User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Effect of vitamin D supplementation in early psychosis
Low vitamin D is common in patients with first-episode psychosis (FEP), but supplementation does not appear to improve mental or physical symptoms, new data show.
“Previous work, our own and others, has shown that people with psychosis, even soon after their first diagnosis, have low vitamin D levels, but it was not known whether supplementing with vitamin D in people with early psychosis would improve health outcomes,” study investigator Fiona Gaughran, MD, with the Institute of Psychiatry, Psychology & Neuroscience, King’s College London, told this news organization.
“While we did not demonstrate a benefit of supplementation over 6 months, these very high rates of vitamin deficiency and insufficiency may have longer-term negative health impacts which we have not measured, so raising awareness of the need to optimize vitamin D in people with psychosis is important,” said Dr. Gaughran.
The results of the randomized clinical trial were published online Dec. 28 in JAMA Network Open.
Thoughtful approach, negative result
Participants included 149 adults within 3 years of a first presentation with a functional psychotic disorder. The cohort’s mean age was 28 years, 60% were men, 44% were Black or of other racial and ethnic minority groups, and 56% were White.
Seventy-five participants were randomly assigned to receive 120,000 IU of cholecalciferol or matching placebo administered by the researchers in monthly doses with an oral syringe.
“We chose a dose of 120,000 IU monthly (equivalent to 4,000 IU daily) which was expected to safely increase vitamin D levels. The regimen was discussed with experts with lived experience, and took into account that a daily preparation would add to the significant medication load that people with psychosis already carry,” said Dr. Gaughran.
Vitamin D supplementation as administered in this study was safe and led to a significant increase in 25-hydroxyvitamin D concentrations.
However, at 6 months (mean difference, 3.57; 95% confidence interval, –1.11 to 8.25; P = .13).
There was also no apparent benefit of vitamin D supplementation on any secondary outcome, including the PANSS subscores of global function and depression or cardiometabolic risk factors.
“With respect to clinical practice, we cannot now recommend monthly treatments with 120,000 IU of cholecalciferol in FEP,” the investigators note.
The prevalence of vitamin D insufficiency and deficiency was high in the population – 74.6% overall and 93.4% among ethnic minorities.
“Thus, the sample was well suited to detecting any potential benefits that may have arisen from correcting this. However, even in this subgroup, there was no evidence to support the guiding hypothesis” that vitamin D supplementation would improve outcomes in patients with early psychosis, the researchers note.
They suggest that future studies examine the association of vitamin D with brain-related outcomes based on periods of treatment longer than 6 months and administered as daily rather than bolus treatments.
“Future public health strategies should acknowledge the high prevalence of vitamin D insufficiency and deficiency in people with psychosis and consider any reasonable adjustments which may be needed to address this over and above general population guidance,” said Dr. Gaughran.
The study was funded by the Stanley Medical Research Institute and received support from the National Institute for Health Research Maudsley Biomedical Research Centre, King’s College London, and the NIHR Applied Research Collaboration South London. Dr. Gaughran reported receiving speaking honoraria from Otsuka Lundbeck outside the submitted work. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Low vitamin D is common in patients with first-episode psychosis (FEP), but supplementation does not appear to improve mental or physical symptoms, new data show.
“Previous work, our own and others, has shown that people with psychosis, even soon after their first diagnosis, have low vitamin D levels, but it was not known whether supplementing with vitamin D in people with early psychosis would improve health outcomes,” study investigator Fiona Gaughran, MD, with the Institute of Psychiatry, Psychology & Neuroscience, King’s College London, told this news organization.
“While we did not demonstrate a benefit of supplementation over 6 months, these very high rates of vitamin deficiency and insufficiency may have longer-term negative health impacts which we have not measured, so raising awareness of the need to optimize vitamin D in people with psychosis is important,” said Dr. Gaughran.
The results of the randomized clinical trial were published online Dec. 28 in JAMA Network Open.
Thoughtful approach, negative result
Participants included 149 adults within 3 years of a first presentation with a functional psychotic disorder. The cohort’s mean age was 28 years, 60% were men, 44% were Black or of other racial and ethnic minority groups, and 56% were White.
Seventy-five participants were randomly assigned to receive 120,000 IU of cholecalciferol or matching placebo administered by the researchers in monthly doses with an oral syringe.
“We chose a dose of 120,000 IU monthly (equivalent to 4,000 IU daily) which was expected to safely increase vitamin D levels. The regimen was discussed with experts with lived experience, and took into account that a daily preparation would add to the significant medication load that people with psychosis already carry,” said Dr. Gaughran.
Vitamin D supplementation as administered in this study was safe and led to a significant increase in 25-hydroxyvitamin D concentrations.
However, at 6 months (mean difference, 3.57; 95% confidence interval, –1.11 to 8.25; P = .13).
There was also no apparent benefit of vitamin D supplementation on any secondary outcome, including the PANSS subscores of global function and depression or cardiometabolic risk factors.
“With respect to clinical practice, we cannot now recommend monthly treatments with 120,000 IU of cholecalciferol in FEP,” the investigators note.
The prevalence of vitamin D insufficiency and deficiency was high in the population – 74.6% overall and 93.4% among ethnic minorities.
“Thus, the sample was well suited to detecting any potential benefits that may have arisen from correcting this. However, even in this subgroup, there was no evidence to support the guiding hypothesis” that vitamin D supplementation would improve outcomes in patients with early psychosis, the researchers note.
They suggest that future studies examine the association of vitamin D with brain-related outcomes based on periods of treatment longer than 6 months and administered as daily rather than bolus treatments.
“Future public health strategies should acknowledge the high prevalence of vitamin D insufficiency and deficiency in people with psychosis and consider any reasonable adjustments which may be needed to address this over and above general population guidance,” said Dr. Gaughran.
The study was funded by the Stanley Medical Research Institute and received support from the National Institute for Health Research Maudsley Biomedical Research Centre, King’s College London, and the NIHR Applied Research Collaboration South London. Dr. Gaughran reported receiving speaking honoraria from Otsuka Lundbeck outside the submitted work. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Low vitamin D is common in patients with first-episode psychosis (FEP), but supplementation does not appear to improve mental or physical symptoms, new data show.
“Previous work, our own and others, has shown that people with psychosis, even soon after their first diagnosis, have low vitamin D levels, but it was not known whether supplementing with vitamin D in people with early psychosis would improve health outcomes,” study investigator Fiona Gaughran, MD, with the Institute of Psychiatry, Psychology & Neuroscience, King’s College London, told this news organization.
“While we did not demonstrate a benefit of supplementation over 6 months, these very high rates of vitamin deficiency and insufficiency may have longer-term negative health impacts which we have not measured, so raising awareness of the need to optimize vitamin D in people with psychosis is important,” said Dr. Gaughran.
The results of the randomized clinical trial were published online Dec. 28 in JAMA Network Open.
Thoughtful approach, negative result
Participants included 149 adults within 3 years of a first presentation with a functional psychotic disorder. The cohort’s mean age was 28 years, 60% were men, 44% were Black or of other racial and ethnic minority groups, and 56% were White.
Seventy-five participants were randomly assigned to receive 120,000 IU of cholecalciferol or matching placebo administered by the researchers in monthly doses with an oral syringe.
“We chose a dose of 120,000 IU monthly (equivalent to 4,000 IU daily) which was expected to safely increase vitamin D levels. The regimen was discussed with experts with lived experience, and took into account that a daily preparation would add to the significant medication load that people with psychosis already carry,” said Dr. Gaughran.
Vitamin D supplementation as administered in this study was safe and led to a significant increase in 25-hydroxyvitamin D concentrations.
However, at 6 months (mean difference, 3.57; 95% confidence interval, –1.11 to 8.25; P = .13).
There was also no apparent benefit of vitamin D supplementation on any secondary outcome, including the PANSS subscores of global function and depression or cardiometabolic risk factors.
“With respect to clinical practice, we cannot now recommend monthly treatments with 120,000 IU of cholecalciferol in FEP,” the investigators note.
The prevalence of vitamin D insufficiency and deficiency was high in the population – 74.6% overall and 93.4% among ethnic minorities.
“Thus, the sample was well suited to detecting any potential benefits that may have arisen from correcting this. However, even in this subgroup, there was no evidence to support the guiding hypothesis” that vitamin D supplementation would improve outcomes in patients with early psychosis, the researchers note.
They suggest that future studies examine the association of vitamin D with brain-related outcomes based on periods of treatment longer than 6 months and administered as daily rather than bolus treatments.
“Future public health strategies should acknowledge the high prevalence of vitamin D insufficiency and deficiency in people with psychosis and consider any reasonable adjustments which may be needed to address this over and above general population guidance,” said Dr. Gaughran.
The study was funded by the Stanley Medical Research Institute and received support from the National Institute for Health Research Maudsley Biomedical Research Centre, King’s College London, and the NIHR Applied Research Collaboration South London. Dr. Gaughran reported receiving speaking honoraria from Otsuka Lundbeck outside the submitted work. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Too close for comfort: When the psychiatrist is stalked
Dr. A has been treating Ms. W, a graduate student, for depression. Ms. W made subtle comments expressing her interest in pursuing a romantic relationship with her psychiatrist. Dr. A gently redirected her, and she seemed to respond appropriately. However, over the past 2 weeks, Dr. A has seen Ms. W at a local park and at the grocery store. Today, Dr. A is startled to see Ms. W at her weekly yoga class. Dr. A plans to ask her supervisor for advice.
Dr. M is a child psychiatrist who spoke at his local school board meeting in support of masking requirements for students during COVID-19. During the discussion, Dr. M shared that, as a psychiatrist, he does not believe it is especially distressing for students to wear masks, and that doing so is a necessary public health measure. On leaving, other parents shouted, “We know who you are and where you live!” The next day, his integrated clinic started receiving threatening and harassing messages, including threats to kill him or his staff if they take part in vaccinating children against COVID-19.
Because of their work, mental health professionals—like other health care professionals—face an elevated risk of being harassed or stalked. Stalking often includes online harassment and may escalate to serious physical violence. Stalking is criminal behavior by a patient and should not be constructed as a “failure to manage transference.” This article explores basic strategies to reduce the risk of harassment and stalking, describes how to recognize early behaviors, and outlines basic steps health care professionals and their employers can take to respond to stalking and harassing behaviors.
Although this article is intended for psychiatrists, it is important to note that all health professionals have significant risk for experiencing stalking or harassment. This is due in part, but not exclusively, to our clinical work. Estimates of how many health professionals experience stalking vary substantially depending upon the study, and differences in methodologies limit easy comparison or extrapolation. More thorough reviews have reported ranges from 2% to 70% among physicians; psychiatrists and other mental health professionals appear to be at greater risk than those in other specialties and the general population.1-3 Physicians who are active on social media may also be at elevated risk.4 Unexpected communications from patients and their family members—especially those with threatening, harassing, or sexualized tones, or involving contact outside of a work setting—can be distressing. These behaviors represent potential harbingers of more dangerous behavior, including physical assault, sexual assault, or homicide. Despite their elevated risk, many psychiatrists are unaware of how to prevent or respond to stalking or harassment.
Recognizing harassment and stalking
Repeated and unwanted contact or communication, regardless of intent, may constitute stalking. Legal definitions vary by jurisdiction and may not align with subjective experiences or understanding of what constitutes stalking.5 At its essence, stalking is repeated harassing behaviors likely to provoke fear in the targeted person. FOUR is a helpful mnemonic when conceptualizing the attributes of stalking: Fixated, Obsessive, Unwanted, and Repetitive.6Table 1 lists examples of common stalking behaviors. Stalking and harassing behavior may be from a known source (eg, a patient, coworker, or paramour), a masked source (ie, someone known to the target but who conceals or obscures their identity), or from otherwise unknown persons. Behaviors that persist after the person engaging in the behaviors has clearly been informed that they are unwanted or inappropriate are especially concerning. Stalking may escalate to include physical or sexual assault and, in some cases, homicide.

Stalking duration can vary substantially, as can the factors that lead to the cessation of the behavior. Indicators of increased risk for physical violence include unwanted physical presence/following of the target (“approach behaviors”), having a prior violent intimate relationship, property destruction, explicit threats, and having a prior intimate relationship with the target.7
Stalking contact or communication may be unwanted because of the content (eg, sexualized or threatening tone), location (eg, at a professional’s home), or means (eg, through social media). Stalking behaviors are not appropriate in any relationship, including a clinical relationship. They should not be treated as a “failure to manage transference” or in other victim-blaming ways.
There are multiple typologies for stalking behavior. Common motivations for stalking health professionals include resentment or grievance, misjudgment of social boundaries, and delusional fixation, including erotomania.8 Associated psychopathologies vary significantly and, while some may be more amenable to psychiatric treatment than others, psychiatrists should not feel compelled to treat patients who repeatedly violate boundaries, regardless of intent or comorbidity.
Patients are not the exclusive perpetrators of stalking; a recent study found that 4% of physicians surveyed reported current or recent stalking by a current or former intimate partner.9 When a person who is a victim of intimate partner violence is also stalked as part of the abuse, homicide risk increases.10 Workplace homicides of health care professionals are most likely to be committed by a current or former partner or other personal acquaintance, not by a patient.11 Workplace harassment and stalking of health care professionals is especially concerning because this behavior can escalate and endanger coworkers or clients.
Continue to: Risk awareness: Recognize your exposure...
Risk awareness: Recognize your exposure
About 80% of stalking involves some form of technology—often telephone calls but also online or other “cyber” elements.12 One recent survey found the rate of online harassment, including threats of physical and sexual violence, was >20% among physicians who were active on social media.4 Health professionals may be at greater risk of having patients find their personal information simply because patients routinely search online for information about new clinicians. Personal information about a clinician may be readily visible among professional information in search results, or a curious patient may simply scroll further down in the results. For a potential stalker, clicking on a search result linking to a personal social media page may be far easier than finding a home address and going in person—but the action may be just as distressing or risky for the clinician.13 Additionally, items visible in a clinician’s office—or visible in the background of those providing telehealth services from their home—may inadvertently reveal personal information about the clinician, their home, or their family.
Psychiatrists are often in a special position in relation to patients and times of crises. They may be involved in involuntary commitment—or declining an admission when a patient or family wishes it. They may be present at the time of the revelation of a serious diagnosis, abuse, injury, or death. They may be a mandated reporter of child or elder abuse.2 Additionally, physicians may be engaged in discourse on politically charged public health topics.14 These factors may increase their risk of being stalked.
Conducting an online visibility self-assessment can be a useful way to learn what information others can find. Table 2 outlines the steps for completing this exercise. Searching multiple iterations of your current and former names (with and without degrees, titles, and cities) will yield differing results in various search engines. After establishing a baseline of what information is available online, it can be helpful to periodically repeat this exercise, and to set up automated alerts for your name, number(s), email(s), and address(es).

Basic mitigation strategies
In the modern era, being invisible online is impractical and likely impossible—especially for a health care professional. Instead, it may be prudent to limit your public visibility to professional portals (eg, LinkedIn or Doximity) and maximize privacy settings on other platforms. Another basic strategy is to avoid providing personal contact information (your home address, phone number, or personal email) for professional purposes, such as licensing and credentialing, conference submissions, or journal publications. Be aware that driving a visually distinct vehicle—one with vanity plates or distinct bumper stickers, or an exotic sportscar—can make it easier to be recognized and located. A personally recorded voicemail greeting (vs one recorded by, for example, an office manager) may be inappropriately reinforcing for some stalkers.
Workplaces should have an established safety policy that addresses stalking and harassment of employees. Similarly, patients and others should receive clear education on how to contact different staff, including physicians, with consideration of how and when to use electronic health information portals, office numbers, and emails. Workplaces should not disclose staff schedules. For example, a receptionist should say “I’ll have Dr. Diaz return your call when she can” instead of “Dr. Diaz is not in until tomorrow.” Avoid unnecessary location/name signals (eg, a parking spot labeled “Dr. Diaz”). Consider creating alert words or phrases for staff to use to signal they are concerned about their immediate safety—and provide education and training, including drills, to test emergency responses when the words/phrases are used. Leaders and managers should nurture a workplace culture where people are comfortable seeking support if they feel they may be the target of harassment or stalking. Many larger health care organizations have threat management programs, which can play a critical role in preventing, investigating, and responding to stalking of employees. Increasingly, threat management teams are being identified as a best practice in health care settings.15Table 3 summarizes measures to mitigate risk.
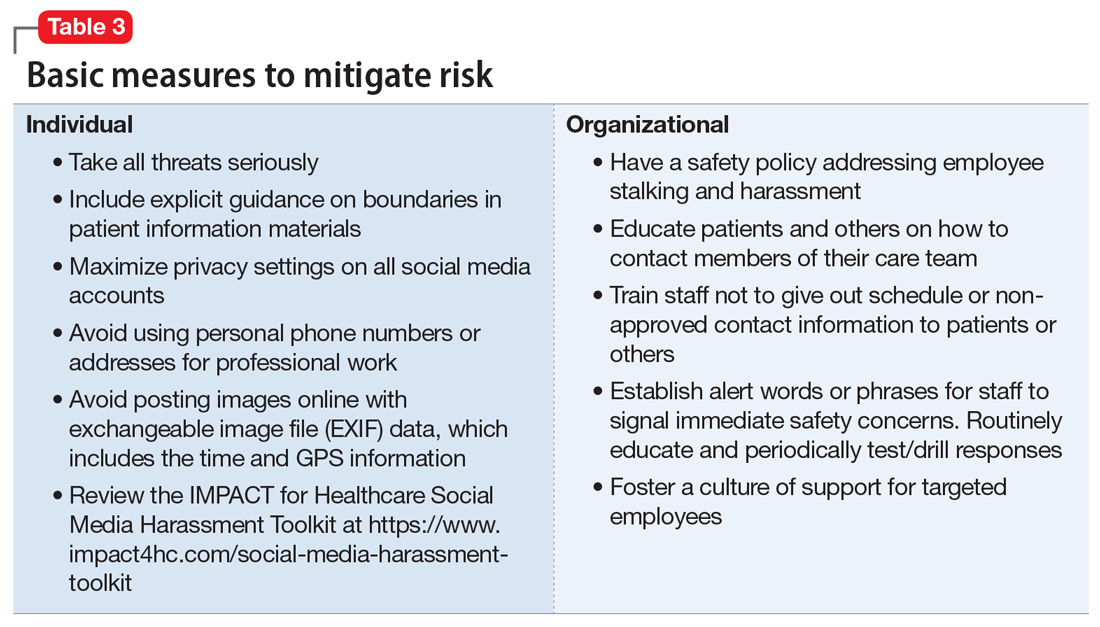
What to do when harassment or stalking occurs
Consulting with subject matter experts is essential. Approach behaviors, stalking patterns, and immediate circumstances vary highly, and so too must responses. A socially inept approach outside of the work setting by a patient may be effectively responded to with a firm explanation of why the behavior was inappropriate and a reiteration of limits. More persistent or serious threats may require taking actions for immediate safety, calling law enforcement or security (who may have the expertise to assist appropriately), or even run/hide/fight measures. Others to notify early on include human resources, supervisors, front desk staff, and coworkers. Although no single measure is always indicated and no single measure will always be effective, consultation with a specialist is always advisable.
Attempting to assess your own risk may be subject to bias and error, even for an experienced forensic psychiatrist. Risk assessment in stalking and harassment cases is complex, nuanced, and beyond the scope of this article; engagement with specialized threat programs or subject matter experts is advisable.15,16 If your medical center or area has police or security officers, engage them early. Risk management, insurers, and legal can also be helpful to consult. Attorneys specializing in harassment, stalking, and domestic violence may be helpful in extreme situations.17Table 417,18 highlights steps to take.
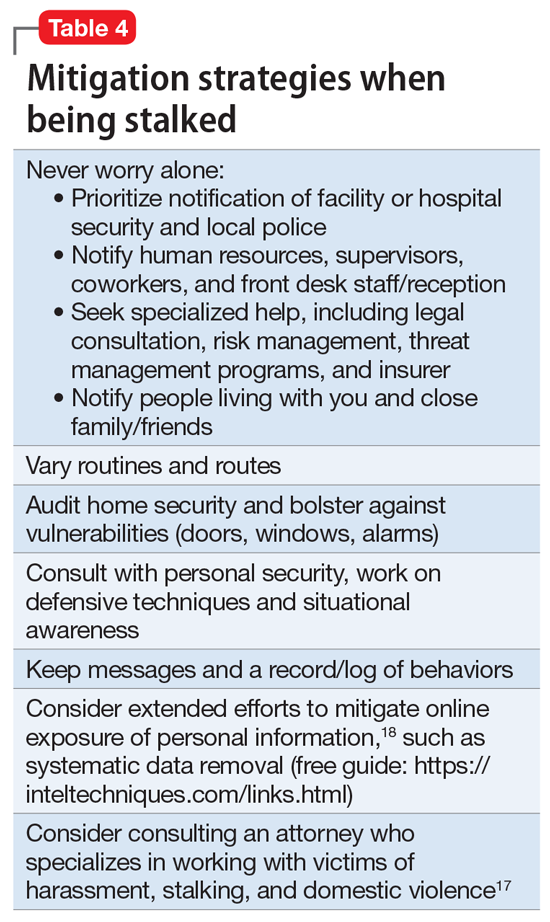
While effective interventions to stop or redirect stalking behavior may vary, some initial considerations include changing established routines (eg, your parking location or daily/weekly patterns such as gym, class, etc.) and letting family and others you live with know what is occurring. Consider implementing and bolstering personal, work, and home security; honing situational awareness skills; and learning advanced situational awareness and self-defense techniques.
Continue to: Clinical documentation and termination of care...
Clinical documentation and termination of care
Repeated and unwanted contact behaviors by a patient may be considered grounds for termination of care by the targeted clinician. Termination may occur through a direct conversation, followed by a mailed letter explaining that the patient’s inappropriate behaviors are the basis for termination. The letter should outline steps for establishing care with another psychiatrist and signing a release to facilitate transfer of records to the next psychiatrist. Ensure that the patient has access to a reasonable supply of medications or refills according to jurisdictional standards for transfer or termination of care.19 While these are common legal standards for termination of care in the United States, clinicians would be well served by appropriate consultation to verify the most appropriate standards for their location.
Documentation of a patient’s behavior should be factual and clear. Under the 21st Century Cures Act, patients often have access to their own electronic records.20 Therefore, clinicians should avoid documenting personal security measures or other information that is not clinically relevant. Communications with legal or risk management should not be documented unless otherwise advised, because such communications may be privileged and may not be clinically relevant.
In some circumstances, continuing to treat a patient who has stalked a member of the current treatment team may be appropriate or necessary. For example, a patient may respond appropriately to redirection after an initial approach behavior and continue to make clinical progress, or may be in a forensic specialty setting with appropriate operational support to continue with treatment.
Ethical dilemmas may arise in underserved areas where there are limited options for psychiatric care and in communicating the reasons for termination to a new clinician. Consultation may help to address these issues. However, as noted before, clinicians should be permitted to discontinue and transfer treatment and should not be compelled to continue to treat a patient who has threatened or harassed them.
Organizational and employer considerations
Victims of stalking have reported that they appreciated explicit support from their supervisor, regular meetings, and measures to reduce potential stalking or violence in the workplace; unsurprisingly, victim blaming and leaving the employee to address the situation on their own were labeled experienced as negative.2 Employers may consider implementing physical security, access controls and panic alarms, and enhancing coworkers’ situational awareness.21 Explicit policies about and attention to reducing workplace violence, including stalking, are always beneficial—and in some settings such policies may be a regulatory requirement.22 Large health care organizations may benefit from developing specialized threat management programs to assist with the evaluation and mitigation of stalking and other workplace violence risks.15,23
Self-care considerations
The impact of stalking can include psychological distress, disruption of work and personal relationships, and false allegations of impropriety. Stalking can make targets feel isolated, violated, and fearful, which makes it challenging to reach out to others for support and safety. It takes time to regain a sense of safety and to find a “new normal,” particularly while experiencing and responding to stalking behavior. Notifying close personal contacts such as family and coworkers about what is occurring (without sharing protected health information) can be helpful for recovery and important for the clinician’s safety. Reaching out for organizational and legal supports is also prudent. It is also important to allow time for, and patience with, a targeted individual’s normal responses, such as decreased work performance, sleep/appetite changes, and hypervigilance, without pathologizing these common stress reactions. Further review of appropriate resources by impacted clinicians is advisable.24-26
1. Nelsen AJ, Johnson RS, Ostermeyer B, et al. The prevalence of physicians who have been stalked: a systematic review. J Am Acad Psychiatry Law. 2015;43(2):177-182.
2. Jutasi C, McEwan TE. Stalking of professionals: a scoping review. Journal of Threat Assessment and Management. 2021;8(3):94-124.
3. Pathé MT, Meloy JR. Commentary: Stalking by patients—psychiatrists’ tales of anger, lust and ignorance. J Am Acad Psychiatry Law. 2013;41(2):200-205.
4. Pendergrast TR, Jain S, Trueger NS, et al. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552.
5. Owens JG. Why definitions matter: stalking victimization in the United States. J Interpers Violence. 2016;31(12):2196-2226.
6. College of Policing. Stalking or harassment. May 2019. Accessed March 8, 2020. https://library.college.police.uk/docs/college-of-policing/Stalking_or_harassment_guidance_200519.pdf
7. McEwan TE, Daffern M, MacKenzie RD, et al. Risk factors for stalking violence, persistence, and recurrence. Journal of Forensic Psychiatry & Psychology. 2017;28(1):3856.
8. Pathé MT, Mullen PE, Purcell R. Patients who stalk doctors: their motives and management. Med J Australia. 2002;176(7):335-338.
9. Reibling ET, Distelberg B, Guptill M, et al. Intimate partner violence experienced by physicians. J Prim Care Community Health. 2020;11:2150132720965077.
10. Matias A, Gonçalves M, Soeiro C, et al. Intimate partner homicide: a meta-analysis of risk factors. Aggression and Violent Behavior. 2019;50:101358.
11. US Bureau of Labor Statistics. Fact sheet. Workplace violence in healthcare, 2018. April 2020. Accessed November 24, 2021. https://www.bls.gov/iif/oshwc/cfoi/workplace-violence-healthcare-2018.htm
12. Truman JL, Morgan RE. Stalking victimization, 2016. Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice. Report No.: NCJ 253526. April 2021. Accessed November 24, 2021. https://bjs.ojp.gov/library/publications/stalking-victimization-2016
13. Reyns BW, Henson B, Fisher BS. Being pursued online: applying cyberlifestyle–routine activities theory to cyberstalking victimization. Criminal Justice and Behavior. 2011;38(11):1149-1169.
14. Stea JN. When promoting knowledge makes you a target. Scientific American Blog Network. March 16, 2020. Accessed November 24, 2021. https://blogs.scientificamerican.com/observations/when-promoting-knowledge-makes-you-a-target/
15. Henkel SJ. Threat assessment strategies to mitigate violence in healthcare. IAHSS Foundation. IAHSS-F RS-19-02. November 2019. Accessed November 24, 2021. https://iahssf.org/assets/IAHSS-Foundation-Threat-Assessment-Strategies-to-Mitigate-Violence-in-Healthcare.pdf
16. McEwan TE. Stalking threat and risk assessment. In: Reid Meloy J, Hoffman J (eds). International Handbook of Threat Assessment. 2nd ed. Oxford University Press; 2021:210-234.
17. Goldberg C. Nobody’s Victim: Fighting Psychos, Stalkers, Pervs, and Trolls. Plume; 2019.
18. Bazzell M. Extreme Privacy: What It Takes to Disappear. 2nd ed. Independently published; 2020.
19. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
20. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program Final Rule (To be codified at 45 CFR 170 and 171). Federal Register. 2020;85(85):25642-25961.
21. Sheridan L, North AC, Scott AJ. Stalking in the workplace. Journal of Threat Assessment and Management. 2019;6(2):61-75.
22. The Joint Commission. Workplace Violence Prevention Standards. R3 Report: Requirement, Rationale, Reference. Issue 30. June 18, 2021. Accessed November 24, 2021. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/wpvp-r3-30_revised_06302021.pdf
23. Terry LP. Threat assessment teams. J Healthc Prot Manage. 2015;31(2):23-35.
24. Pathé M. Surviving Stalking. Cambridge University Press; 2002.
25. Noffsinger S. What stalking victims need to restore their mental and somatic health. Current Psychiatry. 2015;14(6):43-47.
26. Mullen P, Whyte S, McIvor R; Psychiatrists’ Support Service, Royal College of Psychiatry. PSS Information Guide: Stalking. Report No. 11. 2017. Accessed November 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/members/supporting-you/pss/pss-guide-11-stalking.pdf?sfvrsn=2f1c7253_2
Dr. A has been treating Ms. W, a graduate student, for depression. Ms. W made subtle comments expressing her interest in pursuing a romantic relationship with her psychiatrist. Dr. A gently redirected her, and she seemed to respond appropriately. However, over the past 2 weeks, Dr. A has seen Ms. W at a local park and at the grocery store. Today, Dr. A is startled to see Ms. W at her weekly yoga class. Dr. A plans to ask her supervisor for advice.
Dr. M is a child psychiatrist who spoke at his local school board meeting in support of masking requirements for students during COVID-19. During the discussion, Dr. M shared that, as a psychiatrist, he does not believe it is especially distressing for students to wear masks, and that doing so is a necessary public health measure. On leaving, other parents shouted, “We know who you are and where you live!” The next day, his integrated clinic started receiving threatening and harassing messages, including threats to kill him or his staff if they take part in vaccinating children against COVID-19.
Because of their work, mental health professionals—like other health care professionals—face an elevated risk of being harassed or stalked. Stalking often includes online harassment and may escalate to serious physical violence. Stalking is criminal behavior by a patient and should not be constructed as a “failure to manage transference.” This article explores basic strategies to reduce the risk of harassment and stalking, describes how to recognize early behaviors, and outlines basic steps health care professionals and their employers can take to respond to stalking and harassing behaviors.
Although this article is intended for psychiatrists, it is important to note that all health professionals have significant risk for experiencing stalking or harassment. This is due in part, but not exclusively, to our clinical work. Estimates of how many health professionals experience stalking vary substantially depending upon the study, and differences in methodologies limit easy comparison or extrapolation. More thorough reviews have reported ranges from 2% to 70% among physicians; psychiatrists and other mental health professionals appear to be at greater risk than those in other specialties and the general population.1-3 Physicians who are active on social media may also be at elevated risk.4 Unexpected communications from patients and their family members—especially those with threatening, harassing, or sexualized tones, or involving contact outside of a work setting—can be distressing. These behaviors represent potential harbingers of more dangerous behavior, including physical assault, sexual assault, or homicide. Despite their elevated risk, many psychiatrists are unaware of how to prevent or respond to stalking or harassment.
Recognizing harassment and stalking
Repeated and unwanted contact or communication, regardless of intent, may constitute stalking. Legal definitions vary by jurisdiction and may not align with subjective experiences or understanding of what constitutes stalking.5 At its essence, stalking is repeated harassing behaviors likely to provoke fear in the targeted person. FOUR is a helpful mnemonic when conceptualizing the attributes of stalking: Fixated, Obsessive, Unwanted, and Repetitive.6Table 1 lists examples of common stalking behaviors. Stalking and harassing behavior may be from a known source (eg, a patient, coworker, or paramour), a masked source (ie, someone known to the target but who conceals or obscures their identity), or from otherwise unknown persons. Behaviors that persist after the person engaging in the behaviors has clearly been informed that they are unwanted or inappropriate are especially concerning. Stalking may escalate to include physical or sexual assault and, in some cases, homicide.

Stalking duration can vary substantially, as can the factors that lead to the cessation of the behavior. Indicators of increased risk for physical violence include unwanted physical presence/following of the target (“approach behaviors”), having a prior violent intimate relationship, property destruction, explicit threats, and having a prior intimate relationship with the target.7
Stalking contact or communication may be unwanted because of the content (eg, sexualized or threatening tone), location (eg, at a professional’s home), or means (eg, through social media). Stalking behaviors are not appropriate in any relationship, including a clinical relationship. They should not be treated as a “failure to manage transference” or in other victim-blaming ways.
There are multiple typologies for stalking behavior. Common motivations for stalking health professionals include resentment or grievance, misjudgment of social boundaries, and delusional fixation, including erotomania.8 Associated psychopathologies vary significantly and, while some may be more amenable to psychiatric treatment than others, psychiatrists should not feel compelled to treat patients who repeatedly violate boundaries, regardless of intent or comorbidity.
Patients are not the exclusive perpetrators of stalking; a recent study found that 4% of physicians surveyed reported current or recent stalking by a current or former intimate partner.9 When a person who is a victim of intimate partner violence is also stalked as part of the abuse, homicide risk increases.10 Workplace homicides of health care professionals are most likely to be committed by a current or former partner or other personal acquaintance, not by a patient.11 Workplace harassment and stalking of health care professionals is especially concerning because this behavior can escalate and endanger coworkers or clients.
Continue to: Risk awareness: Recognize your exposure...
Risk awareness: Recognize your exposure
About 80% of stalking involves some form of technology—often telephone calls but also online or other “cyber” elements.12 One recent survey found the rate of online harassment, including threats of physical and sexual violence, was >20% among physicians who were active on social media.4 Health professionals may be at greater risk of having patients find their personal information simply because patients routinely search online for information about new clinicians. Personal information about a clinician may be readily visible among professional information in search results, or a curious patient may simply scroll further down in the results. For a potential stalker, clicking on a search result linking to a personal social media page may be far easier than finding a home address and going in person—but the action may be just as distressing or risky for the clinician.13 Additionally, items visible in a clinician’s office—or visible in the background of those providing telehealth services from their home—may inadvertently reveal personal information about the clinician, their home, or their family.
Psychiatrists are often in a special position in relation to patients and times of crises. They may be involved in involuntary commitment—or declining an admission when a patient or family wishes it. They may be present at the time of the revelation of a serious diagnosis, abuse, injury, or death. They may be a mandated reporter of child or elder abuse.2 Additionally, physicians may be engaged in discourse on politically charged public health topics.14 These factors may increase their risk of being stalked.
Conducting an online visibility self-assessment can be a useful way to learn what information others can find. Table 2 outlines the steps for completing this exercise. Searching multiple iterations of your current and former names (with and without degrees, titles, and cities) will yield differing results in various search engines. After establishing a baseline of what information is available online, it can be helpful to periodically repeat this exercise, and to set up automated alerts for your name, number(s), email(s), and address(es).

Basic mitigation strategies
In the modern era, being invisible online is impractical and likely impossible—especially for a health care professional. Instead, it may be prudent to limit your public visibility to professional portals (eg, LinkedIn or Doximity) and maximize privacy settings on other platforms. Another basic strategy is to avoid providing personal contact information (your home address, phone number, or personal email) for professional purposes, such as licensing and credentialing, conference submissions, or journal publications. Be aware that driving a visually distinct vehicle—one with vanity plates or distinct bumper stickers, or an exotic sportscar—can make it easier to be recognized and located. A personally recorded voicemail greeting (vs one recorded by, for example, an office manager) may be inappropriately reinforcing for some stalkers.
Workplaces should have an established safety policy that addresses stalking and harassment of employees. Similarly, patients and others should receive clear education on how to contact different staff, including physicians, with consideration of how and when to use electronic health information portals, office numbers, and emails. Workplaces should not disclose staff schedules. For example, a receptionist should say “I’ll have Dr. Diaz return your call when she can” instead of “Dr. Diaz is not in until tomorrow.” Avoid unnecessary location/name signals (eg, a parking spot labeled “Dr. Diaz”). Consider creating alert words or phrases for staff to use to signal they are concerned about their immediate safety—and provide education and training, including drills, to test emergency responses when the words/phrases are used. Leaders and managers should nurture a workplace culture where people are comfortable seeking support if they feel they may be the target of harassment or stalking. Many larger health care organizations have threat management programs, which can play a critical role in preventing, investigating, and responding to stalking of employees. Increasingly, threat management teams are being identified as a best practice in health care settings.15Table 3 summarizes measures to mitigate risk.

What to do when harassment or stalking occurs
Consulting with subject matter experts is essential. Approach behaviors, stalking patterns, and immediate circumstances vary highly, and so too must responses. A socially inept approach outside of the work setting by a patient may be effectively responded to with a firm explanation of why the behavior was inappropriate and a reiteration of limits. More persistent or serious threats may require taking actions for immediate safety, calling law enforcement or security (who may have the expertise to assist appropriately), or even run/hide/fight measures. Others to notify early on include human resources, supervisors, front desk staff, and coworkers. Although no single measure is always indicated and no single measure will always be effective, consultation with a specialist is always advisable.
Attempting to assess your own risk may be subject to bias and error, even for an experienced forensic psychiatrist. Risk assessment in stalking and harassment cases is complex, nuanced, and beyond the scope of this article; engagement with specialized threat programs or subject matter experts is advisable.15,16 If your medical center or area has police or security officers, engage them early. Risk management, insurers, and legal can also be helpful to consult. Attorneys specializing in harassment, stalking, and domestic violence may be helpful in extreme situations.17Table 417,18 highlights steps to take.

While effective interventions to stop or redirect stalking behavior may vary, some initial considerations include changing established routines (eg, your parking location or daily/weekly patterns such as gym, class, etc.) and letting family and others you live with know what is occurring. Consider implementing and bolstering personal, work, and home security; honing situational awareness skills; and learning advanced situational awareness and self-defense techniques.
Continue to: Clinical documentation and termination of care...
Clinical documentation and termination of care
Repeated and unwanted contact behaviors by a patient may be considered grounds for termination of care by the targeted clinician. Termination may occur through a direct conversation, followed by a mailed letter explaining that the patient’s inappropriate behaviors are the basis for termination. The letter should outline steps for establishing care with another psychiatrist and signing a release to facilitate transfer of records to the next psychiatrist. Ensure that the patient has access to a reasonable supply of medications or refills according to jurisdictional standards for transfer or termination of care.19 While these are common legal standards for termination of care in the United States, clinicians would be well served by appropriate consultation to verify the most appropriate standards for their location.
Documentation of a patient’s behavior should be factual and clear. Under the 21st Century Cures Act, patients often have access to their own electronic records.20 Therefore, clinicians should avoid documenting personal security measures or other information that is not clinically relevant. Communications with legal or risk management should not be documented unless otherwise advised, because such communications may be privileged and may not be clinically relevant.
In some circumstances, continuing to treat a patient who has stalked a member of the current treatment team may be appropriate or necessary. For example, a patient may respond appropriately to redirection after an initial approach behavior and continue to make clinical progress, or may be in a forensic specialty setting with appropriate operational support to continue with treatment.
Ethical dilemmas may arise in underserved areas where there are limited options for psychiatric care and in communicating the reasons for termination to a new clinician. Consultation may help to address these issues. However, as noted before, clinicians should be permitted to discontinue and transfer treatment and should not be compelled to continue to treat a patient who has threatened or harassed them.
Organizational and employer considerations
Victims of stalking have reported that they appreciated explicit support from their supervisor, regular meetings, and measures to reduce potential stalking or violence in the workplace; unsurprisingly, victim blaming and leaving the employee to address the situation on their own were labeled experienced as negative.2 Employers may consider implementing physical security, access controls and panic alarms, and enhancing coworkers’ situational awareness.21 Explicit policies about and attention to reducing workplace violence, including stalking, are always beneficial—and in some settings such policies may be a regulatory requirement.22 Large health care organizations may benefit from developing specialized threat management programs to assist with the evaluation and mitigation of stalking and other workplace violence risks.15,23
Self-care considerations
The impact of stalking can include psychological distress, disruption of work and personal relationships, and false allegations of impropriety. Stalking can make targets feel isolated, violated, and fearful, which makes it challenging to reach out to others for support and safety. It takes time to regain a sense of safety and to find a “new normal,” particularly while experiencing and responding to stalking behavior. Notifying close personal contacts such as family and coworkers about what is occurring (without sharing protected health information) can be helpful for recovery and important for the clinician’s safety. Reaching out for organizational and legal supports is also prudent. It is also important to allow time for, and patience with, a targeted individual’s normal responses, such as decreased work performance, sleep/appetite changes, and hypervigilance, without pathologizing these common stress reactions. Further review of appropriate resources by impacted clinicians is advisable.24-26
Dr. A has been treating Ms. W, a graduate student, for depression. Ms. W made subtle comments expressing her interest in pursuing a romantic relationship with her psychiatrist. Dr. A gently redirected her, and she seemed to respond appropriately. However, over the past 2 weeks, Dr. A has seen Ms. W at a local park and at the grocery store. Today, Dr. A is startled to see Ms. W at her weekly yoga class. Dr. A plans to ask her supervisor for advice.
Dr. M is a child psychiatrist who spoke at his local school board meeting in support of masking requirements for students during COVID-19. During the discussion, Dr. M shared that, as a psychiatrist, he does not believe it is especially distressing for students to wear masks, and that doing so is a necessary public health measure. On leaving, other parents shouted, “We know who you are and where you live!” The next day, his integrated clinic started receiving threatening and harassing messages, including threats to kill him or his staff if they take part in vaccinating children against COVID-19.
Because of their work, mental health professionals—like other health care professionals—face an elevated risk of being harassed or stalked. Stalking often includes online harassment and may escalate to serious physical violence. Stalking is criminal behavior by a patient and should not be constructed as a “failure to manage transference.” This article explores basic strategies to reduce the risk of harassment and stalking, describes how to recognize early behaviors, and outlines basic steps health care professionals and their employers can take to respond to stalking and harassing behaviors.
Although this article is intended for psychiatrists, it is important to note that all health professionals have significant risk for experiencing stalking or harassment. This is due in part, but not exclusively, to our clinical work. Estimates of how many health professionals experience stalking vary substantially depending upon the study, and differences in methodologies limit easy comparison or extrapolation. More thorough reviews have reported ranges from 2% to 70% among physicians; psychiatrists and other mental health professionals appear to be at greater risk than those in other specialties and the general population.1-3 Physicians who are active on social media may also be at elevated risk.4 Unexpected communications from patients and their family members—especially those with threatening, harassing, or sexualized tones, or involving contact outside of a work setting—can be distressing. These behaviors represent potential harbingers of more dangerous behavior, including physical assault, sexual assault, or homicide. Despite their elevated risk, many psychiatrists are unaware of how to prevent or respond to stalking or harassment.
Recognizing harassment and stalking
Repeated and unwanted contact or communication, regardless of intent, may constitute stalking. Legal definitions vary by jurisdiction and may not align with subjective experiences or understanding of what constitutes stalking.5 At its essence, stalking is repeated harassing behaviors likely to provoke fear in the targeted person. FOUR is a helpful mnemonic when conceptualizing the attributes of stalking: Fixated, Obsessive, Unwanted, and Repetitive.6Table 1 lists examples of common stalking behaviors. Stalking and harassing behavior may be from a known source (eg, a patient, coworker, or paramour), a masked source (ie, someone known to the target but who conceals or obscures their identity), or from otherwise unknown persons. Behaviors that persist after the person engaging in the behaviors has clearly been informed that they are unwanted or inappropriate are especially concerning. Stalking may escalate to include physical or sexual assault and, in some cases, homicide.

Stalking duration can vary substantially, as can the factors that lead to the cessation of the behavior. Indicators of increased risk for physical violence include unwanted physical presence/following of the target (“approach behaviors”), having a prior violent intimate relationship, property destruction, explicit threats, and having a prior intimate relationship with the target.7
Stalking contact or communication may be unwanted because of the content (eg, sexualized or threatening tone), location (eg, at a professional’s home), or means (eg, through social media). Stalking behaviors are not appropriate in any relationship, including a clinical relationship. They should not be treated as a “failure to manage transference” or in other victim-blaming ways.
There are multiple typologies for stalking behavior. Common motivations for stalking health professionals include resentment or grievance, misjudgment of social boundaries, and delusional fixation, including erotomania.8 Associated psychopathologies vary significantly and, while some may be more amenable to psychiatric treatment than others, psychiatrists should not feel compelled to treat patients who repeatedly violate boundaries, regardless of intent or comorbidity.
Patients are not the exclusive perpetrators of stalking; a recent study found that 4% of physicians surveyed reported current or recent stalking by a current or former intimate partner.9 When a person who is a victim of intimate partner violence is also stalked as part of the abuse, homicide risk increases.10 Workplace homicides of health care professionals are most likely to be committed by a current or former partner or other personal acquaintance, not by a patient.11 Workplace harassment and stalking of health care professionals is especially concerning because this behavior can escalate and endanger coworkers or clients.
Continue to: Risk awareness: Recognize your exposure...
Risk awareness: Recognize your exposure
About 80% of stalking involves some form of technology—often telephone calls but also online or other “cyber” elements.12 One recent survey found the rate of online harassment, including threats of physical and sexual violence, was >20% among physicians who were active on social media.4 Health professionals may be at greater risk of having patients find their personal information simply because patients routinely search online for information about new clinicians. Personal information about a clinician may be readily visible among professional information in search results, or a curious patient may simply scroll further down in the results. For a potential stalker, clicking on a search result linking to a personal social media page may be far easier than finding a home address and going in person—but the action may be just as distressing or risky for the clinician.13 Additionally, items visible in a clinician’s office—or visible in the background of those providing telehealth services from their home—may inadvertently reveal personal information about the clinician, their home, or their family.
Psychiatrists are often in a special position in relation to patients and times of crises. They may be involved in involuntary commitment—or declining an admission when a patient or family wishes it. They may be present at the time of the revelation of a serious diagnosis, abuse, injury, or death. They may be a mandated reporter of child or elder abuse.2 Additionally, physicians may be engaged in discourse on politically charged public health topics.14 These factors may increase their risk of being stalked.
Conducting an online visibility self-assessment can be a useful way to learn what information others can find. Table 2 outlines the steps for completing this exercise. Searching multiple iterations of your current and former names (with and without degrees, titles, and cities) will yield differing results in various search engines. After establishing a baseline of what information is available online, it can be helpful to periodically repeat this exercise, and to set up automated alerts for your name, number(s), email(s), and address(es).

Basic mitigation strategies
In the modern era, being invisible online is impractical and likely impossible—especially for a health care professional. Instead, it may be prudent to limit your public visibility to professional portals (eg, LinkedIn or Doximity) and maximize privacy settings on other platforms. Another basic strategy is to avoid providing personal contact information (your home address, phone number, or personal email) for professional purposes, such as licensing and credentialing, conference submissions, or journal publications. Be aware that driving a visually distinct vehicle—one with vanity plates or distinct bumper stickers, or an exotic sportscar—can make it easier to be recognized and located. A personally recorded voicemail greeting (vs one recorded by, for example, an office manager) may be inappropriately reinforcing for some stalkers.
Workplaces should have an established safety policy that addresses stalking and harassment of employees. Similarly, patients and others should receive clear education on how to contact different staff, including physicians, with consideration of how and when to use electronic health information portals, office numbers, and emails. Workplaces should not disclose staff schedules. For example, a receptionist should say “I’ll have Dr. Diaz return your call when she can” instead of “Dr. Diaz is not in until tomorrow.” Avoid unnecessary location/name signals (eg, a parking spot labeled “Dr. Diaz”). Consider creating alert words or phrases for staff to use to signal they are concerned about their immediate safety—and provide education and training, including drills, to test emergency responses when the words/phrases are used. Leaders and managers should nurture a workplace culture where people are comfortable seeking support if they feel they may be the target of harassment or stalking. Many larger health care organizations have threat management programs, which can play a critical role in preventing, investigating, and responding to stalking of employees. Increasingly, threat management teams are being identified as a best practice in health care settings.15Table 3 summarizes measures to mitigate risk.

What to do when harassment or stalking occurs
Consulting with subject matter experts is essential. Approach behaviors, stalking patterns, and immediate circumstances vary highly, and so too must responses. A socially inept approach outside of the work setting by a patient may be effectively responded to with a firm explanation of why the behavior was inappropriate and a reiteration of limits. More persistent or serious threats may require taking actions for immediate safety, calling law enforcement or security (who may have the expertise to assist appropriately), or even run/hide/fight measures. Others to notify early on include human resources, supervisors, front desk staff, and coworkers. Although no single measure is always indicated and no single measure will always be effective, consultation with a specialist is always advisable.
Attempting to assess your own risk may be subject to bias and error, even for an experienced forensic psychiatrist. Risk assessment in stalking and harassment cases is complex, nuanced, and beyond the scope of this article; engagement with specialized threat programs or subject matter experts is advisable.15,16 If your medical center or area has police or security officers, engage them early. Risk management, insurers, and legal can also be helpful to consult. Attorneys specializing in harassment, stalking, and domestic violence may be helpful in extreme situations.17Table 417,18 highlights steps to take.

While effective interventions to stop or redirect stalking behavior may vary, some initial considerations include changing established routines (eg, your parking location or daily/weekly patterns such as gym, class, etc.) and letting family and others you live with know what is occurring. Consider implementing and bolstering personal, work, and home security; honing situational awareness skills; and learning advanced situational awareness and self-defense techniques.
Continue to: Clinical documentation and termination of care...
Clinical documentation and termination of care
Repeated and unwanted contact behaviors by a patient may be considered grounds for termination of care by the targeted clinician. Termination may occur through a direct conversation, followed by a mailed letter explaining that the patient’s inappropriate behaviors are the basis for termination. The letter should outline steps for establishing care with another psychiatrist and signing a release to facilitate transfer of records to the next psychiatrist. Ensure that the patient has access to a reasonable supply of medications or refills according to jurisdictional standards for transfer or termination of care.19 While these are common legal standards for termination of care in the United States, clinicians would be well served by appropriate consultation to verify the most appropriate standards for their location.
Documentation of a patient’s behavior should be factual and clear. Under the 21st Century Cures Act, patients often have access to their own electronic records.20 Therefore, clinicians should avoid documenting personal security measures or other information that is not clinically relevant. Communications with legal or risk management should not be documented unless otherwise advised, because such communications may be privileged and may not be clinically relevant.
In some circumstances, continuing to treat a patient who has stalked a member of the current treatment team may be appropriate or necessary. For example, a patient may respond appropriately to redirection after an initial approach behavior and continue to make clinical progress, or may be in a forensic specialty setting with appropriate operational support to continue with treatment.
Ethical dilemmas may arise in underserved areas where there are limited options for psychiatric care and in communicating the reasons for termination to a new clinician. Consultation may help to address these issues. However, as noted before, clinicians should be permitted to discontinue and transfer treatment and should not be compelled to continue to treat a patient who has threatened or harassed them.
Organizational and employer considerations
Victims of stalking have reported that they appreciated explicit support from their supervisor, regular meetings, and measures to reduce potential stalking or violence in the workplace; unsurprisingly, victim blaming and leaving the employee to address the situation on their own were labeled experienced as negative.2 Employers may consider implementing physical security, access controls and panic alarms, and enhancing coworkers’ situational awareness.21 Explicit policies about and attention to reducing workplace violence, including stalking, are always beneficial—and in some settings such policies may be a regulatory requirement.22 Large health care organizations may benefit from developing specialized threat management programs to assist with the evaluation and mitigation of stalking and other workplace violence risks.15,23
Self-care considerations
The impact of stalking can include psychological distress, disruption of work and personal relationships, and false allegations of impropriety. Stalking can make targets feel isolated, violated, and fearful, which makes it challenging to reach out to others for support and safety. It takes time to regain a sense of safety and to find a “new normal,” particularly while experiencing and responding to stalking behavior. Notifying close personal contacts such as family and coworkers about what is occurring (without sharing protected health information) can be helpful for recovery and important for the clinician’s safety. Reaching out for organizational and legal supports is also prudent. It is also important to allow time for, and patience with, a targeted individual’s normal responses, such as decreased work performance, sleep/appetite changes, and hypervigilance, without pathologizing these common stress reactions. Further review of appropriate resources by impacted clinicians is advisable.24-26
1. Nelsen AJ, Johnson RS, Ostermeyer B, et al. The prevalence of physicians who have been stalked: a systematic review. J Am Acad Psychiatry Law. 2015;43(2):177-182.
2. Jutasi C, McEwan TE. Stalking of professionals: a scoping review. Journal of Threat Assessment and Management. 2021;8(3):94-124.
3. Pathé MT, Meloy JR. Commentary: Stalking by patients—psychiatrists’ tales of anger, lust and ignorance. J Am Acad Psychiatry Law. 2013;41(2):200-205.
4. Pendergrast TR, Jain S, Trueger NS, et al. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552.
5. Owens JG. Why definitions matter: stalking victimization in the United States. J Interpers Violence. 2016;31(12):2196-2226.
6. College of Policing. Stalking or harassment. May 2019. Accessed March 8, 2020. https://library.college.police.uk/docs/college-of-policing/Stalking_or_harassment_guidance_200519.pdf
7. McEwan TE, Daffern M, MacKenzie RD, et al. Risk factors for stalking violence, persistence, and recurrence. Journal of Forensic Psychiatry & Psychology. 2017;28(1):3856.
8. Pathé MT, Mullen PE, Purcell R. Patients who stalk doctors: their motives and management. Med J Australia. 2002;176(7):335-338.
9. Reibling ET, Distelberg B, Guptill M, et al. Intimate partner violence experienced by physicians. J Prim Care Community Health. 2020;11:2150132720965077.
10. Matias A, Gonçalves M, Soeiro C, et al. Intimate partner homicide: a meta-analysis of risk factors. Aggression and Violent Behavior. 2019;50:101358.
11. US Bureau of Labor Statistics. Fact sheet. Workplace violence in healthcare, 2018. April 2020. Accessed November 24, 2021. https://www.bls.gov/iif/oshwc/cfoi/workplace-violence-healthcare-2018.htm
12. Truman JL, Morgan RE. Stalking victimization, 2016. Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice. Report No.: NCJ 253526. April 2021. Accessed November 24, 2021. https://bjs.ojp.gov/library/publications/stalking-victimization-2016
13. Reyns BW, Henson B, Fisher BS. Being pursued online: applying cyberlifestyle–routine activities theory to cyberstalking victimization. Criminal Justice and Behavior. 2011;38(11):1149-1169.
14. Stea JN. When promoting knowledge makes you a target. Scientific American Blog Network. March 16, 2020. Accessed November 24, 2021. https://blogs.scientificamerican.com/observations/when-promoting-knowledge-makes-you-a-target/
15. Henkel SJ. Threat assessment strategies to mitigate violence in healthcare. IAHSS Foundation. IAHSS-F RS-19-02. November 2019. Accessed November 24, 2021. https://iahssf.org/assets/IAHSS-Foundation-Threat-Assessment-Strategies-to-Mitigate-Violence-in-Healthcare.pdf
16. McEwan TE. Stalking threat and risk assessment. In: Reid Meloy J, Hoffman J (eds). International Handbook of Threat Assessment. 2nd ed. Oxford University Press; 2021:210-234.
17. Goldberg C. Nobody’s Victim: Fighting Psychos, Stalkers, Pervs, and Trolls. Plume; 2019.
18. Bazzell M. Extreme Privacy: What It Takes to Disappear. 2nd ed. Independently published; 2020.
19. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
20. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program Final Rule (To be codified at 45 CFR 170 and 171). Federal Register. 2020;85(85):25642-25961.
21. Sheridan L, North AC, Scott AJ. Stalking in the workplace. Journal of Threat Assessment and Management. 2019;6(2):61-75.
22. The Joint Commission. Workplace Violence Prevention Standards. R3 Report: Requirement, Rationale, Reference. Issue 30. June 18, 2021. Accessed November 24, 2021. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/wpvp-r3-30_revised_06302021.pdf
23. Terry LP. Threat assessment teams. J Healthc Prot Manage. 2015;31(2):23-35.
24. Pathé M. Surviving Stalking. Cambridge University Press; 2002.
25. Noffsinger S. What stalking victims need to restore their mental and somatic health. Current Psychiatry. 2015;14(6):43-47.
26. Mullen P, Whyte S, McIvor R; Psychiatrists’ Support Service, Royal College of Psychiatry. PSS Information Guide: Stalking. Report No. 11. 2017. Accessed November 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/members/supporting-you/pss/pss-guide-11-stalking.pdf?sfvrsn=2f1c7253_2
1. Nelsen AJ, Johnson RS, Ostermeyer B, et al. The prevalence of physicians who have been stalked: a systematic review. J Am Acad Psychiatry Law. 2015;43(2):177-182.
2. Jutasi C, McEwan TE. Stalking of professionals: a scoping review. Journal of Threat Assessment and Management. 2021;8(3):94-124.
3. Pathé MT, Meloy JR. Commentary: Stalking by patients—psychiatrists’ tales of anger, lust and ignorance. J Am Acad Psychiatry Law. 2013;41(2):200-205.
4. Pendergrast TR, Jain S, Trueger NS, et al. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552.
5. Owens JG. Why definitions matter: stalking victimization in the United States. J Interpers Violence. 2016;31(12):2196-2226.
6. College of Policing. Stalking or harassment. May 2019. Accessed March 8, 2020. https://library.college.police.uk/docs/college-of-policing/Stalking_or_harassment_guidance_200519.pdf
7. McEwan TE, Daffern M, MacKenzie RD, et al. Risk factors for stalking violence, persistence, and recurrence. Journal of Forensic Psychiatry & Psychology. 2017;28(1):3856.
8. Pathé MT, Mullen PE, Purcell R. Patients who stalk doctors: their motives and management. Med J Australia. 2002;176(7):335-338.
9. Reibling ET, Distelberg B, Guptill M, et al. Intimate partner violence experienced by physicians. J Prim Care Community Health. 2020;11:2150132720965077.
10. Matias A, Gonçalves M, Soeiro C, et al. Intimate partner homicide: a meta-analysis of risk factors. Aggression and Violent Behavior. 2019;50:101358.
11. US Bureau of Labor Statistics. Fact sheet. Workplace violence in healthcare, 2018. April 2020. Accessed November 24, 2021. https://www.bls.gov/iif/oshwc/cfoi/workplace-violence-healthcare-2018.htm
12. Truman JL, Morgan RE. Stalking victimization, 2016. Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice. Report No.: NCJ 253526. April 2021. Accessed November 24, 2021. https://bjs.ojp.gov/library/publications/stalking-victimization-2016
13. Reyns BW, Henson B, Fisher BS. Being pursued online: applying cyberlifestyle–routine activities theory to cyberstalking victimization. Criminal Justice and Behavior. 2011;38(11):1149-1169.
14. Stea JN. When promoting knowledge makes you a target. Scientific American Blog Network. March 16, 2020. Accessed November 24, 2021. https://blogs.scientificamerican.com/observations/when-promoting-knowledge-makes-you-a-target/
15. Henkel SJ. Threat assessment strategies to mitigate violence in healthcare. IAHSS Foundation. IAHSS-F RS-19-02. November 2019. Accessed November 24, 2021. https://iahssf.org/assets/IAHSS-Foundation-Threat-Assessment-Strategies-to-Mitigate-Violence-in-Healthcare.pdf
16. McEwan TE. Stalking threat and risk assessment. In: Reid Meloy J, Hoffman J (eds). International Handbook of Threat Assessment. 2nd ed. Oxford University Press; 2021:210-234.
17. Goldberg C. Nobody’s Victim: Fighting Psychos, Stalkers, Pervs, and Trolls. Plume; 2019.
18. Bazzell M. Extreme Privacy: What It Takes to Disappear. 2nd ed. Independently published; 2020.
19. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
20. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program Final Rule (To be codified at 45 CFR 170 and 171). Federal Register. 2020;85(85):25642-25961.
21. Sheridan L, North AC, Scott AJ. Stalking in the workplace. Journal of Threat Assessment and Management. 2019;6(2):61-75.
22. The Joint Commission. Workplace Violence Prevention Standards. R3 Report: Requirement, Rationale, Reference. Issue 30. June 18, 2021. Accessed November 24, 2021. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/wpvp-r3-30_revised_06302021.pdf
23. Terry LP. Threat assessment teams. J Healthc Prot Manage. 2015;31(2):23-35.
24. Pathé M. Surviving Stalking. Cambridge University Press; 2002.
25. Noffsinger S. What stalking victims need to restore their mental and somatic health. Current Psychiatry. 2015;14(6):43-47.
26. Mullen P, Whyte S, McIvor R; Psychiatrists’ Support Service, Royal College of Psychiatry. PSS Information Guide: Stalking. Report No. 11. 2017. Accessed November 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/members/supporting-you/pss/pss-guide-11-stalking.pdf?sfvrsn=2f1c7253_2
Travel/school disruptions as COVID-19 cases grow in 2022
As the United States enters a third year of the COVID-19 pandemic,
The United States is reporting a 7-day average of more than 386,000 cases after several record-breaking days, according to the data tracker by the New York Times. The United States surpassed 585,000 cases on Dec. 30, setting a new record before the New Year’s holiday.
New York, Washington, D.C., and other states along the East Coast are leading the national surge. New York reported more than 85,000 new cases on the last day of 2021, marking the highest 1-day total in the state since the pandemic began.
“As we fight the winter surge, we need to keep the most vulnerable among us in mind – do what you can to keep others in your community safe from COVID-19,” New York Gov. Kathy Hochul said in a statement on Jan. 1, 2022.
“Wear a mask, wash your hands, and take advantage of the best tool we have at our disposal – the vaccine,” she said.
The 2021 winter surge in the United States peaked around Jan. 12, which may suggest that the country has a week or so before the current wave reaches its height and begins to drop, the newspaper reported.
In the meantime, people are dealing with disruptions as they return from holiday travel and begin the new year. Airlines canceled more than 2,700 flights on Jan. 1 and more than 1,900 flights on Jan. 2, bringing the total since Christmas Eve to more than 14,000 canceled flights.
About half of cancellations were connected to wintry weather at key airline hubs in Chicago and Denver, the newspaper reported, as well as ongoing flight crew shortages caused by the Omicron variant.
More disruptions could continue, the Federal Aviation Administration warned, as an increasing number of its air-traffic control employees test positive for COVID-19.
“To maintain safety, traffic volumes at some facilities could be reduced, which might result in delays during busy periods,” an FAA spokesman told The Wall Street Journal.
The current COVID-19 surge will also affect businesses and schools as the new year begins. A growing number of universities are opting to start the next semester with remote instruction.
American University, Duke University, and Michigan State University announced in recent days that they would delay in-person classes to slow the spread of the coronavirus on campus. They will begin classes online on Jan. 10 and return to campus the following week or later.
“I realize that students prefer to be in person, and so do I. But it is important that we do so in a safe manner,” Samuel Stanley Jr., MD, president of Michigan State University, said in a statement on New Year’s Eve.
K-12 school districts are deciding how to adapt as well. Some districts are bringing back mask requirements, and some are ramping up testing. Others are moving to remote learning – and signaling the need for flexibility as the Omicron variant brings new surprises.
“Change has been the only constant in this fight,” Roger Leon, the superintendent for Newark (N.J.) Public Schools, wrote in a note to parents. He announced on Dec. 30, 2021, that students would learn remotely for at least the first 2 weeks of the new year.
This continues “to be a brutal, relentless, and ruthless virus that rears its ugly head at inopportune times,” he said.
A version of this article first appeared on WebMD.com.
As the United States enters a third year of the COVID-19 pandemic,
The United States is reporting a 7-day average of more than 386,000 cases after several record-breaking days, according to the data tracker by the New York Times. The United States surpassed 585,000 cases on Dec. 30, setting a new record before the New Year’s holiday.
New York, Washington, D.C., and other states along the East Coast are leading the national surge. New York reported more than 85,000 new cases on the last day of 2021, marking the highest 1-day total in the state since the pandemic began.
“As we fight the winter surge, we need to keep the most vulnerable among us in mind – do what you can to keep others in your community safe from COVID-19,” New York Gov. Kathy Hochul said in a statement on Jan. 1, 2022.
“Wear a mask, wash your hands, and take advantage of the best tool we have at our disposal – the vaccine,” she said.
The 2021 winter surge in the United States peaked around Jan. 12, which may suggest that the country has a week or so before the current wave reaches its height and begins to drop, the newspaper reported.
In the meantime, people are dealing with disruptions as they return from holiday travel and begin the new year. Airlines canceled more than 2,700 flights on Jan. 1 and more than 1,900 flights on Jan. 2, bringing the total since Christmas Eve to more than 14,000 canceled flights.
About half of cancellations were connected to wintry weather at key airline hubs in Chicago and Denver, the newspaper reported, as well as ongoing flight crew shortages caused by the Omicron variant.
More disruptions could continue, the Federal Aviation Administration warned, as an increasing number of its air-traffic control employees test positive for COVID-19.
“To maintain safety, traffic volumes at some facilities could be reduced, which might result in delays during busy periods,” an FAA spokesman told The Wall Street Journal.
The current COVID-19 surge will also affect businesses and schools as the new year begins. A growing number of universities are opting to start the next semester with remote instruction.
American University, Duke University, and Michigan State University announced in recent days that they would delay in-person classes to slow the spread of the coronavirus on campus. They will begin classes online on Jan. 10 and return to campus the following week or later.
“I realize that students prefer to be in person, and so do I. But it is important that we do so in a safe manner,” Samuel Stanley Jr., MD, president of Michigan State University, said in a statement on New Year’s Eve.
K-12 school districts are deciding how to adapt as well. Some districts are bringing back mask requirements, and some are ramping up testing. Others are moving to remote learning – and signaling the need for flexibility as the Omicron variant brings new surprises.
“Change has been the only constant in this fight,” Roger Leon, the superintendent for Newark (N.J.) Public Schools, wrote in a note to parents. He announced on Dec. 30, 2021, that students would learn remotely for at least the first 2 weeks of the new year.
This continues “to be a brutal, relentless, and ruthless virus that rears its ugly head at inopportune times,” he said.
A version of this article first appeared on WebMD.com.
As the United States enters a third year of the COVID-19 pandemic,
The United States is reporting a 7-day average of more than 386,000 cases after several record-breaking days, according to the data tracker by the New York Times. The United States surpassed 585,000 cases on Dec. 30, setting a new record before the New Year’s holiday.
New York, Washington, D.C., and other states along the East Coast are leading the national surge. New York reported more than 85,000 new cases on the last day of 2021, marking the highest 1-day total in the state since the pandemic began.
“As we fight the winter surge, we need to keep the most vulnerable among us in mind – do what you can to keep others in your community safe from COVID-19,” New York Gov. Kathy Hochul said in a statement on Jan. 1, 2022.
“Wear a mask, wash your hands, and take advantage of the best tool we have at our disposal – the vaccine,” she said.
The 2021 winter surge in the United States peaked around Jan. 12, which may suggest that the country has a week or so before the current wave reaches its height and begins to drop, the newspaper reported.
In the meantime, people are dealing with disruptions as they return from holiday travel and begin the new year. Airlines canceled more than 2,700 flights on Jan. 1 and more than 1,900 flights on Jan. 2, bringing the total since Christmas Eve to more than 14,000 canceled flights.
About half of cancellations were connected to wintry weather at key airline hubs in Chicago and Denver, the newspaper reported, as well as ongoing flight crew shortages caused by the Omicron variant.
More disruptions could continue, the Federal Aviation Administration warned, as an increasing number of its air-traffic control employees test positive for COVID-19.
“To maintain safety, traffic volumes at some facilities could be reduced, which might result in delays during busy periods,” an FAA spokesman told The Wall Street Journal.
The current COVID-19 surge will also affect businesses and schools as the new year begins. A growing number of universities are opting to start the next semester with remote instruction.
American University, Duke University, and Michigan State University announced in recent days that they would delay in-person classes to slow the spread of the coronavirus on campus. They will begin classes online on Jan. 10 and return to campus the following week or later.
“I realize that students prefer to be in person, and so do I. But it is important that we do so in a safe manner,” Samuel Stanley Jr., MD, president of Michigan State University, said in a statement on New Year’s Eve.
K-12 school districts are deciding how to adapt as well. Some districts are bringing back mask requirements, and some are ramping up testing. Others are moving to remote learning – and signaling the need for flexibility as the Omicron variant brings new surprises.
“Change has been the only constant in this fight,” Roger Leon, the superintendent for Newark (N.J.) Public Schools, wrote in a note to parents. He announced on Dec. 30, 2021, that students would learn remotely for at least the first 2 weeks of the new year.
This continues “to be a brutal, relentless, and ruthless virus that rears its ugly head at inopportune times,” he said.
A version of this article first appeared on WebMD.com.
Pandemic screen time linked to anxiety, depression in older kids
However, the study doesn’t definitively prove that screen time is harmful, and an expert challenged the conclusions.
Still, the findings highlight the potential harms of excessive screen time, especially in the context of pandemic-era virtual learning. Clinicians “really need to advocate for policies that would be protective for children to reduce their screen time and social isolation and increase their involvement with school, sports, and academic activities,” Catherine S. Birken, MD, a pediatrician at the University of Toronto and study coauthor said in an interview.
The study appeared Dec. 28, 2021, in the journal JAMA Network Open (doi: 10.1001/jamanetworkopen.2021.40875).
Dr. Birken and colleagues launched the study to examine whether heightened levels of screen time during the pandemic disrupted mental health in kids. In particular, they wanted to break down different types of screen time, such as virtual learning, watching television, and playing video games.
“The bulk of the literature is supportive of a strong relationship between screen time and mental health symptoms like anxiety,” Dr. Birken said.
For the study, the researchers surveyed parents to track the screen time of 2,026 children between May 2020 and April 2021.
In a cohort of 532 younger children (average age, 5.9 years; 52% male; 58% of European ancestry), the researchers linked each extra daily hour of TV or use of digital media to worse behavior, as measured by the Strengths and Difficulties Questionnaire: 0.22 in an adjusted model for children aged 2-4;(95% confidence interval, 0.10-0.35; P < .001) and 0.07 in an adjusted model in those aged 4 and older (95% CI, 0.02-0.11; P = .007).
However, the researchers observed no statistically significant links to more anxiety/depression or hyperactivity/inattention in this group of children.
Among 1,494 older kids (mean age, 11.3; 57% male; 58% of European ancestry), researchers linked greater daily use of TV or digital media to higher levels of depression symptoms in a dose-dependent relationship, Dr. Birken said (1 hour: beta, 0.21; 95% CI, –1.28 to 0.78; 2-3 hours: beta, 1.81; 95% CI, 0.29-3.33; 4-5 hours: beta, 2.80; 95% CI, 1.15-4.44; 6-8 hours: beta, 5.16; 95% CI, 3.32-7.01; 9 hours: beta, 5.42; 95% CI, 3.30-7.54; overall P < .001).
“Similarly, higher TV or digital media time per day was associated with higher levels of anxiety symptoms,” the researchers reported. “TV or digital media time per day was also significantly associated with differences in symptoms of irritability, inattention, and hyperactivity/inattention.”
More time spent learning virtually was associated with higher levels of depression and anxiety in both groups of children, according to the researchers. Whether this finding reflects an effect of screens themselves or because the children most exposed to virtual learning may also have been the most exposed to the stressful disruptiveness of the pandemic is unclear.
The researchers also found “insufficient evidence” to link more virtual learning to irritability, inattention and hyperactivity, inattention, and hyperactivity/impulsivity in adjusted models.
Video chatting did not appear to have a protective effect, Dr. Birken said. The researchers also specifically analyzed children with autism and found no link between more screen time and various mental health/conduct problems.
Is it possible that kids with more anxiety, depression, and isolation simply turn to screens because they’re anxious, depressed, and isolated? Dr. Birken said the researchers adjusted the findings to account for previous mental health problems. And she noted that the study linked more pandemic-era virtual learning to more depression/anxiety. It’s “hard to imagine” how more mental health problems would cause more virtual learning.
Bad news or bad stats?
Chris Ferguson, PhD, a professor of psychology at Stetson University. DeLand, Fla., who studies screen time, criticized the study in an interview. “The observed effects are so tiny, it’s impossible to know if they are real or a false-positive artifact common to social science research,” he said. “Ultimately, this study is better evidence about how many scholars are bad at statistics than anything having to do with kids and screens.”
Dr. Ferguson said that the results may be confounded because kids turn to screens to reduce their anxiety. “For the most part, screens were a godsend during COVID-19,” he said. “They helped kids stay inside and gave them something to do while social distancing and allowed them to keep in touch with friends and families. Honestly, what else were we expecting kids to do, stare at the wallpaper?”
Children with depression and anxiety often retreat into screens or books to escape the unpleasantries of real life. “That doesn’t mean the screens or books are the culprits,” he said.
Instead of focusing on screen time, Dr. Ferguson suggested parents consider these factors: “Keeping in mind not every kid is a genius, is your kid doing about as well in school as you’d expect, given their natural ability? Are they getting at least some exercise every day? Are they getting adequate sleep? Are they able to socialize with friends in some context, either in real life or online? Are they happy?”
The study was funded by the Canadian Institutes of Health Research, the Center for Brain & Mental Health at The Hospital for Sick Children, the Ontario Ministry of Health, and the Miner’s Lamp Innovation Fund in Prevention and Early Detection of Severe Mental Illness at the University of Toronto. The study authors reported various financial relationships. Dr. Ferguson reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
However, the study doesn’t definitively prove that screen time is harmful, and an expert challenged the conclusions.
Still, the findings highlight the potential harms of excessive screen time, especially in the context of pandemic-era virtual learning. Clinicians “really need to advocate for policies that would be protective for children to reduce their screen time and social isolation and increase their involvement with school, sports, and academic activities,” Catherine S. Birken, MD, a pediatrician at the University of Toronto and study coauthor said in an interview.
The study appeared Dec. 28, 2021, in the journal JAMA Network Open (doi: 10.1001/jamanetworkopen.2021.40875).
Dr. Birken and colleagues launched the study to examine whether heightened levels of screen time during the pandemic disrupted mental health in kids. In particular, they wanted to break down different types of screen time, such as virtual learning, watching television, and playing video games.
“The bulk of the literature is supportive of a strong relationship between screen time and mental health symptoms like anxiety,” Dr. Birken said.
For the study, the researchers surveyed parents to track the screen time of 2,026 children between May 2020 and April 2021.
In a cohort of 532 younger children (average age, 5.9 years; 52% male; 58% of European ancestry), the researchers linked each extra daily hour of TV or use of digital media to worse behavior, as measured by the Strengths and Difficulties Questionnaire: 0.22 in an adjusted model for children aged 2-4;(95% confidence interval, 0.10-0.35; P < .001) and 0.07 in an adjusted model in those aged 4 and older (95% CI, 0.02-0.11; P = .007).
However, the researchers observed no statistically significant links to more anxiety/depression or hyperactivity/inattention in this group of children.
Among 1,494 older kids (mean age, 11.3; 57% male; 58% of European ancestry), researchers linked greater daily use of TV or digital media to higher levels of depression symptoms in a dose-dependent relationship, Dr. Birken said (1 hour: beta, 0.21; 95% CI, –1.28 to 0.78; 2-3 hours: beta, 1.81; 95% CI, 0.29-3.33; 4-5 hours: beta, 2.80; 95% CI, 1.15-4.44; 6-8 hours: beta, 5.16; 95% CI, 3.32-7.01; 9 hours: beta, 5.42; 95% CI, 3.30-7.54; overall P < .001).
“Similarly, higher TV or digital media time per day was associated with higher levels of anxiety symptoms,” the researchers reported. “TV or digital media time per day was also significantly associated with differences in symptoms of irritability, inattention, and hyperactivity/inattention.”
More time spent learning virtually was associated with higher levels of depression and anxiety in both groups of children, according to the researchers. Whether this finding reflects an effect of screens themselves or because the children most exposed to virtual learning may also have been the most exposed to the stressful disruptiveness of the pandemic is unclear.
The researchers also found “insufficient evidence” to link more virtual learning to irritability, inattention and hyperactivity, inattention, and hyperactivity/impulsivity in adjusted models.
Video chatting did not appear to have a protective effect, Dr. Birken said. The researchers also specifically analyzed children with autism and found no link between more screen time and various mental health/conduct problems.
Is it possible that kids with more anxiety, depression, and isolation simply turn to screens because they’re anxious, depressed, and isolated? Dr. Birken said the researchers adjusted the findings to account for previous mental health problems. And she noted that the study linked more pandemic-era virtual learning to more depression/anxiety. It’s “hard to imagine” how more mental health problems would cause more virtual learning.
Bad news or bad stats?
Chris Ferguson, PhD, a professor of psychology at Stetson University. DeLand, Fla., who studies screen time, criticized the study in an interview. “The observed effects are so tiny, it’s impossible to know if they are real or a false-positive artifact common to social science research,” he said. “Ultimately, this study is better evidence about how many scholars are bad at statistics than anything having to do with kids and screens.”
Dr. Ferguson said that the results may be confounded because kids turn to screens to reduce their anxiety. “For the most part, screens were a godsend during COVID-19,” he said. “They helped kids stay inside and gave them something to do while social distancing and allowed them to keep in touch with friends and families. Honestly, what else were we expecting kids to do, stare at the wallpaper?”
Children with depression and anxiety often retreat into screens or books to escape the unpleasantries of real life. “That doesn’t mean the screens or books are the culprits,” he said.
Instead of focusing on screen time, Dr. Ferguson suggested parents consider these factors: “Keeping in mind not every kid is a genius, is your kid doing about as well in school as you’d expect, given their natural ability? Are they getting at least some exercise every day? Are they getting adequate sleep? Are they able to socialize with friends in some context, either in real life or online? Are they happy?”
The study was funded by the Canadian Institutes of Health Research, the Center for Brain & Mental Health at The Hospital for Sick Children, the Ontario Ministry of Health, and the Miner’s Lamp Innovation Fund in Prevention and Early Detection of Severe Mental Illness at the University of Toronto. The study authors reported various financial relationships. Dr. Ferguson reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
However, the study doesn’t definitively prove that screen time is harmful, and an expert challenged the conclusions.
Still, the findings highlight the potential harms of excessive screen time, especially in the context of pandemic-era virtual learning. Clinicians “really need to advocate for policies that would be protective for children to reduce their screen time and social isolation and increase their involvement with school, sports, and academic activities,” Catherine S. Birken, MD, a pediatrician at the University of Toronto and study coauthor said in an interview.
The study appeared Dec. 28, 2021, in the journal JAMA Network Open (doi: 10.1001/jamanetworkopen.2021.40875).
Dr. Birken and colleagues launched the study to examine whether heightened levels of screen time during the pandemic disrupted mental health in kids. In particular, they wanted to break down different types of screen time, such as virtual learning, watching television, and playing video games.
“The bulk of the literature is supportive of a strong relationship between screen time and mental health symptoms like anxiety,” Dr. Birken said.
For the study, the researchers surveyed parents to track the screen time of 2,026 children between May 2020 and April 2021.
In a cohort of 532 younger children (average age, 5.9 years; 52% male; 58% of European ancestry), the researchers linked each extra daily hour of TV or use of digital media to worse behavior, as measured by the Strengths and Difficulties Questionnaire: 0.22 in an adjusted model for children aged 2-4;(95% confidence interval, 0.10-0.35; P < .001) and 0.07 in an adjusted model in those aged 4 and older (95% CI, 0.02-0.11; P = .007).
However, the researchers observed no statistically significant links to more anxiety/depression or hyperactivity/inattention in this group of children.
Among 1,494 older kids (mean age, 11.3; 57% male; 58% of European ancestry), researchers linked greater daily use of TV or digital media to higher levels of depression symptoms in a dose-dependent relationship, Dr. Birken said (1 hour: beta, 0.21; 95% CI, –1.28 to 0.78; 2-3 hours: beta, 1.81; 95% CI, 0.29-3.33; 4-5 hours: beta, 2.80; 95% CI, 1.15-4.44; 6-8 hours: beta, 5.16; 95% CI, 3.32-7.01; 9 hours: beta, 5.42; 95% CI, 3.30-7.54; overall P < .001).
“Similarly, higher TV or digital media time per day was associated with higher levels of anxiety symptoms,” the researchers reported. “TV or digital media time per day was also significantly associated with differences in symptoms of irritability, inattention, and hyperactivity/inattention.”
More time spent learning virtually was associated with higher levels of depression and anxiety in both groups of children, according to the researchers. Whether this finding reflects an effect of screens themselves or because the children most exposed to virtual learning may also have been the most exposed to the stressful disruptiveness of the pandemic is unclear.
The researchers also found “insufficient evidence” to link more virtual learning to irritability, inattention and hyperactivity, inattention, and hyperactivity/impulsivity in adjusted models.
Video chatting did not appear to have a protective effect, Dr. Birken said. The researchers also specifically analyzed children with autism and found no link between more screen time and various mental health/conduct problems.
Is it possible that kids with more anxiety, depression, and isolation simply turn to screens because they’re anxious, depressed, and isolated? Dr. Birken said the researchers adjusted the findings to account for previous mental health problems. And she noted that the study linked more pandemic-era virtual learning to more depression/anxiety. It’s “hard to imagine” how more mental health problems would cause more virtual learning.
Bad news or bad stats?
Chris Ferguson, PhD, a professor of psychology at Stetson University. DeLand, Fla., who studies screen time, criticized the study in an interview. “The observed effects are so tiny, it’s impossible to know if they are real or a false-positive artifact common to social science research,” he said. “Ultimately, this study is better evidence about how many scholars are bad at statistics than anything having to do with kids and screens.”
Dr. Ferguson said that the results may be confounded because kids turn to screens to reduce their anxiety. “For the most part, screens were a godsend during COVID-19,” he said. “They helped kids stay inside and gave them something to do while social distancing and allowed them to keep in touch with friends and families. Honestly, what else were we expecting kids to do, stare at the wallpaper?”
Children with depression and anxiety often retreat into screens or books to escape the unpleasantries of real life. “That doesn’t mean the screens or books are the culprits,” he said.
Instead of focusing on screen time, Dr. Ferguson suggested parents consider these factors: “Keeping in mind not every kid is a genius, is your kid doing about as well in school as you’d expect, given their natural ability? Are they getting at least some exercise every day? Are they getting adequate sleep? Are they able to socialize with friends in some context, either in real life or online? Are they happy?”
The study was funded by the Canadian Institutes of Health Research, the Center for Brain & Mental Health at The Hospital for Sick Children, the Ontario Ministry of Health, and the Miner’s Lamp Innovation Fund in Prevention and Early Detection of Severe Mental Illness at the University of Toronto. The study authors reported various financial relationships. Dr. Ferguson reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Medicaid implements waivers for some clinical trial coverage
Federal officials will allow some flexibility in meeting new requirements on covering the costs of clinical trials for people enrolled in Medicaid, seeking to accommodate states where legislatures will not meet in time to make needed changes in rules.
Congress in 2020 ordered U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology (ASCO) and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
The mandate went into effect on Jan. 1, but the Centers for Medicare & Medicaid Services will allow accommodations in terms of implementation time for states that have not yet been able to make needed legislative changes, Daniel Tsai, deputy administrator and director of the Center for Medicaid and CHIP Services, wrote in a Dec. 7 letter. Mr. Tsai’s letter doesn’t mention specific states. The CMS did not immediately respond to a request seeking information on the states expected to apply for waivers.
Medicaid has in recent years been a rare large U.S. insurance program that does not cover the costs of clinical trials. The Affordable Care Act of 2010 mandated this coverage for people in private insurance plans. The federal government in 2000 decided that Medicare would do so.
‘A hidden opportunity’
A perspective article last May in the New England Journal of Medicine referred to the new Medicaid mandate on clinical trials as a “hidden opportunity,” referring to its genesis as an add-on in a massive federal spending package enacted in December 2020.
In the article, Samuel U. Takvorian, MD, MSHP, of the University of Pennsylvania, Philadelphia, and coauthors noted that rates of participation in clinical trials remain low for racial and ethnic minority groups, due in part to the lack of Medicaid coverage.
“For example, non-Hispanic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials – a gap that has widened over time,” Dr. Takvorian and coauthors wrote. “Inequities in enrollment have also manifested during the COVID-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of COVID-19 therapeutics.”
In October, researchers from the Arthur G. James Cancer Hospital and Ohio State University, Columbus, published results of a retrospective study of patients with stage I-IV pancreatic cancer that also found inequities in enrollment. Mariam F. Eskander, MD, MPH, and coauthors reported what they found by examining records for 1,127 patients (0.4%) enrolled in clinical trials and 301,340 (99.6%) who did not enroll. They found that enrollment in trials increased over the study period, but not for Black patients or patients on Medicaid.
In an interview, Dr. Eskander said the new Medicaid policy will remove a major obstacle to participation in clinical trials. An oncologist, Dr. Eskander said she is looking forward to being able to help more of her patients get access to experimental medicines and treatments.
But that may not be enough to draw more people with low incomes into these studies, said Dr. Eskander, who is now at Rutgers Cancer Institute of New Jersey in New Brunswick. She urges greater use of patient navigators to help people on Medicaid understand the resources available to them, as well as broad use of Medicaid’s nonemergency medical transportation (NEMT) benefit.
“Some patients will be offered clinical trial enrollment and some will accept, but I really worry about the challenges low-income people face with things like transportation and getting time off work,” she said.
A version of this article first appeared on Medscape.com.
Federal officials will allow some flexibility in meeting new requirements on covering the costs of clinical trials for people enrolled in Medicaid, seeking to accommodate states where legislatures will not meet in time to make needed changes in rules.
Congress in 2020 ordered U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology (ASCO) and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
The mandate went into effect on Jan. 1, but the Centers for Medicare & Medicaid Services will allow accommodations in terms of implementation time for states that have not yet been able to make needed legislative changes, Daniel Tsai, deputy administrator and director of the Center for Medicaid and CHIP Services, wrote in a Dec. 7 letter. Mr. Tsai’s letter doesn’t mention specific states. The CMS did not immediately respond to a request seeking information on the states expected to apply for waivers.
Medicaid has in recent years been a rare large U.S. insurance program that does not cover the costs of clinical trials. The Affordable Care Act of 2010 mandated this coverage for people in private insurance plans. The federal government in 2000 decided that Medicare would do so.
‘A hidden opportunity’
A perspective article last May in the New England Journal of Medicine referred to the new Medicaid mandate on clinical trials as a “hidden opportunity,” referring to its genesis as an add-on in a massive federal spending package enacted in December 2020.
In the article, Samuel U. Takvorian, MD, MSHP, of the University of Pennsylvania, Philadelphia, and coauthors noted that rates of participation in clinical trials remain low for racial and ethnic minority groups, due in part to the lack of Medicaid coverage.
“For example, non-Hispanic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials – a gap that has widened over time,” Dr. Takvorian and coauthors wrote. “Inequities in enrollment have also manifested during the COVID-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of COVID-19 therapeutics.”
In October, researchers from the Arthur G. James Cancer Hospital and Ohio State University, Columbus, published results of a retrospective study of patients with stage I-IV pancreatic cancer that also found inequities in enrollment. Mariam F. Eskander, MD, MPH, and coauthors reported what they found by examining records for 1,127 patients (0.4%) enrolled in clinical trials and 301,340 (99.6%) who did not enroll. They found that enrollment in trials increased over the study period, but not for Black patients or patients on Medicaid.
In an interview, Dr. Eskander said the new Medicaid policy will remove a major obstacle to participation in clinical trials. An oncologist, Dr. Eskander said she is looking forward to being able to help more of her patients get access to experimental medicines and treatments.
But that may not be enough to draw more people with low incomes into these studies, said Dr. Eskander, who is now at Rutgers Cancer Institute of New Jersey in New Brunswick. She urges greater use of patient navigators to help people on Medicaid understand the resources available to them, as well as broad use of Medicaid’s nonemergency medical transportation (NEMT) benefit.
“Some patients will be offered clinical trial enrollment and some will accept, but I really worry about the challenges low-income people face with things like transportation and getting time off work,” she said.
A version of this article first appeared on Medscape.com.
Federal officials will allow some flexibility in meeting new requirements on covering the costs of clinical trials for people enrolled in Medicaid, seeking to accommodate states where legislatures will not meet in time to make needed changes in rules.
Congress in 2020 ordered U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology (ASCO) and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
The mandate went into effect on Jan. 1, but the Centers for Medicare & Medicaid Services will allow accommodations in terms of implementation time for states that have not yet been able to make needed legislative changes, Daniel Tsai, deputy administrator and director of the Center for Medicaid and CHIP Services, wrote in a Dec. 7 letter. Mr. Tsai’s letter doesn’t mention specific states. The CMS did not immediately respond to a request seeking information on the states expected to apply for waivers.
Medicaid has in recent years been a rare large U.S. insurance program that does not cover the costs of clinical trials. The Affordable Care Act of 2010 mandated this coverage for people in private insurance plans. The federal government in 2000 decided that Medicare would do so.
‘A hidden opportunity’
A perspective article last May in the New England Journal of Medicine referred to the new Medicaid mandate on clinical trials as a “hidden opportunity,” referring to its genesis as an add-on in a massive federal spending package enacted in December 2020.
In the article, Samuel U. Takvorian, MD, MSHP, of the University of Pennsylvania, Philadelphia, and coauthors noted that rates of participation in clinical trials remain low for racial and ethnic minority groups, due in part to the lack of Medicaid coverage.
“For example, non-Hispanic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials – a gap that has widened over time,” Dr. Takvorian and coauthors wrote. “Inequities in enrollment have also manifested during the COVID-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of COVID-19 therapeutics.”
In October, researchers from the Arthur G. James Cancer Hospital and Ohio State University, Columbus, published results of a retrospective study of patients with stage I-IV pancreatic cancer that also found inequities in enrollment. Mariam F. Eskander, MD, MPH, and coauthors reported what they found by examining records for 1,127 patients (0.4%) enrolled in clinical trials and 301,340 (99.6%) who did not enroll. They found that enrollment in trials increased over the study period, but not for Black patients or patients on Medicaid.
In an interview, Dr. Eskander said the new Medicaid policy will remove a major obstacle to participation in clinical trials. An oncologist, Dr. Eskander said she is looking forward to being able to help more of her patients get access to experimental medicines and treatments.
But that may not be enough to draw more people with low incomes into these studies, said Dr. Eskander, who is now at Rutgers Cancer Institute of New Jersey in New Brunswick. She urges greater use of patient navigators to help people on Medicaid understand the resources available to them, as well as broad use of Medicaid’s nonemergency medical transportation (NEMT) benefit.
“Some patients will be offered clinical trial enrollment and some will accept, but I really worry about the challenges low-income people face with things like transportation and getting time off work,” she said.
A version of this article first appeared on Medscape.com.
Were these true medical miracles? Doctors disagree
It was a freezing December day, and two young brothers were playing outside near a swimming pool when the younger boy, a 3-year-old toddler, fell into the water.
The 7-year-old immediately jumped into the pool to save his brother and was able to pull the toddler to the pool steps where the boy’s head was above water. But the icy temperatures overcame the older brother and he drifted underwater.
“Despite being at the forefront of medicine, what we don’t understand often exceeds what we do understand,” said Harley Rotbart, MD, author of “Miracles We Have Seen” (Health Communications: Deerfield Beach, Fla., 2016).
Paramedics arrived to find both boys unconscious and rushed them to the Children’s Hospital of Philadelphia. The younger boy regained consciousness in the ICU and recovered. The 7-year-old, however, was unresponsive and remained in a coma, said Dr. Rotbart a pediatrician and author based in Denver.
Family members stayed at the boy’s bedside and prayed. But after several weeks, the child’s condition remained unchanged. His parents began to discuss ending life support and organ donation. Then late one night, as Dr. Rotbart sat reading to the unconscious patient, the little boy squeezed his hand. In disbelief, Dr. Rotbart told all of his colleagues about the squeeze the next morning. Everyone attributed the movement to an involuntary muscle spasm, he said. After all, every test and scan showed the boy had no brain function.
But later that day, the child grasped another staff member’s hand. Shortly after that, he squeezed in response to a command. Dr. Rotbart and his staff were stunned, but cautious about feeling too much hope.
Days later, the child opened his eyes. Then, he smiled. His parents were overjoyed.
“When he walked out of the hospital more than 2 months after the near-drowning and his heroic rescue of his little brother, we all cheered and cried,” Dr. Rotbart wrote in his book. “We cried many times in the weeks preceding, and I still cry whenever I recall this story.”
The experience, which happened years ago when Dr. Rotbart was a trainee, has stayed with the pediatrician his entire career.
“His awakening was seemingly impossible – and then it happened,” Dr. Rotbart said. “Despite being at the forefront of medicine and science, what we don’t understand often exceeds what we do understand. And even when we think we understand, we are frequently proven wrong.”
For many, Dr. Rotbart’s experience raises questions about the existence of medical miracles.
Do physicians believe in medical miracles? The answers are diverse.
“I have no doubt that extraordinary outcomes happen where patients who are overwhelmingly expected not to survive, do,” says Eric Beam, MD, a hospitalist based in San Diego. “That’s one of the reasons we choose our words very carefully in our conversations with patients and their families and remember that nothing is 0%, and nothing is 100%. But doctors tend to treat situations that are 99.9% as absolute. I don’t think you can practice medicine with the hope or expectation that every case you see has the potential to beat the odds – or be a medical miracle.”
Disappearing cancer hailed as ‘miracle’
In 2003, physicians projected that Joseph Rick, 40, had just a few months to live. His mucosal melanoma had spread throughout his body, progressing even after several surgeries, radiation therapy, and a combination of chemotherapy agents, recalled Antoni Ribas, MD, PhD, an oncologist and director of the tumor immunology program at Jonsson Comprehensive Cancer Center in Los Angeles.
Mr. Rick’s melanoma had spread to his intestines with traces on his stomach and bladder. Tumors were present on his liver, lungs, and pancreas. Rick bought a grave and prepared for the worst, he recounted in a Cancer Research Institute video. But his fate took a turn when he enrolled in an experimental drug trial in December 2003. The phase 1 trial was for a new immune modulating antibody, called an anti–CTLA-4 antibody, said Dr. Ribas, who conducted the trial.
Over the next few weeks and months, all areas of Rick’s melanoma metastases disappeared. By 2009, he was in remission. He has lived the rest of his life with no evidence of melanoma, according to Dr. Ribas.
Mr. Rick’s case has been referenced throughout literature and news stories as a “medical miracle” and a “cancer miracle.”
Does Dr. Ribas think the case was a medical miracle?
“The response in Joseph Rick was what happened in 10%-15% of patients who received anti-CTLA-4 therapy,” Dr. Ribas said. “These were not miracles. These patients responded because their immune system trying to attack the cancer had been stuck at the CTLA-4 checkpoint. Blocking this checkpoint allowed their immune system to proceed to attack and kill cancer cells anywhere in the body.”
The scientific basis of this therapy was work by University of Texas MD Anderson Cancer Center immunologist James Allison, PhD, that had been done 5 years earlier in mouse models, where giving an anti–CTLA-4 antibody to mice allowed them to reject several implanted cancers, Dr. Ribas explained. Dr. Allison received the 2018 Nobel Prize in Physiology or Medicine for this work, subsequently opening the door for what we now call “immune checkpoint blockade therapy for cancer.” Dr. Ribas added.
“We tend to call miracles good things that we do not understand how they happened,” Dr. Ribas said. “From the human observation perspective, there have been plenty of medical miracles. However, each one has a specific biological mechanism that led to improvement in a patient. In cancer treatment, early studies using the immune system resulted in occasional patients having tumor responses and long-term benefits.
“With the increased understanding of how the immune system interacts with cancers, which is based on remarkable progress in understanding how the immune system works generated over the past several decades, these ‘miracles’ become specific mechanisms leading to response to cancer, which can then be replicated in other patients.”
Patient defies odds after 45 minutes without heartbeat
Florida ob.gyn. Michael Fleischer, MD, had just performed a routine repeat cesarean birth, delivering a healthy baby girl. His patient, Ruby, had a history of high blood pressure but medication taken during the pregnancy had kept her levels stabilized.
In the waiting room, Dr. Fleischer informed Ruby’s large family of the good news. He was planning to head home early that day when he heard his name being called over the hospital’s loudspeaker. Ruby had stopped breathing.
“The anesthesiologist was with her and had immediately intubated her,” Dr. Fleischer said. “We checked to make sure there was no problems or bleeding from the C-section, but everything was completely fine. However, we couldn’t keep her blood pressure stable.”
Dr. Fleischer suspected the respiratory arrest was caused by either an amniotic fluid embolism or a pulmonary embolism. Intubation continued and physicians gave Ruby medication to stabilize her blood pressure. Then suddenly, Ruby’s heart stopped.
Dr. Fleischer and other doctors began compressions, which they continued for 30 minutes. They shocked Ruby with defibrillator paddles multiple times, but there was no change.
“I was already thinking, this is hopeless, there’s nothing we can do,” he said. “The writing is on the wall. She’s going to die.”
Dr. Fleischer spoke to Ruby’s family and explained the tragic turn of events. Relatives were distraught and tearfully visited Ruby to say their goodbyes. They prayed and cried. Eventually, physicians ceased compressions. Ruby had gone 45 minutes without a pulse. The EKG was still showing some irregularity, FDr. leischer said, but no rhythm. Physicians kept Ruby intubated as they waited for the background electrical activity to fade. As they watched the screen in anguish, there was suddenly a blip on the heart rate monitor. Then another and another. Within seconds, Ruby’s heart went back into sinus rhythm.
“We were in disbelief,” Dr. Fleischer said. “We did some tests and put her in the ICU, and she was fine. Usually, after doing compressions on anyone, you’d have bruising or broken ribs. She had nothing. She just woke up and said: ‘What am I doing here? Let me go see my baby.’ ”
Ruby fully recovered, and 3 days later, she went home with her newborn.
While the recovery was unbelievable, Dr. Fleischer stopped short of calling it a medical miracle. There were scientific contributors to her survival: she was immediately intubated when she stopped breathing and compressions were started as soon as her heart stopped.
However, Dr. Fleischer said the fact that lifesaving measures had ended, and Ruby revived on her own was indeed, miraculous.
“It wasn’t like we were doing compressions and brought her back,” he said. “I can scientifically explain things in my mind, except for that. That when we finally stopped and took our hands off her, that’s when something changed. That’s when she came back.”
How do ‘medical miracles’ impact physicians?
When Dr. Rotbart was writing his book, which includes physician essays from across the world, he was struck by how many of the events happened decades earlier.
“This is another testament to the powerful impact these experiences have on those witnessing them,” he said. “In many cases, physicians describing events occurring years ago noted that those early memories served to give them hope as they encountered new, seemingly hopeless cases in subsequent years. Some contributors wrote that the ‘miracle experience’ actually directed them in their choice of specialty and has influenced much of their professional decision-making throughout their careers. Others draw on those miraculous moments at times when they themselves feel hopeless in the face of adversity and tragedy.”
Dr. Fleischer said that, although Ruby’s story has stayed with him, his mindset or practice style didn’t necessarily change after the experience.
“I’m not sure if it’s affected me because I haven’t been in that situation again,” he said. “I’m in the middle. I would never rule out anything, but I’m not going to base how I practice on the hope for a medical miracle.”
In a recent opinion piece for the New York Times, pulmonary and critical care physician, Daniela Lamas, MD, wrote about the sometimes negative effects of miracle cases on physicians. Such experiences for instance, can lead to a greater drive to beat the odds in future cases, which can sometimes lead to false hope, protracted critical care admissions, and futile procedures.
“After all, in most cases in the ICU, our initial prognoses are correct,” she wrote. “So there’s a risk to standing at the bedside, thinking about that one patient who made it home despite our predictions. We can give that experience too much weight in influencing our decisions and recommendations.”
Dr. Beam said unexpected outcomes – particularly in the age of COVID-19 – can certainly make physicians think differently about life-sustaining measures and when to discuss end-of-life care with family members. In his own practice, Dr. Beam has encountered unexpected COVID recoveries. Now, he generally gives extremely ill COVID patients a little more time to see if their bodies recover.
“It remains true that people who are really sick with COVID, who are on ventilated or who are requiring a lot of up respiratory support, they don’t do well on average,” he said. “But it is [also] true that there are a handful of people who get to that point and do come back to 80% or 90% of where they were. It makes you think twice.”
What to do when parents hope for a miracle
In his palliative care practice, Nashville, Tenn., surgeon Myrick Shinall Jr., MD, PhD, regularly encounters families and patients who wish for a medical miracle.
“It happens pretty often from a palliative care perspective,” he said. “What I have experienced the most is a patient with a severe brain injury who we don’t believe is recoverable. The medical team is discussing with the family that it is probably time to discontinue the ventilator. In those situations, families will often talk about wanting us to continue on [our life-sustaining efforts] in the hopes that a miracle will happen.”
Dr. Shinall and Trevor Bibler, PhD, recently authored two articles about best practices for responding to patients who hope for a miracle. The first one, published in the American Journal of Bioethics, is directed toward bioethicists; the second article, in the Journal of Pain and Symptom Management, targets clinicians.
A primary takeaway from the papers is that health professionals should recognize that hope for a miracle may mean different things to different people, said Dr. Bibler, an ethicist and assistant professor at Baylor College of Medicine, Houston. Some patients may have an innocuous hope for a miracle without a religious connotation, whereas others may have a firm conviction in their idea of God, their spirituality, and a concrete vision of the miracle.
“To hear that a family or patient is hoping for a miracle, one shouldn’t assume they already know what the patient or the family might mean by that,” Dr. Bibler said. “If a patient were to say, ‘I hope for a miracle,’ you might ask: ‘What do you mean by a miracle?’ Health professionals should feel empowered to ask that question.”
Health care professionals should explore a patient’s hope for a miracle, be nonjudgmental, ask clarifying questions, restate what the patient has said, and delve into the patient’s world view on death and dying, according to Dr. Bibler’s analyses. In some cases, it may be helpful to include a chaplain or the presence of a theology outsider in discussions.
When his patients and their families raise the subject of miracles, Dr. Shinall said he inquires what a miracle would look like in their opinion and tries to gauge how much of the assertion is a general hope compared with a firm belief.
“I try to work with them to make sure they understand doctors’ decisions and recommendations are based on what we know and can predict from our medical experience,” he said. “And that there’s nothing we’re going to do to prevent a miracle from happening, but that that can’t be our medical plan – to wait for a miracle.”
Despite the many patients and families Dr. Shinall has encountered who hope for a miracle, he has never experienced a case that he would describe as a medical miracle, he said.
Dr. Rotbart believes all physicians struggle with finding balance in how far to push in hope of a miracle and when to let go.
“Miracles, whether they happen to us, or we hear of them from colleagues or we read about them, should humble us as physicians,” he said. “I have come to believe that what we don’t know or don’t understand about medicine, medical miracles, or life in general, isn‘t necessarily cause for fear, and can even be reason for hope.
“Medicine has come a long way since Hippocrates’ theory of The Four Humors and The Four Temperaments, yet we still have much to learn about the workings of the human body. As physicians, we should take comfort in how much we don’t know because that allows us to share hope with our patients and, occasionally, makes medical miracles possible.”
A version of this article first appeared on Medscape.com.
It was a freezing December day, and two young brothers were playing outside near a swimming pool when the younger boy, a 3-year-old toddler, fell into the water.
The 7-year-old immediately jumped into the pool to save his brother and was able to pull the toddler to the pool steps where the boy’s head was above water. But the icy temperatures overcame the older brother and he drifted underwater.
“Despite being at the forefront of medicine, what we don’t understand often exceeds what we do understand,” said Harley Rotbart, MD, author of “Miracles We Have Seen” (Health Communications: Deerfield Beach, Fla., 2016).
Paramedics arrived to find both boys unconscious and rushed them to the Children’s Hospital of Philadelphia. The younger boy regained consciousness in the ICU and recovered. The 7-year-old, however, was unresponsive and remained in a coma, said Dr. Rotbart a pediatrician and author based in Denver.
Family members stayed at the boy’s bedside and prayed. But after several weeks, the child’s condition remained unchanged. His parents began to discuss ending life support and organ donation. Then late one night, as Dr. Rotbart sat reading to the unconscious patient, the little boy squeezed his hand. In disbelief, Dr. Rotbart told all of his colleagues about the squeeze the next morning. Everyone attributed the movement to an involuntary muscle spasm, he said. After all, every test and scan showed the boy had no brain function.
But later that day, the child grasped another staff member’s hand. Shortly after that, he squeezed in response to a command. Dr. Rotbart and his staff were stunned, but cautious about feeling too much hope.
Days later, the child opened his eyes. Then, he smiled. His parents were overjoyed.
“When he walked out of the hospital more than 2 months after the near-drowning and his heroic rescue of his little brother, we all cheered and cried,” Dr. Rotbart wrote in his book. “We cried many times in the weeks preceding, and I still cry whenever I recall this story.”
The experience, which happened years ago when Dr. Rotbart was a trainee, has stayed with the pediatrician his entire career.
“His awakening was seemingly impossible – and then it happened,” Dr. Rotbart said. “Despite being at the forefront of medicine and science, what we don’t understand often exceeds what we do understand. And even when we think we understand, we are frequently proven wrong.”
For many, Dr. Rotbart’s experience raises questions about the existence of medical miracles.
Do physicians believe in medical miracles? The answers are diverse.
“I have no doubt that extraordinary outcomes happen where patients who are overwhelmingly expected not to survive, do,” says Eric Beam, MD, a hospitalist based in San Diego. “That’s one of the reasons we choose our words very carefully in our conversations with patients and their families and remember that nothing is 0%, and nothing is 100%. But doctors tend to treat situations that are 99.9% as absolute. I don’t think you can practice medicine with the hope or expectation that every case you see has the potential to beat the odds – or be a medical miracle.”
Disappearing cancer hailed as ‘miracle’
In 2003, physicians projected that Joseph Rick, 40, had just a few months to live. His mucosal melanoma had spread throughout his body, progressing even after several surgeries, radiation therapy, and a combination of chemotherapy agents, recalled Antoni Ribas, MD, PhD, an oncologist and director of the tumor immunology program at Jonsson Comprehensive Cancer Center in Los Angeles.
Mr. Rick’s melanoma had spread to his intestines with traces on his stomach and bladder. Tumors were present on his liver, lungs, and pancreas. Rick bought a grave and prepared for the worst, he recounted in a Cancer Research Institute video. But his fate took a turn when he enrolled in an experimental drug trial in December 2003. The phase 1 trial was for a new immune modulating antibody, called an anti–CTLA-4 antibody, said Dr. Ribas, who conducted the trial.
Over the next few weeks and months, all areas of Rick’s melanoma metastases disappeared. By 2009, he was in remission. He has lived the rest of his life with no evidence of melanoma, according to Dr. Ribas.
Mr. Rick’s case has been referenced throughout literature and news stories as a “medical miracle” and a “cancer miracle.”
Does Dr. Ribas think the case was a medical miracle?
“The response in Joseph Rick was what happened in 10%-15% of patients who received anti-CTLA-4 therapy,” Dr. Ribas said. “These were not miracles. These patients responded because their immune system trying to attack the cancer had been stuck at the CTLA-4 checkpoint. Blocking this checkpoint allowed their immune system to proceed to attack and kill cancer cells anywhere in the body.”
The scientific basis of this therapy was work by University of Texas MD Anderson Cancer Center immunologist James Allison, PhD, that had been done 5 years earlier in mouse models, where giving an anti–CTLA-4 antibody to mice allowed them to reject several implanted cancers, Dr. Ribas explained. Dr. Allison received the 2018 Nobel Prize in Physiology or Medicine for this work, subsequently opening the door for what we now call “immune checkpoint blockade therapy for cancer.” Dr. Ribas added.
“We tend to call miracles good things that we do not understand how they happened,” Dr. Ribas said. “From the human observation perspective, there have been plenty of medical miracles. However, each one has a specific biological mechanism that led to improvement in a patient. In cancer treatment, early studies using the immune system resulted in occasional patients having tumor responses and long-term benefits.
“With the increased understanding of how the immune system interacts with cancers, which is based on remarkable progress in understanding how the immune system works generated over the past several decades, these ‘miracles’ become specific mechanisms leading to response to cancer, which can then be replicated in other patients.”
Patient defies odds after 45 minutes without heartbeat
Florida ob.gyn. Michael Fleischer, MD, had just performed a routine repeat cesarean birth, delivering a healthy baby girl. His patient, Ruby, had a history of high blood pressure but medication taken during the pregnancy had kept her levels stabilized.
In the waiting room, Dr. Fleischer informed Ruby’s large family of the good news. He was planning to head home early that day when he heard his name being called over the hospital’s loudspeaker. Ruby had stopped breathing.
“The anesthesiologist was with her and had immediately intubated her,” Dr. Fleischer said. “We checked to make sure there was no problems or bleeding from the C-section, but everything was completely fine. However, we couldn’t keep her blood pressure stable.”
Dr. Fleischer suspected the respiratory arrest was caused by either an amniotic fluid embolism or a pulmonary embolism. Intubation continued and physicians gave Ruby medication to stabilize her blood pressure. Then suddenly, Ruby’s heart stopped.
Dr. Fleischer and other doctors began compressions, which they continued for 30 minutes. They shocked Ruby with defibrillator paddles multiple times, but there was no change.
“I was already thinking, this is hopeless, there’s nothing we can do,” he said. “The writing is on the wall. She’s going to die.”
Dr. Fleischer spoke to Ruby’s family and explained the tragic turn of events. Relatives were distraught and tearfully visited Ruby to say their goodbyes. They prayed and cried. Eventually, physicians ceased compressions. Ruby had gone 45 minutes without a pulse. The EKG was still showing some irregularity, FDr. leischer said, but no rhythm. Physicians kept Ruby intubated as they waited for the background electrical activity to fade. As they watched the screen in anguish, there was suddenly a blip on the heart rate monitor. Then another and another. Within seconds, Ruby’s heart went back into sinus rhythm.
“We were in disbelief,” Dr. Fleischer said. “We did some tests and put her in the ICU, and she was fine. Usually, after doing compressions on anyone, you’d have bruising or broken ribs. She had nothing. She just woke up and said: ‘What am I doing here? Let me go see my baby.’ ”
Ruby fully recovered, and 3 days later, she went home with her newborn.
While the recovery was unbelievable, Dr. Fleischer stopped short of calling it a medical miracle. There were scientific contributors to her survival: she was immediately intubated when she stopped breathing and compressions were started as soon as her heart stopped.
However, Dr. Fleischer said the fact that lifesaving measures had ended, and Ruby revived on her own was indeed, miraculous.
“It wasn’t like we were doing compressions and brought her back,” he said. “I can scientifically explain things in my mind, except for that. That when we finally stopped and took our hands off her, that’s when something changed. That’s when she came back.”
How do ‘medical miracles’ impact physicians?
When Dr. Rotbart was writing his book, which includes physician essays from across the world, he was struck by how many of the events happened decades earlier.
“This is another testament to the powerful impact these experiences have on those witnessing them,” he said. “In many cases, physicians describing events occurring years ago noted that those early memories served to give them hope as they encountered new, seemingly hopeless cases in subsequent years. Some contributors wrote that the ‘miracle experience’ actually directed them in their choice of specialty and has influenced much of their professional decision-making throughout their careers. Others draw on those miraculous moments at times when they themselves feel hopeless in the face of adversity and tragedy.”
Dr. Fleischer said that, although Ruby’s story has stayed with him, his mindset or practice style didn’t necessarily change after the experience.
“I’m not sure if it’s affected me because I haven’t been in that situation again,” he said. “I’m in the middle. I would never rule out anything, but I’m not going to base how I practice on the hope for a medical miracle.”
In a recent opinion piece for the New York Times, pulmonary and critical care physician, Daniela Lamas, MD, wrote about the sometimes negative effects of miracle cases on physicians. Such experiences for instance, can lead to a greater drive to beat the odds in future cases, which can sometimes lead to false hope, protracted critical care admissions, and futile procedures.
“After all, in most cases in the ICU, our initial prognoses are correct,” she wrote. “So there’s a risk to standing at the bedside, thinking about that one patient who made it home despite our predictions. We can give that experience too much weight in influencing our decisions and recommendations.”
Dr. Beam said unexpected outcomes – particularly in the age of COVID-19 – can certainly make physicians think differently about life-sustaining measures and when to discuss end-of-life care with family members. In his own practice, Dr. Beam has encountered unexpected COVID recoveries. Now, he generally gives extremely ill COVID patients a little more time to see if their bodies recover.
“It remains true that people who are really sick with COVID, who are on ventilated or who are requiring a lot of up respiratory support, they don’t do well on average,” he said. “But it is [also] true that there are a handful of people who get to that point and do come back to 80% or 90% of where they were. It makes you think twice.”
What to do when parents hope for a miracle
In his palliative care practice, Nashville, Tenn., surgeon Myrick Shinall Jr., MD, PhD, regularly encounters families and patients who wish for a medical miracle.
“It happens pretty often from a palliative care perspective,” he said. “What I have experienced the most is a patient with a severe brain injury who we don’t believe is recoverable. The medical team is discussing with the family that it is probably time to discontinue the ventilator. In those situations, families will often talk about wanting us to continue on [our life-sustaining efforts] in the hopes that a miracle will happen.”
Dr. Shinall and Trevor Bibler, PhD, recently authored two articles about best practices for responding to patients who hope for a miracle. The first one, published in the American Journal of Bioethics, is directed toward bioethicists; the second article, in the Journal of Pain and Symptom Management, targets clinicians.
A primary takeaway from the papers is that health professionals should recognize that hope for a miracle may mean different things to different people, said Dr. Bibler, an ethicist and assistant professor at Baylor College of Medicine, Houston. Some patients may have an innocuous hope for a miracle without a religious connotation, whereas others may have a firm conviction in their idea of God, their spirituality, and a concrete vision of the miracle.
“To hear that a family or patient is hoping for a miracle, one shouldn’t assume they already know what the patient or the family might mean by that,” Dr. Bibler said. “If a patient were to say, ‘I hope for a miracle,’ you might ask: ‘What do you mean by a miracle?’ Health professionals should feel empowered to ask that question.”
Health care professionals should explore a patient’s hope for a miracle, be nonjudgmental, ask clarifying questions, restate what the patient has said, and delve into the patient’s world view on death and dying, according to Dr. Bibler’s analyses. In some cases, it may be helpful to include a chaplain or the presence of a theology outsider in discussions.
When his patients and their families raise the subject of miracles, Dr. Shinall said he inquires what a miracle would look like in their opinion and tries to gauge how much of the assertion is a general hope compared with a firm belief.
“I try to work with them to make sure they understand doctors’ decisions and recommendations are based on what we know and can predict from our medical experience,” he said. “And that there’s nothing we’re going to do to prevent a miracle from happening, but that that can’t be our medical plan – to wait for a miracle.”
Despite the many patients and families Dr. Shinall has encountered who hope for a miracle, he has never experienced a case that he would describe as a medical miracle, he said.
Dr. Rotbart believes all physicians struggle with finding balance in how far to push in hope of a miracle and when to let go.
“Miracles, whether they happen to us, or we hear of them from colleagues or we read about them, should humble us as physicians,” he said. “I have come to believe that what we don’t know or don’t understand about medicine, medical miracles, or life in general, isn‘t necessarily cause for fear, and can even be reason for hope.
“Medicine has come a long way since Hippocrates’ theory of The Four Humors and The Four Temperaments, yet we still have much to learn about the workings of the human body. As physicians, we should take comfort in how much we don’t know because that allows us to share hope with our patients and, occasionally, makes medical miracles possible.”
A version of this article first appeared on Medscape.com.
It was a freezing December day, and two young brothers were playing outside near a swimming pool when the younger boy, a 3-year-old toddler, fell into the water.
The 7-year-old immediately jumped into the pool to save his brother and was able to pull the toddler to the pool steps where the boy’s head was above water. But the icy temperatures overcame the older brother and he drifted underwater.
“Despite being at the forefront of medicine, what we don’t understand often exceeds what we do understand,” said Harley Rotbart, MD, author of “Miracles We Have Seen” (Health Communications: Deerfield Beach, Fla., 2016).
Paramedics arrived to find both boys unconscious and rushed them to the Children’s Hospital of Philadelphia. The younger boy regained consciousness in the ICU and recovered. The 7-year-old, however, was unresponsive and remained in a coma, said Dr. Rotbart a pediatrician and author based in Denver.
Family members stayed at the boy’s bedside and prayed. But after several weeks, the child’s condition remained unchanged. His parents began to discuss ending life support and organ donation. Then late one night, as Dr. Rotbart sat reading to the unconscious patient, the little boy squeezed his hand. In disbelief, Dr. Rotbart told all of his colleagues about the squeeze the next morning. Everyone attributed the movement to an involuntary muscle spasm, he said. After all, every test and scan showed the boy had no brain function.
But later that day, the child grasped another staff member’s hand. Shortly after that, he squeezed in response to a command. Dr. Rotbart and his staff were stunned, but cautious about feeling too much hope.
Days later, the child opened his eyes. Then, he smiled. His parents were overjoyed.
“When he walked out of the hospital more than 2 months after the near-drowning and his heroic rescue of his little brother, we all cheered and cried,” Dr. Rotbart wrote in his book. “We cried many times in the weeks preceding, and I still cry whenever I recall this story.”
The experience, which happened years ago when Dr. Rotbart was a trainee, has stayed with the pediatrician his entire career.
“His awakening was seemingly impossible – and then it happened,” Dr. Rotbart said. “Despite being at the forefront of medicine and science, what we don’t understand often exceeds what we do understand. And even when we think we understand, we are frequently proven wrong.”
For many, Dr. Rotbart’s experience raises questions about the existence of medical miracles.
Do physicians believe in medical miracles? The answers are diverse.
“I have no doubt that extraordinary outcomes happen where patients who are overwhelmingly expected not to survive, do,” says Eric Beam, MD, a hospitalist based in San Diego. “That’s one of the reasons we choose our words very carefully in our conversations with patients and their families and remember that nothing is 0%, and nothing is 100%. But doctors tend to treat situations that are 99.9% as absolute. I don’t think you can practice medicine with the hope or expectation that every case you see has the potential to beat the odds – or be a medical miracle.”
Disappearing cancer hailed as ‘miracle’
In 2003, physicians projected that Joseph Rick, 40, had just a few months to live. His mucosal melanoma had spread throughout his body, progressing even after several surgeries, radiation therapy, and a combination of chemotherapy agents, recalled Antoni Ribas, MD, PhD, an oncologist and director of the tumor immunology program at Jonsson Comprehensive Cancer Center in Los Angeles.
Mr. Rick’s melanoma had spread to his intestines with traces on his stomach and bladder. Tumors were present on his liver, lungs, and pancreas. Rick bought a grave and prepared for the worst, he recounted in a Cancer Research Institute video. But his fate took a turn when he enrolled in an experimental drug trial in December 2003. The phase 1 trial was for a new immune modulating antibody, called an anti–CTLA-4 antibody, said Dr. Ribas, who conducted the trial.
Over the next few weeks and months, all areas of Rick’s melanoma metastases disappeared. By 2009, he was in remission. He has lived the rest of his life with no evidence of melanoma, according to Dr. Ribas.
Mr. Rick’s case has been referenced throughout literature and news stories as a “medical miracle” and a “cancer miracle.”
Does Dr. Ribas think the case was a medical miracle?
“The response in Joseph Rick was what happened in 10%-15% of patients who received anti-CTLA-4 therapy,” Dr. Ribas said. “These were not miracles. These patients responded because their immune system trying to attack the cancer had been stuck at the CTLA-4 checkpoint. Blocking this checkpoint allowed their immune system to proceed to attack and kill cancer cells anywhere in the body.”
The scientific basis of this therapy was work by University of Texas MD Anderson Cancer Center immunologist James Allison, PhD, that had been done 5 years earlier in mouse models, where giving an anti–CTLA-4 antibody to mice allowed them to reject several implanted cancers, Dr. Ribas explained. Dr. Allison received the 2018 Nobel Prize in Physiology or Medicine for this work, subsequently opening the door for what we now call “immune checkpoint blockade therapy for cancer.” Dr. Ribas added.
“We tend to call miracles good things that we do not understand how they happened,” Dr. Ribas said. “From the human observation perspective, there have been plenty of medical miracles. However, each one has a specific biological mechanism that led to improvement in a patient. In cancer treatment, early studies using the immune system resulted in occasional patients having tumor responses and long-term benefits.
“With the increased understanding of how the immune system interacts with cancers, which is based on remarkable progress in understanding how the immune system works generated over the past several decades, these ‘miracles’ become specific mechanisms leading to response to cancer, which can then be replicated in other patients.”
Patient defies odds after 45 minutes without heartbeat
Florida ob.gyn. Michael Fleischer, MD, had just performed a routine repeat cesarean birth, delivering a healthy baby girl. His patient, Ruby, had a history of high blood pressure but medication taken during the pregnancy had kept her levels stabilized.
In the waiting room, Dr. Fleischer informed Ruby’s large family of the good news. He was planning to head home early that day when he heard his name being called over the hospital’s loudspeaker. Ruby had stopped breathing.
“The anesthesiologist was with her and had immediately intubated her,” Dr. Fleischer said. “We checked to make sure there was no problems or bleeding from the C-section, but everything was completely fine. However, we couldn’t keep her blood pressure stable.”
Dr. Fleischer suspected the respiratory arrest was caused by either an amniotic fluid embolism or a pulmonary embolism. Intubation continued and physicians gave Ruby medication to stabilize her blood pressure. Then suddenly, Ruby’s heart stopped.
Dr. Fleischer and other doctors began compressions, which they continued for 30 minutes. They shocked Ruby with defibrillator paddles multiple times, but there was no change.
“I was already thinking, this is hopeless, there’s nothing we can do,” he said. “The writing is on the wall. She’s going to die.”
Dr. Fleischer spoke to Ruby’s family and explained the tragic turn of events. Relatives were distraught and tearfully visited Ruby to say their goodbyes. They prayed and cried. Eventually, physicians ceased compressions. Ruby had gone 45 minutes without a pulse. The EKG was still showing some irregularity, FDr. leischer said, but no rhythm. Physicians kept Ruby intubated as they waited for the background electrical activity to fade. As they watched the screen in anguish, there was suddenly a blip on the heart rate monitor. Then another and another. Within seconds, Ruby’s heart went back into sinus rhythm.
“We were in disbelief,” Dr. Fleischer said. “We did some tests and put her in the ICU, and she was fine. Usually, after doing compressions on anyone, you’d have bruising or broken ribs. She had nothing. She just woke up and said: ‘What am I doing here? Let me go see my baby.’ ”
Ruby fully recovered, and 3 days later, she went home with her newborn.
While the recovery was unbelievable, Dr. Fleischer stopped short of calling it a medical miracle. There were scientific contributors to her survival: she was immediately intubated when she stopped breathing and compressions were started as soon as her heart stopped.
However, Dr. Fleischer said the fact that lifesaving measures had ended, and Ruby revived on her own was indeed, miraculous.
“It wasn’t like we were doing compressions and brought her back,” he said. “I can scientifically explain things in my mind, except for that. That when we finally stopped and took our hands off her, that’s when something changed. That’s when she came back.”
How do ‘medical miracles’ impact physicians?
When Dr. Rotbart was writing his book, which includes physician essays from across the world, he was struck by how many of the events happened decades earlier.
“This is another testament to the powerful impact these experiences have on those witnessing them,” he said. “In many cases, physicians describing events occurring years ago noted that those early memories served to give them hope as they encountered new, seemingly hopeless cases in subsequent years. Some contributors wrote that the ‘miracle experience’ actually directed them in their choice of specialty and has influenced much of their professional decision-making throughout their careers. Others draw on those miraculous moments at times when they themselves feel hopeless in the face of adversity and tragedy.”
Dr. Fleischer said that, although Ruby’s story has stayed with him, his mindset or practice style didn’t necessarily change after the experience.
“I’m not sure if it’s affected me because I haven’t been in that situation again,” he said. “I’m in the middle. I would never rule out anything, but I’m not going to base how I practice on the hope for a medical miracle.”
In a recent opinion piece for the New York Times, pulmonary and critical care physician, Daniela Lamas, MD, wrote about the sometimes negative effects of miracle cases on physicians. Such experiences for instance, can lead to a greater drive to beat the odds in future cases, which can sometimes lead to false hope, protracted critical care admissions, and futile procedures.
“After all, in most cases in the ICU, our initial prognoses are correct,” she wrote. “So there’s a risk to standing at the bedside, thinking about that one patient who made it home despite our predictions. We can give that experience too much weight in influencing our decisions and recommendations.”
Dr. Beam said unexpected outcomes – particularly in the age of COVID-19 – can certainly make physicians think differently about life-sustaining measures and when to discuss end-of-life care with family members. In his own practice, Dr. Beam has encountered unexpected COVID recoveries. Now, he generally gives extremely ill COVID patients a little more time to see if their bodies recover.
“It remains true that people who are really sick with COVID, who are on ventilated or who are requiring a lot of up respiratory support, they don’t do well on average,” he said. “But it is [also] true that there are a handful of people who get to that point and do come back to 80% or 90% of where they were. It makes you think twice.”
What to do when parents hope for a miracle
In his palliative care practice, Nashville, Tenn., surgeon Myrick Shinall Jr., MD, PhD, regularly encounters families and patients who wish for a medical miracle.
“It happens pretty often from a palliative care perspective,” he said. “What I have experienced the most is a patient with a severe brain injury who we don’t believe is recoverable. The medical team is discussing with the family that it is probably time to discontinue the ventilator. In those situations, families will often talk about wanting us to continue on [our life-sustaining efforts] in the hopes that a miracle will happen.”
Dr. Shinall and Trevor Bibler, PhD, recently authored two articles about best practices for responding to patients who hope for a miracle. The first one, published in the American Journal of Bioethics, is directed toward bioethicists; the second article, in the Journal of Pain and Symptom Management, targets clinicians.
A primary takeaway from the papers is that health professionals should recognize that hope for a miracle may mean different things to different people, said Dr. Bibler, an ethicist and assistant professor at Baylor College of Medicine, Houston. Some patients may have an innocuous hope for a miracle without a religious connotation, whereas others may have a firm conviction in their idea of God, their spirituality, and a concrete vision of the miracle.
“To hear that a family or patient is hoping for a miracle, one shouldn’t assume they already know what the patient or the family might mean by that,” Dr. Bibler said. “If a patient were to say, ‘I hope for a miracle,’ you might ask: ‘What do you mean by a miracle?’ Health professionals should feel empowered to ask that question.”
Health care professionals should explore a patient’s hope for a miracle, be nonjudgmental, ask clarifying questions, restate what the patient has said, and delve into the patient’s world view on death and dying, according to Dr. Bibler’s analyses. In some cases, it may be helpful to include a chaplain or the presence of a theology outsider in discussions.
When his patients and their families raise the subject of miracles, Dr. Shinall said he inquires what a miracle would look like in their opinion and tries to gauge how much of the assertion is a general hope compared with a firm belief.
“I try to work with them to make sure they understand doctors’ decisions and recommendations are based on what we know and can predict from our medical experience,” he said. “And that there’s nothing we’re going to do to prevent a miracle from happening, but that that can’t be our medical plan – to wait for a miracle.”
Despite the many patients and families Dr. Shinall has encountered who hope for a miracle, he has never experienced a case that he would describe as a medical miracle, he said.
Dr. Rotbart believes all physicians struggle with finding balance in how far to push in hope of a miracle and when to let go.
“Miracles, whether they happen to us, or we hear of them from colleagues or we read about them, should humble us as physicians,” he said. “I have come to believe that what we don’t know or don’t understand about medicine, medical miracles, or life in general, isn‘t necessarily cause for fear, and can even be reason for hope.
“Medicine has come a long way since Hippocrates’ theory of The Four Humors and The Four Temperaments, yet we still have much to learn about the workings of the human body. As physicians, we should take comfort in how much we don’t know because that allows us to share hope with our patients and, occasionally, makes medical miracles possible.”
A version of this article first appeared on Medscape.com.
Pediatric insomnia: Treatment
Children and adolescents who do not receive sufficient sleep can experience worsening inattention, daytime fatigue, and cognitive and behavioral difficulties. Assessment and treatment of insomnia and other sleep difficulties in young patients is critical as poor sleep increases their risk for depression, self-harm, and suicide.
In Part 1 of this article (Pediatric insomnia: Assessment and diagnosis,
Psychotherapeutic interventions
Regardless of the source of a child’s insomnia or co-occurring disorders, healthy sleep practices are the first line behavioral treatment, including for youth with attention-deficit/hyperactivity disorder (ADHD), anxiety disorders, obsessive-compulsive disorder, and depressive disorders.
Healthy sleep practices/sleep hygiene
Developmentally appropriate bedtimes and routines (Table). Helping children establish a regular, consistent bedtime is key in promoting healthy sleep. Ideally, the bedtime routine involves 3 to 4 activities each night in the same order, and these activities should be relaxing and soothing (eg, taking a bath, putting on pajamas, reading books). Setting age-appropriate bedtimes also is important. If an older child is asked to go to bed at 8 pm but cannot fall asleep for an hour, they may not have insomnia but instead a developmentally inappropriate bedtime. Several studies found that children younger than age 10 should go to bed no later than 9 pm. Bedtimes later than 9 pm for young children are correlated with shorter sleep duration.1

Consistent sleep schedules. Another important aspect of healthy sleep is working with parents to enforce a consistent bedtime and wake-up time, including weekdays and weekends. Ideally, bedtime on weekdays and weekends should not vary by more than 1 hour. Helping children wake up at the same time each day helps to set and regulate their circadian rhythm. Keeping these schedules consistent on vacations and school holidays also is helpful. For adolescents, the weekday/weekend bedtimes can vary by up to 2 hours because adolescents have a delayed circadian rhythm and wake-up times for high school can be early.
Environmental factors. An important piece of parental education is stimulus control and the ingredients of healthy sleep. Healthy sleep ingredients include a dark, quiet, consistent, and cool bedroom; a comfortable bed, the child feeling safe, and limited environmental stimuli.
Continue to: Cognitive-behavioral therapy for insomnia...
Cognitive-behavioral therapy for insomnia
Relaxation. Pediatric patients can be taught relaxation, mindfulness, meditation, and progressive muscle relaxation techniques to help lower overall stress. This can be especially helpful for youth with sleep disorders or anxiety. Guided relaxation apps are popular among children and teens, and various apps offer soothing sounds, deep breathing, progressive muscle relaxation, and guided imagery. This can be taught in psychotherapy sessions and used at home to promote gains in between sessions.
Stimulus control. Stimulus control involves using the bed exclusively for sleep and avoiding nonsleep activities in bed (eg, reading, watching television, using a computer, worrying). These activities promote wakefulness and insomnia. This may mean the child does not get into bed until they cannot keep their eyes open, even if that delays bedtime. If the child is still awake within 15 to 20 minutes, they should be encouraged to get out of bed and engage in a nonstimulating activity such as meditation, reading, or sitting quietly in the dark or low light. This recommendation can run counter to parents’ intuition that children with sleep problems should go to bed earlier. Using the bed only for sleep conditions the child to falling asleep or being asleep when in bed.
Sleep restriction. Sleep restriction involves restricting sleep to a set number of hours in order to increase their sleep efficiency (time slept in bed divided by total time spent in bed x 100). Restricting sleep to 6 to 7 hours increases sleep efficiency, consolidates sleep, and extinguishes the association of being awake in bed. For older adolescents, sleep restriction may help to limit their time in bed to either falling asleep or being asleep. This is intended to be used as a short-term strategy and only after other sleep hygiene measures (bedtime routine, environmental factors, etc) have been put into place for several weeks. While this strategy sounds unappealing to most individuals with insomnia, it can lead to lasting change due to the use of behavioral conditioning in bed. Because sleep restriction can lead to significant daytime sleepiness and impairment during the day, sleep should not be restricted to <6 hours a day for children and adolescents. Once the adolescent is sleeping more consistently and sleep efficiency reaches 85% or higher, time in bed for sleep is increased.2
Cognitive restructuring. Some children and adolescents develop maladaptive thoughts about sleep that further promote insomnia. These thoughts might include “I will never get to sleep,” “I am going to have a terrible day if I cannot fall asleep,” or “I will fail my test tomorrow if I am unable to sleep.” Such maladaptive thoughts are often untrue but promote wakefulness in the early or middle part of the night. Cognitive restructuring involves helping the child identify each problematic thought, challenge how accurate each thought is with evidence, and replace the problematic thought with a more helpful thought. For instance, an adolescent can recognize that even if they have a sleepless night, their catastrophic outcome (eg, “I will not be able to function”) is likely untrue. A psychologist can help review evidence for this, including previous times when the adolescent has not slept well and managed to get through the next day.
When is pharmacologic treatment needed?
Pharmacologic treatment may be indicated if a child does not show significant improvement following behavioral intervention (Figure). However, it is critical to exclude other primary causes of dyssomnia (eg, obstructive sleep apnea, iron deficiency anemia) before pursuing pharmacotherapy, because pharmacotherapy could mask an underlying disorder. Moreover, while there is relatively limited evidence for psychopharmacologic interventions for sleep difficulties in children and adolescents, a large survey of child and adolescent psychiatrists (N = 1,273) suggested that medications were considered for one-quarter of pediatric patients with insomnia.3 Further, patients with specific comorbidities such as neurodevelopmental disorders may be more likely to be prescribed soporifics.4

Continue to: What is the evidence for pharmacotherapy?...
What is the evidence for pharmacotherapy?
Antihistamines. Histamine antagonists—which promote sleep by blocking the wakefulness-promoting and circadian-related effects of histamine—are the most commonly used medications to treat pediatric insomnia, despite a dearth of data from prospective trials.5,6 In 1 small study, Russo et al7 found diphenhydramine, 1 mg/kg at bedtime, reduced sleep latency and nighttime awakenings, and increased sleep duration in patients ages 2 to 12; similar effects have been observed in pediatric burn patients.8 There are some limited data for other H1 antagonists (eg, hydroxyzine) in pediatric insomnia.9-11
Alpha-2 agonists increase rapid eye movement sleep via dose-dependent downregulation of noradrenergic signaling12 and thus have been commonly prescribed for insomnia in children and adolescents. In fact, the nonselective alpha-2 agonist clonidine is among the most prescribed medications for youth with insomnia, and may be efficacious in youth with neurodevelopmental disorders and ADHD.13 In small retrospective studies, clonidine decreased sleep latency and nighttime awakenings in addition to increasing sleep duration.14 Also, clonidine was well tolerated but associated with daytime somnolence. Guanfacine—a selective alpha-2 agonist—is also commonly prescribed for insomnia in youth, although results of trials have been equivocal.15 Given the more rapid absorption and shorter Tmax of clonidine relative to guanfacine, the former may be preferred as a soporific.
Melatonin and melatonin agonists. The primary regulator of the sleep-wake cycle is melatonin, an endogenous hormone produced by the pineal gland in response to changes in retinal light perception. Exogenous melatonin supplementation may be the preferred initial pharmacotherapy for sleep-onset insomnia due to its chronobiotic properties.16 In clinical studies, both immediate-release17,18 and extended-release19 melatonin reduced sleep-onset latency and increased total sleep duration in pediatric patients, although the increase in total duration of sleep was greater with extended-release preparations. Additionally, tolerability data for melatonin in pediatric patients are encouraging. A 2-year randomized trial of prolonged-release melatonin for insomnia in pediatric patients found no adverse effects with regard to growth, body mass index, or pubertal development.20 Additionally, significant improvements in sleep quality, sleep patterns, and caregiver satisfaction were maintained throughout the trial, and no withdrawal symptoms were observed upon discontinuation.
Melatonin may have a particularly important role in circadian rhythm sleep disorders. In this regard, low-dose melatonin (0.5 mg), when timed relative to the endogenous dim light melatonin onset (DLMO), is more effective in shifting sleep phase than higher doses, which suggests that timing may have greater impact than dosage.21 Data regarding melatonin administration with respect to DLMO suggest that the optimal administration time is 4 to 6 hours before a child’s preferred bedtime, and doses of 0.5 to 1 mg have been effective when given in this window.22 Variation across studies has contributed to a lack of consensus regarding pediatric melatonin dosing. For example, .05 mg/kg may be a minimal effective dose when given 1 to 2 hours before bedtime18; however, in surveys doses vary considerably, with typical doses of 2.5 to 3 mg for prepubertal children and 5 mg for adolescents.5 Of note, in patients with decreased cytochrome P450 (CYP) 1A2 activity, lack of diurnal variation in melatonin serum concentration may decrease the effectiveness of melatonin.16Ramelteon is a potent agonist of the melatonin MT1 and MT2 receptors, with a significantly higher binding affinity than melatonin in vitro. In case reports, ramelteon was well-tolerated, improved delayed sleep onset, and decreased nighttime awakenings.23
Zolpidem, eszopiclone and zaleplon. Studies of selective GABAergic modulators and benzodiazepines have not produced positive results in prospective trials of youth with insomnia. Zolpidem was studied in children and adolescents (N = 201) with ADHD; although sleep latency did not differ between zolpidem and placebo, some significant improvements were observed in adolescents.24 Zolpidem was generally well tolerated, with approximately 10% of youth discontinuing due to adverse effects. Additionally, eszopiclone—which has a longer duration of action compared with zolpidem—has been studied in children and adolescents with ADHD (N = 486) who were also evaluated with a sleep study. No differences were observed between placebo and eszopiclone in terms of sleep latency and approximately 10% of patients discontinued treatment as a result of adverse events.25 We were unable to locate any prospective trials of zaleplon or benzodiazepine receptor agonists for insomnia in youth, although some reports suggest that clonazepam may have a possible role for specific parasomnias.26,27Dual orexin receptor antagonists. Suvorexant, an antagonist of the wakefulness-promoting neuropeptide orexin, improved subjective sleep quality in a prospective trial of adolescents with insomnia (N = 30), although dropout was high (44%).28 Of those patients, reasons for discontinuation included loss to follow-up, lack of effectiveness, and abnormal dreams. We were unable to locate any trials of lemborexant in pediatric patients.
Atypical antidepressants. Trazodone is commonly prescribed for insomnia in pediatric patients with comorbid mood or anxiety disorders. In open-label studies of children and toddlers, trazodone may be well-tolerated and improve sleep.29 Additionally, development of a physiologically based pharmacokinetic model to inform trazodone dosing for youth with insomnia is underway.30 Some studies in adolescents with depression suggest that caution should be used when combining trazodone with medications that inhibit CYP2D6. In the Treatment of SSRI-Resistant Depression in Adolescents study, none of the patients who were treated with trazodone (vs other soporifics) improved.31 This may relate to CYP2D6 interactions and accumulation of methyl-chloro-piperazine (mCPP), a trazodone metabolite that is associated with dysphoria, irritability, and depression.31 This finding has been replicated in a separate cohort of depressed adolescents.32
Because of its antihistaminergic effects, mirtazapine has been used to treat insomnia in adults. One open-label study of mirtazapine in children and young adults ages 3 to 23 with neurodevelopmental disorders suggested that mirtazapine improved behavioral symptoms and insomnia, and was associated with few treatment-limiting adverse effects.33
Tricyclic antidepressants. In a retrospective study of youth with insomnia who failed behavioral interventions and melatonin (N = 29), doxepin, a potent H1 antagonist, improved subjective sleep in one-half of patients.34
Continue to: Consultation with pediatric sleep medicine specialists...
Consultation with pediatric sleep medicine specialists
It often will behoove the psychiatric clinician to review their concerns with a behavioral sleep medicine specialist or a psychologist with specific expertise in the psychotherapeutic treatment of sleep who can provide important guidance regarding the key aspects of treatment. When discussing a particular patient’s presentation with the pediatric behavioral sleep psychologist/specialist, consider the following questions:
- Is the child’s sleep disorder the primary problem, or is the child’s insomnia secondary to another diagnosis (psychiatric or nonpsychiatric)?
- What are the primary sleep-related problems the child/family presents with? How long have the symptoms been present?
- Is the sleep disorder interfering with the child’s functioning, either academically or socially? Does the child’s sleep problem interfere with other family members’ sleep?
- Does the child have a comorbid psychological conditions such as ADHD, depression, or anxiety, and/or is undergoing treatment for these disorders, which may play a role in the sleep problem?
- Is a sleep study warranted?
A sleep study, also known as polysomnography (PSG), is a diagnostic test in which physiologic parameters are continuously recorded during a period of sleep via electroencephalography, electromyography, electrooculogram, electrocardiogram, airflow sensors, pulse oximeter, body position monitors, and video. In 2012, the American Academy of Sleep Medicine published evidenced-based practice parameters for respiratory and nonrespiratory indications for PSG.35 It is most commonly indicated to rule out obstructive sleep apnea in pediatric patients who exhibit snoring, gasping, irregular breathing, witnessed apneic events, unusual head positioning, or other signs of obstructive breathing during sleep. Nonrespiratory indications for PSG include children suspected of having periodic limb movement disorder and suspected narcolepsy. Children with frequent parasomnias, epilepsy, or nocturnal enuresis should be clinically screened for presence of comorbid sleep disorders, and PSG would be indicated if there are concerns about a possible sleep-disordered breathing disorder. PSG is also recommended for children with hypersomnia, to differentiate a parasomnia from sleep-related epilepsy, and for children suspected of having restless leg syndrome.36 PSG is not typically indicated in the initial evaluation of insomnia (unless there is evidence of a comorbid sleep disorder), circadian rhythm disorders (ie, delayed sleep phase syndrome), or for evaluation of children with sleep-related bruxism.3 Special considerations for PSG in children include ensuring a parent or guardian is always with the child, providing developmentally appropriate sleeping arrangements, arranging family tours of the sleep lab prior to the study, and accommodating for earlier bedtimes.37
Bottom Line
Techniques to promote healthy sleep in pediatric patients include behavioral interventions such as setting a developmentally appropriate bedtime and a consistent wake time, establishing bedtime routines, and encouraging relaxation/ wind-down period before bed. Cognitive-behavioral therapy for insomnia (CBT-I) may include cognitive restructuring of anxious thoughts, relaxation training, stimulus control, and sleep restriction. Use of medications may be indicated for children and teens who have not responded to CBT-I or soporific dosing of melatonin.
1. Mindell JA, Li AM, Sadeh A, et al. Bedtime routines for young children: a dose-dependent association with sleep outcomes. Sleep. 2015;38(5):717-722.
2. Kansagra S. Sleep disorders in adolescents. Pediatrics. 2020;145(Suppl 2):S204-S209.
3. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569.
4. Bruni O, Angriman M, Melegari MG, et al. Pharmacotherapeutic management of sleep disorders in children with neurodevelopmental disorders. Expert Opin Pharmacother. 2019;20(18):2257-2271.
5. Owens JA, Rosen CL, Mindell JA, et al. Use of pharmacotherapy for insomnia in child psychiatry practice: a national survey. Sleep Med. 2010;11(7):692-700.
6. Schnoes CJ, Kuhn BR, Workman EF, et al. Pediatric prescribing practices for clonidine and other pharmacologic agents for children with sleep disturbance. Clin Pediatr (Phila). 2006;45(3):229-238.
7. Russo RM, Gururaj VJ, Allen JE. The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J Clin Pharmacol. 1976;16(5-6):284-288.
8. Yangzom N, Gottschlich MM, Ossege J, et al. The effect of diphenhydramine on sleep in pediatric burn patients: a secondary analysis. J Burn Care Res. 2015;36(2):266-271.
9. Ghanizadeh A, Zare S. A preliminary randomised double-blind placebo-controlled clinical trial of hydroxyzine for treating sleep bruxism in children. J Oral Rehabil. 2013;40(6):413-417.
10. Bektas O, Arıca B, Teber S, et al. Chloral hydrate and/or hydroxyzine for sedation in pediatric EEG recording. Brain Dev. 2014;36(2):130-136.
11. Ottaviano S, Giannotti F, Cortesi F. The effect of niaprazine on some common sleep disorders in children. A double-blind clinical trial by means of continuous home-videorecorded sleep. Childs Nerv Syst. 1991;7(6):332-335.
12. Nguyen M, Tharani S, Rahmani M, et al. A review of the use of clonidine as a sleep aid in the child and adolescent population. Clin Pediatr (Phila). 2014;53(3):211-216.
13. Prince JB, Wilens TE, Biederman J, et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry. 1996;35(5):599-605.

14. Ingrassia A, Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders--a case series. Eur Child Adolesc Psychiatry. 2005;14(1):34-40.
15. Politte LC, Scahill L, Figueroa J, et al. A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: an analysis of secondary outcome measures. Neuropsychopharmacology. 2018;43(8):1772-1778.
16. Bruni O, Alonso-Alconada D, Besag F, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19(2):122-1233.
17. Jain SV, Horn PS, Simakajornboon N, et al. Melatonin improves sleep in children with epilepsy: a randomized, double-blind, crossover study. Sleep Med. 2015;16(5):637-644.
18. van Geijlswijk IM, van der Heijden KB, Egberts AC, et al. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl). 2010;212(3):379-391.
19. Gringras P, Nir T, Breddy J, et al. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(11):948-957.e4.
20. Malow BA, Findling RL, Schroder CM, et al. Sleep, growth, and puberty after two years of prolonged-release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252-261.e3.
21. Burgess HJ, Emens JS. Drugs used in circadian sleep-wake rhythm disturbances. Sleep Med Clin. 2020;15(2):301-310.
22. Arns M, Kooij JJS, Coogan AN. Review: identification and management of circadian rhythm sleep disorders as a transdiagnostic feature in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry. 2021;60(9):1085-1095.
23. Kawabe K, Horiuchi F, Oka Y, et al. The melatonin receptor agonist ramelteon effectively treats insomnia and behavioral symptoms in autistic disorder. Case Rep Psychiatry. 2014;2014:561071.
24. Blumer JL, Findling RL, Shih WJ, et al. Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention-deficit/hyperactivity disorder in children 6 to 17 years of age. Pediatrics. 2009;123(5):e770-e776.
25. Sangal RB, Blumer JL, Lankford DA, et al. Eszopiclone for insomnia associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;134(4):e1095-e1103.
26. Arens R, Wright B, Elliott J, et al. Periodic limb movement in sleep in children with Williams syndrome. J Pediatr. 1998;133(5):670-674.
27. Thirumalai SS, Shubin RA, Robinson R. Rapid eye movement sleep behavior disorder in children with autism. J Child Neurol. 2002;17(3):173-178.
28. Kawabe K, Horiuchi F, Ochi M, et al. Suvorexant for the treatment of insomnia in adolescents. J Child Adolesc Psychopharmacol. 2017;27(9):792-795.
29. Pranzatelli MR, Tate ED, Dukart WS, et al. Sleep disturbance and rage attacks in opsoclonus-myoclonus syndrome: Response to trazodone. J Pediatr. 2005;147(3):372-378.
30. Oggianu L, Ke AB, Chetty M, et al. Estimation of an appropriate dose of trazodone for pediatric insomnia and the potential for a trazodone-atomoxetine interaction. CPT Pharmacometrics Syst Pharmacol. 2020;9(2):77-86.
31. Shamseddeen W, Clarke G, Keller MB, et al. Adjunctive sleep medications and depression outcome in the treatment of serotonin-selective reuptake inhibitor resistant depression in adolescents study. J Child Adolesc Psychopharmacol. 2012;22(1):29-36.
32. Sultan MA, Courtney DB. Adjunctive trazodone and depression outcome in adolescents treated with serotonin re-uptake inhibitors. J Can Acad Child Adolesc Psychiatry. 2017;26(3):233-240.
33. Posey DJ, Guenin KD, Kohn AE, et al. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2001;11(3):267-277.
34. Shah YD, Stringel V, Pavkovic I, et al. Doxepin in children and adolescents with symptoms of insomnia: a single-center experience. J Clin Sleep Med. 2020;16(5):743-747.
35. Aurora RN, Lamm CI, Zak RS, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012;35(11):1467-1473.
36. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24.
37. Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393-406.
Children and adolescents who do not receive sufficient sleep can experience worsening inattention, daytime fatigue, and cognitive and behavioral difficulties. Assessment and treatment of insomnia and other sleep difficulties in young patients is critical as poor sleep increases their risk for depression, self-harm, and suicide.
In Part 1 of this article (Pediatric insomnia: Assessment and diagnosis,
Psychotherapeutic interventions
Regardless of the source of a child’s insomnia or co-occurring disorders, healthy sleep practices are the first line behavioral treatment, including for youth with attention-deficit/hyperactivity disorder (ADHD), anxiety disorders, obsessive-compulsive disorder, and depressive disorders.
Healthy sleep practices/sleep hygiene
Developmentally appropriate bedtimes and routines (Table). Helping children establish a regular, consistent bedtime is key in promoting healthy sleep. Ideally, the bedtime routine involves 3 to 4 activities each night in the same order, and these activities should be relaxing and soothing (eg, taking a bath, putting on pajamas, reading books). Setting age-appropriate bedtimes also is important. If an older child is asked to go to bed at 8 pm but cannot fall asleep for an hour, they may not have insomnia but instead a developmentally inappropriate bedtime. Several studies found that children younger than age 10 should go to bed no later than 9 pm. Bedtimes later than 9 pm for young children are correlated with shorter sleep duration.1

Consistent sleep schedules. Another important aspect of healthy sleep is working with parents to enforce a consistent bedtime and wake-up time, including weekdays and weekends. Ideally, bedtime on weekdays and weekends should not vary by more than 1 hour. Helping children wake up at the same time each day helps to set and regulate their circadian rhythm. Keeping these schedules consistent on vacations and school holidays also is helpful. For adolescents, the weekday/weekend bedtimes can vary by up to 2 hours because adolescents have a delayed circadian rhythm and wake-up times for high school can be early.
Environmental factors. An important piece of parental education is stimulus control and the ingredients of healthy sleep. Healthy sleep ingredients include a dark, quiet, consistent, and cool bedroom; a comfortable bed, the child feeling safe, and limited environmental stimuli.
Continue to: Cognitive-behavioral therapy for insomnia...
Cognitive-behavioral therapy for insomnia
Relaxation. Pediatric patients can be taught relaxation, mindfulness, meditation, and progressive muscle relaxation techniques to help lower overall stress. This can be especially helpful for youth with sleep disorders or anxiety. Guided relaxation apps are popular among children and teens, and various apps offer soothing sounds, deep breathing, progressive muscle relaxation, and guided imagery. This can be taught in psychotherapy sessions and used at home to promote gains in between sessions.
Stimulus control. Stimulus control involves using the bed exclusively for sleep and avoiding nonsleep activities in bed (eg, reading, watching television, using a computer, worrying). These activities promote wakefulness and insomnia. This may mean the child does not get into bed until they cannot keep their eyes open, even if that delays bedtime. If the child is still awake within 15 to 20 minutes, they should be encouraged to get out of bed and engage in a nonstimulating activity such as meditation, reading, or sitting quietly in the dark or low light. This recommendation can run counter to parents’ intuition that children with sleep problems should go to bed earlier. Using the bed only for sleep conditions the child to falling asleep or being asleep when in bed.
Sleep restriction. Sleep restriction involves restricting sleep to a set number of hours in order to increase their sleep efficiency (time slept in bed divided by total time spent in bed x 100). Restricting sleep to 6 to 7 hours increases sleep efficiency, consolidates sleep, and extinguishes the association of being awake in bed. For older adolescents, sleep restriction may help to limit their time in bed to either falling asleep or being asleep. This is intended to be used as a short-term strategy and only after other sleep hygiene measures (bedtime routine, environmental factors, etc) have been put into place for several weeks. While this strategy sounds unappealing to most individuals with insomnia, it can lead to lasting change due to the use of behavioral conditioning in bed. Because sleep restriction can lead to significant daytime sleepiness and impairment during the day, sleep should not be restricted to <6 hours a day for children and adolescents. Once the adolescent is sleeping more consistently and sleep efficiency reaches 85% or higher, time in bed for sleep is increased.2
Cognitive restructuring. Some children and adolescents develop maladaptive thoughts about sleep that further promote insomnia. These thoughts might include “I will never get to sleep,” “I am going to have a terrible day if I cannot fall asleep,” or “I will fail my test tomorrow if I am unable to sleep.” Such maladaptive thoughts are often untrue but promote wakefulness in the early or middle part of the night. Cognitive restructuring involves helping the child identify each problematic thought, challenge how accurate each thought is with evidence, and replace the problematic thought with a more helpful thought. For instance, an adolescent can recognize that even if they have a sleepless night, their catastrophic outcome (eg, “I will not be able to function”) is likely untrue. A psychologist can help review evidence for this, including previous times when the adolescent has not slept well and managed to get through the next day.
When is pharmacologic treatment needed?
Pharmacologic treatment may be indicated if a child does not show significant improvement following behavioral intervention (Figure). However, it is critical to exclude other primary causes of dyssomnia (eg, obstructive sleep apnea, iron deficiency anemia) before pursuing pharmacotherapy, because pharmacotherapy could mask an underlying disorder. Moreover, while there is relatively limited evidence for psychopharmacologic interventions for sleep difficulties in children and adolescents, a large survey of child and adolescent psychiatrists (N = 1,273) suggested that medications were considered for one-quarter of pediatric patients with insomnia.3 Further, patients with specific comorbidities such as neurodevelopmental disorders may be more likely to be prescribed soporifics.4

Continue to: What is the evidence for pharmacotherapy?...
What is the evidence for pharmacotherapy?
Antihistamines. Histamine antagonists—which promote sleep by blocking the wakefulness-promoting and circadian-related effects of histamine—are the most commonly used medications to treat pediatric insomnia, despite a dearth of data from prospective trials.5,6 In 1 small study, Russo et al7 found diphenhydramine, 1 mg/kg at bedtime, reduced sleep latency and nighttime awakenings, and increased sleep duration in patients ages 2 to 12; similar effects have been observed in pediatric burn patients.8 There are some limited data for other H1 antagonists (eg, hydroxyzine) in pediatric insomnia.9-11
Alpha-2 agonists increase rapid eye movement sleep via dose-dependent downregulation of noradrenergic signaling12 and thus have been commonly prescribed for insomnia in children and adolescents. In fact, the nonselective alpha-2 agonist clonidine is among the most prescribed medications for youth with insomnia, and may be efficacious in youth with neurodevelopmental disorders and ADHD.13 In small retrospective studies, clonidine decreased sleep latency and nighttime awakenings in addition to increasing sleep duration.14 Also, clonidine was well tolerated but associated with daytime somnolence. Guanfacine—a selective alpha-2 agonist—is also commonly prescribed for insomnia in youth, although results of trials have been equivocal.15 Given the more rapid absorption and shorter Tmax of clonidine relative to guanfacine, the former may be preferred as a soporific.
Melatonin and melatonin agonists. The primary regulator of the sleep-wake cycle is melatonin, an endogenous hormone produced by the pineal gland in response to changes in retinal light perception. Exogenous melatonin supplementation may be the preferred initial pharmacotherapy for sleep-onset insomnia due to its chronobiotic properties.16 In clinical studies, both immediate-release17,18 and extended-release19 melatonin reduced sleep-onset latency and increased total sleep duration in pediatric patients, although the increase in total duration of sleep was greater with extended-release preparations. Additionally, tolerability data for melatonin in pediatric patients are encouraging. A 2-year randomized trial of prolonged-release melatonin for insomnia in pediatric patients found no adverse effects with regard to growth, body mass index, or pubertal development.20 Additionally, significant improvements in sleep quality, sleep patterns, and caregiver satisfaction were maintained throughout the trial, and no withdrawal symptoms were observed upon discontinuation.
Melatonin may have a particularly important role in circadian rhythm sleep disorders. In this regard, low-dose melatonin (0.5 mg), when timed relative to the endogenous dim light melatonin onset (DLMO), is more effective in shifting sleep phase than higher doses, which suggests that timing may have greater impact than dosage.21 Data regarding melatonin administration with respect to DLMO suggest that the optimal administration time is 4 to 6 hours before a child’s preferred bedtime, and doses of 0.5 to 1 mg have been effective when given in this window.22 Variation across studies has contributed to a lack of consensus regarding pediatric melatonin dosing. For example, .05 mg/kg may be a minimal effective dose when given 1 to 2 hours before bedtime18; however, in surveys doses vary considerably, with typical doses of 2.5 to 3 mg for prepubertal children and 5 mg for adolescents.5 Of note, in patients with decreased cytochrome P450 (CYP) 1A2 activity, lack of diurnal variation in melatonin serum concentration may decrease the effectiveness of melatonin.16Ramelteon is a potent agonist of the melatonin MT1 and MT2 receptors, with a significantly higher binding affinity than melatonin in vitro. In case reports, ramelteon was well-tolerated, improved delayed sleep onset, and decreased nighttime awakenings.23
Zolpidem, eszopiclone and zaleplon. Studies of selective GABAergic modulators and benzodiazepines have not produced positive results in prospective trials of youth with insomnia. Zolpidem was studied in children and adolescents (N = 201) with ADHD; although sleep latency did not differ between zolpidem and placebo, some significant improvements were observed in adolescents.24 Zolpidem was generally well tolerated, with approximately 10% of youth discontinuing due to adverse effects. Additionally, eszopiclone—which has a longer duration of action compared with zolpidem—has been studied in children and adolescents with ADHD (N = 486) who were also evaluated with a sleep study. No differences were observed between placebo and eszopiclone in terms of sleep latency and approximately 10% of patients discontinued treatment as a result of adverse events.25 We were unable to locate any prospective trials of zaleplon or benzodiazepine receptor agonists for insomnia in youth, although some reports suggest that clonazepam may have a possible role for specific parasomnias.26,27Dual orexin receptor antagonists. Suvorexant, an antagonist of the wakefulness-promoting neuropeptide orexin, improved subjective sleep quality in a prospective trial of adolescents with insomnia (N = 30), although dropout was high (44%).28 Of those patients, reasons for discontinuation included loss to follow-up, lack of effectiveness, and abnormal dreams. We were unable to locate any trials of lemborexant in pediatric patients.
Atypical antidepressants. Trazodone is commonly prescribed for insomnia in pediatric patients with comorbid mood or anxiety disorders. In open-label studies of children and toddlers, trazodone may be well-tolerated and improve sleep.29 Additionally, development of a physiologically based pharmacokinetic model to inform trazodone dosing for youth with insomnia is underway.30 Some studies in adolescents with depression suggest that caution should be used when combining trazodone with medications that inhibit CYP2D6. In the Treatment of SSRI-Resistant Depression in Adolescents study, none of the patients who were treated with trazodone (vs other soporifics) improved.31 This may relate to CYP2D6 interactions and accumulation of methyl-chloro-piperazine (mCPP), a trazodone metabolite that is associated with dysphoria, irritability, and depression.31 This finding has been replicated in a separate cohort of depressed adolescents.32
Because of its antihistaminergic effects, mirtazapine has been used to treat insomnia in adults. One open-label study of mirtazapine in children and young adults ages 3 to 23 with neurodevelopmental disorders suggested that mirtazapine improved behavioral symptoms and insomnia, and was associated with few treatment-limiting adverse effects.33
Tricyclic antidepressants. In a retrospective study of youth with insomnia who failed behavioral interventions and melatonin (N = 29), doxepin, a potent H1 antagonist, improved subjective sleep in one-half of patients.34
Continue to: Consultation with pediatric sleep medicine specialists...
Consultation with pediatric sleep medicine specialists
It often will behoove the psychiatric clinician to review their concerns with a behavioral sleep medicine specialist or a psychologist with specific expertise in the psychotherapeutic treatment of sleep who can provide important guidance regarding the key aspects of treatment. When discussing a particular patient’s presentation with the pediatric behavioral sleep psychologist/specialist, consider the following questions:
- Is the child’s sleep disorder the primary problem, or is the child’s insomnia secondary to another diagnosis (psychiatric or nonpsychiatric)?
- What are the primary sleep-related problems the child/family presents with? How long have the symptoms been present?
- Is the sleep disorder interfering with the child’s functioning, either academically or socially? Does the child’s sleep problem interfere with other family members’ sleep?
- Does the child have a comorbid psychological conditions such as ADHD, depression, or anxiety, and/or is undergoing treatment for these disorders, which may play a role in the sleep problem?
- Is a sleep study warranted?
A sleep study, also known as polysomnography (PSG), is a diagnostic test in which physiologic parameters are continuously recorded during a period of sleep via electroencephalography, electromyography, electrooculogram, electrocardiogram, airflow sensors, pulse oximeter, body position monitors, and video. In 2012, the American Academy of Sleep Medicine published evidenced-based practice parameters for respiratory and nonrespiratory indications for PSG.35 It is most commonly indicated to rule out obstructive sleep apnea in pediatric patients who exhibit snoring, gasping, irregular breathing, witnessed apneic events, unusual head positioning, or other signs of obstructive breathing during sleep. Nonrespiratory indications for PSG include children suspected of having periodic limb movement disorder and suspected narcolepsy. Children with frequent parasomnias, epilepsy, or nocturnal enuresis should be clinically screened for presence of comorbid sleep disorders, and PSG would be indicated if there are concerns about a possible sleep-disordered breathing disorder. PSG is also recommended for children with hypersomnia, to differentiate a parasomnia from sleep-related epilepsy, and for children suspected of having restless leg syndrome.36 PSG is not typically indicated in the initial evaluation of insomnia (unless there is evidence of a comorbid sleep disorder), circadian rhythm disorders (ie, delayed sleep phase syndrome), or for evaluation of children with sleep-related bruxism.3 Special considerations for PSG in children include ensuring a parent or guardian is always with the child, providing developmentally appropriate sleeping arrangements, arranging family tours of the sleep lab prior to the study, and accommodating for earlier bedtimes.37
Bottom Line
Techniques to promote healthy sleep in pediatric patients include behavioral interventions such as setting a developmentally appropriate bedtime and a consistent wake time, establishing bedtime routines, and encouraging relaxation/ wind-down period before bed. Cognitive-behavioral therapy for insomnia (CBT-I) may include cognitive restructuring of anxious thoughts, relaxation training, stimulus control, and sleep restriction. Use of medications may be indicated for children and teens who have not responded to CBT-I or soporific dosing of melatonin.
Children and adolescents who do not receive sufficient sleep can experience worsening inattention, daytime fatigue, and cognitive and behavioral difficulties. Assessment and treatment of insomnia and other sleep difficulties in young patients is critical as poor sleep increases their risk for depression, self-harm, and suicide.
In Part 1 of this article (Pediatric insomnia: Assessment and diagnosis,
Psychotherapeutic interventions
Regardless of the source of a child’s insomnia or co-occurring disorders, healthy sleep practices are the first line behavioral treatment, including for youth with attention-deficit/hyperactivity disorder (ADHD), anxiety disorders, obsessive-compulsive disorder, and depressive disorders.
Healthy sleep practices/sleep hygiene
Developmentally appropriate bedtimes and routines (Table). Helping children establish a regular, consistent bedtime is key in promoting healthy sleep. Ideally, the bedtime routine involves 3 to 4 activities each night in the same order, and these activities should be relaxing and soothing (eg, taking a bath, putting on pajamas, reading books). Setting age-appropriate bedtimes also is important. If an older child is asked to go to bed at 8 pm but cannot fall asleep for an hour, they may not have insomnia but instead a developmentally inappropriate bedtime. Several studies found that children younger than age 10 should go to bed no later than 9 pm. Bedtimes later than 9 pm for young children are correlated with shorter sleep duration.1

Consistent sleep schedules. Another important aspect of healthy sleep is working with parents to enforce a consistent bedtime and wake-up time, including weekdays and weekends. Ideally, bedtime on weekdays and weekends should not vary by more than 1 hour. Helping children wake up at the same time each day helps to set and regulate their circadian rhythm. Keeping these schedules consistent on vacations and school holidays also is helpful. For adolescents, the weekday/weekend bedtimes can vary by up to 2 hours because adolescents have a delayed circadian rhythm and wake-up times for high school can be early.
Environmental factors. An important piece of parental education is stimulus control and the ingredients of healthy sleep. Healthy sleep ingredients include a dark, quiet, consistent, and cool bedroom; a comfortable bed, the child feeling safe, and limited environmental stimuli.
Continue to: Cognitive-behavioral therapy for insomnia...
Cognitive-behavioral therapy for insomnia
Relaxation. Pediatric patients can be taught relaxation, mindfulness, meditation, and progressive muscle relaxation techniques to help lower overall stress. This can be especially helpful for youth with sleep disorders or anxiety. Guided relaxation apps are popular among children and teens, and various apps offer soothing sounds, deep breathing, progressive muscle relaxation, and guided imagery. This can be taught in psychotherapy sessions and used at home to promote gains in between sessions.
Stimulus control. Stimulus control involves using the bed exclusively for sleep and avoiding nonsleep activities in bed (eg, reading, watching television, using a computer, worrying). These activities promote wakefulness and insomnia. This may mean the child does not get into bed until they cannot keep their eyes open, even if that delays bedtime. If the child is still awake within 15 to 20 minutes, they should be encouraged to get out of bed and engage in a nonstimulating activity such as meditation, reading, or sitting quietly in the dark or low light. This recommendation can run counter to parents’ intuition that children with sleep problems should go to bed earlier. Using the bed only for sleep conditions the child to falling asleep or being asleep when in bed.
Sleep restriction. Sleep restriction involves restricting sleep to a set number of hours in order to increase their sleep efficiency (time slept in bed divided by total time spent in bed x 100). Restricting sleep to 6 to 7 hours increases sleep efficiency, consolidates sleep, and extinguishes the association of being awake in bed. For older adolescents, sleep restriction may help to limit their time in bed to either falling asleep or being asleep. This is intended to be used as a short-term strategy and only after other sleep hygiene measures (bedtime routine, environmental factors, etc) have been put into place for several weeks. While this strategy sounds unappealing to most individuals with insomnia, it can lead to lasting change due to the use of behavioral conditioning in bed. Because sleep restriction can lead to significant daytime sleepiness and impairment during the day, sleep should not be restricted to <6 hours a day for children and adolescents. Once the adolescent is sleeping more consistently and sleep efficiency reaches 85% or higher, time in bed for sleep is increased.2
Cognitive restructuring. Some children and adolescents develop maladaptive thoughts about sleep that further promote insomnia. These thoughts might include “I will never get to sleep,” “I am going to have a terrible day if I cannot fall asleep,” or “I will fail my test tomorrow if I am unable to sleep.” Such maladaptive thoughts are often untrue but promote wakefulness in the early or middle part of the night. Cognitive restructuring involves helping the child identify each problematic thought, challenge how accurate each thought is with evidence, and replace the problematic thought with a more helpful thought. For instance, an adolescent can recognize that even if they have a sleepless night, their catastrophic outcome (eg, “I will not be able to function”) is likely untrue. A psychologist can help review evidence for this, including previous times when the adolescent has not slept well and managed to get through the next day.
When is pharmacologic treatment needed?
Pharmacologic treatment may be indicated if a child does not show significant improvement following behavioral intervention (Figure). However, it is critical to exclude other primary causes of dyssomnia (eg, obstructive sleep apnea, iron deficiency anemia) before pursuing pharmacotherapy, because pharmacotherapy could mask an underlying disorder. Moreover, while there is relatively limited evidence for psychopharmacologic interventions for sleep difficulties in children and adolescents, a large survey of child and adolescent psychiatrists (N = 1,273) suggested that medications were considered for one-quarter of pediatric patients with insomnia.3 Further, patients with specific comorbidities such as neurodevelopmental disorders may be more likely to be prescribed soporifics.4

Continue to: What is the evidence for pharmacotherapy?...
What is the evidence for pharmacotherapy?
Antihistamines. Histamine antagonists—which promote sleep by blocking the wakefulness-promoting and circadian-related effects of histamine—are the most commonly used medications to treat pediatric insomnia, despite a dearth of data from prospective trials.5,6 In 1 small study, Russo et al7 found diphenhydramine, 1 mg/kg at bedtime, reduced sleep latency and nighttime awakenings, and increased sleep duration in patients ages 2 to 12; similar effects have been observed in pediatric burn patients.8 There are some limited data for other H1 antagonists (eg, hydroxyzine) in pediatric insomnia.9-11
Alpha-2 agonists increase rapid eye movement sleep via dose-dependent downregulation of noradrenergic signaling12 and thus have been commonly prescribed for insomnia in children and adolescents. In fact, the nonselective alpha-2 agonist clonidine is among the most prescribed medications for youth with insomnia, and may be efficacious in youth with neurodevelopmental disorders and ADHD.13 In small retrospective studies, clonidine decreased sleep latency and nighttime awakenings in addition to increasing sleep duration.14 Also, clonidine was well tolerated but associated with daytime somnolence. Guanfacine—a selective alpha-2 agonist—is also commonly prescribed for insomnia in youth, although results of trials have been equivocal.15 Given the more rapid absorption and shorter Tmax of clonidine relative to guanfacine, the former may be preferred as a soporific.
Melatonin and melatonin agonists. The primary regulator of the sleep-wake cycle is melatonin, an endogenous hormone produced by the pineal gland in response to changes in retinal light perception. Exogenous melatonin supplementation may be the preferred initial pharmacotherapy for sleep-onset insomnia due to its chronobiotic properties.16 In clinical studies, both immediate-release17,18 and extended-release19 melatonin reduced sleep-onset latency and increased total sleep duration in pediatric patients, although the increase in total duration of sleep was greater with extended-release preparations. Additionally, tolerability data for melatonin in pediatric patients are encouraging. A 2-year randomized trial of prolonged-release melatonin for insomnia in pediatric patients found no adverse effects with regard to growth, body mass index, or pubertal development.20 Additionally, significant improvements in sleep quality, sleep patterns, and caregiver satisfaction were maintained throughout the trial, and no withdrawal symptoms were observed upon discontinuation.
Melatonin may have a particularly important role in circadian rhythm sleep disorders. In this regard, low-dose melatonin (0.5 mg), when timed relative to the endogenous dim light melatonin onset (DLMO), is more effective in shifting sleep phase than higher doses, which suggests that timing may have greater impact than dosage.21 Data regarding melatonin administration with respect to DLMO suggest that the optimal administration time is 4 to 6 hours before a child’s preferred bedtime, and doses of 0.5 to 1 mg have been effective when given in this window.22 Variation across studies has contributed to a lack of consensus regarding pediatric melatonin dosing. For example, .05 mg/kg may be a minimal effective dose when given 1 to 2 hours before bedtime18; however, in surveys doses vary considerably, with typical doses of 2.5 to 3 mg for prepubertal children and 5 mg for adolescents.5 Of note, in patients with decreased cytochrome P450 (CYP) 1A2 activity, lack of diurnal variation in melatonin serum concentration may decrease the effectiveness of melatonin.16Ramelteon is a potent agonist of the melatonin MT1 and MT2 receptors, with a significantly higher binding affinity than melatonin in vitro. In case reports, ramelteon was well-tolerated, improved delayed sleep onset, and decreased nighttime awakenings.23
Zolpidem, eszopiclone and zaleplon. Studies of selective GABAergic modulators and benzodiazepines have not produced positive results in prospective trials of youth with insomnia. Zolpidem was studied in children and adolescents (N = 201) with ADHD; although sleep latency did not differ between zolpidem and placebo, some significant improvements were observed in adolescents.24 Zolpidem was generally well tolerated, with approximately 10% of youth discontinuing due to adverse effects. Additionally, eszopiclone—which has a longer duration of action compared with zolpidem—has been studied in children and adolescents with ADHD (N = 486) who were also evaluated with a sleep study. No differences were observed between placebo and eszopiclone in terms of sleep latency and approximately 10% of patients discontinued treatment as a result of adverse events.25 We were unable to locate any prospective trials of zaleplon or benzodiazepine receptor agonists for insomnia in youth, although some reports suggest that clonazepam may have a possible role for specific parasomnias.26,27Dual orexin receptor antagonists. Suvorexant, an antagonist of the wakefulness-promoting neuropeptide orexin, improved subjective sleep quality in a prospective trial of adolescents with insomnia (N = 30), although dropout was high (44%).28 Of those patients, reasons for discontinuation included loss to follow-up, lack of effectiveness, and abnormal dreams. We were unable to locate any trials of lemborexant in pediatric patients.
Atypical antidepressants. Trazodone is commonly prescribed for insomnia in pediatric patients with comorbid mood or anxiety disorders. In open-label studies of children and toddlers, trazodone may be well-tolerated and improve sleep.29 Additionally, development of a physiologically based pharmacokinetic model to inform trazodone dosing for youth with insomnia is underway.30 Some studies in adolescents with depression suggest that caution should be used when combining trazodone with medications that inhibit CYP2D6. In the Treatment of SSRI-Resistant Depression in Adolescents study, none of the patients who were treated with trazodone (vs other soporifics) improved.31 This may relate to CYP2D6 interactions and accumulation of methyl-chloro-piperazine (mCPP), a trazodone metabolite that is associated with dysphoria, irritability, and depression.31 This finding has been replicated in a separate cohort of depressed adolescents.32
Because of its antihistaminergic effects, mirtazapine has been used to treat insomnia in adults. One open-label study of mirtazapine in children and young adults ages 3 to 23 with neurodevelopmental disorders suggested that mirtazapine improved behavioral symptoms and insomnia, and was associated with few treatment-limiting adverse effects.33
Tricyclic antidepressants. In a retrospective study of youth with insomnia who failed behavioral interventions and melatonin (N = 29), doxepin, a potent H1 antagonist, improved subjective sleep in one-half of patients.34
Continue to: Consultation with pediatric sleep medicine specialists...
Consultation with pediatric sleep medicine specialists
It often will behoove the psychiatric clinician to review their concerns with a behavioral sleep medicine specialist or a psychologist with specific expertise in the psychotherapeutic treatment of sleep who can provide important guidance regarding the key aspects of treatment. When discussing a particular patient’s presentation with the pediatric behavioral sleep psychologist/specialist, consider the following questions:
- Is the child’s sleep disorder the primary problem, or is the child’s insomnia secondary to another diagnosis (psychiatric or nonpsychiatric)?
- What are the primary sleep-related problems the child/family presents with? How long have the symptoms been present?
- Is the sleep disorder interfering with the child’s functioning, either academically or socially? Does the child’s sleep problem interfere with other family members’ sleep?
- Does the child have a comorbid psychological conditions such as ADHD, depression, or anxiety, and/or is undergoing treatment for these disorders, which may play a role in the sleep problem?
- Is a sleep study warranted?
A sleep study, also known as polysomnography (PSG), is a diagnostic test in which physiologic parameters are continuously recorded during a period of sleep via electroencephalography, electromyography, electrooculogram, electrocardiogram, airflow sensors, pulse oximeter, body position monitors, and video. In 2012, the American Academy of Sleep Medicine published evidenced-based practice parameters for respiratory and nonrespiratory indications for PSG.35 It is most commonly indicated to rule out obstructive sleep apnea in pediatric patients who exhibit snoring, gasping, irregular breathing, witnessed apneic events, unusual head positioning, or other signs of obstructive breathing during sleep. Nonrespiratory indications for PSG include children suspected of having periodic limb movement disorder and suspected narcolepsy. Children with frequent parasomnias, epilepsy, or nocturnal enuresis should be clinically screened for presence of comorbid sleep disorders, and PSG would be indicated if there are concerns about a possible sleep-disordered breathing disorder. PSG is also recommended for children with hypersomnia, to differentiate a parasomnia from sleep-related epilepsy, and for children suspected of having restless leg syndrome.36 PSG is not typically indicated in the initial evaluation of insomnia (unless there is evidence of a comorbid sleep disorder), circadian rhythm disorders (ie, delayed sleep phase syndrome), or for evaluation of children with sleep-related bruxism.3 Special considerations for PSG in children include ensuring a parent or guardian is always with the child, providing developmentally appropriate sleeping arrangements, arranging family tours of the sleep lab prior to the study, and accommodating for earlier bedtimes.37
Bottom Line
Techniques to promote healthy sleep in pediatric patients include behavioral interventions such as setting a developmentally appropriate bedtime and a consistent wake time, establishing bedtime routines, and encouraging relaxation/ wind-down period before bed. Cognitive-behavioral therapy for insomnia (CBT-I) may include cognitive restructuring of anxious thoughts, relaxation training, stimulus control, and sleep restriction. Use of medications may be indicated for children and teens who have not responded to CBT-I or soporific dosing of melatonin.
1. Mindell JA, Li AM, Sadeh A, et al. Bedtime routines for young children: a dose-dependent association with sleep outcomes. Sleep. 2015;38(5):717-722.
2. Kansagra S. Sleep disorders in adolescents. Pediatrics. 2020;145(Suppl 2):S204-S209.
3. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569.
4. Bruni O, Angriman M, Melegari MG, et al. Pharmacotherapeutic management of sleep disorders in children with neurodevelopmental disorders. Expert Opin Pharmacother. 2019;20(18):2257-2271.
5. Owens JA, Rosen CL, Mindell JA, et al. Use of pharmacotherapy for insomnia in child psychiatry practice: a national survey. Sleep Med. 2010;11(7):692-700.
6. Schnoes CJ, Kuhn BR, Workman EF, et al. Pediatric prescribing practices for clonidine and other pharmacologic agents for children with sleep disturbance. Clin Pediatr (Phila). 2006;45(3):229-238.
7. Russo RM, Gururaj VJ, Allen JE. The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J Clin Pharmacol. 1976;16(5-6):284-288.
8. Yangzom N, Gottschlich MM, Ossege J, et al. The effect of diphenhydramine on sleep in pediatric burn patients: a secondary analysis. J Burn Care Res. 2015;36(2):266-271.
9. Ghanizadeh A, Zare S. A preliminary randomised double-blind placebo-controlled clinical trial of hydroxyzine for treating sleep bruxism in children. J Oral Rehabil. 2013;40(6):413-417.
10. Bektas O, Arıca B, Teber S, et al. Chloral hydrate and/or hydroxyzine for sedation in pediatric EEG recording. Brain Dev. 2014;36(2):130-136.
11. Ottaviano S, Giannotti F, Cortesi F. The effect of niaprazine on some common sleep disorders in children. A double-blind clinical trial by means of continuous home-videorecorded sleep. Childs Nerv Syst. 1991;7(6):332-335.
12. Nguyen M, Tharani S, Rahmani M, et al. A review of the use of clonidine as a sleep aid in the child and adolescent population. Clin Pediatr (Phila). 2014;53(3):211-216.
13. Prince JB, Wilens TE, Biederman J, et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry. 1996;35(5):599-605.

14. Ingrassia A, Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders--a case series. Eur Child Adolesc Psychiatry. 2005;14(1):34-40.
15. Politte LC, Scahill L, Figueroa J, et al. A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: an analysis of secondary outcome measures. Neuropsychopharmacology. 2018;43(8):1772-1778.
16. Bruni O, Alonso-Alconada D, Besag F, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19(2):122-1233.
17. Jain SV, Horn PS, Simakajornboon N, et al. Melatonin improves sleep in children with epilepsy: a randomized, double-blind, crossover study. Sleep Med. 2015;16(5):637-644.
18. van Geijlswijk IM, van der Heijden KB, Egberts AC, et al. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl). 2010;212(3):379-391.
19. Gringras P, Nir T, Breddy J, et al. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(11):948-957.e4.
20. Malow BA, Findling RL, Schroder CM, et al. Sleep, growth, and puberty after two years of prolonged-release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252-261.e3.
21. Burgess HJ, Emens JS. Drugs used in circadian sleep-wake rhythm disturbances. Sleep Med Clin. 2020;15(2):301-310.
22. Arns M, Kooij JJS, Coogan AN. Review: identification and management of circadian rhythm sleep disorders as a transdiagnostic feature in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry. 2021;60(9):1085-1095.
23. Kawabe K, Horiuchi F, Oka Y, et al. The melatonin receptor agonist ramelteon effectively treats insomnia and behavioral symptoms in autistic disorder. Case Rep Psychiatry. 2014;2014:561071.
24. Blumer JL, Findling RL, Shih WJ, et al. Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention-deficit/hyperactivity disorder in children 6 to 17 years of age. Pediatrics. 2009;123(5):e770-e776.
25. Sangal RB, Blumer JL, Lankford DA, et al. Eszopiclone for insomnia associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;134(4):e1095-e1103.
26. Arens R, Wright B, Elliott J, et al. Periodic limb movement in sleep in children with Williams syndrome. J Pediatr. 1998;133(5):670-674.
27. Thirumalai SS, Shubin RA, Robinson R. Rapid eye movement sleep behavior disorder in children with autism. J Child Neurol. 2002;17(3):173-178.
28. Kawabe K, Horiuchi F, Ochi M, et al. Suvorexant for the treatment of insomnia in adolescents. J Child Adolesc Psychopharmacol. 2017;27(9):792-795.
29. Pranzatelli MR, Tate ED, Dukart WS, et al. Sleep disturbance and rage attacks in opsoclonus-myoclonus syndrome: Response to trazodone. J Pediatr. 2005;147(3):372-378.
30. Oggianu L, Ke AB, Chetty M, et al. Estimation of an appropriate dose of trazodone for pediatric insomnia and the potential for a trazodone-atomoxetine interaction. CPT Pharmacometrics Syst Pharmacol. 2020;9(2):77-86.
31. Shamseddeen W, Clarke G, Keller MB, et al. Adjunctive sleep medications and depression outcome in the treatment of serotonin-selective reuptake inhibitor resistant depression in adolescents study. J Child Adolesc Psychopharmacol. 2012;22(1):29-36.
32. Sultan MA, Courtney DB. Adjunctive trazodone and depression outcome in adolescents treated with serotonin re-uptake inhibitors. J Can Acad Child Adolesc Psychiatry. 2017;26(3):233-240.
33. Posey DJ, Guenin KD, Kohn AE, et al. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2001;11(3):267-277.
34. Shah YD, Stringel V, Pavkovic I, et al. Doxepin in children and adolescents with symptoms of insomnia: a single-center experience. J Clin Sleep Med. 2020;16(5):743-747.
35. Aurora RN, Lamm CI, Zak RS, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012;35(11):1467-1473.
36. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24.
37. Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393-406.
1. Mindell JA, Li AM, Sadeh A, et al. Bedtime routines for young children: a dose-dependent association with sleep outcomes. Sleep. 2015;38(5):717-722.
2. Kansagra S. Sleep disorders in adolescents. Pediatrics. 2020;145(Suppl 2):S204-S209.
3. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569.
4. Bruni O, Angriman M, Melegari MG, et al. Pharmacotherapeutic management of sleep disorders in children with neurodevelopmental disorders. Expert Opin Pharmacother. 2019;20(18):2257-2271.
5. Owens JA, Rosen CL, Mindell JA, et al. Use of pharmacotherapy for insomnia in child psychiatry practice: a national survey. Sleep Med. 2010;11(7):692-700.
6. Schnoes CJ, Kuhn BR, Workman EF, et al. Pediatric prescribing practices for clonidine and other pharmacologic agents for children with sleep disturbance. Clin Pediatr (Phila). 2006;45(3):229-238.
7. Russo RM, Gururaj VJ, Allen JE. The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J Clin Pharmacol. 1976;16(5-6):284-288.
8. Yangzom N, Gottschlich MM, Ossege J, et al. The effect of diphenhydramine on sleep in pediatric burn patients: a secondary analysis. J Burn Care Res. 2015;36(2):266-271.
9. Ghanizadeh A, Zare S. A preliminary randomised double-blind placebo-controlled clinical trial of hydroxyzine for treating sleep bruxism in children. J Oral Rehabil. 2013;40(6):413-417.
10. Bektas O, Arıca B, Teber S, et al. Chloral hydrate and/or hydroxyzine for sedation in pediatric EEG recording. Brain Dev. 2014;36(2):130-136.
11. Ottaviano S, Giannotti F, Cortesi F. The effect of niaprazine on some common sleep disorders in children. A double-blind clinical trial by means of continuous home-videorecorded sleep. Childs Nerv Syst. 1991;7(6):332-335.
12. Nguyen M, Tharani S, Rahmani M, et al. A review of the use of clonidine as a sleep aid in the child and adolescent population. Clin Pediatr (Phila). 2014;53(3):211-216.
13. Prince JB, Wilens TE, Biederman J, et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry. 1996;35(5):599-605.

14. Ingrassia A, Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders--a case series. Eur Child Adolesc Psychiatry. 2005;14(1):34-40.
15. Politte LC, Scahill L, Figueroa J, et al. A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: an analysis of secondary outcome measures. Neuropsychopharmacology. 2018;43(8):1772-1778.
16. Bruni O, Alonso-Alconada D, Besag F, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19(2):122-1233.
17. Jain SV, Horn PS, Simakajornboon N, et al. Melatonin improves sleep in children with epilepsy: a randomized, double-blind, crossover study. Sleep Med. 2015;16(5):637-644.
18. van Geijlswijk IM, van der Heijden KB, Egberts AC, et al. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl). 2010;212(3):379-391.
19. Gringras P, Nir T, Breddy J, et al. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(11):948-957.e4.
20. Malow BA, Findling RL, Schroder CM, et al. Sleep, growth, and puberty after two years of prolonged-release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252-261.e3.
21. Burgess HJ, Emens JS. Drugs used in circadian sleep-wake rhythm disturbances. Sleep Med Clin. 2020;15(2):301-310.
22. Arns M, Kooij JJS, Coogan AN. Review: identification and management of circadian rhythm sleep disorders as a transdiagnostic feature in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry. 2021;60(9):1085-1095.
23. Kawabe K, Horiuchi F, Oka Y, et al. The melatonin receptor agonist ramelteon effectively treats insomnia and behavioral symptoms in autistic disorder. Case Rep Psychiatry. 2014;2014:561071.
24. Blumer JL, Findling RL, Shih WJ, et al. Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention-deficit/hyperactivity disorder in children 6 to 17 years of age. Pediatrics. 2009;123(5):e770-e776.
25. Sangal RB, Blumer JL, Lankford DA, et al. Eszopiclone for insomnia associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;134(4):e1095-e1103.
26. Arens R, Wright B, Elliott J, et al. Periodic limb movement in sleep in children with Williams syndrome. J Pediatr. 1998;133(5):670-674.
27. Thirumalai SS, Shubin RA, Robinson R. Rapid eye movement sleep behavior disorder in children with autism. J Child Neurol. 2002;17(3):173-178.
28. Kawabe K, Horiuchi F, Ochi M, et al. Suvorexant for the treatment of insomnia in adolescents. J Child Adolesc Psychopharmacol. 2017;27(9):792-795.
29. Pranzatelli MR, Tate ED, Dukart WS, et al. Sleep disturbance and rage attacks in opsoclonus-myoclonus syndrome: Response to trazodone. J Pediatr. 2005;147(3):372-378.
30. Oggianu L, Ke AB, Chetty M, et al. Estimation of an appropriate dose of trazodone for pediatric insomnia and the potential for a trazodone-atomoxetine interaction. CPT Pharmacometrics Syst Pharmacol. 2020;9(2):77-86.
31. Shamseddeen W, Clarke G, Keller MB, et al. Adjunctive sleep medications and depression outcome in the treatment of serotonin-selective reuptake inhibitor resistant depression in adolescents study. J Child Adolesc Psychopharmacol. 2012;22(1):29-36.
32. Sultan MA, Courtney DB. Adjunctive trazodone and depression outcome in adolescents treated with serotonin re-uptake inhibitors. J Can Acad Child Adolesc Psychiatry. 2017;26(3):233-240.
33. Posey DJ, Guenin KD, Kohn AE, et al. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2001;11(3):267-277.
34. Shah YD, Stringel V, Pavkovic I, et al. Doxepin in children and adolescents with symptoms of insomnia: a single-center experience. J Clin Sleep Med. 2020;16(5):743-747.
35. Aurora RN, Lamm CI, Zak RS, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012;35(11):1467-1473.
36. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24.
37. Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393-406.
Confusing messages on COVID taking a psychological toll
The Centers for Disease Control and Prevention’s decision to shorten the length of isolation time for asymptomatic Americans with COVID-19, regardless of their vaccination status, to 5 days from 10 days is confusing. I hope the agency reconsiders this decision.
After all, one of the CDC’s key messages during this pandemic has been that even people with asymptomatic COVID who have been vaccinated and boosted can transmit the disease. So it seems to me that the Dec. 27, 2021, recommendation about shortening the isolation time for COVID-19–positive people, like the agency’s earlier guidance encouraging people who are vaccinated to stop wearing masks while in indoor settings, runs contrary to good public health principles.
As an expert in human behavior, I am worried about the impact of these confusing messages on the psyche of people in general, as well as on our patients.
Mental health impact
Soon after the United States went on lockdown in March 2020, I wrote about the likelihood of a pandemic of PTSD, anxiety, and depression that would occur in the wake of rising COVID-19 rates. Well, it happened.
Many people have felt a sense of existential despair, depression, and anxiety. As we head into year No. 3 of disruption of our daily lives – and face the loss of more than 825,000 Americans to COVID – we continue to navigate this uncertainty. And now we must deal with Omicron, a variant that is so highly transmissible that it is apparently able to, in some cases, evade two-dose regimens of mRNA vaccines, boosters, and immunity from past infections, according to a report from Imperial College London. Yet, we are being told by some that Omicron might be less severe, compared with other variants. I worry that this assessment is misleading. In that same report, the Imperial College said it “found no evidence” that Omicron is less virulent than Delta, based on the risk of hospitalization and symptom status.
Meanwhile, animal studies suggest that the Omicron variant might lead to less lung damage than previous variants. A preprint article that is being considered for publication by a Nature Portfolio journal suggests that hamsters and mice infected with the Omicron variant do not have as much lung damage as those infected with other variants. More data need to come in for us to get a true understanding of Omicron’s virulence and transmissibility. We should keep an eye on Israel, which is launching a clinical trial of a second booster, or fourth mRNA shot.
As clinicians, we should give our patients and other people with whom we come in contact a sense of hope. In addition to urging people to get boosters, let’s tell them to err on the side of safety when it comes to this pandemic. That means encouraging them to remain isolated for longer than 5 days – until they test negative for COVID. It also means encouraging patients to wear high-quality face masks while inside public spaces – even in the absence of mandates. I have found it heartbreaking to watch televised broadcasts of sporting events held at some stadiums across the country where masks are not being worn. This absence of face coverings is counterintuitive at a time when some Broadway shows are closing. Even the great Radio City Rockettes shut down their holiday shows early in December 2021 because of COVID.
And, as I’ve argued before, we must not give up on unvaccinated people. I have had success in changing the minds of a few patients and some acquaintances with gentle, respectful prodding and vaccine education.
I would also like to see public health principles implemented in our schools and colleges. To protect the health of our children and young adults, we must continue to be nimble – which means school districts should implement layered prevention strategies, as the CDC recommends. This includes not only encouraging eligible staff members and students to get vaccinated, but requiring face masks inside school facilities, maintaining a physical distance of at least 3 feet, “screening testing, ventilation, handwashing, and staying home when sick.”
Furthermore, in deciding whether schools should remain open or be closed after positive COVID cases are discovered, officials should look at the vaccine demographics of that particular school. For example, if 15% of students are vaccinated in one school and 70% are vaccinated in another, the judgment would be different. Of course, it’s clearly best for schools to remain open, but perhaps closing them temporarily – perhaps for a week or 10 days – should be on the table if infection rates reach a certain level.
Now that we know more and have the benefit of getting more than 200 million Americans fully vaccinated, we can be far more selective about closings and openings. An important part of our strategy must be to communicate honestly with the public about which measures are best for safety. As a key tenet of cognitive-behavioral therapy tells us, “all-or-nothing” thinking is not productive. That should also be the case with our approach to managing COVID-19.
We don’t know the future of the pandemic. Yes, it will end, and possibly COVID will become endemic – like the flu. However, in the meantime, in addition to promoting vaccinations and boosters, we must rigorously encourage our patients to follow public health standards of masking, social distancing, and closing down businesses – and schools – temporarily.
This pandemic has taken a horrendous mental health toll on all of us – especially our patients and frontline health care workers. I’ve spoken with numerous people who were anxious, depressed, and showed signs of PTSD in early 2020; after they got vaccinated, COVID spread diminished, and as public health protocols began to lift, so did their spirits. Clearly for some, the benefit of psychiatric/psychological care centering on the pandemic has proven invaluable. In some ways, the pandemic has brought to the surface the importance of mental health care and removed some of the stigma from mental illness. And that’s a good thing.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
The Centers for Disease Control and Prevention’s decision to shorten the length of isolation time for asymptomatic Americans with COVID-19, regardless of their vaccination status, to 5 days from 10 days is confusing. I hope the agency reconsiders this decision.
After all, one of the CDC’s key messages during this pandemic has been that even people with asymptomatic COVID who have been vaccinated and boosted can transmit the disease. So it seems to me that the Dec. 27, 2021, recommendation about shortening the isolation time for COVID-19–positive people, like the agency’s earlier guidance encouraging people who are vaccinated to stop wearing masks while in indoor settings, runs contrary to good public health principles.
As an expert in human behavior, I am worried about the impact of these confusing messages on the psyche of people in general, as well as on our patients.
Mental health impact
Soon after the United States went on lockdown in March 2020, I wrote about the likelihood of a pandemic of PTSD, anxiety, and depression that would occur in the wake of rising COVID-19 rates. Well, it happened.
Many people have felt a sense of existential despair, depression, and anxiety. As we head into year No. 3 of disruption of our daily lives – and face the loss of more than 825,000 Americans to COVID – we continue to navigate this uncertainty. And now we must deal with Omicron, a variant that is so highly transmissible that it is apparently able to, in some cases, evade two-dose regimens of mRNA vaccines, boosters, and immunity from past infections, according to a report from Imperial College London. Yet, we are being told by some that Omicron might be less severe, compared with other variants. I worry that this assessment is misleading. In that same report, the Imperial College said it “found no evidence” that Omicron is less virulent than Delta, based on the risk of hospitalization and symptom status.
Meanwhile, animal studies suggest that the Omicron variant might lead to less lung damage than previous variants. A preprint article that is being considered for publication by a Nature Portfolio journal suggests that hamsters and mice infected with the Omicron variant do not have as much lung damage as those infected with other variants. More data need to come in for us to get a true understanding of Omicron’s virulence and transmissibility. We should keep an eye on Israel, which is launching a clinical trial of a second booster, or fourth mRNA shot.
As clinicians, we should give our patients and other people with whom we come in contact a sense of hope. In addition to urging people to get boosters, let’s tell them to err on the side of safety when it comes to this pandemic. That means encouraging them to remain isolated for longer than 5 days – until they test negative for COVID. It also means encouraging patients to wear high-quality face masks while inside public spaces – even in the absence of mandates. I have found it heartbreaking to watch televised broadcasts of sporting events held at some stadiums across the country where masks are not being worn. This absence of face coverings is counterintuitive at a time when some Broadway shows are closing. Even the great Radio City Rockettes shut down their holiday shows early in December 2021 because of COVID.
And, as I’ve argued before, we must not give up on unvaccinated people. I have had success in changing the minds of a few patients and some acquaintances with gentle, respectful prodding and vaccine education.
I would also like to see public health principles implemented in our schools and colleges. To protect the health of our children and young adults, we must continue to be nimble – which means school districts should implement layered prevention strategies, as the CDC recommends. This includes not only encouraging eligible staff members and students to get vaccinated, but requiring face masks inside school facilities, maintaining a physical distance of at least 3 feet, “screening testing, ventilation, handwashing, and staying home when sick.”
Furthermore, in deciding whether schools should remain open or be closed after positive COVID cases are discovered, officials should look at the vaccine demographics of that particular school. For example, if 15% of students are vaccinated in one school and 70% are vaccinated in another, the judgment would be different. Of course, it’s clearly best for schools to remain open, but perhaps closing them temporarily – perhaps for a week or 10 days – should be on the table if infection rates reach a certain level.
Now that we know more and have the benefit of getting more than 200 million Americans fully vaccinated, we can be far more selective about closings and openings. An important part of our strategy must be to communicate honestly with the public about which measures are best for safety. As a key tenet of cognitive-behavioral therapy tells us, “all-or-nothing” thinking is not productive. That should also be the case with our approach to managing COVID-19.
We don’t know the future of the pandemic. Yes, it will end, and possibly COVID will become endemic – like the flu. However, in the meantime, in addition to promoting vaccinations and boosters, we must rigorously encourage our patients to follow public health standards of masking, social distancing, and closing down businesses – and schools – temporarily.
This pandemic has taken a horrendous mental health toll on all of us – especially our patients and frontline health care workers. I’ve spoken with numerous people who were anxious, depressed, and showed signs of PTSD in early 2020; after they got vaccinated, COVID spread diminished, and as public health protocols began to lift, so did their spirits. Clearly for some, the benefit of psychiatric/psychological care centering on the pandemic has proven invaluable. In some ways, the pandemic has brought to the surface the importance of mental health care and removed some of the stigma from mental illness. And that’s a good thing.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
The Centers for Disease Control and Prevention’s decision to shorten the length of isolation time for asymptomatic Americans with COVID-19, regardless of their vaccination status, to 5 days from 10 days is confusing. I hope the agency reconsiders this decision.
After all, one of the CDC’s key messages during this pandemic has been that even people with asymptomatic COVID who have been vaccinated and boosted can transmit the disease. So it seems to me that the Dec. 27, 2021, recommendation about shortening the isolation time for COVID-19–positive people, like the agency’s earlier guidance encouraging people who are vaccinated to stop wearing masks while in indoor settings, runs contrary to good public health principles.
As an expert in human behavior, I am worried about the impact of these confusing messages on the psyche of people in general, as well as on our patients.
Mental health impact
Soon after the United States went on lockdown in March 2020, I wrote about the likelihood of a pandemic of PTSD, anxiety, and depression that would occur in the wake of rising COVID-19 rates. Well, it happened.
Many people have felt a sense of existential despair, depression, and anxiety. As we head into year No. 3 of disruption of our daily lives – and face the loss of more than 825,000 Americans to COVID – we continue to navigate this uncertainty. And now we must deal with Omicron, a variant that is so highly transmissible that it is apparently able to, in some cases, evade two-dose regimens of mRNA vaccines, boosters, and immunity from past infections, according to a report from Imperial College London. Yet, we are being told by some that Omicron might be less severe, compared with other variants. I worry that this assessment is misleading. In that same report, the Imperial College said it “found no evidence” that Omicron is less virulent than Delta, based on the risk of hospitalization and symptom status.
Meanwhile, animal studies suggest that the Omicron variant might lead to less lung damage than previous variants. A preprint article that is being considered for publication by a Nature Portfolio journal suggests that hamsters and mice infected with the Omicron variant do not have as much lung damage as those infected with other variants. More data need to come in for us to get a true understanding of Omicron’s virulence and transmissibility. We should keep an eye on Israel, which is launching a clinical trial of a second booster, or fourth mRNA shot.
As clinicians, we should give our patients and other people with whom we come in contact a sense of hope. In addition to urging people to get boosters, let’s tell them to err on the side of safety when it comes to this pandemic. That means encouraging them to remain isolated for longer than 5 days – until they test negative for COVID. It also means encouraging patients to wear high-quality face masks while inside public spaces – even in the absence of mandates. I have found it heartbreaking to watch televised broadcasts of sporting events held at some stadiums across the country where masks are not being worn. This absence of face coverings is counterintuitive at a time when some Broadway shows are closing. Even the great Radio City Rockettes shut down their holiday shows early in December 2021 because of COVID.
And, as I’ve argued before, we must not give up on unvaccinated people. I have had success in changing the minds of a few patients and some acquaintances with gentle, respectful prodding and vaccine education.
I would also like to see public health principles implemented in our schools and colleges. To protect the health of our children and young adults, we must continue to be nimble – which means school districts should implement layered prevention strategies, as the CDC recommends. This includes not only encouraging eligible staff members and students to get vaccinated, but requiring face masks inside school facilities, maintaining a physical distance of at least 3 feet, “screening testing, ventilation, handwashing, and staying home when sick.”
Furthermore, in deciding whether schools should remain open or be closed after positive COVID cases are discovered, officials should look at the vaccine demographics of that particular school. For example, if 15% of students are vaccinated in one school and 70% are vaccinated in another, the judgment would be different. Of course, it’s clearly best for schools to remain open, but perhaps closing them temporarily – perhaps for a week or 10 days – should be on the table if infection rates reach a certain level.
Now that we know more and have the benefit of getting more than 200 million Americans fully vaccinated, we can be far more selective about closings and openings. An important part of our strategy must be to communicate honestly with the public about which measures are best for safety. As a key tenet of cognitive-behavioral therapy tells us, “all-or-nothing” thinking is not productive. That should also be the case with our approach to managing COVID-19.
We don’t know the future of the pandemic. Yes, it will end, and possibly COVID will become endemic – like the flu. However, in the meantime, in addition to promoting vaccinations and boosters, we must rigorously encourage our patients to follow public health standards of masking, social distancing, and closing down businesses – and schools – temporarily.
This pandemic has taken a horrendous mental health toll on all of us – especially our patients and frontline health care workers. I’ve spoken with numerous people who were anxious, depressed, and showed signs of PTSD in early 2020; after they got vaccinated, COVID spread diminished, and as public health protocols began to lift, so did their spirits. Clearly for some, the benefit of psychiatric/psychological care centering on the pandemic has proven invaluable. In some ways, the pandemic has brought to the surface the importance of mental health care and removed some of the stigma from mental illness. And that’s a good thing.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Olanzapine-samidorphan combination for schizophrenia or bipolar I disorder
Approved by the FDA on May 28, 2021, olanzapine-samidorphan combination (OSC) (Lybalvi, manufactured and distributed by Alkermes, Inc. Waltham, MA USA) is intended to help mitigate some of the weight gain that can be anticipated with the use of olanzapine alone (Table).1-3 Olanzapine (Zyprexa, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA) is a second-generation antipsychotic that has been available for a quarter century.4 Although highly efficacious,5,6 olanzapine has been associated with weight gain, at times substantial, as well as disturbances in glucose and lipid metabolism.7 The addition of samidorphan, an opioid antagonist, to olanzapine in a single tablet may act to decrease the amount of long-term weight gain that can be expected for some patients taking olanzapine alone, consequently minimizing the anticipated increase in waist circumference (a proxy for the measurement of burden imposed by metabolically active adipose tissue). Approval of OSC for the treatment of schizophrenia was based on 2 pivotal randomized controlled trials and their extension studies.8-11 Approval of OSC for bipolar I disorder (acute treatment of manic/mixed episodes as a monotherapy or adjunctive to lithium or valproate, and as a monotherapy maintenance treatment) was based on legacy studies conducted with olanzapine, after establishing that samidorphan does not alter the pharmacokinetics of olanzapine, including in combination with lithium or valproate.3,12,13 OSC should be distinguished from a different combination product, olanzapine-fluoxetine combination (Symbyax, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA), approved for acute depressive episodes associated with bipolar I disorder and for treatment-resistant depression.14

OSC offers the potential to consider olanzapine earlier in the treatment of schizophrenia or bipolar I disorder, especially among practitioners who might otherwise be hesitant to prescribe this agent because of concerns over the risk of excessive weight gain.
OSC is available in 4 dosage strengths containing 5 mg, 10 mg, 15 mg, or 20 mg of olanzapine; all tablets contain 10 mg of samidorphan.2 The recommended starting dose for OSC mirrors the language contained in the legacy olanzapine product label.4 For schizophrenia, the recommended initial dose (olanzapine/samidorphan) is 5 mg/10 mg or 10 mg/10 mg once daily. For bipolar I manic or mixed episodes, the recommended starting dose for monotherapy is 10 mg/10 mg or 15 mg/10 mg, and for use with lithium or valproate, 10 mg/10 mg. For all indications, the recommended target dose can be 10 mg/10 mg, 15 mg/10 mg, or 20 mg/10 mg, with 5 mg/10 mg as an additional potential dose for maintenance monotherapy of bipolar I disorder. The maximum dose is 20 mg/10 mg once daily. Because the amount of samidorphan in each tablet is fixed at 10 mg, combining tablets of OSC, or cutting OSC tablets in half, is not advisable.
Continue to: How it works...
How it works
Product labeling notes that olanzapine is an atypical antipsychotic, that its efficacy in schizophrenia or bipolar I disorder could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism, and that the mechanism of action of samidorphan could be mediated through opioid receptor antagonism.2
The pharmacodynamic profile of olanzapine is complex.2 It binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki = 4, 11, and 5 nM, respectively), dopamine D1-4 (Ki = 11-31 nM), histamine H1 (Ki = 7 nM), and adrenergic alpha-1 receptors (Ki = 19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki = 57 nM) and muscarinic M1-5 (Ki = 73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to gamma aminobutyric acid type A (GABA-A), benzodiazepine, and beta-adrenergic receptors (Ki >10 µM). Olanzapine’s muscarinic receptor affinity can explain why olanzapine can be associated with constipation, dry mouth, and tachycardia, all adverse reactions possibly related to cholinergic antagonism. Thus, OSC should be used with caution in patients with a current diagnosis or prior history of urinary retention, clinically significant prostatic hypertrophy, constipation, or a history of paralytic ileus or related conditions; a potential drug-drug interaction can be anticipated with concomitant use of anticholinergic medications.2 Other pharmacodynamic drug-drug interactions that can occur with the olanzapine component of OSC include the possibility that diazepam, alcohol, or other CNS-acting drugs may potentiate orthostatic hypotension, and there may be a need to reduce the dosage of concomitantly prescribed antihypertensive drugs in patients being treated for hypertension. Moreover, OSC is not recommended in patients receiving levodopa and dopamine agonists.
Samidorphan binds to the mu-, kappa-, and delta-opioid receptors (Ki = .052, .23, and 2.7 nM, respectively).2 Samidorphan is an antagonist at the mu-opioid receptors with partial agonist activity at kappa- and delta-opioid receptors. A major human metabolite of samidorphan (N-dealkylated) binds to the mu-, kappa-, and delta-opioid receptors (Ki = .26, 23, and 56 nM, respectively), and functions as a mu-opioid receptor agonist. The N-oxide major human metabolite binds to mu-, kappa-, and delta-opioid receptors (Ki = 8, 110, and 280 nM, respectively) and functions as a mu-opioid receptor antagonist. This profile differs from that of other opioid antagonists such as naltrexone.15,16
OSC is not a scheduled drug subject to the Controlled Substances Act. Because samidorphan functions as an opioid antagonist, OSC is contraindicated in patients using opioids or undergoing acute opioid withdrawal.2
Regarding cardiac electrophysiology, OSC was not observed to prolong the electrocardiogram QTc interval to any clinically relevant extent when tested at doses up to 30 mg/30 mg (1.5 times and 3 times the maximum recommended daily dosage of olanzapine and samidorphan, respectively).17
Clinical pharmacokinetics
The pharmacokinetics of both olanzapine and samidorphan are linear over the clinical dose range and there is no pharmacokinetic interaction between olanzapine and samidorphan after oral administration of OSC.2 Coadministration of OSC with lithium or valproate does not have a clinically significant effect on systemic exposure of lithium or valproate.13 OSC steady-state concentrations of olanzapine and samidorphan are reached within 7 days, with accumulation at steady state being 2-fold for olanzapine and 1.3-fold for samidorphan (at 5 days). Elimination half-life for olanzapine is 35 to 52 hours, and for samidorphan, 7 to 11 hours. Olanzapine is metabolized primarily via UGT1A4 and CYP1A2, whereas samidorphan is primarily metabolized by CYP3A4. Consequently, concomitant use of OSC with strong CYP3A4 inducers is not recommended. The recommendation regarding CYP1A2 modulators and OSC are similar to those for olanzapine2,4: consider reducing the dosage of the olanzapine component in OSC when used concomitantly with strong CYP1A2 inhibitors, and consider increasing the dosage of the olanzapine component in OSC when used concomitantly with CYP1A2 inducers. Because cigarette smoke contains polycyclic aromatic hydrocarbons that act as CYP1A2 inducers,18 olanzapine clearance is much higher in smokers than in nonsmokers.2 This translates to potentially clinically relevant differences when optimizing the dose. In a study of patients with schizophrenia, olanzapine concentrations were lower in self-reported smokers (16.5, 34.2, and 60.9 ng/mL) than in self-reported nonsmokers (25.6, 43.4, and 113.2 ng/mL) for dosages of 10, 20, and 40 mg/d, respectively.19 In contrast, samidorphan pharmacokinetics are not affected by smoking status.2
No dose adjustment of OSC is needed in patients with hepatic or renal impairment; however, OSC is not recommended for patients with end-stage renal disease because this has not been specifically studied.2
Continue to: Efficacy...
Efficacy
The efficacy of OSC in the treatment of schizophrenia in adults is supported, in part, by the extensive legacy of studies of orally administered olanzapine.2 For OSC specifically, acute efficacy was primarily demonstrated in a randomized, double-blind, phase 3, 4-week study establishing superiority vs placebo in acutely exacerbated patients with schizophrenia.8 Mitigation of weight gain was assessed separately in a randomized, double-blind, phase 3, 24-week study comparing OSC with olanzapine in non-acute outpatients with schizophrenia.10 Both of these 2 trials were accompanied by 52-week open-label extension studies.9,11
The 4-week study evaluated the antipsychotic efficacy of OSC in 401 patients experiencing an acute exacerbation or relapse of schizophrenia who required inpatient treatment.8 Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score ≥80, with a score ≥4 on at least 3 of selected positive symptoms, and a Clinical Global Impression-Severity (CGI-S) score ≥4 at baseline and screening. Patients were required to be inpatients for the first 2 weeks of the study, and were encouraged to remain as inpatients for all 4 weeks. Patients were randomized to receive OSC, olanzapine, or placebo. Dosing was once-daily and flexible based on clinical response and tolerability for the first 2 weeks of the study, and fixed thereafter. Patients assigned to OSC could receive 10 mg/10 mg or 20 mg/10 mg, and patients randomized to olanzapine could receive 10 mg or 20 mg. The study compared OSC with placebo, with olanzapine serving as an active control. Treatment with OSC resulted in significant improvements in symptoms compared with placebo at Week 4, as measured by changes in PANSS total scores from baseline. Improvement in PANSS scores with OSC relative to placebo was similar to that observed with olanzapine. The antipsychotic efficacy of OSC relative to placebo was also supported by improvements in CGI-S scores. Thus, the inclusion of samidorphan in OSC did not negatively impact the antipsychotic efficacy of olanzapine.
In the 24-week study, 561 patients were randomized to OSC or olanzapine.10 There was no placebo control. Patients were treated with doses of OSC 10 mg/10 mg or 20 mg/10 mg, or with doses of olanzapine 10 mg or 20 mg. Dosing was flexible for the first 4 weeks of the study and fixed thereafter. Eligible patients were age 18 to 55 years (younger than the 4-week study, where the maximum age was 70 years), with a body mass index of 18 to 30 kg/m2 (lower than the upper limit of 40 kg/m2 used in the 4-week study). In contrast to the acutely exacerbated patients in the 4-week study, patients were required to have a PANSS total score of 50 to 90, CGI-S score ≤4, and symptoms suitable for outpatient treatment. The co-primary endpoints were percent change from baseline in body weight and proportion of patients who gained ≥10% body weight at Week 24. Treatment with OSC or olanzapine resulted in similar improvements in PANSS total and CGI-S scores, but treatment with OSC was associated with statistically significantly less weight gain than treatment with olanzapine, and with a smaller proportion of patients who gained ≥10% body weight. The least squares mean percent weight change from baseline to the end of treatment was 4.2% with OSC vs 6.6% with olanzapine. Although patients treated with OSC or olanzapine had similar weight gain for the first 4 weeks of treatment, OSC weight gain stabilized after approximately the 6th week, whereas patients who received olanzapine continued to gain weight throughout the remainder of the treatment period. The risk of gaining ≥10% body weight from baseline was reduced by 50% with OSC compared with olanzapine. Moreover, the odds of gaining ≥7% body weight from baseline at Week 24 were also reduced by 50% for OSC compared with olanzapine. OSC was also associated with smaller increases in waist circumference compared with olanzapine, which was observable as early as Week 1. The risk of experiencing a 5-cm increase in waist circumference was 50% lower for patients treated with OSC vs olanzapine, a relevant threshold in assessing risk of all-cause mortality and cardiovascular disease.20 However, changes in metabolic laboratory parameters in patients treated with OSC or olanzapine were generally small and were similar between groups. In addition, there were little differences between the 2 treatment groups in metabolic parameter changes considered to be of potential clinical significance, based on commonly used thresholds.
Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to OSC is unknown.For bipolar I manic or mixed episodes, the use of OSC as monotherapy or in combination with lithium or valproate, as well as for maintenance monotherapy, was approved based on legacy clinical trials with olanzapine, as described in product labeling,2,4 as well as pharmacokinetic data evidencing that OSC did not have a clinically significant effect on the pharmacokinetics of lithium or valproate.13 A study is in progress to evaluate the effect of OSC compared with olanzapine on body weight in young adults with schizophrenia, schizophreniform, or bipolar I disorder who are early in their illness (ClinicalTrials.gov identifier: NCT03187769).
Overall tolerability and safety
The systemic safety and tolerability profile for OSC would be expected to be similar to that for olanzapine, unless there are adverse events that are specifically related to the samidorphan component. In the 4-week acute study described above,8 adverse events that occurred at least twice the rate of placebo with OSC included increased weight (18.7%, 14.3%, 3.0%, for OSC, olanzapine, and placebo, respectively), somnolence (9.0%, 9.8%, 2.2%), dry mouth (7.5%, 5.3%, 0.7%), and headache (6.0%, 5.3%, 3.0%). In the 24-week study,10 which did not have a placebo control, the most commonly reported adverse events (≥10% of patients) were increased weight (24.8% vs 36.2% for OSC vs olanzapine), somnolence (21.2% vs 18.1%), dry mouth (12.8% vs 8.0%), and increased appetite (10.9% vs 12.3%). In both studies, rates of discontinuation due to adverse events were low and similar between groups (in the 4-week study, 1.5% for OSC, 2.3% for olanzapine, and 5.2% for placebo; in the 24-week study, 12.0% for OSC and 9.8% for olanzapine).
In the 2 open-label, phase 3, 52-week extension studies,9,11 long-term tolerability was evidenced by low rates discontinuation due to adverse events (≤6%). Neither extension study reported any clinically meaningful changes over time in hematology, biochemistry, vital signs, or electrocardiogram parameters.3 In addition to durability of antipsychotic response as evidenced by sustained improvements in PANSS and CGI-S scores over time, waist circumference and weight remained stable, and the observed long-term changes in weight were consistent with weight changes observed with other second-generation antipsychotics.3 Long-term changes in metabolic laboratory parameter values were small and remained stable, and there was little change in glycosylated hemoglobin (hemoglobin A1c) values, which suggests that glycemic control was maintained with long-term OSC treatment.3 Caveats to consider are that the extension studies were open label without comparators, and they may have selected for patients who responded favorably to OSC treatment in the preceding studies.3Warnings and precautions in OSC product labeling are generally similar to those for other second-generation antipsychotics,21 other than warnings and precautions specifically related to samidorphan being an opioid antagonist, and special mention of “Drug Reaction with Eosinophilia and Systemic Symptoms” and “Anticholinergic (Antimuscarinic) Effects” warnings, which also are contained in the olanzapine legacy label.2,4
Summary
Olanzapine has a plethora of evidence supporting its robust efficacy profile5,6; however, its use is stymied by an unfavorable weight and metabolic profile.7 OSC may help mitigate at least some of the weight gain that would be expected with the use of olanzapine alone in the long-term treatment of patients with schizophrenia or bipolar I disorder. The addition of samidorphan does not deleteriously affect the efficacy of olanzapine, but decreases the risk of gaining ≥10% or ≥7% of baseline body weight by approximately 50% compared with olanzapine alone. Increase in waist circumference, a proxy for how much metabolically active fat one has, is lower with OSC than it is with olanzapine. Because samidorphan is an opioid receptor antagonist, OSC is contraindicated in patients using opioids and in those undergoing acute opioid withdrawal. Dosage strengths available for OSC parallel those for olanzapine, and all strengths including the same fixed dose of samidorphan—10 mg—so advise patients not to double up on the tablets, and to not split them.
Related Resource
• Olanzapine and samidorphan (Lybalvi) prescribing information. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
Drug Brand Names
Diazepam • Valium
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Olanzapine-fluoxetine combination • Symbyax
Olanzapine-samidorphan combination • Lybalvi
Valproate • Depakote, Depakene
Bottom Line
Olanzapine-samidorphan combination (OSC) is intended to mitigate some of the weight gain anticipated when using olanzapine alone. For clinicians who have prescribed olanzapine and have seen its therapeutic benefits, OSC will be a welcome addition to the therapeutic armamentarium. For practitioners who may have avoided olanzapine entirely, OSC can provide another means of offering this therapeutic option and counter “olanzapine hesitancy.”
1. US Food and Drug Administration. NDA 213378 approval letter. May 28, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000Approv.pdf
2. Alkermes, Inc. LYBALVI™ (olanzapine and samidorphan) tablets, for oral use. Prescribing information. May 2021. Accessed November 24, 2021. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
3. Citrome L, Graham C, Simmons A, et al. An evidence-based review of OLZ/SAM for treatment of adults with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2021;17:2885-2904.
4. Eli Lilly and Company. ZYPREXA (olanzapine) tablet for oral use; ZYPREXA ZYDIS (olanzapine) tablet, orally disintegrating for oral use; ZYPREXA intramuscular (olanzapine) injection, powder, for solution for intramuscular use. Prescribing information. February 2021. Accessed November 24, 2021. https://pi.lilly.com/us/zyprexa-pi.pdf
5. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsychiatr Dis Treat. 2019;15:2559-2569.
6. Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132(1):80-90.
7. Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455-482.
8. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769.
9. Yagoda S, Graham C, Simmons A, et al. Long-term safety and durability of effect with a combination of olanzapine and samidorphan in patients with schizophrenia: results from a 1-year open-label extension study. CNS Spectr. 2021;26(4):383-392.
10. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168-1178.
11. Kahn RS, Silverman BL, DiPetrillo L, et al. A phase 3, multicenter study to assess the 1-year safety and tolerability of a combination of olanzapine and samidorphan in patients with schizophrenia: results from the ENLIGHTEN-2 long-term extension. Schizophr Res. 2021;232:45-53.
12. US Food and Drug Administration. Drug approval package: Lybalvi. June 26, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000TOC.cfm
13. Sun L, Yagoda S, Yao B, et al. Combination of olanzapine and samidorphan has no clinically significant effect on the pharmacokinetics of lithium or valproate. Clin Drug Investig. 2020;40(1):55-64.
14. Eli Lilly and Company. SYMBYAX (olanzapine and fluoxetine) capsules for oral use. Prescribing information. September 2021. Accessed November 24, 2021. https://pi.lilly.com/us/symbyax-pi.pdf
15. Wentland MP, Lu Q, Lou R, et al. Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett. 2005;15(8):2107-2110.
16. Lee MW, Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother. 2009;10(11):1841-1845.
17. Sun L, Yagoda S, Xue H, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109881.
18. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
19. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278-283.
20. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335-345.
21. Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599-1613.
Approved by the FDA on May 28, 2021, olanzapine-samidorphan combination (OSC) (Lybalvi, manufactured and distributed by Alkermes, Inc. Waltham, MA USA) is intended to help mitigate some of the weight gain that can be anticipated with the use of olanzapine alone (Table).1-3 Olanzapine (Zyprexa, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA) is a second-generation antipsychotic that has been available for a quarter century.4 Although highly efficacious,5,6 olanzapine has been associated with weight gain, at times substantial, as well as disturbances in glucose and lipid metabolism.7 The addition of samidorphan, an opioid antagonist, to olanzapine in a single tablet may act to decrease the amount of long-term weight gain that can be expected for some patients taking olanzapine alone, consequently minimizing the anticipated increase in waist circumference (a proxy for the measurement of burden imposed by metabolically active adipose tissue). Approval of OSC for the treatment of schizophrenia was based on 2 pivotal randomized controlled trials and their extension studies.8-11 Approval of OSC for bipolar I disorder (acute treatment of manic/mixed episodes as a monotherapy or adjunctive to lithium or valproate, and as a monotherapy maintenance treatment) was based on legacy studies conducted with olanzapine, after establishing that samidorphan does not alter the pharmacokinetics of olanzapine, including in combination with lithium or valproate.3,12,13 OSC should be distinguished from a different combination product, olanzapine-fluoxetine combination (Symbyax, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA), approved for acute depressive episodes associated with bipolar I disorder and for treatment-resistant depression.14

OSC offers the potential to consider olanzapine earlier in the treatment of schizophrenia or bipolar I disorder, especially among practitioners who might otherwise be hesitant to prescribe this agent because of concerns over the risk of excessive weight gain.
OSC is available in 4 dosage strengths containing 5 mg, 10 mg, 15 mg, or 20 mg of olanzapine; all tablets contain 10 mg of samidorphan.2 The recommended starting dose for OSC mirrors the language contained in the legacy olanzapine product label.4 For schizophrenia, the recommended initial dose (olanzapine/samidorphan) is 5 mg/10 mg or 10 mg/10 mg once daily. For bipolar I manic or mixed episodes, the recommended starting dose for monotherapy is 10 mg/10 mg or 15 mg/10 mg, and for use with lithium or valproate, 10 mg/10 mg. For all indications, the recommended target dose can be 10 mg/10 mg, 15 mg/10 mg, or 20 mg/10 mg, with 5 mg/10 mg as an additional potential dose for maintenance monotherapy of bipolar I disorder. The maximum dose is 20 mg/10 mg once daily. Because the amount of samidorphan in each tablet is fixed at 10 mg, combining tablets of OSC, or cutting OSC tablets in half, is not advisable.
Continue to: How it works...
How it works
Product labeling notes that olanzapine is an atypical antipsychotic, that its efficacy in schizophrenia or bipolar I disorder could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism, and that the mechanism of action of samidorphan could be mediated through opioid receptor antagonism.2
The pharmacodynamic profile of olanzapine is complex.2 It binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki = 4, 11, and 5 nM, respectively), dopamine D1-4 (Ki = 11-31 nM), histamine H1 (Ki = 7 nM), and adrenergic alpha-1 receptors (Ki = 19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki = 57 nM) and muscarinic M1-5 (Ki = 73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to gamma aminobutyric acid type A (GABA-A), benzodiazepine, and beta-adrenergic receptors (Ki >10 µM). Olanzapine’s muscarinic receptor affinity can explain why olanzapine can be associated with constipation, dry mouth, and tachycardia, all adverse reactions possibly related to cholinergic antagonism. Thus, OSC should be used with caution in patients with a current diagnosis or prior history of urinary retention, clinically significant prostatic hypertrophy, constipation, or a history of paralytic ileus or related conditions; a potential drug-drug interaction can be anticipated with concomitant use of anticholinergic medications.2 Other pharmacodynamic drug-drug interactions that can occur with the olanzapine component of OSC include the possibility that diazepam, alcohol, or other CNS-acting drugs may potentiate orthostatic hypotension, and there may be a need to reduce the dosage of concomitantly prescribed antihypertensive drugs in patients being treated for hypertension. Moreover, OSC is not recommended in patients receiving levodopa and dopamine agonists.
Samidorphan binds to the mu-, kappa-, and delta-opioid receptors (Ki = .052, .23, and 2.7 nM, respectively).2 Samidorphan is an antagonist at the mu-opioid receptors with partial agonist activity at kappa- and delta-opioid receptors. A major human metabolite of samidorphan (N-dealkylated) binds to the mu-, kappa-, and delta-opioid receptors (Ki = .26, 23, and 56 nM, respectively), and functions as a mu-opioid receptor agonist. The N-oxide major human metabolite binds to mu-, kappa-, and delta-opioid receptors (Ki = 8, 110, and 280 nM, respectively) and functions as a mu-opioid receptor antagonist. This profile differs from that of other opioid antagonists such as naltrexone.15,16
OSC is not a scheduled drug subject to the Controlled Substances Act. Because samidorphan functions as an opioid antagonist, OSC is contraindicated in patients using opioids or undergoing acute opioid withdrawal.2
Regarding cardiac electrophysiology, OSC was not observed to prolong the electrocardiogram QTc interval to any clinically relevant extent when tested at doses up to 30 mg/30 mg (1.5 times and 3 times the maximum recommended daily dosage of olanzapine and samidorphan, respectively).17
Clinical pharmacokinetics
The pharmacokinetics of both olanzapine and samidorphan are linear over the clinical dose range and there is no pharmacokinetic interaction between olanzapine and samidorphan after oral administration of OSC.2 Coadministration of OSC with lithium or valproate does not have a clinically significant effect on systemic exposure of lithium or valproate.13 OSC steady-state concentrations of olanzapine and samidorphan are reached within 7 days, with accumulation at steady state being 2-fold for olanzapine and 1.3-fold for samidorphan (at 5 days). Elimination half-life for olanzapine is 35 to 52 hours, and for samidorphan, 7 to 11 hours. Olanzapine is metabolized primarily via UGT1A4 and CYP1A2, whereas samidorphan is primarily metabolized by CYP3A4. Consequently, concomitant use of OSC with strong CYP3A4 inducers is not recommended. The recommendation regarding CYP1A2 modulators and OSC are similar to those for olanzapine2,4: consider reducing the dosage of the olanzapine component in OSC when used concomitantly with strong CYP1A2 inhibitors, and consider increasing the dosage of the olanzapine component in OSC when used concomitantly with CYP1A2 inducers. Because cigarette smoke contains polycyclic aromatic hydrocarbons that act as CYP1A2 inducers,18 olanzapine clearance is much higher in smokers than in nonsmokers.2 This translates to potentially clinically relevant differences when optimizing the dose. In a study of patients with schizophrenia, olanzapine concentrations were lower in self-reported smokers (16.5, 34.2, and 60.9 ng/mL) than in self-reported nonsmokers (25.6, 43.4, and 113.2 ng/mL) for dosages of 10, 20, and 40 mg/d, respectively.19 In contrast, samidorphan pharmacokinetics are not affected by smoking status.2
No dose adjustment of OSC is needed in patients with hepatic or renal impairment; however, OSC is not recommended for patients with end-stage renal disease because this has not been specifically studied.2
Continue to: Efficacy...
Efficacy
The efficacy of OSC in the treatment of schizophrenia in adults is supported, in part, by the extensive legacy of studies of orally administered olanzapine.2 For OSC specifically, acute efficacy was primarily demonstrated in a randomized, double-blind, phase 3, 4-week study establishing superiority vs placebo in acutely exacerbated patients with schizophrenia.8 Mitigation of weight gain was assessed separately in a randomized, double-blind, phase 3, 24-week study comparing OSC with olanzapine in non-acute outpatients with schizophrenia.10 Both of these 2 trials were accompanied by 52-week open-label extension studies.9,11
The 4-week study evaluated the antipsychotic efficacy of OSC in 401 patients experiencing an acute exacerbation or relapse of schizophrenia who required inpatient treatment.8 Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score ≥80, with a score ≥4 on at least 3 of selected positive symptoms, and a Clinical Global Impression-Severity (CGI-S) score ≥4 at baseline and screening. Patients were required to be inpatients for the first 2 weeks of the study, and were encouraged to remain as inpatients for all 4 weeks. Patients were randomized to receive OSC, olanzapine, or placebo. Dosing was once-daily and flexible based on clinical response and tolerability for the first 2 weeks of the study, and fixed thereafter. Patients assigned to OSC could receive 10 mg/10 mg or 20 mg/10 mg, and patients randomized to olanzapine could receive 10 mg or 20 mg. The study compared OSC with placebo, with olanzapine serving as an active control. Treatment with OSC resulted in significant improvements in symptoms compared with placebo at Week 4, as measured by changes in PANSS total scores from baseline. Improvement in PANSS scores with OSC relative to placebo was similar to that observed with olanzapine. The antipsychotic efficacy of OSC relative to placebo was also supported by improvements in CGI-S scores. Thus, the inclusion of samidorphan in OSC did not negatively impact the antipsychotic efficacy of olanzapine.
In the 24-week study, 561 patients were randomized to OSC or olanzapine.10 There was no placebo control. Patients were treated with doses of OSC 10 mg/10 mg or 20 mg/10 mg, or with doses of olanzapine 10 mg or 20 mg. Dosing was flexible for the first 4 weeks of the study and fixed thereafter. Eligible patients were age 18 to 55 years (younger than the 4-week study, where the maximum age was 70 years), with a body mass index of 18 to 30 kg/m2 (lower than the upper limit of 40 kg/m2 used in the 4-week study). In contrast to the acutely exacerbated patients in the 4-week study, patients were required to have a PANSS total score of 50 to 90, CGI-S score ≤4, and symptoms suitable for outpatient treatment. The co-primary endpoints were percent change from baseline in body weight and proportion of patients who gained ≥10% body weight at Week 24. Treatment with OSC or olanzapine resulted in similar improvements in PANSS total and CGI-S scores, but treatment with OSC was associated with statistically significantly less weight gain than treatment with olanzapine, and with a smaller proportion of patients who gained ≥10% body weight. The least squares mean percent weight change from baseline to the end of treatment was 4.2% with OSC vs 6.6% with olanzapine. Although patients treated with OSC or olanzapine had similar weight gain for the first 4 weeks of treatment, OSC weight gain stabilized after approximately the 6th week, whereas patients who received olanzapine continued to gain weight throughout the remainder of the treatment period. The risk of gaining ≥10% body weight from baseline was reduced by 50% with OSC compared with olanzapine. Moreover, the odds of gaining ≥7% body weight from baseline at Week 24 were also reduced by 50% for OSC compared with olanzapine. OSC was also associated with smaller increases in waist circumference compared with olanzapine, which was observable as early as Week 1. The risk of experiencing a 5-cm increase in waist circumference was 50% lower for patients treated with OSC vs olanzapine, a relevant threshold in assessing risk of all-cause mortality and cardiovascular disease.20 However, changes in metabolic laboratory parameters in patients treated with OSC or olanzapine were generally small and were similar between groups. In addition, there were little differences between the 2 treatment groups in metabolic parameter changes considered to be of potential clinical significance, based on commonly used thresholds.
Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to OSC is unknown.For bipolar I manic or mixed episodes, the use of OSC as monotherapy or in combination with lithium or valproate, as well as for maintenance monotherapy, was approved based on legacy clinical trials with olanzapine, as described in product labeling,2,4 as well as pharmacokinetic data evidencing that OSC did not have a clinically significant effect on the pharmacokinetics of lithium or valproate.13 A study is in progress to evaluate the effect of OSC compared with olanzapine on body weight in young adults with schizophrenia, schizophreniform, or bipolar I disorder who are early in their illness (ClinicalTrials.gov identifier: NCT03187769).
Overall tolerability and safety
The systemic safety and tolerability profile for OSC would be expected to be similar to that for olanzapine, unless there are adverse events that are specifically related to the samidorphan component. In the 4-week acute study described above,8 adverse events that occurred at least twice the rate of placebo with OSC included increased weight (18.7%, 14.3%, 3.0%, for OSC, olanzapine, and placebo, respectively), somnolence (9.0%, 9.8%, 2.2%), dry mouth (7.5%, 5.3%, 0.7%), and headache (6.0%, 5.3%, 3.0%). In the 24-week study,10 which did not have a placebo control, the most commonly reported adverse events (≥10% of patients) were increased weight (24.8% vs 36.2% for OSC vs olanzapine), somnolence (21.2% vs 18.1%), dry mouth (12.8% vs 8.0%), and increased appetite (10.9% vs 12.3%). In both studies, rates of discontinuation due to adverse events were low and similar between groups (in the 4-week study, 1.5% for OSC, 2.3% for olanzapine, and 5.2% for placebo; in the 24-week study, 12.0% for OSC and 9.8% for olanzapine).
In the 2 open-label, phase 3, 52-week extension studies,9,11 long-term tolerability was evidenced by low rates discontinuation due to adverse events (≤6%). Neither extension study reported any clinically meaningful changes over time in hematology, biochemistry, vital signs, or electrocardiogram parameters.3 In addition to durability of antipsychotic response as evidenced by sustained improvements in PANSS and CGI-S scores over time, waist circumference and weight remained stable, and the observed long-term changes in weight were consistent with weight changes observed with other second-generation antipsychotics.3 Long-term changes in metabolic laboratory parameter values were small and remained stable, and there was little change in glycosylated hemoglobin (hemoglobin A1c) values, which suggests that glycemic control was maintained with long-term OSC treatment.3 Caveats to consider are that the extension studies were open label without comparators, and they may have selected for patients who responded favorably to OSC treatment in the preceding studies.3Warnings and precautions in OSC product labeling are generally similar to those for other second-generation antipsychotics,21 other than warnings and precautions specifically related to samidorphan being an opioid antagonist, and special mention of “Drug Reaction with Eosinophilia and Systemic Symptoms” and “Anticholinergic (Antimuscarinic) Effects” warnings, which also are contained in the olanzapine legacy label.2,4
Summary
Olanzapine has a plethora of evidence supporting its robust efficacy profile5,6; however, its use is stymied by an unfavorable weight and metabolic profile.7 OSC may help mitigate at least some of the weight gain that would be expected with the use of olanzapine alone in the long-term treatment of patients with schizophrenia or bipolar I disorder. The addition of samidorphan does not deleteriously affect the efficacy of olanzapine, but decreases the risk of gaining ≥10% or ≥7% of baseline body weight by approximately 50% compared with olanzapine alone. Increase in waist circumference, a proxy for how much metabolically active fat one has, is lower with OSC than it is with olanzapine. Because samidorphan is an opioid receptor antagonist, OSC is contraindicated in patients using opioids and in those undergoing acute opioid withdrawal. Dosage strengths available for OSC parallel those for olanzapine, and all strengths including the same fixed dose of samidorphan—10 mg—so advise patients not to double up on the tablets, and to not split them.
Related Resource
• Olanzapine and samidorphan (Lybalvi) prescribing information. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
Drug Brand Names
Diazepam • Valium
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Olanzapine-fluoxetine combination • Symbyax
Olanzapine-samidorphan combination • Lybalvi
Valproate • Depakote, Depakene
Bottom Line
Olanzapine-samidorphan combination (OSC) is intended to mitigate some of the weight gain anticipated when using olanzapine alone. For clinicians who have prescribed olanzapine and have seen its therapeutic benefits, OSC will be a welcome addition to the therapeutic armamentarium. For practitioners who may have avoided olanzapine entirely, OSC can provide another means of offering this therapeutic option and counter “olanzapine hesitancy.”
Approved by the FDA on May 28, 2021, olanzapine-samidorphan combination (OSC) (Lybalvi, manufactured and distributed by Alkermes, Inc. Waltham, MA USA) is intended to help mitigate some of the weight gain that can be anticipated with the use of olanzapine alone (Table).1-3 Olanzapine (Zyprexa, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA) is a second-generation antipsychotic that has been available for a quarter century.4 Although highly efficacious,5,6 olanzapine has been associated with weight gain, at times substantial, as well as disturbances in glucose and lipid metabolism.7 The addition of samidorphan, an opioid antagonist, to olanzapine in a single tablet may act to decrease the amount of long-term weight gain that can be expected for some patients taking olanzapine alone, consequently minimizing the anticipated increase in waist circumference (a proxy for the measurement of burden imposed by metabolically active adipose tissue). Approval of OSC for the treatment of schizophrenia was based on 2 pivotal randomized controlled trials and their extension studies.8-11 Approval of OSC for bipolar I disorder (acute treatment of manic/mixed episodes as a monotherapy or adjunctive to lithium or valproate, and as a monotherapy maintenance treatment) was based on legacy studies conducted with olanzapine, after establishing that samidorphan does not alter the pharmacokinetics of olanzapine, including in combination with lithium or valproate.3,12,13 OSC should be distinguished from a different combination product, olanzapine-fluoxetine combination (Symbyax, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA), approved for acute depressive episodes associated with bipolar I disorder and for treatment-resistant depression.14

OSC offers the potential to consider olanzapine earlier in the treatment of schizophrenia or bipolar I disorder, especially among practitioners who might otherwise be hesitant to prescribe this agent because of concerns over the risk of excessive weight gain.
OSC is available in 4 dosage strengths containing 5 mg, 10 mg, 15 mg, or 20 mg of olanzapine; all tablets contain 10 mg of samidorphan.2 The recommended starting dose for OSC mirrors the language contained in the legacy olanzapine product label.4 For schizophrenia, the recommended initial dose (olanzapine/samidorphan) is 5 mg/10 mg or 10 mg/10 mg once daily. For bipolar I manic or mixed episodes, the recommended starting dose for monotherapy is 10 mg/10 mg or 15 mg/10 mg, and for use with lithium or valproate, 10 mg/10 mg. For all indications, the recommended target dose can be 10 mg/10 mg, 15 mg/10 mg, or 20 mg/10 mg, with 5 mg/10 mg as an additional potential dose for maintenance monotherapy of bipolar I disorder. The maximum dose is 20 mg/10 mg once daily. Because the amount of samidorphan in each tablet is fixed at 10 mg, combining tablets of OSC, or cutting OSC tablets in half, is not advisable.
Continue to: How it works...
How it works
Product labeling notes that olanzapine is an atypical antipsychotic, that its efficacy in schizophrenia or bipolar I disorder could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism, and that the mechanism of action of samidorphan could be mediated through opioid receptor antagonism.2
The pharmacodynamic profile of olanzapine is complex.2 It binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki = 4, 11, and 5 nM, respectively), dopamine D1-4 (Ki = 11-31 nM), histamine H1 (Ki = 7 nM), and adrenergic alpha-1 receptors (Ki = 19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki = 57 nM) and muscarinic M1-5 (Ki = 73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to gamma aminobutyric acid type A (GABA-A), benzodiazepine, and beta-adrenergic receptors (Ki >10 µM). Olanzapine’s muscarinic receptor affinity can explain why olanzapine can be associated with constipation, dry mouth, and tachycardia, all adverse reactions possibly related to cholinergic antagonism. Thus, OSC should be used with caution in patients with a current diagnosis or prior history of urinary retention, clinically significant prostatic hypertrophy, constipation, or a history of paralytic ileus or related conditions; a potential drug-drug interaction can be anticipated with concomitant use of anticholinergic medications.2 Other pharmacodynamic drug-drug interactions that can occur with the olanzapine component of OSC include the possibility that diazepam, alcohol, or other CNS-acting drugs may potentiate orthostatic hypotension, and there may be a need to reduce the dosage of concomitantly prescribed antihypertensive drugs in patients being treated for hypertension. Moreover, OSC is not recommended in patients receiving levodopa and dopamine agonists.
Samidorphan binds to the mu-, kappa-, and delta-opioid receptors (Ki = .052, .23, and 2.7 nM, respectively).2 Samidorphan is an antagonist at the mu-opioid receptors with partial agonist activity at kappa- and delta-opioid receptors. A major human metabolite of samidorphan (N-dealkylated) binds to the mu-, kappa-, and delta-opioid receptors (Ki = .26, 23, and 56 nM, respectively), and functions as a mu-opioid receptor agonist. The N-oxide major human metabolite binds to mu-, kappa-, and delta-opioid receptors (Ki = 8, 110, and 280 nM, respectively) and functions as a mu-opioid receptor antagonist. This profile differs from that of other opioid antagonists such as naltrexone.15,16
OSC is not a scheduled drug subject to the Controlled Substances Act. Because samidorphan functions as an opioid antagonist, OSC is contraindicated in patients using opioids or undergoing acute opioid withdrawal.2
Regarding cardiac electrophysiology, OSC was not observed to prolong the electrocardiogram QTc interval to any clinically relevant extent when tested at doses up to 30 mg/30 mg (1.5 times and 3 times the maximum recommended daily dosage of olanzapine and samidorphan, respectively).17
Clinical pharmacokinetics
The pharmacokinetics of both olanzapine and samidorphan are linear over the clinical dose range and there is no pharmacokinetic interaction between olanzapine and samidorphan after oral administration of OSC.2 Coadministration of OSC with lithium or valproate does not have a clinically significant effect on systemic exposure of lithium or valproate.13 OSC steady-state concentrations of olanzapine and samidorphan are reached within 7 days, with accumulation at steady state being 2-fold for olanzapine and 1.3-fold for samidorphan (at 5 days). Elimination half-life for olanzapine is 35 to 52 hours, and for samidorphan, 7 to 11 hours. Olanzapine is metabolized primarily via UGT1A4 and CYP1A2, whereas samidorphan is primarily metabolized by CYP3A4. Consequently, concomitant use of OSC with strong CYP3A4 inducers is not recommended. The recommendation regarding CYP1A2 modulators and OSC are similar to those for olanzapine2,4: consider reducing the dosage of the olanzapine component in OSC when used concomitantly with strong CYP1A2 inhibitors, and consider increasing the dosage of the olanzapine component in OSC when used concomitantly with CYP1A2 inducers. Because cigarette smoke contains polycyclic aromatic hydrocarbons that act as CYP1A2 inducers,18 olanzapine clearance is much higher in smokers than in nonsmokers.2 This translates to potentially clinically relevant differences when optimizing the dose. In a study of patients with schizophrenia, olanzapine concentrations were lower in self-reported smokers (16.5, 34.2, and 60.9 ng/mL) than in self-reported nonsmokers (25.6, 43.4, and 113.2 ng/mL) for dosages of 10, 20, and 40 mg/d, respectively.19 In contrast, samidorphan pharmacokinetics are not affected by smoking status.2
No dose adjustment of OSC is needed in patients with hepatic or renal impairment; however, OSC is not recommended for patients with end-stage renal disease because this has not been specifically studied.2
Continue to: Efficacy...
Efficacy
The efficacy of OSC in the treatment of schizophrenia in adults is supported, in part, by the extensive legacy of studies of orally administered olanzapine.2 For OSC specifically, acute efficacy was primarily demonstrated in a randomized, double-blind, phase 3, 4-week study establishing superiority vs placebo in acutely exacerbated patients with schizophrenia.8 Mitigation of weight gain was assessed separately in a randomized, double-blind, phase 3, 24-week study comparing OSC with olanzapine in non-acute outpatients with schizophrenia.10 Both of these 2 trials were accompanied by 52-week open-label extension studies.9,11
The 4-week study evaluated the antipsychotic efficacy of OSC in 401 patients experiencing an acute exacerbation or relapse of schizophrenia who required inpatient treatment.8 Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score ≥80, with a score ≥4 on at least 3 of selected positive symptoms, and a Clinical Global Impression-Severity (CGI-S) score ≥4 at baseline and screening. Patients were required to be inpatients for the first 2 weeks of the study, and were encouraged to remain as inpatients for all 4 weeks. Patients were randomized to receive OSC, olanzapine, or placebo. Dosing was once-daily and flexible based on clinical response and tolerability for the first 2 weeks of the study, and fixed thereafter. Patients assigned to OSC could receive 10 mg/10 mg or 20 mg/10 mg, and patients randomized to olanzapine could receive 10 mg or 20 mg. The study compared OSC with placebo, with olanzapine serving as an active control. Treatment with OSC resulted in significant improvements in symptoms compared with placebo at Week 4, as measured by changes in PANSS total scores from baseline. Improvement in PANSS scores with OSC relative to placebo was similar to that observed with olanzapine. The antipsychotic efficacy of OSC relative to placebo was also supported by improvements in CGI-S scores. Thus, the inclusion of samidorphan in OSC did not negatively impact the antipsychotic efficacy of olanzapine.
In the 24-week study, 561 patients were randomized to OSC or olanzapine.10 There was no placebo control. Patients were treated with doses of OSC 10 mg/10 mg or 20 mg/10 mg, or with doses of olanzapine 10 mg or 20 mg. Dosing was flexible for the first 4 weeks of the study and fixed thereafter. Eligible patients were age 18 to 55 years (younger than the 4-week study, where the maximum age was 70 years), with a body mass index of 18 to 30 kg/m2 (lower than the upper limit of 40 kg/m2 used in the 4-week study). In contrast to the acutely exacerbated patients in the 4-week study, patients were required to have a PANSS total score of 50 to 90, CGI-S score ≤4, and symptoms suitable for outpatient treatment. The co-primary endpoints were percent change from baseline in body weight and proportion of patients who gained ≥10% body weight at Week 24. Treatment with OSC or olanzapine resulted in similar improvements in PANSS total and CGI-S scores, but treatment with OSC was associated with statistically significantly less weight gain than treatment with olanzapine, and with a smaller proportion of patients who gained ≥10% body weight. The least squares mean percent weight change from baseline to the end of treatment was 4.2% with OSC vs 6.6% with olanzapine. Although patients treated with OSC or olanzapine had similar weight gain for the first 4 weeks of treatment, OSC weight gain stabilized after approximately the 6th week, whereas patients who received olanzapine continued to gain weight throughout the remainder of the treatment period. The risk of gaining ≥10% body weight from baseline was reduced by 50% with OSC compared with olanzapine. Moreover, the odds of gaining ≥7% body weight from baseline at Week 24 were also reduced by 50% for OSC compared with olanzapine. OSC was also associated with smaller increases in waist circumference compared with olanzapine, which was observable as early as Week 1. The risk of experiencing a 5-cm increase in waist circumference was 50% lower for patients treated with OSC vs olanzapine, a relevant threshold in assessing risk of all-cause mortality and cardiovascular disease.20 However, changes in metabolic laboratory parameters in patients treated with OSC or olanzapine were generally small and were similar between groups. In addition, there were little differences between the 2 treatment groups in metabolic parameter changes considered to be of potential clinical significance, based on commonly used thresholds.
Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to OSC is unknown.For bipolar I manic or mixed episodes, the use of OSC as monotherapy or in combination with lithium or valproate, as well as for maintenance monotherapy, was approved based on legacy clinical trials with olanzapine, as described in product labeling,2,4 as well as pharmacokinetic data evidencing that OSC did not have a clinically significant effect on the pharmacokinetics of lithium or valproate.13 A study is in progress to evaluate the effect of OSC compared with olanzapine on body weight in young adults with schizophrenia, schizophreniform, or bipolar I disorder who are early in their illness (ClinicalTrials.gov identifier: NCT03187769).
Overall tolerability and safety
The systemic safety and tolerability profile for OSC would be expected to be similar to that for olanzapine, unless there are adverse events that are specifically related to the samidorphan component. In the 4-week acute study described above,8 adverse events that occurred at least twice the rate of placebo with OSC included increased weight (18.7%, 14.3%, 3.0%, for OSC, olanzapine, and placebo, respectively), somnolence (9.0%, 9.8%, 2.2%), dry mouth (7.5%, 5.3%, 0.7%), and headache (6.0%, 5.3%, 3.0%). In the 24-week study,10 which did not have a placebo control, the most commonly reported adverse events (≥10% of patients) were increased weight (24.8% vs 36.2% for OSC vs olanzapine), somnolence (21.2% vs 18.1%), dry mouth (12.8% vs 8.0%), and increased appetite (10.9% vs 12.3%). In both studies, rates of discontinuation due to adverse events were low and similar between groups (in the 4-week study, 1.5% for OSC, 2.3% for olanzapine, and 5.2% for placebo; in the 24-week study, 12.0% for OSC and 9.8% for olanzapine).
In the 2 open-label, phase 3, 52-week extension studies,9,11 long-term tolerability was evidenced by low rates discontinuation due to adverse events (≤6%). Neither extension study reported any clinically meaningful changes over time in hematology, biochemistry, vital signs, or electrocardiogram parameters.3 In addition to durability of antipsychotic response as evidenced by sustained improvements in PANSS and CGI-S scores over time, waist circumference and weight remained stable, and the observed long-term changes in weight were consistent with weight changes observed with other second-generation antipsychotics.3 Long-term changes in metabolic laboratory parameter values were small and remained stable, and there was little change in glycosylated hemoglobin (hemoglobin A1c) values, which suggests that glycemic control was maintained with long-term OSC treatment.3 Caveats to consider are that the extension studies were open label without comparators, and they may have selected for patients who responded favorably to OSC treatment in the preceding studies.3Warnings and precautions in OSC product labeling are generally similar to those for other second-generation antipsychotics,21 other than warnings and precautions specifically related to samidorphan being an opioid antagonist, and special mention of “Drug Reaction with Eosinophilia and Systemic Symptoms” and “Anticholinergic (Antimuscarinic) Effects” warnings, which also are contained in the olanzapine legacy label.2,4
Summary
Olanzapine has a plethora of evidence supporting its robust efficacy profile5,6; however, its use is stymied by an unfavorable weight and metabolic profile.7 OSC may help mitigate at least some of the weight gain that would be expected with the use of olanzapine alone in the long-term treatment of patients with schizophrenia or bipolar I disorder. The addition of samidorphan does not deleteriously affect the efficacy of olanzapine, but decreases the risk of gaining ≥10% or ≥7% of baseline body weight by approximately 50% compared with olanzapine alone. Increase in waist circumference, a proxy for how much metabolically active fat one has, is lower with OSC than it is with olanzapine. Because samidorphan is an opioid receptor antagonist, OSC is contraindicated in patients using opioids and in those undergoing acute opioid withdrawal. Dosage strengths available for OSC parallel those for olanzapine, and all strengths including the same fixed dose of samidorphan—10 mg—so advise patients not to double up on the tablets, and to not split them.
Related Resource
• Olanzapine and samidorphan (Lybalvi) prescribing information. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
Drug Brand Names
Diazepam • Valium
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Olanzapine-fluoxetine combination • Symbyax
Olanzapine-samidorphan combination • Lybalvi
Valproate • Depakote, Depakene
Bottom Line
Olanzapine-samidorphan combination (OSC) is intended to mitigate some of the weight gain anticipated when using olanzapine alone. For clinicians who have prescribed olanzapine and have seen its therapeutic benefits, OSC will be a welcome addition to the therapeutic armamentarium. For practitioners who may have avoided olanzapine entirely, OSC can provide another means of offering this therapeutic option and counter “olanzapine hesitancy.”
1. US Food and Drug Administration. NDA 213378 approval letter. May 28, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000Approv.pdf
2. Alkermes, Inc. LYBALVI™ (olanzapine and samidorphan) tablets, for oral use. Prescribing information. May 2021. Accessed November 24, 2021. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
3. Citrome L, Graham C, Simmons A, et al. An evidence-based review of OLZ/SAM for treatment of adults with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2021;17:2885-2904.
4. Eli Lilly and Company. ZYPREXA (olanzapine) tablet for oral use; ZYPREXA ZYDIS (olanzapine) tablet, orally disintegrating for oral use; ZYPREXA intramuscular (olanzapine) injection, powder, for solution for intramuscular use. Prescribing information. February 2021. Accessed November 24, 2021. https://pi.lilly.com/us/zyprexa-pi.pdf
5. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsychiatr Dis Treat. 2019;15:2559-2569.
6. Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132(1):80-90.
7. Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455-482.
8. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769.
9. Yagoda S, Graham C, Simmons A, et al. Long-term safety and durability of effect with a combination of olanzapine and samidorphan in patients with schizophrenia: results from a 1-year open-label extension study. CNS Spectr. 2021;26(4):383-392.
10. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168-1178.
11. Kahn RS, Silverman BL, DiPetrillo L, et al. A phase 3, multicenter study to assess the 1-year safety and tolerability of a combination of olanzapine and samidorphan in patients with schizophrenia: results from the ENLIGHTEN-2 long-term extension. Schizophr Res. 2021;232:45-53.
12. US Food and Drug Administration. Drug approval package: Lybalvi. June 26, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000TOC.cfm
13. Sun L, Yagoda S, Yao B, et al. Combination of olanzapine and samidorphan has no clinically significant effect on the pharmacokinetics of lithium or valproate. Clin Drug Investig. 2020;40(1):55-64.
14. Eli Lilly and Company. SYMBYAX (olanzapine and fluoxetine) capsules for oral use. Prescribing information. September 2021. Accessed November 24, 2021. https://pi.lilly.com/us/symbyax-pi.pdf
15. Wentland MP, Lu Q, Lou R, et al. Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett. 2005;15(8):2107-2110.
16. Lee MW, Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother. 2009;10(11):1841-1845.
17. Sun L, Yagoda S, Xue H, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109881.
18. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
19. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278-283.
20. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335-345.
21. Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599-1613.
1. US Food and Drug Administration. NDA 213378 approval letter. May 28, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000Approv.pdf
2. Alkermes, Inc. LYBALVI™ (olanzapine and samidorphan) tablets, for oral use. Prescribing information. May 2021. Accessed November 24, 2021. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
3. Citrome L, Graham C, Simmons A, et al. An evidence-based review of OLZ/SAM for treatment of adults with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2021;17:2885-2904.
4. Eli Lilly and Company. ZYPREXA (olanzapine) tablet for oral use; ZYPREXA ZYDIS (olanzapine) tablet, orally disintegrating for oral use; ZYPREXA intramuscular (olanzapine) injection, powder, for solution for intramuscular use. Prescribing information. February 2021. Accessed November 24, 2021. https://pi.lilly.com/us/zyprexa-pi.pdf
5. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsychiatr Dis Treat. 2019;15:2559-2569.
6. Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132(1):80-90.
7. Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455-482.
8. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769.
9. Yagoda S, Graham C, Simmons A, et al. Long-term safety and durability of effect with a combination of olanzapine and samidorphan in patients with schizophrenia: results from a 1-year open-label extension study. CNS Spectr. 2021;26(4):383-392.
10. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168-1178.
11. Kahn RS, Silverman BL, DiPetrillo L, et al. A phase 3, multicenter study to assess the 1-year safety and tolerability of a combination of olanzapine and samidorphan in patients with schizophrenia: results from the ENLIGHTEN-2 long-term extension. Schizophr Res. 2021;232:45-53.
12. US Food and Drug Administration. Drug approval package: Lybalvi. June 26, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000TOC.cfm
13. Sun L, Yagoda S, Yao B, et al. Combination of olanzapine and samidorphan has no clinically significant effect on the pharmacokinetics of lithium or valproate. Clin Drug Investig. 2020;40(1):55-64.
14. Eli Lilly and Company. SYMBYAX (olanzapine and fluoxetine) capsules for oral use. Prescribing information. September 2021. Accessed November 24, 2021. https://pi.lilly.com/us/symbyax-pi.pdf
15. Wentland MP, Lu Q, Lou R, et al. Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett. 2005;15(8):2107-2110.
16. Lee MW, Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother. 2009;10(11):1841-1845.
17. Sun L, Yagoda S, Xue H, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109881.
18. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
19. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278-283.
20. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335-345.
21. Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599-1613.
New understanding of suicide attempts emerges
even in the absence of a psychiatric disorder.
This finding suggests the genetic underpinnings of suicide attempts are partially shared and partially distinct from those of related psychiatric disorders, the investigators note.
“This study brings us a step closer to understanding the neurobiology of suicidality, with the ultimate goal of developing new treatments and prevention strategies,” Niamh Mullins, PhD, department of psychiatry, department of genetics and genomic sciences, Icahn School of Medicine at Mount Sinai in New York, said in an interview.
The study was published online in Biological Psychiatry.
Largest study to date
In the largest genetic association study of suicide attempt published to date, the researchers conducted a genome-wide association study (GWAS) of 29,782 suicide attempt cases and 519,961 controls in the International Suicide Genetics Consortium (ISGC).
Two loci reached genome-wide significance for suicide attempt – the major histocompatibility complex and an intergenic locus on chromosome 7, the latter of which remained associated with suicide attempt after conditioning on psychiatric disorders and was replicated in an independent cohort of over 14,000 veterans in the Million Veteran Program.
“This is the first replicated genetic locus that contributes more to suicide attempt than related psychiatric disorders,” Dr. Mullins said.
“The study found overlap in the genetic basis of suicide attempt and that of related psychiatric disorders, particularly major depression, but also with that of nonpsychiatric risk factors such as smoking, pain, risk-taking behavior, sleep disturbances, and poorer general health,” Dr. Mullins said.
“These genetic relationships between suicide attempt and nonpsychiatric risk factors were not a by-product of comorbid psychiatric illness, suggesting that there is some shared biological basis between suicide attempt and nonpsychiatric risk factors,” she added.
Dr. Mullins cautioned that the findings do not have any immediate impact on patient care.
“The ultimate goal of this research is to gain insight into the underlying biological pathways involved in suicide attempts or suicidal thoughts, providing potential avenues to treatments and prevention strategies,” she said.
“The study findings also point to the importance of studying the potential direct causal paths between these risk factors and suicide attempt in patients with and without psychiatric illness,” Douglas Ruderfer, PhD, of Vanderbilt University Medical Center, Nashville, Tenn., cofounder and cochair of the consortium and senior author of the paper, added in a news release.
A version of this article first appeared on Medscape.com.
even in the absence of a psychiatric disorder.
This finding suggests the genetic underpinnings of suicide attempts are partially shared and partially distinct from those of related psychiatric disorders, the investigators note.
“This study brings us a step closer to understanding the neurobiology of suicidality, with the ultimate goal of developing new treatments and prevention strategies,” Niamh Mullins, PhD, department of psychiatry, department of genetics and genomic sciences, Icahn School of Medicine at Mount Sinai in New York, said in an interview.
The study was published online in Biological Psychiatry.
Largest study to date
In the largest genetic association study of suicide attempt published to date, the researchers conducted a genome-wide association study (GWAS) of 29,782 suicide attempt cases and 519,961 controls in the International Suicide Genetics Consortium (ISGC).
Two loci reached genome-wide significance for suicide attempt – the major histocompatibility complex and an intergenic locus on chromosome 7, the latter of which remained associated with suicide attempt after conditioning on psychiatric disorders and was replicated in an independent cohort of over 14,000 veterans in the Million Veteran Program.
“This is the first replicated genetic locus that contributes more to suicide attempt than related psychiatric disorders,” Dr. Mullins said.
“The study found overlap in the genetic basis of suicide attempt and that of related psychiatric disorders, particularly major depression, but also with that of nonpsychiatric risk factors such as smoking, pain, risk-taking behavior, sleep disturbances, and poorer general health,” Dr. Mullins said.
“These genetic relationships between suicide attempt and nonpsychiatric risk factors were not a by-product of comorbid psychiatric illness, suggesting that there is some shared biological basis between suicide attempt and nonpsychiatric risk factors,” she added.
Dr. Mullins cautioned that the findings do not have any immediate impact on patient care.
“The ultimate goal of this research is to gain insight into the underlying biological pathways involved in suicide attempts or suicidal thoughts, providing potential avenues to treatments and prevention strategies,” she said.
“The study findings also point to the importance of studying the potential direct causal paths between these risk factors and suicide attempt in patients with and without psychiatric illness,” Douglas Ruderfer, PhD, of Vanderbilt University Medical Center, Nashville, Tenn., cofounder and cochair of the consortium and senior author of the paper, added in a news release.
A version of this article first appeared on Medscape.com.
even in the absence of a psychiatric disorder.
This finding suggests the genetic underpinnings of suicide attempts are partially shared and partially distinct from those of related psychiatric disorders, the investigators note.
“This study brings us a step closer to understanding the neurobiology of suicidality, with the ultimate goal of developing new treatments and prevention strategies,” Niamh Mullins, PhD, department of psychiatry, department of genetics and genomic sciences, Icahn School of Medicine at Mount Sinai in New York, said in an interview.
The study was published online in Biological Psychiatry.
Largest study to date
In the largest genetic association study of suicide attempt published to date, the researchers conducted a genome-wide association study (GWAS) of 29,782 suicide attempt cases and 519,961 controls in the International Suicide Genetics Consortium (ISGC).
Two loci reached genome-wide significance for suicide attempt – the major histocompatibility complex and an intergenic locus on chromosome 7, the latter of which remained associated with suicide attempt after conditioning on psychiatric disorders and was replicated in an independent cohort of over 14,000 veterans in the Million Veteran Program.
“This is the first replicated genetic locus that contributes more to suicide attempt than related psychiatric disorders,” Dr. Mullins said.
“The study found overlap in the genetic basis of suicide attempt and that of related psychiatric disorders, particularly major depression, but also with that of nonpsychiatric risk factors such as smoking, pain, risk-taking behavior, sleep disturbances, and poorer general health,” Dr. Mullins said.
“These genetic relationships between suicide attempt and nonpsychiatric risk factors were not a by-product of comorbid psychiatric illness, suggesting that there is some shared biological basis between suicide attempt and nonpsychiatric risk factors,” she added.
Dr. Mullins cautioned that the findings do not have any immediate impact on patient care.
“The ultimate goal of this research is to gain insight into the underlying biological pathways involved in suicide attempts or suicidal thoughts, providing potential avenues to treatments and prevention strategies,” she said.
“The study findings also point to the importance of studying the potential direct causal paths between these risk factors and suicide attempt in patients with and without psychiatric illness,” Douglas Ruderfer, PhD, of Vanderbilt University Medical Center, Nashville, Tenn., cofounder and cochair of the consortium and senior author of the paper, added in a news release.
A version of this article first appeared on Medscape.com.
FROM BIOLOGICAL PSYCHIATRY




