User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Double Masking and Decontamination: A Doctor's COVID-19 Routine

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about the PPE and decontamination processes she's using. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
PPE Routine at the Hospital
Dr Gary Ferenchick: You've developed your own PPE and decontamination routines. It's about protecting yourself at work but also about protecting your loved ones by not carrying the virus home. Could you walk us through it? I'll show it on the screen.
Dr Hannah Ferenchick: At work I wear scrubs, and I try to minimize any additional clothing. I don't wear a jacket over my scrubs, and I don't wear any T-shirts under my scrubs. If I'm going to be in a situation that might involve exposure to patient secretions or bodily fluids, then I also wear shoe covers.
Because so many of our patients are infected and we may be called upon at any time to do an aerosol-generating procedure, in the ED we have all taken to wearing N95 masks for our entire shift. I wear a fitted N95 mask. I cover that with a surgical mask.
We are anticipating N95 shortages because our use of the masks has increased exponentially. Every hospital has to think about how to protect their healthcare workers while conserving PPE. We cover the N95 mask with a surgical mask, so that if there is any soiling or droplets reaching the mask, we are able to change the surgical mask and continue to use the same N95.
In addition, eye protection is important. Generally throughout the shift I wear my own goggles. If I'm going to be involved in any procedure with the potential for aerosolization (intubation, performing CPR, bronchoscopy) then I wear a creation of my own, which is a welder's shield.
Many of our providers have chosen to use their own equipment, although we are still able to use hospital-provided equipment. There is probably no difference in effectiveness between these devices.
Cell Phones and Stethoscopes
I carry a personal cell phone at work (which I often use to look things up, use the calculator, and for other purposes), and I'm cognizant that when I touch it, I am potentially transmitting pathogens to my phone or its cover. So I've taken to keeping my phone in a plastic sandwich bag, which I disinfect a couple of times throughout the shift. The phone still works normally.
After my shift, in my "decontamination phase," I remove the phone from the plastic bag and disinfect the phone again.
I try to avoid bringing objects into the vicinity of the patient. That's different from my normal routine—I usually like to write down what the patient has told me—but unfortunately, carrying pen and paper or a clipboard into a patient's room is not feasible at this point. During this time, I've also avoided using my personal stethoscope.
There's also transmission risk associated with shared equipment. We share hospital-provided phones and they must be disinfected. We are each disinfecting our own workspaces: computer, keyboard, mouse, and countertop.
Obviously you are trying to minimize any contact with your mouth or face. You don't want to rub your eyes, touch your nose, or eat anything with your hands while you are at work. The assumption is that you are doing very frequent hand hygiene.
Decontamination Routine
One of our concerns as healthcare providers is the possibility that we could, either asymptomatically or through the objects that we use at work, be bringing the disease home. We want to protect the people who may be at higher risk just because they live with a healthcare provider. These are the decontamination practices I've developed for my own situation, taken from best practices and suggestions from others.
I remove my dirty scrubs and leave them at work, and I change into a clean pair of scrubs or clean clothes. I disinfect any inanimate objects that my hands may have touched during the shift using alcohol, sanitizer wipes, bleach wipes, or hospital-grade chemical wipes.
To keep those objects clean after disinfecting, I place them in clean plastic bags away from other objects (eg, a wallet or purse) that may not be easy to disinfect. Then I store those bags in the trunk of my car for my next shift, so I'm not taking them into my home.
I also change my shoes, leaving my work shoes in the trunk of my car, and wear another pair of shoes into the house.
When I get home, I basically do everything again. I disinfect my phone, I wash my hands, and I shower immediately. At that point, I consider myself sufficiently "disinfected."
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about the PPE and decontamination processes she's using. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
PPE Routine at the Hospital
Dr Gary Ferenchick: You've developed your own PPE and decontamination routines. It's about protecting yourself at work but also about protecting your loved ones by not carrying the virus home. Could you walk us through it? I'll show it on the screen.
Dr Hannah Ferenchick: At work I wear scrubs, and I try to minimize any additional clothing. I don't wear a jacket over my scrubs, and I don't wear any T-shirts under my scrubs. If I'm going to be in a situation that might involve exposure to patient secretions or bodily fluids, then I also wear shoe covers.
Because so many of our patients are infected and we may be called upon at any time to do an aerosol-generating procedure, in the ED we have all taken to wearing N95 masks for our entire shift. I wear a fitted N95 mask. I cover that with a surgical mask.
We are anticipating N95 shortages because our use of the masks has increased exponentially. Every hospital has to think about how to protect their healthcare workers while conserving PPE. We cover the N95 mask with a surgical mask, so that if there is any soiling or droplets reaching the mask, we are able to change the surgical mask and continue to use the same N95.
In addition, eye protection is important. Generally throughout the shift I wear my own goggles. If I'm going to be involved in any procedure with the potential for aerosolization (intubation, performing CPR, bronchoscopy) then I wear a creation of my own, which is a welder's shield.
Many of our providers have chosen to use their own equipment, although we are still able to use hospital-provided equipment. There is probably no difference in effectiveness between these devices.
Cell Phones and Stethoscopes
I carry a personal cell phone at work (which I often use to look things up, use the calculator, and for other purposes), and I'm cognizant that when I touch it, I am potentially transmitting pathogens to my phone or its cover. So I've taken to keeping my phone in a plastic sandwich bag, which I disinfect a couple of times throughout the shift. The phone still works normally.
After my shift, in my "decontamination phase," I remove the phone from the plastic bag and disinfect the phone again.
I try to avoid bringing objects into the vicinity of the patient. That's different from my normal routine—I usually like to write down what the patient has told me—but unfortunately, carrying pen and paper or a clipboard into a patient's room is not feasible at this point. During this time, I've also avoided using my personal stethoscope.
There's also transmission risk associated with shared equipment. We share hospital-provided phones and they must be disinfected. We are each disinfecting our own workspaces: computer, keyboard, mouse, and countertop.
Obviously you are trying to minimize any contact with your mouth or face. You don't want to rub your eyes, touch your nose, or eat anything with your hands while you are at work. The assumption is that you are doing very frequent hand hygiene.
Decontamination Routine
One of our concerns as healthcare providers is the possibility that we could, either asymptomatically or through the objects that we use at work, be bringing the disease home. We want to protect the people who may be at higher risk just because they live with a healthcare provider. These are the decontamination practices I've developed for my own situation, taken from best practices and suggestions from others.
I remove my dirty scrubs and leave them at work, and I change into a clean pair of scrubs or clean clothes. I disinfect any inanimate objects that my hands may have touched during the shift using alcohol, sanitizer wipes, bleach wipes, or hospital-grade chemical wipes.
To keep those objects clean after disinfecting, I place them in clean plastic bags away from other objects (eg, a wallet or purse) that may not be easy to disinfect. Then I store those bags in the trunk of my car for my next shift, so I'm not taking them into my home.
I also change my shoes, leaving my work shoes in the trunk of my car, and wear another pair of shoes into the house.
When I get home, I basically do everything again. I disinfect my phone, I wash my hands, and I shower immediately. At that point, I consider myself sufficiently "disinfected."
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about the PPE and decontamination processes she's using. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
PPE Routine at the Hospital
Dr Gary Ferenchick: You've developed your own PPE and decontamination routines. It's about protecting yourself at work but also about protecting your loved ones by not carrying the virus home. Could you walk us through it? I'll show it on the screen.
Dr Hannah Ferenchick: At work I wear scrubs, and I try to minimize any additional clothing. I don't wear a jacket over my scrubs, and I don't wear any T-shirts under my scrubs. If I'm going to be in a situation that might involve exposure to patient secretions or bodily fluids, then I also wear shoe covers.
Because so many of our patients are infected and we may be called upon at any time to do an aerosol-generating procedure, in the ED we have all taken to wearing N95 masks for our entire shift. I wear a fitted N95 mask. I cover that with a surgical mask.
We are anticipating N95 shortages because our use of the masks has increased exponentially. Every hospital has to think about how to protect their healthcare workers while conserving PPE. We cover the N95 mask with a surgical mask, so that if there is any soiling or droplets reaching the mask, we are able to change the surgical mask and continue to use the same N95.
In addition, eye protection is important. Generally throughout the shift I wear my own goggles. If I'm going to be involved in any procedure with the potential for aerosolization (intubation, performing CPR, bronchoscopy) then I wear a creation of my own, which is a welder's shield.
Many of our providers have chosen to use their own equipment, although we are still able to use hospital-provided equipment. There is probably no difference in effectiveness between these devices.
Cell Phones and Stethoscopes
I carry a personal cell phone at work (which I often use to look things up, use the calculator, and for other purposes), and I'm cognizant that when I touch it, I am potentially transmitting pathogens to my phone or its cover. So I've taken to keeping my phone in a plastic sandwich bag, which I disinfect a couple of times throughout the shift. The phone still works normally.
After my shift, in my "decontamination phase," I remove the phone from the plastic bag and disinfect the phone again.
I try to avoid bringing objects into the vicinity of the patient. That's different from my normal routine—I usually like to write down what the patient has told me—but unfortunately, carrying pen and paper or a clipboard into a patient's room is not feasible at this point. During this time, I've also avoided using my personal stethoscope.
There's also transmission risk associated with shared equipment. We share hospital-provided phones and they must be disinfected. We are each disinfecting our own workspaces: computer, keyboard, mouse, and countertop.
Obviously you are trying to minimize any contact with your mouth or face. You don't want to rub your eyes, touch your nose, or eat anything with your hands while you are at work. The assumption is that you are doing very frequent hand hygiene.
Decontamination Routine
One of our concerns as healthcare providers is the possibility that we could, either asymptomatically or through the objects that we use at work, be bringing the disease home. We want to protect the people who may be at higher risk just because they live with a healthcare provider. These are the decontamination practices I've developed for my own situation, taken from best practices and suggestions from others.
I remove my dirty scrubs and leave them at work, and I change into a clean pair of scrubs or clean clothes. I disinfect any inanimate objects that my hands may have touched during the shift using alcohol, sanitizer wipes, bleach wipes, or hospital-grade chemical wipes.
To keep those objects clean after disinfecting, I place them in clean plastic bags away from other objects (eg, a wallet or purse) that may not be easy to disinfect. Then I store those bags in the trunk of my car for my next shift, so I'm not taking them into my home.
I also change my shoes, leaving my work shoes in the trunk of my car, and wear another pair of shoes into the house.
When I get home, I basically do everything again. I disinfect my phone, I wash my hands, and I shower immediately. At that point, I consider myself sufficiently "disinfected."
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.
Artisanal CBD may provide less seizure control than pharmaceutical CBD
preliminary results of a small study indicate.
Given the widespread use of artisanal CBD products, Nathan T. Cohen, MD, pediatric epilepsy fellow, Children’s National Hospital, Washington, DC, and his colleagues wanted to know how these products differ from pharmaceutical grade CBD with respect to seizure control.
“One of the challenges or questions we have is whether there is any information that would guide us and suggest patients transition from artisanal to pharmaceutical grade CBD,” Dr. Cohen, who is lead author of the study, told Medscape Medical News.
The findings were released February 27 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology. The AAN canceled the meeting and released abstracts and access to presenters for press coverage. The study received no outside funding.
In addition to helping relieve anxiety and stress, CBD, one of many constituents of Cannabis sativa, has antiseizure properties. The US Food and Drug Administration (FDA) has approved a pharmaceutical CBD (Epidiolex, GW Pharmaceuticals) for the management of seizures associated with Lennox-Gastaut syndrome (LGS) and Dravet syndrome.
This purified oral CBD prescription product does not contain tetrahydrocannabinol (THC), the component of marijuana that produces a “high.”
Popular products
Artisanal CBD, which has been around since the late 1970s, is manufactured using variable amounts of CBD and THC. Artisanal products, which typically come in the form of oils that are swallowed, are available in dispensaries and elsewhere, depending on the legal status in individual states.
These artisanal formulations are popular among patients with epilepsy and their families. On the basis of the advertising he sees, Dr. Cohen estimates there are at least 100 artisanal CBD products, but he was quick to stress he’s not an expert on artisanal CBD.
He noted that some families are “searching for an alternative treatment” to help control their child’s seizures, and if the seizure syndrome isn’t LGS or Dravet, “then technically, they don’t qualify for prescription-strength CBD,” said Dr. Cohen.
The current study was a retrospective chart review and included patients with epilepsy who underwent treatment with artisanal or pharmaceutical CBD for whom serum CBD levels were available.
In addition to CBD levels, the researchers had information on patients’ date of birth, gender, epilepsy diagnosis, artisanal or pharmaceutical CBD dose, seizure history, and side effects, among other things.
The analysis included 31 patients (48% female; mean age, about 10 years). Of these, 32% had LGS, 6% had Dravet, and the rest had other epilepsy syndromes.
Of the total, 22 patients participated in a pharmaceutical CBD expanded-access program. The remaining nine patients received artisanal CBD.
The mean serum CBD level was 30.1 ng/mL in the artisanal group and 124 ng/mL in the pharmaceutical group.
Dr. Cohen noted that artisanal products contain lower amounts of CBD because they’re not purified, and they may contain other compounds derived from marijuana.
At the last follow-up, which was a median of 11.8 months, patients who took artisanal CBD had a 70% increase in overall seizures. Dr. Cohen pointed out that some of the hundreds of compounds in marijuana could be “pro-convulsant.”
Some seizure free
The prescription CBD group experienced a 39% reduction in seizures. “Some of these kids had up to hundreds of seizures a day and went down to tens, and some kids became seizure free,” said Dr. Cohen.
Because the study was “looking back in time,” the investigators couldn’t determine whether age, type of epilepsy, or other factors affected seizure control in the two groups, said Dr. Cohen. “One of the limitations of a retrospective study is that we’re not able to control for those factors,” he said.
Eleven patients—all in the prescription CBD group—reported adverse effects, including somnolence, emesis, diarrhea, and diminished appetite; six discontinued CBD because of side effects.
Dr. Cohen said he’s not aware of any study that has compared artisanal products “head to head” with pharmaceutical grade CBD. “The whole point of this study was to ask the question, Is there a difference between the groups?, and these new data would suggest that there may be.”
The results appear to support giving encouragement to patients to transition from artisanal to pharmaceutical CBD if appropriate. “Anytime you’re giving your child a medication that has not been produced under the stringent guidelines that all pharmaceutical FDA-approved medications undergo, you don’t know exactly what’s in the product, and not knowing is a potential issue,” said Dr. Cohen.
The findings need to be studied in a more controlled setting “to make sure they’re valid,” said Dr. Cohen. Because this is “a very hot topic,” he’s keen to see what further research his colleagues would be interested in pursuing.
Commenting on the research, Joseph Sirven, MD, a neurologist in Scottsdale, Arizona, said this is an important study.
“It highlights one of the most common questions that I receive almost on a daily basis in my neurology practice,” he said.
Most people think that dispensary-based CBD is the same as prescription-based CBD, said Dr. Sirven. “Technically and theoretically, they certainly could be; however, what this study highlights is that in practice, they are not the same.”
He stressed that prescription CBD has to meet certain quality standards. “That means that whatever the ingredient list states about the concentration of CBD in the product has to be within the product, which is why the FDA approved it. It is, in essence, a quality control issue.”
A dispensary-based product does not need to meet such stringent standards and so “is subject to whatever the manufacturer chooses to put in the product,” said Dr. Sirven.
The study received no outside funding. Drs. Cohen and Sirven reported no relevant financial relationships.
This article first appeared on Medscape.com.
preliminary results of a small study indicate.
Given the widespread use of artisanal CBD products, Nathan T. Cohen, MD, pediatric epilepsy fellow, Children’s National Hospital, Washington, DC, and his colleagues wanted to know how these products differ from pharmaceutical grade CBD with respect to seizure control.
“One of the challenges or questions we have is whether there is any information that would guide us and suggest patients transition from artisanal to pharmaceutical grade CBD,” Dr. Cohen, who is lead author of the study, told Medscape Medical News.
The findings were released February 27 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology. The AAN canceled the meeting and released abstracts and access to presenters for press coverage. The study received no outside funding.
In addition to helping relieve anxiety and stress, CBD, one of many constituents of Cannabis sativa, has antiseizure properties. The US Food and Drug Administration (FDA) has approved a pharmaceutical CBD (Epidiolex, GW Pharmaceuticals) for the management of seizures associated with Lennox-Gastaut syndrome (LGS) and Dravet syndrome.
This purified oral CBD prescription product does not contain tetrahydrocannabinol (THC), the component of marijuana that produces a “high.”
Popular products
Artisanal CBD, which has been around since the late 1970s, is manufactured using variable amounts of CBD and THC. Artisanal products, which typically come in the form of oils that are swallowed, are available in dispensaries and elsewhere, depending on the legal status in individual states.
These artisanal formulations are popular among patients with epilepsy and their families. On the basis of the advertising he sees, Dr. Cohen estimates there are at least 100 artisanal CBD products, but he was quick to stress he’s not an expert on artisanal CBD.
He noted that some families are “searching for an alternative treatment” to help control their child’s seizures, and if the seizure syndrome isn’t LGS or Dravet, “then technically, they don’t qualify for prescription-strength CBD,” said Dr. Cohen.
The current study was a retrospective chart review and included patients with epilepsy who underwent treatment with artisanal or pharmaceutical CBD for whom serum CBD levels were available.
In addition to CBD levels, the researchers had information on patients’ date of birth, gender, epilepsy diagnosis, artisanal or pharmaceutical CBD dose, seizure history, and side effects, among other things.
The analysis included 31 patients (48% female; mean age, about 10 years). Of these, 32% had LGS, 6% had Dravet, and the rest had other epilepsy syndromes.
Of the total, 22 patients participated in a pharmaceutical CBD expanded-access program. The remaining nine patients received artisanal CBD.
The mean serum CBD level was 30.1 ng/mL in the artisanal group and 124 ng/mL in the pharmaceutical group.
Dr. Cohen noted that artisanal products contain lower amounts of CBD because they’re not purified, and they may contain other compounds derived from marijuana.
At the last follow-up, which was a median of 11.8 months, patients who took artisanal CBD had a 70% increase in overall seizures. Dr. Cohen pointed out that some of the hundreds of compounds in marijuana could be “pro-convulsant.”
Some seizure free
The prescription CBD group experienced a 39% reduction in seizures. “Some of these kids had up to hundreds of seizures a day and went down to tens, and some kids became seizure free,” said Dr. Cohen.
Because the study was “looking back in time,” the investigators couldn’t determine whether age, type of epilepsy, or other factors affected seizure control in the two groups, said Dr. Cohen. “One of the limitations of a retrospective study is that we’re not able to control for those factors,” he said.
Eleven patients—all in the prescription CBD group—reported adverse effects, including somnolence, emesis, diarrhea, and diminished appetite; six discontinued CBD because of side effects.
Dr. Cohen said he’s not aware of any study that has compared artisanal products “head to head” with pharmaceutical grade CBD. “The whole point of this study was to ask the question, Is there a difference between the groups?, and these new data would suggest that there may be.”
The results appear to support giving encouragement to patients to transition from artisanal to pharmaceutical CBD if appropriate. “Anytime you’re giving your child a medication that has not been produced under the stringent guidelines that all pharmaceutical FDA-approved medications undergo, you don’t know exactly what’s in the product, and not knowing is a potential issue,” said Dr. Cohen.
The findings need to be studied in a more controlled setting “to make sure they’re valid,” said Dr. Cohen. Because this is “a very hot topic,” he’s keen to see what further research his colleagues would be interested in pursuing.
Commenting on the research, Joseph Sirven, MD, a neurologist in Scottsdale, Arizona, said this is an important study.
“It highlights one of the most common questions that I receive almost on a daily basis in my neurology practice,” he said.
Most people think that dispensary-based CBD is the same as prescription-based CBD, said Dr. Sirven. “Technically and theoretically, they certainly could be; however, what this study highlights is that in practice, they are not the same.”
He stressed that prescription CBD has to meet certain quality standards. “That means that whatever the ingredient list states about the concentration of CBD in the product has to be within the product, which is why the FDA approved it. It is, in essence, a quality control issue.”
A dispensary-based product does not need to meet such stringent standards and so “is subject to whatever the manufacturer chooses to put in the product,” said Dr. Sirven.
The study received no outside funding. Drs. Cohen and Sirven reported no relevant financial relationships.
This article first appeared on Medscape.com.
preliminary results of a small study indicate.
Given the widespread use of artisanal CBD products, Nathan T. Cohen, MD, pediatric epilepsy fellow, Children’s National Hospital, Washington, DC, and his colleagues wanted to know how these products differ from pharmaceutical grade CBD with respect to seizure control.
“One of the challenges or questions we have is whether there is any information that would guide us and suggest patients transition from artisanal to pharmaceutical grade CBD,” Dr. Cohen, who is lead author of the study, told Medscape Medical News.
The findings were released February 27 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology. The AAN canceled the meeting and released abstracts and access to presenters for press coverage. The study received no outside funding.
In addition to helping relieve anxiety and stress, CBD, one of many constituents of Cannabis sativa, has antiseizure properties. The US Food and Drug Administration (FDA) has approved a pharmaceutical CBD (Epidiolex, GW Pharmaceuticals) for the management of seizures associated with Lennox-Gastaut syndrome (LGS) and Dravet syndrome.
This purified oral CBD prescription product does not contain tetrahydrocannabinol (THC), the component of marijuana that produces a “high.”
Popular products
Artisanal CBD, which has been around since the late 1970s, is manufactured using variable amounts of CBD and THC. Artisanal products, which typically come in the form of oils that are swallowed, are available in dispensaries and elsewhere, depending on the legal status in individual states.
These artisanal formulations are popular among patients with epilepsy and their families. On the basis of the advertising he sees, Dr. Cohen estimates there are at least 100 artisanal CBD products, but he was quick to stress he’s not an expert on artisanal CBD.
He noted that some families are “searching for an alternative treatment” to help control their child’s seizures, and if the seizure syndrome isn’t LGS or Dravet, “then technically, they don’t qualify for prescription-strength CBD,” said Dr. Cohen.
The current study was a retrospective chart review and included patients with epilepsy who underwent treatment with artisanal or pharmaceutical CBD for whom serum CBD levels were available.
In addition to CBD levels, the researchers had information on patients’ date of birth, gender, epilepsy diagnosis, artisanal or pharmaceutical CBD dose, seizure history, and side effects, among other things.
The analysis included 31 patients (48% female; mean age, about 10 years). Of these, 32% had LGS, 6% had Dravet, and the rest had other epilepsy syndromes.
Of the total, 22 patients participated in a pharmaceutical CBD expanded-access program. The remaining nine patients received artisanal CBD.
The mean serum CBD level was 30.1 ng/mL in the artisanal group and 124 ng/mL in the pharmaceutical group.
Dr. Cohen noted that artisanal products contain lower amounts of CBD because they’re not purified, and they may contain other compounds derived from marijuana.
At the last follow-up, which was a median of 11.8 months, patients who took artisanal CBD had a 70% increase in overall seizures. Dr. Cohen pointed out that some of the hundreds of compounds in marijuana could be “pro-convulsant.”
Some seizure free
The prescription CBD group experienced a 39% reduction in seizures. “Some of these kids had up to hundreds of seizures a day and went down to tens, and some kids became seizure free,” said Dr. Cohen.
Because the study was “looking back in time,” the investigators couldn’t determine whether age, type of epilepsy, or other factors affected seizure control in the two groups, said Dr. Cohen. “One of the limitations of a retrospective study is that we’re not able to control for those factors,” he said.
Eleven patients—all in the prescription CBD group—reported adverse effects, including somnolence, emesis, diarrhea, and diminished appetite; six discontinued CBD because of side effects.
Dr. Cohen said he’s not aware of any study that has compared artisanal products “head to head” with pharmaceutical grade CBD. “The whole point of this study was to ask the question, Is there a difference between the groups?, and these new data would suggest that there may be.”
The results appear to support giving encouragement to patients to transition from artisanal to pharmaceutical CBD if appropriate. “Anytime you’re giving your child a medication that has not been produced under the stringent guidelines that all pharmaceutical FDA-approved medications undergo, you don’t know exactly what’s in the product, and not knowing is a potential issue,” said Dr. Cohen.
The findings need to be studied in a more controlled setting “to make sure they’re valid,” said Dr. Cohen. Because this is “a very hot topic,” he’s keen to see what further research his colleagues would be interested in pursuing.
Commenting on the research, Joseph Sirven, MD, a neurologist in Scottsdale, Arizona, said this is an important study.
“It highlights one of the most common questions that I receive almost on a daily basis in my neurology practice,” he said.
Most people think that dispensary-based CBD is the same as prescription-based CBD, said Dr. Sirven. “Technically and theoretically, they certainly could be; however, what this study highlights is that in practice, they are not the same.”
He stressed that prescription CBD has to meet certain quality standards. “That means that whatever the ingredient list states about the concentration of CBD in the product has to be within the product, which is why the FDA approved it. It is, in essence, a quality control issue.”
A dispensary-based product does not need to meet such stringent standards and so “is subject to whatever the manufacturer chooses to put in the product,” said Dr. Sirven.
The study received no outside funding. Drs. Cohen and Sirven reported no relevant financial relationships.
This article first appeared on Medscape.com.
Signature STEMI sign may be less diagnostic in the COVID-19 age
The signature electrocardiographic sign indicating ST-segment-elevation MI may be a less-consistent indicator of actual STEMI at a time when patients with COVID-19 have come to overwhelm many hospital ICUs.
Many of the 18 such patients identified at six New York City hospitals who showed ST-segment elevation on their 12-lead ECG in the city’s first month of fighting the pandemic turned out to be free of either obstructive coronary artery disease by angiography or of regional wall-motion abnormalities (RWMA) by ECG, according to a letter published in the New England Journal of Medicine.
Those 10 patients in the 18-case series were said to have noncoronary myocardial injury, perhaps from myocarditis – a prevalent feature of severe COVID-19 – and the remaining 8 patients with obstructive coronary artery disease, RWMA, or both were diagnosed with STEMI. Of the latter patients, six went to the cath lab and five of those underwent percutaneous coronary intervention, Sripal Bangalore, MD, MHA, of New York University, and colleagues reported.
In an interview, Dr. Bangalore framed the case-series report as a caution against substituting fibrinolytic therapy for primary percutaneous coronary intervention in patients with STE while hospitals are unusually burdened by the COVID-19 pandemic and invasive procedures intensify the threat of SARS-CoV-2 exposure to clinicians.
The strategy was recently advanced as an option for highly selected patients in a statement from the American College of Cardiology and Society for Cardiovascular Angiography and Interventions (SCAI).
“During the COVID-19 pandemic, one of the main reasons fibrinolytic therapy has been pushed is to reduce the exposure to the cath-lab staff,” Dr. Bangalore observed. “But if you pursue that route, it’s problematic because more than half may not have obstructive disease and fibrinolytic therapy may not help. And if you give them fibrinolytics, you’re potentially increasing their risk of bleeding complications.
“The take-home from these 18 patients is that it’s very difficult to guess who is going to have obstructive disease and who is going to have nonobstructive disease,” Dr. Bangalore said. “Maybe we should assess these patients with not just an ECG but with a quick echo, then make a decision. Our practice so far has been to take these patients to the cath lab.”
The ACC/SCAI statement proposed that “fibrinolysis can be considered an option for the relatively stable STEMI patient with active COVID-19” after careful consideration of possible patient benefit versus the risks of cath-lab personnel exposure to the virus.
Only six patients in the current series, including five in the STEMI group, are reported to have had chest pain at about the time of STE, observed Michael J. Blaha, MD, MPH, of Johns Hopkins Hospital, Baltimore.
So, he said in an interview, “one of their points is that you have to take ST elevations with a grain of salt in this [COVID-19] era, because there are a lot of people presenting with ST elevations in the absence of chest pain.”
That, and the high prevalence of nonobstructive disease in the series, indeed argues against the use of fibrinolytic therapy in such patients, Dr. Blaha said.
Normally, when there is STE, “the pretest probability of STEMI is so high, and if you can’t make it to the cath lab for some reason, sure, it makes sense to give lytics.” However, he said, “COVID-19 is changing the clinical landscape. Now, with a variety of virus-mediated myocardial injury presentations, including myocarditis, the pretest probability of MI is lower.”
The current report “confirms that, in the COVID era, ST elevations are not diagnostic for MI and must be considered within the totality of clinical evidence, and a conservative approach to going to the cath lab is probably warranted,” Dr. Blaha said in an interview.
However, with the reduced pretest probability of STE for STEMI, he agreed, “I almost don’t see any scenario where I’d be comfortable, based on ECG changes alone, giving lytics at this time.”
Dr. Bangalore pointed out that all of the 18 patients in the series had elevated levels of the fibrin degradation product D-dimer, a biomarker that reflects ongoing hemostatic activation. Levels were higher in the 8 patients who ultimately received a STEMI diagnosis than in the remaining 10 patients.
But COVID-19 patients in general may have elevated D-dimer and “a lot of microthrombi,” he said. “So the question is, are those microthrombi also causal for any of the ECG changes we are also seeing?”
Aside from microthrombi, global hypoxia and myocarditis could be other potential causes of STE in COVID-19 patients in the absence of STEMI, Dr. Bangalore proposed. “At this point we just generally don’t know.”
Dr. Bangalore reported no conflicts; disclosures for the other authors are available at nejm.org. Dr. Blaha disclosed receiving grants from Amgen and serving on advisory boards for Amgen and other pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
The signature electrocardiographic sign indicating ST-segment-elevation MI may be a less-consistent indicator of actual STEMI at a time when patients with COVID-19 have come to overwhelm many hospital ICUs.
Many of the 18 such patients identified at six New York City hospitals who showed ST-segment elevation on their 12-lead ECG in the city’s first month of fighting the pandemic turned out to be free of either obstructive coronary artery disease by angiography or of regional wall-motion abnormalities (RWMA) by ECG, according to a letter published in the New England Journal of Medicine.
Those 10 patients in the 18-case series were said to have noncoronary myocardial injury, perhaps from myocarditis – a prevalent feature of severe COVID-19 – and the remaining 8 patients with obstructive coronary artery disease, RWMA, or both were diagnosed with STEMI. Of the latter patients, six went to the cath lab and five of those underwent percutaneous coronary intervention, Sripal Bangalore, MD, MHA, of New York University, and colleagues reported.
In an interview, Dr. Bangalore framed the case-series report as a caution against substituting fibrinolytic therapy for primary percutaneous coronary intervention in patients with STE while hospitals are unusually burdened by the COVID-19 pandemic and invasive procedures intensify the threat of SARS-CoV-2 exposure to clinicians.
The strategy was recently advanced as an option for highly selected patients in a statement from the American College of Cardiology and Society for Cardiovascular Angiography and Interventions (SCAI).
“During the COVID-19 pandemic, one of the main reasons fibrinolytic therapy has been pushed is to reduce the exposure to the cath-lab staff,” Dr. Bangalore observed. “But if you pursue that route, it’s problematic because more than half may not have obstructive disease and fibrinolytic therapy may not help. And if you give them fibrinolytics, you’re potentially increasing their risk of bleeding complications.
“The take-home from these 18 patients is that it’s very difficult to guess who is going to have obstructive disease and who is going to have nonobstructive disease,” Dr. Bangalore said. “Maybe we should assess these patients with not just an ECG but with a quick echo, then make a decision. Our practice so far has been to take these patients to the cath lab.”
The ACC/SCAI statement proposed that “fibrinolysis can be considered an option for the relatively stable STEMI patient with active COVID-19” after careful consideration of possible patient benefit versus the risks of cath-lab personnel exposure to the virus.
Only six patients in the current series, including five in the STEMI group, are reported to have had chest pain at about the time of STE, observed Michael J. Blaha, MD, MPH, of Johns Hopkins Hospital, Baltimore.
So, he said in an interview, “one of their points is that you have to take ST elevations with a grain of salt in this [COVID-19] era, because there are a lot of people presenting with ST elevations in the absence of chest pain.”
That, and the high prevalence of nonobstructive disease in the series, indeed argues against the use of fibrinolytic therapy in such patients, Dr. Blaha said.
Normally, when there is STE, “the pretest probability of STEMI is so high, and if you can’t make it to the cath lab for some reason, sure, it makes sense to give lytics.” However, he said, “COVID-19 is changing the clinical landscape. Now, with a variety of virus-mediated myocardial injury presentations, including myocarditis, the pretest probability of MI is lower.”
The current report “confirms that, in the COVID era, ST elevations are not diagnostic for MI and must be considered within the totality of clinical evidence, and a conservative approach to going to the cath lab is probably warranted,” Dr. Blaha said in an interview.
However, with the reduced pretest probability of STE for STEMI, he agreed, “I almost don’t see any scenario where I’d be comfortable, based on ECG changes alone, giving lytics at this time.”
Dr. Bangalore pointed out that all of the 18 patients in the series had elevated levels of the fibrin degradation product D-dimer, a biomarker that reflects ongoing hemostatic activation. Levels were higher in the 8 patients who ultimately received a STEMI diagnosis than in the remaining 10 patients.
But COVID-19 patients in general may have elevated D-dimer and “a lot of microthrombi,” he said. “So the question is, are those microthrombi also causal for any of the ECG changes we are also seeing?”
Aside from microthrombi, global hypoxia and myocarditis could be other potential causes of STE in COVID-19 patients in the absence of STEMI, Dr. Bangalore proposed. “At this point we just generally don’t know.”
Dr. Bangalore reported no conflicts; disclosures for the other authors are available at nejm.org. Dr. Blaha disclosed receiving grants from Amgen and serving on advisory boards for Amgen and other pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
The signature electrocardiographic sign indicating ST-segment-elevation MI may be a less-consistent indicator of actual STEMI at a time when patients with COVID-19 have come to overwhelm many hospital ICUs.
Many of the 18 such patients identified at six New York City hospitals who showed ST-segment elevation on their 12-lead ECG in the city’s first month of fighting the pandemic turned out to be free of either obstructive coronary artery disease by angiography or of regional wall-motion abnormalities (RWMA) by ECG, according to a letter published in the New England Journal of Medicine.
Those 10 patients in the 18-case series were said to have noncoronary myocardial injury, perhaps from myocarditis – a prevalent feature of severe COVID-19 – and the remaining 8 patients with obstructive coronary artery disease, RWMA, or both were diagnosed with STEMI. Of the latter patients, six went to the cath lab and five of those underwent percutaneous coronary intervention, Sripal Bangalore, MD, MHA, of New York University, and colleagues reported.
In an interview, Dr. Bangalore framed the case-series report as a caution against substituting fibrinolytic therapy for primary percutaneous coronary intervention in patients with STE while hospitals are unusually burdened by the COVID-19 pandemic and invasive procedures intensify the threat of SARS-CoV-2 exposure to clinicians.
The strategy was recently advanced as an option for highly selected patients in a statement from the American College of Cardiology and Society for Cardiovascular Angiography and Interventions (SCAI).
“During the COVID-19 pandemic, one of the main reasons fibrinolytic therapy has been pushed is to reduce the exposure to the cath-lab staff,” Dr. Bangalore observed. “But if you pursue that route, it’s problematic because more than half may not have obstructive disease and fibrinolytic therapy may not help. And if you give them fibrinolytics, you’re potentially increasing their risk of bleeding complications.
“The take-home from these 18 patients is that it’s very difficult to guess who is going to have obstructive disease and who is going to have nonobstructive disease,” Dr. Bangalore said. “Maybe we should assess these patients with not just an ECG but with a quick echo, then make a decision. Our practice so far has been to take these patients to the cath lab.”
The ACC/SCAI statement proposed that “fibrinolysis can be considered an option for the relatively stable STEMI patient with active COVID-19” after careful consideration of possible patient benefit versus the risks of cath-lab personnel exposure to the virus.
Only six patients in the current series, including five in the STEMI group, are reported to have had chest pain at about the time of STE, observed Michael J. Blaha, MD, MPH, of Johns Hopkins Hospital, Baltimore.
So, he said in an interview, “one of their points is that you have to take ST elevations with a grain of salt in this [COVID-19] era, because there are a lot of people presenting with ST elevations in the absence of chest pain.”
That, and the high prevalence of nonobstructive disease in the series, indeed argues against the use of fibrinolytic therapy in such patients, Dr. Blaha said.
Normally, when there is STE, “the pretest probability of STEMI is so high, and if you can’t make it to the cath lab for some reason, sure, it makes sense to give lytics.” However, he said, “COVID-19 is changing the clinical landscape. Now, with a variety of virus-mediated myocardial injury presentations, including myocarditis, the pretest probability of MI is lower.”
The current report “confirms that, in the COVID era, ST elevations are not diagnostic for MI and must be considered within the totality of clinical evidence, and a conservative approach to going to the cath lab is probably warranted,” Dr. Blaha said in an interview.
However, with the reduced pretest probability of STE for STEMI, he agreed, “I almost don’t see any scenario where I’d be comfortable, based on ECG changes alone, giving lytics at this time.”
Dr. Bangalore pointed out that all of the 18 patients in the series had elevated levels of the fibrin degradation product D-dimer, a biomarker that reflects ongoing hemostatic activation. Levels were higher in the 8 patients who ultimately received a STEMI diagnosis than in the remaining 10 patients.
But COVID-19 patients in general may have elevated D-dimer and “a lot of microthrombi,” he said. “So the question is, are those microthrombi also causal for any of the ECG changes we are also seeing?”
Aside from microthrombi, global hypoxia and myocarditis could be other potential causes of STE in COVID-19 patients in the absence of STEMI, Dr. Bangalore proposed. “At this point we just generally don’t know.”
Dr. Bangalore reported no conflicts; disclosures for the other authors are available at nejm.org. Dr. Blaha disclosed receiving grants from Amgen and serving on advisory boards for Amgen and other pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
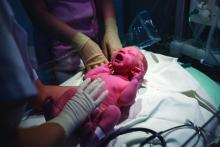
Management of infants born to mothers with COVID-19
Initial guidance for pediatric hospitalists
Clinical question: How should we care for newborns born to mothers with COVID-19?
Background: Around the United States, the SARS-CoV-2 virus is infecting pregnant mothers and causing COVID-19. Current limited data demonstrates that children under the age of 1 year are at risk for severe disease. Clinicians are caring for infants born to mothers with COVID-19 during the pandemic with minimal guidance.
Study design: Clinical practice guidelines.
Synopsis: The American Academy of Pediatrics’ Committee on Fetus and Newborn, Section on Neonatal and Perinatal Medicine and Committee of Infectious Diseases developed guidelines of care for infants born to COVID-19 mothers to help clinicians care for newborns using limited data published before March 30, 2020.
- Neonates should be considered persons under investigation (PUIs) if they are born to mothers with diagnosed COVID-19 or with COVID-19 tests pending at the time of delivery.
- Neonatal clinicians should attend deliveries based on their center’s policies. If clinicians are required to perform stabilization they should use airborne, droplet, and contact personal protective equipment (PPE). This includes, gown, gloves, eye protection (goggles or face shield), and N95 respirator mask or an air-purifying respirator.
- Mother and newborn should be separated to minimize the infant’s risk of postnatal infection.
- Well newborns born at or near term may be admitted to areas physically separated from newborns unaffected by maternal COVID-19. Alternatively, a mother may room-in with her infant with 6 feet of separation between mother and infant. Newborn PUIs should be bathed as soon as possible.
- Newborns requiring intensive care should be admitted to a single negative-pressure room. Alternatively, COVID-19–exposed infants should be grouped with a minimum of 6 feet of separation, or placed in air temperature-controlled isolettes.
- Until the newborn PUI’s virologic status is known, clinical staff caring for the infant should use droplet and contact PPE. This includes gown, gloves, eye protection (goggles or face shield), and a standard surgical mask. Airborne, droplet, and contact precautions should be used for infants requiring CPAP or any form of mechanical ventilation.
- COVID-19–positive mothers who want to breastfeed may feed expressed breast milk using proper breast and hand hygiene or directly breastfeed their infants wearing a mask while practicing proper breast and hand hygiene.
- If testing is available, newborns should be tested for SARS-CoV-2 using molecular arrays. If testing is unavailable, clinicians may monitor newborns clinically. Infants should be tested if they require prolonged intensive care.
- Optimal timing and extent of testing is unknown. Tests should be performed around 24 hours of life and 48 hours of life. If discharge is planned for a well appearing infant before 48 hours of life, the clinician may choose not to do the 48-hour test. A single swab should be taken from the throat followed by the nasopharynx to perform the test.
- Newborns should receive all newborn care, including circumcision if requested.
- Infants who are asymptomatic with positive or pending SARS-CoV-2 tests may be discharged home with plans for frequent outpatient follow-up through 14 days after birth. Infants with negative SARS-CoV-2 testing should be discharged to the care of a noninfected caregiver. If the mother lives in the same household, she must keep a distance of 6 feet as often as possible. When not possible, the mother should wear a mask and practice hand hygiene. The mother may resume caring for her infant normally when she has been afebrile for more than 72 hours (without antipyretics) and has been asymptomatic for 7 days. Alternatively, the mother may resume care if she has two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
- Visitation to infants requiring intensive care should be limited for mothers with COVID-19 until her fever has resolved for more than 72 hours and has improvement of respiratory symptoms and has had two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
Bottom line: Clinicians should protect themselves with contact and droplet PPE at all times until the infant’s viral status is known. Clinicians should use airborne, contact, and droplet PPE when resuscitating the infant and/or when using CPAP/mechanical ventilation. Mothers should be encouraged to feed their infants expressed breast milk while practicing proper hygiene or directly breastfeed while wearing a mask and practicing proper hygiene. Viral testing of every infant born to a mother with COVID-19 should be performed after the infant is 24 hours old. Mothers should resume caring for their infants normally after they have met criteria suggesting they are no longer actively infected.
Article citation: Puopolo KM, Hudak ML, Kimberlin DW, Cummings J. Initial Guidance: Management of Infants born to Mothers with COVID-19. 2020 Apr 2. https://downloads.aap.org/AAP/PDF/COVID%2019%20Initial%20Newborn%20Guidance.pdf. Accessed Apr 2, 2020.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the Pediatrics Editor for The Hospitalist.
Initial guidance for pediatric hospitalists
Initial guidance for pediatric hospitalists
Clinical question: How should we care for newborns born to mothers with COVID-19?
Background: Around the United States, the SARS-CoV-2 virus is infecting pregnant mothers and causing COVID-19. Current limited data demonstrates that children under the age of 1 year are at risk for severe disease. Clinicians are caring for infants born to mothers with COVID-19 during the pandemic with minimal guidance.
Study design: Clinical practice guidelines.
Synopsis: The American Academy of Pediatrics’ Committee on Fetus and Newborn, Section on Neonatal and Perinatal Medicine and Committee of Infectious Diseases developed guidelines of care for infants born to COVID-19 mothers to help clinicians care for newborns using limited data published before March 30, 2020.
- Neonates should be considered persons under investigation (PUIs) if they are born to mothers with diagnosed COVID-19 or with COVID-19 tests pending at the time of delivery.
- Neonatal clinicians should attend deliveries based on their center’s policies. If clinicians are required to perform stabilization they should use airborne, droplet, and contact personal protective equipment (PPE). This includes, gown, gloves, eye protection (goggles or face shield), and N95 respirator mask or an air-purifying respirator.
- Mother and newborn should be separated to minimize the infant’s risk of postnatal infection.
- Well newborns born at or near term may be admitted to areas physically separated from newborns unaffected by maternal COVID-19. Alternatively, a mother may room-in with her infant with 6 feet of separation between mother and infant. Newborn PUIs should be bathed as soon as possible.
- Newborns requiring intensive care should be admitted to a single negative-pressure room. Alternatively, COVID-19–exposed infants should be grouped with a minimum of 6 feet of separation, or placed in air temperature-controlled isolettes.
- Until the newborn PUI’s virologic status is known, clinical staff caring for the infant should use droplet and contact PPE. This includes gown, gloves, eye protection (goggles or face shield), and a standard surgical mask. Airborne, droplet, and contact precautions should be used for infants requiring CPAP or any form of mechanical ventilation.
- COVID-19–positive mothers who want to breastfeed may feed expressed breast milk using proper breast and hand hygiene or directly breastfeed their infants wearing a mask while practicing proper breast and hand hygiene.
- If testing is available, newborns should be tested for SARS-CoV-2 using molecular arrays. If testing is unavailable, clinicians may monitor newborns clinically. Infants should be tested if they require prolonged intensive care.
- Optimal timing and extent of testing is unknown. Tests should be performed around 24 hours of life and 48 hours of life. If discharge is planned for a well appearing infant before 48 hours of life, the clinician may choose not to do the 48-hour test. A single swab should be taken from the throat followed by the nasopharynx to perform the test.
- Newborns should receive all newborn care, including circumcision if requested.
- Infants who are asymptomatic with positive or pending SARS-CoV-2 tests may be discharged home with plans for frequent outpatient follow-up through 14 days after birth. Infants with negative SARS-CoV-2 testing should be discharged to the care of a noninfected caregiver. If the mother lives in the same household, she must keep a distance of 6 feet as often as possible. When not possible, the mother should wear a mask and practice hand hygiene. The mother may resume caring for her infant normally when she has been afebrile for more than 72 hours (without antipyretics) and has been asymptomatic for 7 days. Alternatively, the mother may resume care if she has two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
- Visitation to infants requiring intensive care should be limited for mothers with COVID-19 until her fever has resolved for more than 72 hours and has improvement of respiratory symptoms and has had two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
Bottom line: Clinicians should protect themselves with contact and droplet PPE at all times until the infant’s viral status is known. Clinicians should use airborne, contact, and droplet PPE when resuscitating the infant and/or when using CPAP/mechanical ventilation. Mothers should be encouraged to feed their infants expressed breast milk while practicing proper hygiene or directly breastfeed while wearing a mask and practicing proper hygiene. Viral testing of every infant born to a mother with COVID-19 should be performed after the infant is 24 hours old. Mothers should resume caring for their infants normally after they have met criteria suggesting they are no longer actively infected.
Article citation: Puopolo KM, Hudak ML, Kimberlin DW, Cummings J. Initial Guidance: Management of Infants born to Mothers with COVID-19. 2020 Apr 2. https://downloads.aap.org/AAP/PDF/COVID%2019%20Initial%20Newborn%20Guidance.pdf. Accessed Apr 2, 2020.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the Pediatrics Editor for The Hospitalist.
Clinical question: How should we care for newborns born to mothers with COVID-19?
Background: Around the United States, the SARS-CoV-2 virus is infecting pregnant mothers and causing COVID-19. Current limited data demonstrates that children under the age of 1 year are at risk for severe disease. Clinicians are caring for infants born to mothers with COVID-19 during the pandemic with minimal guidance.
Study design: Clinical practice guidelines.
Synopsis: The American Academy of Pediatrics’ Committee on Fetus and Newborn, Section on Neonatal and Perinatal Medicine and Committee of Infectious Diseases developed guidelines of care for infants born to COVID-19 mothers to help clinicians care for newborns using limited data published before March 30, 2020.
- Neonates should be considered persons under investigation (PUIs) if they are born to mothers with diagnosed COVID-19 or with COVID-19 tests pending at the time of delivery.
- Neonatal clinicians should attend deliveries based on their center’s policies. If clinicians are required to perform stabilization they should use airborne, droplet, and contact personal protective equipment (PPE). This includes, gown, gloves, eye protection (goggles or face shield), and N95 respirator mask or an air-purifying respirator.
- Mother and newborn should be separated to minimize the infant’s risk of postnatal infection.
- Well newborns born at or near term may be admitted to areas physically separated from newborns unaffected by maternal COVID-19. Alternatively, a mother may room-in with her infant with 6 feet of separation between mother and infant. Newborn PUIs should be bathed as soon as possible.
- Newborns requiring intensive care should be admitted to a single negative-pressure room. Alternatively, COVID-19–exposed infants should be grouped with a minimum of 6 feet of separation, or placed in air temperature-controlled isolettes.
- Until the newborn PUI’s virologic status is known, clinical staff caring for the infant should use droplet and contact PPE. This includes gown, gloves, eye protection (goggles or face shield), and a standard surgical mask. Airborne, droplet, and contact precautions should be used for infants requiring CPAP or any form of mechanical ventilation.
- COVID-19–positive mothers who want to breastfeed may feed expressed breast milk using proper breast and hand hygiene or directly breastfeed their infants wearing a mask while practicing proper breast and hand hygiene.
- If testing is available, newborns should be tested for SARS-CoV-2 using molecular arrays. If testing is unavailable, clinicians may monitor newborns clinically. Infants should be tested if they require prolonged intensive care.
- Optimal timing and extent of testing is unknown. Tests should be performed around 24 hours of life and 48 hours of life. If discharge is planned for a well appearing infant before 48 hours of life, the clinician may choose not to do the 48-hour test. A single swab should be taken from the throat followed by the nasopharynx to perform the test.
- Newborns should receive all newborn care, including circumcision if requested.
- Infants who are asymptomatic with positive or pending SARS-CoV-2 tests may be discharged home with plans for frequent outpatient follow-up through 14 days after birth. Infants with negative SARS-CoV-2 testing should be discharged to the care of a noninfected caregiver. If the mother lives in the same household, she must keep a distance of 6 feet as often as possible. When not possible, the mother should wear a mask and practice hand hygiene. The mother may resume caring for her infant normally when she has been afebrile for more than 72 hours (without antipyretics) and has been asymptomatic for 7 days. Alternatively, the mother may resume care if she has two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
- Visitation to infants requiring intensive care should be limited for mothers with COVID-19 until her fever has resolved for more than 72 hours and has improvement of respiratory symptoms and has had two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
Bottom line: Clinicians should protect themselves with contact and droplet PPE at all times until the infant’s viral status is known. Clinicians should use airborne, contact, and droplet PPE when resuscitating the infant and/or when using CPAP/mechanical ventilation. Mothers should be encouraged to feed their infants expressed breast milk while practicing proper hygiene or directly breastfeed while wearing a mask and practicing proper hygiene. Viral testing of every infant born to a mother with COVID-19 should be performed after the infant is 24 hours old. Mothers should resume caring for their infants normally after they have met criteria suggesting they are no longer actively infected.
Article citation: Puopolo KM, Hudak ML, Kimberlin DW, Cummings J. Initial Guidance: Management of Infants born to Mothers with COVID-19. 2020 Apr 2. https://downloads.aap.org/AAP/PDF/COVID%2019%20Initial%20Newborn%20Guidance.pdf. Accessed Apr 2, 2020.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the Pediatrics Editor for The Hospitalist.
Get triage plans in place before COVID-19 surge hits, critical care experts say
, according to authors of recent reports that offer advice on how to prepare for surges in demand.
Even modest numbers of critically ill COVID-19 patients have already rapidly overwhelmed existing hospital capacity in hard-hit areas including Italy, Spain, and New York City, said authors of an expert panel report released in CHEST.
“The ethical burden this places on hospitals, health systems, and society is enormous,” said Ryan C. Maves, MD, FCCP, of the Naval Medical Center in San Diego, lead author of the expert panel report from the Task Force for Mass Critical Care and the American College of Chest Physicians (CHEST).
Triage decisions could be especially daunting for resource-intensive therapies such as extracorporeal membrane oxygenation (ECMO), as physicians may be forced to decide when and if to offer such support after demand outstrips a hospital’s ability to provide it.
“ECMO requires a lot of specialized capability to initiate on a patient, and then, it requires a lot of specialized capability to maintain and do safely,” said Steven P. Keller, MD, of the division of emergency critical care medicine in the department of emergency medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Those resource requirements can present a challenge to health care systems already overtaxed by COVID-19, according to Dr. Keller, coauthor of a guidance document in Annals of the American Thoracic Society. The guidance suggests a pandemic approach to ECMO response that’s tiered depending on the intensity of the surge over usual hospital volumes.
Mild surges call for a focus on increasing ECMO capacity, while a moderate surge may indicate a need to focus on allocating scarce resources, and a major surge may signal the need to limit or defer use of scarce resources, according to the guidance.
“If your health care system is stretched from a resource standpoint, at what point do you say, ‘we don’t even have the capability to even safely do ECMO, and so, perhaps we should not even be offering the support’?” Dr. Keller said in an interview. “That’s what we tried to get at in the paper – helping institutions think about how to prepare for that pandemic, and then when to make decisions on when it should and should not be offered.”
Critical care guidance for COVID-19
The guidance from the Task Force for Mass Critical Care and CHEST offers nine specific actions that authors suggest as part of a framework for communities to establish the infrastructure needed to triage critical care resources and “equitably” meet the needs of the largest number of COVID-19 patients.
“It is the goal of the task force to minimize the need for allocation of scarce resources as much as possible,” the authors stated.
The framework starts with surge planning that includes an inventory of intensive care unit resources such as ventilators, beds, supplies, and staff that could be marshaled to meet a surge in demand, followed by establishing “identification triggers” for triage initiation by a regional authority, should clinical demand reach a crisis stage.
The next step is preparing the triage system, which includes creating a committee at the regional level, identifying members of tertiary triage teams and the support structures they will need, and preparing and distributing training materials.
Agreeing on a triage protocol is important to ensure equitable targeting of resources, and how to allocate limited life-sustaining measures needs to be considered, according to the panel of experts. They also recommend adaptations to the standards of care such as modification of end-of-life care policies, support for health care workers, family, and the public, and consideration of pediatric issues including transport, concentration of care at specific centers, and potential increases in age thresholds to accommodate surges.
Barriers to triage?
When asked about potential barriers to rolling out a triage plan, Dr. Maves said the first is acknowledging the possible need for such a plan: “It is a difficult concept for most in critical care to accept – the idea that we may not be able to provide an individual patient with interventions that we consider routine,” he said.
Beyond acknowledging need, other potential barriers to successful implementation include the limited evidence base to support development of these protocols, as well as the need to address public trust.
“If a triage system is perceived as unjust or biased, or if people think that triage favors or excludes certain groups unfairly, it will undermine any system,” Dr. Maves said. “Making sure the public both understands and has input into system development is critical if we are going to be able to make this work.”
Dr. Maves and coauthors reported that some of the authors of their guidance are United States government employees or military service members, and that their opinions and assertions do not reflect the official views or position of those institutions. Dr. Keller reported no disclosures related to the ECMO guidance.
SOURCES: Maves RC et al. Chest. 2020 Apr 11. pii: S0012-3692(20)30691-7. doi: 10.1016/j.chest.2020.03.063; Seethara R and Keller SP. Ann Am Thorac Soc. 2020 Apr 15. doi: 10.1513/AnnalsATS.202003-233PS.
, according to authors of recent reports that offer advice on how to prepare for surges in demand.
Even modest numbers of critically ill COVID-19 patients have already rapidly overwhelmed existing hospital capacity in hard-hit areas including Italy, Spain, and New York City, said authors of an expert panel report released in CHEST.
“The ethical burden this places on hospitals, health systems, and society is enormous,” said Ryan C. Maves, MD, FCCP, of the Naval Medical Center in San Diego, lead author of the expert panel report from the Task Force for Mass Critical Care and the American College of Chest Physicians (CHEST).
Triage decisions could be especially daunting for resource-intensive therapies such as extracorporeal membrane oxygenation (ECMO), as physicians may be forced to decide when and if to offer such support after demand outstrips a hospital’s ability to provide it.
“ECMO requires a lot of specialized capability to initiate on a patient, and then, it requires a lot of specialized capability to maintain and do safely,” said Steven P. Keller, MD, of the division of emergency critical care medicine in the department of emergency medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Those resource requirements can present a challenge to health care systems already overtaxed by COVID-19, according to Dr. Keller, coauthor of a guidance document in Annals of the American Thoracic Society. The guidance suggests a pandemic approach to ECMO response that’s tiered depending on the intensity of the surge over usual hospital volumes.
Mild surges call for a focus on increasing ECMO capacity, while a moderate surge may indicate a need to focus on allocating scarce resources, and a major surge may signal the need to limit or defer use of scarce resources, according to the guidance.
“If your health care system is stretched from a resource standpoint, at what point do you say, ‘we don’t even have the capability to even safely do ECMO, and so, perhaps we should not even be offering the support’?” Dr. Keller said in an interview. “That’s what we tried to get at in the paper – helping institutions think about how to prepare for that pandemic, and then when to make decisions on when it should and should not be offered.”
Critical care guidance for COVID-19
The guidance from the Task Force for Mass Critical Care and CHEST offers nine specific actions that authors suggest as part of a framework for communities to establish the infrastructure needed to triage critical care resources and “equitably” meet the needs of the largest number of COVID-19 patients.
“It is the goal of the task force to minimize the need for allocation of scarce resources as much as possible,” the authors stated.
The framework starts with surge planning that includes an inventory of intensive care unit resources such as ventilators, beds, supplies, and staff that could be marshaled to meet a surge in demand, followed by establishing “identification triggers” for triage initiation by a regional authority, should clinical demand reach a crisis stage.
The next step is preparing the triage system, which includes creating a committee at the regional level, identifying members of tertiary triage teams and the support structures they will need, and preparing and distributing training materials.
Agreeing on a triage protocol is important to ensure equitable targeting of resources, and how to allocate limited life-sustaining measures needs to be considered, according to the panel of experts. They also recommend adaptations to the standards of care such as modification of end-of-life care policies, support for health care workers, family, and the public, and consideration of pediatric issues including transport, concentration of care at specific centers, and potential increases in age thresholds to accommodate surges.
Barriers to triage?
When asked about potential barriers to rolling out a triage plan, Dr. Maves said the first is acknowledging the possible need for such a plan: “It is a difficult concept for most in critical care to accept – the idea that we may not be able to provide an individual patient with interventions that we consider routine,” he said.
Beyond acknowledging need, other potential barriers to successful implementation include the limited evidence base to support development of these protocols, as well as the need to address public trust.
“If a triage system is perceived as unjust or biased, or if people think that triage favors or excludes certain groups unfairly, it will undermine any system,” Dr. Maves said. “Making sure the public both understands and has input into system development is critical if we are going to be able to make this work.”
Dr. Maves and coauthors reported that some of the authors of their guidance are United States government employees or military service members, and that their opinions and assertions do not reflect the official views or position of those institutions. Dr. Keller reported no disclosures related to the ECMO guidance.
SOURCES: Maves RC et al. Chest. 2020 Apr 11. pii: S0012-3692(20)30691-7. doi: 10.1016/j.chest.2020.03.063; Seethara R and Keller SP. Ann Am Thorac Soc. 2020 Apr 15. doi: 10.1513/AnnalsATS.202003-233PS.
, according to authors of recent reports that offer advice on how to prepare for surges in demand.
Even modest numbers of critically ill COVID-19 patients have already rapidly overwhelmed existing hospital capacity in hard-hit areas including Italy, Spain, and New York City, said authors of an expert panel report released in CHEST.
“The ethical burden this places on hospitals, health systems, and society is enormous,” said Ryan C. Maves, MD, FCCP, of the Naval Medical Center in San Diego, lead author of the expert panel report from the Task Force for Mass Critical Care and the American College of Chest Physicians (CHEST).
Triage decisions could be especially daunting for resource-intensive therapies such as extracorporeal membrane oxygenation (ECMO), as physicians may be forced to decide when and if to offer such support after demand outstrips a hospital’s ability to provide it.
“ECMO requires a lot of specialized capability to initiate on a patient, and then, it requires a lot of specialized capability to maintain and do safely,” said Steven P. Keller, MD, of the division of emergency critical care medicine in the department of emergency medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Those resource requirements can present a challenge to health care systems already overtaxed by COVID-19, according to Dr. Keller, coauthor of a guidance document in Annals of the American Thoracic Society. The guidance suggests a pandemic approach to ECMO response that’s tiered depending on the intensity of the surge over usual hospital volumes.
Mild surges call for a focus on increasing ECMO capacity, while a moderate surge may indicate a need to focus on allocating scarce resources, and a major surge may signal the need to limit or defer use of scarce resources, according to the guidance.
“If your health care system is stretched from a resource standpoint, at what point do you say, ‘we don’t even have the capability to even safely do ECMO, and so, perhaps we should not even be offering the support’?” Dr. Keller said in an interview. “That’s what we tried to get at in the paper – helping institutions think about how to prepare for that pandemic, and then when to make decisions on when it should and should not be offered.”
Critical care guidance for COVID-19
The guidance from the Task Force for Mass Critical Care and CHEST offers nine specific actions that authors suggest as part of a framework for communities to establish the infrastructure needed to triage critical care resources and “equitably” meet the needs of the largest number of COVID-19 patients.
“It is the goal of the task force to minimize the need for allocation of scarce resources as much as possible,” the authors stated.
The framework starts with surge planning that includes an inventory of intensive care unit resources such as ventilators, beds, supplies, and staff that could be marshaled to meet a surge in demand, followed by establishing “identification triggers” for triage initiation by a regional authority, should clinical demand reach a crisis stage.
The next step is preparing the triage system, which includes creating a committee at the regional level, identifying members of tertiary triage teams and the support structures they will need, and preparing and distributing training materials.
Agreeing on a triage protocol is important to ensure equitable targeting of resources, and how to allocate limited life-sustaining measures needs to be considered, according to the panel of experts. They also recommend adaptations to the standards of care such as modification of end-of-life care policies, support for health care workers, family, and the public, and consideration of pediatric issues including transport, concentration of care at specific centers, and potential increases in age thresholds to accommodate surges.
Barriers to triage?
When asked about potential barriers to rolling out a triage plan, Dr. Maves said the first is acknowledging the possible need for such a plan: “It is a difficult concept for most in critical care to accept – the idea that we may not be able to provide an individual patient with interventions that we consider routine,” he said.
Beyond acknowledging need, other potential barriers to successful implementation include the limited evidence base to support development of these protocols, as well as the need to address public trust.
“If a triage system is perceived as unjust or biased, or if people think that triage favors or excludes certain groups unfairly, it will undermine any system,” Dr. Maves said. “Making sure the public both understands and has input into system development is critical if we are going to be able to make this work.”
Dr. Maves and coauthors reported that some of the authors of their guidance are United States government employees or military service members, and that their opinions and assertions do not reflect the official views or position of those institutions. Dr. Keller reported no disclosures related to the ECMO guidance.
SOURCES: Maves RC et al. Chest. 2020 Apr 11. pii: S0012-3692(20)30691-7. doi: 10.1016/j.chest.2020.03.063; Seethara R and Keller SP. Ann Am Thorac Soc. 2020 Apr 15. doi: 10.1513/AnnalsATS.202003-233PS.
FROM CHEST AND ANNALS OF THE AMERICAN THORACIC SOCIETY
ACEI/ARBs linked with survival in hypertensive, Chinese COVID-19 patients
Hospitalized COVID-19 patients with hypertension and on treatment with an renin-angiotensin system inhibiting drug had significantly better survival, compared with similar hypertensive patients not on these drugs, in observational, propensity score–matched analyses that drew from a pool of more than 3,430 patients hospitalized at any of nine Chinese hospitals during December 2019–February 2020.
“Among patients with hypertension hospitalized with COVID-19, inpatient treatment with ACEI [ACE inhibitor]/ARB [angiotensin receptor blocker] was associated with lower risk of all-cause mortality, compared with ACEI/ARB nonusers, during 28 days of follow-up. While study interpretation needs to consider the potential for residual confounders, it is unlikely that inpatient ACEI/ARB would be associated with an increased risk of mortality,” wrote Peng Zhang, MD, a cardiology researcher at Renmin Hospital of Wuhan University, China, and coauthors in Circulations Research, buttressing recent recommendations from several medical societies to maintain COVID-19 patients on these drugs.
“Our findings in this paper provide evidence supporting continuous use of ACEI/ARB for patients with hypertension infected with SARS-COV-2,” wrote the authors, backing up recent recommendations from cardiology societies that called for not stopping ACEI/ARB prescriptions in patients at risk for contracting or already have COVID-19 infection, including a statement from the American College of Cardiology, American Heart Association, and Heart Failure Society of America, and also guidance from the European Society of Cardiology.
The study included 1,128 patients with a history of hypertension, including 188 (17%) who received an ACEI/ARB drug during hospitalization. During 28-day follow-up, 99 died (9%), including 7 deaths among the 188 patients (4%) on an ACEI/ARB drug and 92 deaths among the 940 other hypertensive patients (10%).
The authors ran several analyses to try to adjust for the influence of possible confounders. A mixed-effect Cox model with four adjusted variables showed that treatment with an ACEI/ARB drug was tied to a statistically significant 58% lower death rate, compared with patients not receiving these drugs.
The researchers also ran several propensity score–adjusted analyses. One matched 174 of the patients who received an ACEI/ARB drug with 522 who did not, and comparing these two matched arms showed that ACEI/ARB use was linked with a statistically significant 63% cut in mortality, compared with patients not getting these drugs. A second propensity score–matched analysis first excluded the 383 patients who were hypertensive but received no antihypertensive medication during hospitalization. From the remaining 745 patients who received at least one antihypertensive medication, the authors identified 181 patients who received an ACEI/ARB and propensity-score matched them with 181 hypertensive patients on a different medication class, finding that ACEI/ARB use linked with a statistically significant 71% lower rate of all-cause mortality.
Additional analyses also showed that patients with hypertension had a statistically significant, 41% increased rate of all-cause death, compared with patients without hypertension, and another propensity score–matched analysis showed that among hypertensives treatment with an ACEI/ARB drug was linked with a statistically significant 68% reduced rate of septic shock.
Although this report was received with caution and some skepticism, it was also acknowledged as a step forward in the creation of an evidence base addressing ACEI/ARB treatment during COVID-19 infection.
“These drugs are lifesaving and should not be discontinued” for patients with hypertension, heart failure, and other cardiovascular disease, commented Gian Paolo Rossi, MD, professor and chair of medicine and director of the high blood pressure unit at the University of Padua (Italy). The analysis by Zhang and associates included the largest number of hospitalized COVID-19 patients with hypertension yet reported to assess the impact of treatment with ACEI/ARB drugs, and adds important evidence in favor of continuing these drugs in patients who develop COVID-19 infection, Dr. Rossi said in an interview. He recently coauthored a review that argued against ACEI/ARB discontinuation in COVID-19 patients based on previously reported evidence (Elife. 2020 Apr 6. doi: 10.7554/eLife.57278).
But other researchers take a wary view of the potential impact of ACEI/ARB agents. “If ACEI/ARB therapy increases ACE2 and the virus down-regulates it, and because ACE2 is the viral entry port into cells, why would ACE2-mediated down-regulation of the renin-angiotensin-aldosterone system lead to amelioration of [COVID-19] disease?” asked Laurence W. Busse, MD, a critical care physician at Emory University, Atlanta. “A number of issues could potentially confound the results, including the definition of COVID-19 and imbalance of antiviral therapy,” added Dr. Busse, who recently coauthored an editorial that posited using angiotensin II (Giapreza), an approved vasopressor drug, as an alternative renin-angiotensin system intervention for COVID-19 patients including both those in shock as well as potentially those not in shock (Crit Care. 2020 Apr 7. doi: 10.1186/s13054-020-02862-1). Despite these caveats, the new Chinese findings reported by Dr. Zhang and associates “are hypothesis generating and worth further exploration.”
The authors of an editorial that accompanied the Zhang study in Circulation Research made similar points. “While the investigators used standard techniques to attempt to reduce bias in this observational study via propensity matching, it is not a randomized study and the residual confounding inherent to this approach renders the conclusions hypothesis generating at best,” wrote Ravi V. Shah, MD, and two coauthors in the editorial (Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317174). They also agreed with the several society statements that have supported continued use of ACEI/ARB drugs in COVID-19 patients. “Withdrawal of these medications in the context of those conditions in which they have proven benefit (e.g., heart failure with reduced left ventricular ejection fraction) may actually inflict more harm than good,” they warned. “In the end we must rely on randomized clinical science,” and while this level of evidence is currently lacking, “the study by Zhang and colleagues is a direct step toward that goal.”
Dr. Zhang and coauthors had no commercial disclosures. Dr. Rossi and Dr. Busse had no disclosures. The authors of the Circulation Research editorial reported several disclosures.
SOURCE: Zhang P et al. Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317134.
Hospitalized COVID-19 patients with hypertension and on treatment with an renin-angiotensin system inhibiting drug had significantly better survival, compared with similar hypertensive patients not on these drugs, in observational, propensity score–matched analyses that drew from a pool of more than 3,430 patients hospitalized at any of nine Chinese hospitals during December 2019–February 2020.
“Among patients with hypertension hospitalized with COVID-19, inpatient treatment with ACEI [ACE inhibitor]/ARB [angiotensin receptor blocker] was associated with lower risk of all-cause mortality, compared with ACEI/ARB nonusers, during 28 days of follow-up. While study interpretation needs to consider the potential for residual confounders, it is unlikely that inpatient ACEI/ARB would be associated with an increased risk of mortality,” wrote Peng Zhang, MD, a cardiology researcher at Renmin Hospital of Wuhan University, China, and coauthors in Circulations Research, buttressing recent recommendations from several medical societies to maintain COVID-19 patients on these drugs.
“Our findings in this paper provide evidence supporting continuous use of ACEI/ARB for patients with hypertension infected with SARS-COV-2,” wrote the authors, backing up recent recommendations from cardiology societies that called for not stopping ACEI/ARB prescriptions in patients at risk for contracting or already have COVID-19 infection, including a statement from the American College of Cardiology, American Heart Association, and Heart Failure Society of America, and also guidance from the European Society of Cardiology.
The study included 1,128 patients with a history of hypertension, including 188 (17%) who received an ACEI/ARB drug during hospitalization. During 28-day follow-up, 99 died (9%), including 7 deaths among the 188 patients (4%) on an ACEI/ARB drug and 92 deaths among the 940 other hypertensive patients (10%).
The authors ran several analyses to try to adjust for the influence of possible confounders. A mixed-effect Cox model with four adjusted variables showed that treatment with an ACEI/ARB drug was tied to a statistically significant 58% lower death rate, compared with patients not receiving these drugs.
The researchers also ran several propensity score–adjusted analyses. One matched 174 of the patients who received an ACEI/ARB drug with 522 who did not, and comparing these two matched arms showed that ACEI/ARB use was linked with a statistically significant 63% cut in mortality, compared with patients not getting these drugs. A second propensity score–matched analysis first excluded the 383 patients who were hypertensive but received no antihypertensive medication during hospitalization. From the remaining 745 patients who received at least one antihypertensive medication, the authors identified 181 patients who received an ACEI/ARB and propensity-score matched them with 181 hypertensive patients on a different medication class, finding that ACEI/ARB use linked with a statistically significant 71% lower rate of all-cause mortality.
Additional analyses also showed that patients with hypertension had a statistically significant, 41% increased rate of all-cause death, compared with patients without hypertension, and another propensity score–matched analysis showed that among hypertensives treatment with an ACEI/ARB drug was linked with a statistically significant 68% reduced rate of septic shock.
Although this report was received with caution and some skepticism, it was also acknowledged as a step forward in the creation of an evidence base addressing ACEI/ARB treatment during COVID-19 infection.
“These drugs are lifesaving and should not be discontinued” for patients with hypertension, heart failure, and other cardiovascular disease, commented Gian Paolo Rossi, MD, professor and chair of medicine and director of the high blood pressure unit at the University of Padua (Italy). The analysis by Zhang and associates included the largest number of hospitalized COVID-19 patients with hypertension yet reported to assess the impact of treatment with ACEI/ARB drugs, and adds important evidence in favor of continuing these drugs in patients who develop COVID-19 infection, Dr. Rossi said in an interview. He recently coauthored a review that argued against ACEI/ARB discontinuation in COVID-19 patients based on previously reported evidence (Elife. 2020 Apr 6. doi: 10.7554/eLife.57278).
But other researchers take a wary view of the potential impact of ACEI/ARB agents. “If ACEI/ARB therapy increases ACE2 and the virus down-regulates it, and because ACE2 is the viral entry port into cells, why would ACE2-mediated down-regulation of the renin-angiotensin-aldosterone system lead to amelioration of [COVID-19] disease?” asked Laurence W. Busse, MD, a critical care physician at Emory University, Atlanta. “A number of issues could potentially confound the results, including the definition of COVID-19 and imbalance of antiviral therapy,” added Dr. Busse, who recently coauthored an editorial that posited using angiotensin II (Giapreza), an approved vasopressor drug, as an alternative renin-angiotensin system intervention for COVID-19 patients including both those in shock as well as potentially those not in shock (Crit Care. 2020 Apr 7. doi: 10.1186/s13054-020-02862-1). Despite these caveats, the new Chinese findings reported by Dr. Zhang and associates “are hypothesis generating and worth further exploration.”
The authors of an editorial that accompanied the Zhang study in Circulation Research made similar points. “While the investigators used standard techniques to attempt to reduce bias in this observational study via propensity matching, it is not a randomized study and the residual confounding inherent to this approach renders the conclusions hypothesis generating at best,” wrote Ravi V. Shah, MD, and two coauthors in the editorial (Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317174). They also agreed with the several society statements that have supported continued use of ACEI/ARB drugs in COVID-19 patients. “Withdrawal of these medications in the context of those conditions in which they have proven benefit (e.g., heart failure with reduced left ventricular ejection fraction) may actually inflict more harm than good,” they warned. “In the end we must rely on randomized clinical science,” and while this level of evidence is currently lacking, “the study by Zhang and colleagues is a direct step toward that goal.”
Dr. Zhang and coauthors had no commercial disclosures. Dr. Rossi and Dr. Busse had no disclosures. The authors of the Circulation Research editorial reported several disclosures.
SOURCE: Zhang P et al. Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317134.
Hospitalized COVID-19 patients with hypertension and on treatment with an renin-angiotensin system inhibiting drug had significantly better survival, compared with similar hypertensive patients not on these drugs, in observational, propensity score–matched analyses that drew from a pool of more than 3,430 patients hospitalized at any of nine Chinese hospitals during December 2019–February 2020.
“Among patients with hypertension hospitalized with COVID-19, inpatient treatment with ACEI [ACE inhibitor]/ARB [angiotensin receptor blocker] was associated with lower risk of all-cause mortality, compared with ACEI/ARB nonusers, during 28 days of follow-up. While study interpretation needs to consider the potential for residual confounders, it is unlikely that inpatient ACEI/ARB would be associated with an increased risk of mortality,” wrote Peng Zhang, MD, a cardiology researcher at Renmin Hospital of Wuhan University, China, and coauthors in Circulations Research, buttressing recent recommendations from several medical societies to maintain COVID-19 patients on these drugs.
“Our findings in this paper provide evidence supporting continuous use of ACEI/ARB for patients with hypertension infected with SARS-COV-2,” wrote the authors, backing up recent recommendations from cardiology societies that called for not stopping ACEI/ARB prescriptions in patients at risk for contracting or already have COVID-19 infection, including a statement from the American College of Cardiology, American Heart Association, and Heart Failure Society of America, and also guidance from the European Society of Cardiology.
The study included 1,128 patients with a history of hypertension, including 188 (17%) who received an ACEI/ARB drug during hospitalization. During 28-day follow-up, 99 died (9%), including 7 deaths among the 188 patients (4%) on an ACEI/ARB drug and 92 deaths among the 940 other hypertensive patients (10%).
The authors ran several analyses to try to adjust for the influence of possible confounders. A mixed-effect Cox model with four adjusted variables showed that treatment with an ACEI/ARB drug was tied to a statistically significant 58% lower death rate, compared with patients not receiving these drugs.
The researchers also ran several propensity score–adjusted analyses. One matched 174 of the patients who received an ACEI/ARB drug with 522 who did not, and comparing these two matched arms showed that ACEI/ARB use was linked with a statistically significant 63% cut in mortality, compared with patients not getting these drugs. A second propensity score–matched analysis first excluded the 383 patients who were hypertensive but received no antihypertensive medication during hospitalization. From the remaining 745 patients who received at least one antihypertensive medication, the authors identified 181 patients who received an ACEI/ARB and propensity-score matched them with 181 hypertensive patients on a different medication class, finding that ACEI/ARB use linked with a statistically significant 71% lower rate of all-cause mortality.
Additional analyses also showed that patients with hypertension had a statistically significant, 41% increased rate of all-cause death, compared with patients without hypertension, and another propensity score–matched analysis showed that among hypertensives treatment with an ACEI/ARB drug was linked with a statistically significant 68% reduced rate of septic shock.
Although this report was received with caution and some skepticism, it was also acknowledged as a step forward in the creation of an evidence base addressing ACEI/ARB treatment during COVID-19 infection.
“These drugs are lifesaving and should not be discontinued” for patients with hypertension, heart failure, and other cardiovascular disease, commented Gian Paolo Rossi, MD, professor and chair of medicine and director of the high blood pressure unit at the University of Padua (Italy). The analysis by Zhang and associates included the largest number of hospitalized COVID-19 patients with hypertension yet reported to assess the impact of treatment with ACEI/ARB drugs, and adds important evidence in favor of continuing these drugs in patients who develop COVID-19 infection, Dr. Rossi said in an interview. He recently coauthored a review that argued against ACEI/ARB discontinuation in COVID-19 patients based on previously reported evidence (Elife. 2020 Apr 6. doi: 10.7554/eLife.57278).
But other researchers take a wary view of the potential impact of ACEI/ARB agents. “If ACEI/ARB therapy increases ACE2 and the virus down-regulates it, and because ACE2 is the viral entry port into cells, why would ACE2-mediated down-regulation of the renin-angiotensin-aldosterone system lead to amelioration of [COVID-19] disease?” asked Laurence W. Busse, MD, a critical care physician at Emory University, Atlanta. “A number of issues could potentially confound the results, including the definition of COVID-19 and imbalance of antiviral therapy,” added Dr. Busse, who recently coauthored an editorial that posited using angiotensin II (Giapreza), an approved vasopressor drug, as an alternative renin-angiotensin system intervention for COVID-19 patients including both those in shock as well as potentially those not in shock (Crit Care. 2020 Apr 7. doi: 10.1186/s13054-020-02862-1). Despite these caveats, the new Chinese findings reported by Dr. Zhang and associates “are hypothesis generating and worth further exploration.”
The authors of an editorial that accompanied the Zhang study in Circulation Research made similar points. “While the investigators used standard techniques to attempt to reduce bias in this observational study via propensity matching, it is not a randomized study and the residual confounding inherent to this approach renders the conclusions hypothesis generating at best,” wrote Ravi V. Shah, MD, and two coauthors in the editorial (Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317174). They also agreed with the several society statements that have supported continued use of ACEI/ARB drugs in COVID-19 patients. “Withdrawal of these medications in the context of those conditions in which they have proven benefit (e.g., heart failure with reduced left ventricular ejection fraction) may actually inflict more harm than good,” they warned. “In the end we must rely on randomized clinical science,” and while this level of evidence is currently lacking, “the study by Zhang and colleagues is a direct step toward that goal.”
Dr. Zhang and coauthors had no commercial disclosures. Dr. Rossi and Dr. Busse had no disclosures. The authors of the Circulation Research editorial reported several disclosures.
SOURCE: Zhang P et al. Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317134.
FROM CIRCULATION RESEARCH
COVID-19 strikes hard at state-run veterans nursing homes
In early March, 35 residents in the Life Care Center in Kirkland, Washington, died due to complications associated with COVID-19. And that facility thus became the first example of how extremely vulnerable nursing home residents are to COVID-19. Since then, around the US, thousands of nursing home residents have died from complications of the virus. US Department of Veterans Affairs (VA) nursing homes, while rated high in VA health inspection reports, have not been exempt.
As of April 21, the VA had confirmed > 5,500 coronavirus cases in 50 states, the District of Columbia, and Puerto Rico. More than 350 veterans have died of COVID-19, according to VA data. The VA calculates its rates by health care system or VA medical center and does not provide separate data for the community living centers (CLCs).
The VA initiated an isolation strategy on March 10 that suspended most new admissions and barred outsiders from all of its 134 nursing homes. The only exception to the rule was when a patient was expected to die soon. The VA has taken other precautions as well, including extra screening and directing patients to use telehealth where possible.
State-run long-term care facilities for veterans have been hard hit across the country. At the Soldiers’ Home in Holyoke, Massachusetts, which is run by the state of Massachusetts, 5 of 11 veterans who died recently tested positive for COVID-19. At the 4 state-run nursing homes in Alabama, as of April 14, 45 people were confirmed positive and 2 residents had died. The largest outbreak was in the Bill Nichols State Veterans Home in Alexander City. Alabama State Rep. Ed Oliver and Commissioner Kent Davis, of the Alabama Department of Veterans Affairs (ADVA), are looking into how the outbreak started and whether it could have been prevented. “We have reports of lack of hand sanitizers, and those are the things we’re looking at right now,” Rep. Oliver said. The ADVA says residents who test positive are isolated for treatment, and infected employees are prohibited from entering the homes.
States have deployed National Guard troops to facilities following large scale outbreaks and multiple deaths. Pennsylvania deployed 30 National Guard troops to its Southeastern Veterans Center facility in Spring City after at least 10 veterans had died and at least 19 health care workers had tested positive for the virus. The facility is 1 of 6 extended-care facilities run by the Pennsylvania Department of Military and Veterans Affairs. In New Jersey, 40 National Guard troops, 25 New Jersey Department of Health nurses, and 90 VA nurses were deployed to 2 of its veterans facilities amid worsening outbreaks. At the Paramus facility, 155 residents had tested positive and 39 had died, and at the home in Edison, 86 veterans had tested positive and 25 died; 6 more died at a third state facility.
However, reporting remains inconsistent across many states and facilities. Only on April 19 did the Centers for Medicare and Medicaid Services (CMS) order nursing home facilities to inform residents and families about COVID-19 cases inside. This followed similar orders in New Jersey, New York, California, Washington, and other states.
“Nursing homes have been ground zero for COVID-19,” said CMS Administrator Seema Verma in a written statement. “Nursing home reporting to the [Centers for Disease Control and Prevention] is a critical component of the go-forward national COVID-19 surveillance system and to efforts to reopen America.”
In early March, 35 residents in the Life Care Center in Kirkland, Washington, died due to complications associated with COVID-19. And that facility thus became the first example of how extremely vulnerable nursing home residents are to COVID-19. Since then, around the US, thousands of nursing home residents have died from complications of the virus. US Department of Veterans Affairs (VA) nursing homes, while rated high in VA health inspection reports, have not been exempt.
As of April 21, the VA had confirmed > 5,500 coronavirus cases in 50 states, the District of Columbia, and Puerto Rico. More than 350 veterans have died of COVID-19, according to VA data. The VA calculates its rates by health care system or VA medical center and does not provide separate data for the community living centers (CLCs).
The VA initiated an isolation strategy on March 10 that suspended most new admissions and barred outsiders from all of its 134 nursing homes. The only exception to the rule was when a patient was expected to die soon. The VA has taken other precautions as well, including extra screening and directing patients to use telehealth where possible.
State-run long-term care facilities for veterans have been hard hit across the country. At the Soldiers’ Home in Holyoke, Massachusetts, which is run by the state of Massachusetts, 5 of 11 veterans who died recently tested positive for COVID-19. At the 4 state-run nursing homes in Alabama, as of April 14, 45 people were confirmed positive and 2 residents had died. The largest outbreak was in the Bill Nichols State Veterans Home in Alexander City. Alabama State Rep. Ed Oliver and Commissioner Kent Davis, of the Alabama Department of Veterans Affairs (ADVA), are looking into how the outbreak started and whether it could have been prevented. “We have reports of lack of hand sanitizers, and those are the things we’re looking at right now,” Rep. Oliver said. The ADVA says residents who test positive are isolated for treatment, and infected employees are prohibited from entering the homes.
States have deployed National Guard troops to facilities following large scale outbreaks and multiple deaths. Pennsylvania deployed 30 National Guard troops to its Southeastern Veterans Center facility in Spring City after at least 10 veterans had died and at least 19 health care workers had tested positive for the virus. The facility is 1 of 6 extended-care facilities run by the Pennsylvania Department of Military and Veterans Affairs. In New Jersey, 40 National Guard troops, 25 New Jersey Department of Health nurses, and 90 VA nurses were deployed to 2 of its veterans facilities amid worsening outbreaks. At the Paramus facility, 155 residents had tested positive and 39 had died, and at the home in Edison, 86 veterans had tested positive and 25 died; 6 more died at a third state facility.
However, reporting remains inconsistent across many states and facilities. Only on April 19 did the Centers for Medicare and Medicaid Services (CMS) order nursing home facilities to inform residents and families about COVID-19 cases inside. This followed similar orders in New Jersey, New York, California, Washington, and other states.
“Nursing homes have been ground zero for COVID-19,” said CMS Administrator Seema Verma in a written statement. “Nursing home reporting to the [Centers for Disease Control and Prevention] is a critical component of the go-forward national COVID-19 surveillance system and to efforts to reopen America.”
In early March, 35 residents in the Life Care Center in Kirkland, Washington, died due to complications associated with COVID-19. And that facility thus became the first example of how extremely vulnerable nursing home residents are to COVID-19. Since then, around the US, thousands of nursing home residents have died from complications of the virus. US Department of Veterans Affairs (VA) nursing homes, while rated high in VA health inspection reports, have not been exempt.
As of April 21, the VA had confirmed > 5,500 coronavirus cases in 50 states, the District of Columbia, and Puerto Rico. More than 350 veterans have died of COVID-19, according to VA data. The VA calculates its rates by health care system or VA medical center and does not provide separate data for the community living centers (CLCs).
The VA initiated an isolation strategy on March 10 that suspended most new admissions and barred outsiders from all of its 134 nursing homes. The only exception to the rule was when a patient was expected to die soon. The VA has taken other precautions as well, including extra screening and directing patients to use telehealth where possible.
State-run long-term care facilities for veterans have been hard hit across the country. At the Soldiers’ Home in Holyoke, Massachusetts, which is run by the state of Massachusetts, 5 of 11 veterans who died recently tested positive for COVID-19. At the 4 state-run nursing homes in Alabama, as of April 14, 45 people were confirmed positive and 2 residents had died. The largest outbreak was in the Bill Nichols State Veterans Home in Alexander City. Alabama State Rep. Ed Oliver and Commissioner Kent Davis, of the Alabama Department of Veterans Affairs (ADVA), are looking into how the outbreak started and whether it could have been prevented. “We have reports of lack of hand sanitizers, and those are the things we’re looking at right now,” Rep. Oliver said. The ADVA says residents who test positive are isolated for treatment, and infected employees are prohibited from entering the homes.
States have deployed National Guard troops to facilities following large scale outbreaks and multiple deaths. Pennsylvania deployed 30 National Guard troops to its Southeastern Veterans Center facility in Spring City after at least 10 veterans had died and at least 19 health care workers had tested positive for the virus. The facility is 1 of 6 extended-care facilities run by the Pennsylvania Department of Military and Veterans Affairs. In New Jersey, 40 National Guard troops, 25 New Jersey Department of Health nurses, and 90 VA nurses were deployed to 2 of its veterans facilities amid worsening outbreaks. At the Paramus facility, 155 residents had tested positive and 39 had died, and at the home in Edison, 86 veterans had tested positive and 25 died; 6 more died at a third state facility.
However, reporting remains inconsistent across many states and facilities. Only on April 19 did the Centers for Medicare and Medicaid Services (CMS) order nursing home facilities to inform residents and families about COVID-19 cases inside. This followed similar orders in New Jersey, New York, California, Washington, and other states.
“Nursing homes have been ground zero for COVID-19,” said CMS Administrator Seema Verma in a written statement. “Nursing home reporting to the [Centers for Disease Control and Prevention] is a critical component of the go-forward national COVID-19 surveillance system and to efforts to reopen America.”
Doctors push back on treating COVID-19 as HAPE
For Luanne Freer, MD, an expert in high-altitude pulmonary edema (HAPE) and founder and director of Everest ER, a nonprofit seasonal clinic at the Mt. Everest base camp in Nepal (elevation, 17,600 ft), a sudden flurry of messages and questions she received about a possible COVID-19/HAPE link was startling.
“That’s why it kind of poked me in the eye,” she said, referencing her extensive experience treating HAPE, which she described as a pressure-related phenomenon. “My goodness, they are so completely different.”
Dr. Freer, an emergency physician, reached out to several pulmonary intensivists with experience treating both HAPE and COVID-19 to gauge their reactions, and within 36 hours, they had drafted their response. In the commentary, published in High Altitude Medicine & Biology, the clinicians note that the comparison between HAPE and COVID-19 is potentially risky.
“As a group of physicians who have in some cases cared for patients with COVID-19 and in all cases cared for patients with HAPE and studied its pathophysiology and management, we feel it important to correct this misconception, as continued amplification of this message could have adverse effects on management of these patients,” they wrote.
The suggestion that COVID-19 lung injury sometimes looks more like HAPE than like acute respiratory distress syndrome (ARDS) appeared in a journal review article in late March and was put forth by medical professionals on social media where it gained traction in recent weeks and was amplified in multiple media outlets, including this one.
“With COVID, we don’t understand everything that’s going on, but we know for sure it’s an inflammatory process – not a pressure-related problem,” Dr. Freer said. “I thought ... this could be so dangerous to load the medicines that we use when we’re treating HAPE onto patients with COVID-19.”
The pathophysiological mechanisms in HAPE are different than those in other respiratory syndromes, including those associated with COVID-19, said Andrew M. Luks, MD, of the UW Medicine, Seattle, and the first author on the commentary.
“HAPE is a noncardiogenic form of pulmonary edema, as are ARDS due to bacteria or viral pneumonia, re-expansion pulmonary edema, immersion pulmonary edema, negative pressure pulmonary edema, and neurogenic pulmonary edema,” Dr. Luks, Dr. Freer, and colleagues wrote in the commentary, explaining that all of these entities cause varying degrees of hypoxemia and diffuse bilateral opacities on chest imaging. “Importantly, in all of these cases, edema accumulates in the interstitial and alveolar spaces of the lung as a result of imbalance in Starling forces.”
A difference between these entities, however, is “the mechanism by which that imbalance develops,” they noted.
The excessive and uneven hypoxic pulmonary vasoconstriction that leads to a marked increase in pulmonary artery pressure, subsequent lung overperfusion, increased pulmonary capillary hydrostatic pressure, and leakage of fluid from the vascular space into the alveolar space as seen in HAPE, is a “fundamentally different phenomenon than what is seen in COVID-19-related ARDS, which involves viral-mediated inflammatory responses as the primary pathophysiological mechanism,” they added.
The authors described several other differences between the conditions, ultimately noting that “understanding the distinction between the pathophysiological mechanisms of these entities is critical for patient management.”
In HAPE, supplemental oxygen alone may be sufficient; in COVID-19, it may improve hypoxemia but won’t resolve the underlying inflammation or injury, they explained, adding that “only good supportive care including mechanical ventilation, quite often for long periods of time, allows some patients to survive until their disease resolves.”
Further, HAPE can be prevented or treated with pulmonary vasodilators such a nifedipine or sildenafil, which decrease pulmonary artery pressure and, as a result lower pulmonary capillary hydrostatic pressure, they said.
Use of such medications for COVID-19 might decrease pulmonary artery pressure and improve right ventricular function in COVID-19, but “by releasing hypoxic pulmonary vasoconstriction and increasing perfusion to nonventilated regions of the lung, they could also worsen ventilation-perfusion mismatch” and thereby worsen hypoxemia, they explained, adding that the treatments can also cause or worsen hypotension.
Efforts to share observations and experience are important in medicine, but sometimes, as in this circumstance, “they get out there, spread around – like a brushfire almost – and get [unwarranted] face validity,” Dr. Luks said, noting that in response to information circulating about COVID-19 and HAPE, he has already heard medical professionals floating the idea of treating COVID-19 with treatments used for HAPE.
It’s true that some COVID-19 lung injury cases are behaving differently than typical ARDS, he said, adding that presentation can vary.
“But trying to equate HAPE and COVID-19 is just wrong,” he said. “HAPE and COVID-19 may share several features ...but those are features that are shared by a lot of different forms of respiratory failure.”
In a recent video interview, WebMD’s chief medical officer John Whyte, MD, spoke with a New York City physician trained in critical care and emergency medicine, Cameron Kyle-Sidell, MD, who raised the need to consider different respiratory protocols for COVID-19, noting that standard protocols were falling short in many cases.
“What we’re seeing ... is something unusual, it’s something that we are not used to,” Dr. Kyle-Sidell of Maimonides Medical Center said in that interview, stressing that the presentation differed from that seen in typical ARDS. “The patterns I was seeing did not make sense.”
Like others, he noted that COVID-19 patients were presenting with illness that clinically looked more like HAPE, but that the pathophysiology is not necessary similar to HAPE.
At around the same time, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues, published a letter to the editor in the American Journal of Respiratory and Critical Care Medicine stressing that the ARDS presentation in COVID-19 patients is atypical and requires a patient physiology–driven treatment approach, rather than a standard protocol–driven approach. Dr. Gattinoni and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation.
Dr. Luks agreed that “some patients with COVID-19 do not have the same physiologic derangements that we see in a lot of other people with ARDS.”
“[Dr. Gattinoni] is making the point that we need to treat these people differently ... and I think that’s a valid point, and honestly, that’s a point that applied even before COVID-19,” he said. “Most of the things that we see in clinical practice – there’s a lot of heterogeneity between patients, and you have to be prepared to tailor your therapy in light of the differences that you’re picking up from your observations at the bedside and other data that you’re getting on the patient.”
The main concern Dr. Luks and his coauthors wanted to convey, they said, is making sure that the anecdotal experiences and observations of clinicians struggling to find answers don’t spiral out of control without proper vetting, thereby leading to patient harm.
“In this challenging time, we must identify the best means to care for these critically ill patients. That approach should be grounded in sound pulmonary physiology, clinical experience and, when available, evidence from clinical studies,” they concluded.
Dr. Luks and Dr. Freer reported having no financial disclosures.
For Luanne Freer, MD, an expert in high-altitude pulmonary edema (HAPE) and founder and director of Everest ER, a nonprofit seasonal clinic at the Mt. Everest base camp in Nepal (elevation, 17,600 ft), a sudden flurry of messages and questions she received about a possible COVID-19/HAPE link was startling.
“That’s why it kind of poked me in the eye,” she said, referencing her extensive experience treating HAPE, which she described as a pressure-related phenomenon. “My goodness, they are so completely different.”
Dr. Freer, an emergency physician, reached out to several pulmonary intensivists with experience treating both HAPE and COVID-19 to gauge their reactions, and within 36 hours, they had drafted their response. In the commentary, published in High Altitude Medicine & Biology, the clinicians note that the comparison between HAPE and COVID-19 is potentially risky.
“As a group of physicians who have in some cases cared for patients with COVID-19 and in all cases cared for patients with HAPE and studied its pathophysiology and management, we feel it important to correct this misconception, as continued amplification of this message could have adverse effects on management of these patients,” they wrote.
The suggestion that COVID-19 lung injury sometimes looks more like HAPE than like acute respiratory distress syndrome (ARDS) appeared in a journal review article in late March and was put forth by medical professionals on social media where it gained traction in recent weeks and was amplified in multiple media outlets, including this one.
“With COVID, we don’t understand everything that’s going on, but we know for sure it’s an inflammatory process – not a pressure-related problem,” Dr. Freer said. “I thought ... this could be so dangerous to load the medicines that we use when we’re treating HAPE onto patients with COVID-19.”
The pathophysiological mechanisms in HAPE are different than those in other respiratory syndromes, including those associated with COVID-19, said Andrew M. Luks, MD, of the UW Medicine, Seattle, and the first author on the commentary.
“HAPE is a noncardiogenic form of pulmonary edema, as are ARDS due to bacteria or viral pneumonia, re-expansion pulmonary edema, immersion pulmonary edema, negative pressure pulmonary edema, and neurogenic pulmonary edema,” Dr. Luks, Dr. Freer, and colleagues wrote in the commentary, explaining that all of these entities cause varying degrees of hypoxemia and diffuse bilateral opacities on chest imaging. “Importantly, in all of these cases, edema accumulates in the interstitial and alveolar spaces of the lung as a result of imbalance in Starling forces.”
A difference between these entities, however, is “the mechanism by which that imbalance develops,” they noted.
The excessive and uneven hypoxic pulmonary vasoconstriction that leads to a marked increase in pulmonary artery pressure, subsequent lung overperfusion, increased pulmonary capillary hydrostatic pressure, and leakage of fluid from the vascular space into the alveolar space as seen in HAPE, is a “fundamentally different phenomenon than what is seen in COVID-19-related ARDS, which involves viral-mediated inflammatory responses as the primary pathophysiological mechanism,” they added.
The authors described several other differences between the conditions, ultimately noting that “understanding the distinction between the pathophysiological mechanisms of these entities is critical for patient management.”
In HAPE, supplemental oxygen alone may be sufficient; in COVID-19, it may improve hypoxemia but won’t resolve the underlying inflammation or injury, they explained, adding that “only good supportive care including mechanical ventilation, quite often for long periods of time, allows some patients to survive until their disease resolves.”
Further, HAPE can be prevented or treated with pulmonary vasodilators such a nifedipine or sildenafil, which decrease pulmonary artery pressure and, as a result lower pulmonary capillary hydrostatic pressure, they said.
Use of such medications for COVID-19 might decrease pulmonary artery pressure and improve right ventricular function in COVID-19, but “by releasing hypoxic pulmonary vasoconstriction and increasing perfusion to nonventilated regions of the lung, they could also worsen ventilation-perfusion mismatch” and thereby worsen hypoxemia, they explained, adding that the treatments can also cause or worsen hypotension.
Efforts to share observations and experience are important in medicine, but sometimes, as in this circumstance, “they get out there, spread around – like a brushfire almost – and get [unwarranted] face validity,” Dr. Luks said, noting that in response to information circulating about COVID-19 and HAPE, he has already heard medical professionals floating the idea of treating COVID-19 with treatments used for HAPE.
It’s true that some COVID-19 lung injury cases are behaving differently than typical ARDS, he said, adding that presentation can vary.
“But trying to equate HAPE and COVID-19 is just wrong,” he said. “HAPE and COVID-19 may share several features ...but those are features that are shared by a lot of different forms of respiratory failure.”
In a recent video interview, WebMD’s chief medical officer John Whyte, MD, spoke with a New York City physician trained in critical care and emergency medicine, Cameron Kyle-Sidell, MD, who raised the need to consider different respiratory protocols for COVID-19, noting that standard protocols were falling short in many cases.
“What we’re seeing ... is something unusual, it’s something that we are not used to,” Dr. Kyle-Sidell of Maimonides Medical Center said in that interview, stressing that the presentation differed from that seen in typical ARDS. “The patterns I was seeing did not make sense.”
Like others, he noted that COVID-19 patients were presenting with illness that clinically looked more like HAPE, but that the pathophysiology is not necessary similar to HAPE.
At around the same time, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues, published a letter to the editor in the American Journal of Respiratory and Critical Care Medicine stressing that the ARDS presentation in COVID-19 patients is atypical and requires a patient physiology–driven treatment approach, rather than a standard protocol–driven approach. Dr. Gattinoni and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation.
Dr. Luks agreed that “some patients with COVID-19 do not have the same physiologic derangements that we see in a lot of other people with ARDS.”
“[Dr. Gattinoni] is making the point that we need to treat these people differently ... and I think that’s a valid point, and honestly, that’s a point that applied even before COVID-19,” he said. “Most of the things that we see in clinical practice – there’s a lot of heterogeneity between patients, and you have to be prepared to tailor your therapy in light of the differences that you’re picking up from your observations at the bedside and other data that you’re getting on the patient.”
The main concern Dr. Luks and his coauthors wanted to convey, they said, is making sure that the anecdotal experiences and observations of clinicians struggling to find answers don’t spiral out of control without proper vetting, thereby leading to patient harm.
“In this challenging time, we must identify the best means to care for these critically ill patients. That approach should be grounded in sound pulmonary physiology, clinical experience and, when available, evidence from clinical studies,” they concluded.
Dr. Luks and Dr. Freer reported having no financial disclosures.
For Luanne Freer, MD, an expert in high-altitude pulmonary edema (HAPE) and founder and director of Everest ER, a nonprofit seasonal clinic at the Mt. Everest base camp in Nepal (elevation, 17,600 ft), a sudden flurry of messages and questions she received about a possible COVID-19/HAPE link was startling.
“That’s why it kind of poked me in the eye,” she said, referencing her extensive experience treating HAPE, which she described as a pressure-related phenomenon. “My goodness, they are so completely different.”
Dr. Freer, an emergency physician, reached out to several pulmonary intensivists with experience treating both HAPE and COVID-19 to gauge their reactions, and within 36 hours, they had drafted their response. In the commentary, published in High Altitude Medicine & Biology, the clinicians note that the comparison between HAPE and COVID-19 is potentially risky.
“As a group of physicians who have in some cases cared for patients with COVID-19 and in all cases cared for patients with HAPE and studied its pathophysiology and management, we feel it important to correct this misconception, as continued amplification of this message could have adverse effects on management of these patients,” they wrote.
The suggestion that COVID-19 lung injury sometimes looks more like HAPE than like acute respiratory distress syndrome (ARDS) appeared in a journal review article in late March and was put forth by medical professionals on social media where it gained traction in recent weeks and was amplified in multiple media outlets, including this one.
“With COVID, we don’t understand everything that’s going on, but we know for sure it’s an inflammatory process – not a pressure-related problem,” Dr. Freer said. “I thought ... this could be so dangerous to load the medicines that we use when we’re treating HAPE onto patients with COVID-19.”
The pathophysiological mechanisms in HAPE are different than those in other respiratory syndromes, including those associated with COVID-19, said Andrew M. Luks, MD, of the UW Medicine, Seattle, and the first author on the commentary.
“HAPE is a noncardiogenic form of pulmonary edema, as are ARDS due to bacteria or viral pneumonia, re-expansion pulmonary edema, immersion pulmonary edema, negative pressure pulmonary edema, and neurogenic pulmonary edema,” Dr. Luks, Dr. Freer, and colleagues wrote in the commentary, explaining that all of these entities cause varying degrees of hypoxemia and diffuse bilateral opacities on chest imaging. “Importantly, in all of these cases, edema accumulates in the interstitial and alveolar spaces of the lung as a result of imbalance in Starling forces.”
A difference between these entities, however, is “the mechanism by which that imbalance develops,” they noted.
The excessive and uneven hypoxic pulmonary vasoconstriction that leads to a marked increase in pulmonary artery pressure, subsequent lung overperfusion, increased pulmonary capillary hydrostatic pressure, and leakage of fluid from the vascular space into the alveolar space as seen in HAPE, is a “fundamentally different phenomenon than what is seen in COVID-19-related ARDS, which involves viral-mediated inflammatory responses as the primary pathophysiological mechanism,” they added.
The authors described several other differences between the conditions, ultimately noting that “understanding the distinction between the pathophysiological mechanisms of these entities is critical for patient management.”
In HAPE, supplemental oxygen alone may be sufficient; in COVID-19, it may improve hypoxemia but won’t resolve the underlying inflammation or injury, they explained, adding that “only good supportive care including mechanical ventilation, quite often for long periods of time, allows some patients to survive until their disease resolves.”
Further, HAPE can be prevented or treated with pulmonary vasodilators such a nifedipine or sildenafil, which decrease pulmonary artery pressure and, as a result lower pulmonary capillary hydrostatic pressure, they said.
Use of such medications for COVID-19 might decrease pulmonary artery pressure and improve right ventricular function in COVID-19, but “by releasing hypoxic pulmonary vasoconstriction and increasing perfusion to nonventilated regions of the lung, they could also worsen ventilation-perfusion mismatch” and thereby worsen hypoxemia, they explained, adding that the treatments can also cause or worsen hypotension.
Efforts to share observations and experience are important in medicine, but sometimes, as in this circumstance, “they get out there, spread around – like a brushfire almost – and get [unwarranted] face validity,” Dr. Luks said, noting that in response to information circulating about COVID-19 and HAPE, he has already heard medical professionals floating the idea of treating COVID-19 with treatments used for HAPE.
It’s true that some COVID-19 lung injury cases are behaving differently than typical ARDS, he said, adding that presentation can vary.
“But trying to equate HAPE and COVID-19 is just wrong,” he said. “HAPE and COVID-19 may share several features ...but those are features that are shared by a lot of different forms of respiratory failure.”
In a recent video interview, WebMD’s chief medical officer John Whyte, MD, spoke with a New York City physician trained in critical care and emergency medicine, Cameron Kyle-Sidell, MD, who raised the need to consider different respiratory protocols for COVID-19, noting that standard protocols were falling short in many cases.
“What we’re seeing ... is something unusual, it’s something that we are not used to,” Dr. Kyle-Sidell of Maimonides Medical Center said in that interview, stressing that the presentation differed from that seen in typical ARDS. “The patterns I was seeing did not make sense.”
Like others, he noted that COVID-19 patients were presenting with illness that clinically looked more like HAPE, but that the pathophysiology is not necessary similar to HAPE.
At around the same time, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues, published a letter to the editor in the American Journal of Respiratory and Critical Care Medicine stressing that the ARDS presentation in COVID-19 patients is atypical and requires a patient physiology–driven treatment approach, rather than a standard protocol–driven approach. Dr. Gattinoni and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation.
Dr. Luks agreed that “some patients with COVID-19 do not have the same physiologic derangements that we see in a lot of other people with ARDS.”
“[Dr. Gattinoni] is making the point that we need to treat these people differently ... and I think that’s a valid point, and honestly, that’s a point that applied even before COVID-19,” he said. “Most of the things that we see in clinical practice – there’s a lot of heterogeneity between patients, and you have to be prepared to tailor your therapy in light of the differences that you’re picking up from your observations at the bedside and other data that you’re getting on the patient.”
The main concern Dr. Luks and his coauthors wanted to convey, they said, is making sure that the anecdotal experiences and observations of clinicians struggling to find answers don’t spiral out of control without proper vetting, thereby leading to patient harm.
“In this challenging time, we must identify the best means to care for these critically ill patients. That approach should be grounded in sound pulmonary physiology, clinical experience and, when available, evidence from clinical studies,” they concluded.
Dr. Luks and Dr. Freer reported having no financial disclosures.
COVID-19: New programs can provide money to keep your practice running
Family physician Frank Maselli, MD, saw approximately 30 patients a day in his office in the Bronx before COVID-19. But New York City has become a hot spot for the virus that has claimed the lives of the lives of more than 40,000 people nationwide.
Now Maselli and the other 10 physicians in the practice each treat only eight or nine patients a day via telemedicine. He spends most of his time on the phone answering patients’ questions about COVID-19 symptoms and potential exposure. Although he tries to bill for telemedicine and phone calls, he says many commercial payers reject the claims because their processing systems aren’t updated to reflect new coverage policies. He has enough cash in reserve to cover two payrolls, but he knows he needs a backup plan if patient volumes continue to decrease indefinitely.
“Our doctors will take a pay cut before we let people go,” says Maselli. “So far we’re OK because we’re getting paid for things we did two months ago before all of this happened.”
Ninety-seven percent of medical practices have experienced a negative financial impact directly or indirectly related to the COVID-19 pandemic, according to new data from the Medical Group Management Association (MGMA). On average, practices report a 60% decrease in patient volume and a 55% decrease in revenue since the beginning of the public health emergency.
Four options for financial assistance
However, there are ways to offset revenue loss and remain financially viable during the economic uncertainty of the COVID-19 pandemic. Options include the US Small Business Administration’s (SBA) Paycheck Protection Program; the SBA’s Emergency Economic Injury Disaster Loan (EIDL); Medicare’s advanced payment program; and an SBA Coronavirus Economic Stabilization Act (CESA) loan. These are in addition to several other strategies aimed at reducing costs and improving revenue.
1. Maselli, for example, applied for the Paycheck Protection Program, a short-term loan that helps small businesses (i.e., for physician offices, those with an annual revenue of under $12 million) keep staff employed during the COVID-19 crisis. The loan covers a variety of costs, including payroll, rent, utilities, mortgage interest, and interest on any other debt obligations incurred before February 15 of this year.
“We have no idea if this is coming and when, but it would be a big help,” he adds.
(As of press time, the Paycheck Protection Program had stopped accepting applications, having reached the limit of its $349 billion budget. Congress must now agree on legislation to add additional funding to assist small businesses.)
Practices can take out a loan of up to 2.5 times the average monthly payroll (excluding payroll for those making more than $100,000 annually) with a cap of $10 million. For example, if the average monthly payroll is $10,000 – and no employees earn more than $100,000 annually – the maximum loan amount is $25,000.
Practices approved for this loan can expect to receive the funds from their SBA-approved lender within 10 calendar days of the date of loan approval. Although it’s technically a loan, the good news is that it doesn’t need to be repaid if the practice complies with all of the loan requirements – particularly these two: The practice uses at least 75% of the loan specifically for payroll, and the practice keeps employees on the payroll (or rehire, when necessary) for 8 weeks after the loan origination date.
Forgiveness is reduced if full-time headcount declines, or if salaries and wages decrease. If a practice does need to repay all or a portion of the loan, it must do so within 2 years at an interest rate of 1%, and payments are deferred for 6 months.
Andrew D. McDonald, FACHE, practice leader of health care consulting at LBMC Healthcare, says it behooves practices to apply for this loan because it’s essentially free money during a time when revenue may be at an all-time low. “While the devil is in the details, on the surface, the paycheck protection funds appear to be a no-brainer. However, each practice will need to confirm with their lender that it’s a solid decision.”
One challenge with this loan is that some banks weren’t necessarily ready to accept applications on April 3, and many continue to lag behind in processing these applications.
2. A second option is the SBA’s EIDL, a low-interest, long-term loan (capped at 3.75% for small businesses) that practices with 500 or fewer employees can use to pay fixed debts, payrolls, accounts payable, and other bills that could have been paid had the disaster not occurred. Borrowers can ask for up to $2 million, and the maximum term of this loan is 30 years, though the overall process for obtaining these loans will depend on the lender.
Practices have until December 16 to apply for this loan. They can also apply for an expedited disbursement (i.e., an Economic Injury Disaster Advance) of up to $10,000 that’s paid within 3 days of the request.
3. A third option is Medicare’s COVID-19 advanced payment program. Under this program, eligible physicians are those who:
- Billed Medicare for claims within 180 days prior to the date of the request
- Are financially solvent (i.e., aren’t in bankruptcy)
- Are free from any active medical review or program integrity investigations
- Are in good standing with Medicare (i.e., don’t have an outstanding delinquent Medicare overpayment)
If physicians meet this criteria, they can ask their Medicare Administrative Contractor (MAC) to provide an advanced payment of up to 100% of the Medicare payment amount based on a 3-month lookback period.
Once requested, MACs will issue payment within 7 calendar days from the date of the request. Repayment will occur in the form of automatic recoupments beginning 120 days after the advanced payment is received. Medicare has already approved more than 21,000 requests totaling more than $51 billion. CMS has provided a fact sheet to learn more about how to request an accelerated payment.
“The key is that you need to repay this, so you want to set a reasonable goal,” says Sarah Hostetter, senior consultant at Advisory Board, a health care research & data consulting firm. She says practices should consider what they’ll realistically be able to repay within 120 days.
4. A fourth option – specifically for mid-size practices – is a CESA loan, the details of which have yet to be announced, that will enable practices to access funds with an annualized rate no greater than 2% and with no principal or interest due for at least 6 months. The CARES Act, signed into law 3 weeks ago, provides $454 billion for this program.
Selecting the right option for your practice
Which singular option – or combination of options – is best for your practice? McDonald says to ask these questions:
- How well are patient volumes holding up?
- How well are physicians pivoting to telehealth?
- What is the overall economic loss?
- What are the available liquid assets, and how long can the practice maintain its financial viability over the next couple of months and beyond?
Cheryl Mongillo, MBA, administrative director of two independent family practices in Delaware, applied for both the Paycheck Protection Program and Medicare advanced payments because she’s worried about being able to pay staff while also covering costs related to personal protective equipment, medical waste, and cleaning, all of which have tripled since the pandemic began. One of the practices includes one physician and four nurse practitioners. The other includes five physicians and three nurse practitioners. In total, both practices employ 35 additional staff.
“I want our staff to know how much we care about them. My hope is that after this is over, our business will pick back up pretty quickly,” she adds. “However, until I can get the business back, I needed something to keep us afloat.”
Others are being more cautious. Crystal Bruning, practice manager at an Ob/Gyn clinic in Orlando, Florida, says her practice applied for the Paycheck Protection Program but is waiting another month or so before deciding whether it will also take advantage of Medicare advanced payments.
The practice is still trying to assess the true financial impact of its 30% reduction in patient volume. Bruning says the advanced payments wouldn’t amount to much anyway because only 10% of the practice’s patients have Medicare.
Making tough financial decisions while awaiting assistance
Kansas-based family physician Jennifer Bacani McKenney, MD, says she hasn’t paid herself a salary in weeks because of the revenue loss her practice has incurred.
“I want to make sure we can pay [all 12] employees,” she says. “In my family, we have two incomes, and we’re pretty good at saving money. However, I know not every physician can afford to do this.”
Although McKenney’s practice has seen a 75% reduction in patient volume, staff continue to provide virtual visits – including Zoom-based nursing home visits – phone visits, and in-person visits for acute illnesses. They also provide curbside immunizations. Still, long-term revenue loss is a concern. “I have a threshold in mind based on what we have in reserves,” she says. “If we hit that point, we would need to talk about a loan or Medicare advanced payments.”
Arkansas-based family physician Lonnie S. Robinson, MD, says he immediately applied for the Paycheck Protection Program after it was announced. “We also made sure we had a line of credit with our local bank during the very first discussions about what the pandemic would mean for our revenue streams,” he says.
However, because he’s in a rural area of the state, he continues to struggle with telemedicine due to broadband and connectivity challenges. Cash flow is another challenge because a lot of insurance companies are waiving copayments.
“I didn’t realize the amount of money we collect immediately from the patient,” he says. “This was a substantial revenue stream, and it was immediate revenue – not revenue waiting on a claim to be paid.”
Illinois-based family physician Deborah L. Winiger, MD, says she also applied for the Paycheck Protection Program but in the meantime had to reduce staff hours by a third because her patient volume dropped by more than half. She will also encourage staff to pursue temporary positions at a local hospital if the federal funds don’t materialize.
Kelly Shackleton, practice manager at a New York-based internal medicine practice, says she laid off 7 of her 16 staff members (including lab technicians, licensed practical nurses, billers, a referral specialist, and a file clerk) due to a 70% decrease in patient volume.
“I didn’t lay them all off at once,” she says. “I kept them until things were all caught up in each department. I plan to get them all back when the time is right, but I want to be sure to keep the practice afloat so they have a place to return to.” If the Paycheck Protection Program for which she applied comes through, then she will rehire them. She also applied for Medicare advanced payments and increased the practice’s line of credit.
Bill properly – and for everything you are still doing
Accurate and complete coding is critical during this time of financial instability, says Maselli. “I keep telling doctors to bill for everything they do,” he says. This includes phone calls between patients and physicians or other qualified healthcare providers (CPT codes 99441-99443). Note that these are time-based codes, requiring a minimum of five minutes of medical discussion.
Remote physiologic monitoring (including monitoring a patient’s oxygen saturation levels using pulse oximetry), virtual check-ins, and online digital evaluation and management services are also covered by Medicare and some commercial payers.
Other good news is that the Centers for Medicare & Medicaid Services added more than 80 additional services that providers can furnish using telehealth, including new patient office visits, home visits, prolonged office visits, smoking and tobacco cessation counseling, annual depression and alcohol screenings, advanced care planning, and much more.
Mongillo, the family practice administrator in Delaware, agrees that physicians need to bill for as many services as possible. At one of the family medicine practices she manages, physicians perform wellness visits, when appropriate, if patients are already coming into the office for another ailment.
Also look for ways to cut costs. For example, Mongillo was able to renegotiate the practice’s telemedicine contract after she received several proposals from other vendors offering three months of complimentary service. Shackleton discontinued provider dictation services to save money.
Physicians need to take a hard look at what’s going on to help them sustain their business through times of uncertainty, says Advisory Board’s Hostetter. “Now is the time to evaluate options and figure out what’s right for your practice,” she adds.
This article first appeared on Medscape.com.
Family physician Frank Maselli, MD, saw approximately 30 patients a day in his office in the Bronx before COVID-19. But New York City has become a hot spot for the virus that has claimed the lives of the lives of more than 40,000 people nationwide.
Now Maselli and the other 10 physicians in the practice each treat only eight or nine patients a day via telemedicine. He spends most of his time on the phone answering patients’ questions about COVID-19 symptoms and potential exposure. Although he tries to bill for telemedicine and phone calls, he says many commercial payers reject the claims because their processing systems aren’t updated to reflect new coverage policies. He has enough cash in reserve to cover two payrolls, but he knows he needs a backup plan if patient volumes continue to decrease indefinitely.
“Our doctors will take a pay cut before we let people go,” says Maselli. “So far we’re OK because we’re getting paid for things we did two months ago before all of this happened.”
Ninety-seven percent of medical practices have experienced a negative financial impact directly or indirectly related to the COVID-19 pandemic, according to new data from the Medical Group Management Association (MGMA). On average, practices report a 60% decrease in patient volume and a 55% decrease in revenue since the beginning of the public health emergency.
Four options for financial assistance
However, there are ways to offset revenue loss and remain financially viable during the economic uncertainty of the COVID-19 pandemic. Options include the US Small Business Administration’s (SBA) Paycheck Protection Program; the SBA’s Emergency Economic Injury Disaster Loan (EIDL); Medicare’s advanced payment program; and an SBA Coronavirus Economic Stabilization Act (CESA) loan. These are in addition to several other strategies aimed at reducing costs and improving revenue.
1. Maselli, for example, applied for the Paycheck Protection Program, a short-term loan that helps small businesses (i.e., for physician offices, those with an annual revenue of under $12 million) keep staff employed during the COVID-19 crisis. The loan covers a variety of costs, including payroll, rent, utilities, mortgage interest, and interest on any other debt obligations incurred before February 15 of this year.
“We have no idea if this is coming and when, but it would be a big help,” he adds.
(As of press time, the Paycheck Protection Program had stopped accepting applications, having reached the limit of its $349 billion budget. Congress must now agree on legislation to add additional funding to assist small businesses.)
Practices can take out a loan of up to 2.5 times the average monthly payroll (excluding payroll for those making more than $100,000 annually) with a cap of $10 million. For example, if the average monthly payroll is $10,000 – and no employees earn more than $100,000 annually – the maximum loan amount is $25,000.
Practices approved for this loan can expect to receive the funds from their SBA-approved lender within 10 calendar days of the date of loan approval. Although it’s technically a loan, the good news is that it doesn’t need to be repaid if the practice complies with all of the loan requirements – particularly these two: The practice uses at least 75% of the loan specifically for payroll, and the practice keeps employees on the payroll (or rehire, when necessary) for 8 weeks after the loan origination date.
Forgiveness is reduced if full-time headcount declines, or if salaries and wages decrease. If a practice does need to repay all or a portion of the loan, it must do so within 2 years at an interest rate of 1%, and payments are deferred for 6 months.
Andrew D. McDonald, FACHE, practice leader of health care consulting at LBMC Healthcare, says it behooves practices to apply for this loan because it’s essentially free money during a time when revenue may be at an all-time low. “While the devil is in the details, on the surface, the paycheck protection funds appear to be a no-brainer. However, each practice will need to confirm with their lender that it’s a solid decision.”
One challenge with this loan is that some banks weren’t necessarily ready to accept applications on April 3, and many continue to lag behind in processing these applications.
2. A second option is the SBA’s EIDL, a low-interest, long-term loan (capped at 3.75% for small businesses) that practices with 500 or fewer employees can use to pay fixed debts, payrolls, accounts payable, and other bills that could have been paid had the disaster not occurred. Borrowers can ask for up to $2 million, and the maximum term of this loan is 30 years, though the overall process for obtaining these loans will depend on the lender.
Practices have until December 16 to apply for this loan. They can also apply for an expedited disbursement (i.e., an Economic Injury Disaster Advance) of up to $10,000 that’s paid within 3 days of the request.
3. A third option is Medicare’s COVID-19 advanced payment program. Under this program, eligible physicians are those who:
- Billed Medicare for claims within 180 days prior to the date of the request
- Are financially solvent (i.e., aren’t in bankruptcy)
- Are free from any active medical review or program integrity investigations
- Are in good standing with Medicare (i.e., don’t have an outstanding delinquent Medicare overpayment)
If physicians meet this criteria, they can ask their Medicare Administrative Contractor (MAC) to provide an advanced payment of up to 100% of the Medicare payment amount based on a 3-month lookback period.
Once requested, MACs will issue payment within 7 calendar days from the date of the request. Repayment will occur in the form of automatic recoupments beginning 120 days after the advanced payment is received. Medicare has already approved more than 21,000 requests totaling more than $51 billion. CMS has provided a fact sheet to learn more about how to request an accelerated payment.
“The key is that you need to repay this, so you want to set a reasonable goal,” says Sarah Hostetter, senior consultant at Advisory Board, a health care research & data consulting firm. She says practices should consider what they’ll realistically be able to repay within 120 days.
4. A fourth option – specifically for mid-size practices – is a CESA loan, the details of which have yet to be announced, that will enable practices to access funds with an annualized rate no greater than 2% and with no principal or interest due for at least 6 months. The CARES Act, signed into law 3 weeks ago, provides $454 billion for this program.
Selecting the right option for your practice
Which singular option – or combination of options – is best for your practice? McDonald says to ask these questions:
- How well are patient volumes holding up?
- How well are physicians pivoting to telehealth?
- What is the overall economic loss?
- What are the available liquid assets, and how long can the practice maintain its financial viability over the next couple of months and beyond?
Cheryl Mongillo, MBA, administrative director of two independent family practices in Delaware, applied for both the Paycheck Protection Program and Medicare advanced payments because she’s worried about being able to pay staff while also covering costs related to personal protective equipment, medical waste, and cleaning, all of which have tripled since the pandemic began. One of the practices includes one physician and four nurse practitioners. The other includes five physicians and three nurse practitioners. In total, both practices employ 35 additional staff.
“I want our staff to know how much we care about them. My hope is that after this is over, our business will pick back up pretty quickly,” she adds. “However, until I can get the business back, I needed something to keep us afloat.”
Others are being more cautious. Crystal Bruning, practice manager at an Ob/Gyn clinic in Orlando, Florida, says her practice applied for the Paycheck Protection Program but is waiting another month or so before deciding whether it will also take advantage of Medicare advanced payments.
The practice is still trying to assess the true financial impact of its 30% reduction in patient volume. Bruning says the advanced payments wouldn’t amount to much anyway because only 10% of the practice’s patients have Medicare.
Making tough financial decisions while awaiting assistance
Kansas-based family physician Jennifer Bacani McKenney, MD, says she hasn’t paid herself a salary in weeks because of the revenue loss her practice has incurred.
“I want to make sure we can pay [all 12] employees,” she says. “In my family, we have two incomes, and we’re pretty good at saving money. However, I know not every physician can afford to do this.”
Although McKenney’s practice has seen a 75% reduction in patient volume, staff continue to provide virtual visits – including Zoom-based nursing home visits – phone visits, and in-person visits for acute illnesses. They also provide curbside immunizations. Still, long-term revenue loss is a concern. “I have a threshold in mind based on what we have in reserves,” she says. “If we hit that point, we would need to talk about a loan or Medicare advanced payments.”
Arkansas-based family physician Lonnie S. Robinson, MD, says he immediately applied for the Paycheck Protection Program after it was announced. “We also made sure we had a line of credit with our local bank during the very first discussions about what the pandemic would mean for our revenue streams,” he says.
However, because he’s in a rural area of the state, he continues to struggle with telemedicine due to broadband and connectivity challenges. Cash flow is another challenge because a lot of insurance companies are waiving copayments.
“I didn’t realize the amount of money we collect immediately from the patient,” he says. “This was a substantial revenue stream, and it was immediate revenue – not revenue waiting on a claim to be paid.”
Illinois-based family physician Deborah L. Winiger, MD, says she also applied for the Paycheck Protection Program but in the meantime had to reduce staff hours by a third because her patient volume dropped by more than half. She will also encourage staff to pursue temporary positions at a local hospital if the federal funds don’t materialize.
Kelly Shackleton, practice manager at a New York-based internal medicine practice, says she laid off 7 of her 16 staff members (including lab technicians, licensed practical nurses, billers, a referral specialist, and a file clerk) due to a 70% decrease in patient volume.
“I didn’t lay them all off at once,” she says. “I kept them until things were all caught up in each department. I plan to get them all back when the time is right, but I want to be sure to keep the practice afloat so they have a place to return to.” If the Paycheck Protection Program for which she applied comes through, then she will rehire them. She also applied for Medicare advanced payments and increased the practice’s line of credit.
Bill properly – and for everything you are still doing
Accurate and complete coding is critical during this time of financial instability, says Maselli. “I keep telling doctors to bill for everything they do,” he says. This includes phone calls between patients and physicians or other qualified healthcare providers (CPT codes 99441-99443). Note that these are time-based codes, requiring a minimum of five minutes of medical discussion.
Remote physiologic monitoring (including monitoring a patient’s oxygen saturation levels using pulse oximetry), virtual check-ins, and online digital evaluation and management services are also covered by Medicare and some commercial payers.
Other good news is that the Centers for Medicare & Medicaid Services added more than 80 additional services that providers can furnish using telehealth, including new patient office visits, home visits, prolonged office visits, smoking and tobacco cessation counseling, annual depression and alcohol screenings, advanced care planning, and much more.
Mongillo, the family practice administrator in Delaware, agrees that physicians need to bill for as many services as possible. At one of the family medicine practices she manages, physicians perform wellness visits, when appropriate, if patients are already coming into the office for another ailment.
Also look for ways to cut costs. For example, Mongillo was able to renegotiate the practice’s telemedicine contract after she received several proposals from other vendors offering three months of complimentary service. Shackleton discontinued provider dictation services to save money.
Physicians need to take a hard look at what’s going on to help them sustain their business through times of uncertainty, says Advisory Board’s Hostetter. “Now is the time to evaluate options and figure out what’s right for your practice,” she adds.
This article first appeared on Medscape.com.
Family physician Frank Maselli, MD, saw approximately 30 patients a day in his office in the Bronx before COVID-19. But New York City has become a hot spot for the virus that has claimed the lives of the lives of more than 40,000 people nationwide.
Now Maselli and the other 10 physicians in the practice each treat only eight or nine patients a day via telemedicine. He spends most of his time on the phone answering patients’ questions about COVID-19 symptoms and potential exposure. Although he tries to bill for telemedicine and phone calls, he says many commercial payers reject the claims because their processing systems aren’t updated to reflect new coverage policies. He has enough cash in reserve to cover two payrolls, but he knows he needs a backup plan if patient volumes continue to decrease indefinitely.
“Our doctors will take a pay cut before we let people go,” says Maselli. “So far we’re OK because we’re getting paid for things we did two months ago before all of this happened.”
Ninety-seven percent of medical practices have experienced a negative financial impact directly or indirectly related to the COVID-19 pandemic, according to new data from the Medical Group Management Association (MGMA). On average, practices report a 60% decrease in patient volume and a 55% decrease in revenue since the beginning of the public health emergency.
Four options for financial assistance
However, there are ways to offset revenue loss and remain financially viable during the economic uncertainty of the COVID-19 pandemic. Options include the US Small Business Administration’s (SBA) Paycheck Protection Program; the SBA’s Emergency Economic Injury Disaster Loan (EIDL); Medicare’s advanced payment program; and an SBA Coronavirus Economic Stabilization Act (CESA) loan. These are in addition to several other strategies aimed at reducing costs and improving revenue.
1. Maselli, for example, applied for the Paycheck Protection Program, a short-term loan that helps small businesses (i.e., for physician offices, those with an annual revenue of under $12 million) keep staff employed during the COVID-19 crisis. The loan covers a variety of costs, including payroll, rent, utilities, mortgage interest, and interest on any other debt obligations incurred before February 15 of this year.
“We have no idea if this is coming and when, but it would be a big help,” he adds.
(As of press time, the Paycheck Protection Program had stopped accepting applications, having reached the limit of its $349 billion budget. Congress must now agree on legislation to add additional funding to assist small businesses.)
Practices can take out a loan of up to 2.5 times the average monthly payroll (excluding payroll for those making more than $100,000 annually) with a cap of $10 million. For example, if the average monthly payroll is $10,000 – and no employees earn more than $100,000 annually – the maximum loan amount is $25,000.
Practices approved for this loan can expect to receive the funds from their SBA-approved lender within 10 calendar days of the date of loan approval. Although it’s technically a loan, the good news is that it doesn’t need to be repaid if the practice complies with all of the loan requirements – particularly these two: The practice uses at least 75% of the loan specifically for payroll, and the practice keeps employees on the payroll (or rehire, when necessary) for 8 weeks after the loan origination date.
Forgiveness is reduced if full-time headcount declines, or if salaries and wages decrease. If a practice does need to repay all or a portion of the loan, it must do so within 2 years at an interest rate of 1%, and payments are deferred for 6 months.
Andrew D. McDonald, FACHE, practice leader of health care consulting at LBMC Healthcare, says it behooves practices to apply for this loan because it’s essentially free money during a time when revenue may be at an all-time low. “While the devil is in the details, on the surface, the paycheck protection funds appear to be a no-brainer. However, each practice will need to confirm with their lender that it’s a solid decision.”
One challenge with this loan is that some banks weren’t necessarily ready to accept applications on April 3, and many continue to lag behind in processing these applications.
2. A second option is the SBA’s EIDL, a low-interest, long-term loan (capped at 3.75% for small businesses) that practices with 500 or fewer employees can use to pay fixed debts, payrolls, accounts payable, and other bills that could have been paid had the disaster not occurred. Borrowers can ask for up to $2 million, and the maximum term of this loan is 30 years, though the overall process for obtaining these loans will depend on the lender.
Practices have until December 16 to apply for this loan. They can also apply for an expedited disbursement (i.e., an Economic Injury Disaster Advance) of up to $10,000 that’s paid within 3 days of the request.
3. A third option is Medicare’s COVID-19 advanced payment program. Under this program, eligible physicians are those who:
- Billed Medicare for claims within 180 days prior to the date of the request
- Are financially solvent (i.e., aren’t in bankruptcy)
- Are free from any active medical review or program integrity investigations
- Are in good standing with Medicare (i.e., don’t have an outstanding delinquent Medicare overpayment)
If physicians meet this criteria, they can ask their Medicare Administrative Contractor (MAC) to provide an advanced payment of up to 100% of the Medicare payment amount based on a 3-month lookback period.
Once requested, MACs will issue payment within 7 calendar days from the date of the request. Repayment will occur in the form of automatic recoupments beginning 120 days after the advanced payment is received. Medicare has already approved more than 21,000 requests totaling more than $51 billion. CMS has provided a fact sheet to learn more about how to request an accelerated payment.
“The key is that you need to repay this, so you want to set a reasonable goal,” says Sarah Hostetter, senior consultant at Advisory Board, a health care research & data consulting firm. She says practices should consider what they’ll realistically be able to repay within 120 days.
4. A fourth option – specifically for mid-size practices – is a CESA loan, the details of which have yet to be announced, that will enable practices to access funds with an annualized rate no greater than 2% and with no principal or interest due for at least 6 months. The CARES Act, signed into law 3 weeks ago, provides $454 billion for this program.
Selecting the right option for your practice
Which singular option – or combination of options – is best for your practice? McDonald says to ask these questions:
- How well are patient volumes holding up?
- How well are physicians pivoting to telehealth?
- What is the overall economic loss?
- What are the available liquid assets, and how long can the practice maintain its financial viability over the next couple of months and beyond?
Cheryl Mongillo, MBA, administrative director of two independent family practices in Delaware, applied for both the Paycheck Protection Program and Medicare advanced payments because she’s worried about being able to pay staff while also covering costs related to personal protective equipment, medical waste, and cleaning, all of which have tripled since the pandemic began. One of the practices includes one physician and four nurse practitioners. The other includes five physicians and three nurse practitioners. In total, both practices employ 35 additional staff.
“I want our staff to know how much we care about them. My hope is that after this is over, our business will pick back up pretty quickly,” she adds. “However, until I can get the business back, I needed something to keep us afloat.”
Others are being more cautious. Crystal Bruning, practice manager at an Ob/Gyn clinic in Orlando, Florida, says her practice applied for the Paycheck Protection Program but is waiting another month or so before deciding whether it will also take advantage of Medicare advanced payments.
The practice is still trying to assess the true financial impact of its 30% reduction in patient volume. Bruning says the advanced payments wouldn’t amount to much anyway because only 10% of the practice’s patients have Medicare.
Making tough financial decisions while awaiting assistance
Kansas-based family physician Jennifer Bacani McKenney, MD, says she hasn’t paid herself a salary in weeks because of the revenue loss her practice has incurred.
“I want to make sure we can pay [all 12] employees,” she says. “In my family, we have two incomes, and we’re pretty good at saving money. However, I know not every physician can afford to do this.”
Although McKenney’s practice has seen a 75% reduction in patient volume, staff continue to provide virtual visits – including Zoom-based nursing home visits – phone visits, and in-person visits for acute illnesses. They also provide curbside immunizations. Still, long-term revenue loss is a concern. “I have a threshold in mind based on what we have in reserves,” she says. “If we hit that point, we would need to talk about a loan or Medicare advanced payments.”
Arkansas-based family physician Lonnie S. Robinson, MD, says he immediately applied for the Paycheck Protection Program after it was announced. “We also made sure we had a line of credit with our local bank during the very first discussions about what the pandemic would mean for our revenue streams,” he says.
However, because he’s in a rural area of the state, he continues to struggle with telemedicine due to broadband and connectivity challenges. Cash flow is another challenge because a lot of insurance companies are waiving copayments.
“I didn’t realize the amount of money we collect immediately from the patient,” he says. “This was a substantial revenue stream, and it was immediate revenue – not revenue waiting on a claim to be paid.”
Illinois-based family physician Deborah L. Winiger, MD, says she also applied for the Paycheck Protection Program but in the meantime had to reduce staff hours by a third because her patient volume dropped by more than half. She will also encourage staff to pursue temporary positions at a local hospital if the federal funds don’t materialize.
Kelly Shackleton, practice manager at a New York-based internal medicine practice, says she laid off 7 of her 16 staff members (including lab technicians, licensed practical nurses, billers, a referral specialist, and a file clerk) due to a 70% decrease in patient volume.
“I didn’t lay them all off at once,” she says. “I kept them until things were all caught up in each department. I plan to get them all back when the time is right, but I want to be sure to keep the practice afloat so they have a place to return to.” If the Paycheck Protection Program for which she applied comes through, then she will rehire them. She also applied for Medicare advanced payments and increased the practice’s line of credit.
Bill properly – and for everything you are still doing
Accurate and complete coding is critical during this time of financial instability, says Maselli. “I keep telling doctors to bill for everything they do,” he says. This includes phone calls between patients and physicians or other qualified healthcare providers (CPT codes 99441-99443). Note that these are time-based codes, requiring a minimum of five minutes of medical discussion.
Remote physiologic monitoring (including monitoring a patient’s oxygen saturation levels using pulse oximetry), virtual check-ins, and online digital evaluation and management services are also covered by Medicare and some commercial payers.
Other good news is that the Centers for Medicare & Medicaid Services added more than 80 additional services that providers can furnish using telehealth, including new patient office visits, home visits, prolonged office visits, smoking and tobacco cessation counseling, annual depression and alcohol screenings, advanced care planning, and much more.
Mongillo, the family practice administrator in Delaware, agrees that physicians need to bill for as many services as possible. At one of the family medicine practices she manages, physicians perform wellness visits, when appropriate, if patients are already coming into the office for another ailment.
Also look for ways to cut costs. For example, Mongillo was able to renegotiate the practice’s telemedicine contract after she received several proposals from other vendors offering three months of complimentary service. Shackleton discontinued provider dictation services to save money.
Physicians need to take a hard look at what’s going on to help them sustain their business through times of uncertainty, says Advisory Board’s Hostetter. “Now is the time to evaluate options and figure out what’s right for your practice,” she adds.
This article first appeared on Medscape.com.
Overcoming COVID-related stress
As a department chief managing during this crisis, everyone greets me sympathetically: “This must be so stressful for you! Are you doing OK?” “Um, I’m great,” I answer contritely. Yes, this is hard, yet I feel fine. But why? Shouldn’t I be fretting the damage done by the COVID cyclone? Our operations are smashed and our staff scrambled, my family and friends are out of work; these are difficult times. But a harmful effect on my health or yours is not inevitable. There are things we can do to inoculate ourselves.
No doubt, exercise (if you can find weights!), eating well, sleeping, and meditating help, but they are secondary. None of these protect much if you still believe stress is killing you. You must first reframe what is happening. Health psychologist Kelly McGonigal, PhD, from Stanford (Calif.) University, is a world expert on this topic. If you’ve not seen her TED talk about stress, then watch it now. She teaches how stress is indeed harmful to your health – but only if you believe it to be so. Many studies have borne this out. One showed that people who reported high stress in the previous year were 43% more likely to die than those who did not. But that risk held only when they believed stress was harmful to them. Those who did not think that stress was harmful not only fared better but also had the lowest likelihood of death, lower even than those who reported little stress! So it wasn’t the stress that mattered, it was the physiologic response to it. And that you can control.
Changing your beliefs is no easy feat. There is work to be done, Dr. McGonigal would argue. You must not only reframe our stress as healthful, but also act in ways to make this true. This is easier for us as physicians. First, we understand better than most that difficulty is a normal part of life. We have countless stories of hardship, tragedy, pain and suffering from the work we do. The pandemic may be extraordinary in breadth, but not in depth. We’ve seen worse happen to patients. Second, we have firsthand experience that suffering ends and often leads to strength and resilience. Even in our own lives, it was by traveling through the extraordinary stress of medical school and residency that we arrived here.
Cortisol increases when we are under duress. So does oxytocin. The former gets most of the press, the latter is more interesting. That oxytocin release during stress conferred survival benefits to us as a species: When a threat arrived, we not only ran, but also grabbed the kids, too! Oxytocin is the “tend and befriend” compliment to cortisol’s “fight or flight.” Focusing on this priming to strengthen social ties, listen, spend (Zoom) time together, and provide emotional support is key to our recovery. Even small acts of giving for our staff, friends, family, and strangers can significantly shift consequences of this stress from harmful to beneficial.
Last year, my uncle died in a tragic accident. My aunt, who is alone, is now also isolated. She’s lost her partner, her guardian, and she is afraid. Rather than succumb to the stress, she imagined something she could do to wrest some control. Last week, she filled her minivan with pink and yellow tulips bunched in bouquets and tied with handwritten notes of encouragement. She then drove up and down the streets in her North Attleboro, Mass., neighborhood and left the flowers on doorsteps until her van was empty. She did so to share with them the bit of joy that spring brings, she says, and to encourage people to stay inside!
This is a difficult time for us, and yet even more difficult for others. Perhaps the best we can do is to find ways to bring a bit of joy or comfort to others.
“In some ways suffering ceases to be suffering at the moment it finds a meaning, such as the meaning of a sacrifice.” – Viktor Frankl
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. He had no relevant disclosures. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
As a department chief managing during this crisis, everyone greets me sympathetically: “This must be so stressful for you! Are you doing OK?” “Um, I’m great,” I answer contritely. Yes, this is hard, yet I feel fine. But why? Shouldn’t I be fretting the damage done by the COVID cyclone? Our operations are smashed and our staff scrambled, my family and friends are out of work; these are difficult times. But a harmful effect on my health or yours is not inevitable. There are things we can do to inoculate ourselves.
No doubt, exercise (if you can find weights!), eating well, sleeping, and meditating help, but they are secondary. None of these protect much if you still believe stress is killing you. You must first reframe what is happening. Health psychologist Kelly McGonigal, PhD, from Stanford (Calif.) University, is a world expert on this topic. If you’ve not seen her TED talk about stress, then watch it now. She teaches how stress is indeed harmful to your health – but only if you believe it to be so. Many studies have borne this out. One showed that people who reported high stress in the previous year were 43% more likely to die than those who did not. But that risk held only when they believed stress was harmful to them. Those who did not think that stress was harmful not only fared better but also had the lowest likelihood of death, lower even than those who reported little stress! So it wasn’t the stress that mattered, it was the physiologic response to it. And that you can control.
Changing your beliefs is no easy feat. There is work to be done, Dr. McGonigal would argue. You must not only reframe our stress as healthful, but also act in ways to make this true. This is easier for us as physicians. First, we understand better than most that difficulty is a normal part of life. We have countless stories of hardship, tragedy, pain and suffering from the work we do. The pandemic may be extraordinary in breadth, but not in depth. We’ve seen worse happen to patients. Second, we have firsthand experience that suffering ends and often leads to strength and resilience. Even in our own lives, it was by traveling through the extraordinary stress of medical school and residency that we arrived here.
Cortisol increases when we are under duress. So does oxytocin. The former gets most of the press, the latter is more interesting. That oxytocin release during stress conferred survival benefits to us as a species: When a threat arrived, we not only ran, but also grabbed the kids, too! Oxytocin is the “tend and befriend” compliment to cortisol’s “fight or flight.” Focusing on this priming to strengthen social ties, listen, spend (Zoom) time together, and provide emotional support is key to our recovery. Even small acts of giving for our staff, friends, family, and strangers can significantly shift consequences of this stress from harmful to beneficial.
Last year, my uncle died in a tragic accident. My aunt, who is alone, is now also isolated. She’s lost her partner, her guardian, and she is afraid. Rather than succumb to the stress, she imagined something she could do to wrest some control. Last week, she filled her minivan with pink and yellow tulips bunched in bouquets and tied with handwritten notes of encouragement. She then drove up and down the streets in her North Attleboro, Mass., neighborhood and left the flowers on doorsteps until her van was empty. She did so to share with them the bit of joy that spring brings, she says, and to encourage people to stay inside!
This is a difficult time for us, and yet even more difficult for others. Perhaps the best we can do is to find ways to bring a bit of joy or comfort to others.
“In some ways suffering ceases to be suffering at the moment it finds a meaning, such as the meaning of a sacrifice.” – Viktor Frankl
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. He had no relevant disclosures. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
As a department chief managing during this crisis, everyone greets me sympathetically: “This must be so stressful for you! Are you doing OK?” “Um, I’m great,” I answer contritely. Yes, this is hard, yet I feel fine. But why? Shouldn’t I be fretting the damage done by the COVID cyclone? Our operations are smashed and our staff scrambled, my family and friends are out of work; these are difficult times. But a harmful effect on my health or yours is not inevitable. There are things we can do to inoculate ourselves.
No doubt, exercise (if you can find weights!), eating well, sleeping, and meditating help, but they are secondary. None of these protect much if you still believe stress is killing you. You must first reframe what is happening. Health psychologist Kelly McGonigal, PhD, from Stanford (Calif.) University, is a world expert on this topic. If you’ve not seen her TED talk about stress, then watch it now. She teaches how stress is indeed harmful to your health – but only if you believe it to be so. Many studies have borne this out. One showed that people who reported high stress in the previous year were 43% more likely to die than those who did not. But that risk held only when they believed stress was harmful to them. Those who did not think that stress was harmful not only fared better but also had the lowest likelihood of death, lower even than those who reported little stress! So it wasn’t the stress that mattered, it was the physiologic response to it. And that you can control.
Changing your beliefs is no easy feat. There is work to be done, Dr. McGonigal would argue. You must not only reframe our stress as healthful, but also act in ways to make this true. This is easier for us as physicians. First, we understand better than most that difficulty is a normal part of life. We have countless stories of hardship, tragedy, pain and suffering from the work we do. The pandemic may be extraordinary in breadth, but not in depth. We’ve seen worse happen to patients. Second, we have firsthand experience that suffering ends and often leads to strength and resilience. Even in our own lives, it was by traveling through the extraordinary stress of medical school and residency that we arrived here.
Cortisol increases when we are under duress. So does oxytocin. The former gets most of the press, the latter is more interesting. That oxytocin release during stress conferred survival benefits to us as a species: When a threat arrived, we not only ran, but also grabbed the kids, too! Oxytocin is the “tend and befriend” compliment to cortisol’s “fight or flight.” Focusing on this priming to strengthen social ties, listen, spend (Zoom) time together, and provide emotional support is key to our recovery. Even small acts of giving for our staff, friends, family, and strangers can significantly shift consequences of this stress from harmful to beneficial.
Last year, my uncle died in a tragic accident. My aunt, who is alone, is now also isolated. She’s lost her partner, her guardian, and she is afraid. Rather than succumb to the stress, she imagined something she could do to wrest some control. Last week, she filled her minivan with pink and yellow tulips bunched in bouquets and tied with handwritten notes of encouragement. She then drove up and down the streets in her North Attleboro, Mass., neighborhood and left the flowers on doorsteps until her van was empty. She did so to share with them the bit of joy that spring brings, she says, and to encourage people to stay inside!
This is a difficult time for us, and yet even more difficult for others. Perhaps the best we can do is to find ways to bring a bit of joy or comfort to others.
“In some ways suffering ceases to be suffering at the moment it finds a meaning, such as the meaning of a sacrifice.” – Viktor Frankl
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. He had no relevant disclosures. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].