User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
FDA warns against cooking chicken in NyQuil
Called the “sleepy chicken challenge,” the trend tells people to cook chicken in NyQuil or similar over-the-counter cough and cold medications, which include ingredients such as acetaminophen, dextromethorphan, and doxylamine.
“The challenge sounds silly and unappetizing – and it is. But it could also be very unsafe,” the FDA said. “Boiling a medication can make it much more concentrated and change its properties in other ways.”
Even if someone doesn’t plan to eat the chicken, inhaling the vapors of the medication while it cooks could cause high levels of the drug to enter the body.
“It could also hurt your lungs,” the FDA said. “Put simply: Someone could take a dangerously high amount of the cough and cold medicine without even realizing it.”
This isn’t the first time that social media challenges involving medicine have gone viral. In a 2020 TikTok challenge, people were encouraged to take large doses of the allergy medicine diphenhydramine, called the “Benadryl challenge,” to cause hallucinations. The FDA received several reports of teens who were hospitalized or died, and it issued a warning about taking high doses of the drug.
“These video challenges, which often target youths, can harm people – and even cause death,” the FDA said. “Nonprescription (also called over-the-counter or OTC) drugs are readily available in many homes, making these challenges even more risky.”
In the latest warning, the FDA provided several ways for parents to make it less likely for children to do the social media challenges, such as locking up prescription and over-the-counter medications to prevent accidental overdoses. The FDA also encouraged parents and guardians to have open conversations with their children.
“Sit down with your children and discuss the dangers of misusing drugs and how social media trends can lead to real, sometimes irreversible, damage,” the FDA said. “Remind your children that overdoses can occur with OTC drugs as well as with prescription drugs.”
Following the FDA warning, the American Academy of Pediatrics also issued an advisory about social media trends. Some challenges, such as the ALS ice bucket challenge or the mannequin challenge, can be fun and positive activities. But medication-related challenges, such as the sleepy chicken and Benadryl challenges, can cause serious heart problems, seizures, coma, and even death.
“Teens’ brains are still developing. The part of the brain that handles rational thought, the prefrontal cortex, is not fully developed until the mid-20s,” the American Academy of Pediatrics said. “This means teens are naturally more impulsive and likely to act before thinking through all of the ramifications.”
Social media rewards outrageous behavior, it wrote, and the more outrageous the behavior, the more likely someone will get more engagement online.
“It’s a quick moving, impulsive environment, and the fear of losing out is real for teens,” the academy said. “What they will focus on is that a popular kid in class did this and got hundreds of likes and comments.”
The academy suggested that parents and guardians talk with teens about which challenges are trending on social media and at school.
“Sometimes kids are more willing to talk about their peers than themselves,” it said. “Asking questions about school trends, friends and fads may yield more answers than direct questions about their own activities.”
A version of this article first appeared on WebMD.com.
Called the “sleepy chicken challenge,” the trend tells people to cook chicken in NyQuil or similar over-the-counter cough and cold medications, which include ingredients such as acetaminophen, dextromethorphan, and doxylamine.
“The challenge sounds silly and unappetizing – and it is. But it could also be very unsafe,” the FDA said. “Boiling a medication can make it much more concentrated and change its properties in other ways.”
Even if someone doesn’t plan to eat the chicken, inhaling the vapors of the medication while it cooks could cause high levels of the drug to enter the body.
“It could also hurt your lungs,” the FDA said. “Put simply: Someone could take a dangerously high amount of the cough and cold medicine without even realizing it.”
This isn’t the first time that social media challenges involving medicine have gone viral. In a 2020 TikTok challenge, people were encouraged to take large doses of the allergy medicine diphenhydramine, called the “Benadryl challenge,” to cause hallucinations. The FDA received several reports of teens who were hospitalized or died, and it issued a warning about taking high doses of the drug.
“These video challenges, which often target youths, can harm people – and even cause death,” the FDA said. “Nonprescription (also called over-the-counter or OTC) drugs are readily available in many homes, making these challenges even more risky.”
In the latest warning, the FDA provided several ways for parents to make it less likely for children to do the social media challenges, such as locking up prescription and over-the-counter medications to prevent accidental overdoses. The FDA also encouraged parents and guardians to have open conversations with their children.
“Sit down with your children and discuss the dangers of misusing drugs and how social media trends can lead to real, sometimes irreversible, damage,” the FDA said. “Remind your children that overdoses can occur with OTC drugs as well as with prescription drugs.”
Following the FDA warning, the American Academy of Pediatrics also issued an advisory about social media trends. Some challenges, such as the ALS ice bucket challenge or the mannequin challenge, can be fun and positive activities. But medication-related challenges, such as the sleepy chicken and Benadryl challenges, can cause serious heart problems, seizures, coma, and even death.
“Teens’ brains are still developing. The part of the brain that handles rational thought, the prefrontal cortex, is not fully developed until the mid-20s,” the American Academy of Pediatrics said. “This means teens are naturally more impulsive and likely to act before thinking through all of the ramifications.”
Social media rewards outrageous behavior, it wrote, and the more outrageous the behavior, the more likely someone will get more engagement online.
“It’s a quick moving, impulsive environment, and the fear of losing out is real for teens,” the academy said. “What they will focus on is that a popular kid in class did this and got hundreds of likes and comments.”
The academy suggested that parents and guardians talk with teens about which challenges are trending on social media and at school.
“Sometimes kids are more willing to talk about their peers than themselves,” it said. “Asking questions about school trends, friends and fads may yield more answers than direct questions about their own activities.”
A version of this article first appeared on WebMD.com.
Called the “sleepy chicken challenge,” the trend tells people to cook chicken in NyQuil or similar over-the-counter cough and cold medications, which include ingredients such as acetaminophen, dextromethorphan, and doxylamine.
“The challenge sounds silly and unappetizing – and it is. But it could also be very unsafe,” the FDA said. “Boiling a medication can make it much more concentrated and change its properties in other ways.”
Even if someone doesn’t plan to eat the chicken, inhaling the vapors of the medication while it cooks could cause high levels of the drug to enter the body.
“It could also hurt your lungs,” the FDA said. “Put simply: Someone could take a dangerously high amount of the cough and cold medicine without even realizing it.”
This isn’t the first time that social media challenges involving medicine have gone viral. In a 2020 TikTok challenge, people were encouraged to take large doses of the allergy medicine diphenhydramine, called the “Benadryl challenge,” to cause hallucinations. The FDA received several reports of teens who were hospitalized or died, and it issued a warning about taking high doses of the drug.
“These video challenges, which often target youths, can harm people – and even cause death,” the FDA said. “Nonprescription (also called over-the-counter or OTC) drugs are readily available in many homes, making these challenges even more risky.”
In the latest warning, the FDA provided several ways for parents to make it less likely for children to do the social media challenges, such as locking up prescription and over-the-counter medications to prevent accidental overdoses. The FDA also encouraged parents and guardians to have open conversations with their children.
“Sit down with your children and discuss the dangers of misusing drugs and how social media trends can lead to real, sometimes irreversible, damage,” the FDA said. “Remind your children that overdoses can occur with OTC drugs as well as with prescription drugs.”
Following the FDA warning, the American Academy of Pediatrics also issued an advisory about social media trends. Some challenges, such as the ALS ice bucket challenge or the mannequin challenge, can be fun and positive activities. But medication-related challenges, such as the sleepy chicken and Benadryl challenges, can cause serious heart problems, seizures, coma, and even death.
“Teens’ brains are still developing. The part of the brain that handles rational thought, the prefrontal cortex, is not fully developed until the mid-20s,” the American Academy of Pediatrics said. “This means teens are naturally more impulsive and likely to act before thinking through all of the ramifications.”
Social media rewards outrageous behavior, it wrote, and the more outrageous the behavior, the more likely someone will get more engagement online.
“It’s a quick moving, impulsive environment, and the fear of losing out is real for teens,” the academy said. “What they will focus on is that a popular kid in class did this and got hundreds of likes and comments.”
The academy suggested that parents and guardians talk with teens about which challenges are trending on social media and at school.
“Sometimes kids are more willing to talk about their peers than themselves,” it said. “Asking questions about school trends, friends and fads may yield more answers than direct questions about their own activities.”
A version of this article first appeared on WebMD.com.
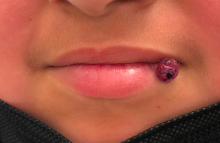
A 10-year-old with a red bump on her lower lip
The patient’s history and examination are consistent with a diagnosis of pyogenic granuloma. Specifically, the history of rapid growth, friable nature, associated bleeding, and hemorrhagic crusting point to pyogenic granuloma as the most likely diagnosis.
Pyogenic granuloma is an acquired benign vascular growth of the skin or mucous membranes.1 It most frequently occurs in children and young adults and most commonly affects the skin of the head, trunk, and extremities.2 Common mucosal sites include the gingiva, lips, and tongue.2 The etiology of pyogenic granuloma is unknown, though it is thought to be a process akin to the overgrowth of granulation tissue.3,4 Expression of angiogenic factors and subsequent vascular hyperplasia are also implicated as key players in the pathogenesis of pyogenic granuloma.1,4 In addition, several associated factors and inciting triggers have been proposed including trauma, infections, and hormonal fluctuations.3-5 However, the majority of patients do not report predisposing factors or a history of prior trauma at the site.3,6
Clinically, pyogenic granuloma usually presents as a painless, erythematous, dome-shaped friable papule or nodule that easily bleeds and may ulcerate. It typically undergoes a period of growth over weeks to months followed by stabilization. Occasionally, pyogenic granulomas will spontaneously involute, though most do not.7 Pyogenic granuloma may occur within an existing capillary malformation, such as a port wine stain, spontaneously or as a sequela of laser treatment.8,9 Diagnosis of pyogenic granuloma can typically be made clinically on the basis of history and exam. Dermoscopic evaluation of pyogenic granuloma will reveal a homogeneous papule with a surrounding white-brown collarette, and potentially white intersecting lines.10 Histopathologic evaluation may be necessary to differentiate lesions from conditions that may mimic pyogenic granuloma.
What’s on the differential?
The differential diagnosis for pyogenic granuloma consists of Spitz nevus, cherry hemangioma, amelanotic melanoma, and glomus tumor.
Spitz nevus
Spitz nevus (spindle and epithelial cell nevus) is a benign melanocytic lesion that classically appears as a sharply circumscribed, smooth, dome-shaped, pink-red, or brown papule or plaque. There is typically a history of rapid growth over several months followed by stabilization. It usually presents in childhood or adolescence and is most commonly located on the face and extremities. While there are similarities in the appearance of Spitz nevi and pyogenic granuloma, Spitz nevi are not usually friable nor associated with bleeding as in our patient. Furthermore, on dermoscopy, Spitz nevus typically exhibits a starburst pattern with regularly distributed dotted vessels, or a peripheral globular pattern with reticular depigmentation. The definitive diagnosis of Spitz nevi relies on histopathologic evaluation, which is critical for discriminating Spitz nevi from melanoma.
Cherry hemangioma
Cherry angiomas are the most common type of acquired benign vascular proliferation. They present as small, bright red or violaceous macules or papules. However, they typically appear in early to midadulthood and increase in number with age. The age of our patient and solitary presentation of the lesion make this diagnosis unlikely. In addition, cherry angiomas are not usually associated with bleeding. It is important to note that, depending on the age of the patient, pyogenic granuloma may also be confused with infantile hemangioma. Infantile hemangiomas may become bright red papules, nodules, or plaques that appear in early infancy. They characteristically involute, which does not typically happen with pyogenic granuloma.
Amelanotic melanoma
Amelanotic melanoma is an uncommon variant of melanoma with little to no pigmentation. It may appear as a skin-colored to light-brown, pink, or red macule, papule, or nodule. The lesion may be asymmetric with irregular and well-defined borders. The variable and uncharacteristic appearance of this melanoma variant makes it diagnostically challenging and it is often confused with benign lesions including pyogenic granuloma. Dermoscopy can help distinguish amelanotic melanoma from other benign conditions, and will reveal areas of pink to white, polymorphous vessels and crystalline structures. However, ultimately biopsy and histopathological evaluation is necessary for accurate diagnosis.
Glomus tumor
Glomus tumors are rare, benign neoplasms originating from cells of the glomus body that presents as a red-purple, vascular papule or nodule. They are usually found in areas rich in glomus bodies, such as the subungual regions, fingertips, palms, wrists, and forearms. Glomus tumors are typically associated with tenderness, paroxysmal pain, and cold sensitivity. They do not bleed or ulcerate. While pyogenic granuloma may be confused for glomus tumor when present on the fingers or extremities, the location of the lesion in our patient is not consistent with a diagnosis of glomus tumor.
Management and disease course
Management with procedural or topical interventions is usually pursued for pyogenic granuloma because of frequent bleeding and ulceration of lesions. The most common approach is simple excision by a scoop or shave technique, with or without curettage and most commonly with electrocautery of the base. Other options include full-thickness excision, destruction with laser therapy, cryotherapy, or topical treatments (for example, timolol).11 Lesion recurrence can occur with both surgical and nonsurgical management.11 Regardless of management technique, it is useful to obtain histopathologic evaluation of tissue for accurate diagnosis.
Our patient underwent surgical destruction of her lower-lip lesion with shave excision followed by electrocautery. The surgical specimen was sent for pathology, which confirmed the diagnosis of pyogenic granuloma. The patient experienced no complications from the procedure and did not have recurrence of the lesion.
Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Ms. Sui nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Lin RL and Janniger CK. Cutis. 2004 Oct;74(4):229-33.
2. Harris MN et al. J Am Acad Dermatol. 2000 Jun;42(6):1012-6.
3. Pagliai KA and Cohen BA. Pediatr Dermatol. 2004 Jan-Feb;21(1):10-3.
4. Kamal R et al. J Oral Maxillofac Pathol. 2012 Jan;16(1):79-82.
5. Requena L and Sangueza OP. J Am Acad Dermatol. 1997 Dec;37(6):887-919.
6. Patrice SJ et al. Pediatr Dermatol. 1991 Dec;8(4):267-76.
7. Luba MC et al. Am Fam Physician. 2003 Feb 15;67(4):729-38.
8. Swerlick RA and Cooper PH. J Am Acad Dermatol. 1983 May;8(5):627-30.
9. Sheehan DJ and Lesher JL Jr. Cutis. 2004 Mar;73(3):175-80.
10. Zaballos P et al. Br J Dermatol. 2006 Jun;154(6):1108-11.
11. Lee J et al. J Plast Reconstr Aesthet Surg. 2011 Sep;64(9):1216-20. .
The patient’s history and examination are consistent with a diagnosis of pyogenic granuloma. Specifically, the history of rapid growth, friable nature, associated bleeding, and hemorrhagic crusting point to pyogenic granuloma as the most likely diagnosis.
Pyogenic granuloma is an acquired benign vascular growth of the skin or mucous membranes.1 It most frequently occurs in children and young adults and most commonly affects the skin of the head, trunk, and extremities.2 Common mucosal sites include the gingiva, lips, and tongue.2 The etiology of pyogenic granuloma is unknown, though it is thought to be a process akin to the overgrowth of granulation tissue.3,4 Expression of angiogenic factors and subsequent vascular hyperplasia are also implicated as key players in the pathogenesis of pyogenic granuloma.1,4 In addition, several associated factors and inciting triggers have been proposed including trauma, infections, and hormonal fluctuations.3-5 However, the majority of patients do not report predisposing factors or a history of prior trauma at the site.3,6
Clinically, pyogenic granuloma usually presents as a painless, erythematous, dome-shaped friable papule or nodule that easily bleeds and may ulcerate. It typically undergoes a period of growth over weeks to months followed by stabilization. Occasionally, pyogenic granulomas will spontaneously involute, though most do not.7 Pyogenic granuloma may occur within an existing capillary malformation, such as a port wine stain, spontaneously or as a sequela of laser treatment.8,9 Diagnosis of pyogenic granuloma can typically be made clinically on the basis of history and exam. Dermoscopic evaluation of pyogenic granuloma will reveal a homogeneous papule with a surrounding white-brown collarette, and potentially white intersecting lines.10 Histopathologic evaluation may be necessary to differentiate lesions from conditions that may mimic pyogenic granuloma.
What’s on the differential?
The differential diagnosis for pyogenic granuloma consists of Spitz nevus, cherry hemangioma, amelanotic melanoma, and glomus tumor.
Spitz nevus
Spitz nevus (spindle and epithelial cell nevus) is a benign melanocytic lesion that classically appears as a sharply circumscribed, smooth, dome-shaped, pink-red, or brown papule or plaque. There is typically a history of rapid growth over several months followed by stabilization. It usually presents in childhood or adolescence and is most commonly located on the face and extremities. While there are similarities in the appearance of Spitz nevi and pyogenic granuloma, Spitz nevi are not usually friable nor associated with bleeding as in our patient. Furthermore, on dermoscopy, Spitz nevus typically exhibits a starburst pattern with regularly distributed dotted vessels, or a peripheral globular pattern with reticular depigmentation. The definitive diagnosis of Spitz nevi relies on histopathologic evaluation, which is critical for discriminating Spitz nevi from melanoma.
Cherry hemangioma
Cherry angiomas are the most common type of acquired benign vascular proliferation. They present as small, bright red or violaceous macules or papules. However, they typically appear in early to midadulthood and increase in number with age. The age of our patient and solitary presentation of the lesion make this diagnosis unlikely. In addition, cherry angiomas are not usually associated with bleeding. It is important to note that, depending on the age of the patient, pyogenic granuloma may also be confused with infantile hemangioma. Infantile hemangiomas may become bright red papules, nodules, or plaques that appear in early infancy. They characteristically involute, which does not typically happen with pyogenic granuloma.
Amelanotic melanoma
Amelanotic melanoma is an uncommon variant of melanoma with little to no pigmentation. It may appear as a skin-colored to light-brown, pink, or red macule, papule, or nodule. The lesion may be asymmetric with irregular and well-defined borders. The variable and uncharacteristic appearance of this melanoma variant makes it diagnostically challenging and it is often confused with benign lesions including pyogenic granuloma. Dermoscopy can help distinguish amelanotic melanoma from other benign conditions, and will reveal areas of pink to white, polymorphous vessels and crystalline structures. However, ultimately biopsy and histopathological evaluation is necessary for accurate diagnosis.
Glomus tumor
Glomus tumors are rare, benign neoplasms originating from cells of the glomus body that presents as a red-purple, vascular papule or nodule. They are usually found in areas rich in glomus bodies, such as the subungual regions, fingertips, palms, wrists, and forearms. Glomus tumors are typically associated with tenderness, paroxysmal pain, and cold sensitivity. They do not bleed or ulcerate. While pyogenic granuloma may be confused for glomus tumor when present on the fingers or extremities, the location of the lesion in our patient is not consistent with a diagnosis of glomus tumor.
Management and disease course
Management with procedural or topical interventions is usually pursued for pyogenic granuloma because of frequent bleeding and ulceration of lesions. The most common approach is simple excision by a scoop or shave technique, with or without curettage and most commonly with electrocautery of the base. Other options include full-thickness excision, destruction with laser therapy, cryotherapy, or topical treatments (for example, timolol).11 Lesion recurrence can occur with both surgical and nonsurgical management.11 Regardless of management technique, it is useful to obtain histopathologic evaluation of tissue for accurate diagnosis.
Our patient underwent surgical destruction of her lower-lip lesion with shave excision followed by electrocautery. The surgical specimen was sent for pathology, which confirmed the diagnosis of pyogenic granuloma. The patient experienced no complications from the procedure and did not have recurrence of the lesion.
Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Ms. Sui nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Lin RL and Janniger CK. Cutis. 2004 Oct;74(4):229-33.
2. Harris MN et al. J Am Acad Dermatol. 2000 Jun;42(6):1012-6.
3. Pagliai KA and Cohen BA. Pediatr Dermatol. 2004 Jan-Feb;21(1):10-3.
4. Kamal R et al. J Oral Maxillofac Pathol. 2012 Jan;16(1):79-82.
5. Requena L and Sangueza OP. J Am Acad Dermatol. 1997 Dec;37(6):887-919.
6. Patrice SJ et al. Pediatr Dermatol. 1991 Dec;8(4):267-76.
7. Luba MC et al. Am Fam Physician. 2003 Feb 15;67(4):729-38.
8. Swerlick RA and Cooper PH. J Am Acad Dermatol. 1983 May;8(5):627-30.
9. Sheehan DJ and Lesher JL Jr. Cutis. 2004 Mar;73(3):175-80.
10. Zaballos P et al. Br J Dermatol. 2006 Jun;154(6):1108-11.
11. Lee J et al. J Plast Reconstr Aesthet Surg. 2011 Sep;64(9):1216-20. .
The patient’s history and examination are consistent with a diagnosis of pyogenic granuloma. Specifically, the history of rapid growth, friable nature, associated bleeding, and hemorrhagic crusting point to pyogenic granuloma as the most likely diagnosis.
Pyogenic granuloma is an acquired benign vascular growth of the skin or mucous membranes.1 It most frequently occurs in children and young adults and most commonly affects the skin of the head, trunk, and extremities.2 Common mucosal sites include the gingiva, lips, and tongue.2 The etiology of pyogenic granuloma is unknown, though it is thought to be a process akin to the overgrowth of granulation tissue.3,4 Expression of angiogenic factors and subsequent vascular hyperplasia are also implicated as key players in the pathogenesis of pyogenic granuloma.1,4 In addition, several associated factors and inciting triggers have been proposed including trauma, infections, and hormonal fluctuations.3-5 However, the majority of patients do not report predisposing factors or a history of prior trauma at the site.3,6
Clinically, pyogenic granuloma usually presents as a painless, erythematous, dome-shaped friable papule or nodule that easily bleeds and may ulcerate. It typically undergoes a period of growth over weeks to months followed by stabilization. Occasionally, pyogenic granulomas will spontaneously involute, though most do not.7 Pyogenic granuloma may occur within an existing capillary malformation, such as a port wine stain, spontaneously or as a sequela of laser treatment.8,9 Diagnosis of pyogenic granuloma can typically be made clinically on the basis of history and exam. Dermoscopic evaluation of pyogenic granuloma will reveal a homogeneous papule with a surrounding white-brown collarette, and potentially white intersecting lines.10 Histopathologic evaluation may be necessary to differentiate lesions from conditions that may mimic pyogenic granuloma.
What’s on the differential?
The differential diagnosis for pyogenic granuloma consists of Spitz nevus, cherry hemangioma, amelanotic melanoma, and glomus tumor.
Spitz nevus
Spitz nevus (spindle and epithelial cell nevus) is a benign melanocytic lesion that classically appears as a sharply circumscribed, smooth, dome-shaped, pink-red, or brown papule or plaque. There is typically a history of rapid growth over several months followed by stabilization. It usually presents in childhood or adolescence and is most commonly located on the face and extremities. While there are similarities in the appearance of Spitz nevi and pyogenic granuloma, Spitz nevi are not usually friable nor associated with bleeding as in our patient. Furthermore, on dermoscopy, Spitz nevus typically exhibits a starburst pattern with regularly distributed dotted vessels, or a peripheral globular pattern with reticular depigmentation. The definitive diagnosis of Spitz nevi relies on histopathologic evaluation, which is critical for discriminating Spitz nevi from melanoma.
Cherry hemangioma
Cherry angiomas are the most common type of acquired benign vascular proliferation. They present as small, bright red or violaceous macules or papules. However, they typically appear in early to midadulthood and increase in number with age. The age of our patient and solitary presentation of the lesion make this diagnosis unlikely. In addition, cherry angiomas are not usually associated with bleeding. It is important to note that, depending on the age of the patient, pyogenic granuloma may also be confused with infantile hemangioma. Infantile hemangiomas may become bright red papules, nodules, or plaques that appear in early infancy. They characteristically involute, which does not typically happen with pyogenic granuloma.
Amelanotic melanoma
Amelanotic melanoma is an uncommon variant of melanoma with little to no pigmentation. It may appear as a skin-colored to light-brown, pink, or red macule, papule, or nodule. The lesion may be asymmetric with irregular and well-defined borders. The variable and uncharacteristic appearance of this melanoma variant makes it diagnostically challenging and it is often confused with benign lesions including pyogenic granuloma. Dermoscopy can help distinguish amelanotic melanoma from other benign conditions, and will reveal areas of pink to white, polymorphous vessels and crystalline structures. However, ultimately biopsy and histopathological evaluation is necessary for accurate diagnosis.
Glomus tumor
Glomus tumors are rare, benign neoplasms originating from cells of the glomus body that presents as a red-purple, vascular papule or nodule. They are usually found in areas rich in glomus bodies, such as the subungual regions, fingertips, palms, wrists, and forearms. Glomus tumors are typically associated with tenderness, paroxysmal pain, and cold sensitivity. They do not bleed or ulcerate. While pyogenic granuloma may be confused for glomus tumor when present on the fingers or extremities, the location of the lesion in our patient is not consistent with a diagnosis of glomus tumor.
Management and disease course
Management with procedural or topical interventions is usually pursued for pyogenic granuloma because of frequent bleeding and ulceration of lesions. The most common approach is simple excision by a scoop or shave technique, with or without curettage and most commonly with electrocautery of the base. Other options include full-thickness excision, destruction with laser therapy, cryotherapy, or topical treatments (for example, timolol).11 Lesion recurrence can occur with both surgical and nonsurgical management.11 Regardless of management technique, it is useful to obtain histopathologic evaluation of tissue for accurate diagnosis.
Our patient underwent surgical destruction of her lower-lip lesion with shave excision followed by electrocautery. The surgical specimen was sent for pathology, which confirmed the diagnosis of pyogenic granuloma. The patient experienced no complications from the procedure and did not have recurrence of the lesion.
Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Ms. Sui nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Lin RL and Janniger CK. Cutis. 2004 Oct;74(4):229-33.
2. Harris MN et al. J Am Acad Dermatol. 2000 Jun;42(6):1012-6.
3. Pagliai KA and Cohen BA. Pediatr Dermatol. 2004 Jan-Feb;21(1):10-3.
4. Kamal R et al. J Oral Maxillofac Pathol. 2012 Jan;16(1):79-82.
5. Requena L and Sangueza OP. J Am Acad Dermatol. 1997 Dec;37(6):887-919.
6. Patrice SJ et al. Pediatr Dermatol. 1991 Dec;8(4):267-76.
7. Luba MC et al. Am Fam Physician. 2003 Feb 15;67(4):729-38.
8. Swerlick RA and Cooper PH. J Am Acad Dermatol. 1983 May;8(5):627-30.
9. Sheehan DJ and Lesher JL Jr. Cutis. 2004 Mar;73(3):175-80.
10. Zaballos P et al. Br J Dermatol. 2006 Jun;154(6):1108-11.
11. Lee J et al. J Plast Reconstr Aesthet Surg. 2011 Sep;64(9):1216-20. .
Early bird gets the worm, night owl gets the diabetes
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
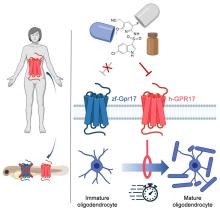
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Formula may be right for infants, but experts warn that toddlers don’t need it
Formulas for toddlers are a burgeoning business in the United States: Sales of the drinks more than doubled in recent years as companies convinced parents that their little ones needed the liquid boost. But many experts warn that these products, designed for children ages 1-3, fill no nutritional needs beyond what is available in a typical toddler diet, are subject to less regulation than infant formula, and are expensive.
In addition, some parents feed the toddler versions to infants even though they do not meet federal standards for infant formula and may not provide babies with adequate nutrients to sustain their growth.
Pediatricians and federal health officials say that when most children turn 1, they can begin drinking cow milk or an unsweetened plant-based milk substitute. In a 2019 “consensus” statement, the American Academy of Pediatrics and other health and nutrition organizations recommended against using toddler formulas, saying “they offer no unique nutritional value beyond what could be obtained with healthy foods; furthermore, they may contribute added sugars to the diet.” The toddler formulas often contain sweeteners and fats that add calories.
Some of the same companies that produce infant formula – including Enfamil, Gerber, and Similac – also make toddler formulas, as do some smaller, boutique brands that advertise that they have organic or other special qualities. Toddler formulas are available nearly everywhere infant formulas are sold and are marketed as providing extra nutrients to help children’s brain, immune system, and eye development, among other benefits. They are different from medical formulas prescribed for children with specific needs.
A 2020 study found that sales of toddler formula in the United States rose to $92 million in 2015 from $39 million in 2006.
Parents are often confused by the marketing for the formulas, according to a study led by Jennifer Harris, PhD, a marketing and public health researcher at the University of Connecticut, Hartford. She found that 60% of caregivers falsely believed toddler formulas have nutrients that toddlers can’t get from other foods.
Anthony Porto, MD, MPH, a pediatric gastroenterologist and pediatrics professor at Yale University, New Haven, Conn., said he is concerned these products could be giving toddlers more nutrients and calories than they need. Unlike what’s designed for infants, toddler formula has no nutritional regulations: Experts say standardizing a supplement to toddlers’ diets is impossible because no two children are alike.
In focus groups, Dr. Harris said, parents report feeding their children toddler formula to fill nutritional gaps when a child isn’t eating enough, a common concern among parents.
“Infants are often voracious eaters,” said Stephen Daniels, MD, chair of pediatrics at Children’s Hospital Colorado, Aurora. But at around a year of age, children’s growth plateaus, he said, and “they’re suddenly not hungry in the way they used to be anymore.” That can worry parents, he added, but “it’s a completely normal phenomenon.”
If parents have concerns about their children’s diet, Dr. Daniels said, they should consult a pediatrician or family doctor.
Blanche Lincoln, president of the Infant Nutrition Council of America, which represents the makers of Enfamil, Gerber, Similac, and store brands, said in an email that the toddler formulas can be helpful because they can fill “nutritional gaps during this period of transition to table foods.” Ms. Lincoln, a former U.S. senator from Arkansas, said the drinks “help contribute to the specific nutritional needs of toddlers by providing energy and important nutrients, as well as essential vitamins and minerals during this important period of growth and development.”
But toddler formula isn’t being ingested by toddlers alone – it’s also being fed to infants. In a recent study, Dr. Porto and colleagues found that 5% of infants’ parents reported giving their babies drinks marketed for the older age group. And Dr. Harris’ research indicated that 22% of parents of infants older than 6 months had fed their babies toddler formula in the previous month. Both studies were conducted before the recent infant formula shortage, which may have exacerbated the problem.
“Infant formulas and toddler formulas tend to be next to each other in the supermarket,” Dr. Harris said. “They look similar, but the toddler formulas are cheaper than the infant formulas. So people confuse them, and they grab the wrong one. Or they think: ‘Oh, this one is less expensive. I’ll get this one instead.’ ”
According to an email from Food and Drug Administration spokesperson Lindsay Haake, toddler drinks do not meet the definition of infant formula, so they are not subject to the same requirements. That means they do not have to undergo the clinical trials and pathogen safety testing that the infant versions do. “Unlike infant formulas, toddler formulas are not necessary to meet the nutritional needs of their intended consumers,” Ms. Haake said.
In a statement to KHN, the Infant Nutrition Council of America said: “Toddler drinks have a distinctive use and nutritional makeup from infant formula; the two are not interchangeable. The labeling of toddler nutritional drinks explicitly identifies the product as a toddler drink intended for children 12 months and older on the front of the package label.”
However, several expensive toddler formula brands made by smaller companies – often advertised as being made from goat milk, A2 whole milk (which lacks one common milk protein), or vegan ingredients that aren’t soy – do meet nutritional requirements for infants, and some advertise that.
Dr. Harris argued that this confuses parents, too, and shouldn’t be allowed. Just because a toddler formula has the nutritional ingredients required by the FDA for infant formula doesn’t mean it has met the other tests required of infant formula.
Federal regulators have not forced any of the companies to withdraw those products. In an email, FDA spokesperson Marianna Naum said: “The FDA does not comment on potential compliance actions.”
One company, Nature’s One, whose toddler formulas are named “Baby’s Only,” received warning letters a decade ago from the FDA about marketing them for infants. That case was closed in 2016. The company’s website says that Baby’s Only formula “meets nutrient requirements for infant” and that “Baby’s Only Organic® can be served up to 3 years of age.” Critics say that language implies the formula is fine for babies younger than 1. The company’s website and its Instagram account feature customer testimonials from parents who report feeding the formula to their infants, as well as pictures of infants drinking it.
Jay Highman, CEO and president of Nature’s One, said that Baby’s Only is clearly labeled as a toddler formula and that the back of the can states that “Baby’s Only is intended for a toddler 1 year of age or older OR when directed by a health care professional.” He also said that since the company launched in 1999, its formulas have met all the nutritional, manufacturing, and safety standards required of infant formula even though they don’t have to. “We behaved like we are an infant formula, but we were selling it as a toddler formula.”
He said that the clinical trials required by the FDA are a huge barrier to bringing a new infant formula to market and that many other countries don’t require a clinical trial. Baby’s Only recently completed a clinical trial, and the company expects to be able to sell it as an infant formula soon.
Yet pediatricians and nutritional experts continue to caution parents about using the toddler drinks. “There’s no question that infant formula is very important in the first year of life,” Dr. Daniels said. But he doesn’t recommend the toddler version “because it’s not that useful, because it’s confusing, because it’s expensive.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Formulas for toddlers are a burgeoning business in the United States: Sales of the drinks more than doubled in recent years as companies convinced parents that their little ones needed the liquid boost. But many experts warn that these products, designed for children ages 1-3, fill no nutritional needs beyond what is available in a typical toddler diet, are subject to less regulation than infant formula, and are expensive.
In addition, some parents feed the toddler versions to infants even though they do not meet federal standards for infant formula and may not provide babies with adequate nutrients to sustain their growth.
Pediatricians and federal health officials say that when most children turn 1, they can begin drinking cow milk or an unsweetened plant-based milk substitute. In a 2019 “consensus” statement, the American Academy of Pediatrics and other health and nutrition organizations recommended against using toddler formulas, saying “they offer no unique nutritional value beyond what could be obtained with healthy foods; furthermore, they may contribute added sugars to the diet.” The toddler formulas often contain sweeteners and fats that add calories.
Some of the same companies that produce infant formula – including Enfamil, Gerber, and Similac – also make toddler formulas, as do some smaller, boutique brands that advertise that they have organic or other special qualities. Toddler formulas are available nearly everywhere infant formulas are sold and are marketed as providing extra nutrients to help children’s brain, immune system, and eye development, among other benefits. They are different from medical formulas prescribed for children with specific needs.
A 2020 study found that sales of toddler formula in the United States rose to $92 million in 2015 from $39 million in 2006.
Parents are often confused by the marketing for the formulas, according to a study led by Jennifer Harris, PhD, a marketing and public health researcher at the University of Connecticut, Hartford. She found that 60% of caregivers falsely believed toddler formulas have nutrients that toddlers can’t get from other foods.
Anthony Porto, MD, MPH, a pediatric gastroenterologist and pediatrics professor at Yale University, New Haven, Conn., said he is concerned these products could be giving toddlers more nutrients and calories than they need. Unlike what’s designed for infants, toddler formula has no nutritional regulations: Experts say standardizing a supplement to toddlers’ diets is impossible because no two children are alike.
In focus groups, Dr. Harris said, parents report feeding their children toddler formula to fill nutritional gaps when a child isn’t eating enough, a common concern among parents.
“Infants are often voracious eaters,” said Stephen Daniels, MD, chair of pediatrics at Children’s Hospital Colorado, Aurora. But at around a year of age, children’s growth plateaus, he said, and “they’re suddenly not hungry in the way they used to be anymore.” That can worry parents, he added, but “it’s a completely normal phenomenon.”
If parents have concerns about their children’s diet, Dr. Daniels said, they should consult a pediatrician or family doctor.
Blanche Lincoln, president of the Infant Nutrition Council of America, which represents the makers of Enfamil, Gerber, Similac, and store brands, said in an email that the toddler formulas can be helpful because they can fill “nutritional gaps during this period of transition to table foods.” Ms. Lincoln, a former U.S. senator from Arkansas, said the drinks “help contribute to the specific nutritional needs of toddlers by providing energy and important nutrients, as well as essential vitamins and minerals during this important period of growth and development.”
But toddler formula isn’t being ingested by toddlers alone – it’s also being fed to infants. In a recent study, Dr. Porto and colleagues found that 5% of infants’ parents reported giving their babies drinks marketed for the older age group. And Dr. Harris’ research indicated that 22% of parents of infants older than 6 months had fed their babies toddler formula in the previous month. Both studies were conducted before the recent infant formula shortage, which may have exacerbated the problem.
“Infant formulas and toddler formulas tend to be next to each other in the supermarket,” Dr. Harris said. “They look similar, but the toddler formulas are cheaper than the infant formulas. So people confuse them, and they grab the wrong one. Or they think: ‘Oh, this one is less expensive. I’ll get this one instead.’ ”
According to an email from Food and Drug Administration spokesperson Lindsay Haake, toddler drinks do not meet the definition of infant formula, so they are not subject to the same requirements. That means they do not have to undergo the clinical trials and pathogen safety testing that the infant versions do. “Unlike infant formulas, toddler formulas are not necessary to meet the nutritional needs of their intended consumers,” Ms. Haake said.
In a statement to KHN, the Infant Nutrition Council of America said: “Toddler drinks have a distinctive use and nutritional makeup from infant formula; the two are not interchangeable. The labeling of toddler nutritional drinks explicitly identifies the product as a toddler drink intended for children 12 months and older on the front of the package label.”
However, several expensive toddler formula brands made by smaller companies – often advertised as being made from goat milk, A2 whole milk (which lacks one common milk protein), or vegan ingredients that aren’t soy – do meet nutritional requirements for infants, and some advertise that.
Dr. Harris argued that this confuses parents, too, and shouldn’t be allowed. Just because a toddler formula has the nutritional ingredients required by the FDA for infant formula doesn’t mean it has met the other tests required of infant formula.
Federal regulators have not forced any of the companies to withdraw those products. In an email, FDA spokesperson Marianna Naum said: “The FDA does not comment on potential compliance actions.”
One company, Nature’s One, whose toddler formulas are named “Baby’s Only,” received warning letters a decade ago from the FDA about marketing them for infants. That case was closed in 2016. The company’s website says that Baby’s Only formula “meets nutrient requirements for infant” and that “Baby’s Only Organic® can be served up to 3 years of age.” Critics say that language implies the formula is fine for babies younger than 1. The company’s website and its Instagram account feature customer testimonials from parents who report feeding the formula to their infants, as well as pictures of infants drinking it.
Jay Highman, CEO and president of Nature’s One, said that Baby’s Only is clearly labeled as a toddler formula and that the back of the can states that “Baby’s Only is intended for a toddler 1 year of age or older OR when directed by a health care professional.” He also said that since the company launched in 1999, its formulas have met all the nutritional, manufacturing, and safety standards required of infant formula even though they don’t have to. “We behaved like we are an infant formula, but we were selling it as a toddler formula.”
He said that the clinical trials required by the FDA are a huge barrier to bringing a new infant formula to market and that many other countries don’t require a clinical trial. Baby’s Only recently completed a clinical trial, and the company expects to be able to sell it as an infant formula soon.
Yet pediatricians and nutritional experts continue to caution parents about using the toddler drinks. “There’s no question that infant formula is very important in the first year of life,” Dr. Daniels said. But he doesn’t recommend the toddler version “because it’s not that useful, because it’s confusing, because it’s expensive.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Formulas for toddlers are a burgeoning business in the United States: Sales of the drinks more than doubled in recent years as companies convinced parents that their little ones needed the liquid boost. But many experts warn that these products, designed for children ages 1-3, fill no nutritional needs beyond what is available in a typical toddler diet, are subject to less regulation than infant formula, and are expensive.
In addition, some parents feed the toddler versions to infants even though they do not meet federal standards for infant formula and may not provide babies with adequate nutrients to sustain their growth.
Pediatricians and federal health officials say that when most children turn 1, they can begin drinking cow milk or an unsweetened plant-based milk substitute. In a 2019 “consensus” statement, the American Academy of Pediatrics and other health and nutrition organizations recommended against using toddler formulas, saying “they offer no unique nutritional value beyond what could be obtained with healthy foods; furthermore, they may contribute added sugars to the diet.” The toddler formulas often contain sweeteners and fats that add calories.
Some of the same companies that produce infant formula – including Enfamil, Gerber, and Similac – also make toddler formulas, as do some smaller, boutique brands that advertise that they have organic or other special qualities. Toddler formulas are available nearly everywhere infant formulas are sold and are marketed as providing extra nutrients to help children’s brain, immune system, and eye development, among other benefits. They are different from medical formulas prescribed for children with specific needs.
A 2020 study found that sales of toddler formula in the United States rose to $92 million in 2015 from $39 million in 2006.
Parents are often confused by the marketing for the formulas, according to a study led by Jennifer Harris, PhD, a marketing and public health researcher at the University of Connecticut, Hartford. She found that 60% of caregivers falsely believed toddler formulas have nutrients that toddlers can’t get from other foods.
Anthony Porto, MD, MPH, a pediatric gastroenterologist and pediatrics professor at Yale University, New Haven, Conn., said he is concerned these products could be giving toddlers more nutrients and calories than they need. Unlike what’s designed for infants, toddler formula has no nutritional regulations: Experts say standardizing a supplement to toddlers’ diets is impossible because no two children are alike.
In focus groups, Dr. Harris said, parents report feeding their children toddler formula to fill nutritional gaps when a child isn’t eating enough, a common concern among parents.
“Infants are often voracious eaters,” said Stephen Daniels, MD, chair of pediatrics at Children’s Hospital Colorado, Aurora. But at around a year of age, children’s growth plateaus, he said, and “they’re suddenly not hungry in the way they used to be anymore.” That can worry parents, he added, but “it’s a completely normal phenomenon.”
If parents have concerns about their children’s diet, Dr. Daniels said, they should consult a pediatrician or family doctor.
Blanche Lincoln, president of the Infant Nutrition Council of America, which represents the makers of Enfamil, Gerber, Similac, and store brands, said in an email that the toddler formulas can be helpful because they can fill “nutritional gaps during this period of transition to table foods.” Ms. Lincoln, a former U.S. senator from Arkansas, said the drinks “help contribute to the specific nutritional needs of toddlers by providing energy and important nutrients, as well as essential vitamins and minerals during this important period of growth and development.”
But toddler formula isn’t being ingested by toddlers alone – it’s also being fed to infants. In a recent study, Dr. Porto and colleagues found that 5% of infants’ parents reported giving their babies drinks marketed for the older age group. And Dr. Harris’ research indicated that 22% of parents of infants older than 6 months had fed their babies toddler formula in the previous month. Both studies were conducted before the recent infant formula shortage, which may have exacerbated the problem.
“Infant formulas and toddler formulas tend to be next to each other in the supermarket,” Dr. Harris said. “They look similar, but the toddler formulas are cheaper than the infant formulas. So people confuse them, and they grab the wrong one. Or they think: ‘Oh, this one is less expensive. I’ll get this one instead.’ ”
According to an email from Food and Drug Administration spokesperson Lindsay Haake, toddler drinks do not meet the definition of infant formula, so they are not subject to the same requirements. That means they do not have to undergo the clinical trials and pathogen safety testing that the infant versions do. “Unlike infant formulas, toddler formulas are not necessary to meet the nutritional needs of their intended consumers,” Ms. Haake said.
In a statement to KHN, the Infant Nutrition Council of America said: “Toddler drinks have a distinctive use and nutritional makeup from infant formula; the two are not interchangeable. The labeling of toddler nutritional drinks explicitly identifies the product as a toddler drink intended for children 12 months and older on the front of the package label.”
However, several expensive toddler formula brands made by smaller companies – often advertised as being made from goat milk, A2 whole milk (which lacks one common milk protein), or vegan ingredients that aren’t soy – do meet nutritional requirements for infants, and some advertise that.
Dr. Harris argued that this confuses parents, too, and shouldn’t be allowed. Just because a toddler formula has the nutritional ingredients required by the FDA for infant formula doesn’t mean it has met the other tests required of infant formula.
Federal regulators have not forced any of the companies to withdraw those products. In an email, FDA spokesperson Marianna Naum said: “The FDA does not comment on potential compliance actions.”
One company, Nature’s One, whose toddler formulas are named “Baby’s Only,” received warning letters a decade ago from the FDA about marketing them for infants. That case was closed in 2016. The company’s website says that Baby’s Only formula “meets nutrient requirements for infant” and that “Baby’s Only Organic® can be served up to 3 years of age.” Critics say that language implies the formula is fine for babies younger than 1. The company’s website and its Instagram account feature customer testimonials from parents who report feeding the formula to their infants, as well as pictures of infants drinking it.
Jay Highman, CEO and president of Nature’s One, said that Baby’s Only is clearly labeled as a toddler formula and that the back of the can states that “Baby’s Only is intended for a toddler 1 year of age or older OR when directed by a health care professional.” He also said that since the company launched in 1999, its formulas have met all the nutritional, manufacturing, and safety standards required of infant formula even though they don’t have to. “We behaved like we are an infant formula, but we were selling it as a toddler formula.”
He said that the clinical trials required by the FDA are a huge barrier to bringing a new infant formula to market and that many other countries don’t require a clinical trial. Baby’s Only recently completed a clinical trial, and the company expects to be able to sell it as an infant formula soon.
Yet pediatricians and nutritional experts continue to caution parents about using the toddler drinks. “There’s no question that infant formula is very important in the first year of life,” Dr. Daniels said. But he doesn’t recommend the toddler version “because it’s not that useful, because it’s confusing, because it’s expensive.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Could exercise improve bone health in youth with type 1 diabetes?
In a small cross-sectional study of 10- to 16-year-old girls with and without type 1 diabetes, both groups were equally physically active, based on their replies to the bone-specific physical activity questionnaire (BPAQ).
However, among the more sedentary girls (with BPAQ scores below the median), those with type 1 diabetes had worse markers of bone health in imaging tests compared with the girls without diabetes.
the researchers summarize in a poster presented at the annual meeting of the American Society of Bone and Mineral Research.
However, this is early research and further study is needed, the group cautions.
“Ongoing studies with objective measures of physical activity as well as interventional studies will clarify whether increasing physical activity may improve bone health and reduce fracture risk in this vulnerable group,” they conclude.
“If you look at the sedentary kids, there’s a big discrepancy between the kids who have diabetes and the control kids, and that’s if we’re looking at radius or tibia or trabecular bone density or estimated failure load,” senior author Deborah M. Mitchell, MD, said in an interview at the poster session.
However, “when we look at the kids who are more physically active, we’re really not seeing as much difference [in bone health] between the kids with and without diabetes,” said Dr. Mitchell, a pediatric endocrinologist at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
But she also acknowledged, “There’s all sorts of caveats, including that this is retrospective questionnaire data.”
However, if further, rigorous studies confirm these findings, “physical activity is potentially a really effective means of improving bone quality in kids with type 1 diabetes.”
“This study suggests that bone-loading physical activity can substantially improve skeletal health in children with [type 1 diabetes] and should provide hope for patients and their families that they can take some action to prevent or mitigate the effects of diabetes on bone,” coauthor and incoming ASBMR President Mary L. Bouxsein, PhD, told this news organization in an email.
“We interpret these data as an important reason to advocate for increased time in moderate to vigorous bone-loading activity,” said Dr. Bouxsein, professor, department of orthopedic surgery, Harvard Medical School, Boston, “though the ‘dose’ in terms of hours per day or episodes per week to promote optimal bone health is still to be determined.”
“Ongoing debate,” “need stronger proof”
Asked for comment, Laura K. Bachrach, MD, who was not involved with the research, noted: “Activity benefits the development of bone strength through effects on bone geometry more than ‘density,’ and conversely, lack of physical activity can compromise gains in cortical bone diameter and thickness.”
However, “there is ongoing debate about the impact of type 1 diabetes on bone health and the factor(s) determining risk,” Dr. Bachrach, a pediatric endocrinologist at Stanford Children’s Health, Palo Alto, Calif., told this news organization in an email.
The current findings suggest “that physical activity in adolescent girls provided protection against potential adverse effects of type 1 diabetes,” said Dr. Bachrach, who spoke about bone fragility in childhood in a video commentary in 2021.
Study strengths, she noted, “include the rigor and expertise of the investigators, use of multiple surrogate measures that capture bone geometry/microarchitecture, as well as the inclusion of healthy local controls.”
“The study is limited by the cross-sectional design and subjects who opted, or not, to be active,” she added. “Stronger proof of the protective effects of activity on bone health in type 1 diabetes would require a randomized longitudinal intervention study, as alluded to by the authors of the study.”
Hypothesis: Those with type 1 diabetes acquire less bone mass in early 20s
The excess fracture risk in children with type 1 diabetes has been previously reported and is 14%-35% higher than the fracture risk in children without diabetes, Dr. Bouxsein explained. And “between 30% to 50% of kids [with type 1 diabetes] will have a fracture before the age of 18, so the excess fracture risk in diabetes is not clinically obvious,” she added.
However, “several lines of evidence strongly suggest that bone mass and microarchitecture at the time of peak bone mass (early 20s) is a major determinant of fracture risk throughout the lifespan,” she noted.
“Our hypothesis,” Dr. Bouxsein said, “is that the metabolic disruptions of diabetes, when they are present during the acquisition of peak bone mass, interfere with optimal bone development, and therefore may contribute to increased fracture risk later in life.”
Dr. Bachrach agreed that “peak bone strength is achieved by early adulthood, making childhood and adolescence important times to optimize bone health,” and that “peak bone strength is a predictor of lifetime risk of osteoporosis.”
“The diagnosis of pediatric osteoporosis is made when a child or teen sustains a vertebral fracture or femur fracture with minimal or no trauma,” she explained. “The diagnosis can also be made in a pediatric patient with low BMD [bone mineral density] for age in combination with a history of several long-bone fractures.”
Dr. Mitchell noted that type 1 diabetes is associated with a higher risk of fractures, which is sixfold in adults. In another study, she said, the group showed that in 10- to 16-year-old girls who’ve only had diabetes for a few years, “trabecular bone density is lower, they have lower estimated failure load, and longitudinally when we follow them, at least at the radius, we’re seeing bone loss at a relatively young age when we wouldn’t be expecting to see bone loss.”
80 girls enrolled, half had type 1 diabetes
Researchers enrolled 36 girls with type 1 diabetes and 44 girls without type 1 diabetes (controls) who were a mean age of 14.7 years and most (92%) were White. The girls with and without diabetes had similar rates of previous fractures (44% and 51%).
Those with diabetes had been diagnosed at a mean age of 9 years and had had diabetes for a mean of 4.6 years.
Researchers calculated participants’ total BPAQ scores based on type, duration, and frequency of bone-loading activities.
Participants had dual-energy X-ray absorptiometry scans to determine areal bone mineral density (BMD) at the total hip, femoral neck, lumbar spine, and whole body less head.
They also had high-resolution peripheral quantitative computed tomography at the distal tibia and radius to determine volumetric BMD, bone microarchitecture, and estimated bone strength (calculated using microfinite element analysis).
The two groups had similar total BPAQ scores (57.3 and 64.6), with a median score of 49.
BPAQ scores were positively associated with areal BMD at all sites (whole body, lumbar spine, total hip, femoral neck, and 1/3 radius) and with trabecular BMD and estimated failure load at the distal radius and tibia (P < .05 for all, adjusted for bone age).
Among participants with low physical activity (BPAQ below the median), compared with controls, those with type 1 diabetes had 6.6% lower aerial BMD at the lumbar spine (0.868 vs. 0.929 g/cm3; P = .04), 8% lower trabecular volumetric BMD at the distal radius (128.5 vs. 156.8 mg/cm3; P = .01), and 12% lower estimated failure load. Results at the distal tibia were similar.
Next steps
“More observational studies in males and females across a broader age spectrum would be helpful,” Dr. Bachrach noted. “The ‘gold standard’ model would be a long-term randomized controlled activity intervention study.”
“Further studies are underway [in girls and boys] using objective measures of activity including accelerometry and longitudinal observation to help confirm the findings from the current study,” Dr. Bouxsein said. “Ultimately, trials of activity interventions in children with [type 1 diabetes] will be the gold standard to determine to what extent physical activity can mitigate bone disease in [type 1 diabetes],” she agreed.
The study authors and Dr. Bachrach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a small cross-sectional study of 10- to 16-year-old girls with and without type 1 diabetes, both groups were equally physically active, based on their replies to the bone-specific physical activity questionnaire (BPAQ).
However, among the more sedentary girls (with BPAQ scores below the median), those with type 1 diabetes had worse markers of bone health in imaging tests compared with the girls without diabetes.
the researchers summarize in a poster presented at the annual meeting of the American Society of Bone and Mineral Research.
However, this is early research and further study is needed, the group cautions.
“Ongoing studies with objective measures of physical activity as well as interventional studies will clarify whether increasing physical activity may improve bone health and reduce fracture risk in this vulnerable group,” they conclude.
“If you look at the sedentary kids, there’s a big discrepancy between the kids who have diabetes and the control kids, and that’s if we’re looking at radius or tibia or trabecular bone density or estimated failure load,” senior author Deborah M. Mitchell, MD, said in an interview at the poster session.
However, “when we look at the kids who are more physically active, we’re really not seeing as much difference [in bone health] between the kids with and without diabetes,” said Dr. Mitchell, a pediatric endocrinologist at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
But she also acknowledged, “There’s all sorts of caveats, including that this is retrospective questionnaire data.”
However, if further, rigorous studies confirm these findings, “physical activity is potentially a really effective means of improving bone quality in kids with type 1 diabetes.”
“This study suggests that bone-loading physical activity can substantially improve skeletal health in children with [type 1 diabetes] and should provide hope for patients and their families that they can take some action to prevent or mitigate the effects of diabetes on bone,” coauthor and incoming ASBMR President Mary L. Bouxsein, PhD, told this news organization in an email.
“We interpret these data as an important reason to advocate for increased time in moderate to vigorous bone-loading activity,” said Dr. Bouxsein, professor, department of orthopedic surgery, Harvard Medical School, Boston, “though the ‘dose’ in terms of hours per day or episodes per week to promote optimal bone health is still to be determined.”
“Ongoing debate,” “need stronger proof”
Asked for comment, Laura K. Bachrach, MD, who was not involved with the research, noted: “Activity benefits the development of bone strength through effects on bone geometry more than ‘density,’ and conversely, lack of physical activity can compromise gains in cortical bone diameter and thickness.”
However, “there is ongoing debate about the impact of type 1 diabetes on bone health and the factor(s) determining risk,” Dr. Bachrach, a pediatric endocrinologist at Stanford Children’s Health, Palo Alto, Calif., told this news organization in an email.
The current findings suggest “that physical activity in adolescent girls provided protection against potential adverse effects of type 1 diabetes,” said Dr. Bachrach, who spoke about bone fragility in childhood in a video commentary in 2021.
Study strengths, she noted, “include the rigor and expertise of the investigators, use of multiple surrogate measures that capture bone geometry/microarchitecture, as well as the inclusion of healthy local controls.”
“The study is limited by the cross-sectional design and subjects who opted, or not, to be active,” she added. “Stronger proof of the protective effects of activity on bone health in type 1 diabetes would require a randomized longitudinal intervention study, as alluded to by the authors of the study.”
Hypothesis: Those with type 1 diabetes acquire less bone mass in early 20s
The excess fracture risk in children with type 1 diabetes has been previously reported and is 14%-35% higher than the fracture risk in children without diabetes, Dr. Bouxsein explained. And “between 30% to 50% of kids [with type 1 diabetes] will have a fracture before the age of 18, so the excess fracture risk in diabetes is not clinically obvious,” she added.
However, “several lines of evidence strongly suggest that bone mass and microarchitecture at the time of peak bone mass (early 20s) is a major determinant of fracture risk throughout the lifespan,” she noted.
“Our hypothesis,” Dr. Bouxsein said, “is that the metabolic disruptions of diabetes, when they are present during the acquisition of peak bone mass, interfere with optimal bone development, and therefore may contribute to increased fracture risk later in life.”
Dr. Bachrach agreed that “peak bone strength is achieved by early adulthood, making childhood and adolescence important times to optimize bone health,” and that “peak bone strength is a predictor of lifetime risk of osteoporosis.”
“The diagnosis of pediatric osteoporosis is made when a child or teen sustains a vertebral fracture or femur fracture with minimal or no trauma,” she explained. “The diagnosis can also be made in a pediatric patient with low BMD [bone mineral density] for age in combination with a history of several long-bone fractures.”
Dr. Mitchell noted that type 1 diabetes is associated with a higher risk of fractures, which is sixfold in adults. In another study, she said, the group showed that in 10- to 16-year-old girls who’ve only had diabetes for a few years, “trabecular bone density is lower, they have lower estimated failure load, and longitudinally when we follow them, at least at the radius, we’re seeing bone loss at a relatively young age when we wouldn’t be expecting to see bone loss.”
80 girls enrolled, half had type 1 diabetes
Researchers enrolled 36 girls with type 1 diabetes and 44 girls without type 1 diabetes (controls) who were a mean age of 14.7 years and most (92%) were White. The girls with and without diabetes had similar rates of previous fractures (44% and 51%).
Those with diabetes had been diagnosed at a mean age of 9 years and had had diabetes for a mean of 4.6 years.
Researchers calculated participants’ total BPAQ scores based on type, duration, and frequency of bone-loading activities.
Participants had dual-energy X-ray absorptiometry scans to determine areal bone mineral density (BMD) at the total hip, femoral neck, lumbar spine, and whole body less head.
They also had high-resolution peripheral quantitative computed tomography at the distal tibia and radius to determine volumetric BMD, bone microarchitecture, and estimated bone strength (calculated using microfinite element analysis).
The two groups had similar total BPAQ scores (57.3 and 64.6), with a median score of 49.
BPAQ scores were positively associated with areal BMD at all sites (whole body, lumbar spine, total hip, femoral neck, and 1/3 radius) and with trabecular BMD and estimated failure load at the distal radius and tibia (P < .05 for all, adjusted for bone age).
Among participants with low physical activity (BPAQ below the median), compared with controls, those with type 1 diabetes had 6.6% lower aerial BMD at the lumbar spine (0.868 vs. 0.929 g/cm3; P = .04), 8% lower trabecular volumetric BMD at the distal radius (128.5 vs. 156.8 mg/cm3; P = .01), and 12% lower estimated failure load. Results at the distal tibia were similar.
Next steps
“More observational studies in males and females across a broader age spectrum would be helpful,” Dr. Bachrach noted. “The ‘gold standard’ model would be a long-term randomized controlled activity intervention study.”
“Further studies are underway [in girls and boys] using objective measures of activity including accelerometry and longitudinal observation to help confirm the findings from the current study,” Dr. Bouxsein said. “Ultimately, trials of activity interventions in children with [type 1 diabetes] will be the gold standard to determine to what extent physical activity can mitigate bone disease in [type 1 diabetes],” she agreed.
The study authors and Dr. Bachrach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a small cross-sectional study of 10- to 16-year-old girls with and without type 1 diabetes, both groups were equally physically active, based on their replies to the bone-specific physical activity questionnaire (BPAQ).
However, among the more sedentary girls (with BPAQ scores below the median), those with type 1 diabetes had worse markers of bone health in imaging tests compared with the girls without diabetes.
the researchers summarize in a poster presented at the annual meeting of the American Society of Bone and Mineral Research.
However, this is early research and further study is needed, the group cautions.
“Ongoing studies with objective measures of physical activity as well as interventional studies will clarify whether increasing physical activity may improve bone health and reduce fracture risk in this vulnerable group,” they conclude.
“If you look at the sedentary kids, there’s a big discrepancy between the kids who have diabetes and the control kids, and that’s if we’re looking at radius or tibia or trabecular bone density or estimated failure load,” senior author Deborah M. Mitchell, MD, said in an interview at the poster session.
However, “when we look at the kids who are more physically active, we’re really not seeing as much difference [in bone health] between the kids with and without diabetes,” said Dr. Mitchell, a pediatric endocrinologist at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
But she also acknowledged, “There’s all sorts of caveats, including that this is retrospective questionnaire data.”
However, if further, rigorous studies confirm these findings, “physical activity is potentially a really effective means of improving bone quality in kids with type 1 diabetes.”
“This study suggests that bone-loading physical activity can substantially improve skeletal health in children with [type 1 diabetes] and should provide hope for patients and their families that they can take some action to prevent or mitigate the effects of diabetes on bone,” coauthor and incoming ASBMR President Mary L. Bouxsein, PhD, told this news organization in an email.
“We interpret these data as an important reason to advocate for increased time in moderate to vigorous bone-loading activity,” said Dr. Bouxsein, professor, department of orthopedic surgery, Harvard Medical School, Boston, “though the ‘dose’ in terms of hours per day or episodes per week to promote optimal bone health is still to be determined.”
“Ongoing debate,” “need stronger proof”
Asked for comment, Laura K. Bachrach, MD, who was not involved with the research, noted: “Activity benefits the development of bone strength through effects on bone geometry more than ‘density,’ and conversely, lack of physical activity can compromise gains in cortical bone diameter and thickness.”
However, “there is ongoing debate about the impact of type 1 diabetes on bone health and the factor(s) determining risk,” Dr. Bachrach, a pediatric endocrinologist at Stanford Children’s Health, Palo Alto, Calif., told this news organization in an email.
The current findings suggest “that physical activity in adolescent girls provided protection against potential adverse effects of type 1 diabetes,” said Dr. Bachrach, who spoke about bone fragility in childhood in a video commentary in 2021.
Study strengths, she noted, “include the rigor and expertise of the investigators, use of multiple surrogate measures that capture bone geometry/microarchitecture, as well as the inclusion of healthy local controls.”
“The study is limited by the cross-sectional design and subjects who opted, or not, to be active,” she added. “Stronger proof of the protective effects of activity on bone health in type 1 diabetes would require a randomized longitudinal intervention study, as alluded to by the authors of the study.”
Hypothesis: Those with type 1 diabetes acquire less bone mass in early 20s
The excess fracture risk in children with type 1 diabetes has been previously reported and is 14%-35% higher than the fracture risk in children without diabetes, Dr. Bouxsein explained. And “between 30% to 50% of kids [with type 1 diabetes] will have a fracture before the age of 18, so the excess fracture risk in diabetes is not clinically obvious,” she added.
However, “several lines of evidence strongly suggest that bone mass and microarchitecture at the time of peak bone mass (early 20s) is a major determinant of fracture risk throughout the lifespan,” she noted.
“Our hypothesis,” Dr. Bouxsein said, “is that the metabolic disruptions of diabetes, when they are present during the acquisition of peak bone mass, interfere with optimal bone development, and therefore may contribute to increased fracture risk later in life.”
Dr. Bachrach agreed that “peak bone strength is achieved by early adulthood, making childhood and adolescence important times to optimize bone health,” and that “peak bone strength is a predictor of lifetime risk of osteoporosis.”
“The diagnosis of pediatric osteoporosis is made when a child or teen sustains a vertebral fracture or femur fracture with minimal or no trauma,” she explained. “The diagnosis can also be made in a pediatric patient with low BMD [bone mineral density] for age in combination with a history of several long-bone fractures.”
Dr. Mitchell noted that type 1 diabetes is associated with a higher risk of fractures, which is sixfold in adults. In another study, she said, the group showed that in 10- to 16-year-old girls who’ve only had diabetes for a few years, “trabecular bone density is lower, they have lower estimated failure load, and longitudinally when we follow them, at least at the radius, we’re seeing bone loss at a relatively young age when we wouldn’t be expecting to see bone loss.”
80 girls enrolled, half had type 1 diabetes
Researchers enrolled 36 girls with type 1 diabetes and 44 girls without type 1 diabetes (controls) who were a mean age of 14.7 years and most (92%) were White. The girls with and without diabetes had similar rates of previous fractures (44% and 51%).
Those with diabetes had been diagnosed at a mean age of 9 years and had had diabetes for a mean of 4.6 years.
Researchers calculated participants’ total BPAQ scores based on type, duration, and frequency of bone-loading activities.
Participants had dual-energy X-ray absorptiometry scans to determine areal bone mineral density (BMD) at the total hip, femoral neck, lumbar spine, and whole body less head.
They also had high-resolution peripheral quantitative computed tomography at the distal tibia and radius to determine volumetric BMD, bone microarchitecture, and estimated bone strength (calculated using microfinite element analysis).
The two groups had similar total BPAQ scores (57.3 and 64.6), with a median score of 49.
BPAQ scores were positively associated with areal BMD at all sites (whole body, lumbar spine, total hip, femoral neck, and 1/3 radius) and with trabecular BMD and estimated failure load at the distal radius and tibia (P < .05 for all, adjusted for bone age).
Among participants with low physical activity (BPAQ below the median), compared with controls, those with type 1 diabetes had 6.6% lower aerial BMD at the lumbar spine (0.868 vs. 0.929 g/cm3; P = .04), 8% lower trabecular volumetric BMD at the distal radius (128.5 vs. 156.8 mg/cm3; P = .01), and 12% lower estimated failure load. Results at the distal tibia were similar.
Next steps
“More observational studies in males and females across a broader age spectrum would be helpful,” Dr. Bachrach noted. “The ‘gold standard’ model would be a long-term randomized controlled activity intervention study.”
“Further studies are underway [in girls and boys] using objective measures of activity including accelerometry and longitudinal observation to help confirm the findings from the current study,” Dr. Bouxsein said. “Ultimately, trials of activity interventions in children with [type 1 diabetes] will be the gold standard to determine to what extent physical activity can mitigate bone disease in [type 1 diabetes],” she agreed.
The study authors and Dr. Bachrach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2022
Children born from frozen embryos may have increased cancer risk
, a large registry study suggests.
The results, however, “should be interpreted cautiously,” the authors noted, given the low number of cancer cases reported among children born using FET.
Still, the findings do “raise concerns considering the increasing use of FET, in particular freeze-all strategies without clear medical indications,” the authors concluded.
The study was published online in PLOS Medicine.
The number of children born after FET has increased globally and even exceeds the number of those born after fresh embryo transfer in many countries. In the United States, for instance, the FET rate has doubled since 2015; FETs constituted almost 80% of all embryo transfers using assisted reproductive technology (ART) without a donor in 2019.
Despite the benefits associated with FET, which include improved embryo survival and higher live birth rates, some previous research has hinted at a higher risk of childhood cancer in this population.
In the current study, researchers from the University of Gothenburg, Sweden, wanted to better understand the risk of childhood cancer following FET. The investigators analyzed data from 171,774 children born via ART, including 22,630 born after FET, as well as roughly 7.7 million children born after spontaneous conception in Denmark, Finland, Norway, and Sweden.
After a mean follow-up of about 10 years, the incidence rate of cancer diagnosed before age 18 years was 16.7 per 100,000 person-years for children born after spontaneous conception (16,184 cases) and 19.3 per 100,000 person-years for children born after ART (329 cases).
The researchers found no increased risk of cancer before age 18 years in the group of children conceived via ART compared with those conceived spontaneously.
However, children born after FET had a significantly higher risk of cancer compared with children born after fresh embryo transfer (adjusted hazard ratio [aHR], 1.59) and spontaneous conception (aHR, 1.65). Specifically with regard to ART, the incidence rate for those born after FET was 30.1 per 100,000 person-years – 48 total cases – compared with 18.8 per 1000,000 person-years after fresh embryo transfer.
Adjustment for macrosomia, birth weight, or major birth defects influenced the association only marginally.
For specific cancer types, children born after FET had more than a twofold higher risk for leukemia in comparison with those born after fresh embryo transfer (aHR, 2.25) and spontaneous conception (aHR, 2.22).
Still, the authors said these results should be interpreted “cautiously,” given the small number of children diagnosed with cancer after FET. The researchers also acknowledged that they do not know why children born after FET would face a higher risk of cancer.
These findings, however, do align with those from a 2019 Dutch population-based study. In the Dutch study, which included more than 24,000 ART-conceived children and more than 23,000 naturally conceived children, the risk of cancer after ART was not higher overall, but it was greater when only those conceived after FET were considered (aHR 1.80); this increased risk, however, was not statistically significant.
“Since the use of FET is substantially increasing, it is important to tease out whether the increased cancer risk is a true risk increase due to the ART procedures using FET, or due to chance or confounding by other factors,” authors of the 2019 Dutch study, Mandy Spaan, PhD, and Flora E. van Leeuwen, PhD, said in an interview.
“But, as childhood cancer is (fortunately) a rare disease, it is very difficult to study this research question among ART children due to limited numbers,” said Dr. Spaan and Dr. van Leeuwen, who are with the Netherlands Cancer Institute.
Given this, the two experts call for additional large population-based cohort studies to investigate the risk of cancer after ART, especially FET, and for a subsequent analysis that pools these data. They hope this strategy “will lead to reliable estimates” and provide information on the risks of FET in comparison with approaches that involve fresh embryos.
The current study had no commercial funding. The study authors as well as Dr. Spaan and Dr. van Leeuwen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large registry study suggests.
The results, however, “should be interpreted cautiously,” the authors noted, given the low number of cancer cases reported among children born using FET.
Still, the findings do “raise concerns considering the increasing use of FET, in particular freeze-all strategies without clear medical indications,” the authors concluded.
The study was published online in PLOS Medicine.
The number of children born after FET has increased globally and even exceeds the number of those born after fresh embryo transfer in many countries. In the United States, for instance, the FET rate has doubled since 2015; FETs constituted almost 80% of all embryo transfers using assisted reproductive technology (ART) without a donor in 2019.
Despite the benefits associated with FET, which include improved embryo survival and higher live birth rates, some previous research has hinted at a higher risk of childhood cancer in this population.
In the current study, researchers from the University of Gothenburg, Sweden, wanted to better understand the risk of childhood cancer following FET. The investigators analyzed data from 171,774 children born via ART, including 22,630 born after FET, as well as roughly 7.7 million children born after spontaneous conception in Denmark, Finland, Norway, and Sweden.
After a mean follow-up of about 10 years, the incidence rate of cancer diagnosed before age 18 years was 16.7 per 100,000 person-years for children born after spontaneous conception (16,184 cases) and 19.3 per 100,000 person-years for children born after ART (329 cases).
The researchers found no increased risk of cancer before age 18 years in the group of children conceived via ART compared with those conceived spontaneously.
However, children born after FET had a significantly higher risk of cancer compared with children born after fresh embryo transfer (adjusted hazard ratio [aHR], 1.59) and spontaneous conception (aHR, 1.65). Specifically with regard to ART, the incidence rate for those born after FET was 30.1 per 100,000 person-years – 48 total cases – compared with 18.8 per 1000,000 person-years after fresh embryo transfer.
Adjustment for macrosomia, birth weight, or major birth defects influenced the association only marginally.
For specific cancer types, children born after FET had more than a twofold higher risk for leukemia in comparison with those born after fresh embryo transfer (aHR, 2.25) and spontaneous conception (aHR, 2.22).
Still, the authors said these results should be interpreted “cautiously,” given the small number of children diagnosed with cancer after FET. The researchers also acknowledged that they do not know why children born after FET would face a higher risk of cancer.
These findings, however, do align with those from a 2019 Dutch population-based study. In the Dutch study, which included more than 24,000 ART-conceived children and more than 23,000 naturally conceived children, the risk of cancer after ART was not higher overall, but it was greater when only those conceived after FET were considered (aHR 1.80); this increased risk, however, was not statistically significant.
“Since the use of FET is substantially increasing, it is important to tease out whether the increased cancer risk is a true risk increase due to the ART procedures using FET, or due to chance or confounding by other factors,” authors of the 2019 Dutch study, Mandy Spaan, PhD, and Flora E. van Leeuwen, PhD, said in an interview.
“But, as childhood cancer is (fortunately) a rare disease, it is very difficult to study this research question among ART children due to limited numbers,” said Dr. Spaan and Dr. van Leeuwen, who are with the Netherlands Cancer Institute.
Given this, the two experts call for additional large population-based cohort studies to investigate the risk of cancer after ART, especially FET, and for a subsequent analysis that pools these data. They hope this strategy “will lead to reliable estimates” and provide information on the risks of FET in comparison with approaches that involve fresh embryos.
The current study had no commercial funding. The study authors as well as Dr. Spaan and Dr. van Leeuwen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large registry study suggests.
The results, however, “should be interpreted cautiously,” the authors noted, given the low number of cancer cases reported among children born using FET.
Still, the findings do “raise concerns considering the increasing use of FET, in particular freeze-all strategies without clear medical indications,” the authors concluded.
The study was published online in PLOS Medicine.
The number of children born after FET has increased globally and even exceeds the number of those born after fresh embryo transfer in many countries. In the United States, for instance, the FET rate has doubled since 2015; FETs constituted almost 80% of all embryo transfers using assisted reproductive technology (ART) without a donor in 2019.
Despite the benefits associated with FET, which include improved embryo survival and higher live birth rates, some previous research has hinted at a higher risk of childhood cancer in this population.
In the current study, researchers from the University of Gothenburg, Sweden, wanted to better understand the risk of childhood cancer following FET. The investigators analyzed data from 171,774 children born via ART, including 22,630 born after FET, as well as roughly 7.7 million children born after spontaneous conception in Denmark, Finland, Norway, and Sweden.
After a mean follow-up of about 10 years, the incidence rate of cancer diagnosed before age 18 years was 16.7 per 100,000 person-years for children born after spontaneous conception (16,184 cases) and 19.3 per 100,000 person-years for children born after ART (329 cases).
The researchers found no increased risk of cancer before age 18 years in the group of children conceived via ART compared with those conceived spontaneously.
However, children born after FET had a significantly higher risk of cancer compared with children born after fresh embryo transfer (adjusted hazard ratio [aHR], 1.59) and spontaneous conception (aHR, 1.65). Specifically with regard to ART, the incidence rate for those born after FET was 30.1 per 100,000 person-years – 48 total cases – compared with 18.8 per 1000,000 person-years after fresh embryo transfer.
Adjustment for macrosomia, birth weight, or major birth defects influenced the association only marginally.
For specific cancer types, children born after FET had more than a twofold higher risk for leukemia in comparison with those born after fresh embryo transfer (aHR, 2.25) and spontaneous conception (aHR, 2.22).
Still, the authors said these results should be interpreted “cautiously,” given the small number of children diagnosed with cancer after FET. The researchers also acknowledged that they do not know why children born after FET would face a higher risk of cancer.
These findings, however, do align with those from a 2019 Dutch population-based study. In the Dutch study, which included more than 24,000 ART-conceived children and more than 23,000 naturally conceived children, the risk of cancer after ART was not higher overall, but it was greater when only those conceived after FET were considered (aHR 1.80); this increased risk, however, was not statistically significant.
“Since the use of FET is substantially increasing, it is important to tease out whether the increased cancer risk is a true risk increase due to the ART procedures using FET, or due to chance or confounding by other factors,” authors of the 2019 Dutch study, Mandy Spaan, PhD, and Flora E. van Leeuwen, PhD, said in an interview.
“But, as childhood cancer is (fortunately) a rare disease, it is very difficult to study this research question among ART children due to limited numbers,” said Dr. Spaan and Dr. van Leeuwen, who are with the Netherlands Cancer Institute.
Given this, the two experts call for additional large population-based cohort studies to investigate the risk of cancer after ART, especially FET, and for a subsequent analysis that pools these data. They hope this strategy “will lead to reliable estimates” and provide information on the risks of FET in comparison with approaches that involve fresh embryos.
The current study had no commercial funding. The study authors as well as Dr. Spaan and Dr. van Leeuwen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS MEDICINE
Children with sickle cell anemia not getting treatments, screening
Fewer than half of children aged 2-16 years with sickle cell anemia are receiving recommended annual screening for stroke, a common complication of the disease, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
Many of these children also are not receiving the recommended medication, hydroxyurea, which can reduce pain and acute chest syndrome and improve anemia and quality of life, according to the report released Sept. 20.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD), which is a red blood cell disorder that primarily affects Black and African American people in the United States. It is associated with severe complications such as stroke, vison damage, frequent infections, and delayed growth, and a reduction in lifespan of more than 20 years.
SCD affects approximately 100,000 Americans and SCA accounts for about 75% of those cases.
Physician remembers her patients’ pain
In a briefing to reporters in advance of the report’s release, Debra Houry, MD, MPH, the CDC’s acting principal deputy director, recalled “long, tough nights with these young sickle cell warriors” in her career as an emergency department physician.
“[S]eeing children and teens suffering from the severe pain that often accompanies sickle cell anemia was heartbreaking,” she said.
She asked health care providers to confront racism as they build better systems for ensuring optimal treatment for children and adolescents with SCA.
“Health care providers can educate themselves, their colleagues, and their institutions about the specialized needs of people with sickle cell anemia, including how racism inhibits optimal care,” Dr. Houry said.
She said people with SCA report difficulty accessing care and when they do, they often report feeling stigmatized.
Lead author of the report, Laura Schieve, PhD, an epidemiologist with CDC’s National Center on Birth Defects and Developmental Disabilities, and colleagues looked at data from more than 3,300 children with SCA who were continuously enrolled in Medicaid during 2019. The data came from the IBM MarketScan Multi-State Medicaid Database.
Key recommendations issued in 2014
In 2014, the National Heart, Lung, and Blood Institute (NHLBI) issued two key recommendations to prevent or reduce complications in children and adolescents with SCA.
One was annual screening of children and adolescents aged 2-16 years with transcranial Doppler (TCD) ultrasound to identify those at risk for stroke. The second was offering hydroxyurea therapy, which keeps red blood cells from sickling and blocking small blood vessels, to children and adolescents who were at least 9 months old to reduce pain and the risk for several life-threatening complications.
The researchers, however, found that in 2019, only 47% and 38% of children and adolescents aged 2-9 and 10-16 years, respectively, had TCD screening and 38% and 53% of children and adolescents aged 2-9 years and 10-16 years, respectively, used hydroxyurea.
“These complications are preventable – not inevitable. We must do more to help lessen the pain and complications associated with this disease by increasing the number of children who are screened for stroke and using the medication that can help reduce painful episodes,” said Karen Remley, MD, MPH, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a press release.
Bridging the gap
Providers, parents, health systems, and governmental agencies all have roles in bringing evidence-based recommended care to young SCA patients, Dr. Houry noted.
Community organizations can also help connect families with resources and tools to increase understanding.
Dr. Schieve pointed to access barriers in that families may have trouble traveling to specialized centers where the TCD screening is given. In addition, appointments for the screening may be limited.
Children taking hydroxyurea must be monitored for the proper dosage of medication, she explained, and that can be logistically challenging as well.
Providers report they often don’t get timely information back from TCD screening programs to keep up with which children need their annual screening.
Overall, the nation lacks providers with expertise in SCD and that can lead to symptoms being dismissed, Dr. Schieve said.
Hematologists and others have a role in advocating for patients with governmental entities to raise awareness of this issue, she added.
It’s also important that electronic health records give prompts and provide information so that all providers who care for a child can track screening and medication for the condition, Dr. Schieve and Dr. Houry said.
New funding for sickle cell data collection
Recent funding to the CDC Sickle Cell Data Collection Program may help more people get appropriate care, Dr. Houry said.
The program is currently active in 11 states and collects data from people all over the United States with SCD to study trends and treatment access for those with the disease.
The data help drive decisions such as where new sickle cell clinics are needed.
“We will expand to more states serving more people affected by this disease,” Dr. Houry said.
The authors declared no relevant financial relationships.
Fewer than half of children aged 2-16 years with sickle cell anemia are receiving recommended annual screening for stroke, a common complication of the disease, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
Many of these children also are not receiving the recommended medication, hydroxyurea, which can reduce pain and acute chest syndrome and improve anemia and quality of life, according to the report released Sept. 20.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD), which is a red blood cell disorder that primarily affects Black and African American people in the United States. It is associated with severe complications such as stroke, vison damage, frequent infections, and delayed growth, and a reduction in lifespan of more than 20 years.
SCD affects approximately 100,000 Americans and SCA accounts for about 75% of those cases.
Physician remembers her patients’ pain
In a briefing to reporters in advance of the report’s release, Debra Houry, MD, MPH, the CDC’s acting principal deputy director, recalled “long, tough nights with these young sickle cell warriors” in her career as an emergency department physician.
“[S]eeing children and teens suffering from the severe pain that often accompanies sickle cell anemia was heartbreaking,” she said.
She asked health care providers to confront racism as they build better systems for ensuring optimal treatment for children and adolescents with SCA.
“Health care providers can educate themselves, their colleagues, and their institutions about the specialized needs of people with sickle cell anemia, including how racism inhibits optimal care,” Dr. Houry said.
She said people with SCA report difficulty accessing care and when they do, they often report feeling stigmatized.
Lead author of the report, Laura Schieve, PhD, an epidemiologist with CDC’s National Center on Birth Defects and Developmental Disabilities, and colleagues looked at data from more than 3,300 children with SCA who were continuously enrolled in Medicaid during 2019. The data came from the IBM MarketScan Multi-State Medicaid Database.
Key recommendations issued in 2014
In 2014, the National Heart, Lung, and Blood Institute (NHLBI) issued two key recommendations to prevent or reduce complications in children and adolescents with SCA.
One was annual screening of children and adolescents aged 2-16 years with transcranial Doppler (TCD) ultrasound to identify those at risk for stroke. The second was offering hydroxyurea therapy, which keeps red blood cells from sickling and blocking small blood vessels, to children and adolescents who were at least 9 months old to reduce pain and the risk for several life-threatening complications.
The researchers, however, found that in 2019, only 47% and 38% of children and adolescents aged 2-9 and 10-16 years, respectively, had TCD screening and 38% and 53% of children and adolescents aged 2-9 years and 10-16 years, respectively, used hydroxyurea.
“These complications are preventable – not inevitable. We must do more to help lessen the pain and complications associated with this disease by increasing the number of children who are screened for stroke and using the medication that can help reduce painful episodes,” said Karen Remley, MD, MPH, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a press release.
Bridging the gap
Providers, parents, health systems, and governmental agencies all have roles in bringing evidence-based recommended care to young SCA patients, Dr. Houry noted.
Community organizations can also help connect families with resources and tools to increase understanding.
Dr. Schieve pointed to access barriers in that families may have trouble traveling to specialized centers where the TCD screening is given. In addition, appointments for the screening may be limited.
Children taking hydroxyurea must be monitored for the proper dosage of medication, she explained, and that can be logistically challenging as well.
Providers report they often don’t get timely information back from TCD screening programs to keep up with which children need their annual screening.
Overall, the nation lacks providers with expertise in SCD and that can lead to symptoms being dismissed, Dr. Schieve said.
Hematologists and others have a role in advocating for patients with governmental entities to raise awareness of this issue, she added.
It’s also important that electronic health records give prompts and provide information so that all providers who care for a child can track screening and medication for the condition, Dr. Schieve and Dr. Houry said.
New funding for sickle cell data collection
Recent funding to the CDC Sickle Cell Data Collection Program may help more people get appropriate care, Dr. Houry said.
The program is currently active in 11 states and collects data from people all over the United States with SCD to study trends and treatment access for those with the disease.
The data help drive decisions such as where new sickle cell clinics are needed.
“We will expand to more states serving more people affected by this disease,” Dr. Houry said.
The authors declared no relevant financial relationships.
Fewer than half of children aged 2-16 years with sickle cell anemia are receiving recommended annual screening for stroke, a common complication of the disease, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
Many of these children also are not receiving the recommended medication, hydroxyurea, which can reduce pain and acute chest syndrome and improve anemia and quality of life, according to the report released Sept. 20.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD), which is a red blood cell disorder that primarily affects Black and African American people in the United States. It is associated with severe complications such as stroke, vison damage, frequent infections, and delayed growth, and a reduction in lifespan of more than 20 years.
SCD affects approximately 100,000 Americans and SCA accounts for about 75% of those cases.
Physician remembers her patients’ pain
In a briefing to reporters in advance of the report’s release, Debra Houry, MD, MPH, the CDC’s acting principal deputy director, recalled “long, tough nights with these young sickle cell warriors” in her career as an emergency department physician.
“[S]eeing children and teens suffering from the severe pain that often accompanies sickle cell anemia was heartbreaking,” she said.
She asked health care providers to confront racism as they build better systems for ensuring optimal treatment for children and adolescents with SCA.
“Health care providers can educate themselves, their colleagues, and their institutions about the specialized needs of people with sickle cell anemia, including how racism inhibits optimal care,” Dr. Houry said.
She said people with SCA report difficulty accessing care and when they do, they often report feeling stigmatized.
Lead author of the report, Laura Schieve, PhD, an epidemiologist with CDC’s National Center on Birth Defects and Developmental Disabilities, and colleagues looked at data from more than 3,300 children with SCA who were continuously enrolled in Medicaid during 2019. The data came from the IBM MarketScan Multi-State Medicaid Database.
Key recommendations issued in 2014
In 2014, the National Heart, Lung, and Blood Institute (NHLBI) issued two key recommendations to prevent or reduce complications in children and adolescents with SCA.
One was annual screening of children and adolescents aged 2-16 years with transcranial Doppler (TCD) ultrasound to identify those at risk for stroke. The second was offering hydroxyurea therapy, which keeps red blood cells from sickling and blocking small blood vessels, to children and adolescents who were at least 9 months old to reduce pain and the risk for several life-threatening complications.
The researchers, however, found that in 2019, only 47% and 38% of children and adolescents aged 2-9 and 10-16 years, respectively, had TCD screening and 38% and 53% of children and adolescents aged 2-9 years and 10-16 years, respectively, used hydroxyurea.
“These complications are preventable – not inevitable. We must do more to help lessen the pain and complications associated with this disease by increasing the number of children who are screened for stroke and using the medication that can help reduce painful episodes,” said Karen Remley, MD, MPH, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a press release.
Bridging the gap
Providers, parents, health systems, and governmental agencies all have roles in bringing evidence-based recommended care to young SCA patients, Dr. Houry noted.
Community organizations can also help connect families with resources and tools to increase understanding.
Dr. Schieve pointed to access barriers in that families may have trouble traveling to specialized centers where the TCD screening is given. In addition, appointments for the screening may be limited.
Children taking hydroxyurea must be monitored for the proper dosage of medication, she explained, and that can be logistically challenging as well.
Providers report they often don’t get timely information back from TCD screening programs to keep up with which children need their annual screening.
Overall, the nation lacks providers with expertise in SCD and that can lead to symptoms being dismissed, Dr. Schieve said.
Hematologists and others have a role in advocating for patients with governmental entities to raise awareness of this issue, she added.
It’s also important that electronic health records give prompts and provide information so that all providers who care for a child can track screening and medication for the condition, Dr. Schieve and Dr. Houry said.
New funding for sickle cell data collection
Recent funding to the CDC Sickle Cell Data Collection Program may help more people get appropriate care, Dr. Houry said.
The program is currently active in 11 states and collects data from people all over the United States with SCD to study trends and treatment access for those with the disease.
The data help drive decisions such as where new sickle cell clinics are needed.
“We will expand to more states serving more people affected by this disease,” Dr. Houry said.
The authors declared no relevant financial relationships.
FROM THE MMWR
Children and COVID: Weekly cases drop to lowest level since April
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.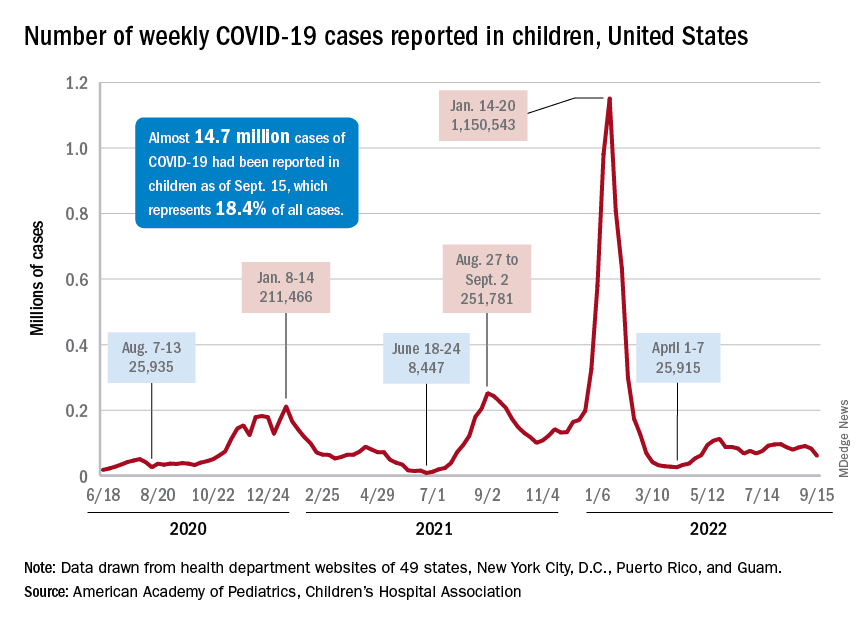
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.
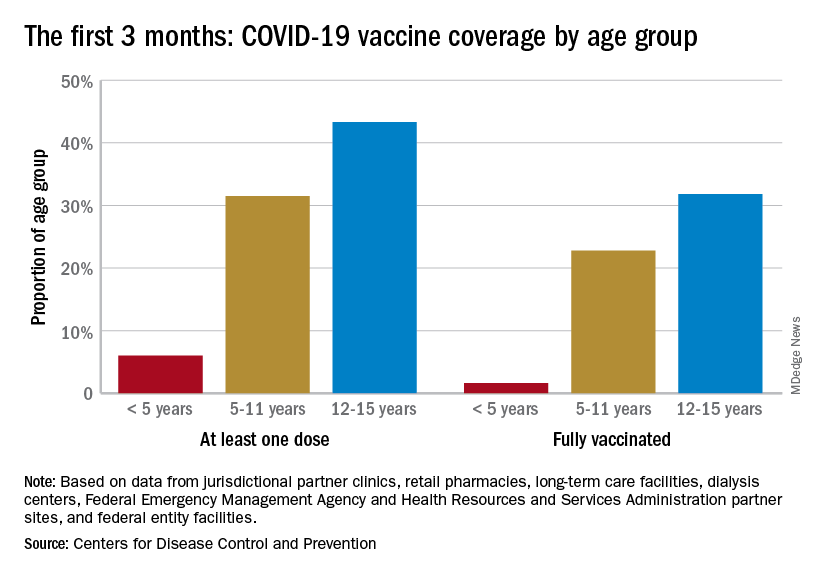
In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.

In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.

In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
Experts debate infant chiropractic care on TikTok
Several chiropractors in the United States are posting TikTok videos of themselves working with newborns, babies, and toddlers, often promoting treatments that aren’t backed by science, according to The Washington Post.
The videos include various devices and treatments, such as vibrating handheld massagers, spinal adjustments, and body movements, which are meant to address colic, constipation, reflux, musculoskeletal problems, and even trauma that babies experience during childbirth.
Chiropractors say the treatments are safe and gentle for babies and are unlike the more strenuous movements associated with adult chiropractic care. However, some doctors have said the videos are concerning because babies have softer bones and looser joints.
“Ultimately, there is no way you’re going to get an improvement in a newborn from a manipulation,” Sean Tabaie, MD, an orthopedic surgeon at Children’s National Hospital, Washington, told the newspaper.
Dr. Tabaie said his colleagues are shocked when he sends them Instagram or TikTok videos of chiropractic clinics treating infants.
“The only thing that you might possibly cause is harm,” he said.
Generally, chiropractors are licensed health professionals who use stretching, pressure, and joint manipulation on the spine to treat patients. Although chiropractic care is typically seen as an “alternative therapy,” some data in adults suggest that chiropractic treatments can help some conditions, such as low back pain.
“To my knowledge, there is little to no evidence that chiropractic care changes the natural history of any disease or condition,” Anthony Stans, MD, a pediatric orthopedic surgeon at Mayo Clinic Children’s Center, Rochester, Minn., told the newspaper. Stans said he would caution parents and recommend against chiropractic treatment for babies.
For some parents, the treatments and TikTok videos seem appealing because they promise relief for problems that traditional medicine can’t always address, especially colic, the newspaper reported. Colic, which features intense and prolonged crying in an otherwise healthy baby, tends to resolve over time without treatment.
Recent studies have attempted to study chiropractic care in infants. In a 2021 study, researchers in Denmark conducted a randomized controlled trial with 186 babies to test light pressure treatments. Although excessive crying was reduced by half an hour in the group that received treatment, the findings weren’t statistically significant in the end.
In a new study, researchers in Spain conducted a randomized trial with 58 babies to test “light touch manual therapy.” The babies who received treatment appeared to cry significantly less, but the parents weren’t “blinded” and were aware of the study’s treatment conditions, which can bias the results.
However, it can be challenging to “get that level of evidence” to support manual therapies such as chiropractic care, Joy Weydert, MD, director of pediatric integrative medicine at the University of Arizona, Tucson, told the newspaper. Certain treatments could help reduce the discomfort of colic or reflux, which can be difficult to measures in infants, she said.
The American Academy of Pediatrics told The Post that it doesn’t have an “official policy” on chiropractic care for infants or toddlers. At the same time, a 2017 report released by the organization concluded that “high-quality evidence” is lacking for spinal manipulation in children.
The American Chiropractic Association said chiropractic treatments are safe and effective for children, yet more research is needed to prove they work.
“We still haven’t been able to demonstrate in the research the effectiveness that we’ve seen clinically,” Jennifer Brocker, president of the group’s Council on Chiropractic Pediatrics, told the newspaper.
“We can’t really say for sure what’s happening,” she said. “It’s sort of like a black box. But what we do know is that, clinically, what we’re doing is effective because we see a change in the symptoms of the child.”
A version of this article first appeared on WebMD.com.
Several chiropractors in the United States are posting TikTok videos of themselves working with newborns, babies, and toddlers, often promoting treatments that aren’t backed by science, according to The Washington Post.
The videos include various devices and treatments, such as vibrating handheld massagers, spinal adjustments, and body movements, which are meant to address colic, constipation, reflux, musculoskeletal problems, and even trauma that babies experience during childbirth.
Chiropractors say the treatments are safe and gentle for babies and are unlike the more strenuous movements associated with adult chiropractic care. However, some doctors have said the videos are concerning because babies have softer bones and looser joints.
“Ultimately, there is no way you’re going to get an improvement in a newborn from a manipulation,” Sean Tabaie, MD, an orthopedic surgeon at Children’s National Hospital, Washington, told the newspaper.
Dr. Tabaie said his colleagues are shocked when he sends them Instagram or TikTok videos of chiropractic clinics treating infants.
“The only thing that you might possibly cause is harm,” he said.
Generally, chiropractors are licensed health professionals who use stretching, pressure, and joint manipulation on the spine to treat patients. Although chiropractic care is typically seen as an “alternative therapy,” some data in adults suggest that chiropractic treatments can help some conditions, such as low back pain.
“To my knowledge, there is little to no evidence that chiropractic care changes the natural history of any disease or condition,” Anthony Stans, MD, a pediatric orthopedic surgeon at Mayo Clinic Children’s Center, Rochester, Minn., told the newspaper. Stans said he would caution parents and recommend against chiropractic treatment for babies.
For some parents, the treatments and TikTok videos seem appealing because they promise relief for problems that traditional medicine can’t always address, especially colic, the newspaper reported. Colic, which features intense and prolonged crying in an otherwise healthy baby, tends to resolve over time without treatment.
Recent studies have attempted to study chiropractic care in infants. In a 2021 study, researchers in Denmark conducted a randomized controlled trial with 186 babies to test light pressure treatments. Although excessive crying was reduced by half an hour in the group that received treatment, the findings weren’t statistically significant in the end.
In a new study, researchers in Spain conducted a randomized trial with 58 babies to test “light touch manual therapy.” The babies who received treatment appeared to cry significantly less, but the parents weren’t “blinded” and were aware of the study’s treatment conditions, which can bias the results.
However, it can be challenging to “get that level of evidence” to support manual therapies such as chiropractic care, Joy Weydert, MD, director of pediatric integrative medicine at the University of Arizona, Tucson, told the newspaper. Certain treatments could help reduce the discomfort of colic or reflux, which can be difficult to measures in infants, she said.
The American Academy of Pediatrics told The Post that it doesn’t have an “official policy” on chiropractic care for infants or toddlers. At the same time, a 2017 report released by the organization concluded that “high-quality evidence” is lacking for spinal manipulation in children.
The American Chiropractic Association said chiropractic treatments are safe and effective for children, yet more research is needed to prove they work.
“We still haven’t been able to demonstrate in the research the effectiveness that we’ve seen clinically,” Jennifer Brocker, president of the group’s Council on Chiropractic Pediatrics, told the newspaper.
“We can’t really say for sure what’s happening,” she said. “It’s sort of like a black box. But what we do know is that, clinically, what we’re doing is effective because we see a change in the symptoms of the child.”
A version of this article first appeared on WebMD.com.
Several chiropractors in the United States are posting TikTok videos of themselves working with newborns, babies, and toddlers, often promoting treatments that aren’t backed by science, according to The Washington Post.
The videos include various devices and treatments, such as vibrating handheld massagers, spinal adjustments, and body movements, which are meant to address colic, constipation, reflux, musculoskeletal problems, and even trauma that babies experience during childbirth.
Chiropractors say the treatments are safe and gentle for babies and are unlike the more strenuous movements associated with adult chiropractic care. However, some doctors have said the videos are concerning because babies have softer bones and looser joints.
“Ultimately, there is no way you’re going to get an improvement in a newborn from a manipulation,” Sean Tabaie, MD, an orthopedic surgeon at Children’s National Hospital, Washington, told the newspaper.
Dr. Tabaie said his colleagues are shocked when he sends them Instagram or TikTok videos of chiropractic clinics treating infants.
“The only thing that you might possibly cause is harm,” he said.
Generally, chiropractors are licensed health professionals who use stretching, pressure, and joint manipulation on the spine to treat patients. Although chiropractic care is typically seen as an “alternative therapy,” some data in adults suggest that chiropractic treatments can help some conditions, such as low back pain.
“To my knowledge, there is little to no evidence that chiropractic care changes the natural history of any disease or condition,” Anthony Stans, MD, a pediatric orthopedic surgeon at Mayo Clinic Children’s Center, Rochester, Minn., told the newspaper. Stans said he would caution parents and recommend against chiropractic treatment for babies.
For some parents, the treatments and TikTok videos seem appealing because they promise relief for problems that traditional medicine can’t always address, especially colic, the newspaper reported. Colic, which features intense and prolonged crying in an otherwise healthy baby, tends to resolve over time without treatment.
Recent studies have attempted to study chiropractic care in infants. In a 2021 study, researchers in Denmark conducted a randomized controlled trial with 186 babies to test light pressure treatments. Although excessive crying was reduced by half an hour in the group that received treatment, the findings weren’t statistically significant in the end.
In a new study, researchers in Spain conducted a randomized trial with 58 babies to test “light touch manual therapy.” The babies who received treatment appeared to cry significantly less, but the parents weren’t “blinded” and were aware of the study’s treatment conditions, which can bias the results.
However, it can be challenging to “get that level of evidence” to support manual therapies such as chiropractic care, Joy Weydert, MD, director of pediatric integrative medicine at the University of Arizona, Tucson, told the newspaper. Certain treatments could help reduce the discomfort of colic or reflux, which can be difficult to measures in infants, she said.
The American Academy of Pediatrics told The Post that it doesn’t have an “official policy” on chiropractic care for infants or toddlers. At the same time, a 2017 report released by the organization concluded that “high-quality evidence” is lacking for spinal manipulation in children.
The American Chiropractic Association said chiropractic treatments are safe and effective for children, yet more research is needed to prove they work.
“We still haven’t been able to demonstrate in the research the effectiveness that we’ve seen clinically,” Jennifer Brocker, president of the group’s Council on Chiropractic Pediatrics, told the newspaper.
“We can’t really say for sure what’s happening,” she said. “It’s sort of like a black box. But what we do know is that, clinically, what we’re doing is effective because we see a change in the symptoms of the child.”
A version of this article first appeared on WebMD.com.
Apremilast alleviates severe psoriasis in some children, data show
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
FROM EADV 2022