User login
Dehydration in terminal illness: Which path forward?
CASE 1
A 94-year-old white woman, who had been in excellent health (other than pernicious anemia, treated with monthly cyanocobalamin injections), suddenly developed gastrointestinal distress 2 weeks earlier. A work-up performed by her physician revealed advanced pancreatic cancer.
Over the next 2 weeks, she experienced pain and nausea. A left-sided fistula developed externally at her flank that drained feces and induced considerable discomfort. An indwelling drain was placed, which provided some relief, but the patient’s dyspepsia, pain, and nausea escalated.
One month into her disease course, an oncologist reported on her potential treatment options and prognosis. Her life expectancy was about 3 months without treatment. This could be extended by 1 to 2 months with extensive surgical and chemotherapeutic interventions, but would further diminish her quality of life. The patient declined further treatment.
Her clinical status declined, and her quality of life significantly deteriorated. At 3 months, she felt life had lost meaning and was not worth living. She began asking for a morphine overdose, stating a desire to end her life.
After several discussions with the oncologist, one of the patient’s adult children suggested that her mother stop eating and drinking in order to diminish discomfort and hasten her demise. This plan was adopted, and the patient declined food and drank only enough to swish for oral comfort.
CASE 2
An 83-year-old woman with advanced Parkinson’s disease had become increasingly disabled. Her gait and motor skills were dramatically and progressively compromised. Pharmacotherapy yielded only transient improvement and considerable adverse effects of choreiform hyperkinesia and hallucinations, which were troublesome and embarrassing. Her social, physical, and personal well-being declined to the point that she was placed in a nursing home.
Despite this help, worsening parkinsonism progressively diminished her physical capacity. She became largely bedridden and developed decubitus ulcerations, especially at the coccyx, which produced severe pain and distress.
Continue to: The confluence of pain...
The confluence of pain, bedfastness, constipation, and social isolation yielded a loss of interest and joy in life. The patient required assistance with almost every aspect of daily life, including eating. As the illness progressed, she prayed at night that God would “take her.” Each morning, she spoke of disappointment upon reawakening. She overtly expressed her lack of desire to live to her family. Medical interventions were increasingly ineffective.
After repeated family and physician discussions had focused on her death wishes, one adult daughter recommended her mother stop eating and drinking; her food intake was already minimal. Although she did not endorse this plan verbally, the patient’s oral intake significantly diminished. Within 2 weeks, her physical state had declined, and she died one night during sleep.
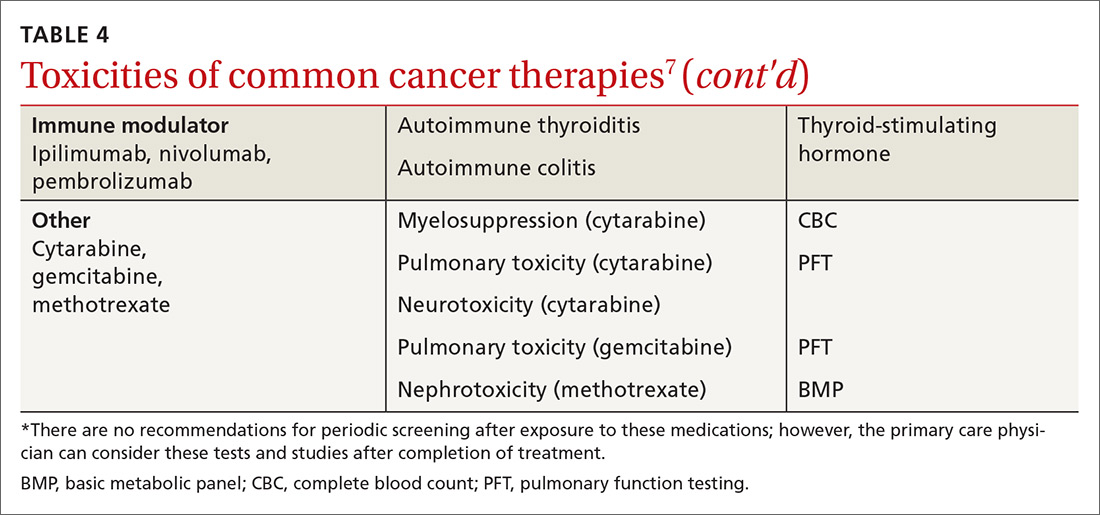
Adequate hydration is stressed in physician education and practice. A conventional expectation to normalize fluid balance is important to restore health and improve well-being. In addition to being good medical practice, it can also show patients (and their families) that we care about their well-being.1-3
Treating dehydration in individuals with terminal illness is controversial from both medical and ethical standpoints. While the natural tendency of physicians is to restore full hydration to their patients, in select cases of imminent death, being fully hydrated may prolong discomfort.1,2 Emphasis in this population should be consistently placed on improving comfort care and quality of life, rather than prolonging life or delaying death.3-5
Continue to: A multifactorial, patient-based decision
A multifactorial, patient-based decision
Years ago, before the advent of hospitalizing people with terminal illnesses, dying at home amongst loved ones was believed to be peaceful. Nevertheless, questions arise about the practical vs ethical approach to caring for patients with terminal illness.2 Sometimes it is difficult to find a balance between potential health care benefits and the burdens induced by medical, legal, moral, and/or social pressures. Our medical communities and the general population uphold preserving dignity at the end of life, which is supported by organizations such as Compassion & Choices (a nonprofit group that seeks to improve and expand options for end of life care; https://www.compassionandchoices.org).
Allowing for voluntary, patient-determined dehydration in those with terminal illness can offer greater comfort than maintaining the physiologic degrees of fluid balance. There are 3 key considerations to bear in mind:
- Hydration is usually a standard part of quality medical care.1
- Selectively allowing dehydration in patients who are dying can facilitate comfort.1-5
- Dehydration may be a deliberate strategy to hasten death.6
When is dehydration appropriate?
Hydration is not favored whenever doing so may increase discomfort and prolong pain without meaningful life.3 In people with terminal illness, hydration may reduce quality of life.7
The data support dehydration in certain patients. A randomized controlled trial involving 129 patients receiving hospice care compared parenteral hydration with placebo, documenting that rehydration did not improve symptoms or quality of life; there was no significant difference between patients who were hydrated and those who were dehydrated.7 In fact, dehydration may even yield greater patient comfort.8
Case reports, retrospective chart reviews, and testimonials from health care professionals have reported that being less hydrated can diminish nausea, vomiting, diarrhea, ascites, edema, and urinary or bowel incontinence, with less skin breakdown.8 Hydration, on the other hand, may exacerbate dyspnea, coughing, and choking, increasing the need for suctioning.
Continue to: A component of palliative care
A component of palliative care. When death is imminent, palliation becomes key. Pain may be more manageable with less fluids, an important goal for this population.6,8 Dehydration is associated with an accumulation of opioids throughout body fluid volumes, which may decrease pain, consciousness, and/or agony.2 Pharmacotherapies might also have greater efficacy in a dehydrated patient.9 In addition, tissue shrinkage might mitigate pain from tumors, especially those in confined spaces.8
Hospice care and palliative medicine confirm that routine hydration is not always advisable; allowing for dehydration is a conventional practice, especially in older adults with terminal illness.7 However, do not deny access to liquids if a patient wants them, and never force unwanted fluids by any route.8 Facilitate oral care in the form of swishing fluids, elective drinking, or providing mouth lubrication for any patients selectively allowed to become dehydrated.3,8
The role of the physician in decision-making
Patients with terminal illness sometimes do not want fluids and may actively decline food and drink.10 This can be emotionally distressing for family members and/or caregivers to witness. Physicians can address this concern by compassionately explaining: “I know you are concerned that your relative is not eating or drinking, but there is no indication that hydration or parenteral feeding will improve function or quality of life.”10 This can generate a discussion between physicians and families by acknowledging concerns, relieving distress, and leading to what is ultimately best for the patient.
Implications for practice: Individualized autonomy
Physicians must identify patients who wish to die by purposely becoming dehydrated and uphold the important physician obligation to hydrate those with a recoverable illness. Allowing for a moderate degree of dehydration might provide greater comfort in select people with terminal illness. Some individuals for whom life has lost meaning may choose dehydration as a means to hasten their departure.4-6 Allowing individualized autonomy over life and death choices is part of a physician’s obligation to their patients. It can be difficult for caregivers, but it is medically indicated to comply with a patient’s desire for comfort when death is imminent.
Providing palliation as a priority over treatment is sometimes challenging, but comfort care takes preference and is always coordinated with the person’s own wishes. Facilitating dehydration removes assisted-suicide issues or requests and thus affords everyone involved more emotional comfort. An advantage of this method is that a decisional patient maintains full control over the direction of their choices and helps preserve dignity during the end of life.
CORRESPONDENCE
Steven Lippmann, MD, Department of Psychiatry, University of Louisville School of Medicine, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected]
1. Burge FI. Dehydration and provision of fluids in palliative care. What is the evidence? Can Fam Physician. 1996;42:2383-2388.
2. Printz LA. Is withholding hydration a valid comfort measure in the terminally ill? Geriatrics. 1988;43:84-88.
3. Lippmann S. Palliative dehydration. Prim Care Companion CNS Disord. 2015;17: doi: 10.4088/PCC.15101797.
4. Bernat JL, Gert B, Mogielnicki RP. Patient refusal of hydration and nutrition: an alternative to physician-assisted suicide or voluntary active euthanasia. Arch Intern Med. 1993;153:2723-2728.
5. Sullivan RJ. Accepting death without artificial nutrition or hydration. J Gen Intern Med.1993;8:220-224.
6. Miller FG, Meier DE. Voluntary death: a comparison of terminal dehydration and physician-assisted suicide. Ann Intern Med. 1998;128:559-562.
7. Bruera E, Hui D, Dalal S, et al. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol. 2013;31:111-118.
8. Forrow L, Smith HS. Pain management in end of life: palliative care. In: Warfield CA, Bajwa ZH, ed. Principles and Practice of Pain Management. 2nd ed. New York, NY: McGraw-Hill; 2004.
9. Zerwekh JV. The dehydration question. Nursing. 1983;13:47-51.
10. Bailey F, Harman S. Palliative care: The last hours and days of life. www.uptodate.com. September, 2016. Accessed on September 11, 2018.
CASE 1
A 94-year-old white woman, who had been in excellent health (other than pernicious anemia, treated with monthly cyanocobalamin injections), suddenly developed gastrointestinal distress 2 weeks earlier. A work-up performed by her physician revealed advanced pancreatic cancer.
Over the next 2 weeks, she experienced pain and nausea. A left-sided fistula developed externally at her flank that drained feces and induced considerable discomfort. An indwelling drain was placed, which provided some relief, but the patient’s dyspepsia, pain, and nausea escalated.
One month into her disease course, an oncologist reported on her potential treatment options and prognosis. Her life expectancy was about 3 months without treatment. This could be extended by 1 to 2 months with extensive surgical and chemotherapeutic interventions, but would further diminish her quality of life. The patient declined further treatment.
Her clinical status declined, and her quality of life significantly deteriorated. At 3 months, she felt life had lost meaning and was not worth living. She began asking for a morphine overdose, stating a desire to end her life.
After several discussions with the oncologist, one of the patient’s adult children suggested that her mother stop eating and drinking in order to diminish discomfort and hasten her demise. This plan was adopted, and the patient declined food and drank only enough to swish for oral comfort.
CASE 2
An 83-year-old woman with advanced Parkinson’s disease had become increasingly disabled. Her gait and motor skills were dramatically and progressively compromised. Pharmacotherapy yielded only transient improvement and considerable adverse effects of choreiform hyperkinesia and hallucinations, which were troublesome and embarrassing. Her social, physical, and personal well-being declined to the point that she was placed in a nursing home.
Despite this help, worsening parkinsonism progressively diminished her physical capacity. She became largely bedridden and developed decubitus ulcerations, especially at the coccyx, which produced severe pain and distress.
Continue to: The confluence of pain...
The confluence of pain, bedfastness, constipation, and social isolation yielded a loss of interest and joy in life. The patient required assistance with almost every aspect of daily life, including eating. As the illness progressed, she prayed at night that God would “take her.” Each morning, she spoke of disappointment upon reawakening. She overtly expressed her lack of desire to live to her family. Medical interventions were increasingly ineffective.
After repeated family and physician discussions had focused on her death wishes, one adult daughter recommended her mother stop eating and drinking; her food intake was already minimal. Although she did not endorse this plan verbally, the patient’s oral intake significantly diminished. Within 2 weeks, her physical state had declined, and she died one night during sleep.
Adequate hydration is stressed in physician education and practice. A conventional expectation to normalize fluid balance is important to restore health and improve well-being. In addition to being good medical practice, it can also show patients (and their families) that we care about their well-being.1-3
Treating dehydration in individuals with terminal illness is controversial from both medical and ethical standpoints. While the natural tendency of physicians is to restore full hydration to their patients, in select cases of imminent death, being fully hydrated may prolong discomfort.1,2 Emphasis in this population should be consistently placed on improving comfort care and quality of life, rather than prolonging life or delaying death.3-5
Continue to: A multifactorial, patient-based decision
A multifactorial, patient-based decision
Years ago, before the advent of hospitalizing people with terminal illnesses, dying at home amongst loved ones was believed to be peaceful. Nevertheless, questions arise about the practical vs ethical approach to caring for patients with terminal illness.2 Sometimes it is difficult to find a balance between potential health care benefits and the burdens induced by medical, legal, moral, and/or social pressures. Our medical communities and the general population uphold preserving dignity at the end of life, which is supported by organizations such as Compassion & Choices (a nonprofit group that seeks to improve and expand options for end of life care; https://www.compassionandchoices.org).
Allowing for voluntary, patient-determined dehydration in those with terminal illness can offer greater comfort than maintaining the physiologic degrees of fluid balance. There are 3 key considerations to bear in mind:
- Hydration is usually a standard part of quality medical care.1
- Selectively allowing dehydration in patients who are dying can facilitate comfort.1-5
- Dehydration may be a deliberate strategy to hasten death.6
When is dehydration appropriate?
Hydration is not favored whenever doing so may increase discomfort and prolong pain without meaningful life.3 In people with terminal illness, hydration may reduce quality of life.7
The data support dehydration in certain patients. A randomized controlled trial involving 129 patients receiving hospice care compared parenteral hydration with placebo, documenting that rehydration did not improve symptoms or quality of life; there was no significant difference between patients who were hydrated and those who were dehydrated.7 In fact, dehydration may even yield greater patient comfort.8
Case reports, retrospective chart reviews, and testimonials from health care professionals have reported that being less hydrated can diminish nausea, vomiting, diarrhea, ascites, edema, and urinary or bowel incontinence, with less skin breakdown.8 Hydration, on the other hand, may exacerbate dyspnea, coughing, and choking, increasing the need for suctioning.
Continue to: A component of palliative care
A component of palliative care. When death is imminent, palliation becomes key. Pain may be more manageable with less fluids, an important goal for this population.6,8 Dehydration is associated with an accumulation of opioids throughout body fluid volumes, which may decrease pain, consciousness, and/or agony.2 Pharmacotherapies might also have greater efficacy in a dehydrated patient.9 In addition, tissue shrinkage might mitigate pain from tumors, especially those in confined spaces.8
Hospice care and palliative medicine confirm that routine hydration is not always advisable; allowing for dehydration is a conventional practice, especially in older adults with terminal illness.7 However, do not deny access to liquids if a patient wants them, and never force unwanted fluids by any route.8 Facilitate oral care in the form of swishing fluids, elective drinking, or providing mouth lubrication for any patients selectively allowed to become dehydrated.3,8
The role of the physician in decision-making
Patients with terminal illness sometimes do not want fluids and may actively decline food and drink.10 This can be emotionally distressing for family members and/or caregivers to witness. Physicians can address this concern by compassionately explaining: “I know you are concerned that your relative is not eating or drinking, but there is no indication that hydration or parenteral feeding will improve function or quality of life.”10 This can generate a discussion between physicians and families by acknowledging concerns, relieving distress, and leading to what is ultimately best for the patient.
Implications for practice: Individualized autonomy
Physicians must identify patients who wish to die by purposely becoming dehydrated and uphold the important physician obligation to hydrate those with a recoverable illness. Allowing for a moderate degree of dehydration might provide greater comfort in select people with terminal illness. Some individuals for whom life has lost meaning may choose dehydration as a means to hasten their departure.4-6 Allowing individualized autonomy over life and death choices is part of a physician’s obligation to their patients. It can be difficult for caregivers, but it is medically indicated to comply with a patient’s desire for comfort when death is imminent.
Providing palliation as a priority over treatment is sometimes challenging, but comfort care takes preference and is always coordinated with the person’s own wishes. Facilitating dehydration removes assisted-suicide issues or requests and thus affords everyone involved more emotional comfort. An advantage of this method is that a decisional patient maintains full control over the direction of their choices and helps preserve dignity during the end of life.
CORRESPONDENCE
Steven Lippmann, MD, Department of Psychiatry, University of Louisville School of Medicine, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected]
CASE 1
A 94-year-old white woman, who had been in excellent health (other than pernicious anemia, treated with monthly cyanocobalamin injections), suddenly developed gastrointestinal distress 2 weeks earlier. A work-up performed by her physician revealed advanced pancreatic cancer.
Over the next 2 weeks, she experienced pain and nausea. A left-sided fistula developed externally at her flank that drained feces and induced considerable discomfort. An indwelling drain was placed, which provided some relief, but the patient’s dyspepsia, pain, and nausea escalated.
One month into her disease course, an oncologist reported on her potential treatment options and prognosis. Her life expectancy was about 3 months without treatment. This could be extended by 1 to 2 months with extensive surgical and chemotherapeutic interventions, but would further diminish her quality of life. The patient declined further treatment.
Her clinical status declined, and her quality of life significantly deteriorated. At 3 months, she felt life had lost meaning and was not worth living. She began asking for a morphine overdose, stating a desire to end her life.
After several discussions with the oncologist, one of the patient’s adult children suggested that her mother stop eating and drinking in order to diminish discomfort and hasten her demise. This plan was adopted, and the patient declined food and drank only enough to swish for oral comfort.
CASE 2
An 83-year-old woman with advanced Parkinson’s disease had become increasingly disabled. Her gait and motor skills were dramatically and progressively compromised. Pharmacotherapy yielded only transient improvement and considerable adverse effects of choreiform hyperkinesia and hallucinations, which were troublesome and embarrassing. Her social, physical, and personal well-being declined to the point that she was placed in a nursing home.
Despite this help, worsening parkinsonism progressively diminished her physical capacity. She became largely bedridden and developed decubitus ulcerations, especially at the coccyx, which produced severe pain and distress.
Continue to: The confluence of pain...
The confluence of pain, bedfastness, constipation, and social isolation yielded a loss of interest and joy in life. The patient required assistance with almost every aspect of daily life, including eating. As the illness progressed, she prayed at night that God would “take her.” Each morning, she spoke of disappointment upon reawakening. She overtly expressed her lack of desire to live to her family. Medical interventions were increasingly ineffective.
After repeated family and physician discussions had focused on her death wishes, one adult daughter recommended her mother stop eating and drinking; her food intake was already minimal. Although she did not endorse this plan verbally, the patient’s oral intake significantly diminished. Within 2 weeks, her physical state had declined, and she died one night during sleep.
Adequate hydration is stressed in physician education and practice. A conventional expectation to normalize fluid balance is important to restore health and improve well-being. In addition to being good medical practice, it can also show patients (and their families) that we care about their well-being.1-3
Treating dehydration in individuals with terminal illness is controversial from both medical and ethical standpoints. While the natural tendency of physicians is to restore full hydration to their patients, in select cases of imminent death, being fully hydrated may prolong discomfort.1,2 Emphasis in this population should be consistently placed on improving comfort care and quality of life, rather than prolonging life or delaying death.3-5
Continue to: A multifactorial, patient-based decision
A multifactorial, patient-based decision
Years ago, before the advent of hospitalizing people with terminal illnesses, dying at home amongst loved ones was believed to be peaceful. Nevertheless, questions arise about the practical vs ethical approach to caring for patients with terminal illness.2 Sometimes it is difficult to find a balance between potential health care benefits and the burdens induced by medical, legal, moral, and/or social pressures. Our medical communities and the general population uphold preserving dignity at the end of life, which is supported by organizations such as Compassion & Choices (a nonprofit group that seeks to improve and expand options for end of life care; https://www.compassionandchoices.org).
Allowing for voluntary, patient-determined dehydration in those with terminal illness can offer greater comfort than maintaining the physiologic degrees of fluid balance. There are 3 key considerations to bear in mind:
- Hydration is usually a standard part of quality medical care.1
- Selectively allowing dehydration in patients who are dying can facilitate comfort.1-5
- Dehydration may be a deliberate strategy to hasten death.6
When is dehydration appropriate?
Hydration is not favored whenever doing so may increase discomfort and prolong pain without meaningful life.3 In people with terminal illness, hydration may reduce quality of life.7
The data support dehydration in certain patients. A randomized controlled trial involving 129 patients receiving hospice care compared parenteral hydration with placebo, documenting that rehydration did not improve symptoms or quality of life; there was no significant difference between patients who were hydrated and those who were dehydrated.7 In fact, dehydration may even yield greater patient comfort.8
Case reports, retrospective chart reviews, and testimonials from health care professionals have reported that being less hydrated can diminish nausea, vomiting, diarrhea, ascites, edema, and urinary or bowel incontinence, with less skin breakdown.8 Hydration, on the other hand, may exacerbate dyspnea, coughing, and choking, increasing the need for suctioning.
Continue to: A component of palliative care
A component of palliative care. When death is imminent, palliation becomes key. Pain may be more manageable with less fluids, an important goal for this population.6,8 Dehydration is associated with an accumulation of opioids throughout body fluid volumes, which may decrease pain, consciousness, and/or agony.2 Pharmacotherapies might also have greater efficacy in a dehydrated patient.9 In addition, tissue shrinkage might mitigate pain from tumors, especially those in confined spaces.8
Hospice care and palliative medicine confirm that routine hydration is not always advisable; allowing for dehydration is a conventional practice, especially in older adults with terminal illness.7 However, do not deny access to liquids if a patient wants them, and never force unwanted fluids by any route.8 Facilitate oral care in the form of swishing fluids, elective drinking, or providing mouth lubrication for any patients selectively allowed to become dehydrated.3,8
The role of the physician in decision-making
Patients with terminal illness sometimes do not want fluids and may actively decline food and drink.10 This can be emotionally distressing for family members and/or caregivers to witness. Physicians can address this concern by compassionately explaining: “I know you are concerned that your relative is not eating or drinking, but there is no indication that hydration or parenteral feeding will improve function or quality of life.”10 This can generate a discussion between physicians and families by acknowledging concerns, relieving distress, and leading to what is ultimately best for the patient.
Implications for practice: Individualized autonomy
Physicians must identify patients who wish to die by purposely becoming dehydrated and uphold the important physician obligation to hydrate those with a recoverable illness. Allowing for a moderate degree of dehydration might provide greater comfort in select people with terminal illness. Some individuals for whom life has lost meaning may choose dehydration as a means to hasten their departure.4-6 Allowing individualized autonomy over life and death choices is part of a physician’s obligation to their patients. It can be difficult for caregivers, but it is medically indicated to comply with a patient’s desire for comfort when death is imminent.
Providing palliation as a priority over treatment is sometimes challenging, but comfort care takes preference and is always coordinated with the person’s own wishes. Facilitating dehydration removes assisted-suicide issues or requests and thus affords everyone involved more emotional comfort. An advantage of this method is that a decisional patient maintains full control over the direction of their choices and helps preserve dignity during the end of life.
CORRESPONDENCE
Steven Lippmann, MD, Department of Psychiatry, University of Louisville School of Medicine, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected]
1. Burge FI. Dehydration and provision of fluids in palliative care. What is the evidence? Can Fam Physician. 1996;42:2383-2388.
2. Printz LA. Is withholding hydration a valid comfort measure in the terminally ill? Geriatrics. 1988;43:84-88.
3. Lippmann S. Palliative dehydration. Prim Care Companion CNS Disord. 2015;17: doi: 10.4088/PCC.15101797.
4. Bernat JL, Gert B, Mogielnicki RP. Patient refusal of hydration and nutrition: an alternative to physician-assisted suicide or voluntary active euthanasia. Arch Intern Med. 1993;153:2723-2728.
5. Sullivan RJ. Accepting death without artificial nutrition or hydration. J Gen Intern Med.1993;8:220-224.
6. Miller FG, Meier DE. Voluntary death: a comparison of terminal dehydration and physician-assisted suicide. Ann Intern Med. 1998;128:559-562.
7. Bruera E, Hui D, Dalal S, et al. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol. 2013;31:111-118.
8. Forrow L, Smith HS. Pain management in end of life: palliative care. In: Warfield CA, Bajwa ZH, ed. Principles and Practice of Pain Management. 2nd ed. New York, NY: McGraw-Hill; 2004.
9. Zerwekh JV. The dehydration question. Nursing. 1983;13:47-51.
10. Bailey F, Harman S. Palliative care: The last hours and days of life. www.uptodate.com. September, 2016. Accessed on September 11, 2018.
1. Burge FI. Dehydration and provision of fluids in palliative care. What is the evidence? Can Fam Physician. 1996;42:2383-2388.
2. Printz LA. Is withholding hydration a valid comfort measure in the terminally ill? Geriatrics. 1988;43:84-88.
3. Lippmann S. Palliative dehydration. Prim Care Companion CNS Disord. 2015;17: doi: 10.4088/PCC.15101797.
4. Bernat JL, Gert B, Mogielnicki RP. Patient refusal of hydration and nutrition: an alternative to physician-assisted suicide or voluntary active euthanasia. Arch Intern Med. 1993;153:2723-2728.
5. Sullivan RJ. Accepting death without artificial nutrition or hydration. J Gen Intern Med.1993;8:220-224.
6. Miller FG, Meier DE. Voluntary death: a comparison of terminal dehydration and physician-assisted suicide. Ann Intern Med. 1998;128:559-562.
7. Bruera E, Hui D, Dalal S, et al. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol. 2013;31:111-118.
8. Forrow L, Smith HS. Pain management in end of life: palliative care. In: Warfield CA, Bajwa ZH, ed. Principles and Practice of Pain Management. 2nd ed. New York, NY: McGraw-Hill; 2004.
9. Zerwekh JV. The dehydration question. Nursing. 1983;13:47-51.
10. Bailey F, Harman S. Palliative care: The last hours and days of life. www.uptodate.com. September, 2016. Accessed on September 11, 2018.
Strategies for caring for the well cancer survivor
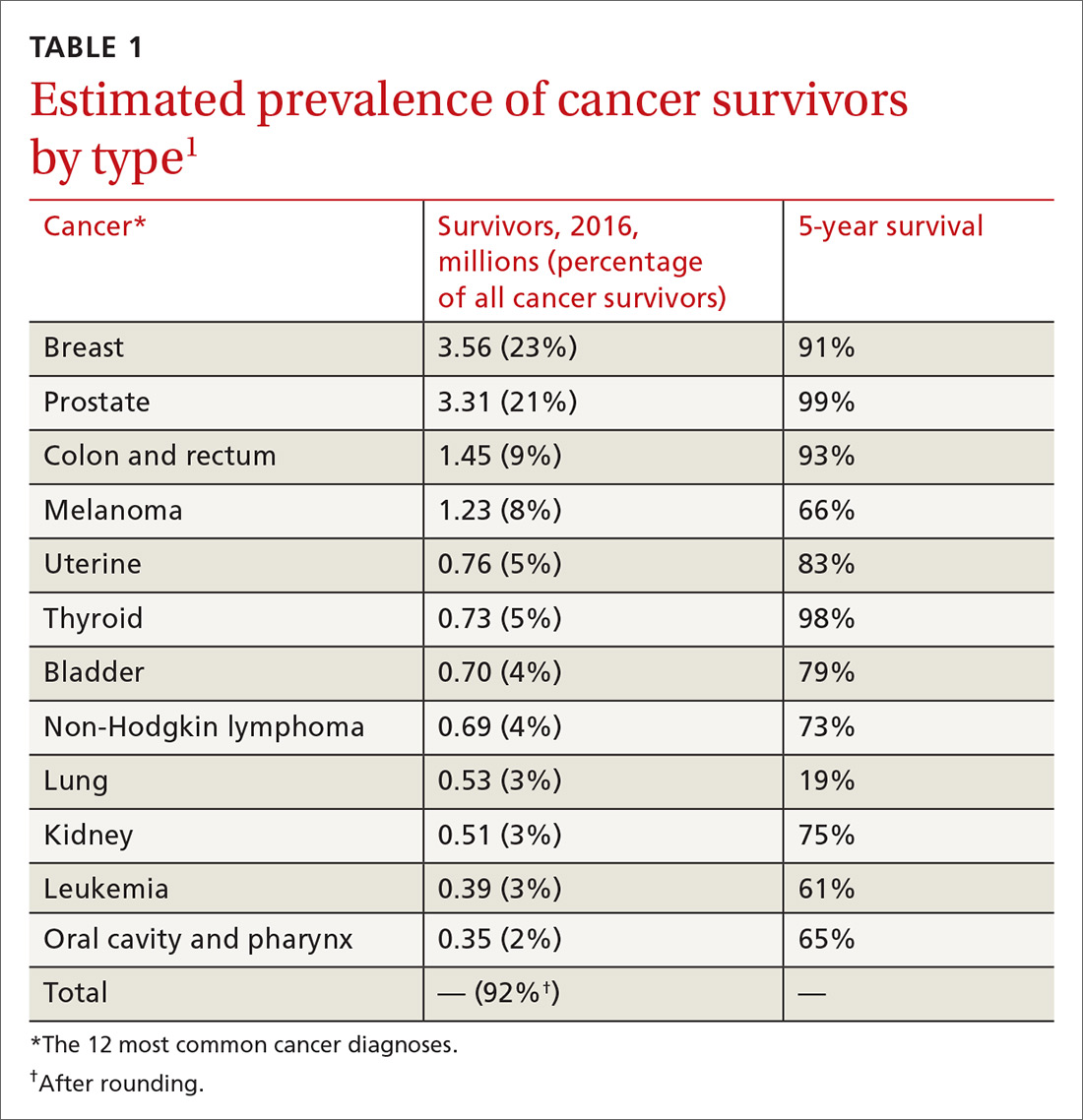
Cancer survivors represent a rapidly increasing population. In 1971, there were 3 million cancer survivors; this number increased to 15.5 million in 2016 and will reach 20 million by 2026.1TABLE 11 shows the percentage of survivors by type of cancer. Cancer survivors tend to be older,* comprising nearly 1 of every 5 people older than 65 years.2
The Institute of Medicine (IOM) identified 3 key characteristics of cancer survivors3:
- Trajectories of survivorship are variable; many cancer patients have periods of relative health between episodes of their disease.
- Survivors require careful cancer monitoring; in addition to the risk that their primary cancer will recur, they have an elevated risk for another, second cancer.
- Both cancer and its treatments increase the risk of other medical and psychiatric problems.
Family physicians (FPs) have optimal skills for navigating the chronic risks and health concerns of the well cancer survivor. This article reviews the primary care management of the functional cancer survivor, focusing on the management of chronic conditions and preventive care.
Survivorship follows any of 6 paths
Cancer survivorship is increasing in importance as treatment has steadily reduced mortality. Six trajectories of cancer survivors have been identified1:
- living cancer-free after treatment with minimal effects
- living cancer-free but suffering serious treatment complications
- Suffering late recurrence
- Developing a second cancer
- Living with intermittent cancer recurrences
- Living with cancer continuously.
Only patients in the last 2 groups are likely to be managed primarily by oncologists.
Survivors look to their FPs for ongoing care
Cancer survivors routinely see their primary care physician after initial treatment. A study of 30,000 Canadian breast cancer survivors demonstrated that follow-up care was limited to an oncologist in only 2%; 84% saw a primary care provider and an oncologist; and 14% saw a primary care provider only.4 A study of colorectal cancer survivors showed that primary care visits increased in each of the 5 years after diagnosis, during which time oncology visits decreased steadily5; in that study, primary care physicians delivered more preventive care than oncologists did.5 Similar to what is done in other chronic conditions, the various effects of cancer are best managed as a whole.
The IOM recommends that cancer survivor care comprise 4 elements2:
- coordination between oncologist and primary care physician
- surveillance for recurrence or spread of existing cancer
- screening for new cancer
- intervention for the effects of cancer and treatment.
Continue to: The following discussion summarizes...
The following discussion summarizes evidence and recommendations for each element of the IOM recommendations for survivor care.
Implementing the 4 elements of cancer survivor care
1. Coordinate care through a unified survivorship care plan
The IOM has noted that the needs of cancer survivors are rarely met2; communication between oncology and primary care is often deficient during transition of care. The IOM has recommended that oncologists provide a survivorship care plan that details the cancer (ie, tumor characteristics), the type of treatment (ie, enrollment in a clinical trial; medical, surgical, or radiation), support services, and follow-up recommendations for the primary care provider. (Examples of elements of a survivorship care plan can be found at www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan6 and http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/7).
Regrettably, survivorship care plans have been rarely and poorly employed. Studies show that fewer than one-half of oncologists provide a plan, and that when they do, the plan often lacks recommended information.8,9 Survivorship care plans may soon become common practice, however; the Commission on Cancer of the American College of Surgeons has required their use in all certified cancer centers since 2015.10
2. Provide surveillance of existing cancer
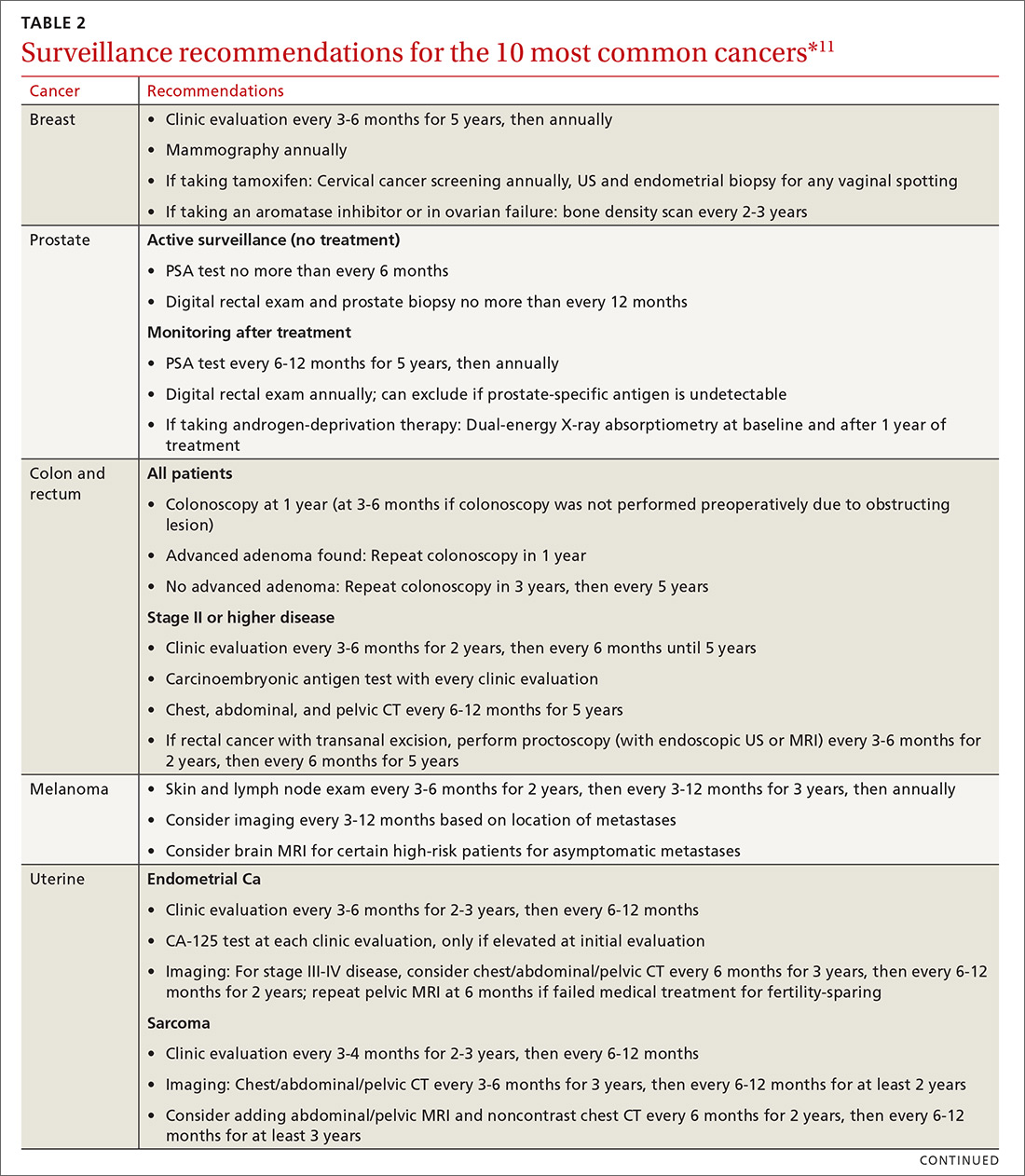
Cancer follow-up is challenging after the initial treatment phase. Although there are many conflicting guidelines for surveillance after cancer, guidelines of the National Comprehensive Cancer Network (NCCN) (summarized in TABLE 211 for the 10 most common cancers in survivors) are the ones generally accepted.12,13
Although individual surveillance recommendations are based on limited evidence, studies confirm the importance of surveillance. A systematic review showed that surveillance mammography after breast cancer reduces breast cancer mortality by 36%.14 A study showed that bladder cancer recurrence diagnosed by surveillance instead of by symptoms led to a 35% increase in 5-year survival.15
Continue to: Yet adherence to cancer surveillance...
Yet adherence to cancer surveillance recommendations is poor. A study of patients with colon cancer demonstrated that only 12% met all recommended surveillance guidelines.16 A study of patients with bladder cancer after radical cystectomy showed that only 9% met recommended surveillance more than 2 years after diagnosis.17 Those dismal statistics may be the result of provider oversight—not patient reluctance.
In the colon cancer study, for example, compliance with follow-up colonoscopy was 80% but compliance with carcinoembryonic antigen testing was only 22%.16 In the bladder cancer study, follow-up urine cytology was obtained in only 23% of patients, although 75% completed recommended imaging.17
Although surveillance remains the oncologist’s responsibility, visits to the FP provide an opportunity to review surveillance and order needed laboratory testing and other studies, including imaging.
3. Screen for new cancers
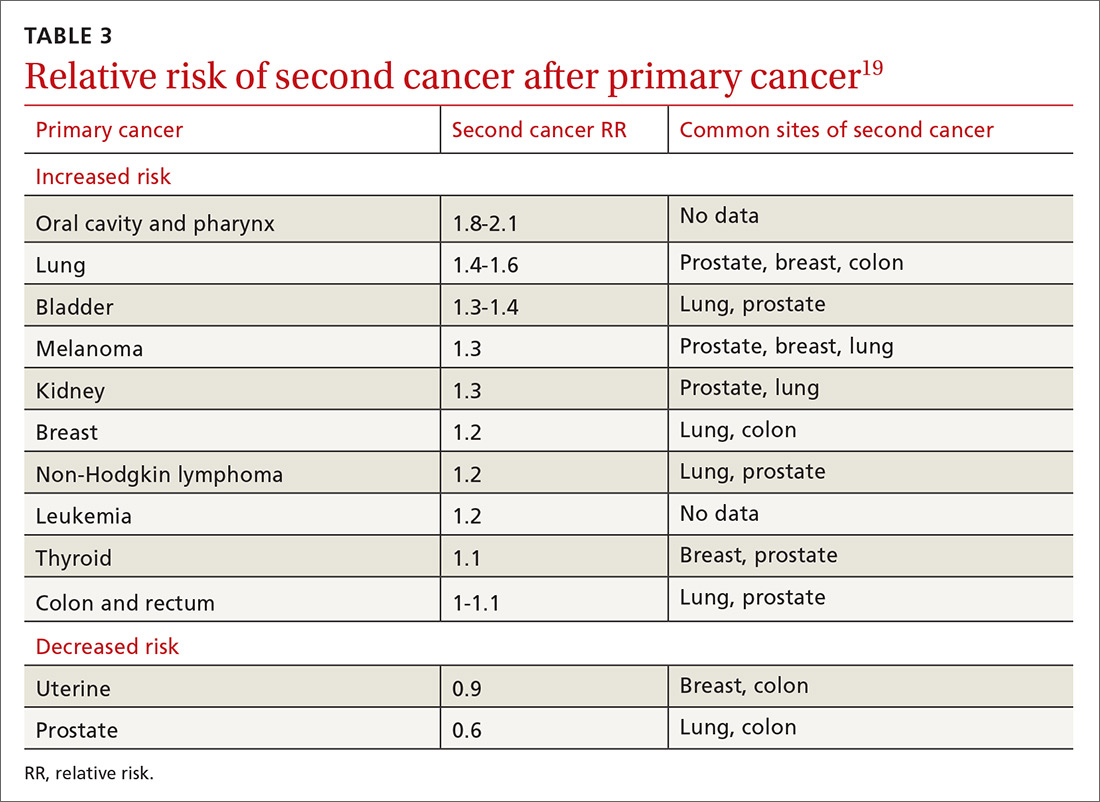
The risk of a second cancer is elevated for cancer survivors compared with the risk of a primary cancer in the healthy general population; some survivors have a lifetime risk of a second cancer as high as 36%.18 Risk varies by cancer type (TABLE 319). Some of this variation is due to the impact of smoking: Smoking-related cancers have the highest risk of second malignancy.19 Genetic predisposition to malignant transformation is also theorized to contribute to increased risk. Second malignancies are dangerous; 55% of patients die of the second cancer compared with only 13% of their initial cancer.19
Studies show that cancer survivors display varying adherence with recommended screening for second cancers. In a study of Latina cancer survivors, depressive symptoms were associated with lower screening compliance.20 A study of survivors of hematologic cancer showed a low rate of cancer screening and high fear of cancer recurrence—suggesting avoidance due to fear.21 Other studies, however, show similar or increased compliance with screening in cancer survivors.22,23 A meta-analysis of 19 studies determined that, overall, cancer survivors receive 25% to 38% more recommended screening than the general population.24
Continue to: Few guidelines exist to guide FPs...
Few guidelines exist to guide FPs in adjusting screening for the cancer survivor. For women who received radiation therapy for a tumor in the chest, for example, the recommendation offered by several groups is to start breast cancer screening 8 to 10 years after treatment or by 30 years of age, and to consider combining magnetic resonance imaging and mammography.25 Recommendations for breast cancer screening do not account for a history of other gynecologic cancers unless genetic markers are present.25 On the other hand, the impact of a history of cancer on the risk of prostate cancer and on screening decisions has not been studied,26 and cervical cancer screening guidelines, which recommend that screening continue after 65 years of age for patients who are immunocompromised, do not address a history of other cancer.27
4. Manage the effects of both the cancer and the treatment
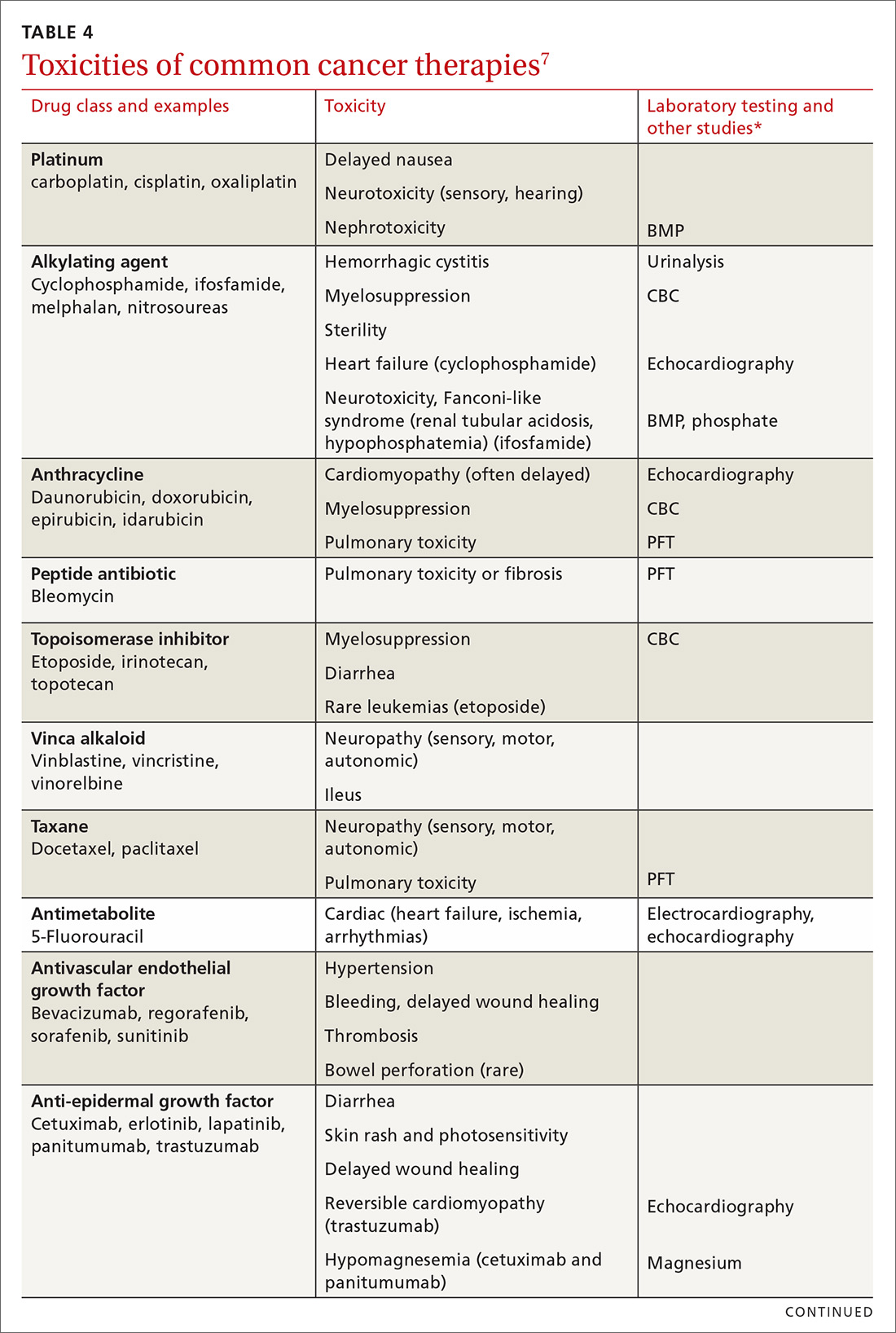
Medical issues faced by cancer survivors are familiar to FPs, but there are some specific recommendations regarding evaluation and treatment that stand in contrast to what would be considered for a healthy, or non-cancer, patient. For example, each chemotherapeutic agent has characteristic adverse effects; TABLE 47 lists the principal adverse effects of common agents and recommendations for testing when these problems develop. Common long-term problems in cancer survivors include fatigue, chronic pain, cognitive dysfunction, psychiatric illness, and cardiovascular disease. Although these symptoms and manifestations are common, the physician must be careful: New or changing symptoms could signal the spread or recurrence of disease. Fear of recurrence can lead patients to exaggerate or minimize symptoms.
Fatigue is the most common symptom seen in cancer survivors during treatment and following remission.28 More than 40% of cancer survivors report significant fatigue.29 Although fatigue is concerning for cancer recurrence, other causes are common in cancer survivors. Both depression and anxiety commonly present with worsened fatigue.30 Sleep disturbances are common, even without a psychiatric diagnosis.31 Effects of treatment, including nausea, anemia, heart failure, and medication adverse effects can cause or worsen fatigue. Pain is associated with fatigue, but to a lesser extent than are depression, anxiety, and nausea.32
Pharmacotherapy of cancer-related fatigue is challenging. Psychostimulants have been most studied. A recent systematic review shows that methylphenidate produces mild or moderate improvement in fatigue, whereas modafanil has minimal effectiveness.33 Antidepressants have not been shown to relieve fatigue.33
A recent meta-analysis showed that nonpharmaceutical treatments for cancer-related fatigue are more effective than pharmacotherapy. In this review, both exercise and pharmacotherapy had a mild-to-moderate effect on fatigue.35 Exercise is best studied in this regard, and has shown the most consistent results.31
Continue to: Chronic pain
Chronic pain. Pain is common in cancer survivors: As many as 40% experience pain for years after initial therapy.36 Treatment of some cancers—eg, thoracotomy (80%), amputation (50%-80%), neck dissection (52%), and surgical management of breast cancer (63%)—increase the likelihood of chronic pain.37 Reports of pain in cancer survivors that should be considered red flags that might signal recurrence of cancer include new or worsening pain; pain worse at night or when recumbent; new neurologic symptoms; and general symptoms of systemic illness37 (TABLE 537).

Management of pain is best approached by its cause, with neurologic, rheumatologic (including myofascial pain and arthralgia), lymphatic, and genital causes most common.37 Across all types of pain, complete relief is unlikely; functional goals provide a more effective target.
For neuropathic cancer pain, duloxetine is the only medication with evidence of benefit; anticonvulsant and topical medications are recommended on the basis of the findings of studies of noncancer pain.38 There are few data on the value of treatments for cancer-related rheumatologic and lymphatic pain, although exercise has shown benefit in both types.38 For dyspareunia and sexual dysfunction (common after gynecologic and nongynecologic cancers), vaginal lubricants and pelvic-floor physiotherapy have shown benefit.39 There is significant overlap in psychiatric comorbidities, sleep, and pain, and addressing all of a patient’s problems can reduce pain and improve function.40
Opioids are often prescribed for pain in cancer survivors. Cancer survivors have a higher rate of opioid prescribing compared with that of non-cancer patients, even 10 years after diagnosis.41 Guidelines of the Centers for Disease Control and Prevention for using opioids to manage chronic pain specifically exclude cancer patients.42 Regrettably, there is no evidence that opioids have long-term efficacy in chronic pain; in fact, evidence is accumulating that chronic opioid therapy exacerbates chronic pain.43
Cognitive dysfunction is present in 17% to 75% of cancer survivors as memory disturbance, psychological disorder, sleep dysfunction, or impairment of executive functioning.44 Cognitive deficits appear to be secondary to both cancer and treatment modalities45; as many as one-third of patients have cognitive dysfunction prior to receiving chemotherapy.46
Continue to: Chemotherapies that are more likely...
Chemotherapies that are more likely to cause cognitive symptoms include methotrexate, 5-fluorouracil, cyclophosphamide, and hormone antagonists.47 More powerful regimens and repetitive chemotherapy regimens tend to cause more cognitive effects.47
Cognitive training interventions show evidence of likely benefit,44,48 leading to recommendations for self-treatment strategies, such as written lists, wordplay, crossword puzzles, jigsaw puzzles, playing a musical instrument, and new hobbies. Small studies suggest a benefit from cognitive behavioral therapy.44,49 A study of breast cancer survivors showed that yoga led to improvement in patient-reported cognitive dysfunction.50 Physical exercise yields cognitive benefit in healthy older adults and is supported by limited evidence in cancer survivors.51
There is no effective pharmacotherapy for cancer- and cancer chemotherapy-related cognitive dysfunction unless a treatable underlying cause is found.44 Symptoms tend to subside with time after completion of chemotherapy, which might be reassuring to patients and families.45
Psychiatric problems. The most common psychiatric issues in cancer survivors are anxiety and depression; the prevalence of anxiety is nearly double that of depression.52 Anxiety often presents as fear of a recurrence of cancer or a feeling of lack of control over present or future circumstances.53 Screening for anxiety and depression is recommended at each visit, using standardized screening questionnaires.54
A small study suggests that psychiatric treatment reduces the risk of early mortality.55 Small studies also suggest that mindfulness-based therapy and cognitive behavioral therapy delivered by telehealth offer benefit.56 A meta-analysis shows that exercise interventions improve depression and anxiety in breast cancer patients.57
Continue to: There are few studies of pharmacotherapy...
There are few studies of pharmacotherapy of anxiety or depression in cancer survivors56; it is known that cancer survivors are nearly twice as likely as the general population to be taking medical therapy for anxiety and depression.58 A Cochrane systematic review of 7 small studies showed uncertain improvement in depressive symptoms in patients with cancer from antidepressant medication; however, an earlier systematic review did show benefit.59,60
In a trial of patients without depression who were being treated for head and neck cancer, escitalopram, 20 mg/d, reduced the risk of subsequent depression compared with placebo.61 A study of 420 breast cancer survivors showed that 300 mg/d and 900 mg/d dosages of gabapentin were both superior to placebo, and nearly equivalent to each other, at reducing anxiety scores.62 In both studies, however, the evidence is nonetheless insufficient to make specific recommendations about these medications.
Cardiac risk
Among chemotherapeutic agents, anthracyclines, such as doxorubicin, cause the most rapid and striking myocyte damage. This damage is dose-dependent and nearly irreversible, with 98% of injury occurring within the first year of chemotherapy.64 More than one half of cancer patients taking an anthracycline have cardiac dysfunction on imaging; 5% will be in overt heart failure 10 to 20 years, or longer, after chemotherapy.63 Following monitoring at 1 year post-therapy, regular cardiac imaging is not recommended in the absence of symptoms.62
Because other cardiotoxic chemotherapeutic agents cause partially reversible damage, imaging is not recommended in the absence of symptoms in patients taking those agents.64
Continue to: Radiation therapy to the chest leads...
Radiation therapy to the chest leads to many cardiac complications, including cardiomyopathy, valvular disease, pericardial disease, and arrhythmias. Development of cardiomyopathy can be delayed 20 to 30 years after radiation; screening echocardiography is therefore recommended every 5 to 10 years after radiation therapy.65 Recent adjustments to the dosages and delivery of radiation therapy should reduce cardiac damage, but will require decades to validate.63
For patients at risk of cardiovascular disease prior to treatment of cancer, there is evidence to support preventive treatment with angiotensin II-receptor antagonists, beta-blockers, and statins to prevent cardiomyopathy.63 Treatment of diagnosed cardiomyopathy and heart failure follows standard guidelines, with significant emphasis on aerobic exercise and smoking cessation.63
Cancer survivorship care: Your critical role
Cancer survivors constitute a large population who frequent the practices of primary care physicians. Primary care visits provide an opportunity to monitor key elements of survivorship, including surveillance of the current cancer and screening for second cancers. Similar to what is seen with diabetes and coronary artery disease, cancer increases cardiac risk, which requires preventive care and chronic management. FPs are well placed to treat common issues in cancer survivors—issues that mirror concerns seen in the general population.
CORRESPONDENCE
Michael J. Arnold, MD, CDR, USN, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected].
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted with the editing of the manuscript.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016-2017. Atlanta, GA: American Cancer Society; 2016. www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html. Accessed July 25, 2018.
2. Survivorship. NCCN Guidelines (version 1.2017). Fort Washington, PA: National Comprehensive Cancer Network; 2017. www.nccn.org/professionals/physician_gls/default.aspx#supportive. Accessed July 26, 2018.
3. Kendall C, Decker KM, Groome PA, et al. Use of physician services during the survivorship phase: a multi-province study of women diagnosed with breast cancer. Curr Oncolog. 2017;24:81-89.
4. Snyder CF, Earle CC, Herbert RJ, et al. Preventive care for colorectal cancer survivors: a 5-year longitudinal study. J Clin Oncol. 2008;26:1073-1079.
5. Hewitt M, Greenfield S, Stovall E (eds); Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington DC: The National Academies Press; 2006. www.nap.edu/read/11468/chapter/1. Accessed July 25, 2018.
6. Survivorship care plan. New York, NY: Memorial Sloan Kettering Cancer Center. www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan. Accessed August 11, 2018.
7. Fuentes AC, Lambird JE, George TJ, et al. Cancer survivor’s history and physical. South Med J. 2017;110:37-44. http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/. Accessed July 26, 2018.
8. Salz T, Oeffinger KC, McCabe MS, et al. Survivorship care plans in research and practice. CA Cancer J Clin. 2012;62:101-117.
9. Birken SA, Mayer DK, Weiner BJ. Survivorship care plans: prevalence and barriers to use. J Cancer Educ. 2013;28:290-296.
10. American College of Surgeons Commission on Cancer. Cancer program standards 2012: Ensuring patient-centered care. V1.2.1. www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed July 25, 2018.
11. NCCN guidelines for treatment of cancer by site. NCCN Guidelines (version 1.2018). Fort Washington, PA: National Comprehensive Cancer Network; 2018. www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed July 25, 2018.
12. Spronka I, Korevaar JC, Burgers JS, et al. Review of guidance on recurrence risk management for general practitioners in breast cancer, colorectal cancer and melanoma guidelines. Family Pract. 2017;34:154-160.
13. Merkow RP, Korenstein D, Yeahia R, et al. Quality of cancer surveillance clinical practice guidelines: specificity and consistency of recommendations. JAMA Intern Med. 2017;177:701-709.
14. Muradali D, Kennedy EB, Eisen A, et al. Breast screening for survivors of breast cancer: a systematic review. Prev Med. 2017;103:70-75.
15. Giannarini G, Kessler TM, Thoeny HC, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486-494.
16. Sisler JJ, Seo B, Katz A, et al. Concordance with ASCO guidelines for surveillance after colorectal cancer treatment: a population-based analysis. J Oncol Pract. 2012;8:e69-e79.
17. Ehdaie B. Atoria CL, Lowrance WT, et al. Adherence to surveillance guidelines after radical cystectomy: a population-based analysis. Urol Oncol. 2014;32:779-784.
18. Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354-1365.
19. Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122:3075-3086.
20. Holder AE, Ramirez AG, Gallion K. Depressive symptoms in Latina breast cancer survivors: a barrier to cancer screening. Health Psycholog. 2014;33:242-248.
21. Dyer G, Larsen SR, Gilroy N, et al. Adherence to cancer screening guidelines in Australian survivors of allogenic blood and marrow transplantation (BMT). Cancer Med. 2016;5:1702-1716.
22. Mandelzweig L, Chetrit A, Amitai T, et al. Primary prevention and screening practices among long-term breast cancer survivors. Cancer Causes Control. 2017;28:657-666.
23. Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16:207-214.
24. Uhlig A, Mei J, Baik I, et al. Screening utilization among cancer survivors: a meta-analysis. J Public Health (Oxf). 2018;40:129-137.
25. Hilal T, Rudy DW. Radiation-induced breast cancer: the question of early breast cancer screening in Hodgkin’s lymphoma survivors. Oxf Med Case Reports. 2016;2016:17-18.
26. Lin K, Croswell JM, Koenig H, et al. Prostate-specific antigen-based screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force [Internet]. Evidence Syntheses No. 90. AHRQ Publication No. 12-05160-EF-1. Rockville, MD: Agency for Healthcare Research and Quality (US); October 2011. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0032900/. Accessed July 25, 2018.
27. US Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
28. Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1:4-10.
29. Jung JY, Lee JM, Kim MS, et al. Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology. 2018;27:465-470.
30. Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatment. Nat Rev Clin Oncol. 2014;11:597-609.
31. Medysky ME, Temesi J, Culos-Reed SN, et al. Exercise, sleep and cancer-related fatigue: are they related? Neurophysiol Clin. 2017;47:111-122.
32. Oh HS, Sea WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid Based Nurs. 2011;8:191-201.
33. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2016;25:970-979.
34. Escalante CP, Manzullo EF. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(suppl 2):S412-S416.
35. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961-968.
36. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
37. Davies PS. Chronic pain management in the cancer survivor: tips for primary care providers. Nurse Pract. 2013;39:28-38.
38. Boland EG, Ahmedzai SH. Persistent pain in cancer survivors. Curr Opin Support Palliat Care. 2017;11:181-190.
39. Sears CS, Robinson JW, Walker LM. A comprehensive review of sexual health concerns after cancer treatment and the biopsychosocial treatment options available to female patients. Eur J Cancer Care (Engl). 2017;27:e12738.
40. Schou Bredal I, Smeby NA, Ottesen S, et al. Chronic pain in breast cancer survivors: comparison of psychological, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manage. 2014;48:852-862.
41. Sutradhar R, Lokku A, Barbera L. Cancer survivorship and opioid prescribing rates: a population-based matched cohort study among individuals with and without a history of cancer. Cancer. 2017;123:4286-4293.
42. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
43. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
44. Von Ah D. Cognitive changes associated with cancer and cancer treatment: state of the science. Clin J Oncol Nurs. 2015;19:47-56.
45. Moore HC. An overview of chemotherapy-related cognitive dysfunction, or ‘chemobrain’. Oncology (Williston Park). 2014;28:797-804.
46. Asher A. Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil. 2011;90(suppl):S16-S26.
47. Joly F, Rigal O, Noal S, et al. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psychooncology. 2011;20:1251-1258.
48. Attention, thinking or memory problems. American Society of Clinical Oncology Cancer.Net. April 2018. www.cancer.net/navigating-cancer-care/side-effects/attention-thinking-or-memory-problems. Accessed July 25, 2018.
49. Kucherer S, Ferguson RJ. Cognitive behavioral therapy for cancer-related cognitive dysfunction. Curr Opin Support Palliat Care. 2017;11:46-51.
50. Derry HM, Jaremka LM, Bennet JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24:958-966.
51. Treanor CJ, McMenamin UC, O’Neill RF, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst Rev. 2016 Aug 16;(8):CD011325.
52. Mitchell AJ, Ferguson DW, Gill J, et al. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721-732.
53. Inhestern L, Beierlein V, Bultmann JC, et al. Anxiety and depression in working-age cancer survivors: a register-based study. BMC Cancer. 2017;17:347.
54. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for adult cancer survivors: highlighting ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015:188-194.
55. Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450-3458.
56. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin N Am. 2017;101:1099-1113.
57. Zhu G, Zhang X, Wang Y, et al. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trials. Onco Targets Ther. 2016;9:2153-2168.
58. Hawkins NA, Soman A, Lunsford N, et al. Use of medications for treating anxiety and depression in cancer survivors in the United States. J Clin Oncol. 2017;35:78-85.
59. Ostuzzi G, Matcham F, Dauchy S, et al. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. 2015 June 1;(6):CD011006.
60. Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:140.
61. Lydiatt WM, Bessette D, Schmid KK, et al. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer: randomized, double-blind, placebo-controlled clinical trial. JAMA Otolaryngol Head Neck Surg. 2013;139:678-686.
62. Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136:479-486.
63. Okwuosa TM, Anzevino S, Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J. 2017;93:82-90.
64. Plana, JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911-939.
65. Lancellotti, P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013-1032.
Cancer survivors represent a rapidly increasing population. In 1971, there were 3 million cancer survivors; this number increased to 15.5 million in 2016 and will reach 20 million by 2026.1TABLE 11 shows the percentage of survivors by type of cancer. Cancer survivors tend to be older,* comprising nearly 1 of every 5 people older than 65 years.2

The Institute of Medicine (IOM) identified 3 key characteristics of cancer survivors3:
- Trajectories of survivorship are variable; many cancer patients have periods of relative health between episodes of their disease.
- Survivors require careful cancer monitoring; in addition to the risk that their primary cancer will recur, they have an elevated risk for another, second cancer.
- Both cancer and its treatments increase the risk of other medical and psychiatric problems.
Family physicians (FPs) have optimal skills for navigating the chronic risks and health concerns of the well cancer survivor. This article reviews the primary care management of the functional cancer survivor, focusing on the management of chronic conditions and preventive care.
Survivorship follows any of 6 paths
Cancer survivorship is increasing in importance as treatment has steadily reduced mortality. Six trajectories of cancer survivors have been identified1:
- living cancer-free after treatment with minimal effects
- living cancer-free but suffering serious treatment complications
- Suffering late recurrence
- Developing a second cancer
- Living with intermittent cancer recurrences
- Living with cancer continuously.
Only patients in the last 2 groups are likely to be managed primarily by oncologists.
Survivors look to their FPs for ongoing care
Cancer survivors routinely see their primary care physician after initial treatment. A study of 30,000 Canadian breast cancer survivors demonstrated that follow-up care was limited to an oncologist in only 2%; 84% saw a primary care provider and an oncologist; and 14% saw a primary care provider only.4 A study of colorectal cancer survivors showed that primary care visits increased in each of the 5 years after diagnosis, during which time oncology visits decreased steadily5; in that study, primary care physicians delivered more preventive care than oncologists did.5 Similar to what is done in other chronic conditions, the various effects of cancer are best managed as a whole.
The IOM recommends that cancer survivor care comprise 4 elements2:
- coordination between oncologist and primary care physician
- surveillance for recurrence or spread of existing cancer
- screening for new cancer
- intervention for the effects of cancer and treatment.
Continue to: The following discussion summarizes...
The following discussion summarizes evidence and recommendations for each element of the IOM recommendations for survivor care.
Implementing the 4 elements of cancer survivor care
1. Coordinate care through a unified survivorship care plan
The IOM has noted that the needs of cancer survivors are rarely met2; communication between oncology and primary care is often deficient during transition of care. The IOM has recommended that oncologists provide a survivorship care plan that details the cancer (ie, tumor characteristics), the type of treatment (ie, enrollment in a clinical trial; medical, surgical, or radiation), support services, and follow-up recommendations for the primary care provider. (Examples of elements of a survivorship care plan can be found at www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan6 and http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/7).
Regrettably, survivorship care plans have been rarely and poorly employed. Studies show that fewer than one-half of oncologists provide a plan, and that when they do, the plan often lacks recommended information.8,9 Survivorship care plans may soon become common practice, however; the Commission on Cancer of the American College of Surgeons has required their use in all certified cancer centers since 2015.10
2. Provide surveillance of existing cancer

Cancer follow-up is challenging after the initial treatment phase. Although there are many conflicting guidelines for surveillance after cancer, guidelines of the National Comprehensive Cancer Network (NCCN) (summarized in TABLE 211 for the 10 most common cancers in survivors) are the ones generally accepted.12,13

Although individual surveillance recommendations are based on limited evidence, studies confirm the importance of surveillance. A systematic review showed that surveillance mammography after breast cancer reduces breast cancer mortality by 36%.14 A study showed that bladder cancer recurrence diagnosed by surveillance instead of by symptoms led to a 35% increase in 5-year survival.15

Continue to: Yet adherence to cancer surveillance...
Yet adherence to cancer surveillance recommendations is poor. A study of patients with colon cancer demonstrated that only 12% met all recommended surveillance guidelines.16 A study of patients with bladder cancer after radical cystectomy showed that only 9% met recommended surveillance more than 2 years after diagnosis.17 Those dismal statistics may be the result of provider oversight—not patient reluctance.
In the colon cancer study, for example, compliance with follow-up colonoscopy was 80% but compliance with carcinoembryonic antigen testing was only 22%.16 In the bladder cancer study, follow-up urine cytology was obtained in only 23% of patients, although 75% completed recommended imaging.17
Although surveillance remains the oncologist’s responsibility, visits to the FP provide an opportunity to review surveillance and order needed laboratory testing and other studies, including imaging.
3. Screen for new cancers
The risk of a second cancer is elevated for cancer survivors compared with the risk of a primary cancer in the healthy general population; some survivors have a lifetime risk of a second cancer as high as 36%.18 Risk varies by cancer type (TABLE 319). Some of this variation is due to the impact of smoking: Smoking-related cancers have the highest risk of second malignancy.19 Genetic predisposition to malignant transformation is also theorized to contribute to increased risk. Second malignancies are dangerous; 55% of patients die of the second cancer compared with only 13% of their initial cancer.19

Studies show that cancer survivors display varying adherence with recommended screening for second cancers. In a study of Latina cancer survivors, depressive symptoms were associated with lower screening compliance.20 A study of survivors of hematologic cancer showed a low rate of cancer screening and high fear of cancer recurrence—suggesting avoidance due to fear.21 Other studies, however, show similar or increased compliance with screening in cancer survivors.22,23 A meta-analysis of 19 studies determined that, overall, cancer survivors receive 25% to 38% more recommended screening than the general population.24
Continue to: Few guidelines exist to guide FPs...
Few guidelines exist to guide FPs in adjusting screening for the cancer survivor. For women who received radiation therapy for a tumor in the chest, for example, the recommendation offered by several groups is to start breast cancer screening 8 to 10 years after treatment or by 30 years of age, and to consider combining magnetic resonance imaging and mammography.25 Recommendations for breast cancer screening do not account for a history of other gynecologic cancers unless genetic markers are present.25 On the other hand, the impact of a history of cancer on the risk of prostate cancer and on screening decisions has not been studied,26 and cervical cancer screening guidelines, which recommend that screening continue after 65 years of age for patients who are immunocompromised, do not address a history of other cancer.27
4. Manage the effects of both the cancer and the treatment
Medical issues faced by cancer survivors are familiar to FPs, but there are some specific recommendations regarding evaluation and treatment that stand in contrast to what would be considered for a healthy, or non-cancer, patient. For example, each chemotherapeutic agent has characteristic adverse effects; TABLE 47 lists the principal adverse effects of common agents and recommendations for testing when these problems develop. Common long-term problems in cancer survivors include fatigue, chronic pain, cognitive dysfunction, psychiatric illness, and cardiovascular disease. Although these symptoms and manifestations are common, the physician must be careful: New or changing symptoms could signal the spread or recurrence of disease. Fear of recurrence can lead patients to exaggerate or minimize symptoms.

Fatigue is the most common symptom seen in cancer survivors during treatment and following remission.28 More than 40% of cancer survivors report significant fatigue.29 Although fatigue is concerning for cancer recurrence, other causes are common in cancer survivors. Both depression and anxiety commonly present with worsened fatigue.30 Sleep disturbances are common, even without a psychiatric diagnosis.31 Effects of treatment, including nausea, anemia, heart failure, and medication adverse effects can cause or worsen fatigue. Pain is associated with fatigue, but to a lesser extent than are depression, anxiety, and nausea.32

Pharmacotherapy of cancer-related fatigue is challenging. Psychostimulants have been most studied. A recent systematic review shows that methylphenidate produces mild or moderate improvement in fatigue, whereas modafanil has minimal effectiveness.33 Antidepressants have not been shown to relieve fatigue.33
A recent meta-analysis showed that nonpharmaceutical treatments for cancer-related fatigue are more effective than pharmacotherapy. In this review, both exercise and pharmacotherapy had a mild-to-moderate effect on fatigue.35 Exercise is best studied in this regard, and has shown the most consistent results.31
Continue to: Chronic pain
Chronic pain. Pain is common in cancer survivors: As many as 40% experience pain for years after initial therapy.36 Treatment of some cancers—eg, thoracotomy (80%), amputation (50%-80%), neck dissection (52%), and surgical management of breast cancer (63%)—increase the likelihood of chronic pain.37 Reports of pain in cancer survivors that should be considered red flags that might signal recurrence of cancer include new or worsening pain; pain worse at night or when recumbent; new neurologic symptoms; and general symptoms of systemic illness37 (TABLE 537).

Management of pain is best approached by its cause, with neurologic, rheumatologic (including myofascial pain and arthralgia), lymphatic, and genital causes most common.37 Across all types of pain, complete relief is unlikely; functional goals provide a more effective target.
For neuropathic cancer pain, duloxetine is the only medication with evidence of benefit; anticonvulsant and topical medications are recommended on the basis of the findings of studies of noncancer pain.38 There are few data on the value of treatments for cancer-related rheumatologic and lymphatic pain, although exercise has shown benefit in both types.38 For dyspareunia and sexual dysfunction (common after gynecologic and nongynecologic cancers), vaginal lubricants and pelvic-floor physiotherapy have shown benefit.39 There is significant overlap in psychiatric comorbidities, sleep, and pain, and addressing all of a patient’s problems can reduce pain and improve function.40
Opioids are often prescribed for pain in cancer survivors. Cancer survivors have a higher rate of opioid prescribing compared with that of non-cancer patients, even 10 years after diagnosis.41 Guidelines of the Centers for Disease Control and Prevention for using opioids to manage chronic pain specifically exclude cancer patients.42 Regrettably, there is no evidence that opioids have long-term efficacy in chronic pain; in fact, evidence is accumulating that chronic opioid therapy exacerbates chronic pain.43
Cognitive dysfunction is present in 17% to 75% of cancer survivors as memory disturbance, psychological disorder, sleep dysfunction, or impairment of executive functioning.44 Cognitive deficits appear to be secondary to both cancer and treatment modalities45; as many as one-third of patients have cognitive dysfunction prior to receiving chemotherapy.46
Continue to: Chemotherapies that are more likely...
Chemotherapies that are more likely to cause cognitive symptoms include methotrexate, 5-fluorouracil, cyclophosphamide, and hormone antagonists.47 More powerful regimens and repetitive chemotherapy regimens tend to cause more cognitive effects.47
Cognitive training interventions show evidence of likely benefit,44,48 leading to recommendations for self-treatment strategies, such as written lists, wordplay, crossword puzzles, jigsaw puzzles, playing a musical instrument, and new hobbies. Small studies suggest a benefit from cognitive behavioral therapy.44,49 A study of breast cancer survivors showed that yoga led to improvement in patient-reported cognitive dysfunction.50 Physical exercise yields cognitive benefit in healthy older adults and is supported by limited evidence in cancer survivors.51
There is no effective pharmacotherapy for cancer- and cancer chemotherapy-related cognitive dysfunction unless a treatable underlying cause is found.44 Symptoms tend to subside with time after completion of chemotherapy, which might be reassuring to patients and families.45
Psychiatric problems. The most common psychiatric issues in cancer survivors are anxiety and depression; the prevalence of anxiety is nearly double that of depression.52 Anxiety often presents as fear of a recurrence of cancer or a feeling of lack of control over present or future circumstances.53 Screening for anxiety and depression is recommended at each visit, using standardized screening questionnaires.54
A small study suggests that psychiatric treatment reduces the risk of early mortality.55 Small studies also suggest that mindfulness-based therapy and cognitive behavioral therapy delivered by telehealth offer benefit.56 A meta-analysis shows that exercise interventions improve depression and anxiety in breast cancer patients.57
Continue to: There are few studies of pharmacotherapy...
There are few studies of pharmacotherapy of anxiety or depression in cancer survivors56; it is known that cancer survivors are nearly twice as likely as the general population to be taking medical therapy for anxiety and depression.58 A Cochrane systematic review of 7 small studies showed uncertain improvement in depressive symptoms in patients with cancer from antidepressant medication; however, an earlier systematic review did show benefit.59,60
In a trial of patients without depression who were being treated for head and neck cancer, escitalopram, 20 mg/d, reduced the risk of subsequent depression compared with placebo.61 A study of 420 breast cancer survivors showed that 300 mg/d and 900 mg/d dosages of gabapentin were both superior to placebo, and nearly equivalent to each other, at reducing anxiety scores.62 In both studies, however, the evidence is nonetheless insufficient to make specific recommendations about these medications.
Cardiac risk
Among chemotherapeutic agents, anthracyclines, such as doxorubicin, cause the most rapid and striking myocyte damage. This damage is dose-dependent and nearly irreversible, with 98% of injury occurring within the first year of chemotherapy.64 More than one half of cancer patients taking an anthracycline have cardiac dysfunction on imaging; 5% will be in overt heart failure 10 to 20 years, or longer, after chemotherapy.63 Following monitoring at 1 year post-therapy, regular cardiac imaging is not recommended in the absence of symptoms.62
Because other cardiotoxic chemotherapeutic agents cause partially reversible damage, imaging is not recommended in the absence of symptoms in patients taking those agents.64
Continue to: Radiation therapy to the chest leads...
Radiation therapy to the chest leads to many cardiac complications, including cardiomyopathy, valvular disease, pericardial disease, and arrhythmias. Development of cardiomyopathy can be delayed 20 to 30 years after radiation; screening echocardiography is therefore recommended every 5 to 10 years after radiation therapy.65 Recent adjustments to the dosages and delivery of radiation therapy should reduce cardiac damage, but will require decades to validate.63
For patients at risk of cardiovascular disease prior to treatment of cancer, there is evidence to support preventive treatment with angiotensin II-receptor antagonists, beta-blockers, and statins to prevent cardiomyopathy.63 Treatment of diagnosed cardiomyopathy and heart failure follows standard guidelines, with significant emphasis on aerobic exercise and smoking cessation.63
Cancer survivorship care: Your critical role
Cancer survivors constitute a large population who frequent the practices of primary care physicians. Primary care visits provide an opportunity to monitor key elements of survivorship, including surveillance of the current cancer and screening for second cancers. Similar to what is seen with diabetes and coronary artery disease, cancer increases cardiac risk, which requires preventive care and chronic management. FPs are well placed to treat common issues in cancer survivors—issues that mirror concerns seen in the general population.
CORRESPONDENCE
Michael J. Arnold, MD, CDR, USN, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected].
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted with the editing of the manuscript.
Cancer survivors represent a rapidly increasing population. In 1971, there were 3 million cancer survivors; this number increased to 15.5 million in 2016 and will reach 20 million by 2026.1TABLE 11 shows the percentage of survivors by type of cancer. Cancer survivors tend to be older,* comprising nearly 1 of every 5 people older than 65 years.2

The Institute of Medicine (IOM) identified 3 key characteristics of cancer survivors3:
- Trajectories of survivorship are variable; many cancer patients have periods of relative health between episodes of their disease.
- Survivors require careful cancer monitoring; in addition to the risk that their primary cancer will recur, they have an elevated risk for another, second cancer.
- Both cancer and its treatments increase the risk of other medical and psychiatric problems.
Family physicians (FPs) have optimal skills for navigating the chronic risks and health concerns of the well cancer survivor. This article reviews the primary care management of the functional cancer survivor, focusing on the management of chronic conditions and preventive care.
Survivorship follows any of 6 paths
Cancer survivorship is increasing in importance as treatment has steadily reduced mortality. Six trajectories of cancer survivors have been identified1:
- living cancer-free after treatment with minimal effects
- living cancer-free but suffering serious treatment complications
- Suffering late recurrence
- Developing a second cancer
- Living with intermittent cancer recurrences
- Living with cancer continuously.
Only patients in the last 2 groups are likely to be managed primarily by oncologists.
Survivors look to their FPs for ongoing care
Cancer survivors routinely see their primary care physician after initial treatment. A study of 30,000 Canadian breast cancer survivors demonstrated that follow-up care was limited to an oncologist in only 2%; 84% saw a primary care provider and an oncologist; and 14% saw a primary care provider only.4 A study of colorectal cancer survivors showed that primary care visits increased in each of the 5 years after diagnosis, during which time oncology visits decreased steadily5; in that study, primary care physicians delivered more preventive care than oncologists did.5 Similar to what is done in other chronic conditions, the various effects of cancer are best managed as a whole.
The IOM recommends that cancer survivor care comprise 4 elements2:
- coordination between oncologist and primary care physician
- surveillance for recurrence or spread of existing cancer
- screening for new cancer
- intervention for the effects of cancer and treatment.
Continue to: The following discussion summarizes...
The following discussion summarizes evidence and recommendations for each element of the IOM recommendations for survivor care.
Implementing the 4 elements of cancer survivor care
1. Coordinate care through a unified survivorship care plan
The IOM has noted that the needs of cancer survivors are rarely met2; communication between oncology and primary care is often deficient during transition of care. The IOM has recommended that oncologists provide a survivorship care plan that details the cancer (ie, tumor characteristics), the type of treatment (ie, enrollment in a clinical trial; medical, surgical, or radiation), support services, and follow-up recommendations for the primary care provider. (Examples of elements of a survivorship care plan can be found at www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan6 and http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/7).
Regrettably, survivorship care plans have been rarely and poorly employed. Studies show that fewer than one-half of oncologists provide a plan, and that when they do, the plan often lacks recommended information.8,9 Survivorship care plans may soon become common practice, however; the Commission on Cancer of the American College of Surgeons has required their use in all certified cancer centers since 2015.10
2. Provide surveillance of existing cancer

Cancer follow-up is challenging after the initial treatment phase. Although there are many conflicting guidelines for surveillance after cancer, guidelines of the National Comprehensive Cancer Network (NCCN) (summarized in TABLE 211 for the 10 most common cancers in survivors) are the ones generally accepted.12,13

Although individual surveillance recommendations are based on limited evidence, studies confirm the importance of surveillance. A systematic review showed that surveillance mammography after breast cancer reduces breast cancer mortality by 36%.14 A study showed that bladder cancer recurrence diagnosed by surveillance instead of by symptoms led to a 35% increase in 5-year survival.15

Continue to: Yet adherence to cancer surveillance...
Yet adherence to cancer surveillance recommendations is poor. A study of patients with colon cancer demonstrated that only 12% met all recommended surveillance guidelines.16 A study of patients with bladder cancer after radical cystectomy showed that only 9% met recommended surveillance more than 2 years after diagnosis.17 Those dismal statistics may be the result of provider oversight—not patient reluctance.
In the colon cancer study, for example, compliance with follow-up colonoscopy was 80% but compliance with carcinoembryonic antigen testing was only 22%.16 In the bladder cancer study, follow-up urine cytology was obtained in only 23% of patients, although 75% completed recommended imaging.17
Although surveillance remains the oncologist’s responsibility, visits to the FP provide an opportunity to review surveillance and order needed laboratory testing and other studies, including imaging.
3. Screen for new cancers
The risk of a second cancer is elevated for cancer survivors compared with the risk of a primary cancer in the healthy general population; some survivors have a lifetime risk of a second cancer as high as 36%.18 Risk varies by cancer type (TABLE 319). Some of this variation is due to the impact of smoking: Smoking-related cancers have the highest risk of second malignancy.19 Genetic predisposition to malignant transformation is also theorized to contribute to increased risk. Second malignancies are dangerous; 55% of patients die of the second cancer compared with only 13% of their initial cancer.19

Studies show that cancer survivors display varying adherence with recommended screening for second cancers. In a study of Latina cancer survivors, depressive symptoms were associated with lower screening compliance.20 A study of survivors of hematologic cancer showed a low rate of cancer screening and high fear of cancer recurrence—suggesting avoidance due to fear.21 Other studies, however, show similar or increased compliance with screening in cancer survivors.22,23 A meta-analysis of 19 studies determined that, overall, cancer survivors receive 25% to 38% more recommended screening than the general population.24
Continue to: Few guidelines exist to guide FPs...
Few guidelines exist to guide FPs in adjusting screening for the cancer survivor. For women who received radiation therapy for a tumor in the chest, for example, the recommendation offered by several groups is to start breast cancer screening 8 to 10 years after treatment or by 30 years of age, and to consider combining magnetic resonance imaging and mammography.25 Recommendations for breast cancer screening do not account for a history of other gynecologic cancers unless genetic markers are present.25 On the other hand, the impact of a history of cancer on the risk of prostate cancer and on screening decisions has not been studied,26 and cervical cancer screening guidelines, which recommend that screening continue after 65 years of age for patients who are immunocompromised, do not address a history of other cancer.27
4. Manage the effects of both the cancer and the treatment
Medical issues faced by cancer survivors are familiar to FPs, but there are some specific recommendations regarding evaluation and treatment that stand in contrast to what would be considered for a healthy, or non-cancer, patient. For example, each chemotherapeutic agent has characteristic adverse effects; TABLE 47 lists the principal adverse effects of common agents and recommendations for testing when these problems develop. Common long-term problems in cancer survivors include fatigue, chronic pain, cognitive dysfunction, psychiatric illness, and cardiovascular disease. Although these symptoms and manifestations are common, the physician must be careful: New or changing symptoms could signal the spread or recurrence of disease. Fear of recurrence can lead patients to exaggerate or minimize symptoms.

Fatigue is the most common symptom seen in cancer survivors during treatment and following remission.28 More than 40% of cancer survivors report significant fatigue.29 Although fatigue is concerning for cancer recurrence, other causes are common in cancer survivors. Both depression and anxiety commonly present with worsened fatigue.30 Sleep disturbances are common, even without a psychiatric diagnosis.31 Effects of treatment, including nausea, anemia, heart failure, and medication adverse effects can cause or worsen fatigue. Pain is associated with fatigue, but to a lesser extent than are depression, anxiety, and nausea.32

Pharmacotherapy of cancer-related fatigue is challenging. Psychostimulants have been most studied. A recent systematic review shows that methylphenidate produces mild or moderate improvement in fatigue, whereas modafanil has minimal effectiveness.33 Antidepressants have not been shown to relieve fatigue.33
A recent meta-analysis showed that nonpharmaceutical treatments for cancer-related fatigue are more effective than pharmacotherapy. In this review, both exercise and pharmacotherapy had a mild-to-moderate effect on fatigue.35 Exercise is best studied in this regard, and has shown the most consistent results.31
Continue to: Chronic pain
Chronic pain. Pain is common in cancer survivors: As many as 40% experience pain for years after initial therapy.36 Treatment of some cancers—eg, thoracotomy (80%), amputation (50%-80%), neck dissection (52%), and surgical management of breast cancer (63%)—increase the likelihood of chronic pain.37 Reports of pain in cancer survivors that should be considered red flags that might signal recurrence of cancer include new or worsening pain; pain worse at night or when recumbent; new neurologic symptoms; and general symptoms of systemic illness37 (TABLE 537).

Management of pain is best approached by its cause, with neurologic, rheumatologic (including myofascial pain and arthralgia), lymphatic, and genital causes most common.37 Across all types of pain, complete relief is unlikely; functional goals provide a more effective target.
For neuropathic cancer pain, duloxetine is the only medication with evidence of benefit; anticonvulsant and topical medications are recommended on the basis of the findings of studies of noncancer pain.38 There are few data on the value of treatments for cancer-related rheumatologic and lymphatic pain, although exercise has shown benefit in both types.38 For dyspareunia and sexual dysfunction (common after gynecologic and nongynecologic cancers), vaginal lubricants and pelvic-floor physiotherapy have shown benefit.39 There is significant overlap in psychiatric comorbidities, sleep, and pain, and addressing all of a patient’s problems can reduce pain and improve function.40
Opioids are often prescribed for pain in cancer survivors. Cancer survivors have a higher rate of opioid prescribing compared with that of non-cancer patients, even 10 years after diagnosis.41 Guidelines of the Centers for Disease Control and Prevention for using opioids to manage chronic pain specifically exclude cancer patients.42 Regrettably, there is no evidence that opioids have long-term efficacy in chronic pain; in fact, evidence is accumulating that chronic opioid therapy exacerbates chronic pain.43
Cognitive dysfunction is present in 17% to 75% of cancer survivors as memory disturbance, psychological disorder, sleep dysfunction, or impairment of executive functioning.44 Cognitive deficits appear to be secondary to both cancer and treatment modalities45; as many as one-third of patients have cognitive dysfunction prior to receiving chemotherapy.46
Continue to: Chemotherapies that are more likely...
Chemotherapies that are more likely to cause cognitive symptoms include methotrexate, 5-fluorouracil, cyclophosphamide, and hormone antagonists.47 More powerful regimens and repetitive chemotherapy regimens tend to cause more cognitive effects.47
Cognitive training interventions show evidence of likely benefit,44,48 leading to recommendations for self-treatment strategies, such as written lists, wordplay, crossword puzzles, jigsaw puzzles, playing a musical instrument, and new hobbies. Small studies suggest a benefit from cognitive behavioral therapy.44,49 A study of breast cancer survivors showed that yoga led to improvement in patient-reported cognitive dysfunction.50 Physical exercise yields cognitive benefit in healthy older adults and is supported by limited evidence in cancer survivors.51
There is no effective pharmacotherapy for cancer- and cancer chemotherapy-related cognitive dysfunction unless a treatable underlying cause is found.44 Symptoms tend to subside with time after completion of chemotherapy, which might be reassuring to patients and families.45
Psychiatric problems. The most common psychiatric issues in cancer survivors are anxiety and depression; the prevalence of anxiety is nearly double that of depression.52 Anxiety often presents as fear of a recurrence of cancer or a feeling of lack of control over present or future circumstances.53 Screening for anxiety and depression is recommended at each visit, using standardized screening questionnaires.54
A small study suggests that psychiatric treatment reduces the risk of early mortality.55 Small studies also suggest that mindfulness-based therapy and cognitive behavioral therapy delivered by telehealth offer benefit.56 A meta-analysis shows that exercise interventions improve depression and anxiety in breast cancer patients.57
Continue to: There are few studies of pharmacotherapy...
There are few studies of pharmacotherapy of anxiety or depression in cancer survivors56; it is known that cancer survivors are nearly twice as likely as the general population to be taking medical therapy for anxiety and depression.58 A Cochrane systematic review of 7 small studies showed uncertain improvement in depressive symptoms in patients with cancer from antidepressant medication; however, an earlier systematic review did show benefit.59,60
In a trial of patients without depression who were being treated for head and neck cancer, escitalopram, 20 mg/d, reduced the risk of subsequent depression compared with placebo.61 A study of 420 breast cancer survivors showed that 300 mg/d and 900 mg/d dosages of gabapentin were both superior to placebo, and nearly equivalent to each other, at reducing anxiety scores.62 In both studies, however, the evidence is nonetheless insufficient to make specific recommendations about these medications.
Cardiac risk
Among chemotherapeutic agents, anthracyclines, such as doxorubicin, cause the most rapid and striking myocyte damage. This damage is dose-dependent and nearly irreversible, with 98% of injury occurring within the first year of chemotherapy.64 More than one half of cancer patients taking an anthracycline have cardiac dysfunction on imaging; 5% will be in overt heart failure 10 to 20 years, or longer, after chemotherapy.63 Following monitoring at 1 year post-therapy, regular cardiac imaging is not recommended in the absence of symptoms.62
Because other cardiotoxic chemotherapeutic agents cause partially reversible damage, imaging is not recommended in the absence of symptoms in patients taking those agents.64
Continue to: Radiation therapy to the chest leads...
Radiation therapy to the chest leads to many cardiac complications, including cardiomyopathy, valvular disease, pericardial disease, and arrhythmias. Development of cardiomyopathy can be delayed 20 to 30 years after radiation; screening echocardiography is therefore recommended every 5 to 10 years after radiation therapy.65 Recent adjustments to the dosages and delivery of radiation therapy should reduce cardiac damage, but will require decades to validate.63
For patients at risk of cardiovascular disease prior to treatment of cancer, there is evidence to support preventive treatment with angiotensin II-receptor antagonists, beta-blockers, and statins to prevent cardiomyopathy.63 Treatment of diagnosed cardiomyopathy and heart failure follows standard guidelines, with significant emphasis on aerobic exercise and smoking cessation.63
Cancer survivorship care: Your critical role
Cancer survivors constitute a large population who frequent the practices of primary care physicians. Primary care visits provide an opportunity to monitor key elements of survivorship, including surveillance of the current cancer and screening for second cancers. Similar to what is seen with diabetes and coronary artery disease, cancer increases cardiac risk, which requires preventive care and chronic management. FPs are well placed to treat common issues in cancer survivors—issues that mirror concerns seen in the general population.
CORRESPONDENCE
Michael J. Arnold, MD, CDR, USN, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected].
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted with the editing of the manuscript.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016-2017. Atlanta, GA: American Cancer Society; 2016. www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html. Accessed July 25, 2018.
2. Survivorship. NCCN Guidelines (version 1.2017). Fort Washington, PA: National Comprehensive Cancer Network; 2017. www.nccn.org/professionals/physician_gls/default.aspx#supportive. Accessed July 26, 2018.
3. Kendall C, Decker KM, Groome PA, et al. Use of physician services during the survivorship phase: a multi-province study of women diagnosed with breast cancer. Curr Oncolog. 2017;24:81-89.
4. Snyder CF, Earle CC, Herbert RJ, et al. Preventive care for colorectal cancer survivors: a 5-year longitudinal study. J Clin Oncol. 2008;26:1073-1079.
5. Hewitt M, Greenfield S, Stovall E (eds); Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington DC: The National Academies Press; 2006. www.nap.edu/read/11468/chapter/1. Accessed July 25, 2018.
6. Survivorship care plan. New York, NY: Memorial Sloan Kettering Cancer Center. www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan. Accessed August 11, 2018.
7. Fuentes AC, Lambird JE, George TJ, et al. Cancer survivor’s history and physical. South Med J. 2017;110:37-44. http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/. Accessed July 26, 2018.
8. Salz T, Oeffinger KC, McCabe MS, et al. Survivorship care plans in research and practice. CA Cancer J Clin. 2012;62:101-117.
9. Birken SA, Mayer DK, Weiner BJ. Survivorship care plans: prevalence and barriers to use. J Cancer Educ. 2013;28:290-296.
10. American College of Surgeons Commission on Cancer. Cancer program standards 2012: Ensuring patient-centered care. V1.2.1. www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed July 25, 2018.
11. NCCN guidelines for treatment of cancer by site. NCCN Guidelines (version 1.2018). Fort Washington, PA: National Comprehensive Cancer Network; 2018. www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed July 25, 2018.
12. Spronka I, Korevaar JC, Burgers JS, et al. Review of guidance on recurrence risk management for general practitioners in breast cancer, colorectal cancer and melanoma guidelines. Family Pract. 2017;34:154-160.
13. Merkow RP, Korenstein D, Yeahia R, et al. Quality of cancer surveillance clinical practice guidelines: specificity and consistency of recommendations. JAMA Intern Med. 2017;177:701-709.
14. Muradali D, Kennedy EB, Eisen A, et al. Breast screening for survivors of breast cancer: a systematic review. Prev Med. 2017;103:70-75.
15. Giannarini G, Kessler TM, Thoeny HC, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486-494.
16. Sisler JJ, Seo B, Katz A, et al. Concordance with ASCO guidelines for surveillance after colorectal cancer treatment: a population-based analysis. J Oncol Pract. 2012;8:e69-e79.
17. Ehdaie B. Atoria CL, Lowrance WT, et al. Adherence to surveillance guidelines after radical cystectomy: a population-based analysis. Urol Oncol. 2014;32:779-784.
18. Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354-1365.
19. Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122:3075-3086.
20. Holder AE, Ramirez AG, Gallion K. Depressive symptoms in Latina breast cancer survivors: a barrier to cancer screening. Health Psycholog. 2014;33:242-248.
21. Dyer G, Larsen SR, Gilroy N, et al. Adherence to cancer screening guidelines in Australian survivors of allogenic blood and marrow transplantation (BMT). Cancer Med. 2016;5:1702-1716.
22. Mandelzweig L, Chetrit A, Amitai T, et al. Primary prevention and screening practices among long-term breast cancer survivors. Cancer Causes Control. 2017;28:657-666.
23. Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16:207-214.
24. Uhlig A, Mei J, Baik I, et al. Screening utilization among cancer survivors: a meta-analysis. J Public Health (Oxf). 2018;40:129-137.
25. Hilal T, Rudy DW. Radiation-induced breast cancer: the question of early breast cancer screening in Hodgkin’s lymphoma survivors. Oxf Med Case Reports. 2016;2016:17-18.
26. Lin K, Croswell JM, Koenig H, et al. Prostate-specific antigen-based screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force [Internet]. Evidence Syntheses No. 90. AHRQ Publication No. 12-05160-EF-1. Rockville, MD: Agency for Healthcare Research and Quality (US); October 2011. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0032900/. Accessed July 25, 2018.
27. US Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
28. Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1:4-10.
29. Jung JY, Lee JM, Kim MS, et al. Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology. 2018;27:465-470.
30. Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatment. Nat Rev Clin Oncol. 2014;11:597-609.
31. Medysky ME, Temesi J, Culos-Reed SN, et al. Exercise, sleep and cancer-related fatigue: are they related? Neurophysiol Clin. 2017;47:111-122.
32. Oh HS, Sea WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid Based Nurs. 2011;8:191-201.
33. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2016;25:970-979.
34. Escalante CP, Manzullo EF. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(suppl 2):S412-S416.
35. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961-968.
36. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
37. Davies PS. Chronic pain management in the cancer survivor: tips for primary care providers. Nurse Pract. 2013;39:28-38.
38. Boland EG, Ahmedzai SH. Persistent pain in cancer survivors. Curr Opin Support Palliat Care. 2017;11:181-190.
39. Sears CS, Robinson JW, Walker LM. A comprehensive review of sexual health concerns after cancer treatment and the biopsychosocial treatment options available to female patients. Eur J Cancer Care (Engl). 2017;27:e12738.
40. Schou Bredal I, Smeby NA, Ottesen S, et al. Chronic pain in breast cancer survivors: comparison of psychological, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manage. 2014;48:852-862.
41. Sutradhar R, Lokku A, Barbera L. Cancer survivorship and opioid prescribing rates: a population-based matched cohort study among individuals with and without a history of cancer. Cancer. 2017;123:4286-4293.
42. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
43. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
44. Von Ah D. Cognitive changes associated with cancer and cancer treatment: state of the science. Clin J Oncol Nurs. 2015;19:47-56.
45. Moore HC. An overview of chemotherapy-related cognitive dysfunction, or ‘chemobrain’. Oncology (Williston Park). 2014;28:797-804.
46. Asher A. Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil. 2011;90(suppl):S16-S26.
47. Joly F, Rigal O, Noal S, et al. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psychooncology. 2011;20:1251-1258.
48. Attention, thinking or memory problems. American Society of Clinical Oncology Cancer.Net. April 2018. www.cancer.net/navigating-cancer-care/side-effects/attention-thinking-or-memory-problems. Accessed July 25, 2018.
49. Kucherer S, Ferguson RJ. Cognitive behavioral therapy for cancer-related cognitive dysfunction. Curr Opin Support Palliat Care. 2017;11:46-51.
50. Derry HM, Jaremka LM, Bennet JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24:958-966.
51. Treanor CJ, McMenamin UC, O’Neill RF, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst Rev. 2016 Aug 16;(8):CD011325.
52. Mitchell AJ, Ferguson DW, Gill J, et al. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721-732.
53. Inhestern L, Beierlein V, Bultmann JC, et al. Anxiety and depression in working-age cancer survivors: a register-based study. BMC Cancer. 2017;17:347.
54. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for adult cancer survivors: highlighting ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015:188-194.
55. Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450-3458.
56. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin N Am. 2017;101:1099-1113.
57. Zhu G, Zhang X, Wang Y, et al. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trials. Onco Targets Ther. 2016;9:2153-2168.
58. Hawkins NA, Soman A, Lunsford N, et al. Use of medications for treating anxiety and depression in cancer survivors in the United States. J Clin Oncol. 2017;35:78-85.
59. Ostuzzi G, Matcham F, Dauchy S, et al. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. 2015 June 1;(6):CD011006.
60. Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:140.
61. Lydiatt WM, Bessette D, Schmid KK, et al. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer: randomized, double-blind, placebo-controlled clinical trial. JAMA Otolaryngol Head Neck Surg. 2013;139:678-686.
62. Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136:479-486.
63. Okwuosa TM, Anzevino S, Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J. 2017;93:82-90.
64. Plana, JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911-939.
65. Lancellotti, P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013-1032.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016-2017. Atlanta, GA: American Cancer Society; 2016. www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html. Accessed July 25, 2018.
2. Survivorship. NCCN Guidelines (version 1.2017). Fort Washington, PA: National Comprehensive Cancer Network; 2017. www.nccn.org/professionals/physician_gls/default.aspx#supportive. Accessed July 26, 2018.
3. Kendall C, Decker KM, Groome PA, et al. Use of physician services during the survivorship phase: a multi-province study of women diagnosed with breast cancer. Curr Oncolog. 2017;24:81-89.
4. Snyder CF, Earle CC, Herbert RJ, et al. Preventive care for colorectal cancer survivors: a 5-year longitudinal study. J Clin Oncol. 2008;26:1073-1079.
5. Hewitt M, Greenfield S, Stovall E (eds); Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington DC: The National Academies Press; 2006. www.nap.edu/read/11468/chapter/1. Accessed July 25, 2018.
6. Survivorship care plan. New York, NY: Memorial Sloan Kettering Cancer Center. www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan. Accessed August 11, 2018.
7. Fuentes AC, Lambird JE, George TJ, et al. Cancer survivor’s history and physical. South Med J. 2017;110:37-44. http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/. Accessed July 26, 2018.
8. Salz T, Oeffinger KC, McCabe MS, et al. Survivorship care plans in research and practice. CA Cancer J Clin. 2012;62:101-117.
9. Birken SA, Mayer DK, Weiner BJ. Survivorship care plans: prevalence and barriers to use. J Cancer Educ. 2013;28:290-296.
10. American College of Surgeons Commission on Cancer. Cancer program standards 2012: Ensuring patient-centered care. V1.2.1. www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed July 25, 2018.
11. NCCN guidelines for treatment of cancer by site. NCCN Guidelines (version 1.2018). Fort Washington, PA: National Comprehensive Cancer Network; 2018. www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed July 25, 2018.
12. Spronka I, Korevaar JC, Burgers JS, et al. Review of guidance on recurrence risk management for general practitioners in breast cancer, colorectal cancer and melanoma guidelines. Family Pract. 2017;34:154-160.
13. Merkow RP, Korenstein D, Yeahia R, et al. Quality of cancer surveillance clinical practice guidelines: specificity and consistency of recommendations. JAMA Intern Med. 2017;177:701-709.
14. Muradali D, Kennedy EB, Eisen A, et al. Breast screening for survivors of breast cancer: a systematic review. Prev Med. 2017;103:70-75.
15. Giannarini G, Kessler TM, Thoeny HC, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486-494.
16. Sisler JJ, Seo B, Katz A, et al. Concordance with ASCO guidelines for surveillance after colorectal cancer treatment: a population-based analysis. J Oncol Pract. 2012;8:e69-e79.
17. Ehdaie B. Atoria CL, Lowrance WT, et al. Adherence to surveillance guidelines after radical cystectomy: a population-based analysis. Urol Oncol. 2014;32:779-784.
18. Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354-1365.
19. Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122:3075-3086.
20. Holder AE, Ramirez AG, Gallion K. Depressive symptoms in Latina breast cancer survivors: a barrier to cancer screening. Health Psycholog. 2014;33:242-248.
21. Dyer G, Larsen SR, Gilroy N, et al. Adherence to cancer screening guidelines in Australian survivors of allogenic blood and marrow transplantation (BMT). Cancer Med. 2016;5:1702-1716.
22. Mandelzweig L, Chetrit A, Amitai T, et al. Primary prevention and screening practices among long-term breast cancer survivors. Cancer Causes Control. 2017;28:657-666.
23. Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16:207-214.
24. Uhlig A, Mei J, Baik I, et al. Screening utilization among cancer survivors: a meta-analysis. J Public Health (Oxf). 2018;40:129-137.
25. Hilal T, Rudy DW. Radiation-induced breast cancer: the question of early breast cancer screening in Hodgkin’s lymphoma survivors. Oxf Med Case Reports. 2016;2016:17-18.
26. Lin K, Croswell JM, Koenig H, et al. Prostate-specific antigen-based screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force [Internet]. Evidence Syntheses No. 90. AHRQ Publication No. 12-05160-EF-1. Rockville, MD: Agency for Healthcare Research and Quality (US); October 2011. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0032900/. Accessed July 25, 2018.
27. US Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
28. Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1:4-10.
29. Jung JY, Lee JM, Kim MS, et al. Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology. 2018;27:465-470.
30. Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatment. Nat Rev Clin Oncol. 2014;11:597-609.
31. Medysky ME, Temesi J, Culos-Reed SN, et al. Exercise, sleep and cancer-related fatigue: are they related? Neurophysiol Clin. 2017;47:111-122.
32. Oh HS, Sea WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid Based Nurs. 2011;8:191-201.
33. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2016;25:970-979.
34. Escalante CP, Manzullo EF. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(suppl 2):S412-S416.
35. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961-968.
36. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
37. Davies PS. Chronic pain management in the cancer survivor: tips for primary care providers. Nurse Pract. 2013;39:28-38.
38. Boland EG, Ahmedzai SH. Persistent pain in cancer survivors. Curr Opin Support Palliat Care. 2017;11:181-190.
39. Sears CS, Robinson JW, Walker LM. A comprehensive review of sexual health concerns after cancer treatment and the biopsychosocial treatment options available to female patients. Eur J Cancer Care (Engl). 2017;27:e12738.
40. Schou Bredal I, Smeby NA, Ottesen S, et al. Chronic pain in breast cancer survivors: comparison of psychological, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manage. 2014;48:852-862.
41. Sutradhar R, Lokku A, Barbera L. Cancer survivorship and opioid prescribing rates: a population-based matched cohort study among individuals with and without a history of cancer. Cancer. 2017;123:4286-4293.
42. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
43. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
44. Von Ah D. Cognitive changes associated with cancer and cancer treatment: state of the science. Clin J Oncol Nurs. 2015;19:47-56.
45. Moore HC. An overview of chemotherapy-related cognitive dysfunction, or ‘chemobrain’. Oncology (Williston Park). 2014;28:797-804.
46. Asher A. Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil. 2011;90(suppl):S16-S26.
47. Joly F, Rigal O, Noal S, et al. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psychooncology. 2011;20:1251-1258.
48. Attention, thinking or memory problems. American Society of Clinical Oncology Cancer.Net. April 2018. www.cancer.net/navigating-cancer-care/side-effects/attention-thinking-or-memory-problems. Accessed July 25, 2018.
49. Kucherer S, Ferguson RJ. Cognitive behavioral therapy for cancer-related cognitive dysfunction. Curr Opin Support Palliat Care. 2017;11:46-51.
50. Derry HM, Jaremka LM, Bennet JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24:958-966.
51. Treanor CJ, McMenamin UC, O’Neill RF, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst Rev. 2016 Aug 16;(8):CD011325.
52. Mitchell AJ, Ferguson DW, Gill J, et al. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721-732.
53. Inhestern L, Beierlein V, Bultmann JC, et al. Anxiety and depression in working-age cancer survivors: a register-based study. BMC Cancer. 2017;17:347.
54. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for adult cancer survivors: highlighting ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015:188-194.
55. Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450-3458.
56. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin N Am. 2017;101:1099-1113.
57. Zhu G, Zhang X, Wang Y, et al. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trials. Onco Targets Ther. 2016;9:2153-2168.
58. Hawkins NA, Soman A, Lunsford N, et al. Use of medications for treating anxiety and depression in cancer survivors in the United States. J Clin Oncol. 2017;35:78-85.
59. Ostuzzi G, Matcham F, Dauchy S, et al. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. 2015 June 1;(6):CD011006.
60. Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:140.
61. Lydiatt WM, Bessette D, Schmid KK, et al. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer: randomized, double-blind, placebo-controlled clinical trial. JAMA Otolaryngol Head Neck Surg. 2013;139:678-686.
62. Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136:479-486.
63. Okwuosa TM, Anzevino S, Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J. 2017;93:82-90.
64. Plana, JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911-939.
65. Lancellotti, P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013-1032.
PRACTICE RECOMMENDATIONS
› Provide normal age-related cancer screening for cancer survivors because of their high risk of a second cancer. B
› Strongly encourage lifestyle changes for cancer survivors, especially smoking cessation. B
› Recommend exercise, which alleviates pain, depression, anxiety, and (more effectively than any other intervention) fatigue, for cancer survivors. B
› Remain vigilant for the development in cancer survivors of cardiovascular disease, including heart failure, which can appear long after therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
*Cancer survivor care in the pediatric patients, including application of a survivorship care plan (also discussed later in this article), is reviewed in “Partnering to optimize care of childhood cancer survivors,” The Journal of Family Practice, April 2017.
Take these steps to improve your flu season preparedness
Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).
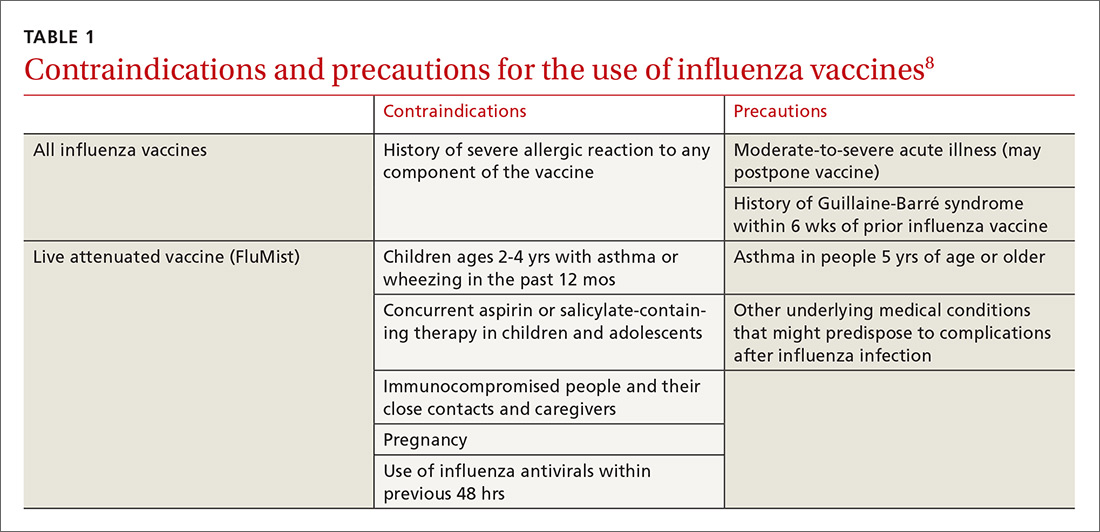
Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.
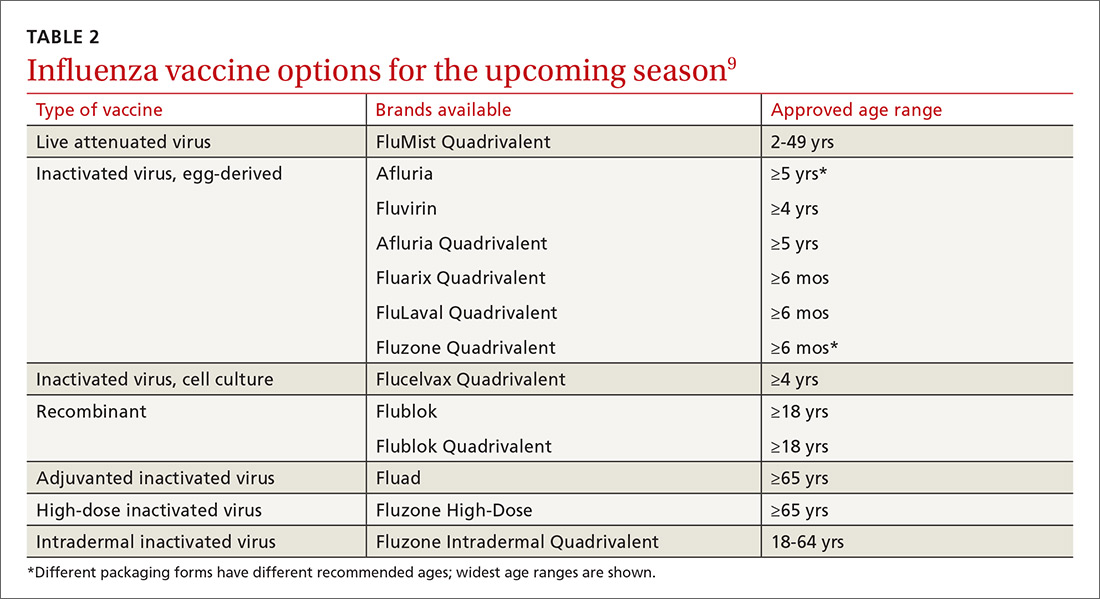
Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
[polldaddy:10124269]
Continue to: Strategy 2
Strategy 2: Make use of chemoprophylaxis
Preventive use of antiviral medication (chemoprophylaxis) may be a useful adjunct or alternative to vaccination in certain circumstances: if the patient is at high risk for complications, has been exposed to someone with influenza, has contraindications to vaccination, or received the vaccine within the past 2 weeks. The CDC also suggests that chemoprophylaxis be considered for those with immune deficiencies or who are otherwise immunosuppressed after exposure.14 Antivirals can also be used to control outbreaks in long-term care facilities; in these cases, the recommendedregimen is daily administration for at least 2 weeks, continuing until at least 7 days after the identification of the last case.14 Oseltamivir (Tamiflu) and zanamivir (Relenza) are the recommended prophylactic agents; a related intravenous medication, peramivir (Rapivab), is recommend for treatment only (see Table 314).
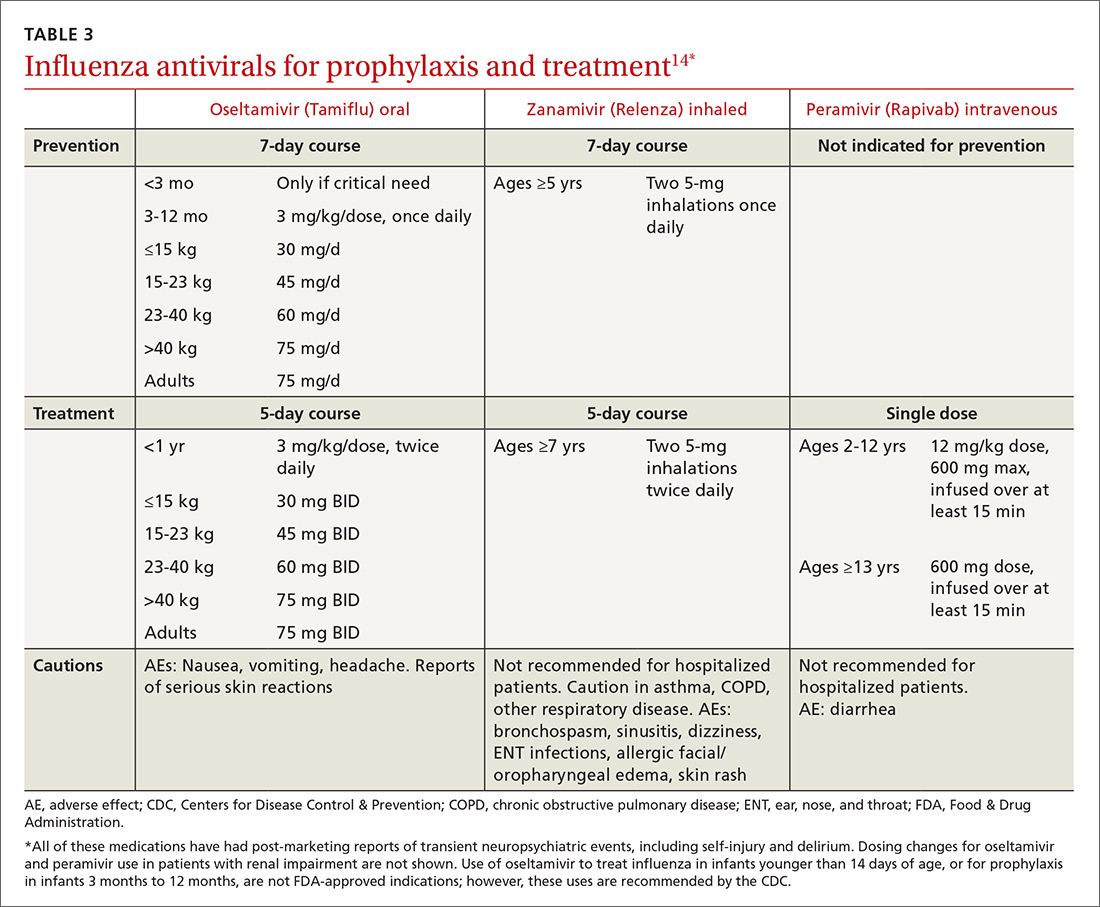
Strategy 3: Prevent comorbidities and opportunistic infections
Morbidity associated with influenza often comes from secondary infection. Pneumonia is among the most common complications, so influenza season is a good time to ensure that patients are appropriately vaccinated against pneumococcus, as well. Pneumococcal conjugate vaccine (Prevnar or PCV13) is recommended for children younger than 2 years of age, to be administered in a series of 4 doses: at 2, 4, 6, and 12-15 months. Vaccination with PCV13 is also recommended for those ages 65 or older, to be followed at least one year later with pneumococcal polysaccharide vaccine (Pneumovax or PPSV23).15 Additional doses of PCV13, PPSV23, or both may be indicated, depending on health status.
Strategy 4: Encourage good hygiene
The availability of immunizations and antivirals does not replace good hygiene. Frequent handwashing reduces the transmission of respiratory viruses, including influenza.16 Few studies have evaluated the use of alcohol-based hand sanitizers, but available evidence suggests they are effective in lowering viral transmission.16
Barriers, such as masks, gloves, and gowns, are helpful for health care workers.16 Surgical masks are often considered more comfortable to wear than N95 respirators. It may therefore be welcome news that when a 2009 randomized study assessed their use by hospital-based nurses, masks were non-inferior in protecting these health care workers against influenza.17
Presenteeism, the practice of going to work while sick, should be discouraged. People at risk for influenza may wish to avoid crowds during flu season; those with symptoms should be encouraged to stay home and limit contact with others.
Continue to: Treatment
Treatment
Strategy 1: Make prompt use of antivirals
Despite available preventive measures, tens of millions of people in the United States develop influenza every year. Use of antiviral medication, begun early in the course of illness, can reduce the duration of symptoms and may reduce the risk for complications.
The neuraminidase inhibitor (NI) group of antivirals—oseltamivir, zanamivir, and peramivir—is effective against influenza types A and B and current resistance rates are low.
The adamantine family of antivirals, amantadine and rimantadine, treat type A only. Since the circulating influenza strains in the past several seasons have demonstrated resistance >99%, these medications are not currently recommended.14
NIs reduce the duration of influenza symptoms by 10% to 20%, shortening the illness by 6 to 24 hours.18,19 In otherwise healthy patients, this benefit must be balanced against the increased risk for nausea and vomiting (oseltamivir), bronchospasm and sinusitis (zanamivir), and diarrhea (peramivir). In adults, NIs reduce the risk for lower respiratory tract complications and hospitalization. A 2015 meta-analysis by Dobson et al found a relative risk for hospitalization among those prescribed oseltamivir vs placebo of 37%.18
In the past, antivirals were used only in high-risk patients, such as children younger than 2 years, adults older than 65 years, and those with chronic health conditions.14 Now, antivirals are recommended for those who are at higher risk for complications (see Table 4), those with “severe, complicated, or progressive illness,” and hospitalized patients.14
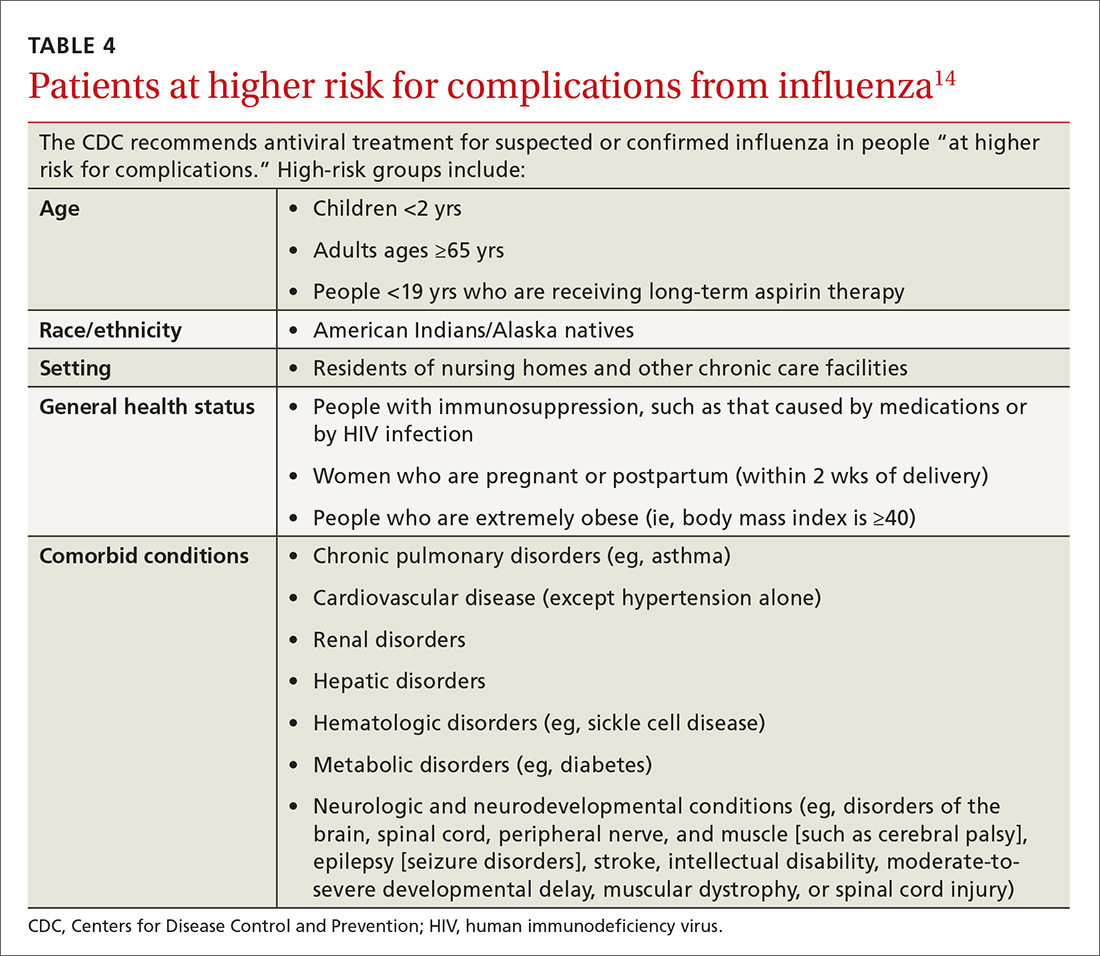
Continue to: Antiviral treatment may have some value...
Antiviral treatment may have some value for hospitalized patients when started even 5 days after symptom onset. Treatment may be extended beyond the usual recommendations (5 days for oseltamivir or zanamivir) in immunosuppressed patients or the critically ill. Additionally, recent guidelines include consideration of antiviral treatment in outpatients who are at normal risk if treatment can be started within 48 hours of symptom onset.14
The CDC currently recommends use of oseltamivir rather than other antivirals for most hospitalized patients, based on the availability of data on its use in this setting.14 Intravenous peramivir is recommended for patients who cannot tolerate or absorb oral medication; inhaled zanamivir or IV peramivir are preferred for patients with end-stage renal disease who are not undergoing dialysis (see Table 3).14
Strategy 2: Exercise caution when it comes to supportive care
There are other medications that may offer symptom relief or prevent complications, especially when antivirals are contraindicated or unavailable.
Corticosteroids are recommended as part of the treatment of community-acquired pneumonia,20 but their role in influenza is controversial. A 2016 Cochrane review21 found no randomized controlled trials on the topic. Although the balance of available data from observational studies indicated that use of corticosteroids was associated with increased mortality, the authors also noted that all the studies included in their meta-analysis were of “very low quality.” They concluded that “the use of steroids in influenza remains a clinical judgement call.”
Statins may be associated with improved outcomes in influenza and pneumonia. Studies thus far have given contradictory results,22,23 and a planned Cochrane review of the question has been withdrawn.24
Continue to: Over-the-counter medications...
Over-the-counter medications, such as aspirin, acetaminophen, and ibuprofen are often used to manage the fever and myalgia associated with influenza. Patients should be cautioned against using the same ingredient in multiple different branded medications. Acetaminophen, for example, is not limited to Tylenol-branded products. To avoid Reye’s syndrome, children and teens with febrile illness, such as influenza, should not use aspirin.
CORRESPONDENCE
Jennifer L. Hamilton, MD, PhD, Drexel Family Medicine, 10 Shurs Lane, Suite 301, Philadelphia, PA 19127; [email protected].
1. CDC. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Published June 8, 2018. Accessed August 22, 2018.
2. CDC. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Published April 19, 2017. https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed Setptember 18, 2018.
3. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Published February 15, 2018. Accessed August 22, 2018.
4. Sah P, Medlock J, Fitzpatrick MC, et al. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018:201802479.
5. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244.
6. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:158–160.
7. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:156–157.
8. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1-20.
9. CDC. Influenza vaccines—United States, 2017–18 influenza season. https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Published May 16, 2018. Accessed August 22, 2018.
10. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643–645.
11. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. AAP News. May 21, 2018. http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 22, 2018.
12. Glinka ER, Smith DM, Johns ST. Timing matters—influenza vaccination to HIV-infected patients. HIV Med. 2016;17:601-604.
13. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18:20388.
14. CDC. Influenza antiviral medications: summary for clinicians. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Published May 11, 2018. Accessed August 22, 2018.
15. CDC. Pneumococcal vaccination summary: who and when to vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Published February 28, 2018. Accessed August 22, 2018.
16. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207.
17. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-1871.
18. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737.
19. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258.
20. Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management. Am Fam Physician. 2016;94:698-706.
21. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406.
22. Brassard P, Wu JW, Ernst P, et al. The effect of statins on influenza-like illness morbidity and mortality. Pharmacoepidemiol Drug Saf. 2017;26:63-70.
23. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417-435.
24. Khandaker G, Rashid H, Chow MY, et al. Statins for influenza and pneumonia. Cochrane Database Syst Rev. January 9, 2017 [withdrawn].
Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).

Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.

Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
[polldaddy:10124269]
Continue to: Strategy 2
Strategy 2: Make use of chemoprophylaxis
Preventive use of antiviral medication (chemoprophylaxis) may be a useful adjunct or alternative to vaccination in certain circumstances: if the patient is at high risk for complications, has been exposed to someone with influenza, has contraindications to vaccination, or received the vaccine within the past 2 weeks. The CDC also suggests that chemoprophylaxis be considered for those with immune deficiencies or who are otherwise immunosuppressed after exposure.14 Antivirals can also be used to control outbreaks in long-term care facilities; in these cases, the recommendedregimen is daily administration for at least 2 weeks, continuing until at least 7 days after the identification of the last case.14 Oseltamivir (Tamiflu) and zanamivir (Relenza) are the recommended prophylactic agents; a related intravenous medication, peramivir (Rapivab), is recommend for treatment only (see Table 314).

Strategy 3: Prevent comorbidities and opportunistic infections
Morbidity associated with influenza often comes from secondary infection. Pneumonia is among the most common complications, so influenza season is a good time to ensure that patients are appropriately vaccinated against pneumococcus, as well. Pneumococcal conjugate vaccine (Prevnar or PCV13) is recommended for children younger than 2 years of age, to be administered in a series of 4 doses: at 2, 4, 6, and 12-15 months. Vaccination with PCV13 is also recommended for those ages 65 or older, to be followed at least one year later with pneumococcal polysaccharide vaccine (Pneumovax or PPSV23).15 Additional doses of PCV13, PPSV23, or both may be indicated, depending on health status.
Strategy 4: Encourage good hygiene
The availability of immunizations and antivirals does not replace good hygiene. Frequent handwashing reduces the transmission of respiratory viruses, including influenza.16 Few studies have evaluated the use of alcohol-based hand sanitizers, but available evidence suggests they are effective in lowering viral transmission.16
Barriers, such as masks, gloves, and gowns, are helpful for health care workers.16 Surgical masks are often considered more comfortable to wear than N95 respirators. It may therefore be welcome news that when a 2009 randomized study assessed their use by hospital-based nurses, masks were non-inferior in protecting these health care workers against influenza.17
Presenteeism, the practice of going to work while sick, should be discouraged. People at risk for influenza may wish to avoid crowds during flu season; those with symptoms should be encouraged to stay home and limit contact with others.
Continue to: Treatment
Treatment
Strategy 1: Make prompt use of antivirals
Despite available preventive measures, tens of millions of people in the United States develop influenza every year. Use of antiviral medication, begun early in the course of illness, can reduce the duration of symptoms and may reduce the risk for complications.
The neuraminidase inhibitor (NI) group of antivirals—oseltamivir, zanamivir, and peramivir—is effective against influenza types A and B and current resistance rates are low.
The adamantine family of antivirals, amantadine and rimantadine, treat type A only. Since the circulating influenza strains in the past several seasons have demonstrated resistance >99%, these medications are not currently recommended.14
NIs reduce the duration of influenza symptoms by 10% to 20%, shortening the illness by 6 to 24 hours.18,19 In otherwise healthy patients, this benefit must be balanced against the increased risk for nausea and vomiting (oseltamivir), bronchospasm and sinusitis (zanamivir), and diarrhea (peramivir). In adults, NIs reduce the risk for lower respiratory tract complications and hospitalization. A 2015 meta-analysis by Dobson et al found a relative risk for hospitalization among those prescribed oseltamivir vs placebo of 37%.18
In the past, antivirals were used only in high-risk patients, such as children younger than 2 years, adults older than 65 years, and those with chronic health conditions.14 Now, antivirals are recommended for those who are at higher risk for complications (see Table 4), those with “severe, complicated, or progressive illness,” and hospitalized patients.14

Continue to: Antiviral treatment may have some value...
Antiviral treatment may have some value for hospitalized patients when started even 5 days after symptom onset. Treatment may be extended beyond the usual recommendations (5 days for oseltamivir or zanamivir) in immunosuppressed patients or the critically ill. Additionally, recent guidelines include consideration of antiviral treatment in outpatients who are at normal risk if treatment can be started within 48 hours of symptom onset.14
The CDC currently recommends use of oseltamivir rather than other antivirals for most hospitalized patients, based on the availability of data on its use in this setting.14 Intravenous peramivir is recommended for patients who cannot tolerate or absorb oral medication; inhaled zanamivir or IV peramivir are preferred for patients with end-stage renal disease who are not undergoing dialysis (see Table 3).14
Strategy 2: Exercise caution when it comes to supportive care
There are other medications that may offer symptom relief or prevent complications, especially when antivirals are contraindicated or unavailable.
Corticosteroids are recommended as part of the treatment of community-acquired pneumonia,20 but their role in influenza is controversial. A 2016 Cochrane review21 found no randomized controlled trials on the topic. Although the balance of available data from observational studies indicated that use of corticosteroids was associated with increased mortality, the authors also noted that all the studies included in their meta-analysis were of “very low quality.” They concluded that “the use of steroids in influenza remains a clinical judgement call.”
Statins may be associated with improved outcomes in influenza and pneumonia. Studies thus far have given contradictory results,22,23 and a planned Cochrane review of the question has been withdrawn.24
Continue to: Over-the-counter medications...
Over-the-counter medications, such as aspirin, acetaminophen, and ibuprofen are often used to manage the fever and myalgia associated with influenza. Patients should be cautioned against using the same ingredient in multiple different branded medications. Acetaminophen, for example, is not limited to Tylenol-branded products. To avoid Reye’s syndrome, children and teens with febrile illness, such as influenza, should not use aspirin.
CORRESPONDENCE
Jennifer L. Hamilton, MD, PhD, Drexel Family Medicine, 10 Shurs Lane, Suite 301, Philadelphia, PA 19127; [email protected].
Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).

Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.

Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
[polldaddy:10124269]
Continue to: Strategy 2
Strategy 2: Make use of chemoprophylaxis
Preventive use of antiviral medication (chemoprophylaxis) may be a useful adjunct or alternative to vaccination in certain circumstances: if the patient is at high risk for complications, has been exposed to someone with influenza, has contraindications to vaccination, or received the vaccine within the past 2 weeks. The CDC also suggests that chemoprophylaxis be considered for those with immune deficiencies or who are otherwise immunosuppressed after exposure.14 Antivirals can also be used to control outbreaks in long-term care facilities; in these cases, the recommendedregimen is daily administration for at least 2 weeks, continuing until at least 7 days after the identification of the last case.14 Oseltamivir (Tamiflu) and zanamivir (Relenza) are the recommended prophylactic agents; a related intravenous medication, peramivir (Rapivab), is recommend for treatment only (see Table 314).

Strategy 3: Prevent comorbidities and opportunistic infections
Morbidity associated with influenza often comes from secondary infection. Pneumonia is among the most common complications, so influenza season is a good time to ensure that patients are appropriately vaccinated against pneumococcus, as well. Pneumococcal conjugate vaccine (Prevnar or PCV13) is recommended for children younger than 2 years of age, to be administered in a series of 4 doses: at 2, 4, 6, and 12-15 months. Vaccination with PCV13 is also recommended for those ages 65 or older, to be followed at least one year later with pneumococcal polysaccharide vaccine (Pneumovax or PPSV23).15 Additional doses of PCV13, PPSV23, or both may be indicated, depending on health status.
Strategy 4: Encourage good hygiene
The availability of immunizations and antivirals does not replace good hygiene. Frequent handwashing reduces the transmission of respiratory viruses, including influenza.16 Few studies have evaluated the use of alcohol-based hand sanitizers, but available evidence suggests they are effective in lowering viral transmission.16
Barriers, such as masks, gloves, and gowns, are helpful for health care workers.16 Surgical masks are often considered more comfortable to wear than N95 respirators. It may therefore be welcome news that when a 2009 randomized study assessed their use by hospital-based nurses, masks were non-inferior in protecting these health care workers against influenza.17
Presenteeism, the practice of going to work while sick, should be discouraged. People at risk for influenza may wish to avoid crowds during flu season; those with symptoms should be encouraged to stay home and limit contact with others.
Continue to: Treatment
Treatment
Strategy 1: Make prompt use of antivirals
Despite available preventive measures, tens of millions of people in the United States develop influenza every year. Use of antiviral medication, begun early in the course of illness, can reduce the duration of symptoms and may reduce the risk for complications.
The neuraminidase inhibitor (NI) group of antivirals—oseltamivir, zanamivir, and peramivir—is effective against influenza types A and B and current resistance rates are low.
The adamantine family of antivirals, amantadine and rimantadine, treat type A only. Since the circulating influenza strains in the past several seasons have demonstrated resistance >99%, these medications are not currently recommended.14
NIs reduce the duration of influenza symptoms by 10% to 20%, shortening the illness by 6 to 24 hours.18,19 In otherwise healthy patients, this benefit must be balanced against the increased risk for nausea and vomiting (oseltamivir), bronchospasm and sinusitis (zanamivir), and diarrhea (peramivir). In adults, NIs reduce the risk for lower respiratory tract complications and hospitalization. A 2015 meta-analysis by Dobson et al found a relative risk for hospitalization among those prescribed oseltamivir vs placebo of 37%.18
In the past, antivirals were used only in high-risk patients, such as children younger than 2 years, adults older than 65 years, and those with chronic health conditions.14 Now, antivirals are recommended for those who are at higher risk for complications (see Table 4), those with “severe, complicated, or progressive illness,” and hospitalized patients.14

Continue to: Antiviral treatment may have some value...
Antiviral treatment may have some value for hospitalized patients when started even 5 days after symptom onset. Treatment may be extended beyond the usual recommendations (5 days for oseltamivir or zanamivir) in immunosuppressed patients or the critically ill. Additionally, recent guidelines include consideration of antiviral treatment in outpatients who are at normal risk if treatment can be started within 48 hours of symptom onset.14
The CDC currently recommends use of oseltamivir rather than other antivirals for most hospitalized patients, based on the availability of data on its use in this setting.14 Intravenous peramivir is recommended for patients who cannot tolerate or absorb oral medication; inhaled zanamivir or IV peramivir are preferred for patients with end-stage renal disease who are not undergoing dialysis (see Table 3).14
Strategy 2: Exercise caution when it comes to supportive care
There are other medications that may offer symptom relief or prevent complications, especially when antivirals are contraindicated or unavailable.
Corticosteroids are recommended as part of the treatment of community-acquired pneumonia,20 but their role in influenza is controversial. A 2016 Cochrane review21 found no randomized controlled trials on the topic. Although the balance of available data from observational studies indicated that use of corticosteroids was associated with increased mortality, the authors also noted that all the studies included in their meta-analysis were of “very low quality.” They concluded that “the use of steroids in influenza remains a clinical judgement call.”
Statins may be associated with improved outcomes in influenza and pneumonia. Studies thus far have given contradictory results,22,23 and a planned Cochrane review of the question has been withdrawn.24
Continue to: Over-the-counter medications...
Over-the-counter medications, such as aspirin, acetaminophen, and ibuprofen are often used to manage the fever and myalgia associated with influenza. Patients should be cautioned against using the same ingredient in multiple different branded medications. Acetaminophen, for example, is not limited to Tylenol-branded products. To avoid Reye’s syndrome, children and teens with febrile illness, such as influenza, should not use aspirin.
CORRESPONDENCE
Jennifer L. Hamilton, MD, PhD, Drexel Family Medicine, 10 Shurs Lane, Suite 301, Philadelphia, PA 19127; [email protected].
1. CDC. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Published June 8, 2018. Accessed August 22, 2018.
2. CDC. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Published April 19, 2017. https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed Setptember 18, 2018.
3. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Published February 15, 2018. Accessed August 22, 2018.
4. Sah P, Medlock J, Fitzpatrick MC, et al. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018:201802479.
5. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244.
6. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:158–160.
7. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:156–157.
8. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1-20.
9. CDC. Influenza vaccines—United States, 2017–18 influenza season. https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Published May 16, 2018. Accessed August 22, 2018.
10. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643–645.
11. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. AAP News. May 21, 2018. http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 22, 2018.
12. Glinka ER, Smith DM, Johns ST. Timing matters—influenza vaccination to HIV-infected patients. HIV Med. 2016;17:601-604.
13. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18:20388.
14. CDC. Influenza antiviral medications: summary for clinicians. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Published May 11, 2018. Accessed August 22, 2018.
15. CDC. Pneumococcal vaccination summary: who and when to vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Published February 28, 2018. Accessed August 22, 2018.
16. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207.
17. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-1871.
18. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737.
19. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258.
20. Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management. Am Fam Physician. 2016;94:698-706.
21. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406.
22. Brassard P, Wu JW, Ernst P, et al. The effect of statins on influenza-like illness morbidity and mortality. Pharmacoepidemiol Drug Saf. 2017;26:63-70.
23. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417-435.
24. Khandaker G, Rashid H, Chow MY, et al. Statins for influenza and pneumonia. Cochrane Database Syst Rev. January 9, 2017 [withdrawn].
1. CDC. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Published June 8, 2018. Accessed August 22, 2018.
2. CDC. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Published April 19, 2017. https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed Setptember 18, 2018.
3. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Published February 15, 2018. Accessed August 22, 2018.
4. Sah P, Medlock J, Fitzpatrick MC, et al. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018:201802479.
5. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244.
6. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:158–160.
7. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:156–157.
8. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1-20.
9. CDC. Influenza vaccines—United States, 2017–18 influenza season. https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Published May 16, 2018. Accessed August 22, 2018.
10. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643–645.
11. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. AAP News. May 21, 2018. http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 22, 2018.
12. Glinka ER, Smith DM, Johns ST. Timing matters—influenza vaccination to HIV-infected patients. HIV Med. 2016;17:601-604.
13. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18:20388.
14. CDC. Influenza antiviral medications: summary for clinicians. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Published May 11, 2018. Accessed August 22, 2018.
15. CDC. Pneumococcal vaccination summary: who and when to vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Published February 28, 2018. Accessed August 22, 2018.
16. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207.
17. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-1871.
18. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737.
19. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258.
20. Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management. Am Fam Physician. 2016;94:698-706.
21. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406.
22. Brassard P, Wu JW, Ernst P, et al. The effect of statins on influenza-like illness morbidity and mortality. Pharmacoepidemiol Drug Saf. 2017;26:63-70.
23. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417-435.
24. Khandaker G, Rashid H, Chow MY, et al. Statins for influenza and pneumonia. Cochrane Database Syst Rev. January 9, 2017 [withdrawn].
PRACTICE RECOMMENDATIONS
› Recommend influenza vaccination for all patients at least 6 months old unless a specific contraindication exists. A
› Recommend pneumococcal vaccination to appropriate patients to reduce the risk for a common complication of influenza. A
› Encourage hygiene-based measures to limit infection, including frequent handwashing or use of a hand sanitizer. B
› Prescribe oseltamivir to hospitalized influenza patients to limit the duration and severity of infection. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Obesity: When to consider surgery
Patients with overweight and obesity are at increased risk of multiple morbidities, including cardiovascular disease, stroke, type 2 diabetes (T2D), osteoarthritis, obstructive sleep apnea (OSA), and all-cause mortality.1 Even modest weight loss—5% to 10%—can lead to a clinically relevant reduction in this risk of disease.2,3 The American Academy of Family Physicians recognizes obesity as a disease, and recommends screening of all adults for obesity and referral for those with body mass index (BMI)* ≥30 to intensive, multicomponent behavioral interventions.4,5
For some patients, diet, exercise, and behavioral modifications are sufficient; for the great majority, however, weight loss achieved by lifestyle modification is counteracted by metabolic adaptations that promote weight regain.6 For patients with obesity who are unable to achieve or maintain sufficient weight loss to improve health outcomes with lifestyle modification alone, options include pharmacotherapy, devices, endoscopic bariatric therapies, and bariatric surgery.
Bariatric surgery is the most effective of these treatments, due to its association with significant and sustained weight loss, reduction in obesity-related comorbidities, and improved quality of life.1,7 Furthermore, compared with usual care, bariatric surgery is associated with a reduced number of cardiovascular deaths, a lower incidence of cardiovascular events in adults with obesity, and a long-term reduction in overall mortality.8-10
What are the options? Who is a candidate?
The 3 most common bariatric procedures in the United States are sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), and laparoscopic adjustable gastric band (LAGB).11 SG and RYGB are performed more often than the LAGB, consequent to greater efficacy and fewer complications.12 Weight loss is maximal at 1 to 2 years, and is estimated to be 15% of total body weight for LAGB; 25% for SG; and 35% for RYGB.13,14
Not all patients are candidates for bariatric surgery. Contraindications include chronic obstructive pulmonary disease or respiratory dysfunction, poor cardiac reserve, nonadherence to medical treatment, and severe psychological disorders.15 Because some patients have difficulty maintaining weight loss following bariatric surgery and, on average, patients regain at least some weight, patients must understand that long-term lifestyle changes and follow-up are critical to the success of bariatric surgery.16
When should bariatric surgery be considered?
American Heart Association/American College of Cardiology/The Obesity Society guidelines16 conceptualize 2 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 who have obesity-related comorbid conditions and are motivated to lose weight but have not responded to behavioral treatment, with or without pharmacotherapy, to achieve sufficient weight loss for target health goals.
American Association of Clinical Endocrinologists guidelines17 conceptualize 3 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 with 1 or more severe obesity-related complications
- adults with BMI 30-34.9 with diabetes or metabolic syndrome (evidence for this recommendation is limited).
Continue to: The 3 illustrative vignettes presented...
The 3 illustrative vignettes presented in this article offer examples of patients with obesity who could benefit from bariatric surgery. Each has been unable to achieve or maintain sufficient weight loss to improve health outcomes with nonsurgical interventions alone.
CASE 1
Sleep apnea persists despite weight loss
Robin W, a 50-year-old woman with class-II obesity (5’8”; 250 lb; BMI, 38 ), OSA requiring continuous positive airway pressure (CPAP), hyperlipidemia, hypertension, and iron-deficiency anemia secondary to menorrhagia, and taking an iron supplement, presents for weight management. She has lost 50 lb, reducing her BMI from 45.6 with behavioral modifications and pharmacotherapy, but she has been unsuccessful at achieving further weight loss despite a reduced-calorie diet and at least 30 minutes of physical activity most days.
Ms. W is frustrated that she has reached a weight plateau; she is motivated to lose more weight. Her goal is to improve her weight-related comorbid conditions and reduce her medication requirement. Despite the initial weight loss, she continues to require CPAP therapy for OSA and remains on 3 medications for hypertension. She does not have cardiac or respiratory disease, psychiatric diagnoses, or a history of gastroesophageal reflux disease (GERD).
Is bariatric surgery a reasonable option for Ms. W? If so, which procedure would you recommend?
Good option for Ms. W: Sleeve gastrectomy
It is reasonable to consider bariatric surgery—in particular, SG—for this patient with class-II obesity and multiple weight-related comorbid conditions because she has been unable to achieve further weight loss with more conservative measures.
Continue to: How does the procedure work?
How does the procedure work? SG removes a large portion of the stomach along the greater curvature, reducing the organ to approximately 15% to 25% of its original size.18 The procedure leaves the pyloric valve intact and does not involve removal or bypass of the intestines.
How appealing and successful is it? The majority of patients who undergo SG experience significant weight loss; studies report approximately 25% total body weight loss after 1 to 2 years.14 Furthermore, most patients with T2D experience resolution of, or improvement in, disease markers.19 Because SG leaves the pylorus intact, there are fewer restrictions on what a patient can eat after surgery, compared with RYGB. With further weight loss, Ms. W may experience improvement in, or resolution of, hypertension, hyperlipidemia, and OSA.
The SG procedure itself is simpler than some other bariatric procedures and presents less risk of malabsorption because the intestines are left intact. Patients who undergo SG report feeling less hungry because the fundus of the stomach, which secretes ghrelin (the so-called hunger hormone), is removed.18,20
What are special considerations, including candidacy? Patients with GERD are not ideal candidates for this procedure because exacerbation of the disease is a potential associated adverse event. SG is a reasonable surgical option for Ms. W because the procedure is less likely to exacerbate her nutritional deficiency (iron-deficiency anemia), compared to RYGB, and she does not have a history of GERD.
What are the complications? Complications of SG occur at a lower rate than they do with RYGB, which is associated with a greater risk of nutritional deficiency.18 Common early complications of SG include leaking, bleeding, stenosis, GERD, and vomiting due to excessive eating. Late complications include stomach expansion by 12 months, leading to decreased restriction.15 Unlike RYGB and LAGB, SG is not reversible.
Continue to: CASE 2
CASE 2
Severe obesity, polypharmacy for type 2 diabetes
Anne P, a 42-year-old woman with class-III obesity (5’6”; 290 lb; BMI, 46.8 kg/m2), presents to discuss bariatric surgery. Comorbidities include T2D, for which she takes metformin, a glucagon-like peptide-1 (GLP-1) receptor agonist, and a sodium–glucose cotransporter-2 (SGLT-2) inhibitor; GERD; hypertension, for which she takes an angiotensin-converting enzyme inhibitor and a calcium-channel blocker; hyperlipidemia, for which she takes a statin; and osteoarthritis.
Ms. P lost 30 pounds—reducing her BMI from 51.6—when the sulfonylurea and thiazolidinedione she was taking were switched to the GLP-1 receptor agonist and the SGLT2 inhibitor. She also made behavioral modifications, including 30 minutes a day of physical activity and a reduced-calorie meal plan under the guidance of a dietitian.
However, Ms. P has been unable to lose more weight or reduce her hemoglobin A1c (HbA1c) level below 8%. Her goal is to avoid the need to take insulin (which several members of her family take), lower her HbA1c level, and decrease her medication requirement.
Ms. P does not have cardiac or respiratory disease or psychiatric diagnoses. Which surgical intervention would you recommend for her?
Good option for Ms. P: Roux-en-Y gastric bypass
RYGB is a reasonable option for a patient with class-III obesity and multiple comorbidities, including poorly controlled T2D and GERD, who has failed conservative measures but wants to lose more weight, reduce her HbA1c, reduce her medication requirement, and avoid the need for insulin.
Continue to: How does the procedure work?
How does the procedure work? RYGB constructs a small pouch from the proximal portion of the stomach and attaches it directly to the jejunum, thus bypassing part of the stomach and duodenum. The procedure is effective for weight loss because it is both restrictive and malabsorptive: patients not only eat smaller portions, but cannot absorb all they eat. Other mechanisms attributed to RYGB that are hypothesized to promote weight loss include21:
- alteration of endogenous gut hormones, which promotes postprandial satiety
- increased levels of bile acids, which promotes alteration of the gut microbiome
- intestinal hypertrophy.
How successful is it? RYGB is associated with significant total body weight loss of approximately 35% at 2 years.9 The procedure has been shown to produce superior outcomes in reducing comorbid disease compared to other bariatric procedures or medical therapy alone. Of the procedures discussed in this article, RYGB is associated with the greatest reduction in triglycerides, HbA1c, and use of diabetes medications, including insulin.22
What are special considerations, including candidacy? For patients with mild or moderate T2D (calculated using the Individualized Metabolic Surgery Score [http://riskcalc.org/Metabolic_Surgery_Score/], which categorizes patients by number of diabetes medications, insulin use, duration of diabetes before surgery, and HbA1c), RYGB is recommended over SG because it leads to greater long-term remission of T2D.
RYGB is associated with a lower rate of GERD than SG and can even alleviate GERD in patients who have the disease. Furthermore, for patients with limited pancreatic beta cell reserve, RYBG and SG have similarly low efficacy for T2D remission; SG is therefore recommended over RYGB in this specific circumstance, given its slightly lower risk profile.23
What are the complications? Patients who undergo any bariatric surgical procedure require long-term follow-up and vitamin supplementation, but those who undergo RYGB require stricter dietary adherence after the procedure; lifelong vitamin (D, B12, folic acid, and thiamine), iron, and calcium supplementation; and long-term follow-up to reduce the risk and severity of complications and to monitor for nutritional deficiencies.7 As such, patients who have shown poor adherence to medical treatment are not good candidates for the procedure.
Continue to: Early complications include...
Early complications include leak, stricture, obstruction, and failure of the staple partition of the upper stomach. Late complications include nutritional deficiencies, as noted, and ulceration of the anastomosis. Dumping syndrome (overly rapid transit of food from the stomach into the small intestine) can develop early or late; early dumping leads to osmotic diarrhea and abdominal cramping, and late dumping leads to reactive hypoglycemia.15
Technically, RYGB is a reversible procedure, although generally it is reversed only in extreme circumstances.
CASE 3
Fatty liver disease, hesitation to undergo surgery
Walt Z, a 35 year-old-man with class-II obesity (5’10”; 265 lb; BMI, 38 kg/m2), T2D, and hepatic steatosis, presents for weight management. He has been able to lose modest weight over the years with behavioral modifications, but has been unsuccessful in maintaining that loss. He requests referral to a bariatric surgeon but is concerned about the permanence and invasiveness of most bariatric procedures.
Which surgical intervention would you recommend for this patient?
Good option for Mr. Z: Laparoscopic adjustable gastric band
Given that Mr. Z is a candidate for a surgical intervention but does not want a permanent or invasive procedure, LAGB is a reasonable option.
Continue to: How does the procedure work?
How does the procedure work? LAGB is a reversible procedure in which an inflatable band is placed around the fundus of the stomach to create a small pouch. The band can be adjusted to regulate food intake by adding or removing saline through a subcutaneous access port.
How appealing and successful is it? LAGB results in approximately 15% total body weight loss at 2 years.13 Because the procedure is purely restrictive, it carries a reduced risk of nutritional deficiency associated more commonly with malabsorptive procedures.
What are special considerations, including candidacy? As noted, Mr. Z expressed concern about the permanence and invasiveness of most bariatric procedures, and therefore wants to undergo a reversible procedure; LAGB can be a reasonable option for such a patient. Patients who want a reversible or minimally invasive procedure should also be made aware that endoscopic bariatric therapies and other devices are being developed to fill the treatment gap in the management of obesity.
What are the complications? Although LAGB is the least invasive procedure discussed here, it is associated with the highest rate of complications—most commonly, complications associated with the band itself (eg, nausea, vomiting, obstruction, band erosion or migration, esophageal dysmotility leading to acid reflux) and failure to lose weight.7 LAGB also requires more postoperative visits than other procedures, to optimize band tightness. A high number of bands are removed eventually because of complications or inadequate weight loss, or both.13,24
Shared decision-making and dialogue are essential to overcome obstacles
Despite the known benefits of bariatric surgery, including greater reduction in the risk and severity of obesity-related comorbid conditions than seen with other interventions and a long-term reduction in overall mortality when compared with usual care, fewer than 1% of eligible patients undergo a weight-loss procedure.25 Likely, this is due to:
- limited patient knowledge of the health benefits of surgery
- limited provider comfort recommending surgery
- inadequate insurance coverage, which might, in part, be due to a lack of prospective studies comparing various bariatric procedures.18
Continue to: Ultimately, the decision whether to undergo a bariatric procedure...
Ultimately, the decision whether to undergo a bariatric procedure, and which one(s) to consider, should be the product of a thorough conversation between patient and provider.
CORRESPONDENCE
Sarah R. Barenbaum, MD, Department of Internal Medicine, New York–Presbyterian Hospital/Weill Cornell Medical College, 530 East 70th Street, M-507, New York, NY 10021; [email protected]
1. Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
3. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
4. American Academy of Family Physicians. Clinical preventive service recommendation: Obesity. www.aafp.org/patient-care/clinical-recommendations/all/obesity.html. Accessed August 22, 2018.
5. American Academy of Family Physicians: USPSTF draft recommendation: Intensive behavioral interventions recommended for obesity. www.aafp.org/news/health-of-the-public/20180221uspstfobesity.html. Published February 21, 2018. Accessed August 22, 2018.
6. Saunders KH, Shukla AP, Igel LI, Aronne LJ. Obesity: When to consider medication. J Fam Pract. 2017;66:608-616.
7. Roux CW, Heneghan HM. Bariatric surgery for obesity. Med Clin North Am. 2018;102:165-182.
8. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56-65.
9. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
10. Reges O, Greenland P, Dicker D, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y, gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA. 2018;319:279-290.
11. Lee JH, Nguyen QN, Le QA. Comparative effectiveness of 3 bariatric surgery procedures: Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:997-1002.
12. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Published June 2018. Accessed August 22, 2018.
13. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153:427-434.
14. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254-266.
15. Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641.
16. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
17. Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for comprehensive medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1-203.
18. Carlin Am, Zeni Tm, English WJ, et al; Michigan Bariatric Surgery Collaborative. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791-797.
19. Gill RS, Birch DW, Shi X, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707-713.
20. Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg. 2008;247:401-407.
21. Abdeen G, le Roux CW. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Obes Surg. 2016;26:410-421.
22. Schauer PR, Bhatt DL, Kirwan JP et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
23. Aminian A, Brethauer SA, Andalib A, et al. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg. 2017;266:4:650-657.
24. Smetana GW, Jones DB, Wee CC. Beyond the guidelines: Should this patient have weight loss surgery? Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2017;166:808-817.
25. Wolfe BM, Morton JM. Weighing in on bariatric surgery: procedure use, readmission rates, and mortality [editorial]. JAMA. 2005;294:1960-1963.
Patients with overweight and obesity are at increased risk of multiple morbidities, including cardiovascular disease, stroke, type 2 diabetes (T2D), osteoarthritis, obstructive sleep apnea (OSA), and all-cause mortality.1 Even modest weight loss—5% to 10%—can lead to a clinically relevant reduction in this risk of disease.2,3 The American Academy of Family Physicians recognizes obesity as a disease, and recommends screening of all adults for obesity and referral for those with body mass index (BMI)* ≥30 to intensive, multicomponent behavioral interventions.4,5

For some patients, diet, exercise, and behavioral modifications are sufficient; for the great majority, however, weight loss achieved by lifestyle modification is counteracted by metabolic adaptations that promote weight regain.6 For patients with obesity who are unable to achieve or maintain sufficient weight loss to improve health outcomes with lifestyle modification alone, options include pharmacotherapy, devices, endoscopic bariatric therapies, and bariatric surgery.
Bariatric surgery is the most effective of these treatments, due to its association with significant and sustained weight loss, reduction in obesity-related comorbidities, and improved quality of life.1,7 Furthermore, compared with usual care, bariatric surgery is associated with a reduced number of cardiovascular deaths, a lower incidence of cardiovascular events in adults with obesity, and a long-term reduction in overall mortality.8-10
What are the options? Who is a candidate?
The 3 most common bariatric procedures in the United States are sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), and laparoscopic adjustable gastric band (LAGB).11 SG and RYGB are performed more often than the LAGB, consequent to greater efficacy and fewer complications.12 Weight loss is maximal at 1 to 2 years, and is estimated to be 15% of total body weight for LAGB; 25% for SG; and 35% for RYGB.13,14
Not all patients are candidates for bariatric surgery. Contraindications include chronic obstructive pulmonary disease or respiratory dysfunction, poor cardiac reserve, nonadherence to medical treatment, and severe psychological disorders.15 Because some patients have difficulty maintaining weight loss following bariatric surgery and, on average, patients regain at least some weight, patients must understand that long-term lifestyle changes and follow-up are critical to the success of bariatric surgery.16
When should bariatric surgery be considered?
American Heart Association/American College of Cardiology/The Obesity Society guidelines16 conceptualize 2 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 who have obesity-related comorbid conditions and are motivated to lose weight but have not responded to behavioral treatment, with or without pharmacotherapy, to achieve sufficient weight loss for target health goals.
American Association of Clinical Endocrinologists guidelines17 conceptualize 3 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 with 1 or more severe obesity-related complications
- adults with BMI 30-34.9 with diabetes or metabolic syndrome (evidence for this recommendation is limited).
Continue to: The 3 illustrative vignettes presented...
The 3 illustrative vignettes presented in this article offer examples of patients with obesity who could benefit from bariatric surgery. Each has been unable to achieve or maintain sufficient weight loss to improve health outcomes with nonsurgical interventions alone.
CASE 1
Sleep apnea persists despite weight loss
Robin W, a 50-year-old woman with class-II obesity (5’8”; 250 lb; BMI, 38 ), OSA requiring continuous positive airway pressure (CPAP), hyperlipidemia, hypertension, and iron-deficiency anemia secondary to menorrhagia, and taking an iron supplement, presents for weight management. She has lost 50 lb, reducing her BMI from 45.6 with behavioral modifications and pharmacotherapy, but she has been unsuccessful at achieving further weight loss despite a reduced-calorie diet and at least 30 minutes of physical activity most days.
Ms. W is frustrated that she has reached a weight plateau; she is motivated to lose more weight. Her goal is to improve her weight-related comorbid conditions and reduce her medication requirement. Despite the initial weight loss, she continues to require CPAP therapy for OSA and remains on 3 medications for hypertension. She does not have cardiac or respiratory disease, psychiatric diagnoses, or a history of gastroesophageal reflux disease (GERD).
Is bariatric surgery a reasonable option for Ms. W? If so, which procedure would you recommend?
Good option for Ms. W: Sleeve gastrectomy
It is reasonable to consider bariatric surgery—in particular, SG—for this patient with class-II obesity and multiple weight-related comorbid conditions because she has been unable to achieve further weight loss with more conservative measures.
Continue to: How does the procedure work?
How does the procedure work? SG removes a large portion of the stomach along the greater curvature, reducing the organ to approximately 15% to 25% of its original size.18 The procedure leaves the pyloric valve intact and does not involve removal or bypass of the intestines.
How appealing and successful is it? The majority of patients who undergo SG experience significant weight loss; studies report approximately 25% total body weight loss after 1 to 2 years.14 Furthermore, most patients with T2D experience resolution of, or improvement in, disease markers.19 Because SG leaves the pylorus intact, there are fewer restrictions on what a patient can eat after surgery, compared with RYGB. With further weight loss, Ms. W may experience improvement in, or resolution of, hypertension, hyperlipidemia, and OSA.
The SG procedure itself is simpler than some other bariatric procedures and presents less risk of malabsorption because the intestines are left intact. Patients who undergo SG report feeling less hungry because the fundus of the stomach, which secretes ghrelin (the so-called hunger hormone), is removed.18,20
What are special considerations, including candidacy? Patients with GERD are not ideal candidates for this procedure because exacerbation of the disease is a potential associated adverse event. SG is a reasonable surgical option for Ms. W because the procedure is less likely to exacerbate her nutritional deficiency (iron-deficiency anemia), compared to RYGB, and she does not have a history of GERD.
What are the complications? Complications of SG occur at a lower rate than they do with RYGB, which is associated with a greater risk of nutritional deficiency.18 Common early complications of SG include leaking, bleeding, stenosis, GERD, and vomiting due to excessive eating. Late complications include stomach expansion by 12 months, leading to decreased restriction.15 Unlike RYGB and LAGB, SG is not reversible.
Continue to: CASE 2
CASE 2
Severe obesity, polypharmacy for type 2 diabetes
Anne P, a 42-year-old woman with class-III obesity (5’6”; 290 lb; BMI, 46.8 kg/m2), presents to discuss bariatric surgery. Comorbidities include T2D, for which she takes metformin, a glucagon-like peptide-1 (GLP-1) receptor agonist, and a sodium–glucose cotransporter-2 (SGLT-2) inhibitor; GERD; hypertension, for which she takes an angiotensin-converting enzyme inhibitor and a calcium-channel blocker; hyperlipidemia, for which she takes a statin; and osteoarthritis.
Ms. P lost 30 pounds—reducing her BMI from 51.6—when the sulfonylurea and thiazolidinedione she was taking were switched to the GLP-1 receptor agonist and the SGLT2 inhibitor. She also made behavioral modifications, including 30 minutes a day of physical activity and a reduced-calorie meal plan under the guidance of a dietitian.
However, Ms. P has been unable to lose more weight or reduce her hemoglobin A1c (HbA1c) level below 8%. Her goal is to avoid the need to take insulin (which several members of her family take), lower her HbA1c level, and decrease her medication requirement.
Ms. P does not have cardiac or respiratory disease or psychiatric diagnoses. Which surgical intervention would you recommend for her?
Good option for Ms. P: Roux-en-Y gastric bypass
RYGB is a reasonable option for a patient with class-III obesity and multiple comorbidities, including poorly controlled T2D and GERD, who has failed conservative measures but wants to lose more weight, reduce her HbA1c, reduce her medication requirement, and avoid the need for insulin.
Continue to: How does the procedure work?
How does the procedure work? RYGB constructs a small pouch from the proximal portion of the stomach and attaches it directly to the jejunum, thus bypassing part of the stomach and duodenum. The procedure is effective for weight loss because it is both restrictive and malabsorptive: patients not only eat smaller portions, but cannot absorb all they eat. Other mechanisms attributed to RYGB that are hypothesized to promote weight loss include21:
- alteration of endogenous gut hormones, which promotes postprandial satiety
- increased levels of bile acids, which promotes alteration of the gut microbiome
- intestinal hypertrophy.
How successful is it? RYGB is associated with significant total body weight loss of approximately 35% at 2 years.9 The procedure has been shown to produce superior outcomes in reducing comorbid disease compared to other bariatric procedures or medical therapy alone. Of the procedures discussed in this article, RYGB is associated with the greatest reduction in triglycerides, HbA1c, and use of diabetes medications, including insulin.22
What are special considerations, including candidacy? For patients with mild or moderate T2D (calculated using the Individualized Metabolic Surgery Score [http://riskcalc.org/Metabolic_Surgery_Score/], which categorizes patients by number of diabetes medications, insulin use, duration of diabetes before surgery, and HbA1c), RYGB is recommended over SG because it leads to greater long-term remission of T2D.
RYGB is associated with a lower rate of GERD than SG and can even alleviate GERD in patients who have the disease. Furthermore, for patients with limited pancreatic beta cell reserve, RYBG and SG have similarly low efficacy for T2D remission; SG is therefore recommended over RYGB in this specific circumstance, given its slightly lower risk profile.23
What are the complications? Patients who undergo any bariatric surgical procedure require long-term follow-up and vitamin supplementation, but those who undergo RYGB require stricter dietary adherence after the procedure; lifelong vitamin (D, B12, folic acid, and thiamine), iron, and calcium supplementation; and long-term follow-up to reduce the risk and severity of complications and to monitor for nutritional deficiencies.7 As such, patients who have shown poor adherence to medical treatment are not good candidates for the procedure.
Continue to: Early complications include...
Early complications include leak, stricture, obstruction, and failure of the staple partition of the upper stomach. Late complications include nutritional deficiencies, as noted, and ulceration of the anastomosis. Dumping syndrome (overly rapid transit of food from the stomach into the small intestine) can develop early or late; early dumping leads to osmotic diarrhea and abdominal cramping, and late dumping leads to reactive hypoglycemia.15
Technically, RYGB is a reversible procedure, although generally it is reversed only in extreme circumstances.
CASE 3
Fatty liver disease, hesitation to undergo surgery
Walt Z, a 35 year-old-man with class-II obesity (5’10”; 265 lb; BMI, 38 kg/m2), T2D, and hepatic steatosis, presents for weight management. He has been able to lose modest weight over the years with behavioral modifications, but has been unsuccessful in maintaining that loss. He requests referral to a bariatric surgeon but is concerned about the permanence and invasiveness of most bariatric procedures.
Which surgical intervention would you recommend for this patient?
Good option for Mr. Z: Laparoscopic adjustable gastric band
Given that Mr. Z is a candidate for a surgical intervention but does not want a permanent or invasive procedure, LAGB is a reasonable option.
Continue to: How does the procedure work?
How does the procedure work? LAGB is a reversible procedure in which an inflatable band is placed around the fundus of the stomach to create a small pouch. The band can be adjusted to regulate food intake by adding or removing saline through a subcutaneous access port.
How appealing and successful is it? LAGB results in approximately 15% total body weight loss at 2 years.13 Because the procedure is purely restrictive, it carries a reduced risk of nutritional deficiency associated more commonly with malabsorptive procedures.
What are special considerations, including candidacy? As noted, Mr. Z expressed concern about the permanence and invasiveness of most bariatric procedures, and therefore wants to undergo a reversible procedure; LAGB can be a reasonable option for such a patient. Patients who want a reversible or minimally invasive procedure should also be made aware that endoscopic bariatric therapies and other devices are being developed to fill the treatment gap in the management of obesity.
What are the complications? Although LAGB is the least invasive procedure discussed here, it is associated with the highest rate of complications—most commonly, complications associated with the band itself (eg, nausea, vomiting, obstruction, band erosion or migration, esophageal dysmotility leading to acid reflux) and failure to lose weight.7 LAGB also requires more postoperative visits than other procedures, to optimize band tightness. A high number of bands are removed eventually because of complications or inadequate weight loss, or both.13,24
Shared decision-making and dialogue are essential to overcome obstacles
Despite the known benefits of bariatric surgery, including greater reduction in the risk and severity of obesity-related comorbid conditions than seen with other interventions and a long-term reduction in overall mortality when compared with usual care, fewer than 1% of eligible patients undergo a weight-loss procedure.25 Likely, this is due to:
- limited patient knowledge of the health benefits of surgery
- limited provider comfort recommending surgery
- inadequate insurance coverage, which might, in part, be due to a lack of prospective studies comparing various bariatric procedures.18
Continue to: Ultimately, the decision whether to undergo a bariatric procedure...
Ultimately, the decision whether to undergo a bariatric procedure, and which one(s) to consider, should be the product of a thorough conversation between patient and provider.
CORRESPONDENCE
Sarah R. Barenbaum, MD, Department of Internal Medicine, New York–Presbyterian Hospital/Weill Cornell Medical College, 530 East 70th Street, M-507, New York, NY 10021; [email protected]
Patients with overweight and obesity are at increased risk of multiple morbidities, including cardiovascular disease, stroke, type 2 diabetes (T2D), osteoarthritis, obstructive sleep apnea (OSA), and all-cause mortality.1 Even modest weight loss—5% to 10%—can lead to a clinically relevant reduction in this risk of disease.2,3 The American Academy of Family Physicians recognizes obesity as a disease, and recommends screening of all adults for obesity and referral for those with body mass index (BMI)* ≥30 to intensive, multicomponent behavioral interventions.4,5

For some patients, diet, exercise, and behavioral modifications are sufficient; for the great majority, however, weight loss achieved by lifestyle modification is counteracted by metabolic adaptations that promote weight regain.6 For patients with obesity who are unable to achieve or maintain sufficient weight loss to improve health outcomes with lifestyle modification alone, options include pharmacotherapy, devices, endoscopic bariatric therapies, and bariatric surgery.
Bariatric surgery is the most effective of these treatments, due to its association with significant and sustained weight loss, reduction in obesity-related comorbidities, and improved quality of life.1,7 Furthermore, compared with usual care, bariatric surgery is associated with a reduced number of cardiovascular deaths, a lower incidence of cardiovascular events in adults with obesity, and a long-term reduction in overall mortality.8-10
What are the options? Who is a candidate?
The 3 most common bariatric procedures in the United States are sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), and laparoscopic adjustable gastric band (LAGB).11 SG and RYGB are performed more often than the LAGB, consequent to greater efficacy and fewer complications.12 Weight loss is maximal at 1 to 2 years, and is estimated to be 15% of total body weight for LAGB; 25% for SG; and 35% for RYGB.13,14
Not all patients are candidates for bariatric surgery. Contraindications include chronic obstructive pulmonary disease or respiratory dysfunction, poor cardiac reserve, nonadherence to medical treatment, and severe psychological disorders.15 Because some patients have difficulty maintaining weight loss following bariatric surgery and, on average, patients regain at least some weight, patients must understand that long-term lifestyle changes and follow-up are critical to the success of bariatric surgery.16
When should bariatric surgery be considered?
American Heart Association/American College of Cardiology/The Obesity Society guidelines16 conceptualize 2 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 who have obesity-related comorbid conditions and are motivated to lose weight but have not responded to behavioral treatment, with or without pharmacotherapy, to achieve sufficient weight loss for target health goals.
American Association of Clinical Endocrinologists guidelines17 conceptualize 3 indications for bariatric surgery:
- adults with BMI ≥40
- adults with BMI ≥35 with 1 or more severe obesity-related complications
- adults with BMI 30-34.9 with diabetes or metabolic syndrome (evidence for this recommendation is limited).
Continue to: The 3 illustrative vignettes presented...
The 3 illustrative vignettes presented in this article offer examples of patients with obesity who could benefit from bariatric surgery. Each has been unable to achieve or maintain sufficient weight loss to improve health outcomes with nonsurgical interventions alone.
CASE 1
Sleep apnea persists despite weight loss
Robin W, a 50-year-old woman with class-II obesity (5’8”; 250 lb; BMI, 38 ), OSA requiring continuous positive airway pressure (CPAP), hyperlipidemia, hypertension, and iron-deficiency anemia secondary to menorrhagia, and taking an iron supplement, presents for weight management. She has lost 50 lb, reducing her BMI from 45.6 with behavioral modifications and pharmacotherapy, but she has been unsuccessful at achieving further weight loss despite a reduced-calorie diet and at least 30 minutes of physical activity most days.
Ms. W is frustrated that she has reached a weight plateau; she is motivated to lose more weight. Her goal is to improve her weight-related comorbid conditions and reduce her medication requirement. Despite the initial weight loss, she continues to require CPAP therapy for OSA and remains on 3 medications for hypertension. She does not have cardiac or respiratory disease, psychiatric diagnoses, or a history of gastroesophageal reflux disease (GERD).
Is bariatric surgery a reasonable option for Ms. W? If so, which procedure would you recommend?
Good option for Ms. W: Sleeve gastrectomy
It is reasonable to consider bariatric surgery—in particular, SG—for this patient with class-II obesity and multiple weight-related comorbid conditions because she has been unable to achieve further weight loss with more conservative measures.
Continue to: How does the procedure work?
How does the procedure work? SG removes a large portion of the stomach along the greater curvature, reducing the organ to approximately 15% to 25% of its original size.18 The procedure leaves the pyloric valve intact and does not involve removal or bypass of the intestines.
How appealing and successful is it? The majority of patients who undergo SG experience significant weight loss; studies report approximately 25% total body weight loss after 1 to 2 years.14 Furthermore, most patients with T2D experience resolution of, or improvement in, disease markers.19 Because SG leaves the pylorus intact, there are fewer restrictions on what a patient can eat after surgery, compared with RYGB. With further weight loss, Ms. W may experience improvement in, or resolution of, hypertension, hyperlipidemia, and OSA.
The SG procedure itself is simpler than some other bariatric procedures and presents less risk of malabsorption because the intestines are left intact. Patients who undergo SG report feeling less hungry because the fundus of the stomach, which secretes ghrelin (the so-called hunger hormone), is removed.18,20
What are special considerations, including candidacy? Patients with GERD are not ideal candidates for this procedure because exacerbation of the disease is a potential associated adverse event. SG is a reasonable surgical option for Ms. W because the procedure is less likely to exacerbate her nutritional deficiency (iron-deficiency anemia), compared to RYGB, and she does not have a history of GERD.
What are the complications? Complications of SG occur at a lower rate than they do with RYGB, which is associated with a greater risk of nutritional deficiency.18 Common early complications of SG include leaking, bleeding, stenosis, GERD, and vomiting due to excessive eating. Late complications include stomach expansion by 12 months, leading to decreased restriction.15 Unlike RYGB and LAGB, SG is not reversible.
Continue to: CASE 2
CASE 2
Severe obesity, polypharmacy for type 2 diabetes
Anne P, a 42-year-old woman with class-III obesity (5’6”; 290 lb; BMI, 46.8 kg/m2), presents to discuss bariatric surgery. Comorbidities include T2D, for which she takes metformin, a glucagon-like peptide-1 (GLP-1) receptor agonist, and a sodium–glucose cotransporter-2 (SGLT-2) inhibitor; GERD; hypertension, for which she takes an angiotensin-converting enzyme inhibitor and a calcium-channel blocker; hyperlipidemia, for which she takes a statin; and osteoarthritis.
Ms. P lost 30 pounds—reducing her BMI from 51.6—when the sulfonylurea and thiazolidinedione she was taking were switched to the GLP-1 receptor agonist and the SGLT2 inhibitor. She also made behavioral modifications, including 30 minutes a day of physical activity and a reduced-calorie meal plan under the guidance of a dietitian.
However, Ms. P has been unable to lose more weight or reduce her hemoglobin A1c (HbA1c) level below 8%. Her goal is to avoid the need to take insulin (which several members of her family take), lower her HbA1c level, and decrease her medication requirement.
Ms. P does not have cardiac or respiratory disease or psychiatric diagnoses. Which surgical intervention would you recommend for her?
Good option for Ms. P: Roux-en-Y gastric bypass
RYGB is a reasonable option for a patient with class-III obesity and multiple comorbidities, including poorly controlled T2D and GERD, who has failed conservative measures but wants to lose more weight, reduce her HbA1c, reduce her medication requirement, and avoid the need for insulin.
Continue to: How does the procedure work?
How does the procedure work? RYGB constructs a small pouch from the proximal portion of the stomach and attaches it directly to the jejunum, thus bypassing part of the stomach and duodenum. The procedure is effective for weight loss because it is both restrictive and malabsorptive: patients not only eat smaller portions, but cannot absorb all they eat. Other mechanisms attributed to RYGB that are hypothesized to promote weight loss include21:
- alteration of endogenous gut hormones, which promotes postprandial satiety
- increased levels of bile acids, which promotes alteration of the gut microbiome
- intestinal hypertrophy.
How successful is it? RYGB is associated with significant total body weight loss of approximately 35% at 2 years.9 The procedure has been shown to produce superior outcomes in reducing comorbid disease compared to other bariatric procedures or medical therapy alone. Of the procedures discussed in this article, RYGB is associated with the greatest reduction in triglycerides, HbA1c, and use of diabetes medications, including insulin.22
What are special considerations, including candidacy? For patients with mild or moderate T2D (calculated using the Individualized Metabolic Surgery Score [http://riskcalc.org/Metabolic_Surgery_Score/], which categorizes patients by number of diabetes medications, insulin use, duration of diabetes before surgery, and HbA1c), RYGB is recommended over SG because it leads to greater long-term remission of T2D.
RYGB is associated with a lower rate of GERD than SG and can even alleviate GERD in patients who have the disease. Furthermore, for patients with limited pancreatic beta cell reserve, RYBG and SG have similarly low efficacy for T2D remission; SG is therefore recommended over RYGB in this specific circumstance, given its slightly lower risk profile.23
What are the complications? Patients who undergo any bariatric surgical procedure require long-term follow-up and vitamin supplementation, but those who undergo RYGB require stricter dietary adherence after the procedure; lifelong vitamin (D, B12, folic acid, and thiamine), iron, and calcium supplementation; and long-term follow-up to reduce the risk and severity of complications and to monitor for nutritional deficiencies.7 As such, patients who have shown poor adherence to medical treatment are not good candidates for the procedure.
Continue to: Early complications include...
Early complications include leak, stricture, obstruction, and failure of the staple partition of the upper stomach. Late complications include nutritional deficiencies, as noted, and ulceration of the anastomosis. Dumping syndrome (overly rapid transit of food from the stomach into the small intestine) can develop early or late; early dumping leads to osmotic diarrhea and abdominal cramping, and late dumping leads to reactive hypoglycemia.15
Technically, RYGB is a reversible procedure, although generally it is reversed only in extreme circumstances.
CASE 3
Fatty liver disease, hesitation to undergo surgery
Walt Z, a 35 year-old-man with class-II obesity (5’10”; 265 lb; BMI, 38 kg/m2), T2D, and hepatic steatosis, presents for weight management. He has been able to lose modest weight over the years with behavioral modifications, but has been unsuccessful in maintaining that loss. He requests referral to a bariatric surgeon but is concerned about the permanence and invasiveness of most bariatric procedures.
Which surgical intervention would you recommend for this patient?
Good option for Mr. Z: Laparoscopic adjustable gastric band
Given that Mr. Z is a candidate for a surgical intervention but does not want a permanent or invasive procedure, LAGB is a reasonable option.
Continue to: How does the procedure work?
How does the procedure work? LAGB is a reversible procedure in which an inflatable band is placed around the fundus of the stomach to create a small pouch. The band can be adjusted to regulate food intake by adding or removing saline through a subcutaneous access port.
How appealing and successful is it? LAGB results in approximately 15% total body weight loss at 2 years.13 Because the procedure is purely restrictive, it carries a reduced risk of nutritional deficiency associated more commonly with malabsorptive procedures.
What are special considerations, including candidacy? As noted, Mr. Z expressed concern about the permanence and invasiveness of most bariatric procedures, and therefore wants to undergo a reversible procedure; LAGB can be a reasonable option for such a patient. Patients who want a reversible or minimally invasive procedure should also be made aware that endoscopic bariatric therapies and other devices are being developed to fill the treatment gap in the management of obesity.
What are the complications? Although LAGB is the least invasive procedure discussed here, it is associated with the highest rate of complications—most commonly, complications associated with the band itself (eg, nausea, vomiting, obstruction, band erosion or migration, esophageal dysmotility leading to acid reflux) and failure to lose weight.7 LAGB also requires more postoperative visits than other procedures, to optimize band tightness. A high number of bands are removed eventually because of complications or inadequate weight loss, or both.13,24
Shared decision-making and dialogue are essential to overcome obstacles
Despite the known benefits of bariatric surgery, including greater reduction in the risk and severity of obesity-related comorbid conditions than seen with other interventions and a long-term reduction in overall mortality when compared with usual care, fewer than 1% of eligible patients undergo a weight-loss procedure.25 Likely, this is due to:
- limited patient knowledge of the health benefits of surgery
- limited provider comfort recommending surgery
- inadequate insurance coverage, which might, in part, be due to a lack of prospective studies comparing various bariatric procedures.18
Continue to: Ultimately, the decision whether to undergo a bariatric procedure...
Ultimately, the decision whether to undergo a bariatric procedure, and which one(s) to consider, should be the product of a thorough conversation between patient and provider.
CORRESPONDENCE
Sarah R. Barenbaum, MD, Department of Internal Medicine, New York–Presbyterian Hospital/Weill Cornell Medical College, 530 East 70th Street, M-507, New York, NY 10021; [email protected]
1. Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
3. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
4. American Academy of Family Physicians. Clinical preventive service recommendation: Obesity. www.aafp.org/patient-care/clinical-recommendations/all/obesity.html. Accessed August 22, 2018.
5. American Academy of Family Physicians: USPSTF draft recommendation: Intensive behavioral interventions recommended for obesity. www.aafp.org/news/health-of-the-public/20180221uspstfobesity.html. Published February 21, 2018. Accessed August 22, 2018.
6. Saunders KH, Shukla AP, Igel LI, Aronne LJ. Obesity: When to consider medication. J Fam Pract. 2017;66:608-616.
7. Roux CW, Heneghan HM. Bariatric surgery for obesity. Med Clin North Am. 2018;102:165-182.
8. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56-65.
9. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
10. Reges O, Greenland P, Dicker D, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y, gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA. 2018;319:279-290.
11. Lee JH, Nguyen QN, Le QA. Comparative effectiveness of 3 bariatric surgery procedures: Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:997-1002.
12. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Published June 2018. Accessed August 22, 2018.
13. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153:427-434.
14. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254-266.
15. Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641.
16. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
17. Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for comprehensive medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1-203.
18. Carlin Am, Zeni Tm, English WJ, et al; Michigan Bariatric Surgery Collaborative. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791-797.
19. Gill RS, Birch DW, Shi X, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707-713.
20. Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg. 2008;247:401-407.
21. Abdeen G, le Roux CW. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Obes Surg. 2016;26:410-421.
22. Schauer PR, Bhatt DL, Kirwan JP et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
23. Aminian A, Brethauer SA, Andalib A, et al. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg. 2017;266:4:650-657.
24. Smetana GW, Jones DB, Wee CC. Beyond the guidelines: Should this patient have weight loss surgery? Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2017;166:808-817.
25. Wolfe BM, Morton JM. Weighing in on bariatric surgery: procedure use, readmission rates, and mortality [editorial]. JAMA. 2005;294:1960-1963.
1. Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
3. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
4. American Academy of Family Physicians. Clinical preventive service recommendation: Obesity. www.aafp.org/patient-care/clinical-recommendations/all/obesity.html. Accessed August 22, 2018.
5. American Academy of Family Physicians: USPSTF draft recommendation: Intensive behavioral interventions recommended for obesity. www.aafp.org/news/health-of-the-public/20180221uspstfobesity.html. Published February 21, 2018. Accessed August 22, 2018.
6. Saunders KH, Shukla AP, Igel LI, Aronne LJ. Obesity: When to consider medication. J Fam Pract. 2017;66:608-616.
7. Roux CW, Heneghan HM. Bariatric surgery for obesity. Med Clin North Am. 2018;102:165-182.
8. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56-65.
9. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
10. Reges O, Greenland P, Dicker D, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y, gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA. 2018;319:279-290.
11. Lee JH, Nguyen QN, Le QA. Comparative effectiveness of 3 bariatric surgery procedures: Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:997-1002.
12. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Published June 2018. Accessed August 22, 2018.
13. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153:427-434.
14. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254-266.
15. Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641.
16. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
17. Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for comprehensive medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1-203.
18. Carlin Am, Zeni Tm, English WJ, et al; Michigan Bariatric Surgery Collaborative. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791-797.
19. Gill RS, Birch DW, Shi X, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707-713.
20. Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg. 2008;247:401-407.
21. Abdeen G, le Roux CW. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Obes Surg. 2016;26:410-421.
22. Schauer PR, Bhatt DL, Kirwan JP et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
23. Aminian A, Brethauer SA, Andalib A, et al. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg. 2017;266:4:650-657.
24. Smetana GW, Jones DB, Wee CC. Beyond the guidelines: Should this patient have weight loss surgery? Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2017;166:808-817.
25. Wolfe BM, Morton JM. Weighing in on bariatric surgery: procedure use, readmission rates, and mortality [editorial]. JAMA. 2005;294:1960-1963.
PRACTICE RECOMMENDATIONS
Among adult patients with body mass index* ≥40, or ≥35 with obesity-related comorbid conditions:
› Consider bariatric surgery in those who are motivated to lose weight but who have not responded to lifestyle modification with or without pharmacotherapy in order to achieve sufficient and sustained weight loss. A
› Consider bariatric surgery to help patients achieve target health goals and reduce/improve obesity-related comorbidities. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
*Calculated as weight in kilograms divided by height in meters squared.
CV risk prediction tools: Imperfect, Yes, but are they serviceable?
Prevention of cardiovascular disease (CVD) requires timely identification of people who are at increased risk in order to target effective dietary, lifestyle, or pharmacotherapeutic intervention—or a combination of the 3. Risk factors for CVD are well understood, but the relative impact of each factor on an individual’s overall risk is difficult to accurately quantify, making a validated CVD risk calculator an important clinical tool.
Despite numerous available CVD risk calculators, one best tool has yet to emerge. This state of affairs has limited the ability of front-line providers who are tasked with primary prevention of CVD—including family physicians (FPs)—to provide the best evidence-based recommendations to patients.
Implications of CVD risk assessment
Baseline CVD risk assessment is the cornerstone of recommendations for primary prevention of CVD, including aspirin and statin therapy. Interventions to lower CVD risk are of greatest benefit to those at highest risk at initiation of therapy. Overall, statins reduce the risk of a first cardiovascular event in otherwise healthy people by approximately 25% over 10 years.1 Because relative risk reduction is fairly consistent across different levels of absolute risk, a 25% relative reduction confers more actual benefit if risk starts at, say, 40% than at 10%.2 In that example, the same 25% reduction in relative risk results in 1) an absolute risk reduction of 10% when risk starts at 40%, compared to an absolute risk reduction of 2.5% when risk starts at 10% and 2) a number needed to treat (NNT) of, respectively, 10 and 40 (over 10 years).
Identifying a person with an elevated risk of developing CVD has multiple implications. Ideally, that patient is motivated to pursue positive therapeutic lifestyle modifications and make changes that positively affect long-term CVD risk. Conversely, that asymptomatic person identified as at elevated risk also becomes a patient with a medical problem that might adversely affect insurance premiums and self-esteem, and may trigger the use of medications with cost and potential adverse effects. Although the benefit of preventive therapy is greater for a patient at higher risk of disease, the harm of a therapy is relatively constant across all risk groups. Accurately discriminating high and low risk of CVD is, therefore, imperative.
The venerable Framingham risk score
Cardiovascular risk prediction has its roots in the late 1940s, when primary risk factors for CVD were not well-understood, with the inception of the Framingham Heart Study. (A greater understanding of CVD risk today notwithstanding, coronary artery disease [CAD] remains the leading cause of death among American adults.) In the late 1940s, blood pressure (BP) was recognized as the single most useful variable for identifying people at high risk of CVD; other variables were understood to be predictive as well. A composite score—the Framingham Risk Score (FRS)—was thereby developed to calculate the probability that CVD would occur over 8 years in a person who was initially free of such disease.3
The original FRS included glucose intolerance and left ventricular hypertrophy (LVH) identified by electrocardiography (EKG) in its algorithm.3 Other, older algorithms also include a family history of premature CVD. In each risk calculator, these variables are treated as dichotomous (Yes or No), but actual risk associated with each variable is in fact more along a continuum. It is now well-recognized that the sensitivity of EKG for accurately detecting LVH is relatively low; more recent algorithms no longer include this component. A family history of premature CVD variably contributes to an individual’s CVD risk; however, its true impact is nearly impossible to accurately quantify, so this variable is also not included in more modern risk calculators.
Caution: The FRS has meaningful limitations
Although the original Framingham cohort has been expanded multiple times since its inception, clinicians and researchers continue to express concern that the predominantly white, middle-class Framingham, Massachusetts, population might not be representative of the United States in general—which would limit the accuracy of the FRS predictive tool when it is applied to a more diverse population. Furthermore, cholesterol-lowering medications were not available when the FRS was first developed. The FRS, therefore, might not accurately estimate risk in more modern populations, in whom aggressive modification of CVD risk factors has resulted in a lower overall rate of atherosclerotic CVD than when the FRS was developed.4
Continue to: Although demographic changes have increasingly...
Although demographic changes have increasingly led to an extension of primary prevention strategies for CAD to elderly people, the FRS has been demonstrated to perform less well in patients older than 70 years, particularly men.5 An ideal CAD prediction model for elderly people should take into account that, with growing age and frailty, CAD events may be increasingly preempted by death from competing non-coronary causes. In addition, the predictive association of typical CVD risk factors diminishes with increasing age.6,7 Koller and colleagues developed a CAD risk prediction model that accounted for death from non-coronary causes and was validated specifically in patients 65 years and older. Koller’s prediction model provided well-calibrated risk estimates, but it was still not substantially more accurate than the FRS—illustrating the overall difficulty in predicting CAD risk in elderly people.8
Alternative risk calculators have come on the scene
Over the past 2 decades, numerous models have been developed in an attempt to overcome the perceived shortcomings of the FRS. A recent systematic review identified 363 prediction models described in the medical literature prior to July 2013.9 The usefulness of most models remains unclear, however, owing to:
- methodological shortcomings,
- considerable heterogeneity in the definitions of outcomes, and
- lack of external validation.
Even models that are well-validated for a specific population suffer from lack of applicability to a broad multinational population.
In the United Kingdom (UK), electronic health record systems now have the QRISK2 tool embedded to calculate 10-year CVD risk. This algorithm incorporates multiple traditional and nontraditional risk factors (TABLE10). With the inclusion of additional risk factors and validation performed in a population similar to the one from which the algorithm was derived, QRISK2 predicts CVD risk in the UK population more accurately than the modified FRS does.10 It is not clear, however, whether the same algorithm can be applied to the general US population.
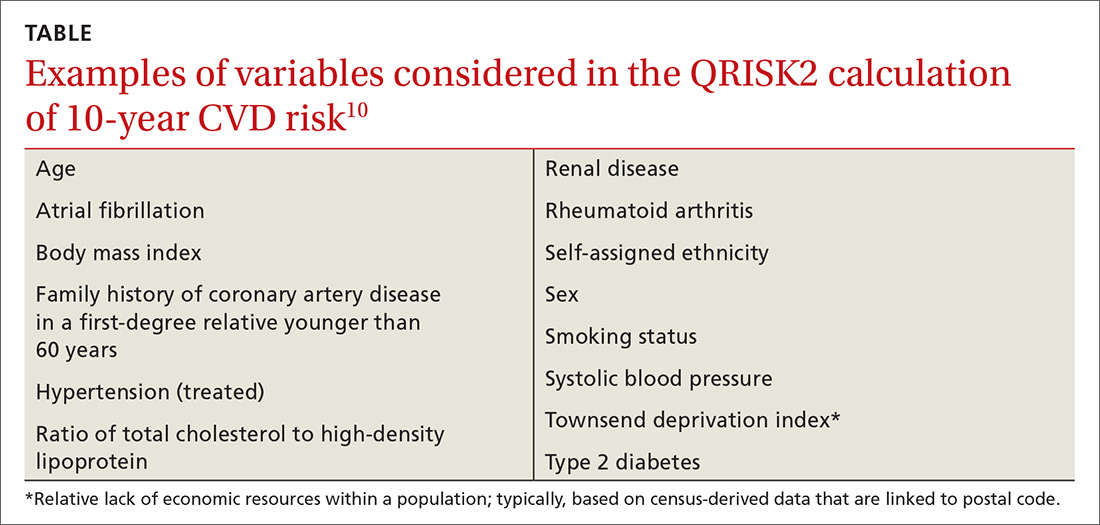
New tool: 2013 ACC/AHA pooled cohort risk equations
In the context of multiple imperfect CVD risk-prediction algorithms, the American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Practice Guidelines published the 2013 Pooled Cohort Risk (PCR) equations to predict 10-year risk of a first atherosclerotic CVD event. The Task Force acknowledged concern that the FRS is based on a cohort that might not accurately represent the general US population. Accordingly, PCR equations were developed from 5 large National Institutes of Health (NIH)-funded cohorts: the Framingham Heart Study, the Framingham Offspring Study, the Atherosclerosis Risk in Communities study, the Cardiovascular Health Study, and the Coronary Artery Risk Development in Young Adults Study.
Continue to: The resulting CVD risk calculator incorporates...
The resulting CVD risk calculator incorporates 4 risk equations: 1 each for African-American and non-Hispanic white males and females.11 Of note, PCR equations are typically used to estimate 10-year CVD risk, but they can be modified to estimate risk over any period. The associated Guideline on the Assessment of Cardiovascular Risk recommends statin therapy for primary prevention of CVD in patients with a predicted 10-year risk ≥7.5% and consideration of statin therapy for patients with a predicted 10-year risk between 5% and 7.5%.12
In late 2016, the US Preventive Services Task Force (USPSTF) recommended low- to moderate-dosage statin therapy in adults 40 to 75 years of age without a history of CVD but with at least 1 CVD risk factor (dyslipidemia, diabetes, hypertension, or smoking), and a PCR-calculated 10-year CVD risk of ≥10%. For people with a PCR-calculated risk of 7.5% to 10%, the USPSTF recommended that clinicians “selectively offer” low- to moderate-dosage statin therapy, noting a smaller likelihood of benefit and uncertainty in an individual’s risk prediction.13
Pooled cohort risk equations have predictive validity
Estimates are that nearly 50% of US adults and as many as 65% of European adults would be candidates for statin therapy if, using PCR equations, the 2013 ACC/AHA guidelines were broadly applied.14 Since PCR equations were released, multiple groups have attempted to evaluate the predictive validity of the algorithm in various populations, with mixed findings.
Data from the 1999-2010 NHANES—the National Health and Nutrition Examination Survey—were used to calculate estimated CVD risk for patients free of atherosclerotic CVD at baseline. Risk prediction using PCR equations was compared to true all-cause and CVD mortality using the National Center for Health Statistics National Death Index. In this large, US adult population without CVD at baseline, PCR-estimated CVD risk was significantly associated with all-cause and CVD-specific mortality risk.15
In a community-based primary prevention cohort, 39% of participants were found statin-eligible—ie, they had an estimated 10-year CVD risk ≥7.5%—by ACC/AHA guidelines, compared with 14% found statin-eligible by the guidelines of the National Cholesterol Education Program’s 2004 updated “Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III).” Despite the larger percentage, participants who were statin-eligible by ACC/AHA guidelines had an increased hazard ratio for incident CVD compared with those who were statin-eligible by ATP III; investigators concluded that ACC/AHA guidelines using PCR equations were associated with greater accuracy and efficiency in identifying increased risk of incident CVD.16
Continue to: Pooled cohort risk equations might overestimate CVD risk
Pooled cohort risk equations might overestimate CVD risk
In contrast, a more recent study followed a large, integrated US health-care delivery system population over 5 years, starting in 2008.17 In this group of adults without diabetes, PCR equations substantially overestimated actual 5-year risk of CVD in both sexes and across multiple socioeconomic strata. Similar overestimation of CVD risk was demonstrated in non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, and Hispanic subjects. The latter 2 ethnic groups are considered “white or other” in the atherosclerotic CVD risk equation, raising additional concern that PCR equations may not be accurate for broad, multiethnic application.17 The ACC/AHA Cardiovascular Risk Assessment guideline recognizes this concern, as well, noting that PCR equations may overestimate risk for Hispanic and Asian Americans.12
Predicted 10-year CVD risk using PCR equations was compared with observed event rates in 3 large-scale primary prevention cohorts: the Women’s Health Study, the Physicians’ Health Study, and the Women’s Health Initiative Observational Study.18 In each cohort, the ACC/AHA risk prediction algorithm overestimated observed risk by 75% to 150%. The authors concluded that 40% to 50% of the 33 million middle-aged Americans deemed statin-eligible by ACC/AHA guidelines may not have actual CVD risk that exceeds the 7.5% threshold recommended for statin treatment.18
Therefore, the discrimination of PCR equations—their ability to differentiate between individuals who are more or less likely to develop clinical CVD—is good. The calibration of the equations—the difference between predicted and observed risk—is not as good, however: PCR equations appear to overestimate actual risk in many groups.15
Additional limitations to pooled cohort risk equations
The predictive value of PCR equations is hampered by several factors:
- Despite expansion of the studied cohorts beyond the original Framingham population, the groups still include people screened for study participation or enrolled in clinical trials. The generalizability of this study population to the diverse population treated in a typical clinical practice is, potentially, limited.
- Use of strategies for primary prevention of CVD (eg, statin therapy, antiplatelet therapy, BP control, blood glucose control) continues to increase. Lowering the risk of CVD in the general population with a broad primary prevention approach effectively widens the gap between observed and equation-predicted CVD risk—and thus strengthens the impression of overestimation of risk by PCR equations.
- Lack of comprehensive surveillance in some studies may result in underassessment of CVD events. In this case, PCR equations would, again, appear to overestimate risk.19
Novel tools are available; their use is qualified
First, newer risk markers offer additional options for improving risk prediction offered by the ACC/AHA PCR equations: Coronary artery calcium, ankle-brachial index, high-sensitivity C-reactive protein, and a family history of CAD are all independently associated with incident CAD. ACC/AHA guidelines suggest that assessment of 1 or more of these variables might be considered an adjunct when risk assessment using PCR equations alone does not offer information for making a clear treatment decision.12
Continue to: Of the 4 risk markers...
Of the 4 risk markers, coronary artery calcium provides the most significant increase in discrimination compared to the FRS alone; comparative data using PCR equations is unavailable.20 ACC/AHA guidelines specifically recommend against routine measurement of carotid intima-media thickness for assessment of risk of a first atherosclerotic event.12
Second, a revised set of PCR equations offers improved discrimination and calibration compared to the 2013 PCR equations. A National Institutes of Health (NIH)-sponsored group updated the equations’ cohort by 1) eliminating the original Framingham Heart Study (FHS) data, which was first collected in 1948, and 2) adding data from the Jackson Heart Study and the Multi-Ethnic Study of Atherosclerosis (MESA). Both new cohorts include patient data from 2000 to 2012. Additionally, the NIH group modified the statistical methods used to derive PCR equations. Although these revised PCR equations offer a substantially more accurate estimate of CVD risk, they have not yet been validated for routine clinical use.21
Bottom line: In prediction there persists imperfection
It is widely held that CVD risk prediction, with subsequent treatment to reduce identified risk, is an important component of an overall strategy to reduce the burden of CVD. Cardiovascular risk factors, such as BP and lipid values, do show limited improvement among populations in which systematic screening is practiced, but the true impact of systematic CVD risk assessment alone for healthy people has yet to be demonstrated in terms of hard clinical outcomes.22
CVD risk prediction is most widely used to inform recommendations for statin treatment. However, ACC/AHA PCR equations might substantially overestimate CVD risk and lead to expanded use of statins in patient populations for which such treatment has less potential benefit. Nonetheless, PCR equations do offer good discrimination between higher-risk and lower-risk people.
CVD risk prediction remains an imperfect science—science that is best used as an adjunct to discussion of comprehensive CVD risk factor modification with the individual patient.
CORRESPONDENCE
Jonathon M. Firnhaber, MD, Brody School of Medicine, East Carolina University, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD004816.
2. Holt T. Predicting cardiovascular disease. BMJ. 2016;353:i2621.
3. Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46-51.
4. Preiss D, Kristensen SL. The new pooled cohort equations risk calculator. Can J Cardiol. 2015;31:613-619.
5. Koller MT, Steyerberg EW, Wolbers M, et al. Validity of the Framingham point scores in the elderly: results from the Rotterdam study. Am Heart J. 2007;154:87-93.
6. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245-1249.
7. Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367-372.
8. Koller MT, Leening MJ, Wolbers M, et al. Development and validation of a coronary risk prediction model for older U.S. and European persons in the Cardiovascular Health Study and the Rotterdam Study. Ann Intern Med. 2012;157:389-397.
9. Damen JA, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416.
10. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336:1475–1482.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Amer Coll Cardiol. 2014;63:2889-2934.
12. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
13. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007.
14. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. New Engl J Med. 2014;370:1422-1431.
15. Loprinzi PD, Addoh O. Predictive validity of the American College of Cardiology/American Heart Association pooled cohort equations in predicting all-cause and cardiovascular disease–specific mortality in a national prospective cohort study of adults in the United States. Mayo Clin Proc. 2016;91:763-769.
16. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314:134-141.
17. Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67:2118-2130.
18. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762-1765.
19. Cook NR, Ridker PM. Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA Intern Med. 2014;174:1964-1971.
20. Yeboah J, McClelland RJ, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788-795.
21. Yadlowsky S, Hayward RA, Sussman JB, et al. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann Intern Med. 2018;169:20-29.
22. Dyakova M, Shantikumar S, Colquitt J, et al. Systematic versus opportunistic risk assessment for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2016 Jan 29;(1):CD010411.
Prevention of cardiovascular disease (CVD) requires timely identification of people who are at increased risk in order to target effective dietary, lifestyle, or pharmacotherapeutic intervention—or a combination of the 3. Risk factors for CVD are well understood, but the relative impact of each factor on an individual’s overall risk is difficult to accurately quantify, making a validated CVD risk calculator an important clinical tool.
Despite numerous available CVD risk calculators, one best tool has yet to emerge. This state of affairs has limited the ability of front-line providers who are tasked with primary prevention of CVD—including family physicians (FPs)—to provide the best evidence-based recommendations to patients.
Implications of CVD risk assessment
Baseline CVD risk assessment is the cornerstone of recommendations for primary prevention of CVD, including aspirin and statin therapy. Interventions to lower CVD risk are of greatest benefit to those at highest risk at initiation of therapy. Overall, statins reduce the risk of a first cardiovascular event in otherwise healthy people by approximately 25% over 10 years.1 Because relative risk reduction is fairly consistent across different levels of absolute risk, a 25% relative reduction confers more actual benefit if risk starts at, say, 40% than at 10%.2 In that example, the same 25% reduction in relative risk results in 1) an absolute risk reduction of 10% when risk starts at 40%, compared to an absolute risk reduction of 2.5% when risk starts at 10% and 2) a number needed to treat (NNT) of, respectively, 10 and 40 (over 10 years).
Identifying a person with an elevated risk of developing CVD has multiple implications. Ideally, that patient is motivated to pursue positive therapeutic lifestyle modifications and make changes that positively affect long-term CVD risk. Conversely, that asymptomatic person identified as at elevated risk also becomes a patient with a medical problem that might adversely affect insurance premiums and self-esteem, and may trigger the use of medications with cost and potential adverse effects. Although the benefit of preventive therapy is greater for a patient at higher risk of disease, the harm of a therapy is relatively constant across all risk groups. Accurately discriminating high and low risk of CVD is, therefore, imperative.
The venerable Framingham risk score
Cardiovascular risk prediction has its roots in the late 1940s, when primary risk factors for CVD were not well-understood, with the inception of the Framingham Heart Study. (A greater understanding of CVD risk today notwithstanding, coronary artery disease [CAD] remains the leading cause of death among American adults.) In the late 1940s, blood pressure (BP) was recognized as the single most useful variable for identifying people at high risk of CVD; other variables were understood to be predictive as well. A composite score—the Framingham Risk Score (FRS)—was thereby developed to calculate the probability that CVD would occur over 8 years in a person who was initially free of such disease.3
The original FRS included glucose intolerance and left ventricular hypertrophy (LVH) identified by electrocardiography (EKG) in its algorithm.3 Other, older algorithms also include a family history of premature CVD. In each risk calculator, these variables are treated as dichotomous (Yes or No), but actual risk associated with each variable is in fact more along a continuum. It is now well-recognized that the sensitivity of EKG for accurately detecting LVH is relatively low; more recent algorithms no longer include this component. A family history of premature CVD variably contributes to an individual’s CVD risk; however, its true impact is nearly impossible to accurately quantify, so this variable is also not included in more modern risk calculators.
Caution: The FRS has meaningful limitations
Although the original Framingham cohort has been expanded multiple times since its inception, clinicians and researchers continue to express concern that the predominantly white, middle-class Framingham, Massachusetts, population might not be representative of the United States in general—which would limit the accuracy of the FRS predictive tool when it is applied to a more diverse population. Furthermore, cholesterol-lowering medications were not available when the FRS was first developed. The FRS, therefore, might not accurately estimate risk in more modern populations, in whom aggressive modification of CVD risk factors has resulted in a lower overall rate of atherosclerotic CVD than when the FRS was developed.4
Continue to: Although demographic changes have increasingly...
Although demographic changes have increasingly led to an extension of primary prevention strategies for CAD to elderly people, the FRS has been demonstrated to perform less well in patients older than 70 years, particularly men.5 An ideal CAD prediction model for elderly people should take into account that, with growing age and frailty, CAD events may be increasingly preempted by death from competing non-coronary causes. In addition, the predictive association of typical CVD risk factors diminishes with increasing age.6,7 Koller and colleagues developed a CAD risk prediction model that accounted for death from non-coronary causes and was validated specifically in patients 65 years and older. Koller’s prediction model provided well-calibrated risk estimates, but it was still not substantially more accurate than the FRS—illustrating the overall difficulty in predicting CAD risk in elderly people.8
Alternative risk calculators have come on the scene
Over the past 2 decades, numerous models have been developed in an attempt to overcome the perceived shortcomings of the FRS. A recent systematic review identified 363 prediction models described in the medical literature prior to July 2013.9 The usefulness of most models remains unclear, however, owing to:
- methodological shortcomings,
- considerable heterogeneity in the definitions of outcomes, and
- lack of external validation.
Even models that are well-validated for a specific population suffer from lack of applicability to a broad multinational population.
In the United Kingdom (UK), electronic health record systems now have the QRISK2 tool embedded to calculate 10-year CVD risk. This algorithm incorporates multiple traditional and nontraditional risk factors (TABLE10). With the inclusion of additional risk factors and validation performed in a population similar to the one from which the algorithm was derived, QRISK2 predicts CVD risk in the UK population more accurately than the modified FRS does.10 It is not clear, however, whether the same algorithm can be applied to the general US population.

New tool: 2013 ACC/AHA pooled cohort risk equations
In the context of multiple imperfect CVD risk-prediction algorithms, the American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Practice Guidelines published the 2013 Pooled Cohort Risk (PCR) equations to predict 10-year risk of a first atherosclerotic CVD event. The Task Force acknowledged concern that the FRS is based on a cohort that might not accurately represent the general US population. Accordingly, PCR equations were developed from 5 large National Institutes of Health (NIH)-funded cohorts: the Framingham Heart Study, the Framingham Offspring Study, the Atherosclerosis Risk in Communities study, the Cardiovascular Health Study, and the Coronary Artery Risk Development in Young Adults Study.
Continue to: The resulting CVD risk calculator incorporates...
The resulting CVD risk calculator incorporates 4 risk equations: 1 each for African-American and non-Hispanic white males and females.11 Of note, PCR equations are typically used to estimate 10-year CVD risk, but they can be modified to estimate risk over any period. The associated Guideline on the Assessment of Cardiovascular Risk recommends statin therapy for primary prevention of CVD in patients with a predicted 10-year risk ≥7.5% and consideration of statin therapy for patients with a predicted 10-year risk between 5% and 7.5%.12
In late 2016, the US Preventive Services Task Force (USPSTF) recommended low- to moderate-dosage statin therapy in adults 40 to 75 years of age without a history of CVD but with at least 1 CVD risk factor (dyslipidemia, diabetes, hypertension, or smoking), and a PCR-calculated 10-year CVD risk of ≥10%. For people with a PCR-calculated risk of 7.5% to 10%, the USPSTF recommended that clinicians “selectively offer” low- to moderate-dosage statin therapy, noting a smaller likelihood of benefit and uncertainty in an individual’s risk prediction.13
Pooled cohort risk equations have predictive validity
Estimates are that nearly 50% of US adults and as many as 65% of European adults would be candidates for statin therapy if, using PCR equations, the 2013 ACC/AHA guidelines were broadly applied.14 Since PCR equations were released, multiple groups have attempted to evaluate the predictive validity of the algorithm in various populations, with mixed findings.
Data from the 1999-2010 NHANES—the National Health and Nutrition Examination Survey—were used to calculate estimated CVD risk for patients free of atherosclerotic CVD at baseline. Risk prediction using PCR equations was compared to true all-cause and CVD mortality using the National Center for Health Statistics National Death Index. In this large, US adult population without CVD at baseline, PCR-estimated CVD risk was significantly associated with all-cause and CVD-specific mortality risk.15
In a community-based primary prevention cohort, 39% of participants were found statin-eligible—ie, they had an estimated 10-year CVD risk ≥7.5%—by ACC/AHA guidelines, compared with 14% found statin-eligible by the guidelines of the National Cholesterol Education Program’s 2004 updated “Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III).” Despite the larger percentage, participants who were statin-eligible by ACC/AHA guidelines had an increased hazard ratio for incident CVD compared with those who were statin-eligible by ATP III; investigators concluded that ACC/AHA guidelines using PCR equations were associated with greater accuracy and efficiency in identifying increased risk of incident CVD.16
Continue to: Pooled cohort risk equations might overestimate CVD risk
Pooled cohort risk equations might overestimate CVD risk
In contrast, a more recent study followed a large, integrated US health-care delivery system population over 5 years, starting in 2008.17 In this group of adults without diabetes, PCR equations substantially overestimated actual 5-year risk of CVD in both sexes and across multiple socioeconomic strata. Similar overestimation of CVD risk was demonstrated in non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, and Hispanic subjects. The latter 2 ethnic groups are considered “white or other” in the atherosclerotic CVD risk equation, raising additional concern that PCR equations may not be accurate for broad, multiethnic application.17 The ACC/AHA Cardiovascular Risk Assessment guideline recognizes this concern, as well, noting that PCR equations may overestimate risk for Hispanic and Asian Americans.12
Predicted 10-year CVD risk using PCR equations was compared with observed event rates in 3 large-scale primary prevention cohorts: the Women’s Health Study, the Physicians’ Health Study, and the Women’s Health Initiative Observational Study.18 In each cohort, the ACC/AHA risk prediction algorithm overestimated observed risk by 75% to 150%. The authors concluded that 40% to 50% of the 33 million middle-aged Americans deemed statin-eligible by ACC/AHA guidelines may not have actual CVD risk that exceeds the 7.5% threshold recommended for statin treatment.18
Therefore, the discrimination of PCR equations—their ability to differentiate between individuals who are more or less likely to develop clinical CVD—is good. The calibration of the equations—the difference between predicted and observed risk—is not as good, however: PCR equations appear to overestimate actual risk in many groups.15
Additional limitations to pooled cohort risk equations
The predictive value of PCR equations is hampered by several factors:
- Despite expansion of the studied cohorts beyond the original Framingham population, the groups still include people screened for study participation or enrolled in clinical trials. The generalizability of this study population to the diverse population treated in a typical clinical practice is, potentially, limited.
- Use of strategies for primary prevention of CVD (eg, statin therapy, antiplatelet therapy, BP control, blood glucose control) continues to increase. Lowering the risk of CVD in the general population with a broad primary prevention approach effectively widens the gap between observed and equation-predicted CVD risk—and thus strengthens the impression of overestimation of risk by PCR equations.
- Lack of comprehensive surveillance in some studies may result in underassessment of CVD events. In this case, PCR equations would, again, appear to overestimate risk.19
Novel tools are available; their use is qualified
First, newer risk markers offer additional options for improving risk prediction offered by the ACC/AHA PCR equations: Coronary artery calcium, ankle-brachial index, high-sensitivity C-reactive protein, and a family history of CAD are all independently associated with incident CAD. ACC/AHA guidelines suggest that assessment of 1 or more of these variables might be considered an adjunct when risk assessment using PCR equations alone does not offer information for making a clear treatment decision.12
Continue to: Of the 4 risk markers...
Of the 4 risk markers, coronary artery calcium provides the most significant increase in discrimination compared to the FRS alone; comparative data using PCR equations is unavailable.20 ACC/AHA guidelines specifically recommend against routine measurement of carotid intima-media thickness for assessment of risk of a first atherosclerotic event.12
Second, a revised set of PCR equations offers improved discrimination and calibration compared to the 2013 PCR equations. A National Institutes of Health (NIH)-sponsored group updated the equations’ cohort by 1) eliminating the original Framingham Heart Study (FHS) data, which was first collected in 1948, and 2) adding data from the Jackson Heart Study and the Multi-Ethnic Study of Atherosclerosis (MESA). Both new cohorts include patient data from 2000 to 2012. Additionally, the NIH group modified the statistical methods used to derive PCR equations. Although these revised PCR equations offer a substantially more accurate estimate of CVD risk, they have not yet been validated for routine clinical use.21
Bottom line: In prediction there persists imperfection
It is widely held that CVD risk prediction, with subsequent treatment to reduce identified risk, is an important component of an overall strategy to reduce the burden of CVD. Cardiovascular risk factors, such as BP and lipid values, do show limited improvement among populations in which systematic screening is practiced, but the true impact of systematic CVD risk assessment alone for healthy people has yet to be demonstrated in terms of hard clinical outcomes.22
CVD risk prediction is most widely used to inform recommendations for statin treatment. However, ACC/AHA PCR equations might substantially overestimate CVD risk and lead to expanded use of statins in patient populations for which such treatment has less potential benefit. Nonetheless, PCR equations do offer good discrimination between higher-risk and lower-risk people.
CVD risk prediction remains an imperfect science—science that is best used as an adjunct to discussion of comprehensive CVD risk factor modification with the individual patient.
CORRESPONDENCE
Jonathon M. Firnhaber, MD, Brody School of Medicine, East Carolina University, 101 Heart Drive, Greenville, NC 27834; [email protected].
Prevention of cardiovascular disease (CVD) requires timely identification of people who are at increased risk in order to target effective dietary, lifestyle, or pharmacotherapeutic intervention—or a combination of the 3. Risk factors for CVD are well understood, but the relative impact of each factor on an individual’s overall risk is difficult to accurately quantify, making a validated CVD risk calculator an important clinical tool.
Despite numerous available CVD risk calculators, one best tool has yet to emerge. This state of affairs has limited the ability of front-line providers who are tasked with primary prevention of CVD—including family physicians (FPs)—to provide the best evidence-based recommendations to patients.
Implications of CVD risk assessment
Baseline CVD risk assessment is the cornerstone of recommendations for primary prevention of CVD, including aspirin and statin therapy. Interventions to lower CVD risk are of greatest benefit to those at highest risk at initiation of therapy. Overall, statins reduce the risk of a first cardiovascular event in otherwise healthy people by approximately 25% over 10 years.1 Because relative risk reduction is fairly consistent across different levels of absolute risk, a 25% relative reduction confers more actual benefit if risk starts at, say, 40% than at 10%.2 In that example, the same 25% reduction in relative risk results in 1) an absolute risk reduction of 10% when risk starts at 40%, compared to an absolute risk reduction of 2.5% when risk starts at 10% and 2) a number needed to treat (NNT) of, respectively, 10 and 40 (over 10 years).
Identifying a person with an elevated risk of developing CVD has multiple implications. Ideally, that patient is motivated to pursue positive therapeutic lifestyle modifications and make changes that positively affect long-term CVD risk. Conversely, that asymptomatic person identified as at elevated risk also becomes a patient with a medical problem that might adversely affect insurance premiums and self-esteem, and may trigger the use of medications with cost and potential adverse effects. Although the benefit of preventive therapy is greater for a patient at higher risk of disease, the harm of a therapy is relatively constant across all risk groups. Accurately discriminating high and low risk of CVD is, therefore, imperative.
The venerable Framingham risk score
Cardiovascular risk prediction has its roots in the late 1940s, when primary risk factors for CVD were not well-understood, with the inception of the Framingham Heart Study. (A greater understanding of CVD risk today notwithstanding, coronary artery disease [CAD] remains the leading cause of death among American adults.) In the late 1940s, blood pressure (BP) was recognized as the single most useful variable for identifying people at high risk of CVD; other variables were understood to be predictive as well. A composite score—the Framingham Risk Score (FRS)—was thereby developed to calculate the probability that CVD would occur over 8 years in a person who was initially free of such disease.3
The original FRS included glucose intolerance and left ventricular hypertrophy (LVH) identified by electrocardiography (EKG) in its algorithm.3 Other, older algorithms also include a family history of premature CVD. In each risk calculator, these variables are treated as dichotomous (Yes or No), but actual risk associated with each variable is in fact more along a continuum. It is now well-recognized that the sensitivity of EKG for accurately detecting LVH is relatively low; more recent algorithms no longer include this component. A family history of premature CVD variably contributes to an individual’s CVD risk; however, its true impact is nearly impossible to accurately quantify, so this variable is also not included in more modern risk calculators.
Caution: The FRS has meaningful limitations
Although the original Framingham cohort has been expanded multiple times since its inception, clinicians and researchers continue to express concern that the predominantly white, middle-class Framingham, Massachusetts, population might not be representative of the United States in general—which would limit the accuracy of the FRS predictive tool when it is applied to a more diverse population. Furthermore, cholesterol-lowering medications were not available when the FRS was first developed. The FRS, therefore, might not accurately estimate risk in more modern populations, in whom aggressive modification of CVD risk factors has resulted in a lower overall rate of atherosclerotic CVD than when the FRS was developed.4
Continue to: Although demographic changes have increasingly...
Although demographic changes have increasingly led to an extension of primary prevention strategies for CAD to elderly people, the FRS has been demonstrated to perform less well in patients older than 70 years, particularly men.5 An ideal CAD prediction model for elderly people should take into account that, with growing age and frailty, CAD events may be increasingly preempted by death from competing non-coronary causes. In addition, the predictive association of typical CVD risk factors diminishes with increasing age.6,7 Koller and colleagues developed a CAD risk prediction model that accounted for death from non-coronary causes and was validated specifically in patients 65 years and older. Koller’s prediction model provided well-calibrated risk estimates, but it was still not substantially more accurate than the FRS—illustrating the overall difficulty in predicting CAD risk in elderly people.8
Alternative risk calculators have come on the scene
Over the past 2 decades, numerous models have been developed in an attempt to overcome the perceived shortcomings of the FRS. A recent systematic review identified 363 prediction models described in the medical literature prior to July 2013.9 The usefulness of most models remains unclear, however, owing to:
- methodological shortcomings,
- considerable heterogeneity in the definitions of outcomes, and
- lack of external validation.
Even models that are well-validated for a specific population suffer from lack of applicability to a broad multinational population.
In the United Kingdom (UK), electronic health record systems now have the QRISK2 tool embedded to calculate 10-year CVD risk. This algorithm incorporates multiple traditional and nontraditional risk factors (TABLE10). With the inclusion of additional risk factors and validation performed in a population similar to the one from which the algorithm was derived, QRISK2 predicts CVD risk in the UK population more accurately than the modified FRS does.10 It is not clear, however, whether the same algorithm can be applied to the general US population.

New tool: 2013 ACC/AHA pooled cohort risk equations
In the context of multiple imperfect CVD risk-prediction algorithms, the American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Practice Guidelines published the 2013 Pooled Cohort Risk (PCR) equations to predict 10-year risk of a first atherosclerotic CVD event. The Task Force acknowledged concern that the FRS is based on a cohort that might not accurately represent the general US population. Accordingly, PCR equations were developed from 5 large National Institutes of Health (NIH)-funded cohorts: the Framingham Heart Study, the Framingham Offspring Study, the Atherosclerosis Risk in Communities study, the Cardiovascular Health Study, and the Coronary Artery Risk Development in Young Adults Study.
Continue to: The resulting CVD risk calculator incorporates...
The resulting CVD risk calculator incorporates 4 risk equations: 1 each for African-American and non-Hispanic white males and females.11 Of note, PCR equations are typically used to estimate 10-year CVD risk, but they can be modified to estimate risk over any period. The associated Guideline on the Assessment of Cardiovascular Risk recommends statin therapy for primary prevention of CVD in patients with a predicted 10-year risk ≥7.5% and consideration of statin therapy for patients with a predicted 10-year risk between 5% and 7.5%.12
In late 2016, the US Preventive Services Task Force (USPSTF) recommended low- to moderate-dosage statin therapy in adults 40 to 75 years of age without a history of CVD but with at least 1 CVD risk factor (dyslipidemia, diabetes, hypertension, or smoking), and a PCR-calculated 10-year CVD risk of ≥10%. For people with a PCR-calculated risk of 7.5% to 10%, the USPSTF recommended that clinicians “selectively offer” low- to moderate-dosage statin therapy, noting a smaller likelihood of benefit and uncertainty in an individual’s risk prediction.13
Pooled cohort risk equations have predictive validity
Estimates are that nearly 50% of US adults and as many as 65% of European adults would be candidates for statin therapy if, using PCR equations, the 2013 ACC/AHA guidelines were broadly applied.14 Since PCR equations were released, multiple groups have attempted to evaluate the predictive validity of the algorithm in various populations, with mixed findings.
Data from the 1999-2010 NHANES—the National Health and Nutrition Examination Survey—were used to calculate estimated CVD risk for patients free of atherosclerotic CVD at baseline. Risk prediction using PCR equations was compared to true all-cause and CVD mortality using the National Center for Health Statistics National Death Index. In this large, US adult population without CVD at baseline, PCR-estimated CVD risk was significantly associated with all-cause and CVD-specific mortality risk.15
In a community-based primary prevention cohort, 39% of participants were found statin-eligible—ie, they had an estimated 10-year CVD risk ≥7.5%—by ACC/AHA guidelines, compared with 14% found statin-eligible by the guidelines of the National Cholesterol Education Program’s 2004 updated “Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III).” Despite the larger percentage, participants who were statin-eligible by ACC/AHA guidelines had an increased hazard ratio for incident CVD compared with those who were statin-eligible by ATP III; investigators concluded that ACC/AHA guidelines using PCR equations were associated with greater accuracy and efficiency in identifying increased risk of incident CVD.16
Continue to: Pooled cohort risk equations might overestimate CVD risk
Pooled cohort risk equations might overestimate CVD risk
In contrast, a more recent study followed a large, integrated US health-care delivery system population over 5 years, starting in 2008.17 In this group of adults without diabetes, PCR equations substantially overestimated actual 5-year risk of CVD in both sexes and across multiple socioeconomic strata. Similar overestimation of CVD risk was demonstrated in non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, and Hispanic subjects. The latter 2 ethnic groups are considered “white or other” in the atherosclerotic CVD risk equation, raising additional concern that PCR equations may not be accurate for broad, multiethnic application.17 The ACC/AHA Cardiovascular Risk Assessment guideline recognizes this concern, as well, noting that PCR equations may overestimate risk for Hispanic and Asian Americans.12
Predicted 10-year CVD risk using PCR equations was compared with observed event rates in 3 large-scale primary prevention cohorts: the Women’s Health Study, the Physicians’ Health Study, and the Women’s Health Initiative Observational Study.18 In each cohort, the ACC/AHA risk prediction algorithm overestimated observed risk by 75% to 150%. The authors concluded that 40% to 50% of the 33 million middle-aged Americans deemed statin-eligible by ACC/AHA guidelines may not have actual CVD risk that exceeds the 7.5% threshold recommended for statin treatment.18
Therefore, the discrimination of PCR equations—their ability to differentiate between individuals who are more or less likely to develop clinical CVD—is good. The calibration of the equations—the difference between predicted and observed risk—is not as good, however: PCR equations appear to overestimate actual risk in many groups.15
Additional limitations to pooled cohort risk equations
The predictive value of PCR equations is hampered by several factors:
- Despite expansion of the studied cohorts beyond the original Framingham population, the groups still include people screened for study participation or enrolled in clinical trials. The generalizability of this study population to the diverse population treated in a typical clinical practice is, potentially, limited.
- Use of strategies for primary prevention of CVD (eg, statin therapy, antiplatelet therapy, BP control, blood glucose control) continues to increase. Lowering the risk of CVD in the general population with a broad primary prevention approach effectively widens the gap between observed and equation-predicted CVD risk—and thus strengthens the impression of overestimation of risk by PCR equations.
- Lack of comprehensive surveillance in some studies may result in underassessment of CVD events. In this case, PCR equations would, again, appear to overestimate risk.19
Novel tools are available; their use is qualified
First, newer risk markers offer additional options for improving risk prediction offered by the ACC/AHA PCR equations: Coronary artery calcium, ankle-brachial index, high-sensitivity C-reactive protein, and a family history of CAD are all independently associated with incident CAD. ACC/AHA guidelines suggest that assessment of 1 or more of these variables might be considered an adjunct when risk assessment using PCR equations alone does not offer information for making a clear treatment decision.12
Continue to: Of the 4 risk markers...
Of the 4 risk markers, coronary artery calcium provides the most significant increase in discrimination compared to the FRS alone; comparative data using PCR equations is unavailable.20 ACC/AHA guidelines specifically recommend against routine measurement of carotid intima-media thickness for assessment of risk of a first atherosclerotic event.12
Second, a revised set of PCR equations offers improved discrimination and calibration compared to the 2013 PCR equations. A National Institutes of Health (NIH)-sponsored group updated the equations’ cohort by 1) eliminating the original Framingham Heart Study (FHS) data, which was first collected in 1948, and 2) adding data from the Jackson Heart Study and the Multi-Ethnic Study of Atherosclerosis (MESA). Both new cohorts include patient data from 2000 to 2012. Additionally, the NIH group modified the statistical methods used to derive PCR equations. Although these revised PCR equations offer a substantially more accurate estimate of CVD risk, they have not yet been validated for routine clinical use.21
Bottom line: In prediction there persists imperfection
It is widely held that CVD risk prediction, with subsequent treatment to reduce identified risk, is an important component of an overall strategy to reduce the burden of CVD. Cardiovascular risk factors, such as BP and lipid values, do show limited improvement among populations in which systematic screening is practiced, but the true impact of systematic CVD risk assessment alone for healthy people has yet to be demonstrated in terms of hard clinical outcomes.22
CVD risk prediction is most widely used to inform recommendations for statin treatment. However, ACC/AHA PCR equations might substantially overestimate CVD risk and lead to expanded use of statins in patient populations for which such treatment has less potential benefit. Nonetheless, PCR equations do offer good discrimination between higher-risk and lower-risk people.
CVD risk prediction remains an imperfect science—science that is best used as an adjunct to discussion of comprehensive CVD risk factor modification with the individual patient.
CORRESPONDENCE
Jonathon M. Firnhaber, MD, Brody School of Medicine, East Carolina University, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD004816.
2. Holt T. Predicting cardiovascular disease. BMJ. 2016;353:i2621.
3. Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46-51.
4. Preiss D, Kristensen SL. The new pooled cohort equations risk calculator. Can J Cardiol. 2015;31:613-619.
5. Koller MT, Steyerberg EW, Wolbers M, et al. Validity of the Framingham point scores in the elderly: results from the Rotterdam study. Am Heart J. 2007;154:87-93.
6. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245-1249.
7. Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367-372.
8. Koller MT, Leening MJ, Wolbers M, et al. Development and validation of a coronary risk prediction model for older U.S. and European persons in the Cardiovascular Health Study and the Rotterdam Study. Ann Intern Med. 2012;157:389-397.
9. Damen JA, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416.
10. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336:1475–1482.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Amer Coll Cardiol. 2014;63:2889-2934.
12. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
13. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007.
14. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. New Engl J Med. 2014;370:1422-1431.
15. Loprinzi PD, Addoh O. Predictive validity of the American College of Cardiology/American Heart Association pooled cohort equations in predicting all-cause and cardiovascular disease–specific mortality in a national prospective cohort study of adults in the United States. Mayo Clin Proc. 2016;91:763-769.
16. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314:134-141.
17. Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67:2118-2130.
18. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762-1765.
19. Cook NR, Ridker PM. Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA Intern Med. 2014;174:1964-1971.
20. Yeboah J, McClelland RJ, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788-795.
21. Yadlowsky S, Hayward RA, Sussman JB, et al. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann Intern Med. 2018;169:20-29.
22. Dyakova M, Shantikumar S, Colquitt J, et al. Systematic versus opportunistic risk assessment for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2016 Jan 29;(1):CD010411.
1. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD004816.
2. Holt T. Predicting cardiovascular disease. BMJ. 2016;353:i2621.
3. Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46-51.
4. Preiss D, Kristensen SL. The new pooled cohort equations risk calculator. Can J Cardiol. 2015;31:613-619.
5. Koller MT, Steyerberg EW, Wolbers M, et al. Validity of the Framingham point scores in the elderly: results from the Rotterdam study. Am Heart J. 2007;154:87-93.
6. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245-1249.
7. Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367-372.
8. Koller MT, Leening MJ, Wolbers M, et al. Development and validation of a coronary risk prediction model for older U.S. and European persons in the Cardiovascular Health Study and the Rotterdam Study. Ann Intern Med. 2012;157:389-397.
9. Damen JA, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416.
10. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336:1475–1482.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Amer Coll Cardiol. 2014;63:2889-2934.
12. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
13. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007.
14. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. New Engl J Med. 2014;370:1422-1431.
15. Loprinzi PD, Addoh O. Predictive validity of the American College of Cardiology/American Heart Association pooled cohort equations in predicting all-cause and cardiovascular disease–specific mortality in a national prospective cohort study of adults in the United States. Mayo Clin Proc. 2016;91:763-769.
16. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314:134-141.
17. Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67:2118-2130.
18. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762-1765.
19. Cook NR, Ridker PM. Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA Intern Med. 2014;174:1964-1971.
20. Yeboah J, McClelland RJ, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788-795.
21. Yadlowsky S, Hayward RA, Sussman JB, et al. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann Intern Med. 2018;169:20-29.
22. Dyakova M, Shantikumar S, Colquitt J, et al. Systematic versus opportunistic risk assessment for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2016 Jan 29;(1):CD010411.
PRACTICE RECOMMENDATIONS
› Avoid the inclination to think that there is 1 best tool for accurately estimating an asymptomatic patient’s risk of cardiovascular disease (CVD). C
› Be mindful that 2013 ACC/AHA Pooled Cohort Risk equations can overestimate CVD risk depending on multiple factors, including the population being evaluated (even though the equations might be the most generalizable of available CVD risk calculators). C
› Consider using one of the newer CVD risk markers to further inform treatment recommendations when quantitative risk assessment does not offer information for making a clear treatment decision. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Consider these exercises for chronic musculoskeletal conditions
Regular exercise confers several well-established benefits. In such conditions as coronary heart disease, stroke, heart failure, and diabetes, exercise has led to a reduction in mortality similar to that seen with pharmacotherapy.1 For patients with chronic musculoskeletal conditions, the benefits of exercise-based interventions are measurably reduced pain and improved daily function.2 However, prescribing of exercise is often neglected, with preference given to pharmacologic or surgical interventions.3 In part, the disregard of exercise as therapy results from unfamiliarity with appropriate exercise prescriptions,3 which include various forms of aerobic exercise, strength training, and stretching to increase flexibility (TABLE).
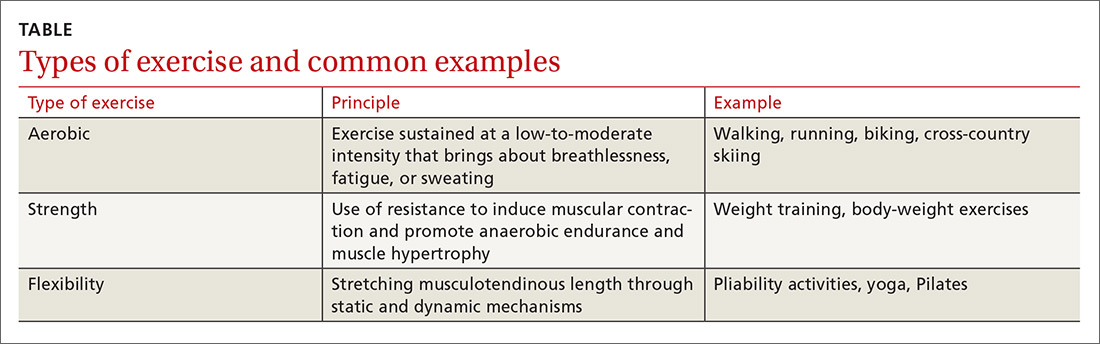
As is true of many therapeutic modalities, exercise must be tailored to the condition and to a patient’s preferences to optimize its benefits. In this review, we describe exercise regimens well suited for common musculoskeletal conditions, examine the effectiveness of exercise in each condition, and provide examples for use in treating patients.
Osteoarthritis of the hip and knee
Osteoarthritis (OA), one of the most common chronic joint diseases, erodes the articular cartilage and subchondral bone of a synovial joint, eventually leading to joint failure. Pain and diminished muscle strength restrict physical activity and can lead to decreased fitness and impaired muscle function. Exercise helps reduce pain and improve muscle function and quality of life in patients with hip or knee OA regardless of age, disease severity, or level of pain and dysfunction.2
Knee exercises. Activities suitable for patients with OA include muscle strengthening, aerobic conditioning, and range-of-motion (ROM) exercises.4-6 A 2015 Cochrane review of OA of the knee showed that exercise reduced pain and improved physical function and quality of life in patients who completed a treatment program, and that pain relief persisted up to 6 months after intervention.5
When designing an exercise prescription for patients with knee OA, consider quadriceps strengthening with an initial period of supervision, which may provide greater pain relief than nonspecific, unsupervised lower limb exercises.4 Enhanced strength of the lower limb may lessen force through the knee, thereby decreasing pain and improving overall physical function.7 Simple, teachable exercises include squats, step-ups, knee extension/flexion while sitting in a chair, and hip abduction/adduction while standing or lying down. Elastic bands, dumbbells, or cuff weights may be used to increase resistance.
Hip exercises. Exercise can significantly reduce pain and improve function for up to 6 months for patients with mild-to-moderate symptomatic hip OA.6 Types of exercise for hip OA include strength training of hip and core muscles, functional exercises that imitate movements in daily activities, and flexibility training. These exercises help reduce pain and increase ROM. Exercise should include resistance training and should not exceed the limit for acceptable pain.8
Aquatic therapy is also appropriate for exercise and strength training and can decrease pain and disability and improve quality of life.9 Supervised physical therapy, including strength training, manual therapy, and balance training, are important for reducing pain and improving function. Physical therapy can also enhance adherence to a prescribed exercise program.10
Continue to: Appropriate exercise prescriptions...
Appropriate exercise prescriptions for patients with knee or hip OA should focus on low-impact activities that can improve strength, flexibility, and function (FIGURE 1). A typical regimen would be 30 or more cumulative minutes daily of stationary cycling, water-based exercises, or strength training, 3 to 5 days per week. Individualize workout intensity for each patient, emphasizing that high-intensity, low-impact effort may yield greater strength gains and take less time to perform.11 A high-intensity exercise prescription focusing on quadriceps, hip, and core strengthening may consist of 3 sets of 8 repetitions with resistance set at 40% of the maximum resistance against which the patient can perform 1 repetition.7
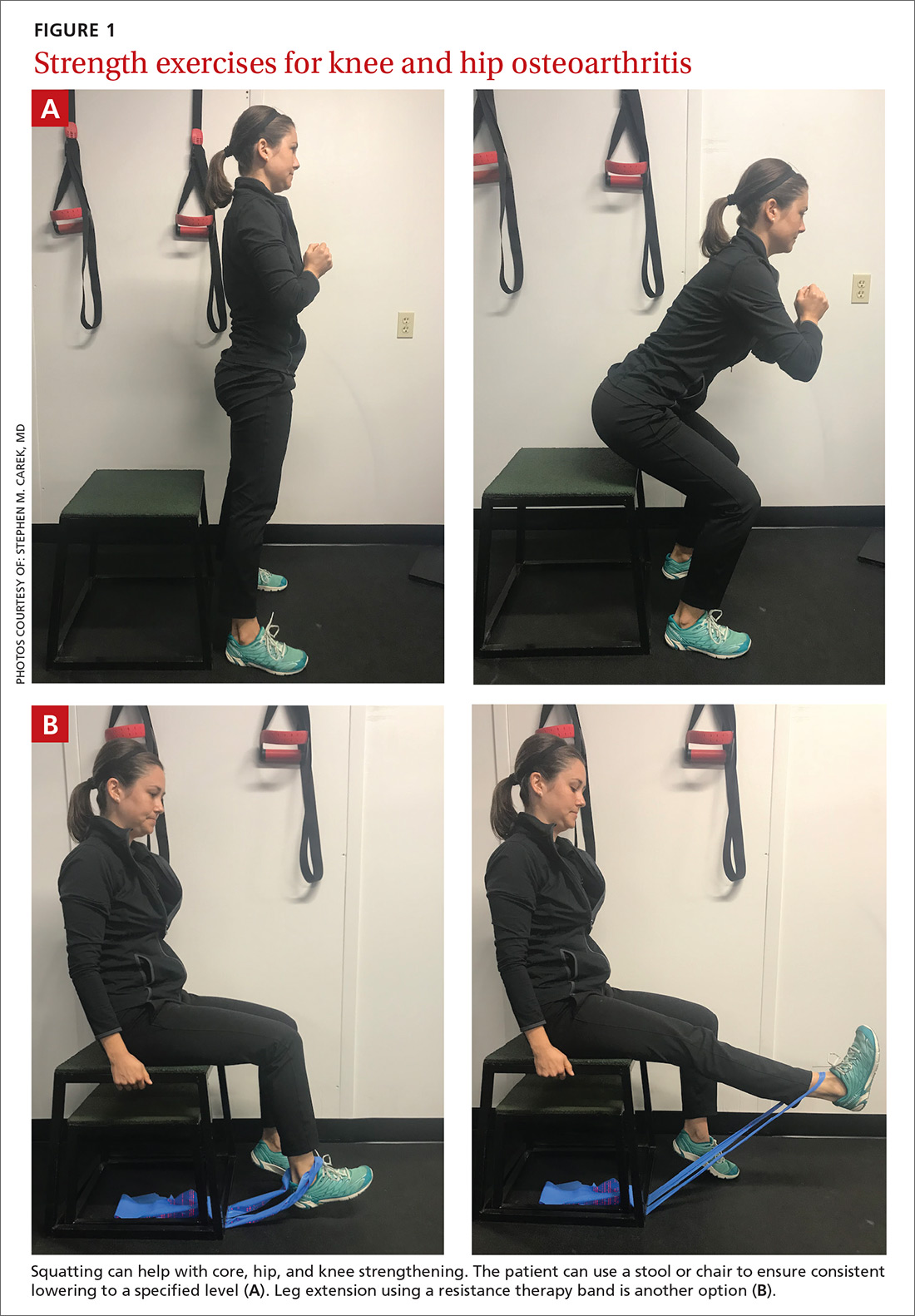
Barriers to exercise in knee and hip OA include negative patient and provider perspectives on exercise and patients’ fear that increased activity may actually worsen OA.12 Depending on a patient’s personal preferences, ways to overcome these barriers and encourage adherence might be supervised exercises in an individual or group setting or audiotapes or videos of recommended exercises.10
Chronic low back pain
Chronic low back pain (LBP) is a large socioeconomic burden in the United States, with upward of $100 billion per year accounted for in health care costs and decreased worker productivity.13 The etiology of chronic LBP can be multifactorial and due to any of several conditions such as degenerative disc disease, spinal stenosis, spondylolisthesis, and facet arthropathy. Treatment is difficult, given that many common interventions—medications, massage, manipulation—have limited efficacy.14 However, for patients with nonspecific chronic LBP, exercise is an effective intervention for reducing pain and improving physical function.15
An effective approach is to design an exercise regimen for the individual by type, duration, and frequency of activity, administered under supervision to encourage adherence.16 Appropriate exercises emphasize resistance, strength training, and core stabilization, often focusing on whole body and trunk motion (FIGURE 2).17
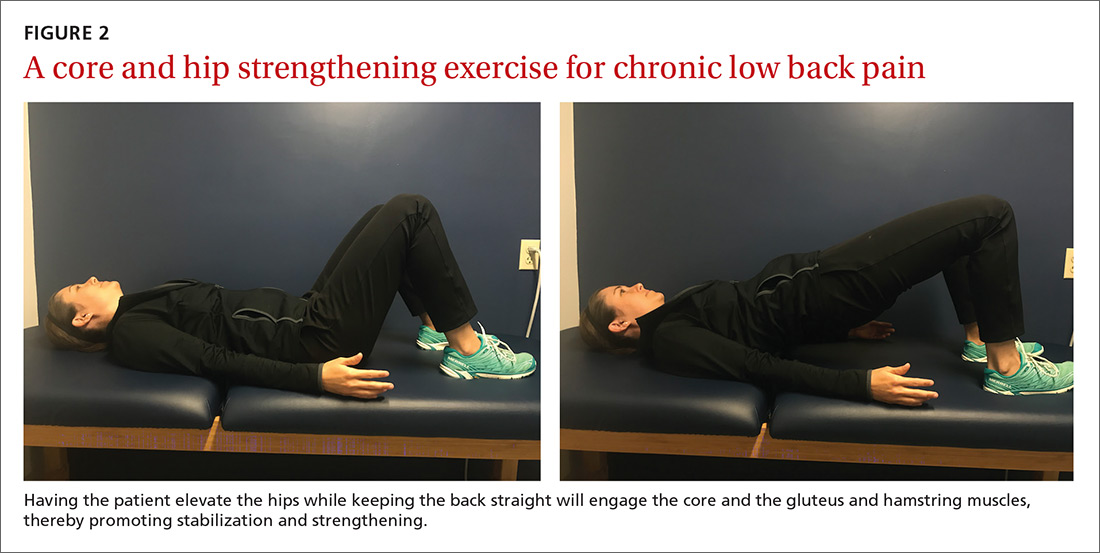
Although yoga or Pilates classes may have a small effect on function, no high-quality evidence exists for their superiority to other forms of exercise.18,19 Back School, a therapeutic program that includes education on anatomy and biomechanics, optimal posture, ergonomics, and back exercises, has limited, low-quality evidence for treatment comparisons.20 Aerobic exercise, including treadmill, elliptical, or cycling exercises or walking outdoors can reduce pain and improve physical and psychologic functioning.21
Continue to: The most common reported adverse effect...
The most common reported adverse effect of exercise is a temporary exacerbation of back pain. However, having patients continue daily activities within the permitted limits of pain leads to more rapid recovery than rest or back-mobilizing exercises.15,22,23
Cautions. Exercise is contraindicated in patients with LBP arising from a serious medical condition, such as fracture, infection, cancer, or cauda equina syndrome.24 Importantly, exercise interventions recommended for acute LBP have not shown benefit for chronic LBP.
Chronic shoulder pain
With a prevalence ranging from 7% to 26% in the general population,25 chronic shoulder pain often interferes with essential activities of daily living. The etiology of chronic shoulder pain is broad and most commonly involves disorders of the rotator cuff, which functions in both motion and dynamic stabilization of the shoulder. The common term “rotator cuff pain syndrome” can cover such disorders as subacromial impingement syndrome, rotator cuff tendinopathy or tendinitis, partial or full thickness rotator cuff tears, calcific tendinitis, and subacromial bursitis. These pathologies may have overlapping presentations. Manual therapy and exercise, usually delivered as a component of structured physical therapy, focus on stretches and other exercises to increase ROM, stability, and strength of the rotator cuff musculature.26
A 2016 Cochrane review that evaluated manual therapy and exercise for chronic shoulder pain yielded limited high-quality evidence for effectiveness compared with placebo.27 Five trials found no important differences between manual therapy and exercise compared with glucocorticoid injection relative to overall pain, function, active shoulder abduction, and quality of life from 4 weeks up to 12 months.27 But compared with placebo, exercise has been more effective in reducing reported pain, especially in the context of strengthening regimens focused on flexion, extension, and internal and external rotation.28
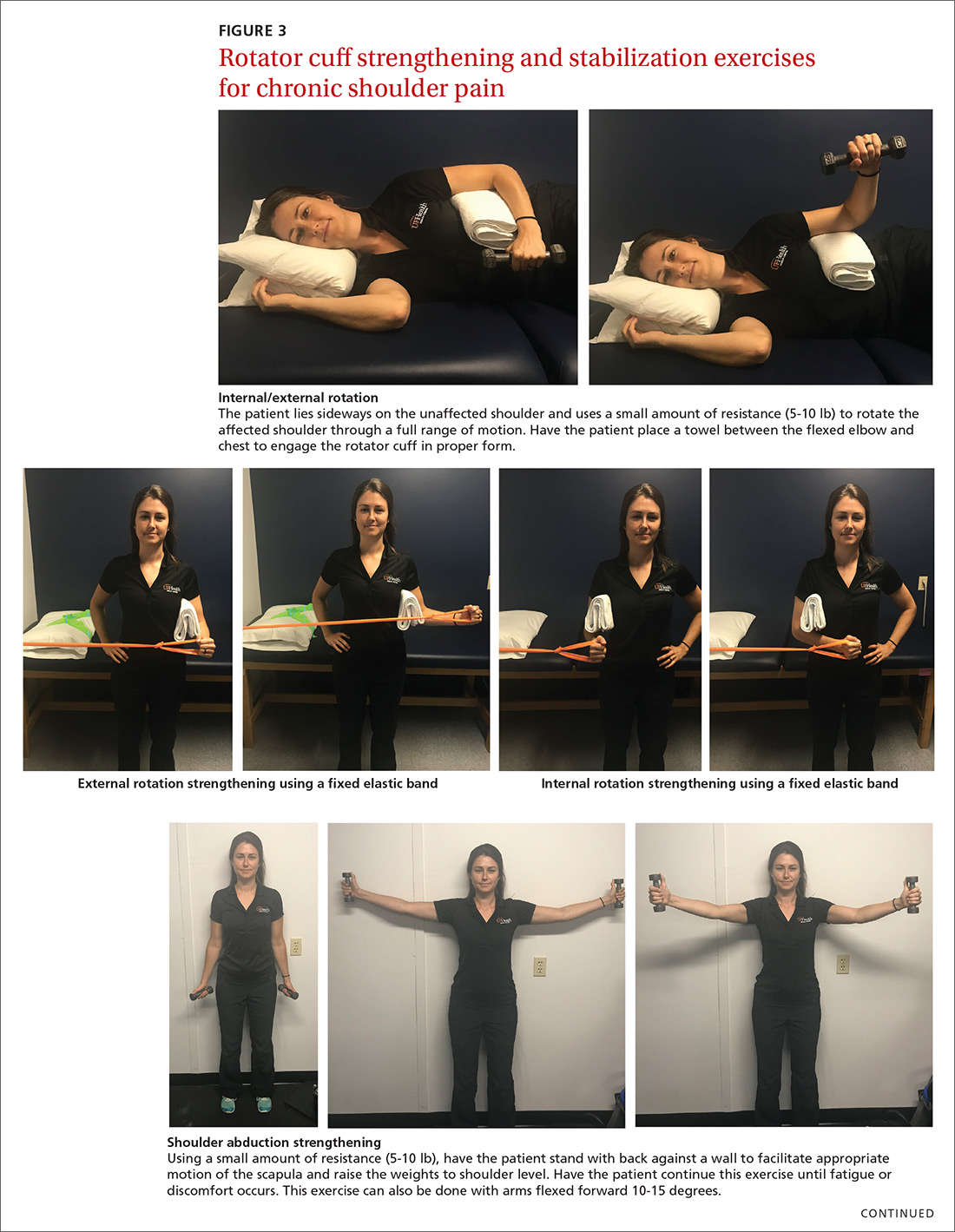
For subacromial impingement syndrome, a 2017 meta-analysis found that a generalized exercise program relieves pain and improves function, ROM, and strength.29 A generalized shoulder-strengthening program includes exercises that focus on internal and external rotation, horizontal abduction, and shoulder stabilization (FIGURE 3). These exercises can be completed with 3 sets of 15 to 20 repetitions, which create a fatigue response that improves strength and targets local muscular endurance.30
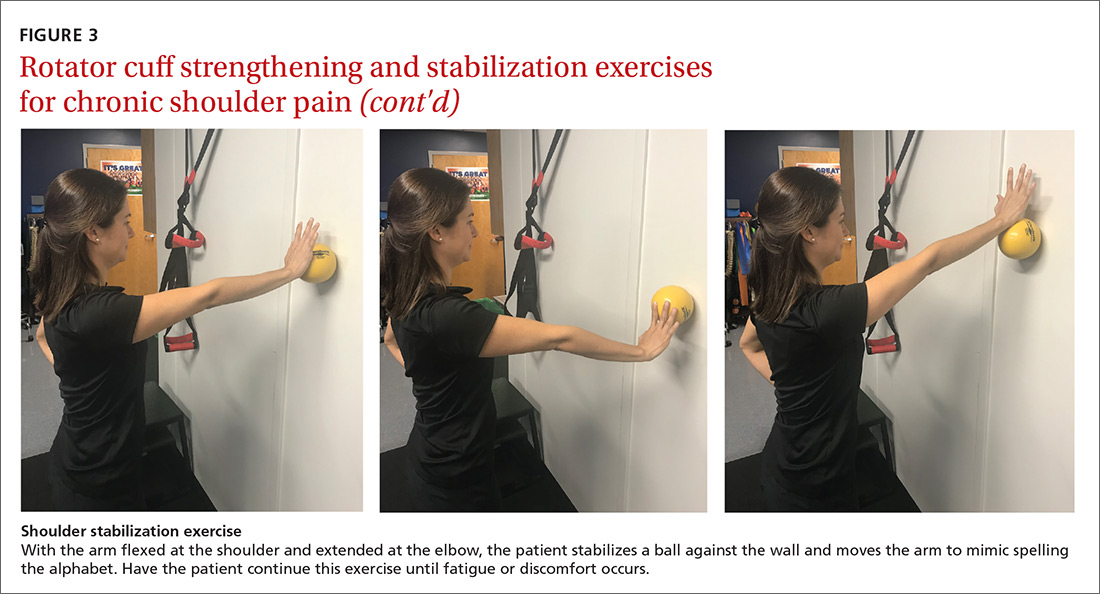
Continue to: Achilles tendinopathy
Achilles tendinopathy
Achilles tendinopathy (also referred to as chronic Achilles tendinitis) is a degenerative condition of the Achilles tendon related to overuse that leads to pain, swelling, and impaired performance. It accounts for approximately 18% of injuries in runners and 4% of all patients presenting to sports medicine clinics.31 Eccentric muscle loading has become the dominant conservative intervention strategy for chronic Achilles tendinopathy.
For chronic tendinopathies, eccentric exercises subject greater force than concentric exercises through a controlled lengthening of a muscle-tendon unit, resulting in a greater remodeling stimulus of the tendon.32 Classically, the Alfredson protocol has been used to treat chronic Achilles tendinopathy. This program of eccentric heel-drop exercises recommends completion of 180 eccentric repetitions a day for up to 12 weeks (FIGURE 4).33 Exercises are performed slowly, and load can be increased when exercises are performed without pain or perhaps with mild nondisabling pain.
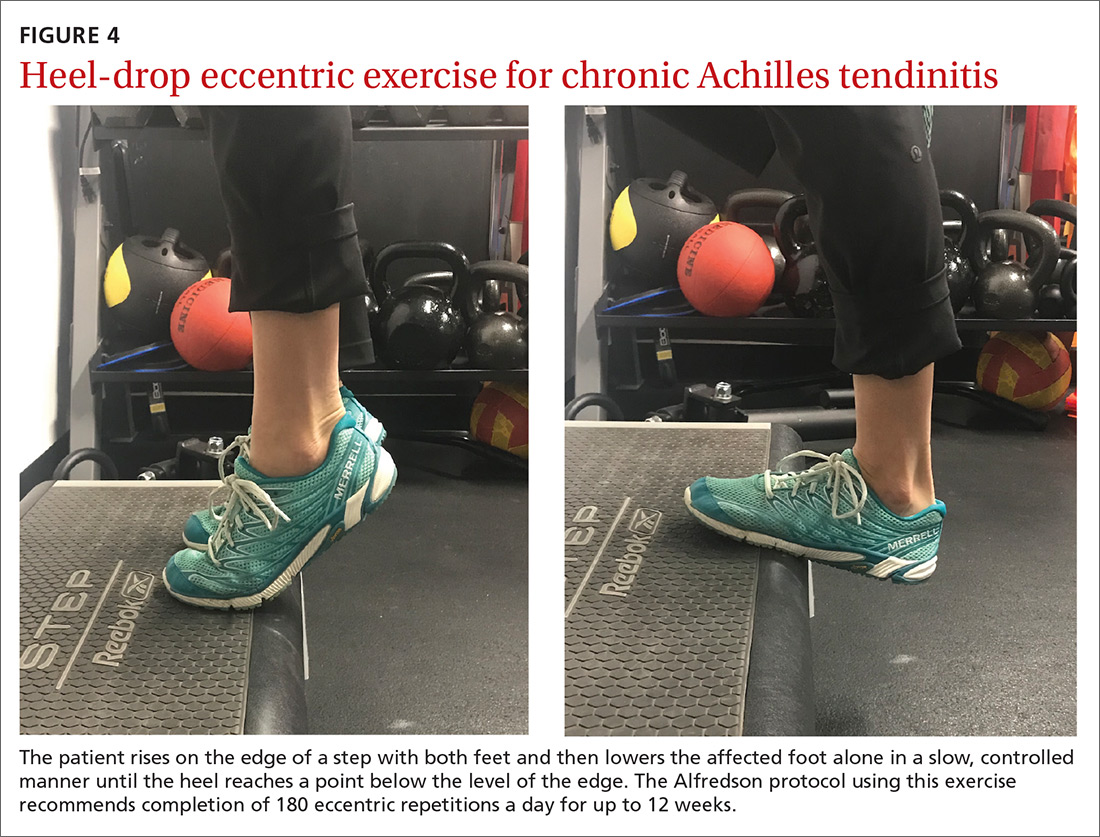
A variation of this protocol has allowed a gradual escalation of repetitions over a week up to the recommended 180 repetitions, and has shown improvements in pain reduction and function similar to that achieved with the primary protocol.34 Additionally, a 6-week “do as tolerated” program of eccentric exercises did not lead to lesser improvement for individuals with midportion Achilles tendinopathy.35
Several systematic reviews have supported the use of eccentric exercises for chronic Achilles tendinopathy,31,36,37 but no specific protocol or exercise regimen has demonstrated superiority. However, with the Alfredson protocol, improvement in pain and function in patients with chronic Achilles tendinopathy has persisted for up to 5 years.38
Lateral epicondylitis
Lateral epicondylitis (also called lateral epicondylosis or “tennis elbow”) is a disabling musculoskeletal condition that leads to pain and tenderness around the extensor mass of the lateral elbow. It is caused by microtrauma to the tendon, usually sustained through repetitive movement in a sporting activity, industrial work, or hobby. Affecting up to 3% of the US population, lateral epicondylitis is associated with pain and functional disability, as well as emotional and psychosocial consequences.39
Continue to: Proposed treatment and rehabilitation options...
Proposed treatment and rehabilitation options for patients with lateral epicondylitis have included massage, manipulation, taping, acupuncture, orthotic devices, ultrasound, activity modification, and rest. Exercise programs incorporating eccentric muscle activity are becoming increasingly popular for such conditions as Achilles and patellar tendinopathies, and they may translate well to other chronic tendinopathies, such as lateral epicondylitis.32
An eccentric exercise program for lateral epicondylitis, either in isolation or as an adjunct to other therapies, has decreased pain and improved function and grip strength from baseline measures.40 Compared with a standard exercise regimen without eccentric strength training, use of eccentric training improves such clinical measures as pain intensity and disability status, as it decreases tendon thickness and aids in recovering homogenous tendon structure.41
A sample exercise. The patient may sit in a chair and, with the forearm flexed and pronated over the edge of a table, grasp some form of resistance (bucket of water, training weight, resistance band) (FIGURE 5). The nonaffected hand can be used to help lift the affected wrist into full extension and then removed to allow lowering of the hand over several seconds into flexion. This activity can be performed in sets of 8 to 12 repetitions, 2 to 3 times a day, until the patient’s pain and function have improved.42
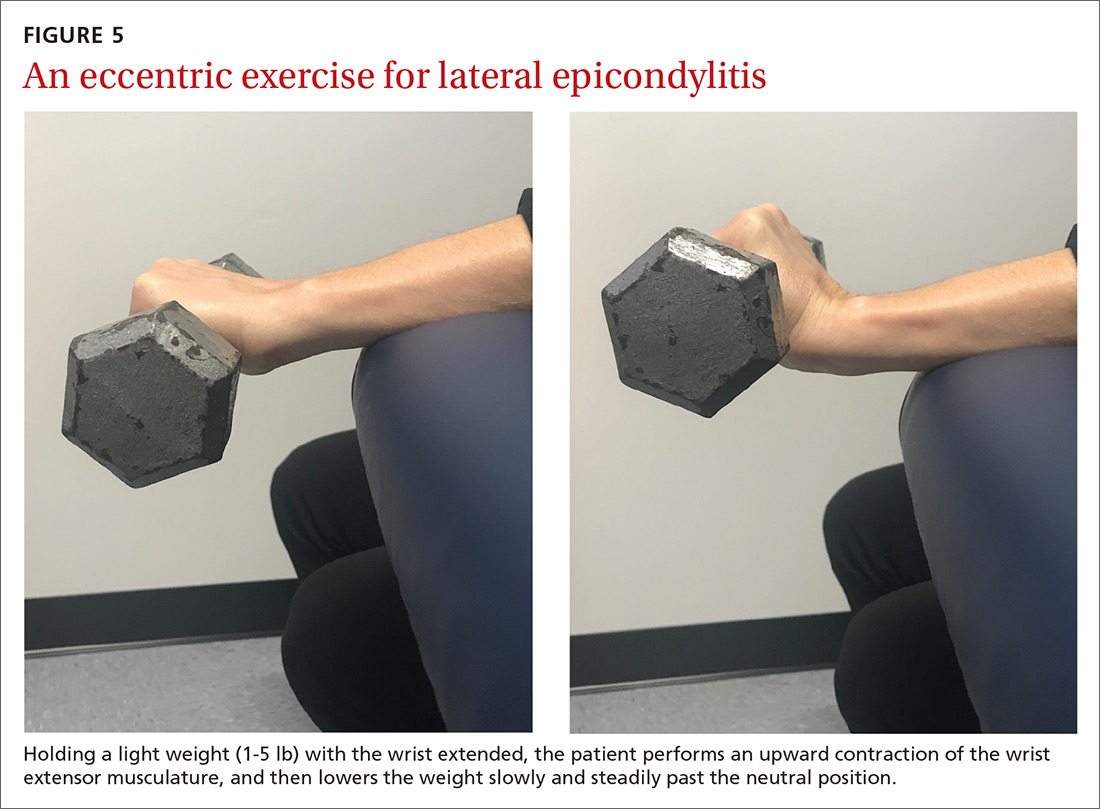
Overcoming barriers to exercise
A major concern across all studies assessing the therapeutic value of exercise is patient compliance and adherence to prescribed programs. Compliance and adherence are affected in part by psychosocial factors such as low literacy and poor social support. From a physician’s perspective, direct and indirect costs of treatment and rehabilitation of chronic musculoskeletal conditions may discourage the prescribing of supervised physical therapy.3
Steps to consider in overcoming these barriers would be advising an exercise regimen that requires only an initial period of supervision; educating patients about the benefits of an exercise program; exploring a patient’s expectations, beliefs, and fears; and developing strategies for long-term adherence.16 Supervision through physical therapy is often suggested. However, significant barriers may exist that impede a patient’s ability to attend or participate, in which case physician observation in the course of regularly scheduled clinical examinations could be considered.
Continue to: When prescribing exercises...
When prescribing exercises, be sure to address patient expectations regarding pain, duration, and limitations of exercise. It would be helpful for patients to know, for instance, that working through mild-to-moderate pain during exercise has been shown to shorten post-exercise recovery time and, in the short-term, improve relief from pain.43
Tailoring specific exercise prescriptions for a patient will make the regimen more satisfying for the individual and optimize adherence, which in turn will increase the potential for pain reduction and improved function. Secondary benefits would likely be weight loss and prevention (or regression) of cardiovascular disease. Continued evaluation by the physician or physical therapist should be part of ongoing management, as well as “refresher courses” to ensure understanding of, and adherence to, the exercise program. The potential benefits and limited risks of exercise, if done properly, make it a primary intervention for specific musculoskeletal conditions.
CORRESPONDENCE
Peter J. Carek, MD, MS, Department of Community Health and Family Medicine, College of Medicine, University of Florida, PO Box 100237, Gainesville, FL 32610-0237; [email protected].
1. Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577.
2. Babatunde OO, Jordan JL, van der Windt DA, et al. Effective treatment options for musculoskeletal pain in primary care: a systematic overview of current evidence. PLoS One. 2017;12:e0178621.
3. Persson G, Brorsson A, Ekvall Hansson E, et al. Physical activity on prescription (PAP) from the general practitioner’s perspective - a qualitative study. BMC Fam Pract. 2013;14:128.
4. Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622-636.
5. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376.
6. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;(4):CD007912.
7. Vincent KR, Vincent HK. Resistance exercise for knee osteoarthritis. PM R. 2012;4(suppl 5):S45-S52.
8. Fernandes L, Storheim K, Nordsletten L, et al. Development of a therapeutic exercise program for patients with osteoarthritis of the hip. Phys Ther. 2010;90:592-601.
9. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
10. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010;(1):CD005956.
11. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
12. Brakke R, Singh J, Sullivan W. Physical therapy in persons with osteoarthritis. PM R. 2012;4:S53-S58.
13. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21-24.
14. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
15. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;(3):CD000335.
16. Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776-785.
17. Searle A, Spink M, Ho A, et al. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. 2015;29:1155-1167.
18. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;(7):CD010265.
19. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(1):CD010671.
20. Parreira P, Heymans MW, van Tulder MW, et al. Back Schools for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(8):CD011674.
21. Meng XG, Yue SW. Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil. 2015;94:358-365.
22. Malmivaara A, Häkkinen U, Aro T, et al. The treatment of acute low back pain--bed rest, exercises, or ordinary activity? N Engl J Med. 1995;332:351-355.
23. van Tulder M, Malmivaara A, Esmail R, Koes B. Exercise therapy for low back pain: a systematic review within the framework of the cochrane collaboration back review group. Spine (Phila Pa 1976). 2000;25:2784-2796.
24. Hoffmann TC, Maher CG, Briffa T, et al. Prescribing exercise interventions for patients with chronic conditions. CMAJ. 2016;188:510-518.
25. Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33:73-81.
26. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18:138-160.
27. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
28. van den Dolder PA, Ferreira PH, Refshauge KM. Effectiveness of soft tissue massage and exercise for the treatment of non-specific shoulder pain: a systematic review with meta-analysis. Br J Sports Med. 2014;48:1216-1226.
29. Shire AR, Stæhr TAB, Overby JB, et al. Specific or general exercise strategy for subacromial impingement syndrome-does it matter? A systematic literature review and meta analysis. BMC Musculoskelet Disord. 2017;18:158.
30. Ellenbecker TS, Cools A. Rehabilitation of shoulder impingement syndrome and rotator cuff injuries: an evidence-based review. Br J Sports Med. 2010;44:319-327.
31. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
32. Rees JD, Wolman RL, Wilson A. Eccentric exercises; why do they work, what are the problems and how can we improve them? Br J Sports Med. 2009;43:242-246.
33. Alfredson H, Pietilä T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26:360-366.
34. Rompe JD, Nafe B, Furia JP, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35:374-383.
35. Stevens M, Tan CW. Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther. 2014;44:59-67.
36. Habets B, van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports. 2015;25:3-15.
37. Malliaras P, Barton CJ, Reeves ND, et al. Achilles and patellar tendinopathy loading programmes : a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013;43:267-286.
38. van der Plas A, de Jonge S, de Vos RJ, et al. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46:214-218.
39. Alizadehkhaiyat O, Fisher AC, Kemp GJ, et al. Pain, functional disability, and psychologic status in tennis elbow. Clin J Pain. 2007;23:482-489.
40. Cullinane FL, Boocock MG, Trevelyan FC. Is eccentric exercise an effective treatment for lateral epicondylitis? A systematic review. Clin Rehabil. 2014;28:3-19.
41. Croisier JL, Foidart-Dessalle M, Tinant F, et al. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy. Br J Sports Med. 2007;41:269-275.
42. Söderberg J, Grooten WJ, Ang BO. Effects of eccentric training on hand strength in subjects with lateral epicondylalgia: a randomized-controlled trial. Scand J Med Sci Sports. 2012;22:797-803.
43. Smith BE, Hendrick P, Smith TO, et al. Should exercises be painful in the management of chronic musculoskeletal pain? A systematic review and meta-analysis. Br J Sports Med. 2017;51:1679-1687.
Regular exercise confers several well-established benefits. In such conditions as coronary heart disease, stroke, heart failure, and diabetes, exercise has led to a reduction in mortality similar to that seen with pharmacotherapy.1 For patients with chronic musculoskeletal conditions, the benefits of exercise-based interventions are measurably reduced pain and improved daily function.2 However, prescribing of exercise is often neglected, with preference given to pharmacologic or surgical interventions.3 In part, the disregard of exercise as therapy results from unfamiliarity with appropriate exercise prescriptions,3 which include various forms of aerobic exercise, strength training, and stretching to increase flexibility (TABLE).

As is true of many therapeutic modalities, exercise must be tailored to the condition and to a patient’s preferences to optimize its benefits. In this review, we describe exercise regimens well suited for common musculoskeletal conditions, examine the effectiveness of exercise in each condition, and provide examples for use in treating patients.
Osteoarthritis of the hip and knee
Osteoarthritis (OA), one of the most common chronic joint diseases, erodes the articular cartilage and subchondral bone of a synovial joint, eventually leading to joint failure. Pain and diminished muscle strength restrict physical activity and can lead to decreased fitness and impaired muscle function. Exercise helps reduce pain and improve muscle function and quality of life in patients with hip or knee OA regardless of age, disease severity, or level of pain and dysfunction.2
Knee exercises. Activities suitable for patients with OA include muscle strengthening, aerobic conditioning, and range-of-motion (ROM) exercises.4-6 A 2015 Cochrane review of OA of the knee showed that exercise reduced pain and improved physical function and quality of life in patients who completed a treatment program, and that pain relief persisted up to 6 months after intervention.5
When designing an exercise prescription for patients with knee OA, consider quadriceps strengthening with an initial period of supervision, which may provide greater pain relief than nonspecific, unsupervised lower limb exercises.4 Enhanced strength of the lower limb may lessen force through the knee, thereby decreasing pain and improving overall physical function.7 Simple, teachable exercises include squats, step-ups, knee extension/flexion while sitting in a chair, and hip abduction/adduction while standing or lying down. Elastic bands, dumbbells, or cuff weights may be used to increase resistance.
Hip exercises. Exercise can significantly reduce pain and improve function for up to 6 months for patients with mild-to-moderate symptomatic hip OA.6 Types of exercise for hip OA include strength training of hip and core muscles, functional exercises that imitate movements in daily activities, and flexibility training. These exercises help reduce pain and increase ROM. Exercise should include resistance training and should not exceed the limit for acceptable pain.8
Aquatic therapy is also appropriate for exercise and strength training and can decrease pain and disability and improve quality of life.9 Supervised physical therapy, including strength training, manual therapy, and balance training, are important for reducing pain and improving function. Physical therapy can also enhance adherence to a prescribed exercise program.10
Continue to: Appropriate exercise prescriptions...
Appropriate exercise prescriptions for patients with knee or hip OA should focus on low-impact activities that can improve strength, flexibility, and function (FIGURE 1). A typical regimen would be 30 or more cumulative minutes daily of stationary cycling, water-based exercises, or strength training, 3 to 5 days per week. Individualize workout intensity for each patient, emphasizing that high-intensity, low-impact effort may yield greater strength gains and take less time to perform.11 A high-intensity exercise prescription focusing on quadriceps, hip, and core strengthening may consist of 3 sets of 8 repetitions with resistance set at 40% of the maximum resistance against which the patient can perform 1 repetition.7

Barriers to exercise in knee and hip OA include negative patient and provider perspectives on exercise and patients’ fear that increased activity may actually worsen OA.12 Depending on a patient’s personal preferences, ways to overcome these barriers and encourage adherence might be supervised exercises in an individual or group setting or audiotapes or videos of recommended exercises.10
Chronic low back pain
Chronic low back pain (LBP) is a large socioeconomic burden in the United States, with upward of $100 billion per year accounted for in health care costs and decreased worker productivity.13 The etiology of chronic LBP can be multifactorial and due to any of several conditions such as degenerative disc disease, spinal stenosis, spondylolisthesis, and facet arthropathy. Treatment is difficult, given that many common interventions—medications, massage, manipulation—have limited efficacy.14 However, for patients with nonspecific chronic LBP, exercise is an effective intervention for reducing pain and improving physical function.15
An effective approach is to design an exercise regimen for the individual by type, duration, and frequency of activity, administered under supervision to encourage adherence.16 Appropriate exercises emphasize resistance, strength training, and core stabilization, often focusing on whole body and trunk motion (FIGURE 2).17

Although yoga or Pilates classes may have a small effect on function, no high-quality evidence exists for their superiority to other forms of exercise.18,19 Back School, a therapeutic program that includes education on anatomy and biomechanics, optimal posture, ergonomics, and back exercises, has limited, low-quality evidence for treatment comparisons.20 Aerobic exercise, including treadmill, elliptical, or cycling exercises or walking outdoors can reduce pain and improve physical and psychologic functioning.21
Continue to: The most common reported adverse effect...
The most common reported adverse effect of exercise is a temporary exacerbation of back pain. However, having patients continue daily activities within the permitted limits of pain leads to more rapid recovery than rest or back-mobilizing exercises.15,22,23
Cautions. Exercise is contraindicated in patients with LBP arising from a serious medical condition, such as fracture, infection, cancer, or cauda equina syndrome.24 Importantly, exercise interventions recommended for acute LBP have not shown benefit for chronic LBP.
Chronic shoulder pain
With a prevalence ranging from 7% to 26% in the general population,25 chronic shoulder pain often interferes with essential activities of daily living. The etiology of chronic shoulder pain is broad and most commonly involves disorders of the rotator cuff, which functions in both motion and dynamic stabilization of the shoulder. The common term “rotator cuff pain syndrome” can cover such disorders as subacromial impingement syndrome, rotator cuff tendinopathy or tendinitis, partial or full thickness rotator cuff tears, calcific tendinitis, and subacromial bursitis. These pathologies may have overlapping presentations. Manual therapy and exercise, usually delivered as a component of structured physical therapy, focus on stretches and other exercises to increase ROM, stability, and strength of the rotator cuff musculature.26
A 2016 Cochrane review that evaluated manual therapy and exercise for chronic shoulder pain yielded limited high-quality evidence for effectiveness compared with placebo.27 Five trials found no important differences between manual therapy and exercise compared with glucocorticoid injection relative to overall pain, function, active shoulder abduction, and quality of life from 4 weeks up to 12 months.27 But compared with placebo, exercise has been more effective in reducing reported pain, especially in the context of strengthening regimens focused on flexion, extension, and internal and external rotation.28

For subacromial impingement syndrome, a 2017 meta-analysis found that a generalized exercise program relieves pain and improves function, ROM, and strength.29 A generalized shoulder-strengthening program includes exercises that focus on internal and external rotation, horizontal abduction, and shoulder stabilization (FIGURE 3). These exercises can be completed with 3 sets of 15 to 20 repetitions, which create a fatigue response that improves strength and targets local muscular endurance.30

Continue to: Achilles tendinopathy
Achilles tendinopathy
Achilles tendinopathy (also referred to as chronic Achilles tendinitis) is a degenerative condition of the Achilles tendon related to overuse that leads to pain, swelling, and impaired performance. It accounts for approximately 18% of injuries in runners and 4% of all patients presenting to sports medicine clinics.31 Eccentric muscle loading has become the dominant conservative intervention strategy for chronic Achilles tendinopathy.
For chronic tendinopathies, eccentric exercises subject greater force than concentric exercises through a controlled lengthening of a muscle-tendon unit, resulting in a greater remodeling stimulus of the tendon.32 Classically, the Alfredson protocol has been used to treat chronic Achilles tendinopathy. This program of eccentric heel-drop exercises recommends completion of 180 eccentric repetitions a day for up to 12 weeks (FIGURE 4).33 Exercises are performed slowly, and load can be increased when exercises are performed without pain or perhaps with mild nondisabling pain.

A variation of this protocol has allowed a gradual escalation of repetitions over a week up to the recommended 180 repetitions, and has shown improvements in pain reduction and function similar to that achieved with the primary protocol.34 Additionally, a 6-week “do as tolerated” program of eccentric exercises did not lead to lesser improvement for individuals with midportion Achilles tendinopathy.35
Several systematic reviews have supported the use of eccentric exercises for chronic Achilles tendinopathy,31,36,37 but no specific protocol or exercise regimen has demonstrated superiority. However, with the Alfredson protocol, improvement in pain and function in patients with chronic Achilles tendinopathy has persisted for up to 5 years.38
Lateral epicondylitis
Lateral epicondylitis (also called lateral epicondylosis or “tennis elbow”) is a disabling musculoskeletal condition that leads to pain and tenderness around the extensor mass of the lateral elbow. It is caused by microtrauma to the tendon, usually sustained through repetitive movement in a sporting activity, industrial work, or hobby. Affecting up to 3% of the US population, lateral epicondylitis is associated with pain and functional disability, as well as emotional and psychosocial consequences.39
Continue to: Proposed treatment and rehabilitation options...
Proposed treatment and rehabilitation options for patients with lateral epicondylitis have included massage, manipulation, taping, acupuncture, orthotic devices, ultrasound, activity modification, and rest. Exercise programs incorporating eccentric muscle activity are becoming increasingly popular for such conditions as Achilles and patellar tendinopathies, and they may translate well to other chronic tendinopathies, such as lateral epicondylitis.32
An eccentric exercise program for lateral epicondylitis, either in isolation or as an adjunct to other therapies, has decreased pain and improved function and grip strength from baseline measures.40 Compared with a standard exercise regimen without eccentric strength training, use of eccentric training improves such clinical measures as pain intensity and disability status, as it decreases tendon thickness and aids in recovering homogenous tendon structure.41
A sample exercise. The patient may sit in a chair and, with the forearm flexed and pronated over the edge of a table, grasp some form of resistance (bucket of water, training weight, resistance band) (FIGURE 5). The nonaffected hand can be used to help lift the affected wrist into full extension and then removed to allow lowering of the hand over several seconds into flexion. This activity can be performed in sets of 8 to 12 repetitions, 2 to 3 times a day, until the patient’s pain and function have improved.42

Overcoming barriers to exercise
A major concern across all studies assessing the therapeutic value of exercise is patient compliance and adherence to prescribed programs. Compliance and adherence are affected in part by psychosocial factors such as low literacy and poor social support. From a physician’s perspective, direct and indirect costs of treatment and rehabilitation of chronic musculoskeletal conditions may discourage the prescribing of supervised physical therapy.3
Steps to consider in overcoming these barriers would be advising an exercise regimen that requires only an initial period of supervision; educating patients about the benefits of an exercise program; exploring a patient’s expectations, beliefs, and fears; and developing strategies for long-term adherence.16 Supervision through physical therapy is often suggested. However, significant barriers may exist that impede a patient’s ability to attend or participate, in which case physician observation in the course of regularly scheduled clinical examinations could be considered.
Continue to: When prescribing exercises...
When prescribing exercises, be sure to address patient expectations regarding pain, duration, and limitations of exercise. It would be helpful for patients to know, for instance, that working through mild-to-moderate pain during exercise has been shown to shorten post-exercise recovery time and, in the short-term, improve relief from pain.43
Tailoring specific exercise prescriptions for a patient will make the regimen more satisfying for the individual and optimize adherence, which in turn will increase the potential for pain reduction and improved function. Secondary benefits would likely be weight loss and prevention (or regression) of cardiovascular disease. Continued evaluation by the physician or physical therapist should be part of ongoing management, as well as “refresher courses” to ensure understanding of, and adherence to, the exercise program. The potential benefits and limited risks of exercise, if done properly, make it a primary intervention for specific musculoskeletal conditions.
CORRESPONDENCE
Peter J. Carek, MD, MS, Department of Community Health and Family Medicine, College of Medicine, University of Florida, PO Box 100237, Gainesville, FL 32610-0237; [email protected].
Regular exercise confers several well-established benefits. In such conditions as coronary heart disease, stroke, heart failure, and diabetes, exercise has led to a reduction in mortality similar to that seen with pharmacotherapy.1 For patients with chronic musculoskeletal conditions, the benefits of exercise-based interventions are measurably reduced pain and improved daily function.2 However, prescribing of exercise is often neglected, with preference given to pharmacologic or surgical interventions.3 In part, the disregard of exercise as therapy results from unfamiliarity with appropriate exercise prescriptions,3 which include various forms of aerobic exercise, strength training, and stretching to increase flexibility (TABLE).

As is true of many therapeutic modalities, exercise must be tailored to the condition and to a patient’s preferences to optimize its benefits. In this review, we describe exercise regimens well suited for common musculoskeletal conditions, examine the effectiveness of exercise in each condition, and provide examples for use in treating patients.
Osteoarthritis of the hip and knee
Osteoarthritis (OA), one of the most common chronic joint diseases, erodes the articular cartilage and subchondral bone of a synovial joint, eventually leading to joint failure. Pain and diminished muscle strength restrict physical activity and can lead to decreased fitness and impaired muscle function. Exercise helps reduce pain and improve muscle function and quality of life in patients with hip or knee OA regardless of age, disease severity, or level of pain and dysfunction.2
Knee exercises. Activities suitable for patients with OA include muscle strengthening, aerobic conditioning, and range-of-motion (ROM) exercises.4-6 A 2015 Cochrane review of OA of the knee showed that exercise reduced pain and improved physical function and quality of life in patients who completed a treatment program, and that pain relief persisted up to 6 months after intervention.5
When designing an exercise prescription for patients with knee OA, consider quadriceps strengthening with an initial period of supervision, which may provide greater pain relief than nonspecific, unsupervised lower limb exercises.4 Enhanced strength of the lower limb may lessen force through the knee, thereby decreasing pain and improving overall physical function.7 Simple, teachable exercises include squats, step-ups, knee extension/flexion while sitting in a chair, and hip abduction/adduction while standing or lying down. Elastic bands, dumbbells, or cuff weights may be used to increase resistance.
Hip exercises. Exercise can significantly reduce pain and improve function for up to 6 months for patients with mild-to-moderate symptomatic hip OA.6 Types of exercise for hip OA include strength training of hip and core muscles, functional exercises that imitate movements in daily activities, and flexibility training. These exercises help reduce pain and increase ROM. Exercise should include resistance training and should not exceed the limit for acceptable pain.8
Aquatic therapy is also appropriate for exercise and strength training and can decrease pain and disability and improve quality of life.9 Supervised physical therapy, including strength training, manual therapy, and balance training, are important for reducing pain and improving function. Physical therapy can also enhance adherence to a prescribed exercise program.10
Continue to: Appropriate exercise prescriptions...
Appropriate exercise prescriptions for patients with knee or hip OA should focus on low-impact activities that can improve strength, flexibility, and function (FIGURE 1). A typical regimen would be 30 or more cumulative minutes daily of stationary cycling, water-based exercises, or strength training, 3 to 5 days per week. Individualize workout intensity for each patient, emphasizing that high-intensity, low-impact effort may yield greater strength gains and take less time to perform.11 A high-intensity exercise prescription focusing on quadriceps, hip, and core strengthening may consist of 3 sets of 8 repetitions with resistance set at 40% of the maximum resistance against which the patient can perform 1 repetition.7

Barriers to exercise in knee and hip OA include negative patient and provider perspectives on exercise and patients’ fear that increased activity may actually worsen OA.12 Depending on a patient’s personal preferences, ways to overcome these barriers and encourage adherence might be supervised exercises in an individual or group setting or audiotapes or videos of recommended exercises.10
Chronic low back pain
Chronic low back pain (LBP) is a large socioeconomic burden in the United States, with upward of $100 billion per year accounted for in health care costs and decreased worker productivity.13 The etiology of chronic LBP can be multifactorial and due to any of several conditions such as degenerative disc disease, spinal stenosis, spondylolisthesis, and facet arthropathy. Treatment is difficult, given that many common interventions—medications, massage, manipulation—have limited efficacy.14 However, for patients with nonspecific chronic LBP, exercise is an effective intervention for reducing pain and improving physical function.15
An effective approach is to design an exercise regimen for the individual by type, duration, and frequency of activity, administered under supervision to encourage adherence.16 Appropriate exercises emphasize resistance, strength training, and core stabilization, often focusing on whole body and trunk motion (FIGURE 2).17

Although yoga or Pilates classes may have a small effect on function, no high-quality evidence exists for their superiority to other forms of exercise.18,19 Back School, a therapeutic program that includes education on anatomy and biomechanics, optimal posture, ergonomics, and back exercises, has limited, low-quality evidence for treatment comparisons.20 Aerobic exercise, including treadmill, elliptical, or cycling exercises or walking outdoors can reduce pain and improve physical and psychologic functioning.21
Continue to: The most common reported adverse effect...
The most common reported adverse effect of exercise is a temporary exacerbation of back pain. However, having patients continue daily activities within the permitted limits of pain leads to more rapid recovery than rest or back-mobilizing exercises.15,22,23
Cautions. Exercise is contraindicated in patients with LBP arising from a serious medical condition, such as fracture, infection, cancer, or cauda equina syndrome.24 Importantly, exercise interventions recommended for acute LBP have not shown benefit for chronic LBP.
Chronic shoulder pain
With a prevalence ranging from 7% to 26% in the general population,25 chronic shoulder pain often interferes with essential activities of daily living. The etiology of chronic shoulder pain is broad and most commonly involves disorders of the rotator cuff, which functions in both motion and dynamic stabilization of the shoulder. The common term “rotator cuff pain syndrome” can cover such disorders as subacromial impingement syndrome, rotator cuff tendinopathy or tendinitis, partial or full thickness rotator cuff tears, calcific tendinitis, and subacromial bursitis. These pathologies may have overlapping presentations. Manual therapy and exercise, usually delivered as a component of structured physical therapy, focus on stretches and other exercises to increase ROM, stability, and strength of the rotator cuff musculature.26
A 2016 Cochrane review that evaluated manual therapy and exercise for chronic shoulder pain yielded limited high-quality evidence for effectiveness compared with placebo.27 Five trials found no important differences between manual therapy and exercise compared with glucocorticoid injection relative to overall pain, function, active shoulder abduction, and quality of life from 4 weeks up to 12 months.27 But compared with placebo, exercise has been more effective in reducing reported pain, especially in the context of strengthening regimens focused on flexion, extension, and internal and external rotation.28

For subacromial impingement syndrome, a 2017 meta-analysis found that a generalized exercise program relieves pain and improves function, ROM, and strength.29 A generalized shoulder-strengthening program includes exercises that focus on internal and external rotation, horizontal abduction, and shoulder stabilization (FIGURE 3). These exercises can be completed with 3 sets of 15 to 20 repetitions, which create a fatigue response that improves strength and targets local muscular endurance.30

Continue to: Achilles tendinopathy
Achilles tendinopathy
Achilles tendinopathy (also referred to as chronic Achilles tendinitis) is a degenerative condition of the Achilles tendon related to overuse that leads to pain, swelling, and impaired performance. It accounts for approximately 18% of injuries in runners and 4% of all patients presenting to sports medicine clinics.31 Eccentric muscle loading has become the dominant conservative intervention strategy for chronic Achilles tendinopathy.
For chronic tendinopathies, eccentric exercises subject greater force than concentric exercises through a controlled lengthening of a muscle-tendon unit, resulting in a greater remodeling stimulus of the tendon.32 Classically, the Alfredson protocol has been used to treat chronic Achilles tendinopathy. This program of eccentric heel-drop exercises recommends completion of 180 eccentric repetitions a day for up to 12 weeks (FIGURE 4).33 Exercises are performed slowly, and load can be increased when exercises are performed without pain or perhaps with mild nondisabling pain.

A variation of this protocol has allowed a gradual escalation of repetitions over a week up to the recommended 180 repetitions, and has shown improvements in pain reduction and function similar to that achieved with the primary protocol.34 Additionally, a 6-week “do as tolerated” program of eccentric exercises did not lead to lesser improvement for individuals with midportion Achilles tendinopathy.35
Several systematic reviews have supported the use of eccentric exercises for chronic Achilles tendinopathy,31,36,37 but no specific protocol or exercise regimen has demonstrated superiority. However, with the Alfredson protocol, improvement in pain and function in patients with chronic Achilles tendinopathy has persisted for up to 5 years.38
Lateral epicondylitis
Lateral epicondylitis (also called lateral epicondylosis or “tennis elbow”) is a disabling musculoskeletal condition that leads to pain and tenderness around the extensor mass of the lateral elbow. It is caused by microtrauma to the tendon, usually sustained through repetitive movement in a sporting activity, industrial work, or hobby. Affecting up to 3% of the US population, lateral epicondylitis is associated with pain and functional disability, as well as emotional and psychosocial consequences.39
Continue to: Proposed treatment and rehabilitation options...
Proposed treatment and rehabilitation options for patients with lateral epicondylitis have included massage, manipulation, taping, acupuncture, orthotic devices, ultrasound, activity modification, and rest. Exercise programs incorporating eccentric muscle activity are becoming increasingly popular for such conditions as Achilles and patellar tendinopathies, and they may translate well to other chronic tendinopathies, such as lateral epicondylitis.32
An eccentric exercise program for lateral epicondylitis, either in isolation or as an adjunct to other therapies, has decreased pain and improved function and grip strength from baseline measures.40 Compared with a standard exercise regimen without eccentric strength training, use of eccentric training improves such clinical measures as pain intensity and disability status, as it decreases tendon thickness and aids in recovering homogenous tendon structure.41
A sample exercise. The patient may sit in a chair and, with the forearm flexed and pronated over the edge of a table, grasp some form of resistance (bucket of water, training weight, resistance band) (FIGURE 5). The nonaffected hand can be used to help lift the affected wrist into full extension and then removed to allow lowering of the hand over several seconds into flexion. This activity can be performed in sets of 8 to 12 repetitions, 2 to 3 times a day, until the patient’s pain and function have improved.42

Overcoming barriers to exercise
A major concern across all studies assessing the therapeutic value of exercise is patient compliance and adherence to prescribed programs. Compliance and adherence are affected in part by psychosocial factors such as low literacy and poor social support. From a physician’s perspective, direct and indirect costs of treatment and rehabilitation of chronic musculoskeletal conditions may discourage the prescribing of supervised physical therapy.3
Steps to consider in overcoming these barriers would be advising an exercise regimen that requires only an initial period of supervision; educating patients about the benefits of an exercise program; exploring a patient’s expectations, beliefs, and fears; and developing strategies for long-term adherence.16 Supervision through physical therapy is often suggested. However, significant barriers may exist that impede a patient’s ability to attend or participate, in which case physician observation in the course of regularly scheduled clinical examinations could be considered.
Continue to: When prescribing exercises...
When prescribing exercises, be sure to address patient expectations regarding pain, duration, and limitations of exercise. It would be helpful for patients to know, for instance, that working through mild-to-moderate pain during exercise has been shown to shorten post-exercise recovery time and, in the short-term, improve relief from pain.43
Tailoring specific exercise prescriptions for a patient will make the regimen more satisfying for the individual and optimize adherence, which in turn will increase the potential for pain reduction and improved function. Secondary benefits would likely be weight loss and prevention (or regression) of cardiovascular disease. Continued evaluation by the physician or physical therapist should be part of ongoing management, as well as “refresher courses” to ensure understanding of, and adherence to, the exercise program. The potential benefits and limited risks of exercise, if done properly, make it a primary intervention for specific musculoskeletal conditions.
CORRESPONDENCE
Peter J. Carek, MD, MS, Department of Community Health and Family Medicine, College of Medicine, University of Florida, PO Box 100237, Gainesville, FL 32610-0237; [email protected].
1. Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577.
2. Babatunde OO, Jordan JL, van der Windt DA, et al. Effective treatment options for musculoskeletal pain in primary care: a systematic overview of current evidence. PLoS One. 2017;12:e0178621.
3. Persson G, Brorsson A, Ekvall Hansson E, et al. Physical activity on prescription (PAP) from the general practitioner’s perspective - a qualitative study. BMC Fam Pract. 2013;14:128.
4. Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622-636.
5. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376.
6. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;(4):CD007912.
7. Vincent KR, Vincent HK. Resistance exercise for knee osteoarthritis. PM R. 2012;4(suppl 5):S45-S52.
8. Fernandes L, Storheim K, Nordsletten L, et al. Development of a therapeutic exercise program for patients with osteoarthritis of the hip. Phys Ther. 2010;90:592-601.
9. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
10. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010;(1):CD005956.
11. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
12. Brakke R, Singh J, Sullivan W. Physical therapy in persons with osteoarthritis. PM R. 2012;4:S53-S58.
13. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21-24.
14. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
15. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;(3):CD000335.
16. Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776-785.
17. Searle A, Spink M, Ho A, et al. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. 2015;29:1155-1167.
18. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;(7):CD010265.
19. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(1):CD010671.
20. Parreira P, Heymans MW, van Tulder MW, et al. Back Schools for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(8):CD011674.
21. Meng XG, Yue SW. Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil. 2015;94:358-365.
22. Malmivaara A, Häkkinen U, Aro T, et al. The treatment of acute low back pain--bed rest, exercises, or ordinary activity? N Engl J Med. 1995;332:351-355.
23. van Tulder M, Malmivaara A, Esmail R, Koes B. Exercise therapy for low back pain: a systematic review within the framework of the cochrane collaboration back review group. Spine (Phila Pa 1976). 2000;25:2784-2796.
24. Hoffmann TC, Maher CG, Briffa T, et al. Prescribing exercise interventions for patients with chronic conditions. CMAJ. 2016;188:510-518.
25. Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33:73-81.
26. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18:138-160.
27. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
28. van den Dolder PA, Ferreira PH, Refshauge KM. Effectiveness of soft tissue massage and exercise for the treatment of non-specific shoulder pain: a systematic review with meta-analysis. Br J Sports Med. 2014;48:1216-1226.
29. Shire AR, Stæhr TAB, Overby JB, et al. Specific or general exercise strategy for subacromial impingement syndrome-does it matter? A systematic literature review and meta analysis. BMC Musculoskelet Disord. 2017;18:158.
30. Ellenbecker TS, Cools A. Rehabilitation of shoulder impingement syndrome and rotator cuff injuries: an evidence-based review. Br J Sports Med. 2010;44:319-327.
31. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
32. Rees JD, Wolman RL, Wilson A. Eccentric exercises; why do they work, what are the problems and how can we improve them? Br J Sports Med. 2009;43:242-246.
33. Alfredson H, Pietilä T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26:360-366.
34. Rompe JD, Nafe B, Furia JP, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35:374-383.
35. Stevens M, Tan CW. Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther. 2014;44:59-67.
36. Habets B, van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports. 2015;25:3-15.
37. Malliaras P, Barton CJ, Reeves ND, et al. Achilles and patellar tendinopathy loading programmes : a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013;43:267-286.
38. van der Plas A, de Jonge S, de Vos RJ, et al. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46:214-218.
39. Alizadehkhaiyat O, Fisher AC, Kemp GJ, et al. Pain, functional disability, and psychologic status in tennis elbow. Clin J Pain. 2007;23:482-489.
40. Cullinane FL, Boocock MG, Trevelyan FC. Is eccentric exercise an effective treatment for lateral epicondylitis? A systematic review. Clin Rehabil. 2014;28:3-19.
41. Croisier JL, Foidart-Dessalle M, Tinant F, et al. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy. Br J Sports Med. 2007;41:269-275.
42. Söderberg J, Grooten WJ, Ang BO. Effects of eccentric training on hand strength in subjects with lateral epicondylalgia: a randomized-controlled trial. Scand J Med Sci Sports. 2012;22:797-803.
43. Smith BE, Hendrick P, Smith TO, et al. Should exercises be painful in the management of chronic musculoskeletal pain? A systematic review and meta-analysis. Br J Sports Med. 2017;51:1679-1687.
1. Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577.
2. Babatunde OO, Jordan JL, van der Windt DA, et al. Effective treatment options for musculoskeletal pain in primary care: a systematic overview of current evidence. PLoS One. 2017;12:e0178621.
3. Persson G, Brorsson A, Ekvall Hansson E, et al. Physical activity on prescription (PAP) from the general practitioner’s perspective - a qualitative study. BMC Fam Pract. 2013;14:128.
4. Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622-636.
5. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376.
6. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;(4):CD007912.
7. Vincent KR, Vincent HK. Resistance exercise for knee osteoarthritis. PM R. 2012;4(suppl 5):S45-S52.
8. Fernandes L, Storheim K, Nordsletten L, et al. Development of a therapeutic exercise program for patients with osteoarthritis of the hip. Phys Ther. 2010;90:592-601.
9. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
10. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010;(1):CD005956.
11. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
12. Brakke R, Singh J, Sullivan W. Physical therapy in persons with osteoarthritis. PM R. 2012;4:S53-S58.
13. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21-24.
14. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
15. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;(3):CD000335.
16. Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776-785.
17. Searle A, Spink M, Ho A, et al. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. 2015;29:1155-1167.
18. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;(7):CD010265.
19. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(1):CD010671.
20. Parreira P, Heymans MW, van Tulder MW, et al. Back Schools for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(8):CD011674.
21. Meng XG, Yue SW. Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil. 2015;94:358-365.
22. Malmivaara A, Häkkinen U, Aro T, et al. The treatment of acute low back pain--bed rest, exercises, or ordinary activity? N Engl J Med. 1995;332:351-355.
23. van Tulder M, Malmivaara A, Esmail R, Koes B. Exercise therapy for low back pain: a systematic review within the framework of the cochrane collaboration back review group. Spine (Phila Pa 1976). 2000;25:2784-2796.
24. Hoffmann TC, Maher CG, Briffa T, et al. Prescribing exercise interventions for patients with chronic conditions. CMAJ. 2016;188:510-518.
25. Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33:73-81.
26. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18:138-160.
27. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
28. van den Dolder PA, Ferreira PH, Refshauge KM. Effectiveness of soft tissue massage and exercise for the treatment of non-specific shoulder pain: a systematic review with meta-analysis. Br J Sports Med. 2014;48:1216-1226.
29. Shire AR, Stæhr TAB, Overby JB, et al. Specific or general exercise strategy for subacromial impingement syndrome-does it matter? A systematic literature review and meta analysis. BMC Musculoskelet Disord. 2017;18:158.
30. Ellenbecker TS, Cools A. Rehabilitation of shoulder impingement syndrome and rotator cuff injuries: an evidence-based review. Br J Sports Med. 2010;44:319-327.
31. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
32. Rees JD, Wolman RL, Wilson A. Eccentric exercises; why do they work, what are the problems and how can we improve them? Br J Sports Med. 2009;43:242-246.
33. Alfredson H, Pietilä T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26:360-366.
34. Rompe JD, Nafe B, Furia JP, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35:374-383.
35. Stevens M, Tan CW. Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther. 2014;44:59-67.
36. Habets B, van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports. 2015;25:3-15.
37. Malliaras P, Barton CJ, Reeves ND, et al. Achilles and patellar tendinopathy loading programmes : a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013;43:267-286.
38. van der Plas A, de Jonge S, de Vos RJ, et al. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46:214-218.
39. Alizadehkhaiyat O, Fisher AC, Kemp GJ, et al. Pain, functional disability, and psychologic status in tennis elbow. Clin J Pain. 2007;23:482-489.
40. Cullinane FL, Boocock MG, Trevelyan FC. Is eccentric exercise an effective treatment for lateral epicondylitis? A systematic review. Clin Rehabil. 2014;28:3-19.
41. Croisier JL, Foidart-Dessalle M, Tinant F, et al. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy. Br J Sports Med. 2007;41:269-275.
42. Söderberg J, Grooten WJ, Ang BO. Effects of eccentric training on hand strength in subjects with lateral epicondylalgia: a randomized-controlled trial. Scand J Med Sci Sports. 2012;22:797-803.
43. Smith BE, Hendrick P, Smith TO, et al. Should exercises be painful in the management of chronic musculoskeletal pain? A systematic review and meta-analysis. Br J Sports Med. 2017;51:1679-1687.
From The Journal of Family Practice | 2018;67(9):534-538,540-543.
PRACTICE RECOMMENDATIONS
› Consider quadriceps strengthening for knee osteoarthritis with an initial period of supervision, which can provide greater pain relief than nonspecific, unsupervised lower limb exercises. B
› Consider a generalized exercise program for subacromial impingement syndrome, to relieve shoulder pain and improve function, range of motion, and strength. A
› Bear in mind that the Alfredson protocol for Achilles tendinopathy has yielded improvement in pain and function for up to 5 years, although other exercise regimens have also proven initially effective. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Buprenorphine to treat opioid use disorder: A practical guide
Opioids were involved in 42,249 deaths in the United States in 2016, and opioid overdoses have quintupled since 1999.1 Among the causes behind these statistics is increased opiate prescribing by physicians—with primary care providers accounting for about one half of opiate prescriptions.2 As a result, the Centers for Disease Control and Prevention has issued a 4-part response for physicians,3 which includes careful opiate prescribing, expanded access to naloxone, prevention of opioid use disorder (OUD), and expanded use of medication-assisted treatment (MAT) of addiction—with the goal of preventing and managing OUD.
CASE
Fred R, a 55-year-old man who has been taking oxycodone, 70 mg/d, for chronic pain for longer than 10 years, visits your clinic for a prescription refill. His prescription monitoring program confirms the long history of regular oxycodone use, with the dosage escalating over the past 6 months. He recently was discharged from the hospital after an overdose of opiates.
Mr. R admits to using heroin after running out of oxycodone. He is in mild withdrawal, with a score of 8 (of a possible 48) on the Clinical Opioid Withdrawal Scale4 (COWS, which assigns point values to 11 common symptoms to gauge the severity of opioid withdrawal and, by inference, the patient’s degree of physical dependence). You determine that Mr. R is frightened about his use of oxycodone and would like to stop; he has tried to stop several times on his own but always relapses when withdrawal becomes severe.
How would you proceed with the care of this patient?
What is OUD? How is the diagnosis made?
OUD is a combination of cognitive, behavioral, and physiologic symptoms arising from continued use of opioids despite significant health, legal, or relationship problems related to their use. The disorder is diagnosed based on specific criteria provided in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)(TABLE 1)5 and is revealed by 1) a careful history that delineates a problematic pattern of opioid use, 2) physical examination, and 3) urine toxicology screen.
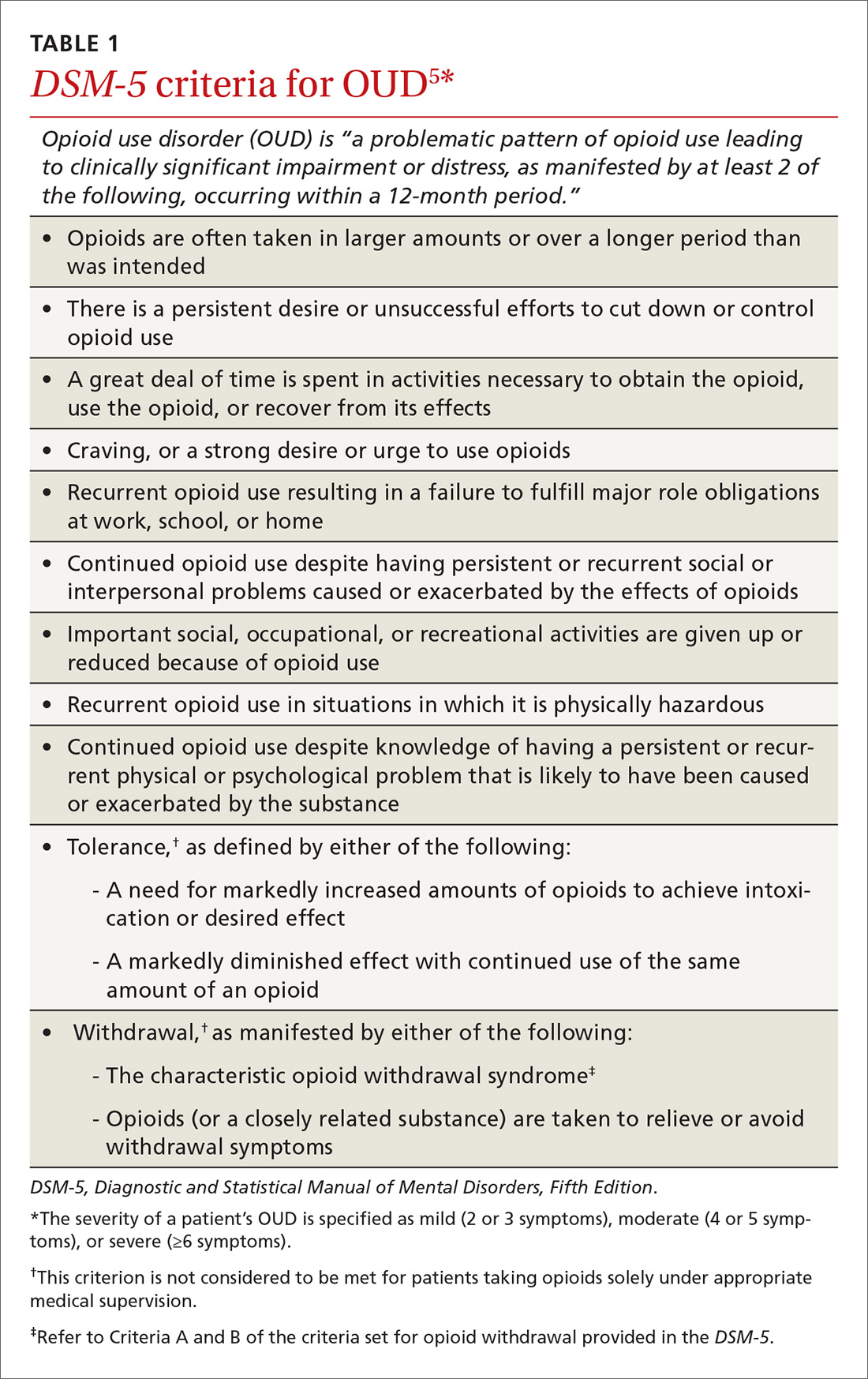
Identification of acute opioid intoxication can also be useful when working up a patient in whom OUD is suspected; findings of acute opioid intoxication on physical examination include constricted pupils, head-nodding, excessive sleepiness, and drooping eyelids. Other physical signs of illicit opioid use include track marks around veins of the arm, evidence of repeated trauma, and stigmata of liver dysfunction. Withdrawal can present as agitation, rhinorrhea, dilated pupils, nausea, diarrhea, yawning, and gooseflesh. The COWS, which, as noted in the case, assigns point values to withdrawal symptoms, can be helpful in determining the severity of withdrawal.4
What is the differential Dx of OUD?
When OUD is likely, but not clearly diagnosable, on the basis of findings, consider a mental health disorder: depressive disorder, bipolar disorder, attention deficit–hyperactivity disorder, personality disorder, and polysubstance use disorder. Concurrent diagnosis of substance abuse and a mental health disorder is common; treatment requires that both disorders be addressed simultaneously.6 Assessing for use or abuse of, and addiction to, other substances is vital to ensure proper diagnosis and effective therapy. Polysubstance dependence can be more difficult to treat than single-substance abuse or addiction alone.
Continue to: How is OUD treated?
How is OUD treated?
This article reviews MAT with buprenorphine; other MAT options include methadone and naltrexone. Regardless of the indicated agent chosen, MAT has been shown to be superior to abstinence alone or abstinence with counseling interventions in maintaining sobriety.7
Evidence of efficacy. In a longitudinal cohort study of patients who received MAT with buprenorphine initiated in general practice, patients in whom buprenorphine therapy was interrupted had a greatly increased risk of all-cause mortality (hazard ratio=29.04; 95% confidence interval, 10.04-83.99).8 The study highlights the harm-reduction treatment philosophy of MAT with buprenorphine: The regimen can be used to keep a patient alive while working toward sobriety.
We encourage physicians to treat addiction as they would any chronic disease. The strategy includes anticipating relapse, engaging support systems (eg, family, counselors, social groups, Alcoholics Anonymous, Narcotics Anonymous [NA]), and working with the patient to obtain a higher level of care, as indicated.
Pharmacology and induction. Alone or in combination with naloxone, buprenorphine can be used as in-office-based MAT. Buprenorphine is a partial opiate agonist that binds tightly to opioid receptors and can block the effects of other opiates. An advantage of buprenorphine is its low likelihood of overdose, due to the drug’s so-called ceiling effect at a dosage of 24 mg/d;9 dosages above this amount have little increased medication effect.
Dosing of buprenorphine is variable from patient to patient, with a maximum dosage of 24 mg/d. Therapy can be initiated safely at home, although some physicians prefer in-office induction. It is important that the patient be in moderate withdrawal (as determined by the score on the COWS) before initiation, because buprenorphine, as a partial agonist, can precipitate withdrawal by displacing full opiate agonists from opioid receptors.
Continue to: In our experience...
In our experience, a common induction method is to give 2 to 4 mg buprenorphine, followed by a 1-hour assessment of withdrawal symptoms. This can be repeated for multiple doses until withdrawal is relieved, usually with a maximum dosage of 6 to 8 mg in the initial 1 or 2 days of treatment. Rapid reassessment is required after induction, preferably in 1 to 3 days. Dosing should be gradually increased in 2- to 4-mg increments until 1) the patient has no withdrawal symptoms in a 24-hour period and 2) craving for opiates is adequately controlled.
Note: Primary care physicians must complete an 8-hour online training course to obtain a US Drug Enforcement Administration waiver to prescribe buprenorphine.
How should coordination of care be approached?
Actual prescribing and monitoring of buprenorphine is not complex, but many physicians are intimidated by the perceived difficulty of coordination of care. The American Society of Addiction Medicine's national practice guideline recommends that buprenorphine and other MAT protocols be offered as a part of a comprehensive treatment plan that includes psychosocial treatment.7 This combination leads to the greatest potential for ongoing remission of OUD. Although many primary care clinics do not have chemical dependency counseling available at their primary location, partnering with community organizations and other mental health resources can meet this need. Coordination of care with home services, behavioral health, and psychiatry is common in primary care, and is no different for OUD.
There are administrative requirements for a clinic that offers MAT (TABLE 2),7 including tracking of numbers of patients who are taking buprenorphine. During the first year of prescribing buprenorphine, a physician or other provider is permitted to care for only 30 patients; once the first year has passed, that provider can apply to care for as many as 100 patients. In addition, the Drug Enforcement Administration might conduct site visits to ensure that proper documentation and tracking of patients is being undertaken. These requirements can seem daunting, but careful monitoring of patient panels can alleviate concerns. For clinics that use an electronic medical record, we recommend developing the capability to pull lists by either buprenorphine prescriptions or diagnosis codes.
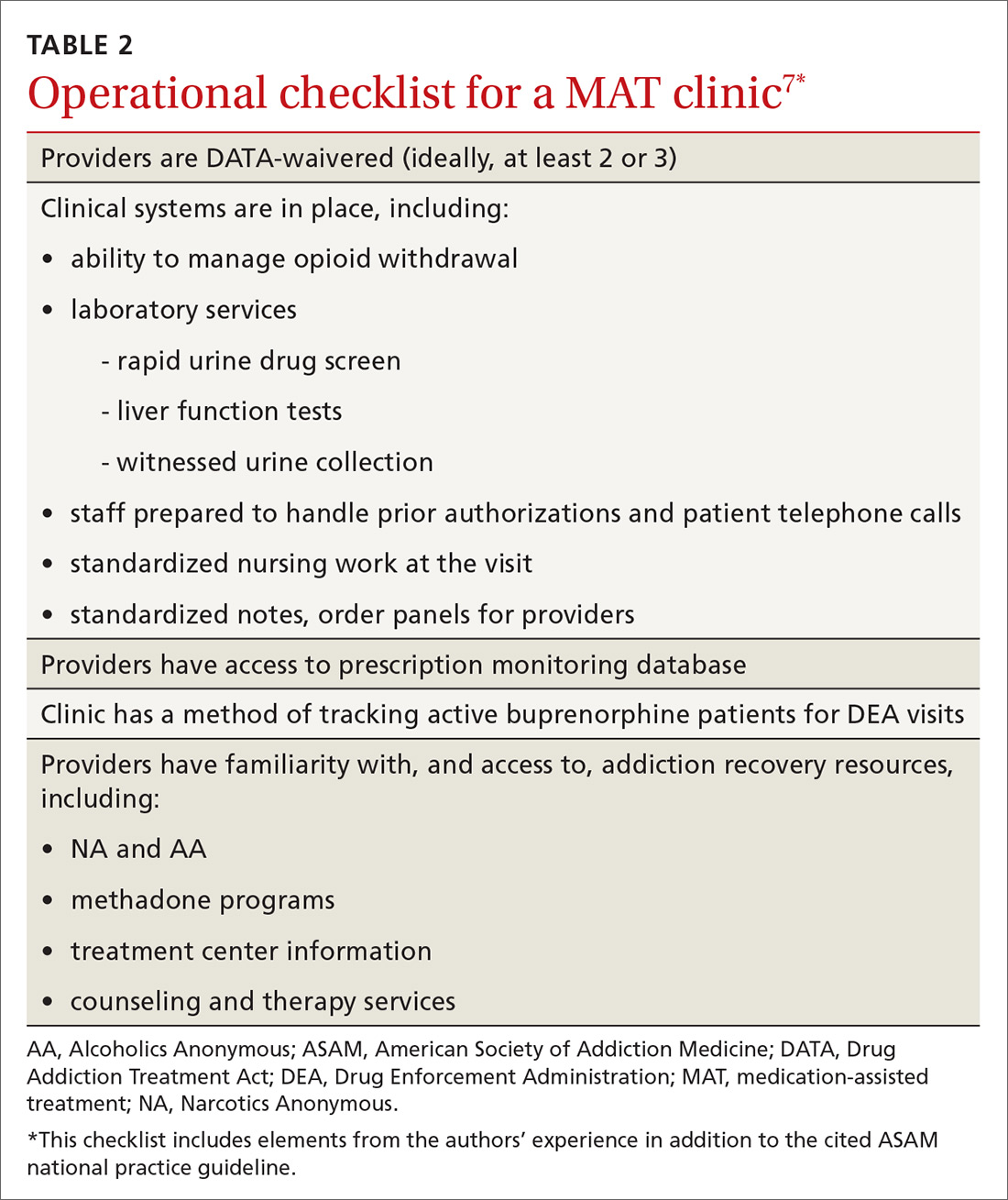
Continue to: CASE
CASE
After you and Mr. R discuss his addiction, you decide to initiate treatment that includes buprenorphine. You have a specimen collected for a urine toxicology screen and blood drawn for a baseline liver function panel, hepatitis panel, and human immunodeficiency virus screen, and provide him with resources (nearby treatment center, an NA meeting location) for treating OUD. You write a prescription for #8 buprenorphine and naloxone, 2 mg/0.5 mg films, and instruct Mr. R to: take 1 film when withdrawal symptoms become worse; wait 1 hour; and take another film if he is still experiencing withdrawal symptoms. He can repeat this dosing regimen until he reaches 8 mg/d of buprenorphine (4 films). You schedule follow-up in 2 days.
At follow-up, the patient reports that taking 3 films alleviated withdrawal symptoms, but that symptoms returned approximately 12 hours later, at which time he took the fourth film. This helped him through until the next day, when he again took 3 films in the morning and 1 film in the late evening. He feels that this regimen is helping relieve withdrawal symptoms and cravings. You provide a prescription for buprenorphine and naloxone, 8 mg/2 mg daily, and request a follow-up visit in 5 days.
At the next visit, Mr. R reports that he still has cravings for oxycodone. You increase the dosage of buprenorphine and naloxone to 12 mg/3 mg daily.
At the next visit, he reports no longer having cravings.
You continue to monitor Mr. R with urine drug screening and discussion of his recovery with the help of his family and support network. After 3 months of consistent visits, he fails to show up for his every-2-or-3-week appointment.
Continue to: Four days later...
Four days later, Mr. R shows up at the clinic, apologizing for missing the appointment and assuring you that this won’t happen again. Rapid urine drug screening is positive for morphine. When confronted, he admits using heroin. He reports that his cravings had increased, for which he took buprenorphine and naloxone above the prescribed dosage, and ran out of films early. He then used heroin 3 times to prevent withdrawal.
Mr. R admits that he has been having cravings for oxycodone since the start of treatment for addiction, but thought he was strong enough to overcome the cravings. He feels disappointed and embarrassed about this; he wants to continue with buprenorphine, he tells you, but worries that you will refuse to continue seeing him now.
Using shared decision-making, you opt to increase the buprenorphine dosage by 4 mg (to 16 mg/d—ie, 2 films of buprenorphine and naloxone, 8 mg/2 mg) to alleviate cravings. You instruct him to engage his support network, including his family and NA sponsor, and to start outpatient group therapy. He tells you that he is willing to go back to weekly clinic visits until he is stabilized.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota Medical School Twin Cities, Department of Family Medicine and Community Health, 1020 West Broadway Avenue, Minneapolis, MN 55411; [email protected].
1. Centers for Disease Control and Prevention. Opioid overdose. December 19, 2017. Available at: www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed June 22, 2018.
2. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51:870-878.
3. Centers for Disease Control and Prevention. Overdose prevention. August 31, 2017. Available at: www.cdc.gov/drugoverdose/prevention/index.html. Accessed June 29, 2018.
4. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259. Available at: www.drugabuse.gov/sites/default/files/files/ClinicalOpiateWithdrawalScale.pdf. Accessed June 22, 2018.
5. Opioid use disorder: Diagnostic criteria. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013. Available at: http://pcssnow.org/wp-content/uploads/2014/02/5B-DSM-5-Opioid-Use-Disorder-Diagnostic-Criteria.pdf. Accessed June 23, 2018.
6. Brunette MF, Mueser KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67(Suppl 7):10-17.
7. Kampman K, Abraham A, Dugosh K, et al; ASAM Quality Improvement Council. The ASAM National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Chevy Chase, MD: American Society of Addiction Medicine; 2015. Available at: www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf. Accessed June 22, 2018.
8. Depouy J, Palmaro A, Fatséas M, et al. Mortality associated with time in and out of buprenorphine treatment in French office-based general practice: A 7-year cohort study. Ann Fam Med. 2017;15:355-358.
9. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580.
Opioids were involved in 42,249 deaths in the United States in 2016, and opioid overdoses have quintupled since 1999.1 Among the causes behind these statistics is increased opiate prescribing by physicians—with primary care providers accounting for about one half of opiate prescriptions.2 As a result, the Centers for Disease Control and Prevention has issued a 4-part response for physicians,3 which includes careful opiate prescribing, expanded access to naloxone, prevention of opioid use disorder (OUD), and expanded use of medication-assisted treatment (MAT) of addiction—with the goal of preventing and managing OUD.
CASE
Fred R, a 55-year-old man who has been taking oxycodone, 70 mg/d, for chronic pain for longer than 10 years, visits your clinic for a prescription refill. His prescription monitoring program confirms the long history of regular oxycodone use, with the dosage escalating over the past 6 months. He recently was discharged from the hospital after an overdose of opiates.
Mr. R admits to using heroin after running out of oxycodone. He is in mild withdrawal, with a score of 8 (of a possible 48) on the Clinical Opioid Withdrawal Scale4 (COWS, which assigns point values to 11 common symptoms to gauge the severity of opioid withdrawal and, by inference, the patient’s degree of physical dependence). You determine that Mr. R is frightened about his use of oxycodone and would like to stop; he has tried to stop several times on his own but always relapses when withdrawal becomes severe.
How would you proceed with the care of this patient?
What is OUD? How is the diagnosis made?
OUD is a combination of cognitive, behavioral, and physiologic symptoms arising from continued use of opioids despite significant health, legal, or relationship problems related to their use. The disorder is diagnosed based on specific criteria provided in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)(TABLE 1)5 and is revealed by 1) a careful history that delineates a problematic pattern of opioid use, 2) physical examination, and 3) urine toxicology screen.

Identification of acute opioid intoxication can also be useful when working up a patient in whom OUD is suspected; findings of acute opioid intoxication on physical examination include constricted pupils, head-nodding, excessive sleepiness, and drooping eyelids. Other physical signs of illicit opioid use include track marks around veins of the arm, evidence of repeated trauma, and stigmata of liver dysfunction. Withdrawal can present as agitation, rhinorrhea, dilated pupils, nausea, diarrhea, yawning, and gooseflesh. The COWS, which, as noted in the case, assigns point values to withdrawal symptoms, can be helpful in determining the severity of withdrawal.4
What is the differential Dx of OUD?
When OUD is likely, but not clearly diagnosable, on the basis of findings, consider a mental health disorder: depressive disorder, bipolar disorder, attention deficit–hyperactivity disorder, personality disorder, and polysubstance use disorder. Concurrent diagnosis of substance abuse and a mental health disorder is common; treatment requires that both disorders be addressed simultaneously.6 Assessing for use or abuse of, and addiction to, other substances is vital to ensure proper diagnosis and effective therapy. Polysubstance dependence can be more difficult to treat than single-substance abuse or addiction alone.
Continue to: How is OUD treated?
How is OUD treated?
This article reviews MAT with buprenorphine; other MAT options include methadone and naltrexone. Regardless of the indicated agent chosen, MAT has been shown to be superior to abstinence alone or abstinence with counseling interventions in maintaining sobriety.7
Evidence of efficacy. In a longitudinal cohort study of patients who received MAT with buprenorphine initiated in general practice, patients in whom buprenorphine therapy was interrupted had a greatly increased risk of all-cause mortality (hazard ratio=29.04; 95% confidence interval, 10.04-83.99).8 The study highlights the harm-reduction treatment philosophy of MAT with buprenorphine: The regimen can be used to keep a patient alive while working toward sobriety.
We encourage physicians to treat addiction as they would any chronic disease. The strategy includes anticipating relapse, engaging support systems (eg, family, counselors, social groups, Alcoholics Anonymous, Narcotics Anonymous [NA]), and working with the patient to obtain a higher level of care, as indicated.
Pharmacology and induction. Alone or in combination with naloxone, buprenorphine can be used as in-office-based MAT. Buprenorphine is a partial opiate agonist that binds tightly to opioid receptors and can block the effects of other opiates. An advantage of buprenorphine is its low likelihood of overdose, due to the drug’s so-called ceiling effect at a dosage of 24 mg/d;9 dosages above this amount have little increased medication effect.

Dosing of buprenorphine is variable from patient to patient, with a maximum dosage of 24 mg/d. Therapy can be initiated safely at home, although some physicians prefer in-office induction. It is important that the patient be in moderate withdrawal (as determined by the score on the COWS) before initiation, because buprenorphine, as a partial agonist, can precipitate withdrawal by displacing full opiate agonists from opioid receptors.
Continue to: In our experience...
In our experience, a common induction method is to give 2 to 4 mg buprenorphine, followed by a 1-hour assessment of withdrawal symptoms. This can be repeated for multiple doses until withdrawal is relieved, usually with a maximum dosage of 6 to 8 mg in the initial 1 or 2 days of treatment. Rapid reassessment is required after induction, preferably in 1 to 3 days. Dosing should be gradually increased in 2- to 4-mg increments until 1) the patient has no withdrawal symptoms in a 24-hour period and 2) craving for opiates is adequately controlled.
Note: Primary care physicians must complete an 8-hour online training course to obtain a US Drug Enforcement Administration waiver to prescribe buprenorphine.
How should coordination of care be approached?
Actual prescribing and monitoring of buprenorphine is not complex, but many physicians are intimidated by the perceived difficulty of coordination of care. The American Society of Addiction Medicine's national practice guideline recommends that buprenorphine and other MAT protocols be offered as a part of a comprehensive treatment plan that includes psychosocial treatment.7 This combination leads to the greatest potential for ongoing remission of OUD. Although many primary care clinics do not have chemical dependency counseling available at their primary location, partnering with community organizations and other mental health resources can meet this need. Coordination of care with home services, behavioral health, and psychiatry is common in primary care, and is no different for OUD.
There are administrative requirements for a clinic that offers MAT (TABLE 2),7 including tracking of numbers of patients who are taking buprenorphine. During the first year of prescribing buprenorphine, a physician or other provider is permitted to care for only 30 patients; once the first year has passed, that provider can apply to care for as many as 100 patients. In addition, the Drug Enforcement Administration might conduct site visits to ensure that proper documentation and tracking of patients is being undertaken. These requirements can seem daunting, but careful monitoring of patient panels can alleviate concerns. For clinics that use an electronic medical record, we recommend developing the capability to pull lists by either buprenorphine prescriptions or diagnosis codes.

Continue to: CASE
CASE
After you and Mr. R discuss his addiction, you decide to initiate treatment that includes buprenorphine. You have a specimen collected for a urine toxicology screen and blood drawn for a baseline liver function panel, hepatitis panel, and human immunodeficiency virus screen, and provide him with resources (nearby treatment center, an NA meeting location) for treating OUD. You write a prescription for #8 buprenorphine and naloxone, 2 mg/0.5 mg films, and instruct Mr. R to: take 1 film when withdrawal symptoms become worse; wait 1 hour; and take another film if he is still experiencing withdrawal symptoms. He can repeat this dosing regimen until he reaches 8 mg/d of buprenorphine (4 films). You schedule follow-up in 2 days.
At follow-up, the patient reports that taking 3 films alleviated withdrawal symptoms, but that symptoms returned approximately 12 hours later, at which time he took the fourth film. This helped him through until the next day, when he again took 3 films in the morning and 1 film in the late evening. He feels that this regimen is helping relieve withdrawal symptoms and cravings. You provide a prescription for buprenorphine and naloxone, 8 mg/2 mg daily, and request a follow-up visit in 5 days.
At the next visit, Mr. R reports that he still has cravings for oxycodone. You increase the dosage of buprenorphine and naloxone to 12 mg/3 mg daily.
At the next visit, he reports no longer having cravings.
You continue to monitor Mr. R with urine drug screening and discussion of his recovery with the help of his family and support network. After 3 months of consistent visits, he fails to show up for his every-2-or-3-week appointment.
Continue to: Four days later...
Four days later, Mr. R shows up at the clinic, apologizing for missing the appointment and assuring you that this won’t happen again. Rapid urine drug screening is positive for morphine. When confronted, he admits using heroin. He reports that his cravings had increased, for which he took buprenorphine and naloxone above the prescribed dosage, and ran out of films early. He then used heroin 3 times to prevent withdrawal.
Mr. R admits that he has been having cravings for oxycodone since the start of treatment for addiction, but thought he was strong enough to overcome the cravings. He feels disappointed and embarrassed about this; he wants to continue with buprenorphine, he tells you, but worries that you will refuse to continue seeing him now.
Using shared decision-making, you opt to increase the buprenorphine dosage by 4 mg (to 16 mg/d—ie, 2 films of buprenorphine and naloxone, 8 mg/2 mg) to alleviate cravings. You instruct him to engage his support network, including his family and NA sponsor, and to start outpatient group therapy. He tells you that he is willing to go back to weekly clinic visits until he is stabilized.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota Medical School Twin Cities, Department of Family Medicine and Community Health, 1020 West Broadway Avenue, Minneapolis, MN 55411; [email protected].
Opioids were involved in 42,249 deaths in the United States in 2016, and opioid overdoses have quintupled since 1999.1 Among the causes behind these statistics is increased opiate prescribing by physicians—with primary care providers accounting for about one half of opiate prescriptions.2 As a result, the Centers for Disease Control and Prevention has issued a 4-part response for physicians,3 which includes careful opiate prescribing, expanded access to naloxone, prevention of opioid use disorder (OUD), and expanded use of medication-assisted treatment (MAT) of addiction—with the goal of preventing and managing OUD.
CASE
Fred R, a 55-year-old man who has been taking oxycodone, 70 mg/d, for chronic pain for longer than 10 years, visits your clinic for a prescription refill. His prescription monitoring program confirms the long history of regular oxycodone use, with the dosage escalating over the past 6 months. He recently was discharged from the hospital after an overdose of opiates.
Mr. R admits to using heroin after running out of oxycodone. He is in mild withdrawal, with a score of 8 (of a possible 48) on the Clinical Opioid Withdrawal Scale4 (COWS, which assigns point values to 11 common symptoms to gauge the severity of opioid withdrawal and, by inference, the patient’s degree of physical dependence). You determine that Mr. R is frightened about his use of oxycodone and would like to stop; he has tried to stop several times on his own but always relapses when withdrawal becomes severe.
How would you proceed with the care of this patient?
What is OUD? How is the diagnosis made?
OUD is a combination of cognitive, behavioral, and physiologic symptoms arising from continued use of opioids despite significant health, legal, or relationship problems related to their use. The disorder is diagnosed based on specific criteria provided in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)(TABLE 1)5 and is revealed by 1) a careful history that delineates a problematic pattern of opioid use, 2) physical examination, and 3) urine toxicology screen.

Identification of acute opioid intoxication can also be useful when working up a patient in whom OUD is suspected; findings of acute opioid intoxication on physical examination include constricted pupils, head-nodding, excessive sleepiness, and drooping eyelids. Other physical signs of illicit opioid use include track marks around veins of the arm, evidence of repeated trauma, and stigmata of liver dysfunction. Withdrawal can present as agitation, rhinorrhea, dilated pupils, nausea, diarrhea, yawning, and gooseflesh. The COWS, which, as noted in the case, assigns point values to withdrawal symptoms, can be helpful in determining the severity of withdrawal.4
What is the differential Dx of OUD?
When OUD is likely, but not clearly diagnosable, on the basis of findings, consider a mental health disorder: depressive disorder, bipolar disorder, attention deficit–hyperactivity disorder, personality disorder, and polysubstance use disorder. Concurrent diagnosis of substance abuse and a mental health disorder is common; treatment requires that both disorders be addressed simultaneously.6 Assessing for use or abuse of, and addiction to, other substances is vital to ensure proper diagnosis and effective therapy. Polysubstance dependence can be more difficult to treat than single-substance abuse or addiction alone.
Continue to: How is OUD treated?
How is OUD treated?
This article reviews MAT with buprenorphine; other MAT options include methadone and naltrexone. Regardless of the indicated agent chosen, MAT has been shown to be superior to abstinence alone or abstinence with counseling interventions in maintaining sobriety.7
Evidence of efficacy. In a longitudinal cohort study of patients who received MAT with buprenorphine initiated in general practice, patients in whom buprenorphine therapy was interrupted had a greatly increased risk of all-cause mortality (hazard ratio=29.04; 95% confidence interval, 10.04-83.99).8 The study highlights the harm-reduction treatment philosophy of MAT with buprenorphine: The regimen can be used to keep a patient alive while working toward sobriety.
We encourage physicians to treat addiction as they would any chronic disease. The strategy includes anticipating relapse, engaging support systems (eg, family, counselors, social groups, Alcoholics Anonymous, Narcotics Anonymous [NA]), and working with the patient to obtain a higher level of care, as indicated.
Pharmacology and induction. Alone or in combination with naloxone, buprenorphine can be used as in-office-based MAT. Buprenorphine is a partial opiate agonist that binds tightly to opioid receptors and can block the effects of other opiates. An advantage of buprenorphine is its low likelihood of overdose, due to the drug’s so-called ceiling effect at a dosage of 24 mg/d;9 dosages above this amount have little increased medication effect.

Dosing of buprenorphine is variable from patient to patient, with a maximum dosage of 24 mg/d. Therapy can be initiated safely at home, although some physicians prefer in-office induction. It is important that the patient be in moderate withdrawal (as determined by the score on the COWS) before initiation, because buprenorphine, as a partial agonist, can precipitate withdrawal by displacing full opiate agonists from opioid receptors.
Continue to: In our experience...
In our experience, a common induction method is to give 2 to 4 mg buprenorphine, followed by a 1-hour assessment of withdrawal symptoms. This can be repeated for multiple doses until withdrawal is relieved, usually with a maximum dosage of 6 to 8 mg in the initial 1 or 2 days of treatment. Rapid reassessment is required after induction, preferably in 1 to 3 days. Dosing should be gradually increased in 2- to 4-mg increments until 1) the patient has no withdrawal symptoms in a 24-hour period and 2) craving for opiates is adequately controlled.
Note: Primary care physicians must complete an 8-hour online training course to obtain a US Drug Enforcement Administration waiver to prescribe buprenorphine.
How should coordination of care be approached?
Actual prescribing and monitoring of buprenorphine is not complex, but many physicians are intimidated by the perceived difficulty of coordination of care. The American Society of Addiction Medicine's national practice guideline recommends that buprenorphine and other MAT protocols be offered as a part of a comprehensive treatment plan that includes psychosocial treatment.7 This combination leads to the greatest potential for ongoing remission of OUD. Although many primary care clinics do not have chemical dependency counseling available at their primary location, partnering with community organizations and other mental health resources can meet this need. Coordination of care with home services, behavioral health, and psychiatry is common in primary care, and is no different for OUD.
There are administrative requirements for a clinic that offers MAT (TABLE 2),7 including tracking of numbers of patients who are taking buprenorphine. During the first year of prescribing buprenorphine, a physician or other provider is permitted to care for only 30 patients; once the first year has passed, that provider can apply to care for as many as 100 patients. In addition, the Drug Enforcement Administration might conduct site visits to ensure that proper documentation and tracking of patients is being undertaken. These requirements can seem daunting, but careful monitoring of patient panels can alleviate concerns. For clinics that use an electronic medical record, we recommend developing the capability to pull lists by either buprenorphine prescriptions or diagnosis codes.

Continue to: CASE
CASE
After you and Mr. R discuss his addiction, you decide to initiate treatment that includes buprenorphine. You have a specimen collected for a urine toxicology screen and blood drawn for a baseline liver function panel, hepatitis panel, and human immunodeficiency virus screen, and provide him with resources (nearby treatment center, an NA meeting location) for treating OUD. You write a prescription for #8 buprenorphine and naloxone, 2 mg/0.5 mg films, and instruct Mr. R to: take 1 film when withdrawal symptoms become worse; wait 1 hour; and take another film if he is still experiencing withdrawal symptoms. He can repeat this dosing regimen until he reaches 8 mg/d of buprenorphine (4 films). You schedule follow-up in 2 days.
At follow-up, the patient reports that taking 3 films alleviated withdrawal symptoms, but that symptoms returned approximately 12 hours later, at which time he took the fourth film. This helped him through until the next day, when he again took 3 films in the morning and 1 film in the late evening. He feels that this regimen is helping relieve withdrawal symptoms and cravings. You provide a prescription for buprenorphine and naloxone, 8 mg/2 mg daily, and request a follow-up visit in 5 days.
At the next visit, Mr. R reports that he still has cravings for oxycodone. You increase the dosage of buprenorphine and naloxone to 12 mg/3 mg daily.
At the next visit, he reports no longer having cravings.
You continue to monitor Mr. R with urine drug screening and discussion of his recovery with the help of his family and support network. After 3 months of consistent visits, he fails to show up for his every-2-or-3-week appointment.
Continue to: Four days later...
Four days later, Mr. R shows up at the clinic, apologizing for missing the appointment and assuring you that this won’t happen again. Rapid urine drug screening is positive for morphine. When confronted, he admits using heroin. He reports that his cravings had increased, for which he took buprenorphine and naloxone above the prescribed dosage, and ran out of films early. He then used heroin 3 times to prevent withdrawal.
Mr. R admits that he has been having cravings for oxycodone since the start of treatment for addiction, but thought he was strong enough to overcome the cravings. He feels disappointed and embarrassed about this; he wants to continue with buprenorphine, he tells you, but worries that you will refuse to continue seeing him now.
Using shared decision-making, you opt to increase the buprenorphine dosage by 4 mg (to 16 mg/d—ie, 2 films of buprenorphine and naloxone, 8 mg/2 mg) to alleviate cravings. You instruct him to engage his support network, including his family and NA sponsor, and to start outpatient group therapy. He tells you that he is willing to go back to weekly clinic visits until he is stabilized.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota Medical School Twin Cities, Department of Family Medicine and Community Health, 1020 West Broadway Avenue, Minneapolis, MN 55411; [email protected].
1. Centers for Disease Control and Prevention. Opioid overdose. December 19, 2017. Available at: www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed June 22, 2018.
2. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51:870-878.
3. Centers for Disease Control and Prevention. Overdose prevention. August 31, 2017. Available at: www.cdc.gov/drugoverdose/prevention/index.html. Accessed June 29, 2018.
4. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259. Available at: www.drugabuse.gov/sites/default/files/files/ClinicalOpiateWithdrawalScale.pdf. Accessed June 22, 2018.
5. Opioid use disorder: Diagnostic criteria. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013. Available at: http://pcssnow.org/wp-content/uploads/2014/02/5B-DSM-5-Opioid-Use-Disorder-Diagnostic-Criteria.pdf. Accessed June 23, 2018.
6. Brunette MF, Mueser KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67(Suppl 7):10-17.
7. Kampman K, Abraham A, Dugosh K, et al; ASAM Quality Improvement Council. The ASAM National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Chevy Chase, MD: American Society of Addiction Medicine; 2015. Available at: www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf. Accessed June 22, 2018.
8. Depouy J, Palmaro A, Fatséas M, et al. Mortality associated with time in and out of buprenorphine treatment in French office-based general practice: A 7-year cohort study. Ann Fam Med. 2017;15:355-358.
9. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580.
1. Centers for Disease Control and Prevention. Opioid overdose. December 19, 2017. Available at: www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed June 22, 2018.
2. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51:870-878.
3. Centers for Disease Control and Prevention. Overdose prevention. August 31, 2017. Available at: www.cdc.gov/drugoverdose/prevention/index.html. Accessed June 29, 2018.
4. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259. Available at: www.drugabuse.gov/sites/default/files/files/ClinicalOpiateWithdrawalScale.pdf. Accessed June 22, 2018.
5. Opioid use disorder: Diagnostic criteria. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013. Available at: http://pcssnow.org/wp-content/uploads/2014/02/5B-DSM-5-Opioid-Use-Disorder-Diagnostic-Criteria.pdf. Accessed June 23, 2018.
6. Brunette MF, Mueser KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67(Suppl 7):10-17.
7. Kampman K, Abraham A, Dugosh K, et al; ASAM Quality Improvement Council. The ASAM National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Chevy Chase, MD: American Society of Addiction Medicine; 2015. Available at: www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf. Accessed June 22, 2018.
8. Depouy J, Palmaro A, Fatséas M, et al. Mortality associated with time in and out of buprenorphine treatment in French office-based general practice: A 7-year cohort study. Ann Fam Med. 2017;15:355-358.
9. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580.
PRACTICE RECOMMENDATIONS
› Use signs of intoxication, signs of withdrawal, urine drug screening, and diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, to screen for, and diagnose, opioid use disorder. C
› Offer and institute medication-assisted treatment when appropriate to reduce the risk of opioid-related and overall mortality in patients with opioid use disorder. A
› Identify and treat comorbid psychiatric disorders in patients with opioid use disorder, which provides benefit during treatment of the disorder. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series