User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Looking for a healthy meat substitute? Consider the potato
Boil ‘em, mash ‘em, include ‘em in a balanced diet
It’s kind of funny that, even though potatoes are vegetables and vegetables are generally considered to be healthy foods, not many people think of potatoes as being particularly good for you. And that’s hardly surprising since we usually either consume them in the form of French fries or potato chips, neither of which are known for their healthiness.
In fact, some previous research shows that potatoes are a food to avoid, particularly for people with insulin resistance. However, a new study from England goes against the grain and asserts that the potato is perfectly fine for insulin-resistant individuals and filled with valuable nutrients and health benefits. Which is great news for the state of Idaho and the potato organization funding the research. Of course there’s a potato organization.
For the study, a group of obese, overweight, or insulin-resistant individuals received a diet of either beans, peas, and meat or fish or white potatoes with meat or fish for 8 weeks; both diets were heavy in fruits and vegetables and both diets replaced about 40% of typical meat consumption with either beans and peas or potatoes. By the end of the study, those on the potato diet experienced health benefits equivalent to those on the bean and pea diet, including losing roughly equivalent amounts of weight and similarly reducing the body’s insulin response.
The researchers noted that, because people tend to eat the same amount of food no matter what, replacing something like meat with dense, low-calorie potatoes meant study participants could eat normally yet consume much fewer calories. So you could make a delicious, healthy stew without the brace of conies and the nice fish, which would make Smeagol very happy.
You won’t have ‘monkeypox’ to kick around anymore
It’s true. No more monkeypox. It’s gone. It’s history. Adios. The World Health Organization said that the disease formerly known as monkeypox will now be called mpox. What? You didn’t think it had been cured, did you? You did? Really? Silly readers.
“Mpox will become a preferred term, replacing monkeypox, after a transition period of 1 year. This serves to mitigate the concerns raised by experts about confusion caused by a name change in the midst of a global outbreak,” WHO said in a statement announcing the change.
The stigma attached to the name was the main problem. New York City Health Commissioner Dr. Ashwin Vasan had sent a letter to WHO earlier this year, according to CNN, saying that there was “growing concern for the potentially devastating and stigmatizing effects that the messaging around the ‘monkeypox’ virus can have on … vulnerable communities.”
We here at LOTME applaud the fight against stigmas of any sort, but we sensed there was more to this name change business, so our dedicated team of investigative journalists went into action. Sure enough, while rooting through WHO Director-General Tedros Adhanom Ghebreyesus’s garbage, we found a list of the names that had been rejected in favor of mpox:
- K-pop (already taken)
- Keeping up with the Kardashi-pox
- Trumpox
- Pox the magic dragon
- Monkey plague (didn’t really solve the problem)
- Hockey pox
- Mission mpoxible
- Jurassic Pox
- The pox that refreshes
- Debbie
Feet catch what the ears miss
The spectrum of frequencies that can be heard by human ears varies from person to person. Then there’s the matter of personal taste in music and volume level. But what really gets people moving? A new study shows that it’s more about the frequency of the sound than the volume.
For the study, participants at a concert by electronic music duo Orphx at LIVELab – a research performance center on the McMaster University campus in Hamilton, Ont., that was specifically designed to study music and dance – filled out questionnaires before and after the show. They also wore motion-capture headbands to detect their movement throughout the concert. During the show the researchers turned very-low-frequency (VLF) sounds (8-37 Hz) on and off every 2.5 minutes. Movement speed was calculated during on and off periods.
Although the effects of subliminal messaging aren’t new, past studies have shown that participants were mostly aware of the messaging. In this study, the researchers found that the subjects’ movements increased by 11.8% when the VLF sounds were on, but without their awareness. The researchers and the participants attributed movement to the bass, as lower pitches tend to elicit stronger neural responses and thus movement, compared with higher pitches.
“Our whole sense of the beat is mediated by the vestibular system but nobody’s really, I think, effectively confirmed that,” Jonathan Cannon, an assistant professor of psychology, neuroscience, and behavior at McMaster who not involved in the study, told Live Science.
Not to say this study didn’t have its limitations, such as the effect of the surrounding crowd or vibrations of the floor influencing the need to dance. But it definitely makes you wonder about what’s actually playing in your favorite song.
Uncle Leonid wants you
Do you like to travel? Are you a bit of a thrill seeker? Do you have any extra socks? If you’re a physician who answered yes to those three questions, then we’ve got an opportunity for you.
Leonid Slutsky, leader of Russia’s populist Liberal Democratic Party and chairman of the foreign relations committee in the lower house of Russia’s parliament – yes, that Leonid Slutsky – recently made a bit of a recruiting pitch, although that’s not how ABC News described it.
Mr. Slutsky, a strong supporter of his country’s war against Ukraine, recently told the mothers of Russian soldiers “that the whole world is watching us. We are the largest state and when we do not have socks, shorts, doctors, intelligence, communications, or simply care for our children, questions arise that will be very difficult to answer.”
It’s probably not what he meant, but the lack of intelligence is pretty clear.
Boil ‘em, mash ‘em, include ‘em in a balanced diet
It’s kind of funny that, even though potatoes are vegetables and vegetables are generally considered to be healthy foods, not many people think of potatoes as being particularly good for you. And that’s hardly surprising since we usually either consume them in the form of French fries or potato chips, neither of which are known for their healthiness.
In fact, some previous research shows that potatoes are a food to avoid, particularly for people with insulin resistance. However, a new study from England goes against the grain and asserts that the potato is perfectly fine for insulin-resistant individuals and filled with valuable nutrients and health benefits. Which is great news for the state of Idaho and the potato organization funding the research. Of course there’s a potato organization.
For the study, a group of obese, overweight, or insulin-resistant individuals received a diet of either beans, peas, and meat or fish or white potatoes with meat or fish for 8 weeks; both diets were heavy in fruits and vegetables and both diets replaced about 40% of typical meat consumption with either beans and peas or potatoes. By the end of the study, those on the potato diet experienced health benefits equivalent to those on the bean and pea diet, including losing roughly equivalent amounts of weight and similarly reducing the body’s insulin response.
The researchers noted that, because people tend to eat the same amount of food no matter what, replacing something like meat with dense, low-calorie potatoes meant study participants could eat normally yet consume much fewer calories. So you could make a delicious, healthy stew without the brace of conies and the nice fish, which would make Smeagol very happy.
You won’t have ‘monkeypox’ to kick around anymore
It’s true. No more monkeypox. It’s gone. It’s history. Adios. The World Health Organization said that the disease formerly known as monkeypox will now be called mpox. What? You didn’t think it had been cured, did you? You did? Really? Silly readers.
“Mpox will become a preferred term, replacing monkeypox, after a transition period of 1 year. This serves to mitigate the concerns raised by experts about confusion caused by a name change in the midst of a global outbreak,” WHO said in a statement announcing the change.
The stigma attached to the name was the main problem. New York City Health Commissioner Dr. Ashwin Vasan had sent a letter to WHO earlier this year, according to CNN, saying that there was “growing concern for the potentially devastating and stigmatizing effects that the messaging around the ‘monkeypox’ virus can have on … vulnerable communities.”
We here at LOTME applaud the fight against stigmas of any sort, but we sensed there was more to this name change business, so our dedicated team of investigative journalists went into action. Sure enough, while rooting through WHO Director-General Tedros Adhanom Ghebreyesus’s garbage, we found a list of the names that had been rejected in favor of mpox:
- K-pop (already taken)
- Keeping up with the Kardashi-pox
- Trumpox
- Pox the magic dragon
- Monkey plague (didn’t really solve the problem)
- Hockey pox
- Mission mpoxible
- Jurassic Pox
- The pox that refreshes
- Debbie
Feet catch what the ears miss
The spectrum of frequencies that can be heard by human ears varies from person to person. Then there’s the matter of personal taste in music and volume level. But what really gets people moving? A new study shows that it’s more about the frequency of the sound than the volume.
For the study, participants at a concert by electronic music duo Orphx at LIVELab – a research performance center on the McMaster University campus in Hamilton, Ont., that was specifically designed to study music and dance – filled out questionnaires before and after the show. They also wore motion-capture headbands to detect their movement throughout the concert. During the show the researchers turned very-low-frequency (VLF) sounds (8-37 Hz) on and off every 2.5 minutes. Movement speed was calculated during on and off periods.
Although the effects of subliminal messaging aren’t new, past studies have shown that participants were mostly aware of the messaging. In this study, the researchers found that the subjects’ movements increased by 11.8% when the VLF sounds were on, but without their awareness. The researchers and the participants attributed movement to the bass, as lower pitches tend to elicit stronger neural responses and thus movement, compared with higher pitches.
“Our whole sense of the beat is mediated by the vestibular system but nobody’s really, I think, effectively confirmed that,” Jonathan Cannon, an assistant professor of psychology, neuroscience, and behavior at McMaster who not involved in the study, told Live Science.
Not to say this study didn’t have its limitations, such as the effect of the surrounding crowd or vibrations of the floor influencing the need to dance. But it definitely makes you wonder about what’s actually playing in your favorite song.
Uncle Leonid wants you
Do you like to travel? Are you a bit of a thrill seeker? Do you have any extra socks? If you’re a physician who answered yes to those three questions, then we’ve got an opportunity for you.
Leonid Slutsky, leader of Russia’s populist Liberal Democratic Party and chairman of the foreign relations committee in the lower house of Russia’s parliament – yes, that Leonid Slutsky – recently made a bit of a recruiting pitch, although that’s not how ABC News described it.
Mr. Slutsky, a strong supporter of his country’s war against Ukraine, recently told the mothers of Russian soldiers “that the whole world is watching us. We are the largest state and when we do not have socks, shorts, doctors, intelligence, communications, or simply care for our children, questions arise that will be very difficult to answer.”
It’s probably not what he meant, but the lack of intelligence is pretty clear.
Boil ‘em, mash ‘em, include ‘em in a balanced diet
It’s kind of funny that, even though potatoes are vegetables and vegetables are generally considered to be healthy foods, not many people think of potatoes as being particularly good for you. And that’s hardly surprising since we usually either consume them in the form of French fries or potato chips, neither of which are known for their healthiness.
In fact, some previous research shows that potatoes are a food to avoid, particularly for people with insulin resistance. However, a new study from England goes against the grain and asserts that the potato is perfectly fine for insulin-resistant individuals and filled with valuable nutrients and health benefits. Which is great news for the state of Idaho and the potato organization funding the research. Of course there’s a potato organization.
For the study, a group of obese, overweight, or insulin-resistant individuals received a diet of either beans, peas, and meat or fish or white potatoes with meat or fish for 8 weeks; both diets were heavy in fruits and vegetables and both diets replaced about 40% of typical meat consumption with either beans and peas or potatoes. By the end of the study, those on the potato diet experienced health benefits equivalent to those on the bean and pea diet, including losing roughly equivalent amounts of weight and similarly reducing the body’s insulin response.
The researchers noted that, because people tend to eat the same amount of food no matter what, replacing something like meat with dense, low-calorie potatoes meant study participants could eat normally yet consume much fewer calories. So you could make a delicious, healthy stew without the brace of conies and the nice fish, which would make Smeagol very happy.
You won’t have ‘monkeypox’ to kick around anymore
It’s true. No more monkeypox. It’s gone. It’s history. Adios. The World Health Organization said that the disease formerly known as monkeypox will now be called mpox. What? You didn’t think it had been cured, did you? You did? Really? Silly readers.
“Mpox will become a preferred term, replacing monkeypox, after a transition period of 1 year. This serves to mitigate the concerns raised by experts about confusion caused by a name change in the midst of a global outbreak,” WHO said in a statement announcing the change.
The stigma attached to the name was the main problem. New York City Health Commissioner Dr. Ashwin Vasan had sent a letter to WHO earlier this year, according to CNN, saying that there was “growing concern for the potentially devastating and stigmatizing effects that the messaging around the ‘monkeypox’ virus can have on … vulnerable communities.”
We here at LOTME applaud the fight against stigmas of any sort, but we sensed there was more to this name change business, so our dedicated team of investigative journalists went into action. Sure enough, while rooting through WHO Director-General Tedros Adhanom Ghebreyesus’s garbage, we found a list of the names that had been rejected in favor of mpox:
- K-pop (already taken)
- Keeping up with the Kardashi-pox
- Trumpox
- Pox the magic dragon
- Monkey plague (didn’t really solve the problem)
- Hockey pox
- Mission mpoxible
- Jurassic Pox
- The pox that refreshes
- Debbie
Feet catch what the ears miss
The spectrum of frequencies that can be heard by human ears varies from person to person. Then there’s the matter of personal taste in music and volume level. But what really gets people moving? A new study shows that it’s more about the frequency of the sound than the volume.
For the study, participants at a concert by electronic music duo Orphx at LIVELab – a research performance center on the McMaster University campus in Hamilton, Ont., that was specifically designed to study music and dance – filled out questionnaires before and after the show. They also wore motion-capture headbands to detect their movement throughout the concert. During the show the researchers turned very-low-frequency (VLF) sounds (8-37 Hz) on and off every 2.5 minutes. Movement speed was calculated during on and off periods.
Although the effects of subliminal messaging aren’t new, past studies have shown that participants were mostly aware of the messaging. In this study, the researchers found that the subjects’ movements increased by 11.8% when the VLF sounds were on, but without their awareness. The researchers and the participants attributed movement to the bass, as lower pitches tend to elicit stronger neural responses and thus movement, compared with higher pitches.
“Our whole sense of the beat is mediated by the vestibular system but nobody’s really, I think, effectively confirmed that,” Jonathan Cannon, an assistant professor of psychology, neuroscience, and behavior at McMaster who not involved in the study, told Live Science.
Not to say this study didn’t have its limitations, such as the effect of the surrounding crowd or vibrations of the floor influencing the need to dance. But it definitely makes you wonder about what’s actually playing in your favorite song.
Uncle Leonid wants you
Do you like to travel? Are you a bit of a thrill seeker? Do you have any extra socks? If you’re a physician who answered yes to those three questions, then we’ve got an opportunity for you.
Leonid Slutsky, leader of Russia’s populist Liberal Democratic Party and chairman of the foreign relations committee in the lower house of Russia’s parliament – yes, that Leonid Slutsky – recently made a bit of a recruiting pitch, although that’s not how ABC News described it.
Mr. Slutsky, a strong supporter of his country’s war against Ukraine, recently told the mothers of Russian soldiers “that the whole world is watching us. We are the largest state and when we do not have socks, shorts, doctors, intelligence, communications, or simply care for our children, questions arise that will be very difficult to answer.”
It’s probably not what he meant, but the lack of intelligence is pretty clear.
Psychedelics for treating psychiatric disorders: Are they safe?
Psychedelics are a class of substances known to produce alterations in consciousness and perception. In the last 2 decades, psychedelic research has garnered increasing attention from scientists, therapists, entrepreneurs, and the public. While many of these compounds remain illegal in the United States and in many parts of the world (Box1), a recent resurrection of psychedelic research has motivated the FDA to designate multiple psychedelic compounds as “breakthrough therapies,” thereby expediting the investigation, development, and review of psychedelic treatments.
Box
The legal landscape of psychedelics is rapidly evolving. Psilocybin use has been decriminalized in many cities in the United States (such as Denver), and some states (such as Oregon) have legalized it for therapeutic use.
It is important to understand the difference between decriminalization and legalization. Decriminalization means the substance is still prohibited under existing laws, but the legal system will choose not to enforce the prohibition. Legalization is the rescinding of laws prohibiting the use of the substance. In the United States, these laws may be state or federal. Despite psilocybin legalization for therapeutic use in Oregon and decriminalization in various cities, psychedelics currently remain illegal under federal law.
Source: Reference 1
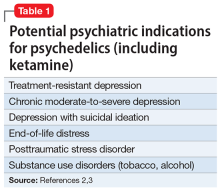
There is growing evidence that psychedelics may be efficacious for treating a range of psychiatric disorders. Potential clinical indications for psychedelics include some forms of depression, posttraumatic stress disorder (PTSD), and substance use disorders (Table 12,3). In most instances, the clinical use of psychedelics is being investigated and offered in the context of psychedelic-assisted psychotherapy, though ketamine is a prominent exception. Ketamine and esketamine are already being used to treat depression, and FDA approval is anticipated for other psychedelics.
This article examines the adverse effect profile of classical (psilocybin [“mushrooms”], lysergic acid diethylamide [LSD], and N,N-dimethyltryptamine [DMT]/ayahuasca) and nonclassical (the entactogen 3,4-methylenedioxymethamphetamine [MDMA, known as “ecstasy”] and the dissociative anesthetic ketamine) psychedelics.
Psilocybin
Psilocybin is typically administered as a single dose of 10 to 30 mg and used in conjunction with preintegration and postintegration psychotherapy. Administration of psilocybin typically produces perceptual distortions and mind-altering effects, which are mediated through 5-HT2A brain receptor agonistic action.4 The acute effects last approximately 6 hours.5 While psilocybin has generated promising results in early clinical trials,3 the adverse effects of these agents have received less attention.
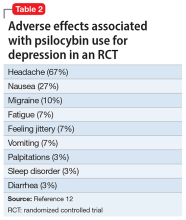
The adverse effect profile of psilocybin in adults appears promising but its powerful psychoactive effects necessitate cautious use.6 It has a very wide therapeutic index, and in a recent meta-analysis of psilocybin for depression, no serious adverse effects were reported in any of the 7 included studies.7 Common adverse effects in the context of clinical use include anxiety, dysphoria, confusion, and an increase in blood pressure and heart rate.6 Due to potential cardiac effects, psilocybin is contraindicated in individuals with cardiovascular and cerebrovascular disease.8 In recreational/nonclinical use, reactions such as suicidality, violence, convulsions, panic attacks, paranoia, confusion, prolonged dissociation, and mania have been reported.9,10 Animal and human studies indicate the risk of abuse and physical dependence is low. Major national surveys indicate low rates of abuse, treatment-seeking, and harm.11 In a recent 6-week randomized controlled trial (RCT) of psilocybin vs escitalopram for depression,12 no serious adverse events were reported. Adverse events reported in the psilocybin group in this trial are listed in Table 2.12
A recent phase 2 double-blind trial of single-dose psilocybin (1 mg, 10 mg, and 25 mg) for treatment-resistant depression (N = 233) sheds more light on the risk of adverse effects.13 The percentage of individuals experiencing adverse effects on Day 1 of administration was high: 61% in the 25 mg psilocybin group. Headache, nausea, fatigue, and dizziness were the most common effects. The incidence of any adverse event in the 25 mg group was 56% from Day 2 to Week 3, and 29% from Week 3 to Week 12. Suicidal ideation, suicidal behavior, or self-injury occurred in all 3 dose groups. Overall, 14% in the 25 mg group, 17% in the 10 mg group, and 9% in the 1 mg group showed worsening of suicidality from baseline to Week 3. Suicidal behavior was reported by 3 individuals in the 25 mg group after Week 3. The new-onset or worsening of preexisting suicidality with psilocybin reported in this study requires further investigation.
Lysergic acid diethylamide
LSD is similar to psilocybin in its agonistic action at the 5-HT2A brain receptors.4 It is typically administered as a single 100 to 200 μg dose and is used in conjunction with preintegration and postintegration psychotherapy.14 Its acute effects last approximately 12 hours.15
Continue to: Like psilocybin...
Like psilocybin, LSD has a wide therapeutic index. Commonly reported adverse effects of LSD are increased anxiety, dysphoria, and confusion. LSD can also lead to physiological adverse effects, such as increased blood pressure and heart rate, and thus is contraindicated in patients with severe heart disease.6 In a systematic review of the therapeutic use of LSD that included 567 participants,16 2 cases of serious adverse events were reported: a tonic-clonic seizure in a patient with a prior history of seizures, and a case of prolonged psychosis in a 21-year-old with a history of psychotic disorder.
Though few psychedelic studies have examined the adverse effects of these agents in older adults, a recent phase 1 study that recruited 48 healthy older adults (age 55 to 75) found that, compared to placebo, low doses (5 to 20 μg) of LSD 2 times a week for 3 weeks had similar adverse effects, cognitive impairment, or balance impairment.17 The only adverse effect noted to be different between the placebo group and active treatment groups was headache (50% for LSD 10 μg, 25% for LSD 20 μg, and 8% for placebo). Because the dose range (5 to 20 μg) used in this study was substantially lower than the typical therapeutic dose range of 100 to 200 μg, these results should not be interpreted as supporting the safety of LSD at higher doses in older adults.
DMT/ayahuasca
Ayahuasca is a plant-based psychedelic that contains an admixture of substances, including DMT, which acts as a 5-HT2A receptor agonist. In addition to DMT, ayahuasca also contains the alkaloid harmaline, which acts as a monoamine inhibitor. Use of ayahuasca can therefore pose a particular risk for individuals taking other serotonergic or noradrenergic medications or substances. The acute effects of DMT last approximately 4 hours,18 and acute administration of ayahuasca leads to a transient modified state of consciousness that is characterized by introspection, visions, enhanced emotions, and recall of personal memories.19 Research shows ayahuasca has been dosed at approximately 0.36 mg/kg of DMT for 1 dosing session alongside 6 2-hour therapy sessions.20
A recent review by Orsolini et al21 consolidated 40 preclinical, observational, and experimental studies of ayahuasca, and this compound appeared to be safe and well-tolerated; the most common adverse effects were transient emesis and nausea. In an RCT by Palhano-Fontes et al,20 nausea was observed in 71% of participants in the ayahuasca group (vs 26% placebo), vomiting in 57% of participants (vs 0% placebo), and restlessness in 50% of participants (vs 20% placebo). The authors noted that for some participants the ayahuasca session “was not necessarily a pleasant experience,” and was accompanied by psychological distress.20 Vomiting is traditionally viewed as an expected part of the purging process of ayahuasca religious ceremonies. Another review found that there appears to be good long-term tolerability of ayahuasca consumption among individuals who use this compound in religious ceremonies.22
MDMA
Entactogens (or empathogens) are a class of psychoactive substances that produce experiences of emotional openness and connection. MDMA is an entactogen known to release serotonin, norepinephrine, and dopamine by inhibiting reuptake.23 This process leads to the stimulation of neurohormonal signaling of oxytocin, cortisol, and other signaling molecules such as brain-derived neurotrophic factor.24 Memory reconsolidation and fear extinction may also play a therapeutic role, enabled by reduced activity in the amygdala and insula, and increased connectivity between the amygdala and hippocampus.24 MDMA has been reported to enhance feelings of well-being and increase prosocial behavior.25 In the therapeutic setting, MDMA has been generally dosed at 75 to 125 mg in 2 to 3 sessions alongside 10 therapy sessions. Administration of MDMA gives the user a subjective experience of energy and distortions in time and perception.26 These acute effects last approximately 2 to 4 hours.27
Continue to: A meta-analysis...
A meta-analysis of 5 RCTs of MDMA-assisted therapy for PTSD in adults demonstrated that MDMA was well-tolerated, and few serious adverse events were reported.28 Two trials from 2018 that were included in this meta-analysis—Mithoefer et al29 and Ot’alora et al30—illustrate the incidence of specific adverse effects. In a randomized, double-blind trial of 26 veterans and first responders with chronic PTSD, Mithoefer et al29 found the most commonly reported reactions during experimental sessions with MDMA were anxiety (81%), headache (69%), fatigue (62%), muscle tension (62%), and jaw clenching or tight jaw (50%). The most commonly reported reactions during 7 days of contact were fatigue (88%), anxiety (73%), insomnia (69%), headache (46%), muscle tension (46%), and increased irritability (46%). One instance of suicidal ideation was severe enough to require psychiatric hospitalization (this was the only instance of suicidal ideation among the 106 patients in the meta-analysis by Bahji et al28); the patient subsequently completed the trial. Transient elevation in pulse, blood pressure, and body temperature were noted during sessions that did not require medical intervention.29 Ot’alora et al30 found similar common adverse reactions: anxiety, dizziness, fatigue, headache, jaw clenching, muscle tension, and irritability. There were no serious adverse effects.
While the use of MDMA in controlled interventional settings has resulted in relatively few adverse events, robust literature describes the risks associated with the nonclinical/recreational use of MDMA. In cases of MDMA toxicity, death has been reported.31 Acutely, MDMA may lead to sympathomimetic effects, including serotonin syndrome.31 Longer-term studies of MDMA users have found chronic recreational use to be associated with worse sleep, poor mood, anxiety disturbances, memory deficits, and attention problems.32 MDMA has also been found to have moderate potential for abuse.33
Ketamine/esketamine
Ketamine is a dissociative anesthetic with some hallucinogenic effects. It is an N-methyl-
Esketamine, the S(+)-enantiomer of ketamine, is also an NDMA antagonist. It has been developed as an intranasal formulation, typically dosed between 56 and 84 mg 2 times a week for 1 month, once a week for the following month, and once every 1 to 2 weeks thereafter.35 In most ketamine and esketamine trials, these compounds have been used without psychotherapy, although some interventions have integrated psychotherapy with ketamine treatment.36
Bennett et al37 elaborated on 3 paradigms for ketamine treatment: biochemical, psychotherapeutic, and psychedelic. The biochemical model examines the neurobiological effects of the medication. The psychotherapeutic model views ketamine as a way of assisting the psychotherapy process. The psychedelic model utilizes ketamine’s dissociative and psychedelic properties to induce an altered state of consciousness for therapeutic purposes and psychospiritual exploration.
Continue to: A systematic review...
A systematic review of the common adverse effects associated with ketamine use in clinical trials for depression reported dissociation, sedation, perceptual disturbances, anxiety, agitation, euphoria, hypertension, tachycardia, headache, and dizziness.38 Adverse effects experienced with esketamine in clinical trials include dissociation, dizziness, sedation, hypertension, hypoesthesia, gastrointestinal symptoms, and euphoric mood (Table 339). A recent systemic review found both ketamine and esketamine demonstrated higher adverse events than control conditions. IV ketamine also demonstrated lower dropouts and adverse events when compared to intranasal esketamine.40
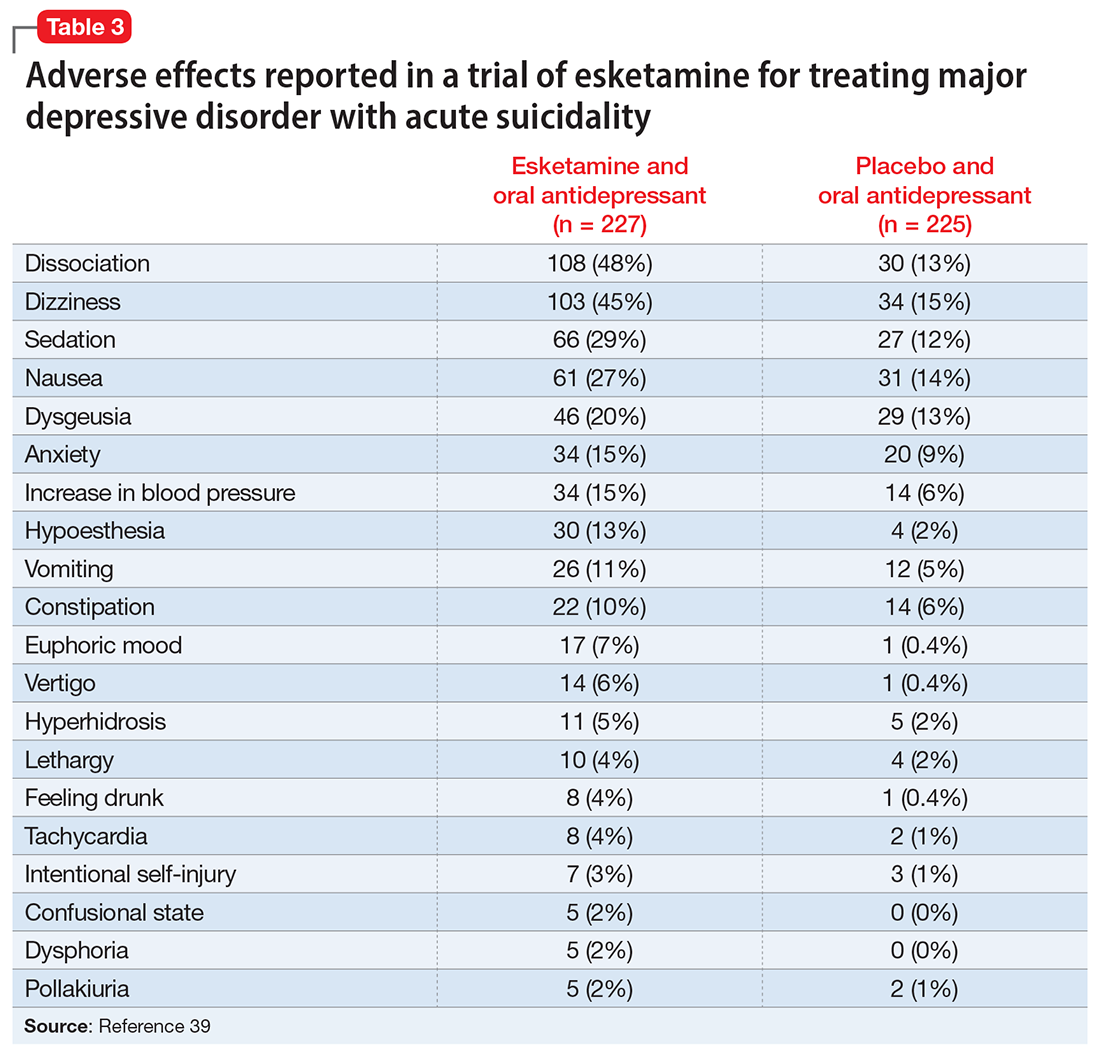
Nonclinical/recreational use of ketamine is notable for urinary toxicity; 20% to 30% of frequent users of ketamine experience urinary problems that can range from ketamine-induced cystitis to hydronephrosis and kidney failure.41 Liver toxicity has also been reported with chronic use of high-dose ketamine. Ketamine is liable to abuse, dependence, and tolerance. There is evidence that nonclinical use of ketamine may lead to morbidity; impairment of memory, cognition, and attention; and urinary, gastric, and hepatic pathology.42
The FDA prescribing information for esketamine lists aneurysmal vascular disease, arteriovenous malformation, and intracerebral hemorrhage as contraindications.39 Patients with cardiovascular and cerebrovascular conditions and risk factors may be at increased risk of adverse effects due to an increase in blood pressure. Esketamine can impair attention, judgment, thinking, reaction speed, and motor skills. Other adverse effects of esketamine noted in the prescribing information include dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, vomiting, feeling drunk, and euphoric mood.39A study of postmarketing safety concerns with esketamine using reports submitted to the FDA Adverse Event Reporting System (FAERS) revealed signals for suicidal ideation (reporting odds ratio [ROR] 24.03; 95% CI, 18.72 to 30.84), and completed suicide (ROR 5.75; 95% CI, 3.18 to 10.41).43 The signals for suicidal and self-injurious ideation remained significant when compared to venlafaxine in the FAERS database, while suicide attempts and fatal suicide attempts were no longer significant.43 Concerns regarding acute ketamine withdrawal have also been described in case reports.44
Other safety considerations of psychedelics
Hallucinogen persisting perception disorder
Hallucinogen persisting perception disorder (HPPD) is a rare condition associated with hallucinogen use. It is characterized by the recurrence of perceptual disturbances that an individual experienced while using hallucinogenic substances that creates significant distress or impairment.45 Because HPPD is a rare disorder, the exact prevalence is not well characterized, but DSM-5 suggests it is approximately 4.2%.46 HPPD is associated with numerous psychoactive substances, including psilocybin, ayahuasca, MDMA, and ketamine, but is most associated with LSD.45 HPPD is more likely to arise in individuals with histories of psychiatric illness or substance use disorders.47
Serotonin toxicity and other serotonergic interactions
Serotonin toxicity is a risk of serotonergic psychedelics, particularly when such agents are used in combination with serotonergic psychotropic medications. The most severe manifestation of serotonin toxicity is serotonin syndrome, which manifests as a life-threatening condition characterized by myoclonus, rigidity, agitation, delirium, and unstable cardiovascular functioning. Many psychedelic compounds have transient serotonin-related adverse effects, but serotonin toxicity due to psychedelic use is rare.48 Due to their mechanism of action, classical psychedelics are relatively safe in combination with monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors. MDMA is a serotonin-releasing agent that has a higher risk of serotonin syndrome or hypertensive crisis when used in combination with MAOIs.48
Boundary violations in psychedelic-assisted psychotherapy
A key task facing psychedelic research is to establish parameters for the safe and ethical use of these agents. This is particularly relevant given the hype that surrounds the psychedelic resurgence and what we know about the controversial history of these substances. Anderson et al49 argued that “psychedelics can have lingering effects that include increased suggestibility and affective instability, as well as altered ego structure, social behaviour, and philosophical worldview. Stated simply, psychedelics can induce a vulnerable state both during and after treatment sessions.”
Continue to: Psychedelic treatment...
Psychedelic treatments such as psilocybin and MDMA are typically offered within the context of psychedelic-assisted psychotherapy, and some researchers have raised concerns regarding boundary violations,50 given the patients’ particularly vulnerable states. In addition to concerns about sexual harassment, the financial exploitation of older adults is also a possible risk.51
Caveats to consider
Novel psychedelics therapies have demonstrated promising preliminary results for a broad range of psychiatric indications, including depression, end-of-life distress, substance use disorders, PTSD, and improving well-being. To date, psychedelics are generally well-tolerated in adults in clinical trials.
However, when it comes to adverse effects, there are challenges in regards to interpreting the psychedelic state.52 Some consider any unpleasant or unsettling psychedelic experience as an adverse reaction, while others consider it part of the therapeutic process. This is exemplified by the case of vomiting during ayahuasca ceremonies, which is generally considered part of the ritual. In such instances, it is essential to obtain informed consent and ensure participants are aware of these aspects of the experience. Compared to substances such as alcohol, opioids, and cocaine, psychedelics are remarkably safe from a physiological perspective, especially with regards to the risks of toxicity, mortality, and dependence.53 Their psychological safety is less established, and more caution and research is needed. The high incidence of adverse effects and suicidality noted in the recent phase 2 trial of psilocybin in treatment resistant depression are a reminder of this.13
There is uncertainty regarding the magnitude of risk in real-world clinical practice, particularly regarding addiction, suicidality, and precipitation or worsening of psychotic disorders. For example, note the extensive exclusion criteria used in the psilocybin vs escitalopram RCT by Carhart-Harris et al12: currently or previously diagnosed psychotic disorder, immediate family member with a diagnosed psychotic disorder, significant medical comorbidity (eg, diabetes, epilepsy, severe cardiovascular disease, hepatic or renal failure), history of suicide attempts requiring hospitalization, history of mania, pregnancy, and abnormal QT interval prolongation, among others. It would be prudent to keep these contraindications in mind regarding the clinical use of psychedelics in the future. This is particularly important in older adults because such patients often have substantial medical comorbidities and are at greater risk for adverse effects. For ketamine, research has implicated the role of mu opioid agonism in mediating ketamine’s antidepressant effects.54 This raises concerns about abuse, dependence, and addiction, especially with long-term use. There are also concerns regarding protracted withdrawal symptoms and associated suicidality.55
The therapeutic use of psychedelics is an exciting and promising avenue, with ongoing research and a rapidly evolving literature. An attitude of cautious optimism is warranted, but efficacy and safety should be demonstrated in well-designed and rigorous trials with adequate long-term follow-up before routine clinical use is recommended.
Bottom Line
In clinical trials for psychiatric disorders, psychedelics have been associated with a range of cognitive, psychiatric, and psychoactive adverse effects but generally have been well-tolerated, with a low incidence of serious adverse effects.
Related Resources
- American Psychiatric Association. Position Statement on the Use of Psychedelic and Empathogenic Agents for Mental Health Conditions. Updated July 2022. Accessed October 24, 2022. https://www.psychiatry.org/getattachment/d5c13619-ca1f-491f-a7a8-b7141c800904/Position-Use-of-Psychedelic-Empathogenic-Agents.pdf
- Johns Hopkins Center for Psychedelic & Consciousness Research. https://hopkinspsychedelic.org/
- Multidisciplinary Association for Psychedelic Studies (MAPS). https://maps.org/
Drug Brand Names
Esketamine • Spravato
Ketamine • Ketalar
Venlafaxine • Effexor
1. The current legal status of psychedelics in the United States. Investing News Network. August 23, 2022. Accessed August 26, 2022. https://investingnews.com/legal-status-of-psychedelics-in-the-united-states/
2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
3. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. 2021;78(2):121-122.
4. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
5. Hasler F, Grimberg U, Benz MA et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145-156.
6. Johnson MW, Hendricks PS, Barrett FS, et al. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. 2019;197:83-102.
7. Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26-34.
8. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
9. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15(4):283-300.
10. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423-429.
11. Johnson MW, Griffiths RR, Hendricks PS, et al. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143-166.
12. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl Med. 2021;384(15):1402-1411.
13. Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant Episode of major depression. N Engl J Med. 2022;387(18):1637-1648.
14. Galvão-Coelho NL, Marx W, Gonzalez M, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology (Berl). 2021;238(2):341-354.
15. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
16. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry. 2020;10:943.
17. Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl). 2020;237(3):841-853.
18. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol. 2016;7:35.
19. Domínguez-Clavé E, Solar J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull. 2016;126(Pt 1):89-101.
20. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
21. Orsolini L, Chiappini S, Papanti D, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol: Clin Exp. 2020;35(3):e2728.
22. Durante Í, Dos Santos RG, Bouso JC, et al. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2021;43(4):362-369.
23. Sessa B, Higbed L, Nutt D. A review of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry. 2019;10:138.
24. Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Progress Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228.
25. Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affective Neurosc. 2014;9(11):1645-1652.
26. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917-928.
27. Dumont GJ, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20(2):176-187.
28. Bahji A, Forsyth A, Groll D, et al. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109735.
29. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
30. Ot’alora GM, Grigsby J, Poulter B, et al. 3,4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295-1307.
31. Steinkellner T, Freissmuth M, Sitte HH, et al. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392(1-2):103-115.
32. Montoya AG, Sorrentino R, Lukas SE, et al. Long-term neuropsychiatric consequences of “ecstasy” (MDMA): a review. Harvard Rev Psychiatry. 2002;10(4):212-220.
33. Yazar‐Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194-196.
34. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
35. Thase M, Connolly KR. Ketamine and esketamine for treating unipolar depression in adults: administration, efficacy, and adverse effects. Wolters Kluwer; 2019. Accessed August 26, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects
36. Dore J, Turnispeed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198.
37. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
38. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78.
39. U.S. Food and Drug Administration. SPRAVATO® (esketamine). Prescribing information. Janssen; 2020. Accessed August 26, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf
40. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affective Disord. 2021;278:542-555.
41. Castellani D, Pirola GM, Gubbiotti M, et al. What urologists need to know about ketamine-induced uropathy: a systematic review. Neurourol Urodyn. 2020;39(4):1049-1062.
42. Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582-586.
43. Gastaldon, C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Psychother Psychosom. 2021;90(1):41-48.
44. Roxas N, Ahuja C, Isom J, et al. A potential case of acute ketamine withdrawal: clinical implications for the treatment of refractory depression. Am J Psychiatry. 2021;178(7):588-591.
45. Orsolini L, Papanti GD, De Berardis D, et al. The “Endless Trip” among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatry Association; 2013.
47. Martinotti G, Santacroce R, Pettorruso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8(3):47.
48. Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022;239(6):1881-1891.
49. Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry, 2020;7(10):829-830.
50. Goldhill O. Psychedelic therapy has a sexual abuse problem. QUARTZ. March 3, 2020. Accessed August 26, 2022. https://qz.com/1809184/psychedelic-therapy-has-a-sexual-abuse-problem-3/
51. Goldhill O. A psychedelic therapist allegedly took millions from a Holocaust survivor, highlighting worries about elders taking hallucinogens. STAT News. April 21, 2022. Accessed August 26, 2022. https://www.statnews.com/2022/04/21/psychedelic-therapist-allegedly-took-millions-from-holocaust-survivor-highlighting-worries-about-elders-taking-hallucinogens/
52. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577-595.
53. Nutt D. Drugs Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge Ltd; 2012.
54. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
55. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422-424.
Psychedelics are a class of substances known to produce alterations in consciousness and perception. In the last 2 decades, psychedelic research has garnered increasing attention from scientists, therapists, entrepreneurs, and the public. While many of these compounds remain illegal in the United States and in many parts of the world (Box1), a recent resurrection of psychedelic research has motivated the FDA to designate multiple psychedelic compounds as “breakthrough therapies,” thereby expediting the investigation, development, and review of psychedelic treatments.
Box
The legal landscape of psychedelics is rapidly evolving. Psilocybin use has been decriminalized in many cities in the United States (such as Denver), and some states (such as Oregon) have legalized it for therapeutic use.
It is important to understand the difference between decriminalization and legalization. Decriminalization means the substance is still prohibited under existing laws, but the legal system will choose not to enforce the prohibition. Legalization is the rescinding of laws prohibiting the use of the substance. In the United States, these laws may be state or federal. Despite psilocybin legalization for therapeutic use in Oregon and decriminalization in various cities, psychedelics currently remain illegal under federal law.
Source: Reference 1
There is growing evidence that psychedelics may be efficacious for treating a range of psychiatric disorders. Potential clinical indications for psychedelics include some forms of depression, posttraumatic stress disorder (PTSD), and substance use disorders (Table 12,3). In most instances, the clinical use of psychedelics is being investigated and offered in the context of psychedelic-assisted psychotherapy, though ketamine is a prominent exception. Ketamine and esketamine are already being used to treat depression, and FDA approval is anticipated for other psychedelics.
This article examines the adverse effect profile of classical (psilocybin [“mushrooms”], lysergic acid diethylamide [LSD], and N,N-dimethyltryptamine [DMT]/ayahuasca) and nonclassical (the entactogen 3,4-methylenedioxymethamphetamine [MDMA, known as “ecstasy”] and the dissociative anesthetic ketamine) psychedelics.
Psilocybin
Psilocybin is typically administered as a single dose of 10 to 30 mg and used in conjunction with preintegration and postintegration psychotherapy. Administration of psilocybin typically produces perceptual distortions and mind-altering effects, which are mediated through 5-HT2A brain receptor agonistic action.4 The acute effects last approximately 6 hours.5 While psilocybin has generated promising results in early clinical trials,3 the adverse effects of these agents have received less attention.
The adverse effect profile of psilocybin in adults appears promising but its powerful psychoactive effects necessitate cautious use.6 It has a very wide therapeutic index, and in a recent meta-analysis of psilocybin for depression, no serious adverse effects were reported in any of the 7 included studies.7 Common adverse effects in the context of clinical use include anxiety, dysphoria, confusion, and an increase in blood pressure and heart rate.6 Due to potential cardiac effects, psilocybin is contraindicated in individuals with cardiovascular and cerebrovascular disease.8 In recreational/nonclinical use, reactions such as suicidality, violence, convulsions, panic attacks, paranoia, confusion, prolonged dissociation, and mania have been reported.9,10 Animal and human studies indicate the risk of abuse and physical dependence is low. Major national surveys indicate low rates of abuse, treatment-seeking, and harm.11 In a recent 6-week randomized controlled trial (RCT) of psilocybin vs escitalopram for depression,12 no serious adverse events were reported. Adverse events reported in the psilocybin group in this trial are listed in Table 2.12
A recent phase 2 double-blind trial of single-dose psilocybin (1 mg, 10 mg, and 25 mg) for treatment-resistant depression (N = 233) sheds more light on the risk of adverse effects.13 The percentage of individuals experiencing adverse effects on Day 1 of administration was high: 61% in the 25 mg psilocybin group. Headache, nausea, fatigue, and dizziness were the most common effects. The incidence of any adverse event in the 25 mg group was 56% from Day 2 to Week 3, and 29% from Week 3 to Week 12. Suicidal ideation, suicidal behavior, or self-injury occurred in all 3 dose groups. Overall, 14% in the 25 mg group, 17% in the 10 mg group, and 9% in the 1 mg group showed worsening of suicidality from baseline to Week 3. Suicidal behavior was reported by 3 individuals in the 25 mg group after Week 3. The new-onset or worsening of preexisting suicidality with psilocybin reported in this study requires further investigation.
Lysergic acid diethylamide
LSD is similar to psilocybin in its agonistic action at the 5-HT2A brain receptors.4 It is typically administered as a single 100 to 200 μg dose and is used in conjunction with preintegration and postintegration psychotherapy.14 Its acute effects last approximately 12 hours.15
Continue to: Like psilocybin...
Like psilocybin, LSD has a wide therapeutic index. Commonly reported adverse effects of LSD are increased anxiety, dysphoria, and confusion. LSD can also lead to physiological adverse effects, such as increased blood pressure and heart rate, and thus is contraindicated in patients with severe heart disease.6 In a systematic review of the therapeutic use of LSD that included 567 participants,16 2 cases of serious adverse events were reported: a tonic-clonic seizure in a patient with a prior history of seizures, and a case of prolonged psychosis in a 21-year-old with a history of psychotic disorder.
Though few psychedelic studies have examined the adverse effects of these agents in older adults, a recent phase 1 study that recruited 48 healthy older adults (age 55 to 75) found that, compared to placebo, low doses (5 to 20 μg) of LSD 2 times a week for 3 weeks had similar adverse effects, cognitive impairment, or balance impairment.17 The only adverse effect noted to be different between the placebo group and active treatment groups was headache (50% for LSD 10 μg, 25% for LSD 20 μg, and 8% for placebo). Because the dose range (5 to 20 μg) used in this study was substantially lower than the typical therapeutic dose range of 100 to 200 μg, these results should not be interpreted as supporting the safety of LSD at higher doses in older adults.
DMT/ayahuasca
Ayahuasca is a plant-based psychedelic that contains an admixture of substances, including DMT, which acts as a 5-HT2A receptor agonist. In addition to DMT, ayahuasca also contains the alkaloid harmaline, which acts as a monoamine inhibitor. Use of ayahuasca can therefore pose a particular risk for individuals taking other serotonergic or noradrenergic medications or substances. The acute effects of DMT last approximately 4 hours,18 and acute administration of ayahuasca leads to a transient modified state of consciousness that is characterized by introspection, visions, enhanced emotions, and recall of personal memories.19 Research shows ayahuasca has been dosed at approximately 0.36 mg/kg of DMT for 1 dosing session alongside 6 2-hour therapy sessions.20
A recent review by Orsolini et al21 consolidated 40 preclinical, observational, and experimental studies of ayahuasca, and this compound appeared to be safe and well-tolerated; the most common adverse effects were transient emesis and nausea. In an RCT by Palhano-Fontes et al,20 nausea was observed in 71% of participants in the ayahuasca group (vs 26% placebo), vomiting in 57% of participants (vs 0% placebo), and restlessness in 50% of participants (vs 20% placebo). The authors noted that for some participants the ayahuasca session “was not necessarily a pleasant experience,” and was accompanied by psychological distress.20 Vomiting is traditionally viewed as an expected part of the purging process of ayahuasca religious ceremonies. Another review found that there appears to be good long-term tolerability of ayahuasca consumption among individuals who use this compound in religious ceremonies.22
MDMA
Entactogens (or empathogens) are a class of psychoactive substances that produce experiences of emotional openness and connection. MDMA is an entactogen known to release serotonin, norepinephrine, and dopamine by inhibiting reuptake.23 This process leads to the stimulation of neurohormonal signaling of oxytocin, cortisol, and other signaling molecules such as brain-derived neurotrophic factor.24 Memory reconsolidation and fear extinction may also play a therapeutic role, enabled by reduced activity in the amygdala and insula, and increased connectivity between the amygdala and hippocampus.24 MDMA has been reported to enhance feelings of well-being and increase prosocial behavior.25 In the therapeutic setting, MDMA has been generally dosed at 75 to 125 mg in 2 to 3 sessions alongside 10 therapy sessions. Administration of MDMA gives the user a subjective experience of energy and distortions in time and perception.26 These acute effects last approximately 2 to 4 hours.27
Continue to: A meta-analysis...
A meta-analysis of 5 RCTs of MDMA-assisted therapy for PTSD in adults demonstrated that MDMA was well-tolerated, and few serious adverse events were reported.28 Two trials from 2018 that were included in this meta-analysis—Mithoefer et al29 and Ot’alora et al30—illustrate the incidence of specific adverse effects. In a randomized, double-blind trial of 26 veterans and first responders with chronic PTSD, Mithoefer et al29 found the most commonly reported reactions during experimental sessions with MDMA were anxiety (81%), headache (69%), fatigue (62%), muscle tension (62%), and jaw clenching or tight jaw (50%). The most commonly reported reactions during 7 days of contact were fatigue (88%), anxiety (73%), insomnia (69%), headache (46%), muscle tension (46%), and increased irritability (46%). One instance of suicidal ideation was severe enough to require psychiatric hospitalization (this was the only instance of suicidal ideation among the 106 patients in the meta-analysis by Bahji et al28); the patient subsequently completed the trial. Transient elevation in pulse, blood pressure, and body temperature were noted during sessions that did not require medical intervention.29 Ot’alora et al30 found similar common adverse reactions: anxiety, dizziness, fatigue, headache, jaw clenching, muscle tension, and irritability. There were no serious adverse effects.
While the use of MDMA in controlled interventional settings has resulted in relatively few adverse events, robust literature describes the risks associated with the nonclinical/recreational use of MDMA. In cases of MDMA toxicity, death has been reported.31 Acutely, MDMA may lead to sympathomimetic effects, including serotonin syndrome.31 Longer-term studies of MDMA users have found chronic recreational use to be associated with worse sleep, poor mood, anxiety disturbances, memory deficits, and attention problems.32 MDMA has also been found to have moderate potential for abuse.33
Ketamine/esketamine
Ketamine is a dissociative anesthetic with some hallucinogenic effects. It is an N-methyl-
Esketamine, the S(+)-enantiomer of ketamine, is also an NDMA antagonist. It has been developed as an intranasal formulation, typically dosed between 56 and 84 mg 2 times a week for 1 month, once a week for the following month, and once every 1 to 2 weeks thereafter.35 In most ketamine and esketamine trials, these compounds have been used without psychotherapy, although some interventions have integrated psychotherapy with ketamine treatment.36
Bennett et al37 elaborated on 3 paradigms for ketamine treatment: biochemical, psychotherapeutic, and psychedelic. The biochemical model examines the neurobiological effects of the medication. The psychotherapeutic model views ketamine as a way of assisting the psychotherapy process. The psychedelic model utilizes ketamine’s dissociative and psychedelic properties to induce an altered state of consciousness for therapeutic purposes and psychospiritual exploration.
Continue to: A systematic review...
A systematic review of the common adverse effects associated with ketamine use in clinical trials for depression reported dissociation, sedation, perceptual disturbances, anxiety, agitation, euphoria, hypertension, tachycardia, headache, and dizziness.38 Adverse effects experienced with esketamine in clinical trials include dissociation, dizziness, sedation, hypertension, hypoesthesia, gastrointestinal symptoms, and euphoric mood (Table 339). A recent systemic review found both ketamine and esketamine demonstrated higher adverse events than control conditions. IV ketamine also demonstrated lower dropouts and adverse events when compared to intranasal esketamine.40

Nonclinical/recreational use of ketamine is notable for urinary toxicity; 20% to 30% of frequent users of ketamine experience urinary problems that can range from ketamine-induced cystitis to hydronephrosis and kidney failure.41 Liver toxicity has also been reported with chronic use of high-dose ketamine. Ketamine is liable to abuse, dependence, and tolerance. There is evidence that nonclinical use of ketamine may lead to morbidity; impairment of memory, cognition, and attention; and urinary, gastric, and hepatic pathology.42
The FDA prescribing information for esketamine lists aneurysmal vascular disease, arteriovenous malformation, and intracerebral hemorrhage as contraindications.39 Patients with cardiovascular and cerebrovascular conditions and risk factors may be at increased risk of adverse effects due to an increase in blood pressure. Esketamine can impair attention, judgment, thinking, reaction speed, and motor skills. Other adverse effects of esketamine noted in the prescribing information include dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, vomiting, feeling drunk, and euphoric mood.39A study of postmarketing safety concerns with esketamine using reports submitted to the FDA Adverse Event Reporting System (FAERS) revealed signals for suicidal ideation (reporting odds ratio [ROR] 24.03; 95% CI, 18.72 to 30.84), and completed suicide (ROR 5.75; 95% CI, 3.18 to 10.41).43 The signals for suicidal and self-injurious ideation remained significant when compared to venlafaxine in the FAERS database, while suicide attempts and fatal suicide attempts were no longer significant.43 Concerns regarding acute ketamine withdrawal have also been described in case reports.44
Other safety considerations of psychedelics
Hallucinogen persisting perception disorder
Hallucinogen persisting perception disorder (HPPD) is a rare condition associated with hallucinogen use. It is characterized by the recurrence of perceptual disturbances that an individual experienced while using hallucinogenic substances that creates significant distress or impairment.45 Because HPPD is a rare disorder, the exact prevalence is not well characterized, but DSM-5 suggests it is approximately 4.2%.46 HPPD is associated with numerous psychoactive substances, including psilocybin, ayahuasca, MDMA, and ketamine, but is most associated with LSD.45 HPPD is more likely to arise in individuals with histories of psychiatric illness or substance use disorders.47
Serotonin toxicity and other serotonergic interactions
Serotonin toxicity is a risk of serotonergic psychedelics, particularly when such agents are used in combination with serotonergic psychotropic medications. The most severe manifestation of serotonin toxicity is serotonin syndrome, which manifests as a life-threatening condition characterized by myoclonus, rigidity, agitation, delirium, and unstable cardiovascular functioning. Many psychedelic compounds have transient serotonin-related adverse effects, but serotonin toxicity due to psychedelic use is rare.48 Due to their mechanism of action, classical psychedelics are relatively safe in combination with monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors. MDMA is a serotonin-releasing agent that has a higher risk of serotonin syndrome or hypertensive crisis when used in combination with MAOIs.48
Boundary violations in psychedelic-assisted psychotherapy
A key task facing psychedelic research is to establish parameters for the safe and ethical use of these agents. This is particularly relevant given the hype that surrounds the psychedelic resurgence and what we know about the controversial history of these substances. Anderson et al49 argued that “psychedelics can have lingering effects that include increased suggestibility and affective instability, as well as altered ego structure, social behaviour, and philosophical worldview. Stated simply, psychedelics can induce a vulnerable state both during and after treatment sessions.”
Continue to: Psychedelic treatment...
Psychedelic treatments such as psilocybin and MDMA are typically offered within the context of psychedelic-assisted psychotherapy, and some researchers have raised concerns regarding boundary violations,50 given the patients’ particularly vulnerable states. In addition to concerns about sexual harassment, the financial exploitation of older adults is also a possible risk.51
Caveats to consider
Novel psychedelics therapies have demonstrated promising preliminary results for a broad range of psychiatric indications, including depression, end-of-life distress, substance use disorders, PTSD, and improving well-being. To date, psychedelics are generally well-tolerated in adults in clinical trials.
However, when it comes to adverse effects, there are challenges in regards to interpreting the psychedelic state.52 Some consider any unpleasant or unsettling psychedelic experience as an adverse reaction, while others consider it part of the therapeutic process. This is exemplified by the case of vomiting during ayahuasca ceremonies, which is generally considered part of the ritual. In such instances, it is essential to obtain informed consent and ensure participants are aware of these aspects of the experience. Compared to substances such as alcohol, opioids, and cocaine, psychedelics are remarkably safe from a physiological perspective, especially with regards to the risks of toxicity, mortality, and dependence.53 Their psychological safety is less established, and more caution and research is needed. The high incidence of adverse effects and suicidality noted in the recent phase 2 trial of psilocybin in treatment resistant depression are a reminder of this.13
There is uncertainty regarding the magnitude of risk in real-world clinical practice, particularly regarding addiction, suicidality, and precipitation or worsening of psychotic disorders. For example, note the extensive exclusion criteria used in the psilocybin vs escitalopram RCT by Carhart-Harris et al12: currently or previously diagnosed psychotic disorder, immediate family member with a diagnosed psychotic disorder, significant medical comorbidity (eg, diabetes, epilepsy, severe cardiovascular disease, hepatic or renal failure), history of suicide attempts requiring hospitalization, history of mania, pregnancy, and abnormal QT interval prolongation, among others. It would be prudent to keep these contraindications in mind regarding the clinical use of psychedelics in the future. This is particularly important in older adults because such patients often have substantial medical comorbidities and are at greater risk for adverse effects. For ketamine, research has implicated the role of mu opioid agonism in mediating ketamine’s antidepressant effects.54 This raises concerns about abuse, dependence, and addiction, especially with long-term use. There are also concerns regarding protracted withdrawal symptoms and associated suicidality.55
The therapeutic use of psychedelics is an exciting and promising avenue, with ongoing research and a rapidly evolving literature. An attitude of cautious optimism is warranted, but efficacy and safety should be demonstrated in well-designed and rigorous trials with adequate long-term follow-up before routine clinical use is recommended.
Bottom Line
In clinical trials for psychiatric disorders, psychedelics have been associated with a range of cognitive, psychiatric, and psychoactive adverse effects but generally have been well-tolerated, with a low incidence of serious adverse effects.
Related Resources
- American Psychiatric Association. Position Statement on the Use of Psychedelic and Empathogenic Agents for Mental Health Conditions. Updated July 2022. Accessed October 24, 2022. https://www.psychiatry.org/getattachment/d5c13619-ca1f-491f-a7a8-b7141c800904/Position-Use-of-Psychedelic-Empathogenic-Agents.pdf
- Johns Hopkins Center for Psychedelic & Consciousness Research. https://hopkinspsychedelic.org/
- Multidisciplinary Association for Psychedelic Studies (MAPS). https://maps.org/
Drug Brand Names
Esketamine • Spravato
Ketamine • Ketalar
Venlafaxine • Effexor
Psychedelics are a class of substances known to produce alterations in consciousness and perception. In the last 2 decades, psychedelic research has garnered increasing attention from scientists, therapists, entrepreneurs, and the public. While many of these compounds remain illegal in the United States and in many parts of the world (Box1), a recent resurrection of psychedelic research has motivated the FDA to designate multiple psychedelic compounds as “breakthrough therapies,” thereby expediting the investigation, development, and review of psychedelic treatments.
Box
The legal landscape of psychedelics is rapidly evolving. Psilocybin use has been decriminalized in many cities in the United States (such as Denver), and some states (such as Oregon) have legalized it for therapeutic use.
It is important to understand the difference between decriminalization and legalization. Decriminalization means the substance is still prohibited under existing laws, but the legal system will choose not to enforce the prohibition. Legalization is the rescinding of laws prohibiting the use of the substance. In the United States, these laws may be state or federal. Despite psilocybin legalization for therapeutic use in Oregon and decriminalization in various cities, psychedelics currently remain illegal under federal law.
Source: Reference 1
There is growing evidence that psychedelics may be efficacious for treating a range of psychiatric disorders. Potential clinical indications for psychedelics include some forms of depression, posttraumatic stress disorder (PTSD), and substance use disorders (Table 12,3). In most instances, the clinical use of psychedelics is being investigated and offered in the context of psychedelic-assisted psychotherapy, though ketamine is a prominent exception. Ketamine and esketamine are already being used to treat depression, and FDA approval is anticipated for other psychedelics.
This article examines the adverse effect profile of classical (psilocybin [“mushrooms”], lysergic acid diethylamide [LSD], and N,N-dimethyltryptamine [DMT]/ayahuasca) and nonclassical (the entactogen 3,4-methylenedioxymethamphetamine [MDMA, known as “ecstasy”] and the dissociative anesthetic ketamine) psychedelics.
Psilocybin
Psilocybin is typically administered as a single dose of 10 to 30 mg and used in conjunction with preintegration and postintegration psychotherapy. Administration of psilocybin typically produces perceptual distortions and mind-altering effects, which are mediated through 5-HT2A brain receptor agonistic action.4 The acute effects last approximately 6 hours.5 While psilocybin has generated promising results in early clinical trials,3 the adverse effects of these agents have received less attention.
The adverse effect profile of psilocybin in adults appears promising but its powerful psychoactive effects necessitate cautious use.6 It has a very wide therapeutic index, and in a recent meta-analysis of psilocybin for depression, no serious adverse effects were reported in any of the 7 included studies.7 Common adverse effects in the context of clinical use include anxiety, dysphoria, confusion, and an increase in blood pressure and heart rate.6 Due to potential cardiac effects, psilocybin is contraindicated in individuals with cardiovascular and cerebrovascular disease.8 In recreational/nonclinical use, reactions such as suicidality, violence, convulsions, panic attacks, paranoia, confusion, prolonged dissociation, and mania have been reported.9,10 Animal and human studies indicate the risk of abuse and physical dependence is low. Major national surveys indicate low rates of abuse, treatment-seeking, and harm.11 In a recent 6-week randomized controlled trial (RCT) of psilocybin vs escitalopram for depression,12 no serious adverse events were reported. Adverse events reported in the psilocybin group in this trial are listed in Table 2.12
A recent phase 2 double-blind trial of single-dose psilocybin (1 mg, 10 mg, and 25 mg) for treatment-resistant depression (N = 233) sheds more light on the risk of adverse effects.13 The percentage of individuals experiencing adverse effects on Day 1 of administration was high: 61% in the 25 mg psilocybin group. Headache, nausea, fatigue, and dizziness were the most common effects. The incidence of any adverse event in the 25 mg group was 56% from Day 2 to Week 3, and 29% from Week 3 to Week 12. Suicidal ideation, suicidal behavior, or self-injury occurred in all 3 dose groups. Overall, 14% in the 25 mg group, 17% in the 10 mg group, and 9% in the 1 mg group showed worsening of suicidality from baseline to Week 3. Suicidal behavior was reported by 3 individuals in the 25 mg group after Week 3. The new-onset or worsening of preexisting suicidality with psilocybin reported in this study requires further investigation.
Lysergic acid diethylamide
LSD is similar to psilocybin in its agonistic action at the 5-HT2A brain receptors.4 It is typically administered as a single 100 to 200 μg dose and is used in conjunction with preintegration and postintegration psychotherapy.14 Its acute effects last approximately 12 hours.15
Continue to: Like psilocybin...
Like psilocybin, LSD has a wide therapeutic index. Commonly reported adverse effects of LSD are increased anxiety, dysphoria, and confusion. LSD can also lead to physiological adverse effects, such as increased blood pressure and heart rate, and thus is contraindicated in patients with severe heart disease.6 In a systematic review of the therapeutic use of LSD that included 567 participants,16 2 cases of serious adverse events were reported: a tonic-clonic seizure in a patient with a prior history of seizures, and a case of prolonged psychosis in a 21-year-old with a history of psychotic disorder.
Though few psychedelic studies have examined the adverse effects of these agents in older adults, a recent phase 1 study that recruited 48 healthy older adults (age 55 to 75) found that, compared to placebo, low doses (5 to 20 μg) of LSD 2 times a week for 3 weeks had similar adverse effects, cognitive impairment, or balance impairment.17 The only adverse effect noted to be different between the placebo group and active treatment groups was headache (50% for LSD 10 μg, 25% for LSD 20 μg, and 8% for placebo). Because the dose range (5 to 20 μg) used in this study was substantially lower than the typical therapeutic dose range of 100 to 200 μg, these results should not be interpreted as supporting the safety of LSD at higher doses in older adults.
DMT/ayahuasca
Ayahuasca is a plant-based psychedelic that contains an admixture of substances, including DMT, which acts as a 5-HT2A receptor agonist. In addition to DMT, ayahuasca also contains the alkaloid harmaline, which acts as a monoamine inhibitor. Use of ayahuasca can therefore pose a particular risk for individuals taking other serotonergic or noradrenergic medications or substances. The acute effects of DMT last approximately 4 hours,18 and acute administration of ayahuasca leads to a transient modified state of consciousness that is characterized by introspection, visions, enhanced emotions, and recall of personal memories.19 Research shows ayahuasca has been dosed at approximately 0.36 mg/kg of DMT for 1 dosing session alongside 6 2-hour therapy sessions.20
A recent review by Orsolini et al21 consolidated 40 preclinical, observational, and experimental studies of ayahuasca, and this compound appeared to be safe and well-tolerated; the most common adverse effects were transient emesis and nausea. In an RCT by Palhano-Fontes et al,20 nausea was observed in 71% of participants in the ayahuasca group (vs 26% placebo), vomiting in 57% of participants (vs 0% placebo), and restlessness in 50% of participants (vs 20% placebo). The authors noted that for some participants the ayahuasca session “was not necessarily a pleasant experience,” and was accompanied by psychological distress.20 Vomiting is traditionally viewed as an expected part of the purging process of ayahuasca religious ceremonies. Another review found that there appears to be good long-term tolerability of ayahuasca consumption among individuals who use this compound in religious ceremonies.22
MDMA
Entactogens (or empathogens) are a class of psychoactive substances that produce experiences of emotional openness and connection. MDMA is an entactogen known to release serotonin, norepinephrine, and dopamine by inhibiting reuptake.23 This process leads to the stimulation of neurohormonal signaling of oxytocin, cortisol, and other signaling molecules such as brain-derived neurotrophic factor.24 Memory reconsolidation and fear extinction may also play a therapeutic role, enabled by reduced activity in the amygdala and insula, and increased connectivity between the amygdala and hippocampus.24 MDMA has been reported to enhance feelings of well-being and increase prosocial behavior.25 In the therapeutic setting, MDMA has been generally dosed at 75 to 125 mg in 2 to 3 sessions alongside 10 therapy sessions. Administration of MDMA gives the user a subjective experience of energy and distortions in time and perception.26 These acute effects last approximately 2 to 4 hours.27
Continue to: A meta-analysis...
A meta-analysis of 5 RCTs of MDMA-assisted therapy for PTSD in adults demonstrated that MDMA was well-tolerated, and few serious adverse events were reported.28 Two trials from 2018 that were included in this meta-analysis—Mithoefer et al29 and Ot’alora et al30—illustrate the incidence of specific adverse effects. In a randomized, double-blind trial of 26 veterans and first responders with chronic PTSD, Mithoefer et al29 found the most commonly reported reactions during experimental sessions with MDMA were anxiety (81%), headache (69%), fatigue (62%), muscle tension (62%), and jaw clenching or tight jaw (50%). The most commonly reported reactions during 7 days of contact were fatigue (88%), anxiety (73%), insomnia (69%), headache (46%), muscle tension (46%), and increased irritability (46%). One instance of suicidal ideation was severe enough to require psychiatric hospitalization (this was the only instance of suicidal ideation among the 106 patients in the meta-analysis by Bahji et al28); the patient subsequently completed the trial. Transient elevation in pulse, blood pressure, and body temperature were noted during sessions that did not require medical intervention.29 Ot’alora et al30 found similar common adverse reactions: anxiety, dizziness, fatigue, headache, jaw clenching, muscle tension, and irritability. There were no serious adverse effects.
While the use of MDMA in controlled interventional settings has resulted in relatively few adverse events, robust literature describes the risks associated with the nonclinical/recreational use of MDMA. In cases of MDMA toxicity, death has been reported.31 Acutely, MDMA may lead to sympathomimetic effects, including serotonin syndrome.31 Longer-term studies of MDMA users have found chronic recreational use to be associated with worse sleep, poor mood, anxiety disturbances, memory deficits, and attention problems.32 MDMA has also been found to have moderate potential for abuse.33
Ketamine/esketamine
Ketamine is a dissociative anesthetic with some hallucinogenic effects. It is an N-methyl-
Esketamine, the S(+)-enantiomer of ketamine, is also an NDMA antagonist. It has been developed as an intranasal formulation, typically dosed between 56 and 84 mg 2 times a week for 1 month, once a week for the following month, and once every 1 to 2 weeks thereafter.35 In most ketamine and esketamine trials, these compounds have been used without psychotherapy, although some interventions have integrated psychotherapy with ketamine treatment.36
Bennett et al37 elaborated on 3 paradigms for ketamine treatment: biochemical, psychotherapeutic, and psychedelic. The biochemical model examines the neurobiological effects of the medication. The psychotherapeutic model views ketamine as a way of assisting the psychotherapy process. The psychedelic model utilizes ketamine’s dissociative and psychedelic properties to induce an altered state of consciousness for therapeutic purposes and psychospiritual exploration.
Continue to: A systematic review...
A systematic review of the common adverse effects associated with ketamine use in clinical trials for depression reported dissociation, sedation, perceptual disturbances, anxiety, agitation, euphoria, hypertension, tachycardia, headache, and dizziness.38 Adverse effects experienced with esketamine in clinical trials include dissociation, dizziness, sedation, hypertension, hypoesthesia, gastrointestinal symptoms, and euphoric mood (Table 339). A recent systemic review found both ketamine and esketamine demonstrated higher adverse events than control conditions. IV ketamine also demonstrated lower dropouts and adverse events when compared to intranasal esketamine.40

Nonclinical/recreational use of ketamine is notable for urinary toxicity; 20% to 30% of frequent users of ketamine experience urinary problems that can range from ketamine-induced cystitis to hydronephrosis and kidney failure.41 Liver toxicity has also been reported with chronic use of high-dose ketamine. Ketamine is liable to abuse, dependence, and tolerance. There is evidence that nonclinical use of ketamine may lead to morbidity; impairment of memory, cognition, and attention; and urinary, gastric, and hepatic pathology.42
The FDA prescribing information for esketamine lists aneurysmal vascular disease, arteriovenous malformation, and intracerebral hemorrhage as contraindications.39 Patients with cardiovascular and cerebrovascular conditions and risk factors may be at increased risk of adverse effects due to an increase in blood pressure. Esketamine can impair attention, judgment, thinking, reaction speed, and motor skills. Other adverse effects of esketamine noted in the prescribing information include dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, vomiting, feeling drunk, and euphoric mood.39A study of postmarketing safety concerns with esketamine using reports submitted to the FDA Adverse Event Reporting System (FAERS) revealed signals for suicidal ideation (reporting odds ratio [ROR] 24.03; 95% CI, 18.72 to 30.84), and completed suicide (ROR 5.75; 95% CI, 3.18 to 10.41).43 The signals for suicidal and self-injurious ideation remained significant when compared to venlafaxine in the FAERS database, while suicide attempts and fatal suicide attempts were no longer significant.43 Concerns regarding acute ketamine withdrawal have also been described in case reports.44
Other safety considerations of psychedelics
Hallucinogen persisting perception disorder
Hallucinogen persisting perception disorder (HPPD) is a rare condition associated with hallucinogen use. It is characterized by the recurrence of perceptual disturbances that an individual experienced while using hallucinogenic substances that creates significant distress or impairment.45 Because HPPD is a rare disorder, the exact prevalence is not well characterized, but DSM-5 suggests it is approximately 4.2%.46 HPPD is associated with numerous psychoactive substances, including psilocybin, ayahuasca, MDMA, and ketamine, but is most associated with LSD.45 HPPD is more likely to arise in individuals with histories of psychiatric illness or substance use disorders.47
Serotonin toxicity and other serotonergic interactions
Serotonin toxicity is a risk of serotonergic psychedelics, particularly when such agents are used in combination with serotonergic psychotropic medications. The most severe manifestation of serotonin toxicity is serotonin syndrome, which manifests as a life-threatening condition characterized by myoclonus, rigidity, agitation, delirium, and unstable cardiovascular functioning. Many psychedelic compounds have transient serotonin-related adverse effects, but serotonin toxicity due to psychedelic use is rare.48 Due to their mechanism of action, classical psychedelics are relatively safe in combination with monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors. MDMA is a serotonin-releasing agent that has a higher risk of serotonin syndrome or hypertensive crisis when used in combination with MAOIs.48
Boundary violations in psychedelic-assisted psychotherapy
A key task facing psychedelic research is to establish parameters for the safe and ethical use of these agents. This is particularly relevant given the hype that surrounds the psychedelic resurgence and what we know about the controversial history of these substances. Anderson et al49 argued that “psychedelics can have lingering effects that include increased suggestibility and affective instability, as well as altered ego structure, social behaviour, and philosophical worldview. Stated simply, psychedelics can induce a vulnerable state both during and after treatment sessions.”
Continue to: Psychedelic treatment...
Psychedelic treatments such as psilocybin and MDMA are typically offered within the context of psychedelic-assisted psychotherapy, and some researchers have raised concerns regarding boundary violations,50 given the patients’ particularly vulnerable states. In addition to concerns about sexual harassment, the financial exploitation of older adults is also a possible risk.51
Caveats to consider
Novel psychedelics therapies have demonstrated promising preliminary results for a broad range of psychiatric indications, including depression, end-of-life distress, substance use disorders, PTSD, and improving well-being. To date, psychedelics are generally well-tolerated in adults in clinical trials.
However, when it comes to adverse effects, there are challenges in regards to interpreting the psychedelic state.52 Some consider any unpleasant or unsettling psychedelic experience as an adverse reaction, while others consider it part of the therapeutic process. This is exemplified by the case of vomiting during ayahuasca ceremonies, which is generally considered part of the ritual. In such instances, it is essential to obtain informed consent and ensure participants are aware of these aspects of the experience. Compared to substances such as alcohol, opioids, and cocaine, psychedelics are remarkably safe from a physiological perspective, especially with regards to the risks of toxicity, mortality, and dependence.53 Their psychological safety is less established, and more caution and research is needed. The high incidence of adverse effects and suicidality noted in the recent phase 2 trial of psilocybin in treatment resistant depression are a reminder of this.13
There is uncertainty regarding the magnitude of risk in real-world clinical practice, particularly regarding addiction, suicidality, and precipitation or worsening of psychotic disorders. For example, note the extensive exclusion criteria used in the psilocybin vs escitalopram RCT by Carhart-Harris et al12: currently or previously diagnosed psychotic disorder, immediate family member with a diagnosed psychotic disorder, significant medical comorbidity (eg, diabetes, epilepsy, severe cardiovascular disease, hepatic or renal failure), history of suicide attempts requiring hospitalization, history of mania, pregnancy, and abnormal QT interval prolongation, among others. It would be prudent to keep these contraindications in mind regarding the clinical use of psychedelics in the future. This is particularly important in older adults because such patients often have substantial medical comorbidities and are at greater risk for adverse effects. For ketamine, research has implicated the role of mu opioid agonism in mediating ketamine’s antidepressant effects.54 This raises concerns about abuse, dependence, and addiction, especially with long-term use. There are also concerns regarding protracted withdrawal symptoms and associated suicidality.55
The therapeutic use of psychedelics is an exciting and promising avenue, with ongoing research and a rapidly evolving literature. An attitude of cautious optimism is warranted, but efficacy and safety should be demonstrated in well-designed and rigorous trials with adequate long-term follow-up before routine clinical use is recommended.
Bottom Line
In clinical trials for psychiatric disorders, psychedelics have been associated with a range of cognitive, psychiatric, and psychoactive adverse effects but generally have been well-tolerated, with a low incidence of serious adverse effects.
Related Resources
- American Psychiatric Association. Position Statement on the Use of Psychedelic and Empathogenic Agents for Mental Health Conditions. Updated July 2022. Accessed October 24, 2022. https://www.psychiatry.org/getattachment/d5c13619-ca1f-491f-a7a8-b7141c800904/Position-Use-of-Psychedelic-Empathogenic-Agents.pdf
- Johns Hopkins Center for Psychedelic & Consciousness Research. https://hopkinspsychedelic.org/
- Multidisciplinary Association for Psychedelic Studies (MAPS). https://maps.org/
Drug Brand Names
Esketamine • Spravato
Ketamine • Ketalar
Venlafaxine • Effexor
1. The current legal status of psychedelics in the United States. Investing News Network. August 23, 2022. Accessed August 26, 2022. https://investingnews.com/legal-status-of-psychedelics-in-the-united-states/
2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
3. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. 2021;78(2):121-122.
4. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
5. Hasler F, Grimberg U, Benz MA et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145-156.
6. Johnson MW, Hendricks PS, Barrett FS, et al. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. 2019;197:83-102.
7. Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26-34.
8. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
9. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15(4):283-300.
10. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423-429.
11. Johnson MW, Griffiths RR, Hendricks PS, et al. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143-166.
12. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl Med. 2021;384(15):1402-1411.
13. Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant Episode of major depression. N Engl J Med. 2022;387(18):1637-1648.
14. Galvão-Coelho NL, Marx W, Gonzalez M, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology (Berl). 2021;238(2):341-354.
15. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
16. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry. 2020;10:943.
17. Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl). 2020;237(3):841-853.
18. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol. 2016;7:35.
19. Domínguez-Clavé E, Solar J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull. 2016;126(Pt 1):89-101.
20. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
21. Orsolini L, Chiappini S, Papanti D, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol: Clin Exp. 2020;35(3):e2728.
22. Durante Í, Dos Santos RG, Bouso JC, et al. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2021;43(4):362-369.
23. Sessa B, Higbed L, Nutt D. A review of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry. 2019;10:138.
24. Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Progress Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228.
25. Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affective Neurosc. 2014;9(11):1645-1652.
26. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917-928.
27. Dumont GJ, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20(2):176-187.
28. Bahji A, Forsyth A, Groll D, et al. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109735.
29. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
30. Ot’alora GM, Grigsby J, Poulter B, et al. 3,4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295-1307.
31. Steinkellner T, Freissmuth M, Sitte HH, et al. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392(1-2):103-115.
32. Montoya AG, Sorrentino R, Lukas SE, et al. Long-term neuropsychiatric consequences of “ecstasy” (MDMA): a review. Harvard Rev Psychiatry. 2002;10(4):212-220.
33. Yazar‐Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194-196.
34. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
35. Thase M, Connolly KR. Ketamine and esketamine for treating unipolar depression in adults: administration, efficacy, and adverse effects. Wolters Kluwer; 2019. Accessed August 26, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects
36. Dore J, Turnispeed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198.
37. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
38. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78.
39. U.S. Food and Drug Administration. SPRAVATO® (esketamine). Prescribing information. Janssen; 2020. Accessed August 26, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf
40. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affective Disord. 2021;278:542-555.
41. Castellani D, Pirola GM, Gubbiotti M, et al. What urologists need to know about ketamine-induced uropathy: a systematic review. Neurourol Urodyn. 2020;39(4):1049-1062.
42. Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582-586.
43. Gastaldon, C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Psychother Psychosom. 2021;90(1):41-48.
44. Roxas N, Ahuja C, Isom J, et al. A potential case of acute ketamine withdrawal: clinical implications for the treatment of refractory depression. Am J Psychiatry. 2021;178(7):588-591.
45. Orsolini L, Papanti GD, De Berardis D, et al. The “Endless Trip” among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatry Association; 2013.
47. Martinotti G, Santacroce R, Pettorruso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8(3):47.
48. Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022;239(6):1881-1891.
49. Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry, 2020;7(10):829-830.
50. Goldhill O. Psychedelic therapy has a sexual abuse problem. QUARTZ. March 3, 2020. Accessed August 26, 2022. https://qz.com/1809184/psychedelic-therapy-has-a-sexual-abuse-problem-3/
51. Goldhill O. A psychedelic therapist allegedly took millions from a Holocaust survivor, highlighting worries about elders taking hallucinogens. STAT News. April 21, 2022. Accessed August 26, 2022. https://www.statnews.com/2022/04/21/psychedelic-therapist-allegedly-took-millions-from-holocaust-survivor-highlighting-worries-about-elders-taking-hallucinogens/
52. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577-595.
53. Nutt D. Drugs Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge Ltd; 2012.
54. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
55. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422-424.
1. The current legal status of psychedelics in the United States. Investing News Network. August 23, 2022. Accessed August 26, 2022. https://investingnews.com/legal-status-of-psychedelics-in-the-united-states/
2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
3. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. 2021;78(2):121-122.
4. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
5. Hasler F, Grimberg U, Benz MA et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145-156.
6. Johnson MW, Hendricks PS, Barrett FS, et al. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. 2019;197:83-102.
7. Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26-34.
8. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
9. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15(4):283-300.
10. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423-429.
11. Johnson MW, Griffiths RR, Hendricks PS, et al. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143-166.
12. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl Med. 2021;384(15):1402-1411.
13. Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant Episode of major depression. N Engl J Med. 2022;387(18):1637-1648.
14. Galvão-Coelho NL, Marx W, Gonzalez M, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology (Berl). 2021;238(2):341-354.
15. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
16. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry. 2020;10:943.
17. Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl). 2020;237(3):841-853.
18. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol. 2016;7:35.
19. Domínguez-Clavé E, Solar J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull. 2016;126(Pt 1):89-101.
20. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
21. Orsolini L, Chiappini S, Papanti D, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol: Clin Exp. 2020;35(3):e2728.
22. Durante Í, Dos Santos RG, Bouso JC, et al. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2021;43(4):362-369.
23. Sessa B, Higbed L, Nutt D. A review of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry. 2019;10:138.
24. Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Progress Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228.
25. Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affective Neurosc. 2014;9(11):1645-1652.
26. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917-928.
27. Dumont GJ, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20(2):176-187.
28. Bahji A, Forsyth A, Groll D, et al. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109735.
29. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
30. Ot’alora GM, Grigsby J, Poulter B, et al. 3,4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295-1307.
31. Steinkellner T, Freissmuth M, Sitte HH, et al. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392(1-2):103-115.
32. Montoya AG, Sorrentino R, Lukas SE, et al. Long-term neuropsychiatric consequences of “ecstasy” (MDMA): a review. Harvard Rev Psychiatry. 2002;10(4):212-220.
33. Yazar‐Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194-196.
34. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
35. Thase M, Connolly KR. Ketamine and esketamine for treating unipolar depression in adults: administration, efficacy, and adverse effects. Wolters Kluwer; 2019. Accessed August 26, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects
36. Dore J, Turnispeed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198.
37. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
38. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78.
39. U.S. Food and Drug Administration. SPRAVATO® (esketamine). Prescribing information. Janssen; 2020. Accessed August 26, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf
40. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affective Disord. 2021;278:542-555.
41. Castellani D, Pirola GM, Gubbiotti M, et al. What urologists need to know about ketamine-induced uropathy: a systematic review. Neurourol Urodyn. 2020;39(4):1049-1062.
42. Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582-586.
43. Gastaldon, C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Psychother Psychosom. 2021;90(1):41-48.
44. Roxas N, Ahuja C, Isom J, et al. A potential case of acute ketamine withdrawal: clinical implications for the treatment of refractory depression. Am J Psychiatry. 2021;178(7):588-591.
45. Orsolini L, Papanti GD, De Berardis D, et al. The “Endless Trip” among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatry Association; 2013.
47. Martinotti G, Santacroce R, Pettorruso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8(3):47.
48. Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022;239(6):1881-1891.
49. Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry, 2020;7(10):829-830.
50. Goldhill O. Psychedelic therapy has a sexual abuse problem. QUARTZ. March 3, 2020. Accessed August 26, 2022. https://qz.com/1809184/psychedelic-therapy-has-a-sexual-abuse-problem-3/
51. Goldhill O. A psychedelic therapist allegedly took millions from a Holocaust survivor, highlighting worries about elders taking hallucinogens. STAT News. April 21, 2022. Accessed August 26, 2022. https://www.statnews.com/2022/04/21/psychedelic-therapist-allegedly-took-millions-from-holocaust-survivor-highlighting-worries-about-elders-taking-hallucinogens/
52. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577-595.
53. Nutt D. Drugs Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge Ltd; 2012.
54. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
55. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422-424.
Resilience and mind-body interventions in late-life depression
Resilience has been defined as the ability to adapt and thrive in the face of adversity, acute stress, or trauma.1 Originally conceived as an inborn trait characteristic, resilience is now conceptualized as a dynamic, multidimensional capacity influenced by the interactions between internal factors (eg, personality, cognitive capacity, physical health) and environmental resources (eg, social status, financial stability).2,3 Resilience in older adults (typically defined as age ≥65) can improve the prognosis and outcomes for physical and mental conditions.4 The construct is closely aligned with “successful aging” and can be fostered in older adults, leading to improved physical and mental health and well-being.5
While initially resilience was conceptualized as the opposite of depressive states, recent research has identified resilience in the context of major depressive disorder (MDD) as the net effects of various psychosocial and biological variables that decrease the risk of onset, relapse, or depressive illness severity and increase the probability or speed of recovery.6 Late-life depression (LLD) in adults age >65 is a common and debilitating disease, often leading to decreased psychological well-being, increased cognitive decline, and excess mortality.7,8 LLD is associated with several factors, such as cerebrovascular disease, neurodegenerative disease, and inflammation, all of which could contribute to brain vulnerability and an increased risk of depression.9 Physical and cognitive engagement, physical activity, and high brain reserve have been shown to confer resilience to affective and cognitive changes in older adults, despite brain vulnerability.9
The greatest levels of resilience have been observed in individuals in their fifth decade of life and later,4,10 with high levels of resilience significantly contributing to longevity5; however, little is known about which factors contribute to heterogeneity in resilience characteristics and outcomes.4 Furthermore, the concept of resilience continues to raise numerous questions, including:
- how resilience should be measured or defined
- what factors promote or deter the development of resilience
- the effects of resilience on various health and psychological outcomes
- which interventions are effective in enhancing resilience in older adults.4
In this article, we describe resilience in older adults with LLD, its clinical and neurocognitive correlates, and underlying neurobiological and immunological biomarkers. We also examine resilience-building interventions, such as mind-body therapies (MBTs), that have been shown to enhance resilience by promoting positive perceptions of difficult experiences and challenges.
Clinical and neurocognitive correlates of resilience
Resilience varies substantially among older adults with LLD as well as across the lifespan of an individual.11 Identifying clinical components and predictors of resilience may usefully inform the development and testing of interventions to prevent and treat LLD.11 One tool widely used to measure resilience—the self-report Connor-Davidson Resilience Scale (CD-RISC)12— has been found to have clinically relevant characteristics.1,11 Using data from 337 older adults with LLD, Laird et al11 performed an exploratory factor analysis of the CD-RISC and found a 4-factor model:
- grit
- adaptive coping self-efficacy
- accommodative coping self-efficacy
- spirituality.1,11
Having a strong sense of purpose and not being easily discouraged by failure were items characteristic of grit.1,11 The preference to take the lead in problem-solving was typical of items loading on adaptive coping self-efficacy, while accommodative coping self-efficacy measured flexibility, cognitive reframing, a sense of humor, and acceptance in the face of uncontrollable stress.1,11 Finally, the belief that “things happen for a reason” and that “sometimes fate or God can help me” are characteristics of spirituality. 1,11 Using a multivariate model, the greatest variance in total resilience scores was explained by less depression, less apathy, higher quality of life, non-White race, and, somewhat counterintuitively, greater medical comorbidity.1,11 Thus, interventions designed to help older adults cultivate grit, active coping, accommodative coping, and spirituality may enhance resilience in LLD.
Resilience may also be positively associated with cognitive functioning and could be neuroprotective in LLD.13 Laird et al13 investigated associations between baseline resilience and several domains of neurocognitive functioning in 288 older adults with LLD. Several positive associations were found between measured language performance and total resilience, active coping, and accommodative coping.13 Additionally, total resilience and accommodative coping were significantly associated with a lower self-reported frequency of forgetfulness, a subjective measure of memory used in this study.13 Together, these results suggest that interventions targeting language might be useful to improve coping in LLD.13 Another interesting finding was that the resilience subdomain of spirituality was negatively associated with memory, language, and executive functioning performance.13 A distinction must be made between religious attendance (eg, regular attendance at religious institutions) vs religious beliefs, which may account for the previously reported associations between spirituality and improved cognition.13
Continue to: Self-reported resilience...
Self-reported resilience may also predict greater responsivity to antidepressant medication in patients with LLD.14 Older adults with LLD and greater self-reported baseline resilience were more likely to experience improvement or remission from depression with antidepressant treatment.14 This is congruent with conceptualizations of resilience as “the ability to adapt to and recover from stress.”14,15 Of the 4 identified resilience factors (grit, adaptive coping, accommodative coping, and spirituality), it appears that accommodative coping predicts LLD treatment response and remission.14 The unique ability to accommodate is associated with better mental health outcomes in the face of uncontrollable stress.14,16-18 Older adults appear to engage in more accommodative coping due to frequent uncontrollable stress and aging-related physiological changes (eg, sleep changes, chronic pain, declining cognition). This could make accommodative coping especially important in this population.14,19
The Figure, adapted from Weisenbach et al,9 exhibits factors that contribute to LLD, including cerebrovascular disease, neurodegeneration, and chronic inflammation, all of which can lead to a vulnerable aging brain that is at higher risk for depression, particularly within the context of stress. Clinical and neurocognitive factors associated with resilience can help buffer vulnerable brains from developing depression.
Neurobiological biomarkers of resilience in
Gross anatomical indicators: Findings from neuroimaging
The neurobiology underlying psychological resilience involves brain networks associated with stress response, negative affect, and emotional control.19 Increased amygdala reactivity and amygdala frontal connectivity are often implicated in neurobiological models of resilience.20 Leaver et al20 correlated psychological resilience measures with amygdala function in 48 depressed vs nondepressed individuals using functional magnetic resonance imaging. Specifically, they targeted the basolateral, centromedial, and superficial nuclei groups of the amygdala while comparing the 2 groups based on resilience scores (CD-RISC), depressive symptom severity, and depression status.20 A significant correlation was identified between resilience and connectivity between the superficial group of amygdala nuclei and the ventral default mode network (VDMN).20 High levels of psychological resilience were associated with lower basal amygdala activity and decreased connectivity between amygdala nuclei and the VDMN.20 Additionally, lower depressive symptoms were associated with higher connectivity between the amygdalae and the dorsal frontal networks.20 These results suggest a complex relationship between amygdala activity, dorsal frontal regions, resilience, and LLD.20
Vlasova et al21 further addressed the multifactorial character of psychological resilience. The associations between the 4 factors of resilience and the regional integrity of white matter in older adults with LLD were examined using diffusion-weighted MRI.21 Grit was found to be associated with greater white matter integrity in the genu of the corpus callosum and cingulum bundle in LLD.21 There was also a positive association between grit and fractional anisotropy (FA) in the callosal region connecting the prefrontal cortex and FA in the cingulum fibers.21 However, results regarding the FA in the cingulum fibers did not survive correction for multiple comparisons and should be considered with caution, pending further research.21
Continue to: Stress response biomarkers of resilience
Stress response biomarkers of resilience
Stress response biomarkers include endocrine, immune, and inflammatory indices. Stress has been identified as a factor in inflammatory responses. Stress-related overstimulation of the HPA axis may increase the risk of LLD.22 Numerous studies have demonstrated an association between increased levels of peripheral proinflammatory cytokines and depressive symptoms in older adults.23 Interleukin-6 (IL-6) has been increasingly linked with depressive symptoms and poor memory performance in older adults.9 There also appears to be an interaction of inflammatory and vascular processes predisposing to LLD, as increased levels of IL-6 and C-reactive protein have been associated with higher white matter pathology.9 Additionally, proinflammatory cytokines impact monoamine neurotransmitter pathways, leading to a reduction in tryptophan and serotonin synthesis, disruption of glucocorticoid receptors, and a decrease in hippocampal neurotrophic support.9 Alexopoulos et al24 further explain that a prolonged CNS immune response can affect emotional and cognitive network functions related to LLD and has a role in the etiology of depressive symptoms in older adults.
Cardiovascular comorbidity and autonomic nervous system dysfunction
Many studies have revealed evidence of a bidirectional association between cardiovascular disease and depression.25 Dysregulation of the autonomic nervous system (ANS) is an underlying mechanism that could explain the link between cardiovascular risk and MDD via heart rate variability (HRV), though research examining age-related capacities provide conflicting data.25,26 HRV is a surrogate index of resting cardiac vagal outflow that represents the ability of the ANS to adapt to psychological, social, and physical environmental changes.27 Higher overall HRV is associated with greater self-regulating capacity, including behavioral, cognitive, and emotional control.28 Additionally, higher HRV may serve as a biomarker of resilience to the development of stress-related disorders such as MDD. Recent studies have shown an overall reduction in HRV in older adults with LLD.29 When high- and low-frequency HRV were investigated separately, only low-frequency HRV was significantly reduced in patients with depression.29 One explanation is that older adults with depression have impaired or reduced baroreflex sensitivity and gain, which is often associated with an increased risk of mortality following cardiac events.30 More research is needed to examine the complex processes required to better characterize the correlation between resilience in cardiovascular disease and autonomic dysfunction.
The Box6,31,32 describes the relationship between markers of cellular health and resilience.
Box
Among the biomarkers of resilience, telomere length and telomerase activity serve as biomarkers of biological aging that can differ from the chronological age and mark successful anti-aging, stress-reducing strategies.31 Telomerase, the cellular enzyme that regulates the health of cells when they reproduce (preserving the telomeres, repetitive DNA strands at the ends of chromosomes), is associated with overall cell health and cellular biological age.31 When telomerase is reduced, the telomeres in a cell are clipped, causing the cells to age more rapidly as the telomeres get shorter through the process of cellular reproduction.31 Psychological stress may play a significant role in telomerase production and subsequent telomere length.32 Lavretsky et al32 evaluated the effect of brief daily yogic meditation on depressive symptoms and immune cell telomerase activity in a family of dementia caregivers with mild depressive symptoms. Brief daily meditation practice led to significant lower levels of depressive symptoms that was accompanied by an increase in telomerase activity, suggesting improvement in stress-induced cellular aging.6,32
Mind-body therapies
There is increasing interest in improving older adults’ physical and emotional well-being while promoting resilience through stress-reducing lifestyle interventions such as MBTs.33 Because MBTs are often considered a natural and safer option compared to conventional medicine, these interventions are rapidly gaining popularity in the United States.33,34 According to a 2017 National Health Survey, there were 5% to 10% increases in the use of yoga, meditation, and chiropractic care from 2012 to 2017, with growing evidence supporting MBTs as minimally invasive, cost-effective approaches for managing stress and neurocognitive disorders.35 In contrast to pharmacologic approaches, MBTs can be used to train individuals to self-regulate in the face of adversity and stress, thus increasing their resilience.
MBTs can be divided into mindful movement exercises and meditative practices. Mindful movement exercises include yoga, tai chi, and qigong. Meditative practices that do not include movement include progressive relaxation, mindfulness, meditation, and acceptance therapies. On average, both mindful movement exercise (eg, yoga) and multicomponent mindfulness-based interventions (eg, mindfulness-based cognitive therapy, mindfulness-based stress reduction [MBSR], and mindfulness-based relapse prevention) can be as effective as other active treatments for psychiatric disorders such as MDD, anxiety, and substance use disorders.36,37 MBSR specifically has been shown to increase empathy, self-control, self-compassion, relationship quality, mindfulness, and spirituality as well as decrease rumination in healthy older adults.38 This suggests that MBSR can help strengthen the 4 factors of resilience.
Continue to: Research has also begun...
Research has also begun to evaluate the neurobiological mechanisms by which meditative therapies enhance resilience in mental health disorders, and several promising mechanistic domains (neural, hormonal, immune, cellular, and cardiovascular) have been identified.39 The physical yoga discipline includes asanas (postures), pranayama (breathing techniques), and dhyana (meditation). With the inclusion of mindfulness training, yoga involves the practice of meditation as well as the dynamic combination of proprioceptive and interoceptive awareness, resulting in both attention and profound focus.40 Dedicated yoga practice allows an individual to develop skills to withdraw the senses (pratyahara), concentrate the mind (dharana), and establish unwavering awareness (dhyana).41 The physical and cognitive benefits associated with yoga and mindfulness may be due to mechanisms including pranayama and activation of the parasympathetic nervous system; meditative or contemplative practices; increased body perception; stronger functional connectivity within the basal ganglia; or neuroplastic effects of increased grey matter volume and amygdala with regional enlargement.41 The new learning aspect of yoga practice may contribute to enhancing or improving various aspects of cognition, although the mechanisms are yet to be clarified.
Continued research in this area will promote the integration of MBTs into mainstream clinical practice and help alleviate the increased chronic health burden of an aging population. In the face of the COVID-19 pandemic, public interest in improving resilience and mental health42 can be supported by MBTs that can improve coping with the stress of the pandemic and enhance critical organ function (eg, lungs, heart, brain).43,44 As a result of these limitations, many resources and health care services have used telehealth and virtual platforms to adapt to these challenges and continue offering MBTs.45
Enhancing resilience to improve clinical outcomes
Increasing our understanding of clinical, neurocognitive, and neurobiological markers of resilience in older adults with and without depression could inform the development of interventions that treat and prevent mood and cognitive disorders of aging. Furthermore, stress reduction, decreased inflammation, and improved emotional regulation may have direct neuroplastic effects on the brain, leading to greater resilience. Complementary use of MBTs combined with standard antidepressant treatment may allow for additional improvement in clinical outcomes of LLD, including resilience, quality of life, general health, and cognitive function. Additional research testing the efficacy of those interventions designed to improve resilience in older adults with mood and mental disorders is needed.
Bottom Line
Identifying the clinical, neurocognitive, and neurobiological biomarkers of resilience in late-life depression could aid in the development of targeted interventions that treat and prevent mood and cognitive disorders of aging. Mind-body interventions can help boost resilience and improve outcomes in geriatric patients with mood and cognitive disorders.
Related Resources
- Lavretsky H. Resilience and Aging: Research and Practice. Johns Hopkins University Press; 2014.
- Lavretsky H, Sajatovic M, Reynolds CF, eds. Complementary and Integrative Therapies for Mental Health and Aging. Oxford University Press; 2016.
- Eyre HA, Berk M, Lavretsky H, et al, eds. Convergence Mental Health: A Transdisciplinary Approach to Innovation. Oxford University Press; 2021.
- UCLA Jane & Terry Semel Institute for Neuroscience & Human Behavior. Late-life Depression, Stress, and Wellness Research Program. https://www.semel.ucla.edu/latelife
1. Reynolds CF. Promoting resilience, reducing depression in older adults. Int Psychogeriatr. 2019;31(2):169-171.
2. Windle G. What is resilience? A review and concept analysis. Rev Clin Gerontol. 2011;21(2):152-169.
3. Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338(6103):79-82.
4. Dunn LB, Predescu I. Resilience: a rich concept in need of research comment on: “Neurocognitive correlates of resilience in late-life depression” (by Laird et al.). Am J Geriatr Psychiatry. 2019;27(1):18-20.
5. Harmell AL, Kamat R, Jeste DV, et al. Resilience-building interventions for successful and positive aging. In: Lavretsky H, Sajatovic M, Reynolds C III, eds. Complementary and Integrative Therapies for Mental Health and Aging. Oxford University Press; 2015:305-316.
6. Laird KT, Krause B, Funes C, et al. Psychobiological factors of resilience and depression in late life. Transl Psychiatry. 2019;9(1):88.
7. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
8. Callahan CM, Wolinsky FD, Stump TE, et al. Mortality, symptoms, and functional impairment in late-life depression. J Gen Intern Med. 1998;13(11):746-752.
9. Weisenbach SL, Kumar A. Current understanding of the neurobiology and longitudinal course of geriatric depression. Curr Psychiatry Rep. 2014;16(9):463.
10. Southwick SM, Litz BT, Charney D, et al. Resilience and Mental Health: Challenges Across the Lifespan. Cambridge University Press; 2011.
11. Laird KT, Lavretsky H, Paholpak P, et al. Clinical correlates of resilience factors in geriatric depression. Int Psychogeriatr. 2019;31(2):193-202.
12. Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
13. Laird KT, Lavretsky H, Wu P, et al. Neurocognitive correlates of resilience in late-life depression. Am J Geriatr Psychiatry. 2019;27(1):12-17.
14. Laird KT, Lavretsky H, St Cyr N, et al. Resilience predicts remission in antidepressant treatment of geriatric depression. Int J Geriatr Psychiatry. 2018;33(12):1596-1603.
15. Waugh CE, Koster EH. A resilience framework for promoting stable remission from depression. Clin Psychol Rev. 2015;41:49-60.
16. Boerner K. Adaptation to disability among middle-aged and older adults: the role of assimilative and accommodative coping. J Gerontol B Psychol Sci Soc Sci. 2004;59(1):P35-P42.
17. Zakowski SG, Hall MH, Klein LC, et al. Appraised control, coping, and stress in a community sample: a test of the goodness-of-fit hypothesis. Ann Behav Med. 2001;23(3):158-165.
18. Cheng C, Lau HB, Chan MP. Coping flexibility and psychological adjustment to stressful life changes: a meta-analytic review. Psychol Bull. 2014;140(6):1582-1607.
19. Stokes SA, Gordon SE. Common stressors experienced by the well elderly. Clinical implications. J Gerontol Nurs. 2003;29(5):38-46.
20. Leaver AM, Yang H, Siddarth P, et al. Resilience and amygdala function in older healthy and depressed adults. J Affect Disord. 2018;237:27-34.
21. Vlasova RM, Siddarth P, Krause B, et al. Resilience and white matter integrity in geriatric depression. Am J Geriatr Psychiatry. 2018;26(8):874-883.
22. Chopra K, Kumar B, Kuhad A. Pathobiological targets of depression. Expert Opin Ther Targets. 2011;15(4):379-400.
23. Martínez-Cengotitabengoa M, Carrascón L, O’Brien JT, et al. Peripheral inflammatory parameters in late-life depression: a systematic review. Int J Mol Sci. 2016;17(12):2022.
24. Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26(11):1109-1118.
25. Carney RM, Freedland KE, Sheline YI, et al. Depression and coronary heart disease: a review for cardiologists. Clin Cardiol. 1997;20(3):196-200.
26. Carney RM, Freedland KE, Steinmeyer BC, et al. Nighttime heart rate predicts response to depression treatment in patients with coronary heart disease. J Affect Disord. 2016;200:165-171.
27. Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psych. 2006;10(3):229-240.
28. Holzman JB, Bridgett DJ. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci Biobehav Rev. 2017;74(Pt A):233-255.
29. Brown L, Karmakar C, Gray R, et al. Heart rate variability alterations in late life depression: a meta-analysis. J Affect Disord. 2018;235:456-466.
30. La Rovere MT, Bigger JT Jr, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351(1901):478-484.
31. Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. 2021;184(2):306-322.
32. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
33. Siddiqui MJ, Min CS, Verma RK, et al. Role of complementary and alternative medicine in geriatric care: a mini review. Pharmacogn Rev. 2014;8(16):81-87.
34. Nguyen SA, Lavretsky H. Emerging complementary and integrative therapies for geriatric mental health. Curr Treat Options Psychiatry. 2020;7(4):447-470.
35. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
36. Hofmann SG, Gómez AF. Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am. 2017;40(4):739-749.
37. Ramadas E, de Lima MP, Caetano T, et al. Effectiveness of mindfulness-based relapse prevention in individuals with substance use disorders: a systematic review. Behav Sci (Basel). 2021;11(10):133.
38. Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J Altern Complement Med. 2009;15(5):593-600.
39. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
40. Chobe S, Chobe M, Metri K, et al. Impact of yoga on cognition and mental health among elderly: a systematic review. Complement Ther Med. 2020;52:102421.
41. Brunner D, Abramovitch A, Etherton J. A yoga program for cognitive enhancement. PLoS One. 2017;12(8):e0182366.
42. Dai J, Sang X, Menhas R, et al. The influence of COVID-19 pandemic on physical health-psychological health, physical activity, and overall well-being: the mediating role of emotional regulation. Front Psychol. 2021;12:667461.
43. Grolli RE, Mingoti MED, Bertollo AG, et al. Impact of COVID-19 in the mental health in elderly: psychological and biological updates. Mol Neurobiol. 2021;58(5):1905-1916.
44. Johansson A, Mohamed MS, Moulin TC, et al. Neurological manifestations of COVID-19: a comprehensive literature review and discussion of mechanisms. J Neuroimmunol. 2021;358:577658.
45. Pandya SP. Older women and wellbeing through the pandemic: examining the effect of daily online yoga lessons. Health Care Women Int. 2021;42(11):1255-1278.
Resilience has been defined as the ability to adapt and thrive in the face of adversity, acute stress, or trauma.1 Originally conceived as an inborn trait characteristic, resilience is now conceptualized as a dynamic, multidimensional capacity influenced by the interactions between internal factors (eg, personality, cognitive capacity, physical health) and environmental resources (eg, social status, financial stability).2,3 Resilience in older adults (typically defined as age ≥65) can improve the prognosis and outcomes for physical and mental conditions.4 The construct is closely aligned with “successful aging” and can be fostered in older adults, leading to improved physical and mental health and well-being.5
While initially resilience was conceptualized as the opposite of depressive states, recent research has identified resilience in the context of major depressive disorder (MDD) as the net effects of various psychosocial and biological variables that decrease the risk of onset, relapse, or depressive illness severity and increase the probability or speed of recovery.6 Late-life depression (LLD) in adults age >65 is a common and debilitating disease, often leading to decreased psychological well-being, increased cognitive decline, and excess mortality.7,8 LLD is associated with several factors, such as cerebrovascular disease, neurodegenerative disease, and inflammation, all of which could contribute to brain vulnerability and an increased risk of depression.9 Physical and cognitive engagement, physical activity, and high brain reserve have been shown to confer resilience to affective and cognitive changes in older adults, despite brain vulnerability.9
The greatest levels of resilience have been observed in individuals in their fifth decade of life and later,4,10 with high levels of resilience significantly contributing to longevity5; however, little is known about which factors contribute to heterogeneity in resilience characteristics and outcomes.4 Furthermore, the concept of resilience continues to raise numerous questions, including:
- how resilience should be measured or defined
- what factors promote or deter the development of resilience
- the effects of resilience on various health and psychological outcomes
- which interventions are effective in enhancing resilience in older adults.4
In this article, we describe resilience in older adults with LLD, its clinical and neurocognitive correlates, and underlying neurobiological and immunological biomarkers. We also examine resilience-building interventions, such as mind-body therapies (MBTs), that have been shown to enhance resilience by promoting positive perceptions of difficult experiences and challenges.
Clinical and neurocognitive correlates of resilience
Resilience varies substantially among older adults with LLD as well as across the lifespan of an individual.11 Identifying clinical components and predictors of resilience may usefully inform the development and testing of interventions to prevent and treat LLD.11 One tool widely used to measure resilience—the self-report Connor-Davidson Resilience Scale (CD-RISC)12— has been found to have clinically relevant characteristics.1,11 Using data from 337 older adults with LLD, Laird et al11 performed an exploratory factor analysis of the CD-RISC and found a 4-factor model:
- grit
- adaptive coping self-efficacy
- accommodative coping self-efficacy
- spirituality.1,11
Having a strong sense of purpose and not being easily discouraged by failure were items characteristic of grit.1,11 The preference to take the lead in problem-solving was typical of items loading on adaptive coping self-efficacy, while accommodative coping self-efficacy measured flexibility, cognitive reframing, a sense of humor, and acceptance in the face of uncontrollable stress.1,11 Finally, the belief that “things happen for a reason” and that “sometimes fate or God can help me” are characteristics of spirituality. 1,11 Using a multivariate model, the greatest variance in total resilience scores was explained by less depression, less apathy, higher quality of life, non-White race, and, somewhat counterintuitively, greater medical comorbidity.1,11 Thus, interventions designed to help older adults cultivate grit, active coping, accommodative coping, and spirituality may enhance resilience in LLD.
Resilience may also be positively associated with cognitive functioning and could be neuroprotective in LLD.13 Laird et al13 investigated associations between baseline resilience and several domains of neurocognitive functioning in 288 older adults with LLD. Several positive associations were found between measured language performance and total resilience, active coping, and accommodative coping.13 Additionally, total resilience and accommodative coping were significantly associated with a lower self-reported frequency of forgetfulness, a subjective measure of memory used in this study.13 Together, these results suggest that interventions targeting language might be useful to improve coping in LLD.13 Another interesting finding was that the resilience subdomain of spirituality was negatively associated with memory, language, and executive functioning performance.13 A distinction must be made between religious attendance (eg, regular attendance at religious institutions) vs religious beliefs, which may account for the previously reported associations between spirituality and improved cognition.13
Continue to: Self-reported resilience...
Self-reported resilience may also predict greater responsivity to antidepressant medication in patients with LLD.14 Older adults with LLD and greater self-reported baseline resilience were more likely to experience improvement or remission from depression with antidepressant treatment.14 This is congruent with conceptualizations of resilience as “the ability to adapt to and recover from stress.”14,15 Of the 4 identified resilience factors (grit, adaptive coping, accommodative coping, and spirituality), it appears that accommodative coping predicts LLD treatment response and remission.14 The unique ability to accommodate is associated with better mental health outcomes in the face of uncontrollable stress.14,16-18 Older adults appear to engage in more accommodative coping due to frequent uncontrollable stress and aging-related physiological changes (eg, sleep changes, chronic pain, declining cognition). This could make accommodative coping especially important in this population.14,19
The Figure, adapted from Weisenbach et al,9 exhibits factors that contribute to LLD, including cerebrovascular disease, neurodegeneration, and chronic inflammation, all of which can lead to a vulnerable aging brain that is at higher risk for depression, particularly within the context of stress. Clinical and neurocognitive factors associated with resilience can help buffer vulnerable brains from developing depression.

Neurobiological biomarkers of resilience in
Gross anatomical indicators: Findings from neuroimaging
The neurobiology underlying psychological resilience involves brain networks associated with stress response, negative affect, and emotional control.19 Increased amygdala reactivity and amygdala frontal connectivity are often implicated in neurobiological models of resilience.20 Leaver et al20 correlated psychological resilience measures with amygdala function in 48 depressed vs nondepressed individuals using functional magnetic resonance imaging. Specifically, they targeted the basolateral, centromedial, and superficial nuclei groups of the amygdala while comparing the 2 groups based on resilience scores (CD-RISC), depressive symptom severity, and depression status.20 A significant correlation was identified between resilience and connectivity between the superficial group of amygdala nuclei and the ventral default mode network (VDMN).20 High levels of psychological resilience were associated with lower basal amygdala activity and decreased connectivity between amygdala nuclei and the VDMN.20 Additionally, lower depressive symptoms were associated with higher connectivity between the amygdalae and the dorsal frontal networks.20 These results suggest a complex relationship between amygdala activity, dorsal frontal regions, resilience, and LLD.20
Vlasova et al21 further addressed the multifactorial character of psychological resilience. The associations between the 4 factors of resilience and the regional integrity of white matter in older adults with LLD were examined using diffusion-weighted MRI.21 Grit was found to be associated with greater white matter integrity in the genu of the corpus callosum and cingulum bundle in LLD.21 There was also a positive association between grit and fractional anisotropy (FA) in the callosal region connecting the prefrontal cortex and FA in the cingulum fibers.21 However, results regarding the FA in the cingulum fibers did not survive correction for multiple comparisons and should be considered with caution, pending further research.21
Continue to: Stress response biomarkers of resilience
Stress response biomarkers of resilience
Stress response biomarkers include endocrine, immune, and inflammatory indices. Stress has been identified as a factor in inflammatory responses. Stress-related overstimulation of the HPA axis may increase the risk of LLD.22 Numerous studies have demonstrated an association between increased levels of peripheral proinflammatory cytokines and depressive symptoms in older adults.23 Interleukin-6 (IL-6) has been increasingly linked with depressive symptoms and poor memory performance in older adults.9 There also appears to be an interaction of inflammatory and vascular processes predisposing to LLD, as increased levels of IL-6 and C-reactive protein have been associated with higher white matter pathology.9 Additionally, proinflammatory cytokines impact monoamine neurotransmitter pathways, leading to a reduction in tryptophan and serotonin synthesis, disruption of glucocorticoid receptors, and a decrease in hippocampal neurotrophic support.9 Alexopoulos et al24 further explain that a prolonged CNS immune response can affect emotional and cognitive network functions related to LLD and has a role in the etiology of depressive symptoms in older adults.
Cardiovascular comorbidity and autonomic nervous system dysfunction
Many studies have revealed evidence of a bidirectional association between cardiovascular disease and depression.25 Dysregulation of the autonomic nervous system (ANS) is an underlying mechanism that could explain the link between cardiovascular risk and MDD via heart rate variability (HRV), though research examining age-related capacities provide conflicting data.25,26 HRV is a surrogate index of resting cardiac vagal outflow that represents the ability of the ANS to adapt to psychological, social, and physical environmental changes.27 Higher overall HRV is associated with greater self-regulating capacity, including behavioral, cognitive, and emotional control.28 Additionally, higher HRV may serve as a biomarker of resilience to the development of stress-related disorders such as MDD. Recent studies have shown an overall reduction in HRV in older adults with LLD.29 When high- and low-frequency HRV were investigated separately, only low-frequency HRV was significantly reduced in patients with depression.29 One explanation is that older adults with depression have impaired or reduced baroreflex sensitivity and gain, which is often associated with an increased risk of mortality following cardiac events.30 More research is needed to examine the complex processes required to better characterize the correlation between resilience in cardiovascular disease and autonomic dysfunction.
The Box6,31,32 describes the relationship between markers of cellular health and resilience.
Box
Among the biomarkers of resilience, telomere length and telomerase activity serve as biomarkers of biological aging that can differ from the chronological age and mark successful anti-aging, stress-reducing strategies.31 Telomerase, the cellular enzyme that regulates the health of cells when they reproduce (preserving the telomeres, repetitive DNA strands at the ends of chromosomes), is associated with overall cell health and cellular biological age.31 When telomerase is reduced, the telomeres in a cell are clipped, causing the cells to age more rapidly as the telomeres get shorter through the process of cellular reproduction.31 Psychological stress may play a significant role in telomerase production and subsequent telomere length.32 Lavretsky et al32 evaluated the effect of brief daily yogic meditation on depressive symptoms and immune cell telomerase activity in a family of dementia caregivers with mild depressive symptoms. Brief daily meditation practice led to significant lower levels of depressive symptoms that was accompanied by an increase in telomerase activity, suggesting improvement in stress-induced cellular aging.6,32
Mind-body therapies
There is increasing interest in improving older adults’ physical and emotional well-being while promoting resilience through stress-reducing lifestyle interventions such as MBTs.33 Because MBTs are often considered a natural and safer option compared to conventional medicine, these interventions are rapidly gaining popularity in the United States.33,34 According to a 2017 National Health Survey, there were 5% to 10% increases in the use of yoga, meditation, and chiropractic care from 2012 to 2017, with growing evidence supporting MBTs as minimally invasive, cost-effective approaches for managing stress and neurocognitive disorders.35 In contrast to pharmacologic approaches, MBTs can be used to train individuals to self-regulate in the face of adversity and stress, thus increasing their resilience.
MBTs can be divided into mindful movement exercises and meditative practices. Mindful movement exercises include yoga, tai chi, and qigong. Meditative practices that do not include movement include progressive relaxation, mindfulness, meditation, and acceptance therapies. On average, both mindful movement exercise (eg, yoga) and multicomponent mindfulness-based interventions (eg, mindfulness-based cognitive therapy, mindfulness-based stress reduction [MBSR], and mindfulness-based relapse prevention) can be as effective as other active treatments for psychiatric disorders such as MDD, anxiety, and substance use disorders.36,37 MBSR specifically has been shown to increase empathy, self-control, self-compassion, relationship quality, mindfulness, and spirituality as well as decrease rumination in healthy older adults.38 This suggests that MBSR can help strengthen the 4 factors of resilience.
Continue to: Research has also begun...
Research has also begun to evaluate the neurobiological mechanisms by which meditative therapies enhance resilience in mental health disorders, and several promising mechanistic domains (neural, hormonal, immune, cellular, and cardiovascular) have been identified.39 The physical yoga discipline includes asanas (postures), pranayama (breathing techniques), and dhyana (meditation). With the inclusion of mindfulness training, yoga involves the practice of meditation as well as the dynamic combination of proprioceptive and interoceptive awareness, resulting in both attention and profound focus.40 Dedicated yoga practice allows an individual to develop skills to withdraw the senses (pratyahara), concentrate the mind (dharana), and establish unwavering awareness (dhyana).41 The physical and cognitive benefits associated with yoga and mindfulness may be due to mechanisms including pranayama and activation of the parasympathetic nervous system; meditative or contemplative practices; increased body perception; stronger functional connectivity within the basal ganglia; or neuroplastic effects of increased grey matter volume and amygdala with regional enlargement.41 The new learning aspect of yoga practice may contribute to enhancing or improving various aspects of cognition, although the mechanisms are yet to be clarified.
Continued research in this area will promote the integration of MBTs into mainstream clinical practice and help alleviate the increased chronic health burden of an aging population. In the face of the COVID-19 pandemic, public interest in improving resilience and mental health42 can be supported by MBTs that can improve coping with the stress of the pandemic and enhance critical organ function (eg, lungs, heart, brain).43,44 As a result of these limitations, many resources and health care services have used telehealth and virtual platforms to adapt to these challenges and continue offering MBTs.45
Enhancing resilience to improve clinical outcomes
Increasing our understanding of clinical, neurocognitive, and neurobiological markers of resilience in older adults with and without depression could inform the development of interventions that treat and prevent mood and cognitive disorders of aging. Furthermore, stress reduction, decreased inflammation, and improved emotional regulation may have direct neuroplastic effects on the brain, leading to greater resilience. Complementary use of MBTs combined with standard antidepressant treatment may allow for additional improvement in clinical outcomes of LLD, including resilience, quality of life, general health, and cognitive function. Additional research testing the efficacy of those interventions designed to improve resilience in older adults with mood and mental disorders is needed.
Bottom Line
Identifying the clinical, neurocognitive, and neurobiological biomarkers of resilience in late-life depression could aid in the development of targeted interventions that treat and prevent mood and cognitive disorders of aging. Mind-body interventions can help boost resilience and improve outcomes in geriatric patients with mood and cognitive disorders.
Related Resources
- Lavretsky H. Resilience and Aging: Research and Practice. Johns Hopkins University Press; 2014.
- Lavretsky H, Sajatovic M, Reynolds CF, eds. Complementary and Integrative Therapies for Mental Health and Aging. Oxford University Press; 2016.
- Eyre HA, Berk M, Lavretsky H, et al, eds. Convergence Mental Health: A Transdisciplinary Approach to Innovation. Oxford University Press; 2021.
- UCLA Jane & Terry Semel Institute for Neuroscience & Human Behavior. Late-life Depression, Stress, and Wellness Research Program. https://www.semel.ucla.edu/latelife
Resilience has been defined as the ability to adapt and thrive in the face of adversity, acute stress, or trauma.1 Originally conceived as an inborn trait characteristic, resilience is now conceptualized as a dynamic, multidimensional capacity influenced by the interactions between internal factors (eg, personality, cognitive capacity, physical health) and environmental resources (eg, social status, financial stability).2,3 Resilience in older adults (typically defined as age ≥65) can improve the prognosis and outcomes for physical and mental conditions.4 The construct is closely aligned with “successful aging” and can be fostered in older adults, leading to improved physical and mental health and well-being.5
While initially resilience was conceptualized as the opposite of depressive states, recent research has identified resilience in the context of major depressive disorder (MDD) as the net effects of various psychosocial and biological variables that decrease the risk of onset, relapse, or depressive illness severity and increase the probability or speed of recovery.6 Late-life depression (LLD) in adults age >65 is a common and debilitating disease, often leading to decreased psychological well-being, increased cognitive decline, and excess mortality.7,8 LLD is associated with several factors, such as cerebrovascular disease, neurodegenerative disease, and inflammation, all of which could contribute to brain vulnerability and an increased risk of depression.9 Physical and cognitive engagement, physical activity, and high brain reserve have been shown to confer resilience to affective and cognitive changes in older adults, despite brain vulnerability.9
The greatest levels of resilience have been observed in individuals in their fifth decade of life and later,4,10 with high levels of resilience significantly contributing to longevity5; however, little is known about which factors contribute to heterogeneity in resilience characteristics and outcomes.4 Furthermore, the concept of resilience continues to raise numerous questions, including:
- how resilience should be measured or defined
- what factors promote or deter the development of resilience
- the effects of resilience on various health and psychological outcomes
- which interventions are effective in enhancing resilience in older adults.4
In this article, we describe resilience in older adults with LLD, its clinical and neurocognitive correlates, and underlying neurobiological and immunological biomarkers. We also examine resilience-building interventions, such as mind-body therapies (MBTs), that have been shown to enhance resilience by promoting positive perceptions of difficult experiences and challenges.
Clinical and neurocognitive correlates of resilience
Resilience varies substantially among older adults with LLD as well as across the lifespan of an individual.11 Identifying clinical components and predictors of resilience may usefully inform the development and testing of interventions to prevent and treat LLD.11 One tool widely used to measure resilience—the self-report Connor-Davidson Resilience Scale (CD-RISC)12— has been found to have clinically relevant characteristics.1,11 Using data from 337 older adults with LLD, Laird et al11 performed an exploratory factor analysis of the CD-RISC and found a 4-factor model:
- grit
- adaptive coping self-efficacy
- accommodative coping self-efficacy
- spirituality.1,11
Having a strong sense of purpose and not being easily discouraged by failure were items characteristic of grit.1,11 The preference to take the lead in problem-solving was typical of items loading on adaptive coping self-efficacy, while accommodative coping self-efficacy measured flexibility, cognitive reframing, a sense of humor, and acceptance in the face of uncontrollable stress.1,11 Finally, the belief that “things happen for a reason” and that “sometimes fate or God can help me” are characteristics of spirituality. 1,11 Using a multivariate model, the greatest variance in total resilience scores was explained by less depression, less apathy, higher quality of life, non-White race, and, somewhat counterintuitively, greater medical comorbidity.1,11 Thus, interventions designed to help older adults cultivate grit, active coping, accommodative coping, and spirituality may enhance resilience in LLD.
Resilience may also be positively associated with cognitive functioning and could be neuroprotective in LLD.13 Laird et al13 investigated associations between baseline resilience and several domains of neurocognitive functioning in 288 older adults with LLD. Several positive associations were found between measured language performance and total resilience, active coping, and accommodative coping.13 Additionally, total resilience and accommodative coping were significantly associated with a lower self-reported frequency of forgetfulness, a subjective measure of memory used in this study.13 Together, these results suggest that interventions targeting language might be useful to improve coping in LLD.13 Another interesting finding was that the resilience subdomain of spirituality was negatively associated with memory, language, and executive functioning performance.13 A distinction must be made between religious attendance (eg, regular attendance at religious institutions) vs religious beliefs, which may account for the previously reported associations between spirituality and improved cognition.13
Continue to: Self-reported resilience...
Self-reported resilience may also predict greater responsivity to antidepressant medication in patients with LLD.14 Older adults with LLD and greater self-reported baseline resilience were more likely to experience improvement or remission from depression with antidepressant treatment.14 This is congruent with conceptualizations of resilience as “the ability to adapt to and recover from stress.”14,15 Of the 4 identified resilience factors (grit, adaptive coping, accommodative coping, and spirituality), it appears that accommodative coping predicts LLD treatment response and remission.14 The unique ability to accommodate is associated with better mental health outcomes in the face of uncontrollable stress.14,16-18 Older adults appear to engage in more accommodative coping due to frequent uncontrollable stress and aging-related physiological changes (eg, sleep changes, chronic pain, declining cognition). This could make accommodative coping especially important in this population.14,19
The Figure, adapted from Weisenbach et al,9 exhibits factors that contribute to LLD, including cerebrovascular disease, neurodegeneration, and chronic inflammation, all of which can lead to a vulnerable aging brain that is at higher risk for depression, particularly within the context of stress. Clinical and neurocognitive factors associated with resilience can help buffer vulnerable brains from developing depression.

Neurobiological biomarkers of resilience in
Gross anatomical indicators: Findings from neuroimaging
The neurobiology underlying psychological resilience involves brain networks associated with stress response, negative affect, and emotional control.19 Increased amygdala reactivity and amygdala frontal connectivity are often implicated in neurobiological models of resilience.20 Leaver et al20 correlated psychological resilience measures with amygdala function in 48 depressed vs nondepressed individuals using functional magnetic resonance imaging. Specifically, they targeted the basolateral, centromedial, and superficial nuclei groups of the amygdala while comparing the 2 groups based on resilience scores (CD-RISC), depressive symptom severity, and depression status.20 A significant correlation was identified between resilience and connectivity between the superficial group of amygdala nuclei and the ventral default mode network (VDMN).20 High levels of psychological resilience were associated with lower basal amygdala activity and decreased connectivity between amygdala nuclei and the VDMN.20 Additionally, lower depressive symptoms were associated with higher connectivity between the amygdalae and the dorsal frontal networks.20 These results suggest a complex relationship between amygdala activity, dorsal frontal regions, resilience, and LLD.20
Vlasova et al21 further addressed the multifactorial character of psychological resilience. The associations between the 4 factors of resilience and the regional integrity of white matter in older adults with LLD were examined using diffusion-weighted MRI.21 Grit was found to be associated with greater white matter integrity in the genu of the corpus callosum and cingulum bundle in LLD.21 There was also a positive association between grit and fractional anisotropy (FA) in the callosal region connecting the prefrontal cortex and FA in the cingulum fibers.21 However, results regarding the FA in the cingulum fibers did not survive correction for multiple comparisons and should be considered with caution, pending further research.21
Continue to: Stress response biomarkers of resilience
Stress response biomarkers of resilience
Stress response biomarkers include endocrine, immune, and inflammatory indices. Stress has been identified as a factor in inflammatory responses. Stress-related overstimulation of the HPA axis may increase the risk of LLD.22 Numerous studies have demonstrated an association between increased levels of peripheral proinflammatory cytokines and depressive symptoms in older adults.23 Interleukin-6 (IL-6) has been increasingly linked with depressive symptoms and poor memory performance in older adults.9 There also appears to be an interaction of inflammatory and vascular processes predisposing to LLD, as increased levels of IL-6 and C-reactive protein have been associated with higher white matter pathology.9 Additionally, proinflammatory cytokines impact monoamine neurotransmitter pathways, leading to a reduction in tryptophan and serotonin synthesis, disruption of glucocorticoid receptors, and a decrease in hippocampal neurotrophic support.9 Alexopoulos et al24 further explain that a prolonged CNS immune response can affect emotional and cognitive network functions related to LLD and has a role in the etiology of depressive symptoms in older adults.
Cardiovascular comorbidity and autonomic nervous system dysfunction
Many studies have revealed evidence of a bidirectional association between cardiovascular disease and depression.25 Dysregulation of the autonomic nervous system (ANS) is an underlying mechanism that could explain the link between cardiovascular risk and MDD via heart rate variability (HRV), though research examining age-related capacities provide conflicting data.25,26 HRV is a surrogate index of resting cardiac vagal outflow that represents the ability of the ANS to adapt to psychological, social, and physical environmental changes.27 Higher overall HRV is associated with greater self-regulating capacity, including behavioral, cognitive, and emotional control.28 Additionally, higher HRV may serve as a biomarker of resilience to the development of stress-related disorders such as MDD. Recent studies have shown an overall reduction in HRV in older adults with LLD.29 When high- and low-frequency HRV were investigated separately, only low-frequency HRV was significantly reduced in patients with depression.29 One explanation is that older adults with depression have impaired or reduced baroreflex sensitivity and gain, which is often associated with an increased risk of mortality following cardiac events.30 More research is needed to examine the complex processes required to better characterize the correlation between resilience in cardiovascular disease and autonomic dysfunction.
The Box6,31,32 describes the relationship between markers of cellular health and resilience.
Box
Among the biomarkers of resilience, telomere length and telomerase activity serve as biomarkers of biological aging that can differ from the chronological age and mark successful anti-aging, stress-reducing strategies.31 Telomerase, the cellular enzyme that regulates the health of cells when they reproduce (preserving the telomeres, repetitive DNA strands at the ends of chromosomes), is associated with overall cell health and cellular biological age.31 When telomerase is reduced, the telomeres in a cell are clipped, causing the cells to age more rapidly as the telomeres get shorter through the process of cellular reproduction.31 Psychological stress may play a significant role in telomerase production and subsequent telomere length.32 Lavretsky et al32 evaluated the effect of brief daily yogic meditation on depressive symptoms and immune cell telomerase activity in a family of dementia caregivers with mild depressive symptoms. Brief daily meditation practice led to significant lower levels of depressive symptoms that was accompanied by an increase in telomerase activity, suggesting improvement in stress-induced cellular aging.6,32
Mind-body therapies
There is increasing interest in improving older adults’ physical and emotional well-being while promoting resilience through stress-reducing lifestyle interventions such as MBTs.33 Because MBTs are often considered a natural and safer option compared to conventional medicine, these interventions are rapidly gaining popularity in the United States.33,34 According to a 2017 National Health Survey, there were 5% to 10% increases in the use of yoga, meditation, and chiropractic care from 2012 to 2017, with growing evidence supporting MBTs as minimally invasive, cost-effective approaches for managing stress and neurocognitive disorders.35 In contrast to pharmacologic approaches, MBTs can be used to train individuals to self-regulate in the face of adversity and stress, thus increasing their resilience.
MBTs can be divided into mindful movement exercises and meditative practices. Mindful movement exercises include yoga, tai chi, and qigong. Meditative practices that do not include movement include progressive relaxation, mindfulness, meditation, and acceptance therapies. On average, both mindful movement exercise (eg, yoga) and multicomponent mindfulness-based interventions (eg, mindfulness-based cognitive therapy, mindfulness-based stress reduction [MBSR], and mindfulness-based relapse prevention) can be as effective as other active treatments for psychiatric disorders such as MDD, anxiety, and substance use disorders.36,37 MBSR specifically has been shown to increase empathy, self-control, self-compassion, relationship quality, mindfulness, and spirituality as well as decrease rumination in healthy older adults.38 This suggests that MBSR can help strengthen the 4 factors of resilience.
Continue to: Research has also begun...
Research has also begun to evaluate the neurobiological mechanisms by which meditative therapies enhance resilience in mental health disorders, and several promising mechanistic domains (neural, hormonal, immune, cellular, and cardiovascular) have been identified.39 The physical yoga discipline includes asanas (postures), pranayama (breathing techniques), and dhyana (meditation). With the inclusion of mindfulness training, yoga involves the practice of meditation as well as the dynamic combination of proprioceptive and interoceptive awareness, resulting in both attention and profound focus.40 Dedicated yoga practice allows an individual to develop skills to withdraw the senses (pratyahara), concentrate the mind (dharana), and establish unwavering awareness (dhyana).41 The physical and cognitive benefits associated with yoga and mindfulness may be due to mechanisms including pranayama and activation of the parasympathetic nervous system; meditative or contemplative practices; increased body perception; stronger functional connectivity within the basal ganglia; or neuroplastic effects of increased grey matter volume and amygdala with regional enlargement.41 The new learning aspect of yoga practice may contribute to enhancing or improving various aspects of cognition, although the mechanisms are yet to be clarified.
Continued research in this area will promote the integration of MBTs into mainstream clinical practice and help alleviate the increased chronic health burden of an aging population. In the face of the COVID-19 pandemic, public interest in improving resilience and mental health42 can be supported by MBTs that can improve coping with the stress of the pandemic and enhance critical organ function (eg, lungs, heart, brain).43,44 As a result of these limitations, many resources and health care services have used telehealth and virtual platforms to adapt to these challenges and continue offering MBTs.45
Enhancing resilience to improve clinical outcomes
Increasing our understanding of clinical, neurocognitive, and neurobiological markers of resilience in older adults with and without depression could inform the development of interventions that treat and prevent mood and cognitive disorders of aging. Furthermore, stress reduction, decreased inflammation, and improved emotional regulation may have direct neuroplastic effects on the brain, leading to greater resilience. Complementary use of MBTs combined with standard antidepressant treatment may allow for additional improvement in clinical outcomes of LLD, including resilience, quality of life, general health, and cognitive function. Additional research testing the efficacy of those interventions designed to improve resilience in older adults with mood and mental disorders is needed.
Bottom Line
Identifying the clinical, neurocognitive, and neurobiological biomarkers of resilience in late-life depression could aid in the development of targeted interventions that treat and prevent mood and cognitive disorders of aging. Mind-body interventions can help boost resilience and improve outcomes in geriatric patients with mood and cognitive disorders.
Related Resources
- Lavretsky H. Resilience and Aging: Research and Practice. Johns Hopkins University Press; 2014.
- Lavretsky H, Sajatovic M, Reynolds CF, eds. Complementary and Integrative Therapies for Mental Health and Aging. Oxford University Press; 2016.
- Eyre HA, Berk M, Lavretsky H, et al, eds. Convergence Mental Health: A Transdisciplinary Approach to Innovation. Oxford University Press; 2021.
- UCLA Jane & Terry Semel Institute for Neuroscience & Human Behavior. Late-life Depression, Stress, and Wellness Research Program. https://www.semel.ucla.edu/latelife
1. Reynolds CF. Promoting resilience, reducing depression in older adults. Int Psychogeriatr. 2019;31(2):169-171.
2. Windle G. What is resilience? A review and concept analysis. Rev Clin Gerontol. 2011;21(2):152-169.
3. Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338(6103):79-82.
4. Dunn LB, Predescu I. Resilience: a rich concept in need of research comment on: “Neurocognitive correlates of resilience in late-life depression” (by Laird et al.). Am J Geriatr Psychiatry. 2019;27(1):18-20.
5. Harmell AL, Kamat R, Jeste DV, et al. Resilience-building interventions for successful and positive aging. In: Lavretsky H, Sajatovic M, Reynolds C III, eds. Complementary and Integrative Therapies for Mental Health and Aging. Oxford University Press; 2015:305-316.
6. Laird KT, Krause B, Funes C, et al. Psychobiological factors of resilience and depression in late life. Transl Psychiatry. 2019;9(1):88.
7. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
8. Callahan CM, Wolinsky FD, Stump TE, et al. Mortality, symptoms, and functional impairment in late-life depression. J Gen Intern Med. 1998;13(11):746-752.
9. Weisenbach SL, Kumar A. Current understanding of the neurobiology and longitudinal course of geriatric depression. Curr Psychiatry Rep. 2014;16(9):463.
10. Southwick SM, Litz BT, Charney D, et al. Resilience and Mental Health: Challenges Across the Lifespan. Cambridge University Press; 2011.
11. Laird KT, Lavretsky H, Paholpak P, et al. Clinical correlates of resilience factors in geriatric depression. Int Psychogeriatr. 2019;31(2):193-202.
12. Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
13. Laird KT, Lavretsky H, Wu P, et al. Neurocognitive correlates of resilience in late-life depression. Am J Geriatr Psychiatry. 2019;27(1):12-17.
14. Laird KT, Lavretsky H, St Cyr N, et al. Resilience predicts remission in antidepressant treatment of geriatric depression. Int J Geriatr Psychiatry. 2018;33(12):1596-1603.
15. Waugh CE, Koster EH. A resilience framework for promoting stable remission from depression. Clin Psychol Rev. 2015;41:49-60.
16. Boerner K. Adaptation to disability among middle-aged and older adults: the role of assimilative and accommodative coping. J Gerontol B Psychol Sci Soc Sci. 2004;59(1):P35-P42.
17. Zakowski SG, Hall MH, Klein LC, et al. Appraised control, coping, and stress in a community sample: a test of the goodness-of-fit hypothesis. Ann Behav Med. 2001;23(3):158-165.
18. Cheng C, Lau HB, Chan MP. Coping flexibility and psychological adjustment to stressful life changes: a meta-analytic review. Psychol Bull. 2014;140(6):1582-1607.
19. Stokes SA, Gordon SE. Common stressors experienced by the well elderly. Clinical implications. J Gerontol Nurs. 2003;29(5):38-46.
20. Leaver AM, Yang H, Siddarth P, et al. Resilience and amygdala function in older healthy and depressed adults. J Affect Disord. 2018;237:27-34.
21. Vlasova RM, Siddarth P, Krause B, et al. Resilience and white matter integrity in geriatric depression. Am J Geriatr Psychiatry. 2018;26(8):874-883.
22. Chopra K, Kumar B, Kuhad A. Pathobiological targets of depression. Expert Opin Ther Targets. 2011;15(4):379-400.
23. Martínez-Cengotitabengoa M, Carrascón L, O’Brien JT, et al. Peripheral inflammatory parameters in late-life depression: a systematic review. Int J Mol Sci. 2016;17(12):2022.
24. Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26(11):1109-1118.
25. Carney RM, Freedland KE, Sheline YI, et al. Depression and coronary heart disease: a review for cardiologists. Clin Cardiol. 1997;20(3):196-200.
26. Carney RM, Freedland KE, Steinmeyer BC, et al. Nighttime heart rate predicts response to depression treatment in patients with coronary heart disease. J Affect Disord. 2016;200:165-171.
27. Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psych. 2006;10(3):229-240.
28. Holzman JB, Bridgett DJ. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci Biobehav Rev. 2017;74(Pt A):233-255.
29. Brown L, Karmakar C, Gray R, et al. Heart rate variability alterations in late life depression: a meta-analysis. J Affect Disord. 2018;235:456-466.
30. La Rovere MT, Bigger JT Jr, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351(1901):478-484.
31. Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. 2021;184(2):306-322.
32. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
33. Siddiqui MJ, Min CS, Verma RK, et al. Role of complementary and alternative medicine in geriatric care: a mini review. Pharmacogn Rev. 2014;8(16):81-87.
34. Nguyen SA, Lavretsky H. Emerging complementary and integrative therapies for geriatric mental health. Curr Treat Options Psychiatry. 2020;7(4):447-470.
35. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
36. Hofmann SG, Gómez AF. Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am. 2017;40(4):739-749.
37. Ramadas E, de Lima MP, Caetano T, et al. Effectiveness of mindfulness-based relapse prevention in individuals with substance use disorders: a systematic review. Behav Sci (Basel). 2021;11(10):133.
38. Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J Altern Complement Med. 2009;15(5):593-600.
39. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
40. Chobe S, Chobe M, Metri K, et al. Impact of yoga on cognition and mental health among elderly: a systematic review. Complement Ther Med. 2020;52:102421.
41. Brunner D, Abramovitch A, Etherton J. A yoga program for cognitive enhancement. PLoS One. 2017;12(8):e0182366.
42. Dai J, Sang X, Menhas R, et al. The influence of COVID-19 pandemic on physical health-psychological health, physical activity, and overall well-being: the mediating role of emotional regulation. Front Psychol. 2021;12:667461.
43. Grolli RE, Mingoti MED, Bertollo AG, et al. Impact of COVID-19 in the mental health in elderly: psychological and biological updates. Mol Neurobiol. 2021;58(5):1905-1916.
44. Johansson A, Mohamed MS, Moulin TC, et al. Neurological manifestations of COVID-19: a comprehensive literature review and discussion of mechanisms. J Neuroimmunol. 2021;358:577658.
45. Pandya SP. Older women and wellbeing through the pandemic: examining the effect of daily online yoga lessons. Health Care Women Int. 2021;42(11):1255-1278.
1. Reynolds CF. Promoting resilience, reducing depression in older adults. Int Psychogeriatr. 2019;31(2):169-171.
2. Windle G. What is resilience? A review and concept analysis. Rev Clin Gerontol. 2011;21(2):152-169.
3. Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338(6103):79-82.
4. Dunn LB, Predescu I. Resilience: a rich concept in need of research comment on: “Neurocognitive correlates of resilience in late-life depression” (by Laird et al.). Am J Geriatr Psychiatry. 2019;27(1):18-20.
5. Harmell AL, Kamat R, Jeste DV, et al. Resilience-building interventions for successful and positive aging. In: Lavretsky H, Sajatovic M, Reynolds C III, eds. Complementary and Integrative Therapies for Mental Health and Aging. Oxford University Press; 2015:305-316.
6. Laird KT, Krause B, Funes C, et al. Psychobiological factors of resilience and depression in late life. Transl Psychiatry. 2019;9(1):88.
7. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
8. Callahan CM, Wolinsky FD, Stump TE, et al. Mortality, symptoms, and functional impairment in late-life depression. J Gen Intern Med. 1998;13(11):746-752.
9. Weisenbach SL, Kumar A. Current understanding of the neurobiology and longitudinal course of geriatric depression. Curr Psychiatry Rep. 2014;16(9):463.
10. Southwick SM, Litz BT, Charney D, et al. Resilience and Mental Health: Challenges Across the Lifespan. Cambridge University Press; 2011.
11. Laird KT, Lavretsky H, Paholpak P, et al. Clinical correlates of resilience factors in geriatric depression. Int Psychogeriatr. 2019;31(2):193-202.
12. Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
13. Laird KT, Lavretsky H, Wu P, et al. Neurocognitive correlates of resilience in late-life depression. Am J Geriatr Psychiatry. 2019;27(1):12-17.
14. Laird KT, Lavretsky H, St Cyr N, et al. Resilience predicts remission in antidepressant treatment of geriatric depression. Int J Geriatr Psychiatry. 2018;33(12):1596-1603.
15. Waugh CE, Koster EH. A resilience framework for promoting stable remission from depression. Clin Psychol Rev. 2015;41:49-60.
16. Boerner K. Adaptation to disability among middle-aged and older adults: the role of assimilative and accommodative coping. J Gerontol B Psychol Sci Soc Sci. 2004;59(1):P35-P42.
17. Zakowski SG, Hall MH, Klein LC, et al. Appraised control, coping, and stress in a community sample: a test of the goodness-of-fit hypothesis. Ann Behav Med. 2001;23(3):158-165.
18. Cheng C, Lau HB, Chan MP. Coping flexibility and psychological adjustment to stressful life changes: a meta-analytic review. Psychol Bull. 2014;140(6):1582-1607.
19. Stokes SA, Gordon SE. Common stressors experienced by the well elderly. Clinical implications. J Gerontol Nurs. 2003;29(5):38-46.
20. Leaver AM, Yang H, Siddarth P, et al. Resilience and amygdala function in older healthy and depressed adults. J Affect Disord. 2018;237:27-34.
21. Vlasova RM, Siddarth P, Krause B, et al. Resilience and white matter integrity in geriatric depression. Am J Geriatr Psychiatry. 2018;26(8):874-883.
22. Chopra K, Kumar B, Kuhad A. Pathobiological targets of depression. Expert Opin Ther Targets. 2011;15(4):379-400.
23. Martínez-Cengotitabengoa M, Carrascón L, O’Brien JT, et al. Peripheral inflammatory parameters in late-life depression: a systematic review. Int J Mol Sci. 2016;17(12):2022.
24. Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26(11):1109-1118.
25. Carney RM, Freedland KE, Sheline YI, et al. Depression and coronary heart disease: a review for cardiologists. Clin Cardiol. 1997;20(3):196-200.
26. Carney RM, Freedland KE, Steinmeyer BC, et al. Nighttime heart rate predicts response to depression treatment in patients with coronary heart disease. J Affect Disord. 2016;200:165-171.
27. Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psych. 2006;10(3):229-240.
28. Holzman JB, Bridgett DJ. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci Biobehav Rev. 2017;74(Pt A):233-255.
29. Brown L, Karmakar C, Gray R, et al. Heart rate variability alterations in late life depression: a meta-analysis. J Affect Disord. 2018;235:456-466.
30. La Rovere MT, Bigger JT Jr, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351(1901):478-484.
31. Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. 2021;184(2):306-322.
32. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
33. Siddiqui MJ, Min CS, Verma RK, et al. Role of complementary and alternative medicine in geriatric care: a mini review. Pharmacogn Rev. 2014;8(16):81-87.
34. Nguyen SA, Lavretsky H. Emerging complementary and integrative therapies for geriatric mental health. Curr Treat Options Psychiatry. 2020;7(4):447-470.
35. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
36. Hofmann SG, Gómez AF. Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am. 2017;40(4):739-749.
37. Ramadas E, de Lima MP, Caetano T, et al. Effectiveness of mindfulness-based relapse prevention in individuals with substance use disorders: a systematic review. Behav Sci (Basel). 2021;11(10):133.
38. Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J Altern Complement Med. 2009;15(5):593-600.
39. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
40. Chobe S, Chobe M, Metri K, et al. Impact of yoga on cognition and mental health among elderly: a systematic review. Complement Ther Med. 2020;52:102421.
41. Brunner D, Abramovitch A, Etherton J. A yoga program for cognitive enhancement. PLoS One. 2017;12(8):e0182366.
42. Dai J, Sang X, Menhas R, et al. The influence of COVID-19 pandemic on physical health-psychological health, physical activity, and overall well-being: the mediating role of emotional regulation. Front Psychol. 2021;12:667461.
43. Grolli RE, Mingoti MED, Bertollo AG, et al. Impact of COVID-19 in the mental health in elderly: psychological and biological updates. Mol Neurobiol. 2021;58(5):1905-1916.
44. Johansson A, Mohamed MS, Moulin TC, et al. Neurological manifestations of COVID-19: a comprehensive literature review and discussion of mechanisms. J Neuroimmunol. 2021;358:577658.
45. Pandya SP. Older women and wellbeing through the pandemic: examining the effect of daily online yoga lessons. Health Care Women Int. 2021;42(11):1255-1278.
Lithium-associated hypercalcemia: Monitoring and management
Hypercalcemia is a well-known but underrecognized adverse effect of lithium. Most patients with lithium-associated hypercalcemia (LAH) have either nonspecific symptoms (eg, persistent tiredness, constipation, polyuria, polydipsia) or no symptoms. Clinically, LAH differs from primary hyperparathyroidism, though the management protocol of these 2 conditions is almost the same. In this article, we discuss how lithium can affect calcium and parathyroid hormone (PTH) levels and how LAH and lithium-associated hyperparathyroidism (LAHP) differs from primary hyperparathyroidism. We also outline a suggested approach to monitoring and management.
An insidious problem
Due to the varying definitions and methods used to assess hypercalcemia, the reported prevalence of LAH varies from 4.3% to 80%.1 McKnight et al2 conducted a systematic review and meta-analysis of studies of the relationship between lithium and parathyroid function that included 14 case-control studies, 36 case reports, and 6 cross-sectional studies without a control group. They found that the levels of calcium and PTH were 10% higher in lithium-treated patients than in controls.2
Pathophysiology. Lithium is known to increase both calcium and PTH levels. PTH is responsible for calcium homeostasis. It is secreted in response to low calcium levels, which it increases by its action on bones, intestines, and kidneys. Vitamin D also plays a crucial role in calcium homeostasis. A deficiency of vitamin D triggers a compensatory increase in PTH to maintain calcium levels.3
Calcium and PTH levels increase soon after administration of lithium, but the rise is usually mild and insidious. In a small proportion of patients who receive long-term lithium treatment, calcium levels can exceed the normal range. Patients who develop LAH typically have serum calcium levels slightly above the normal range and PTH levels ranging from the higher side of the normal range to several times the upper limit of the normal range. Patients might also experience elevated PTH levels without any increase in calcium levels. Lithium can affect calcium and PTH levels in multiple ways. For instance, it increases the reabsorption of calcium in the kidney as well as the reset point of calcium-sensing receptors. Therefore, only higher levels of calcium can inhibit the release of PTH. Hence, in cases where the PTH level is within the normal range, it is generally higher than would be expected for a given serum calcium level. Lithium can also directly affect the parathyroid glands and can lead to either single-nodule or multimodule hyperplasia.4
Long-term lithium use can cause chronic kidney disease (CKD), which in turn leads to vitamin D deficiency and hyperparathyroidism. However, secondary hyperparathyroidism with CKD is usually seen in the more advanced stages of CKD, and is associated with low-to-normal calcium levels (as opposed to the high levels seen in LAH).3-5
Lithium-associated hyperparathyroidism
Primary hyperparathyroidism is the most common cause of hypercalcemia. Its prevalence ranges from 1 to 7 cases per 1,000 adults. The incidence of LAH/LAHP is 4- to 6-fold higher compared to the general population.6 Similar to LAH/LAHP, primary hyperparathyroidism is more common in older adults (age >60) and females. Hence, some researchers have suggested that lithium probably unmasks hyperparathyroidism in patients who are susceptible to primary hyperparathyroidism.3
Look for these clinical manifestations
Symptoms of primary hyperparathyroidism are related to high calcium and PTH levels. They are commonly described as “painful bones, renal stones, abdominal groans (due to hypercalcemia-induced ileus), and psychic moans (lethargy, poor concentration, depression).” Common adverse outcomes associated with primary hyperparathyroidism are renal stones, high risk of fracture, constipation, peptic ulcer, and pancreatitis.3,7
Continue: In contrast...
In contrast, LAHP is characterized by mild, intermittent, and/or persistent hypercalcemia and mildly increased PTH (Table 1).1,3,4 In some patients, it could improve without active intervention. Because lithium increases the absorption of urinary calcium, it is associated with hypocalciuria and a lower risk of renal stones. Additionally, lithium has osteoprotective effects and has not been associated with an increased risk of fracture. Some researchers have suggested that the presentation of LAHP is more like familial hypocalciuric hypercalcemia (FHC), which is also associated with hypocalciuria. FHC is a benign condition and does not require active intervention.3,4 Similar to those with FHC, many patients with LAHP may live with chronic asymptomatic hypercalcemia without any significant adverse outcome.
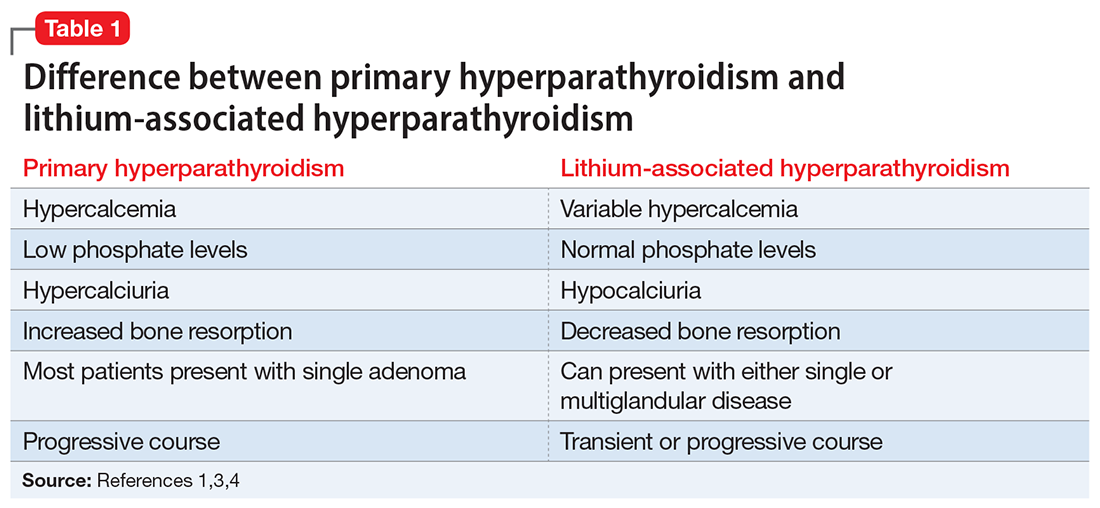
A suggested approach to monitoring
In most cases, LAH is an insidious adverse effect that is usually detected on blood tests after many years of lithium therapy.8 For patients starting lithium therapy, International Society of Bipolar Disorder guidelines recommend testing calcium levels at baseline, 6 months, and annually thereafter, or as clinically indicated, to detect and monitor hypercalcemia and hyperparathyroidism. However, these guidelines do not provide any recommendations regarding how to manage abnormal findings.9
Clinical laboratories report both total and adjusted calcium values. The adjusted calcium value takes into account albumin levels. This is a way to compensate for an abnormal concentration of albumin (establishing what a patient’s total calcium concentration would be if the albumin concentration was normal). Table 25 shows the categorization of adjusted calcium values. For patients receiving lithium, some researchers have suggested monitoring PTH as well as calcium.1
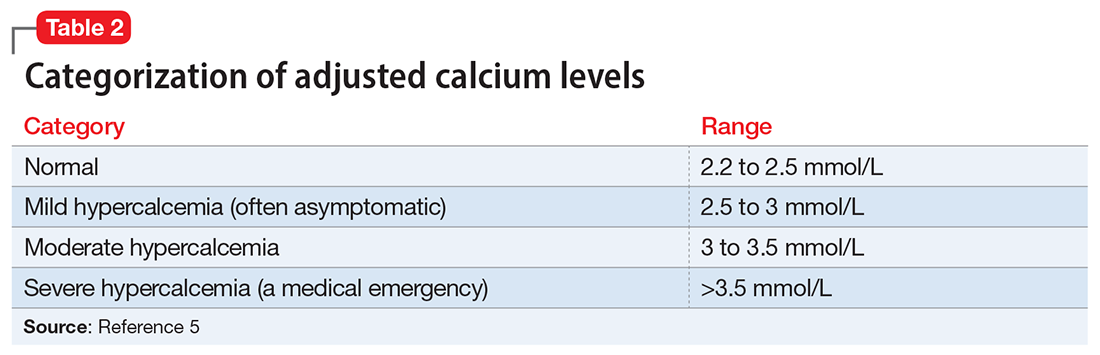
The Figure outlines our proposed approach to monitoring for LAH in patients receiving lithium. An isolated high value of calcium could be due to prolonged venous stasis if a tourniquet is used for phlebotomy. In such instances, the calcium level should be tested again without a tourniquet.10 If the repeat blood test shows elevated calcium levels, then both PTH and serum calcium should be tested.
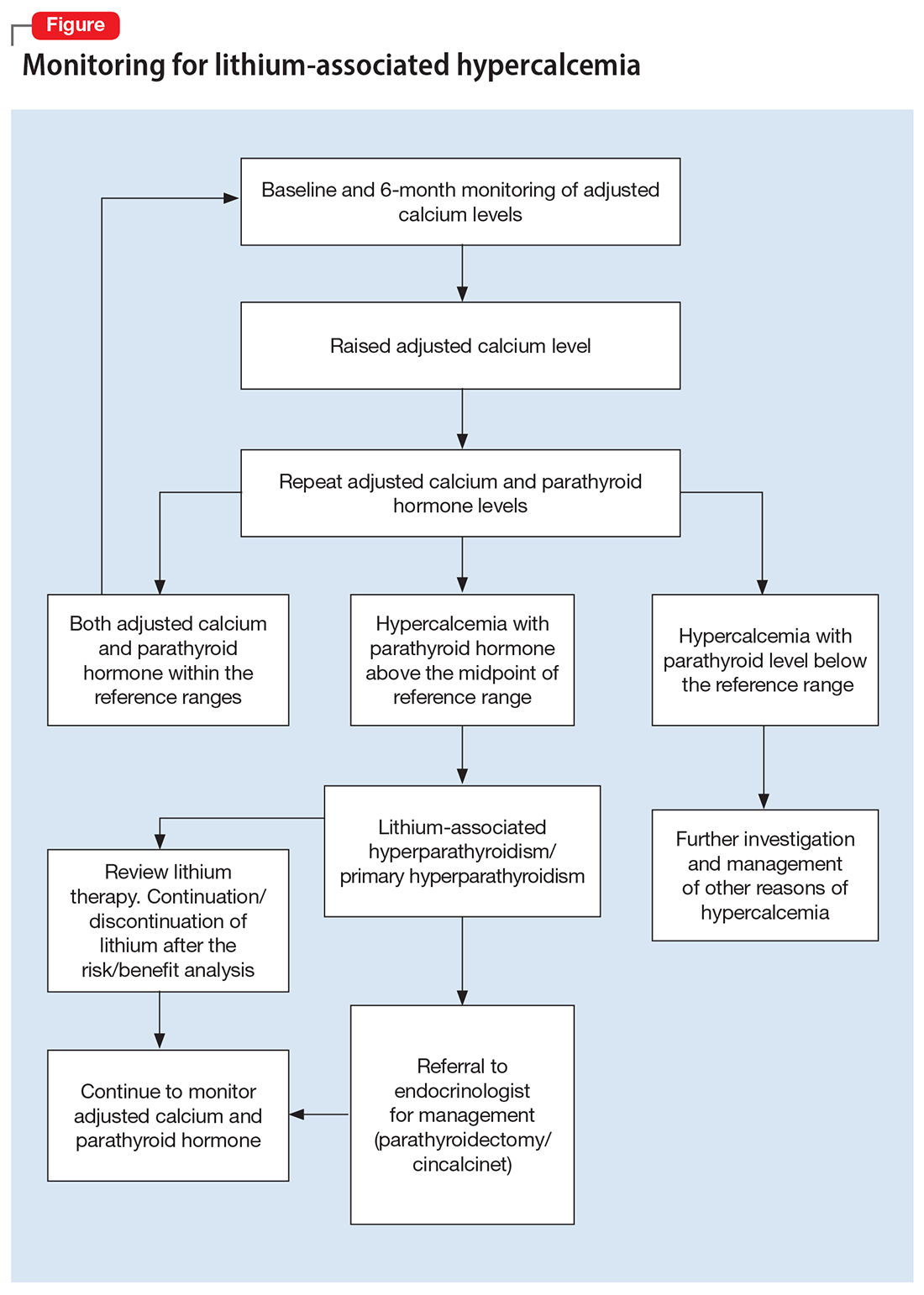
If the PTH level is higher than the midpoint of the reference range, LAH should be suspected, though sometimes hypercalcemia can present without raised PTH. LAH has also been reported to cause a transient increase in calcium levels. If hypercalcemia frequently recurs, PTH levels should be monitored. If PTH is suppressed, then the raised calcium levels are probably secondary to something other than lithium; common reasons for this include the use of vitamin D supplements or thiazide diuretics, or malignancies such as multiple myeloma.3,5,8
Continue to: Treatment
Treatment: Continue lithium?
There are several options for treating LAH. Lithium may be continued or discontinued following close monitoring of calcium and PTH levels, with or without active interventions such as surgery or pharmacotherapy, and as deemed appropriate after consultation with an endocrinologist. The decision should be informed by evaluating the risks and benefits to the patient’s physical and mental health. LAH can be reversed by discontinuing lithium, but this might not be the case in patients receiving long-term lithium therapy, especially if their elevated calcium levels are associated with parathyroid adenomas or hyperplasia. Hence, close monitoring of calcium and PTH is required even after discontinuing lithium.3,8
Surgical treatment. The primary treatment of LAH and primary hyperparathyroidism is parathyroidectomy. The possibility of recovery after parathyroidectomy for primary hyperparathyroidism is 60% to 80%, though a small proportion of patients might experience recurrence. This figure might be higher for LAH, because it is more likely to affect multiple glands.1,11 Other potential complications of parathyroidectomy are recurrent laryngeal nerve injury causing paralysis of vocal cords leading to hoarseness of voice, stridor, or aspiration, and local hematoma and hypocalcemia (requiring vitamin D and/or calcium supplements).12
Pharmacotherapy. Cinacalcet is a calcimimetic drug that decreases the reset point of the calcium-sensing receptor. It can be used if a patient is not suitable for or apprehensive about surgical intervention.1,8
Bottom Line
Calcium levels should be regularly monitored in patients receiving lithium. If calcium levels are persistently high, parathyroid hormone levels should also be measured. Management of lithium-associated hypercalcemia includes watchful waiting, discontinuing lithium, parathyroidectomy, and pharmacotherapy with cinacalcet.
Related Resources
- Laski M, Foreman R, Hancock H, et al. Lithium: an underutilized element. Current Psychiatry. 2021;20(12):27-30,34. doi:10.12788/cp.0193
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Cinacalcet • Sensipar
1. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg. 2018;42(2):415-424.
2. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
3. Shapiro HI, Davis KA. Hypercalcemia and “primary” hyperparathyroidism during lithium therapy. Am J Psychiatry. 2015;172(1):12-15.
4. Lerena VS, León NS, Sosa S, et al. Lithium and endocrine dysfunction. Medicina (B Aires). 2022;82(1):130-137.
5. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959-1966.
6. Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98(3):1122-1129.
7. Dandurand K, Ali DS, Khan AA. Primary hyperparathyroidism: a narrative review of diagnosis and medical management. J Clin Med. 2021;10(8):1604.
8. Mifsud S, Cilia K, Mifsud EL, et al. Lithium-associated hyperparathyroidism. Br J Hosp Med (Lond). 2020;81(11):1-9.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. Mieebi WM, Solomon AE, Wabote AP. The effect of tourniquet application on serum calcium and inorganic phosphorus determination. Journal of Health, Medicine and Nursing. 2019;65:51-54.
11. Awad SS, Miskulin J, Thompson N. Parathyroid adenomas versus four-gland hyperplasia as the cause of primary hyperparathyroidism in patients with prolonged lithium therapy. World J Surg. 2003;27(4):486-488.
12. Farndon JR. Postoperative complications of parathyroidectomy. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem-Oriented. Zuckschwerdt; 2001. Accessed October 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK6967
Hypercalcemia is a well-known but underrecognized adverse effect of lithium. Most patients with lithium-associated hypercalcemia (LAH) have either nonspecific symptoms (eg, persistent tiredness, constipation, polyuria, polydipsia) or no symptoms. Clinically, LAH differs from primary hyperparathyroidism, though the management protocol of these 2 conditions is almost the same. In this article, we discuss how lithium can affect calcium and parathyroid hormone (PTH) levels and how LAH and lithium-associated hyperparathyroidism (LAHP) differs from primary hyperparathyroidism. We also outline a suggested approach to monitoring and management.
An insidious problem
Due to the varying definitions and methods used to assess hypercalcemia, the reported prevalence of LAH varies from 4.3% to 80%.1 McKnight et al2 conducted a systematic review and meta-analysis of studies of the relationship between lithium and parathyroid function that included 14 case-control studies, 36 case reports, and 6 cross-sectional studies without a control group. They found that the levels of calcium and PTH were 10% higher in lithium-treated patients than in controls.2
Pathophysiology. Lithium is known to increase both calcium and PTH levels. PTH is responsible for calcium homeostasis. It is secreted in response to low calcium levels, which it increases by its action on bones, intestines, and kidneys. Vitamin D also plays a crucial role in calcium homeostasis. A deficiency of vitamin D triggers a compensatory increase in PTH to maintain calcium levels.3
Calcium and PTH levels increase soon after administration of lithium, but the rise is usually mild and insidious. In a small proportion of patients who receive long-term lithium treatment, calcium levels can exceed the normal range. Patients who develop LAH typically have serum calcium levels slightly above the normal range and PTH levels ranging from the higher side of the normal range to several times the upper limit of the normal range. Patients might also experience elevated PTH levels without any increase in calcium levels. Lithium can affect calcium and PTH levels in multiple ways. For instance, it increases the reabsorption of calcium in the kidney as well as the reset point of calcium-sensing receptors. Therefore, only higher levels of calcium can inhibit the release of PTH. Hence, in cases where the PTH level is within the normal range, it is generally higher than would be expected for a given serum calcium level. Lithium can also directly affect the parathyroid glands and can lead to either single-nodule or multimodule hyperplasia.4
Long-term lithium use can cause chronic kidney disease (CKD), which in turn leads to vitamin D deficiency and hyperparathyroidism. However, secondary hyperparathyroidism with CKD is usually seen in the more advanced stages of CKD, and is associated with low-to-normal calcium levels (as opposed to the high levels seen in LAH).3-5
Lithium-associated hyperparathyroidism
Primary hyperparathyroidism is the most common cause of hypercalcemia. Its prevalence ranges from 1 to 7 cases per 1,000 adults. The incidence of LAH/LAHP is 4- to 6-fold higher compared to the general population.6 Similar to LAH/LAHP, primary hyperparathyroidism is more common in older adults (age >60) and females. Hence, some researchers have suggested that lithium probably unmasks hyperparathyroidism in patients who are susceptible to primary hyperparathyroidism.3
Look for these clinical manifestations
Symptoms of primary hyperparathyroidism are related to high calcium and PTH levels. They are commonly described as “painful bones, renal stones, abdominal groans (due to hypercalcemia-induced ileus), and psychic moans (lethargy, poor concentration, depression).” Common adverse outcomes associated with primary hyperparathyroidism are renal stones, high risk of fracture, constipation, peptic ulcer, and pancreatitis.3,7
Continue: In contrast...
In contrast, LAHP is characterized by mild, intermittent, and/or persistent hypercalcemia and mildly increased PTH (Table 1).1,3,4 In some patients, it could improve without active intervention. Because lithium increases the absorption of urinary calcium, it is associated with hypocalciuria and a lower risk of renal stones. Additionally, lithium has osteoprotective effects and has not been associated with an increased risk of fracture. Some researchers have suggested that the presentation of LAHP is more like familial hypocalciuric hypercalcemia (FHC), which is also associated with hypocalciuria. FHC is a benign condition and does not require active intervention.3,4 Similar to those with FHC, many patients with LAHP may live with chronic asymptomatic hypercalcemia without any significant adverse outcome.

A suggested approach to monitoring
In most cases, LAH is an insidious adverse effect that is usually detected on blood tests after many years of lithium therapy.8 For patients starting lithium therapy, International Society of Bipolar Disorder guidelines recommend testing calcium levels at baseline, 6 months, and annually thereafter, or as clinically indicated, to detect and monitor hypercalcemia and hyperparathyroidism. However, these guidelines do not provide any recommendations regarding how to manage abnormal findings.9
Clinical laboratories report both total and adjusted calcium values. The adjusted calcium value takes into account albumin levels. This is a way to compensate for an abnormal concentration of albumin (establishing what a patient’s total calcium concentration would be if the albumin concentration was normal). Table 25 shows the categorization of adjusted calcium values. For patients receiving lithium, some researchers have suggested monitoring PTH as well as calcium.1

The Figure outlines our proposed approach to monitoring for LAH in patients receiving lithium. An isolated high value of calcium could be due to prolonged venous stasis if a tourniquet is used for phlebotomy. In such instances, the calcium level should be tested again without a tourniquet.10 If the repeat blood test shows elevated calcium levels, then both PTH and serum calcium should be tested.

If the PTH level is higher than the midpoint of the reference range, LAH should be suspected, though sometimes hypercalcemia can present without raised PTH. LAH has also been reported to cause a transient increase in calcium levels. If hypercalcemia frequently recurs, PTH levels should be monitored. If PTH is suppressed, then the raised calcium levels are probably secondary to something other than lithium; common reasons for this include the use of vitamin D supplements or thiazide diuretics, or malignancies such as multiple myeloma.3,5,8
Continue to: Treatment
Treatment: Continue lithium?
There are several options for treating LAH. Lithium may be continued or discontinued following close monitoring of calcium and PTH levels, with or without active interventions such as surgery or pharmacotherapy, and as deemed appropriate after consultation with an endocrinologist. The decision should be informed by evaluating the risks and benefits to the patient’s physical and mental health. LAH can be reversed by discontinuing lithium, but this might not be the case in patients receiving long-term lithium therapy, especially if their elevated calcium levels are associated with parathyroid adenomas or hyperplasia. Hence, close monitoring of calcium and PTH is required even after discontinuing lithium.3,8
Surgical treatment. The primary treatment of LAH and primary hyperparathyroidism is parathyroidectomy. The possibility of recovery after parathyroidectomy for primary hyperparathyroidism is 60% to 80%, though a small proportion of patients might experience recurrence. This figure might be higher for LAH, because it is more likely to affect multiple glands.1,11 Other potential complications of parathyroidectomy are recurrent laryngeal nerve injury causing paralysis of vocal cords leading to hoarseness of voice, stridor, or aspiration, and local hematoma and hypocalcemia (requiring vitamin D and/or calcium supplements).12
Pharmacotherapy. Cinacalcet is a calcimimetic drug that decreases the reset point of the calcium-sensing receptor. It can be used if a patient is not suitable for or apprehensive about surgical intervention.1,8
Bottom Line
Calcium levels should be regularly monitored in patients receiving lithium. If calcium levels are persistently high, parathyroid hormone levels should also be measured. Management of lithium-associated hypercalcemia includes watchful waiting, discontinuing lithium, parathyroidectomy, and pharmacotherapy with cinacalcet.
Related Resources
- Laski M, Foreman R, Hancock H, et al. Lithium: an underutilized element. Current Psychiatry. 2021;20(12):27-30,34. doi:10.12788/cp.0193
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Cinacalcet • Sensipar
Hypercalcemia is a well-known but underrecognized adverse effect of lithium. Most patients with lithium-associated hypercalcemia (LAH) have either nonspecific symptoms (eg, persistent tiredness, constipation, polyuria, polydipsia) or no symptoms. Clinically, LAH differs from primary hyperparathyroidism, though the management protocol of these 2 conditions is almost the same. In this article, we discuss how lithium can affect calcium and parathyroid hormone (PTH) levels and how LAH and lithium-associated hyperparathyroidism (LAHP) differs from primary hyperparathyroidism. We also outline a suggested approach to monitoring and management.
An insidious problem
Due to the varying definitions and methods used to assess hypercalcemia, the reported prevalence of LAH varies from 4.3% to 80%.1 McKnight et al2 conducted a systematic review and meta-analysis of studies of the relationship between lithium and parathyroid function that included 14 case-control studies, 36 case reports, and 6 cross-sectional studies without a control group. They found that the levels of calcium and PTH were 10% higher in lithium-treated patients than in controls.2
Pathophysiology. Lithium is known to increase both calcium and PTH levels. PTH is responsible for calcium homeostasis. It is secreted in response to low calcium levels, which it increases by its action on bones, intestines, and kidneys. Vitamin D also plays a crucial role in calcium homeostasis. A deficiency of vitamin D triggers a compensatory increase in PTH to maintain calcium levels.3
Calcium and PTH levels increase soon after administration of lithium, but the rise is usually mild and insidious. In a small proportion of patients who receive long-term lithium treatment, calcium levels can exceed the normal range. Patients who develop LAH typically have serum calcium levels slightly above the normal range and PTH levels ranging from the higher side of the normal range to several times the upper limit of the normal range. Patients might also experience elevated PTH levels without any increase in calcium levels. Lithium can affect calcium and PTH levels in multiple ways. For instance, it increases the reabsorption of calcium in the kidney as well as the reset point of calcium-sensing receptors. Therefore, only higher levels of calcium can inhibit the release of PTH. Hence, in cases where the PTH level is within the normal range, it is generally higher than would be expected for a given serum calcium level. Lithium can also directly affect the parathyroid glands and can lead to either single-nodule or multimodule hyperplasia.4
Long-term lithium use can cause chronic kidney disease (CKD), which in turn leads to vitamin D deficiency and hyperparathyroidism. However, secondary hyperparathyroidism with CKD is usually seen in the more advanced stages of CKD, and is associated with low-to-normal calcium levels (as opposed to the high levels seen in LAH).3-5
Lithium-associated hyperparathyroidism
Primary hyperparathyroidism is the most common cause of hypercalcemia. Its prevalence ranges from 1 to 7 cases per 1,000 adults. The incidence of LAH/LAHP is 4- to 6-fold higher compared to the general population.6 Similar to LAH/LAHP, primary hyperparathyroidism is more common in older adults (age >60) and females. Hence, some researchers have suggested that lithium probably unmasks hyperparathyroidism in patients who are susceptible to primary hyperparathyroidism.3
Look for these clinical manifestations
Symptoms of primary hyperparathyroidism are related to high calcium and PTH levels. They are commonly described as “painful bones, renal stones, abdominal groans (due to hypercalcemia-induced ileus), and psychic moans (lethargy, poor concentration, depression).” Common adverse outcomes associated with primary hyperparathyroidism are renal stones, high risk of fracture, constipation, peptic ulcer, and pancreatitis.3,7
Continue: In contrast...
In contrast, LAHP is characterized by mild, intermittent, and/or persistent hypercalcemia and mildly increased PTH (Table 1).1,3,4 In some patients, it could improve without active intervention. Because lithium increases the absorption of urinary calcium, it is associated with hypocalciuria and a lower risk of renal stones. Additionally, lithium has osteoprotective effects and has not been associated with an increased risk of fracture. Some researchers have suggested that the presentation of LAHP is more like familial hypocalciuric hypercalcemia (FHC), which is also associated with hypocalciuria. FHC is a benign condition and does not require active intervention.3,4 Similar to those with FHC, many patients with LAHP may live with chronic asymptomatic hypercalcemia without any significant adverse outcome.

A suggested approach to monitoring
In most cases, LAH is an insidious adverse effect that is usually detected on blood tests after many years of lithium therapy.8 For patients starting lithium therapy, International Society of Bipolar Disorder guidelines recommend testing calcium levels at baseline, 6 months, and annually thereafter, or as clinically indicated, to detect and monitor hypercalcemia and hyperparathyroidism. However, these guidelines do not provide any recommendations regarding how to manage abnormal findings.9
Clinical laboratories report both total and adjusted calcium values. The adjusted calcium value takes into account albumin levels. This is a way to compensate for an abnormal concentration of albumin (establishing what a patient’s total calcium concentration would be if the albumin concentration was normal). Table 25 shows the categorization of adjusted calcium values. For patients receiving lithium, some researchers have suggested monitoring PTH as well as calcium.1

The Figure outlines our proposed approach to monitoring for LAH in patients receiving lithium. An isolated high value of calcium could be due to prolonged venous stasis if a tourniquet is used for phlebotomy. In such instances, the calcium level should be tested again without a tourniquet.10 If the repeat blood test shows elevated calcium levels, then both PTH and serum calcium should be tested.

If the PTH level is higher than the midpoint of the reference range, LAH should be suspected, though sometimes hypercalcemia can present without raised PTH. LAH has also been reported to cause a transient increase in calcium levels. If hypercalcemia frequently recurs, PTH levels should be monitored. If PTH is suppressed, then the raised calcium levels are probably secondary to something other than lithium; common reasons for this include the use of vitamin D supplements or thiazide diuretics, or malignancies such as multiple myeloma.3,5,8
Continue to: Treatment
Treatment: Continue lithium?
There are several options for treating LAH. Lithium may be continued or discontinued following close monitoring of calcium and PTH levels, with or without active interventions such as surgery or pharmacotherapy, and as deemed appropriate after consultation with an endocrinologist. The decision should be informed by evaluating the risks and benefits to the patient’s physical and mental health. LAH can be reversed by discontinuing lithium, but this might not be the case in patients receiving long-term lithium therapy, especially if their elevated calcium levels are associated with parathyroid adenomas or hyperplasia. Hence, close monitoring of calcium and PTH is required even after discontinuing lithium.3,8
Surgical treatment. The primary treatment of LAH and primary hyperparathyroidism is parathyroidectomy. The possibility of recovery after parathyroidectomy for primary hyperparathyroidism is 60% to 80%, though a small proportion of patients might experience recurrence. This figure might be higher for LAH, because it is more likely to affect multiple glands.1,11 Other potential complications of parathyroidectomy are recurrent laryngeal nerve injury causing paralysis of vocal cords leading to hoarseness of voice, stridor, or aspiration, and local hematoma and hypocalcemia (requiring vitamin D and/or calcium supplements).12
Pharmacotherapy. Cinacalcet is a calcimimetic drug that decreases the reset point of the calcium-sensing receptor. It can be used if a patient is not suitable for or apprehensive about surgical intervention.1,8
Bottom Line
Calcium levels should be regularly monitored in patients receiving lithium. If calcium levels are persistently high, parathyroid hormone levels should also be measured. Management of lithium-associated hypercalcemia includes watchful waiting, discontinuing lithium, parathyroidectomy, and pharmacotherapy with cinacalcet.
Related Resources
- Laski M, Foreman R, Hancock H, et al. Lithium: an underutilized element. Current Psychiatry. 2021;20(12):27-30,34. doi:10.12788/cp.0193
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Cinacalcet • Sensipar
1. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg. 2018;42(2):415-424.
2. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
3. Shapiro HI, Davis KA. Hypercalcemia and “primary” hyperparathyroidism during lithium therapy. Am J Psychiatry. 2015;172(1):12-15.
4. Lerena VS, León NS, Sosa S, et al. Lithium and endocrine dysfunction. Medicina (B Aires). 2022;82(1):130-137.
5. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959-1966.
6. Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98(3):1122-1129.
7. Dandurand K, Ali DS, Khan AA. Primary hyperparathyroidism: a narrative review of diagnosis and medical management. J Clin Med. 2021;10(8):1604.
8. Mifsud S, Cilia K, Mifsud EL, et al. Lithium-associated hyperparathyroidism. Br J Hosp Med (Lond). 2020;81(11):1-9.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. Mieebi WM, Solomon AE, Wabote AP. The effect of tourniquet application on serum calcium and inorganic phosphorus determination. Journal of Health, Medicine and Nursing. 2019;65:51-54.
11. Awad SS, Miskulin J, Thompson N. Parathyroid adenomas versus four-gland hyperplasia as the cause of primary hyperparathyroidism in patients with prolonged lithium therapy. World J Surg. 2003;27(4):486-488.
12. Farndon JR. Postoperative complications of parathyroidectomy. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem-Oriented. Zuckschwerdt; 2001. Accessed October 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK6967
1. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg. 2018;42(2):415-424.
2. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
3. Shapiro HI, Davis KA. Hypercalcemia and “primary” hyperparathyroidism during lithium therapy. Am J Psychiatry. 2015;172(1):12-15.
4. Lerena VS, León NS, Sosa S, et al. Lithium and endocrine dysfunction. Medicina (B Aires). 2022;82(1):130-137.
5. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959-1966.
6. Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98(3):1122-1129.
7. Dandurand K, Ali DS, Khan AA. Primary hyperparathyroidism: a narrative review of diagnosis and medical management. J Clin Med. 2021;10(8):1604.
8. Mifsud S, Cilia K, Mifsud EL, et al. Lithium-associated hyperparathyroidism. Br J Hosp Med (Lond). 2020;81(11):1-9.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. Mieebi WM, Solomon AE, Wabote AP. The effect of tourniquet application on serum calcium and inorganic phosphorus determination. Journal of Health, Medicine and Nursing. 2019;65:51-54.
11. Awad SS, Miskulin J, Thompson N. Parathyroid adenomas versus four-gland hyperplasia as the cause of primary hyperparathyroidism in patients with prolonged lithium therapy. World J Surg. 2003;27(4):486-488.
12. Farndon JR. Postoperative complications of parathyroidectomy. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem-Oriented. Zuckschwerdt; 2001. Accessed October 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK6967
Optimal psychiatric treatment: Target the brain and avoid the body
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.
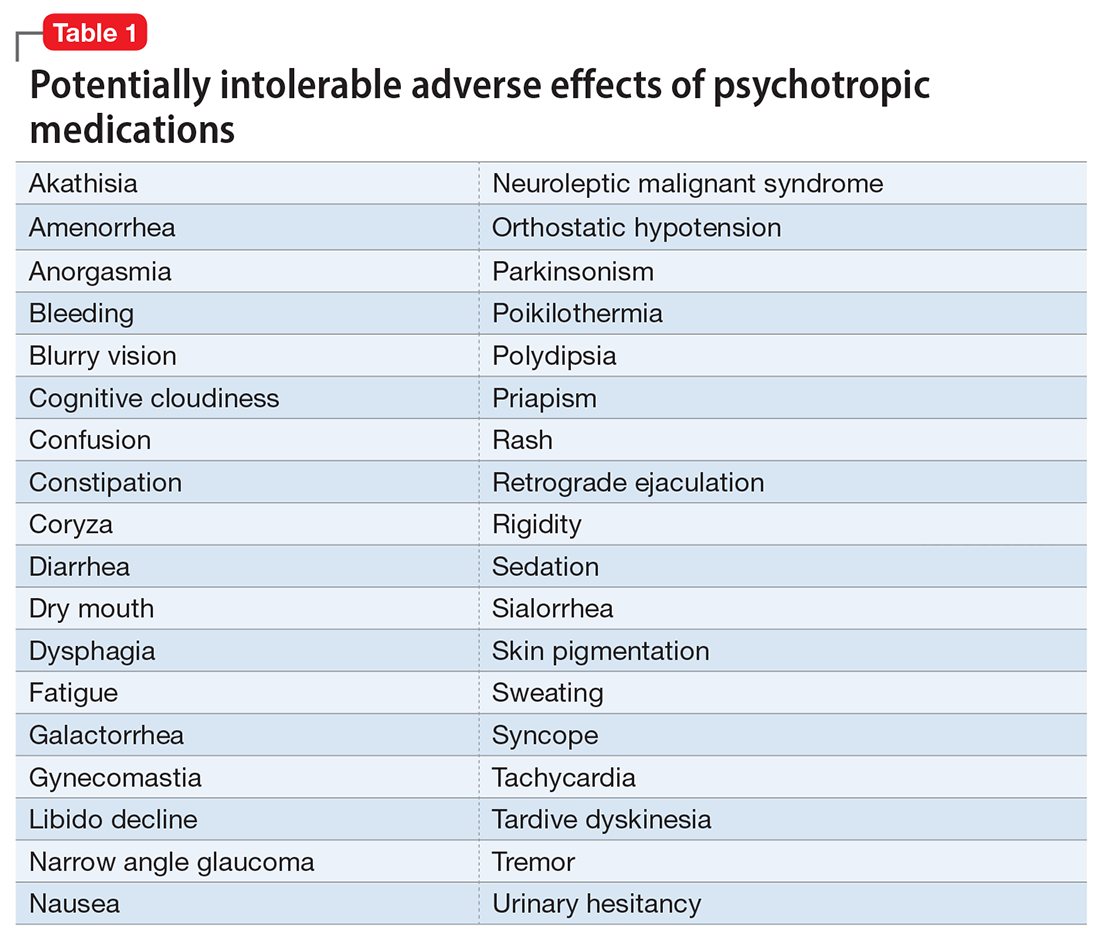
However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.
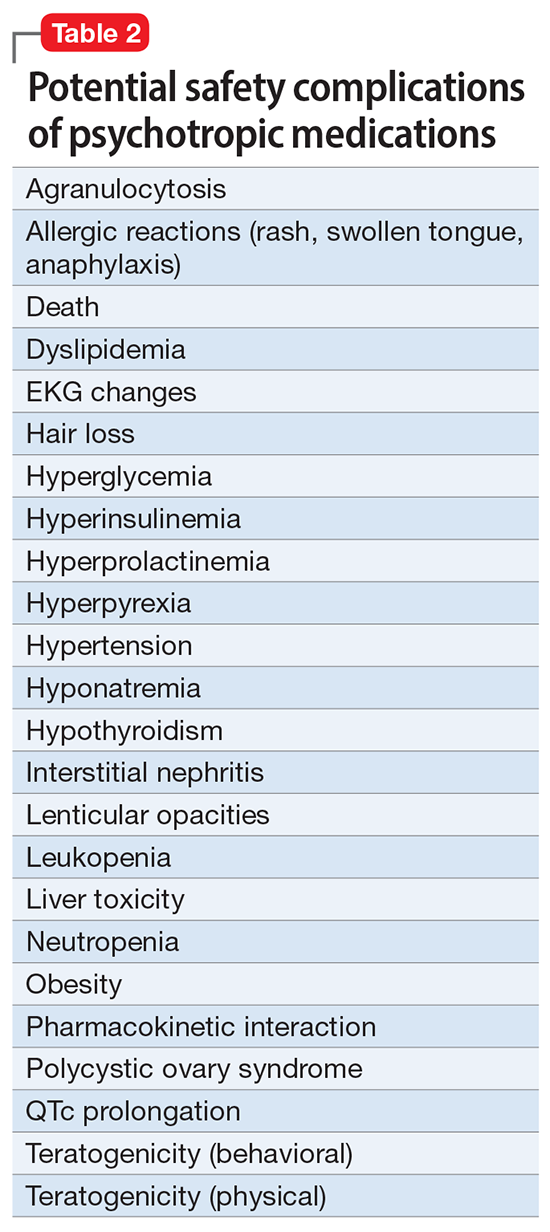
Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17
A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.

However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.

Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17

A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.

However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.

Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17

A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
More on social entropy
As leaders of the American Psychiatric Association, we received dozens of communications from members who were shocked by the discriminatory and transphobic commentary in the recent editorial “The accelerating societal entropy undermines mental health” (
Specifically, citing “lack of certainty about gender identity in children and adults” as an indicator of societal turmoil that undermines mental health is contrary to the scientific understanding of gender identity. Physicians have professional obligations to advance patients’ well-being and do no harm.
The medical profession, including psychiatry, is at a critical juncture in coming to terms with and dismantling its longstanding history of systemic racism and discrimination. Authors and editors must be aware that harmful and divisive language negatively affects mental health, especially for people who have been subject to discrimination individually and/or as members of historically excluded and/or minoritized groups.
In publishing this editorial,
Rebecca W. Brendel, MD, JD, DFAPA
President
American Psychiatric Association
Saul Levin, MD, MPA, FRCP-E, FRCPsych
CEO and Medical Director
American Psychiatric Association
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Dr. Nasrallah responds
I regret that the sentence about gender identity in my October editorial was regarded as transphobic and harmful. While the phrasing reflected my patients’ comments to me, I realize my unfortunate choice of words deeply offended individuals who are transgender, who have been subjected to ongoing discrimination and prejudice.
I apologize to our readers; to my American Psychiatric Association LGBTQAI+ friends, colleagues, and relatives; and to the LGBTQAI+ community at large. The sentence has been deleted from the online version of my editorial. This has been a teachable moment for me.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: More on psychiatric documentation
More on psychiatric documentation
Dr. Joshi’s helpful discussion of clinical documentation strategies (“Medical record documentation: What to do, and what to avoid,”
The mental health record may not always be as confidential as psychiatrists think (or hope) it is. The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, for example, generally does not distinguish between medical and mental health information, nor does it provide special rules for the latter (although certain state laws may do so). HIPAA provides added protections for “psychotherapy notes,” but this category explicitly excludes progress notes that discuss treatment modalities, diagnosis, and clinical milestones. To retain their protected status, psychotherapists’ private, “desk-drawer memory joggers” must never be comingled with the patient chart.1 For mental health professionals, this distinction underscores the importance of keeping personal details broad in the progress note; scandalous or embarrassing narratives recounted in the medical record itself are routinely accessible to the patient and may be lawfully disclosed to others under specified circumstances.
In addition to avoiding speculation and including patient quotes when appropriate, documenting objectively and nonjudgmentally means annotating facts and observations that helped the clinician arrive at their conclusion. For example, “patient appears intoxicated” is less helpful than noting the patient’s slurred speech, impaired gait and/or coordination, and alcohol odor.
Clinical care and its associated documentation are so intertwined that they can become virtually indistinguishable. In a medical malpractice case, the burden is on the plaintiff to prove their injury resulted from substandard care. Some courts, however, have held that missing or incomplete records can effectively shift the burden from the recipient to the provider of care to show that the treatment at issue was rendered non-negligently.2 Statutes of limitations restricting the amount of time in which a patient can sue after an adverse event are sometimes triggered by the date on which they knew or should have known of the alleged malpractice.3 One of the best ways of ascertaining this date, and starting the statute of limitations clock, can be a clear annotation in the medical record that the patient was apprised of an unanticipated outcome or iatrogenic harm. In this way, a timely and thorough note can be critical not just to defending the physician’s quality of care, but potentially to precluding a cognizable lawsuit altogether.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
Disclosures
The views expressed are those of the author and do not necessarily reflect those of any government agency, nor do they constitute individualized legal advice. The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
References
1. 45 CFR Parts 160 and 164, Subparts A and E.
2. Valcin v Public Health Trust, 473 So. 2d 1297 (1984).
3. US v Kubrick, 444 US 111 (1979).
As leaders of the American Psychiatric Association, we received dozens of communications from members who were shocked by the discriminatory and transphobic commentary in the recent editorial “The accelerating societal entropy undermines mental health” (
Specifically, citing “lack of certainty about gender identity in children and adults” as an indicator of societal turmoil that undermines mental health is contrary to the scientific understanding of gender identity. Physicians have professional obligations to advance patients’ well-being and do no harm.
The medical profession, including psychiatry, is at a critical juncture in coming to terms with and dismantling its longstanding history of systemic racism and discrimination. Authors and editors must be aware that harmful and divisive language negatively affects mental health, especially for people who have been subject to discrimination individually and/or as members of historically excluded and/or minoritized groups.
In publishing this editorial,
Rebecca W. Brendel, MD, JD, DFAPA
President
American Psychiatric Association
Saul Levin, MD, MPA, FRCP-E, FRCPsych
CEO and Medical Director
American Psychiatric Association
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Dr. Nasrallah responds
I regret that the sentence about gender identity in my October editorial was regarded as transphobic and harmful. While the phrasing reflected my patients’ comments to me, I realize my unfortunate choice of words deeply offended individuals who are transgender, who have been subjected to ongoing discrimination and prejudice.
I apologize to our readers; to my American Psychiatric Association LGBTQAI+ friends, colleagues, and relatives; and to the LGBTQAI+ community at large. The sentence has been deleted from the online version of my editorial. This has been a teachable moment for me.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: More on psychiatric documentation
More on psychiatric documentation
Dr. Joshi’s helpful discussion of clinical documentation strategies (“Medical record documentation: What to do, and what to avoid,”
The mental health record may not always be as confidential as psychiatrists think (or hope) it is. The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, for example, generally does not distinguish between medical and mental health information, nor does it provide special rules for the latter (although certain state laws may do so). HIPAA provides added protections for “psychotherapy notes,” but this category explicitly excludes progress notes that discuss treatment modalities, diagnosis, and clinical milestones. To retain their protected status, psychotherapists’ private, “desk-drawer memory joggers” must never be comingled with the patient chart.1 For mental health professionals, this distinction underscores the importance of keeping personal details broad in the progress note; scandalous or embarrassing narratives recounted in the medical record itself are routinely accessible to the patient and may be lawfully disclosed to others under specified circumstances.
In addition to avoiding speculation and including patient quotes when appropriate, documenting objectively and nonjudgmentally means annotating facts and observations that helped the clinician arrive at their conclusion. For example, “patient appears intoxicated” is less helpful than noting the patient’s slurred speech, impaired gait and/or coordination, and alcohol odor.
Clinical care and its associated documentation are so intertwined that they can become virtually indistinguishable. In a medical malpractice case, the burden is on the plaintiff to prove their injury resulted from substandard care. Some courts, however, have held that missing or incomplete records can effectively shift the burden from the recipient to the provider of care to show that the treatment at issue was rendered non-negligently.2 Statutes of limitations restricting the amount of time in which a patient can sue after an adverse event are sometimes triggered by the date on which they knew or should have known of the alleged malpractice.3 One of the best ways of ascertaining this date, and starting the statute of limitations clock, can be a clear annotation in the medical record that the patient was apprised of an unanticipated outcome or iatrogenic harm. In this way, a timely and thorough note can be critical not just to defending the physician’s quality of care, but potentially to precluding a cognizable lawsuit altogether.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
Disclosures
The views expressed are those of the author and do not necessarily reflect those of any government agency, nor do they constitute individualized legal advice. The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
References
1. 45 CFR Parts 160 and 164, Subparts A and E.
2. Valcin v Public Health Trust, 473 So. 2d 1297 (1984).
3. US v Kubrick, 444 US 111 (1979).
As leaders of the American Psychiatric Association, we received dozens of communications from members who were shocked by the discriminatory and transphobic commentary in the recent editorial “The accelerating societal entropy undermines mental health” (
Specifically, citing “lack of certainty about gender identity in children and adults” as an indicator of societal turmoil that undermines mental health is contrary to the scientific understanding of gender identity. Physicians have professional obligations to advance patients’ well-being and do no harm.
The medical profession, including psychiatry, is at a critical juncture in coming to terms with and dismantling its longstanding history of systemic racism and discrimination. Authors and editors must be aware that harmful and divisive language negatively affects mental health, especially for people who have been subject to discrimination individually and/or as members of historically excluded and/or minoritized groups.
In publishing this editorial,
Rebecca W. Brendel, MD, JD, DFAPA
President
American Psychiatric Association
Saul Levin, MD, MPA, FRCP-E, FRCPsych
CEO and Medical Director
American Psychiatric Association
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Dr. Nasrallah responds
I regret that the sentence about gender identity in my October editorial was regarded as transphobic and harmful. While the phrasing reflected my patients’ comments to me, I realize my unfortunate choice of words deeply offended individuals who are transgender, who have been subjected to ongoing discrimination and prejudice.
I apologize to our readers; to my American Psychiatric Association LGBTQAI+ friends, colleagues, and relatives; and to the LGBTQAI+ community at large. The sentence has been deleted from the online version of my editorial. This has been a teachable moment for me.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: More on psychiatric documentation
More on psychiatric documentation
Dr. Joshi’s helpful discussion of clinical documentation strategies (“Medical record documentation: What to do, and what to avoid,”
The mental health record may not always be as confidential as psychiatrists think (or hope) it is. The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, for example, generally does not distinguish between medical and mental health information, nor does it provide special rules for the latter (although certain state laws may do so). HIPAA provides added protections for “psychotherapy notes,” but this category explicitly excludes progress notes that discuss treatment modalities, diagnosis, and clinical milestones. To retain their protected status, psychotherapists’ private, “desk-drawer memory joggers” must never be comingled with the patient chart.1 For mental health professionals, this distinction underscores the importance of keeping personal details broad in the progress note; scandalous or embarrassing narratives recounted in the medical record itself are routinely accessible to the patient and may be lawfully disclosed to others under specified circumstances.
In addition to avoiding speculation and including patient quotes when appropriate, documenting objectively and nonjudgmentally means annotating facts and observations that helped the clinician arrive at their conclusion. For example, “patient appears intoxicated” is less helpful than noting the patient’s slurred speech, impaired gait and/or coordination, and alcohol odor.
Clinical care and its associated documentation are so intertwined that they can become virtually indistinguishable. In a medical malpractice case, the burden is on the plaintiff to prove their injury resulted from substandard care. Some courts, however, have held that missing or incomplete records can effectively shift the burden from the recipient to the provider of care to show that the treatment at issue was rendered non-negligently.2 Statutes of limitations restricting the amount of time in which a patient can sue after an adverse event are sometimes triggered by the date on which they knew or should have known of the alleged malpractice.3 One of the best ways of ascertaining this date, and starting the statute of limitations clock, can be a clear annotation in the medical record that the patient was apprised of an unanticipated outcome or iatrogenic harm. In this way, a timely and thorough note can be critical not just to defending the physician’s quality of care, but potentially to precluding a cognizable lawsuit altogether.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
Disclosures
The views expressed are those of the author and do not necessarily reflect those of any government agency, nor do they constitute individualized legal advice. The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
References
1. 45 CFR Parts 160 and 164, Subparts A and E.
2. Valcin v Public Health Trust, 473 So. 2d 1297 (1984).
3. US v Kubrick, 444 US 111 (1979).
Transitioning patients with opioid use disorder from methadone to buprenorphine
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.