User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Rest dyspnea dims as acute heart failure treatment target
PARIS – During the most recent pharmaceutical generation, drug development for heart failure largely focused on acute heart failure, and specifically on patients with rest dyspnea as the primary manifestation of their acute heart failure decompensation events.
That has now changed, agreed heart failure experts as they debated the upshot of sobering results from two neutral trials that failed to show a midterm mortality benefit in patients hospitalized for acute heart failure who underwent aggressive management of their congestion using 2 days of intravenous treatment with either of two potent vasodilating drugs. Results first reported in November 2016 failed to show a survival benefit from ularitide in the 2,100-patient TRUE-AHF (Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure) trial (N Engl J Med. 2017 May 18;376[20]:1956-64). And results reported at a meeting of the Heart Failure Association of the ESC failed to show a survival benefit from serelaxin in more than 6,500 acute heart failure patients in the RELAX-AHF-2 (Efficacy, Safety and Tolerability of Serelaxin When Added to Standard Therapy in AHF) trial.
The failure of a 2-day infusion of serelaxin to produce a significant reduction in cardiovascular death in RELAX-AHF-2 was especially surprising because the predecessor trial, RELAX-AHF, which randomized only 1,160 patients and used a surrogate endpoint of dyspnea improvement, had shown significant benefit that hinted more clinically meaningful benefits might also result from serelaxin treatment (Lancet. 2013 Jan 5;381[9860]:29-39). The disappointing serelaxin and ularitide results also culminate a series of studies using several different agents or procedures to treat acute decompensated heart failure patients that all failed to produce a reduction in deaths.
“This is a sea change; make no mistake. We will need a more targeted, selective approach. It was always a daunting proposition to believe that short-term infusion could have an effect 6 months later. We were misled by the analogy [of acute heart failure] to acute coronary syndrome,” said Dr. Ruschitzka, professor of medicine at the University of Zürich.
The right time to intervene
Meeting attendees offered several hypotheses to explain why the acute ularitide and serelaxin trials both failed to show a mortality benefit, with timing of treatment the most common denominator.
Acute heart failure “is an event, not a disease,” declared Milton Packer, MD, lead investigator of TRUE-AHF, during a session devoted to vasodilator treatment of acute heart failure. Acute heart failure decompensations “are fluctuations in a chronic disease. It doesn’t matter what you do during the episode – it matters what you do between acute episodes. We focus all our attention on which vasodilator and which dose of Lasix [furosemide], but we send patients home on inadequate chronic therapy. It doesn’t matter what you do to the dyspnea, the shortness of breath will get better. Do we need a new drug that makes dyspnea go away an hour sooner and doesn’t cost a fortune? What really matters is what patients do between acute episodes and how to prevent them, ” said Dr. Packer, distinguished scholar in cardiovascular science at Baylor University Medical Center in Dallas.
Dr. Packer strongly urged clinicians to put heart failure patients on the full regimen of guideline-directed drugs and at full dosages, a step he thinks would go a long way toward preventing a majority of decompensation episodes. “Chronic heart failure treatment has improved dramatically, but implementation is abysmal,” he said.
Of course, at this phase of their disease heart failure patients are usually at home, which more or less demands that the treatments they take are oral or at least delivered by subcutaneous injection.
“We’ve had a mismatch of candidate drugs, which have mostly been IV infusions, with a clinical setting where an IV infusion is challenging to use.”
“We are killing good drugs by the way we’re testing them,” commented Javed Butler, MD, who bemoaned the ignominious outcome of serelaxin treatment in RELAX-AHF-2. “The available data show it makes no sense to treat for just 2 days. We should take true worsening heart failure patients, those who are truly failing standard treatment, and look at new chronic oral therapies to try on them.” Oral drugs similar to serelaxin and ularitide could be used chronically, suggested Dr. Butler, professor of medicine and chief of cardiology at Stony Brook (N.Y.) School of Medicine.
Wrong patients with the wrong presentation
Perhaps just as big a flaw of the acute heart failure trials has been their target patient population, patients with rest dyspnea at the time of admission. “Why do we think that dyspnea is a clinically relevant symptom for acute heart failure?” Dr. Packer asked.
Dr. Cleland and his associates analyzed data on 116,752 hospitalizations for acute heart failure in England and Wales during April 2007–March 2013, a database that included more than 90% of hospitals for these regions. “We found that a large proportion of admitted patients did not have breathlessness at rest as their primary reason for seeking hospitalization. For about half the patients, moderate or severe peripheral edema was the main problem,” he reported. Roughly a third of patients had rest dyspnea as their main symptom.
An unadjusted analysis also showed a stronger link between peripheral edema and the rate of mortality during a median follow-up of about a year following hospitalization, compared with rest dyspnea. Compared with the lowest-risk subgroup, the patients with severe peripheral edema (18% of the population) had more than twice the mortality. In contrast, the patients with the most severe rest dyspnea and no evidence at all of peripheral edema, just 6% of the population, had a 50% higher mortality rate than the lowest-risk patients.
”It’s peripheral edema rather than breathlessness that is the important determinant of length of stay and prognosis. The disastrous neutral trials for acute heart failure have all targeted the breathless subset of patients. Maybe a reason for the failures has been that they’ve been treating a problem that does not exist. The trials have looked at the wrong patients,” Dr. Cleland said.
‘We’ve told the wrong story to industry” about the importance of rest dyspnea to acute heart failure patients. “When we say acute heart failure, we mean an ambulance and oxygen and the emergency department and rapid IV treatment. That’s breathlessness. Patients with peripheral edema usually get driven in and walk from the car to a wheelchair and they wait 4 hours to be seen. I think that, following the TRUE-AHF and RELAX-AHF-2 results, we’ll see a radical change.”
But just because the focus should be on peripheral edema rather than dyspnea, that doesn’t mean better drugs aren’t needed, Dr. Cleland added.
“We need better treatments to deal with congestion. Once a patient is congested, we are not very good at getting rid of it. We depend on diuretics, which we don’t use properly. Ultimately I’d like to see agents as adjuncts to diuretics, to produce better kidney function.” But treatments for breathlessness are decent as they now exist: furosemide plus oxygen. When a simple, cheap drug works 80% of the time, it is really hard to improve on that.” The real unmet needs for treating acute decompensated heart failure are patients with rest dyspnea who don’t respond to conventional treatment, and especially patients with gross peripheral edema plus low blood pressure and renal dysfunction for whom no good treatments have been developed, Dr. Cleland said.
Another flaw in the patient selection criteria for the acute heart failure studies has been the focus on patients with elevated blood pressures, the logical target for vasodilator drugs that lower blood pressure, noted Dr. Felker. “But these are the patients at lowest risk. We’ve had a mismatch between the patients with the biggest need and the patients we actually study, which relates to the types of drugs we’ve developed.”
Acute heart failure remains a target
Despite all the talk of refocusing attention on chronic heart failure and peripheral edema, at least one expert remained steadfast in talking up the importance of new acute interventions.
Part of the problem in believing that existing treatments can adequately manage heart failure and prevent acute decompensations is that about 75% of acute heart failure episodes either occur as the first presentation of heart failure, or occur in patients with heart failure with preserved ejection fraction (HFpEF), a type of heart failure that, until recently, had no chronic drug regimens with widely acknowledged efficacy for HFpEF. (The 2017 U.S. heart failure management guidelines update listed aldosterone receptor antagonists as a class IIb recommendation for treating HFpEF, the first time guidelines have sanctioned a drug class for treating HFpEF [Circulation. 2017 Apr 28. doi: 10.1161/CIR.0000000000000509].)
“For patients with heart failure with reduced ejection fraction, you can give optimal treatments and [decompensations] are prevented,” noted Dr. Mebazaa, a professor of anesthesiology and resuscitation at Lariboisière Hospital in Paris. “But for the huge number of HFpEF patients we have nothing. Acute heart failure will remain prevalent, and we still don’t have the right drugs to use on these patients.”
The TRUE-AHF trial was sponsored by Cardiorentis. RELAX-AHF-2 was sponsored by Novartis. Dr. Ruschitzka has been a speaker on behalf of Novartis, and has been a speaker for or consultant to several companies and was a coinvestigator for TRUE-AHF and received fees from Cardiorentis for his participation. Dr. Packer is a consultant to and stockholder in Cardiorentis and has been a consultant to several other companies. Dr. Felker has been a consultant to Novartis and several other companies and was a coinvestigator on RELAX-AHF-2. Dr. Butler has been a consultant to several companies. Dr. Cleland has received research support from Novartis, and he has been a consultant to and received research support from several other companies. Dr. Mebazaa has received honoraria from Novartis and Cardiorentis as well as from several other companies and was a coinvestigator on TRUE-AHF.
[email protected]
On Twitter @mitchelzoler
PARIS – During the most recent pharmaceutical generation, drug development for heart failure largely focused on acute heart failure, and specifically on patients with rest dyspnea as the primary manifestation of their acute heart failure decompensation events.
That has now changed, agreed heart failure experts as they debated the upshot of sobering results from two neutral trials that failed to show a midterm mortality benefit in patients hospitalized for acute heart failure who underwent aggressive management of their congestion using 2 days of intravenous treatment with either of two potent vasodilating drugs. Results first reported in November 2016 failed to show a survival benefit from ularitide in the 2,100-patient TRUE-AHF (Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure) trial (N Engl J Med. 2017 May 18;376[20]:1956-64). And results reported at a meeting of the Heart Failure Association of the ESC failed to show a survival benefit from serelaxin in more than 6,500 acute heart failure patients in the RELAX-AHF-2 (Efficacy, Safety and Tolerability of Serelaxin When Added to Standard Therapy in AHF) trial.
The failure of a 2-day infusion of serelaxin to produce a significant reduction in cardiovascular death in RELAX-AHF-2 was especially surprising because the predecessor trial, RELAX-AHF, which randomized only 1,160 patients and used a surrogate endpoint of dyspnea improvement, had shown significant benefit that hinted more clinically meaningful benefits might also result from serelaxin treatment (Lancet. 2013 Jan 5;381[9860]:29-39). The disappointing serelaxin and ularitide results also culminate a series of studies using several different agents or procedures to treat acute decompensated heart failure patients that all failed to produce a reduction in deaths.
“This is a sea change; make no mistake. We will need a more targeted, selective approach. It was always a daunting proposition to believe that short-term infusion could have an effect 6 months later. We were misled by the analogy [of acute heart failure] to acute coronary syndrome,” said Dr. Ruschitzka, professor of medicine at the University of Zürich.
The right time to intervene
Meeting attendees offered several hypotheses to explain why the acute ularitide and serelaxin trials both failed to show a mortality benefit, with timing of treatment the most common denominator.
Acute heart failure “is an event, not a disease,” declared Milton Packer, MD, lead investigator of TRUE-AHF, during a session devoted to vasodilator treatment of acute heart failure. Acute heart failure decompensations “are fluctuations in a chronic disease. It doesn’t matter what you do during the episode – it matters what you do between acute episodes. We focus all our attention on which vasodilator and which dose of Lasix [furosemide], but we send patients home on inadequate chronic therapy. It doesn’t matter what you do to the dyspnea, the shortness of breath will get better. Do we need a new drug that makes dyspnea go away an hour sooner and doesn’t cost a fortune? What really matters is what patients do between acute episodes and how to prevent them, ” said Dr. Packer, distinguished scholar in cardiovascular science at Baylor University Medical Center in Dallas.
Dr. Packer strongly urged clinicians to put heart failure patients on the full regimen of guideline-directed drugs and at full dosages, a step he thinks would go a long way toward preventing a majority of decompensation episodes. “Chronic heart failure treatment has improved dramatically, but implementation is abysmal,” he said.
Of course, at this phase of their disease heart failure patients are usually at home, which more or less demands that the treatments they take are oral or at least delivered by subcutaneous injection.
“We’ve had a mismatch of candidate drugs, which have mostly been IV infusions, with a clinical setting where an IV infusion is challenging to use.”
“We are killing good drugs by the way we’re testing them,” commented Javed Butler, MD, who bemoaned the ignominious outcome of serelaxin treatment in RELAX-AHF-2. “The available data show it makes no sense to treat for just 2 days. We should take true worsening heart failure patients, those who are truly failing standard treatment, and look at new chronic oral therapies to try on them.” Oral drugs similar to serelaxin and ularitide could be used chronically, suggested Dr. Butler, professor of medicine and chief of cardiology at Stony Brook (N.Y.) School of Medicine.
Wrong patients with the wrong presentation
Perhaps just as big a flaw of the acute heart failure trials has been their target patient population, patients with rest dyspnea at the time of admission. “Why do we think that dyspnea is a clinically relevant symptom for acute heart failure?” Dr. Packer asked.
Dr. Cleland and his associates analyzed data on 116,752 hospitalizations for acute heart failure in England and Wales during April 2007–March 2013, a database that included more than 90% of hospitals for these regions. “We found that a large proportion of admitted patients did not have breathlessness at rest as their primary reason for seeking hospitalization. For about half the patients, moderate or severe peripheral edema was the main problem,” he reported. Roughly a third of patients had rest dyspnea as their main symptom.
An unadjusted analysis also showed a stronger link between peripheral edema and the rate of mortality during a median follow-up of about a year following hospitalization, compared with rest dyspnea. Compared with the lowest-risk subgroup, the patients with severe peripheral edema (18% of the population) had more than twice the mortality. In contrast, the patients with the most severe rest dyspnea and no evidence at all of peripheral edema, just 6% of the population, had a 50% higher mortality rate than the lowest-risk patients.
”It’s peripheral edema rather than breathlessness that is the important determinant of length of stay and prognosis. The disastrous neutral trials for acute heart failure have all targeted the breathless subset of patients. Maybe a reason for the failures has been that they’ve been treating a problem that does not exist. The trials have looked at the wrong patients,” Dr. Cleland said.
‘We’ve told the wrong story to industry” about the importance of rest dyspnea to acute heart failure patients. “When we say acute heart failure, we mean an ambulance and oxygen and the emergency department and rapid IV treatment. That’s breathlessness. Patients with peripheral edema usually get driven in and walk from the car to a wheelchair and they wait 4 hours to be seen. I think that, following the TRUE-AHF and RELAX-AHF-2 results, we’ll see a radical change.”
But just because the focus should be on peripheral edema rather than dyspnea, that doesn’t mean better drugs aren’t needed, Dr. Cleland added.
“We need better treatments to deal with congestion. Once a patient is congested, we are not very good at getting rid of it. We depend on diuretics, which we don’t use properly. Ultimately I’d like to see agents as adjuncts to diuretics, to produce better kidney function.” But treatments for breathlessness are decent as they now exist: furosemide plus oxygen. When a simple, cheap drug works 80% of the time, it is really hard to improve on that.” The real unmet needs for treating acute decompensated heart failure are patients with rest dyspnea who don’t respond to conventional treatment, and especially patients with gross peripheral edema plus low blood pressure and renal dysfunction for whom no good treatments have been developed, Dr. Cleland said.
Another flaw in the patient selection criteria for the acute heart failure studies has been the focus on patients with elevated blood pressures, the logical target for vasodilator drugs that lower blood pressure, noted Dr. Felker. “But these are the patients at lowest risk. We’ve had a mismatch between the patients with the biggest need and the patients we actually study, which relates to the types of drugs we’ve developed.”
Acute heart failure remains a target
Despite all the talk of refocusing attention on chronic heart failure and peripheral edema, at least one expert remained steadfast in talking up the importance of new acute interventions.
Part of the problem in believing that existing treatments can adequately manage heart failure and prevent acute decompensations is that about 75% of acute heart failure episodes either occur as the first presentation of heart failure, or occur in patients with heart failure with preserved ejection fraction (HFpEF), a type of heart failure that, until recently, had no chronic drug regimens with widely acknowledged efficacy for HFpEF. (The 2017 U.S. heart failure management guidelines update listed aldosterone receptor antagonists as a class IIb recommendation for treating HFpEF, the first time guidelines have sanctioned a drug class for treating HFpEF [Circulation. 2017 Apr 28. doi: 10.1161/CIR.0000000000000509].)
“For patients with heart failure with reduced ejection fraction, you can give optimal treatments and [decompensations] are prevented,” noted Dr. Mebazaa, a professor of anesthesiology and resuscitation at Lariboisière Hospital in Paris. “But for the huge number of HFpEF patients we have nothing. Acute heart failure will remain prevalent, and we still don’t have the right drugs to use on these patients.”
The TRUE-AHF trial was sponsored by Cardiorentis. RELAX-AHF-2 was sponsored by Novartis. Dr. Ruschitzka has been a speaker on behalf of Novartis, and has been a speaker for or consultant to several companies and was a coinvestigator for TRUE-AHF and received fees from Cardiorentis for his participation. Dr. Packer is a consultant to and stockholder in Cardiorentis and has been a consultant to several other companies. Dr. Felker has been a consultant to Novartis and several other companies and was a coinvestigator on RELAX-AHF-2. Dr. Butler has been a consultant to several companies. Dr. Cleland has received research support from Novartis, and he has been a consultant to and received research support from several other companies. Dr. Mebazaa has received honoraria from Novartis and Cardiorentis as well as from several other companies and was a coinvestigator on TRUE-AHF.
[email protected]
On Twitter @mitchelzoler
PARIS – During the most recent pharmaceutical generation, drug development for heart failure largely focused on acute heart failure, and specifically on patients with rest dyspnea as the primary manifestation of their acute heart failure decompensation events.
That has now changed, agreed heart failure experts as they debated the upshot of sobering results from two neutral trials that failed to show a midterm mortality benefit in patients hospitalized for acute heart failure who underwent aggressive management of their congestion using 2 days of intravenous treatment with either of two potent vasodilating drugs. Results first reported in November 2016 failed to show a survival benefit from ularitide in the 2,100-patient TRUE-AHF (Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure) trial (N Engl J Med. 2017 May 18;376[20]:1956-64). And results reported at a meeting of the Heart Failure Association of the ESC failed to show a survival benefit from serelaxin in more than 6,500 acute heart failure patients in the RELAX-AHF-2 (Efficacy, Safety and Tolerability of Serelaxin When Added to Standard Therapy in AHF) trial.
The failure of a 2-day infusion of serelaxin to produce a significant reduction in cardiovascular death in RELAX-AHF-2 was especially surprising because the predecessor trial, RELAX-AHF, which randomized only 1,160 patients and used a surrogate endpoint of dyspnea improvement, had shown significant benefit that hinted more clinically meaningful benefits might also result from serelaxin treatment (Lancet. 2013 Jan 5;381[9860]:29-39). The disappointing serelaxin and ularitide results also culminate a series of studies using several different agents or procedures to treat acute decompensated heart failure patients that all failed to produce a reduction in deaths.
“This is a sea change; make no mistake. We will need a more targeted, selective approach. It was always a daunting proposition to believe that short-term infusion could have an effect 6 months later. We were misled by the analogy [of acute heart failure] to acute coronary syndrome,” said Dr. Ruschitzka, professor of medicine at the University of Zürich.
The right time to intervene
Meeting attendees offered several hypotheses to explain why the acute ularitide and serelaxin trials both failed to show a mortality benefit, with timing of treatment the most common denominator.
Acute heart failure “is an event, not a disease,” declared Milton Packer, MD, lead investigator of TRUE-AHF, during a session devoted to vasodilator treatment of acute heart failure. Acute heart failure decompensations “are fluctuations in a chronic disease. It doesn’t matter what you do during the episode – it matters what you do between acute episodes. We focus all our attention on which vasodilator and which dose of Lasix [furosemide], but we send patients home on inadequate chronic therapy. It doesn’t matter what you do to the dyspnea, the shortness of breath will get better. Do we need a new drug that makes dyspnea go away an hour sooner and doesn’t cost a fortune? What really matters is what patients do between acute episodes and how to prevent them, ” said Dr. Packer, distinguished scholar in cardiovascular science at Baylor University Medical Center in Dallas.
Dr. Packer strongly urged clinicians to put heart failure patients on the full regimen of guideline-directed drugs and at full dosages, a step he thinks would go a long way toward preventing a majority of decompensation episodes. “Chronic heart failure treatment has improved dramatically, but implementation is abysmal,” he said.
Of course, at this phase of their disease heart failure patients are usually at home, which more or less demands that the treatments they take are oral or at least delivered by subcutaneous injection.
“We’ve had a mismatch of candidate drugs, which have mostly been IV infusions, with a clinical setting where an IV infusion is challenging to use.”
“We are killing good drugs by the way we’re testing them,” commented Javed Butler, MD, who bemoaned the ignominious outcome of serelaxin treatment in RELAX-AHF-2. “The available data show it makes no sense to treat for just 2 days. We should take true worsening heart failure patients, those who are truly failing standard treatment, and look at new chronic oral therapies to try on them.” Oral drugs similar to serelaxin and ularitide could be used chronically, suggested Dr. Butler, professor of medicine and chief of cardiology at Stony Brook (N.Y.) School of Medicine.
Wrong patients with the wrong presentation
Perhaps just as big a flaw of the acute heart failure trials has been their target patient population, patients with rest dyspnea at the time of admission. “Why do we think that dyspnea is a clinically relevant symptom for acute heart failure?” Dr. Packer asked.
Dr. Cleland and his associates analyzed data on 116,752 hospitalizations for acute heart failure in England and Wales during April 2007–March 2013, a database that included more than 90% of hospitals for these regions. “We found that a large proportion of admitted patients did not have breathlessness at rest as their primary reason for seeking hospitalization. For about half the patients, moderate or severe peripheral edema was the main problem,” he reported. Roughly a third of patients had rest dyspnea as their main symptom.
An unadjusted analysis also showed a stronger link between peripheral edema and the rate of mortality during a median follow-up of about a year following hospitalization, compared with rest dyspnea. Compared with the lowest-risk subgroup, the patients with severe peripheral edema (18% of the population) had more than twice the mortality. In contrast, the patients with the most severe rest dyspnea and no evidence at all of peripheral edema, just 6% of the population, had a 50% higher mortality rate than the lowest-risk patients.
”It’s peripheral edema rather than breathlessness that is the important determinant of length of stay and prognosis. The disastrous neutral trials for acute heart failure have all targeted the breathless subset of patients. Maybe a reason for the failures has been that they’ve been treating a problem that does not exist. The trials have looked at the wrong patients,” Dr. Cleland said.
‘We’ve told the wrong story to industry” about the importance of rest dyspnea to acute heart failure patients. “When we say acute heart failure, we mean an ambulance and oxygen and the emergency department and rapid IV treatment. That’s breathlessness. Patients with peripheral edema usually get driven in and walk from the car to a wheelchair and they wait 4 hours to be seen. I think that, following the TRUE-AHF and RELAX-AHF-2 results, we’ll see a radical change.”
But just because the focus should be on peripheral edema rather than dyspnea, that doesn’t mean better drugs aren’t needed, Dr. Cleland added.
“We need better treatments to deal with congestion. Once a patient is congested, we are not very good at getting rid of it. We depend on diuretics, which we don’t use properly. Ultimately I’d like to see agents as adjuncts to diuretics, to produce better kidney function.” But treatments for breathlessness are decent as they now exist: furosemide plus oxygen. When a simple, cheap drug works 80% of the time, it is really hard to improve on that.” The real unmet needs for treating acute decompensated heart failure are patients with rest dyspnea who don’t respond to conventional treatment, and especially patients with gross peripheral edema plus low blood pressure and renal dysfunction for whom no good treatments have been developed, Dr. Cleland said.
Another flaw in the patient selection criteria for the acute heart failure studies has been the focus on patients with elevated blood pressures, the logical target for vasodilator drugs that lower blood pressure, noted Dr. Felker. “But these are the patients at lowest risk. We’ve had a mismatch between the patients with the biggest need and the patients we actually study, which relates to the types of drugs we’ve developed.”
Acute heart failure remains a target
Despite all the talk of refocusing attention on chronic heart failure and peripheral edema, at least one expert remained steadfast in talking up the importance of new acute interventions.
Part of the problem in believing that existing treatments can adequately manage heart failure and prevent acute decompensations is that about 75% of acute heart failure episodes either occur as the first presentation of heart failure, or occur in patients with heart failure with preserved ejection fraction (HFpEF), a type of heart failure that, until recently, had no chronic drug regimens with widely acknowledged efficacy for HFpEF. (The 2017 U.S. heart failure management guidelines update listed aldosterone receptor antagonists as a class IIb recommendation for treating HFpEF, the first time guidelines have sanctioned a drug class for treating HFpEF [Circulation. 2017 Apr 28. doi: 10.1161/CIR.0000000000000509].)
“For patients with heart failure with reduced ejection fraction, you can give optimal treatments and [decompensations] are prevented,” noted Dr. Mebazaa, a professor of anesthesiology and resuscitation at Lariboisière Hospital in Paris. “But for the huge number of HFpEF patients we have nothing. Acute heart failure will remain prevalent, and we still don’t have the right drugs to use on these patients.”
The TRUE-AHF trial was sponsored by Cardiorentis. RELAX-AHF-2 was sponsored by Novartis. Dr. Ruschitzka has been a speaker on behalf of Novartis, and has been a speaker for or consultant to several companies and was a coinvestigator for TRUE-AHF and received fees from Cardiorentis for his participation. Dr. Packer is a consultant to and stockholder in Cardiorentis and has been a consultant to several other companies. Dr. Felker has been a consultant to Novartis and several other companies and was a coinvestigator on RELAX-AHF-2. Dr. Butler has been a consultant to several companies. Dr. Cleland has received research support from Novartis, and he has been a consultant to and received research support from several other companies. Dr. Mebazaa has received honoraria from Novartis and Cardiorentis as well as from several other companies and was a coinvestigator on TRUE-AHF.
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM HEART FAILURE 2017
Teens’ overall tobacco use falls, but e-cigs now most popular product
Twenty percent of surveyed high school students and 7% of middle school students were using some kind of tobacco product in 2016 – and e-cigarettes were the most commonly used product among those groups, according to an analysis from federal researchers.
, although combustible tobacco product use declined in both groups, said Ahmed Jamal, MD, of the Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and his associates. That was determined using data from the 2011-2016 National Youth Tobacco Surveys, which assessed tobacco use in the past 30 days by groups of middle school and high school students numbering from more than 17,000 to almost 25,000.
High school boys had higher use of any tobacco product, two or more tobacco products, cigars, smokeless tobacco, and pipe tobacco than did high school girls. The most commonly used tobacco product among non-Hispanic white (13.7%) and Hispanic high school students (10%) were e-cigarettes; cigars were the most commonly used tobacco product among non-Hispanic black high school students (9.5%).
Seven percent of middle school students had used any tobacco product within the past 30 days, and 3% had used two or more tobacco products. Similar to high school students, e-cigarettes were the most commonly used tobacco product among middle school students (4.3%), followed by cigarettes (2%), cigars (2%), smokeless tobacco (2%), hookahs (2%), pipe tobacco (0.7%), and bidis (0.3%).
Use of any tobacco product among middle school boys was 8.3%, and among middle school girls use was 6%. Hispanic middle school students reported higher use of any tobacco product, were more likely to use two or more tobacco products, and said they used hookahs more often than did non-Hispanic white middle school students.
Looking at the changes from 2011 to 2016, current use of any tobacco product did not change significantly among high school students (falling from 24% to 20%), but there was a reduction in current use of any combustible tobacco product (22%-14%) and in use of two or more tobacco products (12%-9.6%) over this time period.
By product type, there were increases in current use of e-cigarettes (from 1.5% to 11.3%) and hookahs (from 4.1% to 4.8%) among high school students, as well as decreases in current use of cigarettes (from 16% to 8%), cigars (from 12% to 8%), smokeless tobacco (from 8% to 6%), pipe tobacco (from 4% to 1%), and bidis (from 2% to 0.5%).
Among middle school students during 2011-2016, there was a rise in current use of e-cigarettes (from 0.6% to 4%), and in current use of hookahs (from 1% to 2%). There was a drop in current use of any combustible tobacco products (from 6% to 4%), cigarettes (from 4% to 2%), cigars (from 3.5% to 2.2%), and pipe tobacco (from 2% to 0.7%).
“Since February 2014, the Food and Drug Administration’s first national tobacco public education campaign, the Real Cost, has broadcasted tobacco education advertising designed for youths aged 12-17 years; the campaign was associated with an estimated 348,398 U.S. youths who did not initiate cigarette smoking during February 2014–March 2016,” the investigators said. “Continued implementation of these strategies can help prevent and further reduce the use of all forms of tobacco product among U.S. youths.”
Read more in MMWR (2017 Jun 16;66[23]:597-603).
Twenty percent of surveyed high school students and 7% of middle school students were using some kind of tobacco product in 2016 – and e-cigarettes were the most commonly used product among those groups, according to an analysis from federal researchers.
, although combustible tobacco product use declined in both groups, said Ahmed Jamal, MD, of the Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and his associates. That was determined using data from the 2011-2016 National Youth Tobacco Surveys, which assessed tobacco use in the past 30 days by groups of middle school and high school students numbering from more than 17,000 to almost 25,000.
High school boys had higher use of any tobacco product, two or more tobacco products, cigars, smokeless tobacco, and pipe tobacco than did high school girls. The most commonly used tobacco product among non-Hispanic white (13.7%) and Hispanic high school students (10%) were e-cigarettes; cigars were the most commonly used tobacco product among non-Hispanic black high school students (9.5%).
Seven percent of middle school students had used any tobacco product within the past 30 days, and 3% had used two or more tobacco products. Similar to high school students, e-cigarettes were the most commonly used tobacco product among middle school students (4.3%), followed by cigarettes (2%), cigars (2%), smokeless tobacco (2%), hookahs (2%), pipe tobacco (0.7%), and bidis (0.3%).
Use of any tobacco product among middle school boys was 8.3%, and among middle school girls use was 6%. Hispanic middle school students reported higher use of any tobacco product, were more likely to use two or more tobacco products, and said they used hookahs more often than did non-Hispanic white middle school students.
Looking at the changes from 2011 to 2016, current use of any tobacco product did not change significantly among high school students (falling from 24% to 20%), but there was a reduction in current use of any combustible tobacco product (22%-14%) and in use of two or more tobacco products (12%-9.6%) over this time period.
By product type, there were increases in current use of e-cigarettes (from 1.5% to 11.3%) and hookahs (from 4.1% to 4.8%) among high school students, as well as decreases in current use of cigarettes (from 16% to 8%), cigars (from 12% to 8%), smokeless tobacco (from 8% to 6%), pipe tobacco (from 4% to 1%), and bidis (from 2% to 0.5%).
Among middle school students during 2011-2016, there was a rise in current use of e-cigarettes (from 0.6% to 4%), and in current use of hookahs (from 1% to 2%). There was a drop in current use of any combustible tobacco products (from 6% to 4%), cigarettes (from 4% to 2%), cigars (from 3.5% to 2.2%), and pipe tobacco (from 2% to 0.7%).
“Since February 2014, the Food and Drug Administration’s first national tobacco public education campaign, the Real Cost, has broadcasted tobacco education advertising designed for youths aged 12-17 years; the campaign was associated with an estimated 348,398 U.S. youths who did not initiate cigarette smoking during February 2014–March 2016,” the investigators said. “Continued implementation of these strategies can help prevent and further reduce the use of all forms of tobacco product among U.S. youths.”
Read more in MMWR (2017 Jun 16;66[23]:597-603).
Twenty percent of surveyed high school students and 7% of middle school students were using some kind of tobacco product in 2016 – and e-cigarettes were the most commonly used product among those groups, according to an analysis from federal researchers.
, although combustible tobacco product use declined in both groups, said Ahmed Jamal, MD, of the Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and his associates. That was determined using data from the 2011-2016 National Youth Tobacco Surveys, which assessed tobacco use in the past 30 days by groups of middle school and high school students numbering from more than 17,000 to almost 25,000.
High school boys had higher use of any tobacco product, two or more tobacco products, cigars, smokeless tobacco, and pipe tobacco than did high school girls. The most commonly used tobacco product among non-Hispanic white (13.7%) and Hispanic high school students (10%) were e-cigarettes; cigars were the most commonly used tobacco product among non-Hispanic black high school students (9.5%).
Seven percent of middle school students had used any tobacco product within the past 30 days, and 3% had used two or more tobacco products. Similar to high school students, e-cigarettes were the most commonly used tobacco product among middle school students (4.3%), followed by cigarettes (2%), cigars (2%), smokeless tobacco (2%), hookahs (2%), pipe tobacco (0.7%), and bidis (0.3%).
Use of any tobacco product among middle school boys was 8.3%, and among middle school girls use was 6%. Hispanic middle school students reported higher use of any tobacco product, were more likely to use two or more tobacco products, and said they used hookahs more often than did non-Hispanic white middle school students.
Looking at the changes from 2011 to 2016, current use of any tobacco product did not change significantly among high school students (falling from 24% to 20%), but there was a reduction in current use of any combustible tobacco product (22%-14%) and in use of two or more tobacco products (12%-9.6%) over this time period.
By product type, there were increases in current use of e-cigarettes (from 1.5% to 11.3%) and hookahs (from 4.1% to 4.8%) among high school students, as well as decreases in current use of cigarettes (from 16% to 8%), cigars (from 12% to 8%), smokeless tobacco (from 8% to 6%), pipe tobacco (from 4% to 1%), and bidis (from 2% to 0.5%).
Among middle school students during 2011-2016, there was a rise in current use of e-cigarettes (from 0.6% to 4%), and in current use of hookahs (from 1% to 2%). There was a drop in current use of any combustible tobacco products (from 6% to 4%), cigarettes (from 4% to 2%), cigars (from 3.5% to 2.2%), and pipe tobacco (from 2% to 0.7%).
“Since February 2014, the Food and Drug Administration’s first national tobacco public education campaign, the Real Cost, has broadcasted tobacco education advertising designed for youths aged 12-17 years; the campaign was associated with an estimated 348,398 U.S. youths who did not initiate cigarette smoking during February 2014–March 2016,” the investigators said. “Continued implementation of these strategies can help prevent and further reduce the use of all forms of tobacco product among U.S. youths.”
Read more in MMWR (2017 Jun 16;66[23]:597-603).
FROM MMWR
Most arrhythmia clinic patients have undetected OSA
BOSTON – In a study of patients without a previous diagnosis of obstructive sleep apnea (OSA), 85% of participants in outpatient arrhythmia clinics had undetected OSA.
The study, which also excluded patients who had ever been treated for OSA, was presented by Colin Shapiro, MD, of the Department of Psychiatry, Toronto Western Hospital, University of Toronto, at the annual meeting of the Associated Professional Sleep Societies.
A binary logistic regression analysis showed that only age and male gender were significant predictors of OSA.
Along with a home sleep study, researchers tested 75 nonselected consecutive patients (mean age of 64 years; 72% male) from three outpatient arrhythmia clinics for symptoms indicative of OSA using the Epworth Sleepiness Scale (ESS), the Fatigue Severity Scale (FSS), the Non-Restorative Sleep Scale (NRSS), and other questionnaires.
On the ESS, 32% of patients had a score of 8 or greater, indicating higher than normal daytime sleepiness. Almost half (47%) of patients had a high level of fatigue on the FSS, and symptoms of nonrestorative sleep were detected in 15% (NRSS score greater than or equal to 46).
Dr. Shapiro noted that “high scores suggestive of daytime sleepiness, fatigue, or insomnia did not particularly predict the presence of OSA in patients with arrhythmia.” He concluded that, “with a hit rate of 85%, just about every patient with an arrhythmia should have a sleep study.”
Dr. Shapiro informed attendees at the annual meeting of the Professional Sleep Societies that he was presenting in place of his student and the abstract’s first author, Dr. Asmaa M. Abumuamar, MD, who was denied a visa to attend the meeting. Dr. Abumuamar is from the Toronto Western Research Institute, University of Toronto.
Dr. Shapiro reported that Dr. Abumuamar has no conflicts of interest. Dr. Shapiro reported that he is an investor in the company that supplied the home sleep testing apparatus.
BOSTON – In a study of patients without a previous diagnosis of obstructive sleep apnea (OSA), 85% of participants in outpatient arrhythmia clinics had undetected OSA.
The study, which also excluded patients who had ever been treated for OSA, was presented by Colin Shapiro, MD, of the Department of Psychiatry, Toronto Western Hospital, University of Toronto, at the annual meeting of the Associated Professional Sleep Societies.
A binary logistic regression analysis showed that only age and male gender were significant predictors of OSA.
Along with a home sleep study, researchers tested 75 nonselected consecutive patients (mean age of 64 years; 72% male) from three outpatient arrhythmia clinics for symptoms indicative of OSA using the Epworth Sleepiness Scale (ESS), the Fatigue Severity Scale (FSS), the Non-Restorative Sleep Scale (NRSS), and other questionnaires.
On the ESS, 32% of patients had a score of 8 or greater, indicating higher than normal daytime sleepiness. Almost half (47%) of patients had a high level of fatigue on the FSS, and symptoms of nonrestorative sleep were detected in 15% (NRSS score greater than or equal to 46).
Dr. Shapiro noted that “high scores suggestive of daytime sleepiness, fatigue, or insomnia did not particularly predict the presence of OSA in patients with arrhythmia.” He concluded that, “with a hit rate of 85%, just about every patient with an arrhythmia should have a sleep study.”
Dr. Shapiro informed attendees at the annual meeting of the Professional Sleep Societies that he was presenting in place of his student and the abstract’s first author, Dr. Asmaa M. Abumuamar, MD, who was denied a visa to attend the meeting. Dr. Abumuamar is from the Toronto Western Research Institute, University of Toronto.
Dr. Shapiro reported that Dr. Abumuamar has no conflicts of interest. Dr. Shapiro reported that he is an investor in the company that supplied the home sleep testing apparatus.
BOSTON – In a study of patients without a previous diagnosis of obstructive sleep apnea (OSA), 85% of participants in outpatient arrhythmia clinics had undetected OSA.
The study, which also excluded patients who had ever been treated for OSA, was presented by Colin Shapiro, MD, of the Department of Psychiatry, Toronto Western Hospital, University of Toronto, at the annual meeting of the Associated Professional Sleep Societies.
A binary logistic regression analysis showed that only age and male gender were significant predictors of OSA.
Along with a home sleep study, researchers tested 75 nonselected consecutive patients (mean age of 64 years; 72% male) from three outpatient arrhythmia clinics for symptoms indicative of OSA using the Epworth Sleepiness Scale (ESS), the Fatigue Severity Scale (FSS), the Non-Restorative Sleep Scale (NRSS), and other questionnaires.
On the ESS, 32% of patients had a score of 8 or greater, indicating higher than normal daytime sleepiness. Almost half (47%) of patients had a high level of fatigue on the FSS, and symptoms of nonrestorative sleep were detected in 15% (NRSS score greater than or equal to 46).
Dr. Shapiro noted that “high scores suggestive of daytime sleepiness, fatigue, or insomnia did not particularly predict the presence of OSA in patients with arrhythmia.” He concluded that, “with a hit rate of 85%, just about every patient with an arrhythmia should have a sleep study.”
Dr. Shapiro informed attendees at the annual meeting of the Professional Sleep Societies that he was presenting in place of his student and the abstract’s first author, Dr. Asmaa M. Abumuamar, MD, who was denied a visa to attend the meeting. Dr. Abumuamar is from the Toronto Western Research Institute, University of Toronto.
Dr. Shapiro reported that Dr. Abumuamar has no conflicts of interest. Dr. Shapiro reported that he is an investor in the company that supplied the home sleep testing apparatus.
AT SLEEP 2017
Key clinical point: The majority of patients attending an outpatient arrhythmia clinic had undetected OSA.
Major finding: Of the participants, 91% of males and 71% of females with no history of OSA were found to have the disorder (85% of total).
Data source: A screening study using a randomized, unselected population drawn from outpatient arrhythmia clinics. Those with a diagnoses of OSA were excluded.
Disclosures: Dr. Shapiro reported that Dr. Abumuamar has no conflicts of interest. Dr. Shapiro reported that he is an investor in the company that supplied the home sleep testing apparatus.
Frequent bronchiectasis exacerbations linked to higher mortality
WASHINGTON – Bronchiectasis patients with three or more exacerbations per year had twice the mortality during 5-year follow-up as patients with no recent exacerbations, in a prospective registry of nearly 2,600 European bronchiectasis patients.
A multivariate analysis showed this statistically significant doubled death rate after adjustment for baseline demographic and clinical differences between patients with no exacerbations during the year before they entered the registry, James D. Chalmers, MD, said at an international conference of the American Thoracic Society.
This 37% prevalence contrasted with a 19% U.S. prevalence of bronchiectasis patients having two or more exacerbations per year among 2,114 patients enrolled in a 13-center U.S. registry that was reported during the same session by Timothy R. Aksamit, MD, a pulmonologist at the Mayo Clinic in Rochester, Minn. During his talk, Dr. Aksamit suggested that the European cohort might include more patients with asthma, chronic obstructive pulmonary disease, or other complications in addition to bronchiectasis. Dr. Aksamit contended that the U.S. registry tried to exclusively enroll patients with bronchiectasis and no other disorder, possibly explaining the prevalence difference between Europe and the United States.
The European registry included patients with bronchiectasis seen in 10 centers in seven European countries and Israel. They averaged 67 years of age. While more than a third had a history of at least three exacerbations a year, one-quarter had no exacerbations during the year before they entered the study.
The U.S. registry reported by Dr. Aksamit had 2-year follow-up data for 1,049 of the enrolled patients, a subgroup that closely matched the entire population initially enrolled. The 2-year follow-up showed an overall average exacerbation rate of 0.75 episodes per year, but this was driven largely by the subgroup of patients who entered the registry with a history of two or more exacerbations per year, who then averaged about 2.6 exacerbations during follow-up. In contrast, patients who entered the registry with a history of fewer than two exacerbations per year averaged fewer than a third of an exacerbation per year during follow-up, Dr. Aksamit reported.
The European bronchiectasis registry was partially funded by Bayer. Dr. Chalmers has been a consultant to Bayer and to AstraZeneca, Basilea, Grifols, Napp, and Raptor and has received research funding from Aradigm, AstraZeneca, Bayer, GlaxoSmithKline, and Pfizer. Dr. Aksamit had no disclosures.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – Bronchiectasis patients with three or more exacerbations per year had twice the mortality during 5-year follow-up as patients with no recent exacerbations, in a prospective registry of nearly 2,600 European bronchiectasis patients.
A multivariate analysis showed this statistically significant doubled death rate after adjustment for baseline demographic and clinical differences between patients with no exacerbations during the year before they entered the registry, James D. Chalmers, MD, said at an international conference of the American Thoracic Society.
This 37% prevalence contrasted with a 19% U.S. prevalence of bronchiectasis patients having two or more exacerbations per year among 2,114 patients enrolled in a 13-center U.S. registry that was reported during the same session by Timothy R. Aksamit, MD, a pulmonologist at the Mayo Clinic in Rochester, Minn. During his talk, Dr. Aksamit suggested that the European cohort might include more patients with asthma, chronic obstructive pulmonary disease, or other complications in addition to bronchiectasis. Dr. Aksamit contended that the U.S. registry tried to exclusively enroll patients with bronchiectasis and no other disorder, possibly explaining the prevalence difference between Europe and the United States.
The European registry included patients with bronchiectasis seen in 10 centers in seven European countries and Israel. They averaged 67 years of age. While more than a third had a history of at least three exacerbations a year, one-quarter had no exacerbations during the year before they entered the study.
The U.S. registry reported by Dr. Aksamit had 2-year follow-up data for 1,049 of the enrolled patients, a subgroup that closely matched the entire population initially enrolled. The 2-year follow-up showed an overall average exacerbation rate of 0.75 episodes per year, but this was driven largely by the subgroup of patients who entered the registry with a history of two or more exacerbations per year, who then averaged about 2.6 exacerbations during follow-up. In contrast, patients who entered the registry with a history of fewer than two exacerbations per year averaged fewer than a third of an exacerbation per year during follow-up, Dr. Aksamit reported.
The European bronchiectasis registry was partially funded by Bayer. Dr. Chalmers has been a consultant to Bayer and to AstraZeneca, Basilea, Grifols, Napp, and Raptor and has received research funding from Aradigm, AstraZeneca, Bayer, GlaxoSmithKline, and Pfizer. Dr. Aksamit had no disclosures.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – Bronchiectasis patients with three or more exacerbations per year had twice the mortality during 5-year follow-up as patients with no recent exacerbations, in a prospective registry of nearly 2,600 European bronchiectasis patients.
A multivariate analysis showed this statistically significant doubled death rate after adjustment for baseline demographic and clinical differences between patients with no exacerbations during the year before they entered the registry, James D. Chalmers, MD, said at an international conference of the American Thoracic Society.
This 37% prevalence contrasted with a 19% U.S. prevalence of bronchiectasis patients having two or more exacerbations per year among 2,114 patients enrolled in a 13-center U.S. registry that was reported during the same session by Timothy R. Aksamit, MD, a pulmonologist at the Mayo Clinic in Rochester, Minn. During his talk, Dr. Aksamit suggested that the European cohort might include more patients with asthma, chronic obstructive pulmonary disease, or other complications in addition to bronchiectasis. Dr. Aksamit contended that the U.S. registry tried to exclusively enroll patients with bronchiectasis and no other disorder, possibly explaining the prevalence difference between Europe and the United States.
The European registry included patients with bronchiectasis seen in 10 centers in seven European countries and Israel. They averaged 67 years of age. While more than a third had a history of at least three exacerbations a year, one-quarter had no exacerbations during the year before they entered the study.
The U.S. registry reported by Dr. Aksamit had 2-year follow-up data for 1,049 of the enrolled patients, a subgroup that closely matched the entire population initially enrolled. The 2-year follow-up showed an overall average exacerbation rate of 0.75 episodes per year, but this was driven largely by the subgroup of patients who entered the registry with a history of two or more exacerbations per year, who then averaged about 2.6 exacerbations during follow-up. In contrast, patients who entered the registry with a history of fewer than two exacerbations per year averaged fewer than a third of an exacerbation per year during follow-up, Dr. Aksamit reported.
The European bronchiectasis registry was partially funded by Bayer. Dr. Chalmers has been a consultant to Bayer and to AstraZeneca, Basilea, Grifols, Napp, and Raptor and has received research funding from Aradigm, AstraZeneca, Bayer, GlaxoSmithKline, and Pfizer. Dr. Aksamit had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: Patients with frequent exacerbations had twice the mortality rate, compared with bronchiectasis patients with no recent exacerbations.
Data source: A registry of 2,596 bronchiectasis patients from 10 centers in Europe and Israel.
Disclosures: The European bronchiectasis registry was partially funded by Bayer. Dr. Chalmers has been a consultant to Bayer and to AstraZeneca, Basilea, Grifols, Napp, and Raptor and has received research funding from Aradigm, AstraZeneca, Bayer, GlaxoSmithKline, and Pfizer. Dr. Aksamit had no disclosures.
Telemonitoring with feedback improves CPAP
BOSTON – Remote monitoring of continuous positive airway pressure (CPAP) use with feedback messaging to patients improves adherence but only when patients opt to receive continual feedback on their usage, according to a study.
Dennis Hwang, MD, medical director of Kaiser Permanent Fontana Medical Center in California and his colleagues designed the four-arm Tele-OSA study to evaluate the impact of two automated telemedicine interventions: an obstructive sleep apnea (OSA) education program (provided by Emmi Solutions) and a CPAP remote monitoring system with automated patient feedback (U-Sleep, ResMed). Dr. Hwang, who is also cochair of sleep medicine at Southern California Permanente Medical Group, presented his findings at the annual meeting of the Associated Professional Sleep Societies.
CPAP adherence was compared at 3 months and 1 year for patients in all four groups. Dr. Hwang reported findings from 556 patients who completed one-year follow-up.
At 90 days, patients assigned to either of the telemonitoring arms had significantly higher CPAP usage than those who did not receive telemonitoring.
However, at 3 months when the study protocol called for the automated messaging to be turned off, CPAP adherence dropped off. By 8 months, adherence in patients using the telemonitoring system was no different from that in those who never received the automated messaging. That would have been the end of the story, except that there was a glitch in the system.
“Perhaps serendipitously, we had a group of patients, about one-third, for whom we inadvertently did not turn off the messaging,” explained Dr. Hwang. “In these patients who continued to receive feedback, CPAP usage remained elevated throughout the course of the year and, at 12 months, was significantly higher than in the patients who were not receiving any kind of messaging.”
Dr. Hwang added that the telemonitoring required no additional provider intervention, “suggesting that this could be a cost-effective strategy.”
Only one-third of patients (66.7%) assigned to one of the tele-education groups viewed the video. Additionally, the researchers found that, whether patients used the tele-education alone or in combination with the telemonitoring, tele-education use had no impact on 90-day compliance with CPAP.
Dr. Hwang received support from the American Sleep Medicine Foundation and ResMed Science.
BOSTON – Remote monitoring of continuous positive airway pressure (CPAP) use with feedback messaging to patients improves adherence but only when patients opt to receive continual feedback on their usage, according to a study.
Dennis Hwang, MD, medical director of Kaiser Permanent Fontana Medical Center in California and his colleagues designed the four-arm Tele-OSA study to evaluate the impact of two automated telemedicine interventions: an obstructive sleep apnea (OSA) education program (provided by Emmi Solutions) and a CPAP remote monitoring system with automated patient feedback (U-Sleep, ResMed). Dr. Hwang, who is also cochair of sleep medicine at Southern California Permanente Medical Group, presented his findings at the annual meeting of the Associated Professional Sleep Societies.
CPAP adherence was compared at 3 months and 1 year for patients in all four groups. Dr. Hwang reported findings from 556 patients who completed one-year follow-up.
At 90 days, patients assigned to either of the telemonitoring arms had significantly higher CPAP usage than those who did not receive telemonitoring.
However, at 3 months when the study protocol called for the automated messaging to be turned off, CPAP adherence dropped off. By 8 months, adherence in patients using the telemonitoring system was no different from that in those who never received the automated messaging. That would have been the end of the story, except that there was a glitch in the system.
“Perhaps serendipitously, we had a group of patients, about one-third, for whom we inadvertently did not turn off the messaging,” explained Dr. Hwang. “In these patients who continued to receive feedback, CPAP usage remained elevated throughout the course of the year and, at 12 months, was significantly higher than in the patients who were not receiving any kind of messaging.”
Dr. Hwang added that the telemonitoring required no additional provider intervention, “suggesting that this could be a cost-effective strategy.”
Only one-third of patients (66.7%) assigned to one of the tele-education groups viewed the video. Additionally, the researchers found that, whether patients used the tele-education alone or in combination with the telemonitoring, tele-education use had no impact on 90-day compliance with CPAP.
Dr. Hwang received support from the American Sleep Medicine Foundation and ResMed Science.
BOSTON – Remote monitoring of continuous positive airway pressure (CPAP) use with feedback messaging to patients improves adherence but only when patients opt to receive continual feedback on their usage, according to a study.
Dennis Hwang, MD, medical director of Kaiser Permanent Fontana Medical Center in California and his colleagues designed the four-arm Tele-OSA study to evaluate the impact of two automated telemedicine interventions: an obstructive sleep apnea (OSA) education program (provided by Emmi Solutions) and a CPAP remote monitoring system with automated patient feedback (U-Sleep, ResMed). Dr. Hwang, who is also cochair of sleep medicine at Southern California Permanente Medical Group, presented his findings at the annual meeting of the Associated Professional Sleep Societies.
CPAP adherence was compared at 3 months and 1 year for patients in all four groups. Dr. Hwang reported findings from 556 patients who completed one-year follow-up.
At 90 days, patients assigned to either of the telemonitoring arms had significantly higher CPAP usage than those who did not receive telemonitoring.
However, at 3 months when the study protocol called for the automated messaging to be turned off, CPAP adherence dropped off. By 8 months, adherence in patients using the telemonitoring system was no different from that in those who never received the automated messaging. That would have been the end of the story, except that there was a glitch in the system.
“Perhaps serendipitously, we had a group of patients, about one-third, for whom we inadvertently did not turn off the messaging,” explained Dr. Hwang. “In these patients who continued to receive feedback, CPAP usage remained elevated throughout the course of the year and, at 12 months, was significantly higher than in the patients who were not receiving any kind of messaging.”
Dr. Hwang added that the telemonitoring required no additional provider intervention, “suggesting that this could be a cost-effective strategy.”
Only one-third of patients (66.7%) assigned to one of the tele-education groups viewed the video. Additionally, the researchers found that, whether patients used the tele-education alone or in combination with the telemonitoring, tele-education use had no impact on 90-day compliance with CPAP.
Dr. Hwang received support from the American Sleep Medicine Foundation and ResMed Science.
AT SLEEP 2017
Key clinical point: CPAP telemonitoring with feedback messaging improved CPAP adherence with no additional provider intervention needed.
Major finding: CPAP adherence was significantly higher at 3 months in patients who had remote monitoring of usage with subsequent continuous automated feedback. At one year, usage was only higher in those patients who continued to receive messaging during usage of the telemonitoring system and not in those who had the messaging component “turned off.”
Data source: A four-arm, randomized clinical trial that tested tele-education and CPAP telemonitoring interventions in 1,455 patients.
Disclosures: Dr. Hwang received support from the American Sleep Medicine Foundation and ResMed Science Center.
Hypertonic saline for bronchiolitis found ineffective in large RCT study
MADRID – Giving nebulized hypertonic saline (NHS) to infants who present to pediatric emergency departments with a first episode of moderate to severe acute bronchiolitis did not reduce their hospitalization rate in the randomized, multicenter, double-blind GUERANDE trial.
Moreover, mild adverse events – mainly worsening cough – were significantly more frequent in the NHS recipients than in controls given nebulized normal saline, Christele Gras-le Guen, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Some previous studies have suggested a modest benefit, but they were underpowered to draw meaningful conclusions. GUERANDE, a 777-patient randomized trial conducted in 24 French pediatric emergency departments, was the first quality study large enough to determine whether the therapy results in fewer hospital admissions, she said.
In GUERANDE, infants with a first episode of acute bronchiolitis were given two 20-minute nebulizations of 3% hypertonic saline or 0.9% normal saline 20 minutes apart.
The primary outcome was the rate of hospital admission within 24 hours after enrollment. The rate was 48% in the NHS group and 52% in controls, a nonsignificant difference which shrunk even more after controlling for age, oxygen saturation, and respiratory syncytial virus infection status.
No serious adverse events occurred in GUERANDE. However, the rate of mild adverse events was 9% in the NHS group, significantly higher than the 4% rate in controls. Notably, cough without respiratory distress occurred 30 times in 26 infants in the NHS group, compared with 4 times in 3 control subjects.
Dr. Gras-le Guin reported having no financial conflicts regarding the GUERANDE study, funded by the French Health Ministry.
MADRID – Giving nebulized hypertonic saline (NHS) to infants who present to pediatric emergency departments with a first episode of moderate to severe acute bronchiolitis did not reduce their hospitalization rate in the randomized, multicenter, double-blind GUERANDE trial.
Moreover, mild adverse events – mainly worsening cough – were significantly more frequent in the NHS recipients than in controls given nebulized normal saline, Christele Gras-le Guen, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Some previous studies have suggested a modest benefit, but they were underpowered to draw meaningful conclusions. GUERANDE, a 777-patient randomized trial conducted in 24 French pediatric emergency departments, was the first quality study large enough to determine whether the therapy results in fewer hospital admissions, she said.
In GUERANDE, infants with a first episode of acute bronchiolitis were given two 20-minute nebulizations of 3% hypertonic saline or 0.9% normal saline 20 minutes apart.
The primary outcome was the rate of hospital admission within 24 hours after enrollment. The rate was 48% in the NHS group and 52% in controls, a nonsignificant difference which shrunk even more after controlling for age, oxygen saturation, and respiratory syncytial virus infection status.
No serious adverse events occurred in GUERANDE. However, the rate of mild adverse events was 9% in the NHS group, significantly higher than the 4% rate in controls. Notably, cough without respiratory distress occurred 30 times in 26 infants in the NHS group, compared with 4 times in 3 control subjects.
Dr. Gras-le Guin reported having no financial conflicts regarding the GUERANDE study, funded by the French Health Ministry.
MADRID – Giving nebulized hypertonic saline (NHS) to infants who present to pediatric emergency departments with a first episode of moderate to severe acute bronchiolitis did not reduce their hospitalization rate in the randomized, multicenter, double-blind GUERANDE trial.
Moreover, mild adverse events – mainly worsening cough – were significantly more frequent in the NHS recipients than in controls given nebulized normal saline, Christele Gras-le Guen, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Some previous studies have suggested a modest benefit, but they were underpowered to draw meaningful conclusions. GUERANDE, a 777-patient randomized trial conducted in 24 French pediatric emergency departments, was the first quality study large enough to determine whether the therapy results in fewer hospital admissions, she said.
In GUERANDE, infants with a first episode of acute bronchiolitis were given two 20-minute nebulizations of 3% hypertonic saline or 0.9% normal saline 20 minutes apart.
The primary outcome was the rate of hospital admission within 24 hours after enrollment. The rate was 48% in the NHS group and 52% in controls, a nonsignificant difference which shrunk even more after controlling for age, oxygen saturation, and respiratory syncytial virus infection status.
No serious adverse events occurred in GUERANDE. However, the rate of mild adverse events was 9% in the NHS group, significantly higher than the 4% rate in controls. Notably, cough without respiratory distress occurred 30 times in 26 infants in the NHS group, compared with 4 times in 3 control subjects.
Dr. Gras-le Guin reported having no financial conflicts regarding the GUERANDE study, funded by the French Health Ministry.
AT ESPID 2017
Key clinical point:
Major finding: The rate of hospitalization following administration of nebulized hypertonic saline to infants with a first episode of acute bronchiolitis was 48%, not significantly different from the 52% rate in controls.
Data source: This was a randomized, multicenter, double-blind, controlled clinical trial including 777 infants with acute bronchiolitis.
Disclosures: The GUERANDE study was funded by the French Health Ministry. The presenter reported having no financial conflicts.
Monoclonal antibody holds promise for S. aureus pneumonia
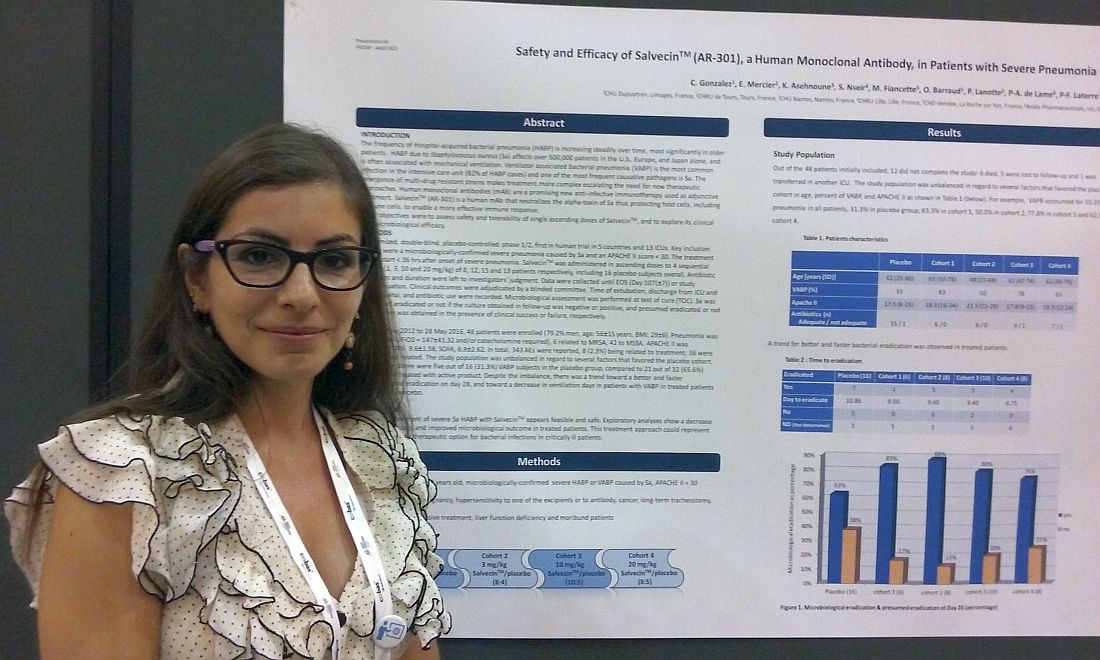
NEW ORLEANS – Monoclonal antibody therapies have already upended treatment strategies in cancer, dermatology, and multiple inflammatory diseases, and infectious disease may be next.
That’s because , according to a new study. The monoclonal antibody attacks the alpha-toxin secreted by S. aureus, thereby helping to protect immune cells.
“We know S. aureus pneumonia is a big problem. There is a lot of antibiotic resistance, and that is why we need new treatments,” Celine Gonzalez, MD, of the Dupuytren Central University Hospital in Limoges, France, said in an interview.
“Animal studies have shown the monoclonal antibody seems to be useful. This is the first in-human study to use a monoclonal antibody to treat hospital-acquired pneumonia due to Staphylococcus aureus,” Dr. Gonzalez said in a late-breaking poster presentation at the annual meeting of the American Society for Microbiology.
Treatment started within 36 hours of onset of severe pneumonia. Severity was based on a mean PaO2/FiO2 of 147 and/or a need for catecholamine. Six cases of pneumonia were related to MRSA and the remaining 42 to methicillin-susceptible S. aureus. The mean APACHE II score was 18.7, the mean Clinical Pulmonary Infection Score was 9.6, and the mean Sequential Organ Failure Assessment score was 6.9.
Participants were recruited from 13 ICUs in four countries. About 80% of participants were men. Their mean age was 56 years, and mean body mass index was 29 kg/m2. Concurrent antibiotic treatment choice and duration were at the investigator’s discretion.
S. aureus infection was considered eradicated if a follow-up culture was negative, a result achieved by 63% of the 16 placebo patients and 75%-88% of the AR-301-dosage groups. Eradication was also based on observed clinical success in the absence of a confirmatory culture. This was achieved by 38% in the placebo group and 13%-25% of the monoclonal antibody cohorts. A total of seven placebo patients and 15 AR-301 patients met eradication by these criteria.
Side effects were primarily minor and transient, Dr. Gonzalez said. Of the 343 total adverse events reported, only 8 (2.3%) were considered treatment related, she added.
“In infectious disease, it’s the beginning” for monoclonal antibody therapy, Dr. Gonzalez said. “But, it appears to be the future because … it is a more specific treatment, and there is no resistance.”
The study suggests adjunctive treatment with AR-301 appears safe for treatment of hospital-acquired bacterial pneumonia, she noted. The next step will be to confirm the findings in a larger, follow-up study that includes more efficacy outcomes, Dr. Gonzalez added.
Dr. Gonzalez reported having no relevant disclosures. The study’s principle investigator is a scientific advisor for Aridis Pharmaceuticals, which is developing AR-301.
NEW ORLEANS – Monoclonal antibody therapies have already upended treatment strategies in cancer, dermatology, and multiple inflammatory diseases, and infectious disease may be next.
That’s because , according to a new study. The monoclonal antibody attacks the alpha-toxin secreted by S. aureus, thereby helping to protect immune cells.
“We know S. aureus pneumonia is a big problem. There is a lot of antibiotic resistance, and that is why we need new treatments,” Celine Gonzalez, MD, of the Dupuytren Central University Hospital in Limoges, France, said in an interview.
“Animal studies have shown the monoclonal antibody seems to be useful. This is the first in-human study to use a monoclonal antibody to treat hospital-acquired pneumonia due to Staphylococcus aureus,” Dr. Gonzalez said in a late-breaking poster presentation at the annual meeting of the American Society for Microbiology.
Treatment started within 36 hours of onset of severe pneumonia. Severity was based on a mean PaO2/FiO2 of 147 and/or a need for catecholamine. Six cases of pneumonia were related to MRSA and the remaining 42 to methicillin-susceptible S. aureus. The mean APACHE II score was 18.7, the mean Clinical Pulmonary Infection Score was 9.6, and the mean Sequential Organ Failure Assessment score was 6.9.
Participants were recruited from 13 ICUs in four countries. About 80% of participants were men. Their mean age was 56 years, and mean body mass index was 29 kg/m2. Concurrent antibiotic treatment choice and duration were at the investigator’s discretion.
S. aureus infection was considered eradicated if a follow-up culture was negative, a result achieved by 63% of the 16 placebo patients and 75%-88% of the AR-301-dosage groups. Eradication was also based on observed clinical success in the absence of a confirmatory culture. This was achieved by 38% in the placebo group and 13%-25% of the monoclonal antibody cohorts. A total of seven placebo patients and 15 AR-301 patients met eradication by these criteria.
Side effects were primarily minor and transient, Dr. Gonzalez said. Of the 343 total adverse events reported, only 8 (2.3%) were considered treatment related, she added.
“In infectious disease, it’s the beginning” for monoclonal antibody therapy, Dr. Gonzalez said. “But, it appears to be the future because … it is a more specific treatment, and there is no resistance.”
The study suggests adjunctive treatment with AR-301 appears safe for treatment of hospital-acquired bacterial pneumonia, she noted. The next step will be to confirm the findings in a larger, follow-up study that includes more efficacy outcomes, Dr. Gonzalez added.
Dr. Gonzalez reported having no relevant disclosures. The study’s principle investigator is a scientific advisor for Aridis Pharmaceuticals, which is developing AR-301.
NEW ORLEANS – Monoclonal antibody therapies have already upended treatment strategies in cancer, dermatology, and multiple inflammatory diseases, and infectious disease may be next.
That’s because , according to a new study. The monoclonal antibody attacks the alpha-toxin secreted by S. aureus, thereby helping to protect immune cells.
“We know S. aureus pneumonia is a big problem. There is a lot of antibiotic resistance, and that is why we need new treatments,” Celine Gonzalez, MD, of the Dupuytren Central University Hospital in Limoges, France, said in an interview.
“Animal studies have shown the monoclonal antibody seems to be useful. This is the first in-human study to use a monoclonal antibody to treat hospital-acquired pneumonia due to Staphylococcus aureus,” Dr. Gonzalez said in a late-breaking poster presentation at the annual meeting of the American Society for Microbiology.
Treatment started within 36 hours of onset of severe pneumonia. Severity was based on a mean PaO2/FiO2 of 147 and/or a need for catecholamine. Six cases of pneumonia were related to MRSA and the remaining 42 to methicillin-susceptible S. aureus. The mean APACHE II score was 18.7, the mean Clinical Pulmonary Infection Score was 9.6, and the mean Sequential Organ Failure Assessment score was 6.9.
Participants were recruited from 13 ICUs in four countries. About 80% of participants were men. Their mean age was 56 years, and mean body mass index was 29 kg/m2. Concurrent antibiotic treatment choice and duration were at the investigator’s discretion.
S. aureus infection was considered eradicated if a follow-up culture was negative, a result achieved by 63% of the 16 placebo patients and 75%-88% of the AR-301-dosage groups. Eradication was also based on observed clinical success in the absence of a confirmatory culture. This was achieved by 38% in the placebo group and 13%-25% of the monoclonal antibody cohorts. A total of seven placebo patients and 15 AR-301 patients met eradication by these criteria.
Side effects were primarily minor and transient, Dr. Gonzalez said. Of the 343 total adverse events reported, only 8 (2.3%) were considered treatment related, she added.
“In infectious disease, it’s the beginning” for monoclonal antibody therapy, Dr. Gonzalez said. “But, it appears to be the future because … it is a more specific treatment, and there is no resistance.”
The study suggests adjunctive treatment with AR-301 appears safe for treatment of hospital-acquired bacterial pneumonia, she noted. The next step will be to confirm the findings in a larger, follow-up study that includes more efficacy outcomes, Dr. Gonzalez added.
Dr. Gonzalez reported having no relevant disclosures. The study’s principle investigator is a scientific advisor for Aridis Pharmaceuticals, which is developing AR-301.
AT ASM MICROBE 2017
Key clinical point: A monoclonal antibody that neutralizes the alpha-toxin secreted by S. aureus appeared safe and effective in an early trial.
Major finding: AR-301 appeared safe, with 8 of 343 adverse events, or 2.3%, considered treatment related.
Data source: Randomized, double-blind, placebo-controlled, international study in 48 patients from 13 ICUs.
Disclosures: Dr. Gonzalez reported having no relevant disclosures. The study’s principle investigator is a scientific advisor for Aridis Pharmaceuticals, which is developing AR-301.
Patients report issues with home O2
WASHINGTON – Patient education in the use of home oxygen halves the number of system use issues reported by patients, based on results of a survey of nearly 2,000 patients.
Pulmonary clinicians and patients report “intolerable barriers to home oxygen services,” lead researcher Susan S. Jacobs, RN, MS, said in a poster session at an international conference of the American Thoracic Society. These barriers include insufficient oxygen supply, inadequate and physically unmanageable portable options, and equipment malfunction.
“We’ve demonstrated that, if the patients are educated by a health care professional, the problems with oxygen go down, Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University, said in an interview. “While physicians can provide oxygen for their patients, the patient oxygen education will most likely lie with the nurses and respiratory therapists.”
Of patients who responded to the survey question "Do you have oxygen problems?" 51% (899) said yes*. On average, these patients said they had experienced 3.5 types of problems with their systems.
Patients who were educated by a health care professional reported fewer problems and were more likely to report having no problems with their oxygen system. Of the patients who received oxygen therapy instruction from a health care professional, 76 (57%) did not report having any issues with their system. In contrast, of the patients who received no instruction, 116 (64%) said they had problems with their oxygen.
Most survey participants (1,113 patients) received oxygen therapy instruction from an oxygen delivery person instead of a health care professional. This group’s opinions about their oxygen systems were split, with 51% (563 patients) experiencing issues with their systems. The other 49% reported no problems.
Survey participants most frequently complained that their equipment was not working; 499 selected this response to the question, “What types of oxygen problems do you have?”
Many patients also reported being unable to spend as much time out of their homes as they wanted. This limitation resulted from their lack of access to functioning, manageable, high flow, portable oxygen systems, according to the researchers. Further, 43% of patients reported that their portable system limited their activity outside the home frequently or all of the time.
“Most of the reported problems were related to respondents not having portable systems that let them be out of their house for more than 2 to 4 hours or [to systems that] were too heavy for the patients to lift up and down their stairs and out of their cars, and they had problems operating them,” said Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University.
The survey respondents also reported experiencing delivery problems, not being able to change the company providing them with oxygen, receiving incorrect or delayed orders from a physician, or being unable to get liquid oxygen. These responses were provided by 267, 177, 166, and 68 patients, respectively.
“There is a lot of confusion for the physicians as well as the nurses about what types of systems the patients can use [and] the pros and cons of each system. There’s lots of confusion and time spent about getting the initial orders right, getting them set up with a supplier, and ensuring the patient gets the equipment that was ordered. There is a lot of back and forth, which results in a delay to the patient, and the patients are upset because they are waiting for their oxygen supply,” she explained. “So, I think that physicians are very much wanting clarification to streamline the process and identify what patient systems are appropriate, which are high flow, [and] what their patients’ needs are to help physicians spend less time on this and help the patients get their oxygen set up in a timely manner.”
The study participants came from all 50 states and were 64 years of age on average and mostly women. A high percentage (39%) of the sample had chronic obstructive pulmonary disease, while 26% had interstitial lung diseases, 18% had pulmonary arterial hypertension, 8% had alpha-1 antitrypsin deficiency, and 4% had lymphangioleiomyomatosis.
Ms. Jacobs noted that she thought patients would benefit from greater physician knowledge of their prescribing options.
“A physician can dictate exactly what system they want. ... You can try to give [patients] a lighter system, a backpack, a smaller tank, more tanks per week, depending on their lifestyle and their needs. But physicians, a lot of times, like all of us and our patients, [are] not aware of all these choices,” she said, during the interview.
An online resource providing all of the pros and cons of the different types of portable oxygen systems that would be appropriate for physicians, nurses, and patients, as well as an examination of the quality standards of the oxygen suppliers, are needed, she noted
Ms. Jacobs reported no financial disclosures.
*This article was corrected June 16, 2017
WASHINGTON – Patient education in the use of home oxygen halves the number of system use issues reported by patients, based on results of a survey of nearly 2,000 patients.
Pulmonary clinicians and patients report “intolerable barriers to home oxygen services,” lead researcher Susan S. Jacobs, RN, MS, said in a poster session at an international conference of the American Thoracic Society. These barriers include insufficient oxygen supply, inadequate and physically unmanageable portable options, and equipment malfunction.
“We’ve demonstrated that, if the patients are educated by a health care professional, the problems with oxygen go down, Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University, said in an interview. “While physicians can provide oxygen for their patients, the patient oxygen education will most likely lie with the nurses and respiratory therapists.”
Of patients who responded to the survey question "Do you have oxygen problems?" 51% (899) said yes*. On average, these patients said they had experienced 3.5 types of problems with their systems.
Patients who were educated by a health care professional reported fewer problems and were more likely to report having no problems with their oxygen system. Of the patients who received oxygen therapy instruction from a health care professional, 76 (57%) did not report having any issues with their system. In contrast, of the patients who received no instruction, 116 (64%) said they had problems with their oxygen.
Most survey participants (1,113 patients) received oxygen therapy instruction from an oxygen delivery person instead of a health care professional. This group’s opinions about their oxygen systems were split, with 51% (563 patients) experiencing issues with their systems. The other 49% reported no problems.
Survey participants most frequently complained that their equipment was not working; 499 selected this response to the question, “What types of oxygen problems do you have?”
Many patients also reported being unable to spend as much time out of their homes as they wanted. This limitation resulted from their lack of access to functioning, manageable, high flow, portable oxygen systems, according to the researchers. Further, 43% of patients reported that their portable system limited their activity outside the home frequently or all of the time.
“Most of the reported problems were related to respondents not having portable systems that let them be out of their house for more than 2 to 4 hours or [to systems that] were too heavy for the patients to lift up and down their stairs and out of their cars, and they had problems operating them,” said Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University.
The survey respondents also reported experiencing delivery problems, not being able to change the company providing them with oxygen, receiving incorrect or delayed orders from a physician, or being unable to get liquid oxygen. These responses were provided by 267, 177, 166, and 68 patients, respectively.
“There is a lot of confusion for the physicians as well as the nurses about what types of systems the patients can use [and] the pros and cons of each system. There’s lots of confusion and time spent about getting the initial orders right, getting them set up with a supplier, and ensuring the patient gets the equipment that was ordered. There is a lot of back and forth, which results in a delay to the patient, and the patients are upset because they are waiting for their oxygen supply,” she explained. “So, I think that physicians are very much wanting clarification to streamline the process and identify what patient systems are appropriate, which are high flow, [and] what their patients’ needs are to help physicians spend less time on this and help the patients get their oxygen set up in a timely manner.”
The study participants came from all 50 states and were 64 years of age on average and mostly women. A high percentage (39%) of the sample had chronic obstructive pulmonary disease, while 26% had interstitial lung diseases, 18% had pulmonary arterial hypertension, 8% had alpha-1 antitrypsin deficiency, and 4% had lymphangioleiomyomatosis.
Ms. Jacobs noted that she thought patients would benefit from greater physician knowledge of their prescribing options.
“A physician can dictate exactly what system they want. ... You can try to give [patients] a lighter system, a backpack, a smaller tank, more tanks per week, depending on their lifestyle and their needs. But physicians, a lot of times, like all of us and our patients, [are] not aware of all these choices,” she said, during the interview.
An online resource providing all of the pros and cons of the different types of portable oxygen systems that would be appropriate for physicians, nurses, and patients, as well as an examination of the quality standards of the oxygen suppliers, are needed, she noted
Ms. Jacobs reported no financial disclosures.
*This article was corrected June 16, 2017
WASHINGTON – Patient education in the use of home oxygen halves the number of system use issues reported by patients, based on results of a survey of nearly 2,000 patients.
Pulmonary clinicians and patients report “intolerable barriers to home oxygen services,” lead researcher Susan S. Jacobs, RN, MS, said in a poster session at an international conference of the American Thoracic Society. These barriers include insufficient oxygen supply, inadequate and physically unmanageable portable options, and equipment malfunction.
“We’ve demonstrated that, if the patients are educated by a health care professional, the problems with oxygen go down, Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University, said in an interview. “While physicians can provide oxygen for their patients, the patient oxygen education will most likely lie with the nurses and respiratory therapists.”
Of patients who responded to the survey question "Do you have oxygen problems?" 51% (899) said yes*. On average, these patients said they had experienced 3.5 types of problems with their systems.
Patients who were educated by a health care professional reported fewer problems and were more likely to report having no problems with their oxygen system. Of the patients who received oxygen therapy instruction from a health care professional, 76 (57%) did not report having any issues with their system. In contrast, of the patients who received no instruction, 116 (64%) said they had problems with their oxygen.
Most survey participants (1,113 patients) received oxygen therapy instruction from an oxygen delivery person instead of a health care professional. This group’s opinions about their oxygen systems were split, with 51% (563 patients) experiencing issues with their systems. The other 49% reported no problems.
Survey participants most frequently complained that their equipment was not working; 499 selected this response to the question, “What types of oxygen problems do you have?”
Many patients also reported being unable to spend as much time out of their homes as they wanted. This limitation resulted from their lack of access to functioning, manageable, high flow, portable oxygen systems, according to the researchers. Further, 43% of patients reported that their portable system limited their activity outside the home frequently or all of the time.
“Most of the reported problems were related to respondents not having portable systems that let them be out of their house for more than 2 to 4 hours or [to systems that] were too heavy for the patients to lift up and down their stairs and out of their cars, and they had problems operating them,” said Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University.
The survey respondents also reported experiencing delivery problems, not being able to change the company providing them with oxygen, receiving incorrect or delayed orders from a physician, or being unable to get liquid oxygen. These responses were provided by 267, 177, 166, and 68 patients, respectively.
“There is a lot of confusion for the physicians as well as the nurses about what types of systems the patients can use [and] the pros and cons of each system. There’s lots of confusion and time spent about getting the initial orders right, getting them set up with a supplier, and ensuring the patient gets the equipment that was ordered. There is a lot of back and forth, which results in a delay to the patient, and the patients are upset because they are waiting for their oxygen supply,” she explained. “So, I think that physicians are very much wanting clarification to streamline the process and identify what patient systems are appropriate, which are high flow, [and] what their patients’ needs are to help physicians spend less time on this and help the patients get their oxygen set up in a timely manner.”
The study participants came from all 50 states and were 64 years of age on average and mostly women. A high percentage (39%) of the sample had chronic obstructive pulmonary disease, while 26% had interstitial lung diseases, 18% had pulmonary arterial hypertension, 8% had alpha-1 antitrypsin deficiency, and 4% had lymphangioleiomyomatosis.
Ms. Jacobs noted that she thought patients would benefit from greater physician knowledge of their prescribing options.
“A physician can dictate exactly what system they want. ... You can try to give [patients] a lighter system, a backpack, a smaller tank, more tanks per week, depending on their lifestyle and their needs. But physicians, a lot of times, like all of us and our patients, [are] not aware of all these choices,” she said, during the interview.
An online resource providing all of the pros and cons of the different types of portable oxygen systems that would be appropriate for physicians, nurses, and patients, as well as an examination of the quality standards of the oxygen suppliers, are needed, she noted
Ms. Jacobs reported no financial disclosures.
*This article was corrected June 16, 2017
AT ATS 2017
Key clinical point:
Major finding: Patients reported experiencing an average of 3.5 types of problems with their home oxygen systems.
Data source: An analysis of 1,926 home-oxygen users’ responses to an online, 60-question survey.
Disclosures: Ms. Jacobs reported no financial disclosures.
Prophylaxis prevents PCP in rheumatic disease patients
MADRID – The benefits of primary prophylaxis for pneumocystis pneumonia (PCP) outweighed the risks of treatment in patients taking prolonged, high-dose corticosteroids for various rheumatic diseases in a study presented at the European Congress of Rheumatology.
In a single-center, retrospective cohort study of 1,522 corticosteroid treatment episodes in 1,092 patients with a variety of rheumatic conditions given over a 12-year follow-up period, the estimated incidence of PCP was 2.37 per 100 person-years.
Significantly fewer cases of PCP occurred at 1 year, however, in the 262 patients who were cotreated with the antibiotic combination of trimethoprim and sulfamethoxazole (TMP-SMX), than in the 1,260 patients who received no such antibiotic prophylaxis in addition to their steroid therapy.
The adjusted hazard ratio (HR) for no PCP at 1 year of follow-up in the prophylaxis group, versus the no prophylaxis group, was 0.096 (P = .022).
The TMP-SMX combination also significantly reduced the mortality associated with PCP, with an adjusted HR of 0.09, versus no prophylaxis (P = .023).
“Pneumocystis pneumonia is a major opportunistic infection in immunocompromised patients associated with high morbidity and mortality,” explained the presenting study investigator Jun Won Park, MD, of Seoul National University Hospital in the Republic of Korea.
Dr. Park added that corticosteroid therapy was an important risk factor for PCP but that the risk-benefit ratio had not been evaluated sufficiently in patients with rheumatic diseases and that there was “different opinion among rheumatologists regarding [the value of] PCP prophylaxis.”
The current study aimed to see if primary antibiotic prophylaxis could prevent PCP in patients with rheumatic diseases, which included patients with systemic lupus erythematosus (SLE), dermatomyositis, rheumatoid arthritis, and Behçet’s disease.
For inclusion, patients had to have been treated with prednisolone at a dose of 30 mg/day or more (or its equivalent) for at least 4 weeks and observed for 1 year. Patients with a prior history of PCP or conditions associated with this opportunistic infection, such as HIV, cancer, or solid organ or hematopoietic stem cell transplantation, were excluded.
Dr. Park reported that PCP prophylaxis was given at the discretion of the treating physician, and the mean duration of TMP-SMX was 230 days.
In the prophylaxis group, 34 adverse drug reactions occurred. Two of these reactions were serious – one case of pancytopenia and one case of Steven’s Johnson syndrome – but both resolved after the antibiotic treatment was discontinued.
A sensitivity analysis was performed, giving consistent results, and a risk-benefit analysis showed that the number needed to treat to prevent one case of PCP was 52, considering all rheumatic disease studied, while the number needed to cause one serious adverse drug reaction was 131.
Taken together, these results suggest a role for TMP-SMX as primary prophylaxis for PCP in patients with rheumatic diseases who need prolonged treatment with high-dose corticosteroids, Dr. Park said.
Dr. Park reported having no relevant financial disclosures.
MADRID – The benefits of primary prophylaxis for pneumocystis pneumonia (PCP) outweighed the risks of treatment in patients taking prolonged, high-dose corticosteroids for various rheumatic diseases in a study presented at the European Congress of Rheumatology.
In a single-center, retrospective cohort study of 1,522 corticosteroid treatment episodes in 1,092 patients with a variety of rheumatic conditions given over a 12-year follow-up period, the estimated incidence of PCP was 2.37 per 100 person-years.
Significantly fewer cases of PCP occurred at 1 year, however, in the 262 patients who were cotreated with the antibiotic combination of trimethoprim and sulfamethoxazole (TMP-SMX), than in the 1,260 patients who received no such antibiotic prophylaxis in addition to their steroid therapy.
The adjusted hazard ratio (HR) for no PCP at 1 year of follow-up in the prophylaxis group, versus the no prophylaxis group, was 0.096 (P = .022).
The TMP-SMX combination also significantly reduced the mortality associated with PCP, with an adjusted HR of 0.09, versus no prophylaxis (P = .023).
“Pneumocystis pneumonia is a major opportunistic infection in immunocompromised patients associated with high morbidity and mortality,” explained the presenting study investigator Jun Won Park, MD, of Seoul National University Hospital in the Republic of Korea.
Dr. Park added that corticosteroid therapy was an important risk factor for PCP but that the risk-benefit ratio had not been evaluated sufficiently in patients with rheumatic diseases and that there was “different opinion among rheumatologists regarding [the value of] PCP prophylaxis.”
The current study aimed to see if primary antibiotic prophylaxis could prevent PCP in patients with rheumatic diseases, which included patients with systemic lupus erythematosus (SLE), dermatomyositis, rheumatoid arthritis, and Behçet’s disease.
For inclusion, patients had to have been treated with prednisolone at a dose of 30 mg/day or more (or its equivalent) for at least 4 weeks and observed for 1 year. Patients with a prior history of PCP or conditions associated with this opportunistic infection, such as HIV, cancer, or solid organ or hematopoietic stem cell transplantation, were excluded.
Dr. Park reported that PCP prophylaxis was given at the discretion of the treating physician, and the mean duration of TMP-SMX was 230 days.
In the prophylaxis group, 34 adverse drug reactions occurred. Two of these reactions were serious – one case of pancytopenia and one case of Steven’s Johnson syndrome – but both resolved after the antibiotic treatment was discontinued.
A sensitivity analysis was performed, giving consistent results, and a risk-benefit analysis showed that the number needed to treat to prevent one case of PCP was 52, considering all rheumatic disease studied, while the number needed to cause one serious adverse drug reaction was 131.
Taken together, these results suggest a role for TMP-SMX as primary prophylaxis for PCP in patients with rheumatic diseases who need prolonged treatment with high-dose corticosteroids, Dr. Park said.
Dr. Park reported having no relevant financial disclosures.
MADRID – The benefits of primary prophylaxis for pneumocystis pneumonia (PCP) outweighed the risks of treatment in patients taking prolonged, high-dose corticosteroids for various rheumatic diseases in a study presented at the European Congress of Rheumatology.
In a single-center, retrospective cohort study of 1,522 corticosteroid treatment episodes in 1,092 patients with a variety of rheumatic conditions given over a 12-year follow-up period, the estimated incidence of PCP was 2.37 per 100 person-years.
Significantly fewer cases of PCP occurred at 1 year, however, in the 262 patients who were cotreated with the antibiotic combination of trimethoprim and sulfamethoxazole (TMP-SMX), than in the 1,260 patients who received no such antibiotic prophylaxis in addition to their steroid therapy.
The adjusted hazard ratio (HR) for no PCP at 1 year of follow-up in the prophylaxis group, versus the no prophylaxis group, was 0.096 (P = .022).
The TMP-SMX combination also significantly reduced the mortality associated with PCP, with an adjusted HR of 0.09, versus no prophylaxis (P = .023).
“Pneumocystis pneumonia is a major opportunistic infection in immunocompromised patients associated with high morbidity and mortality,” explained the presenting study investigator Jun Won Park, MD, of Seoul National University Hospital in the Republic of Korea.
Dr. Park added that corticosteroid therapy was an important risk factor for PCP but that the risk-benefit ratio had not been evaluated sufficiently in patients with rheumatic diseases and that there was “different opinion among rheumatologists regarding [the value of] PCP prophylaxis.”
The current study aimed to see if primary antibiotic prophylaxis could prevent PCP in patients with rheumatic diseases, which included patients with systemic lupus erythematosus (SLE), dermatomyositis, rheumatoid arthritis, and Behçet’s disease.
For inclusion, patients had to have been treated with prednisolone at a dose of 30 mg/day or more (or its equivalent) for at least 4 weeks and observed for 1 year. Patients with a prior history of PCP or conditions associated with this opportunistic infection, such as HIV, cancer, or solid organ or hematopoietic stem cell transplantation, were excluded.
Dr. Park reported that PCP prophylaxis was given at the discretion of the treating physician, and the mean duration of TMP-SMX was 230 days.
In the prophylaxis group, 34 adverse drug reactions occurred. Two of these reactions were serious – one case of pancytopenia and one case of Steven’s Johnson syndrome – but both resolved after the antibiotic treatment was discontinued.
A sensitivity analysis was performed, giving consistent results, and a risk-benefit analysis showed that the number needed to treat to prevent one case of PCP was 52, considering all rheumatic disease studied, while the number needed to cause one serious adverse drug reaction was 131.
Taken together, these results suggest a role for TMP-SMX as primary prophylaxis for PCP in patients with rheumatic diseases who need prolonged treatment with high-dose corticosteroids, Dr. Park said.
Dr. Park reported having no relevant financial disclosures.
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: The adjusted hazard ratio (HR) for no PCP at 1 year of follow-up in the prophylaxis group, versus the no prophylaxis group, was 0.096 (P = .022).
Data source: A single-center, retrospective cohort study of 1,522 episodes of prolonged, high-dose steroid use in 1,092 patients with various rheumatic diseases.
Disclosures: Dr. Park reported having no relevant financial disclosures.
OSA in pregnancy linked to congenital anomalies
BOSTON – Newborns exposed to obstructive sleep apnea (OSA) in utero are at a higher risk of being diagnosed with congenital anomalies, according to a new study presented at the annual meeting of the Associated Professional Sleep Societies.
The researchers’ analysis covered data from more than 1.4 million births during 2010-2014. Circulatory, musculoskeletal, and central nervous systems were among the types of anomalies they saw in the 17.3% of babies born to mothers who had OSA during pregnancy. These babies were also more likely to require intensive care at birth, compared with those born to mothers who had not been diagnosed with OSA.
Additionally, the investigators found that the 0.1% of women who had a diagnosis of OSA were 2.76 times more likely to have babies that required some kind of resuscitative effort at birth. Specifically, 0.5% of the newborns of the mothers with OSA required resuscitation, compared with 0.1% of the other group’s babies. The newborns of women with OSA were also 2.25 times more likely to have a longer hospital stay.
Mothers with OSA were older and more likely to be non-Hispanic black and have a diagnosis of obesity, tobacco use, and drug use but not alcohol use.
“We can’t say for sure that sleep apnea is causing these outcomes,” said abstract presenter and principal investigator Ghada Bourjeily, MD, of Brown University and Miriam Hospital, both in Providence, R.I., in an interview.
“We know that women who have sleep apnea also often have other morbidities, so we don’t know what might have contributed to the congenital outcomes,” said Dr. Bourjeily. “We also don’t know if treating sleep apnea can reverse or prevent birth complications or even maternal complications, like preeclampsia or gestational diabetes.”
Ongoing studies are looking at maternal continuous positive airway pressure therapy use and neonatal outcomes, but “they are nothing to write home about yet,” she said.
“This is an underdiagnosed condition and it’s probably undercoded too, but we know from another study that the prevalence of OSA in the first trimester in an all-comers population that was screened for the condition is 4%,” said Dr. Bourjeily. “If another 3% of [the study participants] actually had OSA, then all of these findings are potentially underestimated.”
The majority of OSA in pregnant women that has been identified in prospective studies is mild and not necessarily something that most physicians would treat, she noted. “In our study, the ones who were diagnosed were those who probably went to their doctors and complained of sleepiness or loud snoring.”
The researchers also determined that the newborns of mothers with sleep apnea were more likely to be admitted to an intensive care unit (25.3% vs. 8.1%) or a special care nursery (34.9% vs. 13.6%).
A diagnosis of OSA was established when a diagnosis code for OSA was present on the delivery discharge record. Maternal and infant outcomes were collected for ICD-9 and procedural codes.
Dr. Bourjeily received research equipment support from Respironics.
BOSTON – Newborns exposed to obstructive sleep apnea (OSA) in utero are at a higher risk of being diagnosed with congenital anomalies, according to a new study presented at the annual meeting of the Associated Professional Sleep Societies.
The researchers’ analysis covered data from more than 1.4 million births during 2010-2014. Circulatory, musculoskeletal, and central nervous systems were among the types of anomalies they saw in the 17.3% of babies born to mothers who had OSA during pregnancy. These babies were also more likely to require intensive care at birth, compared with those born to mothers who had not been diagnosed with OSA.
Additionally, the investigators found that the 0.1% of women who had a diagnosis of OSA were 2.76 times more likely to have babies that required some kind of resuscitative effort at birth. Specifically, 0.5% of the newborns of the mothers with OSA required resuscitation, compared with 0.1% of the other group’s babies. The newborns of women with OSA were also 2.25 times more likely to have a longer hospital stay.
Mothers with OSA were older and more likely to be non-Hispanic black and have a diagnosis of obesity, tobacco use, and drug use but not alcohol use.
“We can’t say for sure that sleep apnea is causing these outcomes,” said abstract presenter and principal investigator Ghada Bourjeily, MD, of Brown University and Miriam Hospital, both in Providence, R.I., in an interview.
“We know that women who have sleep apnea also often have other morbidities, so we don’t know what might have contributed to the congenital outcomes,” said Dr. Bourjeily. “We also don’t know if treating sleep apnea can reverse or prevent birth complications or even maternal complications, like preeclampsia or gestational diabetes.”
Ongoing studies are looking at maternal continuous positive airway pressure therapy use and neonatal outcomes, but “they are nothing to write home about yet,” she said.
“This is an underdiagnosed condition and it’s probably undercoded too, but we know from another study that the prevalence of OSA in the first trimester in an all-comers population that was screened for the condition is 4%,” said Dr. Bourjeily. “If another 3% of [the study participants] actually had OSA, then all of these findings are potentially underestimated.”
The majority of OSA in pregnant women that has been identified in prospective studies is mild and not necessarily something that most physicians would treat, she noted. “In our study, the ones who were diagnosed were those who probably went to their doctors and complained of sleepiness or loud snoring.”
The researchers also determined that the newborns of mothers with sleep apnea were more likely to be admitted to an intensive care unit (25.3% vs. 8.1%) or a special care nursery (34.9% vs. 13.6%).
A diagnosis of OSA was established when a diagnosis code for OSA was present on the delivery discharge record. Maternal and infant outcomes were collected for ICD-9 and procedural codes.
Dr. Bourjeily received research equipment support from Respironics.
BOSTON – Newborns exposed to obstructive sleep apnea (OSA) in utero are at a higher risk of being diagnosed with congenital anomalies, according to a new study presented at the annual meeting of the Associated Professional Sleep Societies.
The researchers’ analysis covered data from more than 1.4 million births during 2010-2014. Circulatory, musculoskeletal, and central nervous systems were among the types of anomalies they saw in the 17.3% of babies born to mothers who had OSA during pregnancy. These babies were also more likely to require intensive care at birth, compared with those born to mothers who had not been diagnosed with OSA.
Additionally, the investigators found that the 0.1% of women who had a diagnosis of OSA were 2.76 times more likely to have babies that required some kind of resuscitative effort at birth. Specifically, 0.5% of the newborns of the mothers with OSA required resuscitation, compared with 0.1% of the other group’s babies. The newborns of women with OSA were also 2.25 times more likely to have a longer hospital stay.
Mothers with OSA were older and more likely to be non-Hispanic black and have a diagnosis of obesity, tobacco use, and drug use but not alcohol use.
“We can’t say for sure that sleep apnea is causing these outcomes,” said abstract presenter and principal investigator Ghada Bourjeily, MD, of Brown University and Miriam Hospital, both in Providence, R.I., in an interview.
“We know that women who have sleep apnea also often have other morbidities, so we don’t know what might have contributed to the congenital outcomes,” said Dr. Bourjeily. “We also don’t know if treating sleep apnea can reverse or prevent birth complications or even maternal complications, like preeclampsia or gestational diabetes.”
Ongoing studies are looking at maternal continuous positive airway pressure therapy use and neonatal outcomes, but “they are nothing to write home about yet,” she said.
“This is an underdiagnosed condition and it’s probably undercoded too, but we know from another study that the prevalence of OSA in the first trimester in an all-comers population that was screened for the condition is 4%,” said Dr. Bourjeily. “If another 3% of [the study participants] actually had OSA, then all of these findings are potentially underestimated.”
The majority of OSA in pregnant women that has been identified in prospective studies is mild and not necessarily something that most physicians would treat, she noted. “In our study, the ones who were diagnosed were those who probably went to their doctors and complained of sleepiness or loud snoring.”
The researchers also determined that the newborns of mothers with sleep apnea were more likely to be admitted to an intensive care unit (25.3% vs. 8.1%) or a special care nursery (34.9% vs. 13.6%).
A diagnosis of OSA was established when a diagnosis code for OSA was present on the delivery discharge record. Maternal and infant outcomes were collected for ICD-9 and procedural codes.
Dr. Bourjeily received research equipment support from Respironics.
AT SLEEP 2017
Key clinical point: This large cohort study is the first study to show an increased risk of congenital anomalies and resuscitation at birth in newborns born to mothers with diagnosed obstructive sleep apnea (OSA).
Major finding: Of babies born to a mother with OSA, 17.3% had a congenital anomaly, compared with 10.6% of those born to mothers without OSA (P less than .001). This difference remained significant after adjusting for potential confounders.
Data source: A national cohort study including more than 1.4 million linked maternal and newborn records with a delivery hospitalization during 2010-2014.
Disclosures: Dr. Bourjeily received research equipment support from Respironics.