User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
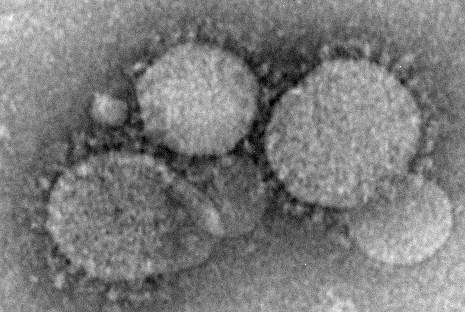
Prevention measures critical in avoiding global spread of MERS
Although currently considered an “endemic, low-level public health threat,” Middle East respiratory syndrome coronavirus (MERS-CoV) has the potential to mutate to have an increased capacity for human transmission, making preventive measures among health care workers crucial, reported Dr. Alimuddin Zumla and his colleagues from University College London, England.
As no drug treatment exists for the disease, which can range from mild to severe with acute respiratory distress syndrome and organ failure leading to death, clinicians are strongly urged to put several preventive measures in place to avoid its spread in health care facilities, the authors said. Recommended measures include droplet precautions such as wearing a surgical mask, using eye protection, and taking contact and respiratory precautions, Dr. Zumla and his colleagues said in the report.
MERS cases reported to the World Health Organization as of May 31, 2015, total 1,149, including 431 deaths, according to a Lancet press release.
The disease is believed to be spread through direct or indirect contact with dromedary camels, and most cases have been concentrated in Saudi Arabia, with added cases reported in Europe, the United States, and Asia in individuals who traveled from the Middle East, or their contacts, the authors said. The exact mechanism of transmission remains unknown.
“Although interhuman transmission is still inefficient, health authorities, governments, and the research community should be prepared for the emergence of a MERS-CoV with increased capacity for transmission and pandemic potential,” they said.
Read the full article here.
Although currently considered an “endemic, low-level public health threat,” Middle East respiratory syndrome coronavirus (MERS-CoV) has the potential to mutate to have an increased capacity for human transmission, making preventive measures among health care workers crucial, reported Dr. Alimuddin Zumla and his colleagues from University College London, England.
As no drug treatment exists for the disease, which can range from mild to severe with acute respiratory distress syndrome and organ failure leading to death, clinicians are strongly urged to put several preventive measures in place to avoid its spread in health care facilities, the authors said. Recommended measures include droplet precautions such as wearing a surgical mask, using eye protection, and taking contact and respiratory precautions, Dr. Zumla and his colleagues said in the report.
MERS cases reported to the World Health Organization as of May 31, 2015, total 1,149, including 431 deaths, according to a Lancet press release.
The disease is believed to be spread through direct or indirect contact with dromedary camels, and most cases have been concentrated in Saudi Arabia, with added cases reported in Europe, the United States, and Asia in individuals who traveled from the Middle East, or their contacts, the authors said. The exact mechanism of transmission remains unknown.
“Although interhuman transmission is still inefficient, health authorities, governments, and the research community should be prepared for the emergence of a MERS-CoV with increased capacity for transmission and pandemic potential,” they said.
Read the full article here.
Although currently considered an “endemic, low-level public health threat,” Middle East respiratory syndrome coronavirus (MERS-CoV) has the potential to mutate to have an increased capacity for human transmission, making preventive measures among health care workers crucial, reported Dr. Alimuddin Zumla and his colleagues from University College London, England.
As no drug treatment exists for the disease, which can range from mild to severe with acute respiratory distress syndrome and organ failure leading to death, clinicians are strongly urged to put several preventive measures in place to avoid its spread in health care facilities, the authors said. Recommended measures include droplet precautions such as wearing a surgical mask, using eye protection, and taking contact and respiratory precautions, Dr. Zumla and his colleagues said in the report.
MERS cases reported to the World Health Organization as of May 31, 2015, total 1,149, including 431 deaths, according to a Lancet press release.
The disease is believed to be spread through direct or indirect contact with dromedary camels, and most cases have been concentrated in Saudi Arabia, with added cases reported in Europe, the United States, and Asia in individuals who traveled from the Middle East, or their contacts, the authors said. The exact mechanism of transmission remains unknown.
“Although interhuman transmission is still inefficient, health authorities, governments, and the research community should be prepared for the emergence of a MERS-CoV with increased capacity for transmission and pandemic potential,” they said.
Read the full article here.
Asthma exacerbations in pregnancy linked to obesity, weight gain
DENVER – Obesity and weight gain of more than 5 kg during the first trimester in women with asthma are significant predictors of asthma exacerbation during pregnancy, according to findings from the Management of Asthma during Pregnancy (MAP) study.
Of 1,018 women in the prospective study, 370 experienced a total of 407 asthma exacerbations. The risk of exacerbation was greatest in those with body mass index over 30 kg/m2 (odds ratio, 2.2) and in those with gestational weight gain of more than 5 kg in the first trimester (OR, 8.2), Zarqa Ali of Hvidovre Hospital, Copenhagen, and colleagues reported in a poster at an international conference of the American Thoracic Society.
Other risk factors for exacerbation included previous asthma exacerbations (OR, 1.3), uncontrolled asthma (OR, 2), inhaled corticosteroid treatment (OR, 5.8), and current and former smoking (OR, 1.9), the investigators said.
Risk factors for severe exacerbation of asthma, which occurred in 157 women, included gestational weight gain of greater than 5 kg (OR, 4.1), and age (OR, 1.1).
After excluding women with a prepregnancy body mass index above the mean of 24.1, risk factors for exacerbation included first-trimester weight gain greater than 5 kg (OR, 13.1) and lower prepregnancy body weight (OR, 0.9), they noted.
Women included in the study had a diagnosis of asthma, had been prescribed rescue bronchodilator therapy, had their first outpatient clinic visit within the first 18 weeks of pregnancy, and were seen for scheduled visits about every 4 weeks during pregnancy and for 3 months post partum. The women had a mean age of 31 years, 73% had never smoked, and their mean forced expiratory volume in 1 second (FEV1) % predicted was 92, and mean exhaled nitric oxide was 20 ppb.
FEV1% predicted was significantly greater at baseline in those without exacerbations vs. with exacerbations (93 vs. 90), and exhaled nitric oxide was significantly lower in the groups, respectively (18 vs. 23 ppb).
The findings are important, as acute exacerbations of asthma during pregnancy may be the most significant risk factor for unfavorable pregnancy outcomes in women with asthma, the investigators said, noting that exacerbations occur in about 30% of pregnant women with asthma and are associated with complications such as preterm delivery, low birth weight, excessive antepartum hemorrhage, and cesarean delivery.
Risk factors for exacerbation identified in prior studies include severe asthma, viral infections, nonadherence to controller medication, tobacco exposure, and possibly fetal gender and maternal body mass index, the investigators said.
The investigators reported having no disclosures.
DENVER – Obesity and weight gain of more than 5 kg during the first trimester in women with asthma are significant predictors of asthma exacerbation during pregnancy, according to findings from the Management of Asthma during Pregnancy (MAP) study.
Of 1,018 women in the prospective study, 370 experienced a total of 407 asthma exacerbations. The risk of exacerbation was greatest in those with body mass index over 30 kg/m2 (odds ratio, 2.2) and in those with gestational weight gain of more than 5 kg in the first trimester (OR, 8.2), Zarqa Ali of Hvidovre Hospital, Copenhagen, and colleagues reported in a poster at an international conference of the American Thoracic Society.
Other risk factors for exacerbation included previous asthma exacerbations (OR, 1.3), uncontrolled asthma (OR, 2), inhaled corticosteroid treatment (OR, 5.8), and current and former smoking (OR, 1.9), the investigators said.
Risk factors for severe exacerbation of asthma, which occurred in 157 women, included gestational weight gain of greater than 5 kg (OR, 4.1), and age (OR, 1.1).
After excluding women with a prepregnancy body mass index above the mean of 24.1, risk factors for exacerbation included first-trimester weight gain greater than 5 kg (OR, 13.1) and lower prepregnancy body weight (OR, 0.9), they noted.
Women included in the study had a diagnosis of asthma, had been prescribed rescue bronchodilator therapy, had their first outpatient clinic visit within the first 18 weeks of pregnancy, and were seen for scheduled visits about every 4 weeks during pregnancy and for 3 months post partum. The women had a mean age of 31 years, 73% had never smoked, and their mean forced expiratory volume in 1 second (FEV1) % predicted was 92, and mean exhaled nitric oxide was 20 ppb.
FEV1% predicted was significantly greater at baseline in those without exacerbations vs. with exacerbations (93 vs. 90), and exhaled nitric oxide was significantly lower in the groups, respectively (18 vs. 23 ppb).
The findings are important, as acute exacerbations of asthma during pregnancy may be the most significant risk factor for unfavorable pregnancy outcomes in women with asthma, the investigators said, noting that exacerbations occur in about 30% of pregnant women with asthma and are associated with complications such as preterm delivery, low birth weight, excessive antepartum hemorrhage, and cesarean delivery.
Risk factors for exacerbation identified in prior studies include severe asthma, viral infections, nonadherence to controller medication, tobacco exposure, and possibly fetal gender and maternal body mass index, the investigators said.
The investigators reported having no disclosures.
DENVER – Obesity and weight gain of more than 5 kg during the first trimester in women with asthma are significant predictors of asthma exacerbation during pregnancy, according to findings from the Management of Asthma during Pregnancy (MAP) study.
Of 1,018 women in the prospective study, 370 experienced a total of 407 asthma exacerbations. The risk of exacerbation was greatest in those with body mass index over 30 kg/m2 (odds ratio, 2.2) and in those with gestational weight gain of more than 5 kg in the first trimester (OR, 8.2), Zarqa Ali of Hvidovre Hospital, Copenhagen, and colleagues reported in a poster at an international conference of the American Thoracic Society.
Other risk factors for exacerbation included previous asthma exacerbations (OR, 1.3), uncontrolled asthma (OR, 2), inhaled corticosteroid treatment (OR, 5.8), and current and former smoking (OR, 1.9), the investigators said.
Risk factors for severe exacerbation of asthma, which occurred in 157 women, included gestational weight gain of greater than 5 kg (OR, 4.1), and age (OR, 1.1).
After excluding women with a prepregnancy body mass index above the mean of 24.1, risk factors for exacerbation included first-trimester weight gain greater than 5 kg (OR, 13.1) and lower prepregnancy body weight (OR, 0.9), they noted.
Women included in the study had a diagnosis of asthma, had been prescribed rescue bronchodilator therapy, had their first outpatient clinic visit within the first 18 weeks of pregnancy, and were seen for scheduled visits about every 4 weeks during pregnancy and for 3 months post partum. The women had a mean age of 31 years, 73% had never smoked, and their mean forced expiratory volume in 1 second (FEV1) % predicted was 92, and mean exhaled nitric oxide was 20 ppb.
FEV1% predicted was significantly greater at baseline in those without exacerbations vs. with exacerbations (93 vs. 90), and exhaled nitric oxide was significantly lower in the groups, respectively (18 vs. 23 ppb).
The findings are important, as acute exacerbations of asthma during pregnancy may be the most significant risk factor for unfavorable pregnancy outcomes in women with asthma, the investigators said, noting that exacerbations occur in about 30% of pregnant women with asthma and are associated with complications such as preterm delivery, low birth weight, excessive antepartum hemorrhage, and cesarean delivery.
Risk factors for exacerbation identified in prior studies include severe asthma, viral infections, nonadherence to controller medication, tobacco exposure, and possibly fetal gender and maternal body mass index, the investigators said.
The investigators reported having no disclosures.
AT ATS 2015
Key clinical point: Baseline weight and gestational weight gain predict asthma exacerbations in pregnancy.
Major finding: Asthma exacerbation risk was greatest in those with body mass index over 30 kg/m2 and with gestational weight gain of more than 5 kg in the first trimester.
Data source: The prospective MAP study involving 1,018 women.
Disclosures: The investigators reported having no disclosures.
Nivolumab transforms practice for advanced, refractory nonsquamous NSCLC
CHICAGO – Nivolumab reduced the risk of death by nearly a third over docetaxel for patients with advanced, refractory nonsquamous non–small cell lung cancer, results of CheckMate 057 showed.
The primary endpoint of median overall survival was 12.2 months for those receiving the PD-1 immune checkpoint inhibitor nivolumab and 9.4 months for those given docetaxel (hazard ratio, 0.73; P = .0015), study author Dr. Luis Paz-Ares reported at the annual meeting of the American Society of Clinical Oncology.
At 1 year, 51% of the nivolumab (Opdivo) group were alive vs. 39% of the docetaxel (Taxotere) group.
The survival advantage was seen across most subgroups, except never smokers and those whose tumors were positive for epidermal growth factor receptor (EGFR) mutations.
The magnitude of the overall survival benefit in patients with PD-L1–positive tumors, however, was “unprecedented in this setting” and ranged from 17.2 months to 19.4 months, Dr. Paz-Ares of the Hospital Universitario Virgen Del Rocio, Seville, Spain, said.
Treatment options for patients with nonsquamous histology who progress following platinum-based doublet chemotherapy are limited. Typical response rates in this context are about 10%, and median overall survival is about 8-10 months, he said.
Discussant Dr. Roy Herbst, chief of medical oncology at Yale Comprehensive Cancer Center in New Haven, Conn., said, “This is a positive randomized phase III trial with a primary endpoint for all comers. The trial sets a new standard for the treatment of previously treated disease … and nivolumab is significantly less toxic than docetaxel.”
Check Mate 057 randomly assigned 292 patients to nivolumab 3 mg/kg every 2 weeks and 290 patients to docetaxel 75 mg/kg every 3 weeks until disease progression or unacceptable toxicity occurred. Patients were stratified by prior maintenance therapy and line of therapy. PD-L1 expression was measured in pretreatment (archival or recent) tumor biopsies.
The objective response rate was significantly higher for patients receiving nivolumab than docetaxel (19% vs. 12%; P = .0246; odds ratio, 1.72), Dr. Paz-Ares said.
Most responses were partial (18% vs. 12%), with only one complete response to nivolumab. The median duration of response was 17.2 months with nivolumab vs. 5.6 months with docetaxel.
Progression-free survival (PFS) was similar between the nivolumab and docetaxel groups (2.3 months vs. 4.2 months; HR, 0.92; P = .39), he said, explaining that progression was more rapid with nivolumab during the first 6 months before slowing to a 1-year PFS rate of 19% vs. 8% for docetaxel.
PD-L1 expression emerged as a significant predictor of objective response rate, PFS, and overall survival, with objective response rates as much as three times higher with nivolumab than docetaxel for patients with high PD-L1 expression, Dr. Paz-Ares said.
Using three predefined cut points of ≥1%, ≥5%, and ≥10% PD-L1 expression, overall survival was 17.2 months, 18.2 months, and 19.4 months with nivolumab vs. 9.0 months, 8.1 months, and 8 months with docetaxel, respectively.
Dr. Herbst described the PD-L1 biomarker as intriguing, but said for now it is only hypothesis generating and should not be used for patient selection. PD-L1 expression was not prospectively stratified in the study, and was not available for 22% of patients, and while it does improve objective response rate, PFS, and overall survival, even patients with less than 1% expression appear to have at least equal activity to that of docetaxel with less toxicity, he noted.
Adverse events of any grade were reported in 69% of patients receiving nivolumab and 88% receiving docetaxel. More importantly, grade 3-4 events occurred in 10% vs. 54%, Dr. Paz-Ares said. The most common events with nivolumab were fatigue, nausea, and decreased appetite.
Notably, the dose intensity delivered was higher for nivolumab than for docetaxel (83% vs. 66%), and 42% of nivolumab patients vs. 50% of docetaxel patients received subsequent systemic therapy, suggesting little influence of further treatment on survival.
In a separate presentation at ASCO, nivolumab reduced the risk of death by 41%, compared with docetaxel, in previously treated advanced squamous NSCLC (HR, 0.59; P = .00025) in the phase III Check Mate 017 study.
Nivolumab received a second indication in March 2015 for use in metastatic squamous NSCLC following failure with platinum-based chemotherapy
Bristol-Myers Squibb sponsored the study. Dr. Paz-Ares reported honoraria from Bristol-Myers Squibb, Roche/Genentech, Lilly, Pfizer, Boehringer, and Clovis. Dr. Herbst reported honoraria from Boehringer Ingelheim, Celgene, Lilly, Merck, NovaRx, and Pfizer; a consulting or advisory role with Biothera, DiaTech Oncology, Koltan Pharmaceuticals, N-of-One, and Quintiles; and research funding from Genentech/Roche and GlaxoSmithKline.
On Twitter @pwendl
CHICAGO – Nivolumab reduced the risk of death by nearly a third over docetaxel for patients with advanced, refractory nonsquamous non–small cell lung cancer, results of CheckMate 057 showed.
The primary endpoint of median overall survival was 12.2 months for those receiving the PD-1 immune checkpoint inhibitor nivolumab and 9.4 months for those given docetaxel (hazard ratio, 0.73; P = .0015), study author Dr. Luis Paz-Ares reported at the annual meeting of the American Society of Clinical Oncology.
At 1 year, 51% of the nivolumab (Opdivo) group were alive vs. 39% of the docetaxel (Taxotere) group.
The survival advantage was seen across most subgroups, except never smokers and those whose tumors were positive for epidermal growth factor receptor (EGFR) mutations.
The magnitude of the overall survival benefit in patients with PD-L1–positive tumors, however, was “unprecedented in this setting” and ranged from 17.2 months to 19.4 months, Dr. Paz-Ares of the Hospital Universitario Virgen Del Rocio, Seville, Spain, said.
Treatment options for patients with nonsquamous histology who progress following platinum-based doublet chemotherapy are limited. Typical response rates in this context are about 10%, and median overall survival is about 8-10 months, he said.
Discussant Dr. Roy Herbst, chief of medical oncology at Yale Comprehensive Cancer Center in New Haven, Conn., said, “This is a positive randomized phase III trial with a primary endpoint for all comers. The trial sets a new standard for the treatment of previously treated disease … and nivolumab is significantly less toxic than docetaxel.”
Check Mate 057 randomly assigned 292 patients to nivolumab 3 mg/kg every 2 weeks and 290 patients to docetaxel 75 mg/kg every 3 weeks until disease progression or unacceptable toxicity occurred. Patients were stratified by prior maintenance therapy and line of therapy. PD-L1 expression was measured in pretreatment (archival or recent) tumor biopsies.
The objective response rate was significantly higher for patients receiving nivolumab than docetaxel (19% vs. 12%; P = .0246; odds ratio, 1.72), Dr. Paz-Ares said.
Most responses were partial (18% vs. 12%), with only one complete response to nivolumab. The median duration of response was 17.2 months with nivolumab vs. 5.6 months with docetaxel.
Progression-free survival (PFS) was similar between the nivolumab and docetaxel groups (2.3 months vs. 4.2 months; HR, 0.92; P = .39), he said, explaining that progression was more rapid with nivolumab during the first 6 months before slowing to a 1-year PFS rate of 19% vs. 8% for docetaxel.
PD-L1 expression emerged as a significant predictor of objective response rate, PFS, and overall survival, with objective response rates as much as three times higher with nivolumab than docetaxel for patients with high PD-L1 expression, Dr. Paz-Ares said.
Using three predefined cut points of ≥1%, ≥5%, and ≥10% PD-L1 expression, overall survival was 17.2 months, 18.2 months, and 19.4 months with nivolumab vs. 9.0 months, 8.1 months, and 8 months with docetaxel, respectively.
Dr. Herbst described the PD-L1 biomarker as intriguing, but said for now it is only hypothesis generating and should not be used for patient selection. PD-L1 expression was not prospectively stratified in the study, and was not available for 22% of patients, and while it does improve objective response rate, PFS, and overall survival, even patients with less than 1% expression appear to have at least equal activity to that of docetaxel with less toxicity, he noted.
Adverse events of any grade were reported in 69% of patients receiving nivolumab and 88% receiving docetaxel. More importantly, grade 3-4 events occurred in 10% vs. 54%, Dr. Paz-Ares said. The most common events with nivolumab were fatigue, nausea, and decreased appetite.
Notably, the dose intensity delivered was higher for nivolumab than for docetaxel (83% vs. 66%), and 42% of nivolumab patients vs. 50% of docetaxel patients received subsequent systemic therapy, suggesting little influence of further treatment on survival.
In a separate presentation at ASCO, nivolumab reduced the risk of death by 41%, compared with docetaxel, in previously treated advanced squamous NSCLC (HR, 0.59; P = .00025) in the phase III Check Mate 017 study.
Nivolumab received a second indication in March 2015 for use in metastatic squamous NSCLC following failure with platinum-based chemotherapy
Bristol-Myers Squibb sponsored the study. Dr. Paz-Ares reported honoraria from Bristol-Myers Squibb, Roche/Genentech, Lilly, Pfizer, Boehringer, and Clovis. Dr. Herbst reported honoraria from Boehringer Ingelheim, Celgene, Lilly, Merck, NovaRx, and Pfizer; a consulting or advisory role with Biothera, DiaTech Oncology, Koltan Pharmaceuticals, N-of-One, and Quintiles; and research funding from Genentech/Roche and GlaxoSmithKline.
On Twitter @pwendl
CHICAGO – Nivolumab reduced the risk of death by nearly a third over docetaxel for patients with advanced, refractory nonsquamous non–small cell lung cancer, results of CheckMate 057 showed.
The primary endpoint of median overall survival was 12.2 months for those receiving the PD-1 immune checkpoint inhibitor nivolumab and 9.4 months for those given docetaxel (hazard ratio, 0.73; P = .0015), study author Dr. Luis Paz-Ares reported at the annual meeting of the American Society of Clinical Oncology.
At 1 year, 51% of the nivolumab (Opdivo) group were alive vs. 39% of the docetaxel (Taxotere) group.
The survival advantage was seen across most subgroups, except never smokers and those whose tumors were positive for epidermal growth factor receptor (EGFR) mutations.
The magnitude of the overall survival benefit in patients with PD-L1–positive tumors, however, was “unprecedented in this setting” and ranged from 17.2 months to 19.4 months, Dr. Paz-Ares of the Hospital Universitario Virgen Del Rocio, Seville, Spain, said.
Treatment options for patients with nonsquamous histology who progress following platinum-based doublet chemotherapy are limited. Typical response rates in this context are about 10%, and median overall survival is about 8-10 months, he said.
Discussant Dr. Roy Herbst, chief of medical oncology at Yale Comprehensive Cancer Center in New Haven, Conn., said, “This is a positive randomized phase III trial with a primary endpoint for all comers. The trial sets a new standard for the treatment of previously treated disease … and nivolumab is significantly less toxic than docetaxel.”
Check Mate 057 randomly assigned 292 patients to nivolumab 3 mg/kg every 2 weeks and 290 patients to docetaxel 75 mg/kg every 3 weeks until disease progression or unacceptable toxicity occurred. Patients were stratified by prior maintenance therapy and line of therapy. PD-L1 expression was measured in pretreatment (archival or recent) tumor biopsies.
The objective response rate was significantly higher for patients receiving nivolumab than docetaxel (19% vs. 12%; P = .0246; odds ratio, 1.72), Dr. Paz-Ares said.
Most responses were partial (18% vs. 12%), with only one complete response to nivolumab. The median duration of response was 17.2 months with nivolumab vs. 5.6 months with docetaxel.
Progression-free survival (PFS) was similar between the nivolumab and docetaxel groups (2.3 months vs. 4.2 months; HR, 0.92; P = .39), he said, explaining that progression was more rapid with nivolumab during the first 6 months before slowing to a 1-year PFS rate of 19% vs. 8% for docetaxel.
PD-L1 expression emerged as a significant predictor of objective response rate, PFS, and overall survival, with objective response rates as much as three times higher with nivolumab than docetaxel for patients with high PD-L1 expression, Dr. Paz-Ares said.
Using three predefined cut points of ≥1%, ≥5%, and ≥10% PD-L1 expression, overall survival was 17.2 months, 18.2 months, and 19.4 months with nivolumab vs. 9.0 months, 8.1 months, and 8 months with docetaxel, respectively.
Dr. Herbst described the PD-L1 biomarker as intriguing, but said for now it is only hypothesis generating and should not be used for patient selection. PD-L1 expression was not prospectively stratified in the study, and was not available for 22% of patients, and while it does improve objective response rate, PFS, and overall survival, even patients with less than 1% expression appear to have at least equal activity to that of docetaxel with less toxicity, he noted.
Adverse events of any grade were reported in 69% of patients receiving nivolumab and 88% receiving docetaxel. More importantly, grade 3-4 events occurred in 10% vs. 54%, Dr. Paz-Ares said. The most common events with nivolumab were fatigue, nausea, and decreased appetite.
Notably, the dose intensity delivered was higher for nivolumab than for docetaxel (83% vs. 66%), and 42% of nivolumab patients vs. 50% of docetaxel patients received subsequent systemic therapy, suggesting little influence of further treatment on survival.
In a separate presentation at ASCO, nivolumab reduced the risk of death by 41%, compared with docetaxel, in previously treated advanced squamous NSCLC (HR, 0.59; P = .00025) in the phase III Check Mate 017 study.
Nivolumab received a second indication in March 2015 for use in metastatic squamous NSCLC following failure with platinum-based chemotherapy
Bristol-Myers Squibb sponsored the study. Dr. Paz-Ares reported honoraria from Bristol-Myers Squibb, Roche/Genentech, Lilly, Pfizer, Boehringer, and Clovis. Dr. Herbst reported honoraria from Boehringer Ingelheim, Celgene, Lilly, Merck, NovaRx, and Pfizer; a consulting or advisory role with Biothera, DiaTech Oncology, Koltan Pharmaceuticals, N-of-One, and Quintiles; and research funding from Genentech/Roche and GlaxoSmithKline.
On Twitter @pwendl
AT 2015 ASCO ANNUAL MEETING
Key clinical point: Nivolumab provided superior overall survival vs. docetaxel and should be considered the new standard of care for previously treated nonsquamous NSCLC.
Major finding: The median overall survival was 12.2 months with nivolumab vs. 9.4 months with docetaxel (HR, 0.73; P = .0015).
Data source: A phase III randomized study in 582 patients with nonsquamous NSCLC that progressed after platinum chemotherapy.
Disclosures: Bristol-Myers Squibb sponsored the study. Dr. Paz-Ares reported honoraria from Bristol-Myers Squibb, Roche/Genentech, Lilly, Pfizer, Boehringer, and Clovis. Dr. Herbst reported honoraria from Boehringer Ingelheim, Celgene, Lilly, Merck, NovaRx, and Pfizer; a consulting or advisory role with Biothera, DiaTech Oncology, Koltan Pharmaceuticals, N-of-One, and Quintiles; and research funding from Genentech/Roche and GlaxoSmithKline.
Oral device reduced obstructive sleep apnea, not sleepiness
An oral appliance that advances a patient’s lower jaw reduced episodes of obstructive sleep apnea, snoring, and restless legs symptoms, according to a report published online June 1 in JAMA Internal Medicine.
The device, however, failed to improve daytime sleepiness or quality of life in a Swedish study of adults who had daytime sleepiness and either snoring or mild to moderate sleep apnea, said Marie Marklund, Ph.D., D.D.S., of the department of odontology at Umeå (Sweden) University and her associates (JAMA Intern. Med. 2015 June 1 [doi:10.1001/jamainternmed.2015.2051]).
Previous studies of oral appliances have focused on patients with more severe sleep apnea and have yielded conflicting results, particularly regarding daytime sleepiness.
A total of 91 patients who were randomly assigned to receive either a placebo device (46 patients) or an oral appliance individually made by a dental technician using separate plaster casts of the upper and lower teeth (45 participants) completed the study. The device’s elastomer pieces fitted over the teeth and were connected with a screw that allowed continuous gradual advancement of the lower jaw by 6-7 mm. Holding the lower mandible forward improves breathing during sleep.
After 4 months of follow-up, at-home overnight polysomnography showed “a clear, significant treatment effect”: the mean apnea-hypopnea index (AHI) was 6.7 in the active-treatment group, compared with 16.7 in the placebo group. A total of 49% of the patients receiving active treatment had an AHI lower than 5, compared with only 11% of those using the placebo device, for an odds ratio of 7.8 and a number needed to treat of 3.
Snoring and symptoms of restless legs also were significantly less frequent with the active treatment, Dr. Marklund and her associates said.
In addition, 73% of patients who used oral appliances said that their expectations of treatment were either “totally” or “sufficiently” fulfilled, compared with only 11% of those who used placebo devices. And 89% of patients who used oral appliances said they would continue the treatment after completing the study, compared with only 52% of those who used the sham device.
However, daytime sleepiness, measured subjectively using the Epworth Sleepiness Scale and the Karolinska Sleepiness Scale and measured objectively using the Oxford Sleep Resistance test, did not differ significantly between the two study groups. The number of days with headaches, the intensity of headaches, the presence of nasal congestion, difficulty falling asleep, nighttime awakenings, nightmares, and reaction times also were not significantly different, nor were scores on a quality of life measure.
The study was supported by grants from the Swedish Research Council, the Swedish Heart and Lung Foundation, and the County Council of Vasterbotten. Dr. Marklund and her associates reported no conflicts of interest.
It appears that patients generally prefer these devices to continuous positive airway pressure (CPAP) therapy. Better adherence to an oral appliance may outweigh the fact that it is not as effective as CPAP. Long-term studies comparing the two approaches are warranted.
The benefits of the mandibular advancement devices used in this study cannot be translated automatically to other devices, because there is a huge variety of these appliances on the market.
The extent of the protrusion of the lower jaw, the stability of the material, and the structural design of the devices vary widely. Several experts currently recommend avoiding the less sophisticated appliances that are not tailored to the individual’s jaw and oral cavity and instead using only customized adjustable appliances made by a trained specialist.
Dr. Winfried J. Randerath is with the pneumonology clinic and the Allergology Center for Sleep Medicine and Respiratory Care at Bethanien Hospital in Solingen, Germany. He reported having no relevant financial disclosures. He has, however, received speaking fees and research funds from companies that produce positive airway pressure devices: Heinen und Lowenstein, Resmed, Respironics, and Weinmann. Dr. Randerath made these remarks in an invited commentary (JAMA Intern. Med. 2015 June 1 [doi:10.1001/jamainternmed.2015.2059]).
It appears that patients generally prefer these devices to continuous positive airway pressure (CPAP) therapy. Better adherence to an oral appliance may outweigh the fact that it is not as effective as CPAP. Long-term studies comparing the two approaches are warranted.
The benefits of the mandibular advancement devices used in this study cannot be translated automatically to other devices, because there is a huge variety of these appliances on the market.
The extent of the protrusion of the lower jaw, the stability of the material, and the structural design of the devices vary widely. Several experts currently recommend avoiding the less sophisticated appliances that are not tailored to the individual’s jaw and oral cavity and instead using only customized adjustable appliances made by a trained specialist.
Dr. Winfried J. Randerath is with the pneumonology clinic and the Allergology Center for Sleep Medicine and Respiratory Care at Bethanien Hospital in Solingen, Germany. He reported having no relevant financial disclosures. He has, however, received speaking fees and research funds from companies that produce positive airway pressure devices: Heinen und Lowenstein, Resmed, Respironics, and Weinmann. Dr. Randerath made these remarks in an invited commentary (JAMA Intern. Med. 2015 June 1 [doi:10.1001/jamainternmed.2015.2059]).
It appears that patients generally prefer these devices to continuous positive airway pressure (CPAP) therapy. Better adherence to an oral appliance may outweigh the fact that it is not as effective as CPAP. Long-term studies comparing the two approaches are warranted.
The benefits of the mandibular advancement devices used in this study cannot be translated automatically to other devices, because there is a huge variety of these appliances on the market.
The extent of the protrusion of the lower jaw, the stability of the material, and the structural design of the devices vary widely. Several experts currently recommend avoiding the less sophisticated appliances that are not tailored to the individual’s jaw and oral cavity and instead using only customized adjustable appliances made by a trained specialist.
Dr. Winfried J. Randerath is with the pneumonology clinic and the Allergology Center for Sleep Medicine and Respiratory Care at Bethanien Hospital in Solingen, Germany. He reported having no relevant financial disclosures. He has, however, received speaking fees and research funds from companies that produce positive airway pressure devices: Heinen und Lowenstein, Resmed, Respironics, and Weinmann. Dr. Randerath made these remarks in an invited commentary (JAMA Intern. Med. 2015 June 1 [doi:10.1001/jamainternmed.2015.2059]).
An oral appliance that advances a patient’s lower jaw reduced episodes of obstructive sleep apnea, snoring, and restless legs symptoms, according to a report published online June 1 in JAMA Internal Medicine.
The device, however, failed to improve daytime sleepiness or quality of life in a Swedish study of adults who had daytime sleepiness and either snoring or mild to moderate sleep apnea, said Marie Marklund, Ph.D., D.D.S., of the department of odontology at Umeå (Sweden) University and her associates (JAMA Intern. Med. 2015 June 1 [doi:10.1001/jamainternmed.2015.2051]).
Previous studies of oral appliances have focused on patients with more severe sleep apnea and have yielded conflicting results, particularly regarding daytime sleepiness.
A total of 91 patients who were randomly assigned to receive either a placebo device (46 patients) or an oral appliance individually made by a dental technician using separate plaster casts of the upper and lower teeth (45 participants) completed the study. The device’s elastomer pieces fitted over the teeth and were connected with a screw that allowed continuous gradual advancement of the lower jaw by 6-7 mm. Holding the lower mandible forward improves breathing during sleep.
After 4 months of follow-up, at-home overnight polysomnography showed “a clear, significant treatment effect”: the mean apnea-hypopnea index (AHI) was 6.7 in the active-treatment group, compared with 16.7 in the placebo group. A total of 49% of the patients receiving active treatment had an AHI lower than 5, compared with only 11% of those using the placebo device, for an odds ratio of 7.8 and a number needed to treat of 3.
Snoring and symptoms of restless legs also were significantly less frequent with the active treatment, Dr. Marklund and her associates said.
In addition, 73% of patients who used oral appliances said that their expectations of treatment were either “totally” or “sufficiently” fulfilled, compared with only 11% of those who used placebo devices. And 89% of patients who used oral appliances said they would continue the treatment after completing the study, compared with only 52% of those who used the sham device.
However, daytime sleepiness, measured subjectively using the Epworth Sleepiness Scale and the Karolinska Sleepiness Scale and measured objectively using the Oxford Sleep Resistance test, did not differ significantly between the two study groups. The number of days with headaches, the intensity of headaches, the presence of nasal congestion, difficulty falling asleep, nighttime awakenings, nightmares, and reaction times also were not significantly different, nor were scores on a quality of life measure.
The study was supported by grants from the Swedish Research Council, the Swedish Heart and Lung Foundation, and the County Council of Vasterbotten. Dr. Marklund and her associates reported no conflicts of interest.
An oral appliance that advances a patient’s lower jaw reduced episodes of obstructive sleep apnea, snoring, and restless legs symptoms, according to a report published online June 1 in JAMA Internal Medicine.
The device, however, failed to improve daytime sleepiness or quality of life in a Swedish study of adults who had daytime sleepiness and either snoring or mild to moderate sleep apnea, said Marie Marklund, Ph.D., D.D.S., of the department of odontology at Umeå (Sweden) University and her associates (JAMA Intern. Med. 2015 June 1 [doi:10.1001/jamainternmed.2015.2051]).
Previous studies of oral appliances have focused on patients with more severe sleep apnea and have yielded conflicting results, particularly regarding daytime sleepiness.
A total of 91 patients who were randomly assigned to receive either a placebo device (46 patients) or an oral appliance individually made by a dental technician using separate plaster casts of the upper and lower teeth (45 participants) completed the study. The device’s elastomer pieces fitted over the teeth and were connected with a screw that allowed continuous gradual advancement of the lower jaw by 6-7 mm. Holding the lower mandible forward improves breathing during sleep.
After 4 months of follow-up, at-home overnight polysomnography showed “a clear, significant treatment effect”: the mean apnea-hypopnea index (AHI) was 6.7 in the active-treatment group, compared with 16.7 in the placebo group. A total of 49% of the patients receiving active treatment had an AHI lower than 5, compared with only 11% of those using the placebo device, for an odds ratio of 7.8 and a number needed to treat of 3.
Snoring and symptoms of restless legs also were significantly less frequent with the active treatment, Dr. Marklund and her associates said.
In addition, 73% of patients who used oral appliances said that their expectations of treatment were either “totally” or “sufficiently” fulfilled, compared with only 11% of those who used placebo devices. And 89% of patients who used oral appliances said they would continue the treatment after completing the study, compared with only 52% of those who used the sham device.
However, daytime sleepiness, measured subjectively using the Epworth Sleepiness Scale and the Karolinska Sleepiness Scale and measured objectively using the Oxford Sleep Resistance test, did not differ significantly between the two study groups. The number of days with headaches, the intensity of headaches, the presence of nasal congestion, difficulty falling asleep, nighttime awakenings, nightmares, and reaction times also were not significantly different, nor were scores on a quality of life measure.
The study was supported by grants from the Swedish Research Council, the Swedish Heart and Lung Foundation, and the County Council of Vasterbotten. Dr. Marklund and her associates reported no conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Key clinical point: An oral appliance to advance the lower jaw reduced apneic episodes, snoring, and restless legs symptoms.
Major finding: Half of the patients receiving active treatment had an apnea-hypopnea index lower than 5, compared with only 11% of those using the placebo device, for an odds ratio of 7.8 and a number-needed-to-treat of 3.
Data source: A randomized, single-blind trial comparing a customized oral appliance against a placebo device in 96 adults with daytime sleepiness and either snoring or mild to moderate obstructive sleep apnea.
Disclosures: This study was supported by grants from the Swedish Research Council, the Swedish Heart and Lung Foundation, and the County Council of Vasterbotten. Dr. Marklund and her associates reported no conflicts of interest.
CDC investigating accidental anthrax shipment to labs
The Centers for Disease Control and Prevention is investigating a shipment of anthrax mistakenly sent to labs in the United States and abroad from the Department of Defense, the agency said in a May 30 announcement.
The presence of anthrax was confirmed after a laboratory working with the DOD reported being able to grow live Bacillus anthracis bacteria, although an inactive agent was expected. The lab was working with the DOD to develop a diagnostic test to identify biological threats, the CDC reported.
The accidental shipment is not believed to pose a risk to the public, the CDC said. Samples are being sent to the CDC or Laboratory Response Network labs for testing, and CDC officials are performing onsite investigations at the laboratories involved.
The Centers for Disease Control and Prevention is investigating a shipment of anthrax mistakenly sent to labs in the United States and abroad from the Department of Defense, the agency said in a May 30 announcement.
The presence of anthrax was confirmed after a laboratory working with the DOD reported being able to grow live Bacillus anthracis bacteria, although an inactive agent was expected. The lab was working with the DOD to develop a diagnostic test to identify biological threats, the CDC reported.
The accidental shipment is not believed to pose a risk to the public, the CDC said. Samples are being sent to the CDC or Laboratory Response Network labs for testing, and CDC officials are performing onsite investigations at the laboratories involved.
The Centers for Disease Control and Prevention is investigating a shipment of anthrax mistakenly sent to labs in the United States and abroad from the Department of Defense, the agency said in a May 30 announcement.
The presence of anthrax was confirmed after a laboratory working with the DOD reported being able to grow live Bacillus anthracis bacteria, although an inactive agent was expected. The lab was working with the DOD to develop a diagnostic test to identify biological threats, the CDC reported.
The accidental shipment is not believed to pose a risk to the public, the CDC said. Samples are being sent to the CDC or Laboratory Response Network labs for testing, and CDC officials are performing onsite investigations at the laboratories involved.
June 2015: Click for Credit
Here are 6 articles in the June issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. ACCP and CTS Issue Joint Guideline on COPD Exacerbations
To take the posttest, go to: http://bit.ly/1EIadmv
VITALS
Key clinical point: The American College of Chest Physicians and the Canadian Thoracic Society have issued a guideline for prevention of acute exacerbations of COPD.
Major finding: COPD exacerbations are acute, trajectory changing, and often deadly manifestations of a chronic disease.
Data source: A comprehensive literature review on prevention of acute COPD exacerbations and a compilation of 33 recommendations and suggestions for clinicians in clinical practice.
Disclosures: The American College of Chest Physicians, the Canadian Thoracic Society, and the American Thoracic Society supported the project. Dr Criner reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
2. GI Symptoms Common in Parkinson Disease and Related Disorders
To take the posttest, go to: http://bit.ly/1AZSaXA
VITALS
Key clinical point: GI symptoms were linked with specific motor and nonmotor features of Parkinson disease and related disorders.
Major finding: Constipation was the most common symptom among all three parkinsonian disorders.
Data source: Multicenter, cross-sectional survey of 473 patients with Parkinson disease, atypical parkinsonism, or vascular parkinsonism.
Disclosures: The Collaborative Clinical Research Fund of Boramae Medical Center partially funded the work. The authors declared no relevant conflicts of interest.
3. Aerosolized Measles Vaccine Inferior to Subcutaneous
To take the posttest, go to: http://bit.ly/1RKJizC
VITALS
Key clinical point: An aerosolized measles vaccine was immunogenic but inferior to the subcutaneous vaccine at inducing seropositivity among babies residing in rural India.
Major finding: The primary endpoint—seropositivity for antibodies against measles at 91 days after vaccination—was 85.4% for aerosolized vaccine and 94.6% for subcutaneous.
Data source: An open-label, randomized noninferiority trial comparing aerosolized vs subcutaneous measles vaccination in 2,004 infants ages 9 to 11.9 months in villages in India.
Disclosures: This study was funded by the Bill and Melinda Gates Foundation. The Serum Institute of India provided vaccines free of charge, and Aerogen provided the delivery devices free of charge. Dr Low reported several grants plus monies paid to her institution from the World Health Organization for projects about vaccines and sexually transmitted infections; her associates reported ties to the Serum Institute of India, Aerogen, and Dance Biopharm. One associate has a patent pending on an aerosol device licensed to Novartis and another has a patent pending related to vaccine nebulizers.
4. Unrecognized Diabetes Common in Acute MI
To take the posttest, go to: http://bit.ly/1IB9sC8
VITALS
Key clinical point: Many patients presenting with acute MI had unrecognized diabetes, which, in most cases, remained undiagnosed, untreated, and unrecorded.
Major finding: Of 2,854 (10%) patients enrolled in an MI registry, 287 had A1C levels of 6.5% or higher on routine laboratory testing during hospitalization for acute MI, but treating physicians recognized only 101 of these cases of diabetes (35%).
Data source: A retrospective cohort study involving 2,854 adults presenting with acute MI to 24 US medical centers in a 3.5-year period.
Disclosures: This study was sponsored by the National Heart, Lung, and Blood Institute and supported by a research grant from Genentech. Dr Arnold reported receiving honoraria from Novartis; her associates reported ties to numerous industry sources.
5. Methotrexate and Biologics Linked to Higher Zoster Risk in Psoriasis
To take the posttest, go to: http://bit.ly/1AZScyF
VITALS
Key clinical point: The combination of methotrexate and biologics for the treatment of psoriasis may increase risk for herpes zoster.
Major finding: Combination therapy with both biologic medications and methotrexate was associated with a significant 66% increase in the incidence of herpes zoster over more than 11 years of follow-up.
Data source: Analysis of medical records for 95,941 patients with psoriasis.
Disclosures: One author reported consultancies and research grants from a range of pharmaceutical companies. There were no other disclosures.
6. ACP: Avoid ECG, MPI Cardiac Screening in Low-risk Patients
To take the posttest, go to: http://bit.ly/1e3NLha
Here are 6 articles in the June issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. ACCP and CTS Issue Joint Guideline on COPD Exacerbations
To take the posttest, go to: http://bit.ly/1EIadmv
VITALS
Key clinical point: The American College of Chest Physicians and the Canadian Thoracic Society have issued a guideline for prevention of acute exacerbations of COPD.
Major finding: COPD exacerbations are acute, trajectory changing, and often deadly manifestations of a chronic disease.
Data source: A comprehensive literature review on prevention of acute COPD exacerbations and a compilation of 33 recommendations and suggestions for clinicians in clinical practice.
Disclosures: The American College of Chest Physicians, the Canadian Thoracic Society, and the American Thoracic Society supported the project. Dr Criner reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
2. GI Symptoms Common in Parkinson Disease and Related Disorders
To take the posttest, go to: http://bit.ly/1AZSaXA
VITALS
Key clinical point: GI symptoms were linked with specific motor and nonmotor features of Parkinson disease and related disorders.
Major finding: Constipation was the most common symptom among all three parkinsonian disorders.
Data source: Multicenter, cross-sectional survey of 473 patients with Parkinson disease, atypical parkinsonism, or vascular parkinsonism.
Disclosures: The Collaborative Clinical Research Fund of Boramae Medical Center partially funded the work. The authors declared no relevant conflicts of interest.
3. Aerosolized Measles Vaccine Inferior to Subcutaneous
To take the posttest, go to: http://bit.ly/1RKJizC
VITALS
Key clinical point: An aerosolized measles vaccine was immunogenic but inferior to the subcutaneous vaccine at inducing seropositivity among babies residing in rural India.
Major finding: The primary endpoint—seropositivity for antibodies against measles at 91 days after vaccination—was 85.4% for aerosolized vaccine and 94.6% for subcutaneous.
Data source: An open-label, randomized noninferiority trial comparing aerosolized vs subcutaneous measles vaccination in 2,004 infants ages 9 to 11.9 months in villages in India.
Disclosures: This study was funded by the Bill and Melinda Gates Foundation. The Serum Institute of India provided vaccines free of charge, and Aerogen provided the delivery devices free of charge. Dr Low reported several grants plus monies paid to her institution from the World Health Organization for projects about vaccines and sexually transmitted infections; her associates reported ties to the Serum Institute of India, Aerogen, and Dance Biopharm. One associate has a patent pending on an aerosol device licensed to Novartis and another has a patent pending related to vaccine nebulizers.
4. Unrecognized Diabetes Common in Acute MI
To take the posttest, go to: http://bit.ly/1IB9sC8
VITALS
Key clinical point: Many patients presenting with acute MI had unrecognized diabetes, which, in most cases, remained undiagnosed, untreated, and unrecorded.
Major finding: Of 2,854 (10%) patients enrolled in an MI registry, 287 had A1C levels of 6.5% or higher on routine laboratory testing during hospitalization for acute MI, but treating physicians recognized only 101 of these cases of diabetes (35%).
Data source: A retrospective cohort study involving 2,854 adults presenting with acute MI to 24 US medical centers in a 3.5-year period.
Disclosures: This study was sponsored by the National Heart, Lung, and Blood Institute and supported by a research grant from Genentech. Dr Arnold reported receiving honoraria from Novartis; her associates reported ties to numerous industry sources.
5. Methotrexate and Biologics Linked to Higher Zoster Risk in Psoriasis
To take the posttest, go to: http://bit.ly/1AZScyF
VITALS
Key clinical point: The combination of methotrexate and biologics for the treatment of psoriasis may increase risk for herpes zoster.
Major finding: Combination therapy with both biologic medications and methotrexate was associated with a significant 66% increase in the incidence of herpes zoster over more than 11 years of follow-up.
Data source: Analysis of medical records for 95,941 patients with psoriasis.
Disclosures: One author reported consultancies and research grants from a range of pharmaceutical companies. There were no other disclosures.
6. ACP: Avoid ECG, MPI Cardiac Screening in Low-risk Patients
To take the posttest, go to: http://bit.ly/1e3NLha
Here are 6 articles in the June issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. ACCP and CTS Issue Joint Guideline on COPD Exacerbations
To take the posttest, go to: http://bit.ly/1EIadmv
VITALS
Key clinical point: The American College of Chest Physicians and the Canadian Thoracic Society have issued a guideline for prevention of acute exacerbations of COPD.
Major finding: COPD exacerbations are acute, trajectory changing, and often deadly manifestations of a chronic disease.
Data source: A comprehensive literature review on prevention of acute COPD exacerbations and a compilation of 33 recommendations and suggestions for clinicians in clinical practice.
Disclosures: The American College of Chest Physicians, the Canadian Thoracic Society, and the American Thoracic Society supported the project. Dr Criner reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
2. GI Symptoms Common in Parkinson Disease and Related Disorders
To take the posttest, go to: http://bit.ly/1AZSaXA
VITALS
Key clinical point: GI symptoms were linked with specific motor and nonmotor features of Parkinson disease and related disorders.
Major finding: Constipation was the most common symptom among all three parkinsonian disorders.
Data source: Multicenter, cross-sectional survey of 473 patients with Parkinson disease, atypical parkinsonism, or vascular parkinsonism.
Disclosures: The Collaborative Clinical Research Fund of Boramae Medical Center partially funded the work. The authors declared no relevant conflicts of interest.
3. Aerosolized Measles Vaccine Inferior to Subcutaneous
To take the posttest, go to: http://bit.ly/1RKJizC
VITALS
Key clinical point: An aerosolized measles vaccine was immunogenic but inferior to the subcutaneous vaccine at inducing seropositivity among babies residing in rural India.
Major finding: The primary endpoint—seropositivity for antibodies against measles at 91 days after vaccination—was 85.4% for aerosolized vaccine and 94.6% for subcutaneous.
Data source: An open-label, randomized noninferiority trial comparing aerosolized vs subcutaneous measles vaccination in 2,004 infants ages 9 to 11.9 months in villages in India.
Disclosures: This study was funded by the Bill and Melinda Gates Foundation. The Serum Institute of India provided vaccines free of charge, and Aerogen provided the delivery devices free of charge. Dr Low reported several grants plus monies paid to her institution from the World Health Organization for projects about vaccines and sexually transmitted infections; her associates reported ties to the Serum Institute of India, Aerogen, and Dance Biopharm. One associate has a patent pending on an aerosol device licensed to Novartis and another has a patent pending related to vaccine nebulizers.
4. Unrecognized Diabetes Common in Acute MI
To take the posttest, go to: http://bit.ly/1IB9sC8
VITALS
Key clinical point: Many patients presenting with acute MI had unrecognized diabetes, which, in most cases, remained undiagnosed, untreated, and unrecorded.
Major finding: Of 2,854 (10%) patients enrolled in an MI registry, 287 had A1C levels of 6.5% or higher on routine laboratory testing during hospitalization for acute MI, but treating physicians recognized only 101 of these cases of diabetes (35%).
Data source: A retrospective cohort study involving 2,854 adults presenting with acute MI to 24 US medical centers in a 3.5-year period.
Disclosures: This study was sponsored by the National Heart, Lung, and Blood Institute and supported by a research grant from Genentech. Dr Arnold reported receiving honoraria from Novartis; her associates reported ties to numerous industry sources.
5. Methotrexate and Biologics Linked to Higher Zoster Risk in Psoriasis
To take the posttest, go to: http://bit.ly/1AZScyF
VITALS
Key clinical point: The combination of methotrexate and biologics for the treatment of psoriasis may increase risk for herpes zoster.
Major finding: Combination therapy with both biologic medications and methotrexate was associated with a significant 66% increase in the incidence of herpes zoster over more than 11 years of follow-up.
Data source: Analysis of medical records for 95,941 patients with psoriasis.
Disclosures: One author reported consultancies and research grants from a range of pharmaceutical companies. There were no other disclosures.
6. ACP: Avoid ECG, MPI Cardiac Screening in Low-risk Patients
To take the posttest, go to: http://bit.ly/1e3NLha
Is it safe to add long-acting β-2 agonists to inhaled corticosteroids in patients with persistent asthma?
Possibly. Long-acting β-2 agonists (LABAs) used in combination with inhaled corticosteroids (ICS) don’t appear to increase all-cause mortality or serious adverse events in patients with persistent asthma compared with ICS alone. Studies showing an increase in catastrophic events had serious methodologic issues. A large surveillance study is ongoing (strength of recommendation: A, meta-analysis of randomized controlled trials [RCTs]).
No significant difference in combination therapy vs ICS alone
In 2013, a Cochrane review analyzed the risk of mortality and nonfatal serious adverse events in patients treated with the LABA salmeterol in combination with ICS, compared with patients receiving the same dose of ICS alone.1 The review included 35 RCTs of moderate quality with 13,447 adolescents and adults and 5 RCTs with 1862 children. Patients had all stages of asthma; mean study duration was 34 weeks in adult trials and 15 weeks in trials of children.
Seven deaths from all causes occurred in both the salmeterol-plus-ICS group and the ICS-alone group (35 trials, N=13,447; Peto odds ratio [OR]=0.90; 95% confidence interval [CI], 0.31-2.6). No deaths in children and no asthma-related deaths occurred in any study participants (40 trials, N=15,309).
Adults treated with ICS alone showed no significant difference from adults receiving combination therapy in the frequency of serious adverse events (defined as life threatening, requiring hospitalization or prolongation of existing hospitalization, or resulting in persistent or significant disability or incapacity). Adults on ICS had 21 events per 1000 compared with 24 per 1000 in adults on combination treatment (35 trials, N=13,447; Peto OR=1.2; 95% CI, 0.91-1.4).
Asthma-related serious adverse events were reported in 29 of 6986 adults in the combination group and 23 of 6461 in the ICS-alone group, a nonsignificant difference (35 trials, N=13,447; Peto OR=1.1; 95% CI, 0.65-1.9).
Only one serious asthma-related adverse event occurred in each group of children (ICS- and combination-treated); (5 trials, N=1862; Peto OR=0.99; 95% CI, 0.6-16). Because the number of events was so small and the results were so imprecise, a relative increase in all-cause mortality or nonfatal adverse events can’t be completely ruled out.
Inconsistent dosages mar trials that show more catastrophic events
A systematic review of 7 RCTs with 7253 asthmatic patients compared LABA plus ICS or ICS alone at various doses. All of the trials included at least one catastrophic event, defined as an asthma-related intubation or death.2 The mean ages of the patients varied from 11 to 48 years, and the length of the studies from 12 to 52 weeks. The risk of catastrophic events was greater in the LABA plus ICS groups than ICS alone (OR=3.7; 95% CI, 1.4-9.6).
Only one of the 7 trials was included in the 2013 Cochrane review. The others were excluded because the control groups used different doses of ICS than the LABA-plus-ICS groups. In one trial, for example, the ICS group used 4 times the dose of budesonide used in the LABA-plus-ICS group. The difference in outcomes may therefore reflect the variation in ICS dose rather than the presence or absence of LABA.
Because of these conflicting results, the US Food and Drug Administration has mandated continued evaluation of LABAs by manufacturers.3 Five clinical trials that are multinational, randomized, double-blind, and lasting at least 6 months will evaluate the safety of LABAs plus fixed-dose ICS compared with fixed-dose ICS alone. A total of 6200 children and 46,800 adults will be enrolled in the studies, whose results should be available in 2017.
1. Cates CJ, Jaeschke R, Schmidt S, et al. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Syst Rev. 2013;(3):CD006922.
2. Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123:322-328.
3. Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteroids for treating asthma. N Engl J Med. 2011;364:2473-2475.
Possibly. Long-acting β-2 agonists (LABAs) used in combination with inhaled corticosteroids (ICS) don’t appear to increase all-cause mortality or serious adverse events in patients with persistent asthma compared with ICS alone. Studies showing an increase in catastrophic events had serious methodologic issues. A large surveillance study is ongoing (strength of recommendation: A, meta-analysis of randomized controlled trials [RCTs]).
No significant difference in combination therapy vs ICS alone
In 2013, a Cochrane review analyzed the risk of mortality and nonfatal serious adverse events in patients treated with the LABA salmeterol in combination with ICS, compared with patients receiving the same dose of ICS alone.1 The review included 35 RCTs of moderate quality with 13,447 adolescents and adults and 5 RCTs with 1862 children. Patients had all stages of asthma; mean study duration was 34 weeks in adult trials and 15 weeks in trials of children.
Seven deaths from all causes occurred in both the salmeterol-plus-ICS group and the ICS-alone group (35 trials, N=13,447; Peto odds ratio [OR]=0.90; 95% confidence interval [CI], 0.31-2.6). No deaths in children and no asthma-related deaths occurred in any study participants (40 trials, N=15,309).
Adults treated with ICS alone showed no significant difference from adults receiving combination therapy in the frequency of serious adverse events (defined as life threatening, requiring hospitalization or prolongation of existing hospitalization, or resulting in persistent or significant disability or incapacity). Adults on ICS had 21 events per 1000 compared with 24 per 1000 in adults on combination treatment (35 trials, N=13,447; Peto OR=1.2; 95% CI, 0.91-1.4).
Asthma-related serious adverse events were reported in 29 of 6986 adults in the combination group and 23 of 6461 in the ICS-alone group, a nonsignificant difference (35 trials, N=13,447; Peto OR=1.1; 95% CI, 0.65-1.9).
Only one serious asthma-related adverse event occurred in each group of children (ICS- and combination-treated); (5 trials, N=1862; Peto OR=0.99; 95% CI, 0.6-16). Because the number of events was so small and the results were so imprecise, a relative increase in all-cause mortality or nonfatal adverse events can’t be completely ruled out.
Inconsistent dosages mar trials that show more catastrophic events
A systematic review of 7 RCTs with 7253 asthmatic patients compared LABA plus ICS or ICS alone at various doses. All of the trials included at least one catastrophic event, defined as an asthma-related intubation or death.2 The mean ages of the patients varied from 11 to 48 years, and the length of the studies from 12 to 52 weeks. The risk of catastrophic events was greater in the LABA plus ICS groups than ICS alone (OR=3.7; 95% CI, 1.4-9.6).
Only one of the 7 trials was included in the 2013 Cochrane review. The others were excluded because the control groups used different doses of ICS than the LABA-plus-ICS groups. In one trial, for example, the ICS group used 4 times the dose of budesonide used in the LABA-plus-ICS group. The difference in outcomes may therefore reflect the variation in ICS dose rather than the presence or absence of LABA.
Because of these conflicting results, the US Food and Drug Administration has mandated continued evaluation of LABAs by manufacturers.3 Five clinical trials that are multinational, randomized, double-blind, and lasting at least 6 months will evaluate the safety of LABAs plus fixed-dose ICS compared with fixed-dose ICS alone. A total of 6200 children and 46,800 adults will be enrolled in the studies, whose results should be available in 2017.
Possibly. Long-acting β-2 agonists (LABAs) used in combination with inhaled corticosteroids (ICS) don’t appear to increase all-cause mortality or serious adverse events in patients with persistent asthma compared with ICS alone. Studies showing an increase in catastrophic events had serious methodologic issues. A large surveillance study is ongoing (strength of recommendation: A, meta-analysis of randomized controlled trials [RCTs]).
No significant difference in combination therapy vs ICS alone
In 2013, a Cochrane review analyzed the risk of mortality and nonfatal serious adverse events in patients treated with the LABA salmeterol in combination with ICS, compared with patients receiving the same dose of ICS alone.1 The review included 35 RCTs of moderate quality with 13,447 adolescents and adults and 5 RCTs with 1862 children. Patients had all stages of asthma; mean study duration was 34 weeks in adult trials and 15 weeks in trials of children.
Seven deaths from all causes occurred in both the salmeterol-plus-ICS group and the ICS-alone group (35 trials, N=13,447; Peto odds ratio [OR]=0.90; 95% confidence interval [CI], 0.31-2.6). No deaths in children and no asthma-related deaths occurred in any study participants (40 trials, N=15,309).
Adults treated with ICS alone showed no significant difference from adults receiving combination therapy in the frequency of serious adverse events (defined as life threatening, requiring hospitalization or prolongation of existing hospitalization, or resulting in persistent or significant disability or incapacity). Adults on ICS had 21 events per 1000 compared with 24 per 1000 in adults on combination treatment (35 trials, N=13,447; Peto OR=1.2; 95% CI, 0.91-1.4).
Asthma-related serious adverse events were reported in 29 of 6986 adults in the combination group and 23 of 6461 in the ICS-alone group, a nonsignificant difference (35 trials, N=13,447; Peto OR=1.1; 95% CI, 0.65-1.9).
Only one serious asthma-related adverse event occurred in each group of children (ICS- and combination-treated); (5 trials, N=1862; Peto OR=0.99; 95% CI, 0.6-16). Because the number of events was so small and the results were so imprecise, a relative increase in all-cause mortality or nonfatal adverse events can’t be completely ruled out.
Inconsistent dosages mar trials that show more catastrophic events
A systematic review of 7 RCTs with 7253 asthmatic patients compared LABA plus ICS or ICS alone at various doses. All of the trials included at least one catastrophic event, defined as an asthma-related intubation or death.2 The mean ages of the patients varied from 11 to 48 years, and the length of the studies from 12 to 52 weeks. The risk of catastrophic events was greater in the LABA plus ICS groups than ICS alone (OR=3.7; 95% CI, 1.4-9.6).
Only one of the 7 trials was included in the 2013 Cochrane review. The others were excluded because the control groups used different doses of ICS than the LABA-plus-ICS groups. In one trial, for example, the ICS group used 4 times the dose of budesonide used in the LABA-plus-ICS group. The difference in outcomes may therefore reflect the variation in ICS dose rather than the presence or absence of LABA.
Because of these conflicting results, the US Food and Drug Administration has mandated continued evaluation of LABAs by manufacturers.3 Five clinical trials that are multinational, randomized, double-blind, and lasting at least 6 months will evaluate the safety of LABAs plus fixed-dose ICS compared with fixed-dose ICS alone. A total of 6200 children and 46,800 adults will be enrolled in the studies, whose results should be available in 2017.
1. Cates CJ, Jaeschke R, Schmidt S, et al. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Syst Rev. 2013;(3):CD006922.
2. Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123:322-328.
3. Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteroids for treating asthma. N Engl J Med. 2011;364:2473-2475.
1. Cates CJ, Jaeschke R, Schmidt S, et al. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Syst Rev. 2013;(3):CD006922.
2. Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123:322-328.
3. Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteroids for treating asthma. N Engl J Med. 2011;364:2473-2475.
Evidence-based answers from the Family Physicians Inquiries Network
FDA approves sirolimus for rare lung disease
The Food and Drug Administration has approved Rapamune for the treatment of lymphangioleiomyomatosis (LAM), a rare, progressive lung disease, the agency announced May 28.
LAM primarily affects women of childbearing age and is characterized by abnormal growth of smooth muscle cells that can cause destruction of the lung, limiting the delivery of oxygen to the body.
Rapamune (sirolimus) was originally approved in 1999 as an immunosuppressive agent to help prevent organ rejection in patients 13 years and older who were receiving kidney transplants. The drug is available as both a tablet and an oral solution.
In a clinical trial, researchers measured the safety and efficacy of the drug for treatment of LAM by comparing Rapamune with placebo in 89 patients for a 12-month treatment period, followed by a 12-month observation period.
During the 12-month treatment period, the difference in the average decrease in FEV1 (the rate of change in how much air a person can exhale during a forced breath in 1 second) was approximately 153 mL. After discontinuation of Rapamune, the decline in lung function resumed at a rate similar to the placebo group.
Because there are no available alternatives approved for treatment of LAM, Rapamune received orphan product designation from the FDA, which provides grants for clinical studies on safety and/or effectiveness of drugs used to treat rare diseases such as LAM.
Common side effects associated with Rapamune for the treatment of LAM include mouth and lip ulcers, diarrhea, abdominal pain, nausea, sore throat, acne, chest pain, leg swelling, upper respiratory tract infection, headache, dizziness, muscle pain, and elevated cholesterol.
Rapamune is manufactured by Wyeth Pharmaceuticals, a subsidiary of Pfizer.
For more safety information, please visit the FDA website.
The Food and Drug Administration has approved Rapamune for the treatment of lymphangioleiomyomatosis (LAM), a rare, progressive lung disease, the agency announced May 28.
LAM primarily affects women of childbearing age and is characterized by abnormal growth of smooth muscle cells that can cause destruction of the lung, limiting the delivery of oxygen to the body.
Rapamune (sirolimus) was originally approved in 1999 as an immunosuppressive agent to help prevent organ rejection in patients 13 years and older who were receiving kidney transplants. The drug is available as both a tablet and an oral solution.
In a clinical trial, researchers measured the safety and efficacy of the drug for treatment of LAM by comparing Rapamune with placebo in 89 patients for a 12-month treatment period, followed by a 12-month observation period.
During the 12-month treatment period, the difference in the average decrease in FEV1 (the rate of change in how much air a person can exhale during a forced breath in 1 second) was approximately 153 mL. After discontinuation of Rapamune, the decline in lung function resumed at a rate similar to the placebo group.
Because there are no available alternatives approved for treatment of LAM, Rapamune received orphan product designation from the FDA, which provides grants for clinical studies on safety and/or effectiveness of drugs used to treat rare diseases such as LAM.
Common side effects associated with Rapamune for the treatment of LAM include mouth and lip ulcers, diarrhea, abdominal pain, nausea, sore throat, acne, chest pain, leg swelling, upper respiratory tract infection, headache, dizziness, muscle pain, and elevated cholesterol.
Rapamune is manufactured by Wyeth Pharmaceuticals, a subsidiary of Pfizer.
For more safety information, please visit the FDA website.
The Food and Drug Administration has approved Rapamune for the treatment of lymphangioleiomyomatosis (LAM), a rare, progressive lung disease, the agency announced May 28.
LAM primarily affects women of childbearing age and is characterized by abnormal growth of smooth muscle cells that can cause destruction of the lung, limiting the delivery of oxygen to the body.
Rapamune (sirolimus) was originally approved in 1999 as an immunosuppressive agent to help prevent organ rejection in patients 13 years and older who were receiving kidney transplants. The drug is available as both a tablet and an oral solution.
In a clinical trial, researchers measured the safety and efficacy of the drug for treatment of LAM by comparing Rapamune with placebo in 89 patients for a 12-month treatment period, followed by a 12-month observation period.
During the 12-month treatment period, the difference in the average decrease in FEV1 (the rate of change in how much air a person can exhale during a forced breath in 1 second) was approximately 153 mL. After discontinuation of Rapamune, the decline in lung function resumed at a rate similar to the placebo group.
Because there are no available alternatives approved for treatment of LAM, Rapamune received orphan product designation from the FDA, which provides grants for clinical studies on safety and/or effectiveness of drugs used to treat rare diseases such as LAM.
Common side effects associated with Rapamune for the treatment of LAM include mouth and lip ulcers, diarrhea, abdominal pain, nausea, sore throat, acne, chest pain, leg swelling, upper respiratory tract infection, headache, dizziness, muscle pain, and elevated cholesterol.
Rapamune is manufactured by Wyeth Pharmaceuticals, a subsidiary of Pfizer.
For more safety information, please visit the FDA website.
Exercise-induced Anaphylaxis
Anaphylaxis is a relatively common occurrence for many adolescents. As primary care doctors, we normally see the patient after the acute phase, and then are required to do the detective work to figure out the causes of the episode. The cause may be obvious, but many times we have to hope for another occurrence with similar circumstances to identify it. Surprisingly, the cause may not be what you think. Factors that contribute to an anaphylaxis response may be related to activity, timing of food ingestion, an environmental factor, or medication.
Let’s look at just one type, exercise-induced anaphylaxis. It’s divided into two categories: food dependent and nonfood dependent. Both are described as an induction of itching, urticaria, and fatigue, with progression to angioedema and hypotension, associated with exercise (J. Allergy Clin. Immunol. 1980;66:106-11).
Food-dependent exercise-induced anaphylaxis occurs when exercise is started 30 minutes after ingesting food. This may be difficult to identify because patients react to the food only if they exercise, so food is usually eliminated as a cause. Wheat and wheat flour are common culprits for this type of reaction because of the omega-5 gliadin, which is the protein in gluten (J. Allergy Clin. Immunol. 1991;87:34-40). In one study, larger amounts of the suspected agent were given; hives and angioedema did start to occur in 20% of patients challenged, which suggested that there was likely a baseline allergy to the food, and exercise itself might be a cofactor in augmentation of the allergic reaction.
In nonfood-dependent exercise-induced anaphylaxis, symptoms of itching, urticaria, and fatigue can occur 5-30 minutes after the start of exercise. Although bronchospasm is rare, it can occur along with angioedema, nausea, vomiting, and hypotension, and can even be fatal if exercise continues. If exercise is stopped, it usually resolves. However, many people try to push through it, which only worsens the symptoms.
Cofactors associated with nonfood-dependent exercise-induced anaphylaxis are ingestion of alcohol and an NSAID several hours beforehand. These agents also might be overlooked if well tolerated independently (Br. J. Dermatol. 2001;145:336-9).
Timing of the episode also plays a role. Premenstrual syndrome can be a factor in augmentation of anaphylaxis, so it also should be considered. Knowing the date of the last menstrual cycle and identifying if the anaphylaxis is episodic will identify premenstrual syndrome as a cause.
The work-up should include standard allergy testing and determination of tryptase levels. Skin testing is essential to identify offending agents, and is rarely negative. If a food is suspected and skin testing is negative, repeat the skin testing in 6 months. In one study, wheat extract was found to be positive in only 29% of persons suspected of having a wheat allergy, but when the paste of wheat flour was tested, 80% were identified. The ImmunoCAP Test also was found to have a sensitivity of 80%, so it is a valuable test to try along with the skin prick.
Tryptase levels should be evaluated because in nonfood-dependent exercise-induced anaphylaxis, these levels are slightly elevated at the time of the anaphylaxis, but return to normal. A patient with mastocytosis, a group of disorders characterized by pathologic mast cells infiltrating the skin, will consistently have elevated tryptase levels. Seasonal allergies associated with pollen, and asthma bronchospasm also should be considered as causes.
Although these exercise-induced anaphylaxis episodes can occur at any age, they are most frequent in the adolescent age group, probably because that’s the time most of this population are involved in organized sports. Upon presentation, a careful detailed history will help to identify the cause of anaphylaxis and result in quicker resolution.
Treatment includes avoidance of the offending agent if identified and an antihistamine, and if symptoms do occur, ceasing exercise immediately to avoid a full-blown anaphylactic reaction.
Dr. Pearce is a pediatrician in Frankfort, Ill.
Anaphylaxis is a relatively common occurrence for many adolescents. As primary care doctors, we normally see the patient after the acute phase, and then are required to do the detective work to figure out the causes of the episode. The cause may be obvious, but many times we have to hope for another occurrence with similar circumstances to identify it. Surprisingly, the cause may not be what you think. Factors that contribute to an anaphylaxis response may be related to activity, timing of food ingestion, an environmental factor, or medication.
Let’s look at just one type, exercise-induced anaphylaxis. It’s divided into two categories: food dependent and nonfood dependent. Both are described as an induction of itching, urticaria, and fatigue, with progression to angioedema and hypotension, associated with exercise (J. Allergy Clin. Immunol. 1980;66:106-11).
Food-dependent exercise-induced anaphylaxis occurs when exercise is started 30 minutes after ingesting food. This may be difficult to identify because patients react to the food only if they exercise, so food is usually eliminated as a cause. Wheat and wheat flour are common culprits for this type of reaction because of the omega-5 gliadin, which is the protein in gluten (J. Allergy Clin. Immunol. 1991;87:34-40). In one study, larger amounts of the suspected agent were given; hives and angioedema did start to occur in 20% of patients challenged, which suggested that there was likely a baseline allergy to the food, and exercise itself might be a cofactor in augmentation of the allergic reaction.
In nonfood-dependent exercise-induced anaphylaxis, symptoms of itching, urticaria, and fatigue can occur 5-30 minutes after the start of exercise. Although bronchospasm is rare, it can occur along with angioedema, nausea, vomiting, and hypotension, and can even be fatal if exercise continues. If exercise is stopped, it usually resolves. However, many people try to push through it, which only worsens the symptoms.
Cofactors associated with nonfood-dependent exercise-induced anaphylaxis are ingestion of alcohol and an NSAID several hours beforehand. These agents also might be overlooked if well tolerated independently (Br. J. Dermatol. 2001;145:336-9).
Timing of the episode also plays a role. Premenstrual syndrome can be a factor in augmentation of anaphylaxis, so it also should be considered. Knowing the date of the last menstrual cycle and identifying if the anaphylaxis is episodic will identify premenstrual syndrome as a cause.
The work-up should include standard allergy testing and determination of tryptase levels. Skin testing is essential to identify offending agents, and is rarely negative. If a food is suspected and skin testing is negative, repeat the skin testing in 6 months. In one study, wheat extract was found to be positive in only 29% of persons suspected of having a wheat allergy, but when the paste of wheat flour was tested, 80% were identified. The ImmunoCAP Test also was found to have a sensitivity of 80%, so it is a valuable test to try along with the skin prick.
Tryptase levels should be evaluated because in nonfood-dependent exercise-induced anaphylaxis, these levels are slightly elevated at the time of the anaphylaxis, but return to normal. A patient with mastocytosis, a group of disorders characterized by pathologic mast cells infiltrating the skin, will consistently have elevated tryptase levels. Seasonal allergies associated with pollen, and asthma bronchospasm also should be considered as causes.
Although these exercise-induced anaphylaxis episodes can occur at any age, they are most frequent in the adolescent age group, probably because that’s the time most of this population are involved in organized sports. Upon presentation, a careful detailed history will help to identify the cause of anaphylaxis and result in quicker resolution.
Treatment includes avoidance of the offending agent if identified and an antihistamine, and if symptoms do occur, ceasing exercise immediately to avoid a full-blown anaphylactic reaction.
Dr. Pearce is a pediatrician in Frankfort, Ill.
Anaphylaxis is a relatively common occurrence for many adolescents. As primary care doctors, we normally see the patient after the acute phase, and then are required to do the detective work to figure out the causes of the episode. The cause may be obvious, but many times we have to hope for another occurrence with similar circumstances to identify it. Surprisingly, the cause may not be what you think. Factors that contribute to an anaphylaxis response may be related to activity, timing of food ingestion, an environmental factor, or medication.
Let’s look at just one type, exercise-induced anaphylaxis. It’s divided into two categories: food dependent and nonfood dependent. Both are described as an induction of itching, urticaria, and fatigue, with progression to angioedema and hypotension, associated with exercise (J. Allergy Clin. Immunol. 1980;66:106-11).
Food-dependent exercise-induced anaphylaxis occurs when exercise is started 30 minutes after ingesting food. This may be difficult to identify because patients react to the food only if they exercise, so food is usually eliminated as a cause. Wheat and wheat flour are common culprits for this type of reaction because of the omega-5 gliadin, which is the protein in gluten (J. Allergy Clin. Immunol. 1991;87:34-40). In one study, larger amounts of the suspected agent were given; hives and angioedema did start to occur in 20% of patients challenged, which suggested that there was likely a baseline allergy to the food, and exercise itself might be a cofactor in augmentation of the allergic reaction.
In nonfood-dependent exercise-induced anaphylaxis, symptoms of itching, urticaria, and fatigue can occur 5-30 minutes after the start of exercise. Although bronchospasm is rare, it can occur along with angioedema, nausea, vomiting, and hypotension, and can even be fatal if exercise continues. If exercise is stopped, it usually resolves. However, many people try to push through it, which only worsens the symptoms.
Cofactors associated with nonfood-dependent exercise-induced anaphylaxis are ingestion of alcohol and an NSAID several hours beforehand. These agents also might be overlooked if well tolerated independently (Br. J. Dermatol. 2001;145:336-9).
Timing of the episode also plays a role. Premenstrual syndrome can be a factor in augmentation of anaphylaxis, so it also should be considered. Knowing the date of the last menstrual cycle and identifying if the anaphylaxis is episodic will identify premenstrual syndrome as a cause.
The work-up should include standard allergy testing and determination of tryptase levels. Skin testing is essential to identify offending agents, and is rarely negative. If a food is suspected and skin testing is negative, repeat the skin testing in 6 months. In one study, wheat extract was found to be positive in only 29% of persons suspected of having a wheat allergy, but when the paste of wheat flour was tested, 80% were identified. The ImmunoCAP Test also was found to have a sensitivity of 80%, so it is a valuable test to try along with the skin prick.
Tryptase levels should be evaluated because in nonfood-dependent exercise-induced anaphylaxis, these levels are slightly elevated at the time of the anaphylaxis, but return to normal. A patient with mastocytosis, a group of disorders characterized by pathologic mast cells infiltrating the skin, will consistently have elevated tryptase levels. Seasonal allergies associated with pollen, and asthma bronchospasm also should be considered as causes.
Although these exercise-induced anaphylaxis episodes can occur at any age, they are most frequent in the adolescent age group, probably because that’s the time most of this population are involved in organized sports. Upon presentation, a careful detailed history will help to identify the cause of anaphylaxis and result in quicker resolution.
Treatment includes avoidance of the offending agent if identified and an antihistamine, and if symptoms do occur, ceasing exercise immediately to avoid a full-blown anaphylactic reaction.
Dr. Pearce is a pediatrician in Frankfort, Ill.
Exercise-induced anaphylaxis
Anaphylaxis is a relatively common occurrence for many adolescents. As primary care doctors, we normally see the patient after the acute phase, and then are required to do the detective work to figure out the causes of the episode. The cause may be obvious, but many times we have to hope for another occurrence with similar circumstances to identify it. Surprisingly, the cause may not be what you think. Factors that contribute to an anaphylaxis response may be related to activity, timing of food ingestion, an environmental factor, or medication.
Let’s look at just one type, exercise-induced anaphylaxis. It’s divided into two categories: food dependent and nonfood dependent. Both are described as an induction of itching, urticaria, and fatigue, with progression to angioedema and hypotension, associated with exercise (J. Allergy Clin. Immunol. 1980;66:106-11).
Food-dependent exercise-induced anaphylaxis occurs when exercise is started 30 minutes after ingesting food. This may be difficult to identify because patients react to the food only if they exercise, so food is usually eliminated as a cause. Wheat and wheat flour are common culprits for this type of reaction because of the omega-5 gliadin, which is the protein in gluten (J. Allergy Clin. Immunol. 1991;87:34-40). In one study, larger amounts of the suspected agent were given; hives and angioedema did start to occur in 20% of patients challenged, which suggested that there was likely a baseline allergy to the food, and exercise itself might be a cofactor in augmentation of the allergic reaction.
In nonfood-dependent exercise-induced anaphylaxis, symptoms of itching, urticaria, and fatigue can occur 5-30 minutes after the start of exercise. Although bronchospasm is rare, it can occur along with angioedema, nausea, vomiting, and hypotension, and can even be fatal if exercise continues. If exercise is stopped, it usually resolves. However, many people try to push through it, which only worsens the symptoms.
Cofactors associated with nonfood-dependent exercise-induced anaphylaxis are ingestion of alcohol and an NSAID several hours beforehand. These agents also might be overlooked if well tolerated independently (Br. J. Dermatol. 2001;145:336-9).
Timing of the episode also plays a role. Premenstrual syndrome can be a factor in augmentation of anaphylaxis, so it also should be considered. Knowing the date of the last menstrual cycle and identifying if the anaphylaxis is episodic will identify premenstrual syndrome as a cause.
The work-up should include standard allergy testing and determination of tryptase levels. Skin testing is essential to identify offending agents, and is rarely negative. If a food is suspected and skin testing is negative, repeat the skin testing in 6 months. In one study, wheat extract was found to be positive in only 29% of persons suspected of having a wheat allergy, but when the paste of wheat flour was tested, 80% were identified. The ImmunoCAP Test also was found to have a sensitivity of 80%, so it is a valuable test to try along with the skin prick.
Tryptase levels should be evaluated because in nonfood-dependent exercise-induced anaphylaxis, these levels are slightly elevated at the time of the anaphylaxis, but return to normal. A patient with mastocytosis, a group of disorders characterized by pathologic mast cells infiltrating the skin, will consistently have elevated tryptase levels. Seasonal allergies associated with pollen, and asthma bronchospasm also should be considered as causes.
Although these exercise-induced anaphylaxis episodes can occur at any age, they are most frequent in the adolescent age group, probably because that’s the time most of this population are involved in organized sports. Upon presentation, a careful detailed history will help to identify the cause of anaphylaxis and result in quicker resolution.
Treatment includes avoidance of the offending agent if identified and an antihistamine, and if symptoms do occur, ceasing exercise immediately to avoid a full-blown anaphylactic reaction.
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected].
Anaphylaxis is a relatively common occurrence for many adolescents. As primary care doctors, we normally see the patient after the acute phase, and then are required to do the detective work to figure out the causes of the episode. The cause may be obvious, but many times we have to hope for another occurrence with similar circumstances to identify it. Surprisingly, the cause may not be what you think. Factors that contribute to an anaphylaxis response may be related to activity, timing of food ingestion, an environmental factor, or medication.
Let’s look at just one type, exercise-induced anaphylaxis. It’s divided into two categories: food dependent and nonfood dependent. Both are described as an induction of itching, urticaria, and fatigue, with progression to angioedema and hypotension, associated with exercise (J. Allergy Clin. Immunol. 1980;66:106-11).
Food-dependent exercise-induced anaphylaxis occurs when exercise is started 30 minutes after ingesting food. This may be difficult to identify because patients react to the food only if they exercise, so food is usually eliminated as a cause. Wheat and wheat flour are common culprits for this type of reaction because of the omega-5 gliadin, which is the protein in gluten (J. Allergy Clin. Immunol. 1991;87:34-40). In one study, larger amounts of the suspected agent were given; hives and angioedema did start to occur in 20% of patients challenged, which suggested that there was likely a baseline allergy to the food, and exercise itself might be a cofactor in augmentation of the allergic reaction.
In nonfood-dependent exercise-induced anaphylaxis, symptoms of itching, urticaria, and fatigue can occur 5-30 minutes after the start of exercise. Although bronchospasm is rare, it can occur along with angioedema, nausea, vomiting, and hypotension, and can even be fatal if exercise continues. If exercise is stopped, it usually resolves. However, many people try to push through it, which only worsens the symptoms.
Cofactors associated with nonfood-dependent exercise-induced anaphylaxis are ingestion of alcohol and an NSAID several hours beforehand. These agents also might be overlooked if well tolerated independently (Br. J. Dermatol. 2001;145:336-9).
Timing of the episode also plays a role. Premenstrual syndrome can be a factor in augmentation of anaphylaxis, so it also should be considered. Knowing the date of the last menstrual cycle and identifying if the anaphylaxis is episodic will identify premenstrual syndrome as a cause.
The work-up should include standard allergy testing and determination of tryptase levels. Skin testing is essential to identify offending agents, and is rarely negative. If a food is suspected and skin testing is negative, repeat the skin testing in 6 months. In one study, wheat extract was found to be positive in only 29% of persons suspected of having a wheat allergy, but when the paste of wheat flour was tested, 80% were identified. The ImmunoCAP Test also was found to have a sensitivity of 80%, so it is a valuable test to try along with the skin prick.
Tryptase levels should be evaluated because in nonfood-dependent exercise-induced anaphylaxis, these levels are slightly elevated at the time of the anaphylaxis, but return to normal. A patient with mastocytosis, a group of disorders characterized by pathologic mast cells infiltrating the skin, will consistently have elevated tryptase levels. Seasonal allergies associated with pollen, and asthma bronchospasm also should be considered as causes.
Although these exercise-induced anaphylaxis episodes can occur at any age, they are most frequent in the adolescent age group, probably because that’s the time most of this population are involved in organized sports. Upon presentation, a careful detailed history will help to identify the cause of anaphylaxis and result in quicker resolution.
Treatment includes avoidance of the offending agent if identified and an antihistamine, and if symptoms do occur, ceasing exercise immediately to avoid a full-blown anaphylactic reaction.
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected].
Anaphylaxis is a relatively common occurrence for many adolescents. As primary care doctors, we normally see the patient after the acute phase, and then are required to do the detective work to figure out the causes of the episode. The cause may be obvious, but many times we have to hope for another occurrence with similar circumstances to identify it. Surprisingly, the cause may not be what you think. Factors that contribute to an anaphylaxis response may be related to activity, timing of food ingestion, an environmental factor, or medication.
Let’s look at just one type, exercise-induced anaphylaxis. It’s divided into two categories: food dependent and nonfood dependent. Both are described as an induction of itching, urticaria, and fatigue, with progression to angioedema and hypotension, associated with exercise (J. Allergy Clin. Immunol. 1980;66:106-11).
Food-dependent exercise-induced anaphylaxis occurs when exercise is started 30 minutes after ingesting food. This may be difficult to identify because patients react to the food only if they exercise, so food is usually eliminated as a cause. Wheat and wheat flour are common culprits for this type of reaction because of the omega-5 gliadin, which is the protein in gluten (J. Allergy Clin. Immunol. 1991;87:34-40). In one study, larger amounts of the suspected agent were given; hives and angioedema did start to occur in 20% of patients challenged, which suggested that there was likely a baseline allergy to the food, and exercise itself might be a cofactor in augmentation of the allergic reaction.
In nonfood-dependent exercise-induced anaphylaxis, symptoms of itching, urticaria, and fatigue can occur 5-30 minutes after the start of exercise. Although bronchospasm is rare, it can occur along with angioedema, nausea, vomiting, and hypotension, and can even be fatal if exercise continues. If exercise is stopped, it usually resolves. However, many people try to push through it, which only worsens the symptoms.
Cofactors associated with nonfood-dependent exercise-induced anaphylaxis are ingestion of alcohol and an NSAID several hours beforehand. These agents also might be overlooked if well tolerated independently (Br. J. Dermatol. 2001;145:336-9).
Timing of the episode also plays a role. Premenstrual syndrome can be a factor in augmentation of anaphylaxis, so it also should be considered. Knowing the date of the last menstrual cycle and identifying if the anaphylaxis is episodic will identify premenstrual syndrome as a cause.
The work-up should include standard allergy testing and determination of tryptase levels. Skin testing is essential to identify offending agents, and is rarely negative. If a food is suspected and skin testing is negative, repeat the skin testing in 6 months. In one study, wheat extract was found to be positive in only 29% of persons suspected of having a wheat allergy, but when the paste of wheat flour was tested, 80% were identified. The ImmunoCAP Test also was found to have a sensitivity of 80%, so it is a valuable test to try along with the skin prick.
Tryptase levels should be evaluated because in nonfood-dependent exercise-induced anaphylaxis, these levels are slightly elevated at the time of the anaphylaxis, but return to normal. A patient with mastocytosis, a group of disorders characterized by pathologic mast cells infiltrating the skin, will consistently have elevated tryptase levels. Seasonal allergies associated with pollen, and asthma bronchospasm also should be considered as causes.
Although these exercise-induced anaphylaxis episodes can occur at any age, they are most frequent in the adolescent age group, probably because that’s the time most of this population are involved in organized sports. Upon presentation, a careful detailed history will help to identify the cause of anaphylaxis and result in quicker resolution.
Treatment includes avoidance of the offending agent if identified and an antihistamine, and if symptoms do occur, ceasing exercise immediately to avoid a full-blown anaphylactic reaction.
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected].