User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
An overlooked cause of catatonia
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
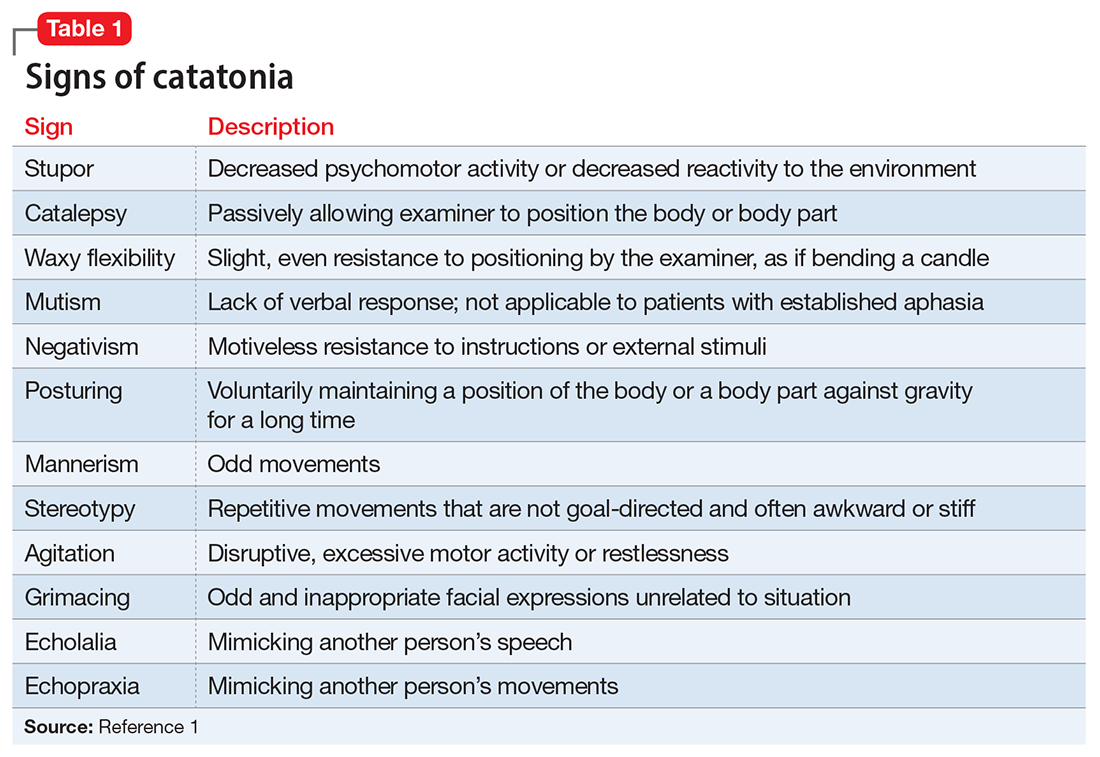
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.
EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
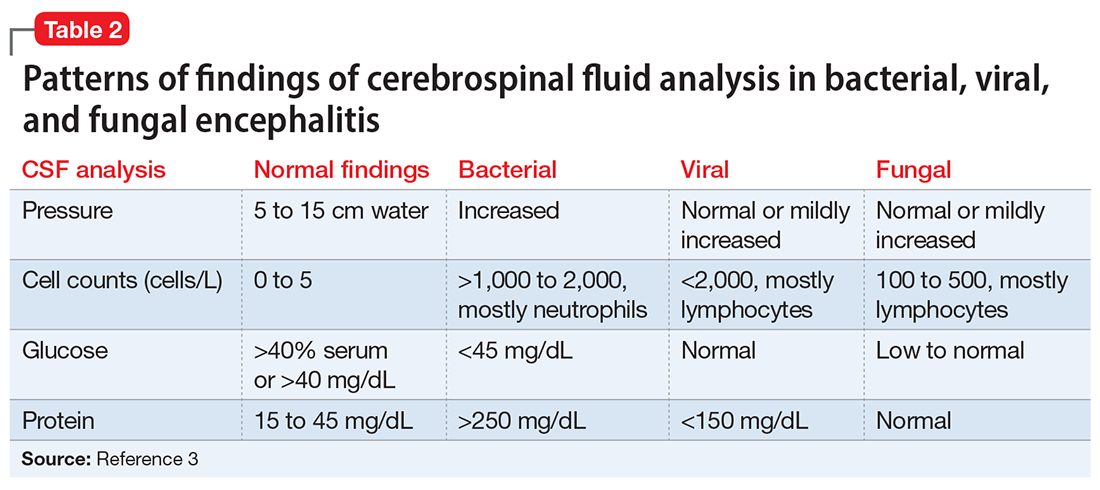
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).
[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.
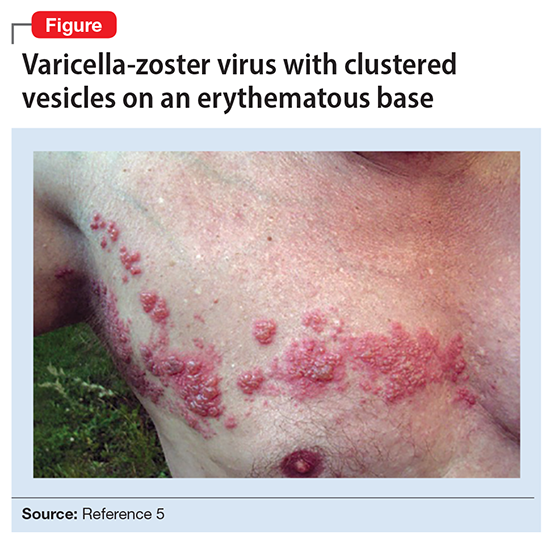
Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
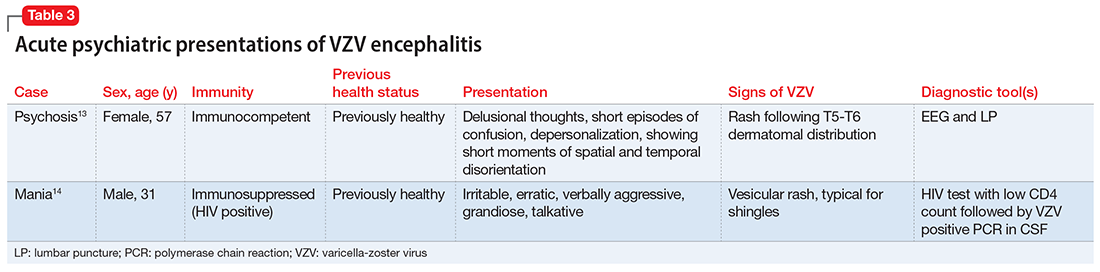
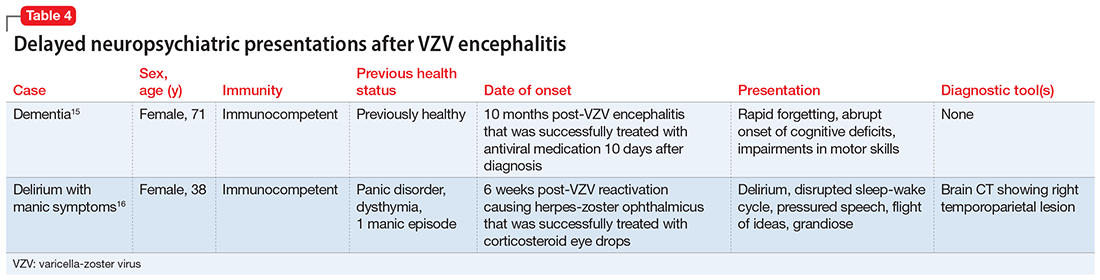
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16
To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-
Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.

EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).

[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.

Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16

To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-

Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.

EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).

[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.

Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16

To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-

Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
Scurvy in psychiatric patients: An easy-to-miss diagnosis
Two years ago, I cared for Ms. L, a woman in her late 40s who had a history of generalized anxiety disorder and major depressive disorder. Unable to work and highly distressed throughout the day, Ms. L was admitted to our psychiatric unit due to her functional decompensation and symptom severity.
Ms. L was extremely focused on physical symptoms. She had rigid rules regarding which beauty products she could and could not use (she insisted most soaps gave her a rash, though she did not have any clear documentation of this) as well as the types of food she could and could not eat due to fear of an allergic reaction (skin testing was negative for the foods she claimed were problematic, though this did not change her selective eating habits). By the time she was admitted to our unit, in addition to outpatient mental health, she was being treated by internal medicine, allergy and immunology, and dermatology, with largely equivocal objective findings.
During her psychiatric admission intake, Ms. L mentioned that due to her fear of anaphylaxis, she hadn’t eaten any fruits or vegetables for at least 2 years. As a result, I ordered testing of her vitamin C level.
Three days following admission, Ms. L requested to be discharged because she said she needed to care for her pet. She reported feeling less anxious, and because the treatment team felt she did not meet the criteria for an involuntary hold, she was discharged. A week later, the results of her vitamin C level came back, indicating a severe deficiency (<0.1 mg/dL; reference range: 0.3 to 2.7 mg/dL). I contacted her outpatient team, and vitamin C supplementation was started immediately.
Notes from Ms. L’s subsequent outpatient mental health visits indicated improvement in her somatic symptoms (less perseveration), although over the next year her scores on the Generalized Anxiety Disorder-7 and Patient Health Questionnaire-9 scales were largely unchanged (fluctuating within the range of 11 to 17 and 12 to 17, respectively). One year later, Ms. L stopped taking vitamin C supplements because she was afraid she was becoming allergic to them, though there was no objective evidence to support this belief. Her vitamin C levels were within the normal range at the time and have not been rechecked since then.
Ms. L’s obsession with “healthy eating” led to numerous red herrings for clinicians, as she was anxious about every food. Countertransference and feelings of frustration may have also led clinicians in multiple specialties to miss the diagnosis of scurvy. Vitamin C supplementation did not result in remission of Ms. L’s symptoms, which reflects the complexity and severity of her comorbid psychiatric illnesses. However, a decrease in her perseveration on somatic symptoms afforded increased opportunities to address her other psychiatric diagnoses. Ms. L eventually enrolled in an eating disorders program, which was beneficial to her.
Keep scurvy in the differential Dx
Symptoms of scurvy include malaise; lethargy; anemia; myalgia; bone pain; easy bruising; petechiae and perifollicular hemorrhages (due to capillary fragility); gum disease; mood changes; and depression.1 In later stages, the presentation can progress to edema; jaundice; hemolysis and spontaneous bleeding; neuropathy; fever; convulsions; and death.
1. Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician. 2008;54(10):1403-1406.
2. Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23(8):1281-1284.
3. Meisel K, Daggubati S, Josephson SA. Scurvy in the 21st century? Vitamin C deficiency presenting to the neurologist. Neurol Clin Pract. 2015;5(6):491-493.
Two years ago, I cared for Ms. L, a woman in her late 40s who had a history of generalized anxiety disorder and major depressive disorder. Unable to work and highly distressed throughout the day, Ms. L was admitted to our psychiatric unit due to her functional decompensation and symptom severity.
Ms. L was extremely focused on physical symptoms. She had rigid rules regarding which beauty products she could and could not use (she insisted most soaps gave her a rash, though she did not have any clear documentation of this) as well as the types of food she could and could not eat due to fear of an allergic reaction (skin testing was negative for the foods she claimed were problematic, though this did not change her selective eating habits). By the time she was admitted to our unit, in addition to outpatient mental health, she was being treated by internal medicine, allergy and immunology, and dermatology, with largely equivocal objective findings.
During her psychiatric admission intake, Ms. L mentioned that due to her fear of anaphylaxis, she hadn’t eaten any fruits or vegetables for at least 2 years. As a result, I ordered testing of her vitamin C level.
Three days following admission, Ms. L requested to be discharged because she said she needed to care for her pet. She reported feeling less anxious, and because the treatment team felt she did not meet the criteria for an involuntary hold, she was discharged. A week later, the results of her vitamin C level came back, indicating a severe deficiency (<0.1 mg/dL; reference range: 0.3 to 2.7 mg/dL). I contacted her outpatient team, and vitamin C supplementation was started immediately.
Notes from Ms. L’s subsequent outpatient mental health visits indicated improvement in her somatic symptoms (less perseveration), although over the next year her scores on the Generalized Anxiety Disorder-7 and Patient Health Questionnaire-9 scales were largely unchanged (fluctuating within the range of 11 to 17 and 12 to 17, respectively). One year later, Ms. L stopped taking vitamin C supplements because she was afraid she was becoming allergic to them, though there was no objective evidence to support this belief. Her vitamin C levels were within the normal range at the time and have not been rechecked since then.
Ms. L’s obsession with “healthy eating” led to numerous red herrings for clinicians, as she was anxious about every food. Countertransference and feelings of frustration may have also led clinicians in multiple specialties to miss the diagnosis of scurvy. Vitamin C supplementation did not result in remission of Ms. L’s symptoms, which reflects the complexity and severity of her comorbid psychiatric illnesses. However, a decrease in her perseveration on somatic symptoms afforded increased opportunities to address her other psychiatric diagnoses. Ms. L eventually enrolled in an eating disorders program, which was beneficial to her.
Keep scurvy in the differential Dx
Symptoms of scurvy include malaise; lethargy; anemia; myalgia; bone pain; easy bruising; petechiae and perifollicular hemorrhages (due to capillary fragility); gum disease; mood changes; and depression.1 In later stages, the presentation can progress to edema; jaundice; hemolysis and spontaneous bleeding; neuropathy; fever; convulsions; and death.
Two years ago, I cared for Ms. L, a woman in her late 40s who had a history of generalized anxiety disorder and major depressive disorder. Unable to work and highly distressed throughout the day, Ms. L was admitted to our psychiatric unit due to her functional decompensation and symptom severity.
Ms. L was extremely focused on physical symptoms. She had rigid rules regarding which beauty products she could and could not use (she insisted most soaps gave her a rash, though she did not have any clear documentation of this) as well as the types of food she could and could not eat due to fear of an allergic reaction (skin testing was negative for the foods she claimed were problematic, though this did not change her selective eating habits). By the time she was admitted to our unit, in addition to outpatient mental health, she was being treated by internal medicine, allergy and immunology, and dermatology, with largely equivocal objective findings.
During her psychiatric admission intake, Ms. L mentioned that due to her fear of anaphylaxis, she hadn’t eaten any fruits or vegetables for at least 2 years. As a result, I ordered testing of her vitamin C level.
Three days following admission, Ms. L requested to be discharged because she said she needed to care for her pet. She reported feeling less anxious, and because the treatment team felt she did not meet the criteria for an involuntary hold, she was discharged. A week later, the results of her vitamin C level came back, indicating a severe deficiency (<0.1 mg/dL; reference range: 0.3 to 2.7 mg/dL). I contacted her outpatient team, and vitamin C supplementation was started immediately.
Notes from Ms. L’s subsequent outpatient mental health visits indicated improvement in her somatic symptoms (less perseveration), although over the next year her scores on the Generalized Anxiety Disorder-7 and Patient Health Questionnaire-9 scales were largely unchanged (fluctuating within the range of 11 to 17 and 12 to 17, respectively). One year later, Ms. L stopped taking vitamin C supplements because she was afraid she was becoming allergic to them, though there was no objective evidence to support this belief. Her vitamin C levels were within the normal range at the time and have not been rechecked since then.
Ms. L’s obsession with “healthy eating” led to numerous red herrings for clinicians, as she was anxious about every food. Countertransference and feelings of frustration may have also led clinicians in multiple specialties to miss the diagnosis of scurvy. Vitamin C supplementation did not result in remission of Ms. L’s symptoms, which reflects the complexity and severity of her comorbid psychiatric illnesses. However, a decrease in her perseveration on somatic symptoms afforded increased opportunities to address her other psychiatric diagnoses. Ms. L eventually enrolled in an eating disorders program, which was beneficial to her.
Keep scurvy in the differential Dx
Symptoms of scurvy include malaise; lethargy; anemia; myalgia; bone pain; easy bruising; petechiae and perifollicular hemorrhages (due to capillary fragility); gum disease; mood changes; and depression.1 In later stages, the presentation can progress to edema; jaundice; hemolysis and spontaneous bleeding; neuropathy; fever; convulsions; and death.
1. Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician. 2008;54(10):1403-1406.
2. Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23(8):1281-1284.
3. Meisel K, Daggubati S, Josephson SA. Scurvy in the 21st century? Vitamin C deficiency presenting to the neurologist. Neurol Clin Pract. 2015;5(6):491-493.
1. Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician. 2008;54(10):1403-1406.
2. Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23(8):1281-1284.
3. Meisel K, Daggubati S, Josephson SA. Scurvy in the 21st century? Vitamin C deficiency presenting to the neurologist. Neurol Clin Pract. 2015;5(6):491-493.
Breast cancer screening in women receiving antipsychotics
Women with severe mental illness (SMI) are more likely to develop breast cancer and often have more advanced stages of breast cancer when it is detected.1 Antipsychotics have a wide variety of FDA-approved indications and many important life-saving properties. However, patients treated with antipsychotic medications that increase prolactin levels require special consideration with regards to referral for breast cancer screening. Although no clear causal link between antipsychotic use and breast cancer has been established, antipsychotics that raise serum prolactin levels (haloperidol, iloperidone, lurasidone, olanzapine, paliperidone, risperidone) are associated with a higher risk of breast cancer than antipsychotics that produce smaller increases in prolactin levels (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, quetiapine, and ziprasidone).2,3 Risperidone and paliperidone have the highest propensities to increase prolactin (45 to >100 ng/mL), whereas other second-generation antipsychotics are associated with only modest elevations.4 Prolonged exposure to high serum prolactin levels should be avoided in women due to the increased risk for breast cancer.2,3 Although there are no clear rules regarding which number or cluster of personal risk factors necessitates a further risk assessment for breast cancer, women receiving antipsychotics (especially those age ≥40) can be referred for further assessment. An individualized, patient-centered approach should be used.
Recognize risk factors
Patients with SMI often need to take a regimen of medications, including antipsychotics, for weeks or months to stabilize their symptoms. Once a woman with SMI is stabilized, consider referral to a clinic that can comprehensively assess for breast cancer risk. Nonmodifiable risk factors include older age, certain genetic mutations (BRCA1 and BRCA2), early menarche, late menopause, high breast tissue density as detected by mammography, a family history of breast cancer, and exposure to radiation.5,6 Modifiable risk factors include physical inactivity, being overweight or obese, hormonal exposure, drinking alcohol, and the presence of certain factors in the patient’s reproductive history (first pregnancy after age 30, not breastfeeding, and never having a full-term pregnancy).2,3 When making such referrals, it is important to avoid making the patient feel alarmed or frightened of antipsychotics. Instead, explain that a referral for breast cancer screening is routine.
When to refer
All women age ≥40 should be offered a referral to a clinic that can provide screening mammography. If a woman has pain, detects a lump in her breast, has a bloody discharge from the nipple, or has changes in the shape or texture of the nipple or breast, a more urgent referral should be made.4 The most important thing to remember is that early breast lesion detection can be life-saving and can avert the need for more invasive surgeries as well as exposure to chemotherapy and radiation.
What to do when prolactin is elevated
Ongoing monitoring of serum prolactin levels can help ensure that the patient’s levels remain in a normal range (<25 ng/mL).2,3,5,6 If hyperprolactinemia is detected, consider switching to an antipsychotic less likely to increase prolactin. Alternatively, the addition of aripiprazole/brexpiprazole or a dopamine agonist as combination therapy can be considered to rapidly restore normal prolactin levels.2 Such changes should be carefully considered because patients may decompensate if antipsychotics are abruptly switched. An individualized risk vs benefit analysis is necessary for any patient in this situation. Risks include not only the recurrence of psychiatric symptoms but also a potential loss of their current level of functioning. Patients may need to continue to take an antipsychotic that is more likely to increase prolactin, in which case close monitoring is advised as well as collaboration with other physicians and members of the patient’s care team. Involving the patient’s support system is helpful.
1. Weinstein LC, Stefancic A, Cunningham AT, et al. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):134-151.
2. Rahman T, Sahrmann JM, Olsen MA, et al. Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol. 2022;42(1):7-16.
3. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
4. Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421-453.
5. Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. Breast cancer. Accessed June 1, 2022. https://www.cdc.gov/cancer/breast/index.htm
6. Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361-1366.
Women with severe mental illness (SMI) are more likely to develop breast cancer and often have more advanced stages of breast cancer when it is detected.1 Antipsychotics have a wide variety of FDA-approved indications and many important life-saving properties. However, patients treated with antipsychotic medications that increase prolactin levels require special consideration with regards to referral for breast cancer screening. Although no clear causal link between antipsychotic use and breast cancer has been established, antipsychotics that raise serum prolactin levels (haloperidol, iloperidone, lurasidone, olanzapine, paliperidone, risperidone) are associated with a higher risk of breast cancer than antipsychotics that produce smaller increases in prolactin levels (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, quetiapine, and ziprasidone).2,3 Risperidone and paliperidone have the highest propensities to increase prolactin (45 to >100 ng/mL), whereas other second-generation antipsychotics are associated with only modest elevations.4 Prolonged exposure to high serum prolactin levels should be avoided in women due to the increased risk for breast cancer.2,3 Although there are no clear rules regarding which number or cluster of personal risk factors necessitates a further risk assessment for breast cancer, women receiving antipsychotics (especially those age ≥40) can be referred for further assessment. An individualized, patient-centered approach should be used.
Recognize risk factors
Patients with SMI often need to take a regimen of medications, including antipsychotics, for weeks or months to stabilize their symptoms. Once a woman with SMI is stabilized, consider referral to a clinic that can comprehensively assess for breast cancer risk. Nonmodifiable risk factors include older age, certain genetic mutations (BRCA1 and BRCA2), early menarche, late menopause, high breast tissue density as detected by mammography, a family history of breast cancer, and exposure to radiation.5,6 Modifiable risk factors include physical inactivity, being overweight or obese, hormonal exposure, drinking alcohol, and the presence of certain factors in the patient’s reproductive history (first pregnancy after age 30, not breastfeeding, and never having a full-term pregnancy).2,3 When making such referrals, it is important to avoid making the patient feel alarmed or frightened of antipsychotics. Instead, explain that a referral for breast cancer screening is routine.
When to refer
All women age ≥40 should be offered a referral to a clinic that can provide screening mammography. If a woman has pain, detects a lump in her breast, has a bloody discharge from the nipple, or has changes in the shape or texture of the nipple or breast, a more urgent referral should be made.4 The most important thing to remember is that early breast lesion detection can be life-saving and can avert the need for more invasive surgeries as well as exposure to chemotherapy and radiation.
What to do when prolactin is elevated
Ongoing monitoring of serum prolactin levels can help ensure that the patient’s levels remain in a normal range (<25 ng/mL).2,3,5,6 If hyperprolactinemia is detected, consider switching to an antipsychotic less likely to increase prolactin. Alternatively, the addition of aripiprazole/brexpiprazole or a dopamine agonist as combination therapy can be considered to rapidly restore normal prolactin levels.2 Such changes should be carefully considered because patients may decompensate if antipsychotics are abruptly switched. An individualized risk vs benefit analysis is necessary for any patient in this situation. Risks include not only the recurrence of psychiatric symptoms but also a potential loss of their current level of functioning. Patients may need to continue to take an antipsychotic that is more likely to increase prolactin, in which case close monitoring is advised as well as collaboration with other physicians and members of the patient’s care team. Involving the patient’s support system is helpful.
Women with severe mental illness (SMI) are more likely to develop breast cancer and often have more advanced stages of breast cancer when it is detected.1 Antipsychotics have a wide variety of FDA-approved indications and many important life-saving properties. However, patients treated with antipsychotic medications that increase prolactin levels require special consideration with regards to referral for breast cancer screening. Although no clear causal link between antipsychotic use and breast cancer has been established, antipsychotics that raise serum prolactin levels (haloperidol, iloperidone, lurasidone, olanzapine, paliperidone, risperidone) are associated with a higher risk of breast cancer than antipsychotics that produce smaller increases in prolactin levels (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, quetiapine, and ziprasidone).2,3 Risperidone and paliperidone have the highest propensities to increase prolactin (45 to >100 ng/mL), whereas other second-generation antipsychotics are associated with only modest elevations.4 Prolonged exposure to high serum prolactin levels should be avoided in women due to the increased risk for breast cancer.2,3 Although there are no clear rules regarding which number or cluster of personal risk factors necessitates a further risk assessment for breast cancer, women receiving antipsychotics (especially those age ≥40) can be referred for further assessment. An individualized, patient-centered approach should be used.
Recognize risk factors
Patients with SMI often need to take a regimen of medications, including antipsychotics, for weeks or months to stabilize their symptoms. Once a woman with SMI is stabilized, consider referral to a clinic that can comprehensively assess for breast cancer risk. Nonmodifiable risk factors include older age, certain genetic mutations (BRCA1 and BRCA2), early menarche, late menopause, high breast tissue density as detected by mammography, a family history of breast cancer, and exposure to radiation.5,6 Modifiable risk factors include physical inactivity, being overweight or obese, hormonal exposure, drinking alcohol, and the presence of certain factors in the patient’s reproductive history (first pregnancy after age 30, not breastfeeding, and never having a full-term pregnancy).2,3 When making such referrals, it is important to avoid making the patient feel alarmed or frightened of antipsychotics. Instead, explain that a referral for breast cancer screening is routine.
When to refer
All women age ≥40 should be offered a referral to a clinic that can provide screening mammography. If a woman has pain, detects a lump in her breast, has a bloody discharge from the nipple, or has changes in the shape or texture of the nipple or breast, a more urgent referral should be made.4 The most important thing to remember is that early breast lesion detection can be life-saving and can avert the need for more invasive surgeries as well as exposure to chemotherapy and radiation.
What to do when prolactin is elevated
Ongoing monitoring of serum prolactin levels can help ensure that the patient’s levels remain in a normal range (<25 ng/mL).2,3,5,6 If hyperprolactinemia is detected, consider switching to an antipsychotic less likely to increase prolactin. Alternatively, the addition of aripiprazole/brexpiprazole or a dopamine agonist as combination therapy can be considered to rapidly restore normal prolactin levels.2 Such changes should be carefully considered because patients may decompensate if antipsychotics are abruptly switched. An individualized risk vs benefit analysis is necessary for any patient in this situation. Risks include not only the recurrence of psychiatric symptoms but also a potential loss of their current level of functioning. Patients may need to continue to take an antipsychotic that is more likely to increase prolactin, in which case close monitoring is advised as well as collaboration with other physicians and members of the patient’s care team. Involving the patient’s support system is helpful.
1. Weinstein LC, Stefancic A, Cunningham AT, et al. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):134-151.
2. Rahman T, Sahrmann JM, Olsen MA, et al. Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol. 2022;42(1):7-16.
3. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
4. Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421-453.
5. Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. Breast cancer. Accessed June 1, 2022. https://www.cdc.gov/cancer/breast/index.htm
6. Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361-1366.
1. Weinstein LC, Stefancic A, Cunningham AT, et al. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):134-151.
2. Rahman T, Sahrmann JM, Olsen MA, et al. Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol. 2022;42(1):7-16.
3. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
4. Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421-453.
5. Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. Breast cancer. Accessed June 1, 2022. https://www.cdc.gov/cancer/breast/index.htm
6. Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361-1366.
Should residents be taught how to prescribe monoamine oxidase inhibitors?
What else can I offer this patient?
This thought passed through my mind as the patient’s desperation grew palpable. He had experienced intractable major depressive disorder (MDD) for years and had exhausted multiple classes of antidepressants, trying various combinations without any relief.
The previous resident had arranged for intranasal ketamine treatment, but the patient was unable to receive it due to lack of transportation. As I combed through the list of the dozens of medications the patient previously had been prescribed, I noticed the absence of a certain class of agents: monoamine oxidase inhibitors (MAOIs).
My knowledge of MAOIs stemmed from medical school, where the dietary restrictions, potential for hypertensive crisis, and capricious drug-drug interactions were heavily emphasized while their value was minimized. I did not have any practical experience with these medications, and even the attending physician disclosed he had not prescribed an MAOI in more than 30 years. Nonetheless, both the attending physician and patient agreed that the patient would try one.
Following a washout period, the patient began tranylcypromine. After taking tranylcypromine 40 mg/d for 3 months, he reported he felt like a weight had been lifted off his chest. He felt less irritable and depressed, more energetic, and more hopeful for the future. He also felt that his symptoms were improving for the first time in many years.
An older but still potentially helpful class of medications
MDD is one of the leading causes of disability in the United States, affecting millions of people. Its economic burden is estimated to be more than $200 billion, with a large contingent consisting of direct medical cost and suicide-related costs.1 MDD is often recurrent—60% of patients experience another episode within 5 years.2 Most of these patients are classified as having treatment-resistant depression (TRD), which typically is defined as the failure to respond to 2 different medications given at adequate doses for a sufficient duration.3 The Sequenced Treatment Alternatives to Relieve Depression trial suggested that after each medication failure, depression becomes increasingly difficult to treat, with many patients developing TRD.4 For some patients with TRD, MAOIs may be a powerful and beneficial option.5,6 Studies have shown that MAOIs (at adequate doses) can be effective in approximately one-half of patients with TRD. Patients with anxious, endogenous, or atypical depression may also respond to MAOIs.7
MAOIs were among the earliest antidepressants on the market, starting in the late 1950s with isocarboxazid, phenelzine, tranylcypromine, and selegiline. The use of MAOIs as a treatment for depression was serendipitously discovered when iproniazid, a tuberculosis drug, was observed to have mood-elevating adverse effects that were explained by its monoamine oxidase (MAO) inhibitory properties.8 This sparked the hypothesis that a deficiency in serotonin, norepinephrine, and dopamine played a central role in depressive disorders. MAOs encompass a class of enzymes that metabolize catecholamines, which include the previously mentioned neurotransmitters and the trace amine tyramine. The MAO isoenzymes also inhabit many tissues, including the central and peripheral nervous system, liver, and intestines.
There are 2 subtypes of MAOs: MAO-A and MAO-B. MAO-A inhibits tyramine, serotonin, norepinephrine, and dopamine. MAO-B is mainly responsible for the degradation of dopamine, which makes MAO-B inhibitors (ie, rasagiline) useful in treating Parkinson disease.9
Continue to: For most psychiatrists...
For most psychiatrists, MAOIs have fallen out of favor due to their discomfort with their potential adverse effects and drug-drug interactions, the dietary restrictions patients must face, and the perception that newer medications have fewer adverse effects.10 Prescribing an MAOI requires the clinician to remain vigilant of any new medication the patient is taking that may potentiate intrasynaptic serotonin, which may include certain antibiotics or analgesics, causing serotonin syndrome. Close monitoring of the patient’s diet also is necessary so the patient avoids foods rich in tyramine that may trigger a hypertensive crisis. This is because excess tyramine can precipitate an increase in catecholamine release, causing a dangerous increase in blood pressure. However, many foods have safe levels of tyramine (<6 mg/serving), although the perception of tyramine levels in modern foods remains overestimated.5
Residents need to know how to use MAOIs
Psychiatrists should weigh the risks and benefits prior to prescribing any new medication, and MAOIs should be no exception. A patient’s enduring pain is often overshadowed by the potential for adverse effects, which occasionally is overemphasized. Other treatments for severe psychiatric illnesses (such as lithium and clozapine) are also declining due to these agents’ requirement for cumbersome monitoring and potential for adverse effects despite evidence of their superior efficacy and antisuicidal properties.11,12
Fortunately, there are many novel therapies available that can be effective for patients with TRD, including transcranial magnetic stimulation, ketamine, and vagal nerve stimulation. However, as psychiatrists, especially during training, our armamentarium should be equipped with all modalities of psychopharmacology. Training and teaching residents to prescribe MAOIs safely and effectively may add a glimmer of hope for an otherwise hopeless patient.
1. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653-665.
2. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-191.
3. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145.
4. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
5. Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
6. Amsterdam JD, Shults J. MAOI efficacy and safety in advanced stage treatment-resistant depression--a retrospective study. J Affect Disord. 2005;89(1-3):183-188.
7. Amsterdam JD, Hornig-Rohan M. Treatment algorithms in treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):371-386.
8. Ramachandraih CT, Subramanyam N, Bar KJ, et al. Antidepressants: from MAOIs to SSRIs and more. Indian J Psychiatry. 2011;53(2):180-182.
9. Tipton KF. 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm (Vienna). 2018;125(11):1519-1551.
10. Gillman PK, Feinberg SS, Fochtmann LJ. Revitalizing monoamine oxidase inhibitors: a call for action. CNS Spectr. 2020;25(4):452-454.
11. Kelly DL, Wehring HJ, Vyas G. Current status of clozapine in the United States. Shanghai Arch Psychiatry. 2012;24(2):110-113.
12. Tibrewal P, Ng T, Bastiampillai T, et al. Why is lithium use declining? Asian J Psychiatr. 2019;43:219-220.
What else can I offer this patient?
This thought passed through my mind as the patient’s desperation grew palpable. He had experienced intractable major depressive disorder (MDD) for years and had exhausted multiple classes of antidepressants, trying various combinations without any relief.
The previous resident had arranged for intranasal ketamine treatment, but the patient was unable to receive it due to lack of transportation. As I combed through the list of the dozens of medications the patient previously had been prescribed, I noticed the absence of a certain class of agents: monoamine oxidase inhibitors (MAOIs).
My knowledge of MAOIs stemmed from medical school, where the dietary restrictions, potential for hypertensive crisis, and capricious drug-drug interactions were heavily emphasized while their value was minimized. I did not have any practical experience with these medications, and even the attending physician disclosed he had not prescribed an MAOI in more than 30 years. Nonetheless, both the attending physician and patient agreed that the patient would try one.
Following a washout period, the patient began tranylcypromine. After taking tranylcypromine 40 mg/d for 3 months, he reported he felt like a weight had been lifted off his chest. He felt less irritable and depressed, more energetic, and more hopeful for the future. He also felt that his symptoms were improving for the first time in many years.
An older but still potentially helpful class of medications
MDD is one of the leading causes of disability in the United States, affecting millions of people. Its economic burden is estimated to be more than $200 billion, with a large contingent consisting of direct medical cost and suicide-related costs.1 MDD is often recurrent—60% of patients experience another episode within 5 years.2 Most of these patients are classified as having treatment-resistant depression (TRD), which typically is defined as the failure to respond to 2 different medications given at adequate doses for a sufficient duration.3 The Sequenced Treatment Alternatives to Relieve Depression trial suggested that after each medication failure, depression becomes increasingly difficult to treat, with many patients developing TRD.4 For some patients with TRD, MAOIs may be a powerful and beneficial option.5,6 Studies have shown that MAOIs (at adequate doses) can be effective in approximately one-half of patients with TRD. Patients with anxious, endogenous, or atypical depression may also respond to MAOIs.7
MAOIs were among the earliest antidepressants on the market, starting in the late 1950s with isocarboxazid, phenelzine, tranylcypromine, and selegiline. The use of MAOIs as a treatment for depression was serendipitously discovered when iproniazid, a tuberculosis drug, was observed to have mood-elevating adverse effects that were explained by its monoamine oxidase (MAO) inhibitory properties.8 This sparked the hypothesis that a deficiency in serotonin, norepinephrine, and dopamine played a central role in depressive disorders. MAOs encompass a class of enzymes that metabolize catecholamines, which include the previously mentioned neurotransmitters and the trace amine tyramine. The MAO isoenzymes also inhabit many tissues, including the central and peripheral nervous system, liver, and intestines.
There are 2 subtypes of MAOs: MAO-A and MAO-B. MAO-A inhibits tyramine, serotonin, norepinephrine, and dopamine. MAO-B is mainly responsible for the degradation of dopamine, which makes MAO-B inhibitors (ie, rasagiline) useful in treating Parkinson disease.9
Continue to: For most psychiatrists...
For most psychiatrists, MAOIs have fallen out of favor due to their discomfort with their potential adverse effects and drug-drug interactions, the dietary restrictions patients must face, and the perception that newer medications have fewer adverse effects.10 Prescribing an MAOI requires the clinician to remain vigilant of any new medication the patient is taking that may potentiate intrasynaptic serotonin, which may include certain antibiotics or analgesics, causing serotonin syndrome. Close monitoring of the patient’s diet also is necessary so the patient avoids foods rich in tyramine that may trigger a hypertensive crisis. This is because excess tyramine can precipitate an increase in catecholamine release, causing a dangerous increase in blood pressure. However, many foods have safe levels of tyramine (<6 mg/serving), although the perception of tyramine levels in modern foods remains overestimated.5
Residents need to know how to use MAOIs
Psychiatrists should weigh the risks and benefits prior to prescribing any new medication, and MAOIs should be no exception. A patient’s enduring pain is often overshadowed by the potential for adverse effects, which occasionally is overemphasized. Other treatments for severe psychiatric illnesses (such as lithium and clozapine) are also declining due to these agents’ requirement for cumbersome monitoring and potential for adverse effects despite evidence of their superior efficacy and antisuicidal properties.11,12
Fortunately, there are many novel therapies available that can be effective for patients with TRD, including transcranial magnetic stimulation, ketamine, and vagal nerve stimulation. However, as psychiatrists, especially during training, our armamentarium should be equipped with all modalities of psychopharmacology. Training and teaching residents to prescribe MAOIs safely and effectively may add a glimmer of hope for an otherwise hopeless patient.
What else can I offer this patient?
This thought passed through my mind as the patient’s desperation grew palpable. He had experienced intractable major depressive disorder (MDD) for years and had exhausted multiple classes of antidepressants, trying various combinations without any relief.
The previous resident had arranged for intranasal ketamine treatment, but the patient was unable to receive it due to lack of transportation. As I combed through the list of the dozens of medications the patient previously had been prescribed, I noticed the absence of a certain class of agents: monoamine oxidase inhibitors (MAOIs).
My knowledge of MAOIs stemmed from medical school, where the dietary restrictions, potential for hypertensive crisis, and capricious drug-drug interactions were heavily emphasized while their value was minimized. I did not have any practical experience with these medications, and even the attending physician disclosed he had not prescribed an MAOI in more than 30 years. Nonetheless, both the attending physician and patient agreed that the patient would try one.
Following a washout period, the patient began tranylcypromine. After taking tranylcypromine 40 mg/d for 3 months, he reported he felt like a weight had been lifted off his chest. He felt less irritable and depressed, more energetic, and more hopeful for the future. He also felt that his symptoms were improving for the first time in many years.
An older but still potentially helpful class of medications
MDD is one of the leading causes of disability in the United States, affecting millions of people. Its economic burden is estimated to be more than $200 billion, with a large contingent consisting of direct medical cost and suicide-related costs.1 MDD is often recurrent—60% of patients experience another episode within 5 years.2 Most of these patients are classified as having treatment-resistant depression (TRD), which typically is defined as the failure to respond to 2 different medications given at adequate doses for a sufficient duration.3 The Sequenced Treatment Alternatives to Relieve Depression trial suggested that after each medication failure, depression becomes increasingly difficult to treat, with many patients developing TRD.4 For some patients with TRD, MAOIs may be a powerful and beneficial option.5,6 Studies have shown that MAOIs (at adequate doses) can be effective in approximately one-half of patients with TRD. Patients with anxious, endogenous, or atypical depression may also respond to MAOIs.7
MAOIs were among the earliest antidepressants on the market, starting in the late 1950s with isocarboxazid, phenelzine, tranylcypromine, and selegiline. The use of MAOIs as a treatment for depression was serendipitously discovered when iproniazid, a tuberculosis drug, was observed to have mood-elevating adverse effects that were explained by its monoamine oxidase (MAO) inhibitory properties.8 This sparked the hypothesis that a deficiency in serotonin, norepinephrine, and dopamine played a central role in depressive disorders. MAOs encompass a class of enzymes that metabolize catecholamines, which include the previously mentioned neurotransmitters and the trace amine tyramine. The MAO isoenzymes also inhabit many tissues, including the central and peripheral nervous system, liver, and intestines.
There are 2 subtypes of MAOs: MAO-A and MAO-B. MAO-A inhibits tyramine, serotonin, norepinephrine, and dopamine. MAO-B is mainly responsible for the degradation of dopamine, which makes MAO-B inhibitors (ie, rasagiline) useful in treating Parkinson disease.9
Continue to: For most psychiatrists...
For most psychiatrists, MAOIs have fallen out of favor due to their discomfort with their potential adverse effects and drug-drug interactions, the dietary restrictions patients must face, and the perception that newer medications have fewer adverse effects.10 Prescribing an MAOI requires the clinician to remain vigilant of any new medication the patient is taking that may potentiate intrasynaptic serotonin, which may include certain antibiotics or analgesics, causing serotonin syndrome. Close monitoring of the patient’s diet also is necessary so the patient avoids foods rich in tyramine that may trigger a hypertensive crisis. This is because excess tyramine can precipitate an increase in catecholamine release, causing a dangerous increase in blood pressure. However, many foods have safe levels of tyramine (<6 mg/serving), although the perception of tyramine levels in modern foods remains overestimated.5
Residents need to know how to use MAOIs
Psychiatrists should weigh the risks and benefits prior to prescribing any new medication, and MAOIs should be no exception. A patient’s enduring pain is often overshadowed by the potential for adverse effects, which occasionally is overemphasized. Other treatments for severe psychiatric illnesses (such as lithium and clozapine) are also declining due to these agents’ requirement for cumbersome monitoring and potential for adverse effects despite evidence of their superior efficacy and antisuicidal properties.11,12
Fortunately, there are many novel therapies available that can be effective for patients with TRD, including transcranial magnetic stimulation, ketamine, and vagal nerve stimulation. However, as psychiatrists, especially during training, our armamentarium should be equipped with all modalities of psychopharmacology. Training and teaching residents to prescribe MAOIs safely and effectively may add a glimmer of hope for an otherwise hopeless patient.
1. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653-665.
2. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-191.
3. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145.
4. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
5. Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
6. Amsterdam JD, Shults J. MAOI efficacy and safety in advanced stage treatment-resistant depression--a retrospective study. J Affect Disord. 2005;89(1-3):183-188.
7. Amsterdam JD, Hornig-Rohan M. Treatment algorithms in treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):371-386.
8. Ramachandraih CT, Subramanyam N, Bar KJ, et al. Antidepressants: from MAOIs to SSRIs and more. Indian J Psychiatry. 2011;53(2):180-182.
9. Tipton KF. 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm (Vienna). 2018;125(11):1519-1551.
10. Gillman PK, Feinberg SS, Fochtmann LJ. Revitalizing monoamine oxidase inhibitors: a call for action. CNS Spectr. 2020;25(4):452-454.
11. Kelly DL, Wehring HJ, Vyas G. Current status of clozapine in the United States. Shanghai Arch Psychiatry. 2012;24(2):110-113.
12. Tibrewal P, Ng T, Bastiampillai T, et al. Why is lithium use declining? Asian J Psychiatr. 2019;43:219-220.
1. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653-665.
2. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-191.
3. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145.
4. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
5. Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
6. Amsterdam JD, Shults J. MAOI efficacy and safety in advanced stage treatment-resistant depression--a retrospective study. J Affect Disord. 2005;89(1-3):183-188.
7. Amsterdam JD, Hornig-Rohan M. Treatment algorithms in treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):371-386.
8. Ramachandraih CT, Subramanyam N, Bar KJ, et al. Antidepressants: from MAOIs to SSRIs and more. Indian J Psychiatry. 2011;53(2):180-182.
9. Tipton KF. 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm (Vienna). 2018;125(11):1519-1551.
10. Gillman PK, Feinberg SS, Fochtmann LJ. Revitalizing monoamine oxidase inhibitors: a call for action. CNS Spectr. 2020;25(4):452-454.
11. Kelly DL, Wehring HJ, Vyas G. Current status of clozapine in the United States. Shanghai Arch Psychiatry. 2012;24(2):110-113.
12. Tibrewal P, Ng T, Bastiampillai T, et al. Why is lithium use declining? Asian J Psychiatr. 2019;43:219-220.
What my Grandma’s schizophrenia taught me
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Grandma was sitting in her chair in the corner of the living room, and her eyes were wide, filled with fear and suspicion as she glanced between me, Mom, and Papa. “They are out to get me,” she said, slightly frantic. She glanced down at her right hand, fixated on a spot on the dorsum. Gingerly lifting her arm, she angled her hand toward my mom’s face. “You see that? They have been conducting experiments on me. I AM THE QUEEN,” she sobbed, “and you are planning together” she said, directing her attention to Papa and me. In that moment, Grandma was convinced Papa and I were conspiring to assassinate her. It hurt to see my grandmother look at me with genuine fear in her eyes. It was overwhelming to watch her deteriorate from the person I had been accustomed to for most of my life to the paranoid individual shaking in front of me.
This was the first time I had really observed my grandmother experiencing acute psychosis. My mom explained to me at a young age that my grandmother had an illness in her mind. I noticed that compared to other people in my life, my grandmother seemed to express less emotion and changed topics in conversations frequently, but by having an understanding provided by my mother, my brother and I didn’t think much of it; that was just Grandma. She would occasionally talk about her experiences with hearing voices or people on the television talking about her. For the most part, though, she was stable; she was able to carry out cleaning, cooking, and watching her favorite shows.
That was until she turned 65 and started on Medicare for insurance. The government required her to trial a less expensive medication and wanted her family practitioner to adjust the medications she had been on for years. This decision was made by people unfamiliar with my grandmother and her story. As a result, my family struggled alongside Grandma for over a month as she battled hallucinations and labile emotions. Living in rural Ohio, she had no access to a psychiatrist or other mental health professional during this period. The adjustments to her medications, changes in her insurance coverage, and lack of consistent psychiatric care led to a deterioration of her stability. This was the only time in my life that I saw Grandma at a place where she would have needed to be hospitalized if the symptoms lasted much longer. I spent evenings sitting with her in that dark and scary place, listening, sympathizing, and challenging her distortions of reality. This experience laid the foundation for my growing passion for providing care and advocating for people experiencing mental illness. I observed firsthand how the absence of consistent, compassionate, and informed care could lead to psychiatric hospitalization.
In the past, my grandfather hid my grandmother’s diagnosis from those around them. This approach prevented my uncle from disclosing the same information to my cousins. I observed how they would look at her with confusion and sometimes fear, which was rooted in a lack of understanding. This desire to hide Grandma’s schizophrenia stemmed from the marginalization society imposed upon her. There were sneers, comments regarding lack of religious faith, and expressions that she was not trying hard enough. My grandparents decided together to inform their church of my grandmother’s illness. The results were astounding. People looked at my grandmother not with confusion but with sympathy and would go out of their way to check on her. Knowledge is power, and awareness can break down stigma. Seeing the difference knowledge could have on a church community further solidified my desire to educate not only patients and their family members but also communities.
Access is another huge barrier my grandmother has faced. There is a lack of referring and awareness as well as large geographic disparities of psychiatrists around my hometown. My grandmother has also had struggles with being able to pay for services, medication, and therapy. This shows the desperate need for more mental health professionals who are competent and knowledgeable in how social determinants of health impact outcomes. These factors contributed to my decision to pursue a Master of Public Health degree. I aspire to use this background to prevent what happened to my Grandma from happening to other patients and to be an advocate for enhanced access to services, improving community mental health and awareness, and promoting continuity of care to increase treatment compliance. That is what my Grandma has fostered in me as a future psychiatrist.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Grandma was sitting in her chair in the corner of the living room, and her eyes were wide, filled with fear and suspicion as she glanced between me, Mom, and Papa. “They are out to get me,” she said, slightly frantic. She glanced down at her right hand, fixated on a spot on the dorsum. Gingerly lifting her arm, she angled her hand toward my mom’s face. “You see that? They have been conducting experiments on me. I AM THE QUEEN,” she sobbed, “and you are planning together” she said, directing her attention to Papa and me. In that moment, Grandma was convinced Papa and I were conspiring to assassinate her. It hurt to see my grandmother look at me with genuine fear in her eyes. It was overwhelming to watch her deteriorate from the person I had been accustomed to for most of my life to the paranoid individual shaking in front of me.
This was the first time I had really observed my grandmother experiencing acute psychosis. My mom explained to me at a young age that my grandmother had an illness in her mind. I noticed that compared to other people in my life, my grandmother seemed to express less emotion and changed topics in conversations frequently, but by having an understanding provided by my mother, my brother and I didn’t think much of it; that was just Grandma. She would occasionally talk about her experiences with hearing voices or people on the television talking about her. For the most part, though, she was stable; she was able to carry out cleaning, cooking, and watching her favorite shows.
That was until she turned 65 and started on Medicare for insurance. The government required her to trial a less expensive medication and wanted her family practitioner to adjust the medications she had been on for years. This decision was made by people unfamiliar with my grandmother and her story. As a result, my family struggled alongside Grandma for over a month as she battled hallucinations and labile emotions. Living in rural Ohio, she had no access to a psychiatrist or other mental health professional during this period. The adjustments to her medications, changes in her insurance coverage, and lack of consistent psychiatric care led to a deterioration of her stability. This was the only time in my life that I saw Grandma at a place where she would have needed to be hospitalized if the symptoms lasted much longer. I spent evenings sitting with her in that dark and scary place, listening, sympathizing, and challenging her distortions of reality. This experience laid the foundation for my growing passion for providing care and advocating for people experiencing mental illness. I observed firsthand how the absence of consistent, compassionate, and informed care could lead to psychiatric hospitalization.
In the past, my grandfather hid my grandmother’s diagnosis from those around them. This approach prevented my uncle from disclosing the same information to my cousins. I observed how they would look at her with confusion and sometimes fear, which was rooted in a lack of understanding. This desire to hide Grandma’s schizophrenia stemmed from the marginalization society imposed upon her. There were sneers, comments regarding lack of religious faith, and expressions that she was not trying hard enough. My grandparents decided together to inform their church of my grandmother’s illness. The results were astounding. People looked at my grandmother not with confusion but with sympathy and would go out of their way to check on her. Knowledge is power, and awareness can break down stigma. Seeing the difference knowledge could have on a church community further solidified my desire to educate not only patients and their family members but also communities.
Access is another huge barrier my grandmother has faced. There is a lack of referring and awareness as well as large geographic disparities of psychiatrists around my hometown. My grandmother has also had struggles with being able to pay for services, medication, and therapy. This shows the desperate need for more mental health professionals who are competent and knowledgeable in how social determinants of health impact outcomes. These factors contributed to my decision to pursue a Master of Public Health degree. I aspire to use this background to prevent what happened to my Grandma from happening to other patients and to be an advocate for enhanced access to services, improving community mental health and awareness, and promoting continuity of care to increase treatment compliance. That is what my Grandma has fostered in me as a future psychiatrist.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Grandma was sitting in her chair in the corner of the living room, and her eyes were wide, filled with fear and suspicion as she glanced between me, Mom, and Papa. “They are out to get me,” she said, slightly frantic. She glanced down at her right hand, fixated on a spot on the dorsum. Gingerly lifting her arm, she angled her hand toward my mom’s face. “You see that? They have been conducting experiments on me. I AM THE QUEEN,” she sobbed, “and you are planning together” she said, directing her attention to Papa and me. In that moment, Grandma was convinced Papa and I were conspiring to assassinate her. It hurt to see my grandmother look at me with genuine fear in her eyes. It was overwhelming to watch her deteriorate from the person I had been accustomed to for most of my life to the paranoid individual shaking in front of me.
This was the first time I had really observed my grandmother experiencing acute psychosis. My mom explained to me at a young age that my grandmother had an illness in her mind. I noticed that compared to other people in my life, my grandmother seemed to express less emotion and changed topics in conversations frequently, but by having an understanding provided by my mother, my brother and I didn’t think much of it; that was just Grandma. She would occasionally talk about her experiences with hearing voices or people on the television talking about her. For the most part, though, she was stable; she was able to carry out cleaning, cooking, and watching her favorite shows.
That was until she turned 65 and started on Medicare for insurance. The government required her to trial a less expensive medication and wanted her family practitioner to adjust the medications she had been on for years. This decision was made by people unfamiliar with my grandmother and her story. As a result, my family struggled alongside Grandma for over a month as she battled hallucinations and labile emotions. Living in rural Ohio, she had no access to a psychiatrist or other mental health professional during this period. The adjustments to her medications, changes in her insurance coverage, and lack of consistent psychiatric care led to a deterioration of her stability. This was the only time in my life that I saw Grandma at a place where she would have needed to be hospitalized if the symptoms lasted much longer. I spent evenings sitting with her in that dark and scary place, listening, sympathizing, and challenging her distortions of reality. This experience laid the foundation for my growing passion for providing care and advocating for people experiencing mental illness. I observed firsthand how the absence of consistent, compassionate, and informed care could lead to psychiatric hospitalization.
In the past, my grandfather hid my grandmother’s diagnosis from those around them. This approach prevented my uncle from disclosing the same information to my cousins. I observed how they would look at her with confusion and sometimes fear, which was rooted in a lack of understanding. This desire to hide Grandma’s schizophrenia stemmed from the marginalization society imposed upon her. There were sneers, comments regarding lack of religious faith, and expressions that she was not trying hard enough. My grandparents decided together to inform their church of my grandmother’s illness. The results were astounding. People looked at my grandmother not with confusion but with sympathy and would go out of their way to check on her. Knowledge is power, and awareness can break down stigma. Seeing the difference knowledge could have on a church community further solidified my desire to educate not only patients and their family members but also communities.
Access is another huge barrier my grandmother has faced. There is a lack of referring and awareness as well as large geographic disparities of psychiatrists around my hometown. My grandmother has also had struggles with being able to pay for services, medication, and therapy. This shows the desperate need for more mental health professionals who are competent and knowledgeable in how social determinants of health impact outcomes. These factors contributed to my decision to pursue a Master of Public Health degree. I aspire to use this background to prevent what happened to my Grandma from happening to other patients and to be an advocate for enhanced access to services, improving community mental health and awareness, and promoting continuity of care to increase treatment compliance. That is what my Grandma has fostered in me as a future psychiatrist.
‘Modest’ benefit for lecanemab in Alzheimer’s disease, but adverse events are common
SAN FRANCISCO –
In the CLARITY AD trial, adverse events (AEs) were common compared with placebo, including amyloid-related edema and effusions; and a recent news report linked a second death to the drug.
Moving forward, “longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer’s disease,” wrote Christopher H. van Dyck, MD, Yale University, New Haven, Conn., and colleagues.
The full trial findings were presented at the Clinical Trials on Alzheimer’s Disease (CTAD) conference, with simultaneous publication on Nov. 29 in the New England Journal of Medicine.
Complications in the field
The phase 3 trial of lecanemab has been closely watched in AD circles, especially considering positive early data released in September and reported by this news organization at that time.
The Food and Drug Administration is expected to make a decision about possible approval of the drug in January 2023. Only one other antiamyloid treatment, the highly controversial and expensive aducanumab (Aduhelm), is currently approved by the FDA.
For the new 18-month, randomized, double-blind CLARITY AD trial, researchers enrolled 1,795 patients aged 50-90 years (average age, 71 years) with early AD. All were randomly assigned to receive either a placebo (n = 898) or intravenous lecanemab, a humanized immunoglobulin G1 (IgG1) monoclonal antibody that selectively targets amyloid beta (A-beta) protofibrils, at 10 mg/kg of body weight every 2 weeks (n = 897).
The study ran from 2019 to 2021. The participants (52% women, 20% non-White) were recruited in North America, Europe, and Asia. Safety data included all participants, and the modified intention-to-treat group included 1,734 participants, with 859 receiving lecanemab and 875 receiving placebo.
The primary endpoint was the Clinical Dementia Rating–Sum of Boxes (CDR-SB). Scores from 0.5 to 6 are signs of early AD, according to the study. The mean baseline score for both groups was 3.2. The adjusted mean change at 18 months was 1.21 for lecanemab versus 1.66 for placebo (difference, –0.45; 95% confidence interval [CI], –0.67 to –0.23; P < .001).
As Dr. van Dyck noted in his presentation at the CTAD meting, this represents a 27% slowing of the decline in the lecanemab group.
The published findings do not speculate about how this difference would affect the day-to-day life of participants who took the drug, although it does refer to “modestly less decline” of cognition/function in the lecanemab group.
Other measurements that suggest cognitive improvements in the lecanemab group versus placebo include the Alzheimer’s Disease Assessment Scale–Cognitive Subscale score (mean difference, –1.44; 95% CI, –2.27 to –0.61), the Alzheimer’s Disease Composite Score (mean difference, –0.05; 95% CI, –.074 to –.027,), and the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment score (mean difference, 2.0; 95% CI, 1.2-2.8; all, P < .001).
Overall, Dr. van Dyck said, “Lecanemab met the primary and secondary endpoints versus placebo at 18 months, with highly significant differences starting at 6 months.”
In a substudy of 698 participants, results showed that amyloid burden fell at a higher rate in the lecanemab group than in the placebo group (difference, –59.1 centiloids; 95% CI, –62.6 to –55.6).
“Lecanemab has high selectivity for soluble aggregated species of A-beta as compared with monomeric amyloid, with moderate selectivity for fibrillar amyloid; this profile is considered to target the most toxic pathologic amyloid species,” the researchers wrote.
Concerning AE data
With respect to AEs, deaths occurred in both groups (0.7% in those who took lecanemab and 0.8% in those who took the placebo). The researchers did not attribute any deaths to the drug. However, according to a report in the journal Science published Nov. 27, a 65-year-old woman who was taking the drug as part of a clinical trial “recently died from a massive brain hemorrhage that some researchers link to the drug.”
The woman, the second person “whose death was linked to lecanemab,” died after suffering a stroke. Researchers summarized a case report as saying that the drug “contributed to her brain hemorrhage after biweekly infusions of lecanemab inflamed and weakened the blood vessels.”
Eisai, which sponsored the new trial, told Science that “all the available safety information indicates that lecanemab therapy is not associated with an increased risk of death overall or from any specific cause.”
In a CTAD presentation, study coauthor Marwan Sabbagh, MD, Barrow Neurological Institute, Phoenix, said two hemorrhage-related deaths occurred in an open-label extension. One was in the context of a tissue plasminogen activator treatment for a stroke, which fits with the description of the case in the Science report. “Causality with lecanemab is a little difficult ...,” he said. “Patients on anticoagulation might need further consideration.”
In the CLARITY AD Trial, serious AEs occurred in 14% of the lecanemab group, leading to discontinuation 6.9% of the time, and in 11.3% of the placebo group, leading to discontinuation 2.9% of the time, the investigators reported.
They added that, in the lecanemab group, the most common AEs, defined as affecting more than 10% of participants, were infusion-related reactions (26.4% vs. 7.4% for placebo); amyloid-related imaging abnormalities with cerebral microhemorrhages, cerebral macrohemorrhages, or superficial siderosis (17.3% vs. 9%, respectively); amyloid-related imaging abnormalities with edema or effusions (12.6% vs. 1.7%); headache (11.1% vs. 8.1%); and falls (10.4% vs. 9.6%).
In addition, macrohemorrhage was reported in 0.6% of the lecanemab group and 0.1% of the placebo group.
Cautious optimism
In separate interviews, two Alzheimer’s specialists who weren’t involved in the study praised the trial and described the findings as “exciting.” But they also highlighted its limitations.
Alvaro Pascual-Leone, MD, PhD, professor of neurology at Harvard Medical School and chief medical officer of Linus Health, said the study represents impressive progress after 60-plus trials examining anti-amyloid monoclonal antibodies. “This is the first trial that shows a clinical benefit that can be measured,” he said.
However, it’s unclear whether the changes “are really going to make a difference in people’s lives,” he said. The drug is likely to be expensive, owing to the large investment needed for research, he added, and patients will have to undergo costly testing, such as PET scans and spinal taps.
Still, “this could be a valuable adjunct to the armamentarium we have,” which includes interventions such as lifestyle changes, he said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, noted that the trial reached its primary and secondary endpoints and that the drug had what he called a “modest” effect on cognition.
However, the drugmaker will need to explore the adverse effects, he said, especially among patients with atrial fibrillation who take anticoagulants. And, he said, medicine is still far from the ultimate goal – fully reversing cognitive decline.
Michael Weiner, MD, president of the CTAD22 Scientific Committee, noted in a press release that there is “growing evidence” that some antiamyloid therapies, “especially lecanemab and donanemab” have shown promising results.
“Unfortunately, these treatments are also associated with abnormal differences seen in imaging, including brain swelling and bleeding in the brain,” said Dr. Weiner, professor of radiology, medicine, and neurology at the University of California, San Francisco.
“There is considerable controversy concerning the significance and impact of these findings, including whether or not governments and medical insurance will provide financial coverage for such treatments,” he added.
Rave reviews from the Alzheimer’s Association
In a statement, the Alzheimer’s Association raved about lecanemab and declared that the FDA should approve lecanemab on an accelerated basis. The study “confirms this treatment can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease ...” the association said, adding that “it could mean many months more of recognizing their spouse, children and grandchildren.”
The association, which is a staunch supporter of aducanumab, called on the Centers for Medicare & Medicaid Services to cover the drug if the FDA approves it. The association’s statement did not address the drug’s potential high cost, the adverse effects, or the two reported deaths.
The trial was supported by Eisai (regulatory sponsor) with partial funding from Biogen. Dr. van Dyck reports having received research grants from Biogen, Eisai, Biohaven, Cerevel Therapeutics, Eli Lilly, Genentech, Janssen, Novartis, and UCB. He has been a consultant to Cerevel, Eisai, Ono Pharmaceutical, and Roche. Relevant financial relationships for the other investigators are fully listed in the original article.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO –
In the CLARITY AD trial, adverse events (AEs) were common compared with placebo, including amyloid-related edema and effusions; and a recent news report linked a second death to the drug.
Moving forward, “longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer’s disease,” wrote Christopher H. van Dyck, MD, Yale University, New Haven, Conn., and colleagues.
The full trial findings were presented at the Clinical Trials on Alzheimer’s Disease (CTAD) conference, with simultaneous publication on Nov. 29 in the New England Journal of Medicine.
Complications in the field
The phase 3 trial of lecanemab has been closely watched in AD circles, especially considering positive early data released in September and reported by this news organization at that time.
The Food and Drug Administration is expected to make a decision about possible approval of the drug in January 2023. Only one other antiamyloid treatment, the highly controversial and expensive aducanumab (Aduhelm), is currently approved by the FDA.
For the new 18-month, randomized, double-blind CLARITY AD trial, researchers enrolled 1,795 patients aged 50-90 years (average age, 71 years) with early AD. All were randomly assigned to receive either a placebo (n = 898) or intravenous lecanemab, a humanized immunoglobulin G1 (IgG1) monoclonal antibody that selectively targets amyloid beta (A-beta) protofibrils, at 10 mg/kg of body weight every 2 weeks (n = 897).
The study ran from 2019 to 2021. The participants (52% women, 20% non-White) were recruited in North America, Europe, and Asia. Safety data included all participants, and the modified intention-to-treat group included 1,734 participants, with 859 receiving lecanemab and 875 receiving placebo.
The primary endpoint was the Clinical Dementia Rating–Sum of Boxes (CDR-SB). Scores from 0.5 to 6 are signs of early AD, according to the study. The mean baseline score for both groups was 3.2. The adjusted mean change at 18 months was 1.21 for lecanemab versus 1.66 for placebo (difference, –0.45; 95% confidence interval [CI], –0.67 to –0.23; P < .001).
As Dr. van Dyck noted in his presentation at the CTAD meting, this represents a 27% slowing of the decline in the lecanemab group.
The published findings do not speculate about how this difference would affect the day-to-day life of participants who took the drug, although it does refer to “modestly less decline” of cognition/function in the lecanemab group.
Other measurements that suggest cognitive improvements in the lecanemab group versus placebo include the Alzheimer’s Disease Assessment Scale–Cognitive Subscale score (mean difference, –1.44; 95% CI, –2.27 to –0.61), the Alzheimer’s Disease Composite Score (mean difference, –0.05; 95% CI, –.074 to –.027,), and the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment score (mean difference, 2.0; 95% CI, 1.2-2.8; all, P < .001).
Overall, Dr. van Dyck said, “Lecanemab met the primary and secondary endpoints versus placebo at 18 months, with highly significant differences starting at 6 months.”
In a substudy of 698 participants, results showed that amyloid burden fell at a higher rate in the lecanemab group than in the placebo group (difference, –59.1 centiloids; 95% CI, –62.6 to –55.6).
“Lecanemab has high selectivity for soluble aggregated species of A-beta as compared with monomeric amyloid, with moderate selectivity for fibrillar amyloid; this profile is considered to target the most toxic pathologic amyloid species,” the researchers wrote.
Concerning AE data
With respect to AEs, deaths occurred in both groups (0.7% in those who took lecanemab and 0.8% in those who took the placebo). The researchers did not attribute any deaths to the drug. However, according to a report in the journal Science published Nov. 27, a 65-year-old woman who was taking the drug as part of a clinical trial “recently died from a massive brain hemorrhage that some researchers link to the drug.”
The woman, the second person “whose death was linked to lecanemab,” died after suffering a stroke. Researchers summarized a case report as saying that the drug “contributed to her brain hemorrhage after biweekly infusions of lecanemab inflamed and weakened the blood vessels.”
Eisai, which sponsored the new trial, told Science that “all the available safety information indicates that lecanemab therapy is not associated with an increased risk of death overall or from any specific cause.”
In a CTAD presentation, study coauthor Marwan Sabbagh, MD, Barrow Neurological Institute, Phoenix, said two hemorrhage-related deaths occurred in an open-label extension. One was in the context of a tissue plasminogen activator treatment for a stroke, which fits with the description of the case in the Science report. “Causality with lecanemab is a little difficult ...,” he said. “Patients on anticoagulation might need further consideration.”
In the CLARITY AD Trial, serious AEs occurred in 14% of the lecanemab group, leading to discontinuation 6.9% of the time, and in 11.3% of the placebo group, leading to discontinuation 2.9% of the time, the investigators reported.
They added that, in the lecanemab group, the most common AEs, defined as affecting more than 10% of participants, were infusion-related reactions (26.4% vs. 7.4% for placebo); amyloid-related imaging abnormalities with cerebral microhemorrhages, cerebral macrohemorrhages, or superficial siderosis (17.3% vs. 9%, respectively); amyloid-related imaging abnormalities with edema or effusions (12.6% vs. 1.7%); headache (11.1% vs. 8.1%); and falls (10.4% vs. 9.6%).
In addition, macrohemorrhage was reported in 0.6% of the lecanemab group and 0.1% of the placebo group.
Cautious optimism
In separate interviews, two Alzheimer’s specialists who weren’t involved in the study praised the trial and described the findings as “exciting.” But they also highlighted its limitations.
Alvaro Pascual-Leone, MD, PhD, professor of neurology at Harvard Medical School and chief medical officer of Linus Health, said the study represents impressive progress after 60-plus trials examining anti-amyloid monoclonal antibodies. “This is the first trial that shows a clinical benefit that can be measured,” he said.
However, it’s unclear whether the changes “are really going to make a difference in people’s lives,” he said. The drug is likely to be expensive, owing to the large investment needed for research, he added, and patients will have to undergo costly testing, such as PET scans and spinal taps.
Still, “this could be a valuable adjunct to the armamentarium we have,” which includes interventions such as lifestyle changes, he said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, noted that the trial reached its primary and secondary endpoints and that the drug had what he called a “modest” effect on cognition.
However, the drugmaker will need to explore the adverse effects, he said, especially among patients with atrial fibrillation who take anticoagulants. And, he said, medicine is still far from the ultimate goal – fully reversing cognitive decline.
Michael Weiner, MD, president of the CTAD22 Scientific Committee, noted in a press release that there is “growing evidence” that some antiamyloid therapies, “especially lecanemab and donanemab” have shown promising results.
“Unfortunately, these treatments are also associated with abnormal differences seen in imaging, including brain swelling and bleeding in the brain,” said Dr. Weiner, professor of radiology, medicine, and neurology at the University of California, San Francisco.
“There is considerable controversy concerning the significance and impact of these findings, including whether or not governments and medical insurance will provide financial coverage for such treatments,” he added.
Rave reviews from the Alzheimer’s Association
In a statement, the Alzheimer’s Association raved about lecanemab and declared that the FDA should approve lecanemab on an accelerated basis. The study “confirms this treatment can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease ...” the association said, adding that “it could mean many months more of recognizing their spouse, children and grandchildren.”
The association, which is a staunch supporter of aducanumab, called on the Centers for Medicare & Medicaid Services to cover the drug if the FDA approves it. The association’s statement did not address the drug’s potential high cost, the adverse effects, or the two reported deaths.
The trial was supported by Eisai (regulatory sponsor) with partial funding from Biogen. Dr. van Dyck reports having received research grants from Biogen, Eisai, Biohaven, Cerevel Therapeutics, Eli Lilly, Genentech, Janssen, Novartis, and UCB. He has been a consultant to Cerevel, Eisai, Ono Pharmaceutical, and Roche. Relevant financial relationships for the other investigators are fully listed in the original article.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO –
In the CLARITY AD trial, adverse events (AEs) were common compared with placebo, including amyloid-related edema and effusions; and a recent news report linked a second death to the drug.
Moving forward, “longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer’s disease,” wrote Christopher H. van Dyck, MD, Yale University, New Haven, Conn., and colleagues.
The full trial findings were presented at the Clinical Trials on Alzheimer’s Disease (CTAD) conference, with simultaneous publication on Nov. 29 in the New England Journal of Medicine.
Complications in the field
The phase 3 trial of lecanemab has been closely watched in AD circles, especially considering positive early data released in September and reported by this news organization at that time.
The Food and Drug Administration is expected to make a decision about possible approval of the drug in January 2023. Only one other antiamyloid treatment, the highly controversial and expensive aducanumab (Aduhelm), is currently approved by the FDA.
For the new 18-month, randomized, double-blind CLARITY AD trial, researchers enrolled 1,795 patients aged 50-90 years (average age, 71 years) with early AD. All were randomly assigned to receive either a placebo (n = 898) or intravenous lecanemab, a humanized immunoglobulin G1 (IgG1) monoclonal antibody that selectively targets amyloid beta (A-beta) protofibrils, at 10 mg/kg of body weight every 2 weeks (n = 897).
The study ran from 2019 to 2021. The participants (52% women, 20% non-White) were recruited in North America, Europe, and Asia. Safety data included all participants, and the modified intention-to-treat group included 1,734 participants, with 859 receiving lecanemab and 875 receiving placebo.
The primary endpoint was the Clinical Dementia Rating–Sum of Boxes (CDR-SB). Scores from 0.5 to 6 are signs of early AD, according to the study. The mean baseline score for both groups was 3.2. The adjusted mean change at 18 months was 1.21 for lecanemab versus 1.66 for placebo (difference, –0.45; 95% confidence interval [CI], –0.67 to –0.23; P < .001).
As Dr. van Dyck noted in his presentation at the CTAD meting, this represents a 27% slowing of the decline in the lecanemab group.
The published findings do not speculate about how this difference would affect the day-to-day life of participants who took the drug, although it does refer to “modestly less decline” of cognition/function in the lecanemab group.
Other measurements that suggest cognitive improvements in the lecanemab group versus placebo include the Alzheimer’s Disease Assessment Scale–Cognitive Subscale score (mean difference, –1.44; 95% CI, –2.27 to –0.61), the Alzheimer’s Disease Composite Score (mean difference, –0.05; 95% CI, –.074 to –.027,), and the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment score (mean difference, 2.0; 95% CI, 1.2-2.8; all, P < .001).
Overall, Dr. van Dyck said, “Lecanemab met the primary and secondary endpoints versus placebo at 18 months, with highly significant differences starting at 6 months.”
In a substudy of 698 participants, results showed that amyloid burden fell at a higher rate in the lecanemab group than in the placebo group (difference, –59.1 centiloids; 95% CI, –62.6 to –55.6).
“Lecanemab has high selectivity for soluble aggregated species of A-beta as compared with monomeric amyloid, with moderate selectivity for fibrillar amyloid; this profile is considered to target the most toxic pathologic amyloid species,” the researchers wrote.
Concerning AE data
With respect to AEs, deaths occurred in both groups (0.7% in those who took lecanemab and 0.8% in those who took the placebo). The researchers did not attribute any deaths to the drug. However, according to a report in the journal Science published Nov. 27, a 65-year-old woman who was taking the drug as part of a clinical trial “recently died from a massive brain hemorrhage that some researchers link to the drug.”
The woman, the second person “whose death was linked to lecanemab,” died after suffering a stroke. Researchers summarized a case report as saying that the drug “contributed to her brain hemorrhage after biweekly infusions of lecanemab inflamed and weakened the blood vessels.”
Eisai, which sponsored the new trial, told Science that “all the available safety information indicates that lecanemab therapy is not associated with an increased risk of death overall or from any specific cause.”
In a CTAD presentation, study coauthor Marwan Sabbagh, MD, Barrow Neurological Institute, Phoenix, said two hemorrhage-related deaths occurred in an open-label extension. One was in the context of a tissue plasminogen activator treatment for a stroke, which fits with the description of the case in the Science report. “Causality with lecanemab is a little difficult ...,” he said. “Patients on anticoagulation might need further consideration.”
In the CLARITY AD Trial, serious AEs occurred in 14% of the lecanemab group, leading to discontinuation 6.9% of the time, and in 11.3% of the placebo group, leading to discontinuation 2.9% of the time, the investigators reported.
They added that, in the lecanemab group, the most common AEs, defined as affecting more than 10% of participants, were infusion-related reactions (26.4% vs. 7.4% for placebo); amyloid-related imaging abnormalities with cerebral microhemorrhages, cerebral macrohemorrhages, or superficial siderosis (17.3% vs. 9%, respectively); amyloid-related imaging abnormalities with edema or effusions (12.6% vs. 1.7%); headache (11.1% vs. 8.1%); and falls (10.4% vs. 9.6%).
In addition, macrohemorrhage was reported in 0.6% of the lecanemab group and 0.1% of the placebo group.
Cautious optimism
In separate interviews, two Alzheimer’s specialists who weren’t involved in the study praised the trial and described the findings as “exciting.” But they also highlighted its limitations.
Alvaro Pascual-Leone, MD, PhD, professor of neurology at Harvard Medical School and chief medical officer of Linus Health, said the study represents impressive progress after 60-plus trials examining anti-amyloid monoclonal antibodies. “This is the first trial that shows a clinical benefit that can be measured,” he said.
However, it’s unclear whether the changes “are really going to make a difference in people’s lives,” he said. The drug is likely to be expensive, owing to the large investment needed for research, he added, and patients will have to undergo costly testing, such as PET scans and spinal taps.
Still, “this could be a valuable adjunct to the armamentarium we have,” which includes interventions such as lifestyle changes, he said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, noted that the trial reached its primary and secondary endpoints and that the drug had what he called a “modest” effect on cognition.
However, the drugmaker will need to explore the adverse effects, he said, especially among patients with atrial fibrillation who take anticoagulants. And, he said, medicine is still far from the ultimate goal – fully reversing cognitive decline.
Michael Weiner, MD, president of the CTAD22 Scientific Committee, noted in a press release that there is “growing evidence” that some antiamyloid therapies, “especially lecanemab and donanemab” have shown promising results.
“Unfortunately, these treatments are also associated with abnormal differences seen in imaging, including brain swelling and bleeding in the brain,” said Dr. Weiner, professor of radiology, medicine, and neurology at the University of California, San Francisco.
“There is considerable controversy concerning the significance and impact of these findings, including whether or not governments and medical insurance will provide financial coverage for such treatments,” he added.
Rave reviews from the Alzheimer’s Association
In a statement, the Alzheimer’s Association raved about lecanemab and declared that the FDA should approve lecanemab on an accelerated basis. The study “confirms this treatment can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease ...” the association said, adding that “it could mean many months more of recognizing their spouse, children and grandchildren.”
The association, which is a staunch supporter of aducanumab, called on the Centers for Medicare & Medicaid Services to cover the drug if the FDA approves it. The association’s statement did not address the drug’s potential high cost, the adverse effects, or the two reported deaths.
The trial was supported by Eisai (regulatory sponsor) with partial funding from Biogen. Dr. van Dyck reports having received research grants from Biogen, Eisai, Biohaven, Cerevel Therapeutics, Eli Lilly, Genentech, Janssen, Novartis, and UCB. He has been a consultant to Cerevel, Eisai, Ono Pharmaceutical, and Roche. Relevant financial relationships for the other investigators are fully listed in the original article.
A version of this article first appeared on Medscape.com.
AT CTAD 2022
Buprenorphine linked with lower risk for neonatal harms than methadone
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Is it long COVID, or dementia, or both?
In early September, about a week after recovering from COVID-19, Barri Sanders went to the bank to pay a bill. But by mistake, she transferred a large amount of money from the wrong account.
“I’m talking about $20,000,” she said. “I had to go back [later] and fix it.”
Ms. Sanders, 83, had not had confusion like that before. Suddenly, the Albuquerque, N.M., resident found herself looking up from a book and not remembering what she had just read. She would stand up from her chair and forget what she meant to do.
“I kind of thought it was just the aging process,” she said. Combined with sudden balance issues, insomnia, and a nagging postnasal drip, the overall effect was “subtle, but scary,” she said.
After 5 days of this, she went to bed and slept the whole night through. She woke up in the morning to find her balanced restored, her sinuses clear, and the mental fog gone. What she’d had, she realized, wasn’t a rapid start of dementia, but rather a mercifully short form of long COVID.
Somewhere between 22% and 32% of people who recover from COVID-19 get “brain fog,” a nonscientific term used to describe slow or sluggish thinking. While this is disturbing at any age, And some scientists are starting to confirm what doctors, patients, and their families can already see: Older patients who have had COVID-19 have a higher risk of getting dementia or, if they already have mental confusion, the illness may worsen their condition.
British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry that people who recovered from COVID-19 had a higher risk of problems with their thinking and dementia even after 2 years had passed.
Another 2022 study, published in JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills. Those who survived a mild infection were at a higher risk for early onset decline, the study found.
Eran Metzger, MD, assistant professor of psychiatry at Beth Israel Deaconess Medical Center in Boston, said he’s noticed that COVID-19 makes some older patients confused, and their brains don’t regain their former clarity.
“We see a stepwise decline in their cognition during the COVID episode, and then they never get back up to their baseline,” said Dr. Metzger, medical director at Hebrew SeniorLife.
New research is beginning to back up such findings.
People who got COVID-19 were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in the journal Nature Medicine , which analyzed the health care databases of the U.S. Department of Veterans Affairs.
Joshua Cahan, MD, a cognitive neurologist at Northwestern University, Chicago, advises caution about applying such a specific label simply from a patient’s medical chart. After all, he noted, few patients get tested to confirm that they have the proteins linked to Alzheimer’s.
“Probably the most appropriate conclusion from that is that there’s an increased risk of dementia after a COVID infection,” he said, “but we don’t know whether it’s truly Alzheimer’s disease or not.”
There could be a number of reasons why COVID-19 triggers a decline in thinking skills, says Michelle Monje, MD, a neuroscientist and neuro-oncologist at Stanford (Calif.) University.
In a paper published in the journal Neuron, Dr. Monje and her coauthor, Akiko Iwasaki, PhD, professor of immunobiology at Yale University, New Haven, Conn., propose possible triggers for brain fog caused by COVID: inflammation in the lungs and respiratory passages that leads to inflammation and dysregulation of the central nervous system; autoimmune reactions that damage the central nervous system; brain infection directly caused by the coronavirus (though, they note, this appears rare); a reactivation of an Epstein-Barr virus, which can lead to neuroinflammation; triggered by the coronavirus; and/or complications from severe cases of COVID-19, possibly involving periods of low blood oxygen and multi-organ failure.
Scientific understanding of brain fog is “part of an emerging picture that inflammation elsewhere in the body can be transmitted to become inflammation in the brain,” Dr. Monje said. “And once there’s inflammation in the brain … that can dysregulate other cell types that normally support healthy cognitive function.”
One issue with the concept of brain fog is that, like the term itself, the condition can be tough to define for doctors and patients alike and difficult, if not impossible, to capture on common cognition tests.
These days, patients often arrive at the Center of Excellence for Alzheimer’s Disease, in Syracuse, N.Y., complaining that they “don’t feel the same” as they did before contracting COVID-19, said Sharon Brangman, MD, the center’s director and the chair of the geriatrics department at Upstate Medical University.
But the evidence of diminished cognition just isn’t there.
“There’s nothing that we can find, objectively, that’s wrong with them,” she said. “They’re not severe enough to score low on mental status testing.”
But specialized, directed testing can find some probable signs, said Dr. Cahan, who evaluates patient cognition in a long COVID clinic at Northwestern University.
He often finds that his long COVID patients score in the low normal range on cognitive testing.
“Patients do have a complaint that something’s changed, and we don’t have prior testing,” he said. “So it’s possible that they were maybe in the high normal range or the superior range, but you just don’t know.”
He said he has seen very high-performing people, such as lawyers, executives, PhDs, and other professionals, who have tests that might be interpreted as normal, but given their level of achievement, “you would expect [higher scores].”
Like Ms. Sanders, many of those who do have muddled thinking after a COVID infection return to their former mental status. A study published in the journal Brain Communications found that people who had recovered from COVID-19, even if they had a mild illness, were significantly more likely to have memory and other cognition issues in the months after infection. But after 9 months, the former COVID patients had returned to their normal level of cognition, the team at Britain’s University of Oxford reported.
Notably, though, the average age of the people in the study was 28.6.
At the Northwestern clinic, Dr. Cahan treats patients who have struggled with COVID-induced cognition issues for months or even years. A rehabilitation program involves working with patients to come up with ways to compensate for cognitive deficits – such as making lists – as well as brain exercises, Dr. Cahan said. Over time, patients may achieve a 75% to 85% improvement, he said.
Dr. Monje hopes that one day, science will come up with ways to fully reverse the decline.
“I think what is likely the most common contributor to brain fog is this neuroinflammation, causing dysfunction of other cell types,” she said. “And, at least in the laboratory, we can rescue that in mouse models of chemotherapy brain fog, which gives me hope that we can rescue that for people.”
A version of this article first appeared on WebMD.com.
In early September, about a week after recovering from COVID-19, Barri Sanders went to the bank to pay a bill. But by mistake, she transferred a large amount of money from the wrong account.
“I’m talking about $20,000,” she said. “I had to go back [later] and fix it.”
Ms. Sanders, 83, had not had confusion like that before. Suddenly, the Albuquerque, N.M., resident found herself looking up from a book and not remembering what she had just read. She would stand up from her chair and forget what she meant to do.
“I kind of thought it was just the aging process,” she said. Combined with sudden balance issues, insomnia, and a nagging postnasal drip, the overall effect was “subtle, but scary,” she said.
After 5 days of this, she went to bed and slept the whole night through. She woke up in the morning to find her balanced restored, her sinuses clear, and the mental fog gone. What she’d had, she realized, wasn’t a rapid start of dementia, but rather a mercifully short form of long COVID.
Somewhere between 22% and 32% of people who recover from COVID-19 get “brain fog,” a nonscientific term used to describe slow or sluggish thinking. While this is disturbing at any age, And some scientists are starting to confirm what doctors, patients, and their families can already see: Older patients who have had COVID-19 have a higher risk of getting dementia or, if they already have mental confusion, the illness may worsen their condition.
British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry that people who recovered from COVID-19 had a higher risk of problems with their thinking and dementia even after 2 years had passed.
Another 2022 study, published in JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills. Those who survived a mild infection were at a higher risk for early onset decline, the study found.
Eran Metzger, MD, assistant professor of psychiatry at Beth Israel Deaconess Medical Center in Boston, said he’s noticed that COVID-19 makes some older patients confused, and their brains don’t regain their former clarity.
“We see a stepwise decline in their cognition during the COVID episode, and then they never get back up to their baseline,” said Dr. Metzger, medical director at Hebrew SeniorLife.
New research is beginning to back up such findings.
People who got COVID-19 were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in the journal Nature Medicine , which analyzed the health care databases of the U.S. Department of Veterans Affairs.
Joshua Cahan, MD, a cognitive neurologist at Northwestern University, Chicago, advises caution about applying such a specific label simply from a patient’s medical chart. After all, he noted, few patients get tested to confirm that they have the proteins linked to Alzheimer’s.
“Probably the most appropriate conclusion from that is that there’s an increased risk of dementia after a COVID infection,” he said, “but we don’t know whether it’s truly Alzheimer’s disease or not.”
There could be a number of reasons why COVID-19 triggers a decline in thinking skills, says Michelle Monje, MD, a neuroscientist and neuro-oncologist at Stanford (Calif.) University.
In a paper published in the journal Neuron, Dr. Monje and her coauthor, Akiko Iwasaki, PhD, professor of immunobiology at Yale University, New Haven, Conn., propose possible triggers for brain fog caused by COVID: inflammation in the lungs and respiratory passages that leads to inflammation and dysregulation of the central nervous system; autoimmune reactions that damage the central nervous system; brain infection directly caused by the coronavirus (though, they note, this appears rare); a reactivation of an Epstein-Barr virus, which can lead to neuroinflammation; triggered by the coronavirus; and/or complications from severe cases of COVID-19, possibly involving periods of low blood oxygen and multi-organ failure.
Scientific understanding of brain fog is “part of an emerging picture that inflammation elsewhere in the body can be transmitted to become inflammation in the brain,” Dr. Monje said. “And once there’s inflammation in the brain … that can dysregulate other cell types that normally support healthy cognitive function.”
One issue with the concept of brain fog is that, like the term itself, the condition can be tough to define for doctors and patients alike and difficult, if not impossible, to capture on common cognition tests.
These days, patients often arrive at the Center of Excellence for Alzheimer’s Disease, in Syracuse, N.Y., complaining that they “don’t feel the same” as they did before contracting COVID-19, said Sharon Brangman, MD, the center’s director and the chair of the geriatrics department at Upstate Medical University.
But the evidence of diminished cognition just isn’t there.
“There’s nothing that we can find, objectively, that’s wrong with them,” she said. “They’re not severe enough to score low on mental status testing.”
But specialized, directed testing can find some probable signs, said Dr. Cahan, who evaluates patient cognition in a long COVID clinic at Northwestern University.
He often finds that his long COVID patients score in the low normal range on cognitive testing.
“Patients do have a complaint that something’s changed, and we don’t have prior testing,” he said. “So it’s possible that they were maybe in the high normal range or the superior range, but you just don’t know.”
He said he has seen very high-performing people, such as lawyers, executives, PhDs, and other professionals, who have tests that might be interpreted as normal, but given their level of achievement, “you would expect [higher scores].”
Like Ms. Sanders, many of those who do have muddled thinking after a COVID infection return to their former mental status. A study published in the journal Brain Communications found that people who had recovered from COVID-19, even if they had a mild illness, were significantly more likely to have memory and other cognition issues in the months after infection. But after 9 months, the former COVID patients had returned to their normal level of cognition, the team at Britain’s University of Oxford reported.
Notably, though, the average age of the people in the study was 28.6.
At the Northwestern clinic, Dr. Cahan treats patients who have struggled with COVID-induced cognition issues for months or even years. A rehabilitation program involves working with patients to come up with ways to compensate for cognitive deficits – such as making lists – as well as brain exercises, Dr. Cahan said. Over time, patients may achieve a 75% to 85% improvement, he said.
Dr. Monje hopes that one day, science will come up with ways to fully reverse the decline.
“I think what is likely the most common contributor to brain fog is this neuroinflammation, causing dysfunction of other cell types,” she said. “And, at least in the laboratory, we can rescue that in mouse models of chemotherapy brain fog, which gives me hope that we can rescue that for people.”
A version of this article first appeared on WebMD.com.
In early September, about a week after recovering from COVID-19, Barri Sanders went to the bank to pay a bill. But by mistake, she transferred a large amount of money from the wrong account.
“I’m talking about $20,000,” she said. “I had to go back [later] and fix it.”
Ms. Sanders, 83, had not had confusion like that before. Suddenly, the Albuquerque, N.M., resident found herself looking up from a book and not remembering what she had just read. She would stand up from her chair and forget what she meant to do.
“I kind of thought it was just the aging process,” she said. Combined with sudden balance issues, insomnia, and a nagging postnasal drip, the overall effect was “subtle, but scary,” she said.
After 5 days of this, she went to bed and slept the whole night through. She woke up in the morning to find her balanced restored, her sinuses clear, and the mental fog gone. What she’d had, she realized, wasn’t a rapid start of dementia, but rather a mercifully short form of long COVID.
Somewhere between 22% and 32% of people who recover from COVID-19 get “brain fog,” a nonscientific term used to describe slow or sluggish thinking. While this is disturbing at any age, And some scientists are starting to confirm what doctors, patients, and their families can already see: Older patients who have had COVID-19 have a higher risk of getting dementia or, if they already have mental confusion, the illness may worsen their condition.
British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry that people who recovered from COVID-19 had a higher risk of problems with their thinking and dementia even after 2 years had passed.
Another 2022 study, published in JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills. Those who survived a mild infection were at a higher risk for early onset decline, the study found.
Eran Metzger, MD, assistant professor of psychiatry at Beth Israel Deaconess Medical Center in Boston, said he’s noticed that COVID-19 makes some older patients confused, and their brains don’t regain their former clarity.
“We see a stepwise decline in their cognition during the COVID episode, and then they never get back up to their baseline,” said Dr. Metzger, medical director at Hebrew SeniorLife.
New research is beginning to back up such findings.
People who got COVID-19 were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in the journal Nature Medicine , which analyzed the health care databases of the U.S. Department of Veterans Affairs.
Joshua Cahan, MD, a cognitive neurologist at Northwestern University, Chicago, advises caution about applying such a specific label simply from a patient’s medical chart. After all, he noted, few patients get tested to confirm that they have the proteins linked to Alzheimer’s.
“Probably the most appropriate conclusion from that is that there’s an increased risk of dementia after a COVID infection,” he said, “but we don’t know whether it’s truly Alzheimer’s disease or not.”
There could be a number of reasons why COVID-19 triggers a decline in thinking skills, says Michelle Monje, MD, a neuroscientist and neuro-oncologist at Stanford (Calif.) University.
In a paper published in the journal Neuron, Dr. Monje and her coauthor, Akiko Iwasaki, PhD, professor of immunobiology at Yale University, New Haven, Conn., propose possible triggers for brain fog caused by COVID: inflammation in the lungs and respiratory passages that leads to inflammation and dysregulation of the central nervous system; autoimmune reactions that damage the central nervous system; brain infection directly caused by the coronavirus (though, they note, this appears rare); a reactivation of an Epstein-Barr virus, which can lead to neuroinflammation; triggered by the coronavirus; and/or complications from severe cases of COVID-19, possibly involving periods of low blood oxygen and multi-organ failure.
Scientific understanding of brain fog is “part of an emerging picture that inflammation elsewhere in the body can be transmitted to become inflammation in the brain,” Dr. Monje said. “And once there’s inflammation in the brain … that can dysregulate other cell types that normally support healthy cognitive function.”
One issue with the concept of brain fog is that, like the term itself, the condition can be tough to define for doctors and patients alike and difficult, if not impossible, to capture on common cognition tests.
These days, patients often arrive at the Center of Excellence for Alzheimer’s Disease, in Syracuse, N.Y., complaining that they “don’t feel the same” as they did before contracting COVID-19, said Sharon Brangman, MD, the center’s director and the chair of the geriatrics department at Upstate Medical University.
But the evidence of diminished cognition just isn’t there.
“There’s nothing that we can find, objectively, that’s wrong with them,” she said. “They’re not severe enough to score low on mental status testing.”
But specialized, directed testing can find some probable signs, said Dr. Cahan, who evaluates patient cognition in a long COVID clinic at Northwestern University.
He often finds that his long COVID patients score in the low normal range on cognitive testing.
“Patients do have a complaint that something’s changed, and we don’t have prior testing,” he said. “So it’s possible that they were maybe in the high normal range or the superior range, but you just don’t know.”
He said he has seen very high-performing people, such as lawyers, executives, PhDs, and other professionals, who have tests that might be interpreted as normal, but given their level of achievement, “you would expect [higher scores].”
Like Ms. Sanders, many of those who do have muddled thinking after a COVID infection return to their former mental status. A study published in the journal Brain Communications found that people who had recovered from COVID-19, even if they had a mild illness, were significantly more likely to have memory and other cognition issues in the months after infection. But after 9 months, the former COVID patients had returned to their normal level of cognition, the team at Britain’s University of Oxford reported.
Notably, though, the average age of the people in the study was 28.6.
At the Northwestern clinic, Dr. Cahan treats patients who have struggled with COVID-induced cognition issues for months or even years. A rehabilitation program involves working with patients to come up with ways to compensate for cognitive deficits – such as making lists – as well as brain exercises, Dr. Cahan said. Over time, patients may achieve a 75% to 85% improvement, he said.
Dr. Monje hopes that one day, science will come up with ways to fully reverse the decline.
“I think what is likely the most common contributor to brain fog is this neuroinflammation, causing dysfunction of other cell types,” she said. “And, at least in the laboratory, we can rescue that in mouse models of chemotherapy brain fog, which gives me hope that we can rescue that for people.”
A version of this article first appeared on WebMD.com.
Don’t call me ‘Dr.,’ say some physicians – but most prefer the title
When Mark Cucuzzella, MD, meets a new patient at the West Virginia Medical School clinic, he introduces himself as “Mark.” For one thing, says Dr. Cucuzzella, his last name is a mouthful. For another, the 56-year-old general practitioner asserts that getting on a first-name basis with his patients is integral to delivering the best care.
“I’m trying to break down the old paternalistic barriers of the doctor/patient relationship,” he says. “Titles create an environment where the doctors are making all the decisions and not involving the patient in any course of action.”
Aniruddh Setya, MD, has a different take on informality between patients and doctors: It’s not OK. “I am not your friend,” says the 35-year-old pediatrician from Florida-based KIDZ Medical Services. “There has to be a level of respect for the education and accomplishment of being a physician.”
published in JAMA Network Open. But that doesn’t mean most physicians support the practice. In fact, some doctors contend that it can be harmful, particularly to female physicians.
“My concern is that untitling (so termed by Amy Diehl, PhD, and Leanne Dzubinski, PhD) intrudes upon important professional boundaries and might be correlated with diminishing the value of someone’s time,” says Leah Witt, MD, a geriatrician at UCSF Health, San Francisco. Dr. Witt, along with colleague Lekshmi Santhosh, MD, a pulmonologist, offered commentary on the study results. “Studies have shown that women physicians get more patient portal messages, spend more time in the electronic health record, and have longer visits,” Dr. Witt said. “Dr. Santhosh and I wonder if untitling is a signifier of this diminished value of our time, and an assumption of increased ease of access leading to this higher workload.”
To compile the results reported in JAMA Network Open, Mayo Clinic researchers analyzed more than 90,000 emails from patients to doctors over the course of 3 years, beginning in 2018. Of those emails, more than 32% included the physician’s first name in greeting or salutation. For women physicians, the odds were twice as high that their titles would be omitted in the correspondence. The same holds true for doctors of osteopathic medicine (DOs) compared with MDs, and primary care physicians had similar odds for a title drop compared with specialists.
Dr. Witt says the findings are not surprising. “They match my experience as a woman in medicine, as Dr. Santhosh and I write in our commentary,” she says. “We think the findings could easily be replicated at other centers.”
Indeed, research on 321 speaker introductions at a medical rounds found that when female physicians introduced other physicians, they usually applied the doctor title. When the job of introducing colleagues fell to male physicians, however, the stats fell to 72.4% for male peers and only 49.2% when introducing female peers.
The Mayo Clinic study authors identified the pitfalls of patients who informally address their doctors. They wrote, “Untitling may have a negative impact on physicians, demonstrate lack of respect, and can lead to reduction in formality of the physician/patient relationship or workplace.”
Physician preferences vary
Although the results of the Mayo Clinic analysis didn’t and couldn’t address physician sentiments on patient informality, Dr. Setya observes that American culture is becoming less formal. “I’ve been practicing for over 10 years, and the number of people who consider doctors as equals is growing,” he says. “This has been particularly true over the last couple of years.”
This change was documented in 2015. Add in the pandemic and an entire society that is now accustomed to working from home in sweats, and it’s not a stretch to understand why some patients have become less formal in many settings. The 2015 article noted, however, that most physicians prefer to keep titles in the mix.
Perhaps most troublesome, says Dr. Setya, is that patients forgo asking whether it’s OK to use his first name and simply assume it’s acceptable. “It bothers me,” he says. “I became a doctor for more than the money.”
He suspects that his cultural background (Dr. Setya is of Indian descent) plays a role in how strongly he feels about patient-doctor informality. “As a British colony, Indian culture dictates that you pay respect to elders and to accomplishment,” he points out. “America is far looser when it comes to salutations.”
Dr. Cucuzzella largely agrees with Dr. Setya, but has a different view of the role culture plays in how physicians prefer to be addressed. “If your last name is difficult to pronounce, it can put the patient at ease if you give them an option,” he says. “I like my patients to feel comfortable and have a friendly conversation, so I don’t ask them to try to manage my last name.”
When patients revert to using Dr. Cucuzzella’s last name and title, this often breaks down along generational lines, Dr. Cucuzzella has found: Older patients might drop his title, whereas younger patients might keep it as a sign of respect. In some cases, Dr. Cucuzzella tries to bridge this gap, and offers the option of “Dr. Mark.” In his small West Virginia community, this is how people often refer to him.
Dr. Setya says that most of the older physicians he works with still prefer that patients and younger colleagues use their title, but he has witnessed exceptions to this. “My boss in residence hated to be called ‘Sir’ or ‘Doctor,’ ” he says. “In a situation like that, it is reasonable to ask, ‘How can I address you?’ But it has to be mutually agreed upon.”
Dr. Cucuzzella cites informality as the preferred mode for older patients. “If I have a 70-year-old patient, it seems natural they shouldn’t use my title,” he says. “They are worthy of equality in the community. If I’m talking to a retired CEO or state delegate, it’s uncomfortable if they call me doctor.”
Moreover, Dr. Cucuzzella maintains that establishing a less formal environment with patients leads to better outcomes. “Shared decision-making is a basic human right,” he says. “In 2022, doctors shouldn’t make decisions without patient input, unless it’s an emergency situation. Removing the title barriers makes that easier.”
How to handle informality
If you fall more in line with Dr. Setya, there are strategies you can use to try to keep formality in your doctor-patient relationships. Dr. Setya’s approach is indirect. “I don’t correct a patient if they use my first name, because that might seem hostile,” he says. “But I alert them in the way I address them back. A Sir, a Mrs., or a Mr. needs to go both ways.”
This particularly holds true in pediatrics, Dr. Setya has found. He has witnessed many colleagues addressing parents as “Mommy and Daddy,” something he says lacks respect and sets too informal a tone. “It’s almost universal that parents don’t like that, and we need to act accordingly.”
Dr. Witt also avoids directly correcting patients, but struggles when they drop her title. “The standard signature I use to sign every patient portal message I respond to includes my first and last name and credentials,” she says. “I maintain formality in most circumstances with that standard reply.”
Beneath the surface, however, Dr. Witt wishes it were easier. “I have struggled with answering the question, ‘Is it OK if I call you Leah?’ she says. “I want to keep our interaction anchored in professionalism without sacrificing the warmth I think is important to a productive patient-physician relationship. For this reason, I tend to say yes to this request, even though I’d rather patients didn’t make such requests.”
In the Fast Company article by Amy Diehl, PhD, and Leanne Dzubinski, PhD, on the topic of untitling professional women, the authors suggest several actions, beginning with leadership that sets expectations on the topic. They also suggest that physicians use polite corrections if patients untitle them. Supplying positive reinforcement when patients include your title can help, too. If all else fails, you can call out the offensive untitling. More often than not, especially with female physicians, the patient is demonstrating an unconscious bias rather than something deliberate.
Opinions vary on the topic of untitling, and ultimately each physician must make the decision for themselves. But creating informal cultures in an organization can have unintended consequences, especially for female peers.
Says Dr. Witt, “We all want to give our patients the best care we can, but professional boundaries are critical to time management, equitable care, and maintaining work-life balance. I would love to see a study that examines untitling by self-reported race and/or ethnicity of physicians, because we know that women of color experience higher rates of burnout and depression, and I wonder if untitling may be part of this.”
A version of this article first appeared on Medscape.com.
When Mark Cucuzzella, MD, meets a new patient at the West Virginia Medical School clinic, he introduces himself as “Mark.” For one thing, says Dr. Cucuzzella, his last name is a mouthful. For another, the 56-year-old general practitioner asserts that getting on a first-name basis with his patients is integral to delivering the best care.
“I’m trying to break down the old paternalistic barriers of the doctor/patient relationship,” he says. “Titles create an environment where the doctors are making all the decisions and not involving the patient in any course of action.”
Aniruddh Setya, MD, has a different take on informality between patients and doctors: It’s not OK. “I am not your friend,” says the 35-year-old pediatrician from Florida-based KIDZ Medical Services. “There has to be a level of respect for the education and accomplishment of being a physician.”
published in JAMA Network Open. But that doesn’t mean most physicians support the practice. In fact, some doctors contend that it can be harmful, particularly to female physicians.
“My concern is that untitling (so termed by Amy Diehl, PhD, and Leanne Dzubinski, PhD) intrudes upon important professional boundaries and might be correlated with diminishing the value of someone’s time,” says Leah Witt, MD, a geriatrician at UCSF Health, San Francisco. Dr. Witt, along with colleague Lekshmi Santhosh, MD, a pulmonologist, offered commentary on the study results. “Studies have shown that women physicians get more patient portal messages, spend more time in the electronic health record, and have longer visits,” Dr. Witt said. “Dr. Santhosh and I wonder if untitling is a signifier of this diminished value of our time, and an assumption of increased ease of access leading to this higher workload.”
To compile the results reported in JAMA Network Open, Mayo Clinic researchers analyzed more than 90,000 emails from patients to doctors over the course of 3 years, beginning in 2018. Of those emails, more than 32% included the physician’s first name in greeting or salutation. For women physicians, the odds were twice as high that their titles would be omitted in the correspondence. The same holds true for doctors of osteopathic medicine (DOs) compared with MDs, and primary care physicians had similar odds for a title drop compared with specialists.
Dr. Witt says the findings are not surprising. “They match my experience as a woman in medicine, as Dr. Santhosh and I write in our commentary,” she says. “We think the findings could easily be replicated at other centers.”
Indeed, research on 321 speaker introductions at a medical rounds found that when female physicians introduced other physicians, they usually applied the doctor title. When the job of introducing colleagues fell to male physicians, however, the stats fell to 72.4% for male peers and only 49.2% when introducing female peers.
The Mayo Clinic study authors identified the pitfalls of patients who informally address their doctors. They wrote, “Untitling may have a negative impact on physicians, demonstrate lack of respect, and can lead to reduction in formality of the physician/patient relationship or workplace.”
Physician preferences vary
Although the results of the Mayo Clinic analysis didn’t and couldn’t address physician sentiments on patient informality, Dr. Setya observes that American culture is becoming less formal. “I’ve been practicing for over 10 years, and the number of people who consider doctors as equals is growing,” he says. “This has been particularly true over the last couple of years.”
This change was documented in 2015. Add in the pandemic and an entire society that is now accustomed to working from home in sweats, and it’s not a stretch to understand why some patients have become less formal in many settings. The 2015 article noted, however, that most physicians prefer to keep titles in the mix.
Perhaps most troublesome, says Dr. Setya, is that patients forgo asking whether it’s OK to use his first name and simply assume it’s acceptable. “It bothers me,” he says. “I became a doctor for more than the money.”
He suspects that his cultural background (Dr. Setya is of Indian descent) plays a role in how strongly he feels about patient-doctor informality. “As a British colony, Indian culture dictates that you pay respect to elders and to accomplishment,” he points out. “America is far looser when it comes to salutations.”
Dr. Cucuzzella largely agrees with Dr. Setya, but has a different view of the role culture plays in how physicians prefer to be addressed. “If your last name is difficult to pronounce, it can put the patient at ease if you give them an option,” he says. “I like my patients to feel comfortable and have a friendly conversation, so I don’t ask them to try to manage my last name.”
When patients revert to using Dr. Cucuzzella’s last name and title, this often breaks down along generational lines, Dr. Cucuzzella has found: Older patients might drop his title, whereas younger patients might keep it as a sign of respect. In some cases, Dr. Cucuzzella tries to bridge this gap, and offers the option of “Dr. Mark.” In his small West Virginia community, this is how people often refer to him.
Dr. Setya says that most of the older physicians he works with still prefer that patients and younger colleagues use their title, but he has witnessed exceptions to this. “My boss in residence hated to be called ‘Sir’ or ‘Doctor,’ ” he says. “In a situation like that, it is reasonable to ask, ‘How can I address you?’ But it has to be mutually agreed upon.”
Dr. Cucuzzella cites informality as the preferred mode for older patients. “If I have a 70-year-old patient, it seems natural they shouldn’t use my title,” he says. “They are worthy of equality in the community. If I’m talking to a retired CEO or state delegate, it’s uncomfortable if they call me doctor.”
Moreover, Dr. Cucuzzella maintains that establishing a less formal environment with patients leads to better outcomes. “Shared decision-making is a basic human right,” he says. “In 2022, doctors shouldn’t make decisions without patient input, unless it’s an emergency situation. Removing the title barriers makes that easier.”
How to handle informality
If you fall more in line with Dr. Setya, there are strategies you can use to try to keep formality in your doctor-patient relationships. Dr. Setya’s approach is indirect. “I don’t correct a patient if they use my first name, because that might seem hostile,” he says. “But I alert them in the way I address them back. A Sir, a Mrs., or a Mr. needs to go both ways.”
This particularly holds true in pediatrics, Dr. Setya has found. He has witnessed many colleagues addressing parents as “Mommy and Daddy,” something he says lacks respect and sets too informal a tone. “It’s almost universal that parents don’t like that, and we need to act accordingly.”
Dr. Witt also avoids directly correcting patients, but struggles when they drop her title. “The standard signature I use to sign every patient portal message I respond to includes my first and last name and credentials,” she says. “I maintain formality in most circumstances with that standard reply.”
Beneath the surface, however, Dr. Witt wishes it were easier. “I have struggled with answering the question, ‘Is it OK if I call you Leah?’ she says. “I want to keep our interaction anchored in professionalism without sacrificing the warmth I think is important to a productive patient-physician relationship. For this reason, I tend to say yes to this request, even though I’d rather patients didn’t make such requests.”
In the Fast Company article by Amy Diehl, PhD, and Leanne Dzubinski, PhD, on the topic of untitling professional women, the authors suggest several actions, beginning with leadership that sets expectations on the topic. They also suggest that physicians use polite corrections if patients untitle them. Supplying positive reinforcement when patients include your title can help, too. If all else fails, you can call out the offensive untitling. More often than not, especially with female physicians, the patient is demonstrating an unconscious bias rather than something deliberate.
Opinions vary on the topic of untitling, and ultimately each physician must make the decision for themselves. But creating informal cultures in an organization can have unintended consequences, especially for female peers.
Says Dr. Witt, “We all want to give our patients the best care we can, but professional boundaries are critical to time management, equitable care, and maintaining work-life balance. I would love to see a study that examines untitling by self-reported race and/or ethnicity of physicians, because we know that women of color experience higher rates of burnout and depression, and I wonder if untitling may be part of this.”
A version of this article first appeared on Medscape.com.
When Mark Cucuzzella, MD, meets a new patient at the West Virginia Medical School clinic, he introduces himself as “Mark.” For one thing, says Dr. Cucuzzella, his last name is a mouthful. For another, the 56-year-old general practitioner asserts that getting on a first-name basis with his patients is integral to delivering the best care.
“I’m trying to break down the old paternalistic barriers of the doctor/patient relationship,” he says. “Titles create an environment where the doctors are making all the decisions and not involving the patient in any course of action.”
Aniruddh Setya, MD, has a different take on informality between patients and doctors: It’s not OK. “I am not your friend,” says the 35-year-old pediatrician from Florida-based KIDZ Medical Services. “There has to be a level of respect for the education and accomplishment of being a physician.”
published in JAMA Network Open. But that doesn’t mean most physicians support the practice. In fact, some doctors contend that it can be harmful, particularly to female physicians.
“My concern is that untitling (so termed by Amy Diehl, PhD, and Leanne Dzubinski, PhD) intrudes upon important professional boundaries and might be correlated with diminishing the value of someone’s time,” says Leah Witt, MD, a geriatrician at UCSF Health, San Francisco. Dr. Witt, along with colleague Lekshmi Santhosh, MD, a pulmonologist, offered commentary on the study results. “Studies have shown that women physicians get more patient portal messages, spend more time in the electronic health record, and have longer visits,” Dr. Witt said. “Dr. Santhosh and I wonder if untitling is a signifier of this diminished value of our time, and an assumption of increased ease of access leading to this higher workload.”
To compile the results reported in JAMA Network Open, Mayo Clinic researchers analyzed more than 90,000 emails from patients to doctors over the course of 3 years, beginning in 2018. Of those emails, more than 32% included the physician’s first name in greeting or salutation. For women physicians, the odds were twice as high that their titles would be omitted in the correspondence. The same holds true for doctors of osteopathic medicine (DOs) compared with MDs, and primary care physicians had similar odds for a title drop compared with specialists.
Dr. Witt says the findings are not surprising. “They match my experience as a woman in medicine, as Dr. Santhosh and I write in our commentary,” she says. “We think the findings could easily be replicated at other centers.”
Indeed, research on 321 speaker introductions at a medical rounds found that when female physicians introduced other physicians, they usually applied the doctor title. When the job of introducing colleagues fell to male physicians, however, the stats fell to 72.4% for male peers and only 49.2% when introducing female peers.
The Mayo Clinic study authors identified the pitfalls of patients who informally address their doctors. They wrote, “Untitling may have a negative impact on physicians, demonstrate lack of respect, and can lead to reduction in formality of the physician/patient relationship or workplace.”
Physician preferences vary
Although the results of the Mayo Clinic analysis didn’t and couldn’t address physician sentiments on patient informality, Dr. Setya observes that American culture is becoming less formal. “I’ve been practicing for over 10 years, and the number of people who consider doctors as equals is growing,” he says. “This has been particularly true over the last couple of years.”
This change was documented in 2015. Add in the pandemic and an entire society that is now accustomed to working from home in sweats, and it’s not a stretch to understand why some patients have become less formal in many settings. The 2015 article noted, however, that most physicians prefer to keep titles in the mix.
Perhaps most troublesome, says Dr. Setya, is that patients forgo asking whether it’s OK to use his first name and simply assume it’s acceptable. “It bothers me,” he says. “I became a doctor for more than the money.”
He suspects that his cultural background (Dr. Setya is of Indian descent) plays a role in how strongly he feels about patient-doctor informality. “As a British colony, Indian culture dictates that you pay respect to elders and to accomplishment,” he points out. “America is far looser when it comes to salutations.”
Dr. Cucuzzella largely agrees with Dr. Setya, but has a different view of the role culture plays in how physicians prefer to be addressed. “If your last name is difficult to pronounce, it can put the patient at ease if you give them an option,” he says. “I like my patients to feel comfortable and have a friendly conversation, so I don’t ask them to try to manage my last name.”
When patients revert to using Dr. Cucuzzella’s last name and title, this often breaks down along generational lines, Dr. Cucuzzella has found: Older patients might drop his title, whereas younger patients might keep it as a sign of respect. In some cases, Dr. Cucuzzella tries to bridge this gap, and offers the option of “Dr. Mark.” In his small West Virginia community, this is how people often refer to him.
Dr. Setya says that most of the older physicians he works with still prefer that patients and younger colleagues use their title, but he has witnessed exceptions to this. “My boss in residence hated to be called ‘Sir’ or ‘Doctor,’ ” he says. “In a situation like that, it is reasonable to ask, ‘How can I address you?’ But it has to be mutually agreed upon.”
Dr. Cucuzzella cites informality as the preferred mode for older patients. “If I have a 70-year-old patient, it seems natural they shouldn’t use my title,” he says. “They are worthy of equality in the community. If I’m talking to a retired CEO or state delegate, it’s uncomfortable if they call me doctor.”
Moreover, Dr. Cucuzzella maintains that establishing a less formal environment with patients leads to better outcomes. “Shared decision-making is a basic human right,” he says. “In 2022, doctors shouldn’t make decisions without patient input, unless it’s an emergency situation. Removing the title barriers makes that easier.”
How to handle informality
If you fall more in line with Dr. Setya, there are strategies you can use to try to keep formality in your doctor-patient relationships. Dr. Setya’s approach is indirect. “I don’t correct a patient if they use my first name, because that might seem hostile,” he says. “But I alert them in the way I address them back. A Sir, a Mrs., or a Mr. needs to go both ways.”
This particularly holds true in pediatrics, Dr. Setya has found. He has witnessed many colleagues addressing parents as “Mommy and Daddy,” something he says lacks respect and sets too informal a tone. “It’s almost universal that parents don’t like that, and we need to act accordingly.”
Dr. Witt also avoids directly correcting patients, but struggles when they drop her title. “The standard signature I use to sign every patient portal message I respond to includes my first and last name and credentials,” she says. “I maintain formality in most circumstances with that standard reply.”
Beneath the surface, however, Dr. Witt wishes it were easier. “I have struggled with answering the question, ‘Is it OK if I call you Leah?’ she says. “I want to keep our interaction anchored in professionalism without sacrificing the warmth I think is important to a productive patient-physician relationship. For this reason, I tend to say yes to this request, even though I’d rather patients didn’t make such requests.”
In the Fast Company article by Amy Diehl, PhD, and Leanne Dzubinski, PhD, on the topic of untitling professional women, the authors suggest several actions, beginning with leadership that sets expectations on the topic. They also suggest that physicians use polite corrections if patients untitle them. Supplying positive reinforcement when patients include your title can help, too. If all else fails, you can call out the offensive untitling. More often than not, especially with female physicians, the patient is demonstrating an unconscious bias rather than something deliberate.
Opinions vary on the topic of untitling, and ultimately each physician must make the decision for themselves. But creating informal cultures in an organization can have unintended consequences, especially for female peers.
Says Dr. Witt, “We all want to give our patients the best care we can, but professional boundaries are critical to time management, equitable care, and maintaining work-life balance. I would love to see a study that examines untitling by self-reported race and/or ethnicity of physicians, because we know that women of color experience higher rates of burnout and depression, and I wonder if untitling may be part of this.”
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Recommending exercise for migraine: Just do it
with high-intensity aerobics coming in second, and both beating top-line migraine medications topiramate and amitriptyline, new research suggests.
The new results should encourage clinicians to recommend patients with migraine engage in strength-training exercise whenever possible, study investigator Yohannes W. Woldeamanuel, MD, a physician-scientist and instructor, department of neurology and neurological sciences, Stanford (Calif.) University, told this news organization.
“Exercise is something patients can do all their lives and use it to prevent migraine attacks instead of taking daily medications or repetitive injections that have several adverse effects.”
The findings were published online in the Journal of Headache and Pain.
Head-to-head comparison
Several clinical trials have shown exercise is effective for migraine management, but to date, there have been no head-to-head comparisons of strength training and aerobic exercise, said Dr. Woldeamanuel.
This new study used a systematic review with network meta-analysis (NMA), which compares multiple interventions and ranks the efficacy of each one.
After a literature search, researchers included 21 clinical trials with an exercise regimen arm and a comparison control arm. All study data reported monthly frequency of migraine at baseline and at the end of the intervention.
The total combined sample size was 1,195 patients with migraine, who were a mean age of 35.5 years, with a female-to-male ratio of 6.7:1. All studies used International Classification of Headache Disorders (ICHD) criteria for migraine diagnosis.
The NMA provided 27 pairwise comparisons and 8 indirect comparisons. The pairwise comparisons provided direct evidence between the different interventions.
Researchers combined strength training, including weightlifting, with resistance training. Both modalities target muscles, while aerobic exercise targets cardiovascular health.
The average number of weeks was 9.3, 9.3, and 10.7, and the average number of hours per session for strength/resistance training, high-intensity aerobic exercise, and moderate-intensity aerobic exercise interventions was 50, 56, and 45.3, respectively.
The analysis showed all exercise interventions were more effective than the placebo groups in reducing the frequency of migraine. In terms of ranking, strength training came out on top, with a mean difference in monthly migraine days of −3.55 (95% confidence interval, −6.15 to −0.95) between the active and placebo groups.
Next was high-intensity aerobic exercise (−3.13; 95% CI, −5.28 to −0.97) and moderate-intensity aerobic exercise (−2.18; 95% CI, −3.25 to −1.11), followed by topiramate, placebo, and then amitriptyline.
Strength/resistance training was superior possibly because it targets muscle strengthening, particularly major muscles in the neck and shoulder area, which can be a source of the pain trigger, said Dr. Woldeamanuel. He added neck pain is highly comorbid with migraine.
Interestingly, patients doing exercises that focus on unaffected muscles – for example, squats – still get the benefits of less migraine burden, said Dr. Woldeamanuel.
Training recommendations
Strength training also increases or preserves lean muscle mass, which is associated with reduced migraine frequency. Research shows preservation of lean body mass combats central sensitization in various pain syndromes, said Dr. Woldeamanuel.
The superior effects of high- versus moderate-intensity aerobic exercise may be due to recruitment of endogenous molecules involved in exercise-mediated hypoalgesia (pain reduction).
The most common pathways are the opioid and endocannabinoid systems, although other systems are also likely involved, said Dr. Woldeamanuel. He noted migraine has been linked to a deficiency of both opioidergic and endocannabinoidergic signaling.
Dr. Woldeamanuel commented on the difficulty of comparing exercise interventions for patients with chronic versus episodic migraine, as many studies include both.
However, the two studies with moderate-intensity aerobic exercise exclusively involving patients with chronic migraine showed large effect sizes (Cohen’s d) of 0.80 and 1.10 in reducing monthly headache frequency.
Based on these new results and their own experience, the researchers recommend strength training start with 50% of repetition maximum (RM) with 2-3 sets of 12-15 repetitions three times a week along with 10 minutes of warm-up, stretching, and cool-down, totaling 45-60 minutes per session. Weight/resistance load can then be increased weekly by 5% of RM if the patient is capable of successfully completing three sets.
They also recommend including active recovery days (low-intensity exercise) between training days. All major muscles, including neck, shoulder, and upper limb muscles, should be trained in a rotation.
For high-intensity aerobic exercise, the authors recommend starting with interval training at 55% VO2max (maximum respiratory capacity), or 50% HRmax (maximal heart rate) for 45-60 minutes per session, including 10 minutes of warm-up and cool-down, three times per week. The intensity can then be increased by 5%-10% each week to reach a maximum target of 80%-90% by week 12.
It is best for patients to start with a trainer for guidance and supervision, but once they master the routines, they can do the exercises independently, said Dr. Woldeamanuel.
Managing flare-ups
Headache flare-ups are normal during exercise, which may be caused by “boom and bust cycles” – exercising excessively when feeling good then completely stopping when feeling bad, said Dr. Woldeamanuel. He noted these flare-ups don’t mean “there’s something wrong with the brain or there’s some injury to muscles.”
The best way to manage such flare-ups is to use a pacing strategy that involves “not going overboard on good days and avoiding excessive rest on bad days,” the investigators note.
Dr. Woldeamanuel noted exercise is a lifestyle-based intervention; it not only helps reduce migraine attacks but also helps control other known comorbidities such as obesity and hypertension.
In a comment, Elizabeth Loder, MD, vice-chair, academic affairs, department of neurology, Brigham and Women’s Hospital, and professor of neurology, Harvard Medical School, both in Boston, said, “It’s useful to collect and summarize all of these studies, and to focus on helping patients and doctors understand the possible value of different kinds of exercise.”
The review was “well done,” said Dr. Loder, adding the researchers “have looked carefully at the quality of included studies.”
The study received support from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. Dr. Woldeamanuel has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
with high-intensity aerobics coming in second, and both beating top-line migraine medications topiramate and amitriptyline, new research suggests.
The new results should encourage clinicians to recommend patients with migraine engage in strength-training exercise whenever possible, study investigator Yohannes W. Woldeamanuel, MD, a physician-scientist and instructor, department of neurology and neurological sciences, Stanford (Calif.) University, told this news organization.
“Exercise is something patients can do all their lives and use it to prevent migraine attacks instead of taking daily medications or repetitive injections that have several adverse effects.”
The findings were published online in the Journal of Headache and Pain.
Head-to-head comparison
Several clinical trials have shown exercise is effective for migraine management, but to date, there have been no head-to-head comparisons of strength training and aerobic exercise, said Dr. Woldeamanuel.
This new study used a systematic review with network meta-analysis (NMA), which compares multiple interventions and ranks the efficacy of each one.
After a literature search, researchers included 21 clinical trials with an exercise regimen arm and a comparison control arm. All study data reported monthly frequency of migraine at baseline and at the end of the intervention.
The total combined sample size was 1,195 patients with migraine, who were a mean age of 35.5 years, with a female-to-male ratio of 6.7:1. All studies used International Classification of Headache Disorders (ICHD) criteria for migraine diagnosis.
The NMA provided 27 pairwise comparisons and 8 indirect comparisons. The pairwise comparisons provided direct evidence between the different interventions.
Researchers combined strength training, including weightlifting, with resistance training. Both modalities target muscles, while aerobic exercise targets cardiovascular health.
The average number of weeks was 9.3, 9.3, and 10.7, and the average number of hours per session for strength/resistance training, high-intensity aerobic exercise, and moderate-intensity aerobic exercise interventions was 50, 56, and 45.3, respectively.
The analysis showed all exercise interventions were more effective than the placebo groups in reducing the frequency of migraine. In terms of ranking, strength training came out on top, with a mean difference in monthly migraine days of −3.55 (95% confidence interval, −6.15 to −0.95) between the active and placebo groups.
Next was high-intensity aerobic exercise (−3.13; 95% CI, −5.28 to −0.97) and moderate-intensity aerobic exercise (−2.18; 95% CI, −3.25 to −1.11), followed by topiramate, placebo, and then amitriptyline.
Strength/resistance training was superior possibly because it targets muscle strengthening, particularly major muscles in the neck and shoulder area, which can be a source of the pain trigger, said Dr. Woldeamanuel. He added neck pain is highly comorbid with migraine.
Interestingly, patients doing exercises that focus on unaffected muscles – for example, squats – still get the benefits of less migraine burden, said Dr. Woldeamanuel.
Training recommendations
Strength training also increases or preserves lean muscle mass, which is associated with reduced migraine frequency. Research shows preservation of lean body mass combats central sensitization in various pain syndromes, said Dr. Woldeamanuel.
The superior effects of high- versus moderate-intensity aerobic exercise may be due to recruitment of endogenous molecules involved in exercise-mediated hypoalgesia (pain reduction).
The most common pathways are the opioid and endocannabinoid systems, although other systems are also likely involved, said Dr. Woldeamanuel. He noted migraine has been linked to a deficiency of both opioidergic and endocannabinoidergic signaling.
Dr. Woldeamanuel commented on the difficulty of comparing exercise interventions for patients with chronic versus episodic migraine, as many studies include both.
However, the two studies with moderate-intensity aerobic exercise exclusively involving patients with chronic migraine showed large effect sizes (Cohen’s d) of 0.80 and 1.10 in reducing monthly headache frequency.
Based on these new results and their own experience, the researchers recommend strength training start with 50% of repetition maximum (RM) with 2-3 sets of 12-15 repetitions three times a week along with 10 minutes of warm-up, stretching, and cool-down, totaling 45-60 minutes per session. Weight/resistance load can then be increased weekly by 5% of RM if the patient is capable of successfully completing three sets.
They also recommend including active recovery days (low-intensity exercise) between training days. All major muscles, including neck, shoulder, and upper limb muscles, should be trained in a rotation.
For high-intensity aerobic exercise, the authors recommend starting with interval training at 55% VO2max (maximum respiratory capacity), or 50% HRmax (maximal heart rate) for 45-60 minutes per session, including 10 minutes of warm-up and cool-down, three times per week. The intensity can then be increased by 5%-10% each week to reach a maximum target of 80%-90% by week 12.
It is best for patients to start with a trainer for guidance and supervision, but once they master the routines, they can do the exercises independently, said Dr. Woldeamanuel.
Managing flare-ups
Headache flare-ups are normal during exercise, which may be caused by “boom and bust cycles” – exercising excessively when feeling good then completely stopping when feeling bad, said Dr. Woldeamanuel. He noted these flare-ups don’t mean “there’s something wrong with the brain or there’s some injury to muscles.”
The best way to manage such flare-ups is to use a pacing strategy that involves “not going overboard on good days and avoiding excessive rest on bad days,” the investigators note.
Dr. Woldeamanuel noted exercise is a lifestyle-based intervention; it not only helps reduce migraine attacks but also helps control other known comorbidities such as obesity and hypertension.
In a comment, Elizabeth Loder, MD, vice-chair, academic affairs, department of neurology, Brigham and Women’s Hospital, and professor of neurology, Harvard Medical School, both in Boston, said, “It’s useful to collect and summarize all of these studies, and to focus on helping patients and doctors understand the possible value of different kinds of exercise.”
The review was “well done,” said Dr. Loder, adding the researchers “have looked carefully at the quality of included studies.”
The study received support from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. Dr. Woldeamanuel has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
with high-intensity aerobics coming in second, and both beating top-line migraine medications topiramate and amitriptyline, new research suggests.
The new results should encourage clinicians to recommend patients with migraine engage in strength-training exercise whenever possible, study investigator Yohannes W. Woldeamanuel, MD, a physician-scientist and instructor, department of neurology and neurological sciences, Stanford (Calif.) University, told this news organization.
“Exercise is something patients can do all their lives and use it to prevent migraine attacks instead of taking daily medications or repetitive injections that have several adverse effects.”
The findings were published online in the Journal of Headache and Pain.
Head-to-head comparison
Several clinical trials have shown exercise is effective for migraine management, but to date, there have been no head-to-head comparisons of strength training and aerobic exercise, said Dr. Woldeamanuel.
This new study used a systematic review with network meta-analysis (NMA), which compares multiple interventions and ranks the efficacy of each one.
After a literature search, researchers included 21 clinical trials with an exercise regimen arm and a comparison control arm. All study data reported monthly frequency of migraine at baseline and at the end of the intervention.
The total combined sample size was 1,195 patients with migraine, who were a mean age of 35.5 years, with a female-to-male ratio of 6.7:1. All studies used International Classification of Headache Disorders (ICHD) criteria for migraine diagnosis.
The NMA provided 27 pairwise comparisons and 8 indirect comparisons. The pairwise comparisons provided direct evidence between the different interventions.
Researchers combined strength training, including weightlifting, with resistance training. Both modalities target muscles, while aerobic exercise targets cardiovascular health.
The average number of weeks was 9.3, 9.3, and 10.7, and the average number of hours per session for strength/resistance training, high-intensity aerobic exercise, and moderate-intensity aerobic exercise interventions was 50, 56, and 45.3, respectively.
The analysis showed all exercise interventions were more effective than the placebo groups in reducing the frequency of migraine. In terms of ranking, strength training came out on top, with a mean difference in monthly migraine days of −3.55 (95% confidence interval, −6.15 to −0.95) between the active and placebo groups.
Next was high-intensity aerobic exercise (−3.13; 95% CI, −5.28 to −0.97) and moderate-intensity aerobic exercise (−2.18; 95% CI, −3.25 to −1.11), followed by topiramate, placebo, and then amitriptyline.
Strength/resistance training was superior possibly because it targets muscle strengthening, particularly major muscles in the neck and shoulder area, which can be a source of the pain trigger, said Dr. Woldeamanuel. He added neck pain is highly comorbid with migraine.
Interestingly, patients doing exercises that focus on unaffected muscles – for example, squats – still get the benefits of less migraine burden, said Dr. Woldeamanuel.
Training recommendations
Strength training also increases or preserves lean muscle mass, which is associated with reduced migraine frequency. Research shows preservation of lean body mass combats central sensitization in various pain syndromes, said Dr. Woldeamanuel.
The superior effects of high- versus moderate-intensity aerobic exercise may be due to recruitment of endogenous molecules involved in exercise-mediated hypoalgesia (pain reduction).
The most common pathways are the opioid and endocannabinoid systems, although other systems are also likely involved, said Dr. Woldeamanuel. He noted migraine has been linked to a deficiency of both opioidergic and endocannabinoidergic signaling.
Dr. Woldeamanuel commented on the difficulty of comparing exercise interventions for patients with chronic versus episodic migraine, as many studies include both.
However, the two studies with moderate-intensity aerobic exercise exclusively involving patients with chronic migraine showed large effect sizes (Cohen’s d) of 0.80 and 1.10 in reducing monthly headache frequency.
Based on these new results and their own experience, the researchers recommend strength training start with 50% of repetition maximum (RM) with 2-3 sets of 12-15 repetitions three times a week along with 10 minutes of warm-up, stretching, and cool-down, totaling 45-60 minutes per session. Weight/resistance load can then be increased weekly by 5% of RM if the patient is capable of successfully completing three sets.
They also recommend including active recovery days (low-intensity exercise) between training days. All major muscles, including neck, shoulder, and upper limb muscles, should be trained in a rotation.
For high-intensity aerobic exercise, the authors recommend starting with interval training at 55% VO2max (maximum respiratory capacity), or 50% HRmax (maximal heart rate) for 45-60 minutes per session, including 10 minutes of warm-up and cool-down, three times per week. The intensity can then be increased by 5%-10% each week to reach a maximum target of 80%-90% by week 12.
It is best for patients to start with a trainer for guidance and supervision, but once they master the routines, they can do the exercises independently, said Dr. Woldeamanuel.
Managing flare-ups
Headache flare-ups are normal during exercise, which may be caused by “boom and bust cycles” – exercising excessively when feeling good then completely stopping when feeling bad, said Dr. Woldeamanuel. He noted these flare-ups don’t mean “there’s something wrong with the brain or there’s some injury to muscles.”
The best way to manage such flare-ups is to use a pacing strategy that involves “not going overboard on good days and avoiding excessive rest on bad days,” the investigators note.
Dr. Woldeamanuel noted exercise is a lifestyle-based intervention; it not only helps reduce migraine attacks but also helps control other known comorbidities such as obesity and hypertension.
In a comment, Elizabeth Loder, MD, vice-chair, academic affairs, department of neurology, Brigham and Women’s Hospital, and professor of neurology, Harvard Medical School, both in Boston, said, “It’s useful to collect and summarize all of these studies, and to focus on helping patients and doctors understand the possible value of different kinds of exercise.”
The review was “well done,” said Dr. Loder, adding the researchers “have looked carefully at the quality of included studies.”
The study received support from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. Dr. Woldeamanuel has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF HEADACHE AND PAIN