User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
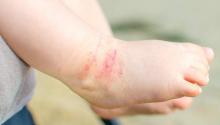
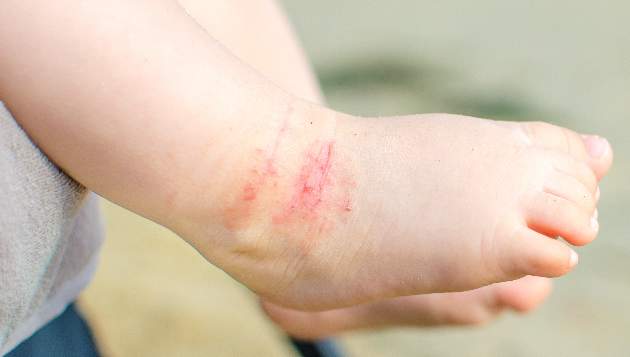
New-onset pediatric AD phenotype differs from adult AD
The skin phenotype of new-onset pediatric atopic dermatitis differs substantially from that in adult AD, according to an assessment of biopsy findings in infants and children.
The study findings have important therapeutic implications, especially in light of the fact that much of the work in this area has been based on adult biomarkers, reflecting “decades of disease activity and chronic use of immunosuppressants in adults,” the investigators reported. Little is known about alterations in early lesions in children, which limits the advancement of targeted therapies, Hitokazu Esaki, MD, of the Icahn School of Medicine at Mount Sinai in New York City, and colleagues reported online in the Journal of Allergy and Clinical Immunology (2016 Sep 22. doi: 10.1016/j.jaci.2016.07.013).
To characterize early pediatric AD skin phenotype, the investigators assessed lesional and nonlesional biopsies from 19 children under age 5 years (mean, 1.3 years) within 6 months of moderate to severe disease onset, as well as those from age-matched controls and adults, and found that, compared with adult AD, early AD involves comparable or greater epidermal hyperplasia and cellular infiltration, similar strong activation of Th2 and Th22 axes, and some Th1 skewing.
In addition, early AD involves significantly higher induction of Th17-related cytokines, compared with adult AD. Expression of filaggrin – an abundant barrier differentiation protein – was similar in AD and healthy children, whereas down-regulation is characteristic in adult AD, the investigators noted.
Nonlesional skin biopsies from the children showed both higher levels of inflammation and epidermal proliferation markers, they said.
The “surprising findings” of an early multicytokine response in new-onset pediatric AD, characterized by marked Th17, Th9, Th2, and Th22 activation, suggest that targeting of multiple cytokine axes may be needed in children with early-onset AD, one of the lead authors on the study, Emma Guttman-Yassky, MD, also of the Icahn School of Medicine at Mount Sinai, said in an interview.
Dr. Guttman-Yassky, who noted that the study was conducted in close collaboration with Amy S. Paller, MD, of Northwestern University, Chicago, explained that early AD, compared with adult AD, involves differential immune skewing and barrier responses with features that are in some ways comparable to those of psoriasis – particularly with respect to the consistently higher levels of Th17-related mediators in childhood AD, as psoriasis is considered a Th17-centered disease.
Further, the findings with respect to filaggrin represent another important aspect of the study, she said, noting that they represent a possible challenge to the notion that filaggrin is integral to disease elicitation and instigation of the “atopic march.”
The study findings may suggest novel targets for pediatric AD, and they also suggest a need for early immune intervention, not only to treat the AD, but also to prevent the atopic march, she said.
“These findings are likely to result in both different understanding of AD onset and distinct treatment approaches for infants and children,” she and her colleagues concluded.
This work was funded by a research grant from the LEO Foundation. Individual authors were supported by grants from the National Center for Advancing Translational Sciences and the National Institutes of Health Clinical and Translational Science Award program. Dr. Esaki reported having no disclosures. Dr. Guttman-Yassky reported financial relationships with numerous pharmaceutical companies.
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease. Severe AD places a huge burden on patients, their families, and society in terms of health care dollars spent and lost work days. Considering the prevalence of AD, both families and dermatologists find it understandably frustrating that we have limited and often ineffective tools to treat severe AD in children. Change may be around the corner.
This study by Esaki et al. sheds critical light on the pathogenesis of early onset AD in children and we hope it will set the stage to revolutionize the treatment of AD using the paradigm of psoriasis as a model. Using lesional and nonlesional biopsies from 19 children under age 5 obtained during the first 6 months of onset of AD, Esaki et al. have demonstrated that children with AD have a multicytokine inflammatory infiltrate with Th17 predominance. This sets the stage for biologics focused on the Th17 pathway in these children, although multimodal therapy to address different cytokines may ultimately be required.
The investigators also found that children with AD had similar filaggrin expression compared to control children, implying that atopic dermatitis is at its heart an immunologic disorder rather than a barrier defect although we will likely continue to learn more about this fine balance.
As pediatric dermatologists on the front line caring for patients with severe AD, we welcome further studies and especially look forward to effective treatments for our patients who might finally experience relief of itch, clear skin, and a good night’s sleep.
A. Yasmine Kirkorian, MD, and Kalyani Marathe, MD, are pediatric dermatologists at Children’s National Health System, in the departments of dermatology and pediatrics at George Washington University, Washington, DC. They are on the editorial advisory board of Dermatology News. They had no disclosures.
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease. Severe AD places a huge burden on patients, their families, and society in terms of health care dollars spent and lost work days. Considering the prevalence of AD, both families and dermatologists find it understandably frustrating that we have limited and often ineffective tools to treat severe AD in children. Change may be around the corner.
This study by Esaki et al. sheds critical light on the pathogenesis of early onset AD in children and we hope it will set the stage to revolutionize the treatment of AD using the paradigm of psoriasis as a model. Using lesional and nonlesional biopsies from 19 children under age 5 obtained during the first 6 months of onset of AD, Esaki et al. have demonstrated that children with AD have a multicytokine inflammatory infiltrate with Th17 predominance. This sets the stage for biologics focused on the Th17 pathway in these children, although multimodal therapy to address different cytokines may ultimately be required.
The investigators also found that children with AD had similar filaggrin expression compared to control children, implying that atopic dermatitis is at its heart an immunologic disorder rather than a barrier defect although we will likely continue to learn more about this fine balance.
As pediatric dermatologists on the front line caring for patients with severe AD, we welcome further studies and especially look forward to effective treatments for our patients who might finally experience relief of itch, clear skin, and a good night’s sleep.
A. Yasmine Kirkorian, MD, and Kalyani Marathe, MD, are pediatric dermatologists at Children’s National Health System, in the departments of dermatology and pediatrics at George Washington University, Washington, DC. They are on the editorial advisory board of Dermatology News. They had no disclosures.
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease. Severe AD places a huge burden on patients, their families, and society in terms of health care dollars spent and lost work days. Considering the prevalence of AD, both families and dermatologists find it understandably frustrating that we have limited and often ineffective tools to treat severe AD in children. Change may be around the corner.
This study by Esaki et al. sheds critical light on the pathogenesis of early onset AD in children and we hope it will set the stage to revolutionize the treatment of AD using the paradigm of psoriasis as a model. Using lesional and nonlesional biopsies from 19 children under age 5 obtained during the first 6 months of onset of AD, Esaki et al. have demonstrated that children with AD have a multicytokine inflammatory infiltrate with Th17 predominance. This sets the stage for biologics focused on the Th17 pathway in these children, although multimodal therapy to address different cytokines may ultimately be required.
The investigators also found that children with AD had similar filaggrin expression compared to control children, implying that atopic dermatitis is at its heart an immunologic disorder rather than a barrier defect although we will likely continue to learn more about this fine balance.
As pediatric dermatologists on the front line caring for patients with severe AD, we welcome further studies and especially look forward to effective treatments for our patients who might finally experience relief of itch, clear skin, and a good night’s sleep.
A. Yasmine Kirkorian, MD, and Kalyani Marathe, MD, are pediatric dermatologists at Children’s National Health System, in the departments of dermatology and pediatrics at George Washington University, Washington, DC. They are on the editorial advisory board of Dermatology News. They had no disclosures.
The skin phenotype of new-onset pediatric atopic dermatitis differs substantially from that in adult AD, according to an assessment of biopsy findings in infants and children.
The study findings have important therapeutic implications, especially in light of the fact that much of the work in this area has been based on adult biomarkers, reflecting “decades of disease activity and chronic use of immunosuppressants in adults,” the investigators reported. Little is known about alterations in early lesions in children, which limits the advancement of targeted therapies, Hitokazu Esaki, MD, of the Icahn School of Medicine at Mount Sinai in New York City, and colleagues reported online in the Journal of Allergy and Clinical Immunology (2016 Sep 22. doi: 10.1016/j.jaci.2016.07.013).
To characterize early pediatric AD skin phenotype, the investigators assessed lesional and nonlesional biopsies from 19 children under age 5 years (mean, 1.3 years) within 6 months of moderate to severe disease onset, as well as those from age-matched controls and adults, and found that, compared with adult AD, early AD involves comparable or greater epidermal hyperplasia and cellular infiltration, similar strong activation of Th2 and Th22 axes, and some Th1 skewing.
In addition, early AD involves significantly higher induction of Th17-related cytokines, compared with adult AD. Expression of filaggrin – an abundant barrier differentiation protein – was similar in AD and healthy children, whereas down-regulation is characteristic in adult AD, the investigators noted.
Nonlesional skin biopsies from the children showed both higher levels of inflammation and epidermal proliferation markers, they said.
The “surprising findings” of an early multicytokine response in new-onset pediatric AD, characterized by marked Th17, Th9, Th2, and Th22 activation, suggest that targeting of multiple cytokine axes may be needed in children with early-onset AD, one of the lead authors on the study, Emma Guttman-Yassky, MD, also of the Icahn School of Medicine at Mount Sinai, said in an interview.
Dr. Guttman-Yassky, who noted that the study was conducted in close collaboration with Amy S. Paller, MD, of Northwestern University, Chicago, explained that early AD, compared with adult AD, involves differential immune skewing and barrier responses with features that are in some ways comparable to those of psoriasis – particularly with respect to the consistently higher levels of Th17-related mediators in childhood AD, as psoriasis is considered a Th17-centered disease.
Further, the findings with respect to filaggrin represent another important aspect of the study, she said, noting that they represent a possible challenge to the notion that filaggrin is integral to disease elicitation and instigation of the “atopic march.”
The study findings may suggest novel targets for pediatric AD, and they also suggest a need for early immune intervention, not only to treat the AD, but also to prevent the atopic march, she said.
“These findings are likely to result in both different understanding of AD onset and distinct treatment approaches for infants and children,” she and her colleagues concluded.
This work was funded by a research grant from the LEO Foundation. Individual authors were supported by grants from the National Center for Advancing Translational Sciences and the National Institutes of Health Clinical and Translational Science Award program. Dr. Esaki reported having no disclosures. Dr. Guttman-Yassky reported financial relationships with numerous pharmaceutical companies.
The skin phenotype of new-onset pediatric atopic dermatitis differs substantially from that in adult AD, according to an assessment of biopsy findings in infants and children.
The study findings have important therapeutic implications, especially in light of the fact that much of the work in this area has been based on adult biomarkers, reflecting “decades of disease activity and chronic use of immunosuppressants in adults,” the investigators reported. Little is known about alterations in early lesions in children, which limits the advancement of targeted therapies, Hitokazu Esaki, MD, of the Icahn School of Medicine at Mount Sinai in New York City, and colleagues reported online in the Journal of Allergy and Clinical Immunology (2016 Sep 22. doi: 10.1016/j.jaci.2016.07.013).
To characterize early pediatric AD skin phenotype, the investigators assessed lesional and nonlesional biopsies from 19 children under age 5 years (mean, 1.3 years) within 6 months of moderate to severe disease onset, as well as those from age-matched controls and adults, and found that, compared with adult AD, early AD involves comparable or greater epidermal hyperplasia and cellular infiltration, similar strong activation of Th2 and Th22 axes, and some Th1 skewing.
In addition, early AD involves significantly higher induction of Th17-related cytokines, compared with adult AD. Expression of filaggrin – an abundant barrier differentiation protein – was similar in AD and healthy children, whereas down-regulation is characteristic in adult AD, the investigators noted.
Nonlesional skin biopsies from the children showed both higher levels of inflammation and epidermal proliferation markers, they said.
The “surprising findings” of an early multicytokine response in new-onset pediatric AD, characterized by marked Th17, Th9, Th2, and Th22 activation, suggest that targeting of multiple cytokine axes may be needed in children with early-onset AD, one of the lead authors on the study, Emma Guttman-Yassky, MD, also of the Icahn School of Medicine at Mount Sinai, said in an interview.
Dr. Guttman-Yassky, who noted that the study was conducted in close collaboration with Amy S. Paller, MD, of Northwestern University, Chicago, explained that early AD, compared with adult AD, involves differential immune skewing and barrier responses with features that are in some ways comparable to those of psoriasis – particularly with respect to the consistently higher levels of Th17-related mediators in childhood AD, as psoriasis is considered a Th17-centered disease.
Further, the findings with respect to filaggrin represent another important aspect of the study, she said, noting that they represent a possible challenge to the notion that filaggrin is integral to disease elicitation and instigation of the “atopic march.”
The study findings may suggest novel targets for pediatric AD, and they also suggest a need for early immune intervention, not only to treat the AD, but also to prevent the atopic march, she said.
“These findings are likely to result in both different understanding of AD onset and distinct treatment approaches for infants and children,” she and her colleagues concluded.
This work was funded by a research grant from the LEO Foundation. Individual authors were supported by grants from the National Center for Advancing Translational Sciences and the National Institutes of Health Clinical and Translational Science Award program. Dr. Esaki reported having no disclosures. Dr. Guttman-Yassky reported financial relationships with numerous pharmaceutical companies.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Key clinical point: The skin phenotype of new-onset pediatric atopic dermatitis differs substantially from that in adult AD, which has important therapeutic implications, according to a study of biopsy findings in infants and children.
Major finding: Early AD involves significantly higher induction of Th17-related cytokines, compared with adult AD.
Data source: An analysis of biopsies from 19 children with AD.
Disclosures: This work was funded by a research grant from the LEO Foundation. Individual authors were supported by grants from the National Center for Advancing Translational Sciences and the National Institutes of Health Clinical and Translational Science Award program. Dr. Esaki reported having no disclosures. Dr. Guttman-Yassky reported financial relationships with numerous pharmaceutical companies.
Empiric warfarin adjustment cut drug-drug interactions with antimicrobials
BOSTON – A medication management strategy to minimize the effect of drug-drug interactions (DDIs) between warfarin and common antimicrobials resulted in significantly greater time within therapeutic range for anticoagulation, as well as a numerically smaller, but nonsignificant, number of bleeding events.
After implementation of a comprehensive inpatient and postdischarge guideline to manage DDIs between warfarin and 16 antibiotics, antivirals, or antifungal medications, patients’ in-hospital time within therapeutic range (TWTR) increased to 72% from 50% preimplementation (P = .043). Warfarin TWTR also improved across care transitions after the guidelines were implemented, rising to 70% from 46% (P = .012). No bleeding events occurred in the group studied after the guidelines were instituted, compared with four events in the comparator preguidelines group (P = .11).
Nghi Ha, PharmD, MPH, and his collaborators sought to determine whether formalizing a process to manage potentially dangerous antimicrobial-warfarin DDIs made a difference in achieving more TWTR for patients, as determined by international normalized ratio (INR) values. Dr. Ha, a clinical pharmacist at University of Michigan Health System, Ann Arbor, presented the results during a poster session at the annual meeting of the American Society for Microbiology.
Secondary outcome measures studied by Dr. Ha and his associates included the incidence of thrombosis or major bleeding events, as well as tracking documentation of medications and the anticoagulation plan in progress and discharge notes.
Patients were included if they were at least 18 years old, and if they were on 3 days or more of an antimicrobial with potential for DDI with warfarin. Patients who were also newly on other medications with the potential for significant DDI with warfarin were excluded to minimize the potential for confounding.
Dr. Ha and his collaborators characterized the study as a retrospective, single-center, quasi-experimental design of a pharmacist-run anticoagulation service. The study examined endpoints before and after implementation of comprehensive guidelines, and included 78 preguideline and 31 postguideline patients.
The guidelines drafted by the investigators and tested in their study included empiric adjustment of warfarin dosing for patients who were placed on an antibiotic with high potential to increase INR. These included many azoles and sulfamethoxazole/trimethoprim, for which initial warfarin doses were empirically reduced 20%-30% for patients whose INRs were therapeutic at the start of antimicrobial therapy. For ciprofloxacin, erythromycin, clarithromycin, and isoniazid, the guidelines recommended initial empiric warfarin dose reductions of 10%-15%.
Patients whose INRs were subtherapeutic at the beginning of therapy and who received these antimicrobials were continued on their maintenance warfarin dosing, but were monitored for rising INRs over the first 48 hours, for consideration of dosing adjustment as needed. Individuals with supratherapeutic INRs at the beginning of antimicrobial dosing had their warfarin doses reduced or held by a more aggressive algorithm based on their initial INR, and based on the potential of the antimicrobial to increase INR.
On discharge, patients were either reverted to their previous warfarin regimen if they had been stable on that regimen, or had their inpatient warfarin dosing increased by 10%-20%.
Drugs that were deemed to have moderate potential to increase INR included doxycycline, levofloxacin, moxifloxacin, quinupristin/dalfopristin, telaprevir, boceprevir, and simeprevir. For these medications, the protocol recommended no initial dose adjustment, but recommended monitoring of INR to consider a dose reduction if needed. On hospital discharge, patients who had been on these medications were to resume their previous warfarin dosing.
Antimicrobials with potential to decrease INR included nafcillin, for which the protocol recommended empiric warfarin dose increases of 25%-50%, starting 3-5 days after nafcillin was begun. Patients on rifampin or rifabutin were to increase their warfarin by 20%-30%, also 3-5 days after beginning the antibiotics. Patients on ritonavir alone, or any protease inhibitor given for HIV along with ritonavir, were closely monitored, but no empiric dosing adjustments were made.
Patients with initial subtherapeutic INRs had dosing increased by 30%-50% for nafcillin and 20%-30% for rifampin and rifabutin. A stepped algorithm for dose adjustment or withholding was also developed for these medications to treat patients with initial supratherapeutic INRs. Patients on these medications were to resume their previous warfarin dosing, with monitoring and adjustment if they had not been previously stable.
Documentation of antimicrobial-warfarin DDI in the anticoagulation service discharge summary improved significantly once the guidelines were implemented (40% compared with 14%, P = .02). There was not a significant improvement in DDI documentation in daily progress notes.
The comprehensive intervention included the formulation of guidelines and requirements to document the medication interaction in the medical chart. Other interventions included training for clinical pharmacists and the development of pocket cards and flyers to educate team members about the new guidelines. The electronic health record had triggers built and implemented to prompt consideration of warfarin/antimicrobial DDIs as well.
Dr. Ha and his coauthors noted that the uncontrolled nature of the pre-post study design was one limitation of the study. Also, the real-world design of the study meant that investigators could not control for diet, comorbidities, and other factors that have the potential to affect INR. “Implementing a process to identify high-risk antimicrobial-warfarin DDIs and provide guidelines for empiric warfarin dose adjustment … can improve INR time within therapeutic range,” noted Dr. Ha and his coauthors.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – A medication management strategy to minimize the effect of drug-drug interactions (DDIs) between warfarin and common antimicrobials resulted in significantly greater time within therapeutic range for anticoagulation, as well as a numerically smaller, but nonsignificant, number of bleeding events.
After implementation of a comprehensive inpatient and postdischarge guideline to manage DDIs between warfarin and 16 antibiotics, antivirals, or antifungal medications, patients’ in-hospital time within therapeutic range (TWTR) increased to 72% from 50% preimplementation (P = .043). Warfarin TWTR also improved across care transitions after the guidelines were implemented, rising to 70% from 46% (P = .012). No bleeding events occurred in the group studied after the guidelines were instituted, compared with four events in the comparator preguidelines group (P = .11).
Nghi Ha, PharmD, MPH, and his collaborators sought to determine whether formalizing a process to manage potentially dangerous antimicrobial-warfarin DDIs made a difference in achieving more TWTR for patients, as determined by international normalized ratio (INR) values. Dr. Ha, a clinical pharmacist at University of Michigan Health System, Ann Arbor, presented the results during a poster session at the annual meeting of the American Society for Microbiology.
Secondary outcome measures studied by Dr. Ha and his associates included the incidence of thrombosis or major bleeding events, as well as tracking documentation of medications and the anticoagulation plan in progress and discharge notes.
Patients were included if they were at least 18 years old, and if they were on 3 days or more of an antimicrobial with potential for DDI with warfarin. Patients who were also newly on other medications with the potential for significant DDI with warfarin were excluded to minimize the potential for confounding.
Dr. Ha and his collaborators characterized the study as a retrospective, single-center, quasi-experimental design of a pharmacist-run anticoagulation service. The study examined endpoints before and after implementation of comprehensive guidelines, and included 78 preguideline and 31 postguideline patients.
The guidelines drafted by the investigators and tested in their study included empiric adjustment of warfarin dosing for patients who were placed on an antibiotic with high potential to increase INR. These included many azoles and sulfamethoxazole/trimethoprim, for which initial warfarin doses were empirically reduced 20%-30% for patients whose INRs were therapeutic at the start of antimicrobial therapy. For ciprofloxacin, erythromycin, clarithromycin, and isoniazid, the guidelines recommended initial empiric warfarin dose reductions of 10%-15%.
Patients whose INRs were subtherapeutic at the beginning of therapy and who received these antimicrobials were continued on their maintenance warfarin dosing, but were monitored for rising INRs over the first 48 hours, for consideration of dosing adjustment as needed. Individuals with supratherapeutic INRs at the beginning of antimicrobial dosing had their warfarin doses reduced or held by a more aggressive algorithm based on their initial INR, and based on the potential of the antimicrobial to increase INR.
On discharge, patients were either reverted to their previous warfarin regimen if they had been stable on that regimen, or had their inpatient warfarin dosing increased by 10%-20%.
Drugs that were deemed to have moderate potential to increase INR included doxycycline, levofloxacin, moxifloxacin, quinupristin/dalfopristin, telaprevir, boceprevir, and simeprevir. For these medications, the protocol recommended no initial dose adjustment, but recommended monitoring of INR to consider a dose reduction if needed. On hospital discharge, patients who had been on these medications were to resume their previous warfarin dosing.
Antimicrobials with potential to decrease INR included nafcillin, for which the protocol recommended empiric warfarin dose increases of 25%-50%, starting 3-5 days after nafcillin was begun. Patients on rifampin or rifabutin were to increase their warfarin by 20%-30%, also 3-5 days after beginning the antibiotics. Patients on ritonavir alone, or any protease inhibitor given for HIV along with ritonavir, were closely monitored, but no empiric dosing adjustments were made.
Patients with initial subtherapeutic INRs had dosing increased by 30%-50% for nafcillin and 20%-30% for rifampin and rifabutin. A stepped algorithm for dose adjustment or withholding was also developed for these medications to treat patients with initial supratherapeutic INRs. Patients on these medications were to resume their previous warfarin dosing, with monitoring and adjustment if they had not been previously stable.
Documentation of antimicrobial-warfarin DDI in the anticoagulation service discharge summary improved significantly once the guidelines were implemented (40% compared with 14%, P = .02). There was not a significant improvement in DDI documentation in daily progress notes.
The comprehensive intervention included the formulation of guidelines and requirements to document the medication interaction in the medical chart. Other interventions included training for clinical pharmacists and the development of pocket cards and flyers to educate team members about the new guidelines. The electronic health record had triggers built and implemented to prompt consideration of warfarin/antimicrobial DDIs as well.
Dr. Ha and his coauthors noted that the uncontrolled nature of the pre-post study design was one limitation of the study. Also, the real-world design of the study meant that investigators could not control for diet, comorbidities, and other factors that have the potential to affect INR. “Implementing a process to identify high-risk antimicrobial-warfarin DDIs and provide guidelines for empiric warfarin dose adjustment … can improve INR time within therapeutic range,” noted Dr. Ha and his coauthors.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – A medication management strategy to minimize the effect of drug-drug interactions (DDIs) between warfarin and common antimicrobials resulted in significantly greater time within therapeutic range for anticoagulation, as well as a numerically smaller, but nonsignificant, number of bleeding events.
After implementation of a comprehensive inpatient and postdischarge guideline to manage DDIs between warfarin and 16 antibiotics, antivirals, or antifungal medications, patients’ in-hospital time within therapeutic range (TWTR) increased to 72% from 50% preimplementation (P = .043). Warfarin TWTR also improved across care transitions after the guidelines were implemented, rising to 70% from 46% (P = .012). No bleeding events occurred in the group studied after the guidelines were instituted, compared with four events in the comparator preguidelines group (P = .11).
Nghi Ha, PharmD, MPH, and his collaborators sought to determine whether formalizing a process to manage potentially dangerous antimicrobial-warfarin DDIs made a difference in achieving more TWTR for patients, as determined by international normalized ratio (INR) values. Dr. Ha, a clinical pharmacist at University of Michigan Health System, Ann Arbor, presented the results during a poster session at the annual meeting of the American Society for Microbiology.
Secondary outcome measures studied by Dr. Ha and his associates included the incidence of thrombosis or major bleeding events, as well as tracking documentation of medications and the anticoagulation plan in progress and discharge notes.
Patients were included if they were at least 18 years old, and if they were on 3 days or more of an antimicrobial with potential for DDI with warfarin. Patients who were also newly on other medications with the potential for significant DDI with warfarin were excluded to minimize the potential for confounding.
Dr. Ha and his collaborators characterized the study as a retrospective, single-center, quasi-experimental design of a pharmacist-run anticoagulation service. The study examined endpoints before and after implementation of comprehensive guidelines, and included 78 preguideline and 31 postguideline patients.
The guidelines drafted by the investigators and tested in their study included empiric adjustment of warfarin dosing for patients who were placed on an antibiotic with high potential to increase INR. These included many azoles and sulfamethoxazole/trimethoprim, for which initial warfarin doses were empirically reduced 20%-30% for patients whose INRs were therapeutic at the start of antimicrobial therapy. For ciprofloxacin, erythromycin, clarithromycin, and isoniazid, the guidelines recommended initial empiric warfarin dose reductions of 10%-15%.
Patients whose INRs were subtherapeutic at the beginning of therapy and who received these antimicrobials were continued on their maintenance warfarin dosing, but were monitored for rising INRs over the first 48 hours, for consideration of dosing adjustment as needed. Individuals with supratherapeutic INRs at the beginning of antimicrobial dosing had their warfarin doses reduced or held by a more aggressive algorithm based on their initial INR, and based on the potential of the antimicrobial to increase INR.
On discharge, patients were either reverted to their previous warfarin regimen if they had been stable on that regimen, or had their inpatient warfarin dosing increased by 10%-20%.
Drugs that were deemed to have moderate potential to increase INR included doxycycline, levofloxacin, moxifloxacin, quinupristin/dalfopristin, telaprevir, boceprevir, and simeprevir. For these medications, the protocol recommended no initial dose adjustment, but recommended monitoring of INR to consider a dose reduction if needed. On hospital discharge, patients who had been on these medications were to resume their previous warfarin dosing.
Antimicrobials with potential to decrease INR included nafcillin, for which the protocol recommended empiric warfarin dose increases of 25%-50%, starting 3-5 days after nafcillin was begun. Patients on rifampin or rifabutin were to increase their warfarin by 20%-30%, also 3-5 days after beginning the antibiotics. Patients on ritonavir alone, or any protease inhibitor given for HIV along with ritonavir, were closely monitored, but no empiric dosing adjustments were made.
Patients with initial subtherapeutic INRs had dosing increased by 30%-50% for nafcillin and 20%-30% for rifampin and rifabutin. A stepped algorithm for dose adjustment or withholding was also developed for these medications to treat patients with initial supratherapeutic INRs. Patients on these medications were to resume their previous warfarin dosing, with monitoring and adjustment if they had not been previously stable.
Documentation of antimicrobial-warfarin DDI in the anticoagulation service discharge summary improved significantly once the guidelines were implemented (40% compared with 14%, P = .02). There was not a significant improvement in DDI documentation in daily progress notes.
The comprehensive intervention included the formulation of guidelines and requirements to document the medication interaction in the medical chart. Other interventions included training for clinical pharmacists and the development of pocket cards and flyers to educate team members about the new guidelines. The electronic health record had triggers built and implemented to prompt consideration of warfarin/antimicrobial DDIs as well.
Dr. Ha and his coauthors noted that the uncontrolled nature of the pre-post study design was one limitation of the study. Also, the real-world design of the study meant that investigators could not control for diet, comorbidities, and other factors that have the potential to affect INR. “Implementing a process to identify high-risk antimicrobial-warfarin DDIs and provide guidelines for empiric warfarin dose adjustment … can improve INR time within therapeutic range,” noted Dr. Ha and his coauthors.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
AT ASM MICROBE 2016
Key clinical point: Clinical guidelines with empiric warfarin adjustments improved time within therapeutic range (TWTR) for inpatients on antimicrobials.
Major finding: In-hospital TWTR increased to 72% from 50% before implementation of clinical guidelines (P = .043).
Data source: Retrospective, single-center study of inpatients on warfarin and antimicrobial with potential for DDI before (n = 78) and after (n = 31) implementation of a comprehensive clinical guideline.
Disclosures: The study investigators reported no outside sources of funding and no disclosures.
Artificial intelligence beats standard algorithms for diagnosing lung disease
LONDON – In a proof-of-principle study, artificial intelligence (AI) led more frequently to the correct diagnosis of underlying lung disease than did physicians’ use of standard algorithms, such as those recommended by the American Thoracic Society and the European Respiratory Society, according to late-breaker data presented at the annual congress of the European Respiratory Society.
“The beauty of this approach is that artificial intelligence can simulate the complex reasoning that clinicians use to reach their diagnosis but in a more standardized and objective fashion, so it removes any bias,” reported Wim Janssens, MD, PhD, of the division of medicine and respiratory rehabilitation at University of Leuven (Belgium).
The AI employed in this study was based on a subfield of computer science that relies on patterns within statistics to build decision trees. Often called machine learning, this type of AI grows smarter as it learns from the patterns it finds in the data provided.
In this case, the AI was designed to provide diagnoses for lung diseases based on patterns drawn from clinical and lung function data. The computer-based choices were compared to diagnoses reached by clinicians. The final diagnoses were validated by a consensus of expert clinicians.
“The computer-based choices were in almost all cases better than the choices made by standard diagnostic algorithms,” reported Marko Topalovic, PhD, a researcher in AI who is affiliated with the University of Leuven. Dr. Topalovic presented the data at the ERS.
The study involved 968 patients presenting with lung symptoms to a pulmonary clinic for the first time. Standard clinical data, such as smoking history, body mass index, and age, were collected. Lung function studies conducted in all patients included spirometry, body plethysmography, and airway diffusion, although participating clinicians were permitted to order additional tests at their own discretion. Clinical diagnoses were separated into 10 predefined disease groups.
The average accuracy of clinicians’ diagnoses was 38%. The clinicians were best at identifying chronic obstructive pulmonary disease (COPD), having accurately diagnosed 74% of the cases of this disease. For other disease groups, the clinician’s accuracy rarely exceeded 50%.
The diagnoses made by AI, on the other hand, on average, were 68% accurate. For diagnosing COPD, the AI achieved a positive predictive value of 83% and a sensitivity of 78%. The positive predictive value and sensitivity of AI for asthma (66% and 82%, respectively) and interstitial lung disease (52% and 59%) were both significantly greater than those achieved by the clinicians.
When findings are ambiguous or there are anomalies in the clinical picture, a final clinical diagnosis can be challenging, according to Dr. Janssens. He suggested that automation eliminates the potential for bias, which often occurs when clinicians inadvertently give more weight to one clinical variable relative to another.
The decision-making system tested in this study was characterized as “a first step to automated interpretation of lung function,” Dr. Topalovic said. He added that he expects the AI to improve as it receives more data.
“Not only do we think this system can help nonexperienced clinicians, but it will help experts reach a diagnosis more quickly,” Dr. Topalovic said. Noting that this same approach is being pursued in other fields of medicine, he said he thinks adding AI to respiratory medicine will reduce the number of redundant tests and, in other ways, introduce opportunities for efficiencies and reduced costs.
Dr. Topalovic and Dr. Janssens reported no relevant financial relationships.
LONDON – In a proof-of-principle study, artificial intelligence (AI) led more frequently to the correct diagnosis of underlying lung disease than did physicians’ use of standard algorithms, such as those recommended by the American Thoracic Society and the European Respiratory Society, according to late-breaker data presented at the annual congress of the European Respiratory Society.
“The beauty of this approach is that artificial intelligence can simulate the complex reasoning that clinicians use to reach their diagnosis but in a more standardized and objective fashion, so it removes any bias,” reported Wim Janssens, MD, PhD, of the division of medicine and respiratory rehabilitation at University of Leuven (Belgium).
The AI employed in this study was based on a subfield of computer science that relies on patterns within statistics to build decision trees. Often called machine learning, this type of AI grows smarter as it learns from the patterns it finds in the data provided.
In this case, the AI was designed to provide diagnoses for lung diseases based on patterns drawn from clinical and lung function data. The computer-based choices were compared to diagnoses reached by clinicians. The final diagnoses were validated by a consensus of expert clinicians.
“The computer-based choices were in almost all cases better than the choices made by standard diagnostic algorithms,” reported Marko Topalovic, PhD, a researcher in AI who is affiliated with the University of Leuven. Dr. Topalovic presented the data at the ERS.
The study involved 968 patients presenting with lung symptoms to a pulmonary clinic for the first time. Standard clinical data, such as smoking history, body mass index, and age, were collected. Lung function studies conducted in all patients included spirometry, body plethysmography, and airway diffusion, although participating clinicians were permitted to order additional tests at their own discretion. Clinical diagnoses were separated into 10 predefined disease groups.
The average accuracy of clinicians’ diagnoses was 38%. The clinicians were best at identifying chronic obstructive pulmonary disease (COPD), having accurately diagnosed 74% of the cases of this disease. For other disease groups, the clinician’s accuracy rarely exceeded 50%.
The diagnoses made by AI, on the other hand, on average, were 68% accurate. For diagnosing COPD, the AI achieved a positive predictive value of 83% and a sensitivity of 78%. The positive predictive value and sensitivity of AI for asthma (66% and 82%, respectively) and interstitial lung disease (52% and 59%) were both significantly greater than those achieved by the clinicians.
When findings are ambiguous or there are anomalies in the clinical picture, a final clinical diagnosis can be challenging, according to Dr. Janssens. He suggested that automation eliminates the potential for bias, which often occurs when clinicians inadvertently give more weight to one clinical variable relative to another.
The decision-making system tested in this study was characterized as “a first step to automated interpretation of lung function,” Dr. Topalovic said. He added that he expects the AI to improve as it receives more data.
“Not only do we think this system can help nonexperienced clinicians, but it will help experts reach a diagnosis more quickly,” Dr. Topalovic said. Noting that this same approach is being pursued in other fields of medicine, he said he thinks adding AI to respiratory medicine will reduce the number of redundant tests and, in other ways, introduce opportunities for efficiencies and reduced costs.
Dr. Topalovic and Dr. Janssens reported no relevant financial relationships.
LONDON – In a proof-of-principle study, artificial intelligence (AI) led more frequently to the correct diagnosis of underlying lung disease than did physicians’ use of standard algorithms, such as those recommended by the American Thoracic Society and the European Respiratory Society, according to late-breaker data presented at the annual congress of the European Respiratory Society.
“The beauty of this approach is that artificial intelligence can simulate the complex reasoning that clinicians use to reach their diagnosis but in a more standardized and objective fashion, so it removes any bias,” reported Wim Janssens, MD, PhD, of the division of medicine and respiratory rehabilitation at University of Leuven (Belgium).
The AI employed in this study was based on a subfield of computer science that relies on patterns within statistics to build decision trees. Often called machine learning, this type of AI grows smarter as it learns from the patterns it finds in the data provided.
In this case, the AI was designed to provide diagnoses for lung diseases based on patterns drawn from clinical and lung function data. The computer-based choices were compared to diagnoses reached by clinicians. The final diagnoses were validated by a consensus of expert clinicians.
“The computer-based choices were in almost all cases better than the choices made by standard diagnostic algorithms,” reported Marko Topalovic, PhD, a researcher in AI who is affiliated with the University of Leuven. Dr. Topalovic presented the data at the ERS.
The study involved 968 patients presenting with lung symptoms to a pulmonary clinic for the first time. Standard clinical data, such as smoking history, body mass index, and age, were collected. Lung function studies conducted in all patients included spirometry, body plethysmography, and airway diffusion, although participating clinicians were permitted to order additional tests at their own discretion. Clinical diagnoses were separated into 10 predefined disease groups.
The average accuracy of clinicians’ diagnoses was 38%. The clinicians were best at identifying chronic obstructive pulmonary disease (COPD), having accurately diagnosed 74% of the cases of this disease. For other disease groups, the clinician’s accuracy rarely exceeded 50%.
The diagnoses made by AI, on the other hand, on average, were 68% accurate. For diagnosing COPD, the AI achieved a positive predictive value of 83% and a sensitivity of 78%. The positive predictive value and sensitivity of AI for asthma (66% and 82%, respectively) and interstitial lung disease (52% and 59%) were both significantly greater than those achieved by the clinicians.
When findings are ambiguous or there are anomalies in the clinical picture, a final clinical diagnosis can be challenging, according to Dr. Janssens. He suggested that automation eliminates the potential for bias, which often occurs when clinicians inadvertently give more weight to one clinical variable relative to another.
The decision-making system tested in this study was characterized as “a first step to automated interpretation of lung function,” Dr. Topalovic said. He added that he expects the AI to improve as it receives more data.
“Not only do we think this system can help nonexperienced clinicians, but it will help experts reach a diagnosis more quickly,” Dr. Topalovic said. Noting that this same approach is being pursued in other fields of medicine, he said he thinks adding AI to respiratory medicine will reduce the number of redundant tests and, in other ways, introduce opportunities for efficiencies and reduced costs.
Dr. Topalovic and Dr. Janssens reported no relevant financial relationships.
AT THE ERS CONGRESS 2016
Key clinical point: When given the same clinical information, artificial intelligence is more likely than are clinicians to diagnose lung diseases correctly.
Major finding: The diagnostic label was correct in 38% of cases with standard algorithms, versus 68% with artificial intelligence.
Data source: Prospective study.
Disclosures: Dr. Marko Topalovic and Dr. Wim Janssens reported no relevant financial relationships.
EpiPen cost increases far exceed overall medical inflation
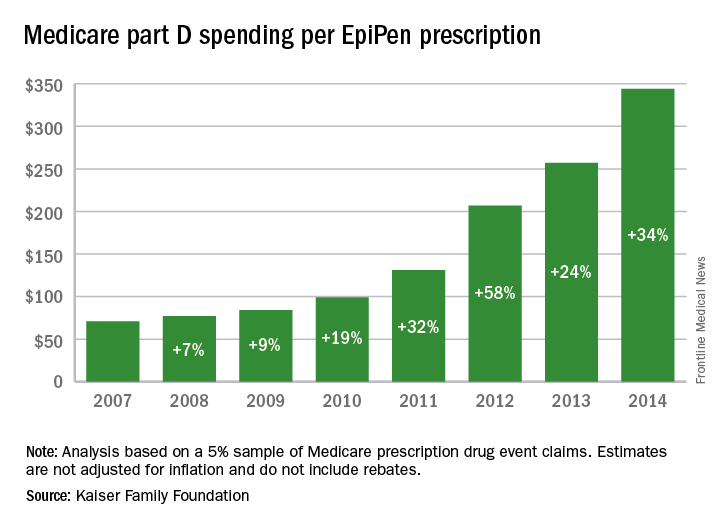
Total Medicare part D spending on EpiPen auto-injectors rose from $7.0 million in 2007 to $87.9 million in 2014 – an increase of 1,151%, according to an analysis released Sept. 20 by the Kaiser Family Foundation.
The number of EpiPen users also increased over that time, however, bringing with it a commensurate 159% rise in the number of prescriptions. Those two trends took the average cost of a single EpiPen prescription from $71 in 2007 to $344 in 2014, the Kaiser analysis showed.
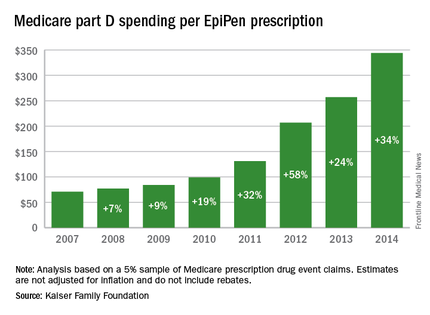
That increase in cost per prescription did not fail to at least double overall medical care price inflation for each year from 2008 to 2014. In 2008, when the two trends were closest together, the EpiPen cost per prescription rose 7.4% from the year before, compared with 3.7% for overall medical spending. In 2014, Medicare part D’s cost for an EpiPen prescription rose 34% from the year before, which was 14 times higher than the 2.4% increase in total medical spending, Kaiser noted.
The analysis was based on a 5% sample of Medicare prescription drug event claims and included beneficiaries who had a least 1 month of part D coverage and one EpiPen prescription during the year. Estimates are not adjusted for inflation and do not include any possible manufacturer discounts, Kaiser said.
Total Medicare part D spending on EpiPen auto-injectors rose from $7.0 million in 2007 to $87.9 million in 2014 – an increase of 1,151%, according to an analysis released Sept. 20 by the Kaiser Family Foundation.
The number of EpiPen users also increased over that time, however, bringing with it a commensurate 159% rise in the number of prescriptions. Those two trends took the average cost of a single EpiPen prescription from $71 in 2007 to $344 in 2014, the Kaiser analysis showed.

That increase in cost per prescription did not fail to at least double overall medical care price inflation for each year from 2008 to 2014. In 2008, when the two trends were closest together, the EpiPen cost per prescription rose 7.4% from the year before, compared with 3.7% for overall medical spending. In 2014, Medicare part D’s cost for an EpiPen prescription rose 34% from the year before, which was 14 times higher than the 2.4% increase in total medical spending, Kaiser noted.
The analysis was based on a 5% sample of Medicare prescription drug event claims and included beneficiaries who had a least 1 month of part D coverage and one EpiPen prescription during the year. Estimates are not adjusted for inflation and do not include any possible manufacturer discounts, Kaiser said.
Total Medicare part D spending on EpiPen auto-injectors rose from $7.0 million in 2007 to $87.9 million in 2014 – an increase of 1,151%, according to an analysis released Sept. 20 by the Kaiser Family Foundation.
The number of EpiPen users also increased over that time, however, bringing with it a commensurate 159% rise in the number of prescriptions. Those two trends took the average cost of a single EpiPen prescription from $71 in 2007 to $344 in 2014, the Kaiser analysis showed.

That increase in cost per prescription did not fail to at least double overall medical care price inflation for each year from 2008 to 2014. In 2008, when the two trends were closest together, the EpiPen cost per prescription rose 7.4% from the year before, compared with 3.7% for overall medical spending. In 2014, Medicare part D’s cost for an EpiPen prescription rose 34% from the year before, which was 14 times higher than the 2.4% increase in total medical spending, Kaiser noted.
The analysis was based on a 5% sample of Medicare prescription drug event claims and included beneficiaries who had a least 1 month of part D coverage and one EpiPen prescription during the year. Estimates are not adjusted for inflation and do not include any possible manufacturer discounts, Kaiser said.
ICS/LABA exacerbation benefit outweighs pneumonia risk
LONDON – The benefit of a fixed-dose inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) combination in reducing exacerbations of chronic obstructive pulmonary disease (COPD) far outweighed any risk for pneumonia in a post hoc analysis of the 48-week FORWARD study.
Although there were 13 extra pneumonia events when a fixed-dose combination of beclometasone diproprionate and formoterol fumarate (Foster, Chiesi Farmaceutici SpA) was used as compared to formoterol fumarate alone, there were 123 fewer moderate to severe COPD exacerbations over a 342-day analysis period.
“Analysis of pneumonia and exacerbation cumulative number of events shows that the number of incident pneumonia remains very small relative to that of moderate to severe exacerbations,” Massimo Corradi, MD, of the University of Parma (Italy), reported at the annual congress of the European Respiratory Society.
Dr. Corradi added that the new analysis confirms that the ICS/LABA combination has a “positive risk-benefit balance over LABA monotherapy, supporting [the argument that] the benefits of adding an ICS to a bronchodilator significantly outweigh potential risks.”
The FORWARD study was a two-arm trial designed to compare the efficacy and safety of fixed-dose treatment with beclometasone diproprionate and formoterol fumarate versus formoterol fumarate alone in 1,199 patients with severe COPD.
For inclusion in the study, patients had to have a post-bronchodilator forced expiratory volume in 1 second below 50% of predicted and a forced vital capacity ratio of less than 0.7. They also had to have a smoking history of 10 pack-years or more, and a history of at least one COPD exacerbation in the previous 12 months that had required treatment or hospitalization (Eur Respir J. 2013;41[1]:12-7).
After a 2-week run-in period, where all patients received a 24-mcg dose of formoterol fumarate, patients were randomized to continue treatment with formoterol fumarate or to receive the fixed-dose combination of beclometasone diproprionate 400 mcg and beclometasone diproprionate 24 mcg for 48 weeks.
A total of 1,186 patients, most of whom were male (69%) with a mean age of 64 years, formed the intention-to-treat population.
Published results (Respir Med. 2014;108[8]:1153-62) showed that the combination of the ICS beclometasone diproprionate and the LABA formoterol fumarate (Chiesi Farmaceutici SpA) was associated with a 28% reduction in the annual rate of moderate to severe exacerbations versus the LABA alone.
The adjusted rate of exacerbations per patient per year was 0.80 in patients treated with the ICS/LABA combination versus 1.12 for those treated with just the LABA, with an adjusted rate ratio of 0.72 (P less than .001).
The published data also showed that pneumonia was reported by 23 patients (3.8%) treated with the ICS/LABA and by 11 (1.8%) treated with the LABA only.
For the new analysis, Dr. Corradi and his coinvestigators looked at the cases of pneumonia and COPD exacerbations in more detail, plotting out the cumulative number of events over time and also characterizing the types of pneumonia in more detail.
All patients had a chest x-ray to confirm the presence of pneumonia, he said, noting that overall there were 35 cases of pneumonia, 24 occurring in patients treated with the fixed-dose beclometasone diproprionate and formoterol fumarate combination and 11 in patients treated only with formoterol fumarate.
Of these cases, 25 required in-hospital treatment – 16 patients in the ICS/LABA arm and 9 in the LABA-only arm. There were three instances of patients acquiring pneumonia in hospital – two in the ICS/LABA and one in the LABA-only arm.
There were also two fatal cases of pneumonia – one in each treatment group. Neither were thought to be related to either of the treatments.
These findings are in line with a recent review of the use of ICS for COPD by the European Medicines Agency (EMA/488280/2016), which noted that “overall the benefits of inhaled corticosteroid medicines in treating COPD continue to outweigh their risks and there should be no change to the way in which these medicines are used.”
The European Medicines Agency advised that patients and clinicians need to “be alert for signs and symptoms of pneumonia, bearing in mind that the clinical features of pneumonia overlap with those of a worsening (exacerbation) of the underlying disease.”
Dr. Corradi has received speaker fees from Chiesi Farmaceutici SpA, which funded the FORWARD study, and his coauthors are employees of the company.
LONDON – The benefit of a fixed-dose inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) combination in reducing exacerbations of chronic obstructive pulmonary disease (COPD) far outweighed any risk for pneumonia in a post hoc analysis of the 48-week FORWARD study.
Although there were 13 extra pneumonia events when a fixed-dose combination of beclometasone diproprionate and formoterol fumarate (Foster, Chiesi Farmaceutici SpA) was used as compared to formoterol fumarate alone, there were 123 fewer moderate to severe COPD exacerbations over a 342-day analysis period.
“Analysis of pneumonia and exacerbation cumulative number of events shows that the number of incident pneumonia remains very small relative to that of moderate to severe exacerbations,” Massimo Corradi, MD, of the University of Parma (Italy), reported at the annual congress of the European Respiratory Society.
Dr. Corradi added that the new analysis confirms that the ICS/LABA combination has a “positive risk-benefit balance over LABA monotherapy, supporting [the argument that] the benefits of adding an ICS to a bronchodilator significantly outweigh potential risks.”
The FORWARD study was a two-arm trial designed to compare the efficacy and safety of fixed-dose treatment with beclometasone diproprionate and formoterol fumarate versus formoterol fumarate alone in 1,199 patients with severe COPD.
For inclusion in the study, patients had to have a post-bronchodilator forced expiratory volume in 1 second below 50% of predicted and a forced vital capacity ratio of less than 0.7. They also had to have a smoking history of 10 pack-years or more, and a history of at least one COPD exacerbation in the previous 12 months that had required treatment or hospitalization (Eur Respir J. 2013;41[1]:12-7).
After a 2-week run-in period, where all patients received a 24-mcg dose of formoterol fumarate, patients were randomized to continue treatment with formoterol fumarate or to receive the fixed-dose combination of beclometasone diproprionate 400 mcg and beclometasone diproprionate 24 mcg for 48 weeks.
A total of 1,186 patients, most of whom were male (69%) with a mean age of 64 years, formed the intention-to-treat population.
Published results (Respir Med. 2014;108[8]:1153-62) showed that the combination of the ICS beclometasone diproprionate and the LABA formoterol fumarate (Chiesi Farmaceutici SpA) was associated with a 28% reduction in the annual rate of moderate to severe exacerbations versus the LABA alone.
The adjusted rate of exacerbations per patient per year was 0.80 in patients treated with the ICS/LABA combination versus 1.12 for those treated with just the LABA, with an adjusted rate ratio of 0.72 (P less than .001).
The published data also showed that pneumonia was reported by 23 patients (3.8%) treated with the ICS/LABA and by 11 (1.8%) treated with the LABA only.
For the new analysis, Dr. Corradi and his coinvestigators looked at the cases of pneumonia and COPD exacerbations in more detail, plotting out the cumulative number of events over time and also characterizing the types of pneumonia in more detail.
All patients had a chest x-ray to confirm the presence of pneumonia, he said, noting that overall there were 35 cases of pneumonia, 24 occurring in patients treated with the fixed-dose beclometasone diproprionate and formoterol fumarate combination and 11 in patients treated only with formoterol fumarate.
Of these cases, 25 required in-hospital treatment – 16 patients in the ICS/LABA arm and 9 in the LABA-only arm. There were three instances of patients acquiring pneumonia in hospital – two in the ICS/LABA and one in the LABA-only arm.
There were also two fatal cases of pneumonia – one in each treatment group. Neither were thought to be related to either of the treatments.
These findings are in line with a recent review of the use of ICS for COPD by the European Medicines Agency (EMA/488280/2016), which noted that “overall the benefits of inhaled corticosteroid medicines in treating COPD continue to outweigh their risks and there should be no change to the way in which these medicines are used.”
The European Medicines Agency advised that patients and clinicians need to “be alert for signs and symptoms of pneumonia, bearing in mind that the clinical features of pneumonia overlap with those of a worsening (exacerbation) of the underlying disease.”
Dr. Corradi has received speaker fees from Chiesi Farmaceutici SpA, which funded the FORWARD study, and his coauthors are employees of the company.
LONDON – The benefit of a fixed-dose inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) combination in reducing exacerbations of chronic obstructive pulmonary disease (COPD) far outweighed any risk for pneumonia in a post hoc analysis of the 48-week FORWARD study.
Although there were 13 extra pneumonia events when a fixed-dose combination of beclometasone diproprionate and formoterol fumarate (Foster, Chiesi Farmaceutici SpA) was used as compared to formoterol fumarate alone, there were 123 fewer moderate to severe COPD exacerbations over a 342-day analysis period.
“Analysis of pneumonia and exacerbation cumulative number of events shows that the number of incident pneumonia remains very small relative to that of moderate to severe exacerbations,” Massimo Corradi, MD, of the University of Parma (Italy), reported at the annual congress of the European Respiratory Society.
Dr. Corradi added that the new analysis confirms that the ICS/LABA combination has a “positive risk-benefit balance over LABA monotherapy, supporting [the argument that] the benefits of adding an ICS to a bronchodilator significantly outweigh potential risks.”
The FORWARD study was a two-arm trial designed to compare the efficacy and safety of fixed-dose treatment with beclometasone diproprionate and formoterol fumarate versus formoterol fumarate alone in 1,199 patients with severe COPD.
For inclusion in the study, patients had to have a post-bronchodilator forced expiratory volume in 1 second below 50% of predicted and a forced vital capacity ratio of less than 0.7. They also had to have a smoking history of 10 pack-years or more, and a history of at least one COPD exacerbation in the previous 12 months that had required treatment or hospitalization (Eur Respir J. 2013;41[1]:12-7).
After a 2-week run-in period, where all patients received a 24-mcg dose of formoterol fumarate, patients were randomized to continue treatment with formoterol fumarate or to receive the fixed-dose combination of beclometasone diproprionate 400 mcg and beclometasone diproprionate 24 mcg for 48 weeks.
A total of 1,186 patients, most of whom were male (69%) with a mean age of 64 years, formed the intention-to-treat population.
Published results (Respir Med. 2014;108[8]:1153-62) showed that the combination of the ICS beclometasone diproprionate and the LABA formoterol fumarate (Chiesi Farmaceutici SpA) was associated with a 28% reduction in the annual rate of moderate to severe exacerbations versus the LABA alone.
The adjusted rate of exacerbations per patient per year was 0.80 in patients treated with the ICS/LABA combination versus 1.12 for those treated with just the LABA, with an adjusted rate ratio of 0.72 (P less than .001).
The published data also showed that pneumonia was reported by 23 patients (3.8%) treated with the ICS/LABA and by 11 (1.8%) treated with the LABA only.
For the new analysis, Dr. Corradi and his coinvestigators looked at the cases of pneumonia and COPD exacerbations in more detail, plotting out the cumulative number of events over time and also characterizing the types of pneumonia in more detail.
All patients had a chest x-ray to confirm the presence of pneumonia, he said, noting that overall there were 35 cases of pneumonia, 24 occurring in patients treated with the fixed-dose beclometasone diproprionate and formoterol fumarate combination and 11 in patients treated only with formoterol fumarate.
Of these cases, 25 required in-hospital treatment – 16 patients in the ICS/LABA arm and 9 in the LABA-only arm. There were three instances of patients acquiring pneumonia in hospital – two in the ICS/LABA and one in the LABA-only arm.
There were also two fatal cases of pneumonia – one in each treatment group. Neither were thought to be related to either of the treatments.
These findings are in line with a recent review of the use of ICS for COPD by the European Medicines Agency (EMA/488280/2016), which noted that “overall the benefits of inhaled corticosteroid medicines in treating COPD continue to outweigh their risks and there should be no change to the way in which these medicines are used.”
The European Medicines Agency advised that patients and clinicians need to “be alert for signs and symptoms of pneumonia, bearing in mind that the clinical features of pneumonia overlap with those of a worsening (exacerbation) of the underlying disease.”
Dr. Corradi has received speaker fees from Chiesi Farmaceutici SpA, which funded the FORWARD study, and his coauthors are employees of the company.
AT THE ERS CONGRESS 2016
Key clinical point: The risk for pneumonia associated with drugs containing inhaled corticosteroids (ICS) is outweighed by the reduction in chronic obstructive pulmonary disease (COPD) exacerbations that can be achieved.
Major finding: There were 13 extra pneumonia events but 123 fewer COPD exacerbations when a fixed dose ICS/long-acting beta-agonist (LABA) combination was used versus a LABA alone.
Data source: Post hoc analysis of the FORWARD study, a multicenter, randomized, double-blind, active-controlled 48-week trial of a fixed-dose ICS/LABA combination versus LABA for reducing exacerbations in 1,186 patients with COPD.
Disclosures: Dr. Corradi has received speaker fees from Chiesi Farmaceutici SpA, which funded the FORWARD study, and his coauthors are employees of the company.
ACOS definitions under fire
LONDON – A study comparing patient data with six definitions of the Asthma-COPD Overlap Syndrome (ACOS) found only one of the patients analyzed met all definitions. This provoked an animated discussion at the annual congress of the European Respiratory Society about the utility of ACOS as a clinical entity.
Of 864 patients diagnosed with chronic obstructive pulmonary disease (COPD) or asthma drawn from the Netherlands Epidemiology of Obesity cohort (a population-based study with 5,784 patients), 39.1% (338 patients) met at least one of the definitions of ACOS, while 0.1% (one patient) met the criteria for all six definitions.
When this finding was presented, the ERS audience first laughed and then applauded. At the end of the presentation, long lines formed at the microphones. Every comment made was hostile to the concept of ACOS.
“Let us bring ACOS to an honorable death,” said one audience member. His point, reiterated by all who commented subsequently, was that ACOS confuses efforts to treat the underlying respiratory symptoms. Even in those who have both asthma and COPD, the speaker, like other members of the audience, said he considered the diagnosis of ACOS unhelpful.
The six definitions in the study included the latest and just published consensus definition from the ERS (Eur Respir J. 2016;48[3]:664-73). According to the ERS definition, the key features of ACOS are age greater than 40 years, long-term history of asthma (since childhood or early adulthood), and significant exposure to cigarette or biomass smoke.
The other definitions analyzed included a medical history of both asthma and COPD, a self-reported history of both asthma and COPD, and a record of the proportion of a person’s vital capacity that he/she is able to expire in 1 second of forced expiration of less than 0.7 plus a record of fractionated nitric oxide concentration in exhaled breath of greater than or equal to 45 parts per billion.
Although attempted, a Venn diagram that would show overlapping subsets of patients that fell into these definitions “was not possible,” according to Tobias Bonten, MD, University of Leiden, the Netherlands.
Asthma duration was just over 10 years in those identified as having ACOS by medical history alone (registry-based definitions), just over 20 years in those with a medical history and objective evidence of impaired lung function, but about 40 years in those with a self-report of both asthma and COPD.
One area that all groups created by the ACOS definitions did have in common was demographic variables, such as median age, proportion of patients defined as overweight or obese by body mass index, and proportion who were current smokers.
Members of the audience acknowledged the importance of considering the coexistence of asthma and COPD, but expressed skepticism about the value of ACOS as a separate entity in the clinic.
“ACOS is something like the emperor’s new clothes,” one audience member said during the discussion. “It is important to identify asthma patients with obstruction because they have reduced lung function that should be treated more actively, but I find the definition [of ACOS] unnecessary,” he said.
A similar conclusion was drawn in a review article devoted to ACOS published last year (N Engl J Med. 2015;373[13]:1241-9). “It is premature to recommend the designation of ACOS as a disease entity,” the authors wrote.
This is a position widely shared by clinicians, judging from audience comments provoked by this demonstration.
For the sake of time, the moderators were forced to end the discussion with significant lines of clinicians at the microphone.
“It is quite clear that ACOS should die,” said one of the last speakers given a chance to voice an opinion. He suggested that the coexistence of asthma and COPD is something that “quite clearly can happen,” but he objected to definitions he said are unhelpful for clinical care.
Dr. Bonten reported no relevant financial relationships.
LONDON – A study comparing patient data with six definitions of the Asthma-COPD Overlap Syndrome (ACOS) found only one of the patients analyzed met all definitions. This provoked an animated discussion at the annual congress of the European Respiratory Society about the utility of ACOS as a clinical entity.
Of 864 patients diagnosed with chronic obstructive pulmonary disease (COPD) or asthma drawn from the Netherlands Epidemiology of Obesity cohort (a population-based study with 5,784 patients), 39.1% (338 patients) met at least one of the definitions of ACOS, while 0.1% (one patient) met the criteria for all six definitions.
When this finding was presented, the ERS audience first laughed and then applauded. At the end of the presentation, long lines formed at the microphones. Every comment made was hostile to the concept of ACOS.
“Let us bring ACOS to an honorable death,” said one audience member. His point, reiterated by all who commented subsequently, was that ACOS confuses efforts to treat the underlying respiratory symptoms. Even in those who have both asthma and COPD, the speaker, like other members of the audience, said he considered the diagnosis of ACOS unhelpful.
The six definitions in the study included the latest and just published consensus definition from the ERS (Eur Respir J. 2016;48[3]:664-73). According to the ERS definition, the key features of ACOS are age greater than 40 years, long-term history of asthma (since childhood or early adulthood), and significant exposure to cigarette or biomass smoke.
The other definitions analyzed included a medical history of both asthma and COPD, a self-reported history of both asthma and COPD, and a record of the proportion of a person’s vital capacity that he/she is able to expire in 1 second of forced expiration of less than 0.7 plus a record of fractionated nitric oxide concentration in exhaled breath of greater than or equal to 45 parts per billion.
Although attempted, a Venn diagram that would show overlapping subsets of patients that fell into these definitions “was not possible,” according to Tobias Bonten, MD, University of Leiden, the Netherlands.
Asthma duration was just over 10 years in those identified as having ACOS by medical history alone (registry-based definitions), just over 20 years in those with a medical history and objective evidence of impaired lung function, but about 40 years in those with a self-report of both asthma and COPD.
One area that all groups created by the ACOS definitions did have in common was demographic variables, such as median age, proportion of patients defined as overweight or obese by body mass index, and proportion who were current smokers.
Members of the audience acknowledged the importance of considering the coexistence of asthma and COPD, but expressed skepticism about the value of ACOS as a separate entity in the clinic.
“ACOS is something like the emperor’s new clothes,” one audience member said during the discussion. “It is important to identify asthma patients with obstruction because they have reduced lung function that should be treated more actively, but I find the definition [of ACOS] unnecessary,” he said.
A similar conclusion was drawn in a review article devoted to ACOS published last year (N Engl J Med. 2015;373[13]:1241-9). “It is premature to recommend the designation of ACOS as a disease entity,” the authors wrote.
This is a position widely shared by clinicians, judging from audience comments provoked by this demonstration.
For the sake of time, the moderators were forced to end the discussion with significant lines of clinicians at the microphone.
“It is quite clear that ACOS should die,” said one of the last speakers given a chance to voice an opinion. He suggested that the coexistence of asthma and COPD is something that “quite clearly can happen,” but he objected to definitions he said are unhelpful for clinical care.
Dr. Bonten reported no relevant financial relationships.
LONDON – A study comparing patient data with six definitions of the Asthma-COPD Overlap Syndrome (ACOS) found only one of the patients analyzed met all definitions. This provoked an animated discussion at the annual congress of the European Respiratory Society about the utility of ACOS as a clinical entity.
Of 864 patients diagnosed with chronic obstructive pulmonary disease (COPD) or asthma drawn from the Netherlands Epidemiology of Obesity cohort (a population-based study with 5,784 patients), 39.1% (338 patients) met at least one of the definitions of ACOS, while 0.1% (one patient) met the criteria for all six definitions.
When this finding was presented, the ERS audience first laughed and then applauded. At the end of the presentation, long lines formed at the microphones. Every comment made was hostile to the concept of ACOS.
“Let us bring ACOS to an honorable death,” said one audience member. His point, reiterated by all who commented subsequently, was that ACOS confuses efforts to treat the underlying respiratory symptoms. Even in those who have both asthma and COPD, the speaker, like other members of the audience, said he considered the diagnosis of ACOS unhelpful.
The six definitions in the study included the latest and just published consensus definition from the ERS (Eur Respir J. 2016;48[3]:664-73). According to the ERS definition, the key features of ACOS are age greater than 40 years, long-term history of asthma (since childhood or early adulthood), and significant exposure to cigarette or biomass smoke.
The other definitions analyzed included a medical history of both asthma and COPD, a self-reported history of both asthma and COPD, and a record of the proportion of a person’s vital capacity that he/she is able to expire in 1 second of forced expiration of less than 0.7 plus a record of fractionated nitric oxide concentration in exhaled breath of greater than or equal to 45 parts per billion.
Although attempted, a Venn diagram that would show overlapping subsets of patients that fell into these definitions “was not possible,” according to Tobias Bonten, MD, University of Leiden, the Netherlands.
Asthma duration was just over 10 years in those identified as having ACOS by medical history alone (registry-based definitions), just over 20 years in those with a medical history and objective evidence of impaired lung function, but about 40 years in those with a self-report of both asthma and COPD.
One area that all groups created by the ACOS definitions did have in common was demographic variables, such as median age, proportion of patients defined as overweight or obese by body mass index, and proportion who were current smokers.
Members of the audience acknowledged the importance of considering the coexistence of asthma and COPD, but expressed skepticism about the value of ACOS as a separate entity in the clinic.
“ACOS is something like the emperor’s new clothes,” one audience member said during the discussion. “It is important to identify asthma patients with obstruction because they have reduced lung function that should be treated more actively, but I find the definition [of ACOS] unnecessary,” he said.
A similar conclusion was drawn in a review article devoted to ACOS published last year (N Engl J Med. 2015;373[13]:1241-9). “It is premature to recommend the designation of ACOS as a disease entity,” the authors wrote.
This is a position widely shared by clinicians, judging from audience comments provoked by this demonstration.
For the sake of time, the moderators were forced to end the discussion with significant lines of clinicians at the microphone.
“It is quite clear that ACOS should die,” said one of the last speakers given a chance to voice an opinion. He suggested that the coexistence of asthma and COPD is something that “quite clearly can happen,” but he objected to definitions he said are unhelpful for clinical care.
Dr. Bonten reported no relevant financial relationships.
AT THE ERS CONGRESS 2016
Key clinical point: A study comparing current definitions of Asthma-COPD Overlap Syndrome (ACOS) provoked criticism of the very concept.
Major finding: When six definitions of ACOS were compared in a population-based study, only 1 (0.1%) of 864 possible candidates met all criteria of all definitions.
Data source: Population-based cohort study.
Disclosures: Dr. Bonten reported no relevant financial relationships.
New method proposed for phenotyping COPD patients
LONDON – Based on readily available clinical data, patients with chronic obstructive pulmonary disease (COPD) can be placed into five phenotypes with different characteristics and risk profiles, according to data generated by a cluster analysis and presented at the European Respiratory Society International Congress 2016.
The algorithm that places patients into these phenotypes was developed from a cluster analysis, reported Pierre-Regis Burgel, MD, PhD, professor of respiratory medicine, Université Réné Descartes, Paris. Mortality rates at 3 years for these phenotypes ranged from 2.6% to 49.5%
“We think that this could be the basis for recognizing clinically distinct COPD phenotypes and designing better tailored management,” Dr. Burgel explained. “We also think it has potential use in routine clinical assessment.”
To create these phenotypes, data from 2,049 COPD patients enrolled in a French-Belgian collaborative cohort were evaluated with Classification And Regression Tree (CART) analyses. The five phenotypes were derived from symptom burden, respiratory function, relative age, and presence of comorbidities.
Based on these characteristics, “a set of clinical rules” to phenotype patients was developed, according to Dr. Burgel. This algorithm was then further validated with the 3,651 COPD patients enrolled in the prospective COPD Cohorts Collaborative International Assessment (3CIA) initiative.
The two initial branches in the algorithm are created by dividing patients into those with and without cardiovascular comorbidities or diabetes. In those without cardiovascular disease, the phenotypes are defined by relative symptom severity, using cut-off scores from the modified Medical Research Council (mMRC) dyspnea assessment tool and relative degrees of lung function impairment as measured with forced expiratory volume in 1 second (FEV1).
In those with cardiovascular disease or diabetes, mMRC-defined symptoms and FEV1-defined lung function impairment also create decision points in the algorithm, but age and body mass index (BMI) are additional variables that direct patients to a specific phenotype. Class 4 and 5 are reached in the absence of cardiovascular disease or diabetes only, while cardiovascular disease is a prerequisite to reach Classes 4 and 5. Class 2 is the only phenotype that can be reached through the algorithm irrespective of the presence or absence of cardiovascular disease.
Using this algorithm, each of the phenotypes was associated with similar relative hierarchy in mortality in the two cohorts, even though mortality rates for each phenotype were consistently lower in the 3CIA group.
For class 1, which was reached by patients with cardiovascular disease or diabetes, the greatest symptom burden, and the lowest lung function, the mortality rates at 3 years were 49.5% and 23.2% for the French-Belgian and 3CIA cohorts, respectively.
For class 4, which was also defined by the greatest symptom burden and the lowest lung function without cardiovascular disease or diabetes the mortality rates were 45.3% and 27.3%, respectively. The lowest mortality rates, which were 2.6% and 4.0%, respectively, were found in the class 5 phenotype, which contained patients with a low symptom burden (mMRC less than or equal to 1), relatively good lung function (FEV1 greater than or equal to 60%), and no history of cardiovascular disease or diabetes.
In classes 2 and 3, the mortality rates fell in between those of the lowest- and highest-risk phenotypes. Specifically, these 3-year mortality rates were 22.9% and 24%, respectively, in the French-Belgian cohort, and 11.1% and 14.1%, respectively, in the 3CIA cohort.
The consistency of the hierarchy of outcomes in the two cohorts provides the basis for suggesting that these phenotypes are effective for categorizing relative risk, according to Dr. Burgel. He believes that the phenotypes are clinically important, and he emphasized that the algorithm relies on clinical information that is already routinely collected and readily accessible.
“There is growing awareness that COPD phenotypes are important and are likely to be valuable in managing patients,” Dr. Burgel explained. “We feel that we have created simple rules for allocating patients that we think will be useful for research and for clinical application.”
Dr. Burgel reported financial relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Pfizer, and Vertex.
LONDON – Based on readily available clinical data, patients with chronic obstructive pulmonary disease (COPD) can be placed into five phenotypes with different characteristics and risk profiles, according to data generated by a cluster analysis and presented at the European Respiratory Society International Congress 2016.
The algorithm that places patients into these phenotypes was developed from a cluster analysis, reported Pierre-Regis Burgel, MD, PhD, professor of respiratory medicine, Université Réné Descartes, Paris. Mortality rates at 3 years for these phenotypes ranged from 2.6% to 49.5%
“We think that this could be the basis for recognizing clinically distinct COPD phenotypes and designing better tailored management,” Dr. Burgel explained. “We also think it has potential use in routine clinical assessment.”
To create these phenotypes, data from 2,049 COPD patients enrolled in a French-Belgian collaborative cohort were evaluated with Classification And Regression Tree (CART) analyses. The five phenotypes were derived from symptom burden, respiratory function, relative age, and presence of comorbidities.
Based on these characteristics, “a set of clinical rules” to phenotype patients was developed, according to Dr. Burgel. This algorithm was then further validated with the 3,651 COPD patients enrolled in the prospective COPD Cohorts Collaborative International Assessment (3CIA) initiative.
The two initial branches in the algorithm are created by dividing patients into those with and without cardiovascular comorbidities or diabetes. In those without cardiovascular disease, the phenotypes are defined by relative symptom severity, using cut-off scores from the modified Medical Research Council (mMRC) dyspnea assessment tool and relative degrees of lung function impairment as measured with forced expiratory volume in 1 second (FEV1).
In those with cardiovascular disease or diabetes, mMRC-defined symptoms and FEV1-defined lung function impairment also create decision points in the algorithm, but age and body mass index (BMI) are additional variables that direct patients to a specific phenotype. Class 4 and 5 are reached in the absence of cardiovascular disease or diabetes only, while cardiovascular disease is a prerequisite to reach Classes 4 and 5. Class 2 is the only phenotype that can be reached through the algorithm irrespective of the presence or absence of cardiovascular disease.
Using this algorithm, each of the phenotypes was associated with similar relative hierarchy in mortality in the two cohorts, even though mortality rates for each phenotype were consistently lower in the 3CIA group.
For class 1, which was reached by patients with cardiovascular disease or diabetes, the greatest symptom burden, and the lowest lung function, the mortality rates at 3 years were 49.5% and 23.2% for the French-Belgian and 3CIA cohorts, respectively.
For class 4, which was also defined by the greatest symptom burden and the lowest lung function without cardiovascular disease or diabetes the mortality rates were 45.3% and 27.3%, respectively. The lowest mortality rates, which were 2.6% and 4.0%, respectively, were found in the class 5 phenotype, which contained patients with a low symptom burden (mMRC less than or equal to 1), relatively good lung function (FEV1 greater than or equal to 60%), and no history of cardiovascular disease or diabetes.
In classes 2 and 3, the mortality rates fell in between those of the lowest- and highest-risk phenotypes. Specifically, these 3-year mortality rates were 22.9% and 24%, respectively, in the French-Belgian cohort, and 11.1% and 14.1%, respectively, in the 3CIA cohort.
The consistency of the hierarchy of outcomes in the two cohorts provides the basis for suggesting that these phenotypes are effective for categorizing relative risk, according to Dr. Burgel. He believes that the phenotypes are clinically important, and he emphasized that the algorithm relies on clinical information that is already routinely collected and readily accessible.
“There is growing awareness that COPD phenotypes are important and are likely to be valuable in managing patients,” Dr. Burgel explained. “We feel that we have created simple rules for allocating patients that we think will be useful for research and for clinical application.”
Dr. Burgel reported financial relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Pfizer, and Vertex.
LONDON – Based on readily available clinical data, patients with chronic obstructive pulmonary disease (COPD) can be placed into five phenotypes with different characteristics and risk profiles, according to data generated by a cluster analysis and presented at the European Respiratory Society International Congress 2016.
The algorithm that places patients into these phenotypes was developed from a cluster analysis, reported Pierre-Regis Burgel, MD, PhD, professor of respiratory medicine, Université Réné Descartes, Paris. Mortality rates at 3 years for these phenotypes ranged from 2.6% to 49.5%
“We think that this could be the basis for recognizing clinically distinct COPD phenotypes and designing better tailored management,” Dr. Burgel explained. “We also think it has potential use in routine clinical assessment.”
To create these phenotypes, data from 2,049 COPD patients enrolled in a French-Belgian collaborative cohort were evaluated with Classification And Regression Tree (CART) analyses. The five phenotypes were derived from symptom burden, respiratory function, relative age, and presence of comorbidities.
Based on these characteristics, “a set of clinical rules” to phenotype patients was developed, according to Dr. Burgel. This algorithm was then further validated with the 3,651 COPD patients enrolled in the prospective COPD Cohorts Collaborative International Assessment (3CIA) initiative.
The two initial branches in the algorithm are created by dividing patients into those with and without cardiovascular comorbidities or diabetes. In those without cardiovascular disease, the phenotypes are defined by relative symptom severity, using cut-off scores from the modified Medical Research Council (mMRC) dyspnea assessment tool and relative degrees of lung function impairment as measured with forced expiratory volume in 1 second (FEV1).
In those with cardiovascular disease or diabetes, mMRC-defined symptoms and FEV1-defined lung function impairment also create decision points in the algorithm, but age and body mass index (BMI) are additional variables that direct patients to a specific phenotype. Class 4 and 5 are reached in the absence of cardiovascular disease or diabetes only, while cardiovascular disease is a prerequisite to reach Classes 4 and 5. Class 2 is the only phenotype that can be reached through the algorithm irrespective of the presence or absence of cardiovascular disease.
Using this algorithm, each of the phenotypes was associated with similar relative hierarchy in mortality in the two cohorts, even though mortality rates for each phenotype were consistently lower in the 3CIA group.
For class 1, which was reached by patients with cardiovascular disease or diabetes, the greatest symptom burden, and the lowest lung function, the mortality rates at 3 years were 49.5% and 23.2% for the French-Belgian and 3CIA cohorts, respectively.
For class 4, which was also defined by the greatest symptom burden and the lowest lung function without cardiovascular disease or diabetes the mortality rates were 45.3% and 27.3%, respectively. The lowest mortality rates, which were 2.6% and 4.0%, respectively, were found in the class 5 phenotype, which contained patients with a low symptom burden (mMRC less than or equal to 1), relatively good lung function (FEV1 greater than or equal to 60%), and no history of cardiovascular disease or diabetes.
In classes 2 and 3, the mortality rates fell in between those of the lowest- and highest-risk phenotypes. Specifically, these 3-year mortality rates were 22.9% and 24%, respectively, in the French-Belgian cohort, and 11.1% and 14.1%, respectively, in the 3CIA cohort.
The consistency of the hierarchy of outcomes in the two cohorts provides the basis for suggesting that these phenotypes are effective for categorizing relative risk, according to Dr. Burgel. He believes that the phenotypes are clinically important, and he emphasized that the algorithm relies on clinical information that is already routinely collected and readily accessible.
“There is growing awareness that COPD phenotypes are important and are likely to be valuable in managing patients,” Dr. Burgel explained. “We feel that we have created simple rules for allocating patients that we think will be useful for research and for clinical application.”
Dr. Burgel reported financial relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Pfizer, and Vertex.
AT THE ERS CONGRESS 2016
Key clinical point: A relatively simple method proposed for identifying COPD phenotypes has implications for clinical care as well as research.
Major finding: Five phenotypes were identified with mortality rates at 3 years ranging from 2.6% to 49.5%
Data source: Cohort analyses.
Disclosures: Dr. Burgel reported financial relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Pfizer, and Vertex.
Analyses strengthen FLAME’s findings
LONDON – In chronic obstructive pulmonary disease (COPD), the advantage of a long-acting beta agonist (LABA) plus a long-acting muscarinic antagonist (LAMA) over a LABA plus an inhaled corticosteroid (ICS) was observed in every subgroup in the FLAME trial evaluated, according to post hoc analyses presented at the annual congress of the European Respiratory Society.
“We thought that we might not see the difference in the COPD patients with more severe disease, but the advantage was consistent even among those who entered the trial on triple therapy,” reported Jadwiga A. Wedzicha, MD, professor of respiratory medicine at the National Heart and Lung Institute, Imperial College, London.
FLAME, the recently published study that compared LABA/LAMA to LABA/ICS, was planned as a noninferiority study with the underlying hypothesis that LABA/LAMA would perform as well as LABA/ICS for the primary outcome of annual rate of COPD exacerbations (N Engl J Med. 2016;374:2222-34). Instead, the 11% lower rate of exacerbations for LABA/LAMA proved statistically significant (P = .003).
Six post hoc FLAME analyses were presented at the 2016 ERS Congress to further explore this result. All supported the main result. In addition to evaluating those who entered the trial on a LABA/LAMA/ICS triple-therapy combination, the analyses covered a broad array of subgroups defined by age, smoking history, and COPD severity as defined by Global Initiative for Chronic Obstructive Lung Disease (GOLD) classifications.
In FLAME, 3,362 COPD patients who had at least one exacerbation in the preceding year were randomized to the LABA indacaterol (110 mcg) plus the LAMA glycopyrronium (50 mcg) once daily or the combination of the LABA salmeterol (50 mcg) and the ICS fluticasone (500 mcg) twice daily. In addition to the relative advantage on the primary outcome of any exacerbation, the LABA/LAMA combination also significantly reduced the rate of moderate to severe exacerbations (P less than .001), and it extended the times to the first moderate to severe exacerbation (P less than .001) and the first severe exacerbation (P = .046), according to the published data.
In the post hoc analyses, the advantage of LABA/LAMA relative to LAMA/ICS was remarkably consistent. For example, in stratifications made for age (less than 55 years, 55 to less than 65 years, 65-75 years, and greater than or equal to 75 years) at least a numerical advantage of LABA/LAMA was seen in all age groups for prevention of any exacerbation, and the difference reached statistical significance for those in the age group 55 to greater than 65 years. For prevention of moderate to severe exacerbations, the treatments were found to be equivalent for individuals younger than 55 years, but LABA/LAMA was statistically superior for the other three age categories.
For ex-smokers, unlike current smokers, the numerical advantage of LABA/LAMA over LABA/ICS for reduction in the rate ratio of all exacerbations did not reach statistical significance, but the LABA/LAMA combination did provide a statistically significant advantage for both ex-smokers and current smokers for moderate to severe exacerbations.
For patients with two or more exacerbations in the year prior to enrollment in FLAME, the relative degree of protection was of magnitude similar to that of patients with only one exacerbation even though the relative advantage in those with multiple prior exacerbations did not reach statistical significance. However, the lack of significance was likely due to the relatively small number of patients in this subpopulation, according to Dr. Wedzicha.
Similarly, the LABA/LAMA combination was at least numerically superior to LABA/ICS for all exacerbations and for moderate to severe exacerbations across GOLD classifications with one exception. When compared for relative protection against moderate to severe exacerbations, there was a slight and nonsignificant disadvantage for LABA/LAMA, but, again, Dr. Wedzicha reported, “the number of patients in this subgroup was quite small.”
In another FLAME post hoc analysis, the odds ratio (OR) for exacerbations among the 1,893 patients (56.3%) who were on ICS at study entry was found to be almost identical to the OR among those who were not. Specifically, the ORs for all exacerbations and moderate to severe exacerbations were 0.88 (P = .008) and 0.86 (P = .018), respectively, for those previously treated with ICS and 0.88 (P = .021) and 0.78 (P = .002), respectively, for those who had not been treated with ICS.
The LABA/LAMA combination was also superior to LABA/ICS for improvements in quality of life, which was measured via the St. George’s Respiratory Questionnaire. With an improvement of at least four units on the St. George’s Respiratory Questionnaire defined as clinically meaningful, 49.5% of LABA/LAMA patients versus 43.8% of LABA/ICS patients (P less than .024) benefited on this measure.
Overall, the results from the FLAME post hoc analyses have demonstrated “remarkable consistency,” Dr. Wedzicha reported. Taken together, she said the data “imply that LABA/LAMA is the first choice of treatment for COPD patients at risk of exacerbation.”
LONDON – In chronic obstructive pulmonary disease (COPD), the advantage of a long-acting beta agonist (LABA) plus a long-acting muscarinic antagonist (LAMA) over a LABA plus an inhaled corticosteroid (ICS) was observed in every subgroup in the FLAME trial evaluated, according to post hoc analyses presented at the annual congress of the European Respiratory Society.
“We thought that we might not see the difference in the COPD patients with more severe disease, but the advantage was consistent even among those who entered the trial on triple therapy,” reported Jadwiga A. Wedzicha, MD, professor of respiratory medicine at the National Heart and Lung Institute, Imperial College, London.
FLAME, the recently published study that compared LABA/LAMA to LABA/ICS, was planned as a noninferiority study with the underlying hypothesis that LABA/LAMA would perform as well as LABA/ICS for the primary outcome of annual rate of COPD exacerbations (N Engl J Med. 2016;374:2222-34). Instead, the 11% lower rate of exacerbations for LABA/LAMA proved statistically significant (P = .003).
Six post hoc FLAME analyses were presented at the 2016 ERS Congress to further explore this result. All supported the main result. In addition to evaluating those who entered the trial on a LABA/LAMA/ICS triple-therapy combination, the analyses covered a broad array of subgroups defined by age, smoking history, and COPD severity as defined by Global Initiative for Chronic Obstructive Lung Disease (GOLD) classifications.
In FLAME, 3,362 COPD patients who had at least one exacerbation in the preceding year were randomized to the LABA indacaterol (110 mcg) plus the LAMA glycopyrronium (50 mcg) once daily or the combination of the LABA salmeterol (50 mcg) and the ICS fluticasone (500 mcg) twice daily. In addition to the relative advantage on the primary outcome of any exacerbation, the LABA/LAMA combination also significantly reduced the rate of moderate to severe exacerbations (P less than .001), and it extended the times to the first moderate to severe exacerbation (P less than .001) and the first severe exacerbation (P = .046), according to the published data.
In the post hoc analyses, the advantage of LABA/LAMA relative to LAMA/ICS was remarkably consistent. For example, in stratifications made for age (less than 55 years, 55 to less than 65 years, 65-75 years, and greater than or equal to 75 years) at least a numerical advantage of LABA/LAMA was seen in all age groups for prevention of any exacerbation, and the difference reached statistical significance for those in the age group 55 to greater than 65 years. For prevention of moderate to severe exacerbations, the treatments were found to be equivalent for individuals younger than 55 years, but LABA/LAMA was statistically superior for the other three age categories.
For ex-smokers, unlike current smokers, the numerical advantage of LABA/LAMA over LABA/ICS for reduction in the rate ratio of all exacerbations did not reach statistical significance, but the LABA/LAMA combination did provide a statistically significant advantage for both ex-smokers and current smokers for moderate to severe exacerbations.
For patients with two or more exacerbations in the year prior to enrollment in FLAME, the relative degree of protection was of magnitude similar to that of patients with only one exacerbation even though the relative advantage in those with multiple prior exacerbations did not reach statistical significance. However, the lack of significance was likely due to the relatively small number of patients in this subpopulation, according to Dr. Wedzicha.
Similarly, the LABA/LAMA combination was at least numerically superior to LABA/ICS for all exacerbations and for moderate to severe exacerbations across GOLD classifications with one exception. When compared for relative protection against moderate to severe exacerbations, there was a slight and nonsignificant disadvantage for LABA/LAMA, but, again, Dr. Wedzicha reported, “the number of patients in this subgroup was quite small.”
In another FLAME post hoc analysis, the odds ratio (OR) for exacerbations among the 1,893 patients (56.3%) who were on ICS at study entry was found to be almost identical to the OR among those who were not. Specifically, the ORs for all exacerbations and moderate to severe exacerbations were 0.88 (P = .008) and 0.86 (P = .018), respectively, for those previously treated with ICS and 0.88 (P = .021) and 0.78 (P = .002), respectively, for those who had not been treated with ICS.
The LABA/LAMA combination was also superior to LABA/ICS for improvements in quality of life, which was measured via the St. George’s Respiratory Questionnaire. With an improvement of at least four units on the St. George’s Respiratory Questionnaire defined as clinically meaningful, 49.5% of LABA/LAMA patients versus 43.8% of LABA/ICS patients (P less than .024) benefited on this measure.
Overall, the results from the FLAME post hoc analyses have demonstrated “remarkable consistency,” Dr. Wedzicha reported. Taken together, she said the data “imply that LABA/LAMA is the first choice of treatment for COPD patients at risk of exacerbation.”
LONDON – In chronic obstructive pulmonary disease (COPD), the advantage of a long-acting beta agonist (LABA) plus a long-acting muscarinic antagonist (LAMA) over a LABA plus an inhaled corticosteroid (ICS) was observed in every subgroup in the FLAME trial evaluated, according to post hoc analyses presented at the annual congress of the European Respiratory Society.
“We thought that we might not see the difference in the COPD patients with more severe disease, but the advantage was consistent even among those who entered the trial on triple therapy,” reported Jadwiga A. Wedzicha, MD, professor of respiratory medicine at the National Heart and Lung Institute, Imperial College, London.
FLAME, the recently published study that compared LABA/LAMA to LABA/ICS, was planned as a noninferiority study with the underlying hypothesis that LABA/LAMA would perform as well as LABA/ICS for the primary outcome of annual rate of COPD exacerbations (N Engl J Med. 2016;374:2222-34). Instead, the 11% lower rate of exacerbations for LABA/LAMA proved statistically significant (P = .003).
Six post hoc FLAME analyses were presented at the 2016 ERS Congress to further explore this result. All supported the main result. In addition to evaluating those who entered the trial on a LABA/LAMA/ICS triple-therapy combination, the analyses covered a broad array of subgroups defined by age, smoking history, and COPD severity as defined by Global Initiative for Chronic Obstructive Lung Disease (GOLD) classifications.
In FLAME, 3,362 COPD patients who had at least one exacerbation in the preceding year were randomized to the LABA indacaterol (110 mcg) plus the LAMA glycopyrronium (50 mcg) once daily or the combination of the LABA salmeterol (50 mcg) and the ICS fluticasone (500 mcg) twice daily. In addition to the relative advantage on the primary outcome of any exacerbation, the LABA/LAMA combination also significantly reduced the rate of moderate to severe exacerbations (P less than .001), and it extended the times to the first moderate to severe exacerbation (P less than .001) and the first severe exacerbation (P = .046), according to the published data.
In the post hoc analyses, the advantage of LABA/LAMA relative to LAMA/ICS was remarkably consistent. For example, in stratifications made for age (less than 55 years, 55 to less than 65 years, 65-75 years, and greater than or equal to 75 years) at least a numerical advantage of LABA/LAMA was seen in all age groups for prevention of any exacerbation, and the difference reached statistical significance for those in the age group 55 to greater than 65 years. For prevention of moderate to severe exacerbations, the treatments were found to be equivalent for individuals younger than 55 years, but LABA/LAMA was statistically superior for the other three age categories.
For ex-smokers, unlike current smokers, the numerical advantage of LABA/LAMA over LABA/ICS for reduction in the rate ratio of all exacerbations did not reach statistical significance, but the LABA/LAMA combination did provide a statistically significant advantage for both ex-smokers and current smokers for moderate to severe exacerbations.
For patients with two or more exacerbations in the year prior to enrollment in FLAME, the relative degree of protection was of magnitude similar to that of patients with only one exacerbation even though the relative advantage in those with multiple prior exacerbations did not reach statistical significance. However, the lack of significance was likely due to the relatively small number of patients in this subpopulation, according to Dr. Wedzicha.
Similarly, the LABA/LAMA combination was at least numerically superior to LABA/ICS for all exacerbations and for moderate to severe exacerbations across GOLD classifications with one exception. When compared for relative protection against moderate to severe exacerbations, there was a slight and nonsignificant disadvantage for LABA/LAMA, but, again, Dr. Wedzicha reported, “the number of patients in this subgroup was quite small.”
In another FLAME post hoc analysis, the odds ratio (OR) for exacerbations among the 1,893 patients (56.3%) who were on ICS at study entry was found to be almost identical to the OR among those who were not. Specifically, the ORs for all exacerbations and moderate to severe exacerbations were 0.88 (P = .008) and 0.86 (P = .018), respectively, for those previously treated with ICS and 0.88 (P = .021) and 0.78 (P = .002), respectively, for those who had not been treated with ICS.
The LABA/LAMA combination was also superior to LABA/ICS for improvements in quality of life, which was measured via the St. George’s Respiratory Questionnaire. With an improvement of at least four units on the St. George’s Respiratory Questionnaire defined as clinically meaningful, 49.5% of LABA/LAMA patients versus 43.8% of LABA/ICS patients (P less than .024) benefited on this measure.
Overall, the results from the FLAME post hoc analyses have demonstrated “remarkable consistency,” Dr. Wedzicha reported. Taken together, she said the data “imply that LABA/LAMA is the first choice of treatment for COPD patients at risk of exacerbation.”
At THE ERS CONGRESS 2016
Key clinical point: The advantage of a LABA/LAMA combination over LABA/ICS in COPD patients persists regardless of patient subgroup.
Major finding: In a series of post hoc analyses from the FLAME trial, the advantage of LABA/LAMA was observed in every subgroup evaluated.
Data source: Post hoc analyses of phase III trial.
Disclosures: Dr Wedzicha reported financial relationships with Bayer, Chiesi, Boehringer Ingelheim, GlaxoSmithKline, Johnson & Johnson, Novartis, Pfizer, Takeda, and Vifor Pharma
Decision rule identifies unprovoked VTE patients who can halt anticoagulation
ROME – Half of all women who experience a first unprovoked venous thromboembolism (VTE) can safely be spared lifelong anticoagulation through application of the newly validated HERDOO2 decision rule, Marc A. Rodger, MD, reported at the annual congress of the European Society of Cardiology.
“We’ve validated that a simple, memorable decision rule on anticoagulation applied at the clinically relevant time point works. And it is the only clinical decision rule that has now been prospectively validated,” said Dr. Rodger, professor of medicine, chief and chair of the division of hematology, and head of the thrombosis program at the University of Ottawa.
He presented the results of the validation study, known as the REVERSE II study, which included 2,779 patients with a first unprovoked VTE at 44 centers in seven countries. The full name of the decision rule is “Men Continue and HERDOO2,” a name that says it all: the rule posits that all men as well as those women with a HERDOO2 (Hyperpigmentation, Edema, Redness, d-dimer, Obesity, Older age, 2 or more points) score of at least 2 out of a possible 4 points need to stay on anticoagulation indefinitely because their risk of a recurrent VTE off-therapy clearly exceeds that of a bleeding event on-therapy. In contrast, women with a HERDOO2 score of 0 or 1 can safely stop anticoagulation after the standard 3-6 months of acute short-term therapy.
“Sorry, gentlemen, but we could find no low-risk group of men. They were all high risk,” he said. “But 50% of women with unprovoked vein blood clots can be spared the burdens, costs, and risks of lifelong blood thinners.”
Dr. Rodger and coinvestigators began work on developing a multivariate clinical decision rule in 2001. They examined 69 risk predictors, eventually winnowing down to a manageable four potent risk predictors identified by the acronym HERDOO2.
The derivation study was published 8 years ago (CMAJ. 2008;Aug 26;179[5]:417-26). It showed that women with a HERDOO2 score of 2 or more as well as all men had roughly a 14% rate of recurrent VTE in the first year after stopping anticoagulation, while women with a score of 0 or 1 had about a 1.6% risk. The International Society on Thrombosis and Haemostasis suggests that it’s safe to discontinue anticoagulants if the risk of recurrent thrombosis at 1 year off-therapy is less than 5%, given the significant risk of serious bleeding on-therapy and the fact that a serious bleed event is two to three times more likely than a VTE to be fatal.
Dr. Rodger and coinvestigators recognized that a clinical decision rule needs to be externally validated before it’s ready for prime-time use in clinical practice. Thus, they conducted the REVERSE II study, in which the decision rule was applied after the 2,799 participants had been on anticoagulation for 5-12 months. All had a first proximal deep vein thrombosis and/or a segmental or greater pulmonary embolism. Patients were still on anticoagulation at the time the rule was applied, which is why the cut point for a positive d-dimer test in HERDOO2 is 250 mcg/L, half of the threshold value for a positive test in patients not on anticoagulation.
They identified 631 women as low risk, with a HERDOO2 score of 0 or 1. They and their physicians were instructed to stop anticoagulation at that time. The 2,148 high-risk subjects – that is, all of the men and the high-risk women – were advised to remain on anticoagulation. The primary study endpoint was the rate of recurrent VTE in the 12 months following testing and patient guidance. The lost-to-follow-up rate was 2.2%.
The recurrent VTE rate was 3% in the 591 low-risk women who discontinued anticoagulants and zero in 31 others who elected to stay on medication. In the high-risk group identified by the HERDOO2 rule, the recurrent VTE rate at 12 months was 8.1% in the 323 who opted to discontinue anticoagulants and just 1.6% in 1,802 who continued on therapy as advised, a finding that underscores the effectiveness of selectively applied long-term anticoagulation therapy, he continued.
The recurrent VTE rate among the 291 women with a HERDOO2 score of 0 or 1 who were on exogenous estrogen was 1.4%, while in high-risk women taking estrogen the rate was more than doubled at 3.1%. But in women aged 50-64 identified by the HERDOO2 rule as being low risk, the actual recurrent VTE rate was 5.7%, a finding that raised a red flag for the investigators.
“There may be an evolution of the HERDOO2 decision rule to a lower age cut point. But that’s something that requires further study in postmenopausal women,” according to Dr. Rodger.
The investigators defined a first unprovoked VTE as one occurring in the absence during the previous 90 days of major surgery, a fracture or cast, more than 3 days of immobilization, or malignancy within the last 5 years.
Venous thromboembolism is the second most common cardiovascular disorder and the third most common cause of cardiovascular death. Unprovoked VTEs account for half of all VTEs. Their management has been a controversial subject. Both the American College of Chest Physicians and the European Society of Cardiology recommend continuing anticoagulation indefinitely in patients who aren’t at high bleeding risk.
“But this is a relatively weak 2B recommendation because of the tightly balanced competing risks of recurrent thrombosis off anticoagulation and major bleeding on anticoagulation,” Dr. Rodger said. He added that he considers REVERSE II to be practice changing, and predicted that once the results are published the guidelines will be revised.
Discussant Giancarlo Agnelli, MD, was a tough critic who gave fair warning.
“I am friends with many of the authors of this paper, and in this country we are usually gentle with enemies and nasty with friends,” declared Dr. Agnelli, professor of internal medicine and director of internal and cardiovascular medicine and the stroke unit at the University of Perugia, Italy.
He didn’t find the REVERSE II study or the HERDOO2 rule persuasive. On the plus side, he said, the HERDOO2 rule has now been validated, unlike the proposed DASH and Vienna rules. And it was tested in a diverse multinational patient population. But the fact that the HERDOO2 rule is only applicable in women is a major limitation. And REVERSE II was not a randomized trial, Dr. Agnelli noted.
Moreover, 1 year of follow-up seems insufficient, he continued. He cited a French multicenter trial in which patients with a first unprovoked VTE received 6 months of anticoagulants and were then randomized to another 18 months of anticoagulation or placebo. During that 18 months, the group on anticoagulants had a significantly lower rate of the composite endpoint comprised of recurrent VTE or major bleeding, but once that period was over they experienced catchup. By the time the study ended at 42 months, the two study arms didn’t differ significantly in the composite endpoint (JAMA. 2015 Jul 7;314[1]:31-40).
More broadly, Dr. Agnelli also questioned the need for an anticoagulation discontinuation rule in the contemporary era of new oral anticoagulants (NOACs). He was lead investigator in the AMPLIFY study, a major randomized trial of fixed-dose apixaban (Eliquis) versus conventional therapy with subcutaneous enoxaparin (Lovenox) bridging to warfarin in 5,395 patients with acute VTE. The NOAC was associated with a 69% reduction in the relative risk of bleeding and was noninferior to standard therapy in the risk of recurrent VTE (N Engl J Med. 2013 Aug 29;369[9]:799-808).
“Why should we think about withholding anticoagulation in some patients when we now have such a safe approach?” he asked.
Dr. Rodger reported receiving research grants from the French government as well as from Biomerieux, which funded the REVERSE II study. Dr. Agnelli reported having no financial conflicts.
ROME – Half of all women who experience a first unprovoked venous thromboembolism (VTE) can safely be spared lifelong anticoagulation through application of the newly validated HERDOO2 decision rule, Marc A. Rodger, MD, reported at the annual congress of the European Society of Cardiology.
“We’ve validated that a simple, memorable decision rule on anticoagulation applied at the clinically relevant time point works. And it is the only clinical decision rule that has now been prospectively validated,” said Dr. Rodger, professor of medicine, chief and chair of the division of hematology, and head of the thrombosis program at the University of Ottawa.
He presented the results of the validation study, known as the REVERSE II study, which included 2,779 patients with a first unprovoked VTE at 44 centers in seven countries. The full name of the decision rule is “Men Continue and HERDOO2,” a name that says it all: the rule posits that all men as well as those women with a HERDOO2 (Hyperpigmentation, Edema, Redness, d-dimer, Obesity, Older age, 2 or more points) score of at least 2 out of a possible 4 points need to stay on anticoagulation indefinitely because their risk of a recurrent VTE off-therapy clearly exceeds that of a bleeding event on-therapy. In contrast, women with a HERDOO2 score of 0 or 1 can safely stop anticoagulation after the standard 3-6 months of acute short-term therapy.
“Sorry, gentlemen, but we could find no low-risk group of men. They were all high risk,” he said. “But 50% of women with unprovoked vein blood clots can be spared the burdens, costs, and risks of lifelong blood thinners.”
Dr. Rodger and coinvestigators began work on developing a multivariate clinical decision rule in 2001. They examined 69 risk predictors, eventually winnowing down to a manageable four potent risk predictors identified by the acronym HERDOO2.
The derivation study was published 8 years ago (CMAJ. 2008;Aug 26;179[5]:417-26). It showed that women with a HERDOO2 score of 2 or more as well as all men had roughly a 14% rate of recurrent VTE in the first year after stopping anticoagulation, while women with a score of 0 or 1 had about a 1.6% risk. The International Society on Thrombosis and Haemostasis suggests that it’s safe to discontinue anticoagulants if the risk of recurrent thrombosis at 1 year off-therapy is less than 5%, given the significant risk of serious bleeding on-therapy and the fact that a serious bleed event is two to three times more likely than a VTE to be fatal.
Dr. Rodger and coinvestigators recognized that a clinical decision rule needs to be externally validated before it’s ready for prime-time use in clinical practice. Thus, they conducted the REVERSE II study, in which the decision rule was applied after the 2,799 participants had been on anticoagulation for 5-12 months. All had a first proximal deep vein thrombosis and/or a segmental or greater pulmonary embolism. Patients were still on anticoagulation at the time the rule was applied, which is why the cut point for a positive d-dimer test in HERDOO2 is 250 mcg/L, half of the threshold value for a positive test in patients not on anticoagulation.
They identified 631 women as low risk, with a HERDOO2 score of 0 or 1. They and their physicians were instructed to stop anticoagulation at that time. The 2,148 high-risk subjects – that is, all of the men and the high-risk women – were advised to remain on anticoagulation. The primary study endpoint was the rate of recurrent VTE in the 12 months following testing and patient guidance. The lost-to-follow-up rate was 2.2%.
The recurrent VTE rate was 3% in the 591 low-risk women who discontinued anticoagulants and zero in 31 others who elected to stay on medication. In the high-risk group identified by the HERDOO2 rule, the recurrent VTE rate at 12 months was 8.1% in the 323 who opted to discontinue anticoagulants and just 1.6% in 1,802 who continued on therapy as advised, a finding that underscores the effectiveness of selectively applied long-term anticoagulation therapy, he continued.
The recurrent VTE rate among the 291 women with a HERDOO2 score of 0 or 1 who were on exogenous estrogen was 1.4%, while in high-risk women taking estrogen the rate was more than doubled at 3.1%. But in women aged 50-64 identified by the HERDOO2 rule as being low risk, the actual recurrent VTE rate was 5.7%, a finding that raised a red flag for the investigators.
“There may be an evolution of the HERDOO2 decision rule to a lower age cut point. But that’s something that requires further study in postmenopausal women,” according to Dr. Rodger.
The investigators defined a first unprovoked VTE as one occurring in the absence during the previous 90 days of major surgery, a fracture or cast, more than 3 days of immobilization, or malignancy within the last 5 years.
Venous thromboembolism is the second most common cardiovascular disorder and the third most common cause of cardiovascular death. Unprovoked VTEs account for half of all VTEs. Their management has been a controversial subject. Both the American College of Chest Physicians and the European Society of Cardiology recommend continuing anticoagulation indefinitely in patients who aren’t at high bleeding risk.
“But this is a relatively weak 2B recommendation because of the tightly balanced competing risks of recurrent thrombosis off anticoagulation and major bleeding on anticoagulation,” Dr. Rodger said. He added that he considers REVERSE II to be practice changing, and predicted that once the results are published the guidelines will be revised.
Discussant Giancarlo Agnelli, MD, was a tough critic who gave fair warning.
“I am friends with many of the authors of this paper, and in this country we are usually gentle with enemies and nasty with friends,” declared Dr. Agnelli, professor of internal medicine and director of internal and cardiovascular medicine and the stroke unit at the University of Perugia, Italy.
He didn’t find the REVERSE II study or the HERDOO2 rule persuasive. On the plus side, he said, the HERDOO2 rule has now been validated, unlike the proposed DASH and Vienna rules. And it was tested in a diverse multinational patient population. But the fact that the HERDOO2 rule is only applicable in women is a major limitation. And REVERSE II was not a randomized trial, Dr. Agnelli noted.
Moreover, 1 year of follow-up seems insufficient, he continued. He cited a French multicenter trial in which patients with a first unprovoked VTE received 6 months of anticoagulants and were then randomized to another 18 months of anticoagulation or placebo. During that 18 months, the group on anticoagulants had a significantly lower rate of the composite endpoint comprised of recurrent VTE or major bleeding, but once that period was over they experienced catchup. By the time the study ended at 42 months, the two study arms didn’t differ significantly in the composite endpoint (JAMA. 2015 Jul 7;314[1]:31-40).
More broadly, Dr. Agnelli also questioned the need for an anticoagulation discontinuation rule in the contemporary era of new oral anticoagulants (NOACs). He was lead investigator in the AMPLIFY study, a major randomized trial of fixed-dose apixaban (Eliquis) versus conventional therapy with subcutaneous enoxaparin (Lovenox) bridging to warfarin in 5,395 patients with acute VTE. The NOAC was associated with a 69% reduction in the relative risk of bleeding and was noninferior to standard therapy in the risk of recurrent VTE (N Engl J Med. 2013 Aug 29;369[9]:799-808).
“Why should we think about withholding anticoagulation in some patients when we now have such a safe approach?” he asked.
Dr. Rodger reported receiving research grants from the French government as well as from Biomerieux, which funded the REVERSE II study. Dr. Agnelli reported having no financial conflicts.
ROME – Half of all women who experience a first unprovoked venous thromboembolism (VTE) can safely be spared lifelong anticoagulation through application of the newly validated HERDOO2 decision rule, Marc A. Rodger, MD, reported at the annual congress of the European Society of Cardiology.
“We’ve validated that a simple, memorable decision rule on anticoagulation applied at the clinically relevant time point works. And it is the only clinical decision rule that has now been prospectively validated,” said Dr. Rodger, professor of medicine, chief and chair of the division of hematology, and head of the thrombosis program at the University of Ottawa.
He presented the results of the validation study, known as the REVERSE II study, which included 2,779 patients with a first unprovoked VTE at 44 centers in seven countries. The full name of the decision rule is “Men Continue and HERDOO2,” a name that says it all: the rule posits that all men as well as those women with a HERDOO2 (Hyperpigmentation, Edema, Redness, d-dimer, Obesity, Older age, 2 or more points) score of at least 2 out of a possible 4 points need to stay on anticoagulation indefinitely because their risk of a recurrent VTE off-therapy clearly exceeds that of a bleeding event on-therapy. In contrast, women with a HERDOO2 score of 0 or 1 can safely stop anticoagulation after the standard 3-6 months of acute short-term therapy.
“Sorry, gentlemen, but we could find no low-risk group of men. They were all high risk,” he said. “But 50% of women with unprovoked vein blood clots can be spared the burdens, costs, and risks of lifelong blood thinners.”
Dr. Rodger and coinvestigators began work on developing a multivariate clinical decision rule in 2001. They examined 69 risk predictors, eventually winnowing down to a manageable four potent risk predictors identified by the acronym HERDOO2.
The derivation study was published 8 years ago (CMAJ. 2008;Aug 26;179[5]:417-26). It showed that women with a HERDOO2 score of 2 or more as well as all men had roughly a 14% rate of recurrent VTE in the first year after stopping anticoagulation, while women with a score of 0 or 1 had about a 1.6% risk. The International Society on Thrombosis and Haemostasis suggests that it’s safe to discontinue anticoagulants if the risk of recurrent thrombosis at 1 year off-therapy is less than 5%, given the significant risk of serious bleeding on-therapy and the fact that a serious bleed event is two to three times more likely than a VTE to be fatal.
Dr. Rodger and coinvestigators recognized that a clinical decision rule needs to be externally validated before it’s ready for prime-time use in clinical practice. Thus, they conducted the REVERSE II study, in which the decision rule was applied after the 2,799 participants had been on anticoagulation for 5-12 months. All had a first proximal deep vein thrombosis and/or a segmental or greater pulmonary embolism. Patients were still on anticoagulation at the time the rule was applied, which is why the cut point for a positive d-dimer test in HERDOO2 is 250 mcg/L, half of the threshold value for a positive test in patients not on anticoagulation.
They identified 631 women as low risk, with a HERDOO2 score of 0 or 1. They and their physicians were instructed to stop anticoagulation at that time. The 2,148 high-risk subjects – that is, all of the men and the high-risk women – were advised to remain on anticoagulation. The primary study endpoint was the rate of recurrent VTE in the 12 months following testing and patient guidance. The lost-to-follow-up rate was 2.2%.
The recurrent VTE rate was 3% in the 591 low-risk women who discontinued anticoagulants and zero in 31 others who elected to stay on medication. In the high-risk group identified by the HERDOO2 rule, the recurrent VTE rate at 12 months was 8.1% in the 323 who opted to discontinue anticoagulants and just 1.6% in 1,802 who continued on therapy as advised, a finding that underscores the effectiveness of selectively applied long-term anticoagulation therapy, he continued.
The recurrent VTE rate among the 291 women with a HERDOO2 score of 0 or 1 who were on exogenous estrogen was 1.4%, while in high-risk women taking estrogen the rate was more than doubled at 3.1%. But in women aged 50-64 identified by the HERDOO2 rule as being low risk, the actual recurrent VTE rate was 5.7%, a finding that raised a red flag for the investigators.
“There may be an evolution of the HERDOO2 decision rule to a lower age cut point. But that’s something that requires further study in postmenopausal women,” according to Dr. Rodger.
The investigators defined a first unprovoked VTE as one occurring in the absence during the previous 90 days of major surgery, a fracture or cast, more than 3 days of immobilization, or malignancy within the last 5 years.
Venous thromboembolism is the second most common cardiovascular disorder and the third most common cause of cardiovascular death. Unprovoked VTEs account for half of all VTEs. Their management has been a controversial subject. Both the American College of Chest Physicians and the European Society of Cardiology recommend continuing anticoagulation indefinitely in patients who aren’t at high bleeding risk.
“But this is a relatively weak 2B recommendation because of the tightly balanced competing risks of recurrent thrombosis off anticoagulation and major bleeding on anticoagulation,” Dr. Rodger said. He added that he considers REVERSE II to be practice changing, and predicted that once the results are published the guidelines will be revised.
Discussant Giancarlo Agnelli, MD, was a tough critic who gave fair warning.
“I am friends with many of the authors of this paper, and in this country we are usually gentle with enemies and nasty with friends,” declared Dr. Agnelli, professor of internal medicine and director of internal and cardiovascular medicine and the stroke unit at the University of Perugia, Italy.
He didn’t find the REVERSE II study or the HERDOO2 rule persuasive. On the plus side, he said, the HERDOO2 rule has now been validated, unlike the proposed DASH and Vienna rules. And it was tested in a diverse multinational patient population. But the fact that the HERDOO2 rule is only applicable in women is a major limitation. And REVERSE II was not a randomized trial, Dr. Agnelli noted.
Moreover, 1 year of follow-up seems insufficient, he continued. He cited a French multicenter trial in which patients with a first unprovoked VTE received 6 months of anticoagulants and were then randomized to another 18 months of anticoagulation or placebo. During that 18 months, the group on anticoagulants had a significantly lower rate of the composite endpoint comprised of recurrent VTE or major bleeding, but once that period was over they experienced catchup. By the time the study ended at 42 months, the two study arms didn’t differ significantly in the composite endpoint (JAMA. 2015 Jul 7;314[1]:31-40).
More broadly, Dr. Agnelli also questioned the need for an anticoagulation discontinuation rule in the contemporary era of new oral anticoagulants (NOACs). He was lead investigator in the AMPLIFY study, a major randomized trial of fixed-dose apixaban (Eliquis) versus conventional therapy with subcutaneous enoxaparin (Lovenox) bridging to warfarin in 5,395 patients with acute VTE. The NOAC was associated with a 69% reduction in the relative risk of bleeding and was noninferior to standard therapy in the risk of recurrent VTE (N Engl J Med. 2013 Aug 29;369[9]:799-808).
“Why should we think about withholding anticoagulation in some patients when we now have such a safe approach?” he asked.
Dr. Rodger reported receiving research grants from the French government as well as from Biomerieux, which funded the REVERSE II study. Dr. Agnelli reported having no financial conflicts.
AT THE ESC CONGRESS 2016
Key clinical point: Half of women who have a first unprovoked venous thromboembolism can safely be spared lifelong anticoagulation through application of the newly validated HERDOO2 decision rule.
Major finding: Women with a first unprovoked venous thromboembolism identified as being at low risk of recurrence on the basis of the HERDOO2 decision rule had a 3% recurrence rate in the year after stopping anticoagulation therapy, while those identified as high risk had an 8.1% recurrence rate if they discontinued anticoagulants.
Data source: This was a prospective, multinational, observational study involving 2,779 patients with a first unprovoked venous thromboembolism.
Disclosures: The presenter reported receiving research grants from the French government as well as from Biomerieux, which funded the REVERSE II study.
Simtuzumab did not help IPF patients
LONDON – Despite very promising activity in animal models of idiopathic pulmonary fibrosis (IPF), a monoclonal antibody targeted at an enzyme considered to be important to collagen cross-linking did not produce any improvement in progression-free survival (PFS), according to results of a multicenter study presented at the annual congress of the European Respiratory Society.
“This was such a negative study, there is no point in doing another,” reported Ganesh Raghu, MD, director of the Pulmonary Fibrosis Program at the University of Washington Medical Center, Seattle.
The focus of this study was simtuzumab, a monoclonal antibody targeted at lysyl oxidase like 2 (LOXL2), an enzyme which catalyzes a step in the formation of collagen crosslinks, which are thought to be important in fibrosis formation. Simtuzumab has been entered into clinical trials for treatment of several forms of fibrosis, including fibrosis in the liver.
“In animal models, simtuzumab has demonstrated efficacy in reducing fibrosis when administered prior to fibrosis formation or after the process has already begun,” Dr. Raghu explained. He said a large trial was initiated in IPF because the agent seemed so promising and because a large study was thought to be the best strategy to arrive at a definitive answer regarding safety and efficacy.
The drug was found safe but not effective. The independent data monitoring and safety committee terminated the trial early for futility.
In the study, 544 IPF patients were randomized to 125 mg simtuzumab or placebo administered subcutaneously once weekly. The primary endpoint was PFS, but there were a large number of secondary endpoints including hospitalization for progressive disease, change in 6-minute walk distance (6MWD), and overall survival.
For the endpoint of PFS, “there was absolutely no difference” between the groups receiving simtuzumab or placebo. When the patients were stratified for demonstrating above or below median expression of LOXL2, which was a prespecified analysis for the trial, there was still no difference between groups. Even when those in the top quarter percentile of LOXL2 expression were compared with those with less [expression of the enzyme], there was still “absolutely no difference.”
There was also no significant evidence of benefit for simtuzumab observed on key secondary endpoints, such as overall survival. When patients were stratified by baseline lung function as expressed by percentage of predicted forced expiratory volume in 1 second (FEV1), there was no signal of benefit for those with severe, moderate, or mild impairment.
One criticism of this study raised after the presentation was that patients with 26% or greater of predicted FEV1 were permitted into the study. It was suggested that such patients would be expected to already have a high degree of fibrosis and therefore would be less likely to benefit from an antifibrosis therapy. Dr. Raghu acknowledged this criticism, but he said it was important to include patients with advanced disease in order to generate an adequate event rate. Even with inclusion of patients with severe lung impairment, the mortality rate was less than 10%.
He concluded that there was no signal of benefit even among those with the greatest expression of the target.
“We absolutely need better markers for IPF,” Dr. Raghu maintained. While other members of the LOXL family of enzymes may still prove to be valuable markers of IPF risk and targets of therapy, these data appear to rule out a therapeutic role for blocking LOXL2.
Dr. Raghu is a consultant for Boehringer Ingelheim, Biogen, FibroGen, Gilead, Janssen, MedImmune, Promedior, Sanofi-Aventis, and Veracyte.
LONDON – Despite very promising activity in animal models of idiopathic pulmonary fibrosis (IPF), a monoclonal antibody targeted at an enzyme considered to be important to collagen cross-linking did not produce any improvement in progression-free survival (PFS), according to results of a multicenter study presented at the annual congress of the European Respiratory Society.
“This was such a negative study, there is no point in doing another,” reported Ganesh Raghu, MD, director of the Pulmonary Fibrosis Program at the University of Washington Medical Center, Seattle.
The focus of this study was simtuzumab, a monoclonal antibody targeted at lysyl oxidase like 2 (LOXL2), an enzyme which catalyzes a step in the formation of collagen crosslinks, which are thought to be important in fibrosis formation. Simtuzumab has been entered into clinical trials for treatment of several forms of fibrosis, including fibrosis in the liver.
“In animal models, simtuzumab has demonstrated efficacy in reducing fibrosis when administered prior to fibrosis formation or after the process has already begun,” Dr. Raghu explained. He said a large trial was initiated in IPF because the agent seemed so promising and because a large study was thought to be the best strategy to arrive at a definitive answer regarding safety and efficacy.
The drug was found safe but not effective. The independent data monitoring and safety committee terminated the trial early for futility.
In the study, 544 IPF patients were randomized to 125 mg simtuzumab or placebo administered subcutaneously once weekly. The primary endpoint was PFS, but there were a large number of secondary endpoints including hospitalization for progressive disease, change in 6-minute walk distance (6MWD), and overall survival.
For the endpoint of PFS, “there was absolutely no difference” between the groups receiving simtuzumab or placebo. When the patients were stratified for demonstrating above or below median expression of LOXL2, which was a prespecified analysis for the trial, there was still no difference between groups. Even when those in the top quarter percentile of LOXL2 expression were compared with those with less [expression of the enzyme], there was still “absolutely no difference.”
There was also no significant evidence of benefit for simtuzumab observed on key secondary endpoints, such as overall survival. When patients were stratified by baseline lung function as expressed by percentage of predicted forced expiratory volume in 1 second (FEV1), there was no signal of benefit for those with severe, moderate, or mild impairment.
One criticism of this study raised after the presentation was that patients with 26% or greater of predicted FEV1 were permitted into the study. It was suggested that such patients would be expected to already have a high degree of fibrosis and therefore would be less likely to benefit from an antifibrosis therapy. Dr. Raghu acknowledged this criticism, but he said it was important to include patients with advanced disease in order to generate an adequate event rate. Even with inclusion of patients with severe lung impairment, the mortality rate was less than 10%.
He concluded that there was no signal of benefit even among those with the greatest expression of the target.
“We absolutely need better markers for IPF,” Dr. Raghu maintained. While other members of the LOXL family of enzymes may still prove to be valuable markers of IPF risk and targets of therapy, these data appear to rule out a therapeutic role for blocking LOXL2.
Dr. Raghu is a consultant for Boehringer Ingelheim, Biogen, FibroGen, Gilead, Janssen, MedImmune, Promedior, Sanofi-Aventis, and Veracyte.
LONDON – Despite very promising activity in animal models of idiopathic pulmonary fibrosis (IPF), a monoclonal antibody targeted at an enzyme considered to be important to collagen cross-linking did not produce any improvement in progression-free survival (PFS), according to results of a multicenter study presented at the annual congress of the European Respiratory Society.
“This was such a negative study, there is no point in doing another,” reported Ganesh Raghu, MD, director of the Pulmonary Fibrosis Program at the University of Washington Medical Center, Seattle.
The focus of this study was simtuzumab, a monoclonal antibody targeted at lysyl oxidase like 2 (LOXL2), an enzyme which catalyzes a step in the formation of collagen crosslinks, which are thought to be important in fibrosis formation. Simtuzumab has been entered into clinical trials for treatment of several forms of fibrosis, including fibrosis in the liver.
“In animal models, simtuzumab has demonstrated efficacy in reducing fibrosis when administered prior to fibrosis formation or after the process has already begun,” Dr. Raghu explained. He said a large trial was initiated in IPF because the agent seemed so promising and because a large study was thought to be the best strategy to arrive at a definitive answer regarding safety and efficacy.
The drug was found safe but not effective. The independent data monitoring and safety committee terminated the trial early for futility.
In the study, 544 IPF patients were randomized to 125 mg simtuzumab or placebo administered subcutaneously once weekly. The primary endpoint was PFS, but there were a large number of secondary endpoints including hospitalization for progressive disease, change in 6-minute walk distance (6MWD), and overall survival.
For the endpoint of PFS, “there was absolutely no difference” between the groups receiving simtuzumab or placebo. When the patients were stratified for demonstrating above or below median expression of LOXL2, which was a prespecified analysis for the trial, there was still no difference between groups. Even when those in the top quarter percentile of LOXL2 expression were compared with those with less [expression of the enzyme], there was still “absolutely no difference.”
There was also no significant evidence of benefit for simtuzumab observed on key secondary endpoints, such as overall survival. When patients were stratified by baseline lung function as expressed by percentage of predicted forced expiratory volume in 1 second (FEV1), there was no signal of benefit for those with severe, moderate, or mild impairment.
One criticism of this study raised after the presentation was that patients with 26% or greater of predicted FEV1 were permitted into the study. It was suggested that such patients would be expected to already have a high degree of fibrosis and therefore would be less likely to benefit from an antifibrosis therapy. Dr. Raghu acknowledged this criticism, but he said it was important to include patients with advanced disease in order to generate an adequate event rate. Even with inclusion of patients with severe lung impairment, the mortality rate was less than 10%.
He concluded that there was no signal of benefit even among those with the greatest expression of the target.
“We absolutely need better markers for IPF,” Dr. Raghu maintained. While other members of the LOXL family of enzymes may still prove to be valuable markers of IPF risk and targets of therapy, these data appear to rule out a therapeutic role for blocking LOXL2.
Dr. Raghu is a consultant for Boehringer Ingelheim, Biogen, FibroGen, Gilead, Janssen, MedImmune, Promedior, Sanofi-Aventis, and Veracyte.
AT THE ERS CONGRESS 2016
Key clinical point: A large multicenter trial with simtuzumab in idiopathic pulmonary fibrosis failed to generate a hint of benefit.
Major finding: In this study, efficacy was not seen even in those with high expression of the simtuzumab target, lysyl oxidase like 2 (LOXL2).
Data source: Phase II multicenter, placebo-controlled trial.
Disclosures: Dr. Raghu is a consultant for Boehringer Ingelheim, Biogen, FibroGen, Gilead, Janssen, MedImmune, Promedior, Sanofi-Aventis, and Veracyte.