid size returned to pretreatment size within 9 months of discontinuation of suppressive therapy.5
Many experts argue against the use of suppressive therapy in long-standing goiters, citing less response from these patients, along with concern about side effects and possible oversuppression, but the evidence in this area is limited. Patients who are treated with thyroxine should be followed for possible side effects of the medication, including arrhythmia and osteopenia, particularly in elderly patients and those who take the medication for long periods.
Recommendations of others
Guidelines from the American Association of Clinical Endocrinology’s Task Force on Thyroid Nodules, released in 2006, recommends ultrasound be used routinely in the case of multinodular goiter to assist with diagnosis, detect suspicious nodules that may require biopsy, and to serve as an objective baseline measure. This group recommends against use of suppression therapy in long-standing goiters.6
A detailed history and exam, confirmation of euthyroid status, and imaging when appropriate is the best approach to euthyroid patients with thyroid enlargement in regions where goiters are not endemic. Ultrasound imaging is recommended in any case of diagnostic uncertainty. Evaluate dominant or suspicious nodules further, while diffuse goiters without symptoms require no further evaluation and can be followed clinically (strength of recommendation [SOR]: C, expert opinion).
Suppressive therapy with levothyroxine can be used to decrease thyroid size for cosmetic reasons or in the case of mild local symptoms, although response is variable (SOR: B, based on small placebo-controlled trials). Patients with severe local symptoms should receive further evaluation and possible surgical management.
A patient with a euthyroid goiter; an opportunity to do no harm
John P. Langlois, MD
University of North Carolina School of Medicine, Chapel Hill
One of my patients is a 95-year-old woman who has spent most of the last 95 years taking very good care of herself. She has a small, slightly asymmetrical goiter that is probably only noticeable to her and to me (when she regularly calls my attention to it). One of the precepts of medicine is to “first do no harm,” and care of the patient with goiter is an excellent opportunity to practice this approach.
An initial ultrasound can give you good reassurance that the goiter is benign. Treatment of a goiter with thyroxine has questionable positive value and puts the patient at considerable risk for the problems of hyperthyroidism such as cardiac dysrhythmias and especially osteoporosis. So I keep tabs on her thyroid with a periodic thyroid-stimulating hormone (TSH) test and regular exams, and we spend a few moments at nearly every visit talking about how the best treatment can often be no treatment at all.
Evidence summary
Prevalence and types
In areas where iodine supplementation is routine, the prevalence of goiter is estimated to be 4% to 7%; however, autopsy studies show a 50% prevalence of nodules, most of which are multinodular goiter.1 Multinodular goiter is diagnosed in up to 5% of the general population and can be classified as euthyroid (“nontoxic”), hypothyroid, or hyperthyroid (“toxic”).1 Multinodular goiter is the most common diagnosis in cases of euthyroid goiter, but other conditions such as diffuse goiter (often idiopathic), thyroiditis, and neoplasms can also present in a euthyroid state.
Diagnosis: Exam and imaging
Studies show variable correlation between physical exam findings and findings on imaging studies. In a retrospective chart review, ultrasound findings differed from clinical exam findings in 63% of cases.2 Thyroid ultrasound is less expensive and less invasive than other imaging modalities, provides excellent visualization of thyroid structure and the nature of cysts and nodules, and allows for estimation of thyroid size.
Computed tomography and magnetic resonance imaging perform better for visualization of extension of thyroid tissue substernally and are also preferred for evaluation of the neck in cases of severe local complications of goiter, such as compressive symptoms.3
Abnormal left lobe of the thyroid gland
The malignant potential in multinodular goiter (2%–4%) is similar to that of solitary nodules (4%–6%).3 Therefore, any dominant nodule in a multinodular goiter should be evaluated the same way one would evaluate a solitary nodule.
Treatment and follow-up
Patients with a reassuring initial work-up can be followed clinically and should be assessed with serial clinical evaluations. No evidence could be found regarding optimal intervals for examination and testing; yearly exams and TSH testing are considered adequate by some experts.4
Because a few non-benign conditions such as thyroiditis and neoplasm can sometimes present in a euthyroid state, the clinician should be alert for any physical exam or laboratory changes. If any changes occur, then further workup is indicated.
Suppressive therapy with thyroxine is an option for decreasing thyroid size in euthyroid goiter, but this therapy remains controversial. One placebo-controlled trial of thyroid suppression in nontoxic multinodular goiter showed regression of thyroid size with suppressive therapy (58% reduction in size in treatment group vs 5% reduction in control group). However, not all goiters responded to this therapy, and the thyroid size returned to pretreatment size within 9 months of discontinuation of suppressive therapy.5
Many experts argue against the use of suppressive therapy in long-standing goiters, citing less response from these patients, along with concern about side effects and possible oversuppression, but the evidence in this area is limited. Patients who are treated with thyroxine should be followed for possible side effects of the medication, including arrhythmia and osteopenia, particularly in elderly patients and those who take the medication for long periods.
Recommendations of others
Guidelines from the American Association of Clinical Endocrinology’s Task Force on Thyroid Nodules, released in 2006, recommends ultrasound be used routinely in the case of multinodular goiter to assist with diagnosis, detect suspicious nodules that may require biopsy, and to serve as an objective baseline measure. This group recommends against use of suppression therapy in long-standing goiters.6
1. Day T, Chu A, Hoang K. Multinodular goiter. Otolaryngol Clin N Am 2003;36:35-54.
2. Marqusee E, Benson C, Frates M, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Int Med 2000;133:691-700.
3. Hurley D, Gharib H. Evaluation and management of multinodular goiter. Otolaryngol Clin N Am 1996;29:527-540.
4. Supit E, Peiris A. Cost-effective management of thyroid nodules and nodular thyroid goiters. Southern Med J 2002;95:514-519.
5. Berghout A, et al. Comparison of placebo with L-thyroxine alone or carbimazole for treatment of sporadic non-toxic goiter. Lancet 1990;336:193-197.
6. AACE/AME Task Force on Thyroid Nodules. AACE/AME medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract 2006;12:53-102.
1. Day T, Chu A, Hoang K. Multinodular goiter. Otolaryngol Clin N Am 2003;36:35-54.
2. Marqusee E, Benson C, Frates M, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Int Med 2000;133:691-700.
3. Hurley D, Gharib H. Evaluation and management of multinodular goiter. Otolaryngol Clin N Am 1996;29:527-540.
4. Supit E, Peiris A. Cost-effective management of thyroid nodules and nodular thyroid goiters. Southern Med J 2002;95:514-519.
5. Berghout A, et al. Comparison of placebo with L-thyroxine alone or carbimazole for treatment of sporadic non-toxic goiter. Lancet 1990;336:193-197.
6. AACE/AME Task Force on Thyroid Nodules. AACE/AME medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract 2006;12:53-102.
Evidence-based answers from the Family Physicians Inquiries Network
History, exam, and labs: Is one enough to diagnose acute adult appendicitis?
No, none of the 3—history, exam, or labs— is sufficiently accurate to diagnose acute appendicitis (strength of recommendation [SOR]: A, based on meta-analysis of high-quality studies). When combined, the following tests are helpful: an elevated C-reactive protein (CRP), elevated total white blood cell (WBC) count, elevated percentage of polymorphonuclear leukocyte (PMN) cells (left shift), and the presence of guarding or rebound on physical examination. The combination of any 2 of these tests yields a very high positive likelihood ratio (LR+), but the absence of these does not exclude appendicitis (SOR: A, based on meta-analysis of high-quality studies).
2 inexpensive tests can lower costs in low-probability presentations
Fereshteh Gerayli, MD
East Tennessee State University, Johnson City
Unlike physicians in other parts of the world, us physicians rely heavily on imaging studies to diagnose acute appendicitis. This has decreased the rate of negative appendectomies by 15% to 20%. However, the liberal and indiscriminate use of imaging studies increases medical costs while diminishing physicians’ clinical diagnostic skills.
The systematic review our authors cited demonstrated a high likelihood ratio for the presence of appendicitis by combining 2 inexpensive tests. Adding a thorough history and physical exam and a clinical scoring system can further enhance our clinical diagnosis. Considering the cost and the wide range of diagnostic accuracy of imaging studies (which depend on the experience of the reader), it is reasonable to skip CT scan in low probability presentations.
Evidence summary
Radiographic imaging to rule out appendicitis has become more commonplace, but it comes with an increased financial cost and additional delay in surgical intervention. Knowing the accuracy of common diagnostic tests may reduce the need for confirmatory imaging studies that increase both cost and time to surgery.
High levels of 2 or more inflammatory values are helpful
A meta-analysis of patients hospitalized for suspected acute appendicitis analyzed 28 different diagnostic variables in 24 studies.1 Variables included WBC, granulocyte count, PMN proportion, CRP level, and body temperature; histopathology was the gold standard. In no circumstance did an isolated elevation of any 1 factor result in a significant LR+. In addition, the absence of any 1 variable failed to yield a LR– <0.01 (low enough to exclude appendicitis).
Clinicians inherently combine multiple variables when evaluating patients, and when evaluating patients with abdominal pain, this technique can result in identification of adequate likelihood ratios (TABLE).1 In general, when 2 or more of the aforementioned inflammatory variables are present, the diagnosis of acute appendicitis is likely. When all markers of inflammation are normal, though acute appendicitis is less likely, the power is insufficient to exclude it as a possible diagnosis.
The value of CRP in the evaluation of suspected appendicitis was confirmed in a retrospective evaluation of 566 patients who underwent appendectomies.2 The sensitivity and specificity of the test improved depending on the duration of symptoms for both appendicitis and ruptured appendicitis. For appendicitis, CRP levels >1.4, 4.0, and 10.5 on Days 1, 2, and 3 had sensitivities/specificities of 0.38/0.81, 0.63/0.78, and 0.72/0.83, respectively. For ruptured appendicitis, levels of 3.3, 8.5, and 12.0 on Days 1, 2, and 3 had sensitivities/specificities of 0.77/0.89, 0.70/0.95, and 0.90/0.96, respectively.
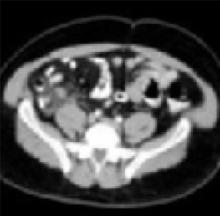
Enlarged appendix with inflammatory changes to mesenteric fatIn a series of 439 patients with symptoms suggestive of acute appendicitis, those with confirmed appendicitis (n=101) had a mean WBC count of 14.8 K/μL (95% CI, 13.9–15.8) and a mean neutrophil percentage of 82 (95% CI, 80–84).1 In contrast, those without appendicitis (n=338) had a mean WBC count of 9.2 K/μL (95% CI, 9.0–9.4) and a mean neutrophil percentage of 68 (95% CI, 66–70).
TABLE
How much do the inflammatory markers tell us? A look at likelihood ratios for appendicitis
| COMBINATION OF TESTS | LIKELIHOOD RATIOS | |
|---|---|---|
| POSITIVE (>10=STRONG EVIDENCE FOR DIAGNOSIS) | NEGATIVE (<0.1=EVIDENCE AGAINST DIAGNOSIS) | |
| WBC >10.0 × 109/L CRP >8 mg/L | 23.32 (95% CI, 6.87–84.79) | 0.03 (95% CI, 0.00–0.14) |
| WBC >10.0 × 109/L PMN cells >70% CRP >12 mg/L | 20.85 (95% CI, 5.47–80.27) | 0.03 (95% CI, 0.01–0.16) |
| Guarding/rebound tenderness WBC >10.0×109 | 11.34 (95% CI, 6.65–19.56) | 0.14 (95% CI, 0.08–0.24) |
| WBC, white blood cell count; CRP, C-reactive protein; PMN, polymorphonuclear leukocyte; CI, confidence interval. | ||
| Source: Andersson, Br J Surg 2004.1 | ||
Recommendations from others
A review of medical and professional associations revealed no official guidelines regarding the evaluation of suspected acute appendicitis. Surgical textbooks confirm that the diagnosis of acute appendicitis is made primarily by history and examination, with help from laboratory and radiographic studies.3
1. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg 2004;91:28-37.
2. Birkhahn R, Briggs M, Datillo PA, Van Deusen SK, Gaeta TJ. Classifying patient suspected of appendicitis with regard to likelihood. Am J Surgery 2006;191:497-502.
3. Townsend CM, Sabiston DC. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders, 2004:1381–1395.
No, none of the 3—history, exam, or labs— is sufficiently accurate to diagnose acute appendicitis (strength of recommendation [SOR]: A, based on meta-analysis of high-quality studies). When combined, the following tests are helpful: an elevated C-reactive protein (CRP), elevated total white blood cell (WBC) count, elevated percentage of polymorphonuclear leukocyte (PMN) cells (left shift), and the presence of guarding or rebound on physical examination. The combination of any 2 of these tests yields a very high positive likelihood ratio (LR+), but the absence of these does not exclude appendicitis (SOR: A, based on meta-analysis of high-quality studies).
2 inexpensive tests can lower costs in low-probability presentations
Fereshteh Gerayli, MD
East Tennessee State University, Johnson City
Unlike physicians in other parts of the world, us physicians rely heavily on imaging studies to diagnose acute appendicitis. This has decreased the rate of negative appendectomies by 15% to 20%. However, the liberal and indiscriminate use of imaging studies increases medical costs while diminishing physicians’ clinical diagnostic skills.
The systematic review our authors cited demonstrated a high likelihood ratio for the presence of appendicitis by combining 2 inexpensive tests. Adding a thorough history and physical exam and a clinical scoring system can further enhance our clinical diagnosis. Considering the cost and the wide range of diagnostic accuracy of imaging studies (which depend on the experience of the reader), it is reasonable to skip CT scan in low probability presentations.
Evidence summary
Radiographic imaging to rule out appendicitis has become more commonplace, but it comes with an increased financial cost and additional delay in surgical intervention. Knowing the accuracy of common diagnostic tests may reduce the need for confirmatory imaging studies that increase both cost and time to surgery.
High levels of 2 or more inflammatory values are helpful
A meta-analysis of patients hospitalized for suspected acute appendicitis analyzed 28 different diagnostic variables in 24 studies.1 Variables included WBC, granulocyte count, PMN proportion, CRP level, and body temperature; histopathology was the gold standard. In no circumstance did an isolated elevation of any 1 factor result in a significant LR+. In addition, the absence of any 1 variable failed to yield a LR– <0.01 (low enough to exclude appendicitis).
Clinicians inherently combine multiple variables when evaluating patients, and when evaluating patients with abdominal pain, this technique can result in identification of adequate likelihood ratios (TABLE).1 In general, when 2 or more of the aforementioned inflammatory variables are present, the diagnosis of acute appendicitis is likely. When all markers of inflammation are normal, though acute appendicitis is less likely, the power is insufficient to exclude it as a possible diagnosis.
The value of CRP in the evaluation of suspected appendicitis was confirmed in a retrospective evaluation of 566 patients who underwent appendectomies.2 The sensitivity and specificity of the test improved depending on the duration of symptoms for both appendicitis and ruptured appendicitis. For appendicitis, CRP levels >1.4, 4.0, and 10.5 on Days 1, 2, and 3 had sensitivities/specificities of 0.38/0.81, 0.63/0.78, and 0.72/0.83, respectively. For ruptured appendicitis, levels of 3.3, 8.5, and 12.0 on Days 1, 2, and 3 had sensitivities/specificities of 0.77/0.89, 0.70/0.95, and 0.90/0.96, respectively.
Enlarged appendix with inflammatory changes to mesenteric fatIn a series of 439 patients with symptoms suggestive of acute appendicitis, those with confirmed appendicitis (n=101) had a mean WBC count of 14.8 K/μL (95% CI, 13.9–15.8) and a mean neutrophil percentage of 82 (95% CI, 80–84).1 In contrast, those without appendicitis (n=338) had a mean WBC count of 9.2 K/μL (95% CI, 9.0–9.4) and a mean neutrophil percentage of 68 (95% CI, 66–70).
TABLE
How much do the inflammatory markers tell us? A look at likelihood ratios for appendicitis
| COMBINATION OF TESTS | LIKELIHOOD RATIOS | |
|---|---|---|
| POSITIVE (>10=STRONG EVIDENCE FOR DIAGNOSIS) | NEGATIVE (<0.1=EVIDENCE AGAINST DIAGNOSIS) | |
| WBC >10.0 × 109/L CRP >8 mg/L | 23.32 (95% CI, 6.87–84.79) | 0.03 (95% CI, 0.00–0.14) |
| WBC >10.0 × 109/L PMN cells >70% CRP >12 mg/L | 20.85 (95% CI, 5.47–80.27) | 0.03 (95% CI, 0.01–0.16) |
| Guarding/rebound tenderness WBC >10.0×109 | 11.34 (95% CI, 6.65–19.56) | 0.14 (95% CI, 0.08–0.24) |
| WBC, white blood cell count; CRP, C-reactive protein; PMN, polymorphonuclear leukocyte; CI, confidence interval. | ||
| Source: Andersson, Br J Surg 2004.1 | ||
Recommendations from others
A review of medical and professional associations revealed no official guidelines regarding the evaluation of suspected acute appendicitis. Surgical textbooks confirm that the diagnosis of acute appendicitis is made primarily by history and examination, with help from laboratory and radiographic studies.3
No, none of the 3—history, exam, or labs— is sufficiently accurate to diagnose acute appendicitis (strength of recommendation [SOR]: A, based on meta-analysis of high-quality studies). When combined, the following tests are helpful: an elevated C-reactive protein (CRP), elevated total white blood cell (WBC) count, elevated percentage of polymorphonuclear leukocyte (PMN) cells (left shift), and the presence of guarding or rebound on physical examination. The combination of any 2 of these tests yields a very high positive likelihood ratio (LR+), but the absence of these does not exclude appendicitis (SOR: A, based on meta-analysis of high-quality studies).
2 inexpensive tests can lower costs in low-probability presentations
Fereshteh Gerayli, MD
East Tennessee State University, Johnson City
Unlike physicians in other parts of the world, us physicians rely heavily on imaging studies to diagnose acute appendicitis. This has decreased the rate of negative appendectomies by 15% to 20%. However, the liberal and indiscriminate use of imaging studies increases medical costs while diminishing physicians’ clinical diagnostic skills.
The systematic review our authors cited demonstrated a high likelihood ratio for the presence of appendicitis by combining 2 inexpensive tests. Adding a thorough history and physical exam and a clinical scoring system can further enhance our clinical diagnosis. Considering the cost and the wide range of diagnostic accuracy of imaging studies (which depend on the experience of the reader), it is reasonable to skip CT scan in low probability presentations.
Evidence summary
Radiographic imaging to rule out appendicitis has become more commonplace, but it comes with an increased financial cost and additional delay in surgical intervention. Knowing the accuracy of common diagnostic tests may reduce the need for confirmatory imaging studies that increase both cost and time to surgery.
High levels of 2 or more inflammatory values are helpful
A meta-analysis of patients hospitalized for suspected acute appendicitis analyzed 28 different diagnostic variables in 24 studies.1 Variables included WBC, granulocyte count, PMN proportion, CRP level, and body temperature; histopathology was the gold standard. In no circumstance did an isolated elevation of any 1 factor result in a significant LR+. In addition, the absence of any 1 variable failed to yield a LR– <0.01 (low enough to exclude appendicitis).
Clinicians inherently combine multiple variables when evaluating patients, and when evaluating patients with abdominal pain, this technique can result in identification of adequate likelihood ratios (TABLE).1 In general, when 2 or more of the aforementioned inflammatory variables are present, the diagnosis of acute appendicitis is likely. When all markers of inflammation are normal, though acute appendicitis is less likely, the power is insufficient to exclude it as a possible diagnosis.
The value of CRP in the evaluation of suspected appendicitis was confirmed in a retrospective evaluation of 566 patients who underwent appendectomies.2 The sensitivity and specificity of the test improved depending on the duration of symptoms for both appendicitis and ruptured appendicitis. For appendicitis, CRP levels >1.4, 4.0, and 10.5 on Days 1, 2, and 3 had sensitivities/specificities of 0.38/0.81, 0.63/0.78, and 0.72/0.83, respectively. For ruptured appendicitis, levels of 3.3, 8.5, and 12.0 on Days 1, 2, and 3 had sensitivities/specificities of 0.77/0.89, 0.70/0.95, and 0.90/0.96, respectively.
Enlarged appendix with inflammatory changes to mesenteric fatIn a series of 439 patients with symptoms suggestive of acute appendicitis, those with confirmed appendicitis (n=101) had a mean WBC count of 14.8 K/μL (95% CI, 13.9–15.8) and a mean neutrophil percentage of 82 (95% CI, 80–84).1 In contrast, those without appendicitis (n=338) had a mean WBC count of 9.2 K/μL (95% CI, 9.0–9.4) and a mean neutrophil percentage of 68 (95% CI, 66–70).
TABLE
How much do the inflammatory markers tell us? A look at likelihood ratios for appendicitis
| COMBINATION OF TESTS | LIKELIHOOD RATIOS | |
|---|---|---|
| POSITIVE (>10=STRONG EVIDENCE FOR DIAGNOSIS) | NEGATIVE (<0.1=EVIDENCE AGAINST DIAGNOSIS) | |
| WBC >10.0 × 109/L CRP >8 mg/L | 23.32 (95% CI, 6.87–84.79) | 0.03 (95% CI, 0.00–0.14) |
| WBC >10.0 × 109/L PMN cells >70% CRP >12 mg/L | 20.85 (95% CI, 5.47–80.27) | 0.03 (95% CI, 0.01–0.16) |
| Guarding/rebound tenderness WBC >10.0×109 | 11.34 (95% CI, 6.65–19.56) | 0.14 (95% CI, 0.08–0.24) |
| WBC, white blood cell count; CRP, C-reactive protein; PMN, polymorphonuclear leukocyte; CI, confidence interval. | ||
| Source: Andersson, Br J Surg 2004.1 | ||
Recommendations from others
A review of medical and professional associations revealed no official guidelines regarding the evaluation of suspected acute appendicitis. Surgical textbooks confirm that the diagnosis of acute appendicitis is made primarily by history and examination, with help from laboratory and radiographic studies.3
1. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg 2004;91:28-37.
2. Birkhahn R, Briggs M, Datillo PA, Van Deusen SK, Gaeta TJ. Classifying patient suspected of appendicitis with regard to likelihood. Am J Surgery 2006;191:497-502.
3. Townsend CM, Sabiston DC. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders, 2004:1381–1395.
1. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg 2004;91:28-37.
2. Birkhahn R, Briggs M, Datillo PA, Van Deusen SK, Gaeta TJ. Classifying patient suspected of appendicitis with regard to likelihood. Am J Surgery 2006;191:497-502.
3. Townsend CM, Sabiston DC. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders, 2004:1381–1395.
Evidence-based answers from the Family Physicians Inquiries Network
What hormonal contraception is most effective for obese women?
Depot medroxyprogesterone acetate (DMPA; Depo-Provera) and the combination contraceptive vaginal ring (NuvaRing) are most effective for obese women because they don’t appear to be affected by body weight (strength of recommendation [SOR]: B, consistent cohort studies).
On the other hand, women using the combination contraceptive patch (Ortho Evra) who weigh ≥90 kg may experience decreased contraceptive efficacy (SOR: A, meta-analysis). Obese women using oral contraceptives may also have an increased risk of pregnancy (SOR: B, inconsistent cohort studies). Data are not available on the levonorgestrel intrauterine system’s (Mirena) efficacy in obese women.
Obese women may have higher rates of pregnancy with OCs
Ronald Januchowski, DO
Spartanburg Regional Family Medicine Program, Spartanburg, SC
This answer shows that we need to provide more guidance to obese patients during contraceptive counseling. In our practice, we may have to develop contraceptive information sheets for overweight women.
I don’t think this will prevent me from prescribing oral contraceptives for obese women, but it will cause me to pause a bit. This question makes me wonder whether official recommendations in other drug classes for obese patients are coming in the near future.
Evidence summary
There is a theoretical risk of decreased hormonal contraceptive efficacy for obese women (defined as those having a body-mass index [BMI] ≥30 kg/m2) due to increased metabolism of the hormones resulting in lower serum levels. With the growing epidemic of obesity, concern over the efficacy of hormonal contraception has grown. At this time, however, only a few published studies evaluating contraception have specifically examined the effect of body weight on efficacy.
Pregnancy risk doubled among heavier patients on OCs
Some studies have shown a possible association between obesity and higher rates of pregnancy among women using oral contraceptives for birth control.
One retrospective cohort analysis found that women weighing >70.5 kg had an increased risk of pregnancy compared with women of lower weight (relative risk [RR]=1.6; 95% confidence interval [CI], 1.1–2.4), after controlling for parity.1 Pill compliance was not accounted for in this study. A follow-up case-control study demonstrated that the risk of pregnancy for consistent pill users doubled for women with a BMI >27.3 (odds ratio [OR]=2.17; 95% CI, 1.38–3.41); results were similar for those with a BMI >32.2 (OR=2.2; 95% CI, 1.18–4.20).2
Another large cohort study did not find any association between failure of the oral contraceptive pill or progestin-only pill and obesity; however, the total number of pregnancies among obese women was too small to achieve statistical significance.3 In a randomized trial studying the efficacy of an extended-cycle oral contraceptive (Seasonale), no woman weighing >90 kg became pregnant.4
When it comes to the combination contraceptive patch, the data show a significant association between baseline body weight and pregnancy. In an analysis of pooled data, 5 of 15 pregnancies occurred in a subgroup of women with a baseline body weight ≥90 kg. Less than 3% of the study population weighed more than 90 kg. Specific data for this subgroup were not presented in the study results, so measures of effect cannot be calculated. The mechanism of the decreased efficacy of the combined contraceptive patch for obese women is unclear.5
DMPA and vaginal ring may be a better option for obese women
Data suggest that increased body weight does not decrease the efficacy of DMPA. In 2 large open-label studies, no pregnancies were observed, regardless of BMI.6 Similarly, the efficacy of the contraceptive vaginal ring does not appear to be affected by body weight, but the mean BMI in intent-to-treat population studies was only 22.9±2.9.7
A secondary analysis of the contraceptive vaginal ring efficacy trials did not show an increased pregnancy rate among heavier women.8 Of note: A higher body weight appeared to be associated with increased likelihood of ovulation using the contraceptive vaginal ring, though it did not lead to any pregnancies in a multicenter study.9
The data on the levonorgestrel intrauterine system do not examine weight and efficacy.10
Recommendations from others
The World Health Organization generally recommends hormonal contraceptives as safe for obese women. The group acknowledges that data are limited regarding effectiveness of oral contraceptives, and efficacy may be lower for the combination contraceptive patch when used by obese women.11
The American College of Obstetrics and Gynecology (ACOG) suggests that despite the possibility of higher failure rates with oral and transdermal contraception, motivated obese women should still be encouraged to use these methods preferentially over known less effective methods.12 In addition, ACOG also notes that no higher rates of pregnancy are observed among overweight women using DMPA.
1. Holt VL, Cushing-Haugen KL, Daling JR. Body weight and the risk of oral contraceptive failure. Obstet Gynecol 2002;99:820-827.
2. Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Daling JR. Body Mass Index, weight, and oral contraceptive failure risk. Obstet Gynecol 2005;105:46-52.
3. Vessey M, Painter R. Oral contraceptive failures and body weight: Findings in a large cohort study. J Fam Plan Reprod Health Care 2001;27:90-91.
4. Anderson FD, Hait H. A multi-center, randomized study of an extended cycle oral contraceptive. Contraception 2003;68:89-106.
5. Zieman M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril 2002;77 (Suppl 2):S13-S18.
6. Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004;70:269-275.
7. Dieben TO, Roumen JM, Apter D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol 2002;100:585-593.
8. Westhoff C. Higher body weight does not affect NuvaRing’s efficacy [abstract]. Obstet Gynecol 2005;105(Suppl 4):56S.-
9. Weisberg E, Fraser I, Lacarra M, et al. Efficacy, bleeding patterns, and side effects of a 1-year contraceptive vaginal ring. Contraception 1999;59:311-318
10. Luukkainen T, Allonen H, Haukkamaa M, et al. Effective contraception with the levonorgestrel-releasing device: 12 month report of a multi-center study. Contraception 1987;36:169-179.
11. World Health Organization. Improving Access to Quality Care in Family Planning Eligibility Criteria for Contraceptive Use. Geneva: WHO; 2004.
12. ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006;107:1453-1472.
Depot medroxyprogesterone acetate (DMPA; Depo-Provera) and the combination contraceptive vaginal ring (NuvaRing) are most effective for obese women because they don’t appear to be affected by body weight (strength of recommendation [SOR]: B, consistent cohort studies).
On the other hand, women using the combination contraceptive patch (Ortho Evra) who weigh ≥90 kg may experience decreased contraceptive efficacy (SOR: A, meta-analysis). Obese women using oral contraceptives may also have an increased risk of pregnancy (SOR: B, inconsistent cohort studies). Data are not available on the levonorgestrel intrauterine system’s (Mirena) efficacy in obese women.
Obese women may have higher rates of pregnancy with OCs
Ronald Januchowski, DO
Spartanburg Regional Family Medicine Program, Spartanburg, SC
This answer shows that we need to provide more guidance to obese patients during contraceptive counseling. In our practice, we may have to develop contraceptive information sheets for overweight women.
I don’t think this will prevent me from prescribing oral contraceptives for obese women, but it will cause me to pause a bit. This question makes me wonder whether official recommendations in other drug classes for obese patients are coming in the near future.
Evidence summary
There is a theoretical risk of decreased hormonal contraceptive efficacy for obese women (defined as those having a body-mass index [BMI] ≥30 kg/m2) due to increased metabolism of the hormones resulting in lower serum levels. With the growing epidemic of obesity, concern over the efficacy of hormonal contraception has grown. At this time, however, only a few published studies evaluating contraception have specifically examined the effect of body weight on efficacy.
Pregnancy risk doubled among heavier patients on OCs
Some studies have shown a possible association between obesity and higher rates of pregnancy among women using oral contraceptives for birth control.
One retrospective cohort analysis found that women weighing >70.5 kg had an increased risk of pregnancy compared with women of lower weight (relative risk [RR]=1.6; 95% confidence interval [CI], 1.1–2.4), after controlling for parity.1 Pill compliance was not accounted for in this study. A follow-up case-control study demonstrated that the risk of pregnancy for consistent pill users doubled for women with a BMI >27.3 (odds ratio [OR]=2.17; 95% CI, 1.38–3.41); results were similar for those with a BMI >32.2 (OR=2.2; 95% CI, 1.18–4.20).2
Another large cohort study did not find any association between failure of the oral contraceptive pill or progestin-only pill and obesity; however, the total number of pregnancies among obese women was too small to achieve statistical significance.3 In a randomized trial studying the efficacy of an extended-cycle oral contraceptive (Seasonale), no woman weighing >90 kg became pregnant.4
When it comes to the combination contraceptive patch, the data show a significant association between baseline body weight and pregnancy. In an analysis of pooled data, 5 of 15 pregnancies occurred in a subgroup of women with a baseline body weight ≥90 kg. Less than 3% of the study population weighed more than 90 kg. Specific data for this subgroup were not presented in the study results, so measures of effect cannot be calculated. The mechanism of the decreased efficacy of the combined contraceptive patch for obese women is unclear.5
DMPA and vaginal ring may be a better option for obese women
Data suggest that increased body weight does not decrease the efficacy of DMPA. In 2 large open-label studies, no pregnancies were observed, regardless of BMI.6 Similarly, the efficacy of the contraceptive vaginal ring does not appear to be affected by body weight, but the mean BMI in intent-to-treat population studies was only 22.9±2.9.7
A secondary analysis of the contraceptive vaginal ring efficacy trials did not show an increased pregnancy rate among heavier women.8 Of note: A higher body weight appeared to be associated with increased likelihood of ovulation using the contraceptive vaginal ring, though it did not lead to any pregnancies in a multicenter study.9
The data on the levonorgestrel intrauterine system do not examine weight and efficacy.10
Recommendations from others
The World Health Organization generally recommends hormonal contraceptives as safe for obese women. The group acknowledges that data are limited regarding effectiveness of oral contraceptives, and efficacy may be lower for the combination contraceptive patch when used by obese women.11
The American College of Obstetrics and Gynecology (ACOG) suggests that despite the possibility of higher failure rates with oral and transdermal contraception, motivated obese women should still be encouraged to use these methods preferentially over known less effective methods.12 In addition, ACOG also notes that no higher rates of pregnancy are observed among overweight women using DMPA.
Depot medroxyprogesterone acetate (DMPA; Depo-Provera) and the combination contraceptive vaginal ring (NuvaRing) are most effective for obese women because they don’t appear to be affected by body weight (strength of recommendation [SOR]: B, consistent cohort studies).
On the other hand, women using the combination contraceptive patch (Ortho Evra) who weigh ≥90 kg may experience decreased contraceptive efficacy (SOR: A, meta-analysis). Obese women using oral contraceptives may also have an increased risk of pregnancy (SOR: B, inconsistent cohort studies). Data are not available on the levonorgestrel intrauterine system’s (Mirena) efficacy in obese women.
Obese women may have higher rates of pregnancy with OCs
Ronald Januchowski, DO
Spartanburg Regional Family Medicine Program, Spartanburg, SC
This answer shows that we need to provide more guidance to obese patients during contraceptive counseling. In our practice, we may have to develop contraceptive information sheets for overweight women.
I don’t think this will prevent me from prescribing oral contraceptives for obese women, but it will cause me to pause a bit. This question makes me wonder whether official recommendations in other drug classes for obese patients are coming in the near future.
Evidence summary
There is a theoretical risk of decreased hormonal contraceptive efficacy for obese women (defined as those having a body-mass index [BMI] ≥30 kg/m2) due to increased metabolism of the hormones resulting in lower serum levels. With the growing epidemic of obesity, concern over the efficacy of hormonal contraception has grown. At this time, however, only a few published studies evaluating contraception have specifically examined the effect of body weight on efficacy.
Pregnancy risk doubled among heavier patients on OCs
Some studies have shown a possible association between obesity and higher rates of pregnancy among women using oral contraceptives for birth control.
One retrospective cohort analysis found that women weighing >70.5 kg had an increased risk of pregnancy compared with women of lower weight (relative risk [RR]=1.6; 95% confidence interval [CI], 1.1–2.4), after controlling for parity.1 Pill compliance was not accounted for in this study. A follow-up case-control study demonstrated that the risk of pregnancy for consistent pill users doubled for women with a BMI >27.3 (odds ratio [OR]=2.17; 95% CI, 1.38–3.41); results were similar for those with a BMI >32.2 (OR=2.2; 95% CI, 1.18–4.20).2
Another large cohort study did not find any association between failure of the oral contraceptive pill or progestin-only pill and obesity; however, the total number of pregnancies among obese women was too small to achieve statistical significance.3 In a randomized trial studying the efficacy of an extended-cycle oral contraceptive (Seasonale), no woman weighing >90 kg became pregnant.4
When it comes to the combination contraceptive patch, the data show a significant association between baseline body weight and pregnancy. In an analysis of pooled data, 5 of 15 pregnancies occurred in a subgroup of women with a baseline body weight ≥90 kg. Less than 3% of the study population weighed more than 90 kg. Specific data for this subgroup were not presented in the study results, so measures of effect cannot be calculated. The mechanism of the decreased efficacy of the combined contraceptive patch for obese women is unclear.5
DMPA and vaginal ring may be a better option for obese women
Data suggest that increased body weight does not decrease the efficacy of DMPA. In 2 large open-label studies, no pregnancies were observed, regardless of BMI.6 Similarly, the efficacy of the contraceptive vaginal ring does not appear to be affected by body weight, but the mean BMI in intent-to-treat population studies was only 22.9±2.9.7
A secondary analysis of the contraceptive vaginal ring efficacy trials did not show an increased pregnancy rate among heavier women.8 Of note: A higher body weight appeared to be associated with increased likelihood of ovulation using the contraceptive vaginal ring, though it did not lead to any pregnancies in a multicenter study.9
The data on the levonorgestrel intrauterine system do not examine weight and efficacy.10
Recommendations from others
The World Health Organization generally recommends hormonal contraceptives as safe for obese women. The group acknowledges that data are limited regarding effectiveness of oral contraceptives, and efficacy may be lower for the combination contraceptive patch when used by obese women.11
The American College of Obstetrics and Gynecology (ACOG) suggests that despite the possibility of higher failure rates with oral and transdermal contraception, motivated obese women should still be encouraged to use these methods preferentially over known less effective methods.12 In addition, ACOG also notes that no higher rates of pregnancy are observed among overweight women using DMPA.
1. Holt VL, Cushing-Haugen KL, Daling JR. Body weight and the risk of oral contraceptive failure. Obstet Gynecol 2002;99:820-827.
2. Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Daling JR. Body Mass Index, weight, and oral contraceptive failure risk. Obstet Gynecol 2005;105:46-52.
3. Vessey M, Painter R. Oral contraceptive failures and body weight: Findings in a large cohort study. J Fam Plan Reprod Health Care 2001;27:90-91.
4. Anderson FD, Hait H. A multi-center, randomized study of an extended cycle oral contraceptive. Contraception 2003;68:89-106.
5. Zieman M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril 2002;77 (Suppl 2):S13-S18.
6. Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004;70:269-275.
7. Dieben TO, Roumen JM, Apter D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol 2002;100:585-593.
8. Westhoff C. Higher body weight does not affect NuvaRing’s efficacy [abstract]. Obstet Gynecol 2005;105(Suppl 4):56S.-
9. Weisberg E, Fraser I, Lacarra M, et al. Efficacy, bleeding patterns, and side effects of a 1-year contraceptive vaginal ring. Contraception 1999;59:311-318
10. Luukkainen T, Allonen H, Haukkamaa M, et al. Effective contraception with the levonorgestrel-releasing device: 12 month report of a multi-center study. Contraception 1987;36:169-179.
11. World Health Organization. Improving Access to Quality Care in Family Planning Eligibility Criteria for Contraceptive Use. Geneva: WHO; 2004.
12. ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006;107:1453-1472.
1. Holt VL, Cushing-Haugen KL, Daling JR. Body weight and the risk of oral contraceptive failure. Obstet Gynecol 2002;99:820-827.
2. Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Daling JR. Body Mass Index, weight, and oral contraceptive failure risk. Obstet Gynecol 2005;105:46-52.
3. Vessey M, Painter R. Oral contraceptive failures and body weight: Findings in a large cohort study. J Fam Plan Reprod Health Care 2001;27:90-91.
4. Anderson FD, Hait H. A multi-center, randomized study of an extended cycle oral contraceptive. Contraception 2003;68:89-106.
5. Zieman M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril 2002;77 (Suppl 2):S13-S18.
6. Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004;70:269-275.
7. Dieben TO, Roumen JM, Apter D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol 2002;100:585-593.
8. Westhoff C. Higher body weight does not affect NuvaRing’s efficacy [abstract]. Obstet Gynecol 2005;105(Suppl 4):56S.-
9. Weisberg E, Fraser I, Lacarra M, et al. Efficacy, bleeding patterns, and side effects of a 1-year contraceptive vaginal ring. Contraception 1999;59:311-318
10. Luukkainen T, Allonen H, Haukkamaa M, et al. Effective contraception with the levonorgestrel-releasing device: 12 month report of a multi-center study. Contraception 1987;36:169-179.
11. World Health Organization. Improving Access to Quality Care in Family Planning Eligibility Criteria for Contraceptive Use. Geneva: WHO; 2004.
12. ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006;107:1453-1472.
Evidence-based answers from the Family Physicians Inquiries Network
What treatment approach to intrapartum maternal fever has the best fetal outcomes?
A combination of beta-lactam and aminoglycoside antibiotics are the recommended empiric agents for the treatment of acute chorioamnionitis, given that no head-to-head trials exist (strength of recommendation [SOR]: C, based on expert opinion). Intrapartum antibiotic treatment is not superior to postpartum antibiotics for reducing neonatal sepsis and pneumonia (SOR: C, based on patient-oriented, underpowered randomized trials).
Carefully follow laboring patients with fever for other signs of chorioamnionitis
Jon O. Neher, MD
Valley Family Medicine, Renton, Wash
The data on the best antibiotic treatment of clinical chorioamnionitis remains as slim as ever, it appears. But since experts continue to recommend potentially toxic gentamicin as part of therapy, you should carefully monitor laboring patients at term who develop a fever for the development of other diagnostic signs of chorioamnionitis. While maternal and fetal tachycardia are frequently caused by conditions other than infection, their appearance in a febrile gravida should prompt full chorioamnionitis therapy (even in patients already on empiric antibiotics for group B streptococci). With epidural anesthesia, uterine tenderness is an unreliable sign of infection. Purulent amniotic fluid is a late sign and rarely contributes clinically.
Evidence summary
Acute chorioamnionitis (or intra-amniotic infection) poses a high risk of maternal and neonatal morbidity. Neonatal sepsis or pneumonia occurs in up to 24% of infants born to mothers with chorioamnionitis;1 1% to 2% of pregnancies complicated by chorioamnionitis end in neonatal death.1,2
Acute chorioamnionitis is defined as intrapartum maternal fever and maternal tachycardia, fetal tachycardia, uterine tenderness, or purulent amniotic fluid.1,3 Antibiotic treatment of acute chorioamnionitis is widely accepted, yet in vivo studies to determine the most effective empiric antibiotic regimens are lacking.
Intrapartum antibiotics probably reduce sepsis
Although few well-designed trials stand out, a Cochrane review4 summarizing 2 relevant studies is available. Gibbs et al3 performed an underpowered, randomized comparative trial of intrapartum vs postpartum treatment of chorioamnionitis, with both groups (45 patients total) receiving ampicillin 2 g IV every 6 hours plus gentamicin 1.5 mg/kg IV every 8 hours.3 Those women who underwent cesarean section also received clindamycin 900 mg IV every 8 hours starting at cord clamping. In this study, investigators reported neonatal sepsis was significantly reduced with intrapartum treatment (0 vs 21%; P=.03, number needed to treat=4.8), as were neonatal hospital stays (3.8 vs 5.7 days; P=.02), regardless of delivery method. The study had been planned for 92 patients; it was stopped early (n=48) after an interim analysis.
Because of the small sample size, other findings from the study must be viewed with caution. Intrapartum treatment with antibiotics was associated with a “significant” clinical reduction in neonatal sepsis (relative risk [RR]=0.08; 95% confidence interval [CI], 0.00–1.44) and pneumonia (RR=0.15; 95% CI, 0.01–2.92) compared with treatment given immediately postpartum; however, neither value was truly statistically significant according to the Cochrane review.4
The research suggests a potential benefit to adding clindamycin to ampicillin and gentamicin. In an effort to test this, 1 study randomized 133 women into 2 arms—treatment with ampicillin, gentamicin, and clindamycin compared with ampicillin and gentamicin alone—and found no additional benefit in regards to neonatal sepsis (RR=2.16; 95% CI, 0.20–23.21) or neonatal death (RR=0.72; 95% CI, 0.12–4.16).1 There was a trend towards a decrease in the incidence of postpartum endometritis in women who received ampicillin, gentamicin, and clindamycin, but this did not reach statistical significance (RR=0.54; 95% CI, 0.19–1.49).4
Recommendations from others
A 2002 bulletin from American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics5 recommended the combination of ampicillin 2 gm IV every 4 to 6 hours or penicillin 5 million units IV every 4 to 6 hours, plus an aminoglycoside (such as gentamicin 1.5 mg/kg IV every 8 hours), since this regimen provides appropriate coverage for typical organisms associated with acute chorioamnionitis. At the time the bulletin was published, the use of single daily dosing of aminoglycoside did not have sufficient studies to back its use. In addition, ACOG recommends adding clindamycin, metronidazole, or an extended-spectrum third-generation cephalosporin to the treatment regimen if cesarean section is required, to provide coverage for anaerobic organisms. They recommend clindamycin 900 mg IV every 8 hours to replace amoxicillin in penicillin-allergic patients. The Nottingham Guideline Development Group recommends amoxicillin 2 gm IV initially then 1 gm every 8 hours, and in place of gentamicin, recommends metronidazole 500 mg IV, every 8 hours (or 1 gm PR twice a day).6 Both recommendations suggest clindamycin 900 mg IV every 8 hours to replace amoxicillin in penicillin-allergic patients. For patients with nonanaphylactic reactions to penicillin, they recommend cefotaxime 1 g IV every 8 hours.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. Maberry MC, Gilstrap LC, 3rd. Intrapartum antibiotic therapy for suspected intraamniotic infection: impact on the fetus and neonate. Clin Obstet Gyn 1991;34:345-351.
2. Hauth JC, Gilstrap LC, Hankins GD, Conner KD. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gyn 1985;66:59-62.
3. Gibbs RS, Dinsmoor MJ, Newton ER, et al. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gyn 1988;72:823-828.
4. Hopkins L, Smaill F. Antibiotic regimens for management of intraamniotic infection. Cochrane Database Syst Rev 2002;(3):CD003254.-
5. American College of Obstetricians and Gynecologists, American Academy of Pediatrics. Guidelines for Perinatal Care. 5th ed. Washington, DC: ACOG;2002:165-166.
6. Hayman R, Kean L. Guidelines for the Prevention of Neonatal Group B Streptococcal Infection. Nottingham: Nottingham City Hospital, National Health Service; 2002. Revised 2005. Available at: www.nuh.nhs.uk/nch/antibiotics. Accessed on March 30, 2007.
A combination of beta-lactam and aminoglycoside antibiotics are the recommended empiric agents for the treatment of acute chorioamnionitis, given that no head-to-head trials exist (strength of recommendation [SOR]: C, based on expert opinion). Intrapartum antibiotic treatment is not superior to postpartum antibiotics for reducing neonatal sepsis and pneumonia (SOR: C, based on patient-oriented, underpowered randomized trials).
Carefully follow laboring patients with fever for other signs of chorioamnionitis
Jon O. Neher, MD
Valley Family Medicine, Renton, Wash
The data on the best antibiotic treatment of clinical chorioamnionitis remains as slim as ever, it appears. But since experts continue to recommend potentially toxic gentamicin as part of therapy, you should carefully monitor laboring patients at term who develop a fever for the development of other diagnostic signs of chorioamnionitis. While maternal and fetal tachycardia are frequently caused by conditions other than infection, their appearance in a febrile gravida should prompt full chorioamnionitis therapy (even in patients already on empiric antibiotics for group B streptococci). With epidural anesthesia, uterine tenderness is an unreliable sign of infection. Purulent amniotic fluid is a late sign and rarely contributes clinically.
Evidence summary
Acute chorioamnionitis (or intra-amniotic infection) poses a high risk of maternal and neonatal morbidity. Neonatal sepsis or pneumonia occurs in up to 24% of infants born to mothers with chorioamnionitis;1 1% to 2% of pregnancies complicated by chorioamnionitis end in neonatal death.1,2
Acute chorioamnionitis is defined as intrapartum maternal fever and maternal tachycardia, fetal tachycardia, uterine tenderness, or purulent amniotic fluid.1,3 Antibiotic treatment of acute chorioamnionitis is widely accepted, yet in vivo studies to determine the most effective empiric antibiotic regimens are lacking.
Intrapartum antibiotics probably reduce sepsis
Although few well-designed trials stand out, a Cochrane review4 summarizing 2 relevant studies is available. Gibbs et al3 performed an underpowered, randomized comparative trial of intrapartum vs postpartum treatment of chorioamnionitis, with both groups (45 patients total) receiving ampicillin 2 g IV every 6 hours plus gentamicin 1.5 mg/kg IV every 8 hours.3 Those women who underwent cesarean section also received clindamycin 900 mg IV every 8 hours starting at cord clamping. In this study, investigators reported neonatal sepsis was significantly reduced with intrapartum treatment (0 vs 21%; P=.03, number needed to treat=4.8), as were neonatal hospital stays (3.8 vs 5.7 days; P=.02), regardless of delivery method. The study had been planned for 92 patients; it was stopped early (n=48) after an interim analysis.
Because of the small sample size, other findings from the study must be viewed with caution. Intrapartum treatment with antibiotics was associated with a “significant” clinical reduction in neonatal sepsis (relative risk [RR]=0.08; 95% confidence interval [CI], 0.00–1.44) and pneumonia (RR=0.15; 95% CI, 0.01–2.92) compared with treatment given immediately postpartum; however, neither value was truly statistically significant according to the Cochrane review.4
The research suggests a potential benefit to adding clindamycin to ampicillin and gentamicin. In an effort to test this, 1 study randomized 133 women into 2 arms—treatment with ampicillin, gentamicin, and clindamycin compared with ampicillin and gentamicin alone—and found no additional benefit in regards to neonatal sepsis (RR=2.16; 95% CI, 0.20–23.21) or neonatal death (RR=0.72; 95% CI, 0.12–4.16).1 There was a trend towards a decrease in the incidence of postpartum endometritis in women who received ampicillin, gentamicin, and clindamycin, but this did not reach statistical significance (RR=0.54; 95% CI, 0.19–1.49).4
Recommendations from others
A 2002 bulletin from American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics5 recommended the combination of ampicillin 2 gm IV every 4 to 6 hours or penicillin 5 million units IV every 4 to 6 hours, plus an aminoglycoside (such as gentamicin 1.5 mg/kg IV every 8 hours), since this regimen provides appropriate coverage for typical organisms associated with acute chorioamnionitis. At the time the bulletin was published, the use of single daily dosing of aminoglycoside did not have sufficient studies to back its use. In addition, ACOG recommends adding clindamycin, metronidazole, or an extended-spectrum third-generation cephalosporin to the treatment regimen if cesarean section is required, to provide coverage for anaerobic organisms. They recommend clindamycin 900 mg IV every 8 hours to replace amoxicillin in penicillin-allergic patients. The Nottingham Guideline Development Group recommends amoxicillin 2 gm IV initially then 1 gm every 8 hours, and in place of gentamicin, recommends metronidazole 500 mg IV, every 8 hours (or 1 gm PR twice a day).6 Both recommendations suggest clindamycin 900 mg IV every 8 hours to replace amoxicillin in penicillin-allergic patients. For patients with nonanaphylactic reactions to penicillin, they recommend cefotaxime 1 g IV every 8 hours.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
A combination of beta-lactam and aminoglycoside antibiotics are the recommended empiric agents for the treatment of acute chorioamnionitis, given that no head-to-head trials exist (strength of recommendation [SOR]: C, based on expert opinion). Intrapartum antibiotic treatment is not superior to postpartum antibiotics for reducing neonatal sepsis and pneumonia (SOR: C, based on patient-oriented, underpowered randomized trials).
Carefully follow laboring patients with fever for other signs of chorioamnionitis
Jon O. Neher, MD
Valley Family Medicine, Renton, Wash
The data on the best antibiotic treatment of clinical chorioamnionitis remains as slim as ever, it appears. But since experts continue to recommend potentially toxic gentamicin as part of therapy, you should carefully monitor laboring patients at term who develop a fever for the development of other diagnostic signs of chorioamnionitis. While maternal and fetal tachycardia are frequently caused by conditions other than infection, their appearance in a febrile gravida should prompt full chorioamnionitis therapy (even in patients already on empiric antibiotics for group B streptococci). With epidural anesthesia, uterine tenderness is an unreliable sign of infection. Purulent amniotic fluid is a late sign and rarely contributes clinically.
Evidence summary
Acute chorioamnionitis (or intra-amniotic infection) poses a high risk of maternal and neonatal morbidity. Neonatal sepsis or pneumonia occurs in up to 24% of infants born to mothers with chorioamnionitis;1 1% to 2% of pregnancies complicated by chorioamnionitis end in neonatal death.1,2
Acute chorioamnionitis is defined as intrapartum maternal fever and maternal tachycardia, fetal tachycardia, uterine tenderness, or purulent amniotic fluid.1,3 Antibiotic treatment of acute chorioamnionitis is widely accepted, yet in vivo studies to determine the most effective empiric antibiotic regimens are lacking.
Intrapartum antibiotics probably reduce sepsis
Although few well-designed trials stand out, a Cochrane review4 summarizing 2 relevant studies is available. Gibbs et al3 performed an underpowered, randomized comparative trial of intrapartum vs postpartum treatment of chorioamnionitis, with both groups (45 patients total) receiving ampicillin 2 g IV every 6 hours plus gentamicin 1.5 mg/kg IV every 8 hours.3 Those women who underwent cesarean section also received clindamycin 900 mg IV every 8 hours starting at cord clamping. In this study, investigators reported neonatal sepsis was significantly reduced with intrapartum treatment (0 vs 21%; P=.03, number needed to treat=4.8), as were neonatal hospital stays (3.8 vs 5.7 days; P=.02), regardless of delivery method. The study had been planned for 92 patients; it was stopped early (n=48) after an interim analysis.
Because of the small sample size, other findings from the study must be viewed with caution. Intrapartum treatment with antibiotics was associated with a “significant” clinical reduction in neonatal sepsis (relative risk [RR]=0.08; 95% confidence interval [CI], 0.00–1.44) and pneumonia (RR=0.15; 95% CI, 0.01–2.92) compared with treatment given immediately postpartum; however, neither value was truly statistically significant according to the Cochrane review.4
The research suggests a potential benefit to adding clindamycin to ampicillin and gentamicin. In an effort to test this, 1 study randomized 133 women into 2 arms—treatment with ampicillin, gentamicin, and clindamycin compared with ampicillin and gentamicin alone—and found no additional benefit in regards to neonatal sepsis (RR=2.16; 95% CI, 0.20–23.21) or neonatal death (RR=0.72; 95% CI, 0.12–4.16).1 There was a trend towards a decrease in the incidence of postpartum endometritis in women who received ampicillin, gentamicin, and clindamycin, but this did not reach statistical significance (RR=0.54; 95% CI, 0.19–1.49).4
Recommendations from others
A 2002 bulletin from American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics5 recommended the combination of ampicillin 2 gm IV every 4 to 6 hours or penicillin 5 million units IV every 4 to 6 hours, plus an aminoglycoside (such as gentamicin 1.5 mg/kg IV every 8 hours), since this regimen provides appropriate coverage for typical organisms associated with acute chorioamnionitis. At the time the bulletin was published, the use of single daily dosing of aminoglycoside did not have sufficient studies to back its use. In addition, ACOG recommends adding clindamycin, metronidazole, or an extended-spectrum third-generation cephalosporin to the treatment regimen if cesarean section is required, to provide coverage for anaerobic organisms. They recommend clindamycin 900 mg IV every 8 hours to replace amoxicillin in penicillin-allergic patients. The Nottingham Guideline Development Group recommends amoxicillin 2 gm IV initially then 1 gm every 8 hours, and in place of gentamicin, recommends metronidazole 500 mg IV, every 8 hours (or 1 gm PR twice a day).6 Both recommendations suggest clindamycin 900 mg IV every 8 hours to replace amoxicillin in penicillin-allergic patients. For patients with nonanaphylactic reactions to penicillin, they recommend cefotaxime 1 g IV every 8 hours.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. Maberry MC, Gilstrap LC, 3rd. Intrapartum antibiotic therapy for suspected intraamniotic infection: impact on the fetus and neonate. Clin Obstet Gyn 1991;34:345-351.
2. Hauth JC, Gilstrap LC, Hankins GD, Conner KD. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gyn 1985;66:59-62.
3. Gibbs RS, Dinsmoor MJ, Newton ER, et al. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gyn 1988;72:823-828.
4. Hopkins L, Smaill F. Antibiotic regimens for management of intraamniotic infection. Cochrane Database Syst Rev 2002;(3):CD003254.-
5. American College of Obstetricians and Gynecologists, American Academy of Pediatrics. Guidelines for Perinatal Care. 5th ed. Washington, DC: ACOG;2002:165-166.
6. Hayman R, Kean L. Guidelines for the Prevention of Neonatal Group B Streptococcal Infection. Nottingham: Nottingham City Hospital, National Health Service; 2002. Revised 2005. Available at: www.nuh.nhs.uk/nch/antibiotics. Accessed on March 30, 2007.
1. Maberry MC, Gilstrap LC, 3rd. Intrapartum antibiotic therapy for suspected intraamniotic infection: impact on the fetus and neonate. Clin Obstet Gyn 1991;34:345-351.
2. Hauth JC, Gilstrap LC, Hankins GD, Conner KD. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gyn 1985;66:59-62.
3. Gibbs RS, Dinsmoor MJ, Newton ER, et al. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gyn 1988;72:823-828.
4. Hopkins L, Smaill F. Antibiotic regimens for management of intraamniotic infection. Cochrane Database Syst Rev 2002;(3):CD003254.-
5. American College of Obstetricians and Gynecologists, American Academy of Pediatrics. Guidelines for Perinatal Care. 5th ed. Washington, DC: ACOG;2002:165-166.
6. Hayman R, Kean L. Guidelines for the Prevention of Neonatal Group B Streptococcal Infection. Nottingham: Nottingham City Hospital, National Health Service; 2002. Revised 2005. Available at: www.nuh.nhs.uk/nch/antibiotics. Accessed on March 30, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
Which patients with suspected exposure to pertussis should receive prophylaxis?
Only high-risk close contacts of known cases should receive prophylactic antibiotics, according to the Centers for Disease Control and Prevention (CDC). The CDC defines high-risk as (1) infants who are <12 months, (2) those especially vulnerable to the complications of pertussis, or (3) those, such as health care workers, in close contact with high-risk individuals (strength of recommendation [SOR]: C, based on consensus/expert opinion). Evidence is insufficient to support a benefit of prophylactic antibiotic treatment for all household pertussis contacts to prevent the development or spread of illness (SOR: B, based on a systematic review of studies).
Give special attention to high-risk close contacts, especially infants
Jose Rodriguez, MD
Florida State University College of Medicine, Tallahassee
Recently, in the medical college where I teach, a student came down with pertussis. Several weeks after the onset of symptoms, she was diagnosed and determined to be no longer contagious. When she coughed in class, however, I worried that she could have infected us all. No one received prophylactic antibiotics. To date, I do not know of anyone who was in close contact with this student who has come down with the illness. However, direct special attention to high-risk close contacts, especially infants, as they can have devastating results from infection.
Evidence summary
A Cochrane review1 of antibiotic use for pertussis prophylaxis, including studies published through 2002, found only 2 randomized, well-controlled trials (RCTs) that compared attack rates between contacts receiving placebo or antibiotic therapy. Neither trial included infants under age 6 months. The reviewers concluded that evidence was insufficient to determine a treatment benefit. The larger study2 included 310 household or family contacts, randomized by household to 10 days of erythromycin estolate or placebo. Positive cultures or clinical pertussis developed in 4.8% of treated contacts and 6.1% of controls (relative risk [RR]=0.8; 95% confidence interval [CI], 0.3–2.2). Adverse side effects occurred in 34% of the erythromycin group and 16% of controls (RR=2.2; 95% CI, 1.4–3.3; number needed to harm=5.6).
Focus on those at high risk
Despite the paucity of RCTs, the CDC and other public health agency guidelines recommend postexposure prophylaxis for certain close contacts to protect high-risk individuals, defined as those who could develop severe disease or experience adverse outcomes if pertussis developed.3-6
High-risk individuals include:
- Infants <1 year old
- Pregnant women in their third trimester
- the immunocompromised
- those with underlying medical condition such as chronic lung disease, respiratory insufficiency, or cystic fibrosis
- those who have close contact with any of the above high-risk individuals (eg, household members or health-care workers providing face-to-face care).
Close contact is defined as:
- confinement in a closed space for >1 hour with a known case, or
- direct contact with respiratory, oral, or nasal secretions from a symptomatic person, or
- face-to-face exposure within 3 feet of a symptomatic patient.
TABLE
Recommendations for pertussis prophylaxis
| ORGANIZATION | RECOMMENDATION |
|---|---|
| Canadian guidelines4 | Reserve prophylaxis for:
|
| Public Health Seattle and King County5 | Prophylax only high-risk individuals with
|
| CDC6 | During institutional outbreaks
|
Clinical trials involving such patients have not been conducted.6,7 Maintenance of active vaccination status is an effective means to prevent the spread of pertussis among the general population and has been suggested as a means to control local outbreaks,6 though it has no role in immediate postexposure prophylaxis for an individual. In one RCT, no (0/60) fully immunized child in a household with pertussis developed whooping cough, with or without antibiotic prophylaxis. Among unimmunized children, pertussis developed in 4/20 receiving erythromycin prophylaxis and 2/11 receiving placebo.
Macrolides (erythromycin, clarithromycin, or azithromycin) are recommended for postexposure prophylaxis. Trimethoprim-sulfamethoxazole is a second-line agent.5 A short course of erythromycin (7 days), azithromycin (3–5 days), or clarithromycin (7 days) is as effective as a 2-week course of erythromycin in eradicating Bordetella pertussis from the nasopharynx.
Recommendations from others
Recommendations from others are in the TABLE.
1. Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2005;(1):CDC004404.-
2. Halperin SA, Bortolussi R, Langley JM, Eastwood BJ, De Serres G. A randomized, placebo-controlled trial of erythromycin estolate chemoprophylaxis for household contacts of children with culture-positive bordetella pertussis infection. Pediatrics 1999;104(4):e42.-
3. Dodhia H, Crowcroft NS, Bramley JC, Miller E. UK guidelines for use of erythromycin chemoprophylaxis in persons exposed to pertussis. J Pub Health Med 2002;24:200-206.
4. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis 2005. Atlanta, Ga: Centers for Disease Control and Prevention. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5414a1.htm?s_cud=rr5414a1_e. Accessed on March 30, 2007.
5. Dodhia H, Miller E. Review of the evidence for the use of erythromycin in the management of persons exposed to pertussis. Epidemiol Infect 1998;120:143-149.
6. Grob PR. Prophylactic erythromycin for whooping-cough contacts. Lancet 1981;1(8223):772.-
7. Tubbs C, Niemi H, Mayo HG, Warren M. What is the best treatment for pertussis? J Fam Pract 2005;54:1096-1098.
Only high-risk close contacts of known cases should receive prophylactic antibiotics, according to the Centers for Disease Control and Prevention (CDC). The CDC defines high-risk as (1) infants who are <12 months, (2) those especially vulnerable to the complications of pertussis, or (3) those, such as health care workers, in close contact with high-risk individuals (strength of recommendation [SOR]: C, based on consensus/expert opinion). Evidence is insufficient to support a benefit of prophylactic antibiotic treatment for all household pertussis contacts to prevent the development or spread of illness (SOR: B, based on a systematic review of studies).
Give special attention to high-risk close contacts, especially infants
Jose Rodriguez, MD
Florida State University College of Medicine, Tallahassee
Recently, in the medical college where I teach, a student came down with pertussis. Several weeks after the onset of symptoms, she was diagnosed and determined to be no longer contagious. When she coughed in class, however, I worried that she could have infected us all. No one received prophylactic antibiotics. To date, I do not know of anyone who was in close contact with this student who has come down with the illness. However, direct special attention to high-risk close contacts, especially infants, as they can have devastating results from infection.
Evidence summary
A Cochrane review1 of antibiotic use for pertussis prophylaxis, including studies published through 2002, found only 2 randomized, well-controlled trials (RCTs) that compared attack rates between contacts receiving placebo or antibiotic therapy. Neither trial included infants under age 6 months. The reviewers concluded that evidence was insufficient to determine a treatment benefit. The larger study2 included 310 household or family contacts, randomized by household to 10 days of erythromycin estolate or placebo. Positive cultures or clinical pertussis developed in 4.8% of treated contacts and 6.1% of controls (relative risk [RR]=0.8; 95% confidence interval [CI], 0.3–2.2). Adverse side effects occurred in 34% of the erythromycin group and 16% of controls (RR=2.2; 95% CI, 1.4–3.3; number needed to harm=5.6).
Focus on those at high risk
Despite the paucity of RCTs, the CDC and other public health agency guidelines recommend postexposure prophylaxis for certain close contacts to protect high-risk individuals, defined as those who could develop severe disease or experience adverse outcomes if pertussis developed.3-6
High-risk individuals include:
- Infants <1 year old
- Pregnant women in their third trimester
- the immunocompromised
- those with underlying medical condition such as chronic lung disease, respiratory insufficiency, or cystic fibrosis
- those who have close contact with any of the above high-risk individuals (eg, household members or health-care workers providing face-to-face care).
Close contact is defined as:
- confinement in a closed space for >1 hour with a known case, or
- direct contact with respiratory, oral, or nasal secretions from a symptomatic person, or
- face-to-face exposure within 3 feet of a symptomatic patient.
TABLE
Recommendations for pertussis prophylaxis
| ORGANIZATION | RECOMMENDATION |
|---|---|
| Canadian guidelines4 | Reserve prophylaxis for:
|
| Public Health Seattle and King County5 | Prophylax only high-risk individuals with
|
| CDC6 | During institutional outbreaks
|
Clinical trials involving such patients have not been conducted.6,7 Maintenance of active vaccination status is an effective means to prevent the spread of pertussis among the general population and has been suggested as a means to control local outbreaks,6 though it has no role in immediate postexposure prophylaxis for an individual. In one RCT, no (0/60) fully immunized child in a household with pertussis developed whooping cough, with or without antibiotic prophylaxis. Among unimmunized children, pertussis developed in 4/20 receiving erythromycin prophylaxis and 2/11 receiving placebo.
Macrolides (erythromycin, clarithromycin, or azithromycin) are recommended for postexposure prophylaxis. Trimethoprim-sulfamethoxazole is a second-line agent.5 A short course of erythromycin (7 days), azithromycin (3–5 days), or clarithromycin (7 days) is as effective as a 2-week course of erythromycin in eradicating Bordetella pertussis from the nasopharynx.
Recommendations from others
Recommendations from others are in the TABLE.
Only high-risk close contacts of known cases should receive prophylactic antibiotics, according to the Centers for Disease Control and Prevention (CDC). The CDC defines high-risk as (1) infants who are <12 months, (2) those especially vulnerable to the complications of pertussis, or (3) those, such as health care workers, in close contact with high-risk individuals (strength of recommendation [SOR]: C, based on consensus/expert opinion). Evidence is insufficient to support a benefit of prophylactic antibiotic treatment for all household pertussis contacts to prevent the development or spread of illness (SOR: B, based on a systematic review of studies).
Give special attention to high-risk close contacts, especially infants
Jose Rodriguez, MD
Florida State University College of Medicine, Tallahassee
Recently, in the medical college where I teach, a student came down with pertussis. Several weeks after the onset of symptoms, she was diagnosed and determined to be no longer contagious. When she coughed in class, however, I worried that she could have infected us all. No one received prophylactic antibiotics. To date, I do not know of anyone who was in close contact with this student who has come down with the illness. However, direct special attention to high-risk close contacts, especially infants, as they can have devastating results from infection.
Evidence summary
A Cochrane review1 of antibiotic use for pertussis prophylaxis, including studies published through 2002, found only 2 randomized, well-controlled trials (RCTs) that compared attack rates between contacts receiving placebo or antibiotic therapy. Neither trial included infants under age 6 months. The reviewers concluded that evidence was insufficient to determine a treatment benefit. The larger study2 included 310 household or family contacts, randomized by household to 10 days of erythromycin estolate or placebo. Positive cultures or clinical pertussis developed in 4.8% of treated contacts and 6.1% of controls (relative risk [RR]=0.8; 95% confidence interval [CI], 0.3–2.2). Adverse side effects occurred in 34% of the erythromycin group and 16% of controls (RR=2.2; 95% CI, 1.4–3.3; number needed to harm=5.6).
Focus on those at high risk
Despite the paucity of RCTs, the CDC and other public health agency guidelines recommend postexposure prophylaxis for certain close contacts to protect high-risk individuals, defined as those who could develop severe disease or experience adverse outcomes if pertussis developed.3-6
High-risk individuals include:
- Infants <1 year old
- Pregnant women in their third trimester
- the immunocompromised
- those with underlying medical condition such as chronic lung disease, respiratory insufficiency, or cystic fibrosis
- those who have close contact with any of the above high-risk individuals (eg, household members or health-care workers providing face-to-face care).
Close contact is defined as:
- confinement in a closed space for >1 hour with a known case, or
- direct contact with respiratory, oral, or nasal secretions from a symptomatic person, or
- face-to-face exposure within 3 feet of a symptomatic patient.
TABLE
Recommendations for pertussis prophylaxis
| ORGANIZATION | RECOMMENDATION |
|---|---|
| Canadian guidelines4 | Reserve prophylaxis for:
|
| Public Health Seattle and King County5 | Prophylax only high-risk individuals with
|
| CDC6 | During institutional outbreaks
|
Clinical trials involving such patients have not been conducted.6,7 Maintenance of active vaccination status is an effective means to prevent the spread of pertussis among the general population and has been suggested as a means to control local outbreaks,6 though it has no role in immediate postexposure prophylaxis for an individual. In one RCT, no (0/60) fully immunized child in a household with pertussis developed whooping cough, with or without antibiotic prophylaxis. Among unimmunized children, pertussis developed in 4/20 receiving erythromycin prophylaxis and 2/11 receiving placebo.
Macrolides (erythromycin, clarithromycin, or azithromycin) are recommended for postexposure prophylaxis. Trimethoprim-sulfamethoxazole is a second-line agent.5 A short course of erythromycin (7 days), azithromycin (3–5 days), or clarithromycin (7 days) is as effective as a 2-week course of erythromycin in eradicating Bordetella pertussis from the nasopharynx.
Recommendations from others
Recommendations from others are in the TABLE.
1. Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2005;(1):CDC004404.-
2. Halperin SA, Bortolussi R, Langley JM, Eastwood BJ, De Serres G. A randomized, placebo-controlled trial of erythromycin estolate chemoprophylaxis for household contacts of children with culture-positive bordetella pertussis infection. Pediatrics 1999;104(4):e42.-
3. Dodhia H, Crowcroft NS, Bramley JC, Miller E. UK guidelines for use of erythromycin chemoprophylaxis in persons exposed to pertussis. J Pub Health Med 2002;24:200-206.
4. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis 2005. Atlanta, Ga: Centers for Disease Control and Prevention. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5414a1.htm?s_cud=rr5414a1_e. Accessed on March 30, 2007.
5. Dodhia H, Miller E. Review of the evidence for the use of erythromycin in the management of persons exposed to pertussis. Epidemiol Infect 1998;120:143-149.
6. Grob PR. Prophylactic erythromycin for whooping-cough contacts. Lancet 1981;1(8223):772.-
7. Tubbs C, Niemi H, Mayo HG, Warren M. What is the best treatment for pertussis? J Fam Pract 2005;54:1096-1098.
1. Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2005;(1):CDC004404.-
2. Halperin SA, Bortolussi R, Langley JM, Eastwood BJ, De Serres G. A randomized, placebo-controlled trial of erythromycin estolate chemoprophylaxis for household contacts of children with culture-positive bordetella pertussis infection. Pediatrics 1999;104(4):e42.-
3. Dodhia H, Crowcroft NS, Bramley JC, Miller E. UK guidelines for use of erythromycin chemoprophylaxis in persons exposed to pertussis. J Pub Health Med 2002;24:200-206.
4. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis 2005. Atlanta, Ga: Centers for Disease Control and Prevention. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5414a1.htm?s_cud=rr5414a1_e. Accessed on March 30, 2007.
5. Dodhia H, Miller E. Review of the evidence for the use of erythromycin in the management of persons exposed to pertussis. Epidemiol Infect 1998;120:143-149.
6. Grob PR. Prophylactic erythromycin for whooping-cough contacts. Lancet 1981;1(8223):772.-
7. Tubbs C, Niemi H, Mayo HG, Warren M. What is the best treatment for pertussis? J Fam Pract 2005;54:1096-1098.
Evidence-based answers from the Family Physicians Inquiries Network
What are the indications for meningococcal vaccination?
Routine vaccination with the meningococcal conjugate vaccine MCV4 (Menactra) is indicated for all US adolescents entering high school and for college freshmen living in dormitories (strength of recommendation [SOR]: B, based on observational studies). For convenience, MCV4 can be given at the 11- to 12-year-old visit.
High-risk individuals (ages 2 and older) who should receive meningococcal vaccine (MCV4 or the unconjugated polysaccharide vaccine [MPSV4]) include those with terminal complement deficiencies, asplenia, or HIV, as well as military recruits, laboratory personnel exposed to aerosolized meningococci, and travelers to areas hyperendemic or epidemic for Neisseria meningitides (SOR: C, based on consensus guidelines). Routine vaccination of infants and toddlers with conjugate vaccine may be more cost-effective than targeting adolescents, but conjugate meningococcal vaccine for this age group is not yet available in the US (SOR: B, based on cohort studies).
The vaccine is available and efficacious—use it well
Mark Stephens, MD
Uniformed Services University, Bethesda, Md
As a junior military medical officer, my first assignment was in San Diego, California near the former Naval Training Center (NTC). The NTC was the site of one of the last major outbreaks of meningococcal disease in a military barracks setting. I recall with alacrity the rapidity with which this disease overcomes its host, and the overwhelming morbidity (and mortality) the disease leaves behind if treatment is delayed. This is truly a “not-to-be-missed” diagnosis.
The historical parallels between smallpox and meningococcal disease are striking. Each is spread primarily by respiratory means, particularly in close quarters. While meningococcal disease is amenable to antibiotic treatment when recognized early (contrary to smallpox), the principles of high-risk “herd” immunization hold true for both conditions. By focusing on high-risk groups and adhering to ACIP recommendations, control of meningococcal disease is within the grasp of modern medical science. The vaccine is available. The vaccine is efficacious. Use it well.
Evidence summary
Two meningococcal vaccines are currently available in the US: tetravalent polysaccharide vaccine (MPSV4) and tetravalent polysaccharide-protein conjugate vaccine (MCV4). Both protect against serogroups A, C, Y, and W-135, but not against serogroup B, which is the most prevalent. A vaccine for serogroup B is under development.
MPSV4 is licensed for ages 2 years and up, but its poor immunogenicity in infants, lack of memory and booster response, and relatively short duration of protection have restricted its use. MCV4 is licensed for 11- to 55-year-olds and is the preferred vaccine in this age group, since it provides longer duration of immunity and reduces nasopharyngeal carriage.1
Infants and freshman are especially vulnerable
Using active community surveillance from 1991 to 2002, Centers for Disease Control and Prevention (CDC) data2 found annual rates of meningococcal disease in the US of 0.5 to 1.1 per 100,000. The highest rates were found in children under age 2. Infants younger than 12 months of age were especially vulnerable (rate 9/100,000), with more than 50% of cases caused by serogroup B.
A 1998–1999 prospective surveillance study3 including 50 state health departments and 231 college health centers identified 96 cases of meningococcal disease in college students (incidence 0.7/100,000). Freshmen living in dormitories had an elevated risk of meningococcal disease compared with other undergraduates or nonstudents of the same age (incidence 5.1/100,000; adjusted relative risk=3.6 [95% confidence interval [CI], 1.6–8.5). Sixty-eight percent had illness due to a vaccine-preventable serogroup.
Using CDC incidence data, a cost-effectiveness model4 compared hypothetical vaccination strategies targeting US infants (3 doses), toddlers (1 dose), or 11-year-olds (1 dose). Routine MCV4 vaccination of all 11-year-olds would prevent 270 cases and 36 deaths in this cohort over their next 22 years. For a toddler cohort, vaccination would prevent 385 cases and 33 deaths; for infants, 447 cases and 36 deaths. Conjugate meningococcal vaccines for serogroups A and C have been tested and used in children in other countries, and appear safe and effective, but are not yet available in the US. An application has been submitted for FDA approval of MCV4 for 2- to 10-year-olds.
Herd immunity may expand benefit of vaccination
A British study compared attack rates for meningococcal C disease in children from infancy to age 18 before and 1 to 2 years after the institution of a nationwide meningococcal serogroup C conjugate vaccination. Vaccine coverage ranged from 66% (adolescents) to 87% (schoolchildren), and vaccine efficacy was 94% to 96%. Incidence of meningococcal C disease in the unvaccinated children also decreased by 52% to 67% (from 4.08/100,000 to 1.36/100,000).5
Vaccinating adolescents may be particularly helpful for building herd immunity. A Norwegian study of nasopharyngeal meningococcal carriage among 943 unimmunized individuals ages 2 months to 95 years found a carriage rate of 28% among 15- to 24-year-olds, compared with 9.6% overall.6
High hospitalization rates in US military recruits during 1964 to 1970 (25.2/100,000) led to the development of the meningococcal polysaccharide vaccine. Since 1971, all new military recruits have received polysaccharide meningococcal vaccine, and for the period 1990 to 1998 the hospitalization rate for meningococcal disease among active duty service members had decreased by 98% (to 0.51/100,000).7
Recommendations from others
The Advisory Committee on Immunization Practices,2 American Academy of Pediatrics,8 American Academy of Family Physicians,9 and American College Association10 recommendations are summarized in the TABLE. Recommendations for vaccination during meningococcal disease outbreaks can be found at www.cdc.gov.2
TABLE
Who should get vaccinated—and when
| TARGET POPULATION | VACCINE TYPE |
|---|---|
| Children 2–10 years at increased risk* | MPSV4† |
| Adolescents 11–12 years | MCV4 |
| Adolescents at high school entry or 15 years of age without prior vaccination | MCV4 |
| College freshmen planning to reside in dormitories | MCV4‡ |
| Patients ages 11–55 at increased risk* | MCV4‡ |
| Patients older than 55 years at increased risk* | MPSV4 |
| Microbiologist, lab personnel exposed to N meningitides | MCV4‡ |
| Military recruits | MCV4‡ |
| *“Increased risk” is defined by terminal complement deficiency, anatomic or functional asplenia, travel to endemic areas, HIV infection (optional). | |
| †May be repeated every 3 to 5 years if increased risk continues. | |
| ‡MPSV4 is an acceptable alternative. | |
| MPSV4, meningococcal polysaccharide vaccine; MCV4, meningococcal polysaccharide diphtheria toxoid conjugate vaccine. | |
| Adapted from Harrison, Clinical Microbiology Reviews 2006;1 Kimmel, Am Fam Physician 2005.9 | |
1. Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev 2006;19:142-164.
2. Bilukha OO, Rosenstein N. National Center for Infectious Diseases; Center for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54:1-21. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5407a1.htm. Accessed on April 19, 2007.
3. Bruce MG, Rosenstein NE, Capparella JM, et al. Risk factors for meningococcal disease in college students. JAMA 2001;286:688-693.
4. Shepard CW, Ortega-Sanchez IR, Scott II RD, Rosenstein NE, ABCs Team. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 2005;115:1220-1232.
5. Ramsay ME, Andrews NJ, Trotter CL, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: data analysis. Br Med J 2003;326:365-366.
6. Caugant DA, Hoiby EA, Magnus P, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiology 1994;32:323-330.
7. US Department of Health and Human Services. Meningococcal disease and college students: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2000;49(RR-07):11-20.
8. American Academy of Pediatrics Committee on Infectious Diseases. Prevention and control of meningococcal disease: recommendations for use of meningococcal vaccines in pediatric patients. Pediatrics 2005;116:496-505.
9. Kimmel SR. Prevention of meningococcal disease. Am Fam Physician 2005;72:2049-2056.
10. American College Health Association. ACHA guidelines: recommendations for institutional prematriculation immunizations, 2006. Available at www.acha.org/info_resources/RIPIstatement.pdf. Accessed on April 3, 2007.
Routine vaccination with the meningococcal conjugate vaccine MCV4 (Menactra) is indicated for all US adolescents entering high school and for college freshmen living in dormitories (strength of recommendation [SOR]: B, based on observational studies). For convenience, MCV4 can be given at the 11- to 12-year-old visit.
High-risk individuals (ages 2 and older) who should receive meningococcal vaccine (MCV4 or the unconjugated polysaccharide vaccine [MPSV4]) include those with terminal complement deficiencies, asplenia, or HIV, as well as military recruits, laboratory personnel exposed to aerosolized meningococci, and travelers to areas hyperendemic or epidemic for Neisseria meningitides (SOR: C, based on consensus guidelines). Routine vaccination of infants and toddlers with conjugate vaccine may be more cost-effective than targeting adolescents, but conjugate meningococcal vaccine for this age group is not yet available in the US (SOR: B, based on cohort studies).
The vaccine is available and efficacious—use it well
Mark Stephens, MD
Uniformed Services University, Bethesda, Md
As a junior military medical officer, my first assignment was in San Diego, California near the former Naval Training Center (NTC). The NTC was the site of one of the last major outbreaks of meningococcal disease in a military barracks setting. I recall with alacrity the rapidity with which this disease overcomes its host, and the overwhelming morbidity (and mortality) the disease leaves behind if treatment is delayed. This is truly a “not-to-be-missed” diagnosis.
The historical parallels between smallpox and meningococcal disease are striking. Each is spread primarily by respiratory means, particularly in close quarters. While meningococcal disease is amenable to antibiotic treatment when recognized early (contrary to smallpox), the principles of high-risk “herd” immunization hold true for both conditions. By focusing on high-risk groups and adhering to ACIP recommendations, control of meningococcal disease is within the grasp of modern medical science. The vaccine is available. The vaccine is efficacious. Use it well.
Evidence summary
Two meningococcal vaccines are currently available in the US: tetravalent polysaccharide vaccine (MPSV4) and tetravalent polysaccharide-protein conjugate vaccine (MCV4). Both protect against serogroups A, C, Y, and W-135, but not against serogroup B, which is the most prevalent. A vaccine for serogroup B is under development.
MPSV4 is licensed for ages 2 years and up, but its poor immunogenicity in infants, lack of memory and booster response, and relatively short duration of protection have restricted its use. MCV4 is licensed for 11- to 55-year-olds and is the preferred vaccine in this age group, since it provides longer duration of immunity and reduces nasopharyngeal carriage.1
Infants and freshman are especially vulnerable
Using active community surveillance from 1991 to 2002, Centers for Disease Control and Prevention (CDC) data2 found annual rates of meningococcal disease in the US of 0.5 to 1.1 per 100,000. The highest rates were found in children under age 2. Infants younger than 12 months of age were especially vulnerable (rate 9/100,000), with more than 50% of cases caused by serogroup B.
A 1998–1999 prospective surveillance study3 including 50 state health departments and 231 college health centers identified 96 cases of meningococcal disease in college students (incidence 0.7/100,000). Freshmen living in dormitories had an elevated risk of meningococcal disease compared with other undergraduates or nonstudents of the same age (incidence 5.1/100,000; adjusted relative risk=3.6 [95% confidence interval [CI], 1.6–8.5). Sixty-eight percent had illness due to a vaccine-preventable serogroup.
Using CDC incidence data, a cost-effectiveness model4 compared hypothetical vaccination strategies targeting US infants (3 doses), toddlers (1 dose), or 11-year-olds (1 dose). Routine MCV4 vaccination of all 11-year-olds would prevent 270 cases and 36 deaths in this cohort over their next 22 years. For a toddler cohort, vaccination would prevent 385 cases and 33 deaths; for infants, 447 cases and 36 deaths. Conjugate meningococcal vaccines for serogroups A and C have been tested and used in children in other countries, and appear safe and effective, but are not yet available in the US. An application has been submitted for FDA approval of MCV4 for 2- to 10-year-olds.
Herd immunity may expand benefit of vaccination
A British study compared attack rates for meningococcal C disease in children from infancy to age 18 before and 1 to 2 years after the institution of a nationwide meningococcal serogroup C conjugate vaccination. Vaccine coverage ranged from 66% (adolescents) to 87% (schoolchildren), and vaccine efficacy was 94% to 96%. Incidence of meningococcal C disease in the unvaccinated children also decreased by 52% to 67% (from 4.08/100,000 to 1.36/100,000).5
Vaccinating adolescents may be particularly helpful for building herd immunity. A Norwegian study of nasopharyngeal meningococcal carriage among 943 unimmunized individuals ages 2 months to 95 years found a carriage rate of 28% among 15- to 24-year-olds, compared with 9.6% overall.6
High hospitalization rates in US military recruits during 1964 to 1970 (25.2/100,000) led to the development of the meningococcal polysaccharide vaccine. Since 1971, all new military recruits have received polysaccharide meningococcal vaccine, and for the period 1990 to 1998 the hospitalization rate for meningococcal disease among active duty service members had decreased by 98% (to 0.51/100,000).7
Recommendations from others
The Advisory Committee on Immunization Practices,2 American Academy of Pediatrics,8 American Academy of Family Physicians,9 and American College Association10 recommendations are summarized in the TABLE. Recommendations for vaccination during meningococcal disease outbreaks can be found at www.cdc.gov.2
TABLE
Who should get vaccinated—and when
| TARGET POPULATION | VACCINE TYPE |
|---|---|
| Children 2–10 years at increased risk* | MPSV4† |
| Adolescents 11–12 years | MCV4 |
| Adolescents at high school entry or 15 years of age without prior vaccination | MCV4 |
| College freshmen planning to reside in dormitories | MCV4‡ |
| Patients ages 11–55 at increased risk* | MCV4‡ |
| Patients older than 55 years at increased risk* | MPSV4 |
| Microbiologist, lab personnel exposed to N meningitides | MCV4‡ |
| Military recruits | MCV4‡ |
| *“Increased risk” is defined by terminal complement deficiency, anatomic or functional asplenia, travel to endemic areas, HIV infection (optional). | |
| †May be repeated every 3 to 5 years if increased risk continues. | |
| ‡MPSV4 is an acceptable alternative. | |
| MPSV4, meningococcal polysaccharide vaccine; MCV4, meningococcal polysaccharide diphtheria toxoid conjugate vaccine. | |
| Adapted from Harrison, Clinical Microbiology Reviews 2006;1 Kimmel, Am Fam Physician 2005.9 | |
Routine vaccination with the meningococcal conjugate vaccine MCV4 (Menactra) is indicated for all US adolescents entering high school and for college freshmen living in dormitories (strength of recommendation [SOR]: B, based on observational studies). For convenience, MCV4 can be given at the 11- to 12-year-old visit.
High-risk individuals (ages 2 and older) who should receive meningococcal vaccine (MCV4 or the unconjugated polysaccharide vaccine [MPSV4]) include those with terminal complement deficiencies, asplenia, or HIV, as well as military recruits, laboratory personnel exposed to aerosolized meningococci, and travelers to areas hyperendemic or epidemic for Neisseria meningitides (SOR: C, based on consensus guidelines). Routine vaccination of infants and toddlers with conjugate vaccine may be more cost-effective than targeting adolescents, but conjugate meningococcal vaccine for this age group is not yet available in the US (SOR: B, based on cohort studies).
The vaccine is available and efficacious—use it well
Mark Stephens, MD
Uniformed Services University, Bethesda, Md
As a junior military medical officer, my first assignment was in San Diego, California near the former Naval Training Center (NTC). The NTC was the site of one of the last major outbreaks of meningococcal disease in a military barracks setting. I recall with alacrity the rapidity with which this disease overcomes its host, and the overwhelming morbidity (and mortality) the disease leaves behind if treatment is delayed. This is truly a “not-to-be-missed” diagnosis.
The historical parallels between smallpox and meningococcal disease are striking. Each is spread primarily by respiratory means, particularly in close quarters. While meningococcal disease is amenable to antibiotic treatment when recognized early (contrary to smallpox), the principles of high-risk “herd” immunization hold true for both conditions. By focusing on high-risk groups and adhering to ACIP recommendations, control of meningococcal disease is within the grasp of modern medical science. The vaccine is available. The vaccine is efficacious. Use it well.
Evidence summary
Two meningococcal vaccines are currently available in the US: tetravalent polysaccharide vaccine (MPSV4) and tetravalent polysaccharide-protein conjugate vaccine (MCV4). Both protect against serogroups A, C, Y, and W-135, but not against serogroup B, which is the most prevalent. A vaccine for serogroup B is under development.
MPSV4 is licensed for ages 2 years and up, but its poor immunogenicity in infants, lack of memory and booster response, and relatively short duration of protection have restricted its use. MCV4 is licensed for 11- to 55-year-olds and is the preferred vaccine in this age group, since it provides longer duration of immunity and reduces nasopharyngeal carriage.1
Infants and freshman are especially vulnerable
Using active community surveillance from 1991 to 2002, Centers for Disease Control and Prevention (CDC) data2 found annual rates of meningococcal disease in the US of 0.5 to 1.1 per 100,000. The highest rates were found in children under age 2. Infants younger than 12 months of age were especially vulnerable (rate 9/100,000), with more than 50% of cases caused by serogroup B.
A 1998–1999 prospective surveillance study3 including 50 state health departments and 231 college health centers identified 96 cases of meningococcal disease in college students (incidence 0.7/100,000). Freshmen living in dormitories had an elevated risk of meningococcal disease compared with other undergraduates or nonstudents of the same age (incidence 5.1/100,000; adjusted relative risk=3.6 [95% confidence interval [CI], 1.6–8.5). Sixty-eight percent had illness due to a vaccine-preventable serogroup.
Using CDC incidence data, a cost-effectiveness model4 compared hypothetical vaccination strategies targeting US infants (3 doses), toddlers (1 dose), or 11-year-olds (1 dose). Routine MCV4 vaccination of all 11-year-olds would prevent 270 cases and 36 deaths in this cohort over their next 22 years. For a toddler cohort, vaccination would prevent 385 cases and 33 deaths; for infants, 447 cases and 36 deaths. Conjugate meningococcal vaccines for serogroups A and C have been tested and used in children in other countries, and appear safe and effective, but are not yet available in the US. An application has been submitted for FDA approval of MCV4 for 2- to 10-year-olds.
Herd immunity may expand benefit of vaccination
A British study compared attack rates for meningococcal C disease in children from infancy to age 18 before and 1 to 2 years after the institution of a nationwide meningococcal serogroup C conjugate vaccination. Vaccine coverage ranged from 66% (adolescents) to 87% (schoolchildren), and vaccine efficacy was 94% to 96%. Incidence of meningococcal C disease in the unvaccinated children also decreased by 52% to 67% (from 4.08/100,000 to 1.36/100,000).5
Vaccinating adolescents may be particularly helpful for building herd immunity. A Norwegian study of nasopharyngeal meningococcal carriage among 943 unimmunized individuals ages 2 months to 95 years found a carriage rate of 28% among 15- to 24-year-olds, compared with 9.6% overall.6
High hospitalization rates in US military recruits during 1964 to 1970 (25.2/100,000) led to the development of the meningococcal polysaccharide vaccine. Since 1971, all new military recruits have received polysaccharide meningococcal vaccine, and for the period 1990 to 1998 the hospitalization rate for meningococcal disease among active duty service members had decreased by 98% (to 0.51/100,000).7
Recommendations from others
The Advisory Committee on Immunization Practices,2 American Academy of Pediatrics,8 American Academy of Family Physicians,9 and American College Association10 recommendations are summarized in the TABLE. Recommendations for vaccination during meningococcal disease outbreaks can be found at www.cdc.gov.2
TABLE
Who should get vaccinated—and when
| TARGET POPULATION | VACCINE TYPE |
|---|---|
| Children 2–10 years at increased risk* | MPSV4† |
| Adolescents 11–12 years | MCV4 |
| Adolescents at high school entry or 15 years of age without prior vaccination | MCV4 |
| College freshmen planning to reside in dormitories | MCV4‡ |
| Patients ages 11–55 at increased risk* | MCV4‡ |
| Patients older than 55 years at increased risk* | MPSV4 |
| Microbiologist, lab personnel exposed to N meningitides | MCV4‡ |
| Military recruits | MCV4‡ |
| *“Increased risk” is defined by terminal complement deficiency, anatomic or functional asplenia, travel to endemic areas, HIV infection (optional). | |
| †May be repeated every 3 to 5 years if increased risk continues. | |
| ‡MPSV4 is an acceptable alternative. | |
| MPSV4, meningococcal polysaccharide vaccine; MCV4, meningococcal polysaccharide diphtheria toxoid conjugate vaccine. | |
| Adapted from Harrison, Clinical Microbiology Reviews 2006;1 Kimmel, Am Fam Physician 2005.9 | |
1. Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev 2006;19:142-164.
2. Bilukha OO, Rosenstein N. National Center for Infectious Diseases; Center for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54:1-21. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5407a1.htm. Accessed on April 19, 2007.
3. Bruce MG, Rosenstein NE, Capparella JM, et al. Risk factors for meningococcal disease in college students. JAMA 2001;286:688-693.
4. Shepard CW, Ortega-Sanchez IR, Scott II RD, Rosenstein NE, ABCs Team. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 2005;115:1220-1232.
5. Ramsay ME, Andrews NJ, Trotter CL, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: data analysis. Br Med J 2003;326:365-366.
6. Caugant DA, Hoiby EA, Magnus P, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiology 1994;32:323-330.
7. US Department of Health and Human Services. Meningococcal disease and college students: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2000;49(RR-07):11-20.
8. American Academy of Pediatrics Committee on Infectious Diseases. Prevention and control of meningococcal disease: recommendations for use of meningococcal vaccines in pediatric patients. Pediatrics 2005;116:496-505.
9. Kimmel SR. Prevention of meningococcal disease. Am Fam Physician 2005;72:2049-2056.
10. American College Health Association. ACHA guidelines: recommendations for institutional prematriculation immunizations, 2006. Available at www.acha.org/info_resources/RIPIstatement.pdf. Accessed on April 3, 2007.
1. Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev 2006;19:142-164.
2. Bilukha OO, Rosenstein N. National Center for Infectious Diseases; Center for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54:1-21. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5407a1.htm. Accessed on April 19, 2007.
3. Bruce MG, Rosenstein NE, Capparella JM, et al. Risk factors for meningococcal disease in college students. JAMA 2001;286:688-693.
4. Shepard CW, Ortega-Sanchez IR, Scott II RD, Rosenstein NE, ABCs Team. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 2005;115:1220-1232.
5. Ramsay ME, Andrews NJ, Trotter CL, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: data analysis. Br Med J 2003;326:365-366.
6. Caugant DA, Hoiby EA, Magnus P, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiology 1994;32:323-330.
7. US Department of Health and Human Services. Meningococcal disease and college students: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2000;49(RR-07):11-20.
8. American Academy of Pediatrics Committee on Infectious Diseases. Prevention and control of meningococcal disease: recommendations for use of meningococcal vaccines in pediatric patients. Pediatrics 2005;116:496-505.
9. Kimmel SR. Prevention of meningococcal disease. Am Fam Physician 2005;72:2049-2056.
10. American College Health Association. ACHA guidelines: recommendations for institutional prematriculation immunizations, 2006. Available at www.acha.org/info_resources/RIPIstatement.pdf. Accessed on April 3, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
Can you differentiate bacterial from viral pediatric infections based on the CBC?
No—the complete blood count (CBC) alone does not have adequate sensitivity or specificity to tell bacterial from viral infections (strength of recommendation [SOR]: B, cohort studies). When used in conjunction with other clinical parameters in validated decision-making algorithms, the CBC can help detect serious bacterial infections in pediatric patients with fever (SOR: B, cohort studies).
There’s no substitute for history, physical exam, and good judgment
John D. Hallgren, MD
Uniformed Services University of the Health Sciences, RAF Menwith Hill, United Kingdom
Viral vs bacterial—often these are surrogate terms for minor vs serious illness. This review is a great lesson in likelihood ratios. Based on the low likelihood ratio, a CBC alone does not shift our suspicion greatly for serious bacterial infections in intermediate-risk patients; however, if you combine it with a clinical decision rule, it can greatly help decision-making, as evidenced by negative predictive values of 99% and above.
In contrast, we don’t need the CBC to tell us that an adult with the sniffles has a rhino/corona/whatevervirus, nor do we need it to tell us that a febrile, lethargic child with a petechial rash has a life-threatening bacteremia. If you enjoy the muck and the mess of primary care as much as I do, this inquiry should provide you with the validation that there’s no substitute for the history, physical exam, and judgment of a good clinician.
Evidence summary
For acutely febrile patients, the presence of an elevated white blood cell (WBC) count with elevated band forms has dogmatically been thought of as a marker for bacterial infection.1 Current literature, however, does not support this.2
Neisseria meningitides
A retrospective study of 5353 infants ages 3 to 89 days presenting to the emergency department for evaluation of fever showed that 3 of 4 infants ultimately diagnosed with bacterial meningitis would have been missed if the WBC count alone were used to predict which infants need a lumbar puncture.3 A prospective study of 2492 children ages 3 to 24 months presenting to the emergency department with acute fever and an absolute WBC count >15,000/mm3 revealed that neither a polymorphonuclear count of >10,000/mm3 (>66% segmented forms) nor a band count of >500/mm3 was associated with an increased likelihood of occult bacterial infection.4 Other studies show that the WBC alone is poorly discriminatory for identifying either bacteremia or meningitis.5,6
To improve the diagnostic utility of the CBC, other studies have examined individual components of the white blood cell differential count (TABLE 1). In particular, the use of the absolute neutrophil count (ANC) has been proposed as a superior marker of serious bacterial infection.7 A review of 6579 outpatients aged 3 to 36 months presenting to the emergency department with temperatures of 39°C or higher showed an ANC of >10,000/mm3 as more predictive of occult pneumococcal bacteremia than an elevated WBC count (>15,000/mm3) alone.8 Another retrospective review of more than 10,000 patients aged 3 to 36 months presenting to the emergency department used logistic regression to identify predictors of bacteremia. In this study, ANC (>9500/mm3) and WBC (>14,300/mm3) were of equal sensitivity (75%) and specificity (75%) in identifying serious bacterial infection.9 Finally, the band count alone does not accurately predict serious bacterial infection.10
In summary, the CBC cannot be used in isolation to differentiate bacterial from viral illness. The CBC can, however, augment clinical data from the history and physical examination to predict the likelihood of serious bacterial illness. As a result, numerous diagnostic criteria, each incorporating elements of the CBC, have been developed in an attempt to accurately differentiate bacterial from viral illness in acutely febrile patients, most typically children (TABLE 2). These criteria differ by age of the patient, clinical testing recommendations, indications for antibiotic therapy, as well as WBC cutoffs.
TABLE 1
WBC markers: How good are they at predicting serious bacterial infection?9,18,19
| VARIABLE | CUTOFF | SENSITIVITY | SPECIFICITY | LR (95% CI) |
|---|---|---|---|---|
| White blood cell count | 15,000/mm3 | 64%–82% | 67%–75% | 1.9–2.7 (1.1–3.8) |
| Absolute neutrophil count | 10,000/mm3 | 64%–76% | 76%–81% | 3.0–3.3 (1.6–6.2) |
| LR, likelihood ratio; CI, confidence interval. | ||||
TABLE 2
Clinical criteria for predicting serious bacterial infection in febrile children
| CRITERION | ROCHESTER CRITERIA11 | BOSTON CRITERIA12 | PHILADELPHIA CRITERIA13 |
|---|---|---|---|
| Predictive value | 98.9% PV–in ruling out serious bacterial infection | 95% PV+ to identify serious bacterial infection | 100% PV–in ruling out serious bacterial infection |
| Age | <60 days | 1–3 mos Present to emergency dept. with fever ≥38.0°C | 29–56 days Present with fever ≥38.2°C |
| Appearance | Well-appearing Previously healthy No evidence of infection (skin, bone, joint, soft tissue or ear) | Healthy appearing No ear, soft tissue, joint or bone infection on exam | Well-appearing |
| White blood cell count | WBC 5–15,000/mm3 Bands ≤1,500/mm3 | Peripheral WBC ≤20,000/mm3 | WBC ≤15,000/mm3 Band-to-neutrophil ratio of ≤0.2 |
| Urinalysis | ≤10 WBC/hpf of centrifuged urine | Urinalysis ≤10 WBC/hpf | Urinalysis ≤10 WBC/hpf |
| Other tests | If diarrhea, ≤5 WBC/hpf of stool smear | CSF WBC ≤10/hpf | CSF WBC ≤8/hpf with negative gram stain If watery diarrhea, few or no WBC/hpf on stool smear |
| WBC, white blood cell count; hpf, high-powered field; CSF, cerebrospinal fluid; PV, predictive value | |||
Recommendations from others
The American College of Emergency Physicians recommends considering antibiotic therapy for previously healthy, well-appearing children ages 3 to 36 months who present with a fever without a clinical source and a WBC count >15,000/mm3.3,14
The University of Cincinnati Evidence-Based Clinical Practice Guidelines for fever of uncertain source in children ages 2 to 36 months recommends obtaining a CBC for any child who is ill-appearing or at high risk for bacteremia (determined by the clinicians’ judgment). A WBC of ≥15,000/mm3 or ANC >10,000/mm3 provide support for antibiotic therapy.15 The 1993 American Academy of Pediatrics guidelines for fever ≥39°C without a source in children ages 3 months to 3 years recommends a CBC; if the WBC count ≥15,000/mm3, they recommend a blood culture and treatment with antibiotics pending culture results.3,16
It is important to note that in the age of Haemophilus influenza and Streptococcus pneumonia vaccination, the rate of occult bacteremia in febrile children presenting without a source has fallen from 3% to 10% to 1% or less.17 A lower prevalence reduces the utility of routine CBC or blood culture in the evaluation of immunized, febrile children. Parameters such as procalcitonin, interleukin-6, interleukin-8, interleukin-1 receptor antagonist and C-reactive protein show future promise as biochemical markers for identifying serious bacterial infections.18
1. Wile MJ, Homer LD, Gaehler S, Phillips S, Millan J. Manual differential cell counts help predict bacterial infection. A multivariate analysis. Am J Clin Pathol 2001;115:644-649.
2. Seebach JD, Morant R, Ruegg R, Seifert B, Fehr J. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious disease. Am J Clin Pathol 1997;107:582-591.
3. Bonsu BK, Harper MB. Utility of the peripheral blood white blood cell count for identifying sick young infants who need lumbar puncture. Ann Emerg Med 2003;41:206-214.
4. Kramer MS, Tange SM, Mills EL, Ciampi A, Bernstein ML, Drummond KN. Role of the complete blood count in detecting occult focal bacterial infection in the young febrile child. J Clin Epidemiol 1993;46:349-357.
5. Brown L, Shaw T, Wittlake WA. Does leucocytosis identify bacterial infections in febrile neonates presenting to the emergency department? Emerg Med J 2005;22:256-259.
6. Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics 2006;117:1094-1100.
7. Gombos MM, Bienkowski RS, Gochman RF, Billett HH. The absolute neutrophil count: is it the best indicator for occult bacteremia in infants? Am J Clin Pathol 1998;109:221-225.
8. Kuppermann N, Fleisher GR, Jaffe DM. Predictors of occult pneumococcal bacteremia in young febrile children. Ann Emerg Med 1998;31:679-687.
9. Isaacman DJ, Shults J, Gross TK, Davis PH, Harper M. Predictors of bacteremia in febrile children 3 to 36 months of age. Pediatrics 2000;106:977-982.
10. Cornbleet PJ. Clinical utility of the band count. Clin Lab Med 2002;22:101-136.
11. Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr 1985;107:855-860.
12. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr 1992;120:22-27.
13. Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics 1999;103:627-631.
14. American College of emergency Physicians. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med 2003;42:530-545.
15. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical practice guideline for fever of uncertain source in children in 2 to 36 months of age. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2003.
16. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med 1993;22:1198-1210.
17. Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine. Arch Pediatr Adolesc Med 2004;158:671-675.
18. Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics 2001;108:1275-1279.
19. Pratt A, Attia MW. Duration of fever and markers of serious bacterial infection in young febrile children. Pediatr Int 2007;49:31-35.
No—the complete blood count (CBC) alone does not have adequate sensitivity or specificity to tell bacterial from viral infections (strength of recommendation [SOR]: B, cohort studies). When used in conjunction with other clinical parameters in validated decision-making algorithms, the CBC can help detect serious bacterial infections in pediatric patients with fever (SOR: B, cohort studies).
There’s no substitute for history, physical exam, and good judgment
John D. Hallgren, MD
Uniformed Services University of the Health Sciences, RAF Menwith Hill, United Kingdom
Viral vs bacterial—often these are surrogate terms for minor vs serious illness. This review is a great lesson in likelihood ratios. Based on the low likelihood ratio, a CBC alone does not shift our suspicion greatly for serious bacterial infections in intermediate-risk patients; however, if you combine it with a clinical decision rule, it can greatly help decision-making, as evidenced by negative predictive values of 99% and above.
In contrast, we don’t need the CBC to tell us that an adult with the sniffles has a rhino/corona/whatevervirus, nor do we need it to tell us that a febrile, lethargic child with a petechial rash has a life-threatening bacteremia. If you enjoy the muck and the mess of primary care as much as I do, this inquiry should provide you with the validation that there’s no substitute for the history, physical exam, and judgment of a good clinician.
Evidence summary
For acutely febrile patients, the presence of an elevated white blood cell (WBC) count with elevated band forms has dogmatically been thought of as a marker for bacterial infection.1 Current literature, however, does not support this.2
Neisseria meningitides
A retrospective study of 5353 infants ages 3 to 89 days presenting to the emergency department for evaluation of fever showed that 3 of 4 infants ultimately diagnosed with bacterial meningitis would have been missed if the WBC count alone were used to predict which infants need a lumbar puncture.3 A prospective study of 2492 children ages 3 to 24 months presenting to the emergency department with acute fever and an absolute WBC count >15,000/mm3 revealed that neither a polymorphonuclear count of >10,000/mm3 (>66% segmented forms) nor a band count of >500/mm3 was associated with an increased likelihood of occult bacterial infection.4 Other studies show that the WBC alone is poorly discriminatory for identifying either bacteremia or meningitis.5,6
To improve the diagnostic utility of the CBC, other studies have examined individual components of the white blood cell differential count (TABLE 1). In particular, the use of the absolute neutrophil count (ANC) has been proposed as a superior marker of serious bacterial infection.7 A review of 6579 outpatients aged 3 to 36 months presenting to the emergency department with temperatures of 39°C or higher showed an ANC of >10,000/mm3 as more predictive of occult pneumococcal bacteremia than an elevated WBC count (>15,000/mm3) alone.8 Another retrospective review of more than 10,000 patients aged 3 to 36 months presenting to the emergency department used logistic regression to identify predictors of bacteremia. In this study, ANC (>9500/mm3) and WBC (>14,300/mm3) were of equal sensitivity (75%) and specificity (75%) in identifying serious bacterial infection.9 Finally, the band count alone does not accurately predict serious bacterial infection.10
In summary, the CBC cannot be used in isolation to differentiate bacterial from viral illness. The CBC can, however, augment clinical data from the history and physical examination to predict the likelihood of serious bacterial illness. As a result, numerous diagnostic criteria, each incorporating elements of the CBC, have been developed in an attempt to accurately differentiate bacterial from viral illness in acutely febrile patients, most typically children (TABLE 2). These criteria differ by age of the patient, clinical testing recommendations, indications for antibiotic therapy, as well as WBC cutoffs.
TABLE 1
WBC markers: How good are they at predicting serious bacterial infection?9,18,19
| VARIABLE | CUTOFF | SENSITIVITY | SPECIFICITY | LR (95% CI) |
|---|---|---|---|---|
| White blood cell count | 15,000/mm3 | 64%–82% | 67%–75% | 1.9–2.7 (1.1–3.8) |
| Absolute neutrophil count | 10,000/mm3 | 64%–76% | 76%–81% | 3.0–3.3 (1.6–6.2) |
| LR, likelihood ratio; CI, confidence interval. | ||||
TABLE 2
Clinical criteria for predicting serious bacterial infection in febrile children
| CRITERION | ROCHESTER CRITERIA11 | BOSTON CRITERIA12 | PHILADELPHIA CRITERIA13 |
|---|---|---|---|
| Predictive value | 98.9% PV–in ruling out serious bacterial infection | 95% PV+ to identify serious bacterial infection | 100% PV–in ruling out serious bacterial infection |
| Age | <60 days | 1–3 mos Present to emergency dept. with fever ≥38.0°C | 29–56 days Present with fever ≥38.2°C |
| Appearance | Well-appearing Previously healthy No evidence of infection (skin, bone, joint, soft tissue or ear) | Healthy appearing No ear, soft tissue, joint or bone infection on exam | Well-appearing |
| White blood cell count | WBC 5–15,000/mm3 Bands ≤1,500/mm3 | Peripheral WBC ≤20,000/mm3 | WBC ≤15,000/mm3 Band-to-neutrophil ratio of ≤0.2 |
| Urinalysis | ≤10 WBC/hpf of centrifuged urine | Urinalysis ≤10 WBC/hpf | Urinalysis ≤10 WBC/hpf |
| Other tests | If diarrhea, ≤5 WBC/hpf of stool smear | CSF WBC ≤10/hpf | CSF WBC ≤8/hpf with negative gram stain If watery diarrhea, few or no WBC/hpf on stool smear |
| WBC, white blood cell count; hpf, high-powered field; CSF, cerebrospinal fluid; PV, predictive value | |||
Recommendations from others
The American College of Emergency Physicians recommends considering antibiotic therapy for previously healthy, well-appearing children ages 3 to 36 months who present with a fever without a clinical source and a WBC count >15,000/mm3.3,14
The University of Cincinnati Evidence-Based Clinical Practice Guidelines for fever of uncertain source in children ages 2 to 36 months recommends obtaining a CBC for any child who is ill-appearing or at high risk for bacteremia (determined by the clinicians’ judgment). A WBC of ≥15,000/mm3 or ANC >10,000/mm3 provide support for antibiotic therapy.15 The 1993 American Academy of Pediatrics guidelines for fever ≥39°C without a source in children ages 3 months to 3 years recommends a CBC; if the WBC count ≥15,000/mm3, they recommend a blood culture and treatment with antibiotics pending culture results.3,16
It is important to note that in the age of Haemophilus influenza and Streptococcus pneumonia vaccination, the rate of occult bacteremia in febrile children presenting without a source has fallen from 3% to 10% to 1% or less.17 A lower prevalence reduces the utility of routine CBC or blood culture in the evaluation of immunized, febrile children. Parameters such as procalcitonin, interleukin-6, interleukin-8, interleukin-1 receptor antagonist and C-reactive protein show future promise as biochemical markers for identifying serious bacterial infections.18
No—the complete blood count (CBC) alone does not have adequate sensitivity or specificity to tell bacterial from viral infections (strength of recommendation [SOR]: B, cohort studies). When used in conjunction with other clinical parameters in validated decision-making algorithms, the CBC can help detect serious bacterial infections in pediatric patients with fever (SOR: B, cohort studies).
There’s no substitute for history, physical exam, and good judgment
John D. Hallgren, MD
Uniformed Services University of the Health Sciences, RAF Menwith Hill, United Kingdom
Viral vs bacterial—often these are surrogate terms for minor vs serious illness. This review is a great lesson in likelihood ratios. Based on the low likelihood ratio, a CBC alone does not shift our suspicion greatly for serious bacterial infections in intermediate-risk patients; however, if you combine it with a clinical decision rule, it can greatly help decision-making, as evidenced by negative predictive values of 99% and above.
In contrast, we don’t need the CBC to tell us that an adult with the sniffles has a rhino/corona/whatevervirus, nor do we need it to tell us that a febrile, lethargic child with a petechial rash has a life-threatening bacteremia. If you enjoy the muck and the mess of primary care as much as I do, this inquiry should provide you with the validation that there’s no substitute for the history, physical exam, and judgment of a good clinician.
Evidence summary
For acutely febrile patients, the presence of an elevated white blood cell (WBC) count with elevated band forms has dogmatically been thought of as a marker for bacterial infection.1 Current literature, however, does not support this.2
Neisseria meningitides
A retrospective study of 5353 infants ages 3 to 89 days presenting to the emergency department for evaluation of fever showed that 3 of 4 infants ultimately diagnosed with bacterial meningitis would have been missed if the WBC count alone were used to predict which infants need a lumbar puncture.3 A prospective study of 2492 children ages 3 to 24 months presenting to the emergency department with acute fever and an absolute WBC count >15,000/mm3 revealed that neither a polymorphonuclear count of >10,000/mm3 (>66% segmented forms) nor a band count of >500/mm3 was associated with an increased likelihood of occult bacterial infection.4 Other studies show that the WBC alone is poorly discriminatory for identifying either bacteremia or meningitis.5,6
To improve the diagnostic utility of the CBC, other studies have examined individual components of the white blood cell differential count (TABLE 1). In particular, the use of the absolute neutrophil count (ANC) has been proposed as a superior marker of serious bacterial infection.7 A review of 6579 outpatients aged 3 to 36 months presenting to the emergency department with temperatures of 39°C or higher showed an ANC of >10,000/mm3 as more predictive of occult pneumococcal bacteremia than an elevated WBC count (>15,000/mm3) alone.8 Another retrospective review of more than 10,000 patients aged 3 to 36 months presenting to the emergency department used logistic regression to identify predictors of bacteremia. In this study, ANC (>9500/mm3) and WBC (>14,300/mm3) were of equal sensitivity (75%) and specificity (75%) in identifying serious bacterial infection.9 Finally, the band count alone does not accurately predict serious bacterial infection.10
In summary, the CBC cannot be used in isolation to differentiate bacterial from viral illness. The CBC can, however, augment clinical data from the history and physical examination to predict the likelihood of serious bacterial illness. As a result, numerous diagnostic criteria, each incorporating elements of the CBC, have been developed in an attempt to accurately differentiate bacterial from viral illness in acutely febrile patients, most typically children (TABLE 2). These criteria differ by age of the patient, clinical testing recommendations, indications for antibiotic therapy, as well as WBC cutoffs.
TABLE 1
WBC markers: How good are they at predicting serious bacterial infection?9,18,19
| VARIABLE | CUTOFF | SENSITIVITY | SPECIFICITY | LR (95% CI) |
|---|---|---|---|---|
| White blood cell count | 15,000/mm3 | 64%–82% | 67%–75% | 1.9–2.7 (1.1–3.8) |
| Absolute neutrophil count | 10,000/mm3 | 64%–76% | 76%–81% | 3.0–3.3 (1.6–6.2) |
| LR, likelihood ratio; CI, confidence interval. | ||||
TABLE 2
Clinical criteria for predicting serious bacterial infection in febrile children
| CRITERION | ROCHESTER CRITERIA11 | BOSTON CRITERIA12 | PHILADELPHIA CRITERIA13 |
|---|---|---|---|
| Predictive value | 98.9% PV–in ruling out serious bacterial infection | 95% PV+ to identify serious bacterial infection | 100% PV–in ruling out serious bacterial infection |
| Age | <60 days | 1–3 mos Present to emergency dept. with fever ≥38.0°C | 29–56 days Present with fever ≥38.2°C |
| Appearance | Well-appearing Previously healthy No evidence of infection (skin, bone, joint, soft tissue or ear) | Healthy appearing No ear, soft tissue, joint or bone infection on exam | Well-appearing |
| White blood cell count | WBC 5–15,000/mm3 Bands ≤1,500/mm3 | Peripheral WBC ≤20,000/mm3 | WBC ≤15,000/mm3 Band-to-neutrophil ratio of ≤0.2 |
| Urinalysis | ≤10 WBC/hpf of centrifuged urine | Urinalysis ≤10 WBC/hpf | Urinalysis ≤10 WBC/hpf |
| Other tests | If diarrhea, ≤5 WBC/hpf of stool smear | CSF WBC ≤10/hpf | CSF WBC ≤8/hpf with negative gram stain If watery diarrhea, few or no WBC/hpf on stool smear |
| WBC, white blood cell count; hpf, high-powered field; CSF, cerebrospinal fluid; PV, predictive value | |||
Recommendations from others
The American College of Emergency Physicians recommends considering antibiotic therapy for previously healthy, well-appearing children ages 3 to 36 months who present with a fever without a clinical source and a WBC count >15,000/mm3.3,14
The University of Cincinnati Evidence-Based Clinical Practice Guidelines for fever of uncertain source in children ages 2 to 36 months recommends obtaining a CBC for any child who is ill-appearing or at high risk for bacteremia (determined by the clinicians’ judgment). A WBC of ≥15,000/mm3 or ANC >10,000/mm3 provide support for antibiotic therapy.15 The 1993 American Academy of Pediatrics guidelines for fever ≥39°C without a source in children ages 3 months to 3 years recommends a CBC; if the WBC count ≥15,000/mm3, they recommend a blood culture and treatment with antibiotics pending culture results.3,16
It is important to note that in the age of Haemophilus influenza and Streptococcus pneumonia vaccination, the rate of occult bacteremia in febrile children presenting without a source has fallen from 3% to 10% to 1% or less.17 A lower prevalence reduces the utility of routine CBC or blood culture in the evaluation of immunized, febrile children. Parameters such as procalcitonin, interleukin-6, interleukin-8, interleukin-1 receptor antagonist and C-reactive protein show future promise as biochemical markers for identifying serious bacterial infections.18
1. Wile MJ, Homer LD, Gaehler S, Phillips S, Millan J. Manual differential cell counts help predict bacterial infection. A multivariate analysis. Am J Clin Pathol 2001;115:644-649.
2. Seebach JD, Morant R, Ruegg R, Seifert B, Fehr J. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious disease. Am J Clin Pathol 1997;107:582-591.
3. Bonsu BK, Harper MB. Utility of the peripheral blood white blood cell count for identifying sick young infants who need lumbar puncture. Ann Emerg Med 2003;41:206-214.
4. Kramer MS, Tange SM, Mills EL, Ciampi A, Bernstein ML, Drummond KN. Role of the complete blood count in detecting occult focal bacterial infection in the young febrile child. J Clin Epidemiol 1993;46:349-357.
5. Brown L, Shaw T, Wittlake WA. Does leucocytosis identify bacterial infections in febrile neonates presenting to the emergency department? Emerg Med J 2005;22:256-259.
6. Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics 2006;117:1094-1100.
7. Gombos MM, Bienkowski RS, Gochman RF, Billett HH. The absolute neutrophil count: is it the best indicator for occult bacteremia in infants? Am J Clin Pathol 1998;109:221-225.
8. Kuppermann N, Fleisher GR, Jaffe DM. Predictors of occult pneumococcal bacteremia in young febrile children. Ann Emerg Med 1998;31:679-687.
9. Isaacman DJ, Shults J, Gross TK, Davis PH, Harper M. Predictors of bacteremia in febrile children 3 to 36 months of age. Pediatrics 2000;106:977-982.
10. Cornbleet PJ. Clinical utility of the band count. Clin Lab Med 2002;22:101-136.
11. Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr 1985;107:855-860.
12. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr 1992;120:22-27.
13. Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics 1999;103:627-631.
14. American College of emergency Physicians. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med 2003;42:530-545.
15. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical practice guideline for fever of uncertain source in children in 2 to 36 months of age. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2003.
16. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med 1993;22:1198-1210.
17. Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine. Arch Pediatr Adolesc Med 2004;158:671-675.
18. Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics 2001;108:1275-1279.
19. Pratt A, Attia MW. Duration of fever and markers of serious bacterial infection in young febrile children. Pediatr Int 2007;49:31-35.
1. Wile MJ, Homer LD, Gaehler S, Phillips S, Millan J. Manual differential cell counts help predict bacterial infection. A multivariate analysis. Am J Clin Pathol 2001;115:644-649.
2. Seebach JD, Morant R, Ruegg R, Seifert B, Fehr J. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious disease. Am J Clin Pathol 1997;107:582-591.
3. Bonsu BK, Harper MB. Utility of the peripheral blood white blood cell count for identifying sick young infants who need lumbar puncture. Ann Emerg Med 2003;41:206-214.
4. Kramer MS, Tange SM, Mills EL, Ciampi A, Bernstein ML, Drummond KN. Role of the complete blood count in detecting occult focal bacterial infection in the young febrile child. J Clin Epidemiol 1993;46:349-357.
5. Brown L, Shaw T, Wittlake WA. Does leucocytosis identify bacterial infections in febrile neonates presenting to the emergency department? Emerg Med J 2005;22:256-259.
6. Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics 2006;117:1094-1100.
7. Gombos MM, Bienkowski RS, Gochman RF, Billett HH. The absolute neutrophil count: is it the best indicator for occult bacteremia in infants? Am J Clin Pathol 1998;109:221-225.
8. Kuppermann N, Fleisher GR, Jaffe DM. Predictors of occult pneumococcal bacteremia in young febrile children. Ann Emerg Med 1998;31:679-687.
9. Isaacman DJ, Shults J, Gross TK, Davis PH, Harper M. Predictors of bacteremia in febrile children 3 to 36 months of age. Pediatrics 2000;106:977-982.
10. Cornbleet PJ. Clinical utility of the band count. Clin Lab Med 2002;22:101-136.
11. Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr 1985;107:855-860.
12. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr 1992;120:22-27.
13. Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics 1999;103:627-631.
14. American College of emergency Physicians. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med 2003;42:530-545.
15. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical practice guideline for fever of uncertain source in children in 2 to 36 months of age. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2003.
16. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med 1993;22:1198-1210.
17. Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine. Arch Pediatr Adolesc Med 2004;158:671-675.
18. Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics 2001;108:1275-1279.
19. Pratt A, Attia MW. Duration of fever and markers of serious bacterial infection in young febrile children. Pediatr Int 2007;49:31-35.
Evidence-based answers from the Family Physicians Inquiries Network
Are any alternative therapies effective in treating asthma?
Yes, some are. Acupuncture relieves subjective symptoms of asthma and reduces medication use in mild to moderate asthma (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials [RCTs] of variable quality). Herbal medications, such as Ginkgo biloba, appear to improve lung function, while herbs such as Tylophora indica and Tsumura saiboku-to may decrease asthma symptoms (SOR: B, based on systematic review of RCTs with poor methodology). No evidence, however, supports the use of room air ionizers, manual therapy, homeopathy, or mind-body therapy for treatment of asthma (SOR: A, based on systematic reviews and meta-analyses of RCTs and individual RCTs).
Though this research is interesting, we should adhere to current guidelines
Vincent Lo, MD
San Joaquin General Hospital, French Camp, Calif
Guidelines for the diagnosis and management of asthma are widely disseminated by the National Asthma Education and Prevention Program through its Expert Panel Reports (updated in 2002).1 Nevertheless, nearly 500,000 hospitalizations, 2 million emergency department visits, and 5000 deaths were reported annually in the US among those who have asthma.2 Furthermore, a significant difference in asthma prevalence, health care use, and mortality was found among different ethnic groups.1
Poor patient understanding of asthma control, nonadherence to medication regimens, cultural beliefs, and disparity of access to the health care system, together with physicians’ lack of close monitoring and inadequate compliance with national asthma guidelines, contribute to suboptimal control of chronic asthma. Family physicians must guide and empower their patients with the knowledge and responsibility of how to manage their asthma. For now, we should adhere to current national guidelines of management of asthma and avoid routine recommendation of any complimentary alternative treatments.
Evidence summary
Although complementary and alternative medicine (CAM) therapies are widely used, the overall body of research into CAM for asthma is still small and of limited quality. Interpreting the research is hampered by lack of standardized therapeutic approaches, lack of accepted methods for appropriate trials, and the fact that many CAM treatments are used as part of a multi-pronged, individualized approach to treatment in actual practice. Our search found 4 good-quality systematic reviews of RCTs, 1 good-quality systematic review of randomized trials, and 1 small additional pilot RCT of various CAM treatments for asthma.
Acupuncture and herbals provide some benefit
While a Cochrane review of 11 RCTs with variable trial quality and a total of 324 participants found that acupuncture had no significant effect on pulmonary function or global assessment of well-being, the review noted that some studies reported significant positive changes in daily symptoms, reductions in medication use, and improved quality of life. This suggests that some patients with mild to moderate asthma may benefit from acupuncture.3 In 1 RCT, improvement in general well-being was reported by 79% of 38 patients receiving acupuncture compared with 47% of 18 patients in the control group.4
When it comes to herbal remedies, a good-quality systematic review5 of 17 trials, with overall poor methodological quality and a total of 1445 participants, reported significant improvements in clinically relevant measures with 6 different herbal medicines.
- Ginkgo biloba liquor increased forced expiratory volume in 1 second (FEV1) by 10% at 4 weeks and by a more clinically relevant 15% at 8 weeks (significantly greater than placebo, P<.05).
- Invigorating Kidney for Preventing Asthma (IKPA) tablets increased FEV1 by 30% at 3 months compared with 17% in controls (P<.05).
- Wenyang Tonglulo Mixture (WTM) improved FEV1 by 30% at 8 weeks compared with a 16% increase in the control group using oral salbutamol and inhaled beclomethasone (P<.05).
- Dried ivy extract, thought to work as both a secretolytic and bronchospasmolytic, reduced airway resistance in children by 23.6% compared with placebo (P=.036).
- Tylophora indica (a rare herb also known as Indian ipecac) provided significant improvement in nocturnal dyspnea when compared with controls (P<.01) in a study that relied on patients’ symptom diaries.
- Tsumura saiboku-to (TJ-96) provided patients in one RCT with significant, but unspecified, asthma symptom relief when compared with those in a control group (P<.01).5
Other therapies didn’t quite make the grade
Homeopathy. A Cochrane review of 6 RCTs of mixed quality, with a total of 556 patients, concluded the evidence is insufficient to evaluate the possible role of homeopathy for the treatment of asthma, due to heterogeneity of interventions, patient populations, and outcome assessments. Each study evaluated a different homeopathic remedy, making any overall assessment difficult.
The review notes there have been only limited attempts to study a complete “package of care,” which includes the in-depth, one-on-one consultation, treatment, and follow-up that characterizes most homeopathic treatment in practice.6
Room air ionizers. A Cochrane review of 6 good-quality trials with a total of 106 participants reported no significant effect of room air ionizers on pulmonary function measures, symptoms, or medication use.7
Manual therapy. A Cochrane review8 of 3 moderate- to poor-quality RCTs with 156 participants reported no significant effect of chiropractic spinal manipulation (2 trials) or massage therapy (1 trial) on lung function, asthma symptoms, or medication use.
Mind-body therapy. A pilot RCT9 with 33 adults found a nonsignificant reduction in medication use among the subjects practicing mental imagery, but no overall effect on lung function or quality-of-life measures.
Recommendations from others
The New Zealand Guideline Group (NZGG)10 gives a Grade B recommendation for Buteyko Breathing Techniques as an intervention that may be helpful in reducing acute exacerbation medication use and improving patient quality of life. However, the NZGG did not find other benefits to this intervention and noted that it might be costly for the patient to obtain training in these techniques. The NZGG further recommends as a good practice point that healthcare professionals be open to the use of CAM therapies and that such therapies be tried by patients who are interested in them, with monitoring and self-assessment to assist patients in determining which therapies are of value.
1. Guidelines for the diagnosis and management of asthma. Update on selected topics 2002. Available at: www.nhlbi.nih.gov/guidelines/asthma/index.htm. Accessed on March 30, 2007.
2. Mannino DM, Home DW, Akinbami LJ, Morrman JE, Guynn C, Redd SC. Surveillance of Asthma—1980–1999. MMWR Surveill Summ 2002;51:1-13.
3. McCarney RW, Brinkhaus B, Lasserson TJ, Linde K. Acupuncture for chronic asthma. Cochrane Database Syst Rev 2004;(1):CD000008.-
4. Joos S, Schott C, Zou H, Daniel V, Martin E. Immunomodulatory effects of acupuncture in the treatment of allergic asthma: a randomized controlled study. J Altern Complementary Med 2000;6:519-525.
5. Huntley A, Ernst E. Herbal medicines for asthma: a systemic review. Thorax 2000;55:925-929.
6. McCarney RW, Linde K, Lasserson TJ. Homeopathy for chronic asthma. Cochrane Database Syst Rev 2004;(1):CD000353.-
7. Blackhall K, Appleton S, Cates FJ. Ionisers for chronic asthma. Cochrane Database Syst Rev 2003;(3):CD002986.-
8. Hondras MA, Jones LK, Jones AP. Manual therapy for asthma. Cochrane Database Syst Rev 2005;(2):CD001002.-
9. Epstein GN, Halper JP, Barrett EA, et al. A pilot study of mind-body changes in adults with asthma who practice mental imagery. Alternative Therapies 2004;10:66-71.
10. New Zealand Guidelines Group (NZGG) The diagnosis and treatment of adult asthma. Best Practice Evidence-Based Guideline. Wellington, NZ: NZGG; 2007. Available at: www.nzgg.org.nz/guidelines/0003/Full_text_Guideline.pdf. Accessed on March 30, 2007.
Yes, some are. Acupuncture relieves subjective symptoms of asthma and reduces medication use in mild to moderate asthma (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials [RCTs] of variable quality). Herbal medications, such as Ginkgo biloba, appear to improve lung function, while herbs such as Tylophora indica and Tsumura saiboku-to may decrease asthma symptoms (SOR: B, based on systematic review of RCTs with poor methodology). No evidence, however, supports the use of room air ionizers, manual therapy, homeopathy, or mind-body therapy for treatment of asthma (SOR: A, based on systematic reviews and meta-analyses of RCTs and individual RCTs).
Though this research is interesting, we should adhere to current guidelines
Vincent Lo, MD
San Joaquin General Hospital, French Camp, Calif
Guidelines for the diagnosis and management of asthma are widely disseminated by the National Asthma Education and Prevention Program through its Expert Panel Reports (updated in 2002).1 Nevertheless, nearly 500,000 hospitalizations, 2 million emergency department visits, and 5000 deaths were reported annually in the US among those who have asthma.2 Furthermore, a significant difference in asthma prevalence, health care use, and mortality was found among different ethnic groups.1
Poor patient understanding of asthma control, nonadherence to medication regimens, cultural beliefs, and disparity of access to the health care system, together with physicians’ lack of close monitoring and inadequate compliance with national asthma guidelines, contribute to suboptimal control of chronic asthma. Family physicians must guide and empower their patients with the knowledge and responsibility of how to manage their asthma. For now, we should adhere to current national guidelines of management of asthma and avoid routine recommendation of any complimentary alternative treatments.
Evidence summary
Although complementary and alternative medicine (CAM) therapies are widely used, the overall body of research into CAM for asthma is still small and of limited quality. Interpreting the research is hampered by lack of standardized therapeutic approaches, lack of accepted methods for appropriate trials, and the fact that many CAM treatments are used as part of a multi-pronged, individualized approach to treatment in actual practice. Our search found 4 good-quality systematic reviews of RCTs, 1 good-quality systematic review of randomized trials, and 1 small additional pilot RCT of various CAM treatments for asthma.
Acupuncture and herbals provide some benefit
While a Cochrane review of 11 RCTs with variable trial quality and a total of 324 participants found that acupuncture had no significant effect on pulmonary function or global assessment of well-being, the review noted that some studies reported significant positive changes in daily symptoms, reductions in medication use, and improved quality of life. This suggests that some patients with mild to moderate asthma may benefit from acupuncture.3 In 1 RCT, improvement in general well-being was reported by 79% of 38 patients receiving acupuncture compared with 47% of 18 patients in the control group.4
When it comes to herbal remedies, a good-quality systematic review5 of 17 trials, with overall poor methodological quality and a total of 1445 participants, reported significant improvements in clinically relevant measures with 6 different herbal medicines.
- Ginkgo biloba liquor increased forced expiratory volume in 1 second (FEV1) by 10% at 4 weeks and by a more clinically relevant 15% at 8 weeks (significantly greater than placebo, P<.05).
- Invigorating Kidney for Preventing Asthma (IKPA) tablets increased FEV1 by 30% at 3 months compared with 17% in controls (P<.05).
- Wenyang Tonglulo Mixture (WTM) improved FEV1 by 30% at 8 weeks compared with a 16% increase in the control group using oral salbutamol and inhaled beclomethasone (P<.05).
- Dried ivy extract, thought to work as both a secretolytic and bronchospasmolytic, reduced airway resistance in children by 23.6% compared with placebo (P=.036).
- Tylophora indica (a rare herb also known as Indian ipecac) provided significant improvement in nocturnal dyspnea when compared with controls (P<.01) in a study that relied on patients’ symptom diaries.
- Tsumura saiboku-to (TJ-96) provided patients in one RCT with significant, but unspecified, asthma symptom relief when compared with those in a control group (P<.01).5
Other therapies didn’t quite make the grade
Homeopathy. A Cochrane review of 6 RCTs of mixed quality, with a total of 556 patients, concluded the evidence is insufficient to evaluate the possible role of homeopathy for the treatment of asthma, due to heterogeneity of interventions, patient populations, and outcome assessments. Each study evaluated a different homeopathic remedy, making any overall assessment difficult.
The review notes there have been only limited attempts to study a complete “package of care,” which includes the in-depth, one-on-one consultation, treatment, and follow-up that characterizes most homeopathic treatment in practice.6
Room air ionizers. A Cochrane review of 6 good-quality trials with a total of 106 participants reported no significant effect of room air ionizers on pulmonary function measures, symptoms, or medication use.7
Manual therapy. A Cochrane review8 of 3 moderate- to poor-quality RCTs with 156 participants reported no significant effect of chiropractic spinal manipulation (2 trials) or massage therapy (1 trial) on lung function, asthma symptoms, or medication use.
Mind-body therapy. A pilot RCT9 with 33 adults found a nonsignificant reduction in medication use among the subjects practicing mental imagery, but no overall effect on lung function or quality-of-life measures.
Recommendations from others
The New Zealand Guideline Group (NZGG)10 gives a Grade B recommendation for Buteyko Breathing Techniques as an intervention that may be helpful in reducing acute exacerbation medication use and improving patient quality of life. However, the NZGG did not find other benefits to this intervention and noted that it might be costly for the patient to obtain training in these techniques. The NZGG further recommends as a good practice point that healthcare professionals be open to the use of CAM therapies and that such therapies be tried by patients who are interested in them, with monitoring and self-assessment to assist patients in determining which therapies are of value.
Yes, some are. Acupuncture relieves subjective symptoms of asthma and reduces medication use in mild to moderate asthma (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials [RCTs] of variable quality). Herbal medications, such as Ginkgo biloba, appear to improve lung function, while herbs such as Tylophora indica and Tsumura saiboku-to may decrease asthma symptoms (SOR: B, based on systematic review of RCTs with poor methodology). No evidence, however, supports the use of room air ionizers, manual therapy, homeopathy, or mind-body therapy for treatment of asthma (SOR: A, based on systematic reviews and meta-analyses of RCTs and individual RCTs).
Though this research is interesting, we should adhere to current guidelines
Vincent Lo, MD
San Joaquin General Hospital, French Camp, Calif
Guidelines for the diagnosis and management of asthma are widely disseminated by the National Asthma Education and Prevention Program through its Expert Panel Reports (updated in 2002).1 Nevertheless, nearly 500,000 hospitalizations, 2 million emergency department visits, and 5000 deaths were reported annually in the US among those who have asthma.2 Furthermore, a significant difference in asthma prevalence, health care use, and mortality was found among different ethnic groups.1
Poor patient understanding of asthma control, nonadherence to medication regimens, cultural beliefs, and disparity of access to the health care system, together with physicians’ lack of close monitoring and inadequate compliance with national asthma guidelines, contribute to suboptimal control of chronic asthma. Family physicians must guide and empower their patients with the knowledge and responsibility of how to manage their asthma. For now, we should adhere to current national guidelines of management of asthma and avoid routine recommendation of any complimentary alternative treatments.
Evidence summary
Although complementary and alternative medicine (CAM) therapies are widely used, the overall body of research into CAM for asthma is still small and of limited quality. Interpreting the research is hampered by lack of standardized therapeutic approaches, lack of accepted methods for appropriate trials, and the fact that many CAM treatments are used as part of a multi-pronged, individualized approach to treatment in actual practice. Our search found 4 good-quality systematic reviews of RCTs, 1 good-quality systematic review of randomized trials, and 1 small additional pilot RCT of various CAM treatments for asthma.
Acupuncture and herbals provide some benefit
While a Cochrane review of 11 RCTs with variable trial quality and a total of 324 participants found that acupuncture had no significant effect on pulmonary function or global assessment of well-being, the review noted that some studies reported significant positive changes in daily symptoms, reductions in medication use, and improved quality of life. This suggests that some patients with mild to moderate asthma may benefit from acupuncture.3 In 1 RCT, improvement in general well-being was reported by 79% of 38 patients receiving acupuncture compared with 47% of 18 patients in the control group.4
When it comes to herbal remedies, a good-quality systematic review5 of 17 trials, with overall poor methodological quality and a total of 1445 participants, reported significant improvements in clinically relevant measures with 6 different herbal medicines.
- Ginkgo biloba liquor increased forced expiratory volume in 1 second (FEV1) by 10% at 4 weeks and by a more clinically relevant 15% at 8 weeks (significantly greater than placebo, P<.05).
- Invigorating Kidney for Preventing Asthma (IKPA) tablets increased FEV1 by 30% at 3 months compared with 17% in controls (P<.05).
- Wenyang Tonglulo Mixture (WTM) improved FEV1 by 30% at 8 weeks compared with a 16% increase in the control group using oral salbutamol and inhaled beclomethasone (P<.05).
- Dried ivy extract, thought to work as both a secretolytic and bronchospasmolytic, reduced airway resistance in children by 23.6% compared with placebo (P=.036).
- Tylophora indica (a rare herb also known as Indian ipecac) provided significant improvement in nocturnal dyspnea when compared with controls (P<.01) in a study that relied on patients’ symptom diaries.
- Tsumura saiboku-to (TJ-96) provided patients in one RCT with significant, but unspecified, asthma symptom relief when compared with those in a control group (P<.01).5
Other therapies didn’t quite make the grade
Homeopathy. A Cochrane review of 6 RCTs of mixed quality, with a total of 556 patients, concluded the evidence is insufficient to evaluate the possible role of homeopathy for the treatment of asthma, due to heterogeneity of interventions, patient populations, and outcome assessments. Each study evaluated a different homeopathic remedy, making any overall assessment difficult.
The review notes there have been only limited attempts to study a complete “package of care,” which includes the in-depth, one-on-one consultation, treatment, and follow-up that characterizes most homeopathic treatment in practice.6
Room air ionizers. A Cochrane review of 6 good-quality trials with a total of 106 participants reported no significant effect of room air ionizers on pulmonary function measures, symptoms, or medication use.7
Manual therapy. A Cochrane review8 of 3 moderate- to poor-quality RCTs with 156 participants reported no significant effect of chiropractic spinal manipulation (2 trials) or massage therapy (1 trial) on lung function, asthma symptoms, or medication use.
Mind-body therapy. A pilot RCT9 with 33 adults found a nonsignificant reduction in medication use among the subjects practicing mental imagery, but no overall effect on lung function or quality-of-life measures.
Recommendations from others
The New Zealand Guideline Group (NZGG)10 gives a Grade B recommendation for Buteyko Breathing Techniques as an intervention that may be helpful in reducing acute exacerbation medication use and improving patient quality of life. However, the NZGG did not find other benefits to this intervention and noted that it might be costly for the patient to obtain training in these techniques. The NZGG further recommends as a good practice point that healthcare professionals be open to the use of CAM therapies and that such therapies be tried by patients who are interested in them, with monitoring and self-assessment to assist patients in determining which therapies are of value.
1. Guidelines for the diagnosis and management of asthma. Update on selected topics 2002. Available at: www.nhlbi.nih.gov/guidelines/asthma/index.htm. Accessed on March 30, 2007.
2. Mannino DM, Home DW, Akinbami LJ, Morrman JE, Guynn C, Redd SC. Surveillance of Asthma—1980–1999. MMWR Surveill Summ 2002;51:1-13.
3. McCarney RW, Brinkhaus B, Lasserson TJ, Linde K. Acupuncture for chronic asthma. Cochrane Database Syst Rev 2004;(1):CD000008.-
4. Joos S, Schott C, Zou H, Daniel V, Martin E. Immunomodulatory effects of acupuncture in the treatment of allergic asthma: a randomized controlled study. J Altern Complementary Med 2000;6:519-525.
5. Huntley A, Ernst E. Herbal medicines for asthma: a systemic review. Thorax 2000;55:925-929.
6. McCarney RW, Linde K, Lasserson TJ. Homeopathy for chronic asthma. Cochrane Database Syst Rev 2004;(1):CD000353.-
7. Blackhall K, Appleton S, Cates FJ. Ionisers for chronic asthma. Cochrane Database Syst Rev 2003;(3):CD002986.-
8. Hondras MA, Jones LK, Jones AP. Manual therapy for asthma. Cochrane Database Syst Rev 2005;(2):CD001002.-
9. Epstein GN, Halper JP, Barrett EA, et al. A pilot study of mind-body changes in adults with asthma who practice mental imagery. Alternative Therapies 2004;10:66-71.
10. New Zealand Guidelines Group (NZGG) The diagnosis and treatment of adult asthma. Best Practice Evidence-Based Guideline. Wellington, NZ: NZGG; 2007. Available at: www.nzgg.org.nz/guidelines/0003/Full_text_Guideline.pdf. Accessed on March 30, 2007.
1. Guidelines for the diagnosis and management of asthma. Update on selected topics 2002. Available at: www.nhlbi.nih.gov/guidelines/asthma/index.htm. Accessed on March 30, 2007.
2. Mannino DM, Home DW, Akinbami LJ, Morrman JE, Guynn C, Redd SC. Surveillance of Asthma—1980–1999. MMWR Surveill Summ 2002;51:1-13.
3. McCarney RW, Brinkhaus B, Lasserson TJ, Linde K. Acupuncture for chronic asthma. Cochrane Database Syst Rev 2004;(1):CD000008.-
4. Joos S, Schott C, Zou H, Daniel V, Martin E. Immunomodulatory effects of acupuncture in the treatment of allergic asthma: a randomized controlled study. J Altern Complementary Med 2000;6:519-525.
5. Huntley A, Ernst E. Herbal medicines for asthma: a systemic review. Thorax 2000;55:925-929.
6. McCarney RW, Linde K, Lasserson TJ. Homeopathy for chronic asthma. Cochrane Database Syst Rev 2004;(1):CD000353.-
7. Blackhall K, Appleton S, Cates FJ. Ionisers for chronic asthma. Cochrane Database Syst Rev 2003;(3):CD002986.-
8. Hondras MA, Jones LK, Jones AP. Manual therapy for asthma. Cochrane Database Syst Rev 2005;(2):CD001002.-
9. Epstein GN, Halper JP, Barrett EA, et al. A pilot study of mind-body changes in adults with asthma who practice mental imagery. Alternative Therapies 2004;10:66-71.
10. New Zealand Guidelines Group (NZGG) The diagnosis and treatment of adult asthma. Best Practice Evidence-Based Guideline. Wellington, NZ: NZGG; 2007. Available at: www.nzgg.org.nz/guidelines/0003/Full_text_Guideline.pdf. Accessed on March 30, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
Should you restrict your cardiac patient from driving?
That depends, of course, on your patient’s particular condition, but your decision can be guided by various cardiovascular society consensus conferences, such as the one from the Canadian Cardiovascular society (TABLE), since no evidence-based guidelines exist. It seems sensible to say, though, that impairment of consciousness associated with any heart disease needs further evaluation, with a complete restriction of driving for at least 6 months (strength of recommendation [SOR]: C, based on expert opinion and extrapolation from observational studies).
Helpful guide stratifies risk
Drew Malloy, MD
University of California Santa Cruz Student Health Service
This review points out the lack of evidence for a common clinical problem. Evidence is scant, but the TABLE helps the busy clinician stratify risks for different types of heart disease and provides some rational basis for the duration of restrictions. The existing expert consensus guidelines are sensible and useful when discussing this important issue with patients and their families after diagnosis of heart disease. However, to be quite frank, when I am driving I am more worried about the teenager on a cell phone behind the wheel of an SUV than my grandmother with an ICD.
Evidence summary
Our search identified no randomized controlled trials, no systematic reviews, 6 observational studies, and 3 consensus panel guidelines on risks from driving and cardiovascular disease. No studies deal specifically with coronary artery disease, congestive heart failure, or valvular heart disease and the risk of motor vehicle crashes for patients with these conditions. A population-based case control study of 5204 male drivers ages 45 to 70 in Quebec found no increased risk of crash for drivers with unspecified cardiovascular disease.1
Car accidents among ICD patients are low
The most studied patients are those with life-threatening ventricular arrhythmias—particularly those with implantable cardioverter-defibrillators (ICDs). Based on observational studies of patients and their physicians, patients with ventricular arrhythmias treated with ICDs do not have an increased risk of motor vehicle crashes.2-4 The largest of the studies2 prospectively and anonymously surveyed 627 patients from the Antiarrhythmics vs Implantable Defibrillators Trial. During follow-up, 2% of patients had a syncopal episode while driving, and 11% had dizziness or palpitations that required stopping the vehicle.
Of the 55 car crashes that occurred during 1619 patient-years after resumption of driving, 11% were preceded by a possible symptom of arrhythmia (0.4% per patient per year). The annual incidence of car accidents for patients with an ICD was 3.4% per patient-year. This is substantially lower than the 7.1% rate among the general driving population in the US.
Recommendations from others
Expert panel guidelines regarding fitness to drive for patients with heart disease are available from the Canadian Cardiovascular Society (CCS),5 the European Society of Cardiology, the American Heart Association, the North American Society of Pacing and Physiology,6 and the Cardiac Society of Australia and New Zealand.7 The 2004 CCS guidelines are the most recent and include a “Risk of Harm” formula that attempts to assign a quantitative level of risk to drivers with heart disease. These guidelines appear sensible but are not evidence-based (TABLE).
TABLE
Should your heart patient get behind the wheel? A helpful guide
| CONDITIONS | TIME TO RESUME DRIVING |
|---|---|
| Coronary artery disease | |
| Coronary bypass graft | 1 month after discharge |
| ST elevation myocardial infarction | 1 month after discharge |
| Unstable angina | |
| – PCI during hospital stay | 48 hours after PCI |
| – PCI not done during hospital stay | 7 days after discharge |
| Ventricular arrhythmias | |
| Non-sustained VT with no loss of consciousness | No restriction |
| VF or unstable VT | 6 months after event |
| Implantable cardioverter defibrillator | |
| For VF or VT with decreased level of consciousness | 6 months after event |
| Rhythm disturbances | |
| Atrial flutter (without impaired level of consciousness) | No restriction |
| Supraventricular tachycardia | No restriction |
| Atrial fibrillation | No restriction |
| Heart block | |
| First- and second-degree atrioventricular block, Mobitz Type 1 (without impairment of consciousness) | No restriction |
| Second-degree atrioventricular block, Mobitz Type II | No driving |
| Permanent pacemaker | |
| All patients | 1 week after implant; normal pacer function; no impaired level of consciousness |
| Congestive heart failure | |
| NYHA Classes I–III | No restriction |
| NYHA Classes IV | No driving |
| Adapted from Canadian Cardiovascular Society Consensus Conference 2003.5 | |
| PCI, percutaneous coronary intervention; VF, ventricular fibrillation; | |
| VT, ventricular tachycardia; NYHA, New York Heart Association | |
1. Guibert R, Potvin L, Ciampi A, Loiselle J, Philibert L, Franco ED. Are drivers with CVD more at risk for motor vehicle crashes? Study of men aged 45 to 70. Can Fam Physician 1998;44:770-776.
2. Akiyama T, Powell JL, Mitchell LB, Ehlert FA, Baessler C. Resumption of driving after life-threatening ventricular tachyarrhythmia. N Engl J Med 2001;345:391-397.
3. Trappe HJ, Wenzlaff P, Grellman G. Should patients with implantable cardioverter defibrillators be allowed to drive? Observations in 291 patients from a single center over an 11-year period. J Interv Card Electrophysiol 1998;2:193-201.
4. Curtis AB, Conti JB, Tucker KJ, Kubilis PS, Reilly RE, Woodard DA. Motor vehicle accidents in patients with an implantable cardioverter-defibrillator. J Am Coll Cardiol 1995;26:180-184.
5. CCS Consensus Conference 2003: Assessment of the cardiac patient fitness to drive and fly—executive summary. Can J Cardiol 2004;20:1313-1323.
6. Epstein AE, Miles WM, Benditt DG, et al. Personal and public safety issues related to arrhythmias that may affect consciousness: Implications for regulation and physician recommendations. A medical/scientific statement from the American Heart Association and the North American Society of Pacing and Electrophysiology. Circulation 1996;94:1147-1166.
7. Cardiac Society of Australia and New Zealand. Cardiovascular Disease and Driving 2002. Available at: www.csanz.edu.au/guidelines/practice/Drivegl2002.pdf. Accessed on April 3, 2007.
That depends, of course, on your patient’s particular condition, but your decision can be guided by various cardiovascular society consensus conferences, such as the one from the Canadian Cardiovascular society (TABLE), since no evidence-based guidelines exist. It seems sensible to say, though, that impairment of consciousness associated with any heart disease needs further evaluation, with a complete restriction of driving for at least 6 months (strength of recommendation [SOR]: C, based on expert opinion and extrapolation from observational studies).
Helpful guide stratifies risk
Drew Malloy, MD
University of California Santa Cruz Student Health Service
This review points out the lack of evidence for a common clinical problem. Evidence is scant, but the TABLE helps the busy clinician stratify risks for different types of heart disease and provides some rational basis for the duration of restrictions. The existing expert consensus guidelines are sensible and useful when discussing this important issue with patients and their families after diagnosis of heart disease. However, to be quite frank, when I am driving I am more worried about the teenager on a cell phone behind the wheel of an SUV than my grandmother with an ICD.
Evidence summary
Our search identified no randomized controlled trials, no systematic reviews, 6 observational studies, and 3 consensus panel guidelines on risks from driving and cardiovascular disease. No studies deal specifically with coronary artery disease, congestive heart failure, or valvular heart disease and the risk of motor vehicle crashes for patients with these conditions. A population-based case control study of 5204 male drivers ages 45 to 70 in Quebec found no increased risk of crash for drivers with unspecified cardiovascular disease.1
Car accidents among ICD patients are low
The most studied patients are those with life-threatening ventricular arrhythmias—particularly those with implantable cardioverter-defibrillators (ICDs). Based on observational studies of patients and their physicians, patients with ventricular arrhythmias treated with ICDs do not have an increased risk of motor vehicle crashes.2-4 The largest of the studies2 prospectively and anonymously surveyed 627 patients from the Antiarrhythmics vs Implantable Defibrillators Trial. During follow-up, 2% of patients had a syncopal episode while driving, and 11% had dizziness or palpitations that required stopping the vehicle.
Of the 55 car crashes that occurred during 1619 patient-years after resumption of driving, 11% were preceded by a possible symptom of arrhythmia (0.4% per patient per year). The annual incidence of car accidents for patients with an ICD was 3.4% per patient-year. This is substantially lower than the 7.1% rate among the general driving population in the US.
Recommendations from others
Expert panel guidelines regarding fitness to drive for patients with heart disease are available from the Canadian Cardiovascular Society (CCS),5 the European Society of Cardiology, the American Heart Association, the North American Society of Pacing and Physiology,6 and the Cardiac Society of Australia and New Zealand.7 The 2004 CCS guidelines are the most recent and include a “Risk of Harm” formula that attempts to assign a quantitative level of risk to drivers with heart disease. These guidelines appear sensible but are not evidence-based (TABLE).
TABLE
Should your heart patient get behind the wheel? A helpful guide
| CONDITIONS | TIME TO RESUME DRIVING |
|---|---|
| Coronary artery disease | |
| Coronary bypass graft | 1 month after discharge |
| ST elevation myocardial infarction | 1 month after discharge |
| Unstable angina | |
| – PCI during hospital stay | 48 hours after PCI |
| – PCI not done during hospital stay | 7 days after discharge |
| Ventricular arrhythmias | |
| Non-sustained VT with no loss of consciousness | No restriction |
| VF or unstable VT | 6 months after event |
| Implantable cardioverter defibrillator | |
| For VF or VT with decreased level of consciousness | 6 months after event |
| Rhythm disturbances | |
| Atrial flutter (without impaired level of consciousness) | No restriction |
| Supraventricular tachycardia | No restriction |
| Atrial fibrillation | No restriction |
| Heart block | |
| First- and second-degree atrioventricular block, Mobitz Type 1 (without impairment of consciousness) | No restriction |
| Second-degree atrioventricular block, Mobitz Type II | No driving |
| Permanent pacemaker | |
| All patients | 1 week after implant; normal pacer function; no impaired level of consciousness |
| Congestive heart failure | |
| NYHA Classes I–III | No restriction |
| NYHA Classes IV | No driving |
| Adapted from Canadian Cardiovascular Society Consensus Conference 2003.5 | |
| PCI, percutaneous coronary intervention; VF, ventricular fibrillation; | |
| VT, ventricular tachycardia; NYHA, New York Heart Association | |
That depends, of course, on your patient’s particular condition, but your decision can be guided by various cardiovascular society consensus conferences, such as the one from the Canadian Cardiovascular society (TABLE), since no evidence-based guidelines exist. It seems sensible to say, though, that impairment of consciousness associated with any heart disease needs further evaluation, with a complete restriction of driving for at least 6 months (strength of recommendation [SOR]: C, based on expert opinion and extrapolation from observational studies).
Helpful guide stratifies risk
Drew Malloy, MD
University of California Santa Cruz Student Health Service
This review points out the lack of evidence for a common clinical problem. Evidence is scant, but the TABLE helps the busy clinician stratify risks for different types of heart disease and provides some rational basis for the duration of restrictions. The existing expert consensus guidelines are sensible and useful when discussing this important issue with patients and their families after diagnosis of heart disease. However, to be quite frank, when I am driving I am more worried about the teenager on a cell phone behind the wheel of an SUV than my grandmother with an ICD.
Evidence summary
Our search identified no randomized controlled trials, no systematic reviews, 6 observational studies, and 3 consensus panel guidelines on risks from driving and cardiovascular disease. No studies deal specifically with coronary artery disease, congestive heart failure, or valvular heart disease and the risk of motor vehicle crashes for patients with these conditions. A population-based case control study of 5204 male drivers ages 45 to 70 in Quebec found no increased risk of crash for drivers with unspecified cardiovascular disease.1
Car accidents among ICD patients are low
The most studied patients are those with life-threatening ventricular arrhythmias—particularly those with implantable cardioverter-defibrillators (ICDs). Based on observational studies of patients and their physicians, patients with ventricular arrhythmias treated with ICDs do not have an increased risk of motor vehicle crashes.2-4 The largest of the studies2 prospectively and anonymously surveyed 627 patients from the Antiarrhythmics vs Implantable Defibrillators Trial. During follow-up, 2% of patients had a syncopal episode while driving, and 11% had dizziness or palpitations that required stopping the vehicle.
Of the 55 car crashes that occurred during 1619 patient-years after resumption of driving, 11% were preceded by a possible symptom of arrhythmia (0.4% per patient per year). The annual incidence of car accidents for patients with an ICD was 3.4% per patient-year. This is substantially lower than the 7.1% rate among the general driving population in the US.
Recommendations from others
Expert panel guidelines regarding fitness to drive for patients with heart disease are available from the Canadian Cardiovascular Society (CCS),5 the European Society of Cardiology, the American Heart Association, the North American Society of Pacing and Physiology,6 and the Cardiac Society of Australia and New Zealand.7 The 2004 CCS guidelines are the most recent and include a “Risk of Harm” formula that attempts to assign a quantitative level of risk to drivers with heart disease. These guidelines appear sensible but are not evidence-based (TABLE).
TABLE
Should your heart patient get behind the wheel? A helpful guide
| CONDITIONS | TIME TO RESUME DRIVING |
|---|---|
| Coronary artery disease | |
| Coronary bypass graft | 1 month after discharge |
| ST elevation myocardial infarction | 1 month after discharge |
| Unstable angina | |
| – PCI during hospital stay | 48 hours after PCI |
| – PCI not done during hospital stay | 7 days after discharge |
| Ventricular arrhythmias | |
| Non-sustained VT with no loss of consciousness | No restriction |
| VF or unstable VT | 6 months after event |
| Implantable cardioverter defibrillator | |
| For VF or VT with decreased level of consciousness | 6 months after event |
| Rhythm disturbances | |
| Atrial flutter (without impaired level of consciousness) | No restriction |
| Supraventricular tachycardia | No restriction |
| Atrial fibrillation | No restriction |
| Heart block | |
| First- and second-degree atrioventricular block, Mobitz Type 1 (without impairment of consciousness) | No restriction |
| Second-degree atrioventricular block, Mobitz Type II | No driving |
| Permanent pacemaker | |
| All patients | 1 week after implant; normal pacer function; no impaired level of consciousness |
| Congestive heart failure | |
| NYHA Classes I–III | No restriction |
| NYHA Classes IV | No driving |
| Adapted from Canadian Cardiovascular Society Consensus Conference 2003.5 | |
| PCI, percutaneous coronary intervention; VF, ventricular fibrillation; | |
| VT, ventricular tachycardia; NYHA, New York Heart Association | |
1. Guibert R, Potvin L, Ciampi A, Loiselle J, Philibert L, Franco ED. Are drivers with CVD more at risk for motor vehicle crashes? Study of men aged 45 to 70. Can Fam Physician 1998;44:770-776.
2. Akiyama T, Powell JL, Mitchell LB, Ehlert FA, Baessler C. Resumption of driving after life-threatening ventricular tachyarrhythmia. N Engl J Med 2001;345:391-397.
3. Trappe HJ, Wenzlaff P, Grellman G. Should patients with implantable cardioverter defibrillators be allowed to drive? Observations in 291 patients from a single center over an 11-year period. J Interv Card Electrophysiol 1998;2:193-201.
4. Curtis AB, Conti JB, Tucker KJ, Kubilis PS, Reilly RE, Woodard DA. Motor vehicle accidents in patients with an implantable cardioverter-defibrillator. J Am Coll Cardiol 1995;26:180-184.
5. CCS Consensus Conference 2003: Assessment of the cardiac patient fitness to drive and fly—executive summary. Can J Cardiol 2004;20:1313-1323.
6. Epstein AE, Miles WM, Benditt DG, et al. Personal and public safety issues related to arrhythmias that may affect consciousness: Implications for regulation and physician recommendations. A medical/scientific statement from the American Heart Association and the North American Society of Pacing and Electrophysiology. Circulation 1996;94:1147-1166.
7. Cardiac Society of Australia and New Zealand. Cardiovascular Disease and Driving 2002. Available at: www.csanz.edu.au/guidelines/practice/Drivegl2002.pdf. Accessed on April 3, 2007.
1. Guibert R, Potvin L, Ciampi A, Loiselle J, Philibert L, Franco ED. Are drivers with CVD more at risk for motor vehicle crashes? Study of men aged 45 to 70. Can Fam Physician 1998;44:770-776.
2. Akiyama T, Powell JL, Mitchell LB, Ehlert FA, Baessler C. Resumption of driving after life-threatening ventricular tachyarrhythmia. N Engl J Med 2001;345:391-397.
3. Trappe HJ, Wenzlaff P, Grellman G. Should patients with implantable cardioverter defibrillators be allowed to drive? Observations in 291 patients from a single center over an 11-year period. J Interv Card Electrophysiol 1998;2:193-201.
4. Curtis AB, Conti JB, Tucker KJ, Kubilis PS, Reilly RE, Woodard DA. Motor vehicle accidents in patients with an implantable cardioverter-defibrillator. J Am Coll Cardiol 1995;26:180-184.
5. CCS Consensus Conference 2003: Assessment of the cardiac patient fitness to drive and fly—executive summary. Can J Cardiol 2004;20:1313-1323.
6. Epstein AE, Miles WM, Benditt DG, et al. Personal and public safety issues related to arrhythmias that may affect consciousness: Implications for regulation and physician recommendations. A medical/scientific statement from the American Heart Association and the North American Society of Pacing and Electrophysiology. Circulation 1996;94:1147-1166.
7. Cardiac Society of Australia and New Zealand. Cardiovascular Disease and Driving 2002. Available at: www.csanz.edu.au/guidelines/practice/Drivegl2002.pdf. Accessed on April 3, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
When (and how) should you evaluate a child for obstructive sleep apnea?
Well-child visits are the appropriate time to screen all children for a history of snoring and apnea (strength of recommendation [SOR]: C, based on expert opinion). Children should be further evaluated for obstructive sleep apnea if parents note habitual nightly snoring, especially if accompanied by pauses, snorts, or gasps (SOR: C, based on case series and expert opinion). Single overnight polysomnography is the test of choice to evaluate a child for obstructive sleep apnea, since it can’t be excluded by history or physical exam. Other evaluation methods for sleep apnea lack either adequate sensitivity or specificity (SOR: C, based on case series, systematic review of case series, and expert opinion).
Add a question about snoring to your parental questionnaire
John D. Hallgren, MD
Uniformed Services University of the Health Sciences, RAF Menwith Hill, United Kingdom
How many of us routinely ask about snoring or sleep apnea at a wellness visit for our young patients? Here we learn that asking a simple question—“Does your child snore heavily most nights or seem to stop breathing when he or she sleeps?”—improves our ability to recognize obstructive sleep apnea in children.
In addition, a relatively simple, one-time, noninvasive test tells us whether the child has obstructive sleep apnea—or not. In light of this information, I plan on putting a revised well-child visit questionnaire that asks about snoring/apnea in my waiting room right away.
Evidence summary
Experts advise screening all children for obstructive sleep apnea by asking about snoring and sleep apnea at routine health supervision visits.1 Obstructive sleep apnea among children has an overall prevalence of 2% but occurs in approximately 13% to 25% of those who snore regularly and heavily.1,2
Obstructive sleep apnea is unlikely in the absence of habitual snoring (expert opinion), and snoring loudness does not correlate with severity. Children with obstructive sleep apnea tend to exhibit symptoms typical of attention-deficit and hyperactivity disorder rather than daytime somnolence.2
Adenotonsillar hypertrophy may be to blame
The most common cause of obstructive sleep apnea is adenotonsillar hypertrophy. Additional risk factors include obesity, allergic rhinitis, neuromuscular disease, craniofacial anomalies, Down syndrome, premature birth, and family history.1
A prospective case-series of 62 consecutive children referred to a sleep clinic for suspected obstructive sleep apnea correlated nightly snoring (relative risk [RR]=10.67; 95% confidence interval [CI], 2.72–41.8) and observed apnea (RR=3.43; 95% CI, 1.42–6.76) with obstructive sleep apnea diagnosed by polysomnography.3 Children with underlying neurological or craniofacial problems were excluded. A retrospective, nonconsecutive cohort study of 50 children previously diagnosed with obstructive sleep apnea found that all presented with “continuous heavy snoring, interrupted by pauses and associated with snorts.”4
Don’t count on the physical exam and history
Experts consider overnight polysomnography to be the gold standard test for obstructive sleep apnea.2 Physical examination alone usually has normal results. A systematic review of 12 studies including 1058 children under age 18 compared evaluation by polysomnography with clinical evaluation by history and physical examination alone. Clinical evaluation had a positive predictive value (PPV) of 55.8% (95% CI, 42.1%–69.6%); no single component demonstrated sensitivity and specificity more than 65%.5
Symptom questionnaires or scales did not correlate well with polysomnography results in 3 studies (which excluded children with craniofacial anomalies, significant medical problems, or prior upper airway surgery). The best study—a prospective, randomized, investigator-blinded, controlled trial of 59 consecutive children clinically diagnosed with obstructive sleep apnea—compared a “clinical assessment score” (comprised of standardized history, physical exam, voice recording, sleep audio recording, lateral neck radiograph, and echocardiogram) with overnight polysomnography. The majority of children had mild obstructive sleep apnea. Overall, the clinical assessment score did not correlate well with polysomnography (PPV=48%).6
Avoid sequential and abbreviated tests
Sequential overnight polysomnography did not dramatically improve sensitivity or specificity over a single test in 2 prospective studies of children with suspected obstructive sleep apnea. The larger study recruited 70 consecutive children (ages 2 to 17 years) from a sleep lab and performed polysomnography on 2 consecutive nights. The first test correctly identified obstructive sleep apnea in 64 children; the second identified 6 additional cases, all of which were “mild.”7
Abbreviated polysomnographies have inadequate sensitivity. A retrospective chart review included 143 children between 1 and 18 years of age with adenotonsillar hypertrophy referred for overnight polysomnography after normal or mildly abnormal “nap polysomnography.” Mildly abnormal nap polysomnography predicted abnormal overnight polysomnography (PPV=77%; P<.0001), but normal nap polysomnography were not predictive of normal overnight polysomnography (NPV=49%; P=.8).8
Video recordings have low specificity
A prospective cohort study of home sleep video recordings included 58 consecutive children who were 2 to 6 years of age with snoring or labored breathing during sleep. Parents filmed 30 minutes of sleep during “worst breathing” episodes. An expert investigator evaluated the recordings using both a standardized scoring system and subjective impression, and compared these with overnight polysomnography results, finding 94% sensitivity and 68% specificity.9
Two prospective trials compared standardized scoring of home sleep audio recordings with overnight polysomnography testing. The best study, an investigator-blinded RCT, scored 20-minute recordings of “worst breathing” in 59 consecutive children referred for snoring or nocturnal breathing problems, 47% of whom had sleep apnea. Results: PPV=62%, NPV=83%, sensitivity=88%, specificity=52%.7
Abnormal pulse ox is highly predictive
A retrospective, cross-sectional study of 349 children between the ages of 6 months and 18 years determined that abnormal home pulse oximetry studies were highly predictive of obstructive sleep apnea on polysomnography (positive likelihood ratio [LR+]=19.4; CIs not given; posttest probability=97%), but that inconclusive or normal pulse oximetry studies were not predictive of negative polysomnographies (LR+=0.58; CIs not given; posttest probability 47%). Children had been referred for suspected obstructive sleep apnea, and the pretest probability was 60%. Oximetry records were evaluated by a sleep laboratory physician blinded to clinical and polysomnography data.10
Tonsillar-pharyngeal ratio misses mild cases
A prospective cohort study of 35 children compared the tonsillar-pharyngeal ratio measured from lateral neck x-rays to overnight polysomnography. X-ray measurements predicted moderate or severe obstructive sleep apnea on polysomnography (sensitivity=96%, specificity=82%, PPV=92%, NPV=90%), but did not accurately differentiate normal children from those with mild obstructive sleep apnea.11
Recommendations from others
The American Academy of Pediatrics recommends that children be screened during well child visits for regular snoring or apnea episodes during sleep. Children with positive screens should have an overnight polysomnography. Children with confirmed obstructive sleep apnea should be referred to a sleep medicine specialist to consider continuous positive airway pressure therapy during sleep, or to an otolaryngologist for possible surgery (tonsillectomy or adenoidectomy).2
1. Li AM, Chan DF, Fok TF, Wing YK. Childhood obstructive sleep apnoea: an update. Hong Kong Med J 2004;10:406-413.
2. Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome, American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109:704-712.
3. Chau KW, Ng DKK, Kwok CKL, Chow PY, Ho JCS. Clinical risk factors for obstructive sleep apnoea in children. Singapore Med J 2003;44:570-573.
4. Guilleminault C, Korobkin R, Winkle R. A review of 50 children with obstructive sleep apnea syndrome. Lung 1981;159:275-287.
5. Brietzke SE, Katz ES, Roberson DW. Can history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypopnea syndrome? A systematic review of the literature. Otolaryngol Head Neck Surg 2004;131:827-832.
6. Goldstein NA, Pugazhendhi V, Rao SM, et al. Clinical assessment of pediatric obstructive sleep apnea. Pediatrics 2004;114:33-43.
7. Verhulst SL, Schrauwen N, De Backer WA, Desager KN. First night effect for polysomnographic data in children and adolescents with suspected sleep disordered breathing. Arch Dis Child 2006;91:233-237.
8. Saeed MM, Keens TG, Stabile MW, Bolokowicz J, Davidson Ward SL. Should children with suspected obstructive sleep apnea syndrome and normal nap sleep studies have overnight sleep studies? Chest 2000;118:360-365.
9. Sivan Y, Kornecki A, Schonfeld T. Screening obstructive sleep apnoea syndrome by home videotape recording in children. Eur Respir J 1996;9:2127-2131.
10. Brouillette RT, Morielli A, Leimanis A, Waters KA, Luciano R, Ducharme FM. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics 2000;105:405-412.
11. Li AM, Wong E, Kew J, Hui S, Fok TF. Use of tonsil size in the evaluation of obstructive sleep apnoea. Arch Dis Child 2002;87:156-159.
Well-child visits are the appropriate time to screen all children for a history of snoring and apnea (strength of recommendation [SOR]: C, based on expert opinion). Children should be further evaluated for obstructive sleep apnea if parents note habitual nightly snoring, especially if accompanied by pauses, snorts, or gasps (SOR: C, based on case series and expert opinion). Single overnight polysomnography is the test of choice to evaluate a child for obstructive sleep apnea, since it can’t be excluded by history or physical exam. Other evaluation methods for sleep apnea lack either adequate sensitivity or specificity (SOR: C, based on case series, systematic review of case series, and expert opinion).
Add a question about snoring to your parental questionnaire
John D. Hallgren, MD
Uniformed Services University of the Health Sciences, RAF Menwith Hill, United Kingdom
How many of us routinely ask about snoring or sleep apnea at a wellness visit for our young patients? Here we learn that asking a simple question—“Does your child snore heavily most nights or seem to stop breathing when he or she sleeps?”—improves our ability to recognize obstructive sleep apnea in children.
In addition, a relatively simple, one-time, noninvasive test tells us whether the child has obstructive sleep apnea—or not. In light of this information, I plan on putting a revised well-child visit questionnaire that asks about snoring/apnea in my waiting room right away.
Evidence summary
Experts advise screening all children for obstructive sleep apnea by asking about snoring and sleep apnea at routine health supervision visits.1 Obstructive sleep apnea among children has an overall prevalence of 2% but occurs in approximately 13% to 25% of those who snore regularly and heavily.1,2
Obstructive sleep apnea is unlikely in the absence of habitual snoring (expert opinion), and snoring loudness does not correlate with severity. Children with obstructive sleep apnea tend to exhibit symptoms typical of attention-deficit and hyperactivity disorder rather than daytime somnolence.2
Adenotonsillar hypertrophy may be to blame
The most common cause of obstructive sleep apnea is adenotonsillar hypertrophy. Additional risk factors include obesity, allergic rhinitis, neuromuscular disease, craniofacial anomalies, Down syndrome, premature birth, and family history.1
A prospective case-series of 62 consecutive children referred to a sleep clinic for suspected obstructive sleep apnea correlated nightly snoring (relative risk [RR]=10.67; 95% confidence interval [CI], 2.72–41.8) and observed apnea (RR=3.43; 95% CI, 1.42–6.76) with obstructive sleep apnea diagnosed by polysomnography.3 Children with underlying neurological or craniofacial problems were excluded. A retrospective, nonconsecutive cohort study of 50 children previously diagnosed with obstructive sleep apnea found that all presented with “continuous heavy snoring, interrupted by pauses and associated with snorts.”4
Don’t count on the physical exam and history
Experts consider overnight polysomnography to be the gold standard test for obstructive sleep apnea.2 Physical examination alone usually has normal results. A systematic review of 12 studies including 1058 children under age 18 compared evaluation by polysomnography with clinical evaluation by history and physical examination alone. Clinical evaluation had a positive predictive value (PPV) of 55.8% (95% CI, 42.1%–69.6%); no single component demonstrated sensitivity and specificity more than 65%.5
Symptom questionnaires or scales did not correlate well with polysomnography results in 3 studies (which excluded children with craniofacial anomalies, significant medical problems, or prior upper airway surgery). The best study—a prospective, randomized, investigator-blinded, controlled trial of 59 consecutive children clinically diagnosed with obstructive sleep apnea—compared a “clinical assessment score” (comprised of standardized history, physical exam, voice recording, sleep audio recording, lateral neck radiograph, and echocardiogram) with overnight polysomnography. The majority of children had mild obstructive sleep apnea. Overall, the clinical assessment score did not correlate well with polysomnography (PPV=48%).6
Avoid sequential and abbreviated tests
Sequential overnight polysomnography did not dramatically improve sensitivity or specificity over a single test in 2 prospective studies of children with suspected obstructive sleep apnea. The larger study recruited 70 consecutive children (ages 2 to 17 years) from a sleep lab and performed polysomnography on 2 consecutive nights. The first test correctly identified obstructive sleep apnea in 64 children; the second identified 6 additional cases, all of which were “mild.”7
Abbreviated polysomnographies have inadequate sensitivity. A retrospective chart review included 143 children between 1 and 18 years of age with adenotonsillar hypertrophy referred for overnight polysomnography after normal or mildly abnormal “nap polysomnography.” Mildly abnormal nap polysomnography predicted abnormal overnight polysomnography (PPV=77%; P<.0001), but normal nap polysomnography were not predictive of normal overnight polysomnography (NPV=49%; P=.8).8
Video recordings have low specificity
A prospective cohort study of home sleep video recordings included 58 consecutive children who were 2 to 6 years of age with snoring or labored breathing during sleep. Parents filmed 30 minutes of sleep during “worst breathing” episodes. An expert investigator evaluated the recordings using both a standardized scoring system and subjective impression, and compared these with overnight polysomnography results, finding 94% sensitivity and 68% specificity.9
Two prospective trials compared standardized scoring of home sleep audio recordings with overnight polysomnography testing. The best study, an investigator-blinded RCT, scored 20-minute recordings of “worst breathing” in 59 consecutive children referred for snoring or nocturnal breathing problems, 47% of whom had sleep apnea. Results: PPV=62%, NPV=83%, sensitivity=88%, specificity=52%.7
Abnormal pulse ox is highly predictive
A retrospective, cross-sectional study of 349 children between the ages of 6 months and 18 years determined that abnormal home pulse oximetry studies were highly predictive of obstructive sleep apnea on polysomnography (positive likelihood ratio [LR+]=19.4; CIs not given; posttest probability=97%), but that inconclusive or normal pulse oximetry studies were not predictive of negative polysomnographies (LR+=0.58; CIs not given; posttest probability 47%). Children had been referred for suspected obstructive sleep apnea, and the pretest probability was 60%. Oximetry records were evaluated by a sleep laboratory physician blinded to clinical and polysomnography data.10
Tonsillar-pharyngeal ratio misses mild cases
A prospective cohort study of 35 children compared the tonsillar-pharyngeal ratio measured from lateral neck x-rays to overnight polysomnography. X-ray measurements predicted moderate or severe obstructive sleep apnea on polysomnography (sensitivity=96%, specificity=82%, PPV=92%, NPV=90%), but did not accurately differentiate normal children from those with mild obstructive sleep apnea.11
Recommendations from others
The American Academy of Pediatrics recommends that children be screened during well child visits for regular snoring or apnea episodes during sleep. Children with positive screens should have an overnight polysomnography. Children with confirmed obstructive sleep apnea should be referred to a sleep medicine specialist to consider continuous positive airway pressure therapy during sleep, or to an otolaryngologist for possible surgery (tonsillectomy or adenoidectomy).2
Well-child visits are the appropriate time to screen all children for a history of snoring and apnea (strength of recommendation [SOR]: C, based on expert opinion). Children should be further evaluated for obstructive sleep apnea if parents note habitual nightly snoring, especially if accompanied by pauses, snorts, or gasps (SOR: C, based on case series and expert opinion). Single overnight polysomnography is the test of choice to evaluate a child for obstructive sleep apnea, since it can’t be excluded by history or physical exam. Other evaluation methods for sleep apnea lack either adequate sensitivity or specificity (SOR: C, based on case series, systematic review of case series, and expert opinion).
Add a question about snoring to your parental questionnaire
John D. Hallgren, MD
Uniformed Services University of the Health Sciences, RAF Menwith Hill, United Kingdom
How many of us routinely ask about snoring or sleep apnea at a wellness visit for our young patients? Here we learn that asking a simple question—“Does your child snore heavily most nights or seem to stop breathing when he or she sleeps?”—improves our ability to recognize obstructive sleep apnea in children.
In addition, a relatively simple, one-time, noninvasive test tells us whether the child has obstructive sleep apnea—or not. In light of this information, I plan on putting a revised well-child visit questionnaire that asks about snoring/apnea in my waiting room right away.
Evidence summary
Experts advise screening all children for obstructive sleep apnea by asking about snoring and sleep apnea at routine health supervision visits.1 Obstructive sleep apnea among children has an overall prevalence of 2% but occurs in approximately 13% to 25% of those who snore regularly and heavily.1,2
Obstructive sleep apnea is unlikely in the absence of habitual snoring (expert opinion), and snoring loudness does not correlate with severity. Children with obstructive sleep apnea tend to exhibit symptoms typical of attention-deficit and hyperactivity disorder rather than daytime somnolence.2
Adenotonsillar hypertrophy may be to blame
The most common cause of obstructive sleep apnea is adenotonsillar hypertrophy. Additional risk factors include obesity, allergic rhinitis, neuromuscular disease, craniofacial anomalies, Down syndrome, premature birth, and family history.1
A prospective case-series of 62 consecutive children referred to a sleep clinic for suspected obstructive sleep apnea correlated nightly snoring (relative risk [RR]=10.67; 95% confidence interval [CI], 2.72–41.8) and observed apnea (RR=3.43; 95% CI, 1.42–6.76) with obstructive sleep apnea diagnosed by polysomnography.3 Children with underlying neurological or craniofacial problems were excluded. A retrospective, nonconsecutive cohort study of 50 children previously diagnosed with obstructive sleep apnea found that all presented with “continuous heavy snoring, interrupted by pauses and associated with snorts.”4
Don’t count on the physical exam and history
Experts consider overnight polysomnography to be the gold standard test for obstructive sleep apnea.2 Physical examination alone usually has normal results. A systematic review of 12 studies including 1058 children under age 18 compared evaluation by polysomnography with clinical evaluation by history and physical examination alone. Clinical evaluation had a positive predictive value (PPV) of 55.8% (95% CI, 42.1%–69.6%); no single component demonstrated sensitivity and specificity more than 65%.5
Symptom questionnaires or scales did not correlate well with polysomnography results in 3 studies (which excluded children with craniofacial anomalies, significant medical problems, or prior upper airway surgery). The best study—a prospective, randomized, investigator-blinded, controlled trial of 59 consecutive children clinically diagnosed with obstructive sleep apnea—compared a “clinical assessment score” (comprised of standardized history, physical exam, voice recording, sleep audio recording, lateral neck radiograph, and echocardiogram) with overnight polysomnography. The majority of children had mild obstructive sleep apnea. Overall, the clinical assessment score did not correlate well with polysomnography (PPV=48%).6
Avoid sequential and abbreviated tests
Sequential overnight polysomnography did not dramatically improve sensitivity or specificity over a single test in 2 prospective studies of children with suspected obstructive sleep apnea. The larger study recruited 70 consecutive children (ages 2 to 17 years) from a sleep lab and performed polysomnography on 2 consecutive nights. The first test correctly identified obstructive sleep apnea in 64 children; the second identified 6 additional cases, all of which were “mild.”7
Abbreviated polysomnographies have inadequate sensitivity. A retrospective chart review included 143 children between 1 and 18 years of age with adenotonsillar hypertrophy referred for overnight polysomnography after normal or mildly abnormal “nap polysomnography.” Mildly abnormal nap polysomnography predicted abnormal overnight polysomnography (PPV=77%; P<.0001), but normal nap polysomnography were not predictive of normal overnight polysomnography (NPV=49%; P=.8).8
Video recordings have low specificity
A prospective cohort study of home sleep video recordings included 58 consecutive children who were 2 to 6 years of age with snoring or labored breathing during sleep. Parents filmed 30 minutes of sleep during “worst breathing” episodes. An expert investigator evaluated the recordings using both a standardized scoring system and subjective impression, and compared these with overnight polysomnography results, finding 94% sensitivity and 68% specificity.9
Two prospective trials compared standardized scoring of home sleep audio recordings with overnight polysomnography testing. The best study, an investigator-blinded RCT, scored 20-minute recordings of “worst breathing” in 59 consecutive children referred for snoring or nocturnal breathing problems, 47% of whom had sleep apnea. Results: PPV=62%, NPV=83%, sensitivity=88%, specificity=52%.7
Abnormal pulse ox is highly predictive
A retrospective, cross-sectional study of 349 children between the ages of 6 months and 18 years determined that abnormal home pulse oximetry studies were highly predictive of obstructive sleep apnea on polysomnography (positive likelihood ratio [LR+]=19.4; CIs not given; posttest probability=97%), but that inconclusive or normal pulse oximetry studies were not predictive of negative polysomnographies (LR+=0.58; CIs not given; posttest probability 47%). Children had been referred for suspected obstructive sleep apnea, and the pretest probability was 60%. Oximetry records were evaluated by a sleep laboratory physician blinded to clinical and polysomnography data.10
Tonsillar-pharyngeal ratio misses mild cases
A prospective cohort study of 35 children compared the tonsillar-pharyngeal ratio measured from lateral neck x-rays to overnight polysomnography. X-ray measurements predicted moderate or severe obstructive sleep apnea on polysomnography (sensitivity=96%, specificity=82%, PPV=92%, NPV=90%), but did not accurately differentiate normal children from those with mild obstructive sleep apnea.11
Recommendations from others
The American Academy of Pediatrics recommends that children be screened during well child visits for regular snoring or apnea episodes during sleep. Children with positive screens should have an overnight polysomnography. Children with confirmed obstructive sleep apnea should be referred to a sleep medicine specialist to consider continuous positive airway pressure therapy during sleep, or to an otolaryngologist for possible surgery (tonsillectomy or adenoidectomy).2
1. Li AM, Chan DF, Fok TF, Wing YK. Childhood obstructive sleep apnoea: an update. Hong Kong Med J 2004;10:406-413.
2. Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome, American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109:704-712.
3. Chau KW, Ng DKK, Kwok CKL, Chow PY, Ho JCS. Clinical risk factors for obstructive sleep apnoea in children. Singapore Med J 2003;44:570-573.
4. Guilleminault C, Korobkin R, Winkle R. A review of 50 children with obstructive sleep apnea syndrome. Lung 1981;159:275-287.
5. Brietzke SE, Katz ES, Roberson DW. Can history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypopnea syndrome? A systematic review of the literature. Otolaryngol Head Neck Surg 2004;131:827-832.
6. Goldstein NA, Pugazhendhi V, Rao SM, et al. Clinical assessment of pediatric obstructive sleep apnea. Pediatrics 2004;114:33-43.
7. Verhulst SL, Schrauwen N, De Backer WA, Desager KN. First night effect for polysomnographic data in children and adolescents with suspected sleep disordered breathing. Arch Dis Child 2006;91:233-237.
8. Saeed MM, Keens TG, Stabile MW, Bolokowicz J, Davidson Ward SL. Should children with suspected obstructive sleep apnea syndrome and normal nap sleep studies have overnight sleep studies? Chest 2000;118:360-365.
9. Sivan Y, Kornecki A, Schonfeld T. Screening obstructive sleep apnoea syndrome by home videotape recording in children. Eur Respir J 1996;9:2127-2131.
10. Brouillette RT, Morielli A, Leimanis A, Waters KA, Luciano R, Ducharme FM. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics 2000;105:405-412.
11. Li AM, Wong E, Kew J, Hui S, Fok TF. Use of tonsil size in the evaluation of obstructive sleep apnoea. Arch Dis Child 2002;87:156-159.
1. Li AM, Chan DF, Fok TF, Wing YK. Childhood obstructive sleep apnoea: an update. Hong Kong Med J 2004;10:406-413.
2. Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome, American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109:704-712.
3. Chau KW, Ng DKK, Kwok CKL, Chow PY, Ho JCS. Clinical risk factors for obstructive sleep apnoea in children. Singapore Med J 2003;44:570-573.
4. Guilleminault C, Korobkin R, Winkle R. A review of 50 children with obstructive sleep apnea syndrome. Lung 1981;159:275-287.
5. Brietzke SE, Katz ES, Roberson DW. Can history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypopnea syndrome? A systematic review of the literature. Otolaryngol Head Neck Surg 2004;131:827-832.
6. Goldstein NA, Pugazhendhi V, Rao SM, et al. Clinical assessment of pediatric obstructive sleep apnea. Pediatrics 2004;114:33-43.
7. Verhulst SL, Schrauwen N, De Backer WA, Desager KN. First night effect for polysomnographic data in children and adolescents with suspected sleep disordered breathing. Arch Dis Child 2006;91:233-237.
8. Saeed MM, Keens TG, Stabile MW, Bolokowicz J, Davidson Ward SL. Should children with suspected obstructive sleep apnea syndrome and normal nap sleep studies have overnight sleep studies? Chest 2000;118:360-365.
9. Sivan Y, Kornecki A, Schonfeld T. Screening obstructive sleep apnoea syndrome by home videotape recording in children. Eur Respir J 1996;9:2127-2131.
10. Brouillette RT, Morielli A, Leimanis A, Waters KA, Luciano R, Ducharme FM. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics 2000;105:405-412.
11. Li AM, Wong E, Kew J, Hui S, Fok TF. Use of tonsil size in the evaluation of obstructive sleep apnoea. Arch Dis Child 2002;87:156-159.
Evidence-based answers from the Family Physicians Inquiries Network